User login
Keeping up with the ever-changing immunization schedules recommended by the Centers for Disease Control and Prevention (CDC)’s Advisory Committee on Immunization Practices (ACIP) can be difficult. The most recent changes are the interim recommendations from the February 2011 ACIP meeting pertaining to tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis (Tdap) vaccine immunization and postexposure prophylaxis (PEP) for health care personnel. Updated schedules for routine immunization of children and adults that incorporate additions and changes made in the preceding year were published by the CDC in February.1,2
ACIP widens the scope of pertussis prevention
The past decade has seen an increase in pertussis cases, including an increase in the number of cases among infants and adolescents (FIGURE). In 2010, California reported 8383 cases, including 10 infant deaths. This was the highest number and rate of cases reported in more than 50 years.3 Other states have also experienced recent increases.
This evolving epidemiology of pertussis has prompted ACIP to recommend a routine single Tdap dose for adolescents between the ages of 11 and 18 years who have completed the recommended DTP/DTaP (diphtheria and tetanus toxoids and pertussis/diphtheria and tetanus toxoids and acellular pertussis) vaccination series and for adults ages 19 to 64 years. ACIP also recommends a single dose for children ages 7 to 10 if they are not fully vaccinated against pertussis and for adults 65 and older who have not previously received Tdap and who are in close contact with infants. The last 2 are off-label recommendations. ACIP has also eliminated any recommended interval between the time of vaccination with tetanus or diphtheriatoxoid (Td) containing vaccine and the administration of Tdap.4
FIGURE
Reported pertussis incidence by age group, 1990-2009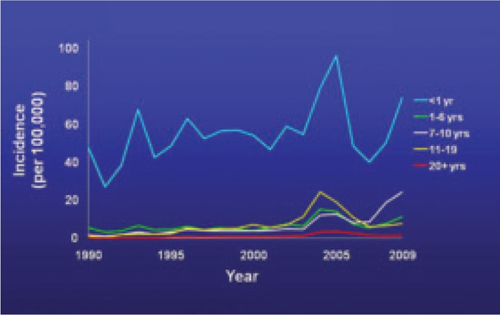
Source: Centers for Disease Control and Prevention. Pertussis (whooping cough): surveillance and reporting. Available at: www.cdc.gov/pertussis/surv-reporting.html. Accessed March 21, 2011.
2 new recommendations for clinician postexposure prophylaxis
Interim recommendations from the most recent ACIP meeting in February 20115 re-emphasize that health care personnel should receive Tdap and recommend that health care facilities take steps to increase adherence, including providing the vaccine at no cost.5
Since health care personnel are at increased risk of exposure to pertussis, ACIP also made 2 recommendations for PEP.
- All health care personnel (vaccinated or not) in close contact with a pertussis patient (as defined in TABLE 1) who are likely to expose patients at high risk for complications from pertussis (infants <1 year of age and those with certain immunodeficiency conditions, chronic lung disease, respiratory insufficiency, or cystic fibrosis) should receive PEP.
- Exposed personnel who do not work with high-risk patients should receive PEP or be monitored daily for 21 days, treated at first signs of infection, and excluded from patient contact for 5 days if symptoms develop. The antimicrobials and doses for treatment and prevention of pertussis have been published in the Morbidity and Mortality Weekly Report.6 Options for PEP include azithromycin, clarithromycin, erythromycin, and trimethoprim-sulfamethoxazole.6
TABLE 1
Definition of close contact with a pertussis patient
|
| Source: Centers for Disease Control and Prevention. MMWR Morb Mortal Wkly Rep. 2005.6 |
Coming soon: Complete vaccine recommendations for health care workers
Recent experience with pertussis (and influenza) has highlighted the need for health care personnel to be vaccinated against infectious diseases to protect themselves, their patients, and their families. To that end, ACIP plans to publish a compendium later this year that brings together all recommendations regarding immunizations for health care personnel. When it becomes available, family physicians will be able to refer to this document to ensure that they and their staff are immunized in line with CDC recommendations.
The latest on influenza vaccine, PCV13, MCV4, hepatitis B, and HPV
The most notable additions to the routine schedules ACIP announced during the past year are universal, yearly influenza immunization from the age of 6 months on and the replacement of the 7-valent pneumococcal conjugate vaccine (PCV7) with a 13-valent product (PCV13) for infants and children. Details of these recommendations, including how to transition from PCV7 to PCV13, were published late last year by the CDC and described in another Practice Alert.7-9
In addition, changes were made in the schedules for meningococcal conjugate vaccine. A 2-dose primary series, instead of a single dose, of MCV4 is now recommended for those with compromised immunity. A booster of MCV4 is now recommended at age 16 for those vaccinated at 11 or 12 years, and at age 16 to 18 for those vaccinated at 13 to 15 years.10 The MCV4 recommendations are summarized in TABLE 2.
More schedule details in the footnotes. The new schedules contain a number of clarifications in the footnotes that:1,11
- explain the spacing of the 3-dose primary series for hepatitis B vaccine (HepB) for infants if they do not receive a dose immediately after birth
- clarify the circumstances in which children younger than age 9 need 2 doses of influenza vaccine
- describe the availability of both a quadrivalent human papillomavirus vaccine (HPV4) and a bivalent vaccine (HPV2) to prevent precancerous cervical lesions and cancer
- list the option for using HPV4 for males for the prevention of genital warts.
TABLE 2
Meningococcal conjugate vaccine recommendations by risk group, ACIP 2010
| Risk group | Primary series | Booster dose |
|---|---|---|
| Individuals ages 11-18 years | 1 dose, preferably at age 11 or 12 years |
|
| HIV-infected individuals ages 11-18 years | 2 doses, 2 months apart |
|
| Individuals ages 2-55 years with persistent complement component deficiency such as C5-C9, properdin, or factor D, or functional or anatomic asplenia | 2 doses, 2 months apart |
|
| Individuals ages 2-55 years with prolonged increased risk of exposure, such as microbiologists routinely working with Neisseria meningitidis and travelers to, or residents of, countries where meningococcal disease is hyperendemic or epidemic | 1 dose |
|
| Source: Centers for Disease Control and Prevention. MMWR Morb Mortal Wkly Rep. 2011.10 | ||
1. Centers for Disease Control and Prevention. Recommended immunization schedules for persons aged 0 through 18 years—United States, 2011. MMWR Morb Mortal Wkly Rep QuickGuide. 2011;60(5):1-4.
2. Centers for Disease Control and Prevention. Recommended adult immunization schedule-United States, 2011. MMWR Morb Mortal Wkly Rep. 2011;60(4):1-4.
3. Centers for Disease Control and Prevention. Pertussis (whooping cough): outbreaks. Available at: http://www.cdc.gov/pertussis/outbreaks.html. Accessed March 19, 2011.
4. Centers for Disease Control and Prevention. Updated recommendations for use of tetanus toxoid, reduced diphtheria toxoid and acellular pertussis (Tdap) vaccine from the Advisory Committee on Immunization Practices, 2010. MMWR Morb Mortal Wkly Rep. 2011;60(1):13-15.
5. Centers for Disease Control and Prevention. ACIP presentation slides: February 2011 meeting. Available at www.cdc.gov/vaccines/recs/acip/slides-feb11.htm#pertussis. Accessed March 19, 2011.
6. Centers for Disease Control and Prevention. Recommended antimicrobial agents for treatment and postexposure prophylaxis of pertussis: 2005 CDC guidelines. MMWR Recomm Rep. 2005;54(RR-14):1-16.
7. Prevention and control of influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2010. MMWR Recomm Rep. 2010;59(RR-8):1-62.
8. Centers for Disease Control and Prevention. Prevention of pneumococcal disease among infants and children—use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine—recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2010;59(RR-11):1-18.
9. Campos-Outcalt D. Your guide to the new pneumococcal vaccine for children. J Fam Pract. 2010;59:394-398.
10. Centers for Disease Control and Prevention. Updated recommendations for use of meningococcal conjugate vaccines—Advisory Committee on Immunization Practices (ACIP), 2010. MMWR Morb Mortal Wkly Rep. 2011;60:72-76.
11. Centers for Disease Control and Prevention. FDA licensure of bivalent human papillomavirus vaccine (HPV2, Cervarix) for use in females and updated HPV vaccination recommendations from the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep. 2010;59:626-629.
Keeping up with the ever-changing immunization schedules recommended by the Centers for Disease Control and Prevention (CDC)’s Advisory Committee on Immunization Practices (ACIP) can be difficult. The most recent changes are the interim recommendations from the February 2011 ACIP meeting pertaining to tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis (Tdap) vaccine immunization and postexposure prophylaxis (PEP) for health care personnel. Updated schedules for routine immunization of children and adults that incorporate additions and changes made in the preceding year were published by the CDC in February.1,2
ACIP widens the scope of pertussis prevention
The past decade has seen an increase in pertussis cases, including an increase in the number of cases among infants and adolescents (FIGURE). In 2010, California reported 8383 cases, including 10 infant deaths. This was the highest number and rate of cases reported in more than 50 years.3 Other states have also experienced recent increases.
This evolving epidemiology of pertussis has prompted ACIP to recommend a routine single Tdap dose for adolescents between the ages of 11 and 18 years who have completed the recommended DTP/DTaP (diphtheria and tetanus toxoids and pertussis/diphtheria and tetanus toxoids and acellular pertussis) vaccination series and for adults ages 19 to 64 years. ACIP also recommends a single dose for children ages 7 to 10 if they are not fully vaccinated against pertussis and for adults 65 and older who have not previously received Tdap and who are in close contact with infants. The last 2 are off-label recommendations. ACIP has also eliminated any recommended interval between the time of vaccination with tetanus or diphtheriatoxoid (Td) containing vaccine and the administration of Tdap.4
FIGURE
Reported pertussis incidence by age group, 1990-2009
Source: Centers for Disease Control and Prevention. Pertussis (whooping cough): surveillance and reporting. Available at: www.cdc.gov/pertussis/surv-reporting.html. Accessed March 21, 2011.
2 new recommendations for clinician postexposure prophylaxis
Interim recommendations from the most recent ACIP meeting in February 20115 re-emphasize that health care personnel should receive Tdap and recommend that health care facilities take steps to increase adherence, including providing the vaccine at no cost.5
Since health care personnel are at increased risk of exposure to pertussis, ACIP also made 2 recommendations for PEP.
- All health care personnel (vaccinated or not) in close contact with a pertussis patient (as defined in TABLE 1) who are likely to expose patients at high risk for complications from pertussis (infants <1 year of age and those with certain immunodeficiency conditions, chronic lung disease, respiratory insufficiency, or cystic fibrosis) should receive PEP.
- Exposed personnel who do not work with high-risk patients should receive PEP or be monitored daily for 21 days, treated at first signs of infection, and excluded from patient contact for 5 days if symptoms develop. The antimicrobials and doses for treatment and prevention of pertussis have been published in the Morbidity and Mortality Weekly Report.6 Options for PEP include azithromycin, clarithromycin, erythromycin, and trimethoprim-sulfamethoxazole.6
TABLE 1
Definition of close contact with a pertussis patient
|
| Source: Centers for Disease Control and Prevention. MMWR Morb Mortal Wkly Rep. 2005.6 |
Coming soon: Complete vaccine recommendations for health care workers
Recent experience with pertussis (and influenza) has highlighted the need for health care personnel to be vaccinated against infectious diseases to protect themselves, their patients, and their families. To that end, ACIP plans to publish a compendium later this year that brings together all recommendations regarding immunizations for health care personnel. When it becomes available, family physicians will be able to refer to this document to ensure that they and their staff are immunized in line with CDC recommendations.
The latest on influenza vaccine, PCV13, MCV4, hepatitis B, and HPV
The most notable additions to the routine schedules ACIP announced during the past year are universal, yearly influenza immunization from the age of 6 months on and the replacement of the 7-valent pneumococcal conjugate vaccine (PCV7) with a 13-valent product (PCV13) for infants and children. Details of these recommendations, including how to transition from PCV7 to PCV13, were published late last year by the CDC and described in another Practice Alert.7-9
In addition, changes were made in the schedules for meningococcal conjugate vaccine. A 2-dose primary series, instead of a single dose, of MCV4 is now recommended for those with compromised immunity. A booster of MCV4 is now recommended at age 16 for those vaccinated at 11 or 12 years, and at age 16 to 18 for those vaccinated at 13 to 15 years.10 The MCV4 recommendations are summarized in TABLE 2.
More schedule details in the footnotes. The new schedules contain a number of clarifications in the footnotes that:1,11
- explain the spacing of the 3-dose primary series for hepatitis B vaccine (HepB) for infants if they do not receive a dose immediately after birth
- clarify the circumstances in which children younger than age 9 need 2 doses of influenza vaccine
- describe the availability of both a quadrivalent human papillomavirus vaccine (HPV4) and a bivalent vaccine (HPV2) to prevent precancerous cervical lesions and cancer
- list the option for using HPV4 for males for the prevention of genital warts.
TABLE 2
Meningococcal conjugate vaccine recommendations by risk group, ACIP 2010
| Risk group | Primary series | Booster dose |
|---|---|---|
| Individuals ages 11-18 years | 1 dose, preferably at age 11 or 12 years |
|
| HIV-infected individuals ages 11-18 years | 2 doses, 2 months apart |
|
| Individuals ages 2-55 years with persistent complement component deficiency such as C5-C9, properdin, or factor D, or functional or anatomic asplenia | 2 doses, 2 months apart |
|
| Individuals ages 2-55 years with prolonged increased risk of exposure, such as microbiologists routinely working with Neisseria meningitidis and travelers to, or residents of, countries where meningococcal disease is hyperendemic or epidemic | 1 dose |
|
| Source: Centers for Disease Control and Prevention. MMWR Morb Mortal Wkly Rep. 2011.10 | ||
Keeping up with the ever-changing immunization schedules recommended by the Centers for Disease Control and Prevention (CDC)’s Advisory Committee on Immunization Practices (ACIP) can be difficult. The most recent changes are the interim recommendations from the February 2011 ACIP meeting pertaining to tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis (Tdap) vaccine immunization and postexposure prophylaxis (PEP) for health care personnel. Updated schedules for routine immunization of children and adults that incorporate additions and changes made in the preceding year were published by the CDC in February.1,2
ACIP widens the scope of pertussis prevention
The past decade has seen an increase in pertussis cases, including an increase in the number of cases among infants and adolescents (FIGURE). In 2010, California reported 8383 cases, including 10 infant deaths. This was the highest number and rate of cases reported in more than 50 years.3 Other states have also experienced recent increases.
This evolving epidemiology of pertussis has prompted ACIP to recommend a routine single Tdap dose for adolescents between the ages of 11 and 18 years who have completed the recommended DTP/DTaP (diphtheria and tetanus toxoids and pertussis/diphtheria and tetanus toxoids and acellular pertussis) vaccination series and for adults ages 19 to 64 years. ACIP also recommends a single dose for children ages 7 to 10 if they are not fully vaccinated against pertussis and for adults 65 and older who have not previously received Tdap and who are in close contact with infants. The last 2 are off-label recommendations. ACIP has also eliminated any recommended interval between the time of vaccination with tetanus or diphtheriatoxoid (Td) containing vaccine and the administration of Tdap.4
FIGURE
Reported pertussis incidence by age group, 1990-2009
Source: Centers for Disease Control and Prevention. Pertussis (whooping cough): surveillance and reporting. Available at: www.cdc.gov/pertussis/surv-reporting.html. Accessed March 21, 2011.
2 new recommendations for clinician postexposure prophylaxis
Interim recommendations from the most recent ACIP meeting in February 20115 re-emphasize that health care personnel should receive Tdap and recommend that health care facilities take steps to increase adherence, including providing the vaccine at no cost.5
Since health care personnel are at increased risk of exposure to pertussis, ACIP also made 2 recommendations for PEP.
- All health care personnel (vaccinated or not) in close contact with a pertussis patient (as defined in TABLE 1) who are likely to expose patients at high risk for complications from pertussis (infants <1 year of age and those with certain immunodeficiency conditions, chronic lung disease, respiratory insufficiency, or cystic fibrosis) should receive PEP.
- Exposed personnel who do not work with high-risk patients should receive PEP or be monitored daily for 21 days, treated at first signs of infection, and excluded from patient contact for 5 days if symptoms develop. The antimicrobials and doses for treatment and prevention of pertussis have been published in the Morbidity and Mortality Weekly Report.6 Options for PEP include azithromycin, clarithromycin, erythromycin, and trimethoprim-sulfamethoxazole.6
TABLE 1
Definition of close contact with a pertussis patient
|
| Source: Centers for Disease Control and Prevention. MMWR Morb Mortal Wkly Rep. 2005.6 |
Coming soon: Complete vaccine recommendations for health care workers
Recent experience with pertussis (and influenza) has highlighted the need for health care personnel to be vaccinated against infectious diseases to protect themselves, their patients, and their families. To that end, ACIP plans to publish a compendium later this year that brings together all recommendations regarding immunizations for health care personnel. When it becomes available, family physicians will be able to refer to this document to ensure that they and their staff are immunized in line with CDC recommendations.
The latest on influenza vaccine, PCV13, MCV4, hepatitis B, and HPV
The most notable additions to the routine schedules ACIP announced during the past year are universal, yearly influenza immunization from the age of 6 months on and the replacement of the 7-valent pneumococcal conjugate vaccine (PCV7) with a 13-valent product (PCV13) for infants and children. Details of these recommendations, including how to transition from PCV7 to PCV13, were published late last year by the CDC and described in another Practice Alert.7-9
In addition, changes were made in the schedules for meningococcal conjugate vaccine. A 2-dose primary series, instead of a single dose, of MCV4 is now recommended for those with compromised immunity. A booster of MCV4 is now recommended at age 16 for those vaccinated at 11 or 12 years, and at age 16 to 18 for those vaccinated at 13 to 15 years.10 The MCV4 recommendations are summarized in TABLE 2.
More schedule details in the footnotes. The new schedules contain a number of clarifications in the footnotes that:1,11
- explain the spacing of the 3-dose primary series for hepatitis B vaccine (HepB) for infants if they do not receive a dose immediately after birth
- clarify the circumstances in which children younger than age 9 need 2 doses of influenza vaccine
- describe the availability of both a quadrivalent human papillomavirus vaccine (HPV4) and a bivalent vaccine (HPV2) to prevent precancerous cervical lesions and cancer
- list the option for using HPV4 for males for the prevention of genital warts.
TABLE 2
Meningococcal conjugate vaccine recommendations by risk group, ACIP 2010
| Risk group | Primary series | Booster dose |
|---|---|---|
| Individuals ages 11-18 years | 1 dose, preferably at age 11 or 12 years |
|
| HIV-infected individuals ages 11-18 years | 2 doses, 2 months apart |
|
| Individuals ages 2-55 years with persistent complement component deficiency such as C5-C9, properdin, or factor D, or functional or anatomic asplenia | 2 doses, 2 months apart |
|
| Individuals ages 2-55 years with prolonged increased risk of exposure, such as microbiologists routinely working with Neisseria meningitidis and travelers to, or residents of, countries where meningococcal disease is hyperendemic or epidemic | 1 dose |
|
| Source: Centers for Disease Control and Prevention. MMWR Morb Mortal Wkly Rep. 2011.10 | ||
1. Centers for Disease Control and Prevention. Recommended immunization schedules for persons aged 0 through 18 years—United States, 2011. MMWR Morb Mortal Wkly Rep QuickGuide. 2011;60(5):1-4.
2. Centers for Disease Control and Prevention. Recommended adult immunization schedule-United States, 2011. MMWR Morb Mortal Wkly Rep. 2011;60(4):1-4.
3. Centers for Disease Control and Prevention. Pertussis (whooping cough): outbreaks. Available at: http://www.cdc.gov/pertussis/outbreaks.html. Accessed March 19, 2011.
4. Centers for Disease Control and Prevention. Updated recommendations for use of tetanus toxoid, reduced diphtheria toxoid and acellular pertussis (Tdap) vaccine from the Advisory Committee on Immunization Practices, 2010. MMWR Morb Mortal Wkly Rep. 2011;60(1):13-15.
5. Centers for Disease Control and Prevention. ACIP presentation slides: February 2011 meeting. Available at www.cdc.gov/vaccines/recs/acip/slides-feb11.htm#pertussis. Accessed March 19, 2011.
6. Centers for Disease Control and Prevention. Recommended antimicrobial agents for treatment and postexposure prophylaxis of pertussis: 2005 CDC guidelines. MMWR Recomm Rep. 2005;54(RR-14):1-16.
7. Prevention and control of influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2010. MMWR Recomm Rep. 2010;59(RR-8):1-62.
8. Centers for Disease Control and Prevention. Prevention of pneumococcal disease among infants and children—use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine—recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2010;59(RR-11):1-18.
9. Campos-Outcalt D. Your guide to the new pneumococcal vaccine for children. J Fam Pract. 2010;59:394-398.
10. Centers for Disease Control and Prevention. Updated recommendations for use of meningococcal conjugate vaccines—Advisory Committee on Immunization Practices (ACIP), 2010. MMWR Morb Mortal Wkly Rep. 2011;60:72-76.
11. Centers for Disease Control and Prevention. FDA licensure of bivalent human papillomavirus vaccine (HPV2, Cervarix) for use in females and updated HPV vaccination recommendations from the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep. 2010;59:626-629.
1. Centers for Disease Control and Prevention. Recommended immunization schedules for persons aged 0 through 18 years—United States, 2011. MMWR Morb Mortal Wkly Rep QuickGuide. 2011;60(5):1-4.
2. Centers for Disease Control and Prevention. Recommended adult immunization schedule-United States, 2011. MMWR Morb Mortal Wkly Rep. 2011;60(4):1-4.
3. Centers for Disease Control and Prevention. Pertussis (whooping cough): outbreaks. Available at: http://www.cdc.gov/pertussis/outbreaks.html. Accessed March 19, 2011.
4. Centers for Disease Control and Prevention. Updated recommendations for use of tetanus toxoid, reduced diphtheria toxoid and acellular pertussis (Tdap) vaccine from the Advisory Committee on Immunization Practices, 2010. MMWR Morb Mortal Wkly Rep. 2011;60(1):13-15.
5. Centers for Disease Control and Prevention. ACIP presentation slides: February 2011 meeting. Available at www.cdc.gov/vaccines/recs/acip/slides-feb11.htm#pertussis. Accessed March 19, 2011.
6. Centers for Disease Control and Prevention. Recommended antimicrobial agents for treatment and postexposure prophylaxis of pertussis: 2005 CDC guidelines. MMWR Recomm Rep. 2005;54(RR-14):1-16.
7. Prevention and control of influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2010. MMWR Recomm Rep. 2010;59(RR-8):1-62.
8. Centers for Disease Control and Prevention. Prevention of pneumococcal disease among infants and children—use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine—recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2010;59(RR-11):1-18.
9. Campos-Outcalt D. Your guide to the new pneumococcal vaccine for children. J Fam Pract. 2010;59:394-398.
10. Centers for Disease Control and Prevention. Updated recommendations for use of meningococcal conjugate vaccines—Advisory Committee on Immunization Practices (ACIP), 2010. MMWR Morb Mortal Wkly Rep. 2011;60:72-76.
11. Centers for Disease Control and Prevention. FDA licensure of bivalent human papillomavirus vaccine (HPV2, Cervarix) for use in females and updated HPV vaccination recommendations from the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep. 2010;59:626-629.