User login
The past 10 years have seen marked advances in vaccine research, resulting in more products being available. In 1983 the childhood vaccination schedule included protection against seven diseases: polio, tetanus, diphtheria, pertussis, measles, mumps, and rubella. The schedule in 2010 includes protection against organisms that cause seven more: Haemophilus influenzae, hepatitis A, hepatitis B, influenza, meningococcus, pneumococcus, and varicella.1 In addition, new vaccine products are available for adolescents, offering protection against meningococcus, seasonal influenza, and human papillomavirus (HPV) and extending the length of protection against pertussis. For adults, a vaccine now protects against shingles, and several products offer boosting of pertussis immunity.
This rapid growth in the number of recommended vaccine products has made it challenging for practicing physicians to stay current on and to implement the ever-changing recommendations. The purpose of this article is to summarize the additions and changes over the past 3 years to the schedules of recommended vaccines for children, adolescents, and adults.
VACCINE UPDATE FOR CHILDREN
The recent changes to the childhood immunization schedule have added protection against rotavirus and seasonal influenza and have expanded the protection against hepatitis A and varicella.
Rotavirus vaccination for infants
Rotavirus is the leading cause of infectious gastroenteritis in infants. It causes significant morbidity and expense, accounting for 2.7 million episodes per year in the United States, 410,000 outpatient or office visits, 201,000 to 272,000 emergency department visits, 55,000 to 70,000 hospitalizations, and 20 to 60 deaths.2 Although the number of deaths in the United States is not large, rotavirus is a leading cause of infant deaths around the world.
Rotavirus vaccination is challenging because of the time frame in which the series needs to be given. The first dose has to be given after 6 weeks of age but before 15 weeks of age, and the last dose should be given before 8 months of age, with a minimum of 4 weeks between doses. It is preferable to use the same product to finish the series. They can be used interchangeably, but this then requires three total doses.
The effectiveness of the vaccine in preventing rotavirus gastroenteritis in the first year after vaccination was greater than 80% in most studies and approached 100% in preventing serious gastroenteritis.2
Those vaccinated appear to have a slightly higher rate of diarrhea and vomiting in the first 42 days after vaccination. Safety monitoring after the products were licensed has not shown an increased rate of intussusception with either product.
The only contraindication to the vaccines is a serious allergic reaction to them or to one of their components. They should be used with caution in patients who have suppressed immunity, acute gastroenteritis, preexisting gastrointestinal disease, or previous intussusception.
Seasonal influenza vaccine extended to ages 5–18
Gradually, we seem to be moving toward vaccinating everyone every year against seasonal influenza. Previously, vaccination was recommended for children age 6 months through 4 years; in 2008, the Advisory Committee on Immunization Practices (ACIP) extended the recommendation to the age group 5 through 18 years.3
Two types of seasonal influenza vaccine are available: trivalent influenza vaccine (TIV), which contains killed virus and is given by injection, and live-attenuated seasonal influenza vaccine (LAIV), which is given by nasal spray. Both contain the same three seasonal influenza antigens, selected each year by a team of experts. TIV is licensed for those age 6 months and older, and LAIV is licensed for ages 2 through 49 years.4
Since LAIV contains a live-attenuated virus, it should not be used in anyone who has a chronic illness (including those under the age of 5 with recurrent wheezing, those with suppressed immunity, and those with a history of Guillain-Barré syndrome); in pregnant women; or those who have close contact with anyone who is immune-suppressed. The injection is contraindicated for those who have had a serious allergic reaction to eggs.
Children younger than 9 years should receive two doses of either type of vaccine the first year they are vaccinated. Those who receive only one dose the first year they are vaccinated should receive two doses the next year. If they fail to receive two doses in the next year, only a single dose is recommended after that. This is a slight modification of the previous recommendation that only one dose be given the second year if only one dose was given the first year.5
Hepatitis A vaccine at age 12–23 months
An inactivated hepatitis A vaccine (HepA) was first licensed in 1995; another was licensed in 1996. Recommendations for their use have been revised periodically, and their widespread use has resulted in a marked reduction in the incidence of hepatitis A virus infection.
The current recommendation is that all children be vaccinated at age 12 to 23 months. In addition, in areas of high prevalence, vaccine is recommended for older children who have not been vaccinated. Other target groups are those at higher risk of hepatitis A, including travelers to endemic areas, users of illicit drugs, and men who have sex with men.6 Indications for vaccination before travel, after exposure to hepatitis A infection, and in families of international adoptees are covered later in this paper in a discussion about vaccinations in adults.
Varicella at 12–15 months and 4–6 years, with catch-up for others
Before varicella vaccine was licensed in 1995, 4 million cases of varicella infection (chickenpox) were reported in the United States each year, resulting in thousands of hospitalizations and more than 100 deaths. The vaccine is now widely used, with a coverage rate of 88%, and it has proven to be 85% effective.7 The result was a marked decrease in the incidence of varicella and in varicella-related hospitalizations and deaths.
In spite of this success, the number of varicella cases has remained constant over the past few years, and sporadic outbreaks continue to occur, predominantly in schools, even schools in which a high percentage of the children are vaccinated.7,8 These outbreaks have involved infections in unvaccinated children and also “breakthrough disease” in children who have been vaccinated. If someone who has received one dose of vaccine is exposed to varicella, the risk of a breakthrough infection is about 15%.9 A two-dose series of varicella vaccine reduces the risk by about 75%.7 Breakthrough disease is usually milder than infection in the unvaccinated, with fewer skin lesions, milder symptoms, and fewer complications, but those affected are still infectious to others.
In 2005 and 2006, this ongoing risk of varicella prompted the ACIP to consider and recommend several new control measures:
- Two doses of varicella vaccine for all children, the first dose at age 12 to 15 months and the second at age 4 to 6 years—the same schedule as for immunization against measles, mumps, and rubella
- Two doses of varicella vaccine, the second given 4 to 8 weeks after the first, for all adolescents and adults who have no evidence of immunity
- A catch-up second dose for everyone who received one dose previously
- Screening for varicella immunity in pregnant women and postpartum vaccination with two doses for those who are not immune, the first dose given before discharge and the second dose 4 to 8 weeks later.
VACCINE UPDATE FOR ADOLESCENTS
A number of vaccines are now available and recommended for routine use in adolescents.9 These include HPV vaccine for girls, quadrivalent meningococcal conjugate vaccine (MCV4), and combined tetanus toxoid, reduced-dose diphtheria toxoid, and acellular pertussis (Tdap). All these are now recommended routinely at age 11 or 12. Seasonal influenza vaccine is recommended annually through age 18.
Meningococcal conjugate vaccine for all at age 11–18
There is some evidence that MCV4 may be linked to a small risk of Guillain-Barré syndrome. Although this link has not been conclusively proven, a history of Guillain-Barré syndrome calls for caution in using MCV4. For those who have a history of this syndrome but need protection against meningococcal infection, the MPSV4 is an alternative.11
Pertussis: A Tdap booster at age 11–18
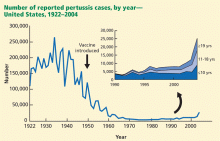
In addition, a greater percentage of cases now occurs in adolescents and young adults. Half of reported cases are now in those age 10 years and older. Most nonimmunized or incompletely immunized infants who develop pertussis were exposed to the disease by older household members, not by same-age cohorts. Since the disease presents as nonspecific cough in adolescents, it is often not diagnosed, and the incidence is probably much higher than the reported number of cases would indicate.
These trends were cause for public health concern and led to the development of pertussis-containing vaccine products for adolescents and adults. Two Tdap products are available: one is licensed for those ages 10 to 64 (Boostrix), the other for ages 11 to 64 (Adacel). Since 2005, the ACIP has recommended a single dose of Tdap for those age 11 to 18, preferably at 11 or 12 years.12 The optimal interval from the last tetanus-diphtheria shot is 5 years, but a shorter interval is acceptable. Thereafter, boosters with the tetanus toxoid and reduced-dose diphtheria toxoid (Td) vaccine are recommended every 10 years. If an adolescent has not previously received a complete series of a tetanus-diphtheria product, he or she should be given the recommended number of doses, only one of which should be Tdap, the others Td. The number and timing of doses can be found at www.cdc.gov/mmwr/preview/mmwrhtml/rr55e223a5.htm.
Human papillomavirus vaccination for girls age 11–12
HPV is sexually transmitted and causes genital warts, cervical cancer, and other oral, anal, and genital cancers.
HPV is the most common sexually transmitted infection in the United States, with over 6 million new cases each year.13 A study in 2003 to 2004 using HPV DNA typing of cervicovaginal swab specimens in a sample of women between the ages of 14 and 59 found an overall point prevalence of 26.8% of any HPV type.14 Those between 20 and 24 years had the highest prevalence at 44.8%. Those ages 14 to 19 had a prevalence of 24.5%. Several studies have reported a similar age-related increase in HPV prevalence.15,16
One survey found that nearly 25% of girls in the United States are sexually active by age 15, 40% by age 16, and 70% by age 18.17 The 2005 Behavioral Risk Survey found that nearly 4% of girls were sexually active before age 13, and by the ninth grade 5.7% of those who were sexually active had had four or more partners.18 To receive the full benefit from the HPV vaccine, it should be given before this risk of acquiring HPV occurs.
A quadrivalent HPV vaccine (HPV4) was first licensed in the United States in 2006 for use in girls and women 9 to 26 years old to prevent cervical, vulvar, and vaginal precancerous lesions and cancer, and for prevention of anogenital warts. It contains viral proteins from HPV types 6, 11, 16, and 18, the types currently responsible for 70% of cervical cancers and 90% of anogenital warts. The vaccine is prepared in a yeast substrate and contains an aluminum-based adjuvant.
HPV4 has proven highly effective in women ages 16 to 26 not previously exposed to the four HPV types in the vaccine. The end points used in these studies were cervical intraepithelial neoplasia grade 2 or 3, adenocarcinoma in situ, anogenital warts, and vulvar and vaginal intraepithelial neoplasms.13,19,20 The vaccine’s effectiveness has been 98% to 100% after 3 to 5 years. These trials are ongoing.
The vaccine’s efficacy in women with current or past HPV infection is less certain. Studies of this question have included only small numbers, and the confidence intervals are large and include 0. In intention-to-treat studies, its efficacy has been 39% to 46% for prevention of cervical intraepithelial neoplasia grade 2 or 3 or adenocarcinoma in situ caused by HPV-16 or HPV-18, 69% for prevention of HPV-16- or HPV-18-related vaginal intraepithelial neoplasia, and 68.5% for vaccine-type-related warts.13
The most common adverse effects of HPV4 have included redness, pain, and swelling at the injection site, which occur in about 20% of recipients.13 There is an increased risk of syncope immediately after the vaccine is given, and observation for 15 minutes after injection is recommended. A recent study suggested a link between the vaccine and venous thromboembolism. 21 The rate was 2 per million doses, and because many of the recipients also were taking oral contraceptives, their venous thromboembolism has not yet been definitively proven to be caused by the vaccine.
HPV4 is contraindicated in those who have experienced a severe allergic reaction to a previous dose or who have an allergy to a vaccine component. Vaccination should be deferred in those with moderate or severe acute illnesses.
In June 2006, the ACIP13 made the following recommendations for HPV4:
- Girls ages 11 to 12 years should be routinely vaccinated with three doses
- The series can start as early as age 9 years
- Women and girls age 13 to 26 who have not been previously vaccinated should receive catch-up vaccination
- Neither Papanicolaou (Pap) testing nor HPV screening is necessary before vaccination
- HPV4 can be given with other age-appropriate vaccines
- Vaccination does not change the recommendations for cervical cancer screening
- The recommendations remain the same regardless of abnormal Pap tests, positive HPV DNA tests, or warts.
There have been two very recent developments regarding HPV vaccines.
A bivalent vaccine (HPV2) has been licensed in the United States and approved for use in girls and women ages 10 to 25 for prevention of cervical cancer and precancerous lesions. It contains antigens against HPV-16 and HPV-18 but does not provide protection against genital warts. The ACIP has stated no preference for the bivalent or the quadrivalent vaccine for the prevention of cervical cancer and precancerous lesions.
HPV4 has also gained licensure for use in boys and men age 9 to 26 for the prevention of genital warts. The ACIP has not recommended it for routine use, leaving the decision to patients and physicians after weighing the potential benefits and costs.
VACCINE UPDATE FOR ADULTS
Four vaccines are now routinely recommended for adults:
- Seasonal influenza vaccine starting at age 50
- Pneumococcal polysaccharide vaccine (PPSV23) starting at age 65
- Herpes zoster vaccine starting at age 60
- A diphtheria and tetanus toxoid product every 10 years, with Tdap given once.22
The rest of the adult schedule is based on catch-up (measles, mumps, rubella, varicella) or risk (hepatitis A and B and meningococccal disease). Seasonal influenza and pneumococcal vaccinations are also recommended before ages 50 and 65, respectively, for those with certain risk conditions. The complete adult immunization schedule can be found on the US Centers for Disease Control and Prevention (CDC) Web site.22
One dose of Tdap instead of the next Td booster
The CDC now recommends that a single dose of Tdap should replace the next dose of Td for adults ages 19 to 64 as part of the every-10-year tetanus-diphtheria boosting recommendation and if indicated for wound management. 23 In addition, a single dose of Tdap should be given to adults who have close contact with infants less than 6 months of age. The optimal interval between this Tdap shot and the last Td booster is 2 years or greater, but shorter intervals are acceptable. Women of childbearing age should receive Tdap preconception or postpartum if they have not previously received it. Tdap is not approved for use during pregnancy. Health care workers should also receive a dose of Tdap if they have never received it previously and if their last Td booster was more than 2 years ago, although less than 2 years is acceptable.
Contraindications to Tdap include anaphylaxis to a vaccine component and encephalopathy occurring within 7 days of previously receiving a pertussis vaccine.
Herpes zoster vaccine for those age 60 and older
Shingles causes considerable morbidity in older adults. The lifetime risk is 25%, and onefourth of those with shingles develop postherpetic neuralgia.
Herpes zoster vaccine is a live-attenuated vaccine that requires only a single injection. It is licensed for use in those ages 60 and older, and the ACIP recommends its routine use.24 Its effectiveness is approximately 50% and is inversely related to age. The number of patients who need to be vaccinated to prevent one lifetime case of shingles is 17.
Contraindications to this vaccine include a prior anaphylactic reaction to gelatin or neomycin, compromised immunity due to disease or to immune-suppressive therapy including high-dose corticosteroids, and active tuberculosis.
Payment for this vaccine by Medicare is through Part D, creating some administrative difficulties for physicians’ offices.
Pneumococcal vaccination extended to smokers and people with asthma
The ACIP recently added two new groups for whom PPSV23 is recommended: smokers and those with asthma.25 Smoking poses as much of a risk for pneumococcal pneumonia as do diabetes and other chronic illnesses that are currently indications for the vaccine. The number needed to vaccinate to prevent one case of pneumonia among smokers is 10,000 in people ages 18 to 44, and 4,000 in those ages 45 to 64.26
The ACIP also clarified the recommendation for a second dose of PPSV23.25 A second dose 5 years after the first is recommended for those who have immune suppression, sickle cell disease, or asplenia. People over age 65 should receive a second dose if they were vaccinated more than 5 years previously and before age 65.
New uses for hepatitis A vaccine
A combined hepatitis A and hepatitis B vaccine (Twinrix) has received approval for an alternate, four-dose schedule at 0, 7, 21 days, and 12 months.27 It has previously only been approved for a three-dose schedule at 0, 1, and 6 months. The new alternative schedule allows greater protection for travelers who need to depart within less than 1 month.
For unvaccinated people who are acutely exposed to hepatitis A virus and for those traveling to areas of high prevalence who do not have time to complete the two doses of hepatitis A vaccine, the only prevention available until recently has been immune globulin. This has changed: hepatitis A vaccine can now be used in both groups. The new recommendations for postexposure prophylaxis is that either a single dose of hepatitis A vaccine or use of immune globulin is acceptable.28 In ages 12 months to 40 years, vaccine is preferred. For those over age 40, immune globulin is preferred, but vaccine is acceptable. For children younger than 12 months, the immune-suppressed, and those with chronic liver disease, immune globulin should be used.
Those traveling or working in countries with high rates of hepatitis A can be protected with either hepatitis A vaccine or immune globulin. A single dose of the vaccine is sufficient for healthy people, with a second dose at the recommended interval to complete the series. Those younger than 12 months and those who choose not to receive the vaccine, including those who are allergic to it, should be offered immune globulin. Both immune globulin and hepatitis A vaccine should be considered for certain patients who plan to travel within 2 weeks of the first vaccine dose, ie, those over age 40, those with compromised immunity, and those with chronic liver disease or other chronic conditions.
Hepatitis A vaccine is now also recommended for all unvaccinated people who anticipate close personal contact with an international adoptee during the first 60 days following arrival from countries with high or intermediate hepatitis A endemicity.29 The first dose should be given as soon as the adoption is planned and ideally at least 2 weeks before the child arrives.
- Centers for Disease Control and Prevention (CDC). Recommended immunization schedule for persons aged 0 through 6 years—United States 2009. www.cdc.gov/vaccines/recs/schedules/downloads/child/2009/09_0-6yrs_schedule_pr.pdf. Accessed March 6, 2010.
- Cortese MM, Parashar UDCenters for Disease Control and Prevention (CDC). Prevention of rotavirus gastroenteritis among infants and children: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2009; 58( RR-2):1–25.
- Fiore AE, Shay DK, Broder K, et al., Centers for Disease Control and Prevention (CDC). Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2009. MMWR Recomm Rep 2009; 58( RR–8):1–52.
- Centers for Disease Control and Prevention (CDC). Notice to readers: expansion of use of live attenuated influenza vaccine (FluMist®) to children aged 2–4 years and other FluMist changes for the 2007–08 influenza season. MMWR Morb Mortal Wkly Rep 2007; 56( 46):1217–1219.
- Fiore AE, Shay DK, Haber PCenters for Disease Control and Prevention (CDC). Prevention and control of influenza: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2007. MMWR Recomm Rep 2007; 56( RR–6):1–54.
- Fiore AE, Wasley A, Bell BPAdvisory Committee on Immunization Practices (ACIP). Prevention of hepatitis A through active or passive immunization: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2006; 55( RR–7):1–23.
- Marin M, Güris D, Chaves SS, Schmid S, Seward JFAdvisory Committee on Immunization Practices. Prevention of varicella: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2007; 56( RR–4):1–40.
- Centers for Disease Control and Prevention (CDC). Varicella disease. www.cdc.gov/vaccines/vpd-vac/varicella/dis-faqs-clinic.htm. Accessed March 4, 2010.
- Centers for Disease Control and Prevention (CDC). 2009 child & adolescent immunization schedules. www.cdc.gov/vaccines/recs/schedules/child-schedule.htm. Accessed March 4, 2010.
- Centers for Disease Control and Prevention (CDC) Advisory Committee on Immunization Practices. Revised recommendations of the Advisory Committee on Immunization Practices to vaccinate all persons aged 11-18 years with meningococcal conjugate vaccine. MMWR Morb Mortal Wkly Rep 2007; 56( 31):794–795.
- Centers for Disease Control and Prevention (CDC). Update: Guillain-Barré syndrome among recipients of menactra meningococcal conjugate vaccine—United States, June 2005–September 2006. MMWR Morb Mortal Wkly Rep 2006; 55( 41):1120–1124.
- Broder KR, Cortese MM, Iskander JK, et al., Advisory Committee on Immunization Practices (ACIP). Preventing tetanus, diphtheria, and pertussis among adolescents: use of tetanus toxoid, reduced diphtheria toxoid and acellular pertussis vaccines recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2006; 55( RR–3):1–34.
- Markowitz LE, Dunne EF, Saraiya M, Lawson HW, Chesson H, Unger ERCenters for Disease Control and Prevention (CDC). Quadrivalent human papillomavirus vaccine: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2007; 56( RR–2):1–24.
- Dunne EF, Unger ER, Sternberg M, et al Prevalence of HPV infection among females in the United States. JAMA 2007; 297:813–819.
- Trottier H, Franco EL. The epidemiology of genital human papillomavirus infection. Vaccine 2006; 24( suppl 1):S1–S15.
- Stone KM, Karem KL, Sternberg MR, et al Seroprevalence of human papillomavirus type 16 infection in the United States. J Infect Dis 2002; 186:1396–1402.
- Abma JC, Martinez GM, Mosher WD, Dawson BS. Teenagers in the United States: sexual activity, contraceptive use, and childbearing, 2002. Vital Health Stat 23 2004; 24:1–48.
- Eaton DK, Kann L, Kinchen S, et al Youth risk behavior surveillance—United States, 2005. MMWR Surveill Summ 2006; 55:1–108.
- Human papillomavirus vaccines. WHO position paper. Wkly Epidemiol Rec 2009; 84:118–131.
- Rambout L, Hopkins L, Hutton B, Fergusson D. Prophylactic vaccination against human papillomavirus infection and disease in women: a systematic review of randomized controlled trials. CMAJ 2007; 177:469–479.
- Slade BA, Leidel L, Vellozzi C, et al Postlicensure safety surveillance for quadrivalent human papillomavirus recombinant vaccine. JAMA 2009; 302:750–757.
- Centers for Disease Control (CDC). Adult immunization schedule. http://www.cdc.gov/vaccines/recs/schedules/adult-schedule.htm. Accessed March 4, 2010.
- Kretsinger K, Broder KR, Cortese MM, et al., Centers for Disease Control and Prevention. Preventing tetanus, diphtheria, and pertussis among adults: use of tetanus toxoid, reduced diphtheria toxoid and acellular pertussis vaccine recommendations of the Advisory Committee on Immunization Practices (ACIP) and recommendation of ACIP, supported by the Healthcare Infection Control Practices Advisory Committee (HICPAC), for use of Tdap among health-care personnel. MMWR Recomm Rep 2006; 55( RR–17):1–37.
- Harpaz R, Ortega-Sanchez IR, Seward JFAdvisory Committee on Immunization Practices (ACIP). Prevention of herpes zoster: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2008; 57( RR–5):1–30.
- Centers for Disease Control (CDC). ACIP provisional recommendations for use of pneumococcal vaccines. www.cdc.gov/vaccines/recs/provisional/downloads/pneumo-Oct-2008-508.pdf. Accessed March 4, 2010.
- Centers for Disease Control and Prevention (CDC). Summary Report: October 22–23, 2008; Atlanta, Georgia. www.cdc.gov/vaccines/recs/ACIP/downloads/min=archive/min-oct08.pdf. Accessed March 6, 2010.
- CDC. Notice to readers: FDA approval of an alternate dosing schedule for a combined hepatitis A and B vaccine (Twinrix®). MMWR Morb Mortal Wkly Rep 2007; 56( 40);1057.
- Advisory Committee on Immunization Practices (ACIP) Centers for Disease Control and Prevention (CDC). Update: prevention of hepatitis A after exposure to hepatitis A virus and in international travelers. Updated recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 2007; 56( 41):1080–1084.
- Centers for Disease Control and Prevention (CDC). Updated recommendations from the Advisory Committee on Immunization Practices (ACIP) for use of hepatitis A vaccine in close contacts of newly arriving international adoptees. MMWR 2009: 58:1006–1007.
The past 10 years have seen marked advances in vaccine research, resulting in more products being available. In 1983 the childhood vaccination schedule included protection against seven diseases: polio, tetanus, diphtheria, pertussis, measles, mumps, and rubella. The schedule in 2010 includes protection against organisms that cause seven more: Haemophilus influenzae, hepatitis A, hepatitis B, influenza, meningococcus, pneumococcus, and varicella.1 In addition, new vaccine products are available for adolescents, offering protection against meningococcus, seasonal influenza, and human papillomavirus (HPV) and extending the length of protection against pertussis. For adults, a vaccine now protects against shingles, and several products offer boosting of pertussis immunity.
This rapid growth in the number of recommended vaccine products has made it challenging for practicing physicians to stay current on and to implement the ever-changing recommendations. The purpose of this article is to summarize the additions and changes over the past 3 years to the schedules of recommended vaccines for children, adolescents, and adults.

VACCINE UPDATE FOR CHILDREN
The recent changes to the childhood immunization schedule have added protection against rotavirus and seasonal influenza and have expanded the protection against hepatitis A and varicella.
Rotavirus vaccination for infants
Rotavirus is the leading cause of infectious gastroenteritis in infants. It causes significant morbidity and expense, accounting for 2.7 million episodes per year in the United States, 410,000 outpatient or office visits, 201,000 to 272,000 emergency department visits, 55,000 to 70,000 hospitalizations, and 20 to 60 deaths.2 Although the number of deaths in the United States is not large, rotavirus is a leading cause of infant deaths around the world.
Rotavirus vaccination is challenging because of the time frame in which the series needs to be given. The first dose has to be given after 6 weeks of age but before 15 weeks of age, and the last dose should be given before 8 months of age, with a minimum of 4 weeks between doses. It is preferable to use the same product to finish the series. They can be used interchangeably, but this then requires three total doses.
The effectiveness of the vaccine in preventing rotavirus gastroenteritis in the first year after vaccination was greater than 80% in most studies and approached 100% in preventing serious gastroenteritis.2
Those vaccinated appear to have a slightly higher rate of diarrhea and vomiting in the first 42 days after vaccination. Safety monitoring after the products were licensed has not shown an increased rate of intussusception with either product.
The only contraindication to the vaccines is a serious allergic reaction to them or to one of their components. They should be used with caution in patients who have suppressed immunity, acute gastroenteritis, preexisting gastrointestinal disease, or previous intussusception.
Seasonal influenza vaccine extended to ages 5–18
Gradually, we seem to be moving toward vaccinating everyone every year against seasonal influenza. Previously, vaccination was recommended for children age 6 months through 4 years; in 2008, the Advisory Committee on Immunization Practices (ACIP) extended the recommendation to the age group 5 through 18 years.3
Two types of seasonal influenza vaccine are available: trivalent influenza vaccine (TIV), which contains killed virus and is given by injection, and live-attenuated seasonal influenza vaccine (LAIV), which is given by nasal spray. Both contain the same three seasonal influenza antigens, selected each year by a team of experts. TIV is licensed for those age 6 months and older, and LAIV is licensed for ages 2 through 49 years.4
Since LAIV contains a live-attenuated virus, it should not be used in anyone who has a chronic illness (including those under the age of 5 with recurrent wheezing, those with suppressed immunity, and those with a history of Guillain-Barré syndrome); in pregnant women; or those who have close contact with anyone who is immune-suppressed. The injection is contraindicated for those who have had a serious allergic reaction to eggs.
Children younger than 9 years should receive two doses of either type of vaccine the first year they are vaccinated. Those who receive only one dose the first year they are vaccinated should receive two doses the next year. If they fail to receive two doses in the next year, only a single dose is recommended after that. This is a slight modification of the previous recommendation that only one dose be given the second year if only one dose was given the first year.5
Hepatitis A vaccine at age 12–23 months
An inactivated hepatitis A vaccine (HepA) was first licensed in 1995; another was licensed in 1996. Recommendations for their use have been revised periodically, and their widespread use has resulted in a marked reduction in the incidence of hepatitis A virus infection.
The current recommendation is that all children be vaccinated at age 12 to 23 months. In addition, in areas of high prevalence, vaccine is recommended for older children who have not been vaccinated. Other target groups are those at higher risk of hepatitis A, including travelers to endemic areas, users of illicit drugs, and men who have sex with men.6 Indications for vaccination before travel, after exposure to hepatitis A infection, and in families of international adoptees are covered later in this paper in a discussion about vaccinations in adults.
Varicella at 12–15 months and 4–6 years, with catch-up for others
Before varicella vaccine was licensed in 1995, 4 million cases of varicella infection (chickenpox) were reported in the United States each year, resulting in thousands of hospitalizations and more than 100 deaths. The vaccine is now widely used, with a coverage rate of 88%, and it has proven to be 85% effective.7 The result was a marked decrease in the incidence of varicella and in varicella-related hospitalizations and deaths.
In spite of this success, the number of varicella cases has remained constant over the past few years, and sporadic outbreaks continue to occur, predominantly in schools, even schools in which a high percentage of the children are vaccinated.7,8 These outbreaks have involved infections in unvaccinated children and also “breakthrough disease” in children who have been vaccinated. If someone who has received one dose of vaccine is exposed to varicella, the risk of a breakthrough infection is about 15%.9 A two-dose series of varicella vaccine reduces the risk by about 75%.7 Breakthrough disease is usually milder than infection in the unvaccinated, with fewer skin lesions, milder symptoms, and fewer complications, but those affected are still infectious to others.
In 2005 and 2006, this ongoing risk of varicella prompted the ACIP to consider and recommend several new control measures:
- Two doses of varicella vaccine for all children, the first dose at age 12 to 15 months and the second at age 4 to 6 years—the same schedule as for immunization against measles, mumps, and rubella
- Two doses of varicella vaccine, the second given 4 to 8 weeks after the first, for all adolescents and adults who have no evidence of immunity
- A catch-up second dose for everyone who received one dose previously
- Screening for varicella immunity in pregnant women and postpartum vaccination with two doses for those who are not immune, the first dose given before discharge and the second dose 4 to 8 weeks later.
VACCINE UPDATE FOR ADOLESCENTS
A number of vaccines are now available and recommended for routine use in adolescents.9 These include HPV vaccine for girls, quadrivalent meningococcal conjugate vaccine (MCV4), and combined tetanus toxoid, reduced-dose diphtheria toxoid, and acellular pertussis (Tdap). All these are now recommended routinely at age 11 or 12. Seasonal influenza vaccine is recommended annually through age 18.
Meningococcal conjugate vaccine for all at age 11–18
There is some evidence that MCV4 may be linked to a small risk of Guillain-Barré syndrome. Although this link has not been conclusively proven, a history of Guillain-Barré syndrome calls for caution in using MCV4. For those who have a history of this syndrome but need protection against meningococcal infection, the MPSV4 is an alternative.11
Pertussis: A Tdap booster at age 11–18
In addition, a greater percentage of cases now occurs in adolescents and young adults. Half of reported cases are now in those age 10 years and older. Most nonimmunized or incompletely immunized infants who develop pertussis were exposed to the disease by older household members, not by same-age cohorts. Since the disease presents as nonspecific cough in adolescents, it is often not diagnosed, and the incidence is probably much higher than the reported number of cases would indicate.
These trends were cause for public health concern and led to the development of pertussis-containing vaccine products for adolescents and adults. Two Tdap products are available: one is licensed for those ages 10 to 64 (Boostrix), the other for ages 11 to 64 (Adacel). Since 2005, the ACIP has recommended a single dose of Tdap for those age 11 to 18, preferably at 11 or 12 years.12 The optimal interval from the last tetanus-diphtheria shot is 5 years, but a shorter interval is acceptable. Thereafter, boosters with the tetanus toxoid and reduced-dose diphtheria toxoid (Td) vaccine are recommended every 10 years. If an adolescent has not previously received a complete series of a tetanus-diphtheria product, he or she should be given the recommended number of doses, only one of which should be Tdap, the others Td. The number and timing of doses can be found at www.cdc.gov/mmwr/preview/mmwrhtml/rr55e223a5.htm.
Human papillomavirus vaccination for girls age 11–12
HPV is sexually transmitted and causes genital warts, cervical cancer, and other oral, anal, and genital cancers.
HPV is the most common sexually transmitted infection in the United States, with over 6 million new cases each year.13 A study in 2003 to 2004 using HPV DNA typing of cervicovaginal swab specimens in a sample of women between the ages of 14 and 59 found an overall point prevalence of 26.8% of any HPV type.14 Those between 20 and 24 years had the highest prevalence at 44.8%. Those ages 14 to 19 had a prevalence of 24.5%. Several studies have reported a similar age-related increase in HPV prevalence.15,16
One survey found that nearly 25% of girls in the United States are sexually active by age 15, 40% by age 16, and 70% by age 18.17 The 2005 Behavioral Risk Survey found that nearly 4% of girls were sexually active before age 13, and by the ninth grade 5.7% of those who were sexually active had had four or more partners.18 To receive the full benefit from the HPV vaccine, it should be given before this risk of acquiring HPV occurs.
A quadrivalent HPV vaccine (HPV4) was first licensed in the United States in 2006 for use in girls and women 9 to 26 years old to prevent cervical, vulvar, and vaginal precancerous lesions and cancer, and for prevention of anogenital warts. It contains viral proteins from HPV types 6, 11, 16, and 18, the types currently responsible for 70% of cervical cancers and 90% of anogenital warts. The vaccine is prepared in a yeast substrate and contains an aluminum-based adjuvant.
HPV4 has proven highly effective in women ages 16 to 26 not previously exposed to the four HPV types in the vaccine. The end points used in these studies were cervical intraepithelial neoplasia grade 2 or 3, adenocarcinoma in situ, anogenital warts, and vulvar and vaginal intraepithelial neoplasms.13,19,20 The vaccine’s effectiveness has been 98% to 100% after 3 to 5 years. These trials are ongoing.
The vaccine’s efficacy in women with current or past HPV infection is less certain. Studies of this question have included only small numbers, and the confidence intervals are large and include 0. In intention-to-treat studies, its efficacy has been 39% to 46% for prevention of cervical intraepithelial neoplasia grade 2 or 3 or adenocarcinoma in situ caused by HPV-16 or HPV-18, 69% for prevention of HPV-16- or HPV-18-related vaginal intraepithelial neoplasia, and 68.5% for vaccine-type-related warts.13
The most common adverse effects of HPV4 have included redness, pain, and swelling at the injection site, which occur in about 20% of recipients.13 There is an increased risk of syncope immediately after the vaccine is given, and observation for 15 minutes after injection is recommended. A recent study suggested a link between the vaccine and venous thromboembolism. 21 The rate was 2 per million doses, and because many of the recipients also were taking oral contraceptives, their venous thromboembolism has not yet been definitively proven to be caused by the vaccine.
HPV4 is contraindicated in those who have experienced a severe allergic reaction to a previous dose or who have an allergy to a vaccine component. Vaccination should be deferred in those with moderate or severe acute illnesses.
In June 2006, the ACIP13 made the following recommendations for HPV4:
- Girls ages 11 to 12 years should be routinely vaccinated with three doses
- The series can start as early as age 9 years
- Women and girls age 13 to 26 who have not been previously vaccinated should receive catch-up vaccination
- Neither Papanicolaou (Pap) testing nor HPV screening is necessary before vaccination
- HPV4 can be given with other age-appropriate vaccines
- Vaccination does not change the recommendations for cervical cancer screening
- The recommendations remain the same regardless of abnormal Pap tests, positive HPV DNA tests, or warts.
There have been two very recent developments regarding HPV vaccines.
A bivalent vaccine (HPV2) has been licensed in the United States and approved for use in girls and women ages 10 to 25 for prevention of cervical cancer and precancerous lesions. It contains antigens against HPV-16 and HPV-18 but does not provide protection against genital warts. The ACIP has stated no preference for the bivalent or the quadrivalent vaccine for the prevention of cervical cancer and precancerous lesions.
HPV4 has also gained licensure for use in boys and men age 9 to 26 for the prevention of genital warts. The ACIP has not recommended it for routine use, leaving the decision to patients and physicians after weighing the potential benefits and costs.
VACCINE UPDATE FOR ADULTS
Four vaccines are now routinely recommended for adults:
- Seasonal influenza vaccine starting at age 50
- Pneumococcal polysaccharide vaccine (PPSV23) starting at age 65
- Herpes zoster vaccine starting at age 60
- A diphtheria and tetanus toxoid product every 10 years, with Tdap given once.22
The rest of the adult schedule is based on catch-up (measles, mumps, rubella, varicella) or risk (hepatitis A and B and meningococccal disease). Seasonal influenza and pneumococcal vaccinations are also recommended before ages 50 and 65, respectively, for those with certain risk conditions. The complete adult immunization schedule can be found on the US Centers for Disease Control and Prevention (CDC) Web site.22
One dose of Tdap instead of the next Td booster
The CDC now recommends that a single dose of Tdap should replace the next dose of Td for adults ages 19 to 64 as part of the every-10-year tetanus-diphtheria boosting recommendation and if indicated for wound management. 23 In addition, a single dose of Tdap should be given to adults who have close contact with infants less than 6 months of age. The optimal interval between this Tdap shot and the last Td booster is 2 years or greater, but shorter intervals are acceptable. Women of childbearing age should receive Tdap preconception or postpartum if they have not previously received it. Tdap is not approved for use during pregnancy. Health care workers should also receive a dose of Tdap if they have never received it previously and if their last Td booster was more than 2 years ago, although less than 2 years is acceptable.
Contraindications to Tdap include anaphylaxis to a vaccine component and encephalopathy occurring within 7 days of previously receiving a pertussis vaccine.
Herpes zoster vaccine for those age 60 and older
Shingles causes considerable morbidity in older adults. The lifetime risk is 25%, and onefourth of those with shingles develop postherpetic neuralgia.
Herpes zoster vaccine is a live-attenuated vaccine that requires only a single injection. It is licensed for use in those ages 60 and older, and the ACIP recommends its routine use.24 Its effectiveness is approximately 50% and is inversely related to age. The number of patients who need to be vaccinated to prevent one lifetime case of shingles is 17.
Contraindications to this vaccine include a prior anaphylactic reaction to gelatin or neomycin, compromised immunity due to disease or to immune-suppressive therapy including high-dose corticosteroids, and active tuberculosis.
Payment for this vaccine by Medicare is through Part D, creating some administrative difficulties for physicians’ offices.
Pneumococcal vaccination extended to smokers and people with asthma
The ACIP recently added two new groups for whom PPSV23 is recommended: smokers and those with asthma.25 Smoking poses as much of a risk for pneumococcal pneumonia as do diabetes and other chronic illnesses that are currently indications for the vaccine. The number needed to vaccinate to prevent one case of pneumonia among smokers is 10,000 in people ages 18 to 44, and 4,000 in those ages 45 to 64.26
The ACIP also clarified the recommendation for a second dose of PPSV23.25 A second dose 5 years after the first is recommended for those who have immune suppression, sickle cell disease, or asplenia. People over age 65 should receive a second dose if they were vaccinated more than 5 years previously and before age 65.
New uses for hepatitis A vaccine
A combined hepatitis A and hepatitis B vaccine (Twinrix) has received approval for an alternate, four-dose schedule at 0, 7, 21 days, and 12 months.27 It has previously only been approved for a three-dose schedule at 0, 1, and 6 months. The new alternative schedule allows greater protection for travelers who need to depart within less than 1 month.
For unvaccinated people who are acutely exposed to hepatitis A virus and for those traveling to areas of high prevalence who do not have time to complete the two doses of hepatitis A vaccine, the only prevention available until recently has been immune globulin. This has changed: hepatitis A vaccine can now be used in both groups. The new recommendations for postexposure prophylaxis is that either a single dose of hepatitis A vaccine or use of immune globulin is acceptable.28 In ages 12 months to 40 years, vaccine is preferred. For those over age 40, immune globulin is preferred, but vaccine is acceptable. For children younger than 12 months, the immune-suppressed, and those with chronic liver disease, immune globulin should be used.
Those traveling or working in countries with high rates of hepatitis A can be protected with either hepatitis A vaccine or immune globulin. A single dose of the vaccine is sufficient for healthy people, with a second dose at the recommended interval to complete the series. Those younger than 12 months and those who choose not to receive the vaccine, including those who are allergic to it, should be offered immune globulin. Both immune globulin and hepatitis A vaccine should be considered for certain patients who plan to travel within 2 weeks of the first vaccine dose, ie, those over age 40, those with compromised immunity, and those with chronic liver disease or other chronic conditions.
Hepatitis A vaccine is now also recommended for all unvaccinated people who anticipate close personal contact with an international adoptee during the first 60 days following arrival from countries with high or intermediate hepatitis A endemicity.29 The first dose should be given as soon as the adoption is planned and ideally at least 2 weeks before the child arrives.
The past 10 years have seen marked advances in vaccine research, resulting in more products being available. In 1983 the childhood vaccination schedule included protection against seven diseases: polio, tetanus, diphtheria, pertussis, measles, mumps, and rubella. The schedule in 2010 includes protection against organisms that cause seven more: Haemophilus influenzae, hepatitis A, hepatitis B, influenza, meningococcus, pneumococcus, and varicella.1 In addition, new vaccine products are available for adolescents, offering protection against meningococcus, seasonal influenza, and human papillomavirus (HPV) and extending the length of protection against pertussis. For adults, a vaccine now protects against shingles, and several products offer boosting of pertussis immunity.
This rapid growth in the number of recommended vaccine products has made it challenging for practicing physicians to stay current on and to implement the ever-changing recommendations. The purpose of this article is to summarize the additions and changes over the past 3 years to the schedules of recommended vaccines for children, adolescents, and adults.

VACCINE UPDATE FOR CHILDREN
The recent changes to the childhood immunization schedule have added protection against rotavirus and seasonal influenza and have expanded the protection against hepatitis A and varicella.
Rotavirus vaccination for infants
Rotavirus is the leading cause of infectious gastroenteritis in infants. It causes significant morbidity and expense, accounting for 2.7 million episodes per year in the United States, 410,000 outpatient or office visits, 201,000 to 272,000 emergency department visits, 55,000 to 70,000 hospitalizations, and 20 to 60 deaths.2 Although the number of deaths in the United States is not large, rotavirus is a leading cause of infant deaths around the world.
Rotavirus vaccination is challenging because of the time frame in which the series needs to be given. The first dose has to be given after 6 weeks of age but before 15 weeks of age, and the last dose should be given before 8 months of age, with a minimum of 4 weeks between doses. It is preferable to use the same product to finish the series. They can be used interchangeably, but this then requires three total doses.
The effectiveness of the vaccine in preventing rotavirus gastroenteritis in the first year after vaccination was greater than 80% in most studies and approached 100% in preventing serious gastroenteritis.2
Those vaccinated appear to have a slightly higher rate of diarrhea and vomiting in the first 42 days after vaccination. Safety monitoring after the products were licensed has not shown an increased rate of intussusception with either product.
The only contraindication to the vaccines is a serious allergic reaction to them or to one of their components. They should be used with caution in patients who have suppressed immunity, acute gastroenteritis, preexisting gastrointestinal disease, or previous intussusception.
Seasonal influenza vaccine extended to ages 5–18
Gradually, we seem to be moving toward vaccinating everyone every year against seasonal influenza. Previously, vaccination was recommended for children age 6 months through 4 years; in 2008, the Advisory Committee on Immunization Practices (ACIP) extended the recommendation to the age group 5 through 18 years.3
Two types of seasonal influenza vaccine are available: trivalent influenza vaccine (TIV), which contains killed virus and is given by injection, and live-attenuated seasonal influenza vaccine (LAIV), which is given by nasal spray. Both contain the same three seasonal influenza antigens, selected each year by a team of experts. TIV is licensed for those age 6 months and older, and LAIV is licensed for ages 2 through 49 years.4
Since LAIV contains a live-attenuated virus, it should not be used in anyone who has a chronic illness (including those under the age of 5 with recurrent wheezing, those with suppressed immunity, and those with a history of Guillain-Barré syndrome); in pregnant women; or those who have close contact with anyone who is immune-suppressed. The injection is contraindicated for those who have had a serious allergic reaction to eggs.
Children younger than 9 years should receive two doses of either type of vaccine the first year they are vaccinated. Those who receive only one dose the first year they are vaccinated should receive two doses the next year. If they fail to receive two doses in the next year, only a single dose is recommended after that. This is a slight modification of the previous recommendation that only one dose be given the second year if only one dose was given the first year.5
Hepatitis A vaccine at age 12–23 months
An inactivated hepatitis A vaccine (HepA) was first licensed in 1995; another was licensed in 1996. Recommendations for their use have been revised periodically, and their widespread use has resulted in a marked reduction in the incidence of hepatitis A virus infection.
The current recommendation is that all children be vaccinated at age 12 to 23 months. In addition, in areas of high prevalence, vaccine is recommended for older children who have not been vaccinated. Other target groups are those at higher risk of hepatitis A, including travelers to endemic areas, users of illicit drugs, and men who have sex with men.6 Indications for vaccination before travel, after exposure to hepatitis A infection, and in families of international adoptees are covered later in this paper in a discussion about vaccinations in adults.
Varicella at 12–15 months and 4–6 years, with catch-up for others
Before varicella vaccine was licensed in 1995, 4 million cases of varicella infection (chickenpox) were reported in the United States each year, resulting in thousands of hospitalizations and more than 100 deaths. The vaccine is now widely used, with a coverage rate of 88%, and it has proven to be 85% effective.7 The result was a marked decrease in the incidence of varicella and in varicella-related hospitalizations and deaths.
In spite of this success, the number of varicella cases has remained constant over the past few years, and sporadic outbreaks continue to occur, predominantly in schools, even schools in which a high percentage of the children are vaccinated.7,8 These outbreaks have involved infections in unvaccinated children and also “breakthrough disease” in children who have been vaccinated. If someone who has received one dose of vaccine is exposed to varicella, the risk of a breakthrough infection is about 15%.9 A two-dose series of varicella vaccine reduces the risk by about 75%.7 Breakthrough disease is usually milder than infection in the unvaccinated, with fewer skin lesions, milder symptoms, and fewer complications, but those affected are still infectious to others.
In 2005 and 2006, this ongoing risk of varicella prompted the ACIP to consider and recommend several new control measures:
- Two doses of varicella vaccine for all children, the first dose at age 12 to 15 months and the second at age 4 to 6 years—the same schedule as for immunization against measles, mumps, and rubella
- Two doses of varicella vaccine, the second given 4 to 8 weeks after the first, for all adolescents and adults who have no evidence of immunity
- A catch-up second dose for everyone who received one dose previously
- Screening for varicella immunity in pregnant women and postpartum vaccination with two doses for those who are not immune, the first dose given before discharge and the second dose 4 to 8 weeks later.
VACCINE UPDATE FOR ADOLESCENTS
A number of vaccines are now available and recommended for routine use in adolescents.9 These include HPV vaccine for girls, quadrivalent meningococcal conjugate vaccine (MCV4), and combined tetanus toxoid, reduced-dose diphtheria toxoid, and acellular pertussis (Tdap). All these are now recommended routinely at age 11 or 12. Seasonal influenza vaccine is recommended annually through age 18.
Meningococcal conjugate vaccine for all at age 11–18
There is some evidence that MCV4 may be linked to a small risk of Guillain-Barré syndrome. Although this link has not been conclusively proven, a history of Guillain-Barré syndrome calls for caution in using MCV4. For those who have a history of this syndrome but need protection against meningococcal infection, the MPSV4 is an alternative.11
Pertussis: A Tdap booster at age 11–18
In addition, a greater percentage of cases now occurs in adolescents and young adults. Half of reported cases are now in those age 10 years and older. Most nonimmunized or incompletely immunized infants who develop pertussis were exposed to the disease by older household members, not by same-age cohorts. Since the disease presents as nonspecific cough in adolescents, it is often not diagnosed, and the incidence is probably much higher than the reported number of cases would indicate.
These trends were cause for public health concern and led to the development of pertussis-containing vaccine products for adolescents and adults. Two Tdap products are available: one is licensed for those ages 10 to 64 (Boostrix), the other for ages 11 to 64 (Adacel). Since 2005, the ACIP has recommended a single dose of Tdap for those age 11 to 18, preferably at 11 or 12 years.12 The optimal interval from the last tetanus-diphtheria shot is 5 years, but a shorter interval is acceptable. Thereafter, boosters with the tetanus toxoid and reduced-dose diphtheria toxoid (Td) vaccine are recommended every 10 years. If an adolescent has not previously received a complete series of a tetanus-diphtheria product, he or she should be given the recommended number of doses, only one of which should be Tdap, the others Td. The number and timing of doses can be found at www.cdc.gov/mmwr/preview/mmwrhtml/rr55e223a5.htm.
Human papillomavirus vaccination for girls age 11–12
HPV is sexually transmitted and causes genital warts, cervical cancer, and other oral, anal, and genital cancers.
HPV is the most common sexually transmitted infection in the United States, with over 6 million new cases each year.13 A study in 2003 to 2004 using HPV DNA typing of cervicovaginal swab specimens in a sample of women between the ages of 14 and 59 found an overall point prevalence of 26.8% of any HPV type.14 Those between 20 and 24 years had the highest prevalence at 44.8%. Those ages 14 to 19 had a prevalence of 24.5%. Several studies have reported a similar age-related increase in HPV prevalence.15,16
One survey found that nearly 25% of girls in the United States are sexually active by age 15, 40% by age 16, and 70% by age 18.17 The 2005 Behavioral Risk Survey found that nearly 4% of girls were sexually active before age 13, and by the ninth grade 5.7% of those who were sexually active had had four or more partners.18 To receive the full benefit from the HPV vaccine, it should be given before this risk of acquiring HPV occurs.
A quadrivalent HPV vaccine (HPV4) was first licensed in the United States in 2006 for use in girls and women 9 to 26 years old to prevent cervical, vulvar, and vaginal precancerous lesions and cancer, and for prevention of anogenital warts. It contains viral proteins from HPV types 6, 11, 16, and 18, the types currently responsible for 70% of cervical cancers and 90% of anogenital warts. The vaccine is prepared in a yeast substrate and contains an aluminum-based adjuvant.
HPV4 has proven highly effective in women ages 16 to 26 not previously exposed to the four HPV types in the vaccine. The end points used in these studies were cervical intraepithelial neoplasia grade 2 or 3, adenocarcinoma in situ, anogenital warts, and vulvar and vaginal intraepithelial neoplasms.13,19,20 The vaccine’s effectiveness has been 98% to 100% after 3 to 5 years. These trials are ongoing.
The vaccine’s efficacy in women with current or past HPV infection is less certain. Studies of this question have included only small numbers, and the confidence intervals are large and include 0. In intention-to-treat studies, its efficacy has been 39% to 46% for prevention of cervical intraepithelial neoplasia grade 2 or 3 or adenocarcinoma in situ caused by HPV-16 or HPV-18, 69% for prevention of HPV-16- or HPV-18-related vaginal intraepithelial neoplasia, and 68.5% for vaccine-type-related warts.13
The most common adverse effects of HPV4 have included redness, pain, and swelling at the injection site, which occur in about 20% of recipients.13 There is an increased risk of syncope immediately after the vaccine is given, and observation for 15 minutes after injection is recommended. A recent study suggested a link between the vaccine and venous thromboembolism. 21 The rate was 2 per million doses, and because many of the recipients also were taking oral contraceptives, their venous thromboembolism has not yet been definitively proven to be caused by the vaccine.
HPV4 is contraindicated in those who have experienced a severe allergic reaction to a previous dose or who have an allergy to a vaccine component. Vaccination should be deferred in those with moderate or severe acute illnesses.
In June 2006, the ACIP13 made the following recommendations for HPV4:
- Girls ages 11 to 12 years should be routinely vaccinated with three doses
- The series can start as early as age 9 years
- Women and girls age 13 to 26 who have not been previously vaccinated should receive catch-up vaccination
- Neither Papanicolaou (Pap) testing nor HPV screening is necessary before vaccination
- HPV4 can be given with other age-appropriate vaccines
- Vaccination does not change the recommendations for cervical cancer screening
- The recommendations remain the same regardless of abnormal Pap tests, positive HPV DNA tests, or warts.
There have been two very recent developments regarding HPV vaccines.
A bivalent vaccine (HPV2) has been licensed in the United States and approved for use in girls and women ages 10 to 25 for prevention of cervical cancer and precancerous lesions. It contains antigens against HPV-16 and HPV-18 but does not provide protection against genital warts. The ACIP has stated no preference for the bivalent or the quadrivalent vaccine for the prevention of cervical cancer and precancerous lesions.
HPV4 has also gained licensure for use in boys and men age 9 to 26 for the prevention of genital warts. The ACIP has not recommended it for routine use, leaving the decision to patients and physicians after weighing the potential benefits and costs.
VACCINE UPDATE FOR ADULTS
Four vaccines are now routinely recommended for adults:
- Seasonal influenza vaccine starting at age 50
- Pneumococcal polysaccharide vaccine (PPSV23) starting at age 65
- Herpes zoster vaccine starting at age 60
- A diphtheria and tetanus toxoid product every 10 years, with Tdap given once.22
The rest of the adult schedule is based on catch-up (measles, mumps, rubella, varicella) or risk (hepatitis A and B and meningococccal disease). Seasonal influenza and pneumococcal vaccinations are also recommended before ages 50 and 65, respectively, for those with certain risk conditions. The complete adult immunization schedule can be found on the US Centers for Disease Control and Prevention (CDC) Web site.22
One dose of Tdap instead of the next Td booster
The CDC now recommends that a single dose of Tdap should replace the next dose of Td for adults ages 19 to 64 as part of the every-10-year tetanus-diphtheria boosting recommendation and if indicated for wound management. 23 In addition, a single dose of Tdap should be given to adults who have close contact with infants less than 6 months of age. The optimal interval between this Tdap shot and the last Td booster is 2 years or greater, but shorter intervals are acceptable. Women of childbearing age should receive Tdap preconception or postpartum if they have not previously received it. Tdap is not approved for use during pregnancy. Health care workers should also receive a dose of Tdap if they have never received it previously and if their last Td booster was more than 2 years ago, although less than 2 years is acceptable.
Contraindications to Tdap include anaphylaxis to a vaccine component and encephalopathy occurring within 7 days of previously receiving a pertussis vaccine.
Herpes zoster vaccine for those age 60 and older
Shingles causes considerable morbidity in older adults. The lifetime risk is 25%, and onefourth of those with shingles develop postherpetic neuralgia.
Herpes zoster vaccine is a live-attenuated vaccine that requires only a single injection. It is licensed for use in those ages 60 and older, and the ACIP recommends its routine use.24 Its effectiveness is approximately 50% and is inversely related to age. The number of patients who need to be vaccinated to prevent one lifetime case of shingles is 17.
Contraindications to this vaccine include a prior anaphylactic reaction to gelatin or neomycin, compromised immunity due to disease or to immune-suppressive therapy including high-dose corticosteroids, and active tuberculosis.
Payment for this vaccine by Medicare is through Part D, creating some administrative difficulties for physicians’ offices.
Pneumococcal vaccination extended to smokers and people with asthma
The ACIP recently added two new groups for whom PPSV23 is recommended: smokers and those with asthma.25 Smoking poses as much of a risk for pneumococcal pneumonia as do diabetes and other chronic illnesses that are currently indications for the vaccine. The number needed to vaccinate to prevent one case of pneumonia among smokers is 10,000 in people ages 18 to 44, and 4,000 in those ages 45 to 64.26
The ACIP also clarified the recommendation for a second dose of PPSV23.25 A second dose 5 years after the first is recommended for those who have immune suppression, sickle cell disease, or asplenia. People over age 65 should receive a second dose if they were vaccinated more than 5 years previously and before age 65.
New uses for hepatitis A vaccine
A combined hepatitis A and hepatitis B vaccine (Twinrix) has received approval for an alternate, four-dose schedule at 0, 7, 21 days, and 12 months.27 It has previously only been approved for a three-dose schedule at 0, 1, and 6 months. The new alternative schedule allows greater protection for travelers who need to depart within less than 1 month.
For unvaccinated people who are acutely exposed to hepatitis A virus and for those traveling to areas of high prevalence who do not have time to complete the two doses of hepatitis A vaccine, the only prevention available until recently has been immune globulin. This has changed: hepatitis A vaccine can now be used in both groups. The new recommendations for postexposure prophylaxis is that either a single dose of hepatitis A vaccine or use of immune globulin is acceptable.28 In ages 12 months to 40 years, vaccine is preferred. For those over age 40, immune globulin is preferred, but vaccine is acceptable. For children younger than 12 months, the immune-suppressed, and those with chronic liver disease, immune globulin should be used.
Those traveling or working in countries with high rates of hepatitis A can be protected with either hepatitis A vaccine or immune globulin. A single dose of the vaccine is sufficient for healthy people, with a second dose at the recommended interval to complete the series. Those younger than 12 months and those who choose not to receive the vaccine, including those who are allergic to it, should be offered immune globulin. Both immune globulin and hepatitis A vaccine should be considered for certain patients who plan to travel within 2 weeks of the first vaccine dose, ie, those over age 40, those with compromised immunity, and those with chronic liver disease or other chronic conditions.
Hepatitis A vaccine is now also recommended for all unvaccinated people who anticipate close personal contact with an international adoptee during the first 60 days following arrival from countries with high or intermediate hepatitis A endemicity.29 The first dose should be given as soon as the adoption is planned and ideally at least 2 weeks before the child arrives.
- Centers for Disease Control and Prevention (CDC). Recommended immunization schedule for persons aged 0 through 6 years—United States 2009. www.cdc.gov/vaccines/recs/schedules/downloads/child/2009/09_0-6yrs_schedule_pr.pdf. Accessed March 6, 2010.
- Cortese MM, Parashar UDCenters for Disease Control and Prevention (CDC). Prevention of rotavirus gastroenteritis among infants and children: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2009; 58( RR-2):1–25.
- Fiore AE, Shay DK, Broder K, et al., Centers for Disease Control and Prevention (CDC). Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2009. MMWR Recomm Rep 2009; 58( RR–8):1–52.
- Centers for Disease Control and Prevention (CDC). Notice to readers: expansion of use of live attenuated influenza vaccine (FluMist®) to children aged 2–4 years and other FluMist changes for the 2007–08 influenza season. MMWR Morb Mortal Wkly Rep 2007; 56( 46):1217–1219.
- Fiore AE, Shay DK, Haber PCenters for Disease Control and Prevention (CDC). Prevention and control of influenza: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2007. MMWR Recomm Rep 2007; 56( RR–6):1–54.
- Fiore AE, Wasley A, Bell BPAdvisory Committee on Immunization Practices (ACIP). Prevention of hepatitis A through active or passive immunization: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2006; 55( RR–7):1–23.
- Marin M, Güris D, Chaves SS, Schmid S, Seward JFAdvisory Committee on Immunization Practices. Prevention of varicella: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2007; 56( RR–4):1–40.
- Centers for Disease Control and Prevention (CDC). Varicella disease. www.cdc.gov/vaccines/vpd-vac/varicella/dis-faqs-clinic.htm. Accessed March 4, 2010.
- Centers for Disease Control and Prevention (CDC). 2009 child & adolescent immunization schedules. www.cdc.gov/vaccines/recs/schedules/child-schedule.htm. Accessed March 4, 2010.
- Centers for Disease Control and Prevention (CDC) Advisory Committee on Immunization Practices. Revised recommendations of the Advisory Committee on Immunization Practices to vaccinate all persons aged 11-18 years with meningococcal conjugate vaccine. MMWR Morb Mortal Wkly Rep 2007; 56( 31):794–795.
- Centers for Disease Control and Prevention (CDC). Update: Guillain-Barré syndrome among recipients of menactra meningococcal conjugate vaccine—United States, June 2005–September 2006. MMWR Morb Mortal Wkly Rep 2006; 55( 41):1120–1124.
- Broder KR, Cortese MM, Iskander JK, et al., Advisory Committee on Immunization Practices (ACIP). Preventing tetanus, diphtheria, and pertussis among adolescents: use of tetanus toxoid, reduced diphtheria toxoid and acellular pertussis vaccines recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2006; 55( RR–3):1–34.
- Markowitz LE, Dunne EF, Saraiya M, Lawson HW, Chesson H, Unger ERCenters for Disease Control and Prevention (CDC). Quadrivalent human papillomavirus vaccine: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2007; 56( RR–2):1–24.
- Dunne EF, Unger ER, Sternberg M, et al Prevalence of HPV infection among females in the United States. JAMA 2007; 297:813–819.
- Trottier H, Franco EL. The epidemiology of genital human papillomavirus infection. Vaccine 2006; 24( suppl 1):S1–S15.
- Stone KM, Karem KL, Sternberg MR, et al Seroprevalence of human papillomavirus type 16 infection in the United States. J Infect Dis 2002; 186:1396–1402.
- Abma JC, Martinez GM, Mosher WD, Dawson BS. Teenagers in the United States: sexual activity, contraceptive use, and childbearing, 2002. Vital Health Stat 23 2004; 24:1–48.
- Eaton DK, Kann L, Kinchen S, et al Youth risk behavior surveillance—United States, 2005. MMWR Surveill Summ 2006; 55:1–108.
- Human papillomavirus vaccines. WHO position paper. Wkly Epidemiol Rec 2009; 84:118–131.
- Rambout L, Hopkins L, Hutton B, Fergusson D. Prophylactic vaccination against human papillomavirus infection and disease in women: a systematic review of randomized controlled trials. CMAJ 2007; 177:469–479.
- Slade BA, Leidel L, Vellozzi C, et al Postlicensure safety surveillance for quadrivalent human papillomavirus recombinant vaccine. JAMA 2009; 302:750–757.
- Centers for Disease Control (CDC). Adult immunization schedule. http://www.cdc.gov/vaccines/recs/schedules/adult-schedule.htm. Accessed March 4, 2010.
- Kretsinger K, Broder KR, Cortese MM, et al., Centers for Disease Control and Prevention. Preventing tetanus, diphtheria, and pertussis among adults: use of tetanus toxoid, reduced diphtheria toxoid and acellular pertussis vaccine recommendations of the Advisory Committee on Immunization Practices (ACIP) and recommendation of ACIP, supported by the Healthcare Infection Control Practices Advisory Committee (HICPAC), for use of Tdap among health-care personnel. MMWR Recomm Rep 2006; 55( RR–17):1–37.
- Harpaz R, Ortega-Sanchez IR, Seward JFAdvisory Committee on Immunization Practices (ACIP). Prevention of herpes zoster: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2008; 57( RR–5):1–30.
- Centers for Disease Control (CDC). ACIP provisional recommendations for use of pneumococcal vaccines. www.cdc.gov/vaccines/recs/provisional/downloads/pneumo-Oct-2008-508.pdf. Accessed March 4, 2010.
- Centers for Disease Control and Prevention (CDC). Summary Report: October 22–23, 2008; Atlanta, Georgia. www.cdc.gov/vaccines/recs/ACIP/downloads/min=archive/min-oct08.pdf. Accessed March 6, 2010.
- CDC. Notice to readers: FDA approval of an alternate dosing schedule for a combined hepatitis A and B vaccine (Twinrix®). MMWR Morb Mortal Wkly Rep 2007; 56( 40);1057.
- Advisory Committee on Immunization Practices (ACIP) Centers for Disease Control and Prevention (CDC). Update: prevention of hepatitis A after exposure to hepatitis A virus and in international travelers. Updated recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 2007; 56( 41):1080–1084.
- Centers for Disease Control and Prevention (CDC). Updated recommendations from the Advisory Committee on Immunization Practices (ACIP) for use of hepatitis A vaccine in close contacts of newly arriving international adoptees. MMWR 2009: 58:1006–1007.
- Centers for Disease Control and Prevention (CDC). Recommended immunization schedule for persons aged 0 through 6 years—United States 2009. www.cdc.gov/vaccines/recs/schedules/downloads/child/2009/09_0-6yrs_schedule_pr.pdf. Accessed March 6, 2010.
- Cortese MM, Parashar UDCenters for Disease Control and Prevention (CDC). Prevention of rotavirus gastroenteritis among infants and children: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2009; 58( RR-2):1–25.
- Fiore AE, Shay DK, Broder K, et al., Centers for Disease Control and Prevention (CDC). Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2009. MMWR Recomm Rep 2009; 58( RR–8):1–52.
- Centers for Disease Control and Prevention (CDC). Notice to readers: expansion of use of live attenuated influenza vaccine (FluMist®) to children aged 2–4 years and other FluMist changes for the 2007–08 influenza season. MMWR Morb Mortal Wkly Rep 2007; 56( 46):1217–1219.
- Fiore AE, Shay DK, Haber PCenters for Disease Control and Prevention (CDC). Prevention and control of influenza: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2007. MMWR Recomm Rep 2007; 56( RR–6):1–54.
- Fiore AE, Wasley A, Bell BPAdvisory Committee on Immunization Practices (ACIP). Prevention of hepatitis A through active or passive immunization: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2006; 55( RR–7):1–23.
- Marin M, Güris D, Chaves SS, Schmid S, Seward JFAdvisory Committee on Immunization Practices. Prevention of varicella: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2007; 56( RR–4):1–40.
- Centers for Disease Control and Prevention (CDC). Varicella disease. www.cdc.gov/vaccines/vpd-vac/varicella/dis-faqs-clinic.htm. Accessed March 4, 2010.
- Centers for Disease Control and Prevention (CDC). 2009 child & adolescent immunization schedules. www.cdc.gov/vaccines/recs/schedules/child-schedule.htm. Accessed March 4, 2010.
- Centers for Disease Control and Prevention (CDC) Advisory Committee on Immunization Practices. Revised recommendations of the Advisory Committee on Immunization Practices to vaccinate all persons aged 11-18 years with meningococcal conjugate vaccine. MMWR Morb Mortal Wkly Rep 2007; 56( 31):794–795.
- Centers for Disease Control and Prevention (CDC). Update: Guillain-Barré syndrome among recipients of menactra meningococcal conjugate vaccine—United States, June 2005–September 2006. MMWR Morb Mortal Wkly Rep 2006; 55( 41):1120–1124.
- Broder KR, Cortese MM, Iskander JK, et al., Advisory Committee on Immunization Practices (ACIP). Preventing tetanus, diphtheria, and pertussis among adolescents: use of tetanus toxoid, reduced diphtheria toxoid and acellular pertussis vaccines recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2006; 55( RR–3):1–34.
- Markowitz LE, Dunne EF, Saraiya M, Lawson HW, Chesson H, Unger ERCenters for Disease Control and Prevention (CDC). Quadrivalent human papillomavirus vaccine: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2007; 56( RR–2):1–24.
- Dunne EF, Unger ER, Sternberg M, et al Prevalence of HPV infection among females in the United States. JAMA 2007; 297:813–819.
- Trottier H, Franco EL. The epidemiology of genital human papillomavirus infection. Vaccine 2006; 24( suppl 1):S1–S15.
- Stone KM, Karem KL, Sternberg MR, et al Seroprevalence of human papillomavirus type 16 infection in the United States. J Infect Dis 2002; 186:1396–1402.
- Abma JC, Martinez GM, Mosher WD, Dawson BS. Teenagers in the United States: sexual activity, contraceptive use, and childbearing, 2002. Vital Health Stat 23 2004; 24:1–48.
- Eaton DK, Kann L, Kinchen S, et al Youth risk behavior surveillance—United States, 2005. MMWR Surveill Summ 2006; 55:1–108.
- Human papillomavirus vaccines. WHO position paper. Wkly Epidemiol Rec 2009; 84:118–131.
- Rambout L, Hopkins L, Hutton B, Fergusson D. Prophylactic vaccination against human papillomavirus infection and disease in women: a systematic review of randomized controlled trials. CMAJ 2007; 177:469–479.
- Slade BA, Leidel L, Vellozzi C, et al Postlicensure safety surveillance for quadrivalent human papillomavirus recombinant vaccine. JAMA 2009; 302:750–757.
- Centers for Disease Control (CDC). Adult immunization schedule. http://www.cdc.gov/vaccines/recs/schedules/adult-schedule.htm. Accessed March 4, 2010.
- Kretsinger K, Broder KR, Cortese MM, et al., Centers for Disease Control and Prevention. Preventing tetanus, diphtheria, and pertussis among adults: use of tetanus toxoid, reduced diphtheria toxoid and acellular pertussis vaccine recommendations of the Advisory Committee on Immunization Practices (ACIP) and recommendation of ACIP, supported by the Healthcare Infection Control Practices Advisory Committee (HICPAC), for use of Tdap among health-care personnel. MMWR Recomm Rep 2006; 55( RR–17):1–37.
- Harpaz R, Ortega-Sanchez IR, Seward JFAdvisory Committee on Immunization Practices (ACIP). Prevention of herpes zoster: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2008; 57( RR–5):1–30.
- Centers for Disease Control (CDC). ACIP provisional recommendations for use of pneumococcal vaccines. www.cdc.gov/vaccines/recs/provisional/downloads/pneumo-Oct-2008-508.pdf. Accessed March 4, 2010.
- Centers for Disease Control and Prevention (CDC). Summary Report: October 22–23, 2008; Atlanta, Georgia. www.cdc.gov/vaccines/recs/ACIP/downloads/min=archive/min-oct08.pdf. Accessed March 6, 2010.
- CDC. Notice to readers: FDA approval of an alternate dosing schedule for a combined hepatitis A and B vaccine (Twinrix®). MMWR Morb Mortal Wkly Rep 2007; 56( 40);1057.
- Advisory Committee on Immunization Practices (ACIP) Centers for Disease Control and Prevention (CDC). Update: prevention of hepatitis A after exposure to hepatitis A virus and in international travelers. Updated recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 2007; 56( 41):1080–1084.
- Centers for Disease Control and Prevention (CDC). Updated recommendations from the Advisory Committee on Immunization Practices (ACIP) for use of hepatitis A vaccine in close contacts of newly arriving international adoptees. MMWR 2009: 58:1006–1007.
KEY POINTS
- New recommendations for infants and children:
- Rotavirus vaccination for infants
- Seasonal influenza vaccine yearly at ages 5–18
- Hepatitis A vaccine at age 12–23 months
- Varicella vaccine at 12–15 months and again at 4–6 years, with catch-up for others.
- New recommendations for adolescents:
- Meningococcus quadrivalent conjugate vaccine for all at age 11 or 12 and catch-up through age 18
- A shot of tetanus toxoid, reduced-dose diphtheria toxoid, and acellular pertussis vaccine (Tdap) at age 11 or 12 and catch-up through age 18
- Human papillomavirus vaccine (three doses) for girls at age 11 or 12 and catch-up through age 26.
- New recommendations for adults:
- One dose of Tdap instead of the next tetanus-diphtheria booster
- Herpes zoster vaccine at age 60 or older
- Pneumococcal vaccination extended to smokers and people with asthma, with a second dose 5 years after the first for people who have immune suppression, sickle cell disease, or asplenia.