User login
One of your office personnel receives a superficial stick from a needle while putting it into a sharps disposal container. Is postexposure prophylaxis (PEP) for HIV warranted?
Another health care worker receives a major blood splash into her eye after dropping a blood tube taken from a source of unknown HIV status. Is PEP called for in this instance?
A child who was rifling through a trash bin accidentally poked himself with an improperly disposed hypodermic needle. Should he be given PEP?
In most cases, HIV PEP is given only to healthcare workers if the settings make exposure to HIV-infected persons likely. Otherwise, it is usually deemed unnecessary. However, a decision for or against PEP is complicated.
Occupational and nonoccupational exposure to HIV can produce fear, anxiety, and stress. Information on the exposure risk is frequently incomplete, the risk of infection is usually low, the degree of protection offered by PEP is not fully defined, and the potential for side effects from the medications is significant.
This article distills the Centers for Disease Control and Prevention’s most recent guidance on HIV PEP.
HIV on the rise again
Antiretroviral therapy has markedly reduced mortality from HIV/AIDS, but the incidence of new cases, after declining in the 1990s, has gradually increased since 2000.1 As described in a previous article in the Journal of Family Practice,2 efforts to control HIV now focus on increased testing of those persons at risk, behavior modification to reduce the chances of infected persons exposing others, and treating HIV-positive pregnant women and providing postnatal prophylaxis to their newly born infants.
Exposure to HIV can occur occupationally, during a sexual assault, or from the failure of barrier protection during sex. Though these types of exposure are not major contributors to HIV incidence, and postexposure prophylaxis is not expected to play a major role in reducing the incidence of disease, it is available to persons potentially exposed to HIV, and it is beneficial to know when it is and is not indicated. Evidence for possible effectiveness of PEP comes from studies of postnatal prophylaxis, animal studies, case control studies and case reports.3
The Centers for Disease Control and Prevention (CDC) has developed 2 sets of recommendations for PEP that take into consideration the type and severity of the exposure and characteristics of the source of the exposure (TABLE 1).3,4
TABLE 1
Recommended HIV postexposure prophylaxis for percutanous injuries and membrane/nonintact skin exposures
| For percutaneous injuries | |||||
| EXPOSURE TYPE | INFECTION STATUS OF SOURCE | ||||
| HIV-POSITIVE, CLASS 1* | HIV-POSITIVE, CLASS 2* | SOURCE OF UNKNOWN HIV STATUS† | UNKNOWN SOURCE‡ | HIV-NEGATIVE | |
| Less severe (eg, solid needle or supercficial injury) | Recommend basic 2-drug PEP | Recommend expanded ≥3-drug PEP | Generally, no PEP warranted however, consider basic 2-drug PEP¶ for source with HIV risk factors** | Generally, no PEP warranted; however, consider basic 2-drug PEP¶ in settings in which exposure to HIV-infected persons is likely | No PEP warranted |
| More severe (large-bore hollow needle, deep puncture wound, blood on device, needle used in artery/vein) | Recommend expanded ≥3-drug PEP | Recommend expanded ≥3-drug PEP | Generally, no PEP warranted; however, consider basic 2-drug¶ for source with HIV risk factors** | Generally, no PEP warranted; however, consider basic 2-drug PEP¶ in settings in which exposure to HIV infected persons is likely | No PEP warranted |
| For mucous membrane and nonintact skin exposures†† | |||||
| Small volume (eg, a few drops) | Consider basic 2-drug PEP¶ | Recommend basic 2-drug PEP | Generally, no PEP warranted** | Generally, no PEP warranted | No PEP warranted |
| Large volume (eg, a major blood splash) | Recommend basic 2-drug PEP | Recommend expended ≥3-drug PEP | Generally, no PEP warranted; however, consider basic 2-drug PEP¶ for source with HIV risk factors** | Generally, no PEP warranted; however, consider basic 2-drug PEP¶ in settings in which exposure to HIV-infected persons is likely | No PEP warranted |
| *HIV-positive, class 1—asymptomatic HIV infection or known low viral load (eg, <1500 ribonucleic acid copies/mL). HIV-positive, class 2—symptomatic HIV infection, AIDS, acute seroconversion, or known high viral load. If drug resistance is a concern, obtain expert consultation. Initiation of PEP should not be delayed pending expert consultation, and, because expert consultation alone cannot substitute for face-to-face counseling, resources should be available to provide immediate evaluation and follow-up care for all exposures. | |||||
| †For example, deceased source person with no samples available for HIV testing. | |||||
| ‡For example, a needle from a sharps container or splash from inappropriately disposed blood. | |||||
| ¶The recommendation “consider PEP” indicates that PEP is optional; a decision to initiate PEP should be based on a discussion between the exposed person and the treating clinician regarding the risks versus benefits of PEP. | |||||
| **If PEP is offered and administered and the source is later determined to be HIV-negative, PEP should be discontinued. | |||||
| ††For skin exposures, follow-up is indicated only if evidence exists of compromised skin integrity (eg, dermatitis, abrasion, or open wound). | |||||
| Source: Centers for Disease Control and Prevention 2005.4 | |||||
Occupational exposures to HIV
Occupational exposure to HIV can result from a needlestick injury, cut with a sharp object, or contact with potentially infectious body fluids to mucous membranes or skin that is not intact (chapped, cut, abraded, inflamed). Body fluids that are considered potentially infectious are listed in TABLE 2, along with fluids not considered to be infectious.
The risk of contracting HIV from an occupational exposure is determined by several factors, but is generally low. The risk of infection after a needle-stick injury with exposure to infected blood is estimated at 0.3%; after a mucous membrane exposure, 0.09%. The risk after exposure to nonintact skin is probably even lower. Risk increases with the quantity of blood exposed to, a needle-stick injury directly into a vein or artery, and deep injuries.
TABLE 2
Which body fluids are infectious?
| BODY FLUIDS POTENTIALLY INFECTIOUS FOR HIV | |
|
|
| BODY FLUIDS NOT CONSIDERED TO BE INFECTIOUS UNLESS THEY ARE VISIBLY BLOODY | |
|
|
Who should and should not receive PEP
TABLE 1 details recommended treatment responses to specific types of exposure (eg, puncture wound) and the status of the exposure source.4 In situations unlikely to result in disease transmission (superficial injury and source patient with unknown HIV status), no PEP is generally warranted due to the low risk of infection and potential toxicity of antiretrovirals.
Treatment particulars
Start postexposure prophylaxis, when indicated, as soon as possible following exposure and continue it for 4 weeks. Obtain baseline test results for HIV at the time of exposure and periodically for 6 months. The CDC recommends testing at 6 weeks, 12 weeks, and 6 months, whether or not PEP is provided.
Testing and monitoring for hepatitis B and C may also be indicated.
Advise patients on PEP to use precautions in avoiding the possibility of secondary transmission, especially in the first 3 months following exposure. Monitor for drug toxicity every week or 2 while giving PEP. Because of the complexity of potential PEP regimens and the risk for drug toxicity, you may want to take advantage of several national sources of consultation, such as the PEPline (www.ucsf.edu/hivcntr/Hotlines/PEPline or 888-448-4911) or the HIV/AIDS Treatment Information Service (aidsinfo.nih.gov)—especially with questions about potential drug resistance or if the exposed person is pregnant.
When risk is real but low, 2-drug PEP is recommended (TABLE 1), usually 2 nucleoside reverse transcriptase inhibitors (NRTI) or 1 NRTI and 1 nucleotide reverse transcriptase inhibitor (NtRTI).
For those at higher risk, 3 or more antiviral regimens are recommended, achieved by adding a protease inhibitor to one of the recommended 2-drug regimens. The potential antiviral combinations in the basic 2-drug and expanded PEP regimens, along with potential side effects and toxicities of antiviral medications are described in TABLE W1, available online at www.jfponline.com.4
Nonoccupational exposures rarely require PEP
There are many unresolved questions regarding PEP for nonoccupational exposures. The lack of definitive evidence of its effectiveness, its unknown influence on risk-taking behavior, and the potential to aggravate viral resistance have led CDC to recommend that PEP be used only infrequently and not continuously for those whose behavior results in frequent exposures.3 Those who continue to participate in high risk activities should be referred for risk-reduction behavioral counseling.
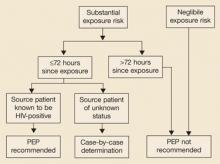
The risk of HIV transmission varies by route and source of exposure (TABLE 3). The CDC has developed an algorithm based on these variables (FIGURE) to help you decide whether to initiate PEP. Two situations that cause concerns but pose little known risk of infection are bites and needlestick injuries from discarded needles; PEP is rarely indicated for either.
As with occupational exposure PEP, those receiving nonoccupational PEP should be evaluated at baseline for HIV infection. In addition, consider evaluating them for other STD’s and pregnancy.
As with occupational exposure, start nonoccupational PEP as soon as possible and continue it for 28 days. Nonoccupational PEP is not recommended if time after exposure is more than 72 hours. A 3-drug regimen is recommended by the CDC for nonoccupational exposures, even though evidence is lacking that it provides superior benefit over 2 drugs (see TABLE W1 at www.jfponline.com).
Follow-up recommendations for those provided nonoccupational PEP are the same as for occupational PEP, and testing for other STDs and hepatitis B and C is also recommended (TABLE 4).
FIGURE
Evaluation and treatment of possible nonoccupational HIV exposures
Source: Centers for Disease Control and Prevention 2005.3
TABLE 3
Estimated per-act risk for acquisition of HIV, by exposure route*
| EXPOSURE ROUTE | RISK PER 10,000 EXPOSURES TO AN INFECTED SOURCE |
|---|---|
| Blood transfusion | 9000 |
| Needle-sharing injection-drug use | 67 |
| Receptive anal intercourse | 50 |
| Percutaneous needle stick | 30 |
| Receptive penile-vaginal intercourse | 10 |
| Insertive anal intercourse | 6.5 |
| Insertive penile-vaginal intercourse | 5 |
| Receptive oral intercourse† | 1 |
| Insertive oral intercourse† | 0.5 |
| *Estimates of risk for transmission from sexual exposures assume no condom use. | |
| †Source refers to oral intercourse performed on a man. | |
| Source: Centers for Disease Control and Prevention 2005.3 | |
TABLE 4
Recommended laboratory evaluation for nonoccupational postexposure prophylaxis of HIV infection
| TEST | BASELINE | DURING PEP* | 4 TO 6 WEEKS AFTER EXPOSURE | 3 MONTHS AFTER EXPOSURE | 6 MONTHS AFTER EXPOSURE |
|---|---|---|---|---|---|
| HIV antibody testing | E, S† | E | E | E | |
| Complete blood count with differential | E | E | |||
| Serum liver enzymes | E | E | |||
| Blood urea nitrogen/creatinine | E | E | |||
| Sexually transmitted diseases screen (gonorrhea, chlamydia, syphilis) | E, S | E‡ | E‡ | ||
| Hepatitis B serology | E, S | E‡ | E‡ | ||
| Hepatitis C serology | E, S | E | E | ||
| Pregnancy test (for women of reproductive age) | E | E‡ | E‡ | ||
| HIV viral load | S | E§ | E§ | E§ | |
| HIV resistance testing | S | E§ | E§ | E§ | |
| CD4+T lymphocyte count | S | E§ | E§ | E§ | |
| PEP, postexposure prophylaxis; E, exposed patient; S, source. | |||||
| *Other specific tests might be indicated dependent on the antiretrovirals prescribed. Literature pertaining to individual agents should be consulted. | |||||
| †HIV antibody testing of the source patient is indicated for sources of unknown serostatus. | |||||
| ‡Additional testing for pregnancy, sexually transmitted diseases, and hepatitis B should be performed as clinically indicated. | |||||
| §If determined to be HIV infected on follow-up testing; perform as clinically indicated once diagnosed. | |||||
Conclusion
When there is uncertainty whether PEP is recommended, start patients on a PEP regimen while the situation is sorted out. Fortunately, joint patient-physician decision making can be assisted by the physician consultation resources mentioned previously. Keep in mind that, depending on the circumstances of the exposure, HIV transmission is only one concern among others, including infectious diseases, pregnancy, and emotional/psychological aspects resulting from the incident.
CORRESPONDENCE
Doug Campos-Outcalt, MD,MPA, 4001 North Third Street #415, Phoenix, AZ 85012. E-mail: dougco@u.arizona.edu
REFERENCE
1. Centers for Disease Control and Prevention (CDC). Trends in HIV/AIDS Diagnoses—33 States, 2001-2004. MMWR Morb Mortal Wkly Rep 2005;54:1149-1153.Available at: www.cdc.gov/mmwr/preview/mmwrhtml/mm5445a1.htm. Accessed on June 8, 2006.
2. Campos-Outcalt D. HIV prevention enters a new era. J Fam Pract 2004;53:563-566.
3. CDC. Antiretroviral postexposure prophylaxis after sexual, injection-drug use, or other nonoccupational exposures to HIV in the United States. MMWR Recomm Rep 2005;54(RR-2).-Available at: www.cdc.gov/mmwr/preview/mmwrhtml/rr5409a1.htm. Accessed on June 8, 2006.
4. CDC. Updated U.S. public health service guidelines for the management of occupational exposures to HIV and recommendations for postexposure prophylaxis. MMWR Recomm Rep 2005;54(RR-9).-Available at: www.cdc.gov/mmwr/preview/mmwrhtml/rr5409a1.htm. Accessed on June 8, 2006.
5. US Department of Health and Human Services. Guideline for the Use of Antiretroviral Agents in HIV-Infected Adults and Adolescents. October 29, 2004 revision. Available at: aidsinfo.nih.gov/guidelines.default_db2,asp?id+50. Accessed on June 8, 2006.
One of your office personnel receives a superficial stick from a needle while putting it into a sharps disposal container. Is postexposure prophylaxis (PEP) for HIV warranted?
Another health care worker receives a major blood splash into her eye after dropping a blood tube taken from a source of unknown HIV status. Is PEP called for in this instance?
A child who was rifling through a trash bin accidentally poked himself with an improperly disposed hypodermic needle. Should he be given PEP?
In most cases, HIV PEP is given only to healthcare workers if the settings make exposure to HIV-infected persons likely. Otherwise, it is usually deemed unnecessary. However, a decision for or against PEP is complicated.
Occupational and nonoccupational exposure to HIV can produce fear, anxiety, and stress. Information on the exposure risk is frequently incomplete, the risk of infection is usually low, the degree of protection offered by PEP is not fully defined, and the potential for side effects from the medications is significant.
This article distills the Centers for Disease Control and Prevention’s most recent guidance on HIV PEP.
HIV on the rise again
Antiretroviral therapy has markedly reduced mortality from HIV/AIDS, but the incidence of new cases, after declining in the 1990s, has gradually increased since 2000.1 As described in a previous article in the Journal of Family Practice,2 efforts to control HIV now focus on increased testing of those persons at risk, behavior modification to reduce the chances of infected persons exposing others, and treating HIV-positive pregnant women and providing postnatal prophylaxis to their newly born infants.
Exposure to HIV can occur occupationally, during a sexual assault, or from the failure of barrier protection during sex. Though these types of exposure are not major contributors to HIV incidence, and postexposure prophylaxis is not expected to play a major role in reducing the incidence of disease, it is available to persons potentially exposed to HIV, and it is beneficial to know when it is and is not indicated. Evidence for possible effectiveness of PEP comes from studies of postnatal prophylaxis, animal studies, case control studies and case reports.3
The Centers for Disease Control and Prevention (CDC) has developed 2 sets of recommendations for PEP that take into consideration the type and severity of the exposure and characteristics of the source of the exposure (TABLE 1).3,4
TABLE 1
Recommended HIV postexposure prophylaxis for percutanous injuries and membrane/nonintact skin exposures
| For percutaneous injuries | |||||
| EXPOSURE TYPE | INFECTION STATUS OF SOURCE | ||||
| HIV-POSITIVE, CLASS 1* | HIV-POSITIVE, CLASS 2* | SOURCE OF UNKNOWN HIV STATUS† | UNKNOWN SOURCE‡ | HIV-NEGATIVE | |
| Less severe (eg, solid needle or supercficial injury) | Recommend basic 2-drug PEP | Recommend expanded ≥3-drug PEP | Generally, no PEP warranted however, consider basic 2-drug PEP¶ for source with HIV risk factors** | Generally, no PEP warranted; however, consider basic 2-drug PEP¶ in settings in which exposure to HIV-infected persons is likely | No PEP warranted |
| More severe (large-bore hollow needle, deep puncture wound, blood on device, needle used in artery/vein) | Recommend expanded ≥3-drug PEP | Recommend expanded ≥3-drug PEP | Generally, no PEP warranted; however, consider basic 2-drug¶ for source with HIV risk factors** | Generally, no PEP warranted; however, consider basic 2-drug PEP¶ in settings in which exposure to HIV infected persons is likely | No PEP warranted |
| For mucous membrane and nonintact skin exposures†† | |||||
| Small volume (eg, a few drops) | Consider basic 2-drug PEP¶ | Recommend basic 2-drug PEP | Generally, no PEP warranted** | Generally, no PEP warranted | No PEP warranted |
| Large volume (eg, a major blood splash) | Recommend basic 2-drug PEP | Recommend expended ≥3-drug PEP | Generally, no PEP warranted; however, consider basic 2-drug PEP¶ for source with HIV risk factors** | Generally, no PEP warranted; however, consider basic 2-drug PEP¶ in settings in which exposure to HIV-infected persons is likely | No PEP warranted |
| *HIV-positive, class 1—asymptomatic HIV infection or known low viral load (eg, <1500 ribonucleic acid copies/mL). HIV-positive, class 2—symptomatic HIV infection, AIDS, acute seroconversion, or known high viral load. If drug resistance is a concern, obtain expert consultation. Initiation of PEP should not be delayed pending expert consultation, and, because expert consultation alone cannot substitute for face-to-face counseling, resources should be available to provide immediate evaluation and follow-up care for all exposures. | |||||
| †For example, deceased source person with no samples available for HIV testing. | |||||
| ‡For example, a needle from a sharps container or splash from inappropriately disposed blood. | |||||
| ¶The recommendation “consider PEP” indicates that PEP is optional; a decision to initiate PEP should be based on a discussion between the exposed person and the treating clinician regarding the risks versus benefits of PEP. | |||||
| **If PEP is offered and administered and the source is later determined to be HIV-negative, PEP should be discontinued. | |||||
| ††For skin exposures, follow-up is indicated only if evidence exists of compromised skin integrity (eg, dermatitis, abrasion, or open wound). | |||||
| Source: Centers for Disease Control and Prevention 2005.4 | |||||
Occupational exposures to HIV
Occupational exposure to HIV can result from a needlestick injury, cut with a sharp object, or contact with potentially infectious body fluids to mucous membranes or skin that is not intact (chapped, cut, abraded, inflamed). Body fluids that are considered potentially infectious are listed in TABLE 2, along with fluids not considered to be infectious.
The risk of contracting HIV from an occupational exposure is determined by several factors, but is generally low. The risk of infection after a needle-stick injury with exposure to infected blood is estimated at 0.3%; after a mucous membrane exposure, 0.09%. The risk after exposure to nonintact skin is probably even lower. Risk increases with the quantity of blood exposed to, a needle-stick injury directly into a vein or artery, and deep injuries.
TABLE 2
Which body fluids are infectious?
| BODY FLUIDS POTENTIALLY INFECTIOUS FOR HIV | |
|
|
| BODY FLUIDS NOT CONSIDERED TO BE INFECTIOUS UNLESS THEY ARE VISIBLY BLOODY | |
|
|
Who should and should not receive PEP
TABLE 1 details recommended treatment responses to specific types of exposure (eg, puncture wound) and the status of the exposure source.4 In situations unlikely to result in disease transmission (superficial injury and source patient with unknown HIV status), no PEP is generally warranted due to the low risk of infection and potential toxicity of antiretrovirals.
Treatment particulars
Start postexposure prophylaxis, when indicated, as soon as possible following exposure and continue it for 4 weeks. Obtain baseline test results for HIV at the time of exposure and periodically for 6 months. The CDC recommends testing at 6 weeks, 12 weeks, and 6 months, whether or not PEP is provided.
Testing and monitoring for hepatitis B and C may also be indicated.
Advise patients on PEP to use precautions in avoiding the possibility of secondary transmission, especially in the first 3 months following exposure. Monitor for drug toxicity every week or 2 while giving PEP. Because of the complexity of potential PEP regimens and the risk for drug toxicity, you may want to take advantage of several national sources of consultation, such as the PEPline (www.ucsf.edu/hivcntr/Hotlines/PEPline or 888-448-4911) or the HIV/AIDS Treatment Information Service (aidsinfo.nih.gov)—especially with questions about potential drug resistance or if the exposed person is pregnant.
When risk is real but low, 2-drug PEP is recommended (TABLE 1), usually 2 nucleoside reverse transcriptase inhibitors (NRTI) or 1 NRTI and 1 nucleotide reverse transcriptase inhibitor (NtRTI).
For those at higher risk, 3 or more antiviral regimens are recommended, achieved by adding a protease inhibitor to one of the recommended 2-drug regimens. The potential antiviral combinations in the basic 2-drug and expanded PEP regimens, along with potential side effects and toxicities of antiviral medications are described in TABLE W1, available online at www.jfponline.com.4
Nonoccupational exposures rarely require PEP
There are many unresolved questions regarding PEP for nonoccupational exposures. The lack of definitive evidence of its effectiveness, its unknown influence on risk-taking behavior, and the potential to aggravate viral resistance have led CDC to recommend that PEP be used only infrequently and not continuously for those whose behavior results in frequent exposures.3 Those who continue to participate in high risk activities should be referred for risk-reduction behavioral counseling.
The risk of HIV transmission varies by route and source of exposure (TABLE 3). The CDC has developed an algorithm based on these variables (FIGURE) to help you decide whether to initiate PEP. Two situations that cause concerns but pose little known risk of infection are bites and needlestick injuries from discarded needles; PEP is rarely indicated for either.
As with occupational exposure PEP, those receiving nonoccupational PEP should be evaluated at baseline for HIV infection. In addition, consider evaluating them for other STD’s and pregnancy.
As with occupational exposure, start nonoccupational PEP as soon as possible and continue it for 28 days. Nonoccupational PEP is not recommended if time after exposure is more than 72 hours. A 3-drug regimen is recommended by the CDC for nonoccupational exposures, even though evidence is lacking that it provides superior benefit over 2 drugs (see TABLE W1 at www.jfponline.com).
Follow-up recommendations for those provided nonoccupational PEP are the same as for occupational PEP, and testing for other STDs and hepatitis B and C is also recommended (TABLE 4).
FIGURE
Evaluation and treatment of possible nonoccupational HIV exposures
Source: Centers for Disease Control and Prevention 2005.3
TABLE 3
Estimated per-act risk for acquisition of HIV, by exposure route*
| EXPOSURE ROUTE | RISK PER 10,000 EXPOSURES TO AN INFECTED SOURCE |
|---|---|
| Blood transfusion | 9000 |
| Needle-sharing injection-drug use | 67 |
| Receptive anal intercourse | 50 |
| Percutaneous needle stick | 30 |
| Receptive penile-vaginal intercourse | 10 |
| Insertive anal intercourse | 6.5 |
| Insertive penile-vaginal intercourse | 5 |
| Receptive oral intercourse† | 1 |
| Insertive oral intercourse† | 0.5 |
| *Estimates of risk for transmission from sexual exposures assume no condom use. | |
| †Source refers to oral intercourse performed on a man. | |
| Source: Centers for Disease Control and Prevention 2005.3 | |
TABLE 4
Recommended laboratory evaluation for nonoccupational postexposure prophylaxis of HIV infection
| TEST | BASELINE | DURING PEP* | 4 TO 6 WEEKS AFTER EXPOSURE | 3 MONTHS AFTER EXPOSURE | 6 MONTHS AFTER EXPOSURE |
|---|---|---|---|---|---|
| HIV antibody testing | E, S† | E | E | E | |
| Complete blood count with differential | E | E | |||
| Serum liver enzymes | E | E | |||
| Blood urea nitrogen/creatinine | E | E | |||
| Sexually transmitted diseases screen (gonorrhea, chlamydia, syphilis) | E, S | E‡ | E‡ | ||
| Hepatitis B serology | E, S | E‡ | E‡ | ||
| Hepatitis C serology | E, S | E | E | ||
| Pregnancy test (for women of reproductive age) | E | E‡ | E‡ | ||
| HIV viral load | S | E§ | E§ | E§ | |
| HIV resistance testing | S | E§ | E§ | E§ | |
| CD4+T lymphocyte count | S | E§ | E§ | E§ | |
| PEP, postexposure prophylaxis; E, exposed patient; S, source. | |||||
| *Other specific tests might be indicated dependent on the antiretrovirals prescribed. Literature pertaining to individual agents should be consulted. | |||||
| †HIV antibody testing of the source patient is indicated for sources of unknown serostatus. | |||||
| ‡Additional testing for pregnancy, sexually transmitted diseases, and hepatitis B should be performed as clinically indicated. | |||||
| §If determined to be HIV infected on follow-up testing; perform as clinically indicated once diagnosed. | |||||
Conclusion
When there is uncertainty whether PEP is recommended, start patients on a PEP regimen while the situation is sorted out. Fortunately, joint patient-physician decision making can be assisted by the physician consultation resources mentioned previously. Keep in mind that, depending on the circumstances of the exposure, HIV transmission is only one concern among others, including infectious diseases, pregnancy, and emotional/psychological aspects resulting from the incident.
CORRESPONDENCE
Doug Campos-Outcalt, MD,MPA, 4001 North Third Street #415, Phoenix, AZ 85012. E-mail: dougco@u.arizona.edu
One of your office personnel receives a superficial stick from a needle while putting it into a sharps disposal container. Is postexposure prophylaxis (PEP) for HIV warranted?
Another health care worker receives a major blood splash into her eye after dropping a blood tube taken from a source of unknown HIV status. Is PEP called for in this instance?
A child who was rifling through a trash bin accidentally poked himself with an improperly disposed hypodermic needle. Should he be given PEP?
In most cases, HIV PEP is given only to healthcare workers if the settings make exposure to HIV-infected persons likely. Otherwise, it is usually deemed unnecessary. However, a decision for or against PEP is complicated.
Occupational and nonoccupational exposure to HIV can produce fear, anxiety, and stress. Information on the exposure risk is frequently incomplete, the risk of infection is usually low, the degree of protection offered by PEP is not fully defined, and the potential for side effects from the medications is significant.
This article distills the Centers for Disease Control and Prevention’s most recent guidance on HIV PEP.
HIV on the rise again
Antiretroviral therapy has markedly reduced mortality from HIV/AIDS, but the incidence of new cases, after declining in the 1990s, has gradually increased since 2000.1 As described in a previous article in the Journal of Family Practice,2 efforts to control HIV now focus on increased testing of those persons at risk, behavior modification to reduce the chances of infected persons exposing others, and treating HIV-positive pregnant women and providing postnatal prophylaxis to their newly born infants.
Exposure to HIV can occur occupationally, during a sexual assault, or from the failure of barrier protection during sex. Though these types of exposure are not major contributors to HIV incidence, and postexposure prophylaxis is not expected to play a major role in reducing the incidence of disease, it is available to persons potentially exposed to HIV, and it is beneficial to know when it is and is not indicated. Evidence for possible effectiveness of PEP comes from studies of postnatal prophylaxis, animal studies, case control studies and case reports.3
The Centers for Disease Control and Prevention (CDC) has developed 2 sets of recommendations for PEP that take into consideration the type and severity of the exposure and characteristics of the source of the exposure (TABLE 1).3,4
TABLE 1
Recommended HIV postexposure prophylaxis for percutanous injuries and membrane/nonintact skin exposures
| For percutaneous injuries | |||||
| EXPOSURE TYPE | INFECTION STATUS OF SOURCE | ||||
| HIV-POSITIVE, CLASS 1* | HIV-POSITIVE, CLASS 2* | SOURCE OF UNKNOWN HIV STATUS† | UNKNOWN SOURCE‡ | HIV-NEGATIVE | |
| Less severe (eg, solid needle or supercficial injury) | Recommend basic 2-drug PEP | Recommend expanded ≥3-drug PEP | Generally, no PEP warranted however, consider basic 2-drug PEP¶ for source with HIV risk factors** | Generally, no PEP warranted; however, consider basic 2-drug PEP¶ in settings in which exposure to HIV-infected persons is likely | No PEP warranted |
| More severe (large-bore hollow needle, deep puncture wound, blood on device, needle used in artery/vein) | Recommend expanded ≥3-drug PEP | Recommend expanded ≥3-drug PEP | Generally, no PEP warranted; however, consider basic 2-drug¶ for source with HIV risk factors** | Generally, no PEP warranted; however, consider basic 2-drug PEP¶ in settings in which exposure to HIV infected persons is likely | No PEP warranted |
| For mucous membrane and nonintact skin exposures†† | |||||
| Small volume (eg, a few drops) | Consider basic 2-drug PEP¶ | Recommend basic 2-drug PEP | Generally, no PEP warranted** | Generally, no PEP warranted | No PEP warranted |
| Large volume (eg, a major blood splash) | Recommend basic 2-drug PEP | Recommend expended ≥3-drug PEP | Generally, no PEP warranted; however, consider basic 2-drug PEP¶ for source with HIV risk factors** | Generally, no PEP warranted; however, consider basic 2-drug PEP¶ in settings in which exposure to HIV-infected persons is likely | No PEP warranted |
| *HIV-positive, class 1—asymptomatic HIV infection or known low viral load (eg, <1500 ribonucleic acid copies/mL). HIV-positive, class 2—symptomatic HIV infection, AIDS, acute seroconversion, or known high viral load. If drug resistance is a concern, obtain expert consultation. Initiation of PEP should not be delayed pending expert consultation, and, because expert consultation alone cannot substitute for face-to-face counseling, resources should be available to provide immediate evaluation and follow-up care for all exposures. | |||||
| †For example, deceased source person with no samples available for HIV testing. | |||||
| ‡For example, a needle from a sharps container or splash from inappropriately disposed blood. | |||||
| ¶The recommendation “consider PEP” indicates that PEP is optional; a decision to initiate PEP should be based on a discussion between the exposed person and the treating clinician regarding the risks versus benefits of PEP. | |||||
| **If PEP is offered and administered and the source is later determined to be HIV-negative, PEP should be discontinued. | |||||
| ††For skin exposures, follow-up is indicated only if evidence exists of compromised skin integrity (eg, dermatitis, abrasion, or open wound). | |||||
| Source: Centers for Disease Control and Prevention 2005.4 | |||||
Occupational exposures to HIV
Occupational exposure to HIV can result from a needlestick injury, cut with a sharp object, or contact with potentially infectious body fluids to mucous membranes or skin that is not intact (chapped, cut, abraded, inflamed). Body fluids that are considered potentially infectious are listed in TABLE 2, along with fluids not considered to be infectious.
The risk of contracting HIV from an occupational exposure is determined by several factors, but is generally low. The risk of infection after a needle-stick injury with exposure to infected blood is estimated at 0.3%; after a mucous membrane exposure, 0.09%. The risk after exposure to nonintact skin is probably even lower. Risk increases with the quantity of blood exposed to, a needle-stick injury directly into a vein or artery, and deep injuries.
TABLE 2
Which body fluids are infectious?
| BODY FLUIDS POTENTIALLY INFECTIOUS FOR HIV | |
|
|
| BODY FLUIDS NOT CONSIDERED TO BE INFECTIOUS UNLESS THEY ARE VISIBLY BLOODY | |
|
|
Who should and should not receive PEP
TABLE 1 details recommended treatment responses to specific types of exposure (eg, puncture wound) and the status of the exposure source.4 In situations unlikely to result in disease transmission (superficial injury and source patient with unknown HIV status), no PEP is generally warranted due to the low risk of infection and potential toxicity of antiretrovirals.
Treatment particulars
Start postexposure prophylaxis, when indicated, as soon as possible following exposure and continue it for 4 weeks. Obtain baseline test results for HIV at the time of exposure and periodically for 6 months. The CDC recommends testing at 6 weeks, 12 weeks, and 6 months, whether or not PEP is provided.
Testing and monitoring for hepatitis B and C may also be indicated.
Advise patients on PEP to use precautions in avoiding the possibility of secondary transmission, especially in the first 3 months following exposure. Monitor for drug toxicity every week or 2 while giving PEP. Because of the complexity of potential PEP regimens and the risk for drug toxicity, you may want to take advantage of several national sources of consultation, such as the PEPline (www.ucsf.edu/hivcntr/Hotlines/PEPline or 888-448-4911) or the HIV/AIDS Treatment Information Service (aidsinfo.nih.gov)—especially with questions about potential drug resistance or if the exposed person is pregnant.
When risk is real but low, 2-drug PEP is recommended (TABLE 1), usually 2 nucleoside reverse transcriptase inhibitors (NRTI) or 1 NRTI and 1 nucleotide reverse transcriptase inhibitor (NtRTI).
For those at higher risk, 3 or more antiviral regimens are recommended, achieved by adding a protease inhibitor to one of the recommended 2-drug regimens. The potential antiviral combinations in the basic 2-drug and expanded PEP regimens, along with potential side effects and toxicities of antiviral medications are described in TABLE W1, available online at www.jfponline.com.4
Nonoccupational exposures rarely require PEP
There are many unresolved questions regarding PEP for nonoccupational exposures. The lack of definitive evidence of its effectiveness, its unknown influence on risk-taking behavior, and the potential to aggravate viral resistance have led CDC to recommend that PEP be used only infrequently and not continuously for those whose behavior results in frequent exposures.3 Those who continue to participate in high risk activities should be referred for risk-reduction behavioral counseling.
The risk of HIV transmission varies by route and source of exposure (TABLE 3). The CDC has developed an algorithm based on these variables (FIGURE) to help you decide whether to initiate PEP. Two situations that cause concerns but pose little known risk of infection are bites and needlestick injuries from discarded needles; PEP is rarely indicated for either.
As with occupational exposure PEP, those receiving nonoccupational PEP should be evaluated at baseline for HIV infection. In addition, consider evaluating them for other STD’s and pregnancy.
As with occupational exposure, start nonoccupational PEP as soon as possible and continue it for 28 days. Nonoccupational PEP is not recommended if time after exposure is more than 72 hours. A 3-drug regimen is recommended by the CDC for nonoccupational exposures, even though evidence is lacking that it provides superior benefit over 2 drugs (see TABLE W1 at www.jfponline.com).
Follow-up recommendations for those provided nonoccupational PEP are the same as for occupational PEP, and testing for other STDs and hepatitis B and C is also recommended (TABLE 4).
FIGURE
Evaluation and treatment of possible nonoccupational HIV exposures
Source: Centers for Disease Control and Prevention 2005.3
TABLE 3
Estimated per-act risk for acquisition of HIV, by exposure route*
| EXPOSURE ROUTE | RISK PER 10,000 EXPOSURES TO AN INFECTED SOURCE |
|---|---|
| Blood transfusion | 9000 |
| Needle-sharing injection-drug use | 67 |
| Receptive anal intercourse | 50 |
| Percutaneous needle stick | 30 |
| Receptive penile-vaginal intercourse | 10 |
| Insertive anal intercourse | 6.5 |
| Insertive penile-vaginal intercourse | 5 |
| Receptive oral intercourse† | 1 |
| Insertive oral intercourse† | 0.5 |
| *Estimates of risk for transmission from sexual exposures assume no condom use. | |
| †Source refers to oral intercourse performed on a man. | |
| Source: Centers for Disease Control and Prevention 2005.3 | |
TABLE 4
Recommended laboratory evaluation for nonoccupational postexposure prophylaxis of HIV infection
| TEST | BASELINE | DURING PEP* | 4 TO 6 WEEKS AFTER EXPOSURE | 3 MONTHS AFTER EXPOSURE | 6 MONTHS AFTER EXPOSURE |
|---|---|---|---|---|---|
| HIV antibody testing | E, S† | E | E | E | |
| Complete blood count with differential | E | E | |||
| Serum liver enzymes | E | E | |||
| Blood urea nitrogen/creatinine | E | E | |||
| Sexually transmitted diseases screen (gonorrhea, chlamydia, syphilis) | E, S | E‡ | E‡ | ||
| Hepatitis B serology | E, S | E‡ | E‡ | ||
| Hepatitis C serology | E, S | E | E | ||
| Pregnancy test (for women of reproductive age) | E | E‡ | E‡ | ||
| HIV viral load | S | E§ | E§ | E§ | |
| HIV resistance testing | S | E§ | E§ | E§ | |
| CD4+T lymphocyte count | S | E§ | E§ | E§ | |
| PEP, postexposure prophylaxis; E, exposed patient; S, source. | |||||
| *Other specific tests might be indicated dependent on the antiretrovirals prescribed. Literature pertaining to individual agents should be consulted. | |||||
| †HIV antibody testing of the source patient is indicated for sources of unknown serostatus. | |||||
| ‡Additional testing for pregnancy, sexually transmitted diseases, and hepatitis B should be performed as clinically indicated. | |||||
| §If determined to be HIV infected on follow-up testing; perform as clinically indicated once diagnosed. | |||||
Conclusion
When there is uncertainty whether PEP is recommended, start patients on a PEP regimen while the situation is sorted out. Fortunately, joint patient-physician decision making can be assisted by the physician consultation resources mentioned previously. Keep in mind that, depending on the circumstances of the exposure, HIV transmission is only one concern among others, including infectious diseases, pregnancy, and emotional/psychological aspects resulting from the incident.
CORRESPONDENCE
Doug Campos-Outcalt, MD,MPA, 4001 North Third Street #415, Phoenix, AZ 85012. E-mail: dougco@u.arizona.edu
REFERENCE
1. Centers for Disease Control and Prevention (CDC). Trends in HIV/AIDS Diagnoses—33 States, 2001-2004. MMWR Morb Mortal Wkly Rep 2005;54:1149-1153.Available at: www.cdc.gov/mmwr/preview/mmwrhtml/mm5445a1.htm. Accessed on June 8, 2006.
2. Campos-Outcalt D. HIV prevention enters a new era. J Fam Pract 2004;53:563-566.
3. CDC. Antiretroviral postexposure prophylaxis after sexual, injection-drug use, or other nonoccupational exposures to HIV in the United States. MMWR Recomm Rep 2005;54(RR-2).-Available at: www.cdc.gov/mmwr/preview/mmwrhtml/rr5409a1.htm. Accessed on June 8, 2006.
4. CDC. Updated U.S. public health service guidelines for the management of occupational exposures to HIV and recommendations for postexposure prophylaxis. MMWR Recomm Rep 2005;54(RR-9).-Available at: www.cdc.gov/mmwr/preview/mmwrhtml/rr5409a1.htm. Accessed on June 8, 2006.
5. US Department of Health and Human Services. Guideline for the Use of Antiretroviral Agents in HIV-Infected Adults and Adolescents. October 29, 2004 revision. Available at: aidsinfo.nih.gov/guidelines.default_db2,asp?id+50. Accessed on June 8, 2006.
REFERENCE
1. Centers for Disease Control and Prevention (CDC). Trends in HIV/AIDS Diagnoses—33 States, 2001-2004. MMWR Morb Mortal Wkly Rep 2005;54:1149-1153.Available at: www.cdc.gov/mmwr/preview/mmwrhtml/mm5445a1.htm. Accessed on June 8, 2006.
2. Campos-Outcalt D. HIV prevention enters a new era. J Fam Pract 2004;53:563-566.
3. CDC. Antiretroviral postexposure prophylaxis after sexual, injection-drug use, or other nonoccupational exposures to HIV in the United States. MMWR Recomm Rep 2005;54(RR-2).-Available at: www.cdc.gov/mmwr/preview/mmwrhtml/rr5409a1.htm. Accessed on June 8, 2006.
4. CDC. Updated U.S. public health service guidelines for the management of occupational exposures to HIV and recommendations for postexposure prophylaxis. MMWR Recomm Rep 2005;54(RR-9).-Available at: www.cdc.gov/mmwr/preview/mmwrhtml/rr5409a1.htm. Accessed on June 8, 2006.
5. US Department of Health and Human Services. Guideline for the Use of Antiretroviral Agents in HIV-Infected Adults and Adolescents. October 29, 2004 revision. Available at: aidsinfo.nih.gov/guidelines.default_db2,asp?id+50. Accessed on June 8, 2006.