User login
ACIP immunization update
Keeping up with the ever-changing immunization schedules recommended by the Centers for Disease Control and Prevention (CDC)’s Advisory Committee on Immunization Practices (ACIP) can be difficult. The most recent changes are the interim recommendations from the February 2011 ACIP meeting pertaining to tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis (Tdap) vaccine immunization and postexposure prophylaxis (PEP) for health care personnel. Updated schedules for routine immunization of children and adults that incorporate additions and changes made in the preceding year were published by the CDC in February.1,2
ACIP widens the scope of pertussis prevention
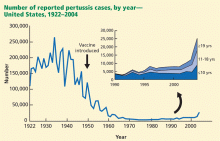
The past decade has seen an increase in pertussis cases, including an increase in the number of cases among infants and adolescents (FIGURE). In 2010, California reported 8383 cases, including 10 infant deaths. This was the highest number and rate of cases reported in more than 50 years.3 Other states have also experienced recent increases.
This evolving epidemiology of pertussis has prompted ACIP to recommend a routine single Tdap dose for adolescents between the ages of 11 and 18 years who have completed the recommended DTP/DTaP (diphtheria and tetanus toxoids and pertussis/diphtheria and tetanus toxoids and acellular pertussis) vaccination series and for adults ages 19 to 64 years. ACIP also recommends a single dose for children ages 7 to 10 if they are not fully vaccinated against pertussis and for adults 65 and older who have not previously received Tdap and who are in close contact with infants. The last 2 are off-label recommendations. ACIP has also eliminated any recommended interval between the time of vaccination with tetanus or diphtheriatoxoid (Td) containing vaccine and the administration of Tdap.4
FIGURE
Reported pertussis incidence by age group, 1990-2009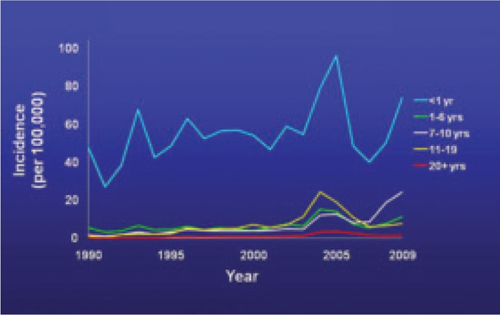
Source: Centers for Disease Control and Prevention. Pertussis (whooping cough): surveillance and reporting. Available at: www.cdc.gov/pertussis/surv-reporting.html. Accessed March 21, 2011.
2 new recommendations for clinician postexposure prophylaxis
Interim recommendations from the most recent ACIP meeting in February 20115 re-emphasize that health care personnel should receive Tdap and recommend that health care facilities take steps to increase adherence, including providing the vaccine at no cost.5
Since health care personnel are at increased risk of exposure to pertussis, ACIP also made 2 recommendations for PEP.
- All health care personnel (vaccinated or not) in close contact with a pertussis patient (as defined in TABLE 1) who are likely to expose patients at high risk for complications from pertussis (infants <1 year of age and those with certain immunodeficiency conditions, chronic lung disease, respiratory insufficiency, or cystic fibrosis) should receive PEP.
- Exposed personnel who do not work with high-risk patients should receive PEP or be monitored daily for 21 days, treated at first signs of infection, and excluded from patient contact for 5 days if symptoms develop. The antimicrobials and doses for treatment and prevention of pertussis have been published in the Morbidity and Mortality Weekly Report.6 Options for PEP include azithromycin, clarithromycin, erythromycin, and trimethoprim-sulfamethoxazole.6
TABLE 1
Definition of close contact with a pertussis patient
|
| Source: Centers for Disease Control and Prevention. MMWR Morb Mortal Wkly Rep. 2005.6 |
Coming soon: Complete vaccine recommendations for health care workers
Recent experience with pertussis (and influenza) has highlighted the need for health care personnel to be vaccinated against infectious diseases to protect themselves, their patients, and their families. To that end, ACIP plans to publish a compendium later this year that brings together all recommendations regarding immunizations for health care personnel. When it becomes available, family physicians will be able to refer to this document to ensure that they and their staff are immunized in line with CDC recommendations.
The latest on influenza vaccine, PCV13, MCV4, hepatitis B, and HPV
The most notable additions to the routine schedules ACIP announced during the past year are universal, yearly influenza immunization from the age of 6 months on and the replacement of the 7-valent pneumococcal conjugate vaccine (PCV7) with a 13-valent product (PCV13) for infants and children. Details of these recommendations, including how to transition from PCV7 to PCV13, were published late last year by the CDC and described in another Practice Alert.7-9
In addition, changes were made in the schedules for meningococcal conjugate vaccine. A 2-dose primary series, instead of a single dose, of MCV4 is now recommended for those with compromised immunity. A booster of MCV4 is now recommended at age 16 for those vaccinated at 11 or 12 years, and at age 16 to 18 for those vaccinated at 13 to 15 years.10 The MCV4 recommendations are summarized in TABLE 2.
More schedule details in the footnotes. The new schedules contain a number of clarifications in the footnotes that:1,11
- explain the spacing of the 3-dose primary series for hepatitis B vaccine (HepB) for infants if they do not receive a dose immediately after birth
- clarify the circumstances in which children younger than age 9 need 2 doses of influenza vaccine
- describe the availability of both a quadrivalent human papillomavirus vaccine (HPV4) and a bivalent vaccine (HPV2) to prevent precancerous cervical lesions and cancer
- list the option for using HPV4 for males for the prevention of genital warts.
TABLE 2
Meningococcal conjugate vaccine recommendations by risk group, ACIP 2010
| Risk group | Primary series | Booster dose |
|---|---|---|
| Individuals ages 11-18 years | 1 dose, preferably at age 11 or 12 years |
|
| HIV-infected individuals ages 11-18 years | 2 doses, 2 months apart |
|
| Individuals ages 2-55 years with persistent complement component deficiency such as C5-C9, properdin, or factor D, or functional or anatomic asplenia | 2 doses, 2 months apart |
|
| Individuals ages 2-55 years with prolonged increased risk of exposure, such as microbiologists routinely working with Neisseria meningitidis and travelers to, or residents of, countries where meningococcal disease is hyperendemic or epidemic | 1 dose |
|
| Source: Centers for Disease Control and Prevention. MMWR Morb Mortal Wkly Rep. 2011.10 | ||
1. Centers for Disease Control and Prevention. Recommended immunization schedules for persons aged 0 through 18 years—United States, 2011. MMWR Morb Mortal Wkly Rep QuickGuide. 2011;60(5):1-4.
2. Centers for Disease Control and Prevention. Recommended adult immunization schedule-United States, 2011. MMWR Morb Mortal Wkly Rep. 2011;60(4):1-4.
3. Centers for Disease Control and Prevention. Pertussis (whooping cough): outbreaks. Available at: http://www.cdc.gov/pertussis/outbreaks.html. Accessed March 19, 2011.
4. Centers for Disease Control and Prevention. Updated recommendations for use of tetanus toxoid, reduced diphtheria toxoid and acellular pertussis (Tdap) vaccine from the Advisory Committee on Immunization Practices, 2010. MMWR Morb Mortal Wkly Rep. 2011;60(1):13-15.
5. Centers for Disease Control and Prevention. ACIP presentation slides: February 2011 meeting. Available at www.cdc.gov/vaccines/recs/acip/slides-feb11.htm#pertussis. Accessed March 19, 2011.
6. Centers for Disease Control and Prevention. Recommended antimicrobial agents for treatment and postexposure prophylaxis of pertussis: 2005 CDC guidelines. MMWR Recomm Rep. 2005;54(RR-14):1-16.
7. Prevention and control of influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2010. MMWR Recomm Rep. 2010;59(RR-8):1-62.
8. Centers for Disease Control and Prevention. Prevention of pneumococcal disease among infants and children—use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine—recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2010;59(RR-11):1-18.
9. Campos-Outcalt D. Your guide to the new pneumococcal vaccine for children. J Fam Pract. 2010;59:394-398.
10. Centers for Disease Control and Prevention. Updated recommendations for use of meningococcal conjugate vaccines—Advisory Committee on Immunization Practices (ACIP), 2010. MMWR Morb Mortal Wkly Rep. 2011;60:72-76.
11. Centers for Disease Control and Prevention. FDA licensure of bivalent human papillomavirus vaccine (HPV2, Cervarix) for use in females and updated HPV vaccination recommendations from the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep. 2010;59:626-629.
Keeping up with the ever-changing immunization schedules recommended by the Centers for Disease Control and Prevention (CDC)’s Advisory Committee on Immunization Practices (ACIP) can be difficult. The most recent changes are the interim recommendations from the February 2011 ACIP meeting pertaining to tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis (Tdap) vaccine immunization and postexposure prophylaxis (PEP) for health care personnel. Updated schedules for routine immunization of children and adults that incorporate additions and changes made in the preceding year were published by the CDC in February.1,2
ACIP widens the scope of pertussis prevention
The past decade has seen an increase in pertussis cases, including an increase in the number of cases among infants and adolescents (FIGURE). In 2010, California reported 8383 cases, including 10 infant deaths. This was the highest number and rate of cases reported in more than 50 years.3 Other states have also experienced recent increases.
This evolving epidemiology of pertussis has prompted ACIP to recommend a routine single Tdap dose for adolescents between the ages of 11 and 18 years who have completed the recommended DTP/DTaP (diphtheria and tetanus toxoids and pertussis/diphtheria and tetanus toxoids and acellular pertussis) vaccination series and for adults ages 19 to 64 years. ACIP also recommends a single dose for children ages 7 to 10 if they are not fully vaccinated against pertussis and for adults 65 and older who have not previously received Tdap and who are in close contact with infants. The last 2 are off-label recommendations. ACIP has also eliminated any recommended interval between the time of vaccination with tetanus or diphtheriatoxoid (Td) containing vaccine and the administration of Tdap.4
FIGURE
Reported pertussis incidence by age group, 1990-2009
Source: Centers for Disease Control and Prevention. Pertussis (whooping cough): surveillance and reporting. Available at: www.cdc.gov/pertussis/surv-reporting.html. Accessed March 21, 2011.
2 new recommendations for clinician postexposure prophylaxis
Interim recommendations from the most recent ACIP meeting in February 20115 re-emphasize that health care personnel should receive Tdap and recommend that health care facilities take steps to increase adherence, including providing the vaccine at no cost.5
Since health care personnel are at increased risk of exposure to pertussis, ACIP also made 2 recommendations for PEP.
- All health care personnel (vaccinated or not) in close contact with a pertussis patient (as defined in TABLE 1) who are likely to expose patients at high risk for complications from pertussis (infants <1 year of age and those with certain immunodeficiency conditions, chronic lung disease, respiratory insufficiency, or cystic fibrosis) should receive PEP.
- Exposed personnel who do not work with high-risk patients should receive PEP or be monitored daily for 21 days, treated at first signs of infection, and excluded from patient contact for 5 days if symptoms develop. The antimicrobials and doses for treatment and prevention of pertussis have been published in the Morbidity and Mortality Weekly Report.6 Options for PEP include azithromycin, clarithromycin, erythromycin, and trimethoprim-sulfamethoxazole.6
TABLE 1
Definition of close contact with a pertussis patient
|
| Source: Centers for Disease Control and Prevention. MMWR Morb Mortal Wkly Rep. 2005.6 |
Coming soon: Complete vaccine recommendations for health care workers
Recent experience with pertussis (and influenza) has highlighted the need for health care personnel to be vaccinated against infectious diseases to protect themselves, their patients, and their families. To that end, ACIP plans to publish a compendium later this year that brings together all recommendations regarding immunizations for health care personnel. When it becomes available, family physicians will be able to refer to this document to ensure that they and their staff are immunized in line with CDC recommendations.
The latest on influenza vaccine, PCV13, MCV4, hepatitis B, and HPV
The most notable additions to the routine schedules ACIP announced during the past year are universal, yearly influenza immunization from the age of 6 months on and the replacement of the 7-valent pneumococcal conjugate vaccine (PCV7) with a 13-valent product (PCV13) for infants and children. Details of these recommendations, including how to transition from PCV7 to PCV13, were published late last year by the CDC and described in another Practice Alert.7-9
In addition, changes were made in the schedules for meningococcal conjugate vaccine. A 2-dose primary series, instead of a single dose, of MCV4 is now recommended for those with compromised immunity. A booster of MCV4 is now recommended at age 16 for those vaccinated at 11 or 12 years, and at age 16 to 18 for those vaccinated at 13 to 15 years.10 The MCV4 recommendations are summarized in TABLE 2.
More schedule details in the footnotes. The new schedules contain a number of clarifications in the footnotes that:1,11
- explain the spacing of the 3-dose primary series for hepatitis B vaccine (HepB) for infants if they do not receive a dose immediately after birth
- clarify the circumstances in which children younger than age 9 need 2 doses of influenza vaccine
- describe the availability of both a quadrivalent human papillomavirus vaccine (HPV4) and a bivalent vaccine (HPV2) to prevent precancerous cervical lesions and cancer
- list the option for using HPV4 for males for the prevention of genital warts.
TABLE 2
Meningococcal conjugate vaccine recommendations by risk group, ACIP 2010
| Risk group | Primary series | Booster dose |
|---|---|---|
| Individuals ages 11-18 years | 1 dose, preferably at age 11 or 12 years |
|
| HIV-infected individuals ages 11-18 years | 2 doses, 2 months apart |
|
| Individuals ages 2-55 years with persistent complement component deficiency such as C5-C9, properdin, or factor D, or functional or anatomic asplenia | 2 doses, 2 months apart |
|
| Individuals ages 2-55 years with prolonged increased risk of exposure, such as microbiologists routinely working with Neisseria meningitidis and travelers to, or residents of, countries where meningococcal disease is hyperendemic or epidemic | 1 dose |
|
| Source: Centers for Disease Control and Prevention. MMWR Morb Mortal Wkly Rep. 2011.10 | ||
Keeping up with the ever-changing immunization schedules recommended by the Centers for Disease Control and Prevention (CDC)’s Advisory Committee on Immunization Practices (ACIP) can be difficult. The most recent changes are the interim recommendations from the February 2011 ACIP meeting pertaining to tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis (Tdap) vaccine immunization and postexposure prophylaxis (PEP) for health care personnel. Updated schedules for routine immunization of children and adults that incorporate additions and changes made in the preceding year were published by the CDC in February.1,2
ACIP widens the scope of pertussis prevention
The past decade has seen an increase in pertussis cases, including an increase in the number of cases among infants and adolescents (FIGURE). In 2010, California reported 8383 cases, including 10 infant deaths. This was the highest number and rate of cases reported in more than 50 years.3 Other states have also experienced recent increases.
This evolving epidemiology of pertussis has prompted ACIP to recommend a routine single Tdap dose for adolescents between the ages of 11 and 18 years who have completed the recommended DTP/DTaP (diphtheria and tetanus toxoids and pertussis/diphtheria and tetanus toxoids and acellular pertussis) vaccination series and for adults ages 19 to 64 years. ACIP also recommends a single dose for children ages 7 to 10 if they are not fully vaccinated against pertussis and for adults 65 and older who have not previously received Tdap and who are in close contact with infants. The last 2 are off-label recommendations. ACIP has also eliminated any recommended interval between the time of vaccination with tetanus or diphtheriatoxoid (Td) containing vaccine and the administration of Tdap.4
FIGURE
Reported pertussis incidence by age group, 1990-2009
Source: Centers for Disease Control and Prevention. Pertussis (whooping cough): surveillance and reporting. Available at: www.cdc.gov/pertussis/surv-reporting.html. Accessed March 21, 2011.
2 new recommendations for clinician postexposure prophylaxis
Interim recommendations from the most recent ACIP meeting in February 20115 re-emphasize that health care personnel should receive Tdap and recommend that health care facilities take steps to increase adherence, including providing the vaccine at no cost.5
Since health care personnel are at increased risk of exposure to pertussis, ACIP also made 2 recommendations for PEP.
- All health care personnel (vaccinated or not) in close contact with a pertussis patient (as defined in TABLE 1) who are likely to expose patients at high risk for complications from pertussis (infants <1 year of age and those with certain immunodeficiency conditions, chronic lung disease, respiratory insufficiency, or cystic fibrosis) should receive PEP.
- Exposed personnel who do not work with high-risk patients should receive PEP or be monitored daily for 21 days, treated at first signs of infection, and excluded from patient contact for 5 days if symptoms develop. The antimicrobials and doses for treatment and prevention of pertussis have been published in the Morbidity and Mortality Weekly Report.6 Options for PEP include azithromycin, clarithromycin, erythromycin, and trimethoprim-sulfamethoxazole.6
TABLE 1
Definition of close contact with a pertussis patient
|
| Source: Centers for Disease Control and Prevention. MMWR Morb Mortal Wkly Rep. 2005.6 |
Coming soon: Complete vaccine recommendations for health care workers
Recent experience with pertussis (and influenza) has highlighted the need for health care personnel to be vaccinated against infectious diseases to protect themselves, their patients, and their families. To that end, ACIP plans to publish a compendium later this year that brings together all recommendations regarding immunizations for health care personnel. When it becomes available, family physicians will be able to refer to this document to ensure that they and their staff are immunized in line with CDC recommendations.
The latest on influenza vaccine, PCV13, MCV4, hepatitis B, and HPV
The most notable additions to the routine schedules ACIP announced during the past year are universal, yearly influenza immunization from the age of 6 months on and the replacement of the 7-valent pneumococcal conjugate vaccine (PCV7) with a 13-valent product (PCV13) for infants and children. Details of these recommendations, including how to transition from PCV7 to PCV13, were published late last year by the CDC and described in another Practice Alert.7-9
In addition, changes were made in the schedules for meningococcal conjugate vaccine. A 2-dose primary series, instead of a single dose, of MCV4 is now recommended for those with compromised immunity. A booster of MCV4 is now recommended at age 16 for those vaccinated at 11 or 12 years, and at age 16 to 18 for those vaccinated at 13 to 15 years.10 The MCV4 recommendations are summarized in TABLE 2.
More schedule details in the footnotes. The new schedules contain a number of clarifications in the footnotes that:1,11
- explain the spacing of the 3-dose primary series for hepatitis B vaccine (HepB) for infants if they do not receive a dose immediately after birth
- clarify the circumstances in which children younger than age 9 need 2 doses of influenza vaccine
- describe the availability of both a quadrivalent human papillomavirus vaccine (HPV4) and a bivalent vaccine (HPV2) to prevent precancerous cervical lesions and cancer
- list the option for using HPV4 for males for the prevention of genital warts.
TABLE 2
Meningococcal conjugate vaccine recommendations by risk group, ACIP 2010
| Risk group | Primary series | Booster dose |
|---|---|---|
| Individuals ages 11-18 years | 1 dose, preferably at age 11 or 12 years |
|
| HIV-infected individuals ages 11-18 years | 2 doses, 2 months apart |
|
| Individuals ages 2-55 years with persistent complement component deficiency such as C5-C9, properdin, or factor D, or functional or anatomic asplenia | 2 doses, 2 months apart |
|
| Individuals ages 2-55 years with prolonged increased risk of exposure, such as microbiologists routinely working with Neisseria meningitidis and travelers to, or residents of, countries where meningococcal disease is hyperendemic or epidemic | 1 dose |
|
| Source: Centers for Disease Control and Prevention. MMWR Morb Mortal Wkly Rep. 2011.10 | ||
1. Centers for Disease Control and Prevention. Recommended immunization schedules for persons aged 0 through 18 years—United States, 2011. MMWR Morb Mortal Wkly Rep QuickGuide. 2011;60(5):1-4.
2. Centers for Disease Control and Prevention. Recommended adult immunization schedule-United States, 2011. MMWR Morb Mortal Wkly Rep. 2011;60(4):1-4.
3. Centers for Disease Control and Prevention. Pertussis (whooping cough): outbreaks. Available at: http://www.cdc.gov/pertussis/outbreaks.html. Accessed March 19, 2011.
4. Centers for Disease Control and Prevention. Updated recommendations for use of tetanus toxoid, reduced diphtheria toxoid and acellular pertussis (Tdap) vaccine from the Advisory Committee on Immunization Practices, 2010. MMWR Morb Mortal Wkly Rep. 2011;60(1):13-15.
5. Centers for Disease Control and Prevention. ACIP presentation slides: February 2011 meeting. Available at www.cdc.gov/vaccines/recs/acip/slides-feb11.htm#pertussis. Accessed March 19, 2011.
6. Centers for Disease Control and Prevention. Recommended antimicrobial agents for treatment and postexposure prophylaxis of pertussis: 2005 CDC guidelines. MMWR Recomm Rep. 2005;54(RR-14):1-16.
7. Prevention and control of influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2010. MMWR Recomm Rep. 2010;59(RR-8):1-62.
8. Centers for Disease Control and Prevention. Prevention of pneumococcal disease among infants and children—use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine—recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2010;59(RR-11):1-18.
9. Campos-Outcalt D. Your guide to the new pneumococcal vaccine for children. J Fam Pract. 2010;59:394-398.
10. Centers for Disease Control and Prevention. Updated recommendations for use of meningococcal conjugate vaccines—Advisory Committee on Immunization Practices (ACIP), 2010. MMWR Morb Mortal Wkly Rep. 2011;60:72-76.
11. Centers for Disease Control and Prevention. FDA licensure of bivalent human papillomavirus vaccine (HPV2, Cervarix) for use in females and updated HPV vaccination recommendations from the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep. 2010;59:626-629.
1. Centers for Disease Control and Prevention. Recommended immunization schedules for persons aged 0 through 18 years—United States, 2011. MMWR Morb Mortal Wkly Rep QuickGuide. 2011;60(5):1-4.
2. Centers for Disease Control and Prevention. Recommended adult immunization schedule-United States, 2011. MMWR Morb Mortal Wkly Rep. 2011;60(4):1-4.
3. Centers for Disease Control and Prevention. Pertussis (whooping cough): outbreaks. Available at: http://www.cdc.gov/pertussis/outbreaks.html. Accessed March 19, 2011.
4. Centers for Disease Control and Prevention. Updated recommendations for use of tetanus toxoid, reduced diphtheria toxoid and acellular pertussis (Tdap) vaccine from the Advisory Committee on Immunization Practices, 2010. MMWR Morb Mortal Wkly Rep. 2011;60(1):13-15.
5. Centers for Disease Control and Prevention. ACIP presentation slides: February 2011 meeting. Available at www.cdc.gov/vaccines/recs/acip/slides-feb11.htm#pertussis. Accessed March 19, 2011.
6. Centers for Disease Control and Prevention. Recommended antimicrobial agents for treatment and postexposure prophylaxis of pertussis: 2005 CDC guidelines. MMWR Recomm Rep. 2005;54(RR-14):1-16.
7. Prevention and control of influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2010. MMWR Recomm Rep. 2010;59(RR-8):1-62.
8. Centers for Disease Control and Prevention. Prevention of pneumococcal disease among infants and children—use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine—recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2010;59(RR-11):1-18.
9. Campos-Outcalt D. Your guide to the new pneumococcal vaccine for children. J Fam Pract. 2010;59:394-398.
10. Centers for Disease Control and Prevention. Updated recommendations for use of meningococcal conjugate vaccines—Advisory Committee on Immunization Practices (ACIP), 2010. MMWR Morb Mortal Wkly Rep. 2011;60:72-76.
11. Centers for Disease Control and Prevention. FDA licensure of bivalent human papillomavirus vaccine (HPV2, Cervarix) for use in females and updated HPV vaccination recommendations from the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep. 2010;59:626-629.
CDC update: Guidelines for treating STDs
In 2010, the CDC released an update of its Sexually Transmitted Diseases (STD) Treatment Guidelines,1 which were last updated in 2006. The guidelines are widely viewed as the most authoritative source of information on the diagnosis, treatment, and follow-up of STDs, and they are the standard for publicly and privately funded clinics focusing on sexual health.
What’s new
Some of the notable changes made since the last update in 2006 appear in TABLE 1.1,2
Uncomplicated gonorrhea. Cephalosporins are the only class of antibiotic recommended as first-line treatment for gonorrhea. (In a 2007 recommendation revision, the CDC opted to no longer recommend quinolone antibiotics for the treatment of gonorrhea, because of widespread bacterial resistance.3) Preference is now given to ceftriaxone because of its proven effectiveness against pharyngeal infection, which is often asymptomatic, difficult to detect, and difficult to eradicate. Additionally, the 2010 update has increased the recommended dose of ceftriazone from 125 to 250 mg intramuscularly. The larger dose is more effective against pharyngeal infection; it is also a safeguard against decreased bacterial susceptibility to cephalosporins, which, although still very low, has been reported in more cases recently.
The guidelines still recommend that azithromycin, 1 g orally in a single dose, be given with ceftriaxone because of the relatively high rate of co-infection with Chlamydia trachomatis and the potential for azithromycin to assist with eradicating any gonorrhea with decreased susceptibility to ceftriaxone.
Pelvic inflammatory disease. Quinolones have also been removed from the list of options for outpatient treatment of pelvic inflammatory disease. All recommended regimens now specify a parenteral cephalosporin as a single injection with doxycycline 100 mg PO twice a day for 14 days, with or without metronidazole 500 mg PO twice a day for 14 days.
Bacterial vaginosis. Tinidazole, 2 g orally once a day for 2 days or 1 g orally once a day for 5 days, is now an alternative agent for bacterial vaginosis. However, preferred treatments remain metronidazole 500 mg orally twice a day for 7 days, metronidazole gel intravaginally once a day for 5 days, or clindamycin cream intravaginally at bedtime for 7 days.
Newborn gonococcal eye infection. A relatively minor change is the elimination of tetracycline as prophylaxis for newborn gonococcal eye infections, leaving only erythromycin ointment to prevent the condition.
TABLE 1
2010 vs 2006: How have the CDC recommendations for STD treatment changed?1,2
Uncomplicated gonococcal infections of the cervix, urethra, rectum, and pharynx
|
| Pelvic inflammatory disease Parenteral regimens
|
Bacterial vaginosis
|
Prophylaxis for gonococcal eye infection in a newborn
|
Single-dose therapy preferred among equivalent options
Single-dose therapy (TABLE 2), while often more expensive than other options, increases compliance and helps ensure treatment completion. Single-dose therapy administered in your office is essentially directly observed treatment, an intervention that has become the standard of care for other diseases such as tuberculosis. If the single-dose agent is as effective as alternative medications, directly observed on-site administration is the preferred option for treating STDs.
TABLE 2
Single-dose therapies for specific STDs1
| Infection or condition | Single-dose therapy |
|---|---|
| Candida | Miconazole 1200 mg vaginal suppository or Tioconazole 6.5% ointment 5 g intravaginally or Butoconazole 2% cream 5 g intravaginally or Fluconazole 150 mg PO |
| Cervicitis | Azithromycin 1 g PO |
| Chancroid | Azithromycin 1 g PO or Ceftriaxone 250 mg IM |
| Chlamydia urogenital infection | Azithromycin 1 g PO |
| Gonorrhea: conjunctivitis | Ceftriaxone 1 g IM |
| Gonorrhea: uncomplicated infection of the cervix, urethra, rectum | Ceftriaxone 250 mg IM (preferred) or Cefixime 400 mg PO or Single-dose injectable cephalosporin plus Azithromycin 1 g PO |
| Gonorrhea: uncomplicated infection of the pharynx | Ceftriaxone 250 mg IM plus Azithromycin 1 g PO |
| Nongonococcal urethritis | Azithromycin 1 g PO |
| Post-sexual assault prophylaxis | Ceftriaxone 250 mg IM or Cefixime 400 mg PO plus Metronidazole 2 g PO plus Azithromycin 1 g PO |
| Recurrent, persistent nongonococcal urethritis | Metronidazole 2 g PO or Tinidazole 2 g PO plus Azithromycin 1 g PO (if not used for initial episode) |
| Syphilis: primary, secondary, and early latent | Benzathine penicillin G 2.4 million units IM |
| Trichomoniasis | Metronidazole 2 g PO or Tinidazole 2 g PO |
Other guideline recommendations
The CDC’s STD treatment guidelines contain a wealth of useful information beyond treatment advice: recommended methods of confirming diagnoses, analyses of the usefulness of various diagnostic tests, recommendations on how to manage sex partners of those infected, guidance on STD prevention counseling, and considerations for special populations and circumstances.
Additionally, there is a section on screening for STDs reflecting recommendations of the US Preventive Services Task Force (USPSTF); it also includes recommendations from the American College of Obstetricians and Gynecologists. In at least one instance, though, the USPSTF recommendation on screening for HIV infection contradicts other CDC sources.4,5 Also included is guidance on using vaccines to prevent hepatitis A, hepatitis B, and human papillomavirus (HPV), which follows the recommendations of the Advisory Committee on Immunization Practices. When to use DNA testing to detect HPV is described briefly.
A shortcoming of the CDC guidelines
Although the CDC’s STD guidelines remain the most authoritative source of information on the diagnosis and treatment of STDs, they do not seem to use a consistent method for assessing and describing the strength of the evidence behind the recommendations, which family physicians have come to expect. (However, it is sometimes possible to discern the type and strength of evidence for a particular recommendation from the written discussion.)
The new guidelines state that a series of papers to be published in Clinical Infectious Diseases will describe more fully the evidence behind some of the recommendations and include evidence tables. However, in future guideline updates, it would be helpful if the CDC were to include a brief description of the quantity and strength of evidence alongside each recommended treatment option in the tables.
How best to keep up to date
Although the new guidelines summarize the current status of recommendations on the diagnosis, treatment, and prevention of STDs and are a useful resource for family physicians, we cannot stay current simply by referring to them alone over the next 4 to 5 years until a new edition is published. As new evidence develops, changes in recommendations will be published in the Morbidity and Mortality Weekly Report.
Case in point: new interim HIV recommendations. Interim recommendations were recently released on pre-exposure prophylaxis for men who have sex with men.6 (For more on these recommendations, check out this month’s audiocast at jfponline.com.) Final recommendations are expected later this year. Recommendations for post-exposure prophylaxis to prevent HIV infection are also expected soon.
1. Workowski KA, Berman S. Centers for Disease Control and Prevention (CDC). Sexually transmitted diseases treatment guidelines, 2010. MMWR Recomm Rep. 2010;59(RR-12):1-110.
2. Centers for Disease Control and Prevention, Workowski KA, Berman SM. Sexually transmitted diseases treatment guidelines, 2006. MMWR Recomm Rep. 2006;55(RR-11):1-94.
3. Campos-Outcalt D. Practice alert: CDC no longer recommends quinolones for treatment of gonorrhea. J Fam Pract. 2007;56:554-558.
4. Branson BM, Handsfield HH, Lampe MA, et al. for the Centers for Disease Control and Prevention (CDC). Revised recommendations for HIV testing of adults, adolescents, and pregnant women in health-care settings. MMWR Recomm Rep. 2006;55(RR-14):1-17.
5. Campos-Outcalt D. Time to revise your HIV testing routine. J Fam Pract. 2007;56:283-284.
6. Centers for Disease Control and Prevention (CDC). Interim guidance: preexposure prophylaxis for the prevention of HIV infection in men who have sex with men. MMWR Morb Mortal Wkly Rep. 2011;60:65-68.
In 2010, the CDC released an update of its Sexually Transmitted Diseases (STD) Treatment Guidelines,1 which were last updated in 2006. The guidelines are widely viewed as the most authoritative source of information on the diagnosis, treatment, and follow-up of STDs, and they are the standard for publicly and privately funded clinics focusing on sexual health.
What’s new
Some of the notable changes made since the last update in 2006 appear in TABLE 1.1,2
Uncomplicated gonorrhea. Cephalosporins are the only class of antibiotic recommended as first-line treatment for gonorrhea. (In a 2007 recommendation revision, the CDC opted to no longer recommend quinolone antibiotics for the treatment of gonorrhea, because of widespread bacterial resistance.3) Preference is now given to ceftriaxone because of its proven effectiveness against pharyngeal infection, which is often asymptomatic, difficult to detect, and difficult to eradicate. Additionally, the 2010 update has increased the recommended dose of ceftriazone from 125 to 250 mg intramuscularly. The larger dose is more effective against pharyngeal infection; it is also a safeguard against decreased bacterial susceptibility to cephalosporins, which, although still very low, has been reported in more cases recently.
The guidelines still recommend that azithromycin, 1 g orally in a single dose, be given with ceftriaxone because of the relatively high rate of co-infection with Chlamydia trachomatis and the potential for azithromycin to assist with eradicating any gonorrhea with decreased susceptibility to ceftriaxone.
Pelvic inflammatory disease. Quinolones have also been removed from the list of options for outpatient treatment of pelvic inflammatory disease. All recommended regimens now specify a parenteral cephalosporin as a single injection with doxycycline 100 mg PO twice a day for 14 days, with or without metronidazole 500 mg PO twice a day for 14 days.
Bacterial vaginosis. Tinidazole, 2 g orally once a day for 2 days or 1 g orally once a day for 5 days, is now an alternative agent for bacterial vaginosis. However, preferred treatments remain metronidazole 500 mg orally twice a day for 7 days, metronidazole gel intravaginally once a day for 5 days, or clindamycin cream intravaginally at bedtime for 7 days.
Newborn gonococcal eye infection. A relatively minor change is the elimination of tetracycline as prophylaxis for newborn gonococcal eye infections, leaving only erythromycin ointment to prevent the condition.
TABLE 1
2010 vs 2006: How have the CDC recommendations for STD treatment changed?1,2
Uncomplicated gonococcal infections of the cervix, urethra, rectum, and pharynx
|
| Pelvic inflammatory disease Parenteral regimens
|
Bacterial vaginosis
|
Prophylaxis for gonococcal eye infection in a newborn
|
Single-dose therapy preferred among equivalent options
Single-dose therapy (TABLE 2), while often more expensive than other options, increases compliance and helps ensure treatment completion. Single-dose therapy administered in your office is essentially directly observed treatment, an intervention that has become the standard of care for other diseases such as tuberculosis. If the single-dose agent is as effective as alternative medications, directly observed on-site administration is the preferred option for treating STDs.
TABLE 2
Single-dose therapies for specific STDs1
| Infection or condition | Single-dose therapy |
|---|---|
| Candida | Miconazole 1200 mg vaginal suppository or Tioconazole 6.5% ointment 5 g intravaginally or Butoconazole 2% cream 5 g intravaginally or Fluconazole 150 mg PO |
| Cervicitis | Azithromycin 1 g PO |
| Chancroid | Azithromycin 1 g PO or Ceftriaxone 250 mg IM |
| Chlamydia urogenital infection | Azithromycin 1 g PO |
| Gonorrhea: conjunctivitis | Ceftriaxone 1 g IM |
| Gonorrhea: uncomplicated infection of the cervix, urethra, rectum | Ceftriaxone 250 mg IM (preferred) or Cefixime 400 mg PO or Single-dose injectable cephalosporin plus Azithromycin 1 g PO |
| Gonorrhea: uncomplicated infection of the pharynx | Ceftriaxone 250 mg IM plus Azithromycin 1 g PO |
| Nongonococcal urethritis | Azithromycin 1 g PO |
| Post-sexual assault prophylaxis | Ceftriaxone 250 mg IM or Cefixime 400 mg PO plus Metronidazole 2 g PO plus Azithromycin 1 g PO |
| Recurrent, persistent nongonococcal urethritis | Metronidazole 2 g PO or Tinidazole 2 g PO plus Azithromycin 1 g PO (if not used for initial episode) |
| Syphilis: primary, secondary, and early latent | Benzathine penicillin G 2.4 million units IM |
| Trichomoniasis | Metronidazole 2 g PO or Tinidazole 2 g PO |
Other guideline recommendations
The CDC’s STD treatment guidelines contain a wealth of useful information beyond treatment advice: recommended methods of confirming diagnoses, analyses of the usefulness of various diagnostic tests, recommendations on how to manage sex partners of those infected, guidance on STD prevention counseling, and considerations for special populations and circumstances.
Additionally, there is a section on screening for STDs reflecting recommendations of the US Preventive Services Task Force (USPSTF); it also includes recommendations from the American College of Obstetricians and Gynecologists. In at least one instance, though, the USPSTF recommendation on screening for HIV infection contradicts other CDC sources.4,5 Also included is guidance on using vaccines to prevent hepatitis A, hepatitis B, and human papillomavirus (HPV), which follows the recommendations of the Advisory Committee on Immunization Practices. When to use DNA testing to detect HPV is described briefly.
A shortcoming of the CDC guidelines
Although the CDC’s STD guidelines remain the most authoritative source of information on the diagnosis and treatment of STDs, they do not seem to use a consistent method for assessing and describing the strength of the evidence behind the recommendations, which family physicians have come to expect. (However, it is sometimes possible to discern the type and strength of evidence for a particular recommendation from the written discussion.)
The new guidelines state that a series of papers to be published in Clinical Infectious Diseases will describe more fully the evidence behind some of the recommendations and include evidence tables. However, in future guideline updates, it would be helpful if the CDC were to include a brief description of the quantity and strength of evidence alongside each recommended treatment option in the tables.
How best to keep up to date
Although the new guidelines summarize the current status of recommendations on the diagnosis, treatment, and prevention of STDs and are a useful resource for family physicians, we cannot stay current simply by referring to them alone over the next 4 to 5 years until a new edition is published. As new evidence develops, changes in recommendations will be published in the Morbidity and Mortality Weekly Report.
Case in point: new interim HIV recommendations. Interim recommendations were recently released on pre-exposure prophylaxis for men who have sex with men.6 (For more on these recommendations, check out this month’s audiocast at jfponline.com.) Final recommendations are expected later this year. Recommendations for post-exposure prophylaxis to prevent HIV infection are also expected soon.
In 2010, the CDC released an update of its Sexually Transmitted Diseases (STD) Treatment Guidelines,1 which were last updated in 2006. The guidelines are widely viewed as the most authoritative source of information on the diagnosis, treatment, and follow-up of STDs, and they are the standard for publicly and privately funded clinics focusing on sexual health.
What’s new
Some of the notable changes made since the last update in 2006 appear in TABLE 1.1,2
Uncomplicated gonorrhea. Cephalosporins are the only class of antibiotic recommended as first-line treatment for gonorrhea. (In a 2007 recommendation revision, the CDC opted to no longer recommend quinolone antibiotics for the treatment of gonorrhea, because of widespread bacterial resistance.3) Preference is now given to ceftriaxone because of its proven effectiveness against pharyngeal infection, which is often asymptomatic, difficult to detect, and difficult to eradicate. Additionally, the 2010 update has increased the recommended dose of ceftriazone from 125 to 250 mg intramuscularly. The larger dose is more effective against pharyngeal infection; it is also a safeguard against decreased bacterial susceptibility to cephalosporins, which, although still very low, has been reported in more cases recently.
The guidelines still recommend that azithromycin, 1 g orally in a single dose, be given with ceftriaxone because of the relatively high rate of co-infection with Chlamydia trachomatis and the potential for azithromycin to assist with eradicating any gonorrhea with decreased susceptibility to ceftriaxone.
Pelvic inflammatory disease. Quinolones have also been removed from the list of options for outpatient treatment of pelvic inflammatory disease. All recommended regimens now specify a parenteral cephalosporin as a single injection with doxycycline 100 mg PO twice a day for 14 days, with or without metronidazole 500 mg PO twice a day for 14 days.
Bacterial vaginosis. Tinidazole, 2 g orally once a day for 2 days or 1 g orally once a day for 5 days, is now an alternative agent for bacterial vaginosis. However, preferred treatments remain metronidazole 500 mg orally twice a day for 7 days, metronidazole gel intravaginally once a day for 5 days, or clindamycin cream intravaginally at bedtime for 7 days.
Newborn gonococcal eye infection. A relatively minor change is the elimination of tetracycline as prophylaxis for newborn gonococcal eye infections, leaving only erythromycin ointment to prevent the condition.
TABLE 1
2010 vs 2006: How have the CDC recommendations for STD treatment changed?1,2
Uncomplicated gonococcal infections of the cervix, urethra, rectum, and pharynx
|
| Pelvic inflammatory disease Parenteral regimens
|
Bacterial vaginosis
|
Prophylaxis for gonococcal eye infection in a newborn
|
Single-dose therapy preferred among equivalent options
Single-dose therapy (TABLE 2), while often more expensive than other options, increases compliance and helps ensure treatment completion. Single-dose therapy administered in your office is essentially directly observed treatment, an intervention that has become the standard of care for other diseases such as tuberculosis. If the single-dose agent is as effective as alternative medications, directly observed on-site administration is the preferred option for treating STDs.
TABLE 2
Single-dose therapies for specific STDs1
| Infection or condition | Single-dose therapy |
|---|---|
| Candida | Miconazole 1200 mg vaginal suppository or Tioconazole 6.5% ointment 5 g intravaginally or Butoconazole 2% cream 5 g intravaginally or Fluconazole 150 mg PO |
| Cervicitis | Azithromycin 1 g PO |
| Chancroid | Azithromycin 1 g PO or Ceftriaxone 250 mg IM |
| Chlamydia urogenital infection | Azithromycin 1 g PO |
| Gonorrhea: conjunctivitis | Ceftriaxone 1 g IM |
| Gonorrhea: uncomplicated infection of the cervix, urethra, rectum | Ceftriaxone 250 mg IM (preferred) or Cefixime 400 mg PO or Single-dose injectable cephalosporin plus Azithromycin 1 g PO |
| Gonorrhea: uncomplicated infection of the pharynx | Ceftriaxone 250 mg IM plus Azithromycin 1 g PO |
| Nongonococcal urethritis | Azithromycin 1 g PO |
| Post-sexual assault prophylaxis | Ceftriaxone 250 mg IM or Cefixime 400 mg PO plus Metronidazole 2 g PO plus Azithromycin 1 g PO |
| Recurrent, persistent nongonococcal urethritis | Metronidazole 2 g PO or Tinidazole 2 g PO plus Azithromycin 1 g PO (if not used for initial episode) |
| Syphilis: primary, secondary, and early latent | Benzathine penicillin G 2.4 million units IM |
| Trichomoniasis | Metronidazole 2 g PO or Tinidazole 2 g PO |
Other guideline recommendations
The CDC’s STD treatment guidelines contain a wealth of useful information beyond treatment advice: recommended methods of confirming diagnoses, analyses of the usefulness of various diagnostic tests, recommendations on how to manage sex partners of those infected, guidance on STD prevention counseling, and considerations for special populations and circumstances.
Additionally, there is a section on screening for STDs reflecting recommendations of the US Preventive Services Task Force (USPSTF); it also includes recommendations from the American College of Obstetricians and Gynecologists. In at least one instance, though, the USPSTF recommendation on screening for HIV infection contradicts other CDC sources.4,5 Also included is guidance on using vaccines to prevent hepatitis A, hepatitis B, and human papillomavirus (HPV), which follows the recommendations of the Advisory Committee on Immunization Practices. When to use DNA testing to detect HPV is described briefly.
A shortcoming of the CDC guidelines
Although the CDC’s STD guidelines remain the most authoritative source of information on the diagnosis and treatment of STDs, they do not seem to use a consistent method for assessing and describing the strength of the evidence behind the recommendations, which family physicians have come to expect. (However, it is sometimes possible to discern the type and strength of evidence for a particular recommendation from the written discussion.)
The new guidelines state that a series of papers to be published in Clinical Infectious Diseases will describe more fully the evidence behind some of the recommendations and include evidence tables. However, in future guideline updates, it would be helpful if the CDC were to include a brief description of the quantity and strength of evidence alongside each recommended treatment option in the tables.
How best to keep up to date
Although the new guidelines summarize the current status of recommendations on the diagnosis, treatment, and prevention of STDs and are a useful resource for family physicians, we cannot stay current simply by referring to them alone over the next 4 to 5 years until a new edition is published. As new evidence develops, changes in recommendations will be published in the Morbidity and Mortality Weekly Report.
Case in point: new interim HIV recommendations. Interim recommendations were recently released on pre-exposure prophylaxis for men who have sex with men.6 (For more on these recommendations, check out this month’s audiocast at jfponline.com.) Final recommendations are expected later this year. Recommendations for post-exposure prophylaxis to prevent HIV infection are also expected soon.
1. Workowski KA, Berman S. Centers for Disease Control and Prevention (CDC). Sexually transmitted diseases treatment guidelines, 2010. MMWR Recomm Rep. 2010;59(RR-12):1-110.
2. Centers for Disease Control and Prevention, Workowski KA, Berman SM. Sexually transmitted diseases treatment guidelines, 2006. MMWR Recomm Rep. 2006;55(RR-11):1-94.
3. Campos-Outcalt D. Practice alert: CDC no longer recommends quinolones for treatment of gonorrhea. J Fam Pract. 2007;56:554-558.
4. Branson BM, Handsfield HH, Lampe MA, et al. for the Centers for Disease Control and Prevention (CDC). Revised recommendations for HIV testing of adults, adolescents, and pregnant women in health-care settings. MMWR Recomm Rep. 2006;55(RR-14):1-17.
5. Campos-Outcalt D. Time to revise your HIV testing routine. J Fam Pract. 2007;56:283-284.
6. Centers for Disease Control and Prevention (CDC). Interim guidance: preexposure prophylaxis for the prevention of HIV infection in men who have sex with men. MMWR Morb Mortal Wkly Rep. 2011;60:65-68.
1. Workowski KA, Berman S. Centers for Disease Control and Prevention (CDC). Sexually transmitted diseases treatment guidelines, 2010. MMWR Recomm Rep. 2010;59(RR-12):1-110.
2. Centers for Disease Control and Prevention, Workowski KA, Berman SM. Sexually transmitted diseases treatment guidelines, 2006. MMWR Recomm Rep. 2006;55(RR-11):1-94.
3. Campos-Outcalt D. Practice alert: CDC no longer recommends quinolones for treatment of gonorrhea. J Fam Pract. 2007;56:554-558.
4. Branson BM, Handsfield HH, Lampe MA, et al. for the Centers for Disease Control and Prevention (CDC). Revised recommendations for HIV testing of adults, adolescents, and pregnant women in health-care settings. MMWR Recomm Rep. 2006;55(RR-14):1-17.
5. Campos-Outcalt D. Time to revise your HIV testing routine. J Fam Pract. 2007;56:283-284.
6. Centers for Disease Control and Prevention (CDC). Interim guidance: preexposure prophylaxis for the prevention of HIV infection in men who have sex with men. MMWR Morb Mortal Wkly Rep. 2011;60:65-68.
ACIP update: 2 new recommendations for meningococcal vaccine
At its October 2010 meeting, the Advisory Committee on Immunization Practices (ACIP) made 2 additions to its recommendations for quadrivalent meningococcal conjugate vaccine (MCV4), based on evolving knowledge of the vaccine and its duration of protection.
- A booster dose at age 16 has been added to the routine schedule for those vaccinated at ages 11 to 12 years. A booster dose has also been added for those vaccinated at ages 13 to 15 years, although the recommended timing of this booster had not been finalized at press time.
- A 2-dose primary series, 2 months apart, is now recommended for patients at higher risk of meningococcal disease. The high-risk category includes those with persistent complement component deficiency, asplenia, or human immunodeficiency virus (HIV). High-risk patients who were previously vaccinated should receive a booster dose at the earliest opportunity and continue to receive boosters at the appropriate interval (3-5 years).
Meningitis is rare but serious
Meningococcal meningitis is a potentially devastating disease in adolescents and young adults. It has a case fatality rate of about 20%, and the sequelae for survivors can be severe: 3.1% require limb amputations and another 10.9% suffer neurological deficits.1 Thankfully, meningococcal disease is rare, occurring at rates below 1 in 200,000 in the 11- to 15-year-old age group and less than 1 in 100,000 in the 16- to 21-year-old age group.2
Routine immunization with MCV4 is recommended for adolescents
In 2007, ACIP recommended routine use of MCV4 for adolescents between the ages of 11 and 18 years. The recommendation gave preference to immunization at ages 11 to 12 years, along with the other adolescent vaccines given at that time.3 Updated recommendations in effect in 2010 state that those at highest risk for meningococcal infection (those with functional or anatomic asplenia, C3 complement deficiency, or HIV infection) should be vaccinated with MCV4 starting at age 2 and revaccinated every 3 years if last vaccinated at 2 to 6 years, and every 5 years if last vaccinated at or after age 7.4,5 TABLE 1 lists the recommendations for MCV4 in place prior to the October 2010 ACIP meeting.
Two MCV4 products are licensed for use in the United States: Menactra (Sanofi Pasteur) and Menveo (Novartis). Both contain antigens against 4 serotypes, A, C, Y, and W-135. Neither protects against type B, which causes a majority of the disease in infants.6 In recent years, serotype A disease has become extremely rare in the United States.6 MCV4 coverage for adolescents ages 13 to 17 years is increasing, going from 41.8% in 2008 to 53.6% in 2009.7
TABLE 1
Recommendations for MCV4 prior to October 20103-5
|
|
|
| MCV4, meningococcal conjugate vaccine. |
The new recommendations: One is more controversial than the other
The recommendation for a 2-dose primary series in high-risk groups was not controversial. The same conditions that place individuals at highest risk for meningococcal infection also result in a less robust response to a single dose of the vaccine, and a 2-dose series is needed to achieve protective antibody levels in a high proportion of those vaccine recipients.8 This recommendation will affect relatively few patients.
The recommendation for booster doses in the general adolescent population generated a lot more debate. Studies performed since the licensure of MCV4 have shown that levels of protective antibodies decline over time. Five years after vaccination, 50% of vaccine recipients have levels below that considered fully protective.2 One small case-control study of 107 cases suggested that the number of years from receipt of the vaccine was a risk factor for meningococcal disease.2
However, rates of meningococcal meningitis in adolescents have been declining over the past few years (TABLE 2), and there are no surveillance data to support the conclusion that teens vaccinated at ages 11 to 12 years are at increased risk as they age. In addition, the number of cases is very low (TABLE 3) and the cost benefit analysis of a booster dose of MCV4 is very unfavorable.1,2
TABLE 2
Rates* of serogroup C, Y, and W-135 meningococcal disease†
| Age group (y) | ||
|---|---|---|
| Year | 11-19 | ≥20 |
| 2004-2005 | 0.23 | 0.16 |
| 2006-2007 | 0.27 | 0.22 |
| 2008-2009 | 0.14 | 0.21 |
| *Annual rate per 100,000. †Serogroup A disease is too rare for inclusion here. Source: Cohn A. Advisory Committee on Immunization Practices Meeting; October 27, 2010.2 | ||
TABLE 3
Average annual number of cases of C, Y, and W-135 meningococcal disease
| Age group (y) | 2000-2004 | 2005-2009 | Change |
|---|---|---|---|
| 11-14 | 46 | 12 | -74% |
| 15-18 | 106 | 77 | -27% |
| 19-22 | 62 | 52 | -16% |
| Total (11-22) | 214 | 141 | -34% |
| Source: Cohn A. Advisory Committee on Immunization Practices Meeting; October 27, 2010.2 | |||
ACIP weighed the options for a booster dose
Three options were presented at the October 2010 ACIP meeting:
- Option 1: No change to the current recommendation for vaccination of 11- to 12-year-olds. Wait and see what happens to disease incidence over several more years.
- Option 2: Move the age of vaccination to 15 years with no booster. This would allow protection to persist through the years of highest risk (16-21 years).
- Option 3: Keep the recommendation for vaccination at ages 11 to 12 years, and add a booster dose at age 16.
The first option was the least cost effective: $281,000/quality-adjusted life year (QALY). The second option was the most cost effective at $121,000/QALY. The last option came out in the middle: $157,000/QALY, but it would save the most lives—9 more per year compared with Option 2.1 There is, however, a caveat with regard to the cost-effectiveness estimates. The numbers were obtained using incidence data from the year 2000; incidence has declined since then, and cost-effectiveness estimates would be much less favorable using today’s rates.
These issues were discussed at length, and the decision to add a booster dose at age 16 was made on a close vote. This decision illustrates how difficult vaccine policy-making has become in recent years, when choices must be made about recommending safe, effective, and expensive vaccines to prevent illnesses that are both rare and serious.
The new MCV4 recommendations will be added to the child immunization schedule for 2011.
The take-home message for family physicians is to strive to increase the proportion of 11- to 12-year-olds who are fully vaccinated and in 2011 to begin to advise those who are between the ages of 16 and 20 years of the recommendation for a booster dose of MCV4.
1. Ortega-Sanchez I. Cost-effectiveness of meningococcal vaccination strategies for adolescents in the United States. Presented at: Advisory Committee on Immunization Practices Meeting; October 27, 2010; Atlanta, Ga.
2. Cohn A. Optimizing the adolescent meningococcal vaccination program. Presented at: Advisory Committee on Immunization Practices Meeting; October 27, 2010; Atlanta, Ga.
3. CDC. Revised recommendations of the Advisory Committee on Immunization Practices to vaccinate all persons aged 11-18 years with meningococcal conjugate vaccine. MMWR Morb Mortal Wkly Rep. 2007;56:794-795.
4. CDC.Updated recommendation from the Advisory Committee on Immunization Practices for revaccination of persons at prolonged increased risk for meningococcal disease. MMWR Morb Mortal Wkly Rep. 2009;58:1042-1043.
5. CDC. Recommended immunization schedules for persons aged 0 through 18 years—United States, 2010. MMWR Morb Mortal Wkly Rep. 2010;58:1-4.
6. Schaffner W, Harrison LH, Kaplan SL, et al. The changing epidemiology of meningococcal disease among U.S. children, adolescents and young adults. National Foundation for Infectious Diseases. November 2004. Available at: www.nfid.org/pdf/meningitis/FINALChanging_Epidemiology_of_Meningococcal_Disease.pdf. Accessed November 4, 2010.
7. CDC. National, state, and local area vaccination coverage among adolescents aged 13-17 years—United States, 2009. MMWR Morb Mortal Wkly Rep. 2010;59:1018-1023.
8. Cohn A. Rationale and proposed recommendations for two dose primary vaccination for persons at increased risk for meningococcal disease. Presented at: Advisory Committee on Immunization Practices Meeting; October 27, 2010; Atlanta, Ga.
At its October 2010 meeting, the Advisory Committee on Immunization Practices (ACIP) made 2 additions to its recommendations for quadrivalent meningococcal conjugate vaccine (MCV4), based on evolving knowledge of the vaccine and its duration of protection.
- A booster dose at age 16 has been added to the routine schedule for those vaccinated at ages 11 to 12 years. A booster dose has also been added for those vaccinated at ages 13 to 15 years, although the recommended timing of this booster had not been finalized at press time.
- A 2-dose primary series, 2 months apart, is now recommended for patients at higher risk of meningococcal disease. The high-risk category includes those with persistent complement component deficiency, asplenia, or human immunodeficiency virus (HIV). High-risk patients who were previously vaccinated should receive a booster dose at the earliest opportunity and continue to receive boosters at the appropriate interval (3-5 years).
Meningitis is rare but serious
Meningococcal meningitis is a potentially devastating disease in adolescents and young adults. It has a case fatality rate of about 20%, and the sequelae for survivors can be severe: 3.1% require limb amputations and another 10.9% suffer neurological deficits.1 Thankfully, meningococcal disease is rare, occurring at rates below 1 in 200,000 in the 11- to 15-year-old age group and less than 1 in 100,000 in the 16- to 21-year-old age group.2
Routine immunization with MCV4 is recommended for adolescents
In 2007, ACIP recommended routine use of MCV4 for adolescents between the ages of 11 and 18 years. The recommendation gave preference to immunization at ages 11 to 12 years, along with the other adolescent vaccines given at that time.3 Updated recommendations in effect in 2010 state that those at highest risk for meningococcal infection (those with functional or anatomic asplenia, C3 complement deficiency, or HIV infection) should be vaccinated with MCV4 starting at age 2 and revaccinated every 3 years if last vaccinated at 2 to 6 years, and every 5 years if last vaccinated at or after age 7.4,5 TABLE 1 lists the recommendations for MCV4 in place prior to the October 2010 ACIP meeting.
Two MCV4 products are licensed for use in the United States: Menactra (Sanofi Pasteur) and Menveo (Novartis). Both contain antigens against 4 serotypes, A, C, Y, and W-135. Neither protects against type B, which causes a majority of the disease in infants.6 In recent years, serotype A disease has become extremely rare in the United States.6 MCV4 coverage for adolescents ages 13 to 17 years is increasing, going from 41.8% in 2008 to 53.6% in 2009.7
TABLE 1
Recommendations for MCV4 prior to October 20103-5
|
|
|
| MCV4, meningococcal conjugate vaccine. |
The new recommendations: One is more controversial than the other
The recommendation for a 2-dose primary series in high-risk groups was not controversial. The same conditions that place individuals at highest risk for meningococcal infection also result in a less robust response to a single dose of the vaccine, and a 2-dose series is needed to achieve protective antibody levels in a high proportion of those vaccine recipients.8 This recommendation will affect relatively few patients.
The recommendation for booster doses in the general adolescent population generated a lot more debate. Studies performed since the licensure of MCV4 have shown that levels of protective antibodies decline over time. Five years after vaccination, 50% of vaccine recipients have levels below that considered fully protective.2 One small case-control study of 107 cases suggested that the number of years from receipt of the vaccine was a risk factor for meningococcal disease.2
However, rates of meningococcal meningitis in adolescents have been declining over the past few years (TABLE 2), and there are no surveillance data to support the conclusion that teens vaccinated at ages 11 to 12 years are at increased risk as they age. In addition, the number of cases is very low (TABLE 3) and the cost benefit analysis of a booster dose of MCV4 is very unfavorable.1,2
TABLE 2
Rates* of serogroup C, Y, and W-135 meningococcal disease†
| Age group (y) | ||
|---|---|---|
| Year | 11-19 | ≥20 |
| 2004-2005 | 0.23 | 0.16 |
| 2006-2007 | 0.27 | 0.22 |
| 2008-2009 | 0.14 | 0.21 |
| *Annual rate per 100,000. †Serogroup A disease is too rare for inclusion here. Source: Cohn A. Advisory Committee on Immunization Practices Meeting; October 27, 2010.2 | ||
TABLE 3
Average annual number of cases of C, Y, and W-135 meningococcal disease
| Age group (y) | 2000-2004 | 2005-2009 | Change |
|---|---|---|---|
| 11-14 | 46 | 12 | -74% |
| 15-18 | 106 | 77 | -27% |
| 19-22 | 62 | 52 | -16% |
| Total (11-22) | 214 | 141 | -34% |
| Source: Cohn A. Advisory Committee on Immunization Practices Meeting; October 27, 2010.2 | |||
ACIP weighed the options for a booster dose
Three options were presented at the October 2010 ACIP meeting:
- Option 1: No change to the current recommendation for vaccination of 11- to 12-year-olds. Wait and see what happens to disease incidence over several more years.
- Option 2: Move the age of vaccination to 15 years with no booster. This would allow protection to persist through the years of highest risk (16-21 years).
- Option 3: Keep the recommendation for vaccination at ages 11 to 12 years, and add a booster dose at age 16.
The first option was the least cost effective: $281,000/quality-adjusted life year (QALY). The second option was the most cost effective at $121,000/QALY. The last option came out in the middle: $157,000/QALY, but it would save the most lives—9 more per year compared with Option 2.1 There is, however, a caveat with regard to the cost-effectiveness estimates. The numbers were obtained using incidence data from the year 2000; incidence has declined since then, and cost-effectiveness estimates would be much less favorable using today’s rates.
These issues were discussed at length, and the decision to add a booster dose at age 16 was made on a close vote. This decision illustrates how difficult vaccine policy-making has become in recent years, when choices must be made about recommending safe, effective, and expensive vaccines to prevent illnesses that are both rare and serious.
The new MCV4 recommendations will be added to the child immunization schedule for 2011.
The take-home message for family physicians is to strive to increase the proportion of 11- to 12-year-olds who are fully vaccinated and in 2011 to begin to advise those who are between the ages of 16 and 20 years of the recommendation for a booster dose of MCV4.
At its October 2010 meeting, the Advisory Committee on Immunization Practices (ACIP) made 2 additions to its recommendations for quadrivalent meningococcal conjugate vaccine (MCV4), based on evolving knowledge of the vaccine and its duration of protection.
- A booster dose at age 16 has been added to the routine schedule for those vaccinated at ages 11 to 12 years. A booster dose has also been added for those vaccinated at ages 13 to 15 years, although the recommended timing of this booster had not been finalized at press time.
- A 2-dose primary series, 2 months apart, is now recommended for patients at higher risk of meningococcal disease. The high-risk category includes those with persistent complement component deficiency, asplenia, or human immunodeficiency virus (HIV). High-risk patients who were previously vaccinated should receive a booster dose at the earliest opportunity and continue to receive boosters at the appropriate interval (3-5 years).
Meningitis is rare but serious
Meningococcal meningitis is a potentially devastating disease in adolescents and young adults. It has a case fatality rate of about 20%, and the sequelae for survivors can be severe: 3.1% require limb amputations and another 10.9% suffer neurological deficits.1 Thankfully, meningococcal disease is rare, occurring at rates below 1 in 200,000 in the 11- to 15-year-old age group and less than 1 in 100,000 in the 16- to 21-year-old age group.2
Routine immunization with MCV4 is recommended for adolescents
In 2007, ACIP recommended routine use of MCV4 for adolescents between the ages of 11 and 18 years. The recommendation gave preference to immunization at ages 11 to 12 years, along with the other adolescent vaccines given at that time.3 Updated recommendations in effect in 2010 state that those at highest risk for meningococcal infection (those with functional or anatomic asplenia, C3 complement deficiency, or HIV infection) should be vaccinated with MCV4 starting at age 2 and revaccinated every 3 years if last vaccinated at 2 to 6 years, and every 5 years if last vaccinated at or after age 7.4,5 TABLE 1 lists the recommendations for MCV4 in place prior to the October 2010 ACIP meeting.
Two MCV4 products are licensed for use in the United States: Menactra (Sanofi Pasteur) and Menveo (Novartis). Both contain antigens against 4 serotypes, A, C, Y, and W-135. Neither protects against type B, which causes a majority of the disease in infants.6 In recent years, serotype A disease has become extremely rare in the United States.6 MCV4 coverage for adolescents ages 13 to 17 years is increasing, going from 41.8% in 2008 to 53.6% in 2009.7
TABLE 1
Recommendations for MCV4 prior to October 20103-5
|
|
|
| MCV4, meningococcal conjugate vaccine. |
The new recommendations: One is more controversial than the other
The recommendation for a 2-dose primary series in high-risk groups was not controversial. The same conditions that place individuals at highest risk for meningococcal infection also result in a less robust response to a single dose of the vaccine, and a 2-dose series is needed to achieve protective antibody levels in a high proportion of those vaccine recipients.8 This recommendation will affect relatively few patients.
The recommendation for booster doses in the general adolescent population generated a lot more debate. Studies performed since the licensure of MCV4 have shown that levels of protective antibodies decline over time. Five years after vaccination, 50% of vaccine recipients have levels below that considered fully protective.2 One small case-control study of 107 cases suggested that the number of years from receipt of the vaccine was a risk factor for meningococcal disease.2
However, rates of meningococcal meningitis in adolescents have been declining over the past few years (TABLE 2), and there are no surveillance data to support the conclusion that teens vaccinated at ages 11 to 12 years are at increased risk as they age. In addition, the number of cases is very low (TABLE 3) and the cost benefit analysis of a booster dose of MCV4 is very unfavorable.1,2
TABLE 2
Rates* of serogroup C, Y, and W-135 meningococcal disease†
| Age group (y) | ||
|---|---|---|
| Year | 11-19 | ≥20 |
| 2004-2005 | 0.23 | 0.16 |
| 2006-2007 | 0.27 | 0.22 |
| 2008-2009 | 0.14 | 0.21 |
| *Annual rate per 100,000. †Serogroup A disease is too rare for inclusion here. Source: Cohn A. Advisory Committee on Immunization Practices Meeting; October 27, 2010.2 | ||
TABLE 3
Average annual number of cases of C, Y, and W-135 meningococcal disease
| Age group (y) | 2000-2004 | 2005-2009 | Change |
|---|---|---|---|
| 11-14 | 46 | 12 | -74% |
| 15-18 | 106 | 77 | -27% |
| 19-22 | 62 | 52 | -16% |
| Total (11-22) | 214 | 141 | -34% |
| Source: Cohn A. Advisory Committee on Immunization Practices Meeting; October 27, 2010.2 | |||
ACIP weighed the options for a booster dose
Three options were presented at the October 2010 ACIP meeting:
- Option 1: No change to the current recommendation for vaccination of 11- to 12-year-olds. Wait and see what happens to disease incidence over several more years.
- Option 2: Move the age of vaccination to 15 years with no booster. This would allow protection to persist through the years of highest risk (16-21 years).
- Option 3: Keep the recommendation for vaccination at ages 11 to 12 years, and add a booster dose at age 16.
The first option was the least cost effective: $281,000/quality-adjusted life year (QALY). The second option was the most cost effective at $121,000/QALY. The last option came out in the middle: $157,000/QALY, but it would save the most lives—9 more per year compared with Option 2.1 There is, however, a caveat with regard to the cost-effectiveness estimates. The numbers were obtained using incidence data from the year 2000; incidence has declined since then, and cost-effectiveness estimates would be much less favorable using today’s rates.
These issues were discussed at length, and the decision to add a booster dose at age 16 was made on a close vote. This decision illustrates how difficult vaccine policy-making has become in recent years, when choices must be made about recommending safe, effective, and expensive vaccines to prevent illnesses that are both rare and serious.
The new MCV4 recommendations will be added to the child immunization schedule for 2011.
The take-home message for family physicians is to strive to increase the proportion of 11- to 12-year-olds who are fully vaccinated and in 2011 to begin to advise those who are between the ages of 16 and 20 years of the recommendation for a booster dose of MCV4.
1. Ortega-Sanchez I. Cost-effectiveness of meningococcal vaccination strategies for adolescents in the United States. Presented at: Advisory Committee on Immunization Practices Meeting; October 27, 2010; Atlanta, Ga.
2. Cohn A. Optimizing the adolescent meningococcal vaccination program. Presented at: Advisory Committee on Immunization Practices Meeting; October 27, 2010; Atlanta, Ga.
3. CDC. Revised recommendations of the Advisory Committee on Immunization Practices to vaccinate all persons aged 11-18 years with meningococcal conjugate vaccine. MMWR Morb Mortal Wkly Rep. 2007;56:794-795.
4. CDC.Updated recommendation from the Advisory Committee on Immunization Practices for revaccination of persons at prolonged increased risk for meningococcal disease. MMWR Morb Mortal Wkly Rep. 2009;58:1042-1043.
5. CDC. Recommended immunization schedules for persons aged 0 through 18 years—United States, 2010. MMWR Morb Mortal Wkly Rep. 2010;58:1-4.
6. Schaffner W, Harrison LH, Kaplan SL, et al. The changing epidemiology of meningococcal disease among U.S. children, adolescents and young adults. National Foundation for Infectious Diseases. November 2004. Available at: www.nfid.org/pdf/meningitis/FINALChanging_Epidemiology_of_Meningococcal_Disease.pdf. Accessed November 4, 2010.
7. CDC. National, state, and local area vaccination coverage among adolescents aged 13-17 years—United States, 2009. MMWR Morb Mortal Wkly Rep. 2010;59:1018-1023.
8. Cohn A. Rationale and proposed recommendations for two dose primary vaccination for persons at increased risk for meningococcal disease. Presented at: Advisory Committee on Immunization Practices Meeting; October 27, 2010; Atlanta, Ga.
1. Ortega-Sanchez I. Cost-effectiveness of meningococcal vaccination strategies for adolescents in the United States. Presented at: Advisory Committee on Immunization Practices Meeting; October 27, 2010; Atlanta, Ga.
2. Cohn A. Optimizing the adolescent meningococcal vaccination program. Presented at: Advisory Committee on Immunization Practices Meeting; October 27, 2010; Atlanta, Ga.
3. CDC. Revised recommendations of the Advisory Committee on Immunization Practices to vaccinate all persons aged 11-18 years with meningococcal conjugate vaccine. MMWR Morb Mortal Wkly Rep. 2007;56:794-795.
4. CDC.Updated recommendation from the Advisory Committee on Immunization Practices for revaccination of persons at prolonged increased risk for meningococcal disease. MMWR Morb Mortal Wkly Rep. 2009;58:1042-1043.
5. CDC. Recommended immunization schedules for persons aged 0 through 18 years—United States, 2010. MMWR Morb Mortal Wkly Rep. 2010;58:1-4.
6. Schaffner W, Harrison LH, Kaplan SL, et al. The changing epidemiology of meningococcal disease among U.S. children, adolescents and young adults. National Foundation for Infectious Diseases. November 2004. Available at: www.nfid.org/pdf/meningitis/FINALChanging_Epidemiology_of_Meningococcal_Disease.pdf. Accessed November 4, 2010.
7. CDC. National, state, and local area vaccination coverage among adolescents aged 13-17 years—United States, 2009. MMWR Morb Mortal Wkly Rep. 2010;59:1018-1023.
8. Cohn A. Rationale and proposed recommendations for two dose primary vaccination for persons at increased risk for meningococcal disease. Presented at: Advisory Committee on Immunization Practices Meeting; October 27, 2010; Atlanta, Ga.
Flu season’s almost here: Are you ready?
Influenza pandemics like the one we had last year are uncommon, and mounting an effective response was a difficult challenge. The pandemic hit early and hard. Physicians and the public health system responded well, administering a seasonal flu vaccine as well as a new H1N1 vaccine that was approved, produced, and distributed in record time. Before the end of the season, approximately 30% of the population had received an H1N1 vaccine and 40% a seasonal vaccine.1
What happened last year
The influenza attack rate in 2009-2010 exceeded that of a normal influenza season and the age groups most affected were also different, with those over the age of 65 largely spared.2 Virtually all the influenza last year was caused by the pandemic H1N1 strain.2 Fortuitously, the virus was not especially virulent and the death rates were below what was initially expected. TABLE 1 lists the population death rates that occurred for different age groups.2 Most of the more than 2000 deaths were among those with high-risk conditions.3 Those conditions are listed in TABLE 2.
There were, however, 269 deaths by late March among children, which far exceeded the number of deaths in this age group for the previous 3 influenza seasons.2 For the most part, these higher mortality rates were due to higher attack rates, rather than higher case fatality rates. This is evident from hospitalization rates for children younger than age 5, which exceeded those of other age groups, as shown in FIGURE 1.
TABLE 1
2009-2010 Influenza death rates by age
| Age group, years | Death rate/100,000 |
|---|---|
| 0-4 | 0.43 |
| 5-18 | 0.36 |
| 19-24 | 0.54 |
| 25-49 | 0.87 |
| 50-64 | 1.56 |
| ≥65 | 0.95 |
| Source: CDC. MMWR Morb Mortal Wkly Rep. 2010.2 | |
TABLE 2
Individuals at higher risk for influenza complications (or who may spread infection to those at higher risk)
|
| Source: CDC. MMWR Morb Mortal Wkly Rep. 2010.4 |
FIGURE 1
Cumulative lab-confirmed hospitalization rate by age group, 2009 H1N1, April 2009-February 13, 2010*
*Based on 35 states reporting (n=49,516).
Source: Finelli L, et al. Available at http://www.cdc.gov/vaccines/recs/acip/downloads/mtg-slides-feb10/05-2-flu-vac.pdf. 2010.3
The task will be simpler this year
While it’s not possible to predict what will happen in the upcoming season, 2 developments should simplify the family physician’s task of adhering to official recommendations:
- Only 1 vaccine formulation will be available, and
- For the first time, the recommendation is to vaccinate everyone who does not have a contraindication.4
The vaccine for the 2010-2011 season will contain 3 antigens: the pandemic H1N1 virus, an H3N2 A strain (A/Perth/16/2009), and a B virus (B/Brisbane/60/2008).2 The decision on which antigens to include is made 6 months in advance of the start of the next flu season and is based on information about the most common influenza antigens circulating worldwide at that time.
Immunization for all
This year’s recommendation to immunize everyone who does not have a contraindication is a major change from the age- and risk-based recommendations of past years. The universal recommendation is the culmination of the incremental expansions of recommendation categories that occurred over the past decade, which resulted in suboptimal immunization rates.1 In 2009, only 40% to 50% of adults for whom the seasonal vaccine was recommended received it.5 While the annual influenza vaccine recommendation is now universal, those who should be specially targeted include those in TABLE 2. Most public health authorities believe children should also receive special emphasis because of the high transmission rate among school-age children and their home contacts. Next, of course, come health care workers, who should be vaccinated to protect ourselves, our families, and our patients.4,6
Antivirals for treatment and prevention
There are 2 uses for antivirals to combat influenza: treatment of those infected and chemoprevention for those exposed to someone infected. Treatment is recommended for those with confirmed or suspected influenza who have severe, complicated, or progressive illness or who are hospitalized.7 Treatment should be strongly considered for anyone at higher risk for complications and death from influenza.7
Chemoprevention is now being deemphasized because of a concern for possible development of antiviral resistance. It should be considered for those in the high-risk categories (TABLE 2) with a documented exposure.7
Which antiviral to use will depend on which influenza strains are circulating and their resistance patterns. So far, H1N1 has remained largely sensitive to both neuraminidase inhibitors: oseltamivir and zanamivir. However, oseltamivir resistance has been documented in a few cases and will be monitored carefully.
Family physicians will need to stay informed by state and local health departments about circulating strains and resistance patterns. The latest Centers for Disease Control and Prevention (CDC) guidance on antiviral therapy can be consulted for dosage and other details on the 4 antiviral drugs licensed in the United States.7
What you must know about vaccine safety
Because of increasing public awareness of safety issues, family physicians will frequently need to address patients’ questions about the safety of this year’s vaccine. Last year, multiple reporting systems including the Vaccine Adverse Event Reporting System (VAERS), Vaccine Safety Datalink (VSD) Project, the Defense Medical Surveillance System (DMSS), and others, extensively monitored adverse events that could potentially be linked to the H1N1 vaccine.8 Three so-called weak signals—indications of a possible link to a rare, but statistically significant adverse event—were received.
The 3 signals were for Guillain-Barré syndrome (GBS), Bell’s palsy, and thrombocytopenia/idiopathic thrombocytopenic purpura. The status of the investigation of each potential link to the vaccine can be found on the National Vaccine Advisory Committee (NVAC) safety Web site at http://www.hhs.gov/nvpo/nvac/reports/index.html.
The GBS signal has been investigated the most aggressively because this adverse reaction has been linked to the so-called swine flu vaccine of 1976. One analysis has been published in the Morbidity and Mortality Weekly Report.9 Whether GBS has a causal link to the H1N1 vaccine remains in doubt. In the worst-case scenario, if causation is determined, it appears that the vaccine would account for no more than 1 excess case of GBS per million doses.9
In Western Australia, there has been a recent report of an excess of fever and febrile seizures in children 6 months to 5 years of age, and fever in children 5 to 9 years of age who received seasonal influenza vaccine. The rate of febrile seizures in children younger than age 3 was 7 per 1000, which is 7 times the rate normally expected. These adverse reactions were associated with only 1 vaccine product, Fluvax, and Fluvax Junior, manufactured by CSL Biotherapies.10 The CSL product is marketed in the United States by Merck & Co. under the brand name Afluria.
The Advisory Committee on Immunization Practices (ACIP) has issued the following recommendations:11
- Afluria should not be used in children ages 6 months through 8 years. The exception: children who are ages 5 through 8 years who are considered to be at high risk for influenza complications and for whom no other trivalent inactivated vaccine is available.
- Other age-appropriate, licensed seasonal influenza vaccine formulations should be used for prevention of influenza in children ages 6 months through 8 years.
High-dose vaccine for elderly patients
A new seasonal influenza vaccine (Fluzone High-Dose, manufactured by Sanofi Pasteur) is now available for use in people who are 65 years of age and older.12 Fluzone High-Dose contains 4 times the amount of influenza antigen as other inactivated seasonal influenza vaccines. Fluzone High-Dose vaccine produces higher antibody levels in the elderly but also a higher frequency of local reactions. Studies are being conducted to see if the vaccine results in better patient outcomes. ACIP does not state a preference for any of the available influenza vaccines for those who are 65 years of age and older.12
Children younger than age 9: One dose or two?
The new recommendations for deciding if a child under the age of 9 years should receive 1 or 2 doses of the vaccine run counter to the trend for simplification in influenza vaccine recommendations. The decision depends on the child’s past immunization history for both seasonal and H1N1 vaccines. To be fully vaccinated with only 1 dose this year, a child must have previously received at least 1 dose of H1N1 vaccine and 2 doses of seasonal vaccine. FIGURE 2 illustrates the process you need to go through to make the dosage choice. When the child’s immunization history is unknown or uncertain, give 2 doses, separated by 4 weeks.4
FIGURE 2
Children younger than 9: Ask 4 questions
Source: CDC. MMWR Morb Mortal Wkly Rep. 2010.4
1. Singleton JA. H1N1 vaccination coverage: updated interim results February 24, 2010. ACIP presentation slides, February 2010 meeting. Available at: http://www.cdc.gov/vaccines/recs/acip/downloads/mtg-slides-feb10/05-4-flu-vac.pdf. Accessed July 16, 2010.
2. CDC. Update: influenza activity—United States, August 30, 2009-March 27, 2010, and composition of the 2010-11 influenza vaccine. MMWR Morb Mortal Wkly Rep. 2010;59:423-438.
3. Finelli L, Brammer L, Kniss K, et al. Influenza epidemiology and surveillance. ACIP Presentation slides, February 2010 meeting. Available at: http://www.cdc.gov/vaccines/recs/acip/downloads/mtg-slides-feb10/05-2-flu-vac.pdf. Accessed July 26, 2010.
4. CDC. Prevention and control of influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2010. MMWR Morb Mortal Wkly Rep. July 29, 2010 (early release);1-62.
5. Harris KM, Maurer J, Uscher-Pines L. Seasonal influenza vaccine use by adults in the US: a snapshot as of mid-November 2009. Available at: http://www.rand.org/pubs/occasional_papers/OP289/. Accessed July 16, 2010.
6. Fiore A. Influenza vaccine workgroup discussions and recommendations, November 2009-February 2010. ACIP presentation slides, February 2010 meeting. Available at: http://www.cdc.gov/vaccines/recs/acip/downloads/mtg-slides-feb10/05-7-flu-vac.pdf. Accessed July 26, 2010.
7. CDC. Updated interim recommendations for the use of antiviral medications in the treatment and prevention of influenza for the 2009-2010 season. Available at: http://www.cdc.gov/H1N1flu/recommendations.htm. Accessed July 16, 2010.
8. National Vaccine Advisory Committee Report on 2009 H1N1 Vaccine Safety Risk Assessment. June 2010. Available at: http://www.hhs.gov/nvpo/nvac/reports/vsrawg_repot_may2010.html. Accessed July 16, 2010.
9. CDC. Preliminary results: surveillance for Guillain-Barré syndrome after receipt of influenza A (H1N1) 2009 monovalent vaccine—United States, 2009–2010. MMWR Morb Mortal Wkly Rep. 2010;59:657-661.
10. McNeil M. Febrile seizures in Australia and CDC monitoring plan for 2010-2011 seasonal influenza vaccine. Available at: www.cdc.gov/vaccines/recs/acip/downloads/mtg-slides-jun10/10-8-flu.pdf. Accessed August 19, 2010.
11. CDC. Media statement: ACIP recommendation for use of CSL influenza vaccine. August 6, 2010. Available at: http://www.cdc.gov/media/pressrel/2010/s100806.htm?s_cid=mediarel_s100806. Accessed August 6, 2010.
12. CDC. Licensure of a high-dose inactivated influenza vaccine for persons aged ≥65 years (Fluzone High-Dose) and guidance for use—United States, 2010. MMWR Morb Mortal Wkly Rep. 2010;59:485-486.
Influenza pandemics like the one we had last year are uncommon, and mounting an effective response was a difficult challenge. The pandemic hit early and hard. Physicians and the public health system responded well, administering a seasonal flu vaccine as well as a new H1N1 vaccine that was approved, produced, and distributed in record time. Before the end of the season, approximately 30% of the population had received an H1N1 vaccine and 40% a seasonal vaccine.1
What happened last year
The influenza attack rate in 2009-2010 exceeded that of a normal influenza season and the age groups most affected were also different, with those over the age of 65 largely spared.2 Virtually all the influenza last year was caused by the pandemic H1N1 strain.2 Fortuitously, the virus was not especially virulent and the death rates were below what was initially expected. TABLE 1 lists the population death rates that occurred for different age groups.2 Most of the more than 2000 deaths were among those with high-risk conditions.3 Those conditions are listed in TABLE 2.
There were, however, 269 deaths by late March among children, which far exceeded the number of deaths in this age group for the previous 3 influenza seasons.2 For the most part, these higher mortality rates were due to higher attack rates, rather than higher case fatality rates. This is evident from hospitalization rates for children younger than age 5, which exceeded those of other age groups, as shown in FIGURE 1.
TABLE 1
2009-2010 Influenza death rates by age
| Age group, years | Death rate/100,000 |
|---|---|
| 0-4 | 0.43 |
| 5-18 | 0.36 |
| 19-24 | 0.54 |
| 25-49 | 0.87 |
| 50-64 | 1.56 |
| ≥65 | 0.95 |
| Source: CDC. MMWR Morb Mortal Wkly Rep. 2010.2 | |
TABLE 2
Individuals at higher risk for influenza complications (or who may spread infection to those at higher risk)
|
| Source: CDC. MMWR Morb Mortal Wkly Rep. 2010.4 |
FIGURE 1
Cumulative lab-confirmed hospitalization rate by age group, 2009 H1N1, April 2009-February 13, 2010*
*Based on 35 states reporting (n=49,516).
Source: Finelli L, et al. Available at http://www.cdc.gov/vaccines/recs/acip/downloads/mtg-slides-feb10/05-2-flu-vac.pdf. 2010.3
The task will be simpler this year
While it’s not possible to predict what will happen in the upcoming season, 2 developments should simplify the family physician’s task of adhering to official recommendations:
- Only 1 vaccine formulation will be available, and
- For the first time, the recommendation is to vaccinate everyone who does not have a contraindication.4
The vaccine for the 2010-2011 season will contain 3 antigens: the pandemic H1N1 virus, an H3N2 A strain (A/Perth/16/2009), and a B virus (B/Brisbane/60/2008).2 The decision on which antigens to include is made 6 months in advance of the start of the next flu season and is based on information about the most common influenza antigens circulating worldwide at that time.
Immunization for all
This year’s recommendation to immunize everyone who does not have a contraindication is a major change from the age- and risk-based recommendations of past years. The universal recommendation is the culmination of the incremental expansions of recommendation categories that occurred over the past decade, which resulted in suboptimal immunization rates.1 In 2009, only 40% to 50% of adults for whom the seasonal vaccine was recommended received it.5 While the annual influenza vaccine recommendation is now universal, those who should be specially targeted include those in TABLE 2. Most public health authorities believe children should also receive special emphasis because of the high transmission rate among school-age children and their home contacts. Next, of course, come health care workers, who should be vaccinated to protect ourselves, our families, and our patients.4,6
Antivirals for treatment and prevention
There are 2 uses for antivirals to combat influenza: treatment of those infected and chemoprevention for those exposed to someone infected. Treatment is recommended for those with confirmed or suspected influenza who have severe, complicated, or progressive illness or who are hospitalized.7 Treatment should be strongly considered for anyone at higher risk for complications and death from influenza.7
Chemoprevention is now being deemphasized because of a concern for possible development of antiviral resistance. It should be considered for those in the high-risk categories (TABLE 2) with a documented exposure.7
Which antiviral to use will depend on which influenza strains are circulating and their resistance patterns. So far, H1N1 has remained largely sensitive to both neuraminidase inhibitors: oseltamivir and zanamivir. However, oseltamivir resistance has been documented in a few cases and will be monitored carefully.
Family physicians will need to stay informed by state and local health departments about circulating strains and resistance patterns. The latest Centers for Disease Control and Prevention (CDC) guidance on antiviral therapy can be consulted for dosage and other details on the 4 antiviral drugs licensed in the United States.7
What you must know about vaccine safety
Because of increasing public awareness of safety issues, family physicians will frequently need to address patients’ questions about the safety of this year’s vaccine. Last year, multiple reporting systems including the Vaccine Adverse Event Reporting System (VAERS), Vaccine Safety Datalink (VSD) Project, the Defense Medical Surveillance System (DMSS), and others, extensively monitored adverse events that could potentially be linked to the H1N1 vaccine.8 Three so-called weak signals—indications of a possible link to a rare, but statistically significant adverse event—were received.
The 3 signals were for Guillain-Barré syndrome (GBS), Bell’s palsy, and thrombocytopenia/idiopathic thrombocytopenic purpura. The status of the investigation of each potential link to the vaccine can be found on the National Vaccine Advisory Committee (NVAC) safety Web site at http://www.hhs.gov/nvpo/nvac/reports/index.html.
The GBS signal has been investigated the most aggressively because this adverse reaction has been linked to the so-called swine flu vaccine of 1976. One analysis has been published in the Morbidity and Mortality Weekly Report.9 Whether GBS has a causal link to the H1N1 vaccine remains in doubt. In the worst-case scenario, if causation is determined, it appears that the vaccine would account for no more than 1 excess case of GBS per million doses.9
In Western Australia, there has been a recent report of an excess of fever and febrile seizures in children 6 months to 5 years of age, and fever in children 5 to 9 years of age who received seasonal influenza vaccine. The rate of febrile seizures in children younger than age 3 was 7 per 1000, which is 7 times the rate normally expected. These adverse reactions were associated with only 1 vaccine product, Fluvax, and Fluvax Junior, manufactured by CSL Biotherapies.10 The CSL product is marketed in the United States by Merck & Co. under the brand name Afluria.
The Advisory Committee on Immunization Practices (ACIP) has issued the following recommendations:11
- Afluria should not be used in children ages 6 months through 8 years. The exception: children who are ages 5 through 8 years who are considered to be at high risk for influenza complications and for whom no other trivalent inactivated vaccine is available.
- Other age-appropriate, licensed seasonal influenza vaccine formulations should be used for prevention of influenza in children ages 6 months through 8 years.
High-dose vaccine for elderly patients
A new seasonal influenza vaccine (Fluzone High-Dose, manufactured by Sanofi Pasteur) is now available for use in people who are 65 years of age and older.12 Fluzone High-Dose contains 4 times the amount of influenza antigen as other inactivated seasonal influenza vaccines. Fluzone High-Dose vaccine produces higher antibody levels in the elderly but also a higher frequency of local reactions. Studies are being conducted to see if the vaccine results in better patient outcomes. ACIP does not state a preference for any of the available influenza vaccines for those who are 65 years of age and older.12
Children younger than age 9: One dose or two?
The new recommendations for deciding if a child under the age of 9 years should receive 1 or 2 doses of the vaccine run counter to the trend for simplification in influenza vaccine recommendations. The decision depends on the child’s past immunization history for both seasonal and H1N1 vaccines. To be fully vaccinated with only 1 dose this year, a child must have previously received at least 1 dose of H1N1 vaccine and 2 doses of seasonal vaccine. FIGURE 2 illustrates the process you need to go through to make the dosage choice. When the child’s immunization history is unknown or uncertain, give 2 doses, separated by 4 weeks.4
FIGURE 2
Children younger than 9: Ask 4 questions
Source: CDC. MMWR Morb Mortal Wkly Rep. 2010.4
Influenza pandemics like the one we had last year are uncommon, and mounting an effective response was a difficult challenge. The pandemic hit early and hard. Physicians and the public health system responded well, administering a seasonal flu vaccine as well as a new H1N1 vaccine that was approved, produced, and distributed in record time. Before the end of the season, approximately 30% of the population had received an H1N1 vaccine and 40% a seasonal vaccine.1
What happened last year
The influenza attack rate in 2009-2010 exceeded that of a normal influenza season and the age groups most affected were also different, with those over the age of 65 largely spared.2 Virtually all the influenza last year was caused by the pandemic H1N1 strain.2 Fortuitously, the virus was not especially virulent and the death rates were below what was initially expected. TABLE 1 lists the population death rates that occurred for different age groups.2 Most of the more than 2000 deaths were among those with high-risk conditions.3 Those conditions are listed in TABLE 2.
There were, however, 269 deaths by late March among children, which far exceeded the number of deaths in this age group for the previous 3 influenza seasons.2 For the most part, these higher mortality rates were due to higher attack rates, rather than higher case fatality rates. This is evident from hospitalization rates for children younger than age 5, which exceeded those of other age groups, as shown in FIGURE 1.
TABLE 1
2009-2010 Influenza death rates by age
| Age group, years | Death rate/100,000 |
|---|---|
| 0-4 | 0.43 |
| 5-18 | 0.36 |
| 19-24 | 0.54 |
| 25-49 | 0.87 |
| 50-64 | 1.56 |
| ≥65 | 0.95 |
| Source: CDC. MMWR Morb Mortal Wkly Rep. 2010.2 | |
TABLE 2
Individuals at higher risk for influenza complications (or who may spread infection to those at higher risk)
|
| Source: CDC. MMWR Morb Mortal Wkly Rep. 2010.4 |
FIGURE 1
Cumulative lab-confirmed hospitalization rate by age group, 2009 H1N1, April 2009-February 13, 2010*
*Based on 35 states reporting (n=49,516).
Source: Finelli L, et al. Available at http://www.cdc.gov/vaccines/recs/acip/downloads/mtg-slides-feb10/05-2-flu-vac.pdf. 2010.3
The task will be simpler this year
While it’s not possible to predict what will happen in the upcoming season, 2 developments should simplify the family physician’s task of adhering to official recommendations:
- Only 1 vaccine formulation will be available, and
- For the first time, the recommendation is to vaccinate everyone who does not have a contraindication.4
The vaccine for the 2010-2011 season will contain 3 antigens: the pandemic H1N1 virus, an H3N2 A strain (A/Perth/16/2009), and a B virus (B/Brisbane/60/2008).2 The decision on which antigens to include is made 6 months in advance of the start of the next flu season and is based on information about the most common influenza antigens circulating worldwide at that time.
Immunization for all
This year’s recommendation to immunize everyone who does not have a contraindication is a major change from the age- and risk-based recommendations of past years. The universal recommendation is the culmination of the incremental expansions of recommendation categories that occurred over the past decade, which resulted in suboptimal immunization rates.1 In 2009, only 40% to 50% of adults for whom the seasonal vaccine was recommended received it.5 While the annual influenza vaccine recommendation is now universal, those who should be specially targeted include those in TABLE 2. Most public health authorities believe children should also receive special emphasis because of the high transmission rate among school-age children and their home contacts. Next, of course, come health care workers, who should be vaccinated to protect ourselves, our families, and our patients.4,6
Antivirals for treatment and prevention
There are 2 uses for antivirals to combat influenza: treatment of those infected and chemoprevention for those exposed to someone infected. Treatment is recommended for those with confirmed or suspected influenza who have severe, complicated, or progressive illness or who are hospitalized.7 Treatment should be strongly considered for anyone at higher risk for complications and death from influenza.7
Chemoprevention is now being deemphasized because of a concern for possible development of antiviral resistance. It should be considered for those in the high-risk categories (TABLE 2) with a documented exposure.7
Which antiviral to use will depend on which influenza strains are circulating and their resistance patterns. So far, H1N1 has remained largely sensitive to both neuraminidase inhibitors: oseltamivir and zanamivir. However, oseltamivir resistance has been documented in a few cases and will be monitored carefully.
Family physicians will need to stay informed by state and local health departments about circulating strains and resistance patterns. The latest Centers for Disease Control and Prevention (CDC) guidance on antiviral therapy can be consulted for dosage and other details on the 4 antiviral drugs licensed in the United States.7
What you must know about vaccine safety
Because of increasing public awareness of safety issues, family physicians will frequently need to address patients’ questions about the safety of this year’s vaccine. Last year, multiple reporting systems including the Vaccine Adverse Event Reporting System (VAERS), Vaccine Safety Datalink (VSD) Project, the Defense Medical Surveillance System (DMSS), and others, extensively monitored adverse events that could potentially be linked to the H1N1 vaccine.8 Three so-called weak signals—indications of a possible link to a rare, but statistically significant adverse event—were received.
The 3 signals were for Guillain-Barré syndrome (GBS), Bell’s palsy, and thrombocytopenia/idiopathic thrombocytopenic purpura. The status of the investigation of each potential link to the vaccine can be found on the National Vaccine Advisory Committee (NVAC) safety Web site at http://www.hhs.gov/nvpo/nvac/reports/index.html.
The GBS signal has been investigated the most aggressively because this adverse reaction has been linked to the so-called swine flu vaccine of 1976. One analysis has been published in the Morbidity and Mortality Weekly Report.9 Whether GBS has a causal link to the H1N1 vaccine remains in doubt. In the worst-case scenario, if causation is determined, it appears that the vaccine would account for no more than 1 excess case of GBS per million doses.9
In Western Australia, there has been a recent report of an excess of fever and febrile seizures in children 6 months to 5 years of age, and fever in children 5 to 9 years of age who received seasonal influenza vaccine. The rate of febrile seizures in children younger than age 3 was 7 per 1000, which is 7 times the rate normally expected. These adverse reactions were associated with only 1 vaccine product, Fluvax, and Fluvax Junior, manufactured by CSL Biotherapies.10 The CSL product is marketed in the United States by Merck & Co. under the brand name Afluria.
The Advisory Committee on Immunization Practices (ACIP) has issued the following recommendations:11
- Afluria should not be used in children ages 6 months through 8 years. The exception: children who are ages 5 through 8 years who are considered to be at high risk for influenza complications and for whom no other trivalent inactivated vaccine is available.
- Other age-appropriate, licensed seasonal influenza vaccine formulations should be used for prevention of influenza in children ages 6 months through 8 years.
High-dose vaccine for elderly patients
A new seasonal influenza vaccine (Fluzone High-Dose, manufactured by Sanofi Pasteur) is now available for use in people who are 65 years of age and older.12 Fluzone High-Dose contains 4 times the amount of influenza antigen as other inactivated seasonal influenza vaccines. Fluzone High-Dose vaccine produces higher antibody levels in the elderly but also a higher frequency of local reactions. Studies are being conducted to see if the vaccine results in better patient outcomes. ACIP does not state a preference for any of the available influenza vaccines for those who are 65 years of age and older.12
Children younger than age 9: One dose or two?
The new recommendations for deciding if a child under the age of 9 years should receive 1 or 2 doses of the vaccine run counter to the trend for simplification in influenza vaccine recommendations. The decision depends on the child’s past immunization history for both seasonal and H1N1 vaccines. To be fully vaccinated with only 1 dose this year, a child must have previously received at least 1 dose of H1N1 vaccine and 2 doses of seasonal vaccine. FIGURE 2 illustrates the process you need to go through to make the dosage choice. When the child’s immunization history is unknown or uncertain, give 2 doses, separated by 4 weeks.4
FIGURE 2
Children younger than 9: Ask 4 questions
Source: CDC. MMWR Morb Mortal Wkly Rep. 2010.4
1. Singleton JA. H1N1 vaccination coverage: updated interim results February 24, 2010. ACIP presentation slides, February 2010 meeting. Available at: http://www.cdc.gov/vaccines/recs/acip/downloads/mtg-slides-feb10/05-4-flu-vac.pdf. Accessed July 16, 2010.
2. CDC. Update: influenza activity—United States, August 30, 2009-March 27, 2010, and composition of the 2010-11 influenza vaccine. MMWR Morb Mortal Wkly Rep. 2010;59:423-438.
3. Finelli L, Brammer L, Kniss K, et al. Influenza epidemiology and surveillance. ACIP Presentation slides, February 2010 meeting. Available at: http://www.cdc.gov/vaccines/recs/acip/downloads/mtg-slides-feb10/05-2-flu-vac.pdf. Accessed July 26, 2010.
4. CDC. Prevention and control of influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2010. MMWR Morb Mortal Wkly Rep. July 29, 2010 (early release);1-62.
5. Harris KM, Maurer J, Uscher-Pines L. Seasonal influenza vaccine use by adults in the US: a snapshot as of mid-November 2009. Available at: http://www.rand.org/pubs/occasional_papers/OP289/. Accessed July 16, 2010.
6. Fiore A. Influenza vaccine workgroup discussions and recommendations, November 2009-February 2010. ACIP presentation slides, February 2010 meeting. Available at: http://www.cdc.gov/vaccines/recs/acip/downloads/mtg-slides-feb10/05-7-flu-vac.pdf. Accessed July 26, 2010.
7. CDC. Updated interim recommendations for the use of antiviral medications in the treatment and prevention of influenza for the 2009-2010 season. Available at: http://www.cdc.gov/H1N1flu/recommendations.htm. Accessed July 16, 2010.
8. National Vaccine Advisory Committee Report on 2009 H1N1 Vaccine Safety Risk Assessment. June 2010. Available at: http://www.hhs.gov/nvpo/nvac/reports/vsrawg_repot_may2010.html. Accessed July 16, 2010.
9. CDC. Preliminary results: surveillance for Guillain-Barré syndrome after receipt of influenza A (H1N1) 2009 monovalent vaccine—United States, 2009–2010. MMWR Morb Mortal Wkly Rep. 2010;59:657-661.
10. McNeil M. Febrile seizures in Australia and CDC monitoring plan for 2010-2011 seasonal influenza vaccine. Available at: www.cdc.gov/vaccines/recs/acip/downloads/mtg-slides-jun10/10-8-flu.pdf. Accessed August 19, 2010.
11. CDC. Media statement: ACIP recommendation for use of CSL influenza vaccine. August 6, 2010. Available at: http://www.cdc.gov/media/pressrel/2010/s100806.htm?s_cid=mediarel_s100806. Accessed August 6, 2010.
12. CDC. Licensure of a high-dose inactivated influenza vaccine for persons aged ≥65 years (Fluzone High-Dose) and guidance for use—United States, 2010. MMWR Morb Mortal Wkly Rep. 2010;59:485-486.
1. Singleton JA. H1N1 vaccination coverage: updated interim results February 24, 2010. ACIP presentation slides, February 2010 meeting. Available at: http://www.cdc.gov/vaccines/recs/acip/downloads/mtg-slides-feb10/05-4-flu-vac.pdf. Accessed July 16, 2010.
2. CDC. Update: influenza activity—United States, August 30, 2009-March 27, 2010, and composition of the 2010-11 influenza vaccine. MMWR Morb Mortal Wkly Rep. 2010;59:423-438.
3. Finelli L, Brammer L, Kniss K, et al. Influenza epidemiology and surveillance. ACIP Presentation slides, February 2010 meeting. Available at: http://www.cdc.gov/vaccines/recs/acip/downloads/mtg-slides-feb10/05-2-flu-vac.pdf. Accessed July 26, 2010.
4. CDC. Prevention and control of influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2010. MMWR Morb Mortal Wkly Rep. July 29, 2010 (early release);1-62.
5. Harris KM, Maurer J, Uscher-Pines L. Seasonal influenza vaccine use by adults in the US: a snapshot as of mid-November 2009. Available at: http://www.rand.org/pubs/occasional_papers/OP289/. Accessed July 16, 2010.
6. Fiore A. Influenza vaccine workgroup discussions and recommendations, November 2009-February 2010. ACIP presentation slides, February 2010 meeting. Available at: http://www.cdc.gov/vaccines/recs/acip/downloads/mtg-slides-feb10/05-7-flu-vac.pdf. Accessed July 26, 2010.
7. CDC. Updated interim recommendations for the use of antiviral medications in the treatment and prevention of influenza for the 2009-2010 season. Available at: http://www.cdc.gov/H1N1flu/recommendations.htm. Accessed July 16, 2010.
8. National Vaccine Advisory Committee Report on 2009 H1N1 Vaccine Safety Risk Assessment. June 2010. Available at: http://www.hhs.gov/nvpo/nvac/reports/vsrawg_repot_may2010.html. Accessed July 16, 2010.
9. CDC. Preliminary results: surveillance for Guillain-Barré syndrome after receipt of influenza A (H1N1) 2009 monovalent vaccine—United States, 2009–2010. MMWR Morb Mortal Wkly Rep. 2010;59:657-661.
10. McNeil M. Febrile seizures in Australia and CDC monitoring plan for 2010-2011 seasonal influenza vaccine. Available at: www.cdc.gov/vaccines/recs/acip/downloads/mtg-slides-jun10/10-8-flu.pdf. Accessed August 19, 2010.
11. CDC. Media statement: ACIP recommendation for use of CSL influenza vaccine. August 6, 2010. Available at: http://www.cdc.gov/media/pressrel/2010/s100806.htm?s_cid=mediarel_s100806. Accessed August 6, 2010.
12. CDC. Licensure of a high-dose inactivated influenza vaccine for persons aged ≥65 years (Fluzone High-Dose) and guidance for use—United States, 2010. MMWR Morb Mortal Wkly Rep. 2010;59:485-486.
Your guide to the new pneumococcal vaccine for children
A new, 13-valent pneumococcal conjugate vaccine (PCV13, Prevnar 13), from Wyeth Pharmaceuticals was licensed by the US Food and Drug Administration (FDA) in February for use in all children ages 6 weeks to 59 months. The new vaccine was licensed for the prevention of invasive pneumococcal disease (pneumonia, meningitis, and bacteremia) and otitis media.1 PCV13 is meant to replace the 7-valent PCV7 (Prevnar), and will offer protection against a wider array of pneumococcal serotypes.1
Invasive pneumococcal disease in kids has diminished substantially
Soon after PCV7 was included in the routine child immunization schedule, the incidence of invasive pneumococcal disease (IPD) began to decline.2-5 In 1 study, the annual rate of IPD among children younger than 5 years of age decreased from 98.7 cases/100,000 in 1998–1999 to 22.6 cases/100,000 in 2006-2007.3 This decline was due to a decrease in the rate of disease caused by the 7 vaccine serotypes, from 81.9 cases/100,000 to 0.4 cases/100,000.
However, during that same time period, the rate of IPD caused by nonvaccine serotypes increased from 16.8 cases/100,000 population to 22.1 cases/100,000.3 The percentage of IPD caused by nonvaccine serotypes rose from 20% to 90% among children younger than 5 years of age during that time period.3
Fewer cases in adults, as well
In addition to the decline of IPD in children, there has also been a decline in adults. In those older than age 65, the rate of IPD decreased from 60.1/100,000 to 38.2/100,000 between 1998 and 2007—most likely because routine use of the PCV7 vaccine in children has resulted in decreased carriage and transmission of infection from children to adults.3 As in children, the decline was due to a decreasing incidence of infection from PCV7 vaccine serotypes, from 33.7 cases/100,000 to 3.3 cases/100,000. At the same time, the rate of disease caused by nonvaccine serotypes increased from 26.4 cases/100,000 to 34.9 cases/100,000.3
Nonvaccine serotypes still cause concern
While the overall decline in IPD has been a public health success, the increase in incidence of disease caused by nonvaccine serotypes has been cause for concern. According to an analysis of 2007 data from the Centers for Disease Control and Prevention (CDC)’s Active Bacterial Core surveillance, 64% of IPD cases in children younger than 5 years of age in 2006-2007 were caused by serotypes 1, 3, 5, 6A, 7F, and 19A.6 Several of these replacement serotypes have high levels of resistance to penicillin and erythromycin. This trend is what led to the development of the PCV13, which adds these 6 to the 7 serotypes covered by Prevnar.
The dosing schedule is complicated
The recommended schedule for the older PCV7 vaccine has always been a challenge, because the number of doses depends on the age of the child when first vaccinated.7,8 The introduction of PCV13 adds to the complexity, because many children will be in the midst of a PCV7 series when they make the transition to PCV13.
The Advisory Committee on Immunization Practices (ACIP) recommendations on how many doses of PCV13 a child should receive depend now on the age at which the first PCV vaccine was received (either PCV7 or PCV13), the number of doses of each received, and the presence or absence of high-risk medical conditions. These recommendations are summarized below and illustrated in TABLE 1 and TABLE 2.
TABLE 1
PCV13: Routine vaccination schedule
| Age at first dose | Primary series* | Booster dose† |
|---|---|---|
| 2-6 months | 3 doses | 1 dose, 12-15 months |
| 7-11 months | 2 doses | 1 dose, 12-15 months |
| 12-23 months | 2 doses | None |
| 24-59 months, healthy children | 1 dose | None |
| 24-71 months for children with certain chronic diseases or immunocompromising conditions (see TABLE 3) | 2 doses | None |
| *Minimum interval between doses is 8 weeks, except for children vaccinated at <12 months for whom the minimum interval is 4 weeks. Minimum age for first dose is 6 weeks. | ||
| †Given at least 8 weeks after previous dose. | ||
| Source: CDC. MMWR Morb Mortal Wkly Rep. 2010.1 | ||
TABLE 2
In transition: From PCV7 to PCV13
| Infant series | Booster dose | Supplemental PCV13 dose | ||
|---|---|---|---|---|
| 2 months | 4 months | 6 months | ≥12 months* | 14-59 months† |
| PCV7 | PCV13 | PCV13 | PCV13 | None |
| PCV7 | PCV7 | PCV13 | PCV13 | None |
| PCV7 | PCV7 | PCV7 | PCV13 | None |
| PCV7 | PCV7 | PCV7 | PCV7 | PCV13 |
| *No additional PCV13 doses are indicated for children ages 12-23 months who have received 2 or 3 doses of PCV before age 12 months and at least 1 dose of PCV13 at ≥12 months. | ||||
| †For children with underlying medical conditions (see TABLE 3), a single supplemental PCV13 dose is recommended through age 71 months. | ||||
| Source: CDC. MMWR Morb Mortal Wkly Rep. 2010.1 | ||||
For a child who started PCV7 on time and is in mid series, the recommendation is to simply finish the series with PCV13.
If a child has completed a series of PCV7, the recommendation is to give him or her 1 dose of PCV13 up to age 59 months. (If the child has a chronic underlying medical condition, this age is extended to 71 months.1)
Infants between the ages of 1 and 6 months who have never received any PCV product should complete a series of PCV13 at 2, 4, 6, and 12 to 15 months—the same time line as the PCV7 series.
Children ages 7 to 59 months who have not been vaccinated with PCV7 or PCV13 previously should receive 1 to 3 doses of PCV13, depending on their age at the time when vaccination begins and whether underlying medical conditions are present (TABLE 3).
Healthy children ages 24 to 59 months without previous PCV vaccine should receive 1 dose of PCV13.
Children ages 24 to 71 months without previous PCV vaccine who have a chronic medical condition that increases their risk for pneumococcal disease should receive 2 doses of PCV13, 8 weeks apart.1
TABLE 3
Underlying conditions that place kids at risk for pneumococcal disease
| Risk group | Condition |
|---|---|
| Immunocompetent children | Chronic heart disease* |
| Chronic lung disease† | |
| Diabetes mellitus | |
| Cerebrospinal fluid leaks | |
| Cochlear implant | |
| Children with functional or anatomic asplenia | Sickle cell disease and other hemoglobulinopathies |
| Congenital or acquired asplenia or splenic dysfunction | |
| Children with immunocompromising conditions | HIV infection |
| Chronic renal failure and nephrotic syndrome | |
| Diseases associated with immunosuppressive drugs or radiation therapy, including malignant neoplasms, leukemias, lymphomas, and Hodgkin’s disease; or solid organ transplantation | |
| Congenital immunodeficiency‡ | |
| *Particularly cyanotic congenital heart disease and cardiac failure. | |
| †Including asthma if treated with prolonged high-dose oral corticosteroids. | |
| ‡Includes B- (humoral) or T-lymphocyte deficiency; complement deficiencies, particularly C1, C2, C3, and C4 deficiency; and phagocytic disorders (excluding chronic granulomatous disease). | |
| Source: CDC. MMWR Morb Mortal Wkly Rep. 2010.1 | |
Recommendations for children at higher risk
Provisional recommendations from ACIP advise that children 2 through 18 years of age at increased risk for invasive pneumococcal disease should also receive 23-valent pneumococcal polysaccharide vaccine (PPSV23). Ideally, the child should have received all of the recommended doses of PCV13 before the physician administers PPSV23, with a minimum interval of at least 8 weeks after the last dose of PCV13.
However, some children will have previously received PPSV23. They should also receive the recommended PCV13 doses. A second dose of PPSV23 is recommended 5 years after the first dose of PPSV23 for children who have sickle cell disease, or functional or anatomic asplenia, human immunodeficiency virus (HIV) infection, or other immunocompromising conditions. No more than 2 PPSV23 doses are recommended.9
The ACIP provisional recommendations also say that a single dose of PCV13 may be administered to children ages 6 to 18 years who are at increased risk for IPD because of sickle cell disease, HIV infection or other immunocompromising condition, cochlear implant, or cerebrospinal fluid leaks, regardless of whether they have previously received PCV7 or PPSV23.9 This, however, is an off-label recommendation.
The usual contraindications
PCV13 is contraindicated among individuals known to have a severe allergic reaction to any component of PCV13 or PCV7 or to any diphtheria toxoid-containing vaccine, because the pneumococcal antigens are conjugated to a diphtheria carrier protein.1
A useful vaccine, with its share of challenges
The pneumococcal conjugate vaccine combats infections such as pneumococcal pneumonia and meningitis, which are potentially serious—even though their incidence is relatively low.
The vaccine’s high private-sector cost—reported by the manufacturer to the CDC as $435 for the full, 4-dose series of PCV13—can be a drawback for the family physician trying to keep a full array of vaccine products on hand.10 Eligible low-income and uninsured children can receive free vaccine under the federal Vaccines for Children Program, and providers who choose to enroll in the program can access free vaccines and may charge for the expense of administering them.11
With this hurdle overcome, the remaining challenge for physicians will be to stay on top of the complicated dosing schedule.
1. CDC. Licensure of a 13-valent pneumococcal conjugate vaccine (PCV13) and recommendations for use among children—Advisory Committee on Immunization Practices (ACIP), 2010. MMWR Morb Mortal Wkly Rep. 2010;59:258-261.
2. Hicks LA, Harrison LH, Flannery B, et al. Incidence of pneumococcal disease due to nonpneumococcal conjugate vaccine (PCV7) serotypes in the United States during the era of widespread PCV7 vaccination, 1998-2004. J Infect Dis. 2007;196:1346-1354.
3. Pilishvili T, Lexau C, Farley MM, et al. Sustained reductions in invasive pneumococcal disease in the era of conjugate vaccine. J Infect Dis. 2010;201:32-41.
4. Whitney CG, Farley MM, Hadler J, et al. Decline in invasive pneumococcal disease after the introduction of protein-polysaccharide conjugate vaccine. N Engl J Med. 2003;348:1737-1746.
5. Direct and indirect effects of routine vaccination of children with 7-valent pneumococcal conjugate vaccine on incidence of invasive pneumococcal disease—United States, 1998-2003. MMWR Morb Mortal Wkly Rep. 2005;54:893-897.
6. CDC. Invasive pneumococcal disease in young children before licensure of 13-valent pneumococcal conjugate vaccine—United States, 2007. MMWR Morb Mortal Wkly Rep. 2010;59:253-257.
7. CDC. Preventing pneumococcal disease among infants and young children: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2000;49(RR-9):1-35.
8. CDC. Updated recommendation from the Advisory Committee on Immunization Practices (ACIP) for use of 7-valent pneumococcal conjugate vaccine (PCV7) in children aged 24-59 months who are not completely vaccinated. MMWR Morb Mortal Wkly Rep. 2008;57:343-344.
9. ACIP provisional recommendations for use of 13-valent pneumococcal conjugate vaccine (PCV13) among infants and children. March 3, 2010. Available at: www.cdc.gov/vaccines/recs/provisional/downloads/pcv13-mar-2010-508.pdf. Accessed May 24, 2010.
10. CDC vaccine price list. Available at: www.cdc.gov/vaccines/programs/vfc/cdc-vac-price-list.htm. Accessed May 22, 2010.
11. Vaccines for Children Program. FAQs from providers. Available at www.cdc.gov/vaccines/programs/vfc/providers/faq-hcp. htm. Accessed May 22, 2010.
A new, 13-valent pneumococcal conjugate vaccine (PCV13, Prevnar 13), from Wyeth Pharmaceuticals was licensed by the US Food and Drug Administration (FDA) in February for use in all children ages 6 weeks to 59 months. The new vaccine was licensed for the prevention of invasive pneumococcal disease (pneumonia, meningitis, and bacteremia) and otitis media.1 PCV13 is meant to replace the 7-valent PCV7 (Prevnar), and will offer protection against a wider array of pneumococcal serotypes.1
Invasive pneumococcal disease in kids has diminished substantially
Soon after PCV7 was included in the routine child immunization schedule, the incidence of invasive pneumococcal disease (IPD) began to decline.2-5 In 1 study, the annual rate of IPD among children younger than 5 years of age decreased from 98.7 cases/100,000 in 1998–1999 to 22.6 cases/100,000 in 2006-2007.3 This decline was due to a decrease in the rate of disease caused by the 7 vaccine serotypes, from 81.9 cases/100,000 to 0.4 cases/100,000.
However, during that same time period, the rate of IPD caused by nonvaccine serotypes increased from 16.8 cases/100,000 population to 22.1 cases/100,000.3 The percentage of IPD caused by nonvaccine serotypes rose from 20% to 90% among children younger than 5 years of age during that time period.3
Fewer cases in adults, as well
In addition to the decline of IPD in children, there has also been a decline in adults. In those older than age 65, the rate of IPD decreased from 60.1/100,000 to 38.2/100,000 between 1998 and 2007—most likely because routine use of the PCV7 vaccine in children has resulted in decreased carriage and transmission of infection from children to adults.3 As in children, the decline was due to a decreasing incidence of infection from PCV7 vaccine serotypes, from 33.7 cases/100,000 to 3.3 cases/100,000. At the same time, the rate of disease caused by nonvaccine serotypes increased from 26.4 cases/100,000 to 34.9 cases/100,000.3
Nonvaccine serotypes still cause concern
While the overall decline in IPD has been a public health success, the increase in incidence of disease caused by nonvaccine serotypes has been cause for concern. According to an analysis of 2007 data from the Centers for Disease Control and Prevention (CDC)’s Active Bacterial Core surveillance, 64% of IPD cases in children younger than 5 years of age in 2006-2007 were caused by serotypes 1, 3, 5, 6A, 7F, and 19A.6 Several of these replacement serotypes have high levels of resistance to penicillin and erythromycin. This trend is what led to the development of the PCV13, which adds these 6 to the 7 serotypes covered by Prevnar.
The dosing schedule is complicated
The recommended schedule for the older PCV7 vaccine has always been a challenge, because the number of doses depends on the age of the child when first vaccinated.7,8 The introduction of PCV13 adds to the complexity, because many children will be in the midst of a PCV7 series when they make the transition to PCV13.
The Advisory Committee on Immunization Practices (ACIP) recommendations on how many doses of PCV13 a child should receive depend now on the age at which the first PCV vaccine was received (either PCV7 or PCV13), the number of doses of each received, and the presence or absence of high-risk medical conditions. These recommendations are summarized below and illustrated in TABLE 1 and TABLE 2.
TABLE 1
PCV13: Routine vaccination schedule
| Age at first dose | Primary series* | Booster dose† |
|---|---|---|
| 2-6 months | 3 doses | 1 dose, 12-15 months |
| 7-11 months | 2 doses | 1 dose, 12-15 months |
| 12-23 months | 2 doses | None |
| 24-59 months, healthy children | 1 dose | None |
| 24-71 months for children with certain chronic diseases or immunocompromising conditions (see TABLE 3) | 2 doses | None |
| *Minimum interval between doses is 8 weeks, except for children vaccinated at <12 months for whom the minimum interval is 4 weeks. Minimum age for first dose is 6 weeks. | ||
| †Given at least 8 weeks after previous dose. | ||
| Source: CDC. MMWR Morb Mortal Wkly Rep. 2010.1 | ||
TABLE 2
In transition: From PCV7 to PCV13
| Infant series | Booster dose | Supplemental PCV13 dose | ||
|---|---|---|---|---|
| 2 months | 4 months | 6 months | ≥12 months* | 14-59 months† |
| PCV7 | PCV13 | PCV13 | PCV13 | None |
| PCV7 | PCV7 | PCV13 | PCV13 | None |
| PCV7 | PCV7 | PCV7 | PCV13 | None |
| PCV7 | PCV7 | PCV7 | PCV7 | PCV13 |
| *No additional PCV13 doses are indicated for children ages 12-23 months who have received 2 or 3 doses of PCV before age 12 months and at least 1 dose of PCV13 at ≥12 months. | ||||
| †For children with underlying medical conditions (see TABLE 3), a single supplemental PCV13 dose is recommended through age 71 months. | ||||
| Source: CDC. MMWR Morb Mortal Wkly Rep. 2010.1 | ||||
For a child who started PCV7 on time and is in mid series, the recommendation is to simply finish the series with PCV13.
If a child has completed a series of PCV7, the recommendation is to give him or her 1 dose of PCV13 up to age 59 months. (If the child has a chronic underlying medical condition, this age is extended to 71 months.1)
Infants between the ages of 1 and 6 months who have never received any PCV product should complete a series of PCV13 at 2, 4, 6, and 12 to 15 months—the same time line as the PCV7 series.
Children ages 7 to 59 months who have not been vaccinated with PCV7 or PCV13 previously should receive 1 to 3 doses of PCV13, depending on their age at the time when vaccination begins and whether underlying medical conditions are present (TABLE 3).
Healthy children ages 24 to 59 months without previous PCV vaccine should receive 1 dose of PCV13.
Children ages 24 to 71 months without previous PCV vaccine who have a chronic medical condition that increases their risk for pneumococcal disease should receive 2 doses of PCV13, 8 weeks apart.1
TABLE 3
Underlying conditions that place kids at risk for pneumococcal disease
| Risk group | Condition |
|---|---|
| Immunocompetent children | Chronic heart disease* |
| Chronic lung disease† | |
| Diabetes mellitus | |
| Cerebrospinal fluid leaks | |
| Cochlear implant | |
| Children with functional or anatomic asplenia | Sickle cell disease and other hemoglobulinopathies |
| Congenital or acquired asplenia or splenic dysfunction | |
| Children with immunocompromising conditions | HIV infection |
| Chronic renal failure and nephrotic syndrome | |
| Diseases associated with immunosuppressive drugs or radiation therapy, including malignant neoplasms, leukemias, lymphomas, and Hodgkin’s disease; or solid organ transplantation | |
| Congenital immunodeficiency‡ | |
| *Particularly cyanotic congenital heart disease and cardiac failure. | |
| †Including asthma if treated with prolonged high-dose oral corticosteroids. | |
| ‡Includes B- (humoral) or T-lymphocyte deficiency; complement deficiencies, particularly C1, C2, C3, and C4 deficiency; and phagocytic disorders (excluding chronic granulomatous disease). | |
| Source: CDC. MMWR Morb Mortal Wkly Rep. 2010.1 | |
Recommendations for children at higher risk
Provisional recommendations from ACIP advise that children 2 through 18 years of age at increased risk for invasive pneumococcal disease should also receive 23-valent pneumococcal polysaccharide vaccine (PPSV23). Ideally, the child should have received all of the recommended doses of PCV13 before the physician administers PPSV23, with a minimum interval of at least 8 weeks after the last dose of PCV13.
However, some children will have previously received PPSV23. They should also receive the recommended PCV13 doses. A second dose of PPSV23 is recommended 5 years after the first dose of PPSV23 for children who have sickle cell disease, or functional or anatomic asplenia, human immunodeficiency virus (HIV) infection, or other immunocompromising conditions. No more than 2 PPSV23 doses are recommended.9
The ACIP provisional recommendations also say that a single dose of PCV13 may be administered to children ages 6 to 18 years who are at increased risk for IPD because of sickle cell disease, HIV infection or other immunocompromising condition, cochlear implant, or cerebrospinal fluid leaks, regardless of whether they have previously received PCV7 or PPSV23.9 This, however, is an off-label recommendation.
The usual contraindications
PCV13 is contraindicated among individuals known to have a severe allergic reaction to any component of PCV13 or PCV7 or to any diphtheria toxoid-containing vaccine, because the pneumococcal antigens are conjugated to a diphtheria carrier protein.1
A useful vaccine, with its share of challenges
The pneumococcal conjugate vaccine combats infections such as pneumococcal pneumonia and meningitis, which are potentially serious—even though their incidence is relatively low.
The vaccine’s high private-sector cost—reported by the manufacturer to the CDC as $435 for the full, 4-dose series of PCV13—can be a drawback for the family physician trying to keep a full array of vaccine products on hand.10 Eligible low-income and uninsured children can receive free vaccine under the federal Vaccines for Children Program, and providers who choose to enroll in the program can access free vaccines and may charge for the expense of administering them.11
With this hurdle overcome, the remaining challenge for physicians will be to stay on top of the complicated dosing schedule.
A new, 13-valent pneumococcal conjugate vaccine (PCV13, Prevnar 13), from Wyeth Pharmaceuticals was licensed by the US Food and Drug Administration (FDA) in February for use in all children ages 6 weeks to 59 months. The new vaccine was licensed for the prevention of invasive pneumococcal disease (pneumonia, meningitis, and bacteremia) and otitis media.1 PCV13 is meant to replace the 7-valent PCV7 (Prevnar), and will offer protection against a wider array of pneumococcal serotypes.1
Invasive pneumococcal disease in kids has diminished substantially
Soon after PCV7 was included in the routine child immunization schedule, the incidence of invasive pneumococcal disease (IPD) began to decline.2-5 In 1 study, the annual rate of IPD among children younger than 5 years of age decreased from 98.7 cases/100,000 in 1998–1999 to 22.6 cases/100,000 in 2006-2007.3 This decline was due to a decrease in the rate of disease caused by the 7 vaccine serotypes, from 81.9 cases/100,000 to 0.4 cases/100,000.
However, during that same time period, the rate of IPD caused by nonvaccine serotypes increased from 16.8 cases/100,000 population to 22.1 cases/100,000.3 The percentage of IPD caused by nonvaccine serotypes rose from 20% to 90% among children younger than 5 years of age during that time period.3
Fewer cases in adults, as well
In addition to the decline of IPD in children, there has also been a decline in adults. In those older than age 65, the rate of IPD decreased from 60.1/100,000 to 38.2/100,000 between 1998 and 2007—most likely because routine use of the PCV7 vaccine in children has resulted in decreased carriage and transmission of infection from children to adults.3 As in children, the decline was due to a decreasing incidence of infection from PCV7 vaccine serotypes, from 33.7 cases/100,000 to 3.3 cases/100,000. At the same time, the rate of disease caused by nonvaccine serotypes increased from 26.4 cases/100,000 to 34.9 cases/100,000.3
Nonvaccine serotypes still cause concern
While the overall decline in IPD has been a public health success, the increase in incidence of disease caused by nonvaccine serotypes has been cause for concern. According to an analysis of 2007 data from the Centers for Disease Control and Prevention (CDC)’s Active Bacterial Core surveillance, 64% of IPD cases in children younger than 5 years of age in 2006-2007 were caused by serotypes 1, 3, 5, 6A, 7F, and 19A.6 Several of these replacement serotypes have high levels of resistance to penicillin and erythromycin. This trend is what led to the development of the PCV13, which adds these 6 to the 7 serotypes covered by Prevnar.
The dosing schedule is complicated
The recommended schedule for the older PCV7 vaccine has always been a challenge, because the number of doses depends on the age of the child when first vaccinated.7,8 The introduction of PCV13 adds to the complexity, because many children will be in the midst of a PCV7 series when they make the transition to PCV13.
The Advisory Committee on Immunization Practices (ACIP) recommendations on how many doses of PCV13 a child should receive depend now on the age at which the first PCV vaccine was received (either PCV7 or PCV13), the number of doses of each received, and the presence or absence of high-risk medical conditions. These recommendations are summarized below and illustrated in TABLE 1 and TABLE 2.
TABLE 1
PCV13: Routine vaccination schedule
| Age at first dose | Primary series* | Booster dose† |
|---|---|---|
| 2-6 months | 3 doses | 1 dose, 12-15 months |
| 7-11 months | 2 doses | 1 dose, 12-15 months |
| 12-23 months | 2 doses | None |
| 24-59 months, healthy children | 1 dose | None |
| 24-71 months for children with certain chronic diseases or immunocompromising conditions (see TABLE 3) | 2 doses | None |
| *Minimum interval between doses is 8 weeks, except for children vaccinated at <12 months for whom the minimum interval is 4 weeks. Minimum age for first dose is 6 weeks. | ||
| †Given at least 8 weeks after previous dose. | ||
| Source: CDC. MMWR Morb Mortal Wkly Rep. 2010.1 | ||
TABLE 2
In transition: From PCV7 to PCV13
| Infant series | Booster dose | Supplemental PCV13 dose | ||
|---|---|---|---|---|
| 2 months | 4 months | 6 months | ≥12 months* | 14-59 months† |
| PCV7 | PCV13 | PCV13 | PCV13 | None |
| PCV7 | PCV7 | PCV13 | PCV13 | None |
| PCV7 | PCV7 | PCV7 | PCV13 | None |
| PCV7 | PCV7 | PCV7 | PCV7 | PCV13 |
| *No additional PCV13 doses are indicated for children ages 12-23 months who have received 2 or 3 doses of PCV before age 12 months and at least 1 dose of PCV13 at ≥12 months. | ||||
| †For children with underlying medical conditions (see TABLE 3), a single supplemental PCV13 dose is recommended through age 71 months. | ||||
| Source: CDC. MMWR Morb Mortal Wkly Rep. 2010.1 | ||||
For a child who started PCV7 on time and is in mid series, the recommendation is to simply finish the series with PCV13.
If a child has completed a series of PCV7, the recommendation is to give him or her 1 dose of PCV13 up to age 59 months. (If the child has a chronic underlying medical condition, this age is extended to 71 months.1)
Infants between the ages of 1 and 6 months who have never received any PCV product should complete a series of PCV13 at 2, 4, 6, and 12 to 15 months—the same time line as the PCV7 series.
Children ages 7 to 59 months who have not been vaccinated with PCV7 or PCV13 previously should receive 1 to 3 doses of PCV13, depending on their age at the time when vaccination begins and whether underlying medical conditions are present (TABLE 3).
Healthy children ages 24 to 59 months without previous PCV vaccine should receive 1 dose of PCV13.
Children ages 24 to 71 months without previous PCV vaccine who have a chronic medical condition that increases their risk for pneumococcal disease should receive 2 doses of PCV13, 8 weeks apart.1
TABLE 3
Underlying conditions that place kids at risk for pneumococcal disease
| Risk group | Condition |
|---|---|
| Immunocompetent children | Chronic heart disease* |
| Chronic lung disease† | |
| Diabetes mellitus | |
| Cerebrospinal fluid leaks | |
| Cochlear implant | |
| Children with functional or anatomic asplenia | Sickle cell disease and other hemoglobulinopathies |
| Congenital or acquired asplenia or splenic dysfunction | |
| Children with immunocompromising conditions | HIV infection |
| Chronic renal failure and nephrotic syndrome | |
| Diseases associated with immunosuppressive drugs or radiation therapy, including malignant neoplasms, leukemias, lymphomas, and Hodgkin’s disease; or solid organ transplantation | |
| Congenital immunodeficiency‡ | |
| *Particularly cyanotic congenital heart disease and cardiac failure. | |
| †Including asthma if treated with prolonged high-dose oral corticosteroids. | |
| ‡Includes B- (humoral) or T-lymphocyte deficiency; complement deficiencies, particularly C1, C2, C3, and C4 deficiency; and phagocytic disorders (excluding chronic granulomatous disease). | |
| Source: CDC. MMWR Morb Mortal Wkly Rep. 2010.1 | |
Recommendations for children at higher risk
Provisional recommendations from ACIP advise that children 2 through 18 years of age at increased risk for invasive pneumococcal disease should also receive 23-valent pneumococcal polysaccharide vaccine (PPSV23). Ideally, the child should have received all of the recommended doses of PCV13 before the physician administers PPSV23, with a minimum interval of at least 8 weeks after the last dose of PCV13.
However, some children will have previously received PPSV23. They should also receive the recommended PCV13 doses. A second dose of PPSV23 is recommended 5 years after the first dose of PPSV23 for children who have sickle cell disease, or functional or anatomic asplenia, human immunodeficiency virus (HIV) infection, or other immunocompromising conditions. No more than 2 PPSV23 doses are recommended.9
The ACIP provisional recommendations also say that a single dose of PCV13 may be administered to children ages 6 to 18 years who are at increased risk for IPD because of sickle cell disease, HIV infection or other immunocompromising condition, cochlear implant, or cerebrospinal fluid leaks, regardless of whether they have previously received PCV7 or PPSV23.9 This, however, is an off-label recommendation.
The usual contraindications
PCV13 is contraindicated among individuals known to have a severe allergic reaction to any component of PCV13 or PCV7 or to any diphtheria toxoid-containing vaccine, because the pneumococcal antigens are conjugated to a diphtheria carrier protein.1
A useful vaccine, with its share of challenges
The pneumococcal conjugate vaccine combats infections such as pneumococcal pneumonia and meningitis, which are potentially serious—even though their incidence is relatively low.
The vaccine’s high private-sector cost—reported by the manufacturer to the CDC as $435 for the full, 4-dose series of PCV13—can be a drawback for the family physician trying to keep a full array of vaccine products on hand.10 Eligible low-income and uninsured children can receive free vaccine under the federal Vaccines for Children Program, and providers who choose to enroll in the program can access free vaccines and may charge for the expense of administering them.11
With this hurdle overcome, the remaining challenge for physicians will be to stay on top of the complicated dosing schedule.
1. CDC. Licensure of a 13-valent pneumococcal conjugate vaccine (PCV13) and recommendations for use among children—Advisory Committee on Immunization Practices (ACIP), 2010. MMWR Morb Mortal Wkly Rep. 2010;59:258-261.
2. Hicks LA, Harrison LH, Flannery B, et al. Incidence of pneumococcal disease due to nonpneumococcal conjugate vaccine (PCV7) serotypes in the United States during the era of widespread PCV7 vaccination, 1998-2004. J Infect Dis. 2007;196:1346-1354.
3. Pilishvili T, Lexau C, Farley MM, et al. Sustained reductions in invasive pneumococcal disease in the era of conjugate vaccine. J Infect Dis. 2010;201:32-41.
4. Whitney CG, Farley MM, Hadler J, et al. Decline in invasive pneumococcal disease after the introduction of protein-polysaccharide conjugate vaccine. N Engl J Med. 2003;348:1737-1746.
5. Direct and indirect effects of routine vaccination of children with 7-valent pneumococcal conjugate vaccine on incidence of invasive pneumococcal disease—United States, 1998-2003. MMWR Morb Mortal Wkly Rep. 2005;54:893-897.
6. CDC. Invasive pneumococcal disease in young children before licensure of 13-valent pneumococcal conjugate vaccine—United States, 2007. MMWR Morb Mortal Wkly Rep. 2010;59:253-257.
7. CDC. Preventing pneumococcal disease among infants and young children: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2000;49(RR-9):1-35.
8. CDC. Updated recommendation from the Advisory Committee on Immunization Practices (ACIP) for use of 7-valent pneumococcal conjugate vaccine (PCV7) in children aged 24-59 months who are not completely vaccinated. MMWR Morb Mortal Wkly Rep. 2008;57:343-344.
9. ACIP provisional recommendations for use of 13-valent pneumococcal conjugate vaccine (PCV13) among infants and children. March 3, 2010. Available at: www.cdc.gov/vaccines/recs/provisional/downloads/pcv13-mar-2010-508.pdf. Accessed May 24, 2010.
10. CDC vaccine price list. Available at: www.cdc.gov/vaccines/programs/vfc/cdc-vac-price-list.htm. Accessed May 22, 2010.
11. Vaccines for Children Program. FAQs from providers. Available at www.cdc.gov/vaccines/programs/vfc/providers/faq-hcp. htm. Accessed May 22, 2010.
1. CDC. Licensure of a 13-valent pneumococcal conjugate vaccine (PCV13) and recommendations for use among children—Advisory Committee on Immunization Practices (ACIP), 2010. MMWR Morb Mortal Wkly Rep. 2010;59:258-261.
2. Hicks LA, Harrison LH, Flannery B, et al. Incidence of pneumococcal disease due to nonpneumococcal conjugate vaccine (PCV7) serotypes in the United States during the era of widespread PCV7 vaccination, 1998-2004. J Infect Dis. 2007;196:1346-1354.
3. Pilishvili T, Lexau C, Farley MM, et al. Sustained reductions in invasive pneumococcal disease in the era of conjugate vaccine. J Infect Dis. 2010;201:32-41.
4. Whitney CG, Farley MM, Hadler J, et al. Decline in invasive pneumococcal disease after the introduction of protein-polysaccharide conjugate vaccine. N Engl J Med. 2003;348:1737-1746.
5. Direct and indirect effects of routine vaccination of children with 7-valent pneumococcal conjugate vaccine on incidence of invasive pneumococcal disease—United States, 1998-2003. MMWR Morb Mortal Wkly Rep. 2005;54:893-897.
6. CDC. Invasive pneumococcal disease in young children before licensure of 13-valent pneumococcal conjugate vaccine—United States, 2007. MMWR Morb Mortal Wkly Rep. 2010;59:253-257.
7. CDC. Preventing pneumococcal disease among infants and young children: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2000;49(RR-9):1-35.
8. CDC. Updated recommendation from the Advisory Committee on Immunization Practices (ACIP) for use of 7-valent pneumococcal conjugate vaccine (PCV7) in children aged 24-59 months who are not completely vaccinated. MMWR Morb Mortal Wkly Rep. 2008;57:343-344.
9. ACIP provisional recommendations for use of 13-valent pneumococcal conjugate vaccine (PCV13) among infants and children. March 3, 2010. Available at: www.cdc.gov/vaccines/recs/provisional/downloads/pcv13-mar-2010-508.pdf. Accessed May 24, 2010.
10. CDC vaccine price list. Available at: www.cdc.gov/vaccines/programs/vfc/cdc-vac-price-list.htm. Accessed May 22, 2010.
11. Vaccines for Children Program. FAQs from providers. Available at www.cdc.gov/vaccines/programs/vfc/providers/faq-hcp. htm. Accessed May 22, 2010.
USPSTF recommendations you may have missed amid the breast cancer controversy
Late in 2009, a change in the recommendations of the US Preventive Services Task Force (USPSTF) brought more public attention to this panel than it had ever experienced before. This publicity centered on revised recommendations on breast cancer screening that pointed out that mammograms benefit a few women under 50, but are also associated with some harms. The Task Force recommended that patients and physicians discuss these potential benefits and harms and make an individual decision about whether to have a mammogram.1
Even though the criticism was loud—and harsh—from some sectors, many professional organizations, including the American Academy of Family Physicians, the American College of Physicians, and the American College of Preventive Medicine, came to the defense of the Task Force and its rigorous, evidence-based methodology.2-4 Both the Journal of the American Medical Association and the Annals of Internal Medicine have since published a series of articles and opinions on the controversy, most of them favorable to the Task Force and its methods.2-9
Lost in all the brouhaha were a number of other, less controversial recommendations that the Task Force made in 2009 (and early 2010). You can find them at www.ahrq.gov/clinic/uspstfix.htm. They are categorized by strength of recommendation (TABLE 1) and listed in TABLES 2 and 3. Family physicians should review the A and B recommendations and try to incorporate those into practice. At the same time, we should avoid services in the D category, as the evidence is strong that they are not effective or cause more harm than benefit. The C and I recommendations leave more discretion for physicians and patients to decide on these interventions based on personal values and risks. A C recommendation means the service can benefit some individuals, but the totality of benefit is small. An I recommendation means that evidence is insufficient to evaluate benefits vs harms.
TABLE 1
US Preventive Services Task Force recommendation categories
| Grade | Definition |
|---|---|
| A | The USPSTF recommends the service. There is high certainty that the net benefit is substantial. |
| B | The USPSTF recommends the service. There is high certainty that the net benefit is moderate or there is moderate certainty that the net benefit is moderate to substantial. |
| C | The USPSTF recommends against routinely providing the service. There may be considerations that support providing the service in an individual patient. There is at least moderate certainty that the net benefit is small. |
| D | The USPSTF recommends against the service. There is moderate or high certainty that the service has no net benefit or that the harms outweigh the benefits. |
| I | The USPSTF concludes that the current evidence is insufficient to assess the balance of benefits and harms of the service. Evidence is lacking, of poor quality, or conflicting, and the balance of benefits and harms cannot be determined. |
| Source: Agency for Healthcare Research and Quality. US Preventive Services Task Force (USPSTF) ratings. Available at: http://www.uspreventiveservicestaskforce.org/uspstf07/ratingsv2.htm. Accessed September 5, 2013. | |
TABLE 2
USPSTF recommends FOR
| CARDIOVASCULAR DISEASE PREVENTION |
|
| PREGNANCY |
|
| CANCER SCREENING |
|
| DEPRESSION |
|
| OBESITY |
|
TABLE 3
USPSTF recommends AGAINST routinely
|
| USPSTF recommends AGAINST |
|
| USPSTF indicates the evidence is INSUFFICIENT to assess the balance of benefits and harms of |
|
| Source: Agency for Healthcare Research and Quality. Available at: www.ahrq.gov/clinic/uspstfix.htm. Accessed April 2, 2010. |
The A and B recommendations you may have missed
The major additions to the A and B recommendations pertained to the use of aspirin to prevent cardiovascular disease, routine screening for depression in adults and adolescents, and screening for obesity in children ages 6 and older. The other recommendations in these categories were reaffirmations of previous recommendations (asking about smoking and providing smoking cessation guidance to adults and pregnant women, advising folic acid supplementation for women planning or capable of pregnancy, and screening pregnant women for syphilis and hepatitis B virus) and the more controversial recommendation for biennial rather than annual mammography for women ages 50 to 74.
The use of aspirin to prevent myocardial infarction in men ages 45 to 79 and ischemic strokes in women ages 55 to 79 was endorsed if a patient’s risk of these cardiovascular events exceeds the risk of bleeding from regular aspirin use. The Task Force recommendation statement is available athttp://www.ahrq.gov/clinic/uspstf09/aspirincvd/aspcvdrs.htmand provides links to tools for calculating the risk of a myocardial infarction (MI) and ischemic stroke, as well as 2 tables to compare the risks and benefits of aspirin therapy for prevention.
Screening adults for depression is endorsed if “staff-assisted depression care supports” are in place to assure accurate diagnosis, effective treatment, and follow-up. Such support includes the presence of clinical staff members who can assist the primary care provider with care support or coordination, case management, or mental health treatment. The definition can be accessed athttp://www.ahrq.gov/clinic/uspstf09/adultdepression/addeprrs.htm.
One example in the statement describes “a successful study designed for practices without ready access to mental health specialty care, (in which) office staff recruited, screened, and enrolled participants who screened positive for depression before a clinic visit. If the physician confirmed the depression diagnosis, the participant was scheduled for a return visit with the physician and to meet with the nurse specialist in 1 week. The nurse specialist reassessed the patient’s level of depression, discussed treatment options and preferences, and asked the participant to complete a homework assignment. Participants completed up to 8 additional sessions that followed the same pattern, either by phone or in person.”
Screening for major depressive disorder (MDD) in adolescents 12 to 18 years of age is recommended when systems are in place to ensure accurate diagnosis, psychotherapy (cognitive-behavioral or interpersonal), and follow-up. The Task Force addressed screening for MDD only—not for less severe depression. The instruments the group recommended using included the Patient Health Questionnaire for Adolescents (PHQ-A) and the Beck Depression Inventory-Primary Care Version (BDI-PC).
The recommendation for screening for obesity in children ages 6 and older reflects the difficulty in achieving long-term, sustainable weight loss in this group. Effective comprehensive weight-management programs include counseling and other interventions that target both diet and physical activity. Behavioral interventions and parental involvement are also encouraged. Moderate- to high-intensity programs include more than 25 hours of contact with the child and/or the family over a 6-month period; less than this does not result in sustained improvement.
What about the D and I categories?
Two interventions received a D recommendation: Use of aspirin for stroke prevention in women <55 years and for MI prevention in men <45 years, and teaching breast self-examination (BSE) to women. The BSE recommendation has been misinterpreted as recommending against women performing self-breast exams. The recommendation is against formalized teaching of the procedure by physicians, as this leads to increased false positives and no improvement in outcomes when compared to women performing exams on their own.
The list of interventions receiving an I recommendation include some services that are commonly offered in the belief that they are effective. The Task Force is attempting to develop methodologies to decrease the number of interventions that receive an I recommendation. Currently, about 40% of all recommendations end up in this category, and physicians and patients alike could use more guidance on them. This plethora of recommendations made with insufficient evidence reflects the “ready, shoot, aim” philosophy of American medicine. We tend to accept and adopt new interventions before they are proven effective. The I recommendations are valuable reminders that, while many interventions are in common use, we often do not know as much as we should about their benefits and harms.
1. Agency for Healthcare Research and Quality. Screening for breast cancer. Updated December 2009. Available at: www.ahrq.gov/clinic/uspstf/uspsbrca.htm. Accessed March 17, 2010.
2. Woolf SH. The 2009 breast cancer screening recommendations of the US Preventive Services Task Force. JAMA. 2010;303:162-163.
3. Woloshin S, Schwartz LM. The benefits and harms of mammography screening: understanding the trade-offs. JAMA. 2010;303:164-165.
4. Murphy AM. Mammography screening for breast cancer: a view from 2 worlds. JAMA. 2010;303:166-167.
5. Berg WA. Benefits of screening mammography. JAMA. 2010;303:168-169.
6. DeAngelis CF, Fontanarosa PB. US Preventive Services Task Force and breast cancer screening. JAMA. 2010;303:172-173.
7. Editors’ note on the USPSTF recommendation on screening for breast cancer. February 15, 2010. Available at: http://www.annals.org/content/early/2010/02/12/0003-4819-152-8-201004200-00209.full. Accessed April 7, 2010.
8. Begg CB. Comments and response on the USPSTF recommendation on screening for breast cancer. February 15, 2010. Available at: http://www.annals.org/content/early/2010/02/12/0003-4819-152-8-201004200-00203.full. Accessed April 7, 2010.
9. Jorgensen KJ, Gotzsche PC. The background review for the USPSTF recommendation on screening for breast cancer. February 15, 2010. Available at: http://www.annals.org/content/early/2010/02/12/0003-4819-152-8-201004200-00198.full. Accessed April 7, 2010.
Late in 2009, a change in the recommendations of the US Preventive Services Task Force (USPSTF) brought more public attention to this panel than it had ever experienced before. This publicity centered on revised recommendations on breast cancer screening that pointed out that mammograms benefit a few women under 50, but are also associated with some harms. The Task Force recommended that patients and physicians discuss these potential benefits and harms and make an individual decision about whether to have a mammogram.1
Even though the criticism was loud—and harsh—from some sectors, many professional organizations, including the American Academy of Family Physicians, the American College of Physicians, and the American College of Preventive Medicine, came to the defense of the Task Force and its rigorous, evidence-based methodology.2-4 Both the Journal of the American Medical Association and the Annals of Internal Medicine have since published a series of articles and opinions on the controversy, most of them favorable to the Task Force and its methods.2-9
Lost in all the brouhaha were a number of other, less controversial recommendations that the Task Force made in 2009 (and early 2010). You can find them at www.ahrq.gov/clinic/uspstfix.htm. They are categorized by strength of recommendation (TABLE 1) and listed in TABLES 2 and 3. Family physicians should review the A and B recommendations and try to incorporate those into practice. At the same time, we should avoid services in the D category, as the evidence is strong that they are not effective or cause more harm than benefit. The C and I recommendations leave more discretion for physicians and patients to decide on these interventions based on personal values and risks. A C recommendation means the service can benefit some individuals, but the totality of benefit is small. An I recommendation means that evidence is insufficient to evaluate benefits vs harms.
TABLE 1
US Preventive Services Task Force recommendation categories
| Grade | Definition |
|---|---|
| A | The USPSTF recommends the service. There is high certainty that the net benefit is substantial. |
| B | The USPSTF recommends the service. There is high certainty that the net benefit is moderate or there is moderate certainty that the net benefit is moderate to substantial. |
| C | The USPSTF recommends against routinely providing the service. There may be considerations that support providing the service in an individual patient. There is at least moderate certainty that the net benefit is small. |
| D | The USPSTF recommends against the service. There is moderate or high certainty that the service has no net benefit or that the harms outweigh the benefits. |
| I | The USPSTF concludes that the current evidence is insufficient to assess the balance of benefits and harms of the service. Evidence is lacking, of poor quality, or conflicting, and the balance of benefits and harms cannot be determined. |
| Source: Agency for Healthcare Research and Quality. US Preventive Services Task Force (USPSTF) ratings. Available at: http://www.uspreventiveservicestaskforce.org/uspstf07/ratingsv2.htm. Accessed September 5, 2013. | |
TABLE 2
USPSTF recommends FOR
| CARDIOVASCULAR DISEASE PREVENTION |
|
| PREGNANCY |
|
| CANCER SCREENING |
|
| DEPRESSION |
|
| OBESITY |
|
TABLE 3
USPSTF recommends AGAINST routinely
|
| USPSTF recommends AGAINST |
|
| USPSTF indicates the evidence is INSUFFICIENT to assess the balance of benefits and harms of |
|
| Source: Agency for Healthcare Research and Quality. Available at: www.ahrq.gov/clinic/uspstfix.htm. Accessed April 2, 2010. |
The A and B recommendations you may have missed
The major additions to the A and B recommendations pertained to the use of aspirin to prevent cardiovascular disease, routine screening for depression in adults and adolescents, and screening for obesity in children ages 6 and older. The other recommendations in these categories were reaffirmations of previous recommendations (asking about smoking and providing smoking cessation guidance to adults and pregnant women, advising folic acid supplementation for women planning or capable of pregnancy, and screening pregnant women for syphilis and hepatitis B virus) and the more controversial recommendation for biennial rather than annual mammography for women ages 50 to 74.
The use of aspirin to prevent myocardial infarction in men ages 45 to 79 and ischemic strokes in women ages 55 to 79 was endorsed if a patient’s risk of these cardiovascular events exceeds the risk of bleeding from regular aspirin use. The Task Force recommendation statement is available athttp://www.ahrq.gov/clinic/uspstf09/aspirincvd/aspcvdrs.htmand provides links to tools for calculating the risk of a myocardial infarction (MI) and ischemic stroke, as well as 2 tables to compare the risks and benefits of aspirin therapy for prevention.
Screening adults for depression is endorsed if “staff-assisted depression care supports” are in place to assure accurate diagnosis, effective treatment, and follow-up. Such support includes the presence of clinical staff members who can assist the primary care provider with care support or coordination, case management, or mental health treatment. The definition can be accessed athttp://www.ahrq.gov/clinic/uspstf09/adultdepression/addeprrs.htm.
One example in the statement describes “a successful study designed for practices without ready access to mental health specialty care, (in which) office staff recruited, screened, and enrolled participants who screened positive for depression before a clinic visit. If the physician confirmed the depression diagnosis, the participant was scheduled for a return visit with the physician and to meet with the nurse specialist in 1 week. The nurse specialist reassessed the patient’s level of depression, discussed treatment options and preferences, and asked the participant to complete a homework assignment. Participants completed up to 8 additional sessions that followed the same pattern, either by phone or in person.”
Screening for major depressive disorder (MDD) in adolescents 12 to 18 years of age is recommended when systems are in place to ensure accurate diagnosis, psychotherapy (cognitive-behavioral or interpersonal), and follow-up. The Task Force addressed screening for MDD only—not for less severe depression. The instruments the group recommended using included the Patient Health Questionnaire for Adolescents (PHQ-A) and the Beck Depression Inventory-Primary Care Version (BDI-PC).
The recommendation for screening for obesity in children ages 6 and older reflects the difficulty in achieving long-term, sustainable weight loss in this group. Effective comprehensive weight-management programs include counseling and other interventions that target both diet and physical activity. Behavioral interventions and parental involvement are also encouraged. Moderate- to high-intensity programs include more than 25 hours of contact with the child and/or the family over a 6-month period; less than this does not result in sustained improvement.
What about the D and I categories?
Two interventions received a D recommendation: Use of aspirin for stroke prevention in women <55 years and for MI prevention in men <45 years, and teaching breast self-examination (BSE) to women. The BSE recommendation has been misinterpreted as recommending against women performing self-breast exams. The recommendation is against formalized teaching of the procedure by physicians, as this leads to increased false positives and no improvement in outcomes when compared to women performing exams on their own.
The list of interventions receiving an I recommendation include some services that are commonly offered in the belief that they are effective. The Task Force is attempting to develop methodologies to decrease the number of interventions that receive an I recommendation. Currently, about 40% of all recommendations end up in this category, and physicians and patients alike could use more guidance on them. This plethora of recommendations made with insufficient evidence reflects the “ready, shoot, aim” philosophy of American medicine. We tend to accept and adopt new interventions before they are proven effective. The I recommendations are valuable reminders that, while many interventions are in common use, we often do not know as much as we should about their benefits and harms.
Late in 2009, a change in the recommendations of the US Preventive Services Task Force (USPSTF) brought more public attention to this panel than it had ever experienced before. This publicity centered on revised recommendations on breast cancer screening that pointed out that mammograms benefit a few women under 50, but are also associated with some harms. The Task Force recommended that patients and physicians discuss these potential benefits and harms and make an individual decision about whether to have a mammogram.1
Even though the criticism was loud—and harsh—from some sectors, many professional organizations, including the American Academy of Family Physicians, the American College of Physicians, and the American College of Preventive Medicine, came to the defense of the Task Force and its rigorous, evidence-based methodology.2-4 Both the Journal of the American Medical Association and the Annals of Internal Medicine have since published a series of articles and opinions on the controversy, most of them favorable to the Task Force and its methods.2-9
Lost in all the brouhaha were a number of other, less controversial recommendations that the Task Force made in 2009 (and early 2010). You can find them at www.ahrq.gov/clinic/uspstfix.htm. They are categorized by strength of recommendation (TABLE 1) and listed in TABLES 2 and 3. Family physicians should review the A and B recommendations and try to incorporate those into practice. At the same time, we should avoid services in the D category, as the evidence is strong that they are not effective or cause more harm than benefit. The C and I recommendations leave more discretion for physicians and patients to decide on these interventions based on personal values and risks. A C recommendation means the service can benefit some individuals, but the totality of benefit is small. An I recommendation means that evidence is insufficient to evaluate benefits vs harms.
TABLE 1
US Preventive Services Task Force recommendation categories
| Grade | Definition |
|---|---|
| A | The USPSTF recommends the service. There is high certainty that the net benefit is substantial. |
| B | The USPSTF recommends the service. There is high certainty that the net benefit is moderate or there is moderate certainty that the net benefit is moderate to substantial. |
| C | The USPSTF recommends against routinely providing the service. There may be considerations that support providing the service in an individual patient. There is at least moderate certainty that the net benefit is small. |
| D | The USPSTF recommends against the service. There is moderate or high certainty that the service has no net benefit or that the harms outweigh the benefits. |
| I | The USPSTF concludes that the current evidence is insufficient to assess the balance of benefits and harms of the service. Evidence is lacking, of poor quality, or conflicting, and the balance of benefits and harms cannot be determined. |
| Source: Agency for Healthcare Research and Quality. US Preventive Services Task Force (USPSTF) ratings. Available at: http://www.uspreventiveservicestaskforce.org/uspstf07/ratingsv2.htm. Accessed September 5, 2013. | |
TABLE 2
USPSTF recommends FOR
| CARDIOVASCULAR DISEASE PREVENTION |
|
| PREGNANCY |
|
| CANCER SCREENING |
|
| DEPRESSION |
|
| OBESITY |
|
TABLE 3
USPSTF recommends AGAINST routinely
|
| USPSTF recommends AGAINST |
|
| USPSTF indicates the evidence is INSUFFICIENT to assess the balance of benefits and harms of |
|
| Source: Agency for Healthcare Research and Quality. Available at: www.ahrq.gov/clinic/uspstfix.htm. Accessed April 2, 2010. |
The A and B recommendations you may have missed
The major additions to the A and B recommendations pertained to the use of aspirin to prevent cardiovascular disease, routine screening for depression in adults and adolescents, and screening for obesity in children ages 6 and older. The other recommendations in these categories were reaffirmations of previous recommendations (asking about smoking and providing smoking cessation guidance to adults and pregnant women, advising folic acid supplementation for women planning or capable of pregnancy, and screening pregnant women for syphilis and hepatitis B virus) and the more controversial recommendation for biennial rather than annual mammography for women ages 50 to 74.
The use of aspirin to prevent myocardial infarction in men ages 45 to 79 and ischemic strokes in women ages 55 to 79 was endorsed if a patient’s risk of these cardiovascular events exceeds the risk of bleeding from regular aspirin use. The Task Force recommendation statement is available athttp://www.ahrq.gov/clinic/uspstf09/aspirincvd/aspcvdrs.htmand provides links to tools for calculating the risk of a myocardial infarction (MI) and ischemic stroke, as well as 2 tables to compare the risks and benefits of aspirin therapy for prevention.
Screening adults for depression is endorsed if “staff-assisted depression care supports” are in place to assure accurate diagnosis, effective treatment, and follow-up. Such support includes the presence of clinical staff members who can assist the primary care provider with care support or coordination, case management, or mental health treatment. The definition can be accessed athttp://www.ahrq.gov/clinic/uspstf09/adultdepression/addeprrs.htm.
One example in the statement describes “a successful study designed for practices without ready access to mental health specialty care, (in which) office staff recruited, screened, and enrolled participants who screened positive for depression before a clinic visit. If the physician confirmed the depression diagnosis, the participant was scheduled for a return visit with the physician and to meet with the nurse specialist in 1 week. The nurse specialist reassessed the patient’s level of depression, discussed treatment options and preferences, and asked the participant to complete a homework assignment. Participants completed up to 8 additional sessions that followed the same pattern, either by phone or in person.”
Screening for major depressive disorder (MDD) in adolescents 12 to 18 years of age is recommended when systems are in place to ensure accurate diagnosis, psychotherapy (cognitive-behavioral or interpersonal), and follow-up. The Task Force addressed screening for MDD only—not for less severe depression. The instruments the group recommended using included the Patient Health Questionnaire for Adolescents (PHQ-A) and the Beck Depression Inventory-Primary Care Version (BDI-PC).
The recommendation for screening for obesity in children ages 6 and older reflects the difficulty in achieving long-term, sustainable weight loss in this group. Effective comprehensive weight-management programs include counseling and other interventions that target both diet and physical activity. Behavioral interventions and parental involvement are also encouraged. Moderate- to high-intensity programs include more than 25 hours of contact with the child and/or the family over a 6-month period; less than this does not result in sustained improvement.
What about the D and I categories?
Two interventions received a D recommendation: Use of aspirin for stroke prevention in women <55 years and for MI prevention in men <45 years, and teaching breast self-examination (BSE) to women. The BSE recommendation has been misinterpreted as recommending against women performing self-breast exams. The recommendation is against formalized teaching of the procedure by physicians, as this leads to increased false positives and no improvement in outcomes when compared to women performing exams on their own.
The list of interventions receiving an I recommendation include some services that are commonly offered in the belief that they are effective. The Task Force is attempting to develop methodologies to decrease the number of interventions that receive an I recommendation. Currently, about 40% of all recommendations end up in this category, and physicians and patients alike could use more guidance on them. This plethora of recommendations made with insufficient evidence reflects the “ready, shoot, aim” philosophy of American medicine. We tend to accept and adopt new interventions before they are proven effective. The I recommendations are valuable reminders that, while many interventions are in common use, we often do not know as much as we should about their benefits and harms.
1. Agency for Healthcare Research and Quality. Screening for breast cancer. Updated December 2009. Available at: www.ahrq.gov/clinic/uspstf/uspsbrca.htm. Accessed March 17, 2010.
2. Woolf SH. The 2009 breast cancer screening recommendations of the US Preventive Services Task Force. JAMA. 2010;303:162-163.
3. Woloshin S, Schwartz LM. The benefits and harms of mammography screening: understanding the trade-offs. JAMA. 2010;303:164-165.
4. Murphy AM. Mammography screening for breast cancer: a view from 2 worlds. JAMA. 2010;303:166-167.
5. Berg WA. Benefits of screening mammography. JAMA. 2010;303:168-169.
6. DeAngelis CF, Fontanarosa PB. US Preventive Services Task Force and breast cancer screening. JAMA. 2010;303:172-173.
7. Editors’ note on the USPSTF recommendation on screening for breast cancer. February 15, 2010. Available at: http://www.annals.org/content/early/2010/02/12/0003-4819-152-8-201004200-00209.full. Accessed April 7, 2010.
8. Begg CB. Comments and response on the USPSTF recommendation on screening for breast cancer. February 15, 2010. Available at: http://www.annals.org/content/early/2010/02/12/0003-4819-152-8-201004200-00203.full. Accessed April 7, 2010.
9. Jorgensen KJ, Gotzsche PC. The background review for the USPSTF recommendation on screening for breast cancer. February 15, 2010. Available at: http://www.annals.org/content/early/2010/02/12/0003-4819-152-8-201004200-00198.full. Accessed April 7, 2010.
1. Agency for Healthcare Research and Quality. Screening for breast cancer. Updated December 2009. Available at: www.ahrq.gov/clinic/uspstf/uspsbrca.htm. Accessed March 17, 2010.
2. Woolf SH. The 2009 breast cancer screening recommendations of the US Preventive Services Task Force. JAMA. 2010;303:162-163.
3. Woloshin S, Schwartz LM. The benefits and harms of mammography screening: understanding the trade-offs. JAMA. 2010;303:164-165.
4. Murphy AM. Mammography screening for breast cancer: a view from 2 worlds. JAMA. 2010;303:166-167.
5. Berg WA. Benefits of screening mammography. JAMA. 2010;303:168-169.
6. DeAngelis CF, Fontanarosa PB. US Preventive Services Task Force and breast cancer screening. JAMA. 2010;303:172-173.
7. Editors’ note on the USPSTF recommendation on screening for breast cancer. February 15, 2010. Available at: http://www.annals.org/content/early/2010/02/12/0003-4819-152-8-201004200-00209.full. Accessed April 7, 2010.
8. Begg CB. Comments and response on the USPSTF recommendation on screening for breast cancer. February 15, 2010. Available at: http://www.annals.org/content/early/2010/02/12/0003-4819-152-8-201004200-00203.full. Accessed April 7, 2010.
9. Jorgensen KJ, Gotzsche PC. The background review for the USPSTF recommendation on screening for breast cancer. February 15, 2010. Available at: http://www.annals.org/content/early/2010/02/12/0003-4819-152-8-201004200-00198.full. Accessed April 7, 2010.
Vaccine update 2010: Keeping up with the changes
The past 10 years have seen marked advances in vaccine research, resulting in more products being available. In 1983 the childhood vaccination schedule included protection against seven diseases: polio, tetanus, diphtheria, pertussis, measles, mumps, and rubella. The schedule in 2010 includes protection against organisms that cause seven more: Haemophilus influenzae, hepatitis A, hepatitis B, influenza, meningococcus, pneumococcus, and varicella.1 In addition, new vaccine products are available for adolescents, offering protection against meningococcus, seasonal influenza, and human papillomavirus (HPV) and extending the length of protection against pertussis. For adults, a vaccine now protects against shingles, and several products offer boosting of pertussis immunity.
This rapid growth in the number of recommended vaccine products has made it challenging for practicing physicians to stay current on and to implement the ever-changing recommendations. The purpose of this article is to summarize the additions and changes over the past 3 years to the schedules of recommended vaccines for children, adolescents, and adults.
VACCINE UPDATE FOR CHILDREN
The recent changes to the childhood immunization schedule have added protection against rotavirus and seasonal influenza and have expanded the protection against hepatitis A and varicella.
Rotavirus vaccination for infants
Rotavirus is the leading cause of infectious gastroenteritis in infants. It causes significant morbidity and expense, accounting for 2.7 million episodes per year in the United States, 410,000 outpatient or office visits, 201,000 to 272,000 emergency department visits, 55,000 to 70,000 hospitalizations, and 20 to 60 deaths.2 Although the number of deaths in the United States is not large, rotavirus is a leading cause of infant deaths around the world.
Rotavirus vaccination is challenging because of the time frame in which the series needs to be given. The first dose has to be given after 6 weeks of age but before 15 weeks of age, and the last dose should be given before 8 months of age, with a minimum of 4 weeks between doses. It is preferable to use the same product to finish the series. They can be used interchangeably, but this then requires three total doses.
The effectiveness of the vaccine in preventing rotavirus gastroenteritis in the first year after vaccination was greater than 80% in most studies and approached 100% in preventing serious gastroenteritis.2
Those vaccinated appear to have a slightly higher rate of diarrhea and vomiting in the first 42 days after vaccination. Safety monitoring after the products were licensed has not shown an increased rate of intussusception with either product.
The only contraindication to the vaccines is a serious allergic reaction to them or to one of their components. They should be used with caution in patients who have suppressed immunity, acute gastroenteritis, preexisting gastrointestinal disease, or previous intussusception.
Seasonal influenza vaccine extended to ages 5–18
Gradually, we seem to be moving toward vaccinating everyone every year against seasonal influenza. Previously, vaccination was recommended for children age 6 months through 4 years; in 2008, the Advisory Committee on Immunization Practices (ACIP) extended the recommendation to the age group 5 through 18 years.3
Two types of seasonal influenza vaccine are available: trivalent influenza vaccine (TIV), which contains killed virus and is given by injection, and live-attenuated seasonal influenza vaccine (LAIV), which is given by nasal spray. Both contain the same three seasonal influenza antigens, selected each year by a team of experts. TIV is licensed for those age 6 months and older, and LAIV is licensed for ages 2 through 49 years.4
Since LAIV contains a live-attenuated virus, it should not be used in anyone who has a chronic illness (including those under the age of 5 with recurrent wheezing, those with suppressed immunity, and those with a history of Guillain-Barré syndrome); in pregnant women; or those who have close contact with anyone who is immune-suppressed. The injection is contraindicated for those who have had a serious allergic reaction to eggs.
Children younger than 9 years should receive two doses of either type of vaccine the first year they are vaccinated. Those who receive only one dose the first year they are vaccinated should receive two doses the next year. If they fail to receive two doses in the next year, only a single dose is recommended after that. This is a slight modification of the previous recommendation that only one dose be given the second year if only one dose was given the first year.5
Hepatitis A vaccine at age 12–23 months
An inactivated hepatitis A vaccine (HepA) was first licensed in 1995; another was licensed in 1996. Recommendations for their use have been revised periodically, and their widespread use has resulted in a marked reduction in the incidence of hepatitis A virus infection.
The current recommendation is that all children be vaccinated at age 12 to 23 months. In addition, in areas of high prevalence, vaccine is recommended for older children who have not been vaccinated. Other target groups are those at higher risk of hepatitis A, including travelers to endemic areas, users of illicit drugs, and men who have sex with men.6 Indications for vaccination before travel, after exposure to hepatitis A infection, and in families of international adoptees are covered later in this paper in a discussion about vaccinations in adults.
Varicella at 12–15 months and 4–6 years, with catch-up for others
Before varicella vaccine was licensed in 1995, 4 million cases of varicella infection (chickenpox) were reported in the United States each year, resulting in thousands of hospitalizations and more than 100 deaths. The vaccine is now widely used, with a coverage rate of 88%, and it has proven to be 85% effective.7 The result was a marked decrease in the incidence of varicella and in varicella-related hospitalizations and deaths.
In spite of this success, the number of varicella cases has remained constant over the past few years, and sporadic outbreaks continue to occur, predominantly in schools, even schools in which a high percentage of the children are vaccinated.7,8 These outbreaks have involved infections in unvaccinated children and also “breakthrough disease” in children who have been vaccinated. If someone who has received one dose of vaccine is exposed to varicella, the risk of a breakthrough infection is about 15%.9 A two-dose series of varicella vaccine reduces the risk by about 75%.7 Breakthrough disease is usually milder than infection in the unvaccinated, with fewer skin lesions, milder symptoms, and fewer complications, but those affected are still infectious to others.
In 2005 and 2006, this ongoing risk of varicella prompted the ACIP to consider and recommend several new control measures:
- Two doses of varicella vaccine for all children, the first dose at age 12 to 15 months and the second at age 4 to 6 years—the same schedule as for immunization against measles, mumps, and rubella
- Two doses of varicella vaccine, the second given 4 to 8 weeks after the first, for all adolescents and adults who have no evidence of immunity
- A catch-up second dose for everyone who received one dose previously
- Screening for varicella immunity in pregnant women and postpartum vaccination with two doses for those who are not immune, the first dose given before discharge and the second dose 4 to 8 weeks later.
VACCINE UPDATE FOR ADOLESCENTS
A number of vaccines are now available and recommended for routine use in adolescents.9 These include HPV vaccine for girls, quadrivalent meningococcal conjugate vaccine (MCV4), and combined tetanus toxoid, reduced-dose diphtheria toxoid, and acellular pertussis (Tdap). All these are now recommended routinely at age 11 or 12. Seasonal influenza vaccine is recommended annually through age 18.
Meningococcal conjugate vaccine for all at age 11–18
There is some evidence that MCV4 may be linked to a small risk of Guillain-Barré syndrome. Although this link has not been conclusively proven, a history of Guillain-Barré syndrome calls for caution in using MCV4. For those who have a history of this syndrome but need protection against meningococcal infection, the MPSV4 is an alternative.11
Pertussis: A Tdap booster at age 11–18
In addition, a greater percentage of cases now occurs in adolescents and young adults. Half of reported cases are now in those age 10 years and older. Most nonimmunized or incompletely immunized infants who develop pertussis were exposed to the disease by older household members, not by same-age cohorts. Since the disease presents as nonspecific cough in adolescents, it is often not diagnosed, and the incidence is probably much higher than the reported number of cases would indicate.
These trends were cause for public health concern and led to the development of pertussis-containing vaccine products for adolescents and adults. Two Tdap products are available: one is licensed for those ages 10 to 64 (Boostrix), the other for ages 11 to 64 (Adacel). Since 2005, the ACIP has recommended a single dose of Tdap for those age 11 to 18, preferably at 11 or 12 years.12 The optimal interval from the last tetanus-diphtheria shot is 5 years, but a shorter interval is acceptable. Thereafter, boosters with the tetanus toxoid and reduced-dose diphtheria toxoid (Td) vaccine are recommended every 10 years. If an adolescent has not previously received a complete series of a tetanus-diphtheria product, he or she should be given the recommended number of doses, only one of which should be Tdap, the others Td. The number and timing of doses can be found at www.cdc.gov/mmwr/preview/mmwrhtml/rr55e223a5.htm.
Human papillomavirus vaccination for girls age 11–12
HPV is sexually transmitted and causes genital warts, cervical cancer, and other oral, anal, and genital cancers.
HPV is the most common sexually transmitted infection in the United States, with over 6 million new cases each year.13 A study in 2003 to 2004 using HPV DNA typing of cervicovaginal swab specimens in a sample of women between the ages of 14 and 59 found an overall point prevalence of 26.8% of any HPV type.14 Those between 20 and 24 years had the highest prevalence at 44.8%. Those ages 14 to 19 had a prevalence of 24.5%. Several studies have reported a similar age-related increase in HPV prevalence.15,16
One survey found that nearly 25% of girls in the United States are sexually active by age 15, 40% by age 16, and 70% by age 18.17 The 2005 Behavioral Risk Survey found that nearly 4% of girls were sexually active before age 13, and by the ninth grade 5.7% of those who were sexually active had had four or more partners.18 To receive the full benefit from the HPV vaccine, it should be given before this risk of acquiring HPV occurs.
A quadrivalent HPV vaccine (HPV4) was first licensed in the United States in 2006 for use in girls and women 9 to 26 years old to prevent cervical, vulvar, and vaginal precancerous lesions and cancer, and for prevention of anogenital warts. It contains viral proteins from HPV types 6, 11, 16, and 18, the types currently responsible for 70% of cervical cancers and 90% of anogenital warts. The vaccine is prepared in a yeast substrate and contains an aluminum-based adjuvant.
HPV4 has proven highly effective in women ages 16 to 26 not previously exposed to the four HPV types in the vaccine. The end points used in these studies were cervical intraepithelial neoplasia grade 2 or 3, adenocarcinoma in situ, anogenital warts, and vulvar and vaginal intraepithelial neoplasms.13,19,20 The vaccine’s effectiveness has been 98% to 100% after 3 to 5 years. These trials are ongoing.
The vaccine’s efficacy in women with current or past HPV infection is less certain. Studies of this question have included only small numbers, and the confidence intervals are large and include 0. In intention-to-treat studies, its efficacy has been 39% to 46% for prevention of cervical intraepithelial neoplasia grade 2 or 3 or adenocarcinoma in situ caused by HPV-16 or HPV-18, 69% for prevention of HPV-16- or HPV-18-related vaginal intraepithelial neoplasia, and 68.5% for vaccine-type-related warts.13
The most common adverse effects of HPV4 have included redness, pain, and swelling at the injection site, which occur in about 20% of recipients.13 There is an increased risk of syncope immediately after the vaccine is given, and observation for 15 minutes after injection is recommended. A recent study suggested a link between the vaccine and venous thromboembolism. 21 The rate was 2 per million doses, and because many of the recipients also were taking oral contraceptives, their venous thromboembolism has not yet been definitively proven to be caused by the vaccine.
HPV4 is contraindicated in those who have experienced a severe allergic reaction to a previous dose or who have an allergy to a vaccine component. Vaccination should be deferred in those with moderate or severe acute illnesses.
In June 2006, the ACIP13 made the following recommendations for HPV4:
- Girls ages 11 to 12 years should be routinely vaccinated with three doses
- The series can start as early as age 9 years
- Women and girls age 13 to 26 who have not been previously vaccinated should receive catch-up vaccination
- Neither Papanicolaou (Pap) testing nor HPV screening is necessary before vaccination
- HPV4 can be given with other age-appropriate vaccines
- Vaccination does not change the recommendations for cervical cancer screening
- The recommendations remain the same regardless of abnormal Pap tests, positive HPV DNA tests, or warts.
There have been two very recent developments regarding HPV vaccines.
A bivalent vaccine (HPV2) has been licensed in the United States and approved for use in girls and women ages 10 to 25 for prevention of cervical cancer and precancerous lesions. It contains antigens against HPV-16 and HPV-18 but does not provide protection against genital warts. The ACIP has stated no preference for the bivalent or the quadrivalent vaccine for the prevention of cervical cancer and precancerous lesions.
HPV4 has also gained licensure for use in boys and men age 9 to 26 for the prevention of genital warts. The ACIP has not recommended it for routine use, leaving the decision to patients and physicians after weighing the potential benefits and costs.
VACCINE UPDATE FOR ADULTS
Four vaccines are now routinely recommended for adults:
- Seasonal influenza vaccine starting at age 50
- Pneumococcal polysaccharide vaccine (PPSV23) starting at age 65
- Herpes zoster vaccine starting at age 60
- A diphtheria and tetanus toxoid product every 10 years, with Tdap given once.22
The rest of the adult schedule is based on catch-up (measles, mumps, rubella, varicella) or risk (hepatitis A and B and meningococccal disease). Seasonal influenza and pneumococcal vaccinations are also recommended before ages 50 and 65, respectively, for those with certain risk conditions. The complete adult immunization schedule can be found on the US Centers for Disease Control and Prevention (CDC) Web site.22
One dose of Tdap instead of the next Td booster
The CDC now recommends that a single dose of Tdap should replace the next dose of Td for adults ages 19 to 64 as part of the every-10-year tetanus-diphtheria boosting recommendation and if indicated for wound management. 23 In addition, a single dose of Tdap should be given to adults who have close contact with infants less than 6 months of age. The optimal interval between this Tdap shot and the last Td booster is 2 years or greater, but shorter intervals are acceptable. Women of childbearing age should receive Tdap preconception or postpartum if they have not previously received it. Tdap is not approved for use during pregnancy. Health care workers should also receive a dose of Tdap if they have never received it previously and if their last Td booster was more than 2 years ago, although less than 2 years is acceptable.
Contraindications to Tdap include anaphylaxis to a vaccine component and encephalopathy occurring within 7 days of previously receiving a pertussis vaccine.
Herpes zoster vaccine for those age 60 and older
Shingles causes considerable morbidity in older adults. The lifetime risk is 25%, and onefourth of those with shingles develop postherpetic neuralgia.
Herpes zoster vaccine is a live-attenuated vaccine that requires only a single injection. It is licensed for use in those ages 60 and older, and the ACIP recommends its routine use.24 Its effectiveness is approximately 50% and is inversely related to age. The number of patients who need to be vaccinated to prevent one lifetime case of shingles is 17.
Contraindications to this vaccine include a prior anaphylactic reaction to gelatin or neomycin, compromised immunity due to disease or to immune-suppressive therapy including high-dose corticosteroids, and active tuberculosis.
Payment for this vaccine by Medicare is through Part D, creating some administrative difficulties for physicians’ offices.
Pneumococcal vaccination extended to smokers and people with asthma
The ACIP recently added two new groups for whom PPSV23 is recommended: smokers and those with asthma.25 Smoking poses as much of a risk for pneumococcal pneumonia as do diabetes and other chronic illnesses that are currently indications for the vaccine. The number needed to vaccinate to prevent one case of pneumonia among smokers is 10,000 in people ages 18 to 44, and 4,000 in those ages 45 to 64.26
The ACIP also clarified the recommendation for a second dose of PPSV23.25 A second dose 5 years after the first is recommended for those who have immune suppression, sickle cell disease, or asplenia. People over age 65 should receive a second dose if they were vaccinated more than 5 years previously and before age 65.
New uses for hepatitis A vaccine
A combined hepatitis A and hepatitis B vaccine (Twinrix) has received approval for an alternate, four-dose schedule at 0, 7, 21 days, and 12 months.27 It has previously only been approved for a three-dose schedule at 0, 1, and 6 months. The new alternative schedule allows greater protection for travelers who need to depart within less than 1 month.
For unvaccinated people who are acutely exposed to hepatitis A virus and for those traveling to areas of high prevalence who do not have time to complete the two doses of hepatitis A vaccine, the only prevention available until recently has been immune globulin. This has changed: hepatitis A vaccine can now be used in both groups. The new recommendations for postexposure prophylaxis is that either a single dose of hepatitis A vaccine or use of immune globulin is acceptable.28 In ages 12 months to 40 years, vaccine is preferred. For those over age 40, immune globulin is preferred, but vaccine is acceptable. For children younger than 12 months, the immune-suppressed, and those with chronic liver disease, immune globulin should be used.
Those traveling or working in countries with high rates of hepatitis A can be protected with either hepatitis A vaccine or immune globulin. A single dose of the vaccine is sufficient for healthy people, with a second dose at the recommended interval to complete the series. Those younger than 12 months and those who choose not to receive the vaccine, including those who are allergic to it, should be offered immune globulin. Both immune globulin and hepatitis A vaccine should be considered for certain patients who plan to travel within 2 weeks of the first vaccine dose, ie, those over age 40, those with compromised immunity, and those with chronic liver disease or other chronic conditions.
Hepatitis A vaccine is now also recommended for all unvaccinated people who anticipate close personal contact with an international adoptee during the first 60 days following arrival from countries with high or intermediate hepatitis A endemicity.29 The first dose should be given as soon as the adoption is planned and ideally at least 2 weeks before the child arrives.
- Centers for Disease Control and Prevention (CDC). Recommended immunization schedule for persons aged 0 through 6 years—United States 2009. www.cdc.gov/vaccines/recs/schedules/downloads/child/2009/09_0-6yrs_schedule_pr.pdf. Accessed March 6, 2010.
- Cortese MM, Parashar UDCenters for Disease Control and Prevention (CDC). Prevention of rotavirus gastroenteritis among infants and children: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2009; 58( RR-2):1–25.
- Fiore AE, Shay DK, Broder K, et al., Centers for Disease Control and Prevention (CDC). Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2009. MMWR Recomm Rep 2009; 58( RR–8):1–52.
- Centers for Disease Control and Prevention (CDC). Notice to readers: expansion of use of live attenuated influenza vaccine (FluMist®) to children aged 2–4 years and other FluMist changes for the 2007–08 influenza season. MMWR Morb Mortal Wkly Rep 2007; 56( 46):1217–1219.
- Fiore AE, Shay DK, Haber PCenters for Disease Control and Prevention (CDC). Prevention and control of influenza: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2007. MMWR Recomm Rep 2007; 56( RR–6):1–54.
- Fiore AE, Wasley A, Bell BPAdvisory Committee on Immunization Practices (ACIP). Prevention of hepatitis A through active or passive immunization: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2006; 55( RR–7):1–23.
- Marin M, Güris D, Chaves SS, Schmid S, Seward JFAdvisory Committee on Immunization Practices. Prevention of varicella: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2007; 56( RR–4):1–40.
- Centers for Disease Control and Prevention (CDC). Varicella disease. www.cdc.gov/vaccines/vpd-vac/varicella/dis-faqs-clinic.htm. Accessed March 4, 2010.
- Centers for Disease Control and Prevention (CDC). 2009 child & adolescent immunization schedules. www.cdc.gov/vaccines/recs/schedules/child-schedule.htm. Accessed March 4, 2010.
- Centers for Disease Control and Prevention (CDC) Advisory Committee on Immunization Practices. Revised recommendations of the Advisory Committee on Immunization Practices to vaccinate all persons aged 11-18 years with meningococcal conjugate vaccine. MMWR Morb Mortal Wkly Rep 2007; 56( 31):794–795.
- Centers for Disease Control and Prevention (CDC). Update: Guillain-Barré syndrome among recipients of menactra meningococcal conjugate vaccine—United States, June 2005–September 2006. MMWR Morb Mortal Wkly Rep 2006; 55( 41):1120–1124.
- Broder KR, Cortese MM, Iskander JK, et al., Advisory Committee on Immunization Practices (ACIP). Preventing tetanus, diphtheria, and pertussis among adolescents: use of tetanus toxoid, reduced diphtheria toxoid and acellular pertussis vaccines recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2006; 55( RR–3):1–34.
- Markowitz LE, Dunne EF, Saraiya M, Lawson HW, Chesson H, Unger ERCenters for Disease Control and Prevention (CDC). Quadrivalent human papillomavirus vaccine: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2007; 56( RR–2):1–24.
- Dunne EF, Unger ER, Sternberg M, et al Prevalence of HPV infection among females in the United States. JAMA 2007; 297:813–819.
- Trottier H, Franco EL. The epidemiology of genital human papillomavirus infection. Vaccine 2006; 24( suppl 1):S1–S15.
- Stone KM, Karem KL, Sternberg MR, et al Seroprevalence of human papillomavirus type 16 infection in the United States. J Infect Dis 2002; 186:1396–1402.
- Abma JC, Martinez GM, Mosher WD, Dawson BS. Teenagers in the United States: sexual activity, contraceptive use, and childbearing, 2002. Vital Health Stat 23 2004; 24:1–48.
- Eaton DK, Kann L, Kinchen S, et al Youth risk behavior surveillance—United States, 2005. MMWR Surveill Summ 2006; 55:1–108.
- Human papillomavirus vaccines. WHO position paper. Wkly Epidemiol Rec 2009; 84:118–131.
- Rambout L, Hopkins L, Hutton B, Fergusson D. Prophylactic vaccination against human papillomavirus infection and disease in women: a systematic review of randomized controlled trials. CMAJ 2007; 177:469–479.
- Slade BA, Leidel L, Vellozzi C, et al Postlicensure safety surveillance for quadrivalent human papillomavirus recombinant vaccine. JAMA 2009; 302:750–757.
- Centers for Disease Control (CDC). Adult immunization schedule. http://www.cdc.gov/vaccines/recs/schedules/adult-schedule.htm. Accessed March 4, 2010.
- Kretsinger K, Broder KR, Cortese MM, et al., Centers for Disease Control and Prevention. Preventing tetanus, diphtheria, and pertussis among adults: use of tetanus toxoid, reduced diphtheria toxoid and acellular pertussis vaccine recommendations of the Advisory Committee on Immunization Practices (ACIP) and recommendation of ACIP, supported by the Healthcare Infection Control Practices Advisory Committee (HICPAC), for use of Tdap among health-care personnel. MMWR Recomm Rep 2006; 55( RR–17):1–37.
- Harpaz R, Ortega-Sanchez IR, Seward JFAdvisory Committee on Immunization Practices (ACIP). Prevention of herpes zoster: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2008; 57( RR–5):1–30.
- Centers for Disease Control (CDC). ACIP provisional recommendations for use of pneumococcal vaccines. www.cdc.gov/vaccines/recs/provisional/downloads/pneumo-Oct-2008-508.pdf. Accessed March 4, 2010.
- Centers for Disease Control and Prevention (CDC). Summary Report: October 22–23, 2008; Atlanta, Georgia. www.cdc.gov/vaccines/recs/ACIP/downloads/min=archive/min-oct08.pdf. Accessed March 6, 2010.
- CDC. Notice to readers: FDA approval of an alternate dosing schedule for a combined hepatitis A and B vaccine (Twinrix®). MMWR Morb Mortal Wkly Rep 2007; 56( 40);1057.
- Advisory Committee on Immunization Practices (ACIP) Centers for Disease Control and Prevention (CDC). Update: prevention of hepatitis A after exposure to hepatitis A virus and in international travelers. Updated recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 2007; 56( 41):1080–1084.
- Centers for Disease Control and Prevention (CDC). Updated recommendations from the Advisory Committee on Immunization Practices (ACIP) for use of hepatitis A vaccine in close contacts of newly arriving international adoptees. MMWR 2009: 58:1006–1007.
The past 10 years have seen marked advances in vaccine research, resulting in more products being available. In 1983 the childhood vaccination schedule included protection against seven diseases: polio, tetanus, diphtheria, pertussis, measles, mumps, and rubella. The schedule in 2010 includes protection against organisms that cause seven more: Haemophilus influenzae, hepatitis A, hepatitis B, influenza, meningococcus, pneumococcus, and varicella.1 In addition, new vaccine products are available for adolescents, offering protection against meningococcus, seasonal influenza, and human papillomavirus (HPV) and extending the length of protection against pertussis. For adults, a vaccine now protects against shingles, and several products offer boosting of pertussis immunity.
This rapid growth in the number of recommended vaccine products has made it challenging for practicing physicians to stay current on and to implement the ever-changing recommendations. The purpose of this article is to summarize the additions and changes over the past 3 years to the schedules of recommended vaccines for children, adolescents, and adults.

VACCINE UPDATE FOR CHILDREN
The recent changes to the childhood immunization schedule have added protection against rotavirus and seasonal influenza and have expanded the protection against hepatitis A and varicella.
Rotavirus vaccination for infants
Rotavirus is the leading cause of infectious gastroenteritis in infants. It causes significant morbidity and expense, accounting for 2.7 million episodes per year in the United States, 410,000 outpatient or office visits, 201,000 to 272,000 emergency department visits, 55,000 to 70,000 hospitalizations, and 20 to 60 deaths.2 Although the number of deaths in the United States is not large, rotavirus is a leading cause of infant deaths around the world.
Rotavirus vaccination is challenging because of the time frame in which the series needs to be given. The first dose has to be given after 6 weeks of age but before 15 weeks of age, and the last dose should be given before 8 months of age, with a minimum of 4 weeks between doses. It is preferable to use the same product to finish the series. They can be used interchangeably, but this then requires three total doses.
The effectiveness of the vaccine in preventing rotavirus gastroenteritis in the first year after vaccination was greater than 80% in most studies and approached 100% in preventing serious gastroenteritis.2
Those vaccinated appear to have a slightly higher rate of diarrhea and vomiting in the first 42 days after vaccination. Safety monitoring after the products were licensed has not shown an increased rate of intussusception with either product.
The only contraindication to the vaccines is a serious allergic reaction to them or to one of their components. They should be used with caution in patients who have suppressed immunity, acute gastroenteritis, preexisting gastrointestinal disease, or previous intussusception.
Seasonal influenza vaccine extended to ages 5–18
Gradually, we seem to be moving toward vaccinating everyone every year against seasonal influenza. Previously, vaccination was recommended for children age 6 months through 4 years; in 2008, the Advisory Committee on Immunization Practices (ACIP) extended the recommendation to the age group 5 through 18 years.3
Two types of seasonal influenza vaccine are available: trivalent influenza vaccine (TIV), which contains killed virus and is given by injection, and live-attenuated seasonal influenza vaccine (LAIV), which is given by nasal spray. Both contain the same three seasonal influenza antigens, selected each year by a team of experts. TIV is licensed for those age 6 months and older, and LAIV is licensed for ages 2 through 49 years.4
Since LAIV contains a live-attenuated virus, it should not be used in anyone who has a chronic illness (including those under the age of 5 with recurrent wheezing, those with suppressed immunity, and those with a history of Guillain-Barré syndrome); in pregnant women; or those who have close contact with anyone who is immune-suppressed. The injection is contraindicated for those who have had a serious allergic reaction to eggs.
Children younger than 9 years should receive two doses of either type of vaccine the first year they are vaccinated. Those who receive only one dose the first year they are vaccinated should receive two doses the next year. If they fail to receive two doses in the next year, only a single dose is recommended after that. This is a slight modification of the previous recommendation that only one dose be given the second year if only one dose was given the first year.5
Hepatitis A vaccine at age 12–23 months
An inactivated hepatitis A vaccine (HepA) was first licensed in 1995; another was licensed in 1996. Recommendations for their use have been revised periodically, and their widespread use has resulted in a marked reduction in the incidence of hepatitis A virus infection.
The current recommendation is that all children be vaccinated at age 12 to 23 months. In addition, in areas of high prevalence, vaccine is recommended for older children who have not been vaccinated. Other target groups are those at higher risk of hepatitis A, including travelers to endemic areas, users of illicit drugs, and men who have sex with men.6 Indications for vaccination before travel, after exposure to hepatitis A infection, and in families of international adoptees are covered later in this paper in a discussion about vaccinations in adults.
Varicella at 12–15 months and 4–6 years, with catch-up for others
Before varicella vaccine was licensed in 1995, 4 million cases of varicella infection (chickenpox) were reported in the United States each year, resulting in thousands of hospitalizations and more than 100 deaths. The vaccine is now widely used, with a coverage rate of 88%, and it has proven to be 85% effective.7 The result was a marked decrease in the incidence of varicella and in varicella-related hospitalizations and deaths.
In spite of this success, the number of varicella cases has remained constant over the past few years, and sporadic outbreaks continue to occur, predominantly in schools, even schools in which a high percentage of the children are vaccinated.7,8 These outbreaks have involved infections in unvaccinated children and also “breakthrough disease” in children who have been vaccinated. If someone who has received one dose of vaccine is exposed to varicella, the risk of a breakthrough infection is about 15%.9 A two-dose series of varicella vaccine reduces the risk by about 75%.7 Breakthrough disease is usually milder than infection in the unvaccinated, with fewer skin lesions, milder symptoms, and fewer complications, but those affected are still infectious to others.
In 2005 and 2006, this ongoing risk of varicella prompted the ACIP to consider and recommend several new control measures:
- Two doses of varicella vaccine for all children, the first dose at age 12 to 15 months and the second at age 4 to 6 years—the same schedule as for immunization against measles, mumps, and rubella
- Two doses of varicella vaccine, the second given 4 to 8 weeks after the first, for all adolescents and adults who have no evidence of immunity
- A catch-up second dose for everyone who received one dose previously
- Screening for varicella immunity in pregnant women and postpartum vaccination with two doses for those who are not immune, the first dose given before discharge and the second dose 4 to 8 weeks later.
VACCINE UPDATE FOR ADOLESCENTS
A number of vaccines are now available and recommended for routine use in adolescents.9 These include HPV vaccine for girls, quadrivalent meningococcal conjugate vaccine (MCV4), and combined tetanus toxoid, reduced-dose diphtheria toxoid, and acellular pertussis (Tdap). All these are now recommended routinely at age 11 or 12. Seasonal influenza vaccine is recommended annually through age 18.
Meningococcal conjugate vaccine for all at age 11–18
There is some evidence that MCV4 may be linked to a small risk of Guillain-Barré syndrome. Although this link has not been conclusively proven, a history of Guillain-Barré syndrome calls for caution in using MCV4. For those who have a history of this syndrome but need protection against meningococcal infection, the MPSV4 is an alternative.11
Pertussis: A Tdap booster at age 11–18
In addition, a greater percentage of cases now occurs in adolescents and young adults. Half of reported cases are now in those age 10 years and older. Most nonimmunized or incompletely immunized infants who develop pertussis were exposed to the disease by older household members, not by same-age cohorts. Since the disease presents as nonspecific cough in adolescents, it is often not diagnosed, and the incidence is probably much higher than the reported number of cases would indicate.
These trends were cause for public health concern and led to the development of pertussis-containing vaccine products for adolescents and adults. Two Tdap products are available: one is licensed for those ages 10 to 64 (Boostrix), the other for ages 11 to 64 (Adacel). Since 2005, the ACIP has recommended a single dose of Tdap for those age 11 to 18, preferably at 11 or 12 years.12 The optimal interval from the last tetanus-diphtheria shot is 5 years, but a shorter interval is acceptable. Thereafter, boosters with the tetanus toxoid and reduced-dose diphtheria toxoid (Td) vaccine are recommended every 10 years. If an adolescent has not previously received a complete series of a tetanus-diphtheria product, he or she should be given the recommended number of doses, only one of which should be Tdap, the others Td. The number and timing of doses can be found at www.cdc.gov/mmwr/preview/mmwrhtml/rr55e223a5.htm.
Human papillomavirus vaccination for girls age 11–12
HPV is sexually transmitted and causes genital warts, cervical cancer, and other oral, anal, and genital cancers.
HPV is the most common sexually transmitted infection in the United States, with over 6 million new cases each year.13 A study in 2003 to 2004 using HPV DNA typing of cervicovaginal swab specimens in a sample of women between the ages of 14 and 59 found an overall point prevalence of 26.8% of any HPV type.14 Those between 20 and 24 years had the highest prevalence at 44.8%. Those ages 14 to 19 had a prevalence of 24.5%. Several studies have reported a similar age-related increase in HPV prevalence.15,16
One survey found that nearly 25% of girls in the United States are sexually active by age 15, 40% by age 16, and 70% by age 18.17 The 2005 Behavioral Risk Survey found that nearly 4% of girls were sexually active before age 13, and by the ninth grade 5.7% of those who were sexually active had had four or more partners.18 To receive the full benefit from the HPV vaccine, it should be given before this risk of acquiring HPV occurs.
A quadrivalent HPV vaccine (HPV4) was first licensed in the United States in 2006 for use in girls and women 9 to 26 years old to prevent cervical, vulvar, and vaginal precancerous lesions and cancer, and for prevention of anogenital warts. It contains viral proteins from HPV types 6, 11, 16, and 18, the types currently responsible for 70% of cervical cancers and 90% of anogenital warts. The vaccine is prepared in a yeast substrate and contains an aluminum-based adjuvant.
HPV4 has proven highly effective in women ages 16 to 26 not previously exposed to the four HPV types in the vaccine. The end points used in these studies were cervical intraepithelial neoplasia grade 2 or 3, adenocarcinoma in situ, anogenital warts, and vulvar and vaginal intraepithelial neoplasms.13,19,20 The vaccine’s effectiveness has been 98% to 100% after 3 to 5 years. These trials are ongoing.
The vaccine’s efficacy in women with current or past HPV infection is less certain. Studies of this question have included only small numbers, and the confidence intervals are large and include 0. In intention-to-treat studies, its efficacy has been 39% to 46% for prevention of cervical intraepithelial neoplasia grade 2 or 3 or adenocarcinoma in situ caused by HPV-16 or HPV-18, 69% for prevention of HPV-16- or HPV-18-related vaginal intraepithelial neoplasia, and 68.5% for vaccine-type-related warts.13
The most common adverse effects of HPV4 have included redness, pain, and swelling at the injection site, which occur in about 20% of recipients.13 There is an increased risk of syncope immediately after the vaccine is given, and observation for 15 minutes after injection is recommended. A recent study suggested a link between the vaccine and venous thromboembolism. 21 The rate was 2 per million doses, and because many of the recipients also were taking oral contraceptives, their venous thromboembolism has not yet been definitively proven to be caused by the vaccine.
HPV4 is contraindicated in those who have experienced a severe allergic reaction to a previous dose or who have an allergy to a vaccine component. Vaccination should be deferred in those with moderate or severe acute illnesses.
In June 2006, the ACIP13 made the following recommendations for HPV4:
- Girls ages 11 to 12 years should be routinely vaccinated with three doses
- The series can start as early as age 9 years
- Women and girls age 13 to 26 who have not been previously vaccinated should receive catch-up vaccination
- Neither Papanicolaou (Pap) testing nor HPV screening is necessary before vaccination
- HPV4 can be given with other age-appropriate vaccines
- Vaccination does not change the recommendations for cervical cancer screening
- The recommendations remain the same regardless of abnormal Pap tests, positive HPV DNA tests, or warts.
There have been two very recent developments regarding HPV vaccines.
A bivalent vaccine (HPV2) has been licensed in the United States and approved for use in girls and women ages 10 to 25 for prevention of cervical cancer and precancerous lesions. It contains antigens against HPV-16 and HPV-18 but does not provide protection against genital warts. The ACIP has stated no preference for the bivalent or the quadrivalent vaccine for the prevention of cervical cancer and precancerous lesions.
HPV4 has also gained licensure for use in boys and men age 9 to 26 for the prevention of genital warts. The ACIP has not recommended it for routine use, leaving the decision to patients and physicians after weighing the potential benefits and costs.
VACCINE UPDATE FOR ADULTS
Four vaccines are now routinely recommended for adults:
- Seasonal influenza vaccine starting at age 50
- Pneumococcal polysaccharide vaccine (PPSV23) starting at age 65
- Herpes zoster vaccine starting at age 60
- A diphtheria and tetanus toxoid product every 10 years, with Tdap given once.22
The rest of the adult schedule is based on catch-up (measles, mumps, rubella, varicella) or risk (hepatitis A and B and meningococccal disease). Seasonal influenza and pneumococcal vaccinations are also recommended before ages 50 and 65, respectively, for those with certain risk conditions. The complete adult immunization schedule can be found on the US Centers for Disease Control and Prevention (CDC) Web site.22
One dose of Tdap instead of the next Td booster
The CDC now recommends that a single dose of Tdap should replace the next dose of Td for adults ages 19 to 64 as part of the every-10-year tetanus-diphtheria boosting recommendation and if indicated for wound management. 23 In addition, a single dose of Tdap should be given to adults who have close contact with infants less than 6 months of age. The optimal interval between this Tdap shot and the last Td booster is 2 years or greater, but shorter intervals are acceptable. Women of childbearing age should receive Tdap preconception or postpartum if they have not previously received it. Tdap is not approved for use during pregnancy. Health care workers should also receive a dose of Tdap if they have never received it previously and if their last Td booster was more than 2 years ago, although less than 2 years is acceptable.
Contraindications to Tdap include anaphylaxis to a vaccine component and encephalopathy occurring within 7 days of previously receiving a pertussis vaccine.
Herpes zoster vaccine for those age 60 and older
Shingles causes considerable morbidity in older adults. The lifetime risk is 25%, and onefourth of those with shingles develop postherpetic neuralgia.
Herpes zoster vaccine is a live-attenuated vaccine that requires only a single injection. It is licensed for use in those ages 60 and older, and the ACIP recommends its routine use.24 Its effectiveness is approximately 50% and is inversely related to age. The number of patients who need to be vaccinated to prevent one lifetime case of shingles is 17.
Contraindications to this vaccine include a prior anaphylactic reaction to gelatin or neomycin, compromised immunity due to disease or to immune-suppressive therapy including high-dose corticosteroids, and active tuberculosis.
Payment for this vaccine by Medicare is through Part D, creating some administrative difficulties for physicians’ offices.
Pneumococcal vaccination extended to smokers and people with asthma
The ACIP recently added two new groups for whom PPSV23 is recommended: smokers and those with asthma.25 Smoking poses as much of a risk for pneumococcal pneumonia as do diabetes and other chronic illnesses that are currently indications for the vaccine. The number needed to vaccinate to prevent one case of pneumonia among smokers is 10,000 in people ages 18 to 44, and 4,000 in those ages 45 to 64.26
The ACIP also clarified the recommendation for a second dose of PPSV23.25 A second dose 5 years after the first is recommended for those who have immune suppression, sickle cell disease, or asplenia. People over age 65 should receive a second dose if they were vaccinated more than 5 years previously and before age 65.
New uses for hepatitis A vaccine
A combined hepatitis A and hepatitis B vaccine (Twinrix) has received approval for an alternate, four-dose schedule at 0, 7, 21 days, and 12 months.27 It has previously only been approved for a three-dose schedule at 0, 1, and 6 months. The new alternative schedule allows greater protection for travelers who need to depart within less than 1 month.
For unvaccinated people who are acutely exposed to hepatitis A virus and for those traveling to areas of high prevalence who do not have time to complete the two doses of hepatitis A vaccine, the only prevention available until recently has been immune globulin. This has changed: hepatitis A vaccine can now be used in both groups. The new recommendations for postexposure prophylaxis is that either a single dose of hepatitis A vaccine or use of immune globulin is acceptable.28 In ages 12 months to 40 years, vaccine is preferred. For those over age 40, immune globulin is preferred, but vaccine is acceptable. For children younger than 12 months, the immune-suppressed, and those with chronic liver disease, immune globulin should be used.
Those traveling or working in countries with high rates of hepatitis A can be protected with either hepatitis A vaccine or immune globulin. A single dose of the vaccine is sufficient for healthy people, with a second dose at the recommended interval to complete the series. Those younger than 12 months and those who choose not to receive the vaccine, including those who are allergic to it, should be offered immune globulin. Both immune globulin and hepatitis A vaccine should be considered for certain patients who plan to travel within 2 weeks of the first vaccine dose, ie, those over age 40, those with compromised immunity, and those with chronic liver disease or other chronic conditions.
Hepatitis A vaccine is now also recommended for all unvaccinated people who anticipate close personal contact with an international adoptee during the first 60 days following arrival from countries with high or intermediate hepatitis A endemicity.29 The first dose should be given as soon as the adoption is planned and ideally at least 2 weeks before the child arrives.
The past 10 years have seen marked advances in vaccine research, resulting in more products being available. In 1983 the childhood vaccination schedule included protection against seven diseases: polio, tetanus, diphtheria, pertussis, measles, mumps, and rubella. The schedule in 2010 includes protection against organisms that cause seven more: Haemophilus influenzae, hepatitis A, hepatitis B, influenza, meningococcus, pneumococcus, and varicella.1 In addition, new vaccine products are available for adolescents, offering protection against meningococcus, seasonal influenza, and human papillomavirus (HPV) and extending the length of protection against pertussis. For adults, a vaccine now protects against shingles, and several products offer boosting of pertussis immunity.
This rapid growth in the number of recommended vaccine products has made it challenging for practicing physicians to stay current on and to implement the ever-changing recommendations. The purpose of this article is to summarize the additions and changes over the past 3 years to the schedules of recommended vaccines for children, adolescents, and adults.

VACCINE UPDATE FOR CHILDREN
The recent changes to the childhood immunization schedule have added protection against rotavirus and seasonal influenza and have expanded the protection against hepatitis A and varicella.
Rotavirus vaccination for infants
Rotavirus is the leading cause of infectious gastroenteritis in infants. It causes significant morbidity and expense, accounting for 2.7 million episodes per year in the United States, 410,000 outpatient or office visits, 201,000 to 272,000 emergency department visits, 55,000 to 70,000 hospitalizations, and 20 to 60 deaths.2 Although the number of deaths in the United States is not large, rotavirus is a leading cause of infant deaths around the world.
Rotavirus vaccination is challenging because of the time frame in which the series needs to be given. The first dose has to be given after 6 weeks of age but before 15 weeks of age, and the last dose should be given before 8 months of age, with a minimum of 4 weeks between doses. It is preferable to use the same product to finish the series. They can be used interchangeably, but this then requires three total doses.
The effectiveness of the vaccine in preventing rotavirus gastroenteritis in the first year after vaccination was greater than 80% in most studies and approached 100% in preventing serious gastroenteritis.2
Those vaccinated appear to have a slightly higher rate of diarrhea and vomiting in the first 42 days after vaccination. Safety monitoring after the products were licensed has not shown an increased rate of intussusception with either product.
The only contraindication to the vaccines is a serious allergic reaction to them or to one of their components. They should be used with caution in patients who have suppressed immunity, acute gastroenteritis, preexisting gastrointestinal disease, or previous intussusception.
Seasonal influenza vaccine extended to ages 5–18
Gradually, we seem to be moving toward vaccinating everyone every year against seasonal influenza. Previously, vaccination was recommended for children age 6 months through 4 years; in 2008, the Advisory Committee on Immunization Practices (ACIP) extended the recommendation to the age group 5 through 18 years.3
Two types of seasonal influenza vaccine are available: trivalent influenza vaccine (TIV), which contains killed virus and is given by injection, and live-attenuated seasonal influenza vaccine (LAIV), which is given by nasal spray. Both contain the same three seasonal influenza antigens, selected each year by a team of experts. TIV is licensed for those age 6 months and older, and LAIV is licensed for ages 2 through 49 years.4
Since LAIV contains a live-attenuated virus, it should not be used in anyone who has a chronic illness (including those under the age of 5 with recurrent wheezing, those with suppressed immunity, and those with a history of Guillain-Barré syndrome); in pregnant women; or those who have close contact with anyone who is immune-suppressed. The injection is contraindicated for those who have had a serious allergic reaction to eggs.
Children younger than 9 years should receive two doses of either type of vaccine the first year they are vaccinated. Those who receive only one dose the first year they are vaccinated should receive two doses the next year. If they fail to receive two doses in the next year, only a single dose is recommended after that. This is a slight modification of the previous recommendation that only one dose be given the second year if only one dose was given the first year.5
Hepatitis A vaccine at age 12–23 months
An inactivated hepatitis A vaccine (HepA) was first licensed in 1995; another was licensed in 1996. Recommendations for their use have been revised periodically, and their widespread use has resulted in a marked reduction in the incidence of hepatitis A virus infection.
The current recommendation is that all children be vaccinated at age 12 to 23 months. In addition, in areas of high prevalence, vaccine is recommended for older children who have not been vaccinated. Other target groups are those at higher risk of hepatitis A, including travelers to endemic areas, users of illicit drugs, and men who have sex with men.6 Indications for vaccination before travel, after exposure to hepatitis A infection, and in families of international adoptees are covered later in this paper in a discussion about vaccinations in adults.
Varicella at 12–15 months and 4–6 years, with catch-up for others
Before varicella vaccine was licensed in 1995, 4 million cases of varicella infection (chickenpox) were reported in the United States each year, resulting in thousands of hospitalizations and more than 100 deaths. The vaccine is now widely used, with a coverage rate of 88%, and it has proven to be 85% effective.7 The result was a marked decrease in the incidence of varicella and in varicella-related hospitalizations and deaths.
In spite of this success, the number of varicella cases has remained constant over the past few years, and sporadic outbreaks continue to occur, predominantly in schools, even schools in which a high percentage of the children are vaccinated.7,8 These outbreaks have involved infections in unvaccinated children and also “breakthrough disease” in children who have been vaccinated. If someone who has received one dose of vaccine is exposed to varicella, the risk of a breakthrough infection is about 15%.9 A two-dose series of varicella vaccine reduces the risk by about 75%.7 Breakthrough disease is usually milder than infection in the unvaccinated, with fewer skin lesions, milder symptoms, and fewer complications, but those affected are still infectious to others.
In 2005 and 2006, this ongoing risk of varicella prompted the ACIP to consider and recommend several new control measures:
- Two doses of varicella vaccine for all children, the first dose at age 12 to 15 months and the second at age 4 to 6 years—the same schedule as for immunization against measles, mumps, and rubella
- Two doses of varicella vaccine, the second given 4 to 8 weeks after the first, for all adolescents and adults who have no evidence of immunity
- A catch-up second dose for everyone who received one dose previously
- Screening for varicella immunity in pregnant women and postpartum vaccination with two doses for those who are not immune, the first dose given before discharge and the second dose 4 to 8 weeks later.
VACCINE UPDATE FOR ADOLESCENTS
A number of vaccines are now available and recommended for routine use in adolescents.9 These include HPV vaccine for girls, quadrivalent meningococcal conjugate vaccine (MCV4), and combined tetanus toxoid, reduced-dose diphtheria toxoid, and acellular pertussis (Tdap). All these are now recommended routinely at age 11 or 12. Seasonal influenza vaccine is recommended annually through age 18.
Meningococcal conjugate vaccine for all at age 11–18
There is some evidence that MCV4 may be linked to a small risk of Guillain-Barré syndrome. Although this link has not been conclusively proven, a history of Guillain-Barré syndrome calls for caution in using MCV4. For those who have a history of this syndrome but need protection against meningococcal infection, the MPSV4 is an alternative.11
Pertussis: A Tdap booster at age 11–18
In addition, a greater percentage of cases now occurs in adolescents and young adults. Half of reported cases are now in those age 10 years and older. Most nonimmunized or incompletely immunized infants who develop pertussis were exposed to the disease by older household members, not by same-age cohorts. Since the disease presents as nonspecific cough in adolescents, it is often not diagnosed, and the incidence is probably much higher than the reported number of cases would indicate.
These trends were cause for public health concern and led to the development of pertussis-containing vaccine products for adolescents and adults. Two Tdap products are available: one is licensed for those ages 10 to 64 (Boostrix), the other for ages 11 to 64 (Adacel). Since 2005, the ACIP has recommended a single dose of Tdap for those age 11 to 18, preferably at 11 or 12 years.12 The optimal interval from the last tetanus-diphtheria shot is 5 years, but a shorter interval is acceptable. Thereafter, boosters with the tetanus toxoid and reduced-dose diphtheria toxoid (Td) vaccine are recommended every 10 years. If an adolescent has not previously received a complete series of a tetanus-diphtheria product, he or she should be given the recommended number of doses, only one of which should be Tdap, the others Td. The number and timing of doses can be found at www.cdc.gov/mmwr/preview/mmwrhtml/rr55e223a5.htm.
Human papillomavirus vaccination for girls age 11–12
HPV is sexually transmitted and causes genital warts, cervical cancer, and other oral, anal, and genital cancers.
HPV is the most common sexually transmitted infection in the United States, with over 6 million new cases each year.13 A study in 2003 to 2004 using HPV DNA typing of cervicovaginal swab specimens in a sample of women between the ages of 14 and 59 found an overall point prevalence of 26.8% of any HPV type.14 Those between 20 and 24 years had the highest prevalence at 44.8%. Those ages 14 to 19 had a prevalence of 24.5%. Several studies have reported a similar age-related increase in HPV prevalence.15,16
One survey found that nearly 25% of girls in the United States are sexually active by age 15, 40% by age 16, and 70% by age 18.17 The 2005 Behavioral Risk Survey found that nearly 4% of girls were sexually active before age 13, and by the ninth grade 5.7% of those who were sexually active had had four or more partners.18 To receive the full benefit from the HPV vaccine, it should be given before this risk of acquiring HPV occurs.
A quadrivalent HPV vaccine (HPV4) was first licensed in the United States in 2006 for use in girls and women 9 to 26 years old to prevent cervical, vulvar, and vaginal precancerous lesions and cancer, and for prevention of anogenital warts. It contains viral proteins from HPV types 6, 11, 16, and 18, the types currently responsible for 70% of cervical cancers and 90% of anogenital warts. The vaccine is prepared in a yeast substrate and contains an aluminum-based adjuvant.
HPV4 has proven highly effective in women ages 16 to 26 not previously exposed to the four HPV types in the vaccine. The end points used in these studies were cervical intraepithelial neoplasia grade 2 or 3, adenocarcinoma in situ, anogenital warts, and vulvar and vaginal intraepithelial neoplasms.13,19,20 The vaccine’s effectiveness has been 98% to 100% after 3 to 5 years. These trials are ongoing.
The vaccine’s efficacy in women with current or past HPV infection is less certain. Studies of this question have included only small numbers, and the confidence intervals are large and include 0. In intention-to-treat studies, its efficacy has been 39% to 46% for prevention of cervical intraepithelial neoplasia grade 2 or 3 or adenocarcinoma in situ caused by HPV-16 or HPV-18, 69% for prevention of HPV-16- or HPV-18-related vaginal intraepithelial neoplasia, and 68.5% for vaccine-type-related warts.13
The most common adverse effects of HPV4 have included redness, pain, and swelling at the injection site, which occur in about 20% of recipients.13 There is an increased risk of syncope immediately after the vaccine is given, and observation for 15 minutes after injection is recommended. A recent study suggested a link between the vaccine and venous thromboembolism. 21 The rate was 2 per million doses, and because many of the recipients also were taking oral contraceptives, their venous thromboembolism has not yet been definitively proven to be caused by the vaccine.
HPV4 is contraindicated in those who have experienced a severe allergic reaction to a previous dose or who have an allergy to a vaccine component. Vaccination should be deferred in those with moderate or severe acute illnesses.
In June 2006, the ACIP13 made the following recommendations for HPV4:
- Girls ages 11 to 12 years should be routinely vaccinated with three doses
- The series can start as early as age 9 years
- Women and girls age 13 to 26 who have not been previously vaccinated should receive catch-up vaccination
- Neither Papanicolaou (Pap) testing nor HPV screening is necessary before vaccination
- HPV4 can be given with other age-appropriate vaccines
- Vaccination does not change the recommendations for cervical cancer screening
- The recommendations remain the same regardless of abnormal Pap tests, positive HPV DNA tests, or warts.
There have been two very recent developments regarding HPV vaccines.
A bivalent vaccine (HPV2) has been licensed in the United States and approved for use in girls and women ages 10 to 25 for prevention of cervical cancer and precancerous lesions. It contains antigens against HPV-16 and HPV-18 but does not provide protection against genital warts. The ACIP has stated no preference for the bivalent or the quadrivalent vaccine for the prevention of cervical cancer and precancerous lesions.
HPV4 has also gained licensure for use in boys and men age 9 to 26 for the prevention of genital warts. The ACIP has not recommended it for routine use, leaving the decision to patients and physicians after weighing the potential benefits and costs.
VACCINE UPDATE FOR ADULTS
Four vaccines are now routinely recommended for adults:
- Seasonal influenza vaccine starting at age 50
- Pneumococcal polysaccharide vaccine (PPSV23) starting at age 65
- Herpes zoster vaccine starting at age 60
- A diphtheria and tetanus toxoid product every 10 years, with Tdap given once.22
The rest of the adult schedule is based on catch-up (measles, mumps, rubella, varicella) or risk (hepatitis A and B and meningococccal disease). Seasonal influenza and pneumococcal vaccinations are also recommended before ages 50 and 65, respectively, for those with certain risk conditions. The complete adult immunization schedule can be found on the US Centers for Disease Control and Prevention (CDC) Web site.22
One dose of Tdap instead of the next Td booster
The CDC now recommends that a single dose of Tdap should replace the next dose of Td for adults ages 19 to 64 as part of the every-10-year tetanus-diphtheria boosting recommendation and if indicated for wound management. 23 In addition, a single dose of Tdap should be given to adults who have close contact with infants less than 6 months of age. The optimal interval between this Tdap shot and the last Td booster is 2 years or greater, but shorter intervals are acceptable. Women of childbearing age should receive Tdap preconception or postpartum if they have not previously received it. Tdap is not approved for use during pregnancy. Health care workers should also receive a dose of Tdap if they have never received it previously and if their last Td booster was more than 2 years ago, although less than 2 years is acceptable.
Contraindications to Tdap include anaphylaxis to a vaccine component and encephalopathy occurring within 7 days of previously receiving a pertussis vaccine.
Herpes zoster vaccine for those age 60 and older
Shingles causes considerable morbidity in older adults. The lifetime risk is 25%, and onefourth of those with shingles develop postherpetic neuralgia.
Herpes zoster vaccine is a live-attenuated vaccine that requires only a single injection. It is licensed for use in those ages 60 and older, and the ACIP recommends its routine use.24 Its effectiveness is approximately 50% and is inversely related to age. The number of patients who need to be vaccinated to prevent one lifetime case of shingles is 17.
Contraindications to this vaccine include a prior anaphylactic reaction to gelatin or neomycin, compromised immunity due to disease or to immune-suppressive therapy including high-dose corticosteroids, and active tuberculosis.
Payment for this vaccine by Medicare is through Part D, creating some administrative difficulties for physicians’ offices.
Pneumococcal vaccination extended to smokers and people with asthma
The ACIP recently added two new groups for whom PPSV23 is recommended: smokers and those with asthma.25 Smoking poses as much of a risk for pneumococcal pneumonia as do diabetes and other chronic illnesses that are currently indications for the vaccine. The number needed to vaccinate to prevent one case of pneumonia among smokers is 10,000 in people ages 18 to 44, and 4,000 in those ages 45 to 64.26
The ACIP also clarified the recommendation for a second dose of PPSV23.25 A second dose 5 years after the first is recommended for those who have immune suppression, sickle cell disease, or asplenia. People over age 65 should receive a second dose if they were vaccinated more than 5 years previously and before age 65.
New uses for hepatitis A vaccine
A combined hepatitis A and hepatitis B vaccine (Twinrix) has received approval for an alternate, four-dose schedule at 0, 7, 21 days, and 12 months.27 It has previously only been approved for a three-dose schedule at 0, 1, and 6 months. The new alternative schedule allows greater protection for travelers who need to depart within less than 1 month.
For unvaccinated people who are acutely exposed to hepatitis A virus and for those traveling to areas of high prevalence who do not have time to complete the two doses of hepatitis A vaccine, the only prevention available until recently has been immune globulin. This has changed: hepatitis A vaccine can now be used in both groups. The new recommendations for postexposure prophylaxis is that either a single dose of hepatitis A vaccine or use of immune globulin is acceptable.28 In ages 12 months to 40 years, vaccine is preferred. For those over age 40, immune globulin is preferred, but vaccine is acceptable. For children younger than 12 months, the immune-suppressed, and those with chronic liver disease, immune globulin should be used.
Those traveling or working in countries with high rates of hepatitis A can be protected with either hepatitis A vaccine or immune globulin. A single dose of the vaccine is sufficient for healthy people, with a second dose at the recommended interval to complete the series. Those younger than 12 months and those who choose not to receive the vaccine, including those who are allergic to it, should be offered immune globulin. Both immune globulin and hepatitis A vaccine should be considered for certain patients who plan to travel within 2 weeks of the first vaccine dose, ie, those over age 40, those with compromised immunity, and those with chronic liver disease or other chronic conditions.
Hepatitis A vaccine is now also recommended for all unvaccinated people who anticipate close personal contact with an international adoptee during the first 60 days following arrival from countries with high or intermediate hepatitis A endemicity.29 The first dose should be given as soon as the adoption is planned and ideally at least 2 weeks before the child arrives.
- Centers for Disease Control and Prevention (CDC). Recommended immunization schedule for persons aged 0 through 6 years—United States 2009. www.cdc.gov/vaccines/recs/schedules/downloads/child/2009/09_0-6yrs_schedule_pr.pdf. Accessed March 6, 2010.
- Cortese MM, Parashar UDCenters for Disease Control and Prevention (CDC). Prevention of rotavirus gastroenteritis among infants and children: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2009; 58( RR-2):1–25.
- Fiore AE, Shay DK, Broder K, et al., Centers for Disease Control and Prevention (CDC). Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2009. MMWR Recomm Rep 2009; 58( RR–8):1–52.
- Centers for Disease Control and Prevention (CDC). Notice to readers: expansion of use of live attenuated influenza vaccine (FluMist®) to children aged 2–4 years and other FluMist changes for the 2007–08 influenza season. MMWR Morb Mortal Wkly Rep 2007; 56( 46):1217–1219.
- Fiore AE, Shay DK, Haber PCenters for Disease Control and Prevention (CDC). Prevention and control of influenza: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2007. MMWR Recomm Rep 2007; 56( RR–6):1–54.
- Fiore AE, Wasley A, Bell BPAdvisory Committee on Immunization Practices (ACIP). Prevention of hepatitis A through active or passive immunization: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2006; 55( RR–7):1–23.
- Marin M, Güris D, Chaves SS, Schmid S, Seward JFAdvisory Committee on Immunization Practices. Prevention of varicella: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2007; 56( RR–4):1–40.
- Centers for Disease Control and Prevention (CDC). Varicella disease. www.cdc.gov/vaccines/vpd-vac/varicella/dis-faqs-clinic.htm. Accessed March 4, 2010.
- Centers for Disease Control and Prevention (CDC). 2009 child & adolescent immunization schedules. www.cdc.gov/vaccines/recs/schedules/child-schedule.htm. Accessed March 4, 2010.
- Centers for Disease Control and Prevention (CDC) Advisory Committee on Immunization Practices. Revised recommendations of the Advisory Committee on Immunization Practices to vaccinate all persons aged 11-18 years with meningococcal conjugate vaccine. MMWR Morb Mortal Wkly Rep 2007; 56( 31):794–795.
- Centers for Disease Control and Prevention (CDC). Update: Guillain-Barré syndrome among recipients of menactra meningococcal conjugate vaccine—United States, June 2005–September 2006. MMWR Morb Mortal Wkly Rep 2006; 55( 41):1120–1124.
- Broder KR, Cortese MM, Iskander JK, et al., Advisory Committee on Immunization Practices (ACIP). Preventing tetanus, diphtheria, and pertussis among adolescents: use of tetanus toxoid, reduced diphtheria toxoid and acellular pertussis vaccines recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2006; 55( RR–3):1–34.
- Markowitz LE, Dunne EF, Saraiya M, Lawson HW, Chesson H, Unger ERCenters for Disease Control and Prevention (CDC). Quadrivalent human papillomavirus vaccine: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2007; 56( RR–2):1–24.
- Dunne EF, Unger ER, Sternberg M, et al Prevalence of HPV infection among females in the United States. JAMA 2007; 297:813–819.
- Trottier H, Franco EL. The epidemiology of genital human papillomavirus infection. Vaccine 2006; 24( suppl 1):S1–S15.
- Stone KM, Karem KL, Sternberg MR, et al Seroprevalence of human papillomavirus type 16 infection in the United States. J Infect Dis 2002; 186:1396–1402.
- Abma JC, Martinez GM, Mosher WD, Dawson BS. Teenagers in the United States: sexual activity, contraceptive use, and childbearing, 2002. Vital Health Stat 23 2004; 24:1–48.
- Eaton DK, Kann L, Kinchen S, et al Youth risk behavior surveillance—United States, 2005. MMWR Surveill Summ 2006; 55:1–108.
- Human papillomavirus vaccines. WHO position paper. Wkly Epidemiol Rec 2009; 84:118–131.
- Rambout L, Hopkins L, Hutton B, Fergusson D. Prophylactic vaccination against human papillomavirus infection and disease in women: a systematic review of randomized controlled trials. CMAJ 2007; 177:469–479.
- Slade BA, Leidel L, Vellozzi C, et al Postlicensure safety surveillance for quadrivalent human papillomavirus recombinant vaccine. JAMA 2009; 302:750–757.
- Centers for Disease Control (CDC). Adult immunization schedule. http://www.cdc.gov/vaccines/recs/schedules/adult-schedule.htm. Accessed March 4, 2010.
- Kretsinger K, Broder KR, Cortese MM, et al., Centers for Disease Control and Prevention. Preventing tetanus, diphtheria, and pertussis among adults: use of tetanus toxoid, reduced diphtheria toxoid and acellular pertussis vaccine recommendations of the Advisory Committee on Immunization Practices (ACIP) and recommendation of ACIP, supported by the Healthcare Infection Control Practices Advisory Committee (HICPAC), for use of Tdap among health-care personnel. MMWR Recomm Rep 2006; 55( RR–17):1–37.
- Harpaz R, Ortega-Sanchez IR, Seward JFAdvisory Committee on Immunization Practices (ACIP). Prevention of herpes zoster: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2008; 57( RR–5):1–30.
- Centers for Disease Control (CDC). ACIP provisional recommendations for use of pneumococcal vaccines. www.cdc.gov/vaccines/recs/provisional/downloads/pneumo-Oct-2008-508.pdf. Accessed March 4, 2010.
- Centers for Disease Control and Prevention (CDC). Summary Report: October 22–23, 2008; Atlanta, Georgia. www.cdc.gov/vaccines/recs/ACIP/downloads/min=archive/min-oct08.pdf. Accessed March 6, 2010.
- CDC. Notice to readers: FDA approval of an alternate dosing schedule for a combined hepatitis A and B vaccine (Twinrix®). MMWR Morb Mortal Wkly Rep 2007; 56( 40);1057.
- Advisory Committee on Immunization Practices (ACIP) Centers for Disease Control and Prevention (CDC). Update: prevention of hepatitis A after exposure to hepatitis A virus and in international travelers. Updated recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 2007; 56( 41):1080–1084.
- Centers for Disease Control and Prevention (CDC). Updated recommendations from the Advisory Committee on Immunization Practices (ACIP) for use of hepatitis A vaccine in close contacts of newly arriving international adoptees. MMWR 2009: 58:1006–1007.
- Centers for Disease Control and Prevention (CDC). Recommended immunization schedule for persons aged 0 through 6 years—United States 2009. www.cdc.gov/vaccines/recs/schedules/downloads/child/2009/09_0-6yrs_schedule_pr.pdf. Accessed March 6, 2010.
- Cortese MM, Parashar UDCenters for Disease Control and Prevention (CDC). Prevention of rotavirus gastroenteritis among infants and children: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2009; 58( RR-2):1–25.
- Fiore AE, Shay DK, Broder K, et al., Centers for Disease Control and Prevention (CDC). Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2009. MMWR Recomm Rep 2009; 58( RR–8):1–52.
- Centers for Disease Control and Prevention (CDC). Notice to readers: expansion of use of live attenuated influenza vaccine (FluMist®) to children aged 2–4 years and other FluMist changes for the 2007–08 influenza season. MMWR Morb Mortal Wkly Rep 2007; 56( 46):1217–1219.
- Fiore AE, Shay DK, Haber PCenters for Disease Control and Prevention (CDC). Prevention and control of influenza: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2007. MMWR Recomm Rep 2007; 56( RR–6):1–54.
- Fiore AE, Wasley A, Bell BPAdvisory Committee on Immunization Practices (ACIP). Prevention of hepatitis A through active or passive immunization: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2006; 55( RR–7):1–23.
- Marin M, Güris D, Chaves SS, Schmid S, Seward JFAdvisory Committee on Immunization Practices. Prevention of varicella: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2007; 56( RR–4):1–40.
- Centers for Disease Control and Prevention (CDC). Varicella disease. www.cdc.gov/vaccines/vpd-vac/varicella/dis-faqs-clinic.htm. Accessed March 4, 2010.
- Centers for Disease Control and Prevention (CDC). 2009 child & adolescent immunization schedules. www.cdc.gov/vaccines/recs/schedules/child-schedule.htm. Accessed March 4, 2010.
- Centers for Disease Control and Prevention (CDC) Advisory Committee on Immunization Practices. Revised recommendations of the Advisory Committee on Immunization Practices to vaccinate all persons aged 11-18 years with meningococcal conjugate vaccine. MMWR Morb Mortal Wkly Rep 2007; 56( 31):794–795.
- Centers for Disease Control and Prevention (CDC). Update: Guillain-Barré syndrome among recipients of menactra meningococcal conjugate vaccine—United States, June 2005–September 2006. MMWR Morb Mortal Wkly Rep 2006; 55( 41):1120–1124.
- Broder KR, Cortese MM, Iskander JK, et al., Advisory Committee on Immunization Practices (ACIP). Preventing tetanus, diphtheria, and pertussis among adolescents: use of tetanus toxoid, reduced diphtheria toxoid and acellular pertussis vaccines recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2006; 55( RR–3):1–34.
- Markowitz LE, Dunne EF, Saraiya M, Lawson HW, Chesson H, Unger ERCenters for Disease Control and Prevention (CDC). Quadrivalent human papillomavirus vaccine: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2007; 56( RR–2):1–24.
- Dunne EF, Unger ER, Sternberg M, et al Prevalence of HPV infection among females in the United States. JAMA 2007; 297:813–819.
- Trottier H, Franco EL. The epidemiology of genital human papillomavirus infection. Vaccine 2006; 24( suppl 1):S1–S15.
- Stone KM, Karem KL, Sternberg MR, et al Seroprevalence of human papillomavirus type 16 infection in the United States. J Infect Dis 2002; 186:1396–1402.
- Abma JC, Martinez GM, Mosher WD, Dawson BS. Teenagers in the United States: sexual activity, contraceptive use, and childbearing, 2002. Vital Health Stat 23 2004; 24:1–48.
- Eaton DK, Kann L, Kinchen S, et al Youth risk behavior surveillance—United States, 2005. MMWR Surveill Summ 2006; 55:1–108.
- Human papillomavirus vaccines. WHO position paper. Wkly Epidemiol Rec 2009; 84:118–131.
- Rambout L, Hopkins L, Hutton B, Fergusson D. Prophylactic vaccination against human papillomavirus infection and disease in women: a systematic review of randomized controlled trials. CMAJ 2007; 177:469–479.
- Slade BA, Leidel L, Vellozzi C, et al Postlicensure safety surveillance for quadrivalent human papillomavirus recombinant vaccine. JAMA 2009; 302:750–757.
- Centers for Disease Control (CDC). Adult immunization schedule. http://www.cdc.gov/vaccines/recs/schedules/adult-schedule.htm. Accessed March 4, 2010.
- Kretsinger K, Broder KR, Cortese MM, et al., Centers for Disease Control and Prevention. Preventing tetanus, diphtheria, and pertussis among adults: use of tetanus toxoid, reduced diphtheria toxoid and acellular pertussis vaccine recommendations of the Advisory Committee on Immunization Practices (ACIP) and recommendation of ACIP, supported by the Healthcare Infection Control Practices Advisory Committee (HICPAC), for use of Tdap among health-care personnel. MMWR Recomm Rep 2006; 55( RR–17):1–37.
- Harpaz R, Ortega-Sanchez IR, Seward JFAdvisory Committee on Immunization Practices (ACIP). Prevention of herpes zoster: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2008; 57( RR–5):1–30.
- Centers for Disease Control (CDC). ACIP provisional recommendations for use of pneumococcal vaccines. www.cdc.gov/vaccines/recs/provisional/downloads/pneumo-Oct-2008-508.pdf. Accessed March 4, 2010.
- Centers for Disease Control and Prevention (CDC). Summary Report: October 22–23, 2008; Atlanta, Georgia. www.cdc.gov/vaccines/recs/ACIP/downloads/min=archive/min-oct08.pdf. Accessed March 6, 2010.
- CDC. Notice to readers: FDA approval of an alternate dosing schedule for a combined hepatitis A and B vaccine (Twinrix®). MMWR Morb Mortal Wkly Rep 2007; 56( 40);1057.
- Advisory Committee on Immunization Practices (ACIP) Centers for Disease Control and Prevention (CDC). Update: prevention of hepatitis A after exposure to hepatitis A virus and in international travelers. Updated recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 2007; 56( 41):1080–1084.
- Centers for Disease Control and Prevention (CDC). Updated recommendations from the Advisory Committee on Immunization Practices (ACIP) for use of hepatitis A vaccine in close contacts of newly arriving international adoptees. MMWR 2009: 58:1006–1007.
KEY POINTS
- New recommendations for infants and children:
- Rotavirus vaccination for infants
- Seasonal influenza vaccine yearly at ages 5–18
- Hepatitis A vaccine at age 12–23 months
- Varicella vaccine at 12–15 months and again at 4–6 years, with catch-up for others.
- New recommendations for adolescents:
- Meningococcus quadrivalent conjugate vaccine for all at age 11 or 12 and catch-up through age 18
- A shot of tetanus toxoid, reduced-dose diphtheria toxoid, and acellular pertussis vaccine (Tdap) at age 11 or 12 and catch-up through age 18
- Human papillomavirus vaccine (three doses) for girls at age 11 or 12 and catch-up through age 26.
- New recommendations for adults:
- One dose of Tdap instead of the next tetanus-diphtheria booster
- Herpes zoster vaccine at age 60 or older
- Pneumococcal vaccination extended to smokers and people with asthma, with a second dose 5 years after the first for people who have immune suppression, sickle cell disease, or asplenia.
ACIP immunization update
The Advisory Committee on Immunization Practices (ACIP) made a number of major new recommendations last year. These new recommendations address:
- expanded use of hepatitis A virus (HAV) vaccine
- preferences for combination vaccines
- timing of poliovirus vaccine doses
- resumption of the normal Haemophilus influenzae Type b (Hib) schedule, as shortages have resolved
- the use of a new bivalent human papilloma virus (HPV2) vaccine in women and quadrivalent (HPV4) vaccine in men
- a reduced-dose schedule for rabies postexposure prophylaxis
- proof of immunity against mumps, measles, and rubella for health care workers
- recommendations for meningococcal vaccine boosters.
Adoptive families need more protection against HAV
Each year, approximately 18,000 children are adopted from foreign countries, almost all of them born in countries with high or intermediate rates of HAV, 85% of them under 5 years of age.1 Identifying adoptees with an acute HAV infection is problematic, because in this age group, fewer than 10% of infected children manifest jaundice.1 The Centers for Disease Control and Prevention (CDC) has recorded a small number of cases of acute HAV infection traced back to exposure to adoptees, and there is some evidence that 1% to 6% of new international adoptees have acute, and infectious, HAV.1
In response to these data, the most recent ACIP recommendation expands indications for HAV vaccine to include anyone who will be in close personal contact—living in the same household or providing regular babysitting—with an adoptee from any country with high or intermediate endemic rates of HAV. The vaccine should be given within the first 60 days of the adoptee’s arrival in the United States.1The first dose of the 2-dose series should be given as soon as the adoption is planned, ideally 2 or more weeks before exposure to the adoptee.
This new recommendation adds to earlier expansions of indications for HAV vaccine, which include universal use in children, use in postexposure prophylaxis, and preexposure protection for travelers.2,3
ACIP still prefers combination vaccines, with caveats
Increasing numbers of vaccine products with multiple antigens have reduced the number of injections needed to complete the recommended childhood immunization schedule. These new products also create a situation in which parents and physicians have to choose between using the combination products or staying with component vaccines that contain fewer antigens, but necessitate a larger number of injections.
When ACIP considered this dilemma, committee members gave the general preference to combination vaccines. At the same time, the committee acknowledged that many considerations—storage, costs, number of injections, vaccine availability, vaccination status, likelihood of improved coverage, likelihood of return visits, patient preference, and the potential for adverse events—factor into the decision.4
MMRV is a special case. One combination product received special attention because of the potential for increased rates of febrile seizures. Combined measles, mumps, rubella, and varicella (MMRV) vaccine is currently in short supply, but when the supply improves it will provide 1 less injection to immunize against 4 childhood viral infections at each of 2 visits. However, there is good evidence that in children 1 to 2 years of age who are receiving the first dose of MMRV, there is an additional incidence of febrile seizures of 1 in every 2300 to 2600, compared with children receiving separate doses of MMR and varicella vaccines.5 There is no increased risk for older children or for the second dose.
ACIP considered this risk and recommends discussing the benefits and risks of MMR and varicella separately vs using the MMRV combination vaccine. The committee notes: “Use of MMR and varicella vaccines avoids [the] increased risk for fever and febrile seizures following MMRV vaccine.”5
IPV combination dosing is clarified
The inclusion of inactivated poliovirus (IPV) antigen into new combination vaccine products has caused some confusion over the recommended dosing schedule of polio vaccine. ACIP has now clarified that for the recommended 4-dose IPV schedule, the fourth dose should be administered after age 4 and at least 6 months after dose 3. In addition, the minimal intervals (4 weeks) in the first 6 months of life should be used only for those traveling overseas.6
Resume normal Hib schedule
With the licensure of a new Hib product (Hiberix, GlaxoSmithKline) for the booster dose of Hib starting at age 15 months, the supply of Hib vaccine has stabilized. Supply is now adequate to resume all 4 doses in the routine schedule and to recall all children who had their booster dose deferred. Children can be vaccinated with Hib through the age of 59 months (prior to their fifth birthday).7
2 HPV vaccines are now available
With the licensure of an HPV2 vaccine for use in women in the United States (Cervarix, GlaxoSmithKline), 2 HPV vaccine products are now available for use.8 An HPV4 vaccine (Gardasil, Merck & Co.) was licensed in 2006. The TABLE compares the composition, dosing schedules, and precaution for these 2 products. Each requires 3 doses, but the age ranges and dosing schedules are slightly different. The HPV4 vaccine contains antigens against HPV types 16 and 18, which cause 70% of cervical cancers and precancerous lesions, and types 6 and 11, which cause 90% of anogenital warts.9
The HPV2 vaccine contains antigens for HPV types 16 and 18 only and does not protect against warts. The bivalent product appears to produce a higher level of antibody response and may provide better cross protection against other HPV types. ACIP compared effectiveness studies of both vaccines and decided to show no preference for either vaccine for the prevention of cervical cancer and precancerous lesions.
TABLE
HPV vaccines: A side-by-side comparison
| HPV4 | HPV2 | |
|---|---|---|
| Year licensed | 2006 | 2009 |
| Virus-like particle types | 6, 11, 16, 18 | 16,18 |
| Hypersensitivity-related contraindication | Yeast | Latex |
| Schedule | 0, 2, 6 months | 0, 1, 6 months |
| Age range | 9-26 years | 10-25 years |
The recommendation is for routine vaccination with an HPV product for all adolescent girls ages 11 to 12, with catch-up through age 26. If a female wants protection against anogenital warts, HPV4 is recommended. It is preferable to complete a 3-dose series with the same product, but if this is not possible, a series can be completed with the other product. The HPV4 vaccine is made using yeast, and prefilled HPV2 syringes contain latex. Hypersensitivity to these substances is a contraindication to their use. Patients who receive either vaccine should be observed for 15 minutes after the injection to prevent injury from syncope.
HPV4 in men. The HPV4 vaccine has now been licensed in the United States for use in males ages 9 to 26 to prevent anogenital warts. It may also protect against HPV-caused cancers (oral, genital, and anal), but the proof of that is still lacking. ACIP debated whether to recommend HPV4 for boys routinely at age 11 to 12 and decided against this. Instead the group voted for a “permissive” recommendation that states HPV4 may be given to adolescents and young men ages 9 to 26 to prevent warts and that protection is better if it is administered before exposure.10 This allows vaccine use in young males to be provided in the Vaccines for Children Program, but falls short of including it in the routine vaccine schedules.
The reasons for not recommending HPV4 routinely in young men were the cost and the perception that anogenital warts are primarily a cosmetic problem, although it was acknowledged that they can cause serious psychological morbidity. ACIP acknowledged that using HPV4 in men might lead to more protection for women because viral spread would be reduced, but stated that much more protection for women would be gained from a higher level of vaccination among women. As the evidence of protection against HPV-related cancers in men is gathered, ACIP will probably revisit this recommendation.
For a more detailed discussion of the issues posed by these 2 vaccines, see “The case for HPV immunization” in the Journal of Family Practice, December 2009.11
Rabies vaccine: 4 doses are sufficient
Due to a threatened shortage of rabies vaccine, ACIP commissioned a study to determine if a 4-dose series might be as effective as the licensed 5-dose series. The results showed that a reduced-dose series achieved equivalent antibody levels, so ACIP voted to recommend 4 doses of vaccine at days 0, 3, 7, and 14 postexposure.12 The vaccine should be part of a 3-pronged approach to prevent rabies after an exposure, along with rabies immune globulin administration and wound cleaning.13 The 4-dose schedule differs from the rabies vaccine package inserts and the FDA licensure information.
Tougher immunity criteria for health care personnel
Prior to 2009, criteria for proof of immunity to measles, mumps, or rubella among health care workers included serologic testing, history of 2 vaccines after age 1, physician-diagnosed disease, or being born prior to 1957. The new criteria require laboratory confirmation of a physician diagnosis and add a footnote to the “born before 1957” criterion that states: Institutions with unvaccinated health care workers who lack laboratory evidence of immunity should consider vaccinating them with 2 doses of MMR (for measles and mumps) and 1 dose of MMR (for rubella). In an outbreak, the new standards recommend inoculating unvaccinated health care personnel who do not have serological proof of immunity with 2 doses for outbreaks of measles or mumps and 1 dose during an outbreak of rubella.14,15
Meningococcal booster for those at high risk
ACIP now recommends quadrivalent meningococcal conjugate vaccine (MCV4) for all teens ages 11 to 18 years and for anyone 2 to 55 years of age who is at increased risk for meningococcal disease.16 MCV4 is licensed as a single dose.
Because of the high risk for meningococcal disease among certain groups of people, as well as limited data on duration of protection, ACIP now recommends that individuals previously vaccinated with either MCV4 or meningococcal polysaccharide vaccine (MPSV4) who are at prolonged increased risk be revaccinated with MCV4.
Those who were previously vaccinated at 7 years of age or older should be revaccinated 5 years after their previous meningococcal vaccine; individuals who were previously vaccinated at ages 2 to 6 years should be revaccinated 3 years after their previous meningococcal vaccine.
Individuals at prolonged risk for meningococcal disease are those with complement component deficiencies or anatomic or functional asplenia, microbiologists who routinely work with Neisseria meningitides, and travelers to countries where meningococcal disease is hyperendemic or epidemic.
College freshmen living in dormitories who were previously vaccinated with MCV4 do not need to be revaccinated. However, college freshmen living in dormitories who were vaccinated with MPSV4 ≥5 years previously should be vaccinated with MCV4.
New pneumococcal vaccine with more coverage
A new pneumococcal conjugate vaccine (PCV13) for infants and children will be licensed soon. It will replace the PCV7 vaccine now recommended routinely. ACIP will make recommendations on how to introduce PCV13 into a schedule for infants and children who are in the middle of a PCV7 series, and for catch-up vaccination for children who have completed a PCV7 series.
The new vaccine will provide added protection against an additional 6 types of pneumococcal bacteria, and will replace the older product immediately after licensure. It is unclear what will become of unused supplies of PCV7. Physicians who need to order PCV7 in this interim period before the new vaccine is licensed will be faced with difficult choices. The options include ordering only small quantities or trying to get an advance commitment from the manufacturers to take back any unused vaccine.
1. Centers for Disease Control and Prevention. Updated recommendations from the ACIP for use of hepatitis A vaccine in close contacts of newly arriving international adoptees. MMWR Morbid Mortal Wkly Rep. 2009;58:1006-1007.Available at: www.cdc.gov/mmwr/preview/mmwrhtml/mm5836a4.htm. Accessed January 19, 2010.
2. Centers for Disease Control and Prevention. Update: prevention of hepatitis A after exposure to hepatitis A virus and in international travelers. Updated recommendations of the ACIP. MMWR Morbid Mortal Wkly Rep. 2007;56:1080-1084.Available at: www.cdc.gov/mmwr/preview/mmwrhtml/mm5641a3.htm. Accessed January 19, 2010.
3. Centers for Disease Control and Prevention. Prevention of hepatitis A through active or passive immunization: recommendation of the ACIP. MMWR Recomm Rep. 2006;55(RR-7):1-23.Available at: www.cdc.gov/mmwr/preview/mmwrhtml/rr5507a1.htm. Accessed January 19, 2010.
4. ACIP provisional recommendations for the use of combination vaccines. August 28, 2009. Available at: www.cdc.gov/vaccines/recs/provisional/downloads/combo-vax-Aug2009-508.pdf. Accessed January 18, 2010.
5. ACIP provisional recommendations for use of measles, mumps, rubella and varicella (MMRV) vaccine. October 20, 2009. Available at: www.cdc.gov/vaccines/recs/provisional/downloads/mmrv-oct2009-508.pdf. Accessed January 19, 2010.
6. Centers for Disease Control and Prevention. Update recommendations of the ACIP regarding routine poliovirus vaccination. MMWR Morbid Mortal Wkly Rep. 2009;58:829-830.Available at: www.cdc.gov/mmwr/preview/mmwrhtml/mm5830a3.htm?s-cid=mr. Accessed January 20, 2010.
7. Centers for Disease Control and Prevention. Provider letter, July 30, 2009. Available at: www.cdc.gov/vaccines/vac-gen/shortages/downloads/Hib-hcp-ltr-7-30-09.pdf. Accessed February 15, 2010.
8. ACIP provisional recommendations for HPV vaccine. December 1, 2009. Available at: www.cdc.gov/vaccines/recs/provisional/downloads/hpv-vac-dec2009-508.pdf. Accessed January 18, 2010.
9. Centers for Disease Control and Prevention. Quadrivalent human papillomavirus vaccine: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep. 2007;56(RR-2):1-24.Available at: http://www.cdc.gov/mmwr/pdf/rr/rr5602.pdf. Accessed February 2, 2010.
10. Meeting of the Advisory Committee on Immunization Practices. October 21-22, 2009, Atlanta, GA. Available at: www.cdc.gov/vaccines/recs/ACIP/livemeeting-Oct09.htm#hpv. Accessed January 21, 2010.
11. Campos-Outcalt D. The case for HPV immunization. J Fam Pract. 2009;58:660-664.
12. ACIP provisional recommendations for the prevention of human rabies. July 10, 2009. Available at: www.cdc.gov/vaccines/recs/provisional/downloads/rabies-July2009-508.pdf. Accessed January 28, 2010.
13. Centers for Disease Control and Prevention. Human rabies prevention—United States, 2008: Recommendations of the ACIP. MMWR Morbid Mortal Wkly Rep. 2008;57(early release):1-26, 28.Available at: www.cdc.gov/mmwr/preview/mmwrhtml/rr57e507a1.htm. Accessed January 28, 2010.
14. Advisory Committee on Immunization Practices summary report. June 24-26, 2009. Available at: http://www.cdc.gov/vaccines/recs/ACIP/downloads/min-jun09.pdf. Accessed January 28, 2010.
15. ACIP provisional recommendations for measles-mumps-rubella (MMR) “evidence of immunity” requirements for healthcare personnel. August 28, 2009. Available at:www.cdc.gov/vaccines/recs/provisional/downloads/mmr-evidence-immunity-Aug2009-508.pdf. Accessed January 28, 2010.
16. Centers for Disease Control and Prevention. Updated recommendation from ACIP for revaccination of persons at prolonged increased risk for meningococcal disease. MMWR Morb Mortal Wkly Rep. 2009;58:1042-1043.Available at: www.cdc.gov/mmwr/preview/mmwrhtml/mm5837a4.htm. Accessed January 20, 2010.
The Advisory Committee on Immunization Practices (ACIP) made a number of major new recommendations last year. These new recommendations address:
- expanded use of hepatitis A virus (HAV) vaccine
- preferences for combination vaccines
- timing of poliovirus vaccine doses
- resumption of the normal Haemophilus influenzae Type b (Hib) schedule, as shortages have resolved
- the use of a new bivalent human papilloma virus (HPV2) vaccine in women and quadrivalent (HPV4) vaccine in men
- a reduced-dose schedule for rabies postexposure prophylaxis
- proof of immunity against mumps, measles, and rubella for health care workers
- recommendations for meningococcal vaccine boosters.
Adoptive families need more protection against HAV
Each year, approximately 18,000 children are adopted from foreign countries, almost all of them born in countries with high or intermediate rates of HAV, 85% of them under 5 years of age.1 Identifying adoptees with an acute HAV infection is problematic, because in this age group, fewer than 10% of infected children manifest jaundice.1 The Centers for Disease Control and Prevention (CDC) has recorded a small number of cases of acute HAV infection traced back to exposure to adoptees, and there is some evidence that 1% to 6% of new international adoptees have acute, and infectious, HAV.1
In response to these data, the most recent ACIP recommendation expands indications for HAV vaccine to include anyone who will be in close personal contact—living in the same household or providing regular babysitting—with an adoptee from any country with high or intermediate endemic rates of HAV. The vaccine should be given within the first 60 days of the adoptee’s arrival in the United States.1The first dose of the 2-dose series should be given as soon as the adoption is planned, ideally 2 or more weeks before exposure to the adoptee.
This new recommendation adds to earlier expansions of indications for HAV vaccine, which include universal use in children, use in postexposure prophylaxis, and preexposure protection for travelers.2,3
ACIP still prefers combination vaccines, with caveats
Increasing numbers of vaccine products with multiple antigens have reduced the number of injections needed to complete the recommended childhood immunization schedule. These new products also create a situation in which parents and physicians have to choose between using the combination products or staying with component vaccines that contain fewer antigens, but necessitate a larger number of injections.
When ACIP considered this dilemma, committee members gave the general preference to combination vaccines. At the same time, the committee acknowledged that many considerations—storage, costs, number of injections, vaccine availability, vaccination status, likelihood of improved coverage, likelihood of return visits, patient preference, and the potential for adverse events—factor into the decision.4
MMRV is a special case. One combination product received special attention because of the potential for increased rates of febrile seizures. Combined measles, mumps, rubella, and varicella (MMRV) vaccine is currently in short supply, but when the supply improves it will provide 1 less injection to immunize against 4 childhood viral infections at each of 2 visits. However, there is good evidence that in children 1 to 2 years of age who are receiving the first dose of MMRV, there is an additional incidence of febrile seizures of 1 in every 2300 to 2600, compared with children receiving separate doses of MMR and varicella vaccines.5 There is no increased risk for older children or for the second dose.
ACIP considered this risk and recommends discussing the benefits and risks of MMR and varicella separately vs using the MMRV combination vaccine. The committee notes: “Use of MMR and varicella vaccines avoids [the] increased risk for fever and febrile seizures following MMRV vaccine.”5
IPV combination dosing is clarified
The inclusion of inactivated poliovirus (IPV) antigen into new combination vaccine products has caused some confusion over the recommended dosing schedule of polio vaccine. ACIP has now clarified that for the recommended 4-dose IPV schedule, the fourth dose should be administered after age 4 and at least 6 months after dose 3. In addition, the minimal intervals (4 weeks) in the first 6 months of life should be used only for those traveling overseas.6
Resume normal Hib schedule
With the licensure of a new Hib product (Hiberix, GlaxoSmithKline) for the booster dose of Hib starting at age 15 months, the supply of Hib vaccine has stabilized. Supply is now adequate to resume all 4 doses in the routine schedule and to recall all children who had their booster dose deferred. Children can be vaccinated with Hib through the age of 59 months (prior to their fifth birthday).7
2 HPV vaccines are now available
With the licensure of an HPV2 vaccine for use in women in the United States (Cervarix, GlaxoSmithKline), 2 HPV vaccine products are now available for use.8 An HPV4 vaccine (Gardasil, Merck & Co.) was licensed in 2006. The TABLE compares the composition, dosing schedules, and precaution for these 2 products. Each requires 3 doses, but the age ranges and dosing schedules are slightly different. The HPV4 vaccine contains antigens against HPV types 16 and 18, which cause 70% of cervical cancers and precancerous lesions, and types 6 and 11, which cause 90% of anogenital warts.9
The HPV2 vaccine contains antigens for HPV types 16 and 18 only and does not protect against warts. The bivalent product appears to produce a higher level of antibody response and may provide better cross protection against other HPV types. ACIP compared effectiveness studies of both vaccines and decided to show no preference for either vaccine for the prevention of cervical cancer and precancerous lesions.
TABLE
HPV vaccines: A side-by-side comparison
| HPV4 | HPV2 | |
|---|---|---|
| Year licensed | 2006 | 2009 |
| Virus-like particle types | 6, 11, 16, 18 | 16,18 |
| Hypersensitivity-related contraindication | Yeast | Latex |
| Schedule | 0, 2, 6 months | 0, 1, 6 months |
| Age range | 9-26 years | 10-25 years |
The recommendation is for routine vaccination with an HPV product for all adolescent girls ages 11 to 12, with catch-up through age 26. If a female wants protection against anogenital warts, HPV4 is recommended. It is preferable to complete a 3-dose series with the same product, but if this is not possible, a series can be completed with the other product. The HPV4 vaccine is made using yeast, and prefilled HPV2 syringes contain latex. Hypersensitivity to these substances is a contraindication to their use. Patients who receive either vaccine should be observed for 15 minutes after the injection to prevent injury from syncope.
HPV4 in men. The HPV4 vaccine has now been licensed in the United States for use in males ages 9 to 26 to prevent anogenital warts. It may also protect against HPV-caused cancers (oral, genital, and anal), but the proof of that is still lacking. ACIP debated whether to recommend HPV4 for boys routinely at age 11 to 12 and decided against this. Instead the group voted for a “permissive” recommendation that states HPV4 may be given to adolescents and young men ages 9 to 26 to prevent warts and that protection is better if it is administered before exposure.10 This allows vaccine use in young males to be provided in the Vaccines for Children Program, but falls short of including it in the routine vaccine schedules.
The reasons for not recommending HPV4 routinely in young men were the cost and the perception that anogenital warts are primarily a cosmetic problem, although it was acknowledged that they can cause serious psychological morbidity. ACIP acknowledged that using HPV4 in men might lead to more protection for women because viral spread would be reduced, but stated that much more protection for women would be gained from a higher level of vaccination among women. As the evidence of protection against HPV-related cancers in men is gathered, ACIP will probably revisit this recommendation.
For a more detailed discussion of the issues posed by these 2 vaccines, see “The case for HPV immunization” in the Journal of Family Practice, December 2009.11
Rabies vaccine: 4 doses are sufficient
Due to a threatened shortage of rabies vaccine, ACIP commissioned a study to determine if a 4-dose series might be as effective as the licensed 5-dose series. The results showed that a reduced-dose series achieved equivalent antibody levels, so ACIP voted to recommend 4 doses of vaccine at days 0, 3, 7, and 14 postexposure.12 The vaccine should be part of a 3-pronged approach to prevent rabies after an exposure, along with rabies immune globulin administration and wound cleaning.13 The 4-dose schedule differs from the rabies vaccine package inserts and the FDA licensure information.
Tougher immunity criteria for health care personnel
Prior to 2009, criteria for proof of immunity to measles, mumps, or rubella among health care workers included serologic testing, history of 2 vaccines after age 1, physician-diagnosed disease, or being born prior to 1957. The new criteria require laboratory confirmation of a physician diagnosis and add a footnote to the “born before 1957” criterion that states: Institutions with unvaccinated health care workers who lack laboratory evidence of immunity should consider vaccinating them with 2 doses of MMR (for measles and mumps) and 1 dose of MMR (for rubella). In an outbreak, the new standards recommend inoculating unvaccinated health care personnel who do not have serological proof of immunity with 2 doses for outbreaks of measles or mumps and 1 dose during an outbreak of rubella.14,15
Meningococcal booster for those at high risk
ACIP now recommends quadrivalent meningococcal conjugate vaccine (MCV4) for all teens ages 11 to 18 years and for anyone 2 to 55 years of age who is at increased risk for meningococcal disease.16 MCV4 is licensed as a single dose.
Because of the high risk for meningococcal disease among certain groups of people, as well as limited data on duration of protection, ACIP now recommends that individuals previously vaccinated with either MCV4 or meningococcal polysaccharide vaccine (MPSV4) who are at prolonged increased risk be revaccinated with MCV4.
Those who were previously vaccinated at 7 years of age or older should be revaccinated 5 years after their previous meningococcal vaccine; individuals who were previously vaccinated at ages 2 to 6 years should be revaccinated 3 years after their previous meningococcal vaccine.
Individuals at prolonged risk for meningococcal disease are those with complement component deficiencies or anatomic or functional asplenia, microbiologists who routinely work with Neisseria meningitides, and travelers to countries where meningococcal disease is hyperendemic or epidemic.
College freshmen living in dormitories who were previously vaccinated with MCV4 do not need to be revaccinated. However, college freshmen living in dormitories who were vaccinated with MPSV4 ≥5 years previously should be vaccinated with MCV4.
New pneumococcal vaccine with more coverage
A new pneumococcal conjugate vaccine (PCV13) for infants and children will be licensed soon. It will replace the PCV7 vaccine now recommended routinely. ACIP will make recommendations on how to introduce PCV13 into a schedule for infants and children who are in the middle of a PCV7 series, and for catch-up vaccination for children who have completed a PCV7 series.
The new vaccine will provide added protection against an additional 6 types of pneumococcal bacteria, and will replace the older product immediately after licensure. It is unclear what will become of unused supplies of PCV7. Physicians who need to order PCV7 in this interim period before the new vaccine is licensed will be faced with difficult choices. The options include ordering only small quantities or trying to get an advance commitment from the manufacturers to take back any unused vaccine.
The Advisory Committee on Immunization Practices (ACIP) made a number of major new recommendations last year. These new recommendations address:
- expanded use of hepatitis A virus (HAV) vaccine
- preferences for combination vaccines
- timing of poliovirus vaccine doses
- resumption of the normal Haemophilus influenzae Type b (Hib) schedule, as shortages have resolved
- the use of a new bivalent human papilloma virus (HPV2) vaccine in women and quadrivalent (HPV4) vaccine in men
- a reduced-dose schedule for rabies postexposure prophylaxis
- proof of immunity against mumps, measles, and rubella for health care workers
- recommendations for meningococcal vaccine boosters.
Adoptive families need more protection against HAV
Each year, approximately 18,000 children are adopted from foreign countries, almost all of them born in countries with high or intermediate rates of HAV, 85% of them under 5 years of age.1 Identifying adoptees with an acute HAV infection is problematic, because in this age group, fewer than 10% of infected children manifest jaundice.1 The Centers for Disease Control and Prevention (CDC) has recorded a small number of cases of acute HAV infection traced back to exposure to adoptees, and there is some evidence that 1% to 6% of new international adoptees have acute, and infectious, HAV.1
In response to these data, the most recent ACIP recommendation expands indications for HAV vaccine to include anyone who will be in close personal contact—living in the same household or providing regular babysitting—with an adoptee from any country with high or intermediate endemic rates of HAV. The vaccine should be given within the first 60 days of the adoptee’s arrival in the United States.1The first dose of the 2-dose series should be given as soon as the adoption is planned, ideally 2 or more weeks before exposure to the adoptee.
This new recommendation adds to earlier expansions of indications for HAV vaccine, which include universal use in children, use in postexposure prophylaxis, and preexposure protection for travelers.2,3
ACIP still prefers combination vaccines, with caveats
Increasing numbers of vaccine products with multiple antigens have reduced the number of injections needed to complete the recommended childhood immunization schedule. These new products also create a situation in which parents and physicians have to choose between using the combination products or staying with component vaccines that contain fewer antigens, but necessitate a larger number of injections.
When ACIP considered this dilemma, committee members gave the general preference to combination vaccines. At the same time, the committee acknowledged that many considerations—storage, costs, number of injections, vaccine availability, vaccination status, likelihood of improved coverage, likelihood of return visits, patient preference, and the potential for adverse events—factor into the decision.4
MMRV is a special case. One combination product received special attention because of the potential for increased rates of febrile seizures. Combined measles, mumps, rubella, and varicella (MMRV) vaccine is currently in short supply, but when the supply improves it will provide 1 less injection to immunize against 4 childhood viral infections at each of 2 visits. However, there is good evidence that in children 1 to 2 years of age who are receiving the first dose of MMRV, there is an additional incidence of febrile seizures of 1 in every 2300 to 2600, compared with children receiving separate doses of MMR and varicella vaccines.5 There is no increased risk for older children or for the second dose.
ACIP considered this risk and recommends discussing the benefits and risks of MMR and varicella separately vs using the MMRV combination vaccine. The committee notes: “Use of MMR and varicella vaccines avoids [the] increased risk for fever and febrile seizures following MMRV vaccine.”5
IPV combination dosing is clarified
The inclusion of inactivated poliovirus (IPV) antigen into new combination vaccine products has caused some confusion over the recommended dosing schedule of polio vaccine. ACIP has now clarified that for the recommended 4-dose IPV schedule, the fourth dose should be administered after age 4 and at least 6 months after dose 3. In addition, the minimal intervals (4 weeks) in the first 6 months of life should be used only for those traveling overseas.6
Resume normal Hib schedule
With the licensure of a new Hib product (Hiberix, GlaxoSmithKline) for the booster dose of Hib starting at age 15 months, the supply of Hib vaccine has stabilized. Supply is now adequate to resume all 4 doses in the routine schedule and to recall all children who had their booster dose deferred. Children can be vaccinated with Hib through the age of 59 months (prior to their fifth birthday).7
2 HPV vaccines are now available
With the licensure of an HPV2 vaccine for use in women in the United States (Cervarix, GlaxoSmithKline), 2 HPV vaccine products are now available for use.8 An HPV4 vaccine (Gardasil, Merck & Co.) was licensed in 2006. The TABLE compares the composition, dosing schedules, and precaution for these 2 products. Each requires 3 doses, but the age ranges and dosing schedules are slightly different. The HPV4 vaccine contains antigens against HPV types 16 and 18, which cause 70% of cervical cancers and precancerous lesions, and types 6 and 11, which cause 90% of anogenital warts.9
The HPV2 vaccine contains antigens for HPV types 16 and 18 only and does not protect against warts. The bivalent product appears to produce a higher level of antibody response and may provide better cross protection against other HPV types. ACIP compared effectiveness studies of both vaccines and decided to show no preference for either vaccine for the prevention of cervical cancer and precancerous lesions.
TABLE
HPV vaccines: A side-by-side comparison
| HPV4 | HPV2 | |
|---|---|---|
| Year licensed | 2006 | 2009 |
| Virus-like particle types | 6, 11, 16, 18 | 16,18 |
| Hypersensitivity-related contraindication | Yeast | Latex |
| Schedule | 0, 2, 6 months | 0, 1, 6 months |
| Age range | 9-26 years | 10-25 years |
The recommendation is for routine vaccination with an HPV product for all adolescent girls ages 11 to 12, with catch-up through age 26. If a female wants protection against anogenital warts, HPV4 is recommended. It is preferable to complete a 3-dose series with the same product, but if this is not possible, a series can be completed with the other product. The HPV4 vaccine is made using yeast, and prefilled HPV2 syringes contain latex. Hypersensitivity to these substances is a contraindication to their use. Patients who receive either vaccine should be observed for 15 minutes after the injection to prevent injury from syncope.
HPV4 in men. The HPV4 vaccine has now been licensed in the United States for use in males ages 9 to 26 to prevent anogenital warts. It may also protect against HPV-caused cancers (oral, genital, and anal), but the proof of that is still lacking. ACIP debated whether to recommend HPV4 for boys routinely at age 11 to 12 and decided against this. Instead the group voted for a “permissive” recommendation that states HPV4 may be given to adolescents and young men ages 9 to 26 to prevent warts and that protection is better if it is administered before exposure.10 This allows vaccine use in young males to be provided in the Vaccines for Children Program, but falls short of including it in the routine vaccine schedules.
The reasons for not recommending HPV4 routinely in young men were the cost and the perception that anogenital warts are primarily a cosmetic problem, although it was acknowledged that they can cause serious psychological morbidity. ACIP acknowledged that using HPV4 in men might lead to more protection for women because viral spread would be reduced, but stated that much more protection for women would be gained from a higher level of vaccination among women. As the evidence of protection against HPV-related cancers in men is gathered, ACIP will probably revisit this recommendation.
For a more detailed discussion of the issues posed by these 2 vaccines, see “The case for HPV immunization” in the Journal of Family Practice, December 2009.11
Rabies vaccine: 4 doses are sufficient
Due to a threatened shortage of rabies vaccine, ACIP commissioned a study to determine if a 4-dose series might be as effective as the licensed 5-dose series. The results showed that a reduced-dose series achieved equivalent antibody levels, so ACIP voted to recommend 4 doses of vaccine at days 0, 3, 7, and 14 postexposure.12 The vaccine should be part of a 3-pronged approach to prevent rabies after an exposure, along with rabies immune globulin administration and wound cleaning.13 The 4-dose schedule differs from the rabies vaccine package inserts and the FDA licensure information.
Tougher immunity criteria for health care personnel
Prior to 2009, criteria for proof of immunity to measles, mumps, or rubella among health care workers included serologic testing, history of 2 vaccines after age 1, physician-diagnosed disease, or being born prior to 1957. The new criteria require laboratory confirmation of a physician diagnosis and add a footnote to the “born before 1957” criterion that states: Institutions with unvaccinated health care workers who lack laboratory evidence of immunity should consider vaccinating them with 2 doses of MMR (for measles and mumps) and 1 dose of MMR (for rubella). In an outbreak, the new standards recommend inoculating unvaccinated health care personnel who do not have serological proof of immunity with 2 doses for outbreaks of measles or mumps and 1 dose during an outbreak of rubella.14,15
Meningococcal booster for those at high risk
ACIP now recommends quadrivalent meningococcal conjugate vaccine (MCV4) for all teens ages 11 to 18 years and for anyone 2 to 55 years of age who is at increased risk for meningococcal disease.16 MCV4 is licensed as a single dose.
Because of the high risk for meningococcal disease among certain groups of people, as well as limited data on duration of protection, ACIP now recommends that individuals previously vaccinated with either MCV4 or meningococcal polysaccharide vaccine (MPSV4) who are at prolonged increased risk be revaccinated with MCV4.
Those who were previously vaccinated at 7 years of age or older should be revaccinated 5 years after their previous meningococcal vaccine; individuals who were previously vaccinated at ages 2 to 6 years should be revaccinated 3 years after their previous meningococcal vaccine.
Individuals at prolonged risk for meningococcal disease are those with complement component deficiencies or anatomic or functional asplenia, microbiologists who routinely work with Neisseria meningitides, and travelers to countries where meningococcal disease is hyperendemic or epidemic.
College freshmen living in dormitories who were previously vaccinated with MCV4 do not need to be revaccinated. However, college freshmen living in dormitories who were vaccinated with MPSV4 ≥5 years previously should be vaccinated with MCV4.
New pneumococcal vaccine with more coverage
A new pneumococcal conjugate vaccine (PCV13) for infants and children will be licensed soon. It will replace the PCV7 vaccine now recommended routinely. ACIP will make recommendations on how to introduce PCV13 into a schedule for infants and children who are in the middle of a PCV7 series, and for catch-up vaccination for children who have completed a PCV7 series.
The new vaccine will provide added protection against an additional 6 types of pneumococcal bacteria, and will replace the older product immediately after licensure. It is unclear what will become of unused supplies of PCV7. Physicians who need to order PCV7 in this interim period before the new vaccine is licensed will be faced with difficult choices. The options include ordering only small quantities or trying to get an advance commitment from the manufacturers to take back any unused vaccine.
1. Centers for Disease Control and Prevention. Updated recommendations from the ACIP for use of hepatitis A vaccine in close contacts of newly arriving international adoptees. MMWR Morbid Mortal Wkly Rep. 2009;58:1006-1007.Available at: www.cdc.gov/mmwr/preview/mmwrhtml/mm5836a4.htm. Accessed January 19, 2010.
2. Centers for Disease Control and Prevention. Update: prevention of hepatitis A after exposure to hepatitis A virus and in international travelers. Updated recommendations of the ACIP. MMWR Morbid Mortal Wkly Rep. 2007;56:1080-1084.Available at: www.cdc.gov/mmwr/preview/mmwrhtml/mm5641a3.htm. Accessed January 19, 2010.
3. Centers for Disease Control and Prevention. Prevention of hepatitis A through active or passive immunization: recommendation of the ACIP. MMWR Recomm Rep. 2006;55(RR-7):1-23.Available at: www.cdc.gov/mmwr/preview/mmwrhtml/rr5507a1.htm. Accessed January 19, 2010.
4. ACIP provisional recommendations for the use of combination vaccines. August 28, 2009. Available at: www.cdc.gov/vaccines/recs/provisional/downloads/combo-vax-Aug2009-508.pdf. Accessed January 18, 2010.
5. ACIP provisional recommendations for use of measles, mumps, rubella and varicella (MMRV) vaccine. October 20, 2009. Available at: www.cdc.gov/vaccines/recs/provisional/downloads/mmrv-oct2009-508.pdf. Accessed January 19, 2010.
6. Centers for Disease Control and Prevention. Update recommendations of the ACIP regarding routine poliovirus vaccination. MMWR Morbid Mortal Wkly Rep. 2009;58:829-830.Available at: www.cdc.gov/mmwr/preview/mmwrhtml/mm5830a3.htm?s-cid=mr. Accessed January 20, 2010.
7. Centers for Disease Control and Prevention. Provider letter, July 30, 2009. Available at: www.cdc.gov/vaccines/vac-gen/shortages/downloads/Hib-hcp-ltr-7-30-09.pdf. Accessed February 15, 2010.
8. ACIP provisional recommendations for HPV vaccine. December 1, 2009. Available at: www.cdc.gov/vaccines/recs/provisional/downloads/hpv-vac-dec2009-508.pdf. Accessed January 18, 2010.
9. Centers for Disease Control and Prevention. Quadrivalent human papillomavirus vaccine: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep. 2007;56(RR-2):1-24.Available at: http://www.cdc.gov/mmwr/pdf/rr/rr5602.pdf. Accessed February 2, 2010.
10. Meeting of the Advisory Committee on Immunization Practices. October 21-22, 2009, Atlanta, GA. Available at: www.cdc.gov/vaccines/recs/ACIP/livemeeting-Oct09.htm#hpv. Accessed January 21, 2010.
11. Campos-Outcalt D. The case for HPV immunization. J Fam Pract. 2009;58:660-664.
12. ACIP provisional recommendations for the prevention of human rabies. July 10, 2009. Available at: www.cdc.gov/vaccines/recs/provisional/downloads/rabies-July2009-508.pdf. Accessed January 28, 2010.
13. Centers for Disease Control and Prevention. Human rabies prevention—United States, 2008: Recommendations of the ACIP. MMWR Morbid Mortal Wkly Rep. 2008;57(early release):1-26, 28.Available at: www.cdc.gov/mmwr/preview/mmwrhtml/rr57e507a1.htm. Accessed January 28, 2010.
14. Advisory Committee on Immunization Practices summary report. June 24-26, 2009. Available at: http://www.cdc.gov/vaccines/recs/ACIP/downloads/min-jun09.pdf. Accessed January 28, 2010.
15. ACIP provisional recommendations for measles-mumps-rubella (MMR) “evidence of immunity” requirements for healthcare personnel. August 28, 2009. Available at:www.cdc.gov/vaccines/recs/provisional/downloads/mmr-evidence-immunity-Aug2009-508.pdf. Accessed January 28, 2010.
16. Centers for Disease Control and Prevention. Updated recommendation from ACIP for revaccination of persons at prolonged increased risk for meningococcal disease. MMWR Morb Mortal Wkly Rep. 2009;58:1042-1043.Available at: www.cdc.gov/mmwr/preview/mmwrhtml/mm5837a4.htm. Accessed January 20, 2010.
1. Centers for Disease Control and Prevention. Updated recommendations from the ACIP for use of hepatitis A vaccine in close contacts of newly arriving international adoptees. MMWR Morbid Mortal Wkly Rep. 2009;58:1006-1007.Available at: www.cdc.gov/mmwr/preview/mmwrhtml/mm5836a4.htm. Accessed January 19, 2010.
2. Centers for Disease Control and Prevention. Update: prevention of hepatitis A after exposure to hepatitis A virus and in international travelers. Updated recommendations of the ACIP. MMWR Morbid Mortal Wkly Rep. 2007;56:1080-1084.Available at: www.cdc.gov/mmwr/preview/mmwrhtml/mm5641a3.htm. Accessed January 19, 2010.
3. Centers for Disease Control and Prevention. Prevention of hepatitis A through active or passive immunization: recommendation of the ACIP. MMWR Recomm Rep. 2006;55(RR-7):1-23.Available at: www.cdc.gov/mmwr/preview/mmwrhtml/rr5507a1.htm. Accessed January 19, 2010.
4. ACIP provisional recommendations for the use of combination vaccines. August 28, 2009. Available at: www.cdc.gov/vaccines/recs/provisional/downloads/combo-vax-Aug2009-508.pdf. Accessed January 18, 2010.
5. ACIP provisional recommendations for use of measles, mumps, rubella and varicella (MMRV) vaccine. October 20, 2009. Available at: www.cdc.gov/vaccines/recs/provisional/downloads/mmrv-oct2009-508.pdf. Accessed January 19, 2010.
6. Centers for Disease Control and Prevention. Update recommendations of the ACIP regarding routine poliovirus vaccination. MMWR Morbid Mortal Wkly Rep. 2009;58:829-830.Available at: www.cdc.gov/mmwr/preview/mmwrhtml/mm5830a3.htm?s-cid=mr. Accessed January 20, 2010.
7. Centers for Disease Control and Prevention. Provider letter, July 30, 2009. Available at: www.cdc.gov/vaccines/vac-gen/shortages/downloads/Hib-hcp-ltr-7-30-09.pdf. Accessed February 15, 2010.
8. ACIP provisional recommendations for HPV vaccine. December 1, 2009. Available at: www.cdc.gov/vaccines/recs/provisional/downloads/hpv-vac-dec2009-508.pdf. Accessed January 18, 2010.
9. Centers for Disease Control and Prevention. Quadrivalent human papillomavirus vaccine: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep. 2007;56(RR-2):1-24.Available at: http://www.cdc.gov/mmwr/pdf/rr/rr5602.pdf. Accessed February 2, 2010.
10. Meeting of the Advisory Committee on Immunization Practices. October 21-22, 2009, Atlanta, GA. Available at: www.cdc.gov/vaccines/recs/ACIP/livemeeting-Oct09.htm#hpv. Accessed January 21, 2010.
11. Campos-Outcalt D. The case for HPV immunization. J Fam Pract. 2009;58:660-664.
12. ACIP provisional recommendations for the prevention of human rabies. July 10, 2009. Available at: www.cdc.gov/vaccines/recs/provisional/downloads/rabies-July2009-508.pdf. Accessed January 28, 2010.
13. Centers for Disease Control and Prevention. Human rabies prevention—United States, 2008: Recommendations of the ACIP. MMWR Morbid Mortal Wkly Rep. 2008;57(early release):1-26, 28.Available at: www.cdc.gov/mmwr/preview/mmwrhtml/rr57e507a1.htm. Accessed January 28, 2010.
14. Advisory Committee on Immunization Practices summary report. June 24-26, 2009. Available at: http://www.cdc.gov/vaccines/recs/ACIP/downloads/min-jun09.pdf. Accessed January 28, 2010.
15. ACIP provisional recommendations for measles-mumps-rubella (MMR) “evidence of immunity” requirements for healthcare personnel. August 28, 2009. Available at:www.cdc.gov/vaccines/recs/provisional/downloads/mmr-evidence-immunity-Aug2009-508.pdf. Accessed January 28, 2010.
16. Centers for Disease Control and Prevention. Updated recommendation from ACIP for revaccination of persons at prolonged increased risk for meningococcal disease. MMWR Morb Mortal Wkly Rep. 2009;58:1042-1043.Available at: www.cdc.gov/mmwr/preview/mmwrhtml/mm5837a4.htm. Accessed January 20, 2010.
The case for HPV immunization
The first quadrivalent human papillomavirus vaccine (HPV4) was licensed in the United States in 2006 (Gardasil, Merck & Co., Inc.).1 It contains viral proteins from HPV types 18, 16, 11, and 6, the types currently responsible for 70% of cervical cancers and 90% of anogenital warts.2 The vaccine is licensed for use in females ages 9 to 26 years for the prevention of cervical, vulvar, and vaginal precancerous lesions and cancer, and for the prevention of anogenital warts.1 It was recently licensed in the United States for the prevention of anogenital warts in males, as it has been in other countries.2,3
HPV and cancer: Quantifying the threat
Human papillomavirus (HPV) is responsible for cancers at several anatomical sites, including the cervix, anus, oral mucosa, vulva, vagina, and penis.1 The rate of cervical cancer in the United States has declined markedly since the introduction of screening programs using cervical cytology testing.1 This decline has been predominantly in squamous cell carcinomas, not adenocarcinomas, which are located in the endocervix and harder to detect.1
There are still around 12,000 cases of cervical cancer diagnosed each year in the United States, for an incidence of 8.1/100,000 women, and 3924 cervical cancer-related deaths.1 In addition, 7% to 10% of the 50 million cervical cytology tests done each year require some form of follow-up. Of these, 2 million to 3 million findings requiring follow-up are atypical squamous cells of undetermined significance (ASC-US) and 1.25 million are low-grade squamous intraepithelial lesions.1
There were more than 4000 cases of anal cancer recorded in 2003, a rate of 1.6/100,000 in women and 1.3/100,000 in men. In contrast to the trend in cervical cancer rates, anal cancer rates are increasing.4 It is not known how many incident cases of genital and anal warts there are annually, but some estimates place the number as high as 1 million. Lifetime cumulative risk has been estimated at 10%.5
Global morbidity and mortality from HPV is considerable, with 500,000 cases of cervical cancer and 260,000 cervical cancerrelated deaths reported worldwide in 2005.2 Rates are highest in developing countries in Latin America, Africa, and Asia.2
The vaccine is effective in women
HPV4 has proven to be highly effective in women ages 15 to 26 who have not been previously infected with the HPV types in the vaccine. Effectiveness has been 98% to 100% after 3 to 5 years in these women, using such end points as moderate and severe cervical intraepithelial neoplasia (CIN2 and CIN3), endocervical adenocarcinoma in situ (AIS), anogenital warts, and vulvar and vaginal intraepithelial neoplasia.1,2,6 These trials are ongoing.
Efficacy among women with current or past HPV infection is less certain. Studies of this question have included only small numbers and the confidence intervals have been large and included 0. In intention-to-treat studies, efficacy has been 39% to 46% for prevention of CIN2 or 3 and AIS caused by HPV 16 and 18, 69% for prevention of HPV 16/18-related vaginal intraepithelial neoplasia, and 68.5% for vaccine type-related warts.1
Who should be vaccinated?
According to the June 2006 recommendations of the Advisory Committee on Immunization Practices (ACIP) of the Centers for Disease Control and Prevention (CDC), immunization with 3 doses of HPV4 should be routine for girls between the ages of 11 and 12. Vaccination may be started in girls as young as age 9 and can also be done for females between the ages of 13 and 26.1 The ACIP recommendations are summarized in TABLE 1.
The World Health Organization (WHO) qualifies its recommendations a bit. “Routine HPV vaccination,” notes WHO, “should be included in national immunization programs provided that:
- prevention of cervical cancer or other HPV-related diseases, or both, constitutes a public health priority,
- vaccine introduction is programmatically feasible,
- sustainable financing can be secured, and
- the cost effectiveness of vaccination strategies in the country or region are considered.”2
WHO also says the vaccine is most effective prior to HPV infection and that, based on the age of initiation of sexual activity, the target population is most likely to be females 9 to 13 years of age. WHO does not recommend vaccination in males.2
In the United States, most professional organizations, including the American Academy of Family Physicians, have adopted recommendations in line with those of ACIP. One exception is the American Cancer Society (ACS), which takes issue with ACIP’s recommendations for the 19- to 26-year age group. The ACS position is that the evidence is insufficient to recommend for or against routine use of the HPV vaccine for this age group.7
TABLE 1
ACIP HPV4 recommendations1
|
| ACIP, Advisory Committee on Immunization Practices |
Some doubts among parents and physicians
Recent national vaccine survey data show that only 25% of females ages 13 to 17 had received 1 or more doses of HPV4 vaccine.8 Young women appear to be interested in the vaccine and in possibly receiving it, but they tend to underestimate their risk of contracting HPV.9,10 Some parents are concerned that the vaccine may encourage risk-taking behavior.11 Physicians report that some parents fear the vaccine is too new to be fully evaluated and are concerned that insurance may not cover the cost of the 3-shot series.12
Physician attitudes toward the vaccine are generally positive. Close to 90% of family physicians and 98% of pediatricians administer the vaccine in their practices. Eighty percent strongly recommend it to 13- to 15-year-olds, and 50% recommend it to 11- to 12-year-olds.
A small minority of family physicians has misconceptions regarding the vaccine:
- 15% believe an HPV test should be ordered before vaccination
- 19% believe the vaccine should not be given to those diagnosed with HPV
- 31% believe a pregnancy test should be ordered before administering the vaccine.12
Safety concerns, minor and major
Clinical trials conducted by the vaccine manufacturer demonstrated slightly higher rates of some systemic adverse reactions in the vaccinated group compared with placebo groups (TABLE 2). Data on adverse reactions at the injection site also showed somewhat higher percentages in the vaccine group. These trials were not large enough to detect severe, rare adverse reactions.
The CDC and the US Food and Drug Administration (FDA) collaboratively operate a passive reporting system, the Vaccine Adverse Events Reporting System (VAERS), as a way of conducting surveillance for these rare events. The manufacturer is required to report suspected adverse events to VAERS, but providers and consumers can also report any suspected adverse events.
There are problems with VAERS. Because it is a passive system, some adverse events may not be reported. At the same time, some events reported by consumers and physicians may be coincidental occurrences not caused by the vaccine. To complicate matters further, patients often receive more than 1 vaccine at the same time, so that attributing any particular adverse reaction to a single vaccine is problematic. These imperfections in VAERS should lead to caution in interpreting reports received on any 1 vaccine.
A recent article published in the Journal of the American Medical Association (JAMA) described the reports on the HPV4 vaccine received by the VAERS for the first 2½ years after licensure.13 Slightly more than 23 million doses had been distributed during this time, and 12,424 adverse events were reported. The most common were syncope (1847), dizziness (1763), nausea (1170), headache (957), and injection site reactions (926). Of all these reported events, 772 reactions were classified as serious, and 32 vaccine recipients died. Investigation of the deaths revealed that the mean time from vaccine to the death was 47 days, the deaths were caused by a variety of underlying conditions, and 4 deaths remained unexplained.
The only 2 serious adverse events that appeared to occur more frequently than background rates were venous thrombotic events, at 1 per 500,000 doses, and syncope, at a rate of 8.2 per 100,000 doses. The syncopal events were concentrated among the 11- to 18-year-olds and resulted in 293 falls and 200 head injuries. The authors of the JAMA article caution about attributing any cause and effect to the venous thromboembolism findings because of the high rates of oral contraceptive use in this age group, which increases the risk of this condition. Studies are ongoing to try to sort out these issues.
TABLE 2
HPV4 systemic adverse events in females, ages 9-23 years1
| Adverse events occurring 1 to 15 days post-vaccination | HPV4 recipients (N=5088) | Placebo recipients (N=3790) |
|---|---|---|
| Pyrexia | 13.0% | 11.2% |
| Nausea | 6.7% | 6.6% |
| Nasopharyngitis | 6.4% | 6.4% |
| Dizziness | 4.0% | 3.7% |
| Diarrhea | 3.6% | 3.5% |
| Vomiting | 2.4% | 1.9% |
| Myalgia | 2.0% | 2.0% |
| Cough | 2.0% | 1.5% |
| Toothache | 1.5% | 1.4% |
| Upper respiratory tract infection | 1.5% | 1.5% |
| Malaise | 1.4% | 1.2% |
| Arthralgia | 1.2% | 0.9% |
| Insomnia | 1.2% | 0.9% |
| Nasal congestion | 1.1% | 0.9% |
New developments: HPV4 for boys, licensing a bivalent vaccine
At its meeting in October 2009, ACIP decided to approve HPV4 for the prevention of anogenital warts in boys and young men ages 9 to 26.14 The potential benefits of using the HPV vaccine in males include reduced incidence of anogenital warts, possible reduction in HPV-related cancers, and reduced transmission of the HPV viruses in the vaccine to women and other men. The ACIP panel did not recommend routine immunization, however, leaving it up to physicians and patients to decide whether the vaccine is worthwhile. The advisory group said it would take up the question of the vaccine’s effectiveness in preventing HPV-related male cancers at future meetings.
At the same meeting, ACIP also voted to recommend Cervarix, the bivalent HPV vaccine from GlaxoSmithKline, for routine use in girls 11 and 12 years of age for the prevention of cancer and precancerous lesions.14 This vaccine contains antigens against HPV types 16 and 18 and does not provide protection against genital warts. Cervarix has been licensed in other countries and, to date, has demonstrated effectiveness comparable to that of the HPV4 against HPV 16- and 18-related outcomes.1,2,6
The availability of 2 HPV vaccines, 1 against both warts and cervical cancer and the other against cervical cancer only, will present some challenging ethical and practical issues for ACIP, as well as for states and physicians.
Unresolved issues
Some critics of the vaccine have pointed out that neither HPV vaccine has yet been proven to prevent cervical cancer. Because the amount of time it takes HPV infection to progress to cervical cancer is, on average, 10 to 20 years, vaccine trials will need to be continued for years to establish this point. However, high-grade cervical lesions and genital warts are outcomes important to patients on their own and are associated with considerable morbidity. It is unknown how continued use of the vaccine will affect the epidemiology of HPV infection and the incidence of HPV types not affected by the vaccine.
Safety monitoring of the vaccine continues. At this time it appears that syncopal episodes occur at increased rates shortly after administration of the HPV4 vaccine, and vaccine providers are encouraged to follow ACIP recommendations of a 15-minute waiting period after the administration of the vaccine.13 Ongoing studies will continue to look at potential rare adverse reactions and determine if the vaccine is truly a cause of venous thromboembolic events.
The approved age range for the use of HPV4 in women for the prevention of cancer, precancerous lesions, and warts may be expanded above 26 years. The benefit among women of this age will be less than for younger women, because of the higher probability of previous exposure to HPV. ACIP will need to decide on whether the vaccine should be routinely or selectively recommended above age 26.
1. Centers for Disease Control and Prevention. Quadrivalent human papillomavirus vaccine: Recommendations of the Advisory Committee on Immunization Practices. March 23, 2007. Available at: http://www.cdc.gov/mmwr/pdf/rr/rr5602.pdf. Accessed November 3, 2009.
2. World Health Organization. Human papillomavirus vaccines. WHO position paper. Weekly Epidemiological Record. 2009;84(15):118-131.Available at: http://www.who.int/wer/2009/wer8415.pdf. Accessed October 27, 2009.
3. U.S. Food and Drug Administration. October 16, 2009 Approval letter—Gardasil. Available at: www.fda.gov/BiologicsBloodVaccines/Vaccines/ApprovedProducts/ucm186991.htm. Accessed October 31, 2009.
4. Johnson LG, Madeleine MM, Newcomer LM, et al. Anal cancer incidence and survival; the surveillance, epidemiology and end results experience, 1973-2000. Cancer. 2004;101:281-288.
5. Trottier H, Franco EL. The epidemiology of genital human papillomavirus infection. Vaccine. 2006;24:1-15.
6. Rambout L, Hopkins L, Hutton B, et al. Prophylactic vaccination against human papillomavirus infection and disease in women: a systematic review of randomized controlled trials. CMAJ. 2007;177:469-479.
7. Saslow D, Castle PE, Cox JT, et al. American Cancer Society guideline for human papillomavirus vaccine use to prevent cervical cancer and its precursors. CA Cancer J Clin. 2007;57:7-28.
8. Centers for Disease Control and Prevention. Vaccination coverage among adolescents aged 13-17 years, United States 2007. MMWR Morb Mortal Wkly Rep. 2008;57:1100-1103.
9. Fisher R, Darrow DH, Tranter M, et al. Human papillomavirus vaccine: recommendations, issues and controversies. Curr Opin Pediatr. 2008;20:441-445.
10. Gerend MA, Magloire ZF. Awareness, knowledge and beliefs about human papillomavirus in a racially diverse sample of young adults. J Adolesc Health. 2008;42:237-242.
11. Advisory Committee on Immunization Practices: summary report, October 22-23, 2008, Atlanta, Ga. Available at: http://www.cdc.gov/vaccines/recs/ACIP/downloads/min-oct08.pdf. Accessed April 27, 2009.
12. Daley M. HPV vaccination practices. A national survey of physicians 18 months post licensure. Presentation at the October 2008 ACIP meeting. Available at: http://cdc.confex.com/cdc/nic2009/webprogram/Paper18003.html. Accessed November 3, 2009.
13. Slade BA, Leidel L, Vellozzi C, et al. Postlicensure safety surveillance for quadrivalent human papillomavirus recombinant vaccine. JAMA. 2009;302:750-757.
14. Meeting of the Advisory Committee on Immunization Practices; October 21-22, 2009; Atlanta, Ga.
The first quadrivalent human papillomavirus vaccine (HPV4) was licensed in the United States in 2006 (Gardasil, Merck & Co., Inc.).1 It contains viral proteins from HPV types 18, 16, 11, and 6, the types currently responsible for 70% of cervical cancers and 90% of anogenital warts.2 The vaccine is licensed for use in females ages 9 to 26 years for the prevention of cervical, vulvar, and vaginal precancerous lesions and cancer, and for the prevention of anogenital warts.1 It was recently licensed in the United States for the prevention of anogenital warts in males, as it has been in other countries.2,3
HPV and cancer: Quantifying the threat
Human papillomavirus (HPV) is responsible for cancers at several anatomical sites, including the cervix, anus, oral mucosa, vulva, vagina, and penis.1 The rate of cervical cancer in the United States has declined markedly since the introduction of screening programs using cervical cytology testing.1 This decline has been predominantly in squamous cell carcinomas, not adenocarcinomas, which are located in the endocervix and harder to detect.1
There are still around 12,000 cases of cervical cancer diagnosed each year in the United States, for an incidence of 8.1/100,000 women, and 3924 cervical cancer-related deaths.1 In addition, 7% to 10% of the 50 million cervical cytology tests done each year require some form of follow-up. Of these, 2 million to 3 million findings requiring follow-up are atypical squamous cells of undetermined significance (ASC-US) and 1.25 million are low-grade squamous intraepithelial lesions.1
There were more than 4000 cases of anal cancer recorded in 2003, a rate of 1.6/100,000 in women and 1.3/100,000 in men. In contrast to the trend in cervical cancer rates, anal cancer rates are increasing.4 It is not known how many incident cases of genital and anal warts there are annually, but some estimates place the number as high as 1 million. Lifetime cumulative risk has been estimated at 10%.5
Global morbidity and mortality from HPV is considerable, with 500,000 cases of cervical cancer and 260,000 cervical cancerrelated deaths reported worldwide in 2005.2 Rates are highest in developing countries in Latin America, Africa, and Asia.2
The vaccine is effective in women
HPV4 has proven to be highly effective in women ages 15 to 26 who have not been previously infected with the HPV types in the vaccine. Effectiveness has been 98% to 100% after 3 to 5 years in these women, using such end points as moderate and severe cervical intraepithelial neoplasia (CIN2 and CIN3), endocervical adenocarcinoma in situ (AIS), anogenital warts, and vulvar and vaginal intraepithelial neoplasia.1,2,6 These trials are ongoing.
Efficacy among women with current or past HPV infection is less certain. Studies of this question have included only small numbers and the confidence intervals have been large and included 0. In intention-to-treat studies, efficacy has been 39% to 46% for prevention of CIN2 or 3 and AIS caused by HPV 16 and 18, 69% for prevention of HPV 16/18-related vaginal intraepithelial neoplasia, and 68.5% for vaccine type-related warts.1
Who should be vaccinated?
According to the June 2006 recommendations of the Advisory Committee on Immunization Practices (ACIP) of the Centers for Disease Control and Prevention (CDC), immunization with 3 doses of HPV4 should be routine for girls between the ages of 11 and 12. Vaccination may be started in girls as young as age 9 and can also be done for females between the ages of 13 and 26.1 The ACIP recommendations are summarized in TABLE 1.
The World Health Organization (WHO) qualifies its recommendations a bit. “Routine HPV vaccination,” notes WHO, “should be included in national immunization programs provided that:
- prevention of cervical cancer or other HPV-related diseases, or both, constitutes a public health priority,
- vaccine introduction is programmatically feasible,
- sustainable financing can be secured, and
- the cost effectiveness of vaccination strategies in the country or region are considered.”2
WHO also says the vaccine is most effective prior to HPV infection and that, based on the age of initiation of sexual activity, the target population is most likely to be females 9 to 13 years of age. WHO does not recommend vaccination in males.2
In the United States, most professional organizations, including the American Academy of Family Physicians, have adopted recommendations in line with those of ACIP. One exception is the American Cancer Society (ACS), which takes issue with ACIP’s recommendations for the 19- to 26-year age group. The ACS position is that the evidence is insufficient to recommend for or against routine use of the HPV vaccine for this age group.7
TABLE 1
ACIP HPV4 recommendations1
|
| ACIP, Advisory Committee on Immunization Practices |
Some doubts among parents and physicians
Recent national vaccine survey data show that only 25% of females ages 13 to 17 had received 1 or more doses of HPV4 vaccine.8 Young women appear to be interested in the vaccine and in possibly receiving it, but they tend to underestimate their risk of contracting HPV.9,10 Some parents are concerned that the vaccine may encourage risk-taking behavior.11 Physicians report that some parents fear the vaccine is too new to be fully evaluated and are concerned that insurance may not cover the cost of the 3-shot series.12
Physician attitudes toward the vaccine are generally positive. Close to 90% of family physicians and 98% of pediatricians administer the vaccine in their practices. Eighty percent strongly recommend it to 13- to 15-year-olds, and 50% recommend it to 11- to 12-year-olds.
A small minority of family physicians has misconceptions regarding the vaccine:
- 15% believe an HPV test should be ordered before vaccination
- 19% believe the vaccine should not be given to those diagnosed with HPV
- 31% believe a pregnancy test should be ordered before administering the vaccine.12
Safety concerns, minor and major
Clinical trials conducted by the vaccine manufacturer demonstrated slightly higher rates of some systemic adverse reactions in the vaccinated group compared with placebo groups (TABLE 2). Data on adverse reactions at the injection site also showed somewhat higher percentages in the vaccine group. These trials were not large enough to detect severe, rare adverse reactions.
The CDC and the US Food and Drug Administration (FDA) collaboratively operate a passive reporting system, the Vaccine Adverse Events Reporting System (VAERS), as a way of conducting surveillance for these rare events. The manufacturer is required to report suspected adverse events to VAERS, but providers and consumers can also report any suspected adverse events.
There are problems with VAERS. Because it is a passive system, some adverse events may not be reported. At the same time, some events reported by consumers and physicians may be coincidental occurrences not caused by the vaccine. To complicate matters further, patients often receive more than 1 vaccine at the same time, so that attributing any particular adverse reaction to a single vaccine is problematic. These imperfections in VAERS should lead to caution in interpreting reports received on any 1 vaccine.
A recent article published in the Journal of the American Medical Association (JAMA) described the reports on the HPV4 vaccine received by the VAERS for the first 2½ years after licensure.13 Slightly more than 23 million doses had been distributed during this time, and 12,424 adverse events were reported. The most common were syncope (1847), dizziness (1763), nausea (1170), headache (957), and injection site reactions (926). Of all these reported events, 772 reactions were classified as serious, and 32 vaccine recipients died. Investigation of the deaths revealed that the mean time from vaccine to the death was 47 days, the deaths were caused by a variety of underlying conditions, and 4 deaths remained unexplained.
The only 2 serious adverse events that appeared to occur more frequently than background rates were venous thrombotic events, at 1 per 500,000 doses, and syncope, at a rate of 8.2 per 100,000 doses. The syncopal events were concentrated among the 11- to 18-year-olds and resulted in 293 falls and 200 head injuries. The authors of the JAMA article caution about attributing any cause and effect to the venous thromboembolism findings because of the high rates of oral contraceptive use in this age group, which increases the risk of this condition. Studies are ongoing to try to sort out these issues.
TABLE 2
HPV4 systemic adverse events in females, ages 9-23 years1
| Adverse events occurring 1 to 15 days post-vaccination | HPV4 recipients (N=5088) | Placebo recipients (N=3790) |
|---|---|---|
| Pyrexia | 13.0% | 11.2% |
| Nausea | 6.7% | 6.6% |
| Nasopharyngitis | 6.4% | 6.4% |
| Dizziness | 4.0% | 3.7% |
| Diarrhea | 3.6% | 3.5% |
| Vomiting | 2.4% | 1.9% |
| Myalgia | 2.0% | 2.0% |
| Cough | 2.0% | 1.5% |
| Toothache | 1.5% | 1.4% |
| Upper respiratory tract infection | 1.5% | 1.5% |
| Malaise | 1.4% | 1.2% |
| Arthralgia | 1.2% | 0.9% |
| Insomnia | 1.2% | 0.9% |
| Nasal congestion | 1.1% | 0.9% |
New developments: HPV4 for boys, licensing a bivalent vaccine
At its meeting in October 2009, ACIP decided to approve HPV4 for the prevention of anogenital warts in boys and young men ages 9 to 26.14 The potential benefits of using the HPV vaccine in males include reduced incidence of anogenital warts, possible reduction in HPV-related cancers, and reduced transmission of the HPV viruses in the vaccine to women and other men. The ACIP panel did not recommend routine immunization, however, leaving it up to physicians and patients to decide whether the vaccine is worthwhile. The advisory group said it would take up the question of the vaccine’s effectiveness in preventing HPV-related male cancers at future meetings.
At the same meeting, ACIP also voted to recommend Cervarix, the bivalent HPV vaccine from GlaxoSmithKline, for routine use in girls 11 and 12 years of age for the prevention of cancer and precancerous lesions.14 This vaccine contains antigens against HPV types 16 and 18 and does not provide protection against genital warts. Cervarix has been licensed in other countries and, to date, has demonstrated effectiveness comparable to that of the HPV4 against HPV 16- and 18-related outcomes.1,2,6
The availability of 2 HPV vaccines, 1 against both warts and cervical cancer and the other against cervical cancer only, will present some challenging ethical and practical issues for ACIP, as well as for states and physicians.
Unresolved issues
Some critics of the vaccine have pointed out that neither HPV vaccine has yet been proven to prevent cervical cancer. Because the amount of time it takes HPV infection to progress to cervical cancer is, on average, 10 to 20 years, vaccine trials will need to be continued for years to establish this point. However, high-grade cervical lesions and genital warts are outcomes important to patients on their own and are associated with considerable morbidity. It is unknown how continued use of the vaccine will affect the epidemiology of HPV infection and the incidence of HPV types not affected by the vaccine.
Safety monitoring of the vaccine continues. At this time it appears that syncopal episodes occur at increased rates shortly after administration of the HPV4 vaccine, and vaccine providers are encouraged to follow ACIP recommendations of a 15-minute waiting period after the administration of the vaccine.13 Ongoing studies will continue to look at potential rare adverse reactions and determine if the vaccine is truly a cause of venous thromboembolic events.
The approved age range for the use of HPV4 in women for the prevention of cancer, precancerous lesions, and warts may be expanded above 26 years. The benefit among women of this age will be less than for younger women, because of the higher probability of previous exposure to HPV. ACIP will need to decide on whether the vaccine should be routinely or selectively recommended above age 26.
The first quadrivalent human papillomavirus vaccine (HPV4) was licensed in the United States in 2006 (Gardasil, Merck & Co., Inc.).1 It contains viral proteins from HPV types 18, 16, 11, and 6, the types currently responsible for 70% of cervical cancers and 90% of anogenital warts.2 The vaccine is licensed for use in females ages 9 to 26 years for the prevention of cervical, vulvar, and vaginal precancerous lesions and cancer, and for the prevention of anogenital warts.1 It was recently licensed in the United States for the prevention of anogenital warts in males, as it has been in other countries.2,3
HPV and cancer: Quantifying the threat
Human papillomavirus (HPV) is responsible for cancers at several anatomical sites, including the cervix, anus, oral mucosa, vulva, vagina, and penis.1 The rate of cervical cancer in the United States has declined markedly since the introduction of screening programs using cervical cytology testing.1 This decline has been predominantly in squamous cell carcinomas, not adenocarcinomas, which are located in the endocervix and harder to detect.1
There are still around 12,000 cases of cervical cancer diagnosed each year in the United States, for an incidence of 8.1/100,000 women, and 3924 cervical cancer-related deaths.1 In addition, 7% to 10% of the 50 million cervical cytology tests done each year require some form of follow-up. Of these, 2 million to 3 million findings requiring follow-up are atypical squamous cells of undetermined significance (ASC-US) and 1.25 million are low-grade squamous intraepithelial lesions.1
There were more than 4000 cases of anal cancer recorded in 2003, a rate of 1.6/100,000 in women and 1.3/100,000 in men. In contrast to the trend in cervical cancer rates, anal cancer rates are increasing.4 It is not known how many incident cases of genital and anal warts there are annually, but some estimates place the number as high as 1 million. Lifetime cumulative risk has been estimated at 10%.5
Global morbidity and mortality from HPV is considerable, with 500,000 cases of cervical cancer and 260,000 cervical cancerrelated deaths reported worldwide in 2005.2 Rates are highest in developing countries in Latin America, Africa, and Asia.2
The vaccine is effective in women
HPV4 has proven to be highly effective in women ages 15 to 26 who have not been previously infected with the HPV types in the vaccine. Effectiveness has been 98% to 100% after 3 to 5 years in these women, using such end points as moderate and severe cervical intraepithelial neoplasia (CIN2 and CIN3), endocervical adenocarcinoma in situ (AIS), anogenital warts, and vulvar and vaginal intraepithelial neoplasia.1,2,6 These trials are ongoing.
Efficacy among women with current or past HPV infection is less certain. Studies of this question have included only small numbers and the confidence intervals have been large and included 0. In intention-to-treat studies, efficacy has been 39% to 46% for prevention of CIN2 or 3 and AIS caused by HPV 16 and 18, 69% for prevention of HPV 16/18-related vaginal intraepithelial neoplasia, and 68.5% for vaccine type-related warts.1
Who should be vaccinated?
According to the June 2006 recommendations of the Advisory Committee on Immunization Practices (ACIP) of the Centers for Disease Control and Prevention (CDC), immunization with 3 doses of HPV4 should be routine for girls between the ages of 11 and 12. Vaccination may be started in girls as young as age 9 and can also be done for females between the ages of 13 and 26.1 The ACIP recommendations are summarized in TABLE 1.
The World Health Organization (WHO) qualifies its recommendations a bit. “Routine HPV vaccination,” notes WHO, “should be included in national immunization programs provided that:
- prevention of cervical cancer or other HPV-related diseases, or both, constitutes a public health priority,
- vaccine introduction is programmatically feasible,
- sustainable financing can be secured, and
- the cost effectiveness of vaccination strategies in the country or region are considered.”2
WHO also says the vaccine is most effective prior to HPV infection and that, based on the age of initiation of sexual activity, the target population is most likely to be females 9 to 13 years of age. WHO does not recommend vaccination in males.2
In the United States, most professional organizations, including the American Academy of Family Physicians, have adopted recommendations in line with those of ACIP. One exception is the American Cancer Society (ACS), which takes issue with ACIP’s recommendations for the 19- to 26-year age group. The ACS position is that the evidence is insufficient to recommend for or against routine use of the HPV vaccine for this age group.7
TABLE 1
ACIP HPV4 recommendations1
|
| ACIP, Advisory Committee on Immunization Practices |
Some doubts among parents and physicians
Recent national vaccine survey data show that only 25% of females ages 13 to 17 had received 1 or more doses of HPV4 vaccine.8 Young women appear to be interested in the vaccine and in possibly receiving it, but they tend to underestimate their risk of contracting HPV.9,10 Some parents are concerned that the vaccine may encourage risk-taking behavior.11 Physicians report that some parents fear the vaccine is too new to be fully evaluated and are concerned that insurance may not cover the cost of the 3-shot series.12
Physician attitudes toward the vaccine are generally positive. Close to 90% of family physicians and 98% of pediatricians administer the vaccine in their practices. Eighty percent strongly recommend it to 13- to 15-year-olds, and 50% recommend it to 11- to 12-year-olds.
A small minority of family physicians has misconceptions regarding the vaccine:
- 15% believe an HPV test should be ordered before vaccination
- 19% believe the vaccine should not be given to those diagnosed with HPV
- 31% believe a pregnancy test should be ordered before administering the vaccine.12
Safety concerns, minor and major
Clinical trials conducted by the vaccine manufacturer demonstrated slightly higher rates of some systemic adverse reactions in the vaccinated group compared with placebo groups (TABLE 2). Data on adverse reactions at the injection site also showed somewhat higher percentages in the vaccine group. These trials were not large enough to detect severe, rare adverse reactions.
The CDC and the US Food and Drug Administration (FDA) collaboratively operate a passive reporting system, the Vaccine Adverse Events Reporting System (VAERS), as a way of conducting surveillance for these rare events. The manufacturer is required to report suspected adverse events to VAERS, but providers and consumers can also report any suspected adverse events.
There are problems with VAERS. Because it is a passive system, some adverse events may not be reported. At the same time, some events reported by consumers and physicians may be coincidental occurrences not caused by the vaccine. To complicate matters further, patients often receive more than 1 vaccine at the same time, so that attributing any particular adverse reaction to a single vaccine is problematic. These imperfections in VAERS should lead to caution in interpreting reports received on any 1 vaccine.
A recent article published in the Journal of the American Medical Association (JAMA) described the reports on the HPV4 vaccine received by the VAERS for the first 2½ years after licensure.13 Slightly more than 23 million doses had been distributed during this time, and 12,424 adverse events were reported. The most common were syncope (1847), dizziness (1763), nausea (1170), headache (957), and injection site reactions (926). Of all these reported events, 772 reactions were classified as serious, and 32 vaccine recipients died. Investigation of the deaths revealed that the mean time from vaccine to the death was 47 days, the deaths were caused by a variety of underlying conditions, and 4 deaths remained unexplained.
The only 2 serious adverse events that appeared to occur more frequently than background rates were venous thrombotic events, at 1 per 500,000 doses, and syncope, at a rate of 8.2 per 100,000 doses. The syncopal events were concentrated among the 11- to 18-year-olds and resulted in 293 falls and 200 head injuries. The authors of the JAMA article caution about attributing any cause and effect to the venous thromboembolism findings because of the high rates of oral contraceptive use in this age group, which increases the risk of this condition. Studies are ongoing to try to sort out these issues.
TABLE 2
HPV4 systemic adverse events in females, ages 9-23 years1
| Adverse events occurring 1 to 15 days post-vaccination | HPV4 recipients (N=5088) | Placebo recipients (N=3790) |
|---|---|---|
| Pyrexia | 13.0% | 11.2% |
| Nausea | 6.7% | 6.6% |
| Nasopharyngitis | 6.4% | 6.4% |
| Dizziness | 4.0% | 3.7% |
| Diarrhea | 3.6% | 3.5% |
| Vomiting | 2.4% | 1.9% |
| Myalgia | 2.0% | 2.0% |
| Cough | 2.0% | 1.5% |
| Toothache | 1.5% | 1.4% |
| Upper respiratory tract infection | 1.5% | 1.5% |
| Malaise | 1.4% | 1.2% |
| Arthralgia | 1.2% | 0.9% |
| Insomnia | 1.2% | 0.9% |
| Nasal congestion | 1.1% | 0.9% |
New developments: HPV4 for boys, licensing a bivalent vaccine
At its meeting in October 2009, ACIP decided to approve HPV4 for the prevention of anogenital warts in boys and young men ages 9 to 26.14 The potential benefits of using the HPV vaccine in males include reduced incidence of anogenital warts, possible reduction in HPV-related cancers, and reduced transmission of the HPV viruses in the vaccine to women and other men. The ACIP panel did not recommend routine immunization, however, leaving it up to physicians and patients to decide whether the vaccine is worthwhile. The advisory group said it would take up the question of the vaccine’s effectiveness in preventing HPV-related male cancers at future meetings.
At the same meeting, ACIP also voted to recommend Cervarix, the bivalent HPV vaccine from GlaxoSmithKline, for routine use in girls 11 and 12 years of age for the prevention of cancer and precancerous lesions.14 This vaccine contains antigens against HPV types 16 and 18 and does not provide protection against genital warts. Cervarix has been licensed in other countries and, to date, has demonstrated effectiveness comparable to that of the HPV4 against HPV 16- and 18-related outcomes.1,2,6
The availability of 2 HPV vaccines, 1 against both warts and cervical cancer and the other against cervical cancer only, will present some challenging ethical and practical issues for ACIP, as well as for states and physicians.
Unresolved issues
Some critics of the vaccine have pointed out that neither HPV vaccine has yet been proven to prevent cervical cancer. Because the amount of time it takes HPV infection to progress to cervical cancer is, on average, 10 to 20 years, vaccine trials will need to be continued for years to establish this point. However, high-grade cervical lesions and genital warts are outcomes important to patients on their own and are associated with considerable morbidity. It is unknown how continued use of the vaccine will affect the epidemiology of HPV infection and the incidence of HPV types not affected by the vaccine.
Safety monitoring of the vaccine continues. At this time it appears that syncopal episodes occur at increased rates shortly after administration of the HPV4 vaccine, and vaccine providers are encouraged to follow ACIP recommendations of a 15-minute waiting period after the administration of the vaccine.13 Ongoing studies will continue to look at potential rare adverse reactions and determine if the vaccine is truly a cause of venous thromboembolic events.
The approved age range for the use of HPV4 in women for the prevention of cancer, precancerous lesions, and warts may be expanded above 26 years. The benefit among women of this age will be less than for younger women, because of the higher probability of previous exposure to HPV. ACIP will need to decide on whether the vaccine should be routinely or selectively recommended above age 26.
1. Centers for Disease Control and Prevention. Quadrivalent human papillomavirus vaccine: Recommendations of the Advisory Committee on Immunization Practices. March 23, 2007. Available at: http://www.cdc.gov/mmwr/pdf/rr/rr5602.pdf. Accessed November 3, 2009.
2. World Health Organization. Human papillomavirus vaccines. WHO position paper. Weekly Epidemiological Record. 2009;84(15):118-131.Available at: http://www.who.int/wer/2009/wer8415.pdf. Accessed October 27, 2009.
3. U.S. Food and Drug Administration. October 16, 2009 Approval letter—Gardasil. Available at: www.fda.gov/BiologicsBloodVaccines/Vaccines/ApprovedProducts/ucm186991.htm. Accessed October 31, 2009.
4. Johnson LG, Madeleine MM, Newcomer LM, et al. Anal cancer incidence and survival; the surveillance, epidemiology and end results experience, 1973-2000. Cancer. 2004;101:281-288.
5. Trottier H, Franco EL. The epidemiology of genital human papillomavirus infection. Vaccine. 2006;24:1-15.
6. Rambout L, Hopkins L, Hutton B, et al. Prophylactic vaccination against human papillomavirus infection and disease in women: a systematic review of randomized controlled trials. CMAJ. 2007;177:469-479.
7. Saslow D, Castle PE, Cox JT, et al. American Cancer Society guideline for human papillomavirus vaccine use to prevent cervical cancer and its precursors. CA Cancer J Clin. 2007;57:7-28.
8. Centers for Disease Control and Prevention. Vaccination coverage among adolescents aged 13-17 years, United States 2007. MMWR Morb Mortal Wkly Rep. 2008;57:1100-1103.
9. Fisher R, Darrow DH, Tranter M, et al. Human papillomavirus vaccine: recommendations, issues and controversies. Curr Opin Pediatr. 2008;20:441-445.
10. Gerend MA, Magloire ZF. Awareness, knowledge and beliefs about human papillomavirus in a racially diverse sample of young adults. J Adolesc Health. 2008;42:237-242.
11. Advisory Committee on Immunization Practices: summary report, October 22-23, 2008, Atlanta, Ga. Available at: http://www.cdc.gov/vaccines/recs/ACIP/downloads/min-oct08.pdf. Accessed April 27, 2009.
12. Daley M. HPV vaccination practices. A national survey of physicians 18 months post licensure. Presentation at the October 2008 ACIP meeting. Available at: http://cdc.confex.com/cdc/nic2009/webprogram/Paper18003.html. Accessed November 3, 2009.
13. Slade BA, Leidel L, Vellozzi C, et al. Postlicensure safety surveillance for quadrivalent human papillomavirus recombinant vaccine. JAMA. 2009;302:750-757.
14. Meeting of the Advisory Committee on Immunization Practices; October 21-22, 2009; Atlanta, Ga.
1. Centers for Disease Control and Prevention. Quadrivalent human papillomavirus vaccine: Recommendations of the Advisory Committee on Immunization Practices. March 23, 2007. Available at: http://www.cdc.gov/mmwr/pdf/rr/rr5602.pdf. Accessed November 3, 2009.
2. World Health Organization. Human papillomavirus vaccines. WHO position paper. Weekly Epidemiological Record. 2009;84(15):118-131.Available at: http://www.who.int/wer/2009/wer8415.pdf. Accessed October 27, 2009.
3. U.S. Food and Drug Administration. October 16, 2009 Approval letter—Gardasil. Available at: www.fda.gov/BiologicsBloodVaccines/Vaccines/ApprovedProducts/ucm186991.htm. Accessed October 31, 2009.
4. Johnson LG, Madeleine MM, Newcomer LM, et al. Anal cancer incidence and survival; the surveillance, epidemiology and end results experience, 1973-2000. Cancer. 2004;101:281-288.
5. Trottier H, Franco EL. The epidemiology of genital human papillomavirus infection. Vaccine. 2006;24:1-15.
6. Rambout L, Hopkins L, Hutton B, et al. Prophylactic vaccination against human papillomavirus infection and disease in women: a systematic review of randomized controlled trials. CMAJ. 2007;177:469-479.
7. Saslow D, Castle PE, Cox JT, et al. American Cancer Society guideline for human papillomavirus vaccine use to prevent cervical cancer and its precursors. CA Cancer J Clin. 2007;57:7-28.
8. Centers for Disease Control and Prevention. Vaccination coverage among adolescents aged 13-17 years, United States 2007. MMWR Morb Mortal Wkly Rep. 2008;57:1100-1103.
9. Fisher R, Darrow DH, Tranter M, et al. Human papillomavirus vaccine: recommendations, issues and controversies. Curr Opin Pediatr. 2008;20:441-445.
10. Gerend MA, Magloire ZF. Awareness, knowledge and beliefs about human papillomavirus in a racially diverse sample of young adults. J Adolesc Health. 2008;42:237-242.
11. Advisory Committee on Immunization Practices: summary report, October 22-23, 2008, Atlanta, Ga. Available at: http://www.cdc.gov/vaccines/recs/ACIP/downloads/min-oct08.pdf. Accessed April 27, 2009.
12. Daley M. HPV vaccination practices. A national survey of physicians 18 months post licensure. Presentation at the October 2008 ACIP meeting. Available at: http://cdc.confex.com/cdc/nic2009/webprogram/Paper18003.html. Accessed November 3, 2009.
13. Slade BA, Leidel L, Vellozzi C, et al. Postlicensure safety surveillance for quadrivalent human papillomavirus recombinant vaccine. JAMA. 2009;302:750-757.
14. Meeting of the Advisory Committee on Immunization Practices; October 21-22, 2009; Atlanta, Ga.
Pandemic and seasonal flu: What you need to know
This coming flu season will be interesting—and confusing. As of August 6, 2009, the Centers for Disease Control and Prevention (CDC) reported 6506 hospitalized cases and 436 deaths from the pandemic H1N1 flu virus since the first US cases were reported in April 2009.1 (Reporting on individual confirmed and probable cases has been discontinued.) On July 31, the World Health Organization reported pandemic influenza in 168 countries, with 162,380 reported cases and 1154 deaths.2 At the same time the pandemic was developing, the seasonal flu of 2009—a relatively mild year—was tapering off. The pandemic influenza has continued to cause widespread disease in the United States throughout the summer, a somewhat unusual pattern for influenza.
So far, pandemic H1N1 flu is relatively benign, treatable
The pandemic virus, though highly infectious, has had a low case fatality rate up to now. Deaths have occurred predominantly in those with underlying medical conditions that put them at high risk of infection. Attack rates for those older than age 65 have been lower than expected, indicating that this age group may have some immunity based on past infection. The pandemic virus so far has been sensitive to both oseltamivir (Tamiflu) and zanamivir (Relenza). The resistance patterns of the key viruses from last flu season showed that the H1N1 seasonal virus was resistant to oseltamivir but sensitive to zanamivir and the adamantanes (rimantadine and amantadine), while the H3N2 virus that circulated last year was sensitive to oseltamivir.3
Fall flu season: Be prepared
So, what can you expect this fall? With pandemic H1N1 still causing illness and strains of seasonal virus circulating elsewhere in the world, no one knows for sure. But it is very likely that we will experience much higher rates of pandemic influenza once schools reopen and children begin to congregate. It is also likely we will have pandemic influenza circulating along with seasonal influenza viruses this fall and into 2010.
Immunize for seasonal flu, now
The 2009-2010 seasonal influenza vaccine will contain antigens from 3 strains: a nonpandemic H1N1 influenza A strain, an H3N2 influenza A strain, and an influenza B strain.4 These 3 antigens will be in all seasonal influenza vaccine products, whether they are the trivalent influenza vaccine given by injection or the live attenuated influenza vaccine provided as a nasal spray. The CDC is recommending immunization against seasonal influenza as soon as the vaccine is available.
The groups for whom seasonal influenza vaccine is recommended have not changed from last year. The recommendations are summarized in the TABLE.
TABLE
Who should get seasonal flu vaccine, 2009-2010?
| All children and adolescents ages 6 months through 18 years |
| Adults ≥50 years of age |
| Individuals at risk for medical complications |
| Women who will be pregnant during the influenza season |
| Adults and children who have chronic pulmonary (including asthma), cardiovascular (except hypertension), renal, hepatic, hematologic, or metabolic disorders (including diabetes mellitus) |
| Adults and children who have immunosuppression (including immunosuppression caused by medications or by HIV) |
| Adults and children who have any condition (eg, cognitive dysfunction, spinal cord injury, seizure disorder, or other neuromuscular disorder) that can compromise respiratory function or the handling of respiratory secretions or increase the risk for aspiration |
| Residents of nursing homes and other chronic-care facilities |
| Individuals who live with, or care for, people at high risk for influenza-related complications |
| Health care personnel |
| Healthy household contacts (including children) and caregivers of children <5 years of age and adults ≥50 years |
| Healthy household contacts (including children) of individuals with medical conditions that put them at higher risk for severe complications from influenza. |
| Source: Centers for Disease Control and Prevention. MMWR Recomm Rep. 2009.4 |
Pandemic flu vaccine will be available in the fall
The vaccine for pandemic H1N1 is being produced, and the Department of Health and Human Services is projecting it to be available starting in mid- to late October. The supply will be limited at first, with increasing quantities produced as time progresses. The intent is to produce 600 million doses, or 2 per US resident, since 2 doses will be required.
Who should get the vaccine for pandemic H1N1? At its meeting at the end of July, the Advisory Committee on Immunization Practices (ACIP) recommended that vaccination efforts focus on 5 key populations:
- pregnant women
- people who live with, or care for, children <6 months of age
- health care and emergency services personnel
- individuals between the ages of 6 months and 24 years
- individuals 25 to 64 years of age who are at higher risk for novel H1N1 because of chronic health disorders or compromised immune systems.
In the event of initial shortages of the vaccine, the first 3 groups listed above should be given priority, along with children 6 months through 4 years of age and children 5 through 18 years who have chronic medical conditions.5 In the event of a vaccine surplus (due to low demand and/or faster-than-expected supply), prioritization will not apply and the vaccine should be administered to anyone requesting it who does not have a contraindication.
It is not known how the pandemic influenza vaccine will be distributed and administered. The extent of involvement by physician offices and clinics is undetermined and may vary by locale. There may be extensive use of mass immunization clinics and school clinics to administer the vaccine quickly. Administration will be complicated by the need for 2 doses for protection and a perception by the public that the pandemic virus is not a major concern.
Medical practices may be administering 2 influenza vaccines with different dose requirements: a single dose for seasonal influenza vaccine (except for children <9 years who are being vaccinated for the first time; they get 2 doses), and 2 doses for pandemic vaccine.
Antivirals protect vulnerable patients
Antiviral medications can be used for chemoprophylaxis, both to prevent infection in patients with a high-risk medical condition who are not, or cannot be, vaccinated (chemoprevention), and for post-exposure prophylaxis (PEP) for those who are at risk for complications or want to avoid illness. PEP is time limited (5 days), while chemoprevention may be needed for the duration of potential exposure during an outbreak or epidemic.
PEP should be considered for residents in an assisted living facility during an influenza outbreak, and for individuals who are at higher risk for influenza-related complications and who have had recent household or other close contact with a person with laboratory-confirmed influenza. Chemoprevention is an option with limited applicability at this time. If the pandemic virus were to become more virulent, it might be considered for health care workers until they had received 2 doses of vaccine.
Follow recommendations for antiviral treatment
Because resistance patterns differ among flu viruses, the decision on which antiviral or combination of antivirals to use depends on the predominant viruses circulating in the community and on laboratory tests from the infected patient to determine the influenza type involved. Current recommendations for seasonal influenza can be found at http://www2a.cdc.gov/han/ArchiveSys/ViewMsgV.asp?AlertNum=00279, and recommendations for pandemic influenza are at http://www.cdc.gov/h1n1flu/recommendations.htm#table1. These recommendations may change as the season progresses and viral resistance patterns are determined.
Consider antiviral treatment for those at high risk for complications from the virus. These include anyone hospitalized for influenza, children <5 years of age (especially those <2 years), adults ≥65 years of age, and individuals with the following conditions:
- chronic pulmonary (including asthma), cardiovascular (except hypertension), renal, hepatic, hematologic (including sickle cell disease), neurologic, neuromuscular, or metabolic disorders (including diabetes mellitus)
- immunosuppression, including that caused by medications or by HIV
- pregnant women
- individuals <19 years of age who are receiving long-term aspirin therapy
- residents of nursing homes and other chronic-care facilities.
The evidence for antiviral effectiveness is strongest if it is given within the first 48 hours of symptom onset, although in hospitalized patients, there is some evidence of effectiveness if started after this time.
Be diligent about infection control
Physicians and other health care workers will need to practice good infection control this flu season. This has been the topic of a previous Practice Alert.6 All health care workers should be fully immunized against influenza—seasonal and pandemic. In addition, each clinical practice should plan on implementing policies to prevent the spread of infection within the clinic or office. Such policies might include scheduling patients with respiratory illnesses for later in the day, separating patients with respiratory illnesses from other patients, requiring patients to cover their nose and mouth when they cough or sneeze, and providing tissues and hand sanitizers for patients and staff.
Physicians and staff will need to take measures to protect themselves from infection by frequent hand washing, avoiding work when ill, and using personal protective equipment when there is potential exposure to respiratory droplets.7 It will also be important to teach families to follow infection control practices at home whenever a household member has an influenza-like illness. Recommendations for home care can be found at www.cdc.gov/h1n1flu/guidance_homecare.htm/?x_cid=ccu071309_HomeCareGuidance_e.
Stay on top of the situation
As this influenza season progresses, keeping current about influenza recommendations will be crucial. The 3 issues to say on top of are:
- Who should receive the vaccine for pandemic influenza and where will it be administered?
- What influenza viruses are circulating in the community?
- What is happening to antiviral resistance patterns and how are changes in these patterns affecting recommendations for treatment and chemoprophylaxis?
Web sites that will keep you up to date
- The CDC influenza Web site: http://www.cdc.gov/flu
- Your local and state public health department Web sites
- The American Academy of Family Physicians (AAFP) Web site: http://www.aafp.org/online/en/home.html.
CORRESPONDENCE
Doug Campos-Outcalt, MD, MPA, 550 E. Van Buren, Phoenix, AZ 85004; dougco@u.arizona.edu
1. CDC. Novel H1N1 flu situation update: August 6, 2009. Available at: http://www.cdc.gov/h1n1flu/update.htm. Accessed August 12, 2009.
2. WHO. Pandemic (H1N1) 2009-update 60. July 31, 2009. Available at: http://www.who.int/csr/don/2009_08_04/en/index.html. Accessed August 5, 2009.
3. CDC. CDC issues interim recommendations for the use of influenza antiviral medications in the setting of oseltamivir resistance among circulating influenza A (H1N1) viruses, 2008-09 influenza season [CDC health advisory]. December 19, 2008. Available at: http://www2a.cdc.gov/han/Archivesys/ViewMsgV.asp?AlertNum=00279. Accessed August 5, 2009.
4. CDC. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2009. MMWR Recomm Rep. 2009;58(RR-8):1-52. Available at: http://www.cdc.gov/mmwr/preview/mmwrhtml/rr5808a1.htm. Accessed August 5, 2009.
5. CDC. CDC advisors make recommendations for use of vaccine against novel H1N1 [press release]. July 29, 2009. Available at: http://www.cdc.gov/media/pressrel/2009/r090729b.htm. Accessed August 5, 2009.
6. Campos-Outcalt D. Infection control in the outpatient setting. J Fam Pract. 2004;53:485-488.
7. CDC. 10 steps you can take: actions for novel H1N1 influenza planning and response for medical offices and outpatient facilities. July 14, 2009. Available at: http://www.cdc.gov/h1n1flu/10steps.htm. Accessed August 3, 2009.
This coming flu season will be interesting—and confusing. As of August 6, 2009, the Centers for Disease Control and Prevention (CDC) reported 6506 hospitalized cases and 436 deaths from the pandemic H1N1 flu virus since the first US cases were reported in April 2009.1 (Reporting on individual confirmed and probable cases has been discontinued.) On July 31, the World Health Organization reported pandemic influenza in 168 countries, with 162,380 reported cases and 1154 deaths.2 At the same time the pandemic was developing, the seasonal flu of 2009—a relatively mild year—was tapering off. The pandemic influenza has continued to cause widespread disease in the United States throughout the summer, a somewhat unusual pattern for influenza.
So far, pandemic H1N1 flu is relatively benign, treatable
The pandemic virus, though highly infectious, has had a low case fatality rate up to now. Deaths have occurred predominantly in those with underlying medical conditions that put them at high risk of infection. Attack rates for those older than age 65 have been lower than expected, indicating that this age group may have some immunity based on past infection. The pandemic virus so far has been sensitive to both oseltamivir (Tamiflu) and zanamivir (Relenza). The resistance patterns of the key viruses from last flu season showed that the H1N1 seasonal virus was resistant to oseltamivir but sensitive to zanamivir and the adamantanes (rimantadine and amantadine), while the H3N2 virus that circulated last year was sensitive to oseltamivir.3
Fall flu season: Be prepared
So, what can you expect this fall? With pandemic H1N1 still causing illness and strains of seasonal virus circulating elsewhere in the world, no one knows for sure. But it is very likely that we will experience much higher rates of pandemic influenza once schools reopen and children begin to congregate. It is also likely we will have pandemic influenza circulating along with seasonal influenza viruses this fall and into 2010.
Immunize for seasonal flu, now
The 2009-2010 seasonal influenza vaccine will contain antigens from 3 strains: a nonpandemic H1N1 influenza A strain, an H3N2 influenza A strain, and an influenza B strain.4 These 3 antigens will be in all seasonal influenza vaccine products, whether they are the trivalent influenza vaccine given by injection or the live attenuated influenza vaccine provided as a nasal spray. The CDC is recommending immunization against seasonal influenza as soon as the vaccine is available.
The groups for whom seasonal influenza vaccine is recommended have not changed from last year. The recommendations are summarized in the TABLE.
TABLE
Who should get seasonal flu vaccine, 2009-2010?
| All children and adolescents ages 6 months through 18 years |
| Adults ≥50 years of age |
| Individuals at risk for medical complications |
| Women who will be pregnant during the influenza season |
| Adults and children who have chronic pulmonary (including asthma), cardiovascular (except hypertension), renal, hepatic, hematologic, or metabolic disorders (including diabetes mellitus) |
| Adults and children who have immunosuppression (including immunosuppression caused by medications or by HIV) |
| Adults and children who have any condition (eg, cognitive dysfunction, spinal cord injury, seizure disorder, or other neuromuscular disorder) that can compromise respiratory function or the handling of respiratory secretions or increase the risk for aspiration |
| Residents of nursing homes and other chronic-care facilities |
| Individuals who live with, or care for, people at high risk for influenza-related complications |
| Health care personnel |
| Healthy household contacts (including children) and caregivers of children <5 years of age and adults ≥50 years |
| Healthy household contacts (including children) of individuals with medical conditions that put them at higher risk for severe complications from influenza. |
| Source: Centers for Disease Control and Prevention. MMWR Recomm Rep. 2009.4 |
Pandemic flu vaccine will be available in the fall
The vaccine for pandemic H1N1 is being produced, and the Department of Health and Human Services is projecting it to be available starting in mid- to late October. The supply will be limited at first, with increasing quantities produced as time progresses. The intent is to produce 600 million doses, or 2 per US resident, since 2 doses will be required.
Who should get the vaccine for pandemic H1N1? At its meeting at the end of July, the Advisory Committee on Immunization Practices (ACIP) recommended that vaccination efforts focus on 5 key populations:
- pregnant women
- people who live with, or care for, children <6 months of age
- health care and emergency services personnel
- individuals between the ages of 6 months and 24 years
- individuals 25 to 64 years of age who are at higher risk for novel H1N1 because of chronic health disorders or compromised immune systems.
In the event of initial shortages of the vaccine, the first 3 groups listed above should be given priority, along with children 6 months through 4 years of age and children 5 through 18 years who have chronic medical conditions.5 In the event of a vaccine surplus (due to low demand and/or faster-than-expected supply), prioritization will not apply and the vaccine should be administered to anyone requesting it who does not have a contraindication.
It is not known how the pandemic influenza vaccine will be distributed and administered. The extent of involvement by physician offices and clinics is undetermined and may vary by locale. There may be extensive use of mass immunization clinics and school clinics to administer the vaccine quickly. Administration will be complicated by the need for 2 doses for protection and a perception by the public that the pandemic virus is not a major concern.
Medical practices may be administering 2 influenza vaccines with different dose requirements: a single dose for seasonal influenza vaccine (except for children <9 years who are being vaccinated for the first time; they get 2 doses), and 2 doses for pandemic vaccine.
Antivirals protect vulnerable patients
Antiviral medications can be used for chemoprophylaxis, both to prevent infection in patients with a high-risk medical condition who are not, or cannot be, vaccinated (chemoprevention), and for post-exposure prophylaxis (PEP) for those who are at risk for complications or want to avoid illness. PEP is time limited (5 days), while chemoprevention may be needed for the duration of potential exposure during an outbreak or epidemic.
PEP should be considered for residents in an assisted living facility during an influenza outbreak, and for individuals who are at higher risk for influenza-related complications and who have had recent household or other close contact with a person with laboratory-confirmed influenza. Chemoprevention is an option with limited applicability at this time. If the pandemic virus were to become more virulent, it might be considered for health care workers until they had received 2 doses of vaccine.
Follow recommendations for antiviral treatment
Because resistance patterns differ among flu viruses, the decision on which antiviral or combination of antivirals to use depends on the predominant viruses circulating in the community and on laboratory tests from the infected patient to determine the influenza type involved. Current recommendations for seasonal influenza can be found at http://www2a.cdc.gov/han/ArchiveSys/ViewMsgV.asp?AlertNum=00279, and recommendations for pandemic influenza are at http://www.cdc.gov/h1n1flu/recommendations.htm#table1. These recommendations may change as the season progresses and viral resistance patterns are determined.
Consider antiviral treatment for those at high risk for complications from the virus. These include anyone hospitalized for influenza, children <5 years of age (especially those <2 years), adults ≥65 years of age, and individuals with the following conditions:
- chronic pulmonary (including asthma), cardiovascular (except hypertension), renal, hepatic, hematologic (including sickle cell disease), neurologic, neuromuscular, or metabolic disorders (including diabetes mellitus)
- immunosuppression, including that caused by medications or by HIV
- pregnant women
- individuals <19 years of age who are receiving long-term aspirin therapy
- residents of nursing homes and other chronic-care facilities.
The evidence for antiviral effectiveness is strongest if it is given within the first 48 hours of symptom onset, although in hospitalized patients, there is some evidence of effectiveness if started after this time.
Be diligent about infection control
Physicians and other health care workers will need to practice good infection control this flu season. This has been the topic of a previous Practice Alert.6 All health care workers should be fully immunized against influenza—seasonal and pandemic. In addition, each clinical practice should plan on implementing policies to prevent the spread of infection within the clinic or office. Such policies might include scheduling patients with respiratory illnesses for later in the day, separating patients with respiratory illnesses from other patients, requiring patients to cover their nose and mouth when they cough or sneeze, and providing tissues and hand sanitizers for patients and staff.
Physicians and staff will need to take measures to protect themselves from infection by frequent hand washing, avoiding work when ill, and using personal protective equipment when there is potential exposure to respiratory droplets.7 It will also be important to teach families to follow infection control practices at home whenever a household member has an influenza-like illness. Recommendations for home care can be found at www.cdc.gov/h1n1flu/guidance_homecare.htm/?x_cid=ccu071309_HomeCareGuidance_e.
Stay on top of the situation
As this influenza season progresses, keeping current about influenza recommendations will be crucial. The 3 issues to say on top of are:
- Who should receive the vaccine for pandemic influenza and where will it be administered?
- What influenza viruses are circulating in the community?
- What is happening to antiviral resistance patterns and how are changes in these patterns affecting recommendations for treatment and chemoprophylaxis?
Web sites that will keep you up to date
- The CDC influenza Web site: http://www.cdc.gov/flu
- Your local and state public health department Web sites
- The American Academy of Family Physicians (AAFP) Web site: http://www.aafp.org/online/en/home.html.
CORRESPONDENCE
Doug Campos-Outcalt, MD, MPA, 550 E. Van Buren, Phoenix, AZ 85004; dougco@u.arizona.edu
This coming flu season will be interesting—and confusing. As of August 6, 2009, the Centers for Disease Control and Prevention (CDC) reported 6506 hospitalized cases and 436 deaths from the pandemic H1N1 flu virus since the first US cases were reported in April 2009.1 (Reporting on individual confirmed and probable cases has been discontinued.) On July 31, the World Health Organization reported pandemic influenza in 168 countries, with 162,380 reported cases and 1154 deaths.2 At the same time the pandemic was developing, the seasonal flu of 2009—a relatively mild year—was tapering off. The pandemic influenza has continued to cause widespread disease in the United States throughout the summer, a somewhat unusual pattern for influenza.
So far, pandemic H1N1 flu is relatively benign, treatable
The pandemic virus, though highly infectious, has had a low case fatality rate up to now. Deaths have occurred predominantly in those with underlying medical conditions that put them at high risk of infection. Attack rates for those older than age 65 have been lower than expected, indicating that this age group may have some immunity based on past infection. The pandemic virus so far has been sensitive to both oseltamivir (Tamiflu) and zanamivir (Relenza). The resistance patterns of the key viruses from last flu season showed that the H1N1 seasonal virus was resistant to oseltamivir but sensitive to zanamivir and the adamantanes (rimantadine and amantadine), while the H3N2 virus that circulated last year was sensitive to oseltamivir.3
Fall flu season: Be prepared
So, what can you expect this fall? With pandemic H1N1 still causing illness and strains of seasonal virus circulating elsewhere in the world, no one knows for sure. But it is very likely that we will experience much higher rates of pandemic influenza once schools reopen and children begin to congregate. It is also likely we will have pandemic influenza circulating along with seasonal influenza viruses this fall and into 2010.
Immunize for seasonal flu, now
The 2009-2010 seasonal influenza vaccine will contain antigens from 3 strains: a nonpandemic H1N1 influenza A strain, an H3N2 influenza A strain, and an influenza B strain.4 These 3 antigens will be in all seasonal influenza vaccine products, whether they are the trivalent influenza vaccine given by injection or the live attenuated influenza vaccine provided as a nasal spray. The CDC is recommending immunization against seasonal influenza as soon as the vaccine is available.
The groups for whom seasonal influenza vaccine is recommended have not changed from last year. The recommendations are summarized in the TABLE.
TABLE
Who should get seasonal flu vaccine, 2009-2010?
| All children and adolescents ages 6 months through 18 years |
| Adults ≥50 years of age |
| Individuals at risk for medical complications |
| Women who will be pregnant during the influenza season |
| Adults and children who have chronic pulmonary (including asthma), cardiovascular (except hypertension), renal, hepatic, hematologic, or metabolic disorders (including diabetes mellitus) |
| Adults and children who have immunosuppression (including immunosuppression caused by medications or by HIV) |
| Adults and children who have any condition (eg, cognitive dysfunction, spinal cord injury, seizure disorder, or other neuromuscular disorder) that can compromise respiratory function or the handling of respiratory secretions or increase the risk for aspiration |
| Residents of nursing homes and other chronic-care facilities |
| Individuals who live with, or care for, people at high risk for influenza-related complications |
| Health care personnel |
| Healthy household contacts (including children) and caregivers of children <5 years of age and adults ≥50 years |
| Healthy household contacts (including children) of individuals with medical conditions that put them at higher risk for severe complications from influenza. |
| Source: Centers for Disease Control and Prevention. MMWR Recomm Rep. 2009.4 |
Pandemic flu vaccine will be available in the fall
The vaccine for pandemic H1N1 is being produced, and the Department of Health and Human Services is projecting it to be available starting in mid- to late October. The supply will be limited at first, with increasing quantities produced as time progresses. The intent is to produce 600 million doses, or 2 per US resident, since 2 doses will be required.
Who should get the vaccine for pandemic H1N1? At its meeting at the end of July, the Advisory Committee on Immunization Practices (ACIP) recommended that vaccination efforts focus on 5 key populations:
- pregnant women
- people who live with, or care for, children <6 months of age
- health care and emergency services personnel
- individuals between the ages of 6 months and 24 years
- individuals 25 to 64 years of age who are at higher risk for novel H1N1 because of chronic health disorders or compromised immune systems.
In the event of initial shortages of the vaccine, the first 3 groups listed above should be given priority, along with children 6 months through 4 years of age and children 5 through 18 years who have chronic medical conditions.5 In the event of a vaccine surplus (due to low demand and/or faster-than-expected supply), prioritization will not apply and the vaccine should be administered to anyone requesting it who does not have a contraindication.
It is not known how the pandemic influenza vaccine will be distributed and administered. The extent of involvement by physician offices and clinics is undetermined and may vary by locale. There may be extensive use of mass immunization clinics and school clinics to administer the vaccine quickly. Administration will be complicated by the need for 2 doses for protection and a perception by the public that the pandemic virus is not a major concern.
Medical practices may be administering 2 influenza vaccines with different dose requirements: a single dose for seasonal influenza vaccine (except for children <9 years who are being vaccinated for the first time; they get 2 doses), and 2 doses for pandemic vaccine.
Antivirals protect vulnerable patients
Antiviral medications can be used for chemoprophylaxis, both to prevent infection in patients with a high-risk medical condition who are not, or cannot be, vaccinated (chemoprevention), and for post-exposure prophylaxis (PEP) for those who are at risk for complications or want to avoid illness. PEP is time limited (5 days), while chemoprevention may be needed for the duration of potential exposure during an outbreak or epidemic.
PEP should be considered for residents in an assisted living facility during an influenza outbreak, and for individuals who are at higher risk for influenza-related complications and who have had recent household or other close contact with a person with laboratory-confirmed influenza. Chemoprevention is an option with limited applicability at this time. If the pandemic virus were to become more virulent, it might be considered for health care workers until they had received 2 doses of vaccine.
Follow recommendations for antiviral treatment
Because resistance patterns differ among flu viruses, the decision on which antiviral or combination of antivirals to use depends on the predominant viruses circulating in the community and on laboratory tests from the infected patient to determine the influenza type involved. Current recommendations for seasonal influenza can be found at http://www2a.cdc.gov/han/ArchiveSys/ViewMsgV.asp?AlertNum=00279, and recommendations for pandemic influenza are at http://www.cdc.gov/h1n1flu/recommendations.htm#table1. These recommendations may change as the season progresses and viral resistance patterns are determined.
Consider antiviral treatment for those at high risk for complications from the virus. These include anyone hospitalized for influenza, children <5 years of age (especially those <2 years), adults ≥65 years of age, and individuals with the following conditions:
- chronic pulmonary (including asthma), cardiovascular (except hypertension), renal, hepatic, hematologic (including sickle cell disease), neurologic, neuromuscular, or metabolic disorders (including diabetes mellitus)
- immunosuppression, including that caused by medications or by HIV
- pregnant women
- individuals <19 years of age who are receiving long-term aspirin therapy
- residents of nursing homes and other chronic-care facilities.
The evidence for antiviral effectiveness is strongest if it is given within the first 48 hours of symptom onset, although in hospitalized patients, there is some evidence of effectiveness if started after this time.
Be diligent about infection control
Physicians and other health care workers will need to practice good infection control this flu season. This has been the topic of a previous Practice Alert.6 All health care workers should be fully immunized against influenza—seasonal and pandemic. In addition, each clinical practice should plan on implementing policies to prevent the spread of infection within the clinic or office. Such policies might include scheduling patients with respiratory illnesses for later in the day, separating patients with respiratory illnesses from other patients, requiring patients to cover their nose and mouth when they cough or sneeze, and providing tissues and hand sanitizers for patients and staff.
Physicians and staff will need to take measures to protect themselves from infection by frequent hand washing, avoiding work when ill, and using personal protective equipment when there is potential exposure to respiratory droplets.7 It will also be important to teach families to follow infection control practices at home whenever a household member has an influenza-like illness. Recommendations for home care can be found at www.cdc.gov/h1n1flu/guidance_homecare.htm/?x_cid=ccu071309_HomeCareGuidance_e.
Stay on top of the situation
As this influenza season progresses, keeping current about influenza recommendations will be crucial. The 3 issues to say on top of are:
- Who should receive the vaccine for pandemic influenza and where will it be administered?
- What influenza viruses are circulating in the community?
- What is happening to antiviral resistance patterns and how are changes in these patterns affecting recommendations for treatment and chemoprophylaxis?
Web sites that will keep you up to date
- The CDC influenza Web site: http://www.cdc.gov/flu
- Your local and state public health department Web sites
- The American Academy of Family Physicians (AAFP) Web site: http://www.aafp.org/online/en/home.html.
CORRESPONDENCE
Doug Campos-Outcalt, MD, MPA, 550 E. Van Buren, Phoenix, AZ 85004; dougco@u.arizona.edu
1. CDC. Novel H1N1 flu situation update: August 6, 2009. Available at: http://www.cdc.gov/h1n1flu/update.htm. Accessed August 12, 2009.
2. WHO. Pandemic (H1N1) 2009-update 60. July 31, 2009. Available at: http://www.who.int/csr/don/2009_08_04/en/index.html. Accessed August 5, 2009.
3. CDC. CDC issues interim recommendations for the use of influenza antiviral medications in the setting of oseltamivir resistance among circulating influenza A (H1N1) viruses, 2008-09 influenza season [CDC health advisory]. December 19, 2008. Available at: http://www2a.cdc.gov/han/Archivesys/ViewMsgV.asp?AlertNum=00279. Accessed August 5, 2009.
4. CDC. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2009. MMWR Recomm Rep. 2009;58(RR-8):1-52. Available at: http://www.cdc.gov/mmwr/preview/mmwrhtml/rr5808a1.htm. Accessed August 5, 2009.
5. CDC. CDC advisors make recommendations for use of vaccine against novel H1N1 [press release]. July 29, 2009. Available at: http://www.cdc.gov/media/pressrel/2009/r090729b.htm. Accessed August 5, 2009.
6. Campos-Outcalt D. Infection control in the outpatient setting. J Fam Pract. 2004;53:485-488.
7. CDC. 10 steps you can take: actions for novel H1N1 influenza planning and response for medical offices and outpatient facilities. July 14, 2009. Available at: http://www.cdc.gov/h1n1flu/10steps.htm. Accessed August 3, 2009.
1. CDC. Novel H1N1 flu situation update: August 6, 2009. Available at: http://www.cdc.gov/h1n1flu/update.htm. Accessed August 12, 2009.
2. WHO. Pandemic (H1N1) 2009-update 60. July 31, 2009. Available at: http://www.who.int/csr/don/2009_08_04/en/index.html. Accessed August 5, 2009.
3. CDC. CDC issues interim recommendations for the use of influenza antiviral medications in the setting of oseltamivir resistance among circulating influenza A (H1N1) viruses, 2008-09 influenza season [CDC health advisory]. December 19, 2008. Available at: http://www2a.cdc.gov/han/Archivesys/ViewMsgV.asp?AlertNum=00279. Accessed August 5, 2009.
4. CDC. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2009. MMWR Recomm Rep. 2009;58(RR-8):1-52. Available at: http://www.cdc.gov/mmwr/preview/mmwrhtml/rr5808a1.htm. Accessed August 5, 2009.
5. CDC. CDC advisors make recommendations for use of vaccine against novel H1N1 [press release]. July 29, 2009. Available at: http://www.cdc.gov/media/pressrel/2009/r090729b.htm. Accessed August 5, 2009.
6. Campos-Outcalt D. Infection control in the outpatient setting. J Fam Pract. 2004;53:485-488.
7. CDC. 10 steps you can take: actions for novel H1N1 influenza planning and response for medical offices and outpatient facilities. July 14, 2009. Available at: http://www.cdc.gov/h1n1flu/10steps.htm. Accessed August 3, 2009.