User login
Adult separation anxiety raises suicidality risk
Separation anxiety plays a substantial role in suicidality in patients with mood and anxiety disorders, new research suggests.
Results of a study that included 500 outpatients with mood or anxiety disorders showed adult separation anxiety disorder (ASAD) was more frequent in patients with suicidal thoughts versus those who did not have the disorder. In addition, depression and separation anxiety also significantly predicted lifetime suicide risk.
“This study indicates a substantial role of separation anxiety in predicting suicidal thoughts, both as state-related symptoms ... and as longitudinal dimension symptoms,” say the investigators, led by Stefano Pini, MD, of the department of clinical and experimental medicine, section of psychiatry, University of Pisa (Italy).
“ for reducing suicide risk,” they add.
The study was published in the March/April issue of the Journal of Clinical Psychiatry.
Frequently underdiagnosed
The authors describe a “close link between suicidal behaviors and interpersonal difficulties extending beyond the traditional approach of comprehending suicide as a phenomenon mainly related to depression.”
Previous research indicates that insecure adult attachment style might be associated with a greater likelihood of suicidal thoughts and attempts, and there might be an association between individual abnormal attachment sensitivity and suicide.
“Suicidal ideation or suicide attempts may be associated with disturbances in attachment, which may lead not only to a devastating experience of losing the feeling of interdependence and closeness but also to a rejection of life itself,” the authors suggest.
ASAD may be a “key factor” in understanding the relationship between individual attachment sensitivity to separation and suicidality.
An ASAD diagnosis was traditionally reserved for children and adolescents, but DSM-5 expanded the diagnosis to include adults over 18 years of age because research had “found a later onset to be common,” spanning the life course, even in the absence of a history of separation anxiety in childhood.
“Separation anxiety is an important clinical dimension, often with roots in childhood, but likely to manifest across the lifespan,” the authors note, adding that it is “frequently underdiagnosed.”
The relationship between ASAD and suicidality has not been explored extensively, so the researchers set out to examine the association.
The study included 509 consecutively recruited adult psychiatric outpatients with mood or anxiety disorders as a principle diagnosis.
Participants completed an array of scales, including item 3 on the Hamilton Depression Rating Scale (HDRS), which measures suicidality, as well as the Mood Spectrum Self-Report (MOODS-SR), a questionnaire evaluating lifetime suicidal symptoms.
Three scales were used to measure separation anxiety disorder: The Structured Interview for Separation Anxiety Symptoms in Adulthood/Childhood (SCI-SAS-A/C); the Separation Anxiety Symptom Inventory (SASI); and the Adult Separation Anxiety Scale (ASA-27).
Waxing and waning
Of the total sample, 215 patients were diagnosed with separation anxiety disorder (mean age at onset 15 years). Of the total sample, 19.9% scored ≥ 1 on the HDRS item 3, indicating the presence of suicidality.
Patients with suicidal thoughts more frequently experienced ASAD, compared with those without suicidal thoughts (53.6% vs. 39.6%, respectively, P = .01).
“All measures of adult as well as childhood separation anxiety were significantly elevated in the group of patients with current suicidality, based on HDRS item 3,” the authors report.
Logistic regression found that ASAD, major depression, bipolar I, and bipolar II disorders all predicted suicidal thoughts.
A linear regression model found that depression (P = .001) and ASA-27 separation anxiety (P = .001) significantly predicted lifetime suicide risk, based on the MOODS-SR scale.
In addition, “mediation analysis showed that, besides a direct effect, there is also an indirect effect of depression severity on the MOODS-SR suicidality score through the ASA-27 score, indicating that separation anxiety may act as an important mediating factor in the relationship between depression and suicidality,” the authors state.
The authors observe that separation anxiety “is an important clinical dimension, often with roots in childhood, but likely to wax and wane across the lifespan and even to manifest for the first time during adulthood.”
Treatment target?
Commenting on the study for this news organization, Megan Rogers, PhD, postdoctoral research fellow, Mount Sinai Beth Israel, New York, said the findings “point to symptoms of separation anxiety as a potential indicator of suicidal ideation, and should these findings be replicated and extended through longitudinal research, it suggests that symptoms of separation anxiety may be a relevant treatment target in certain populations to mitigate suicide risk.”
Dr. Rogers, who is the student division director at the American Association of Suicidology and was not involved with the study, said she thinks that studies of suicide have focused more on “individual symptoms of separation anxiety, such as excessive worry about loved ones or distress when anticipating separation from loved ones, rather than on separation anxiety as a categorical diagnosis.”
However, the study has an important take-home message for practicing clinicians, Dr. Rogers said. “In individuals with separation anxiety disorders, particularly those with comorbid mood conditions, it may be worth conducting a more thorough assessment of suicide risk, given the possibility of elevated suicidality in these patients.”
The study was supported in part by the German Research Foundation and the Fondazione Cassa di Risparmio di la Spezia. The authors and Dr. Rogers have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Separation anxiety plays a substantial role in suicidality in patients with mood and anxiety disorders, new research suggests.
Results of a study that included 500 outpatients with mood or anxiety disorders showed adult separation anxiety disorder (ASAD) was more frequent in patients with suicidal thoughts versus those who did not have the disorder. In addition, depression and separation anxiety also significantly predicted lifetime suicide risk.
“This study indicates a substantial role of separation anxiety in predicting suicidal thoughts, both as state-related symptoms ... and as longitudinal dimension symptoms,” say the investigators, led by Stefano Pini, MD, of the department of clinical and experimental medicine, section of psychiatry, University of Pisa (Italy).
“ for reducing suicide risk,” they add.
The study was published in the March/April issue of the Journal of Clinical Psychiatry.
Frequently underdiagnosed
The authors describe a “close link between suicidal behaviors and interpersonal difficulties extending beyond the traditional approach of comprehending suicide as a phenomenon mainly related to depression.”
Previous research indicates that insecure adult attachment style might be associated with a greater likelihood of suicidal thoughts and attempts, and there might be an association between individual abnormal attachment sensitivity and suicide.
“Suicidal ideation or suicide attempts may be associated with disturbances in attachment, which may lead not only to a devastating experience of losing the feeling of interdependence and closeness but also to a rejection of life itself,” the authors suggest.
ASAD may be a “key factor” in understanding the relationship between individual attachment sensitivity to separation and suicidality.
An ASAD diagnosis was traditionally reserved for children and adolescents, but DSM-5 expanded the diagnosis to include adults over 18 years of age because research had “found a later onset to be common,” spanning the life course, even in the absence of a history of separation anxiety in childhood.
“Separation anxiety is an important clinical dimension, often with roots in childhood, but likely to manifest across the lifespan,” the authors note, adding that it is “frequently underdiagnosed.”
The relationship between ASAD and suicidality has not been explored extensively, so the researchers set out to examine the association.
The study included 509 consecutively recruited adult psychiatric outpatients with mood or anxiety disorders as a principle diagnosis.
Participants completed an array of scales, including item 3 on the Hamilton Depression Rating Scale (HDRS), which measures suicidality, as well as the Mood Spectrum Self-Report (MOODS-SR), a questionnaire evaluating lifetime suicidal symptoms.
Three scales were used to measure separation anxiety disorder: The Structured Interview for Separation Anxiety Symptoms in Adulthood/Childhood (SCI-SAS-A/C); the Separation Anxiety Symptom Inventory (SASI); and the Adult Separation Anxiety Scale (ASA-27).
Waxing and waning
Of the total sample, 215 patients were diagnosed with separation anxiety disorder (mean age at onset 15 years). Of the total sample, 19.9% scored ≥ 1 on the HDRS item 3, indicating the presence of suicidality.
Patients with suicidal thoughts more frequently experienced ASAD, compared with those without suicidal thoughts (53.6% vs. 39.6%, respectively, P = .01).
“All measures of adult as well as childhood separation anxiety were significantly elevated in the group of patients with current suicidality, based on HDRS item 3,” the authors report.
Logistic regression found that ASAD, major depression, bipolar I, and bipolar II disorders all predicted suicidal thoughts.
A linear regression model found that depression (P = .001) and ASA-27 separation anxiety (P = .001) significantly predicted lifetime suicide risk, based on the MOODS-SR scale.
In addition, “mediation analysis showed that, besides a direct effect, there is also an indirect effect of depression severity on the MOODS-SR suicidality score through the ASA-27 score, indicating that separation anxiety may act as an important mediating factor in the relationship between depression and suicidality,” the authors state.
The authors observe that separation anxiety “is an important clinical dimension, often with roots in childhood, but likely to wax and wane across the lifespan and even to manifest for the first time during adulthood.”
Treatment target?
Commenting on the study for this news organization, Megan Rogers, PhD, postdoctoral research fellow, Mount Sinai Beth Israel, New York, said the findings “point to symptoms of separation anxiety as a potential indicator of suicidal ideation, and should these findings be replicated and extended through longitudinal research, it suggests that symptoms of separation anxiety may be a relevant treatment target in certain populations to mitigate suicide risk.”
Dr. Rogers, who is the student division director at the American Association of Suicidology and was not involved with the study, said she thinks that studies of suicide have focused more on “individual symptoms of separation anxiety, such as excessive worry about loved ones or distress when anticipating separation from loved ones, rather than on separation anxiety as a categorical diagnosis.”
However, the study has an important take-home message for practicing clinicians, Dr. Rogers said. “In individuals with separation anxiety disorders, particularly those with comorbid mood conditions, it may be worth conducting a more thorough assessment of suicide risk, given the possibility of elevated suicidality in these patients.”
The study was supported in part by the German Research Foundation and the Fondazione Cassa di Risparmio di la Spezia. The authors and Dr. Rogers have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Separation anxiety plays a substantial role in suicidality in patients with mood and anxiety disorders, new research suggests.
Results of a study that included 500 outpatients with mood or anxiety disorders showed adult separation anxiety disorder (ASAD) was more frequent in patients with suicidal thoughts versus those who did not have the disorder. In addition, depression and separation anxiety also significantly predicted lifetime suicide risk.
“This study indicates a substantial role of separation anxiety in predicting suicidal thoughts, both as state-related symptoms ... and as longitudinal dimension symptoms,” say the investigators, led by Stefano Pini, MD, of the department of clinical and experimental medicine, section of psychiatry, University of Pisa (Italy).
“ for reducing suicide risk,” they add.
The study was published in the March/April issue of the Journal of Clinical Psychiatry.
Frequently underdiagnosed
The authors describe a “close link between suicidal behaviors and interpersonal difficulties extending beyond the traditional approach of comprehending suicide as a phenomenon mainly related to depression.”
Previous research indicates that insecure adult attachment style might be associated with a greater likelihood of suicidal thoughts and attempts, and there might be an association between individual abnormal attachment sensitivity and suicide.
“Suicidal ideation or suicide attempts may be associated with disturbances in attachment, which may lead not only to a devastating experience of losing the feeling of interdependence and closeness but also to a rejection of life itself,” the authors suggest.
ASAD may be a “key factor” in understanding the relationship between individual attachment sensitivity to separation and suicidality.
An ASAD diagnosis was traditionally reserved for children and adolescents, but DSM-5 expanded the diagnosis to include adults over 18 years of age because research had “found a later onset to be common,” spanning the life course, even in the absence of a history of separation anxiety in childhood.
“Separation anxiety is an important clinical dimension, often with roots in childhood, but likely to manifest across the lifespan,” the authors note, adding that it is “frequently underdiagnosed.”
The relationship between ASAD and suicidality has not been explored extensively, so the researchers set out to examine the association.
The study included 509 consecutively recruited adult psychiatric outpatients with mood or anxiety disorders as a principle diagnosis.
Participants completed an array of scales, including item 3 on the Hamilton Depression Rating Scale (HDRS), which measures suicidality, as well as the Mood Spectrum Self-Report (MOODS-SR), a questionnaire evaluating lifetime suicidal symptoms.
Three scales were used to measure separation anxiety disorder: The Structured Interview for Separation Anxiety Symptoms in Adulthood/Childhood (SCI-SAS-A/C); the Separation Anxiety Symptom Inventory (SASI); and the Adult Separation Anxiety Scale (ASA-27).
Waxing and waning
Of the total sample, 215 patients were diagnosed with separation anxiety disorder (mean age at onset 15 years). Of the total sample, 19.9% scored ≥ 1 on the HDRS item 3, indicating the presence of suicidality.
Patients with suicidal thoughts more frequently experienced ASAD, compared with those without suicidal thoughts (53.6% vs. 39.6%, respectively, P = .01).
“All measures of adult as well as childhood separation anxiety were significantly elevated in the group of patients with current suicidality, based on HDRS item 3,” the authors report.
Logistic regression found that ASAD, major depression, bipolar I, and bipolar II disorders all predicted suicidal thoughts.
A linear regression model found that depression (P = .001) and ASA-27 separation anxiety (P = .001) significantly predicted lifetime suicide risk, based on the MOODS-SR scale.
In addition, “mediation analysis showed that, besides a direct effect, there is also an indirect effect of depression severity on the MOODS-SR suicidality score through the ASA-27 score, indicating that separation anxiety may act as an important mediating factor in the relationship between depression and suicidality,” the authors state.
The authors observe that separation anxiety “is an important clinical dimension, often with roots in childhood, but likely to wax and wane across the lifespan and even to manifest for the first time during adulthood.”
Treatment target?
Commenting on the study for this news organization, Megan Rogers, PhD, postdoctoral research fellow, Mount Sinai Beth Israel, New York, said the findings “point to symptoms of separation anxiety as a potential indicator of suicidal ideation, and should these findings be replicated and extended through longitudinal research, it suggests that symptoms of separation anxiety may be a relevant treatment target in certain populations to mitigate suicide risk.”
Dr. Rogers, who is the student division director at the American Association of Suicidology and was not involved with the study, said she thinks that studies of suicide have focused more on “individual symptoms of separation anxiety, such as excessive worry about loved ones or distress when anticipating separation from loved ones, rather than on separation anxiety as a categorical diagnosis.”
However, the study has an important take-home message for practicing clinicians, Dr. Rogers said. “In individuals with separation anxiety disorders, particularly those with comorbid mood conditions, it may be worth conducting a more thorough assessment of suicide risk, given the possibility of elevated suicidality in these patients.”
The study was supported in part by the German Research Foundation and the Fondazione Cassa di Risparmio di la Spezia. The authors and Dr. Rogers have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Can exercise prevent cognitive decline in patients with early Parkinson’s disease?
, new research suggests. Investigators found that patients with Parkinson’s disease who were APOE epsilon4 carriers had greater cognitive decline compared with non-APOE epsilon4 carriers, but the findings also revealed higher physical activity appeared to slow cognitive decline in this higher risk group.
“The main finding of the current study is that higher physical activity was related to slower APOE epsilon4-associated cognitive decline in patients with early Parkinson’s disease, which was shown to be robust in sensitivity analyses,” wrote the researchers, led by Ryul Kim, MD, Inha University Hospital, Incheon, Korea.
The study was published online March 31 in Neurology.
Unclear mechanism
The APOE epsilon4 allele is known to be a “major risk factor” for Alzheimer’s disease, but “accumulating evidence shows that this allele also has a potential role in cognitive impairment in Parkinson’s disease,” the authors noted.
Previous research shows physical activity has beneficial effects in patients with Parkinson’s disease, but the mechanisms underlying these effects are “not well understood.” Additional data suggest physical activity modifies the APOE epsilon4 effect on the development and progression of Alzheimer’s disease.
“These observations led us to hypothesize that physical activity also plays a role in modulating the association between APOE [epsilon4] and cognition in Parkinson’s disease,” but no studies have yet reported on this interaction in patients with Parkinson’s disease, the authors noted.
To investigate, they drew on data from the Parkinson’s Progression Markers Initiative (PPMI) – a cohort study conducted to identify Parkinson’s disease progression markers.
The current analysis included 173 patients recently diagnosed with Parkinson’s disease but not yet treated for the condition. The cohort’s mean age was 63.3 ± 10.0 years, age of Parkinson’s disease onset was 59.4 ± 10.0 years, and 68% were male. Of these participants, 46 were APOE epsilon4 carriers.
Dopamine transporter (DAT) activity was assessed using imaging at enrollment and again at years 2 and 4. Cognitive function was assessed at years 2, 3, and 4 using the Montreal Cognitive Assessment (MoCA) test.
Protective effect
Although APOE epsilon4 carriers tended to be younger than noncarriers, the age of Parkinson’s disease onset did not differ between the 2 groups, and there were also no significant differences between the groups in demographic and clinical variables.
There were larger declines in MoCA scores in the APOE epsilon4 carriers versus the noncarriers (0.21 ± 1.40 and 0.08 ± 1.15 respectively).
The APOE epsilon4 allele was associated with a “steeper” rate of cognitive decline, compared with the non-APOE epsilon4 allele (estimate −1.33 [95% confidence interval, −2.12 to −0.47, P = .002).
There was a significant interaction of physical activity, APOE epsilon4, and time: Higher physical activity was associated with slower APOE epsilon4-related cognitive decline (estimate 0.007 [0.003 to 0.011, P = .001).
However, the researchers found no significant main effects of the APOE epsilon4 allele or physical activity on the change in the MoCA score.
“Considering that dopaminergic treatment may affect cognitive function, particularly in the early stage of Parkinson’s disease, we additionally included the levodopa daily equivalent dose (LEDD) and its interaction with time as covariates in the model,” the investigators noted.
They found that the interactive association between physical activity and the APOE epsilon4 allele on cognitive decline remained significant, even when participants who had normal cognitive performance at year 2 were included in the study population or when LEDD variables were included as covariates in the model.
Both high- and low-intensity exercise were significantly associated with slower APOE epsilon4-related cognitive decline.
There was no significant interaction between physical activity and APOE epsilon4 with changes in striatal DAT activities.
“Increased physical activity attenuated APOE epsilon4-related vulnerability to early cognitive decline in patients with Parkinson’s disease,” the authors noted, adding that the effect “did not appear to be mediated by striatal dopamine activity.”
They hypothesized that physical activity may “offer a greater protective effect” on cerebral amyloid accumulation in APOE epsilon4 carriers. It is also possible that physical activity will counteract the negative impact of the APOE epsilon4 allele through improved brain mechanism and decreased neuroinflammation.
‘The next blockbuster drug’
Commenting on the study in an interview, Bastiaan R. Bloem, MD, PhD, director of the center of expertise for Parkinson & movement disorders, Radboud University Medical Center, Nijmegen, Netherlands, said exercise might be seen as “the next blockbuster drug.”
Dr. Bloem, who was not involved in the study, noted there is “quite robust evidence now that exercise acts as symptomatic therapy, like a drug, alleviating sleep [disturbances], depression, constipation, and motor symptoms.”
The study “sheds new light on the idea of exercise as not only alleviating symptoms but actually as a potential disease modifier,” said Dr. Bloem, whose research has focused on the beneficial effects of a rigorous exercise program, combined with tablet-based gamificaton and a reward system in stabilizing motor symptoms in patients with Parkinson’s disease over time.
“The reward system created additional motivation for the patients with Parkinson’s disease who often experience depression and apathy that interfere with motivation,” he said.
The current study has important take-home messages for practicing clinicians. “Physicians should encourage exercise in patients, and patients should also take the lead themselves,” Dr. Bloem said. “It doesn’t matter what type of exercise you do, but it should have an aerobic component, should be safe so the patient doesn’t fall down, should have enough intensity to cause the patient to pant, and should be individualized and enjoyable so the patients stick to it,” he emphasized.
Dr. Bloem noted that yoga and mindfulness are also helpful. “If we’ve learned anything from the COVID-19 crisis, it’s that chronic stress is deleterious to all of us and particularly bad for people with PD, because you need dopamine to be able to handle stress, and the lack of dopamine in people with PD makes them deteriorate faster.”
The study was supported by a research grant of National Research Foundation by the Ministry of Science and ICT (MSIT) in Korea. The authors and Dr. Bloem have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new research suggests. Investigators found that patients with Parkinson’s disease who were APOE epsilon4 carriers had greater cognitive decline compared with non-APOE epsilon4 carriers, but the findings also revealed higher physical activity appeared to slow cognitive decline in this higher risk group.
“The main finding of the current study is that higher physical activity was related to slower APOE epsilon4-associated cognitive decline in patients with early Parkinson’s disease, which was shown to be robust in sensitivity analyses,” wrote the researchers, led by Ryul Kim, MD, Inha University Hospital, Incheon, Korea.
The study was published online March 31 in Neurology.
Unclear mechanism
The APOE epsilon4 allele is known to be a “major risk factor” for Alzheimer’s disease, but “accumulating evidence shows that this allele also has a potential role in cognitive impairment in Parkinson’s disease,” the authors noted.
Previous research shows physical activity has beneficial effects in patients with Parkinson’s disease, but the mechanisms underlying these effects are “not well understood.” Additional data suggest physical activity modifies the APOE epsilon4 effect on the development and progression of Alzheimer’s disease.
“These observations led us to hypothesize that physical activity also plays a role in modulating the association between APOE [epsilon4] and cognition in Parkinson’s disease,” but no studies have yet reported on this interaction in patients with Parkinson’s disease, the authors noted.
To investigate, they drew on data from the Parkinson’s Progression Markers Initiative (PPMI) – a cohort study conducted to identify Parkinson’s disease progression markers.
The current analysis included 173 patients recently diagnosed with Parkinson’s disease but not yet treated for the condition. The cohort’s mean age was 63.3 ± 10.0 years, age of Parkinson’s disease onset was 59.4 ± 10.0 years, and 68% were male. Of these participants, 46 were APOE epsilon4 carriers.
Dopamine transporter (DAT) activity was assessed using imaging at enrollment and again at years 2 and 4. Cognitive function was assessed at years 2, 3, and 4 using the Montreal Cognitive Assessment (MoCA) test.
Protective effect
Although APOE epsilon4 carriers tended to be younger than noncarriers, the age of Parkinson’s disease onset did not differ between the 2 groups, and there were also no significant differences between the groups in demographic and clinical variables.
There were larger declines in MoCA scores in the APOE epsilon4 carriers versus the noncarriers (0.21 ± 1.40 and 0.08 ± 1.15 respectively).
The APOE epsilon4 allele was associated with a “steeper” rate of cognitive decline, compared with the non-APOE epsilon4 allele (estimate −1.33 [95% confidence interval, −2.12 to −0.47, P = .002).
There was a significant interaction of physical activity, APOE epsilon4, and time: Higher physical activity was associated with slower APOE epsilon4-related cognitive decline (estimate 0.007 [0.003 to 0.011, P = .001).
However, the researchers found no significant main effects of the APOE epsilon4 allele or physical activity on the change in the MoCA score.
“Considering that dopaminergic treatment may affect cognitive function, particularly in the early stage of Parkinson’s disease, we additionally included the levodopa daily equivalent dose (LEDD) and its interaction with time as covariates in the model,” the investigators noted.
They found that the interactive association between physical activity and the APOE epsilon4 allele on cognitive decline remained significant, even when participants who had normal cognitive performance at year 2 were included in the study population or when LEDD variables were included as covariates in the model.
Both high- and low-intensity exercise were significantly associated with slower APOE epsilon4-related cognitive decline.
There was no significant interaction between physical activity and APOE epsilon4 with changes in striatal DAT activities.
“Increased physical activity attenuated APOE epsilon4-related vulnerability to early cognitive decline in patients with Parkinson’s disease,” the authors noted, adding that the effect “did not appear to be mediated by striatal dopamine activity.”
They hypothesized that physical activity may “offer a greater protective effect” on cerebral amyloid accumulation in APOE epsilon4 carriers. It is also possible that physical activity will counteract the negative impact of the APOE epsilon4 allele through improved brain mechanism and decreased neuroinflammation.
‘The next blockbuster drug’
Commenting on the study in an interview, Bastiaan R. Bloem, MD, PhD, director of the center of expertise for Parkinson & movement disorders, Radboud University Medical Center, Nijmegen, Netherlands, said exercise might be seen as “the next blockbuster drug.”
Dr. Bloem, who was not involved in the study, noted there is “quite robust evidence now that exercise acts as symptomatic therapy, like a drug, alleviating sleep [disturbances], depression, constipation, and motor symptoms.”
The study “sheds new light on the idea of exercise as not only alleviating symptoms but actually as a potential disease modifier,” said Dr. Bloem, whose research has focused on the beneficial effects of a rigorous exercise program, combined with tablet-based gamificaton and a reward system in stabilizing motor symptoms in patients with Parkinson’s disease over time.
“The reward system created additional motivation for the patients with Parkinson’s disease who often experience depression and apathy that interfere with motivation,” he said.
The current study has important take-home messages for practicing clinicians. “Physicians should encourage exercise in patients, and patients should also take the lead themselves,” Dr. Bloem said. “It doesn’t matter what type of exercise you do, but it should have an aerobic component, should be safe so the patient doesn’t fall down, should have enough intensity to cause the patient to pant, and should be individualized and enjoyable so the patients stick to it,” he emphasized.
Dr. Bloem noted that yoga and mindfulness are also helpful. “If we’ve learned anything from the COVID-19 crisis, it’s that chronic stress is deleterious to all of us and particularly bad for people with PD, because you need dopamine to be able to handle stress, and the lack of dopamine in people with PD makes them deteriorate faster.”
The study was supported by a research grant of National Research Foundation by the Ministry of Science and ICT (MSIT) in Korea. The authors and Dr. Bloem have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new research suggests. Investigators found that patients with Parkinson’s disease who were APOE epsilon4 carriers had greater cognitive decline compared with non-APOE epsilon4 carriers, but the findings also revealed higher physical activity appeared to slow cognitive decline in this higher risk group.
“The main finding of the current study is that higher physical activity was related to slower APOE epsilon4-associated cognitive decline in patients with early Parkinson’s disease, which was shown to be robust in sensitivity analyses,” wrote the researchers, led by Ryul Kim, MD, Inha University Hospital, Incheon, Korea.
The study was published online March 31 in Neurology.
Unclear mechanism
The APOE epsilon4 allele is known to be a “major risk factor” for Alzheimer’s disease, but “accumulating evidence shows that this allele also has a potential role in cognitive impairment in Parkinson’s disease,” the authors noted.
Previous research shows physical activity has beneficial effects in patients with Parkinson’s disease, but the mechanisms underlying these effects are “not well understood.” Additional data suggest physical activity modifies the APOE epsilon4 effect on the development and progression of Alzheimer’s disease.
“These observations led us to hypothesize that physical activity also plays a role in modulating the association between APOE [epsilon4] and cognition in Parkinson’s disease,” but no studies have yet reported on this interaction in patients with Parkinson’s disease, the authors noted.
To investigate, they drew on data from the Parkinson’s Progression Markers Initiative (PPMI) – a cohort study conducted to identify Parkinson’s disease progression markers.
The current analysis included 173 patients recently diagnosed with Parkinson’s disease but not yet treated for the condition. The cohort’s mean age was 63.3 ± 10.0 years, age of Parkinson’s disease onset was 59.4 ± 10.0 years, and 68% were male. Of these participants, 46 were APOE epsilon4 carriers.
Dopamine transporter (DAT) activity was assessed using imaging at enrollment and again at years 2 and 4. Cognitive function was assessed at years 2, 3, and 4 using the Montreal Cognitive Assessment (MoCA) test.
Protective effect
Although APOE epsilon4 carriers tended to be younger than noncarriers, the age of Parkinson’s disease onset did not differ between the 2 groups, and there were also no significant differences between the groups in demographic and clinical variables.
There were larger declines in MoCA scores in the APOE epsilon4 carriers versus the noncarriers (0.21 ± 1.40 and 0.08 ± 1.15 respectively).
The APOE epsilon4 allele was associated with a “steeper” rate of cognitive decline, compared with the non-APOE epsilon4 allele (estimate −1.33 [95% confidence interval, −2.12 to −0.47, P = .002).
There was a significant interaction of physical activity, APOE epsilon4, and time: Higher physical activity was associated with slower APOE epsilon4-related cognitive decline (estimate 0.007 [0.003 to 0.011, P = .001).
However, the researchers found no significant main effects of the APOE epsilon4 allele or physical activity on the change in the MoCA score.
“Considering that dopaminergic treatment may affect cognitive function, particularly in the early stage of Parkinson’s disease, we additionally included the levodopa daily equivalent dose (LEDD) and its interaction with time as covariates in the model,” the investigators noted.
They found that the interactive association between physical activity and the APOE epsilon4 allele on cognitive decline remained significant, even when participants who had normal cognitive performance at year 2 were included in the study population or when LEDD variables were included as covariates in the model.
Both high- and low-intensity exercise were significantly associated with slower APOE epsilon4-related cognitive decline.
There was no significant interaction between physical activity and APOE epsilon4 with changes in striatal DAT activities.
“Increased physical activity attenuated APOE epsilon4-related vulnerability to early cognitive decline in patients with Parkinson’s disease,” the authors noted, adding that the effect “did not appear to be mediated by striatal dopamine activity.”
They hypothesized that physical activity may “offer a greater protective effect” on cerebral amyloid accumulation in APOE epsilon4 carriers. It is also possible that physical activity will counteract the negative impact of the APOE epsilon4 allele through improved brain mechanism and decreased neuroinflammation.
‘The next blockbuster drug’
Commenting on the study in an interview, Bastiaan R. Bloem, MD, PhD, director of the center of expertise for Parkinson & movement disorders, Radboud University Medical Center, Nijmegen, Netherlands, said exercise might be seen as “the next blockbuster drug.”
Dr. Bloem, who was not involved in the study, noted there is “quite robust evidence now that exercise acts as symptomatic therapy, like a drug, alleviating sleep [disturbances], depression, constipation, and motor symptoms.”
The study “sheds new light on the idea of exercise as not only alleviating symptoms but actually as a potential disease modifier,” said Dr. Bloem, whose research has focused on the beneficial effects of a rigorous exercise program, combined with tablet-based gamificaton and a reward system in stabilizing motor symptoms in patients with Parkinson’s disease over time.
“The reward system created additional motivation for the patients with Parkinson’s disease who often experience depression and apathy that interfere with motivation,” he said.
The current study has important take-home messages for practicing clinicians. “Physicians should encourage exercise in patients, and patients should also take the lead themselves,” Dr. Bloem said. “It doesn’t matter what type of exercise you do, but it should have an aerobic component, should be safe so the patient doesn’t fall down, should have enough intensity to cause the patient to pant, and should be individualized and enjoyable so the patients stick to it,” he emphasized.
Dr. Bloem noted that yoga and mindfulness are also helpful. “If we’ve learned anything from the COVID-19 crisis, it’s that chronic stress is deleterious to all of us and particularly bad for people with PD, because you need dopamine to be able to handle stress, and the lack of dopamine in people with PD makes them deteriorate faster.”
The study was supported by a research grant of National Research Foundation by the Ministry of Science and ICT (MSIT) in Korea. The authors and Dr. Bloem have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM NEUROLOGY
Age-related cognitive decline not inevitable?
Investigators found that despite the presence of neuropathologies associated with Alzheimer’s disease (AD), many centenarians maintained high levels of cognitive performance.
“Cognitive decline is not inevitable,” senior author Henne Holstege, PhD, assistant professor, Amsterdam Alzheimer Center and Clinical Genetics, Amsterdam University Medical Center, said in an interview.
“At 100 years or older, high levels of cognitive performance can be maintained for several years, even when individuals are exposed to risk factors associated with cognitive decline,” she said.
The study was published online Jan. 15 in JAMA Network Open.
Escaping cognitive decline
Dr. Holstege said her interest in researching aging and cognitive health was inspired by the “fascinating” story of Hendrikje van Andel-Schipper, who died at age 115 in 2015 “completely cognitively healthy.” Her mother, who died at age 100, also was cognitively intact at the end of her life.
“I wanted to know how it is possible that some people can completely escape all aspects of cognitive decline while reaching extreme ages,” Dr. Holstege said.
To discover the secret to cognitive health in the oldest old, Dr. Holstege initiated the 100-Plus Study, which involved a cohort of healthy centenarians.
The investigators conducted extensive neuropsychological testing and collected blood and fecal samples to examine “the myriad factors that influence physical health, including genetics, neuropathology, blood markers, and the gut microbiome, to explore the molecular and neuropsychologic constellations associated with the escape from cognitive decline.”
The goal of the research was to investigate “to what extent centenarians were able to maintain their cognitive health after study inclusion, and to what extent this was associated with genetic, physical, or neuropathological features,” she said.
The study included 330 centenarians who completed one or more neuropsychological assessments. Neuropathologic studies were available for 44 participants.
To assess baseline cognitive performance, the researchers administered a wide array of neurocognitive tests, as well as the Mini–Mental State Examination, from which mean z scores for cognitive domains were calculated.
Additional factors in the analysis included sex, age, APOE status, cognitive reserve, physical health, and whether participants lived independently.
At autopsy, amyloid-beta (A-beta) level, the level of intracellular accumulation of phosphorylated tau protein in neurofibrillary tangles (NFTs), and the neuritic plaque (NP) load were assessed.
Resilience and cognitive reserve
At baseline, the median age of the centenarians (n = 330, 72.4% women) was 100.5 years (interquartile range, 100.2-101.7). A little over half (56.7%) lived independently, and the majority had good vision (65%) and hearing (56.4%). Most (78.8%) were able to walk independently, and 37.9% had achieved the highest International Standard Classification of Education level of postsecondary education.
The researchers found “varying degrees of neuropathology” in the brains of the 44 donors, including A-beta, NFT, and NPs.
The duration of follow-up in analyzing cognitive trajectories ranged from 0 to 4 years (median, 1.6 years).
Assessments of all cognitive domains showed no decline, with the exception of a “slight” decrement in memory function (beta −.10 SD per year; 95% confidence interval, –.14 to –.05 SD; P < .001).
Cognitive performance was associated with factors of physical health or cognitive reserve, for example, greater independence in performing activities of daily living, as assessed by the Barthel index (beta .37 SD per year; 95% CI, .24-.49; P < .001), or higher educational level (beta .41 SD per year; 95% CI, .29-.53; P < .001).
Despite findings of neuropathologic “hallmarks” of AD post mortem in the brains of the centenarians, these were not associated with cognitive performance or rate of decline.
APOE epsilon-4 or an APOE epsilon-3 alleles also were not significantly associated with cognitive performance or decline, suggesting that the “effects of APOE alleles are exerted before the age of 100 years,” the authors noted.
“Our findings suggest that after reaching age 100 years, cognitive performance remains relatively stable during ensuing years. Therefore, these centenarians might be resilient or resistant against different risk factors of cognitive decline,” the authors wrote. They also speculate that resilience may be attributable to greater cognitive reserve.
“Our preliminary data indicate that approximately 60% of the chance to reach 100 years old is heritable. Therefore, to get a better understanding of which genetic factors associate with the prolonged maintenance of cognitive health, we are looking into which genetic variants occur more commonly in centenarians compared to younger individuals,” said Dr. Holstege.
“Of course, more research needs to be performed to get a better understanding of how such genetic elements might sustain brain health,” she added.
A ‘landmark study’
Commenting on the study in an interview, Thomas Perls, MD, MPH, professor of medicine, Boston University, called it a “landmark” study in research on exceptional longevity in humans.
Dr. Perls, the author of an accompanying editorial, noted that “one cannot absolutely assume a certain level or disability or risk for disease just because a person has achieved extreme age – in fact, if anything, their ability to achieve much older ages likely indicates that they have resistance or resilience to aging-related problems.”
Understanding the mechanism of the resilience could lead to treatment or prevention of AD, said Dr. Perls, who was not involved in the research.
“People have to be careful about ageist myths and attitudes and not have the ageist idea that the older you get, the sicker you get, because many individuals disprove that,” he cautioned.
The study was supported by Stichting Alzheimer Nederland and Stichting Vumc Fonds. Research from the Alzheimer Center Amsterdam is part of the neurodegeneration research program of Amsterdam Neuroscience. Dr. Holstege and Dr. Perls reported having no relevant financial relationships. The other authors’ disclosures are listed on the original article.
A version of this article first appeared on Medscape.com.
Investigators found that despite the presence of neuropathologies associated with Alzheimer’s disease (AD), many centenarians maintained high levels of cognitive performance.
“Cognitive decline is not inevitable,” senior author Henne Holstege, PhD, assistant professor, Amsterdam Alzheimer Center and Clinical Genetics, Amsterdam University Medical Center, said in an interview.
“At 100 years or older, high levels of cognitive performance can be maintained for several years, even when individuals are exposed to risk factors associated with cognitive decline,” she said.
The study was published online Jan. 15 in JAMA Network Open.
Escaping cognitive decline
Dr. Holstege said her interest in researching aging and cognitive health was inspired by the “fascinating” story of Hendrikje van Andel-Schipper, who died at age 115 in 2015 “completely cognitively healthy.” Her mother, who died at age 100, also was cognitively intact at the end of her life.
“I wanted to know how it is possible that some people can completely escape all aspects of cognitive decline while reaching extreme ages,” Dr. Holstege said.
To discover the secret to cognitive health in the oldest old, Dr. Holstege initiated the 100-Plus Study, which involved a cohort of healthy centenarians.
The investigators conducted extensive neuropsychological testing and collected blood and fecal samples to examine “the myriad factors that influence physical health, including genetics, neuropathology, blood markers, and the gut microbiome, to explore the molecular and neuropsychologic constellations associated with the escape from cognitive decline.”
The goal of the research was to investigate “to what extent centenarians were able to maintain their cognitive health after study inclusion, and to what extent this was associated with genetic, physical, or neuropathological features,” she said.
The study included 330 centenarians who completed one or more neuropsychological assessments. Neuropathologic studies were available for 44 participants.
To assess baseline cognitive performance, the researchers administered a wide array of neurocognitive tests, as well as the Mini–Mental State Examination, from which mean z scores for cognitive domains were calculated.
Additional factors in the analysis included sex, age, APOE status, cognitive reserve, physical health, and whether participants lived independently.
At autopsy, amyloid-beta (A-beta) level, the level of intracellular accumulation of phosphorylated tau protein in neurofibrillary tangles (NFTs), and the neuritic plaque (NP) load were assessed.
Resilience and cognitive reserve
At baseline, the median age of the centenarians (n = 330, 72.4% women) was 100.5 years (interquartile range, 100.2-101.7). A little over half (56.7%) lived independently, and the majority had good vision (65%) and hearing (56.4%). Most (78.8%) were able to walk independently, and 37.9% had achieved the highest International Standard Classification of Education level of postsecondary education.
The researchers found “varying degrees of neuropathology” in the brains of the 44 donors, including A-beta, NFT, and NPs.
The duration of follow-up in analyzing cognitive trajectories ranged from 0 to 4 years (median, 1.6 years).
Assessments of all cognitive domains showed no decline, with the exception of a “slight” decrement in memory function (beta −.10 SD per year; 95% confidence interval, –.14 to –.05 SD; P < .001).
Cognitive performance was associated with factors of physical health or cognitive reserve, for example, greater independence in performing activities of daily living, as assessed by the Barthel index (beta .37 SD per year; 95% CI, .24-.49; P < .001), or higher educational level (beta .41 SD per year; 95% CI, .29-.53; P < .001).
Despite findings of neuropathologic “hallmarks” of AD post mortem in the brains of the centenarians, these were not associated with cognitive performance or rate of decline.
APOE epsilon-4 or an APOE epsilon-3 alleles also were not significantly associated with cognitive performance or decline, suggesting that the “effects of APOE alleles are exerted before the age of 100 years,” the authors noted.
“Our findings suggest that after reaching age 100 years, cognitive performance remains relatively stable during ensuing years. Therefore, these centenarians might be resilient or resistant against different risk factors of cognitive decline,” the authors wrote. They also speculate that resilience may be attributable to greater cognitive reserve.
“Our preliminary data indicate that approximately 60% of the chance to reach 100 years old is heritable. Therefore, to get a better understanding of which genetic factors associate with the prolonged maintenance of cognitive health, we are looking into which genetic variants occur more commonly in centenarians compared to younger individuals,” said Dr. Holstege.
“Of course, more research needs to be performed to get a better understanding of how such genetic elements might sustain brain health,” she added.
A ‘landmark study’
Commenting on the study in an interview, Thomas Perls, MD, MPH, professor of medicine, Boston University, called it a “landmark” study in research on exceptional longevity in humans.
Dr. Perls, the author of an accompanying editorial, noted that “one cannot absolutely assume a certain level or disability or risk for disease just because a person has achieved extreme age – in fact, if anything, their ability to achieve much older ages likely indicates that they have resistance or resilience to aging-related problems.”
Understanding the mechanism of the resilience could lead to treatment or prevention of AD, said Dr. Perls, who was not involved in the research.
“People have to be careful about ageist myths and attitudes and not have the ageist idea that the older you get, the sicker you get, because many individuals disprove that,” he cautioned.
The study was supported by Stichting Alzheimer Nederland and Stichting Vumc Fonds. Research from the Alzheimer Center Amsterdam is part of the neurodegeneration research program of Amsterdam Neuroscience. Dr. Holstege and Dr. Perls reported having no relevant financial relationships. The other authors’ disclosures are listed on the original article.
A version of this article first appeared on Medscape.com.
Investigators found that despite the presence of neuropathologies associated with Alzheimer’s disease (AD), many centenarians maintained high levels of cognitive performance.
“Cognitive decline is not inevitable,” senior author Henne Holstege, PhD, assistant professor, Amsterdam Alzheimer Center and Clinical Genetics, Amsterdam University Medical Center, said in an interview.
“At 100 years or older, high levels of cognitive performance can be maintained for several years, even when individuals are exposed to risk factors associated with cognitive decline,” she said.
The study was published online Jan. 15 in JAMA Network Open.
Escaping cognitive decline
Dr. Holstege said her interest in researching aging and cognitive health was inspired by the “fascinating” story of Hendrikje van Andel-Schipper, who died at age 115 in 2015 “completely cognitively healthy.” Her mother, who died at age 100, also was cognitively intact at the end of her life.
“I wanted to know how it is possible that some people can completely escape all aspects of cognitive decline while reaching extreme ages,” Dr. Holstege said.
To discover the secret to cognitive health in the oldest old, Dr. Holstege initiated the 100-Plus Study, which involved a cohort of healthy centenarians.
The investigators conducted extensive neuropsychological testing and collected blood and fecal samples to examine “the myriad factors that influence physical health, including genetics, neuropathology, blood markers, and the gut microbiome, to explore the molecular and neuropsychologic constellations associated with the escape from cognitive decline.”
The goal of the research was to investigate “to what extent centenarians were able to maintain their cognitive health after study inclusion, and to what extent this was associated with genetic, physical, or neuropathological features,” she said.
The study included 330 centenarians who completed one or more neuropsychological assessments. Neuropathologic studies were available for 44 participants.
To assess baseline cognitive performance, the researchers administered a wide array of neurocognitive tests, as well as the Mini–Mental State Examination, from which mean z scores for cognitive domains were calculated.
Additional factors in the analysis included sex, age, APOE status, cognitive reserve, physical health, and whether participants lived independently.
At autopsy, amyloid-beta (A-beta) level, the level of intracellular accumulation of phosphorylated tau protein in neurofibrillary tangles (NFTs), and the neuritic plaque (NP) load were assessed.
Resilience and cognitive reserve
At baseline, the median age of the centenarians (n = 330, 72.4% women) was 100.5 years (interquartile range, 100.2-101.7). A little over half (56.7%) lived independently, and the majority had good vision (65%) and hearing (56.4%). Most (78.8%) were able to walk independently, and 37.9% had achieved the highest International Standard Classification of Education level of postsecondary education.
The researchers found “varying degrees of neuropathology” in the brains of the 44 donors, including A-beta, NFT, and NPs.
The duration of follow-up in analyzing cognitive trajectories ranged from 0 to 4 years (median, 1.6 years).
Assessments of all cognitive domains showed no decline, with the exception of a “slight” decrement in memory function (beta −.10 SD per year; 95% confidence interval, –.14 to –.05 SD; P < .001).
Cognitive performance was associated with factors of physical health or cognitive reserve, for example, greater independence in performing activities of daily living, as assessed by the Barthel index (beta .37 SD per year; 95% CI, .24-.49; P < .001), or higher educational level (beta .41 SD per year; 95% CI, .29-.53; P < .001).
Despite findings of neuropathologic “hallmarks” of AD post mortem in the brains of the centenarians, these were not associated with cognitive performance or rate of decline.
APOE epsilon-4 or an APOE epsilon-3 alleles also were not significantly associated with cognitive performance or decline, suggesting that the “effects of APOE alleles are exerted before the age of 100 years,” the authors noted.
“Our findings suggest that after reaching age 100 years, cognitive performance remains relatively stable during ensuing years. Therefore, these centenarians might be resilient or resistant against different risk factors of cognitive decline,” the authors wrote. They also speculate that resilience may be attributable to greater cognitive reserve.
“Our preliminary data indicate that approximately 60% of the chance to reach 100 years old is heritable. Therefore, to get a better understanding of which genetic factors associate with the prolonged maintenance of cognitive health, we are looking into which genetic variants occur more commonly in centenarians compared to younger individuals,” said Dr. Holstege.
“Of course, more research needs to be performed to get a better understanding of how such genetic elements might sustain brain health,” she added.
A ‘landmark study’
Commenting on the study in an interview, Thomas Perls, MD, MPH, professor of medicine, Boston University, called it a “landmark” study in research on exceptional longevity in humans.
Dr. Perls, the author of an accompanying editorial, noted that “one cannot absolutely assume a certain level or disability or risk for disease just because a person has achieved extreme age – in fact, if anything, their ability to achieve much older ages likely indicates that they have resistance or resilience to aging-related problems.”
Understanding the mechanism of the resilience could lead to treatment or prevention of AD, said Dr. Perls, who was not involved in the research.
“People have to be careful about ageist myths and attitudes and not have the ageist idea that the older you get, the sicker you get, because many individuals disprove that,” he cautioned.
The study was supported by Stichting Alzheimer Nederland and Stichting Vumc Fonds. Research from the Alzheimer Center Amsterdam is part of the neurodegeneration research program of Amsterdam Neuroscience. Dr. Holstege and Dr. Perls reported having no relevant financial relationships. The other authors’ disclosures are listed on the original article.
A version of this article first appeared on Medscape.com.
Encephalopathy common, often lethal in hospitalized patients with COVID-19
, new research shows. Results of a retrospective study show that of almost 4,500 patients with COVID-19, 12% were diagnosed with TME. Of these, 78% developed encephalopathy immediately prior to hospital admission. Septic encephalopathy, hypoxic-ischemic encephalopathy (HIE), and uremia were the most common causes, although multiple causes were present in close to 80% of patients. TME was also associated with a 24% higher risk of in-hospital death.
“We found that close to one in eight patients who were hospitalized with COVID-19 had TME that was not attributed to the effects of sedatives, and that this is incredibly common among these patients who are critically ill” said lead author Jennifer A. Frontera, MD, New York University.
“The general principle of our findings is to be more aggressive in TME; and from a neurologist perspective, the way to do this is to eliminate the effects of sedation, which is a confounder,” she said.
The study was published online March 16 in Neurocritical Care.
Drilling down
“Many neurological complications of COVID-19 are sequelae of severe illness or secondary effects of multisystem organ failure, but our previous work identified TME as the most common neurological complication,” Dr. Frontera said.
Previous research investigating encephalopathy among patients with COVID-19 included patients who may have been sedated or have had a positive Confusion Assessment Method (CAM) result.
“A lot of the delirium literature is effectively heterogeneous because there are a number of patients who are on sedative medication that, if you could turn it off, these patients would return to normal. Some may have underlying neurological issues that can be addressed, but you can›t get to the bottom of this unless you turn off the sedation,” Dr. Frontera noted.
“We wanted to be specific and try to drill down to see what the underlying cause of the encephalopathy was,” she said.
The researchers retrospectively analyzed data on 4,491 patients (≥ 18 years old) with COVID-19 who were admitted to four New York City hospitals between March 1, 2020, and May 20, 2020. Of these, 559 (12%) with TME were compared with 3,932 patients without TME.
The researchers looked at index admissions and included patients who had:
- New changes in mental status or significant worsening of mental status (in patients with baseline abnormal mental status).
- Hyperglycemia or with transient focal neurologic deficits that resolved with glucose correction.
- An adequate washout of sedating medications (when relevant) prior to mental status assessment.
Potential etiologies included electrolyte abnormalities, organ failure, hypertensive encephalopathy, sepsis or active infection, fever, nutritional deficiency, and environmental injury.
Foreign environment
Most (78%) of the 559 patients diagnosed with TME had already developed encephalopathy immediately prior to hospital admission, the authors report. The most common etiologies of TME among hospitalized patients with COVID-19 are listed below.
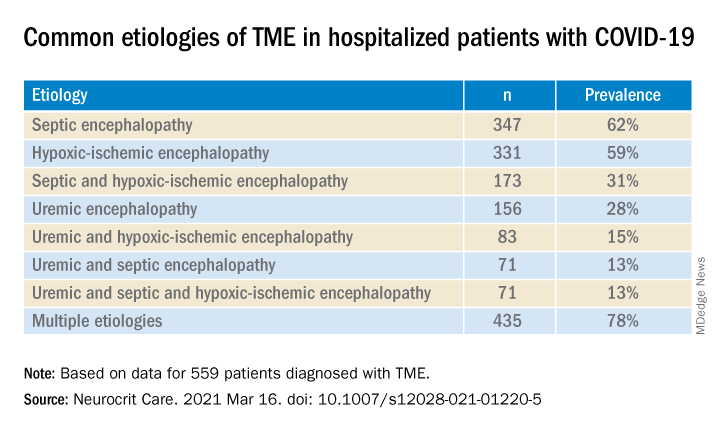
Compared with patients without TME, those with TME – (all Ps < .001):
- Were older (76 vs. 62 years).
- Had higher rates of dementia (27% vs. 3%).
- Had higher rates of psychiatric history (20% vs. 10%).
- Were more often intubated (37% vs. 20%).
- Had a longer length of hospital stay (7.9 vs. 6.0 days).
- Were less often discharged home (25% vs. 66%).
“It’s no surprise that older patients and people with dementia or psychiatric illness are predisposed to becoming encephalopathic,” said Dr. Frontera. “Being in a foreign environment, such as a hospital, or being sleep-deprived in the ICU is likely to make them more confused during their hospital stay.”
Delirium as a symptom
In-hospital mortality or discharge to hospice was considerably higher in the TME versus non-TME patients (44% vs. 18%, respectively).
When the researchers adjusted for confounders (age, sex, race, worse Sequential Organ Failure Assessment score during hospitalization, ventilator status, study week, hospital location, and ICU care level) and excluded patients receiving only comfort care, they found that TME was associated with a 24% increased risk of in-hospital death (30% in patients with TME vs. 16% in those without TME).
The highest mortality risk was associated with hypoxemia, with 42% of patients with HIE dying during hospitalization, compared with 16% of patients without HIE (adjusted hazard ratio 1.56; 95% confidence interval, 1.21-2.00; P = .001).
“Not all patients who are intubated require sedation, but there’s generally a lot of hesitation in reducing or stopping sedation in some patients,” Dr. Frontera observed.
She acknowledged there are “many extremely sick patients whom you can’t ventilate without sedation.”
Nevertheless, “delirium in and of itself does not cause death. It’s a symptom, not a disease, and we have to figure out what causes it. Delirium might not need to be sedated, and it’s more important to see what the causal problem is.”
Independent predictor of death
Commenting on the study, Panayiotis N. Varelas, MD, PhD, vice president of the Neurocritical Care Society, said the study “approached the TME issue better than previously, namely allowing time for sedatives to wear off to have a better sample of patients with this syndrome.”
Dr. Varelas, who is chairman of the department of neurology and professor of neurology at Albany (N.Y.) Medical College, emphasized that TME “is not benign and, in patients with COVID-19, it is an independent predictor of in-hospital mortality.”
“One should take all possible measures … to avoid desaturation and hypotensive episodes and also aggressively treat SAE and uremic encephalopathy in hopes of improving the outcomes,” added Dr. Varelas, who was not involved with the study.
Also commenting on the study, Mitchell Elkind, MD, professor of neurology and epidemiology at Columbia University in New York, who was not associated with the research, said it “nicely distinguishes among the different causes of encephalopathy, including sepsis, hypoxia, and kidney failure … emphasizing just how sick these patients are.”
The study received no direct funding. Individual investigators were supported by grants from the National Institute on Aging and the National Institute of Neurological Disorders and Stroke. The investigators, Dr. Varelas, and Dr. Elkind have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new research shows. Results of a retrospective study show that of almost 4,500 patients with COVID-19, 12% were diagnosed with TME. Of these, 78% developed encephalopathy immediately prior to hospital admission. Septic encephalopathy, hypoxic-ischemic encephalopathy (HIE), and uremia were the most common causes, although multiple causes were present in close to 80% of patients. TME was also associated with a 24% higher risk of in-hospital death.
“We found that close to one in eight patients who were hospitalized with COVID-19 had TME that was not attributed to the effects of sedatives, and that this is incredibly common among these patients who are critically ill” said lead author Jennifer A. Frontera, MD, New York University.
“The general principle of our findings is to be more aggressive in TME; and from a neurologist perspective, the way to do this is to eliminate the effects of sedation, which is a confounder,” she said.
The study was published online March 16 in Neurocritical Care.
Drilling down
“Many neurological complications of COVID-19 are sequelae of severe illness or secondary effects of multisystem organ failure, but our previous work identified TME as the most common neurological complication,” Dr. Frontera said.
Previous research investigating encephalopathy among patients with COVID-19 included patients who may have been sedated or have had a positive Confusion Assessment Method (CAM) result.
“A lot of the delirium literature is effectively heterogeneous because there are a number of patients who are on sedative medication that, if you could turn it off, these patients would return to normal. Some may have underlying neurological issues that can be addressed, but you can›t get to the bottom of this unless you turn off the sedation,” Dr. Frontera noted.
“We wanted to be specific and try to drill down to see what the underlying cause of the encephalopathy was,” she said.
The researchers retrospectively analyzed data on 4,491 patients (≥ 18 years old) with COVID-19 who were admitted to four New York City hospitals between March 1, 2020, and May 20, 2020. Of these, 559 (12%) with TME were compared with 3,932 patients without TME.
The researchers looked at index admissions and included patients who had:
- New changes in mental status or significant worsening of mental status (in patients with baseline abnormal mental status).
- Hyperglycemia or with transient focal neurologic deficits that resolved with glucose correction.
- An adequate washout of sedating medications (when relevant) prior to mental status assessment.
Potential etiologies included electrolyte abnormalities, organ failure, hypertensive encephalopathy, sepsis or active infection, fever, nutritional deficiency, and environmental injury.
Foreign environment
Most (78%) of the 559 patients diagnosed with TME had already developed encephalopathy immediately prior to hospital admission, the authors report. The most common etiologies of TME among hospitalized patients with COVID-19 are listed below.

Compared with patients without TME, those with TME – (all Ps < .001):
- Were older (76 vs. 62 years).
- Had higher rates of dementia (27% vs. 3%).
- Had higher rates of psychiatric history (20% vs. 10%).
- Were more often intubated (37% vs. 20%).
- Had a longer length of hospital stay (7.9 vs. 6.0 days).
- Were less often discharged home (25% vs. 66%).
“It’s no surprise that older patients and people with dementia or psychiatric illness are predisposed to becoming encephalopathic,” said Dr. Frontera. “Being in a foreign environment, such as a hospital, or being sleep-deprived in the ICU is likely to make them more confused during their hospital stay.”
Delirium as a symptom
In-hospital mortality or discharge to hospice was considerably higher in the TME versus non-TME patients (44% vs. 18%, respectively).
When the researchers adjusted for confounders (age, sex, race, worse Sequential Organ Failure Assessment score during hospitalization, ventilator status, study week, hospital location, and ICU care level) and excluded patients receiving only comfort care, they found that TME was associated with a 24% increased risk of in-hospital death (30% in patients with TME vs. 16% in those without TME).
The highest mortality risk was associated with hypoxemia, with 42% of patients with HIE dying during hospitalization, compared with 16% of patients without HIE (adjusted hazard ratio 1.56; 95% confidence interval, 1.21-2.00; P = .001).
“Not all patients who are intubated require sedation, but there’s generally a lot of hesitation in reducing or stopping sedation in some patients,” Dr. Frontera observed.
She acknowledged there are “many extremely sick patients whom you can’t ventilate without sedation.”
Nevertheless, “delirium in and of itself does not cause death. It’s a symptom, not a disease, and we have to figure out what causes it. Delirium might not need to be sedated, and it’s more important to see what the causal problem is.”
Independent predictor of death
Commenting on the study, Panayiotis N. Varelas, MD, PhD, vice president of the Neurocritical Care Society, said the study “approached the TME issue better than previously, namely allowing time for sedatives to wear off to have a better sample of patients with this syndrome.”
Dr. Varelas, who is chairman of the department of neurology and professor of neurology at Albany (N.Y.) Medical College, emphasized that TME “is not benign and, in patients with COVID-19, it is an independent predictor of in-hospital mortality.”
“One should take all possible measures … to avoid desaturation and hypotensive episodes and also aggressively treat SAE and uremic encephalopathy in hopes of improving the outcomes,” added Dr. Varelas, who was not involved with the study.
Also commenting on the study, Mitchell Elkind, MD, professor of neurology and epidemiology at Columbia University in New York, who was not associated with the research, said it “nicely distinguishes among the different causes of encephalopathy, including sepsis, hypoxia, and kidney failure … emphasizing just how sick these patients are.”
The study received no direct funding. Individual investigators were supported by grants from the National Institute on Aging and the National Institute of Neurological Disorders and Stroke. The investigators, Dr. Varelas, and Dr. Elkind have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new research shows. Results of a retrospective study show that of almost 4,500 patients with COVID-19, 12% were diagnosed with TME. Of these, 78% developed encephalopathy immediately prior to hospital admission. Septic encephalopathy, hypoxic-ischemic encephalopathy (HIE), and uremia were the most common causes, although multiple causes were present in close to 80% of patients. TME was also associated with a 24% higher risk of in-hospital death.
“We found that close to one in eight patients who were hospitalized with COVID-19 had TME that was not attributed to the effects of sedatives, and that this is incredibly common among these patients who are critically ill” said lead author Jennifer A. Frontera, MD, New York University.
“The general principle of our findings is to be more aggressive in TME; and from a neurologist perspective, the way to do this is to eliminate the effects of sedation, which is a confounder,” she said.
The study was published online March 16 in Neurocritical Care.
Drilling down
“Many neurological complications of COVID-19 are sequelae of severe illness or secondary effects of multisystem organ failure, but our previous work identified TME as the most common neurological complication,” Dr. Frontera said.
Previous research investigating encephalopathy among patients with COVID-19 included patients who may have been sedated or have had a positive Confusion Assessment Method (CAM) result.
“A lot of the delirium literature is effectively heterogeneous because there are a number of patients who are on sedative medication that, if you could turn it off, these patients would return to normal. Some may have underlying neurological issues that can be addressed, but you can›t get to the bottom of this unless you turn off the sedation,” Dr. Frontera noted.
“We wanted to be specific and try to drill down to see what the underlying cause of the encephalopathy was,” she said.
The researchers retrospectively analyzed data on 4,491 patients (≥ 18 years old) with COVID-19 who were admitted to four New York City hospitals between March 1, 2020, and May 20, 2020. Of these, 559 (12%) with TME were compared with 3,932 patients without TME.
The researchers looked at index admissions and included patients who had:
- New changes in mental status or significant worsening of mental status (in patients with baseline abnormal mental status).
- Hyperglycemia or with transient focal neurologic deficits that resolved with glucose correction.
- An adequate washout of sedating medications (when relevant) prior to mental status assessment.
Potential etiologies included electrolyte abnormalities, organ failure, hypertensive encephalopathy, sepsis or active infection, fever, nutritional deficiency, and environmental injury.
Foreign environment
Most (78%) of the 559 patients diagnosed with TME had already developed encephalopathy immediately prior to hospital admission, the authors report. The most common etiologies of TME among hospitalized patients with COVID-19 are listed below.

Compared with patients without TME, those with TME – (all Ps < .001):
- Were older (76 vs. 62 years).
- Had higher rates of dementia (27% vs. 3%).
- Had higher rates of psychiatric history (20% vs. 10%).
- Were more often intubated (37% vs. 20%).
- Had a longer length of hospital stay (7.9 vs. 6.0 days).
- Were less often discharged home (25% vs. 66%).
“It’s no surprise that older patients and people with dementia or psychiatric illness are predisposed to becoming encephalopathic,” said Dr. Frontera. “Being in a foreign environment, such as a hospital, or being sleep-deprived in the ICU is likely to make them more confused during their hospital stay.”
Delirium as a symptom
In-hospital mortality or discharge to hospice was considerably higher in the TME versus non-TME patients (44% vs. 18%, respectively).
When the researchers adjusted for confounders (age, sex, race, worse Sequential Organ Failure Assessment score during hospitalization, ventilator status, study week, hospital location, and ICU care level) and excluded patients receiving only comfort care, they found that TME was associated with a 24% increased risk of in-hospital death (30% in patients with TME vs. 16% in those without TME).
The highest mortality risk was associated with hypoxemia, with 42% of patients with HIE dying during hospitalization, compared with 16% of patients without HIE (adjusted hazard ratio 1.56; 95% confidence interval, 1.21-2.00; P = .001).
“Not all patients who are intubated require sedation, but there’s generally a lot of hesitation in reducing or stopping sedation in some patients,” Dr. Frontera observed.
She acknowledged there are “many extremely sick patients whom you can’t ventilate without sedation.”
Nevertheless, “delirium in and of itself does not cause death. It’s a symptom, not a disease, and we have to figure out what causes it. Delirium might not need to be sedated, and it’s more important to see what the causal problem is.”
Independent predictor of death
Commenting on the study, Panayiotis N. Varelas, MD, PhD, vice president of the Neurocritical Care Society, said the study “approached the TME issue better than previously, namely allowing time for sedatives to wear off to have a better sample of patients with this syndrome.”
Dr. Varelas, who is chairman of the department of neurology and professor of neurology at Albany (N.Y.) Medical College, emphasized that TME “is not benign and, in patients with COVID-19, it is an independent predictor of in-hospital mortality.”
“One should take all possible measures … to avoid desaturation and hypotensive episodes and also aggressively treat SAE and uremic encephalopathy in hopes of improving the outcomes,” added Dr. Varelas, who was not involved with the study.
Also commenting on the study, Mitchell Elkind, MD, professor of neurology and epidemiology at Columbia University in New York, who was not associated with the research, said it “nicely distinguishes among the different causes of encephalopathy, including sepsis, hypoxia, and kidney failure … emphasizing just how sick these patients are.”
The study received no direct funding. Individual investigators were supported by grants from the National Institute on Aging and the National Institute of Neurological Disorders and Stroke. The investigators, Dr. Varelas, and Dr. Elkind have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM NEUROCRITICAL CARE
New data on worldwide mental health impact of COVID-19
A new survey that assessed the mental health impact of COVID-19 across the globe shows high rates of trauma and clinical mood disorders related to the pandemic.
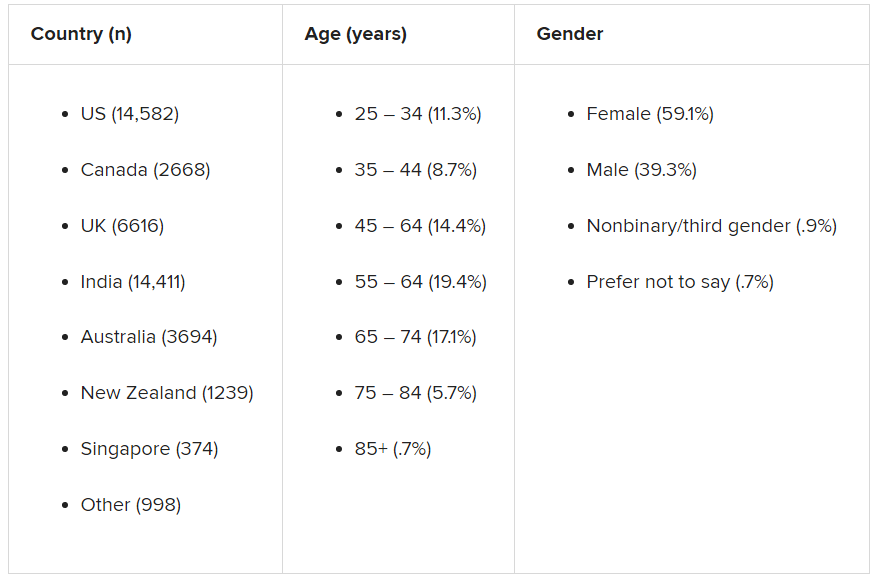
The survey, carried out by Sapien Labs, was conducted in eight English-speaking countries and included 49,000 adults. It showed that 57% of respondents experienced some COVID-19–related adversity or trauma.
Roughly one-quarter showed clinical signs of or were at risk for a mood disorder, and 40% described themselves as “succeeding or thriving.”
Those who reported the poorest mental health were young adults and individuals who experienced financial adversity or were unable to receive care for other medical conditions. Nonbinary gender and not getting enough sleep, exercise, or face-to-face socialization also increased the risk for poorer mental well-being.
“The data suggest that there will be long-term fallout from the pandemic on the mental health front,” Tara Thiagarajan, PhD, Sapien Labs founder and chief scientist, said in a press release.
Novel initiative
Dr. Thiagarajan said in an interview that she was running a company that provided microloans to 30,000 villages in India. The company included a research group the goal of which was to understand what predicts success in an individual and in a particular ecosystem, she said – “Why did some villages succeed and others didn’t?”
Dr. Thiagarajan and associates thought that “something big is happening in our life circumstances that causes changes in our brain and felt that we need to understand what they are and how they affect humanity. This was the impetus for founding Sapien Labs. “
The survey, which is part of the company’s Mental Health Million project, is an ongoing research initiative that makes data freely available to other researchers.
The investigators developed a “free and anonymous assessment tool,” the Mental Health Quotient (MHQ), which “encompasses a comprehensive view of our emotional, social, and cognitive function and capability,” said Dr. Thiagarajan.
The MHQ consists of 47 “elements of mental well-being.” Respondents’ MHQ scores ranged from –100 to +200. Negative scores indicate poorer mental well-being. Respondents were categorized as clinical, at risk, enduring, managing, succeeding, and thriving.
MHQ scores were computed for six “broad dimensions” of mental health: Core cognition, complex cognition, mood and outlook, drive and motivation, social self, and mind-body connection.
Participants were recruited through advertising on Google and Facebook in eight English-speaking countries – Canada, the United States, the United Kingdom, South Africa, Singapore, Australia, New Zealand, and India. The researchers collected demographic information, including age, education, and gender.
First step
The assessment was completed by 48,808 respondents between April 8 and Dec. 31, 2020.
A smaller sample of 2,000 people from the same countries who were polled by the investigators in 2019 was used as a comparator.
Taken together, the overall mental well-being score for 2020 was 8% lower than the score obtained in 2019 from the same countries, and the percentage of respondents who fell into the “clinical” category increased from 14% in 2009 to 26% in 2020.
Residents of Singapore had the highest MHQ score, followed by residents of the United States. At the other extreme, respondents from the United Kingdom and South Africa had the poorest MHQ scores.
“It is important to keep in mind that the English-speaking, Internet-enabled populace is not necessarily representative of each country as a whole,” the authors noted.
Youth hardest hit
whose average MHQ score was 29% lower than those aged at least 65 years.
Worldwide, 70% of respondents aged at least 65 years fell into the categories of “succeeding” or “thriving,” compared with just 17% of those aged 18-24 years.
“We saw a massive trend of diminishing mental well-being in younger individuals, suggesting that some societal force is at play that we need to get to the bottom of,” said Dr. Thiagarajan.
“Young people are still learning how to calibrate themselves in the world, and with age comes maturity, leading to a difference in emotional resilience,” she said.
Highest risk group
Mental well-being was poorest among nonbinary/third-gender respondents. Among those persons, more than 50% were classified as being at clinical risk, in comparison with males and females combined, and their MHQ scores were about 47 points lower.
Nonbinary individuals “are universally doing very poorly, relative to males or females,” said Dr. Thiagarajan. “This is a demographic at very high risk with a lot of suicidal thoughts.”
Respondents who had insufficient sleep, who lacked social interaction, and whose level of exercise was insufficient had lower MHQ scores of an “unexpected magnitude,” compared with their counterparts who had sufficient sleep, more social interaction, and more exercise (a discrepancy of 82, 66, and 46 points, respectively).
Only 3.9% of respondents reported having had COVID-19; 0.7% reported having had a severe case. Yet 57% of respondents reported that the pandemic had had negative consequences with regard to their health or their finances or social situation.
Those who were unable to get care for their other health conditions because of the pandemic (2% of all respondents) reported the worst mental well-being, followed by those who struggled for basic necessities (1.4%).
Reduced household income was associated with a 4% lower score but affected a higher percentage of people (17%). Social isolation was associated with a score of about 20 less. Higher rates of lifetime traumas and adversities were likewise associated with lower scores for mental well-being.
Creative, generous approach
Commenting on the survey results, Ken Duckworth, MD, clinical professor at Harvard Medical School, Boston, and chief medical officer of the National Alliance of Mental Illness, noted that the findings were similar to findings from studies in the United States, which showed disproportionately higher rates of mental health problems in younger individuals. Dr. Duckworth was not involved with the survey.
“The idea that this is an international phenomenon and the broad-stroke finding that younger people are suffering across nations is compelling and important for policymakers to look at,” he said.
Dr. Duckworth noted that although the findings are not “representative” of entire populations in a given country, the report is a “first step in a long journey.”
He described the report as “extremely brilliant, creative, and generous, allowing any academician to get access to the data.”
He saw it “less as a definitive report and more as a directionally informative survey that will yield great fruit over time.”
In a comment, Joshua Morganstein, MD, chair of the American Psychiatric Association’s Committee on the Psychiatric Dimensions of Disaster, said: “One of the important things a document like this highlights is the importance of understanding more where risk [for mental health disorders] is concentrated and what things have occurred or might occur that can buffer against that risk or protect us from it. We see that each nation has similar but also different challenges.”
Dr. Thiagarajan is the founder and chief scientist of Sapien Labs. Her coauthors are employees of Sapien Labs. Dr. Duckworth and Dr. Morganstein disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A new survey that assessed the mental health impact of COVID-19 across the globe shows high rates of trauma and clinical mood disorders related to the pandemic.
The survey, carried out by Sapien Labs, was conducted in eight English-speaking countries and included 49,000 adults. It showed that 57% of respondents experienced some COVID-19–related adversity or trauma.
Roughly one-quarter showed clinical signs of or were at risk for a mood disorder, and 40% described themselves as “succeeding or thriving.”
Those who reported the poorest mental health were young adults and individuals who experienced financial adversity or were unable to receive care for other medical conditions. Nonbinary gender and not getting enough sleep, exercise, or face-to-face socialization also increased the risk for poorer mental well-being.
“The data suggest that there will be long-term fallout from the pandemic on the mental health front,” Tara Thiagarajan, PhD, Sapien Labs founder and chief scientist, said in a press release.
Novel initiative
Dr. Thiagarajan said in an interview that she was running a company that provided microloans to 30,000 villages in India. The company included a research group the goal of which was to understand what predicts success in an individual and in a particular ecosystem, she said – “Why did some villages succeed and others didn’t?”
Dr. Thiagarajan and associates thought that “something big is happening in our life circumstances that causes changes in our brain and felt that we need to understand what they are and how they affect humanity. This was the impetus for founding Sapien Labs. “
The survey, which is part of the company’s Mental Health Million project, is an ongoing research initiative that makes data freely available to other researchers.
The investigators developed a “free and anonymous assessment tool,” the Mental Health Quotient (MHQ), which “encompasses a comprehensive view of our emotional, social, and cognitive function and capability,” said Dr. Thiagarajan.
The MHQ consists of 47 “elements of mental well-being.” Respondents’ MHQ scores ranged from –100 to +200. Negative scores indicate poorer mental well-being. Respondents were categorized as clinical, at risk, enduring, managing, succeeding, and thriving.
MHQ scores were computed for six “broad dimensions” of mental health: Core cognition, complex cognition, mood and outlook, drive and motivation, social self, and mind-body connection.
Participants were recruited through advertising on Google and Facebook in eight English-speaking countries – Canada, the United States, the United Kingdom, South Africa, Singapore, Australia, New Zealand, and India. The researchers collected demographic information, including age, education, and gender.
First step
The assessment was completed by 48,808 respondents between April 8 and Dec. 31, 2020.
A smaller sample of 2,000 people from the same countries who were polled by the investigators in 2019 was used as a comparator.
Taken together, the overall mental well-being score for 2020 was 8% lower than the score obtained in 2019 from the same countries, and the percentage of respondents who fell into the “clinical” category increased from 14% in 2009 to 26% in 2020.
Residents of Singapore had the highest MHQ score, followed by residents of the United States. At the other extreme, respondents from the United Kingdom and South Africa had the poorest MHQ scores.
“It is important to keep in mind that the English-speaking, Internet-enabled populace is not necessarily representative of each country as a whole,” the authors noted.
Youth hardest hit
whose average MHQ score was 29% lower than those aged at least 65 years.
Worldwide, 70% of respondents aged at least 65 years fell into the categories of “succeeding” or “thriving,” compared with just 17% of those aged 18-24 years.
“We saw a massive trend of diminishing mental well-being in younger individuals, suggesting that some societal force is at play that we need to get to the bottom of,” said Dr. Thiagarajan.
“Young people are still learning how to calibrate themselves in the world, and with age comes maturity, leading to a difference in emotional resilience,” she said.
Highest risk group
Mental well-being was poorest among nonbinary/third-gender respondents. Among those persons, more than 50% were classified as being at clinical risk, in comparison with males and females combined, and their MHQ scores were about 47 points lower.
Nonbinary individuals “are universally doing very poorly, relative to males or females,” said Dr. Thiagarajan. “This is a demographic at very high risk with a lot of suicidal thoughts.”
Respondents who had insufficient sleep, who lacked social interaction, and whose level of exercise was insufficient had lower MHQ scores of an “unexpected magnitude,” compared with their counterparts who had sufficient sleep, more social interaction, and more exercise (a discrepancy of 82, 66, and 46 points, respectively).
Only 3.9% of respondents reported having had COVID-19; 0.7% reported having had a severe case. Yet 57% of respondents reported that the pandemic had had negative consequences with regard to their health or their finances or social situation.
Those who were unable to get care for their other health conditions because of the pandemic (2% of all respondents) reported the worst mental well-being, followed by those who struggled for basic necessities (1.4%).
Reduced household income was associated with a 4% lower score but affected a higher percentage of people (17%). Social isolation was associated with a score of about 20 less. Higher rates of lifetime traumas and adversities were likewise associated with lower scores for mental well-being.
Creative, generous approach
Commenting on the survey results, Ken Duckworth, MD, clinical professor at Harvard Medical School, Boston, and chief medical officer of the National Alliance of Mental Illness, noted that the findings were similar to findings from studies in the United States, which showed disproportionately higher rates of mental health problems in younger individuals. Dr. Duckworth was not involved with the survey.
“The idea that this is an international phenomenon and the broad-stroke finding that younger people are suffering across nations is compelling and important for policymakers to look at,” he said.
Dr. Duckworth noted that although the findings are not “representative” of entire populations in a given country, the report is a “first step in a long journey.”
He described the report as “extremely brilliant, creative, and generous, allowing any academician to get access to the data.”
He saw it “less as a definitive report and more as a directionally informative survey that will yield great fruit over time.”
In a comment, Joshua Morganstein, MD, chair of the American Psychiatric Association’s Committee on the Psychiatric Dimensions of Disaster, said: “One of the important things a document like this highlights is the importance of understanding more where risk [for mental health disorders] is concentrated and what things have occurred or might occur that can buffer against that risk or protect us from it. We see that each nation has similar but also different challenges.”
Dr. Thiagarajan is the founder and chief scientist of Sapien Labs. Her coauthors are employees of Sapien Labs. Dr. Duckworth and Dr. Morganstein disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A new survey that assessed the mental health impact of COVID-19 across the globe shows high rates of trauma and clinical mood disorders related to the pandemic.
The survey, carried out by Sapien Labs, was conducted in eight English-speaking countries and included 49,000 adults. It showed that 57% of respondents experienced some COVID-19–related adversity or trauma.
Roughly one-quarter showed clinical signs of or were at risk for a mood disorder, and 40% described themselves as “succeeding or thriving.”
Those who reported the poorest mental health were young adults and individuals who experienced financial adversity or were unable to receive care for other medical conditions. Nonbinary gender and not getting enough sleep, exercise, or face-to-face socialization also increased the risk for poorer mental well-being.
“The data suggest that there will be long-term fallout from the pandemic on the mental health front,” Tara Thiagarajan, PhD, Sapien Labs founder and chief scientist, said in a press release.
Novel initiative
Dr. Thiagarajan said in an interview that she was running a company that provided microloans to 30,000 villages in India. The company included a research group the goal of which was to understand what predicts success in an individual and in a particular ecosystem, she said – “Why did some villages succeed and others didn’t?”
Dr. Thiagarajan and associates thought that “something big is happening in our life circumstances that causes changes in our brain and felt that we need to understand what they are and how they affect humanity. This was the impetus for founding Sapien Labs. “
The survey, which is part of the company’s Mental Health Million project, is an ongoing research initiative that makes data freely available to other researchers.
The investigators developed a “free and anonymous assessment tool,” the Mental Health Quotient (MHQ), which “encompasses a comprehensive view of our emotional, social, and cognitive function and capability,” said Dr. Thiagarajan.
The MHQ consists of 47 “elements of mental well-being.” Respondents’ MHQ scores ranged from –100 to +200. Negative scores indicate poorer mental well-being. Respondents were categorized as clinical, at risk, enduring, managing, succeeding, and thriving.
MHQ scores were computed for six “broad dimensions” of mental health: Core cognition, complex cognition, mood and outlook, drive and motivation, social self, and mind-body connection.
Participants were recruited through advertising on Google and Facebook in eight English-speaking countries – Canada, the United States, the United Kingdom, South Africa, Singapore, Australia, New Zealand, and India. The researchers collected demographic information, including age, education, and gender.
First step
The assessment was completed by 48,808 respondents between April 8 and Dec. 31, 2020.
A smaller sample of 2,000 people from the same countries who were polled by the investigators in 2019 was used as a comparator.
Taken together, the overall mental well-being score for 2020 was 8% lower than the score obtained in 2019 from the same countries, and the percentage of respondents who fell into the “clinical” category increased from 14% in 2009 to 26% in 2020.
Residents of Singapore had the highest MHQ score, followed by residents of the United States. At the other extreme, respondents from the United Kingdom and South Africa had the poorest MHQ scores.
“It is important to keep in mind that the English-speaking, Internet-enabled populace is not necessarily representative of each country as a whole,” the authors noted.
Youth hardest hit
whose average MHQ score was 29% lower than those aged at least 65 years.
Worldwide, 70% of respondents aged at least 65 years fell into the categories of “succeeding” or “thriving,” compared with just 17% of those aged 18-24 years.
“We saw a massive trend of diminishing mental well-being in younger individuals, suggesting that some societal force is at play that we need to get to the bottom of,” said Dr. Thiagarajan.
“Young people are still learning how to calibrate themselves in the world, and with age comes maturity, leading to a difference in emotional resilience,” she said.
Highest risk group
Mental well-being was poorest among nonbinary/third-gender respondents. Among those persons, more than 50% were classified as being at clinical risk, in comparison with males and females combined, and their MHQ scores were about 47 points lower.
Nonbinary individuals “are universally doing very poorly, relative to males or females,” said Dr. Thiagarajan. “This is a demographic at very high risk with a lot of suicidal thoughts.”
Respondents who had insufficient sleep, who lacked social interaction, and whose level of exercise was insufficient had lower MHQ scores of an “unexpected magnitude,” compared with their counterparts who had sufficient sleep, more social interaction, and more exercise (a discrepancy of 82, 66, and 46 points, respectively).
Only 3.9% of respondents reported having had COVID-19; 0.7% reported having had a severe case. Yet 57% of respondents reported that the pandemic had had negative consequences with regard to their health or their finances or social situation.
Those who were unable to get care for their other health conditions because of the pandemic (2% of all respondents) reported the worst mental well-being, followed by those who struggled for basic necessities (1.4%).
Reduced household income was associated with a 4% lower score but affected a higher percentage of people (17%). Social isolation was associated with a score of about 20 less. Higher rates of lifetime traumas and adversities were likewise associated with lower scores for mental well-being.
Creative, generous approach
Commenting on the survey results, Ken Duckworth, MD, clinical professor at Harvard Medical School, Boston, and chief medical officer of the National Alliance of Mental Illness, noted that the findings were similar to findings from studies in the United States, which showed disproportionately higher rates of mental health problems in younger individuals. Dr. Duckworth was not involved with the survey.
“The idea that this is an international phenomenon and the broad-stroke finding that younger people are suffering across nations is compelling and important for policymakers to look at,” he said.
Dr. Duckworth noted that although the findings are not “representative” of entire populations in a given country, the report is a “first step in a long journey.”
He described the report as “extremely brilliant, creative, and generous, allowing any academician to get access to the data.”
He saw it “less as a definitive report and more as a directionally informative survey that will yield great fruit over time.”
In a comment, Joshua Morganstein, MD, chair of the American Psychiatric Association’s Committee on the Psychiatric Dimensions of Disaster, said: “One of the important things a document like this highlights is the importance of understanding more where risk [for mental health disorders] is concentrated and what things have occurred or might occur that can buffer against that risk or protect us from it. We see that each nation has similar but also different challenges.”
Dr. Thiagarajan is the founder and chief scientist of Sapien Labs. Her coauthors are employees of Sapien Labs. Dr. Duckworth and Dr. Morganstein disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Novel therapeutic target for depression identified
An antiseizure medication appears to reduce anhedonia in patients with depression via a novel mechanism that may offer a new therapeutic target for the disorder, new research suggests.
Results of a small, randomized trial show those who received ezogabine (Potiga) experienced a significant reduction in key measures of depression and anhedonia versus placebo.
Participants in the treatment group also showed a trend toward increased response to reward anticipation on functional MRI (fMRI), compared with those treated with placebo, although the effect did not reach statistical significance.
“Our study was the first randomized, placebo-controlled trial to show that a drug affecting this kind of ion channel in the brain can improve depression and anhedonia in patients,” senior investigator James Murrough, MD, PhD, associate professor of psychiatry and neuroscience at the Icahn School of Medicine at Mount Sinai, New York, said in a press release.
“Targeting this channel represents a completely different mechanism of action than any currently available antidepressant treatment,” said Dr. Murrough, who is also director of the Depression and Anxiety Center for Discovery and Treatment at Mount Sinai.
The study was published online March 3 in the American Journal of Psychiatry.
Need for a novel target
“One of the main issues in treating depression is that many of our current antidepressants have similar mechanisms of action,” Dr. Murrough said in an interview. “Once a patient hasn’t responded to currently available agents, it’s hard think of new medications to fill that need.”
This need for a novel target motivated Dr. Murrough and associates to research the KCNQ2/3 potassium channel, which has not been previously studied as a therapeutic target for depression.
The KCNQ2/3 channel controls brain cell excitability and function by controlling the flow of the electrical charge across the cell membrane in the form of potassium ions, Dr. Murrough explained.
Previous research using a chronic social defeat model of depression in mice showed changes in the KCNQ2/3 channel. “This was key to determining whether a mouse showed depressive behavior in the context of stress, or whether the mice were resistant or resilient to stress,” he said.
Mice resistant to stress showed increased markers in brain regions associated with reward, while the less resilient mice showed excessive excitability and dysfunction. Dysfunction in the brain’s reward system leads to anhedonia, a “core feature” of depressive disorders.
This inspired Dr. Murrough’s group to identify ezogabine, a drug that acts on this channel.
“ for addressing depressive symptoms,” Dr. Murrough said
Nonsignificant trend
The researchers studied 45 adults diagnosed with depression who exhibited significant anhedonia and at least moderate illness severity.
Participants were randomly assigned to receive either ezogabine (n = 21, mean age 44 years at enrollment, 28.3% male) or placebo (n = 24, mean age 39 years at enrollment, 50% male). At baseline and following treatment, participants completed the incentive flanker test under fMRI conditions to model brain activity during anticipation of a reward. In addition, clinical measures of depression and anhedonia were assessed at weekly visits.
The study groups did not differ significantly in performance accuracy during the fMRI task at baseline or following treatment. The table below summarizes the percentage of errors in each group, with standard deviation.
Participants in the ezogabine group showed a numerical increase in ventral striatum activation in response to reward anticipation, compared with participants in the placebo group, but this trend was not considered significant.
Heterogeneous condition
In contrast, there were notable improvements from baseline to the final outcome visit in clinical measures of depression and anhedonia in the ezogabine group, compared with the placebo group. Mean (SD) differences in depression scores, based on the MADRS (Montgomery-Åsberg Depression Rating Scale) from baseline to endpoint as follows: mean difference, –7.9 (3.0); effect size, 0.76; response rate, 61.9% (ezogabine) and 37.5% (placebo); remission rate: 38.1% (ezogabine) and 20.8% (placebo)
Compared with placebo, there were also large improvements in hedonic capacity, as measured by the Snaith-Hamilton Pleasure Scale and the anticipatory subscale of the Temporal Experience of Pleasure Scale (t, –4.1; df, 212; P < .001 and t, 3.4; df, 213; P < .001, respectively).
Compared with placebo, ezogabine was associated with “significant benefit” in global illness severity and improvement (Clinical Global Impression–Severity: t, –2.2; df, 214; P = .026 and CGI-Improvement: t, –2.9; d, 214; P = .004, respectively).
Ezogabine was well tolerated. Dizziness and headache were the most common adverse events.
Depression is a “heterogeneous condition” with a single diagnosis encompassing a “large, multifaceted” array of symptoms, Dr. Murrough noted. A growing body of research is focusing on specific components as potential treatment targets. “Our study looked specifically at patients with a diagnosis of depression but high scores on the anhedonia scale and we found that boosting the function of the KCNQ2/3 channel may have a beneficial antidepressant effect by improving anhedonia.”
Potential gain
In a comment, Alan Schatzberg, MD, professor in the department of psychiatry and behavioral sciences at Stanford (Calif.) University, said that “anytime there’s a new treatment with a new mechanism of action for a given condition, there’s a potential gain for the field.”
Dr. Schatzberg, who was not involved with the study, said that ezogabine, with its “potentially new mechanism of action, seems to have an effect and reasonable safety and could be important for patients who may not respond to traditional medications. It might also be important for all sorts of patients, depending on findings of later trials.”
Dr. Murrough said that ezogabine in still in “early stages” of research. “We hope that future studies will look at other agents that would also affect this channel,” he added.
This research was supported by the National Institute of Mental Health. Additional funding was provided by the Friedman Brain Institute at the Icahn School of Medicine at Mount Sinai and the Ehrenkranz Laboratory for Human Resilience, a component of the Depression and Anxiety Center for Discovery and Treatment at the Icahn School of Medicine at Mount Sinai. Dr. Murrough is an inventor of a pending patent application for the use of ezogabine and other KCNQ channel openers to treat depression and related disorders. Dr. Schatzberg disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
An antiseizure medication appears to reduce anhedonia in patients with depression via a novel mechanism that may offer a new therapeutic target for the disorder, new research suggests.
Results of a small, randomized trial show those who received ezogabine (Potiga) experienced a significant reduction in key measures of depression and anhedonia versus placebo.
Participants in the treatment group also showed a trend toward increased response to reward anticipation on functional MRI (fMRI), compared with those treated with placebo, although the effect did not reach statistical significance.
“Our study was the first randomized, placebo-controlled trial to show that a drug affecting this kind of ion channel in the brain can improve depression and anhedonia in patients,” senior investigator James Murrough, MD, PhD, associate professor of psychiatry and neuroscience at the Icahn School of Medicine at Mount Sinai, New York, said in a press release.
“Targeting this channel represents a completely different mechanism of action than any currently available antidepressant treatment,” said Dr. Murrough, who is also director of the Depression and Anxiety Center for Discovery and Treatment at Mount Sinai.
The study was published online March 3 in the American Journal of Psychiatry.
Need for a novel target
“One of the main issues in treating depression is that many of our current antidepressants have similar mechanisms of action,” Dr. Murrough said in an interview. “Once a patient hasn’t responded to currently available agents, it’s hard think of new medications to fill that need.”
This need for a novel target motivated Dr. Murrough and associates to research the KCNQ2/3 potassium channel, which has not been previously studied as a therapeutic target for depression.
The KCNQ2/3 channel controls brain cell excitability and function by controlling the flow of the electrical charge across the cell membrane in the form of potassium ions, Dr. Murrough explained.
Previous research using a chronic social defeat model of depression in mice showed changes in the KCNQ2/3 channel. “This was key to determining whether a mouse showed depressive behavior in the context of stress, or whether the mice were resistant or resilient to stress,” he said.
Mice resistant to stress showed increased markers in brain regions associated with reward, while the less resilient mice showed excessive excitability and dysfunction. Dysfunction in the brain’s reward system leads to anhedonia, a “core feature” of depressive disorders.
This inspired Dr. Murrough’s group to identify ezogabine, a drug that acts on this channel.
“ for addressing depressive symptoms,” Dr. Murrough said
Nonsignificant trend
The researchers studied 45 adults diagnosed with depression who exhibited significant anhedonia and at least moderate illness severity.
Participants were randomly assigned to receive either ezogabine (n = 21, mean age 44 years at enrollment, 28.3% male) or placebo (n = 24, mean age 39 years at enrollment, 50% male). At baseline and following treatment, participants completed the incentive flanker test under fMRI conditions to model brain activity during anticipation of a reward. In addition, clinical measures of depression and anhedonia were assessed at weekly visits.
The study groups did not differ significantly in performance accuracy during the fMRI task at baseline or following treatment. The table below summarizes the percentage of errors in each group, with standard deviation.
Participants in the ezogabine group showed a numerical increase in ventral striatum activation in response to reward anticipation, compared with participants in the placebo group, but this trend was not considered significant.
Heterogeneous condition
In contrast, there were notable improvements from baseline to the final outcome visit in clinical measures of depression and anhedonia in the ezogabine group, compared with the placebo group. Mean (SD) differences in depression scores, based on the MADRS (Montgomery-Åsberg Depression Rating Scale) from baseline to endpoint as follows: mean difference, –7.9 (3.0); effect size, 0.76; response rate, 61.9% (ezogabine) and 37.5% (placebo); remission rate: 38.1% (ezogabine) and 20.8% (placebo)
Compared with placebo, there were also large improvements in hedonic capacity, as measured by the Snaith-Hamilton Pleasure Scale and the anticipatory subscale of the Temporal Experience of Pleasure Scale (t, –4.1; df, 212; P < .001 and t, 3.4; df, 213; P < .001, respectively).
Compared with placebo, ezogabine was associated with “significant benefit” in global illness severity and improvement (Clinical Global Impression–Severity: t, –2.2; df, 214; P = .026 and CGI-Improvement: t, –2.9; d, 214; P = .004, respectively).
Ezogabine was well tolerated. Dizziness and headache were the most common adverse events.
Depression is a “heterogeneous condition” with a single diagnosis encompassing a “large, multifaceted” array of symptoms, Dr. Murrough noted. A growing body of research is focusing on specific components as potential treatment targets. “Our study looked specifically at patients with a diagnosis of depression but high scores on the anhedonia scale and we found that boosting the function of the KCNQ2/3 channel may have a beneficial antidepressant effect by improving anhedonia.”
Potential gain
In a comment, Alan Schatzberg, MD, professor in the department of psychiatry and behavioral sciences at Stanford (Calif.) University, said that “anytime there’s a new treatment with a new mechanism of action for a given condition, there’s a potential gain for the field.”
Dr. Schatzberg, who was not involved with the study, said that ezogabine, with its “potentially new mechanism of action, seems to have an effect and reasonable safety and could be important for patients who may not respond to traditional medications. It might also be important for all sorts of patients, depending on findings of later trials.”
Dr. Murrough said that ezogabine in still in “early stages” of research. “We hope that future studies will look at other agents that would also affect this channel,” he added.
This research was supported by the National Institute of Mental Health. Additional funding was provided by the Friedman Brain Institute at the Icahn School of Medicine at Mount Sinai and the Ehrenkranz Laboratory for Human Resilience, a component of the Depression and Anxiety Center for Discovery and Treatment at the Icahn School of Medicine at Mount Sinai. Dr. Murrough is an inventor of a pending patent application for the use of ezogabine and other KCNQ channel openers to treat depression and related disorders. Dr. Schatzberg disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
An antiseizure medication appears to reduce anhedonia in patients with depression via a novel mechanism that may offer a new therapeutic target for the disorder, new research suggests.
Results of a small, randomized trial show those who received ezogabine (Potiga) experienced a significant reduction in key measures of depression and anhedonia versus placebo.
Participants in the treatment group also showed a trend toward increased response to reward anticipation on functional MRI (fMRI), compared with those treated with placebo, although the effect did not reach statistical significance.
“Our study was the first randomized, placebo-controlled trial to show that a drug affecting this kind of ion channel in the brain can improve depression and anhedonia in patients,” senior investigator James Murrough, MD, PhD, associate professor of psychiatry and neuroscience at the Icahn School of Medicine at Mount Sinai, New York, said in a press release.
“Targeting this channel represents a completely different mechanism of action than any currently available antidepressant treatment,” said Dr. Murrough, who is also director of the Depression and Anxiety Center for Discovery and Treatment at Mount Sinai.
The study was published online March 3 in the American Journal of Psychiatry.
Need for a novel target
“One of the main issues in treating depression is that many of our current antidepressants have similar mechanisms of action,” Dr. Murrough said in an interview. “Once a patient hasn’t responded to currently available agents, it’s hard think of new medications to fill that need.”
This need for a novel target motivated Dr. Murrough and associates to research the KCNQ2/3 potassium channel, which has not been previously studied as a therapeutic target for depression.
The KCNQ2/3 channel controls brain cell excitability and function by controlling the flow of the electrical charge across the cell membrane in the form of potassium ions, Dr. Murrough explained.
Previous research using a chronic social defeat model of depression in mice showed changes in the KCNQ2/3 channel. “This was key to determining whether a mouse showed depressive behavior in the context of stress, or whether the mice were resistant or resilient to stress,” he said.
Mice resistant to stress showed increased markers in brain regions associated with reward, while the less resilient mice showed excessive excitability and dysfunction. Dysfunction in the brain’s reward system leads to anhedonia, a “core feature” of depressive disorders.
This inspired Dr. Murrough’s group to identify ezogabine, a drug that acts on this channel.
“ for addressing depressive symptoms,” Dr. Murrough said
Nonsignificant trend
The researchers studied 45 adults diagnosed with depression who exhibited significant anhedonia and at least moderate illness severity.
Participants were randomly assigned to receive either ezogabine (n = 21, mean age 44 years at enrollment, 28.3% male) or placebo (n = 24, mean age 39 years at enrollment, 50% male). At baseline and following treatment, participants completed the incentive flanker test under fMRI conditions to model brain activity during anticipation of a reward. In addition, clinical measures of depression and anhedonia were assessed at weekly visits.
The study groups did not differ significantly in performance accuracy during the fMRI task at baseline or following treatment. The table below summarizes the percentage of errors in each group, with standard deviation.
Participants in the ezogabine group showed a numerical increase in ventral striatum activation in response to reward anticipation, compared with participants in the placebo group, but this trend was not considered significant.
Heterogeneous condition
In contrast, there were notable improvements from baseline to the final outcome visit in clinical measures of depression and anhedonia in the ezogabine group, compared with the placebo group. Mean (SD) differences in depression scores, based on the MADRS (Montgomery-Åsberg Depression Rating Scale) from baseline to endpoint as follows: mean difference, –7.9 (3.0); effect size, 0.76; response rate, 61.9% (ezogabine) and 37.5% (placebo); remission rate: 38.1% (ezogabine) and 20.8% (placebo)
Compared with placebo, there were also large improvements in hedonic capacity, as measured by the Snaith-Hamilton Pleasure Scale and the anticipatory subscale of the Temporal Experience of Pleasure Scale (t, –4.1; df, 212; P < .001 and t, 3.4; df, 213; P < .001, respectively).
Compared with placebo, ezogabine was associated with “significant benefit” in global illness severity and improvement (Clinical Global Impression–Severity: t, –2.2; df, 214; P = .026 and CGI-Improvement: t, –2.9; d, 214; P = .004, respectively).
Ezogabine was well tolerated. Dizziness and headache were the most common adverse events.
Depression is a “heterogeneous condition” with a single diagnosis encompassing a “large, multifaceted” array of symptoms, Dr. Murrough noted. A growing body of research is focusing on specific components as potential treatment targets. “Our study looked specifically at patients with a diagnosis of depression but high scores on the anhedonia scale and we found that boosting the function of the KCNQ2/3 channel may have a beneficial antidepressant effect by improving anhedonia.”
Potential gain
In a comment, Alan Schatzberg, MD, professor in the department of psychiatry and behavioral sciences at Stanford (Calif.) University, said that “anytime there’s a new treatment with a new mechanism of action for a given condition, there’s a potential gain for the field.”
Dr. Schatzberg, who was not involved with the study, said that ezogabine, with its “potentially new mechanism of action, seems to have an effect and reasonable safety and could be important for patients who may not respond to traditional medications. It might also be important for all sorts of patients, depending on findings of later trials.”
Dr. Murrough said that ezogabine in still in “early stages” of research. “We hope that future studies will look at other agents that would also affect this channel,” he added.
This research was supported by the National Institute of Mental Health. Additional funding was provided by the Friedman Brain Institute at the Icahn School of Medicine at Mount Sinai and the Ehrenkranz Laboratory for Human Resilience, a component of the Depression and Anxiety Center for Discovery and Treatment at the Icahn School of Medicine at Mount Sinai. Dr. Murrough is an inventor of a pending patent application for the use of ezogabine and other KCNQ channel openers to treat depression and related disorders. Dr. Schatzberg disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Core feature of frontotemporal dementia may aid diagnosis
(FTD) in findings that may help physicians make this difficult diagnosis that affects adults in their prime.
“The assessment of WMH can aid differential diagnosis of bvFTD [behavioral-variant FTD] against other neurodegenerative conditions in the absence of vascular risk factors, especially when considering their spatial distribution,” said senior author Ramón Landin-Romero, PhD, Appenzeller Neuroscience Fellow, Frontotemporal Dementia Research Group, University of Sydney.
“Clinicians can ask for specific sequences in routine MRI scans to visually detect WMH,” said Dr. Landin-Romero, who is also a senior lecturer in the School of Psychology and Brain and Mind Center.
The study was published online Feb. 17 in Neurology.
Difficult diagnosis
“FTD is a collection of unrecognized young-onset (before age 65) dementia syndromes that affect people in their prime,” said Dr. Landin-Romero. He added that heterogeneity in progression trajectories and symptoms, which can include changes in behavior and personality, language impairments, and psychosis, make it a difficult disease to diagnose.
“As such, our research was motivated by the need of sensitive and specific biomarkers of FTD, which are urgently needed to aid diagnosis, prognosis, and treatment development,” he said.
Previous research has been limited; there have only been a “handful” of cohort and case studies and studies involving individuals with mutations in one FTD-causative gene.
FTD is genetically and pathologically complex, and there has been no clear correlation between genetic mutations/underlying pathology and clinical presentation, Dr. Landin-Romero said.
WMH are common in older individuals and are linked to increased risk for cognitive impairment and dementia. Traditionally, they have been associated with vascular risk factors, such as smoking and diabetes. “But the presentation of WMH in FTD and its associations with the severity of symptoms and brain atrophy across FTD symptoms remains to be established,” said Dr. Landin-Romero.
Higher disease severity
To explore the possible association, the researchers studied 129 patients with either bvFTD (n = 64; mean age, 64 years) or Alzheimer’s disease (n = 65; mean age, 64.66 years).
Neuropsychological assessments, medical and neurologic examinations, clinical interview, and structural brain MRI were conducted for all patients, who were compared with 66 age-, sex-, and education-matched healthy control persons (mean age, 64.69 years).
Some participants in the FTD, Alzheimer’s disease, and healthy control groups (n = 54, 44, and 26, respectively) also underwent genetic screening. Postmortem pathology findings were available for a small number of FTD and Alzheimer’s disease participants (n = 13 and 5, respectively).
The medical history included lifestyle and cardiovascular risk factors, as well as other health and neurologic conditions and medication history. Hypertension, hypercholesterolemia, diabetes, and smoking were used to assess vascular risk.
The FTD and Alzheimer’s disease groups did not differ with regard to disease duration (3.55 years; standard deviation, 1.75, and 3.24 years; SD, 1.59, respectively). However, disease severity was significantly higher among those with FTD than among those with Alzheimer’s disease, as measured by the FTD Rating Scale Rasch score (–0.52; SD, 1.28, vs. 0.78; SD, 1.55; P < .001).
Compared with healthy controls, patients in the FTD and Alzheimer’s disease groups scored significantly lower on the Addenbrooke’s Cognitive Examination–Revised (ACE-R) or ACE-III scale. Patients with Alzheimer’s disease showed “disproportionately larger deficits” in memory and visuospatial processing, compared with those with FTD, whereas those with FTD performed significantly worse than those with Alzheimer’s disease in the fluency subdomain.
A larger number of patients in the FTD group screened positive for genetic abnormalities than in the Alzheimer’s disease group; no participants in the healthy control group had genetic mutations.
Unexpected findings
Mean WMH volume was significantly higher in participants with FTD than in participants with Alzheimer’s disease and in healthy controls (mean, 0.76 mL, 0.40 mL, and 0.12 mL respectively). These larger volumes contributed to greater disease severity and cortical atrophy. Moreover, disease severity was “found to be a strong predictor of WMH volume in FTD,” the authors stated. Among patients with FTD, WMH volumes did not differ significantly with regard to genetic mutation status or presence of strong family history.
After controlling for age, vascular risk did not significantly predict WMH volume in the FTD group (P = .16); however, that did not hold true in the Alzheimer’s disease group.
Increased WMH were associated with anterior brain regions in FTD and with posterior brain regions in Alzheimer’s disease. In both disorders, higher WMH volume in the corpus callosum was associated with poorer cognitive performance in the domain of attention.
“The spatial distribution of WMH mirrored patterns of brain atrophy in FTD and Alzheimer’s disease, was partially independent of cortical degeneration, and was correlated with cognitive deficits,” said Dr. Landin-Romero.
The findings were not what he and his research colleagues expected. “We were expecting that the amounts of WMH would be similar in FTD and Alzheimer’s disease, but we actually found higher levels in participants with FTD,” he said. Additionally, he anticipated that patients with either FTD or Alzheimer’s disease who had more severe disease would have more WMH, but that finding only held true for people with FTD.
“In sum, our findings show that WMH are a core feature of FTD and Alzheimer’s disease that can contribute to cognitive problems, and not simply as a marker of vascular disease,” said Dr. Landin-Romero.
Major research contribution
Commenting on the study, Jordi Matias-Guiu, PhD, MD, of the department of neurology, Hospital Clinico, San Carlos, Spain, considers the study to be a “great contribution to the field.” Dr. Matias-Guiu, who was not involved with the study, said that WMH “do not necessarily mean vascular pathology, and atrophy may partially explain these abnormalities and should be taken into account in the interpretation of brain MRI.
“WMH are present in both Alzheimer’s disease and FTD and are relevant to cognitive deficits found in these disorders,” he added.
The study was funded by grants from the National Health and Medical Research Council of Australia, the Dementia Research Team, and the ARC Center of Excellence in Cognition and Its Disorders. Dr. Landin-Romero is supported by the Appenzeller Neuroscience Fellowship in Alzheimer’s Disease and the ARC Center of Excellence in Cognition and Its Disorders Memory Program. The other authors’ disclosures are listed on the original article. Dr. Matias-Guiu reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
(FTD) in findings that may help physicians make this difficult diagnosis that affects adults in their prime.
“The assessment of WMH can aid differential diagnosis of bvFTD [behavioral-variant FTD] against other neurodegenerative conditions in the absence of vascular risk factors, especially when considering their spatial distribution,” said senior author Ramón Landin-Romero, PhD, Appenzeller Neuroscience Fellow, Frontotemporal Dementia Research Group, University of Sydney.
“Clinicians can ask for specific sequences in routine MRI scans to visually detect WMH,” said Dr. Landin-Romero, who is also a senior lecturer in the School of Psychology and Brain and Mind Center.
The study was published online Feb. 17 in Neurology.
Difficult diagnosis
“FTD is a collection of unrecognized young-onset (before age 65) dementia syndromes that affect people in their prime,” said Dr. Landin-Romero. He added that heterogeneity in progression trajectories and symptoms, which can include changes in behavior and personality, language impairments, and psychosis, make it a difficult disease to diagnose.
“As such, our research was motivated by the need of sensitive and specific biomarkers of FTD, which are urgently needed to aid diagnosis, prognosis, and treatment development,” he said.
Previous research has been limited; there have only been a “handful” of cohort and case studies and studies involving individuals with mutations in one FTD-causative gene.
FTD is genetically and pathologically complex, and there has been no clear correlation between genetic mutations/underlying pathology and clinical presentation, Dr. Landin-Romero said.
WMH are common in older individuals and are linked to increased risk for cognitive impairment and dementia. Traditionally, they have been associated with vascular risk factors, such as smoking and diabetes. “But the presentation of WMH in FTD and its associations with the severity of symptoms and brain atrophy across FTD symptoms remains to be established,” said Dr. Landin-Romero.
Higher disease severity
To explore the possible association, the researchers studied 129 patients with either bvFTD (n = 64; mean age, 64 years) or Alzheimer’s disease (n = 65; mean age, 64.66 years).
Neuropsychological assessments, medical and neurologic examinations, clinical interview, and structural brain MRI were conducted for all patients, who were compared with 66 age-, sex-, and education-matched healthy control persons (mean age, 64.69 years).
Some participants in the FTD, Alzheimer’s disease, and healthy control groups (n = 54, 44, and 26, respectively) also underwent genetic screening. Postmortem pathology findings were available for a small number of FTD and Alzheimer’s disease participants (n = 13 and 5, respectively).
The medical history included lifestyle and cardiovascular risk factors, as well as other health and neurologic conditions and medication history. Hypertension, hypercholesterolemia, diabetes, and smoking were used to assess vascular risk.
The FTD and Alzheimer’s disease groups did not differ with regard to disease duration (3.55 years; standard deviation, 1.75, and 3.24 years; SD, 1.59, respectively). However, disease severity was significantly higher among those with FTD than among those with Alzheimer’s disease, as measured by the FTD Rating Scale Rasch score (–0.52; SD, 1.28, vs. 0.78; SD, 1.55; P < .001).
Compared with healthy controls, patients in the FTD and Alzheimer’s disease groups scored significantly lower on the Addenbrooke’s Cognitive Examination–Revised (ACE-R) or ACE-III scale. Patients with Alzheimer’s disease showed “disproportionately larger deficits” in memory and visuospatial processing, compared with those with FTD, whereas those with FTD performed significantly worse than those with Alzheimer’s disease in the fluency subdomain.
A larger number of patients in the FTD group screened positive for genetic abnormalities than in the Alzheimer’s disease group; no participants in the healthy control group had genetic mutations.
Unexpected findings
Mean WMH volume was significantly higher in participants with FTD than in participants with Alzheimer’s disease and in healthy controls (mean, 0.76 mL, 0.40 mL, and 0.12 mL respectively). These larger volumes contributed to greater disease severity and cortical atrophy. Moreover, disease severity was “found to be a strong predictor of WMH volume in FTD,” the authors stated. Among patients with FTD, WMH volumes did not differ significantly with regard to genetic mutation status or presence of strong family history.
After controlling for age, vascular risk did not significantly predict WMH volume in the FTD group (P = .16); however, that did not hold true in the Alzheimer’s disease group.
Increased WMH were associated with anterior brain regions in FTD and with posterior brain regions in Alzheimer’s disease. In both disorders, higher WMH volume in the corpus callosum was associated with poorer cognitive performance in the domain of attention.
“The spatial distribution of WMH mirrored patterns of brain atrophy in FTD and Alzheimer’s disease, was partially independent of cortical degeneration, and was correlated with cognitive deficits,” said Dr. Landin-Romero.
The findings were not what he and his research colleagues expected. “We were expecting that the amounts of WMH would be similar in FTD and Alzheimer’s disease, but we actually found higher levels in participants with FTD,” he said. Additionally, he anticipated that patients with either FTD or Alzheimer’s disease who had more severe disease would have more WMH, but that finding only held true for people with FTD.
“In sum, our findings show that WMH are a core feature of FTD and Alzheimer’s disease that can contribute to cognitive problems, and not simply as a marker of vascular disease,” said Dr. Landin-Romero.
Major research contribution
Commenting on the study, Jordi Matias-Guiu, PhD, MD, of the department of neurology, Hospital Clinico, San Carlos, Spain, considers the study to be a “great contribution to the field.” Dr. Matias-Guiu, who was not involved with the study, said that WMH “do not necessarily mean vascular pathology, and atrophy may partially explain these abnormalities and should be taken into account in the interpretation of brain MRI.
“WMH are present in both Alzheimer’s disease and FTD and are relevant to cognitive deficits found in these disorders,” he added.
The study was funded by grants from the National Health and Medical Research Council of Australia, the Dementia Research Team, and the ARC Center of Excellence in Cognition and Its Disorders. Dr. Landin-Romero is supported by the Appenzeller Neuroscience Fellowship in Alzheimer’s Disease and the ARC Center of Excellence in Cognition and Its Disorders Memory Program. The other authors’ disclosures are listed on the original article. Dr. Matias-Guiu reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
(FTD) in findings that may help physicians make this difficult diagnosis that affects adults in their prime.
“The assessment of WMH can aid differential diagnosis of bvFTD [behavioral-variant FTD] against other neurodegenerative conditions in the absence of vascular risk factors, especially when considering their spatial distribution,” said senior author Ramón Landin-Romero, PhD, Appenzeller Neuroscience Fellow, Frontotemporal Dementia Research Group, University of Sydney.
“Clinicians can ask for specific sequences in routine MRI scans to visually detect WMH,” said Dr. Landin-Romero, who is also a senior lecturer in the School of Psychology and Brain and Mind Center.
The study was published online Feb. 17 in Neurology.
Difficult diagnosis
“FTD is a collection of unrecognized young-onset (before age 65) dementia syndromes that affect people in their prime,” said Dr. Landin-Romero. He added that heterogeneity in progression trajectories and symptoms, which can include changes in behavior and personality, language impairments, and psychosis, make it a difficult disease to diagnose.
“As such, our research was motivated by the need of sensitive and specific biomarkers of FTD, which are urgently needed to aid diagnosis, prognosis, and treatment development,” he said.
Previous research has been limited; there have only been a “handful” of cohort and case studies and studies involving individuals with mutations in one FTD-causative gene.
FTD is genetically and pathologically complex, and there has been no clear correlation between genetic mutations/underlying pathology and clinical presentation, Dr. Landin-Romero said.
WMH are common in older individuals and are linked to increased risk for cognitive impairment and dementia. Traditionally, they have been associated with vascular risk factors, such as smoking and diabetes. “But the presentation of WMH in FTD and its associations with the severity of symptoms and brain atrophy across FTD symptoms remains to be established,” said Dr. Landin-Romero.
Higher disease severity
To explore the possible association, the researchers studied 129 patients with either bvFTD (n = 64; mean age, 64 years) or Alzheimer’s disease (n = 65; mean age, 64.66 years).
Neuropsychological assessments, medical and neurologic examinations, clinical interview, and structural brain MRI were conducted for all patients, who were compared with 66 age-, sex-, and education-matched healthy control persons (mean age, 64.69 years).
Some participants in the FTD, Alzheimer’s disease, and healthy control groups (n = 54, 44, and 26, respectively) also underwent genetic screening. Postmortem pathology findings were available for a small number of FTD and Alzheimer’s disease participants (n = 13 and 5, respectively).
The medical history included lifestyle and cardiovascular risk factors, as well as other health and neurologic conditions and medication history. Hypertension, hypercholesterolemia, diabetes, and smoking were used to assess vascular risk.
The FTD and Alzheimer’s disease groups did not differ with regard to disease duration (3.55 years; standard deviation, 1.75, and 3.24 years; SD, 1.59, respectively). However, disease severity was significantly higher among those with FTD than among those with Alzheimer’s disease, as measured by the FTD Rating Scale Rasch score (–0.52; SD, 1.28, vs. 0.78; SD, 1.55; P < .001).
Compared with healthy controls, patients in the FTD and Alzheimer’s disease groups scored significantly lower on the Addenbrooke’s Cognitive Examination–Revised (ACE-R) or ACE-III scale. Patients with Alzheimer’s disease showed “disproportionately larger deficits” in memory and visuospatial processing, compared with those with FTD, whereas those with FTD performed significantly worse than those with Alzheimer’s disease in the fluency subdomain.
A larger number of patients in the FTD group screened positive for genetic abnormalities than in the Alzheimer’s disease group; no participants in the healthy control group had genetic mutations.
Unexpected findings
Mean WMH volume was significantly higher in participants with FTD than in participants with Alzheimer’s disease and in healthy controls (mean, 0.76 mL, 0.40 mL, and 0.12 mL respectively). These larger volumes contributed to greater disease severity and cortical atrophy. Moreover, disease severity was “found to be a strong predictor of WMH volume in FTD,” the authors stated. Among patients with FTD, WMH volumes did not differ significantly with regard to genetic mutation status or presence of strong family history.
After controlling for age, vascular risk did not significantly predict WMH volume in the FTD group (P = .16); however, that did not hold true in the Alzheimer’s disease group.
Increased WMH were associated with anterior brain regions in FTD and with posterior brain regions in Alzheimer’s disease. In both disorders, higher WMH volume in the corpus callosum was associated with poorer cognitive performance in the domain of attention.
“The spatial distribution of WMH mirrored patterns of brain atrophy in FTD and Alzheimer’s disease, was partially independent of cortical degeneration, and was correlated with cognitive deficits,” said Dr. Landin-Romero.
The findings were not what he and his research colleagues expected. “We were expecting that the amounts of WMH would be similar in FTD and Alzheimer’s disease, but we actually found higher levels in participants with FTD,” he said. Additionally, he anticipated that patients with either FTD or Alzheimer’s disease who had more severe disease would have more WMH, but that finding only held true for people with FTD.
“In sum, our findings show that WMH are a core feature of FTD and Alzheimer’s disease that can contribute to cognitive problems, and not simply as a marker of vascular disease,” said Dr. Landin-Romero.
Major research contribution
Commenting on the study, Jordi Matias-Guiu, PhD, MD, of the department of neurology, Hospital Clinico, San Carlos, Spain, considers the study to be a “great contribution to the field.” Dr. Matias-Guiu, who was not involved with the study, said that WMH “do not necessarily mean vascular pathology, and atrophy may partially explain these abnormalities and should be taken into account in the interpretation of brain MRI.
“WMH are present in both Alzheimer’s disease and FTD and are relevant to cognitive deficits found in these disorders,” he added.
The study was funded by grants from the National Health and Medical Research Council of Australia, the Dementia Research Team, and the ARC Center of Excellence in Cognition and Its Disorders. Dr. Landin-Romero is supported by the Appenzeller Neuroscience Fellowship in Alzheimer’s Disease and the ARC Center of Excellence in Cognition and Its Disorders Memory Program. The other authors’ disclosures are listed on the original article. Dr. Matias-Guiu reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Strep A and tic worsening: Final word?
Exposure to Group A streptococcus (GAS) does not appear to worsen symptoms of Tourette syndrome and other chronic tic disorders (CTDs) in children and adolescents, new research suggests.
Investigators studied over 700 children and teenagers with CTDs, one-third of whom also had attention deficit hyperactivity disorder and one-third who had obsessive-compulsive disorder (OCD).
The youngsters were followed for an average of 16 months and evaluated at 4-month intervals to see if they were infected with GAS. Tic severity was monitored through telephone interviews, in-person visits, and parental reports.
A little less than half the children experienced worsening of tics during the study period, but the researchers found no association between these exacerbations and GAS exposure.
There was also no link between GAS and worsening OCD. However, researchers did find an association between GAS exposure and an increase in hyperactivity and impulsivity in patients with ADHD.
“This study does not support GAS exposures as contributing factors for tic exacerbations in children with CTD,” the authors note.
“Specific work-up or active management of GAS infections is unlikely to help modifying the course of tics in CTD and is therefore not recommended,” they conclude.
The study was published online in Neurology.
‘Intense debate’
The association between GAS and CTD stems from the description of Pediatric Autoimmune Neuropsychiatric Disorders Associated with Streptococcal infection (PANDAS) – a condition that is now incorporated in the pediatric acute neuropsychiatric syndromes (PANS), the authors note. Tics constitute an “accompanying feature” of this condition.
However, neither population-based nor longitudinal clinical studies “could definitely establish if tic exacerbations in CTD are associated with GAS infections,” they note.
“The link between streptococcus and tics in children is still a matter of intense debate,” said study author Davide Martino, MD, PhD, director of the Movement Disorders Program at the University of Calgary (Alta.), in a press release.
“We wanted to look at that question, as well as a possible link between strep and behavioral symptoms like obsessive-compulsive disorder and attention deficit hyperactivity disorder,” he said.
The researchers followed 715 children with CTD (mean age 10.7 years, 76.8% male) who were drawn from 16 specialist clinics in nine countries. Almost all (90.8%) had a diagnosis of Tourette syndrome (TS); 31.7% had OCD, and 36.1% had ADHD.
Participants received a throat swab at baseline, and of these, 8.4% tested positive for GAS.
Participants were evaluated over a 16- to 18-month period, consisting of:
- Face-to-face interviews and collection of throat swabs and serum at 4-month intervals.
- Telephone interviews at 4-month intervals, which took place at 2 months between study visit.
- Weekly diaries: Parents were asked to indicate any worsening of tics and focus on detecting the earliest possible tic exacerbation.
Beyond the regularly scheduled visits, parents were instructed to report, by phone or email, any noticeable increase in tic severity and then attend an in-person visit.
Tic exacerbations were defined as an increase of greater than or equal to 6 points on the Yale Global Tic Severity Scale-Total Tic Severity Score (YGTSS-TTS), compared with the previous assessment.
OCD and ADHD symptoms were assessed according to the Yale-Brown Obsessive-Compulsive Scale and the parent-reported Swanson, Nolan, and Pelham-IV (SNAP-IV) questionnaire.
The researchers divided GAS exposures into four categories: new definite exposure; new possible exposure; ongoing definite exposure; and ongoing possible exposure.
Unlikely trigger
During the follow-up period, 43.1% (n = 308) of participants experienced tic exacerbations. Of these, 218 participants experienced one exacerbation, while 90 participants experienced two, three, or four exacerbations.
The researchers did not find a significant association between GAS exposure status and tic exacerbation.
Participants who did develop a GAS-associated exacerbation (n = 49) were younger at study exit (9.63 vs. 11.4 years, P < .0001) and were more likely to be male (46/49 vs. 210/259, Fisher’s = .035), compared with participants who developed a non-GAS-associated tic exacerbation (n = 259).
Additional analyses were adjusted for sex, age at onset, exposure to psychotropic medications, exposures to antibiotics, geographical regions, and number of visits in the time interval of interest. These analyses continued to yield no significant association between new or ongoing concurrent GAS exposure episodes and tic exacerbation events.
Of the children in the study, 103 had a positive throat swab, indicating a new definite GAS exposure, whereas 46 had a positive throat swab indicating an ongoing definite exposure (n = 149 visits). Of these visits, only 20 corresponded to tic exacerbations.
There was also no association between GAS exposure and OCD symptom severity. However, it was associated with longitudinal changes (between 17% and 21%, depending on GAS exposure definition) in the severity of hyperactivity-impulsivity symptoms in children with ADHD.
“It is known that immune activation may concur with tic severity in youth with CTDs and that psychosocial stress levels may predict short-term future tic severity in these patients,” the authors write.
“Our findings suggest that GAS is unlikely to be the main trigger for immune activation in these patients,” they add.
Brick or cornerstone?
Commenting on the study for this news organization, Margo Thienemann, MD, clinical professor of psychiatry, Stanford (Calif.) University, said that in the clinic population they treat, GAS, other pathogens, and other stresses can “each be associated with PANS symptom exacerbations.”
However, these “would not be likely to cause PANS symptoms exacerbations in the vast majority of individuals, only individuals with genetic backgrounds and immunologic dysfunctions creating susceptibility,” said Dr. Thienemann, who also directs the Pediatric Acute-Onset Neuropsychiatric Syndrome (PANS) Clinic at Stanford Children’s Health. She was not involved with the study.
In an accompanying editorial, Andrea Cavanna, MD, PhD, honorary reader in neuropsychiatry, Birmingham (England) Medical School and Keith Coffman, MD, director, Tourette Syndrome Center of Excellence, Children’s Mercy Hospital, Kansas City, Mo., suggest that perhaps the “interaction of psychosocial stress and GAS infections contributes more to tic exacerbation than psychosocial stress alone.”
“Time will tell whether this study stands as another brick – a cornerstone? – in the wall that separates streptococcus from tics,” they write.
The study was supported by the European Union’s Seventh Framework Program. Dr. Martino has received honoraria for lecturing from the Movement Disorders Society, Tourette Syndrome Association of America, and Dystonia Medical Research Foundation Canada; research funding support from Dystonia Medical Research Foundation Canada, the University of Calgary (Alta.), the Michael P. Smith Family, the Owerko Foundation, Ipsen Corporate, the Parkinson Association of Alberta, and the Canadian Institutes for Health Research; and royalties from Springer-Verlag. The other authors’ disclosures are listed in the original article. Dr. Cavanna, Dr. Coffman, and Dr. Thienemann have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Exposure to Group A streptococcus (GAS) does not appear to worsen symptoms of Tourette syndrome and other chronic tic disorders (CTDs) in children and adolescents, new research suggests.
Investigators studied over 700 children and teenagers with CTDs, one-third of whom also had attention deficit hyperactivity disorder and one-third who had obsessive-compulsive disorder (OCD).
The youngsters were followed for an average of 16 months and evaluated at 4-month intervals to see if they were infected with GAS. Tic severity was monitored through telephone interviews, in-person visits, and parental reports.
A little less than half the children experienced worsening of tics during the study period, but the researchers found no association between these exacerbations and GAS exposure.
There was also no link between GAS and worsening OCD. However, researchers did find an association between GAS exposure and an increase in hyperactivity and impulsivity in patients with ADHD.
“This study does not support GAS exposures as contributing factors for tic exacerbations in children with CTD,” the authors note.
“Specific work-up or active management of GAS infections is unlikely to help modifying the course of tics in CTD and is therefore not recommended,” they conclude.
The study was published online in Neurology.
‘Intense debate’
The association between GAS and CTD stems from the description of Pediatric Autoimmune Neuropsychiatric Disorders Associated with Streptococcal infection (PANDAS) – a condition that is now incorporated in the pediatric acute neuropsychiatric syndromes (PANS), the authors note. Tics constitute an “accompanying feature” of this condition.
However, neither population-based nor longitudinal clinical studies “could definitely establish if tic exacerbations in CTD are associated with GAS infections,” they note.
“The link between streptococcus and tics in children is still a matter of intense debate,” said study author Davide Martino, MD, PhD, director of the Movement Disorders Program at the University of Calgary (Alta.), in a press release.
“We wanted to look at that question, as well as a possible link between strep and behavioral symptoms like obsessive-compulsive disorder and attention deficit hyperactivity disorder,” he said.
The researchers followed 715 children with CTD (mean age 10.7 years, 76.8% male) who were drawn from 16 specialist clinics in nine countries. Almost all (90.8%) had a diagnosis of Tourette syndrome (TS); 31.7% had OCD, and 36.1% had ADHD.
Participants received a throat swab at baseline, and of these, 8.4% tested positive for GAS.
Participants were evaluated over a 16- to 18-month period, consisting of:
- Face-to-face interviews and collection of throat swabs and serum at 4-month intervals.
- Telephone interviews at 4-month intervals, which took place at 2 months between study visit.
- Weekly diaries: Parents were asked to indicate any worsening of tics and focus on detecting the earliest possible tic exacerbation.
Beyond the regularly scheduled visits, parents were instructed to report, by phone or email, any noticeable increase in tic severity and then attend an in-person visit.
Tic exacerbations were defined as an increase of greater than or equal to 6 points on the Yale Global Tic Severity Scale-Total Tic Severity Score (YGTSS-TTS), compared with the previous assessment.
OCD and ADHD symptoms were assessed according to the Yale-Brown Obsessive-Compulsive Scale and the parent-reported Swanson, Nolan, and Pelham-IV (SNAP-IV) questionnaire.
The researchers divided GAS exposures into four categories: new definite exposure; new possible exposure; ongoing definite exposure; and ongoing possible exposure.
Unlikely trigger
During the follow-up period, 43.1% (n = 308) of participants experienced tic exacerbations. Of these, 218 participants experienced one exacerbation, while 90 participants experienced two, three, or four exacerbations.
The researchers did not find a significant association between GAS exposure status and tic exacerbation.
Participants who did develop a GAS-associated exacerbation (n = 49) were younger at study exit (9.63 vs. 11.4 years, P < .0001) and were more likely to be male (46/49 vs. 210/259, Fisher’s = .035), compared with participants who developed a non-GAS-associated tic exacerbation (n = 259).
Additional analyses were adjusted for sex, age at onset, exposure to psychotropic medications, exposures to antibiotics, geographical regions, and number of visits in the time interval of interest. These analyses continued to yield no significant association between new or ongoing concurrent GAS exposure episodes and tic exacerbation events.
Of the children in the study, 103 had a positive throat swab, indicating a new definite GAS exposure, whereas 46 had a positive throat swab indicating an ongoing definite exposure (n = 149 visits). Of these visits, only 20 corresponded to tic exacerbations.
There was also no association between GAS exposure and OCD symptom severity. However, it was associated with longitudinal changes (between 17% and 21%, depending on GAS exposure definition) in the severity of hyperactivity-impulsivity symptoms in children with ADHD.
“It is known that immune activation may concur with tic severity in youth with CTDs and that psychosocial stress levels may predict short-term future tic severity in these patients,” the authors write.
“Our findings suggest that GAS is unlikely to be the main trigger for immune activation in these patients,” they add.
Brick or cornerstone?
Commenting on the study for this news organization, Margo Thienemann, MD, clinical professor of psychiatry, Stanford (Calif.) University, said that in the clinic population they treat, GAS, other pathogens, and other stresses can “each be associated with PANS symptom exacerbations.”
However, these “would not be likely to cause PANS symptoms exacerbations in the vast majority of individuals, only individuals with genetic backgrounds and immunologic dysfunctions creating susceptibility,” said Dr. Thienemann, who also directs the Pediatric Acute-Onset Neuropsychiatric Syndrome (PANS) Clinic at Stanford Children’s Health. She was not involved with the study.
In an accompanying editorial, Andrea Cavanna, MD, PhD, honorary reader in neuropsychiatry, Birmingham (England) Medical School and Keith Coffman, MD, director, Tourette Syndrome Center of Excellence, Children’s Mercy Hospital, Kansas City, Mo., suggest that perhaps the “interaction of psychosocial stress and GAS infections contributes more to tic exacerbation than psychosocial stress alone.”
“Time will tell whether this study stands as another brick – a cornerstone? – in the wall that separates streptococcus from tics,” they write.
The study was supported by the European Union’s Seventh Framework Program. Dr. Martino has received honoraria for lecturing from the Movement Disorders Society, Tourette Syndrome Association of America, and Dystonia Medical Research Foundation Canada; research funding support from Dystonia Medical Research Foundation Canada, the University of Calgary (Alta.), the Michael P. Smith Family, the Owerko Foundation, Ipsen Corporate, the Parkinson Association of Alberta, and the Canadian Institutes for Health Research; and royalties from Springer-Verlag. The other authors’ disclosures are listed in the original article. Dr. Cavanna, Dr. Coffman, and Dr. Thienemann have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Exposure to Group A streptococcus (GAS) does not appear to worsen symptoms of Tourette syndrome and other chronic tic disorders (CTDs) in children and adolescents, new research suggests.
Investigators studied over 700 children and teenagers with CTDs, one-third of whom also had attention deficit hyperactivity disorder and one-third who had obsessive-compulsive disorder (OCD).
The youngsters were followed for an average of 16 months and evaluated at 4-month intervals to see if they were infected with GAS. Tic severity was monitored through telephone interviews, in-person visits, and parental reports.
A little less than half the children experienced worsening of tics during the study period, but the researchers found no association between these exacerbations and GAS exposure.
There was also no link between GAS and worsening OCD. However, researchers did find an association between GAS exposure and an increase in hyperactivity and impulsivity in patients with ADHD.
“This study does not support GAS exposures as contributing factors for tic exacerbations in children with CTD,” the authors note.
“Specific work-up or active management of GAS infections is unlikely to help modifying the course of tics in CTD and is therefore not recommended,” they conclude.
The study was published online in Neurology.
‘Intense debate’
The association between GAS and CTD stems from the description of Pediatric Autoimmune Neuropsychiatric Disorders Associated with Streptococcal infection (PANDAS) – a condition that is now incorporated in the pediatric acute neuropsychiatric syndromes (PANS), the authors note. Tics constitute an “accompanying feature” of this condition.
However, neither population-based nor longitudinal clinical studies “could definitely establish if tic exacerbations in CTD are associated with GAS infections,” they note.
“The link between streptococcus and tics in children is still a matter of intense debate,” said study author Davide Martino, MD, PhD, director of the Movement Disorders Program at the University of Calgary (Alta.), in a press release.
“We wanted to look at that question, as well as a possible link between strep and behavioral symptoms like obsessive-compulsive disorder and attention deficit hyperactivity disorder,” he said.
The researchers followed 715 children with CTD (mean age 10.7 years, 76.8% male) who were drawn from 16 specialist clinics in nine countries. Almost all (90.8%) had a diagnosis of Tourette syndrome (TS); 31.7% had OCD, and 36.1% had ADHD.
Participants received a throat swab at baseline, and of these, 8.4% tested positive for GAS.
Participants were evaluated over a 16- to 18-month period, consisting of:
- Face-to-face interviews and collection of throat swabs and serum at 4-month intervals.
- Telephone interviews at 4-month intervals, which took place at 2 months between study visit.
- Weekly diaries: Parents were asked to indicate any worsening of tics and focus on detecting the earliest possible tic exacerbation.
Beyond the regularly scheduled visits, parents were instructed to report, by phone or email, any noticeable increase in tic severity and then attend an in-person visit.
Tic exacerbations were defined as an increase of greater than or equal to 6 points on the Yale Global Tic Severity Scale-Total Tic Severity Score (YGTSS-TTS), compared with the previous assessment.
OCD and ADHD symptoms were assessed according to the Yale-Brown Obsessive-Compulsive Scale and the parent-reported Swanson, Nolan, and Pelham-IV (SNAP-IV) questionnaire.
The researchers divided GAS exposures into four categories: new definite exposure; new possible exposure; ongoing definite exposure; and ongoing possible exposure.
Unlikely trigger
During the follow-up period, 43.1% (n = 308) of participants experienced tic exacerbations. Of these, 218 participants experienced one exacerbation, while 90 participants experienced two, three, or four exacerbations.
The researchers did not find a significant association between GAS exposure status and tic exacerbation.
Participants who did develop a GAS-associated exacerbation (n = 49) were younger at study exit (9.63 vs. 11.4 years, P < .0001) and were more likely to be male (46/49 vs. 210/259, Fisher’s = .035), compared with participants who developed a non-GAS-associated tic exacerbation (n = 259).
Additional analyses were adjusted for sex, age at onset, exposure to psychotropic medications, exposures to antibiotics, geographical regions, and number of visits in the time interval of interest. These analyses continued to yield no significant association between new or ongoing concurrent GAS exposure episodes and tic exacerbation events.
Of the children in the study, 103 had a positive throat swab, indicating a new definite GAS exposure, whereas 46 had a positive throat swab indicating an ongoing definite exposure (n = 149 visits). Of these visits, only 20 corresponded to tic exacerbations.
There was also no association between GAS exposure and OCD symptom severity. However, it was associated with longitudinal changes (between 17% and 21%, depending on GAS exposure definition) in the severity of hyperactivity-impulsivity symptoms in children with ADHD.
“It is known that immune activation may concur with tic severity in youth with CTDs and that psychosocial stress levels may predict short-term future tic severity in these patients,” the authors write.
“Our findings suggest that GAS is unlikely to be the main trigger for immune activation in these patients,” they add.
Brick or cornerstone?
Commenting on the study for this news organization, Margo Thienemann, MD, clinical professor of psychiatry, Stanford (Calif.) University, said that in the clinic population they treat, GAS, other pathogens, and other stresses can “each be associated with PANS symptom exacerbations.”
However, these “would not be likely to cause PANS symptoms exacerbations in the vast majority of individuals, only individuals with genetic backgrounds and immunologic dysfunctions creating susceptibility,” said Dr. Thienemann, who also directs the Pediatric Acute-Onset Neuropsychiatric Syndrome (PANS) Clinic at Stanford Children’s Health. She was not involved with the study.
In an accompanying editorial, Andrea Cavanna, MD, PhD, honorary reader in neuropsychiatry, Birmingham (England) Medical School and Keith Coffman, MD, director, Tourette Syndrome Center of Excellence, Children’s Mercy Hospital, Kansas City, Mo., suggest that perhaps the “interaction of psychosocial stress and GAS infections contributes more to tic exacerbation than psychosocial stress alone.”
“Time will tell whether this study stands as another brick – a cornerstone? – in the wall that separates streptococcus from tics,” they write.
The study was supported by the European Union’s Seventh Framework Program. Dr. Martino has received honoraria for lecturing from the Movement Disorders Society, Tourette Syndrome Association of America, and Dystonia Medical Research Foundation Canada; research funding support from Dystonia Medical Research Foundation Canada, the University of Calgary (Alta.), the Michael P. Smith Family, the Owerko Foundation, Ipsen Corporate, the Parkinson Association of Alberta, and the Canadian Institutes for Health Research; and royalties from Springer-Verlag. The other authors’ disclosures are listed in the original article. Dr. Cavanna, Dr. Coffman, and Dr. Thienemann have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Higher dietary fiber tied to lower depression risk in young women
Higher fiber intake may be associated with decreased risk of depression in premenopausal women, new research suggests.
Investigators analyzed data from close to 6,000 pre- and postmenopausal women. They found that, in premenopausal women, dietary fiber intake was higher among those without depression versus their counterparts with the disorder in a dose-dependent manner. However, there appeared to be no relationship between higher fiber intake and depression risk in postmenopausal women.
“We think the most important finding of our study is that dietary fiber intake was inversely associated with depression in premenopausal but not postmenopausal women,” lead author Yunsun Kim, MD, resident, department of family medicine, Chung-Ang University Hospital, Seoul, South Korea, said in an interview.
“We hope that the findings of this study could form the basis of future investigations to determine the causal relationship between dietary fiber intake and depression,” she added.
The study was published online Dec. 21, 2020, in Menopause.
Gut-brain interaction
The prevalence of depression is twice as high in women, compared with men, which may be attributable to a number of factors, including hormonal status – especially during menstruation and menopause, the authors wrote.
Previous research suggests a potential association between dietary fiber and depression in premenopausal women and between estrogen and gut microbiota. Fiber intake has an impact on gut microbiota, Dr. Kim said.
“We are motivated by the fact that depression provokes disease burden internationally and we would like to find modifiable factors that could prevent depression, especially in women, who are more vulnerable to depression,” she noted.
To investigate, the researchers drew on data from the Korea National Health and Nutrition Examination Survey for 2014, 2016, and 2018. Of the total number of women who met inclusion criteria (n = 5807; mean age, 47.11), roughly half were premenopausal and half were postmenopausal (n = 2,949 [mean age, 36.23 years] and n = 2,868 [mean age, 62.73], respectively).
Dietary fiber intake was assessed using the 24-hour dietary recall method, while depression was assessed using the Patient Health Questionnaire-9. The researchers used the Dietary Reference Intakes for Koreans to define a sufficient intake of dietary fiber (i.e., 12 g/1,000 kcal).
Covariates included chronic diseases, body mass index, medications, smoking status, alcohol use, physical activity, and sociodemographic factors.
When the researchers looked at all participants, they found that the estimated mean dietary fiber intake was significantly higher in women without depression, compared with those with depression (14.07 g/1,000 kcal/d; 95% confidence interval, 13.85-14.29 vs. 12.67 g/1,000 kcal/d; 95% CI, 11.79-13.55; P = .003).
Although the relationship remained significant in premenopausal women, it lost significance in postmenopausal women.
A 5% decrease in the prevalence of depression in premenopausal (but not postmenopausal) women was found in those with an increased intake of dietary fiber – i..e, there was a 1-g increase for every 1,000 kcal of daily energy intake, after adjusting for potential confounders in premenopausal women (OR, 0.949; 95% CI, 0.906-0.993]).
“The inverse relationship between dietary fiber intake and depression could be explained by the gut-brain interactions,” said Dr. Kim.
“Changes in the gut microbiota composition may affect neurotransmission and various neuropsychiatric phenomena in the brain,” she said, noting that previous studies have suggested that dietary fiber intake “may modulate the richness and diversity of the gut microbiota, and this change may promote brain health by affecting neurotransmission.”
Because postmenopausal women experience estrogen depletion, “the decreased interaction between estrogen and the gut microbiota may be related to the insignificant association between dietary fiber intake and depression in postmenopausal women,” she said.
Despite the lack of a significant association between postmenopausal depression and fiber intake, Dr. Kim said she “advises middle-aged women to have dietary fiber–rich diets, regardless of their menopausal status.”
Link between food and mood
In a comment, Stephanie S. Faubion, MD, MBA, a professor and chair of the department of medicine and the Penny and Bill George director of the Mayo Clinic’s Center for Women’s Health in Rochester, Minn., noted the study was cross-sectional and therefore the direction of the association could not be determined and “causality cannot be assumed.”
It is possible that “depressed women are less likely to eat fiber than women without depression. For example, a depressed woman may be more likely to sit on the couch eating Cheetos than shopping for and preparing a healthy meal,” said Dr. Faubion, who is also the medical director of the North American Menopause Society and was not involved with the study.
She noted that other potential confounders, including access to fresh fruits and vegetables or geographic locations could also “impact the findings and .”
Nevertheless, the study does “add to the body of evidence suggesting a link between diet and overall health, including brain health,” Dr. Faubion said.
One take-home message for practicing clinicians is that a healthy diet that includes fiber may benefit women (and men) for a number of reasons and “appears to be linked to mood.”
More research is needed “to determine the pathophysiologic mechanisms (such as potential brain-gut connection that involves the microbiome) that may explain this association,” Dr. Faubion added.
No source of funding listed. Dr. Kim and coauthors, as well as Dr. Faubion, disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Higher fiber intake may be associated with decreased risk of depression in premenopausal women, new research suggests.
Investigators analyzed data from close to 6,000 pre- and postmenopausal women. They found that, in premenopausal women, dietary fiber intake was higher among those without depression versus their counterparts with the disorder in a dose-dependent manner. However, there appeared to be no relationship between higher fiber intake and depression risk in postmenopausal women.
“We think the most important finding of our study is that dietary fiber intake was inversely associated with depression in premenopausal but not postmenopausal women,” lead author Yunsun Kim, MD, resident, department of family medicine, Chung-Ang University Hospital, Seoul, South Korea, said in an interview.
“We hope that the findings of this study could form the basis of future investigations to determine the causal relationship between dietary fiber intake and depression,” she added.
The study was published online Dec. 21, 2020, in Menopause.
Gut-brain interaction
The prevalence of depression is twice as high in women, compared with men, which may be attributable to a number of factors, including hormonal status – especially during menstruation and menopause, the authors wrote.
Previous research suggests a potential association between dietary fiber and depression in premenopausal women and between estrogen and gut microbiota. Fiber intake has an impact on gut microbiota, Dr. Kim said.
“We are motivated by the fact that depression provokes disease burden internationally and we would like to find modifiable factors that could prevent depression, especially in women, who are more vulnerable to depression,” she noted.
To investigate, the researchers drew on data from the Korea National Health and Nutrition Examination Survey for 2014, 2016, and 2018. Of the total number of women who met inclusion criteria (n = 5807; mean age, 47.11), roughly half were premenopausal and half were postmenopausal (n = 2,949 [mean age, 36.23 years] and n = 2,868 [mean age, 62.73], respectively).
Dietary fiber intake was assessed using the 24-hour dietary recall method, while depression was assessed using the Patient Health Questionnaire-9. The researchers used the Dietary Reference Intakes for Koreans to define a sufficient intake of dietary fiber (i.e., 12 g/1,000 kcal).
Covariates included chronic diseases, body mass index, medications, smoking status, alcohol use, physical activity, and sociodemographic factors.
When the researchers looked at all participants, they found that the estimated mean dietary fiber intake was significantly higher in women without depression, compared with those with depression (14.07 g/1,000 kcal/d; 95% confidence interval, 13.85-14.29 vs. 12.67 g/1,000 kcal/d; 95% CI, 11.79-13.55; P = .003).
Although the relationship remained significant in premenopausal women, it lost significance in postmenopausal women.
A 5% decrease in the prevalence of depression in premenopausal (but not postmenopausal) women was found in those with an increased intake of dietary fiber – i..e, there was a 1-g increase for every 1,000 kcal of daily energy intake, after adjusting for potential confounders in premenopausal women (OR, 0.949; 95% CI, 0.906-0.993]).
“The inverse relationship between dietary fiber intake and depression could be explained by the gut-brain interactions,” said Dr. Kim.
“Changes in the gut microbiota composition may affect neurotransmission and various neuropsychiatric phenomena in the brain,” she said, noting that previous studies have suggested that dietary fiber intake “may modulate the richness and diversity of the gut microbiota, and this change may promote brain health by affecting neurotransmission.”
Because postmenopausal women experience estrogen depletion, “the decreased interaction between estrogen and the gut microbiota may be related to the insignificant association between dietary fiber intake and depression in postmenopausal women,” she said.
Despite the lack of a significant association between postmenopausal depression and fiber intake, Dr. Kim said she “advises middle-aged women to have dietary fiber–rich diets, regardless of their menopausal status.”
Link between food and mood
In a comment, Stephanie S. Faubion, MD, MBA, a professor and chair of the department of medicine and the Penny and Bill George director of the Mayo Clinic’s Center for Women’s Health in Rochester, Minn., noted the study was cross-sectional and therefore the direction of the association could not be determined and “causality cannot be assumed.”
It is possible that “depressed women are less likely to eat fiber than women without depression. For example, a depressed woman may be more likely to sit on the couch eating Cheetos than shopping for and preparing a healthy meal,” said Dr. Faubion, who is also the medical director of the North American Menopause Society and was not involved with the study.
She noted that other potential confounders, including access to fresh fruits and vegetables or geographic locations could also “impact the findings and .”
Nevertheless, the study does “add to the body of evidence suggesting a link between diet and overall health, including brain health,” Dr. Faubion said.
One take-home message for practicing clinicians is that a healthy diet that includes fiber may benefit women (and men) for a number of reasons and “appears to be linked to mood.”
More research is needed “to determine the pathophysiologic mechanisms (such as potential brain-gut connection that involves the microbiome) that may explain this association,” Dr. Faubion added.
No source of funding listed. Dr. Kim and coauthors, as well as Dr. Faubion, disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Higher fiber intake may be associated with decreased risk of depression in premenopausal women, new research suggests.
Investigators analyzed data from close to 6,000 pre- and postmenopausal women. They found that, in premenopausal women, dietary fiber intake was higher among those without depression versus their counterparts with the disorder in a dose-dependent manner. However, there appeared to be no relationship between higher fiber intake and depression risk in postmenopausal women.
“We think the most important finding of our study is that dietary fiber intake was inversely associated with depression in premenopausal but not postmenopausal women,” lead author Yunsun Kim, MD, resident, department of family medicine, Chung-Ang University Hospital, Seoul, South Korea, said in an interview.
“We hope that the findings of this study could form the basis of future investigations to determine the causal relationship between dietary fiber intake and depression,” she added.
The study was published online Dec. 21, 2020, in Menopause.
Gut-brain interaction
The prevalence of depression is twice as high in women, compared with men, which may be attributable to a number of factors, including hormonal status – especially during menstruation and menopause, the authors wrote.
Previous research suggests a potential association between dietary fiber and depression in premenopausal women and between estrogen and gut microbiota. Fiber intake has an impact on gut microbiota, Dr. Kim said.
“We are motivated by the fact that depression provokes disease burden internationally and we would like to find modifiable factors that could prevent depression, especially in women, who are more vulnerable to depression,” she noted.
To investigate, the researchers drew on data from the Korea National Health and Nutrition Examination Survey for 2014, 2016, and 2018. Of the total number of women who met inclusion criteria (n = 5807; mean age, 47.11), roughly half were premenopausal and half were postmenopausal (n = 2,949 [mean age, 36.23 years] and n = 2,868 [mean age, 62.73], respectively).
Dietary fiber intake was assessed using the 24-hour dietary recall method, while depression was assessed using the Patient Health Questionnaire-9. The researchers used the Dietary Reference Intakes for Koreans to define a sufficient intake of dietary fiber (i.e., 12 g/1,000 kcal).
Covariates included chronic diseases, body mass index, medications, smoking status, alcohol use, physical activity, and sociodemographic factors.
When the researchers looked at all participants, they found that the estimated mean dietary fiber intake was significantly higher in women without depression, compared with those with depression (14.07 g/1,000 kcal/d; 95% confidence interval, 13.85-14.29 vs. 12.67 g/1,000 kcal/d; 95% CI, 11.79-13.55; P = .003).
Although the relationship remained significant in premenopausal women, it lost significance in postmenopausal women.
A 5% decrease in the prevalence of depression in premenopausal (but not postmenopausal) women was found in those with an increased intake of dietary fiber – i..e, there was a 1-g increase for every 1,000 kcal of daily energy intake, after adjusting for potential confounders in premenopausal women (OR, 0.949; 95% CI, 0.906-0.993]).
“The inverse relationship between dietary fiber intake and depression could be explained by the gut-brain interactions,” said Dr. Kim.
“Changes in the gut microbiota composition may affect neurotransmission and various neuropsychiatric phenomena in the brain,” she said, noting that previous studies have suggested that dietary fiber intake “may modulate the richness and diversity of the gut microbiota, and this change may promote brain health by affecting neurotransmission.”
Because postmenopausal women experience estrogen depletion, “the decreased interaction between estrogen and the gut microbiota may be related to the insignificant association between dietary fiber intake and depression in postmenopausal women,” she said.
Despite the lack of a significant association between postmenopausal depression and fiber intake, Dr. Kim said she “advises middle-aged women to have dietary fiber–rich diets, regardless of their menopausal status.”
Link between food and mood
In a comment, Stephanie S. Faubion, MD, MBA, a professor and chair of the department of medicine and the Penny and Bill George director of the Mayo Clinic’s Center for Women’s Health in Rochester, Minn., noted the study was cross-sectional and therefore the direction of the association could not be determined and “causality cannot be assumed.”
It is possible that “depressed women are less likely to eat fiber than women without depression. For example, a depressed woman may be more likely to sit on the couch eating Cheetos than shopping for and preparing a healthy meal,” said Dr. Faubion, who is also the medical director of the North American Menopause Society and was not involved with the study.
She noted that other potential confounders, including access to fresh fruits and vegetables or geographic locations could also “impact the findings and .”
Nevertheless, the study does “add to the body of evidence suggesting a link between diet and overall health, including brain health,” Dr. Faubion said.
One take-home message for practicing clinicians is that a healthy diet that includes fiber may benefit women (and men) for a number of reasons and “appears to be linked to mood.”
More research is needed “to determine the pathophysiologic mechanisms (such as potential brain-gut connection that involves the microbiome) that may explain this association,” Dr. Faubion added.
No source of funding listed. Dr. Kim and coauthors, as well as Dr. Faubion, disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Cannabis tied to self-harm, death in youth with mood disorders
Adolescents and young adults with mood disorders and cannabis use disorder (CUD) are at significantly increased risk for self-harm, all-cause mortality, homicide, and death by unintentional overdose, new research suggests.
Investigators found the risk for self-harm was three times higher, all-cause mortality was 59% higher, unintentional overdose was 2.5 times higher, and homicide was more than three times higher in those with versus without CUD.
“The take-home message of these findings is that we need to be aware of the perception that cannabis use is harmless, when it’s actually not,” lead author Cynthia Fontanella, PhD, associate professor of psychiatry, Ohio State University Wexner Medical Center, Columbus, said in an interview.
“We need to educate parents and clinicians that there are risks associated with cannabis, including increased risk for self-harm and death, and we need to effectively treat both cannabis use disorder and mood disorders,” she said.
The study was published online Jan. 19, 2021, in JAMA Pediatrics.
Little research in youth
“There has been very little research conducted on CUD in the adolescent population, and most studies have been conducted with adults,” Dr. Fontanella said.
Research on adults has shown that, even in people without mood disorders, cannabis use is associated with the early onset of mood disorders, psychosis, and anxiety disorders and has also been linked with suicidal behavior and increased risk for motor vehicle accidents, Dr. Fontanella said.
“We were motivated to conduct this study because we treat kids with depression and bipolar disorder and we noticed a high prevalence of CUD in this population, so we were curious about what its negative effects might be,” Dr. Fontanella recounted.
The researchers analyzed 7-year data drawn from Ohio Medicaid claims and linked to data from death certificates in 204,780 youths between the ages of 10 and 24 years (mean age was 17.2 years at the time of mood disorder diagnosis). Most were female, non-Hispanic White, enrolled in Medicaid because of poverty, and living in a metropolitan area (65.0%, 66.9%, 87.6%, and 77.1%, respectively).
Participants were followed up to 1 year from diagnosis until the end of enrollment, a self-harm event, or death.
Researchers included demographic, clinical, and treatment factors as covariates.
Close to three-quarters (72.7%) of the cohort had a depressive disorder, followed by unspecified/persistent mood disorder and bipolar disorder (14.9% and 12.4%, respectively). Comorbidities included ADHD (12.4%), anxiety disorder (12.3%), and other mental disorders (13.1%).
One -tenth of the cohort (10.3%) were diagnosed with CUD.
CUD treatment referrals
“Although CUD was associated with suicide in the unadjusted model, it was not significantly associated in adjusted models,” the authors reported.
Dr. Fontanella noted that the risk for these adverse outcomes is greater among those who engage in heavy, frequent use or who use cannabis that has higher-potency tetrahydrocannabinol (THC) content.
Reasons why CUD might be associated with these adverse outcomes are that it can increase impulsivity, poor judgment, and clouded thinking, which may in turn increase the risk for self-harm behaviors, she said.
She recommended that clinicians refer youth with CUD for “effective treatments,” including family-based models and individual approaches, such as cognitive behavioral therapy and motivational enhancement therapy.
Open dialogue
In a comment, Wilfrid Noel Raby, MD, PhD, adjunct clinical professor, Albert Einstein College of Medicine, New York, noted that psychosis can occur in patients with CUD and mood disorders – especially bipolar disorder – but was not included as a study outcome. “I would have liked to see more data about that,” he said.
However, “The trend is that cannabis use is starting at younger and younger ages, which has all kinds of ramifications in terms of cerebral development.”
Christopher Hammond, MD, PhD, assistant professor of psychiatry, Johns Hopkins University, Baltimore, said: “Three major strengths of the study are the size of the sample, its longitudinal analysis, and that the authors controlled for a number of potential confounding variables.”
In light of the findings, Dr. Hammond recommended clinicians and other health professionals who work with young people “should screen for cannabis-related problems in youth with mood disorders.”
Dr. Hammond, who is the director of the Co-occurring Disorders in Adolescents and Young Adults Clinical and Research Program, Johns Hopkins Bayview Medical Center, Baltimore, and was not involved with the study, recommended counseling youth with mood disorders and their parents and families “regarding the potential adverse health effects related to cannabis use.”
He also recommended “open dialogue with youth with and without mental health conditions about misleading reports in the national media and advertising about cannabis’ health benefits.”
The study was funded by the National Institute of Mental Health. Dr. Fontanella reported receiving grants from the National Institute of Mental Health during the conduct of the study. Dr. Raby reported no relevant financial relationships. Dr. Hammond reported receiving research grant funding from the National Institutes of Health, the American Academy of Child & Adolescent Psychiatry, Substance Abuse Mental Health Services Administration, the National Network of Depression Centers, and the Armstrong Institute at Johns Hopkins Bayview and serves as a scientific adviser for the National Courts and Science Institute and as a subject matter expert for SAMHSA related to co-occurring substance use disorders and severe emotional disturbance in youth.
A version of this article first appeared on Medscape.com.
Adolescents and young adults with mood disorders and cannabis use disorder (CUD) are at significantly increased risk for self-harm, all-cause mortality, homicide, and death by unintentional overdose, new research suggests.
Investigators found the risk for self-harm was three times higher, all-cause mortality was 59% higher, unintentional overdose was 2.5 times higher, and homicide was more than three times higher in those with versus without CUD.
“The take-home message of these findings is that we need to be aware of the perception that cannabis use is harmless, when it’s actually not,” lead author Cynthia Fontanella, PhD, associate professor of psychiatry, Ohio State University Wexner Medical Center, Columbus, said in an interview.
“We need to educate parents and clinicians that there are risks associated with cannabis, including increased risk for self-harm and death, and we need to effectively treat both cannabis use disorder and mood disorders,” she said.
The study was published online Jan. 19, 2021, in JAMA Pediatrics.
Little research in youth
“There has been very little research conducted on CUD in the adolescent population, and most studies have been conducted with adults,” Dr. Fontanella said.
Research on adults has shown that, even in people without mood disorders, cannabis use is associated with the early onset of mood disorders, psychosis, and anxiety disorders and has also been linked with suicidal behavior and increased risk for motor vehicle accidents, Dr. Fontanella said.
“We were motivated to conduct this study because we treat kids with depression and bipolar disorder and we noticed a high prevalence of CUD in this population, so we were curious about what its negative effects might be,” Dr. Fontanella recounted.
The researchers analyzed 7-year data drawn from Ohio Medicaid claims and linked to data from death certificates in 204,780 youths between the ages of 10 and 24 years (mean age was 17.2 years at the time of mood disorder diagnosis). Most were female, non-Hispanic White, enrolled in Medicaid because of poverty, and living in a metropolitan area (65.0%, 66.9%, 87.6%, and 77.1%, respectively).
Participants were followed up to 1 year from diagnosis until the end of enrollment, a self-harm event, or death.
Researchers included demographic, clinical, and treatment factors as covariates.
Close to three-quarters (72.7%) of the cohort had a depressive disorder, followed by unspecified/persistent mood disorder and bipolar disorder (14.9% and 12.4%, respectively). Comorbidities included ADHD (12.4%), anxiety disorder (12.3%), and other mental disorders (13.1%).
One -tenth of the cohort (10.3%) were diagnosed with CUD.
CUD treatment referrals
“Although CUD was associated with suicide in the unadjusted model, it was not significantly associated in adjusted models,” the authors reported.
Dr. Fontanella noted that the risk for these adverse outcomes is greater among those who engage in heavy, frequent use or who use cannabis that has higher-potency tetrahydrocannabinol (THC) content.
Reasons why CUD might be associated with these adverse outcomes are that it can increase impulsivity, poor judgment, and clouded thinking, which may in turn increase the risk for self-harm behaviors, she said.
She recommended that clinicians refer youth with CUD for “effective treatments,” including family-based models and individual approaches, such as cognitive behavioral therapy and motivational enhancement therapy.
Open dialogue
In a comment, Wilfrid Noel Raby, MD, PhD, adjunct clinical professor, Albert Einstein College of Medicine, New York, noted that psychosis can occur in patients with CUD and mood disorders – especially bipolar disorder – but was not included as a study outcome. “I would have liked to see more data about that,” he said.
However, “The trend is that cannabis use is starting at younger and younger ages, which has all kinds of ramifications in terms of cerebral development.”
Christopher Hammond, MD, PhD, assistant professor of psychiatry, Johns Hopkins University, Baltimore, said: “Three major strengths of the study are the size of the sample, its longitudinal analysis, and that the authors controlled for a number of potential confounding variables.”
In light of the findings, Dr. Hammond recommended clinicians and other health professionals who work with young people “should screen for cannabis-related problems in youth with mood disorders.”
Dr. Hammond, who is the director of the Co-occurring Disorders in Adolescents and Young Adults Clinical and Research Program, Johns Hopkins Bayview Medical Center, Baltimore, and was not involved with the study, recommended counseling youth with mood disorders and their parents and families “regarding the potential adverse health effects related to cannabis use.”
He also recommended “open dialogue with youth with and without mental health conditions about misleading reports in the national media and advertising about cannabis’ health benefits.”
The study was funded by the National Institute of Mental Health. Dr. Fontanella reported receiving grants from the National Institute of Mental Health during the conduct of the study. Dr. Raby reported no relevant financial relationships. Dr. Hammond reported receiving research grant funding from the National Institutes of Health, the American Academy of Child & Adolescent Psychiatry, Substance Abuse Mental Health Services Administration, the National Network of Depression Centers, and the Armstrong Institute at Johns Hopkins Bayview and serves as a scientific adviser for the National Courts and Science Institute and as a subject matter expert for SAMHSA related to co-occurring substance use disorders and severe emotional disturbance in youth.
A version of this article first appeared on Medscape.com.
Adolescents and young adults with mood disorders and cannabis use disorder (CUD) are at significantly increased risk for self-harm, all-cause mortality, homicide, and death by unintentional overdose, new research suggests.
Investigators found the risk for self-harm was three times higher, all-cause mortality was 59% higher, unintentional overdose was 2.5 times higher, and homicide was more than three times higher in those with versus without CUD.
“The take-home message of these findings is that we need to be aware of the perception that cannabis use is harmless, when it’s actually not,” lead author Cynthia Fontanella, PhD, associate professor of psychiatry, Ohio State University Wexner Medical Center, Columbus, said in an interview.
“We need to educate parents and clinicians that there are risks associated with cannabis, including increased risk for self-harm and death, and we need to effectively treat both cannabis use disorder and mood disorders,” she said.
The study was published online Jan. 19, 2021, in JAMA Pediatrics.
Little research in youth
“There has been very little research conducted on CUD in the adolescent population, and most studies have been conducted with adults,” Dr. Fontanella said.
Research on adults has shown that, even in people without mood disorders, cannabis use is associated with the early onset of mood disorders, psychosis, and anxiety disorders and has also been linked with suicidal behavior and increased risk for motor vehicle accidents, Dr. Fontanella said.
“We were motivated to conduct this study because we treat kids with depression and bipolar disorder and we noticed a high prevalence of CUD in this population, so we were curious about what its negative effects might be,” Dr. Fontanella recounted.
The researchers analyzed 7-year data drawn from Ohio Medicaid claims and linked to data from death certificates in 204,780 youths between the ages of 10 and 24 years (mean age was 17.2 years at the time of mood disorder diagnosis). Most were female, non-Hispanic White, enrolled in Medicaid because of poverty, and living in a metropolitan area (65.0%, 66.9%, 87.6%, and 77.1%, respectively).
Participants were followed up to 1 year from diagnosis until the end of enrollment, a self-harm event, or death.
Researchers included demographic, clinical, and treatment factors as covariates.
Close to three-quarters (72.7%) of the cohort had a depressive disorder, followed by unspecified/persistent mood disorder and bipolar disorder (14.9% and 12.4%, respectively). Comorbidities included ADHD (12.4%), anxiety disorder (12.3%), and other mental disorders (13.1%).
One -tenth of the cohort (10.3%) were diagnosed with CUD.
CUD treatment referrals
“Although CUD was associated with suicide in the unadjusted model, it was not significantly associated in adjusted models,” the authors reported.
Dr. Fontanella noted that the risk for these adverse outcomes is greater among those who engage in heavy, frequent use or who use cannabis that has higher-potency tetrahydrocannabinol (THC) content.
Reasons why CUD might be associated with these adverse outcomes are that it can increase impulsivity, poor judgment, and clouded thinking, which may in turn increase the risk for self-harm behaviors, she said.
She recommended that clinicians refer youth with CUD for “effective treatments,” including family-based models and individual approaches, such as cognitive behavioral therapy and motivational enhancement therapy.
Open dialogue
In a comment, Wilfrid Noel Raby, MD, PhD, adjunct clinical professor, Albert Einstein College of Medicine, New York, noted that psychosis can occur in patients with CUD and mood disorders – especially bipolar disorder – but was not included as a study outcome. “I would have liked to see more data about that,” he said.
However, “The trend is that cannabis use is starting at younger and younger ages, which has all kinds of ramifications in terms of cerebral development.”
Christopher Hammond, MD, PhD, assistant professor of psychiatry, Johns Hopkins University, Baltimore, said: “Three major strengths of the study are the size of the sample, its longitudinal analysis, and that the authors controlled for a number of potential confounding variables.”
In light of the findings, Dr. Hammond recommended clinicians and other health professionals who work with young people “should screen for cannabis-related problems in youth with mood disorders.”
Dr. Hammond, who is the director of the Co-occurring Disorders in Adolescents and Young Adults Clinical and Research Program, Johns Hopkins Bayview Medical Center, Baltimore, and was not involved with the study, recommended counseling youth with mood disorders and their parents and families “regarding the potential adverse health effects related to cannabis use.”
He also recommended “open dialogue with youth with and without mental health conditions about misleading reports in the national media and advertising about cannabis’ health benefits.”
The study was funded by the National Institute of Mental Health. Dr. Fontanella reported receiving grants from the National Institute of Mental Health during the conduct of the study. Dr. Raby reported no relevant financial relationships. Dr. Hammond reported receiving research grant funding from the National Institutes of Health, the American Academy of Child & Adolescent Psychiatry, Substance Abuse Mental Health Services Administration, the National Network of Depression Centers, and the Armstrong Institute at Johns Hopkins Bayview and serves as a scientific adviser for the National Courts and Science Institute and as a subject matter expert for SAMHSA related to co-occurring substance use disorders and severe emotional disturbance in youth.
A version of this article first appeared on Medscape.com.