User login
FDA proposes lower nicotine levels in cigarettes
Nicotine levels in cigarettes could see a significant reduction under regulatory options being considered by the Food and Drug Administration.
Cigarettes “are the only legal consumer product that, when used as intended, will kill half all long-term users,” FDA Commissioner Scott Gottlieb, MD, said in a statement announcing the effort.
The agency is seeking comment on a proposed regulation regarding “a potential maximum nicotine level that would be appropriate for the protection of public health, in light of scientific evidence about the addictive properties of nicotine in cigarettes.” An advance notice of proposed rule making was posted online March 15 and is scheduled for publication in the Federal Register on March 16.
The FDA also is seeking comments on a number of other areas to help inform potential regulatory action down the road, including whether a new standard for lower nicotine levels should be implemented at once or whether a phased-in approach should be taken; whether FDA should specify a method for manufacturers to use in order to detect nicotine levels in their products; and whether the proposed lower level is technically achievable.
The agency also is seeking comment on potential unintended effects of lowering the amount of nicotine in cigarettes, such as turning to other combustible tobacco products such as cigars in conjunction with or as a replacement for cigarette use; increasing the number of cigarettes smoked, or seeking comparable nicotine from noncombustible tobacco sources.
At this time, FDA is not suggesting what the target might be on a specific nicotine level. While the advanced notice asks specifically about the “merits of nicotine levels like 0.3, 0.4, and 0.5 mg nicotine/g of tobacco filler,” it is not suggesting that this is the range being considered.
[polldaddy:9960560]
“Not to prejudge any possible proposed rule that we would do or any possible level, that is the purpose of an advanced proposed rule making, but we share all the science that we are aware of, and we characterize the studies that have been done to date in trying to find out what that right level is,” Mitch Zeller, director of the FDA Center for Tobacco Products, said during a March 15 press call.
He said that the FDA aiming to make sure the level is low enough that it cannot be compensated for by smoking more or inhaling deeper and holding the breath in longer, much like how smokers compensated when they smoked “light” cigarettes in the unregulated market.
Mr. Zeller said that seeking comments on those levels is based on the scientific evidence that is laid out in the advanced notice, but it is not necessarily foreshadowing where the standard will be set.
Drastically reducing the amount of nicotine in cigarettes is expected to significantly lower not only the number of people addicted to cigarettes but also as the negative health effects of nicotine addiction, FDA experts wrote in a perspective piece published March 15 in the New England Journal of Medicine (doi: 10.1065/NEJMsr1714617).
“Our findings show that reducing the nicotine level in cigarettes has the potential to substantially reduce the enormous burden of smoking-related death and disease,” Benjamin J. Apelberg, PhD, director of the Division of Population Health Science, Office of Science, within the FDA Center for Tobacco Products, and his colleagues, wrote in the report.
Modeling for the implementation of a lower nicotine level policy suggests that smoking prevalence will decline from a median of 12.8% in baseline scenario to a median of 10.8% within a year of implementation, with the increase related to smoking cessation.
“We estimate that approximately 5 million additional smokers would quit smoking within a year after implementation of the hypothetical policy,” Dr. Apelberg and his colleagues wrote. “By 2060, smoking prevalence drops from 7.9% in the baseline scenario to 1.4% in the policy scenario.”
Their analysis is based on a nicotine level that is “so low that there would not be enough nicotine available in cigarette tobacco for smokers to sustain addiction,” they noted.
The FDA plans to release two more advanced notices of proposed rule making related to using regulatory levers to reduce cigarette smoking, including one that addresses flavoring in tobacco and one related to the regulation of premium cigars. Other than the 90-day comment period related to the advanced notices, the agency has not set any deadlines on when it will act.
“Legally, we are not going to prejudge how long this will take or what will happen,” Mr. Zeller said. “This is an early step in what could be a potential rule-making process using the product standard authority. We will take a long and hard look at all the comments that come in over the next 90 days and based upon our review of all the information, all the comments that come in, all the feedback that we have, we will then make a decision about taking the next step in the rule-making process.”
Nicotine levels in cigarettes could see a significant reduction under regulatory options being considered by the Food and Drug Administration.
Cigarettes “are the only legal consumer product that, when used as intended, will kill half all long-term users,” FDA Commissioner Scott Gottlieb, MD, said in a statement announcing the effort.
The agency is seeking comment on a proposed regulation regarding “a potential maximum nicotine level that would be appropriate for the protection of public health, in light of scientific evidence about the addictive properties of nicotine in cigarettes.” An advance notice of proposed rule making was posted online March 15 and is scheduled for publication in the Federal Register on March 16.
The FDA also is seeking comments on a number of other areas to help inform potential regulatory action down the road, including whether a new standard for lower nicotine levels should be implemented at once or whether a phased-in approach should be taken; whether FDA should specify a method for manufacturers to use in order to detect nicotine levels in their products; and whether the proposed lower level is technically achievable.
The agency also is seeking comment on potential unintended effects of lowering the amount of nicotine in cigarettes, such as turning to other combustible tobacco products such as cigars in conjunction with or as a replacement for cigarette use; increasing the number of cigarettes smoked, or seeking comparable nicotine from noncombustible tobacco sources.
At this time, FDA is not suggesting what the target might be on a specific nicotine level. While the advanced notice asks specifically about the “merits of nicotine levels like 0.3, 0.4, and 0.5 mg nicotine/g of tobacco filler,” it is not suggesting that this is the range being considered.
[polldaddy:9960560]
“Not to prejudge any possible proposed rule that we would do or any possible level, that is the purpose of an advanced proposed rule making, but we share all the science that we are aware of, and we characterize the studies that have been done to date in trying to find out what that right level is,” Mitch Zeller, director of the FDA Center for Tobacco Products, said during a March 15 press call.
He said that the FDA aiming to make sure the level is low enough that it cannot be compensated for by smoking more or inhaling deeper and holding the breath in longer, much like how smokers compensated when they smoked “light” cigarettes in the unregulated market.
Mr. Zeller said that seeking comments on those levels is based on the scientific evidence that is laid out in the advanced notice, but it is not necessarily foreshadowing where the standard will be set.
Drastically reducing the amount of nicotine in cigarettes is expected to significantly lower not only the number of people addicted to cigarettes but also as the negative health effects of nicotine addiction, FDA experts wrote in a perspective piece published March 15 in the New England Journal of Medicine (doi: 10.1065/NEJMsr1714617).
“Our findings show that reducing the nicotine level in cigarettes has the potential to substantially reduce the enormous burden of smoking-related death and disease,” Benjamin J. Apelberg, PhD, director of the Division of Population Health Science, Office of Science, within the FDA Center for Tobacco Products, and his colleagues, wrote in the report.
Modeling for the implementation of a lower nicotine level policy suggests that smoking prevalence will decline from a median of 12.8% in baseline scenario to a median of 10.8% within a year of implementation, with the increase related to smoking cessation.
“We estimate that approximately 5 million additional smokers would quit smoking within a year after implementation of the hypothetical policy,” Dr. Apelberg and his colleagues wrote. “By 2060, smoking prevalence drops from 7.9% in the baseline scenario to 1.4% in the policy scenario.”
Their analysis is based on a nicotine level that is “so low that there would not be enough nicotine available in cigarette tobacco for smokers to sustain addiction,” they noted.
The FDA plans to release two more advanced notices of proposed rule making related to using regulatory levers to reduce cigarette smoking, including one that addresses flavoring in tobacco and one related to the regulation of premium cigars. Other than the 90-day comment period related to the advanced notices, the agency has not set any deadlines on when it will act.
“Legally, we are not going to prejudge how long this will take or what will happen,” Mr. Zeller said. “This is an early step in what could be a potential rule-making process using the product standard authority. We will take a long and hard look at all the comments that come in over the next 90 days and based upon our review of all the information, all the comments that come in, all the feedback that we have, we will then make a decision about taking the next step in the rule-making process.”
Nicotine levels in cigarettes could see a significant reduction under regulatory options being considered by the Food and Drug Administration.
Cigarettes “are the only legal consumer product that, when used as intended, will kill half all long-term users,” FDA Commissioner Scott Gottlieb, MD, said in a statement announcing the effort.
The agency is seeking comment on a proposed regulation regarding “a potential maximum nicotine level that would be appropriate for the protection of public health, in light of scientific evidence about the addictive properties of nicotine in cigarettes.” An advance notice of proposed rule making was posted online March 15 and is scheduled for publication in the Federal Register on March 16.
The FDA also is seeking comments on a number of other areas to help inform potential regulatory action down the road, including whether a new standard for lower nicotine levels should be implemented at once or whether a phased-in approach should be taken; whether FDA should specify a method for manufacturers to use in order to detect nicotine levels in their products; and whether the proposed lower level is technically achievable.
The agency also is seeking comment on potential unintended effects of lowering the amount of nicotine in cigarettes, such as turning to other combustible tobacco products such as cigars in conjunction with or as a replacement for cigarette use; increasing the number of cigarettes smoked, or seeking comparable nicotine from noncombustible tobacco sources.
At this time, FDA is not suggesting what the target might be on a specific nicotine level. While the advanced notice asks specifically about the “merits of nicotine levels like 0.3, 0.4, and 0.5 mg nicotine/g of tobacco filler,” it is not suggesting that this is the range being considered.
[polldaddy:9960560]
“Not to prejudge any possible proposed rule that we would do or any possible level, that is the purpose of an advanced proposed rule making, but we share all the science that we are aware of, and we characterize the studies that have been done to date in trying to find out what that right level is,” Mitch Zeller, director of the FDA Center for Tobacco Products, said during a March 15 press call.
He said that the FDA aiming to make sure the level is low enough that it cannot be compensated for by smoking more or inhaling deeper and holding the breath in longer, much like how smokers compensated when they smoked “light” cigarettes in the unregulated market.
Mr. Zeller said that seeking comments on those levels is based on the scientific evidence that is laid out in the advanced notice, but it is not necessarily foreshadowing where the standard will be set.
Drastically reducing the amount of nicotine in cigarettes is expected to significantly lower not only the number of people addicted to cigarettes but also as the negative health effects of nicotine addiction, FDA experts wrote in a perspective piece published March 15 in the New England Journal of Medicine (doi: 10.1065/NEJMsr1714617).
“Our findings show that reducing the nicotine level in cigarettes has the potential to substantially reduce the enormous burden of smoking-related death and disease,” Benjamin J. Apelberg, PhD, director of the Division of Population Health Science, Office of Science, within the FDA Center for Tobacco Products, and his colleagues, wrote in the report.
Modeling for the implementation of a lower nicotine level policy suggests that smoking prevalence will decline from a median of 12.8% in baseline scenario to a median of 10.8% within a year of implementation, with the increase related to smoking cessation.
“We estimate that approximately 5 million additional smokers would quit smoking within a year after implementation of the hypothetical policy,” Dr. Apelberg and his colleagues wrote. “By 2060, smoking prevalence drops from 7.9% in the baseline scenario to 1.4% in the policy scenario.”
Their analysis is based on a nicotine level that is “so low that there would not be enough nicotine available in cigarette tobacco for smokers to sustain addiction,” they noted.
The FDA plans to release two more advanced notices of proposed rule making related to using regulatory levers to reduce cigarette smoking, including one that addresses flavoring in tobacco and one related to the regulation of premium cigars. Other than the 90-day comment period related to the advanced notices, the agency has not set any deadlines on when it will act.
“Legally, we are not going to prejudge how long this will take or what will happen,” Mr. Zeller said. “This is an early step in what could be a potential rule-making process using the product standard authority. We will take a long and hard look at all the comments that come in over the next 90 days and based upon our review of all the information, all the comments that come in, all the feedback that we have, we will then make a decision about taking the next step in the rule-making process.”
Payers part of the drug-pricing problem, says FDA commissioner
WASHINGTON –
“Payers are going to have to decide what they want,” Dr. Gottlieb said at the conference sponsored by America’s Health Insurance Plans. “Do they want the short-term profit boost that comes with these rebates or in the long-run assist in their function” to make things better for patients, for providers, and those who pay for care.
It is possible to spur competition while working within the confines of the rebate-based system, he said. “I don’t see these as binary choices. You can have your cake and eat it too. Or in this case your rebates.”
His comments received a quick rebuttal from AHIP.
“There is a lot of blame being pushed around,” Daniel Nam, AHIP executive director of federal programs, said in an interview. “Along with the blame are a lot of distractions, misdirections, and these easy one-off fixes or problems that pop their head up and come and go. ... We try to stay focused on what is the real problem and that is essentially the starting price.”
He noted that if a treatment has a high starting price, such at coming gene therapies that could cost $1 million per patient, “it creates a pressure on the industry and it threatens our health care system to be unsustainable in the long term. What we are worried about is that there is no check on [the pharmaceutical industry’s] ability to set high list prices and even subsequently increase them.”
Mr. Nam noted that there is a fine balance that needs to be achieved to allow manufacturers to profit while at the same time ensuring access to therapies at reasonable prices.
“We believe in the free market and competition, but we feel like there are levers that could bring down the list price very effectively,” such as meaningful competition. “The situation that we have now is really more about the list price being way too high and unchallenged at this moment.”
Mr. Nam also took issue with the characterization that insurers are gaming the rebate system for profit.
“With the rebates, plans and PBMs [pharmacy benefit managers] want the lowest net cost,” he said. “That is our end goal. When you have this accusation that plans manipulate the rebate structure in order to skim off a couple of dollars here and there ... they are giving plans a lot more credit than what they can actually do at the negotiation table.”
He said the rebate system “is not perfect by any means, but I think drawing a conclusion of an imperfect system that [creates] a perverse incentive for insurers is completely distracting from the real problem” of high list prices.
WASHINGTON –
“Payers are going to have to decide what they want,” Dr. Gottlieb said at the conference sponsored by America’s Health Insurance Plans. “Do they want the short-term profit boost that comes with these rebates or in the long-run assist in their function” to make things better for patients, for providers, and those who pay for care.
It is possible to spur competition while working within the confines of the rebate-based system, he said. “I don’t see these as binary choices. You can have your cake and eat it too. Or in this case your rebates.”
His comments received a quick rebuttal from AHIP.
“There is a lot of blame being pushed around,” Daniel Nam, AHIP executive director of federal programs, said in an interview. “Along with the blame are a lot of distractions, misdirections, and these easy one-off fixes or problems that pop their head up and come and go. ... We try to stay focused on what is the real problem and that is essentially the starting price.”
He noted that if a treatment has a high starting price, such at coming gene therapies that could cost $1 million per patient, “it creates a pressure on the industry and it threatens our health care system to be unsustainable in the long term. What we are worried about is that there is no check on [the pharmaceutical industry’s] ability to set high list prices and even subsequently increase them.”
Mr. Nam noted that there is a fine balance that needs to be achieved to allow manufacturers to profit while at the same time ensuring access to therapies at reasonable prices.
“We believe in the free market and competition, but we feel like there are levers that could bring down the list price very effectively,” such as meaningful competition. “The situation that we have now is really more about the list price being way too high and unchallenged at this moment.”
Mr. Nam also took issue with the characterization that insurers are gaming the rebate system for profit.
“With the rebates, plans and PBMs [pharmacy benefit managers] want the lowest net cost,” he said. “That is our end goal. When you have this accusation that plans manipulate the rebate structure in order to skim off a couple of dollars here and there ... they are giving plans a lot more credit than what they can actually do at the negotiation table.”
He said the rebate system “is not perfect by any means, but I think drawing a conclusion of an imperfect system that [creates] a perverse incentive for insurers is completely distracting from the real problem” of high list prices.
WASHINGTON –
“Payers are going to have to decide what they want,” Dr. Gottlieb said at the conference sponsored by America’s Health Insurance Plans. “Do they want the short-term profit boost that comes with these rebates or in the long-run assist in their function” to make things better for patients, for providers, and those who pay for care.
It is possible to spur competition while working within the confines of the rebate-based system, he said. “I don’t see these as binary choices. You can have your cake and eat it too. Or in this case your rebates.”
His comments received a quick rebuttal from AHIP.
“There is a lot of blame being pushed around,” Daniel Nam, AHIP executive director of federal programs, said in an interview. “Along with the blame are a lot of distractions, misdirections, and these easy one-off fixes or problems that pop their head up and come and go. ... We try to stay focused on what is the real problem and that is essentially the starting price.”
He noted that if a treatment has a high starting price, such at coming gene therapies that could cost $1 million per patient, “it creates a pressure on the industry and it threatens our health care system to be unsustainable in the long term. What we are worried about is that there is no check on [the pharmaceutical industry’s] ability to set high list prices and even subsequently increase them.”
Mr. Nam noted that there is a fine balance that needs to be achieved to allow manufacturers to profit while at the same time ensuring access to therapies at reasonable prices.
“We believe in the free market and competition, but we feel like there are levers that could bring down the list price very effectively,” such as meaningful competition. “The situation that we have now is really more about the list price being way too high and unchallenged at this moment.”
Mr. Nam also took issue with the characterization that insurers are gaming the rebate system for profit.
“With the rebates, plans and PBMs [pharmacy benefit managers] want the lowest net cost,” he said. “That is our end goal. When you have this accusation that plans manipulate the rebate structure in order to skim off a couple of dollars here and there ... they are giving plans a lot more credit than what they can actually do at the negotiation table.”
He said the rebate system “is not perfect by any means, but I think drawing a conclusion of an imperfect system that [creates] a perverse incentive for insurers is completely distracting from the real problem” of high list prices.
REPORTING FROM AHIP 2018
HM18 plenaries explore future of hospital medicine
The plenary sessions bookending the Society of Hospital Medicine’s HM18 conference will provide insight into the current state of hospital medicine and a glimpse at the directions in which it is evolving.
Opening the conference will be Kate Goodrich, MD, chief medical officer at the Centers for Medicare & Medicaid Services.
“What I want people to understand is the evolution within our health care system from one where we pay for volume to paying for value, and the role that Medicare can play in that,” Dr. Goodrich said in an interview. “Medicare has traditionally been sort of a passive payer, if you will, a passive payer of claims without a great deal of emphasis on the cost of care and the quality of care. [Now there is] a groundswell of concern nationally, not just here at CMS but nationwide, around the rising cost of care, and our quality of care is not as good as it should be for the amount that we spend.”
Dr. Goodrich said her plenary talk will look at how “that came to be, and then what CMS and other payers in the country are trying to do about it.” She said the United States is in a “truly transformative era in our health care system in changing how we pay for care, in service of better outcomes for patients and lower costs. I would like to give attendees the larger picture, of how we got here and what’s happening both at CMS and nationally to try and reverse some of those trends.”
Closing HM18, as has become tradition at the annual meeting, will be Robert Wachter, MD, MHM, of the University of California San Francisco, who will focus on the broader changes that must happen as the role of the hospitalist continues to evolve.
“I am going to talk about the changes in the world of hospital care and the importance of the field to innovate,” Dr. Wachter said. “To me, there are gravitational forces in the health care world that are making … patients who are in hospitals sicker than they were before. More and more patients are going to be cared for in outpatient settings and at home. We are going start to ... see things like sensors and telemedicine to enable more care outside of the hospital.”
Dr. Wachter said hospital medicine must evolve and mature, to continue to prove that hospitalists are indispensable staff members within the hospital.
“That was why the field became the fastest-growing profession in medical history. We can’t sit on our laurels. We have to continue to innovate,” he said. “Even as the system changes around us, I am confident that we will innovate. My talk will be a pep talk and include reflections on how the world of health care is changing, and what those changes will mean to hospitalists.”
As value-based purchasing programs – and the push to pay for value over volume in Medicare and the private sector – continue to become the norm, the expected trend of sicker, more complex patients entering the hospital is already happening, Dr. Goodrich said. She is experiencing it in her own clinical work, which continues in addition to her role at CMS.
“I can confirm from my own personal experience [that] I have absolutely encountered that exact trend,” she said. “I feel like every time I go in the hospital, my patients are sicker and more complex. That is the population of patients that hospitalists are dealing with. That’s why we are actually in that practice. We enjoy taking care of those types of patients and the challenges they bring, both on a clinical level but I would say also even on a social and economic level.”
Dr. Goodrich said that trend will present one of the key challenges hospitalists face in the future, especially as paying for value entails more two-sided risk.
“In a value-based purchasing world, transitioning to payments based on quality and cost is harder because by nature the sicker patients cost more and it is harder to improve their outcomes. They come to you already quite sick,” she said. “That’s a dilemma that a lot of hospitalists face, wondering ‘How is this going to affect me if I am already seeing the sickest of the sick?’ ”
Dr. Wachter noted that the trend of steering less sick patients to the outpatient setting, as well as other economic factors, would change the nature of hospitalist practice.
“It will be more acuity, more intensity, more complex relationships with your own hospital and often with partner hospitals,” he said. “More of the work will be digitally enabled than it would have been five or ten years ago.”
Integration of data and technology innovation will be a key to better serving this sicker population, Dr. Wachter predicted. We need “to take much fuller advantage than we have so far of the fact that we are all dealing with digital records and the decision support, the data analytics, the artificial intelligence that we get from our computer systems is pretty puny,” he said. “That is partly why physicians don’t love their computers so much. They spend huge amounts of time entering data into computers and don’t get much useful information out of it.”
Dr. Goodrich agreed that this is a challenge.
“How do we make it usable for the average front-line nurse or doctor who didn’t go to school to learn how to code and analyze data?” she asked. “How do we get platforms and analytics that are developed using human-centered design principles to make it very understandable and actionable to the front-end clinician, but also to patients and consumers? What is really needed to truly drive improvement is not just access to the data but usability.” She added that this problem is directly related to the usability of electronic health records. “That is a significant focus right now for the Office of the National Coordinator [of Health Information Technology] – to move away from just [adopting] EHRs, to promoting interoperability and also the usability aspects that exactly gets to the problems we’ve identified.”
Dr. Wachter also warned that too much data could have a negative impact on the delivery of care.
“One of the challenges we face is continuing to stay alert, not turn our brains off, and become increasingly dependent on the computer to give us information,” he said. “How do we avoid the challenges we’ve already seen from things like alert and alarm fatigue as the computer becomes more robust as an information source. There is always the risk it is going to overwhelm us with too much information, and we are going to fall asleep at the switch. Or when the computer says something that really is not right for a patient, we will not be thinking clearly enough to catch it.”
Despite the looming challenges and industry consolidation that are expected, Dr. Wachter doesn’t believe there will be any shortage of demand for hospitalists.
“I think in most circumstances, [hospitalists are a protected] profession, given the complexity, the high variations, and the dependence that it still has on seeing the patient, talking to the patient, and talking to multiple consultants,” he said. “It’s a pretty hard thing to replace with technology. Overall, the job situation is pretty bright.”
The plenary sessions bookending the Society of Hospital Medicine’s HM18 conference will provide insight into the current state of hospital medicine and a glimpse at the directions in which it is evolving.
Opening the conference will be Kate Goodrich, MD, chief medical officer at the Centers for Medicare & Medicaid Services.
“What I want people to understand is the evolution within our health care system from one where we pay for volume to paying for value, and the role that Medicare can play in that,” Dr. Goodrich said in an interview. “Medicare has traditionally been sort of a passive payer, if you will, a passive payer of claims without a great deal of emphasis on the cost of care and the quality of care. [Now there is] a groundswell of concern nationally, not just here at CMS but nationwide, around the rising cost of care, and our quality of care is not as good as it should be for the amount that we spend.”
Dr. Goodrich said her plenary talk will look at how “that came to be, and then what CMS and other payers in the country are trying to do about it.” She said the United States is in a “truly transformative era in our health care system in changing how we pay for care, in service of better outcomes for patients and lower costs. I would like to give attendees the larger picture, of how we got here and what’s happening both at CMS and nationally to try and reverse some of those trends.”
Closing HM18, as has become tradition at the annual meeting, will be Robert Wachter, MD, MHM, of the University of California San Francisco, who will focus on the broader changes that must happen as the role of the hospitalist continues to evolve.
“I am going to talk about the changes in the world of hospital care and the importance of the field to innovate,” Dr. Wachter said. “To me, there are gravitational forces in the health care world that are making … patients who are in hospitals sicker than they were before. More and more patients are going to be cared for in outpatient settings and at home. We are going start to ... see things like sensors and telemedicine to enable more care outside of the hospital.”
Dr. Wachter said hospital medicine must evolve and mature, to continue to prove that hospitalists are indispensable staff members within the hospital.
“That was why the field became the fastest-growing profession in medical history. We can’t sit on our laurels. We have to continue to innovate,” he said. “Even as the system changes around us, I am confident that we will innovate. My talk will be a pep talk and include reflections on how the world of health care is changing, and what those changes will mean to hospitalists.”
As value-based purchasing programs – and the push to pay for value over volume in Medicare and the private sector – continue to become the norm, the expected trend of sicker, more complex patients entering the hospital is already happening, Dr. Goodrich said. She is experiencing it in her own clinical work, which continues in addition to her role at CMS.
“I can confirm from my own personal experience [that] I have absolutely encountered that exact trend,” she said. “I feel like every time I go in the hospital, my patients are sicker and more complex. That is the population of patients that hospitalists are dealing with. That’s why we are actually in that practice. We enjoy taking care of those types of patients and the challenges they bring, both on a clinical level but I would say also even on a social and economic level.”
Dr. Goodrich said that trend will present one of the key challenges hospitalists face in the future, especially as paying for value entails more two-sided risk.
“In a value-based purchasing world, transitioning to payments based on quality and cost is harder because by nature the sicker patients cost more and it is harder to improve their outcomes. They come to you already quite sick,” she said. “That’s a dilemma that a lot of hospitalists face, wondering ‘How is this going to affect me if I am already seeing the sickest of the sick?’ ”
Dr. Wachter noted that the trend of steering less sick patients to the outpatient setting, as well as other economic factors, would change the nature of hospitalist practice.
“It will be more acuity, more intensity, more complex relationships with your own hospital and often with partner hospitals,” he said. “More of the work will be digitally enabled than it would have been five or ten years ago.”
Integration of data and technology innovation will be a key to better serving this sicker population, Dr. Wachter predicted. We need “to take much fuller advantage than we have so far of the fact that we are all dealing with digital records and the decision support, the data analytics, the artificial intelligence that we get from our computer systems is pretty puny,” he said. “That is partly why physicians don’t love their computers so much. They spend huge amounts of time entering data into computers and don’t get much useful information out of it.”
Dr. Goodrich agreed that this is a challenge.
“How do we make it usable for the average front-line nurse or doctor who didn’t go to school to learn how to code and analyze data?” she asked. “How do we get platforms and analytics that are developed using human-centered design principles to make it very understandable and actionable to the front-end clinician, but also to patients and consumers? What is really needed to truly drive improvement is not just access to the data but usability.” She added that this problem is directly related to the usability of electronic health records. “That is a significant focus right now for the Office of the National Coordinator [of Health Information Technology] – to move away from just [adopting] EHRs, to promoting interoperability and also the usability aspects that exactly gets to the problems we’ve identified.”
Dr. Wachter also warned that too much data could have a negative impact on the delivery of care.
“One of the challenges we face is continuing to stay alert, not turn our brains off, and become increasingly dependent on the computer to give us information,” he said. “How do we avoid the challenges we’ve already seen from things like alert and alarm fatigue as the computer becomes more robust as an information source. There is always the risk it is going to overwhelm us with too much information, and we are going to fall asleep at the switch. Or when the computer says something that really is not right for a patient, we will not be thinking clearly enough to catch it.”
Despite the looming challenges and industry consolidation that are expected, Dr. Wachter doesn’t believe there will be any shortage of demand for hospitalists.
“I think in most circumstances, [hospitalists are a protected] profession, given the complexity, the high variations, and the dependence that it still has on seeing the patient, talking to the patient, and talking to multiple consultants,” he said. “It’s a pretty hard thing to replace with technology. Overall, the job situation is pretty bright.”
The plenary sessions bookending the Society of Hospital Medicine’s HM18 conference will provide insight into the current state of hospital medicine and a glimpse at the directions in which it is evolving.
Opening the conference will be Kate Goodrich, MD, chief medical officer at the Centers for Medicare & Medicaid Services.
“What I want people to understand is the evolution within our health care system from one where we pay for volume to paying for value, and the role that Medicare can play in that,” Dr. Goodrich said in an interview. “Medicare has traditionally been sort of a passive payer, if you will, a passive payer of claims without a great deal of emphasis on the cost of care and the quality of care. [Now there is] a groundswell of concern nationally, not just here at CMS but nationwide, around the rising cost of care, and our quality of care is not as good as it should be for the amount that we spend.”
Dr. Goodrich said her plenary talk will look at how “that came to be, and then what CMS and other payers in the country are trying to do about it.” She said the United States is in a “truly transformative era in our health care system in changing how we pay for care, in service of better outcomes for patients and lower costs. I would like to give attendees the larger picture, of how we got here and what’s happening both at CMS and nationally to try and reverse some of those trends.”
Closing HM18, as has become tradition at the annual meeting, will be Robert Wachter, MD, MHM, of the University of California San Francisco, who will focus on the broader changes that must happen as the role of the hospitalist continues to evolve.
“I am going to talk about the changes in the world of hospital care and the importance of the field to innovate,” Dr. Wachter said. “To me, there are gravitational forces in the health care world that are making … patients who are in hospitals sicker than they were before. More and more patients are going to be cared for in outpatient settings and at home. We are going start to ... see things like sensors and telemedicine to enable more care outside of the hospital.”
Dr. Wachter said hospital medicine must evolve and mature, to continue to prove that hospitalists are indispensable staff members within the hospital.
“That was why the field became the fastest-growing profession in medical history. We can’t sit on our laurels. We have to continue to innovate,” he said. “Even as the system changes around us, I am confident that we will innovate. My talk will be a pep talk and include reflections on how the world of health care is changing, and what those changes will mean to hospitalists.”
As value-based purchasing programs – and the push to pay for value over volume in Medicare and the private sector – continue to become the norm, the expected trend of sicker, more complex patients entering the hospital is already happening, Dr. Goodrich said. She is experiencing it in her own clinical work, which continues in addition to her role at CMS.
“I can confirm from my own personal experience [that] I have absolutely encountered that exact trend,” she said. “I feel like every time I go in the hospital, my patients are sicker and more complex. That is the population of patients that hospitalists are dealing with. That’s why we are actually in that practice. We enjoy taking care of those types of patients and the challenges they bring, both on a clinical level but I would say also even on a social and economic level.”
Dr. Goodrich said that trend will present one of the key challenges hospitalists face in the future, especially as paying for value entails more two-sided risk.
“In a value-based purchasing world, transitioning to payments based on quality and cost is harder because by nature the sicker patients cost more and it is harder to improve their outcomes. They come to you already quite sick,” she said. “That’s a dilemma that a lot of hospitalists face, wondering ‘How is this going to affect me if I am already seeing the sickest of the sick?’ ”
Dr. Wachter noted that the trend of steering less sick patients to the outpatient setting, as well as other economic factors, would change the nature of hospitalist practice.
“It will be more acuity, more intensity, more complex relationships with your own hospital and often with partner hospitals,” he said. “More of the work will be digitally enabled than it would have been five or ten years ago.”
Integration of data and technology innovation will be a key to better serving this sicker population, Dr. Wachter predicted. We need “to take much fuller advantage than we have so far of the fact that we are all dealing with digital records and the decision support, the data analytics, the artificial intelligence that we get from our computer systems is pretty puny,” he said. “That is partly why physicians don’t love their computers so much. They spend huge amounts of time entering data into computers and don’t get much useful information out of it.”
Dr. Goodrich agreed that this is a challenge.
“How do we make it usable for the average front-line nurse or doctor who didn’t go to school to learn how to code and analyze data?” she asked. “How do we get platforms and analytics that are developed using human-centered design principles to make it very understandable and actionable to the front-end clinician, but also to patients and consumers? What is really needed to truly drive improvement is not just access to the data but usability.” She added that this problem is directly related to the usability of electronic health records. “That is a significant focus right now for the Office of the National Coordinator [of Health Information Technology] – to move away from just [adopting] EHRs, to promoting interoperability and also the usability aspects that exactly gets to the problems we’ve identified.”
Dr. Wachter also warned that too much data could have a negative impact on the delivery of care.
“One of the challenges we face is continuing to stay alert, not turn our brains off, and become increasingly dependent on the computer to give us information,” he said. “How do we avoid the challenges we’ve already seen from things like alert and alarm fatigue as the computer becomes more robust as an information source. There is always the risk it is going to overwhelm us with too much information, and we are going to fall asleep at the switch. Or when the computer says something that really is not right for a patient, we will not be thinking clearly enough to catch it.”
Despite the looming challenges and industry consolidation that are expected, Dr. Wachter doesn’t believe there will be any shortage of demand for hospitalists.
“I think in most circumstances, [hospitalists are a protected] profession, given the complexity, the high variations, and the dependence that it still has on seeing the patient, talking to the patient, and talking to multiple consultants,” he said. “It’s a pretty hard thing to replace with technology. Overall, the job situation is pretty bright.”
Maternity care: The challenge of paying for value
TriHealth of Cincinnati is testing the waters of value-based payment for maternity care.
The impetus came from a Cincinnati-based employer that contracts with TriHealth under its self-insured health care plan, according to Jennifer Pavelka, project lead for the health system’s Maternity Bundling Care Select Program.
“Employers are looking at the value proposition and are having keen interest in the achievement of the triple aim of optimal outcomes, optimal experience, both with a mindfulness on value, Ms. Pavelka said in an interview. “An employer that had extensive experience with other episodes of care [payments], particularly in the space of knee and hip replacements in the orthopedic world, wanted to dip their toe into a value-based program for maternity.”
“Many forays into value-based payments are very much code driven, saying this code, this service is included in the bundle and this is not,” Ms. Pavelka said. “Part of what makes our work really challenging and exciting is the fact that we have taken in the realm of the methodology and philosophy of bundles, we are implementing a prospective bundled payment model.”
Their engaged physician community has been a key factor in early successes, she added. “What heartens me the most is the degree to which the physicians have really led and informed that evolution of the program and the commitment on the partner side to say first and foremost what does the evidence say, what is clinically appropriate. That has always been our North Star.”
Even with the guidelines of eligibility, patient selection is at the physician’s discretion.
And doctors are helping to shape how women are included in the process.
“We actually have two tiers,” Dr. Marcotte said. “Tier one is the uncomplicated pregnancy and our first project was really to get consensus [around those patients]. There was quite a bit of engagement. We had several meetings at the beginning of our project to help build consensus and we continue to look at that as new evidence comes out to make sure that we are staying consistent with best practice.”
The second tier focuses on more complicated pregnancies.
“There was a reengagement with providers, both our subspecialists in maternal fetal medicine and our generalists [ob.gyns.], to rethink what are the essential elements of best practice care for a patient that has certain complications like hypertension or twins or some of the other things that we included in the tier two clinical care pathways.”
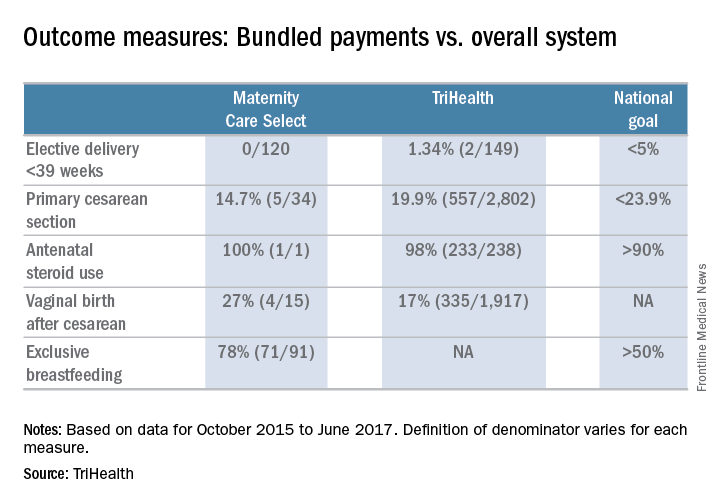
So far, very early results show that those in the bundle are in fact seeing better outcomes, something that in general is expected of a value-based payment model.
The bundled payment program also is opening the door to new service that can be provided to maternity patients.
“We are still relatively early in that journey,” Dr. Marcotte said. “We have a full-time concierge navigator who really works with this population, and we are beginning to make plans to expand that service to more patients beyond just our one population that are in the bundled care package. That’s one concrete area.”
He continued: “I think there is recognition of the need to provide more [patient] education and resources for our entire population that we are working with. Lastly, I think some of our specialized obstetric services for women with complicated pregnancies have gotten a boost. We can additionally provide those kind of services that might be extended beyond just our local community and provide attractive things that can be best in class for even those complicated pregnancies.”
TriHealth really found the key with its early and robust physician involvement, according to Malini Nijagal, MD, associate professor of ob.gyn. and reproductive sciences at the University of California, San Francisco.
“The strong sense that I have is that there is a bit of a disconnect, where it feels on the ground like people believe that value-based payment models may be good for finance and health care expenditures but they are not going to be good for patients and providers,” Dr. Nijagal said in an interview. “I totally disagree.”
Rather, value-based payment models are a way to increase provider autonomy.
“Specific to maternity care and across health care, at this point, insurers are the ones who decide what services we can provide,” she said. “They have their fee schedules and that limits what we can do. What value-based payments and episode payments allow us to do is that, as providers, if we end up saving money from avoiding unnecessary interventions and diagnostics, we then get some of that money back to provide services that may not be covered.”
Dr. Nijagal and her colleagues looked at maternity care bundles and episode payment models in state Medicaid programs, managed care plans, and self-insured employers and found that physicians and other providers can benefit from participation in four ways, by:
- Finding opportunities to improve outcomes and patient experience.
- Developing better quality metrics.
- Reducing waste in the system.
- Creating stronger health care teams.
Value-based payments can “actually bring together hospitals and health care providers onto the same team and the way they do that is by tying the payment together,” Dr. Nijagal said.
The biggest challenge, according to Dr. Nijagal, is that the conversion takes time and the patience to realize that benefits will likely not be fully realized until the system is able to get to a prospective payment model, in which the real innovation can take place (Am J Obstet Gynecol. 2018 Jan 12. doi. org/10.1016/j.ajog.2018.01.014).
“We are having to prioritize just getting providers to agree to doing them and because of that, we are not actually seeing the improvements in quality and cost that we would expect,” Dr. Nijagal said. “For example, a number of the programs that have been rolled out have been the upside risk only, which is superimportant because that is the way you are going to get people to feel more comfortable. It’s a stepping-stone, but you are not going to see the benefits.” And if you can’t prove benefit, it will be harder to get people to move to a two-sided risk model where the real innovation can take place.
To that end, TriHealth provides full transparency with the program so physicians can see how patients in the bundle are doing compared to the overall TriHealth patient population.
“We provide those to them in a dashboard on a monthly basis with transparency so they can see how the other providers in other practices are doing within the system,” Dr, Marcotte said.
TriHealth of Cincinnati is testing the waters of value-based payment for maternity care.
The impetus came from a Cincinnati-based employer that contracts with TriHealth under its self-insured health care plan, according to Jennifer Pavelka, project lead for the health system’s Maternity Bundling Care Select Program.
“Employers are looking at the value proposition and are having keen interest in the achievement of the triple aim of optimal outcomes, optimal experience, both with a mindfulness on value, Ms. Pavelka said in an interview. “An employer that had extensive experience with other episodes of care [payments], particularly in the space of knee and hip replacements in the orthopedic world, wanted to dip their toe into a value-based program for maternity.”
“Many forays into value-based payments are very much code driven, saying this code, this service is included in the bundle and this is not,” Ms. Pavelka said. “Part of what makes our work really challenging and exciting is the fact that we have taken in the realm of the methodology and philosophy of bundles, we are implementing a prospective bundled payment model.”
Their engaged physician community has been a key factor in early successes, she added. “What heartens me the most is the degree to which the physicians have really led and informed that evolution of the program and the commitment on the partner side to say first and foremost what does the evidence say, what is clinically appropriate. That has always been our North Star.”
Even with the guidelines of eligibility, patient selection is at the physician’s discretion.

And doctors are helping to shape how women are included in the process.
“We actually have two tiers,” Dr. Marcotte said. “Tier one is the uncomplicated pregnancy and our first project was really to get consensus [around those patients]. There was quite a bit of engagement. We had several meetings at the beginning of our project to help build consensus and we continue to look at that as new evidence comes out to make sure that we are staying consistent with best practice.”
The second tier focuses on more complicated pregnancies.
“There was a reengagement with providers, both our subspecialists in maternal fetal medicine and our generalists [ob.gyns.], to rethink what are the essential elements of best practice care for a patient that has certain complications like hypertension or twins or some of the other things that we included in the tier two clinical care pathways.”
So far, very early results show that those in the bundle are in fact seeing better outcomes, something that in general is expected of a value-based payment model.
The bundled payment program also is opening the door to new service that can be provided to maternity patients.
“We are still relatively early in that journey,” Dr. Marcotte said. “We have a full-time concierge navigator who really works with this population, and we are beginning to make plans to expand that service to more patients beyond just our one population that are in the bundled care package. That’s one concrete area.”
He continued: “I think there is recognition of the need to provide more [patient] education and resources for our entire population that we are working with. Lastly, I think some of our specialized obstetric services for women with complicated pregnancies have gotten a boost. We can additionally provide those kind of services that might be extended beyond just our local community and provide attractive things that can be best in class for even those complicated pregnancies.”
TriHealth really found the key with its early and robust physician involvement, according to Malini Nijagal, MD, associate professor of ob.gyn. and reproductive sciences at the University of California, San Francisco.
“The strong sense that I have is that there is a bit of a disconnect, where it feels on the ground like people believe that value-based payment models may be good for finance and health care expenditures but they are not going to be good for patients and providers,” Dr. Nijagal said in an interview. “I totally disagree.”
Rather, value-based payment models are a way to increase provider autonomy.
“Specific to maternity care and across health care, at this point, insurers are the ones who decide what services we can provide,” she said. “They have their fee schedules and that limits what we can do. What value-based payments and episode payments allow us to do is that, as providers, if we end up saving money from avoiding unnecessary interventions and diagnostics, we then get some of that money back to provide services that may not be covered.”
Dr. Nijagal and her colleagues looked at maternity care bundles and episode payment models in state Medicaid programs, managed care plans, and self-insured employers and found that physicians and other providers can benefit from participation in four ways, by:
- Finding opportunities to improve outcomes and patient experience.
- Developing better quality metrics.
- Reducing waste in the system.
- Creating stronger health care teams.
Value-based payments can “actually bring together hospitals and health care providers onto the same team and the way they do that is by tying the payment together,” Dr. Nijagal said.
The biggest challenge, according to Dr. Nijagal, is that the conversion takes time and the patience to realize that benefits will likely not be fully realized until the system is able to get to a prospective payment model, in which the real innovation can take place (Am J Obstet Gynecol. 2018 Jan 12. doi. org/10.1016/j.ajog.2018.01.014).
“We are having to prioritize just getting providers to agree to doing them and because of that, we are not actually seeing the improvements in quality and cost that we would expect,” Dr. Nijagal said. “For example, a number of the programs that have been rolled out have been the upside risk only, which is superimportant because that is the way you are going to get people to feel more comfortable. It’s a stepping-stone, but you are not going to see the benefits.” And if you can’t prove benefit, it will be harder to get people to move to a two-sided risk model where the real innovation can take place.
To that end, TriHealth provides full transparency with the program so physicians can see how patients in the bundle are doing compared to the overall TriHealth patient population.
“We provide those to them in a dashboard on a monthly basis with transparency so they can see how the other providers in other practices are doing within the system,” Dr, Marcotte said.
TriHealth of Cincinnati is testing the waters of value-based payment for maternity care.
The impetus came from a Cincinnati-based employer that contracts with TriHealth under its self-insured health care plan, according to Jennifer Pavelka, project lead for the health system’s Maternity Bundling Care Select Program.
“Employers are looking at the value proposition and are having keen interest in the achievement of the triple aim of optimal outcomes, optimal experience, both with a mindfulness on value, Ms. Pavelka said in an interview. “An employer that had extensive experience with other episodes of care [payments], particularly in the space of knee and hip replacements in the orthopedic world, wanted to dip their toe into a value-based program for maternity.”
“Many forays into value-based payments are very much code driven, saying this code, this service is included in the bundle and this is not,” Ms. Pavelka said. “Part of what makes our work really challenging and exciting is the fact that we have taken in the realm of the methodology and philosophy of bundles, we are implementing a prospective bundled payment model.”
Their engaged physician community has been a key factor in early successes, she added. “What heartens me the most is the degree to which the physicians have really led and informed that evolution of the program and the commitment on the partner side to say first and foremost what does the evidence say, what is clinically appropriate. That has always been our North Star.”
Even with the guidelines of eligibility, patient selection is at the physician’s discretion.

And doctors are helping to shape how women are included in the process.
“We actually have two tiers,” Dr. Marcotte said. “Tier one is the uncomplicated pregnancy and our first project was really to get consensus [around those patients]. There was quite a bit of engagement. We had several meetings at the beginning of our project to help build consensus and we continue to look at that as new evidence comes out to make sure that we are staying consistent with best practice.”
The second tier focuses on more complicated pregnancies.
“There was a reengagement with providers, both our subspecialists in maternal fetal medicine and our generalists [ob.gyns.], to rethink what are the essential elements of best practice care for a patient that has certain complications like hypertension or twins or some of the other things that we included in the tier two clinical care pathways.”
So far, very early results show that those in the bundle are in fact seeing better outcomes, something that in general is expected of a value-based payment model.
The bundled payment program also is opening the door to new service that can be provided to maternity patients.
“We are still relatively early in that journey,” Dr. Marcotte said. “We have a full-time concierge navigator who really works with this population, and we are beginning to make plans to expand that service to more patients beyond just our one population that are in the bundled care package. That’s one concrete area.”
He continued: “I think there is recognition of the need to provide more [patient] education and resources for our entire population that we are working with. Lastly, I think some of our specialized obstetric services for women with complicated pregnancies have gotten a boost. We can additionally provide those kind of services that might be extended beyond just our local community and provide attractive things that can be best in class for even those complicated pregnancies.”
TriHealth really found the key with its early and robust physician involvement, according to Malini Nijagal, MD, associate professor of ob.gyn. and reproductive sciences at the University of California, San Francisco.
“The strong sense that I have is that there is a bit of a disconnect, where it feels on the ground like people believe that value-based payment models may be good for finance and health care expenditures but they are not going to be good for patients and providers,” Dr. Nijagal said in an interview. “I totally disagree.”
Rather, value-based payment models are a way to increase provider autonomy.
“Specific to maternity care and across health care, at this point, insurers are the ones who decide what services we can provide,” she said. “They have their fee schedules and that limits what we can do. What value-based payments and episode payments allow us to do is that, as providers, if we end up saving money from avoiding unnecessary interventions and diagnostics, we then get some of that money back to provide services that may not be covered.”
Dr. Nijagal and her colleagues looked at maternity care bundles and episode payment models in state Medicaid programs, managed care plans, and self-insured employers and found that physicians and other providers can benefit from participation in four ways, by:
- Finding opportunities to improve outcomes and patient experience.
- Developing better quality metrics.
- Reducing waste in the system.
- Creating stronger health care teams.
Value-based payments can “actually bring together hospitals and health care providers onto the same team and the way they do that is by tying the payment together,” Dr. Nijagal said.
The biggest challenge, according to Dr. Nijagal, is that the conversion takes time and the patience to realize that benefits will likely not be fully realized until the system is able to get to a prospective payment model, in which the real innovation can take place (Am J Obstet Gynecol. 2018 Jan 12. doi. org/10.1016/j.ajog.2018.01.014).
“We are having to prioritize just getting providers to agree to doing them and because of that, we are not actually seeing the improvements in quality and cost that we would expect,” Dr. Nijagal said. “For example, a number of the programs that have been rolled out have been the upside risk only, which is superimportant because that is the way you are going to get people to feel more comfortable. It’s a stepping-stone, but you are not going to see the benefits.” And if you can’t prove benefit, it will be harder to get people to move to a two-sided risk model where the real innovation can take place.
To that end, TriHealth provides full transparency with the program so physicians can see how patients in the bundle are doing compared to the overall TriHealth patient population.
“We provide those to them in a dashboard on a monthly basis with transparency so they can see how the other providers in other practices are doing within the system,” Dr, Marcotte said.
Hospital chemo carries higher price tag than the office
Commercial insurers are spending nearly twice as much on chemotherapy administered in hospital outpatient departments as they are for therapy administered in a physician’s office, according to an analysis of a decade of claims data.
Commercial insurance data from 283,502 patients who initiated treatment with infused chemotherapy and remained enrolled continuously for 6 months, without receiving infused chemotherapy in the preceding 6 months, revealed that spending at the drug level was “significantly lower in offices vs. in HOPDs [hospital outpatient departments],” Aaron Winn, PhD, of the Medical College of Wisconsin, Milwaukee, and his colleagues wrote in a research letter published Feb. 22 in JAMA Oncology.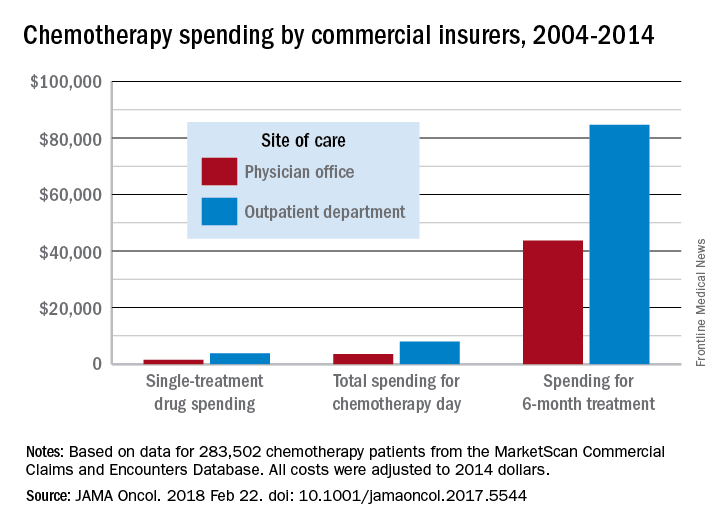
During the review period from Jan. 1, 2004 through Dec. 31, 2014, the rate of commercially-insured patients receiving chemotherapy in HOPDs grew from 6% in 2004 to 43% in 2014. The spending data was adjusted for various factors, including, sex, comorbidity, year of diagnosis, drug administered, and location.
“Shifting the provision of infused chemotherapy from physician offices to HOPDs is increasing and is associated with increased spending for chemotherapy services,” the researchers wrote. “Potential targets for reduction of excess spending can come from private insurers following Medicaid’s lead, which has started to equalize payments across sites of care.”
“I was a little surprised that the site location was converging on 50-50 across the country,” Dr. Carole Miller, director of the Cancer Institute at St. Agnes Hospital, Baltimore, said in an interview, adding that the spending figures were not a surprise.
Dr. Miller noted that another thing the claims data does not capture are the kinds of additional services that patients are receiving in their respective sites of care, which could also account for the difference in total reimbursement. For example, hospital-based cancer centers may offer more social support service given that the patients tend to be older and may have more social service needs as well as more uncompensated care.
David Henry, MD, an oncologist who practices in a community setting that is part of the University of Pennsylvania hospital system, said the data fits with his experience. “Is the care better? I don’t think so. Is the overhead bigger? Sure.”
Part of what makes the care better in the community setting is the patient experience, said Dr. Henry, who serves as editor-in-chief of the Journal of Community and Supportive Oncology, which is published by this news organization.
The office setting can often boast a streamlined experience, he added. In the hospital, the administrative elements and travel across the hospital campus can make an infusion a day-long task, versus going to a community office setting where the total infusion process, including the administrative aspects, can be handled in a few hours.
Dr. Henry acknowledged that the hospital setting does have an advantage in terms of depth of services, including specialists to deal with a variety of tumors.
Dr. Henry noted that the current analysis does not address the shift to paying for value and the move away from fee-for-service payment. Speaking about whether Medicare’s Quality Payment Program can level the playing field in some ways between the two settings of care, he said “the idea has potential” but the success will be determined by how it is implemented.
This article was updated on 2/22/18.
gtwachtman@frontlinemedcom.com
SOURCE: Winn A et al., JAMA Oncol. 2018 Feb 22. doi: 10.1001/jamaoncol.2017.5544.
Commercial insurers are spending nearly twice as much on chemotherapy administered in hospital outpatient departments as they are for therapy administered in a physician’s office, according to an analysis of a decade of claims data.
Commercial insurance data from 283,502 patients who initiated treatment with infused chemotherapy and remained enrolled continuously for 6 months, without receiving infused chemotherapy in the preceding 6 months, revealed that spending at the drug level was “significantly lower in offices vs. in HOPDs [hospital outpatient departments],” Aaron Winn, PhD, of the Medical College of Wisconsin, Milwaukee, and his colleagues wrote in a research letter published Feb. 22 in JAMA Oncology.
During the review period from Jan. 1, 2004 through Dec. 31, 2014, the rate of commercially-insured patients receiving chemotherapy in HOPDs grew from 6% in 2004 to 43% in 2014. The spending data was adjusted for various factors, including, sex, comorbidity, year of diagnosis, drug administered, and location.
“Shifting the provision of infused chemotherapy from physician offices to HOPDs is increasing and is associated with increased spending for chemotherapy services,” the researchers wrote. “Potential targets for reduction of excess spending can come from private insurers following Medicaid’s lead, which has started to equalize payments across sites of care.”
“I was a little surprised that the site location was converging on 50-50 across the country,” Dr. Carole Miller, director of the Cancer Institute at St. Agnes Hospital, Baltimore, said in an interview, adding that the spending figures were not a surprise.
Dr. Miller noted that another thing the claims data does not capture are the kinds of additional services that patients are receiving in their respective sites of care, which could also account for the difference in total reimbursement. For example, hospital-based cancer centers may offer more social support service given that the patients tend to be older and may have more social service needs as well as more uncompensated care.
David Henry, MD, an oncologist who practices in a community setting that is part of the University of Pennsylvania hospital system, said the data fits with his experience. “Is the care better? I don’t think so. Is the overhead bigger? Sure.”
Part of what makes the care better in the community setting is the patient experience, said Dr. Henry, who serves as editor-in-chief of the Journal of Community and Supportive Oncology, which is published by this news organization.
The office setting can often boast a streamlined experience, he added. In the hospital, the administrative elements and travel across the hospital campus can make an infusion a day-long task, versus going to a community office setting where the total infusion process, including the administrative aspects, can be handled in a few hours.
Dr. Henry acknowledged that the hospital setting does have an advantage in terms of depth of services, including specialists to deal with a variety of tumors.
Dr. Henry noted that the current analysis does not address the shift to paying for value and the move away from fee-for-service payment. Speaking about whether Medicare’s Quality Payment Program can level the playing field in some ways between the two settings of care, he said “the idea has potential” but the success will be determined by how it is implemented.
This article was updated on 2/22/18.
gtwachtman@frontlinemedcom.com
SOURCE: Winn A et al., JAMA Oncol. 2018 Feb 22. doi: 10.1001/jamaoncol.2017.5544.
Commercial insurers are spending nearly twice as much on chemotherapy administered in hospital outpatient departments as they are for therapy administered in a physician’s office, according to an analysis of a decade of claims data.
Commercial insurance data from 283,502 patients who initiated treatment with infused chemotherapy and remained enrolled continuously for 6 months, without receiving infused chemotherapy in the preceding 6 months, revealed that spending at the drug level was “significantly lower in offices vs. in HOPDs [hospital outpatient departments],” Aaron Winn, PhD, of the Medical College of Wisconsin, Milwaukee, and his colleagues wrote in a research letter published Feb. 22 in JAMA Oncology.
During the review period from Jan. 1, 2004 through Dec. 31, 2014, the rate of commercially-insured patients receiving chemotherapy in HOPDs grew from 6% in 2004 to 43% in 2014. The spending data was adjusted for various factors, including, sex, comorbidity, year of diagnosis, drug administered, and location.
“Shifting the provision of infused chemotherapy from physician offices to HOPDs is increasing and is associated with increased spending for chemotherapy services,” the researchers wrote. “Potential targets for reduction of excess spending can come from private insurers following Medicaid’s lead, which has started to equalize payments across sites of care.”
“I was a little surprised that the site location was converging on 50-50 across the country,” Dr. Carole Miller, director of the Cancer Institute at St. Agnes Hospital, Baltimore, said in an interview, adding that the spending figures were not a surprise.
Dr. Miller noted that another thing the claims data does not capture are the kinds of additional services that patients are receiving in their respective sites of care, which could also account for the difference in total reimbursement. For example, hospital-based cancer centers may offer more social support service given that the patients tend to be older and may have more social service needs as well as more uncompensated care.
David Henry, MD, an oncologist who practices in a community setting that is part of the University of Pennsylvania hospital system, said the data fits with his experience. “Is the care better? I don’t think so. Is the overhead bigger? Sure.”
Part of what makes the care better in the community setting is the patient experience, said Dr. Henry, who serves as editor-in-chief of the Journal of Community and Supportive Oncology, which is published by this news organization.
The office setting can often boast a streamlined experience, he added. In the hospital, the administrative elements and travel across the hospital campus can make an infusion a day-long task, versus going to a community office setting where the total infusion process, including the administrative aspects, can be handled in a few hours.
Dr. Henry acknowledged that the hospital setting does have an advantage in terms of depth of services, including specialists to deal with a variety of tumors.
Dr. Henry noted that the current analysis does not address the shift to paying for value and the move away from fee-for-service payment. Speaking about whether Medicare’s Quality Payment Program can level the playing field in some ways between the two settings of care, he said “the idea has potential” but the success will be determined by how it is implemented.
This article was updated on 2/22/18.
gtwachtman@frontlinemedcom.com
SOURCE: Winn A et al., JAMA Oncol. 2018 Feb 22. doi: 10.1001/jamaoncol.2017.5544.
FROM JAMA ONCOLOGY
Key clinical point:
Major finding: Total reimbursement during the 6-month treatment episode was lower in offices ($43,700) than in hospital outpatient departments ($84,660).
Study details: An examination of claims data from 283,502 patients who initiated treatment with infused chemotherapy and remained enrolled continuously for 6 months between Jan. 1, 2004, and Dec. 31, 2014.
Disclosures: The researchers reported having no financial conflicts of interest.
Source: Winn A et al., JAMA Oncol. 2018 Feb 22. doi: 10.1001/jamaoncol.2017.5544.
Opioids a focus as HHS Secretary Azar defends White House budget proposal
The opioid abuse epidemic took center stage during a hearing of the House Ways and Means Committee, during which Alex Azar, secretary of the Department of Health & Human Services, defended the White House budget proposal for fiscal year 2019.
The president’s budget, released Feb. 13, allocates $5 billion in new resources over the next 5 years to combat the opioid abuse epidemic
During his Feb. 14 testimony, Mr. Azar said that the budget proposal “brings a new level of commitment to fighting the crisis of opioid addiction and overdose that is stealing more than 100 American lives from us every single day.”
He noted that part of the funding request includes spending $500 million to launch public-private partnership with the National Institutes of Health “to development new addiction treatments, new overdose-reversing drugs, and nonaddictive approaches to pain.”
Mr. Azar called for greater use of nonopioid pain management techniques and better communication between the HHS and the Drug Enforcement Agency to identify doctors who overprescribe opioids.
“I really want to focus on that entry point to working with DEA on ways that we can control pill mills and even just bad practice that has become part of our culture of medicine of giving people excessive numbers of pills when they do not need them,” Mr. Azar said.
The administration’s proposals for addressing the opioid crisis revealed partisan divisions among members of the Ways and Means Committee, as Republican members of the committee hailed the White House proposals while Democrats argued that the budget proposal could make it more difficult for people to fight the crisis.
Noting that many who have opioid use disorders rely on Medicaid for health insurance, Rep. Richard Neal (D-Mass.), the ranking member on the committee, noted that the proposed budget cuts $1.4 trillion from Medicaid and $500 billion from Medicare.
Physician groups were quick to point out that the budget proposal calls for addressing health care issues while simultaneously cutting the necessary funding.
The Infectious Diseases Society of America said in a statement that “while we appreciate continued funding for antimicrobial resistance research and development through the Biomedical Advanced Research and Development Authority, the current investment is insufficient, as evidenced by an antibiotic pipeline that falls far short of projected needs, while pharmaceutical company investments in antibiotic research continue to diminish. Also, the plan would limit CDC [Centers for Disease Control and Prevention] efforts to address and prevent growing resistance to existing antimicrobial drugs with a nearly $25 million cut.”
The White House budget proposal also tackles drug pricing and payment, including a new Medicaid demonstration program that would allow up to five participating states to test the use of drug formularies in the Medicaid program, speed the access to generic medications, and modify the Medicare Part D prescription drug program. Mr. Azar also reiterated his openness to government price negotiation for drugs administered in the physician office under Medicare Part B.
The proposal on drug discounts was praised by the Community Oncology Alliance (COA).
“The White House budget proposal for sharing manufacturer rebates and discounts with seniors in Medicare Part D is also a great idea,” COA said in a statement. “Pharmacy benefit managers have been enriching themselves with these rebates for too long, and their growing scale has resulted in higher drug costs for everyone. COA believes that the proposed Part D change to share rebates and discounts proposed will lower costs for patients, taxpayers, and the government.”
The budget proposal reflects President Trump’s desire to see the Affordable Care Act repealed and is built on that premise, but questions linger as to whether Congress will take up health care legislation again this year.
The opioid abuse epidemic took center stage during a hearing of the House Ways and Means Committee, during which Alex Azar, secretary of the Department of Health & Human Services, defended the White House budget proposal for fiscal year 2019.
The president’s budget, released Feb. 13, allocates $5 billion in new resources over the next 5 years to combat the opioid abuse epidemic
During his Feb. 14 testimony, Mr. Azar said that the budget proposal “brings a new level of commitment to fighting the crisis of opioid addiction and overdose that is stealing more than 100 American lives from us every single day.”
He noted that part of the funding request includes spending $500 million to launch public-private partnership with the National Institutes of Health “to development new addiction treatments, new overdose-reversing drugs, and nonaddictive approaches to pain.”
Mr. Azar called for greater use of nonopioid pain management techniques and better communication between the HHS and the Drug Enforcement Agency to identify doctors who overprescribe opioids.
“I really want to focus on that entry point to working with DEA on ways that we can control pill mills and even just bad practice that has become part of our culture of medicine of giving people excessive numbers of pills when they do not need them,” Mr. Azar said.
The administration’s proposals for addressing the opioid crisis revealed partisan divisions among members of the Ways and Means Committee, as Republican members of the committee hailed the White House proposals while Democrats argued that the budget proposal could make it more difficult for people to fight the crisis.
Noting that many who have opioid use disorders rely on Medicaid for health insurance, Rep. Richard Neal (D-Mass.), the ranking member on the committee, noted that the proposed budget cuts $1.4 trillion from Medicaid and $500 billion from Medicare.
Physician groups were quick to point out that the budget proposal calls for addressing health care issues while simultaneously cutting the necessary funding.
The Infectious Diseases Society of America said in a statement that “while we appreciate continued funding for antimicrobial resistance research and development through the Biomedical Advanced Research and Development Authority, the current investment is insufficient, as evidenced by an antibiotic pipeline that falls far short of projected needs, while pharmaceutical company investments in antibiotic research continue to diminish. Also, the plan would limit CDC [Centers for Disease Control and Prevention] efforts to address and prevent growing resistance to existing antimicrobial drugs with a nearly $25 million cut.”
The White House budget proposal also tackles drug pricing and payment, including a new Medicaid demonstration program that would allow up to five participating states to test the use of drug formularies in the Medicaid program, speed the access to generic medications, and modify the Medicare Part D prescription drug program. Mr. Azar also reiterated his openness to government price negotiation for drugs administered in the physician office under Medicare Part B.
The proposal on drug discounts was praised by the Community Oncology Alliance (COA).
“The White House budget proposal for sharing manufacturer rebates and discounts with seniors in Medicare Part D is also a great idea,” COA said in a statement. “Pharmacy benefit managers have been enriching themselves with these rebates for too long, and their growing scale has resulted in higher drug costs for everyone. COA believes that the proposed Part D change to share rebates and discounts proposed will lower costs for patients, taxpayers, and the government.”
The budget proposal reflects President Trump’s desire to see the Affordable Care Act repealed and is built on that premise, but questions linger as to whether Congress will take up health care legislation again this year.
The opioid abuse epidemic took center stage during a hearing of the House Ways and Means Committee, during which Alex Azar, secretary of the Department of Health & Human Services, defended the White House budget proposal for fiscal year 2019.
The president’s budget, released Feb. 13, allocates $5 billion in new resources over the next 5 years to combat the opioid abuse epidemic
During his Feb. 14 testimony, Mr. Azar said that the budget proposal “brings a new level of commitment to fighting the crisis of opioid addiction and overdose that is stealing more than 100 American lives from us every single day.”
He noted that part of the funding request includes spending $500 million to launch public-private partnership with the National Institutes of Health “to development new addiction treatments, new overdose-reversing drugs, and nonaddictive approaches to pain.”
Mr. Azar called for greater use of nonopioid pain management techniques and better communication between the HHS and the Drug Enforcement Agency to identify doctors who overprescribe opioids.
“I really want to focus on that entry point to working with DEA on ways that we can control pill mills and even just bad practice that has become part of our culture of medicine of giving people excessive numbers of pills when they do not need them,” Mr. Azar said.
The administration’s proposals for addressing the opioid crisis revealed partisan divisions among members of the Ways and Means Committee, as Republican members of the committee hailed the White House proposals while Democrats argued that the budget proposal could make it more difficult for people to fight the crisis.
Noting that many who have opioid use disorders rely on Medicaid for health insurance, Rep. Richard Neal (D-Mass.), the ranking member on the committee, noted that the proposed budget cuts $1.4 trillion from Medicaid and $500 billion from Medicare.
Physician groups were quick to point out that the budget proposal calls for addressing health care issues while simultaneously cutting the necessary funding.
The Infectious Diseases Society of America said in a statement that “while we appreciate continued funding for antimicrobial resistance research and development through the Biomedical Advanced Research and Development Authority, the current investment is insufficient, as evidenced by an antibiotic pipeline that falls far short of projected needs, while pharmaceutical company investments in antibiotic research continue to diminish. Also, the plan would limit CDC [Centers for Disease Control and Prevention] efforts to address and prevent growing resistance to existing antimicrobial drugs with a nearly $25 million cut.”
The White House budget proposal also tackles drug pricing and payment, including a new Medicaid demonstration program that would allow up to five participating states to test the use of drug formularies in the Medicaid program, speed the access to generic medications, and modify the Medicare Part D prescription drug program. Mr. Azar also reiterated his openness to government price negotiation for drugs administered in the physician office under Medicare Part B.
The proposal on drug discounts was praised by the Community Oncology Alliance (COA).
“The White House budget proposal for sharing manufacturer rebates and discounts with seniors in Medicare Part D is also a great idea,” COA said in a statement. “Pharmacy benefit managers have been enriching themselves with these rebates for too long, and their growing scale has resulted in higher drug costs for everyone. COA believes that the proposed Part D change to share rebates and discounts proposed will lower costs for patients, taxpayers, and the government.”
The budget proposal reflects President Trump’s desire to see the Affordable Care Act repealed and is built on that premise, but questions linger as to whether Congress will take up health care legislation again this year.
REPORTING FROM a HOUSE WAYS AND MEANS HEARING
Rheumatologists target prior authorization, Stark Law as barriers to competition
Onerous prior authorization rules, Stark Law, and other issues are creating barriers to health care choice and competition, the American College of Rheumatology recently told the Department of Health & Human Services.
The agency recently issued an informal request for information seeking “input from the public on the extent to which existing state and federal laws, regulations, guidance, requirements, and policies limit choice and competition across all health care markets, and the identification of actions that states or the federal government could take to support the development and operations of a health care system that provides high-quality care at affordable prices for the American people.”
“We believe this is a waste of valuable resources that results in delays in care and does not add value to health care delivery,” the ACR said in its Jan. 25 reply to the request for information. The ACR offered a series of recommendations to make the use of prior authorization by the Centers for Medicare & Medicaid Services more efficient.
Among the requested changes are:
• CMS should require all Medicare Advantage and Part D prescription drug plans to publicly disclose in a searchable electronic format to both patients and physicians all drugs and medical services that are subject to coverage restrictions (prior authorization, step therapy, formulary restrictions, quantity limits), and provide this information to vendors to be displayed in electronic health records.
• CMS should ensure that all utilization management requirements are based on accurate and up-to-date, publicly available clinical criteria and never cost alone.
• CMS should ensure that any “peer-to-peer” reviews utilize physicians from the same specialty/subspecialty as the ordering physician.
• CMS should restrict prior authorization requirements to “outlier” providers whose prescribing or ordering patterns differ significantly from their peers after adjusting for patient mix.
• CMS should not allow Part B services to be subject to prior authorization requirements because this would increase physician time spent on administrative tasks and reduce availability for patient care.
In addition, the ACR expressed concern about the consolidated pharmacy benefit managers market, noting that two PBMs cover more than 170 million Americans, and urged the HHS to “consider policies that require PBMs to be more transparent about their payment practices, including transparency around the true cost of prescription drugs.”
In the area of the Stark Law, the ACR called for the HHS to waive the prohibitions “for physicians seeking to develop and operate alternative payment models (APMs) as was provided to accountable care organizations in the Affordable Care Act. We also recommend removing the ‘volume or value’ prohibition in Stark policy so that physician practices can incentivize physicians to abide by best practices and succeed in new value-based alternative payment models.”
The ACR asked the CMS not to alter policy on assigning unique J-codes to biosimilars, as the unique codes allow for better monitoring of effectiveness and ensure adequate pharmacovigilance.
The physician organization also called for the removal of antitrust exemptions to insurance companies to give “the federal government the ability to intervene in places where insurance monopolies exist or develop.”
One request made – the removal of Part B drug payments from being adjusted by scoring in the Merit-based Incentive Payment System in the Quality Payment Program created by MACRA – was addressed in legislation passed by Congress and signed into law Feb. 9 by President Trump that provided short-term funding for the government and funding and other policy changes in the health care space, among other issues.
Onerous prior authorization rules, Stark Law, and other issues are creating barriers to health care choice and competition, the American College of Rheumatology recently told the Department of Health & Human Services.
The agency recently issued an informal request for information seeking “input from the public on the extent to which existing state and federal laws, regulations, guidance, requirements, and policies limit choice and competition across all health care markets, and the identification of actions that states or the federal government could take to support the development and operations of a health care system that provides high-quality care at affordable prices for the American people.”
“We believe this is a waste of valuable resources that results in delays in care and does not add value to health care delivery,” the ACR said in its Jan. 25 reply to the request for information. The ACR offered a series of recommendations to make the use of prior authorization by the Centers for Medicare & Medicaid Services more efficient.
Among the requested changes are:
• CMS should require all Medicare Advantage and Part D prescription drug plans to publicly disclose in a searchable electronic format to both patients and physicians all drugs and medical services that are subject to coverage restrictions (prior authorization, step therapy, formulary restrictions, quantity limits), and provide this information to vendors to be displayed in electronic health records.
• CMS should ensure that all utilization management requirements are based on accurate and up-to-date, publicly available clinical criteria and never cost alone.
• CMS should ensure that any “peer-to-peer” reviews utilize physicians from the same specialty/subspecialty as the ordering physician.
• CMS should restrict prior authorization requirements to “outlier” providers whose prescribing or ordering patterns differ significantly from their peers after adjusting for patient mix.
• CMS should not allow Part B services to be subject to prior authorization requirements because this would increase physician time spent on administrative tasks and reduce availability for patient care.
In addition, the ACR expressed concern about the consolidated pharmacy benefit managers market, noting that two PBMs cover more than 170 million Americans, and urged the HHS to “consider policies that require PBMs to be more transparent about their payment practices, including transparency around the true cost of prescription drugs.”
In the area of the Stark Law, the ACR called for the HHS to waive the prohibitions “for physicians seeking to develop and operate alternative payment models (APMs) as was provided to accountable care organizations in the Affordable Care Act. We also recommend removing the ‘volume or value’ prohibition in Stark policy so that physician practices can incentivize physicians to abide by best practices and succeed in new value-based alternative payment models.”
The ACR asked the CMS not to alter policy on assigning unique J-codes to biosimilars, as the unique codes allow for better monitoring of effectiveness and ensure adequate pharmacovigilance.
The physician organization also called for the removal of antitrust exemptions to insurance companies to give “the federal government the ability to intervene in places where insurance monopolies exist or develop.”
One request made – the removal of Part B drug payments from being adjusted by scoring in the Merit-based Incentive Payment System in the Quality Payment Program created by MACRA – was addressed in legislation passed by Congress and signed into law Feb. 9 by President Trump that provided short-term funding for the government and funding and other policy changes in the health care space, among other issues.
Onerous prior authorization rules, Stark Law, and other issues are creating barriers to health care choice and competition, the American College of Rheumatology recently told the Department of Health & Human Services.
The agency recently issued an informal request for information seeking “input from the public on the extent to which existing state and federal laws, regulations, guidance, requirements, and policies limit choice and competition across all health care markets, and the identification of actions that states or the federal government could take to support the development and operations of a health care system that provides high-quality care at affordable prices for the American people.”
“We believe this is a waste of valuable resources that results in delays in care and does not add value to health care delivery,” the ACR said in its Jan. 25 reply to the request for information. The ACR offered a series of recommendations to make the use of prior authorization by the Centers for Medicare & Medicaid Services more efficient.
Among the requested changes are:
• CMS should require all Medicare Advantage and Part D prescription drug plans to publicly disclose in a searchable electronic format to both patients and physicians all drugs and medical services that are subject to coverage restrictions (prior authorization, step therapy, formulary restrictions, quantity limits), and provide this information to vendors to be displayed in electronic health records.
• CMS should ensure that all utilization management requirements are based on accurate and up-to-date, publicly available clinical criteria and never cost alone.
• CMS should ensure that any “peer-to-peer” reviews utilize physicians from the same specialty/subspecialty as the ordering physician.
• CMS should restrict prior authorization requirements to “outlier” providers whose prescribing or ordering patterns differ significantly from their peers after adjusting for patient mix.
• CMS should not allow Part B services to be subject to prior authorization requirements because this would increase physician time spent on administrative tasks and reduce availability for patient care.
In addition, the ACR expressed concern about the consolidated pharmacy benefit managers market, noting that two PBMs cover more than 170 million Americans, and urged the HHS to “consider policies that require PBMs to be more transparent about their payment practices, including transparency around the true cost of prescription drugs.”
In the area of the Stark Law, the ACR called for the HHS to waive the prohibitions “for physicians seeking to develop and operate alternative payment models (APMs) as was provided to accountable care organizations in the Affordable Care Act. We also recommend removing the ‘volume or value’ prohibition in Stark policy so that physician practices can incentivize physicians to abide by best practices and succeed in new value-based alternative payment models.”
The ACR asked the CMS not to alter policy on assigning unique J-codes to biosimilars, as the unique codes allow for better monitoring of effectiveness and ensure adequate pharmacovigilance.
The physician organization also called for the removal of antitrust exemptions to insurance companies to give “the federal government the ability to intervene in places where insurance monopolies exist or develop.”
One request made – the removal of Part B drug payments from being adjusted by scoring in the Merit-based Incentive Payment System in the Quality Payment Program created by MACRA – was addressed in legislation passed by Congress and signed into law Feb. 9 by President Trump that provided short-term funding for the government and funding and other policy changes in the health care space, among other issues.
Congress extends CHIP, funds opioid crisis response following temporary shutdown
Congress, despite a second shutdown in less than a month, was able to pass a number of financial extenders to fund key health care programs.
The bipartisan spending bill (H.R. 1892), passed in the early morning hours on Feb. 9 by a 71-28 vote in the Senate (16 Republicans and 12 Democrats voted against it, and Sen. John McCain [R-Ariz.] was not present) and a 240-186 vote in the House (67 Republicans and 119 Democrats voted against and 5 representatives did not vote). President Trump signed the bill later that morning.
The spending bill and continuing resolution to fund the government through March 23 includes $6 billion to fund treatment for opioid addiction and other mental health issues, $2 billion in additional funding for the National Institutes of Health, and 4 additional years of funding for the Children’s Health Insurance Program. The additional CHIP funding extends the program for a total of 10 years.
The funding bill also made a technical correction to the Merit-based Incentive Payment System (MIPS) track of the Medicare Quality Payment Program. It removes Part B drug reimbursement from the MIPS payment adjustment, so any positive or negative change to physician payments based on the MIPS score will only be applied to physician fee schedule payments.
The bill also repeals the Independent Payment Advisory Board, a panel created by the Affordable Care Act that would have the power to slash Medicare spending under certain budget circumstances. That board was never convened.
The funding legislation also accelerates closure of the Medicare Part D “donut hole,” the coverage gap in which beneficiaries must pay 100% of medication costs prior to entering catastrophic coverage.
Just over $7 billion was provided for community health centers and Medicare’s therapy caps were repealed.
While the funding bill was written in the Senate with bipartisan input and received bipartisan support, Sen. Rand Paul (R-Ky.) held up votes over objections to the more than $1 trillion it will add to the nation’s debt, as well as for the fact that there was no opportunity to introduce and vote on amendments, leading to an hours-long government shutdown.
There also were concerns about two issues that could have derailed the vote in the House. Democrats wanted to add language to address immigrants brought to this nation illegally as children, while some Republicans did not want to increase the federal debt. However, there were enough votes to pass the funding legislation.
Congress, despite a second shutdown in less than a month, was able to pass a number of financial extenders to fund key health care programs.
The bipartisan spending bill (H.R. 1892), passed in the early morning hours on Feb. 9 by a 71-28 vote in the Senate (16 Republicans and 12 Democrats voted against it, and Sen. John McCain [R-Ariz.] was not present) and a 240-186 vote in the House (67 Republicans and 119 Democrats voted against and 5 representatives did not vote). President Trump signed the bill later that morning.
The spending bill and continuing resolution to fund the government through March 23 includes $6 billion to fund treatment for opioid addiction and other mental health issues, $2 billion in additional funding for the National Institutes of Health, and 4 additional years of funding for the Children’s Health Insurance Program. The additional CHIP funding extends the program for a total of 10 years.
The funding bill also made a technical correction to the Merit-based Incentive Payment System (MIPS) track of the Medicare Quality Payment Program. It removes Part B drug reimbursement from the MIPS payment adjustment, so any positive or negative change to physician payments based on the MIPS score will only be applied to physician fee schedule payments.
The bill also repeals the Independent Payment Advisory Board, a panel created by the Affordable Care Act that would have the power to slash Medicare spending under certain budget circumstances. That board was never convened.
The funding legislation also accelerates closure of the Medicare Part D “donut hole,” the coverage gap in which beneficiaries must pay 100% of medication costs prior to entering catastrophic coverage.
Just over $7 billion was provided for community health centers and Medicare’s therapy caps were repealed.
While the funding bill was written in the Senate with bipartisan input and received bipartisan support, Sen. Rand Paul (R-Ky.) held up votes over objections to the more than $1 trillion it will add to the nation’s debt, as well as for the fact that there was no opportunity to introduce and vote on amendments, leading to an hours-long government shutdown.
There also were concerns about two issues that could have derailed the vote in the House. Democrats wanted to add language to address immigrants brought to this nation illegally as children, while some Republicans did not want to increase the federal debt. However, there were enough votes to pass the funding legislation.
Congress, despite a second shutdown in less than a month, was able to pass a number of financial extenders to fund key health care programs.
The bipartisan spending bill (H.R. 1892), passed in the early morning hours on Feb. 9 by a 71-28 vote in the Senate (16 Republicans and 12 Democrats voted against it, and Sen. John McCain [R-Ariz.] was not present) and a 240-186 vote in the House (67 Republicans and 119 Democrats voted against and 5 representatives did not vote). President Trump signed the bill later that morning.
The spending bill and continuing resolution to fund the government through March 23 includes $6 billion to fund treatment for opioid addiction and other mental health issues, $2 billion in additional funding for the National Institutes of Health, and 4 additional years of funding for the Children’s Health Insurance Program. The additional CHIP funding extends the program for a total of 10 years.
The funding bill also made a technical correction to the Merit-based Incentive Payment System (MIPS) track of the Medicare Quality Payment Program. It removes Part B drug reimbursement from the MIPS payment adjustment, so any positive or negative change to physician payments based on the MIPS score will only be applied to physician fee schedule payments.
The bill also repeals the Independent Payment Advisory Board, a panel created by the Affordable Care Act that would have the power to slash Medicare spending under certain budget circumstances. That board was never convened.
The funding legislation also accelerates closure of the Medicare Part D “donut hole,” the coverage gap in which beneficiaries must pay 100% of medication costs prior to entering catastrophic coverage.
Just over $7 billion was provided for community health centers and Medicare’s therapy caps were repealed.
While the funding bill was written in the Senate with bipartisan input and received bipartisan support, Sen. Rand Paul (R-Ky.) held up votes over objections to the more than $1 trillion it will add to the nation’s debt, as well as for the fact that there was no opportunity to introduce and vote on amendments, leading to an hours-long government shutdown.
There also were concerns about two issues that could have derailed the vote in the House. Democrats wanted to add language to address immigrants brought to this nation illegally as children, while some Republicans did not want to increase the federal debt. However, there were enough votes to pass the funding legislation.
MedPAC: Medicare hospital readmissions program is working
WASHINGTON – The Medicare Hospital Readmissions Reduction Program is working, according to an original analysis of Medicare claims data presented at a meeting of the Medicare Payment Advisory Commission.
“First, readmissions declined,” MedPAC staff member Jeff Stensland, PhD, said during a congressionally mandated staff report to the commissioners. “Second, while observation stays increased, they did not fully offset the decrease in readmissions. Third, while [emergency department] visits also increased, those increases appear to largely be due to factors other than the readmission program. And fourth, in addition, all the evidence we examined suggests that the readmissions program did not result in increased mortality.”
including a reduction in readmissions and patients spending less time in the hospital with “at least equal outcomes,” Dr. Stensland said at the meeting.
Taxpayers benefited from a $2 billion reduction in spending on readmissions, which will “help extend the viability of the Medicare Trust Fund.” He noted that improvements to the program will be discussed at future MedPAC meetings.
Not all MedPAC commissioners agreed with the staff analysis.
David Nerenz, PhD, of the Henry Ford Health System, Detroit, also was not convinced the program was having an impact, noting that hospital readmissions began to decline even before the program started.
In looking at a graph presented that showed this trend, “I was impressed by the fact that the trend line started coming down all the way to the left side of the graph, and what my eye was impressed with was more just the continuation rather than a change, so I guess I feel cautious saying the program had certain effects because they certainly don’t jump off the graph visually,” Dr. Nerenz said. “I’m not disputing the numbers, but to say just as a clear unqualified conclusion the program reduced readmissions, I’m not so sure.”
It is likely premature to make any firm conclusions about how effectively this program decreases unnecessary utilization of hospitals. However, it is heartening to know that it did not increase mortality. The one variable that would best control readmissions is patient education. What constitutes an emergency requiring hospital evaluation and potential admission is often not explained to the patient by you and me.
It is likely premature to make any firm conclusions about how effectively this program decreases unnecessary utilization of hospitals. However, it is heartening to know that it did not increase mortality. The one variable that would best control readmissions is patient education. What constitutes an emergency requiring hospital evaluation and potential admission is often not explained to the patient by you and me.
It is likely premature to make any firm conclusions about how effectively this program decreases unnecessary utilization of hospitals. However, it is heartening to know that it did not increase mortality. The one variable that would best control readmissions is patient education. What constitutes an emergency requiring hospital evaluation and potential admission is often not explained to the patient by you and me.
WASHINGTON – The Medicare Hospital Readmissions Reduction Program is working, according to an original analysis of Medicare claims data presented at a meeting of the Medicare Payment Advisory Commission.
“First, readmissions declined,” MedPAC staff member Jeff Stensland, PhD, said during a congressionally mandated staff report to the commissioners. “Second, while observation stays increased, they did not fully offset the decrease in readmissions. Third, while [emergency department] visits also increased, those increases appear to largely be due to factors other than the readmission program. And fourth, in addition, all the evidence we examined suggests that the readmissions program did not result in increased mortality.”
including a reduction in readmissions and patients spending less time in the hospital with “at least equal outcomes,” Dr. Stensland said at the meeting.
Taxpayers benefited from a $2 billion reduction in spending on readmissions, which will “help extend the viability of the Medicare Trust Fund.” He noted that improvements to the program will be discussed at future MedPAC meetings.
Not all MedPAC commissioners agreed with the staff analysis.
David Nerenz, PhD, of the Henry Ford Health System, Detroit, also was not convinced the program was having an impact, noting that hospital readmissions began to decline even before the program started.
In looking at a graph presented that showed this trend, “I was impressed by the fact that the trend line started coming down all the way to the left side of the graph, and what my eye was impressed with was more just the continuation rather than a change, so I guess I feel cautious saying the program had certain effects because they certainly don’t jump off the graph visually,” Dr. Nerenz said. “I’m not disputing the numbers, but to say just as a clear unqualified conclusion the program reduced readmissions, I’m not so sure.”
WASHINGTON – The Medicare Hospital Readmissions Reduction Program is working, according to an original analysis of Medicare claims data presented at a meeting of the Medicare Payment Advisory Commission.
“First, readmissions declined,” MedPAC staff member Jeff Stensland, PhD, said during a congressionally mandated staff report to the commissioners. “Second, while observation stays increased, they did not fully offset the decrease in readmissions. Third, while [emergency department] visits also increased, those increases appear to largely be due to factors other than the readmission program. And fourth, in addition, all the evidence we examined suggests that the readmissions program did not result in increased mortality.”
including a reduction in readmissions and patients spending less time in the hospital with “at least equal outcomes,” Dr. Stensland said at the meeting.
Taxpayers benefited from a $2 billion reduction in spending on readmissions, which will “help extend the viability of the Medicare Trust Fund.” He noted that improvements to the program will be discussed at future MedPAC meetings.
Not all MedPAC commissioners agreed with the staff analysis.
David Nerenz, PhD, of the Henry Ford Health System, Detroit, also was not convinced the program was having an impact, noting that hospital readmissions began to decline even before the program started.
In looking at a graph presented that showed this trend, “I was impressed by the fact that the trend line started coming down all the way to the left side of the graph, and what my eye was impressed with was more just the continuation rather than a change, so I guess I feel cautious saying the program had certain effects because they certainly don’t jump off the graph visually,” Dr. Nerenz said. “I’m not disputing the numbers, but to say just as a clear unqualified conclusion the program reduced readmissions, I’m not so sure.”
REPORTING FROM MEDPAC
MedPAC mulls boost to payments for E&M visits
WASHINGTON – Members of the Medicare Payment Advisory Commission, which advises Congress, are weighing whether to recommend a 10% increase in payments for evaluation and management services.
The boost would be offset by a 4.5% decrease in all other payments on the Medicare physician fee schedule.
“The fee schedule underprices ambulatory [evaluation and management] services relative to other services,” MedPAC staff member Ariel Winter said during a Jan. 12 presentation of a draft proposal.
Payments in the physician fee schedule are based on the relative amount of time and intensity required for each service, she noted. “E&M services are labor intensive,” she said. “A clinician takes the patient’s history, examines the patient, engages in medical decision-making and so forth. These activities do not lend themselves to reductions in the time it takes to provide the visit.”
In contrast, “the time needed for other services, such as procedures, often declines over time due to productivity gains in clinical practice and technology,” Ms. Winter continued. “Ideally, the prices for these services would also be reduced to reflect these efficiency gains.”
Ms. Winter pointed out that the payment reduction that captures the efficiency gains rarely happens, and because of budget neutral requirements, it means that “payment rates for ambulatory E&M services are too low relative to other services.”
MedPAC looked at the fee schedule time estimates across four specialties (cardiology, family medicine, orthopedics, and urology) and found that across the board, the fee schedule assumed it took the specialist more time than what was actually needed.
“However, the discrepancy was much greater for the practices that focus on procedures, which suggests that the services they provide may be based on inflated time estimates,” Ms. Winter said. “For example, the hours assumed in the fee schedule were 24% higher than actual hours worked for family medicine, but 64% higher in cardiology and 92% higher in orthopedics.”
To account for this, and psychiatric services, regardless of specialty, resulting in an increase of $2.7 billion in spending on these codes. That would force a reduction of 4.5% in payments for everything else in the fee schedule under budget neutrality requirements.
Based on this change, licensed clinical social workers would see the full 10% increase to their physician fee schedule payments and clinical psychologists would see an 8% increase, based on 2016 Medicare claims. Endocrinology would see a 6.5% increase, family practice a 5.7% increase, and rheumatology a 5.4% increase.
Of the specialties displayed on a presentation slide that showed an increase, internal medicine fared the worst, with a 2% increase. “That is because this specialty performs a lot of services other than ambulatory E&M” and the 4.5% reduction in those other services would have an offsetting effect, Ms. Winter said.
Ms. Winter noted that a number of specialties would see a reduction in their physician fee schedule payments greater than 4% “because they provide very few E&M or psychiatric services, and these include radiology, pathology, physical therapy, and occupational therapy.”
Another option presented to the MedPAC commissioners was offering a special incentive payment equal to a 10% increase to clinicians who bill 60% of their claims for E&M services. That idea, which is similar to the now-expired primary care incentive payment program, would require a 1.7% reduction in other services to maintain budget neutrality.
But MedPAC commissioners questioned the overall aim of this type of payment rebalancing.
“Are we hoping that enhancing payments for primary care will actually improve coordination of care and management of patients?” asked Commissioner Kathy Buto, former vice president of global health policy at Johnson & Johnson. “I don’t think either of these options really does that. So that leads me to ask the question, ‘should we be thinking more about that issue?’”
Commissioner David Nerenz, PhD, director of the Center for Health Policy and Health Services Research at the Henry Ford Health System in Detroit, pointed out that a 10% uptick is not going to solve the problem of payment disparities. “And that doesn’t mean it should not be done. It just means that other things have to be considered and addressed at the same time if ultimately we are talking about people’s choice, for example, to go under primary care.”
Dr. Nerenz also noted that providers such as physical therapists and occupational therapists have to spend their time in face-to-face settings in the same way E&M visits are structured, but because it is billed as a procedure, they would get penalized under the draft proposal.
“I have the same concern about a procedure like colonoscopy,” he added. “You know, for the appropriate people, it’s a lifesaving procedure. Should that payment go down 4.5%? I don’t know.”
Commissioner Rita Redberg, MD, of the University of California San Francisco, suggested that some of these issues would go away on their own as physicians move into advanced alternative payment models in the Quality Payment Program, something MedPAC hopes will happen if their repeal and replacement of the Merit-based Incentive Payment System portion of the Quality Payment Program is adopted.
Commissioner Dana Gelb Safran, ScD, of Blue Cross Blue Shield of Massachusetts, looked at payment adequacy from three perspectives: better equity, better access, and better alignment with value. “I guess on all three counts, I am finding both of these approaches coming up short,” Dr. Safran said. “It maybe helps the most with equity, but then there’s the challenge about how payment actually gets shaped by the organizations providers are a part of,” she said, referring to whether employed physicians would see any benefit trickle down to them in the form of increased salary and benefits. “So I struggle with whether it even accomplished that, but maybe it’s not a bad idea for that.”
MedPAC is in the early stages of developing a recommendation on this subject and more discussions are expected at upcoming meetings. The next scheduled meeting is in March 2018.
WASHINGTON – Members of the Medicare Payment Advisory Commission, which advises Congress, are weighing whether to recommend a 10% increase in payments for evaluation and management services.
The boost would be offset by a 4.5% decrease in all other payments on the Medicare physician fee schedule.
“The fee schedule underprices ambulatory [evaluation and management] services relative to other services,” MedPAC staff member Ariel Winter said during a Jan. 12 presentation of a draft proposal.
Payments in the physician fee schedule are based on the relative amount of time and intensity required for each service, she noted. “E&M services are labor intensive,” she said. “A clinician takes the patient’s history, examines the patient, engages in medical decision-making and so forth. These activities do not lend themselves to reductions in the time it takes to provide the visit.”
In contrast, “the time needed for other services, such as procedures, often declines over time due to productivity gains in clinical practice and technology,” Ms. Winter continued. “Ideally, the prices for these services would also be reduced to reflect these efficiency gains.”
Ms. Winter pointed out that the payment reduction that captures the efficiency gains rarely happens, and because of budget neutral requirements, it means that “payment rates for ambulatory E&M services are too low relative to other services.”
MedPAC looked at the fee schedule time estimates across four specialties (cardiology, family medicine, orthopedics, and urology) and found that across the board, the fee schedule assumed it took the specialist more time than what was actually needed.
“However, the discrepancy was much greater for the practices that focus on procedures, which suggests that the services they provide may be based on inflated time estimates,” Ms. Winter said. “For example, the hours assumed in the fee schedule were 24% higher than actual hours worked for family medicine, but 64% higher in cardiology and 92% higher in orthopedics.”
To account for this, and psychiatric services, regardless of specialty, resulting in an increase of $2.7 billion in spending on these codes. That would force a reduction of 4.5% in payments for everything else in the fee schedule under budget neutrality requirements.
Based on this change, licensed clinical social workers would see the full 10% increase to their physician fee schedule payments and clinical psychologists would see an 8% increase, based on 2016 Medicare claims. Endocrinology would see a 6.5% increase, family practice a 5.7% increase, and rheumatology a 5.4% increase.
Of the specialties displayed on a presentation slide that showed an increase, internal medicine fared the worst, with a 2% increase. “That is because this specialty performs a lot of services other than ambulatory E&M” and the 4.5% reduction in those other services would have an offsetting effect, Ms. Winter said.
Ms. Winter noted that a number of specialties would see a reduction in their physician fee schedule payments greater than 4% “because they provide very few E&M or psychiatric services, and these include radiology, pathology, physical therapy, and occupational therapy.”
Another option presented to the MedPAC commissioners was offering a special incentive payment equal to a 10% increase to clinicians who bill 60% of their claims for E&M services. That idea, which is similar to the now-expired primary care incentive payment program, would require a 1.7% reduction in other services to maintain budget neutrality.
But MedPAC commissioners questioned the overall aim of this type of payment rebalancing.
“Are we hoping that enhancing payments for primary care will actually improve coordination of care and management of patients?” asked Commissioner Kathy Buto, former vice president of global health policy at Johnson & Johnson. “I don’t think either of these options really does that. So that leads me to ask the question, ‘should we be thinking more about that issue?’”
Commissioner David Nerenz, PhD, director of the Center for Health Policy and Health Services Research at the Henry Ford Health System in Detroit, pointed out that a 10% uptick is not going to solve the problem of payment disparities. “And that doesn’t mean it should not be done. It just means that other things have to be considered and addressed at the same time if ultimately we are talking about people’s choice, for example, to go under primary care.”
Dr. Nerenz also noted that providers such as physical therapists and occupational therapists have to spend their time in face-to-face settings in the same way E&M visits are structured, but because it is billed as a procedure, they would get penalized under the draft proposal.
“I have the same concern about a procedure like colonoscopy,” he added. “You know, for the appropriate people, it’s a lifesaving procedure. Should that payment go down 4.5%? I don’t know.”
Commissioner Rita Redberg, MD, of the University of California San Francisco, suggested that some of these issues would go away on their own as physicians move into advanced alternative payment models in the Quality Payment Program, something MedPAC hopes will happen if their repeal and replacement of the Merit-based Incentive Payment System portion of the Quality Payment Program is adopted.
Commissioner Dana Gelb Safran, ScD, of Blue Cross Blue Shield of Massachusetts, looked at payment adequacy from three perspectives: better equity, better access, and better alignment with value. “I guess on all three counts, I am finding both of these approaches coming up short,” Dr. Safran said. “It maybe helps the most with equity, but then there’s the challenge about how payment actually gets shaped by the organizations providers are a part of,” she said, referring to whether employed physicians would see any benefit trickle down to them in the form of increased salary and benefits. “So I struggle with whether it even accomplished that, but maybe it’s not a bad idea for that.”
MedPAC is in the early stages of developing a recommendation on this subject and more discussions are expected at upcoming meetings. The next scheduled meeting is in March 2018.
WASHINGTON – Members of the Medicare Payment Advisory Commission, which advises Congress, are weighing whether to recommend a 10% increase in payments for evaluation and management services.
The boost would be offset by a 4.5% decrease in all other payments on the Medicare physician fee schedule.
“The fee schedule underprices ambulatory [evaluation and management] services relative to other services,” MedPAC staff member Ariel Winter said during a Jan. 12 presentation of a draft proposal.
Payments in the physician fee schedule are based on the relative amount of time and intensity required for each service, she noted. “E&M services are labor intensive,” she said. “A clinician takes the patient’s history, examines the patient, engages in medical decision-making and so forth. These activities do not lend themselves to reductions in the time it takes to provide the visit.”
In contrast, “the time needed for other services, such as procedures, often declines over time due to productivity gains in clinical practice and technology,” Ms. Winter continued. “Ideally, the prices for these services would also be reduced to reflect these efficiency gains.”
Ms. Winter pointed out that the payment reduction that captures the efficiency gains rarely happens, and because of budget neutral requirements, it means that “payment rates for ambulatory E&M services are too low relative to other services.”
MedPAC looked at the fee schedule time estimates across four specialties (cardiology, family medicine, orthopedics, and urology) and found that across the board, the fee schedule assumed it took the specialist more time than what was actually needed.
“However, the discrepancy was much greater for the practices that focus on procedures, which suggests that the services they provide may be based on inflated time estimates,” Ms. Winter said. “For example, the hours assumed in the fee schedule were 24% higher than actual hours worked for family medicine, but 64% higher in cardiology and 92% higher in orthopedics.”
To account for this, and psychiatric services, regardless of specialty, resulting in an increase of $2.7 billion in spending on these codes. That would force a reduction of 4.5% in payments for everything else in the fee schedule under budget neutrality requirements.
Based on this change, licensed clinical social workers would see the full 10% increase to their physician fee schedule payments and clinical psychologists would see an 8% increase, based on 2016 Medicare claims. Endocrinology would see a 6.5% increase, family practice a 5.7% increase, and rheumatology a 5.4% increase.
Of the specialties displayed on a presentation slide that showed an increase, internal medicine fared the worst, with a 2% increase. “That is because this specialty performs a lot of services other than ambulatory E&M” and the 4.5% reduction in those other services would have an offsetting effect, Ms. Winter said.
Ms. Winter noted that a number of specialties would see a reduction in their physician fee schedule payments greater than 4% “because they provide very few E&M or psychiatric services, and these include radiology, pathology, physical therapy, and occupational therapy.”
Another option presented to the MedPAC commissioners was offering a special incentive payment equal to a 10% increase to clinicians who bill 60% of their claims for E&M services. That idea, which is similar to the now-expired primary care incentive payment program, would require a 1.7% reduction in other services to maintain budget neutrality.
But MedPAC commissioners questioned the overall aim of this type of payment rebalancing.
“Are we hoping that enhancing payments for primary care will actually improve coordination of care and management of patients?” asked Commissioner Kathy Buto, former vice president of global health policy at Johnson & Johnson. “I don’t think either of these options really does that. So that leads me to ask the question, ‘should we be thinking more about that issue?’”
Commissioner David Nerenz, PhD, director of the Center for Health Policy and Health Services Research at the Henry Ford Health System in Detroit, pointed out that a 10% uptick is not going to solve the problem of payment disparities. “And that doesn’t mean it should not be done. It just means that other things have to be considered and addressed at the same time if ultimately we are talking about people’s choice, for example, to go under primary care.”
Dr. Nerenz also noted that providers such as physical therapists and occupational therapists have to spend their time in face-to-face settings in the same way E&M visits are structured, but because it is billed as a procedure, they would get penalized under the draft proposal.
“I have the same concern about a procedure like colonoscopy,” he added. “You know, for the appropriate people, it’s a lifesaving procedure. Should that payment go down 4.5%? I don’t know.”
Commissioner Rita Redberg, MD, of the University of California San Francisco, suggested that some of these issues would go away on their own as physicians move into advanced alternative payment models in the Quality Payment Program, something MedPAC hopes will happen if their repeal and replacement of the Merit-based Incentive Payment System portion of the Quality Payment Program is adopted.
Commissioner Dana Gelb Safran, ScD, of Blue Cross Blue Shield of Massachusetts, looked at payment adequacy from three perspectives: better equity, better access, and better alignment with value. “I guess on all three counts, I am finding both of these approaches coming up short,” Dr. Safran said. “It maybe helps the most with equity, but then there’s the challenge about how payment actually gets shaped by the organizations providers are a part of,” she said, referring to whether employed physicians would see any benefit trickle down to them in the form of increased salary and benefits. “So I struggle with whether it even accomplished that, but maybe it’s not a bad idea for that.”
MedPAC is in the early stages of developing a recommendation on this subject and more discussions are expected at upcoming meetings. The next scheduled meeting is in March 2018.
REPORTING FROM A MEDPAC MEETING