User login
Aquatic Antagonists: Sea Cucumbers (Holothuroidea)
Sea cucumbers—commonly known as trepang in Indonesia, namako in Japan, and hai shen in China, where they are treasured as a food delicacy—are sea creatures belonging to the phylum Echinodermata, class Holothuridea, and family Cucumariidae . 1,2 They are an integral part of a variety of marine habitats, serving as cleaners as they filter through sediment for nutrients. They can be found on the ocean floor under hundreds of feet of water or in shallow sandy waters along the coast, but they most commonly are found living among coral reefs. Sea cucumbers look just as they sound—shaped like cucumbers or sausages, ranging from under 1 inch to upwards of 6 feet in length depending on the specific species (Figure 1). They have a group of tentacles around the mouth used for filtering sediment, and they move about the ocean floor on tubular feet protruding through the body wall, similar to a sea star.
Beneficial Properties and Cultural Relevance
Although more than 1200 species of sea cucumbers have been identified thus far, only about 20 of these are edible.2 The most common of the edible species is Stichopus japonicus, which can be found off the coasts of Korea, China, Japan, and Russia. This particular species most commonly is used in traditional dishes and is divided into 3 groups based on the color: red, green, or black. The price and taste of sea cucumbers varies based on the color, with red being the most expensive.2 The body wall of the sea cucumber is cleaned, repeatedly boiled, and dried until edible. It is considered a delicacy, not only in food but also in pharmaceutical forms, as it is comprised of a variety of vitamins, minerals, and other nutrients that are thought to provide anticancer, anticoagulant, antioxidant, antifungal, and anti-inflammatory properties. Components of the body wall include collagen, mucopolysaccharides, peptides, gelatin, glycosaminoglycans, glycosides (including various holotoxins), hydroxylates, saponins, and fatty acids.2 The regenerative properties of the sea cucumber also are important in future biomedical developments.
Toxic Properties
Although sea cucumbers have proven to have many beneficial properties, at least 30 species also produce potent toxins that pose a danger to both humans and other wildlife.3 The toxins are collectively referred to as holothurin; however, specific species actually produce a variety of holothurin toxins with unique chemical structures. Each toxin is a variation of a specific triterpene glycoside called saponins, which are common glycosides in the plant world. Holothurin was the first saponin to be found in animals. The only animals known to contain holothurin are the echinoderms, including sea cucumbers and sea stars.1 Holothurins A and B are the 2 groups of holothurin toxins produced specifically by sea cucumbers. The toxins are composed of roughly 60% glycosides and pigment; 30% free amino acids (alanine, arginine, cysteine, glycine, glutamic acid, histidine, serine, and valine); 5% to 10% insoluble proteins; and 1% cholesterol, salts, and polypeptides.3
Holothurins are concentrated in granules within specialized structures of the sea cucumber called Cuvierian tubules, which freely float in the posterior coelomic cavity of the sea cucumber and are attached at the base of the respiratory tree. It is with these tubules that sea cucumbers utilize a unique defensive mechanism. Upon disturbance, the sea cucumber will turn its posterior end to the threat and squeeze its body in a series of violent contractions, inducing a tear in the cloacal wall.4 The tubules pass through this tear, are autotomized from the attachment point at the respiratory tree, and are finally expelled through the anus onto the predator and into the surrounding waters. The tubules are both sticky on contact and poisonous due to the holothurin, allowing the sea cucumber to crawl away from the threat unscathed. Over time, the tubules will regenerate, allowing the sea cucumber to protect itself again in the face of future danger.
Aside from direct disturbance by a threat, sea cucumbers also are known to undergo evisceration due to high temperatures and oxygen deficiency.3 Species that lack Cuvierian tubules can still produce holothurin toxins, though the toxins are secreted onto the outer surface of the body wall and mainly pose a risk with direct contact undiluted by seawater.5 The toxin induces a neural blockade in other sea creatures through its interaction with ion channels. On Asian islands, sea cucumbers have been exploited for this ability and commonly are thrown into tidal pools by fishermen to paralyze fish for easier capture.1
Effects on Human Skin
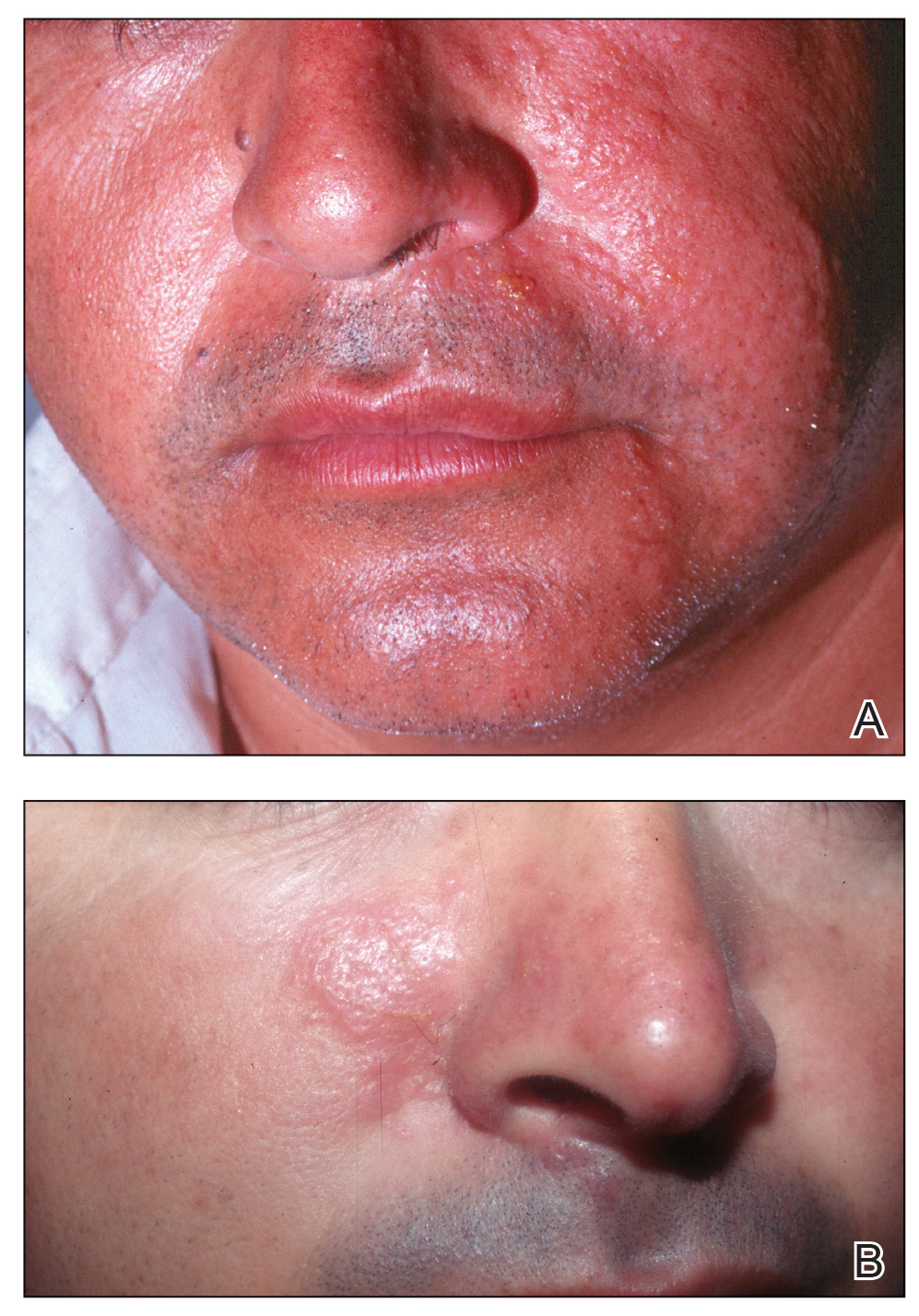
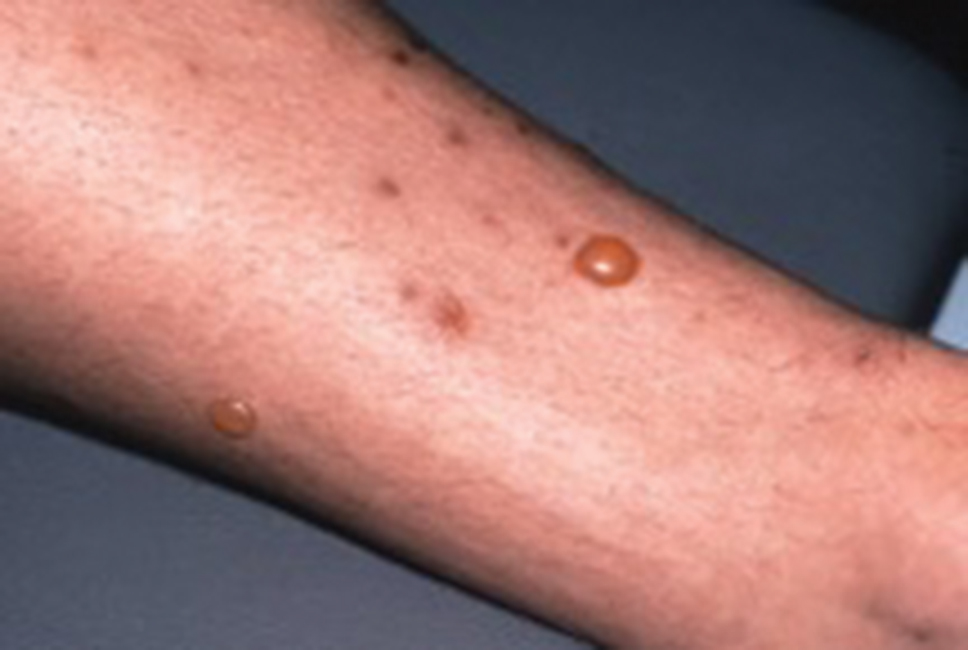
In humans, the holothurin toxins of sea cucumbers cause an acute irritant dermatitis upon contact with the skin.6 Fishermen or divers handling sea cucumbers without gloves may present with an irritant contact dermatitis characterized by marked erythema and swelling (Figure 2).6-8 Additionally, holothurin toxins can cause irritation of the mucous membranes of the eyes and mouth. Contact with the mucous membranes of the eyes can induce a painful conjunctivitis that may result in blindness.6,8 Ingestion of large quantities of sea cucumber can produce an anticoagulant effect, and toxins in some species act similar to cardiac glycosides.3,9
In addition to their own toxins, sea cucumbers also can secrete undigested nematocysts of previously consumed cnidarians through the integument.7,10 In this case, the result of direct contact with the body wall is similar to a jellyfish sting in addition to the irritant contact dermatitis caused by the holothurin toxin.
Treatment and Prevention
Irritant dermatitis resulting from contact with a holothurin toxin is first treated with cleansing of the affected area at the time of exposure with generous amounts of seawater or preferably hot seawater and soap. Most marine toxins are inactivated by heat, but holothurin is partially heat stable. Vinegar or isopropyl alcohol also have been used.9 The result is removal of the slime containing the holothurin toxin rather than deactivation of the toxin. Although this alone may relieve symptoms, dermatitis also may be addressed with topical anesthetics, corticosteroids, or, if a severe reaction has occurred, systemic steroids.9
Conjunctivitis should be addressed with copious irrigation with tap water and topical anesthesia. Following proper irrigation, providers may choose to follow up with fluorescein staining to rule out corneal injury.10
The dermatologic effects of holothurin toxins can be prevented with the use of gloves and diving masks or goggles. Proper protective wear should be utilized not only when directly handling sea cucumbers but also when swimming in water where sea cucumbers may be present. Systemic toxicity can be prevented by proper cooking, as holothurin toxins are only partially heat resistant and also are hydrolyzed into nontoxic products by gastric acid. Additionally, the species of the sea cucumber should be confirmed prior to consumption, as edible species are known to contain less toxin.1
Conclusion
Although sea cucumbers have ecologic, culinary, and pharmaceutical value, they also can pose a threat to both humans and wildlife. The holothurin toxins produced by sea cucumbers cause a painful contact dermatitis and can lead to conjunctivitis and even blindness following eye exposure. Although the toxin is broken down into nontoxic metabolites by gastric acid, large amounts of potent variants can induce systemic effects. Individuals who come in contact with sea cucumbers, such as fishermen and divers, should utilize proper protection including gloves and protective eyewear.
- Burnett K, Fenner P, Williamson J. Venomous and Poisonous Marine Animals: A Medical and Biological Handbook. University of New South Wales Press; 1996.
- Oh GW, Ko SC, Lee DH, et al. Biological activities and biomedical potential of sea cucumber (Stichopus japonicus): a review. Fisheries Aquatic Sci. 2017;20:28.
- Nigrelli RF, Jakowska S. Effects of holothurian, a steroid saponin from the Bahamian sea cucumber (Actinopyga agassizi), on various biological systems. Ann NY Acad Sci. 1960;90:884-892.
- Demeuldre M, Hennebert E, Bonneel M, et al. Mechanical adaptability of sea cucumber Cuvierian tubules involves a mutable collagenous tissue. J Exp Biol. 2017;220:2108-2119.
- Matranga V, ed. Echinodermata: Progress in Molecular and Subcellular Biology. Springer; 2005.
- Tlougan, BE, Podjasek, JO, Adams BB. Aquatic sports dermatoses. part 2—in the water: saltwater dermatoses. Int J Dermatol. 2010;49:994-1002.
- Bonamonte D, Verni P, Filoni A, et al. Dermatitis caused by echinoderms. In: Bonamonte D, Angelini G, eds. Springer; 2016:59-72.
- Haddad V Jr. Medical Emergencies Caused by Aquatic Animals: A Zoological and Clinical Guide. Springer International Publishing; 2016.
- French LK, Horowitz BZ. Marine vertebrates, cnidarians, and mollusks. In: Brent J, Burkhart K, Dargan P, et al, eds. Critical Care Toxicology. Springer; 2017:1-30.
- Smith ML. Skin problems from marine echinoderms. Dermatol Ther. 2002;15:30-33.
Sea cucumbers—commonly known as trepang in Indonesia, namako in Japan, and hai shen in China, where they are treasured as a food delicacy—are sea creatures belonging to the phylum Echinodermata, class Holothuridea, and family Cucumariidae . 1,2 They are an integral part of a variety of marine habitats, serving as cleaners as they filter through sediment for nutrients. They can be found on the ocean floor under hundreds of feet of water or in shallow sandy waters along the coast, but they most commonly are found living among coral reefs. Sea cucumbers look just as they sound—shaped like cucumbers or sausages, ranging from under 1 inch to upwards of 6 feet in length depending on the specific species (Figure 1). They have a group of tentacles around the mouth used for filtering sediment, and they move about the ocean floor on tubular feet protruding through the body wall, similar to a sea star.
Beneficial Properties and Cultural Relevance
Although more than 1200 species of sea cucumbers have been identified thus far, only about 20 of these are edible.2 The most common of the edible species is Stichopus japonicus, which can be found off the coasts of Korea, China, Japan, and Russia. This particular species most commonly is used in traditional dishes and is divided into 3 groups based on the color: red, green, or black. The price and taste of sea cucumbers varies based on the color, with red being the most expensive.2 The body wall of the sea cucumber is cleaned, repeatedly boiled, and dried until edible. It is considered a delicacy, not only in food but also in pharmaceutical forms, as it is comprised of a variety of vitamins, minerals, and other nutrients that are thought to provide anticancer, anticoagulant, antioxidant, antifungal, and anti-inflammatory properties. Components of the body wall include collagen, mucopolysaccharides, peptides, gelatin, glycosaminoglycans, glycosides (including various holotoxins), hydroxylates, saponins, and fatty acids.2 The regenerative properties of the sea cucumber also are important in future biomedical developments.
Toxic Properties
Although sea cucumbers have proven to have many beneficial properties, at least 30 species also produce potent toxins that pose a danger to both humans and other wildlife.3 The toxins are collectively referred to as holothurin; however, specific species actually produce a variety of holothurin toxins with unique chemical structures. Each toxin is a variation of a specific triterpene glycoside called saponins, which are common glycosides in the plant world. Holothurin was the first saponin to be found in animals. The only animals known to contain holothurin are the echinoderms, including sea cucumbers and sea stars.1 Holothurins A and B are the 2 groups of holothurin toxins produced specifically by sea cucumbers. The toxins are composed of roughly 60% glycosides and pigment; 30% free amino acids (alanine, arginine, cysteine, glycine, glutamic acid, histidine, serine, and valine); 5% to 10% insoluble proteins; and 1% cholesterol, salts, and polypeptides.3
Holothurins are concentrated in granules within specialized structures of the sea cucumber called Cuvierian tubules, which freely float in the posterior coelomic cavity of the sea cucumber and are attached at the base of the respiratory tree. It is with these tubules that sea cucumbers utilize a unique defensive mechanism. Upon disturbance, the sea cucumber will turn its posterior end to the threat and squeeze its body in a series of violent contractions, inducing a tear in the cloacal wall.4 The tubules pass through this tear, are autotomized from the attachment point at the respiratory tree, and are finally expelled through the anus onto the predator and into the surrounding waters. The tubules are both sticky on contact and poisonous due to the holothurin, allowing the sea cucumber to crawl away from the threat unscathed. Over time, the tubules will regenerate, allowing the sea cucumber to protect itself again in the face of future danger.
Aside from direct disturbance by a threat, sea cucumbers also are known to undergo evisceration due to high temperatures and oxygen deficiency.3 Species that lack Cuvierian tubules can still produce holothurin toxins, though the toxins are secreted onto the outer surface of the body wall and mainly pose a risk with direct contact undiluted by seawater.5 The toxin induces a neural blockade in other sea creatures through its interaction with ion channels. On Asian islands, sea cucumbers have been exploited for this ability and commonly are thrown into tidal pools by fishermen to paralyze fish for easier capture.1
Effects on Human Skin
In humans, the holothurin toxins of sea cucumbers cause an acute irritant dermatitis upon contact with the skin.6 Fishermen or divers handling sea cucumbers without gloves may present with an irritant contact dermatitis characterized by marked erythema and swelling (Figure 2).6-8 Additionally, holothurin toxins can cause irritation of the mucous membranes of the eyes and mouth. Contact with the mucous membranes of the eyes can induce a painful conjunctivitis that may result in blindness.6,8 Ingestion of large quantities of sea cucumber can produce an anticoagulant effect, and toxins in some species act similar to cardiac glycosides.3,9
In addition to their own toxins, sea cucumbers also can secrete undigested nematocysts of previously consumed cnidarians through the integument.7,10 In this case, the result of direct contact with the body wall is similar to a jellyfish sting in addition to the irritant contact dermatitis caused by the holothurin toxin.
Treatment and Prevention
Irritant dermatitis resulting from contact with a holothurin toxin is first treated with cleansing of the affected area at the time of exposure with generous amounts of seawater or preferably hot seawater and soap. Most marine toxins are inactivated by heat, but holothurin is partially heat stable. Vinegar or isopropyl alcohol also have been used.9 The result is removal of the slime containing the holothurin toxin rather than deactivation of the toxin. Although this alone may relieve symptoms, dermatitis also may be addressed with topical anesthetics, corticosteroids, or, if a severe reaction has occurred, systemic steroids.9
Conjunctivitis should be addressed with copious irrigation with tap water and topical anesthesia. Following proper irrigation, providers may choose to follow up with fluorescein staining to rule out corneal injury.10
The dermatologic effects of holothurin toxins can be prevented with the use of gloves and diving masks or goggles. Proper protective wear should be utilized not only when directly handling sea cucumbers but also when swimming in water where sea cucumbers may be present. Systemic toxicity can be prevented by proper cooking, as holothurin toxins are only partially heat resistant and also are hydrolyzed into nontoxic products by gastric acid. Additionally, the species of the sea cucumber should be confirmed prior to consumption, as edible species are known to contain less toxin.1
Conclusion
Although sea cucumbers have ecologic, culinary, and pharmaceutical value, they also can pose a threat to both humans and wildlife. The holothurin toxins produced by sea cucumbers cause a painful contact dermatitis and can lead to conjunctivitis and even blindness following eye exposure. Although the toxin is broken down into nontoxic metabolites by gastric acid, large amounts of potent variants can induce systemic effects. Individuals who come in contact with sea cucumbers, such as fishermen and divers, should utilize proper protection including gloves and protective eyewear.
Sea cucumbers—commonly known as trepang in Indonesia, namako in Japan, and hai shen in China, where they are treasured as a food delicacy—are sea creatures belonging to the phylum Echinodermata, class Holothuridea, and family Cucumariidae . 1,2 They are an integral part of a variety of marine habitats, serving as cleaners as they filter through sediment for nutrients. They can be found on the ocean floor under hundreds of feet of water or in shallow sandy waters along the coast, but they most commonly are found living among coral reefs. Sea cucumbers look just as they sound—shaped like cucumbers or sausages, ranging from under 1 inch to upwards of 6 feet in length depending on the specific species (Figure 1). They have a group of tentacles around the mouth used for filtering sediment, and they move about the ocean floor on tubular feet protruding through the body wall, similar to a sea star.
Beneficial Properties and Cultural Relevance
Although more than 1200 species of sea cucumbers have been identified thus far, only about 20 of these are edible.2 The most common of the edible species is Stichopus japonicus, which can be found off the coasts of Korea, China, Japan, and Russia. This particular species most commonly is used in traditional dishes and is divided into 3 groups based on the color: red, green, or black. The price and taste of sea cucumbers varies based on the color, with red being the most expensive.2 The body wall of the sea cucumber is cleaned, repeatedly boiled, and dried until edible. It is considered a delicacy, not only in food but also in pharmaceutical forms, as it is comprised of a variety of vitamins, minerals, and other nutrients that are thought to provide anticancer, anticoagulant, antioxidant, antifungal, and anti-inflammatory properties. Components of the body wall include collagen, mucopolysaccharides, peptides, gelatin, glycosaminoglycans, glycosides (including various holotoxins), hydroxylates, saponins, and fatty acids.2 The regenerative properties of the sea cucumber also are important in future biomedical developments.
Toxic Properties
Although sea cucumbers have proven to have many beneficial properties, at least 30 species also produce potent toxins that pose a danger to both humans and other wildlife.3 The toxins are collectively referred to as holothurin; however, specific species actually produce a variety of holothurin toxins with unique chemical structures. Each toxin is a variation of a specific triterpene glycoside called saponins, which are common glycosides in the plant world. Holothurin was the first saponin to be found in animals. The only animals known to contain holothurin are the echinoderms, including sea cucumbers and sea stars.1 Holothurins A and B are the 2 groups of holothurin toxins produced specifically by sea cucumbers. The toxins are composed of roughly 60% glycosides and pigment; 30% free amino acids (alanine, arginine, cysteine, glycine, glutamic acid, histidine, serine, and valine); 5% to 10% insoluble proteins; and 1% cholesterol, salts, and polypeptides.3
Holothurins are concentrated in granules within specialized structures of the sea cucumber called Cuvierian tubules, which freely float in the posterior coelomic cavity of the sea cucumber and are attached at the base of the respiratory tree. It is with these tubules that sea cucumbers utilize a unique defensive mechanism. Upon disturbance, the sea cucumber will turn its posterior end to the threat and squeeze its body in a series of violent contractions, inducing a tear in the cloacal wall.4 The tubules pass through this tear, are autotomized from the attachment point at the respiratory tree, and are finally expelled through the anus onto the predator and into the surrounding waters. The tubules are both sticky on contact and poisonous due to the holothurin, allowing the sea cucumber to crawl away from the threat unscathed. Over time, the tubules will regenerate, allowing the sea cucumber to protect itself again in the face of future danger.
Aside from direct disturbance by a threat, sea cucumbers also are known to undergo evisceration due to high temperatures and oxygen deficiency.3 Species that lack Cuvierian tubules can still produce holothurin toxins, though the toxins are secreted onto the outer surface of the body wall and mainly pose a risk with direct contact undiluted by seawater.5 The toxin induces a neural blockade in other sea creatures through its interaction with ion channels. On Asian islands, sea cucumbers have been exploited for this ability and commonly are thrown into tidal pools by fishermen to paralyze fish for easier capture.1
Effects on Human Skin
In humans, the holothurin toxins of sea cucumbers cause an acute irritant dermatitis upon contact with the skin.6 Fishermen or divers handling sea cucumbers without gloves may present with an irritant contact dermatitis characterized by marked erythema and swelling (Figure 2).6-8 Additionally, holothurin toxins can cause irritation of the mucous membranes of the eyes and mouth. Contact with the mucous membranes of the eyes can induce a painful conjunctivitis that may result in blindness.6,8 Ingestion of large quantities of sea cucumber can produce an anticoagulant effect, and toxins in some species act similar to cardiac glycosides.3,9
In addition to their own toxins, sea cucumbers also can secrete undigested nematocysts of previously consumed cnidarians through the integument.7,10 In this case, the result of direct contact with the body wall is similar to a jellyfish sting in addition to the irritant contact dermatitis caused by the holothurin toxin.
Treatment and Prevention
Irritant dermatitis resulting from contact with a holothurin toxin is first treated with cleansing of the affected area at the time of exposure with generous amounts of seawater or preferably hot seawater and soap. Most marine toxins are inactivated by heat, but holothurin is partially heat stable. Vinegar or isopropyl alcohol also have been used.9 The result is removal of the slime containing the holothurin toxin rather than deactivation of the toxin. Although this alone may relieve symptoms, dermatitis also may be addressed with topical anesthetics, corticosteroids, or, if a severe reaction has occurred, systemic steroids.9
Conjunctivitis should be addressed with copious irrigation with tap water and topical anesthesia. Following proper irrigation, providers may choose to follow up with fluorescein staining to rule out corneal injury.10
The dermatologic effects of holothurin toxins can be prevented with the use of gloves and diving masks or goggles. Proper protective wear should be utilized not only when directly handling sea cucumbers but also when swimming in water where sea cucumbers may be present. Systemic toxicity can be prevented by proper cooking, as holothurin toxins are only partially heat resistant and also are hydrolyzed into nontoxic products by gastric acid. Additionally, the species of the sea cucumber should be confirmed prior to consumption, as edible species are known to contain less toxin.1
Conclusion
Although sea cucumbers have ecologic, culinary, and pharmaceutical value, they also can pose a threat to both humans and wildlife. The holothurin toxins produced by sea cucumbers cause a painful contact dermatitis and can lead to conjunctivitis and even blindness following eye exposure. Although the toxin is broken down into nontoxic metabolites by gastric acid, large amounts of potent variants can induce systemic effects. Individuals who come in contact with sea cucumbers, such as fishermen and divers, should utilize proper protection including gloves and protective eyewear.
- Burnett K, Fenner P, Williamson J. Venomous and Poisonous Marine Animals: A Medical and Biological Handbook. University of New South Wales Press; 1996.
- Oh GW, Ko SC, Lee DH, et al. Biological activities and biomedical potential of sea cucumber (Stichopus japonicus): a review. Fisheries Aquatic Sci. 2017;20:28.
- Nigrelli RF, Jakowska S. Effects of holothurian, a steroid saponin from the Bahamian sea cucumber (Actinopyga agassizi), on various biological systems. Ann NY Acad Sci. 1960;90:884-892.
- Demeuldre M, Hennebert E, Bonneel M, et al. Mechanical adaptability of sea cucumber Cuvierian tubules involves a mutable collagenous tissue. J Exp Biol. 2017;220:2108-2119.
- Matranga V, ed. Echinodermata: Progress in Molecular and Subcellular Biology. Springer; 2005.
- Tlougan, BE, Podjasek, JO, Adams BB. Aquatic sports dermatoses. part 2—in the water: saltwater dermatoses. Int J Dermatol. 2010;49:994-1002.
- Bonamonte D, Verni P, Filoni A, et al. Dermatitis caused by echinoderms. In: Bonamonte D, Angelini G, eds. Springer; 2016:59-72.
- Haddad V Jr. Medical Emergencies Caused by Aquatic Animals: A Zoological and Clinical Guide. Springer International Publishing; 2016.
- French LK, Horowitz BZ. Marine vertebrates, cnidarians, and mollusks. In: Brent J, Burkhart K, Dargan P, et al, eds. Critical Care Toxicology. Springer; 2017:1-30.
- Smith ML. Skin problems from marine echinoderms. Dermatol Ther. 2002;15:30-33.
- Burnett K, Fenner P, Williamson J. Venomous and Poisonous Marine Animals: A Medical and Biological Handbook. University of New South Wales Press; 1996.
- Oh GW, Ko SC, Lee DH, et al. Biological activities and biomedical potential of sea cucumber (Stichopus japonicus): a review. Fisheries Aquatic Sci. 2017;20:28.
- Nigrelli RF, Jakowska S. Effects of holothurian, a steroid saponin from the Bahamian sea cucumber (Actinopyga agassizi), on various biological systems. Ann NY Acad Sci. 1960;90:884-892.
- Demeuldre M, Hennebert E, Bonneel M, et al. Mechanical adaptability of sea cucumber Cuvierian tubules involves a mutable collagenous tissue. J Exp Biol. 2017;220:2108-2119.
- Matranga V, ed. Echinodermata: Progress in Molecular and Subcellular Biology. Springer; 2005.
- Tlougan, BE, Podjasek, JO, Adams BB. Aquatic sports dermatoses. part 2—in the water: saltwater dermatoses. Int J Dermatol. 2010;49:994-1002.
- Bonamonte D, Verni P, Filoni A, et al. Dermatitis caused by echinoderms. In: Bonamonte D, Angelini G, eds. Springer; 2016:59-72.
- Haddad V Jr. Medical Emergencies Caused by Aquatic Animals: A Zoological and Clinical Guide. Springer International Publishing; 2016.
- French LK, Horowitz BZ. Marine vertebrates, cnidarians, and mollusks. In: Brent J, Burkhart K, Dargan P, et al, eds. Critical Care Toxicology. Springer; 2017:1-30.
- Smith ML. Skin problems from marine echinoderms. Dermatol Ther. 2002;15:30-33.
Practice Points
- Sea cucumbers produce a toxin known as holothurin, which is contained in specialized structures called Cuvierian tubules and secreted onto the outer surface of the body wall. Some species also eject portions of their toxic inner organs through the anus as a defensive mechanism.
- In humans, the holothurin toxins cause an acute irritant dermatitis upon contact with the skin and a painful chemical conjunctivitis upon contact with the eyes.
- In addition to their own toxin, sea cucumbers also can secrete undigested nematocysts of previously consumed cnidarians through their integument, causing additional effects on human skin.
- The dermatologic effects of sea cucumbers can be prevented with the use of gloves and swim masks or goggles.
Phacomatosis Pigmentokeratotica Associated With Raynaud Phenomenon, Segmental Nevi, Hyperhidrosis, and Scoliosis
To the Editor:
Phacomatosis pigmentokeratotica (PPK) is a rare epidermal nevus syndrome complicated by multiple extracutaneous anomalies, including skeletal defects and neurologic anomalies. Less common associations include lateral curvature of the spine and hyperhidrosis. We present a patient with PPK and unilateral Raynaud phenomenon in addition to a segmental distribution of melanocytic nevi, hyperhidrosis, and scoliosis.
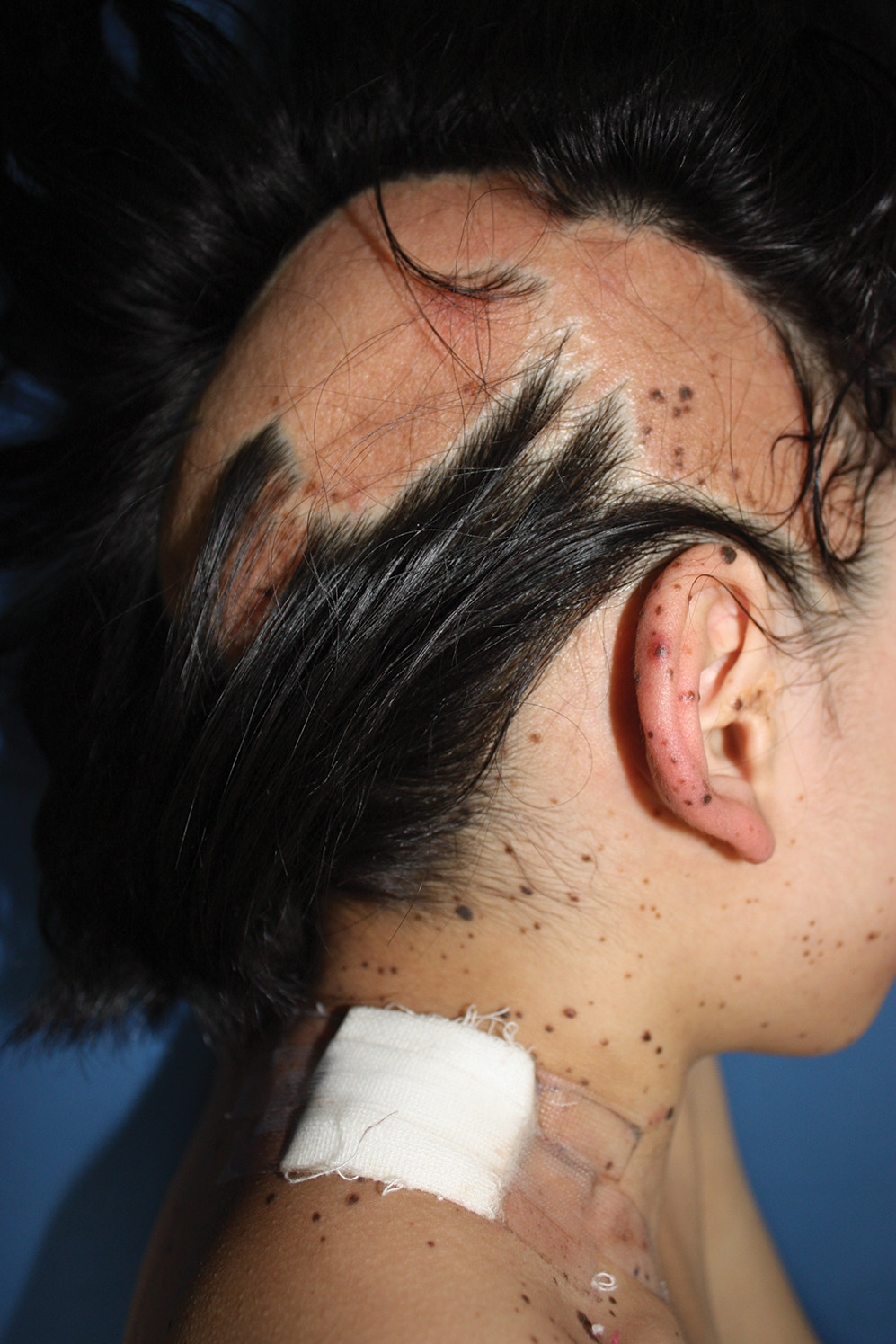
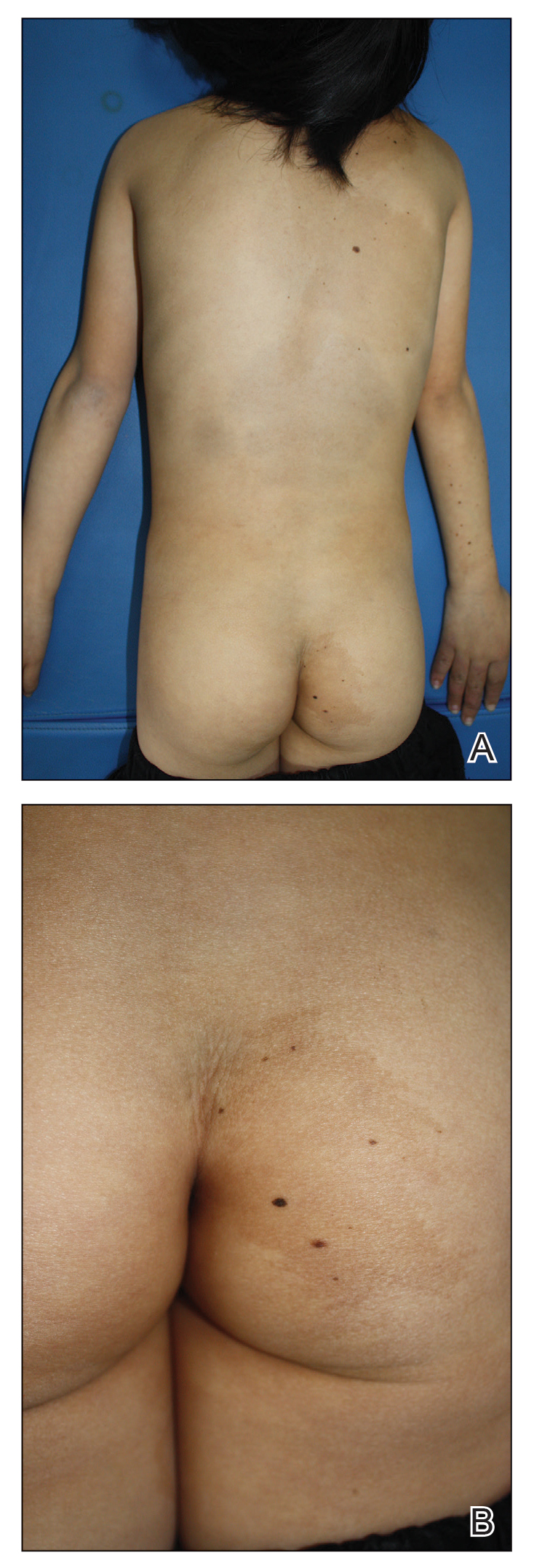
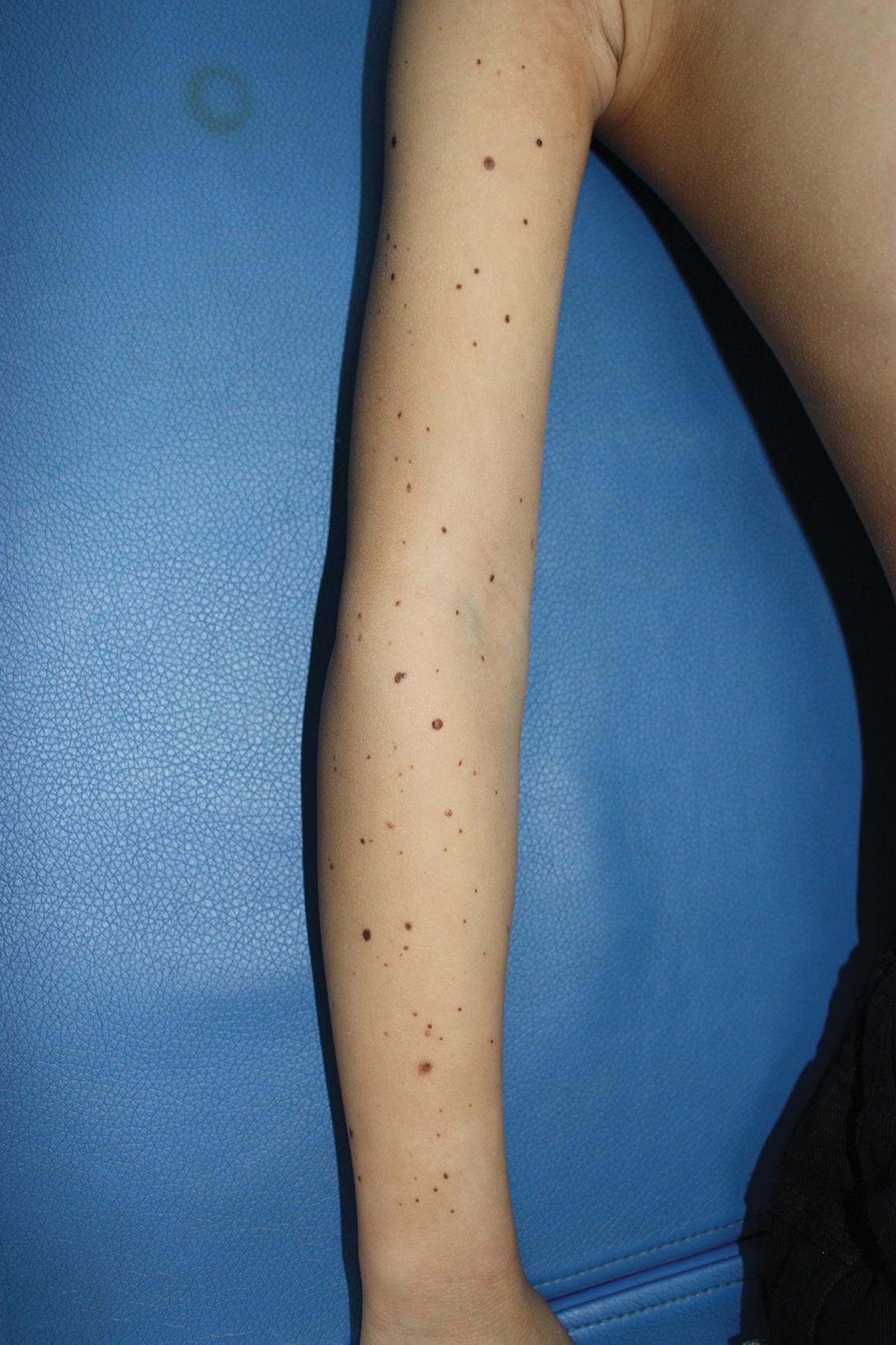
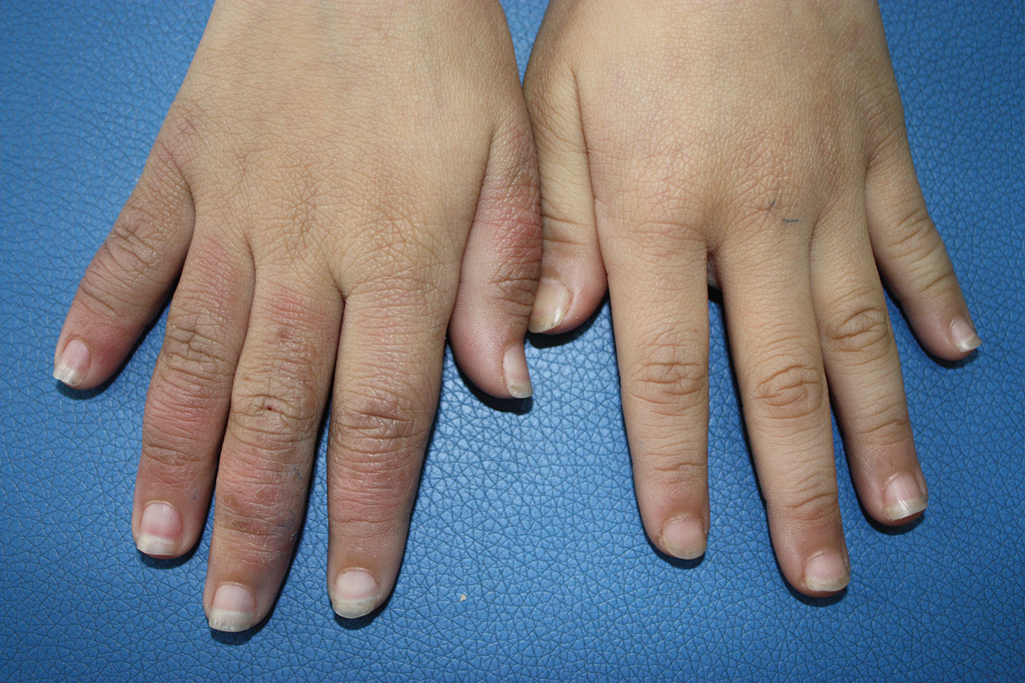
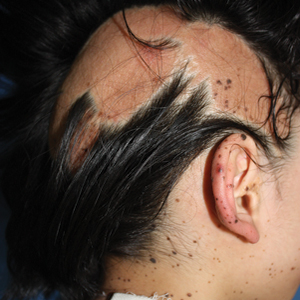
A 9-year-old girl was born with a yellow-orange alopecic plaque on the right side of the scalp (Figure 1). There also were 2 large, irregularly pigmented patches localized on the right side of the upper back and buttock. Over 3 years, numerous papular nevi developed within these pigmented patches and were diagnosed as speckled lentiginous nevi (Figure 2). In addition, numerous nevi of various sizes affected the right face, right shoulder, right arm (Figure 3), and right neck and were clearly demarcated along the midline. Several nevi also were noted within the nevus sebaceous on the right scalp. These skin lesions expanded progressively with age. At 6 years of age, she was diagnosed with hyperhidrosis of the right half of the body, which was most pronounced on the face. Raynaud phenomenon restricted to the right hand also was noted (Figure 4). Upon cold exposure, the digits become pale white, cold, and numb; then blue; and finally red. She lacked other features of connective tissue disease, and autoantibody testing was negative. She also was noted to have an abnormal lateral curvature of the spine (scoliosis). Auditory, ocular, and neurologic examinations were normal. Cranial and cerebral magnetic resonance imaging showed no central nervous system abnormalities. Her family history was negative for nevus spilus, nevus sebaceous, and neurofibromatosis. The clinical findings in our patient led to the diagnosis of PPK.
Phacomatosis pigmentokeratotica is a distinctive epidermal nevus syndrome characterized by the coexistence of a speckled lentiginous nevus, also known as a nevus spilus, and a nevus sebaceous1; PPK frequently is complicated by skeletal, ophthalmic, or neurologic abnormalities.2 Most cases reported are sporadic, and a postzygotic mosaic HRas proto-oncogene, GTPase, HRAS, mutation has been demonstrated in some patients and may contribute to the phenotype of PPK.3,4
Other anomalies have included ichthyosislike diffuse hyperkeratosis, laxity of the hands, pelvic hypoplasia, glaucoma, psychomotor retardation, and hypophosphatemic rickets. These patients also should be monitored for the development of malignant neoplasms within the nevus sebaceous.5 Segmental hyperhidrosis may be seen in association with the nevus spilus component.2
Raynaud phenomenon involving only the right hand was a unique finding in our patient. In 3 years of follow-up, our patient developed no evidence of connective tissue disease or other systemic illness. We speculate that Raynaud phenomenon of the right hand along with hyperhidrosis of the right side of the body could be a result of dysfunction of the autonomic nervous system. We propose that Raynaud phenomenon represents an unusual manifestation of PPK and may broaden the spectrum of extracutaneous anomalies associated with the disease. The finding of segmental nevi outside of the confines of the nevus spilus was another unusual manifestation of mosaicism.
- Happle R, Hoffmann R, Restano L, et al. Phacomatosis pigmentokeratotica: a melanocytic-epidermal twin nevus syndrome. Am J Med Genet. 1996;65:363-365.
- Happle R. The group of epidermal nevus syndromes part I. well defined phenotypes. J Am Acad Dermatol. 2010;63:1-22, 23-24.
- Groesser L, Herschberger E, Sagrera A, et al. Phacomatosis pigmentokeratotica is caused by a postzygotic HRAS mutation in a multipotent progenitor cell. J Invest Dermatol. 2013;133:1998-2003.
- Martin RJ, Arefi M, Splitt M, et al. Phacomatosis pigmentokeratotica and precocious puberty associated with HRAS mutation. Br J Dermatol. 2018;178:289-291.
- Chu GY, Wu CY. Phacomatosis pigmentokeratotica: a follow-up report with fatal outcome. Acta Derm Venereol. 2014;94:467-468.
To the Editor:
Phacomatosis pigmentokeratotica (PPK) is a rare epidermal nevus syndrome complicated by multiple extracutaneous anomalies, including skeletal defects and neurologic anomalies. Less common associations include lateral curvature of the spine and hyperhidrosis. We present a patient with PPK and unilateral Raynaud phenomenon in addition to a segmental distribution of melanocytic nevi, hyperhidrosis, and scoliosis.
A 9-year-old girl was born with a yellow-orange alopecic plaque on the right side of the scalp (Figure 1). There also were 2 large, irregularly pigmented patches localized on the right side of the upper back and buttock. Over 3 years, numerous papular nevi developed within these pigmented patches and were diagnosed as speckled lentiginous nevi (Figure 2). In addition, numerous nevi of various sizes affected the right face, right shoulder, right arm (Figure 3), and right neck and were clearly demarcated along the midline. Several nevi also were noted within the nevus sebaceous on the right scalp. These skin lesions expanded progressively with age. At 6 years of age, she was diagnosed with hyperhidrosis of the right half of the body, which was most pronounced on the face. Raynaud phenomenon restricted to the right hand also was noted (Figure 4). Upon cold exposure, the digits become pale white, cold, and numb; then blue; and finally red. She lacked other features of connective tissue disease, and autoantibody testing was negative. She also was noted to have an abnormal lateral curvature of the spine (scoliosis). Auditory, ocular, and neurologic examinations were normal. Cranial and cerebral magnetic resonance imaging showed no central nervous system abnormalities. Her family history was negative for nevus spilus, nevus sebaceous, and neurofibromatosis. The clinical findings in our patient led to the diagnosis of PPK.
Phacomatosis pigmentokeratotica is a distinctive epidermal nevus syndrome characterized by the coexistence of a speckled lentiginous nevus, also known as a nevus spilus, and a nevus sebaceous1; PPK frequently is complicated by skeletal, ophthalmic, or neurologic abnormalities.2 Most cases reported are sporadic, and a postzygotic mosaic HRas proto-oncogene, GTPase, HRAS, mutation has been demonstrated in some patients and may contribute to the phenotype of PPK.3,4
Other anomalies have included ichthyosislike diffuse hyperkeratosis, laxity of the hands, pelvic hypoplasia, glaucoma, psychomotor retardation, and hypophosphatemic rickets. These patients also should be monitored for the development of malignant neoplasms within the nevus sebaceous.5 Segmental hyperhidrosis may be seen in association with the nevus spilus component.2
Raynaud phenomenon involving only the right hand was a unique finding in our patient. In 3 years of follow-up, our patient developed no evidence of connective tissue disease or other systemic illness. We speculate that Raynaud phenomenon of the right hand along with hyperhidrosis of the right side of the body could be a result of dysfunction of the autonomic nervous system. We propose that Raynaud phenomenon represents an unusual manifestation of PPK and may broaden the spectrum of extracutaneous anomalies associated with the disease. The finding of segmental nevi outside of the confines of the nevus spilus was another unusual manifestation of mosaicism.
To the Editor:
Phacomatosis pigmentokeratotica (PPK) is a rare epidermal nevus syndrome complicated by multiple extracutaneous anomalies, including skeletal defects and neurologic anomalies. Less common associations include lateral curvature of the spine and hyperhidrosis. We present a patient with PPK and unilateral Raynaud phenomenon in addition to a segmental distribution of melanocytic nevi, hyperhidrosis, and scoliosis.
A 9-year-old girl was born with a yellow-orange alopecic plaque on the right side of the scalp (Figure 1). There also were 2 large, irregularly pigmented patches localized on the right side of the upper back and buttock. Over 3 years, numerous papular nevi developed within these pigmented patches and were diagnosed as speckled lentiginous nevi (Figure 2). In addition, numerous nevi of various sizes affected the right face, right shoulder, right arm (Figure 3), and right neck and were clearly demarcated along the midline. Several nevi also were noted within the nevus sebaceous on the right scalp. These skin lesions expanded progressively with age. At 6 years of age, she was diagnosed with hyperhidrosis of the right half of the body, which was most pronounced on the face. Raynaud phenomenon restricted to the right hand also was noted (Figure 4). Upon cold exposure, the digits become pale white, cold, and numb; then blue; and finally red. She lacked other features of connective tissue disease, and autoantibody testing was negative. She also was noted to have an abnormal lateral curvature of the spine (scoliosis). Auditory, ocular, and neurologic examinations were normal. Cranial and cerebral magnetic resonance imaging showed no central nervous system abnormalities. Her family history was negative for nevus spilus, nevus sebaceous, and neurofibromatosis. The clinical findings in our patient led to the diagnosis of PPK.
Phacomatosis pigmentokeratotica is a distinctive epidermal nevus syndrome characterized by the coexistence of a speckled lentiginous nevus, also known as a nevus spilus, and a nevus sebaceous1; PPK frequently is complicated by skeletal, ophthalmic, or neurologic abnormalities.2 Most cases reported are sporadic, and a postzygotic mosaic HRas proto-oncogene, GTPase, HRAS, mutation has been demonstrated in some patients and may contribute to the phenotype of PPK.3,4
Other anomalies have included ichthyosislike diffuse hyperkeratosis, laxity of the hands, pelvic hypoplasia, glaucoma, psychomotor retardation, and hypophosphatemic rickets. These patients also should be monitored for the development of malignant neoplasms within the nevus sebaceous.5 Segmental hyperhidrosis may be seen in association with the nevus spilus component.2
Raynaud phenomenon involving only the right hand was a unique finding in our patient. In 3 years of follow-up, our patient developed no evidence of connective tissue disease or other systemic illness. We speculate that Raynaud phenomenon of the right hand along with hyperhidrosis of the right side of the body could be a result of dysfunction of the autonomic nervous system. We propose that Raynaud phenomenon represents an unusual manifestation of PPK and may broaden the spectrum of extracutaneous anomalies associated with the disease. The finding of segmental nevi outside of the confines of the nevus spilus was another unusual manifestation of mosaicism.
- Happle R, Hoffmann R, Restano L, et al. Phacomatosis pigmentokeratotica: a melanocytic-epidermal twin nevus syndrome. Am J Med Genet. 1996;65:363-365.
- Happle R. The group of epidermal nevus syndromes part I. well defined phenotypes. J Am Acad Dermatol. 2010;63:1-22, 23-24.
- Groesser L, Herschberger E, Sagrera A, et al. Phacomatosis pigmentokeratotica is caused by a postzygotic HRAS mutation in a multipotent progenitor cell. J Invest Dermatol. 2013;133:1998-2003.
- Martin RJ, Arefi M, Splitt M, et al. Phacomatosis pigmentokeratotica and precocious puberty associated with HRAS mutation. Br J Dermatol. 2018;178:289-291.
- Chu GY, Wu CY. Phacomatosis pigmentokeratotica: a follow-up report with fatal outcome. Acta Derm Venereol. 2014;94:467-468.
- Happle R, Hoffmann R, Restano L, et al. Phacomatosis pigmentokeratotica: a melanocytic-epidermal twin nevus syndrome. Am J Med Genet. 1996;65:363-365.
- Happle R. The group of epidermal nevus syndromes part I. well defined phenotypes. J Am Acad Dermatol. 2010;63:1-22, 23-24.
- Groesser L, Herschberger E, Sagrera A, et al. Phacomatosis pigmentokeratotica is caused by a postzygotic HRAS mutation in a multipotent progenitor cell. J Invest Dermatol. 2013;133:1998-2003.
- Martin RJ, Arefi M, Splitt M, et al. Phacomatosis pigmentokeratotica and precocious puberty associated with HRAS mutation. Br J Dermatol. 2018;178:289-291.
- Chu GY, Wu CY. Phacomatosis pigmentokeratotica: a follow-up report with fatal outcome. Acta Derm Venereol. 2014;94:467-468.
Practice Points
- Phacomatosis pigmentokeratotica (PPK) is characterized by the coexistence of speckled lentiginous nevus and nevus sebaceous.
- Raynaud phenomenon may be an unreported association with PPK.
Thick Hyperkeratotic Plaques on the Palms and Soles
The Diagnosis: Keratoderma Climactericum
Keratoderma climactericum was first reported in 1934 by Haxthausen1 as nonpruritic circumscribed hyperkeratosis located mainly on the palms and soles. The initial eruption was described as discrete lesions with an oval or round shape that progressed to less-defined, confluent, hyperkeratotic patches with fissures.1 Keratoderma climactericum also may be referred to as Haxthausen disease and is considered an acquired palmoplantar keratoderma.2
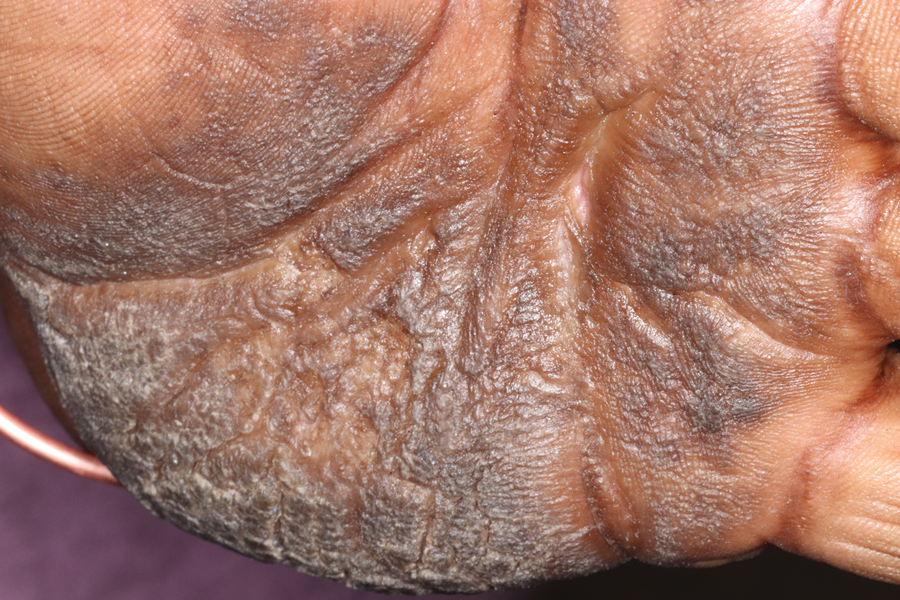
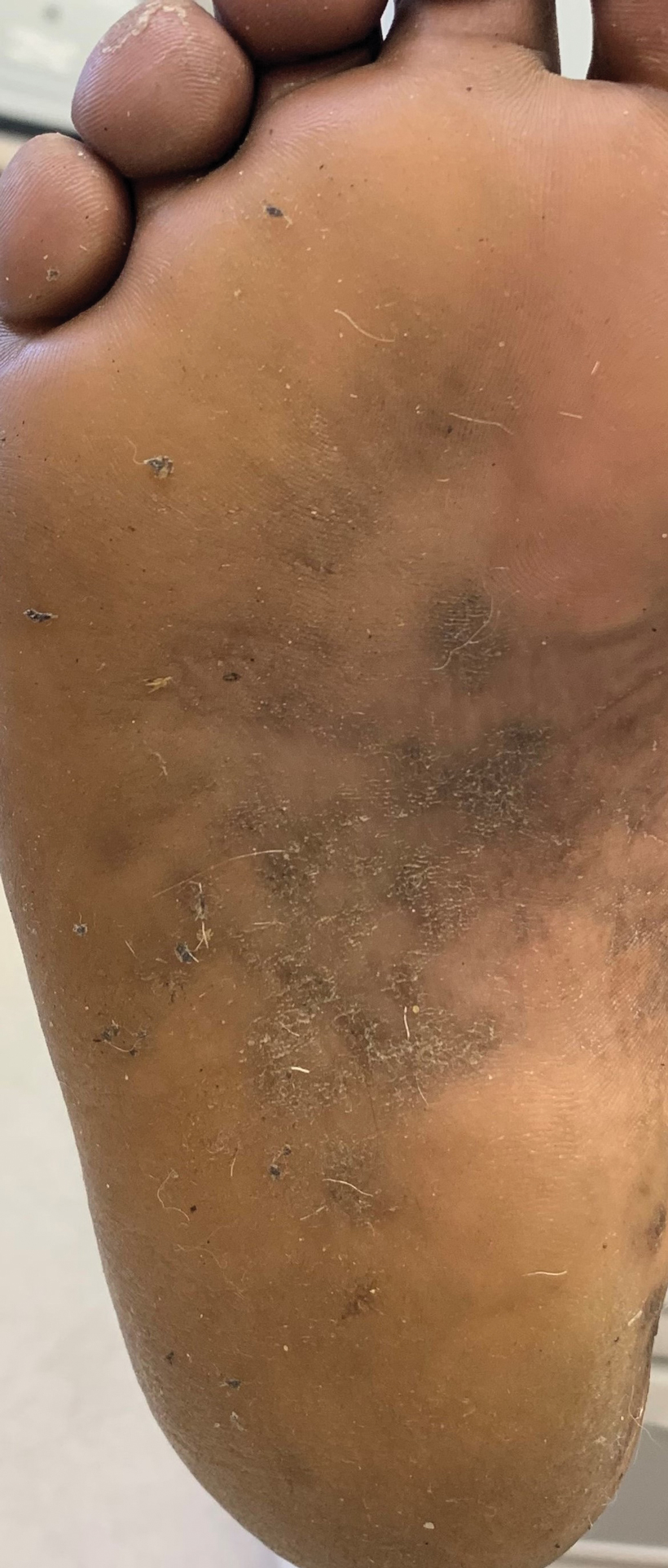
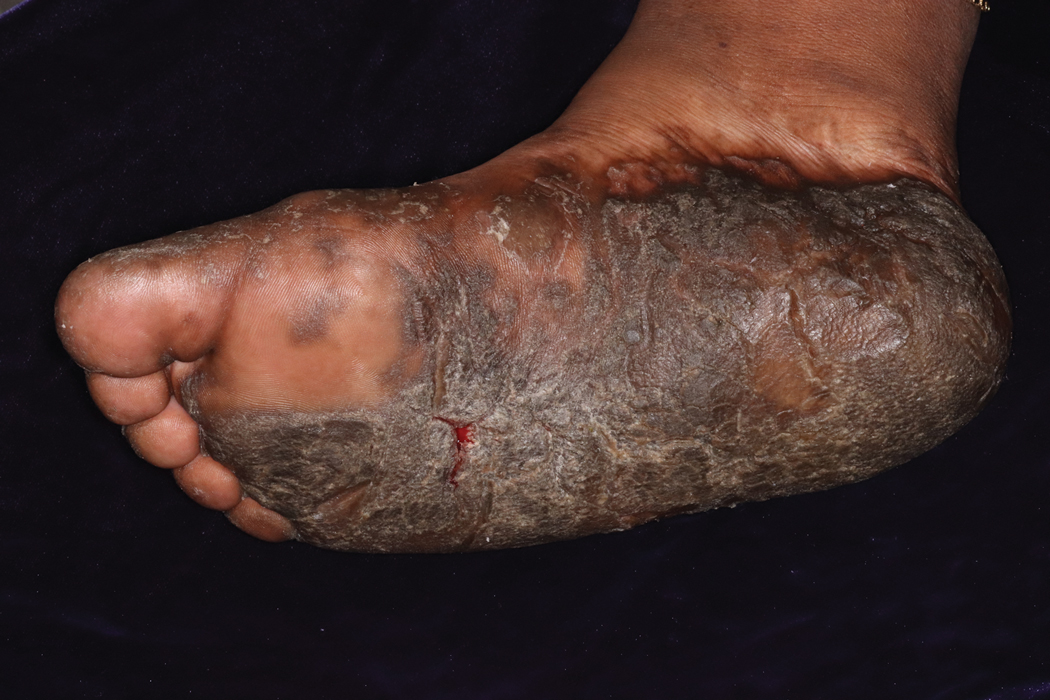
Keratoderma climactericum is a rare dermatologic disorder that presents in women of menopausal age who have no family or personal history of skin disease. Keratoderma climactericum is associated with hypertension and obesity.2 Keratotic lesions usually first occur on the plantar surfaces with eventual development of fissuring and hyperkeratosis that causes painful walking. The keratotic lesions on the plantar surfaces often are nonpruritic and gradually become confluent over time. As the disease progresses, keratotic lesions appear on the central palms, which can lead to confluent hyperkeratosis on the palmar surfaces (Figure 1).2 The exact mechanism of keratoderma climactericum has not been described but is believed to be due to hormonal dysregulation.2
In 1986, Deschamps et al3 presented 10 cases of keratoderma climactericum occurring in menopausal women with an average age of 57 years. The lesions began on the soles at areas of greatest pressure. Histopathology for each patient showed orthokeratotic hyperkeratosis, irregular hyperplasia, interpapillary ridges, and exocytosis of lymphocytes in the epidermis. Seven patients were treated with etretinate, which first led to the removal of palmar lesions, followed by improvement in plantar lesions and pain when walking. There was no association of keratoderma climactericum and sex hormones, as hormone levels were negative or normal for each patient.3
Three cases of keratoderma climactericum following bilateral oophorectomy in young women were reported by Wachtel4 in 1981. Unlike in women of menopausal age, there was no association of keratoderma climactericum with hypertension or obesity. Additionally, the lesions on the palms and soles were more diffusely distributed than in women of menopausal age. Estrogen administration completely reversed each patient's hyperkeratotic palms and soles.4 A definitive pathogenic role of estrogens in the development of keratoderma climactericum has yet to be determined.2
Histopathology is not specific for keratoderma climactericum, making the disease a clinical diagnosis. However, a biopsy may be useful to rule out palmoplantar psoriasis.2 Clinical information such as the age and sex of the patient, distribution of disease, presence of fissuring, and progression of disease from soles to palms should be considered when making a diagnosis of keratoderma climactericum. The differential diagnosis of keratoderma climactericum should include fungal infections, contact dermatitis, irritant dermatitis, psoriasis, atopic dermatitis, underlying malignancy, and pityriasis rubra pilaris.
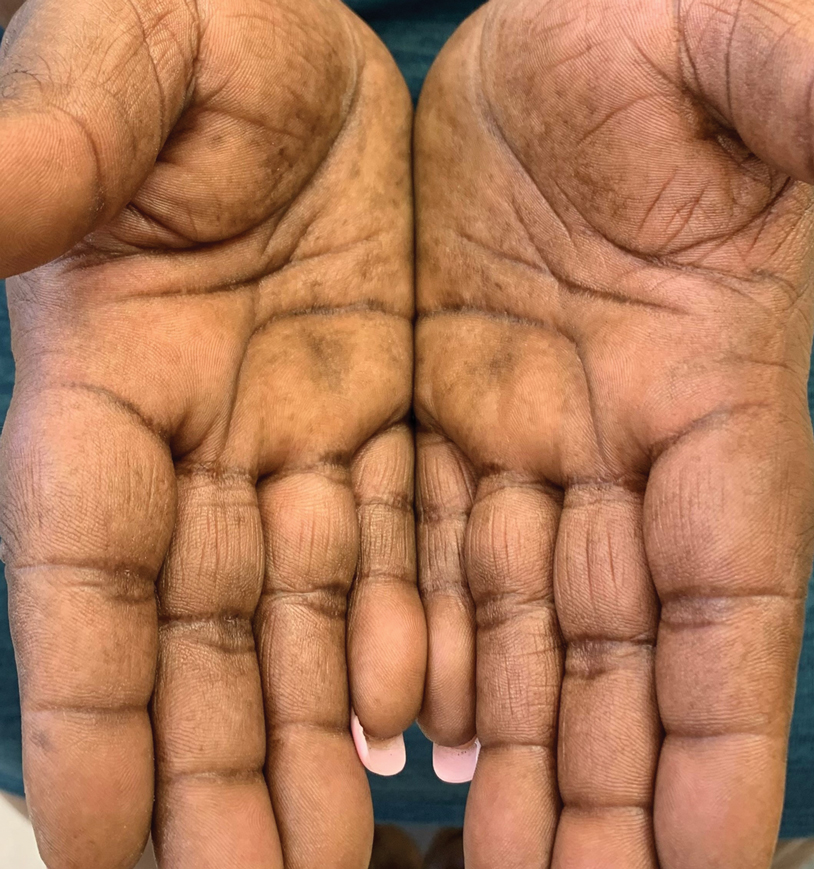
Treatment options for keratoderma climactericum include salicylic acid, emollients, oral retinoids, urea ointments, estriol cream, and topical steroids.5,6 Our patient was prescribed acitretin 25 mg daily and ammonium lactate to apply topically as needed for dry skin. Five months after the initial presentation, fissures and dry skin on the bilateral soles were still present. Ammonium lactate was discontinued, and the patient was prescribed urea cream 40%. Fifteen months after the initial presentation, the patient reported substantial improvement on the hands and feet and noted that she no longer needed the urea cream. Physical examination revealed no presence of hyperkeratosis or fissuring on the palms (Figure 2), and mild hyperkeratosis was present on the plantar surfaces of the feet (Figure 3). The patient continued to use acitretin to prevent disease relapse.
Keratoderma climactericum is an unusual and debilitating condition that occurs in women of menopausal age. It is diagnosed by its specific clinical presentation. More common diagnoses such as tinea and dermatitis should be ruled out before considering keratoderma climactericum.
- Haxthausen H. Keratoderma climactericum. Br J Dermatol. 1934;46:161-167.
- Patel S, Zirwas M, English JC. Acquired palmoplantar keratoderma. Am J Clin Dermatol. 2007;8:1-11.
- Deschamps P, Leroy D, Pedailles S, et al. Keratoderma climactericum (Haxthausen's disease): clinical signs, laboratory findings and etretinate treatment in 10 patients. Dermatologica. 1986;172:258-262.
- Wachtel TJ. Plantar and palmar hyperkeratosis in young castrated women. Int J Dermatol. 1981;20:270-271.
- Bristow I. The management of heel fissures using a steroid impregnated tape (Haelan) in a patient with Keratoderma climactericum. Podiatry Now. 2008;11:22-23.
- Mendes-Bastos P. Plantar keratoderma climactericum: successful improvement with a topical estriol cream. J Cosmet Dermatol. 2018;17:811-813.
The Diagnosis: Keratoderma Climactericum
Keratoderma climactericum was first reported in 1934 by Haxthausen1 as nonpruritic circumscribed hyperkeratosis located mainly on the palms and soles. The initial eruption was described as discrete lesions with an oval or round shape that progressed to less-defined, confluent, hyperkeratotic patches with fissures.1 Keratoderma climactericum also may be referred to as Haxthausen disease and is considered an acquired palmoplantar keratoderma.2
Keratoderma climactericum is a rare dermatologic disorder that presents in women of menopausal age who have no family or personal history of skin disease. Keratoderma climactericum is associated with hypertension and obesity.2 Keratotic lesions usually first occur on the plantar surfaces with eventual development of fissuring and hyperkeratosis that causes painful walking. The keratotic lesions on the plantar surfaces often are nonpruritic and gradually become confluent over time. As the disease progresses, keratotic lesions appear on the central palms, which can lead to confluent hyperkeratosis on the palmar surfaces (Figure 1).2 The exact mechanism of keratoderma climactericum has not been described but is believed to be due to hormonal dysregulation.2
In 1986, Deschamps et al3 presented 10 cases of keratoderma climactericum occurring in menopausal women with an average age of 57 years. The lesions began on the soles at areas of greatest pressure. Histopathology for each patient showed orthokeratotic hyperkeratosis, irregular hyperplasia, interpapillary ridges, and exocytosis of lymphocytes in the epidermis. Seven patients were treated with etretinate, which first led to the removal of palmar lesions, followed by improvement in plantar lesions and pain when walking. There was no association of keratoderma climactericum and sex hormones, as hormone levels were negative or normal for each patient.3
Three cases of keratoderma climactericum following bilateral oophorectomy in young women were reported by Wachtel4 in 1981. Unlike in women of menopausal age, there was no association of keratoderma climactericum with hypertension or obesity. Additionally, the lesions on the palms and soles were more diffusely distributed than in women of menopausal age. Estrogen administration completely reversed each patient's hyperkeratotic palms and soles.4 A definitive pathogenic role of estrogens in the development of keratoderma climactericum has yet to be determined.2
Histopathology is not specific for keratoderma climactericum, making the disease a clinical diagnosis. However, a biopsy may be useful to rule out palmoplantar psoriasis.2 Clinical information such as the age and sex of the patient, distribution of disease, presence of fissuring, and progression of disease from soles to palms should be considered when making a diagnosis of keratoderma climactericum. The differential diagnosis of keratoderma climactericum should include fungal infections, contact dermatitis, irritant dermatitis, psoriasis, atopic dermatitis, underlying malignancy, and pityriasis rubra pilaris.
Treatment options for keratoderma climactericum include salicylic acid, emollients, oral retinoids, urea ointments, estriol cream, and topical steroids.5,6 Our patient was prescribed acitretin 25 mg daily and ammonium lactate to apply topically as needed for dry skin. Five months after the initial presentation, fissures and dry skin on the bilateral soles were still present. Ammonium lactate was discontinued, and the patient was prescribed urea cream 40%. Fifteen months after the initial presentation, the patient reported substantial improvement on the hands and feet and noted that she no longer needed the urea cream. Physical examination revealed no presence of hyperkeratosis or fissuring on the palms (Figure 2), and mild hyperkeratosis was present on the plantar surfaces of the feet (Figure 3). The patient continued to use acitretin to prevent disease relapse.
Keratoderma climactericum is an unusual and debilitating condition that occurs in women of menopausal age. It is diagnosed by its specific clinical presentation. More common diagnoses such as tinea and dermatitis should be ruled out before considering keratoderma climactericum.
The Diagnosis: Keratoderma Climactericum
Keratoderma climactericum was first reported in 1934 by Haxthausen1 as nonpruritic circumscribed hyperkeratosis located mainly on the palms and soles. The initial eruption was described as discrete lesions with an oval or round shape that progressed to less-defined, confluent, hyperkeratotic patches with fissures.1 Keratoderma climactericum also may be referred to as Haxthausen disease and is considered an acquired palmoplantar keratoderma.2
Keratoderma climactericum is a rare dermatologic disorder that presents in women of menopausal age who have no family or personal history of skin disease. Keratoderma climactericum is associated with hypertension and obesity.2 Keratotic lesions usually first occur on the plantar surfaces with eventual development of fissuring and hyperkeratosis that causes painful walking. The keratotic lesions on the plantar surfaces often are nonpruritic and gradually become confluent over time. As the disease progresses, keratotic lesions appear on the central palms, which can lead to confluent hyperkeratosis on the palmar surfaces (Figure 1).2 The exact mechanism of keratoderma climactericum has not been described but is believed to be due to hormonal dysregulation.2
In 1986, Deschamps et al3 presented 10 cases of keratoderma climactericum occurring in menopausal women with an average age of 57 years. The lesions began on the soles at areas of greatest pressure. Histopathology for each patient showed orthokeratotic hyperkeratosis, irregular hyperplasia, interpapillary ridges, and exocytosis of lymphocytes in the epidermis. Seven patients were treated with etretinate, which first led to the removal of palmar lesions, followed by improvement in plantar lesions and pain when walking. There was no association of keratoderma climactericum and sex hormones, as hormone levels were negative or normal for each patient.3
Three cases of keratoderma climactericum following bilateral oophorectomy in young women were reported by Wachtel4 in 1981. Unlike in women of menopausal age, there was no association of keratoderma climactericum with hypertension or obesity. Additionally, the lesions on the palms and soles were more diffusely distributed than in women of menopausal age. Estrogen administration completely reversed each patient's hyperkeratotic palms and soles.4 A definitive pathogenic role of estrogens in the development of keratoderma climactericum has yet to be determined.2
Histopathology is not specific for keratoderma climactericum, making the disease a clinical diagnosis. However, a biopsy may be useful to rule out palmoplantar psoriasis.2 Clinical information such as the age and sex of the patient, distribution of disease, presence of fissuring, and progression of disease from soles to palms should be considered when making a diagnosis of keratoderma climactericum. The differential diagnosis of keratoderma climactericum should include fungal infections, contact dermatitis, irritant dermatitis, psoriasis, atopic dermatitis, underlying malignancy, and pityriasis rubra pilaris.
Treatment options for keratoderma climactericum include salicylic acid, emollients, oral retinoids, urea ointments, estriol cream, and topical steroids.5,6 Our patient was prescribed acitretin 25 mg daily and ammonium lactate to apply topically as needed for dry skin. Five months after the initial presentation, fissures and dry skin on the bilateral soles were still present. Ammonium lactate was discontinued, and the patient was prescribed urea cream 40%. Fifteen months after the initial presentation, the patient reported substantial improvement on the hands and feet and noted that she no longer needed the urea cream. Physical examination revealed no presence of hyperkeratosis or fissuring on the palms (Figure 2), and mild hyperkeratosis was present on the plantar surfaces of the feet (Figure 3). The patient continued to use acitretin to prevent disease relapse.
Keratoderma climactericum is an unusual and debilitating condition that occurs in women of menopausal age. It is diagnosed by its specific clinical presentation. More common diagnoses such as tinea and dermatitis should be ruled out before considering keratoderma climactericum.
- Haxthausen H. Keratoderma climactericum. Br J Dermatol. 1934;46:161-167.
- Patel S, Zirwas M, English JC. Acquired palmoplantar keratoderma. Am J Clin Dermatol. 2007;8:1-11.
- Deschamps P, Leroy D, Pedailles S, et al. Keratoderma climactericum (Haxthausen's disease): clinical signs, laboratory findings and etretinate treatment in 10 patients. Dermatologica. 1986;172:258-262.
- Wachtel TJ. Plantar and palmar hyperkeratosis in young castrated women. Int J Dermatol. 1981;20:270-271.
- Bristow I. The management of heel fissures using a steroid impregnated tape (Haelan) in a patient with Keratoderma climactericum. Podiatry Now. 2008;11:22-23.
- Mendes-Bastos P. Plantar keratoderma climactericum: successful improvement with a topical estriol cream. J Cosmet Dermatol. 2018;17:811-813.
- Haxthausen H. Keratoderma climactericum. Br J Dermatol. 1934;46:161-167.
- Patel S, Zirwas M, English JC. Acquired palmoplantar keratoderma. Am J Clin Dermatol. 2007;8:1-11.
- Deschamps P, Leroy D, Pedailles S, et al. Keratoderma climactericum (Haxthausen's disease): clinical signs, laboratory findings and etretinate treatment in 10 patients. Dermatologica. 1986;172:258-262.
- Wachtel TJ. Plantar and palmar hyperkeratosis in young castrated women. Int J Dermatol. 1981;20:270-271.
- Bristow I. The management of heel fissures using a steroid impregnated tape (Haelan) in a patient with Keratoderma climactericum. Podiatry Now. 2008;11:22-23.
- Mendes-Bastos P. Plantar keratoderma climactericum: successful improvement with a topical estriol cream. J Cosmet Dermatol. 2018;17:811-813.
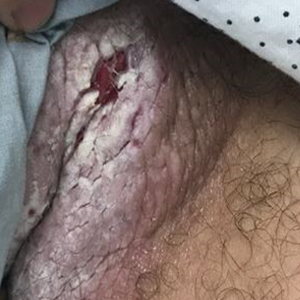
A 52-year-old woman with a history of rheumatoid arthritis presented with a rash on the palms and soles of 7 years' duration that started around the onset of menopause. Physical examination revealed thick hyperkeratotic plaques with multiple deep fissures on the palms and soles. The patient's current medications included methotrexate for rheumatoid arthritis. She previously had been prescribed adalimumab by an outside physician for the rash, which provided no relief, and currently was using urea ointment, which caused a burning sensation on the palms and soles. The patient denied a personal or family history of psoriasis.
Pink Patches With a Hyperpigmented Rim
The Diagnosis: Phytophotodermatitis
A more detailed patient history revealed that there was beer with limes on the boat, but the partygoers neglected to bring a knife. The patient volunteered to tear the limes apart with his bare hands. Because he was clad only in swim trunks, lime juice splattered over various regions of his body.
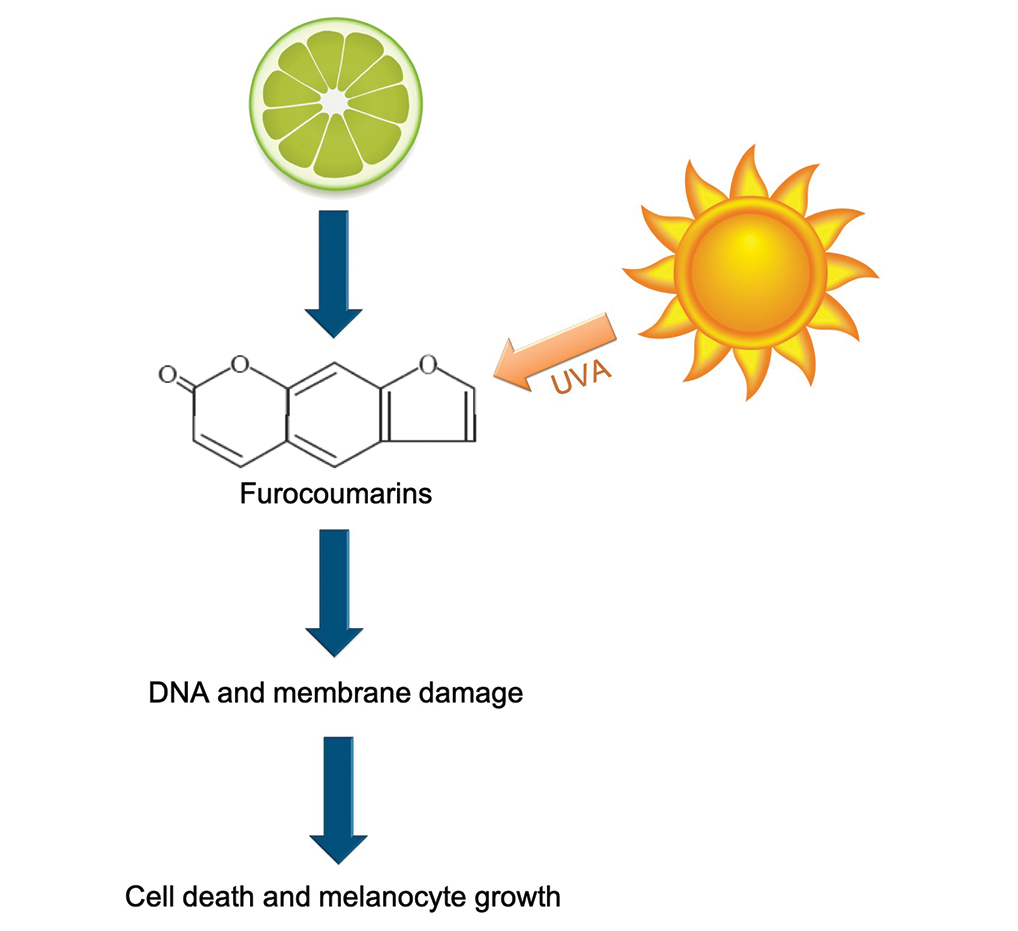
Phytophotodermatitis is a phototoxic blistering rash that follows topical exposure to plant-derived furocoumarins and sunlight. (Figure) Furocoumarins are photosensitizing substances produced by certain plants, possibly as a defense mechanism against predators.1 They cause a nonimmunologic phototoxic reaction when deposited on the skin and exposed to UVA radiation. Exposure to limes is the most common precipitant of phytophotodermatitis, but other potential culprits include lemons, grapefruit, figs, carrots, parsnips, celery, and dill.2
Lesions associated with phytophotodermatitis classically present as painful erythematous patches and bullae in regions of furocoumarin exposure. Affected areas are well demarcated and irregularly shaped and heal with a characteristic hyperpigmented rim. They often have a downward streak pattern from the dripping juice.3 If the furocoumarins are transferred by touch, lesions can appear in the shape of handprints, which may raise alarms for physical abuse in children.4
Photochemical reactions caused by activated furocoumarins cross-link nuclear DNA and damage cell membranes. These changes lead to cellular death resulting in edema and destruction of the epidermis. Other effects include an increase in keratin and thickening of the stratum corneum. The hyperpigmentation is a result of increased concentration of melanosomes and stimulation of melanocytes by activated furocoumarins.5
Management of phytophotodermatitis depends on the severity of skin injury. Mild cases may not require any treatment, whereas the most severe ones require admission to a burn unit for wound care. Anti-inflammatory medications are the mainstay of therapy. Our patient was prescribed desonide cream 0.05% for application to the affected areas. Sunscreen should be applied to prevent worsening of hyperpigmentation, which may take months to years to fade naturally. If hyperpigmentation is cosmetically troubling to the patient, bleaching agents such as hydroquinone and retinoids or Nd:YAG laser can be used to accelerate the resolution of pigment.5
Phototoxicity differs from less common photoallergic reactions caused by preformed antibodies or a delayed cell-mediated response to a trigger. The classic presentation of photoallergy is apruritic, inflammatory, bullous eruption in a sensitized individual.6 Allergic contact dermatitis more commonly is associated with pruritus than pain, and it presents as a papulovesicular eruption that evolves into lichenified plaques.7 Porphyria cutanea tarda would likely be accompanied by other cutaneous features such as hypertrichosis and sclerodermoid plaques with dystrophic calcification, in addition to wine-colored urine-containing porphyrins.8 Bullous fixed drug eruptions develop within 48 hours of exposure to a causative agent. The patient typically would experience pruritus and burning at the site of clearly demarcated erythematous lesions that healed with hyperpigmentation.9 Lesions of bullous lupus erythematosus may appear in areas without sun exposure, and they would be more likely to leave behind hypopigmentation rather than hyperpigmentation.10
- Pathak MA. Phytophotodermatitis. Clin Dermatol. 1986;4:102-121.
- Egan CL, Sterling G. Phytophotodermatitis: a visit to Margaritaville. Cutis. 1993;51:41-42.
- Hankinson A, Lloyd B, Alweis R. Lime-induced phytophotodermatitis [published online ahead of print September 29, 2014]. J Community Hosp Intern Med Perspect. doi:10.3402/jchimp.v4.25090
- Fitzpatrick JK, Kohlwes J. Lime-induced phytophotodermatitis. J Gen Intern Med. 2018;33:975.
- Weber IC, Davis CP, Greeson DM. Phytophotodermatitis: the other "lime" disease. J Emerg Med. 1999;17:235-237.
- Monteiro AF, Rato M, Martins C. Drug-induced photosensitivity: photoallergic and phototoxic reactions. Clin Dermatol. 2016;34:571-581.
- Tan CH, Rasool S, Johnston GA. Contact dermatitis: allergic and irritant. Clin Dermatol. 2014;32:116-124.
- Dawe R. An overview of the cutaneous porphyrias. F1000Res. 2017;6:1906.
- Bandino JP, Wohltmann WE, Bray DW, et al. Naproxen-induced generalized bullous fixed drug eruption. Dermatol Online J. 2009;15:4.
- Contestable JJ, Edhegard KD, Meyerle JH. Bullous systemic lupus erythematosus: a review and update to diagnosis and treatment. Am J Clin Dermatol. 2014;15:517-524.
The Diagnosis: Phytophotodermatitis
A more detailed patient history revealed that there was beer with limes on the boat, but the partygoers neglected to bring a knife. The patient volunteered to tear the limes apart with his bare hands. Because he was clad only in swim trunks, lime juice splattered over various regions of his body.
Phytophotodermatitis is a phototoxic blistering rash that follows topical exposure to plant-derived furocoumarins and sunlight. (Figure) Furocoumarins are photosensitizing substances produced by certain plants, possibly as a defense mechanism against predators.1 They cause a nonimmunologic phototoxic reaction when deposited on the skin and exposed to UVA radiation. Exposure to limes is the most common precipitant of phytophotodermatitis, but other potential culprits include lemons, grapefruit, figs, carrots, parsnips, celery, and dill.2
Lesions associated with phytophotodermatitis classically present as painful erythematous patches and bullae in regions of furocoumarin exposure. Affected areas are well demarcated and irregularly shaped and heal with a characteristic hyperpigmented rim. They often have a downward streak pattern from the dripping juice.3 If the furocoumarins are transferred by touch, lesions can appear in the shape of handprints, which may raise alarms for physical abuse in children.4
Photochemical reactions caused by activated furocoumarins cross-link nuclear DNA and damage cell membranes. These changes lead to cellular death resulting in edema and destruction of the epidermis. Other effects include an increase in keratin and thickening of the stratum corneum. The hyperpigmentation is a result of increased concentration of melanosomes and stimulation of melanocytes by activated furocoumarins.5
Management of phytophotodermatitis depends on the severity of skin injury. Mild cases may not require any treatment, whereas the most severe ones require admission to a burn unit for wound care. Anti-inflammatory medications are the mainstay of therapy. Our patient was prescribed desonide cream 0.05% for application to the affected areas. Sunscreen should be applied to prevent worsening of hyperpigmentation, which may take months to years to fade naturally. If hyperpigmentation is cosmetically troubling to the patient, bleaching agents such as hydroquinone and retinoids or Nd:YAG laser can be used to accelerate the resolution of pigment.5
Phototoxicity differs from less common photoallergic reactions caused by preformed antibodies or a delayed cell-mediated response to a trigger. The classic presentation of photoallergy is apruritic, inflammatory, bullous eruption in a sensitized individual.6 Allergic contact dermatitis more commonly is associated with pruritus than pain, and it presents as a papulovesicular eruption that evolves into lichenified plaques.7 Porphyria cutanea tarda would likely be accompanied by other cutaneous features such as hypertrichosis and sclerodermoid plaques with dystrophic calcification, in addition to wine-colored urine-containing porphyrins.8 Bullous fixed drug eruptions develop within 48 hours of exposure to a causative agent. The patient typically would experience pruritus and burning at the site of clearly demarcated erythematous lesions that healed with hyperpigmentation.9 Lesions of bullous lupus erythematosus may appear in areas without sun exposure, and they would be more likely to leave behind hypopigmentation rather than hyperpigmentation.10
The Diagnosis: Phytophotodermatitis
A more detailed patient history revealed that there was beer with limes on the boat, but the partygoers neglected to bring a knife. The patient volunteered to tear the limes apart with his bare hands. Because he was clad only in swim trunks, lime juice splattered over various regions of his body.
Phytophotodermatitis is a phototoxic blistering rash that follows topical exposure to plant-derived furocoumarins and sunlight. (Figure) Furocoumarins are photosensitizing substances produced by certain plants, possibly as a defense mechanism against predators.1 They cause a nonimmunologic phototoxic reaction when deposited on the skin and exposed to UVA radiation. Exposure to limes is the most common precipitant of phytophotodermatitis, but other potential culprits include lemons, grapefruit, figs, carrots, parsnips, celery, and dill.2
Lesions associated with phytophotodermatitis classically present as painful erythematous patches and bullae in regions of furocoumarin exposure. Affected areas are well demarcated and irregularly shaped and heal with a characteristic hyperpigmented rim. They often have a downward streak pattern from the dripping juice.3 If the furocoumarins are transferred by touch, lesions can appear in the shape of handprints, which may raise alarms for physical abuse in children.4
Photochemical reactions caused by activated furocoumarins cross-link nuclear DNA and damage cell membranes. These changes lead to cellular death resulting in edema and destruction of the epidermis. Other effects include an increase in keratin and thickening of the stratum corneum. The hyperpigmentation is a result of increased concentration of melanosomes and stimulation of melanocytes by activated furocoumarins.5
Management of phytophotodermatitis depends on the severity of skin injury. Mild cases may not require any treatment, whereas the most severe ones require admission to a burn unit for wound care. Anti-inflammatory medications are the mainstay of therapy. Our patient was prescribed desonide cream 0.05% for application to the affected areas. Sunscreen should be applied to prevent worsening of hyperpigmentation, which may take months to years to fade naturally. If hyperpigmentation is cosmetically troubling to the patient, bleaching agents such as hydroquinone and retinoids or Nd:YAG laser can be used to accelerate the resolution of pigment.5
Phototoxicity differs from less common photoallergic reactions caused by preformed antibodies or a delayed cell-mediated response to a trigger. The classic presentation of photoallergy is apruritic, inflammatory, bullous eruption in a sensitized individual.6 Allergic contact dermatitis more commonly is associated with pruritus than pain, and it presents as a papulovesicular eruption that evolves into lichenified plaques.7 Porphyria cutanea tarda would likely be accompanied by other cutaneous features such as hypertrichosis and sclerodermoid plaques with dystrophic calcification, in addition to wine-colored urine-containing porphyrins.8 Bullous fixed drug eruptions develop within 48 hours of exposure to a causative agent. The patient typically would experience pruritus and burning at the site of clearly demarcated erythematous lesions that healed with hyperpigmentation.9 Lesions of bullous lupus erythematosus may appear in areas without sun exposure, and they would be more likely to leave behind hypopigmentation rather than hyperpigmentation.10
- Pathak MA. Phytophotodermatitis. Clin Dermatol. 1986;4:102-121.
- Egan CL, Sterling G. Phytophotodermatitis: a visit to Margaritaville. Cutis. 1993;51:41-42.
- Hankinson A, Lloyd B, Alweis R. Lime-induced phytophotodermatitis [published online ahead of print September 29, 2014]. J Community Hosp Intern Med Perspect. doi:10.3402/jchimp.v4.25090
- Fitzpatrick JK, Kohlwes J. Lime-induced phytophotodermatitis. J Gen Intern Med. 2018;33:975.
- Weber IC, Davis CP, Greeson DM. Phytophotodermatitis: the other "lime" disease. J Emerg Med. 1999;17:235-237.
- Monteiro AF, Rato M, Martins C. Drug-induced photosensitivity: photoallergic and phototoxic reactions. Clin Dermatol. 2016;34:571-581.
- Tan CH, Rasool S, Johnston GA. Contact dermatitis: allergic and irritant. Clin Dermatol. 2014;32:116-124.
- Dawe R. An overview of the cutaneous porphyrias. F1000Res. 2017;6:1906.
- Bandino JP, Wohltmann WE, Bray DW, et al. Naproxen-induced generalized bullous fixed drug eruption. Dermatol Online J. 2009;15:4.
- Contestable JJ, Edhegard KD, Meyerle JH. Bullous systemic lupus erythematosus: a review and update to diagnosis and treatment. Am J Clin Dermatol. 2014;15:517-524.
- Pathak MA. Phytophotodermatitis. Clin Dermatol. 1986;4:102-121.
- Egan CL, Sterling G. Phytophotodermatitis: a visit to Margaritaville. Cutis. 1993;51:41-42.
- Hankinson A, Lloyd B, Alweis R. Lime-induced phytophotodermatitis [published online ahead of print September 29, 2014]. J Community Hosp Intern Med Perspect. doi:10.3402/jchimp.v4.25090
- Fitzpatrick JK, Kohlwes J. Lime-induced phytophotodermatitis. J Gen Intern Med. 2018;33:975.
- Weber IC, Davis CP, Greeson DM. Phytophotodermatitis: the other "lime" disease. J Emerg Med. 1999;17:235-237.
- Monteiro AF, Rato M, Martins C. Drug-induced photosensitivity: photoallergic and phototoxic reactions. Clin Dermatol. 2016;34:571-581.
- Tan CH, Rasool S, Johnston GA. Contact dermatitis: allergic and irritant. Clin Dermatol. 2014;32:116-124.
- Dawe R. An overview of the cutaneous porphyrias. F1000Res. 2017;6:1906.
- Bandino JP, Wohltmann WE, Bray DW, et al. Naproxen-induced generalized bullous fixed drug eruption. Dermatol Online J. 2009;15:4.
- Contestable JJ, Edhegard KD, Meyerle JH. Bullous systemic lupus erythematosus: a review and update to diagnosis and treatment. Am J Clin Dermatol. 2014;15:517-524.
A 25-year-old man presented with a rash on the right hand, chest, abdomen, right thigh, and ankles of 2 weeks’ duration. He reported that the eruption began with bullous lesions following a boat trip. The bullae ruptured over the next several days, and the lesions evolved to the current appearance. Although the patient had experienced pain at the site of active blisters, he denied any current pain, itching, or bleeding from the lesions. No other medical comorbidities were present.
Aquatic Antagonists: Sponge Dermatitis
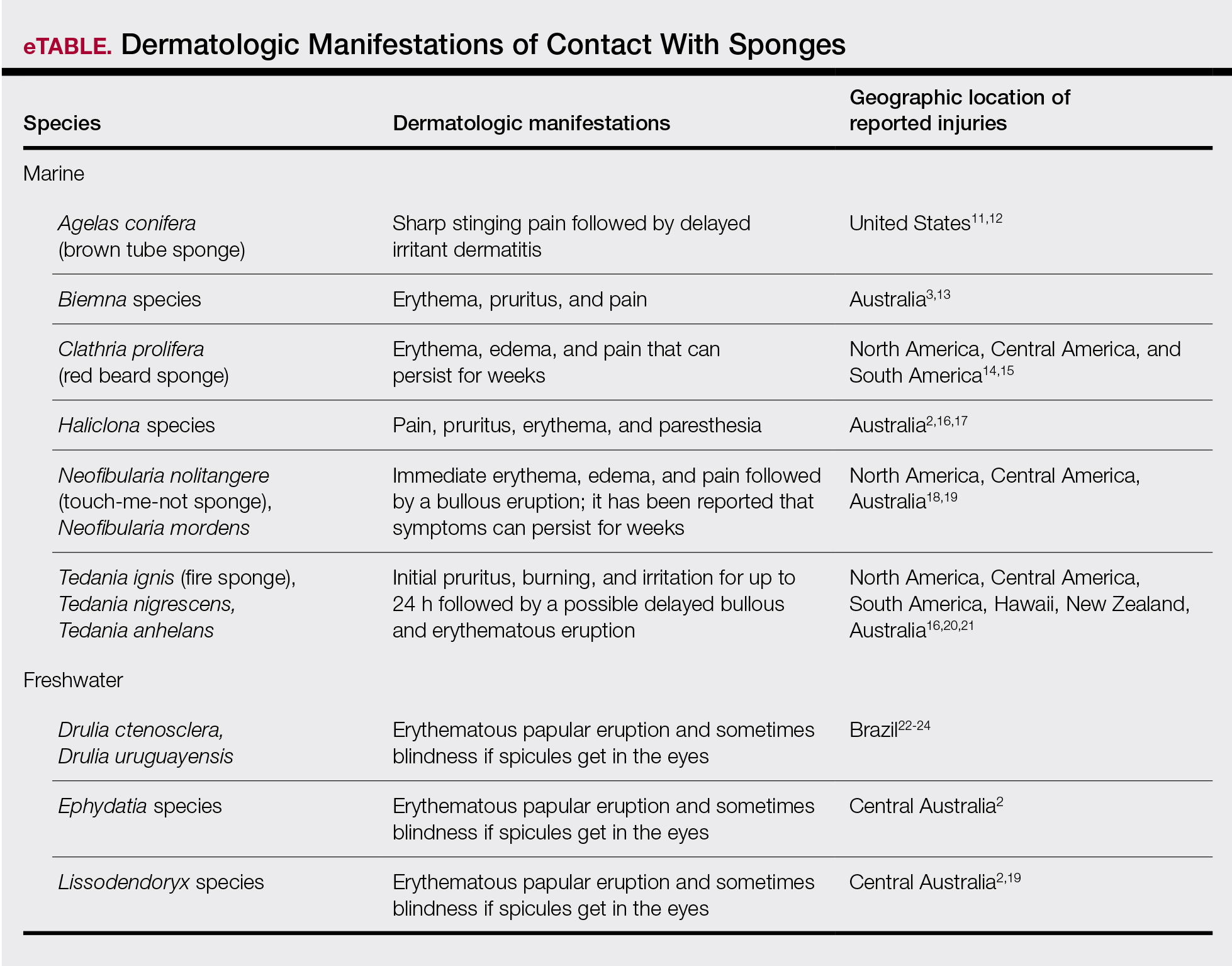
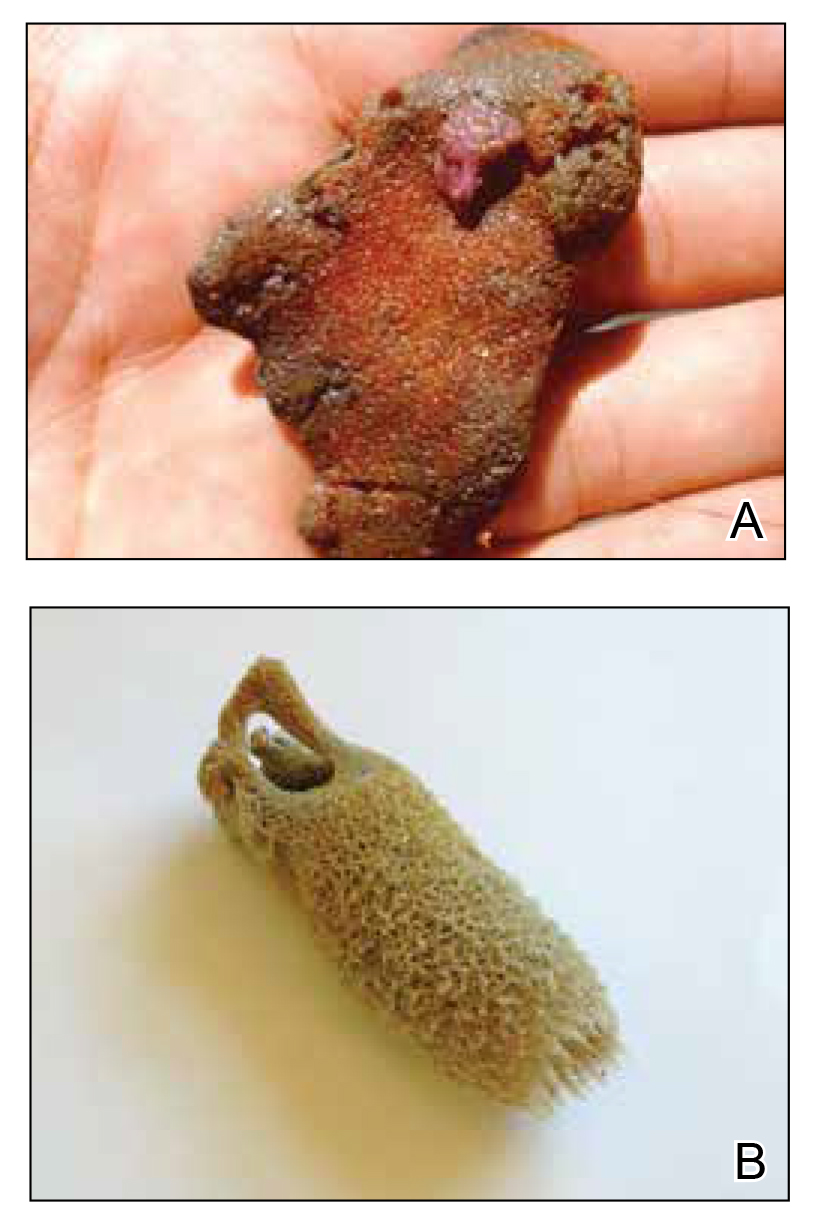
Sponges are among the oldest animals on earth, appearing more than 640 million years ago before the Cambrian explosion, a period when most major animal phyla appeared in the fossil records.1 More than 10,000 species of sponges have been identified worldwide and are distributed from polar to tropical regions in both marine (Figure 1) and freshwater (Figure 2) environments. They inhabit both shallow waters as well as depths of more than 2800 m, with shallower sponges tending to be more vibrantly colored than their deeper counterparts. The wide-ranging habitats of sponges have led to size variations from as small as 0.05 mm to more than 3 m in height.2 Their taxonomic phylum, Porifera (meaning pore bearers), is derived from the millions of pores lining the surface of the sponge that are used to filter planktonic organisms.3 Flagellated epithelioid cells called choanocytes line the internal chambers of sponges, creating a water current that promotes filter feeding as well as nutrient absorption across their microvilli.4 The body walls of many sponges consist of a collagenous skeleton made up of spongin and spicules of silicon dioxide (silica) or calcium carbonate embedded in the spongin connective tissue matrix.5 Bath sponges lack silica spicules.
Sponges have been used in medicine for centuries. The first use in Western culture was recorded in 405
Mechanisms and Symptoms of Injury
Bathing sponges (silk sponges) derived from Spongia officinalis are harmless. Other sponges can exert their damaging effects through a variety of mechanisms that lead to dermatologic manifestations (eTable). Some species of sponges produce and secrete toxic metabolites (eg, crinotoxins) onto the body surface or into the surrounding water. They also are capable of synthesizing a mucous slime that can be irritating to human skin. Direct trauma also can be caused by fragments of the silica or calcium carbonate sponge skeleton penetrating the skin. Stinging members of the phylum Cnidaria can colonize the sponge, leading to injury when a human handles the sponge.25-27
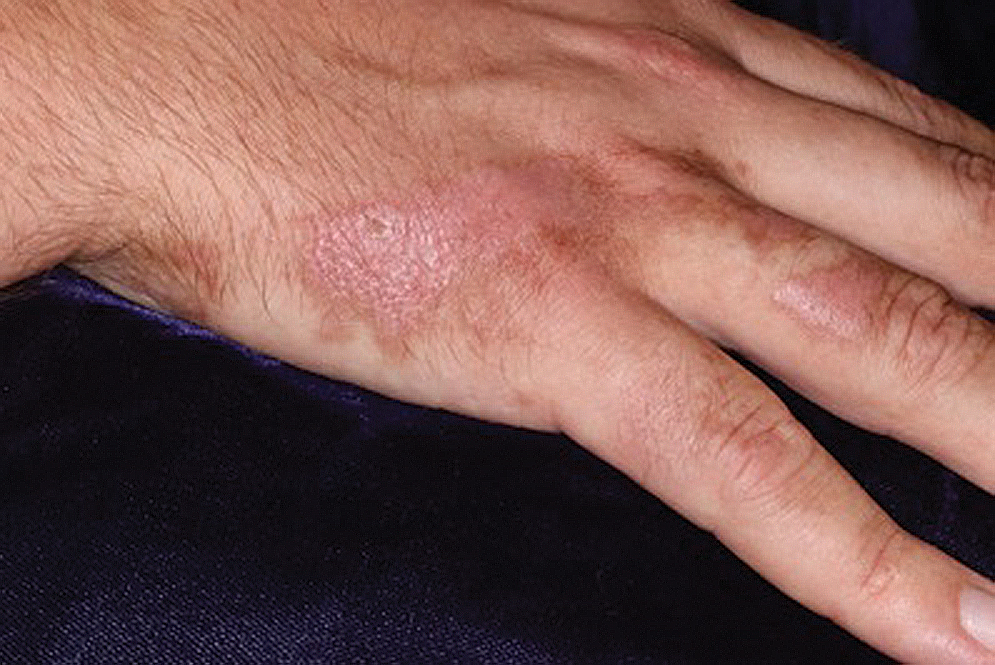
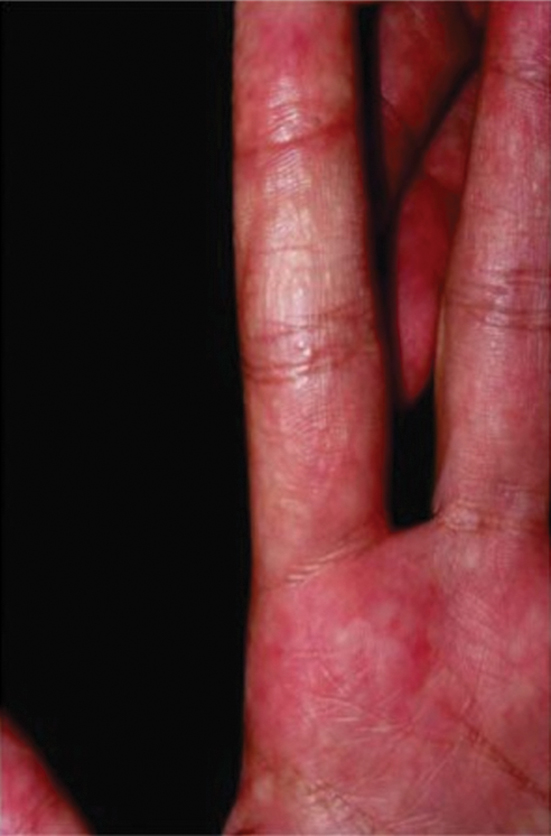
Sponge dermatitis can be divided into 2 major categories: an initial pruritic dermatitis (Figure 3) that occurs within 20 minutes to a few hours after contact and a delayed irritant dermatitis caused by penetration of the spicules and chemical agents into skin.28 Importantly, different species can lead to varying manifestations.
The initial pruritic dermatitis is characterized by itching and burning that progresses to local edema, vesiculation, joint swelling, and stiffness. Because most contact with sponges occurs with handling, joint immobility may ensue within 24 hours of the encounter. Rarely, larger areas of the skin are affected, and fever, chills, malaise, dizziness, nausea, purulent bullae, muscle cramps, and formication may occur.28 Anaphylactic reactions have been described in a small subset of patients. There have even been reports of delayed (ie, 1–2 weeks following exposure) erythema multiforme, livedo reticularis, purpura, and dyshidrotic eczema.16,20,29 The irritant dermatitis caused by spicule trauma is due to a foreign body reaction that can be exacerbated by toxins entering the skin. In severe cases, desquamation, recurrent eczema, and arthralgia can occur.30 In general, more mild cases should self-resolve within 3 to 7 days. Dermatologic conditions also can be caused by organisms that inhabit sponges and as a result produce a dermatitis when the sponge is handled, including sponge divers disease (maladie des plongeurs), a necrotic dermatitis caused by stinging Cnidaria species.31 Dogger Bank itch, first described as a dermatitis caused by sensitization to (2-hydroxyethyl) dimethylsulfoxonium chloride, initially was isolated from the sea chervil (a type of Bryozoan); however, that same chemical also was later found in sponges, producing the same dermatitis after handling the sponge.32 Freshwater sponges also have been reported to be injurious and exist worldwide. In contrast to marine sponges, lesions from freshwater sponges are disseminated pruritic erythematous papules with ulcerations, crusts, and secondary infections.22 The disseminated nature of the dermatitis caused by freshwater sponges is due to contact with the spicules of dead sponges that are dispersed throughout the water rather than from direct handling. Sponge dermatitis occurs mostly in sponge collectors, divers, trawlers, and biology students and has been reported extensively in the United States, Caribbean Islands, Australia, New Zealand, and Brazil.18,27,33,34
Management
Treatment should consist of an initial decontamination; the skin should be dried, and adhesive tape or rubber cement should be utilized to remove any spicules embedded in the skin. Diluted vinegar soaks should be initiated for 10 to 30 minutes on the affected area(s) 3 or 4 times daily.19 The initial decontamination should occur immediately, as delay may lead to persistent purulent bullae that may take months to heal. Topical steroids may be used following the initial decontamination to help relieve inflammation. Antihistamines and nonsteroidal anti-inflammatory drugs may be used to alleviate pruritus and pain, respectively. Severe cases may require systemic glucocorticoids. Additionally, immunization status against tetanus toxoid should be assessed.35 In the event of an anaphylactic reaction, it is important to maintain a patent airway and normalized blood pressure through the use of intramuscular epinephrine.36 Frequent follow-up is warranted, as serious secondary infections can develop.37 Patients also should be counseled on the potential for delayed dermatologic reactions, including erythema multiforme. Contact between humans and coastal environments has been increasing in the last few decades; therefore, an increase in contact with sponges is to be expected.22
- Gold DA, Grabenstatter J, de Mendoza A, et al. Sterol and genomic analyses validate the sponge biomarker hypothesis. Proc Natl Acad Sci U S A. 2016;113:2684-2689.
- Bonamonte D, Filoni A, Verni P, et al. Dermatitis caused by sponges. In: Bonamonte D, Angelini G, eds. Aquatic Dermatology. 2nd ed. Springer; 2016:121-126.
- Marsh LM, Slack-Smith S, Gurry DL. Field Guide to Sea Stingers and Other Venomous and Poisonous Marine Invertebrates. 2nd ed. Western Australian Museum; 2010.
- Eid E, Al-Tawaha M. A Guide to Harmful and Toxic Creatures in the Gulf of Aqaba Jordan. The Royal Marine Conservation Society of Jordan; 2016.
- Reese E, Depenbrock P. Water envenomations and stings. Curr Sports Med Rep. 2014;13:126-131.
- Dormandy TL. Trace element analysis of hair. Br Med J (Clin Res Ed). 1986;293:975-976.
- Voultsiadou E. Sponges: an historical survey of their knowledge in Greek antiquity. J Mar Biol Assoc UK. 2007;87:1757-1763.
- Senthilkumar K, Kim SK. Marine invertebrate natural products for anti-inflammatory and chronic diseases [published online December 31, 2013]. Evid Based Complement Alternat Med. doi:10.1155/2013/572859
- Sagar S, Kaur M, Minneman KP. Antiviral lead compounds from marine sponges. Mar Drugs. 2010;8:2619-2638.
- Usagawa T, Nishimura M, Itoh Y, et al. Preparation of monoclonal antibodies against okadaic acid prepared from the sponge Halichondria okadai. Toxicon. 1989;27:1323-1330.
- Elston DM. Aquatic antagonists: sponge dermatitis. Cutis. 2007;80:279-280.
- Parra-Velandia FJ, Zea S, Van Soest RW. Reef sponges of the genus Agelas (Porifera: Demospongiae) from the Greater Caribbean. Zootaxa. 2014;3794:301-343.
- Hooper JN, Capon RJ, Hodder RA. A new species of toxic marine sponge (Porifera: Demospongiae: Poecilosclerida) from northwest Australia. The Beagle, Records of the Northern Territory Museum of Arts and sciences. 1991;8:27-36.
- Burnett JW, Calton GJ, Morgan RJ. Dermatitis due to stinging sponges. Cutis. 1987;39:476.
- Kizer KW. Marine envenomations. J Toxicol Clin Toxicol. 1983;21:527-555.
- Isbister GK, Hooper JN. Clinical effects of stings by sponges of the genus Tedania and a review of sponge stings worldwide. Toxicon. 2005;46:782-785.
- Fromont J, Abdo DA. New species of Haliclona (Demospongiae: Haplosclerida: Chalinidae) from Western Australia. Zootaxa. 2014;3835:97-109.
- Flachsenberger W, Holmes NJ, Leigh C, et al. Properties of the extract and spicules of the dermatitis inducing sponge Neofibularia mordens Hartman. J Toxicol Clin Toxicol. 1987;25:255-272.
- Southcott RV, Coulter JR. The effects of the southern Australian marine stinging sponges, Neofibularia mordens and Lissodendoryx sp. Med J Aust. 1971;2:895-901.
- Yaffee HS, Stargardter F. Erythema multiforme from Tedania ignis. report of a case and an experimental study of the mechanism of cutaneous irritation from the fire sponge. Arch Dermatol. 1963;87:601-604.
- Yaffee HS. Irritation from red sponge. N Engl J Med. 1970;282:51.
- Haddad V Jr. Environmental dermatology: skin manifestations of injuries caused by invertebrate aquatic animals. An Bras Dermatol. 2013;88:496-506.
- Volkmer-Ribeiro C, Lenzi HL, Orefice F, et al. Freshwater sponge spicules: a new agent of ocular pathology. Mem Inst Oswaldo Cruz. 2006;101:899-903.
- Cruz AA, Alencar VM, Medina NH, et al. Dangerous waters: outbreak of eye lesions caused by fresh water sponge spicules. Eye (Lond). 2013;27:398-402.
- Haddad V Jr. Clinical and therapeutic aspects of envenomations caused by sponges and jellyfish. In: Gopalakrishnakone P, Haddad V Jr, Kem WR, et al, eds. Marine and Freshwater Toxins. Springer; 2016:317-325.
- Haddad V Jr, Lupi O, Lonza JP, et al. Tropical dermatology: marine and aquatic dermatology. J Am Acad Dermatol. 2009;61:733-750.
- Gaastra MT. Aquatic skin disorders. In: Faber WR, Hay RJ, Naafs B, eds. Imported Skin Diseases. 2nd ed. Wiley; 2012:283-292.
- Auerbach P. Envenomation by aquatic invertebrates. In: Auerbach P, ed. Wilderness Medicine. 6th ed. Elsevier Mosby; 2011;1596-1627.
- Sims JK, Irei MY. Human Hawaiian marine sponge poisoning. Hawaii Med J. 1979;38:263-270.
- Haddad V Jr. Aquatic animals of medical importance in Brazil. Rev Soc Bras Med Trop. 2003;36:591-597.
- Tlougan BE, Podjasek JO, Adams BB. Aquatic sports dermatoses. part 2—in the water: saltwater dermatoses. Int J Dermatol. 2010;49:994-1002.
- Warabi K, Nakao Y, Matsunaga S, et al. Dogger Bank itch revisited: isolation of (2-hydroxyethyl) dimethylsulfoxonium chloride as a cytotoxic constituent from the marine sponge Theonella aff. mirabilis. Comp Biochem Physiol B Biochem Mol Biol. 2001;128:27-30.
- Southcott R. Human injuries from invertebrate animals in the Australian seas. Clin Toxicol. 1970;3:617-636.
- Russell FE. Sponge injury—traumatic, toxic or allergic? N Engl J Med. 1970;282:753-754.
- Hornbeak KB, Auerbach PS. Marine envenomation. Emerg Med Clin North Am. 2017;35:321-337.
- Muraro A, Roberts G, Worm M, et al. Anaphylaxis: guidelines from the European Academy of Allergy and Clinical Immunology. Allergy. 2014;69:1026-1045.
- Kizer K, Auerbach P, Dwyer B. Marine envenomations: not just a problem of the tropics. Emerg Med Rep. 1985;6:129-135.
Sponges are among the oldest animals on earth, appearing more than 640 million years ago before the Cambrian explosion, a period when most major animal phyla appeared in the fossil records.1 More than 10,000 species of sponges have been identified worldwide and are distributed from polar to tropical regions in both marine (Figure 1) and freshwater (Figure 2) environments. They inhabit both shallow waters as well as depths of more than 2800 m, with shallower sponges tending to be more vibrantly colored than their deeper counterparts. The wide-ranging habitats of sponges have led to size variations from as small as 0.05 mm to more than 3 m in height.2 Their taxonomic phylum, Porifera (meaning pore bearers), is derived from the millions of pores lining the surface of the sponge that are used to filter planktonic organisms.3 Flagellated epithelioid cells called choanocytes line the internal chambers of sponges, creating a water current that promotes filter feeding as well as nutrient absorption across their microvilli.4 The body walls of many sponges consist of a collagenous skeleton made up of spongin and spicules of silicon dioxide (silica) or calcium carbonate embedded in the spongin connective tissue matrix.5 Bath sponges lack silica spicules.

Sponges have been used in medicine for centuries. The first use in Western culture was recorded in 405
Mechanisms and Symptoms of Injury
Bathing sponges (silk sponges) derived from Spongia officinalis are harmless. Other sponges can exert their damaging effects through a variety of mechanisms that lead to dermatologic manifestations (eTable). Some species of sponges produce and secrete toxic metabolites (eg, crinotoxins) onto the body surface or into the surrounding water. They also are capable of synthesizing a mucous slime that can be irritating to human skin. Direct trauma also can be caused by fragments of the silica or calcium carbonate sponge skeleton penetrating the skin. Stinging members of the phylum Cnidaria can colonize the sponge, leading to injury when a human handles the sponge.25-27
Sponge dermatitis can be divided into 2 major categories: an initial pruritic dermatitis (Figure 3) that occurs within 20 minutes to a few hours after contact and a delayed irritant dermatitis caused by penetration of the spicules and chemical agents into skin.28 Importantly, different species can lead to varying manifestations.
The initial pruritic dermatitis is characterized by itching and burning that progresses to local edema, vesiculation, joint swelling, and stiffness. Because most contact with sponges occurs with handling, joint immobility may ensue within 24 hours of the encounter. Rarely, larger areas of the skin are affected, and fever, chills, malaise, dizziness, nausea, purulent bullae, muscle cramps, and formication may occur.28 Anaphylactic reactions have been described in a small subset of patients. There have even been reports of delayed (ie, 1–2 weeks following exposure) erythema multiforme, livedo reticularis, purpura, and dyshidrotic eczema.16,20,29 The irritant dermatitis caused by spicule trauma is due to a foreign body reaction that can be exacerbated by toxins entering the skin. In severe cases, desquamation, recurrent eczema, and arthralgia can occur.30 In general, more mild cases should self-resolve within 3 to 7 days. Dermatologic conditions also can be caused by organisms that inhabit sponges and as a result produce a dermatitis when the sponge is handled, including sponge divers disease (maladie des plongeurs), a necrotic dermatitis caused by stinging Cnidaria species.31 Dogger Bank itch, first described as a dermatitis caused by sensitization to (2-hydroxyethyl) dimethylsulfoxonium chloride, initially was isolated from the sea chervil (a type of Bryozoan); however, that same chemical also was later found in sponges, producing the same dermatitis after handling the sponge.32 Freshwater sponges also have been reported to be injurious and exist worldwide. In contrast to marine sponges, lesions from freshwater sponges are disseminated pruritic erythematous papules with ulcerations, crusts, and secondary infections.22 The disseminated nature of the dermatitis caused by freshwater sponges is due to contact with the spicules of dead sponges that are dispersed throughout the water rather than from direct handling. Sponge dermatitis occurs mostly in sponge collectors, divers, trawlers, and biology students and has been reported extensively in the United States, Caribbean Islands, Australia, New Zealand, and Brazil.18,27,33,34
Management
Treatment should consist of an initial decontamination; the skin should be dried, and adhesive tape or rubber cement should be utilized to remove any spicules embedded in the skin. Diluted vinegar soaks should be initiated for 10 to 30 minutes on the affected area(s) 3 or 4 times daily.19 The initial decontamination should occur immediately, as delay may lead to persistent purulent bullae that may take months to heal. Topical steroids may be used following the initial decontamination to help relieve inflammation. Antihistamines and nonsteroidal anti-inflammatory drugs may be used to alleviate pruritus and pain, respectively. Severe cases may require systemic glucocorticoids. Additionally, immunization status against tetanus toxoid should be assessed.35 In the event of an anaphylactic reaction, it is important to maintain a patent airway and normalized blood pressure through the use of intramuscular epinephrine.36 Frequent follow-up is warranted, as serious secondary infections can develop.37 Patients also should be counseled on the potential for delayed dermatologic reactions, including erythema multiforme. Contact between humans and coastal environments has been increasing in the last few decades; therefore, an increase in contact with sponges is to be expected.22
Sponges are among the oldest animals on earth, appearing more than 640 million years ago before the Cambrian explosion, a period when most major animal phyla appeared in the fossil records.1 More than 10,000 species of sponges have been identified worldwide and are distributed from polar to tropical regions in both marine (Figure 1) and freshwater (Figure 2) environments. They inhabit both shallow waters as well as depths of more than 2800 m, with shallower sponges tending to be more vibrantly colored than their deeper counterparts. The wide-ranging habitats of sponges have led to size variations from as small as 0.05 mm to more than 3 m in height.2 Their taxonomic phylum, Porifera (meaning pore bearers), is derived from the millions of pores lining the surface of the sponge that are used to filter planktonic organisms.3 Flagellated epithelioid cells called choanocytes line the internal chambers of sponges, creating a water current that promotes filter feeding as well as nutrient absorption across their microvilli.4 The body walls of many sponges consist of a collagenous skeleton made up of spongin and spicules of silicon dioxide (silica) or calcium carbonate embedded in the spongin connective tissue matrix.5 Bath sponges lack silica spicules.

Sponges have been used in medicine for centuries. The first use in Western culture was recorded in 405
Mechanisms and Symptoms of Injury
Bathing sponges (silk sponges) derived from Spongia officinalis are harmless. Other sponges can exert their damaging effects through a variety of mechanisms that lead to dermatologic manifestations (eTable). Some species of sponges produce and secrete toxic metabolites (eg, crinotoxins) onto the body surface or into the surrounding water. They also are capable of synthesizing a mucous slime that can be irritating to human skin. Direct trauma also can be caused by fragments of the silica or calcium carbonate sponge skeleton penetrating the skin. Stinging members of the phylum Cnidaria can colonize the sponge, leading to injury when a human handles the sponge.25-27
Sponge dermatitis can be divided into 2 major categories: an initial pruritic dermatitis (Figure 3) that occurs within 20 minutes to a few hours after contact and a delayed irritant dermatitis caused by penetration of the spicules and chemical agents into skin.28 Importantly, different species can lead to varying manifestations.
The initial pruritic dermatitis is characterized by itching and burning that progresses to local edema, vesiculation, joint swelling, and stiffness. Because most contact with sponges occurs with handling, joint immobility may ensue within 24 hours of the encounter. Rarely, larger areas of the skin are affected, and fever, chills, malaise, dizziness, nausea, purulent bullae, muscle cramps, and formication may occur.28 Anaphylactic reactions have been described in a small subset of patients. There have even been reports of delayed (ie, 1–2 weeks following exposure) erythema multiforme, livedo reticularis, purpura, and dyshidrotic eczema.16,20,29 The irritant dermatitis caused by spicule trauma is due to a foreign body reaction that can be exacerbated by toxins entering the skin. In severe cases, desquamation, recurrent eczema, and arthralgia can occur.30 In general, more mild cases should self-resolve within 3 to 7 days. Dermatologic conditions also can be caused by organisms that inhabit sponges and as a result produce a dermatitis when the sponge is handled, including sponge divers disease (maladie des plongeurs), a necrotic dermatitis caused by stinging Cnidaria species.31 Dogger Bank itch, first described as a dermatitis caused by sensitization to (2-hydroxyethyl) dimethylsulfoxonium chloride, initially was isolated from the sea chervil (a type of Bryozoan); however, that same chemical also was later found in sponges, producing the same dermatitis after handling the sponge.32 Freshwater sponges also have been reported to be injurious and exist worldwide. In contrast to marine sponges, lesions from freshwater sponges are disseminated pruritic erythematous papules with ulcerations, crusts, and secondary infections.22 The disseminated nature of the dermatitis caused by freshwater sponges is due to contact with the spicules of dead sponges that are dispersed throughout the water rather than from direct handling. Sponge dermatitis occurs mostly in sponge collectors, divers, trawlers, and biology students and has been reported extensively in the United States, Caribbean Islands, Australia, New Zealand, and Brazil.18,27,33,34
Management
Treatment should consist of an initial decontamination; the skin should be dried, and adhesive tape or rubber cement should be utilized to remove any spicules embedded in the skin. Diluted vinegar soaks should be initiated for 10 to 30 minutes on the affected area(s) 3 or 4 times daily.19 The initial decontamination should occur immediately, as delay may lead to persistent purulent bullae that may take months to heal. Topical steroids may be used following the initial decontamination to help relieve inflammation. Antihistamines and nonsteroidal anti-inflammatory drugs may be used to alleviate pruritus and pain, respectively. Severe cases may require systemic glucocorticoids. Additionally, immunization status against tetanus toxoid should be assessed.35 In the event of an anaphylactic reaction, it is important to maintain a patent airway and normalized blood pressure through the use of intramuscular epinephrine.36 Frequent follow-up is warranted, as serious secondary infections can develop.37 Patients also should be counseled on the potential for delayed dermatologic reactions, including erythema multiforme. Contact between humans and coastal environments has been increasing in the last few decades; therefore, an increase in contact with sponges is to be expected.22
- Gold DA, Grabenstatter J, de Mendoza A, et al. Sterol and genomic analyses validate the sponge biomarker hypothesis. Proc Natl Acad Sci U S A. 2016;113:2684-2689.
- Bonamonte D, Filoni A, Verni P, et al. Dermatitis caused by sponges. In: Bonamonte D, Angelini G, eds. Aquatic Dermatology. 2nd ed. Springer; 2016:121-126.
- Marsh LM, Slack-Smith S, Gurry DL. Field Guide to Sea Stingers and Other Venomous and Poisonous Marine Invertebrates. 2nd ed. Western Australian Museum; 2010.
- Eid E, Al-Tawaha M. A Guide to Harmful and Toxic Creatures in the Gulf of Aqaba Jordan. The Royal Marine Conservation Society of Jordan; 2016.
- Reese E, Depenbrock P. Water envenomations and stings. Curr Sports Med Rep. 2014;13:126-131.
- Dormandy TL. Trace element analysis of hair. Br Med J (Clin Res Ed). 1986;293:975-976.
- Voultsiadou E. Sponges: an historical survey of their knowledge in Greek antiquity. J Mar Biol Assoc UK. 2007;87:1757-1763.
- Senthilkumar K, Kim SK. Marine invertebrate natural products for anti-inflammatory and chronic diseases [published online December 31, 2013]. Evid Based Complement Alternat Med. doi:10.1155/2013/572859
- Sagar S, Kaur M, Minneman KP. Antiviral lead compounds from marine sponges. Mar Drugs. 2010;8:2619-2638.
- Usagawa T, Nishimura M, Itoh Y, et al. Preparation of monoclonal antibodies against okadaic acid prepared from the sponge Halichondria okadai. Toxicon. 1989;27:1323-1330.
- Elston DM. Aquatic antagonists: sponge dermatitis. Cutis. 2007;80:279-280.
- Parra-Velandia FJ, Zea S, Van Soest RW. Reef sponges of the genus Agelas (Porifera: Demospongiae) from the Greater Caribbean. Zootaxa. 2014;3794:301-343.
- Hooper JN, Capon RJ, Hodder RA. A new species of toxic marine sponge (Porifera: Demospongiae: Poecilosclerida) from northwest Australia. The Beagle, Records of the Northern Territory Museum of Arts and sciences. 1991;8:27-36.
- Burnett JW, Calton GJ, Morgan RJ. Dermatitis due to stinging sponges. Cutis. 1987;39:476.
- Kizer KW. Marine envenomations. J Toxicol Clin Toxicol. 1983;21:527-555.
- Isbister GK, Hooper JN. Clinical effects of stings by sponges of the genus Tedania and a review of sponge stings worldwide. Toxicon. 2005;46:782-785.
- Fromont J, Abdo DA. New species of Haliclona (Demospongiae: Haplosclerida: Chalinidae) from Western Australia. Zootaxa. 2014;3835:97-109.
- Flachsenberger W, Holmes NJ, Leigh C, et al. Properties of the extract and spicules of the dermatitis inducing sponge Neofibularia mordens Hartman. J Toxicol Clin Toxicol. 1987;25:255-272.
- Southcott RV, Coulter JR. The effects of the southern Australian marine stinging sponges, Neofibularia mordens and Lissodendoryx sp. Med J Aust. 1971;2:895-901.
- Yaffee HS, Stargardter F. Erythema multiforme from Tedania ignis. report of a case and an experimental study of the mechanism of cutaneous irritation from the fire sponge. Arch Dermatol. 1963;87:601-604.
- Yaffee HS. Irritation from red sponge. N Engl J Med. 1970;282:51.
- Haddad V Jr. Environmental dermatology: skin manifestations of injuries caused by invertebrate aquatic animals. An Bras Dermatol. 2013;88:496-506.
- Volkmer-Ribeiro C, Lenzi HL, Orefice F, et al. Freshwater sponge spicules: a new agent of ocular pathology. Mem Inst Oswaldo Cruz. 2006;101:899-903.
- Cruz AA, Alencar VM, Medina NH, et al. Dangerous waters: outbreak of eye lesions caused by fresh water sponge spicules. Eye (Lond). 2013;27:398-402.
- Haddad V Jr. Clinical and therapeutic aspects of envenomations caused by sponges and jellyfish. In: Gopalakrishnakone P, Haddad V Jr, Kem WR, et al, eds. Marine and Freshwater Toxins. Springer; 2016:317-325.
- Haddad V Jr, Lupi O, Lonza JP, et al. Tropical dermatology: marine and aquatic dermatology. J Am Acad Dermatol. 2009;61:733-750.
- Gaastra MT. Aquatic skin disorders. In: Faber WR, Hay RJ, Naafs B, eds. Imported Skin Diseases. 2nd ed. Wiley; 2012:283-292.
- Auerbach P. Envenomation by aquatic invertebrates. In: Auerbach P, ed. Wilderness Medicine. 6th ed. Elsevier Mosby; 2011;1596-1627.
- Sims JK, Irei MY. Human Hawaiian marine sponge poisoning. Hawaii Med J. 1979;38:263-270.
- Haddad V Jr. Aquatic animals of medical importance in Brazil. Rev Soc Bras Med Trop. 2003;36:591-597.
- Tlougan BE, Podjasek JO, Adams BB. Aquatic sports dermatoses. part 2—in the water: saltwater dermatoses. Int J Dermatol. 2010;49:994-1002.
- Warabi K, Nakao Y, Matsunaga S, et al. Dogger Bank itch revisited: isolation of (2-hydroxyethyl) dimethylsulfoxonium chloride as a cytotoxic constituent from the marine sponge Theonella aff. mirabilis. Comp Biochem Physiol B Biochem Mol Biol. 2001;128:27-30.
- Southcott R. Human injuries from invertebrate animals in the Australian seas. Clin Toxicol. 1970;3:617-636.
- Russell FE. Sponge injury—traumatic, toxic or allergic? N Engl J Med. 1970;282:753-754.
- Hornbeak KB, Auerbach PS. Marine envenomation. Emerg Med Clin North Am. 2017;35:321-337.
- Muraro A, Roberts G, Worm M, et al. Anaphylaxis: guidelines from the European Academy of Allergy and Clinical Immunology. Allergy. 2014;69:1026-1045.
- Kizer K, Auerbach P, Dwyer B. Marine envenomations: not just a problem of the tropics. Emerg Med Rep. 1985;6:129-135.
- Gold DA, Grabenstatter J, de Mendoza A, et al. Sterol and genomic analyses validate the sponge biomarker hypothesis. Proc Natl Acad Sci U S A. 2016;113:2684-2689.
- Bonamonte D, Filoni A, Verni P, et al. Dermatitis caused by sponges. In: Bonamonte D, Angelini G, eds. Aquatic Dermatology. 2nd ed. Springer; 2016:121-126.
- Marsh LM, Slack-Smith S, Gurry DL. Field Guide to Sea Stingers and Other Venomous and Poisonous Marine Invertebrates. 2nd ed. Western Australian Museum; 2010.
- Eid E, Al-Tawaha M. A Guide to Harmful and Toxic Creatures in the Gulf of Aqaba Jordan. The Royal Marine Conservation Society of Jordan; 2016.
- Reese E, Depenbrock P. Water envenomations and stings. Curr Sports Med Rep. 2014;13:126-131.
- Dormandy TL. Trace element analysis of hair. Br Med J (Clin Res Ed). 1986;293:975-976.
- Voultsiadou E. Sponges: an historical survey of their knowledge in Greek antiquity. J Mar Biol Assoc UK. 2007;87:1757-1763.
- Senthilkumar K, Kim SK. Marine invertebrate natural products for anti-inflammatory and chronic diseases [published online December 31, 2013]. Evid Based Complement Alternat Med. doi:10.1155/2013/572859
- Sagar S, Kaur M, Minneman KP. Antiviral lead compounds from marine sponges. Mar Drugs. 2010;8:2619-2638.
- Usagawa T, Nishimura M, Itoh Y, et al. Preparation of monoclonal antibodies against okadaic acid prepared from the sponge Halichondria okadai. Toxicon. 1989;27:1323-1330.
- Elston DM. Aquatic antagonists: sponge dermatitis. Cutis. 2007;80:279-280.
- Parra-Velandia FJ, Zea S, Van Soest RW. Reef sponges of the genus Agelas (Porifera: Demospongiae) from the Greater Caribbean. Zootaxa. 2014;3794:301-343.
- Hooper JN, Capon RJ, Hodder RA. A new species of toxic marine sponge (Porifera: Demospongiae: Poecilosclerida) from northwest Australia. The Beagle, Records of the Northern Territory Museum of Arts and sciences. 1991;8:27-36.
- Burnett JW, Calton GJ, Morgan RJ. Dermatitis due to stinging sponges. Cutis. 1987;39:476.
- Kizer KW. Marine envenomations. J Toxicol Clin Toxicol. 1983;21:527-555.
- Isbister GK, Hooper JN. Clinical effects of stings by sponges of the genus Tedania and a review of sponge stings worldwide. Toxicon. 2005;46:782-785.
- Fromont J, Abdo DA. New species of Haliclona (Demospongiae: Haplosclerida: Chalinidae) from Western Australia. Zootaxa. 2014;3835:97-109.
- Flachsenberger W, Holmes NJ, Leigh C, et al. Properties of the extract and spicules of the dermatitis inducing sponge Neofibularia mordens Hartman. J Toxicol Clin Toxicol. 1987;25:255-272.
- Southcott RV, Coulter JR. The effects of the southern Australian marine stinging sponges, Neofibularia mordens and Lissodendoryx sp. Med J Aust. 1971;2:895-901.
- Yaffee HS, Stargardter F. Erythema multiforme from Tedania ignis. report of a case and an experimental study of the mechanism of cutaneous irritation from the fire sponge. Arch Dermatol. 1963;87:601-604.
- Yaffee HS. Irritation from red sponge. N Engl J Med. 1970;282:51.
- Haddad V Jr. Environmental dermatology: skin manifestations of injuries caused by invertebrate aquatic animals. An Bras Dermatol. 2013;88:496-506.
- Volkmer-Ribeiro C, Lenzi HL, Orefice F, et al. Freshwater sponge spicules: a new agent of ocular pathology. Mem Inst Oswaldo Cruz. 2006;101:899-903.
- Cruz AA, Alencar VM, Medina NH, et al. Dangerous waters: outbreak of eye lesions caused by fresh water sponge spicules. Eye (Lond). 2013;27:398-402.
- Haddad V Jr. Clinical and therapeutic aspects of envenomations caused by sponges and jellyfish. In: Gopalakrishnakone P, Haddad V Jr, Kem WR, et al, eds. Marine and Freshwater Toxins. Springer; 2016:317-325.
- Haddad V Jr, Lupi O, Lonza JP, et al. Tropical dermatology: marine and aquatic dermatology. J Am Acad Dermatol. 2009;61:733-750.
- Gaastra MT. Aquatic skin disorders. In: Faber WR, Hay RJ, Naafs B, eds. Imported Skin Diseases. 2nd ed. Wiley; 2012:283-292.
- Auerbach P. Envenomation by aquatic invertebrates. In: Auerbach P, ed. Wilderness Medicine. 6th ed. Elsevier Mosby; 2011;1596-1627.
- Sims JK, Irei MY. Human Hawaiian marine sponge poisoning. Hawaii Med J. 1979;38:263-270.
- Haddad V Jr. Aquatic animals of medical importance in Brazil. Rev Soc Bras Med Trop. 2003;36:591-597.
- Tlougan BE, Podjasek JO, Adams BB. Aquatic sports dermatoses. part 2—in the water: saltwater dermatoses. Int J Dermatol. 2010;49:994-1002.
- Warabi K, Nakao Y, Matsunaga S, et al. Dogger Bank itch revisited: isolation of (2-hydroxyethyl) dimethylsulfoxonium chloride as a cytotoxic constituent from the marine sponge Theonella aff. mirabilis. Comp Biochem Physiol B Biochem Mol Biol. 2001;128:27-30.
- Southcott R. Human injuries from invertebrate animals in the Australian seas. Clin Toxicol. 1970;3:617-636.
- Russell FE. Sponge injury—traumatic, toxic or allergic? N Engl J Med. 1970;282:753-754.
- Hornbeak KB, Auerbach PS. Marine envenomation. Emerg Med Clin North Am. 2017;35:321-337.
- Muraro A, Roberts G, Worm M, et al. Anaphylaxis: guidelines from the European Academy of Allergy and Clinical Immunology. Allergy. 2014;69:1026-1045.
- Kizer K, Auerbach P, Dwyer B. Marine envenomations: not just a problem of the tropics. Emerg Med Rep. 1985;6:129-135.
Practice Points
- Sponges exist in both marine and freshwater environments throughout the world.
- Immediate management of sponge dermatitis should include decontamination by removing the sponge spicules with tape or rubber cement followed by dilute vinegar soaks.
- Topical steroids may be used only after initial decontamination. Use of oral steroids may be needed for more severe reactions.
What’s Eating You? Human Flea (Pulex irritans)
Characteristics
The ubiquitous human flea, Pulex irritans, is a hematophagous wingless ectoparasite in the order Siphonaptera (wingless siphon) that survives by consuming the blood of its mammalian and avian hosts. Due to diseases such as the bubonic plague, fleas have claimed more victims than all the wars ever fought; in the 14th century, the Black Death caused more than 200 million deaths. Fleas fossilized in amber have been found to be 200 million years old and closely resemble the modern human flea, demonstrating the resilience of the species.
The adult human flea is a small, reddish brown, laterally compressed, wingless insect that is approximately 2- to 3.5-mm long (females, 2.5–3.5 mm; males, 2–2.5 mm) and enclosed by a tough cuticle. Compared to the dog flea (Ctenocephalides canis) and cat flea (Ctenocephalides felis), P irritans has no combs or ctenidia (Figure 1). Fleas have large powerful hind legs enabling them to jump horizontally or vertically 200 times their body length (equivalent to a 6-foot human jumping 1200 feet) using stored muscle energy in a pad on the hind legs composed of the elastic protein resilin.1 They feed off a wide variety of hosts, including humans, pigs, cats, dogs, goats, sheep, cattle, chickens, owls, foxes, rabbits, mice, and feral cats. The flea’s mouthparts are highly specialized for piercing the skin and sucking its blood meal via direct capillary cannulation.
Life Cycle
There are 4 stages of the flea life cycle: egg, larva, pupa, and adult. Most adult flea species mate on the host; the female will lay an average of 4 to 8 small white eggs on the host after each blood meal, laying more than 400 eggs during her lifetime. The eggs then drop from the host and hatch in approximately 4 to 6 days to become larvae. The active larvae feed on available organic matter in their environment, such as their parents’ feces and detritus, while undergoing 3 molts within 1 week to several months.2 The larva then spins a silken cocoon from modified salivary glands to form the pupa. In favorable conditions, the pupa lasts only a few weeks; however, it can last for a year or more in unfavorable conditions. Triggers for emergence of the adult flea from the pupa include high humidity, warm temperatures, increased levels of carbon dioxide, and vibrations including sound. An adult P irritans flea can live for a few weeks to more than 1.5 years in favorable conditions of lower air temperature, high relative humidity, and access to a host.3
Related Diseases
Pulex irritans can be a vector for several human diseases. Yersinia pestis is a gram-negative bacteria that causes plague, a highly virulent disease that killed millions of people during its 3 largest human pandemics. The black rat (Rattus rattus) and the oriental rat flea (Xenopsylla cheopis) have been implicated as initial vectors; however, transmission may be human-to-human with pneumonic plague, and septicemic plague may be spread via Pulex fleas or body lice.4,5 In 1971, Y pestis was isolated from P irritans on a dog in the home of a plague patient in Kayenta, Arizona.6Yersinia pestis bacterial DNA also was extracted from P irritans during a plague outbreak in Madagascar in 20147 and was implicated in epidemiologic studies of plague in Tanzania from 1986 to 2004, suggesting it also plays a role in endemic disease.8
Bartonellosis is an emerging disease caused by different species of the gram-negative intracellular bacteria of the genus Bartonella transmitted by lice, ticks, and fleas. Bartonella quintana causes trench fever primarily transmitted by the human body louse, Pediculus humanus corporis, and resulted in more than 1 million cases during World War I. Trench fever is characterized by headache, fever, dizziness, and shin pain that lasts 1 to 3 days and recurs in cycles every 4 to 6 days. Other clinical manifestations of B quintana include chronic bacteremia, endocarditis, lymphadenopathy, and bacillary angiomatosis.9Bartonella henselae causes cat scratch fever, characterized by lymphadenopathy, fever, headache, joint pain, and lethargy from infected cat scratches or the bite of an infected flea. Bartonella rochalimae also has been found to cause a trench fever–like bacteremia.10Bartonella species have been found in P irritans, and the flea is implicated as a vector of bartonellosis in humans.11-15
Rickettsioses are worldwide diseases caused by the gram-negative intracellular bacteria of the genus Rickettsia transmitted to humans via hematophagous arthropods. The rickettsiae traditionally have been classified into the spotted fever or typhus groups. The spotted fever group (ie, Rocky Mountain spotted fever, Mediterranean spotted fever) is transmitted via ticks. The typhus group is transmitted via lice (epidemic typhus) and fleas (endemic or murine typhus). Murine typhus can be caused by Rickettsia typhi in warm coastal areas around the world where the main mammal reservoir is the rat and the rat flea vector X cheopis. Clinical signs of infection are abrupt onset of fever, headaches, myalgia, malaise, and chills, with a truncal maculopapular rash progressing peripherally several days after the initial clinical signs. Rash is present in up to 50% of cases.16Rickettsia felis is an emerging flea-borne pathogen causing an acute febrile illness usually transmitted via the cat flea C felis.17Rickettsia species DNA have been found to be present in P irritans from dogs18 and livestock19 and pose a risk for causing rickettsioses in humans.
Environmental Treatment and Prevention
Flea bites present as intense, pruritic, urticarial to vesicular papules that usually are located on the lower extremities but also can be present on exposed areas of the upper extremities and hands (Figure 2). Human fleas infest clothing, and bites can be widespread. Topical antipruritics and corticosteroids can be used for controlling itch and the intense cutaneous inflammatory response. The flea host should be identified in areas of the home, school, farm, work, or local environment. House pets should be examined and treated by a veterinarian. The pet’s bedding should be washed and dried at high temperatures, and carpets and floors should be routinely vacuumed or cleaned to remove eggs, larvae, flea feces, and/or pupae. The killing of adult fleas with insecticidal products (eg, imidacloprid, fipronil, spinosad, selamectin, lufenuron, ivermectin) is the primary method of flea control. Use of insect growth regulators such as pyriproxyfen inhibits adult reproduction and blocks the organogenesis of immature larval stages via hormonal or enzymatic actions.20 The combination of an insecticide and an insect growth regulator appears to be most effective in their synergistic actions against adult fleas and larvae. There have been reports of insecticidal resistance in the flea population, especially with pyrethroids.21,22 A professional exterminator and veterinarian should be consulted. In recalcitrant cases, evaluation for other wild mammals or birds should be performed in unoccupied areas of the home such as the attic, crawl spaces, and basements, as well as inside walls.
Conclusion
The human flea, P irritans, is an important vector in the transmission of human diseases such as the bubonic plague, bartonellosis, and rickettsioses. Flea bites present as intensely pruritic, urticarial to vesicular papules that most commonly present on the lower extremities. Flea bites can be treated with topical steroids, and fleas can be controlled by a combination of insecticidal products and insect growth regulators.
- Burrow M. How fleas jump. J Exp Biol. 2009;18:2881-2883.
- Buckland PC, Sandler JP. A biogeography of the human flea, Pulex irritans L (Siphonaptera: Pulicidae). J Biogeogr. 1989;16:115-120.
- Krasnov BR. Life cycles. In: Krasnov BR, ed. Functional and Evolutional Ecology of Fleas. Cambridge, MA: Cambridge Univ Press; 2008:45-67.
- Dean KR, Krauer F, Walloe L, et al. Human ectoparasites and the spread of plague in Europe during the second pandemic. Proc Natl Acad Sci U S A. 2018;115:1304-1309.
- Hufthammer AK, Walloe L. Rats cannot have been intermediate hosts for Yersinia pestis during medieval plague epidemics in Northern Europe. J Archeol Sci. 2013;40:1752-1759.
- Archibald WS, Kunitz SJ. Detection of plague by testing serums of dogs on the Navajo Reservation. HSMHA Health Rep. 1971;86:377-380.
- Ratovonjato J, Rajerison M, Rahelinirina S, et al. Yersinia pestis in Pulex irritans fleas during plague outbreak, Madagascar. Emerg Infect Dis. 2014;20:1414-1415.
- Laudisoit A, Leirs H, Makundi RH, et al. Plague and the human flea, Tanzania. Emerg Infect Dis. 2007;13:687-693.
- Foucault C, Brouqui P, Raoult D. Bartonella quintana characteristics and clinical management. Emerg Infect Dis. 2006;12:217-223.
- Eremeeva ME, Gerns HL, Lydy SL, et al. Bacteremia, fever, and splenomegaly caused by a newly recognized bartonella species. N Engl J Med. 2007; 356:2381-2387.11.
- Marquez FJ, Millan J, Rodriguez-Liebana JJ, et al. Detection and identification of Bartonella sp. in fleas from carnivorous mammals in Andalusia, Spain. Med Vet Entomol. 2009;23:393-398.
- Perez-Martinez L, Venzal JM, Portillo A, et al. Bartonella rochalimae and other Bartonella spp. in fleas, Chile. Emerg Infect Dis. 2009;15:1150-1152.
- Sofer S, Gutierrez DM, Mumcuoglu KY, et al. Molecular detection of zoonotic bartonellae (B. henselae, B. elizabethae and B. rochalimae) in fleas collected from dogs in Israel. Med Vet Entomol. 2015;29:344-348.
- Zouari S, Khrouf F, M’ghirbi Y, et al. First molecular detection and characterization of zoonotic Bartonella species in fleas infesting domestic animals in Tunisia. Parasit Vectors. 2017;10:436.
- Rolain JM, Bourry, O, Davoust B, et al. Bartonella quintana and Rickettsia felis in Gabon. Emerg Infect Dis. 2005;11:1742-1744.
- Tsioutis C, Zafeiri M, Avramopoulos A, et al. Clinical and laboratory characteristics, epidemiology, and outcomes of murine typhus: a systematic review. Acta Trop. 2017;166:16-24.
- Brown L, Macaluso KR. Rickettsia felis, an emerging flea-borne rickettsiosis. Curr Trop Med Rep. 2016;3:27-39.
- Oteo JA, Portillo A, Potero F, et al. ‘Candidatus Rickettsia asemboensis’ and Wolbachia spp. in Ctenocephalides felis and Pulex irritans fleas removed from dogs in Ecuador. Parasit Vectors. 2014;7:455.
- Ghavami MB, Mirzadeh H, Mohammadi J, et al. Molecular survey of ITS spacer and Rickettsia infection in human flea, Pulex irritans. Parasitol Res. 2018;117:1433-1442.
- Traversa D. Fleas infesting pets in the era of emerging extra-intestinal nematodes. Parasit Vectors. 2013;6:59.
- Rust MK. Insecticide resistance in fleas. Insects. 2016;7:10.
- Ghavami MB, Haghi FP, Alibabaei Z, et al. First report of target site insensitivity to pyrethroids in human flea, Pulex irritans (Siphonaptera: Pulicidae). Pest Biochem Physiol. 2018;146:97-105.
Characteristics
The ubiquitous human flea, Pulex irritans, is a hematophagous wingless ectoparasite in the order Siphonaptera (wingless siphon) that survives by consuming the blood of its mammalian and avian hosts. Due to diseases such as the bubonic plague, fleas have claimed more victims than all the wars ever fought; in the 14th century, the Black Death caused more than 200 million deaths. Fleas fossilized in amber have been found to be 200 million years old and closely resemble the modern human flea, demonstrating the resilience of the species.
The adult human flea is a small, reddish brown, laterally compressed, wingless insect that is approximately 2- to 3.5-mm long (females, 2.5–3.5 mm; males, 2–2.5 mm) and enclosed by a tough cuticle. Compared to the dog flea (Ctenocephalides canis) and cat flea (Ctenocephalides felis), P irritans has no combs or ctenidia (Figure 1). Fleas have large powerful hind legs enabling them to jump horizontally or vertically 200 times their body length (equivalent to a 6-foot human jumping 1200 feet) using stored muscle energy in a pad on the hind legs composed of the elastic protein resilin.1 They feed off a wide variety of hosts, including humans, pigs, cats, dogs, goats, sheep, cattle, chickens, owls, foxes, rabbits, mice, and feral cats. The flea’s mouthparts are highly specialized for piercing the skin and sucking its blood meal via direct capillary cannulation.
Life Cycle
There are 4 stages of the flea life cycle: egg, larva, pupa, and adult. Most adult flea species mate on the host; the female will lay an average of 4 to 8 small white eggs on the host after each blood meal, laying more than 400 eggs during her lifetime. The eggs then drop from the host and hatch in approximately 4 to 6 days to become larvae. The active larvae feed on available organic matter in their environment, such as their parents’ feces and detritus, while undergoing 3 molts within 1 week to several months.2 The larva then spins a silken cocoon from modified salivary glands to form the pupa. In favorable conditions, the pupa lasts only a few weeks; however, it can last for a year or more in unfavorable conditions. Triggers for emergence of the adult flea from the pupa include high humidity, warm temperatures, increased levels of carbon dioxide, and vibrations including sound. An adult P irritans flea can live for a few weeks to more than 1.5 years in favorable conditions of lower air temperature, high relative humidity, and access to a host.3
Related Diseases
Pulex irritans can be a vector for several human diseases. Yersinia pestis is a gram-negative bacteria that causes plague, a highly virulent disease that killed millions of people during its 3 largest human pandemics. The black rat (Rattus rattus) and the oriental rat flea (Xenopsylla cheopis) have been implicated as initial vectors; however, transmission may be human-to-human with pneumonic plague, and septicemic plague may be spread via Pulex fleas or body lice.4,5 In 1971, Y pestis was isolated from P irritans on a dog in the home of a plague patient in Kayenta, Arizona.6Yersinia pestis bacterial DNA also was extracted from P irritans during a plague outbreak in Madagascar in 20147 and was implicated in epidemiologic studies of plague in Tanzania from 1986 to 2004, suggesting it also plays a role in endemic disease.8
Bartonellosis is an emerging disease caused by different species of the gram-negative intracellular bacteria of the genus Bartonella transmitted by lice, ticks, and fleas. Bartonella quintana causes trench fever primarily transmitted by the human body louse, Pediculus humanus corporis, and resulted in more than 1 million cases during World War I. Trench fever is characterized by headache, fever, dizziness, and shin pain that lasts 1 to 3 days and recurs in cycles every 4 to 6 days. Other clinical manifestations of B quintana include chronic bacteremia, endocarditis, lymphadenopathy, and bacillary angiomatosis.9Bartonella henselae causes cat scratch fever, characterized by lymphadenopathy, fever, headache, joint pain, and lethargy from infected cat scratches or the bite of an infected flea. Bartonella rochalimae also has been found to cause a trench fever–like bacteremia.10Bartonella species have been found in P irritans, and the flea is implicated as a vector of bartonellosis in humans.11-15
Rickettsioses are worldwide diseases caused by the gram-negative intracellular bacteria of the genus Rickettsia transmitted to humans via hematophagous arthropods. The rickettsiae traditionally have been classified into the spotted fever or typhus groups. The spotted fever group (ie, Rocky Mountain spotted fever, Mediterranean spotted fever) is transmitted via ticks. The typhus group is transmitted via lice (epidemic typhus) and fleas (endemic or murine typhus). Murine typhus can be caused by Rickettsia typhi in warm coastal areas around the world where the main mammal reservoir is the rat and the rat flea vector X cheopis. Clinical signs of infection are abrupt onset of fever, headaches, myalgia, malaise, and chills, with a truncal maculopapular rash progressing peripherally several days after the initial clinical signs. Rash is present in up to 50% of cases.16Rickettsia felis is an emerging flea-borne pathogen causing an acute febrile illness usually transmitted via the cat flea C felis.17Rickettsia species DNA have been found to be present in P irritans from dogs18 and livestock19 and pose a risk for causing rickettsioses in humans.
Environmental Treatment and Prevention
Flea bites present as intense, pruritic, urticarial to vesicular papules that usually are located on the lower extremities but also can be present on exposed areas of the upper extremities and hands (Figure 2). Human fleas infest clothing, and bites can be widespread. Topical antipruritics and corticosteroids can be used for controlling itch and the intense cutaneous inflammatory response. The flea host should be identified in areas of the home, school, farm, work, or local environment. House pets should be examined and treated by a veterinarian. The pet’s bedding should be washed and dried at high temperatures, and carpets and floors should be routinely vacuumed or cleaned to remove eggs, larvae, flea feces, and/or pupae. The killing of adult fleas with insecticidal products (eg, imidacloprid, fipronil, spinosad, selamectin, lufenuron, ivermectin) is the primary method of flea control. Use of insect growth regulators such as pyriproxyfen inhibits adult reproduction and blocks the organogenesis of immature larval stages via hormonal or enzymatic actions.20 The combination of an insecticide and an insect growth regulator appears to be most effective in their synergistic actions against adult fleas and larvae. There have been reports of insecticidal resistance in the flea population, especially with pyrethroids.21,22 A professional exterminator and veterinarian should be consulted. In recalcitrant cases, evaluation for other wild mammals or birds should be performed in unoccupied areas of the home such as the attic, crawl spaces, and basements, as well as inside walls.
Conclusion
The human flea, P irritans, is an important vector in the transmission of human diseases such as the bubonic plague, bartonellosis, and rickettsioses. Flea bites present as intensely pruritic, urticarial to vesicular papules that most commonly present on the lower extremities. Flea bites can be treated with topical steroids, and fleas can be controlled by a combination of insecticidal products and insect growth regulators.
Characteristics
The ubiquitous human flea, Pulex irritans, is a hematophagous wingless ectoparasite in the order Siphonaptera (wingless siphon) that survives by consuming the blood of its mammalian and avian hosts. Due to diseases such as the bubonic plague, fleas have claimed more victims than all the wars ever fought; in the 14th century, the Black Death caused more than 200 million deaths. Fleas fossilized in amber have been found to be 200 million years old and closely resemble the modern human flea, demonstrating the resilience of the species.
The adult human flea is a small, reddish brown, laterally compressed, wingless insect that is approximately 2- to 3.5-mm long (females, 2.5–3.5 mm; males, 2–2.5 mm) and enclosed by a tough cuticle. Compared to the dog flea (Ctenocephalides canis) and cat flea (Ctenocephalides felis), P irritans has no combs or ctenidia (Figure 1). Fleas have large powerful hind legs enabling them to jump horizontally or vertically 200 times their body length (equivalent to a 6-foot human jumping 1200 feet) using stored muscle energy in a pad on the hind legs composed of the elastic protein resilin.1 They feed off a wide variety of hosts, including humans, pigs, cats, dogs, goats, sheep, cattle, chickens, owls, foxes, rabbits, mice, and feral cats. The flea’s mouthparts are highly specialized for piercing the skin and sucking its blood meal via direct capillary cannulation.
Life Cycle
There are 4 stages of the flea life cycle: egg, larva, pupa, and adult. Most adult flea species mate on the host; the female will lay an average of 4 to 8 small white eggs on the host after each blood meal, laying more than 400 eggs during her lifetime. The eggs then drop from the host and hatch in approximately 4 to 6 days to become larvae. The active larvae feed on available organic matter in their environment, such as their parents’ feces and detritus, while undergoing 3 molts within 1 week to several months.2 The larva then spins a silken cocoon from modified salivary glands to form the pupa. In favorable conditions, the pupa lasts only a few weeks; however, it can last for a year or more in unfavorable conditions. Triggers for emergence of the adult flea from the pupa include high humidity, warm temperatures, increased levels of carbon dioxide, and vibrations including sound. An adult P irritans flea can live for a few weeks to more than 1.5 years in favorable conditions of lower air temperature, high relative humidity, and access to a host.3
Related Diseases
Pulex irritans can be a vector for several human diseases. Yersinia pestis is a gram-negative bacteria that causes plague, a highly virulent disease that killed millions of people during its 3 largest human pandemics. The black rat (Rattus rattus) and the oriental rat flea (Xenopsylla cheopis) have been implicated as initial vectors; however, transmission may be human-to-human with pneumonic plague, and septicemic plague may be spread via Pulex fleas or body lice.4,5 In 1971, Y pestis was isolated from P irritans on a dog in the home of a plague patient in Kayenta, Arizona.6Yersinia pestis bacterial DNA also was extracted from P irritans during a plague outbreak in Madagascar in 20147 and was implicated in epidemiologic studies of plague in Tanzania from 1986 to 2004, suggesting it also plays a role in endemic disease.8
Bartonellosis is an emerging disease caused by different species of the gram-negative intracellular bacteria of the genus Bartonella transmitted by lice, ticks, and fleas. Bartonella quintana causes trench fever primarily transmitted by the human body louse, Pediculus humanus corporis, and resulted in more than 1 million cases during World War I. Trench fever is characterized by headache, fever, dizziness, and shin pain that lasts 1 to 3 days and recurs in cycles every 4 to 6 days. Other clinical manifestations of B quintana include chronic bacteremia, endocarditis, lymphadenopathy, and bacillary angiomatosis.9Bartonella henselae causes cat scratch fever, characterized by lymphadenopathy, fever, headache, joint pain, and lethargy from infected cat scratches or the bite of an infected flea. Bartonella rochalimae also has been found to cause a trench fever–like bacteremia.10Bartonella species have been found in P irritans, and the flea is implicated as a vector of bartonellosis in humans.11-15
Rickettsioses are worldwide diseases caused by the gram-negative intracellular bacteria of the genus Rickettsia transmitted to humans via hematophagous arthropods. The rickettsiae traditionally have been classified into the spotted fever or typhus groups. The spotted fever group (ie, Rocky Mountain spotted fever, Mediterranean spotted fever) is transmitted via ticks. The typhus group is transmitted via lice (epidemic typhus) and fleas (endemic or murine typhus). Murine typhus can be caused by Rickettsia typhi in warm coastal areas around the world where the main mammal reservoir is the rat and the rat flea vector X cheopis. Clinical signs of infection are abrupt onset of fever, headaches, myalgia, malaise, and chills, with a truncal maculopapular rash progressing peripherally several days after the initial clinical signs. Rash is present in up to 50% of cases.16Rickettsia felis is an emerging flea-borne pathogen causing an acute febrile illness usually transmitted via the cat flea C felis.17Rickettsia species DNA have been found to be present in P irritans from dogs18 and livestock19 and pose a risk for causing rickettsioses in humans.
Environmental Treatment and Prevention
Flea bites present as intense, pruritic, urticarial to vesicular papules that usually are located on the lower extremities but also can be present on exposed areas of the upper extremities and hands (Figure 2). Human fleas infest clothing, and bites can be widespread. Topical antipruritics and corticosteroids can be used for controlling itch and the intense cutaneous inflammatory response. The flea host should be identified in areas of the home, school, farm, work, or local environment. House pets should be examined and treated by a veterinarian. The pet’s bedding should be washed and dried at high temperatures, and carpets and floors should be routinely vacuumed or cleaned to remove eggs, larvae, flea feces, and/or pupae. The killing of adult fleas with insecticidal products (eg, imidacloprid, fipronil, spinosad, selamectin, lufenuron, ivermectin) is the primary method of flea control. Use of insect growth regulators such as pyriproxyfen inhibits adult reproduction and blocks the organogenesis of immature larval stages via hormonal or enzymatic actions.20 The combination of an insecticide and an insect growth regulator appears to be most effective in their synergistic actions against adult fleas and larvae. There have been reports of insecticidal resistance in the flea population, especially with pyrethroids.21,22 A professional exterminator and veterinarian should be consulted. In recalcitrant cases, evaluation for other wild mammals or birds should be performed in unoccupied areas of the home such as the attic, crawl spaces, and basements, as well as inside walls.
Conclusion
The human flea, P irritans, is an important vector in the transmission of human diseases such as the bubonic plague, bartonellosis, and rickettsioses. Flea bites present as intensely pruritic, urticarial to vesicular papules that most commonly present on the lower extremities. Flea bites can be treated with topical steroids, and fleas can be controlled by a combination of insecticidal products and insect growth regulators.
- Burrow M. How fleas jump. J Exp Biol. 2009;18:2881-2883.
- Buckland PC, Sandler JP. A biogeography of the human flea, Pulex irritans L (Siphonaptera: Pulicidae). J Biogeogr. 1989;16:115-120.
- Krasnov BR. Life cycles. In: Krasnov BR, ed. Functional and Evolutional Ecology of Fleas. Cambridge, MA: Cambridge Univ Press; 2008:45-67.
- Dean KR, Krauer F, Walloe L, et al. Human ectoparasites and the spread of plague in Europe during the second pandemic. Proc Natl Acad Sci U S A. 2018;115:1304-1309.
- Hufthammer AK, Walloe L. Rats cannot have been intermediate hosts for Yersinia pestis during medieval plague epidemics in Northern Europe. J Archeol Sci. 2013;40:1752-1759.
- Archibald WS, Kunitz SJ. Detection of plague by testing serums of dogs on the Navajo Reservation. HSMHA Health Rep. 1971;86:377-380.
- Ratovonjato J, Rajerison M, Rahelinirina S, et al. Yersinia pestis in Pulex irritans fleas during plague outbreak, Madagascar. Emerg Infect Dis. 2014;20:1414-1415.
- Laudisoit A, Leirs H, Makundi RH, et al. Plague and the human flea, Tanzania. Emerg Infect Dis. 2007;13:687-693.
- Foucault C, Brouqui P, Raoult D. Bartonella quintana characteristics and clinical management. Emerg Infect Dis. 2006;12:217-223.
- Eremeeva ME, Gerns HL, Lydy SL, et al. Bacteremia, fever, and splenomegaly caused by a newly recognized bartonella species. N Engl J Med. 2007; 356:2381-2387.11.
- Marquez FJ, Millan J, Rodriguez-Liebana JJ, et al. Detection and identification of Bartonella sp. in fleas from carnivorous mammals in Andalusia, Spain. Med Vet Entomol. 2009;23:393-398.
- Perez-Martinez L, Venzal JM, Portillo A, et al. Bartonella rochalimae and other Bartonella spp. in fleas, Chile. Emerg Infect Dis. 2009;15:1150-1152.
- Sofer S, Gutierrez DM, Mumcuoglu KY, et al. Molecular detection of zoonotic bartonellae (B. henselae, B. elizabethae and B. rochalimae) in fleas collected from dogs in Israel. Med Vet Entomol. 2015;29:344-348.
- Zouari S, Khrouf F, M’ghirbi Y, et al. First molecular detection and characterization of zoonotic Bartonella species in fleas infesting domestic animals in Tunisia. Parasit Vectors. 2017;10:436.
- Rolain JM, Bourry, O, Davoust B, et al. Bartonella quintana and Rickettsia felis in Gabon. Emerg Infect Dis. 2005;11:1742-1744.
- Tsioutis C, Zafeiri M, Avramopoulos A, et al. Clinical and laboratory characteristics, epidemiology, and outcomes of murine typhus: a systematic review. Acta Trop. 2017;166:16-24.
- Brown L, Macaluso KR. Rickettsia felis, an emerging flea-borne rickettsiosis. Curr Trop Med Rep. 2016;3:27-39.
- Oteo JA, Portillo A, Potero F, et al. ‘Candidatus Rickettsia asemboensis’ and Wolbachia spp. in Ctenocephalides felis and Pulex irritans fleas removed from dogs in Ecuador. Parasit Vectors. 2014;7:455.
- Ghavami MB, Mirzadeh H, Mohammadi J, et al. Molecular survey of ITS spacer and Rickettsia infection in human flea, Pulex irritans. Parasitol Res. 2018;117:1433-1442.
- Traversa D. Fleas infesting pets in the era of emerging extra-intestinal nematodes. Parasit Vectors. 2013;6:59.
- Rust MK. Insecticide resistance in fleas. Insects. 2016;7:10.
- Ghavami MB, Haghi FP, Alibabaei Z, et al. First report of target site insensitivity to pyrethroids in human flea, Pulex irritans (Siphonaptera: Pulicidae). Pest Biochem Physiol. 2018;146:97-105.
- Burrow M. How fleas jump. J Exp Biol. 2009;18:2881-2883.
- Buckland PC, Sandler JP. A biogeography of the human flea, Pulex irritans L (Siphonaptera: Pulicidae). J Biogeogr. 1989;16:115-120.
- Krasnov BR. Life cycles. In: Krasnov BR, ed. Functional and Evolutional Ecology of Fleas. Cambridge, MA: Cambridge Univ Press; 2008:45-67.
- Dean KR, Krauer F, Walloe L, et al. Human ectoparasites and the spread of plague in Europe during the second pandemic. Proc Natl Acad Sci U S A. 2018;115:1304-1309.
- Hufthammer AK, Walloe L. Rats cannot have been intermediate hosts for Yersinia pestis during medieval plague epidemics in Northern Europe. J Archeol Sci. 2013;40:1752-1759.
- Archibald WS, Kunitz SJ. Detection of plague by testing serums of dogs on the Navajo Reservation. HSMHA Health Rep. 1971;86:377-380.
- Ratovonjato J, Rajerison M, Rahelinirina S, et al. Yersinia pestis in Pulex irritans fleas during plague outbreak, Madagascar. Emerg Infect Dis. 2014;20:1414-1415.
- Laudisoit A, Leirs H, Makundi RH, et al. Plague and the human flea, Tanzania. Emerg Infect Dis. 2007;13:687-693.
- Foucault C, Brouqui P, Raoult D. Bartonella quintana characteristics and clinical management. Emerg Infect Dis. 2006;12:217-223.
- Eremeeva ME, Gerns HL, Lydy SL, et al. Bacteremia, fever, and splenomegaly caused by a newly recognized bartonella species. N Engl J Med. 2007; 356:2381-2387.11.
- Marquez FJ, Millan J, Rodriguez-Liebana JJ, et al. Detection and identification of Bartonella sp. in fleas from carnivorous mammals in Andalusia, Spain. Med Vet Entomol. 2009;23:393-398.
- Perez-Martinez L, Venzal JM, Portillo A, et al. Bartonella rochalimae and other Bartonella spp. in fleas, Chile. Emerg Infect Dis. 2009;15:1150-1152.
- Sofer S, Gutierrez DM, Mumcuoglu KY, et al. Molecular detection of zoonotic bartonellae (B. henselae, B. elizabethae and B. rochalimae) in fleas collected from dogs in Israel. Med Vet Entomol. 2015;29:344-348.
- Zouari S, Khrouf F, M’ghirbi Y, et al. First molecular detection and characterization of zoonotic Bartonella species in fleas infesting domestic animals in Tunisia. Parasit Vectors. 2017;10:436.
- Rolain JM, Bourry, O, Davoust B, et al. Bartonella quintana and Rickettsia felis in Gabon. Emerg Infect Dis. 2005;11:1742-1744.
- Tsioutis C, Zafeiri M, Avramopoulos A, et al. Clinical and laboratory characteristics, epidemiology, and outcomes of murine typhus: a systematic review. Acta Trop. 2017;166:16-24.
- Brown L, Macaluso KR. Rickettsia felis, an emerging flea-borne rickettsiosis. Curr Trop Med Rep. 2016;3:27-39.
- Oteo JA, Portillo A, Potero F, et al. ‘Candidatus Rickettsia asemboensis’ and Wolbachia spp. in Ctenocephalides felis and Pulex irritans fleas removed from dogs in Ecuador. Parasit Vectors. 2014;7:455.
- Ghavami MB, Mirzadeh H, Mohammadi J, et al. Molecular survey of ITS spacer and Rickettsia infection in human flea, Pulex irritans. Parasitol Res. 2018;117:1433-1442.
- Traversa D. Fleas infesting pets in the era of emerging extra-intestinal nematodes. Parasit Vectors. 2013;6:59.
- Rust MK. Insecticide resistance in fleas. Insects. 2016;7:10.
- Ghavami MB, Haghi FP, Alibabaei Z, et al. First report of target site insensitivity to pyrethroids in human flea, Pulex irritans (Siphonaptera: Pulicidae). Pest Biochem Physiol. 2018;146:97-105.
Practice Points
- The human flea, Pulex irritans, is a vector for various human diseases including the bubonic plague, bartonellosis, and rickettsioses.
- Presenting symptoms of flea bites include intensely pruritic, urticarial to vesicular papules on exposed areas of skin.
- The primary method of flea control includes a combination of insecticidal products and insect growth regulators.
What’s Eating You? Oriental Rat Flea (Xenopsylla cheopis)
A dult Siphonaptera (fleas) are highly adapted to life on the surface of their hosts. Their small 2- to 10-mm bodies are laterally flattened and wingless. They utilize particularly strong hind legs for jumping up to 150 times their body length and backward-directed spines on their legs and bodies for moving forward through fur, hair, and feathers. Xenopsylla cheopis , the oriental rat flea, lacks pronotal and genal combs and has a mesopleuron divided by internal scleritinization (Figure). These features differentiate the species from its close relatives, Ctenocephalides (cat and dog fleas), which have both sets of combs, as well as Pulex irritans (human flea), which do not have a divided mesopleuron. 1,2
Flea-borne infections are extremely important to public health and are present throughout the world. Further, humidity and warmth are essential for the life cycle of many species of fleas. Predicted global warming likely will increase their distribution, allowing the spread of diseases they carry into previously untouched areas.1 Therefore, it is important to continue to examine species that carry particularly dangerous pathogens, such as X cheopis.
Disease Vector
Xenopsylla cheopis primarily is known for being a vector in the transmission of Yersinia pestis, the etiologic agent of the plague. Plague occurs in 3 forms: bubonic, pneumonic, and septicemic. It has caused major epidemics throughout history, the most widely known being the Black Death, which lasted for 130 years, beginning in the 1330s in China and spreading into Europe where it wiped out one-third of the population. However, bubonic plague is thought to have caused documented outbreaks as early as 320
Between January 2010 and December 2015, 3248 cases of plague in humans were reported, resulting in 584 deaths worldwide.5 It is thought that the plague originated in Central Asia, and this area still is a focus of disease. However, the at-risk population is reduced to breeders and hunters of gerbils and marmots, the main reservoirs in the area. In Africa, 4 countries still regularly report cases, with Madagascar being the most severely affected country in the world.5 The Democratic Republic of the Congo, Uganda, and Tanzania also are affected. The Americas also experience the plague. There are sporadic cases of bubonic plague in the northwest corner of Peru, mostly in rural areas. In the western United States, plague circulates among wild rodents, resulting in several reported cases each year, with the most recent confirmed case noted in California in August 2020.5,6 Further adding to its relevance, Y pestis is one of several organisms most likely to be used as a biologic weapon.3,4
Due to the historical and continued significance of Y pestis, many studies have been performed over the decades regarding its association with X cheopis. It has been discovered that fleas transmit the bacteria to the host in 2 ways. The most well-defined form of transmission occurs after an incubation period of Y pestis in the flea for 7 to 31 days. During this time, the bacteria form a dense biofilm on a valve in the flea foregut—the proventriculus—interfering with its function, which allows regurgitation of the blood and the bacteria it contains into the bite site and consequently disease transmission. The proventriculus can become completely blocked in some fleas, preventing any blood from reaching the midgut and causing starvation. In these scenarios, the flea will make continuous attempts to feed, increasing transmission.7 The hemin storage gene, hms, encoding the second messenger molecule cyclic di-GMP plays a critical role in biofilm formation and proventricular blockage.8 The phosphoheptose isomerase gene, GmhA, also has been elucidated as crucial in late transmission due to its role in biofilm formation.9 Early-phase transmission, or biofilm-independent transmission, has been documented to occur as early as 3 hours after infection of the flea but can occur for up to 4 days.10 Historically, the importance of early-phase transmission has been overlooked. Research has shown that it likely is crucial to the epizootic transmission of the plague.10 As a result, the search has begun for genes that contribute to the maintenance of Y pestis in the flea vector during the first 4 days of colonization. It is thought that a key evolutionary development was the selective loss-of-function mutation in a gene essential for the activity of urease, an enzyme that causes acute oral toxicity and mortality in fleas.11,12 The Yersinia murine toxin gene, Ymt, also allows for early survival of Y pestis in the flea midgut by producing a phospholipase D that protects the bacteria from toxic by-products produced during digestion of blood.11,13 In addition, gene products that function in lipid A modification are crucial for the ability of Y pestis to resist the action of cationic antimicrobial peptides it produces, such as cecropin A and polymyxin B.13
Murine typhus, an acute febrile illness caused by Rickettsia typhi, is another disease that can be spread by oriental rat fleas. It has a worldwide distribution. In the United States, R typhi–induced rickettsia mainly is concentrated in suburban areas of Texas and California where it is thought to be mainly spread by Ctenocephalides, but it also is found in Hawaii where spread by X cheopis has been documented.14,15 The most common symptoms of rickettsia include fever, headache, arthralgia, and a characteristic rash that is pruritic and maculopapular, starting on the trunk and spreading peripherally but sparing the palms and soles. This rash occurs about a week after the onset of fever.14Rickettsia felis also has been isolated in the oriental rat flea. However, only a handful of cases of human disease caused by this bacterium have been reported throughout the world, with clinical similarity to murine typhus likely leading to underestimation of disease prevalence.15Bartonella and other species of bacteria also have been documented to be spread by X cheopis.16 Unfortunately, the interactions of X cheopis with these other bacteria are not as well studied as its relationship with Y pestis.
Adverse Reactions
A flea bite itself can cause discomfort. It begins as a punctate hemorrhagic area that develops a surrounding wheal within 30 minutes. Over the course of 1 to 2 days, a delayed reaction occurs and there is a transition to an extremely pruritic, papular lesion. Bites often occur in clusters and can persist for weeks.1
Prevention and Treatment
Control of host animals via extermination and proper sanitation can secondarily reduce the population of X cheopis. Direct pesticide control of the flea population also has been suggested to reduce flea-borne disease. However, insecticides cause a selective pressure on the flea population, leading to populations that are resistant to them. For example, the flea population in Madagascar developed resistance to DDT (dichlorodiphenyltrichloroethane), dieldrin, deltamethrin, and cyfluthrin after their widespread use.17 Further, a recent study revealed resistance of X cheopis populations to alphacypermethrin, lambda-cyhalothrin, and etofenprox, none of which were used in mass vector control, indicating that some cross-resistance mechanism between these and the extensively used insecticides may exist. With the development of widespread resistance to most pesticides, flea control in endemic areas is difficult. Insecticide targeting to fleas on rodents (eg, rodent bait containing insecticide) can allow for more targeted insecticide treatment, limiting the development of resistance.17 Recent development of a maceration protocol used to detect zoonotic pathogens in fleas in the field also will allow management with pesticides to be targeted geographically and temporally where infected vectors are located.18 Research of the interaction between vector, pathogen, and insect microbiome also should continue, as it may allow for development of biopesticides, limiting the use of chemical pesticides all together. The strategy is based on the introduction of microorganisms that can reduce vector lifespan or their ability to transmit pathogens.17
When flea-transmitted diseases do occur, treatment with antibiotics is advised. Early treatment of the plague with effective antibiotics such as streptomycin, gentamicin, tetracycline, or chloramphenicol for a minimum of 10 days is critical for survival. Additionally, patients with bubonic plague should be isolated for at least 2 days after administration of antibiotics, while patients with the pneumonic form should be isolated for 4 days into therapy to prevent the spread of disease. Prophylactic therapy for individuals who come into contact with infected individuals also is advised.4 Patients with murine typhus typically respond to doxycycline, tetracycline, or fluoroquinolones. The few cases of R felis–induced disease have responded to doxycycline. Of note, short courses of treatment of doxycycline are appropriate and safe in young children. The short (3–7 day) nature of the course limits the chances of teeth staining.14
- Bitam I, Dittmar K, Parola P, et al. Flea and flea-borne diseases. Int J Infect Dis. 2010;14:E667-E676.
- Mathison BA, Pritt BS. Laboratory identification of arthropod ectoparasites. Clin Microbiol Rev. 2014;27:48-67.
- Ligon BL. Plague: a review of its history and potential as a biological weapon. Semin Pediatr Infect Dis. 2006;17:161-170.
- Josko D. Yersinia pestis: still a plague in the 21st century. Clin Lab Sci. 2004;17:25-29.
- Plague around the world, 2010–2015. Wkly Epidemiol Rec. 2016;91:89-93.
- Sullivan K. California confirms first human case of the plague in 5 years: what to know. NBC News website. https://www.nbcnews.com/health/health-news/california-confirms-first-human-case-bubonic-plague-5-years-what-n1237306. Published August 19, 2020. Accessed August 24, 2020.
- Hinnebusch BJ, Bland DM, Bosio CF, et al. Comparative ability of Oropsylla and Xenopsylla cheopis fleas to transmit Yersinia pestis by two different mechanisms. PLOS Negl Trop Dis. 2017;11:e0005276.
- Bobrov AG, Kirillina O, Vadyvaloo V, et al. The Yersinia pestis HmsCDE regulatory system is essential for blockage of the oriental rat flea (Xenopsylla cheopis), a classic plague vector. Environ Microbiol. 2015;17:947-959.
- Darby C, Ananth SL, Tan L, et al. Identification of gmhA, a Yersina pestis gene required for flea blockage, by using a Caenorhabditis elegans biofilm system. Infect Immun. 2005;73:7236-7242.
- Eisen RJ, Dennis DT, Gage KL. The role of early-phase transmission in the spread of Yersinia pestis. J Med Entomol. 2015;52:1183-1192.
- Carniel E. Subtle genetic modifications transformed an enteropathogen into a flea-borne pathogen. Proc Natl Acad Sci U S A. 2014;111:18409-18410.
- Chouikha I, Hinnebusch BJ. Silencing urease: a key evolutionary step that facilitated the adaptation of Yersinia pestis to the flea-borne transmission route. Proc Natl Acad Sci U S A. 2014;111:18709-19714.
- Aoyagi KL, Brooks BD, Bearden SW, et al. LPS modification promotes maintenance of Yersinia pestis in fleas. Microbiology. 2015;161:628-638.
- Civen R, Ngo V. Murine typhus: an unrecognized suburban vectorborne disease. Clin Infect Dis. 2008;46:913-918.
- Eremeeva ME, Warashina WR, Sturgeon MM, et al. Rickettsia typhi and R. felis in rat fleas (Xenopsylla cheopis), Oahu, Hawaii. Emerg Infect Dis. 2018;14:1613-1615.
- Billeter SA, Gundi VAKB, Rood MP, et al. Molecular detection and identification of Bartonella species in Xenopsylla cheopis fleas (Siphonaptera: Pulicidae) collected from Rattus norvecus rats in Los Angeles, California. Appl Environ Microbiol. 2011;77:7850-7852.
- Miarinjara A, Boyer S. Current perspectives on plague vector control in Madagascar: susceptibility status of Xenopsylla cheopis to 12 insecticides. PLOS Negl Trop Dis. 2016;10:e0004414.
- Harrison GF, Scheirer JL, Melanson VR. Development and validation of an arthropod maceration protocol for zoonotic pathogen detection in mosquitoes and fleas. J Vector Ecol. 2014;40:83-89.
A dult Siphonaptera (fleas) are highly adapted to life on the surface of their hosts. Their small 2- to 10-mm bodies are laterally flattened and wingless. They utilize particularly strong hind legs for jumping up to 150 times their body length and backward-directed spines on their legs and bodies for moving forward through fur, hair, and feathers. Xenopsylla cheopis , the oriental rat flea, lacks pronotal and genal combs and has a mesopleuron divided by internal scleritinization (Figure). These features differentiate the species from its close relatives, Ctenocephalides (cat and dog fleas), which have both sets of combs, as well as Pulex irritans (human flea), which do not have a divided mesopleuron. 1,2

Flea-borne infections are extremely important to public health and are present throughout the world. Further, humidity and warmth are essential for the life cycle of many species of fleas. Predicted global warming likely will increase their distribution, allowing the spread of diseases they carry into previously untouched areas.1 Therefore, it is important to continue to examine species that carry particularly dangerous pathogens, such as X cheopis.
Disease Vector
Xenopsylla cheopis primarily is known for being a vector in the transmission of Yersinia pestis, the etiologic agent of the plague. Plague occurs in 3 forms: bubonic, pneumonic, and septicemic. It has caused major epidemics throughout history, the most widely known being the Black Death, which lasted for 130 years, beginning in the 1330s in China and spreading into Europe where it wiped out one-third of the population. However, bubonic plague is thought to have caused documented outbreaks as early as 320
Between January 2010 and December 2015, 3248 cases of plague in humans were reported, resulting in 584 deaths worldwide.5 It is thought that the plague originated in Central Asia, and this area still is a focus of disease. However, the at-risk population is reduced to breeders and hunters of gerbils and marmots, the main reservoirs in the area. In Africa, 4 countries still regularly report cases, with Madagascar being the most severely affected country in the world.5 The Democratic Republic of the Congo, Uganda, and Tanzania also are affected. The Americas also experience the plague. There are sporadic cases of bubonic plague in the northwest corner of Peru, mostly in rural areas. In the western United States, plague circulates among wild rodents, resulting in several reported cases each year, with the most recent confirmed case noted in California in August 2020.5,6 Further adding to its relevance, Y pestis is one of several organisms most likely to be used as a biologic weapon.3,4
Due to the historical and continued significance of Y pestis, many studies have been performed over the decades regarding its association with X cheopis. It has been discovered that fleas transmit the bacteria to the host in 2 ways. The most well-defined form of transmission occurs after an incubation period of Y pestis in the flea for 7 to 31 days. During this time, the bacteria form a dense biofilm on a valve in the flea foregut—the proventriculus—interfering with its function, which allows regurgitation of the blood and the bacteria it contains into the bite site and consequently disease transmission. The proventriculus can become completely blocked in some fleas, preventing any blood from reaching the midgut and causing starvation. In these scenarios, the flea will make continuous attempts to feed, increasing transmission.7 The hemin storage gene, hms, encoding the second messenger molecule cyclic di-GMP plays a critical role in biofilm formation and proventricular blockage.8 The phosphoheptose isomerase gene, GmhA, also has been elucidated as crucial in late transmission due to its role in biofilm formation.9 Early-phase transmission, or biofilm-independent transmission, has been documented to occur as early as 3 hours after infection of the flea but can occur for up to 4 days.10 Historically, the importance of early-phase transmission has been overlooked. Research has shown that it likely is crucial to the epizootic transmission of the plague.10 As a result, the search has begun for genes that contribute to the maintenance of Y pestis in the flea vector during the first 4 days of colonization. It is thought that a key evolutionary development was the selective loss-of-function mutation in a gene essential for the activity of urease, an enzyme that causes acute oral toxicity and mortality in fleas.11,12 The Yersinia murine toxin gene, Ymt, also allows for early survival of Y pestis in the flea midgut by producing a phospholipase D that protects the bacteria from toxic by-products produced during digestion of blood.11,13 In addition, gene products that function in lipid A modification are crucial for the ability of Y pestis to resist the action of cationic antimicrobial peptides it produces, such as cecropin A and polymyxin B.13
Murine typhus, an acute febrile illness caused by Rickettsia typhi, is another disease that can be spread by oriental rat fleas. It has a worldwide distribution. In the United States, R typhi–induced rickettsia mainly is concentrated in suburban areas of Texas and California where it is thought to be mainly spread by Ctenocephalides, but it also is found in Hawaii where spread by X cheopis has been documented.14,15 The most common symptoms of rickettsia include fever, headache, arthralgia, and a characteristic rash that is pruritic and maculopapular, starting on the trunk and spreading peripherally but sparing the palms and soles. This rash occurs about a week after the onset of fever.14Rickettsia felis also has been isolated in the oriental rat flea. However, only a handful of cases of human disease caused by this bacterium have been reported throughout the world, with clinical similarity to murine typhus likely leading to underestimation of disease prevalence.15Bartonella and other species of bacteria also have been documented to be spread by X cheopis.16 Unfortunately, the interactions of X cheopis with these other bacteria are not as well studied as its relationship with Y pestis.
Adverse Reactions
A flea bite itself can cause discomfort. It begins as a punctate hemorrhagic area that develops a surrounding wheal within 30 minutes. Over the course of 1 to 2 days, a delayed reaction occurs and there is a transition to an extremely pruritic, papular lesion. Bites often occur in clusters and can persist for weeks.1
Prevention and Treatment
Control of host animals via extermination and proper sanitation can secondarily reduce the population of X cheopis. Direct pesticide control of the flea population also has been suggested to reduce flea-borne disease. However, insecticides cause a selective pressure on the flea population, leading to populations that are resistant to them. For example, the flea population in Madagascar developed resistance to DDT (dichlorodiphenyltrichloroethane), dieldrin, deltamethrin, and cyfluthrin after their widespread use.17 Further, a recent study revealed resistance of X cheopis populations to alphacypermethrin, lambda-cyhalothrin, and etofenprox, none of which were used in mass vector control, indicating that some cross-resistance mechanism between these and the extensively used insecticides may exist. With the development of widespread resistance to most pesticides, flea control in endemic areas is difficult. Insecticide targeting to fleas on rodents (eg, rodent bait containing insecticide) can allow for more targeted insecticide treatment, limiting the development of resistance.17 Recent development of a maceration protocol used to detect zoonotic pathogens in fleas in the field also will allow management with pesticides to be targeted geographically and temporally where infected vectors are located.18 Research of the interaction between vector, pathogen, and insect microbiome also should continue, as it may allow for development of biopesticides, limiting the use of chemical pesticides all together. The strategy is based on the introduction of microorganisms that can reduce vector lifespan or their ability to transmit pathogens.17
When flea-transmitted diseases do occur, treatment with antibiotics is advised. Early treatment of the plague with effective antibiotics such as streptomycin, gentamicin, tetracycline, or chloramphenicol for a minimum of 10 days is critical for survival. Additionally, patients with bubonic plague should be isolated for at least 2 days after administration of antibiotics, while patients with the pneumonic form should be isolated for 4 days into therapy to prevent the spread of disease. Prophylactic therapy for individuals who come into contact with infected individuals also is advised.4 Patients with murine typhus typically respond to doxycycline, tetracycline, or fluoroquinolones. The few cases of R felis–induced disease have responded to doxycycline. Of note, short courses of treatment of doxycycline are appropriate and safe in young children. The short (3–7 day) nature of the course limits the chances of teeth staining.14
A dult Siphonaptera (fleas) are highly adapted to life on the surface of their hosts. Their small 2- to 10-mm bodies are laterally flattened and wingless. They utilize particularly strong hind legs for jumping up to 150 times their body length and backward-directed spines on their legs and bodies for moving forward through fur, hair, and feathers. Xenopsylla cheopis , the oriental rat flea, lacks pronotal and genal combs and has a mesopleuron divided by internal scleritinization (Figure). These features differentiate the species from its close relatives, Ctenocephalides (cat and dog fleas), which have both sets of combs, as well as Pulex irritans (human flea), which do not have a divided mesopleuron. 1,2

Flea-borne infections are extremely important to public health and are present throughout the world. Further, humidity and warmth are essential for the life cycle of many species of fleas. Predicted global warming likely will increase their distribution, allowing the spread of diseases they carry into previously untouched areas.1 Therefore, it is important to continue to examine species that carry particularly dangerous pathogens, such as X cheopis.
Disease Vector
Xenopsylla cheopis primarily is known for being a vector in the transmission of Yersinia pestis, the etiologic agent of the plague. Plague occurs in 3 forms: bubonic, pneumonic, and septicemic. It has caused major epidemics throughout history, the most widely known being the Black Death, which lasted for 130 years, beginning in the 1330s in China and spreading into Europe where it wiped out one-third of the population. However, bubonic plague is thought to have caused documented outbreaks as early as 320
Between January 2010 and December 2015, 3248 cases of plague in humans were reported, resulting in 584 deaths worldwide.5 It is thought that the plague originated in Central Asia, and this area still is a focus of disease. However, the at-risk population is reduced to breeders and hunters of gerbils and marmots, the main reservoirs in the area. In Africa, 4 countries still regularly report cases, with Madagascar being the most severely affected country in the world.5 The Democratic Republic of the Congo, Uganda, and Tanzania also are affected. The Americas also experience the plague. There are sporadic cases of bubonic plague in the northwest corner of Peru, mostly in rural areas. In the western United States, plague circulates among wild rodents, resulting in several reported cases each year, with the most recent confirmed case noted in California in August 2020.5,6 Further adding to its relevance, Y pestis is one of several organisms most likely to be used as a biologic weapon.3,4
Due to the historical and continued significance of Y pestis, many studies have been performed over the decades regarding its association with X cheopis. It has been discovered that fleas transmit the bacteria to the host in 2 ways. The most well-defined form of transmission occurs after an incubation period of Y pestis in the flea for 7 to 31 days. During this time, the bacteria form a dense biofilm on a valve in the flea foregut—the proventriculus—interfering with its function, which allows regurgitation of the blood and the bacteria it contains into the bite site and consequently disease transmission. The proventriculus can become completely blocked in some fleas, preventing any blood from reaching the midgut and causing starvation. In these scenarios, the flea will make continuous attempts to feed, increasing transmission.7 The hemin storage gene, hms, encoding the second messenger molecule cyclic di-GMP plays a critical role in biofilm formation and proventricular blockage.8 The phosphoheptose isomerase gene, GmhA, also has been elucidated as crucial in late transmission due to its role in biofilm formation.9 Early-phase transmission, or biofilm-independent transmission, has been documented to occur as early as 3 hours after infection of the flea but can occur for up to 4 days.10 Historically, the importance of early-phase transmission has been overlooked. Research has shown that it likely is crucial to the epizootic transmission of the plague.10 As a result, the search has begun for genes that contribute to the maintenance of Y pestis in the flea vector during the first 4 days of colonization. It is thought that a key evolutionary development was the selective loss-of-function mutation in a gene essential for the activity of urease, an enzyme that causes acute oral toxicity and mortality in fleas.11,12 The Yersinia murine toxin gene, Ymt, also allows for early survival of Y pestis in the flea midgut by producing a phospholipase D that protects the bacteria from toxic by-products produced during digestion of blood.11,13 In addition, gene products that function in lipid A modification are crucial for the ability of Y pestis to resist the action of cationic antimicrobial peptides it produces, such as cecropin A and polymyxin B.13
Murine typhus, an acute febrile illness caused by Rickettsia typhi, is another disease that can be spread by oriental rat fleas. It has a worldwide distribution. In the United States, R typhi–induced rickettsia mainly is concentrated in suburban areas of Texas and California where it is thought to be mainly spread by Ctenocephalides, but it also is found in Hawaii where spread by X cheopis has been documented.14,15 The most common symptoms of rickettsia include fever, headache, arthralgia, and a characteristic rash that is pruritic and maculopapular, starting on the trunk and spreading peripherally but sparing the palms and soles. This rash occurs about a week after the onset of fever.14Rickettsia felis also has been isolated in the oriental rat flea. However, only a handful of cases of human disease caused by this bacterium have been reported throughout the world, with clinical similarity to murine typhus likely leading to underestimation of disease prevalence.15Bartonella and other species of bacteria also have been documented to be spread by X cheopis.16 Unfortunately, the interactions of X cheopis with these other bacteria are not as well studied as its relationship with Y pestis.
Adverse Reactions
A flea bite itself can cause discomfort. It begins as a punctate hemorrhagic area that develops a surrounding wheal within 30 minutes. Over the course of 1 to 2 days, a delayed reaction occurs and there is a transition to an extremely pruritic, papular lesion. Bites often occur in clusters and can persist for weeks.1
Prevention and Treatment
Control of host animals via extermination and proper sanitation can secondarily reduce the population of X cheopis. Direct pesticide control of the flea population also has been suggested to reduce flea-borne disease. However, insecticides cause a selective pressure on the flea population, leading to populations that are resistant to them. For example, the flea population in Madagascar developed resistance to DDT (dichlorodiphenyltrichloroethane), dieldrin, deltamethrin, and cyfluthrin after their widespread use.17 Further, a recent study revealed resistance of X cheopis populations to alphacypermethrin, lambda-cyhalothrin, and etofenprox, none of which were used in mass vector control, indicating that some cross-resistance mechanism between these and the extensively used insecticides may exist. With the development of widespread resistance to most pesticides, flea control in endemic areas is difficult. Insecticide targeting to fleas on rodents (eg, rodent bait containing insecticide) can allow for more targeted insecticide treatment, limiting the development of resistance.17 Recent development of a maceration protocol used to detect zoonotic pathogens in fleas in the field also will allow management with pesticides to be targeted geographically and temporally where infected vectors are located.18 Research of the interaction between vector, pathogen, and insect microbiome also should continue, as it may allow for development of biopesticides, limiting the use of chemical pesticides all together. The strategy is based on the introduction of microorganisms that can reduce vector lifespan or their ability to transmit pathogens.17
When flea-transmitted diseases do occur, treatment with antibiotics is advised. Early treatment of the plague with effective antibiotics such as streptomycin, gentamicin, tetracycline, or chloramphenicol for a minimum of 10 days is critical for survival. Additionally, patients with bubonic plague should be isolated for at least 2 days after administration of antibiotics, while patients with the pneumonic form should be isolated for 4 days into therapy to prevent the spread of disease. Prophylactic therapy for individuals who come into contact with infected individuals also is advised.4 Patients with murine typhus typically respond to doxycycline, tetracycline, or fluoroquinolones. The few cases of R felis–induced disease have responded to doxycycline. Of note, short courses of treatment of doxycycline are appropriate and safe in young children. The short (3–7 day) nature of the course limits the chances of teeth staining.14
- Bitam I, Dittmar K, Parola P, et al. Flea and flea-borne diseases. Int J Infect Dis. 2010;14:E667-E676.
- Mathison BA, Pritt BS. Laboratory identification of arthropod ectoparasites. Clin Microbiol Rev. 2014;27:48-67.
- Ligon BL. Plague: a review of its history and potential as a biological weapon. Semin Pediatr Infect Dis. 2006;17:161-170.
- Josko D. Yersinia pestis: still a plague in the 21st century. Clin Lab Sci. 2004;17:25-29.
- Plague around the world, 2010–2015. Wkly Epidemiol Rec. 2016;91:89-93.
- Sullivan K. California confirms first human case of the plague in 5 years: what to know. NBC News website. https://www.nbcnews.com/health/health-news/california-confirms-first-human-case-bubonic-plague-5-years-what-n1237306. Published August 19, 2020. Accessed August 24, 2020.
- Hinnebusch BJ, Bland DM, Bosio CF, et al. Comparative ability of Oropsylla and Xenopsylla cheopis fleas to transmit Yersinia pestis by two different mechanisms. PLOS Negl Trop Dis. 2017;11:e0005276.
- Bobrov AG, Kirillina O, Vadyvaloo V, et al. The Yersinia pestis HmsCDE regulatory system is essential for blockage of the oriental rat flea (Xenopsylla cheopis), a classic plague vector. Environ Microbiol. 2015;17:947-959.
- Darby C, Ananth SL, Tan L, et al. Identification of gmhA, a Yersina pestis gene required for flea blockage, by using a Caenorhabditis elegans biofilm system. Infect Immun. 2005;73:7236-7242.
- Eisen RJ, Dennis DT, Gage KL. The role of early-phase transmission in the spread of Yersinia pestis. J Med Entomol. 2015;52:1183-1192.
- Carniel E. Subtle genetic modifications transformed an enteropathogen into a flea-borne pathogen. Proc Natl Acad Sci U S A. 2014;111:18409-18410.
- Chouikha I, Hinnebusch BJ. Silencing urease: a key evolutionary step that facilitated the adaptation of Yersinia pestis to the flea-borne transmission route. Proc Natl Acad Sci U S A. 2014;111:18709-19714.
- Aoyagi KL, Brooks BD, Bearden SW, et al. LPS modification promotes maintenance of Yersinia pestis in fleas. Microbiology. 2015;161:628-638.
- Civen R, Ngo V. Murine typhus: an unrecognized suburban vectorborne disease. Clin Infect Dis. 2008;46:913-918.
- Eremeeva ME, Warashina WR, Sturgeon MM, et al. Rickettsia typhi and R. felis in rat fleas (Xenopsylla cheopis), Oahu, Hawaii. Emerg Infect Dis. 2018;14:1613-1615.
- Billeter SA, Gundi VAKB, Rood MP, et al. Molecular detection and identification of Bartonella species in Xenopsylla cheopis fleas (Siphonaptera: Pulicidae) collected from Rattus norvecus rats in Los Angeles, California. Appl Environ Microbiol. 2011;77:7850-7852.
- Miarinjara A, Boyer S. Current perspectives on plague vector control in Madagascar: susceptibility status of Xenopsylla cheopis to 12 insecticides. PLOS Negl Trop Dis. 2016;10:e0004414.
- Harrison GF, Scheirer JL, Melanson VR. Development and validation of an arthropod maceration protocol for zoonotic pathogen detection in mosquitoes and fleas. J Vector Ecol. 2014;40:83-89.
- Bitam I, Dittmar K, Parola P, et al. Flea and flea-borne diseases. Int J Infect Dis. 2010;14:E667-E676.
- Mathison BA, Pritt BS. Laboratory identification of arthropod ectoparasites. Clin Microbiol Rev. 2014;27:48-67.
- Ligon BL. Plague: a review of its history and potential as a biological weapon. Semin Pediatr Infect Dis. 2006;17:161-170.
- Josko D. Yersinia pestis: still a plague in the 21st century. Clin Lab Sci. 2004;17:25-29.
- Plague around the world, 2010–2015. Wkly Epidemiol Rec. 2016;91:89-93.
- Sullivan K. California confirms first human case of the plague in 5 years: what to know. NBC News website. https://www.nbcnews.com/health/health-news/california-confirms-first-human-case-bubonic-plague-5-years-what-n1237306. Published August 19, 2020. Accessed August 24, 2020.
- Hinnebusch BJ, Bland DM, Bosio CF, et al. Comparative ability of Oropsylla and Xenopsylla cheopis fleas to transmit Yersinia pestis by two different mechanisms. PLOS Negl Trop Dis. 2017;11:e0005276.
- Bobrov AG, Kirillina O, Vadyvaloo V, et al. The Yersinia pestis HmsCDE regulatory system is essential for blockage of the oriental rat flea (Xenopsylla cheopis), a classic plague vector. Environ Microbiol. 2015;17:947-959.
- Darby C, Ananth SL, Tan L, et al. Identification of gmhA, a Yersina pestis gene required for flea blockage, by using a Caenorhabditis elegans biofilm system. Infect Immun. 2005;73:7236-7242.
- Eisen RJ, Dennis DT, Gage KL. The role of early-phase transmission in the spread of Yersinia pestis. J Med Entomol. 2015;52:1183-1192.
- Carniel E. Subtle genetic modifications transformed an enteropathogen into a flea-borne pathogen. Proc Natl Acad Sci U S A. 2014;111:18409-18410.
- Chouikha I, Hinnebusch BJ. Silencing urease: a key evolutionary step that facilitated the adaptation of Yersinia pestis to the flea-borne transmission route. Proc Natl Acad Sci U S A. 2014;111:18709-19714.
- Aoyagi KL, Brooks BD, Bearden SW, et al. LPS modification promotes maintenance of Yersinia pestis in fleas. Microbiology. 2015;161:628-638.
- Civen R, Ngo V. Murine typhus: an unrecognized suburban vectorborne disease. Clin Infect Dis. 2008;46:913-918.
- Eremeeva ME, Warashina WR, Sturgeon MM, et al. Rickettsia typhi and R. felis in rat fleas (Xenopsylla cheopis), Oahu, Hawaii. Emerg Infect Dis. 2018;14:1613-1615.
- Billeter SA, Gundi VAKB, Rood MP, et al. Molecular detection and identification of Bartonella species in Xenopsylla cheopis fleas (Siphonaptera: Pulicidae) collected from Rattus norvecus rats in Los Angeles, California. Appl Environ Microbiol. 2011;77:7850-7852.
- Miarinjara A, Boyer S. Current perspectives on plague vector control in Madagascar: susceptibility status of Xenopsylla cheopis to 12 insecticides. PLOS Negl Trop Dis. 2016;10:e0004414.
- Harrison GF, Scheirer JL, Melanson VR. Development and validation of an arthropod maceration protocol for zoonotic pathogen detection in mosquitoes and fleas. J Vector Ecol. 2014;40:83-89.
Practice Points
- Xenopsylla cheopis, the oriental rat flea, is most known for carrying Yersinia pestis, the causative agent of the plague; however, it also is a vector for other bacteria, such as Rickettsia typhi, the species responsible for most cases of murine typhus.
- Despite the perception that it largely is a historical illness, modern outbreaks of plague occur in many parts of the world each year. Because fleas thrive in warm humid weather, global warming threatens the spread of the oriental rat flea and its diseases into previously unaffected parts of the world.
- There has been an effort to control oriental rat flea populations, which unfortunately has been complicated by pesticide resistance in many flea populations. It is important to continue to research the oriental rat flea and the bacterial species it carries in the hopes of finding better methods of controlling the pests and therefore decreasing illness in humans.
- Health care providers should be vigilant in identifying symptoms of flea-borne illnesses. If a patient is displaying symptoms, prompt recognition and antibiotic therapy is critical, particularly for the plague.
What’s Eating You? Megalopyge opercularis
Lepidoptera is the second largest order of the class Insecta and comprises approximately 160,000 species of butterflies and moths classified among approximately 124 families and subfamilies. Venomous properties have been identified in 12 of these families, posing a serious threat to human health. 1
The clinical manifestations from Lepidoptera envenomation can range from general systemic symptoms such as fever and abdominal distress; to more complex focal affections including hemorrhage, ophthalmologic lesions, and irritation of the respiratory tracts; to less severe reactions of the skin, which are the most common presentation.1
Terminology
Lepidopterism is the term used to address a clinical spectrum of systemic manifestations from direct contact with venomous butterflies or moths and/or their products.2 Conversely, erucism is a term used to describe localized cutaneous reactions after direct contact with toxins from caterpillars.
Lepidopterism is derived from the Greek roots lepis, meaning scale, and pteron, meaning wing. The term erucism stems from the Latin word eruca, which means larva.2
Ideally, lepidopterism should refer solely to reactions from butterflies and moths—adult forms of insects with scaly wings—while erucism should refer to reactions from contact with caterpillars—the larval form of butterflies and moths.
In common use, lepidopterism can describe any reaction from caterpillars, moths, or adult butterflies, as well as any case of Lepidoptera exposure with only systemic manifestations, regardless of cutaneous findings. Concurrently, erucism has been defined as either any reaction from caterpillars or any skin reaction from contact with caterpillars or moths.2
Because caterpillars are the larval form of butterflies and moths, caterpillar-associated skin reactions also have been conveniently denominated caterpillar dermatitis.1 Henceforth in this article, both terms erucism and caterpillar dermatitis are used interchangeably.
Caterpillar Envenomation
Caterpillars cause the vast majority of adverse events from lepidopteran exposures.2 Envenomation by caterpillars might stand as the world’s most common envenomation given the larvae proximity to humans.3 Although involvement of internal organs (eg, renal failure), cerebral hemorrhage, and joint lesions can occur, skin manifestations are more predominant with the majority of species. Initial localized pain, edema, and erythema usually are present at the site of direct contact and subsequently progress toward maculopapular to bullous lesions, erosions, petechiae, necrosis, and ulceration depending on the offending species.1,4
Megalopyge opercularis
In the United States, more than 50 species of caterpillars have been identified as poisonous or venomous.5 Megalopyge opercularis (Figure 1), the larval form of the flannel moth, is an important cause of caterpillar-associated dermatitis in the southern United States.6,7 Megalopyge opercularis also is commonly known as the puss caterpillar, opossum bug, wooly slug, el perrito, tree asp, or Italian asp.6 This lepidopteran insect is mainly found in the southeastern and southcentral United States, with noted particular abundance in Texas, Louisiana, and Florida.6,8 The puss caterpillar has 2 generations per year; the first develops during the months of June to July, and the second develops from September to October, carrying seasonal health hazards.6,8

Megalopyge opercularis is tapered at the ends and can measure 2.5 to 3.5×1 cm at maturity. It is covered by silky, long-streaked, wavy hairs that may appear single colored or as a mix of colors—from white to gray to brown—forming a mid-dorsal crest.6 Beneath this furry coat, rows of short sharp spines are hidden. Upon contact with the human skin, these spines will break and discharge venom.1,6,8 Toxins contained within the hollow spines are thought to be produced by specialized basal cells, but there still is little knowledge about the dynamics and composition of the venom.1
Clinical Manifestations
The severity of the reaction depends on the caterpillar’s size and the extent of contact.1,4 Contact with M opercularis instantly presents with a throbbing or burning pain that may be followed by localized erythema and rash.1,6 A characteristic gridlike pattern of erythematous macules develops, reflecting each site of puncture from the insect’s spines (Figure 2).8,9 Skin lesions can progress from erythematous macules to hemorrhagic vesicles or pustules, usually self-resolving after a few days. The reaction also can present with radiating pain to regional lymph nodes and numbness of the affected area.1,6,8 Moreover, some patients may report urticaria and pruritus.9
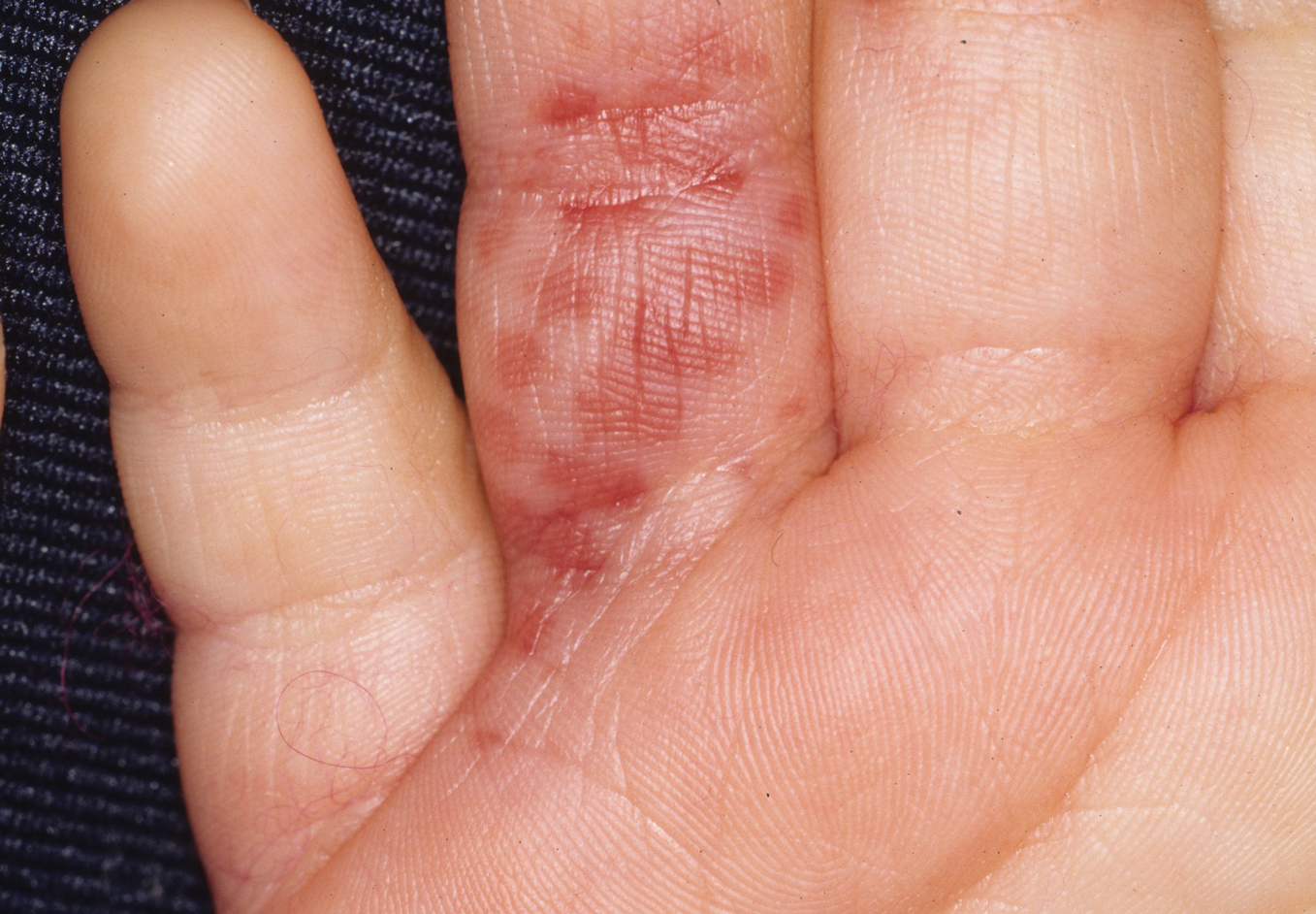
Envenomation by a puss caterpillar also can present with systemic manifestations including fever, headache, nausea, vomiting, shocklike symptoms, and seizures.1,6,7 Anaphylactic reaction is rare but also can present.7 Uncommon cases have been reported with severe abdominal pain and muscle spasm mimicking acute appendicitis and latrodectism, respectively.7,9
Diagnosis
The diagnosis of M opercularis envenomation is made clinically based on the morphology of the skin lesions and a history of probable exposure. Coexistent leukocytosis is likely, but laboratory testing is not warranted, as it is both nonspecific and insensitive.9
Management/Treatment
The most commonly reported immediate approaches to treatment involve attempts to remove the spines from the skin with tape (stripping), application of ice packs over the affected area, oral antihistamines, topical and intralesional anesthetics, regional nerve block, and oral analgesics.6,9 There have been several cases detailing the successful use of parenteral calcium gluconate,5,7 and diazepam has been used to treat severe muscle spasms. Anaphylactic reactions should be managed in a controlled monitored setting with subcutaneous epinephrine.7 Despite their common use, some data suggest that ice packs and mid- to high-potency topical steroids are ineffective.9
Incidence
From 2001 to 2005, a mean average of 94,552 annual cases of animal bites and stings were reported to poison control centers in the United States, of which 2094 were linked to caterpillars in this 5-year period.10 There were 3484 M opercularis caterpillar stings reported to the Texas Poison Center Network from 2000 to 2016.5,6 Given their ability to sting throughout their life cycle, thousands of M opercularis caterpillar stings can occur each year.1,6 Existing literature on M opercularis caterpillar stings mainly involves case reports with affections of the skin and oral mucosa, self-reported envenomation, and case studies.5,6,8
Although multiple health concerns associated with caterpillar envenomation have been reported worldwide, the lack of official epidemiologic reports highly suggests that this problem remains underestimated. There also may be many unreported cases because certain reactions are mild or self-limited and can even go unnoticed.11 Nonetheless, there is an evident rise of cases reported in the United States. According to the 2018 annual report of the American Association of Poison Control Centers, there were 2815 case mentions from caterpillar envenomation.12
In 1921 and 1952, some public schools in Texas were temporarily closed due to outbreaks of puss caterpillar–associated dermatitis.8 Similar outbreaks also have been reported in South Carolina, Virginia, and Oklahoma.9 Emerging data suggest that plant oil products and the pesticide cypermethrin may be helpful in controlling local infestations of the puss caterpillar.8
- Villas-Boas IM, Bonfa G, Tambourgi DV. Venomous caterpillars: from inoculation apparatus to venom composition and envenomation. Toxicon. 2018;153:39-52.
- Hossler EW. Caterpillars and moths: part I. dermatologic manifestations of encounters with Lepidoptera. J Am Acad Dermatol. 2010;62:1-10; quiz 11-12.
- Haddad Junior V, Amorim PC, Haddad Junior WT, et al. Venomous and poisonous arthropods: identification, clinical manifestations of envenomation, and treatments used in human injuries. Rev Soc Bras Med Trop. 2015;48:650-657.
- Haddad V Jr, Cardoso JL, Lupi O, et al. Tropical dermatology: venomous arthropods and human skin: part I. Insecta. J Am Acad Dermatol. 2012;67:331.e1-331.e14; quiz 345.
- Pappano DA, Trout Fryxell R, Warren M. Oral mucosal envenomation of an infant by a puss caterpillar. Pediatr Emerg Care. 2017;33:424-426.
- Forrester MB. Megalopyge opercularis caterpillar stings reported to Texas poison centers. Wilderness Environ Med. 2018;29:215-220.
- Hossler EW. Caterpillars and moths: part II. dermatologic manifestations of encounters with Lepidoptera. J Am Acad Dermatol. 2010;62:13-28; quiz 29-30.
- Eagleman DM. Envenomation by the asp caterpillar (Megalopyge opercularis). Clin Toxicol (Phila). 2008;46:201-205.
- Greene SC, Carey JM. Puss caterpillar envenomation: erucism mimicking appendicitis in a young child [published online May 23, 2018]. Pediatr Emerg Care. doi:10.1097/PEC.0000000000001514.
- Langley RL. Animal bites and stings reported by United States Poison Control Centers, 2001-2005. Wilderness Environ Med. 2008;19:7-14.
- Seldeslachts A, Peigneur S, Tytgat J. Caterpillar venom: a health hazard of the 21st century [published online May 30, 2020]. Biomedicines. doi:10.3390/biomedicines8060143.
- Gummin DD, Mowry JB, Spyker DA, et al. 2018 annual report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 36th annual report. Clin Toxicol (Phila). 2019;57:1220-1413.
Lepidoptera is the second largest order of the class Insecta and comprises approximately 160,000 species of butterflies and moths classified among approximately 124 families and subfamilies. Venomous properties have been identified in 12 of these families, posing a serious threat to human health. 1
The clinical manifestations from Lepidoptera envenomation can range from general systemic symptoms such as fever and abdominal distress; to more complex focal affections including hemorrhage, ophthalmologic lesions, and irritation of the respiratory tracts; to less severe reactions of the skin, which are the most common presentation.1
Terminology
Lepidopterism is the term used to address a clinical spectrum of systemic manifestations from direct contact with venomous butterflies or moths and/or their products.2 Conversely, erucism is a term used to describe localized cutaneous reactions after direct contact with toxins from caterpillars.
Lepidopterism is derived from the Greek roots lepis, meaning scale, and pteron, meaning wing. The term erucism stems from the Latin word eruca, which means larva.2
Ideally, lepidopterism should refer solely to reactions from butterflies and moths—adult forms of insects with scaly wings—while erucism should refer to reactions from contact with caterpillars—the larval form of butterflies and moths.
In common use, lepidopterism can describe any reaction from caterpillars, moths, or adult butterflies, as well as any case of Lepidoptera exposure with only systemic manifestations, regardless of cutaneous findings. Concurrently, erucism has been defined as either any reaction from caterpillars or any skin reaction from contact with caterpillars or moths.2
Because caterpillars are the larval form of butterflies and moths, caterpillar-associated skin reactions also have been conveniently denominated caterpillar dermatitis.1 Henceforth in this article, both terms erucism and caterpillar dermatitis are used interchangeably.
Caterpillar Envenomation
Caterpillars cause the vast majority of adverse events from lepidopteran exposures.2 Envenomation by caterpillars might stand as the world’s most common envenomation given the larvae proximity to humans.3 Although involvement of internal organs (eg, renal failure), cerebral hemorrhage, and joint lesions can occur, skin manifestations are more predominant with the majority of species. Initial localized pain, edema, and erythema usually are present at the site of direct contact and subsequently progress toward maculopapular to bullous lesions, erosions, petechiae, necrosis, and ulceration depending on the offending species.1,4
Megalopyge opercularis
In the United States, more than 50 species of caterpillars have been identified as poisonous or venomous.5 Megalopyge opercularis (Figure 1), the larval form of the flannel moth, is an important cause of caterpillar-associated dermatitis in the southern United States.6,7 Megalopyge opercularis also is commonly known as the puss caterpillar, opossum bug, wooly slug, el perrito, tree asp, or Italian asp.6 This lepidopteran insect is mainly found in the southeastern and southcentral United States, with noted particular abundance in Texas, Louisiana, and Florida.6,8 The puss caterpillar has 2 generations per year; the first develops during the months of June to July, and the second develops from September to October, carrying seasonal health hazards.6,8

Megalopyge opercularis is tapered at the ends and can measure 2.5 to 3.5×1 cm at maturity. It is covered by silky, long-streaked, wavy hairs that may appear single colored or as a mix of colors—from white to gray to brown—forming a mid-dorsal crest.6 Beneath this furry coat, rows of short sharp spines are hidden. Upon contact with the human skin, these spines will break and discharge venom.1,6,8 Toxins contained within the hollow spines are thought to be produced by specialized basal cells, but there still is little knowledge about the dynamics and composition of the venom.1
Clinical Manifestations
The severity of the reaction depends on the caterpillar’s size and the extent of contact.1,4 Contact with M opercularis instantly presents with a throbbing or burning pain that may be followed by localized erythema and rash.1,6 A characteristic gridlike pattern of erythematous macules develops, reflecting each site of puncture from the insect’s spines (Figure 2).8,9 Skin lesions can progress from erythematous macules to hemorrhagic vesicles or pustules, usually self-resolving after a few days. The reaction also can present with radiating pain to regional lymph nodes and numbness of the affected area.1,6,8 Moreover, some patients may report urticaria and pruritus.9

Envenomation by a puss caterpillar also can present with systemic manifestations including fever, headache, nausea, vomiting, shocklike symptoms, and seizures.1,6,7 Anaphylactic reaction is rare but also can present.7 Uncommon cases have been reported with severe abdominal pain and muscle spasm mimicking acute appendicitis and latrodectism, respectively.7,9
Diagnosis
The diagnosis of M opercularis envenomation is made clinically based on the morphology of the skin lesions and a history of probable exposure. Coexistent leukocytosis is likely, but laboratory testing is not warranted, as it is both nonspecific and insensitive.9
Management/Treatment
The most commonly reported immediate approaches to treatment involve attempts to remove the spines from the skin with tape (stripping), application of ice packs over the affected area, oral antihistamines, topical and intralesional anesthetics, regional nerve block, and oral analgesics.6,9 There have been several cases detailing the successful use of parenteral calcium gluconate,5,7 and diazepam has been used to treat severe muscle spasms. Anaphylactic reactions should be managed in a controlled monitored setting with subcutaneous epinephrine.7 Despite their common use, some data suggest that ice packs and mid- to high-potency topical steroids are ineffective.9
Incidence
From 2001 to 2005, a mean average of 94,552 annual cases of animal bites and stings were reported to poison control centers in the United States, of which 2094 were linked to caterpillars in this 5-year period.10 There were 3484 M opercularis caterpillar stings reported to the Texas Poison Center Network from 2000 to 2016.5,6 Given their ability to sting throughout their life cycle, thousands of M opercularis caterpillar stings can occur each year.1,6 Existing literature on M opercularis caterpillar stings mainly involves case reports with affections of the skin and oral mucosa, self-reported envenomation, and case studies.5,6,8
Although multiple health concerns associated with caterpillar envenomation have been reported worldwide, the lack of official epidemiologic reports highly suggests that this problem remains underestimated. There also may be many unreported cases because certain reactions are mild or self-limited and can even go unnoticed.11 Nonetheless, there is an evident rise of cases reported in the United States. According to the 2018 annual report of the American Association of Poison Control Centers, there were 2815 case mentions from caterpillar envenomation.12
In 1921 and 1952, some public schools in Texas were temporarily closed due to outbreaks of puss caterpillar–associated dermatitis.8 Similar outbreaks also have been reported in South Carolina, Virginia, and Oklahoma.9 Emerging data suggest that plant oil products and the pesticide cypermethrin may be helpful in controlling local infestations of the puss caterpillar.8
Lepidoptera is the second largest order of the class Insecta and comprises approximately 160,000 species of butterflies and moths classified among approximately 124 families and subfamilies. Venomous properties have been identified in 12 of these families, posing a serious threat to human health. 1
The clinical manifestations from Lepidoptera envenomation can range from general systemic symptoms such as fever and abdominal distress; to more complex focal affections including hemorrhage, ophthalmologic lesions, and irritation of the respiratory tracts; to less severe reactions of the skin, which are the most common presentation.1
Terminology
Lepidopterism is the term used to address a clinical spectrum of systemic manifestations from direct contact with venomous butterflies or moths and/or their products.2 Conversely, erucism is a term used to describe localized cutaneous reactions after direct contact with toxins from caterpillars.
Lepidopterism is derived from the Greek roots lepis, meaning scale, and pteron, meaning wing. The term erucism stems from the Latin word eruca, which means larva.2
Ideally, lepidopterism should refer solely to reactions from butterflies and moths—adult forms of insects with scaly wings—while erucism should refer to reactions from contact with caterpillars—the larval form of butterflies and moths.
In common use, lepidopterism can describe any reaction from caterpillars, moths, or adult butterflies, as well as any case of Lepidoptera exposure with only systemic manifestations, regardless of cutaneous findings. Concurrently, erucism has been defined as either any reaction from caterpillars or any skin reaction from contact with caterpillars or moths.2
Because caterpillars are the larval form of butterflies and moths, caterpillar-associated skin reactions also have been conveniently denominated caterpillar dermatitis.1 Henceforth in this article, both terms erucism and caterpillar dermatitis are used interchangeably.
Caterpillar Envenomation
Caterpillars cause the vast majority of adverse events from lepidopteran exposures.2 Envenomation by caterpillars might stand as the world’s most common envenomation given the larvae proximity to humans.3 Although involvement of internal organs (eg, renal failure), cerebral hemorrhage, and joint lesions can occur, skin manifestations are more predominant with the majority of species. Initial localized pain, edema, and erythema usually are present at the site of direct contact and subsequently progress toward maculopapular to bullous lesions, erosions, petechiae, necrosis, and ulceration depending on the offending species.1,4
Megalopyge opercularis
In the United States, more than 50 species of caterpillars have been identified as poisonous or venomous.5 Megalopyge opercularis (Figure 1), the larval form of the flannel moth, is an important cause of caterpillar-associated dermatitis in the southern United States.6,7 Megalopyge opercularis also is commonly known as the puss caterpillar, opossum bug, wooly slug, el perrito, tree asp, or Italian asp.6 This lepidopteran insect is mainly found in the southeastern and southcentral United States, with noted particular abundance in Texas, Louisiana, and Florida.6,8 The puss caterpillar has 2 generations per year; the first develops during the months of June to July, and the second develops from September to October, carrying seasonal health hazards.6,8

Megalopyge opercularis is tapered at the ends and can measure 2.5 to 3.5×1 cm at maturity. It is covered by silky, long-streaked, wavy hairs that may appear single colored or as a mix of colors—from white to gray to brown—forming a mid-dorsal crest.6 Beneath this furry coat, rows of short sharp spines are hidden. Upon contact with the human skin, these spines will break and discharge venom.1,6,8 Toxins contained within the hollow spines are thought to be produced by specialized basal cells, but there still is little knowledge about the dynamics and composition of the venom.1
Clinical Manifestations
The severity of the reaction depends on the caterpillar’s size and the extent of contact.1,4 Contact with M opercularis instantly presents with a throbbing or burning pain that may be followed by localized erythema and rash.1,6 A characteristic gridlike pattern of erythematous macules develops, reflecting each site of puncture from the insect’s spines (Figure 2).8,9 Skin lesions can progress from erythematous macules to hemorrhagic vesicles or pustules, usually self-resolving after a few days. The reaction also can present with radiating pain to regional lymph nodes and numbness of the affected area.1,6,8 Moreover, some patients may report urticaria and pruritus.9

Envenomation by a puss caterpillar also can present with systemic manifestations including fever, headache, nausea, vomiting, shocklike symptoms, and seizures.1,6,7 Anaphylactic reaction is rare but also can present.7 Uncommon cases have been reported with severe abdominal pain and muscle spasm mimicking acute appendicitis and latrodectism, respectively.7,9
Diagnosis
The diagnosis of M opercularis envenomation is made clinically based on the morphology of the skin lesions and a history of probable exposure. Coexistent leukocytosis is likely, but laboratory testing is not warranted, as it is both nonspecific and insensitive.9
Management/Treatment
The most commonly reported immediate approaches to treatment involve attempts to remove the spines from the skin with tape (stripping), application of ice packs over the affected area, oral antihistamines, topical and intralesional anesthetics, regional nerve block, and oral analgesics.6,9 There have been several cases detailing the successful use of parenteral calcium gluconate,5,7 and diazepam has been used to treat severe muscle spasms. Anaphylactic reactions should be managed in a controlled monitored setting with subcutaneous epinephrine.7 Despite their common use, some data suggest that ice packs and mid- to high-potency topical steroids are ineffective.9
Incidence
From 2001 to 2005, a mean average of 94,552 annual cases of animal bites and stings were reported to poison control centers in the United States, of which 2094 were linked to caterpillars in this 5-year period.10 There were 3484 M opercularis caterpillar stings reported to the Texas Poison Center Network from 2000 to 2016.5,6 Given their ability to sting throughout their life cycle, thousands of M opercularis caterpillar stings can occur each year.1,6 Existing literature on M opercularis caterpillar stings mainly involves case reports with affections of the skin and oral mucosa, self-reported envenomation, and case studies.5,6,8
Although multiple health concerns associated with caterpillar envenomation have been reported worldwide, the lack of official epidemiologic reports highly suggests that this problem remains underestimated. There also may be many unreported cases because certain reactions are mild or self-limited and can even go unnoticed.11 Nonetheless, there is an evident rise of cases reported in the United States. According to the 2018 annual report of the American Association of Poison Control Centers, there were 2815 case mentions from caterpillar envenomation.12
In 1921 and 1952, some public schools in Texas were temporarily closed due to outbreaks of puss caterpillar–associated dermatitis.8 Similar outbreaks also have been reported in South Carolina, Virginia, and Oklahoma.9 Emerging data suggest that plant oil products and the pesticide cypermethrin may be helpful in controlling local infestations of the puss caterpillar.8
- Villas-Boas IM, Bonfa G, Tambourgi DV. Venomous caterpillars: from inoculation apparatus to venom composition and envenomation. Toxicon. 2018;153:39-52.
- Hossler EW. Caterpillars and moths: part I. dermatologic manifestations of encounters with Lepidoptera. J Am Acad Dermatol. 2010;62:1-10; quiz 11-12.
- Haddad Junior V, Amorim PC, Haddad Junior WT, et al. Venomous and poisonous arthropods: identification, clinical manifestations of envenomation, and treatments used in human injuries. Rev Soc Bras Med Trop. 2015;48:650-657.
- Haddad V Jr, Cardoso JL, Lupi O, et al. Tropical dermatology: venomous arthropods and human skin: part I. Insecta. J Am Acad Dermatol. 2012;67:331.e1-331.e14; quiz 345.
- Pappano DA, Trout Fryxell R, Warren M. Oral mucosal envenomation of an infant by a puss caterpillar. Pediatr Emerg Care. 2017;33:424-426.
- Forrester MB. Megalopyge opercularis caterpillar stings reported to Texas poison centers. Wilderness Environ Med. 2018;29:215-220.
- Hossler EW. Caterpillars and moths: part II. dermatologic manifestations of encounters with Lepidoptera. J Am Acad Dermatol. 2010;62:13-28; quiz 29-30.
- Eagleman DM. Envenomation by the asp caterpillar (Megalopyge opercularis). Clin Toxicol (Phila). 2008;46:201-205.
- Greene SC, Carey JM. Puss caterpillar envenomation: erucism mimicking appendicitis in a young child [published online May 23, 2018]. Pediatr Emerg Care. doi:10.1097/PEC.0000000000001514.
- Langley RL. Animal bites and stings reported by United States Poison Control Centers, 2001-2005. Wilderness Environ Med. 2008;19:7-14.
- Seldeslachts A, Peigneur S, Tytgat J. Caterpillar venom: a health hazard of the 21st century [published online May 30, 2020]. Biomedicines. doi:10.3390/biomedicines8060143.
- Gummin DD, Mowry JB, Spyker DA, et al. 2018 annual report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 36th annual report. Clin Toxicol (Phila). 2019;57:1220-1413.
- Villas-Boas IM, Bonfa G, Tambourgi DV. Venomous caterpillars: from inoculation apparatus to venom composition and envenomation. Toxicon. 2018;153:39-52.
- Hossler EW. Caterpillars and moths: part I. dermatologic manifestations of encounters with Lepidoptera. J Am Acad Dermatol. 2010;62:1-10; quiz 11-12.
- Haddad Junior V, Amorim PC, Haddad Junior WT, et al. Venomous and poisonous arthropods: identification, clinical manifestations of envenomation, and treatments used in human injuries. Rev Soc Bras Med Trop. 2015;48:650-657.
- Haddad V Jr, Cardoso JL, Lupi O, et al. Tropical dermatology: venomous arthropods and human skin: part I. Insecta. J Am Acad Dermatol. 2012;67:331.e1-331.e14; quiz 345.
- Pappano DA, Trout Fryxell R, Warren M. Oral mucosal envenomation of an infant by a puss caterpillar. Pediatr Emerg Care. 2017;33:424-426.
- Forrester MB. Megalopyge opercularis caterpillar stings reported to Texas poison centers. Wilderness Environ Med. 2018;29:215-220.
- Hossler EW. Caterpillars and moths: part II. dermatologic manifestations of encounters with Lepidoptera. J Am Acad Dermatol. 2010;62:13-28; quiz 29-30.
- Eagleman DM. Envenomation by the asp caterpillar (Megalopyge opercularis). Clin Toxicol (Phila). 2008;46:201-205.
- Greene SC, Carey JM. Puss caterpillar envenomation: erucism mimicking appendicitis in a young child [published online May 23, 2018]. Pediatr Emerg Care. doi:10.1097/PEC.0000000000001514.
- Langley RL. Animal bites and stings reported by United States Poison Control Centers, 2001-2005. Wilderness Environ Med. 2008;19:7-14.
- Seldeslachts A, Peigneur S, Tytgat J. Caterpillar venom: a health hazard of the 21st century [published online May 30, 2020]. Biomedicines. doi:10.3390/biomedicines8060143.
- Gummin DD, Mowry JB, Spyker DA, et al. 2018 annual report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 36th annual report. Clin Toxicol (Phila). 2019;57:1220-1413.
Practice Points
- Megalopyge opercularis is the most widely distributed caterpillar species in the Americas, and envenomation by it can occur year-round.
- Skin reactions to M opercularis stings can present as maculopapular dermatitis, eczematous eruptions, or urticarial reactions.
- During the initial presentation, patients experience intense throbbing pain, yet the severity of symptoms depends on the caterpillar’s size and the extent of contact.
- A history of caterpillar exposure helps with diagnosis, and treatment remains empiric.
What’s Eating You? Bark Scorpions (Centruroides exilicauda and Centruroides sculpturatus)
Epidemiology and Identification
Centruroides is a common genus of bark scorpions in the United States with at least 21 species considered to be medically important, including the closely related Centruroides exilicauda and Centruroides sculpturatus.1 Scorpions can be recognized by a bulbous sac and pointed stinger at the end of a tail-like abdomen. They also have long lobsterlike pedipalps (pincers) for grasping their prey. Identifying characteristics for C exilicauda and C sculpturatus include a small, slender, yellow to light brown or tan body typically measuring 1.3 to 7.6 cm in length with a subaculear tooth or tubercle at the base of the stinger, a characteristic that is common to all Centruroides species (Figure).2 Some variability in size has been shown, with smaller scorpions found in increased elevations and cooler temperatures.1,3 Both C exilicauda and C sculpturatus are found in northern Mexico as well as the southwestern United States (eg, Arizona, New Mexico, Texas, California, Nevada).1 They have a preference for residing in or around trees and often are found on the underside of bark, stones, or tables as well as inside shoes or small cracks and crevices. Scorpions typically sting in self-defense, and stings commonly occur when humans attempt to move tables, put on shoes, or walk barefoot in scorpion-infested areas. Most stings occur from the end of spring through the end summer, but many may go unreported.1,4

The venom of the Centruroides genus includes peptides and proteins that play a fundamental role in toxic activity by impairing potassium, sodium, and calcium ion channels.1,3 Toxins have been shown to be species specific, functioning either in capturing prey or deterring predators. Intraspecies variability in toxins has been demonstrated, which may complicate the production of adequate antivenin.3 Many have thought that C exilicauda Wood and C sculpturatus Ewing are the same species, and the names have been used synonymously in the past; however, genetic and biochemical studies of their venom components have shown that they are distinct species and that C sculpturatus is the more dangerous of the two.5 The median lethal dose 50% of C sculpturatus was found to be 22.7 μg in CD1 mice.6
Envenomation and Clinical Manifestations
Stings from C exilicauda and C sculpturatus have been shown to cause fatality in children more often than in adults.7 In the United States, Arizona has the highest frequency of serious symptoms of envenomation as well as the highest hospital and intensive care unit admission rates.6 Envenomation results in an immediate sharp burning pain followed by numbness.4 Wounds can produce some regional lymph node swelling, ecchymosis, paresthesia, and lymphangitis. More often than not, however, wounds have little to no inflammation and are characterized only by pain.4 The puncture wound is too small to be seen, and C exilicauda and C sculpturatus venom do not cause local tissue destruction, an important factor in distinguishing it from other scorpion envenomations.
More severe complications that may follow are caused by the neurotoxin released by Centruroides stings. The toxin components can increase the duration and amplitude of the neuronal action potential and enhance the release of neurotransmitters such as acetylcholine and norepinephrine.8 Stings can lead to cranial nerve dysfunction and somatic skeletal neuromuscular dysfunction as well as autonomic dysfunction, specifically salivation, fever, tongue and muscle fasciculations, opsoclonus, vomiting, bronchoconstriction, diaphoresis, nystagmus, blurred vision, slurred speech, hypertension, rhabdomyolysis, stridor, wheezing, aspiration, anaphylaxis, and tachycardia, leading to cardiac and respiratory compromise.4,8 Some patients have experienced a decreased sense of smell or hearing and decreased fine motor movements.7 Although pancreatitis may occur with scorpion stings, it is not common for C exilicauda.9 Comorbidities such as cardiac disease and substance use disorders contribute to prolonged length of hospital stay and poor outcome.8
Treatment
Most Centruroides stings can be managed at home, but patients with more serious symptoms and children younger than 2 years should be taken to a hospital for treatment.7 If a patient reports only pain but shows no other signs of neurotoxicity, observation and pain relief with rest, ice, and elevation is appropriate management. Patients with severe manifestations have been treated with various combinations of lorazepam, glycopyrrolate, ipratropium bromide, and ondansetron, but the only treatment definitively shown to decrease time to symptom abatement is antivenin.7 It has been demonstrated that C exilicauda and C sculpturatus antivenin is relatively safe.7 Most patients, especially adults, do not die from C exilicauda and C sculpturatus stings; therefore, antivenin more commonly is symptom abating than it is lifesaving.10 In children, time to symptom resolution was decreased to fewer than 4 hours with antivenin, and there is a lower rate of inpatient admission when antivenin is administered.4,10,11 There is a low incidence of anaphylactic reaction after antivenin, but there have been reported cases of self-limited serum sickness after antivenin use that generally can be managed with antihistamines and corticosteroids.4,7
- Gonzalez-Santillan E, Possani LD. North American scorpion species of public health importance with reappraisal of historical epidemiology. Acta Tropica. 2018;187:264-274.
- Goldsmith LA, Katz SI, Gilchrest BA, et al, eds. Fitzpatrick’s Dermatology in General Medicine. 8th ed. New York, NY: McGraw-Hill; 2012.
- Carcamo-Noriega EN, Olamendi-Portugal T, Restano-Cassulini R, et al. Intraspecific variation of Centruroides sculpturatus scorpion venom from two regions of Arizona. Arch Biochem Biophys. 2018;638:52-57.
- Kang AM, Brooks DE. Nationwide scorpion exposures reported to US Poison Control centers from 2005 to 2015. J Med Toxicol. 2017;13:158-165.
- Valdez-Cruz N, Dávila S, Licea A, et al. Biochemical, genetic and physiological characterization of venom components from two species of scorpions: Centruroides exilicauda Wood and Centruroides sculpturatus Ewing. Biochimie. 2004;86:387-396.
- Jiménez-Vargas JM, Quintero-Hernández V, Gonzáles-Morales L, et al. Design and expression of recombinant toxins from Mexican scorpions of the genus Centruroides for production of antivenoms. Toxicon. 2017;128:5-14.
- Hurst NB, Lipe DN, Karpen SR, et al. Centruroides sculpturatus envenomation in three adult patients requiring treatment with antivenom. Clin Toxicol (Phila). 2018;56:294-296.
- O’Connor A, Padilla-Jones A, Ruha A. Severe bark scorpion envenomation in adults. Clin Toxicol. 2018;56:170-174.
- Berg R, Tarantino M. Envenomation by the scorpion Centruroides exilicauda (C sculpturatus): severe and unusual manifestations. Pediatrics. 1991;87:930-933.
- LoVecchio F, McBride C. Scorpion envenomations in young children in central Arizona. J Toxicol Clin Toxicol. 2003;41:937-940.
- Rodrigo C, Gnanathasan A. Management of scorpion envenoming: a systematic review and meta-analysis of controlled clinical trials. Syst Rev. 2017;6:74.
Epidemiology and Identification
Centruroides is a common genus of bark scorpions in the United States with at least 21 species considered to be medically important, including the closely related Centruroides exilicauda and Centruroides sculpturatus.1 Scorpions can be recognized by a bulbous sac and pointed stinger at the end of a tail-like abdomen. They also have long lobsterlike pedipalps (pincers) for grasping their prey. Identifying characteristics for C exilicauda and C sculpturatus include a small, slender, yellow to light brown or tan body typically measuring 1.3 to 7.6 cm in length with a subaculear tooth or tubercle at the base of the stinger, a characteristic that is common to all Centruroides species (Figure).2 Some variability in size has been shown, with smaller scorpions found in increased elevations and cooler temperatures.1,3 Both C exilicauda and C sculpturatus are found in northern Mexico as well as the southwestern United States (eg, Arizona, New Mexico, Texas, California, Nevada).1 They have a preference for residing in or around trees and often are found on the underside of bark, stones, or tables as well as inside shoes or small cracks and crevices. Scorpions typically sting in self-defense, and stings commonly occur when humans attempt to move tables, put on shoes, or walk barefoot in scorpion-infested areas. Most stings occur from the end of spring through the end summer, but many may go unreported.1,4

The venom of the Centruroides genus includes peptides and proteins that play a fundamental role in toxic activity by impairing potassium, sodium, and calcium ion channels.1,3 Toxins have been shown to be species specific, functioning either in capturing prey or deterring predators. Intraspecies variability in toxins has been demonstrated, which may complicate the production of adequate antivenin.3 Many have thought that C exilicauda Wood and C sculpturatus Ewing are the same species, and the names have been used synonymously in the past; however, genetic and biochemical studies of their venom components have shown that they are distinct species and that C sculpturatus is the more dangerous of the two.5 The median lethal dose 50% of C sculpturatus was found to be 22.7 μg in CD1 mice.6
Envenomation and Clinical Manifestations
Stings from C exilicauda and C sculpturatus have been shown to cause fatality in children more often than in adults.7 In the United States, Arizona has the highest frequency of serious symptoms of envenomation as well as the highest hospital and intensive care unit admission rates.6 Envenomation results in an immediate sharp burning pain followed by numbness.4 Wounds can produce some regional lymph node swelling, ecchymosis, paresthesia, and lymphangitis. More often than not, however, wounds have little to no inflammation and are characterized only by pain.4 The puncture wound is too small to be seen, and C exilicauda and C sculpturatus venom do not cause local tissue destruction, an important factor in distinguishing it from other scorpion envenomations.
More severe complications that may follow are caused by the neurotoxin released by Centruroides stings. The toxin components can increase the duration and amplitude of the neuronal action potential and enhance the release of neurotransmitters such as acetylcholine and norepinephrine.8 Stings can lead to cranial nerve dysfunction and somatic skeletal neuromuscular dysfunction as well as autonomic dysfunction, specifically salivation, fever, tongue and muscle fasciculations, opsoclonus, vomiting, bronchoconstriction, diaphoresis, nystagmus, blurred vision, slurred speech, hypertension, rhabdomyolysis, stridor, wheezing, aspiration, anaphylaxis, and tachycardia, leading to cardiac and respiratory compromise.4,8 Some patients have experienced a decreased sense of smell or hearing and decreased fine motor movements.7 Although pancreatitis may occur with scorpion stings, it is not common for C exilicauda.9 Comorbidities such as cardiac disease and substance use disorders contribute to prolonged length of hospital stay and poor outcome.8
Treatment
Most Centruroides stings can be managed at home, but patients with more serious symptoms and children younger than 2 years should be taken to a hospital for treatment.7 If a patient reports only pain but shows no other signs of neurotoxicity, observation and pain relief with rest, ice, and elevation is appropriate management. Patients with severe manifestations have been treated with various combinations of lorazepam, glycopyrrolate, ipratropium bromide, and ondansetron, but the only treatment definitively shown to decrease time to symptom abatement is antivenin.7 It has been demonstrated that C exilicauda and C sculpturatus antivenin is relatively safe.7 Most patients, especially adults, do not die from C exilicauda and C sculpturatus stings; therefore, antivenin more commonly is symptom abating than it is lifesaving.10 In children, time to symptom resolution was decreased to fewer than 4 hours with antivenin, and there is a lower rate of inpatient admission when antivenin is administered.4,10,11 There is a low incidence of anaphylactic reaction after antivenin, but there have been reported cases of self-limited serum sickness after antivenin use that generally can be managed with antihistamines and corticosteroids.4,7
Epidemiology and Identification
Centruroides is a common genus of bark scorpions in the United States with at least 21 species considered to be medically important, including the closely related Centruroides exilicauda and Centruroides sculpturatus.1 Scorpions can be recognized by a bulbous sac and pointed stinger at the end of a tail-like abdomen. They also have long lobsterlike pedipalps (pincers) for grasping their prey. Identifying characteristics for C exilicauda and C sculpturatus include a small, slender, yellow to light brown or tan body typically measuring 1.3 to 7.6 cm in length with a subaculear tooth or tubercle at the base of the stinger, a characteristic that is common to all Centruroides species (Figure).2 Some variability in size has been shown, with smaller scorpions found in increased elevations and cooler temperatures.1,3 Both C exilicauda and C sculpturatus are found in northern Mexico as well as the southwestern United States (eg, Arizona, New Mexico, Texas, California, Nevada).1 They have a preference for residing in or around trees and often are found on the underside of bark, stones, or tables as well as inside shoes or small cracks and crevices. Scorpions typically sting in self-defense, and stings commonly occur when humans attempt to move tables, put on shoes, or walk barefoot in scorpion-infested areas. Most stings occur from the end of spring through the end summer, but many may go unreported.1,4

The venom of the Centruroides genus includes peptides and proteins that play a fundamental role in toxic activity by impairing potassium, sodium, and calcium ion channels.1,3 Toxins have been shown to be species specific, functioning either in capturing prey or deterring predators. Intraspecies variability in toxins has been demonstrated, which may complicate the production of adequate antivenin.3 Many have thought that C exilicauda Wood and C sculpturatus Ewing are the same species, and the names have been used synonymously in the past; however, genetic and biochemical studies of their venom components have shown that they are distinct species and that C sculpturatus is the more dangerous of the two.5 The median lethal dose 50% of C sculpturatus was found to be 22.7 μg in CD1 mice.6
Envenomation and Clinical Manifestations
Stings from C exilicauda and C sculpturatus have been shown to cause fatality in children more often than in adults.7 In the United States, Arizona has the highest frequency of serious symptoms of envenomation as well as the highest hospital and intensive care unit admission rates.6 Envenomation results in an immediate sharp burning pain followed by numbness.4 Wounds can produce some regional lymph node swelling, ecchymosis, paresthesia, and lymphangitis. More often than not, however, wounds have little to no inflammation and are characterized only by pain.4 The puncture wound is too small to be seen, and C exilicauda and C sculpturatus venom do not cause local tissue destruction, an important factor in distinguishing it from other scorpion envenomations.
More severe complications that may follow are caused by the neurotoxin released by Centruroides stings. The toxin components can increase the duration and amplitude of the neuronal action potential and enhance the release of neurotransmitters such as acetylcholine and norepinephrine.8 Stings can lead to cranial nerve dysfunction and somatic skeletal neuromuscular dysfunction as well as autonomic dysfunction, specifically salivation, fever, tongue and muscle fasciculations, opsoclonus, vomiting, bronchoconstriction, diaphoresis, nystagmus, blurred vision, slurred speech, hypertension, rhabdomyolysis, stridor, wheezing, aspiration, anaphylaxis, and tachycardia, leading to cardiac and respiratory compromise.4,8 Some patients have experienced a decreased sense of smell or hearing and decreased fine motor movements.7 Although pancreatitis may occur with scorpion stings, it is not common for C exilicauda.9 Comorbidities such as cardiac disease and substance use disorders contribute to prolonged length of hospital stay and poor outcome.8
Treatment
Most Centruroides stings can be managed at home, but patients with more serious symptoms and children younger than 2 years should be taken to a hospital for treatment.7 If a patient reports only pain but shows no other signs of neurotoxicity, observation and pain relief with rest, ice, and elevation is appropriate management. Patients with severe manifestations have been treated with various combinations of lorazepam, glycopyrrolate, ipratropium bromide, and ondansetron, but the only treatment definitively shown to decrease time to symptom abatement is antivenin.7 It has been demonstrated that C exilicauda and C sculpturatus antivenin is relatively safe.7 Most patients, especially adults, do not die from C exilicauda and C sculpturatus stings; therefore, antivenin more commonly is symptom abating than it is lifesaving.10 In children, time to symptom resolution was decreased to fewer than 4 hours with antivenin, and there is a lower rate of inpatient admission when antivenin is administered.4,10,11 There is a low incidence of anaphylactic reaction after antivenin, but there have been reported cases of self-limited serum sickness after antivenin use that generally can be managed with antihistamines and corticosteroids.4,7
- Gonzalez-Santillan E, Possani LD. North American scorpion species of public health importance with reappraisal of historical epidemiology. Acta Tropica. 2018;187:264-274.
- Goldsmith LA, Katz SI, Gilchrest BA, et al, eds. Fitzpatrick’s Dermatology in General Medicine. 8th ed. New York, NY: McGraw-Hill; 2012.
- Carcamo-Noriega EN, Olamendi-Portugal T, Restano-Cassulini R, et al. Intraspecific variation of Centruroides sculpturatus scorpion venom from two regions of Arizona. Arch Biochem Biophys. 2018;638:52-57.
- Kang AM, Brooks DE. Nationwide scorpion exposures reported to US Poison Control centers from 2005 to 2015. J Med Toxicol. 2017;13:158-165.
- Valdez-Cruz N, Dávila S, Licea A, et al. Biochemical, genetic and physiological characterization of venom components from two species of scorpions: Centruroides exilicauda Wood and Centruroides sculpturatus Ewing. Biochimie. 2004;86:387-396.
- Jiménez-Vargas JM, Quintero-Hernández V, Gonzáles-Morales L, et al. Design and expression of recombinant toxins from Mexican scorpions of the genus Centruroides for production of antivenoms. Toxicon. 2017;128:5-14.
- Hurst NB, Lipe DN, Karpen SR, et al. Centruroides sculpturatus envenomation in three adult patients requiring treatment with antivenom. Clin Toxicol (Phila). 2018;56:294-296.
- O’Connor A, Padilla-Jones A, Ruha A. Severe bark scorpion envenomation in adults. Clin Toxicol. 2018;56:170-174.
- Berg R, Tarantino M. Envenomation by the scorpion Centruroides exilicauda (C sculpturatus): severe and unusual manifestations. Pediatrics. 1991;87:930-933.
- LoVecchio F, McBride C. Scorpion envenomations in young children in central Arizona. J Toxicol Clin Toxicol. 2003;41:937-940.
- Rodrigo C, Gnanathasan A. Management of scorpion envenoming: a systematic review and meta-analysis of controlled clinical trials. Syst Rev. 2017;6:74.
- Gonzalez-Santillan E, Possani LD. North American scorpion species of public health importance with reappraisal of historical epidemiology. Acta Tropica. 2018;187:264-274.
- Goldsmith LA, Katz SI, Gilchrest BA, et al, eds. Fitzpatrick’s Dermatology in General Medicine. 8th ed. New York, NY: McGraw-Hill; 2012.
- Carcamo-Noriega EN, Olamendi-Portugal T, Restano-Cassulini R, et al. Intraspecific variation of Centruroides sculpturatus scorpion venom from two regions of Arizona. Arch Biochem Biophys. 2018;638:52-57.
- Kang AM, Brooks DE. Nationwide scorpion exposures reported to US Poison Control centers from 2005 to 2015. J Med Toxicol. 2017;13:158-165.
- Valdez-Cruz N, Dávila S, Licea A, et al. Biochemical, genetic and physiological characterization of venom components from two species of scorpions: Centruroides exilicauda Wood and Centruroides sculpturatus Ewing. Biochimie. 2004;86:387-396.
- Jiménez-Vargas JM, Quintero-Hernández V, Gonzáles-Morales L, et al. Design and expression of recombinant toxins from Mexican scorpions of the genus Centruroides for production of antivenoms. Toxicon. 2017;128:5-14.
- Hurst NB, Lipe DN, Karpen SR, et al. Centruroides sculpturatus envenomation in three adult patients requiring treatment with antivenom. Clin Toxicol (Phila). 2018;56:294-296.
- O’Connor A, Padilla-Jones A, Ruha A. Severe bark scorpion envenomation in adults. Clin Toxicol. 2018;56:170-174.
- Berg R, Tarantino M. Envenomation by the scorpion Centruroides exilicauda (C sculpturatus): severe and unusual manifestations. Pediatrics. 1991;87:930-933.
- LoVecchio F, McBride C. Scorpion envenomations in young children in central Arizona. J Toxicol Clin Toxicol. 2003;41:937-940.
- Rodrigo C, Gnanathasan A. Management of scorpion envenoming: a systematic review and meta-analysis of controlled clinical trials. Syst Rev. 2017;6:74.
Practice Points
- Centruroides scorpions can inflict painful stings.
- Children are at greatest risk for systemic toxicity.
Tender White Lesions on the Groin
The Diagnosis: Candidal Intertrigo
The biopsy confirmed a diagnosis of severe hyperkeratotic candidal intertrigo with no evidence of Hailey-Hailey disease. Hematoxylin and eosin- stained sections demonstrated irregular acanthosis and variable spongiosis. The stratum corneum was predominantly orthokeratotic with overlying psuedohyphae and yeast fungal elements (Figure 1).
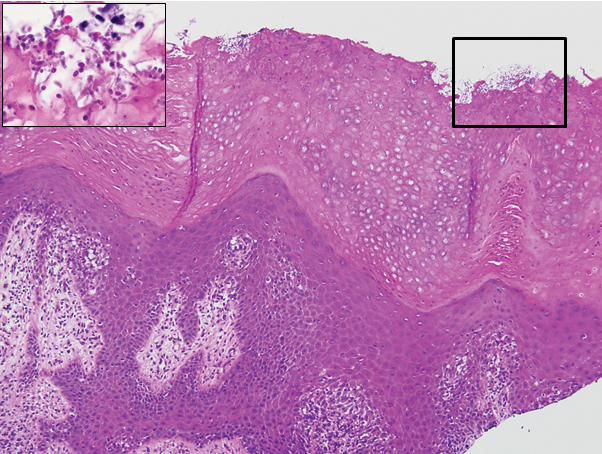
Hyperimmunoglobulinemia E syndrome (HIES), also known as hyper-IgE syndrome or Job syndrome, is a rare immunodeficiency disorder characterized by an eczematous dermatitis-like rash, recurrent skin and lung abscesses, eosinophilia, and elevated serum IgE. Facial asymmetry, prominent forehead, deep-set eyes, broad nose, and roughened facial skin with large pores are characteristic of the sporadic and autosomal-recessive forms. Other common findings include retained primary teeth, hyperextensible joints, and recurrent mucocutaneous candidiasis.1
Although autosomal-dominant and autosomal-recessive inheritance patterns exist, sporadic mutations are the most common cause of HIES.2 Several genes have been implicated depending on the inheritance pattern. The majority of autosomal-dominant cases are associated with inactivating STAT3 (signal transducer and activator of transcription 3) mutations, whereas the majority of autosomal-recessive cases are associated with inactivating DOCK8 (dedicator of cytokinesis 8) mutations.1 Ultimately, all of these mutations lead to an impaired helper T cell (TH17) response, which is crucial for clearing fungal and extracellular bacterial infections.3
Skin eruptions typically are the first manifestation of HIES; they appear within the first week to month of life as papulopustular eruptions on the face and scalp and rapidly generalize to the rest of the body, favoring the shoulders, arms, chest, and buttocks. The pustules then coalesce into crusted plaques that resemble atopic dermatitis, frequently with superimposed Staphylococcus aureus infection. On microscopy, the pustules are folliculocentric and often contain eosinophils, whereas the plaques may contain intraepidermal collections of eosinophils.1
Mucocutaneous candidiasis is seen in approximately 60% of HIES cases and is closely linked to STAT3 inactivating mutations.3 Histologically, there is marked acanthosis with neutrophil exocytosis and abundant yeast and pseudohyphal forms within the stratum corneum (Figure 2).4 Cutaneous candidal infections typically require both oral and topical antifungal agents to clear the infection.3 Most cases of mucocutaneous candidiasis are caused by Candida albicans; however, other known culprits include Candida glabrata, Candida tropicalis, Candida parapsilosis, and Candida krusei.5,6 Of note, species identification and antifungal susceptibility studies may be useful in refractory cases, especially with C glabrata, which is known to acquire resistance to azoles, such as fluconazole, with emerging resistance to echinocandins.6
The differential diagnosis of this groin eruption included Hailey-Hailey disease; pemphigus vegetans, Hallopeau type; tinea cruris; and inverse psoriasis. Hailey-Hailey disease can be complicated by a superimposed candidal infection with similar clinical features, and biopsy may be required for definitive diagnosis. Hailey-Hailey disease typically presents with macerated fissured plaques that resemble macerated tissue paper with red fissures (Figure 3). Biopsy confirms full-thickness acantholysis resembling a dilapidated brick wall with minimal dyskeratosis.1 Pemphigus vegetans is a localized variant of pemphigus vulgaris with a predilection for flexural surfaces. The lesions progress to vegetating erosive plaques.4 The Hallopeau type often is studded with pustules and typically remains more localized than the Neumann type. Direct immunofluorescence demonstrates intercellular deposition of IgG and C3, and routine sections characteristically show pseudoepitheliomatous hyperplasia with intraepidermal eosinophilic microabscesses.1,4 Tinea cruris is characterized by erythematous annular lesions with raised scaly borders spreading down the inner thighs.7 The epidermis is variably spongiotic with parakeratosis, and neutrophils often present in a layered stratum corneum with basketweave keratin above a layer of more compact and eosinophilic keratin. Fungal stains, such as periodic acid-Schiff, will highlight the fungal hyphae within the stratum corneum. The inguinal folds are a typical location for inverse psoriasis, which generally appears as thin, sharply demarcated, shiny red plaques with less scale than plaque psoriasis.1 Psoriasiform hyperplasia with a diminished granular layer and tortuous papillary dermal vessels would be expected histologically.1
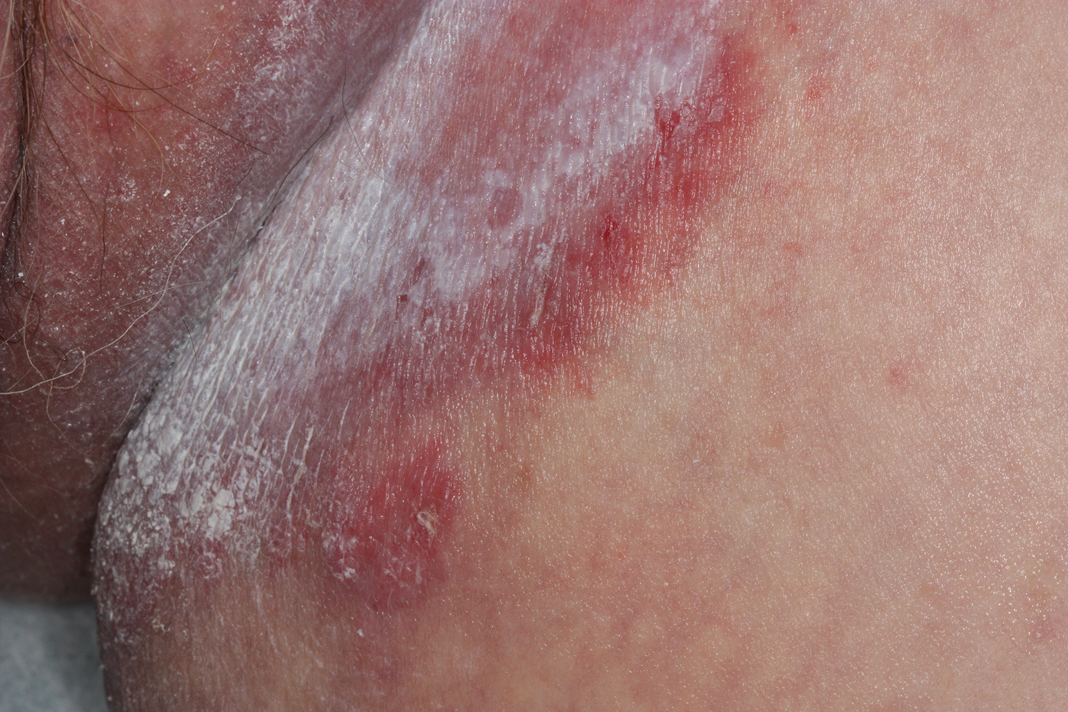
- James WD, Berger TG, Elston DM. Andrews' Diseases of the Skin. 12th ed. Philadelphia, PA: Elsevier; 2016.
- Schwartz RA, Tarlow MM. Dermatologic manifestations of Job syndrome. Medscape website. https://emedicine.medscape.com/article/1050852-overview. Updated April 22, 2019. Accessed March 28, 2020.
- Minegishi Y, Saito M. Cutaneous manifestations of hyper IgE syndrome. Allergol Int. 2012;61:191-196.
- Patterson JW. Weedon's Skin Pathology. 4th ed. China: Churchill Livingstone Elsevier; 2016.
- Pappas PG, Kauffman CA, Andes DR, et al. Executive summary: clinical practice guideline for the management of candidiasis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis. 2016;62:409-417.
- Center for Disease Control and Prevention. Antifungal resistance. https://www.cdc.gov/fungal/antifungal-resistance.html. Updated March 17, 2020. Accessed April 20, 2020.
- Tinea cruris. DermNet NZ website. https://www.dermnetnz.org/topics/tinea-cruris/. Published 2003. Accessed March 28, 2020.
The Diagnosis: Candidal Intertrigo
The biopsy confirmed a diagnosis of severe hyperkeratotic candidal intertrigo with no evidence of Hailey-Hailey disease. Hematoxylin and eosin- stained sections demonstrated irregular acanthosis and variable spongiosis. The stratum corneum was predominantly orthokeratotic with overlying psuedohyphae and yeast fungal elements (Figure 1).

Hyperimmunoglobulinemia E syndrome (HIES), also known as hyper-IgE syndrome or Job syndrome, is a rare immunodeficiency disorder characterized by an eczematous dermatitis-like rash, recurrent skin and lung abscesses, eosinophilia, and elevated serum IgE. Facial asymmetry, prominent forehead, deep-set eyes, broad nose, and roughened facial skin with large pores are characteristic of the sporadic and autosomal-recessive forms. Other common findings include retained primary teeth, hyperextensible joints, and recurrent mucocutaneous candidiasis.1
Although autosomal-dominant and autosomal-recessive inheritance patterns exist, sporadic mutations are the most common cause of HIES.2 Several genes have been implicated depending on the inheritance pattern. The majority of autosomal-dominant cases are associated with inactivating STAT3 (signal transducer and activator of transcription 3) mutations, whereas the majority of autosomal-recessive cases are associated with inactivating DOCK8 (dedicator of cytokinesis 8) mutations.1 Ultimately, all of these mutations lead to an impaired helper T cell (TH17) response, which is crucial for clearing fungal and extracellular bacterial infections.3
Skin eruptions typically are the first manifestation of HIES; they appear within the first week to month of life as papulopustular eruptions on the face and scalp and rapidly generalize to the rest of the body, favoring the shoulders, arms, chest, and buttocks. The pustules then coalesce into crusted plaques that resemble atopic dermatitis, frequently with superimposed Staphylococcus aureus infection. On microscopy, the pustules are folliculocentric and often contain eosinophils, whereas the plaques may contain intraepidermal collections of eosinophils.1
Mucocutaneous candidiasis is seen in approximately 60% of HIES cases and is closely linked to STAT3 inactivating mutations.3 Histologically, there is marked acanthosis with neutrophil exocytosis and abundant yeast and pseudohyphal forms within the stratum corneum (Figure 2).4 Cutaneous candidal infections typically require both oral and topical antifungal agents to clear the infection.3 Most cases of mucocutaneous candidiasis are caused by Candida albicans; however, other known culprits include Candida glabrata, Candida tropicalis, Candida parapsilosis, and Candida krusei.5,6 Of note, species identification and antifungal susceptibility studies may be useful in refractory cases, especially with C glabrata, which is known to acquire resistance to azoles, such as fluconazole, with emerging resistance to echinocandins.6
The differential diagnosis of this groin eruption included Hailey-Hailey disease; pemphigus vegetans, Hallopeau type; tinea cruris; and inverse psoriasis. Hailey-Hailey disease can be complicated by a superimposed candidal infection with similar clinical features, and biopsy may be required for definitive diagnosis. Hailey-Hailey disease typically presents with macerated fissured plaques that resemble macerated tissue paper with red fissures (Figure 3). Biopsy confirms full-thickness acantholysis resembling a dilapidated brick wall with minimal dyskeratosis.1 Pemphigus vegetans is a localized variant of pemphigus vulgaris with a predilection for flexural surfaces. The lesions progress to vegetating erosive plaques.4 The Hallopeau type often is studded with pustules and typically remains more localized than the Neumann type. Direct immunofluorescence demonstrates intercellular deposition of IgG and C3, and routine sections characteristically show pseudoepitheliomatous hyperplasia with intraepidermal eosinophilic microabscesses.1,4 Tinea cruris is characterized by erythematous annular lesions with raised scaly borders spreading down the inner thighs.7 The epidermis is variably spongiotic with parakeratosis, and neutrophils often present in a layered stratum corneum with basketweave keratin above a layer of more compact and eosinophilic keratin. Fungal stains, such as periodic acid-Schiff, will highlight the fungal hyphae within the stratum corneum. The inguinal folds are a typical location for inverse psoriasis, which generally appears as thin, sharply demarcated, shiny red plaques with less scale than plaque psoriasis.1 Psoriasiform hyperplasia with a diminished granular layer and tortuous papillary dermal vessels would be expected histologically.1

The Diagnosis: Candidal Intertrigo
The biopsy confirmed a diagnosis of severe hyperkeratotic candidal intertrigo with no evidence of Hailey-Hailey disease. Hematoxylin and eosin- stained sections demonstrated irregular acanthosis and variable spongiosis. The stratum corneum was predominantly orthokeratotic with overlying psuedohyphae and yeast fungal elements (Figure 1).

Hyperimmunoglobulinemia E syndrome (HIES), also known as hyper-IgE syndrome or Job syndrome, is a rare immunodeficiency disorder characterized by an eczematous dermatitis-like rash, recurrent skin and lung abscesses, eosinophilia, and elevated serum IgE. Facial asymmetry, prominent forehead, deep-set eyes, broad nose, and roughened facial skin with large pores are characteristic of the sporadic and autosomal-recessive forms. Other common findings include retained primary teeth, hyperextensible joints, and recurrent mucocutaneous candidiasis.1
Although autosomal-dominant and autosomal-recessive inheritance patterns exist, sporadic mutations are the most common cause of HIES.2 Several genes have been implicated depending on the inheritance pattern. The majority of autosomal-dominant cases are associated with inactivating STAT3 (signal transducer and activator of transcription 3) mutations, whereas the majority of autosomal-recessive cases are associated with inactivating DOCK8 (dedicator of cytokinesis 8) mutations.1 Ultimately, all of these mutations lead to an impaired helper T cell (TH17) response, which is crucial for clearing fungal and extracellular bacterial infections.3
Skin eruptions typically are the first manifestation of HIES; they appear within the first week to month of life as papulopustular eruptions on the face and scalp and rapidly generalize to the rest of the body, favoring the shoulders, arms, chest, and buttocks. The pustules then coalesce into crusted plaques that resemble atopic dermatitis, frequently with superimposed Staphylococcus aureus infection. On microscopy, the pustules are folliculocentric and often contain eosinophils, whereas the plaques may contain intraepidermal collections of eosinophils.1
Mucocutaneous candidiasis is seen in approximately 60% of HIES cases and is closely linked to STAT3 inactivating mutations.3 Histologically, there is marked acanthosis with neutrophil exocytosis and abundant yeast and pseudohyphal forms within the stratum corneum (Figure 2).4 Cutaneous candidal infections typically require both oral and topical antifungal agents to clear the infection.3 Most cases of mucocutaneous candidiasis are caused by Candida albicans; however, other known culprits include Candida glabrata, Candida tropicalis, Candida parapsilosis, and Candida krusei.5,6 Of note, species identification and antifungal susceptibility studies may be useful in refractory cases, especially with C glabrata, which is known to acquire resistance to azoles, such as fluconazole, with emerging resistance to echinocandins.6
The differential diagnosis of this groin eruption included Hailey-Hailey disease; pemphigus vegetans, Hallopeau type; tinea cruris; and inverse psoriasis. Hailey-Hailey disease can be complicated by a superimposed candidal infection with similar clinical features, and biopsy may be required for definitive diagnosis. Hailey-Hailey disease typically presents with macerated fissured plaques that resemble macerated tissue paper with red fissures (Figure 3). Biopsy confirms full-thickness acantholysis resembling a dilapidated brick wall with minimal dyskeratosis.1 Pemphigus vegetans is a localized variant of pemphigus vulgaris with a predilection for flexural surfaces. The lesions progress to vegetating erosive plaques.4 The Hallopeau type often is studded with pustules and typically remains more localized than the Neumann type. Direct immunofluorescence demonstrates intercellular deposition of IgG and C3, and routine sections characteristically show pseudoepitheliomatous hyperplasia with intraepidermal eosinophilic microabscesses.1,4 Tinea cruris is characterized by erythematous annular lesions with raised scaly borders spreading down the inner thighs.7 The epidermis is variably spongiotic with parakeratosis, and neutrophils often present in a layered stratum corneum with basketweave keratin above a layer of more compact and eosinophilic keratin. Fungal stains, such as periodic acid-Schiff, will highlight the fungal hyphae within the stratum corneum. The inguinal folds are a typical location for inverse psoriasis, which generally appears as thin, sharply demarcated, shiny red plaques with less scale than plaque psoriasis.1 Psoriasiform hyperplasia with a diminished granular layer and tortuous papillary dermal vessels would be expected histologically.1

- James WD, Berger TG, Elston DM. Andrews' Diseases of the Skin. 12th ed. Philadelphia, PA: Elsevier; 2016.
- Schwartz RA, Tarlow MM. Dermatologic manifestations of Job syndrome. Medscape website. https://emedicine.medscape.com/article/1050852-overview. Updated April 22, 2019. Accessed March 28, 2020.
- Minegishi Y, Saito M. Cutaneous manifestations of hyper IgE syndrome. Allergol Int. 2012;61:191-196.
- Patterson JW. Weedon's Skin Pathology. 4th ed. China: Churchill Livingstone Elsevier; 2016.
- Pappas PG, Kauffman CA, Andes DR, et al. Executive summary: clinical practice guideline for the management of candidiasis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis. 2016;62:409-417.
- Center for Disease Control and Prevention. Antifungal resistance. https://www.cdc.gov/fungal/antifungal-resistance.html. Updated March 17, 2020. Accessed April 20, 2020.
- Tinea cruris. DermNet NZ website. https://www.dermnetnz.org/topics/tinea-cruris/. Published 2003. Accessed March 28, 2020.
- James WD, Berger TG, Elston DM. Andrews' Diseases of the Skin. 12th ed. Philadelphia, PA: Elsevier; 2016.
- Schwartz RA, Tarlow MM. Dermatologic manifestations of Job syndrome. Medscape website. https://emedicine.medscape.com/article/1050852-overview. Updated April 22, 2019. Accessed March 28, 2020.
- Minegishi Y, Saito M. Cutaneous manifestations of hyper IgE syndrome. Allergol Int. 2012;61:191-196.
- Patterson JW. Weedon's Skin Pathology. 4th ed. China: Churchill Livingstone Elsevier; 2016.
- Pappas PG, Kauffman CA, Andes DR, et al. Executive summary: clinical practice guideline for the management of candidiasis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis. 2016;62:409-417.
- Center for Disease Control and Prevention. Antifungal resistance. https://www.cdc.gov/fungal/antifungal-resistance.html. Updated March 17, 2020. Accessed April 20, 2020.
- Tinea cruris. DermNet NZ website. https://www.dermnetnz.org/topics/tinea-cruris/. Published 2003. Accessed March 28, 2020.
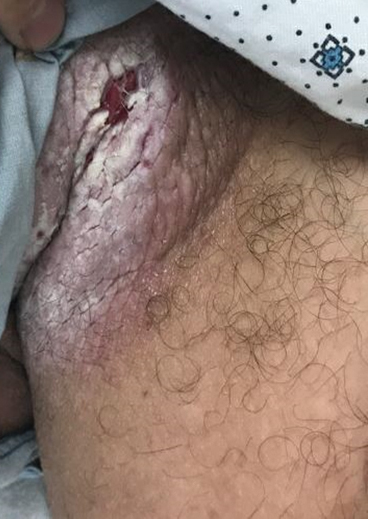
A 28-year-old man with a history of hyperimmunoglobulinemia E syndrome (previously known as Job syndrome), coarse facial features, and multiple skin and soft tissue infections presented to the university dermatology clinic with persistent white, macerated, fissured groin plaques that were present for months. The lesions were tender and pruritic with a burning sensation. Treatment with topical terbinafine and oral fluconazole was attempted without resolution of the eruption. A biopsy of the groin lesion was performed.