User login
Don’t delay hip-fracture surgery
Background: Guidelines from the American College of Surgeons and Canadian Institute for Health recommend hip fracture surgery within 48 hours. However, a time-to-surgery threshold after which mortality and complications are increased has not been determined. This study aims to determine a time to surgery threshold for hip-fracture surgery.
Study design: Retrospective cohort trial.
Setting: 72 hospitals in Ontario, Ca., during April 1, 2009-March 31, 2014.
Synopsis: Of the 42,230 adult patients in this study, 14,174 (33.6%) received hip-fracture surgery within 24 hours of emergency department arrival. A matched patient analysis of early surgery (within 24 hours of ED arrival) vs. delayed surgery determined that patients undergoing early operation experienced lower 30-day mortality (5.8% vs 6.5%) and fewer complications (myocardial infarction, deep vein thrombosis, pulmonary embolism, and pneumonia). Major bleeding was not assessed as a complication. Also omitted from analysis were patients undergoing nonoperative hip-fracture management.
These findings suggest a time to surgery of 24 hours may represent a threshold defining higher risk. Two-thirds of patients in this study surpassed this threshold. Hospitalists seeing patients with hip fracture should balance time delay risks with the need for medical optimization.
Bottom line: Wait time greater than 24 hours for adults undergoing hip fracture surgery is associated with an increased risk of 30-day mortality and complications.
Citation: Pincus D et al. Association between wait time and 30-day mortality in adults undergoing hip fracture surgery. JAMA. 2017 Nov 28;318(20):1994-2003.
Dr. Moulder is assistant professor, University of Virginia Health System.
Background: Guidelines from the American College of Surgeons and Canadian Institute for Health recommend hip fracture surgery within 48 hours. However, a time-to-surgery threshold after which mortality and complications are increased has not been determined. This study aims to determine a time to surgery threshold for hip-fracture surgery.
Study design: Retrospective cohort trial.
Setting: 72 hospitals in Ontario, Ca., during April 1, 2009-March 31, 2014.
Synopsis: Of the 42,230 adult patients in this study, 14,174 (33.6%) received hip-fracture surgery within 24 hours of emergency department arrival. A matched patient analysis of early surgery (within 24 hours of ED arrival) vs. delayed surgery determined that patients undergoing early operation experienced lower 30-day mortality (5.8% vs 6.5%) and fewer complications (myocardial infarction, deep vein thrombosis, pulmonary embolism, and pneumonia). Major bleeding was not assessed as a complication. Also omitted from analysis were patients undergoing nonoperative hip-fracture management.
These findings suggest a time to surgery of 24 hours may represent a threshold defining higher risk. Two-thirds of patients in this study surpassed this threshold. Hospitalists seeing patients with hip fracture should balance time delay risks with the need for medical optimization.
Bottom line: Wait time greater than 24 hours for adults undergoing hip fracture surgery is associated with an increased risk of 30-day mortality and complications.
Citation: Pincus D et al. Association between wait time and 30-day mortality in adults undergoing hip fracture surgery. JAMA. 2017 Nov 28;318(20):1994-2003.
Dr. Moulder is assistant professor, University of Virginia Health System.
Background: Guidelines from the American College of Surgeons and Canadian Institute for Health recommend hip fracture surgery within 48 hours. However, a time-to-surgery threshold after which mortality and complications are increased has not been determined. This study aims to determine a time to surgery threshold for hip-fracture surgery.
Study design: Retrospective cohort trial.
Setting: 72 hospitals in Ontario, Ca., during April 1, 2009-March 31, 2014.
Synopsis: Of the 42,230 adult patients in this study, 14,174 (33.6%) received hip-fracture surgery within 24 hours of emergency department arrival. A matched patient analysis of early surgery (within 24 hours of ED arrival) vs. delayed surgery determined that patients undergoing early operation experienced lower 30-day mortality (5.8% vs 6.5%) and fewer complications (myocardial infarction, deep vein thrombosis, pulmonary embolism, and pneumonia). Major bleeding was not assessed as a complication. Also omitted from analysis were patients undergoing nonoperative hip-fracture management.
These findings suggest a time to surgery of 24 hours may represent a threshold defining higher risk. Two-thirds of patients in this study surpassed this threshold. Hospitalists seeing patients with hip fracture should balance time delay risks with the need for medical optimization.
Bottom line: Wait time greater than 24 hours for adults undergoing hip fracture surgery is associated with an increased risk of 30-day mortality and complications.
Citation: Pincus D et al. Association between wait time and 30-day mortality in adults undergoing hip fracture surgery. JAMA. 2017 Nov 28;318(20):1994-2003.
Dr. Moulder is assistant professor, University of Virginia Health System.
Lower risk of ICH with dabigatran as compared to warfarin in atrial fibrillation
Clinical question: Is dabigatran superior to warfarin with regards to the risk of intracranial hemorrhage and myocardial infarction in patients with atrial fibrillation?
Background: Several studies – including the RELY trial – have revealed a lower rate of intracranial hemorrhage with dabigatran, compared with warfarin, in patients with atrial fibrillation; however, few of these have been on populations generalizable to clinical practice. Furthermore, there are conflicting data on the association of dabigatran with higher rates of myocardial infarction in patients with atrial fibrillation versus warfarin.
Study design: Retrospective cohort study.
Setting: The Sentinel Program (national surveillance system) including 17 collaborating institutions and health care delivery systems.
Synopsis: In 25,289 propensity score–matched pairs of commercially insured patients aged 21 years or older starting dabigatran or warfarin therapy for atrial fibrillation, dabigatran was associated with a lower rate of intracranial hemorrhage (hazard ratio, 0.89; 95% confidence interval, 0.72-1.09), similar rates of ischemic stroke and extracranial hemorrhage, and a potentially higher rate of myocardial infarction (HR, 1.88; 95% CI, 1.22-2.90). However, the association between dabigatran use and myocardial infarction was smaller and not statistically significant in sensitivity analyses. Also, subgroup analyses demonstrated higher rates of gastrointestinal bleeding with dabigatran than did warfarin in patients aged 75 years or older and those with kidney dysfunction.
Although providing further reassuring evidence about bleeding risks – particularly intracranial hemorrhage – associated with dabigatran use, this observational study fails to clarify the association between dabigatran and myocardial infarction.
Bottom line: Dabigatran, compared with warfarin, in adults with atrial fibrillation is associated with a lower risk of intracranial hemorrhage and similar risk of ischemic stroke and extracranial hemorrhage.
Citation: Go AS et al. Outcomes of dabigatran and warfarin for atrial fibrillation in contemporary practice. Ann Intern Med. 2017 Dec.19;167:845-54.
Dr. Mehta is assistant professor, division of hospital medicine, University of Virginia.
Clinical question: Is dabigatran superior to warfarin with regards to the risk of intracranial hemorrhage and myocardial infarction in patients with atrial fibrillation?
Background: Several studies – including the RELY trial – have revealed a lower rate of intracranial hemorrhage with dabigatran, compared with warfarin, in patients with atrial fibrillation; however, few of these have been on populations generalizable to clinical practice. Furthermore, there are conflicting data on the association of dabigatran with higher rates of myocardial infarction in patients with atrial fibrillation versus warfarin.
Study design: Retrospective cohort study.
Setting: The Sentinel Program (national surveillance system) including 17 collaborating institutions and health care delivery systems.
Synopsis: In 25,289 propensity score–matched pairs of commercially insured patients aged 21 years or older starting dabigatran or warfarin therapy for atrial fibrillation, dabigatran was associated with a lower rate of intracranial hemorrhage (hazard ratio, 0.89; 95% confidence interval, 0.72-1.09), similar rates of ischemic stroke and extracranial hemorrhage, and a potentially higher rate of myocardial infarction (HR, 1.88; 95% CI, 1.22-2.90). However, the association between dabigatran use and myocardial infarction was smaller and not statistically significant in sensitivity analyses. Also, subgroup analyses demonstrated higher rates of gastrointestinal bleeding with dabigatran than did warfarin in patients aged 75 years or older and those with kidney dysfunction.
Although providing further reassuring evidence about bleeding risks – particularly intracranial hemorrhage – associated with dabigatran use, this observational study fails to clarify the association between dabigatran and myocardial infarction.
Bottom line: Dabigatran, compared with warfarin, in adults with atrial fibrillation is associated with a lower risk of intracranial hemorrhage and similar risk of ischemic stroke and extracranial hemorrhage.
Citation: Go AS et al. Outcomes of dabigatran and warfarin for atrial fibrillation in contemporary practice. Ann Intern Med. 2017 Dec.19;167:845-54.
Dr. Mehta is assistant professor, division of hospital medicine, University of Virginia.
Clinical question: Is dabigatran superior to warfarin with regards to the risk of intracranial hemorrhage and myocardial infarction in patients with atrial fibrillation?
Background: Several studies – including the RELY trial – have revealed a lower rate of intracranial hemorrhage with dabigatran, compared with warfarin, in patients with atrial fibrillation; however, few of these have been on populations generalizable to clinical practice. Furthermore, there are conflicting data on the association of dabigatran with higher rates of myocardial infarction in patients with atrial fibrillation versus warfarin.
Study design: Retrospective cohort study.
Setting: The Sentinel Program (national surveillance system) including 17 collaborating institutions and health care delivery systems.
Synopsis: In 25,289 propensity score–matched pairs of commercially insured patients aged 21 years or older starting dabigatran or warfarin therapy for atrial fibrillation, dabigatran was associated with a lower rate of intracranial hemorrhage (hazard ratio, 0.89; 95% confidence interval, 0.72-1.09), similar rates of ischemic stroke and extracranial hemorrhage, and a potentially higher rate of myocardial infarction (HR, 1.88; 95% CI, 1.22-2.90). However, the association between dabigatran use and myocardial infarction was smaller and not statistically significant in sensitivity analyses. Also, subgroup analyses demonstrated higher rates of gastrointestinal bleeding with dabigatran than did warfarin in patients aged 75 years or older and those with kidney dysfunction.
Although providing further reassuring evidence about bleeding risks – particularly intracranial hemorrhage – associated with dabigatran use, this observational study fails to clarify the association between dabigatran and myocardial infarction.
Bottom line: Dabigatran, compared with warfarin, in adults with atrial fibrillation is associated with a lower risk of intracranial hemorrhage and similar risk of ischemic stroke and extracranial hemorrhage.
Citation: Go AS et al. Outcomes of dabigatran and warfarin for atrial fibrillation in contemporary practice. Ann Intern Med. 2017 Dec.19;167:845-54.
Dr. Mehta is assistant professor, division of hospital medicine, University of Virginia.
Providers’ prior imaging patterns and ownership of equipment are strong predictors of low-value imaging
Background: For many common conditions, expert guidelines such as Choosing Wisely recommend against ordering specific low-value tests, yet overuse of these tests remains widespread, and unnecessary care may account for up to a third of all medical expenditures. Studies have demonstrated that there is considerable geographic variation in health care usage and higher overall imaging among clinicians who own imaging equipment. No prior study has assessed whether prior ordering patterns of low-value care predict future ordering patterns or whether providers who order low-value imaging in one clinical scenario are more likely to do so in another scenario.
Study design: Retrospective analysis of insurance claims data.
Setting: Medical claims data from a large U.S. commercial health insurer, inclusive of 29 million commercially insured members across all 50 states from January 2010 to December 2014.
Synopsis: Using the claims database, researchers created three unique study samples to examine clinician predictors of low-value imaging. The study involved outpatient visits by patients aged 18-64 years without red-flag symptoms. The first included 1,007,392 visits across 878,720 patients with acute, uncomplicated low-back pain. Physicians who ordered imaging for the prior patient with back pain were 1.8 times more likely to do so again. Similarly, in 492,804 visits by 417,010 patients with headache, clinicians who ordered imaging on the prior patient demonstrated a twofold higher odds of imaging. Physicians who practiced low-value ordering for one condition were 1.8 times more likely to do so for the other. Across all studies, imaging ownership was an independent predictor (odds ratio, 1.8).
As this study analyzed only claims data, some patients may have warranted imaging based on red-flag symptoms that were not documented. Hospitalists designing initiatives to reduce low-value testing should consider targeting specific providers with a history of low-value test use.
Bottom line: Among commercially insured patients, a clinician’s prior imaging pattern and ownership of imaging equipment were strong independent predictors of low-value back pain and headache imaging.
Citation: Hong AS et al. Clinician-level predictors for ordering low-value imaging. JAMA Intern Med. 2017 Nov 1;177(11):1577-85.
Background: For many common conditions, expert guidelines such as Choosing Wisely recommend against ordering specific low-value tests, yet overuse of these tests remains widespread, and unnecessary care may account for up to a third of all medical expenditures. Studies have demonstrated that there is considerable geographic variation in health care usage and higher overall imaging among clinicians who own imaging equipment. No prior study has assessed whether prior ordering patterns of low-value care predict future ordering patterns or whether providers who order low-value imaging in one clinical scenario are more likely to do so in another scenario.
Study design: Retrospective analysis of insurance claims data.
Setting: Medical claims data from a large U.S. commercial health insurer, inclusive of 29 million commercially insured members across all 50 states from January 2010 to December 2014.
Synopsis: Using the claims database, researchers created three unique study samples to examine clinician predictors of low-value imaging. The study involved outpatient visits by patients aged 18-64 years without red-flag symptoms. The first included 1,007,392 visits across 878,720 patients with acute, uncomplicated low-back pain. Physicians who ordered imaging for the prior patient with back pain were 1.8 times more likely to do so again. Similarly, in 492,804 visits by 417,010 patients with headache, clinicians who ordered imaging on the prior patient demonstrated a twofold higher odds of imaging. Physicians who practiced low-value ordering for one condition were 1.8 times more likely to do so for the other. Across all studies, imaging ownership was an independent predictor (odds ratio, 1.8).
As this study analyzed only claims data, some patients may have warranted imaging based on red-flag symptoms that were not documented. Hospitalists designing initiatives to reduce low-value testing should consider targeting specific providers with a history of low-value test use.
Bottom line: Among commercially insured patients, a clinician’s prior imaging pattern and ownership of imaging equipment were strong independent predictors of low-value back pain and headache imaging.
Citation: Hong AS et al. Clinician-level predictors for ordering low-value imaging. JAMA Intern Med. 2017 Nov 1;177(11):1577-85.
Background: For many common conditions, expert guidelines such as Choosing Wisely recommend against ordering specific low-value tests, yet overuse of these tests remains widespread, and unnecessary care may account for up to a third of all medical expenditures. Studies have demonstrated that there is considerable geographic variation in health care usage and higher overall imaging among clinicians who own imaging equipment. No prior study has assessed whether prior ordering patterns of low-value care predict future ordering patterns or whether providers who order low-value imaging in one clinical scenario are more likely to do so in another scenario.
Study design: Retrospective analysis of insurance claims data.
Setting: Medical claims data from a large U.S. commercial health insurer, inclusive of 29 million commercially insured members across all 50 states from January 2010 to December 2014.
Synopsis: Using the claims database, researchers created three unique study samples to examine clinician predictors of low-value imaging. The study involved outpatient visits by patients aged 18-64 years without red-flag symptoms. The first included 1,007,392 visits across 878,720 patients with acute, uncomplicated low-back pain. Physicians who ordered imaging for the prior patient with back pain were 1.8 times more likely to do so again. Similarly, in 492,804 visits by 417,010 patients with headache, clinicians who ordered imaging on the prior patient demonstrated a twofold higher odds of imaging. Physicians who practiced low-value ordering for one condition were 1.8 times more likely to do so for the other. Across all studies, imaging ownership was an independent predictor (odds ratio, 1.8).
As this study analyzed only claims data, some patients may have warranted imaging based on red-flag symptoms that were not documented. Hospitalists designing initiatives to reduce low-value testing should consider targeting specific providers with a history of low-value test use.
Bottom line: Among commercially insured patients, a clinician’s prior imaging pattern and ownership of imaging equipment were strong independent predictors of low-value back pain and headache imaging.
Citation: Hong AS et al. Clinician-level predictors for ordering low-value imaging. JAMA Intern Med. 2017 Nov 1;177(11):1577-85.
High 5-year mortality in patients admitted with heart failure regardless of ejection fraction
Background: Heart failure with preserved EF is a common cause of inpatient admission and previously was thought to carry a better prognosis than heart failure with reduced EF. Recent analysis using data from Get With the Guidelines–Heart Failure (GWTG-HF) registry has shown similarly poor survival rates at 30 days and 1 year when compared with heart failure with reduced EF.
Study design: Multicenter retrospective cohort study.
Setting: 276 hospitals in the GWTG-HF registry during 2005-2009, with 5 years of follow-up through 2014.
Synopsis: A total 39,982 patients who were admitted for heart failure during 2005-2009 were included in the study with stratification into three groups based on ejection fraction; 18,398 (46%) with heart failure with reduced EF; 2,385 (8.2%) with heart failure with borderline EF; and 18,299 (46%) with heart failure with preserved EF. The 5-year mortality rate for the entire cohort was 75.4% with similar mortality rates for patient with preserved EF (75.3%), compared with those with reduced EF (75.7%).
Bottom line: Among patients hospitalized with heart failure, irrespective of their ejection fraction, the 5-year survival rates were equally dismal. Hospitalists may wish to use this information in goals of care discussions.
Citation: Shah KS et al. Heart failure with preserved, borderline, and reduced ejection fraction: 5-year outcomes. J Am Coll Cardiol. 2017 Oct 31. doi: 10.1016/j.jacc.2017.08.074.
Dr. Gomez-Sanchez is a hospitalist at the University of Virginia Medical Center.
Background: Heart failure with preserved EF is a common cause of inpatient admission and previously was thought to carry a better prognosis than heart failure with reduced EF. Recent analysis using data from Get With the Guidelines–Heart Failure (GWTG-HF) registry has shown similarly poor survival rates at 30 days and 1 year when compared with heart failure with reduced EF.
Study design: Multicenter retrospective cohort study.
Setting: 276 hospitals in the GWTG-HF registry during 2005-2009, with 5 years of follow-up through 2014.
Synopsis: A total 39,982 patients who were admitted for heart failure during 2005-2009 were included in the study with stratification into three groups based on ejection fraction; 18,398 (46%) with heart failure with reduced EF; 2,385 (8.2%) with heart failure with borderline EF; and 18,299 (46%) with heart failure with preserved EF. The 5-year mortality rate for the entire cohort was 75.4% with similar mortality rates for patient with preserved EF (75.3%), compared with those with reduced EF (75.7%).
Bottom line: Among patients hospitalized with heart failure, irrespective of their ejection fraction, the 5-year survival rates were equally dismal. Hospitalists may wish to use this information in goals of care discussions.
Citation: Shah KS et al. Heart failure with preserved, borderline, and reduced ejection fraction: 5-year outcomes. J Am Coll Cardiol. 2017 Oct 31. doi: 10.1016/j.jacc.2017.08.074.
Dr. Gomez-Sanchez is a hospitalist at the University of Virginia Medical Center.
Background: Heart failure with preserved EF is a common cause of inpatient admission and previously was thought to carry a better prognosis than heart failure with reduced EF. Recent analysis using data from Get With the Guidelines–Heart Failure (GWTG-HF) registry has shown similarly poor survival rates at 30 days and 1 year when compared with heart failure with reduced EF.
Study design: Multicenter retrospective cohort study.
Setting: 276 hospitals in the GWTG-HF registry during 2005-2009, with 5 years of follow-up through 2014.
Synopsis: A total 39,982 patients who were admitted for heart failure during 2005-2009 were included in the study with stratification into three groups based on ejection fraction; 18,398 (46%) with heart failure with reduced EF; 2,385 (8.2%) with heart failure with borderline EF; and 18,299 (46%) with heart failure with preserved EF. The 5-year mortality rate for the entire cohort was 75.4% with similar mortality rates for patient with preserved EF (75.3%), compared with those with reduced EF (75.7%).
Bottom line: Among patients hospitalized with heart failure, irrespective of their ejection fraction, the 5-year survival rates were equally dismal. Hospitalists may wish to use this information in goals of care discussions.
Citation: Shah KS et al. Heart failure with preserved, borderline, and reduced ejection fraction: 5-year outcomes. J Am Coll Cardiol. 2017 Oct 31. doi: 10.1016/j.jacc.2017.08.074.
Dr. Gomez-Sanchez is a hospitalist at the University of Virginia Medical Center.
Use procalcitonin-guided algorithms to guide antibiotic therapy for acute respiratory infections to improve patient outcomes
Clinical question: How does using procalcitonin levels for adults with acute respiratory infections (ARIs) affect patient outcomes?
Background: While the ARI diagnosis encompasses bacterial, viral, and inflammatory etiologies, as many as 75% of ARIs are treated with antibiotics. Procalcitonin is a biomarker released by tissues in response to bacterial infections. Its production is also inhibited by interferon-gamma, a cytokine released in response to viral infections, therefore, making procalcitonin a biomarker of particular interest to support the use of antibiotic therapy in the treatment of ARIs.
Study design: Cochrane Review.
Setting: Medical wards, intensive care units, primary care clinics, and emergency departments across 12 countries.
Synopsis: The review included 26 randomized control trials of 6,708 immunocompetent adults with ARIs who received antibiotics either based on procalcitonin-guided antibiotic therapy or routine care. Primary endpoints evaluated included all-cause mortality and treatment failure at 30 days. Secondary endpoints were antibiotic use, antibiotic-related side effects, and length of hospital stay. There were significantly fewer deaths in the procalcitonin-guided group than in the control group (286/8.6% vs. 336/10%; adjusted odds ratio, 0.83; 95% confidence interval, 0.70-0.99; P = .037). Treatment failure was not statistically different between the procalcitonin-guided participants and the controls. Of the secondary endpoints, antibiotic use and antibiotic-related side effects were lower in the procalcitonin-guided group (5.7 days vs. 8.1 days; P less than .001; and 16.3% vs. 22.1%; P less than .001). Each of the RCTs had varying algorithms for the use of procalcitonin-guided therapy, so no specific treatment guidelines can be gleaned from this review.
Bottom line: Procalcitonin-guided algorithms are associated with lower mortality, lower antibiotic exposure, and lower antibiotic-related side effects. However, more research is needed to determine best practice algorithms for using procalcitonin levels to guide treatment decisions.
Citation: Schuetz P et al. Procalcitonin to initiate or discontinue antibiotics in acute respiratory tract infections. Cochrane Database Syst Rev. 2017 Oct 12. doi: 10.1002/14651858.cd007498.pub3.
Dr. Sundar is assistant professor of medicine in the division of hospital medicine, Emory University, Atlanta.
Clinical question: How does using procalcitonin levels for adults with acute respiratory infections (ARIs) affect patient outcomes?
Background: While the ARI diagnosis encompasses bacterial, viral, and inflammatory etiologies, as many as 75% of ARIs are treated with antibiotics. Procalcitonin is a biomarker released by tissues in response to bacterial infections. Its production is also inhibited by interferon-gamma, a cytokine released in response to viral infections, therefore, making procalcitonin a biomarker of particular interest to support the use of antibiotic therapy in the treatment of ARIs.
Study design: Cochrane Review.
Setting: Medical wards, intensive care units, primary care clinics, and emergency departments across 12 countries.
Synopsis: The review included 26 randomized control trials of 6,708 immunocompetent adults with ARIs who received antibiotics either based on procalcitonin-guided antibiotic therapy or routine care. Primary endpoints evaluated included all-cause mortality and treatment failure at 30 days. Secondary endpoints were antibiotic use, antibiotic-related side effects, and length of hospital stay. There were significantly fewer deaths in the procalcitonin-guided group than in the control group (286/8.6% vs. 336/10%; adjusted odds ratio, 0.83; 95% confidence interval, 0.70-0.99; P = .037). Treatment failure was not statistically different between the procalcitonin-guided participants and the controls. Of the secondary endpoints, antibiotic use and antibiotic-related side effects were lower in the procalcitonin-guided group (5.7 days vs. 8.1 days; P less than .001; and 16.3% vs. 22.1%; P less than .001). Each of the RCTs had varying algorithms for the use of procalcitonin-guided therapy, so no specific treatment guidelines can be gleaned from this review.
Bottom line: Procalcitonin-guided algorithms are associated with lower mortality, lower antibiotic exposure, and lower antibiotic-related side effects. However, more research is needed to determine best practice algorithms for using procalcitonin levels to guide treatment decisions.
Citation: Schuetz P et al. Procalcitonin to initiate or discontinue antibiotics in acute respiratory tract infections. Cochrane Database Syst Rev. 2017 Oct 12. doi: 10.1002/14651858.cd007498.pub3.
Dr. Sundar is assistant professor of medicine in the division of hospital medicine, Emory University, Atlanta.
Clinical question: How does using procalcitonin levels for adults with acute respiratory infections (ARIs) affect patient outcomes?
Background: While the ARI diagnosis encompasses bacterial, viral, and inflammatory etiologies, as many as 75% of ARIs are treated with antibiotics. Procalcitonin is a biomarker released by tissues in response to bacterial infections. Its production is also inhibited by interferon-gamma, a cytokine released in response to viral infections, therefore, making procalcitonin a biomarker of particular interest to support the use of antibiotic therapy in the treatment of ARIs.
Study design: Cochrane Review.
Setting: Medical wards, intensive care units, primary care clinics, and emergency departments across 12 countries.
Synopsis: The review included 26 randomized control trials of 6,708 immunocompetent adults with ARIs who received antibiotics either based on procalcitonin-guided antibiotic therapy or routine care. Primary endpoints evaluated included all-cause mortality and treatment failure at 30 days. Secondary endpoints were antibiotic use, antibiotic-related side effects, and length of hospital stay. There were significantly fewer deaths in the procalcitonin-guided group than in the control group (286/8.6% vs. 336/10%; adjusted odds ratio, 0.83; 95% confidence interval, 0.70-0.99; P = .037). Treatment failure was not statistically different between the procalcitonin-guided participants and the controls. Of the secondary endpoints, antibiotic use and antibiotic-related side effects were lower in the procalcitonin-guided group (5.7 days vs. 8.1 days; P less than .001; and 16.3% vs. 22.1%; P less than .001). Each of the RCTs had varying algorithms for the use of procalcitonin-guided therapy, so no specific treatment guidelines can be gleaned from this review.
Bottom line: Procalcitonin-guided algorithms are associated with lower mortality, lower antibiotic exposure, and lower antibiotic-related side effects. However, more research is needed to determine best practice algorithms for using procalcitonin levels to guide treatment decisions.
Citation: Schuetz P et al. Procalcitonin to initiate or discontinue antibiotics in acute respiratory tract infections. Cochrane Database Syst Rev. 2017 Oct 12. doi: 10.1002/14651858.cd007498.pub3.
Dr. Sundar is assistant professor of medicine in the division of hospital medicine, Emory University, Atlanta.
Myelodysplastic syndromes: etiologies, evaluation, and therapy
In this interview, Dr David Henry, MD, the Editor-in-Chief of JCSO, and David Steensma, MD, of the Dana-Farber Cancer Institute in Boston, talk about myelodysplasic syndromes, from diagnosis, evaluation, and etiologies, to therapy options and molecular sequencing.
Listen to the podcast below, or click on the PDF icon at the top of this introduction to read a transcript of the interview.
In this interview, Dr David Henry, MD, the Editor-in-Chief of JCSO, and David Steensma, MD, of the Dana-Farber Cancer Institute in Boston, talk about myelodysplasic syndromes, from diagnosis, evaluation, and etiologies, to therapy options and molecular sequencing.
Listen to the podcast below, or click on the PDF icon at the top of this introduction to read a transcript of the interview.
In this interview, Dr David Henry, MD, the Editor-in-Chief of JCSO, and David Steensma, MD, of the Dana-Farber Cancer Institute in Boston, talk about myelodysplasic syndromes, from diagnosis, evaluation, and etiologies, to therapy options and molecular sequencing.
Listen to the podcast below, or click on the PDF icon at the top of this introduction to read a transcript of the interview.
Delaying lumbar punctures for a head CT may result in increased mortality in acute bacterial meningitis
Background: ABM is a diagnosis with high morbidity and mortality. Early antimicrobial and corticosteroid therapy is beneficial. Current practice tends to defer LP prior to imaging when there is potential risk of herniation. Sweden’s guidelines for getting a CT scan prior to LP differ substantially from the Infectious Disease Society of America (IDSA), which recommends obtaining CT in patients with immunocompromised state, history of CNS disease, or impaired mental status.
Study design: Prospective cohort study.
Setting: 815 adult patients (older than 16 years old) in Sweden with confirmed acute bacterial meningitis.
Synopsis: The authors looked at adherence to guidelines for when to obtain a CT prior to LP, as well as compared mortality and neurologic outcomes when an LP was performed promptly versus when delayed for prior neuroimaging. CT neuroimaging was required in much smaller populations under Swedish guidelines (7%), compared with IDSA (65%), with improved mortality and outcomes in patients managed with the Swedish guidelines. Mortality was lower in patients who had a prompt LP than for those who got CT prior to the LP (4% vs. 10%). This mortality benefit was seen even in patients with immunocompromised state or altered mental status, confirming that earlier administration of appropriate therapy is associated with lower mortality. A major limitation is that the study included patients with confirmed meningitis rather than more clinically relevant cases of suspected bacterial meningitis.
Bottom line: Patients with suspected bacterial meningitis should have appropriate antimicrobial and corticosteroid therapy started as soon as possible, regardless of the decision to obtain CT scan prior to performing lumbar puncture.
Citation: Glimaker M et al. Lumbar puncture performed promptly or after neuroimaging in acute bacterial meningitis in adults: a prospective national cohort study evaluating different guidelines. Clin Infect Dis. 2017 Sep 9. doi: 10.1093/cid/cix806 (epub ahead of print).
Dr. Maleque is assistant professor of medicine in the division of hospital medicine, Emory University, Atlanta.
Background: ABM is a diagnosis with high morbidity and mortality. Early antimicrobial and corticosteroid therapy is beneficial. Current practice tends to defer LP prior to imaging when there is potential risk of herniation. Sweden’s guidelines for getting a CT scan prior to LP differ substantially from the Infectious Disease Society of America (IDSA), which recommends obtaining CT in patients with immunocompromised state, history of CNS disease, or impaired mental status.
Study design: Prospective cohort study.
Setting: 815 adult patients (older than 16 years old) in Sweden with confirmed acute bacterial meningitis.
Synopsis: The authors looked at adherence to guidelines for when to obtain a CT prior to LP, as well as compared mortality and neurologic outcomes when an LP was performed promptly versus when delayed for prior neuroimaging. CT neuroimaging was required in much smaller populations under Swedish guidelines (7%), compared with IDSA (65%), with improved mortality and outcomes in patients managed with the Swedish guidelines. Mortality was lower in patients who had a prompt LP than for those who got CT prior to the LP (4% vs. 10%). This mortality benefit was seen even in patients with immunocompromised state or altered mental status, confirming that earlier administration of appropriate therapy is associated with lower mortality. A major limitation is that the study included patients with confirmed meningitis rather than more clinically relevant cases of suspected bacterial meningitis.
Bottom line: Patients with suspected bacterial meningitis should have appropriate antimicrobial and corticosteroid therapy started as soon as possible, regardless of the decision to obtain CT scan prior to performing lumbar puncture.
Citation: Glimaker M et al. Lumbar puncture performed promptly or after neuroimaging in acute bacterial meningitis in adults: a prospective national cohort study evaluating different guidelines. Clin Infect Dis. 2017 Sep 9. doi: 10.1093/cid/cix806 (epub ahead of print).
Dr. Maleque is assistant professor of medicine in the division of hospital medicine, Emory University, Atlanta.
Background: ABM is a diagnosis with high morbidity and mortality. Early antimicrobial and corticosteroid therapy is beneficial. Current practice tends to defer LP prior to imaging when there is potential risk of herniation. Sweden’s guidelines for getting a CT scan prior to LP differ substantially from the Infectious Disease Society of America (IDSA), which recommends obtaining CT in patients with immunocompromised state, history of CNS disease, or impaired mental status.
Study design: Prospective cohort study.
Setting: 815 adult patients (older than 16 years old) in Sweden with confirmed acute bacterial meningitis.
Synopsis: The authors looked at adherence to guidelines for when to obtain a CT prior to LP, as well as compared mortality and neurologic outcomes when an LP was performed promptly versus when delayed for prior neuroimaging. CT neuroimaging was required in much smaller populations under Swedish guidelines (7%), compared with IDSA (65%), with improved mortality and outcomes in patients managed with the Swedish guidelines. Mortality was lower in patients who had a prompt LP than for those who got CT prior to the LP (4% vs. 10%). This mortality benefit was seen even in patients with immunocompromised state or altered mental status, confirming that earlier administration of appropriate therapy is associated with lower mortality. A major limitation is that the study included patients with confirmed meningitis rather than more clinically relevant cases of suspected bacterial meningitis.
Bottom line: Patients with suspected bacterial meningitis should have appropriate antimicrobial and corticosteroid therapy started as soon as possible, regardless of the decision to obtain CT scan prior to performing lumbar puncture.
Citation: Glimaker M et al. Lumbar puncture performed promptly or after neuroimaging in acute bacterial meningitis in adults: a prospective national cohort study evaluating different guidelines. Clin Infect Dis. 2017 Sep 9. doi: 10.1093/cid/cix806 (epub ahead of print).
Dr. Maleque is assistant professor of medicine in the division of hospital medicine, Emory University, Atlanta.
Neuromodulation for Treatment-Refractory PTSD (FULL)
Failure of fear extinction is a core feature of posttraumatic stress disorder (PTSD).1 Recently, it was confirmed that the amygdala and the orbitofrontal cortex are crucial for both fear acquisition and fear extinction.2 The amygdala was found to have neurons active only during fear acquisition, and other neurons active only during fear extinction.3 In essence, the balance of activity between these 2 neuronal populations determines whether if an incoming stimulus is feared or not feared. This balance is under the influence of several cognitive domains, including memory, reward, and executive function.
In PTSD, the equilibrium is shifted heavily toward fear acquisition. The majority of patients spontaneously regain the capacity for fear extinction over time4 or with the help of treatment.5,6 Nonetheless, some patients with severe PTSD seem unable to recover the ability of fear extinction and remain refractory to both standard and novel psychotherapeutic or psychopharmacologic treatments.7 For these patients, direct modulation of the neural activity in the amygdala may permit fear extinction. This article describes the rationale for using deep brain stimulation (DBS) and initial results from the first-ever clinical trial.
Deep Brain Stimulation
Deep brain stimulation involves inserting electrodes in precise cerebral targets and then connecting the leads to a pulse generator (similar to a pacemaker) inserted in a subclavicular pocket. The generator controls the electrical signal (amplitude, pulse width, pulse frequency) delivered to the brain target and can be adjusted with use of a noninvasive programmer. In 1997, the FDA approved DBS for patients with Parkinson disease or essential tremor. Since then, its efficacy in these movement disorders has been confirmed in several studies.8,9
The mechanism by which the small electrical pulses of DBS influence activity is not clear. Clinically, DBS functionally inhibits the activity of local neurons.10 One theory describes “frequency jamming,” a concept similar to cardiac overdrive pacing in which the resultant high-frequency neuronal signal is meaningless and discounted by the rest of the brain.11
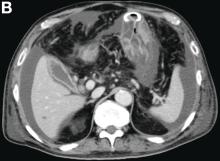
Over the years, DBS has demonstrated a strong safety profile.12 The risks of electrode insertion are mitigated with targeting based on high-quality magnetic resonance imaging (MRI) and computed tomography (Figure). Unlike a destructive lesion, DBS is reversible, and the implanted system can be removed in its entirety. Histologic analyses have shown only a small amount of scarring around the electrode tip.13 In movement disorder treatment, clinical experience has shown that stimulation-related adverse effects (AEs) are reversible with readjustment of stimulation parameters by external programmer.14
Novel Applications of DBS
The advantageous safety profile of DBS has permitted its evaluation in the treatment of other conditions thought to have malfunctioning networks at their core—such as intractable epilepsy (in resective surgery noncandidates).15,16 Although several trials have shown promising results of using DBS for treatment-resistant depression,17 the results of pivotal sham-controlled trials have been mixed.18,19 Obsessive-compulsive disorder, on the other hand, received the FDA humanitarian device exemption designation on the basis of positive long-term results.20 In treatment-resistant depression and obsessive-compulsive disorder, functional neuroimaging has identified DBS targets.21,22 Functional MRI or positron emission tomography (PET) images can be compared at resting state, at symptomatic state, and after treatment response. Nodes hyperactive during a symptomatic state and less active after successful treatment can be targeted with high-frequency DBS to directly reduce the hyperactivity and indirectly modulate or normalize the overall function of the circuit.23
Given the functional MRI and O15 (oxygen-15) PET evidence of amygdala hyperactivity in patients with PTSD having core symptoms,24-26 the authors hypothesized that high-frequency DBS targeting of the amygdala would improve PTSD-associated hyperarousal and reexperiencing symptoms in treatment-refractory patients. Indirect data supporting this hypothesis include a correlation between amygdala hyperactivity of increased intensity and symptom severity measured with the Clinician-Administered PTSD Scale (CAPS),27 and a correlation between reduced pretreatment amygdala hyperactivity and successful cognitive-behavioral treatment.28,29
Preclinical Work
Using a rodent model in which a novel object serves as a cue reminder of foot shocks (traumatic event), the authors tested the hypothesis that amygdala DBS would reduce PTSD-like symptoms.30 When untreated rats were presented with the object in their cage a week after the initial exposure, they immediately buried the object under bedding to avoid being reminded of the shocks. In contrast, rats treated with DBS did not bury the object. In most cases, in fact, they played with it.
The authors also replicated their results but with the addition of rats treated with paroxetine.31 Using the same rodent model, they found DBS superior to paroxetine in treating PTSD-like symptoms. This study had a crossover design: DBS and sham DBS. Briefly, 20 rats received an electrode in the amygdala and were exposed to inescapable shocks in the presence of the cue object. The rats were then randomly assigned to a DBS group (10 rats) or a sham-DBS group (10 rats). After 1 week, behavioral testing showed fear extinction in the DBS group and no improvement in the sham-DBS group. Then the groups were switched: The rats originally treated with DBS received no treatment, and the rats that were originally sham-treated underwent DBS. One week later, behavioral testing showed acquisition of fear extinction in all the rats. These results suggested DBS can be effective even when delayed after establishment of fear persistence and PTSD symptoms. These results also showed that DBS effects persist even after therapy discontinuation.
Similarly, other investigators have reported that the role of the amygdala is not limited to fear acquisition; it extends to fear expression. A lesion in the amygdala can prevent fear expression even if the disruption is performed subsequent to fear-conditioning training.32 This finding is important for humans, as DBS would be initiated during the chronic phase of the disorder, after failure of less invasive treatment options, such as pharmacotherapy and psychotherapy.
Early Clinical Experience
The authors have initiated the first ever clinical trial (NCT02091843) evaluating use of DBS for PTSD and are now recruiting patients. Enrollment is limited to 6 combat veterans with disabling PTSD that has not responded to pharmacotherapy and psychotherapy. This VA-funded single-site study, being conducted at the VA Greater Los Angeles Healthcare System (VAGLAHS), was approved by the VAGLAHS Institutional Review Board and the FDA. The authors have published the 2-year trial’s protocol, which includes an active-versus-sham stimulation phase; continuous electroencephalogram monitoring; baseline and posttreatment 18FDG (fluorodeoxyglucose) PET performed during a resting state vs during investigator-guided exposure to trauma reminders; and extensive psychological and neuropsychological assessments.33 The literature includes only 1 case report on amygdala DBS.34 The authors of that report used DBS of the basolateral nucleus of the amygdala to treat a teenaged boy with severe autism and found that the therapy was safe.
As of this writing, the authors have recruited and implanted 1 patient and reported on his clinical results (including baseline PET) over the first 8 months of stimulation35 and on the electrophysiologic findings over the first year.36 After experiencing extremely severe combat PTSD refractory to pharmacotherapy and psychotherapy treatments for more than 20 years, the patient treated with DBS is now experiencing substantial symptom relief, and his CAPS score (primary outcome measure) has improved by about 40%. He has tolerated continuous stimulation without any serious DBS-related AEs for up to 16 months. Notably, he has not had a single severe combat nightmare in a year—in stark contrast to nightly combat nightmares during the 20-year period leading to the trial. Furthermore, he has not been having any episodes of severe dissociation, which had been a common disabling problem before the trial. He has taken a second trip out of the country, improved his relationships with family, and made strides (albeit limited) in pursuing additional social interactions.
Avoidance remains a major problem. He recently left his job after 7 years, because he prefers a more nature-oriented rather than people-oriented environment. In addition, his interest in intensive psychotherapy has increased, and he has been considering options for spending more time working on his therapy.
Over 15 months of treatment, the patient’s CAPS total and subscale scores have decreased—his symptoms have improved (Table).21 He has had rapid and substantial reductions in recurrence and hyperarousal symptoms but slower improvement in avoidance. Improvements in emotional reactivity would be expected to occur before any change in behavior (eg, avoidance). Patients likely must first recognize changes in emotional reactivity to events before they can engage in a cognitive process to modify learned behavioral responses to those events.
After about 9 months of treatment, all of the study patient’s symptoms were somewhat stabilized, and the authors began making gradual stimulation adjustments to the latest parameters—3.5 V, 60 µs, and 160 Hz for the right electrode and 1.5 V, 60 µs, and 160 Hz for the left electrode—using the contacts in the basolateral nucleus of the amygdala, per postoperative neuroimaging.3
After 15 to 18 months, when improvement peaked at 48% symptom reduction from baseline, the patient experienced psychiatric decompensation (depression, suicide gesture) not attributable to changes in stimulation settings and not associated with exacerbation of PTSD symptoms. Treatment team members and independent psychiatric consultants attributed the decompensation to the patient’s difficulty in changing a long-standing avoidant behavior routine, owing to severe recurrence and hyperarousal symptoms in the past. His persistent inability to overcome avoidance and isolation, despite core PTSD symptom improvement, had left him feeling worthless.
The patient remains in the study but also is participating in other medication and psychotherapy trials and is making a career change. Periodic decompensations may be part of the treatment course as patients reach a more complex and volatile phase of improvement that requires more intensive cognitive reprocessing. If this proves to be the case with other patients enrolling in the study, intensive psychotherapy that addresses cognitive and emotional PTSD symptoms may be needed once there is improvement in intrusive and hyperarousal symptoms.
Conclusion
Deep brain stimulation has been successful in treating Parkinson disease and essential tremor. Physiologically, DBS seems to inhibit specific brain regions’ dysfunctional activity stemming from a disease process. Deep brain stimulation-induced inhibition of a dysfunctional node improves clinical outcomes in movement disorders.
Given the reversibility and positive safety profile of DBS, new applications are being studied. The authors propose that DBS may benefit patients with severe treatment-refractory PTSD. Their first patient’s core PTSD symptoms have improved significantly, as expected, but as in other psychiatric DBS cases, the seriousness and chronicity of his illness may be complicating the course of recovery. The authors plan to recruit 6 patients for this early-phase safety trial.
Click here to read the digital edition.
1. Milad MR, Pitman RK, Ellis CB, et al. Neurobiological basis of failure to recall extinction memory in posttraumatic stress disorder. Biol Psychiatry. 2009;66(12):1075-1082.
2. Marin MF, Song H, VanElzakker MB, et al. Association of resting metabolism in the fear neural network with extinction recall activations and clinical measures in trauma-exposed individuals. Am J Psychiatry. 2016;173(9):930-938.
3. Herry C, Ciocchi S, Senn V, Demmou L, Müller C, Lüthi A. Switching on and off fear by distinct neuronal circuits. Nature. 2008;454(7204):600-606.
4. Morina N, Wicherts JM, Lobbrecht J, Priebe S. Remission from post-traumatic stress disorder in adults: a systematic review and meta-analysis of long term outcome studies. Clin Psychol Rev. 2014;34(3):249-255.
5. Steenkamp MM, Litz BT, Hoge CW, Marmar CR. Psychotherapy for military-related PTSD: a review of randomized clinical trials. JAMA. 2015;314(5):489-500.
6. Hoskins M, Pearce J, Bethell A, et al. Pharmacotherapy for post-traumatic stress disorder: systematic review and meta-analysis. Br J Psychiatry. 2015;206(2):93-100.
7. Koek RJ, Schwartz HN, Scully S, et al. Treatment-refractory posttraumatic stress disorder (TRPTSD): a review and framework for the future. Prog Neuropsychopharmacol Biol Psychiatry. 2016;70:170-218.
8. Wagle Shukla A, Okun MS. State of the art for deep brain stimulation therapy in movement disorders: a clinical and technological perspective. IEEE Rev Biomed Eng. 2016;9:219-233.
9. Weaver FM, Follett K, Stern M, et al; CSP 468 Study Group. Bilateral deep brain stimulation vs best medical therapy for patients with advanced Parkinson disease: a randomized controlled trial. JAMA. 2009;301(1):63-73.
10. Benabid AL, Benazzouz A, Hoffmann D, Limousin P, Krack P, Pollack P. Long-term electrical inhibition of deep brain targets in movement disorders. Mov Disord. 1998;13(suppl 3):119-125.
11. Benabid AL, Wallace B, Mitrofanis J, et al. A putative generalized model of the effects and mechanism of action of high frequency electrical stimulation of the central nervous system. Acta Neurol Belg. 2005;105(3):149-157.
12. Fenoy AJ, Simpson RK Jr. Risks of common complications in deep brain stimulation surgery: management and avoidance. J Neurosurg. 2014;120(1):132-139.
13. DiLorenzo DJ, Jankovic J, Simpson RK, Takei H, Powell SZ. Neurohistopathological findings at the electrode–tissue interface in long-term deep brain stimulation: systematic literature review, case report, and assessment of stimulation threshold safety. Neuromodulation. 2014;17(5):405-418.
14. Revell MA. Deep brain stimulation for movement disorders. Nurs Clin North Am. 2015;50(4):691-701.
15. Fisher R, Salanova V, Witt T, et al; SANTE Study Group. Electrical stimulation of the anterior nucleus of thalamus for treatment of refractory epilepsy. Epilepsia. 2010;51(5):899-908.
16. Salanova V, Witt T, Worth R, et al; SANTE Study Group. Long-term efficacy and safety of thalamic stimulation for drug-resistant partial epilepsy. Neurology. 2015;84(10):1017-1025.
17. Berlim MT, McGirr A, Van den Eynde F, Fleck MP, Giacobbe P. Effectiveness and acceptability of deep brain stimulation (DBS) of the subgenual cingulate cortex for treatment-resistant depression: a systematic review and exploratory meta-analysis. J Affect Disord. 2014;159:31-38.
18. Dougherty DD, Rezai AR, Carpenter LL, et al. A randomized sham-controlled trial of deep brain stimulation of the ventral capsule/ventral striatum for chronic treatment-resistant depression. Biol Psychiatry. 2015;78(4):240-248.
19. Bergfeld IO, Mantione M, Hoogendoorn ML, et al. Deep brain stimulation of the ventral anterior limb of the internal capsule for treatment-resistant depression: a randomized clinical trial. JAMA Psychiatry. 2016;73(5):456-464.
20. Greenberg BD, Malone DA, Friehs GM, et al. Three-year outcomes in deep brain stimulation for highly resistant obsessive-compulsive disorder. Neuropsychopharmacology. 2006;31(11):2384-2393.
21. Mayber HS, Liotti M, Brannan SK, et al. Reciprocal limbic-cortical function and negative mood: converging PET findings in depression and normal sadness. Am J Psychiatry. 1999;156(5):675-682.
22. Rauch SL, Jenike MA, Alpert NM, et al. Regional cerebral blood flow measured during symptom provocation in obsessive-compulsive disorder using oxygen 15-labeled carbon dioxide and positron emission tomography. Arch Gen Psychiatry. 1994;51(1):62-70.
23. Williams NR, Taylor JJ, Lamb K, Hanlon CA, Short EB, George MS. Role of functional imaging in the development and refinement of invasive neuromodulation for psychiatric disorders. World J Radiol. 2014;6(10):756-778.
24. Francati V, Vermetten E, Bremner JD. Functional neuroimaging studies in posttraumatic stress disorder: review of current methods and findings. Depress Anxiety. 2007;24(3):202-218.
25. Shin LM, Orr SP, Carson MA, et al. Regional cerebral blood flow in the amygdala and medial prefrontal cortex during traumatic imagery in male and female Vietnam veterans with PTSD. Arch Gen Psychiatry. 2004;61(2):168-176.
26. Armony JL, Corbo V, Clément MH, Brunet A. Amygdala response in patients with acute PTSD to masked and unmasked emotional facial expressions. Am J Psychiatry. 2005;162(10):1961-1963.
27. Blake DD, Weathers FW, Nagy LM, et al. The development of a Clinician-Administered PTSD Scale. J Trauma Stress. 1995;8(1):75-90.
28. Felmingham K, Kemp A, Williams L, et al. Changes in anterior cingulate and amygdala after cognitive behavior therapy of posttraumatic stress disorder. Psychol Sci. 2007;18(2):127-129.
29. Peres JF, Newberg AB, Mercante JP, et al. Cerebral blood flow changes during retrieval of traumatic memories before and after psychotherapy: a SPECT study. Psychol Med. 2007;37(10):1481-1491.
30. Langevin JP, De Salles AA, Kosoyan HP, Krahl SE. Deep brain stimulation of the amygdala alleviates post-traumatic stress disorder symptoms in a rat model. J Psychiatr Res. 2010;44(16):1241-1245.
31. Stidd DA, Vogelsang K, Krahl SE, Langevin JP, Fellous JM. Amygdala deep brain stimulation is superior to paroxetine treatment in a rat model of posttraumatic stress disorder. Brain Stimul. 2013;6(6):837-844.
32. Anglada-Figueroa D, Quirk GJ. Lesions of the basal amygdala block expression of conditioned fear but not extinction. J Neurosci. 2005;25(42):9680-9685.
33. Koek RJ, Langevin JP, Krahl SE, et al. Deep brain stimulation of the basolateral amygdala for treatment-refractory combat post-traumatic stress disorder (PTSD): study protocol for a pilot randomized controlled trial with blinded, staggered onset of stimulation. Trials. 2014;15:356.
34. Sturm V, Fricke O, Bührle CP, et al. DBS in the basolateral amygdala improves symptoms of autism and related self-injurious behavior: a case report and hypothesis on the pathogenesis of the disorder. Front Hum Neurosci. 2013;6:341.
35. Langevin JP, Koek RJ, Schwartz HN, et al. Deep brain stimulation of the basolateral amygdala for treatment-refractory posttraumatic stress disorder. Biol Psychiatry. 2016;79(10):e82-e84.
36. Langevin JP, Chen JW, Koek RJ, et al. Deep brain stimulation of the basolateral amygdala: targeting technique and electrodiagnostic findings. Brain Sci. 2016;6(3):E28.
Failure of fear extinction is a core feature of posttraumatic stress disorder (PTSD).1 Recently, it was confirmed that the amygdala and the orbitofrontal cortex are crucial for both fear acquisition and fear extinction.2 The amygdala was found to have neurons active only during fear acquisition, and other neurons active only during fear extinction.3 In essence, the balance of activity between these 2 neuronal populations determines whether if an incoming stimulus is feared or not feared. This balance is under the influence of several cognitive domains, including memory, reward, and executive function.
In PTSD, the equilibrium is shifted heavily toward fear acquisition. The majority of patients spontaneously regain the capacity for fear extinction over time4 or with the help of treatment.5,6 Nonetheless, some patients with severe PTSD seem unable to recover the ability of fear extinction and remain refractory to both standard and novel psychotherapeutic or psychopharmacologic treatments.7 For these patients, direct modulation of the neural activity in the amygdala may permit fear extinction. This article describes the rationale for using deep brain stimulation (DBS) and initial results from the first-ever clinical trial.
Deep Brain Stimulation
Deep brain stimulation involves inserting electrodes in precise cerebral targets and then connecting the leads to a pulse generator (similar to a pacemaker) inserted in a subclavicular pocket. The generator controls the electrical signal (amplitude, pulse width, pulse frequency) delivered to the brain target and can be adjusted with use of a noninvasive programmer. In 1997, the FDA approved DBS for patients with Parkinson disease or essential tremor. Since then, its efficacy in these movement disorders has been confirmed in several studies.8,9
The mechanism by which the small electrical pulses of DBS influence activity is not clear. Clinically, DBS functionally inhibits the activity of local neurons.10 One theory describes “frequency jamming,” a concept similar to cardiac overdrive pacing in which the resultant high-frequency neuronal signal is meaningless and discounted by the rest of the brain.11
Over the years, DBS has demonstrated a strong safety profile.12 The risks of electrode insertion are mitigated with targeting based on high-quality magnetic resonance imaging (MRI) and computed tomography (Figure). Unlike a destructive lesion, DBS is reversible, and the implanted system can be removed in its entirety. Histologic analyses have shown only a small amount of scarring around the electrode tip.13 In movement disorder treatment, clinical experience has shown that stimulation-related adverse effects (AEs) are reversible with readjustment of stimulation parameters by external programmer.14
Novel Applications of DBS
The advantageous safety profile of DBS has permitted its evaluation in the treatment of other conditions thought to have malfunctioning networks at their core—such as intractable epilepsy (in resective surgery noncandidates).15,16 Although several trials have shown promising results of using DBS for treatment-resistant depression,17 the results of pivotal sham-controlled trials have been mixed.18,19 Obsessive-compulsive disorder, on the other hand, received the FDA humanitarian device exemption designation on the basis of positive long-term results.20 In treatment-resistant depression and obsessive-compulsive disorder, functional neuroimaging has identified DBS targets.21,22 Functional MRI or positron emission tomography (PET) images can be compared at resting state, at symptomatic state, and after treatment response. Nodes hyperactive during a symptomatic state and less active after successful treatment can be targeted with high-frequency DBS to directly reduce the hyperactivity and indirectly modulate or normalize the overall function of the circuit.23
Given the functional MRI and O15 (oxygen-15) PET evidence of amygdala hyperactivity in patients with PTSD having core symptoms,24-26 the authors hypothesized that high-frequency DBS targeting of the amygdala would improve PTSD-associated hyperarousal and reexperiencing symptoms in treatment-refractory patients. Indirect data supporting this hypothesis include a correlation between amygdala hyperactivity of increased intensity and symptom severity measured with the Clinician-Administered PTSD Scale (CAPS),27 and a correlation between reduced pretreatment amygdala hyperactivity and successful cognitive-behavioral treatment.28,29
Preclinical Work
Using a rodent model in which a novel object serves as a cue reminder of foot shocks (traumatic event), the authors tested the hypothesis that amygdala DBS would reduce PTSD-like symptoms.30 When untreated rats were presented with the object in their cage a week after the initial exposure, they immediately buried the object under bedding to avoid being reminded of the shocks. In contrast, rats treated with DBS did not bury the object. In most cases, in fact, they played with it.
The authors also replicated their results but with the addition of rats treated with paroxetine.31 Using the same rodent model, they found DBS superior to paroxetine in treating PTSD-like symptoms. This study had a crossover design: DBS and sham DBS. Briefly, 20 rats received an electrode in the amygdala and were exposed to inescapable shocks in the presence of the cue object. The rats were then randomly assigned to a DBS group (10 rats) or a sham-DBS group (10 rats). After 1 week, behavioral testing showed fear extinction in the DBS group and no improvement in the sham-DBS group. Then the groups were switched: The rats originally treated with DBS received no treatment, and the rats that were originally sham-treated underwent DBS. One week later, behavioral testing showed acquisition of fear extinction in all the rats. These results suggested DBS can be effective even when delayed after establishment of fear persistence and PTSD symptoms. These results also showed that DBS effects persist even after therapy discontinuation.
Similarly, other investigators have reported that the role of the amygdala is not limited to fear acquisition; it extends to fear expression. A lesion in the amygdala can prevent fear expression even if the disruption is performed subsequent to fear-conditioning training.32 This finding is important for humans, as DBS would be initiated during the chronic phase of the disorder, after failure of less invasive treatment options, such as pharmacotherapy and psychotherapy.
Early Clinical Experience
The authors have initiated the first ever clinical trial (NCT02091843) evaluating use of DBS for PTSD and are now recruiting patients. Enrollment is limited to 6 combat veterans with disabling PTSD that has not responded to pharmacotherapy and psychotherapy. This VA-funded single-site study, being conducted at the VA Greater Los Angeles Healthcare System (VAGLAHS), was approved by the VAGLAHS Institutional Review Board and the FDA. The authors have published the 2-year trial’s protocol, which includes an active-versus-sham stimulation phase; continuous electroencephalogram monitoring; baseline and posttreatment 18FDG (fluorodeoxyglucose) PET performed during a resting state vs during investigator-guided exposure to trauma reminders; and extensive psychological and neuropsychological assessments.33 The literature includes only 1 case report on amygdala DBS.34 The authors of that report used DBS of the basolateral nucleus of the amygdala to treat a teenaged boy with severe autism and found that the therapy was safe.
As of this writing, the authors have recruited and implanted 1 patient and reported on his clinical results (including baseline PET) over the first 8 months of stimulation35 and on the electrophysiologic findings over the first year.36 After experiencing extremely severe combat PTSD refractory to pharmacotherapy and psychotherapy treatments for more than 20 years, the patient treated with DBS is now experiencing substantial symptom relief, and his CAPS score (primary outcome measure) has improved by about 40%. He has tolerated continuous stimulation without any serious DBS-related AEs for up to 16 months. Notably, he has not had a single severe combat nightmare in a year—in stark contrast to nightly combat nightmares during the 20-year period leading to the trial. Furthermore, he has not been having any episodes of severe dissociation, which had been a common disabling problem before the trial. He has taken a second trip out of the country, improved his relationships with family, and made strides (albeit limited) in pursuing additional social interactions.
Avoidance remains a major problem. He recently left his job after 7 years, because he prefers a more nature-oriented rather than people-oriented environment. In addition, his interest in intensive psychotherapy has increased, and he has been considering options for spending more time working on his therapy.
Over 15 months of treatment, the patient’s CAPS total and subscale scores have decreased—his symptoms have improved (Table).21 He has had rapid and substantial reductions in recurrence and hyperarousal symptoms but slower improvement in avoidance. Improvements in emotional reactivity would be expected to occur before any change in behavior (eg, avoidance). Patients likely must first recognize changes in emotional reactivity to events before they can engage in a cognitive process to modify learned behavioral responses to those events.
After about 9 months of treatment, all of the study patient’s symptoms were somewhat stabilized, and the authors began making gradual stimulation adjustments to the latest parameters—3.5 V, 60 µs, and 160 Hz for the right electrode and 1.5 V, 60 µs, and 160 Hz for the left electrode—using the contacts in the basolateral nucleus of the amygdala, per postoperative neuroimaging.3
After 15 to 18 months, when improvement peaked at 48% symptom reduction from baseline, the patient experienced psychiatric decompensation (depression, suicide gesture) not attributable to changes in stimulation settings and not associated with exacerbation of PTSD symptoms. Treatment team members and independent psychiatric consultants attributed the decompensation to the patient’s difficulty in changing a long-standing avoidant behavior routine, owing to severe recurrence and hyperarousal symptoms in the past. His persistent inability to overcome avoidance and isolation, despite core PTSD symptom improvement, had left him feeling worthless.
The patient remains in the study but also is participating in other medication and psychotherapy trials and is making a career change. Periodic decompensations may be part of the treatment course as patients reach a more complex and volatile phase of improvement that requires more intensive cognitive reprocessing. If this proves to be the case with other patients enrolling in the study, intensive psychotherapy that addresses cognitive and emotional PTSD symptoms may be needed once there is improvement in intrusive and hyperarousal symptoms.
Conclusion
Deep brain stimulation has been successful in treating Parkinson disease and essential tremor. Physiologically, DBS seems to inhibit specific brain regions’ dysfunctional activity stemming from a disease process. Deep brain stimulation-induced inhibition of a dysfunctional node improves clinical outcomes in movement disorders.
Given the reversibility and positive safety profile of DBS, new applications are being studied. The authors propose that DBS may benefit patients with severe treatment-refractory PTSD. Their first patient’s core PTSD symptoms have improved significantly, as expected, but as in other psychiatric DBS cases, the seriousness and chronicity of his illness may be complicating the course of recovery. The authors plan to recruit 6 patients for this early-phase safety trial.
Click here to read the digital edition.
Failure of fear extinction is a core feature of posttraumatic stress disorder (PTSD).1 Recently, it was confirmed that the amygdala and the orbitofrontal cortex are crucial for both fear acquisition and fear extinction.2 The amygdala was found to have neurons active only during fear acquisition, and other neurons active only during fear extinction.3 In essence, the balance of activity between these 2 neuronal populations determines whether if an incoming stimulus is feared or not feared. This balance is under the influence of several cognitive domains, including memory, reward, and executive function.
In PTSD, the equilibrium is shifted heavily toward fear acquisition. The majority of patients spontaneously regain the capacity for fear extinction over time4 or with the help of treatment.5,6 Nonetheless, some patients with severe PTSD seem unable to recover the ability of fear extinction and remain refractory to both standard and novel psychotherapeutic or psychopharmacologic treatments.7 For these patients, direct modulation of the neural activity in the amygdala may permit fear extinction. This article describes the rationale for using deep brain stimulation (DBS) and initial results from the first-ever clinical trial.
Deep Brain Stimulation
Deep brain stimulation involves inserting electrodes in precise cerebral targets and then connecting the leads to a pulse generator (similar to a pacemaker) inserted in a subclavicular pocket. The generator controls the electrical signal (amplitude, pulse width, pulse frequency) delivered to the brain target and can be adjusted with use of a noninvasive programmer. In 1997, the FDA approved DBS for patients with Parkinson disease or essential tremor. Since then, its efficacy in these movement disorders has been confirmed in several studies.8,9
The mechanism by which the small electrical pulses of DBS influence activity is not clear. Clinically, DBS functionally inhibits the activity of local neurons.10 One theory describes “frequency jamming,” a concept similar to cardiac overdrive pacing in which the resultant high-frequency neuronal signal is meaningless and discounted by the rest of the brain.11
Over the years, DBS has demonstrated a strong safety profile.12 The risks of electrode insertion are mitigated with targeting based on high-quality magnetic resonance imaging (MRI) and computed tomography (Figure). Unlike a destructive lesion, DBS is reversible, and the implanted system can be removed in its entirety. Histologic analyses have shown only a small amount of scarring around the electrode tip.13 In movement disorder treatment, clinical experience has shown that stimulation-related adverse effects (AEs) are reversible with readjustment of stimulation parameters by external programmer.14
Novel Applications of DBS
The advantageous safety profile of DBS has permitted its evaluation in the treatment of other conditions thought to have malfunctioning networks at their core—such as intractable epilepsy (in resective surgery noncandidates).15,16 Although several trials have shown promising results of using DBS for treatment-resistant depression,17 the results of pivotal sham-controlled trials have been mixed.18,19 Obsessive-compulsive disorder, on the other hand, received the FDA humanitarian device exemption designation on the basis of positive long-term results.20 In treatment-resistant depression and obsessive-compulsive disorder, functional neuroimaging has identified DBS targets.21,22 Functional MRI or positron emission tomography (PET) images can be compared at resting state, at symptomatic state, and after treatment response. Nodes hyperactive during a symptomatic state and less active after successful treatment can be targeted with high-frequency DBS to directly reduce the hyperactivity and indirectly modulate or normalize the overall function of the circuit.23
Given the functional MRI and O15 (oxygen-15) PET evidence of amygdala hyperactivity in patients with PTSD having core symptoms,24-26 the authors hypothesized that high-frequency DBS targeting of the amygdala would improve PTSD-associated hyperarousal and reexperiencing symptoms in treatment-refractory patients. Indirect data supporting this hypothesis include a correlation between amygdala hyperactivity of increased intensity and symptom severity measured with the Clinician-Administered PTSD Scale (CAPS),27 and a correlation between reduced pretreatment amygdala hyperactivity and successful cognitive-behavioral treatment.28,29
Preclinical Work
Using a rodent model in which a novel object serves as a cue reminder of foot shocks (traumatic event), the authors tested the hypothesis that amygdala DBS would reduce PTSD-like symptoms.30 When untreated rats were presented with the object in their cage a week after the initial exposure, they immediately buried the object under bedding to avoid being reminded of the shocks. In contrast, rats treated with DBS did not bury the object. In most cases, in fact, they played with it.
The authors also replicated their results but with the addition of rats treated with paroxetine.31 Using the same rodent model, they found DBS superior to paroxetine in treating PTSD-like symptoms. This study had a crossover design: DBS and sham DBS. Briefly, 20 rats received an electrode in the amygdala and were exposed to inescapable shocks in the presence of the cue object. The rats were then randomly assigned to a DBS group (10 rats) or a sham-DBS group (10 rats). After 1 week, behavioral testing showed fear extinction in the DBS group and no improvement in the sham-DBS group. Then the groups were switched: The rats originally treated with DBS received no treatment, and the rats that were originally sham-treated underwent DBS. One week later, behavioral testing showed acquisition of fear extinction in all the rats. These results suggested DBS can be effective even when delayed after establishment of fear persistence and PTSD symptoms. These results also showed that DBS effects persist even after therapy discontinuation.
Similarly, other investigators have reported that the role of the amygdala is not limited to fear acquisition; it extends to fear expression. A lesion in the amygdala can prevent fear expression even if the disruption is performed subsequent to fear-conditioning training.32 This finding is important for humans, as DBS would be initiated during the chronic phase of the disorder, after failure of less invasive treatment options, such as pharmacotherapy and psychotherapy.
Early Clinical Experience
The authors have initiated the first ever clinical trial (NCT02091843) evaluating use of DBS for PTSD and are now recruiting patients. Enrollment is limited to 6 combat veterans with disabling PTSD that has not responded to pharmacotherapy and psychotherapy. This VA-funded single-site study, being conducted at the VA Greater Los Angeles Healthcare System (VAGLAHS), was approved by the VAGLAHS Institutional Review Board and the FDA. The authors have published the 2-year trial’s protocol, which includes an active-versus-sham stimulation phase; continuous electroencephalogram monitoring; baseline and posttreatment 18FDG (fluorodeoxyglucose) PET performed during a resting state vs during investigator-guided exposure to trauma reminders; and extensive psychological and neuropsychological assessments.33 The literature includes only 1 case report on amygdala DBS.34 The authors of that report used DBS of the basolateral nucleus of the amygdala to treat a teenaged boy with severe autism and found that the therapy was safe.
As of this writing, the authors have recruited and implanted 1 patient and reported on his clinical results (including baseline PET) over the first 8 months of stimulation35 and on the electrophysiologic findings over the first year.36 After experiencing extremely severe combat PTSD refractory to pharmacotherapy and psychotherapy treatments for more than 20 years, the patient treated with DBS is now experiencing substantial symptom relief, and his CAPS score (primary outcome measure) has improved by about 40%. He has tolerated continuous stimulation without any serious DBS-related AEs for up to 16 months. Notably, he has not had a single severe combat nightmare in a year—in stark contrast to nightly combat nightmares during the 20-year period leading to the trial. Furthermore, he has not been having any episodes of severe dissociation, which had been a common disabling problem before the trial. He has taken a second trip out of the country, improved his relationships with family, and made strides (albeit limited) in pursuing additional social interactions.
Avoidance remains a major problem. He recently left his job after 7 years, because he prefers a more nature-oriented rather than people-oriented environment. In addition, his interest in intensive psychotherapy has increased, and he has been considering options for spending more time working on his therapy.
Over 15 months of treatment, the patient’s CAPS total and subscale scores have decreased—his symptoms have improved (Table).21 He has had rapid and substantial reductions in recurrence and hyperarousal symptoms but slower improvement in avoidance. Improvements in emotional reactivity would be expected to occur before any change in behavior (eg, avoidance). Patients likely must first recognize changes in emotional reactivity to events before they can engage in a cognitive process to modify learned behavioral responses to those events.
After about 9 months of treatment, all of the study patient’s symptoms were somewhat stabilized, and the authors began making gradual stimulation adjustments to the latest parameters—3.5 V, 60 µs, and 160 Hz for the right electrode and 1.5 V, 60 µs, and 160 Hz for the left electrode—using the contacts in the basolateral nucleus of the amygdala, per postoperative neuroimaging.3
After 15 to 18 months, when improvement peaked at 48% symptom reduction from baseline, the patient experienced psychiatric decompensation (depression, suicide gesture) not attributable to changes in stimulation settings and not associated with exacerbation of PTSD symptoms. Treatment team members and independent psychiatric consultants attributed the decompensation to the patient’s difficulty in changing a long-standing avoidant behavior routine, owing to severe recurrence and hyperarousal symptoms in the past. His persistent inability to overcome avoidance and isolation, despite core PTSD symptom improvement, had left him feeling worthless.
The patient remains in the study but also is participating in other medication and psychotherapy trials and is making a career change. Periodic decompensations may be part of the treatment course as patients reach a more complex and volatile phase of improvement that requires more intensive cognitive reprocessing. If this proves to be the case with other patients enrolling in the study, intensive psychotherapy that addresses cognitive and emotional PTSD symptoms may be needed once there is improvement in intrusive and hyperarousal symptoms.
Conclusion
Deep brain stimulation has been successful in treating Parkinson disease and essential tremor. Physiologically, DBS seems to inhibit specific brain regions’ dysfunctional activity stemming from a disease process. Deep brain stimulation-induced inhibition of a dysfunctional node improves clinical outcomes in movement disorders.
Given the reversibility and positive safety profile of DBS, new applications are being studied. The authors propose that DBS may benefit patients with severe treatment-refractory PTSD. Their first patient’s core PTSD symptoms have improved significantly, as expected, but as in other psychiatric DBS cases, the seriousness and chronicity of his illness may be complicating the course of recovery. The authors plan to recruit 6 patients for this early-phase safety trial.
Click here to read the digital edition.
1. Milad MR, Pitman RK, Ellis CB, et al. Neurobiological basis of failure to recall extinction memory in posttraumatic stress disorder. Biol Psychiatry. 2009;66(12):1075-1082.
2. Marin MF, Song H, VanElzakker MB, et al. Association of resting metabolism in the fear neural network with extinction recall activations and clinical measures in trauma-exposed individuals. Am J Psychiatry. 2016;173(9):930-938.
3. Herry C, Ciocchi S, Senn V, Demmou L, Müller C, Lüthi A. Switching on and off fear by distinct neuronal circuits. Nature. 2008;454(7204):600-606.
4. Morina N, Wicherts JM, Lobbrecht J, Priebe S. Remission from post-traumatic stress disorder in adults: a systematic review and meta-analysis of long term outcome studies. Clin Psychol Rev. 2014;34(3):249-255.
5. Steenkamp MM, Litz BT, Hoge CW, Marmar CR. Psychotherapy for military-related PTSD: a review of randomized clinical trials. JAMA. 2015;314(5):489-500.
6. Hoskins M, Pearce J, Bethell A, et al. Pharmacotherapy for post-traumatic stress disorder: systematic review and meta-analysis. Br J Psychiatry. 2015;206(2):93-100.
7. Koek RJ, Schwartz HN, Scully S, et al. Treatment-refractory posttraumatic stress disorder (TRPTSD): a review and framework for the future. Prog Neuropsychopharmacol Biol Psychiatry. 2016;70:170-218.
8. Wagle Shukla A, Okun MS. State of the art for deep brain stimulation therapy in movement disorders: a clinical and technological perspective. IEEE Rev Biomed Eng. 2016;9:219-233.
9. Weaver FM, Follett K, Stern M, et al; CSP 468 Study Group. Bilateral deep brain stimulation vs best medical therapy for patients with advanced Parkinson disease: a randomized controlled trial. JAMA. 2009;301(1):63-73.
10. Benabid AL, Benazzouz A, Hoffmann D, Limousin P, Krack P, Pollack P. Long-term electrical inhibition of deep brain targets in movement disorders. Mov Disord. 1998;13(suppl 3):119-125.
11. Benabid AL, Wallace B, Mitrofanis J, et al. A putative generalized model of the effects and mechanism of action of high frequency electrical stimulation of the central nervous system. Acta Neurol Belg. 2005;105(3):149-157.
12. Fenoy AJ, Simpson RK Jr. Risks of common complications in deep brain stimulation surgery: management and avoidance. J Neurosurg. 2014;120(1):132-139.
13. DiLorenzo DJ, Jankovic J, Simpson RK, Takei H, Powell SZ. Neurohistopathological findings at the electrode–tissue interface in long-term deep brain stimulation: systematic literature review, case report, and assessment of stimulation threshold safety. Neuromodulation. 2014;17(5):405-418.
14. Revell MA. Deep brain stimulation for movement disorders. Nurs Clin North Am. 2015;50(4):691-701.
15. Fisher R, Salanova V, Witt T, et al; SANTE Study Group. Electrical stimulation of the anterior nucleus of thalamus for treatment of refractory epilepsy. Epilepsia. 2010;51(5):899-908.
16. Salanova V, Witt T, Worth R, et al; SANTE Study Group. Long-term efficacy and safety of thalamic stimulation for drug-resistant partial epilepsy. Neurology. 2015;84(10):1017-1025.
17. Berlim MT, McGirr A, Van den Eynde F, Fleck MP, Giacobbe P. Effectiveness and acceptability of deep brain stimulation (DBS) of the subgenual cingulate cortex for treatment-resistant depression: a systematic review and exploratory meta-analysis. J Affect Disord. 2014;159:31-38.
18. Dougherty DD, Rezai AR, Carpenter LL, et al. A randomized sham-controlled trial of deep brain stimulation of the ventral capsule/ventral striatum for chronic treatment-resistant depression. Biol Psychiatry. 2015;78(4):240-248.
19. Bergfeld IO, Mantione M, Hoogendoorn ML, et al. Deep brain stimulation of the ventral anterior limb of the internal capsule for treatment-resistant depression: a randomized clinical trial. JAMA Psychiatry. 2016;73(5):456-464.
20. Greenberg BD, Malone DA, Friehs GM, et al. Three-year outcomes in deep brain stimulation for highly resistant obsessive-compulsive disorder. Neuropsychopharmacology. 2006;31(11):2384-2393.
21. Mayber HS, Liotti M, Brannan SK, et al. Reciprocal limbic-cortical function and negative mood: converging PET findings in depression and normal sadness. Am J Psychiatry. 1999;156(5):675-682.
22. Rauch SL, Jenike MA, Alpert NM, et al. Regional cerebral blood flow measured during symptom provocation in obsessive-compulsive disorder using oxygen 15-labeled carbon dioxide and positron emission tomography. Arch Gen Psychiatry. 1994;51(1):62-70.
23. Williams NR, Taylor JJ, Lamb K, Hanlon CA, Short EB, George MS. Role of functional imaging in the development and refinement of invasive neuromodulation for psychiatric disorders. World J Radiol. 2014;6(10):756-778.
24. Francati V, Vermetten E, Bremner JD. Functional neuroimaging studies in posttraumatic stress disorder: review of current methods and findings. Depress Anxiety. 2007;24(3):202-218.
25. Shin LM, Orr SP, Carson MA, et al. Regional cerebral blood flow in the amygdala and medial prefrontal cortex during traumatic imagery in male and female Vietnam veterans with PTSD. Arch Gen Psychiatry. 2004;61(2):168-176.
26. Armony JL, Corbo V, Clément MH, Brunet A. Amygdala response in patients with acute PTSD to masked and unmasked emotional facial expressions. Am J Psychiatry. 2005;162(10):1961-1963.
27. Blake DD, Weathers FW, Nagy LM, et al. The development of a Clinician-Administered PTSD Scale. J Trauma Stress. 1995;8(1):75-90.
28. Felmingham K, Kemp A, Williams L, et al. Changes in anterior cingulate and amygdala after cognitive behavior therapy of posttraumatic stress disorder. Psychol Sci. 2007;18(2):127-129.
29. Peres JF, Newberg AB, Mercante JP, et al. Cerebral blood flow changes during retrieval of traumatic memories before and after psychotherapy: a SPECT study. Psychol Med. 2007;37(10):1481-1491.
30. Langevin JP, De Salles AA, Kosoyan HP, Krahl SE. Deep brain stimulation of the amygdala alleviates post-traumatic stress disorder symptoms in a rat model. J Psychiatr Res. 2010;44(16):1241-1245.
31. Stidd DA, Vogelsang K, Krahl SE, Langevin JP, Fellous JM. Amygdala deep brain stimulation is superior to paroxetine treatment in a rat model of posttraumatic stress disorder. Brain Stimul. 2013;6(6):837-844.
32. Anglada-Figueroa D, Quirk GJ. Lesions of the basal amygdala block expression of conditioned fear but not extinction. J Neurosci. 2005;25(42):9680-9685.
33. Koek RJ, Langevin JP, Krahl SE, et al. Deep brain stimulation of the basolateral amygdala for treatment-refractory combat post-traumatic stress disorder (PTSD): study protocol for a pilot randomized controlled trial with blinded, staggered onset of stimulation. Trials. 2014;15:356.
34. Sturm V, Fricke O, Bührle CP, et al. DBS in the basolateral amygdala improves symptoms of autism and related self-injurious behavior: a case report and hypothesis on the pathogenesis of the disorder. Front Hum Neurosci. 2013;6:341.
35. Langevin JP, Koek RJ, Schwartz HN, et al. Deep brain stimulation of the basolateral amygdala for treatment-refractory posttraumatic stress disorder. Biol Psychiatry. 2016;79(10):e82-e84.
36. Langevin JP, Chen JW, Koek RJ, et al. Deep brain stimulation of the basolateral amygdala: targeting technique and electrodiagnostic findings. Brain Sci. 2016;6(3):E28.
1. Milad MR, Pitman RK, Ellis CB, et al. Neurobiological basis of failure to recall extinction memory in posttraumatic stress disorder. Biol Psychiatry. 2009;66(12):1075-1082.
2. Marin MF, Song H, VanElzakker MB, et al. Association of resting metabolism in the fear neural network with extinction recall activations and clinical measures in trauma-exposed individuals. Am J Psychiatry. 2016;173(9):930-938.
3. Herry C, Ciocchi S, Senn V, Demmou L, Müller C, Lüthi A. Switching on and off fear by distinct neuronal circuits. Nature. 2008;454(7204):600-606.
4. Morina N, Wicherts JM, Lobbrecht J, Priebe S. Remission from post-traumatic stress disorder in adults: a systematic review and meta-analysis of long term outcome studies. Clin Psychol Rev. 2014;34(3):249-255.
5. Steenkamp MM, Litz BT, Hoge CW, Marmar CR. Psychotherapy for military-related PTSD: a review of randomized clinical trials. JAMA. 2015;314(5):489-500.
6. Hoskins M, Pearce J, Bethell A, et al. Pharmacotherapy for post-traumatic stress disorder: systematic review and meta-analysis. Br J Psychiatry. 2015;206(2):93-100.
7. Koek RJ, Schwartz HN, Scully S, et al. Treatment-refractory posttraumatic stress disorder (TRPTSD): a review and framework for the future. Prog Neuropsychopharmacol Biol Psychiatry. 2016;70:170-218.
8. Wagle Shukla A, Okun MS. State of the art for deep brain stimulation therapy in movement disorders: a clinical and technological perspective. IEEE Rev Biomed Eng. 2016;9:219-233.
9. Weaver FM, Follett K, Stern M, et al; CSP 468 Study Group. Bilateral deep brain stimulation vs best medical therapy for patients with advanced Parkinson disease: a randomized controlled trial. JAMA. 2009;301(1):63-73.
10. Benabid AL, Benazzouz A, Hoffmann D, Limousin P, Krack P, Pollack P. Long-term electrical inhibition of deep brain targets in movement disorders. Mov Disord. 1998;13(suppl 3):119-125.
11. Benabid AL, Wallace B, Mitrofanis J, et al. A putative generalized model of the effects and mechanism of action of high frequency electrical stimulation of the central nervous system. Acta Neurol Belg. 2005;105(3):149-157.
12. Fenoy AJ, Simpson RK Jr. Risks of common complications in deep brain stimulation surgery: management and avoidance. J Neurosurg. 2014;120(1):132-139.
13. DiLorenzo DJ, Jankovic J, Simpson RK, Takei H, Powell SZ. Neurohistopathological findings at the electrode–tissue interface in long-term deep brain stimulation: systematic literature review, case report, and assessment of stimulation threshold safety. Neuromodulation. 2014;17(5):405-418.
14. Revell MA. Deep brain stimulation for movement disorders. Nurs Clin North Am. 2015;50(4):691-701.
15. Fisher R, Salanova V, Witt T, et al; SANTE Study Group. Electrical stimulation of the anterior nucleus of thalamus for treatment of refractory epilepsy. Epilepsia. 2010;51(5):899-908.
16. Salanova V, Witt T, Worth R, et al; SANTE Study Group. Long-term efficacy and safety of thalamic stimulation for drug-resistant partial epilepsy. Neurology. 2015;84(10):1017-1025.
17. Berlim MT, McGirr A, Van den Eynde F, Fleck MP, Giacobbe P. Effectiveness and acceptability of deep brain stimulation (DBS) of the subgenual cingulate cortex for treatment-resistant depression: a systematic review and exploratory meta-analysis. J Affect Disord. 2014;159:31-38.
18. Dougherty DD, Rezai AR, Carpenter LL, et al. A randomized sham-controlled trial of deep brain stimulation of the ventral capsule/ventral striatum for chronic treatment-resistant depression. Biol Psychiatry. 2015;78(4):240-248.
19. Bergfeld IO, Mantione M, Hoogendoorn ML, et al. Deep brain stimulation of the ventral anterior limb of the internal capsule for treatment-resistant depression: a randomized clinical trial. JAMA Psychiatry. 2016;73(5):456-464.
20. Greenberg BD, Malone DA, Friehs GM, et al. Three-year outcomes in deep brain stimulation for highly resistant obsessive-compulsive disorder. Neuropsychopharmacology. 2006;31(11):2384-2393.
21. Mayber HS, Liotti M, Brannan SK, et al. Reciprocal limbic-cortical function and negative mood: converging PET findings in depression and normal sadness. Am J Psychiatry. 1999;156(5):675-682.
22. Rauch SL, Jenike MA, Alpert NM, et al. Regional cerebral blood flow measured during symptom provocation in obsessive-compulsive disorder using oxygen 15-labeled carbon dioxide and positron emission tomography. Arch Gen Psychiatry. 1994;51(1):62-70.
23. Williams NR, Taylor JJ, Lamb K, Hanlon CA, Short EB, George MS. Role of functional imaging in the development and refinement of invasive neuromodulation for psychiatric disorders. World J Radiol. 2014;6(10):756-778.
24. Francati V, Vermetten E, Bremner JD. Functional neuroimaging studies in posttraumatic stress disorder: review of current methods and findings. Depress Anxiety. 2007;24(3):202-218.
25. Shin LM, Orr SP, Carson MA, et al. Regional cerebral blood flow in the amygdala and medial prefrontal cortex during traumatic imagery in male and female Vietnam veterans with PTSD. Arch Gen Psychiatry. 2004;61(2):168-176.
26. Armony JL, Corbo V, Clément MH, Brunet A. Amygdala response in patients with acute PTSD to masked and unmasked emotional facial expressions. Am J Psychiatry. 2005;162(10):1961-1963.
27. Blake DD, Weathers FW, Nagy LM, et al. The development of a Clinician-Administered PTSD Scale. J Trauma Stress. 1995;8(1):75-90.
28. Felmingham K, Kemp A, Williams L, et al. Changes in anterior cingulate and amygdala after cognitive behavior therapy of posttraumatic stress disorder. Psychol Sci. 2007;18(2):127-129.
29. Peres JF, Newberg AB, Mercante JP, et al. Cerebral blood flow changes during retrieval of traumatic memories before and after psychotherapy: a SPECT study. Psychol Med. 2007;37(10):1481-1491.
30. Langevin JP, De Salles AA, Kosoyan HP, Krahl SE. Deep brain stimulation of the amygdala alleviates post-traumatic stress disorder symptoms in a rat model. J Psychiatr Res. 2010;44(16):1241-1245.
31. Stidd DA, Vogelsang K, Krahl SE, Langevin JP, Fellous JM. Amygdala deep brain stimulation is superior to paroxetine treatment in a rat model of posttraumatic stress disorder. Brain Stimul. 2013;6(6):837-844.
32. Anglada-Figueroa D, Quirk GJ. Lesions of the basal amygdala block expression of conditioned fear but not extinction. J Neurosci. 2005;25(42):9680-9685.
33. Koek RJ, Langevin JP, Krahl SE, et al. Deep brain stimulation of the basolateral amygdala for treatment-refractory combat post-traumatic stress disorder (PTSD): study protocol for a pilot randomized controlled trial with blinded, staggered onset of stimulation. Trials. 2014;15:356.
34. Sturm V, Fricke O, Bührle CP, et al. DBS in the basolateral amygdala improves symptoms of autism and related self-injurious behavior: a case report and hypothesis on the pathogenesis of the disorder. Front Hum Neurosci. 2013;6:341.
35. Langevin JP, Koek RJ, Schwartz HN, et al. Deep brain stimulation of the basolateral amygdala for treatment-refractory posttraumatic stress disorder. Biol Psychiatry. 2016;79(10):e82-e84.
36. Langevin JP, Chen JW, Koek RJ, et al. Deep brain stimulation of the basolateral amygdala: targeting technique and electrodiagnostic findings. Brain Sci. 2016;6(3):E28.
Defensive medicine’s stranglehold on the realities of practice
In the September 2017 issue of JAMA Neurology, Louis R. Caplan, MD, wrote an excellent editorial, “Patient care is all about stories.” He notes that we all hear from patients about a recurrence of their previous stroke deficits, typically caused by infections, medications, or metabolic changes.
His point is that, telling the difference between true vascular events and recrudescence of old deficits can be difficult, but generally can be gleaned by taking a thorough history. He also notes, quite correctly, that the generic, automated features of modern charting systems often make it harder to get the details you need from previous visits.
Obviously, being able to accurately tell the difference between them can save health care costs, too. In a study in the same issue, Mehmet Topcuoglo, MD, and his colleagues discuss methodologies to differentiate between the causes of recrudescence of stroke-related deficits. Currently, the main approach is to admit patients to the hospital, do a knee-jerk repeat work-up with MRI, magnetic resonance angiogram, and echocardiogram (typically ordered before the neurologist has even been told of the consult) and then conclude that nothing has changed neurologically and that it was all caused by a bladder infection.
Surely, if we had an accurate way of telling the difference between them with a careful history, we’d save a lot of time and money on unnecessary hospital admissions. Right?
It sounds good in principle, but, sadly, the answer is “probably not.”
This is where the idealism of medicine meets the reality of its practice.
In the world of the emergency department, time and resources are limited. Emergency medicine physicians don’t have the luxury of taking a detailed neurologic history, nor are they trained (or expected) to be able to do so. Their job is to decide what is (and isn’t) life-threatening and who does (or doesn’t) need to be admitted.
But probably the main reason why Dr. Topcuoglo and his colleagues’ methodologies will never be implemented is defensive medicine. It’s a heck of lot easier and safer for any doctor – emergency medicine, hospitalist, and neurologist – to admit the patient and order more studies than it is to get served for malpractice and have to defend why you didn’t do that.
People can bemoan defensive medicine and its costs all they want. But, if you’ve been sued, you won’t care. You’ll order any test to protect yourself. Claiming that you followed a guideline from a journal, no matter how well researched it was, will likely be worthless the one time a stroke was missed. It’s easy for a plaintiff’s attorney to find someone to say you fell below the standard of care for doing so.
For an example of where this stands, here’s something from personal experience: One of my patients went to the emergency department for recrudescence of an old left hemiparesis, likely caused by a urinary tract infection. This wasn’t the first time it had happened. A head CT was stable while a urine analysis was abnormal. Because of my schedule, I wasn’t in a position to go see him in the ED in an expedient fashion. The ED physician was planning on admitting him and called to notify me. Knowing the history, I suggested sending him home with treatment for the UTI and to follow up with me the next day.
I thought that seemed reasonable, but the ED doctor didn’t. He said, “If you want to do that, then I am going to document that it’s on your instructions, that you are assuming all responsibility for care and outcome if a stroke is missed, and that I entirely disagree with your decision.”
I’m sure another neurologist might have said, “Okay, tell him to come in here tomorrow,” and hung up, but I really don’t have that kind of fortitude or desire for conflict with another physician. So I backed down and let the person on the scene make the decision. I saw the patient later that day as a consult, all his tests (except the urine analysis in the ED) were fine, and he went home the next day. I’m sure the bill was at least $50,000 (what really got paid is another matter), and defensive medicine had, for better or worse, won out over probability and reason.
Dr. Caplan, quite correctly, emphasizes the importance of taking a careful history, and I absolutely agree with him. Unfortunately, the lack of time in the ED setting, and fears driven by legal consequences, often make a good history irrelevant. Even when it’s done, there are other forces that push it to the background in making medical decisions.
I’m not saying that’s a good thing – it isn’t. But that’s the way it is right now in American medicine, and this aspect of the system shows no sign of changing.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
In the September 2017 issue of JAMA Neurology, Louis R. Caplan, MD, wrote an excellent editorial, “Patient care is all about stories.” He notes that we all hear from patients about a recurrence of their previous stroke deficits, typically caused by infections, medications, or metabolic changes.
His point is that, telling the difference between true vascular events and recrudescence of old deficits can be difficult, but generally can be gleaned by taking a thorough history. He also notes, quite correctly, that the generic, automated features of modern charting systems often make it harder to get the details you need from previous visits.
Obviously, being able to accurately tell the difference between them can save health care costs, too. In a study in the same issue, Mehmet Topcuoglo, MD, and his colleagues discuss methodologies to differentiate between the causes of recrudescence of stroke-related deficits. Currently, the main approach is to admit patients to the hospital, do a knee-jerk repeat work-up with MRI, magnetic resonance angiogram, and echocardiogram (typically ordered before the neurologist has even been told of the consult) and then conclude that nothing has changed neurologically and that it was all caused by a bladder infection.
Surely, if we had an accurate way of telling the difference between them with a careful history, we’d save a lot of time and money on unnecessary hospital admissions. Right?
It sounds good in principle, but, sadly, the answer is “probably not.”
This is where the idealism of medicine meets the reality of its practice.
In the world of the emergency department, time and resources are limited. Emergency medicine physicians don’t have the luxury of taking a detailed neurologic history, nor are they trained (or expected) to be able to do so. Their job is to decide what is (and isn’t) life-threatening and who does (or doesn’t) need to be admitted.
But probably the main reason why Dr. Topcuoglo and his colleagues’ methodologies will never be implemented is defensive medicine. It’s a heck of lot easier and safer for any doctor – emergency medicine, hospitalist, and neurologist – to admit the patient and order more studies than it is to get served for malpractice and have to defend why you didn’t do that.
People can bemoan defensive medicine and its costs all they want. But, if you’ve been sued, you won’t care. You’ll order any test to protect yourself. Claiming that you followed a guideline from a journal, no matter how well researched it was, will likely be worthless the one time a stroke was missed. It’s easy for a plaintiff’s attorney to find someone to say you fell below the standard of care for doing so.
For an example of where this stands, here’s something from personal experience: One of my patients went to the emergency department for recrudescence of an old left hemiparesis, likely caused by a urinary tract infection. This wasn’t the first time it had happened. A head CT was stable while a urine analysis was abnormal. Because of my schedule, I wasn’t in a position to go see him in the ED in an expedient fashion. The ED physician was planning on admitting him and called to notify me. Knowing the history, I suggested sending him home with treatment for the UTI and to follow up with me the next day.
I thought that seemed reasonable, but the ED doctor didn’t. He said, “If you want to do that, then I am going to document that it’s on your instructions, that you are assuming all responsibility for care and outcome if a stroke is missed, and that I entirely disagree with your decision.”
I’m sure another neurologist might have said, “Okay, tell him to come in here tomorrow,” and hung up, but I really don’t have that kind of fortitude or desire for conflict with another physician. So I backed down and let the person on the scene make the decision. I saw the patient later that day as a consult, all his tests (except the urine analysis in the ED) were fine, and he went home the next day. I’m sure the bill was at least $50,000 (what really got paid is another matter), and defensive medicine had, for better or worse, won out over probability and reason.
Dr. Caplan, quite correctly, emphasizes the importance of taking a careful history, and I absolutely agree with him. Unfortunately, the lack of time in the ED setting, and fears driven by legal consequences, often make a good history irrelevant. Even when it’s done, there are other forces that push it to the background in making medical decisions.
I’m not saying that’s a good thing – it isn’t. But that’s the way it is right now in American medicine, and this aspect of the system shows no sign of changing.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
In the September 2017 issue of JAMA Neurology, Louis R. Caplan, MD, wrote an excellent editorial, “Patient care is all about stories.” He notes that we all hear from patients about a recurrence of their previous stroke deficits, typically caused by infections, medications, or metabolic changes.
His point is that, telling the difference between true vascular events and recrudescence of old deficits can be difficult, but generally can be gleaned by taking a thorough history. He also notes, quite correctly, that the generic, automated features of modern charting systems often make it harder to get the details you need from previous visits.
Obviously, being able to accurately tell the difference between them can save health care costs, too. In a study in the same issue, Mehmet Topcuoglo, MD, and his colleagues discuss methodologies to differentiate between the causes of recrudescence of stroke-related deficits. Currently, the main approach is to admit patients to the hospital, do a knee-jerk repeat work-up with MRI, magnetic resonance angiogram, and echocardiogram (typically ordered before the neurologist has even been told of the consult) and then conclude that nothing has changed neurologically and that it was all caused by a bladder infection.
Surely, if we had an accurate way of telling the difference between them with a careful history, we’d save a lot of time and money on unnecessary hospital admissions. Right?
It sounds good in principle, but, sadly, the answer is “probably not.”
This is where the idealism of medicine meets the reality of its practice.
In the world of the emergency department, time and resources are limited. Emergency medicine physicians don’t have the luxury of taking a detailed neurologic history, nor are they trained (or expected) to be able to do so. Their job is to decide what is (and isn’t) life-threatening and who does (or doesn’t) need to be admitted.
But probably the main reason why Dr. Topcuoglo and his colleagues’ methodologies will never be implemented is defensive medicine. It’s a heck of lot easier and safer for any doctor – emergency medicine, hospitalist, and neurologist – to admit the patient and order more studies than it is to get served for malpractice and have to defend why you didn’t do that.
People can bemoan defensive medicine and its costs all they want. But, if you’ve been sued, you won’t care. You’ll order any test to protect yourself. Claiming that you followed a guideline from a journal, no matter how well researched it was, will likely be worthless the one time a stroke was missed. It’s easy for a plaintiff’s attorney to find someone to say you fell below the standard of care for doing so.
For an example of where this stands, here’s something from personal experience: One of my patients went to the emergency department for recrudescence of an old left hemiparesis, likely caused by a urinary tract infection. This wasn’t the first time it had happened. A head CT was stable while a urine analysis was abnormal. Because of my schedule, I wasn’t in a position to go see him in the ED in an expedient fashion. The ED physician was planning on admitting him and called to notify me. Knowing the history, I suggested sending him home with treatment for the UTI and to follow up with me the next day.
I thought that seemed reasonable, but the ED doctor didn’t. He said, “If you want to do that, then I am going to document that it’s on your instructions, that you are assuming all responsibility for care and outcome if a stroke is missed, and that I entirely disagree with your decision.”
I’m sure another neurologist might have said, “Okay, tell him to come in here tomorrow,” and hung up, but I really don’t have that kind of fortitude or desire for conflict with another physician. So I backed down and let the person on the scene make the decision. I saw the patient later that day as a consult, all his tests (except the urine analysis in the ED) were fine, and he went home the next day. I’m sure the bill was at least $50,000 (what really got paid is another matter), and defensive medicine had, for better or worse, won out over probability and reason.
Dr. Caplan, quite correctly, emphasizes the importance of taking a careful history, and I absolutely agree with him. Unfortunately, the lack of time in the ED setting, and fears driven by legal consequences, often make a good history irrelevant. Even when it’s done, there are other forces that push it to the background in making medical decisions.
I’m not saying that’s a good thing – it isn’t. But that’s the way it is right now in American medicine, and this aspect of the system shows no sign of changing.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Clinical Challenges - March 2018 What's your diagnosis?
The diagnosis: Spontaneous gallbladder perforation
References
1. Ausania, F., Guzman Suarez, S., Alvarez Garcia, H. et al. Gallbladder perforation: morbidity, mortality and preoperative risk prediction. Surg Endosc. 2015;29:955-60.
2. Niemeier, O.W. Acute free perforation of the gall-bladder. Ann Surg. 1934;99:922-4.
3. Hyodo, T., Kumano, S., Kushihata, F. et al. CT and MR cholangiography: advantages and pitfalls in perioperative evaluation of biliary tree. Br J Radiol. 2012;85:887-96.
The diagnosis: Spontaneous gallbladder perforation
References
1. Ausania, F., Guzman Suarez, S., Alvarez Garcia, H. et al. Gallbladder perforation: morbidity, mortality and preoperative risk prediction. Surg Endosc. 2015;29:955-60.
2. Niemeier, O.W. Acute free perforation of the gall-bladder. Ann Surg. 1934;99:922-4.
3. Hyodo, T., Kumano, S., Kushihata, F. et al. CT and MR cholangiography: advantages and pitfalls in perioperative evaluation of biliary tree. Br J Radiol. 2012;85:887-96.
The diagnosis: Spontaneous gallbladder perforation
References
1. Ausania, F., Guzman Suarez, S., Alvarez Garcia, H. et al. Gallbladder perforation: morbidity, mortality and preoperative risk prediction. Surg Endosc. 2015;29:955-60.
2. Niemeier, O.W. Acute free perforation of the gall-bladder. Ann Surg. 1934;99:922-4.
3. Hyodo, T., Kumano, S., Kushihata, F. et al. CT and MR cholangiography: advantages and pitfalls in perioperative evaluation of biliary tree. Br J Radiol. 2012;85:887-96.
Published previously in Gastroenterology (2016;151[1]:40-2).
What is your diagnosis and treatment?