User login
Preventive services: The good, the bad, and the unproven
The past 12 months have been busy ones for the United States Preventive Services Task Force (USPSTF), which issued 34 new recommendations since our last Practice Alert on the group’s activity a year ago. Some recommendations address controversial topics, such as cholesterol screening, and several others—on topics such as prostate cancer screening and acceptable tests for detecting colorectal cancer—differ from those of such prominent groups as the American Cancer Society (ACS).
TABLE 1 provides a breakdown of the 5 categories of USPSTF recommendations (A, B, C, D, I). We’ll start with recent D recommendations (TABLE 2), services the Task Force recommends against, to emphasize that some preventive measures—even if they are widely touted—either provide no benefit or cause more harms than benefits.
TABLE 1
USPSTF recommendation categories
| A Recommendation: The Task Force recommends the service. There is high certainty that the net benefit is substantial. |
| B Recommendation: The Task Force recommends this service. There is high certainty that the net benefit is moderate or there is moderate certainty that the net benefit is moderate to substantial. |
| C Recommendation: The Task Force recommends against routinely providing the service. There may be considerations that support providing the service in an individual patient. There is at least moderate certainty that the net benefit is small. |
| D Recommendation: The Task Force recommends against the service. There is moderate or high certainty that the service has no net benefit or that the harms outweigh the benefits. |
| I Statement: The Task Force concludes that the current evidence is insufficient to assess the balance of benefits and harms of the service. Evidence is lacking, of poor quality, or conflicting, and the balance of benefits and harms cannot be determined. |
TABLE 2
The USPSTF recommends AGAINST
|
What not to do
The most notable new D recommendations advise against screening men ≥75 years of age for prostate cancer and against screening for colorectal cancer after age 85. The Task Force also recommends against routine screening for colorectal cancer after age 75, although individual patient considerations may influence your decision about this screen for patients between ages 76 and 85. Bear in mind that the benefits of early detection of colon cancer decline after age 75 because of the time lag between early intervention and benefit and because of competing causes of morbidity and mortality.1
Cancer screening controversies. The recommendations for an age cutoff for prostate and colon cancer screening differ from those of the ACS, which lists no age cutoff for screening for either condition.2 In fact, the Task Force does not recommend screening for prostate cancer at all. Its rationale is that before age 75, the evidence is insufficient to evaluate benefits and harms, and after 75 there is good evidence that screening does more harm than good. The ACS no longer recommends routine prostate cancer screening, but does say that when a patient leaves the decision to the physician, screening should be performed.
Thumbs down on these, too. The Task Force now recommends against using spirometry to screen for chronic obstructive pulmonary disease and against using aspirin for preventing stroke in women <55 years and myocardial infarction (MI) in men <45 years. (See below for a fuller discussion of aspirin as a preventive measure.) The Task Force also recommends against screening for asymptomatic bacteriuria in men and nonpregnant women.
Recommended interventions
Now for the preventive interventions the USPSTF advises you to perform. They include:
Prescribing low-dose aspirin. The most complicated positive recommendations are those for low-dose aspirin to prevent MI in men and stroke in women. Aspirin is effective in preventing these conditions, but carries the risk of major gastrointestinal (GI) bleeding and cerebral hemorrhage. For younger patients, as we’ve seen in the previous section, the Task Force finds the risks of prophylactic low-dose aspirin therapy outweigh the benefits. But for older patients (men between the ages of 45 and 79 years and women ages 55-79), aspirin is recommended when the potential benefit of reducing the incidence of MI in men and stroke in women outweigh the harms. To assist clinicians in weighing the potential benefits and harms, the USPSTF provides a link to a coronary heart disease risk calculator, as well as several tables comparing numbers of prevented heart attacks for men and strokes for women by age and risk category, as well as risks of bleeding complications.3
Screening for hypercholesterolemia. The Task Force’s recommendations for dyslipidemia screening differ markedly from those of the American Heart Association and the Final Report of the National Cholesterol Education Program (NCEP) Expert Panel, which recommend routine screening for all adults starting at age 20 with no age cutoff.4 The USPSTF recommends deferring screening until patients are older, except for those at increased risk of coronary heart disease. This controversy was described in a 2008 Practice Alert.5
Screening for diabetes. The only asymptomatic patients the Task Force recommends screening for diabetes are those with a sustained blood pressure of more than 135/80 mm Hg, treated or untreated. The American Diabetes Association (ADA) would cast a wider net, recommending that you consider screening for prediabetes or diabetes in those ≥45 years of age, particularly in those with a body mass index of ≥25 kg/m2, and in overweight patients <45 years of age who have another risk factor for diabetes.6
Screening for colorectal cancer. The Task Force recommends screening adults starting at age 50 until age 75, using fecal occult blood testing, sigmoidoscopy, or colonoscopy. The ACS also recommends these screening modalities, but adds CT colonography and fecal DNA testing to the list of acceptable methods. The USPSTF found insufficient evidence to evaluate the benefits and harms of these newer tests and expressed concern over the high rate of incidental findings and the unknown long-term effects of radiation from CT colonography.
Screening adolescents. The Task Force is in favor of screening teenagers for major depressive disorder (MDD), as long as systems are in place to provide accurate diagnosis, therapy, and follow-up. High-intensity behavioral counseling for sexually active teens and adults at risk is also endorsed for the prevention of sexually transmitted infections. In both areas, however, the Task Force recognizes that adequately addressing these issues will require more than brief office- or clinic-based interventions.
Caring for pregnant women and newborns. According to the USPSTF, pregnant women should be screened for asymptomatic bacteriuria, advised to take a daily folic acid supplement, counseled about tobacco use, and encouraged to breastfeed. Newborns should be screened for congenital hypothyroidism, phenylketonuria, and hearing loss. These most recent A and B recommendations from the USPSTF are summarized in TABLE 3.
TABLE 3
The USPSTF recommends FOR
| CARDIOVASCULAR DISEASE PREVENTION |
|
| CANCER SCREENING |
|
| PREGNANCY |
|
| NEWBORNS |
|
| ADOLESCENTS |
|
Not proven
When evidence is not available, some organizations are willing to issue guidelines based on expert opinion or consensus. Not so the USPSTF. When the Task Force members find current evidence is not sufficient to make a judgment, they put the intervention into Category I, for Insufficient. The new I recommendations range from aspirin to prevent MI and stroke in those ≥80 years to screening children for MDD and performing whole body skin examinations to detect early manifestations of skin cancer. The new I recommendations are listed in TABLE 4.
TABLE 4
Evidence is INSUFFICIENT to recommend for or against
|
What’s the take-home message?
All of these recent Task Force decisions add substantially to the full set of Task Force recommendations, which can be found at www.ahrq.gov/CLINIC/uspstfix.htm. Given the large number of level A and B recommendations from the Task Force, clinicians are faced with the dilemma of limited time to accomplish all the recommendations. It is reasonable to concentrate on the positive recommendations and avoid performing the interventions recommended against. The interventions in the “I” category are not as clear-cut and clinicians will continue to struggle with them, particularly when other professional organizations recommend them.
CORRESPONDENCE
Doug Campos-Outcalt, MD, MPA, 550 E. Van Buren, Phoenix, AZ 85004; dougco@u.arizona.edu.
1. US Preventive Services Task Force. Screening for colorectal cancer. October 2008. Available at: www.ahrq.gov/clinic/uspstf/uspscolo.htm. Accessed June 3, 2009.
2. American Cancer Society guidelines for early detection of cancer. Last revised May 21, 2009. Available at: http://www.cancer.org/docroot/PED/content/PED_2_3X_ACS_Cancer_Detection_Guidelines_36.asp?sitearea=PED. Accessed June 3, 2009.
3. US Preventive Services Task Force. Aspirin for the prevention of cardiovascular disease: recommendation statement. March 2009. Available at: http://www.ahrq.gov/clinic/uspstf09/aspirincvd/aspcvdrs.htm. Accessed June 3, 2009.
4. National Cholesterol Education Program. Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) Final Report. Circulation. 2002;106:3143-3421.Available at: http://circ.ahajournals.org/cgi/content/full/106/25/3143. Accessed June 3, 2009.
5. Campos-Outcalt D. USPSTF scales back approach to lipid screening for women. J Fam Pract. 2008;57:740-742.
6. American Diabetes Association. Standards of medical care in diabetes—2008. Diabetes Care. 2008;31(suppl 1):S12-S54.
The past 12 months have been busy ones for the United States Preventive Services Task Force (USPSTF), which issued 34 new recommendations since our last Practice Alert on the group’s activity a year ago. Some recommendations address controversial topics, such as cholesterol screening, and several others—on topics such as prostate cancer screening and acceptable tests for detecting colorectal cancer—differ from those of such prominent groups as the American Cancer Society (ACS).
TABLE 1 provides a breakdown of the 5 categories of USPSTF recommendations (A, B, C, D, I). We’ll start with recent D recommendations (TABLE 2), services the Task Force recommends against, to emphasize that some preventive measures—even if they are widely touted—either provide no benefit or cause more harms than benefits.
TABLE 1
USPSTF recommendation categories
| A Recommendation: The Task Force recommends the service. There is high certainty that the net benefit is substantial. |
| B Recommendation: The Task Force recommends this service. There is high certainty that the net benefit is moderate or there is moderate certainty that the net benefit is moderate to substantial. |
| C Recommendation: The Task Force recommends against routinely providing the service. There may be considerations that support providing the service in an individual patient. There is at least moderate certainty that the net benefit is small. |
| D Recommendation: The Task Force recommends against the service. There is moderate or high certainty that the service has no net benefit or that the harms outweigh the benefits. |
| I Statement: The Task Force concludes that the current evidence is insufficient to assess the balance of benefits and harms of the service. Evidence is lacking, of poor quality, or conflicting, and the balance of benefits and harms cannot be determined. |
TABLE 2
The USPSTF recommends AGAINST
|
What not to do
The most notable new D recommendations advise against screening men ≥75 years of age for prostate cancer and against screening for colorectal cancer after age 85. The Task Force also recommends against routine screening for colorectal cancer after age 75, although individual patient considerations may influence your decision about this screen for patients between ages 76 and 85. Bear in mind that the benefits of early detection of colon cancer decline after age 75 because of the time lag between early intervention and benefit and because of competing causes of morbidity and mortality.1
Cancer screening controversies. The recommendations for an age cutoff for prostate and colon cancer screening differ from those of the ACS, which lists no age cutoff for screening for either condition.2 In fact, the Task Force does not recommend screening for prostate cancer at all. Its rationale is that before age 75, the evidence is insufficient to evaluate benefits and harms, and after 75 there is good evidence that screening does more harm than good. The ACS no longer recommends routine prostate cancer screening, but does say that when a patient leaves the decision to the physician, screening should be performed.
Thumbs down on these, too. The Task Force now recommends against using spirometry to screen for chronic obstructive pulmonary disease and against using aspirin for preventing stroke in women <55 years and myocardial infarction (MI) in men <45 years. (See below for a fuller discussion of aspirin as a preventive measure.) The Task Force also recommends against screening for asymptomatic bacteriuria in men and nonpregnant women.
Recommended interventions
Now for the preventive interventions the USPSTF advises you to perform. They include:
Prescribing low-dose aspirin. The most complicated positive recommendations are those for low-dose aspirin to prevent MI in men and stroke in women. Aspirin is effective in preventing these conditions, but carries the risk of major gastrointestinal (GI) bleeding and cerebral hemorrhage. For younger patients, as we’ve seen in the previous section, the Task Force finds the risks of prophylactic low-dose aspirin therapy outweigh the benefits. But for older patients (men between the ages of 45 and 79 years and women ages 55-79), aspirin is recommended when the potential benefit of reducing the incidence of MI in men and stroke in women outweigh the harms. To assist clinicians in weighing the potential benefits and harms, the USPSTF provides a link to a coronary heart disease risk calculator, as well as several tables comparing numbers of prevented heart attacks for men and strokes for women by age and risk category, as well as risks of bleeding complications.3
Screening for hypercholesterolemia. The Task Force’s recommendations for dyslipidemia screening differ markedly from those of the American Heart Association and the Final Report of the National Cholesterol Education Program (NCEP) Expert Panel, which recommend routine screening for all adults starting at age 20 with no age cutoff.4 The USPSTF recommends deferring screening until patients are older, except for those at increased risk of coronary heart disease. This controversy was described in a 2008 Practice Alert.5
Screening for diabetes. The only asymptomatic patients the Task Force recommends screening for diabetes are those with a sustained blood pressure of more than 135/80 mm Hg, treated or untreated. The American Diabetes Association (ADA) would cast a wider net, recommending that you consider screening for prediabetes or diabetes in those ≥45 years of age, particularly in those with a body mass index of ≥25 kg/m2, and in overweight patients <45 years of age who have another risk factor for diabetes.6
Screening for colorectal cancer. The Task Force recommends screening adults starting at age 50 until age 75, using fecal occult blood testing, sigmoidoscopy, or colonoscopy. The ACS also recommends these screening modalities, but adds CT colonography and fecal DNA testing to the list of acceptable methods. The USPSTF found insufficient evidence to evaluate the benefits and harms of these newer tests and expressed concern over the high rate of incidental findings and the unknown long-term effects of radiation from CT colonography.
Screening adolescents. The Task Force is in favor of screening teenagers for major depressive disorder (MDD), as long as systems are in place to provide accurate diagnosis, therapy, and follow-up. High-intensity behavioral counseling for sexually active teens and adults at risk is also endorsed for the prevention of sexually transmitted infections. In both areas, however, the Task Force recognizes that adequately addressing these issues will require more than brief office- or clinic-based interventions.
Caring for pregnant women and newborns. According to the USPSTF, pregnant women should be screened for asymptomatic bacteriuria, advised to take a daily folic acid supplement, counseled about tobacco use, and encouraged to breastfeed. Newborns should be screened for congenital hypothyroidism, phenylketonuria, and hearing loss. These most recent A and B recommendations from the USPSTF are summarized in TABLE 3.
TABLE 3
The USPSTF recommends FOR
| CARDIOVASCULAR DISEASE PREVENTION |
|
| CANCER SCREENING |
|
| PREGNANCY |
|
| NEWBORNS |
|
| ADOLESCENTS |
|
Not proven
When evidence is not available, some organizations are willing to issue guidelines based on expert opinion or consensus. Not so the USPSTF. When the Task Force members find current evidence is not sufficient to make a judgment, they put the intervention into Category I, for Insufficient. The new I recommendations range from aspirin to prevent MI and stroke in those ≥80 years to screening children for MDD and performing whole body skin examinations to detect early manifestations of skin cancer. The new I recommendations are listed in TABLE 4.
TABLE 4
Evidence is INSUFFICIENT to recommend for or against
|
What’s the take-home message?
All of these recent Task Force decisions add substantially to the full set of Task Force recommendations, which can be found at www.ahrq.gov/CLINIC/uspstfix.htm. Given the large number of level A and B recommendations from the Task Force, clinicians are faced with the dilemma of limited time to accomplish all the recommendations. It is reasonable to concentrate on the positive recommendations and avoid performing the interventions recommended against. The interventions in the “I” category are not as clear-cut and clinicians will continue to struggle with them, particularly when other professional organizations recommend them.
CORRESPONDENCE
Doug Campos-Outcalt, MD, MPA, 550 E. Van Buren, Phoenix, AZ 85004; dougco@u.arizona.edu.
The past 12 months have been busy ones for the United States Preventive Services Task Force (USPSTF), which issued 34 new recommendations since our last Practice Alert on the group’s activity a year ago. Some recommendations address controversial topics, such as cholesterol screening, and several others—on topics such as prostate cancer screening and acceptable tests for detecting colorectal cancer—differ from those of such prominent groups as the American Cancer Society (ACS).
TABLE 1 provides a breakdown of the 5 categories of USPSTF recommendations (A, B, C, D, I). We’ll start with recent D recommendations (TABLE 2), services the Task Force recommends against, to emphasize that some preventive measures—even if they are widely touted—either provide no benefit or cause more harms than benefits.
TABLE 1
USPSTF recommendation categories
| A Recommendation: The Task Force recommends the service. There is high certainty that the net benefit is substantial. |
| B Recommendation: The Task Force recommends this service. There is high certainty that the net benefit is moderate or there is moderate certainty that the net benefit is moderate to substantial. |
| C Recommendation: The Task Force recommends against routinely providing the service. There may be considerations that support providing the service in an individual patient. There is at least moderate certainty that the net benefit is small. |
| D Recommendation: The Task Force recommends against the service. There is moderate or high certainty that the service has no net benefit or that the harms outweigh the benefits. |
| I Statement: The Task Force concludes that the current evidence is insufficient to assess the balance of benefits and harms of the service. Evidence is lacking, of poor quality, or conflicting, and the balance of benefits and harms cannot be determined. |
TABLE 2
The USPSTF recommends AGAINST
|
What not to do
The most notable new D recommendations advise against screening men ≥75 years of age for prostate cancer and against screening for colorectal cancer after age 85. The Task Force also recommends against routine screening for colorectal cancer after age 75, although individual patient considerations may influence your decision about this screen for patients between ages 76 and 85. Bear in mind that the benefits of early detection of colon cancer decline after age 75 because of the time lag between early intervention and benefit and because of competing causes of morbidity and mortality.1
Cancer screening controversies. The recommendations for an age cutoff for prostate and colon cancer screening differ from those of the ACS, which lists no age cutoff for screening for either condition.2 In fact, the Task Force does not recommend screening for prostate cancer at all. Its rationale is that before age 75, the evidence is insufficient to evaluate benefits and harms, and after 75 there is good evidence that screening does more harm than good. The ACS no longer recommends routine prostate cancer screening, but does say that when a patient leaves the decision to the physician, screening should be performed.
Thumbs down on these, too. The Task Force now recommends against using spirometry to screen for chronic obstructive pulmonary disease and against using aspirin for preventing stroke in women <55 years and myocardial infarction (MI) in men <45 years. (See below for a fuller discussion of aspirin as a preventive measure.) The Task Force also recommends against screening for asymptomatic bacteriuria in men and nonpregnant women.
Recommended interventions
Now for the preventive interventions the USPSTF advises you to perform. They include:
Prescribing low-dose aspirin. The most complicated positive recommendations are those for low-dose aspirin to prevent MI in men and stroke in women. Aspirin is effective in preventing these conditions, but carries the risk of major gastrointestinal (GI) bleeding and cerebral hemorrhage. For younger patients, as we’ve seen in the previous section, the Task Force finds the risks of prophylactic low-dose aspirin therapy outweigh the benefits. But for older patients (men between the ages of 45 and 79 years and women ages 55-79), aspirin is recommended when the potential benefit of reducing the incidence of MI in men and stroke in women outweigh the harms. To assist clinicians in weighing the potential benefits and harms, the USPSTF provides a link to a coronary heart disease risk calculator, as well as several tables comparing numbers of prevented heart attacks for men and strokes for women by age and risk category, as well as risks of bleeding complications.3
Screening for hypercholesterolemia. The Task Force’s recommendations for dyslipidemia screening differ markedly from those of the American Heart Association and the Final Report of the National Cholesterol Education Program (NCEP) Expert Panel, which recommend routine screening for all adults starting at age 20 with no age cutoff.4 The USPSTF recommends deferring screening until patients are older, except for those at increased risk of coronary heart disease. This controversy was described in a 2008 Practice Alert.5
Screening for diabetes. The only asymptomatic patients the Task Force recommends screening for diabetes are those with a sustained blood pressure of more than 135/80 mm Hg, treated or untreated. The American Diabetes Association (ADA) would cast a wider net, recommending that you consider screening for prediabetes or diabetes in those ≥45 years of age, particularly in those with a body mass index of ≥25 kg/m2, and in overweight patients <45 years of age who have another risk factor for diabetes.6
Screening for colorectal cancer. The Task Force recommends screening adults starting at age 50 until age 75, using fecal occult blood testing, sigmoidoscopy, or colonoscopy. The ACS also recommends these screening modalities, but adds CT colonography and fecal DNA testing to the list of acceptable methods. The USPSTF found insufficient evidence to evaluate the benefits and harms of these newer tests and expressed concern over the high rate of incidental findings and the unknown long-term effects of radiation from CT colonography.
Screening adolescents. The Task Force is in favor of screening teenagers for major depressive disorder (MDD), as long as systems are in place to provide accurate diagnosis, therapy, and follow-up. High-intensity behavioral counseling for sexually active teens and adults at risk is also endorsed for the prevention of sexually transmitted infections. In both areas, however, the Task Force recognizes that adequately addressing these issues will require more than brief office- or clinic-based interventions.
Caring for pregnant women and newborns. According to the USPSTF, pregnant women should be screened for asymptomatic bacteriuria, advised to take a daily folic acid supplement, counseled about tobacco use, and encouraged to breastfeed. Newborns should be screened for congenital hypothyroidism, phenylketonuria, and hearing loss. These most recent A and B recommendations from the USPSTF are summarized in TABLE 3.
TABLE 3
The USPSTF recommends FOR
| CARDIOVASCULAR DISEASE PREVENTION |
|
| CANCER SCREENING |
|
| PREGNANCY |
|
| NEWBORNS |
|
| ADOLESCENTS |
|
Not proven
When evidence is not available, some organizations are willing to issue guidelines based on expert opinion or consensus. Not so the USPSTF. When the Task Force members find current evidence is not sufficient to make a judgment, they put the intervention into Category I, for Insufficient. The new I recommendations range from aspirin to prevent MI and stroke in those ≥80 years to screening children for MDD and performing whole body skin examinations to detect early manifestations of skin cancer. The new I recommendations are listed in TABLE 4.
TABLE 4
Evidence is INSUFFICIENT to recommend for or against
|
What’s the take-home message?
All of these recent Task Force decisions add substantially to the full set of Task Force recommendations, which can be found at www.ahrq.gov/CLINIC/uspstfix.htm. Given the large number of level A and B recommendations from the Task Force, clinicians are faced with the dilemma of limited time to accomplish all the recommendations. It is reasonable to concentrate on the positive recommendations and avoid performing the interventions recommended against. The interventions in the “I” category are not as clear-cut and clinicians will continue to struggle with them, particularly when other professional organizations recommend them.
CORRESPONDENCE
Doug Campos-Outcalt, MD, MPA, 550 E. Van Buren, Phoenix, AZ 85004; dougco@u.arizona.edu.
1. US Preventive Services Task Force. Screening for colorectal cancer. October 2008. Available at: www.ahrq.gov/clinic/uspstf/uspscolo.htm. Accessed June 3, 2009.
2. American Cancer Society guidelines for early detection of cancer. Last revised May 21, 2009. Available at: http://www.cancer.org/docroot/PED/content/PED_2_3X_ACS_Cancer_Detection_Guidelines_36.asp?sitearea=PED. Accessed June 3, 2009.
3. US Preventive Services Task Force. Aspirin for the prevention of cardiovascular disease: recommendation statement. March 2009. Available at: http://www.ahrq.gov/clinic/uspstf09/aspirincvd/aspcvdrs.htm. Accessed June 3, 2009.
4. National Cholesterol Education Program. Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) Final Report. Circulation. 2002;106:3143-3421.Available at: http://circ.ahajournals.org/cgi/content/full/106/25/3143. Accessed June 3, 2009.
5. Campos-Outcalt D. USPSTF scales back approach to lipid screening for women. J Fam Pract. 2008;57:740-742.
6. American Diabetes Association. Standards of medical care in diabetes—2008. Diabetes Care. 2008;31(suppl 1):S12-S54.
1. US Preventive Services Task Force. Screening for colorectal cancer. October 2008. Available at: www.ahrq.gov/clinic/uspstf/uspscolo.htm. Accessed June 3, 2009.
2. American Cancer Society guidelines for early detection of cancer. Last revised May 21, 2009. Available at: http://www.cancer.org/docroot/PED/content/PED_2_3X_ACS_Cancer_Detection_Guidelines_36.asp?sitearea=PED. Accessed June 3, 2009.
3. US Preventive Services Task Force. Aspirin for the prevention of cardiovascular disease: recommendation statement. March 2009. Available at: http://www.ahrq.gov/clinic/uspstf09/aspirincvd/aspcvdrs.htm. Accessed June 3, 2009.
4. National Cholesterol Education Program. Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) Final Report. Circulation. 2002;106:3143-3421.Available at: http://circ.ahajournals.org/cgi/content/full/106/25/3143. Accessed June 3, 2009.
5. Campos-Outcalt D. USPSTF scales back approach to lipid screening for women. J Fam Pract. 2008;57:740-742.
6. American Diabetes Association. Standards of medical care in diabetes—2008. Diabetes Care. 2008;31(suppl 1):S12-S54.
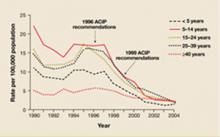
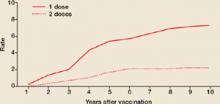
CDC recommendations expand vaccine indications
Highlights of the 2008 recommendations of the CDC’s Advisory Committee on Immunization Practices (ACIP), detailed in the child and adult immunization schedules in the MMWR in January,1,2 include:
- an expansion of the age groups for whom an annual influenza vaccine is recommended;
- expanded indications for the pneumococcal polysaccharide vaccine;
- 2 new combination vaccines for children; and
- a second rotavirus vaccine, with revised recommendations to accommodate both vaccine products.
School-age children should get flu vaccine
Children and adolescents ages 5 through 18 years are now among those who should receive an annual flu vaccine. Previously, routine vaccination was recommended only for adults and children ages 6 months through 59 months.3
Because of the timing of vaccine purchase, ACIP recognizes that routine vaccination of 5- to 18-year-olds may not be possible in some settings until next year. Family physicians who are unable to fully incorporate this new recommendation in the 2008-2009 flu season should immunize children and adolescents who are at high risk for complications of the flu. Included in that group are 5- to 18-year-olds who are on long-term aspirin therapy; have a chronic pulmonary disease, including asthma, or a cardiovascular, renal, hepatic, hematologic, or metabolic disorder; are immunosuppressed; or have a neurological or musculoskeletal disorder that alters respiratory function or the clearance of respiratory secretions. Children and adolescents who live with others at elevated risk—kids younger than 5 years, adults older than 50 years, or individuals with medical conditions that place them at high risk for severe influenza complications—should also be vaccinated.
Pneumococcal vaccine: New indications, clarifications
Two new groups have been added to the list of people for whom the 23-valent pneumococcal polysaccharide vaccine (PPV23) is recommended: asthma patients and smokers. Smoking poses as great a risk for pneumococcal pneumonia as diabetes and other chronic illnesses that had already been noted as indications for the vaccine. The number needed to vaccinate to prevent 1 case of pneumonia in smokers is 10,000 for those between the ages of 18 to 44 years, and 4000 for those ages 45 to 64 years.
A second dose. Also in 2008, ACIP clarified its dosing recommendations for PPV23: A second dose, given 5 years after the first, is recommended for those with immune suppression, sickle cell disease, or asplenia. Individuals who are 65 years of age or older should receive a second dose if they were vaccinated 5 or more years ago and were younger than 65 at the time of primary vaccination.
Not for all Native Americans. The recommendation for the use of PPV23 among the Native American population has changed, too.
Research showing high rates of invasive pneumococcal disease in Native American communities has been performed in only a few locations and cannot be generalized to all Native Americans. Therefore, ACIP has gone from recommending routine use of the vaccine among all Native Americans to a recommendation based on the same risks and age recommendations as the general population and, in communities with high rates of disease, on public health recommendations based on the incidence and epidemiology of disease.
Combination products may mean fewer injections
Two new combination vaccine products—Pentacel4 and Kinrix5—were approved last year. Both can reduce the number of injections required to complete the child immunization recommendations.
Pentacel combines 5 vaccines—diphtheria, tetanus, and pertussis (DTaP), inactivated poliovirus (IPV), and Haemophilus influenzae type b (Hib)—and is licensed for children 6 weeks through 4 years of age. Pentacel has a 4-dose schedule, with vaccine administration at 2, 4, 6, and 15 to 18 months of age. Technically, this 4-dose schedule would fulfill requirements for 4 doses of IPV. However, this could conflict with a state school immunization schedule that requires the last dose of IPV vaccine to be administered when the child is between the ages of 4 and 6 years.6
TABLE
Rotavirus vaccines: An administration guide
| ROTATEQ | ROTARIX | |
|---|---|---|
| No. of doses | 3 | 2 |
| Recommended dosing schedule | 2, 4, and 6 mo of age | 2 and 4 mo of age |
| First dose | 6–14 wk 6 d of age | |
| Dosing interval | ≥4 wk | |
| Final dose | ≤8 mo of age | |
| Source: Centers for Disease Control and Prevention. 2009.1 | ||
Kinrix contains DTaP and IPV. The vaccine is indicated for use as the fifth dose of DTaP and the fourth dose of IPV in children 4 through 6 years of age, following a primary series using Infanrix (DTaP) and Pediarix (DTaP, hepatitis B, and IPV).
Rotavirus vaccines: Now there are 2
There are now 2 licensed rotavirus vaccines: RotaTeq was approved in 2006,7 and Rotarix in 2008.8 ACIP does not express a preference for either product, but has revised its recommendations for rotavirus vaccination to accommodate the new release. Both RotaTeq and Rotarix are live oral vaccines, but they differ in composition and schedule of administration. Rotarix should not be given to infants who are allergic to latex, as its oral applicator contains latex rubber.
Dosing requirements. RotaTeq is administered in a 3-dose series at ages 2, 4, and 6 months; Rotarix is given in a 2-dose series at 2 and 4 months of age (TABLE). The first dose of either vaccine should be administered to children between the ages of 6 weeks and 14 weeks, 6 days. (Previously, 12 weeks was the maximum age for the first dose of rotavirus vaccine.) Neither vaccine series should be initiated in infants who are 15 weeks of age or older. The minimum interval between doses is 4 weeks, and the final dose should be administered by the age of 8 months.
It is best to complete the vaccine series with the same product. If the vaccine used initially is not available, the series can be completed with the other product, but the different number of doses required must be considered. If any dose in the series was RotaTeq or you are unable to determine which rotavirus vaccine was administered previously, a total of 3 doses should be given.
HPV and meningococcal vaccine clarification
Human papilloma virus vaccine. The HPV vaccine is recommended for all females ages 11 through 26 years, but ACIP has indicated that girls as young as 9 years may be vaccinated.1
Three doses are required, with the second and third doses administered 2 and 6 months after the first. Because some providers had been administering the third dose at month 4, ACIP issued a clarification in 2008, noting that there should be a minimum of 24 weeks between the first and third dose.
MCV and MPSV. Meningococcal conjugate vaccine (MCV) is preferred over meningococcal polysaccharide vaccine (MPSV) for those 55 years of age or younger, although MPSV is an acceptable alternative. ACIP clarified recommendations for revaccination, as follows:
Individuals ages 11 to 55 years who were vaccinated with MPSV should consider revaccination with MCV after 5 years, if the risk of meningococcal meningitis persists. Children ages 2 to 10 years should be revaccinated with MCV 3 years after receiving MPSV.
1. Centers for Disease Control and Prevention (CDC). Recommended immunization schedules for persons aged 0 through 18 years—United States, 2009. http://www.cdc.gov/mmwr/preview/mmwrhtml/mm5751a5.htm. Accessed January 20, 2009.
2. CDC. Recommended adult immunization schedule—United States, 2009. http://www.cdc.gov/mmwr/preview/mmwrhtml/mm5753a6.htm. Accessed January 20, 2009.
3. CDC. Recommended immunization schedules for persons aged 0-18 years—United States, 2008. http://cdc.gov/mmwr/preview/mmwrhtml/mm5701a8.htm. Accessed January 19, 2009.
4. US Food and Drug Administration (FDA) Product approval information [memorandum]. Pentacel: recommendations regarding request for partial waiver of pediatric studies. April 25, 2008. http://www.fda.gov/CBER/products/pentacel/pentacel042508mem.htm. Accessed January 27, 2009.
5. FDA Product approval information [approval letter]. Kinrix. June 24, 2008. http://www.fda.gov/cber/approvltr/kinrix062408L.htm. Accessed January 27, 2009.
6. Immunization Action Coalition State information. State mandates on immunization and vaccine-preventable diseases. Polio: 2005-2006 requirements for kindergarten. http://www.immunize.org/laws/polio_kinder.pdf. Accessed February 3, 2009.
7. FDA. FDA approves new vaccine to prevent rotavirus gastroenteritis in infants. February 3, 2006. http://www.fda.gov/bbs/topics/news/2006/NEW01307.html. Accessed January 19, 2009.
8. FDA. FDA approves new vaccine to prevent gastroenteritis caused by rotavirus. April 3, 2008. http://www.fda.gov/bbs/topics/NEWS/2008/NEW01814.html. Accessed January 28, 2009.
Highlights of the 2008 recommendations of the CDC’s Advisory Committee on Immunization Practices (ACIP), detailed in the child and adult immunization schedules in the MMWR in January,1,2 include:
- an expansion of the age groups for whom an annual influenza vaccine is recommended;
- expanded indications for the pneumococcal polysaccharide vaccine;
- 2 new combination vaccines for children; and
- a second rotavirus vaccine, with revised recommendations to accommodate both vaccine products.
School-age children should get flu vaccine
Children and adolescents ages 5 through 18 years are now among those who should receive an annual flu vaccine. Previously, routine vaccination was recommended only for adults and children ages 6 months through 59 months.3
Because of the timing of vaccine purchase, ACIP recognizes that routine vaccination of 5- to 18-year-olds may not be possible in some settings until next year. Family physicians who are unable to fully incorporate this new recommendation in the 2008-2009 flu season should immunize children and adolescents who are at high risk for complications of the flu. Included in that group are 5- to 18-year-olds who are on long-term aspirin therapy; have a chronic pulmonary disease, including asthma, or a cardiovascular, renal, hepatic, hematologic, or metabolic disorder; are immunosuppressed; or have a neurological or musculoskeletal disorder that alters respiratory function or the clearance of respiratory secretions. Children and adolescents who live with others at elevated risk—kids younger than 5 years, adults older than 50 years, or individuals with medical conditions that place them at high risk for severe influenza complications—should also be vaccinated.
Pneumococcal vaccine: New indications, clarifications
Two new groups have been added to the list of people for whom the 23-valent pneumococcal polysaccharide vaccine (PPV23) is recommended: asthma patients and smokers. Smoking poses as great a risk for pneumococcal pneumonia as diabetes and other chronic illnesses that had already been noted as indications for the vaccine. The number needed to vaccinate to prevent 1 case of pneumonia in smokers is 10,000 for those between the ages of 18 to 44 years, and 4000 for those ages 45 to 64 years.
A second dose. Also in 2008, ACIP clarified its dosing recommendations for PPV23: A second dose, given 5 years after the first, is recommended for those with immune suppression, sickle cell disease, or asplenia. Individuals who are 65 years of age or older should receive a second dose if they were vaccinated 5 or more years ago and were younger than 65 at the time of primary vaccination.
Not for all Native Americans. The recommendation for the use of PPV23 among the Native American population has changed, too.
Research showing high rates of invasive pneumococcal disease in Native American communities has been performed in only a few locations and cannot be generalized to all Native Americans. Therefore, ACIP has gone from recommending routine use of the vaccine among all Native Americans to a recommendation based on the same risks and age recommendations as the general population and, in communities with high rates of disease, on public health recommendations based on the incidence and epidemiology of disease.
Combination products may mean fewer injections
Two new combination vaccine products—Pentacel4 and Kinrix5—were approved last year. Both can reduce the number of injections required to complete the child immunization recommendations.
Pentacel combines 5 vaccines—diphtheria, tetanus, and pertussis (DTaP), inactivated poliovirus (IPV), and Haemophilus influenzae type b (Hib)—and is licensed for children 6 weeks through 4 years of age. Pentacel has a 4-dose schedule, with vaccine administration at 2, 4, 6, and 15 to 18 months of age. Technically, this 4-dose schedule would fulfill requirements for 4 doses of IPV. However, this could conflict with a state school immunization schedule that requires the last dose of IPV vaccine to be administered when the child is between the ages of 4 and 6 years.6
TABLE
Rotavirus vaccines: An administration guide
| ROTATEQ | ROTARIX | |
|---|---|---|
| No. of doses | 3 | 2 |
| Recommended dosing schedule | 2, 4, and 6 mo of age | 2 and 4 mo of age |
| First dose | 6–14 wk 6 d of age | |
| Dosing interval | ≥4 wk | |
| Final dose | ≤8 mo of age | |
| Source: Centers for Disease Control and Prevention. 2009.1 | ||
Kinrix contains DTaP and IPV. The vaccine is indicated for use as the fifth dose of DTaP and the fourth dose of IPV in children 4 through 6 years of age, following a primary series using Infanrix (DTaP) and Pediarix (DTaP, hepatitis B, and IPV).
Rotavirus vaccines: Now there are 2
There are now 2 licensed rotavirus vaccines: RotaTeq was approved in 2006,7 and Rotarix in 2008.8 ACIP does not express a preference for either product, but has revised its recommendations for rotavirus vaccination to accommodate the new release. Both RotaTeq and Rotarix are live oral vaccines, but they differ in composition and schedule of administration. Rotarix should not be given to infants who are allergic to latex, as its oral applicator contains latex rubber.
Dosing requirements. RotaTeq is administered in a 3-dose series at ages 2, 4, and 6 months; Rotarix is given in a 2-dose series at 2 and 4 months of age (TABLE). The first dose of either vaccine should be administered to children between the ages of 6 weeks and 14 weeks, 6 days. (Previously, 12 weeks was the maximum age for the first dose of rotavirus vaccine.) Neither vaccine series should be initiated in infants who are 15 weeks of age or older. The minimum interval between doses is 4 weeks, and the final dose should be administered by the age of 8 months.
It is best to complete the vaccine series with the same product. If the vaccine used initially is not available, the series can be completed with the other product, but the different number of doses required must be considered. If any dose in the series was RotaTeq or you are unable to determine which rotavirus vaccine was administered previously, a total of 3 doses should be given.
HPV and meningococcal vaccine clarification
Human papilloma virus vaccine. The HPV vaccine is recommended for all females ages 11 through 26 years, but ACIP has indicated that girls as young as 9 years may be vaccinated.1
Three doses are required, with the second and third doses administered 2 and 6 months after the first. Because some providers had been administering the third dose at month 4, ACIP issued a clarification in 2008, noting that there should be a minimum of 24 weeks between the first and third dose.
MCV and MPSV. Meningococcal conjugate vaccine (MCV) is preferred over meningococcal polysaccharide vaccine (MPSV) for those 55 years of age or younger, although MPSV is an acceptable alternative. ACIP clarified recommendations for revaccination, as follows:
Individuals ages 11 to 55 years who were vaccinated with MPSV should consider revaccination with MCV after 5 years, if the risk of meningococcal meningitis persists. Children ages 2 to 10 years should be revaccinated with MCV 3 years after receiving MPSV.
Highlights of the 2008 recommendations of the CDC’s Advisory Committee on Immunization Practices (ACIP), detailed in the child and adult immunization schedules in the MMWR in January,1,2 include:
- an expansion of the age groups for whom an annual influenza vaccine is recommended;
- expanded indications for the pneumococcal polysaccharide vaccine;
- 2 new combination vaccines for children; and
- a second rotavirus vaccine, with revised recommendations to accommodate both vaccine products.
School-age children should get flu vaccine
Children and adolescents ages 5 through 18 years are now among those who should receive an annual flu vaccine. Previously, routine vaccination was recommended only for adults and children ages 6 months through 59 months.3
Because of the timing of vaccine purchase, ACIP recognizes that routine vaccination of 5- to 18-year-olds may not be possible in some settings until next year. Family physicians who are unable to fully incorporate this new recommendation in the 2008-2009 flu season should immunize children and adolescents who are at high risk for complications of the flu. Included in that group are 5- to 18-year-olds who are on long-term aspirin therapy; have a chronic pulmonary disease, including asthma, or a cardiovascular, renal, hepatic, hematologic, or metabolic disorder; are immunosuppressed; or have a neurological or musculoskeletal disorder that alters respiratory function or the clearance of respiratory secretions. Children and adolescents who live with others at elevated risk—kids younger than 5 years, adults older than 50 years, or individuals with medical conditions that place them at high risk for severe influenza complications—should also be vaccinated.
Pneumococcal vaccine: New indications, clarifications
Two new groups have been added to the list of people for whom the 23-valent pneumococcal polysaccharide vaccine (PPV23) is recommended: asthma patients and smokers. Smoking poses as great a risk for pneumococcal pneumonia as diabetes and other chronic illnesses that had already been noted as indications for the vaccine. The number needed to vaccinate to prevent 1 case of pneumonia in smokers is 10,000 for those between the ages of 18 to 44 years, and 4000 for those ages 45 to 64 years.
A second dose. Also in 2008, ACIP clarified its dosing recommendations for PPV23: A second dose, given 5 years after the first, is recommended for those with immune suppression, sickle cell disease, or asplenia. Individuals who are 65 years of age or older should receive a second dose if they were vaccinated 5 or more years ago and were younger than 65 at the time of primary vaccination.
Not for all Native Americans. The recommendation for the use of PPV23 among the Native American population has changed, too.
Research showing high rates of invasive pneumococcal disease in Native American communities has been performed in only a few locations and cannot be generalized to all Native Americans. Therefore, ACIP has gone from recommending routine use of the vaccine among all Native Americans to a recommendation based on the same risks and age recommendations as the general population and, in communities with high rates of disease, on public health recommendations based on the incidence and epidemiology of disease.
Combination products may mean fewer injections
Two new combination vaccine products—Pentacel4 and Kinrix5—were approved last year. Both can reduce the number of injections required to complete the child immunization recommendations.
Pentacel combines 5 vaccines—diphtheria, tetanus, and pertussis (DTaP), inactivated poliovirus (IPV), and Haemophilus influenzae type b (Hib)—and is licensed for children 6 weeks through 4 years of age. Pentacel has a 4-dose schedule, with vaccine administration at 2, 4, 6, and 15 to 18 months of age. Technically, this 4-dose schedule would fulfill requirements for 4 doses of IPV. However, this could conflict with a state school immunization schedule that requires the last dose of IPV vaccine to be administered when the child is between the ages of 4 and 6 years.6
TABLE
Rotavirus vaccines: An administration guide
| ROTATEQ | ROTARIX | |
|---|---|---|
| No. of doses | 3 | 2 |
| Recommended dosing schedule | 2, 4, and 6 mo of age | 2 and 4 mo of age |
| First dose | 6–14 wk 6 d of age | |
| Dosing interval | ≥4 wk | |
| Final dose | ≤8 mo of age | |
| Source: Centers for Disease Control and Prevention. 2009.1 | ||
Kinrix contains DTaP and IPV. The vaccine is indicated for use as the fifth dose of DTaP and the fourth dose of IPV in children 4 through 6 years of age, following a primary series using Infanrix (DTaP) and Pediarix (DTaP, hepatitis B, and IPV).
Rotavirus vaccines: Now there are 2
There are now 2 licensed rotavirus vaccines: RotaTeq was approved in 2006,7 and Rotarix in 2008.8 ACIP does not express a preference for either product, but has revised its recommendations for rotavirus vaccination to accommodate the new release. Both RotaTeq and Rotarix are live oral vaccines, but they differ in composition and schedule of administration. Rotarix should not be given to infants who are allergic to latex, as its oral applicator contains latex rubber.
Dosing requirements. RotaTeq is administered in a 3-dose series at ages 2, 4, and 6 months; Rotarix is given in a 2-dose series at 2 and 4 months of age (TABLE). The first dose of either vaccine should be administered to children between the ages of 6 weeks and 14 weeks, 6 days. (Previously, 12 weeks was the maximum age for the first dose of rotavirus vaccine.) Neither vaccine series should be initiated in infants who are 15 weeks of age or older. The minimum interval between doses is 4 weeks, and the final dose should be administered by the age of 8 months.
It is best to complete the vaccine series with the same product. If the vaccine used initially is not available, the series can be completed with the other product, but the different number of doses required must be considered. If any dose in the series was RotaTeq or you are unable to determine which rotavirus vaccine was administered previously, a total of 3 doses should be given.
HPV and meningococcal vaccine clarification
Human papilloma virus vaccine. The HPV vaccine is recommended for all females ages 11 through 26 years, but ACIP has indicated that girls as young as 9 years may be vaccinated.1
Three doses are required, with the second and third doses administered 2 and 6 months after the first. Because some providers had been administering the third dose at month 4, ACIP issued a clarification in 2008, noting that there should be a minimum of 24 weeks between the first and third dose.
MCV and MPSV. Meningococcal conjugate vaccine (MCV) is preferred over meningococcal polysaccharide vaccine (MPSV) for those 55 years of age or younger, although MPSV is an acceptable alternative. ACIP clarified recommendations for revaccination, as follows:
Individuals ages 11 to 55 years who were vaccinated with MPSV should consider revaccination with MCV after 5 years, if the risk of meningococcal meningitis persists. Children ages 2 to 10 years should be revaccinated with MCV 3 years after receiving MPSV.
1. Centers for Disease Control and Prevention (CDC). Recommended immunization schedules for persons aged 0 through 18 years—United States, 2009. http://www.cdc.gov/mmwr/preview/mmwrhtml/mm5751a5.htm. Accessed January 20, 2009.
2. CDC. Recommended adult immunization schedule—United States, 2009. http://www.cdc.gov/mmwr/preview/mmwrhtml/mm5753a6.htm. Accessed January 20, 2009.
3. CDC. Recommended immunization schedules for persons aged 0-18 years—United States, 2008. http://cdc.gov/mmwr/preview/mmwrhtml/mm5701a8.htm. Accessed January 19, 2009.
4. US Food and Drug Administration (FDA) Product approval information [memorandum]. Pentacel: recommendations regarding request for partial waiver of pediatric studies. April 25, 2008. http://www.fda.gov/CBER/products/pentacel/pentacel042508mem.htm. Accessed January 27, 2009.
5. FDA Product approval information [approval letter]. Kinrix. June 24, 2008. http://www.fda.gov/cber/approvltr/kinrix062408L.htm. Accessed January 27, 2009.
6. Immunization Action Coalition State information. State mandates on immunization and vaccine-preventable diseases. Polio: 2005-2006 requirements for kindergarten. http://www.immunize.org/laws/polio_kinder.pdf. Accessed February 3, 2009.
7. FDA. FDA approves new vaccine to prevent rotavirus gastroenteritis in infants. February 3, 2006. http://www.fda.gov/bbs/topics/news/2006/NEW01307.html. Accessed January 19, 2009.
8. FDA. FDA approves new vaccine to prevent gastroenteritis caused by rotavirus. April 3, 2008. http://www.fda.gov/bbs/topics/NEWS/2008/NEW01814.html. Accessed January 28, 2009.
1. Centers for Disease Control and Prevention (CDC). Recommended immunization schedules for persons aged 0 through 18 years—United States, 2009. http://www.cdc.gov/mmwr/preview/mmwrhtml/mm5751a5.htm. Accessed January 20, 2009.
2. CDC. Recommended adult immunization schedule—United States, 2009. http://www.cdc.gov/mmwr/preview/mmwrhtml/mm5753a6.htm. Accessed January 20, 2009.
3. CDC. Recommended immunization schedules for persons aged 0-18 years—United States, 2008. http://cdc.gov/mmwr/preview/mmwrhtml/mm5701a8.htm. Accessed January 19, 2009.
4. US Food and Drug Administration (FDA) Product approval information [memorandum]. Pentacel: recommendations regarding request for partial waiver of pediatric studies. April 25, 2008. http://www.fda.gov/CBER/products/pentacel/pentacel042508mem.htm. Accessed January 27, 2009.
5. FDA Product approval information [approval letter]. Kinrix. June 24, 2008. http://www.fda.gov/cber/approvltr/kinrix062408L.htm. Accessed January 27, 2009.
6. Immunization Action Coalition State information. State mandates on immunization and vaccine-preventable diseases. Polio: 2005-2006 requirements for kindergarten. http://www.immunize.org/laws/polio_kinder.pdf. Accessed February 3, 2009.
7. FDA. FDA approves new vaccine to prevent rotavirus gastroenteritis in infants. February 3, 2006. http://www.fda.gov/bbs/topics/news/2006/NEW01307.html. Accessed January 19, 2009.
8. FDA. FDA approves new vaccine to prevent gastroenteritis caused by rotavirus. April 3, 2008. http://www.fda.gov/bbs/topics/NEWS/2008/NEW01814.html. Accessed January 28, 2009.
USPSTF scales back approach to lipid screening for women
When patients reached a certain age (36 for men, 46 for women), it used to mean that it was time, in the eyes of the United States Preventive Services Task Force (USPSTF), to screen for lipid disorders. But that’s changed for female patients.
The USPSTF’s latest recommendations (TABLE 1) on screening for lipid disorders in adults1 call for screening women only when coronary heart disease (CHD) risk factors are present, regardless of their age. (See TABLE 2 for a list of CHD risk factors.) That’s a major shift from the 2001 recommendation, which stated that all women over age 45 should be screened and women ages 20 to 45 should be screened if they were at elevated risk.
The recommendations for men remain the same: All men older than 35 should be screened, as should men who are between the ages of 20 and 35 who have other CHD risks.
TABLE 1
USPSTF lipid disorder screening recommendations at a glance
| Screening men • The United States Preventive Services Task Force (USPSTF) strongly recommends screening men ages 35 and older for lipid disorders. Grade A recommendation |
| • The USPSTF recommends screening men ages 20 to 35 for lipid disorders if they are at increased risk for coronary heart disease (CHD). Grade B recommendation |
| Screening women at increased risk • The USPSTF strongly recommends screening women ages 45 and older for lipid disorders if they are at increased risk for CHD. Grade A recommendation |
| • The USPSTF recommends screening women ages 20 to 45 for lipid disorders if they are at increased risk for CHD. Grade B recommendation |
| Screening young men and all women not at increased risk • The USPSTF makes no recommendation for or against routine screening for lipid disorders in men between the ages of 20 and 35, or in women ages 20 and older who are not at increased risk for CHD. Grade C recommendation |
TABLE 2
Risk factors for CHD
| • Diabetes |
| • Personal history of coronary heart disease (CHD) or noncoronary atherosclerosis (eg, abdominal aortic aneurysm, peripheral artery disease, and carotid artery stenosis) |
| • A family history of cardiovascular disease before age 50 in male relatives or age 60 in female relatives |
| • Tobacco use |
| • Hypertension |
| • Obesity (body mass index ≥30) |
A different approach from NIH and AHA
The revised updated recommendation for women over age 45 was based on 2 systematic evidence reviews2,3 that concluded, while treatment clearly benefits women with other risk factors, benefit has not been proven for women who are otherwise CHD risk free.
The recommendation for women conflicts with those of the National Institutes of Health and the American Heart Association; both recommend screening all adults starting at age 20—regardless of risk.
Screening those without risk isn’t ruled out
It is important to note that the task force is not recommending against screening in women (or men between the ages of 20 and 35) who do not have other CHD risks. The task force makes a C recommendation with wording that states, “The USPSTF makes no recommendation for or against routine provision of [the service]. The USPSTF found at least fair evidence that [the service] can improve health outcomes but concludes that the balance of benefits and harms is too close to justify a general recommendation” (TABLE 3).
The task force chose not to use the new wording for a C recommendation, adopted in 2007, which reads, “The USPSTF recommends against routinely providing the service. There may be considerations that support providing the service in an individual patient. There is at least moderate certainty that the net benefit is small.”
It is also important to realize that a large proportion of women have another CHD risk and will not fall into the C category recommendation.
TABLE 3
USPSTF recommendation categories
| A—Strongly recommended: The United States Preventive Services Task Force (USPSTF) strongly recommends that clinicians provide the service to eligible patients. The USPSTF found good evidence that the service improves important health outcomes and concludes that benefits substantially outweigh harms. |
| B—Recommended: The USPSTF recommends that clinicians provide the service to eligible patients. The USPSTF found at least fair evidence that the service improves important health outcomes and concludes that benefits outweigh harms. |
| C—No recommendation: The USPSTF makes no recommendation for or against routine provision of the service. The USPSTF found at least fair evidence that the service can improve health outcomes but concludes that the balance of benefits and harms is too close to justify a general recommendation. |
| D—Not recommended: The USPSTF recommends against routinely providing the service to asymptomatic patients. The USPSTF found at least fair evidence that the service is ineffective or that harms outweigh benefits. |
| I—Insufficient evidence to make a recommendation: The USPSTF concludes that the evidence is insufficient to recommend for or against routinely providing the service. Evidence that the service is effective is lacking, of poor quality, or conflicting, and the balance of benefits and harms cannot be determined. |
No need to look at triglycerides initially
The task force recommends screening with a fasting or nonfasting serum sample for total cholesterol and high-density lipoprotein cholesterol. The task force does not recommend including a triglyceride level because there is mixed and inclusive evidence that triglyceride levels are independently associated with CHD risk and scant evidence that treating isolated elevated triglyceride levels reduces the occurrence of CHD events. This approach also conflicts with other organizations that recommend screening with fasting lipid profiles that include a triglyceride level.
The task force states that an abnormal initial screen should be confirmed by a repeat test and, if confirmed, a fasting lipid panel should be obtained. Wide adoption of the task force recommendations would result in considerable savings in cost and patient inconvenience by avoiding complete fasting lipid panels as the initial screen.
The optimal frequency of screening is not established and the task force states that every 5 years is reasonable, although more frequent testing might be considered for those with high normal values, and less frequent intervals for those with optimal cholesterol levels and healthy lifestyles.
Treatment: Look beyond lifestyle
The screening recommendations are accompanied by a discussion of clinical considerations and a description of an approach to treatment for those with lipid disorders. The main point the task force makes is that all CHD risks should be addressed, and that lifestyle changes alone rarely reduce elevated cholesterol to an optimal level. (For more on the treatment of hyperlipidemia, see the National Heart, Lung, and Blood Institute’s Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults [Adult Treatment Panel III] at http://www.nhlbi.nih.gov/guidelines/cholesterol/index.htm.)
Time to rethink conventional opinion
The updated task force recommendations are a reminder that many widely used guidelines, including those on the prevention of CHD, are based on a lack of high-level evidence. Thus, it is not surprising that a rigorously evidence-based analysis, as preformed by the USPSTF, will frequently result in recommendations that are at variance with common practice and conventional opinion.
1. U.S. Preventive Services Task Force (USPSTF). Screening for lipid disorders in adults: recommendation statement. June 2008. Available at: http://www.ahrq.gov/clinic/uspstf08/lipid/lipidrs.htm. Accessed September 26, 2008.
2. Grady D, Chaput L, Kristof M. Systematic Review of Lipid Lowering Treatment to Reduce Risk of Coronary Heart Disease in Women. Rockville, Md: Agency for Healthcare Research and Quality; 2003.
3. Helfand M, Carson S. Screening for lipid disorders in adults: selective update of 2001 U.S. Preventive Services Task Force Review. June 2008. AHRQ publication number 08-05114-EF-1. Available at: http://www.ahrq.gov/clinic/uspstf08/lipid/lipides.pdf. Accessed September 26, 2008.
When patients reached a certain age (36 for men, 46 for women), it used to mean that it was time, in the eyes of the United States Preventive Services Task Force (USPSTF), to screen for lipid disorders. But that’s changed for female patients.
The USPSTF’s latest recommendations (TABLE 1) on screening for lipid disorders in adults1 call for screening women only when coronary heart disease (CHD) risk factors are present, regardless of their age. (See TABLE 2 for a list of CHD risk factors.) That’s a major shift from the 2001 recommendation, which stated that all women over age 45 should be screened and women ages 20 to 45 should be screened if they were at elevated risk.
The recommendations for men remain the same: All men older than 35 should be screened, as should men who are between the ages of 20 and 35 who have other CHD risks.
TABLE 1
USPSTF lipid disorder screening recommendations at a glance
| Screening men • The United States Preventive Services Task Force (USPSTF) strongly recommends screening men ages 35 and older for lipid disorders. Grade A recommendation |
| • The USPSTF recommends screening men ages 20 to 35 for lipid disorders if they are at increased risk for coronary heart disease (CHD). Grade B recommendation |
| Screening women at increased risk • The USPSTF strongly recommends screening women ages 45 and older for lipid disorders if they are at increased risk for CHD. Grade A recommendation |
| • The USPSTF recommends screening women ages 20 to 45 for lipid disorders if they are at increased risk for CHD. Grade B recommendation |
| Screening young men and all women not at increased risk • The USPSTF makes no recommendation for or against routine screening for lipid disorders in men between the ages of 20 and 35, or in women ages 20 and older who are not at increased risk for CHD. Grade C recommendation |
TABLE 2
Risk factors for CHD
| • Diabetes |
| • Personal history of coronary heart disease (CHD) or noncoronary atherosclerosis (eg, abdominal aortic aneurysm, peripheral artery disease, and carotid artery stenosis) |
| • A family history of cardiovascular disease before age 50 in male relatives or age 60 in female relatives |
| • Tobacco use |
| • Hypertension |
| • Obesity (body mass index ≥30) |
A different approach from NIH and AHA
The revised updated recommendation for women over age 45 was based on 2 systematic evidence reviews2,3 that concluded, while treatment clearly benefits women with other risk factors, benefit has not been proven for women who are otherwise CHD risk free.
The recommendation for women conflicts with those of the National Institutes of Health and the American Heart Association; both recommend screening all adults starting at age 20—regardless of risk.
Screening those without risk isn’t ruled out
It is important to note that the task force is not recommending against screening in women (or men between the ages of 20 and 35) who do not have other CHD risks. The task force makes a C recommendation with wording that states, “The USPSTF makes no recommendation for or against routine provision of [the service]. The USPSTF found at least fair evidence that [the service] can improve health outcomes but concludes that the balance of benefits and harms is too close to justify a general recommendation” (TABLE 3).
The task force chose not to use the new wording for a C recommendation, adopted in 2007, which reads, “The USPSTF recommends against routinely providing the service. There may be considerations that support providing the service in an individual patient. There is at least moderate certainty that the net benefit is small.”
It is also important to realize that a large proportion of women have another CHD risk and will not fall into the C category recommendation.
TABLE 3
USPSTF recommendation categories
| A—Strongly recommended: The United States Preventive Services Task Force (USPSTF) strongly recommends that clinicians provide the service to eligible patients. The USPSTF found good evidence that the service improves important health outcomes and concludes that benefits substantially outweigh harms. |
| B—Recommended: The USPSTF recommends that clinicians provide the service to eligible patients. The USPSTF found at least fair evidence that the service improves important health outcomes and concludes that benefits outweigh harms. |
| C—No recommendation: The USPSTF makes no recommendation for or against routine provision of the service. The USPSTF found at least fair evidence that the service can improve health outcomes but concludes that the balance of benefits and harms is too close to justify a general recommendation. |
| D—Not recommended: The USPSTF recommends against routinely providing the service to asymptomatic patients. The USPSTF found at least fair evidence that the service is ineffective or that harms outweigh benefits. |
| I—Insufficient evidence to make a recommendation: The USPSTF concludes that the evidence is insufficient to recommend for or against routinely providing the service. Evidence that the service is effective is lacking, of poor quality, or conflicting, and the balance of benefits and harms cannot be determined. |
No need to look at triglycerides initially
The task force recommends screening with a fasting or nonfasting serum sample for total cholesterol and high-density lipoprotein cholesterol. The task force does not recommend including a triglyceride level because there is mixed and inclusive evidence that triglyceride levels are independently associated with CHD risk and scant evidence that treating isolated elevated triglyceride levels reduces the occurrence of CHD events. This approach also conflicts with other organizations that recommend screening with fasting lipid profiles that include a triglyceride level.
The task force states that an abnormal initial screen should be confirmed by a repeat test and, if confirmed, a fasting lipid panel should be obtained. Wide adoption of the task force recommendations would result in considerable savings in cost and patient inconvenience by avoiding complete fasting lipid panels as the initial screen.
The optimal frequency of screening is not established and the task force states that every 5 years is reasonable, although more frequent testing might be considered for those with high normal values, and less frequent intervals for those with optimal cholesterol levels and healthy lifestyles.
Treatment: Look beyond lifestyle
The screening recommendations are accompanied by a discussion of clinical considerations and a description of an approach to treatment for those with lipid disorders. The main point the task force makes is that all CHD risks should be addressed, and that lifestyle changes alone rarely reduce elevated cholesterol to an optimal level. (For more on the treatment of hyperlipidemia, see the National Heart, Lung, and Blood Institute’s Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults [Adult Treatment Panel III] at http://www.nhlbi.nih.gov/guidelines/cholesterol/index.htm.)
Time to rethink conventional opinion
The updated task force recommendations are a reminder that many widely used guidelines, including those on the prevention of CHD, are based on a lack of high-level evidence. Thus, it is not surprising that a rigorously evidence-based analysis, as preformed by the USPSTF, will frequently result in recommendations that are at variance with common practice and conventional opinion.
When patients reached a certain age (36 for men, 46 for women), it used to mean that it was time, in the eyes of the United States Preventive Services Task Force (USPSTF), to screen for lipid disorders. But that’s changed for female patients.
The USPSTF’s latest recommendations (TABLE 1) on screening for lipid disorders in adults1 call for screening women only when coronary heart disease (CHD) risk factors are present, regardless of their age. (See TABLE 2 for a list of CHD risk factors.) That’s a major shift from the 2001 recommendation, which stated that all women over age 45 should be screened and women ages 20 to 45 should be screened if they were at elevated risk.
The recommendations for men remain the same: All men older than 35 should be screened, as should men who are between the ages of 20 and 35 who have other CHD risks.
TABLE 1
USPSTF lipid disorder screening recommendations at a glance
| Screening men • The United States Preventive Services Task Force (USPSTF) strongly recommends screening men ages 35 and older for lipid disorders. Grade A recommendation |
| • The USPSTF recommends screening men ages 20 to 35 for lipid disorders if they are at increased risk for coronary heart disease (CHD). Grade B recommendation |
| Screening women at increased risk • The USPSTF strongly recommends screening women ages 45 and older for lipid disorders if they are at increased risk for CHD. Grade A recommendation |
| • The USPSTF recommends screening women ages 20 to 45 for lipid disorders if they are at increased risk for CHD. Grade B recommendation |
| Screening young men and all women not at increased risk • The USPSTF makes no recommendation for or against routine screening for lipid disorders in men between the ages of 20 and 35, or in women ages 20 and older who are not at increased risk for CHD. Grade C recommendation |
TABLE 2
Risk factors for CHD
| • Diabetes |
| • Personal history of coronary heart disease (CHD) or noncoronary atherosclerosis (eg, abdominal aortic aneurysm, peripheral artery disease, and carotid artery stenosis) |
| • A family history of cardiovascular disease before age 50 in male relatives or age 60 in female relatives |
| • Tobacco use |
| • Hypertension |
| • Obesity (body mass index ≥30) |
A different approach from NIH and AHA
The revised updated recommendation for women over age 45 was based on 2 systematic evidence reviews2,3 that concluded, while treatment clearly benefits women with other risk factors, benefit has not been proven for women who are otherwise CHD risk free.
The recommendation for women conflicts with those of the National Institutes of Health and the American Heart Association; both recommend screening all adults starting at age 20—regardless of risk.
Screening those without risk isn’t ruled out
It is important to note that the task force is not recommending against screening in women (or men between the ages of 20 and 35) who do not have other CHD risks. The task force makes a C recommendation with wording that states, “The USPSTF makes no recommendation for or against routine provision of [the service]. The USPSTF found at least fair evidence that [the service] can improve health outcomes but concludes that the balance of benefits and harms is too close to justify a general recommendation” (TABLE 3).
The task force chose not to use the new wording for a C recommendation, adopted in 2007, which reads, “The USPSTF recommends against routinely providing the service. There may be considerations that support providing the service in an individual patient. There is at least moderate certainty that the net benefit is small.”
It is also important to realize that a large proportion of women have another CHD risk and will not fall into the C category recommendation.
TABLE 3
USPSTF recommendation categories
| A—Strongly recommended: The United States Preventive Services Task Force (USPSTF) strongly recommends that clinicians provide the service to eligible patients. The USPSTF found good evidence that the service improves important health outcomes and concludes that benefits substantially outweigh harms. |
| B—Recommended: The USPSTF recommends that clinicians provide the service to eligible patients. The USPSTF found at least fair evidence that the service improves important health outcomes and concludes that benefits outweigh harms. |
| C—No recommendation: The USPSTF makes no recommendation for or against routine provision of the service. The USPSTF found at least fair evidence that the service can improve health outcomes but concludes that the balance of benefits and harms is too close to justify a general recommendation. |
| D—Not recommended: The USPSTF recommends against routinely providing the service to asymptomatic patients. The USPSTF found at least fair evidence that the service is ineffective or that harms outweigh benefits. |
| I—Insufficient evidence to make a recommendation: The USPSTF concludes that the evidence is insufficient to recommend for or against routinely providing the service. Evidence that the service is effective is lacking, of poor quality, or conflicting, and the balance of benefits and harms cannot be determined. |
No need to look at triglycerides initially
The task force recommends screening with a fasting or nonfasting serum sample for total cholesterol and high-density lipoprotein cholesterol. The task force does not recommend including a triglyceride level because there is mixed and inclusive evidence that triglyceride levels are independently associated with CHD risk and scant evidence that treating isolated elevated triglyceride levels reduces the occurrence of CHD events. This approach also conflicts with other organizations that recommend screening with fasting lipid profiles that include a triglyceride level.
The task force states that an abnormal initial screen should be confirmed by a repeat test and, if confirmed, a fasting lipid panel should be obtained. Wide adoption of the task force recommendations would result in considerable savings in cost and patient inconvenience by avoiding complete fasting lipid panels as the initial screen.
The optimal frequency of screening is not established and the task force states that every 5 years is reasonable, although more frequent testing might be considered for those with high normal values, and less frequent intervals for those with optimal cholesterol levels and healthy lifestyles.
Treatment: Look beyond lifestyle
The screening recommendations are accompanied by a discussion of clinical considerations and a description of an approach to treatment for those with lipid disorders. The main point the task force makes is that all CHD risks should be addressed, and that lifestyle changes alone rarely reduce elevated cholesterol to an optimal level. (For more on the treatment of hyperlipidemia, see the National Heart, Lung, and Blood Institute’s Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults [Adult Treatment Panel III] at http://www.nhlbi.nih.gov/guidelines/cholesterol/index.htm.)
Time to rethink conventional opinion
The updated task force recommendations are a reminder that many widely used guidelines, including those on the prevention of CHD, are based on a lack of high-level evidence. Thus, it is not surprising that a rigorously evidence-based analysis, as preformed by the USPSTF, will frequently result in recommendations that are at variance with common practice and conventional opinion.
1. U.S. Preventive Services Task Force (USPSTF). Screening for lipid disorders in adults: recommendation statement. June 2008. Available at: http://www.ahrq.gov/clinic/uspstf08/lipid/lipidrs.htm. Accessed September 26, 2008.
2. Grady D, Chaput L, Kristof M. Systematic Review of Lipid Lowering Treatment to Reduce Risk of Coronary Heart Disease in Women. Rockville, Md: Agency for Healthcare Research and Quality; 2003.
3. Helfand M, Carson S. Screening for lipid disorders in adults: selective update of 2001 U.S. Preventive Services Task Force Review. June 2008. AHRQ publication number 08-05114-EF-1. Available at: http://www.ahrq.gov/clinic/uspstf08/lipid/lipides.pdf. Accessed September 26, 2008.
1. U.S. Preventive Services Task Force (USPSTF). Screening for lipid disorders in adults: recommendation statement. June 2008. Available at: http://www.ahrq.gov/clinic/uspstf08/lipid/lipidrs.htm. Accessed September 26, 2008.
2. Grady D, Chaput L, Kristof M. Systematic Review of Lipid Lowering Treatment to Reduce Risk of Coronary Heart Disease in Women. Rockville, Md: Agency for Healthcare Research and Quality; 2003.
3. Helfand M, Carson S. Screening for lipid disorders in adults: selective update of 2001 U.S. Preventive Services Task Force Review. June 2008. AHRQ publication number 08-05114-EF-1. Available at: http://www.ahrq.gov/clinic/uspstf08/lipid/lipides.pdf. Accessed September 26, 2008.
CDC: Older kids should get annual flu vaccine, too
The Centers for Disease Control and Prevention (CDC) has made 2 significant changes to its annual recommendations for the prevention of influenza during the 2008-2009 flu season:1
- Annual vaccination is now recommended for all children ages 6 months through 18 years. (Last year, universal influenza vaccination was recommended only for children ages 6 months through 4 years.)
- The live attenuated influenza vaccine (LAIV) can now be used starting at 2 years of age.
Vaccinate older children
The CDC now recommends that 5- to 18-year-olds receive the influenza vaccine annually, and that this routine vaccination start as soon as possible, but no later than the 2009-2010 flu season. In other words, if routine vaccination can be achieved this year it is encouraged, but the CDC recognizes that it may not be possible to achieve in some settings until next year.
If family physicians do not incorporate routine vaccination for those ages 5 to 18 this year, they should still provide it for those in this age group who are at high risk for influenza complications, including those who:
- are on long-term aspirin therapy;
- have chronic pulmonary (including asthma), cardiovascular, renal, hepatic, hematological, or metabolic disorders;
- are immunosuppressed; or
- have disorders that alter respiratory functions or the handling of respiratory secretions.
Children who live in households with others who are at higher risk (children who are <5 years old, adults >50 years, and anyone with a medical condition that places him or her at high risk for severe influenza complications) should also be vaccinated.
LAIV is an option for even younger kids
Last year, the LAIV vaccine was licensed for children starting at age 5. Now, the LAIV can be given to healthy children starting at age 2, as well as to adolescents and adults through age 49. TABLE 1 compares the LAIV with the trivalent influenza vaccine (TIV).
Because LAIV is an attenuated live virus vaccine, some children should not receive it, including those younger than 5 years of age with reactive airway disease (recurrent wheezing or recent wheezing); those with a medical condition that places them at high risk of influenza complications; and those younger than 2 years of age. The TIV can be used in these children, starting at 6 months of age.
Regardless of whether a child receives LAIV or TIV, those younger than 9 years of age who are receiving influenza vaccine for the first time should receive 2 doses 4 weeks apart. If a child received only 1 dose in the first year, the following year he or she should receive 2 doses 4 weeks apart.
TABLE 1
LAIV vs TIV: How the 2 compare
| LAIV | TIV | |
|---|---|---|
| Route of administration | Intranasal spray | Intramuscular injection |
| Type of vaccine | Live attenuated virus | Killed virus |
| Approved age | 2-49 years | ≥6 months |
| Interval between 2 doses recommended for children 6 months to 8 years who are receiving influenza vaccine for the first time | 4 weeks | 4 weeks |
| Use with other live virus vaccines | Simultaneously or separated by 4 weeks | No restrictions |
| Use with influenza antiviral medication | Wait 48 hours after last antiviral dose to administer LAIV; wait 2 weeks after LAIV to administer antivirals | No restrictions |
| Contraindications and precautions | Chronic illness | Anaphylactic hypersensitivity to eggs |
| Chronic aspirin therapy | Moderate-to-severe illness (precaution) | |
| History of Guillain-Barre syndrome | ||
| Pregnancy | ||
| Caregiver to severely immune-suppressed individual | ||
| LAIV, live attenuated influenza vaccine; TIV, trivalent influenza vaccine. | ||
Coverage rates still need to improve
Influenza vaccine and antiviral agents continue to be underutilized. TABLE 2 lists estimated coverage with influenza vaccine for specific groups for whom immunization is recommended. It is particularly important that coverage rates for health care workers—which remain below 50%—be improved. Health care workers are at high risk of exposure to influenza and pose a risk of disease transmission to their families, other staff members, and patients. Family physicians should ensure that they and their staff are vaccinated each year.
Missed opportunities. Many patients for whom influenza vaccine is recommended fail to receive the vaccine because of missed opportunities. Physicians should offer the vaccine starting in October (or as soon as the vaccine supply allows) and continue to offer and encourage it through the entire flu season. Peak influenza activity can occur as late as April and May and occurs after February on average of 1 in every 5 years.
TABLE 2
Immunization is recommended, but what were the coverage rates?*
| POPULATION GROUP | COVERAGE |
|---|---|
| Age 6-23 months | 32.2% |
| Age 2-4 years | 37.9% |
| Age ≥65 years | 65.6% |
| Pregnant women | 13.4% |
| Health care workers | 41.8% |
| Ages 18-64 years with high-risk conditions | 35.3% |
| * Influenza vaccination coverage is for the most recent year surveyed (2005-06 or 2006-07). | |
Autism concerns persist among parents
Despite clear scientific evidence that neither vaccines nor thimerosal preservative cause autism, some parents remain concerned. Some states have passed laws prohibiting the use of any thimerosal-containing vaccines and some parents may request thimerosal-free vaccines. TABLE 3 lists all the influenza vaccines and their thimerosal content.
TABLE 3
Which vaccines contain thimerosal—and how much?
| VACCINE | TRADE NAME | MANUFACTURER | HOW SUPPLIED | MERCURY CONTENT (MCG HG/0.5 ML DOSE) |
|---|---|---|---|---|
| TIV | Fluzone | Sanofi Pasteur | 0.25-mL prefilled syringe | 0 |
| 0.5-mL prefilled syringe | 0 | |||
| 0.5-mL vial | 0 | |||
| 5-mL multidose vial | 25 | |||
| TIV | Fluvirin | Novartis Vaccines | 5-mL multidose vial | 25 |
| 0.5-mL prefilled syringe | ≤1 | |||
| TIV | Fluarix | GlaxoSmithKline | 0.5-mL prefilled syringe | ≤1 |
| TIV | FluLaval | GlaxoSmithKline | 5-mL multidose vial | 25 |
| TIV | Afluria | CSL Biotherapies | 0.5-mL prefilled syringe | 0 |
| 5-mL multidose vial | 24.5 | |||
| LAIV | FluMist | MedImmune | 0.2-mL sprayer | 0 |
Make use of antivirals
Two antiviral medications are licensed and approved for the treatment and prevention of influenza: oseltamivir (Tamiflu) and zanamivir (Relenza). Two others (amantadine and rimantadine) are licensed but not currently recommended due to the high rates of resistance that influenza has developed against them.
Oseltamivir is approved for the treatment and prophylaxis of influenza starting at 1 year of age.
Zanamivir is approved for the treatment of influenza starting at 7 years of age and for prophylaxis starting at 5 years of age.
Treatment, if started within 48 hours of symptom onset, reduces the severity and length of infection and the length of infectiousness. Antiviral prophylaxis should be considered when there is increased influenza activity for those listed in TABLE 4.
TABLE 4
Increased flu activity in the community? Consider antiviral prophylaxis
|
| Note: Recommended antiviral medications (neuraminidase inhibitors) are not licensed for prophylaxis of children <1 year of age (oseltamivir) or <5 years of age (zanamivir). |
Every bit helps
Each year, influenza kills, on average, 36,000 Americans and hospitalizes another 200,000. Much of this morbidity and mortality could be avoided with full utilization of influenza vaccines and antiviral medications. You can contribute to improved public health by assuring that your patients and staff are fully immunized, that office infection control practices are adhered to, and that antiviral prophylaxis is used when indicated.
Reference
1. Prevention and control of influenza. Recommendations of the Advisory Committee on Immunization Practices, 2008. MMWR;57(Early Release: July 17, 2008). Available at: http://www.cdc.gov/mmwr/preview/mmwrhtml/rr57e717a1.htm. Accessed August 25, 2008.
The Centers for Disease Control and Prevention (CDC) has made 2 significant changes to its annual recommendations for the prevention of influenza during the 2008-2009 flu season:1
- Annual vaccination is now recommended for all children ages 6 months through 18 years. (Last year, universal influenza vaccination was recommended only for children ages 6 months through 4 years.)
- The live attenuated influenza vaccine (LAIV) can now be used starting at 2 years of age.
Vaccinate older children
The CDC now recommends that 5- to 18-year-olds receive the influenza vaccine annually, and that this routine vaccination start as soon as possible, but no later than the 2009-2010 flu season. In other words, if routine vaccination can be achieved this year it is encouraged, but the CDC recognizes that it may not be possible to achieve in some settings until next year.
If family physicians do not incorporate routine vaccination for those ages 5 to 18 this year, they should still provide it for those in this age group who are at high risk for influenza complications, including those who:
- are on long-term aspirin therapy;
- have chronic pulmonary (including asthma), cardiovascular, renal, hepatic, hematological, or metabolic disorders;
- are immunosuppressed; or
- have disorders that alter respiratory functions or the handling of respiratory secretions.
Children who live in households with others who are at higher risk (children who are <5 years old, adults >50 years, and anyone with a medical condition that places him or her at high risk for severe influenza complications) should also be vaccinated.
LAIV is an option for even younger kids
Last year, the LAIV vaccine was licensed for children starting at age 5. Now, the LAIV can be given to healthy children starting at age 2, as well as to adolescents and adults through age 49. TABLE 1 compares the LAIV with the trivalent influenza vaccine (TIV).
Because LAIV is an attenuated live virus vaccine, some children should not receive it, including those younger than 5 years of age with reactive airway disease (recurrent wheezing or recent wheezing); those with a medical condition that places them at high risk of influenza complications; and those younger than 2 years of age. The TIV can be used in these children, starting at 6 months of age.
Regardless of whether a child receives LAIV or TIV, those younger than 9 years of age who are receiving influenza vaccine for the first time should receive 2 doses 4 weeks apart. If a child received only 1 dose in the first year, the following year he or she should receive 2 doses 4 weeks apart.
TABLE 1
LAIV vs TIV: How the 2 compare
| LAIV | TIV | |
|---|---|---|
| Route of administration | Intranasal spray | Intramuscular injection |
| Type of vaccine | Live attenuated virus | Killed virus |
| Approved age | 2-49 years | ≥6 months |
| Interval between 2 doses recommended for children 6 months to 8 years who are receiving influenza vaccine for the first time | 4 weeks | 4 weeks |
| Use with other live virus vaccines | Simultaneously or separated by 4 weeks | No restrictions |
| Use with influenza antiviral medication | Wait 48 hours after last antiviral dose to administer LAIV; wait 2 weeks after LAIV to administer antivirals | No restrictions |
| Contraindications and precautions | Chronic illness | Anaphylactic hypersensitivity to eggs |
| Chronic aspirin therapy | Moderate-to-severe illness (precaution) | |
| History of Guillain-Barre syndrome | ||
| Pregnancy | ||
| Caregiver to severely immune-suppressed individual | ||
| LAIV, live attenuated influenza vaccine; TIV, trivalent influenza vaccine. | ||
Coverage rates still need to improve
Influenza vaccine and antiviral agents continue to be underutilized. TABLE 2 lists estimated coverage with influenza vaccine for specific groups for whom immunization is recommended. It is particularly important that coverage rates for health care workers—which remain below 50%—be improved. Health care workers are at high risk of exposure to influenza and pose a risk of disease transmission to their families, other staff members, and patients. Family physicians should ensure that they and their staff are vaccinated each year.
Missed opportunities. Many patients for whom influenza vaccine is recommended fail to receive the vaccine because of missed opportunities. Physicians should offer the vaccine starting in October (or as soon as the vaccine supply allows) and continue to offer and encourage it through the entire flu season. Peak influenza activity can occur as late as April and May and occurs after February on average of 1 in every 5 years.
TABLE 2
Immunization is recommended, but what were the coverage rates?*
| POPULATION GROUP | COVERAGE |
|---|---|
| Age 6-23 months | 32.2% |
| Age 2-4 years | 37.9% |
| Age ≥65 years | 65.6% |
| Pregnant women | 13.4% |
| Health care workers | 41.8% |
| Ages 18-64 years with high-risk conditions | 35.3% |
| * Influenza vaccination coverage is for the most recent year surveyed (2005-06 or 2006-07). | |
Autism concerns persist among parents
Despite clear scientific evidence that neither vaccines nor thimerosal preservative cause autism, some parents remain concerned. Some states have passed laws prohibiting the use of any thimerosal-containing vaccines and some parents may request thimerosal-free vaccines. TABLE 3 lists all the influenza vaccines and their thimerosal content.
TABLE 3
Which vaccines contain thimerosal—and how much?
| VACCINE | TRADE NAME | MANUFACTURER | HOW SUPPLIED | MERCURY CONTENT (MCG HG/0.5 ML DOSE) |
|---|---|---|---|---|
| TIV | Fluzone | Sanofi Pasteur | 0.25-mL prefilled syringe | 0 |
| 0.5-mL prefilled syringe | 0 | |||
| 0.5-mL vial | 0 | |||
| 5-mL multidose vial | 25 | |||
| TIV | Fluvirin | Novartis Vaccines | 5-mL multidose vial | 25 |
| 0.5-mL prefilled syringe | ≤1 | |||
| TIV | Fluarix | GlaxoSmithKline | 0.5-mL prefilled syringe | ≤1 |
| TIV | FluLaval | GlaxoSmithKline | 5-mL multidose vial | 25 |
| TIV | Afluria | CSL Biotherapies | 0.5-mL prefilled syringe | 0 |
| 5-mL multidose vial | 24.5 | |||
| LAIV | FluMist | MedImmune | 0.2-mL sprayer | 0 |
Make use of antivirals
Two antiviral medications are licensed and approved for the treatment and prevention of influenza: oseltamivir (Tamiflu) and zanamivir (Relenza). Two others (amantadine and rimantadine) are licensed but not currently recommended due to the high rates of resistance that influenza has developed against them.
Oseltamivir is approved for the treatment and prophylaxis of influenza starting at 1 year of age.
Zanamivir is approved for the treatment of influenza starting at 7 years of age and for prophylaxis starting at 5 years of age.
Treatment, if started within 48 hours of symptom onset, reduces the severity and length of infection and the length of infectiousness. Antiviral prophylaxis should be considered when there is increased influenza activity for those listed in TABLE 4.
TABLE 4
Increased flu activity in the community? Consider antiviral prophylaxis
|
| Note: Recommended antiviral medications (neuraminidase inhibitors) are not licensed for prophylaxis of children <1 year of age (oseltamivir) or <5 years of age (zanamivir). |
Every bit helps
Each year, influenza kills, on average, 36,000 Americans and hospitalizes another 200,000. Much of this morbidity and mortality could be avoided with full utilization of influenza vaccines and antiviral medications. You can contribute to improved public health by assuring that your patients and staff are fully immunized, that office infection control practices are adhered to, and that antiviral prophylaxis is used when indicated.
The Centers for Disease Control and Prevention (CDC) has made 2 significant changes to its annual recommendations for the prevention of influenza during the 2008-2009 flu season:1
- Annual vaccination is now recommended for all children ages 6 months through 18 years. (Last year, universal influenza vaccination was recommended only for children ages 6 months through 4 years.)
- The live attenuated influenza vaccine (LAIV) can now be used starting at 2 years of age.
Vaccinate older children
The CDC now recommends that 5- to 18-year-olds receive the influenza vaccine annually, and that this routine vaccination start as soon as possible, but no later than the 2009-2010 flu season. In other words, if routine vaccination can be achieved this year it is encouraged, but the CDC recognizes that it may not be possible to achieve in some settings until next year.
If family physicians do not incorporate routine vaccination for those ages 5 to 18 this year, they should still provide it for those in this age group who are at high risk for influenza complications, including those who:
- are on long-term aspirin therapy;
- have chronic pulmonary (including asthma), cardiovascular, renal, hepatic, hematological, or metabolic disorders;
- are immunosuppressed; or
- have disorders that alter respiratory functions or the handling of respiratory secretions.
Children who live in households with others who are at higher risk (children who are <5 years old, adults >50 years, and anyone with a medical condition that places him or her at high risk for severe influenza complications) should also be vaccinated.
LAIV is an option for even younger kids
Last year, the LAIV vaccine was licensed for children starting at age 5. Now, the LAIV can be given to healthy children starting at age 2, as well as to adolescents and adults through age 49. TABLE 1 compares the LAIV with the trivalent influenza vaccine (TIV).
Because LAIV is an attenuated live virus vaccine, some children should not receive it, including those younger than 5 years of age with reactive airway disease (recurrent wheezing or recent wheezing); those with a medical condition that places them at high risk of influenza complications; and those younger than 2 years of age. The TIV can be used in these children, starting at 6 months of age.
Regardless of whether a child receives LAIV or TIV, those younger than 9 years of age who are receiving influenza vaccine for the first time should receive 2 doses 4 weeks apart. If a child received only 1 dose in the first year, the following year he or she should receive 2 doses 4 weeks apart.
TABLE 1
LAIV vs TIV: How the 2 compare
| LAIV | TIV | |
|---|---|---|
| Route of administration | Intranasal spray | Intramuscular injection |
| Type of vaccine | Live attenuated virus | Killed virus |
| Approved age | 2-49 years | ≥6 months |
| Interval between 2 doses recommended for children 6 months to 8 years who are receiving influenza vaccine for the first time | 4 weeks | 4 weeks |
| Use with other live virus vaccines | Simultaneously or separated by 4 weeks | No restrictions |
| Use with influenza antiviral medication | Wait 48 hours after last antiviral dose to administer LAIV; wait 2 weeks after LAIV to administer antivirals | No restrictions |
| Contraindications and precautions | Chronic illness | Anaphylactic hypersensitivity to eggs |
| Chronic aspirin therapy | Moderate-to-severe illness (precaution) | |
| History of Guillain-Barre syndrome | ||
| Pregnancy | ||
| Caregiver to severely immune-suppressed individual | ||
| LAIV, live attenuated influenza vaccine; TIV, trivalent influenza vaccine. | ||
Coverage rates still need to improve
Influenza vaccine and antiviral agents continue to be underutilized. TABLE 2 lists estimated coverage with influenza vaccine for specific groups for whom immunization is recommended. It is particularly important that coverage rates for health care workers—which remain below 50%—be improved. Health care workers are at high risk of exposure to influenza and pose a risk of disease transmission to their families, other staff members, and patients. Family physicians should ensure that they and their staff are vaccinated each year.
Missed opportunities. Many patients for whom influenza vaccine is recommended fail to receive the vaccine because of missed opportunities. Physicians should offer the vaccine starting in October (or as soon as the vaccine supply allows) and continue to offer and encourage it through the entire flu season. Peak influenza activity can occur as late as April and May and occurs after February on average of 1 in every 5 years.
TABLE 2
Immunization is recommended, but what were the coverage rates?*
| POPULATION GROUP | COVERAGE |
|---|---|
| Age 6-23 months | 32.2% |
| Age 2-4 years | 37.9% |
| Age ≥65 years | 65.6% |
| Pregnant women | 13.4% |
| Health care workers | 41.8% |
| Ages 18-64 years with high-risk conditions | 35.3% |
| * Influenza vaccination coverage is for the most recent year surveyed (2005-06 or 2006-07). | |
Autism concerns persist among parents
Despite clear scientific evidence that neither vaccines nor thimerosal preservative cause autism, some parents remain concerned. Some states have passed laws prohibiting the use of any thimerosal-containing vaccines and some parents may request thimerosal-free vaccines. TABLE 3 lists all the influenza vaccines and their thimerosal content.
TABLE 3
Which vaccines contain thimerosal—and how much?
| VACCINE | TRADE NAME | MANUFACTURER | HOW SUPPLIED | MERCURY CONTENT (MCG HG/0.5 ML DOSE) |
|---|---|---|---|---|
| TIV | Fluzone | Sanofi Pasteur | 0.25-mL prefilled syringe | 0 |
| 0.5-mL prefilled syringe | 0 | |||
| 0.5-mL vial | 0 | |||
| 5-mL multidose vial | 25 | |||
| TIV | Fluvirin | Novartis Vaccines | 5-mL multidose vial | 25 |
| 0.5-mL prefilled syringe | ≤1 | |||
| TIV | Fluarix | GlaxoSmithKline | 0.5-mL prefilled syringe | ≤1 |
| TIV | FluLaval | GlaxoSmithKline | 5-mL multidose vial | 25 |
| TIV | Afluria | CSL Biotherapies | 0.5-mL prefilled syringe | 0 |
| 5-mL multidose vial | 24.5 | |||
| LAIV | FluMist | MedImmune | 0.2-mL sprayer | 0 |
Make use of antivirals
Two antiviral medications are licensed and approved for the treatment and prevention of influenza: oseltamivir (Tamiflu) and zanamivir (Relenza). Two others (amantadine and rimantadine) are licensed but not currently recommended due to the high rates of resistance that influenza has developed against them.
Oseltamivir is approved for the treatment and prophylaxis of influenza starting at 1 year of age.
Zanamivir is approved for the treatment of influenza starting at 7 years of age and for prophylaxis starting at 5 years of age.
Treatment, if started within 48 hours of symptom onset, reduces the severity and length of infection and the length of infectiousness. Antiviral prophylaxis should be considered when there is increased influenza activity for those listed in TABLE 4.
TABLE 4
Increased flu activity in the community? Consider antiviral prophylaxis
|
| Note: Recommended antiviral medications (neuraminidase inhibitors) are not licensed for prophylaxis of children <1 year of age (oseltamivir) or <5 years of age (zanamivir). |
Every bit helps
Each year, influenza kills, on average, 36,000 Americans and hospitalizes another 200,000. Much of this morbidity and mortality could be avoided with full utilization of influenza vaccines and antiviral medications. You can contribute to improved public health by assuring that your patients and staff are fully immunized, that office infection control practices are adhered to, and that antiviral prophylaxis is used when indicated.
Reference
1. Prevention and control of influenza. Recommendations of the Advisory Committee on Immunization Practices, 2008. MMWR;57(Early Release: July 17, 2008). Available at: http://www.cdc.gov/mmwr/preview/mmwrhtml/rr57e717a1.htm. Accessed August 25, 2008.
Reference
1. Prevention and control of influenza. Recommendations of the Advisory Committee on Immunization Practices, 2008. MMWR;57(Early Release: July 17, 2008). Available at: http://www.cdc.gov/mmwr/preview/mmwrhtml/rr57e717a1.htm. Accessed August 25, 2008.
Should you screen—or not? The latest recommendations
Not enough time and too many potential tests to do. This is the problem faced daily by family physicians. We want to practice up-to-date preventive medicine, but there’s little time to analyze the latest studies. Thankfully, we can rely on the United States Preventive Services Task Force, the organization with the most rigorous evidence-based approach, to do the legwork for us.1
Last year, and in the early part of this year, the Task Force issued a number of recommendations on topics ranging from hypertension screening to screening for illicit drug use. (See TABLE 1 for a breakdown of the 5 categories of recommendations.)
While some of these recommendations (TABLE 2) were reaffirmations of past recommendations, others included some changes.
The Task Force has:
- dropped the age for routine screening for Chlamydia in sexually active women from 25 years and younger to 24 and younger.
- added a recommendation against the use of aspirin or other nonsteroidal anti-inflammatory drugs (NSAIDs) to prevent colorectal cancer (CRC).
- changed its recommendation on screening for carotid artery stenosis. In 1996, the Task Force noted that the evidence was insufficient to make a recommendation; in 2007 it recommended against such routine screening.
- added recommendations on counseling patients about drinking and driving, as well as on screening for illicit drug use. In both cases, the Task Force says the evidence is insufficient to recommend for or against.
TABLE 1
USPSTF recommendation categories
| A Recommendation: The Task Force recommends the service. There is a high certainty that the net benefit is substantial. |
| B Recommendation: The Task Force recommends the service. There is high certainty that the net benefit is moderate or there is moderate certainty that the net benefit is moderate to substantial. |
| C Recommendation: The Task Force recommends against routinely providing the service. There may be considerations that support providing the service in an individual patient. There is at least moderate certainty that the net benefit is small. |
| D Recommendation: The Task Force recommends against the service. There is moderate or high certainty that the service has no net benefit or that the harms outweigh the benefits. |
| I Recommendation: The Task Force concludes that the current evidence is insufficient to assess the balance of benefits and harms of the service. Evidence is lacking, of poor quality, or conflicting, and the balance of benefits and harms cannot be determined. |
TABLE 2
Summary of new USPSTF recommendations
| A RECOMMENDATIONS |
The USPSTF recommends routinely:
|
| B RECOMMENDATIONS |
The USPSTF recommends routinely:
|
| C RECOMMENDATIONS |
The USPSTF recommends against routine:
|
| D RECOMMENDATIONS |
The USPSTF recommends against routine:
|
| I RECOMMENDATIONS |
The USPSTF concludes that the current evidence is insufficient to recommend for or against routine:
|
Continue to screen for HTN, sickle cell, Chlamydia
The latest A and B recommendations from the Task Force largely reaffirm previous recommendations. These recommendations cover hypertension, sickle cell disease, and Chlamydia.
Hypertension. Screening and treatment of hypertension in adults leads to lower morbidity and mortality from cardiovascular disease and is still recommended.2
Sickle cell disease. Screening newborns for sickle cell disease and treating those affected with oral prophylactic penicillin prevents serious bacterial infections. It also remains a recommended service.3
Chlamydia. Following a review of the evidence, the Task Force reconfirms the benefits of screening for Chlamydia in sexually active young women, but it has changed the age cutoff. In 2001, the Task Force indicated that sexually active women who were 25 years of age and younger should be screened. In 2007, the Task Force dropped the age to 24 and younger.
The latest recommendation reaffirms the need to screen women (above the cutoff) who are at risk—that is, women who have previously had a sexually transmitted infection (STI), those who have a new or multiple sex partners, and those who exchange sex for money or drugs.4 Screening is recommended annually; nucleic acid amplification tests are acceptable, allowing testing of urine or vaginal swabs.
Screening during pregnancy is recommended for the same groups—women who are 24 and younger and older women at risk—at the first prenatal visit and again in the third trimester if risk continues. Chlamydia is the most common bacterial STI, and screening and treatment prevents pelvic inflammatory disease in women and leads to improved pregnancy outcomes.
Interventions that are not recommended
Chemopreventon of colorectal cancer. For the first time, the Task Force issued a recommendation on the use of aspirin or other NSAIDs to prevent CRC. The Task Force does not recommend the routine use of these agents.5 The dosage needed to prevent CRC is higher than that which prevents cardiovascular disease and can cause significant harm.
Aspirin use is associated with gastrointestinal bleeding and hemorrhagic stroke; NSAID use is associated with gastrointestinal bleeding and renal impairment. The Task Force concludes that in the general adult population, potential harms exceed potential benefits.
Screening for carotid artery stenosis. In 1996, the Task Force found insufficient evidence to recommend for or against routine screening for carotid artery stenosis. In 2007, the Task Force made a recommendation against routine screening for carotid artery stenosis.6 Screening with duplex ultrasonography results in frequent false positives. Confirmatory testing with angiography is associated with a 1% rate of stroke. Endarterectomy itself has a death or stroke rate of about 3%.
In the general population, close to 8700 adults would need to be screened to prevent 1 disabling stroke. The Task Force indicates that primary care physicians would have better outcomes by concentrating on optimal management of risk factors for cerebral artery disease.
Screening for bacterial vaginosis among low-risk pregnant women. The final D recommendation pertains to screening for bacterial vaginosis during pregnancy to prevent preterm delivery.7 Pregnant women who have not had a previous preterm delivery are considered at low risk for preterm delivery and there is good evidence that this group does not benefit from screening for, or treatment of, asymptomatic bacterial vaginosis. (A similar recommendation was made in 2001, but it referred to women of “average” risk.)
Insufficient evidence to make a recommendation
Routinely screening men for Chlamydia. While it makes clinical sense to test and treat male partners of women with Chlamydia infection, the Task Force could not find evidence of the effectiveness of routinely screening men as a way to prevent infection in women.4 That said, the Task Force points out that screening men is relatively inexpensive and has negligible harms.
Screening for hyperlipidemia in children. While 50% of children with hyperlipidemia continue to have this disorder as adults, the long-term benefits and harms of early detection and treatment with medications and lipid-lowering diets have not been studied.8 This echoes the position the Task Force took in 1996, when it commented on children as part of an adult hyperlipidemia recommendation.
Physician counseling on drinking and driving. Motor vehicle crashes result in significant morbidity and mortality—especially among adolescents and young adults. Improved car and road design, as well as public health safety efforts, have led to significant improvements in motor vehicle safety. While avoidance of driving under the influence and proper use of occupant restraints are important public health goals, the Task Force, in this first recommendation on the subject, could find no evidence that physician counseling added benefit above those provided by community-wide efforts.9
Screening for bacterial vaginosis in pregnant women at high risk for preterm birth. As mentioned previously, screening low-risk pregnant women for bacterial vaginosis results in no benefit. The issue is less clear cut among women at high risk for a preterm delivery—that is, those who have had one previously.
The evidence regarding screening and treating asymptomatic bacterial vaginosis as a means of preventing preterm delivery in these women is mixed and the Task Force was unable to recommend for or against this practice.7 This reaffirms the Task Force’s 2001 recommendation.
Screening for illicit drug use. The Task Force recognizes that illicit drug use is a major cause of illness and social problems. It would appear to have great potential for early detection and intervention. However, the Task Force, in this first-time recommendation, found that screening tools have not been well studied, nor have the long-term effects of different treatment strategies.10 These are high priority areas for future research.
Correspondence
Doug Campos-Outcalt, MD, MPA, 550 E. Van Buren, Phoenix, AZ 85004; dougco@u.arizona.edu
1. Agency for Healthcare Quality and Research. USPSTF. Available at: http://www.ahrq.gov/clinic/uspstfix.htm. Accessed May 5, 2008.
2. USPSTF. Screening for High Blood Pressure. Available at: http://www.ahrq.gov/clinic/uspstf/uspshype.htm. Accessed May 5, 2008.
3. USPSTF. Screening for Sickle Cell Disease in Newborns. Available at: http://www.ahrq.gov/clinic/uspstf/uspshemo.htm. Accessed May 5, 2008.
4. USPSTF. Screening for Chlamydia Infection. Available at: http://www.ahrq.gov/clinic/uspstf/uspschlm.htm. Accessed May 5, 2008.
5. USPSTF. Aspirin or Nonsteroidal Anti-inflamatory Drugs for the Primary Prevention of Colorectal Cancer. Available at: http://www.ahrq.gov/clinic/uspstf/uspsasco.htm. Accessed May 5, 2008.
6. USPSTF. Screening for Carotid Artery Stenosis. Available at: http://www.ahrq.gov/clinic/uspstf/uspsacas.htm. Accessed May 5, 2008.
7. USPSTF. Screening for Bacterial Vaginosis in Pregnancy. Available at: http://www.ahrq.gov/clinic/uspstf/uspsbvag.htm. Accessed May 5, 2008.
8. USPSTF. Screening for Lipid Disorders in Children. Available at: http://www.ahrq.gov/clinic/uspstf/uspschlip.htm. Accessed May 5, 2008.
9. USPSTF. Counseling About Proper Use of Motor Vehicle Occupant Restraints and Avoidance of Alcohol Use While Driving. Available at: http://www.ahrq.gov/clinic/uspstf/uspsmvin.htm. Accessed May 5, 2008.
10. USPSTF. Screening for Illicit Drug Use. Available at: http://www.ahrq.gov/clinic/uspstf/uspsdrug.htm. Accessed May 5, 2008.
Not enough time and too many potential tests to do. This is the problem faced daily by family physicians. We want to practice up-to-date preventive medicine, but there’s little time to analyze the latest studies. Thankfully, we can rely on the United States Preventive Services Task Force, the organization with the most rigorous evidence-based approach, to do the legwork for us.1
Last year, and in the early part of this year, the Task Force issued a number of recommendations on topics ranging from hypertension screening to screening for illicit drug use. (See TABLE 1 for a breakdown of the 5 categories of recommendations.)
While some of these recommendations (TABLE 2) were reaffirmations of past recommendations, others included some changes.
The Task Force has:
- dropped the age for routine screening for Chlamydia in sexually active women from 25 years and younger to 24 and younger.
- added a recommendation against the use of aspirin or other nonsteroidal anti-inflammatory drugs (NSAIDs) to prevent colorectal cancer (CRC).
- changed its recommendation on screening for carotid artery stenosis. In 1996, the Task Force noted that the evidence was insufficient to make a recommendation; in 2007 it recommended against such routine screening.
- added recommendations on counseling patients about drinking and driving, as well as on screening for illicit drug use. In both cases, the Task Force says the evidence is insufficient to recommend for or against.
TABLE 1
USPSTF recommendation categories
| A Recommendation: The Task Force recommends the service. There is a high certainty that the net benefit is substantial. |
| B Recommendation: The Task Force recommends the service. There is high certainty that the net benefit is moderate or there is moderate certainty that the net benefit is moderate to substantial. |
| C Recommendation: The Task Force recommends against routinely providing the service. There may be considerations that support providing the service in an individual patient. There is at least moderate certainty that the net benefit is small. |
| D Recommendation: The Task Force recommends against the service. There is moderate or high certainty that the service has no net benefit or that the harms outweigh the benefits. |
| I Recommendation: The Task Force concludes that the current evidence is insufficient to assess the balance of benefits and harms of the service. Evidence is lacking, of poor quality, or conflicting, and the balance of benefits and harms cannot be determined. |
TABLE 2
Summary of new USPSTF recommendations
| A RECOMMENDATIONS |
The USPSTF recommends routinely:
|
| B RECOMMENDATIONS |
The USPSTF recommends routinely:
|
| C RECOMMENDATIONS |
The USPSTF recommends against routine:
|
| D RECOMMENDATIONS |
The USPSTF recommends against routine:
|
| I RECOMMENDATIONS |
The USPSTF concludes that the current evidence is insufficient to recommend for or against routine:
|
Continue to screen for HTN, sickle cell, Chlamydia
The latest A and B recommendations from the Task Force largely reaffirm previous recommendations. These recommendations cover hypertension, sickle cell disease, and Chlamydia.
Hypertension. Screening and treatment of hypertension in adults leads to lower morbidity and mortality from cardiovascular disease and is still recommended.2
Sickle cell disease. Screening newborns for sickle cell disease and treating those affected with oral prophylactic penicillin prevents serious bacterial infections. It also remains a recommended service.3
Chlamydia. Following a review of the evidence, the Task Force reconfirms the benefits of screening for Chlamydia in sexually active young women, but it has changed the age cutoff. In 2001, the Task Force indicated that sexually active women who were 25 years of age and younger should be screened. In 2007, the Task Force dropped the age to 24 and younger.
The latest recommendation reaffirms the need to screen women (above the cutoff) who are at risk—that is, women who have previously had a sexually transmitted infection (STI), those who have a new or multiple sex partners, and those who exchange sex for money or drugs.4 Screening is recommended annually; nucleic acid amplification tests are acceptable, allowing testing of urine or vaginal swabs.
Screening during pregnancy is recommended for the same groups—women who are 24 and younger and older women at risk—at the first prenatal visit and again in the third trimester if risk continues. Chlamydia is the most common bacterial STI, and screening and treatment prevents pelvic inflammatory disease in women and leads to improved pregnancy outcomes.
Interventions that are not recommended
Chemopreventon of colorectal cancer. For the first time, the Task Force issued a recommendation on the use of aspirin or other NSAIDs to prevent CRC. The Task Force does not recommend the routine use of these agents.5 The dosage needed to prevent CRC is higher than that which prevents cardiovascular disease and can cause significant harm.
Aspirin use is associated with gastrointestinal bleeding and hemorrhagic stroke; NSAID use is associated with gastrointestinal bleeding and renal impairment. The Task Force concludes that in the general adult population, potential harms exceed potential benefits.
Screening for carotid artery stenosis. In 1996, the Task Force found insufficient evidence to recommend for or against routine screening for carotid artery stenosis. In 2007, the Task Force made a recommendation against routine screening for carotid artery stenosis.6 Screening with duplex ultrasonography results in frequent false positives. Confirmatory testing with angiography is associated with a 1% rate of stroke. Endarterectomy itself has a death or stroke rate of about 3%.
In the general population, close to 8700 adults would need to be screened to prevent 1 disabling stroke. The Task Force indicates that primary care physicians would have better outcomes by concentrating on optimal management of risk factors for cerebral artery disease.
Screening for bacterial vaginosis among low-risk pregnant women. The final D recommendation pertains to screening for bacterial vaginosis during pregnancy to prevent preterm delivery.7 Pregnant women who have not had a previous preterm delivery are considered at low risk for preterm delivery and there is good evidence that this group does not benefit from screening for, or treatment of, asymptomatic bacterial vaginosis. (A similar recommendation was made in 2001, but it referred to women of “average” risk.)
Insufficient evidence to make a recommendation
Routinely screening men for Chlamydia. While it makes clinical sense to test and treat male partners of women with Chlamydia infection, the Task Force could not find evidence of the effectiveness of routinely screening men as a way to prevent infection in women.4 That said, the Task Force points out that screening men is relatively inexpensive and has negligible harms.
Screening for hyperlipidemia in children. While 50% of children with hyperlipidemia continue to have this disorder as adults, the long-term benefits and harms of early detection and treatment with medications and lipid-lowering diets have not been studied.8 This echoes the position the Task Force took in 1996, when it commented on children as part of an adult hyperlipidemia recommendation.
Physician counseling on drinking and driving. Motor vehicle crashes result in significant morbidity and mortality—especially among adolescents and young adults. Improved car and road design, as well as public health safety efforts, have led to significant improvements in motor vehicle safety. While avoidance of driving under the influence and proper use of occupant restraints are important public health goals, the Task Force, in this first recommendation on the subject, could find no evidence that physician counseling added benefit above those provided by community-wide efforts.9
Screening for bacterial vaginosis in pregnant women at high risk for preterm birth. As mentioned previously, screening low-risk pregnant women for bacterial vaginosis results in no benefit. The issue is less clear cut among women at high risk for a preterm delivery—that is, those who have had one previously.
The evidence regarding screening and treating asymptomatic bacterial vaginosis as a means of preventing preterm delivery in these women is mixed and the Task Force was unable to recommend for or against this practice.7 This reaffirms the Task Force’s 2001 recommendation.
Screening for illicit drug use. The Task Force recognizes that illicit drug use is a major cause of illness and social problems. It would appear to have great potential for early detection and intervention. However, the Task Force, in this first-time recommendation, found that screening tools have not been well studied, nor have the long-term effects of different treatment strategies.10 These are high priority areas for future research.
Correspondence
Doug Campos-Outcalt, MD, MPA, 550 E. Van Buren, Phoenix, AZ 85004; dougco@u.arizona.edu
Not enough time and too many potential tests to do. This is the problem faced daily by family physicians. We want to practice up-to-date preventive medicine, but there’s little time to analyze the latest studies. Thankfully, we can rely on the United States Preventive Services Task Force, the organization with the most rigorous evidence-based approach, to do the legwork for us.1
Last year, and in the early part of this year, the Task Force issued a number of recommendations on topics ranging from hypertension screening to screening for illicit drug use. (See TABLE 1 for a breakdown of the 5 categories of recommendations.)
While some of these recommendations (TABLE 2) were reaffirmations of past recommendations, others included some changes.
The Task Force has:
- dropped the age for routine screening for Chlamydia in sexually active women from 25 years and younger to 24 and younger.
- added a recommendation against the use of aspirin or other nonsteroidal anti-inflammatory drugs (NSAIDs) to prevent colorectal cancer (CRC).
- changed its recommendation on screening for carotid artery stenosis. In 1996, the Task Force noted that the evidence was insufficient to make a recommendation; in 2007 it recommended against such routine screening.
- added recommendations on counseling patients about drinking and driving, as well as on screening for illicit drug use. In both cases, the Task Force says the evidence is insufficient to recommend for or against.
TABLE 1
USPSTF recommendation categories
| A Recommendation: The Task Force recommends the service. There is a high certainty that the net benefit is substantial. |
| B Recommendation: The Task Force recommends the service. There is high certainty that the net benefit is moderate or there is moderate certainty that the net benefit is moderate to substantial. |
| C Recommendation: The Task Force recommends against routinely providing the service. There may be considerations that support providing the service in an individual patient. There is at least moderate certainty that the net benefit is small. |
| D Recommendation: The Task Force recommends against the service. There is moderate or high certainty that the service has no net benefit or that the harms outweigh the benefits. |
| I Recommendation: The Task Force concludes that the current evidence is insufficient to assess the balance of benefits and harms of the service. Evidence is lacking, of poor quality, or conflicting, and the balance of benefits and harms cannot be determined. |
TABLE 2
Summary of new USPSTF recommendations
| A RECOMMENDATIONS |
The USPSTF recommends routinely:
|
| B RECOMMENDATIONS |
The USPSTF recommends routinely:
|
| C RECOMMENDATIONS |
The USPSTF recommends against routine:
|
| D RECOMMENDATIONS |
The USPSTF recommends against routine:
|
| I RECOMMENDATIONS |
The USPSTF concludes that the current evidence is insufficient to recommend for or against routine:
|
Continue to screen for HTN, sickle cell, Chlamydia
The latest A and B recommendations from the Task Force largely reaffirm previous recommendations. These recommendations cover hypertension, sickle cell disease, and Chlamydia.
Hypertension. Screening and treatment of hypertension in adults leads to lower morbidity and mortality from cardiovascular disease and is still recommended.2
Sickle cell disease. Screening newborns for sickle cell disease and treating those affected with oral prophylactic penicillin prevents serious bacterial infections. It also remains a recommended service.3
Chlamydia. Following a review of the evidence, the Task Force reconfirms the benefits of screening for Chlamydia in sexually active young women, but it has changed the age cutoff. In 2001, the Task Force indicated that sexually active women who were 25 years of age and younger should be screened. In 2007, the Task Force dropped the age to 24 and younger.
The latest recommendation reaffirms the need to screen women (above the cutoff) who are at risk—that is, women who have previously had a sexually transmitted infection (STI), those who have a new or multiple sex partners, and those who exchange sex for money or drugs.4 Screening is recommended annually; nucleic acid amplification tests are acceptable, allowing testing of urine or vaginal swabs.
Screening during pregnancy is recommended for the same groups—women who are 24 and younger and older women at risk—at the first prenatal visit and again in the third trimester if risk continues. Chlamydia is the most common bacterial STI, and screening and treatment prevents pelvic inflammatory disease in women and leads to improved pregnancy outcomes.
Interventions that are not recommended
Chemopreventon of colorectal cancer. For the first time, the Task Force issued a recommendation on the use of aspirin or other NSAIDs to prevent CRC. The Task Force does not recommend the routine use of these agents.5 The dosage needed to prevent CRC is higher than that which prevents cardiovascular disease and can cause significant harm.
Aspirin use is associated with gastrointestinal bleeding and hemorrhagic stroke; NSAID use is associated with gastrointestinal bleeding and renal impairment. The Task Force concludes that in the general adult population, potential harms exceed potential benefits.
Screening for carotid artery stenosis. In 1996, the Task Force found insufficient evidence to recommend for or against routine screening for carotid artery stenosis. In 2007, the Task Force made a recommendation against routine screening for carotid artery stenosis.6 Screening with duplex ultrasonography results in frequent false positives. Confirmatory testing with angiography is associated with a 1% rate of stroke. Endarterectomy itself has a death or stroke rate of about 3%.
In the general population, close to 8700 adults would need to be screened to prevent 1 disabling stroke. The Task Force indicates that primary care physicians would have better outcomes by concentrating on optimal management of risk factors for cerebral artery disease.
Screening for bacterial vaginosis among low-risk pregnant women. The final D recommendation pertains to screening for bacterial vaginosis during pregnancy to prevent preterm delivery.7 Pregnant women who have not had a previous preterm delivery are considered at low risk for preterm delivery and there is good evidence that this group does not benefit from screening for, or treatment of, asymptomatic bacterial vaginosis. (A similar recommendation was made in 2001, but it referred to women of “average” risk.)
Insufficient evidence to make a recommendation
Routinely screening men for Chlamydia. While it makes clinical sense to test and treat male partners of women with Chlamydia infection, the Task Force could not find evidence of the effectiveness of routinely screening men as a way to prevent infection in women.4 That said, the Task Force points out that screening men is relatively inexpensive and has negligible harms.
Screening for hyperlipidemia in children. While 50% of children with hyperlipidemia continue to have this disorder as adults, the long-term benefits and harms of early detection and treatment with medications and lipid-lowering diets have not been studied.8 This echoes the position the Task Force took in 1996, when it commented on children as part of an adult hyperlipidemia recommendation.
Physician counseling on drinking and driving. Motor vehicle crashes result in significant morbidity and mortality—especially among adolescents and young adults. Improved car and road design, as well as public health safety efforts, have led to significant improvements in motor vehicle safety. While avoidance of driving under the influence and proper use of occupant restraints are important public health goals, the Task Force, in this first recommendation on the subject, could find no evidence that physician counseling added benefit above those provided by community-wide efforts.9
Screening for bacterial vaginosis in pregnant women at high risk for preterm birth. As mentioned previously, screening low-risk pregnant women for bacterial vaginosis results in no benefit. The issue is less clear cut among women at high risk for a preterm delivery—that is, those who have had one previously.
The evidence regarding screening and treating asymptomatic bacterial vaginosis as a means of preventing preterm delivery in these women is mixed and the Task Force was unable to recommend for or against this practice.7 This reaffirms the Task Force’s 2001 recommendation.
Screening for illicit drug use. The Task Force recognizes that illicit drug use is a major cause of illness and social problems. It would appear to have great potential for early detection and intervention. However, the Task Force, in this first-time recommendation, found that screening tools have not been well studied, nor have the long-term effects of different treatment strategies.10 These are high priority areas for future research.
Correspondence
Doug Campos-Outcalt, MD, MPA, 550 E. Van Buren, Phoenix, AZ 85004; dougco@u.arizona.edu
1. Agency for Healthcare Quality and Research. USPSTF. Available at: http://www.ahrq.gov/clinic/uspstfix.htm. Accessed May 5, 2008.
2. USPSTF. Screening for High Blood Pressure. Available at: http://www.ahrq.gov/clinic/uspstf/uspshype.htm. Accessed May 5, 2008.
3. USPSTF. Screening for Sickle Cell Disease in Newborns. Available at: http://www.ahrq.gov/clinic/uspstf/uspshemo.htm. Accessed May 5, 2008.
4. USPSTF. Screening for Chlamydia Infection. Available at: http://www.ahrq.gov/clinic/uspstf/uspschlm.htm. Accessed May 5, 2008.
5. USPSTF. Aspirin or Nonsteroidal Anti-inflamatory Drugs for the Primary Prevention of Colorectal Cancer. Available at: http://www.ahrq.gov/clinic/uspstf/uspsasco.htm. Accessed May 5, 2008.
6. USPSTF. Screening for Carotid Artery Stenosis. Available at: http://www.ahrq.gov/clinic/uspstf/uspsacas.htm. Accessed May 5, 2008.
7. USPSTF. Screening for Bacterial Vaginosis in Pregnancy. Available at: http://www.ahrq.gov/clinic/uspstf/uspsbvag.htm. Accessed May 5, 2008.
8. USPSTF. Screening for Lipid Disorders in Children. Available at: http://www.ahrq.gov/clinic/uspstf/uspschlip.htm. Accessed May 5, 2008.
9. USPSTF. Counseling About Proper Use of Motor Vehicle Occupant Restraints and Avoidance of Alcohol Use While Driving. Available at: http://www.ahrq.gov/clinic/uspstf/uspsmvin.htm. Accessed May 5, 2008.
10. USPSTF. Screening for Illicit Drug Use. Available at: http://www.ahrq.gov/clinic/uspstf/uspsdrug.htm. Accessed May 5, 2008.
1. Agency for Healthcare Quality and Research. USPSTF. Available at: http://www.ahrq.gov/clinic/uspstfix.htm. Accessed May 5, 2008.
2. USPSTF. Screening for High Blood Pressure. Available at: http://www.ahrq.gov/clinic/uspstf/uspshype.htm. Accessed May 5, 2008.
3. USPSTF. Screening for Sickle Cell Disease in Newborns. Available at: http://www.ahrq.gov/clinic/uspstf/uspshemo.htm. Accessed May 5, 2008.
4. USPSTF. Screening for Chlamydia Infection. Available at: http://www.ahrq.gov/clinic/uspstf/uspschlm.htm. Accessed May 5, 2008.
5. USPSTF. Aspirin or Nonsteroidal Anti-inflamatory Drugs for the Primary Prevention of Colorectal Cancer. Available at: http://www.ahrq.gov/clinic/uspstf/uspsasco.htm. Accessed May 5, 2008.
6. USPSTF. Screening for Carotid Artery Stenosis. Available at: http://www.ahrq.gov/clinic/uspstf/uspsacas.htm. Accessed May 5, 2008.
7. USPSTF. Screening for Bacterial Vaginosis in Pregnancy. Available at: http://www.ahrq.gov/clinic/uspstf/uspsbvag.htm. Accessed May 5, 2008.
8. USPSTF. Screening for Lipid Disorders in Children. Available at: http://www.ahrq.gov/clinic/uspstf/uspschlip.htm. Accessed May 5, 2008.
9. USPSTF. Counseling About Proper Use of Motor Vehicle Occupant Restraints and Avoidance of Alcohol Use While Driving. Available at: http://www.ahrq.gov/clinic/uspstf/uspsmvin.htm. Accessed May 5, 2008.
10. USPSTF. Screening for Illicit Drug Use. Available at: http://www.ahrq.gov/clinic/uspstf/uspsdrug.htm. Accessed May 5, 2008.
MEASLES HITS HOME: Sobering lessons from 2 travel-related outbreaks
Inform concerned parents about the safety and effectiveness of vaccines.
2 doses of measles-containing vaccine are 99% effective.
Those exposed who are not immune should be vaccinated or offered immune globulin if the vaccine is contraindicated.
Contraindications
- Primary immune deficiency diseases of T-cell functions
- Acquired immune deficiency from leukemia, lymphoma, or generalized malignancy
- Therapy with corticosteroids: 2 mg/kg prednisone >2 weeks
- Previous anaphylactic reaction to measles vaccine, gelatin, or neomycins
- Pregnancy
Measles is still a threat. Endemic transmission of measles no longer occurs in the United States (or any of the Americas), yet this highly infectious disease is still a threat from importation by visitors from other countries and from US residents who have traveled abroad. Two recent outbreaks (described at left) illustrate these risks.
3 infants too young to be vaccinated contracted measles in their doctor’s office in San Diego, in January 2008. (An infant with measles rash [above] is for illustration only, and does not depict any of the 3.)
What the CDC discovered
The 2 outbreaks of import-linked measles brought home—literally—the sobering facts about vulnerability among US residents. The CDC report 1,2 of its investigation observed:
US travelers can be exposed almost anywhere, developed countries included. The California outbreak started with a visit to Switzerland.
Measles spreads rapidly in susceptible subgroups, unless effective control strategies are used. In California, on 2 consecutive days, 5 school children and 4 children in a doctor’s office were infected; all were unvaccinated.
People not considered at risk can contract measles. Although 2 doses of vaccine are 99% effective, vaccinated individuals, such as the college students, can contract measles. Likewise, people born before 1957 may not be immune, in contrast to the general definition of immunity (see Measles Basics. Case in point: the airline passenger, born in 1954.
Disease can be severe. The 40-year-old salesperson (no documented vaccination) was hospitalized with seizure, 105ºF fever, and pneumonia. One of the infants was hospitalized due to dehydration.
People in routine contact with travelers entering the United States can be exposed to measles—like the airline worker.
CALIFORNIA - A February 22 early-release CDC report1 linked 12 measles cases in California to an unvaccinated 7-year-old boy infected while traveling in Europe with his family in January. He was taken to his pediatrician after onset of rash, and to the emergency department the next day, because of high fever and generalized rash. No isolation precautions were used in the office or hospital.
The boy’s 2 siblings, 5 children at his school, and 4 children at the doctor’s office while he was there contracted measles (3 of whom were infants <12 months of age).
Nearly 10% of the children at the index case’s school were unvaccinated because of personal belief exemptions.
PENNSYLVANIA, MICHIGAN, TEXAS - A young boy from Japan participated in an international sporting event and attended a related sales event in Pennsylvania last August. He was infectious when he left Japan and as he traveled in the United States.
The CDC2 linked a total of 6 additional cases of measles in US-born residents to the index case: another young person from Japan who watched the sporting event; a 53-year-old airline passenger and a 25-year-old airline worker in Michigan; and a corporate sales representative who had met the index patient at the sales event and subsequently made sales visits to Houston-area colleges, where 2 college roommates became infected.
Viral genotyping supported a single chain of transmission, and genetic sequencing linked 6 of the 7 cases.
Take-home lessons for family physicians
Include measles in the differential diagnosis of patients who have fever and rash, especially if they have traveled to another country within the past 3 to 4 weeks. Any patient who meets the definition of measles (fever 101ºF or higher; rash; and at least 1 of the 3 Cs—cough, coryza, conjunctivitis) should be immediately reported to the local health department. The health department will provide instructions for collecting laboratory samples for confirmation; instructions on patient isolation; and assistance with notification and disease control measures for exposed individuals.
Immunize patients and staff. These recurring cases of imported measles underscore the importance of maintaining a high level of immunity. Outbreaks can happen even where immunity is 90% to 95%. When vaccination rates dip below 90%, sustained outbreaks can occur.6
Ensure that staff and patients are all immunized against vaccine-preventable diseases, and inform concerned parents about the safety and effectiveness of vaccines. Parents who refuse to have their children vaccinated place their children at risk and contribute to higher community risk. Communities that have higher rates of non-adherence to vaccine recommendations are more likely to have outbreaks.7,8
Use strict infection control in the office. The recent outbreak in California where 4 children were infected in their physician’s office reinforces the need for strict infection-control practices. Do not allow patients with rash and fever to remain in a common waiting area. Move them to an examination room, preferably an airborne infection isolation room. Keep the door to the examination room closed, and be sure that all health care personnel who come in contact with such patients are immune. Do not use triage rooms for 2 hours after the patient suspected of having measles leaves. Do not send these patients to other health care facilities, such as laboratories, unless infection control measures can be adhered to at those locations. Guidelines on infection control practices in health care settings are available.9,10
Quick response
Quick control of these outbreaks shows the value of the public health infrastructure. Disease surveillance and outbreak response is vital to the public health system, and its value is frequently under-appreciated by physicians and the public.
Fewer than 100 cases of measles occur in the United States each year, and virtually all are linked to imported cases.3 Before vaccine was introduced in 1963, 3 to 4 million cases per year occurred, and caused, on average, 450 deaths, 1000 chronic disabilities, and 28,000 hospitalizations.1 Success in controlling measles is due largely to high levels of coverage with 2 doses of measles-containing vaccine and public health surveillance and disease control.
Measles virus is highly infectious and is spread by airborne droplets and direct contact with nose and throat secretions. The incubation is 7 to 18 days.
Measles begins with fever, cough, coryza, conjunctivitis, and whitish spots on the buccal mucosa (Koplick spots).4 Rash appears on the 3rd to 7th day and lasts 4 to 7 days. It begins on the face but soon becomes generalized. An infected person is contagious from 5 days before the rash until 4 days after the rash appears. The diagnosis of measles can be confirrmed by serum measles IGM, which occurs within 3 days of rash, or a rise in measles IGG between acute and 2-week convalescent serum titers.
Complications: pneumonia (5%), otitis media (10%), and encephalitis 1/1000). Death rates: 1 to 2/1000, varying greatly based on age and nutrition; more severe in the very young and the malnourished. Worldwide, about 500,000 children die from measles each year.5
Immunity is defined as:
- 2 vaccine doses at least 1 month apart, both given after the 1st birthday,
- born before 1957,
- serological evidence, or
- history of physician-diagnosed measles.
1. CDC. Outbreak of measles—San Diego, California, January-February 2008. MMWR. 2008;57:Early Release February 22, 2008.-
2. CDC. Multistate measles outbreak associated with an international youth sporting event—Pennsylvania, Michigan, and Texas, August-September 2007. MMWR. 2008;57:169-173.
3. CDC. Measles—United States, 2005. MMWR. 2006;55:1348-1351.
4. Measles. In: Heyman DL. Control of Communicable Diseases Manual. 18th ed. Washington, DC: American Public Health Association.
5. CDC. Parents’ guide to childhood immunizations. Available at: http://www.cdc.gov/vaccines/vpd-vac/measles/downloads/pg_why_vacc_measles.pdf. Accessed March 17, 2008.
6. Richard JL, Masserey-Spicher V, Santibanez S, Mankertz A. Measles outbreak in Switzerland. Available at: http://www.eurosurveillance.org/edition/v13n08/080221_1.asp. Accessed March 17. 2008.
7. Salmon DA, Haber M, Gangarosa EJ, et al. Health consequences of religious and philosophical exemptions from immunization laws; individual and societal risk of measles. JAMA. 1999;282:47-53
8. Feikin DR, Lezotte DC, Hamman RF, et al. Individual and community risks of measles and pertussis associated with personal exemptions to immunization. JAMA. 2008;284:3145-3150.
9. Siegel JD, Rhinehart E, Jackson M, Chiarello L. Health care infection control practices advisory committee, 2007.Guideline for isolation precautions: preventing transmission of infectious agents in health care settings. Am J Infect Control. 2007;35(suppl 2):S65-164.
10. Campos-Outcalt D. Infection control in outpatient settings. J Fam Pract. 2004;53:485-488.
Inform concerned parents about the safety and effectiveness of vaccines.
2 doses of measles-containing vaccine are 99% effective.
Those exposed who are not immune should be vaccinated or offered immune globulin if the vaccine is contraindicated.
Contraindications
- Primary immune deficiency diseases of T-cell functions
- Acquired immune deficiency from leukemia, lymphoma, or generalized malignancy
- Therapy with corticosteroids: 2 mg/kg prednisone >2 weeks
- Previous anaphylactic reaction to measles vaccine, gelatin, or neomycins
- Pregnancy
Measles is still a threat. Endemic transmission of measles no longer occurs in the United States (or any of the Americas), yet this highly infectious disease is still a threat from importation by visitors from other countries and from US residents who have traveled abroad. Two recent outbreaks (described at left) illustrate these risks.
3 infants too young to be vaccinated contracted measles in their doctor’s office in San Diego, in January 2008. (An infant with measles rash [above] is for illustration only, and does not depict any of the 3.)
What the CDC discovered
The 2 outbreaks of import-linked measles brought home—literally—the sobering facts about vulnerability among US residents. The CDC report 1,2 of its investigation observed:
US travelers can be exposed almost anywhere, developed countries included. The California outbreak started with a visit to Switzerland.
Measles spreads rapidly in susceptible subgroups, unless effective control strategies are used. In California, on 2 consecutive days, 5 school children and 4 children in a doctor’s office were infected; all were unvaccinated.
People not considered at risk can contract measles. Although 2 doses of vaccine are 99% effective, vaccinated individuals, such as the college students, can contract measles. Likewise, people born before 1957 may not be immune, in contrast to the general definition of immunity (see Measles Basics. Case in point: the airline passenger, born in 1954.
Disease can be severe. The 40-year-old salesperson (no documented vaccination) was hospitalized with seizure, 105ºF fever, and pneumonia. One of the infants was hospitalized due to dehydration.
People in routine contact with travelers entering the United States can be exposed to measles—like the airline worker.
CALIFORNIA - A February 22 early-release CDC report1 linked 12 measles cases in California to an unvaccinated 7-year-old boy infected while traveling in Europe with his family in January. He was taken to his pediatrician after onset of rash, and to the emergency department the next day, because of high fever and generalized rash. No isolation precautions were used in the office or hospital.
The boy’s 2 siblings, 5 children at his school, and 4 children at the doctor’s office while he was there contracted measles (3 of whom were infants <12 months of age).
Nearly 10% of the children at the index case’s school were unvaccinated because of personal belief exemptions.
PENNSYLVANIA, MICHIGAN, TEXAS - A young boy from Japan participated in an international sporting event and attended a related sales event in Pennsylvania last August. He was infectious when he left Japan and as he traveled in the United States.
The CDC2 linked a total of 6 additional cases of measles in US-born residents to the index case: another young person from Japan who watched the sporting event; a 53-year-old airline passenger and a 25-year-old airline worker in Michigan; and a corporate sales representative who had met the index patient at the sales event and subsequently made sales visits to Houston-area colleges, where 2 college roommates became infected.
Viral genotyping supported a single chain of transmission, and genetic sequencing linked 6 of the 7 cases.
Take-home lessons for family physicians
Include measles in the differential diagnosis of patients who have fever and rash, especially if they have traveled to another country within the past 3 to 4 weeks. Any patient who meets the definition of measles (fever 101ºF or higher; rash; and at least 1 of the 3 Cs—cough, coryza, conjunctivitis) should be immediately reported to the local health department. The health department will provide instructions for collecting laboratory samples for confirmation; instructions on patient isolation; and assistance with notification and disease control measures for exposed individuals.
Immunize patients and staff. These recurring cases of imported measles underscore the importance of maintaining a high level of immunity. Outbreaks can happen even where immunity is 90% to 95%. When vaccination rates dip below 90%, sustained outbreaks can occur.6
Ensure that staff and patients are all immunized against vaccine-preventable diseases, and inform concerned parents about the safety and effectiveness of vaccines. Parents who refuse to have their children vaccinated place their children at risk and contribute to higher community risk. Communities that have higher rates of non-adherence to vaccine recommendations are more likely to have outbreaks.7,8
Use strict infection control in the office. The recent outbreak in California where 4 children were infected in their physician’s office reinforces the need for strict infection-control practices. Do not allow patients with rash and fever to remain in a common waiting area. Move them to an examination room, preferably an airborne infection isolation room. Keep the door to the examination room closed, and be sure that all health care personnel who come in contact with such patients are immune. Do not use triage rooms for 2 hours after the patient suspected of having measles leaves. Do not send these patients to other health care facilities, such as laboratories, unless infection control measures can be adhered to at those locations. Guidelines on infection control practices in health care settings are available.9,10
Quick response
Quick control of these outbreaks shows the value of the public health infrastructure. Disease surveillance and outbreak response is vital to the public health system, and its value is frequently under-appreciated by physicians and the public.
Fewer than 100 cases of measles occur in the United States each year, and virtually all are linked to imported cases.3 Before vaccine was introduced in 1963, 3 to 4 million cases per year occurred, and caused, on average, 450 deaths, 1000 chronic disabilities, and 28,000 hospitalizations.1 Success in controlling measles is due largely to high levels of coverage with 2 doses of measles-containing vaccine and public health surveillance and disease control.
Measles virus is highly infectious and is spread by airborne droplets and direct contact with nose and throat secretions. The incubation is 7 to 18 days.
Measles begins with fever, cough, coryza, conjunctivitis, and whitish spots on the buccal mucosa (Koplick spots).4 Rash appears on the 3rd to 7th day and lasts 4 to 7 days. It begins on the face but soon becomes generalized. An infected person is contagious from 5 days before the rash until 4 days after the rash appears. The diagnosis of measles can be confirrmed by serum measles IGM, which occurs within 3 days of rash, or a rise in measles IGG between acute and 2-week convalescent serum titers.
Complications: pneumonia (5%), otitis media (10%), and encephalitis 1/1000). Death rates: 1 to 2/1000, varying greatly based on age and nutrition; more severe in the very young and the malnourished. Worldwide, about 500,000 children die from measles each year.5
Immunity is defined as:
- 2 vaccine doses at least 1 month apart, both given after the 1st birthday,
- born before 1957,
- serological evidence, or
- history of physician-diagnosed measles.
Inform concerned parents about the safety and effectiveness of vaccines.
2 doses of measles-containing vaccine are 99% effective.
Those exposed who are not immune should be vaccinated or offered immune globulin if the vaccine is contraindicated.
Contraindications
- Primary immune deficiency diseases of T-cell functions
- Acquired immune deficiency from leukemia, lymphoma, or generalized malignancy
- Therapy with corticosteroids: 2 mg/kg prednisone >2 weeks
- Previous anaphylactic reaction to measles vaccine, gelatin, or neomycins
- Pregnancy
Measles is still a threat. Endemic transmission of measles no longer occurs in the United States (or any of the Americas), yet this highly infectious disease is still a threat from importation by visitors from other countries and from US residents who have traveled abroad. Two recent outbreaks (described at left) illustrate these risks.
3 infants too young to be vaccinated contracted measles in their doctor’s office in San Diego, in January 2008. (An infant with measles rash [above] is for illustration only, and does not depict any of the 3.)
What the CDC discovered
The 2 outbreaks of import-linked measles brought home—literally—the sobering facts about vulnerability among US residents. The CDC report 1,2 of its investigation observed:
US travelers can be exposed almost anywhere, developed countries included. The California outbreak started with a visit to Switzerland.
Measles spreads rapidly in susceptible subgroups, unless effective control strategies are used. In California, on 2 consecutive days, 5 school children and 4 children in a doctor’s office were infected; all were unvaccinated.
People not considered at risk can contract measles. Although 2 doses of vaccine are 99% effective, vaccinated individuals, such as the college students, can contract measles. Likewise, people born before 1957 may not be immune, in contrast to the general definition of immunity (see Measles Basics. Case in point: the airline passenger, born in 1954.
Disease can be severe. The 40-year-old salesperson (no documented vaccination) was hospitalized with seizure, 105ºF fever, and pneumonia. One of the infants was hospitalized due to dehydration.
People in routine contact with travelers entering the United States can be exposed to measles—like the airline worker.
CALIFORNIA - A February 22 early-release CDC report1 linked 12 measles cases in California to an unvaccinated 7-year-old boy infected while traveling in Europe with his family in January. He was taken to his pediatrician after onset of rash, and to the emergency department the next day, because of high fever and generalized rash. No isolation precautions were used in the office or hospital.
The boy’s 2 siblings, 5 children at his school, and 4 children at the doctor’s office while he was there contracted measles (3 of whom were infants <12 months of age).
Nearly 10% of the children at the index case’s school were unvaccinated because of personal belief exemptions.
PENNSYLVANIA, MICHIGAN, TEXAS - A young boy from Japan participated in an international sporting event and attended a related sales event in Pennsylvania last August. He was infectious when he left Japan and as he traveled in the United States.
The CDC2 linked a total of 6 additional cases of measles in US-born residents to the index case: another young person from Japan who watched the sporting event; a 53-year-old airline passenger and a 25-year-old airline worker in Michigan; and a corporate sales representative who had met the index patient at the sales event and subsequently made sales visits to Houston-area colleges, where 2 college roommates became infected.
Viral genotyping supported a single chain of transmission, and genetic sequencing linked 6 of the 7 cases.
Take-home lessons for family physicians
Include measles in the differential diagnosis of patients who have fever and rash, especially if they have traveled to another country within the past 3 to 4 weeks. Any patient who meets the definition of measles (fever 101ºF or higher; rash; and at least 1 of the 3 Cs—cough, coryza, conjunctivitis) should be immediately reported to the local health department. The health department will provide instructions for collecting laboratory samples for confirmation; instructions on patient isolation; and assistance with notification and disease control measures for exposed individuals.
Immunize patients and staff. These recurring cases of imported measles underscore the importance of maintaining a high level of immunity. Outbreaks can happen even where immunity is 90% to 95%. When vaccination rates dip below 90%, sustained outbreaks can occur.6
Ensure that staff and patients are all immunized against vaccine-preventable diseases, and inform concerned parents about the safety and effectiveness of vaccines. Parents who refuse to have their children vaccinated place their children at risk and contribute to higher community risk. Communities that have higher rates of non-adherence to vaccine recommendations are more likely to have outbreaks.7,8
Use strict infection control in the office. The recent outbreak in California where 4 children were infected in their physician’s office reinforces the need for strict infection-control practices. Do not allow patients with rash and fever to remain in a common waiting area. Move them to an examination room, preferably an airborne infection isolation room. Keep the door to the examination room closed, and be sure that all health care personnel who come in contact with such patients are immune. Do not use triage rooms for 2 hours after the patient suspected of having measles leaves. Do not send these patients to other health care facilities, such as laboratories, unless infection control measures can be adhered to at those locations. Guidelines on infection control practices in health care settings are available.9,10
Quick response
Quick control of these outbreaks shows the value of the public health infrastructure. Disease surveillance and outbreak response is vital to the public health system, and its value is frequently under-appreciated by physicians and the public.
Fewer than 100 cases of measles occur in the United States each year, and virtually all are linked to imported cases.3 Before vaccine was introduced in 1963, 3 to 4 million cases per year occurred, and caused, on average, 450 deaths, 1000 chronic disabilities, and 28,000 hospitalizations.1 Success in controlling measles is due largely to high levels of coverage with 2 doses of measles-containing vaccine and public health surveillance and disease control.
Measles virus is highly infectious and is spread by airborne droplets and direct contact with nose and throat secretions. The incubation is 7 to 18 days.
Measles begins with fever, cough, coryza, conjunctivitis, and whitish spots on the buccal mucosa (Koplick spots).4 Rash appears on the 3rd to 7th day and lasts 4 to 7 days. It begins on the face but soon becomes generalized. An infected person is contagious from 5 days before the rash until 4 days after the rash appears. The diagnosis of measles can be confirrmed by serum measles IGM, which occurs within 3 days of rash, or a rise in measles IGG between acute and 2-week convalescent serum titers.
Complications: pneumonia (5%), otitis media (10%), and encephalitis 1/1000). Death rates: 1 to 2/1000, varying greatly based on age and nutrition; more severe in the very young and the malnourished. Worldwide, about 500,000 children die from measles each year.5
Immunity is defined as:
- 2 vaccine doses at least 1 month apart, both given after the 1st birthday,
- born before 1957,
- serological evidence, or
- history of physician-diagnosed measles.
1. CDC. Outbreak of measles—San Diego, California, January-February 2008. MMWR. 2008;57:Early Release February 22, 2008.-
2. CDC. Multistate measles outbreak associated with an international youth sporting event—Pennsylvania, Michigan, and Texas, August-September 2007. MMWR. 2008;57:169-173.
3. CDC. Measles—United States, 2005. MMWR. 2006;55:1348-1351.
4. Measles. In: Heyman DL. Control of Communicable Diseases Manual. 18th ed. Washington, DC: American Public Health Association.
5. CDC. Parents’ guide to childhood immunizations. Available at: http://www.cdc.gov/vaccines/vpd-vac/measles/downloads/pg_why_vacc_measles.pdf. Accessed March 17, 2008.
6. Richard JL, Masserey-Spicher V, Santibanez S, Mankertz A. Measles outbreak in Switzerland. Available at: http://www.eurosurveillance.org/edition/v13n08/080221_1.asp. Accessed March 17. 2008.
7. Salmon DA, Haber M, Gangarosa EJ, et al. Health consequences of religious and philosophical exemptions from immunization laws; individual and societal risk of measles. JAMA. 1999;282:47-53
8. Feikin DR, Lezotte DC, Hamman RF, et al. Individual and community risks of measles and pertussis associated with personal exemptions to immunization. JAMA. 2008;284:3145-3150.
9. Siegel JD, Rhinehart E, Jackson M, Chiarello L. Health care infection control practices advisory committee, 2007.Guideline for isolation precautions: preventing transmission of infectious agents in health care settings. Am J Infect Control. 2007;35(suppl 2):S65-164.
10. Campos-Outcalt D. Infection control in outpatient settings. J Fam Pract. 2004;53:485-488.
1. CDC. Outbreak of measles—San Diego, California, January-February 2008. MMWR. 2008;57:Early Release February 22, 2008.-
2. CDC. Multistate measles outbreak associated with an international youth sporting event—Pennsylvania, Michigan, and Texas, August-September 2007. MMWR. 2008;57:169-173.
3. CDC. Measles—United States, 2005. MMWR. 2006;55:1348-1351.
4. Measles. In: Heyman DL. Control of Communicable Diseases Manual. 18th ed. Washington, DC: American Public Health Association.
5. CDC. Parents’ guide to childhood immunizations. Available at: http://www.cdc.gov/vaccines/vpd-vac/measles/downloads/pg_why_vacc_measles.pdf. Accessed March 17, 2008.
6. Richard JL, Masserey-Spicher V, Santibanez S, Mankertz A. Measles outbreak in Switzerland. Available at: http://www.eurosurveillance.org/edition/v13n08/080221_1.asp. Accessed March 17. 2008.
7. Salmon DA, Haber M, Gangarosa EJ, et al. Health consequences of religious and philosophical exemptions from immunization laws; individual and societal risk of measles. JAMA. 1999;282:47-53
8. Feikin DR, Lezotte DC, Hamman RF, et al. Individual and community risks of measles and pertussis associated with personal exemptions to immunization. JAMA. 2008;284:3145-3150.
9. Siegel JD, Rhinehart E, Jackson M, Chiarello L. Health care infection control practices advisory committee, 2007.Guideline for isolation precautions: preventing transmission of infectious agents in health care settings. Am J Infect Control. 2007;35(suppl 2):S65-164.
10. Campos-Outcalt D. Infection control in outpatient settings. J Fam Pract. 2004;53:485-488.
Vaccine update: New CDC recommendations from 2007
The year 2007 was rather calm, compared to the 3 previous years in regards to new vaccines and vaccine recommendations. Although no breakthrough vaccine products came onto the market in 2007, there were new recommendations and licensure for new age groups for existing vaccines and a recall of some lots of Hib vaccines.
Meningococcal vaccine
Recommendations on the use of the quadrivalent meningococcal conjugate vaccine (MCV4) have evolved since its licensure in 2005 for use in persons 11 to 55 years of age. The first set of recommendations focused on universal vaccination of preteens, aged 11 to 12, those entering high school who had not received the vaccine previously, and others at risk for meningococcal disease including college freshmen living in dormitories.1 The MCV4 was preferred to the older polysaccharide vaccine (MPSV4) which was recommended only for children aged 2 to 10 and adults over age 55 at increased risk.
In 2007, the CDC changed 2 of the 2005 recommendations:
- The first, in August, simplified the recommendations for teens, making MCV4 universally recommended for all those aged 11 to 18 at the earliest opportunity.2
- The second, in December, followed FDA approval for use of MCV4 in children aged 2 to 10 years. The CDC now recommends MCV4 as the preferred vaccine in this age group for those at risk (TABLE 1).3
TABLE 1
Populations at increased risk for meningococcal disease who should receive quadrivalent meningococcal conjugate vaccine
|
If someone at ongoing risk for meningococcal disease has been previously vaccinated with MPSV4, they should be revaccinated 3 years later with MCV4. It is not known if repeat doses of MCV4 will be needed, and if so, after what amount of time.
The MCV4 has been linked to Guillain-Barré syndrome (GBS), and a history of GBS is a precaution for its use. For those with a history of GBS who need protection against meningococcal infection, MPSV4 is an alternative.
Hepatitis A vaccine
Widespread use of inactivated hepatitis A vaccine (HAV), first licensed in 1995, has markedly reduced the incidence of hepatitis A infection (FIGURE). Recommendations for its use have been periodically revised; current recommendations include universal vaccination of all children at age 12 to 23 months, catch-up vaccination in older children in areas of high prevalence, and vaccination of those at increased risk for hepatitis A including travelers to endemic areas, users of illicit drugs and men who have sex with men.4
FIGURE
Reduction in incidence of hepatitis A infection
Source: Prevention of hepatitis A through active or passive immunization: recommendations of the Advisory Committee on Immunization Practices. MMWR Recomm Rep 2006; 55(RR-07).
For those unvaccinated who are acutely exposed to hepatitis A virus and those traveling to areas of high prevalence who do not have time to complete the 2 doses of HAV, the only prevention available until recently has been IG. This has now changed and HAV can be used in both groups. The new recommendation for postexposure prophylaxis is that either a single dose of HAV or use of IG is acceptable.5 At ages 12 months to 40 years, vaccine is preferred. For those over age 40, IG is preferred but vaccine is acceptable. For children less than 12 months, the immune suppressed, and those with chronic liver disease, IG should be used.
Those traveling or working in countries with high rates of hepatitis A can be protected with either HAV or IG. A single dose of HAV is sufficient for healthy people, with a second dose at the recommended interval to complete the series. Those under age 12 months, those who choose not to receive the vaccine, and those who are allergic to the vaccine should be offered IG. Both IG and HAV should be considered for individuals who plan to travel within 2 weeks of the first HAV dose; those over age 40, the immune compromised, and those with chronic liver disease or other chronic medical conditions.
Live attenuated influenza vaccine
FluMist, the live attenuated influenza vaccine (LAIV), which is administered as an intranasal spray, is now approved for use among those 2 to 4 years of age.6 Previously, the LAIV was approved only for healthy, non pregnant persons, 5 to 49 years of age. The LAIV may actually be the preferred product in children as it has been shown to prevent more influenza illness than the trivalent inactivated vaccine (TIV). The LAIV should not be used in anyone with a condition listed in TABLE 2 and should not be administered to children under age 5 who have recurrent wheezing.
FluMist has also been modified in several advantageous ways:
- The dose in the sprayer is now 0.2 mL (previously 0.5 mL). One half of the dose should be administered in each nostril.
- The product no longer has to be stored frozen; it should be kept at 35° to 46°F.
- When 2 doses are needed in children under age 9 being vaccinated for the first time, the interval between doses is now 4 weeks (previously 6 weeks).
TABLE 2
LAIV (FluMist) should not be used in these groups
|
Children under age 9 years who receive only 1 dose of vaccine (either TIV or LAIV) the first year they are vaccinated should receive 2 doses the next year.6 If they fail to receive 2 doses in the next year, only a single dose is recommended after that. This is a slight modification of the previous recommendation that only 1 dose was recommended in this situation.7
Alternative schedule for combined hepatitis A and B vaccine
The FDA approved an alternate, 4-dose schedule for the combined hepatitis A and hepatitis B vaccine (Twinrix): at 0, 7, 21 days, and 12 months.8 It was previously approved only for a 3-dose schedule: at 0, 1, and 6 months. The new alternative schedule allows greater protection for travelers who need to depart in less than a month’s time.
Merck recalls some lots of Hib vaccine
On December 11, 2007, Merck announced a voluntary recall of specific lots of Haemophilus influenza type b (Hib) conjugate vaccine products: 10 lots of a monovalent Hib vaccine, PedvaxHIB, and 2 lots of a combined hepatitis B/Hib vaccine, Comvax.
Consult Merck’s Web site for the lots involved and for instructions on returning vaccine (www.merckvaccines. com/PCHRecall.pdf). The recall was prompted by concern about equipment sterility, although no vaccine has been shown to be contaminated. Children vaccinated with Merck products do not need to be revaccinated or obtain any special follow-up.
Shortage expected. It is unknown when Merck will resume production, but it is not anticipated until at least late in 2008. Other Hib-containing products are produced by Sanofi Pasteur but the supply of these products will not make up for the expected shortage.
Interim recommendations. The recall resulted in interim recommendations from the CDC.9 These recommendations are complicated because the dosing schedule for Hib vaccine differs by the product and the age of receipt of first vaccine when children are not on schedule. TABLE 3 lists the Hib-containing products, the recommended primary series schedule, and booster dose.
TABLE 3
Hib products
| PRIMARY SERIES | BOOSTER | |||
|---|---|---|---|---|
| Merck Products | ||||
| PedvaxHIB | Monovalent Hib vaccine | 2, 4 months | 12–15 months* | |
| Comvax | Combined Hib/hepatitis B vaccine | 2, 4 months | 12–15 months* | |
| Sanofi Pasteur products | ||||
| ActHIB | Monovalent hib vaccine | 2, 4, 6 months | 12–15 months* | |
| TriHIBit | DTaP/Hib vaccine | Not licensed for this age group | 15–18 months* | |
| * Can follow a primary series of any product or serve as the only dose for a child up to 59 months, not previously immunized. | ||||
The main points are:
- Defer the booster dose at age 12 to 15 months until the shortage is resolved, except for high-risk children.
- High-risk children, who should continue to receive the booster at ages 12 to 15 months, include those with asplenia, sickle cell disease, HIV infection, and certain other immune deficiencies and cancers, and American Indian/Alaskan Native children.
- Physicians should keep track of children who have the booster deferred so they can be vaccinated when the supply improves.
- Non-recalled lots of PedvaxHIB and Comvax in the CDC stockpile will be prioritized to providers who care for predominantly American Indian/Alaskan Native children, who are at markedly in creased risk of Hib infection.
- If a child has received only 1 dose of PedvaxHIB or Comvax, their primary series can be completed with ActHIB, but 3 total doses are needed.
Children through age 59 months who are behind schedule should complete a primary series according to published recommendations.10 Physicians should call their local health department if they have any questions about what to do in a specific case.
1. CDC. Prevention and control of meningococcal disease: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2005;54(RR-7):1-21.
2. CDC. Revised recommendations of the Advisory Committee on Immunization Practices to vaccinate all persons aged 11-18 years with meningococcal conjugate vaccine. MMWR Morb Mortal Wkly Rep 2007;56:794-795.
3. CDC. Recommendation from the Advisory Committee on Immunization Practices (ACIP) for use of quadrivalent meningococcal conjugate vaccine (MCV4) in children aged 2-10 years at increased risk for invasive meningococcal disease. MMWR Morb Mortal Wkly Rep 2007;56:1265-1266.
4. CDC. Update: prevention of hepatitis A after exposure to hepatitis A virus and in international travelers. Updated recommendations of the ACIP. MMWR Morb Mortal Wkly Rep 2007;56:1080-1084.
5. Advisory Committee on Immunization Practices (ACIP), Fiore AE, Wasley A, Bell BP. Prevention of hepatitis A through active or passive immunization: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2006;55(RR-07):1-23.
6. CDC. Expansion of use of live attenuated influenza vaccine to children aged 2-4 years and other Flu-Mist changes for the 2007-2008 influenza season. MMWR Morb Mortal Wkly Rep 2007;56:1217-1219.
7. Fiore AE, Shay DK, Haber P, et al. Advisory Committee on Immunization Practices (ACIP), Centers for Disease Control and Prevention (CDC). Prevention and control of influenza: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2007. MMWR Recomm Rep 2007;56(RR-6):1-54.
8. CDC. FDA approval of an alternate dosing schedule for a combined hepatitis A and B vaccine (Twinrix). MMWR Morb Mortal Wkly Rep 2007;56:1057.-
9. CDC. Interim recommendations for the use of Haemophilus influenza Type b (Hib) conjugate vaccines related to the recall of certain lots of Hib-containing vaccines (PedvaxHIB and Comvax). MMWR Morb Mortal Wkly Rep 2007;56:1318-1320.
10. CDC. Catch-up immunization schedule for persons aged 4 months-18 years who start late or are more than one month behind. Available at www.cdc.gov/vaccines/recs/schedules/downloads/child/2007/child-schedule-color-print.pdf. Accessed February 11, 2008.
The year 2007 was rather calm, compared to the 3 previous years in regards to new vaccines and vaccine recommendations. Although no breakthrough vaccine products came onto the market in 2007, there were new recommendations and licensure for new age groups for existing vaccines and a recall of some lots of Hib vaccines.
Meningococcal vaccine
Recommendations on the use of the quadrivalent meningococcal conjugate vaccine (MCV4) have evolved since its licensure in 2005 for use in persons 11 to 55 years of age. The first set of recommendations focused on universal vaccination of preteens, aged 11 to 12, those entering high school who had not received the vaccine previously, and others at risk for meningococcal disease including college freshmen living in dormitories.1 The MCV4 was preferred to the older polysaccharide vaccine (MPSV4) which was recommended only for children aged 2 to 10 and adults over age 55 at increased risk.
In 2007, the CDC changed 2 of the 2005 recommendations:
- The first, in August, simplified the recommendations for teens, making MCV4 universally recommended for all those aged 11 to 18 at the earliest opportunity.2
- The second, in December, followed FDA approval for use of MCV4 in children aged 2 to 10 years. The CDC now recommends MCV4 as the preferred vaccine in this age group for those at risk (TABLE 1).3
TABLE 1
Populations at increased risk for meningococcal disease who should receive quadrivalent meningococcal conjugate vaccine
|
If someone at ongoing risk for meningococcal disease has been previously vaccinated with MPSV4, they should be revaccinated 3 years later with MCV4. It is not known if repeat doses of MCV4 will be needed, and if so, after what amount of time.
The MCV4 has been linked to Guillain-Barré syndrome (GBS), and a history of GBS is a precaution for its use. For those with a history of GBS who need protection against meningococcal infection, MPSV4 is an alternative.
Hepatitis A vaccine
Widespread use of inactivated hepatitis A vaccine (HAV), first licensed in 1995, has markedly reduced the incidence of hepatitis A infection (FIGURE). Recommendations for its use have been periodically revised; current recommendations include universal vaccination of all children at age 12 to 23 months, catch-up vaccination in older children in areas of high prevalence, and vaccination of those at increased risk for hepatitis A including travelers to endemic areas, users of illicit drugs and men who have sex with men.4
FIGURE
Reduction in incidence of hepatitis A infection
Source: Prevention of hepatitis A through active or passive immunization: recommendations of the Advisory Committee on Immunization Practices. MMWR Recomm Rep 2006; 55(RR-07).
For those unvaccinated who are acutely exposed to hepatitis A virus and those traveling to areas of high prevalence who do not have time to complete the 2 doses of HAV, the only prevention available until recently has been IG. This has now changed and HAV can be used in both groups. The new recommendation for postexposure prophylaxis is that either a single dose of HAV or use of IG is acceptable.5 At ages 12 months to 40 years, vaccine is preferred. For those over age 40, IG is preferred but vaccine is acceptable. For children less than 12 months, the immune suppressed, and those with chronic liver disease, IG should be used.
Those traveling or working in countries with high rates of hepatitis A can be protected with either HAV or IG. A single dose of HAV is sufficient for healthy people, with a second dose at the recommended interval to complete the series. Those under age 12 months, those who choose not to receive the vaccine, and those who are allergic to the vaccine should be offered IG. Both IG and HAV should be considered for individuals who plan to travel within 2 weeks of the first HAV dose; those over age 40, the immune compromised, and those with chronic liver disease or other chronic medical conditions.
Live attenuated influenza vaccine
FluMist, the live attenuated influenza vaccine (LAIV), which is administered as an intranasal spray, is now approved for use among those 2 to 4 years of age.6 Previously, the LAIV was approved only for healthy, non pregnant persons, 5 to 49 years of age. The LAIV may actually be the preferred product in children as it has been shown to prevent more influenza illness than the trivalent inactivated vaccine (TIV). The LAIV should not be used in anyone with a condition listed in TABLE 2 and should not be administered to children under age 5 who have recurrent wheezing.
FluMist has also been modified in several advantageous ways:
- The dose in the sprayer is now 0.2 mL (previously 0.5 mL). One half of the dose should be administered in each nostril.
- The product no longer has to be stored frozen; it should be kept at 35° to 46°F.
- When 2 doses are needed in children under age 9 being vaccinated for the first time, the interval between doses is now 4 weeks (previously 6 weeks).
TABLE 2
LAIV (FluMist) should not be used in these groups
|
Children under age 9 years who receive only 1 dose of vaccine (either TIV or LAIV) the first year they are vaccinated should receive 2 doses the next year.6 If they fail to receive 2 doses in the next year, only a single dose is recommended after that. This is a slight modification of the previous recommendation that only 1 dose was recommended in this situation.7
Alternative schedule for combined hepatitis A and B vaccine
The FDA approved an alternate, 4-dose schedule for the combined hepatitis A and hepatitis B vaccine (Twinrix): at 0, 7, 21 days, and 12 months.8 It was previously approved only for a 3-dose schedule: at 0, 1, and 6 months. The new alternative schedule allows greater protection for travelers who need to depart in less than a month’s time.
Merck recalls some lots of Hib vaccine
On December 11, 2007, Merck announced a voluntary recall of specific lots of Haemophilus influenza type b (Hib) conjugate vaccine products: 10 lots of a monovalent Hib vaccine, PedvaxHIB, and 2 lots of a combined hepatitis B/Hib vaccine, Comvax.
Consult Merck’s Web site for the lots involved and for instructions on returning vaccine (www.merckvaccines. com/PCHRecall.pdf). The recall was prompted by concern about equipment sterility, although no vaccine has been shown to be contaminated. Children vaccinated with Merck products do not need to be revaccinated or obtain any special follow-up.
Shortage expected. It is unknown when Merck will resume production, but it is not anticipated until at least late in 2008. Other Hib-containing products are produced by Sanofi Pasteur but the supply of these products will not make up for the expected shortage.
Interim recommendations. The recall resulted in interim recommendations from the CDC.9 These recommendations are complicated because the dosing schedule for Hib vaccine differs by the product and the age of receipt of first vaccine when children are not on schedule. TABLE 3 lists the Hib-containing products, the recommended primary series schedule, and booster dose.
TABLE 3
Hib products
| PRIMARY SERIES | BOOSTER | |||
|---|---|---|---|---|
| Merck Products | ||||
| PedvaxHIB | Monovalent Hib vaccine | 2, 4 months | 12–15 months* | |
| Comvax | Combined Hib/hepatitis B vaccine | 2, 4 months | 12–15 months* | |
| Sanofi Pasteur products | ||||
| ActHIB | Monovalent hib vaccine | 2, 4, 6 months | 12–15 months* | |
| TriHIBit | DTaP/Hib vaccine | Not licensed for this age group | 15–18 months* | |
| * Can follow a primary series of any product or serve as the only dose for a child up to 59 months, not previously immunized. | ||||
The main points are:
- Defer the booster dose at age 12 to 15 months until the shortage is resolved, except for high-risk children.
- High-risk children, who should continue to receive the booster at ages 12 to 15 months, include those with asplenia, sickle cell disease, HIV infection, and certain other immune deficiencies and cancers, and American Indian/Alaskan Native children.
- Physicians should keep track of children who have the booster deferred so they can be vaccinated when the supply improves.
- Non-recalled lots of PedvaxHIB and Comvax in the CDC stockpile will be prioritized to providers who care for predominantly American Indian/Alaskan Native children, who are at markedly in creased risk of Hib infection.
- If a child has received only 1 dose of PedvaxHIB or Comvax, their primary series can be completed with ActHIB, but 3 total doses are needed.
Children through age 59 months who are behind schedule should complete a primary series according to published recommendations.10 Physicians should call their local health department if they have any questions about what to do in a specific case.
The year 2007 was rather calm, compared to the 3 previous years in regards to new vaccines and vaccine recommendations. Although no breakthrough vaccine products came onto the market in 2007, there were new recommendations and licensure for new age groups for existing vaccines and a recall of some lots of Hib vaccines.
Meningococcal vaccine
Recommendations on the use of the quadrivalent meningococcal conjugate vaccine (MCV4) have evolved since its licensure in 2005 for use in persons 11 to 55 years of age. The first set of recommendations focused on universal vaccination of preteens, aged 11 to 12, those entering high school who had not received the vaccine previously, and others at risk for meningococcal disease including college freshmen living in dormitories.1 The MCV4 was preferred to the older polysaccharide vaccine (MPSV4) which was recommended only for children aged 2 to 10 and adults over age 55 at increased risk.
In 2007, the CDC changed 2 of the 2005 recommendations:
- The first, in August, simplified the recommendations for teens, making MCV4 universally recommended for all those aged 11 to 18 at the earliest opportunity.2
- The second, in December, followed FDA approval for use of MCV4 in children aged 2 to 10 years. The CDC now recommends MCV4 as the preferred vaccine in this age group for those at risk (TABLE 1).3
TABLE 1
Populations at increased risk for meningococcal disease who should receive quadrivalent meningococcal conjugate vaccine
|
If someone at ongoing risk for meningococcal disease has been previously vaccinated with MPSV4, they should be revaccinated 3 years later with MCV4. It is not known if repeat doses of MCV4 will be needed, and if so, after what amount of time.
The MCV4 has been linked to Guillain-Barré syndrome (GBS), and a history of GBS is a precaution for its use. For those with a history of GBS who need protection against meningococcal infection, MPSV4 is an alternative.
Hepatitis A vaccine
Widespread use of inactivated hepatitis A vaccine (HAV), first licensed in 1995, has markedly reduced the incidence of hepatitis A infection (FIGURE). Recommendations for its use have been periodically revised; current recommendations include universal vaccination of all children at age 12 to 23 months, catch-up vaccination in older children in areas of high prevalence, and vaccination of those at increased risk for hepatitis A including travelers to endemic areas, users of illicit drugs and men who have sex with men.4
FIGURE
Reduction in incidence of hepatitis A infection
Source: Prevention of hepatitis A through active or passive immunization: recommendations of the Advisory Committee on Immunization Practices. MMWR Recomm Rep 2006; 55(RR-07).
For those unvaccinated who are acutely exposed to hepatitis A virus and those traveling to areas of high prevalence who do not have time to complete the 2 doses of HAV, the only prevention available until recently has been IG. This has now changed and HAV can be used in both groups. The new recommendation for postexposure prophylaxis is that either a single dose of HAV or use of IG is acceptable.5 At ages 12 months to 40 years, vaccine is preferred. For those over age 40, IG is preferred but vaccine is acceptable. For children less than 12 months, the immune suppressed, and those with chronic liver disease, IG should be used.
Those traveling or working in countries with high rates of hepatitis A can be protected with either HAV or IG. A single dose of HAV is sufficient for healthy people, with a second dose at the recommended interval to complete the series. Those under age 12 months, those who choose not to receive the vaccine, and those who are allergic to the vaccine should be offered IG. Both IG and HAV should be considered for individuals who plan to travel within 2 weeks of the first HAV dose; those over age 40, the immune compromised, and those with chronic liver disease or other chronic medical conditions.
Live attenuated influenza vaccine
FluMist, the live attenuated influenza vaccine (LAIV), which is administered as an intranasal spray, is now approved for use among those 2 to 4 years of age.6 Previously, the LAIV was approved only for healthy, non pregnant persons, 5 to 49 years of age. The LAIV may actually be the preferred product in children as it has been shown to prevent more influenza illness than the trivalent inactivated vaccine (TIV). The LAIV should not be used in anyone with a condition listed in TABLE 2 and should not be administered to children under age 5 who have recurrent wheezing.
FluMist has also been modified in several advantageous ways:
- The dose in the sprayer is now 0.2 mL (previously 0.5 mL). One half of the dose should be administered in each nostril.
- The product no longer has to be stored frozen; it should be kept at 35° to 46°F.
- When 2 doses are needed in children under age 9 being vaccinated for the first time, the interval between doses is now 4 weeks (previously 6 weeks).
TABLE 2
LAIV (FluMist) should not be used in these groups
|
Children under age 9 years who receive only 1 dose of vaccine (either TIV or LAIV) the first year they are vaccinated should receive 2 doses the next year.6 If they fail to receive 2 doses in the next year, only a single dose is recommended after that. This is a slight modification of the previous recommendation that only 1 dose was recommended in this situation.7
Alternative schedule for combined hepatitis A and B vaccine
The FDA approved an alternate, 4-dose schedule for the combined hepatitis A and hepatitis B vaccine (Twinrix): at 0, 7, 21 days, and 12 months.8 It was previously approved only for a 3-dose schedule: at 0, 1, and 6 months. The new alternative schedule allows greater protection for travelers who need to depart in less than a month’s time.
Merck recalls some lots of Hib vaccine
On December 11, 2007, Merck announced a voluntary recall of specific lots of Haemophilus influenza type b (Hib) conjugate vaccine products: 10 lots of a monovalent Hib vaccine, PedvaxHIB, and 2 lots of a combined hepatitis B/Hib vaccine, Comvax.
Consult Merck’s Web site for the lots involved and for instructions on returning vaccine (www.merckvaccines. com/PCHRecall.pdf). The recall was prompted by concern about equipment sterility, although no vaccine has been shown to be contaminated. Children vaccinated with Merck products do not need to be revaccinated or obtain any special follow-up.
Shortage expected. It is unknown when Merck will resume production, but it is not anticipated until at least late in 2008. Other Hib-containing products are produced by Sanofi Pasteur but the supply of these products will not make up for the expected shortage.
Interim recommendations. The recall resulted in interim recommendations from the CDC.9 These recommendations are complicated because the dosing schedule for Hib vaccine differs by the product and the age of receipt of first vaccine when children are not on schedule. TABLE 3 lists the Hib-containing products, the recommended primary series schedule, and booster dose.
TABLE 3
Hib products
| PRIMARY SERIES | BOOSTER | |||
|---|---|---|---|---|
| Merck Products | ||||
| PedvaxHIB | Monovalent Hib vaccine | 2, 4 months | 12–15 months* | |
| Comvax | Combined Hib/hepatitis B vaccine | 2, 4 months | 12–15 months* | |
| Sanofi Pasteur products | ||||
| ActHIB | Monovalent hib vaccine | 2, 4, 6 months | 12–15 months* | |
| TriHIBit | DTaP/Hib vaccine | Not licensed for this age group | 15–18 months* | |
| * Can follow a primary series of any product or serve as the only dose for a child up to 59 months, not previously immunized. | ||||
The main points are:
- Defer the booster dose at age 12 to 15 months until the shortage is resolved, except for high-risk children.
- High-risk children, who should continue to receive the booster at ages 12 to 15 months, include those with asplenia, sickle cell disease, HIV infection, and certain other immune deficiencies and cancers, and American Indian/Alaskan Native children.
- Physicians should keep track of children who have the booster deferred so they can be vaccinated when the supply improves.
- Non-recalled lots of PedvaxHIB and Comvax in the CDC stockpile will be prioritized to providers who care for predominantly American Indian/Alaskan Native children, who are at markedly in creased risk of Hib infection.
- If a child has received only 1 dose of PedvaxHIB or Comvax, their primary series can be completed with ActHIB, but 3 total doses are needed.
Children through age 59 months who are behind schedule should complete a primary series according to published recommendations.10 Physicians should call their local health department if they have any questions about what to do in a specific case.
1. CDC. Prevention and control of meningococcal disease: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2005;54(RR-7):1-21.
2. CDC. Revised recommendations of the Advisory Committee on Immunization Practices to vaccinate all persons aged 11-18 years with meningococcal conjugate vaccine. MMWR Morb Mortal Wkly Rep 2007;56:794-795.
3. CDC. Recommendation from the Advisory Committee on Immunization Practices (ACIP) for use of quadrivalent meningococcal conjugate vaccine (MCV4) in children aged 2-10 years at increased risk for invasive meningococcal disease. MMWR Morb Mortal Wkly Rep 2007;56:1265-1266.
4. CDC. Update: prevention of hepatitis A after exposure to hepatitis A virus and in international travelers. Updated recommendations of the ACIP. MMWR Morb Mortal Wkly Rep 2007;56:1080-1084.
5. Advisory Committee on Immunization Practices (ACIP), Fiore AE, Wasley A, Bell BP. Prevention of hepatitis A through active or passive immunization: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2006;55(RR-07):1-23.
6. CDC. Expansion of use of live attenuated influenza vaccine to children aged 2-4 years and other Flu-Mist changes for the 2007-2008 influenza season. MMWR Morb Mortal Wkly Rep 2007;56:1217-1219.
7. Fiore AE, Shay DK, Haber P, et al. Advisory Committee on Immunization Practices (ACIP), Centers for Disease Control and Prevention (CDC). Prevention and control of influenza: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2007. MMWR Recomm Rep 2007;56(RR-6):1-54.
8. CDC. FDA approval of an alternate dosing schedule for a combined hepatitis A and B vaccine (Twinrix). MMWR Morb Mortal Wkly Rep 2007;56:1057.-
9. CDC. Interim recommendations for the use of Haemophilus influenza Type b (Hib) conjugate vaccines related to the recall of certain lots of Hib-containing vaccines (PedvaxHIB and Comvax). MMWR Morb Mortal Wkly Rep 2007;56:1318-1320.
10. CDC. Catch-up immunization schedule for persons aged 4 months-18 years who start late or are more than one month behind. Available at www.cdc.gov/vaccines/recs/schedules/downloads/child/2007/child-schedule-color-print.pdf. Accessed February 11, 2008.
1. CDC. Prevention and control of meningococcal disease: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2005;54(RR-7):1-21.
2. CDC. Revised recommendations of the Advisory Committee on Immunization Practices to vaccinate all persons aged 11-18 years with meningococcal conjugate vaccine. MMWR Morb Mortal Wkly Rep 2007;56:794-795.
3. CDC. Recommendation from the Advisory Committee on Immunization Practices (ACIP) for use of quadrivalent meningococcal conjugate vaccine (MCV4) in children aged 2-10 years at increased risk for invasive meningococcal disease. MMWR Morb Mortal Wkly Rep 2007;56:1265-1266.
4. CDC. Update: prevention of hepatitis A after exposure to hepatitis A virus and in international travelers. Updated recommendations of the ACIP. MMWR Morb Mortal Wkly Rep 2007;56:1080-1084.
5. Advisory Committee on Immunization Practices (ACIP), Fiore AE, Wasley A, Bell BP. Prevention of hepatitis A through active or passive immunization: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2006;55(RR-07):1-23.
6. CDC. Expansion of use of live attenuated influenza vaccine to children aged 2-4 years and other Flu-Mist changes for the 2007-2008 influenza season. MMWR Morb Mortal Wkly Rep 2007;56:1217-1219.
7. Fiore AE, Shay DK, Haber P, et al. Advisory Committee on Immunization Practices (ACIP), Centers for Disease Control and Prevention (CDC). Prevention and control of influenza: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2007. MMWR Recomm Rep 2007;56(RR-6):1-54.
8. CDC. FDA approval of an alternate dosing schedule for a combined hepatitis A and B vaccine (Twinrix). MMWR Morb Mortal Wkly Rep 2007;56:1057.-
9. CDC. Interim recommendations for the use of Haemophilus influenza Type b (Hib) conjugate vaccines related to the recall of certain lots of Hib-containing vaccines (PedvaxHIB and Comvax). MMWR Morb Mortal Wkly Rep 2007;56:1318-1320.
10. CDC. Catch-up immunization schedule for persons aged 4 months-18 years who start late or are more than one month behind. Available at www.cdc.gov/vaccines/recs/schedules/downloads/child/2007/child-schedule-color-print.pdf. Accessed February 11, 2008.
Varicella vaccination: 2 doses now the standard
The varicella vaccine has had tremendous success over the last few years, but its success has stalled.
The widespread use of the varicella vaccine has led to a coverage rate of 88%, and the vaccine has proven to be 85% effective. As a result, between 1995 and 2001 there was an 87% decline in hospitalizations, 66% decline in deaths, and an 87% decline in costs attributed to varicella.
However, the number of varicella cases has remained at a constant level over the past few years and sporadic outbreaks continue to occur in schools—even where high rates of immunization are achieved.1,2
Varicella outbreaks involve both infections in unvaccinated children and “breakthrough disease” in those who have been vaccinated. If a vaccinated person is exposed to varicella, the risk of suffering a breakthrough infection is about 15%.2 A 2-dose series of varicella vaccine reduces the risk by about 75%1 (Figure).
Breakthrough disease is usually milder than infection in the unvaccinated, with fewer skin lesions, milder symptoms, and fewer complications. Those affected, though, are still infectious to others.
It was this ongoing risk of varicella that prompted the Advisory Committee on Immunization Practices (ACIP) to recommend new control measures, reported on in 2007.1
- All children should now receive 2 doses of varicella vaccine. The timing of the first and second dose should correspond with the administration of the MMR vaccine.
- Children older than 6 years of age and adults who previously received only 1 dose of vaccine should receive 1 more dose.
- Health care workers should ensure that they are immune to varicella by blood titers or receiving 2 doses of the vaccine.
- Pregnant women should be screened for immunity to varicella. They should be vaccinated postpartum if they are not immune.
FIGURE
2 doses of varicella vaccine reduce risk of breakthrough infection by about 75%1
Cumulative breakthrough rates for 1 and 2 doses of single-antigen varicella vaccine among children (ages 12 months to 12 years) by number of years after vaccination. Breakthrough rates are per 100 person-years at risk.
ACIP now recommends 2 doses of the vaccine
ACIP recommends the following:
- Universal administration of 2 doses of varicella vaccine; the first at ages 12 to 15 months and the second at age 4 to 6 years. (This is the same schedule as immunization against mumps, measles, and rubella.)
- Two doses of varicella vaccine, 4 to 8 weeks apart, for all adolescents and adults without evidence of immunity. (See “New criteria to prove immunity” at right.)
- A catch-up second dose for everyone who received one dose previously.
- Screening for varicella immunity in pregnant women and postpartum vaccination for those who are not immune, with 2 doses 4 to 8 weeks apart. The first dose should be administered before discharge.
Which HIV patients can get the vaccine?
ACIP has also clarified when HIV patients can be vaccinated, noting that single antigen varicella vaccine can be administered to HIV positive children if their CD4+ Tlymphocyte % is ≥15%. HIV positive adolescents and adults can be vaccinated if their CD4+ T-lymphocyte count ≥200/μL and, if 2 doses are indicated, they should be separated by at least 3 months.
ACIP has approved new criteria for establishing proof of immunity to varicella. ACIP now includes laboratory confirmation of disease or birth in the US prior to 1980 as evidence of immunity. Another change to ACIP’s criteria: A reported varicella history alone does not suffice; it needs to be verified by a provider.
ACIP’s new criteria include:
- Documentation of age appropriate vaccination (1 dose for preschool children ≥12 months of age, and 2 doses, 1 month apart, for school-age children, adolescents, and adults)
- Laboratory evidence of immunity or laboratory confirmation of disease
- A history of varicella disease or varicella zoster verified by a health care provider
- 4. Birth in the US prior to 1980. This criterion does not apply to health care providers, pregnant women, or the immune-suppressed.
2 options: Varivax and ProQuad
Two varicella vaccines contain modified live varicella virus antigen. Varivax, a single antigen vaccine, is approved for use in adults, adolescents, and children ≥12 months of age. The second vaccine, ProQuad, is approved for use in patients who are between 12 months and 12 years of age, and contains 4 viral antigens: mumps, measles, rubella, and varicella.
The quadrivalent MMRV vaccine is currently unavailable, however, and isn’t expected to be available until early 2009.3 Once the supply is stabilized, though, it will facilitate vaccination of children by decreasing the number of injections needed to achieve full immunization status.
29-year-old patient with varicellaThese 2 varicella vaccines should not be confused with the varicella zoster vaccine, Zostavax, which is approved for use in adults who are 60 years of age and older for the prevention of shingles and postherpetic neuralgia.4
- Can the varicella vaccine be co-administered with other childhood vaccines?
Yes. - What if a nonimmune pregnant women is exposed to chicken-pox?
You’ll need to consult your local health department about the possibility of administering varicella immune globulin. - Can the vaccine be administered to mothers who are breastfeeding their babies?
yes. - Can the vaccine be administered to those who live in a household with an immune-suppressed person?
yes, the risk of transmission of vaccine virus is very low. - What if a woman is inadvertently vaccinated while pregnant?
The risk during pregnancy is theoretical and to date, no cases of congenital varicella have resulted from inadvertent vaccination during pregnancy. - Will the vaccine prevent shingles later in life?
No one knows for sure. Surveillance is currently in progress, but long-term results are not available.
Pregnancy precludes vaccination
Varicella vaccine is contraindicated during pregnancy and in those who have had a severe allergic reaction to any vaccine component, including gelatin; have a malignancy of the blood, bone marrow, or lymphatic system; have a congenital or hereditary immunodeficiency; or are receiving systemic immunosuppressive therapy including those on the equivalent of 2 mg/kg, or >20 mg/day, of prednisone.
You should delay giving the vaccine to patients with an acute, severe illness. There is a potential for immune globulin containing products to interfere with the effectiveness of live virus vaccines. As a result, if a patient has received blood, plasma, or immune globulin, you should wait 3 to 11 months before giving the varicella vaccine. These products should also be avoided, if possible, for 2 weeks after the vaccine has been administered.
Avoid using quadrivalent MMRV in patients with HIV infection because it contains a higher quantity of varicella antigen than the single antigen product.
One final precaution: Patients should avoid taking salicylates for 6 weeks following vaccination because of the theoretical risk of Reye’s syndrome.
1. CDC. Prevention of varicella: Recommendations of the Advisory Committee on Immunization Practices. MMWR Recomm Rep 2007; 56(rr-4):1–40. Available at: www.cdc.gov/mmwr/PDF/rr/rr5604.pdf. Accessed on November 27, 2007.
2. CDC. Varicella disease. Available at: www.cdc.gov/vaccines/vpd-vac/varicella/dis-faqs-clinic.htm. Accessed on November 27, 2007.
3. Public Affairs Department, Merck & Co, Inc. Personal communication; December 4, 2007.
4. Zostavax [package insert]. Whitehouse Sation, NJ: Merck & Co, Inc; 2006. Available at: www.fda.gov/cber/label/zostavaxlB.pdf. Accessed on November 27, 2007.
The varicella vaccine has had tremendous success over the last few years, but its success has stalled.
The widespread use of the varicella vaccine has led to a coverage rate of 88%, and the vaccine has proven to be 85% effective. As a result, between 1995 and 2001 there was an 87% decline in hospitalizations, 66% decline in deaths, and an 87% decline in costs attributed to varicella.
However, the number of varicella cases has remained at a constant level over the past few years and sporadic outbreaks continue to occur in schools—even where high rates of immunization are achieved.1,2
Varicella outbreaks involve both infections in unvaccinated children and “breakthrough disease” in those who have been vaccinated. If a vaccinated person is exposed to varicella, the risk of suffering a breakthrough infection is about 15%.2 A 2-dose series of varicella vaccine reduces the risk by about 75%1 (Figure).
Breakthrough disease is usually milder than infection in the unvaccinated, with fewer skin lesions, milder symptoms, and fewer complications. Those affected, though, are still infectious to others.
It was this ongoing risk of varicella that prompted the Advisory Committee on Immunization Practices (ACIP) to recommend new control measures, reported on in 2007.1
- All children should now receive 2 doses of varicella vaccine. The timing of the first and second dose should correspond with the administration of the MMR vaccine.
- Children older than 6 years of age and adults who previously received only 1 dose of vaccine should receive 1 more dose.
- Health care workers should ensure that they are immune to varicella by blood titers or receiving 2 doses of the vaccine.
- Pregnant women should be screened for immunity to varicella. They should be vaccinated postpartum if they are not immune.
FIGURE
2 doses of varicella vaccine reduce risk of breakthrough infection by about 75%1
Cumulative breakthrough rates for 1 and 2 doses of single-antigen varicella vaccine among children (ages 12 months to 12 years) by number of years after vaccination. Breakthrough rates are per 100 person-years at risk.
ACIP now recommends 2 doses of the vaccine
ACIP recommends the following:
- Universal administration of 2 doses of varicella vaccine; the first at ages 12 to 15 months and the second at age 4 to 6 years. (This is the same schedule as immunization against mumps, measles, and rubella.)
- Two doses of varicella vaccine, 4 to 8 weeks apart, for all adolescents and adults without evidence of immunity. (See “New criteria to prove immunity” at right.)
- A catch-up second dose for everyone who received one dose previously.
- Screening for varicella immunity in pregnant women and postpartum vaccination for those who are not immune, with 2 doses 4 to 8 weeks apart. The first dose should be administered before discharge.
Which HIV patients can get the vaccine?
ACIP has also clarified when HIV patients can be vaccinated, noting that single antigen varicella vaccine can be administered to HIV positive children if their CD4+ Tlymphocyte % is ≥15%. HIV positive adolescents and adults can be vaccinated if their CD4+ T-lymphocyte count ≥200/μL and, if 2 doses are indicated, they should be separated by at least 3 months.
ACIP has approved new criteria for establishing proof of immunity to varicella. ACIP now includes laboratory confirmation of disease or birth in the US prior to 1980 as evidence of immunity. Another change to ACIP’s criteria: A reported varicella history alone does not suffice; it needs to be verified by a provider.
ACIP’s new criteria include:
- Documentation of age appropriate vaccination (1 dose for preschool children ≥12 months of age, and 2 doses, 1 month apart, for school-age children, adolescents, and adults)
- Laboratory evidence of immunity or laboratory confirmation of disease
- A history of varicella disease or varicella zoster verified by a health care provider
- 4. Birth in the US prior to 1980. This criterion does not apply to health care providers, pregnant women, or the immune-suppressed.
2 options: Varivax and ProQuad
Two varicella vaccines contain modified live varicella virus antigen. Varivax, a single antigen vaccine, is approved for use in adults, adolescents, and children ≥12 months of age. The second vaccine, ProQuad, is approved for use in patients who are between 12 months and 12 years of age, and contains 4 viral antigens: mumps, measles, rubella, and varicella.
The quadrivalent MMRV vaccine is currently unavailable, however, and isn’t expected to be available until early 2009.3 Once the supply is stabilized, though, it will facilitate vaccination of children by decreasing the number of injections needed to achieve full immunization status.
29-year-old patient with varicellaThese 2 varicella vaccines should not be confused with the varicella zoster vaccine, Zostavax, which is approved for use in adults who are 60 years of age and older for the prevention of shingles and postherpetic neuralgia.4
- Can the varicella vaccine be co-administered with other childhood vaccines?
Yes. - What if a nonimmune pregnant women is exposed to chicken-pox?
You’ll need to consult your local health department about the possibility of administering varicella immune globulin. - Can the vaccine be administered to mothers who are breastfeeding their babies?
yes. - Can the vaccine be administered to those who live in a household with an immune-suppressed person?
yes, the risk of transmission of vaccine virus is very low. - What if a woman is inadvertently vaccinated while pregnant?
The risk during pregnancy is theoretical and to date, no cases of congenital varicella have resulted from inadvertent vaccination during pregnancy. - Will the vaccine prevent shingles later in life?
No one knows for sure. Surveillance is currently in progress, but long-term results are not available.
Pregnancy precludes vaccination
Varicella vaccine is contraindicated during pregnancy and in those who have had a severe allergic reaction to any vaccine component, including gelatin; have a malignancy of the blood, bone marrow, or lymphatic system; have a congenital or hereditary immunodeficiency; or are receiving systemic immunosuppressive therapy including those on the equivalent of 2 mg/kg, or >20 mg/day, of prednisone.
You should delay giving the vaccine to patients with an acute, severe illness. There is a potential for immune globulin containing products to interfere with the effectiveness of live virus vaccines. As a result, if a patient has received blood, plasma, or immune globulin, you should wait 3 to 11 months before giving the varicella vaccine. These products should also be avoided, if possible, for 2 weeks after the vaccine has been administered.
Avoid using quadrivalent MMRV in patients with HIV infection because it contains a higher quantity of varicella antigen than the single antigen product.
One final precaution: Patients should avoid taking salicylates for 6 weeks following vaccination because of the theoretical risk of Reye’s syndrome.
The varicella vaccine has had tremendous success over the last few years, but its success has stalled.
The widespread use of the varicella vaccine has led to a coverage rate of 88%, and the vaccine has proven to be 85% effective. As a result, between 1995 and 2001 there was an 87% decline in hospitalizations, 66% decline in deaths, and an 87% decline in costs attributed to varicella.
However, the number of varicella cases has remained at a constant level over the past few years and sporadic outbreaks continue to occur in schools—even where high rates of immunization are achieved.1,2
Varicella outbreaks involve both infections in unvaccinated children and “breakthrough disease” in those who have been vaccinated. If a vaccinated person is exposed to varicella, the risk of suffering a breakthrough infection is about 15%.2 A 2-dose series of varicella vaccine reduces the risk by about 75%1 (Figure).
Breakthrough disease is usually milder than infection in the unvaccinated, with fewer skin lesions, milder symptoms, and fewer complications. Those affected, though, are still infectious to others.
It was this ongoing risk of varicella that prompted the Advisory Committee on Immunization Practices (ACIP) to recommend new control measures, reported on in 2007.1
- All children should now receive 2 doses of varicella vaccine. The timing of the first and second dose should correspond with the administration of the MMR vaccine.
- Children older than 6 years of age and adults who previously received only 1 dose of vaccine should receive 1 more dose.
- Health care workers should ensure that they are immune to varicella by blood titers or receiving 2 doses of the vaccine.
- Pregnant women should be screened for immunity to varicella. They should be vaccinated postpartum if they are not immune.
FIGURE
2 doses of varicella vaccine reduce risk of breakthrough infection by about 75%1
Cumulative breakthrough rates for 1 and 2 doses of single-antigen varicella vaccine among children (ages 12 months to 12 years) by number of years after vaccination. Breakthrough rates are per 100 person-years at risk.
ACIP now recommends 2 doses of the vaccine
ACIP recommends the following:
- Universal administration of 2 doses of varicella vaccine; the first at ages 12 to 15 months and the second at age 4 to 6 years. (This is the same schedule as immunization against mumps, measles, and rubella.)
- Two doses of varicella vaccine, 4 to 8 weeks apart, for all adolescents and adults without evidence of immunity. (See “New criteria to prove immunity” at right.)
- A catch-up second dose for everyone who received one dose previously.
- Screening for varicella immunity in pregnant women and postpartum vaccination for those who are not immune, with 2 doses 4 to 8 weeks apart. The first dose should be administered before discharge.
Which HIV patients can get the vaccine?
ACIP has also clarified when HIV patients can be vaccinated, noting that single antigen varicella vaccine can be administered to HIV positive children if their CD4+ Tlymphocyte % is ≥15%. HIV positive adolescents and adults can be vaccinated if their CD4+ T-lymphocyte count ≥200/μL and, if 2 doses are indicated, they should be separated by at least 3 months.
ACIP has approved new criteria for establishing proof of immunity to varicella. ACIP now includes laboratory confirmation of disease or birth in the US prior to 1980 as evidence of immunity. Another change to ACIP’s criteria: A reported varicella history alone does not suffice; it needs to be verified by a provider.
ACIP’s new criteria include:
- Documentation of age appropriate vaccination (1 dose for preschool children ≥12 months of age, and 2 doses, 1 month apart, for school-age children, adolescents, and adults)
- Laboratory evidence of immunity or laboratory confirmation of disease
- A history of varicella disease or varicella zoster verified by a health care provider
- 4. Birth in the US prior to 1980. This criterion does not apply to health care providers, pregnant women, or the immune-suppressed.
2 options: Varivax and ProQuad
Two varicella vaccines contain modified live varicella virus antigen. Varivax, a single antigen vaccine, is approved for use in adults, adolescents, and children ≥12 months of age. The second vaccine, ProQuad, is approved for use in patients who are between 12 months and 12 years of age, and contains 4 viral antigens: mumps, measles, rubella, and varicella.
The quadrivalent MMRV vaccine is currently unavailable, however, and isn’t expected to be available until early 2009.3 Once the supply is stabilized, though, it will facilitate vaccination of children by decreasing the number of injections needed to achieve full immunization status.
29-year-old patient with varicellaThese 2 varicella vaccines should not be confused with the varicella zoster vaccine, Zostavax, which is approved for use in adults who are 60 years of age and older for the prevention of shingles and postherpetic neuralgia.4
- Can the varicella vaccine be co-administered with other childhood vaccines?
Yes. - What if a nonimmune pregnant women is exposed to chicken-pox?
You’ll need to consult your local health department about the possibility of administering varicella immune globulin. - Can the vaccine be administered to mothers who are breastfeeding their babies?
yes. - Can the vaccine be administered to those who live in a household with an immune-suppressed person?
yes, the risk of transmission of vaccine virus is very low. - What if a woman is inadvertently vaccinated while pregnant?
The risk during pregnancy is theoretical and to date, no cases of congenital varicella have resulted from inadvertent vaccination during pregnancy. - Will the vaccine prevent shingles later in life?
No one knows for sure. Surveillance is currently in progress, but long-term results are not available.
Pregnancy precludes vaccination
Varicella vaccine is contraindicated during pregnancy and in those who have had a severe allergic reaction to any vaccine component, including gelatin; have a malignancy of the blood, bone marrow, or lymphatic system; have a congenital or hereditary immunodeficiency; or are receiving systemic immunosuppressive therapy including those on the equivalent of 2 mg/kg, or >20 mg/day, of prednisone.
You should delay giving the vaccine to patients with an acute, severe illness. There is a potential for immune globulin containing products to interfere with the effectiveness of live virus vaccines. As a result, if a patient has received blood, plasma, or immune globulin, you should wait 3 to 11 months before giving the varicella vaccine. These products should also be avoided, if possible, for 2 weeks after the vaccine has been administered.
Avoid using quadrivalent MMRV in patients with HIV infection because it contains a higher quantity of varicella antigen than the single antigen product.
One final precaution: Patients should avoid taking salicylates for 6 weeks following vaccination because of the theoretical risk of Reye’s syndrome.
1. CDC. Prevention of varicella: Recommendations of the Advisory Committee on Immunization Practices. MMWR Recomm Rep 2007; 56(rr-4):1–40. Available at: www.cdc.gov/mmwr/PDF/rr/rr5604.pdf. Accessed on November 27, 2007.
2. CDC. Varicella disease. Available at: www.cdc.gov/vaccines/vpd-vac/varicella/dis-faqs-clinic.htm. Accessed on November 27, 2007.
3. Public Affairs Department, Merck & Co, Inc. Personal communication; December 4, 2007.
4. Zostavax [package insert]. Whitehouse Sation, NJ: Merck & Co, Inc; 2006. Available at: www.fda.gov/cber/label/zostavaxlB.pdf. Accessed on November 27, 2007.
1. CDC. Prevention of varicella: Recommendations of the Advisory Committee on Immunization Practices. MMWR Recomm Rep 2007; 56(rr-4):1–40. Available at: www.cdc.gov/mmwr/PDF/rr/rr5604.pdf. Accessed on November 27, 2007.
2. CDC. Varicella disease. Available at: www.cdc.gov/vaccines/vpd-vac/varicella/dis-faqs-clinic.htm. Accessed on November 27, 2007.
3. Public Affairs Department, Merck & Co, Inc. Personal communication; December 4, 2007.
4. Zostavax [package insert]. Whitehouse Sation, NJ: Merck & Co, Inc; 2006. Available at: www.fda.gov/cber/label/zostavaxlB.pdf. Accessed on November 27, 2007.
Flu vaccination rates: How can you do better?
Each year, the flu causes an average of about 36,000 excess deaths and over 200,000 hospitalizations in the US.1,2 Much of this morbidity and mortality is preventable, yet each year, a large proportion of those for whom the vaccine is recommended go unvaccinated (TABLE 1).
TABLE 1
High-risk groups who went unvaccinated with influenza vaccine (2005)
| POPULATION GROUP | PROPORTION UNVACCINATED |
|---|---|
| Household contacts of those at high risk | 83%–91% |
| Pregnant women | 84% |
| Patients, ages 50–64 years | 77% |
| Patients, ages 6–23 months | 67% |
| Those with high-risk medical conditions | 66%–82% |
| Health care workers | 64% |
| Patients, ages ≥65 years | 40% |
Improving rates among health care workers
The recommendations of the Centers for Disease Control and Prevention (CDC) for the 2007–2008 influenza season include a new recommendation that targets health care worker vaccination rates.3 Because of the low rate of vaccination of health care workers, and the potential impact of higher coverage on both worker and patient safety, the CDC now recommends that the level of vaccination coverage be used as one measure of a facility’s patient safety quality program. The CDC also recommends the implementation of policies to encourage acceptance of the vaccine, such as requiring those caregivers who refuse immunization to sign waivers.
Improving rates among patients
To improve vaccination levels among patients, the CDC recommends:
- using reminder/recall systems
- using standing order programs
- administering the vaccine before and during the influenza season to patients during routine health care visits.
For more on improving vaccination coverage, see “Tips to help improve vaccination rates”.
Offer the vaccine to anyone who wants it
While the groups for whom vaccine is recommended are the same as last year (TABLE 2), this year the CDC is emphasizing the importance of:
- offering the vaccine to anyone who wants to reduce their risk of contracting influenza or transmitting the virus to others.
- continuing to offer vaccine to those susceptible throughout the flu season.
A minor change from last year’s recommendations involves children who are 6 months through 8 years of age who receive only 1 dose of vaccine their first year of vaccination. The CDC now recommends that these children receive 2 doses the next year. If they receive only 1 dose 2 years in a row, the CDC recommends only a single dose annually thereafter.
TABLE 2
Who should receive the influenza vaccine?
| Anyone who wants to reduce their risk of contracting the flu or transmitting the virus to others People at high risk for complications from the flu, including:
|
The 2 vaccines: How they differ
The same 2 vaccine types are available this year as last: trivalent influenza vaccine (TIV) and live attenuated influenza vaccine (LAIV). The vaccines include the same viral strain antigens and either can be used annually unless contraindicated (TABLE 3).
The major differences between the 2 vaccine types are:
- LAIV is administered as an intranasal spray while TIV requires an intramuscular injection
- LAIV is approved only for healthy people who are 5 to 49 years of age, whereas TIV is approved for anyone over the age of 6 months
- The interval between 2 doses in children under 9 years of age is 4 weeks for TIV and 6 to 10 weeks for LAIV
- LAIV should not be administered to family members or close contacts of those who are immunosuppressed and require a protective environment, while TIV can be used in this situation
- LAIV, being a live virus vaccine, should be administered simultaneously with, or 4 weeks after, the administration of other live virus vaccines. TIV is not a live virus vaccine, and its timing in relation to other live virus vaccines is not an issue.
TABLE 3
Contraindications and precautions for influenza vaccines
| TIV trivalent influenza vaccine |
| ||
| LAIV live attenuated influenza vaccine |
| ||
Antiviral options remain the same
Once again this year, the CDC does not recommend the use of adamantane antivirals for prophylaxis or treatment of influenza, leaving the 2 neuraminidase inhibitors, oseltamivir (Tamiflu) and zanamivir (Relenza), for these purposes. Treating flu patients with these antivirals shortens the duration of symptoms and may reduce viral shedding.
The earlier the treatment is started, the better the results. There appears to be no—or only minimal—benefit for those with uncomplicated influenza if the treatment is started more than 2 days after the onset of illness.
The Task Force on Community Preventive Services (an independent group, whose members are appointed by the director of the CDC) indicates that there is evidence to support the use of the following methods for improving vaccination rates:4
- Provider reminders, including notations, stickers, or other prompts in clients’ charts that notify staff when a client is due for certain vaccinations, including the influenza vaccine
- A recall system to notify patients when vaccines are due, using telephone messages or mailings. (E-mail messages are not mentioned but should also work)
- Standing orders for adults that allow staff to administer vaccines without the patient seeing the physician
- Assessing provider performance in delivering vaccinations and supplying this data to the provider
- Decreasing out-of-pocket costs for vaccinations.
Consider antiviral prophylaxis for these patients
The CDC recommends that antiviral prophylaxis be considered for those who are susceptible, residing in an area with circulating influenza virus, and who:
- have not been vaccinated or were recently vaccinated (since it takes 2 weeks for immunity to develop after vaccination)
- are unvaccinated and providing care for high-risk individuals
- have a contraindication to the vaccine
- have immune deficiencies and may not respond adequately to the vaccine.
The CDC also recommends prophylaxis for all residents and staff in a long-term care facility where influenza is circulating, without regard to vaccine status. More complete information on indications, dose and duration of antivirals for prophylaxis, and treatment can be found in this year’s CDC recommendations.3
Another flu season approaches
The good news for the coming year is that the government expects that the supply of vaccine will exceed 100 million doses. This should be sufficient, unless unforeseen production problems arise.
Each year millions of doses of influenza vaccine go unused and are discarded. By following the CDC’s recommendations, and those of the Task Force on Community Preventive Services4 (top left), each of us can improve vaccination coverage in our area and minimize the number of hospitalizations and deaths from the flu.
Correspondence
Doug Campos-Outcalt, MD, MPA, 550 e. van buren, Phoenix, AZ 85004; dougco@u.arizona.edu.
1. Thompson WW, Shay DK, Wintraub E, et al. Mortality associated with influenza and respiratory synctial virus in the United States. JAMA 2003;289:179-186.
2. Thompson WW, Shay DK, Wintraub E, et al. Influenza-associated hospitalizations in the United States. JAMA 2004;292:1333-1340.
3. Centers for Disease Control and Prevention. Prevention and control of influenza. Recommendations of the Advisory Committee on Immunization Practices, 2007. MMWR Recomm Rep 2007;56(RR-6).-
4. Centers for Disease Control and Prevention. Vaccines. Guide to Community Preventive Services Web site. Available at: www.thecommunityguide.org/vaccine. Accessed on September 10, 2007.
Each year, the flu causes an average of about 36,000 excess deaths and over 200,000 hospitalizations in the US.1,2 Much of this morbidity and mortality is preventable, yet each year, a large proportion of those for whom the vaccine is recommended go unvaccinated (TABLE 1).
TABLE 1
High-risk groups who went unvaccinated with influenza vaccine (2005)
| POPULATION GROUP | PROPORTION UNVACCINATED |
|---|---|
| Household contacts of those at high risk | 83%–91% |
| Pregnant women | 84% |
| Patients, ages 50–64 years | 77% |
| Patients, ages 6–23 months | 67% |
| Those with high-risk medical conditions | 66%–82% |
| Health care workers | 64% |
| Patients, ages ≥65 years | 40% |
Improving rates among health care workers
The recommendations of the Centers for Disease Control and Prevention (CDC) for the 2007–2008 influenza season include a new recommendation that targets health care worker vaccination rates.3 Because of the low rate of vaccination of health care workers, and the potential impact of higher coverage on both worker and patient safety, the CDC now recommends that the level of vaccination coverage be used as one measure of a facility’s patient safety quality program. The CDC also recommends the implementation of policies to encourage acceptance of the vaccine, such as requiring those caregivers who refuse immunization to sign waivers.
Improving rates among patients
To improve vaccination levels among patients, the CDC recommends:
- using reminder/recall systems
- using standing order programs
- administering the vaccine before and during the influenza season to patients during routine health care visits.
For more on improving vaccination coverage, see “Tips to help improve vaccination rates”.
Offer the vaccine to anyone who wants it
While the groups for whom vaccine is recommended are the same as last year (TABLE 2), this year the CDC is emphasizing the importance of:
- offering the vaccine to anyone who wants to reduce their risk of contracting influenza or transmitting the virus to others.
- continuing to offer vaccine to those susceptible throughout the flu season.
A minor change from last year’s recommendations involves children who are 6 months through 8 years of age who receive only 1 dose of vaccine their first year of vaccination. The CDC now recommends that these children receive 2 doses the next year. If they receive only 1 dose 2 years in a row, the CDC recommends only a single dose annually thereafter.
TABLE 2
Who should receive the influenza vaccine?
| Anyone who wants to reduce their risk of contracting the flu or transmitting the virus to others People at high risk for complications from the flu, including:
|
The 2 vaccines: How they differ
The same 2 vaccine types are available this year as last: trivalent influenza vaccine (TIV) and live attenuated influenza vaccine (LAIV). The vaccines include the same viral strain antigens and either can be used annually unless contraindicated (TABLE 3).
The major differences between the 2 vaccine types are:
- LAIV is administered as an intranasal spray while TIV requires an intramuscular injection
- LAIV is approved only for healthy people who are 5 to 49 years of age, whereas TIV is approved for anyone over the age of 6 months
- The interval between 2 doses in children under 9 years of age is 4 weeks for TIV and 6 to 10 weeks for LAIV
- LAIV should not be administered to family members or close contacts of those who are immunosuppressed and require a protective environment, while TIV can be used in this situation
- LAIV, being a live virus vaccine, should be administered simultaneously with, or 4 weeks after, the administration of other live virus vaccines. TIV is not a live virus vaccine, and its timing in relation to other live virus vaccines is not an issue.
TABLE 3
Contraindications and precautions for influenza vaccines
| TIV trivalent influenza vaccine |
| ||
| LAIV live attenuated influenza vaccine |
| ||
Antiviral options remain the same
Once again this year, the CDC does not recommend the use of adamantane antivirals for prophylaxis or treatment of influenza, leaving the 2 neuraminidase inhibitors, oseltamivir (Tamiflu) and zanamivir (Relenza), for these purposes. Treating flu patients with these antivirals shortens the duration of symptoms and may reduce viral shedding.
The earlier the treatment is started, the better the results. There appears to be no—or only minimal—benefit for those with uncomplicated influenza if the treatment is started more than 2 days after the onset of illness.
The Task Force on Community Preventive Services (an independent group, whose members are appointed by the director of the CDC) indicates that there is evidence to support the use of the following methods for improving vaccination rates:4
- Provider reminders, including notations, stickers, or other prompts in clients’ charts that notify staff when a client is due for certain vaccinations, including the influenza vaccine
- A recall system to notify patients when vaccines are due, using telephone messages or mailings. (E-mail messages are not mentioned but should also work)
- Standing orders for adults that allow staff to administer vaccines without the patient seeing the physician
- Assessing provider performance in delivering vaccinations and supplying this data to the provider
- Decreasing out-of-pocket costs for vaccinations.
Consider antiviral prophylaxis for these patients
The CDC recommends that antiviral prophylaxis be considered for those who are susceptible, residing in an area with circulating influenza virus, and who:
- have not been vaccinated or were recently vaccinated (since it takes 2 weeks for immunity to develop after vaccination)
- are unvaccinated and providing care for high-risk individuals
- have a contraindication to the vaccine
- have immune deficiencies and may not respond adequately to the vaccine.
The CDC also recommends prophylaxis for all residents and staff in a long-term care facility where influenza is circulating, without regard to vaccine status. More complete information on indications, dose and duration of antivirals for prophylaxis, and treatment can be found in this year’s CDC recommendations.3
Another flu season approaches
The good news for the coming year is that the government expects that the supply of vaccine will exceed 100 million doses. This should be sufficient, unless unforeseen production problems arise.
Each year millions of doses of influenza vaccine go unused and are discarded. By following the CDC’s recommendations, and those of the Task Force on Community Preventive Services4 (top left), each of us can improve vaccination coverage in our area and minimize the number of hospitalizations and deaths from the flu.
Correspondence
Doug Campos-Outcalt, MD, MPA, 550 e. van buren, Phoenix, AZ 85004; dougco@u.arizona.edu.
Each year, the flu causes an average of about 36,000 excess deaths and over 200,000 hospitalizations in the US.1,2 Much of this morbidity and mortality is preventable, yet each year, a large proportion of those for whom the vaccine is recommended go unvaccinated (TABLE 1).
TABLE 1
High-risk groups who went unvaccinated with influenza vaccine (2005)
| POPULATION GROUP | PROPORTION UNVACCINATED |
|---|---|
| Household contacts of those at high risk | 83%–91% |
| Pregnant women | 84% |
| Patients, ages 50–64 years | 77% |
| Patients, ages 6–23 months | 67% |
| Those with high-risk medical conditions | 66%–82% |
| Health care workers | 64% |
| Patients, ages ≥65 years | 40% |
Improving rates among health care workers
The recommendations of the Centers for Disease Control and Prevention (CDC) for the 2007–2008 influenza season include a new recommendation that targets health care worker vaccination rates.3 Because of the low rate of vaccination of health care workers, and the potential impact of higher coverage on both worker and patient safety, the CDC now recommends that the level of vaccination coverage be used as one measure of a facility’s patient safety quality program. The CDC also recommends the implementation of policies to encourage acceptance of the vaccine, such as requiring those caregivers who refuse immunization to sign waivers.
Improving rates among patients
To improve vaccination levels among patients, the CDC recommends:
- using reminder/recall systems
- using standing order programs
- administering the vaccine before and during the influenza season to patients during routine health care visits.
For more on improving vaccination coverage, see “Tips to help improve vaccination rates”.
Offer the vaccine to anyone who wants it
While the groups for whom vaccine is recommended are the same as last year (TABLE 2), this year the CDC is emphasizing the importance of:
- offering the vaccine to anyone who wants to reduce their risk of contracting influenza or transmitting the virus to others.
- continuing to offer vaccine to those susceptible throughout the flu season.
A minor change from last year’s recommendations involves children who are 6 months through 8 years of age who receive only 1 dose of vaccine their first year of vaccination. The CDC now recommends that these children receive 2 doses the next year. If they receive only 1 dose 2 years in a row, the CDC recommends only a single dose annually thereafter.
TABLE 2
Who should receive the influenza vaccine?
| Anyone who wants to reduce their risk of contracting the flu or transmitting the virus to others People at high risk for complications from the flu, including:
|
The 2 vaccines: How they differ
The same 2 vaccine types are available this year as last: trivalent influenza vaccine (TIV) and live attenuated influenza vaccine (LAIV). The vaccines include the same viral strain antigens and either can be used annually unless contraindicated (TABLE 3).
The major differences between the 2 vaccine types are:
- LAIV is administered as an intranasal spray while TIV requires an intramuscular injection
- LAIV is approved only for healthy people who are 5 to 49 years of age, whereas TIV is approved for anyone over the age of 6 months
- The interval between 2 doses in children under 9 years of age is 4 weeks for TIV and 6 to 10 weeks for LAIV
- LAIV should not be administered to family members or close contacts of those who are immunosuppressed and require a protective environment, while TIV can be used in this situation
- LAIV, being a live virus vaccine, should be administered simultaneously with, or 4 weeks after, the administration of other live virus vaccines. TIV is not a live virus vaccine, and its timing in relation to other live virus vaccines is not an issue.
TABLE 3
Contraindications and precautions for influenza vaccines
| TIV trivalent influenza vaccine |
| ||
| LAIV live attenuated influenza vaccine |
| ||
Antiviral options remain the same
Once again this year, the CDC does not recommend the use of adamantane antivirals for prophylaxis or treatment of influenza, leaving the 2 neuraminidase inhibitors, oseltamivir (Tamiflu) and zanamivir (Relenza), for these purposes. Treating flu patients with these antivirals shortens the duration of symptoms and may reduce viral shedding.
The earlier the treatment is started, the better the results. There appears to be no—or only minimal—benefit for those with uncomplicated influenza if the treatment is started more than 2 days after the onset of illness.
The Task Force on Community Preventive Services (an independent group, whose members are appointed by the director of the CDC) indicates that there is evidence to support the use of the following methods for improving vaccination rates:4
- Provider reminders, including notations, stickers, or other prompts in clients’ charts that notify staff when a client is due for certain vaccinations, including the influenza vaccine
- A recall system to notify patients when vaccines are due, using telephone messages or mailings. (E-mail messages are not mentioned but should also work)
- Standing orders for adults that allow staff to administer vaccines without the patient seeing the physician
- Assessing provider performance in delivering vaccinations and supplying this data to the provider
- Decreasing out-of-pocket costs for vaccinations.
Consider antiviral prophylaxis for these patients
The CDC recommends that antiviral prophylaxis be considered for those who are susceptible, residing in an area with circulating influenza virus, and who:
- have not been vaccinated or were recently vaccinated (since it takes 2 weeks for immunity to develop after vaccination)
- are unvaccinated and providing care for high-risk individuals
- have a contraindication to the vaccine
- have immune deficiencies and may not respond adequately to the vaccine.
The CDC also recommends prophylaxis for all residents and staff in a long-term care facility where influenza is circulating, without regard to vaccine status. More complete information on indications, dose and duration of antivirals for prophylaxis, and treatment can be found in this year’s CDC recommendations.3
Another flu season approaches
The good news for the coming year is that the government expects that the supply of vaccine will exceed 100 million doses. This should be sufficient, unless unforeseen production problems arise.
Each year millions of doses of influenza vaccine go unused and are discarded. By following the CDC’s recommendations, and those of the Task Force on Community Preventive Services4 (top left), each of us can improve vaccination coverage in our area and minimize the number of hospitalizations and deaths from the flu.
Correspondence
Doug Campos-Outcalt, MD, MPA, 550 e. van buren, Phoenix, AZ 85004; dougco@u.arizona.edu.
1. Thompson WW, Shay DK, Wintraub E, et al. Mortality associated with influenza and respiratory synctial virus in the United States. JAMA 2003;289:179-186.
2. Thompson WW, Shay DK, Wintraub E, et al. Influenza-associated hospitalizations in the United States. JAMA 2004;292:1333-1340.
3. Centers for Disease Control and Prevention. Prevention and control of influenza. Recommendations of the Advisory Committee on Immunization Practices, 2007. MMWR Recomm Rep 2007;56(RR-6).-
4. Centers for Disease Control and Prevention. Vaccines. Guide to Community Preventive Services Web site. Available at: www.thecommunityguide.org/vaccine. Accessed on September 10, 2007.
1. Thompson WW, Shay DK, Wintraub E, et al. Mortality associated with influenza and respiratory synctial virus in the United States. JAMA 2003;289:179-186.
2. Thompson WW, Shay DK, Wintraub E, et al. Influenza-associated hospitalizations in the United States. JAMA 2004;292:1333-1340.
3. Centers for Disease Control and Prevention. Prevention and control of influenza. Recommendations of the Advisory Committee on Immunization Practices, 2007. MMWR Recomm Rep 2007;56(RR-6).-
4. Centers for Disease Control and Prevention. Vaccines. Guide to Community Preventive Services Web site. Available at: www.thecommunityguide.org/vaccine. Accessed on September 10, 2007.
Personalized medicine: The promise, the reality
Genetic tests to guide warfarin dosing could avert 85,000 serious bleeding events and 17,000 strokes annually, according to a report from the AEI-Brookings Joint Center for Regulatory Studies, a Washington, DC, think tank. The report further suggests that by integrating genetic testing into warfarin therapy, American health care spending could be reduced by $1.1 billion annually.1 Unfortunately, the promise of using genetic testing to guide such pharmacological treatment has largely gone unfulfilled.2
Case in point: Genetic testing can tell us whether a patient is likely to be an ultra-rapid metabolizer of warfarin (and need larger doses) or a poor metabolizer (and need lower doses), but there are no guidelines to tell us how to dose accordingly. International normalized ratios (INRs) still need to be ordered and the patient will likely have to pick up the tab for the genetic test ($250), since Medicare and private insurers don’t cover the cost. (See “Warfarin: An ideal, but far from ready, candidate”)
Hints that change may be on the horizon. The government—specifically the Department of Health and Human Services—created the Secretary’s Advisory Committee on Genetics, Health, and Society (SACGHS) to assess how genetic and genomic technologies are being integrated into health care and to identify opportunities and gaps in research. To that end, SACGHS issued a draft report earlier this year that notes that genetic-based treatment has “the potential to yield significant gains in personal health, population health, and cost-effective resource allocation.” Among its many recommendations, SACGHS calls for greater collaboration between the public and private sectors to expand our knowledge of the clinical validity and utility of using genetics to guide treatment.3
A standard of care, potentially. Readying ourselves for the ways that genetics is likely to shape the way we prescribe such drugs as anticoagulants, antidepressants, and antiarrhythmics requires that we step back and assess the progress made so far, and the work that still needs to be done before genetic testing becomes a common occurrence, and perhaps even a standard of care.
The goal: Avert adverse events
The wide variation in the way different people respond to the same dose of medications is a major contributor to the problem of adverse drug reactions. Lazarou and colleagues estimated that 6.7% of hospitalized patients—over 2 million patients in the US—experienced an adverse drug reaction and 0.32% (106,000) had a fatal adverse drug reaction.4
It would appear that warfarin dosing would be a perfect candidate for the clinical use of a pharmacogenetic test. Studies have shown that about 7% of the Caucasian population are poor metabolizers and at increased risk of bleeding from over coagulation and 1% are ultra-rapid metabolizers.4 Despite what we know about the polymorphisms to the CYP2C9 enzyme, which is the primary route of metabolism for warfarin, the package insert on Coumadin still doesn’t contain a recommendation for determining a patient’s genetic profile before initiating treatment.30 Similarly, the chapter on anticoagulation in Applied Therapeutics, a commonly used medical textbook, says nothing about the use of CYP polymorphisms for dosing decisions.13
At issue: Genotyping to guide dosing has not been tested in comparison to the usual monitoring using the international normalized ratio (INR).31 Specifically, the outcomes of bleeding complications and adequate anticoagulation of the 2 methods have not been compared in a clinical trial.
Here’s what we do know: In one study, the presence of specific polymorphisms was associated with a lower maintenance warfarin dose, but not with over-anticoagulation.32 In a review of 4 studies on CYP2C9 polymorphisms and warfarin daily dose, Lee and colleagues found that between the slowest and fastest metabolizers, the difference in dose was, at most, 4 mg/day. These studies did not explore if dosing decisions could accurately be made on genetic classifications and it is unlikely they could because of the wide overlap in maintenance dosages in the different classes.7
To complicate things further, the future use of warfarin in some conditions is problematic because fractionated heparin has been proven in many situations to be as effective and less risky than warfarin, and does not require frequent monitoring with blood tests. All of these unknowns make it unclear how useful genetic tests will be, and whether insurers will pay for them.
Individual response to medications is determined by a host of factors including age, environment, other medications being taken, and genetic differences in drug absorption and metabolism. These genetic differences have spawned the fields of pharmacogenomics and pharmacogenetics.
Pharmacogenomics is the biotechnological science that combines the techniques of medicine, pharmacology, and genomics and is concerned with developing drug therapies to compensate for genetic differences in patients, which cause varied responses to a single therapeutic regimen.
- A good example of pharmacogenomics at work is the use of trastuzumab in addition to chemotherapy for breast cancer patients who are positive for the human epidermal growth factor receptor 2 (HER2) oncogene.5
Pharmacogenetics is the branch of pharmacology that examines the relation of genetic factors to variations in response to drugs.
The use of pharmacogenetics to predict individualized responses to medications and to prevent adverse drug reactions through individualized dosing regimens or avoidance of certain medications hinges on our knowledge of genetic polymorphisms, that is, gene-based differences in drug absorption, distribution, metabolism, or excretion.
Polymorphisms of the cytochrome P450 family of drug metabolizing enzymes have been the most extensively studied. The names of these enzymes are abbreviated by using CYP and then a series of letters and numbers to describe individual enzymes. The 4 most extensively studied CYP enzymes are CYP2A6, CYP2C9, CYP2C19, and CYP2D6. These 4 metabolize an estimated 40% of all drugs; the distributions of polymorphisms of each vary considerably by race/ethnicity.6-8
A sampling of medications and classes of medications where polymorphisms play a significant role in drug metabolism is listed in the TABLE. Three with significant potential for prevention of adverse drug reactions are the antipsychotics, because of the severity of a specific drug adverse reaction, tardive dyskinesia;6,9 warfarin, because of the risk of bleeding complications and its narrow therapeutic index;7,10 and chemotherapeutic agents because of the serious nature of the disease and the potential for tailoring individualized therapies to maximize tumor response to medication and to minimize adverse reactions of very toxic drugs.11
TABLE
Polymorphisms to these enzymes affect drug metabolism
| DRUG/CLASS | ENZYMES |
|---|---|
| Antiarrhythmmics7 | CYP2D6 |
| Antidepressants7 | CYP2D6 |
| Antipsychotics6,9 | CYP2D6, CYP1A2, CYP2C19, CYP3A4 |
| Beta-blockers7 | CYP2D6 |
| Cancer chemotherapy11 | Varies by the agent |
| HMG-CoA reductase inhibitors (statins)29 | CYP2D6, CYP2C9, CYP2C19 |
| Losartan7 | CYP2C9 |
| Neuroleptics7 | CYP2D6 |
| NSAIDs29 | CYP2C9 |
| Phenytoin7 | CYP2C9, CYP2C19 |
| Proton pump inhibitors29 | CYP2C19 |
| Tolbutamide7 | CYP2C9 |
| Warfarin7,10 | CYP2C9 |
Clinical resources reflect an information gap
In spite of the potential for improved patient care, there remains very little clinical application of pharmacogenetic information in primary care practice. Zineh and colleagues reviewed prescribing information in the electronic version of the Physicians’ Desk Reference (PDR) in 2004 and found that only 76 package inserts out of 3382 contained pharmacogenetic information.12 In only 25 was there enough information to affect treatment decisions. Just 5 inserts mentioned that the chance of successful response to treatment could be predicted by genetic testing, and only one insert mentioned that a specific genetic subgroup should not take a drug.
The authors concluded that, generally, the pharmacogenetic information was inadequate to guide drug therapy and the majority of information was available for drugs that are not commonly prescribed. The FDA is addressing this issue by requiring the inclusion of pharmacogenetic information in package inserts more frequently.
Consider, too, the 2005 edition of Applied Therapeutics, a commonly used medical textbook.13 In it there is no mention of the use of pharmacogenetics in managing pharmacological therapies, which tells us that very little teaching on this topic is going on in medical schools and residencies.
But why? Why are clinicians and the tools we rely on so out of sync with the recommendations and expectations of personalized medicine advocates?
Certain conditions will need to exist before testing for CYP polymorphisms will become the standard of care in dosing certain drugs, such as warfarin. (see “Warfarin: An ideal, but far from ready, candidate”.) The most important is that this clinical approach needs to be proven superior to existing methods, such as INR monitoring. Should this occur, guidelines that are evidence-based will include it as a recommendation, physician continuing education courses will cover it, and it will enter into the curricula of medical schools and residency programs. But the process of adopting new, evidence-based best practices has historically been slow.32-36
One variable that may play a part in facilitating more rapid acceptance of genetic testing before warfarin use is litigation. Marchant and colleagues describe potential legal pressures that may drive medicine to adopt more personalized medicine.37 If genetic testing before warfarin use results in better outcomes (fewer catastrophic events or very bad outcomes) and there is plausible evidence that a catastrophic outcome (massive bleeding) could have been avoided with genetic information, then litigation will surely be close behind.
As news of successful litigation spreads, one of two results will likely occur: Either the use of the CYP polymorphism testing will increase, or the movement to use alternative medications, such as fractionated heparin, will accelerate.
There are 5 likely culprits:
- A lack of clinically useful pharmacogenetic tests.
- A lack of test standardization and availability.
- A lack of coverage by third-party payers.
- A low level of physician knowledge about genetic testing.
- A lack of evidence of improved outcomes.
How useful are pharmacogenetic tests?
For a laboratory test to be clinically useful, it should provide information that will influence a therapeutic decision. Decisions that could be influenced by this information include the dose of a particular drug and the potential use of an alternative because of a contraindication or likelihood of a poor outcome based on a particular genetic polymorphism.
The use of pharmacogenetic laboratory information can place a patient into one of several groups:
- ultra-rapid metabolizers, who need a larger dose of medication
- normal metabolizers, often called extensive metabolizers, who do not need dose modifications
- poor metabolizers, who need lower doses.9
Most medications have a wide therapeutic margin of effectiveness and safety. This means that the medication works within a wide range of serum drug levels and is safe at these different levels, making refinement in dosing based on genetic information unnecessary. In medications with narrower therapeutic windows for effectiveness or adverse reactions, there are frequently alternative means of drug level monitoring.
In many instances, the genetic test predictability of a patient’s actual metabolic responses—and resulting drug levels—is poor, leading to the need to monitor drug levels anyway.14-16 This occurs because there is often a great deal of overlap in the response to a medication dose among the different metabolism classifications.9,14,16-18 All of these realities have limited the clinical usefulness of pharmacogenetics up to this point.
Test standardization: Poised to improve?
Genotyping, the determination of the actual genetic makeup of the patient is not always predictive of an individual patient’s metabolic response. In other words, genotype does not always equate to a phenotype. Further complicating matters is the fact that many of the pharmacogenetic studies have been performed at research laboratories and have used tests that are not standardized, or widely available.19-21
The recent commercial availability of pharmacogenetic tests by well-established and reputable laboratories will probably improve both standardization and availability.
An example is the AmpliChip CYP450 test by Roche Diagnostics.22 This microassay-based test identifies 29 CYP-2D6 polymorphisms and 2 CYP-2C19 polymorphisms. These genes affect the metabolism of 25% of currently prescribed drugs. Using this test, patients can be classified as poor, intermediate, extensive, or ultra rapid metabolizers of CYP-2D6 affected drugs, including antidepressants, antiarrhythmics, and antipsychotics. They can also be classified as poor or extensive metabolizers of CYP-2C19 affected drugs, including phenytoin and proton pump inhibitors.
How costly?
The cost of genetic testing will also affect availability. Tests will be widely available only if covered by third-party payers. The AmpliChip test costs between $300 and $500. Clearly, then, both cost and insurance coverage are issues that impede the adoption of pharmacogenetic testing, though there is little written about this in the medical literature.19,23
AAFP explores ways to teach genomics
Physicians who are currently in practice received little or no training in the clinical use of pharmacogenetics or other genetic tests, such as genetic testing for the prediction of cancer risk.24,25 The main resources of pharmacological information for practicing physicians do not contain much, if any, useful genomic information. A recent continuing education monograph for family physicians on clinical genetics mentioned pharmacogenetics only as a promising future technology.26
For its part, the American Academy of Family Physicians has formed a genomics work group and is exploring how to educate family physicians on clinically useful genomic topics.
Evidence-based outcomes are needed
To date there has not been a head-to-head comparison of the outcomes of using clinical pharmacogenetics with those obtained from standard drug level monitoring practices. The CDC has formed a committee modeled after the USPSTF, the Evaluations of Genomics Applications in Practice and Prevention (EGAPP), which will evaluate the effectiveness of genomic clinical tests and make recommendations to physicians on their use.27 The group’s first report, on the use of CYP450 testing in depression, concluded that there is a paucity of good quality data that addresses whether testing for CYP450 polymorphisms in adults entering SSRI treatment leads to improved outcomes.28
In addition, SACGHS, the Department of Health and Human Services’ Committee, recommends in its draft report that HHS “provide resources to identify and address evidentiary gaps in the analytic validity, clinical validity, clinical utility, and cost effectiveness of pharmacogenomics.”3
Outcomes data will undoubtedly be key. With it, pharmacogenetic testing has the potential to grow by leaps and bounds—perhaps even becoming a standard of care in guiding pharmacological therapy. Without it, such testing will remain a promising, but as yet unrealized advance in personalized medicine.
Correspondence
Doug Campos-Outcalt, MD, MPA, 550 E. Van Buren, Phoenix, AZ 85004; dougco@u.arizona.edu.
1. McWilliam A, Lutter R, Nardinelli C. Health care savings from personalized medicine using genetic testing: The case of warfarin. [Working Paper 06-23]. November 2006. Available at: www.aei-brookings.org/admin/authorpdfs/page.php?id=1337&PHPsEssID=7b3a3ae4b30d77cb76223e29535e7590. Accessed on June 27, 2007.
2. Tucker G. Pharmacogenetics—expectations and reality. BMJ 2004;329:4-6.
3. Department of Health and Human Services. Realizing the promise of pharmacogenomics: Opportunities and Challenges [Draft report of the Secretary’s Advisory Committee on Genetics, Health, and Society]. Available for public comment March 23–June 1, 2007. Available at: www4.od.nih.gov/oba/sacghs/SACGHS_Pgx_PCdraft.pdf. Accessed on June 27, 2007.
4. Lazarou J, Pomeranz BH, Corey PN. Incidence of adverse drug reactions in hospitalized patients. JAMA 1998;279:1200-1205.
5. Dent R, Clemons M. Adjuvant trastuzumab for breast cancer. Br Med J 2005;331:1035-1036.
6. Wolf CR, Smith G. Pharmacokinetics. Br Med Bull 1999;55:366-386.
7. Lee CR, Goldstein JA, Pieper JA. Cytochrome P450 2C9 polymorphisms: a comprehensive review of the in-vitro and human data. Pharmacogenetics 2002;12:251-263.
8. Bachman K. Genotyping and phenotyping the cytochrome P450 enzymes. Am J Ther 2002;9:309-316.
9. Dahl ML. Cytochrome P450 phenotyping/genotyping in patients receiving antipsychotics. Clin Pharmacokinet 2002;41:453-470.
10. Daly AK, King BP. Pharmacogenetics of oral anticoagulants. Pharmacogenetics 2003;13:247-252.
11. Van Schaik RHN. Implications of cytochrome P450 genetic polymorphisms on the toxicity of antitumor agents. Ther Drug Monit 2004;26:236-240.
12. Zineh I, Gerhard T, Aquilante CL, Beitelshees AL, Beasley BN, Hartzema AG. Availability of pharmacogenomics-based prescribing information in drug package inserts for currently approved drugs. Pharmacogenomics J 2004;4:354-358.
13. Koda Kimball M, ed. Applied Therapeutics. 8th ed. Baltimore, Md: Lippincott, Williams and Wilkins; 2005.
14. Furuya H, Fernandez-Salguero P, Gregory W, et al. Genetic polymorphism of CYP2C9 and its effect on warfarin maintenance dose requirement in patients undergoing anticoagulation therapy. Pharmacogenetics 1995;5:389-392.
15. Arthur H, Dahl ML, Siwers B. Polymorphic drug metabolism in schizophrenic patients with tardive dyskinesia. J Clin Psychopharmacology 1995;15:211-216.
16. Mulder AB, Van Lijf HJ, Bon MAM, et al. Association of polymorphism in the cytochrome CYP2D6 and the efficacy and tolerability of simvastatin. Clin Pharmacol Ther 2001;70:546-551.
17. Takahashi H, Ehizen H. Pharmacogenetics of warfarin elimination and its clinical implications. Clin Pharmakinetics 2001;40:587-603.
18. Caraco J. Genetic determinants of drug responsiveness and drug interactions. Ther Drug Monit 1998;20:517-524.
19. Logue LJ. Genetic testing coverage and reimbursement: a provider’s dilemma. Clin Leadersh Manag Rev 2003;17:346-350.
20. Schwartz MK. Genetic testing and the clinical laboratory improvement amendments of 1988: present and future. Clin Chem 1999;45:739-745.
21. US Food and Drug Administration. Guidance for industry: Pharmacogenomic data submissions 2003. Available at: www.fda.gov/cber/gdlns/pharmdtasub.pdf. Accessed on July 3, 2007.
22. Howard RH. Personalized drug therapy with pharmacogenetics. Part I; pharmacokinetics. J Psychosoc Nurs Ment Health Serv 2006;44:13-16.
23. Veenstra DL, Higashi MK, Phillips KA. Assessing the cost effectiveness of pharmacogenics. AAPS PharmSci 2000;2:E29.-
24. Gramling R, Trask P, Nash J, Culpepper L. Family physicians’ beliefs about genetic testing. Fam Med 2004;36:691-692.
25. Caulfield TA. The informed gatekeeper? A commentary on genetic tests, marketing pressure and the role of primary care physicians. Health Law Rev 2000;9:14-17.
26. Mueller C, Feero WG. Clinical genetics. AAFP Home study self assessment Program No 317. Leawood, Kan: AAFP; 2005.
27. CDC Activities page. Programs in Brief: Evaluations of Genomics Applications and Prevention (EGAPP). Available at: www.cdc.gov/genomics/activities/pib/egapp.htm. Accessed on June 27, 2007.
28. Agency for Healthcare Research and Quality. Testing for cytochrome P450 polymorphisms in adults with non-psychotic depression treated with selective serotonin reuptake inhibitors (SSRIs) [AHRQ Publication No. 07-E002]. Available at: www.ahrq.gov/downloads/pub/evidence/pdf/cyp450/cyp450.pdf. Accessed on July 3, 2007.
29. Vermes A, Vermes I. Genetic polymorphisms in cytochrome P450 enzymes. Effect on efficacy and tolerability of HMG-CoA reductase inhibitors. Am J Cardiovasc Drugs 2004;4:247-255.
30. Yasar U, Eliasson E, Dahl ML, Johansson I, Ingelman-Sundberg M, Sjöqvist F. Validation of methods for CYP2C9 genotyping of mutant alleles in a swedish population. Biochem Biophys Res Commun 1999;254:628-631.
31. Aithal GP, Day CP, Kesteven PJL, Daly AK. Association of polymorphisms in the cytochrome p450 CYP2C9 with warfarin dose requirement and risk of bleeding complications. Lancet 1999;353:717-719.
32. Taube J, Halsall D, Baglin T. Influence of cytochrome P450 CYP2C9 polymorphisms on warfarin sensitivity and risk of over anticoagulation in patients on long-term therapy. Blood 2000;96:1816-1819.
33. Reeves MJ, Bohm SR, Korzeniewski SJ, Brown MD. Asthma care and management before an emergency department visit in children in western Michigan: how well does care adhere to guidelines? Pediatrics 2006;117:118-126.
34. Rastogi D, Shetty A, Neugebauer R, Harijith A. National heart, lung, and blood institute guidelines and asthma management practices among innercity pediatric primary care providers. Chest 2006;129:619-623.
35. Bishop PB, Wing PC. Knowledge transfer in family physicians managing patients with acute low back pain: a prospective randomized control trial. Spine J 2006;6:282-288.
36. Bauchner H, Marchant CD, Bisbee A, et al. Effectiveness of centers for disease control and prevention recommendations for outcomes of acute otitis media. Pediatrics 2006;117:1009-1017.
37. Marchant GE, Milligan RJ, Wilhelmi B. Legal pressures and incentives for personalized medicine. Personalized Medicine 2007;3:391-399.
Genetic tests to guide warfarin dosing could avert 85,000 serious bleeding events and 17,000 strokes annually, according to a report from the AEI-Brookings Joint Center for Regulatory Studies, a Washington, DC, think tank. The report further suggests that by integrating genetic testing into warfarin therapy, American health care spending could be reduced by $1.1 billion annually.1 Unfortunately, the promise of using genetic testing to guide such pharmacological treatment has largely gone unfulfilled.2
Case in point: Genetic testing can tell us whether a patient is likely to be an ultra-rapid metabolizer of warfarin (and need larger doses) or a poor metabolizer (and need lower doses), but there are no guidelines to tell us how to dose accordingly. International normalized ratios (INRs) still need to be ordered and the patient will likely have to pick up the tab for the genetic test ($250), since Medicare and private insurers don’t cover the cost. (See “Warfarin: An ideal, but far from ready, candidate”)
Hints that change may be on the horizon. The government—specifically the Department of Health and Human Services—created the Secretary’s Advisory Committee on Genetics, Health, and Society (SACGHS) to assess how genetic and genomic technologies are being integrated into health care and to identify opportunities and gaps in research. To that end, SACGHS issued a draft report earlier this year that notes that genetic-based treatment has “the potential to yield significant gains in personal health, population health, and cost-effective resource allocation.” Among its many recommendations, SACGHS calls for greater collaboration between the public and private sectors to expand our knowledge of the clinical validity and utility of using genetics to guide treatment.3
A standard of care, potentially. Readying ourselves for the ways that genetics is likely to shape the way we prescribe such drugs as anticoagulants, antidepressants, and antiarrhythmics requires that we step back and assess the progress made so far, and the work that still needs to be done before genetic testing becomes a common occurrence, and perhaps even a standard of care.
The goal: Avert adverse events
The wide variation in the way different people respond to the same dose of medications is a major contributor to the problem of adverse drug reactions. Lazarou and colleagues estimated that 6.7% of hospitalized patients—over 2 million patients in the US—experienced an adverse drug reaction and 0.32% (106,000) had a fatal adverse drug reaction.4
It would appear that warfarin dosing would be a perfect candidate for the clinical use of a pharmacogenetic test. Studies have shown that about 7% of the Caucasian population are poor metabolizers and at increased risk of bleeding from over coagulation and 1% are ultra-rapid metabolizers.4 Despite what we know about the polymorphisms to the CYP2C9 enzyme, which is the primary route of metabolism for warfarin, the package insert on Coumadin still doesn’t contain a recommendation for determining a patient’s genetic profile before initiating treatment.30 Similarly, the chapter on anticoagulation in Applied Therapeutics, a commonly used medical textbook, says nothing about the use of CYP polymorphisms for dosing decisions.13
At issue: Genotyping to guide dosing has not been tested in comparison to the usual monitoring using the international normalized ratio (INR).31 Specifically, the outcomes of bleeding complications and adequate anticoagulation of the 2 methods have not been compared in a clinical trial.
Here’s what we do know: In one study, the presence of specific polymorphisms was associated with a lower maintenance warfarin dose, but not with over-anticoagulation.32 In a review of 4 studies on CYP2C9 polymorphisms and warfarin daily dose, Lee and colleagues found that between the slowest and fastest metabolizers, the difference in dose was, at most, 4 mg/day. These studies did not explore if dosing decisions could accurately be made on genetic classifications and it is unlikely they could because of the wide overlap in maintenance dosages in the different classes.7
To complicate things further, the future use of warfarin in some conditions is problematic because fractionated heparin has been proven in many situations to be as effective and less risky than warfarin, and does not require frequent monitoring with blood tests. All of these unknowns make it unclear how useful genetic tests will be, and whether insurers will pay for them.
Individual response to medications is determined by a host of factors including age, environment, other medications being taken, and genetic differences in drug absorption and metabolism. These genetic differences have spawned the fields of pharmacogenomics and pharmacogenetics.
Pharmacogenomics is the biotechnological science that combines the techniques of medicine, pharmacology, and genomics and is concerned with developing drug therapies to compensate for genetic differences in patients, which cause varied responses to a single therapeutic regimen.
- A good example of pharmacogenomics at work is the use of trastuzumab in addition to chemotherapy for breast cancer patients who are positive for the human epidermal growth factor receptor 2 (HER2) oncogene.5
Pharmacogenetics is the branch of pharmacology that examines the relation of genetic factors to variations in response to drugs.
The use of pharmacogenetics to predict individualized responses to medications and to prevent adverse drug reactions through individualized dosing regimens or avoidance of certain medications hinges on our knowledge of genetic polymorphisms, that is, gene-based differences in drug absorption, distribution, metabolism, or excretion.
Polymorphisms of the cytochrome P450 family of drug metabolizing enzymes have been the most extensively studied. The names of these enzymes are abbreviated by using CYP and then a series of letters and numbers to describe individual enzymes. The 4 most extensively studied CYP enzymes are CYP2A6, CYP2C9, CYP2C19, and CYP2D6. These 4 metabolize an estimated 40% of all drugs; the distributions of polymorphisms of each vary considerably by race/ethnicity.6-8
A sampling of medications and classes of medications where polymorphisms play a significant role in drug metabolism is listed in the TABLE. Three with significant potential for prevention of adverse drug reactions are the antipsychotics, because of the severity of a specific drug adverse reaction, tardive dyskinesia;6,9 warfarin, because of the risk of bleeding complications and its narrow therapeutic index;7,10 and chemotherapeutic agents because of the serious nature of the disease and the potential for tailoring individualized therapies to maximize tumor response to medication and to minimize adverse reactions of very toxic drugs.11
TABLE
Polymorphisms to these enzymes affect drug metabolism
| DRUG/CLASS | ENZYMES |
|---|---|
| Antiarrhythmmics7 | CYP2D6 |
| Antidepressants7 | CYP2D6 |
| Antipsychotics6,9 | CYP2D6, CYP1A2, CYP2C19, CYP3A4 |
| Beta-blockers7 | CYP2D6 |
| Cancer chemotherapy11 | Varies by the agent |
| HMG-CoA reductase inhibitors (statins)29 | CYP2D6, CYP2C9, CYP2C19 |
| Losartan7 | CYP2C9 |
| Neuroleptics7 | CYP2D6 |
| NSAIDs29 | CYP2C9 |
| Phenytoin7 | CYP2C9, CYP2C19 |
| Proton pump inhibitors29 | CYP2C19 |
| Tolbutamide7 | CYP2C9 |
| Warfarin7,10 | CYP2C9 |
Clinical resources reflect an information gap
In spite of the potential for improved patient care, there remains very little clinical application of pharmacogenetic information in primary care practice. Zineh and colleagues reviewed prescribing information in the electronic version of the Physicians’ Desk Reference (PDR) in 2004 and found that only 76 package inserts out of 3382 contained pharmacogenetic information.12 In only 25 was there enough information to affect treatment decisions. Just 5 inserts mentioned that the chance of successful response to treatment could be predicted by genetic testing, and only one insert mentioned that a specific genetic subgroup should not take a drug.
The authors concluded that, generally, the pharmacogenetic information was inadequate to guide drug therapy and the majority of information was available for drugs that are not commonly prescribed. The FDA is addressing this issue by requiring the inclusion of pharmacogenetic information in package inserts more frequently.
Consider, too, the 2005 edition of Applied Therapeutics, a commonly used medical textbook.13 In it there is no mention of the use of pharmacogenetics in managing pharmacological therapies, which tells us that very little teaching on this topic is going on in medical schools and residencies.
But why? Why are clinicians and the tools we rely on so out of sync with the recommendations and expectations of personalized medicine advocates?
Certain conditions will need to exist before testing for CYP polymorphisms will become the standard of care in dosing certain drugs, such as warfarin. (see “Warfarin: An ideal, but far from ready, candidate”.) The most important is that this clinical approach needs to be proven superior to existing methods, such as INR monitoring. Should this occur, guidelines that are evidence-based will include it as a recommendation, physician continuing education courses will cover it, and it will enter into the curricula of medical schools and residency programs. But the process of adopting new, evidence-based best practices has historically been slow.32-36
One variable that may play a part in facilitating more rapid acceptance of genetic testing before warfarin use is litigation. Marchant and colleagues describe potential legal pressures that may drive medicine to adopt more personalized medicine.37 If genetic testing before warfarin use results in better outcomes (fewer catastrophic events or very bad outcomes) and there is plausible evidence that a catastrophic outcome (massive bleeding) could have been avoided with genetic information, then litigation will surely be close behind.
As news of successful litigation spreads, one of two results will likely occur: Either the use of the CYP polymorphism testing will increase, or the movement to use alternative medications, such as fractionated heparin, will accelerate.
There are 5 likely culprits:
- A lack of clinically useful pharmacogenetic tests.
- A lack of test standardization and availability.
- A lack of coverage by third-party payers.
- A low level of physician knowledge about genetic testing.
- A lack of evidence of improved outcomes.
How useful are pharmacogenetic tests?
For a laboratory test to be clinically useful, it should provide information that will influence a therapeutic decision. Decisions that could be influenced by this information include the dose of a particular drug and the potential use of an alternative because of a contraindication or likelihood of a poor outcome based on a particular genetic polymorphism.
The use of pharmacogenetic laboratory information can place a patient into one of several groups:
- ultra-rapid metabolizers, who need a larger dose of medication
- normal metabolizers, often called extensive metabolizers, who do not need dose modifications
- poor metabolizers, who need lower doses.9
Most medications have a wide therapeutic margin of effectiveness and safety. This means that the medication works within a wide range of serum drug levels and is safe at these different levels, making refinement in dosing based on genetic information unnecessary. In medications with narrower therapeutic windows for effectiveness or adverse reactions, there are frequently alternative means of drug level monitoring.
In many instances, the genetic test predictability of a patient’s actual metabolic responses—and resulting drug levels—is poor, leading to the need to monitor drug levels anyway.14-16 This occurs because there is often a great deal of overlap in the response to a medication dose among the different metabolism classifications.9,14,16-18 All of these realities have limited the clinical usefulness of pharmacogenetics up to this point.
Test standardization: Poised to improve?
Genotyping, the determination of the actual genetic makeup of the patient is not always predictive of an individual patient’s metabolic response. In other words, genotype does not always equate to a phenotype. Further complicating matters is the fact that many of the pharmacogenetic studies have been performed at research laboratories and have used tests that are not standardized, or widely available.19-21
The recent commercial availability of pharmacogenetic tests by well-established and reputable laboratories will probably improve both standardization and availability.
An example is the AmpliChip CYP450 test by Roche Diagnostics.22 This microassay-based test identifies 29 CYP-2D6 polymorphisms and 2 CYP-2C19 polymorphisms. These genes affect the metabolism of 25% of currently prescribed drugs. Using this test, patients can be classified as poor, intermediate, extensive, or ultra rapid metabolizers of CYP-2D6 affected drugs, including antidepressants, antiarrhythmics, and antipsychotics. They can also be classified as poor or extensive metabolizers of CYP-2C19 affected drugs, including phenytoin and proton pump inhibitors.
How costly?
The cost of genetic testing will also affect availability. Tests will be widely available only if covered by third-party payers. The AmpliChip test costs between $300 and $500. Clearly, then, both cost and insurance coverage are issues that impede the adoption of pharmacogenetic testing, though there is little written about this in the medical literature.19,23
AAFP explores ways to teach genomics
Physicians who are currently in practice received little or no training in the clinical use of pharmacogenetics or other genetic tests, such as genetic testing for the prediction of cancer risk.24,25 The main resources of pharmacological information for practicing physicians do not contain much, if any, useful genomic information. A recent continuing education monograph for family physicians on clinical genetics mentioned pharmacogenetics only as a promising future technology.26
For its part, the American Academy of Family Physicians has formed a genomics work group and is exploring how to educate family physicians on clinically useful genomic topics.
Evidence-based outcomes are needed
To date there has not been a head-to-head comparison of the outcomes of using clinical pharmacogenetics with those obtained from standard drug level monitoring practices. The CDC has formed a committee modeled after the USPSTF, the Evaluations of Genomics Applications in Practice and Prevention (EGAPP), which will evaluate the effectiveness of genomic clinical tests and make recommendations to physicians on their use.27 The group’s first report, on the use of CYP450 testing in depression, concluded that there is a paucity of good quality data that addresses whether testing for CYP450 polymorphisms in adults entering SSRI treatment leads to improved outcomes.28
In addition, SACGHS, the Department of Health and Human Services’ Committee, recommends in its draft report that HHS “provide resources to identify and address evidentiary gaps in the analytic validity, clinical validity, clinical utility, and cost effectiveness of pharmacogenomics.”3
Outcomes data will undoubtedly be key. With it, pharmacogenetic testing has the potential to grow by leaps and bounds—perhaps even becoming a standard of care in guiding pharmacological therapy. Without it, such testing will remain a promising, but as yet unrealized advance in personalized medicine.
Correspondence
Doug Campos-Outcalt, MD, MPA, 550 E. Van Buren, Phoenix, AZ 85004; dougco@u.arizona.edu.
Genetic tests to guide warfarin dosing could avert 85,000 serious bleeding events and 17,000 strokes annually, according to a report from the AEI-Brookings Joint Center for Regulatory Studies, a Washington, DC, think tank. The report further suggests that by integrating genetic testing into warfarin therapy, American health care spending could be reduced by $1.1 billion annually.1 Unfortunately, the promise of using genetic testing to guide such pharmacological treatment has largely gone unfulfilled.2
Case in point: Genetic testing can tell us whether a patient is likely to be an ultra-rapid metabolizer of warfarin (and need larger doses) or a poor metabolizer (and need lower doses), but there are no guidelines to tell us how to dose accordingly. International normalized ratios (INRs) still need to be ordered and the patient will likely have to pick up the tab for the genetic test ($250), since Medicare and private insurers don’t cover the cost. (See “Warfarin: An ideal, but far from ready, candidate”)
Hints that change may be on the horizon. The government—specifically the Department of Health and Human Services—created the Secretary’s Advisory Committee on Genetics, Health, and Society (SACGHS) to assess how genetic and genomic technologies are being integrated into health care and to identify opportunities and gaps in research. To that end, SACGHS issued a draft report earlier this year that notes that genetic-based treatment has “the potential to yield significant gains in personal health, population health, and cost-effective resource allocation.” Among its many recommendations, SACGHS calls for greater collaboration between the public and private sectors to expand our knowledge of the clinical validity and utility of using genetics to guide treatment.3
A standard of care, potentially. Readying ourselves for the ways that genetics is likely to shape the way we prescribe such drugs as anticoagulants, antidepressants, and antiarrhythmics requires that we step back and assess the progress made so far, and the work that still needs to be done before genetic testing becomes a common occurrence, and perhaps even a standard of care.
The goal: Avert adverse events
The wide variation in the way different people respond to the same dose of medications is a major contributor to the problem of adverse drug reactions. Lazarou and colleagues estimated that 6.7% of hospitalized patients—over 2 million patients in the US—experienced an adverse drug reaction and 0.32% (106,000) had a fatal adverse drug reaction.4
It would appear that warfarin dosing would be a perfect candidate for the clinical use of a pharmacogenetic test. Studies have shown that about 7% of the Caucasian population are poor metabolizers and at increased risk of bleeding from over coagulation and 1% are ultra-rapid metabolizers.4 Despite what we know about the polymorphisms to the CYP2C9 enzyme, which is the primary route of metabolism for warfarin, the package insert on Coumadin still doesn’t contain a recommendation for determining a patient’s genetic profile before initiating treatment.30 Similarly, the chapter on anticoagulation in Applied Therapeutics, a commonly used medical textbook, says nothing about the use of CYP polymorphisms for dosing decisions.13
At issue: Genotyping to guide dosing has not been tested in comparison to the usual monitoring using the international normalized ratio (INR).31 Specifically, the outcomes of bleeding complications and adequate anticoagulation of the 2 methods have not been compared in a clinical trial.
Here’s what we do know: In one study, the presence of specific polymorphisms was associated with a lower maintenance warfarin dose, but not with over-anticoagulation.32 In a review of 4 studies on CYP2C9 polymorphisms and warfarin daily dose, Lee and colleagues found that between the slowest and fastest metabolizers, the difference in dose was, at most, 4 mg/day. These studies did not explore if dosing decisions could accurately be made on genetic classifications and it is unlikely they could because of the wide overlap in maintenance dosages in the different classes.7
To complicate things further, the future use of warfarin in some conditions is problematic because fractionated heparin has been proven in many situations to be as effective and less risky than warfarin, and does not require frequent monitoring with blood tests. All of these unknowns make it unclear how useful genetic tests will be, and whether insurers will pay for them.
Individual response to medications is determined by a host of factors including age, environment, other medications being taken, and genetic differences in drug absorption and metabolism. These genetic differences have spawned the fields of pharmacogenomics and pharmacogenetics.
Pharmacogenomics is the biotechnological science that combines the techniques of medicine, pharmacology, and genomics and is concerned with developing drug therapies to compensate for genetic differences in patients, which cause varied responses to a single therapeutic regimen.
- A good example of pharmacogenomics at work is the use of trastuzumab in addition to chemotherapy for breast cancer patients who are positive for the human epidermal growth factor receptor 2 (HER2) oncogene.5
Pharmacogenetics is the branch of pharmacology that examines the relation of genetic factors to variations in response to drugs.
The use of pharmacogenetics to predict individualized responses to medications and to prevent adverse drug reactions through individualized dosing regimens or avoidance of certain medications hinges on our knowledge of genetic polymorphisms, that is, gene-based differences in drug absorption, distribution, metabolism, or excretion.
Polymorphisms of the cytochrome P450 family of drug metabolizing enzymes have been the most extensively studied. The names of these enzymes are abbreviated by using CYP and then a series of letters and numbers to describe individual enzymes. The 4 most extensively studied CYP enzymes are CYP2A6, CYP2C9, CYP2C19, and CYP2D6. These 4 metabolize an estimated 40% of all drugs; the distributions of polymorphisms of each vary considerably by race/ethnicity.6-8
A sampling of medications and classes of medications where polymorphisms play a significant role in drug metabolism is listed in the TABLE. Three with significant potential for prevention of adverse drug reactions are the antipsychotics, because of the severity of a specific drug adverse reaction, tardive dyskinesia;6,9 warfarin, because of the risk of bleeding complications and its narrow therapeutic index;7,10 and chemotherapeutic agents because of the serious nature of the disease and the potential for tailoring individualized therapies to maximize tumor response to medication and to minimize adverse reactions of very toxic drugs.11
TABLE
Polymorphisms to these enzymes affect drug metabolism
| DRUG/CLASS | ENZYMES |
|---|---|
| Antiarrhythmmics7 | CYP2D6 |
| Antidepressants7 | CYP2D6 |
| Antipsychotics6,9 | CYP2D6, CYP1A2, CYP2C19, CYP3A4 |
| Beta-blockers7 | CYP2D6 |
| Cancer chemotherapy11 | Varies by the agent |
| HMG-CoA reductase inhibitors (statins)29 | CYP2D6, CYP2C9, CYP2C19 |
| Losartan7 | CYP2C9 |
| Neuroleptics7 | CYP2D6 |
| NSAIDs29 | CYP2C9 |
| Phenytoin7 | CYP2C9, CYP2C19 |
| Proton pump inhibitors29 | CYP2C19 |
| Tolbutamide7 | CYP2C9 |
| Warfarin7,10 | CYP2C9 |
Clinical resources reflect an information gap
In spite of the potential for improved patient care, there remains very little clinical application of pharmacogenetic information in primary care practice. Zineh and colleagues reviewed prescribing information in the electronic version of the Physicians’ Desk Reference (PDR) in 2004 and found that only 76 package inserts out of 3382 contained pharmacogenetic information.12 In only 25 was there enough information to affect treatment decisions. Just 5 inserts mentioned that the chance of successful response to treatment could be predicted by genetic testing, and only one insert mentioned that a specific genetic subgroup should not take a drug.
The authors concluded that, generally, the pharmacogenetic information was inadequate to guide drug therapy and the majority of information was available for drugs that are not commonly prescribed. The FDA is addressing this issue by requiring the inclusion of pharmacogenetic information in package inserts more frequently.
Consider, too, the 2005 edition of Applied Therapeutics, a commonly used medical textbook.13 In it there is no mention of the use of pharmacogenetics in managing pharmacological therapies, which tells us that very little teaching on this topic is going on in medical schools and residencies.
But why? Why are clinicians and the tools we rely on so out of sync with the recommendations and expectations of personalized medicine advocates?
Certain conditions will need to exist before testing for CYP polymorphisms will become the standard of care in dosing certain drugs, such as warfarin. (see “Warfarin: An ideal, but far from ready, candidate”.) The most important is that this clinical approach needs to be proven superior to existing methods, such as INR monitoring. Should this occur, guidelines that are evidence-based will include it as a recommendation, physician continuing education courses will cover it, and it will enter into the curricula of medical schools and residency programs. But the process of adopting new, evidence-based best practices has historically been slow.32-36
One variable that may play a part in facilitating more rapid acceptance of genetic testing before warfarin use is litigation. Marchant and colleagues describe potential legal pressures that may drive medicine to adopt more personalized medicine.37 If genetic testing before warfarin use results in better outcomes (fewer catastrophic events or very bad outcomes) and there is plausible evidence that a catastrophic outcome (massive bleeding) could have been avoided with genetic information, then litigation will surely be close behind.
As news of successful litigation spreads, one of two results will likely occur: Either the use of the CYP polymorphism testing will increase, or the movement to use alternative medications, such as fractionated heparin, will accelerate.
There are 5 likely culprits:
- A lack of clinically useful pharmacogenetic tests.
- A lack of test standardization and availability.
- A lack of coverage by third-party payers.
- A low level of physician knowledge about genetic testing.
- A lack of evidence of improved outcomes.
How useful are pharmacogenetic tests?
For a laboratory test to be clinically useful, it should provide information that will influence a therapeutic decision. Decisions that could be influenced by this information include the dose of a particular drug and the potential use of an alternative because of a contraindication or likelihood of a poor outcome based on a particular genetic polymorphism.
The use of pharmacogenetic laboratory information can place a patient into one of several groups:
- ultra-rapid metabolizers, who need a larger dose of medication
- normal metabolizers, often called extensive metabolizers, who do not need dose modifications
- poor metabolizers, who need lower doses.9
Most medications have a wide therapeutic margin of effectiveness and safety. This means that the medication works within a wide range of serum drug levels and is safe at these different levels, making refinement in dosing based on genetic information unnecessary. In medications with narrower therapeutic windows for effectiveness or adverse reactions, there are frequently alternative means of drug level monitoring.
In many instances, the genetic test predictability of a patient’s actual metabolic responses—and resulting drug levels—is poor, leading to the need to monitor drug levels anyway.14-16 This occurs because there is often a great deal of overlap in the response to a medication dose among the different metabolism classifications.9,14,16-18 All of these realities have limited the clinical usefulness of pharmacogenetics up to this point.
Test standardization: Poised to improve?
Genotyping, the determination of the actual genetic makeup of the patient is not always predictive of an individual patient’s metabolic response. In other words, genotype does not always equate to a phenotype. Further complicating matters is the fact that many of the pharmacogenetic studies have been performed at research laboratories and have used tests that are not standardized, or widely available.19-21
The recent commercial availability of pharmacogenetic tests by well-established and reputable laboratories will probably improve both standardization and availability.
An example is the AmpliChip CYP450 test by Roche Diagnostics.22 This microassay-based test identifies 29 CYP-2D6 polymorphisms and 2 CYP-2C19 polymorphisms. These genes affect the metabolism of 25% of currently prescribed drugs. Using this test, patients can be classified as poor, intermediate, extensive, or ultra rapid metabolizers of CYP-2D6 affected drugs, including antidepressants, antiarrhythmics, and antipsychotics. They can also be classified as poor or extensive metabolizers of CYP-2C19 affected drugs, including phenytoin and proton pump inhibitors.
How costly?
The cost of genetic testing will also affect availability. Tests will be widely available only if covered by third-party payers. The AmpliChip test costs between $300 and $500. Clearly, then, both cost and insurance coverage are issues that impede the adoption of pharmacogenetic testing, though there is little written about this in the medical literature.19,23
AAFP explores ways to teach genomics
Physicians who are currently in practice received little or no training in the clinical use of pharmacogenetics or other genetic tests, such as genetic testing for the prediction of cancer risk.24,25 The main resources of pharmacological information for practicing physicians do not contain much, if any, useful genomic information. A recent continuing education monograph for family physicians on clinical genetics mentioned pharmacogenetics only as a promising future technology.26
For its part, the American Academy of Family Physicians has formed a genomics work group and is exploring how to educate family physicians on clinically useful genomic topics.
Evidence-based outcomes are needed
To date there has not been a head-to-head comparison of the outcomes of using clinical pharmacogenetics with those obtained from standard drug level monitoring practices. The CDC has formed a committee modeled after the USPSTF, the Evaluations of Genomics Applications in Practice and Prevention (EGAPP), which will evaluate the effectiveness of genomic clinical tests and make recommendations to physicians on their use.27 The group’s first report, on the use of CYP450 testing in depression, concluded that there is a paucity of good quality data that addresses whether testing for CYP450 polymorphisms in adults entering SSRI treatment leads to improved outcomes.28
In addition, SACGHS, the Department of Health and Human Services’ Committee, recommends in its draft report that HHS “provide resources to identify and address evidentiary gaps in the analytic validity, clinical validity, clinical utility, and cost effectiveness of pharmacogenomics.”3
Outcomes data will undoubtedly be key. With it, pharmacogenetic testing has the potential to grow by leaps and bounds—perhaps even becoming a standard of care in guiding pharmacological therapy. Without it, such testing will remain a promising, but as yet unrealized advance in personalized medicine.
Correspondence
Doug Campos-Outcalt, MD, MPA, 550 E. Van Buren, Phoenix, AZ 85004; dougco@u.arizona.edu.
1. McWilliam A, Lutter R, Nardinelli C. Health care savings from personalized medicine using genetic testing: The case of warfarin. [Working Paper 06-23]. November 2006. Available at: www.aei-brookings.org/admin/authorpdfs/page.php?id=1337&PHPsEssID=7b3a3ae4b30d77cb76223e29535e7590. Accessed on June 27, 2007.
2. Tucker G. Pharmacogenetics—expectations and reality. BMJ 2004;329:4-6.
3. Department of Health and Human Services. Realizing the promise of pharmacogenomics: Opportunities and Challenges [Draft report of the Secretary’s Advisory Committee on Genetics, Health, and Society]. Available for public comment March 23–June 1, 2007. Available at: www4.od.nih.gov/oba/sacghs/SACGHS_Pgx_PCdraft.pdf. Accessed on June 27, 2007.
4. Lazarou J, Pomeranz BH, Corey PN. Incidence of adverse drug reactions in hospitalized patients. JAMA 1998;279:1200-1205.
5. Dent R, Clemons M. Adjuvant trastuzumab for breast cancer. Br Med J 2005;331:1035-1036.
6. Wolf CR, Smith G. Pharmacokinetics. Br Med Bull 1999;55:366-386.
7. Lee CR, Goldstein JA, Pieper JA. Cytochrome P450 2C9 polymorphisms: a comprehensive review of the in-vitro and human data. Pharmacogenetics 2002;12:251-263.
8. Bachman K. Genotyping and phenotyping the cytochrome P450 enzymes. Am J Ther 2002;9:309-316.
9. Dahl ML. Cytochrome P450 phenotyping/genotyping in patients receiving antipsychotics. Clin Pharmacokinet 2002;41:453-470.
10. Daly AK, King BP. Pharmacogenetics of oral anticoagulants. Pharmacogenetics 2003;13:247-252.
11. Van Schaik RHN. Implications of cytochrome P450 genetic polymorphisms on the toxicity of antitumor agents. Ther Drug Monit 2004;26:236-240.
12. Zineh I, Gerhard T, Aquilante CL, Beitelshees AL, Beasley BN, Hartzema AG. Availability of pharmacogenomics-based prescribing information in drug package inserts for currently approved drugs. Pharmacogenomics J 2004;4:354-358.
13. Koda Kimball M, ed. Applied Therapeutics. 8th ed. Baltimore, Md: Lippincott, Williams and Wilkins; 2005.
14. Furuya H, Fernandez-Salguero P, Gregory W, et al. Genetic polymorphism of CYP2C9 and its effect on warfarin maintenance dose requirement in patients undergoing anticoagulation therapy. Pharmacogenetics 1995;5:389-392.
15. Arthur H, Dahl ML, Siwers B. Polymorphic drug metabolism in schizophrenic patients with tardive dyskinesia. J Clin Psychopharmacology 1995;15:211-216.
16. Mulder AB, Van Lijf HJ, Bon MAM, et al. Association of polymorphism in the cytochrome CYP2D6 and the efficacy and tolerability of simvastatin. Clin Pharmacol Ther 2001;70:546-551.
17. Takahashi H, Ehizen H. Pharmacogenetics of warfarin elimination and its clinical implications. Clin Pharmakinetics 2001;40:587-603.
18. Caraco J. Genetic determinants of drug responsiveness and drug interactions. Ther Drug Monit 1998;20:517-524.
19. Logue LJ. Genetic testing coverage and reimbursement: a provider’s dilemma. Clin Leadersh Manag Rev 2003;17:346-350.
20. Schwartz MK. Genetic testing and the clinical laboratory improvement amendments of 1988: present and future. Clin Chem 1999;45:739-745.
21. US Food and Drug Administration. Guidance for industry: Pharmacogenomic data submissions 2003. Available at: www.fda.gov/cber/gdlns/pharmdtasub.pdf. Accessed on July 3, 2007.
22. Howard RH. Personalized drug therapy with pharmacogenetics. Part I; pharmacokinetics. J Psychosoc Nurs Ment Health Serv 2006;44:13-16.
23. Veenstra DL, Higashi MK, Phillips KA. Assessing the cost effectiveness of pharmacogenics. AAPS PharmSci 2000;2:E29.-
24. Gramling R, Trask P, Nash J, Culpepper L. Family physicians’ beliefs about genetic testing. Fam Med 2004;36:691-692.
25. Caulfield TA. The informed gatekeeper? A commentary on genetic tests, marketing pressure and the role of primary care physicians. Health Law Rev 2000;9:14-17.
26. Mueller C, Feero WG. Clinical genetics. AAFP Home study self assessment Program No 317. Leawood, Kan: AAFP; 2005.
27. CDC Activities page. Programs in Brief: Evaluations of Genomics Applications and Prevention (EGAPP). Available at: www.cdc.gov/genomics/activities/pib/egapp.htm. Accessed on June 27, 2007.
28. Agency for Healthcare Research and Quality. Testing for cytochrome P450 polymorphisms in adults with non-psychotic depression treated with selective serotonin reuptake inhibitors (SSRIs) [AHRQ Publication No. 07-E002]. Available at: www.ahrq.gov/downloads/pub/evidence/pdf/cyp450/cyp450.pdf. Accessed on July 3, 2007.
29. Vermes A, Vermes I. Genetic polymorphisms in cytochrome P450 enzymes. Effect on efficacy and tolerability of HMG-CoA reductase inhibitors. Am J Cardiovasc Drugs 2004;4:247-255.
30. Yasar U, Eliasson E, Dahl ML, Johansson I, Ingelman-Sundberg M, Sjöqvist F. Validation of methods for CYP2C9 genotyping of mutant alleles in a swedish population. Biochem Biophys Res Commun 1999;254:628-631.
31. Aithal GP, Day CP, Kesteven PJL, Daly AK. Association of polymorphisms in the cytochrome p450 CYP2C9 with warfarin dose requirement and risk of bleeding complications. Lancet 1999;353:717-719.
32. Taube J, Halsall D, Baglin T. Influence of cytochrome P450 CYP2C9 polymorphisms on warfarin sensitivity and risk of over anticoagulation in patients on long-term therapy. Blood 2000;96:1816-1819.
33. Reeves MJ, Bohm SR, Korzeniewski SJ, Brown MD. Asthma care and management before an emergency department visit in children in western Michigan: how well does care adhere to guidelines? Pediatrics 2006;117:118-126.
34. Rastogi D, Shetty A, Neugebauer R, Harijith A. National heart, lung, and blood institute guidelines and asthma management practices among innercity pediatric primary care providers. Chest 2006;129:619-623.
35. Bishop PB, Wing PC. Knowledge transfer in family physicians managing patients with acute low back pain: a prospective randomized control trial. Spine J 2006;6:282-288.
36. Bauchner H, Marchant CD, Bisbee A, et al. Effectiveness of centers for disease control and prevention recommendations for outcomes of acute otitis media. Pediatrics 2006;117:1009-1017.
37. Marchant GE, Milligan RJ, Wilhelmi B. Legal pressures and incentives for personalized medicine. Personalized Medicine 2007;3:391-399.
1. McWilliam A, Lutter R, Nardinelli C. Health care savings from personalized medicine using genetic testing: The case of warfarin. [Working Paper 06-23]. November 2006. Available at: www.aei-brookings.org/admin/authorpdfs/page.php?id=1337&PHPsEssID=7b3a3ae4b30d77cb76223e29535e7590. Accessed on June 27, 2007.
2. Tucker G. Pharmacogenetics—expectations and reality. BMJ 2004;329:4-6.
3. Department of Health and Human Services. Realizing the promise of pharmacogenomics: Opportunities and Challenges [Draft report of the Secretary’s Advisory Committee on Genetics, Health, and Society]. Available for public comment March 23–June 1, 2007. Available at: www4.od.nih.gov/oba/sacghs/SACGHS_Pgx_PCdraft.pdf. Accessed on June 27, 2007.
4. Lazarou J, Pomeranz BH, Corey PN. Incidence of adverse drug reactions in hospitalized patients. JAMA 1998;279:1200-1205.
5. Dent R, Clemons M. Adjuvant trastuzumab for breast cancer. Br Med J 2005;331:1035-1036.
6. Wolf CR, Smith G. Pharmacokinetics. Br Med Bull 1999;55:366-386.
7. Lee CR, Goldstein JA, Pieper JA. Cytochrome P450 2C9 polymorphisms: a comprehensive review of the in-vitro and human data. Pharmacogenetics 2002;12:251-263.
8. Bachman K. Genotyping and phenotyping the cytochrome P450 enzymes. Am J Ther 2002;9:309-316.
9. Dahl ML. Cytochrome P450 phenotyping/genotyping in patients receiving antipsychotics. Clin Pharmacokinet 2002;41:453-470.
10. Daly AK, King BP. Pharmacogenetics of oral anticoagulants. Pharmacogenetics 2003;13:247-252.
11. Van Schaik RHN. Implications of cytochrome P450 genetic polymorphisms on the toxicity of antitumor agents. Ther Drug Monit 2004;26:236-240.
12. Zineh I, Gerhard T, Aquilante CL, Beitelshees AL, Beasley BN, Hartzema AG. Availability of pharmacogenomics-based prescribing information in drug package inserts for currently approved drugs. Pharmacogenomics J 2004;4:354-358.
13. Koda Kimball M, ed. Applied Therapeutics. 8th ed. Baltimore, Md: Lippincott, Williams and Wilkins; 2005.
14. Furuya H, Fernandez-Salguero P, Gregory W, et al. Genetic polymorphism of CYP2C9 and its effect on warfarin maintenance dose requirement in patients undergoing anticoagulation therapy. Pharmacogenetics 1995;5:389-392.
15. Arthur H, Dahl ML, Siwers B. Polymorphic drug metabolism in schizophrenic patients with tardive dyskinesia. J Clin Psychopharmacology 1995;15:211-216.
16. Mulder AB, Van Lijf HJ, Bon MAM, et al. Association of polymorphism in the cytochrome CYP2D6 and the efficacy and tolerability of simvastatin. Clin Pharmacol Ther 2001;70:546-551.
17. Takahashi H, Ehizen H. Pharmacogenetics of warfarin elimination and its clinical implications. Clin Pharmakinetics 2001;40:587-603.
18. Caraco J. Genetic determinants of drug responsiveness and drug interactions. Ther Drug Monit 1998;20:517-524.
19. Logue LJ. Genetic testing coverage and reimbursement: a provider’s dilemma. Clin Leadersh Manag Rev 2003;17:346-350.
20. Schwartz MK. Genetic testing and the clinical laboratory improvement amendments of 1988: present and future. Clin Chem 1999;45:739-745.
21. US Food and Drug Administration. Guidance for industry: Pharmacogenomic data submissions 2003. Available at: www.fda.gov/cber/gdlns/pharmdtasub.pdf. Accessed on July 3, 2007.
22. Howard RH. Personalized drug therapy with pharmacogenetics. Part I; pharmacokinetics. J Psychosoc Nurs Ment Health Serv 2006;44:13-16.
23. Veenstra DL, Higashi MK, Phillips KA. Assessing the cost effectiveness of pharmacogenics. AAPS PharmSci 2000;2:E29.-
24. Gramling R, Trask P, Nash J, Culpepper L. Family physicians’ beliefs about genetic testing. Fam Med 2004;36:691-692.
25. Caulfield TA. The informed gatekeeper? A commentary on genetic tests, marketing pressure and the role of primary care physicians. Health Law Rev 2000;9:14-17.
26. Mueller C, Feero WG. Clinical genetics. AAFP Home study self assessment Program No 317. Leawood, Kan: AAFP; 2005.
27. CDC Activities page. Programs in Brief: Evaluations of Genomics Applications and Prevention (EGAPP). Available at: www.cdc.gov/genomics/activities/pib/egapp.htm. Accessed on June 27, 2007.
28. Agency for Healthcare Research and Quality. Testing for cytochrome P450 polymorphisms in adults with non-psychotic depression treated with selective serotonin reuptake inhibitors (SSRIs) [AHRQ Publication No. 07-E002]. Available at: www.ahrq.gov/downloads/pub/evidence/pdf/cyp450/cyp450.pdf. Accessed on July 3, 2007.
29. Vermes A, Vermes I. Genetic polymorphisms in cytochrome P450 enzymes. Effect on efficacy and tolerability of HMG-CoA reductase inhibitors. Am J Cardiovasc Drugs 2004;4:247-255.
30. Yasar U, Eliasson E, Dahl ML, Johansson I, Ingelman-Sundberg M, Sjöqvist F. Validation of methods for CYP2C9 genotyping of mutant alleles in a swedish population. Biochem Biophys Res Commun 1999;254:628-631.
31. Aithal GP, Day CP, Kesteven PJL, Daly AK. Association of polymorphisms in the cytochrome p450 CYP2C9 with warfarin dose requirement and risk of bleeding complications. Lancet 1999;353:717-719.
32. Taube J, Halsall D, Baglin T. Influence of cytochrome P450 CYP2C9 polymorphisms on warfarin sensitivity and risk of over anticoagulation in patients on long-term therapy. Blood 2000;96:1816-1819.
33. Reeves MJ, Bohm SR, Korzeniewski SJ, Brown MD. Asthma care and management before an emergency department visit in children in western Michigan: how well does care adhere to guidelines? Pediatrics 2006;117:118-126.
34. Rastogi D, Shetty A, Neugebauer R, Harijith A. National heart, lung, and blood institute guidelines and asthma management practices among innercity pediatric primary care providers. Chest 2006;129:619-623.
35. Bishop PB, Wing PC. Knowledge transfer in family physicians managing patients with acute low back pain: a prospective randomized control trial. Spine J 2006;6:282-288.
36. Bauchner H, Marchant CD, Bisbee A, et al. Effectiveness of centers for disease control and prevention recommendations for outcomes of acute otitis media. Pediatrics 2006;117:1009-1017.
37. Marchant GE, Milligan RJ, Wilhelmi B. Legal pressures and incentives for personalized medicine. Personalized Medicine 2007;3:391-399.