User login
Analysis: CMS expects no MACRA pay cut for most small practices
Most small practices will see no change in their Medicare payments or perhaps even a bonus for participating in MACRA’s new Quality Payment Program (QPP) beginning in 2017.
Contrary to the initial proposed regulations from the Centers for Medicare & Medicaid Services, analysis of the final regulations shows that 90% of practices with one to nine clinicians should see no impact or even a pay increase in 2019 if they start participating in QPP next year, according to an agency analysis. The only way to fare worse would be to completely opt out of the program.
The sea change is based on two modifications to the proposed rule, according to Walter Gorski, director of regulatory affairs at the American College of Physicians. The first is the new flexibility for QPP participation for 2017. In September, CMS announced that any level of participation in the Merit-Based Incentive Payment System (MIPS) – from reporting “some data” as a low-level test to full participation in an Advanced Alternative Payment Model – would result in no penalty or pay cut for physicians and other health care providers.
The second key is the higher threshold for exemption from reporting requirements. Under the proposed rule, providers who receive Medicare payments of more than $10,000 for 100 and see fewer Medicare patients had to participate; the final rule raises that to $30,000 or more or 100 or fewer Medicare patients. Mr. Gorski said this could result in 30% of physicians being exempt and therefore not having to face the penalties associated with the program.
In comparison, CMS expect 98.5% of practices with more than 100 clinicians to receive either a bonus payment or no bonus/penalty.
Most small practices will see no change in their Medicare payments or perhaps even a bonus for participating in MACRA’s new Quality Payment Program (QPP) beginning in 2017.
Contrary to the initial proposed regulations from the Centers for Medicare & Medicaid Services, analysis of the final regulations shows that 90% of practices with one to nine clinicians should see no impact or even a pay increase in 2019 if they start participating in QPP next year, according to an agency analysis. The only way to fare worse would be to completely opt out of the program.
The sea change is based on two modifications to the proposed rule, according to Walter Gorski, director of regulatory affairs at the American College of Physicians. The first is the new flexibility for QPP participation for 2017. In September, CMS announced that any level of participation in the Merit-Based Incentive Payment System (MIPS) – from reporting “some data” as a low-level test to full participation in an Advanced Alternative Payment Model – would result in no penalty or pay cut for physicians and other health care providers.
The second key is the higher threshold for exemption from reporting requirements. Under the proposed rule, providers who receive Medicare payments of more than $10,000 for 100 and see fewer Medicare patients had to participate; the final rule raises that to $30,000 or more or 100 or fewer Medicare patients. Mr. Gorski said this could result in 30% of physicians being exempt and therefore not having to face the penalties associated with the program.
In comparison, CMS expect 98.5% of practices with more than 100 clinicians to receive either a bonus payment or no bonus/penalty.
Most small practices will see no change in their Medicare payments or perhaps even a bonus for participating in MACRA’s new Quality Payment Program (QPP) beginning in 2017.
Contrary to the initial proposed regulations from the Centers for Medicare & Medicaid Services, analysis of the final regulations shows that 90% of practices with one to nine clinicians should see no impact or even a pay increase in 2019 if they start participating in QPP next year, according to an agency analysis. The only way to fare worse would be to completely opt out of the program.
The sea change is based on two modifications to the proposed rule, according to Walter Gorski, director of regulatory affairs at the American College of Physicians. The first is the new flexibility for QPP participation for 2017. In September, CMS announced that any level of participation in the Merit-Based Incentive Payment System (MIPS) – from reporting “some data” as a low-level test to full participation in an Advanced Alternative Payment Model – would result in no penalty or pay cut for physicians and other health care providers.
The second key is the higher threshold for exemption from reporting requirements. Under the proposed rule, providers who receive Medicare payments of more than $10,000 for 100 and see fewer Medicare patients had to participate; the final rule raises that to $30,000 or more or 100 or fewer Medicare patients. Mr. Gorski said this could result in 30% of physicians being exempt and therefore not having to face the penalties associated with the program.
In comparison, CMS expect 98.5% of practices with more than 100 clinicians to receive either a bonus payment or no bonus/penalty.
Access issues looming as more docs eye exit from clinical practice
Almost half (47.8%) of physicians surveyed are considering a change in how they practice medicine within the next 1-3 years, including cutting back on hours, retiring, switching to cash/concierge practice, working locum tenens, seeking a nonclinical health care job, seeking employment, or working part time.
That’s according to responses to the 2016 Survey of America’s Physicians Practice Patterns & Perspectives, a biennial survey commissioned by The Physicians Foundation and conducted by Merritt Hawkins. A total of 17,236 physicians responded to the survey.
The percentage looking to change is up from 43.6% in the 2014 survey, but down from the 50.2% of physicians who responded the same way in 2012.
Dr. Ray pointed to a number of findings in the survey that could be driving the growth in those looking to make a change.
“If you think about the last year, the clinical, administrative, and financial changes that have occurred have occurred at a whirlwind pace,” Dr. Ray said. “For instance, continued expansion of the Affordable Care Act has included as many as 20 million new people to the rolls at the same time we have a documented physician shortage where 80% of physicians say that they have all the patients that they can take care of. They are either at capacity or they are overextended.”
Other factors influencing physicians’ employment decisions include the transition to value-based payments under the Medicare Access and CHIP Reauthorization Act (MACRA), the transition to ICD-10, the growth of physician employment, the continued sale of private practices to hospitals and health systems, and the “businessification” of heath care.
“If any of these [changes] occurred in a period of time, it would be impactful,” he said. “But to have all occur simultaneously, we say now that to be a physician is to feel the ground shaking under your feet. This is the landscape in which the survey was taken.”
Given the volume of change, it is no surprise that physician morale is low, Dr. Ray said. “One of the first things that jumps out is morale. The question was ‘what best describes your professional morale about your current feelings and the current state of the medical profession?’ and 54% were somewhat or very negative; 46% were positive/somewhat positive/very positive. The very negatives were twice what the very positives were,” he noted.
Inadequate time to deliver quality care also was cited.
“Only 14% [of respondents] said that they have all the time that they need to provide the highest standards of care; 86% said that their time was either always limited, often limited, or sometimes limited,” Dr. Ray said.
Dr. Ray highlighted another question that provides insight into physicians’ dissatisfaction with their practice environment.
The survey asked, “To what degree is patient care in your practice adversely impacted by external factors such as third party authorizations, treatment protocols, EHR design, etc.”
“The critical term here is ‘adversely impacted,’ not just influenced, but adversely impacted,” Dr. Ray said. “Only 2.3% said not at all; 10% total said either not at all or a little bit. The rest said either somewhat, a good deal, or a great deal. In fact, 72% said a good deal or a great deal.”
All of this is leading to physician burnout, he said.
“I think it all bounces back to interference with the doctor/patient relationship and the fact that [physicians] feel like there are distractions within the practice of medicine due to the regulatory environment,” he said “They are not allowed to make decisions like they want to make in the best interest of their patients based on their training and their studying and their special experience with that patient. It creates this barrier with this professional satisfaction that they want to receive from taking care of patients.”
Almost half (47.8%) of physicians surveyed are considering a change in how they practice medicine within the next 1-3 years, including cutting back on hours, retiring, switching to cash/concierge practice, working locum tenens, seeking a nonclinical health care job, seeking employment, or working part time.
That’s according to responses to the 2016 Survey of America’s Physicians Practice Patterns & Perspectives, a biennial survey commissioned by The Physicians Foundation and conducted by Merritt Hawkins. A total of 17,236 physicians responded to the survey.
The percentage looking to change is up from 43.6% in the 2014 survey, but down from the 50.2% of physicians who responded the same way in 2012.
Dr. Ray pointed to a number of findings in the survey that could be driving the growth in those looking to make a change.
“If you think about the last year, the clinical, administrative, and financial changes that have occurred have occurred at a whirlwind pace,” Dr. Ray said. “For instance, continued expansion of the Affordable Care Act has included as many as 20 million new people to the rolls at the same time we have a documented physician shortage where 80% of physicians say that they have all the patients that they can take care of. They are either at capacity or they are overextended.”
Other factors influencing physicians’ employment decisions include the transition to value-based payments under the Medicare Access and CHIP Reauthorization Act (MACRA), the transition to ICD-10, the growth of physician employment, the continued sale of private practices to hospitals and health systems, and the “businessification” of heath care.
“If any of these [changes] occurred in a period of time, it would be impactful,” he said. “But to have all occur simultaneously, we say now that to be a physician is to feel the ground shaking under your feet. This is the landscape in which the survey was taken.”
Given the volume of change, it is no surprise that physician morale is low, Dr. Ray said. “One of the first things that jumps out is morale. The question was ‘what best describes your professional morale about your current feelings and the current state of the medical profession?’ and 54% were somewhat or very negative; 46% were positive/somewhat positive/very positive. The very negatives were twice what the very positives were,” he noted.
Inadequate time to deliver quality care also was cited.
“Only 14% [of respondents] said that they have all the time that they need to provide the highest standards of care; 86% said that their time was either always limited, often limited, or sometimes limited,” Dr. Ray said.
Dr. Ray highlighted another question that provides insight into physicians’ dissatisfaction with their practice environment.
The survey asked, “To what degree is patient care in your practice adversely impacted by external factors such as third party authorizations, treatment protocols, EHR design, etc.”
“The critical term here is ‘adversely impacted,’ not just influenced, but adversely impacted,” Dr. Ray said. “Only 2.3% said not at all; 10% total said either not at all or a little bit. The rest said either somewhat, a good deal, or a great deal. In fact, 72% said a good deal or a great deal.”
All of this is leading to physician burnout, he said.
“I think it all bounces back to interference with the doctor/patient relationship and the fact that [physicians] feel like there are distractions within the practice of medicine due to the regulatory environment,” he said “They are not allowed to make decisions like they want to make in the best interest of their patients based on their training and their studying and their special experience with that patient. It creates this barrier with this professional satisfaction that they want to receive from taking care of patients.”
Almost half (47.8%) of physicians surveyed are considering a change in how they practice medicine within the next 1-3 years, including cutting back on hours, retiring, switching to cash/concierge practice, working locum tenens, seeking a nonclinical health care job, seeking employment, or working part time.
That’s according to responses to the 2016 Survey of America’s Physicians Practice Patterns & Perspectives, a biennial survey commissioned by The Physicians Foundation and conducted by Merritt Hawkins. A total of 17,236 physicians responded to the survey.
The percentage looking to change is up from 43.6% in the 2014 survey, but down from the 50.2% of physicians who responded the same way in 2012.
Dr. Ray pointed to a number of findings in the survey that could be driving the growth in those looking to make a change.
“If you think about the last year, the clinical, administrative, and financial changes that have occurred have occurred at a whirlwind pace,” Dr. Ray said. “For instance, continued expansion of the Affordable Care Act has included as many as 20 million new people to the rolls at the same time we have a documented physician shortage where 80% of physicians say that they have all the patients that they can take care of. They are either at capacity or they are overextended.”
Other factors influencing physicians’ employment decisions include the transition to value-based payments under the Medicare Access and CHIP Reauthorization Act (MACRA), the transition to ICD-10, the growth of physician employment, the continued sale of private practices to hospitals and health systems, and the “businessification” of heath care.
“If any of these [changes] occurred in a period of time, it would be impactful,” he said. “But to have all occur simultaneously, we say now that to be a physician is to feel the ground shaking under your feet. This is the landscape in which the survey was taken.”
Given the volume of change, it is no surprise that physician morale is low, Dr. Ray said. “One of the first things that jumps out is morale. The question was ‘what best describes your professional morale about your current feelings and the current state of the medical profession?’ and 54% were somewhat or very negative; 46% were positive/somewhat positive/very positive. The very negatives were twice what the very positives were,” he noted.
Inadequate time to deliver quality care also was cited.
“Only 14% [of respondents] said that they have all the time that they need to provide the highest standards of care; 86% said that their time was either always limited, often limited, or sometimes limited,” Dr. Ray said.
Dr. Ray highlighted another question that provides insight into physicians’ dissatisfaction with their practice environment.
The survey asked, “To what degree is patient care in your practice adversely impacted by external factors such as third party authorizations, treatment protocols, EHR design, etc.”
“The critical term here is ‘adversely impacted,’ not just influenced, but adversely impacted,” Dr. Ray said. “Only 2.3% said not at all; 10% total said either not at all or a little bit. The rest said either somewhat, a good deal, or a great deal. In fact, 72% said a good deal or a great deal.”
All of this is leading to physician burnout, he said.
“I think it all bounces back to interference with the doctor/patient relationship and the fact that [physicians] feel like there are distractions within the practice of medicine due to the regulatory environment,” he said “They are not allowed to make decisions like they want to make in the best interest of their patients based on their training and their studying and their special experience with that patient. It creates this barrier with this professional satisfaction that they want to receive from taking care of patients.”
CMS offering educational webinars on MACRA
The Centers for Medicare & Medicaid Services is offering a pair of webinars aimed at helping physicians navigate the new regulation that operationalizes the Medicare Access and CHIP Reauthorization Act (MACRA).
The first webinar, scheduled for Oct. 26, will provide an overview of the two components of the Quality Payment Program – the Merit-Based Incentive Payment System (MIPS) and advanced Alternative Payment Models (APMs).
The second webinar, scheduled for Nov. 15, is targeted to Medicare Part B fee-for-service clinicians, office managers and administrators, state and national associations that represent health care providers, and other stakeholders and will feature a question-and-answer session.
The webinars are part of the agency’s ongoing efforts to help educate practitioners on the provisions of the final MACRA regulation, which was issued on Oct. 14. CMS also recently launched a website to help in that regard.
The Centers for Medicare & Medicaid Services is offering a pair of webinars aimed at helping physicians navigate the new regulation that operationalizes the Medicare Access and CHIP Reauthorization Act (MACRA).
The first webinar, scheduled for Oct. 26, will provide an overview of the two components of the Quality Payment Program – the Merit-Based Incentive Payment System (MIPS) and advanced Alternative Payment Models (APMs).
The second webinar, scheduled for Nov. 15, is targeted to Medicare Part B fee-for-service clinicians, office managers and administrators, state and national associations that represent health care providers, and other stakeholders and will feature a question-and-answer session.
The webinars are part of the agency’s ongoing efforts to help educate practitioners on the provisions of the final MACRA regulation, which was issued on Oct. 14. CMS also recently launched a website to help in that regard.
The Centers for Medicare & Medicaid Services is offering a pair of webinars aimed at helping physicians navigate the new regulation that operationalizes the Medicare Access and CHIP Reauthorization Act (MACRA).
The first webinar, scheduled for Oct. 26, will provide an overview of the two components of the Quality Payment Program – the Merit-Based Incentive Payment System (MIPS) and advanced Alternative Payment Models (APMs).
The second webinar, scheduled for Nov. 15, is targeted to Medicare Part B fee-for-service clinicians, office managers and administrators, state and national associations that represent health care providers, and other stakeholders and will feature a question-and-answer session.
The webinars are part of the agency’s ongoing efforts to help educate practitioners on the provisions of the final MACRA regulation, which was issued on Oct. 14. CMS also recently launched a website to help in that regard.
MACRA final rule exempts many more doctors
Physicians who do not have a large Medicare population or who do not bill much to Medicare Part B will get a bit more breathing room to avoid having to participate in MACRA’s Quality Payment Program.
In a final rule posted Oct. 14 that sets out how the Medicare Access and CHIP Reauthorization Act (MACRA) will work, the Centers for Medicare & Medicaid Services increased the threshold for inclusion in the new value-based payment program from the initial proposal of physicians who bill Medicare more than $10,000 per year or treat more than 100 Medicare patients per year to those who bill more than $30,000 per year or provide care to more than 100 Medicare patients per year.
However, agency officials noted that it is committed to helping these small and solo practices become active participants in the Quality Payment Program.
“We heard these concerns and are taking additional steps to aid small practices, including reducing the time and cost to participate, excluding more small practices, increasing the availability of Advanced APMs [Alternative Payment Models] to small practices, allowing practices to begin participation at their own pace, changing one of the qualifications for participation in Advanced APMs to be practice-based as an alternative to total cost–based, and conducting significant technical support and outreach to small practices using $20 million a year over the next 5 years.”
CMS officials estimate that the new threshold will exclude an estimated 380,000 physicians and health care providers, up from about 225,000 under the initially proposed threshold.
Mr. Slavitt added that with these changes, “we estimate that small physicians will have the same level of participation as that of other practice sizes.”
The flexibility of participation was first announced Sept. 8, in a blog post outlining four options for participation in the Quality Payment Program:
• Option 1: Test the quality payment program in 2017 by submitting data without facing any negative payment adjustments. This will give physicians the year to make sure their processes are in place and ready for broader participation in 2018 and beyond.
• Option 2: Delay the start of the performance period and participate for just part of 2017. Depending on how long a physician delays reporting quality information back to CMS, they could still qualify for a smaller bonus payment.
• Option 3: Participate for the entire calendar year as called for by the law and be eligible for the full participation bonuses.
• Option 4: For those who qualify, participate in an Advanced Alternative Payment Model beginning next year.
That said, under the final rule, those who fail to do the bare minimum and report no data in 2017 will face a 4% pay cut in 2019.
“I am sure that is going to impact some providers,” John Feore, director at Avalere Health, said in an interview. “But with the options, you can report on a very small number of measures, one for each of the categories, for a continuous 90-day period and you will be sort of held harmless [and able] to transition over time into the program.”
Mr. Feore said that did not see any surprises in his initial quick scan of the final rule and that he views the increased flexibility as positive.
“CMS is understanding that MACRA is a pretty substantial change,” he said. “They are calling [2017] a transition year. They are even referring to 2018 as a transition year with more details to come. They are responding to stakeholder concerns that it was a little too much too soon and there is varying degrees of readiness.”
Physician organizations were supportive of the final rule, particularly regarding how it addresses the concerns of small/solo practices.
CMS officials “took a significant step last month to address AMA concerns about the original proposal,” American Medical Association President Andrew W. Gurman, MD, said in a statement. “The final rule includes additional steps to help small and rural practices by raising the low-volume threshold exemption, and practices of all sizes will benefit from reduced MIPS reporting requirements. Our initial review indicates that CMS has been responsive to many concerns raised by the AMA.”
American College of Cardiology President Richard A. Chazal, MD, said in a statement that the organization is “encouraged to see that CMS has made several changes in the final rule based on comments by the clinician community.”
The American College of Rheumatology also expressed support.
“Giving providers the flexibility of multiple options for participation in the first and second years will help ensure a smooth transition to the new payment system, and the continued delivery of quality care to Medicare patients living with rheumatic diseases,” the organization said in a statement. “We also appreciated the broadening of exemptions from the program, which will help to protect small practices that already struggle to keep up with administrative burdens, along with the reduction in the number of required measures to be reported.”
The American Osteopathic Association applauded the flexibility being offered, but found it “disappointing that many of those currently in patient-centered medical homes will still not qualify for [APMs] and opportunities to enter other such value-based models remain limited.”
To that end, CMS officials said that the agency is looking into creating an accountable care organization (ACO) “Track 1 Plus” model that would qualify for as an APM. Currently, ACOs that are in Track 1 share savings but do not assume risk. The agency said that the Track 1 Plus model would have organizations assuming some nominal level of risk that would be smaller, compared with those in the Medicare Shared Savings Program (MSSP) Track 2 and Track 3, as well as those that qualify as Next Generation ACOs. CMS plans to have the ACO Track 1 Plus Model ready for the 2018 reporting year.
The National Association of ACOs expressed disappointment that those ACOs that fall in the Track 1 of the MSSP do not qualify as an APM, but it is “incredibly pleased that CMS recognizes the need for a new model and is taking steps to develop a new MSSP Track 1 Plus,” President and CEO Clif Gaus said in a statement.
More CMS-issued information and educational material about the MACRA final rule can be found here.
Physicians who do not have a large Medicare population or who do not bill much to Medicare Part B will get a bit more breathing room to avoid having to participate in MACRA’s Quality Payment Program.
In a final rule posted Oct. 14 that sets out how the Medicare Access and CHIP Reauthorization Act (MACRA) will work, the Centers for Medicare & Medicaid Services increased the threshold for inclusion in the new value-based payment program from the initial proposal of physicians who bill Medicare more than $10,000 per year or treat more than 100 Medicare patients per year to those who bill more than $30,000 per year or provide care to more than 100 Medicare patients per year.
However, agency officials noted that it is committed to helping these small and solo practices become active participants in the Quality Payment Program.
“We heard these concerns and are taking additional steps to aid small practices, including reducing the time and cost to participate, excluding more small practices, increasing the availability of Advanced APMs [Alternative Payment Models] to small practices, allowing practices to begin participation at their own pace, changing one of the qualifications for participation in Advanced APMs to be practice-based as an alternative to total cost–based, and conducting significant technical support and outreach to small practices using $20 million a year over the next 5 years.”
CMS officials estimate that the new threshold will exclude an estimated 380,000 physicians and health care providers, up from about 225,000 under the initially proposed threshold.
Mr. Slavitt added that with these changes, “we estimate that small physicians will have the same level of participation as that of other practice sizes.”
The flexibility of participation was first announced Sept. 8, in a blog post outlining four options for participation in the Quality Payment Program:
• Option 1: Test the quality payment program in 2017 by submitting data without facing any negative payment adjustments. This will give physicians the year to make sure their processes are in place and ready for broader participation in 2018 and beyond.
• Option 2: Delay the start of the performance period and participate for just part of 2017. Depending on how long a physician delays reporting quality information back to CMS, they could still qualify for a smaller bonus payment.
• Option 3: Participate for the entire calendar year as called for by the law and be eligible for the full participation bonuses.
• Option 4: For those who qualify, participate in an Advanced Alternative Payment Model beginning next year.
That said, under the final rule, those who fail to do the bare minimum and report no data in 2017 will face a 4% pay cut in 2019.
“I am sure that is going to impact some providers,” John Feore, director at Avalere Health, said in an interview. “But with the options, you can report on a very small number of measures, one for each of the categories, for a continuous 90-day period and you will be sort of held harmless [and able] to transition over time into the program.”
Mr. Feore said that did not see any surprises in his initial quick scan of the final rule and that he views the increased flexibility as positive.
“CMS is understanding that MACRA is a pretty substantial change,” he said. “They are calling [2017] a transition year. They are even referring to 2018 as a transition year with more details to come. They are responding to stakeholder concerns that it was a little too much too soon and there is varying degrees of readiness.”
Physician organizations were supportive of the final rule, particularly regarding how it addresses the concerns of small/solo practices.
CMS officials “took a significant step last month to address AMA concerns about the original proposal,” American Medical Association President Andrew W. Gurman, MD, said in a statement. “The final rule includes additional steps to help small and rural practices by raising the low-volume threshold exemption, and practices of all sizes will benefit from reduced MIPS reporting requirements. Our initial review indicates that CMS has been responsive to many concerns raised by the AMA.”
American College of Cardiology President Richard A. Chazal, MD, said in a statement that the organization is “encouraged to see that CMS has made several changes in the final rule based on comments by the clinician community.”
The American College of Rheumatology also expressed support.
“Giving providers the flexibility of multiple options for participation in the first and second years will help ensure a smooth transition to the new payment system, and the continued delivery of quality care to Medicare patients living with rheumatic diseases,” the organization said in a statement. “We also appreciated the broadening of exemptions from the program, which will help to protect small practices that already struggle to keep up with administrative burdens, along with the reduction in the number of required measures to be reported.”
The American Osteopathic Association applauded the flexibility being offered, but found it “disappointing that many of those currently in patient-centered medical homes will still not qualify for [APMs] and opportunities to enter other such value-based models remain limited.”
To that end, CMS officials said that the agency is looking into creating an accountable care organization (ACO) “Track 1 Plus” model that would qualify for as an APM. Currently, ACOs that are in Track 1 share savings but do not assume risk. The agency said that the Track 1 Plus model would have organizations assuming some nominal level of risk that would be smaller, compared with those in the Medicare Shared Savings Program (MSSP) Track 2 and Track 3, as well as those that qualify as Next Generation ACOs. CMS plans to have the ACO Track 1 Plus Model ready for the 2018 reporting year.
The National Association of ACOs expressed disappointment that those ACOs that fall in the Track 1 of the MSSP do not qualify as an APM, but it is “incredibly pleased that CMS recognizes the need for a new model and is taking steps to develop a new MSSP Track 1 Plus,” President and CEO Clif Gaus said in a statement.
More CMS-issued information and educational material about the MACRA final rule can be found here.
Physicians who do not have a large Medicare population or who do not bill much to Medicare Part B will get a bit more breathing room to avoid having to participate in MACRA’s Quality Payment Program.
In a final rule posted Oct. 14 that sets out how the Medicare Access and CHIP Reauthorization Act (MACRA) will work, the Centers for Medicare & Medicaid Services increased the threshold for inclusion in the new value-based payment program from the initial proposal of physicians who bill Medicare more than $10,000 per year or treat more than 100 Medicare patients per year to those who bill more than $30,000 per year or provide care to more than 100 Medicare patients per year.
However, agency officials noted that it is committed to helping these small and solo practices become active participants in the Quality Payment Program.
“We heard these concerns and are taking additional steps to aid small practices, including reducing the time and cost to participate, excluding more small practices, increasing the availability of Advanced APMs [Alternative Payment Models] to small practices, allowing practices to begin participation at their own pace, changing one of the qualifications for participation in Advanced APMs to be practice-based as an alternative to total cost–based, and conducting significant technical support and outreach to small practices using $20 million a year over the next 5 years.”
CMS officials estimate that the new threshold will exclude an estimated 380,000 physicians and health care providers, up from about 225,000 under the initially proposed threshold.
Mr. Slavitt added that with these changes, “we estimate that small physicians will have the same level of participation as that of other practice sizes.”
The flexibility of participation was first announced Sept. 8, in a blog post outlining four options for participation in the Quality Payment Program:
• Option 1: Test the quality payment program in 2017 by submitting data without facing any negative payment adjustments. This will give physicians the year to make sure their processes are in place and ready for broader participation in 2018 and beyond.
• Option 2: Delay the start of the performance period and participate for just part of 2017. Depending on how long a physician delays reporting quality information back to CMS, they could still qualify for a smaller bonus payment.
• Option 3: Participate for the entire calendar year as called for by the law and be eligible for the full participation bonuses.
• Option 4: For those who qualify, participate in an Advanced Alternative Payment Model beginning next year.
That said, under the final rule, those who fail to do the bare minimum and report no data in 2017 will face a 4% pay cut in 2019.
“I am sure that is going to impact some providers,” John Feore, director at Avalere Health, said in an interview. “But with the options, you can report on a very small number of measures, one for each of the categories, for a continuous 90-day period and you will be sort of held harmless [and able] to transition over time into the program.”
Mr. Feore said that did not see any surprises in his initial quick scan of the final rule and that he views the increased flexibility as positive.
“CMS is understanding that MACRA is a pretty substantial change,” he said. “They are calling [2017] a transition year. They are even referring to 2018 as a transition year with more details to come. They are responding to stakeholder concerns that it was a little too much too soon and there is varying degrees of readiness.”
Physician organizations were supportive of the final rule, particularly regarding how it addresses the concerns of small/solo practices.
CMS officials “took a significant step last month to address AMA concerns about the original proposal,” American Medical Association President Andrew W. Gurman, MD, said in a statement. “The final rule includes additional steps to help small and rural practices by raising the low-volume threshold exemption, and practices of all sizes will benefit from reduced MIPS reporting requirements. Our initial review indicates that CMS has been responsive to many concerns raised by the AMA.”
American College of Cardiology President Richard A. Chazal, MD, said in a statement that the organization is “encouraged to see that CMS has made several changes in the final rule based on comments by the clinician community.”
The American College of Rheumatology also expressed support.
“Giving providers the flexibility of multiple options for participation in the first and second years will help ensure a smooth transition to the new payment system, and the continued delivery of quality care to Medicare patients living with rheumatic diseases,” the organization said in a statement. “We also appreciated the broadening of exemptions from the program, which will help to protect small practices that already struggle to keep up with administrative burdens, along with the reduction in the number of required measures to be reported.”
The American Osteopathic Association applauded the flexibility being offered, but found it “disappointing that many of those currently in patient-centered medical homes will still not qualify for [APMs] and opportunities to enter other such value-based models remain limited.”
To that end, CMS officials said that the agency is looking into creating an accountable care organization (ACO) “Track 1 Plus” model that would qualify for as an APM. Currently, ACOs that are in Track 1 share savings but do not assume risk. The agency said that the Track 1 Plus model would have organizations assuming some nominal level of risk that would be smaller, compared with those in the Medicare Shared Savings Program (MSSP) Track 2 and Track 3, as well as those that qualify as Next Generation ACOs. CMS plans to have the ACO Track 1 Plus Model ready for the 2018 reporting year.
The National Association of ACOs expressed disappointment that those ACOs that fall in the Track 1 of the MSSP do not qualify as an APM, but it is “incredibly pleased that CMS recognizes the need for a new model and is taking steps to develop a new MSSP Track 1 Plus,” President and CEO Clif Gaus said in a statement.
More CMS-issued information and educational material about the MACRA final rule can be found here.
MACRA options offer flexibility for smaller practices
Federal flexibility in compliance with the first year of MACRA reforms is a win for most physicians.
The American Gastroenterological Association “is pleased that CMS [the Centers for Medicare and Medicaid Services] is providing physicians woth more flexible options for reporting and that, most importantly, no physician will be penalized in 2019 for their reporting in 2017,” the association said in a statement. “Since the release of the MACRA [Medicare Access and CHIP Reauthorization Act of 2015] proposd rule, AGA has been advocating to Congress and CMS that the agency provide physicians with mor eflexibility to coply with the law and have opportunities to succeed in the new payment system.”
Those who choose the Merit-Based Incentive Payment System (MIPS) track now have three options for when they must start reporting data next year. Data reported in 2017 will serve as the benchmark for bonus payments paid in 2019.
Option 1
Report “some data” in 2017. Doctors who report some data to the Quality Payments Program (QPP) – the official name for MACRA-required reforms – will not face a Medicare pay cut. CMS considers even this low level of reporting a test for whether physicians will be ready for more intense MACRA involvement in 2018 and 2019. Exactly how much “some data” is currently is not defined.
Option 2
Participate for part of 2017. Those who choose to report data to QPP for some of the year also will be testing their systems for future MACRA compliance and may end up with a small Medicare pay increase. Again, the duration of reporting was not defined by the CMS at press time.
Option 3
Participate for the full year. Doctors who begin to report data from all parts of QPP on Jan. 1 will be eligible for a “modest” Medicare pay increase in 2019. Data on quality measures, use of technology, and practice improvement must be reported.
For those who are eligible for participation in Advanced Alternative Payment Models (APMs), that track will begin Jan. 1, 2017.
The American Medical Association commended federal officials “for listening to physicians’ concerns about the timeline that was originally proposed for MACRA,” AMA President Andrew Gurman, MD, said in a statement. “The AMA believes the actions that the administration announced today will help give physicians a fair shot in the first year of MACRA implementation. This is the flexibility that physicians were seeking all along.”
Not all see the new flexibility as a good thing, particularly for larger group practices that are ready to fully participate in MACRA as of Jan. 1.
“This flexibility is especially important for small provider groups that may have legitimate logistical issues around MACRA’s reporting requirements,” Donald Fisher, PhD, president and CEO of AMGA, said in a statement. (AMGA was formerly known as the American Medical Group Association.)
“However, our membership is deeply concerned that the creation of these new reporting options will have the unintended result of penalizing the very provider groups that have made the largest investments to meet MACRA’s goals of better quality, improved clinical practice activities, better use of electronic medical records, and lower resource use. These groups have already begun the transition from volume to value, and it is disappointing the rewards for their effort will be compromised rather than rewarded, as was MACRA’s stated purpose by Congress and the administration.”
By offering options for compliance, the CMS could potentially limit the amount of bonus payments in order to meet MACRA’s budget-neutral requirements, according to Chet Speed, vice president of public policy at AMGA. There will be no potential penalties that would offset bonuses for organizations that are performing at a high rate, which could result in having to lower the maximum bonuses an organization would be eligible to receive.
“You’ve compressed rewards to a level where it just penalizes those who have made the investments” in upgrading their systems to prepare for the Jan. 1 start date, Mr. Speed said.
He emphasized that the CMS could still address this and make the full bonus payments available to those who are prepared to participate on Jan. 1, but that will not be known until the final rule is published. He applauded the agency’s efforts in continually reaching out to the physician community throughout the MACRA development process and said he is hopeful there will be resolution to these concerns in the final rule.
Federal flexibility in compliance with the first year of MACRA reforms is a win for most physicians.
The American Gastroenterological Association “is pleased that CMS [the Centers for Medicare and Medicaid Services] is providing physicians woth more flexible options for reporting and that, most importantly, no physician will be penalized in 2019 for their reporting in 2017,” the association said in a statement. “Since the release of the MACRA [Medicare Access and CHIP Reauthorization Act of 2015] proposd rule, AGA has been advocating to Congress and CMS that the agency provide physicians with mor eflexibility to coply with the law and have opportunities to succeed in the new payment system.”
Those who choose the Merit-Based Incentive Payment System (MIPS) track now have three options for when they must start reporting data next year. Data reported in 2017 will serve as the benchmark for bonus payments paid in 2019.
Option 1
Report “some data” in 2017. Doctors who report some data to the Quality Payments Program (QPP) – the official name for MACRA-required reforms – will not face a Medicare pay cut. CMS considers even this low level of reporting a test for whether physicians will be ready for more intense MACRA involvement in 2018 and 2019. Exactly how much “some data” is currently is not defined.
Option 2
Participate for part of 2017. Those who choose to report data to QPP for some of the year also will be testing their systems for future MACRA compliance and may end up with a small Medicare pay increase. Again, the duration of reporting was not defined by the CMS at press time.
Option 3
Participate for the full year. Doctors who begin to report data from all parts of QPP on Jan. 1 will be eligible for a “modest” Medicare pay increase in 2019. Data on quality measures, use of technology, and practice improvement must be reported.
For those who are eligible for participation in Advanced Alternative Payment Models (APMs), that track will begin Jan. 1, 2017.
The American Medical Association commended federal officials “for listening to physicians’ concerns about the timeline that was originally proposed for MACRA,” AMA President Andrew Gurman, MD, said in a statement. “The AMA believes the actions that the administration announced today will help give physicians a fair shot in the first year of MACRA implementation. This is the flexibility that physicians were seeking all along.”
Not all see the new flexibility as a good thing, particularly for larger group practices that are ready to fully participate in MACRA as of Jan. 1.
“This flexibility is especially important for small provider groups that may have legitimate logistical issues around MACRA’s reporting requirements,” Donald Fisher, PhD, president and CEO of AMGA, said in a statement. (AMGA was formerly known as the American Medical Group Association.)
“However, our membership is deeply concerned that the creation of these new reporting options will have the unintended result of penalizing the very provider groups that have made the largest investments to meet MACRA’s goals of better quality, improved clinical practice activities, better use of electronic medical records, and lower resource use. These groups have already begun the transition from volume to value, and it is disappointing the rewards for their effort will be compromised rather than rewarded, as was MACRA’s stated purpose by Congress and the administration.”
By offering options for compliance, the CMS could potentially limit the amount of bonus payments in order to meet MACRA’s budget-neutral requirements, according to Chet Speed, vice president of public policy at AMGA. There will be no potential penalties that would offset bonuses for organizations that are performing at a high rate, which could result in having to lower the maximum bonuses an organization would be eligible to receive.
“You’ve compressed rewards to a level where it just penalizes those who have made the investments” in upgrading their systems to prepare for the Jan. 1 start date, Mr. Speed said.
He emphasized that the CMS could still address this and make the full bonus payments available to those who are prepared to participate on Jan. 1, but that will not be known until the final rule is published. He applauded the agency’s efforts in continually reaching out to the physician community throughout the MACRA development process and said he is hopeful there will be resolution to these concerns in the final rule.
Federal flexibility in compliance with the first year of MACRA reforms is a win for most physicians.
The American Gastroenterological Association “is pleased that CMS [the Centers for Medicare and Medicaid Services] is providing physicians woth more flexible options for reporting and that, most importantly, no physician will be penalized in 2019 for their reporting in 2017,” the association said in a statement. “Since the release of the MACRA [Medicare Access and CHIP Reauthorization Act of 2015] proposd rule, AGA has been advocating to Congress and CMS that the agency provide physicians with mor eflexibility to coply with the law and have opportunities to succeed in the new payment system.”
Those who choose the Merit-Based Incentive Payment System (MIPS) track now have three options for when they must start reporting data next year. Data reported in 2017 will serve as the benchmark for bonus payments paid in 2019.
Option 1
Report “some data” in 2017. Doctors who report some data to the Quality Payments Program (QPP) – the official name for MACRA-required reforms – will not face a Medicare pay cut. CMS considers even this low level of reporting a test for whether physicians will be ready for more intense MACRA involvement in 2018 and 2019. Exactly how much “some data” is currently is not defined.
Option 2
Participate for part of 2017. Those who choose to report data to QPP for some of the year also will be testing their systems for future MACRA compliance and may end up with a small Medicare pay increase. Again, the duration of reporting was not defined by the CMS at press time.
Option 3
Participate for the full year. Doctors who begin to report data from all parts of QPP on Jan. 1 will be eligible for a “modest” Medicare pay increase in 2019. Data on quality measures, use of technology, and practice improvement must be reported.
For those who are eligible for participation in Advanced Alternative Payment Models (APMs), that track will begin Jan. 1, 2017.
The American Medical Association commended federal officials “for listening to physicians’ concerns about the timeline that was originally proposed for MACRA,” AMA President Andrew Gurman, MD, said in a statement. “The AMA believes the actions that the administration announced today will help give physicians a fair shot in the first year of MACRA implementation. This is the flexibility that physicians were seeking all along.”
Not all see the new flexibility as a good thing, particularly for larger group practices that are ready to fully participate in MACRA as of Jan. 1.
“This flexibility is especially important for small provider groups that may have legitimate logistical issues around MACRA’s reporting requirements,” Donald Fisher, PhD, president and CEO of AMGA, said in a statement. (AMGA was formerly known as the American Medical Group Association.)
“However, our membership is deeply concerned that the creation of these new reporting options will have the unintended result of penalizing the very provider groups that have made the largest investments to meet MACRA’s goals of better quality, improved clinical practice activities, better use of electronic medical records, and lower resource use. These groups have already begun the transition from volume to value, and it is disappointing the rewards for their effort will be compromised rather than rewarded, as was MACRA’s stated purpose by Congress and the administration.”
By offering options for compliance, the CMS could potentially limit the amount of bonus payments in order to meet MACRA’s budget-neutral requirements, according to Chet Speed, vice president of public policy at AMGA. There will be no potential penalties that would offset bonuses for organizations that are performing at a high rate, which could result in having to lower the maximum bonuses an organization would be eligible to receive.
“You’ve compressed rewards to a level where it just penalizes those who have made the investments” in upgrading their systems to prepare for the Jan. 1 start date, Mr. Speed said.
He emphasized that the CMS could still address this and make the full bonus payments available to those who are prepared to participate on Jan. 1, but that will not be known until the final rule is published. He applauded the agency’s efforts in continually reaching out to the physician community throughout the MACRA development process and said he is hopeful there will be resolution to these concerns in the final rule.
CMS assures small-practice doctors they have a place in MACRA
WASHINGTON – Fear over how Medicare officials will operationalize the Medicare Access and CHIP Reauthorization Act – known as MACRA – should not be driving physicians in small and solo practices to become employees of large systems, a top federal health official said.
“If people want to sell their practices and they want to [be a] part of integrated care models for other reasons, that’s fine,” Andy Slavitt, acting administrator of the Centers for Medicare & Medicaid Services said during an Oct. 6 event hosted by the Association of Health Care Journalists. “I think we have to make a really big effort though to make sure that they are not being driven there because the world’s getting too complicated ... and they throw up their hands.”
“There are people throwing fear. There are consultants and there are hospitals saying MACRA is going to be impossible,” Mr. Slavitt said. “They don’t know the support that’s going to be provided. It is in certain people’s interest to put the story forward that small physician practices are doomed.”
One aspect of that support has already been announced – that physicians will have flexibility in terms of how much, or how little, they want to participate in the reporting requirements if they are selecting the Merit-based Incentive Payment System in 2017.
While it will be a challenge, Mr. Slavitt said the final MACRA regulations – due out in early November – will support small and solo practices so that they can be successful in the program.
“Health care has become more and more of a business and it becomes harder and harder if you don’t have those kinds of operations. But there are other ways to do it and I think we are going to be successful in a lot of ways,” he said.
WASHINGTON – Fear over how Medicare officials will operationalize the Medicare Access and CHIP Reauthorization Act – known as MACRA – should not be driving physicians in small and solo practices to become employees of large systems, a top federal health official said.
“If people want to sell their practices and they want to [be a] part of integrated care models for other reasons, that’s fine,” Andy Slavitt, acting administrator of the Centers for Medicare & Medicaid Services said during an Oct. 6 event hosted by the Association of Health Care Journalists. “I think we have to make a really big effort though to make sure that they are not being driven there because the world’s getting too complicated ... and they throw up their hands.”
“There are people throwing fear. There are consultants and there are hospitals saying MACRA is going to be impossible,” Mr. Slavitt said. “They don’t know the support that’s going to be provided. It is in certain people’s interest to put the story forward that small physician practices are doomed.”
One aspect of that support has already been announced – that physicians will have flexibility in terms of how much, or how little, they want to participate in the reporting requirements if they are selecting the Merit-based Incentive Payment System in 2017.
While it will be a challenge, Mr. Slavitt said the final MACRA regulations – due out in early November – will support small and solo practices so that they can be successful in the program.
“Health care has become more and more of a business and it becomes harder and harder if you don’t have those kinds of operations. But there are other ways to do it and I think we are going to be successful in a lot of ways,” he said.
WASHINGTON – Fear over how Medicare officials will operationalize the Medicare Access and CHIP Reauthorization Act – known as MACRA – should not be driving physicians in small and solo practices to become employees of large systems, a top federal health official said.
“If people want to sell their practices and they want to [be a] part of integrated care models for other reasons, that’s fine,” Andy Slavitt, acting administrator of the Centers for Medicare & Medicaid Services said during an Oct. 6 event hosted by the Association of Health Care Journalists. “I think we have to make a really big effort though to make sure that they are not being driven there because the world’s getting too complicated ... and they throw up their hands.”
“There are people throwing fear. There are consultants and there are hospitals saying MACRA is going to be impossible,” Mr. Slavitt said. “They don’t know the support that’s going to be provided. It is in certain people’s interest to put the story forward that small physician practices are doomed.”
One aspect of that support has already been announced – that physicians will have flexibility in terms of how much, or how little, they want to participate in the reporting requirements if they are selecting the Merit-based Incentive Payment System in 2017.
While it will be a challenge, Mr. Slavitt said the final MACRA regulations – due out in early November – will support small and solo practices so that they can be successful in the program.
“Health care has become more and more of a business and it becomes harder and harder if you don’t have those kinds of operations. But there are other ways to do it and I think we are going to be successful in a lot of ways,” he said.
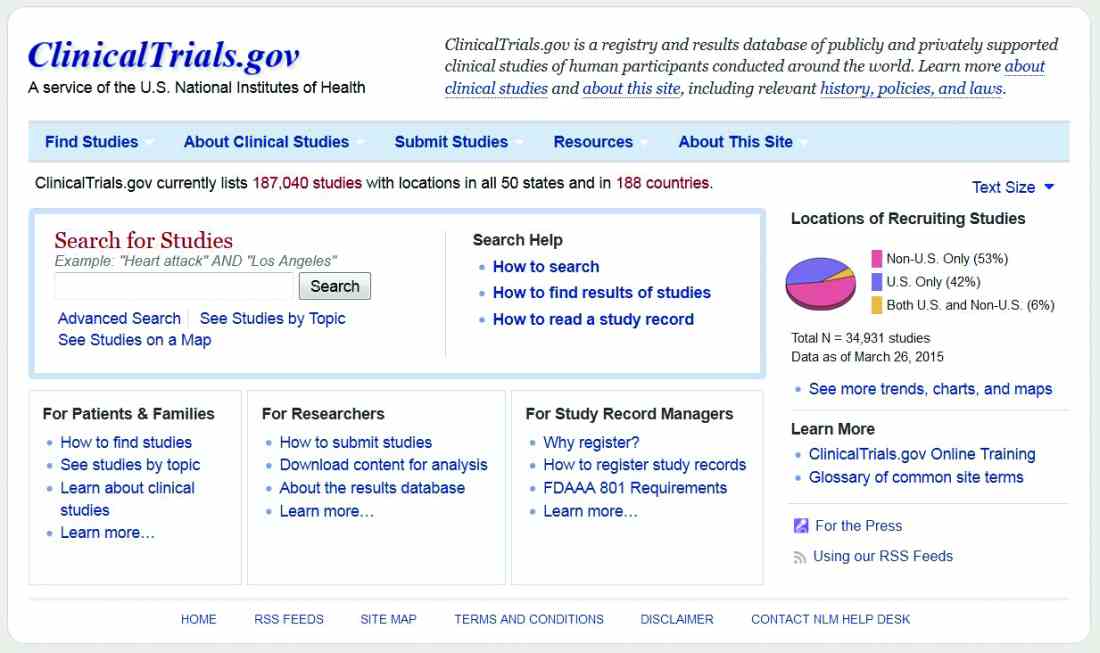
Feds require more transparent reporting of clinical trial results
New federal regulations aim to strengthen the current requirements on reporting of clinical trial information.
The new rule offers a checklist to help determine which clinical trials are subject to regulations and who is responsible for information that appears on ClinicalTrials.gov. In addition, the rule does the following:
• Expands the scope of trials for which results must be submitted to include Food and Drug Administration–regulated products that have not yet been approved, licensed, or cleared.
• Requires additional information be submitted to ClinicalTrials.gov, including demographic information about trial participants.
• Expands adverse-event reporting.
• Adds potential penalties for noncompliance.
Current federal requirements for reporting of clinical trial information were originally enacted as part of the FDA Amendments Act of 2007. However, a 2015 analysis of trials listed on ClinicalTrials.gov from 2007 to 2012 found a woeful lack of compliance: Study results were posted for 13% of trials covered by reporting requirements (N Engl J Med. 2015;372:1031-9).
“Organizations will need to ensure that their systems, procedures, and organizational values all promote complete and timely clinical trial reporting,” wrote Deborah Zarin, MD, of the National Library of Medicine, Bethesda, Md., and her colleagues in a special report on the final rule (N Engl J Med. 2016 Sep 16. doi: 10.1056/NEJMsr1611785). “In the end, the parties responsible for clinical trials will be held accountable by the public.”
The final rule applies to most interventional studies of drugs, biologics, and devices regulated by FDA, but it will not apply to phase I trials of pharmaceuticals or small feasibility studies of devices. It specifies how and when information collected in a clinical trial should be collected and submitted to ClinicalTrials.gov but does not dictate how clinical trials should be designed or conducted.
“Expanding the registration information in ClinicalTrials.gov improves people’s ability to find clinical trials in which they may be able to participate and access investigational therapies,” NIH officials said in a statement. “More information about the scientific results of trials, whether positive or negative, may help inform healthcare providers and patients regarding medical decisions. Additional information will help researchers avoid unnecessary duplication of studies, focus on areas in need of study and improve study design, ultimately advancing the development of clinical interventions.”
“Many U.S. academic medical centers, including those that conduct the most clinical trials, will find that the majority of their clinical trials fall under the [FDA Amendments Act], the NIH policy, or both,” Dr. Zarin and her colleagues wrote. “We hope that sponsors and other relevant entities will go considerably above and beyond the minimum requirements and expectations, making an effort to honor the contributions of all study participants and ensure that others in the scientific community have access to complete and high-quality information about every clinical trial under their stewardship.”
The final rule was published in the Federal Register on Sept. 21 and becomes effective on Jan. 18, 2017.
AGA Resource
The AGA Center for Diagnostics and Therapeutics was created to provide objective, independent guidance to companies, regulators, investors and health-care professionals on the development of new therapies and diagnostics tests for digestive disorders. Learn more at http://www.gastro.org/about/initiatives/aga-center-for-diagnostics-and-therapeutics.
New federal regulations aim to strengthen the current requirements on reporting of clinical trial information.
The new rule offers a checklist to help determine which clinical trials are subject to regulations and who is responsible for information that appears on ClinicalTrials.gov. In addition, the rule does the following:
• Expands the scope of trials for which results must be submitted to include Food and Drug Administration–regulated products that have not yet been approved, licensed, or cleared.
• Requires additional information be submitted to ClinicalTrials.gov, including demographic information about trial participants.
• Expands adverse-event reporting.
• Adds potential penalties for noncompliance.
Current federal requirements for reporting of clinical trial information were originally enacted as part of the FDA Amendments Act of 2007. However, a 2015 analysis of trials listed on ClinicalTrials.gov from 2007 to 2012 found a woeful lack of compliance: Study results were posted for 13% of trials covered by reporting requirements (N Engl J Med. 2015;372:1031-9).
“Organizations will need to ensure that their systems, procedures, and organizational values all promote complete and timely clinical trial reporting,” wrote Deborah Zarin, MD, of the National Library of Medicine, Bethesda, Md., and her colleagues in a special report on the final rule (N Engl J Med. 2016 Sep 16. doi: 10.1056/NEJMsr1611785). “In the end, the parties responsible for clinical trials will be held accountable by the public.”
The final rule applies to most interventional studies of drugs, biologics, and devices regulated by FDA, but it will not apply to phase I trials of pharmaceuticals or small feasibility studies of devices. It specifies how and when information collected in a clinical trial should be collected and submitted to ClinicalTrials.gov but does not dictate how clinical trials should be designed or conducted.
“Expanding the registration information in ClinicalTrials.gov improves people’s ability to find clinical trials in which they may be able to participate and access investigational therapies,” NIH officials said in a statement. “More information about the scientific results of trials, whether positive or negative, may help inform healthcare providers and patients regarding medical decisions. Additional information will help researchers avoid unnecessary duplication of studies, focus on areas in need of study and improve study design, ultimately advancing the development of clinical interventions.”
“Many U.S. academic medical centers, including those that conduct the most clinical trials, will find that the majority of their clinical trials fall under the [FDA Amendments Act], the NIH policy, or both,” Dr. Zarin and her colleagues wrote. “We hope that sponsors and other relevant entities will go considerably above and beyond the minimum requirements and expectations, making an effort to honor the contributions of all study participants and ensure that others in the scientific community have access to complete and high-quality information about every clinical trial under their stewardship.”
The final rule was published in the Federal Register on Sept. 21 and becomes effective on Jan. 18, 2017.
AGA Resource
The AGA Center for Diagnostics and Therapeutics was created to provide objective, independent guidance to companies, regulators, investors and health-care professionals on the development of new therapies and diagnostics tests for digestive disorders. Learn more at http://www.gastro.org/about/initiatives/aga-center-for-diagnostics-and-therapeutics.
New federal regulations aim to strengthen the current requirements on reporting of clinical trial information.
The new rule offers a checklist to help determine which clinical trials are subject to regulations and who is responsible for information that appears on ClinicalTrials.gov. In addition, the rule does the following:
• Expands the scope of trials for which results must be submitted to include Food and Drug Administration–regulated products that have not yet been approved, licensed, or cleared.
• Requires additional information be submitted to ClinicalTrials.gov, including demographic information about trial participants.
• Expands adverse-event reporting.
• Adds potential penalties for noncompliance.
Current federal requirements for reporting of clinical trial information were originally enacted as part of the FDA Amendments Act of 2007. However, a 2015 analysis of trials listed on ClinicalTrials.gov from 2007 to 2012 found a woeful lack of compliance: Study results were posted for 13% of trials covered by reporting requirements (N Engl J Med. 2015;372:1031-9).
“Organizations will need to ensure that their systems, procedures, and organizational values all promote complete and timely clinical trial reporting,” wrote Deborah Zarin, MD, of the National Library of Medicine, Bethesda, Md., and her colleagues in a special report on the final rule (N Engl J Med. 2016 Sep 16. doi: 10.1056/NEJMsr1611785). “In the end, the parties responsible for clinical trials will be held accountable by the public.”
The final rule applies to most interventional studies of drugs, biologics, and devices regulated by FDA, but it will not apply to phase I trials of pharmaceuticals or small feasibility studies of devices. It specifies how and when information collected in a clinical trial should be collected and submitted to ClinicalTrials.gov but does not dictate how clinical trials should be designed or conducted.
“Expanding the registration information in ClinicalTrials.gov improves people’s ability to find clinical trials in which they may be able to participate and access investigational therapies,” NIH officials said in a statement. “More information about the scientific results of trials, whether positive or negative, may help inform healthcare providers and patients regarding medical decisions. Additional information will help researchers avoid unnecessary duplication of studies, focus on areas in need of study and improve study design, ultimately advancing the development of clinical interventions.”
“Many U.S. academic medical centers, including those that conduct the most clinical trials, will find that the majority of their clinical trials fall under the [FDA Amendments Act], the NIH policy, or both,” Dr. Zarin and her colleagues wrote. “We hope that sponsors and other relevant entities will go considerably above and beyond the minimum requirements and expectations, making an effort to honor the contributions of all study participants and ensure that others in the scientific community have access to complete and high-quality information about every clinical trial under their stewardship.”
The final rule was published in the Federal Register on Sept. 21 and becomes effective on Jan. 18, 2017.
AGA Resource
The AGA Center for Diagnostics and Therapeutics was created to provide objective, independent guidance to companies, regulators, investors and health-care professionals on the development of new therapies and diagnostics tests for digestive disorders. Learn more at http://www.gastro.org/about/initiatives/aga-center-for-diagnostics-and-therapeutics.
Feds require more transparent reporting of clinical trial results
New federal regulations aim to strengthen the current requirements on reporting of clinical trial information.
The new rule offers a checklist to help determine which clinical trials are subject to regulations and who is responsible for information that appears on ClinicalTrials.gov. In addition, the rule does the following:
• Expands the scope of trials for which results must be submitted to include Food and Drug Administration–regulated products that have not yet been approved, licensed, or cleared.
• Requires additional information be submitted to ClinicalTrials.gov, including demographic information about trial participants.
• Expands adverse-event reporting.
• Adds potential penalties for noncompliance.
Simultaneously, the National Institutes of Health issued a complimentary policy for all trials it funds, regardless of whether the trials would be subject to the final rule.
Current federal requirements for reporting of clinical trial information were originally enacted as part of the FDA Amendments Act of 2007. However, a 2015 analysis of trials listed on ClinicalTrials.gov from 2007 to 2012 found a woeful lack of compliance: Study results were posted for 13% of trials covered by reporting requirements (N Engl J Med. 2015;372:1031-9).
“Organizations will need to ensure that their systems, procedures, and organizational values all promote complete and timely clinical trial reporting,” wrote Deborah Zarin, MD, of the National Library of Medicine, Bethesda, Md., and her colleagues in a special report on the final rule (N Engl J Med. 2016 Sep 16. doi: 10.1056/NEJMsr1611785). “In the end, the parties responsible for clinical trials will be held accountable by the public.”
The final rule applies to most interventional studies of drugs, biologics, and devices regulated by FDA, but it will not apply to phase I trials of pharmaceuticals or small feasibility studies of devices. It specifies how and when information collected in a clinical trial should be collected and submitted to ClinicalTrials.gov but does not dictate how clinical trials should be designed or conducted.
“Expanding the registration information in ClinicalTrials.gov improves people’s ability to find clinical trials in which they may be able to participate and access investigational therapies,” NIH officials said in a statement. “More information about the scientific results of trials, whether positive or negative, may help inform healthcare providers and patients regarding medical decisions. Additional information will help researchers avoid unnecessary duplication of studies, focus on areas in need of study and improve study design, ultimately advancing the development of clinical interventions.”
“Many U.S. academic medical centers, including those that conduct the most clinical trials, will find that the majority of their clinical trials fall under the [FDA Amendments Act], the NIH policy, or both,” Dr. Zarin and her colleagues wrote. “We hope that sponsors and other relevant entities will go considerably above and beyond the minimum requirements and expectations, making an effort to honor the contributions of all study participants and ensure that others in the scientific community have access to complete and high-quality information about every clinical trial under their stewardship.”
The final rule was published in the Federal Register on Sept. 21 and becomes effective on Jan. 18, 2017.
New federal regulations aim to strengthen the current requirements on reporting of clinical trial information.
The new rule offers a checklist to help determine which clinical trials are subject to regulations and who is responsible for information that appears on ClinicalTrials.gov. In addition, the rule does the following:
• Expands the scope of trials for which results must be submitted to include Food and Drug Administration–regulated products that have not yet been approved, licensed, or cleared.
• Requires additional information be submitted to ClinicalTrials.gov, including demographic information about trial participants.
• Expands adverse-event reporting.
• Adds potential penalties for noncompliance.
Simultaneously, the National Institutes of Health issued a complimentary policy for all trials it funds, regardless of whether the trials would be subject to the final rule.
Current federal requirements for reporting of clinical trial information were originally enacted as part of the FDA Amendments Act of 2007. However, a 2015 analysis of trials listed on ClinicalTrials.gov from 2007 to 2012 found a woeful lack of compliance: Study results were posted for 13% of trials covered by reporting requirements (N Engl J Med. 2015;372:1031-9).
“Organizations will need to ensure that their systems, procedures, and organizational values all promote complete and timely clinical trial reporting,” wrote Deborah Zarin, MD, of the National Library of Medicine, Bethesda, Md., and her colleagues in a special report on the final rule (N Engl J Med. 2016 Sep 16. doi: 10.1056/NEJMsr1611785). “In the end, the parties responsible for clinical trials will be held accountable by the public.”
The final rule applies to most interventional studies of drugs, biologics, and devices regulated by FDA, but it will not apply to phase I trials of pharmaceuticals or small feasibility studies of devices. It specifies how and when information collected in a clinical trial should be collected and submitted to ClinicalTrials.gov but does not dictate how clinical trials should be designed or conducted.
“Expanding the registration information in ClinicalTrials.gov improves people’s ability to find clinical trials in which they may be able to participate and access investigational therapies,” NIH officials said in a statement. “More information about the scientific results of trials, whether positive or negative, may help inform healthcare providers and patients regarding medical decisions. Additional information will help researchers avoid unnecessary duplication of studies, focus on areas in need of study and improve study design, ultimately advancing the development of clinical interventions.”
“Many U.S. academic medical centers, including those that conduct the most clinical trials, will find that the majority of their clinical trials fall under the [FDA Amendments Act], the NIH policy, or both,” Dr. Zarin and her colleagues wrote. “We hope that sponsors and other relevant entities will go considerably above and beyond the minimum requirements and expectations, making an effort to honor the contributions of all study participants and ensure that others in the scientific community have access to complete and high-quality information about every clinical trial under their stewardship.”
The final rule was published in the Federal Register on Sept. 21 and becomes effective on Jan. 18, 2017.
New federal regulations aim to strengthen the current requirements on reporting of clinical trial information.
The new rule offers a checklist to help determine which clinical trials are subject to regulations and who is responsible for information that appears on ClinicalTrials.gov. In addition, the rule does the following:
• Expands the scope of trials for which results must be submitted to include Food and Drug Administration–regulated products that have not yet been approved, licensed, or cleared.
• Requires additional information be submitted to ClinicalTrials.gov, including demographic information about trial participants.
• Expands adverse-event reporting.
• Adds potential penalties for noncompliance.
Simultaneously, the National Institutes of Health issued a complimentary policy for all trials it funds, regardless of whether the trials would be subject to the final rule.
Current federal requirements for reporting of clinical trial information were originally enacted as part of the FDA Amendments Act of 2007. However, a 2015 analysis of trials listed on ClinicalTrials.gov from 2007 to 2012 found a woeful lack of compliance: Study results were posted for 13% of trials covered by reporting requirements (N Engl J Med. 2015;372:1031-9).
“Organizations will need to ensure that their systems, procedures, and organizational values all promote complete and timely clinical trial reporting,” wrote Deborah Zarin, MD, of the National Library of Medicine, Bethesda, Md., and her colleagues in a special report on the final rule (N Engl J Med. 2016 Sep 16. doi: 10.1056/NEJMsr1611785). “In the end, the parties responsible for clinical trials will be held accountable by the public.”
The final rule applies to most interventional studies of drugs, biologics, and devices regulated by FDA, but it will not apply to phase I trials of pharmaceuticals or small feasibility studies of devices. It specifies how and when information collected in a clinical trial should be collected and submitted to ClinicalTrials.gov but does not dictate how clinical trials should be designed or conducted.
“Expanding the registration information in ClinicalTrials.gov improves people’s ability to find clinical trials in which they may be able to participate and access investigational therapies,” NIH officials said in a statement. “More information about the scientific results of trials, whether positive or negative, may help inform healthcare providers and patients regarding medical decisions. Additional information will help researchers avoid unnecessary duplication of studies, focus on areas in need of study and improve study design, ultimately advancing the development of clinical interventions.”
“Many U.S. academic medical centers, including those that conduct the most clinical trials, will find that the majority of their clinical trials fall under the [FDA Amendments Act], the NIH policy, or both,” Dr. Zarin and her colleagues wrote. “We hope that sponsors and other relevant entities will go considerably above and beyond the minimum requirements and expectations, making an effort to honor the contributions of all study participants and ensure that others in the scientific community have access to complete and high-quality information about every clinical trial under their stewardship.”
The final rule was published in the Federal Register on Sept. 21 and becomes effective on Jan. 18, 2017.
EHR woes will get worse before they get better: ONC chief
WASHINGTON – EHR woes may tick up as medical practices begin to move from fee for service to a value-based care model, according to the new federal health IT leader, B. Vindell Washington, MD.
“If you are in an environment where you have, say for example, 20%-25% of your patients that are in an accountable care model and the rest of your entire panel is a fee-for-service model, then you’re really not in a position to really reap the full benefit, and quite frankly you are straddling the fence in terms of both your work flow and patient delivery,” Dr. Washington, National Coordinator for Health Information Technology, said at a Sept. 19 press briefing. That said, “I think that there will be improvements for physicians as we work our way through delivery system reform.”
The Office of the National Coordinator for Health Information Technology (ONC) is working on regulations that would push EHR vendors to publish app interfaces to foster innovation, create more efficient work-flow applications, and improve EHRs in general, said Dr. Washington, former president and chief medical information officer of the Franciscan Missionaries of Our Lady Health System Medical Group, Baton Rogue, La. “This change is not just a change in the tool that folks are using, it is also a change in the system in which they operate.”
Interoperability also should improve as more clinicians move into value-based care models, he said. For example, in Tulsa, Okla., three competing health systems are participating in the Comprehensive Primary Care Initiative demonstration. The health systems involved in the demonstration are seeing unprecedented levels of information sharing between them because participation in CPC requires it.
“Part of this value proposition comes when you take care of groups of patients and you move into more of the CPC+ or medical home or coordinated care models where the sort of larger, longer view and team-based approach to care become more and more prominent,” Dr. Washington said.
He also talked about the value of documenting efforts related to process measures, and the criticism that clinicians are simply checking boxes rather than focusing on outcomes.
Dr. Washington acknowledged that defining and measuring outcomes is a much more difficult task. He noted that improvement in health is the goal. And while the measure may be difficult, what physicians “need to do in health care to lead to those outcomes, to have people have better, healthier, more enriched lives, you end up with measures around.”
As an example, he pointed to preventing diabetic retinopathy or amputation. “Our best guess at how to do that is to keep their hemoglobin A1c in a certain range,” he said, adding that while tracking hemoglobin A1c is the measurement, “what you really want is the [diabetes patient] to have a long life, keep their eyesight, and not lose a limb. It’s that juxtaposition of what you can measure easily versus what the ultimate outcome” is, and physicians will come up with a series of steps in order to achieve the outcome of improved overall health status.
WASHINGTON – EHR woes may tick up as medical practices begin to move from fee for service to a value-based care model, according to the new federal health IT leader, B. Vindell Washington, MD.
“If you are in an environment where you have, say for example, 20%-25% of your patients that are in an accountable care model and the rest of your entire panel is a fee-for-service model, then you’re really not in a position to really reap the full benefit, and quite frankly you are straddling the fence in terms of both your work flow and patient delivery,” Dr. Washington, National Coordinator for Health Information Technology, said at a Sept. 19 press briefing. That said, “I think that there will be improvements for physicians as we work our way through delivery system reform.”
The Office of the National Coordinator for Health Information Technology (ONC) is working on regulations that would push EHR vendors to publish app interfaces to foster innovation, create more efficient work-flow applications, and improve EHRs in general, said Dr. Washington, former president and chief medical information officer of the Franciscan Missionaries of Our Lady Health System Medical Group, Baton Rogue, La. “This change is not just a change in the tool that folks are using, it is also a change in the system in which they operate.”
Interoperability also should improve as more clinicians move into value-based care models, he said. For example, in Tulsa, Okla., three competing health systems are participating in the Comprehensive Primary Care Initiative demonstration. The health systems involved in the demonstration are seeing unprecedented levels of information sharing between them because participation in CPC requires it.
“Part of this value proposition comes when you take care of groups of patients and you move into more of the CPC+ or medical home or coordinated care models where the sort of larger, longer view and team-based approach to care become more and more prominent,” Dr. Washington said.
He also talked about the value of documenting efforts related to process measures, and the criticism that clinicians are simply checking boxes rather than focusing on outcomes.
Dr. Washington acknowledged that defining and measuring outcomes is a much more difficult task. He noted that improvement in health is the goal. And while the measure may be difficult, what physicians “need to do in health care to lead to those outcomes, to have people have better, healthier, more enriched lives, you end up with measures around.”
As an example, he pointed to preventing diabetic retinopathy or amputation. “Our best guess at how to do that is to keep their hemoglobin A1c in a certain range,” he said, adding that while tracking hemoglobin A1c is the measurement, “what you really want is the [diabetes patient] to have a long life, keep their eyesight, and not lose a limb. It’s that juxtaposition of what you can measure easily versus what the ultimate outcome” is, and physicians will come up with a series of steps in order to achieve the outcome of improved overall health status.
WASHINGTON – EHR woes may tick up as medical practices begin to move from fee for service to a value-based care model, according to the new federal health IT leader, B. Vindell Washington, MD.
“If you are in an environment where you have, say for example, 20%-25% of your patients that are in an accountable care model and the rest of your entire panel is a fee-for-service model, then you’re really not in a position to really reap the full benefit, and quite frankly you are straddling the fence in terms of both your work flow and patient delivery,” Dr. Washington, National Coordinator for Health Information Technology, said at a Sept. 19 press briefing. That said, “I think that there will be improvements for physicians as we work our way through delivery system reform.”
The Office of the National Coordinator for Health Information Technology (ONC) is working on regulations that would push EHR vendors to publish app interfaces to foster innovation, create more efficient work-flow applications, and improve EHRs in general, said Dr. Washington, former president and chief medical information officer of the Franciscan Missionaries of Our Lady Health System Medical Group, Baton Rogue, La. “This change is not just a change in the tool that folks are using, it is also a change in the system in which they operate.”
Interoperability also should improve as more clinicians move into value-based care models, he said. For example, in Tulsa, Okla., three competing health systems are participating in the Comprehensive Primary Care Initiative demonstration. The health systems involved in the demonstration are seeing unprecedented levels of information sharing between them because participation in CPC requires it.
“Part of this value proposition comes when you take care of groups of patients and you move into more of the CPC+ or medical home or coordinated care models where the sort of larger, longer view and team-based approach to care become more and more prominent,” Dr. Washington said.
He also talked about the value of documenting efforts related to process measures, and the criticism that clinicians are simply checking boxes rather than focusing on outcomes.
Dr. Washington acknowledged that defining and measuring outcomes is a much more difficult task. He noted that improvement in health is the goal. And while the measure may be difficult, what physicians “need to do in health care to lead to those outcomes, to have people have better, healthier, more enriched lives, you end up with measures around.”
As an example, he pointed to preventing diabetic retinopathy or amputation. “Our best guess at how to do that is to keep their hemoglobin A1c in a certain range,” he said, adding that while tracking hemoglobin A1c is the measurement, “what you really want is the [diabetes patient] to have a long life, keep their eyesight, and not lose a limb. It’s that juxtaposition of what you can measure easily versus what the ultimate outcome” is, and physicians will come up with a series of steps in order to achieve the outcome of improved overall health status.
More than half of high-cost Medicaid children use fewer resources over time
More than half of children who are the costliest to cover in the Medicaid program eventually fall out of that category, according to new research.
A retrospective analysis of 48,743 children aged 1-18 years continuously enrolled in Medicaid during 2009-2013 across 10 states who were in the top 5% of all health care spending in 2010 found that from 2011 to 2013, “54.2% fell below the top 5% and did not return to the top 5% in 2011-2013,” Rishi K. Agrawal, MD, of the Ann and Robert Lurie Children’s Hospital, Chicago, and his colleagues wrote in a study published Sept. 15 online in Pediatrics (2016. doi: 10.1542/peds.2016-0682).
Children who were persistently in the top 5% accounted for 32.9% of the cohort and 12.9% of the children who were in the top 5% during 2010 fell out of and returned to the group of highest cost patients in the following 3 years.
“The highest likelihood of subsequent high spending was observed in older children with many chronic conditions, respiratory, or neuromuscular complex chronic conditions, and those who used home health services,” the authors noted. “Decreased likelihood of subsequent high spending was observed with children with hospital or ED [emergency department] use in 2010.”
“Understanding the clinical attributes of children most likely to experience persistent high resource use might help inform clinical approaches to optimize their health,” Dr. Agrawal and associates continued.
They added that previous literature and the authors’ clinical experiences suggest that those with multiple chronic conditions, especially a neuromuscular condition, “are at risk for underuse of primary care,” and without a primary care physician taking charge of overall care coordination, that burden falls on the families.
“Perhaps population health initiatives (e.g., enhanced medical homes or neighborhoods for children with medical complexity, complex care networks) designed to alleviate these specific issues might benefit children who are the most likely to have persistent high resource use,” Dr. Agrawal and his colleagues wrote.
The authors cited no relevant financial disclosures. Jay G. Berry, MD, was supported by the Agency for Healthcare Research and Quality, and Eyal Cohen, MD, was supported as a Harkness Fellow in Health Care Policy and Practice by the Canadian Foundation for Healthcare Improvement and the Commonwealth Fund.
[A] longitudinal analysis of children’s health care utilization and spending while on Medicaid offers fresh insights and raises new questions about how best to provide supportive coordinated care and mitigate rising costs over time.
Understanding specific aspects of complexity – such as the impact of infancy, risks for newly intensified care needs, and the role of mental health comorbidities – will likely enrich our collective understanding of longitudinal trends in spending. Although illness acuity will always demand our clinical attention, there is growing evidence that illness complexity demands our programmatic attention as well.
Matthew Davis, MD, is a pediatrician and researcher at the Ann and Robert H. Lurie Children’s Hospital, Chicago. His observations are excerpted from a commentary published Sept. 15 online in Pediatrics. He said he had no relevant financial disclosures.
[A] longitudinal analysis of children’s health care utilization and spending while on Medicaid offers fresh insights and raises new questions about how best to provide supportive coordinated care and mitigate rising costs over time.
Understanding specific aspects of complexity – such as the impact of infancy, risks for newly intensified care needs, and the role of mental health comorbidities – will likely enrich our collective understanding of longitudinal trends in spending. Although illness acuity will always demand our clinical attention, there is growing evidence that illness complexity demands our programmatic attention as well.
Matthew Davis, MD, is a pediatrician and researcher at the Ann and Robert H. Lurie Children’s Hospital, Chicago. His observations are excerpted from a commentary published Sept. 15 online in Pediatrics. He said he had no relevant financial disclosures.
[A] longitudinal analysis of children’s health care utilization and spending while on Medicaid offers fresh insights and raises new questions about how best to provide supportive coordinated care and mitigate rising costs over time.
Understanding specific aspects of complexity – such as the impact of infancy, risks for newly intensified care needs, and the role of mental health comorbidities – will likely enrich our collective understanding of longitudinal trends in spending. Although illness acuity will always demand our clinical attention, there is growing evidence that illness complexity demands our programmatic attention as well.
Matthew Davis, MD, is a pediatrician and researcher at the Ann and Robert H. Lurie Children’s Hospital, Chicago. His observations are excerpted from a commentary published Sept. 15 online in Pediatrics. He said he had no relevant financial disclosures.
More than half of children who are the costliest to cover in the Medicaid program eventually fall out of that category, according to new research.
A retrospective analysis of 48,743 children aged 1-18 years continuously enrolled in Medicaid during 2009-2013 across 10 states who were in the top 5% of all health care spending in 2010 found that from 2011 to 2013, “54.2% fell below the top 5% and did not return to the top 5% in 2011-2013,” Rishi K. Agrawal, MD, of the Ann and Robert Lurie Children’s Hospital, Chicago, and his colleagues wrote in a study published Sept. 15 online in Pediatrics (2016. doi: 10.1542/peds.2016-0682).
Children who were persistently in the top 5% accounted for 32.9% of the cohort and 12.9% of the children who were in the top 5% during 2010 fell out of and returned to the group of highest cost patients in the following 3 years.
“The highest likelihood of subsequent high spending was observed in older children with many chronic conditions, respiratory, or neuromuscular complex chronic conditions, and those who used home health services,” the authors noted. “Decreased likelihood of subsequent high spending was observed with children with hospital or ED [emergency department] use in 2010.”
“Understanding the clinical attributes of children most likely to experience persistent high resource use might help inform clinical approaches to optimize their health,” Dr. Agrawal and associates continued.
They added that previous literature and the authors’ clinical experiences suggest that those with multiple chronic conditions, especially a neuromuscular condition, “are at risk for underuse of primary care,” and without a primary care physician taking charge of overall care coordination, that burden falls on the families.
“Perhaps population health initiatives (e.g., enhanced medical homes or neighborhoods for children with medical complexity, complex care networks) designed to alleviate these specific issues might benefit children who are the most likely to have persistent high resource use,” Dr. Agrawal and his colleagues wrote.
The authors cited no relevant financial disclosures. Jay G. Berry, MD, was supported by the Agency for Healthcare Research and Quality, and Eyal Cohen, MD, was supported as a Harkness Fellow in Health Care Policy and Practice by the Canadian Foundation for Healthcare Improvement and the Commonwealth Fund.
More than half of children who are the costliest to cover in the Medicaid program eventually fall out of that category, according to new research.
A retrospective analysis of 48,743 children aged 1-18 years continuously enrolled in Medicaid during 2009-2013 across 10 states who were in the top 5% of all health care spending in 2010 found that from 2011 to 2013, “54.2% fell below the top 5% and did not return to the top 5% in 2011-2013,” Rishi K. Agrawal, MD, of the Ann and Robert Lurie Children’s Hospital, Chicago, and his colleagues wrote in a study published Sept. 15 online in Pediatrics (2016. doi: 10.1542/peds.2016-0682).
Children who were persistently in the top 5% accounted for 32.9% of the cohort and 12.9% of the children who were in the top 5% during 2010 fell out of and returned to the group of highest cost patients in the following 3 years.
“The highest likelihood of subsequent high spending was observed in older children with many chronic conditions, respiratory, or neuromuscular complex chronic conditions, and those who used home health services,” the authors noted. “Decreased likelihood of subsequent high spending was observed with children with hospital or ED [emergency department] use in 2010.”
“Understanding the clinical attributes of children most likely to experience persistent high resource use might help inform clinical approaches to optimize their health,” Dr. Agrawal and associates continued.
They added that previous literature and the authors’ clinical experiences suggest that those with multiple chronic conditions, especially a neuromuscular condition, “are at risk for underuse of primary care,” and without a primary care physician taking charge of overall care coordination, that burden falls on the families.
“Perhaps population health initiatives (e.g., enhanced medical homes or neighborhoods for children with medical complexity, complex care networks) designed to alleviate these specific issues might benefit children who are the most likely to have persistent high resource use,” Dr. Agrawal and his colleagues wrote.
The authors cited no relevant financial disclosures. Jay G. Berry, MD, was supported by the Agency for Healthcare Research and Quality, and Eyal Cohen, MD, was supported as a Harkness Fellow in Health Care Policy and Practice by the Canadian Foundation for Healthcare Improvement and the Commonwealth Fund.
FROM PEDIATRICS