User login
Practice alert: CDC no longer recommends quinolones for treatment of gonorrhea
- The CDC no longer recommends the use of fluoroquinolones for the treatment of gonococcal infections and associated conditions such as pelvic inflammatory disease (PID).
- Consequently, only one class of drugs, the cephalosporins, is still recommended and available for the treatment of gonorrhea.
- The CDC now recommends ceftriaxone, 125 mg IM, in a single dose, as the preferred treatment.
- For patients with cephalosporin allergies, azithromycin, 2 g orally, as a single dose, remains an option. The CDC discourages widespread use, however, because of concerns about resistance.
The Centers for Disease Control and Prevention (CDC) recently released an update to its treatment guidelines for sexually transmitted diseases, stating that fluoroquinolones are no longer recommended for treatment of gonococcal infections.1 This change resulted from a progressive increase in the rate of resistance to quinolones among gonorrhea isolated from publicly funded treatment centers across the country.
The new advisory applies to all quinolones previously recommended: ciprofloxacin, ofloxacin, and levofloxacin.
Epidemiology. Gonorrhea remains common in the United States, with nearly 340,000 cases reported in 2005. Since it is under-reported, estimates are that more than 600,000 cases occur each year.2
Neisseria gonorrhoeae causes infection of the cervix, urethra, rectum, pharynx, and adnexa. It can also cause disseminated disease that can affect joints, heart, and the meninges.
Tracking the spread of resistant cases
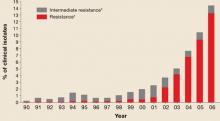
Since the early 1990s, fluoroquinolones have been one of the recommended treatments for gonorrhea because of their availability as effective, single-dose oral regimens. Fluoroquinolone-resistant N gonorrhea began to emerge at the end of the century and has progressed rapidly since. FIGURE 1 illustrates the proportion of fluoroquinolone-resistant N gonorrhea from the CDC’s Gonococcal Isolate Surveillance Project (GISP) by year, from 1990 to 2006.
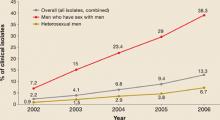
Resistance began to emerge first among gonorrhea isolates from men who have sex with men (MSM), and resistance rates among MSM continue to be higher than in heterosexual men (FIGURE 2).
Geographic trends. In 2000, the CDC recommended that quinolones should no longer be used to treat gonorrhea in persons who contracted the infection in Asia or the Pacific. In 2002, California was added to this list. In 2004, the recommendation against quinolone use was extended to all MSM in the US.
The new recommendation against general use is based on resistance surpassing 5% of total isolates.
FIGURE 1
Percentage of N gonorrhoeae isolates with intermediate resistance or resistance to ciprofloxacin
Data for 2006 are preliminary (January-June only).
* Demonstrating ciprofloxacin minimum inhibitory concentration of 0.125–0.500 mcg/mL.
† Demonstrating ciprofloxacin minimum inhibitory concentration of ≥1.0 mcg/ml.
Source: Update to CDC’s sexually transmitted diseases treatment guidelines, 2006: Fluoroquinalones no longer recommended for treatment of gonococcal infections.
MMWR Recomm Rep 2007; 56:332-336.
FIGURE 2
Progressive increase of fluoroquinolone resistance
Percent of isolates from the CDC Gonococcal Isolate Surveillance Project found to be resistant to fluoroquinalones, 2002 through June 2006
Source: GISP report. Centers for Disease Control and Prevention.
Sexually Transmitted Disease Surveillance 2005 Supplement, Gonoccal Isolate Surveillance Project (GISP) Annual Report 2005. Atlanta, Ga: US Department of Health and Human Services, Centers for Disease Control and Prevention, January 2007.
Ceftriaxone, the default treatment of choice
The loss of quinolones as a recommended gonorrhea treatment leaves only ceftriaxone, 125 mg intramuscularly (IM), as the only readily available treatment for urogenital, anorectal, and pharyngeal gonorrhea. Cefixime 400 mg as a single dose is also recommended, but is not currently available in tablet form in the US. It is available as a suspension with 100 mg per 5 cc.
Other options
Possible oral options include cefpodoxime 400 mg or cefuroxime axetil 1 g. However, neither has the official endorsement of the CDC, and neither appears effective against pharyngeal infection.
Spectinomycin 2 g intramuscularly is recommended for those with cephalosporin allergy—but, like cefixime, it is not currently available in the US, and it also is not considered effective against pharyngeal infection.
Azithromycin 2 g orally as a single dose is currently effective against gonorrhea and is an option for those with cephalosporin allergies. The CDC discourages its widespread use because of concerns about resistance.
New information regarding the availability of spectinomycin and cefixime can be obtained from local health departments or the CDC’s sexually transmitted diseases web site (www.cdc.gov/std).3
Recommended regimens for treatment of gonorrhea
| Uncomplicated gonococcal infections of the cervix, urethra, and rectum* |
| Recommended regimens† |
| Ceftriaxone 125 mg in a single IM dose |
| or |
| Cefixime‡ 400 mg in a single oral dose |
| plus |
| Treatment for chlamydia if chlamydial infection has not been ruled out |
| Uncomplicated gonococcal infections of the pharynx* |
| Recommended regimens |
| Ceftriaxone 125 mg in a single IM dose |
| plus |
| Treatment for chlamydia if chlamydial infection has not been ruled out |
| * For all adult and adolescent patients, regardless of travel history or sexual behavior. For those allergic to penicillins or cephalosporins, or for treatment of disseminated gonococcal infections, PID, and epididymitis, see www.cdc.gov/std/treatment. |
| † Alternative regimens: Spectinomycin 2 g in a single IM dose (not currently available in US) or cephalosporin single-dose regimens. |
| Other single-dose cephalosporin regimens that are considered alternative treatment regimens against uncomplicated urogenital and anorectal gonococcal infections include ceftizoxime 500 mg IM; or cefoxitin 2 g IM, administered with probenecid 1 g orally; or cefotaxime 500 mg IM. Some evidence indicates that cefpodoxime 400 mg and cefuroxime axetil 1 g might be oral alternatives. |
| ‡ 400 mg by suspension; tablets are no longer available in the US. |
| Source: www.cdc.gov/mmwr/PDF/rr/rr5511.pdf.2 |
Associated conditions
Treat for chlamydia if chlamydial infection is not ruled out
The CDC continues to recommend concurrent treatment for chlamydia for all persons who have gonorrhea, unless coinfection has been ruled out.
Therapies for chlamydia include azithromycin 1 g as a single dose or doxycycline 100 mg twice a day for 7 days.
Pelvic inflammatory disease and epididymitis
The treatment of both pelvic inflammatory disease (PID) and epididymitis include an option of ceftriaxone 250 mg IM plus doxycycline for either 7 days (for epididymitis) or 10 days (for PID). There are several parenteral options for PID and disseminated gonorrhea; these can be found on the CDC’s STD web site.3
Should you always retest to ensure a cure?
It is still not necessary to retest patients who have had the recommended treatments. However, patients with persistent symptoms or rapidly recurring symptoms should be retested by cultures so that drug-resistance patterns can be checked if gonorrhea is documented.
Retest for recurrence
Consider retesting all treated patients after 3 to 6 months, since anyone with a sexually transmitted infection is at risk of being reinfected.
Summary
The ongoing challenges with the evolving resistance patterns of gonorrhea illustrate the importance of physicians accurately diagnosing gonorrhea, treating with recommended regimens, reporting positive cases to the local public health department, and assisting with partner evaluation and treatment.
1. CDC. Update to CDC’s sexually transmitted diseases treatment guidelines, 2006: Fluoroquinolones no longer recommended for treatment of gonococcal infections. MMWR Morb Mortal Wkly Rep 2007;56:332-336.Available at: www.cdc.gov/mmwr/pdf/wk/mm5614.pdf. Accessed on June 15, 2007.
2. CDC. Sexually transmitted diseases treatment guidelines, 2006. MMWR Recomm Rep 2006;55(RR-11).-Available at www.cdc.gov/mmwr/PDF/rr/rr5511.pdf. Accessed on June 15, 2007.
3. Updated recommended treatment regimens for gonococcal infections and associated conditions—United States, April 2007. Available at: www.cdc.gov/std/treatment/2006/updated-regimens.htm. Accessed on June 15, 2007.
- The CDC no longer recommends the use of fluoroquinolones for the treatment of gonococcal infections and associated conditions such as pelvic inflammatory disease (PID).
- Consequently, only one class of drugs, the cephalosporins, is still recommended and available for the treatment of gonorrhea.
- The CDC now recommends ceftriaxone, 125 mg IM, in a single dose, as the preferred treatment.
- For patients with cephalosporin allergies, azithromycin, 2 g orally, as a single dose, remains an option. The CDC discourages widespread use, however, because of concerns about resistance.
The Centers for Disease Control and Prevention (CDC) recently released an update to its treatment guidelines for sexually transmitted diseases, stating that fluoroquinolones are no longer recommended for treatment of gonococcal infections.1 This change resulted from a progressive increase in the rate of resistance to quinolones among gonorrhea isolated from publicly funded treatment centers across the country.
The new advisory applies to all quinolones previously recommended: ciprofloxacin, ofloxacin, and levofloxacin.
Epidemiology. Gonorrhea remains common in the United States, with nearly 340,000 cases reported in 2005. Since it is under-reported, estimates are that more than 600,000 cases occur each year.2
Neisseria gonorrhoeae causes infection of the cervix, urethra, rectum, pharynx, and adnexa. It can also cause disseminated disease that can affect joints, heart, and the meninges.
Tracking the spread of resistant cases
Since the early 1990s, fluoroquinolones have been one of the recommended treatments for gonorrhea because of their availability as effective, single-dose oral regimens. Fluoroquinolone-resistant N gonorrhea began to emerge at the end of the century and has progressed rapidly since. FIGURE 1 illustrates the proportion of fluoroquinolone-resistant N gonorrhea from the CDC’s Gonococcal Isolate Surveillance Project (GISP) by year, from 1990 to 2006.
Resistance began to emerge first among gonorrhea isolates from men who have sex with men (MSM), and resistance rates among MSM continue to be higher than in heterosexual men (FIGURE 2).
Geographic trends. In 2000, the CDC recommended that quinolones should no longer be used to treat gonorrhea in persons who contracted the infection in Asia or the Pacific. In 2002, California was added to this list. In 2004, the recommendation against quinolone use was extended to all MSM in the US.
The new recommendation against general use is based on resistance surpassing 5% of total isolates.
FIGURE 1
Percentage of N gonorrhoeae isolates with intermediate resistance or resistance to ciprofloxacin
Data for 2006 are preliminary (January-June only).
* Demonstrating ciprofloxacin minimum inhibitory concentration of 0.125–0.500 mcg/mL.
† Demonstrating ciprofloxacin minimum inhibitory concentration of ≥1.0 mcg/ml.
Source: Update to CDC’s sexually transmitted diseases treatment guidelines, 2006: Fluoroquinalones no longer recommended for treatment of gonococcal infections.
MMWR Recomm Rep 2007; 56:332-336.
FIGURE 2
Progressive increase of fluoroquinolone resistance
Percent of isolates from the CDC Gonococcal Isolate Surveillance Project found to be resistant to fluoroquinalones, 2002 through June 2006
Source: GISP report. Centers for Disease Control and Prevention.
Sexually Transmitted Disease Surveillance 2005 Supplement, Gonoccal Isolate Surveillance Project (GISP) Annual Report 2005. Atlanta, Ga: US Department of Health and Human Services, Centers for Disease Control and Prevention, January 2007.
Ceftriaxone, the default treatment of choice
The loss of quinolones as a recommended gonorrhea treatment leaves only ceftriaxone, 125 mg intramuscularly (IM), as the only readily available treatment for urogenital, anorectal, and pharyngeal gonorrhea. Cefixime 400 mg as a single dose is also recommended, but is not currently available in tablet form in the US. It is available as a suspension with 100 mg per 5 cc.
Other options
Possible oral options include cefpodoxime 400 mg or cefuroxime axetil 1 g. However, neither has the official endorsement of the CDC, and neither appears effective against pharyngeal infection.
Spectinomycin 2 g intramuscularly is recommended for those with cephalosporin allergy—but, like cefixime, it is not currently available in the US, and it also is not considered effective against pharyngeal infection.
Azithromycin 2 g orally as a single dose is currently effective against gonorrhea and is an option for those with cephalosporin allergies. The CDC discourages its widespread use because of concerns about resistance.
New information regarding the availability of spectinomycin and cefixime can be obtained from local health departments or the CDC’s sexually transmitted diseases web site (www.cdc.gov/std).3
Recommended regimens for treatment of gonorrhea
| Uncomplicated gonococcal infections of the cervix, urethra, and rectum* |
| Recommended regimens† |
| Ceftriaxone 125 mg in a single IM dose |
| or |
| Cefixime‡ 400 mg in a single oral dose |
| plus |
| Treatment for chlamydia if chlamydial infection has not been ruled out |
| Uncomplicated gonococcal infections of the pharynx* |
| Recommended regimens |
| Ceftriaxone 125 mg in a single IM dose |
| plus |
| Treatment for chlamydia if chlamydial infection has not been ruled out |
| * For all adult and adolescent patients, regardless of travel history or sexual behavior. For those allergic to penicillins or cephalosporins, or for treatment of disseminated gonococcal infections, PID, and epididymitis, see www.cdc.gov/std/treatment. |
| † Alternative regimens: Spectinomycin 2 g in a single IM dose (not currently available in US) or cephalosporin single-dose regimens. |
| Other single-dose cephalosporin regimens that are considered alternative treatment regimens against uncomplicated urogenital and anorectal gonococcal infections include ceftizoxime 500 mg IM; or cefoxitin 2 g IM, administered with probenecid 1 g orally; or cefotaxime 500 mg IM. Some evidence indicates that cefpodoxime 400 mg and cefuroxime axetil 1 g might be oral alternatives. |
| ‡ 400 mg by suspension; tablets are no longer available in the US. |
| Source: www.cdc.gov/mmwr/PDF/rr/rr5511.pdf.2 |
Associated conditions
Treat for chlamydia if chlamydial infection is not ruled out
The CDC continues to recommend concurrent treatment for chlamydia for all persons who have gonorrhea, unless coinfection has been ruled out.
Therapies for chlamydia include azithromycin 1 g as a single dose or doxycycline 100 mg twice a day for 7 days.
Pelvic inflammatory disease and epididymitis
The treatment of both pelvic inflammatory disease (PID) and epididymitis include an option of ceftriaxone 250 mg IM plus doxycycline for either 7 days (for epididymitis) or 10 days (for PID). There are several parenteral options for PID and disseminated gonorrhea; these can be found on the CDC’s STD web site.3
Should you always retest to ensure a cure?
It is still not necessary to retest patients who have had the recommended treatments. However, patients with persistent symptoms or rapidly recurring symptoms should be retested by cultures so that drug-resistance patterns can be checked if gonorrhea is documented.
Retest for recurrence
Consider retesting all treated patients after 3 to 6 months, since anyone with a sexually transmitted infection is at risk of being reinfected.
Summary
The ongoing challenges with the evolving resistance patterns of gonorrhea illustrate the importance of physicians accurately diagnosing gonorrhea, treating with recommended regimens, reporting positive cases to the local public health department, and assisting with partner evaluation and treatment.
- The CDC no longer recommends the use of fluoroquinolones for the treatment of gonococcal infections and associated conditions such as pelvic inflammatory disease (PID).
- Consequently, only one class of drugs, the cephalosporins, is still recommended and available for the treatment of gonorrhea.
- The CDC now recommends ceftriaxone, 125 mg IM, in a single dose, as the preferred treatment.
- For patients with cephalosporin allergies, azithromycin, 2 g orally, as a single dose, remains an option. The CDC discourages widespread use, however, because of concerns about resistance.
The Centers for Disease Control and Prevention (CDC) recently released an update to its treatment guidelines for sexually transmitted diseases, stating that fluoroquinolones are no longer recommended for treatment of gonococcal infections.1 This change resulted from a progressive increase in the rate of resistance to quinolones among gonorrhea isolated from publicly funded treatment centers across the country.
The new advisory applies to all quinolones previously recommended: ciprofloxacin, ofloxacin, and levofloxacin.
Epidemiology. Gonorrhea remains common in the United States, with nearly 340,000 cases reported in 2005. Since it is under-reported, estimates are that more than 600,000 cases occur each year.2
Neisseria gonorrhoeae causes infection of the cervix, urethra, rectum, pharynx, and adnexa. It can also cause disseminated disease that can affect joints, heart, and the meninges.
Tracking the spread of resistant cases
Since the early 1990s, fluoroquinolones have been one of the recommended treatments for gonorrhea because of their availability as effective, single-dose oral regimens. Fluoroquinolone-resistant N gonorrhea began to emerge at the end of the century and has progressed rapidly since. FIGURE 1 illustrates the proportion of fluoroquinolone-resistant N gonorrhea from the CDC’s Gonococcal Isolate Surveillance Project (GISP) by year, from 1990 to 2006.
Resistance began to emerge first among gonorrhea isolates from men who have sex with men (MSM), and resistance rates among MSM continue to be higher than in heterosexual men (FIGURE 2).
Geographic trends. In 2000, the CDC recommended that quinolones should no longer be used to treat gonorrhea in persons who contracted the infection in Asia or the Pacific. In 2002, California was added to this list. In 2004, the recommendation against quinolone use was extended to all MSM in the US.
The new recommendation against general use is based on resistance surpassing 5% of total isolates.
FIGURE 1
Percentage of N gonorrhoeae isolates with intermediate resistance or resistance to ciprofloxacin
Data for 2006 are preliminary (January-June only).
* Demonstrating ciprofloxacin minimum inhibitory concentration of 0.125–0.500 mcg/mL.
† Demonstrating ciprofloxacin minimum inhibitory concentration of ≥1.0 mcg/ml.
Source: Update to CDC’s sexually transmitted diseases treatment guidelines, 2006: Fluoroquinalones no longer recommended for treatment of gonococcal infections.
MMWR Recomm Rep 2007; 56:332-336.
FIGURE 2
Progressive increase of fluoroquinolone resistance
Percent of isolates from the CDC Gonococcal Isolate Surveillance Project found to be resistant to fluoroquinalones, 2002 through June 2006
Source: GISP report. Centers for Disease Control and Prevention.
Sexually Transmitted Disease Surveillance 2005 Supplement, Gonoccal Isolate Surveillance Project (GISP) Annual Report 2005. Atlanta, Ga: US Department of Health and Human Services, Centers for Disease Control and Prevention, January 2007.
Ceftriaxone, the default treatment of choice
The loss of quinolones as a recommended gonorrhea treatment leaves only ceftriaxone, 125 mg intramuscularly (IM), as the only readily available treatment for urogenital, anorectal, and pharyngeal gonorrhea. Cefixime 400 mg as a single dose is also recommended, but is not currently available in tablet form in the US. It is available as a suspension with 100 mg per 5 cc.
Other options
Possible oral options include cefpodoxime 400 mg or cefuroxime axetil 1 g. However, neither has the official endorsement of the CDC, and neither appears effective against pharyngeal infection.
Spectinomycin 2 g intramuscularly is recommended for those with cephalosporin allergy—but, like cefixime, it is not currently available in the US, and it also is not considered effective against pharyngeal infection.
Azithromycin 2 g orally as a single dose is currently effective against gonorrhea and is an option for those with cephalosporin allergies. The CDC discourages its widespread use because of concerns about resistance.
New information regarding the availability of spectinomycin and cefixime can be obtained from local health departments or the CDC’s sexually transmitted diseases web site (www.cdc.gov/std).3
Recommended regimens for treatment of gonorrhea
| Uncomplicated gonococcal infections of the cervix, urethra, and rectum* |
| Recommended regimens† |
| Ceftriaxone 125 mg in a single IM dose |
| or |
| Cefixime‡ 400 mg in a single oral dose |
| plus |
| Treatment for chlamydia if chlamydial infection has not been ruled out |
| Uncomplicated gonococcal infections of the pharynx* |
| Recommended regimens |
| Ceftriaxone 125 mg in a single IM dose |
| plus |
| Treatment for chlamydia if chlamydial infection has not been ruled out |
| * For all adult and adolescent patients, regardless of travel history or sexual behavior. For those allergic to penicillins or cephalosporins, or for treatment of disseminated gonococcal infections, PID, and epididymitis, see www.cdc.gov/std/treatment. |
| † Alternative regimens: Spectinomycin 2 g in a single IM dose (not currently available in US) or cephalosporin single-dose regimens. |
| Other single-dose cephalosporin regimens that are considered alternative treatment regimens against uncomplicated urogenital and anorectal gonococcal infections include ceftizoxime 500 mg IM; or cefoxitin 2 g IM, administered with probenecid 1 g orally; or cefotaxime 500 mg IM. Some evidence indicates that cefpodoxime 400 mg and cefuroxime axetil 1 g might be oral alternatives. |
| ‡ 400 mg by suspension; tablets are no longer available in the US. |
| Source: www.cdc.gov/mmwr/PDF/rr/rr5511.pdf.2 |
Associated conditions
Treat for chlamydia if chlamydial infection is not ruled out
The CDC continues to recommend concurrent treatment for chlamydia for all persons who have gonorrhea, unless coinfection has been ruled out.
Therapies for chlamydia include azithromycin 1 g as a single dose or doxycycline 100 mg twice a day for 7 days.
Pelvic inflammatory disease and epididymitis
The treatment of both pelvic inflammatory disease (PID) and epididymitis include an option of ceftriaxone 250 mg IM plus doxycycline for either 7 days (for epididymitis) or 10 days (for PID). There are several parenteral options for PID and disseminated gonorrhea; these can be found on the CDC’s STD web site.3
Should you always retest to ensure a cure?
It is still not necessary to retest patients who have had the recommended treatments. However, patients with persistent symptoms or rapidly recurring symptoms should be retested by cultures so that drug-resistance patterns can be checked if gonorrhea is documented.
Retest for recurrence
Consider retesting all treated patients after 3 to 6 months, since anyone with a sexually transmitted infection is at risk of being reinfected.
Summary
The ongoing challenges with the evolving resistance patterns of gonorrhea illustrate the importance of physicians accurately diagnosing gonorrhea, treating with recommended regimens, reporting positive cases to the local public health department, and assisting with partner evaluation and treatment.
1. CDC. Update to CDC’s sexually transmitted diseases treatment guidelines, 2006: Fluoroquinolones no longer recommended for treatment of gonococcal infections. MMWR Morb Mortal Wkly Rep 2007;56:332-336.Available at: www.cdc.gov/mmwr/pdf/wk/mm5614.pdf. Accessed on June 15, 2007.
2. CDC. Sexually transmitted diseases treatment guidelines, 2006. MMWR Recomm Rep 2006;55(RR-11).-Available at www.cdc.gov/mmwr/PDF/rr/rr5511.pdf. Accessed on June 15, 2007.
3. Updated recommended treatment regimens for gonococcal infections and associated conditions—United States, April 2007. Available at: www.cdc.gov/std/treatment/2006/updated-regimens.htm. Accessed on June 15, 2007.
1. CDC. Update to CDC’s sexually transmitted diseases treatment guidelines, 2006: Fluoroquinolones no longer recommended for treatment of gonococcal infections. MMWR Morb Mortal Wkly Rep 2007;56:332-336.Available at: www.cdc.gov/mmwr/pdf/wk/mm5614.pdf. Accessed on June 15, 2007.
2. CDC. Sexually transmitted diseases treatment guidelines, 2006. MMWR Recomm Rep 2006;55(RR-11).-Available at www.cdc.gov/mmwr/PDF/rr/rr5511.pdf. Accessed on June 15, 2007.
3. Updated recommended treatment regimens for gonococcal infections and associated conditions—United States, April 2007. Available at: www.cdc.gov/std/treatment/2006/updated-regimens.htm. Accessed on June 15, 2007.
Screening: New guidance on what and what not to do
One of our key responsibilities is to provide effective preventive services—and avoid performing tests of no value. Since most of us do not have time to keep up with the literature on what services and tests have and have not been proven effective, we depend on trusted authorities to make these assessments for us.
The entity with the most rigorously evidence-based approach is the United States Preventive Services Task Force (USPSTF). (TABLE 1 lists the criteria for their recommendations.) Every year, this Practice Alert summarizes the new recommendations from the task force. The new recommendations in the 6 disease categories discussed here were published by the USPSTF in 2006 and the first quarter of 2007 (TABLE 2).
- Iron deficiency anemia
- Colon cancer chemoprevention
- Genetic screening for hemochromatosis
- Congenital hip dysplasia
- Elevated lead levels
- Speech delay
TABLE 1
The rigor behind the recommendations
| RECOMMENDATION | EVIDENCE | RESULTS OF THE SERVICE |
|---|---|---|
| A Strongly recommends | Good evidence |
|
| B Recommends | At least fair evidence |
|
| C No recommendation | At least fair evidence |
|
| D Recommends against | At least fair evidence |
|
| I Insufficient evidence | Insufficient to recommend for or against |
|
TABLE 2
Summary of new USPSTF recommendations
| B RECOMMENDATIONS |
The USPSTF recommends routine:
|
| D RECOMMENDATIONS |
The USPSTF recommends against routine:
|
| I RECOMMENDATIONS |
The USPSTF concludes that evidence is insufficient to recommend for or against routine:
|
“I” means insufficient evidence
As usual, there are many screening tests that lack evidence either for or against their effectiveness. The Task Force places such tests in the “I” (insufficient evidence) category. Physicians should remember that an “I” recommendation is not the equivalent of a “D” (recommend against).
Screening implies routine testing, and no symptoms
We also need to keep in mind the difference between screening and diagnosis. Screening implies routine testing among asymptomatic patients. Screening recommendations do not apply to symptomatic patients in whom diagnostic testing may be indicated.
1. Iron deficiency anemia
The task force recommends
- routine screening for iron deficiency anemia in asymptomatic pregnant women, and
- iron supplementation for asymptomatic children ages 6 to 12 months who are at increased risk for iron deficiency anemia.1
The task force concludes that the evidence is insufficient to recommend for or against
- routine screening for iron deficiency anemia in asymptomatic children ages 6 to 12 months,
- iron supplementation for asymptomatic children ages 6 to 12 months who are at average risk for iron deficiency anemia,
- iron supplementation for nonanemic pregnant women.1
Iron deficiency anemia is linked to developmental and cognitive abnormalities in children and poorer birth outcomes in pregnant women. The task force felt that the weight of the evidence supports a set of recommendations that includes screening all pregnant women and using iron preparations for those who have deficiency, and using routine iron supplementation for at-risk infants between the ages of 6 and 12 months.
The lack of a recommendation on screening all children was based on concern about the accuracy of hemoglobin as a screening test for iron deficiency and a scarcity of evidence that universal screening results in improved outcomes. Routine iron supplementation was felt to be of proven benefit only for infants at increased risk: those from low socioeconomic backgrounds and premature and low birth weight infants.
The Centers for Disease Control and Prevention (CDC) agrees that screening should be performed in high-risk infants and all pregnant women, but recommends universal iron supplementation during pregnancy.
The American Academy of Pediatrics recommends screening all infants twice (at age 9 to 12 months, and 6 months later) along with dietary interventions to prevent iron deficiency, such as: breast-feeding or the use of iron-fortified formula, and the introduction of iron-rich foods at age 6 months.
2. Colon cancer
The task force recommends against routine use of aspirin and NSAIDs to prevent colorectal cancer in individuals at average risk for colorectal cancer.2
Colon cancer is common and a common cause of cancer mortality,3 and proven secondary preventions are available. Chemoprevention is a potential method of primary prevention; it has some benefits as well as harms. The task force concluded that the documented harms exceed the potential benefits.
- Aspirin taken at the high-dose regimen (350–700 mg/day) needed to protect from colon cancer increases the risks for gastrointestinal bleeding and hemorrhagic stroke. A lower dose of aspirin (75–350 mg/day) is used for chemoprevention in adults who are at increased risk for coronary heart disease but this does not protect against colon cancer.
- Nonsteroidal anti-inflammatory drugs (NSAIDs) may reduce the risk of colorectal cancer, but they also increase the risks of gastrointestinal bleeding and renal injury.
- Cyclooxygenase-2 inhibitors have been linked to increases in coronary artery disease.
3. Hemochromatosis
The task force recommends against routine genetic screening for hereditary hemochromatosis in the asymptomatic general population.4
Hemochromatosis is rare, and only a small proportion of those with the high-risk genotype actually develop the disease. The effectiveness of early intervention is unproven, and the potential for harm from false positives is significant.
The D recommendation does not apply to those with signs and symptoms consistent with hemochromatosis or a strong family history of the disease. Nor does it pertain to non-genetic laboratory tests to identify iron overload (although these also lack proof that they improve outcomes in the general population).
4. Congenital hip dysplasia
The task force concludes that evidence is insufficient to recommend routine screening for developmental dysplasia of the hip in infants as a means to prevent adverse outcomes.5
Physical examination and ultrasonography have limited accuracy in finding hip dysplasia, and there is a high rate of natural resolution (60% to 90%) of hip abnormalities found with these tests. Both surgical and non-surgical treatments lack evidence of effectiveness and are associated with potential for harm from avascular necrosis, high costs, and complications from surgery and anesthesia.
This uncertainty applies only to asymptomatic infants—not to those who have obvious hip dislocations or other hip abnormalities.
5. Elevated lead levels
The task force concludes that evidence is insufficient to recommend for or against routine screening for elevated blood lead levels in asymptomatic children ages 1 to 5 who are at increased risk.6
The task force recommends against routine screening for elevated blood lead levels in:
- asymptomatic children ages 1 to 5 years who are at average risk
- asymptomatic pregnant women.6
The reduction of lead in the environment, especially the reduction of leadbased gasoline, has resulted in a decline in elevated blood lead levels in the United States. The task force’s uncertainty regarding screening at-risk children centered around a lack of evidence of the effectiveness of interventions in decreasing blood lead levels. Other organizations that continue to recommend screening in high-risk children include the CDC and the American Academy of Pediatrics. The main risk factor for elevated blood lead levels is living in housing constructed before 1950.
The recommendation against screening in pregnant women was based on the low prevalence, no evidence for effectiveness of interventions to decrease lead levels, and potential harms from screening. This recommendation agrees with those of other organizations.
Speech delay
The task force concludes that evidence is insufficient to recommend for or against routine use of brief, formal screening instruments in primary care to detect speech and language delay in children up to 5 years of age.7
While speech delay affects 5% to 8% of children under the age of 5, and interventions can result in short-term improvements, long-term benefits have not been studied. It is also unclear whether the brief screening tools used in primary care accurately identify children who will benefit from interventions, or whether the results of early intervention are better than when difficulties are first identified by parents. Overall, the task force felt that we lack sufficient evidence to evaluate overall benefits and harms of brief formal screening tools in primary care among asymptomatic children.
Correspondence
Doug Campos-Outcalt, MD, MPA, 4001 N Third Street #415, Phoenix, AZ 85012; dougco@u.arizona.edu.
1. USPSTF. Screening for iron deficiency anemia—including iron supplementation for children and pregnant women. Available at: www.ahrq.gov/clinic/uspstf/uspsiron.htm. Accessed on May 17, 2007.
2. USPSTF. Routine aspirin and nonsteroidal anti-inflammatory drugs for the primary prevention of colorectal cancer. Available at: www.ahrq.gov/clinic/uspstf/uspsasco.htm. Accessed on May 17, 2007.
3. USPSTF. Screening for colorectal cancer. Available at: www.ahrq.gov/clinic/uspstf/uspscolo.htm. Accessed on May 17, 2007.
4. USPSTF. Screening for hemochromatosis. Available at: www.ahrq.gov/clinic/uspstf/uspshemoch.htm. Accessed on May 17, 2007.
5. USPSTF. Screening for developmental dysplasia of the hip. Available at: www.ahrq.gov/clinic/uspstf/uspshipd.htm. Accessed on May 17, 2007.
6. USPSTF. Screening for elevated blood lead levels in childhood and pregnant women. Available at: www.ahrq.gov/clinic/uspstf/uspslead.htm. Accessed on May 17, 2007.
7. USPSTF. Screening for speech and language delay in preschool children. Available at: www.ahrq.gov/clinic/uspstf/uspschdv.htm. Accessed on May 17, 2007.
One of our key responsibilities is to provide effective preventive services—and avoid performing tests of no value. Since most of us do not have time to keep up with the literature on what services and tests have and have not been proven effective, we depend on trusted authorities to make these assessments for us.
The entity with the most rigorously evidence-based approach is the United States Preventive Services Task Force (USPSTF). (TABLE 1 lists the criteria for their recommendations.) Every year, this Practice Alert summarizes the new recommendations from the task force. The new recommendations in the 6 disease categories discussed here were published by the USPSTF in 2006 and the first quarter of 2007 (TABLE 2).
- Iron deficiency anemia
- Colon cancer chemoprevention
- Genetic screening for hemochromatosis
- Congenital hip dysplasia
- Elevated lead levels
- Speech delay
TABLE 1
The rigor behind the recommendations
| RECOMMENDATION | EVIDENCE | RESULTS OF THE SERVICE |
|---|---|---|
| A Strongly recommends | Good evidence |
|
| B Recommends | At least fair evidence |
|
| C No recommendation | At least fair evidence |
|
| D Recommends against | At least fair evidence |
|
| I Insufficient evidence | Insufficient to recommend for or against |
|
TABLE 2
Summary of new USPSTF recommendations
| B RECOMMENDATIONS |
The USPSTF recommends routine:
|
| D RECOMMENDATIONS |
The USPSTF recommends against routine:
|
| I RECOMMENDATIONS |
The USPSTF concludes that evidence is insufficient to recommend for or against routine:
|
“I” means insufficient evidence
As usual, there are many screening tests that lack evidence either for or against their effectiveness. The Task Force places such tests in the “I” (insufficient evidence) category. Physicians should remember that an “I” recommendation is not the equivalent of a “D” (recommend against).
Screening implies routine testing, and no symptoms
We also need to keep in mind the difference between screening and diagnosis. Screening implies routine testing among asymptomatic patients. Screening recommendations do not apply to symptomatic patients in whom diagnostic testing may be indicated.
1. Iron deficiency anemia
The task force recommends
- routine screening for iron deficiency anemia in asymptomatic pregnant women, and
- iron supplementation for asymptomatic children ages 6 to 12 months who are at increased risk for iron deficiency anemia.1
The task force concludes that the evidence is insufficient to recommend for or against
- routine screening for iron deficiency anemia in asymptomatic children ages 6 to 12 months,
- iron supplementation for asymptomatic children ages 6 to 12 months who are at average risk for iron deficiency anemia,
- iron supplementation for nonanemic pregnant women.1
Iron deficiency anemia is linked to developmental and cognitive abnormalities in children and poorer birth outcomes in pregnant women. The task force felt that the weight of the evidence supports a set of recommendations that includes screening all pregnant women and using iron preparations for those who have deficiency, and using routine iron supplementation for at-risk infants between the ages of 6 and 12 months.
The lack of a recommendation on screening all children was based on concern about the accuracy of hemoglobin as a screening test for iron deficiency and a scarcity of evidence that universal screening results in improved outcomes. Routine iron supplementation was felt to be of proven benefit only for infants at increased risk: those from low socioeconomic backgrounds and premature and low birth weight infants.
The Centers for Disease Control and Prevention (CDC) agrees that screening should be performed in high-risk infants and all pregnant women, but recommends universal iron supplementation during pregnancy.
The American Academy of Pediatrics recommends screening all infants twice (at age 9 to 12 months, and 6 months later) along with dietary interventions to prevent iron deficiency, such as: breast-feeding or the use of iron-fortified formula, and the introduction of iron-rich foods at age 6 months.
2. Colon cancer
The task force recommends against routine use of aspirin and NSAIDs to prevent colorectal cancer in individuals at average risk for colorectal cancer.2
Colon cancer is common and a common cause of cancer mortality,3 and proven secondary preventions are available. Chemoprevention is a potential method of primary prevention; it has some benefits as well as harms. The task force concluded that the documented harms exceed the potential benefits.
- Aspirin taken at the high-dose regimen (350–700 mg/day) needed to protect from colon cancer increases the risks for gastrointestinal bleeding and hemorrhagic stroke. A lower dose of aspirin (75–350 mg/day) is used for chemoprevention in adults who are at increased risk for coronary heart disease but this does not protect against colon cancer.
- Nonsteroidal anti-inflammatory drugs (NSAIDs) may reduce the risk of colorectal cancer, but they also increase the risks of gastrointestinal bleeding and renal injury.
- Cyclooxygenase-2 inhibitors have been linked to increases in coronary artery disease.
3. Hemochromatosis
The task force recommends against routine genetic screening for hereditary hemochromatosis in the asymptomatic general population.4
Hemochromatosis is rare, and only a small proportion of those with the high-risk genotype actually develop the disease. The effectiveness of early intervention is unproven, and the potential for harm from false positives is significant.
The D recommendation does not apply to those with signs and symptoms consistent with hemochromatosis or a strong family history of the disease. Nor does it pertain to non-genetic laboratory tests to identify iron overload (although these also lack proof that they improve outcomes in the general population).
4. Congenital hip dysplasia
The task force concludes that evidence is insufficient to recommend routine screening for developmental dysplasia of the hip in infants as a means to prevent adverse outcomes.5
Physical examination and ultrasonography have limited accuracy in finding hip dysplasia, and there is a high rate of natural resolution (60% to 90%) of hip abnormalities found with these tests. Both surgical and non-surgical treatments lack evidence of effectiveness and are associated with potential for harm from avascular necrosis, high costs, and complications from surgery and anesthesia.
This uncertainty applies only to asymptomatic infants—not to those who have obvious hip dislocations or other hip abnormalities.
5. Elevated lead levels
The task force concludes that evidence is insufficient to recommend for or against routine screening for elevated blood lead levels in asymptomatic children ages 1 to 5 who are at increased risk.6
The task force recommends against routine screening for elevated blood lead levels in:
- asymptomatic children ages 1 to 5 years who are at average risk
- asymptomatic pregnant women.6
The reduction of lead in the environment, especially the reduction of leadbased gasoline, has resulted in a decline in elevated blood lead levels in the United States. The task force’s uncertainty regarding screening at-risk children centered around a lack of evidence of the effectiveness of interventions in decreasing blood lead levels. Other organizations that continue to recommend screening in high-risk children include the CDC and the American Academy of Pediatrics. The main risk factor for elevated blood lead levels is living in housing constructed before 1950.
The recommendation against screening in pregnant women was based on the low prevalence, no evidence for effectiveness of interventions to decrease lead levels, and potential harms from screening. This recommendation agrees with those of other organizations.
Speech delay
The task force concludes that evidence is insufficient to recommend for or against routine use of brief, formal screening instruments in primary care to detect speech and language delay in children up to 5 years of age.7
While speech delay affects 5% to 8% of children under the age of 5, and interventions can result in short-term improvements, long-term benefits have not been studied. It is also unclear whether the brief screening tools used in primary care accurately identify children who will benefit from interventions, or whether the results of early intervention are better than when difficulties are first identified by parents. Overall, the task force felt that we lack sufficient evidence to evaluate overall benefits and harms of brief formal screening tools in primary care among asymptomatic children.
Correspondence
Doug Campos-Outcalt, MD, MPA, 4001 N Third Street #415, Phoenix, AZ 85012; dougco@u.arizona.edu.
One of our key responsibilities is to provide effective preventive services—and avoid performing tests of no value. Since most of us do not have time to keep up with the literature on what services and tests have and have not been proven effective, we depend on trusted authorities to make these assessments for us.
The entity with the most rigorously evidence-based approach is the United States Preventive Services Task Force (USPSTF). (TABLE 1 lists the criteria for their recommendations.) Every year, this Practice Alert summarizes the new recommendations from the task force. The new recommendations in the 6 disease categories discussed here were published by the USPSTF in 2006 and the first quarter of 2007 (TABLE 2).
- Iron deficiency anemia
- Colon cancer chemoprevention
- Genetic screening for hemochromatosis
- Congenital hip dysplasia
- Elevated lead levels
- Speech delay
TABLE 1
The rigor behind the recommendations
| RECOMMENDATION | EVIDENCE | RESULTS OF THE SERVICE |
|---|---|---|
| A Strongly recommends | Good evidence |
|
| B Recommends | At least fair evidence |
|
| C No recommendation | At least fair evidence |
|
| D Recommends against | At least fair evidence |
|
| I Insufficient evidence | Insufficient to recommend for or against |
|
TABLE 2
Summary of new USPSTF recommendations
| B RECOMMENDATIONS |
The USPSTF recommends routine:
|
| D RECOMMENDATIONS |
The USPSTF recommends against routine:
|
| I RECOMMENDATIONS |
The USPSTF concludes that evidence is insufficient to recommend for or against routine:
|
“I” means insufficient evidence
As usual, there are many screening tests that lack evidence either for or against their effectiveness. The Task Force places such tests in the “I” (insufficient evidence) category. Physicians should remember that an “I” recommendation is not the equivalent of a “D” (recommend against).
Screening implies routine testing, and no symptoms
We also need to keep in mind the difference between screening and diagnosis. Screening implies routine testing among asymptomatic patients. Screening recommendations do not apply to symptomatic patients in whom diagnostic testing may be indicated.
1. Iron deficiency anemia
The task force recommends
- routine screening for iron deficiency anemia in asymptomatic pregnant women, and
- iron supplementation for asymptomatic children ages 6 to 12 months who are at increased risk for iron deficiency anemia.1
The task force concludes that the evidence is insufficient to recommend for or against
- routine screening for iron deficiency anemia in asymptomatic children ages 6 to 12 months,
- iron supplementation for asymptomatic children ages 6 to 12 months who are at average risk for iron deficiency anemia,
- iron supplementation for nonanemic pregnant women.1
Iron deficiency anemia is linked to developmental and cognitive abnormalities in children and poorer birth outcomes in pregnant women. The task force felt that the weight of the evidence supports a set of recommendations that includes screening all pregnant women and using iron preparations for those who have deficiency, and using routine iron supplementation for at-risk infants between the ages of 6 and 12 months.
The lack of a recommendation on screening all children was based on concern about the accuracy of hemoglobin as a screening test for iron deficiency and a scarcity of evidence that universal screening results in improved outcomes. Routine iron supplementation was felt to be of proven benefit only for infants at increased risk: those from low socioeconomic backgrounds and premature and low birth weight infants.
The Centers for Disease Control and Prevention (CDC) agrees that screening should be performed in high-risk infants and all pregnant women, but recommends universal iron supplementation during pregnancy.
The American Academy of Pediatrics recommends screening all infants twice (at age 9 to 12 months, and 6 months later) along with dietary interventions to prevent iron deficiency, such as: breast-feeding or the use of iron-fortified formula, and the introduction of iron-rich foods at age 6 months.
2. Colon cancer
The task force recommends against routine use of aspirin and NSAIDs to prevent colorectal cancer in individuals at average risk for colorectal cancer.2
Colon cancer is common and a common cause of cancer mortality,3 and proven secondary preventions are available. Chemoprevention is a potential method of primary prevention; it has some benefits as well as harms. The task force concluded that the documented harms exceed the potential benefits.
- Aspirin taken at the high-dose regimen (350–700 mg/day) needed to protect from colon cancer increases the risks for gastrointestinal bleeding and hemorrhagic stroke. A lower dose of aspirin (75–350 mg/day) is used for chemoprevention in adults who are at increased risk for coronary heart disease but this does not protect against colon cancer.
- Nonsteroidal anti-inflammatory drugs (NSAIDs) may reduce the risk of colorectal cancer, but they also increase the risks of gastrointestinal bleeding and renal injury.
- Cyclooxygenase-2 inhibitors have been linked to increases in coronary artery disease.
3. Hemochromatosis
The task force recommends against routine genetic screening for hereditary hemochromatosis in the asymptomatic general population.4
Hemochromatosis is rare, and only a small proportion of those with the high-risk genotype actually develop the disease. The effectiveness of early intervention is unproven, and the potential for harm from false positives is significant.
The D recommendation does not apply to those with signs and symptoms consistent with hemochromatosis or a strong family history of the disease. Nor does it pertain to non-genetic laboratory tests to identify iron overload (although these also lack proof that they improve outcomes in the general population).
4. Congenital hip dysplasia
The task force concludes that evidence is insufficient to recommend routine screening for developmental dysplasia of the hip in infants as a means to prevent adverse outcomes.5
Physical examination and ultrasonography have limited accuracy in finding hip dysplasia, and there is a high rate of natural resolution (60% to 90%) of hip abnormalities found with these tests. Both surgical and non-surgical treatments lack evidence of effectiveness and are associated with potential for harm from avascular necrosis, high costs, and complications from surgery and anesthesia.
This uncertainty applies only to asymptomatic infants—not to those who have obvious hip dislocations or other hip abnormalities.
5. Elevated lead levels
The task force concludes that evidence is insufficient to recommend for or against routine screening for elevated blood lead levels in asymptomatic children ages 1 to 5 who are at increased risk.6
The task force recommends against routine screening for elevated blood lead levels in:
- asymptomatic children ages 1 to 5 years who are at average risk
- asymptomatic pregnant women.6
The reduction of lead in the environment, especially the reduction of leadbased gasoline, has resulted in a decline in elevated blood lead levels in the United States. The task force’s uncertainty regarding screening at-risk children centered around a lack of evidence of the effectiveness of interventions in decreasing blood lead levels. Other organizations that continue to recommend screening in high-risk children include the CDC and the American Academy of Pediatrics. The main risk factor for elevated blood lead levels is living in housing constructed before 1950.
The recommendation against screening in pregnant women was based on the low prevalence, no evidence for effectiveness of interventions to decrease lead levels, and potential harms from screening. This recommendation agrees with those of other organizations.
Speech delay
The task force concludes that evidence is insufficient to recommend for or against routine use of brief, formal screening instruments in primary care to detect speech and language delay in children up to 5 years of age.7
While speech delay affects 5% to 8% of children under the age of 5, and interventions can result in short-term improvements, long-term benefits have not been studied. It is also unclear whether the brief screening tools used in primary care accurately identify children who will benefit from interventions, or whether the results of early intervention are better than when difficulties are first identified by parents. Overall, the task force felt that we lack sufficient evidence to evaluate overall benefits and harms of brief formal screening tools in primary care among asymptomatic children.
Correspondence
Doug Campos-Outcalt, MD, MPA, 4001 N Third Street #415, Phoenix, AZ 85012; dougco@u.arizona.edu.
1. USPSTF. Screening for iron deficiency anemia—including iron supplementation for children and pregnant women. Available at: www.ahrq.gov/clinic/uspstf/uspsiron.htm. Accessed on May 17, 2007.
2. USPSTF. Routine aspirin and nonsteroidal anti-inflammatory drugs for the primary prevention of colorectal cancer. Available at: www.ahrq.gov/clinic/uspstf/uspsasco.htm. Accessed on May 17, 2007.
3. USPSTF. Screening for colorectal cancer. Available at: www.ahrq.gov/clinic/uspstf/uspscolo.htm. Accessed on May 17, 2007.
4. USPSTF. Screening for hemochromatosis. Available at: www.ahrq.gov/clinic/uspstf/uspshemoch.htm. Accessed on May 17, 2007.
5. USPSTF. Screening for developmental dysplasia of the hip. Available at: www.ahrq.gov/clinic/uspstf/uspshipd.htm. Accessed on May 17, 2007.
6. USPSTF. Screening for elevated blood lead levels in childhood and pregnant women. Available at: www.ahrq.gov/clinic/uspstf/uspslead.htm. Accessed on May 17, 2007.
7. USPSTF. Screening for speech and language delay in preschool children. Available at: www.ahrq.gov/clinic/uspstf/uspschdv.htm. Accessed on May 17, 2007.
1. USPSTF. Screening for iron deficiency anemia—including iron supplementation for children and pregnant women. Available at: www.ahrq.gov/clinic/uspstf/uspsiron.htm. Accessed on May 17, 2007.
2. USPSTF. Routine aspirin and nonsteroidal anti-inflammatory drugs for the primary prevention of colorectal cancer. Available at: www.ahrq.gov/clinic/uspstf/uspsasco.htm. Accessed on May 17, 2007.
3. USPSTF. Screening for colorectal cancer. Available at: www.ahrq.gov/clinic/uspstf/uspscolo.htm. Accessed on May 17, 2007.
4. USPSTF. Screening for hemochromatosis. Available at: www.ahrq.gov/clinic/uspstf/uspshemoch.htm. Accessed on May 17, 2007.
5. USPSTF. Screening for developmental dysplasia of the hip. Available at: www.ahrq.gov/clinic/uspstf/uspshipd.htm. Accessed on May 17, 2007.
6. USPSTF. Screening for elevated blood lead levels in childhood and pregnant women. Available at: www.ahrq.gov/clinic/uspstf/uspslead.htm. Accessed on May 17, 2007.
7. USPSTF. Screening for speech and language delay in preschool children. Available at: www.ahrq.gov/clinic/uspstf/uspschdv.htm. Accessed on May 17, 2007.
Immunization update: Latest recommendations from the CDC
How effective is the new varicella zoster virus vaccine and when should adults receive it?
Who should recieve the new Tdap vaccine?
The answers to these and other immunization-related questions are addressed by the Centers for Disease Control and Prevention (CDC) in a number of recently-issued immunization recommendations. Here, by vaccine, is a quick review of these recommendations. (Several were reviewed in a previous Practice Alert. March 2007 issue of JFPRotavirus vaccine is 74% effective in preventing all rotavirus gastroenteritis and 98% effective in preventing severe rotavirus gastroenteritis.4 It is contraindicated in those who have had a severe allergic reaction to the vaccine and should be used with caution in children with altered immunocompetence, acute gastroenteritis, and moderate-to-severe illness. Even though it’s a modified live virus, it can be used in infants even if someone in the household is pregnant or immune-deficient.
Human papilloma virus
The quadrivalent human papilloma virus (HPV) vaccine was licensed by the US Food and Drug Administration (FDA) in June 2006; the CDC released its recommendations for its use in March 2007.5 The vaccine should be administered routinely to all girls aged 11 to 12 and can be started as early as age 9. The vaccine should also be given to women ages 13 to 26 who have not previously received the vaccine.
HPV is responsible for over 6 million new infections per year, although only a small proportion of these infections involve types that pose high risk for cervical cancer.5,6 The virus is associated with cervical cancer, genital warts, anal cancer, and possibly oral and pharyngeal cancer. TABLE 3 shows the number of each type of cancer that occurs in the US each year and the proportion attributed to HPV. There are over 11,000 new cases of cervical cancer and 3700 deaths from the disease each year.7,8
The HPV vaccine is produced in yeast using recombinant DNA technology and contains virus-like products of 4 HPV subtypes (6, 11, 16, and 18) that are responsible for between 60% and 80% of cervical cancers in the US. It prevents persistent HPV infection, genital warts, and cervical, vaginal and vulvar precancerous lesions due to the 4 subtypes contained. Since the vaccine does not completely protect from cervical cancer, Pap smear testing is still recommended after vaccination.
The vaccine is administered intramuscularly in 3 doses at months 0, 2, and 6. The minimum interval between doses 1 and 2 is 4 weeks and between doses 2 and 3, 12 weeks. It is contraindicated in those with allergies to yeast and other vaccine components. It can be coadministered with other vaccines but should be deferred for moderate to severe illness. The most common side effects are pain, swelling, and redness at the injection site; fever occurs at a rate slightly above placebo. The vaccine has not been tested for safety for use in pregnancy, but inadvertent administration during pregnancy has not led to any documented adverse effects.
TABLE 3
Cancers associated with HPV—US, 2003
| CANCER | CASES | % ATTRIBUTABLE TO ONCOGENIC HPV |
|---|---|---|
| Cervix* | 11,820 | 100 |
| Anus† | 4187 | 90 |
| Vulva† | 3507 | 40 |
| Vagina† | 1070 | 40 |
| Penis† | 1059 | 40 |
| Oral cavity/pharynx† | 29,627 | ≤12 |
| *A total of 70% of these cancers are attributable to HPV types 16 or 18. | ||
| †Majority of these cancers are attributable to HPV type 16. | ||
| Sources: US Cancer Statistics Working Group. United States Cancer Statistics: 2003. Incidence and Mortality. Altanta, Ga: US Department of Health and Human Services, CDC, and the National Cancer Institute; 2006; Parkin M. The global health burden of infection-associated cancers in the year 2002. Int J Cancer 2006; 118:3030–3044. | ||
Correspondence
Doug Campos-Outcalt, MD, MPA, 55 E. Van Buren, Phoenix, AZ 85004. dougco@u.arizona.edu
1. Campos-Outcalt D. Are you up to date with new immunization recommendations? J Fam Pract 2006;55:232-234.
2. CDC. Preventing tetanus, diphtheria and pertussis: use of tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis vaccine. Recommendations of the Advisory Committee on Immunization Practices (ACIP) and Recommendation of ACIP, supported by the Healthcare Infection Control Practices Advisory Committee (HICPAC), for Use of Tdap Among HealthCare Personnel. MMWR Recomm Rep 2005;55(RR-17):1-33.
3. CDC. A comprehensive immunization strategy to eliminate transmission of Hepatitis B Virus infection in the United States. Recommendations of the Advisory Committee on Immunization Practices (ACIP) Part II: Immunization of Adults. MMWR Recomm Rep 2006;55(RR-16):1-25.
4. CDC. Prevention of rotavirus gastroenteritis among infants and children. Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2006;55(RR-12):1-13.
5. CDC. Quadrivalent human papilloma virus vaccine. Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2007;56(RR-2):1-24.
6. Dunne EF, Unger ER, Sternberg M, et al. Prevalence of HPV infection among females in the United States. JAMA 2007;297:813-819.
How effective is the new varicella zoster virus vaccine and when should adults receive it?
Who should recieve the new Tdap vaccine?
The answers to these and other immunization-related questions are addressed by the Centers for Disease Control and Prevention (CDC) in a number of recently-issued immunization recommendations. Here, by vaccine, is a quick review of these recommendations. (Several were reviewed in a previous Practice Alert. March 2007 issue of JFPRotavirus vaccine is 74% effective in preventing all rotavirus gastroenteritis and 98% effective in preventing severe rotavirus gastroenteritis.4 It is contraindicated in those who have had a severe allergic reaction to the vaccine and should be used with caution in children with altered immunocompetence, acute gastroenteritis, and moderate-to-severe illness. Even though it’s a modified live virus, it can be used in infants even if someone in the household is pregnant or immune-deficient.
Human papilloma virus
The quadrivalent human papilloma virus (HPV) vaccine was licensed by the US Food and Drug Administration (FDA) in June 2006; the CDC released its recommendations for its use in March 2007.5 The vaccine should be administered routinely to all girls aged 11 to 12 and can be started as early as age 9. The vaccine should also be given to women ages 13 to 26 who have not previously received the vaccine.
HPV is responsible for over 6 million new infections per year, although only a small proportion of these infections involve types that pose high risk for cervical cancer.5,6 The virus is associated with cervical cancer, genital warts, anal cancer, and possibly oral and pharyngeal cancer. TABLE 3 shows the number of each type of cancer that occurs in the US each year and the proportion attributed to HPV. There are over 11,000 new cases of cervical cancer and 3700 deaths from the disease each year.7,8
The HPV vaccine is produced in yeast using recombinant DNA technology and contains virus-like products of 4 HPV subtypes (6, 11, 16, and 18) that are responsible for between 60% and 80% of cervical cancers in the US. It prevents persistent HPV infection, genital warts, and cervical, vaginal and vulvar precancerous lesions due to the 4 subtypes contained. Since the vaccine does not completely protect from cervical cancer, Pap smear testing is still recommended after vaccination.
The vaccine is administered intramuscularly in 3 doses at months 0, 2, and 6. The minimum interval between doses 1 and 2 is 4 weeks and between doses 2 and 3, 12 weeks. It is contraindicated in those with allergies to yeast and other vaccine components. It can be coadministered with other vaccines but should be deferred for moderate to severe illness. The most common side effects are pain, swelling, and redness at the injection site; fever occurs at a rate slightly above placebo. The vaccine has not been tested for safety for use in pregnancy, but inadvertent administration during pregnancy has not led to any documented adverse effects.
TABLE 3
Cancers associated with HPV—US, 2003
| CANCER | CASES | % ATTRIBUTABLE TO ONCOGENIC HPV |
|---|---|---|
| Cervix* | 11,820 | 100 |
| Anus† | 4187 | 90 |
| Vulva† | 3507 | 40 |
| Vagina† | 1070 | 40 |
| Penis† | 1059 | 40 |
| Oral cavity/pharynx† | 29,627 | ≤12 |
| *A total of 70% of these cancers are attributable to HPV types 16 or 18. | ||
| †Majority of these cancers are attributable to HPV type 16. | ||
| Sources: US Cancer Statistics Working Group. United States Cancer Statistics: 2003. Incidence and Mortality. Altanta, Ga: US Department of Health and Human Services, CDC, and the National Cancer Institute; 2006; Parkin M. The global health burden of infection-associated cancers in the year 2002. Int J Cancer 2006; 118:3030–3044. | ||
Correspondence
Doug Campos-Outcalt, MD, MPA, 55 E. Van Buren, Phoenix, AZ 85004. dougco@u.arizona.edu
How effective is the new varicella zoster virus vaccine and when should adults receive it?
Who should recieve the new Tdap vaccine?
The answers to these and other immunization-related questions are addressed by the Centers for Disease Control and Prevention (CDC) in a number of recently-issued immunization recommendations. Here, by vaccine, is a quick review of these recommendations. (Several were reviewed in a previous Practice Alert. March 2007 issue of JFPRotavirus vaccine is 74% effective in preventing all rotavirus gastroenteritis and 98% effective in preventing severe rotavirus gastroenteritis.4 It is contraindicated in those who have had a severe allergic reaction to the vaccine and should be used with caution in children with altered immunocompetence, acute gastroenteritis, and moderate-to-severe illness. Even though it’s a modified live virus, it can be used in infants even if someone in the household is pregnant or immune-deficient.
Human papilloma virus
The quadrivalent human papilloma virus (HPV) vaccine was licensed by the US Food and Drug Administration (FDA) in June 2006; the CDC released its recommendations for its use in March 2007.5 The vaccine should be administered routinely to all girls aged 11 to 12 and can be started as early as age 9. The vaccine should also be given to women ages 13 to 26 who have not previously received the vaccine.
HPV is responsible for over 6 million new infections per year, although only a small proportion of these infections involve types that pose high risk for cervical cancer.5,6 The virus is associated with cervical cancer, genital warts, anal cancer, and possibly oral and pharyngeal cancer. TABLE 3 shows the number of each type of cancer that occurs in the US each year and the proportion attributed to HPV. There are over 11,000 new cases of cervical cancer and 3700 deaths from the disease each year.7,8
The HPV vaccine is produced in yeast using recombinant DNA technology and contains virus-like products of 4 HPV subtypes (6, 11, 16, and 18) that are responsible for between 60% and 80% of cervical cancers in the US. It prevents persistent HPV infection, genital warts, and cervical, vaginal and vulvar precancerous lesions due to the 4 subtypes contained. Since the vaccine does not completely protect from cervical cancer, Pap smear testing is still recommended after vaccination.
The vaccine is administered intramuscularly in 3 doses at months 0, 2, and 6. The minimum interval between doses 1 and 2 is 4 weeks and between doses 2 and 3, 12 weeks. It is contraindicated in those with allergies to yeast and other vaccine components. It can be coadministered with other vaccines but should be deferred for moderate to severe illness. The most common side effects are pain, swelling, and redness at the injection site; fever occurs at a rate slightly above placebo. The vaccine has not been tested for safety for use in pregnancy, but inadvertent administration during pregnancy has not led to any documented adverse effects.
TABLE 3
Cancers associated with HPV—US, 2003
| CANCER | CASES | % ATTRIBUTABLE TO ONCOGENIC HPV |
|---|---|---|
| Cervix* | 11,820 | 100 |
| Anus† | 4187 | 90 |
| Vulva† | 3507 | 40 |
| Vagina† | 1070 | 40 |
| Penis† | 1059 | 40 |
| Oral cavity/pharynx† | 29,627 | ≤12 |
| *A total of 70% of these cancers are attributable to HPV types 16 or 18. | ||
| †Majority of these cancers are attributable to HPV type 16. | ||
| Sources: US Cancer Statistics Working Group. United States Cancer Statistics: 2003. Incidence and Mortality. Altanta, Ga: US Department of Health and Human Services, CDC, and the National Cancer Institute; 2006; Parkin M. The global health burden of infection-associated cancers in the year 2002. Int J Cancer 2006; 118:3030–3044. | ||
Correspondence
Doug Campos-Outcalt, MD, MPA, 55 E. Van Buren, Phoenix, AZ 85004. dougco@u.arizona.edu
1. Campos-Outcalt D. Are you up to date with new immunization recommendations? J Fam Pract 2006;55:232-234.
2. CDC. Preventing tetanus, diphtheria and pertussis: use of tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis vaccine. Recommendations of the Advisory Committee on Immunization Practices (ACIP) and Recommendation of ACIP, supported by the Healthcare Infection Control Practices Advisory Committee (HICPAC), for Use of Tdap Among HealthCare Personnel. MMWR Recomm Rep 2005;55(RR-17):1-33.
3. CDC. A comprehensive immunization strategy to eliminate transmission of Hepatitis B Virus infection in the United States. Recommendations of the Advisory Committee on Immunization Practices (ACIP) Part II: Immunization of Adults. MMWR Recomm Rep 2006;55(RR-16):1-25.
4. CDC. Prevention of rotavirus gastroenteritis among infants and children. Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2006;55(RR-12):1-13.
5. CDC. Quadrivalent human papilloma virus vaccine. Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2007;56(RR-2):1-24.
6. Dunne EF, Unger ER, Sternberg M, et al. Prevalence of HPV infection among females in the United States. JAMA 2007;297:813-819.
1. Campos-Outcalt D. Are you up to date with new immunization recommendations? J Fam Pract 2006;55:232-234.
2. CDC. Preventing tetanus, diphtheria and pertussis: use of tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis vaccine. Recommendations of the Advisory Committee on Immunization Practices (ACIP) and Recommendation of ACIP, supported by the Healthcare Infection Control Practices Advisory Committee (HICPAC), for Use of Tdap Among HealthCare Personnel. MMWR Recomm Rep 2005;55(RR-17):1-33.
3. CDC. A comprehensive immunization strategy to eliminate transmission of Hepatitis B Virus infection in the United States. Recommendations of the Advisory Committee on Immunization Practices (ACIP) Part II: Immunization of Adults. MMWR Recomm Rep 2006;55(RR-16):1-25.
4. CDC. Prevention of rotavirus gastroenteritis among infants and children. Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2006;55(RR-12):1-13.
5. CDC. Quadrivalent human papilloma virus vaccine. Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2007;56(RR-2):1-24.
6. Dunne EF, Unger ER, Sternberg M, et al. Prevalence of HPV infection among females in the United States. JAMA 2007;297:813-819.
Time to revise your HIV testing routine
The CDC now recommends that clinicians:
- Do HIV testing in all health care settings after the patient is notified that testing will be performed (unless the patient declines).
- Test high-risk patients annually.
- Discontinue use of a separate written consent for HIV testing, if allowed by state law. General consent for medical care should be considered sufficient.
- Drop the requirement that prevention counseling be conducted with HIV testing.
- Include HIV testing in the routine panel of prenatal screening tests for all pregnant women.
- Perform a repeat test on women in their third trimester in regions with elevated rates of HIV infection among pregnant women.
Should all adults and adolescents be screened for HIV? Do all persons at high risk deserve annual screening? The Centers for Disease Control and Prevention thinks so, but the US Preventive Services Task Force takes a less aggressive stance. The 2 agencies looked at the evidence and interpreted it differently—and likewise we must each decide what is best for our own patients and community.
Routine screening is one of several recently revised recommendations from the CDC (at right).1 Though the CDC has historically taken a cautious approach to HIV testing, the winds appear to be changing. The reasons:
- Risk-based screening did not reduce incidence. The previous approach—targeted counseling and testing—has not led to a decline in HIV incidence—it has hovered at around 40,000 cases per year for over a decade.2
- An estimated one fourth of HIV-positive people in the US don’t know their status, and thus are at increased risk of transmitting the disease to others.
- Risk-based screening failed to detect many who are HIV-infected because patients either don’t appreciate—or don’t want to acknowledge—their risks.3,4
- Risk-based screening failed to detect many HIV-infected pregnant women, leading to preventable infection in newborns; routine opt-out testing has been more successful.5
- Highly active antiretroviral therapy has had marked success in reducing mortality from HIV infection. Chemoprophylaxis has proven benefits for preventing certain opportunistic infections.6,7
Removing barriers to testing
The CDC is also advising clinicians that requiring pretest counseling or a separate written consent is a barrier to testing. Clinicians still should inform patients that HIV testing is being conducted and that they have a right to refuse. There is evidence, though, that making the test routine reduces its stigma and increases acceptance.8-11
Evidence also indicates that preventive counseling is very effective in reducing risky behavior among those who are HIV-positive. It’s unclear, however, whether such counseling is effective among those who are HIV-negative.12
Thus, the CDC’s new approach stresses finding those who are infected, getting them medical care, and lowering their risk of transmitting infection to others.
If a pregnant women refuses HIV testing, ask why
The new CDC recommendations take an especially aggressive approach to screening pregnant women, stating that women who refuse testing should be questioned about their reasons for refusal and counseled about the benefits of the test.
The CDC advises repeat testing in the third trimester, in areas of increased risk—which includes 20 states1—and for pregnant women with individual risk factors, as well as those who receive care in facilities with rates of infection of 1 per 1000 women screened. The CDC also urges rapid HIV testing during labor, in women who were not tested during pregnancy, and on newborns whose mothers were not tested during pregnancy or labor.
USPSTF is less aggressive
The USPSTF13 does not recommend for or against testing persons who are not at high risk (TABLE). Both the CDC and the USPSTF recognize that routine screening is probably warranted in populations with HIV prevalence of 1/1000 or greater. However, the CDC recommends routine screening in all settings until there is evidence that the site or population-specific prevalence is lower than this threshold, while the USPSTF simply states that routine screening may be warranted in populations with a prevalence above this level.
TABLE
USPSTF vs CDC recommendations on HIV testing
| GROUP | USPSTF | CDC |
|---|---|---|
| High-risk adolescents | Recommends testing, no frequency mentioned | Recommends annual testing and before starting a new sexual relationship |
| High-risk adults | Recommends testing, no frequency mentioned | Recommends annual testing as well as before starting a new sexual relationship |
| Adolescents not at high risk | No recommendation for or against | Recommends testing, no frequency mentioned, and testing before starting a new sexual relationship. |
| Adults not at high risk | No recommendation for or against | Recommends testing, no frequency mentioned. recommends testing before starting a new sexual relationship. |
| Pregnant women | Recommends testing | Recommends testing at first visit, repeat test in the third trimester in regions with high rates of HIV infection in pregnant women. |
| Written consent | Does not comment about | Recommends against |
The take-away message
It’s time to review both sets of guidelines and adopt HIV testing policies that are most appropriate for your clinical and community situation, and that meet state laws, many of which still require separate written consent and pretest counseling.
Correspondence
Doug Campos-outcalt, MD, MPA, 4001 N. Third Street #415, phoenix, AZ 85012. dougco@u.arizona.edu
1. Branson BM, Handsfield HH, Lampe MA, et al. CDC. Revised recommendations for HIV testing of adults, adolescents, and pregnant women in health care settings. MMWR Recomm Rep 2006;55(RR-14):1-17.Available at: www.cdc.gov/mmwr/preview/mmwrhtml/rr5514a1.htm. Accessed on March 16, 2007.
2. CDC. US HIV and AIDS cases reported through December 2001. HIV/AIDS Surveillance Report 2001;13(2). Available at: www.cdc.gov/hiv/stats/hasr1302.htm. Accessed on March 13, 2007.
3. Institute of Medicine No Time to Lose: Getting More from HIV Prevention. Washington, DC: National Academy Press; 2001.
4. Peterman TA, Todd KA, Mupanduki I. Opportunities for targeting publicly funded human immunodeficiency virus counseling and testing. J Acquir Immune Defic Syndr Hum Retrovirol 1996;12:69-74.
5. CDC. HIV testing among pregnant women—US and Canada, 1998–2001. MMWR Morb Mortal Wkly Rep 2002;51:1013-1016.
6. McNaghten AD, Hanson DL, Jones JL, Dworkin MS, Ward JW. Effects of antiretroviral therapy and opportunistic illness primary chemoprophylaxis on survival after AIDS diagnosis. Adult/Adolescent Spectrum of Disease Group. AIDS 1999;13:1687-1695.
7. Palella FJ, Delaney KM, Moorman AC, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. N Engl J Med 1998;338:853-860.
8. Irwin KL, Valdiserri RO, Holmberg SD. The acceptability of voluntary HIV antibody testing in the United States: a decade of lessons learned. AIDS 1996;10:1707-1717.
9. Hutchinson AB, Corbie-Smith G, Thomas SB, et al. Understanding the patient’s perspective on rapid and routine HIV testing in an inner-city urgent care center. AIDS Educ Prev 2004;16:101-114.
10. Spielberg F, Branson BM, Goldbaum GM, et al. Overcoming barriers to HIV testing: p for new strategies among clients of a needle exchange, a sexually transmitted disease clinic, and sex venues for men who have sex with men. J Acquir Immune Defic Syndr 2003;32:318-328.
11. Copenhaver MM, Fisher JD. Experts outline ways to decrease the decade-long yearly rate of 40,000 new HIV infections in the US. AIDS Behav 2006;10:105-114.
12. Weinhard LS, Carey MP, Johnson BT, Bickham NL. Effects of HIV counseling and testing on sexual risk behavior: a metanalytic review of published research 1985–1997. Am J Public Health 1999;89:1397-1405.
13. USPSTF. Recommendation statement: Screening for HIV. Available at: www.ahrq.gov/clinic/uspstf05/hiv/hivrs.htm#clinical. Accessed on March 13, 2007.
The CDC now recommends that clinicians:
- Do HIV testing in all health care settings after the patient is notified that testing will be performed (unless the patient declines).
- Test high-risk patients annually.
- Discontinue use of a separate written consent for HIV testing, if allowed by state law. General consent for medical care should be considered sufficient.
- Drop the requirement that prevention counseling be conducted with HIV testing.
- Include HIV testing in the routine panel of prenatal screening tests for all pregnant women.
- Perform a repeat test on women in their third trimester in regions with elevated rates of HIV infection among pregnant women.
Should all adults and adolescents be screened for HIV? Do all persons at high risk deserve annual screening? The Centers for Disease Control and Prevention thinks so, but the US Preventive Services Task Force takes a less aggressive stance. The 2 agencies looked at the evidence and interpreted it differently—and likewise we must each decide what is best for our own patients and community.
Routine screening is one of several recently revised recommendations from the CDC (at right).1 Though the CDC has historically taken a cautious approach to HIV testing, the winds appear to be changing. The reasons:
- Risk-based screening did not reduce incidence. The previous approach—targeted counseling and testing—has not led to a decline in HIV incidence—it has hovered at around 40,000 cases per year for over a decade.2
- An estimated one fourth of HIV-positive people in the US don’t know their status, and thus are at increased risk of transmitting the disease to others.
- Risk-based screening failed to detect many who are HIV-infected because patients either don’t appreciate—or don’t want to acknowledge—their risks.3,4
- Risk-based screening failed to detect many HIV-infected pregnant women, leading to preventable infection in newborns; routine opt-out testing has been more successful.5
- Highly active antiretroviral therapy has had marked success in reducing mortality from HIV infection. Chemoprophylaxis has proven benefits for preventing certain opportunistic infections.6,7
Removing barriers to testing
The CDC is also advising clinicians that requiring pretest counseling or a separate written consent is a barrier to testing. Clinicians still should inform patients that HIV testing is being conducted and that they have a right to refuse. There is evidence, though, that making the test routine reduces its stigma and increases acceptance.8-11
Evidence also indicates that preventive counseling is very effective in reducing risky behavior among those who are HIV-positive. It’s unclear, however, whether such counseling is effective among those who are HIV-negative.12
Thus, the CDC’s new approach stresses finding those who are infected, getting them medical care, and lowering their risk of transmitting infection to others.
If a pregnant women refuses HIV testing, ask why
The new CDC recommendations take an especially aggressive approach to screening pregnant women, stating that women who refuse testing should be questioned about their reasons for refusal and counseled about the benefits of the test.
The CDC advises repeat testing in the third trimester, in areas of increased risk—which includes 20 states1—and for pregnant women with individual risk factors, as well as those who receive care in facilities with rates of infection of 1 per 1000 women screened. The CDC also urges rapid HIV testing during labor, in women who were not tested during pregnancy, and on newborns whose mothers were not tested during pregnancy or labor.
USPSTF is less aggressive
The USPSTF13 does not recommend for or against testing persons who are not at high risk (TABLE). Both the CDC and the USPSTF recognize that routine screening is probably warranted in populations with HIV prevalence of 1/1000 or greater. However, the CDC recommends routine screening in all settings until there is evidence that the site or population-specific prevalence is lower than this threshold, while the USPSTF simply states that routine screening may be warranted in populations with a prevalence above this level.
TABLE
USPSTF vs CDC recommendations on HIV testing
| GROUP | USPSTF | CDC |
|---|---|---|
| High-risk adolescents | Recommends testing, no frequency mentioned | Recommends annual testing and before starting a new sexual relationship |
| High-risk adults | Recommends testing, no frequency mentioned | Recommends annual testing as well as before starting a new sexual relationship |
| Adolescents not at high risk | No recommendation for or against | Recommends testing, no frequency mentioned, and testing before starting a new sexual relationship. |
| Adults not at high risk | No recommendation for or against | Recommends testing, no frequency mentioned. recommends testing before starting a new sexual relationship. |
| Pregnant women | Recommends testing | Recommends testing at first visit, repeat test in the third trimester in regions with high rates of HIV infection in pregnant women. |
| Written consent | Does not comment about | Recommends against |
The take-away message
It’s time to review both sets of guidelines and adopt HIV testing policies that are most appropriate for your clinical and community situation, and that meet state laws, many of which still require separate written consent and pretest counseling.
Correspondence
Doug Campos-outcalt, MD, MPA, 4001 N. Third Street #415, phoenix, AZ 85012. dougco@u.arizona.edu
The CDC now recommends that clinicians:
- Do HIV testing in all health care settings after the patient is notified that testing will be performed (unless the patient declines).
- Test high-risk patients annually.
- Discontinue use of a separate written consent for HIV testing, if allowed by state law. General consent for medical care should be considered sufficient.
- Drop the requirement that prevention counseling be conducted with HIV testing.
- Include HIV testing in the routine panel of prenatal screening tests for all pregnant women.
- Perform a repeat test on women in their third trimester in regions with elevated rates of HIV infection among pregnant women.
Should all adults and adolescents be screened for HIV? Do all persons at high risk deserve annual screening? The Centers for Disease Control and Prevention thinks so, but the US Preventive Services Task Force takes a less aggressive stance. The 2 agencies looked at the evidence and interpreted it differently—and likewise we must each decide what is best for our own patients and community.
Routine screening is one of several recently revised recommendations from the CDC (at right).1 Though the CDC has historically taken a cautious approach to HIV testing, the winds appear to be changing. The reasons:
- Risk-based screening did not reduce incidence. The previous approach—targeted counseling and testing—has not led to a decline in HIV incidence—it has hovered at around 40,000 cases per year for over a decade.2
- An estimated one fourth of HIV-positive people in the US don’t know their status, and thus are at increased risk of transmitting the disease to others.
- Risk-based screening failed to detect many who are HIV-infected because patients either don’t appreciate—or don’t want to acknowledge—their risks.3,4
- Risk-based screening failed to detect many HIV-infected pregnant women, leading to preventable infection in newborns; routine opt-out testing has been more successful.5
- Highly active antiretroviral therapy has had marked success in reducing mortality from HIV infection. Chemoprophylaxis has proven benefits for preventing certain opportunistic infections.6,7
Removing barriers to testing
The CDC is also advising clinicians that requiring pretest counseling or a separate written consent is a barrier to testing. Clinicians still should inform patients that HIV testing is being conducted and that they have a right to refuse. There is evidence, though, that making the test routine reduces its stigma and increases acceptance.8-11
Evidence also indicates that preventive counseling is very effective in reducing risky behavior among those who are HIV-positive. It’s unclear, however, whether such counseling is effective among those who are HIV-negative.12
Thus, the CDC’s new approach stresses finding those who are infected, getting them medical care, and lowering their risk of transmitting infection to others.
If a pregnant women refuses HIV testing, ask why
The new CDC recommendations take an especially aggressive approach to screening pregnant women, stating that women who refuse testing should be questioned about their reasons for refusal and counseled about the benefits of the test.
The CDC advises repeat testing in the third trimester, in areas of increased risk—which includes 20 states1—and for pregnant women with individual risk factors, as well as those who receive care in facilities with rates of infection of 1 per 1000 women screened. The CDC also urges rapid HIV testing during labor, in women who were not tested during pregnancy, and on newborns whose mothers were not tested during pregnancy or labor.
USPSTF is less aggressive
The USPSTF13 does not recommend for or against testing persons who are not at high risk (TABLE). Both the CDC and the USPSTF recognize that routine screening is probably warranted in populations with HIV prevalence of 1/1000 or greater. However, the CDC recommends routine screening in all settings until there is evidence that the site or population-specific prevalence is lower than this threshold, while the USPSTF simply states that routine screening may be warranted in populations with a prevalence above this level.
TABLE
USPSTF vs CDC recommendations on HIV testing
| GROUP | USPSTF | CDC |
|---|---|---|
| High-risk adolescents | Recommends testing, no frequency mentioned | Recommends annual testing and before starting a new sexual relationship |
| High-risk adults | Recommends testing, no frequency mentioned | Recommends annual testing as well as before starting a new sexual relationship |
| Adolescents not at high risk | No recommendation for or against | Recommends testing, no frequency mentioned, and testing before starting a new sexual relationship. |
| Adults not at high risk | No recommendation for or against | Recommends testing, no frequency mentioned. recommends testing before starting a new sexual relationship. |
| Pregnant women | Recommends testing | Recommends testing at first visit, repeat test in the third trimester in regions with high rates of HIV infection in pregnant women. |
| Written consent | Does not comment about | Recommends against |
The take-away message
It’s time to review both sets of guidelines and adopt HIV testing policies that are most appropriate for your clinical and community situation, and that meet state laws, many of which still require separate written consent and pretest counseling.
Correspondence
Doug Campos-outcalt, MD, MPA, 4001 N. Third Street #415, phoenix, AZ 85012. dougco@u.arizona.edu
1. Branson BM, Handsfield HH, Lampe MA, et al. CDC. Revised recommendations for HIV testing of adults, adolescents, and pregnant women in health care settings. MMWR Recomm Rep 2006;55(RR-14):1-17.Available at: www.cdc.gov/mmwr/preview/mmwrhtml/rr5514a1.htm. Accessed on March 16, 2007.
2. CDC. US HIV and AIDS cases reported through December 2001. HIV/AIDS Surveillance Report 2001;13(2). Available at: www.cdc.gov/hiv/stats/hasr1302.htm. Accessed on March 13, 2007.
3. Institute of Medicine No Time to Lose: Getting More from HIV Prevention. Washington, DC: National Academy Press; 2001.
4. Peterman TA, Todd KA, Mupanduki I. Opportunities for targeting publicly funded human immunodeficiency virus counseling and testing. J Acquir Immune Defic Syndr Hum Retrovirol 1996;12:69-74.
5. CDC. HIV testing among pregnant women—US and Canada, 1998–2001. MMWR Morb Mortal Wkly Rep 2002;51:1013-1016.
6. McNaghten AD, Hanson DL, Jones JL, Dworkin MS, Ward JW. Effects of antiretroviral therapy and opportunistic illness primary chemoprophylaxis on survival after AIDS diagnosis. Adult/Adolescent Spectrum of Disease Group. AIDS 1999;13:1687-1695.
7. Palella FJ, Delaney KM, Moorman AC, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. N Engl J Med 1998;338:853-860.
8. Irwin KL, Valdiserri RO, Holmberg SD. The acceptability of voluntary HIV antibody testing in the United States: a decade of lessons learned. AIDS 1996;10:1707-1717.
9. Hutchinson AB, Corbie-Smith G, Thomas SB, et al. Understanding the patient’s perspective on rapid and routine HIV testing in an inner-city urgent care center. AIDS Educ Prev 2004;16:101-114.
10. Spielberg F, Branson BM, Goldbaum GM, et al. Overcoming barriers to HIV testing: p for new strategies among clients of a needle exchange, a sexually transmitted disease clinic, and sex venues for men who have sex with men. J Acquir Immune Defic Syndr 2003;32:318-328.
11. Copenhaver MM, Fisher JD. Experts outline ways to decrease the decade-long yearly rate of 40,000 new HIV infections in the US. AIDS Behav 2006;10:105-114.
12. Weinhard LS, Carey MP, Johnson BT, Bickham NL. Effects of HIV counseling and testing on sexual risk behavior: a metanalytic review of published research 1985–1997. Am J Public Health 1999;89:1397-1405.
13. USPSTF. Recommendation statement: Screening for HIV. Available at: www.ahrq.gov/clinic/uspstf05/hiv/hivrs.htm#clinical. Accessed on March 13, 2007.
1. Branson BM, Handsfield HH, Lampe MA, et al. CDC. Revised recommendations for HIV testing of adults, adolescents, and pregnant women in health care settings. MMWR Recomm Rep 2006;55(RR-14):1-17.Available at: www.cdc.gov/mmwr/preview/mmwrhtml/rr5514a1.htm. Accessed on March 16, 2007.
2. CDC. US HIV and AIDS cases reported through December 2001. HIV/AIDS Surveillance Report 2001;13(2). Available at: www.cdc.gov/hiv/stats/hasr1302.htm. Accessed on March 13, 2007.
3. Institute of Medicine No Time to Lose: Getting More from HIV Prevention. Washington, DC: National Academy Press; 2001.
4. Peterman TA, Todd KA, Mupanduki I. Opportunities for targeting publicly funded human immunodeficiency virus counseling and testing. J Acquir Immune Defic Syndr Hum Retrovirol 1996;12:69-74.
5. CDC. HIV testing among pregnant women—US and Canada, 1998–2001. MMWR Morb Mortal Wkly Rep 2002;51:1013-1016.
6. McNaghten AD, Hanson DL, Jones JL, Dworkin MS, Ward JW. Effects of antiretroviral therapy and opportunistic illness primary chemoprophylaxis on survival after AIDS diagnosis. Adult/Adolescent Spectrum of Disease Group. AIDS 1999;13:1687-1695.
7. Palella FJ, Delaney KM, Moorman AC, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. N Engl J Med 1998;338:853-860.
8. Irwin KL, Valdiserri RO, Holmberg SD. The acceptability of voluntary HIV antibody testing in the United States: a decade of lessons learned. AIDS 1996;10:1707-1717.
9. Hutchinson AB, Corbie-Smith G, Thomas SB, et al. Understanding the patient’s perspective on rapid and routine HIV testing in an inner-city urgent care center. AIDS Educ Prev 2004;16:101-114.
10. Spielberg F, Branson BM, Goldbaum GM, et al. Overcoming barriers to HIV testing: p for new strategies among clients of a needle exchange, a sexually transmitted disease clinic, and sex venues for men who have sex with men. J Acquir Immune Defic Syndr 2003;32:318-328.
11. Copenhaver MM, Fisher JD. Experts outline ways to decrease the decade-long yearly rate of 40,000 new HIV infections in the US. AIDS Behav 2006;10:105-114.
12. Weinhard LS, Carey MP, Johnson BT, Bickham NL. Effects of HIV counseling and testing on sexual risk behavior: a metanalytic review of published research 1985–1997. Am J Public Health 1999;89:1397-1405.
13. USPSTF. Recommendation statement: Screening for HIV. Available at: www.ahrq.gov/clinic/uspstf05/hiv/hivrs.htm#clinical. Accessed on March 13, 2007.
The preteen visit: An opportunity for prevention
All early adolescents should visit a physician at age 11 or 12 years to receive a set of recommended vaccines. Two vaccines are recommended for boys in this age group—quadrivalent meningococcal conjugate vaccine (MCV4) and tetanus toxoid, reduced diphtheria, and acellular pertussis vaccine (Tdap). Three vaccines are recommended for girls—MCV4, Tdap, and human papilloma virus (HPV) vaccine.
In addition, 2 doses of varicella vaccine are now recommended before age 5 years; both boys and girls at age 11 or 12 who have received only 1 dose should be given a second. TABLE 1 contains details on each recommended vaccine.
TABLE 1
Vaccines recommended for early adolescents
| VACCINE | ROUTE | SCHEDULE | CONTRAINDICATIONS* | PRECAUTIONS |
|---|---|---|---|---|
| MCV4 | IM | 1 dose | Moderate to severe illness | |
| Tdap | IM | 1 dose, may need other doses of Td to complete a tetanus and diphtheria series | Encephalopathy within 7 days of previous vaccine not attributed to other cause | Hypersensitivity with prior tetanus toxoid |
| Progressive neurological disorder | ||||
| Latex allergy | ||||
| Guillain-Barré syndrome within 6 weeks of a previous dose of tetanus toxoid | ||||
| Acute moderate to severe illness | ||||
| HPV | IM | 3 doses at months 0, 2, and 6 | History of hypersensitivity | Defer for moderate to severe illness to yeast |
| Varicella | SQ | 2 doses 3 months apart (1 month interval is acceptable) | Pregnancy | Moderate to severe illness |
| Severe suppression of cellular immunity | Receipt of antibody containing blood product in the preceding 11 months | |||
| Complete information for each vaccine can be located on the CDC web site at: www.cdc.gov/node.do/id/0900f3ec8005df1f. | ||||
| * All vaccines have as a contraindication a previous anaphylactic reaction to the vaccine or vaccine components. | ||||
| Details on contraindications can be found at: www.cdc.gov/nip/recs/contraindications_vacc.htm#var. | ||||
Meningococcal vaccine
Quadrivalent meningococcal conjugate vaccine (Menactra) contains antigens for 4 meningococcal groups (A, C, Y, W-135), and is licensed for ages 11 to 55 years. The Advisory Committee on Immunization Practices (ACIP) recommends that all preteens receive 1 dose at age 11 or 12. Unvaccinated older children should receive a dose before entering high school; unvaccinated college freshmen living in dorms should also be vaccinated.
Because of a shortage of vaccine, the Centers for Disease Control and Prevention (CDC) had recommended a delay in the implementation of routine vaccination at age 11 and 12. The supply situation has now corrected, and this recommendation has been rescinded.
There have been 17 cases of Guillain-Barré syndrome appearing in adolescents and young adults within 33 days of receiving MCV4. The possibility of a cause-and-effect relationship is under investigation. The CDC recommends that preadolescents and adolescents who have a history of Guillain-Barré should not receive MCV4 unless they are college freshmen who live in dorms.
Tetanus/diphtheria/pertussis vaccine
There are 2 Tdap products, one licensed for ages 10 to 18 years (Boostrix), the other for ages 11 to 64 (Adacel). The ACIP recommends a single dose of Tdap for those aged 11 to 18, preferably at age 11 or 12. The optimal interval from the last tetanus and diphtheria toxoid (TD or Td) is 5 years but a shorter interval is acceptable. Thereafter, Td boosters are recommended every 10 years. If an 11- or 12-year-old has not previously received a complete series of a tetanus toxoid, diphtheria product tetanus and diphtheria vaccines, they should be given the recommended number of doses—only one of which should be Tdap, the others Td. The number and timing of doses can be found at www.cdc.gov/mmwr/preview/mmwrhtml/rr55e223a5.htm.
Human papilloma virus vaccine
The HPV vaccine (Gardasil) is licensed only for females aged 9 to 26 years, and is the first vaccine for the prevention of cervical cancer. It protects against HPV types 6, 11, 16, and 18, which are the cause of approximately 80% of cervical cancers. The ACIP recommends routine administration for all females between ages 9 and 26, preferably before the onset of sexual activity. The vaccine requires 3 doses at months 0, 2, and 6; it can be administered concurrently with MCV4, Tdap, and Td.
Varicella vaccine
Two doses of varicella are now recommended for all children at ages 12 to 15 months and 4 to 6 years—the same as for the measles, mumps, and rubella vaccine (MMR). A new MMRV product (Proquad) could reduce the number of injections needed at these ages.
Adolescents and adults who are not immune to varicella should receive 2 doses of vaccine 3 months apart, or 1 dose if they have been vaccinated with a single dose of varicella vaccine. Immunity to varicella is defined as birth in the US prior to 1980, 2 doses of varicella vaccine, or having had a diagnosed case of chickenpox or shingles.
Other interventions
This expanding list of recommended vaccines should create an incentive for parents to bring their preteen children to visit a physician. The American College of Physicians (ACP) recommendations are built on the assumption that these vaccines should be part of a routine preventive visit at this age group.
If preteens do visit a physician more frequently, it will provide an opportunity for other health care maintenance interventions, such as measuring height, weight, and blood pressure and providing health education on diet, physical activity, and substance abuse. Unfortunately, the evidence base for the effectiveness of preventive interventions at this age is very weak.
TABLE 2 lists the interventions for the age group 11 to 12 years that have been evaluated by the US Preventive Services Task Force (USPSTF). This is not a comprehensive list of all possible preventive interventions for young adolescents, only those that have been evaluated by the USPSTF. Those with either an A (strongly recommend) or B (recommend) recommendation are screening tests related to risks involved with sexual activity and will not apply to all young adolescents. Some of those with a D recommendation (recommend against) will surprise many physicians, as they have historically been included in various screening guidelines.
TABLE 2
US Preventive Services Task Force recommendations on interventions for adolescents
Recommend for
|
Recommend Against
|
Insufficient Evidence to Recommend For or Against
|
Reports in Progress
|
For a listing of vaccine components and contraindications see: www.cdc.gov/nip/recs/contraindications.htm
Vaccine information statements are found at: www.cdc.gov/nip/publications/VIS/default.htm#hpv
For a useful chart with information on all vaccines go to: www.cdc.gov/nip/vaccine/vac-chart-hcp.htm
General information on immunizations is on the CDC web site: www.cdc.gov/mmwr/preview/mmwrhtml/rr5102a1.htm
The United States Preventive Services Task Force lists current recommendations, rationales, and clinical considerations at: www.ahrq.gov/clinic/uspstfix.htm
The bulk of the potential interventions are in the I category (insufficient evidence to recommend for or against) or are the subject of an ongoing evaluation. Many of these receive this rating not because the targeted behavior is in question but because it is unclear how effective physician counseling in a clinical encounter is in changing these behaviors—for example, avoidance of tobacco products and maintenance of ideal weight. Many providers will probably choose to provide young adolescents advice on these topics in spite of the meager evidence available.
CORRESPONDENCE
Doug Campos-Outcalt, MD, MPA, 4001 North Third Street #415, Phoenix, AZ 85012. E-mail: dougco@u.arizona.edu
All early adolescents should visit a physician at age 11 or 12 years to receive a set of recommended vaccines. Two vaccines are recommended for boys in this age group—quadrivalent meningococcal conjugate vaccine (MCV4) and tetanus toxoid, reduced diphtheria, and acellular pertussis vaccine (Tdap). Three vaccines are recommended for girls—MCV4, Tdap, and human papilloma virus (HPV) vaccine.
In addition, 2 doses of varicella vaccine are now recommended before age 5 years; both boys and girls at age 11 or 12 who have received only 1 dose should be given a second. TABLE 1 contains details on each recommended vaccine.
TABLE 1
Vaccines recommended for early adolescents
| VACCINE | ROUTE | SCHEDULE | CONTRAINDICATIONS* | PRECAUTIONS |
|---|---|---|---|---|
| MCV4 | IM | 1 dose | Moderate to severe illness | |
| Tdap | IM | 1 dose, may need other doses of Td to complete a tetanus and diphtheria series | Encephalopathy within 7 days of previous vaccine not attributed to other cause | Hypersensitivity with prior tetanus toxoid |
| Progressive neurological disorder | ||||
| Latex allergy | ||||
| Guillain-Barré syndrome within 6 weeks of a previous dose of tetanus toxoid | ||||
| Acute moderate to severe illness | ||||
| HPV | IM | 3 doses at months 0, 2, and 6 | History of hypersensitivity | Defer for moderate to severe illness to yeast |
| Varicella | SQ | 2 doses 3 months apart (1 month interval is acceptable) | Pregnancy | Moderate to severe illness |
| Severe suppression of cellular immunity | Receipt of antibody containing blood product in the preceding 11 months | |||
| Complete information for each vaccine can be located on the CDC web site at: www.cdc.gov/node.do/id/0900f3ec8005df1f. | ||||
| * All vaccines have as a contraindication a previous anaphylactic reaction to the vaccine or vaccine components. | ||||
| Details on contraindications can be found at: www.cdc.gov/nip/recs/contraindications_vacc.htm#var. | ||||
Meningococcal vaccine
Quadrivalent meningococcal conjugate vaccine (Menactra) contains antigens for 4 meningococcal groups (A, C, Y, W-135), and is licensed for ages 11 to 55 years. The Advisory Committee on Immunization Practices (ACIP) recommends that all preteens receive 1 dose at age 11 or 12. Unvaccinated older children should receive a dose before entering high school; unvaccinated college freshmen living in dorms should also be vaccinated.
Because of a shortage of vaccine, the Centers for Disease Control and Prevention (CDC) had recommended a delay in the implementation of routine vaccination at age 11 and 12. The supply situation has now corrected, and this recommendation has been rescinded.
There have been 17 cases of Guillain-Barré syndrome appearing in adolescents and young adults within 33 days of receiving MCV4. The possibility of a cause-and-effect relationship is under investigation. The CDC recommends that preadolescents and adolescents who have a history of Guillain-Barré should not receive MCV4 unless they are college freshmen who live in dorms.
Tetanus/diphtheria/pertussis vaccine
There are 2 Tdap products, one licensed for ages 10 to 18 years (Boostrix), the other for ages 11 to 64 (Adacel). The ACIP recommends a single dose of Tdap for those aged 11 to 18, preferably at age 11 or 12. The optimal interval from the last tetanus and diphtheria toxoid (TD or Td) is 5 years but a shorter interval is acceptable. Thereafter, Td boosters are recommended every 10 years. If an 11- or 12-year-old has not previously received a complete series of a tetanus toxoid, diphtheria product tetanus and diphtheria vaccines, they should be given the recommended number of doses—only one of which should be Tdap, the others Td. The number and timing of doses can be found at www.cdc.gov/mmwr/preview/mmwrhtml/rr55e223a5.htm.
Human papilloma virus vaccine
The HPV vaccine (Gardasil) is licensed only for females aged 9 to 26 years, and is the first vaccine for the prevention of cervical cancer. It protects against HPV types 6, 11, 16, and 18, which are the cause of approximately 80% of cervical cancers. The ACIP recommends routine administration for all females between ages 9 and 26, preferably before the onset of sexual activity. The vaccine requires 3 doses at months 0, 2, and 6; it can be administered concurrently with MCV4, Tdap, and Td.
Varicella vaccine
Two doses of varicella are now recommended for all children at ages 12 to 15 months and 4 to 6 years—the same as for the measles, mumps, and rubella vaccine (MMR). A new MMRV product (Proquad) could reduce the number of injections needed at these ages.
Adolescents and adults who are not immune to varicella should receive 2 doses of vaccine 3 months apart, or 1 dose if they have been vaccinated with a single dose of varicella vaccine. Immunity to varicella is defined as birth in the US prior to 1980, 2 doses of varicella vaccine, or having had a diagnosed case of chickenpox or shingles.
Other interventions
This expanding list of recommended vaccines should create an incentive for parents to bring their preteen children to visit a physician. The American College of Physicians (ACP) recommendations are built on the assumption that these vaccines should be part of a routine preventive visit at this age group.
If preteens do visit a physician more frequently, it will provide an opportunity for other health care maintenance interventions, such as measuring height, weight, and blood pressure and providing health education on diet, physical activity, and substance abuse. Unfortunately, the evidence base for the effectiveness of preventive interventions at this age is very weak.
TABLE 2 lists the interventions for the age group 11 to 12 years that have been evaluated by the US Preventive Services Task Force (USPSTF). This is not a comprehensive list of all possible preventive interventions for young adolescents, only those that have been evaluated by the USPSTF. Those with either an A (strongly recommend) or B (recommend) recommendation are screening tests related to risks involved with sexual activity and will not apply to all young adolescents. Some of those with a D recommendation (recommend against) will surprise many physicians, as they have historically been included in various screening guidelines.
TABLE 2
US Preventive Services Task Force recommendations on interventions for adolescents
Recommend for
|
Recommend Against
|
Insufficient Evidence to Recommend For or Against
|
Reports in Progress
|
For a listing of vaccine components and contraindications see: www.cdc.gov/nip/recs/contraindications.htm
Vaccine information statements are found at: www.cdc.gov/nip/publications/VIS/default.htm#hpv
For a useful chart with information on all vaccines go to: www.cdc.gov/nip/vaccine/vac-chart-hcp.htm
General information on immunizations is on the CDC web site: www.cdc.gov/mmwr/preview/mmwrhtml/rr5102a1.htm
The United States Preventive Services Task Force lists current recommendations, rationales, and clinical considerations at: www.ahrq.gov/clinic/uspstfix.htm
The bulk of the potential interventions are in the I category (insufficient evidence to recommend for or against) or are the subject of an ongoing evaluation. Many of these receive this rating not because the targeted behavior is in question but because it is unclear how effective physician counseling in a clinical encounter is in changing these behaviors—for example, avoidance of tobacco products and maintenance of ideal weight. Many providers will probably choose to provide young adolescents advice on these topics in spite of the meager evidence available.
CORRESPONDENCE
Doug Campos-Outcalt, MD, MPA, 4001 North Third Street #415, Phoenix, AZ 85012. E-mail: dougco@u.arizona.edu
All early adolescents should visit a physician at age 11 or 12 years to receive a set of recommended vaccines. Two vaccines are recommended for boys in this age group—quadrivalent meningococcal conjugate vaccine (MCV4) and tetanus toxoid, reduced diphtheria, and acellular pertussis vaccine (Tdap). Three vaccines are recommended for girls—MCV4, Tdap, and human papilloma virus (HPV) vaccine.
In addition, 2 doses of varicella vaccine are now recommended before age 5 years; both boys and girls at age 11 or 12 who have received only 1 dose should be given a second. TABLE 1 contains details on each recommended vaccine.
TABLE 1
Vaccines recommended for early adolescents
| VACCINE | ROUTE | SCHEDULE | CONTRAINDICATIONS* | PRECAUTIONS |
|---|---|---|---|---|
| MCV4 | IM | 1 dose | Moderate to severe illness | |
| Tdap | IM | 1 dose, may need other doses of Td to complete a tetanus and diphtheria series | Encephalopathy within 7 days of previous vaccine not attributed to other cause | Hypersensitivity with prior tetanus toxoid |
| Progressive neurological disorder | ||||
| Latex allergy | ||||
| Guillain-Barré syndrome within 6 weeks of a previous dose of tetanus toxoid | ||||
| Acute moderate to severe illness | ||||
| HPV | IM | 3 doses at months 0, 2, and 6 | History of hypersensitivity | Defer for moderate to severe illness to yeast |
| Varicella | SQ | 2 doses 3 months apart (1 month interval is acceptable) | Pregnancy | Moderate to severe illness |
| Severe suppression of cellular immunity | Receipt of antibody containing blood product in the preceding 11 months | |||
| Complete information for each vaccine can be located on the CDC web site at: www.cdc.gov/node.do/id/0900f3ec8005df1f. | ||||
| * All vaccines have as a contraindication a previous anaphylactic reaction to the vaccine or vaccine components. | ||||
| Details on contraindications can be found at: www.cdc.gov/nip/recs/contraindications_vacc.htm#var. | ||||
Meningococcal vaccine
Quadrivalent meningococcal conjugate vaccine (Menactra) contains antigens for 4 meningococcal groups (A, C, Y, W-135), and is licensed for ages 11 to 55 years. The Advisory Committee on Immunization Practices (ACIP) recommends that all preteens receive 1 dose at age 11 or 12. Unvaccinated older children should receive a dose before entering high school; unvaccinated college freshmen living in dorms should also be vaccinated.
Because of a shortage of vaccine, the Centers for Disease Control and Prevention (CDC) had recommended a delay in the implementation of routine vaccination at age 11 and 12. The supply situation has now corrected, and this recommendation has been rescinded.
There have been 17 cases of Guillain-Barré syndrome appearing in adolescents and young adults within 33 days of receiving MCV4. The possibility of a cause-and-effect relationship is under investigation. The CDC recommends that preadolescents and adolescents who have a history of Guillain-Barré should not receive MCV4 unless they are college freshmen who live in dorms.
Tetanus/diphtheria/pertussis vaccine
There are 2 Tdap products, one licensed for ages 10 to 18 years (Boostrix), the other for ages 11 to 64 (Adacel). The ACIP recommends a single dose of Tdap for those aged 11 to 18, preferably at age 11 or 12. The optimal interval from the last tetanus and diphtheria toxoid (TD or Td) is 5 years but a shorter interval is acceptable. Thereafter, Td boosters are recommended every 10 years. If an 11- or 12-year-old has not previously received a complete series of a tetanus toxoid, diphtheria product tetanus and diphtheria vaccines, they should be given the recommended number of doses—only one of which should be Tdap, the others Td. The number and timing of doses can be found at www.cdc.gov/mmwr/preview/mmwrhtml/rr55e223a5.htm.
Human papilloma virus vaccine
The HPV vaccine (Gardasil) is licensed only for females aged 9 to 26 years, and is the first vaccine for the prevention of cervical cancer. It protects against HPV types 6, 11, 16, and 18, which are the cause of approximately 80% of cervical cancers. The ACIP recommends routine administration for all females between ages 9 and 26, preferably before the onset of sexual activity. The vaccine requires 3 doses at months 0, 2, and 6; it can be administered concurrently with MCV4, Tdap, and Td.
Varicella vaccine
Two doses of varicella are now recommended for all children at ages 12 to 15 months and 4 to 6 years—the same as for the measles, mumps, and rubella vaccine (MMR). A new MMRV product (Proquad) could reduce the number of injections needed at these ages.
Adolescents and adults who are not immune to varicella should receive 2 doses of vaccine 3 months apart, or 1 dose if they have been vaccinated with a single dose of varicella vaccine. Immunity to varicella is defined as birth in the US prior to 1980, 2 doses of varicella vaccine, or having had a diagnosed case of chickenpox or shingles.
Other interventions
This expanding list of recommended vaccines should create an incentive for parents to bring their preteen children to visit a physician. The American College of Physicians (ACP) recommendations are built on the assumption that these vaccines should be part of a routine preventive visit at this age group.
If preteens do visit a physician more frequently, it will provide an opportunity for other health care maintenance interventions, such as measuring height, weight, and blood pressure and providing health education on diet, physical activity, and substance abuse. Unfortunately, the evidence base for the effectiveness of preventive interventions at this age is very weak.
TABLE 2 lists the interventions for the age group 11 to 12 years that have been evaluated by the US Preventive Services Task Force (USPSTF). This is not a comprehensive list of all possible preventive interventions for young adolescents, only those that have been evaluated by the USPSTF. Those with either an A (strongly recommend) or B (recommend) recommendation are screening tests related to risks involved with sexual activity and will not apply to all young adolescents. Some of those with a D recommendation (recommend against) will surprise many physicians, as they have historically been included in various screening guidelines.
TABLE 2
US Preventive Services Task Force recommendations on interventions for adolescents
Recommend for
|
Recommend Against
|
Insufficient Evidence to Recommend For or Against
|
Reports in Progress
|
For a listing of vaccine components and contraindications see: www.cdc.gov/nip/recs/contraindications.htm
Vaccine information statements are found at: www.cdc.gov/nip/publications/VIS/default.htm#hpv
For a useful chart with information on all vaccines go to: www.cdc.gov/nip/vaccine/vac-chart-hcp.htm
General information on immunizations is on the CDC web site: www.cdc.gov/mmwr/preview/mmwrhtml/rr5102a1.htm
The United States Preventive Services Task Force lists current recommendations, rationales, and clinical considerations at: www.ahrq.gov/clinic/uspstfix.htm
The bulk of the potential interventions are in the I category (insufficient evidence to recommend for or against) or are the subject of an ongoing evaluation. Many of these receive this rating not because the targeted behavior is in question but because it is unclear how effective physician counseling in a clinical encounter is in changing these behaviors—for example, avoidance of tobacco products and maintenance of ideal weight. Many providers will probably choose to provide young adolescents advice on these topics in spite of the meager evidence available.
CORRESPONDENCE
Doug Campos-Outcalt, MD, MPA, 4001 North Third Street #415, Phoenix, AZ 85012. E-mail: dougco@u.arizona.edu
Important questions before flu season
Influenza season is upon us. If this year is typical, 5% to 20 % of the US population will contract influenza. Of these, 200,000 people will be hospitalized and 36,000 will die. To minimize the effects of seasonal influenza, family physicians rely on annual influenza vaccine and antiviral therapy and prophylaxis. Every year at this time, we need to ask ourselves important questions as we prepare to provide maximum protection for our patients, our communities, and ourselves.
Who should receive an influenza vaccine?—an addition this year
The list of those who should receive an annual influenza vaccine (TABLE 1) is the same as last year, with 1 addition; children between ages 24 and 59 months and their household contacts and out-of-home caregivers.1-2 There are 2 types of vaccines available; trivalent inactivated influenza vaccine (TIA) and live attenuated influenza vaccine (LAIV). The TIA vaccine is administered intramuscularly, the LAIV by nasal spray. The vaccine products available and the ages for which they are licensed are listed in TABLE 2.3
The LAIV can be used for healthy people between the ages of 5 and 49 years; it should not be used for those who are pregnant, have a chronic illness, are caregivers to someone severely immune suppressed, are on chronic aspirin therapy, or have a history of Guillain-Barré syndrome. Neither vaccine should be used for anyone with an anaphylactic hypersensitivity to eggs or has had a severe allergic reaction to a previous vaccine. Vaccine should be postponed for anyone with a moderate-to-severe acute illness.
There are several other considerations for LAIV. Vaccination should be postponed for anyone with nasal congestion since it is not clear the vaccine can be administered correctly. Since LAIV is a live virus vaccine, it should be administered concurrently with, or 4 weeks after, any other live virus vaccines. It should also not be administered concurrently or within 2 weeks of receiving influenza antiviral agents. Finally, it needs to be stored at –15°C or below.
TABLE 1
Who should receive influenza vaccine
| People at high risk for complications from the flu, including: |
| Children aged 6–59 months |
| Pregnant women |
| People aged 50 years and older |
| People who live in nursing homes and other long-term care facilities |
People of any age with the following chronic medical conditions:
|
| People who live with or care for those at high risk for complications from flu, including: |
| Household contacts of persons at high risk for complications from the flu (see above) |
| Household contacts and out of home caregivers of children less than 6 months of age (these children are too young to be vaccinated) |
| Healthcare workers |
TABLE 2
Vaccine products available
| VACCINE | TRADE NAME (MANUFACTURER) | DOSE/PRESENTATION | THIMEROSAL MERCURY CONTENT (MCG/HG/0.5-ML DOSE) | AGE GROUP | NO. OF DOSES | ROUTE |
|---|---|---|---|---|---|---|
| Inactivated | ||||||
| TIV | Fluzone (Sanofi Pasteur) | 0.25-mL prefilled syringe | 0 | 6–35 mos | 1 or 2* | IM† |
| 0.5-mL prefilled syringe | 0 | ≥36 mos | 1 or 2* | IM† | ||
| 0.5-mL vial | 0 | ≥36 mos | 1 or 2* | IM† | ||
| 5.0-mL multidose vial | 25 | ≥6 mos | 1 or 2* | IM† | ||
| TIV | Fluvirin | 0.5-mL prefilled syringe | <1.0 | ≥4 years | 1 or 2* | IM† |
| 5.0-mL multidose vial | 24.5 | ≥4 years | 1 or 2* | IM† | ||
| TIV | FLUARIX (Glaxo-SmithKline) | 0.5-mL prefilled syringe | <1.25 | ≥18 years | 1 | IM† |
| Live, attenuated | ||||||
| LAIV | FluMist (Medimmune) | 0.5-mL sprayer | 0 | 5–49 years | 1 or 2‡ | Intranasal** |
| * Two doses administered at least 1 month apart are recommended for children aged 6 months to <9 years who are receiving influenza vaccine for the first time. | ||||||
| † For adults and older children, the recommended site of vaccination is the deltoid muscle. | ||||||
| The preferred site for infants and young children is the anterolateral aspect of the thigh. | ||||||
| ‡ Two doses administered at least 6 weeks apart are recommended for children aged 5 to <9 years who are receiving influenza vaccine for the first time. | ||||||
| ** One dose equals 0.5 mL, divided equally between each nostril. | ||||||
What issues are specific to ages 6 months to <9 years?
A child being immunized against influenza for the first time before his or her ninth birthday should receive 2 doses—4 weeks apart for TIV, 6 weeks apart for LAIV. The doses can be 2 of either TIV or LAIV or 1 of each. If a child received only 1 dose the prior year and it was their first time to receive the vaccine, they only need 1 dose the current year.
The supply of vaccine for this age group can be problematic, especially for age 3, for whom there is only 1 product licensed. Four of the products available for children contain trace amounts of thimerosal (TABLE 2), with the highest amounts being in multidose vials.3 There have been no proven harmful effects of these amounts of thimerosal, and both the Centers for Disease Control and Prevention (CDC) and the Food and Drug Administration (FDA) continue to recommend these products. Some parents, however, may insist on a thimerosal-free product, and a few states have taken action to limit the use of thimerosal-containing vaccines.
When should we immunize?
Immunization of those at high risk should begin in September (if vaccine is available) or October. This is particularly important for children who need 2 doses for protection. Vaccination in nursing homes is best started in October—early enough to provide protection throughout the season but late enough to provide more assurance immunity will last throughout a late season. Vaccination of all those at risk should continue throughout the whole season, or as long as vaccine supplies last.
Will there be an adequate supply of vaccine?
There will be between 100 million and 120 million doses of vaccine available this year. Based on prior years, this should be an adequate supply.
Information is available on the American Academy of Family Physicians (AAFP) Web site on current vaccine supply issues and how to purchase influenza vaccine (available at: www.aafp.org/online/en/home/clinical/immunizations/flu06/ordering.html). The AAFP Web site will also contain information on vaccine prioritization should a shortage develop.
What is the role of antiviral medications?
The CDC currently recommends that the adamantanes (amantadine [Symmetrel] and rimantadine [Flumadine]) not be used for treatment or prophylaxis of influenza A because of a high rate of resistance documented last flu season. This situation could change as the current influenza season progresses. The remaining antivirals are both neuraminidase inhibitors; oseltamivir (Tamiflu—licensed for use in treatment and prophylaxis beginning at age 1 year) and zanamivir (Relenza—licensed for treatment beginning at age 5 years and prophylaxis at age 7 years).
Chemoprophylaxis is most useful in those whom the vaccine is contraindicated; in the 2 weeks after receipt of a vaccine, which is the time needed for it to be effective (2 weeks after the second dose in children receiving the vaccine for the first time); when the circulating virus does not match the vaccine; in those who are immune-suppressed and may have an inadequate response to the vaccine; and in nursing homes where there is an outbreak, when it should be used for everyone regardless of their vaccine status.
Treatment of those with influenza A can shorten the illness and reduce its severity if started within 2 days of symptoms. Details on antiviral recommendations and doses for treatment and prophylaxis can be found in the annual CDC influenza recommendations.3
Are rapid office lab tests useful?
The gold standard for laboratory confirmation of influenza is viral culture from a nasopharyngeal swab or washing. The time needed for this creates some difficulty initiating antiviral therapy within the two day window. Rapid, office-based tests are available and are listed in TABLE 3.5 Some of these tests are specific for influenza A, others for influenza B, and some are for both. The sensitivities and specificities for each product vary. A negative test in a highly suspicious patient should not rule out the disease, especially in a high prevalence situation. In a low prevalence situation a positive test is more likely to be a false positive than when the virus is causing an outbreak in the community.
TABLE 3
Rapid (<30-minute) laboratory tests available for influenza
| RAPID DIAGNOSTIC TESTS | INFLUENZA TYPE | APPLICATION METHODS |
|---|---|---|
| Directigen Flu A* (Becton-Dickinson) | A | NP swab, throat swab, nasal wash, nasal aspirate |
| Directigen Flu A+B* (Becton-Dickinson) | A and B† | NP swab, throat swab, nasal wash, nasal aspirate |
| Directigen EZ Flu A+B* (Becton-Dickinson) | A and B† | Throat swab, nasal wash, nasal aspirate |
| FLU OIA* (Thermo Electron) | A and B‡ | NP swab, throat swab, nasal aspirate, sputum |
| FLU OIA A/B* (Thermo Electron) | A and B† | NP swab, throat swab, nasal aspirate, sputum |
| XPECT Flu A&B* (Remel) | A and B† | Nasal wash, NP swab, throat swab |
| NOW Influenza A & B* (Binax) | A and B† | Nasal wash, NP swab |
| QuickVue Influenza Test** (Quidel) | A and B‡ | NP swab, nasal wash, nasal aspirate |
| QuickVue Influenza A+B Test** (Quidel) | A and B‡ | NP swab, nasal wash, nasal aspirate |
| SAS Influenza A Test* | A† | NP wash, NP aspirate |
| SAS Influenza B Test* | A† | NP wash, NP aspirate |
| ZstatFlu† (ZymeTx) | A and B‡ | Throat swab |
| Table may not include all test kits approved by the US Food and Drug Administration. | ||
| NP, nasopharyngeal | ||
| * Moderately complex test—requires specific laboratory certification. | ||
| † Distinguishes between influenza A and B virus infections. | ||
| ‡ Does not distinguish between influenza A and B virus infections. | ||
| ** CLIA-waived test. Can be used in any office setting. Requires a certificate of waiver or higher laboratory certification | ||
| Source: Centers for Disease Control and Prevention.5 | ||
CORRESPONDENCE
Doug Campos-Outcalt, MD, MPA, 4001 North Third Street #415, Phoenix, AZ 85012. E-mail: dougco@u.arizona.edu
RESOURCES
1. American Academy of Family Physicians Web site. Clinical Care & Research. Immunization resources. Available at: www.aafp.org/online/en/home/clinical/immunizations.html. Accessed on September 21, 2006. Current immunization recommendations, information on ordering influenza vaccine and steps to take should there be a vaccine shortage.
2. Centers for Disease Control and Prevention (CDC) Web site. Influenza (flu). Available at: www.cdc.gov/flu/. Accessed on September 21, 2006. Information for health professionals and consumers on all aspects of influenza.
3. CDC. Prevention and control of influenza. MMWR Recomm Rep 2006; 55:(RR-10):1-42. Available at: www.cdc.gov/mmwr/preview/mmwrhtml/rr5510a1.htm. Accessed on September 21, 2006. Annual update on recommendations for physicians on influenza diagnosis, treatment and prevention.
4. CDC Web site. Infection guidance for the prevention and control of influenza in acute care facilities. Available at: www.cdc.gov/flu/professionals/infectioncontrol/health-carefacilities.htm. Accessed on September 21, 2006. Information on how to protect staff and patients from the spread of influenza in the office setting.
5. CDC. Prevention and control of influenza: Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2005; 54(RR-8):1-40. Available at: www.cdc.gov/flu/professionals/labdiagnosis.htm. Accessed on September 21, 2006.
Influenza season is upon us. If this year is typical, 5% to 20 % of the US population will contract influenza. Of these, 200,000 people will be hospitalized and 36,000 will die. To minimize the effects of seasonal influenza, family physicians rely on annual influenza vaccine and antiviral therapy and prophylaxis. Every year at this time, we need to ask ourselves important questions as we prepare to provide maximum protection for our patients, our communities, and ourselves.
Who should receive an influenza vaccine?—an addition this year
The list of those who should receive an annual influenza vaccine (TABLE 1) is the same as last year, with 1 addition; children between ages 24 and 59 months and their household contacts and out-of-home caregivers.1-2 There are 2 types of vaccines available; trivalent inactivated influenza vaccine (TIA) and live attenuated influenza vaccine (LAIV). The TIA vaccine is administered intramuscularly, the LAIV by nasal spray. The vaccine products available and the ages for which they are licensed are listed in TABLE 2.3
The LAIV can be used for healthy people between the ages of 5 and 49 years; it should not be used for those who are pregnant, have a chronic illness, are caregivers to someone severely immune suppressed, are on chronic aspirin therapy, or have a history of Guillain-Barré syndrome. Neither vaccine should be used for anyone with an anaphylactic hypersensitivity to eggs or has had a severe allergic reaction to a previous vaccine. Vaccine should be postponed for anyone with a moderate-to-severe acute illness.
There are several other considerations for LAIV. Vaccination should be postponed for anyone with nasal congestion since it is not clear the vaccine can be administered correctly. Since LAIV is a live virus vaccine, it should be administered concurrently with, or 4 weeks after, any other live virus vaccines. It should also not be administered concurrently or within 2 weeks of receiving influenza antiviral agents. Finally, it needs to be stored at –15°C or below.
TABLE 1
Who should receive influenza vaccine
| People at high risk for complications from the flu, including: |
| Children aged 6–59 months |
| Pregnant women |
| People aged 50 years and older |
| People who live in nursing homes and other long-term care facilities |
People of any age with the following chronic medical conditions:
|
| People who live with or care for those at high risk for complications from flu, including: |
| Household contacts of persons at high risk for complications from the flu (see above) |
| Household contacts and out of home caregivers of children less than 6 months of age (these children are too young to be vaccinated) |
| Healthcare workers |
TABLE 2
Vaccine products available
| VACCINE | TRADE NAME (MANUFACTURER) | DOSE/PRESENTATION | THIMEROSAL MERCURY CONTENT (MCG/HG/0.5-ML DOSE) | AGE GROUP | NO. OF DOSES | ROUTE |
|---|---|---|---|---|---|---|
| Inactivated | ||||||
| TIV | Fluzone (Sanofi Pasteur) | 0.25-mL prefilled syringe | 0 | 6–35 mos | 1 or 2* | IM† |
| 0.5-mL prefilled syringe | 0 | ≥36 mos | 1 or 2* | IM† | ||
| 0.5-mL vial | 0 | ≥36 mos | 1 or 2* | IM† | ||
| 5.0-mL multidose vial | 25 | ≥6 mos | 1 or 2* | IM† | ||
| TIV | Fluvirin | 0.5-mL prefilled syringe | <1.0 | ≥4 years | 1 or 2* | IM† |
| 5.0-mL multidose vial | 24.5 | ≥4 years | 1 or 2* | IM† | ||
| TIV | FLUARIX (Glaxo-SmithKline) | 0.5-mL prefilled syringe | <1.25 | ≥18 years | 1 | IM† |
| Live, attenuated | ||||||
| LAIV | FluMist (Medimmune) | 0.5-mL sprayer | 0 | 5–49 years | 1 or 2‡ | Intranasal** |
| * Two doses administered at least 1 month apart are recommended for children aged 6 months to <9 years who are receiving influenza vaccine for the first time. | ||||||
| † For adults and older children, the recommended site of vaccination is the deltoid muscle. | ||||||
| The preferred site for infants and young children is the anterolateral aspect of the thigh. | ||||||
| ‡ Two doses administered at least 6 weeks apart are recommended for children aged 5 to <9 years who are receiving influenza vaccine for the first time. | ||||||
| ** One dose equals 0.5 mL, divided equally between each nostril. | ||||||
What issues are specific to ages 6 months to <9 years?
A child being immunized against influenza for the first time before his or her ninth birthday should receive 2 doses—4 weeks apart for TIV, 6 weeks apart for LAIV. The doses can be 2 of either TIV or LAIV or 1 of each. If a child received only 1 dose the prior year and it was their first time to receive the vaccine, they only need 1 dose the current year.
The supply of vaccine for this age group can be problematic, especially for age 3, for whom there is only 1 product licensed. Four of the products available for children contain trace amounts of thimerosal (TABLE 2), with the highest amounts being in multidose vials.3 There have been no proven harmful effects of these amounts of thimerosal, and both the Centers for Disease Control and Prevention (CDC) and the Food and Drug Administration (FDA) continue to recommend these products. Some parents, however, may insist on a thimerosal-free product, and a few states have taken action to limit the use of thimerosal-containing vaccines.
When should we immunize?
Immunization of those at high risk should begin in September (if vaccine is available) or October. This is particularly important for children who need 2 doses for protection. Vaccination in nursing homes is best started in October—early enough to provide protection throughout the season but late enough to provide more assurance immunity will last throughout a late season. Vaccination of all those at risk should continue throughout the whole season, or as long as vaccine supplies last.
Will there be an adequate supply of vaccine?
There will be between 100 million and 120 million doses of vaccine available this year. Based on prior years, this should be an adequate supply.
Information is available on the American Academy of Family Physicians (AAFP) Web site on current vaccine supply issues and how to purchase influenza vaccine (available at: www.aafp.org/online/en/home/clinical/immunizations/flu06/ordering.html). The AAFP Web site will also contain information on vaccine prioritization should a shortage develop.
What is the role of antiviral medications?
The CDC currently recommends that the adamantanes (amantadine [Symmetrel] and rimantadine [Flumadine]) not be used for treatment or prophylaxis of influenza A because of a high rate of resistance documented last flu season. This situation could change as the current influenza season progresses. The remaining antivirals are both neuraminidase inhibitors; oseltamivir (Tamiflu—licensed for use in treatment and prophylaxis beginning at age 1 year) and zanamivir (Relenza—licensed for treatment beginning at age 5 years and prophylaxis at age 7 years).
Chemoprophylaxis is most useful in those whom the vaccine is contraindicated; in the 2 weeks after receipt of a vaccine, which is the time needed for it to be effective (2 weeks after the second dose in children receiving the vaccine for the first time); when the circulating virus does not match the vaccine; in those who are immune-suppressed and may have an inadequate response to the vaccine; and in nursing homes where there is an outbreak, when it should be used for everyone regardless of their vaccine status.
Treatment of those with influenza A can shorten the illness and reduce its severity if started within 2 days of symptoms. Details on antiviral recommendations and doses for treatment and prophylaxis can be found in the annual CDC influenza recommendations.3
Are rapid office lab tests useful?
The gold standard for laboratory confirmation of influenza is viral culture from a nasopharyngeal swab or washing. The time needed for this creates some difficulty initiating antiviral therapy within the two day window. Rapid, office-based tests are available and are listed in TABLE 3.5 Some of these tests are specific for influenza A, others for influenza B, and some are for both. The sensitivities and specificities for each product vary. A negative test in a highly suspicious patient should not rule out the disease, especially in a high prevalence situation. In a low prevalence situation a positive test is more likely to be a false positive than when the virus is causing an outbreak in the community.
TABLE 3
Rapid (<30-minute) laboratory tests available for influenza
| RAPID DIAGNOSTIC TESTS | INFLUENZA TYPE | APPLICATION METHODS |
|---|---|---|
| Directigen Flu A* (Becton-Dickinson) | A | NP swab, throat swab, nasal wash, nasal aspirate |
| Directigen Flu A+B* (Becton-Dickinson) | A and B† | NP swab, throat swab, nasal wash, nasal aspirate |
| Directigen EZ Flu A+B* (Becton-Dickinson) | A and B† | Throat swab, nasal wash, nasal aspirate |
| FLU OIA* (Thermo Electron) | A and B‡ | NP swab, throat swab, nasal aspirate, sputum |
| FLU OIA A/B* (Thermo Electron) | A and B† | NP swab, throat swab, nasal aspirate, sputum |
| XPECT Flu A&B* (Remel) | A and B† | Nasal wash, NP swab, throat swab |
| NOW Influenza A & B* (Binax) | A and B† | Nasal wash, NP swab |
| QuickVue Influenza Test** (Quidel) | A and B‡ | NP swab, nasal wash, nasal aspirate |
| QuickVue Influenza A+B Test** (Quidel) | A and B‡ | NP swab, nasal wash, nasal aspirate |
| SAS Influenza A Test* | A† | NP wash, NP aspirate |
| SAS Influenza B Test* | A† | NP wash, NP aspirate |
| ZstatFlu† (ZymeTx) | A and B‡ | Throat swab |
| Table may not include all test kits approved by the US Food and Drug Administration. | ||
| NP, nasopharyngeal | ||
| * Moderately complex test—requires specific laboratory certification. | ||
| † Distinguishes between influenza A and B virus infections. | ||
| ‡ Does not distinguish between influenza A and B virus infections. | ||
| ** CLIA-waived test. Can be used in any office setting. Requires a certificate of waiver or higher laboratory certification | ||
| Source: Centers for Disease Control and Prevention.5 | ||
CORRESPONDENCE
Doug Campos-Outcalt, MD, MPA, 4001 North Third Street #415, Phoenix, AZ 85012. E-mail: dougco@u.arizona.edu
Influenza season is upon us. If this year is typical, 5% to 20 % of the US population will contract influenza. Of these, 200,000 people will be hospitalized and 36,000 will die. To minimize the effects of seasonal influenza, family physicians rely on annual influenza vaccine and antiviral therapy and prophylaxis. Every year at this time, we need to ask ourselves important questions as we prepare to provide maximum protection for our patients, our communities, and ourselves.
Who should receive an influenza vaccine?—an addition this year
The list of those who should receive an annual influenza vaccine (TABLE 1) is the same as last year, with 1 addition; children between ages 24 and 59 months and their household contacts and out-of-home caregivers.1-2 There are 2 types of vaccines available; trivalent inactivated influenza vaccine (TIA) and live attenuated influenza vaccine (LAIV). The TIA vaccine is administered intramuscularly, the LAIV by nasal spray. The vaccine products available and the ages for which they are licensed are listed in TABLE 2.3
The LAIV can be used for healthy people between the ages of 5 and 49 years; it should not be used for those who are pregnant, have a chronic illness, are caregivers to someone severely immune suppressed, are on chronic aspirin therapy, or have a history of Guillain-Barré syndrome. Neither vaccine should be used for anyone with an anaphylactic hypersensitivity to eggs or has had a severe allergic reaction to a previous vaccine. Vaccine should be postponed for anyone with a moderate-to-severe acute illness.
There are several other considerations for LAIV. Vaccination should be postponed for anyone with nasal congestion since it is not clear the vaccine can be administered correctly. Since LAIV is a live virus vaccine, it should be administered concurrently with, or 4 weeks after, any other live virus vaccines. It should also not be administered concurrently or within 2 weeks of receiving influenza antiviral agents. Finally, it needs to be stored at –15°C or below.
TABLE 1
Who should receive influenza vaccine
| People at high risk for complications from the flu, including: |
| Children aged 6–59 months |
| Pregnant women |
| People aged 50 years and older |
| People who live in nursing homes and other long-term care facilities |
People of any age with the following chronic medical conditions:
|
| People who live with or care for those at high risk for complications from flu, including: |
| Household contacts of persons at high risk for complications from the flu (see above) |
| Household contacts and out of home caregivers of children less than 6 months of age (these children are too young to be vaccinated) |
| Healthcare workers |
TABLE 2
Vaccine products available
| VACCINE | TRADE NAME (MANUFACTURER) | DOSE/PRESENTATION | THIMEROSAL MERCURY CONTENT (MCG/HG/0.5-ML DOSE) | AGE GROUP | NO. OF DOSES | ROUTE |
|---|---|---|---|---|---|---|
| Inactivated | ||||||
| TIV | Fluzone (Sanofi Pasteur) | 0.25-mL prefilled syringe | 0 | 6–35 mos | 1 or 2* | IM† |
| 0.5-mL prefilled syringe | 0 | ≥36 mos | 1 or 2* | IM† | ||
| 0.5-mL vial | 0 | ≥36 mos | 1 or 2* | IM† | ||
| 5.0-mL multidose vial | 25 | ≥6 mos | 1 or 2* | IM† | ||
| TIV | Fluvirin | 0.5-mL prefilled syringe | <1.0 | ≥4 years | 1 or 2* | IM† |
| 5.0-mL multidose vial | 24.5 | ≥4 years | 1 or 2* | IM† | ||
| TIV | FLUARIX (Glaxo-SmithKline) | 0.5-mL prefilled syringe | <1.25 | ≥18 years | 1 | IM† |
| Live, attenuated | ||||||
| LAIV | FluMist (Medimmune) | 0.5-mL sprayer | 0 | 5–49 years | 1 or 2‡ | Intranasal** |
| * Two doses administered at least 1 month apart are recommended for children aged 6 months to <9 years who are receiving influenza vaccine for the first time. | ||||||
| † For adults and older children, the recommended site of vaccination is the deltoid muscle. | ||||||
| The preferred site for infants and young children is the anterolateral aspect of the thigh. | ||||||
| ‡ Two doses administered at least 6 weeks apart are recommended for children aged 5 to <9 years who are receiving influenza vaccine for the first time. | ||||||
| ** One dose equals 0.5 mL, divided equally between each nostril. | ||||||
What issues are specific to ages 6 months to <9 years?
A child being immunized against influenza for the first time before his or her ninth birthday should receive 2 doses—4 weeks apart for TIV, 6 weeks apart for LAIV. The doses can be 2 of either TIV or LAIV or 1 of each. If a child received only 1 dose the prior year and it was their first time to receive the vaccine, they only need 1 dose the current year.
The supply of vaccine for this age group can be problematic, especially for age 3, for whom there is only 1 product licensed. Four of the products available for children contain trace amounts of thimerosal (TABLE 2), with the highest amounts being in multidose vials.3 There have been no proven harmful effects of these amounts of thimerosal, and both the Centers for Disease Control and Prevention (CDC) and the Food and Drug Administration (FDA) continue to recommend these products. Some parents, however, may insist on a thimerosal-free product, and a few states have taken action to limit the use of thimerosal-containing vaccines.
When should we immunize?
Immunization of those at high risk should begin in September (if vaccine is available) or October. This is particularly important for children who need 2 doses for protection. Vaccination in nursing homes is best started in October—early enough to provide protection throughout the season but late enough to provide more assurance immunity will last throughout a late season. Vaccination of all those at risk should continue throughout the whole season, or as long as vaccine supplies last.
Will there be an adequate supply of vaccine?
There will be between 100 million and 120 million doses of vaccine available this year. Based on prior years, this should be an adequate supply.
Information is available on the American Academy of Family Physicians (AAFP) Web site on current vaccine supply issues and how to purchase influenza vaccine (available at: www.aafp.org/online/en/home/clinical/immunizations/flu06/ordering.html). The AAFP Web site will also contain information on vaccine prioritization should a shortage develop.
What is the role of antiviral medications?
The CDC currently recommends that the adamantanes (amantadine [Symmetrel] and rimantadine [Flumadine]) not be used for treatment or prophylaxis of influenza A because of a high rate of resistance documented last flu season. This situation could change as the current influenza season progresses. The remaining antivirals are both neuraminidase inhibitors; oseltamivir (Tamiflu—licensed for use in treatment and prophylaxis beginning at age 1 year) and zanamivir (Relenza—licensed for treatment beginning at age 5 years and prophylaxis at age 7 years).
Chemoprophylaxis is most useful in those whom the vaccine is contraindicated; in the 2 weeks after receipt of a vaccine, which is the time needed for it to be effective (2 weeks after the second dose in children receiving the vaccine for the first time); when the circulating virus does not match the vaccine; in those who are immune-suppressed and may have an inadequate response to the vaccine; and in nursing homes where there is an outbreak, when it should be used for everyone regardless of their vaccine status.
Treatment of those with influenza A can shorten the illness and reduce its severity if started within 2 days of symptoms. Details on antiviral recommendations and doses for treatment and prophylaxis can be found in the annual CDC influenza recommendations.3
Are rapid office lab tests useful?
The gold standard for laboratory confirmation of influenza is viral culture from a nasopharyngeal swab or washing. The time needed for this creates some difficulty initiating antiviral therapy within the two day window. Rapid, office-based tests are available and are listed in TABLE 3.5 Some of these tests are specific for influenza A, others for influenza B, and some are for both. The sensitivities and specificities for each product vary. A negative test in a highly suspicious patient should not rule out the disease, especially in a high prevalence situation. In a low prevalence situation a positive test is more likely to be a false positive than when the virus is causing an outbreak in the community.
TABLE 3
Rapid (<30-minute) laboratory tests available for influenza
| RAPID DIAGNOSTIC TESTS | INFLUENZA TYPE | APPLICATION METHODS |
|---|---|---|
| Directigen Flu A* (Becton-Dickinson) | A | NP swab, throat swab, nasal wash, nasal aspirate |
| Directigen Flu A+B* (Becton-Dickinson) | A and B† | NP swab, throat swab, nasal wash, nasal aspirate |
| Directigen EZ Flu A+B* (Becton-Dickinson) | A and B† | Throat swab, nasal wash, nasal aspirate |
| FLU OIA* (Thermo Electron) | A and B‡ | NP swab, throat swab, nasal aspirate, sputum |
| FLU OIA A/B* (Thermo Electron) | A and B† | NP swab, throat swab, nasal aspirate, sputum |
| XPECT Flu A&B* (Remel) | A and B† | Nasal wash, NP swab, throat swab |
| NOW Influenza A & B* (Binax) | A and B† | Nasal wash, NP swab |
| QuickVue Influenza Test** (Quidel) | A and B‡ | NP swab, nasal wash, nasal aspirate |
| QuickVue Influenza A+B Test** (Quidel) | A and B‡ | NP swab, nasal wash, nasal aspirate |
| SAS Influenza A Test* | A† | NP wash, NP aspirate |
| SAS Influenza B Test* | A† | NP wash, NP aspirate |
| ZstatFlu† (ZymeTx) | A and B‡ | Throat swab |
| Table may not include all test kits approved by the US Food and Drug Administration. | ||
| NP, nasopharyngeal | ||
| * Moderately complex test—requires specific laboratory certification. | ||
| † Distinguishes between influenza A and B virus infections. | ||
| ‡ Does not distinguish between influenza A and B virus infections. | ||
| ** CLIA-waived test. Can be used in any office setting. Requires a certificate of waiver or higher laboratory certification | ||
| Source: Centers for Disease Control and Prevention.5 | ||
CORRESPONDENCE
Doug Campos-Outcalt, MD, MPA, 4001 North Third Street #415, Phoenix, AZ 85012. E-mail: dougco@u.arizona.edu
RESOURCES
1. American Academy of Family Physicians Web site. Clinical Care & Research. Immunization resources. Available at: www.aafp.org/online/en/home/clinical/immunizations.html. Accessed on September 21, 2006. Current immunization recommendations, information on ordering influenza vaccine and steps to take should there be a vaccine shortage.
2. Centers for Disease Control and Prevention (CDC) Web site. Influenza (flu). Available at: www.cdc.gov/flu/. Accessed on September 21, 2006. Information for health professionals and consumers on all aspects of influenza.
3. CDC. Prevention and control of influenza. MMWR Recomm Rep 2006; 55:(RR-10):1-42. Available at: www.cdc.gov/mmwr/preview/mmwrhtml/rr5510a1.htm. Accessed on September 21, 2006. Annual update on recommendations for physicians on influenza diagnosis, treatment and prevention.
4. CDC Web site. Infection guidance for the prevention and control of influenza in acute care facilities. Available at: www.cdc.gov/flu/professionals/infectioncontrol/health-carefacilities.htm. Accessed on September 21, 2006. Information on how to protect staff and patients from the spread of influenza in the office setting.
5. CDC. Prevention and control of influenza: Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2005; 54(RR-8):1-40. Available at: www.cdc.gov/flu/professionals/labdiagnosis.htm. Accessed on September 21, 2006.
RESOURCES
1. American Academy of Family Physicians Web site. Clinical Care & Research. Immunization resources. Available at: www.aafp.org/online/en/home/clinical/immunizations.html. Accessed on September 21, 2006. Current immunization recommendations, information on ordering influenza vaccine and steps to take should there be a vaccine shortage.
2. Centers for Disease Control and Prevention (CDC) Web site. Influenza (flu). Available at: www.cdc.gov/flu/. Accessed on September 21, 2006. Information for health professionals and consumers on all aspects of influenza.
3. CDC. Prevention and control of influenza. MMWR Recomm Rep 2006; 55:(RR-10):1-42. Available at: www.cdc.gov/mmwr/preview/mmwrhtml/rr5510a1.htm. Accessed on September 21, 2006. Annual update on recommendations for physicians on influenza diagnosis, treatment and prevention.
4. CDC Web site. Infection guidance for the prevention and control of influenza in acute care facilities. Available at: www.cdc.gov/flu/professionals/infectioncontrol/health-carefacilities.htm. Accessed on September 21, 2006. Information on how to protect staff and patients from the spread of influenza in the office setting.
5. CDC. Prevention and control of influenza: Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2005; 54(RR-8):1-40. Available at: www.cdc.gov/flu/professionals/labdiagnosis.htm. Accessed on September 21, 2006.
The Journal of Family Practice ©2006 Dowden Health Media
HIV postexposure prophylaxis: Who should get it?
One of your office personnel receives a superficial stick from a needle while putting it into a sharps disposal container. Is postexposure prophylaxis (PEP) for HIV warranted?
Another health care worker receives a major blood splash into her eye after dropping a blood tube taken from a source of unknown HIV status. Is PEP called for in this instance?
A child who was rifling through a trash bin accidentally poked himself with an improperly disposed hypodermic needle. Should he be given PEP?
In most cases, HIV PEP is given only to healthcare workers if the settings make exposure to HIV-infected persons likely. Otherwise, it is usually deemed unnecessary. However, a decision for or against PEP is complicated.
Occupational and nonoccupational exposure to HIV can produce fear, anxiety, and stress. Information on the exposure risk is frequently incomplete, the risk of infection is usually low, the degree of protection offered by PEP is not fully defined, and the potential for side effects from the medications is significant.
This article distills the Centers for Disease Control and Prevention’s most recent guidance on HIV PEP.
HIV on the rise again
Antiretroviral therapy has markedly reduced mortality from HIV/AIDS, but the incidence of new cases, after declining in the 1990s, has gradually increased since 2000.1 As described in a previous article in the Journal of Family Practice,2 efforts to control HIV now focus on increased testing of those persons at risk, behavior modification to reduce the chances of infected persons exposing others, and treating HIV-positive pregnant women and providing postnatal prophylaxis to their newly born infants.
Exposure to HIV can occur occupationally, during a sexual assault, or from the failure of barrier protection during sex. Though these types of exposure are not major contributors to HIV incidence, and postexposure prophylaxis is not expected to play a major role in reducing the incidence of disease, it is available to persons potentially exposed to HIV, and it is beneficial to know when it is and is not indicated. Evidence for possible effectiveness of PEP comes from studies of postnatal prophylaxis, animal studies, case control studies and case reports.3
The Centers for Disease Control and Prevention (CDC) has developed 2 sets of recommendations for PEP that take into consideration the type and severity of the exposure and characteristics of the source of the exposure (TABLE 1).3,4
TABLE 1
Recommended HIV postexposure prophylaxis for percutanous injuries and membrane/nonintact skin exposures
| For percutaneous injuries | |||||
| EXPOSURE TYPE | INFECTION STATUS OF SOURCE | ||||
| HIV-POSITIVE, CLASS 1* | HIV-POSITIVE, CLASS 2* | SOURCE OF UNKNOWN HIV STATUS† | UNKNOWN SOURCE‡ | HIV-NEGATIVE | |
| Less severe (eg, solid needle or supercficial injury) | Recommend basic 2-drug PEP | Recommend expanded ≥3-drug PEP | Generally, no PEP warranted however, consider basic 2-drug PEP¶ for source with HIV risk factors** | Generally, no PEP warranted; however, consider basic 2-drug PEP¶ in settings in which exposure to HIV-infected persons is likely | No PEP warranted |
| More severe (large-bore hollow needle, deep puncture wound, blood on device, needle used in artery/vein) | Recommend expanded ≥3-drug PEP | Recommend expanded ≥3-drug PEP | Generally, no PEP warranted; however, consider basic 2-drug¶ for source with HIV risk factors** | Generally, no PEP warranted; however, consider basic 2-drug PEP¶ in settings in which exposure to HIV infected persons is likely | No PEP warranted |
| For mucous membrane and nonintact skin exposures†† | |||||
| Small volume (eg, a few drops) | Consider basic 2-drug PEP¶ | Recommend basic 2-drug PEP | Generally, no PEP warranted** | Generally, no PEP warranted | No PEP warranted |
| Large volume (eg, a major blood splash) | Recommend basic 2-drug PEP | Recommend expended ≥3-drug PEP | Generally, no PEP warranted; however, consider basic 2-drug PEP¶ for source with HIV risk factors** | Generally, no PEP warranted; however, consider basic 2-drug PEP¶ in settings in which exposure to HIV-infected persons is likely | No PEP warranted |
| *HIV-positive, class 1—asymptomatic HIV infection or known low viral load (eg, <1500 ribonucleic acid copies/mL). HIV-positive, class 2—symptomatic HIV infection, AIDS, acute seroconversion, or known high viral load. If drug resistance is a concern, obtain expert consultation. Initiation of PEP should not be delayed pending expert consultation, and, because expert consultation alone cannot substitute for face-to-face counseling, resources should be available to provide immediate evaluation and follow-up care for all exposures. | |||||
| †For example, deceased source person with no samples available for HIV testing. | |||||
| ‡For example, a needle from a sharps container or splash from inappropriately disposed blood. | |||||
| ¶The recommendation “consider PEP” indicates that PEP is optional; a decision to initiate PEP should be based on a discussion between the exposed person and the treating clinician regarding the risks versus benefits of PEP. | |||||
| **If PEP is offered and administered and the source is later determined to be HIV-negative, PEP should be discontinued. | |||||
| ††For skin exposures, follow-up is indicated only if evidence exists of compromised skin integrity (eg, dermatitis, abrasion, or open wound). | |||||
| Source: Centers for Disease Control and Prevention 2005.4 | |||||
Occupational exposures to HIV
Occupational exposure to HIV can result from a needlestick injury, cut with a sharp object, or contact with potentially infectious body fluids to mucous membranes or skin that is not intact (chapped, cut, abraded, inflamed). Body fluids that are considered potentially infectious are listed in TABLE 2, along with fluids not considered to be infectious.
The risk of contracting HIV from an occupational exposure is determined by several factors, but is generally low. The risk of infection after a needle-stick injury with exposure to infected blood is estimated at 0.3%; after a mucous membrane exposure, 0.09%. The risk after exposure to nonintact skin is probably even lower. Risk increases with the quantity of blood exposed to, a needle-stick injury directly into a vein or artery, and deep injuries.
TABLE 2
Which body fluids are infectious?
| BODY FLUIDS POTENTIALLY INFECTIOUS FOR HIV | |
|
|
| BODY FLUIDS NOT CONSIDERED TO BE INFECTIOUS UNLESS THEY ARE VISIBLY BLOODY | |
|
|
Who should and should not receive PEP
TABLE 1 details recommended treatment responses to specific types of exposure (eg, puncture wound) and the status of the exposure source.4 In situations unlikely to result in disease transmission (superficial injury and source patient with unknown HIV status), no PEP is generally warranted due to the low risk of infection and potential toxicity of antiretrovirals.
Treatment particulars
Start postexposure prophylaxis, when indicated, as soon as possible following exposure and continue it for 4 weeks. Obtain baseline test results for HIV at the time of exposure and periodically for 6 months. The CDC recommends testing at 6 weeks, 12 weeks, and 6 months, whether or not PEP is provided.
Testing and monitoring for hepatitis B and C may also be indicated.
Advise patients on PEP to use precautions in avoiding the possibility of secondary transmission, especially in the first 3 months following exposure. Monitor for drug toxicity every week or 2 while giving PEP. Because of the complexity of potential PEP regimens and the risk for drug toxicity, you may want to take advantage of several national sources of consultation, such as the PEPline (www.ucsf.edu/hivcntr/Hotlines/PEPline or 888-448-4911) or the HIV/AIDS Treatment Information Service (aidsinfo.nih.gov)—especially with questions about potential drug resistance or if the exposed person is pregnant.
When risk is real but low, 2-drug PEP is recommended (TABLE 1), usually 2 nucleoside reverse transcriptase inhibitors (NRTI) or 1 NRTI and 1 nucleotide reverse transcriptase inhibitor (NtRTI).
For those at higher risk, 3 or more antiviral regimens are recommended, achieved by adding a protease inhibitor to one of the recommended 2-drug regimens. The potential antiviral combinations in the basic 2-drug and expanded PEP regimens, along with potential side effects and toxicities of antiviral medications are described in TABLE W1, available online at www.jfponline.com.4
Nonoccupational exposures rarely require PEP
There are many unresolved questions regarding PEP for nonoccupational exposures. The lack of definitive evidence of its effectiveness, its unknown influence on risk-taking behavior, and the potential to aggravate viral resistance have led CDC to recommend that PEP be used only infrequently and not continuously for those whose behavior results in frequent exposures.3 Those who continue to participate in high risk activities should be referred for risk-reduction behavioral counseling.
The risk of HIV transmission varies by route and source of exposure (TABLE 3). The CDC has developed an algorithm based on these variables (FIGURE) to help you decide whether to initiate PEP. Two situations that cause concerns but pose little known risk of infection are bites and needlestick injuries from discarded needles; PEP is rarely indicated for either.
As with occupational exposure PEP, those receiving nonoccupational PEP should be evaluated at baseline for HIV infection. In addition, consider evaluating them for other STD’s and pregnancy.
As with occupational exposure, start nonoccupational PEP as soon as possible and continue it for 28 days. Nonoccupational PEP is not recommended if time after exposure is more than 72 hours. A 3-drug regimen is recommended by the CDC for nonoccupational exposures, even though evidence is lacking that it provides superior benefit over 2 drugs (see TABLE W1 at www.jfponline.com).
Follow-up recommendations for those provided nonoccupational PEP are the same as for occupational PEP, and testing for other STDs and hepatitis B and C is also recommended (TABLE 4).
FIGURE
Evaluation and treatment of possible nonoccupational HIV exposures
Source: Centers for Disease Control and Prevention 2005.3
TABLE 3
Estimated per-act risk for acquisition of HIV, by exposure route*
| EXPOSURE ROUTE | RISK PER 10,000 EXPOSURES TO AN INFECTED SOURCE |
|---|---|
| Blood transfusion | 9000 |
| Needle-sharing injection-drug use | 67 |
| Receptive anal intercourse | 50 |
| Percutaneous needle stick | 30 |
| Receptive penile-vaginal intercourse | 10 |
| Insertive anal intercourse | 6.5 |
| Insertive penile-vaginal intercourse | 5 |
| Receptive oral intercourse† | 1 |
| Insertive oral intercourse† | 0.5 |
| *Estimates of risk for transmission from sexual exposures assume no condom use. | |
| †Source refers to oral intercourse performed on a man. | |
| Source: Centers for Disease Control and Prevention 2005.3 | |
TABLE 4
Recommended laboratory evaluation for nonoccupational postexposure prophylaxis of HIV infection
| TEST | BASELINE | DURING PEP* | 4 TO 6 WEEKS AFTER EXPOSURE | 3 MONTHS AFTER EXPOSURE | 6 MONTHS AFTER EXPOSURE |
|---|---|---|---|---|---|
| HIV antibody testing | E, S† | E | E | E | |
| Complete blood count with differential | E | E | |||
| Serum liver enzymes | E | E | |||
| Blood urea nitrogen/creatinine | E | E | |||
| Sexually transmitted diseases screen (gonorrhea, chlamydia, syphilis) | E, S | E‡ | E‡ | ||
| Hepatitis B serology | E, S | E‡ | E‡ | ||
| Hepatitis C serology | E, S | E | E | ||
| Pregnancy test (for women of reproductive age) | E | E‡ | E‡ | ||
| HIV viral load | S | E§ | E§ | E§ | |
| HIV resistance testing | S | E§ | E§ | E§ | |
| CD4+T lymphocyte count | S | E§ | E§ | E§ | |
| PEP, postexposure prophylaxis; E, exposed patient; S, source. | |||||
| *Other specific tests might be indicated dependent on the antiretrovirals prescribed. Literature pertaining to individual agents should be consulted. | |||||
| †HIV antibody testing of the source patient is indicated for sources of unknown serostatus. | |||||
| ‡Additional testing for pregnancy, sexually transmitted diseases, and hepatitis B should be performed as clinically indicated. | |||||
| §If determined to be HIV infected on follow-up testing; perform as clinically indicated once diagnosed. | |||||
Conclusion
When there is uncertainty whether PEP is recommended, start patients on a PEP regimen while the situation is sorted out. Fortunately, joint patient-physician decision making can be assisted by the physician consultation resources mentioned previously. Keep in mind that, depending on the circumstances of the exposure, HIV transmission is only one concern among others, including infectious diseases, pregnancy, and emotional/psychological aspects resulting from the incident.
CORRESPONDENCE
Doug Campos-Outcalt, MD,MPA, 4001 North Third Street #415, Phoenix, AZ 85012. E-mail: dougco@u.arizona.edu
REFERENCE
1. Centers for Disease Control and Prevention (CDC). Trends in HIV/AIDS Diagnoses—33 States, 2001-2004. MMWR Morb Mortal Wkly Rep 2005;54:1149-1153.Available at: www.cdc.gov/mmwr/preview/mmwrhtml/mm5445a1.htm. Accessed on June 8, 2006.
2. Campos-Outcalt D. HIV prevention enters a new era. J Fam Pract 2004;53:563-566.
3. CDC. Antiretroviral postexposure prophylaxis after sexual, injection-drug use, or other nonoccupational exposures to HIV in the United States. MMWR Recomm Rep 2005;54(RR-2).-Available at: www.cdc.gov/mmwr/preview/mmwrhtml/rr5409a1.htm. Accessed on June 8, 2006.
4. CDC. Updated U.S. public health service guidelines for the management of occupational exposures to HIV and recommendations for postexposure prophylaxis. MMWR Recomm Rep 2005;54(RR-9).-Available at: www.cdc.gov/mmwr/preview/mmwrhtml/rr5409a1.htm. Accessed on June 8, 2006.
5. US Department of Health and Human Services. Guideline for the Use of Antiretroviral Agents in HIV-Infected Adults and Adolescents. October 29, 2004 revision. Available at: aidsinfo.nih.gov/guidelines.default_db2,asp?id+50. Accessed on June 8, 2006.
One of your office personnel receives a superficial stick from a needle while putting it into a sharps disposal container. Is postexposure prophylaxis (PEP) for HIV warranted?
Another health care worker receives a major blood splash into her eye after dropping a blood tube taken from a source of unknown HIV status. Is PEP called for in this instance?
A child who was rifling through a trash bin accidentally poked himself with an improperly disposed hypodermic needle. Should he be given PEP?
In most cases, HIV PEP is given only to healthcare workers if the settings make exposure to HIV-infected persons likely. Otherwise, it is usually deemed unnecessary. However, a decision for or against PEP is complicated.
Occupational and nonoccupational exposure to HIV can produce fear, anxiety, and stress. Information on the exposure risk is frequently incomplete, the risk of infection is usually low, the degree of protection offered by PEP is not fully defined, and the potential for side effects from the medications is significant.
This article distills the Centers for Disease Control and Prevention’s most recent guidance on HIV PEP.
HIV on the rise again
Antiretroviral therapy has markedly reduced mortality from HIV/AIDS, but the incidence of new cases, after declining in the 1990s, has gradually increased since 2000.1 As described in a previous article in the Journal of Family Practice,2 efforts to control HIV now focus on increased testing of those persons at risk, behavior modification to reduce the chances of infected persons exposing others, and treating HIV-positive pregnant women and providing postnatal prophylaxis to their newly born infants.
Exposure to HIV can occur occupationally, during a sexual assault, or from the failure of barrier protection during sex. Though these types of exposure are not major contributors to HIV incidence, and postexposure prophylaxis is not expected to play a major role in reducing the incidence of disease, it is available to persons potentially exposed to HIV, and it is beneficial to know when it is and is not indicated. Evidence for possible effectiveness of PEP comes from studies of postnatal prophylaxis, animal studies, case control studies and case reports.3
The Centers for Disease Control and Prevention (CDC) has developed 2 sets of recommendations for PEP that take into consideration the type and severity of the exposure and characteristics of the source of the exposure (TABLE 1).3,4
TABLE 1
Recommended HIV postexposure prophylaxis for percutanous injuries and membrane/nonintact skin exposures
| For percutaneous injuries | |||||
| EXPOSURE TYPE | INFECTION STATUS OF SOURCE | ||||
| HIV-POSITIVE, CLASS 1* | HIV-POSITIVE, CLASS 2* | SOURCE OF UNKNOWN HIV STATUS† | UNKNOWN SOURCE‡ | HIV-NEGATIVE | |
| Less severe (eg, solid needle or supercficial injury) | Recommend basic 2-drug PEP | Recommend expanded ≥3-drug PEP | Generally, no PEP warranted however, consider basic 2-drug PEP¶ for source with HIV risk factors** | Generally, no PEP warranted; however, consider basic 2-drug PEP¶ in settings in which exposure to HIV-infected persons is likely | No PEP warranted |
| More severe (large-bore hollow needle, deep puncture wound, blood on device, needle used in artery/vein) | Recommend expanded ≥3-drug PEP | Recommend expanded ≥3-drug PEP | Generally, no PEP warranted; however, consider basic 2-drug¶ for source with HIV risk factors** | Generally, no PEP warranted; however, consider basic 2-drug PEP¶ in settings in which exposure to HIV infected persons is likely | No PEP warranted |
| For mucous membrane and nonintact skin exposures†† | |||||
| Small volume (eg, a few drops) | Consider basic 2-drug PEP¶ | Recommend basic 2-drug PEP | Generally, no PEP warranted** | Generally, no PEP warranted | No PEP warranted |
| Large volume (eg, a major blood splash) | Recommend basic 2-drug PEP | Recommend expended ≥3-drug PEP | Generally, no PEP warranted; however, consider basic 2-drug PEP¶ for source with HIV risk factors** | Generally, no PEP warranted; however, consider basic 2-drug PEP¶ in settings in which exposure to HIV-infected persons is likely | No PEP warranted |
| *HIV-positive, class 1—asymptomatic HIV infection or known low viral load (eg, <1500 ribonucleic acid copies/mL). HIV-positive, class 2—symptomatic HIV infection, AIDS, acute seroconversion, or known high viral load. If drug resistance is a concern, obtain expert consultation. Initiation of PEP should not be delayed pending expert consultation, and, because expert consultation alone cannot substitute for face-to-face counseling, resources should be available to provide immediate evaluation and follow-up care for all exposures. | |||||
| †For example, deceased source person with no samples available for HIV testing. | |||||
| ‡For example, a needle from a sharps container or splash from inappropriately disposed blood. | |||||
| ¶The recommendation “consider PEP” indicates that PEP is optional; a decision to initiate PEP should be based on a discussion between the exposed person and the treating clinician regarding the risks versus benefits of PEP. | |||||
| **If PEP is offered and administered and the source is later determined to be HIV-negative, PEP should be discontinued. | |||||
| ††For skin exposures, follow-up is indicated only if evidence exists of compromised skin integrity (eg, dermatitis, abrasion, or open wound). | |||||
| Source: Centers for Disease Control and Prevention 2005.4 | |||||
Occupational exposures to HIV
Occupational exposure to HIV can result from a needlestick injury, cut with a sharp object, or contact with potentially infectious body fluids to mucous membranes or skin that is not intact (chapped, cut, abraded, inflamed). Body fluids that are considered potentially infectious are listed in TABLE 2, along with fluids not considered to be infectious.
The risk of contracting HIV from an occupational exposure is determined by several factors, but is generally low. The risk of infection after a needle-stick injury with exposure to infected blood is estimated at 0.3%; after a mucous membrane exposure, 0.09%. The risk after exposure to nonintact skin is probably even lower. Risk increases with the quantity of blood exposed to, a needle-stick injury directly into a vein or artery, and deep injuries.
TABLE 2
Which body fluids are infectious?
| BODY FLUIDS POTENTIALLY INFECTIOUS FOR HIV | |
|
|
| BODY FLUIDS NOT CONSIDERED TO BE INFECTIOUS UNLESS THEY ARE VISIBLY BLOODY | |
|
|
Who should and should not receive PEP
TABLE 1 details recommended treatment responses to specific types of exposure (eg, puncture wound) and the status of the exposure source.4 In situations unlikely to result in disease transmission (superficial injury and source patient with unknown HIV status), no PEP is generally warranted due to the low risk of infection and potential toxicity of antiretrovirals.
Treatment particulars
Start postexposure prophylaxis, when indicated, as soon as possible following exposure and continue it for 4 weeks. Obtain baseline test results for HIV at the time of exposure and periodically for 6 months. The CDC recommends testing at 6 weeks, 12 weeks, and 6 months, whether or not PEP is provided.
Testing and monitoring for hepatitis B and C may also be indicated.
Advise patients on PEP to use precautions in avoiding the possibility of secondary transmission, especially in the first 3 months following exposure. Monitor for drug toxicity every week or 2 while giving PEP. Because of the complexity of potential PEP regimens and the risk for drug toxicity, you may want to take advantage of several national sources of consultation, such as the PEPline (www.ucsf.edu/hivcntr/Hotlines/PEPline or 888-448-4911) or the HIV/AIDS Treatment Information Service (aidsinfo.nih.gov)—especially with questions about potential drug resistance or if the exposed person is pregnant.
When risk is real but low, 2-drug PEP is recommended (TABLE 1), usually 2 nucleoside reverse transcriptase inhibitors (NRTI) or 1 NRTI and 1 nucleotide reverse transcriptase inhibitor (NtRTI).
For those at higher risk, 3 or more antiviral regimens are recommended, achieved by adding a protease inhibitor to one of the recommended 2-drug regimens. The potential antiviral combinations in the basic 2-drug and expanded PEP regimens, along with potential side effects and toxicities of antiviral medications are described in TABLE W1, available online at www.jfponline.com.4
Nonoccupational exposures rarely require PEP
There are many unresolved questions regarding PEP for nonoccupational exposures. The lack of definitive evidence of its effectiveness, its unknown influence on risk-taking behavior, and the potential to aggravate viral resistance have led CDC to recommend that PEP be used only infrequently and not continuously for those whose behavior results in frequent exposures.3 Those who continue to participate in high risk activities should be referred for risk-reduction behavioral counseling.
The risk of HIV transmission varies by route and source of exposure (TABLE 3). The CDC has developed an algorithm based on these variables (FIGURE) to help you decide whether to initiate PEP. Two situations that cause concerns but pose little known risk of infection are bites and needlestick injuries from discarded needles; PEP is rarely indicated for either.
As with occupational exposure PEP, those receiving nonoccupational PEP should be evaluated at baseline for HIV infection. In addition, consider evaluating them for other STD’s and pregnancy.
As with occupational exposure, start nonoccupational PEP as soon as possible and continue it for 28 days. Nonoccupational PEP is not recommended if time after exposure is more than 72 hours. A 3-drug regimen is recommended by the CDC for nonoccupational exposures, even though evidence is lacking that it provides superior benefit over 2 drugs (see TABLE W1 at www.jfponline.com).
Follow-up recommendations for those provided nonoccupational PEP are the same as for occupational PEP, and testing for other STDs and hepatitis B and C is also recommended (TABLE 4).
FIGURE
Evaluation and treatment of possible nonoccupational HIV exposures
Source: Centers for Disease Control and Prevention 2005.3
TABLE 3
Estimated per-act risk for acquisition of HIV, by exposure route*
| EXPOSURE ROUTE | RISK PER 10,000 EXPOSURES TO AN INFECTED SOURCE |
|---|---|
| Blood transfusion | 9000 |
| Needle-sharing injection-drug use | 67 |
| Receptive anal intercourse | 50 |
| Percutaneous needle stick | 30 |
| Receptive penile-vaginal intercourse | 10 |
| Insertive anal intercourse | 6.5 |
| Insertive penile-vaginal intercourse | 5 |
| Receptive oral intercourse† | 1 |
| Insertive oral intercourse† | 0.5 |
| *Estimates of risk for transmission from sexual exposures assume no condom use. | |
| †Source refers to oral intercourse performed on a man. | |
| Source: Centers for Disease Control and Prevention 2005.3 | |
TABLE 4
Recommended laboratory evaluation for nonoccupational postexposure prophylaxis of HIV infection
| TEST | BASELINE | DURING PEP* | 4 TO 6 WEEKS AFTER EXPOSURE | 3 MONTHS AFTER EXPOSURE | 6 MONTHS AFTER EXPOSURE |
|---|---|---|---|---|---|
| HIV antibody testing | E, S† | E | E | E | |
| Complete blood count with differential | E | E | |||
| Serum liver enzymes | E | E | |||
| Blood urea nitrogen/creatinine | E | E | |||
| Sexually transmitted diseases screen (gonorrhea, chlamydia, syphilis) | E, S | E‡ | E‡ | ||
| Hepatitis B serology | E, S | E‡ | E‡ | ||
| Hepatitis C serology | E, S | E | E | ||
| Pregnancy test (for women of reproductive age) | E | E‡ | E‡ | ||
| HIV viral load | S | E§ | E§ | E§ | |
| HIV resistance testing | S | E§ | E§ | E§ | |
| CD4+T lymphocyte count | S | E§ | E§ | E§ | |
| PEP, postexposure prophylaxis; E, exposed patient; S, source. | |||||
| *Other specific tests might be indicated dependent on the antiretrovirals prescribed. Literature pertaining to individual agents should be consulted. | |||||
| †HIV antibody testing of the source patient is indicated for sources of unknown serostatus. | |||||
| ‡Additional testing for pregnancy, sexually transmitted diseases, and hepatitis B should be performed as clinically indicated. | |||||
| §If determined to be HIV infected on follow-up testing; perform as clinically indicated once diagnosed. | |||||
Conclusion
When there is uncertainty whether PEP is recommended, start patients on a PEP regimen while the situation is sorted out. Fortunately, joint patient-physician decision making can be assisted by the physician consultation resources mentioned previously. Keep in mind that, depending on the circumstances of the exposure, HIV transmission is only one concern among others, including infectious diseases, pregnancy, and emotional/psychological aspects resulting from the incident.
CORRESPONDENCE
Doug Campos-Outcalt, MD,MPA, 4001 North Third Street #415, Phoenix, AZ 85012. E-mail: dougco@u.arizona.edu
One of your office personnel receives a superficial stick from a needle while putting it into a sharps disposal container. Is postexposure prophylaxis (PEP) for HIV warranted?
Another health care worker receives a major blood splash into her eye after dropping a blood tube taken from a source of unknown HIV status. Is PEP called for in this instance?
A child who was rifling through a trash bin accidentally poked himself with an improperly disposed hypodermic needle. Should he be given PEP?
In most cases, HIV PEP is given only to healthcare workers if the settings make exposure to HIV-infected persons likely. Otherwise, it is usually deemed unnecessary. However, a decision for or against PEP is complicated.
Occupational and nonoccupational exposure to HIV can produce fear, anxiety, and stress. Information on the exposure risk is frequently incomplete, the risk of infection is usually low, the degree of protection offered by PEP is not fully defined, and the potential for side effects from the medications is significant.
This article distills the Centers for Disease Control and Prevention’s most recent guidance on HIV PEP.
HIV on the rise again
Antiretroviral therapy has markedly reduced mortality from HIV/AIDS, but the incidence of new cases, after declining in the 1990s, has gradually increased since 2000.1 As described in a previous article in the Journal of Family Practice,2 efforts to control HIV now focus on increased testing of those persons at risk, behavior modification to reduce the chances of infected persons exposing others, and treating HIV-positive pregnant women and providing postnatal prophylaxis to their newly born infants.
Exposure to HIV can occur occupationally, during a sexual assault, or from the failure of barrier protection during sex. Though these types of exposure are not major contributors to HIV incidence, and postexposure prophylaxis is not expected to play a major role in reducing the incidence of disease, it is available to persons potentially exposed to HIV, and it is beneficial to know when it is and is not indicated. Evidence for possible effectiveness of PEP comes from studies of postnatal prophylaxis, animal studies, case control studies and case reports.3
The Centers for Disease Control and Prevention (CDC) has developed 2 sets of recommendations for PEP that take into consideration the type and severity of the exposure and characteristics of the source of the exposure (TABLE 1).3,4
TABLE 1
Recommended HIV postexposure prophylaxis for percutanous injuries and membrane/nonintact skin exposures
| For percutaneous injuries | |||||
| EXPOSURE TYPE | INFECTION STATUS OF SOURCE | ||||
| HIV-POSITIVE, CLASS 1* | HIV-POSITIVE, CLASS 2* | SOURCE OF UNKNOWN HIV STATUS† | UNKNOWN SOURCE‡ | HIV-NEGATIVE | |
| Less severe (eg, solid needle or supercficial injury) | Recommend basic 2-drug PEP | Recommend expanded ≥3-drug PEP | Generally, no PEP warranted however, consider basic 2-drug PEP¶ for source with HIV risk factors** | Generally, no PEP warranted; however, consider basic 2-drug PEP¶ in settings in which exposure to HIV-infected persons is likely | No PEP warranted |
| More severe (large-bore hollow needle, deep puncture wound, blood on device, needle used in artery/vein) | Recommend expanded ≥3-drug PEP | Recommend expanded ≥3-drug PEP | Generally, no PEP warranted; however, consider basic 2-drug¶ for source with HIV risk factors** | Generally, no PEP warranted; however, consider basic 2-drug PEP¶ in settings in which exposure to HIV infected persons is likely | No PEP warranted |
| For mucous membrane and nonintact skin exposures†† | |||||
| Small volume (eg, a few drops) | Consider basic 2-drug PEP¶ | Recommend basic 2-drug PEP | Generally, no PEP warranted** | Generally, no PEP warranted | No PEP warranted |
| Large volume (eg, a major blood splash) | Recommend basic 2-drug PEP | Recommend expended ≥3-drug PEP | Generally, no PEP warranted; however, consider basic 2-drug PEP¶ for source with HIV risk factors** | Generally, no PEP warranted; however, consider basic 2-drug PEP¶ in settings in which exposure to HIV-infected persons is likely | No PEP warranted |
| *HIV-positive, class 1—asymptomatic HIV infection or known low viral load (eg, <1500 ribonucleic acid copies/mL). HIV-positive, class 2—symptomatic HIV infection, AIDS, acute seroconversion, or known high viral load. If drug resistance is a concern, obtain expert consultation. Initiation of PEP should not be delayed pending expert consultation, and, because expert consultation alone cannot substitute for face-to-face counseling, resources should be available to provide immediate evaluation and follow-up care for all exposures. | |||||
| †For example, deceased source person with no samples available for HIV testing. | |||||
| ‡For example, a needle from a sharps container or splash from inappropriately disposed blood. | |||||
| ¶The recommendation “consider PEP” indicates that PEP is optional; a decision to initiate PEP should be based on a discussion between the exposed person and the treating clinician regarding the risks versus benefits of PEP. | |||||
| **If PEP is offered and administered and the source is later determined to be HIV-negative, PEP should be discontinued. | |||||
| ††For skin exposures, follow-up is indicated only if evidence exists of compromised skin integrity (eg, dermatitis, abrasion, or open wound). | |||||
| Source: Centers for Disease Control and Prevention 2005.4 | |||||
Occupational exposures to HIV
Occupational exposure to HIV can result from a needlestick injury, cut with a sharp object, or contact with potentially infectious body fluids to mucous membranes or skin that is not intact (chapped, cut, abraded, inflamed). Body fluids that are considered potentially infectious are listed in TABLE 2, along with fluids not considered to be infectious.
The risk of contracting HIV from an occupational exposure is determined by several factors, but is generally low. The risk of infection after a needle-stick injury with exposure to infected blood is estimated at 0.3%; after a mucous membrane exposure, 0.09%. The risk after exposure to nonintact skin is probably even lower. Risk increases with the quantity of blood exposed to, a needle-stick injury directly into a vein or artery, and deep injuries.
TABLE 2
Which body fluids are infectious?
| BODY FLUIDS POTENTIALLY INFECTIOUS FOR HIV | |
|
|
| BODY FLUIDS NOT CONSIDERED TO BE INFECTIOUS UNLESS THEY ARE VISIBLY BLOODY | |
|
|
Who should and should not receive PEP
TABLE 1 details recommended treatment responses to specific types of exposure (eg, puncture wound) and the status of the exposure source.4 In situations unlikely to result in disease transmission (superficial injury and source patient with unknown HIV status), no PEP is generally warranted due to the low risk of infection and potential toxicity of antiretrovirals.
Treatment particulars
Start postexposure prophylaxis, when indicated, as soon as possible following exposure and continue it for 4 weeks. Obtain baseline test results for HIV at the time of exposure and periodically for 6 months. The CDC recommends testing at 6 weeks, 12 weeks, and 6 months, whether or not PEP is provided.
Testing and monitoring for hepatitis B and C may also be indicated.
Advise patients on PEP to use precautions in avoiding the possibility of secondary transmission, especially in the first 3 months following exposure. Monitor for drug toxicity every week or 2 while giving PEP. Because of the complexity of potential PEP regimens and the risk for drug toxicity, you may want to take advantage of several national sources of consultation, such as the PEPline (www.ucsf.edu/hivcntr/Hotlines/PEPline or 888-448-4911) or the HIV/AIDS Treatment Information Service (aidsinfo.nih.gov)—especially with questions about potential drug resistance or if the exposed person is pregnant.
When risk is real but low, 2-drug PEP is recommended (TABLE 1), usually 2 nucleoside reverse transcriptase inhibitors (NRTI) or 1 NRTI and 1 nucleotide reverse transcriptase inhibitor (NtRTI).
For those at higher risk, 3 or more antiviral regimens are recommended, achieved by adding a protease inhibitor to one of the recommended 2-drug regimens. The potential antiviral combinations in the basic 2-drug and expanded PEP regimens, along with potential side effects and toxicities of antiviral medications are described in TABLE W1, available online at www.jfponline.com.4
Nonoccupational exposures rarely require PEP
There are many unresolved questions regarding PEP for nonoccupational exposures. The lack of definitive evidence of its effectiveness, its unknown influence on risk-taking behavior, and the potential to aggravate viral resistance have led CDC to recommend that PEP be used only infrequently and not continuously for those whose behavior results in frequent exposures.3 Those who continue to participate in high risk activities should be referred for risk-reduction behavioral counseling.
The risk of HIV transmission varies by route and source of exposure (TABLE 3). The CDC has developed an algorithm based on these variables (FIGURE) to help you decide whether to initiate PEP. Two situations that cause concerns but pose little known risk of infection are bites and needlestick injuries from discarded needles; PEP is rarely indicated for either.
As with occupational exposure PEP, those receiving nonoccupational PEP should be evaluated at baseline for HIV infection. In addition, consider evaluating them for other STD’s and pregnancy.
As with occupational exposure, start nonoccupational PEP as soon as possible and continue it for 28 days. Nonoccupational PEP is not recommended if time after exposure is more than 72 hours. A 3-drug regimen is recommended by the CDC for nonoccupational exposures, even though evidence is lacking that it provides superior benefit over 2 drugs (see TABLE W1 at www.jfponline.com).
Follow-up recommendations for those provided nonoccupational PEP are the same as for occupational PEP, and testing for other STDs and hepatitis B and C is also recommended (TABLE 4).
FIGURE
Evaluation and treatment of possible nonoccupational HIV exposures
Source: Centers for Disease Control and Prevention 2005.3
TABLE 3
Estimated per-act risk for acquisition of HIV, by exposure route*
| EXPOSURE ROUTE | RISK PER 10,000 EXPOSURES TO AN INFECTED SOURCE |
|---|---|
| Blood transfusion | 9000 |
| Needle-sharing injection-drug use | 67 |
| Receptive anal intercourse | 50 |
| Percutaneous needle stick | 30 |
| Receptive penile-vaginal intercourse | 10 |
| Insertive anal intercourse | 6.5 |
| Insertive penile-vaginal intercourse | 5 |
| Receptive oral intercourse† | 1 |
| Insertive oral intercourse† | 0.5 |
| *Estimates of risk for transmission from sexual exposures assume no condom use. | |
| †Source refers to oral intercourse performed on a man. | |
| Source: Centers for Disease Control and Prevention 2005.3 | |
TABLE 4
Recommended laboratory evaluation for nonoccupational postexposure prophylaxis of HIV infection
| TEST | BASELINE | DURING PEP* | 4 TO 6 WEEKS AFTER EXPOSURE | 3 MONTHS AFTER EXPOSURE | 6 MONTHS AFTER EXPOSURE |
|---|---|---|---|---|---|
| HIV antibody testing | E, S† | E | E | E | |
| Complete blood count with differential | E | E | |||
| Serum liver enzymes | E | E | |||
| Blood urea nitrogen/creatinine | E | E | |||
| Sexually transmitted diseases screen (gonorrhea, chlamydia, syphilis) | E, S | E‡ | E‡ | ||
| Hepatitis B serology | E, S | E‡ | E‡ | ||
| Hepatitis C serology | E, S | E | E | ||
| Pregnancy test (for women of reproductive age) | E | E‡ | E‡ | ||
| HIV viral load | S | E§ | E§ | E§ | |
| HIV resistance testing | S | E§ | E§ | E§ | |
| CD4+T lymphocyte count | S | E§ | E§ | E§ | |
| PEP, postexposure prophylaxis; E, exposed patient; S, source. | |||||
| *Other specific tests might be indicated dependent on the antiretrovirals prescribed. Literature pertaining to individual agents should be consulted. | |||||
| †HIV antibody testing of the source patient is indicated for sources of unknown serostatus. | |||||
| ‡Additional testing for pregnancy, sexually transmitted diseases, and hepatitis B should be performed as clinically indicated. | |||||
| §If determined to be HIV infected on follow-up testing; perform as clinically indicated once diagnosed. | |||||
Conclusion
When there is uncertainty whether PEP is recommended, start patients on a PEP regimen while the situation is sorted out. Fortunately, joint patient-physician decision making can be assisted by the physician consultation resources mentioned previously. Keep in mind that, depending on the circumstances of the exposure, HIV transmission is only one concern among others, including infectious diseases, pregnancy, and emotional/psychological aspects resulting from the incident.
CORRESPONDENCE
Doug Campos-Outcalt, MD,MPA, 4001 North Third Street #415, Phoenix, AZ 85012. E-mail: dougco@u.arizona.edu
REFERENCE
1. Centers for Disease Control and Prevention (CDC). Trends in HIV/AIDS Diagnoses—33 States, 2001-2004. MMWR Morb Mortal Wkly Rep 2005;54:1149-1153.Available at: www.cdc.gov/mmwr/preview/mmwrhtml/mm5445a1.htm. Accessed on June 8, 2006.
2. Campos-Outcalt D. HIV prevention enters a new era. J Fam Pract 2004;53:563-566.
3. CDC. Antiretroviral postexposure prophylaxis after sexual, injection-drug use, or other nonoccupational exposures to HIV in the United States. MMWR Recomm Rep 2005;54(RR-2).-Available at: www.cdc.gov/mmwr/preview/mmwrhtml/rr5409a1.htm. Accessed on June 8, 2006.
4. CDC. Updated U.S. public health service guidelines for the management of occupational exposures to HIV and recommendations for postexposure prophylaxis. MMWR Recomm Rep 2005;54(RR-9).-Available at: www.cdc.gov/mmwr/preview/mmwrhtml/rr5409a1.htm. Accessed on June 8, 2006.
5. US Department of Health and Human Services. Guideline for the Use of Antiretroviral Agents in HIV-Infected Adults and Adolescents. October 29, 2004 revision. Available at: aidsinfo.nih.gov/guidelines.default_db2,asp?id+50. Accessed on June 8, 2006.
REFERENCE
1. Centers for Disease Control and Prevention (CDC). Trends in HIV/AIDS Diagnoses—33 States, 2001-2004. MMWR Morb Mortal Wkly Rep 2005;54:1149-1153.Available at: www.cdc.gov/mmwr/preview/mmwrhtml/mm5445a1.htm. Accessed on June 8, 2006.
2. Campos-Outcalt D. HIV prevention enters a new era. J Fam Pract 2004;53:563-566.
3. CDC. Antiretroviral postexposure prophylaxis after sexual, injection-drug use, or other nonoccupational exposures to HIV in the United States. MMWR Recomm Rep 2005;54(RR-2).-Available at: www.cdc.gov/mmwr/preview/mmwrhtml/rr5409a1.htm. Accessed on June 8, 2006.
4. CDC. Updated U.S. public health service guidelines for the management of occupational exposures to HIV and recommendations for postexposure prophylaxis. MMWR Recomm Rep 2005;54(RR-9).-Available at: www.cdc.gov/mmwr/preview/mmwrhtml/rr5409a1.htm. Accessed on June 8, 2006.
5. US Department of Health and Human Services. Guideline for the Use of Antiretroviral Agents in HIV-Infected Adults and Adolescents. October 29, 2004 revision. Available at: aidsinfo.nih.gov/guidelines.default_db2,asp?id+50. Accessed on June 8, 2006.
Mumps epidemic in 2006: Are you prepared to detect and prevent it?
The ongoing mumps epidemic in the Midwestern US is a stark reminder of how fast and easily an epidemic can still occur. It serves as a warning that we need to remain vigilant in maintaining high immunization levels among our patients. The morbidity and mortality resulting from this outbreak will depend on the soundness and response of the public health infrastructure.
We family physicians need to be prepared to detect and prevent mumps among our patients and staff. While it may not be immediately apparent, if we fulfill our roles competently the outbreak will be less severe.
The start of an epidemic
As of May 15, 1765 cases of mumps had been reported to the Iowa Department of Public Health.1 The median age of those infected was 22 years, with more than one third of cases occurring in those aged 16 to 22 years (FIGURE). College students accounted for 20% of cases.
By the middle of April, at least 8 other states were also investigating possible mumps cases. More states will likely become involved: 2 people who were probably infectious traveled on 9 commercial airline flights in a 1-week period in late March.2 The number of fellow travelers infected is under investigation.
In Iowa, investigation has shown that 5% of those infected reported no history of mumps immunization, 13% reported only 1 MMR vaccine, and in 30% the vaccine status was unknown. A majority of cases (51%) had received the recommended 2 MMR vaccines.1 These numbers are consistent with what would be expected with a vaccine that has 90% to 95% effectiveness in a highly immunized population. In such conditions, most cases occur among the vaccinated even though their rate of infection is far lower than the unvaccinated.
The strain of mumps virus isolated in this outbreak is the same one responsible for a recent large outbreak in Great Britain, where there have been more than 70,000 cases in the past 3 years.3 In contrast to measles—which has resulted in few secondary cases and practically no prolonged transmission in the US after importation from abroad4 —this introduction of mumps has resulted in more cases and multiple generations. Given that mumps and measles vaccine are usally given together in MMR, the reasons for this difference are not clear and include several possibilities:
- The mumps vaccine appears to be less effective than measles vaccine. The mumps vaccine is reported to be 80% effective after the first dose and 90% after the second,3 where measles vaccine effectiveness approaches 98% after 2 doses.
- The number of persons with atypical or asymptomatic mumps presentations is significant and complicates control efforts.
- There is possibly a lower level of immunity to mumps in older age groups. The pre-vaccine epidemiology of measles and mumps were different, with virtually everyone born before 1957 having contracted measles naturally. This artificial cutoff date has also been used to set an age limit for mumps vaccine, although those born before 1957 may not be universally immune to mumps from natural infection.
- Later adoption of routine mumps immunization (1970s) vs routine measles immunization (1960s).
FIGURE
Age distribution of mumps cases in Iowa
Source: Data from the Iowa Department of Public Health website.1
The public health response: Controlling the outbreak
Mumps vaccine is not effective until 2 to 4 weeks after it is administered, so it is not useful as postexposure prophylaxis. However, it is effective in stopping population-based outbreaks after 1 or 2 generations. MMR vaccine should be given to all who cannot provide evidence of immunity, which includes laboratory evidence of prior mumps infection, birth before 1957, or history of 1 dose of mumps-containing vaccine. Health care workers and students (including college students) should receive a second dose of vaccine.
At schools with documented cases, students and staff without proof of mumps immunity should be excluded until they receive a mumps vaccine (MMR) or provide proof of immunity. Exclusion should be until 25 days after the onset of parotitis in the last person with mumps at the institution. Anyone with mumps should be isolated until 9 days after onset of symptoms.
Laboratory confirmation of mumps involves positive mumps immunoglobulin M (IgM), a 4-fold rise in mumps IgG titers between acute and 2-week convalescent serum, or detection of virus by culture or polymerase chain reaction (PCR) in urine or throat swab specimens. Laboratory results can be confusing in those previously vaccinated; mumps IgM titers may not be detectable, a 4-fold rise in IgG titers my not occur, and the acute IgG titer may be high. False-positive IgM results can occur because of infection with parainfluenza viruses.7
Transmission of the mumps virus occurs through the saliva or respiratory droplets of an infected person. The incubation period is usually 16 to 18 days but can range from 12 to 25 days. A person is infectious up to 2 days before symptoms begin and until 9 days after. Symptoms include myalgia, malaise, fever, and headache followed by tender swelling of parotid or other salivary glands (sublingual, submaxillary). Up to 50% of those infected can present atypically and 20% can be asymptomatic, which compicates disease control efforts.
Compications of mumps caninclude orchitis (in 20% of postpubertal males), oophoritis, mastitis (30% of postpubertal females), pancreatitis (4%), deafness (5/100,000), encephalitis (2/10,000), and spontaneous abortion 25% of first-trimester pregnancies.5,6
How can you help bring this outbreak under control?
Appropriate steps can be grouped into office infection control practices, diagnosis and reporting, and community infection control measures (TABLE).
Office infection control. Office infection control (subject of a Practice Alert8) is critical so that health care settings do not become a major source of disease transmission. Make sure you and your staff have immunity to mumps. Health care workers should receive 2 doses of MMR vaccine at least a month apart. If mumps occurs in your area, consider requiring proof of immunity, even for those born before 1957.
Make sure that tissues and hand sanitizers are available for patients in the waiting areas, and that signs are posted advising respiratory hygiene. Instruct your front-office staff to ask patients to cover their mouths and noses when they cough and sneeze. Make masks available to any patient who is unable or unwilling to comply; surgical masks are sufficient. Health care staff should be familiar with and use recommended hand sanitation practices.
Don’t let patients with parotid gland swelling sit in the waiting area—place them in an examination room and ask them to wear a mask.
Diagnosis and reporting. When you suspect mumps, collect any specimens requested by the local health department. This probably includes an immediate serum sample for IgM or IgG and possibly a convalescent serum for IgG; it may include a throat swab or urine sample for viral isolation. You should know the phone number of the local health department or have access to their Web site so that current recommendations for specimen collection and analysis can be obtained quickly.
Community infection control. If you suspect a patient has mumps, report it to the local health department and instruct the patient to remain in isolation for 9 days after the start of symptoms. Family members and close contacts should be assisted in assuring they are immune to mumps.
Review with each patient their immunization status; encourage those without documented immunity to mumps to receive the vaccine, if they have no contraindications.
TABLE
Family physicians’ role in controlling mumps
| OFFICE INFECTION CONTROL |
| Post respiratory hygiene notices |
| Make readily available for patients and staff tissues, tissue disposal containers, and hand sanitizers |
| Instruct office staff to request patients use respiratory hygiene |
| Have masks available for those who cannot or refuse to comply with respiratory hygiene |
| Train staff to place patients suspected to have mumps in an examine room immediately and to provide them a mask |
| Instruct staff to use recommended hand sanitation methods |
| Insure that staff are immunized |
| DIAGNOSING AND REPORTING |
| Maintain a high index of suspicion for mumps |
| Collect recommended laboratory specimens |
| COMMUNITY INFECTION CONTROL |
| Report suspected mumps cases to the local public health department |
| Advise those infected to remain in isolation until 9 days after the start of symptoms |
| Help insure families and close contacts of those infected are immunized against mumps |
| Check mumps immunization status of all patients |
1. Iowa mumps update. Available at: www.idph.state.ia.us/adper/common/pdf/mumps/mumps_update_051606.pdf. Accessed on May 16, 2006.
2. Centers for Disease Control and Prevention (CDC). Exposure to mumps during air travel—United States, April 2006. MMWR 2006;55:401-402.
3. CDC Health Advisory. Multi-state mumps outbreak. Available at: www.phppo.cdc.gov/HAN/ArchiveSys/ViewMsgV.asp?AlertNum=00243. Accessed on May 16, 2006.
4. CDC. Measles history. Available at: www.cdc.gov/nip/diseases/measles/history.htm. Accessed on May 16, 2006.
5. Heyman DL. Control of Communicable Diseases Manual 18th ed. Washington, DC: American Public Health Association; 2004.
6. Zimmerman L, Reef S, Wharton M. Mumps. Chapter 7 in Manual for the Surveillance of Vaccine-Preventable Diseases. 3rd ed. Washington, DC: National Immunization Program; 2002. Available at: www.cdc.gov/nip/publications/surv-manual/chpt07_mumps.pdf. Accessed on May 16, 2006.
7. CDC. Laboratory testing for mumps infection. Available at: www.cdc.gov/nip/diseases/mumps/lab-test-faqs.htm. Accessed on May 16, 2006.
8. Campos-Outcalt D. Infection control in the outpatient settings. J Fam Pract 2004;53:485-488.
The ongoing mumps epidemic in the Midwestern US is a stark reminder of how fast and easily an epidemic can still occur. It serves as a warning that we need to remain vigilant in maintaining high immunization levels among our patients. The morbidity and mortality resulting from this outbreak will depend on the soundness and response of the public health infrastructure.
We family physicians need to be prepared to detect and prevent mumps among our patients and staff. While it may not be immediately apparent, if we fulfill our roles competently the outbreak will be less severe.
The start of an epidemic
As of May 15, 1765 cases of mumps had been reported to the Iowa Department of Public Health.1 The median age of those infected was 22 years, with more than one third of cases occurring in those aged 16 to 22 years (FIGURE). College students accounted for 20% of cases.
By the middle of April, at least 8 other states were also investigating possible mumps cases. More states will likely become involved: 2 people who were probably infectious traveled on 9 commercial airline flights in a 1-week period in late March.2 The number of fellow travelers infected is under investigation.
In Iowa, investigation has shown that 5% of those infected reported no history of mumps immunization, 13% reported only 1 MMR vaccine, and in 30% the vaccine status was unknown. A majority of cases (51%) had received the recommended 2 MMR vaccines.1 These numbers are consistent with what would be expected with a vaccine that has 90% to 95% effectiveness in a highly immunized population. In such conditions, most cases occur among the vaccinated even though their rate of infection is far lower than the unvaccinated.
The strain of mumps virus isolated in this outbreak is the same one responsible for a recent large outbreak in Great Britain, where there have been more than 70,000 cases in the past 3 years.3 In contrast to measles—which has resulted in few secondary cases and practically no prolonged transmission in the US after importation from abroad4 —this introduction of mumps has resulted in more cases and multiple generations. Given that mumps and measles vaccine are usally given together in MMR, the reasons for this difference are not clear and include several possibilities:
- The mumps vaccine appears to be less effective than measles vaccine. The mumps vaccine is reported to be 80% effective after the first dose and 90% after the second,3 where measles vaccine effectiveness approaches 98% after 2 doses.
- The number of persons with atypical or asymptomatic mumps presentations is significant and complicates control efforts.
- There is possibly a lower level of immunity to mumps in older age groups. The pre-vaccine epidemiology of measles and mumps were different, with virtually everyone born before 1957 having contracted measles naturally. This artificial cutoff date has also been used to set an age limit for mumps vaccine, although those born before 1957 may not be universally immune to mumps from natural infection.
- Later adoption of routine mumps immunization (1970s) vs routine measles immunization (1960s).
FIGURE
Age distribution of mumps cases in Iowa
Source: Data from the Iowa Department of Public Health website.1
The public health response: Controlling the outbreak
Mumps vaccine is not effective until 2 to 4 weeks after it is administered, so it is not useful as postexposure prophylaxis. However, it is effective in stopping population-based outbreaks after 1 or 2 generations. MMR vaccine should be given to all who cannot provide evidence of immunity, which includes laboratory evidence of prior mumps infection, birth before 1957, or history of 1 dose of mumps-containing vaccine. Health care workers and students (including college students) should receive a second dose of vaccine.
At schools with documented cases, students and staff without proof of mumps immunity should be excluded until they receive a mumps vaccine (MMR) or provide proof of immunity. Exclusion should be until 25 days after the onset of parotitis in the last person with mumps at the institution. Anyone with mumps should be isolated until 9 days after onset of symptoms.
Laboratory confirmation of mumps involves positive mumps immunoglobulin M (IgM), a 4-fold rise in mumps IgG titers between acute and 2-week convalescent serum, or detection of virus by culture or polymerase chain reaction (PCR) in urine or throat swab specimens. Laboratory results can be confusing in those previously vaccinated; mumps IgM titers may not be detectable, a 4-fold rise in IgG titers my not occur, and the acute IgG titer may be high. False-positive IgM results can occur because of infection with parainfluenza viruses.7
Transmission of the mumps virus occurs through the saliva or respiratory droplets of an infected person. The incubation period is usually 16 to 18 days but can range from 12 to 25 days. A person is infectious up to 2 days before symptoms begin and until 9 days after. Symptoms include myalgia, malaise, fever, and headache followed by tender swelling of parotid or other salivary glands (sublingual, submaxillary). Up to 50% of those infected can present atypically and 20% can be asymptomatic, which compicates disease control efforts.
Compications of mumps caninclude orchitis (in 20% of postpubertal males), oophoritis, mastitis (30% of postpubertal females), pancreatitis (4%), deafness (5/100,000), encephalitis (2/10,000), and spontaneous abortion 25% of first-trimester pregnancies.5,6
How can you help bring this outbreak under control?
Appropriate steps can be grouped into office infection control practices, diagnosis and reporting, and community infection control measures (TABLE).
Office infection control. Office infection control (subject of a Practice Alert8) is critical so that health care settings do not become a major source of disease transmission. Make sure you and your staff have immunity to mumps. Health care workers should receive 2 doses of MMR vaccine at least a month apart. If mumps occurs in your area, consider requiring proof of immunity, even for those born before 1957.
Make sure that tissues and hand sanitizers are available for patients in the waiting areas, and that signs are posted advising respiratory hygiene. Instruct your front-office staff to ask patients to cover their mouths and noses when they cough and sneeze. Make masks available to any patient who is unable or unwilling to comply; surgical masks are sufficient. Health care staff should be familiar with and use recommended hand sanitation practices.
Don’t let patients with parotid gland swelling sit in the waiting area—place them in an examination room and ask them to wear a mask.
Diagnosis and reporting. When you suspect mumps, collect any specimens requested by the local health department. This probably includes an immediate serum sample for IgM or IgG and possibly a convalescent serum for IgG; it may include a throat swab or urine sample for viral isolation. You should know the phone number of the local health department or have access to their Web site so that current recommendations for specimen collection and analysis can be obtained quickly.
Community infection control. If you suspect a patient has mumps, report it to the local health department and instruct the patient to remain in isolation for 9 days after the start of symptoms. Family members and close contacts should be assisted in assuring they are immune to mumps.
Review with each patient their immunization status; encourage those without documented immunity to mumps to receive the vaccine, if they have no contraindications.
TABLE
Family physicians’ role in controlling mumps
| OFFICE INFECTION CONTROL |
| Post respiratory hygiene notices |
| Make readily available for patients and staff tissues, tissue disposal containers, and hand sanitizers |
| Instruct office staff to request patients use respiratory hygiene |
| Have masks available for those who cannot or refuse to comply with respiratory hygiene |
| Train staff to place patients suspected to have mumps in an examine room immediately and to provide them a mask |
| Instruct staff to use recommended hand sanitation methods |
| Insure that staff are immunized |
| DIAGNOSING AND REPORTING |
| Maintain a high index of suspicion for mumps |
| Collect recommended laboratory specimens |
| COMMUNITY INFECTION CONTROL |
| Report suspected mumps cases to the local public health department |
| Advise those infected to remain in isolation until 9 days after the start of symptoms |
| Help insure families and close contacts of those infected are immunized against mumps |
| Check mumps immunization status of all patients |
The ongoing mumps epidemic in the Midwestern US is a stark reminder of how fast and easily an epidemic can still occur. It serves as a warning that we need to remain vigilant in maintaining high immunization levels among our patients. The morbidity and mortality resulting from this outbreak will depend on the soundness and response of the public health infrastructure.
We family physicians need to be prepared to detect and prevent mumps among our patients and staff. While it may not be immediately apparent, if we fulfill our roles competently the outbreak will be less severe.
The start of an epidemic
As of May 15, 1765 cases of mumps had been reported to the Iowa Department of Public Health.1 The median age of those infected was 22 years, with more than one third of cases occurring in those aged 16 to 22 years (FIGURE). College students accounted for 20% of cases.
By the middle of April, at least 8 other states were also investigating possible mumps cases. More states will likely become involved: 2 people who were probably infectious traveled on 9 commercial airline flights in a 1-week period in late March.2 The number of fellow travelers infected is under investigation.
In Iowa, investigation has shown that 5% of those infected reported no history of mumps immunization, 13% reported only 1 MMR vaccine, and in 30% the vaccine status was unknown. A majority of cases (51%) had received the recommended 2 MMR vaccines.1 These numbers are consistent with what would be expected with a vaccine that has 90% to 95% effectiveness in a highly immunized population. In such conditions, most cases occur among the vaccinated even though their rate of infection is far lower than the unvaccinated.
The strain of mumps virus isolated in this outbreak is the same one responsible for a recent large outbreak in Great Britain, where there have been more than 70,000 cases in the past 3 years.3 In contrast to measles—which has resulted in few secondary cases and practically no prolonged transmission in the US after importation from abroad4 —this introduction of mumps has resulted in more cases and multiple generations. Given that mumps and measles vaccine are usally given together in MMR, the reasons for this difference are not clear and include several possibilities:
- The mumps vaccine appears to be less effective than measles vaccine. The mumps vaccine is reported to be 80% effective after the first dose and 90% after the second,3 where measles vaccine effectiveness approaches 98% after 2 doses.
- The number of persons with atypical or asymptomatic mumps presentations is significant and complicates control efforts.
- There is possibly a lower level of immunity to mumps in older age groups. The pre-vaccine epidemiology of measles and mumps were different, with virtually everyone born before 1957 having contracted measles naturally. This artificial cutoff date has also been used to set an age limit for mumps vaccine, although those born before 1957 may not be universally immune to mumps from natural infection.
- Later adoption of routine mumps immunization (1970s) vs routine measles immunization (1960s).
FIGURE
Age distribution of mumps cases in Iowa
Source: Data from the Iowa Department of Public Health website.1
The public health response: Controlling the outbreak
Mumps vaccine is not effective until 2 to 4 weeks after it is administered, so it is not useful as postexposure prophylaxis. However, it is effective in stopping population-based outbreaks after 1 or 2 generations. MMR vaccine should be given to all who cannot provide evidence of immunity, which includes laboratory evidence of prior mumps infection, birth before 1957, or history of 1 dose of mumps-containing vaccine. Health care workers and students (including college students) should receive a second dose of vaccine.
At schools with documented cases, students and staff without proof of mumps immunity should be excluded until they receive a mumps vaccine (MMR) or provide proof of immunity. Exclusion should be until 25 days after the onset of parotitis in the last person with mumps at the institution. Anyone with mumps should be isolated until 9 days after onset of symptoms.
Laboratory confirmation of mumps involves positive mumps immunoglobulin M (IgM), a 4-fold rise in mumps IgG titers between acute and 2-week convalescent serum, or detection of virus by culture or polymerase chain reaction (PCR) in urine or throat swab specimens. Laboratory results can be confusing in those previously vaccinated; mumps IgM titers may not be detectable, a 4-fold rise in IgG titers my not occur, and the acute IgG titer may be high. False-positive IgM results can occur because of infection with parainfluenza viruses.7
Transmission of the mumps virus occurs through the saliva or respiratory droplets of an infected person. The incubation period is usually 16 to 18 days but can range from 12 to 25 days. A person is infectious up to 2 days before symptoms begin and until 9 days after. Symptoms include myalgia, malaise, fever, and headache followed by tender swelling of parotid or other salivary glands (sublingual, submaxillary). Up to 50% of those infected can present atypically and 20% can be asymptomatic, which compicates disease control efforts.
Compications of mumps caninclude orchitis (in 20% of postpubertal males), oophoritis, mastitis (30% of postpubertal females), pancreatitis (4%), deafness (5/100,000), encephalitis (2/10,000), and spontaneous abortion 25% of first-trimester pregnancies.5,6
How can you help bring this outbreak under control?
Appropriate steps can be grouped into office infection control practices, diagnosis and reporting, and community infection control measures (TABLE).
Office infection control. Office infection control (subject of a Practice Alert8) is critical so that health care settings do not become a major source of disease transmission. Make sure you and your staff have immunity to mumps. Health care workers should receive 2 doses of MMR vaccine at least a month apart. If mumps occurs in your area, consider requiring proof of immunity, even for those born before 1957.
Make sure that tissues and hand sanitizers are available for patients in the waiting areas, and that signs are posted advising respiratory hygiene. Instruct your front-office staff to ask patients to cover their mouths and noses when they cough and sneeze. Make masks available to any patient who is unable or unwilling to comply; surgical masks are sufficient. Health care staff should be familiar with and use recommended hand sanitation practices.
Don’t let patients with parotid gland swelling sit in the waiting area—place them in an examination room and ask them to wear a mask.
Diagnosis and reporting. When you suspect mumps, collect any specimens requested by the local health department. This probably includes an immediate serum sample for IgM or IgG and possibly a convalescent serum for IgG; it may include a throat swab or urine sample for viral isolation. You should know the phone number of the local health department or have access to their Web site so that current recommendations for specimen collection and analysis can be obtained quickly.
Community infection control. If you suspect a patient has mumps, report it to the local health department and instruct the patient to remain in isolation for 9 days after the start of symptoms. Family members and close contacts should be assisted in assuring they are immune to mumps.
Review with each patient their immunization status; encourage those without documented immunity to mumps to receive the vaccine, if they have no contraindications.
TABLE
Family physicians’ role in controlling mumps
| OFFICE INFECTION CONTROL |
| Post respiratory hygiene notices |
| Make readily available for patients and staff tissues, tissue disposal containers, and hand sanitizers |
| Instruct office staff to request patients use respiratory hygiene |
| Have masks available for those who cannot or refuse to comply with respiratory hygiene |
| Train staff to place patients suspected to have mumps in an examine room immediately and to provide them a mask |
| Instruct staff to use recommended hand sanitation methods |
| Insure that staff are immunized |
| DIAGNOSING AND REPORTING |
| Maintain a high index of suspicion for mumps |
| Collect recommended laboratory specimens |
| COMMUNITY INFECTION CONTROL |
| Report suspected mumps cases to the local public health department |
| Advise those infected to remain in isolation until 9 days after the start of symptoms |
| Help insure families and close contacts of those infected are immunized against mumps |
| Check mumps immunization status of all patients |
1. Iowa mumps update. Available at: www.idph.state.ia.us/adper/common/pdf/mumps/mumps_update_051606.pdf. Accessed on May 16, 2006.
2. Centers for Disease Control and Prevention (CDC). Exposure to mumps during air travel—United States, April 2006. MMWR 2006;55:401-402.
3. CDC Health Advisory. Multi-state mumps outbreak. Available at: www.phppo.cdc.gov/HAN/ArchiveSys/ViewMsgV.asp?AlertNum=00243. Accessed on May 16, 2006.
4. CDC. Measles history. Available at: www.cdc.gov/nip/diseases/measles/history.htm. Accessed on May 16, 2006.
5. Heyman DL. Control of Communicable Diseases Manual 18th ed. Washington, DC: American Public Health Association; 2004.
6. Zimmerman L, Reef S, Wharton M. Mumps. Chapter 7 in Manual for the Surveillance of Vaccine-Preventable Diseases. 3rd ed. Washington, DC: National Immunization Program; 2002. Available at: www.cdc.gov/nip/publications/surv-manual/chpt07_mumps.pdf. Accessed on May 16, 2006.
7. CDC. Laboratory testing for mumps infection. Available at: www.cdc.gov/nip/diseases/mumps/lab-test-faqs.htm. Accessed on May 16, 2006.
8. Campos-Outcalt D. Infection control in the outpatient settings. J Fam Pract 2004;53:485-488.
1. Iowa mumps update. Available at: www.idph.state.ia.us/adper/common/pdf/mumps/mumps_update_051606.pdf. Accessed on May 16, 2006.
2. Centers for Disease Control and Prevention (CDC). Exposure to mumps during air travel—United States, April 2006. MMWR 2006;55:401-402.
3. CDC Health Advisory. Multi-state mumps outbreak. Available at: www.phppo.cdc.gov/HAN/ArchiveSys/ViewMsgV.asp?AlertNum=00243. Accessed on May 16, 2006.
4. CDC. Measles history. Available at: www.cdc.gov/nip/diseases/measles/history.htm. Accessed on May 16, 2006.
5. Heyman DL. Control of Communicable Diseases Manual 18th ed. Washington, DC: American Public Health Association; 2004.
6. Zimmerman L, Reef S, Wharton M. Mumps. Chapter 7 in Manual for the Surveillance of Vaccine-Preventable Diseases. 3rd ed. Washington, DC: National Immunization Program; 2002. Available at: www.cdc.gov/nip/publications/surv-manual/chpt07_mumps.pdf. Accessed on May 16, 2006.
7. CDC. Laboratory testing for mumps infection. Available at: www.cdc.gov/nip/diseases/mumps/lab-test-faqs.htm. Accessed on May 16, 2006.
8. Campos-Outcalt D. Infection control in the outpatient settings. J Fam Pract 2004;53:485-488.
Clarifying the US Preventive Services Task Force’s 2005 recommendations
The United States Preventive Services Task Force (USPSTF) is the most evidence-based and authoritative organization making recommendations on preventive services in the US. During 2005, 20 recommendations were made on a total of 10 conditions. TABLE 1 lists the recommendations made in 2005. TABLE 2 describes the criteria for the recommendations coming from the task force.
Several of these recommendations deserve elaboration.
TABLE 1
USPSTF recommendations made in 2005
| A RECOMMENDATION (STRONGLY RECOMMENDS) |
|
| B RECOMMENDATION (RECOMMENDS) |
|
| C RECOMMENDATION (NO RECOMMENDATION FOR OR AGAINST) |
|
| D RECOMMENDATION (RECOMMENDS AGAINST) |
|
| I RECOMMENDATION (INSUFFICIENT EVIDENCE TO RECOMMEND FOR OR AGAINST) |
|
TABLE 2
Meaning of recommendations by the USPSTF
| A RECOMMENDATION: STRONGLY RECOMMENDS |
| The USPSTF found good evidence that the service improves important health outcomes and concludes that benefits substantially outweigh harms. |
| B RECOMMENDATION: RECOMMENDS |
| The USPSTF found at least fair evidence that the service improves important health outcomes and concludes that benefits outweigh harms. |
| C RECOMMENDATION: NO RECOMMENDATION FOR OR AGAINST |
| The USPSTF found at least fair evidence that the service can improve health outcomes but concludes that the balance of the benefits and harms is too close to justify a general recommendation. |
| D RECOMMENDATION: RECOMMENDS AGAINST |
| The USPSTF found at least fair evidence that the service is ineffective or that harms outweigh benefits. |
| I RECOMMENDATION: INSUFFICIENT EVIDENCE TO RECOMMEND FOR OR AGAINST |
| Evidence that the service is effective is lacking, of poor quality, or conflicting and the balance of benefits and harms cannot be determined. |
Sexually transmitted infections
Among the conditions studied in 2005 were gonorrhea, herpes simplex virus (HSV), and human immunodefeciency virus (HIV). The recommendations were different for each. The task force strongly recommends (A recommendation) screening all pregnant women and all high-risk adolescents and adults for HIV. No recommendation is made regarding HIV screening in adolescents and adults who are not at high risk.
The task force recommends (B recommendation) screening high-risk women for gonorrhea, including those who are pregnant.
It recommends against (D recommendation) screening for gonorrhea in men and women at low risk and believes there is insufficient evidence (I recommendation) to advocate for or against screening men at high risk and pregnant women at low risk. It recommends against screening for HSV during pregnancy and among asymptomatic adolescents and adults.
The Task Force defines high risk a little differently for gonorrhea and HIV. These definitions are listed in TABLE 3.
What the C and I recommendations do and do not mean. Keep several points in mind regarding these recommendations. When no recommendation is made for or against (C recommendation), it signifies there is evidence of some benefit but not clear enough to outweigh harms. An I recommendation means that there is not enough quality evidence to make a recommendation. These 2 recommendations are often misinterpreted as a recommendation against the service, which they are not.
Don’t confuse screening with diagnosis. Screening, by definition, means looking for a condition among asymptomatic persons. Diagnostic tests performed to clarify the cause of symptoms or to improve clinical care are not screening tests. Screening recommendations therefore do not apply in these latter instances. In addition, the task force recognizes that screening recommendations need to be interpreted in light of local epidemiology. In areas of high STD prevalence, more widespread screening might well be justified.
TABLE 3
USPSTF definitions of risk for HIV and gonorrhea
| HIV RISK |
|
| GONORRHEA RISK |
|
Abdominal aortic aneurysms
The rationale behind these recommendations was discussed in a previous Practice Alert.1 The take-home message for physicians is that any male who has ever smoked can possibly benefit from a one-time abdominal ultrasound.
Women’s health conditions
Two sets of recommendations pertain to hormone replacement therapy (HRT) and screening for breast and ovarian cancer.
When to avoid HRT. The task force recommends against using combined estrogen and progestin in postmenopausal women, and against using estrogen to prevent chronic health conditions in postmenopausal women who have had a hysterectomy.
Estrogen and progestin combinations reduce the risk for fractures and colorectal cancer but have no beneficial effect on (and may increase the risk of) coronary heart disease. Other documented harms include increased risk for breast cancer, venous thromboembolism, stroke, cholecystitis, dementia, and lower global cognitive function. Unopposed estrogen reduces the risk for fractures but increases risk for venous thromboembolism, stroke, dementia, and lower global cognitive functioning, and it has no beneficial effect on coronary heart disease.
When to investigate possible BRCA1 or BRCA2 gene mutations. For women with family histories suggestive of mutations of BRCA1 or BRCA2 genes—which place women at markedly higher lifetime risk of breast and ovarian cancer—the task force recommends referral for counseling and possible genetic testing. While the task force acknowledges the unresolved ethical, social, and legal issues, as well as the unknown benefits of chemoprevention and intensive screening, they believe the potential of prophylactic surgery to prevent breast and ovarian cancer is sufficient to make the recommendation.
Note that the recommendation is for referral for genetic counseling in which genetic testing may be considered. It is not a recommendation for testing by itself.
The following elements of a family history place a woman at risk:
- 2 first-degree relatives with breast cancer, 1 of whom received the diagnosis at age 50 years or younger
- A combination of 3 or more first- or second-degree relatives with breast cancer regardless of age at diagnosis
- A combination of both breast and ovarian cancer among first- and second-degree relatives
- A first-degree relative with bilateral breast cancer
- A combination of 2 or more first-or second-degree relatives with ovarian cancer regardless of age at diagnosis
- A first- or second-degree relative with both breast and ovarian cancer at any age
- A history of breast cancer in a male relative.
For Ashkenazi Jewish women, an increased-risk family history includes any first-degree relative (or 2 second-degree relatives on the same side of the family) with breast or ovarian cancer.
For women without a high-risk family history, the task force believes that counseling or routine testing will lead to more harms than benefits and they recommend against it.
Overweight in children and adolescents
The task force acknowledges the increasing prevalence of overweight and obesity in children and adolescents and the consequent adverse health outcomes caused by this condition. As with many unhealthy conditions or habits, however, it is not known whether screening and counseling in the primary care setting reduce child and adolescent obesity.
Remember that the task force is not recommending for or against measuring height, weight, and body-mass index in the office or talking to young patients about their weight. They are simply summarizing the state of the science, which is unclear about whether such efforts by physicians have any effect.
Resources
The complete set of current USPSTF recommendations along with clinical considerations and links to evidence reports can be found on the USPSTF web site, www.ahrq.gov/clinic/uspstfix.htm.
CORRESPONDENCE
Doug Campos-Outcalt, MD,MPA, 4001North Third Street #415, Phoenix, AZ 85012. E-mail: dougco@u.arizona.edu
REFERENCE
1. Campos-Outcalt D. US Preventive Services Task Force: The gold standard of evidence-based prevention. J Fam Pract 2005;54:517-519.
The United States Preventive Services Task Force (USPSTF) is the most evidence-based and authoritative organization making recommendations on preventive services in the US. During 2005, 20 recommendations were made on a total of 10 conditions. TABLE 1 lists the recommendations made in 2005. TABLE 2 describes the criteria for the recommendations coming from the task force.
Several of these recommendations deserve elaboration.
TABLE 1
USPSTF recommendations made in 2005
| A RECOMMENDATION (STRONGLY RECOMMENDS) |
|
| B RECOMMENDATION (RECOMMENDS) |
|
| C RECOMMENDATION (NO RECOMMENDATION FOR OR AGAINST) |
|
| D RECOMMENDATION (RECOMMENDS AGAINST) |
|
| I RECOMMENDATION (INSUFFICIENT EVIDENCE TO RECOMMEND FOR OR AGAINST) |
|
TABLE 2
Meaning of recommendations by the USPSTF
| A RECOMMENDATION: STRONGLY RECOMMENDS |
| The USPSTF found good evidence that the service improves important health outcomes and concludes that benefits substantially outweigh harms. |
| B RECOMMENDATION: RECOMMENDS |
| The USPSTF found at least fair evidence that the service improves important health outcomes and concludes that benefits outweigh harms. |
| C RECOMMENDATION: NO RECOMMENDATION FOR OR AGAINST |
| The USPSTF found at least fair evidence that the service can improve health outcomes but concludes that the balance of the benefits and harms is too close to justify a general recommendation. |
| D RECOMMENDATION: RECOMMENDS AGAINST |
| The USPSTF found at least fair evidence that the service is ineffective or that harms outweigh benefits. |
| I RECOMMENDATION: INSUFFICIENT EVIDENCE TO RECOMMEND FOR OR AGAINST |
| Evidence that the service is effective is lacking, of poor quality, or conflicting and the balance of benefits and harms cannot be determined. |
Sexually transmitted infections
Among the conditions studied in 2005 were gonorrhea, herpes simplex virus (HSV), and human immunodefeciency virus (HIV). The recommendations were different for each. The task force strongly recommends (A recommendation) screening all pregnant women and all high-risk adolescents and adults for HIV. No recommendation is made regarding HIV screening in adolescents and adults who are not at high risk.
The task force recommends (B recommendation) screening high-risk women for gonorrhea, including those who are pregnant.
It recommends against (D recommendation) screening for gonorrhea in men and women at low risk and believes there is insufficient evidence (I recommendation) to advocate for or against screening men at high risk and pregnant women at low risk. It recommends against screening for HSV during pregnancy and among asymptomatic adolescents and adults.
The Task Force defines high risk a little differently for gonorrhea and HIV. These definitions are listed in TABLE 3.
What the C and I recommendations do and do not mean. Keep several points in mind regarding these recommendations. When no recommendation is made for or against (C recommendation), it signifies there is evidence of some benefit but not clear enough to outweigh harms. An I recommendation means that there is not enough quality evidence to make a recommendation. These 2 recommendations are often misinterpreted as a recommendation against the service, which they are not.
Don’t confuse screening with diagnosis. Screening, by definition, means looking for a condition among asymptomatic persons. Diagnostic tests performed to clarify the cause of symptoms or to improve clinical care are not screening tests. Screening recommendations therefore do not apply in these latter instances. In addition, the task force recognizes that screening recommendations need to be interpreted in light of local epidemiology. In areas of high STD prevalence, more widespread screening might well be justified.
TABLE 3
USPSTF definitions of risk for HIV and gonorrhea
| HIV RISK |
|
| GONORRHEA RISK |
|
Abdominal aortic aneurysms
The rationale behind these recommendations was discussed in a previous Practice Alert.1 The take-home message for physicians is that any male who has ever smoked can possibly benefit from a one-time abdominal ultrasound.
Women’s health conditions
Two sets of recommendations pertain to hormone replacement therapy (HRT) and screening for breast and ovarian cancer.
When to avoid HRT. The task force recommends against using combined estrogen and progestin in postmenopausal women, and against using estrogen to prevent chronic health conditions in postmenopausal women who have had a hysterectomy.
Estrogen and progestin combinations reduce the risk for fractures and colorectal cancer but have no beneficial effect on (and may increase the risk of) coronary heart disease. Other documented harms include increased risk for breast cancer, venous thromboembolism, stroke, cholecystitis, dementia, and lower global cognitive function. Unopposed estrogen reduces the risk for fractures but increases risk for venous thromboembolism, stroke, dementia, and lower global cognitive functioning, and it has no beneficial effect on coronary heart disease.
When to investigate possible BRCA1 or BRCA2 gene mutations. For women with family histories suggestive of mutations of BRCA1 or BRCA2 genes—which place women at markedly higher lifetime risk of breast and ovarian cancer—the task force recommends referral for counseling and possible genetic testing. While the task force acknowledges the unresolved ethical, social, and legal issues, as well as the unknown benefits of chemoprevention and intensive screening, they believe the potential of prophylactic surgery to prevent breast and ovarian cancer is sufficient to make the recommendation.
Note that the recommendation is for referral for genetic counseling in which genetic testing may be considered. It is not a recommendation for testing by itself.
The following elements of a family history place a woman at risk:
- 2 first-degree relatives with breast cancer, 1 of whom received the diagnosis at age 50 years or younger
- A combination of 3 or more first- or second-degree relatives with breast cancer regardless of age at diagnosis
- A combination of both breast and ovarian cancer among first- and second-degree relatives
- A first-degree relative with bilateral breast cancer
- A combination of 2 or more first-or second-degree relatives with ovarian cancer regardless of age at diagnosis
- A first- or second-degree relative with both breast and ovarian cancer at any age
- A history of breast cancer in a male relative.
For Ashkenazi Jewish women, an increased-risk family history includes any first-degree relative (or 2 second-degree relatives on the same side of the family) with breast or ovarian cancer.
For women without a high-risk family history, the task force believes that counseling or routine testing will lead to more harms than benefits and they recommend against it.
Overweight in children and adolescents
The task force acknowledges the increasing prevalence of overweight and obesity in children and adolescents and the consequent adverse health outcomes caused by this condition. As with many unhealthy conditions or habits, however, it is not known whether screening and counseling in the primary care setting reduce child and adolescent obesity.
Remember that the task force is not recommending for or against measuring height, weight, and body-mass index in the office or talking to young patients about their weight. They are simply summarizing the state of the science, which is unclear about whether such efforts by physicians have any effect.
Resources
The complete set of current USPSTF recommendations along with clinical considerations and links to evidence reports can be found on the USPSTF web site, www.ahrq.gov/clinic/uspstfix.htm.
CORRESPONDENCE
Doug Campos-Outcalt, MD,MPA, 4001North Third Street #415, Phoenix, AZ 85012. E-mail: dougco@u.arizona.edu
The United States Preventive Services Task Force (USPSTF) is the most evidence-based and authoritative organization making recommendations on preventive services in the US. During 2005, 20 recommendations were made on a total of 10 conditions. TABLE 1 lists the recommendations made in 2005. TABLE 2 describes the criteria for the recommendations coming from the task force.
Several of these recommendations deserve elaboration.
TABLE 1
USPSTF recommendations made in 2005
| A RECOMMENDATION (STRONGLY RECOMMENDS) |
|
| B RECOMMENDATION (RECOMMENDS) |
|
| C RECOMMENDATION (NO RECOMMENDATION FOR OR AGAINST) |
|
| D RECOMMENDATION (RECOMMENDS AGAINST) |
|
| I RECOMMENDATION (INSUFFICIENT EVIDENCE TO RECOMMEND FOR OR AGAINST) |
|
TABLE 2
Meaning of recommendations by the USPSTF
| A RECOMMENDATION: STRONGLY RECOMMENDS |
| The USPSTF found good evidence that the service improves important health outcomes and concludes that benefits substantially outweigh harms. |
| B RECOMMENDATION: RECOMMENDS |
| The USPSTF found at least fair evidence that the service improves important health outcomes and concludes that benefits outweigh harms. |
| C RECOMMENDATION: NO RECOMMENDATION FOR OR AGAINST |
| The USPSTF found at least fair evidence that the service can improve health outcomes but concludes that the balance of the benefits and harms is too close to justify a general recommendation. |
| D RECOMMENDATION: RECOMMENDS AGAINST |
| The USPSTF found at least fair evidence that the service is ineffective or that harms outweigh benefits. |
| I RECOMMENDATION: INSUFFICIENT EVIDENCE TO RECOMMEND FOR OR AGAINST |
| Evidence that the service is effective is lacking, of poor quality, or conflicting and the balance of benefits and harms cannot be determined. |
Sexually transmitted infections
Among the conditions studied in 2005 were gonorrhea, herpes simplex virus (HSV), and human immunodefeciency virus (HIV). The recommendations were different for each. The task force strongly recommends (A recommendation) screening all pregnant women and all high-risk adolescents and adults for HIV. No recommendation is made regarding HIV screening in adolescents and adults who are not at high risk.
The task force recommends (B recommendation) screening high-risk women for gonorrhea, including those who are pregnant.
It recommends against (D recommendation) screening for gonorrhea in men and women at low risk and believes there is insufficient evidence (I recommendation) to advocate for or against screening men at high risk and pregnant women at low risk. It recommends against screening for HSV during pregnancy and among asymptomatic adolescents and adults.
The Task Force defines high risk a little differently for gonorrhea and HIV. These definitions are listed in TABLE 3.
What the C and I recommendations do and do not mean. Keep several points in mind regarding these recommendations. When no recommendation is made for or against (C recommendation), it signifies there is evidence of some benefit but not clear enough to outweigh harms. An I recommendation means that there is not enough quality evidence to make a recommendation. These 2 recommendations are often misinterpreted as a recommendation against the service, which they are not.
Don’t confuse screening with diagnosis. Screening, by definition, means looking for a condition among asymptomatic persons. Diagnostic tests performed to clarify the cause of symptoms or to improve clinical care are not screening tests. Screening recommendations therefore do not apply in these latter instances. In addition, the task force recognizes that screening recommendations need to be interpreted in light of local epidemiology. In areas of high STD prevalence, more widespread screening might well be justified.
TABLE 3
USPSTF definitions of risk for HIV and gonorrhea
| HIV RISK |
|
| GONORRHEA RISK |
|
Abdominal aortic aneurysms
The rationale behind these recommendations was discussed in a previous Practice Alert.1 The take-home message for physicians is that any male who has ever smoked can possibly benefit from a one-time abdominal ultrasound.
Women’s health conditions
Two sets of recommendations pertain to hormone replacement therapy (HRT) and screening for breast and ovarian cancer.
When to avoid HRT. The task force recommends against using combined estrogen and progestin in postmenopausal women, and against using estrogen to prevent chronic health conditions in postmenopausal women who have had a hysterectomy.
Estrogen and progestin combinations reduce the risk for fractures and colorectal cancer but have no beneficial effect on (and may increase the risk of) coronary heart disease. Other documented harms include increased risk for breast cancer, venous thromboembolism, stroke, cholecystitis, dementia, and lower global cognitive function. Unopposed estrogen reduces the risk for fractures but increases risk for venous thromboembolism, stroke, dementia, and lower global cognitive functioning, and it has no beneficial effect on coronary heart disease.
When to investigate possible BRCA1 or BRCA2 gene mutations. For women with family histories suggestive of mutations of BRCA1 or BRCA2 genes—which place women at markedly higher lifetime risk of breast and ovarian cancer—the task force recommends referral for counseling and possible genetic testing. While the task force acknowledges the unresolved ethical, social, and legal issues, as well as the unknown benefits of chemoprevention and intensive screening, they believe the potential of prophylactic surgery to prevent breast and ovarian cancer is sufficient to make the recommendation.
Note that the recommendation is for referral for genetic counseling in which genetic testing may be considered. It is not a recommendation for testing by itself.
The following elements of a family history place a woman at risk:
- 2 first-degree relatives with breast cancer, 1 of whom received the diagnosis at age 50 years or younger
- A combination of 3 or more first- or second-degree relatives with breast cancer regardless of age at diagnosis
- A combination of both breast and ovarian cancer among first- and second-degree relatives
- A first-degree relative with bilateral breast cancer
- A combination of 2 or more first-or second-degree relatives with ovarian cancer regardless of age at diagnosis
- A first- or second-degree relative with both breast and ovarian cancer at any age
- A history of breast cancer in a male relative.
For Ashkenazi Jewish women, an increased-risk family history includes any first-degree relative (or 2 second-degree relatives on the same side of the family) with breast or ovarian cancer.
For women without a high-risk family history, the task force believes that counseling or routine testing will lead to more harms than benefits and they recommend against it.
Overweight in children and adolescents
The task force acknowledges the increasing prevalence of overweight and obesity in children and adolescents and the consequent adverse health outcomes caused by this condition. As with many unhealthy conditions or habits, however, it is not known whether screening and counseling in the primary care setting reduce child and adolescent obesity.
Remember that the task force is not recommending for or against measuring height, weight, and body-mass index in the office or talking to young patients about their weight. They are simply summarizing the state of the science, which is unclear about whether such efforts by physicians have any effect.
Resources
The complete set of current USPSTF recommendations along with clinical considerations and links to evidence reports can be found on the USPSTF web site, www.ahrq.gov/clinic/uspstfix.htm.
CORRESPONDENCE
Doug Campos-Outcalt, MD,MPA, 4001North Third Street #415, Phoenix, AZ 85012. E-mail: dougco@u.arizona.edu
REFERENCE
1. Campos-Outcalt D. US Preventive Services Task Force: The gold standard of evidence-based prevention. J Fam Pract 2005;54:517-519.
REFERENCE
1. Campos-Outcalt D. US Preventive Services Task Force: The gold standard of evidence-based prevention. J Fam Pract 2005;54:517-519.
Are you up to date with new immunization recommendations?
Vaccines are one of the most important and effective tools for protecting the health of the public and family physicians are instrumental in insuring that vaccine recommendations are implemented. With the development of new vaccines come increasingly complex recommendations. Staying current is challenging.
This column describes the most recent changes to the immunization schedules made by the Advisory Committee on Immunization Practices (ACIP) of the Centers for Disease Control and Prevention (CDC).
Hepatitis A
Universal vaccination with hepatitis A virus (HAV) vaccine is now recommended for all children between their first and second birthdays, with 2 doses of vaccine given 6 months apart.
Previously, universal vaccination was recommended only for states and population groups known to have a high prevalence of infection. The recommended age for vaccination varied depending on local circumstances, but the vaccine was not approved for use before the second birthday. Because of the marked success in reducing hepatitis A infection rates in high-prevalence areas, most HAV cases now occur in states without routine vaccination recommendations.
Each year, 5000 to 7000 cases of hepatitis A are reported, and it is estimated that 4 times that number of symptomatic cases occur. The ACIP’s new recommendation is based on recent approval of HAV vaccine for use starting at age 1 year, and on the expected doubling of disease reduction from routine, universal vaccination of children. Moreover, universal vaccination of children is expected to reduce hepatitis A incidence among adults because children often are the source of infection transmission to older family members.
Keep in mind that HAV vaccination for children age 2 to 18 years may still be recommended in your area depending on local circumstances and disease epidemiology.
Hepatitis B
The ACIP recommends that the first dose of hepatitis B vaccine (HepB) routinely be given to newborns before hospital discharge. This first dose in the 3-dose series should be monovalent HepB and should be delayed only if a physician so orders and if the mother is documented to be hepatitis surface antigen negative. This recommendation is the latest addition to the national strategy to eliminate hepatitis B virus transmission in the United States.1
Pertussis
A Practice Alert column last year described the re-emergence of pertussis and the licensure of tetanus and diphtheria toxoid and acellular pertussis (Tdap) products for use in adolescents and adults.2 There are now 5 new recommendations regarding the use of Tdap.
- A single dose of Tdap should replace the next dose of Td for adults aged 19 to 64 years as part of the every-10-year Td boosting schedule.
- A single dose of Tdap should be administered to adults who have close contact with infants less than 6 months of age. The optimal interval between Tdap and the last Td is 2 years or greater, but shorter intervals are acceptable.
- Women of childbearing age should receive Tdap before conception or postpartum, if they have not previously received Tdap. Tdap is not approved for use during pregnancy.
- All adolescents aged 11 to 12 should receive a single dose of Tdap.
- Adolescents aged 13 to 18 should receive Tdap if they received the last Td more than 5 years previously, or in less than 2 years for special circumstances such as close contact with an infant or in an outbreak.
There are only 2 contraindications to the use of Tdap: a history of anaphylactic reaction to a Tdap vaccine component, or a history of encephalopathy within 7 days of receiving a pertussis vaccine that cannot be attributed to another cause. Precautions include Guillain-Barré syndrome less than 6 weeks after a previous dose of tetanus toxoid, moderate or severe acute illness (with or without fever), unstable neurologic condition, or a history of an Arthus hypersensitivity reaction after a dose of tetanus or diphtheria toxoid.
With the new recommendation for Tdap at age 11 to 12 years, 2 vaccines are now indicated for this age group—the other being quadrivalent meningococcal vaccine.3 The ACIP recommends a wellness visit at this age to facilitate these immunizations.
Varicella
Varicella immune globulin is no longer produced. Post-varicella-exposure prophylaxis is recommended for those who do not have varicella immunity and who are likely to get severe varicella disease. These include immunocompromised persons, neonates, premature infants, and pregnant women. The ACIP now recommends intravenous immune globulin for these situations.
ACIP also recommends that pregnant women with no proof of varicella immunity be screened with a blood test during pregnancy, and those found to be nonimmune should receive varicella vaccine postpartum with the second dose 4 to 8 weeks later. Proof of immunity consists of a history of documented varicella infection or herpes zoster, age-appropriate vaccination, being born before 1966, or a positive serology result for varicella antibody.
A measles-mumps-rubella-varicella combination vaccine (MMRV) is now available and can cut down on the number of injections needed to complete child vaccination recommendations. It will also stimulate more discussion about the potential advantages of a second varicella dose for children under age 13 years, which is currently not recommended.
The TABLE summarizes these new vaccine recommendations by age group. The complete immunization schedule for children and adults can be located on the CDC Web site.4,5 These schedules can be printed and placed in clinic setting to assist physicians and staff to competently fulfill one their most important public health functions, insuring the full immunization of their patient populations.
TABLE
Recent immunization recommendations by age group
| INFANTS AND CHILDREN |
| Hepatitis B vaccine (HepB) as monovalent HepB before discharge from the hospital unless order otherwise by a physicians and the mother is documented to be hepatitis B surface antigen negative. |
| Universal, routine vaccination with hepatitis A vaccine between age 1 to 2 years with 2 doses 6 months apart. |
| ADOLESCENTS |
| Tdap at age 11 to 12 years. |
| Tdap at age 13 to 18 years if the last Td was administered >5 years previously and no previous Tdap administered. In special circumstances the interval from the last Td can be less than 5 years. |
| Wellness visit at age 11 to 12 years to administer both Tdap and meningococcal vaccines. |
| ADULTS |
| Tdap to replace the next scheduled Td booster, one time only. |
| Tdap as single dose for adults caring for children less than age 6 months. |
| PREGNANT WOMEN |
| Screen for varicella immunity in those without proof of immunity. Immunize postpartum those nonimmune. |
| Tdap either during preconception period or immediately postpartum, if no Tdap previously received. |
CORRESPONDENCE
Doug Campos-Outcalt, MD, MPA, 4001 North Third Street #415, Phoenix, AZ 85012. E-mail: dougco@u.arizona.edu
1. A Comprehensive Immunization Strategy to Eliminate Transmission of Hepatitis B Virus Infection in the United States. MMWR Recomm Rep 2005; 54(RR-16):1–23. Available at: www.cdc.gov/mmwr/preview/mmwrhtml/rr5416a1.htm?s_cid=rr5416a1_e. Accessed on February 7, 2006.
2. Campos-Outcalt D. Pertussis: A disease re-emerges. J Fam Pract 2005;54:699-703.
3. Campos-Outcalt D. Meningococcal vaccine: new product, new recommendations. J Fam Pract 2005;54:324-326.
4. CDC. Recommended childhood and adolescent immunization schedule. United States, 2006. Available at: www.immunize.org/cdc/child-schedule.pdf. Accessed on February 7, 2006.
5. CDC. Recommended adult immunization schedule, by vaccine and age group. United States, October 2005—September 2006. Available at: www.cdc.gov/nip/recs/adult-schedule.pdf. Accessed on February 7, 2006.
Vaccines are one of the most important and effective tools for protecting the health of the public and family physicians are instrumental in insuring that vaccine recommendations are implemented. With the development of new vaccines come increasingly complex recommendations. Staying current is challenging.
This column describes the most recent changes to the immunization schedules made by the Advisory Committee on Immunization Practices (ACIP) of the Centers for Disease Control and Prevention (CDC).
Hepatitis A
Universal vaccination with hepatitis A virus (HAV) vaccine is now recommended for all children between their first and second birthdays, with 2 doses of vaccine given 6 months apart.
Previously, universal vaccination was recommended only for states and population groups known to have a high prevalence of infection. The recommended age for vaccination varied depending on local circumstances, but the vaccine was not approved for use before the second birthday. Because of the marked success in reducing hepatitis A infection rates in high-prevalence areas, most HAV cases now occur in states without routine vaccination recommendations.
Each year, 5000 to 7000 cases of hepatitis A are reported, and it is estimated that 4 times that number of symptomatic cases occur. The ACIP’s new recommendation is based on recent approval of HAV vaccine for use starting at age 1 year, and on the expected doubling of disease reduction from routine, universal vaccination of children. Moreover, universal vaccination of children is expected to reduce hepatitis A incidence among adults because children often are the source of infection transmission to older family members.
Keep in mind that HAV vaccination for children age 2 to 18 years may still be recommended in your area depending on local circumstances and disease epidemiology.
Hepatitis B
The ACIP recommends that the first dose of hepatitis B vaccine (HepB) routinely be given to newborns before hospital discharge. This first dose in the 3-dose series should be monovalent HepB and should be delayed only if a physician so orders and if the mother is documented to be hepatitis surface antigen negative. This recommendation is the latest addition to the national strategy to eliminate hepatitis B virus transmission in the United States.1
Pertussis
A Practice Alert column last year described the re-emergence of pertussis and the licensure of tetanus and diphtheria toxoid and acellular pertussis (Tdap) products for use in adolescents and adults.2 There are now 5 new recommendations regarding the use of Tdap.
- A single dose of Tdap should replace the next dose of Td for adults aged 19 to 64 years as part of the every-10-year Td boosting schedule.
- A single dose of Tdap should be administered to adults who have close contact with infants less than 6 months of age. The optimal interval between Tdap and the last Td is 2 years or greater, but shorter intervals are acceptable.
- Women of childbearing age should receive Tdap before conception or postpartum, if they have not previously received Tdap. Tdap is not approved for use during pregnancy.
- All adolescents aged 11 to 12 should receive a single dose of Tdap.
- Adolescents aged 13 to 18 should receive Tdap if they received the last Td more than 5 years previously, or in less than 2 years for special circumstances such as close contact with an infant or in an outbreak.
There are only 2 contraindications to the use of Tdap: a history of anaphylactic reaction to a Tdap vaccine component, or a history of encephalopathy within 7 days of receiving a pertussis vaccine that cannot be attributed to another cause. Precautions include Guillain-Barré syndrome less than 6 weeks after a previous dose of tetanus toxoid, moderate or severe acute illness (with or without fever), unstable neurologic condition, or a history of an Arthus hypersensitivity reaction after a dose of tetanus or diphtheria toxoid.
With the new recommendation for Tdap at age 11 to 12 years, 2 vaccines are now indicated for this age group—the other being quadrivalent meningococcal vaccine.3 The ACIP recommends a wellness visit at this age to facilitate these immunizations.
Varicella
Varicella immune globulin is no longer produced. Post-varicella-exposure prophylaxis is recommended for those who do not have varicella immunity and who are likely to get severe varicella disease. These include immunocompromised persons, neonates, premature infants, and pregnant women. The ACIP now recommends intravenous immune globulin for these situations.
ACIP also recommends that pregnant women with no proof of varicella immunity be screened with a blood test during pregnancy, and those found to be nonimmune should receive varicella vaccine postpartum with the second dose 4 to 8 weeks later. Proof of immunity consists of a history of documented varicella infection or herpes zoster, age-appropriate vaccination, being born before 1966, or a positive serology result for varicella antibody.
A measles-mumps-rubella-varicella combination vaccine (MMRV) is now available and can cut down on the number of injections needed to complete child vaccination recommendations. It will also stimulate more discussion about the potential advantages of a second varicella dose for children under age 13 years, which is currently not recommended.
The TABLE summarizes these new vaccine recommendations by age group. The complete immunization schedule for children and adults can be located on the CDC Web site.4,5 These schedules can be printed and placed in clinic setting to assist physicians and staff to competently fulfill one their most important public health functions, insuring the full immunization of their patient populations.
TABLE
Recent immunization recommendations by age group
| INFANTS AND CHILDREN |
| Hepatitis B vaccine (HepB) as monovalent HepB before discharge from the hospital unless order otherwise by a physicians and the mother is documented to be hepatitis B surface antigen negative. |
| Universal, routine vaccination with hepatitis A vaccine between age 1 to 2 years with 2 doses 6 months apart. |
| ADOLESCENTS |
| Tdap at age 11 to 12 years. |
| Tdap at age 13 to 18 years if the last Td was administered >5 years previously and no previous Tdap administered. In special circumstances the interval from the last Td can be less than 5 years. |
| Wellness visit at age 11 to 12 years to administer both Tdap and meningococcal vaccines. |
| ADULTS |
| Tdap to replace the next scheduled Td booster, one time only. |
| Tdap as single dose for adults caring for children less than age 6 months. |
| PREGNANT WOMEN |
| Screen for varicella immunity in those without proof of immunity. Immunize postpartum those nonimmune. |
| Tdap either during preconception period or immediately postpartum, if no Tdap previously received. |
CORRESPONDENCE
Doug Campos-Outcalt, MD, MPA, 4001 North Third Street #415, Phoenix, AZ 85012. E-mail: dougco@u.arizona.edu
Vaccines are one of the most important and effective tools for protecting the health of the public and family physicians are instrumental in insuring that vaccine recommendations are implemented. With the development of new vaccines come increasingly complex recommendations. Staying current is challenging.
This column describes the most recent changes to the immunization schedules made by the Advisory Committee on Immunization Practices (ACIP) of the Centers for Disease Control and Prevention (CDC).
Hepatitis A
Universal vaccination with hepatitis A virus (HAV) vaccine is now recommended for all children between their first and second birthdays, with 2 doses of vaccine given 6 months apart.
Previously, universal vaccination was recommended only for states and population groups known to have a high prevalence of infection. The recommended age for vaccination varied depending on local circumstances, but the vaccine was not approved for use before the second birthday. Because of the marked success in reducing hepatitis A infection rates in high-prevalence areas, most HAV cases now occur in states without routine vaccination recommendations.
Each year, 5000 to 7000 cases of hepatitis A are reported, and it is estimated that 4 times that number of symptomatic cases occur. The ACIP’s new recommendation is based on recent approval of HAV vaccine for use starting at age 1 year, and on the expected doubling of disease reduction from routine, universal vaccination of children. Moreover, universal vaccination of children is expected to reduce hepatitis A incidence among adults because children often are the source of infection transmission to older family members.
Keep in mind that HAV vaccination for children age 2 to 18 years may still be recommended in your area depending on local circumstances and disease epidemiology.
Hepatitis B
The ACIP recommends that the first dose of hepatitis B vaccine (HepB) routinely be given to newborns before hospital discharge. This first dose in the 3-dose series should be monovalent HepB and should be delayed only if a physician so orders and if the mother is documented to be hepatitis surface antigen negative. This recommendation is the latest addition to the national strategy to eliminate hepatitis B virus transmission in the United States.1
Pertussis
A Practice Alert column last year described the re-emergence of pertussis and the licensure of tetanus and diphtheria toxoid and acellular pertussis (Tdap) products for use in adolescents and adults.2 There are now 5 new recommendations regarding the use of Tdap.
- A single dose of Tdap should replace the next dose of Td for adults aged 19 to 64 years as part of the every-10-year Td boosting schedule.
- A single dose of Tdap should be administered to adults who have close contact with infants less than 6 months of age. The optimal interval between Tdap and the last Td is 2 years or greater, but shorter intervals are acceptable.
- Women of childbearing age should receive Tdap before conception or postpartum, if they have not previously received Tdap. Tdap is not approved for use during pregnancy.
- All adolescents aged 11 to 12 should receive a single dose of Tdap.
- Adolescents aged 13 to 18 should receive Tdap if they received the last Td more than 5 years previously, or in less than 2 years for special circumstances such as close contact with an infant or in an outbreak.
There are only 2 contraindications to the use of Tdap: a history of anaphylactic reaction to a Tdap vaccine component, or a history of encephalopathy within 7 days of receiving a pertussis vaccine that cannot be attributed to another cause. Precautions include Guillain-Barré syndrome less than 6 weeks after a previous dose of tetanus toxoid, moderate or severe acute illness (with or without fever), unstable neurologic condition, or a history of an Arthus hypersensitivity reaction after a dose of tetanus or diphtheria toxoid.
With the new recommendation for Tdap at age 11 to 12 years, 2 vaccines are now indicated for this age group—the other being quadrivalent meningococcal vaccine.3 The ACIP recommends a wellness visit at this age to facilitate these immunizations.
Varicella
Varicella immune globulin is no longer produced. Post-varicella-exposure prophylaxis is recommended for those who do not have varicella immunity and who are likely to get severe varicella disease. These include immunocompromised persons, neonates, premature infants, and pregnant women. The ACIP now recommends intravenous immune globulin for these situations.
ACIP also recommends that pregnant women with no proof of varicella immunity be screened with a blood test during pregnancy, and those found to be nonimmune should receive varicella vaccine postpartum with the second dose 4 to 8 weeks later. Proof of immunity consists of a history of documented varicella infection or herpes zoster, age-appropriate vaccination, being born before 1966, or a positive serology result for varicella antibody.
A measles-mumps-rubella-varicella combination vaccine (MMRV) is now available and can cut down on the number of injections needed to complete child vaccination recommendations. It will also stimulate more discussion about the potential advantages of a second varicella dose for children under age 13 years, which is currently not recommended.
The TABLE summarizes these new vaccine recommendations by age group. The complete immunization schedule for children and adults can be located on the CDC Web site.4,5 These schedules can be printed and placed in clinic setting to assist physicians and staff to competently fulfill one their most important public health functions, insuring the full immunization of their patient populations.
TABLE
Recent immunization recommendations by age group
| INFANTS AND CHILDREN |
| Hepatitis B vaccine (HepB) as monovalent HepB before discharge from the hospital unless order otherwise by a physicians and the mother is documented to be hepatitis B surface antigen negative. |
| Universal, routine vaccination with hepatitis A vaccine between age 1 to 2 years with 2 doses 6 months apart. |
| ADOLESCENTS |
| Tdap at age 11 to 12 years. |
| Tdap at age 13 to 18 years if the last Td was administered >5 years previously and no previous Tdap administered. In special circumstances the interval from the last Td can be less than 5 years. |
| Wellness visit at age 11 to 12 years to administer both Tdap and meningococcal vaccines. |
| ADULTS |
| Tdap to replace the next scheduled Td booster, one time only. |
| Tdap as single dose for adults caring for children less than age 6 months. |
| PREGNANT WOMEN |
| Screen for varicella immunity in those without proof of immunity. Immunize postpartum those nonimmune. |
| Tdap either during preconception period or immediately postpartum, if no Tdap previously received. |
CORRESPONDENCE
Doug Campos-Outcalt, MD, MPA, 4001 North Third Street #415, Phoenix, AZ 85012. E-mail: dougco@u.arizona.edu
1. A Comprehensive Immunization Strategy to Eliminate Transmission of Hepatitis B Virus Infection in the United States. MMWR Recomm Rep 2005; 54(RR-16):1–23. Available at: www.cdc.gov/mmwr/preview/mmwrhtml/rr5416a1.htm?s_cid=rr5416a1_e. Accessed on February 7, 2006.
2. Campos-Outcalt D. Pertussis: A disease re-emerges. J Fam Pract 2005;54:699-703.
3. Campos-Outcalt D. Meningococcal vaccine: new product, new recommendations. J Fam Pract 2005;54:324-326.
4. CDC. Recommended childhood and adolescent immunization schedule. United States, 2006. Available at: www.immunize.org/cdc/child-schedule.pdf. Accessed on February 7, 2006.
5. CDC. Recommended adult immunization schedule, by vaccine and age group. United States, October 2005—September 2006. Available at: www.cdc.gov/nip/recs/adult-schedule.pdf. Accessed on February 7, 2006.
1. A Comprehensive Immunization Strategy to Eliminate Transmission of Hepatitis B Virus Infection in the United States. MMWR Recomm Rep 2005; 54(RR-16):1–23. Available at: www.cdc.gov/mmwr/preview/mmwrhtml/rr5416a1.htm?s_cid=rr5416a1_e. Accessed on February 7, 2006.
2. Campos-Outcalt D. Pertussis: A disease re-emerges. J Fam Pract 2005;54:699-703.
3. Campos-Outcalt D. Meningococcal vaccine: new product, new recommendations. J Fam Pract 2005;54:324-326.
4. CDC. Recommended childhood and adolescent immunization schedule. United States, 2006. Available at: www.immunize.org/cdc/child-schedule.pdf. Accessed on February 7, 2006.
5. CDC. Recommended adult immunization schedule, by vaccine and age group. United States, October 2005—September 2006. Available at: www.cdc.gov/nip/recs/adult-schedule.pdf. Accessed on February 7, 2006.