User login
Slavitt to Trump administration: Keep the CMS Innovation Center
WASHINGTON – Acting Administrator Andy Slavitt has some advice for his successors at the CMS: Keep the Center for Medicare & Medicaid Innovation, even if you trash the Affordable Care Act.
The innovation center is vital to the success of the Quality Payment Program, the value-based payment framework set up by the Medicare Access and CHIP Reauthorization Act (MACRA), Mr. Slavitt said Dec. 1 at the National MACRA MIPS/APM Summit.
“MACRA can’t work as well without a CMS Innovation Center that can move quickly to develop and expand new approaches to paying for care,” Mr. Slavitt said. “With changes to the Innovation Center, the advanced alternative payment approaches could slow significantly. We will have a much narrower path with fewer specialty options and approaches, which take in patient and physician feedback. Medicare and commercial payers would then fall further out of alignment, and more importantly, less patients would have access to innovative care methods.”
Mr. Slavitt offered a few other recommendations to the next regime. First, he called on the Trump administration to ensure that the 20 million people who have obtained health care coverage under the ACA do not lose it as a key to continued delivery system reform.
“Build from a foundation of progress, do not head backwards,” Mr. Slavitt advised. “There can be no delivery system reform without building on the foundation of reaching universal coverage.”
To that end, he advised keeping other key ACA provisions, including no-cost preventive care, the elimination of annual and lifetime coverage caps, and the end of pre-existing condition exclusions.
“If we want to fix how care is delivered, so that we’re providing value, then we must ensure that Americans can afford and access quality care at every point in their lives,” he said. “If we lose even some of the coverage gains made under the ACA, or leave people in limbo, people will lose access to regular care and we will drive up long-term costs.”
He also called for more improvements in the health IT space, including a demand for affordable systems and technologies that can exchange data and support quality health care.
“MACRA is an opportunity to move the focus away from paperwork and reporting and toward paying for what works,” Mr. Slavitt said. “For a variety of reasons, EHRs became an industry before they became a useful tool. The technology community must be held accountable ... to make room for new innovators and to give clinicians more freedom and more flexibility to focus on their patients, to practice medicine, and deliver better care.”
President-elect Trump has designated Seema Verma, a health care consultant who helped design the Indiana’s ACA Medicaid expansion, to be the next CMS administrator.
WASHINGTON – Acting Administrator Andy Slavitt has some advice for his successors at the CMS: Keep the Center for Medicare & Medicaid Innovation, even if you trash the Affordable Care Act.
The innovation center is vital to the success of the Quality Payment Program, the value-based payment framework set up by the Medicare Access and CHIP Reauthorization Act (MACRA), Mr. Slavitt said Dec. 1 at the National MACRA MIPS/APM Summit.
“MACRA can’t work as well without a CMS Innovation Center that can move quickly to develop and expand new approaches to paying for care,” Mr. Slavitt said. “With changes to the Innovation Center, the advanced alternative payment approaches could slow significantly. We will have a much narrower path with fewer specialty options and approaches, which take in patient and physician feedback. Medicare and commercial payers would then fall further out of alignment, and more importantly, less patients would have access to innovative care methods.”
Mr. Slavitt offered a few other recommendations to the next regime. First, he called on the Trump administration to ensure that the 20 million people who have obtained health care coverage under the ACA do not lose it as a key to continued delivery system reform.
“Build from a foundation of progress, do not head backwards,” Mr. Slavitt advised. “There can be no delivery system reform without building on the foundation of reaching universal coverage.”
To that end, he advised keeping other key ACA provisions, including no-cost preventive care, the elimination of annual and lifetime coverage caps, and the end of pre-existing condition exclusions.
“If we want to fix how care is delivered, so that we’re providing value, then we must ensure that Americans can afford and access quality care at every point in their lives,” he said. “If we lose even some of the coverage gains made under the ACA, or leave people in limbo, people will lose access to regular care and we will drive up long-term costs.”
He also called for more improvements in the health IT space, including a demand for affordable systems and technologies that can exchange data and support quality health care.
“MACRA is an opportunity to move the focus away from paperwork and reporting and toward paying for what works,” Mr. Slavitt said. “For a variety of reasons, EHRs became an industry before they became a useful tool. The technology community must be held accountable ... to make room for new innovators and to give clinicians more freedom and more flexibility to focus on their patients, to practice medicine, and deliver better care.”
President-elect Trump has designated Seema Verma, a health care consultant who helped design the Indiana’s ACA Medicaid expansion, to be the next CMS administrator.
WASHINGTON – Acting Administrator Andy Slavitt has some advice for his successors at the CMS: Keep the Center for Medicare & Medicaid Innovation, even if you trash the Affordable Care Act.
The innovation center is vital to the success of the Quality Payment Program, the value-based payment framework set up by the Medicare Access and CHIP Reauthorization Act (MACRA), Mr. Slavitt said Dec. 1 at the National MACRA MIPS/APM Summit.
“MACRA can’t work as well without a CMS Innovation Center that can move quickly to develop and expand new approaches to paying for care,” Mr. Slavitt said. “With changes to the Innovation Center, the advanced alternative payment approaches could slow significantly. We will have a much narrower path with fewer specialty options and approaches, which take in patient and physician feedback. Medicare and commercial payers would then fall further out of alignment, and more importantly, less patients would have access to innovative care methods.”
Mr. Slavitt offered a few other recommendations to the next regime. First, he called on the Trump administration to ensure that the 20 million people who have obtained health care coverage under the ACA do not lose it as a key to continued delivery system reform.
“Build from a foundation of progress, do not head backwards,” Mr. Slavitt advised. “There can be no delivery system reform without building on the foundation of reaching universal coverage.”
To that end, he advised keeping other key ACA provisions, including no-cost preventive care, the elimination of annual and lifetime coverage caps, and the end of pre-existing condition exclusions.
“If we want to fix how care is delivered, so that we’re providing value, then we must ensure that Americans can afford and access quality care at every point in their lives,” he said. “If we lose even some of the coverage gains made under the ACA, or leave people in limbo, people will lose access to regular care and we will drive up long-term costs.”
He also called for more improvements in the health IT space, including a demand for affordable systems and technologies that can exchange data and support quality health care.
“MACRA is an opportunity to move the focus away from paperwork and reporting and toward paying for what works,” Mr. Slavitt said. “For a variety of reasons, EHRs became an industry before they became a useful tool. The technology community must be held accountable ... to make room for new innovators and to give clinicians more freedom and more flexibility to focus on their patients, to practice medicine, and deliver better care.”
President-elect Trump has designated Seema Verma, a health care consultant who helped design the Indiana’s ACA Medicaid expansion, to be the next CMS administrator.
AT THE NATIONAL MACRA MIPS/APM SUMMIT
California bucks trend of rising U.S. maternal mortality
While the United States as a whole is seeing an unsettling rise in maternal mortality, California is on a divergent path.
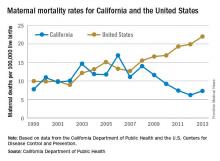
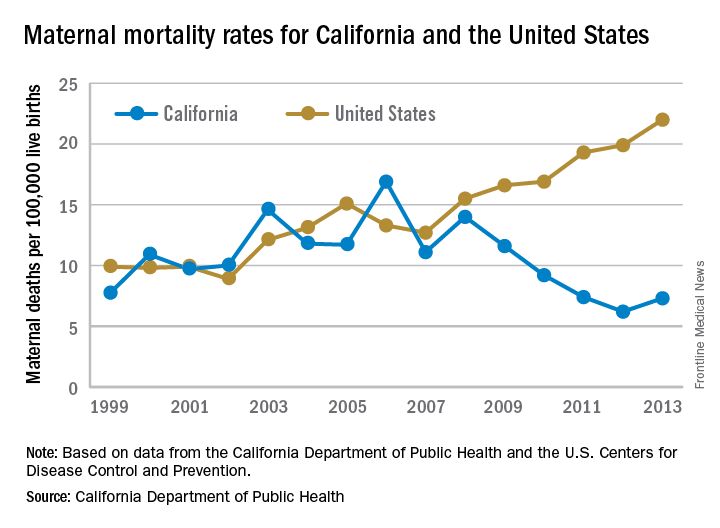
Maternal mortality in the Golden State was tracking at a similar rate with national figures from 1999-2008 when the trend started to change. By 2013, the U.S. maternal mortality rate had grown to 22.0 deaths per 100,000 live births, while California’s rate had dropped to 7.3 per 100,000, according to data from the California Department of Public Health and the U.S. Centers for Disease Control and Prevention.
“We reviewed every maternal death for almost 10 years and through that process, we learned a lot about practices of care and the opportunities to really have intervened,” Elliott Main, MD, medical director of the Collaborative, said in an interview. “There were certain causes of death that had a pretty high chance of preventability and those were hemorrhage and preeclampsia.”
Identifying risk factors
The Collaborative’s research identified a number of risk factors that were significant contributors to maternal morbidity.
Dr. Main noted that obesity and older maternal age are both associated with an increased likelihood of having a cesarean delivery, which is associated with an increased risk of hemorrhage.
“So there are pathways that can get you into more trouble, but again if you are on top of those, you can be proactive and not necessarily have this high rate of complications,” he added.
With data in hand about what was contributing to the risks of maternal mortality, the Collaborative set out to build a series of toolkits or “bundles” to help guide hospitals in limiting complications and responding to emergencies. These toolkits are based on the state’s own data, as well as best practices identified in the medical literature and national guidelines from organizations such as the American College of Obstetricians and Gynecologists.
For example, the hypertension bundle includes information aimed at readiness, recognition and prevention, response, and reporting/system learning. Other bundles, which the Collaborative helped to develop and which are distributed through the Council on Patient Safety in Women’s Health Care, cover areas such as mental health, thromboembolism, hemorrhage, and safe reduction in primary cesarean births.
“It’s all about the implementation of those and that’s where we spent a lot of time in California with what are called quality collaboratives,” he said. “These are generally state-based [efforts], where you put together a consortium of providers, hospitals, and public health and patient advocates, and you work on improving the care for these certain conditions.”
The bundles are not meant to be cookbook medicine, Dr. Main noted, but rather are designed so that they can be customized based on the resources of an individual hospital, whether the facility handles 300 births a year or 5,000.
“There is no such thing as a national protocol for this,” Dr. Main said. “You have to have some flexibility and differences in the protocols but what we really are striving for is that for emergencies, people have standard protocols for the treatment of that emergency.”
Disparities remain
While California is a success story in terms of its overall drop in maternal mortality, racial disparities remain, particularly for African American women.
Maternal mortality among African Americans has dropped 50% in the state, mirroring the overall decline in the state during the 2008-2013 time period, but it is still three to four times higher than for other racial/ethnic groups.
“African American women do have more risk factors. There is more obesity. There is more hypertension and they have more social stresses and so forth, but none of those are reasons that they should die,” Dr. Main said. “They are reasons that they should have more intensive attention and care. If you have an older African American woman with hypertension, you’ve got to be on your toes when you are taking care of her and be a lot more responsive to warning signs than in a 25-year-old white woman that is perfectly healthy.”
“Minorities represent half of all U.S. persons, yet racial and ethnic minorities suffer higher rates of maternal mortality than whites in this country,” said Dr. Howell, who also chairs ACOG’s work group on reduction of peripartum racial disparities. “In fact, black and African Americans are three to four times more likely to die than whites. This is the largest disparity among population perinatal health measures.”
Native Americans, Asians, and some Latinas also have elevated rates of maternal mortality, compared with white women, she noted.
Depending on the city, those rates could be even higher, she said.
“In New York City, a recent publication by our Department of Health reviewed deaths from 2006 to 2010 and they found that black women were 10 times more likely to die than white women,” Dr. Howell said. “It’s also important to remember that for every maternal death, over 100 women experience severe obstetric morbidity or a life-threatening diagnosis or undergo a lifesaving procedure during their delivery hospitalization.”
But there are care tools available to help address this issue, too. The Alliance for Innovation on Maternal Health, which includes the California collaborative and ACOG, has developed a safety bundle that focuses on themes of shared decision making, implicit bias, continuity of care, provider and patient education, and care fragmentation. It also recommends implementation of a disparity dashboard, meaning that hospitals and health systems would stratify their quality results by race and ethnicity to identify and address gaps in care, Dr. Howell said.
gtwachtman@frontlinemedcom.com
While the United States as a whole is seeing an unsettling rise in maternal mortality, California is on a divergent path.
Maternal mortality in the Golden State was tracking at a similar rate with national figures from 1999-2008 when the trend started to change. By 2013, the U.S. maternal mortality rate had grown to 22.0 deaths per 100,000 live births, while California’s rate had dropped to 7.3 per 100,000, according to data from the California Department of Public Health and the U.S. Centers for Disease Control and Prevention.
“We reviewed every maternal death for almost 10 years and through that process, we learned a lot about practices of care and the opportunities to really have intervened,” Elliott Main, MD, medical director of the Collaborative, said in an interview. “There were certain causes of death that had a pretty high chance of preventability and those were hemorrhage and preeclampsia.”
Identifying risk factors
The Collaborative’s research identified a number of risk factors that were significant contributors to maternal morbidity.
Dr. Main noted that obesity and older maternal age are both associated with an increased likelihood of having a cesarean delivery, which is associated with an increased risk of hemorrhage.
“So there are pathways that can get you into more trouble, but again if you are on top of those, you can be proactive and not necessarily have this high rate of complications,” he added.
With data in hand about what was contributing to the risks of maternal mortality, the Collaborative set out to build a series of toolkits or “bundles” to help guide hospitals in limiting complications and responding to emergencies. These toolkits are based on the state’s own data, as well as best practices identified in the medical literature and national guidelines from organizations such as the American College of Obstetricians and Gynecologists.
For example, the hypertension bundle includes information aimed at readiness, recognition and prevention, response, and reporting/system learning. Other bundles, which the Collaborative helped to develop and which are distributed through the Council on Patient Safety in Women’s Health Care, cover areas such as mental health, thromboembolism, hemorrhage, and safe reduction in primary cesarean births.
“It’s all about the implementation of those and that’s where we spent a lot of time in California with what are called quality collaboratives,” he said. “These are generally state-based [efforts], where you put together a consortium of providers, hospitals, and public health and patient advocates, and you work on improving the care for these certain conditions.”
The bundles are not meant to be cookbook medicine, Dr. Main noted, but rather are designed so that they can be customized based on the resources of an individual hospital, whether the facility handles 300 births a year or 5,000.
“There is no such thing as a national protocol for this,” Dr. Main said. “You have to have some flexibility and differences in the protocols but what we really are striving for is that for emergencies, people have standard protocols for the treatment of that emergency.”
Disparities remain
While California is a success story in terms of its overall drop in maternal mortality, racial disparities remain, particularly for African American women.
Maternal mortality among African Americans has dropped 50% in the state, mirroring the overall decline in the state during the 2008-2013 time period, but it is still three to four times higher than for other racial/ethnic groups.
“African American women do have more risk factors. There is more obesity. There is more hypertension and they have more social stresses and so forth, but none of those are reasons that they should die,” Dr. Main said. “They are reasons that they should have more intensive attention and care. If you have an older African American woman with hypertension, you’ve got to be on your toes when you are taking care of her and be a lot more responsive to warning signs than in a 25-year-old white woman that is perfectly healthy.”
“Minorities represent half of all U.S. persons, yet racial and ethnic minorities suffer higher rates of maternal mortality than whites in this country,” said Dr. Howell, who also chairs ACOG’s work group on reduction of peripartum racial disparities. “In fact, black and African Americans are three to four times more likely to die than whites. This is the largest disparity among population perinatal health measures.”
Native Americans, Asians, and some Latinas also have elevated rates of maternal mortality, compared with white women, she noted.
Depending on the city, those rates could be even higher, she said.
“In New York City, a recent publication by our Department of Health reviewed deaths from 2006 to 2010 and they found that black women were 10 times more likely to die than white women,” Dr. Howell said. “It’s also important to remember that for every maternal death, over 100 women experience severe obstetric morbidity or a life-threatening diagnosis or undergo a lifesaving procedure during their delivery hospitalization.”
But there are care tools available to help address this issue, too. The Alliance for Innovation on Maternal Health, which includes the California collaborative and ACOG, has developed a safety bundle that focuses on themes of shared decision making, implicit bias, continuity of care, provider and patient education, and care fragmentation. It also recommends implementation of a disparity dashboard, meaning that hospitals and health systems would stratify their quality results by race and ethnicity to identify and address gaps in care, Dr. Howell said.
gtwachtman@frontlinemedcom.com
While the United States as a whole is seeing an unsettling rise in maternal mortality, California is on a divergent path.
Maternal mortality in the Golden State was tracking at a similar rate with national figures from 1999-2008 when the trend started to change. By 2013, the U.S. maternal mortality rate had grown to 22.0 deaths per 100,000 live births, while California’s rate had dropped to 7.3 per 100,000, according to data from the California Department of Public Health and the U.S. Centers for Disease Control and Prevention.
“We reviewed every maternal death for almost 10 years and through that process, we learned a lot about practices of care and the opportunities to really have intervened,” Elliott Main, MD, medical director of the Collaborative, said in an interview. “There were certain causes of death that had a pretty high chance of preventability and those were hemorrhage and preeclampsia.”
Identifying risk factors
The Collaborative’s research identified a number of risk factors that were significant contributors to maternal morbidity.
Dr. Main noted that obesity and older maternal age are both associated with an increased likelihood of having a cesarean delivery, which is associated with an increased risk of hemorrhage.
“So there are pathways that can get you into more trouble, but again if you are on top of those, you can be proactive and not necessarily have this high rate of complications,” he added.
With data in hand about what was contributing to the risks of maternal mortality, the Collaborative set out to build a series of toolkits or “bundles” to help guide hospitals in limiting complications and responding to emergencies. These toolkits are based on the state’s own data, as well as best practices identified in the medical literature and national guidelines from organizations such as the American College of Obstetricians and Gynecologists.
For example, the hypertension bundle includes information aimed at readiness, recognition and prevention, response, and reporting/system learning. Other bundles, which the Collaborative helped to develop and which are distributed through the Council on Patient Safety in Women’s Health Care, cover areas such as mental health, thromboembolism, hemorrhage, and safe reduction in primary cesarean births.
“It’s all about the implementation of those and that’s where we spent a lot of time in California with what are called quality collaboratives,” he said. “These are generally state-based [efforts], where you put together a consortium of providers, hospitals, and public health and patient advocates, and you work on improving the care for these certain conditions.”
The bundles are not meant to be cookbook medicine, Dr. Main noted, but rather are designed so that they can be customized based on the resources of an individual hospital, whether the facility handles 300 births a year or 5,000.
“There is no such thing as a national protocol for this,” Dr. Main said. “You have to have some flexibility and differences in the protocols but what we really are striving for is that for emergencies, people have standard protocols for the treatment of that emergency.”
Disparities remain
While California is a success story in terms of its overall drop in maternal mortality, racial disparities remain, particularly for African American women.
Maternal mortality among African Americans has dropped 50% in the state, mirroring the overall decline in the state during the 2008-2013 time period, but it is still three to four times higher than for other racial/ethnic groups.
“African American women do have more risk factors. There is more obesity. There is more hypertension and they have more social stresses and so forth, but none of those are reasons that they should die,” Dr. Main said. “They are reasons that they should have more intensive attention and care. If you have an older African American woman with hypertension, you’ve got to be on your toes when you are taking care of her and be a lot more responsive to warning signs than in a 25-year-old white woman that is perfectly healthy.”
“Minorities represent half of all U.S. persons, yet racial and ethnic minorities suffer higher rates of maternal mortality than whites in this country,” said Dr. Howell, who also chairs ACOG’s work group on reduction of peripartum racial disparities. “In fact, black and African Americans are three to four times more likely to die than whites. This is the largest disparity among population perinatal health measures.”
Native Americans, Asians, and some Latinas also have elevated rates of maternal mortality, compared with white women, she noted.
Depending on the city, those rates could be even higher, she said.
“In New York City, a recent publication by our Department of Health reviewed deaths from 2006 to 2010 and they found that black women were 10 times more likely to die than white women,” Dr. Howell said. “It’s also important to remember that for every maternal death, over 100 women experience severe obstetric morbidity or a life-threatening diagnosis or undergo a lifesaving procedure during their delivery hospitalization.”
But there are care tools available to help address this issue, too. The Alliance for Innovation on Maternal Health, which includes the California collaborative and ACOG, has developed a safety bundle that focuses on themes of shared decision making, implicit bias, continuity of care, provider and patient education, and care fragmentation. It also recommends implementation of a disparity dashboard, meaning that hospitals and health systems would stratify their quality results by race and ethnicity to identify and address gaps in care, Dr. Howell said.
gtwachtman@frontlinemedcom.com
Misvalued codes lead to small cut in Medicare fee schedule
Instead of getting a 0.5% pay increase, doctors will lose 0.18% of their Medicare payments because the Centers for Medicare & Medicaid Services could not find enough misvalued services in the 2017 Medicare physician fee schedule.
A health reform law called the Protecting Access to Medicare Act of 2014 (H.R. 4302) was designed to hold down Medicare spending by requiring CMS to identify misvalued codes based on up to 16 criteria. Once identified, the value of those codes was to be lowered by CMS by an amount equal to 1% of the overall fee schedule so that physician pay could be increased by 1%. Codes up for consideration included codes that were the fastest-growing, codes for maturing technologies, codes for services that have undergone a change in service site, and codes with a significant difference in value based on service site.
A total of 19 potentially misvalued codes were found, including biopsy of finger or toe nail; injection of carpal tunnel; change of stomach feeding, accessed through skin; irrigation of vagina and/or application of drug to treat infection; wound closure utilizing tissue adhesive(s) only; and injection of anesthetic agent and/or steroid drug into nerve of foot. Lowering the value of those codes reduced the physician fee schedule by 0.32%, causing the 0.18% pay cut.
It is disappointing that a 0.5% fee schedule increase could not be accomplished, according to officials at the American Academy of Family Physicians.
“What I have to do in my office visit has greatly increased, whereas what it takes [specialists] to do their procedure has decreased. There needs to be an adjustment in the fee schedule,” AAFP President John Meigs, MD, said in an interview. This legislation “was supposed to spur Medicare and CMS to start making some adjustments and with these adjustments, give that tiny little pay increase to the fee schedule. That leads to the frustration of primary care physicians and family medicine physicians. We were promised a tiny pay increase and we are not going to get it.”
“CMS is paying lip service to increasing value of primary care, increasing the value of cognitive-based services, and they did come up with some new codes,” Dr. Meigs said. “They did some things with paying for diabetic patients that are things that they should have been paying for a long time ago. But the administrative hassle and regulatory burden of trying to do the documentation they require to actually get paid for some of these codes have a lot of physicians throwing their hands up saying, ‘it’s not worth my time to spend $5 to make a nickel.’ ”
Instead of getting a 0.5% pay increase, doctors will lose 0.18% of their Medicare payments because the Centers for Medicare & Medicaid Services could not find enough misvalued services in the 2017 Medicare physician fee schedule.
A health reform law called the Protecting Access to Medicare Act of 2014 (H.R. 4302) was designed to hold down Medicare spending by requiring CMS to identify misvalued codes based on up to 16 criteria. Once identified, the value of those codes was to be lowered by CMS by an amount equal to 1% of the overall fee schedule so that physician pay could be increased by 1%. Codes up for consideration included codes that were the fastest-growing, codes for maturing technologies, codes for services that have undergone a change in service site, and codes with a significant difference in value based on service site.
A total of 19 potentially misvalued codes were found, including biopsy of finger or toe nail; injection of carpal tunnel; change of stomach feeding, accessed through skin; irrigation of vagina and/or application of drug to treat infection; wound closure utilizing tissue adhesive(s) only; and injection of anesthetic agent and/or steroid drug into nerve of foot. Lowering the value of those codes reduced the physician fee schedule by 0.32%, causing the 0.18% pay cut.
It is disappointing that a 0.5% fee schedule increase could not be accomplished, according to officials at the American Academy of Family Physicians.
“What I have to do in my office visit has greatly increased, whereas what it takes [specialists] to do their procedure has decreased. There needs to be an adjustment in the fee schedule,” AAFP President John Meigs, MD, said in an interview. This legislation “was supposed to spur Medicare and CMS to start making some adjustments and with these adjustments, give that tiny little pay increase to the fee schedule. That leads to the frustration of primary care physicians and family medicine physicians. We were promised a tiny pay increase and we are not going to get it.”
“CMS is paying lip service to increasing value of primary care, increasing the value of cognitive-based services, and they did come up with some new codes,” Dr. Meigs said. “They did some things with paying for diabetic patients that are things that they should have been paying for a long time ago. But the administrative hassle and regulatory burden of trying to do the documentation they require to actually get paid for some of these codes have a lot of physicians throwing their hands up saying, ‘it’s not worth my time to spend $5 to make a nickel.’ ”
Instead of getting a 0.5% pay increase, doctors will lose 0.18% of their Medicare payments because the Centers for Medicare & Medicaid Services could not find enough misvalued services in the 2017 Medicare physician fee schedule.
A health reform law called the Protecting Access to Medicare Act of 2014 (H.R. 4302) was designed to hold down Medicare spending by requiring CMS to identify misvalued codes based on up to 16 criteria. Once identified, the value of those codes was to be lowered by CMS by an amount equal to 1% of the overall fee schedule so that physician pay could be increased by 1%. Codes up for consideration included codes that were the fastest-growing, codes for maturing technologies, codes for services that have undergone a change in service site, and codes with a significant difference in value based on service site.
A total of 19 potentially misvalued codes were found, including biopsy of finger or toe nail; injection of carpal tunnel; change of stomach feeding, accessed through skin; irrigation of vagina and/or application of drug to treat infection; wound closure utilizing tissue adhesive(s) only; and injection of anesthetic agent and/or steroid drug into nerve of foot. Lowering the value of those codes reduced the physician fee schedule by 0.32%, causing the 0.18% pay cut.
It is disappointing that a 0.5% fee schedule increase could not be accomplished, according to officials at the American Academy of Family Physicians.
“What I have to do in my office visit has greatly increased, whereas what it takes [specialists] to do their procedure has decreased. There needs to be an adjustment in the fee schedule,” AAFP President John Meigs, MD, said in an interview. This legislation “was supposed to spur Medicare and CMS to start making some adjustments and with these adjustments, give that tiny little pay increase to the fee schedule. That leads to the frustration of primary care physicians and family medicine physicians. We were promised a tiny pay increase and we are not going to get it.”
“CMS is paying lip service to increasing value of primary care, increasing the value of cognitive-based services, and they did come up with some new codes,” Dr. Meigs said. “They did some things with paying for diabetic patients that are things that they should have been paying for a long time ago. But the administrative hassle and regulatory burden of trying to do the documentation they require to actually get paid for some of these codes have a lot of physicians throwing their hands up saying, ‘it’s not worth my time to spend $5 to make a nickel.’ ”
Meaningful use: CMS extends 90-day reporting period to 2016, 2017
Physicians who already participate in the EHR incentive program for meaningful use will have to demonstrate they are meaningful users for only 90 days in 2016 and 2017.
The Centers for Medicare & Medicaid Services finalized the 90-day reporting period in its annual update to the hospital outpatient prospective payment system, scheduled for publication in the Federal Register on Nov. 14.
The CMS was preparing to require a full year of reporting in 2017 to be eligible for bonuses under the EHR Incentive Program; but instead extended the 90-day reporting requirement to address concerns about technical functionalities and to help ease the transition to the Merit-based Incentive Payment System (MIPS) created by the 2015 MACRA law, according to an agency fact sheet.
The Outpatient Prospective Payment System (OPPS) final rule also eliminated clinical decision support and computerized order entry objectives and measures for eligible hospitals and critical access hospitals.
Instead of requiring that eligible professionals and hospitals new to the EHR Incentive Program in 2017 to meet Stage 3 requirements, the CMS has provided modified Stage 2 requirements.
Physicians who are new to the EHR Incentive Program in 2017 but are transitioning their practice to MIPS next year can apply for a significant hardship exemption to avoid any penalties to their 2018 Medicare payments.
Physicians who already participate in the EHR incentive program for meaningful use will have to demonstrate they are meaningful users for only 90 days in 2016 and 2017.
The Centers for Medicare & Medicaid Services finalized the 90-day reporting period in its annual update to the hospital outpatient prospective payment system, scheduled for publication in the Federal Register on Nov. 14.
The CMS was preparing to require a full year of reporting in 2017 to be eligible for bonuses under the EHR Incentive Program; but instead extended the 90-day reporting requirement to address concerns about technical functionalities and to help ease the transition to the Merit-based Incentive Payment System (MIPS) created by the 2015 MACRA law, according to an agency fact sheet.
The Outpatient Prospective Payment System (OPPS) final rule also eliminated clinical decision support and computerized order entry objectives and measures for eligible hospitals and critical access hospitals.
Instead of requiring that eligible professionals and hospitals new to the EHR Incentive Program in 2017 to meet Stage 3 requirements, the CMS has provided modified Stage 2 requirements.
Physicians who are new to the EHR Incentive Program in 2017 but are transitioning their practice to MIPS next year can apply for a significant hardship exemption to avoid any penalties to their 2018 Medicare payments.
Physicians who already participate in the EHR incentive program for meaningful use will have to demonstrate they are meaningful users for only 90 days in 2016 and 2017.
The Centers for Medicare & Medicaid Services finalized the 90-day reporting period in its annual update to the hospital outpatient prospective payment system, scheduled for publication in the Federal Register on Nov. 14.
The CMS was preparing to require a full year of reporting in 2017 to be eligible for bonuses under the EHR Incentive Program; but instead extended the 90-day reporting requirement to address concerns about technical functionalities and to help ease the transition to the Merit-based Incentive Payment System (MIPS) created by the 2015 MACRA law, according to an agency fact sheet.
The Outpatient Prospective Payment System (OPPS) final rule also eliminated clinical decision support and computerized order entry objectives and measures for eligible hospitals and critical access hospitals.
Instead of requiring that eligible professionals and hospitals new to the EHR Incentive Program in 2017 to meet Stage 3 requirements, the CMS has provided modified Stage 2 requirements.
Physicians who are new to the EHR Incentive Program in 2017 but are transitioning their practice to MIPS next year can apply for a significant hardship exemption to avoid any penalties to their 2018 Medicare payments.
CMS to pay more for care coordination, prevention
The Centers for Medicare & Medicaid Services plans to spend an extra $140 million on these services next year under the final physician fee schedule, released on Nov. 2 and scheduled for publication in the Federal Register on Nov. 15.
“Clinicians will additionally be able to bill and be paid more appropriately when they spend more time with their patients, serving their patients’ needs outside of the office visit, and better coordinate care,” they wrote.
CMS finalized a number of payment codes that “better identify and value primary care, care management, and cognitive services,” according to an agency fact sheet highlighting key provisions of the final physician fee schedule for 2017.
The coding changes allow for separate payments for non–face-to-face prolonged evaluation and management services; revalue existing codes for face-to-face prolonged services; separate payments for comprehensive assessment and care planning for patients with cognitive impairments such as dementia; and separate payments for chronic care management of complex patients.
“This final decision by CMS means individuals living with Alzheimer’s disease will finally have access to critical care and support services that can improve quality of life for the individual, their family, and caregivers,” Harry Johns, Alzheimer’s Association President and CEO, said in a statement. “Now that care-planning sessions will be available to them, individuals living with the disease will have access to much-needed information on treatments and services.”
“Geriatricians, internists, and family physicians provide core services for the Medicare program, including the kinds of care management and patient-centered care that are described by these new codes,” Mr. Slavitt and Dr. Conway wrote. “Over time, we estimate that the payment increases attributable to these new codes could be as much as 30% and 37%, respectively, to these specialties.”
CMS also finalized other coding changes, including:
- A separate code for moderate sedation services to account for changes in practice trends that report anesthesia separately from certain endoscopic procedures despite payment being built into the overall procedure payment.
- More payments for telehealth services, including for end-stage renal disease-related services for dialysis, advanced care planning; and critical care consultations.
The American College of Physicians applauded the final rule.
“The policies in the rule more accurately recognize the work of primary care physicians and other cognitive specialties to accommodate the changing needs of Medicare beneficiaries,” ACP President Nitin S. Damle, MD, said in a statement.
The Centers for Medicare & Medicaid Services plans to spend an extra $140 million on these services next year under the final physician fee schedule, released on Nov. 2 and scheduled for publication in the Federal Register on Nov. 15.
“Clinicians will additionally be able to bill and be paid more appropriately when they spend more time with their patients, serving their patients’ needs outside of the office visit, and better coordinate care,” they wrote.
CMS finalized a number of payment codes that “better identify and value primary care, care management, and cognitive services,” according to an agency fact sheet highlighting key provisions of the final physician fee schedule for 2017.
The coding changes allow for separate payments for non–face-to-face prolonged evaluation and management services; revalue existing codes for face-to-face prolonged services; separate payments for comprehensive assessment and care planning for patients with cognitive impairments such as dementia; and separate payments for chronic care management of complex patients.
“This final decision by CMS means individuals living with Alzheimer’s disease will finally have access to critical care and support services that can improve quality of life for the individual, their family, and caregivers,” Harry Johns, Alzheimer’s Association President and CEO, said in a statement. “Now that care-planning sessions will be available to them, individuals living with the disease will have access to much-needed information on treatments and services.”
“Geriatricians, internists, and family physicians provide core services for the Medicare program, including the kinds of care management and patient-centered care that are described by these new codes,” Mr. Slavitt and Dr. Conway wrote. “Over time, we estimate that the payment increases attributable to these new codes could be as much as 30% and 37%, respectively, to these specialties.”
CMS also finalized other coding changes, including:
- A separate code for moderate sedation services to account for changes in practice trends that report anesthesia separately from certain endoscopic procedures despite payment being built into the overall procedure payment.
- More payments for telehealth services, including for end-stage renal disease-related services for dialysis, advanced care planning; and critical care consultations.
The American College of Physicians applauded the final rule.
“The policies in the rule more accurately recognize the work of primary care physicians and other cognitive specialties to accommodate the changing needs of Medicare beneficiaries,” ACP President Nitin S. Damle, MD, said in a statement.
The Centers for Medicare & Medicaid Services plans to spend an extra $140 million on these services next year under the final physician fee schedule, released on Nov. 2 and scheduled for publication in the Federal Register on Nov. 15.
“Clinicians will additionally be able to bill and be paid more appropriately when they spend more time with their patients, serving their patients’ needs outside of the office visit, and better coordinate care,” they wrote.
CMS finalized a number of payment codes that “better identify and value primary care, care management, and cognitive services,” according to an agency fact sheet highlighting key provisions of the final physician fee schedule for 2017.
The coding changes allow for separate payments for non–face-to-face prolonged evaluation and management services; revalue existing codes for face-to-face prolonged services; separate payments for comprehensive assessment and care planning for patients with cognitive impairments such as dementia; and separate payments for chronic care management of complex patients.
“This final decision by CMS means individuals living with Alzheimer’s disease will finally have access to critical care and support services that can improve quality of life for the individual, their family, and caregivers,” Harry Johns, Alzheimer’s Association President and CEO, said in a statement. “Now that care-planning sessions will be available to them, individuals living with the disease will have access to much-needed information on treatments and services.”
“Geriatricians, internists, and family physicians provide core services for the Medicare program, including the kinds of care management and patient-centered care that are described by these new codes,” Mr. Slavitt and Dr. Conway wrote. “Over time, we estimate that the payment increases attributable to these new codes could be as much as 30% and 37%, respectively, to these specialties.”
CMS also finalized other coding changes, including:
- A separate code for moderate sedation services to account for changes in practice trends that report anesthesia separately from certain endoscopic procedures despite payment being built into the overall procedure payment.
- More payments for telehealth services, including for end-stage renal disease-related services for dialysis, advanced care planning; and critical care consultations.
The American College of Physicians applauded the final rule.
“The policies in the rule more accurately recognize the work of primary care physicians and other cognitive specialties to accommodate the changing needs of Medicare beneficiaries,” ACP President Nitin S. Damle, MD, said in a statement.
Remembering three giants in vascular surgery
This year the VEITHsymposium will be paying tribute to three influential vascular surgeons who are no longer with us: Dr. Allan Callow, Dr. Calvin Ernst, and Dr. John (Jack) Connolly.
Dr. Jerry Goldstone, MD, will have the honor of offering his thoughts on these extraordinary gentlemen during a session on Wednesday morning.
“I knew all three of these men quite well at various stages of my career,” Dr. Goldstone said.
Dr. Allan Callow, who died Dec. 22, 2015, at the age of 99, was considered a pioneer in vascular surgery. His contributions to vascular surgery include helping to perfect carotid endarterectomy.
He served in the U.S. Navy during World War II, retiring with the rank of rear admiral and was “an excellent speaker and had accumulated a very large personal experience with carotid artery disease which he was most recognized for as a clinician.”
Dr. Goldstone noted the different career path that this “great role model” followed.
“The most inspirational thing was his late-in-life switch to a basic science career,” Dr. Goldstone said. “Most of us in academics have our most intense basic research very early in our careers, but Allan’s late research career is inspiring and makes so much sense for a variety of reasons, not the least of which is it avoids the physical demands of clinical research.” Dr. Callow received an NIH RO1 grant at a time when contemporaries were retiring, Dr. Goldstone added.
Dr. Calvin Ernst “was probably best known as an educator, author and very dynamic Society of Vascular Surgery,” Dr. Goldstone said. “During his years on the SVS council, he was very actively involved in just about every activity that affected vascular surgery.”
Describing him as a “true Michigan guy” who was born in Detroit and attended the University of Michigan for undergraduate and medical school and stayed on for his surgery residency and then joined the faculty, Dr. Goldstone also remembered his accomplishments outside of SVS.
“Cal was a renowned surgeon and educator,” Dr. Goldstone recalled. “He was a prolific writer, authoring more than 300 papers and books, the best known probably being the first four editions of ‘Current Therapy in Vascular Surgery’ with Dr. James Stanley. ... He also served as the second co-editor of the Journal of Vascular Surgery and played a very important role in the early success of that journal.”
Dr. Ernst retired from practice in 2001 and died July 7, 2015 at the age of 81.
Dr. Goldstone noted that Dr. John (Jack) Connolly’s interests in cardiac and vascular surgery were broad and that he achieved world-wide fame for his presentations and lectures.
“He had a very prominent international influence and received countless invitations to speak abroad.”
He received fellowships were at the Royal College of Surgeons of England, Ireland and Edinburgh, as well as honorary membership in the Japan Vascular Society and the Vascular Surgical Society of Great Britain and Ireland. He also was honored by the University of California Irvine in 2012 when the institution established the John E. Connolly Endowed Chair in Surgery.
Dr. Goldstone remembered how “Jack” was “always a presence during my surgical training and career. Like Dr. Callow, he was still giving talks and writing papers well into his last years of life. He was very active internationally and was a vascular ambassador. He was charming, friendly, always willing to help younger surgeons and always had a genuine smile when he saw you.”
“For many,” Dr. Goldstone continued, “Jack will also be remembered for his friendship and unselfish support and mentoring.”
Dr. Connolly died Jan. 20, 2016, at the age of 92.
Session 34: Giants No Longer With Us: A Tribute To Allan Callow, Calvin Ernst And John (Jack) Connolly
Wednesday, 10:16 a.m. - 10:21 a.m.
Location: Grand Ballroom West, 3rd Floor
This year the VEITHsymposium will be paying tribute to three influential vascular surgeons who are no longer with us: Dr. Allan Callow, Dr. Calvin Ernst, and Dr. John (Jack) Connolly.
Dr. Jerry Goldstone, MD, will have the honor of offering his thoughts on these extraordinary gentlemen during a session on Wednesday morning.
“I knew all three of these men quite well at various stages of my career,” Dr. Goldstone said.
Dr. Allan Callow, who died Dec. 22, 2015, at the age of 99, was considered a pioneer in vascular surgery. His contributions to vascular surgery include helping to perfect carotid endarterectomy.
He served in the U.S. Navy during World War II, retiring with the rank of rear admiral and was “an excellent speaker and had accumulated a very large personal experience with carotid artery disease which he was most recognized for as a clinician.”
Dr. Goldstone noted the different career path that this “great role model” followed.
“The most inspirational thing was his late-in-life switch to a basic science career,” Dr. Goldstone said. “Most of us in academics have our most intense basic research very early in our careers, but Allan’s late research career is inspiring and makes so much sense for a variety of reasons, not the least of which is it avoids the physical demands of clinical research.” Dr. Callow received an NIH RO1 grant at a time when contemporaries were retiring, Dr. Goldstone added.
Dr. Calvin Ernst “was probably best known as an educator, author and very dynamic Society of Vascular Surgery,” Dr. Goldstone said. “During his years on the SVS council, he was very actively involved in just about every activity that affected vascular surgery.”
Describing him as a “true Michigan guy” who was born in Detroit and attended the University of Michigan for undergraduate and medical school and stayed on for his surgery residency and then joined the faculty, Dr. Goldstone also remembered his accomplishments outside of SVS.
“Cal was a renowned surgeon and educator,” Dr. Goldstone recalled. “He was a prolific writer, authoring more than 300 papers and books, the best known probably being the first four editions of ‘Current Therapy in Vascular Surgery’ with Dr. James Stanley. ... He also served as the second co-editor of the Journal of Vascular Surgery and played a very important role in the early success of that journal.”
Dr. Ernst retired from practice in 2001 and died July 7, 2015 at the age of 81.
Dr. Goldstone noted that Dr. John (Jack) Connolly’s interests in cardiac and vascular surgery were broad and that he achieved world-wide fame for his presentations and lectures.
“He had a very prominent international influence and received countless invitations to speak abroad.”
He received fellowships were at the Royal College of Surgeons of England, Ireland and Edinburgh, as well as honorary membership in the Japan Vascular Society and the Vascular Surgical Society of Great Britain and Ireland. He also was honored by the University of California Irvine in 2012 when the institution established the John E. Connolly Endowed Chair in Surgery.
Dr. Goldstone remembered how “Jack” was “always a presence during my surgical training and career. Like Dr. Callow, he was still giving talks and writing papers well into his last years of life. He was very active internationally and was a vascular ambassador. He was charming, friendly, always willing to help younger surgeons and always had a genuine smile when he saw you.”
“For many,” Dr. Goldstone continued, “Jack will also be remembered for his friendship and unselfish support and mentoring.”
Dr. Connolly died Jan. 20, 2016, at the age of 92.
Session 34: Giants No Longer With Us: A Tribute To Allan Callow, Calvin Ernst And John (Jack) Connolly
Wednesday, 10:16 a.m. - 10:21 a.m.
Location: Grand Ballroom West, 3rd Floor
This year the VEITHsymposium will be paying tribute to three influential vascular surgeons who are no longer with us: Dr. Allan Callow, Dr. Calvin Ernst, and Dr. John (Jack) Connolly.
Dr. Jerry Goldstone, MD, will have the honor of offering his thoughts on these extraordinary gentlemen during a session on Wednesday morning.
“I knew all three of these men quite well at various stages of my career,” Dr. Goldstone said.
Dr. Allan Callow, who died Dec. 22, 2015, at the age of 99, was considered a pioneer in vascular surgery. His contributions to vascular surgery include helping to perfect carotid endarterectomy.
He served in the U.S. Navy during World War II, retiring with the rank of rear admiral and was “an excellent speaker and had accumulated a very large personal experience with carotid artery disease which he was most recognized for as a clinician.”
Dr. Goldstone noted the different career path that this “great role model” followed.
“The most inspirational thing was his late-in-life switch to a basic science career,” Dr. Goldstone said. “Most of us in academics have our most intense basic research very early in our careers, but Allan’s late research career is inspiring and makes so much sense for a variety of reasons, not the least of which is it avoids the physical demands of clinical research.” Dr. Callow received an NIH RO1 grant at a time when contemporaries were retiring, Dr. Goldstone added.
Dr. Calvin Ernst “was probably best known as an educator, author and very dynamic Society of Vascular Surgery,” Dr. Goldstone said. “During his years on the SVS council, he was very actively involved in just about every activity that affected vascular surgery.”
Describing him as a “true Michigan guy” who was born in Detroit and attended the University of Michigan for undergraduate and medical school and stayed on for his surgery residency and then joined the faculty, Dr. Goldstone also remembered his accomplishments outside of SVS.
“Cal was a renowned surgeon and educator,” Dr. Goldstone recalled. “He was a prolific writer, authoring more than 300 papers and books, the best known probably being the first four editions of ‘Current Therapy in Vascular Surgery’ with Dr. James Stanley. ... He also served as the second co-editor of the Journal of Vascular Surgery and played a very important role in the early success of that journal.”
Dr. Ernst retired from practice in 2001 and died July 7, 2015 at the age of 81.
Dr. Goldstone noted that Dr. John (Jack) Connolly’s interests in cardiac and vascular surgery were broad and that he achieved world-wide fame for his presentations and lectures.
“He had a very prominent international influence and received countless invitations to speak abroad.”
He received fellowships were at the Royal College of Surgeons of England, Ireland and Edinburgh, as well as honorary membership in the Japan Vascular Society and the Vascular Surgical Society of Great Britain and Ireland. He also was honored by the University of California Irvine in 2012 when the institution established the John E. Connolly Endowed Chair in Surgery.
Dr. Goldstone remembered how “Jack” was “always a presence during my surgical training and career. Like Dr. Callow, he was still giving talks and writing papers well into his last years of life. He was very active internationally and was a vascular ambassador. He was charming, friendly, always willing to help younger surgeons and always had a genuine smile when he saw you.”
“For many,” Dr. Goldstone continued, “Jack will also be remembered for his friendship and unselfish support and mentoring.”
Dr. Connolly died Jan. 20, 2016, at the age of 92.
Session 34: Giants No Longer With Us: A Tribute To Allan Callow, Calvin Ernst And John (Jack) Connolly
Wednesday, 10:16 a.m. - 10:21 a.m.
Location: Grand Ballroom West, 3rd Floor
Balancing speed, safety in device approvals
WASHINGTON – Regulators from two continents are slowly inching toward more common ground when it comes to device approvals, a move that should strike a better balance between the time to approval and safety of the products.
It is no surprise that for devices to be approved in the United States, a much more rigorous evidence standard exists than it does in Europe, but the Food and Drug Administration is looking for ways to make changes to allow for quicker approval times without compromising safety.
Meanwhile, across the Atlantic, European regulators are looking for increased evidence requirements that will not stifle innovation.
However, health information technology may help relieve some inefficiencies going forward.
“If we combine quality registries, claims data, the evolving improving electronic health records, and the data warehouses that all of our health systems have, we can conduct prospective, observational, and clinical trials at a dramatically lower cost, answering many more questions per unit of time,” Dr. Califf added.
Other areas that will help improve the efficiency of the U.S. approval process is the work on developing a framework to bring feasibility studies back to America.
“I want to really accelerate that because we are hearing from a lot of people that they want to access the technology early; it’s the right thing to do, and we are hearing from industry that they’d like to work in the United States,” he said.
However, that is going to require one group that is generally more risk adverse to be willing to participate more in the process.
“The main limiting factors at this point in time are the health systems that we are all working in,” Dr. Califf noted. “They are very risk adverse. So while it is nice to argue and advertise that your interventional cardiologists are the best in town on your billboard, protecting those interventional cardiologists to do the high-risk, early studies that are really needed to develop devices is a different matter.”
He called on doctors to go back to their health systems to help better develop those early feasibility pathways to help bring some of that innovation back the United States.
Finally, Dr. Califf addressed the idea of developing evidence that can function not only for device approval, but for payment as well, especially as reimbursement systems are becoming more value driven.
“We are going to move to a system where reimbursement will increasingly move away from fee for service and increasingly gravitate to payment for value,” Dr. Califf said. “In order for this to work, we’ve got to develop the kinds of clinical trials [that include] the calculation of value, so the winners can be promoted on a wider scale and the procedures and technologies that don’t provide incremental value are left behind.”
He continued: “We need your help in figuring out the common source of information so that the FDA can make its decision – which is different from CMS’s decision – but where there’s a continuum of knowledge that allows for products to be approved if they are worthwhile, and then to be paid for and allowed to be marketed.”
Meanwhile, developers are facing a different situation in Europe, where more regulation and more stringent evidence standards could be coming down the pike.
Dr. Fraser noted research that has shown that devices first approved in Europe, compared with their U.S. counterparts, are associated with an increased incidence of recalls or safety alerts.
That being said, he noted that there are several proposed regulations that would increase the evidence requirements related to regulatory approval that manufacturers could face in the coming year, a challenge as it will require some level of harmonization across countries and regulatory systems within each country.
“In Europe, we are on the dawn of a new era regarding medical device assessment, but it is going to pose a very large challenge for our colleagues in the regulatory agencies because of their personnel, funding, and the integration between all these bodies and national systems. There are also tremendous challenges for us as physicians to ensure that we seek the evidence, we assess and contribute to it, and particularly now, that we also routinely take part in new systems for postmarket surveillance,” he said.
WASHINGTON – Regulators from two continents are slowly inching toward more common ground when it comes to device approvals, a move that should strike a better balance between the time to approval and safety of the products.
It is no surprise that for devices to be approved in the United States, a much more rigorous evidence standard exists than it does in Europe, but the Food and Drug Administration is looking for ways to make changes to allow for quicker approval times without compromising safety.
Meanwhile, across the Atlantic, European regulators are looking for increased evidence requirements that will not stifle innovation.
However, health information technology may help relieve some inefficiencies going forward.
“If we combine quality registries, claims data, the evolving improving electronic health records, and the data warehouses that all of our health systems have, we can conduct prospective, observational, and clinical trials at a dramatically lower cost, answering many more questions per unit of time,” Dr. Califf added.
Other areas that will help improve the efficiency of the U.S. approval process is the work on developing a framework to bring feasibility studies back to America.
“I want to really accelerate that because we are hearing from a lot of people that they want to access the technology early; it’s the right thing to do, and we are hearing from industry that they’d like to work in the United States,” he said.
However, that is going to require one group that is generally more risk adverse to be willing to participate more in the process.
“The main limiting factors at this point in time are the health systems that we are all working in,” Dr. Califf noted. “They are very risk adverse. So while it is nice to argue and advertise that your interventional cardiologists are the best in town on your billboard, protecting those interventional cardiologists to do the high-risk, early studies that are really needed to develop devices is a different matter.”
He called on doctors to go back to their health systems to help better develop those early feasibility pathways to help bring some of that innovation back the United States.
Finally, Dr. Califf addressed the idea of developing evidence that can function not only for device approval, but for payment as well, especially as reimbursement systems are becoming more value driven.
“We are going to move to a system where reimbursement will increasingly move away from fee for service and increasingly gravitate to payment for value,” Dr. Califf said. “In order for this to work, we’ve got to develop the kinds of clinical trials [that include] the calculation of value, so the winners can be promoted on a wider scale and the procedures and technologies that don’t provide incremental value are left behind.”
He continued: “We need your help in figuring out the common source of information so that the FDA can make its decision – which is different from CMS’s decision – but where there’s a continuum of knowledge that allows for products to be approved if they are worthwhile, and then to be paid for and allowed to be marketed.”
Meanwhile, developers are facing a different situation in Europe, where more regulation and more stringent evidence standards could be coming down the pike.
Dr. Fraser noted research that has shown that devices first approved in Europe, compared with their U.S. counterparts, are associated with an increased incidence of recalls or safety alerts.
That being said, he noted that there are several proposed regulations that would increase the evidence requirements related to regulatory approval that manufacturers could face in the coming year, a challenge as it will require some level of harmonization across countries and regulatory systems within each country.
“In Europe, we are on the dawn of a new era regarding medical device assessment, but it is going to pose a very large challenge for our colleagues in the regulatory agencies because of their personnel, funding, and the integration between all these bodies and national systems. There are also tremendous challenges for us as physicians to ensure that we seek the evidence, we assess and contribute to it, and particularly now, that we also routinely take part in new systems for postmarket surveillance,” he said.
WASHINGTON – Regulators from two continents are slowly inching toward more common ground when it comes to device approvals, a move that should strike a better balance between the time to approval and safety of the products.
It is no surprise that for devices to be approved in the United States, a much more rigorous evidence standard exists than it does in Europe, but the Food and Drug Administration is looking for ways to make changes to allow for quicker approval times without compromising safety.
Meanwhile, across the Atlantic, European regulators are looking for increased evidence requirements that will not stifle innovation.
However, health information technology may help relieve some inefficiencies going forward.
“If we combine quality registries, claims data, the evolving improving electronic health records, and the data warehouses that all of our health systems have, we can conduct prospective, observational, and clinical trials at a dramatically lower cost, answering many more questions per unit of time,” Dr. Califf added.
Other areas that will help improve the efficiency of the U.S. approval process is the work on developing a framework to bring feasibility studies back to America.
“I want to really accelerate that because we are hearing from a lot of people that they want to access the technology early; it’s the right thing to do, and we are hearing from industry that they’d like to work in the United States,” he said.
However, that is going to require one group that is generally more risk adverse to be willing to participate more in the process.
“The main limiting factors at this point in time are the health systems that we are all working in,” Dr. Califf noted. “They are very risk adverse. So while it is nice to argue and advertise that your interventional cardiologists are the best in town on your billboard, protecting those interventional cardiologists to do the high-risk, early studies that are really needed to develop devices is a different matter.”
He called on doctors to go back to their health systems to help better develop those early feasibility pathways to help bring some of that innovation back the United States.
Finally, Dr. Califf addressed the idea of developing evidence that can function not only for device approval, but for payment as well, especially as reimbursement systems are becoming more value driven.
“We are going to move to a system where reimbursement will increasingly move away from fee for service and increasingly gravitate to payment for value,” Dr. Califf said. “In order for this to work, we’ve got to develop the kinds of clinical trials [that include] the calculation of value, so the winners can be promoted on a wider scale and the procedures and technologies that don’t provide incremental value are left behind.”
He continued: “We need your help in figuring out the common source of information so that the FDA can make its decision – which is different from CMS’s decision – but where there’s a continuum of knowledge that allows for products to be approved if they are worthwhile, and then to be paid for and allowed to be marketed.”
Meanwhile, developers are facing a different situation in Europe, where more regulation and more stringent evidence standards could be coming down the pike.
Dr. Fraser noted research that has shown that devices first approved in Europe, compared with their U.S. counterparts, are associated with an increased incidence of recalls or safety alerts.
That being said, he noted that there are several proposed regulations that would increase the evidence requirements related to regulatory approval that manufacturers could face in the coming year, a challenge as it will require some level of harmonization across countries and regulatory systems within each country.
“In Europe, we are on the dawn of a new era regarding medical device assessment, but it is going to pose a very large challenge for our colleagues in the regulatory agencies because of their personnel, funding, and the integration between all these bodies and national systems. There are also tremendous challenges for us as physicians to ensure that we seek the evidence, we assess and contribute to it, and particularly now, that we also routinely take part in new systems for postmarket surveillance,” he said.
AT TCT 2016
MACRA final rule exempts many more doctors
Physicians who do not have a large Medicare population or who do not bill much to Medicare Part B will get a bit more breathing room to avoid having to participate in MACRA’s Quality Payment Program.
In a final rule posted Oct. 14 that sets out how the Medicare Access and CHIP Reauthorization Act (MACRA) will work, the Centers for Medicare & Medicaid Services increased the threshold for inclusion in the new value-based payment program from the initial proposal of physicians who bill Medicare more than $10,000 per year or treat more than 100 Medicare patients per year to those who bill more than $30,000 per year or provide care to more than 100 Medicare patients per year.
However, agency officials noted that it is committed to helping these small and solo practices become active participants in the Quality Payment Program.
“We heard these concerns and are taking additional steps to aid small practices, including reducing the time and cost to participate, excluding more small practices, increasing the availability of Advanced APMs [Alternative Payment Models] to small practices, allowing practices to begin participation at their own pace, changing one of the qualifications for participation in Advanced APMs to be practice-based as an alternative to total cost–based, and conducting significant technical support and outreach to small practices using $20 million a year over the next 5 years.”
CMS officials estimate that the new threshold will exclude an estimated 380,000 physicians and health care providers, up from about 225,000 under the initially proposed threshold.
Mr. Slavitt added that with these changes, “we estimate that small [practice] physicians will have the same level of participation as that of other practice sizes.”
The flexibility of participation was first announced Sept. 8, in a blog post outlining four options for participation in the Quality Payment Program:
• Option 1: Test the quality payment program in 2017 by submitting data without facing any negative payment adjustments. This will give physicians the year to make sure their processes are in place and ready for broader participation in 2018 and beyond.
• Option 2: Delay the start of the performance period and participate for just part of 2017. Depending on how long a physician delays reporting quality information back to CMS, they could still qualify for a smaller bonus payment.
• Option 3: Participate for the entire calendar year as called for by the law and be eligible for the full participation bonuses.
• Option 4: For those who qualify, participate in an Advanced Alternative Payment Model beginning next year.
That said, under the final rule, those who fail to do the bare minimum and report no data in 2017 will face a 4% pay cut in 2019.
“We are pleased CMS listened to the AGA’s concerns regarding the complexity of MACRA, its implementation, and its impact on small practices, among other issues,” said Timothy C. Wang, MD, AGAF, AGA Institute President, Columbia University, New York. “We believe that designation of 2017 as a transition year will allow GI’s the opportunity to understand the new value-based reimbursement system, and AGA stands ready to guide the GI community to survive and thrive in the new environment.”
“I am sure [the pay cuts are] going to impact some providers,” John Feore, director at Avalere Health, said in an interview. “But with the options, you can report on a very small number of measures, one for each of the categories, for a continuous 90-day period, and you will be sort of held harmless [and able] to transition over time into the program.”
Physician organizations were supportive of the final rule, particularly regarding how it addresses the concerns of small/solo practices.
CMS officials “took a significant step last month to address AMA concerns about the original proposal,” American Medical Association President Andrew W. Gurman, MD, said in a statement. “The final rule includes additional steps to help small and rural practices by raising the low-volume threshold exemption, and practices of all sizes will benefit from reduced MIPS reporting requirements. Our initial review indicates that CMS has been responsive to many concerns raised by the AMA.”
CMS officials said that the agency is looking into creating an accountable care organization (ACO) “Track 1 Plus” model that would qualify as an APM. Currently, ACOs that are in Track 1 share savings but do not assume risk. The agency said that the Track 1 Plus model would have organizations assuming some nominal level of risk that would be smaller, compared with those in the Medicare Shared Savings Program (MSSP) Track 2 and Track 3, as well as those that qualify as Next Generation ACOs. CMS plans to have the ACO Track 1 Plus Model ready for the 2018 reporting year.
Physicians who do not have a large Medicare population or who do not bill much to Medicare Part B will get a bit more breathing room to avoid having to participate in MACRA’s Quality Payment Program.
In a final rule posted Oct. 14 that sets out how the Medicare Access and CHIP Reauthorization Act (MACRA) will work, the Centers for Medicare & Medicaid Services increased the threshold for inclusion in the new value-based payment program from the initial proposal of physicians who bill Medicare more than $10,000 per year or treat more than 100 Medicare patients per year to those who bill more than $30,000 per year or provide care to more than 100 Medicare patients per year.
However, agency officials noted that it is committed to helping these small and solo practices become active participants in the Quality Payment Program.
“We heard these concerns and are taking additional steps to aid small practices, including reducing the time and cost to participate, excluding more small practices, increasing the availability of Advanced APMs [Alternative Payment Models] to small practices, allowing practices to begin participation at their own pace, changing one of the qualifications for participation in Advanced APMs to be practice-based as an alternative to total cost–based, and conducting significant technical support and outreach to small practices using $20 million a year over the next 5 years.”
CMS officials estimate that the new threshold will exclude an estimated 380,000 physicians and health care providers, up from about 225,000 under the initially proposed threshold.
Mr. Slavitt added that with these changes, “we estimate that small [practice] physicians will have the same level of participation as that of other practice sizes.”
The flexibility of participation was first announced Sept. 8, in a blog post outlining four options for participation in the Quality Payment Program:
• Option 1: Test the quality payment program in 2017 by submitting data without facing any negative payment adjustments. This will give physicians the year to make sure their processes are in place and ready for broader participation in 2018 and beyond.
• Option 2: Delay the start of the performance period and participate for just part of 2017. Depending on how long a physician delays reporting quality information back to CMS, they could still qualify for a smaller bonus payment.
• Option 3: Participate for the entire calendar year as called for by the law and be eligible for the full participation bonuses.
• Option 4: For those who qualify, participate in an Advanced Alternative Payment Model beginning next year.
That said, under the final rule, those who fail to do the bare minimum and report no data in 2017 will face a 4% pay cut in 2019.
“We are pleased CMS listened to the AGA’s concerns regarding the complexity of MACRA, its implementation, and its impact on small practices, among other issues,” said Timothy C. Wang, MD, AGAF, AGA Institute President, Columbia University, New York. “We believe that designation of 2017 as a transition year will allow GI’s the opportunity to understand the new value-based reimbursement system, and AGA stands ready to guide the GI community to survive and thrive in the new environment.”
“I am sure [the pay cuts are] going to impact some providers,” John Feore, director at Avalere Health, said in an interview. “But with the options, you can report on a very small number of measures, one for each of the categories, for a continuous 90-day period, and you will be sort of held harmless [and able] to transition over time into the program.”
Physician organizations were supportive of the final rule, particularly regarding how it addresses the concerns of small/solo practices.
CMS officials “took a significant step last month to address AMA concerns about the original proposal,” American Medical Association President Andrew W. Gurman, MD, said in a statement. “The final rule includes additional steps to help small and rural practices by raising the low-volume threshold exemption, and practices of all sizes will benefit from reduced MIPS reporting requirements. Our initial review indicates that CMS has been responsive to many concerns raised by the AMA.”
CMS officials said that the agency is looking into creating an accountable care organization (ACO) “Track 1 Plus” model that would qualify as an APM. Currently, ACOs that are in Track 1 share savings but do not assume risk. The agency said that the Track 1 Plus model would have organizations assuming some nominal level of risk that would be smaller, compared with those in the Medicare Shared Savings Program (MSSP) Track 2 and Track 3, as well as those that qualify as Next Generation ACOs. CMS plans to have the ACO Track 1 Plus Model ready for the 2018 reporting year.
Physicians who do not have a large Medicare population or who do not bill much to Medicare Part B will get a bit more breathing room to avoid having to participate in MACRA’s Quality Payment Program.
In a final rule posted Oct. 14 that sets out how the Medicare Access and CHIP Reauthorization Act (MACRA) will work, the Centers for Medicare & Medicaid Services increased the threshold for inclusion in the new value-based payment program from the initial proposal of physicians who bill Medicare more than $10,000 per year or treat more than 100 Medicare patients per year to those who bill more than $30,000 per year or provide care to more than 100 Medicare patients per year.
However, agency officials noted that it is committed to helping these small and solo practices become active participants in the Quality Payment Program.
“We heard these concerns and are taking additional steps to aid small practices, including reducing the time and cost to participate, excluding more small practices, increasing the availability of Advanced APMs [Alternative Payment Models] to small practices, allowing practices to begin participation at their own pace, changing one of the qualifications for participation in Advanced APMs to be practice-based as an alternative to total cost–based, and conducting significant technical support and outreach to small practices using $20 million a year over the next 5 years.”
CMS officials estimate that the new threshold will exclude an estimated 380,000 physicians and health care providers, up from about 225,000 under the initially proposed threshold.
Mr. Slavitt added that with these changes, “we estimate that small [practice] physicians will have the same level of participation as that of other practice sizes.”
The flexibility of participation was first announced Sept. 8, in a blog post outlining four options for participation in the Quality Payment Program:
• Option 1: Test the quality payment program in 2017 by submitting data without facing any negative payment adjustments. This will give physicians the year to make sure their processes are in place and ready for broader participation in 2018 and beyond.
• Option 2: Delay the start of the performance period and participate for just part of 2017. Depending on how long a physician delays reporting quality information back to CMS, they could still qualify for a smaller bonus payment.
• Option 3: Participate for the entire calendar year as called for by the law and be eligible for the full participation bonuses.
• Option 4: For those who qualify, participate in an Advanced Alternative Payment Model beginning next year.
That said, under the final rule, those who fail to do the bare minimum and report no data in 2017 will face a 4% pay cut in 2019.
“We are pleased CMS listened to the AGA’s concerns regarding the complexity of MACRA, its implementation, and its impact on small practices, among other issues,” said Timothy C. Wang, MD, AGAF, AGA Institute President, Columbia University, New York. “We believe that designation of 2017 as a transition year will allow GI’s the opportunity to understand the new value-based reimbursement system, and AGA stands ready to guide the GI community to survive and thrive in the new environment.”
“I am sure [the pay cuts are] going to impact some providers,” John Feore, director at Avalere Health, said in an interview. “But with the options, you can report on a very small number of measures, one for each of the categories, for a continuous 90-day period, and you will be sort of held harmless [and able] to transition over time into the program.”
Physician organizations were supportive of the final rule, particularly regarding how it addresses the concerns of small/solo practices.
CMS officials “took a significant step last month to address AMA concerns about the original proposal,” American Medical Association President Andrew W. Gurman, MD, said in a statement. “The final rule includes additional steps to help small and rural practices by raising the low-volume threshold exemption, and practices of all sizes will benefit from reduced MIPS reporting requirements. Our initial review indicates that CMS has been responsive to many concerns raised by the AMA.”
CMS officials said that the agency is looking into creating an accountable care organization (ACO) “Track 1 Plus” model that would qualify as an APM. Currently, ACOs that are in Track 1 share savings but do not assume risk. The agency said that the Track 1 Plus model would have organizations assuming some nominal level of risk that would be smaller, compared with those in the Medicare Shared Savings Program (MSSP) Track 2 and Track 3, as well as those that qualify as Next Generation ACOs. CMS plans to have the ACO Track 1 Plus Model ready for the 2018 reporting year.
Growth in hospital-employed physicians shows no signs of slowing
The parade of doctors leaving private practice for an employee position shows no sign of slowing.
In July 2012, an estimated 95,000 physicians (26%) were hospital employed. By July 2015, that number grew to 141,000 (38%), according to study conducted by Avalere for the Physicians Advocacy Institute. The biggest leap occurred between July 2014, when 114,000 physicians were listed as employees, to January 2015, when 133,000 were listed.
“They [most physicians] know a physician or two or a practice that has made that move to become employed, but I think many of them are quite surprised, if not shocked to see the tremendous transition over such a short period of time,” said Matthew Katz, PAI board member and CEO of the Connecticut State Medical Society.
Similarly, the number of hospital-owned physician practices grew from 36,000 (14%) in July 2012 to 67,000 (26%) in July 2015.
Regionally, by July 2015, almost half (49%) of Midwest physicians were hospital employed, while just over a quarter (27%) were in Alaska and Hawaii.
Mr. Katz said that he is hearing that the shift is having an impact on the practice of medicine. While certain aspects, such as physician autonomy, are among common issues raised with employment, he noted that referrals can also be an issue as doctors move from private practice to an employed status.
Many of the physicians he spoke with “have said that it is in some respects limiting them in what they can do and who they can refer to because they are losing their referral base in the community. When physicians become employed, they may no longer be accessible to those community-practicing physicians.”
He continued: “I have not talked to those physicians who have recently become employed to see what they think of their transition, but in talking to the physicians who remain in the community, they are concerned about the loss of those community physicians, the loss of the referral base, the loss of who to send their patients to who are no longer in the community and are now employed and working for the hospital. They seem to, in some respects, have lost touch with some of them once they transitioned to an employment status.”
Kelly Kenney, executive VP at PAI, said that there are some benefits to moving toward hospital-based employment, particularly with the reporting requirements attached to the many quality programs and the IT infrastructure that is a necessary part of it.
“Some physicians who have moved to employment say it just got to be too much,” Ms. Kenney said.
Mr. Katz added that private practice remains an option for most.
“We are still seeing some physicians, at least here in Connecticut and in other places around the country going into private practice trying to make a go of it,” he said. “I don’t think this is the death knell of private practice. I think that the pendulum definitely has swung in one direction, and I believe it will swing back a bit. I think that this will highlight what private practice physicians are facing and the barriers that exist today that did not exist a few years ago.”
He said that he hopes regulators, medical societies, and others step up in education efforts and other assistance to help small and solo practitioners navigate the value-based payment waters and give them the tools to stay in private practice.
Ms. Kenney noted that the flexibility of when physicians can start to meet reporting requirements under MACRA will help.
Mr. Katz added that the Department of Justice can do its part to help the small and solo practices by blocking the any of the mega-insurance mergers that are being proposed. He expressed concern that as insurers get bigger, the ability for small and solo practices to negotiate with them goes away and could be another driver to send physicians into employed situations.
The parade of doctors leaving private practice for an employee position shows no sign of slowing.
In July 2012, an estimated 95,000 physicians (26%) were hospital employed. By July 2015, that number grew to 141,000 (38%), according to study conducted by Avalere for the Physicians Advocacy Institute. The biggest leap occurred between July 2014, when 114,000 physicians were listed as employees, to January 2015, when 133,000 were listed.
“They [most physicians] know a physician or two or a practice that has made that move to become employed, but I think many of them are quite surprised, if not shocked to see the tremendous transition over such a short period of time,” said Matthew Katz, PAI board member and CEO of the Connecticut State Medical Society.
Similarly, the number of hospital-owned physician practices grew from 36,000 (14%) in July 2012 to 67,000 (26%) in July 2015.
Regionally, by July 2015, almost half (49%) of Midwest physicians were hospital employed, while just over a quarter (27%) were in Alaska and Hawaii.
Mr. Katz said that he is hearing that the shift is having an impact on the practice of medicine. While certain aspects, such as physician autonomy, are among common issues raised with employment, he noted that referrals can also be an issue as doctors move from private practice to an employed status.
Many of the physicians he spoke with “have said that it is in some respects limiting them in what they can do and who they can refer to because they are losing their referral base in the community. When physicians become employed, they may no longer be accessible to those community-practicing physicians.”
He continued: “I have not talked to those physicians who have recently become employed to see what they think of their transition, but in talking to the physicians who remain in the community, they are concerned about the loss of those community physicians, the loss of the referral base, the loss of who to send their patients to who are no longer in the community and are now employed and working for the hospital. They seem to, in some respects, have lost touch with some of them once they transitioned to an employment status.”
Kelly Kenney, executive VP at PAI, said that there are some benefits to moving toward hospital-based employment, particularly with the reporting requirements attached to the many quality programs and the IT infrastructure that is a necessary part of it.
“Some physicians who have moved to employment say it just got to be too much,” Ms. Kenney said.
Mr. Katz added that private practice remains an option for most.
“We are still seeing some physicians, at least here in Connecticut and in other places around the country going into private practice trying to make a go of it,” he said. “I don’t think this is the death knell of private practice. I think that the pendulum definitely has swung in one direction, and I believe it will swing back a bit. I think that this will highlight what private practice physicians are facing and the barriers that exist today that did not exist a few years ago.”
He said that he hopes regulators, medical societies, and others step up in education efforts and other assistance to help small and solo practitioners navigate the value-based payment waters and give them the tools to stay in private practice.
Ms. Kenney noted that the flexibility of when physicians can start to meet reporting requirements under MACRA will help.
Mr. Katz added that the Department of Justice can do its part to help the small and solo practices by blocking the any of the mega-insurance mergers that are being proposed. He expressed concern that as insurers get bigger, the ability for small and solo practices to negotiate with them goes away and could be another driver to send physicians into employed situations.
The parade of doctors leaving private practice for an employee position shows no sign of slowing.
In July 2012, an estimated 95,000 physicians (26%) were hospital employed. By July 2015, that number grew to 141,000 (38%), according to study conducted by Avalere for the Physicians Advocacy Institute. The biggest leap occurred between July 2014, when 114,000 physicians were listed as employees, to January 2015, when 133,000 were listed.
“They [most physicians] know a physician or two or a practice that has made that move to become employed, but I think many of them are quite surprised, if not shocked to see the tremendous transition over such a short period of time,” said Matthew Katz, PAI board member and CEO of the Connecticut State Medical Society.
Similarly, the number of hospital-owned physician practices grew from 36,000 (14%) in July 2012 to 67,000 (26%) in July 2015.
Regionally, by July 2015, almost half (49%) of Midwest physicians were hospital employed, while just over a quarter (27%) were in Alaska and Hawaii.
Mr. Katz said that he is hearing that the shift is having an impact on the practice of medicine. While certain aspects, such as physician autonomy, are among common issues raised with employment, he noted that referrals can also be an issue as doctors move from private practice to an employed status.
Many of the physicians he spoke with “have said that it is in some respects limiting them in what they can do and who they can refer to because they are losing their referral base in the community. When physicians become employed, they may no longer be accessible to those community-practicing physicians.”
He continued: “I have not talked to those physicians who have recently become employed to see what they think of their transition, but in talking to the physicians who remain in the community, they are concerned about the loss of those community physicians, the loss of the referral base, the loss of who to send their patients to who are no longer in the community and are now employed and working for the hospital. They seem to, in some respects, have lost touch with some of them once they transitioned to an employment status.”
Kelly Kenney, executive VP at PAI, said that there are some benefits to moving toward hospital-based employment, particularly with the reporting requirements attached to the many quality programs and the IT infrastructure that is a necessary part of it.
“Some physicians who have moved to employment say it just got to be too much,” Ms. Kenney said.
Mr. Katz added that private practice remains an option for most.
“We are still seeing some physicians, at least here in Connecticut and in other places around the country going into private practice trying to make a go of it,” he said. “I don’t think this is the death knell of private practice. I think that the pendulum definitely has swung in one direction, and I believe it will swing back a bit. I think that this will highlight what private practice physicians are facing and the barriers that exist today that did not exist a few years ago.”
He said that he hopes regulators, medical societies, and others step up in education efforts and other assistance to help small and solo practitioners navigate the value-based payment waters and give them the tools to stay in private practice.
Ms. Kenney noted that the flexibility of when physicians can start to meet reporting requirements under MACRA will help.
Mr. Katz added that the Department of Justice can do its part to help the small and solo practices by blocking the any of the mega-insurance mergers that are being proposed. He expressed concern that as insurers get bigger, the ability for small and solo practices to negotiate with them goes away and could be another driver to send physicians into employed situations.
Medicare subsidies eliminate disparities in adherence to hormonal
The low-income subsidy in Medicare Part D is helping to eliminate racial disparities in adherence to hormonal therapy among breast cancer patients.
Investigators identified a nationwide cohort of 25,111 Medicare D enrollees with a breast cancer operation between 2006 and 2007 and at least one prescription filled for oral breast cancer hormonal therapy. Women not receiving the low-income subsidy “were 60%-200% more likely to discontinue hormonal therapy in the first 35 months, compared with low-income subsidy recipients of the same race or ethnicity,” reported Alana Biggers, MD, of University of Illinois at Chicago, and her colleagues, in the Journal of Clinical Oncology.
The study found that overall, it took 14 months for 25% of the cohort to discontinue medication (unadjusted). When the researchers broke it down by race/ethnicity, they found that it took 12 months for 25% of unsubsidized whites to discontinue medication, compared with 24 months for subsidized whites. For unsubsidized blacks, it was 9 months, compared with 24 months for subsidized blacks. For unsubsidized Hispanics, it was 10 months vs. 29 months for subsidized Hispanics.
“Our findings regarding the relatively large difference in persistence in adherence between subsidized and unsubsidized women are consistent with large reductions in out-of-pocket costs for aromatase inhibitors with the subsidy,” the authors state.
More effort should be placed on ensuring that women eligible for the low-income subsidy are enrolled, and legislative and advocacy efforts for those outside of Medicare “should focus on lowering out-of-pocket costs for younger women. Given the high costs of oral oncologic and supportive medications, the impact on disparities of other initiatives to reduce out-of-pocket costs deserves urgent study,” they said.
The authors reported no relevant conflicts of interest related to this study, which was supported by grants from the National Institutes of Health and the American Cancer Society.
The low-income subsidy in Medicare Part D is helping to eliminate racial disparities in adherence to hormonal therapy among breast cancer patients.
Investigators identified a nationwide cohort of 25,111 Medicare D enrollees with a breast cancer operation between 2006 and 2007 and at least one prescription filled for oral breast cancer hormonal therapy. Women not receiving the low-income subsidy “were 60%-200% more likely to discontinue hormonal therapy in the first 35 months, compared with low-income subsidy recipients of the same race or ethnicity,” reported Alana Biggers, MD, of University of Illinois at Chicago, and her colleagues, in the Journal of Clinical Oncology.
The study found that overall, it took 14 months for 25% of the cohort to discontinue medication (unadjusted). When the researchers broke it down by race/ethnicity, they found that it took 12 months for 25% of unsubsidized whites to discontinue medication, compared with 24 months for subsidized whites. For unsubsidized blacks, it was 9 months, compared with 24 months for subsidized blacks. For unsubsidized Hispanics, it was 10 months vs. 29 months for subsidized Hispanics.
“Our findings regarding the relatively large difference in persistence in adherence between subsidized and unsubsidized women are consistent with large reductions in out-of-pocket costs for aromatase inhibitors with the subsidy,” the authors state.
More effort should be placed on ensuring that women eligible for the low-income subsidy are enrolled, and legislative and advocacy efforts for those outside of Medicare “should focus on lowering out-of-pocket costs for younger women. Given the high costs of oral oncologic and supportive medications, the impact on disparities of other initiatives to reduce out-of-pocket costs deserves urgent study,” they said.
The authors reported no relevant conflicts of interest related to this study, which was supported by grants from the National Institutes of Health and the American Cancer Society.
The low-income subsidy in Medicare Part D is helping to eliminate racial disparities in adherence to hormonal therapy among breast cancer patients.
Investigators identified a nationwide cohort of 25,111 Medicare D enrollees with a breast cancer operation between 2006 and 2007 and at least one prescription filled for oral breast cancer hormonal therapy. Women not receiving the low-income subsidy “were 60%-200% more likely to discontinue hormonal therapy in the first 35 months, compared with low-income subsidy recipients of the same race or ethnicity,” reported Alana Biggers, MD, of University of Illinois at Chicago, and her colleagues, in the Journal of Clinical Oncology.
The study found that overall, it took 14 months for 25% of the cohort to discontinue medication (unadjusted). When the researchers broke it down by race/ethnicity, they found that it took 12 months for 25% of unsubsidized whites to discontinue medication, compared with 24 months for subsidized whites. For unsubsidized blacks, it was 9 months, compared with 24 months for subsidized blacks. For unsubsidized Hispanics, it was 10 months vs. 29 months for subsidized Hispanics.
“Our findings regarding the relatively large difference in persistence in adherence between subsidized and unsubsidized women are consistent with large reductions in out-of-pocket costs for aromatase inhibitors with the subsidy,” the authors state.
More effort should be placed on ensuring that women eligible for the low-income subsidy are enrolled, and legislative and advocacy efforts for those outside of Medicare “should focus on lowering out-of-pocket costs for younger women. Given the high costs of oral oncologic and supportive medications, the impact on disparities of other initiatives to reduce out-of-pocket costs deserves urgent study,” they said.
The authors reported no relevant conflicts of interest related to this study, which was supported by grants from the National Institutes of Health and the American Cancer Society.