User login
Bullous Systemic Lupus Erythematosus Successfully Treated With Rituximab
Bullous systemic lupus erythematosus (BSLE) is a rare cutaneous presentation of systemic lupus erythematosus (SLE).1 Although 59% to 85% of SLE patients develop skin-related symptoms, fewer than 5% of SLE patients develop BSLE.1-3 This acquired autoimmune bullous disease, characterized by subepidermal bullae with a neutrophilic infiltrate on histopathology, is precipitated by autoantibodies to type VII collagen. Bullae can appear on both cutaneous and mucosal surfaces but tend to favor the trunk, upper extremities, neck, face, and vermilion border.3
Our case of an 18-year-old black woman with BSLE was originally reported in 2011.4 We update the case to illustrate the heterogeneous presentation of BSLE in a single patient and to expand on the role of rituximab in this disease.
Case Report
An 18-year-old black woman presented with a vesicular eruption of 3 weeks’ duration that started on the trunk and buttocks and progressed to involve the face, oral mucosa, and posterior auricular area. The vesicular eruption was accompanied by fatigue, arthralgia, and myalgia.
Physical examination revealed multiple tense, fluid-filled vesicles, measuring roughly 2 to 3 mm in diameter, over the cheeks, chin, postauricular area, vermilion border, oral mucosa, and left side of the neck and shoulder. Resolved lesions on the trunk and buttocks were marked by superficial crust and postinflammatory hyperpigmentation. Scarring was absent.
Laboratory analysis demonstrated hemolytic anemia with a positive direct antiglobulin test, hypocomplementemia, and an elevated erythrocyte sedimentation rate. Antinuclear antibody testing was positive (titer, 1:640).
Biopsies were taken from the left cheek for hematoxylin and eosin (H&E) staining and direct immunofluorescence (DIF), which revealed subepidermal clefting, few neutrophils, and notable mucin deposition. Direct immunofluorescence showed a broad deposition of IgG, IgA, and IgM, as well as C3 in a ribbonlike pattern at the dermoepidermal junction.
A diagnosis of SLE with BSLE was made. The patient initially was treated with prednisone, hydroxychloroquine, mycophenolate mofetil, and intravenous immunoglobulin, but the cutaneous disease persisted. The bullous eruption resolved with 2 infusions of rituximab (1000 mg) spaced 2 weeks apart.
The patient was in remission on 5 mg of prednisone for 2 years following the initial course of rituximab. However, she developed a flare of SLE, with fatigue, arthralgia, hypocomplementemia, and recurrence of BSLE with tense bullae on the face and lips. The flare resolved with prednisone and a single infusion of rituximab (1000 mg). She was then maintained on hydroxychloroquine (200 mg/d).
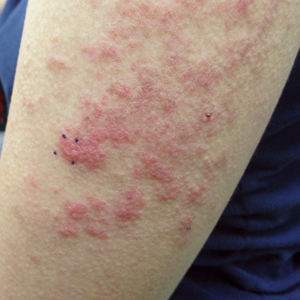
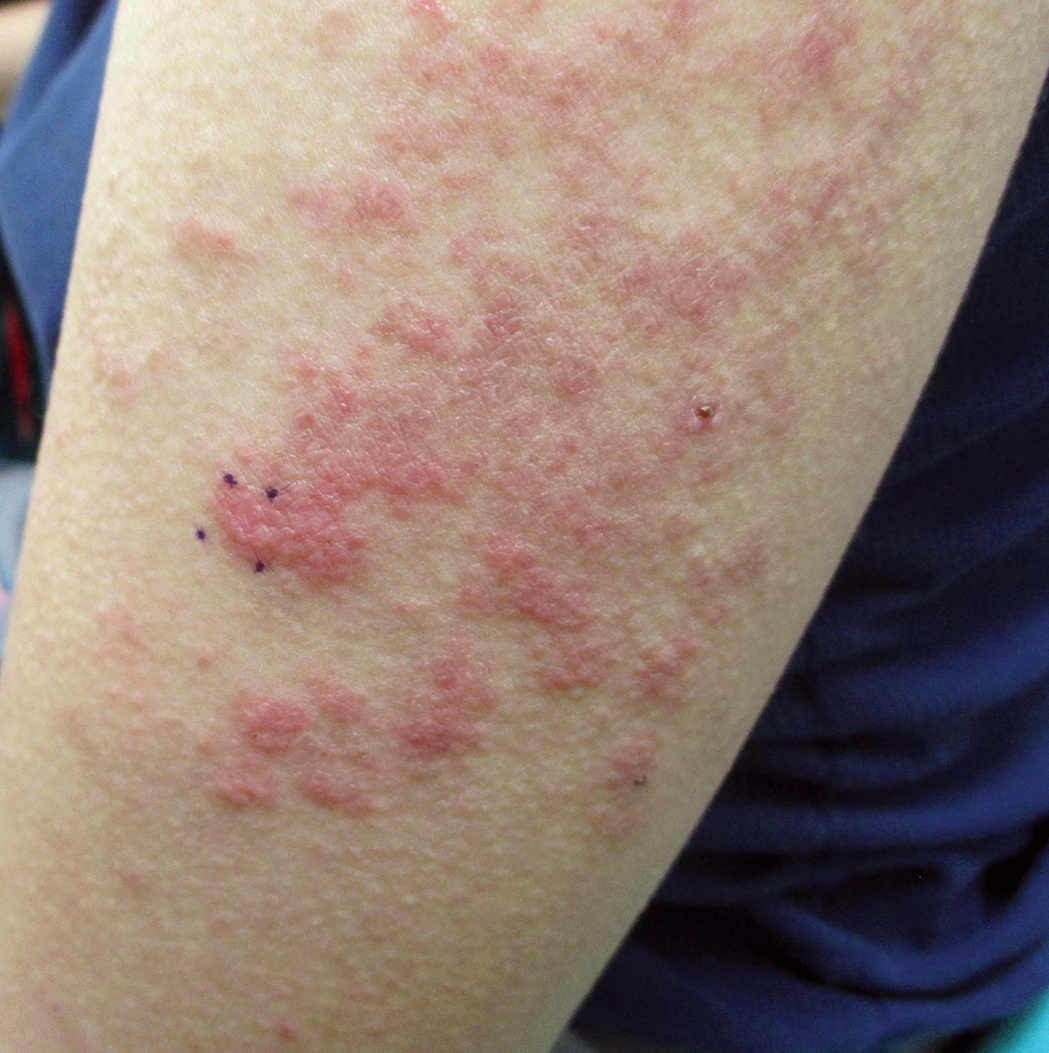
Three years later (5 years after the initial presentation), the patient presented with pruritic erythematous papulovesicles on the bilateral extensor elbows and right knee (Figure 1). The clinical appearance suggested dermatitis herpetiformis (DH).
Punch biopsies were obtained from the right elbow for H&E and DIF testing; the H&E-stained specimen showed lichenoid dermatitis with prominent dermal mucin, consistent with cutaneous lupus erythematosus. Direct immunofluorescence showed prominent linear IgG, linear IgA, and granular IgM along the basement membrane, which were identical to DIF findings of the original eruption.
Further laboratory testing revealed hypocomplementemia, anemia of chronic disease (hemoglobin, 8.4 g/dL [reference range, 14.0–17.5 g/dL]), and an elevated erythrocyte sedimentation rate. Given the clinical appearance of the vesicles, DIF findings, and the corresponding SLE flare, a diagnosis of BSLE was made. Because of the systemic symptoms, skin findings, and laboratory results, azathioprine was started. The cutaneous symptoms were treated and resolved with the addition of triamcinolone ointment 0.1% twice daily.
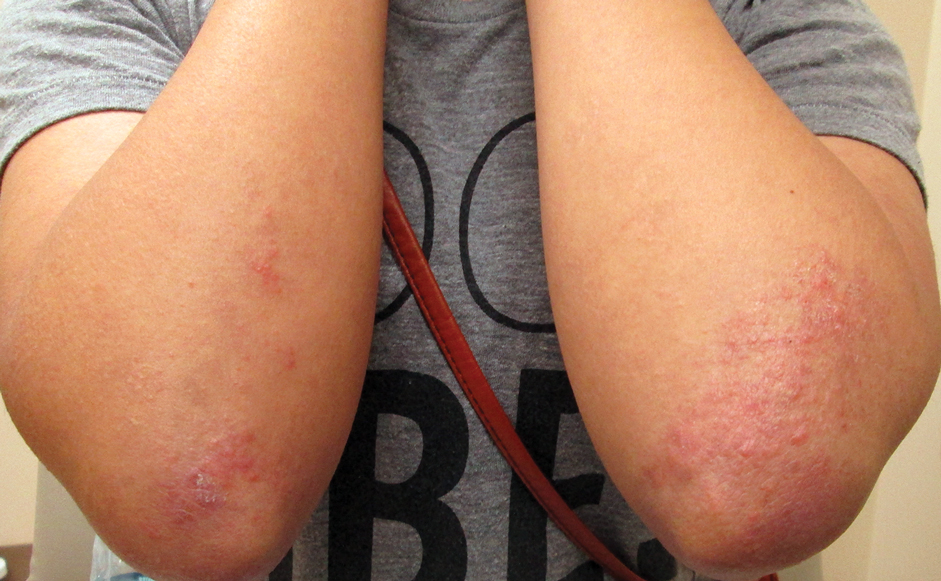
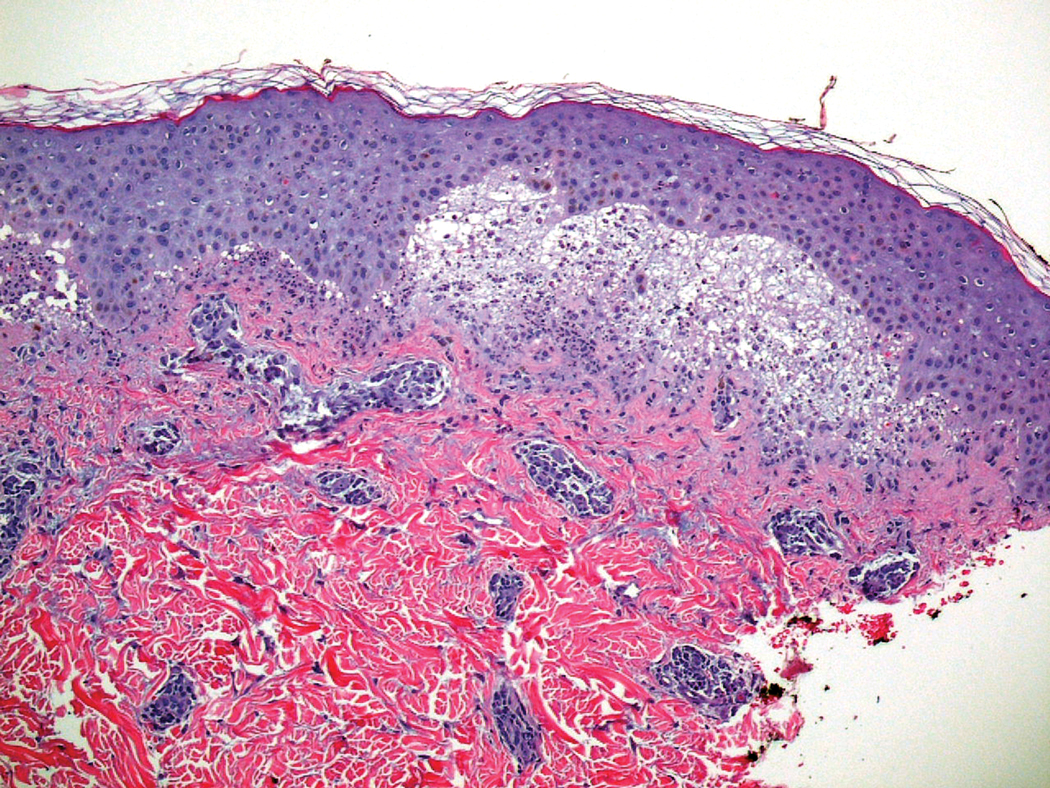
Six months later, the patient presented to our facility with fatigue, arthralgia, and numerous erythematous papules coalescing into a large plaque on the left upper arm (Figure 2). Biopsy showed interface dermatitis with numerous neutrophils and early vesiculation, consistent with BSLE (Figure 3). She underwent another course of 2 infusions of rituximab (1000 mg) administered 2 weeks apart, with resolution of cutaneous and systemic disease.
Comment
Diagnosis of BSLE
Bullous systemic lupus erythematosus is a rare cutaneous complication of SLE. It typically affects young black women in the second to fourth decades of life.1 It is a heterogeneous disorder with several clinical variants reported in the literature, and it can be mistaken for bullous pemphigoid, epidermolysis bullosa acquisita (EBA), linear IgA bullous dermatosis, and DH.1-3 Despite its varying clinical phenotypes, BSLE is associated with autoantibodies to the EBA antigen, type VII collagen.1
Current diagnostic criteria for BSLE, revised in 1995,5 include the following: (1) a diagnosis of SLE, based on criteria outlined by the American College of Rheumatology6; (2) vesicles or bullae, or both, involving but not limited to sun-exposed skin; (3) histopathologic features similar to DH; (4) DIF with IgG or IgM, or both, and IgA at the basement membrane zone; and (5) indirect immunofluorescence testing for circulating autoantibodies against the basement membrane zone, using the salt-split skin technique.
Clinical Presentation of BSLE
The classic phenotype associated with BSLE is similar to our patient’s original eruption, with tense bullae favoring the upper trunk and healing without scarring. The extensor surfaces typically are spared. Another presentation of BSLE is an EBA-like phenotype, with bullae on acral and extensor surfaces that heal with scarring. The EBA-like phenotype usually is more difficult to control. Lesions appearing clinically similar to DH have been reported, either as DH associated with SLE (later postulated to have been BSLE) or as herpetiform BSLE.1,4,7-10
Histopathology of BSLE
The typical histologic appearance of BSLE is similar to DH or linear IgA bullous dermatosis, with a predominantly neutrophilic inflammatory infiltrate in the upper dermis and a subepidermal split. Direct immunofluorescence shows broad deposition of IgG along the basement membrane zone (93% of cases; 60% of which are linear and 40% are granular), with approximately 70% of cases showing positive IgA or IgM, or both, at the basement membrane zone. Indirect immunofluorescence performed on 1 M NaCl salt-split skin showed staining on the dermal side of the split, similar to EBA.11
Treatment Options
Rapid clinical response has been reported with dapsone, usually in combination with other immunosuppresants.1,2 A subset of patients does not respond to dapsone, however, as was the case in our patient who tried dapsone early in the disease course but was not effective. Other therapies including azathioprine, cyclophosphamide, mycophenolate mofetil, and antimalarials have been used with some success.3
Rituximab, an anti-CD20 monoclonal antibody, has been used off label to treat BSLE cases that are resistant to dapsone, corticosteroids, and other immunosuppressants.12 Rituximab functions by depleting CD20+ B cells, thus altering the production of autoantibodies and, in the case of BSLE, reducing the concentration of circulating anti–type VII collagen antibodies. Rituximab was approved by the US Food and Drug Administration in 1997 for the treatment of non–Hodgkin lymphoma and later for chronic lymphocytic leukemia, rheumatoid arthritis, granulomatosis with polyangiitis (Wegener granulomatosis), and microscopic polyangiitis.12 Off-label administration of rituximab to treat autoimmune bullous dermatoses has been increasing, and the drug is now approved by the US Food and Drug Administration to treat pemphigus vulgaris (as of June 2018).13
In 2011, Alsanafi et al12 reported successful treatment of BSLE with rituximab in a 61-year-old black woman who had rapid clearance of skin lesions. Our patient had rapid resolution of cutaneous disease with rituximab after the second infusion in a 2-infusion regimen. Interestingly, rituximab is the only agent that has reliably resulted in resolution of our patient’s cutaneous and systemic disease during multiple episodes.
There is little information in the literature regarding the duration of response to rituximab in BSLE or its use in subsequent flares. Our patient relapsed at 2 years and again 3 years later (5 years after the initial presentation). The original cutaneous outbreak and subsequent relapse had classic clinical and histological findings for BSLE; however, the third cutaneous relapse was more similar to DH, given its distribution and appearance. However, the histopathologic findings were the same at the third relapse as they were at the initial presentation and not reflective of DH. We propose that our patient’s prior treatment with rituximab and ongoing immunosuppression at presentation contributed to the more atypical cutaneous findings observed late in the disease course.
Conclusion
We report this case to highlight the heterogeneity of BSLE, even in a single patient, and to report the time course of treatment with rituximab. Although BSLE is considered a rare cutaneous complication of SLE, it is important to note that BSLE also can present as the initial manifestation of SLE.7 As such, BSLE should always be included in the differential diagnosis for a patient presenting with a bullous eruption and symptoms that suggest SLE.
This case also illustrates the repeated use of rituximab for the treatment of BSLE over a 5-year period and justifies the need for larger population-based studies to demonstrate the efficacy of rituximab in BSLE.
- Contestable JJ, Edhegard KD, Meyerle JH. Bullous systemic lupus erythematosus: a review and update to diagnosis and treatment. Am J Clin Dermatol. 2014;15:517-524.
- Camisa C. Vesiculobullous systemic lupus erythematosus. a report of four cases. J Am Acad Dermatol. 1988;18(1, pt 1):93-100.
- Tincopa M, Puttgen KB, Sule S, et al. Bullous lupus: an unusual initial presentation of systemic lupus erythematosus in an adolescent girl. Pediatr Dermatol. 2010;27:373-376.
- Burke KR, Green BP, Meyerle J. Bullous lupus in an 18-year-old. Pediatr Dermatol. 2011;28:483.
- Yell JA, Allen J, Wojnarowska F, et al. Bullous systemic lupus erythematosus: revised criteria for diagnosis. Br J Dermatol. 1995;132:921-928.
- Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheumat. 1997;40:1725.
- Fujimoto W, Hamada T, Yamada J, et al. Bullous systemic lupus erythematosus as an initial manifestation of SLE. J Dermatol. 2005;32:1021-1027.
- Moncada B. Dermatitis herpetiformis in association with systemic lupus erythematosus. Arch Dermatol. 1974;109:723-725.
- Davies MG, Marks R, Waddington E. Simultaneous systemic lupus erythematosus and dermatitis herpetiformis. Arch Dermatol. 1976;112:1292-1294.
- Burrows N, Bhogal BS, Black MM, et al. Bullous eruption of systemic lupus erythematosus: a clinicopathological study of four cases. Br J Dermatol. 1993;128:332-338.
- Sebaratnam DF, Murrell DF. Bullous systemic lupus erythematosus. Dermatol Clin. 2011;29:649-653.
- Alsanafi S, Kovarik C, Mermelstein AL, et al. Rituximab in the treatment of bullous systemic lupus erythematosus. J Clin Rheumatol. 2011;17:142-144.
- Durable remission of pemphigus with a fixed-dose rituximab protocol. JAMA Dermatol. 2014;150:703-708.
Bullous systemic lupus erythematosus (BSLE) is a rare cutaneous presentation of systemic lupus erythematosus (SLE).1 Although 59% to 85% of SLE patients develop skin-related symptoms, fewer than 5% of SLE patients develop BSLE.1-3 This acquired autoimmune bullous disease, characterized by subepidermal bullae with a neutrophilic infiltrate on histopathology, is precipitated by autoantibodies to type VII collagen. Bullae can appear on both cutaneous and mucosal surfaces but tend to favor the trunk, upper extremities, neck, face, and vermilion border.3
Our case of an 18-year-old black woman with BSLE was originally reported in 2011.4 We update the case to illustrate the heterogeneous presentation of BSLE in a single patient and to expand on the role of rituximab in this disease.
Case Report
An 18-year-old black woman presented with a vesicular eruption of 3 weeks’ duration that started on the trunk and buttocks and progressed to involve the face, oral mucosa, and posterior auricular area. The vesicular eruption was accompanied by fatigue, arthralgia, and myalgia.
Physical examination revealed multiple tense, fluid-filled vesicles, measuring roughly 2 to 3 mm in diameter, over the cheeks, chin, postauricular area, vermilion border, oral mucosa, and left side of the neck and shoulder. Resolved lesions on the trunk and buttocks were marked by superficial crust and postinflammatory hyperpigmentation. Scarring was absent.
Laboratory analysis demonstrated hemolytic anemia with a positive direct antiglobulin test, hypocomplementemia, and an elevated erythrocyte sedimentation rate. Antinuclear antibody testing was positive (titer, 1:640).
Biopsies were taken from the left cheek for hematoxylin and eosin (H&E) staining and direct immunofluorescence (DIF), which revealed subepidermal clefting, few neutrophils, and notable mucin deposition. Direct immunofluorescence showed a broad deposition of IgG, IgA, and IgM, as well as C3 in a ribbonlike pattern at the dermoepidermal junction.
A diagnosis of SLE with BSLE was made. The patient initially was treated with prednisone, hydroxychloroquine, mycophenolate mofetil, and intravenous immunoglobulin, but the cutaneous disease persisted. The bullous eruption resolved with 2 infusions of rituximab (1000 mg) spaced 2 weeks apart.
The patient was in remission on 5 mg of prednisone for 2 years following the initial course of rituximab. However, she developed a flare of SLE, with fatigue, arthralgia, hypocomplementemia, and recurrence of BSLE with tense bullae on the face and lips. The flare resolved with prednisone and a single infusion of rituximab (1000 mg). She was then maintained on hydroxychloroquine (200 mg/d).
Three years later (5 years after the initial presentation), the patient presented with pruritic erythematous papulovesicles on the bilateral extensor elbows and right knee (Figure 1). The clinical appearance suggested dermatitis herpetiformis (DH).

Punch biopsies were obtained from the right elbow for H&E and DIF testing; the H&E-stained specimen showed lichenoid dermatitis with prominent dermal mucin, consistent with cutaneous lupus erythematosus. Direct immunofluorescence showed prominent linear IgG, linear IgA, and granular IgM along the basement membrane, which were identical to DIF findings of the original eruption.
Further laboratory testing revealed hypocomplementemia, anemia of chronic disease (hemoglobin, 8.4 g/dL [reference range, 14.0–17.5 g/dL]), and an elevated erythrocyte sedimentation rate. Given the clinical appearance of the vesicles, DIF findings, and the corresponding SLE flare, a diagnosis of BSLE was made. Because of the systemic symptoms, skin findings, and laboratory results, azathioprine was started. The cutaneous symptoms were treated and resolved with the addition of triamcinolone ointment 0.1% twice daily.
Six months later, the patient presented to our facility with fatigue, arthralgia, and numerous erythematous papules coalescing into a large plaque on the left upper arm (Figure 2). Biopsy showed interface dermatitis with numerous neutrophils and early vesiculation, consistent with BSLE (Figure 3). She underwent another course of 2 infusions of rituximab (1000 mg) administered 2 weeks apart, with resolution of cutaneous and systemic disease.


Comment
Diagnosis of BSLE
Bullous systemic lupus erythematosus is a rare cutaneous complication of SLE. It typically affects young black women in the second to fourth decades of life.1 It is a heterogeneous disorder with several clinical variants reported in the literature, and it can be mistaken for bullous pemphigoid, epidermolysis bullosa acquisita (EBA), linear IgA bullous dermatosis, and DH.1-3 Despite its varying clinical phenotypes, BSLE is associated with autoantibodies to the EBA antigen, type VII collagen.1
Current diagnostic criteria for BSLE, revised in 1995,5 include the following: (1) a diagnosis of SLE, based on criteria outlined by the American College of Rheumatology6; (2) vesicles or bullae, or both, involving but not limited to sun-exposed skin; (3) histopathologic features similar to DH; (4) DIF with IgG or IgM, or both, and IgA at the basement membrane zone; and (5) indirect immunofluorescence testing for circulating autoantibodies against the basement membrane zone, using the salt-split skin technique.
Clinical Presentation of BSLE
The classic phenotype associated with BSLE is similar to our patient’s original eruption, with tense bullae favoring the upper trunk and healing without scarring. The extensor surfaces typically are spared. Another presentation of BSLE is an EBA-like phenotype, with bullae on acral and extensor surfaces that heal with scarring. The EBA-like phenotype usually is more difficult to control. Lesions appearing clinically similar to DH have been reported, either as DH associated with SLE (later postulated to have been BSLE) or as herpetiform BSLE.1,4,7-10
Histopathology of BSLE
The typical histologic appearance of BSLE is similar to DH or linear IgA bullous dermatosis, with a predominantly neutrophilic inflammatory infiltrate in the upper dermis and a subepidermal split. Direct immunofluorescence shows broad deposition of IgG along the basement membrane zone (93% of cases; 60% of which are linear and 40% are granular), with approximately 70% of cases showing positive IgA or IgM, or both, at the basement membrane zone. Indirect immunofluorescence performed on 1 M NaCl salt-split skin showed staining on the dermal side of the split, similar to EBA.11
Treatment Options
Rapid clinical response has been reported with dapsone, usually in combination with other immunosuppresants.1,2 A subset of patients does not respond to dapsone, however, as was the case in our patient who tried dapsone early in the disease course but was not effective. Other therapies including azathioprine, cyclophosphamide, mycophenolate mofetil, and antimalarials have been used with some success.3
Rituximab, an anti-CD20 monoclonal antibody, has been used off label to treat BSLE cases that are resistant to dapsone, corticosteroids, and other immunosuppressants.12 Rituximab functions by depleting CD20+ B cells, thus altering the production of autoantibodies and, in the case of BSLE, reducing the concentration of circulating anti–type VII collagen antibodies. Rituximab was approved by the US Food and Drug Administration in 1997 for the treatment of non–Hodgkin lymphoma and later for chronic lymphocytic leukemia, rheumatoid arthritis, granulomatosis with polyangiitis (Wegener granulomatosis), and microscopic polyangiitis.12 Off-label administration of rituximab to treat autoimmune bullous dermatoses has been increasing, and the drug is now approved by the US Food and Drug Administration to treat pemphigus vulgaris (as of June 2018).13
In 2011, Alsanafi et al12 reported successful treatment of BSLE with rituximab in a 61-year-old black woman who had rapid clearance of skin lesions. Our patient had rapid resolution of cutaneous disease with rituximab after the second infusion in a 2-infusion regimen. Interestingly, rituximab is the only agent that has reliably resulted in resolution of our patient’s cutaneous and systemic disease during multiple episodes.
There is little information in the literature regarding the duration of response to rituximab in BSLE or its use in subsequent flares. Our patient relapsed at 2 years and again 3 years later (5 years after the initial presentation). The original cutaneous outbreak and subsequent relapse had classic clinical and histological findings for BSLE; however, the third cutaneous relapse was more similar to DH, given its distribution and appearance. However, the histopathologic findings were the same at the third relapse as they were at the initial presentation and not reflective of DH. We propose that our patient’s prior treatment with rituximab and ongoing immunosuppression at presentation contributed to the more atypical cutaneous findings observed late in the disease course.
Conclusion
We report this case to highlight the heterogeneity of BSLE, even in a single patient, and to report the time course of treatment with rituximab. Although BSLE is considered a rare cutaneous complication of SLE, it is important to note that BSLE also can present as the initial manifestation of SLE.7 As such, BSLE should always be included in the differential diagnosis for a patient presenting with a bullous eruption and symptoms that suggest SLE.
This case also illustrates the repeated use of rituximab for the treatment of BSLE over a 5-year period and justifies the need for larger population-based studies to demonstrate the efficacy of rituximab in BSLE.
Bullous systemic lupus erythematosus (BSLE) is a rare cutaneous presentation of systemic lupus erythematosus (SLE).1 Although 59% to 85% of SLE patients develop skin-related symptoms, fewer than 5% of SLE patients develop BSLE.1-3 This acquired autoimmune bullous disease, characterized by subepidermal bullae with a neutrophilic infiltrate on histopathology, is precipitated by autoantibodies to type VII collagen. Bullae can appear on both cutaneous and mucosal surfaces but tend to favor the trunk, upper extremities, neck, face, and vermilion border.3
Our case of an 18-year-old black woman with BSLE was originally reported in 2011.4 We update the case to illustrate the heterogeneous presentation of BSLE in a single patient and to expand on the role of rituximab in this disease.
Case Report
An 18-year-old black woman presented with a vesicular eruption of 3 weeks’ duration that started on the trunk and buttocks and progressed to involve the face, oral mucosa, and posterior auricular area. The vesicular eruption was accompanied by fatigue, arthralgia, and myalgia.
Physical examination revealed multiple tense, fluid-filled vesicles, measuring roughly 2 to 3 mm in diameter, over the cheeks, chin, postauricular area, vermilion border, oral mucosa, and left side of the neck and shoulder. Resolved lesions on the trunk and buttocks were marked by superficial crust and postinflammatory hyperpigmentation. Scarring was absent.
Laboratory analysis demonstrated hemolytic anemia with a positive direct antiglobulin test, hypocomplementemia, and an elevated erythrocyte sedimentation rate. Antinuclear antibody testing was positive (titer, 1:640).
Biopsies were taken from the left cheek for hematoxylin and eosin (H&E) staining and direct immunofluorescence (DIF), which revealed subepidermal clefting, few neutrophils, and notable mucin deposition. Direct immunofluorescence showed a broad deposition of IgG, IgA, and IgM, as well as C3 in a ribbonlike pattern at the dermoepidermal junction.
A diagnosis of SLE with BSLE was made. The patient initially was treated with prednisone, hydroxychloroquine, mycophenolate mofetil, and intravenous immunoglobulin, but the cutaneous disease persisted. The bullous eruption resolved with 2 infusions of rituximab (1000 mg) spaced 2 weeks apart.
The patient was in remission on 5 mg of prednisone for 2 years following the initial course of rituximab. However, she developed a flare of SLE, with fatigue, arthralgia, hypocomplementemia, and recurrence of BSLE with tense bullae on the face and lips. The flare resolved with prednisone and a single infusion of rituximab (1000 mg). She was then maintained on hydroxychloroquine (200 mg/d).
Three years later (5 years after the initial presentation), the patient presented with pruritic erythematous papulovesicles on the bilateral extensor elbows and right knee (Figure 1). The clinical appearance suggested dermatitis herpetiformis (DH).

Punch biopsies were obtained from the right elbow for H&E and DIF testing; the H&E-stained specimen showed lichenoid dermatitis with prominent dermal mucin, consistent with cutaneous lupus erythematosus. Direct immunofluorescence showed prominent linear IgG, linear IgA, and granular IgM along the basement membrane, which were identical to DIF findings of the original eruption.
Further laboratory testing revealed hypocomplementemia, anemia of chronic disease (hemoglobin, 8.4 g/dL [reference range, 14.0–17.5 g/dL]), and an elevated erythrocyte sedimentation rate. Given the clinical appearance of the vesicles, DIF findings, and the corresponding SLE flare, a diagnosis of BSLE was made. Because of the systemic symptoms, skin findings, and laboratory results, azathioprine was started. The cutaneous symptoms were treated and resolved with the addition of triamcinolone ointment 0.1% twice daily.
Six months later, the patient presented to our facility with fatigue, arthralgia, and numerous erythematous papules coalescing into a large plaque on the left upper arm (Figure 2). Biopsy showed interface dermatitis with numerous neutrophils and early vesiculation, consistent with BSLE (Figure 3). She underwent another course of 2 infusions of rituximab (1000 mg) administered 2 weeks apart, with resolution of cutaneous and systemic disease.


Comment
Diagnosis of BSLE
Bullous systemic lupus erythematosus is a rare cutaneous complication of SLE. It typically affects young black women in the second to fourth decades of life.1 It is a heterogeneous disorder with several clinical variants reported in the literature, and it can be mistaken for bullous pemphigoid, epidermolysis bullosa acquisita (EBA), linear IgA bullous dermatosis, and DH.1-3 Despite its varying clinical phenotypes, BSLE is associated with autoantibodies to the EBA antigen, type VII collagen.1
Current diagnostic criteria for BSLE, revised in 1995,5 include the following: (1) a diagnosis of SLE, based on criteria outlined by the American College of Rheumatology6; (2) vesicles or bullae, or both, involving but not limited to sun-exposed skin; (3) histopathologic features similar to DH; (4) DIF with IgG or IgM, or both, and IgA at the basement membrane zone; and (5) indirect immunofluorescence testing for circulating autoantibodies against the basement membrane zone, using the salt-split skin technique.
Clinical Presentation of BSLE
The classic phenotype associated with BSLE is similar to our patient’s original eruption, with tense bullae favoring the upper trunk and healing without scarring. The extensor surfaces typically are spared. Another presentation of BSLE is an EBA-like phenotype, with bullae on acral and extensor surfaces that heal with scarring. The EBA-like phenotype usually is more difficult to control. Lesions appearing clinically similar to DH have been reported, either as DH associated with SLE (later postulated to have been BSLE) or as herpetiform BSLE.1,4,7-10
Histopathology of BSLE
The typical histologic appearance of BSLE is similar to DH or linear IgA bullous dermatosis, with a predominantly neutrophilic inflammatory infiltrate in the upper dermis and a subepidermal split. Direct immunofluorescence shows broad deposition of IgG along the basement membrane zone (93% of cases; 60% of which are linear and 40% are granular), with approximately 70% of cases showing positive IgA or IgM, or both, at the basement membrane zone. Indirect immunofluorescence performed on 1 M NaCl salt-split skin showed staining on the dermal side of the split, similar to EBA.11
Treatment Options
Rapid clinical response has been reported with dapsone, usually in combination with other immunosuppresants.1,2 A subset of patients does not respond to dapsone, however, as was the case in our patient who tried dapsone early in the disease course but was not effective. Other therapies including azathioprine, cyclophosphamide, mycophenolate mofetil, and antimalarials have been used with some success.3
Rituximab, an anti-CD20 monoclonal antibody, has been used off label to treat BSLE cases that are resistant to dapsone, corticosteroids, and other immunosuppressants.12 Rituximab functions by depleting CD20+ B cells, thus altering the production of autoantibodies and, in the case of BSLE, reducing the concentration of circulating anti–type VII collagen antibodies. Rituximab was approved by the US Food and Drug Administration in 1997 for the treatment of non–Hodgkin lymphoma and later for chronic lymphocytic leukemia, rheumatoid arthritis, granulomatosis with polyangiitis (Wegener granulomatosis), and microscopic polyangiitis.12 Off-label administration of rituximab to treat autoimmune bullous dermatoses has been increasing, and the drug is now approved by the US Food and Drug Administration to treat pemphigus vulgaris (as of June 2018).13
In 2011, Alsanafi et al12 reported successful treatment of BSLE with rituximab in a 61-year-old black woman who had rapid clearance of skin lesions. Our patient had rapid resolution of cutaneous disease with rituximab after the second infusion in a 2-infusion regimen. Interestingly, rituximab is the only agent that has reliably resulted in resolution of our patient’s cutaneous and systemic disease during multiple episodes.
There is little information in the literature regarding the duration of response to rituximab in BSLE or its use in subsequent flares. Our patient relapsed at 2 years and again 3 years later (5 years after the initial presentation). The original cutaneous outbreak and subsequent relapse had classic clinical and histological findings for BSLE; however, the third cutaneous relapse was more similar to DH, given its distribution and appearance. However, the histopathologic findings were the same at the third relapse as they were at the initial presentation and not reflective of DH. We propose that our patient’s prior treatment with rituximab and ongoing immunosuppression at presentation contributed to the more atypical cutaneous findings observed late in the disease course.
Conclusion
We report this case to highlight the heterogeneity of BSLE, even in a single patient, and to report the time course of treatment with rituximab. Although BSLE is considered a rare cutaneous complication of SLE, it is important to note that BSLE also can present as the initial manifestation of SLE.7 As such, BSLE should always be included in the differential diagnosis for a patient presenting with a bullous eruption and symptoms that suggest SLE.
This case also illustrates the repeated use of rituximab for the treatment of BSLE over a 5-year period and justifies the need for larger population-based studies to demonstrate the efficacy of rituximab in BSLE.
- Contestable JJ, Edhegard KD, Meyerle JH. Bullous systemic lupus erythematosus: a review and update to diagnosis and treatment. Am J Clin Dermatol. 2014;15:517-524.
- Camisa C. Vesiculobullous systemic lupus erythematosus. a report of four cases. J Am Acad Dermatol. 1988;18(1, pt 1):93-100.
- Tincopa M, Puttgen KB, Sule S, et al. Bullous lupus: an unusual initial presentation of systemic lupus erythematosus in an adolescent girl. Pediatr Dermatol. 2010;27:373-376.
- Burke KR, Green BP, Meyerle J. Bullous lupus in an 18-year-old. Pediatr Dermatol. 2011;28:483.
- Yell JA, Allen J, Wojnarowska F, et al. Bullous systemic lupus erythematosus: revised criteria for diagnosis. Br J Dermatol. 1995;132:921-928.
- Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheumat. 1997;40:1725.
- Fujimoto W, Hamada T, Yamada J, et al. Bullous systemic lupus erythematosus as an initial manifestation of SLE. J Dermatol. 2005;32:1021-1027.
- Moncada B. Dermatitis herpetiformis in association with systemic lupus erythematosus. Arch Dermatol. 1974;109:723-725.
- Davies MG, Marks R, Waddington E. Simultaneous systemic lupus erythematosus and dermatitis herpetiformis. Arch Dermatol. 1976;112:1292-1294.
- Burrows N, Bhogal BS, Black MM, et al. Bullous eruption of systemic lupus erythematosus: a clinicopathological study of four cases. Br J Dermatol. 1993;128:332-338.
- Sebaratnam DF, Murrell DF. Bullous systemic lupus erythematosus. Dermatol Clin. 2011;29:649-653.
- Alsanafi S, Kovarik C, Mermelstein AL, et al. Rituximab in the treatment of bullous systemic lupus erythematosus. J Clin Rheumatol. 2011;17:142-144.
- Durable remission of pemphigus with a fixed-dose rituximab protocol. JAMA Dermatol. 2014;150:703-708.
- Contestable JJ, Edhegard KD, Meyerle JH. Bullous systemic lupus erythematosus: a review and update to diagnosis and treatment. Am J Clin Dermatol. 2014;15:517-524.
- Camisa C. Vesiculobullous systemic lupus erythematosus. a report of four cases. J Am Acad Dermatol. 1988;18(1, pt 1):93-100.
- Tincopa M, Puttgen KB, Sule S, et al. Bullous lupus: an unusual initial presentation of systemic lupus erythematosus in an adolescent girl. Pediatr Dermatol. 2010;27:373-376.
- Burke KR, Green BP, Meyerle J. Bullous lupus in an 18-year-old. Pediatr Dermatol. 2011;28:483.
- Yell JA, Allen J, Wojnarowska F, et al. Bullous systemic lupus erythematosus: revised criteria for diagnosis. Br J Dermatol. 1995;132:921-928.
- Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheumat. 1997;40:1725.
- Fujimoto W, Hamada T, Yamada J, et al. Bullous systemic lupus erythematosus as an initial manifestation of SLE. J Dermatol. 2005;32:1021-1027.
- Moncada B. Dermatitis herpetiformis in association with systemic lupus erythematosus. Arch Dermatol. 1974;109:723-725.
- Davies MG, Marks R, Waddington E. Simultaneous systemic lupus erythematosus and dermatitis herpetiformis. Arch Dermatol. 1976;112:1292-1294.
- Burrows N, Bhogal BS, Black MM, et al. Bullous eruption of systemic lupus erythematosus: a clinicopathological study of four cases. Br J Dermatol. 1993;128:332-338.
- Sebaratnam DF, Murrell DF. Bullous systemic lupus erythematosus. Dermatol Clin. 2011;29:649-653.
- Alsanafi S, Kovarik C, Mermelstein AL, et al. Rituximab in the treatment of bullous systemic lupus erythematosus. J Clin Rheumatol. 2011;17:142-144.
- Durable remission of pemphigus with a fixed-dose rituximab protocol. JAMA Dermatol. 2014;150:703-708.
Practice Points
- Bullous systemic lupus erythematosus (BSLE) can present with a waxing and waning course punctuated by flares.
- Different clinical presentations can occur over the disease course.
- Rituximab is a viable treatment option in BSLE.
Heparin-Induced Bullous Hemorrhagic Dermatosis Confined to the Oral Mucosa
Heparin is a naturally occurring anticoagulant and is commonly used to treat or prevent venous thrombosis or the extension of thrombosis.
Adverse effects of heparin administration include bleeding, injection-site pain, and thrombocytopenia. Heparin-induced thrombocytopenia (HIT) is a serious side effect wherein antibodies are formed against platelet antigens and predispose the patient to venous and arterial thrombosis.
Bullous hemorrhagic dermatosis is a poorly understood idiosyncratic drug reaction characterized by tense, blood-filled blisters that arise following the administration of subcutaneous low-molecular-weight heparin or intravenous unfractionated heparin (UFH). First reported in 2006 by Perrinaud et al
Case Report
An 84-year-old man was admitted to the cardiology service with severe substernal chest pain. An electrocardiogram did not show any ST-segment elevations; however, he had elevated troponin T levels. He had a medical history of coronary artery disease complicated by myocardial infarction (MI), as well as ischemic cardiomyopathy, hypertension, hyperlipidemia, ischemic stroke, and pulmonary embolism for which he was on long-term anticoagulation for years with warfarin, aspirin, and clopidogrel. The patient was diagnosed with a non–ST-segment elevation MI. Accordingly, the patient’s warfarin was discontinued, and he was administered a bolus and continuous infusion of UFH. He also was continued on aspirin and clopidogrel. Within 6 hours of initiation of UFH, the patient noted multiple discrete swollen lesions in the mouth. Dermatology consultation and biopsy of the lesions were deferred due to acute management of the patient’s MI.
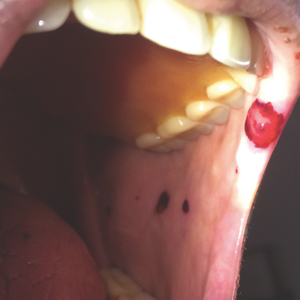
Physical examination revealed a moist oral mucosa with 7 slightly raised, hemorrhagic bullae ranging from 2 to 7 mm in diameter (Figure, A and B). One oral lesion was tense and had become denuded prior to evaluation. Laboratory testing included a normal platelet count (160,000/µL), a nearly therapeutic international normalized ratio (1.9), and a partial thromboplastin time that was initially normal (27 seconds) prior to admission and development of the oral lesions but found to be elevated (176 seconds) after admission and initial UFH bolus.
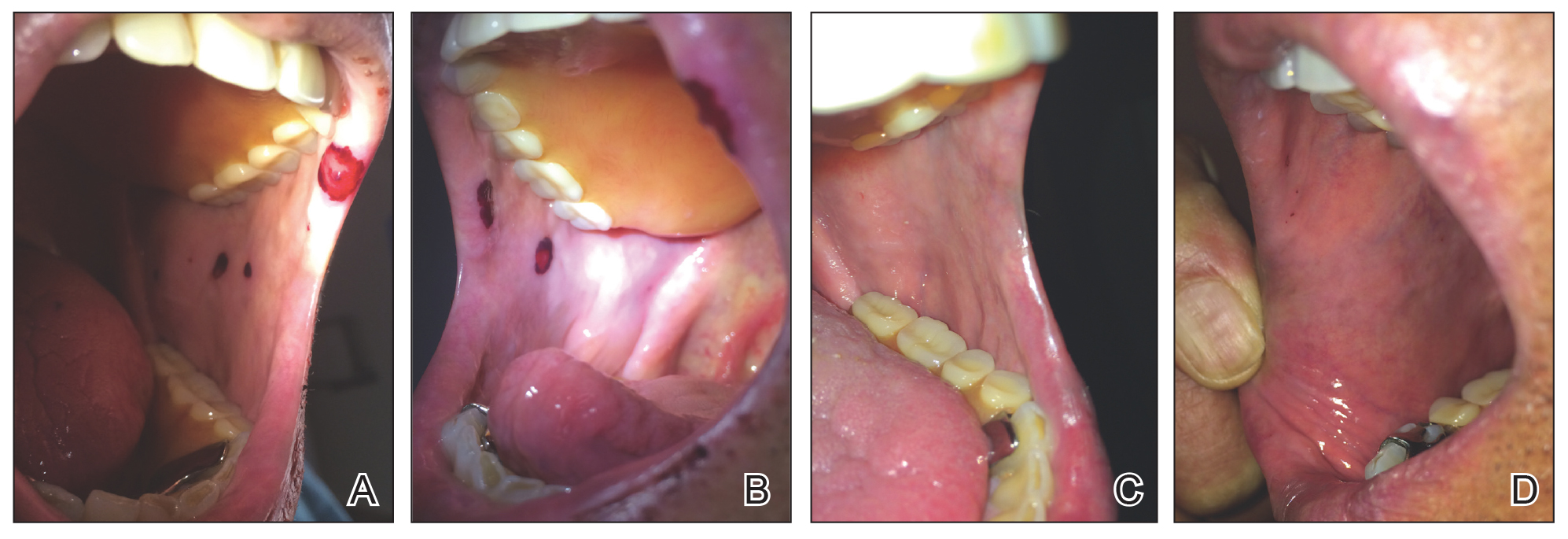
Upon further questioning, the patient revealed a history of similar oral lesions 1 year prior, following exposure to subcutaneous enoxaparin. At that time, formal evaluation by dermatology was deferred due to the rapid resolution of the blisters. Despite these new oral lesions, the patient was continued on a heparin drip for the next 48 hours because of the mortality benefit of heparin in non–ST-segment elevation MI. The patient was discharged from the hospital on a regimen of aspirin, warfarin, and clopidogrel. At 2-week follow-up, the oral lesions had resolved (Figure, C and D).
Comment
Heparin-Induced Skin Lesions
The 2 most common types of heparin-induced skin lesions are delayed-type hypersensitivity reactions and immune-mediated HIT. A 2009 Canadian study found that the overwhelming majority of heparin-induced skin lesions are due to delayed-type hypersensitivity reactions.
Types of HIT
Heparin-induced thrombocytopenia is one of the most serious adverse reactions to heparin administration. There are 2 subtypes of HIT, which differ in their clinical significance and pathophysiology.
Type II HIT is an immune-mediated response caused by the formation of IgG autoantibodies against the heparin–platelet factor 4 complex. Antibody formation and thrombocytopenia typically occur after 4 to 10 days of heparin exposure, and there can be devastating arterial and venous thrombotic complications.
Diagnosis of HIT
Heparin-induced thrombocytopenia should be suspected in patients with a lowered platelet count, particularly if the decrease is more than 50% from baseline, and in patients who develop stroke, MI, pulmonary embolism, or deep vein thrombosis while on heparin. Heparin-induced thrombocytopenia was not observed in our patient, as his platelet count remained stable between 160,000 and 164,000/µL throughout his hospital stay and he did not develop any evidence of thrombosis.
Differential Diagnosis
Our patient’s lesions appeared morphologically similar to
Bullous pemphigoid also was considered given the presence of tense bullae in an elderly patient. However, the rapid and spontaneous resolution of these lesions with complete lack of skin involvement made this diagnosis less likely.12
Heparin-Induced Bullous Hemorrhagic Dermatosis
Because our patient described a similar reaction while taking enoxaparin in the past, this case represents an idiosyncratic drug reaction, possibly from antibodies to a heparin-antigen complex. Heparin-induced bullous hemorrhagic dermatosis is a rarely reported condition with the majority of lesions presenting on the extremities.
Conclusion
We describe a rare side effect of heparin therapy characterized by discrete blisters on the oral mucosa. However, familiarity with the spectrum of reactions to heparin allowed the patient to continue heparin therapy despite this side effect, as the eruption was not life-threatening and the benefit of continuing heparin outweighed this adverse effect.
- Gómez-Outes A, Suárez-Gea ML, Calvo-Rojas G, et al. Discovery of anticoagulant drugs: a historical perspective. Curr Drug Discov Technol. 2012;9:83-104.
- Noti C, Seeberger PH. Chemical approaches to define the structure-activity relationship of heparin-like glycosaminoglycans. Chem Biol. 2005;12:731-756.
- Bakchoul T. An update on heparin-induced thrombocytopenia: diagnosis and management. Expert Opin Drug Saf. 2016;15:787-797.
- Schindewolf M, Schwaner S, Wolter M, et al. Incidence and causes of heparin-induced skin lesions. Can Med Assoc J. 2009;181:477-481.
- Perrinaud A, Jacobi D, Machet MC, et al. Bullous hemorrhagic dermatosis occurring at sites distant from subcutaneous injections of heparin: three cases. J Am Acad Dermatol. 2006;54(2 suppl):S5-S7.
- Naveen KN, Rai V. Bullous hemorrhagic dermatosis: a case report. Indian J Dermatol. 2014;59:423.
- Choudhry S, Fishman PM, Hernandez C. Heparin-induced bullous hemorrhagic dermatosis. Cutis. 2013;91:93-98.
- Villanueva CA, Nájera L, Espinosa P, et al. Bullous hemorrhagic dermatosis at distant sites: a report of 2 new cases due to enoxaparin injection and a review of the literature. Actas Dermosifiliogr. 2012;103:816-819.
- Ahmed I, Majeed A, Powell R. Heparin induced thrombocytopenia: diagnosis and management update. Postgrad Med J. 2007;83:575-582.
- Horie N, Kawano R, Inaba J, et al. Angina bullosa hemorrhagica of the soft palate: a clinical study of 16 cases. J Oral Sci. 2008;50:33-36.
- Rai S, Kaur M, Goel S. Angina bullosa hemorrhagica: report of 2 cases. Indian J Dermatol. 2012;57:503.
- Lawson W. Bullous oral lesions: clues to identifying—and managing—the cause. Consultant. 2013;53:168-176.
Heparin is a naturally occurring anticoagulant and is commonly used to treat or prevent venous thrombosis or the extension of thrombosis.
Adverse effects of heparin administration include bleeding, injection-site pain, and thrombocytopenia. Heparin-induced thrombocytopenia (HIT) is a serious side effect wherein antibodies are formed against platelet antigens and predispose the patient to venous and arterial thrombosis.
Bullous hemorrhagic dermatosis is a poorly understood idiosyncratic drug reaction characterized by tense, blood-filled blisters that arise following the administration of subcutaneous low-molecular-weight heparin or intravenous unfractionated heparin (UFH). First reported in 2006 by Perrinaud et al
Case Report
An 84-year-old man was admitted to the cardiology service with severe substernal chest pain. An electrocardiogram did not show any ST-segment elevations; however, he had elevated troponin T levels. He had a medical history of coronary artery disease complicated by myocardial infarction (MI), as well as ischemic cardiomyopathy, hypertension, hyperlipidemia, ischemic stroke, and pulmonary embolism for which he was on long-term anticoagulation for years with warfarin, aspirin, and clopidogrel. The patient was diagnosed with a non–ST-segment elevation MI. Accordingly, the patient’s warfarin was discontinued, and he was administered a bolus and continuous infusion of UFH. He also was continued on aspirin and clopidogrel. Within 6 hours of initiation of UFH, the patient noted multiple discrete swollen lesions in the mouth. Dermatology consultation and biopsy of the lesions were deferred due to acute management of the patient’s MI.
Physical examination revealed a moist oral mucosa with 7 slightly raised, hemorrhagic bullae ranging from 2 to 7 mm in diameter (Figure, A and B). One oral lesion was tense and had become denuded prior to evaluation. Laboratory testing included a normal platelet count (160,000/µL), a nearly therapeutic international normalized ratio (1.9), and a partial thromboplastin time that was initially normal (27 seconds) prior to admission and development of the oral lesions but found to be elevated (176 seconds) after admission and initial UFH bolus.

Upon further questioning, the patient revealed a history of similar oral lesions 1 year prior, following exposure to subcutaneous enoxaparin. At that time, formal evaluation by dermatology was deferred due to the rapid resolution of the blisters. Despite these new oral lesions, the patient was continued on a heparin drip for the next 48 hours because of the mortality benefit of heparin in non–ST-segment elevation MI. The patient was discharged from the hospital on a regimen of aspirin, warfarin, and clopidogrel. At 2-week follow-up, the oral lesions had resolved (Figure, C and D).
Comment
Heparin-Induced Skin Lesions
The 2 most common types of heparin-induced skin lesions are delayed-type hypersensitivity reactions and immune-mediated HIT. A 2009 Canadian study found that the overwhelming majority of heparin-induced skin lesions are due to delayed-type hypersensitivity reactions.
Types of HIT
Heparin-induced thrombocytopenia is one of the most serious adverse reactions to heparin administration. There are 2 subtypes of HIT, which differ in their clinical significance and pathophysiology.
Type II HIT is an immune-mediated response caused by the formation of IgG autoantibodies against the heparin–platelet factor 4 complex. Antibody formation and thrombocytopenia typically occur after 4 to 10 days of heparin exposure, and there can be devastating arterial and venous thrombotic complications.
Diagnosis of HIT
Heparin-induced thrombocytopenia should be suspected in patients with a lowered platelet count, particularly if the decrease is more than 50% from baseline, and in patients who develop stroke, MI, pulmonary embolism, or deep vein thrombosis while on heparin. Heparin-induced thrombocytopenia was not observed in our patient, as his platelet count remained stable between 160,000 and 164,000/µL throughout his hospital stay and he did not develop any evidence of thrombosis.
Differential Diagnosis
Our patient’s lesions appeared morphologically similar to
Bullous pemphigoid also was considered given the presence of tense bullae in an elderly patient. However, the rapid and spontaneous resolution of these lesions with complete lack of skin involvement made this diagnosis less likely.12
Heparin-Induced Bullous Hemorrhagic Dermatosis
Because our patient described a similar reaction while taking enoxaparin in the past, this case represents an idiosyncratic drug reaction, possibly from antibodies to a heparin-antigen complex. Heparin-induced bullous hemorrhagic dermatosis is a rarely reported condition with the majority of lesions presenting on the extremities.
Conclusion
We describe a rare side effect of heparin therapy characterized by discrete blisters on the oral mucosa. However, familiarity with the spectrum of reactions to heparin allowed the patient to continue heparin therapy despite this side effect, as the eruption was not life-threatening and the benefit of continuing heparin outweighed this adverse effect.
Heparin is a naturally occurring anticoagulant and is commonly used to treat or prevent venous thrombosis or the extension of thrombosis.
Adverse effects of heparin administration include bleeding, injection-site pain, and thrombocytopenia. Heparin-induced thrombocytopenia (HIT) is a serious side effect wherein antibodies are formed against platelet antigens and predispose the patient to venous and arterial thrombosis.
Bullous hemorrhagic dermatosis is a poorly understood idiosyncratic drug reaction characterized by tense, blood-filled blisters that arise following the administration of subcutaneous low-molecular-weight heparin or intravenous unfractionated heparin (UFH). First reported in 2006 by Perrinaud et al
Case Report
An 84-year-old man was admitted to the cardiology service with severe substernal chest pain. An electrocardiogram did not show any ST-segment elevations; however, he had elevated troponin T levels. He had a medical history of coronary artery disease complicated by myocardial infarction (MI), as well as ischemic cardiomyopathy, hypertension, hyperlipidemia, ischemic stroke, and pulmonary embolism for which he was on long-term anticoagulation for years with warfarin, aspirin, and clopidogrel. The patient was diagnosed with a non–ST-segment elevation MI. Accordingly, the patient’s warfarin was discontinued, and he was administered a bolus and continuous infusion of UFH. He also was continued on aspirin and clopidogrel. Within 6 hours of initiation of UFH, the patient noted multiple discrete swollen lesions in the mouth. Dermatology consultation and biopsy of the lesions were deferred due to acute management of the patient’s MI.
Physical examination revealed a moist oral mucosa with 7 slightly raised, hemorrhagic bullae ranging from 2 to 7 mm in diameter (Figure, A and B). One oral lesion was tense and had become denuded prior to evaluation. Laboratory testing included a normal platelet count (160,000/µL), a nearly therapeutic international normalized ratio (1.9), and a partial thromboplastin time that was initially normal (27 seconds) prior to admission and development of the oral lesions but found to be elevated (176 seconds) after admission and initial UFH bolus.

Upon further questioning, the patient revealed a history of similar oral lesions 1 year prior, following exposure to subcutaneous enoxaparin. At that time, formal evaluation by dermatology was deferred due to the rapid resolution of the blisters. Despite these new oral lesions, the patient was continued on a heparin drip for the next 48 hours because of the mortality benefit of heparin in non–ST-segment elevation MI. The patient was discharged from the hospital on a regimen of aspirin, warfarin, and clopidogrel. At 2-week follow-up, the oral lesions had resolved (Figure, C and D).
Comment
Heparin-Induced Skin Lesions
The 2 most common types of heparin-induced skin lesions are delayed-type hypersensitivity reactions and immune-mediated HIT. A 2009 Canadian study found that the overwhelming majority of heparin-induced skin lesions are due to delayed-type hypersensitivity reactions.
Types of HIT
Heparin-induced thrombocytopenia is one of the most serious adverse reactions to heparin administration. There are 2 subtypes of HIT, which differ in their clinical significance and pathophysiology.
Type II HIT is an immune-mediated response caused by the formation of IgG autoantibodies against the heparin–platelet factor 4 complex. Antibody formation and thrombocytopenia typically occur after 4 to 10 days of heparin exposure, and there can be devastating arterial and venous thrombotic complications.
Diagnosis of HIT
Heparin-induced thrombocytopenia should be suspected in patients with a lowered platelet count, particularly if the decrease is more than 50% from baseline, and in patients who develop stroke, MI, pulmonary embolism, or deep vein thrombosis while on heparin. Heparin-induced thrombocytopenia was not observed in our patient, as his platelet count remained stable between 160,000 and 164,000/µL throughout his hospital stay and he did not develop any evidence of thrombosis.
Differential Diagnosis
Our patient’s lesions appeared morphologically similar to
Bullous pemphigoid also was considered given the presence of tense bullae in an elderly patient. However, the rapid and spontaneous resolution of these lesions with complete lack of skin involvement made this diagnosis less likely.12
Heparin-Induced Bullous Hemorrhagic Dermatosis
Because our patient described a similar reaction while taking enoxaparin in the past, this case represents an idiosyncratic drug reaction, possibly from antibodies to a heparin-antigen complex. Heparin-induced bullous hemorrhagic dermatosis is a rarely reported condition with the majority of lesions presenting on the extremities.
Conclusion
We describe a rare side effect of heparin therapy characterized by discrete blisters on the oral mucosa. However, familiarity with the spectrum of reactions to heparin allowed the patient to continue heparin therapy despite this side effect, as the eruption was not life-threatening and the benefit of continuing heparin outweighed this adverse effect.
- Gómez-Outes A, Suárez-Gea ML, Calvo-Rojas G, et al. Discovery of anticoagulant drugs: a historical perspective. Curr Drug Discov Technol. 2012;9:83-104.
- Noti C, Seeberger PH. Chemical approaches to define the structure-activity relationship of heparin-like glycosaminoglycans. Chem Biol. 2005;12:731-756.
- Bakchoul T. An update on heparin-induced thrombocytopenia: diagnosis and management. Expert Opin Drug Saf. 2016;15:787-797.
- Schindewolf M, Schwaner S, Wolter M, et al. Incidence and causes of heparin-induced skin lesions. Can Med Assoc J. 2009;181:477-481.
- Perrinaud A, Jacobi D, Machet MC, et al. Bullous hemorrhagic dermatosis occurring at sites distant from subcutaneous injections of heparin: three cases. J Am Acad Dermatol. 2006;54(2 suppl):S5-S7.
- Naveen KN, Rai V. Bullous hemorrhagic dermatosis: a case report. Indian J Dermatol. 2014;59:423.
- Choudhry S, Fishman PM, Hernandez C. Heparin-induced bullous hemorrhagic dermatosis. Cutis. 2013;91:93-98.
- Villanueva CA, Nájera L, Espinosa P, et al. Bullous hemorrhagic dermatosis at distant sites: a report of 2 new cases due to enoxaparin injection and a review of the literature. Actas Dermosifiliogr. 2012;103:816-819.
- Ahmed I, Majeed A, Powell R. Heparin induced thrombocytopenia: diagnosis and management update. Postgrad Med J. 2007;83:575-582.
- Horie N, Kawano R, Inaba J, et al. Angina bullosa hemorrhagica of the soft palate: a clinical study of 16 cases. J Oral Sci. 2008;50:33-36.
- Rai S, Kaur M, Goel S. Angina bullosa hemorrhagica: report of 2 cases. Indian J Dermatol. 2012;57:503.
- Lawson W. Bullous oral lesions: clues to identifying—and managing—the cause. Consultant. 2013;53:168-176.
- Gómez-Outes A, Suárez-Gea ML, Calvo-Rojas G, et al. Discovery of anticoagulant drugs: a historical perspective. Curr Drug Discov Technol. 2012;9:83-104.
- Noti C, Seeberger PH. Chemical approaches to define the structure-activity relationship of heparin-like glycosaminoglycans. Chem Biol. 2005;12:731-756.
- Bakchoul T. An update on heparin-induced thrombocytopenia: diagnosis and management. Expert Opin Drug Saf. 2016;15:787-797.
- Schindewolf M, Schwaner S, Wolter M, et al. Incidence and causes of heparin-induced skin lesions. Can Med Assoc J. 2009;181:477-481.
- Perrinaud A, Jacobi D, Machet MC, et al. Bullous hemorrhagic dermatosis occurring at sites distant from subcutaneous injections of heparin: three cases. J Am Acad Dermatol. 2006;54(2 suppl):S5-S7.
- Naveen KN, Rai V. Bullous hemorrhagic dermatosis: a case report. Indian J Dermatol. 2014;59:423.
- Choudhry S, Fishman PM, Hernandez C. Heparin-induced bullous hemorrhagic dermatosis. Cutis. 2013;91:93-98.
- Villanueva CA, Nájera L, Espinosa P, et al. Bullous hemorrhagic dermatosis at distant sites: a report of 2 new cases due to enoxaparin injection and a review of the literature. Actas Dermosifiliogr. 2012;103:816-819.
- Ahmed I, Majeed A, Powell R. Heparin induced thrombocytopenia: diagnosis and management update. Postgrad Med J. 2007;83:575-582.
- Horie N, Kawano R, Inaba J, et al. Angina bullosa hemorrhagica of the soft palate: a clinical study of 16 cases. J Oral Sci. 2008;50:33-36.
- Rai S, Kaur M, Goel S. Angina bullosa hemorrhagica: report of 2 cases. Indian J Dermatol. 2012;57:503.
- Lawson W. Bullous oral lesions: clues to identifying—and managing—the cause. Consultant. 2013;53:168-176.
Practice Points
- It is important for physicians to recognize the clinical appearance of cutaneous adverse reactions to heparin, including bullous hemorrhagic dermatosis.
- Heparin-induced bullous hemorrhagic dermatosis tends to self-resolve, even with continuation of unfractionated heparin.
The Dermatologist’s Role in Amputee Skin Care
Limb amputation is a major life-changing event that markedly affects a patient’s quality of life as well as his/her ability to participate in activities of daily living. The most prevalent causes for amputation include vascular diseases, diabetes mellitus, trauma, and cancer, respectively.1,2 For amputees, maintaining prosthetic use is a major physical and psychological undertaking that benefits from a multidisciplinary team approach. Although individuals with lower limb amputations are disproportionately impacted by skin disease due to the increased mechanical forces exerted over the lower limbs, patients with upper limb amputations also develop dermatologic conditions secondary to wearing prostheses.
Approximately 185,000 amputations occur each year in the United States.3 Although amputations resulting from peripheral vascular disease or diabetes mellitus tend to occur in older individuals, amputations in younger patients usually occur from trauma.2 The US military has experienced increasing numbers of amputations from trauma due to the ongoing combat operations in the Middle East. Although improvements in body armor and tactical combat casualty care have reduced the number of preventable deaths, the number of casualties surviving with extremity injuries requiring amputation has increased.4,5 As of October 2017, 1705 US servicemembers underwent major limb amputations, with 1914 lower limb amputations and 302 upper limb amputations. These amputations mainly impacted men aged 21 to 29 years, but female servicemembers also were affected, and a small group of servicemembers had multiple amputations.6
One of the most common medical problems that amputees face during long-term care is skin disease, with approximately 75% of amputees using a lower limb prosthesis experiencing skin problems. In general, amputees experience nearly 65% more dermatologic concerns than the general population.7 In one study of 97 individuals with transfemoral amputations, some of the most common issues associated with socket prosthetics included heat and sweating in the prosthetic socket (72%) as well as sores and skin irritation from the socket (62%).8 Given the high incidence of skin disease on residual limbs, dermatologists are uniquely positioned to keep the amputee in his/her prosthesis and prevent prosthetic abandonment.
Complications Following Amputation
Although US military servicemembers who undergo amputations receive the very best prosthetic devices and rehabilitation resources, they still experience prosthesis abandonment.9 Despite the fact that prosthetic limbs and prosthesis technology have substantially improved over the last 2 decades, one study indicated that the high frequency of problems affecting tissue viability at residual limbs is due to the age-old problem of prosthetic fit.10 In patients with the most advanced prostheses, poor fit still results in mechanical damage to the skin, as the residual limb is exposed to unequal and shearing forces across the amputation site as well as high pressures that cause a vaso-occlusive effect.11,12 Issues with poor fit are especially important for more active patients, as they normally want to immediately return to their vigorous preinjury lifestyles. In these patients, even a properly fitting prosthetic may not be able to overcome the fact that the residual limb skin is not well suited for the mechanical forces generated by the prosthesis and the humid environment of the socket.1,13 Another complicating factor is the dynamic nature of the residual limb. Muscle atrophy, changes in gait, and weight gain or loss can lead to an ill-fitting prosthetic and subsequent skin breakdown.
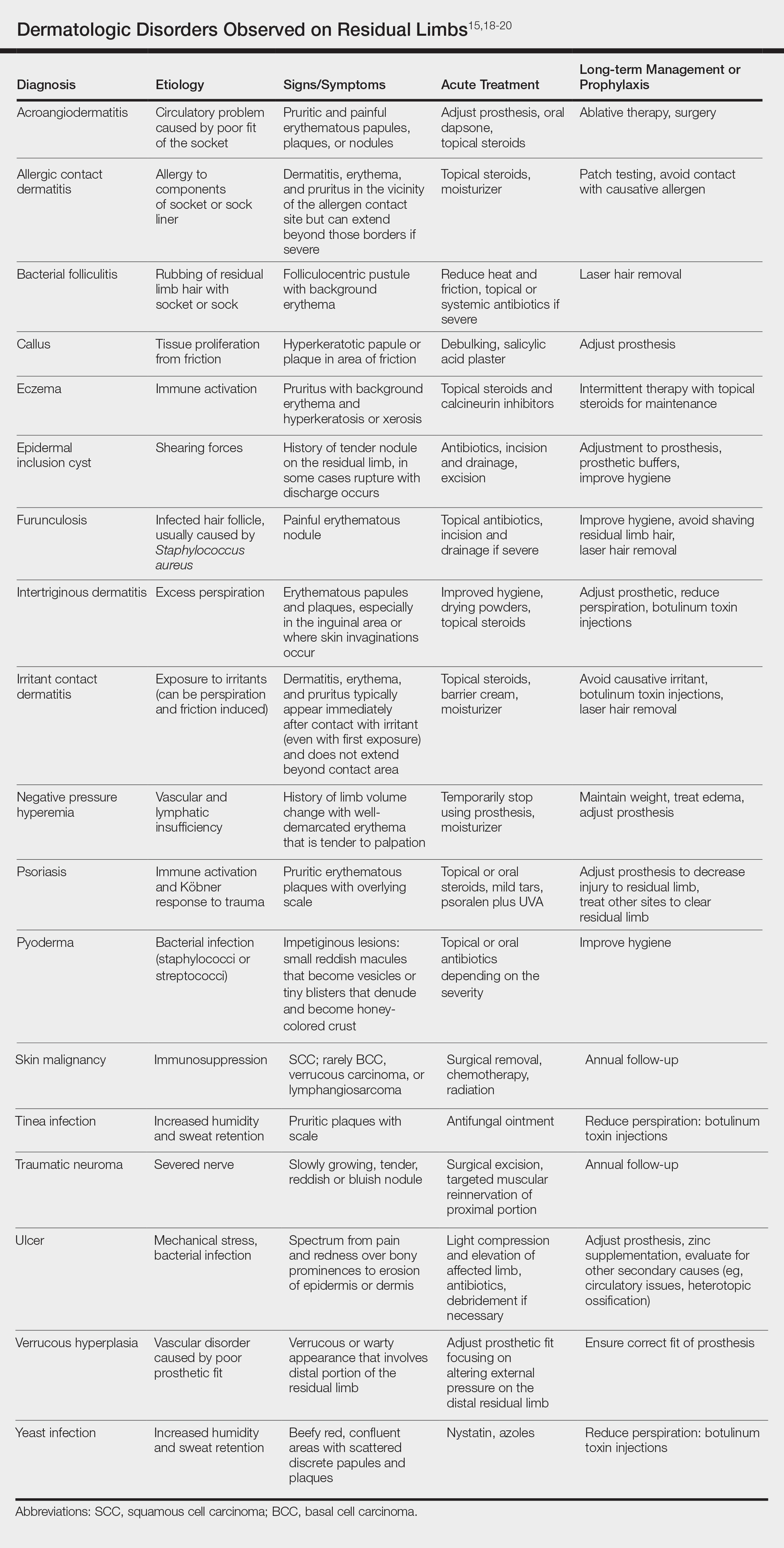
There are many case reports and review articles describing the skin problems in amputees.1,14-17 The Table summarizes these conditions and outlines treatment options for each.15,18-20
Most skin diseases on residual limbs are the result of mechanical skin breakdown, inflammation, infection, or combinations of these processes. Overall, amputees with diabetes mellitus and peripheral vascular disease tend to have skin disease related to poor perfusion, whereas amputees who are active and healthy tend to have conditions related to mechanical stress.7,13,14,17,21,22 Bui et al17 reported ulcers, abscesses, and blisters as the most common skin conditions that occur at the site of residual limbs; however, other less common dermatologic disorders such as skin malignancies, verrucous hyperplasia and carcinoma, granulomatous cutaneous lesions, acroangiodermatitis, and bullous pemphigoid also are seen.23-26 Buikema and Meyerle15 hypothesize that these conditions, as well as the more common skin diseases, are partly from the amputation disrupting blood and lymphatic flow in the residual limb, which causes the site to act as an immunocompromised district that induces dysregulation of neuroimmune regulators.
It is important to note that skin disease on residual limbs is not just an acute problem. Long-term follow-up of 247 traumatic amputees from the Vietnam War showed that almost half of prosthesis users (48.2%) reported a skin problem in the preceding year, more than 38 years after the amputation. Additionally, one-quarter of these individuals experienced skin problems approximately 50% of the time, which unfortunately led to limited use or total abandonment of the prosthesis for the preceding year in 56% of the veterans surveyed.21
Other complications following amputation indirectly lead to skin problems. Heterotopic ossification, or the formation of bone at extraskeletal sites, has been observed in up to 65% of military amputees from recent operations in Iraq and Afghanistan.27,28 If symptomatic, heterotopic ossification can lead to poor prosthetic fit and subsequent skin breakdown. As a result, it has been reported that up to 40% of combat-related lower extremity amputations may require excision of heterotopic ossificiation.29
Amputation also can result in psychologic concerns that indirectly affect skin health. A systematic review by Mckechnie and John30 suggested that despite heterogeneity between studies, even using the lowest figures demonstrated the significance anxiety and depression play in the lives of traumatic amputees. If left untreated, these mental health issues can lead to poor residual limb hygiene and prosthetic maintenance due to reductions in the patient’s energy and motivation. Studies have shown that proper hygiene of residual limbs and silicone liners reduces associated skin problems.19,31
Role of the Dermatologist
Routine care and conservative management of amputee skin problems often are accomplished by prosthetists, primary care physicians, nurses, and physical therapists. In one study, more than 80% of the most common skin problems affecting amputees could be attributed to the prosthesis itself, which highlights the importance of the continued involvement of the prosthetist beyond the initial fitting period.13 However, when a skin problem becomes refractory to conservative management, referral to a dermatologist is prudent; therefore, the dermatologist is an integral member of the multidisciplinary team that provides care for amputees.
The dermatologist often is best positioned to diagnose skin diseases that result from wearing prostheses and is well versed in treatments for short-term and long-term management of skin disease on residual limbs. The dermatologist also can offer prophylactic treatments to decrease sweating and hair growth to prevent potential infections and subsequent skin breakdown. Additionally, proper education on self-care has been shown to decrease the amount of skin problems and increase functional status and quality of life for amputees.32,33 Dermatologists can assist with the patient education process as well as refer amputees to a useful resource from the Amputee Coalition website (www.amputee-coalition.org) to provide specific patient education on how to maintain skin on the residual limb to prevent skin disease.
Current Treatments and Future Directions
Skin disorders affecting residual limbs usually are conditions that dermatologists commonly encounter and are comfortable managing in general practice. Additionally, dermatologists routinely treat hyperhidrosis and conduct laser hair removal, both of which are effective prophylactic adjuncts for amputee skin health. There are a few treatments for reducing residual limb hyperhidrosis that are particularly useful. Although first-line treatment of residual limb hyperhidrosis often is topical aluminum chloride, it requires frequent application and often causes considerable skin irritation when applied to residual limbs. Alternatively, intradermal botulinum toxin has been shown to successfully reduce sweat production in individuals with residual limb hyperhidrosis and is well tolerated.34 A 2017 case report discussed the use of microwave thermal ablation of eccrine coils using a noninvasive 3-step hyperhidrosis treatment system on a bilateral below-the-knee amputee. The authors reported the patient tolerated the procedure well with decreased dermatitis and folliculitis, leading to his ability to wear a prosthetic for longer periods of time.35
Ablative fractional resurfacing with a CO2 laser is another key treatment modality central to amputees, more specifically to traumatic amputees. A CO2 laser can decrease skin tension and increase skin mobility associated with traumatic scars as well as decrease skin vulnerability to biofilms present in chronic wounds on residual limbs. It is believed that the pattern of injury caused by ablative fractional lasers disrupts biofilms and stimulates growth factor secretion and collagen remodeling through the concept of photomicrodebridement.36 The ablative fractional resurfacing approach to scar therapy and chronic wound debridement can result in less skin injury, allowing the amputee to continue rehabilitation and return more quickly to prosthetic use.37
One interesting area of research in amputee care involves the study of novel ways to increase the skin’s ability to adapt to mechanical stress and load bearing and accelerate wound healing on the residual limb. Multiple studies have identified collagen fibril enlargement as an important component of skin adaptation, and biomolecules such as decorin may enhance this process.38-40 The concept of increasing these biomolecules at the correct time during wound healing to strengthen the residual limb tissue currently is being studied.39
Another encouraging area of research is the involvement of fibroblasts in cutaneous wound healing and their role in determining the phenotype of residual limb skin in amputees. The clinical application of autologous fibroblasts is approved by the US Food and Drug Administration for cosmetic use as a filler material and currently is under research for other applications, such as skin regeneration after surgery or manipulating skin characteristics to enhance the durability of residual limbs.41
Future preventative care of amputee skin may rely on tracking residual limb health before severe tissue injury occurs. For instance, Rink et al42 described an approach to monitor residual limb health using noninvasive imaging (eg, hyperspectral imaging, laser speckle imaging) and noninvasive probes that measure oxygenation, perfusion, skin barrier function, and skin hydration to the residual limb. Although these limb surveillance sensors would be employed by prosthetists, the dermatologist, as part of the multispecialty team, also could leverage the data for diagnosis and treatment considerations.
Final Thoughts
The dermatologist is an important member of the multidisciplinary team involved in the care of amputees. Skin disease is prevalent in amputees throughout their lives and often leads to abandonment of prostheses. Although current therapies and preventative treatments are for the most part successful, future research involving advanced technology to monitor skin health, increasing residual limb skin durability at the molecular level, and targeted laser therapies are promising. Through engagement and effective collaboration with the entire multidisciplinary team, dermatologists will have a considerable impact on amputee skin health.
- Dudek NL, Marks MB, Marshall SC, et al. Dermatologic conditions associated with use of a lower-extremity prosthesis. Arch Phys Med Rehabil. 2005;86:659-663.
- Ziegler-Graham K, MacKenzie EJ, Ephraim PL, et al. Estimating the prevalence of limb loss in the United States: 2005 to 2050. Arch Phys Med Rehabil. 2008;89:422-429.
- Kozak LJ. Ambulatory and Inpatient Procedures in the United States, 1995. Hyattsville, MD: US Department of Health and Human Services; 1998.
- Epstein RA, Heinemann AW, McFarland LV. Quality of life for veterans and servicemembers with major traumatic limb loss from Vietnam and OIF/OEF conflicts. J Rehabil Res Dev. 2010;47:373-385.
- Dougherty AL, Mohrle CR, Galarneau MR, et al. Battlefield extremity injuries in Operation Iraqi Freedom. Injury. 2009;40:772-777.
- Farrokhi S, Perez K, Eskridge S, et al. Major deployment-related amputations of lower and upper limbs, active and reserve components, U.S. Armed Forces, 2001-2017. MSMR. 2018;25:10-16.
- Highsmith MJ, Highsmith JT. Identifying and managing skin issues with lower-limb prosthetic use. Amputee Coalition website. https://www.amputee-coalition.org/wp-content/uploads/2015/.../skin_issues_lower.pdf. Accessed January 4, 2019.
- Hagberg K, Brånemark R. Consequences of non-vascular trans-femoral amputation: a survey of quality of life, prosthetic use and problems. Prosthet Orthot Int. 2001;25:186-194.
- Gajewski D, Granville R. The United States Armed Forces Amputee Patient Care Program. J Am Acad Orthop Surg. 2006;14(10 spec no):S183-S187.
- Butler K, Bowen C, Hughes AM, et al. A systematic review of the key factors affecting tissue viability and rehabilitation outcomes of the residual limb in lower extremity traumatic amputees. J Tissue Viability. 2014;23:81-93.
- Mak AF, Zhang M, Boone DA. State-of-the-art research in lower-limb prosthetic biomechanics-socket interface: a review. J Rehabil Res Dev. 2001;38:161-174.
- Silver-Thorn MB, Steege JW. A review of prosthetic interface stress investigations. J Rehabil Res Dev. 1996;33:253-266.
- Dudek NL, Marks MB, Marshall SC. Skin problems in an amputee clinic. Am J Phys Med Rehabil. 2006;85:424-429.
- Meulenbelt HE, Geertzen JH, Dijkstra PU, et al. Skin problems in lower limb amputees: an overview by case reports. J Eur Acad Dermatol Venereol. 2007;21:147-155.
- Buikema KE, Meyerle JH. Amputation stump: privileged harbor for infections, tumors, and immune disorders. Clin Dermatol. 2014;32:670-677.
- Highsmith JT, Highsmith MJ. Common skin pathology in LE prosthesis users. JAAPA. 2007;20:33-36, 47.
- Bui KM, Raugi GJ, Nguyen VQ, et al. Skin problems in individuals with lower-limb loss: literature review and proposed classification system. J Rehabil Res Dev. 2009;46:1085-1090.
- Levy SW. Skin Problems of the Amputee. St. Louis, MO: Warren H. Green Inc; 1983.
- Levy SW, Allende MF, Barnes GH. Skin problems of the leg amputee. Arch Dermatol. 1962;85:65-81.
- Dumanian GA, Potter BK, Mioton LM, et al. Targeted muscle reinnervation treats neuroma and phantom pain in major limb amputees: a randomized clinical trial [published October 26, 2018]. Ann Surg. 2018. doi:10.1097/SLA.0000000000003088.
- Yang NB, Garza LA, Foote CE, et al. High prevalence of stump dermatoses 38 years or more after amputation. Arch Dermatol. 2012;148:1283-1286.
- Meulenbelt HE, Geertzen JH, Jonkman MF, et al. Determinants of skin problems of the stump in lower-limb amputees. Arch Phys Med Rehabil. 2009;90:74-81.
- Lin CH, Ma H, Chung MT, et al. Granulomatous cutaneous lesions associated with risperidone-induced hyperprolactinemia in an amputated upper limb: risperidone-induced cutaneous granulomas. Int J Dermatol. 2012;51:75-78.
- Schwartz RA, Bagley MP, Janniger CK, et al. Verrucous carcinoma of a leg amputation stump. Dermatology. 1991;182:193-195.
- Reilly GD, Boulton AJ, Harrington CI. Stump pemphigoid: a new complication of the amputee. Br Med J. 1983;287:875-876.
- Turan H, Bas¸kan EB, Adim SB, et al. Acroangiodermatitis in a below-knee amputation stump: correspondence. Clin Exp Dermatol. 2011;36:560-561.
- Edwards DS, Kuhn KM, Potter BK, et al. Heterotopic ossification: a review of current understanding, treatment, and future. J Orthop Trauma. 2016;30(suppl 3):S27-S30.
- Potter BK, Burns TC, Lacap AP, et al. Heterotopic ossification following traumatic and combat-related amputations: prevalence, risk factors, and preliminary results of excision. J Bone Joint Surg Am. 2007;89:476-486.
- Tintle SM, Shawen SB, Forsberg JA, et al. Reoperation after combat-related major lower extremity amputations. J Orthop Trauma. 2014;28:232-237.
- Mckechnie PS, John A. Anxiety and depression following traumatic limb amputation: a systematic review. Injury. 2014;45:1859-1866.
- Hachisuka K, Nakamura T, Ohmine S, et al. Hygiene problems of residual limb and silicone liners in transtibial amputees wearing the total surface bearing socket. Arch Phys Med Rehabil. 2001;82:1286-1290.
- Pantera E, Pourtier-Piotte C, Bensoussan L, et al. Patient education after amputation: systematic review and experts’ opinions. Ann Phys Rehabil Med. 2014;57:143-158.
- Blum C, Ehrler S, Isner ME. Assessment of therapeutic education in 135 lower limb amputees. Ann Phys Rehabil Med. 2016;59:E161.
- Pasquina PF, Perry BN, Alphonso AL, et al. Residual limb hyperhidrosis and rimabotulinumtoxinB: a randomized, placebo-controlled study. Arch Phys Med Rehabil. 2015;97:659-664.e2.
- Mula KN, Winston J, Pace S, et al. Use of a microwave device for treatment of amputation residual limb hyperhidrosis. Dermatol Surg. 2017;43:149-152.
- Shumaker PR, Kwan JM, Badiavas EV, et al. Rapid healing of scar-associated chronic wounds after ablative fractional resurfacing. Arch Dermatol. 2012;148:1289-1293.
- Anderson RR, Donelan MB, Hivnor C, et al. Laser treatment of traumatic scars with an emphasis on ablative fractional laser resurfacing: consensus report. JAMA Dermatol. 2014;150:187-193.
- Sanders JE, Mitchell SB, Wang YN, et al. An explant model for the investigation of skin adaptation to mechanical stress. IEEE Trans Biomed Eng. 2002;49(12 pt 2):1626-1631.
- Wang YN, Sanders JE. How does skin adapt to repetitive mechanical stress to become load tolerant? Med Hypotheses. 2003;61:29-35.
- Sanders JE, Goldstein BS. Collagen fibril diameters increase and fibril densities decrease in skin subjected to repetitive compressive and shear stresses. J Biomech. 2001;34:1581-1587.
- Thangapazham R, Darling T, Meyerle J. Alteration of skin properties with autologous dermal fibroblasts. Int J Mol Sci. 2014;15:8407-8427.
- Rink CL, Wernke MM, Powell HM, et al. Standardized approach to quantitatively measure residual limb skin health in individuals with lower limb amputation. Adv Wound Care. 2017;6:225-232.
Limb amputation is a major life-changing event that markedly affects a patient’s quality of life as well as his/her ability to participate in activities of daily living. The most prevalent causes for amputation include vascular diseases, diabetes mellitus, trauma, and cancer, respectively.1,2 For amputees, maintaining prosthetic use is a major physical and psychological undertaking that benefits from a multidisciplinary team approach. Although individuals with lower limb amputations are disproportionately impacted by skin disease due to the increased mechanical forces exerted over the lower limbs, patients with upper limb amputations also develop dermatologic conditions secondary to wearing prostheses.
Approximately 185,000 amputations occur each year in the United States.3 Although amputations resulting from peripheral vascular disease or diabetes mellitus tend to occur in older individuals, amputations in younger patients usually occur from trauma.2 The US military has experienced increasing numbers of amputations from trauma due to the ongoing combat operations in the Middle East. Although improvements in body armor and tactical combat casualty care have reduced the number of preventable deaths, the number of casualties surviving with extremity injuries requiring amputation has increased.4,5 As of October 2017, 1705 US servicemembers underwent major limb amputations, with 1914 lower limb amputations and 302 upper limb amputations. These amputations mainly impacted men aged 21 to 29 years, but female servicemembers also were affected, and a small group of servicemembers had multiple amputations.6
One of the most common medical problems that amputees face during long-term care is skin disease, with approximately 75% of amputees using a lower limb prosthesis experiencing skin problems. In general, amputees experience nearly 65% more dermatologic concerns than the general population.7 In one study of 97 individuals with transfemoral amputations, some of the most common issues associated with socket prosthetics included heat and sweating in the prosthetic socket (72%) as well as sores and skin irritation from the socket (62%).8 Given the high incidence of skin disease on residual limbs, dermatologists are uniquely positioned to keep the amputee in his/her prosthesis and prevent prosthetic abandonment.
Complications Following Amputation
Although US military servicemembers who undergo amputations receive the very best prosthetic devices and rehabilitation resources, they still experience prosthesis abandonment.9 Despite the fact that prosthetic limbs and prosthesis technology have substantially improved over the last 2 decades, one study indicated that the high frequency of problems affecting tissue viability at residual limbs is due to the age-old problem of prosthetic fit.10 In patients with the most advanced prostheses, poor fit still results in mechanical damage to the skin, as the residual limb is exposed to unequal and shearing forces across the amputation site as well as high pressures that cause a vaso-occlusive effect.11,12 Issues with poor fit are especially important for more active patients, as they normally want to immediately return to their vigorous preinjury lifestyles. In these patients, even a properly fitting prosthetic may not be able to overcome the fact that the residual limb skin is not well suited for the mechanical forces generated by the prosthesis and the humid environment of the socket.1,13 Another complicating factor is the dynamic nature of the residual limb. Muscle atrophy, changes in gait, and weight gain or loss can lead to an ill-fitting prosthetic and subsequent skin breakdown.
There are many case reports and review articles describing the skin problems in amputees.1,14-17 The Table summarizes these conditions and outlines treatment options for each.15,18-20

Most skin diseases on residual limbs are the result of mechanical skin breakdown, inflammation, infection, or combinations of these processes. Overall, amputees with diabetes mellitus and peripheral vascular disease tend to have skin disease related to poor perfusion, whereas amputees who are active and healthy tend to have conditions related to mechanical stress.7,13,14,17,21,22 Bui et al17 reported ulcers, abscesses, and blisters as the most common skin conditions that occur at the site of residual limbs; however, other less common dermatologic disorders such as skin malignancies, verrucous hyperplasia and carcinoma, granulomatous cutaneous lesions, acroangiodermatitis, and bullous pemphigoid also are seen.23-26 Buikema and Meyerle15 hypothesize that these conditions, as well as the more common skin diseases, are partly from the amputation disrupting blood and lymphatic flow in the residual limb, which causes the site to act as an immunocompromised district that induces dysregulation of neuroimmune regulators.
It is important to note that skin disease on residual limbs is not just an acute problem. Long-term follow-up of 247 traumatic amputees from the Vietnam War showed that almost half of prosthesis users (48.2%) reported a skin problem in the preceding year, more than 38 years after the amputation. Additionally, one-quarter of these individuals experienced skin problems approximately 50% of the time, which unfortunately led to limited use or total abandonment of the prosthesis for the preceding year in 56% of the veterans surveyed.21
Other complications following amputation indirectly lead to skin problems. Heterotopic ossification, or the formation of bone at extraskeletal sites, has been observed in up to 65% of military amputees from recent operations in Iraq and Afghanistan.27,28 If symptomatic, heterotopic ossification can lead to poor prosthetic fit and subsequent skin breakdown. As a result, it has been reported that up to 40% of combat-related lower extremity amputations may require excision of heterotopic ossificiation.29
Amputation also can result in psychologic concerns that indirectly affect skin health. A systematic review by Mckechnie and John30 suggested that despite heterogeneity between studies, even using the lowest figures demonstrated the significance anxiety and depression play in the lives of traumatic amputees. If left untreated, these mental health issues can lead to poor residual limb hygiene and prosthetic maintenance due to reductions in the patient’s energy and motivation. Studies have shown that proper hygiene of residual limbs and silicone liners reduces associated skin problems.19,31
Role of the Dermatologist
Routine care and conservative management of amputee skin problems often are accomplished by prosthetists, primary care physicians, nurses, and physical therapists. In one study, more than 80% of the most common skin problems affecting amputees could be attributed to the prosthesis itself, which highlights the importance of the continued involvement of the prosthetist beyond the initial fitting period.13 However, when a skin problem becomes refractory to conservative management, referral to a dermatologist is prudent; therefore, the dermatologist is an integral member of the multidisciplinary team that provides care for amputees.
The dermatologist often is best positioned to diagnose skin diseases that result from wearing prostheses and is well versed in treatments for short-term and long-term management of skin disease on residual limbs. The dermatologist also can offer prophylactic treatments to decrease sweating and hair growth to prevent potential infections and subsequent skin breakdown. Additionally, proper education on self-care has been shown to decrease the amount of skin problems and increase functional status and quality of life for amputees.32,33 Dermatologists can assist with the patient education process as well as refer amputees to a useful resource from the Amputee Coalition website (www.amputee-coalition.org) to provide specific patient education on how to maintain skin on the residual limb to prevent skin disease.
Current Treatments and Future Directions
Skin disorders affecting residual limbs usually are conditions that dermatologists commonly encounter and are comfortable managing in general practice. Additionally, dermatologists routinely treat hyperhidrosis and conduct laser hair removal, both of which are effective prophylactic adjuncts for amputee skin health. There are a few treatments for reducing residual limb hyperhidrosis that are particularly useful. Although first-line treatment of residual limb hyperhidrosis often is topical aluminum chloride, it requires frequent application and often causes considerable skin irritation when applied to residual limbs. Alternatively, intradermal botulinum toxin has been shown to successfully reduce sweat production in individuals with residual limb hyperhidrosis and is well tolerated.34 A 2017 case report discussed the use of microwave thermal ablation of eccrine coils using a noninvasive 3-step hyperhidrosis treatment system on a bilateral below-the-knee amputee. The authors reported the patient tolerated the procedure well with decreased dermatitis and folliculitis, leading to his ability to wear a prosthetic for longer periods of time.35
Ablative fractional resurfacing with a CO2 laser is another key treatment modality central to amputees, more specifically to traumatic amputees. A CO2 laser can decrease skin tension and increase skin mobility associated with traumatic scars as well as decrease skin vulnerability to biofilms present in chronic wounds on residual limbs. It is believed that the pattern of injury caused by ablative fractional lasers disrupts biofilms and stimulates growth factor secretion and collagen remodeling through the concept of photomicrodebridement.36 The ablative fractional resurfacing approach to scar therapy and chronic wound debridement can result in less skin injury, allowing the amputee to continue rehabilitation and return more quickly to prosthetic use.37
One interesting area of research in amputee care involves the study of novel ways to increase the skin’s ability to adapt to mechanical stress and load bearing and accelerate wound healing on the residual limb. Multiple studies have identified collagen fibril enlargement as an important component of skin adaptation, and biomolecules such as decorin may enhance this process.38-40 The concept of increasing these biomolecules at the correct time during wound healing to strengthen the residual limb tissue currently is being studied.39
Another encouraging area of research is the involvement of fibroblasts in cutaneous wound healing and their role in determining the phenotype of residual limb skin in amputees. The clinical application of autologous fibroblasts is approved by the US Food and Drug Administration for cosmetic use as a filler material and currently is under research for other applications, such as skin regeneration after surgery or manipulating skin characteristics to enhance the durability of residual limbs.41
Future preventative care of amputee skin may rely on tracking residual limb health before severe tissue injury occurs. For instance, Rink et al42 described an approach to monitor residual limb health using noninvasive imaging (eg, hyperspectral imaging, laser speckle imaging) and noninvasive probes that measure oxygenation, perfusion, skin barrier function, and skin hydration to the residual limb. Although these limb surveillance sensors would be employed by prosthetists, the dermatologist, as part of the multispecialty team, also could leverage the data for diagnosis and treatment considerations.
Final Thoughts
The dermatologist is an important member of the multidisciplinary team involved in the care of amputees. Skin disease is prevalent in amputees throughout their lives and often leads to abandonment of prostheses. Although current therapies and preventative treatments are for the most part successful, future research involving advanced technology to monitor skin health, increasing residual limb skin durability at the molecular level, and targeted laser therapies are promising. Through engagement and effective collaboration with the entire multidisciplinary team, dermatologists will have a considerable impact on amputee skin health.
Limb amputation is a major life-changing event that markedly affects a patient’s quality of life as well as his/her ability to participate in activities of daily living. The most prevalent causes for amputation include vascular diseases, diabetes mellitus, trauma, and cancer, respectively.1,2 For amputees, maintaining prosthetic use is a major physical and psychological undertaking that benefits from a multidisciplinary team approach. Although individuals with lower limb amputations are disproportionately impacted by skin disease due to the increased mechanical forces exerted over the lower limbs, patients with upper limb amputations also develop dermatologic conditions secondary to wearing prostheses.
Approximately 185,000 amputations occur each year in the United States.3 Although amputations resulting from peripheral vascular disease or diabetes mellitus tend to occur in older individuals, amputations in younger patients usually occur from trauma.2 The US military has experienced increasing numbers of amputations from trauma due to the ongoing combat operations in the Middle East. Although improvements in body armor and tactical combat casualty care have reduced the number of preventable deaths, the number of casualties surviving with extremity injuries requiring amputation has increased.4,5 As of October 2017, 1705 US servicemembers underwent major limb amputations, with 1914 lower limb amputations and 302 upper limb amputations. These amputations mainly impacted men aged 21 to 29 years, but female servicemembers also were affected, and a small group of servicemembers had multiple amputations.6
One of the most common medical problems that amputees face during long-term care is skin disease, with approximately 75% of amputees using a lower limb prosthesis experiencing skin problems. In general, amputees experience nearly 65% more dermatologic concerns than the general population.7 In one study of 97 individuals with transfemoral amputations, some of the most common issues associated with socket prosthetics included heat and sweating in the prosthetic socket (72%) as well as sores and skin irritation from the socket (62%).8 Given the high incidence of skin disease on residual limbs, dermatologists are uniquely positioned to keep the amputee in his/her prosthesis and prevent prosthetic abandonment.
Complications Following Amputation
Although US military servicemembers who undergo amputations receive the very best prosthetic devices and rehabilitation resources, they still experience prosthesis abandonment.9 Despite the fact that prosthetic limbs and prosthesis technology have substantially improved over the last 2 decades, one study indicated that the high frequency of problems affecting tissue viability at residual limbs is due to the age-old problem of prosthetic fit.10 In patients with the most advanced prostheses, poor fit still results in mechanical damage to the skin, as the residual limb is exposed to unequal and shearing forces across the amputation site as well as high pressures that cause a vaso-occlusive effect.11,12 Issues with poor fit are especially important for more active patients, as they normally want to immediately return to their vigorous preinjury lifestyles. In these patients, even a properly fitting prosthetic may not be able to overcome the fact that the residual limb skin is not well suited for the mechanical forces generated by the prosthesis and the humid environment of the socket.1,13 Another complicating factor is the dynamic nature of the residual limb. Muscle atrophy, changes in gait, and weight gain or loss can lead to an ill-fitting prosthetic and subsequent skin breakdown.
There are many case reports and review articles describing the skin problems in amputees.1,14-17 The Table summarizes these conditions and outlines treatment options for each.15,18-20

Most skin diseases on residual limbs are the result of mechanical skin breakdown, inflammation, infection, or combinations of these processes. Overall, amputees with diabetes mellitus and peripheral vascular disease tend to have skin disease related to poor perfusion, whereas amputees who are active and healthy tend to have conditions related to mechanical stress.7,13,14,17,21,22 Bui et al17 reported ulcers, abscesses, and blisters as the most common skin conditions that occur at the site of residual limbs; however, other less common dermatologic disorders such as skin malignancies, verrucous hyperplasia and carcinoma, granulomatous cutaneous lesions, acroangiodermatitis, and bullous pemphigoid also are seen.23-26 Buikema and Meyerle15 hypothesize that these conditions, as well as the more common skin diseases, are partly from the amputation disrupting blood and lymphatic flow in the residual limb, which causes the site to act as an immunocompromised district that induces dysregulation of neuroimmune regulators.
It is important to note that skin disease on residual limbs is not just an acute problem. Long-term follow-up of 247 traumatic amputees from the Vietnam War showed that almost half of prosthesis users (48.2%) reported a skin problem in the preceding year, more than 38 years after the amputation. Additionally, one-quarter of these individuals experienced skin problems approximately 50% of the time, which unfortunately led to limited use or total abandonment of the prosthesis for the preceding year in 56% of the veterans surveyed.21
Other complications following amputation indirectly lead to skin problems. Heterotopic ossification, or the formation of bone at extraskeletal sites, has been observed in up to 65% of military amputees from recent operations in Iraq and Afghanistan.27,28 If symptomatic, heterotopic ossification can lead to poor prosthetic fit and subsequent skin breakdown. As a result, it has been reported that up to 40% of combat-related lower extremity amputations may require excision of heterotopic ossificiation.29
Amputation also can result in psychologic concerns that indirectly affect skin health. A systematic review by Mckechnie and John30 suggested that despite heterogeneity between studies, even using the lowest figures demonstrated the significance anxiety and depression play in the lives of traumatic amputees. If left untreated, these mental health issues can lead to poor residual limb hygiene and prosthetic maintenance due to reductions in the patient’s energy and motivation. Studies have shown that proper hygiene of residual limbs and silicone liners reduces associated skin problems.19,31
Role of the Dermatologist
Routine care and conservative management of amputee skin problems often are accomplished by prosthetists, primary care physicians, nurses, and physical therapists. In one study, more than 80% of the most common skin problems affecting amputees could be attributed to the prosthesis itself, which highlights the importance of the continued involvement of the prosthetist beyond the initial fitting period.13 However, when a skin problem becomes refractory to conservative management, referral to a dermatologist is prudent; therefore, the dermatologist is an integral member of the multidisciplinary team that provides care for amputees.
The dermatologist often is best positioned to diagnose skin diseases that result from wearing prostheses and is well versed in treatments for short-term and long-term management of skin disease on residual limbs. The dermatologist also can offer prophylactic treatments to decrease sweating and hair growth to prevent potential infections and subsequent skin breakdown. Additionally, proper education on self-care has been shown to decrease the amount of skin problems and increase functional status and quality of life for amputees.32,33 Dermatologists can assist with the patient education process as well as refer amputees to a useful resource from the Amputee Coalition website (www.amputee-coalition.org) to provide specific patient education on how to maintain skin on the residual limb to prevent skin disease.
Current Treatments and Future Directions
Skin disorders affecting residual limbs usually are conditions that dermatologists commonly encounter and are comfortable managing in general practice. Additionally, dermatologists routinely treat hyperhidrosis and conduct laser hair removal, both of which are effective prophylactic adjuncts for amputee skin health. There are a few treatments for reducing residual limb hyperhidrosis that are particularly useful. Although first-line treatment of residual limb hyperhidrosis often is topical aluminum chloride, it requires frequent application and often causes considerable skin irritation when applied to residual limbs. Alternatively, intradermal botulinum toxin has been shown to successfully reduce sweat production in individuals with residual limb hyperhidrosis and is well tolerated.34 A 2017 case report discussed the use of microwave thermal ablation of eccrine coils using a noninvasive 3-step hyperhidrosis treatment system on a bilateral below-the-knee amputee. The authors reported the patient tolerated the procedure well with decreased dermatitis and folliculitis, leading to his ability to wear a prosthetic for longer periods of time.35
Ablative fractional resurfacing with a CO2 laser is another key treatment modality central to amputees, more specifically to traumatic amputees. A CO2 laser can decrease skin tension and increase skin mobility associated with traumatic scars as well as decrease skin vulnerability to biofilms present in chronic wounds on residual limbs. It is believed that the pattern of injury caused by ablative fractional lasers disrupts biofilms and stimulates growth factor secretion and collagen remodeling through the concept of photomicrodebridement.36 The ablative fractional resurfacing approach to scar therapy and chronic wound debridement can result in less skin injury, allowing the amputee to continue rehabilitation and return more quickly to prosthetic use.37
One interesting area of research in amputee care involves the study of novel ways to increase the skin’s ability to adapt to mechanical stress and load bearing and accelerate wound healing on the residual limb. Multiple studies have identified collagen fibril enlargement as an important component of skin adaptation, and biomolecules such as decorin may enhance this process.38-40 The concept of increasing these biomolecules at the correct time during wound healing to strengthen the residual limb tissue currently is being studied.39
Another encouraging area of research is the involvement of fibroblasts in cutaneous wound healing and their role in determining the phenotype of residual limb skin in amputees. The clinical application of autologous fibroblasts is approved by the US Food and Drug Administration for cosmetic use as a filler material and currently is under research for other applications, such as skin regeneration after surgery or manipulating skin characteristics to enhance the durability of residual limbs.41
Future preventative care of amputee skin may rely on tracking residual limb health before severe tissue injury occurs. For instance, Rink et al42 described an approach to monitor residual limb health using noninvasive imaging (eg, hyperspectral imaging, laser speckle imaging) and noninvasive probes that measure oxygenation, perfusion, skin barrier function, and skin hydration to the residual limb. Although these limb surveillance sensors would be employed by prosthetists, the dermatologist, as part of the multispecialty team, also could leverage the data for diagnosis and treatment considerations.
Final Thoughts
The dermatologist is an important member of the multidisciplinary team involved in the care of amputees. Skin disease is prevalent in amputees throughout their lives and often leads to abandonment of prostheses. Although current therapies and preventative treatments are for the most part successful, future research involving advanced technology to monitor skin health, increasing residual limb skin durability at the molecular level, and targeted laser therapies are promising. Through engagement and effective collaboration with the entire multidisciplinary team, dermatologists will have a considerable impact on amputee skin health.
- Dudek NL, Marks MB, Marshall SC, et al. Dermatologic conditions associated with use of a lower-extremity prosthesis. Arch Phys Med Rehabil. 2005;86:659-663.
- Ziegler-Graham K, MacKenzie EJ, Ephraim PL, et al. Estimating the prevalence of limb loss in the United States: 2005 to 2050. Arch Phys Med Rehabil. 2008;89:422-429.
- Kozak LJ. Ambulatory and Inpatient Procedures in the United States, 1995. Hyattsville, MD: US Department of Health and Human Services; 1998.
- Epstein RA, Heinemann AW, McFarland LV. Quality of life for veterans and servicemembers with major traumatic limb loss from Vietnam and OIF/OEF conflicts. J Rehabil Res Dev. 2010;47:373-385.
- Dougherty AL, Mohrle CR, Galarneau MR, et al. Battlefield extremity injuries in Operation Iraqi Freedom. Injury. 2009;40:772-777.
- Farrokhi S, Perez K, Eskridge S, et al. Major deployment-related amputations of lower and upper limbs, active and reserve components, U.S. Armed Forces, 2001-2017. MSMR. 2018;25:10-16.
- Highsmith MJ, Highsmith JT. Identifying and managing skin issues with lower-limb prosthetic use. Amputee Coalition website. https://www.amputee-coalition.org/wp-content/uploads/2015/.../skin_issues_lower.pdf. Accessed January 4, 2019.
- Hagberg K, Brånemark R. Consequences of non-vascular trans-femoral amputation: a survey of quality of life, prosthetic use and problems. Prosthet Orthot Int. 2001;25:186-194.
- Gajewski D, Granville R. The United States Armed Forces Amputee Patient Care Program. J Am Acad Orthop Surg. 2006;14(10 spec no):S183-S187.
- Butler K, Bowen C, Hughes AM, et al. A systematic review of the key factors affecting tissue viability and rehabilitation outcomes of the residual limb in lower extremity traumatic amputees. J Tissue Viability. 2014;23:81-93.
- Mak AF, Zhang M, Boone DA. State-of-the-art research in lower-limb prosthetic biomechanics-socket interface: a review. J Rehabil Res Dev. 2001;38:161-174.
- Silver-Thorn MB, Steege JW. A review of prosthetic interface stress investigations. J Rehabil Res Dev. 1996;33:253-266.
- Dudek NL, Marks MB, Marshall SC. Skin problems in an amputee clinic. Am J Phys Med Rehabil. 2006;85:424-429.
- Meulenbelt HE, Geertzen JH, Dijkstra PU, et al. Skin problems in lower limb amputees: an overview by case reports. J Eur Acad Dermatol Venereol. 2007;21:147-155.
- Buikema KE, Meyerle JH. Amputation stump: privileged harbor for infections, tumors, and immune disorders. Clin Dermatol. 2014;32:670-677.
- Highsmith JT, Highsmith MJ. Common skin pathology in LE prosthesis users. JAAPA. 2007;20:33-36, 47.
- Bui KM, Raugi GJ, Nguyen VQ, et al. Skin problems in individuals with lower-limb loss: literature review and proposed classification system. J Rehabil Res Dev. 2009;46:1085-1090.
- Levy SW. Skin Problems of the Amputee. St. Louis, MO: Warren H. Green Inc; 1983.
- Levy SW, Allende MF, Barnes GH. Skin problems of the leg amputee. Arch Dermatol. 1962;85:65-81.
- Dumanian GA, Potter BK, Mioton LM, et al. Targeted muscle reinnervation treats neuroma and phantom pain in major limb amputees: a randomized clinical trial [published October 26, 2018]. Ann Surg. 2018. doi:10.1097/SLA.0000000000003088.
- Yang NB, Garza LA, Foote CE, et al. High prevalence of stump dermatoses 38 years or more after amputation. Arch Dermatol. 2012;148:1283-1286.
- Meulenbelt HE, Geertzen JH, Jonkman MF, et al. Determinants of skin problems of the stump in lower-limb amputees. Arch Phys Med Rehabil. 2009;90:74-81.
- Lin CH, Ma H, Chung MT, et al. Granulomatous cutaneous lesions associated with risperidone-induced hyperprolactinemia in an amputated upper limb: risperidone-induced cutaneous granulomas. Int J Dermatol. 2012;51:75-78.
- Schwartz RA, Bagley MP, Janniger CK, et al. Verrucous carcinoma of a leg amputation stump. Dermatology. 1991;182:193-195.
- Reilly GD, Boulton AJ, Harrington CI. Stump pemphigoid: a new complication of the amputee. Br Med J. 1983;287:875-876.
- Turan H, Bas¸kan EB, Adim SB, et al. Acroangiodermatitis in a below-knee amputation stump: correspondence. Clin Exp Dermatol. 2011;36:560-561.
- Edwards DS, Kuhn KM, Potter BK, et al. Heterotopic ossification: a review of current understanding, treatment, and future. J Orthop Trauma. 2016;30(suppl 3):S27-S30.
- Potter BK, Burns TC, Lacap AP, et al. Heterotopic ossification following traumatic and combat-related amputations: prevalence, risk factors, and preliminary results of excision. J Bone Joint Surg Am. 2007;89:476-486.
- Tintle SM, Shawen SB, Forsberg JA, et al. Reoperation after combat-related major lower extremity amputations. J Orthop Trauma. 2014;28:232-237.
- Mckechnie PS, John A. Anxiety and depression following traumatic limb amputation: a systematic review. Injury. 2014;45:1859-1866.
- Hachisuka K, Nakamura T, Ohmine S, et al. Hygiene problems of residual limb and silicone liners in transtibial amputees wearing the total surface bearing socket. Arch Phys Med Rehabil. 2001;82:1286-1290.
- Pantera E, Pourtier-Piotte C, Bensoussan L, et al. Patient education after amputation: systematic review and experts’ opinions. Ann Phys Rehabil Med. 2014;57:143-158.
- Blum C, Ehrler S, Isner ME. Assessment of therapeutic education in 135 lower limb amputees. Ann Phys Rehabil Med. 2016;59:E161.
- Pasquina PF, Perry BN, Alphonso AL, et al. Residual limb hyperhidrosis and rimabotulinumtoxinB: a randomized, placebo-controlled study. Arch Phys Med Rehabil. 2015;97:659-664.e2.
- Mula KN, Winston J, Pace S, et al. Use of a microwave device for treatment of amputation residual limb hyperhidrosis. Dermatol Surg. 2017;43:149-152.
- Shumaker PR, Kwan JM, Badiavas EV, et al. Rapid healing of scar-associated chronic wounds after ablative fractional resurfacing. Arch Dermatol. 2012;148:1289-1293.
- Anderson RR, Donelan MB, Hivnor C, et al. Laser treatment of traumatic scars with an emphasis on ablative fractional laser resurfacing: consensus report. JAMA Dermatol. 2014;150:187-193.
- Sanders JE, Mitchell SB, Wang YN, et al. An explant model for the investigation of skin adaptation to mechanical stress. IEEE Trans Biomed Eng. 2002;49(12 pt 2):1626-1631.
- Wang YN, Sanders JE. How does skin adapt to repetitive mechanical stress to become load tolerant? Med Hypotheses. 2003;61:29-35.
- Sanders JE, Goldstein BS. Collagen fibril diameters increase and fibril densities decrease in skin subjected to repetitive compressive and shear stresses. J Biomech. 2001;34:1581-1587.
- Thangapazham R, Darling T, Meyerle J. Alteration of skin properties with autologous dermal fibroblasts. Int J Mol Sci. 2014;15:8407-8427.
- Rink CL, Wernke MM, Powell HM, et al. Standardized approach to quantitatively measure residual limb skin health in individuals with lower limb amputation. Adv Wound Care. 2017;6:225-232.
- Dudek NL, Marks MB, Marshall SC, et al. Dermatologic conditions associated with use of a lower-extremity prosthesis. Arch Phys Med Rehabil. 2005;86:659-663.
- Ziegler-Graham K, MacKenzie EJ, Ephraim PL, et al. Estimating the prevalence of limb loss in the United States: 2005 to 2050. Arch Phys Med Rehabil. 2008;89:422-429.
- Kozak LJ. Ambulatory and Inpatient Procedures in the United States, 1995. Hyattsville, MD: US Department of Health and Human Services; 1998.
- Epstein RA, Heinemann AW, McFarland LV. Quality of life for veterans and servicemembers with major traumatic limb loss from Vietnam and OIF/OEF conflicts. J Rehabil Res Dev. 2010;47:373-385.
- Dougherty AL, Mohrle CR, Galarneau MR, et al. Battlefield extremity injuries in Operation Iraqi Freedom. Injury. 2009;40:772-777.
- Farrokhi S, Perez K, Eskridge S, et al. Major deployment-related amputations of lower and upper limbs, active and reserve components, U.S. Armed Forces, 2001-2017. MSMR. 2018;25:10-16.
- Highsmith MJ, Highsmith JT. Identifying and managing skin issues with lower-limb prosthetic use. Amputee Coalition website. https://www.amputee-coalition.org/wp-content/uploads/2015/.../skin_issues_lower.pdf. Accessed January 4, 2019.
- Hagberg K, Brånemark R. Consequences of non-vascular trans-femoral amputation: a survey of quality of life, prosthetic use and problems. Prosthet Orthot Int. 2001;25:186-194.
- Gajewski D, Granville R. The United States Armed Forces Amputee Patient Care Program. J Am Acad Orthop Surg. 2006;14(10 spec no):S183-S187.
- Butler K, Bowen C, Hughes AM, et al. A systematic review of the key factors affecting tissue viability and rehabilitation outcomes of the residual limb in lower extremity traumatic amputees. J Tissue Viability. 2014;23:81-93.
- Mak AF, Zhang M, Boone DA. State-of-the-art research in lower-limb prosthetic biomechanics-socket interface: a review. J Rehabil Res Dev. 2001;38:161-174.
- Silver-Thorn MB, Steege JW. A review of prosthetic interface stress investigations. J Rehabil Res Dev. 1996;33:253-266.
- Dudek NL, Marks MB, Marshall SC. Skin problems in an amputee clinic. Am J Phys Med Rehabil. 2006;85:424-429.
- Meulenbelt HE, Geertzen JH, Dijkstra PU, et al. Skin problems in lower limb amputees: an overview by case reports. J Eur Acad Dermatol Venereol. 2007;21:147-155.
- Buikema KE, Meyerle JH. Amputation stump: privileged harbor for infections, tumors, and immune disorders. Clin Dermatol. 2014;32:670-677.
- Highsmith JT, Highsmith MJ. Common skin pathology in LE prosthesis users. JAAPA. 2007;20:33-36, 47.
- Bui KM, Raugi GJ, Nguyen VQ, et al. Skin problems in individuals with lower-limb loss: literature review and proposed classification system. J Rehabil Res Dev. 2009;46:1085-1090.
- Levy SW. Skin Problems of the Amputee. St. Louis, MO: Warren H. Green Inc; 1983.
- Levy SW, Allende MF, Barnes GH. Skin problems of the leg amputee. Arch Dermatol. 1962;85:65-81.
- Dumanian GA, Potter BK, Mioton LM, et al. Targeted muscle reinnervation treats neuroma and phantom pain in major limb amputees: a randomized clinical trial [published October 26, 2018]. Ann Surg. 2018. doi:10.1097/SLA.0000000000003088.
- Yang NB, Garza LA, Foote CE, et al. High prevalence of stump dermatoses 38 years or more after amputation. Arch Dermatol. 2012;148:1283-1286.
- Meulenbelt HE, Geertzen JH, Jonkman MF, et al. Determinants of skin problems of the stump in lower-limb amputees. Arch Phys Med Rehabil. 2009;90:74-81.
- Lin CH, Ma H, Chung MT, et al. Granulomatous cutaneous lesions associated with risperidone-induced hyperprolactinemia in an amputated upper limb: risperidone-induced cutaneous granulomas. Int J Dermatol. 2012;51:75-78.
- Schwartz RA, Bagley MP, Janniger CK, et al. Verrucous carcinoma of a leg amputation stump. Dermatology. 1991;182:193-195.
- Reilly GD, Boulton AJ, Harrington CI. Stump pemphigoid: a new complication of the amputee. Br Med J. 1983;287:875-876.
- Turan H, Bas¸kan EB, Adim SB, et al. Acroangiodermatitis in a below-knee amputation stump: correspondence. Clin Exp Dermatol. 2011;36:560-561.
- Edwards DS, Kuhn KM, Potter BK, et al. Heterotopic ossification: a review of current understanding, treatment, and future. J Orthop Trauma. 2016;30(suppl 3):S27-S30.
- Potter BK, Burns TC, Lacap AP, et al. Heterotopic ossification following traumatic and combat-related amputations: prevalence, risk factors, and preliminary results of excision. J Bone Joint Surg Am. 2007;89:476-486.
- Tintle SM, Shawen SB, Forsberg JA, et al. Reoperation after combat-related major lower extremity amputations. J Orthop Trauma. 2014;28:232-237.
- Mckechnie PS, John A. Anxiety and depression following traumatic limb amputation: a systematic review. Injury. 2014;45:1859-1866.
- Hachisuka K, Nakamura T, Ohmine S, et al. Hygiene problems of residual limb and silicone liners in transtibial amputees wearing the total surface bearing socket. Arch Phys Med Rehabil. 2001;82:1286-1290.
- Pantera E, Pourtier-Piotte C, Bensoussan L, et al. Patient education after amputation: systematic review and experts’ opinions. Ann Phys Rehabil Med. 2014;57:143-158.
- Blum C, Ehrler S, Isner ME. Assessment of therapeutic education in 135 lower limb amputees. Ann Phys Rehabil Med. 2016;59:E161.
- Pasquina PF, Perry BN, Alphonso AL, et al. Residual limb hyperhidrosis and rimabotulinumtoxinB: a randomized, placebo-controlled study. Arch Phys Med Rehabil. 2015;97:659-664.e2.
- Mula KN, Winston J, Pace S, et al. Use of a microwave device for treatment of amputation residual limb hyperhidrosis. Dermatol Surg. 2017;43:149-152.
- Shumaker PR, Kwan JM, Badiavas EV, et al. Rapid healing of scar-associated chronic wounds after ablative fractional resurfacing. Arch Dermatol. 2012;148:1289-1293.
- Anderson RR, Donelan MB, Hivnor C, et al. Laser treatment of traumatic scars with an emphasis on ablative fractional laser resurfacing: consensus report. JAMA Dermatol. 2014;150:187-193.
- Sanders JE, Mitchell SB, Wang YN, et al. An explant model for the investigation of skin adaptation to mechanical stress. IEEE Trans Biomed Eng. 2002;49(12 pt 2):1626-1631.
- Wang YN, Sanders JE. How does skin adapt to repetitive mechanical stress to become load tolerant? Med Hypotheses. 2003;61:29-35.
- Sanders JE, Goldstein BS. Collagen fibril diameters increase and fibril densities decrease in skin subjected to repetitive compressive and shear stresses. J Biomech. 2001;34:1581-1587.
- Thangapazham R, Darling T, Meyerle J. Alteration of skin properties with autologous dermal fibroblasts. Int J Mol Sci. 2014;15:8407-8427.
- Rink CL, Wernke MM, Powell HM, et al. Standardized approach to quantitatively measure residual limb skin health in individuals with lower limb amputation. Adv Wound Care. 2017;6:225-232.
Practice Points
- Amputees have an increased risk for skin disease occurring on residual limbs.
- It is important to educate patients about proper hygiene techniques for residual limbs and prostheses as well as common signs and symptoms of skin disease at the amputation site.
- Amputees should see a dermatologist within the first year after amputation and often benefit from annual follow-up examinations.
- Early referral to a dermatologist for skin disease affecting residual limbs is warranted.
Amantadine-Induced Livedo Reticularis in a Child Treated Off Label for Neurobehavioral Disorders
Livedo reticularis (LR) is a common dermatologic finding consisting of diffuse, reticulated, violaceous patches. It often is a benign physical finding known as cutis marmorata; however, LR can be associated with other medical conditions as well as with the use of some medications.1,2 Amantadine is a common cause of LR in Parkinson disease patients.3,4 We present a rare case of amantadine-induced LR in a pediatric patient and highlight the off-label use of this medication in children.
Case Report
An 8-year-old boy presented with a diffuse rash on the trunk, arms, and legs of 9 months’ duration. The patient denied any associated symptoms as well as alleviating or exacerbating factors. He also denied any changes with temperature. He had no recent international travel and no prior drug allergies. His medical history was remarkable for attention deficit hyperactivity disorder (ADHD), bipolar disorder, and autism spectrum disorder. His previously prescribed medications included atomoxetine, quetiapine, and valproic acid. The only new medication that had been started within the last year was amantadine. Physical examination revealed a diffuse, reticulated, erythematous to violaceous, blanching rash that was most notable on the legs (Figure 1A) but also was present on the trunk (Figure 1B) and arms (Figure 1C). The clinical examination was consistent with LR, which was presumed to be secondary to amantadine use. Given the multiple psychiatric diagnoses and medication history in this young patient, a consultation with child psychiatry was facilitated. His medications and diagnosis were reviewed, and amantadine was discontinued. At a follow-up visit 5 months later, the patient’s LR had improved (Figure 2).
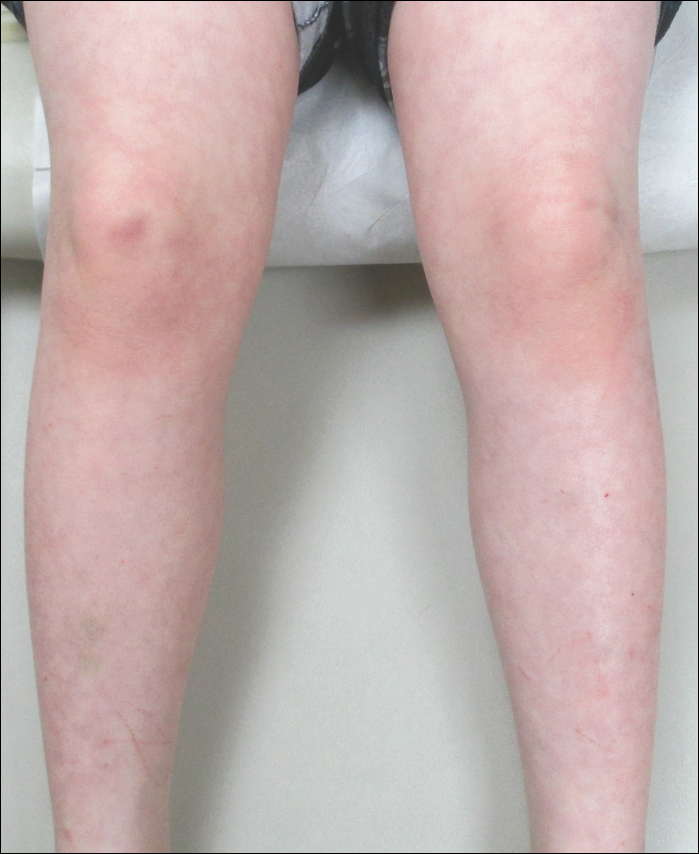
Comment
Amantadine has a well-documented association with LR in patients with Parkinson disease,3,4 which has been reported in up to 40% of those taking amantadine.2 More recently, amantadine has been used off label to treat neurobehavioral disorders in children due to beneficial effects including improvement in attention and concentration, distractibility, and fatigue.5 Our patient was being treated off label with amantadine for ADHD and bipolar disorder. Amantadine acts as a noncompetitive antagonist of the N-methyl-D-aspartate receptor, enhancing dopamine release to reduce symptoms of ADHD.5,6 Additionally, amantadine can cause a depletion of catecholamines in the peripheral nerve terminals, which may lead to dilatation of dermal vessels.4,6 This sequence of events has been proposed as a possible mechanism contributing to amantadine-induced LR, though the pathophysiology is not fully understood.1,3,4
Our case of LR likely was induced by amantadine given the temporal relationship between initiation of the medication, onset of the rash, and the considerable improvement of the rash upon discontinuation of amantadine. Barrera and Browning6 reported another case of amantadine-induced LR in a pediatric patient. Because amantadine is increasingly being used off label to treat childhood neurobehavioral disorders, amantadine-induced LR may become more prevalent in patients who do not have Parkinson disease; therefore, physicians who treat pediatric patients must be aware of this side effect.5
- Quaresma MV, Gomes-Dias AC, Serruya A, et al. Amantadine-induced livedo reticularis: a case report. An Bras Dermatol. 2015;90:745-747.
- Gibbs MB, English JC, Zirwas MJ. Livedo reticularis: an update. J Am Acad Dermatol. 2005;52:1009-1019.
- Silva SB, Miot HA. Case for diagnosis. amantadine-induced livedo reticularis. An Bras Dermatol. 2012;87:319-321.
- Vollum DI, Parkes JD, Doyle D. Livedo reticularis during amantadine treatment. Br Med J. 1971;2:627-628.
- Hosenbocus S, Chahal R. Amantadine: a review of use in child and adolescent psychiatry. J Can Acad Child Adolesc Psychiatry. 2013;22:55-60.
- Barrera F, Browning JC. Likely amantadine-induced livedo reticularis in a child. Pediatr Dermatol. 2012;29:329-330.
Livedo reticularis (LR) is a common dermatologic finding consisting of diffuse, reticulated, violaceous patches. It often is a benign physical finding known as cutis marmorata; however, LR can be associated with other medical conditions as well as with the use of some medications.1,2 Amantadine is a common cause of LR in Parkinson disease patients.3,4 We present a rare case of amantadine-induced LR in a pediatric patient and highlight the off-label use of this medication in children.
Case Report
An 8-year-old boy presented with a diffuse rash on the trunk, arms, and legs of 9 months’ duration. The patient denied any associated symptoms as well as alleviating or exacerbating factors. He also denied any changes with temperature. He had no recent international travel and no prior drug allergies. His medical history was remarkable for attention deficit hyperactivity disorder (ADHD), bipolar disorder, and autism spectrum disorder. His previously prescribed medications included atomoxetine, quetiapine, and valproic acid. The only new medication that had been started within the last year was amantadine. Physical examination revealed a diffuse, reticulated, erythematous to violaceous, blanching rash that was most notable on the legs (Figure 1A) but also was present on the trunk (Figure 1B) and arms (Figure 1C). The clinical examination was consistent with LR, which was presumed to be secondary to amantadine use. Given the multiple psychiatric diagnoses and medication history in this young patient, a consultation with child psychiatry was facilitated. His medications and diagnosis were reviewed, and amantadine was discontinued. At a follow-up visit 5 months later, the patient’s LR had improved (Figure 2).


Comment
Amantadine has a well-documented association with LR in patients with Parkinson disease,3,4 which has been reported in up to 40% of those taking amantadine.2 More recently, amantadine has been used off label to treat neurobehavioral disorders in children due to beneficial effects including improvement in attention and concentration, distractibility, and fatigue.5 Our patient was being treated off label with amantadine for ADHD and bipolar disorder. Amantadine acts as a noncompetitive antagonist of the N-methyl-D-aspartate receptor, enhancing dopamine release to reduce symptoms of ADHD.5,6 Additionally, amantadine can cause a depletion of catecholamines in the peripheral nerve terminals, which may lead to dilatation of dermal vessels.4,6 This sequence of events has been proposed as a possible mechanism contributing to amantadine-induced LR, though the pathophysiology is not fully understood.1,3,4
Our case of LR likely was induced by amantadine given the temporal relationship between initiation of the medication, onset of the rash, and the considerable improvement of the rash upon discontinuation of amantadine. Barrera and Browning6 reported another case of amantadine-induced LR in a pediatric patient. Because amantadine is increasingly being used off label to treat childhood neurobehavioral disorders, amantadine-induced LR may become more prevalent in patients who do not have Parkinson disease; therefore, physicians who treat pediatric patients must be aware of this side effect.5
Livedo reticularis (LR) is a common dermatologic finding consisting of diffuse, reticulated, violaceous patches. It often is a benign physical finding known as cutis marmorata; however, LR can be associated with other medical conditions as well as with the use of some medications.1,2 Amantadine is a common cause of LR in Parkinson disease patients.3,4 We present a rare case of amantadine-induced LR in a pediatric patient and highlight the off-label use of this medication in children.
Case Report
An 8-year-old boy presented with a diffuse rash on the trunk, arms, and legs of 9 months’ duration. The patient denied any associated symptoms as well as alleviating or exacerbating factors. He also denied any changes with temperature. He had no recent international travel and no prior drug allergies. His medical history was remarkable for attention deficit hyperactivity disorder (ADHD), bipolar disorder, and autism spectrum disorder. His previously prescribed medications included atomoxetine, quetiapine, and valproic acid. The only new medication that had been started within the last year was amantadine. Physical examination revealed a diffuse, reticulated, erythematous to violaceous, blanching rash that was most notable on the legs (Figure 1A) but also was present on the trunk (Figure 1B) and arms (Figure 1C). The clinical examination was consistent with LR, which was presumed to be secondary to amantadine use. Given the multiple psychiatric diagnoses and medication history in this young patient, a consultation with child psychiatry was facilitated. His medications and diagnosis were reviewed, and amantadine was discontinued. At a follow-up visit 5 months later, the patient’s LR had improved (Figure 2).


Comment
Amantadine has a well-documented association with LR in patients with Parkinson disease,3,4 which has been reported in up to 40% of those taking amantadine.2 More recently, amantadine has been used off label to treat neurobehavioral disorders in children due to beneficial effects including improvement in attention and concentration, distractibility, and fatigue.5 Our patient was being treated off label with amantadine for ADHD and bipolar disorder. Amantadine acts as a noncompetitive antagonist of the N-methyl-D-aspartate receptor, enhancing dopamine release to reduce symptoms of ADHD.5,6 Additionally, amantadine can cause a depletion of catecholamines in the peripheral nerve terminals, which may lead to dilatation of dermal vessels.4,6 This sequence of events has been proposed as a possible mechanism contributing to amantadine-induced LR, though the pathophysiology is not fully understood.1,3,4
Our case of LR likely was induced by amantadine given the temporal relationship between initiation of the medication, onset of the rash, and the considerable improvement of the rash upon discontinuation of amantadine. Barrera and Browning6 reported another case of amantadine-induced LR in a pediatric patient. Because amantadine is increasingly being used off label to treat childhood neurobehavioral disorders, amantadine-induced LR may become more prevalent in patients who do not have Parkinson disease; therefore, physicians who treat pediatric patients must be aware of this side effect.5
- Quaresma MV, Gomes-Dias AC, Serruya A, et al. Amantadine-induced livedo reticularis: a case report. An Bras Dermatol. 2015;90:745-747.
- Gibbs MB, English JC, Zirwas MJ. Livedo reticularis: an update. J Am Acad Dermatol. 2005;52:1009-1019.
- Silva SB, Miot HA. Case for diagnosis. amantadine-induced livedo reticularis. An Bras Dermatol. 2012;87:319-321.
- Vollum DI, Parkes JD, Doyle D. Livedo reticularis during amantadine treatment. Br Med J. 1971;2:627-628.
- Hosenbocus S, Chahal R. Amantadine: a review of use in child and adolescent psychiatry. J Can Acad Child Adolesc Psychiatry. 2013;22:55-60.
- Barrera F, Browning JC. Likely amantadine-induced livedo reticularis in a child. Pediatr Dermatol. 2012;29:329-330.
- Quaresma MV, Gomes-Dias AC, Serruya A, et al. Amantadine-induced livedo reticularis: a case report. An Bras Dermatol. 2015;90:745-747.
- Gibbs MB, English JC, Zirwas MJ. Livedo reticularis: an update. J Am Acad Dermatol. 2005;52:1009-1019.
- Silva SB, Miot HA. Case for diagnosis. amantadine-induced livedo reticularis. An Bras Dermatol. 2012;87:319-321.
- Vollum DI, Parkes JD, Doyle D. Livedo reticularis during amantadine treatment. Br Med J. 1971;2:627-628.
- Hosenbocus S, Chahal R. Amantadine: a review of use in child and adolescent psychiatry. J Can Acad Child Adolesc Psychiatry. 2013;22:55-60.
- Barrera F, Browning JC. Likely amantadine-induced livedo reticularis in a child. Pediatr Dermatol. 2012;29:329-330.
Practice Points
- Amantadine is a generally well-tolerated medication that is more commonly used for off-label treatment of several pediatric neurobehavioral conditions such as attention deficit hyperactivity disorder, autism spectrum disorders, obsessive compulsive disorder, depression, and others.
- Livedo reticularis has known associations with several medications and diseases; however, the most common presentation is cutis marmorata, a benign condition that typically affects newborns.
Total-Body Photography in Skin Cancer Screening: The Clinical Utility of Standardized Imaging
Skin cancer is an important public health issue in the United States, as 1 in 5 Americans are projected to develop a cutaneous malignancy during their lifetime. Currently, 75% of all skin cancer–related deaths are due to malignant melanomas (MMs), though melanomas account for less than 5% of all skin cancers.1 Early detection of MM is essential, as prognosis depends on tumor stage, particularly the depth of the melanoma.2-4 In general, patients with thin, early-stage melanomas have a more than 96% survival rate, which drops to 14% in late-stage disease.5,6 Five percent to 30% of all melanomas are identified incidentally on total-body skin examinations (TBSEs) performed by a trained provider and thus would not have been caught with only a focused skin examination or patient self-examination.7,8 Nonetheless, the clinical utility of skin cancer screening with TBSEs remains controversial, largely due to the poor quality of data available to establish a notable mortality benefit from skin cancer screening. As a result, obtaining endorsement from the larger medical community, federal government, and health insurance industry to include routine TBSEs as part of a preventive care health care strategy has not occurred. The absence of definitive clinical care guidelines mandating routine TBSEs is one of the greatest barriers preventing access to appropriate dermatologic screening along with the paucity of trained providers; however, standardized total-body photography (TBP) promises to provide a way forward by lowering the costs of dermatologic screening while simultaneously leveraging technology to increase availability.
Impact on Biopsy Efficiency
Current US Preventive Services Task Force (USPSTF) guidelines state that evidence is insufficient to assess the balance of benefits and harms of visual skin examination by a clinician to screen for skin cancer in adults. The USPSTF noted that “[d]irect evidence on the effectiveness of screening in reducing melanoma morbidity and mortality is limited to a single fair-quality ecologic study with important methodological limitations” (ie, the Skin Cancer Research to Provide Evidence for Effectiveness of Screening in Northern Germany [SCREEN] study), and although information on harm is similarly sparse, “[t]he potential for harm clearly exists, including a high rate of unnecessary biopsies, possibly resulting in cosmetic or, more rarely, functional adverse effects, and the risk of overdiagnosis and overtreatment.”9 The majority of suspicious skin lesions excised during screenings are not cancerous. For example, the SCREEN study found that 20 to 55 excisions were performed to detect 1 case of melanoma.10 At that rate, the USPSTF also noted that approximately 4000 excisions would be required to prevent a single death from melanoma.9 Following the lead of the USPSTF, the Patient Protection and Affordable Care Act did not mandate that skin examinations be included as essential preventive coverage in its requirements for insurance coverage of primary care prevention. As such, dermatologists face financial pressure to avoid performing time-consuming TBSEs, regardless of their perceived utility.11
As the USPSTF points out, the value of TBSEs relies on the examiner’s ability to correctly identify malignant lesions and minimize biopsies of benign lesions, a concept known as biopsy efficiency.9 Secondarily, a TBSE is time consuming, and the time required for a dermatologist to complete a TBSE given the high rate of benign findings may not be financially viable. We argue that the routine use of total-body skin imaging may offer a way forward in addressing these issues. Total-body photography involves a photographic system that can allow dermatologists to acquire standardized images that can be used for primary diagnosis and to track individual lesions over time. Nonmedical personnel and medical assistants can be easily trained to use standardized photography devices to quickly obtain high-quality clinical images, thereby greatly reducing the time and cost of obtaining these images. Studies have found that the use of photographic monitoring may improve biopsy efficiency.12-15 A recent study by Truong et al16 found that TBP used to monitor all existing melanocytic lesions on patients substantially reduced the number of biopsies that patients required. These results reflect that most nevi, including clinically atypical nevi, are usually stable and unlikely to exhibit suspicious changes over time.17,18 For this reason, the use of TBP could minimize unnecessary biopsies because clinically suspicious but stable nevi can be objectively documented and followed over time.
Standardized TBP also offers the ability for dermatologists to work synergistically with modern computer technology involving algorithms capable of analyzing high-quality images to autodiagnose or flag concerning lesions that may require biopsy. Esteva et al19 described their development of a deep learning algorithm that relies on a convolutional neural network (CNN). This CNN was trained to identify melanomas using a large data set of clinical dermatologic images and subsequently was able to distinguish MMs from benign nevi at a rate on par with a board-certified dermatologist.19 Widespread use of total-body imaging would create an enormous database of high-resolution images that would be ideally suited to the development of such computerized algorithms, which could then be applied to future images by way of artificial intelligence. Convolutional neural networks that use a single patient’s imaging over time could be developed to assess the change in number or size of benign nevi and identify lesions that are concerning for MM while simultaneously comparing them to a population-based data set.
On a large scale, such a capability would minimize the inefficiency and subjectivity of TBSEs as a tool for identifying malignancy. Currently, dermatologists are only able to track and document a few concerning lesions on a patient’s body, rendering the choice of which lesions require biopsy more subjective. Total-body photography, particularly if used with an algorithm capable of quickly analyzing all the nevi on a person’s body, largely eliminates such subjectivity by creating a standardized set of images that can be tracked over time and flagging concerning lesions prior to the dermatologist examining the patient. In this way, the specialty of dermatology could achieve the same model of objective evaluation of standardized clinical images as those employed in radiology, cardiology, and other clinical disciplines. The additional benefit of such a system would be lower costs, as the images could be acquired by nonmedical personnel and then undergo initial assessment by an algorithm, which would flag concerning lesions, similar to a modern electrocardiogram machine, allowing the dermatologist to use his/her time more efficiently by only focusing on concerning lesions with the confidence that the patient’s entire body has already been rigorously screened.
By using TBP to improve biopsy efficiency and the objectivity of the TBSE as a tool to detect skin cancer, we propose that the benefit-to-harm ratio of the TBSE would remarkably improve. Ultimately, this type of screening would meet the strict requirements to be included in preventive health care strategies and thereby improve access to dermatologic care.
The Use of TBP in the Military
Total-body photography has several specific applications in the military. Standardized imaging has the potential to improve dermatologic care for active-duty soldiers across space and time. First, a large percentage of deployment medical care is devoted to dermatologic issues. From 2008 to 2015, 5% of all medical encounters in the combat theaters of Iraq and Afghanistan involved dermatologic concerns.20 Access to appropriate dermatologic care in a combat theater is important, as poorly controlled dermatologic conditions (eg, psoriasis, eczema) often require evacuation when left untreated. Although current TBP systems may not be portable or durable enough to survive in an austere deployment environment, we propose it would be feasible to have skin imaging booths at larger forward operating bases. The images could then be transported to a remote dermatologist to assess and recommend treatment. The expense of transporting and maintaining the imaging system in country would be offset by the expenses spared by not requiring a dermatologist in country and the reductions in costly medical evacuations from theater.
Although the US military population is younger and generally healthier than the general adult population due to extensive medical screening on admission, age limitations for active-duty service, a mandated active lifestyle, and access to good health care, there are still a substantial number of service members diagnosed with skin cancer each year.21 From 2005 through 2014, MM was the most common non–gender-specific cancer (n=1571); in men, only testicular cancer was more prevalent (1591 vs 1298 cases), and in women, only breast cancer was more prevalent (773 vs 273 cases). Furthermore, from 2004 to 2013, the incidence rates of melanoma have increased by 1.4%, while with other cancer rates have declined during the same time period.21 Thus, TBP as a screening modality across the military population is a promising method for improving detection of skin cancer and reducing morbidity and mortality.
Military medicine often is on the forefront of medical advances in technology, disease understanding, and clinical care due to the unique resources available in the military health care system, which allow investigators the ability to obtain vast amounts of epidemiologic data.22 The military health care system also is unique in its ability to mandate that its members obtain preventive health services. Thus, it would be possible for the military to mandate TBP at accession and retirement, for instance, or more frequently for annual screening. The implementation of such a program would improve the health of the military population and be a public health service by pioneering the use of a standardized TBP system across a large health care system to improve skin cancer detection.
Current Studies in the Military
The Dermatology Service at the Walter Reed National Military Medical Center (WRNMMC)(Bethesda, Maryland) is evaluating the use of a total-body digital skin imaging system under a grant from the Telemedicine and Advanced Technology Research Center of the US Army. The system in use was found to be particularly well suited for military dermatology because it offers standardized TBP processing, produces a report that can be uploaded to the US Department of Defense (DoD) electronic medical record system, and requires relatively brief training for ancillary personnel to operate. Regardless of the platform the DoD ultimately finds most suitable, it is critical that a standard exist for TBP to ensure that uniform data sets are generated to allow military and other DoD dermatologists as well as civilian health care providers to share clinical information. The goal of the current study at WRNMMC is to vet TBP platforms at WRNMMC so the military can then develop standards to procure additional platforms for placement throughout the Military Health System, Military Entrance Processing Stations, operational environments, and collaborating health care systems (eg, the Veterans Health Administration).
Once deployed broadly across the Military Health System, these TBP platforms would be part of a network of telehealth care. For acute dermatologic issues, diagnoses provided via teledermatology platforms can then be managed by health care providers utilizing appropriate clinical practice guidelines or by non–health care providers utilizing general medical knowledge databases. Such a system with TBP information collected at multiple access points across a service member’s career would build a repository of data that would be immensely useful to patients and to clinical research. Of particular interest to military researchers is that TBP data could be used to study which patients require in-person examinations or more careful monitoring; the proper intervals for skin cancer screening; and the assessment of the benefits of TBP in improving morbidity, mortality, and biopsy efficiency in the detection of MM as well as nonmelanoma skin cancers.
Limitations to Progress
Currently, there are multiple limitations to the implementation of TBP as a part of TBSE screening. First, the potential improvement in biopsy efficiency using TBP is predicated on its ability to prove nevi stability over time, but in younger populations, benign nevi are more likely to change or increase in number, which may reduce the biopsy efficiency of screening in a younger population, thereby negating some of the benefit of imaging and CNN assessment. For instance, Truong et al16 found that younger age (<30 years) did not show the same improvement in biopsy efficiency with the use of TBP, which the authors theorized may reflect “the dynamic nature of nevi in younger patients” that has been documented in other studies.23,24 Approximately 65% of the active-duty military population is aged 18 to 30 years, and 98% of accessions to active duty occur in individuals aged 17 to 30 years.25 As such, TBP may not improve biopsy efficiency in the active-duty military population as dramatically as it would across the general population.
A second limitation of the use of TBP in the active-duty military population is the ethics of implementing DoD-wide mandatory TBP. Although the TBP platform will be compliant with the Health Insurance Portability and Accountability Act, mandating that soldiers contribute their TBP to a repository of data that will then be used for research without explicitly requesting their consent is ethically problematic; however, since the 1950s, the DoD has collected serum samples from its service members for force protection and operations reasons as well as for the purpose of research.22,26 Currently, the DoD Serum Repository collects serum samples as part of a mandatory human immunodeficiency virus screening program that evaluates service members every 2 years; this repository of human serum samples is accessible for research purposes without the consent of the individuals being studied.27 These individuals are not informed of potential use of their serum specimens for research purposes and no consent forms or opt-out options are provided. Thus, although there is precedent in the DoD for such mass data collection, it is an ongoing ethical consideration.28
RELATED ARTICLE: Gigapixel Photography for Skin Cancer Surveillance
Finally, although the potential use of TBP and computer algorithms to improve the efficiency and affordability of TBSEs is exciting, there are no existing computer algorithms that we are aware of that can be used with existing TBP platforms in the manner we proposed. However, we feel that computer algorithms, such as the one created by Esteva et al,19 are just the beginning and that the use of artificial intelligence is not far off. Even after the creation of a TBP-compatible algorithm adept at analyzing malignant lesions, however, this technology would need to be further evaluated in the clinical setting to determine its capability and practicality. Current TBP platforms also are limited by their large size, cost, and complexity. As TBP platforms improve, it is likely that more streamlined and less expensive versions of current models will greatly enhance the field of teledermatology, particularly in the military setting.
- Rogers HW, Weinstock MA, Feldman SR, et al. Incidence estimate of nonmelanoma skin cancer (keratinocyte carcinomas) in the U.S. population, 2012. JAMA Dermatol. 2015;151:1081-1086.
- Balch CM, Soong SJ, Atkins MB, et al. An evidence-based staging system for cutaneous melanoma. CA Cancer J Clin. 2004;54:131-149; quiz 182-184.
- Eisemann N, Jansen L, Holleczek B, et al. Up-to-date results on survival of patients with melanoma in Germany [published online July 19, 2012]. Br J Dermatol. 2012;167:606-612.
- MacKie RM, Bray C, Vestey J, et al. Melanoma incidence and mortality in Scotland 1979-2003 [published online May 29, 2007]. Br J Cancer. 2007;96:1772-1777.
- Dickson PV, Gershenwald JE. Staging and prognosis of cutaneous melanoma. Surg Oncol Clin N Am. 2011;20:1-17.
- Balch CM, Gershenwald JE, Soong SL, et al. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol. 2009;27:6199-6206.
- Kingsley-Loso JL, Grey KR, Hanson JL, et al. Incidental lesions found in veterans referred to dermatology: the value of a dermatologic examination [published online January 23, 2015]. J Am Acad Dermatol. 2015;72:651.e1-655.e1.
- Grant-Kels JM, Stoff B. Total body skin exams (TBSEs): saving lives or wasting time? J Am Acad Dermatol. 2017;76:183-185.
- US Preventive Services Task Force; Bibbins-Domingo K, Grossman DC, Curry SJ, et al. Screening for skin cancer: US Preventive Services Task Force recommendation statement. JAMA. 2016;316:429-435.
- Breitbart EW, Waldmann A, Nolte S, et al. Systematic skin cancer screening in Northern Germany. J Am Acad Dermatol. 2012;66:201-211.
- Robinson JK, Halpern AC. Cost-effective melanoma screening. JAMA Dermatol. 2016;152:19-21.
- Feit NE, Dusza SW, Marghoob AA. Melanomas detected with the aid of total cutaneous photography. Br J Dermatol. 2004;150:706-714.
- Haenssle HA, Krueger U, Vente C, et al. Results from an observational trial: digital epiluminescence microscopy follow-up of atypical nevi increases the sensitivity and the chance of success of conventional dermoscopy in detecting melanoma. J Invest Dermatol. 2006;126:980-985.
- Salerni G, Carrera C, Lovatto L, et al. Benefits of total body photography and digital dermatoscopy (“two-step method of digital follow-up”) in the early diagnosis of melanoma in patients at high risk for melanoma. J Am Acad Dermatol. 2012;67:E17-E27.
- Rice ZP, Weiss FJ, DeLong LK, et al. Utilization and rationale for the implementation of total body (digital) photography as an adjunct screening measure for melanoma. Melanoma Res. 2010;20:417-421.
- Truong A, Strazzulla L, March J, et al. Reduction in nevus biopsies in patients monitored by total body photography [published online March 3, 2016]. J Am Acad Dermatol. 2016;75:135.e5-143.e5.
- Lucas CR, Sanders LL, Murray JC, et al. Early melanoma detection: nonuniform dermoscopic features and growth. J Am Acad Dermatol. 2003;48:663-671.
- Fuller SR, Bowen GM, Tanner B, et al. Digital dermoscopic monitoring of atypical nevi in patients at risk for melanoma. Dermatol Surg. 2007;33:1198-1206; discussion 1205-1206.
- Esteva A, Kuprel B, Novoa RA, et al. Dermatologist-level classification of skin cancer with deep neural networks [published online January 25, 2017]. Nature. 2017;542:115-118.
- Defense Medical Epidemiology Database. Military Health System website. http://www.health.mil/Military-Health-Topics/Health-Readiness/Armed-Forces-Health-Surveillance-Branch/Data-Management-and-Technical-Support/Defense-Medical-Epidemiology-Database. Accessed April 10, 2017.
- Lee T, Williams VF, Clark LL. Incident diagnoses of cancers in the active component and cancer-related deaths in the active and reserve components, U.S. Armed Forces, 2005-2014. MSMR. 2016;23:23-31.
- Helmandollar KJ, Meyerle JH. Exploration of modern military research resources. Cutis. 2016;98:231-234.
- Goodson AG, Grossman D. Strategies for early melanoma detection: approaches to the patient with nevi. J Am Acad Dermatol. 2009;60:719-735; quiz 736-738.
- Bajaj S, Dusza SW, Marchetti MA, et al. Growth-curve modeling of nevi with a peripheral globular pattern. JAMA Dermatol. 2015;151:1338-1345.
- Niebuhr DW, Gubata ME, Cowan DN, et al. Accession Medical Standards Analysis & Research Activity (AMSARA) 2011 Annual Report. Silver Spring, MD: Division of Preventive Medicine, Walter Reed Army Institute of Research; 2012.
- Liao SJ. Immunity status of military recruits in 1951 in the United States. I. results of Schick tests. Am J Hyg. 1954;59:262-272.
- Perdue CL, Eick-Cost AA, Rubertone MV. A brief description of the operation of the DoD Serum Repository. Mil Med. 2015;180:10-12.
- Pavlin JA, Welch RA. Ethics, human use, and the Department of Defense Serum Repository. Mil Med. 2015;180:49-56.
Skin cancer is an important public health issue in the United States, as 1 in 5 Americans are projected to develop a cutaneous malignancy during their lifetime. Currently, 75% of all skin cancer–related deaths are due to malignant melanomas (MMs), though melanomas account for less than 5% of all skin cancers.1 Early detection of MM is essential, as prognosis depends on tumor stage, particularly the depth of the melanoma.2-4 In general, patients with thin, early-stage melanomas have a more than 96% survival rate, which drops to 14% in late-stage disease.5,6 Five percent to 30% of all melanomas are identified incidentally on total-body skin examinations (TBSEs) performed by a trained provider and thus would not have been caught with only a focused skin examination or patient self-examination.7,8 Nonetheless, the clinical utility of skin cancer screening with TBSEs remains controversial, largely due to the poor quality of data available to establish a notable mortality benefit from skin cancer screening. As a result, obtaining endorsement from the larger medical community, federal government, and health insurance industry to include routine TBSEs as part of a preventive care health care strategy has not occurred. The absence of definitive clinical care guidelines mandating routine TBSEs is one of the greatest barriers preventing access to appropriate dermatologic screening along with the paucity of trained providers; however, standardized total-body photography (TBP) promises to provide a way forward by lowering the costs of dermatologic screening while simultaneously leveraging technology to increase availability.
Impact on Biopsy Efficiency
Current US Preventive Services Task Force (USPSTF) guidelines state that evidence is insufficient to assess the balance of benefits and harms of visual skin examination by a clinician to screen for skin cancer in adults. The USPSTF noted that “[d]irect evidence on the effectiveness of screening in reducing melanoma morbidity and mortality is limited to a single fair-quality ecologic study with important methodological limitations” (ie, the Skin Cancer Research to Provide Evidence for Effectiveness of Screening in Northern Germany [SCREEN] study), and although information on harm is similarly sparse, “[t]he potential for harm clearly exists, including a high rate of unnecessary biopsies, possibly resulting in cosmetic or, more rarely, functional adverse effects, and the risk of overdiagnosis and overtreatment.”9 The majority of suspicious skin lesions excised during screenings are not cancerous. For example, the SCREEN study found that 20 to 55 excisions were performed to detect 1 case of melanoma.10 At that rate, the USPSTF also noted that approximately 4000 excisions would be required to prevent a single death from melanoma.9 Following the lead of the USPSTF, the Patient Protection and Affordable Care Act did not mandate that skin examinations be included as essential preventive coverage in its requirements for insurance coverage of primary care prevention. As such, dermatologists face financial pressure to avoid performing time-consuming TBSEs, regardless of their perceived utility.11
As the USPSTF points out, the value of TBSEs relies on the examiner’s ability to correctly identify malignant lesions and minimize biopsies of benign lesions, a concept known as biopsy efficiency.9 Secondarily, a TBSE is time consuming, and the time required for a dermatologist to complete a TBSE given the high rate of benign findings may not be financially viable. We argue that the routine use of total-body skin imaging may offer a way forward in addressing these issues. Total-body photography involves a photographic system that can allow dermatologists to acquire standardized images that can be used for primary diagnosis and to track individual lesions over time. Nonmedical personnel and medical assistants can be easily trained to use standardized photography devices to quickly obtain high-quality clinical images, thereby greatly reducing the time and cost of obtaining these images. Studies have found that the use of photographic monitoring may improve biopsy efficiency.12-15 A recent study by Truong et al16 found that TBP used to monitor all existing melanocytic lesions on patients substantially reduced the number of biopsies that patients required. These results reflect that most nevi, including clinically atypical nevi, are usually stable and unlikely to exhibit suspicious changes over time.17,18 For this reason, the use of TBP could minimize unnecessary biopsies because clinically suspicious but stable nevi can be objectively documented and followed over time.
Standardized TBP also offers the ability for dermatologists to work synergistically with modern computer technology involving algorithms capable of analyzing high-quality images to autodiagnose or flag concerning lesions that may require biopsy. Esteva et al19 described their development of a deep learning algorithm that relies on a convolutional neural network (CNN). This CNN was trained to identify melanomas using a large data set of clinical dermatologic images and subsequently was able to distinguish MMs from benign nevi at a rate on par with a board-certified dermatologist.19 Widespread use of total-body imaging would create an enormous database of high-resolution images that would be ideally suited to the development of such computerized algorithms, which could then be applied to future images by way of artificial intelligence. Convolutional neural networks that use a single patient’s imaging over time could be developed to assess the change in number or size of benign nevi and identify lesions that are concerning for MM while simultaneously comparing them to a population-based data set.
On a large scale, such a capability would minimize the inefficiency and subjectivity of TBSEs as a tool for identifying malignancy. Currently, dermatologists are only able to track and document a few concerning lesions on a patient’s body, rendering the choice of which lesions require biopsy more subjective. Total-body photography, particularly if used with an algorithm capable of quickly analyzing all the nevi on a person’s body, largely eliminates such subjectivity by creating a standardized set of images that can be tracked over time and flagging concerning lesions prior to the dermatologist examining the patient. In this way, the specialty of dermatology could achieve the same model of objective evaluation of standardized clinical images as those employed in radiology, cardiology, and other clinical disciplines. The additional benefit of such a system would be lower costs, as the images could be acquired by nonmedical personnel and then undergo initial assessment by an algorithm, which would flag concerning lesions, similar to a modern electrocardiogram machine, allowing the dermatologist to use his/her time more efficiently by only focusing on concerning lesions with the confidence that the patient’s entire body has already been rigorously screened.
By using TBP to improve biopsy efficiency and the objectivity of the TBSE as a tool to detect skin cancer, we propose that the benefit-to-harm ratio of the TBSE would remarkably improve. Ultimately, this type of screening would meet the strict requirements to be included in preventive health care strategies and thereby improve access to dermatologic care.
The Use of TBP in the Military
Total-body photography has several specific applications in the military. Standardized imaging has the potential to improve dermatologic care for active-duty soldiers across space and time. First, a large percentage of deployment medical care is devoted to dermatologic issues. From 2008 to 2015, 5% of all medical encounters in the combat theaters of Iraq and Afghanistan involved dermatologic concerns.20 Access to appropriate dermatologic care in a combat theater is important, as poorly controlled dermatologic conditions (eg, psoriasis, eczema) often require evacuation when left untreated. Although current TBP systems may not be portable or durable enough to survive in an austere deployment environment, we propose it would be feasible to have skin imaging booths at larger forward operating bases. The images could then be transported to a remote dermatologist to assess and recommend treatment. The expense of transporting and maintaining the imaging system in country would be offset by the expenses spared by not requiring a dermatologist in country and the reductions in costly medical evacuations from theater.
Although the US military population is younger and generally healthier than the general adult population due to extensive medical screening on admission, age limitations for active-duty service, a mandated active lifestyle, and access to good health care, there are still a substantial number of service members diagnosed with skin cancer each year.21 From 2005 through 2014, MM was the most common non–gender-specific cancer (n=1571); in men, only testicular cancer was more prevalent (1591 vs 1298 cases), and in women, only breast cancer was more prevalent (773 vs 273 cases). Furthermore, from 2004 to 2013, the incidence rates of melanoma have increased by 1.4%, while with other cancer rates have declined during the same time period.21 Thus, TBP as a screening modality across the military population is a promising method for improving detection of skin cancer and reducing morbidity and mortality.
Military medicine often is on the forefront of medical advances in technology, disease understanding, and clinical care due to the unique resources available in the military health care system, which allow investigators the ability to obtain vast amounts of epidemiologic data.22 The military health care system also is unique in its ability to mandate that its members obtain preventive health services. Thus, it would be possible for the military to mandate TBP at accession and retirement, for instance, or more frequently for annual screening. The implementation of such a program would improve the health of the military population and be a public health service by pioneering the use of a standardized TBP system across a large health care system to improve skin cancer detection.
Current Studies in the Military
The Dermatology Service at the Walter Reed National Military Medical Center (WRNMMC)(Bethesda, Maryland) is evaluating the use of a total-body digital skin imaging system under a grant from the Telemedicine and Advanced Technology Research Center of the US Army. The system in use was found to be particularly well suited for military dermatology because it offers standardized TBP processing, produces a report that can be uploaded to the US Department of Defense (DoD) electronic medical record system, and requires relatively brief training for ancillary personnel to operate. Regardless of the platform the DoD ultimately finds most suitable, it is critical that a standard exist for TBP to ensure that uniform data sets are generated to allow military and other DoD dermatologists as well as civilian health care providers to share clinical information. The goal of the current study at WRNMMC is to vet TBP platforms at WRNMMC so the military can then develop standards to procure additional platforms for placement throughout the Military Health System, Military Entrance Processing Stations, operational environments, and collaborating health care systems (eg, the Veterans Health Administration).
Once deployed broadly across the Military Health System, these TBP platforms would be part of a network of telehealth care. For acute dermatologic issues, diagnoses provided via teledermatology platforms can then be managed by health care providers utilizing appropriate clinical practice guidelines or by non–health care providers utilizing general medical knowledge databases. Such a system with TBP information collected at multiple access points across a service member’s career would build a repository of data that would be immensely useful to patients and to clinical research. Of particular interest to military researchers is that TBP data could be used to study which patients require in-person examinations or more careful monitoring; the proper intervals for skin cancer screening; and the assessment of the benefits of TBP in improving morbidity, mortality, and biopsy efficiency in the detection of MM as well as nonmelanoma skin cancers.
Limitations to Progress
Currently, there are multiple limitations to the implementation of TBP as a part of TBSE screening. First, the potential improvement in biopsy efficiency using TBP is predicated on its ability to prove nevi stability over time, but in younger populations, benign nevi are more likely to change or increase in number, which may reduce the biopsy efficiency of screening in a younger population, thereby negating some of the benefit of imaging and CNN assessment. For instance, Truong et al16 found that younger age (<30 years) did not show the same improvement in biopsy efficiency with the use of TBP, which the authors theorized may reflect “the dynamic nature of nevi in younger patients” that has been documented in other studies.23,24 Approximately 65% of the active-duty military population is aged 18 to 30 years, and 98% of accessions to active duty occur in individuals aged 17 to 30 years.25 As such, TBP may not improve biopsy efficiency in the active-duty military population as dramatically as it would across the general population.
A second limitation of the use of TBP in the active-duty military population is the ethics of implementing DoD-wide mandatory TBP. Although the TBP platform will be compliant with the Health Insurance Portability and Accountability Act, mandating that soldiers contribute their TBP to a repository of data that will then be used for research without explicitly requesting their consent is ethically problematic; however, since the 1950s, the DoD has collected serum samples from its service members for force protection and operations reasons as well as for the purpose of research.22,26 Currently, the DoD Serum Repository collects serum samples as part of a mandatory human immunodeficiency virus screening program that evaluates service members every 2 years; this repository of human serum samples is accessible for research purposes without the consent of the individuals being studied.27 These individuals are not informed of potential use of their serum specimens for research purposes and no consent forms or opt-out options are provided. Thus, although there is precedent in the DoD for such mass data collection, it is an ongoing ethical consideration.28
RELATED ARTICLE: Gigapixel Photography for Skin Cancer Surveillance
Finally, although the potential use of TBP and computer algorithms to improve the efficiency and affordability of TBSEs is exciting, there are no existing computer algorithms that we are aware of that can be used with existing TBP platforms in the manner we proposed. However, we feel that computer algorithms, such as the one created by Esteva et al,19 are just the beginning and that the use of artificial intelligence is not far off. Even after the creation of a TBP-compatible algorithm adept at analyzing malignant lesions, however, this technology would need to be further evaluated in the clinical setting to determine its capability and practicality. Current TBP platforms also are limited by their large size, cost, and complexity. As TBP platforms improve, it is likely that more streamlined and less expensive versions of current models will greatly enhance the field of teledermatology, particularly in the military setting.
Skin cancer is an important public health issue in the United States, as 1 in 5 Americans are projected to develop a cutaneous malignancy during their lifetime. Currently, 75% of all skin cancer–related deaths are due to malignant melanomas (MMs), though melanomas account for less than 5% of all skin cancers.1 Early detection of MM is essential, as prognosis depends on tumor stage, particularly the depth of the melanoma.2-4 In general, patients with thin, early-stage melanomas have a more than 96% survival rate, which drops to 14% in late-stage disease.5,6 Five percent to 30% of all melanomas are identified incidentally on total-body skin examinations (TBSEs) performed by a trained provider and thus would not have been caught with only a focused skin examination or patient self-examination.7,8 Nonetheless, the clinical utility of skin cancer screening with TBSEs remains controversial, largely due to the poor quality of data available to establish a notable mortality benefit from skin cancer screening. As a result, obtaining endorsement from the larger medical community, federal government, and health insurance industry to include routine TBSEs as part of a preventive care health care strategy has not occurred. The absence of definitive clinical care guidelines mandating routine TBSEs is one of the greatest barriers preventing access to appropriate dermatologic screening along with the paucity of trained providers; however, standardized total-body photography (TBP) promises to provide a way forward by lowering the costs of dermatologic screening while simultaneously leveraging technology to increase availability.
Impact on Biopsy Efficiency
Current US Preventive Services Task Force (USPSTF) guidelines state that evidence is insufficient to assess the balance of benefits and harms of visual skin examination by a clinician to screen for skin cancer in adults. The USPSTF noted that “[d]irect evidence on the effectiveness of screening in reducing melanoma morbidity and mortality is limited to a single fair-quality ecologic study with important methodological limitations” (ie, the Skin Cancer Research to Provide Evidence for Effectiveness of Screening in Northern Germany [SCREEN] study), and although information on harm is similarly sparse, “[t]he potential for harm clearly exists, including a high rate of unnecessary biopsies, possibly resulting in cosmetic or, more rarely, functional adverse effects, and the risk of overdiagnosis and overtreatment.”9 The majority of suspicious skin lesions excised during screenings are not cancerous. For example, the SCREEN study found that 20 to 55 excisions were performed to detect 1 case of melanoma.10 At that rate, the USPSTF also noted that approximately 4000 excisions would be required to prevent a single death from melanoma.9 Following the lead of the USPSTF, the Patient Protection and Affordable Care Act did not mandate that skin examinations be included as essential preventive coverage in its requirements for insurance coverage of primary care prevention. As such, dermatologists face financial pressure to avoid performing time-consuming TBSEs, regardless of their perceived utility.11
As the USPSTF points out, the value of TBSEs relies on the examiner’s ability to correctly identify malignant lesions and minimize biopsies of benign lesions, a concept known as biopsy efficiency.9 Secondarily, a TBSE is time consuming, and the time required for a dermatologist to complete a TBSE given the high rate of benign findings may not be financially viable. We argue that the routine use of total-body skin imaging may offer a way forward in addressing these issues. Total-body photography involves a photographic system that can allow dermatologists to acquire standardized images that can be used for primary diagnosis and to track individual lesions over time. Nonmedical personnel and medical assistants can be easily trained to use standardized photography devices to quickly obtain high-quality clinical images, thereby greatly reducing the time and cost of obtaining these images. Studies have found that the use of photographic monitoring may improve biopsy efficiency.12-15 A recent study by Truong et al16 found that TBP used to monitor all existing melanocytic lesions on patients substantially reduced the number of biopsies that patients required. These results reflect that most nevi, including clinically atypical nevi, are usually stable and unlikely to exhibit suspicious changes over time.17,18 For this reason, the use of TBP could minimize unnecessary biopsies because clinically suspicious but stable nevi can be objectively documented and followed over time.
Standardized TBP also offers the ability for dermatologists to work synergistically with modern computer technology involving algorithms capable of analyzing high-quality images to autodiagnose or flag concerning lesions that may require biopsy. Esteva et al19 described their development of a deep learning algorithm that relies on a convolutional neural network (CNN). This CNN was trained to identify melanomas using a large data set of clinical dermatologic images and subsequently was able to distinguish MMs from benign nevi at a rate on par with a board-certified dermatologist.19 Widespread use of total-body imaging would create an enormous database of high-resolution images that would be ideally suited to the development of such computerized algorithms, which could then be applied to future images by way of artificial intelligence. Convolutional neural networks that use a single patient’s imaging over time could be developed to assess the change in number or size of benign nevi and identify lesions that are concerning for MM while simultaneously comparing them to a population-based data set.
On a large scale, such a capability would minimize the inefficiency and subjectivity of TBSEs as a tool for identifying malignancy. Currently, dermatologists are only able to track and document a few concerning lesions on a patient’s body, rendering the choice of which lesions require biopsy more subjective. Total-body photography, particularly if used with an algorithm capable of quickly analyzing all the nevi on a person’s body, largely eliminates such subjectivity by creating a standardized set of images that can be tracked over time and flagging concerning lesions prior to the dermatologist examining the patient. In this way, the specialty of dermatology could achieve the same model of objective evaluation of standardized clinical images as those employed in radiology, cardiology, and other clinical disciplines. The additional benefit of such a system would be lower costs, as the images could be acquired by nonmedical personnel and then undergo initial assessment by an algorithm, which would flag concerning lesions, similar to a modern electrocardiogram machine, allowing the dermatologist to use his/her time more efficiently by only focusing on concerning lesions with the confidence that the patient’s entire body has already been rigorously screened.
By using TBP to improve biopsy efficiency and the objectivity of the TBSE as a tool to detect skin cancer, we propose that the benefit-to-harm ratio of the TBSE would remarkably improve. Ultimately, this type of screening would meet the strict requirements to be included in preventive health care strategies and thereby improve access to dermatologic care.
The Use of TBP in the Military
Total-body photography has several specific applications in the military. Standardized imaging has the potential to improve dermatologic care for active-duty soldiers across space and time. First, a large percentage of deployment medical care is devoted to dermatologic issues. From 2008 to 2015, 5% of all medical encounters in the combat theaters of Iraq and Afghanistan involved dermatologic concerns.20 Access to appropriate dermatologic care in a combat theater is important, as poorly controlled dermatologic conditions (eg, psoriasis, eczema) often require evacuation when left untreated. Although current TBP systems may not be portable or durable enough to survive in an austere deployment environment, we propose it would be feasible to have skin imaging booths at larger forward operating bases. The images could then be transported to a remote dermatologist to assess and recommend treatment. The expense of transporting and maintaining the imaging system in country would be offset by the expenses spared by not requiring a dermatologist in country and the reductions in costly medical evacuations from theater.
Although the US military population is younger and generally healthier than the general adult population due to extensive medical screening on admission, age limitations for active-duty service, a mandated active lifestyle, and access to good health care, there are still a substantial number of service members diagnosed with skin cancer each year.21 From 2005 through 2014, MM was the most common non–gender-specific cancer (n=1571); in men, only testicular cancer was more prevalent (1591 vs 1298 cases), and in women, only breast cancer was more prevalent (773 vs 273 cases). Furthermore, from 2004 to 2013, the incidence rates of melanoma have increased by 1.4%, while with other cancer rates have declined during the same time period.21 Thus, TBP as a screening modality across the military population is a promising method for improving detection of skin cancer and reducing morbidity and mortality.
Military medicine often is on the forefront of medical advances in technology, disease understanding, and clinical care due to the unique resources available in the military health care system, which allow investigators the ability to obtain vast amounts of epidemiologic data.22 The military health care system also is unique in its ability to mandate that its members obtain preventive health services. Thus, it would be possible for the military to mandate TBP at accession and retirement, for instance, or more frequently for annual screening. The implementation of such a program would improve the health of the military population and be a public health service by pioneering the use of a standardized TBP system across a large health care system to improve skin cancer detection.
Current Studies in the Military
The Dermatology Service at the Walter Reed National Military Medical Center (WRNMMC)(Bethesda, Maryland) is evaluating the use of a total-body digital skin imaging system under a grant from the Telemedicine and Advanced Technology Research Center of the US Army. The system in use was found to be particularly well suited for military dermatology because it offers standardized TBP processing, produces a report that can be uploaded to the US Department of Defense (DoD) electronic medical record system, and requires relatively brief training for ancillary personnel to operate. Regardless of the platform the DoD ultimately finds most suitable, it is critical that a standard exist for TBP to ensure that uniform data sets are generated to allow military and other DoD dermatologists as well as civilian health care providers to share clinical information. The goal of the current study at WRNMMC is to vet TBP platforms at WRNMMC so the military can then develop standards to procure additional platforms for placement throughout the Military Health System, Military Entrance Processing Stations, operational environments, and collaborating health care systems (eg, the Veterans Health Administration).
Once deployed broadly across the Military Health System, these TBP platforms would be part of a network of telehealth care. For acute dermatologic issues, diagnoses provided via teledermatology platforms can then be managed by health care providers utilizing appropriate clinical practice guidelines or by non–health care providers utilizing general medical knowledge databases. Such a system with TBP information collected at multiple access points across a service member’s career would build a repository of data that would be immensely useful to patients and to clinical research. Of particular interest to military researchers is that TBP data could be used to study which patients require in-person examinations or more careful monitoring; the proper intervals for skin cancer screening; and the assessment of the benefits of TBP in improving morbidity, mortality, and biopsy efficiency in the detection of MM as well as nonmelanoma skin cancers.
Limitations to Progress
Currently, there are multiple limitations to the implementation of TBP as a part of TBSE screening. First, the potential improvement in biopsy efficiency using TBP is predicated on its ability to prove nevi stability over time, but in younger populations, benign nevi are more likely to change or increase in number, which may reduce the biopsy efficiency of screening in a younger population, thereby negating some of the benefit of imaging and CNN assessment. For instance, Truong et al16 found that younger age (<30 years) did not show the same improvement in biopsy efficiency with the use of TBP, which the authors theorized may reflect “the dynamic nature of nevi in younger patients” that has been documented in other studies.23,24 Approximately 65% of the active-duty military population is aged 18 to 30 years, and 98% of accessions to active duty occur in individuals aged 17 to 30 years.25 As such, TBP may not improve biopsy efficiency in the active-duty military population as dramatically as it would across the general population.
A second limitation of the use of TBP in the active-duty military population is the ethics of implementing DoD-wide mandatory TBP. Although the TBP platform will be compliant with the Health Insurance Portability and Accountability Act, mandating that soldiers contribute their TBP to a repository of data that will then be used for research without explicitly requesting their consent is ethically problematic; however, since the 1950s, the DoD has collected serum samples from its service members for force protection and operations reasons as well as for the purpose of research.22,26 Currently, the DoD Serum Repository collects serum samples as part of a mandatory human immunodeficiency virus screening program that evaluates service members every 2 years; this repository of human serum samples is accessible for research purposes without the consent of the individuals being studied.27 These individuals are not informed of potential use of their serum specimens for research purposes and no consent forms or opt-out options are provided. Thus, although there is precedent in the DoD for such mass data collection, it is an ongoing ethical consideration.28
RELATED ARTICLE: Gigapixel Photography for Skin Cancer Surveillance
Finally, although the potential use of TBP and computer algorithms to improve the efficiency and affordability of TBSEs is exciting, there are no existing computer algorithms that we are aware of that can be used with existing TBP platforms in the manner we proposed. However, we feel that computer algorithms, such as the one created by Esteva et al,19 are just the beginning and that the use of artificial intelligence is not far off. Even after the creation of a TBP-compatible algorithm adept at analyzing malignant lesions, however, this technology would need to be further evaluated in the clinical setting to determine its capability and practicality. Current TBP platforms also are limited by their large size, cost, and complexity. As TBP platforms improve, it is likely that more streamlined and less expensive versions of current models will greatly enhance the field of teledermatology, particularly in the military setting.
- Rogers HW, Weinstock MA, Feldman SR, et al. Incidence estimate of nonmelanoma skin cancer (keratinocyte carcinomas) in the U.S. population, 2012. JAMA Dermatol. 2015;151:1081-1086.
- Balch CM, Soong SJ, Atkins MB, et al. An evidence-based staging system for cutaneous melanoma. CA Cancer J Clin. 2004;54:131-149; quiz 182-184.
- Eisemann N, Jansen L, Holleczek B, et al. Up-to-date results on survival of patients with melanoma in Germany [published online July 19, 2012]. Br J Dermatol. 2012;167:606-612.
- MacKie RM, Bray C, Vestey J, et al. Melanoma incidence and mortality in Scotland 1979-2003 [published online May 29, 2007]. Br J Cancer. 2007;96:1772-1777.
- Dickson PV, Gershenwald JE. Staging and prognosis of cutaneous melanoma. Surg Oncol Clin N Am. 2011;20:1-17.
- Balch CM, Gershenwald JE, Soong SL, et al. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol. 2009;27:6199-6206.
- Kingsley-Loso JL, Grey KR, Hanson JL, et al. Incidental lesions found in veterans referred to dermatology: the value of a dermatologic examination [published online January 23, 2015]. J Am Acad Dermatol. 2015;72:651.e1-655.e1.
- Grant-Kels JM, Stoff B. Total body skin exams (TBSEs): saving lives or wasting time? J Am Acad Dermatol. 2017;76:183-185.
- US Preventive Services Task Force; Bibbins-Domingo K, Grossman DC, Curry SJ, et al. Screening for skin cancer: US Preventive Services Task Force recommendation statement. JAMA. 2016;316:429-435.
- Breitbart EW, Waldmann A, Nolte S, et al. Systematic skin cancer screening in Northern Germany. J Am Acad Dermatol. 2012;66:201-211.
- Robinson JK, Halpern AC. Cost-effective melanoma screening. JAMA Dermatol. 2016;152:19-21.
- Feit NE, Dusza SW, Marghoob AA. Melanomas detected with the aid of total cutaneous photography. Br J Dermatol. 2004;150:706-714.
- Haenssle HA, Krueger U, Vente C, et al. Results from an observational trial: digital epiluminescence microscopy follow-up of atypical nevi increases the sensitivity and the chance of success of conventional dermoscopy in detecting melanoma. J Invest Dermatol. 2006;126:980-985.
- Salerni G, Carrera C, Lovatto L, et al. Benefits of total body photography and digital dermatoscopy (“two-step method of digital follow-up”) in the early diagnosis of melanoma in patients at high risk for melanoma. J Am Acad Dermatol. 2012;67:E17-E27.
- Rice ZP, Weiss FJ, DeLong LK, et al. Utilization and rationale for the implementation of total body (digital) photography as an adjunct screening measure for melanoma. Melanoma Res. 2010;20:417-421.
- Truong A, Strazzulla L, March J, et al. Reduction in nevus biopsies in patients monitored by total body photography [published online March 3, 2016]. J Am Acad Dermatol. 2016;75:135.e5-143.e5.
- Lucas CR, Sanders LL, Murray JC, et al. Early melanoma detection: nonuniform dermoscopic features and growth. J Am Acad Dermatol. 2003;48:663-671.
- Fuller SR, Bowen GM, Tanner B, et al. Digital dermoscopic monitoring of atypical nevi in patients at risk for melanoma. Dermatol Surg. 2007;33:1198-1206; discussion 1205-1206.
- Esteva A, Kuprel B, Novoa RA, et al. Dermatologist-level classification of skin cancer with deep neural networks [published online January 25, 2017]. Nature. 2017;542:115-118.
- Defense Medical Epidemiology Database. Military Health System website. http://www.health.mil/Military-Health-Topics/Health-Readiness/Armed-Forces-Health-Surveillance-Branch/Data-Management-and-Technical-Support/Defense-Medical-Epidemiology-Database. Accessed April 10, 2017.
- Lee T, Williams VF, Clark LL. Incident diagnoses of cancers in the active component and cancer-related deaths in the active and reserve components, U.S. Armed Forces, 2005-2014. MSMR. 2016;23:23-31.
- Helmandollar KJ, Meyerle JH. Exploration of modern military research resources. Cutis. 2016;98:231-234.
- Goodson AG, Grossman D. Strategies for early melanoma detection: approaches to the patient with nevi. J Am Acad Dermatol. 2009;60:719-735; quiz 736-738.
- Bajaj S, Dusza SW, Marchetti MA, et al. Growth-curve modeling of nevi with a peripheral globular pattern. JAMA Dermatol. 2015;151:1338-1345.
- Niebuhr DW, Gubata ME, Cowan DN, et al. Accession Medical Standards Analysis & Research Activity (AMSARA) 2011 Annual Report. Silver Spring, MD: Division of Preventive Medicine, Walter Reed Army Institute of Research; 2012.
- Liao SJ. Immunity status of military recruits in 1951 in the United States. I. results of Schick tests. Am J Hyg. 1954;59:262-272.
- Perdue CL, Eick-Cost AA, Rubertone MV. A brief description of the operation of the DoD Serum Repository. Mil Med. 2015;180:10-12.
- Pavlin JA, Welch RA. Ethics, human use, and the Department of Defense Serum Repository. Mil Med. 2015;180:49-56.
- Rogers HW, Weinstock MA, Feldman SR, et al. Incidence estimate of nonmelanoma skin cancer (keratinocyte carcinomas) in the U.S. population, 2012. JAMA Dermatol. 2015;151:1081-1086.
- Balch CM, Soong SJ, Atkins MB, et al. An evidence-based staging system for cutaneous melanoma. CA Cancer J Clin. 2004;54:131-149; quiz 182-184.
- Eisemann N, Jansen L, Holleczek B, et al. Up-to-date results on survival of patients with melanoma in Germany [published online July 19, 2012]. Br J Dermatol. 2012;167:606-612.
- MacKie RM, Bray C, Vestey J, et al. Melanoma incidence and mortality in Scotland 1979-2003 [published online May 29, 2007]. Br J Cancer. 2007;96:1772-1777.
- Dickson PV, Gershenwald JE. Staging and prognosis of cutaneous melanoma. Surg Oncol Clin N Am. 2011;20:1-17.
- Balch CM, Gershenwald JE, Soong SL, et al. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol. 2009;27:6199-6206.
- Kingsley-Loso JL, Grey KR, Hanson JL, et al. Incidental lesions found in veterans referred to dermatology: the value of a dermatologic examination [published online January 23, 2015]. J Am Acad Dermatol. 2015;72:651.e1-655.e1.
- Grant-Kels JM, Stoff B. Total body skin exams (TBSEs): saving lives or wasting time? J Am Acad Dermatol. 2017;76:183-185.
- US Preventive Services Task Force; Bibbins-Domingo K, Grossman DC, Curry SJ, et al. Screening for skin cancer: US Preventive Services Task Force recommendation statement. JAMA. 2016;316:429-435.
- Breitbart EW, Waldmann A, Nolte S, et al. Systematic skin cancer screening in Northern Germany. J Am Acad Dermatol. 2012;66:201-211.
- Robinson JK, Halpern AC. Cost-effective melanoma screening. JAMA Dermatol. 2016;152:19-21.
- Feit NE, Dusza SW, Marghoob AA. Melanomas detected with the aid of total cutaneous photography. Br J Dermatol. 2004;150:706-714.
- Haenssle HA, Krueger U, Vente C, et al. Results from an observational trial: digital epiluminescence microscopy follow-up of atypical nevi increases the sensitivity and the chance of success of conventional dermoscopy in detecting melanoma. J Invest Dermatol. 2006;126:980-985.
- Salerni G, Carrera C, Lovatto L, et al. Benefits of total body photography and digital dermatoscopy (“two-step method of digital follow-up”) in the early diagnosis of melanoma in patients at high risk for melanoma. J Am Acad Dermatol. 2012;67:E17-E27.
- Rice ZP, Weiss FJ, DeLong LK, et al. Utilization and rationale for the implementation of total body (digital) photography as an adjunct screening measure for melanoma. Melanoma Res. 2010;20:417-421.
- Truong A, Strazzulla L, March J, et al. Reduction in nevus biopsies in patients monitored by total body photography [published online March 3, 2016]. J Am Acad Dermatol. 2016;75:135.e5-143.e5.
- Lucas CR, Sanders LL, Murray JC, et al. Early melanoma detection: nonuniform dermoscopic features and growth. J Am Acad Dermatol. 2003;48:663-671.
- Fuller SR, Bowen GM, Tanner B, et al. Digital dermoscopic monitoring of atypical nevi in patients at risk for melanoma. Dermatol Surg. 2007;33:1198-1206; discussion 1205-1206.
- Esteva A, Kuprel B, Novoa RA, et al. Dermatologist-level classification of skin cancer with deep neural networks [published online January 25, 2017]. Nature. 2017;542:115-118.
- Defense Medical Epidemiology Database. Military Health System website. http://www.health.mil/Military-Health-Topics/Health-Readiness/Armed-Forces-Health-Surveillance-Branch/Data-Management-and-Technical-Support/Defense-Medical-Epidemiology-Database. Accessed April 10, 2017.
- Lee T, Williams VF, Clark LL. Incident diagnoses of cancers in the active component and cancer-related deaths in the active and reserve components, U.S. Armed Forces, 2005-2014. MSMR. 2016;23:23-31.
- Helmandollar KJ, Meyerle JH. Exploration of modern military research resources. Cutis. 2016;98:231-234.
- Goodson AG, Grossman D. Strategies for early melanoma detection: approaches to the patient with nevi. J Am Acad Dermatol. 2009;60:719-735; quiz 736-738.
- Bajaj S, Dusza SW, Marchetti MA, et al. Growth-curve modeling of nevi with a peripheral globular pattern. JAMA Dermatol. 2015;151:1338-1345.
- Niebuhr DW, Gubata ME, Cowan DN, et al. Accession Medical Standards Analysis & Research Activity (AMSARA) 2011 Annual Report. Silver Spring, MD: Division of Preventive Medicine, Walter Reed Army Institute of Research; 2012.
- Liao SJ. Immunity status of military recruits in 1951 in the United States. I. results of Schick tests. Am J Hyg. 1954;59:262-272.
- Perdue CL, Eick-Cost AA, Rubertone MV. A brief description of the operation of the DoD Serum Repository. Mil Med. 2015;180:10-12.
- Pavlin JA, Welch RA. Ethics, human use, and the Department of Defense Serum Repository. Mil Med. 2015;180:49-56.
Practice Points
- Advances in technology have the potential to provide affordable standardized total-body photography platforms.
- Total-body photography augments the clinical examination and plays a role in clinical decision-making.
- Total-body photography has the potential to become a part of the total-body skin examination and increase access to dermatologic care.
Exploration of Modern Military Research Resources
Advances in medical biotechnologies, data-gathering techniques, and -omics technologies have resulted in the broader understanding of disease pathology and treatment and have facilitated the individualization of health care plans to meet the unique needs of each patient. Military medicine often has been on the forefront of medical technology, disease understanding, and clinical care both on and off the battlefield, in large part due to the unique resources available in the military health care system. These resources allow investigators the ability to integrate vast amounts of epidemiologic data with an extensive biological sample database of its service members, which in the modern age has translated into advances in the understanding of melanoma and the treatment of scars.
History of Research in the Military
Starting in the 1950s, the US Department of Defense (DoD) started to collect serum samples of its service members for the purpose of research.1 It was not until 1985 that the DoD implemented a long-term frozen storage system for serum samples obtained through mandatory screening for human immunodeficiency virus (HIV) in service members.2 Subsequently, the Department of Defense Serum Repository (DoDSR) was officially established in 1989 as a central archive for the long-term storage of serum obtained from active-duty and reserve service members in the US Navy, Army, and Marines.2,3 In the mid-1990s, the DoDSR expanded its capabilities to include the storage of serum samples from all military members, including the US Air Force, obtained predeployment and postdeployment.3,4 At that time, a records-keeping system was established, now known as the Defense Medical Surveillance System (DMSS). The creation of the DMSS provided an extensive epidemiologic database that provided valuable information such as demographic data, service records, deployment data, reportable medical events, exposure history, and vaccination records, which could be linked to the serum samples of each service member.2-4 Since 2008, the responsibilities of maintaining the DoDSR and the DMSS were transferred to the Armed Forces Health Surveillance Center (AFHSC).5
There have been several other databases created over the years that provide additional support and resources to military investigators. The Automated Central Tumor Registry and Department of Pathology and Area Laboratory Services both help investigators to track the incidence of specific cancers in the military population and provide them with pathologic specimens. Additionally, electronic medical records including the composite health care system and the Armed Forces Health Longitudinal Technology Application supplemented with insurance claims data accessible from the Military Health System Management and Reporting Tool (M2) database have made it possible to track patient data.
Utilization of Military Research Resources
Today, the DoDSR is a secure facility that maintains more than 56 million serum specimens from more than 11 million individuals in –30°C freezers, making it one of the largest repositories in the world.3,6 Each serum sample is linked with an individual’s DMSS record, providing a way for investigators to study how external factors such as deployment history, occupation, and exposure history relate to an individual’s unique genetic and physiological makeup. Furthermore, these data can be used for seroepidemiologic investigations that contribute to all facets of clinical care. The AFHSC routinely publishes findings related to notifiable diseases, disease outbreaks, and disease trends in a monthly report.7
There are strict guidelines in place that limit access to the DoDSR and service members’ data. Use of the repository for information directly related to a patient’s health care is one reason for access, such as analyzing serum for antibodies and seroconversion to assist in the diagnosis of a disease such as HIV. Another reason would be to obtain information needed for criminal investigations and prosecution. Typically, these types of requests require a judge-issued court order and approval by the Assistant Secretary of Defense for Health Affairs.4 The DoDSR also is used to study force health protection issues, such as infectious disease incidence and disease prevalence in the military population.
Obtaining access to the DoDSR and service members’ data for research purposes requires that the principal investigator be a DoD employee. Each research proposal is reviewed by members of the AFHSC to determine if the DoDSR is able to meet the demands of the project, including having the appropriate number of serum samples and supporting epidemiologic data available. The AFHSC provides a letter of support if it deems the project to be in line with its current resources and capabilities. Each research proposal is then sent to an institutional review board (IRB) to determine if the study is exempt or needs to go through a full IRB review process. A study might be exempt if the investigators are not obtaining data through interaction with living individuals or not having access to any identifiable protected health information associated with the samples.6 Regardless of whether the study is exempt or not exempt, the AFHSC will de-identify each sample before releasing the samples to the investigators by using a coding system to shield the patient’s identity from the investigator.
Resources within the military medical research system provide investigators with access to an extensive biorepository of serum and linked epidemiological data. Samples from the DoDSR have been used in no less than 75 peer-reviewed publications since 1985.8,9 Several of these studies have been influential in expanding knowledge about conditions seen more commonly in the military population such as stress fractures, traumatic brain injuries, posttraumatic stress disorder, and suicide.8 Additionally, DoDSR samples have been used to form military vaccination policies and track both infectious and noninfectious conditions in the military; for example, during the H1N1 influenza virus outbreak of 2009, AFHSC was essential in helping to limit the spread of the virus within the military community by using its data and collaborating with groups such as the Centers for Disease Control and Prevention to develop a plan for disease surveillance and control.5
Several military research resources are currently being used for a melanoma study that aims to assess if specific phenotypic features, melanoma risk alleles, and environmental factors (eg, duty station location, occupation, amount of UV exposure) can be used to develop better screening models to identify individuals who are at risk for developing melanoma. Secondarily, the study aims to determine if recently developed multimarker diagnostic and prognostic assays for melanoma will prove useful in the diagnostic and prognostic assessment of melanocytic neoplasms in the military population. For this study, one of the authors (J.H.M) is utilizing DoDSR serum from 1700 retrospective cases of invasive melanoma and 1700 matched controls. Additionally, the Automated Central Tumor Registry and Department of Pathology and Area Laboratory Services databases are being used to obtain tissue from more than 300 melanoma cases and nevi controls.
Limitations of the Current System
Despite the impressive capabilities of the current system, there are some issues that limit its potential. One such limitation is associated with the way that the serum samples at the DoDSR are utilized. Through 2012, the DoDSR had 54,542,658 serum specimens available, of which only 228,610 (0.42%) had ever been accessed for study.8 With such a wealth of information and relative availability, why are the serum samples not being accessed more frequently for studies? The inherent nature of the DoDSR being a restricted facility and only accessible to DoD-affiliated investigators may contribute, which allows the DoDSR to fulfill its primary purpose of contributing to military-relevant investigations but at the same time limits the number and type of investigations that can be performed. One idea that has been proposed is allowing civilian investigator access to the DoDSR if it can be proven that the research is targeted toward military-relevant issues.8 However, the current AFHSC access guidelines would need revision and would require additional safeguards to ensure that military-protected health information is not compromised. Nonetheless, such a change may result in more extensive use of DoDSR resources in the future.
An ethical issue that needs to be addressed pertains to how the DoDSR permits use of human serum samples for research purposes without getting consent from the individuals being studied. The serum samples are collected as part of mandatory predeployment and postdeployment examinations for HIV screening of all military members. These individuals are not informed of potential use of their serum specimens for research purposes and no consent forms or opt-out options are provided. Although it is true that military members must comply with specific requirements pertaining to military readiness (eg, receiving appropriate vaccinations, drug testing, regular medical screening), it is debated whether they still retain the right as patients to refuse participating in research and clinical trials.10 The AFHSC does have several regulatory steps in place to ensure that military members’ samples are used in an appropriate manner, including requiring a DoD primary investigator, IRB review of every research proposal, and de-identification of samples. At a minimum, giving military members the ability to provide informed consent would ensure that the military system is adhering to evolving human research standards.
The current lack of biological specimens other than serum in the DoDSR is another limitation of the current system. Recent advances in molecular analyses are impacted by expanding -omics techniques, such as epigenomics, transcriptomics, and proteomics. The field of epigenomics is the study of reversible changes to DNA (eg, methylation) associated with specific disease states or following specific environmental exposures.9,11 Transcriptomics, which analyzes messenger RNA transcript levels of expressed genes, and proteomics, which uses expression of proteins, are 2 techniques being used to develop biomarkers associated with specific diseases and environmental exposures.9,11 Serum alone does not provide the high-quality nucleic acids needed for many of these studies to take place. Adding whole-blood specimens or blood spot samples of military service members to the DoDSR would allow researchers to use these techniques to investigate many new biomarkers associated with military-relevant diseases and exposures. These techniques also can be used in the expanding field of personalized medicine so that health care providers are able to tailor all phases of care, including diagnosis and treatment, to an individual’s genetic profile.
Conclusion
The history of research in military medicine has been built on achieving the primary goal of serving those men and women who put their lives in danger to protect this country. In an evolving environment of new technologies that have led to changes in service members’ injuries, exposures, and diseases, military medicine also must adapt. Resources such as the DoDSR and DMSS, which provide investigators with the unique ability to link epidemiological data with serum samples, have been invaluable contributors to this overall mission. As with any large system, there are always improvements that can be made. Improving access to the DoDSR serum samples, educating and obtaining consent from military service members to use their samples in research, and adding specimens to the DoDSR that can be used for -omics techniques are 3 changes that should be considered to maximize
- Liao SJ. Immunity status of military recruits in 1951 in the United States. I. results of Schick tests. Am J Hyg. 1954;59:262-272.
- Rubertone MV, Brundage JF. The defense medical surveillance system and the department of defense serum repository: glimpses of the future of public health surveillance. Am J Public Health. 2002;92:1900-1904.
- Department of Defense Serum Repository. Military Health System and the Defense Health Agency website. http://www.health.mil/Military-Health-Topics/Health-Readiness/Armed-Forces-Health-Surveillance-Branch/Data-Management-and-Technical-Support/Department-of-Defense-Serum-Repository. Accessed August 2, 2016.
- Perdue CL, Eick-Cost AA, Rubertone MV. A brief description of the operation of the DoD serum repository. Mil Med. 2015;180(10 suppl):10-12.
- DeFraites RF. The Armed Forces Health Surveillance Center: enhancing the Military Health System’s public health capabilities. BMC Public Health. 2011;11(suppl 2):S1.
- Pavlin JA, Welch RA. Ethics, human use, and the department of defense serum repository. Mil Med. 2015;180:49-56.
- Defense Medical Surveillance System. Military Health System and the Defense Health Agency website. http://www.health.mil/Military-Health-Topics/Health-Readiness/Armed-Forces-Health-Surveillance-Branch/Data-Management-and-Technical-Support/Defense-Medical-Surveillance-System. Accessed August 2, 2016.
- Perdue CL, Eick-Cost AA, Rubertone MV, et al. Description and utilization of the United States Department of Defense Serum Repository: a review of published studies, 1985-2012. Plos One. 2015;10:1-16.
- Mancuso JD, Mallon TM, Gaydos JC. Maximizing the capabilities of the DoD serum repository to meet current and future needs: report of the needs panel. Mil Med. 2015;180:14-24.
- Department of Defense. Department of Defense Instruction. http://www.dtic.mil/whs/directives/corres/pdf/600014p.pdf. Posted September 26, 2001. Updated October 3, 2013. Accessed August 2, 2016.
- Lindler LE. Building a DoD biorepository for the future: potential benefits and way forward. Mil Med. 2015;180:90-94.
Advances in medical biotechnologies, data-gathering techniques, and -omics technologies have resulted in the broader understanding of disease pathology and treatment and have facilitated the individualization of health care plans to meet the unique needs of each patient. Military medicine often has been on the forefront of medical technology, disease understanding, and clinical care both on and off the battlefield, in large part due to the unique resources available in the military health care system. These resources allow investigators the ability to integrate vast amounts of epidemiologic data with an extensive biological sample database of its service members, which in the modern age has translated into advances in the understanding of melanoma and the treatment of scars.
History of Research in the Military
Starting in the 1950s, the US Department of Defense (DoD) started to collect serum samples of its service members for the purpose of research.1 It was not until 1985 that the DoD implemented a long-term frozen storage system for serum samples obtained through mandatory screening for human immunodeficiency virus (HIV) in service members.2 Subsequently, the Department of Defense Serum Repository (DoDSR) was officially established in 1989 as a central archive for the long-term storage of serum obtained from active-duty and reserve service members in the US Navy, Army, and Marines.2,3 In the mid-1990s, the DoDSR expanded its capabilities to include the storage of serum samples from all military members, including the US Air Force, obtained predeployment and postdeployment.3,4 At that time, a records-keeping system was established, now known as the Defense Medical Surveillance System (DMSS). The creation of the DMSS provided an extensive epidemiologic database that provided valuable information such as demographic data, service records, deployment data, reportable medical events, exposure history, and vaccination records, which could be linked to the serum samples of each service member.2-4 Since 2008, the responsibilities of maintaining the DoDSR and the DMSS were transferred to the Armed Forces Health Surveillance Center (AFHSC).5
There have been several other databases created over the years that provide additional support and resources to military investigators. The Automated Central Tumor Registry and Department of Pathology and Area Laboratory Services both help investigators to track the incidence of specific cancers in the military population and provide them with pathologic specimens. Additionally, electronic medical records including the composite health care system and the Armed Forces Health Longitudinal Technology Application supplemented with insurance claims data accessible from the Military Health System Management and Reporting Tool (M2) database have made it possible to track patient data.
Utilization of Military Research Resources
Today, the DoDSR is a secure facility that maintains more than 56 million serum specimens from more than 11 million individuals in –30°C freezers, making it one of the largest repositories in the world.3,6 Each serum sample is linked with an individual’s DMSS record, providing a way for investigators to study how external factors such as deployment history, occupation, and exposure history relate to an individual’s unique genetic and physiological makeup. Furthermore, these data can be used for seroepidemiologic investigations that contribute to all facets of clinical care. The AFHSC routinely publishes findings related to notifiable diseases, disease outbreaks, and disease trends in a monthly report.7
There are strict guidelines in place that limit access to the DoDSR and service members’ data. Use of the repository for information directly related to a patient’s health care is one reason for access, such as analyzing serum for antibodies and seroconversion to assist in the diagnosis of a disease such as HIV. Another reason would be to obtain information needed for criminal investigations and prosecution. Typically, these types of requests require a judge-issued court order and approval by the Assistant Secretary of Defense for Health Affairs.4 The DoDSR also is used to study force health protection issues, such as infectious disease incidence and disease prevalence in the military population.
Obtaining access to the DoDSR and service members’ data for research purposes requires that the principal investigator be a DoD employee. Each research proposal is reviewed by members of the AFHSC to determine if the DoDSR is able to meet the demands of the project, including having the appropriate number of serum samples and supporting epidemiologic data available. The AFHSC provides a letter of support if it deems the project to be in line with its current resources and capabilities. Each research proposal is then sent to an institutional review board (IRB) to determine if the study is exempt or needs to go through a full IRB review process. A study might be exempt if the investigators are not obtaining data through interaction with living individuals or not having access to any identifiable protected health information associated with the samples.6 Regardless of whether the study is exempt or not exempt, the AFHSC will de-identify each sample before releasing the samples to the investigators by using a coding system to shield the patient’s identity from the investigator.
Resources within the military medical research system provide investigators with access to an extensive biorepository of serum and linked epidemiological data. Samples from the DoDSR have been used in no less than 75 peer-reviewed publications since 1985.8,9 Several of these studies have been influential in expanding knowledge about conditions seen more commonly in the military population such as stress fractures, traumatic brain injuries, posttraumatic stress disorder, and suicide.8 Additionally, DoDSR samples have been used to form military vaccination policies and track both infectious and noninfectious conditions in the military; for example, during the H1N1 influenza virus outbreak of 2009, AFHSC was essential in helping to limit the spread of the virus within the military community by using its data and collaborating with groups such as the Centers for Disease Control and Prevention to develop a plan for disease surveillance and control.5
Several military research resources are currently being used for a melanoma study that aims to assess if specific phenotypic features, melanoma risk alleles, and environmental factors (eg, duty station location, occupation, amount of UV exposure) can be used to develop better screening models to identify individuals who are at risk for developing melanoma. Secondarily, the study aims to determine if recently developed multimarker diagnostic and prognostic assays for melanoma will prove useful in the diagnostic and prognostic assessment of melanocytic neoplasms in the military population. For this study, one of the authors (J.H.M) is utilizing DoDSR serum from 1700 retrospective cases of invasive melanoma and 1700 matched controls. Additionally, the Automated Central Tumor Registry and Department of Pathology and Area Laboratory Services databases are being used to obtain tissue from more than 300 melanoma cases and nevi controls.
Limitations of the Current System
Despite the impressive capabilities of the current system, there are some issues that limit its potential. One such limitation is associated with the way that the serum samples at the DoDSR are utilized. Through 2012, the DoDSR had 54,542,658 serum specimens available, of which only 228,610 (0.42%) had ever been accessed for study.8 With such a wealth of information and relative availability, why are the serum samples not being accessed more frequently for studies? The inherent nature of the DoDSR being a restricted facility and only accessible to DoD-affiliated investigators may contribute, which allows the DoDSR to fulfill its primary purpose of contributing to military-relevant investigations but at the same time limits the number and type of investigations that can be performed. One idea that has been proposed is allowing civilian investigator access to the DoDSR if it can be proven that the research is targeted toward military-relevant issues.8 However, the current AFHSC access guidelines would need revision and would require additional safeguards to ensure that military-protected health information is not compromised. Nonetheless, such a change may result in more extensive use of DoDSR resources in the future.
An ethical issue that needs to be addressed pertains to how the DoDSR permits use of human serum samples for research purposes without getting consent from the individuals being studied. The serum samples are collected as part of mandatory predeployment and postdeployment examinations for HIV screening of all military members. These individuals are not informed of potential use of their serum specimens for research purposes and no consent forms or opt-out options are provided. Although it is true that military members must comply with specific requirements pertaining to military readiness (eg, receiving appropriate vaccinations, drug testing, regular medical screening), it is debated whether they still retain the right as patients to refuse participating in research and clinical trials.10 The AFHSC does have several regulatory steps in place to ensure that military members’ samples are used in an appropriate manner, including requiring a DoD primary investigator, IRB review of every research proposal, and de-identification of samples. At a minimum, giving military members the ability to provide informed consent would ensure that the military system is adhering to evolving human research standards.
The current lack of biological specimens other than serum in the DoDSR is another limitation of the current system. Recent advances in molecular analyses are impacted by expanding -omics techniques, such as epigenomics, transcriptomics, and proteomics. The field of epigenomics is the study of reversible changes to DNA (eg, methylation) associated with specific disease states or following specific environmental exposures.9,11 Transcriptomics, which analyzes messenger RNA transcript levels of expressed genes, and proteomics, which uses expression of proteins, are 2 techniques being used to develop biomarkers associated with specific diseases and environmental exposures.9,11 Serum alone does not provide the high-quality nucleic acids needed for many of these studies to take place. Adding whole-blood specimens or blood spot samples of military service members to the DoDSR would allow researchers to use these techniques to investigate many new biomarkers associated with military-relevant diseases and exposures. These techniques also can be used in the expanding field of personalized medicine so that health care providers are able to tailor all phases of care, including diagnosis and treatment, to an individual’s genetic profile.
Conclusion
The history of research in military medicine has been built on achieving the primary goal of serving those men and women who put their lives in danger to protect this country. In an evolving environment of new technologies that have led to changes in service members’ injuries, exposures, and diseases, military medicine also must adapt. Resources such as the DoDSR and DMSS, which provide investigators with the unique ability to link epidemiological data with serum samples, have been invaluable contributors to this overall mission. As with any large system, there are always improvements that can be made. Improving access to the DoDSR serum samples, educating and obtaining consent from military service members to use their samples in research, and adding specimens to the DoDSR that can be used for -omics techniques are 3 changes that should be considered to maximize
Advances in medical biotechnologies, data-gathering techniques, and -omics technologies have resulted in the broader understanding of disease pathology and treatment and have facilitated the individualization of health care plans to meet the unique needs of each patient. Military medicine often has been on the forefront of medical technology, disease understanding, and clinical care both on and off the battlefield, in large part due to the unique resources available in the military health care system. These resources allow investigators the ability to integrate vast amounts of epidemiologic data with an extensive biological sample database of its service members, which in the modern age has translated into advances in the understanding of melanoma and the treatment of scars.
History of Research in the Military
Starting in the 1950s, the US Department of Defense (DoD) started to collect serum samples of its service members for the purpose of research.1 It was not until 1985 that the DoD implemented a long-term frozen storage system for serum samples obtained through mandatory screening for human immunodeficiency virus (HIV) in service members.2 Subsequently, the Department of Defense Serum Repository (DoDSR) was officially established in 1989 as a central archive for the long-term storage of serum obtained from active-duty and reserve service members in the US Navy, Army, and Marines.2,3 In the mid-1990s, the DoDSR expanded its capabilities to include the storage of serum samples from all military members, including the US Air Force, obtained predeployment and postdeployment.3,4 At that time, a records-keeping system was established, now known as the Defense Medical Surveillance System (DMSS). The creation of the DMSS provided an extensive epidemiologic database that provided valuable information such as demographic data, service records, deployment data, reportable medical events, exposure history, and vaccination records, which could be linked to the serum samples of each service member.2-4 Since 2008, the responsibilities of maintaining the DoDSR and the DMSS were transferred to the Armed Forces Health Surveillance Center (AFHSC).5
There have been several other databases created over the years that provide additional support and resources to military investigators. The Automated Central Tumor Registry and Department of Pathology and Area Laboratory Services both help investigators to track the incidence of specific cancers in the military population and provide them with pathologic specimens. Additionally, electronic medical records including the composite health care system and the Armed Forces Health Longitudinal Technology Application supplemented with insurance claims data accessible from the Military Health System Management and Reporting Tool (M2) database have made it possible to track patient data.
Utilization of Military Research Resources
Today, the DoDSR is a secure facility that maintains more than 56 million serum specimens from more than 11 million individuals in –30°C freezers, making it one of the largest repositories in the world.3,6 Each serum sample is linked with an individual’s DMSS record, providing a way for investigators to study how external factors such as deployment history, occupation, and exposure history relate to an individual’s unique genetic and physiological makeup. Furthermore, these data can be used for seroepidemiologic investigations that contribute to all facets of clinical care. The AFHSC routinely publishes findings related to notifiable diseases, disease outbreaks, and disease trends in a monthly report.7
There are strict guidelines in place that limit access to the DoDSR and service members’ data. Use of the repository for information directly related to a patient’s health care is one reason for access, such as analyzing serum for antibodies and seroconversion to assist in the diagnosis of a disease such as HIV. Another reason would be to obtain information needed for criminal investigations and prosecution. Typically, these types of requests require a judge-issued court order and approval by the Assistant Secretary of Defense for Health Affairs.4 The DoDSR also is used to study force health protection issues, such as infectious disease incidence and disease prevalence in the military population.
Obtaining access to the DoDSR and service members’ data for research purposes requires that the principal investigator be a DoD employee. Each research proposal is reviewed by members of the AFHSC to determine if the DoDSR is able to meet the demands of the project, including having the appropriate number of serum samples and supporting epidemiologic data available. The AFHSC provides a letter of support if it deems the project to be in line with its current resources and capabilities. Each research proposal is then sent to an institutional review board (IRB) to determine if the study is exempt or needs to go through a full IRB review process. A study might be exempt if the investigators are not obtaining data through interaction with living individuals or not having access to any identifiable protected health information associated with the samples.6 Regardless of whether the study is exempt or not exempt, the AFHSC will de-identify each sample before releasing the samples to the investigators by using a coding system to shield the patient’s identity from the investigator.
Resources within the military medical research system provide investigators with access to an extensive biorepository of serum and linked epidemiological data. Samples from the DoDSR have been used in no less than 75 peer-reviewed publications since 1985.8,9 Several of these studies have been influential in expanding knowledge about conditions seen more commonly in the military population such as stress fractures, traumatic brain injuries, posttraumatic stress disorder, and suicide.8 Additionally, DoDSR samples have been used to form military vaccination policies and track both infectious and noninfectious conditions in the military; for example, during the H1N1 influenza virus outbreak of 2009, AFHSC was essential in helping to limit the spread of the virus within the military community by using its data and collaborating with groups such as the Centers for Disease Control and Prevention to develop a plan for disease surveillance and control.5
Several military research resources are currently being used for a melanoma study that aims to assess if specific phenotypic features, melanoma risk alleles, and environmental factors (eg, duty station location, occupation, amount of UV exposure) can be used to develop better screening models to identify individuals who are at risk for developing melanoma. Secondarily, the study aims to determine if recently developed multimarker diagnostic and prognostic assays for melanoma will prove useful in the diagnostic and prognostic assessment of melanocytic neoplasms in the military population. For this study, one of the authors (J.H.M) is utilizing DoDSR serum from 1700 retrospective cases of invasive melanoma and 1700 matched controls. Additionally, the Automated Central Tumor Registry and Department of Pathology and Area Laboratory Services databases are being used to obtain tissue from more than 300 melanoma cases and nevi controls.
Limitations of the Current System
Despite the impressive capabilities of the current system, there are some issues that limit its potential. One such limitation is associated with the way that the serum samples at the DoDSR are utilized. Through 2012, the DoDSR had 54,542,658 serum specimens available, of which only 228,610 (0.42%) had ever been accessed for study.8 With such a wealth of information and relative availability, why are the serum samples not being accessed more frequently for studies? The inherent nature of the DoDSR being a restricted facility and only accessible to DoD-affiliated investigators may contribute, which allows the DoDSR to fulfill its primary purpose of contributing to military-relevant investigations but at the same time limits the number and type of investigations that can be performed. One idea that has been proposed is allowing civilian investigator access to the DoDSR if it can be proven that the research is targeted toward military-relevant issues.8 However, the current AFHSC access guidelines would need revision and would require additional safeguards to ensure that military-protected health information is not compromised. Nonetheless, such a change may result in more extensive use of DoDSR resources in the future.
An ethical issue that needs to be addressed pertains to how the DoDSR permits use of human serum samples for research purposes without getting consent from the individuals being studied. The serum samples are collected as part of mandatory predeployment and postdeployment examinations for HIV screening of all military members. These individuals are not informed of potential use of their serum specimens for research purposes and no consent forms or opt-out options are provided. Although it is true that military members must comply with specific requirements pertaining to military readiness (eg, receiving appropriate vaccinations, drug testing, regular medical screening), it is debated whether they still retain the right as patients to refuse participating in research and clinical trials.10 The AFHSC does have several regulatory steps in place to ensure that military members’ samples are used in an appropriate manner, including requiring a DoD primary investigator, IRB review of every research proposal, and de-identification of samples. At a minimum, giving military members the ability to provide informed consent would ensure that the military system is adhering to evolving human research standards.
The current lack of biological specimens other than serum in the DoDSR is another limitation of the current system. Recent advances in molecular analyses are impacted by expanding -omics techniques, such as epigenomics, transcriptomics, and proteomics. The field of epigenomics is the study of reversible changes to DNA (eg, methylation) associated with specific disease states or following specific environmental exposures.9,11 Transcriptomics, which analyzes messenger RNA transcript levels of expressed genes, and proteomics, which uses expression of proteins, are 2 techniques being used to develop biomarkers associated with specific diseases and environmental exposures.9,11 Serum alone does not provide the high-quality nucleic acids needed for many of these studies to take place. Adding whole-blood specimens or blood spot samples of military service members to the DoDSR would allow researchers to use these techniques to investigate many new biomarkers associated with military-relevant diseases and exposures. These techniques also can be used in the expanding field of personalized medicine so that health care providers are able to tailor all phases of care, including diagnosis and treatment, to an individual’s genetic profile.
Conclusion
The history of research in military medicine has been built on achieving the primary goal of serving those men and women who put their lives in danger to protect this country. In an evolving environment of new technologies that have led to changes in service members’ injuries, exposures, and diseases, military medicine also must adapt. Resources such as the DoDSR and DMSS, which provide investigators with the unique ability to link epidemiological data with serum samples, have been invaluable contributors to this overall mission. As with any large system, there are always improvements that can be made. Improving access to the DoDSR serum samples, educating and obtaining consent from military service members to use their samples in research, and adding specimens to the DoDSR that can be used for -omics techniques are 3 changes that should be considered to maximize
- Liao SJ. Immunity status of military recruits in 1951 in the United States. I. results of Schick tests. Am J Hyg. 1954;59:262-272.
- Rubertone MV, Brundage JF. The defense medical surveillance system and the department of defense serum repository: glimpses of the future of public health surveillance. Am J Public Health. 2002;92:1900-1904.
- Department of Defense Serum Repository. Military Health System and the Defense Health Agency website. http://www.health.mil/Military-Health-Topics/Health-Readiness/Armed-Forces-Health-Surveillance-Branch/Data-Management-and-Technical-Support/Department-of-Defense-Serum-Repository. Accessed August 2, 2016.
- Perdue CL, Eick-Cost AA, Rubertone MV. A brief description of the operation of the DoD serum repository. Mil Med. 2015;180(10 suppl):10-12.
- DeFraites RF. The Armed Forces Health Surveillance Center: enhancing the Military Health System’s public health capabilities. BMC Public Health. 2011;11(suppl 2):S1.
- Pavlin JA, Welch RA. Ethics, human use, and the department of defense serum repository. Mil Med. 2015;180:49-56.
- Defense Medical Surveillance System. Military Health System and the Defense Health Agency website. http://www.health.mil/Military-Health-Topics/Health-Readiness/Armed-Forces-Health-Surveillance-Branch/Data-Management-and-Technical-Support/Defense-Medical-Surveillance-System. Accessed August 2, 2016.
- Perdue CL, Eick-Cost AA, Rubertone MV, et al. Description and utilization of the United States Department of Defense Serum Repository: a review of published studies, 1985-2012. Plos One. 2015;10:1-16.
- Mancuso JD, Mallon TM, Gaydos JC. Maximizing the capabilities of the DoD serum repository to meet current and future needs: report of the needs panel. Mil Med. 2015;180:14-24.
- Department of Defense. Department of Defense Instruction. http://www.dtic.mil/whs/directives/corres/pdf/600014p.pdf. Posted September 26, 2001. Updated October 3, 2013. Accessed August 2, 2016.
- Lindler LE. Building a DoD biorepository for the future: potential benefits and way forward. Mil Med. 2015;180:90-94.
- Liao SJ. Immunity status of military recruits in 1951 in the United States. I. results of Schick tests. Am J Hyg. 1954;59:262-272.
- Rubertone MV, Brundage JF. The defense medical surveillance system and the department of defense serum repository: glimpses of the future of public health surveillance. Am J Public Health. 2002;92:1900-1904.
- Department of Defense Serum Repository. Military Health System and the Defense Health Agency website. http://www.health.mil/Military-Health-Topics/Health-Readiness/Armed-Forces-Health-Surveillance-Branch/Data-Management-and-Technical-Support/Department-of-Defense-Serum-Repository. Accessed August 2, 2016.
- Perdue CL, Eick-Cost AA, Rubertone MV. A brief description of the operation of the DoD serum repository. Mil Med. 2015;180(10 suppl):10-12.
- DeFraites RF. The Armed Forces Health Surveillance Center: enhancing the Military Health System’s public health capabilities. BMC Public Health. 2011;11(suppl 2):S1.
- Pavlin JA, Welch RA. Ethics, human use, and the department of defense serum repository. Mil Med. 2015;180:49-56.
- Defense Medical Surveillance System. Military Health System and the Defense Health Agency website. http://www.health.mil/Military-Health-Topics/Health-Readiness/Armed-Forces-Health-Surveillance-Branch/Data-Management-and-Technical-Support/Defense-Medical-Surveillance-System. Accessed August 2, 2016.
- Perdue CL, Eick-Cost AA, Rubertone MV, et al. Description and utilization of the United States Department of Defense Serum Repository: a review of published studies, 1985-2012. Plos One. 2015;10:1-16.
- Mancuso JD, Mallon TM, Gaydos JC. Maximizing the capabilities of the DoD serum repository to meet current and future needs: report of the needs panel. Mil Med. 2015;180:14-24.
- Department of Defense. Department of Defense Instruction. http://www.dtic.mil/whs/directives/corres/pdf/600014p.pdf. Posted September 26, 2001. Updated October 3, 2013. Accessed August 2, 2016.
- Lindler LE. Building a DoD biorepository for the future: potential benefits and way forward. Mil Med. 2015;180:90-94.
Practice Points
- Large patient databases and tissue repositories are increasingly being used to improve patient care through the use of clinical data, genomics, proteinomics, and metabolomics.
- The US Military has an established electronic medical record as well as tissue and serum repositories that can be leveraged to study melanoma and other dermatologic diseases.