User login
Naltrexone cuts hospitalization, deaths in alcohol use disorder
Naltrexone reduces the risk for hospitalization for alcohol use disorder (AUD), regardless of whether it is used alone or in conjunction with disulfiram or acamprosate, research suggests.
Investigators analyzed 10-year data on more than 125,000 Swedish residents with AUD and found that naltrexone, used as monotherapy or combined with acamprosate or disulfiram, was associated with significantly lower risk for AUD hospitalization or all-cause hospitalization in comparison with patients who did not use AUD medication. The patients ranged in age from 16 to 64 years.
By contrast, benzodiazepines and acamprosate monotherapy were associated with increased risk for AUD hospitalization.
“The take-home message for practicing clinicians would be that especially naltrexone use is associated with favorable treatment outcomes and should be utilized as part of the treatment protocol for AUD,” study investigator Milja Heikkinen, MD, specialist in forensic psychiatry and addiction medicine, University of Eastern Finland, Kuopio, told this news organization.
On the other hand, “benzodiazepines should be avoided and should not be administered other than for alcohol withdrawal symptoms,” she said.
The study was published online Jan. 4 in Addiction.
Real-world data
Previous research has shown that disulfiram, acamprosate, naltrexone, and nalmefene are efficacious in treating AUD, but most studies have been randomized controlled trials or meta-analyses, the authors write.
“Very little is known about overall health outcomes (such as risks of hospitalization and mortality) associated with specific treatments in real-world circumstances,” they write.
“The study was motivated by the fact that, although AUD is a significant public health concern, very little is known, especially about the comparative effectiveness of medications indicated in AUD,” said Dr. Heikkinen.
who had been diagnosed with AUD (62.5% men; mean [standard deviation] age, 38.1 [15.9] years). They followed the cohort over a median of 4.6 years (interquartile range, 2.1-.2 years).
During the follow-up period, roughly one-fourth of patients (25.6) underwent treatment with one or more drugs.

The main outcome measure was AUD-related hospitalization. Secondary outcomes were hospitalization for any cause and for alcohol-related somatic causes; all-cause mortality; and work disability.
Two types of analyses were conducted. The within-individual analyses, designed to eliminate selection bias, compared the use of a medication to periods during which the same individual was not using the medication.
Between-individual analyses (adjusted for sex, age, educational level, number of previous AUD-related hospitalizations, time since first AUD diagnosis, comorbidities, and use of other medications) utilized a “traditional” multivariate-adjusted Cox hazards regression model.
AUD pharmacotherapy ‘underutilized’
Close to one-fourth of patients (23.9%) experienced the main outcome event (AUD-related hospitalization) during the follow-up period.
The within-individual analysis showed that naltrexone – whether used as monotherapy or adjunctively with disulfiram or acamprosate – “was associated with a significantly lower risk of AUD-related hospitalization, compared to those time periods in which the same individual did not use any AUD medication,” the authors report.
By contrast, they state, acamprosate monotherapy and benzodiazepines were associated with a significantly higher risk for AUD-related hospitalization.
Similar results were obtained in the between-individual analysis. Longer duration of naltrexone use was associated with lower risk for AUD-related hospitalization.
The pattern was also found when the outcome was hospitalization for any cause. However, unlike the findings of the within-individual model, the second model found that acamprosate monotherapy was not associated with a higher risk for any-cause hospitalization.
Polytherapy, including combinations of the four AUD medications, as well as disulfiram monotherapy were similarly associated with lower risk for hospitalization for alcohol-related somatic causes.
Of the overall cohort, 6.2% died during the follow-up period. No association was found between disulfiram, acamprosate, nalmefene, and naltrexone use and all-cause mortality. By contrast, benzodiazepine use was associated with a significantly higher mortality rate (hazard ratio, 1.11; 95% confidence interval, 1.04-1.19).
“AUD drugs are underutilized, despite AUD being a significant public health concern,” Dr. Heikkinen noted. On the other hand, benzodiazepine use is “very common.”
‘Ravages’ of benzodiazepines
Commenting on the study in an interview, John Krystal, MD, professor and chair of psychiatry and director of the Center for the Translational Neuroscience of Alcoholism, Yale University, New Haven, Conn., said, “The main message from the study for practicing clinicians is that treatment works.”
Dr. Krystal, who was not involved with the study, noted that “many practicing clinicians are discouraged by the course of their patients with AUD, and this study highlights that naltrexone, perhaps in combination with other medications, may be effective in preventing hospitalization and, presumably, other hospitalization-related complications of AUD.”
Also commenting on the study, Raymond Anton, MD, professor, department of psychiatry and behavioral sciences, Medical University of South Carolina, Charleston, suggested that the “clinical knowledge of the harm of benzodiazepines in those with AUD is reinforced by these findings.”
In fact, the harm of benzodiazepines might be the study’s “most important message ... [a message that was] recently highlighted by the Netflix series “The Queen’s Gambit”, which shows the ravages of using both together, or how one leads to potential addiction with the other,” said Dr. Anton, who was not involved with the study.
The other “big take-home message is that naltrexone should be used more frequently,” said Dr. Anton, distinguished professor of psychiatry at the university and scientific director of the Charleston Alcohol Research Center. He noted that there are “recent data suggesting some clinical and genetic indicators that predict responsiveness to these medications, improving efficacy.”
The study was funded by the Finnish Ministry of Social Affairs and Health. Dr. Heikkinen reports no relevant financial relationships. The other authors’ disclosures are listed on the original article. Dr. Krystal consults for companies currently developing other treatments for AUDs and receives medications to test from AstraZeneca and Novartis for NIAAA-funded research programs. Dr. Anton has consulted for Alkermes, Lipha, and Lundbeck in the past. He is also chair of the Alcohol Clinical Trials Initiative, which is a public-private partnership partially sponsored by several companies and has received grant funding from the National Institutes of Health/National Institute on Alcohol Abuse and Alcoholism to study pharmacotherapies, including naltrexone, nalmefene, and acamprosate.
A version of this article first appeared on Medscape.com.
Naltrexone reduces the risk for hospitalization for alcohol use disorder (AUD), regardless of whether it is used alone or in conjunction with disulfiram or acamprosate, research suggests.
Investigators analyzed 10-year data on more than 125,000 Swedish residents with AUD and found that naltrexone, used as monotherapy or combined with acamprosate or disulfiram, was associated with significantly lower risk for AUD hospitalization or all-cause hospitalization in comparison with patients who did not use AUD medication. The patients ranged in age from 16 to 64 years.
By contrast, benzodiazepines and acamprosate monotherapy were associated with increased risk for AUD hospitalization.
“The take-home message for practicing clinicians would be that especially naltrexone use is associated with favorable treatment outcomes and should be utilized as part of the treatment protocol for AUD,” study investigator Milja Heikkinen, MD, specialist in forensic psychiatry and addiction medicine, University of Eastern Finland, Kuopio, told this news organization.
On the other hand, “benzodiazepines should be avoided and should not be administered other than for alcohol withdrawal symptoms,” she said.
The study was published online Jan. 4 in Addiction.
Real-world data
Previous research has shown that disulfiram, acamprosate, naltrexone, and nalmefene are efficacious in treating AUD, but most studies have been randomized controlled trials or meta-analyses, the authors write.
“Very little is known about overall health outcomes (such as risks of hospitalization and mortality) associated with specific treatments in real-world circumstances,” they write.
“The study was motivated by the fact that, although AUD is a significant public health concern, very little is known, especially about the comparative effectiveness of medications indicated in AUD,” said Dr. Heikkinen.
who had been diagnosed with AUD (62.5% men; mean [standard deviation] age, 38.1 [15.9] years). They followed the cohort over a median of 4.6 years (interquartile range, 2.1-.2 years).
During the follow-up period, roughly one-fourth of patients (25.6) underwent treatment with one or more drugs.

The main outcome measure was AUD-related hospitalization. Secondary outcomes were hospitalization for any cause and for alcohol-related somatic causes; all-cause mortality; and work disability.
Two types of analyses were conducted. The within-individual analyses, designed to eliminate selection bias, compared the use of a medication to periods during which the same individual was not using the medication.
Between-individual analyses (adjusted for sex, age, educational level, number of previous AUD-related hospitalizations, time since first AUD diagnosis, comorbidities, and use of other medications) utilized a “traditional” multivariate-adjusted Cox hazards regression model.
AUD pharmacotherapy ‘underutilized’
Close to one-fourth of patients (23.9%) experienced the main outcome event (AUD-related hospitalization) during the follow-up period.
The within-individual analysis showed that naltrexone – whether used as monotherapy or adjunctively with disulfiram or acamprosate – “was associated with a significantly lower risk of AUD-related hospitalization, compared to those time periods in which the same individual did not use any AUD medication,” the authors report.
By contrast, they state, acamprosate monotherapy and benzodiazepines were associated with a significantly higher risk for AUD-related hospitalization.
Similar results were obtained in the between-individual analysis. Longer duration of naltrexone use was associated with lower risk for AUD-related hospitalization.
The pattern was also found when the outcome was hospitalization for any cause. However, unlike the findings of the within-individual model, the second model found that acamprosate monotherapy was not associated with a higher risk for any-cause hospitalization.
Polytherapy, including combinations of the four AUD medications, as well as disulfiram monotherapy were similarly associated with lower risk for hospitalization for alcohol-related somatic causes.
Of the overall cohort, 6.2% died during the follow-up period. No association was found between disulfiram, acamprosate, nalmefene, and naltrexone use and all-cause mortality. By contrast, benzodiazepine use was associated with a significantly higher mortality rate (hazard ratio, 1.11; 95% confidence interval, 1.04-1.19).
“AUD drugs are underutilized, despite AUD being a significant public health concern,” Dr. Heikkinen noted. On the other hand, benzodiazepine use is “very common.”
‘Ravages’ of benzodiazepines
Commenting on the study in an interview, John Krystal, MD, professor and chair of psychiatry and director of the Center for the Translational Neuroscience of Alcoholism, Yale University, New Haven, Conn., said, “The main message from the study for practicing clinicians is that treatment works.”
Dr. Krystal, who was not involved with the study, noted that “many practicing clinicians are discouraged by the course of their patients with AUD, and this study highlights that naltrexone, perhaps in combination with other medications, may be effective in preventing hospitalization and, presumably, other hospitalization-related complications of AUD.”
Also commenting on the study, Raymond Anton, MD, professor, department of psychiatry and behavioral sciences, Medical University of South Carolina, Charleston, suggested that the “clinical knowledge of the harm of benzodiazepines in those with AUD is reinforced by these findings.”
In fact, the harm of benzodiazepines might be the study’s “most important message ... [a message that was] recently highlighted by the Netflix series “The Queen’s Gambit”, which shows the ravages of using both together, or how one leads to potential addiction with the other,” said Dr. Anton, who was not involved with the study.
The other “big take-home message is that naltrexone should be used more frequently,” said Dr. Anton, distinguished professor of psychiatry at the university and scientific director of the Charleston Alcohol Research Center. He noted that there are “recent data suggesting some clinical and genetic indicators that predict responsiveness to these medications, improving efficacy.”
The study was funded by the Finnish Ministry of Social Affairs and Health. Dr. Heikkinen reports no relevant financial relationships. The other authors’ disclosures are listed on the original article. Dr. Krystal consults for companies currently developing other treatments for AUDs and receives medications to test from AstraZeneca and Novartis for NIAAA-funded research programs. Dr. Anton has consulted for Alkermes, Lipha, and Lundbeck in the past. He is also chair of the Alcohol Clinical Trials Initiative, which is a public-private partnership partially sponsored by several companies and has received grant funding from the National Institutes of Health/National Institute on Alcohol Abuse and Alcoholism to study pharmacotherapies, including naltrexone, nalmefene, and acamprosate.
A version of this article first appeared on Medscape.com.
Naltrexone reduces the risk for hospitalization for alcohol use disorder (AUD), regardless of whether it is used alone or in conjunction with disulfiram or acamprosate, research suggests.
Investigators analyzed 10-year data on more than 125,000 Swedish residents with AUD and found that naltrexone, used as monotherapy or combined with acamprosate or disulfiram, was associated with significantly lower risk for AUD hospitalization or all-cause hospitalization in comparison with patients who did not use AUD medication. The patients ranged in age from 16 to 64 years.
By contrast, benzodiazepines and acamprosate monotherapy were associated with increased risk for AUD hospitalization.
“The take-home message for practicing clinicians would be that especially naltrexone use is associated with favorable treatment outcomes and should be utilized as part of the treatment protocol for AUD,” study investigator Milja Heikkinen, MD, specialist in forensic psychiatry and addiction medicine, University of Eastern Finland, Kuopio, told this news organization.
On the other hand, “benzodiazepines should be avoided and should not be administered other than for alcohol withdrawal symptoms,” she said.
The study was published online Jan. 4 in Addiction.
Real-world data
Previous research has shown that disulfiram, acamprosate, naltrexone, and nalmefene are efficacious in treating AUD, but most studies have been randomized controlled trials or meta-analyses, the authors write.
“Very little is known about overall health outcomes (such as risks of hospitalization and mortality) associated with specific treatments in real-world circumstances,” they write.
“The study was motivated by the fact that, although AUD is a significant public health concern, very little is known, especially about the comparative effectiveness of medications indicated in AUD,” said Dr. Heikkinen.
who had been diagnosed with AUD (62.5% men; mean [standard deviation] age, 38.1 [15.9] years). They followed the cohort over a median of 4.6 years (interquartile range, 2.1-.2 years).
During the follow-up period, roughly one-fourth of patients (25.6) underwent treatment with one or more drugs.

The main outcome measure was AUD-related hospitalization. Secondary outcomes were hospitalization for any cause and for alcohol-related somatic causes; all-cause mortality; and work disability.
Two types of analyses were conducted. The within-individual analyses, designed to eliminate selection bias, compared the use of a medication to periods during which the same individual was not using the medication.
Between-individual analyses (adjusted for sex, age, educational level, number of previous AUD-related hospitalizations, time since first AUD diagnosis, comorbidities, and use of other medications) utilized a “traditional” multivariate-adjusted Cox hazards regression model.
AUD pharmacotherapy ‘underutilized’
Close to one-fourth of patients (23.9%) experienced the main outcome event (AUD-related hospitalization) during the follow-up period.
The within-individual analysis showed that naltrexone – whether used as monotherapy or adjunctively with disulfiram or acamprosate – “was associated with a significantly lower risk of AUD-related hospitalization, compared to those time periods in which the same individual did not use any AUD medication,” the authors report.
By contrast, they state, acamprosate monotherapy and benzodiazepines were associated with a significantly higher risk for AUD-related hospitalization.
Similar results were obtained in the between-individual analysis. Longer duration of naltrexone use was associated with lower risk for AUD-related hospitalization.
The pattern was also found when the outcome was hospitalization for any cause. However, unlike the findings of the within-individual model, the second model found that acamprosate monotherapy was not associated with a higher risk for any-cause hospitalization.
Polytherapy, including combinations of the four AUD medications, as well as disulfiram monotherapy were similarly associated with lower risk for hospitalization for alcohol-related somatic causes.
Of the overall cohort, 6.2% died during the follow-up period. No association was found between disulfiram, acamprosate, nalmefene, and naltrexone use and all-cause mortality. By contrast, benzodiazepine use was associated with a significantly higher mortality rate (hazard ratio, 1.11; 95% confidence interval, 1.04-1.19).
“AUD drugs are underutilized, despite AUD being a significant public health concern,” Dr. Heikkinen noted. On the other hand, benzodiazepine use is “very common.”
‘Ravages’ of benzodiazepines
Commenting on the study in an interview, John Krystal, MD, professor and chair of psychiatry and director of the Center for the Translational Neuroscience of Alcoholism, Yale University, New Haven, Conn., said, “The main message from the study for practicing clinicians is that treatment works.”
Dr. Krystal, who was not involved with the study, noted that “many practicing clinicians are discouraged by the course of their patients with AUD, and this study highlights that naltrexone, perhaps in combination with other medications, may be effective in preventing hospitalization and, presumably, other hospitalization-related complications of AUD.”
Also commenting on the study, Raymond Anton, MD, professor, department of psychiatry and behavioral sciences, Medical University of South Carolina, Charleston, suggested that the “clinical knowledge of the harm of benzodiazepines in those with AUD is reinforced by these findings.”
In fact, the harm of benzodiazepines might be the study’s “most important message ... [a message that was] recently highlighted by the Netflix series “The Queen’s Gambit”, which shows the ravages of using both together, or how one leads to potential addiction with the other,” said Dr. Anton, who was not involved with the study.
The other “big take-home message is that naltrexone should be used more frequently,” said Dr. Anton, distinguished professor of psychiatry at the university and scientific director of the Charleston Alcohol Research Center. He noted that there are “recent data suggesting some clinical and genetic indicators that predict responsiveness to these medications, improving efficacy.”
The study was funded by the Finnish Ministry of Social Affairs and Health. Dr. Heikkinen reports no relevant financial relationships. The other authors’ disclosures are listed on the original article. Dr. Krystal consults for companies currently developing other treatments for AUDs and receives medications to test from AstraZeneca and Novartis for NIAAA-funded research programs. Dr. Anton has consulted for Alkermes, Lipha, and Lundbeck in the past. He is also chair of the Alcohol Clinical Trials Initiative, which is a public-private partnership partially sponsored by several companies and has received grant funding from the National Institutes of Health/National Institute on Alcohol Abuse and Alcoholism to study pharmacotherapies, including naltrexone, nalmefene, and acamprosate.
A version of this article first appeared on Medscape.com.
Machine learning flags key risk factors for suicide attempts
A history of suicidal behaviors or ideation, functional impairment related to mental health disorders, and socioeconomic disadvantage are the three most important risk factors predicting subsequent suicide attempts, new research suggests.
Investigators applied a machine-learning model to data on over 34,500 adults drawn from a large national survey database. After analyzing more than 2,500 survey questions, key areas were identified that yielded the most accurate predictions of who might be at risk for later suicide attempt.
These predictors included experiencing previous suicidal behaviors and ideation or functional impairment because of emotional problems, being at a younger age, having a lower educational achievement, and experiencing a recent financial crisis.
“Our machine learning model confirmed well-known risk factors of suicide attempt, including previous suicidal behavior and depression; and we also identified functional impairment, such as doing activities less carefully or accomplishing less because of emotional problems, as a new important risk,” lead author Angel Garcia de la Garza, PhD candidate in the department of biostatistics, Columbia University, New York, said in an interview.
“We hope our results provide a novel avenue for future suicide risk assessment,” Mr. Garcia de la Garza said.
The findings were published online Jan. 6 in JAMA Psychiatry.
‘Rich’ dataset
Previous research using machine learning approaches to study nonfatal suicide attempt prediction has focused on high-risk patients in clinical treatment. However, more than one-third of individuals making nonfatal suicide attempts do not receive mental health treatment, Mr. Garcia de la Garza noted.
To gain further insight into predictors of suicide risk in nonclinical populations, the researchers turned to the National Epidemiologic Survey on Alcohol and Related Conditions (NESARC), a longitudinal survey of noninstitutionalized U.S. adults.
“We wanted to extend our understanding of suicide attempt risk factors beyond high-risk clinical populations to the general adult population; and the richness of the NESARC dataset provides a unique opportunity to do so,” Mr. Garcia de la Garza said.
The NESARC surveys were conducted in two waves: Wave 1 (2001-2002) and wave 2 (2004-2005), in which participants self-reported nonfatal suicide attempts in the preceding 3 years since wave 1.
Assessment of wave 1 participants was based on the Alcohol Use Disorder and Associated Disabilities Interview Schedule DSM-IV.
“This survey’s extensive assessment instrument contained a detailed evaluation of substance use, psychiatric disorders, and symptoms not routinely available in electronic health records,” Mr. Garcia de la Garza noted.
The wave 1 survey contained 2,805 separate questions. From participants’ responses, the investigators derived 180 variables for three categories: past-year, prior-to-past-year, and lifetime mental disorders.
They then identified 2,978 factors associated with suicide attempts and used a statistical method called balanced random forest to classify suicide attempts at wave 2. Each variable was accorded an “importance score” using identified wave 1 features.
The outcome variable of attempted suicide at any point during the 3 years prior to the wave 2 interview was defined by combining responses to three wave 2 questions:
- In your entire life, did you ever attempt suicide?
- If yes, how old were you the first time?
- If the most recent event occurred within the last 3 years, how old were you during the most recent time?
Suicide risk severity was classified into four groups (low, medium, high, and very high) on the basis of the top-performing risk factors.
A statistical model combining survey design and nonresponse weights enabled estimates to be representative of the U.S. population, based on the 2000 census.
Out-of-fold model prediction assessed performance of the model, using area under receiver operator curve (AUC), sensitivity, and specificity.
Daily functioning
Of all participants, 70.2% (n = 34,653; almost 60% women) completed wave 2 interviews. The weighted mean ages at waves 1 and 2 were 45.1 and 48.2 years, respectively.
Of wave 2 respondents, 0.6% (n = 222) attempted suicide during the preceding 3 years.
Half of those who attempted suicide within the first year were classified as “very high risk,” while 33.2% of those who attempted suicide between the first and second year and 33.3% of those who attempted suicide between the second and third year were classified as “very high risk.”
Among participants who attempted suicide between the third year and follow-up, 16.48% were classified as “very high risk.”
The model accurately captured classification of participants, even across demographic characteristics, such as age, sex, race, and income.
Younger individuals (aged 18-36 years) were at higher risk, compared with older individuals. In addition, women were at higher risk than were men, White participants were at higher risk than were non-White participants, and individuals with lower income were at greater risk than were those with higher income.
The model found that 1.8% of the U.S. population had a 10% or greater risk of a suicide attempt.
The most important risk factors identified were the three questions about previous suicidal ideation or behavior; three items from the 12-Item Short Form Health Survey (feeling downhearted, doing activities less carefully, or accomplishing less because of emotional problems); younger age; lower educational achievement; and recent financial crisis.
“The clinical assessment of suicide risk typically focuses on acute suicidal symptoms, together with depression, anxiety, substance misuse, and recent stressful events,” coinvestigator Mark Olfson, MD, PhD, professor of epidemiology, Columbia University Irving Medical Center, New York, said in an interview.
Dr. Olfson said.
Extra vigilance
Commenting on the study in an interview, April C. Foreman, PhD, an executive board member of the American Association of Suicidology, noted that some of the findings were not surprising.
“When discharging a patient from inpatient care, or seeing them in primary care, bring up mental health concerns proactively and ask whether they have ever attempted suicide or harmed themselves – even a long time ago – just as you ask about a family history of heart disease or cancer, or other health issues,” said Dr. Foreman, chief medical officer of the Kevin and Margaret Hines Foundation.
She noted that half of people who die by suicide have a primary care visit within the preceding month.
“Primary care is a great place to get a suicide history and follow the patient with extra vigilance, just as you would with any other risk factors,” Dr. Foreman said.
The study was funded by the National Institute on Alcohol Abuse and Alcoholism and its Intramural Program. The study authors and Dr. Foreman have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A history of suicidal behaviors or ideation, functional impairment related to mental health disorders, and socioeconomic disadvantage are the three most important risk factors predicting subsequent suicide attempts, new research suggests.
Investigators applied a machine-learning model to data on over 34,500 adults drawn from a large national survey database. After analyzing more than 2,500 survey questions, key areas were identified that yielded the most accurate predictions of who might be at risk for later suicide attempt.
These predictors included experiencing previous suicidal behaviors and ideation or functional impairment because of emotional problems, being at a younger age, having a lower educational achievement, and experiencing a recent financial crisis.
“Our machine learning model confirmed well-known risk factors of suicide attempt, including previous suicidal behavior and depression; and we also identified functional impairment, such as doing activities less carefully or accomplishing less because of emotional problems, as a new important risk,” lead author Angel Garcia de la Garza, PhD candidate in the department of biostatistics, Columbia University, New York, said in an interview.
“We hope our results provide a novel avenue for future suicide risk assessment,” Mr. Garcia de la Garza said.
The findings were published online Jan. 6 in JAMA Psychiatry.
‘Rich’ dataset
Previous research using machine learning approaches to study nonfatal suicide attempt prediction has focused on high-risk patients in clinical treatment. However, more than one-third of individuals making nonfatal suicide attempts do not receive mental health treatment, Mr. Garcia de la Garza noted.
To gain further insight into predictors of suicide risk in nonclinical populations, the researchers turned to the National Epidemiologic Survey on Alcohol and Related Conditions (NESARC), a longitudinal survey of noninstitutionalized U.S. adults.
“We wanted to extend our understanding of suicide attempt risk factors beyond high-risk clinical populations to the general adult population; and the richness of the NESARC dataset provides a unique opportunity to do so,” Mr. Garcia de la Garza said.
The NESARC surveys were conducted in two waves: Wave 1 (2001-2002) and wave 2 (2004-2005), in which participants self-reported nonfatal suicide attempts in the preceding 3 years since wave 1.
Assessment of wave 1 participants was based on the Alcohol Use Disorder and Associated Disabilities Interview Schedule DSM-IV.
“This survey’s extensive assessment instrument contained a detailed evaluation of substance use, psychiatric disorders, and symptoms not routinely available in electronic health records,” Mr. Garcia de la Garza noted.
The wave 1 survey contained 2,805 separate questions. From participants’ responses, the investigators derived 180 variables for three categories: past-year, prior-to-past-year, and lifetime mental disorders.
They then identified 2,978 factors associated with suicide attempts and used a statistical method called balanced random forest to classify suicide attempts at wave 2. Each variable was accorded an “importance score” using identified wave 1 features.
The outcome variable of attempted suicide at any point during the 3 years prior to the wave 2 interview was defined by combining responses to three wave 2 questions:
- In your entire life, did you ever attempt suicide?
- If yes, how old were you the first time?
- If the most recent event occurred within the last 3 years, how old were you during the most recent time?
Suicide risk severity was classified into four groups (low, medium, high, and very high) on the basis of the top-performing risk factors.
A statistical model combining survey design and nonresponse weights enabled estimates to be representative of the U.S. population, based on the 2000 census.
Out-of-fold model prediction assessed performance of the model, using area under receiver operator curve (AUC), sensitivity, and specificity.
Daily functioning
Of all participants, 70.2% (n = 34,653; almost 60% women) completed wave 2 interviews. The weighted mean ages at waves 1 and 2 were 45.1 and 48.2 years, respectively.
Of wave 2 respondents, 0.6% (n = 222) attempted suicide during the preceding 3 years.
Half of those who attempted suicide within the first year were classified as “very high risk,” while 33.2% of those who attempted suicide between the first and second year and 33.3% of those who attempted suicide between the second and third year were classified as “very high risk.”
Among participants who attempted suicide between the third year and follow-up, 16.48% were classified as “very high risk.”
The model accurately captured classification of participants, even across demographic characteristics, such as age, sex, race, and income.
Younger individuals (aged 18-36 years) were at higher risk, compared with older individuals. In addition, women were at higher risk than were men, White participants were at higher risk than were non-White participants, and individuals with lower income were at greater risk than were those with higher income.
The model found that 1.8% of the U.S. population had a 10% or greater risk of a suicide attempt.
The most important risk factors identified were the three questions about previous suicidal ideation or behavior; three items from the 12-Item Short Form Health Survey (feeling downhearted, doing activities less carefully, or accomplishing less because of emotional problems); younger age; lower educational achievement; and recent financial crisis.
“The clinical assessment of suicide risk typically focuses on acute suicidal symptoms, together with depression, anxiety, substance misuse, and recent stressful events,” coinvestigator Mark Olfson, MD, PhD, professor of epidemiology, Columbia University Irving Medical Center, New York, said in an interview.
Dr. Olfson said.
Extra vigilance
Commenting on the study in an interview, April C. Foreman, PhD, an executive board member of the American Association of Suicidology, noted that some of the findings were not surprising.
“When discharging a patient from inpatient care, or seeing them in primary care, bring up mental health concerns proactively and ask whether they have ever attempted suicide or harmed themselves – even a long time ago – just as you ask about a family history of heart disease or cancer, or other health issues,” said Dr. Foreman, chief medical officer of the Kevin and Margaret Hines Foundation.
She noted that half of people who die by suicide have a primary care visit within the preceding month.
“Primary care is a great place to get a suicide history and follow the patient with extra vigilance, just as you would with any other risk factors,” Dr. Foreman said.
The study was funded by the National Institute on Alcohol Abuse and Alcoholism and its Intramural Program. The study authors and Dr. Foreman have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A history of suicidal behaviors or ideation, functional impairment related to mental health disorders, and socioeconomic disadvantage are the three most important risk factors predicting subsequent suicide attempts, new research suggests.
Investigators applied a machine-learning model to data on over 34,500 adults drawn from a large national survey database. After analyzing more than 2,500 survey questions, key areas were identified that yielded the most accurate predictions of who might be at risk for later suicide attempt.
These predictors included experiencing previous suicidal behaviors and ideation or functional impairment because of emotional problems, being at a younger age, having a lower educational achievement, and experiencing a recent financial crisis.
“Our machine learning model confirmed well-known risk factors of suicide attempt, including previous suicidal behavior and depression; and we also identified functional impairment, such as doing activities less carefully or accomplishing less because of emotional problems, as a new important risk,” lead author Angel Garcia de la Garza, PhD candidate in the department of biostatistics, Columbia University, New York, said in an interview.
“We hope our results provide a novel avenue for future suicide risk assessment,” Mr. Garcia de la Garza said.
The findings were published online Jan. 6 in JAMA Psychiatry.
‘Rich’ dataset
Previous research using machine learning approaches to study nonfatal suicide attempt prediction has focused on high-risk patients in clinical treatment. However, more than one-third of individuals making nonfatal suicide attempts do not receive mental health treatment, Mr. Garcia de la Garza noted.
To gain further insight into predictors of suicide risk in nonclinical populations, the researchers turned to the National Epidemiologic Survey on Alcohol and Related Conditions (NESARC), a longitudinal survey of noninstitutionalized U.S. adults.
“We wanted to extend our understanding of suicide attempt risk factors beyond high-risk clinical populations to the general adult population; and the richness of the NESARC dataset provides a unique opportunity to do so,” Mr. Garcia de la Garza said.
The NESARC surveys were conducted in two waves: Wave 1 (2001-2002) and wave 2 (2004-2005), in which participants self-reported nonfatal suicide attempts in the preceding 3 years since wave 1.
Assessment of wave 1 participants was based on the Alcohol Use Disorder and Associated Disabilities Interview Schedule DSM-IV.
“This survey’s extensive assessment instrument contained a detailed evaluation of substance use, psychiatric disorders, and symptoms not routinely available in electronic health records,” Mr. Garcia de la Garza noted.
The wave 1 survey contained 2,805 separate questions. From participants’ responses, the investigators derived 180 variables for three categories: past-year, prior-to-past-year, and lifetime mental disorders.
They then identified 2,978 factors associated with suicide attempts and used a statistical method called balanced random forest to classify suicide attempts at wave 2. Each variable was accorded an “importance score” using identified wave 1 features.
The outcome variable of attempted suicide at any point during the 3 years prior to the wave 2 interview was defined by combining responses to three wave 2 questions:
- In your entire life, did you ever attempt suicide?
- If yes, how old were you the first time?
- If the most recent event occurred within the last 3 years, how old were you during the most recent time?
Suicide risk severity was classified into four groups (low, medium, high, and very high) on the basis of the top-performing risk factors.
A statistical model combining survey design and nonresponse weights enabled estimates to be representative of the U.S. population, based on the 2000 census.
Out-of-fold model prediction assessed performance of the model, using area under receiver operator curve (AUC), sensitivity, and specificity.
Daily functioning
Of all participants, 70.2% (n = 34,653; almost 60% women) completed wave 2 interviews. The weighted mean ages at waves 1 and 2 were 45.1 and 48.2 years, respectively.
Of wave 2 respondents, 0.6% (n = 222) attempted suicide during the preceding 3 years.
Half of those who attempted suicide within the first year were classified as “very high risk,” while 33.2% of those who attempted suicide between the first and second year and 33.3% of those who attempted suicide between the second and third year were classified as “very high risk.”
Among participants who attempted suicide between the third year and follow-up, 16.48% were classified as “very high risk.”
The model accurately captured classification of participants, even across demographic characteristics, such as age, sex, race, and income.
Younger individuals (aged 18-36 years) were at higher risk, compared with older individuals. In addition, women were at higher risk than were men, White participants were at higher risk than were non-White participants, and individuals with lower income were at greater risk than were those with higher income.
The model found that 1.8% of the U.S. population had a 10% or greater risk of a suicide attempt.
The most important risk factors identified were the three questions about previous suicidal ideation or behavior; three items from the 12-Item Short Form Health Survey (feeling downhearted, doing activities less carefully, or accomplishing less because of emotional problems); younger age; lower educational achievement; and recent financial crisis.
“The clinical assessment of suicide risk typically focuses on acute suicidal symptoms, together with depression, anxiety, substance misuse, and recent stressful events,” coinvestigator Mark Olfson, MD, PhD, professor of epidemiology, Columbia University Irving Medical Center, New York, said in an interview.
Dr. Olfson said.
Extra vigilance
Commenting on the study in an interview, April C. Foreman, PhD, an executive board member of the American Association of Suicidology, noted that some of the findings were not surprising.
“When discharging a patient from inpatient care, or seeing them in primary care, bring up mental health concerns proactively and ask whether they have ever attempted suicide or harmed themselves – even a long time ago – just as you ask about a family history of heart disease or cancer, or other health issues,” said Dr. Foreman, chief medical officer of the Kevin and Margaret Hines Foundation.
She noted that half of people who die by suicide have a primary care visit within the preceding month.
“Primary care is a great place to get a suicide history and follow the patient with extra vigilance, just as you would with any other risk factors,” Dr. Foreman said.
The study was funded by the National Institute on Alcohol Abuse and Alcoholism and its Intramural Program. The study authors and Dr. Foreman have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
New evidence shows that COVID-19 invades the brain
, new animal research suggests. Investigators injected spike 1 (S1), which is found on the tufts of the “red spikes” of the virus, into mice and found that it crossed the blood-brain barrier (BBB) and was taken up not only by brain regions and the brain space but also by other organs – specifically, the lungs, spleen, liver, and kidneys.
“We found that the S1 protein, which is the protein COVID-19 uses to ‘grab onto’ cells, crosses the BBB and is a good model of what the virus does when it enters the brain,” lead author William A. Banks, MD, professor of medicine, University of Washington, Seattle, said in an interview.
“When proteins such as the S1 protein become detached from the virus, they can enter the brain and cause mayhem, causing the brain to release cytokines, which, in turn, cause inflammation and subsequent neurotoxicity,” said Dr. Banks, associate chief of staff and a researcher at the Puget Sound Veterans Affairs Healthcare System.
The study was published online in Nature Neuroscience.
Neurologic symptoms
COVID-19 is associated with a variety of central nervous system symptoms, including the loss of taste and smell, headaches, confusion, stroke, and cerebral hemorrhage, the investigators noted.
Dr. Banks explained that SARS-CoV-2 may enter the brain by crossing the BBB, acting directly on the brain centers responsible for other body functions. The respiratory symptoms of COVID-19 may therefore result partly from the invasion of the areas of the brain responsible for respiratory functions, not only from the virus’ action at the site of the lungs.
The researchers set out to assess whether a particular viral protein – S1, which is a subunit of the viral spike protein – could cross the BBB or enter other organs when injected into mice. They found that, when intravenously injected S1 (I-S1) was cleared from the blood, tissues in multiple organs, including the lung, spleen, kidney, and liver, took it up.
Notably, uptake of I-S1 was higher in the liver, “suggesting that this protein is cleared from the blood predominantly by the liver,” Dr. Banks said. In addition, uptake by the lungs is “important, because that’s where many of the effects of the virus are,” he added.
The researchers found that I-S1 in the brains of the mice was “mostly degraded” 30 minutes following injection. “This indicates that I-S1 enters the BBB intact but is eventually degraded in the brain,” they wrote.
Moreover, by 30 minutes, more than half of the I-S1 proteins had crossed the capillary wall and had fully entered into the brain parenchymal and interstitial fluid spaces, as well as other regions.
More severe outcomes in men
The researchers then induced an inflammatory state in the mice through injection of lipopolysaccharide (LPS) and found that inflammation increased I-S1 uptake in both the brain and the lung (where uptake was increased by 101%). “These results show that inflammation could increase S1 toxicity for lung tissue by increasing its uptake,” the authors suggested. Moreover, inflammation also increased the entry of I-S1 into the brain, “likely due to BBB disruption.”
In human beings, male sex and APOE4 genotype are risk factors for both contracting COVID-19 and having a poor outcome, the authors noted. As a result, they examined I-S1 uptake in male and female mice that expressed human APOE3 or APOE4 (induced by a mouse ApoE promoter).
Multiple-comparison tests showed that among male mice that expressed human APOE3, the “fastest I-S1 uptake” was in the olfactory bulb, liver, and kidney. Female mice displayed increased APOE3 uptake in the spleen.
“This observation might relate to the increased susceptibility of men to more severe COVID-19 outcomes,” coauthor Jacob Raber, PhD, professor, departments of behavioral neuroscience, neurology, and radiation medicine, Oregon Health & Science University, Portland, said in a press release.
In addition to intravenous I-S1 injection, the researchers also investigated the effects of intranasal administration. They found that, although it also entered the brain, it did so at levels roughly 10 times lower than those induced by intravenous administration.
“Frightening tricks”
Dr. Banks said his laboratory has studied the BBB in conditions such as Alzheimer’s disease, obesity, diabetes, and HIV. “Our experience with viruses is that they do an incredible number of things and have a frightening number of tricks,” he said. In this case, “the virus is probably causing inflammation by releasing cytokines elsewhere in the body that get into the brain through the BBB.” Conversely, “the virus itself may enter the brain by crossing the BBB and directly cause brain cells to release their own cytokines,” he added.
An additional finding of the study is that, whatever the S1 protein does in the brain is a model for what the entire virus itself does, because these proteins often bring the viruses along with them, he added.
Dr. Banks said the clinical implications of the findings are that antibodies from those who have already had COVID-19 could potentially be directed against S1. Similarly, he added, so can COVID-19 vaccines, which induce production of S1.
“When an antibody locks onto something, it prevents it from crossing the BBB,” Dr. Banks noted.
Confirmatory findings
Commenting on the study, Howard E. Gendelman, MD, Margaret R. Larson Professor of Internal Medicine and Infectious Diseases and professor and chair of the department of pharmacology and experimental neuroscience, University of Nebraska, Omaha, said the study is confirmatory.
“What this paper highlights, and we have known for a long time, is that COVID-19 is a systemic, not only a respiratory, disease involving many organs and tissues and can yield not only pulmonary problems but also a whole host of cardiac, brain, and kidney problems,” he said.
“So the fact that these proteins are getting in [the brain] and are able to induce a reaction in the brain itself, and this is part of the complex progressive nature of COVID-19, is an important finding,” added Dr. Gendelman, director of the center for neurodegenerative disorders at the university. He was not involved with the study.
The study was supported by the Veterans Affairs Puget Sound Healthcare System and by grants from the National Institutes of Health. The authors and Dr. Gendelman have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new animal research suggests. Investigators injected spike 1 (S1), which is found on the tufts of the “red spikes” of the virus, into mice and found that it crossed the blood-brain barrier (BBB) and was taken up not only by brain regions and the brain space but also by other organs – specifically, the lungs, spleen, liver, and kidneys.
“We found that the S1 protein, which is the protein COVID-19 uses to ‘grab onto’ cells, crosses the BBB and is a good model of what the virus does when it enters the brain,” lead author William A. Banks, MD, professor of medicine, University of Washington, Seattle, said in an interview.
“When proteins such as the S1 protein become detached from the virus, they can enter the brain and cause mayhem, causing the brain to release cytokines, which, in turn, cause inflammation and subsequent neurotoxicity,” said Dr. Banks, associate chief of staff and a researcher at the Puget Sound Veterans Affairs Healthcare System.
The study was published online in Nature Neuroscience.
Neurologic symptoms
COVID-19 is associated with a variety of central nervous system symptoms, including the loss of taste and smell, headaches, confusion, stroke, and cerebral hemorrhage, the investigators noted.
Dr. Banks explained that SARS-CoV-2 may enter the brain by crossing the BBB, acting directly on the brain centers responsible for other body functions. The respiratory symptoms of COVID-19 may therefore result partly from the invasion of the areas of the brain responsible for respiratory functions, not only from the virus’ action at the site of the lungs.
The researchers set out to assess whether a particular viral protein – S1, which is a subunit of the viral spike protein – could cross the BBB or enter other organs when injected into mice. They found that, when intravenously injected S1 (I-S1) was cleared from the blood, tissues in multiple organs, including the lung, spleen, kidney, and liver, took it up.
Notably, uptake of I-S1 was higher in the liver, “suggesting that this protein is cleared from the blood predominantly by the liver,” Dr. Banks said. In addition, uptake by the lungs is “important, because that’s where many of the effects of the virus are,” he added.
The researchers found that I-S1 in the brains of the mice was “mostly degraded” 30 minutes following injection. “This indicates that I-S1 enters the BBB intact but is eventually degraded in the brain,” they wrote.
Moreover, by 30 minutes, more than half of the I-S1 proteins had crossed the capillary wall and had fully entered into the brain parenchymal and interstitial fluid spaces, as well as other regions.
More severe outcomes in men
The researchers then induced an inflammatory state in the mice through injection of lipopolysaccharide (LPS) and found that inflammation increased I-S1 uptake in both the brain and the lung (where uptake was increased by 101%). “These results show that inflammation could increase S1 toxicity for lung tissue by increasing its uptake,” the authors suggested. Moreover, inflammation also increased the entry of I-S1 into the brain, “likely due to BBB disruption.”
In human beings, male sex and APOE4 genotype are risk factors for both contracting COVID-19 and having a poor outcome, the authors noted. As a result, they examined I-S1 uptake in male and female mice that expressed human APOE3 or APOE4 (induced by a mouse ApoE promoter).
Multiple-comparison tests showed that among male mice that expressed human APOE3, the “fastest I-S1 uptake” was in the olfactory bulb, liver, and kidney. Female mice displayed increased APOE3 uptake in the spleen.
“This observation might relate to the increased susceptibility of men to more severe COVID-19 outcomes,” coauthor Jacob Raber, PhD, professor, departments of behavioral neuroscience, neurology, and radiation medicine, Oregon Health & Science University, Portland, said in a press release.
In addition to intravenous I-S1 injection, the researchers also investigated the effects of intranasal administration. They found that, although it also entered the brain, it did so at levels roughly 10 times lower than those induced by intravenous administration.
“Frightening tricks”
Dr. Banks said his laboratory has studied the BBB in conditions such as Alzheimer’s disease, obesity, diabetes, and HIV. “Our experience with viruses is that they do an incredible number of things and have a frightening number of tricks,” he said. In this case, “the virus is probably causing inflammation by releasing cytokines elsewhere in the body that get into the brain through the BBB.” Conversely, “the virus itself may enter the brain by crossing the BBB and directly cause brain cells to release their own cytokines,” he added.
An additional finding of the study is that, whatever the S1 protein does in the brain is a model for what the entire virus itself does, because these proteins often bring the viruses along with them, he added.
Dr. Banks said the clinical implications of the findings are that antibodies from those who have already had COVID-19 could potentially be directed against S1. Similarly, he added, so can COVID-19 vaccines, which induce production of S1.
“When an antibody locks onto something, it prevents it from crossing the BBB,” Dr. Banks noted.
Confirmatory findings
Commenting on the study, Howard E. Gendelman, MD, Margaret R. Larson Professor of Internal Medicine and Infectious Diseases and professor and chair of the department of pharmacology and experimental neuroscience, University of Nebraska, Omaha, said the study is confirmatory.
“What this paper highlights, and we have known for a long time, is that COVID-19 is a systemic, not only a respiratory, disease involving many organs and tissues and can yield not only pulmonary problems but also a whole host of cardiac, brain, and kidney problems,” he said.
“So the fact that these proteins are getting in [the brain] and are able to induce a reaction in the brain itself, and this is part of the complex progressive nature of COVID-19, is an important finding,” added Dr. Gendelman, director of the center for neurodegenerative disorders at the university. He was not involved with the study.
The study was supported by the Veterans Affairs Puget Sound Healthcare System and by grants from the National Institutes of Health. The authors and Dr. Gendelman have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new animal research suggests. Investigators injected spike 1 (S1), which is found on the tufts of the “red spikes” of the virus, into mice and found that it crossed the blood-brain barrier (BBB) and was taken up not only by brain regions and the brain space but also by other organs – specifically, the lungs, spleen, liver, and kidneys.
“We found that the S1 protein, which is the protein COVID-19 uses to ‘grab onto’ cells, crosses the BBB and is a good model of what the virus does when it enters the brain,” lead author William A. Banks, MD, professor of medicine, University of Washington, Seattle, said in an interview.
“When proteins such as the S1 protein become detached from the virus, they can enter the brain and cause mayhem, causing the brain to release cytokines, which, in turn, cause inflammation and subsequent neurotoxicity,” said Dr. Banks, associate chief of staff and a researcher at the Puget Sound Veterans Affairs Healthcare System.
The study was published online in Nature Neuroscience.
Neurologic symptoms
COVID-19 is associated with a variety of central nervous system symptoms, including the loss of taste and smell, headaches, confusion, stroke, and cerebral hemorrhage, the investigators noted.
Dr. Banks explained that SARS-CoV-2 may enter the brain by crossing the BBB, acting directly on the brain centers responsible for other body functions. The respiratory symptoms of COVID-19 may therefore result partly from the invasion of the areas of the brain responsible for respiratory functions, not only from the virus’ action at the site of the lungs.
The researchers set out to assess whether a particular viral protein – S1, which is a subunit of the viral spike protein – could cross the BBB or enter other organs when injected into mice. They found that, when intravenously injected S1 (I-S1) was cleared from the blood, tissues in multiple organs, including the lung, spleen, kidney, and liver, took it up.
Notably, uptake of I-S1 was higher in the liver, “suggesting that this protein is cleared from the blood predominantly by the liver,” Dr. Banks said. In addition, uptake by the lungs is “important, because that’s where many of the effects of the virus are,” he added.
The researchers found that I-S1 in the brains of the mice was “mostly degraded” 30 minutes following injection. “This indicates that I-S1 enters the BBB intact but is eventually degraded in the brain,” they wrote.
Moreover, by 30 minutes, more than half of the I-S1 proteins had crossed the capillary wall and had fully entered into the brain parenchymal and interstitial fluid spaces, as well as other regions.
More severe outcomes in men
The researchers then induced an inflammatory state in the mice through injection of lipopolysaccharide (LPS) and found that inflammation increased I-S1 uptake in both the brain and the lung (where uptake was increased by 101%). “These results show that inflammation could increase S1 toxicity for lung tissue by increasing its uptake,” the authors suggested. Moreover, inflammation also increased the entry of I-S1 into the brain, “likely due to BBB disruption.”
In human beings, male sex and APOE4 genotype are risk factors for both contracting COVID-19 and having a poor outcome, the authors noted. As a result, they examined I-S1 uptake in male and female mice that expressed human APOE3 or APOE4 (induced by a mouse ApoE promoter).
Multiple-comparison tests showed that among male mice that expressed human APOE3, the “fastest I-S1 uptake” was in the olfactory bulb, liver, and kidney. Female mice displayed increased APOE3 uptake in the spleen.
“This observation might relate to the increased susceptibility of men to more severe COVID-19 outcomes,” coauthor Jacob Raber, PhD, professor, departments of behavioral neuroscience, neurology, and radiation medicine, Oregon Health & Science University, Portland, said in a press release.
In addition to intravenous I-S1 injection, the researchers also investigated the effects of intranasal administration. They found that, although it also entered the brain, it did so at levels roughly 10 times lower than those induced by intravenous administration.
“Frightening tricks”
Dr. Banks said his laboratory has studied the BBB in conditions such as Alzheimer’s disease, obesity, diabetes, and HIV. “Our experience with viruses is that they do an incredible number of things and have a frightening number of tricks,” he said. In this case, “the virus is probably causing inflammation by releasing cytokines elsewhere in the body that get into the brain through the BBB.” Conversely, “the virus itself may enter the brain by crossing the BBB and directly cause brain cells to release their own cytokines,” he added.
An additional finding of the study is that, whatever the S1 protein does in the brain is a model for what the entire virus itself does, because these proteins often bring the viruses along with them, he added.
Dr. Banks said the clinical implications of the findings are that antibodies from those who have already had COVID-19 could potentially be directed against S1. Similarly, he added, so can COVID-19 vaccines, which induce production of S1.
“When an antibody locks onto something, it prevents it from crossing the BBB,” Dr. Banks noted.
Confirmatory findings
Commenting on the study, Howard E. Gendelman, MD, Margaret R. Larson Professor of Internal Medicine and Infectious Diseases and professor and chair of the department of pharmacology and experimental neuroscience, University of Nebraska, Omaha, said the study is confirmatory.
“What this paper highlights, and we have known for a long time, is that COVID-19 is a systemic, not only a respiratory, disease involving many organs and tissues and can yield not only pulmonary problems but also a whole host of cardiac, brain, and kidney problems,” he said.
“So the fact that these proteins are getting in [the brain] and are able to induce a reaction in the brain itself, and this is part of the complex progressive nature of COVID-19, is an important finding,” added Dr. Gendelman, director of the center for neurodegenerative disorders at the university. He was not involved with the study.
The study was supported by the Veterans Affairs Puget Sound Healthcare System and by grants from the National Institutes of Health. The authors and Dr. Gendelman have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM NATURE NEUROSCIENCE
Global experts map the latest in bipolar management
A new monograph offers a far-reaching update on research and clinical management of bipolar disorders (BDs), including epidemiology, genetics, pathogenesis, psychosocial aspects, and current and investigational therapies.
“I regard this as a ‘global state-of-the-union’ type of paper designed to bring the world up to speed regarding where we’re at and where we’re going in terms of bipolar disorder, to present the changes on the scientific and clinical fronts, and to open up a global conversation about bipolar disorder,” lead author Roger S. McIntyre, MD, professor of psychiatry and pharmacology, University of Toronto, Ontario, Canada, told Medscape Medical News.
“The paper is oriented toward multidisciplinary care, with particular emphasis on primary care, as well as people in healthcare administration and policy, who want a snapshot of where we’re at,” said McIntyre, who is also the head of the Mood Disorders Psychopharmacology Unit and director of the Depression and Bipolar Support Alliance in Chicago, Illinois.
The article was published online December 5 in The Lancet.
Severe, complex
The authors call BPs “a complex group of severe and chronic disorders” that include both BP I and BP II disorders.
“These disorders continue to be the world’s leading causes of disability, morbidity, and mortality, which are significant and getting worse, with studies indicating that bipolar disorders are associated with a loss of roughly 10 to 20 potential years of life,” McIntyre said.
Cardiovascular disease is the most common cause of premature death in people with BD. The second is suicide, the authors state, noting that patients with BDs are roughly 20-30 times more likely to die by suicide compared with the general population. In addition, 30%-50% have a lifetime history of suicide attempts.
BP I is “defined by the presence of a syndromal manic episode,” while BP II is “defined by the presence of a syndromal hypomanic episode and a major depressive episode,” the authors state.
Unlike the DSM-IV-TR, the DSM-5 includes “persistently increased energy or activity, along with elevated, expansive, or irritable mood” in the diagnostic criteria for mania and hypomania, “so diagnosing mania on mood instability alone is no longer sufficient,” the authors note.
In addition, clinicians “should be aware that individuals with BDs presenting with depression will often manifest symptoms of anxiety, agitation, anger-irritability, and attentional disturbance-distractibility (the four A’s), all of which are highly suggestive of mixed features,” they write.
Depression is the “predominant index presentation of BD” and “differentiating BD from major depressive disorder (MDD) is the most common clinical challenge for most clinicians.”
Features suggesting a diagnosis of BD rather than MDD include earlier age of onset, phenomenology (e.g., hyperphagia, hypersomnia, psychosis), higher frequency of affective episodes, comorbidities (e.g., substance use disorders, anxiety disorders, binge eating disorders, and migraines), family history of psychopathology, nonresponse to antidepressants or induction of hypomania, mixed features, and comorbidities
The authors advise “routine and systematic screening for BDs in all patients presenting with depressive symptomatology” and recommend using the Mood Disorders Questionnaire and the Hypomania Checklist.
Additional differential diagnoses include psychiatric disorders involving impulsivity, affective instability, anxiety, cognitive disorganization, depression, and psychosis.
“Futuristic” technology
“Although the pathogenesis of BDs is unknown, approximately 70% of the risk for BDs is heritable,” the authors note. They review recent research into genetic loci associated with BDs, based on genome-wide association studies, and the role of genetics not only in BDs but also in overlapping neurologic and psychiatric conditions, insulin resistance, and endocannabinoid signaling.
Inflammatory disturbances may also be implicated, in part related to “lifestyle and environment exposures” common in BDs such as smoking, poor diet, physical inactivity, and trauma, they suggest.
An “exciting new technology” analyzing “pluripotent” stem cells might illuminate the pathogenesis of BDs and mechanism of action of treatments by shedding light on mitochondrial dysfunction, McIntyre said.
“This interest in stem cells might almost be seen as futuristic. It is currently being used in the laboratory to understand the biology of BD, and it may eventually lead to the development of new therapeutics,” he added.
“Exciting” treatments
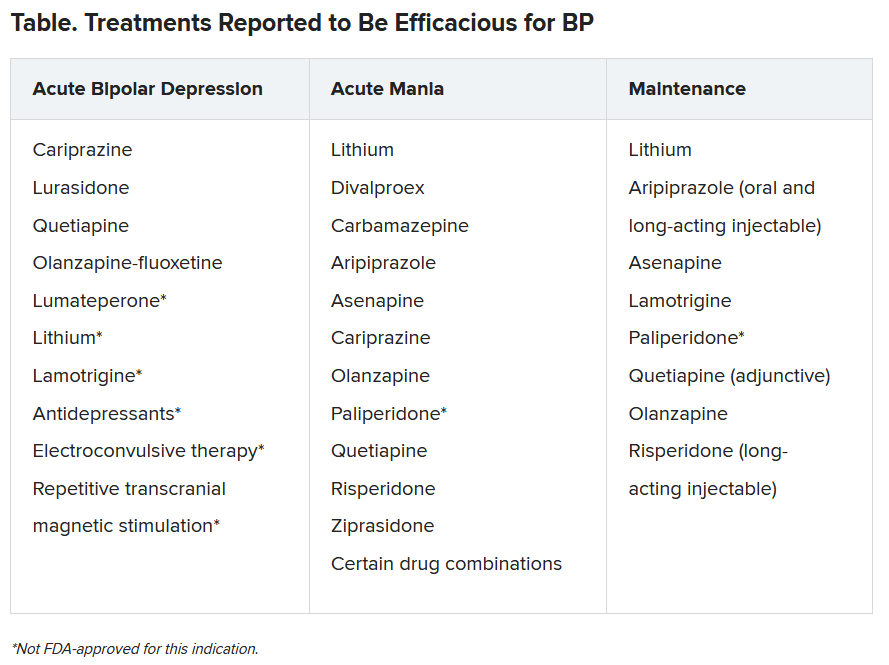
“Our expansive list of treatments and soon-to-be new treatments is very exciting,” said McIntyre.
The authors highlight “ongoing controversy regarding the safe and appropriate use of antidepressants in BD,” cautioning against potential treatment-emergent hypomania and suggesting limited circumstances when antidepressants might be administered.
Lithium remains the “gold standard mood-stabilizing agent” and is “capable of reducing suicidality,” they note.
Nonpharmacologic interventions include patient self-management, compliance, and cognitive enhancement strategies, primary prevention for psychiatric and medical comorbidity, psychosocial treatments and lifestyle interventions during maintenance, as well as surveillance for suicidality during both acute and maintenance phases.
Novel potential treatments include coenzyme Q10, N-acetyl cysteine, statins, nonsteroidal anti-inflammatory drugs, omega-3 fatty acids, incretin-based therapies, insulin, nitrous oxide, ketamine, prebiotics, probiotics, antibiotics, and adjunctive bright light therapy.
The authors caution that these investigational agents “cannot be considered efficacious or safe” in the treatment of BDs at present.
Call to action
Commenting for Medscape Medical News, Michael Thase, MD, professor of psychiatry, Perelman School of Medicine, University of Pennsylvania, Philadelphia, said he is glad that this “stellar group of authors” with “worldwide psychiatric expertise” wrote the article and he hopes it “gets the readership it deserves.”
Thase, who was not an author, said, “One takeaway is that BDs together comprise one of the world’s great public health problems — probably within the top 10.”
Another “has to do with our ability to do more with the tools we have — ie, ensuring diagnosis, implementing treatment, engaging social support, and using proven therapies from both psychopharmacologic and psychosocial domains.”
McIntyre characterized the article as a “public health call to action, incorporating screening, interesting neurobiological insights, an extensive set of treatments, and cool technological capabilities for the future.”
McIntyre has reported receiving grant support from the Stanley Medical Research Institute and the Canadian Institutes of Health Research/Global Alliance for Chronic Disease/Chinese National Natural Research Foundation, and speaker fees from Lundbeck, Janssen, Shire, Purdue, Pfizer, Otsuka, Allergan, Takeda, Neurocrine, Sunovion, Intra-Cellular, Alkermes, and Minerva, and is chief executive officer of Champignon. Disclosures for the other authors are listed in the article. Thase has reported consulting with and receiving research funding from many of the companies that manufacture/sell antidepressants and antipsychotics. He also has reported receiving royalties from the American Psychiatric Press Incorporated, Guilford Publications, Herald House, and W.W. Norton & Company.
A version of this article first appeared on Medscape.com.
A new monograph offers a far-reaching update on research and clinical management of bipolar disorders (BDs), including epidemiology, genetics, pathogenesis, psychosocial aspects, and current and investigational therapies.
“I regard this as a ‘global state-of-the-union’ type of paper designed to bring the world up to speed regarding where we’re at and where we’re going in terms of bipolar disorder, to present the changes on the scientific and clinical fronts, and to open up a global conversation about bipolar disorder,” lead author Roger S. McIntyre, MD, professor of psychiatry and pharmacology, University of Toronto, Ontario, Canada, told Medscape Medical News.
“The paper is oriented toward multidisciplinary care, with particular emphasis on primary care, as well as people in healthcare administration and policy, who want a snapshot of where we’re at,” said McIntyre, who is also the head of the Mood Disorders Psychopharmacology Unit and director of the Depression and Bipolar Support Alliance in Chicago, Illinois.
The article was published online December 5 in The Lancet.
Severe, complex
The authors call BPs “a complex group of severe and chronic disorders” that include both BP I and BP II disorders.
“These disorders continue to be the world’s leading causes of disability, morbidity, and mortality, which are significant and getting worse, with studies indicating that bipolar disorders are associated with a loss of roughly 10 to 20 potential years of life,” McIntyre said.
Cardiovascular disease is the most common cause of premature death in people with BD. The second is suicide, the authors state, noting that patients with BDs are roughly 20-30 times more likely to die by suicide compared with the general population. In addition, 30%-50% have a lifetime history of suicide attempts.
BP I is “defined by the presence of a syndromal manic episode,” while BP II is “defined by the presence of a syndromal hypomanic episode and a major depressive episode,” the authors state.
Unlike the DSM-IV-TR, the DSM-5 includes “persistently increased energy or activity, along with elevated, expansive, or irritable mood” in the diagnostic criteria for mania and hypomania, “so diagnosing mania on mood instability alone is no longer sufficient,” the authors note.
In addition, clinicians “should be aware that individuals with BDs presenting with depression will often manifest symptoms of anxiety, agitation, anger-irritability, and attentional disturbance-distractibility (the four A’s), all of which are highly suggestive of mixed features,” they write.
Depression is the “predominant index presentation of BD” and “differentiating BD from major depressive disorder (MDD) is the most common clinical challenge for most clinicians.”
Features suggesting a diagnosis of BD rather than MDD include earlier age of onset, phenomenology (e.g., hyperphagia, hypersomnia, psychosis), higher frequency of affective episodes, comorbidities (e.g., substance use disorders, anxiety disorders, binge eating disorders, and migraines), family history of psychopathology, nonresponse to antidepressants or induction of hypomania, mixed features, and comorbidities
The authors advise “routine and systematic screening for BDs in all patients presenting with depressive symptomatology” and recommend using the Mood Disorders Questionnaire and the Hypomania Checklist.
Additional differential diagnoses include psychiatric disorders involving impulsivity, affective instability, anxiety, cognitive disorganization, depression, and psychosis.
“Futuristic” technology
“Although the pathogenesis of BDs is unknown, approximately 70% of the risk for BDs is heritable,” the authors note. They review recent research into genetic loci associated with BDs, based on genome-wide association studies, and the role of genetics not only in BDs but also in overlapping neurologic and psychiatric conditions, insulin resistance, and endocannabinoid signaling.
Inflammatory disturbances may also be implicated, in part related to “lifestyle and environment exposures” common in BDs such as smoking, poor diet, physical inactivity, and trauma, they suggest.
An “exciting new technology” analyzing “pluripotent” stem cells might illuminate the pathogenesis of BDs and mechanism of action of treatments by shedding light on mitochondrial dysfunction, McIntyre said.
“This interest in stem cells might almost be seen as futuristic. It is currently being used in the laboratory to understand the biology of BD, and it may eventually lead to the development of new therapeutics,” he added.
“Exciting” treatments
“Our expansive list of treatments and soon-to-be new treatments is very exciting,” said McIntyre.
The authors highlight “ongoing controversy regarding the safe and appropriate use of antidepressants in BD,” cautioning against potential treatment-emergent hypomania and suggesting limited circumstances when antidepressants might be administered.
Lithium remains the “gold standard mood-stabilizing agent” and is “capable of reducing suicidality,” they note.
Nonpharmacologic interventions include patient self-management, compliance, and cognitive enhancement strategies, primary prevention for psychiatric and medical comorbidity, psychosocial treatments and lifestyle interventions during maintenance, as well as surveillance for suicidality during both acute and maintenance phases.
Novel potential treatments include coenzyme Q10, N-acetyl cysteine, statins, nonsteroidal anti-inflammatory drugs, omega-3 fatty acids, incretin-based therapies, insulin, nitrous oxide, ketamine, prebiotics, probiotics, antibiotics, and adjunctive bright light therapy.
The authors caution that these investigational agents “cannot be considered efficacious or safe” in the treatment of BDs at present.
Call to action
Commenting for Medscape Medical News, Michael Thase, MD, professor of psychiatry, Perelman School of Medicine, University of Pennsylvania, Philadelphia, said he is glad that this “stellar group of authors” with “worldwide psychiatric expertise” wrote the article and he hopes it “gets the readership it deserves.”
Thase, who was not an author, said, “One takeaway is that BDs together comprise one of the world’s great public health problems — probably within the top 10.”
Another “has to do with our ability to do more with the tools we have — ie, ensuring diagnosis, implementing treatment, engaging social support, and using proven therapies from both psychopharmacologic and psychosocial domains.”
McIntyre characterized the article as a “public health call to action, incorporating screening, interesting neurobiological insights, an extensive set of treatments, and cool technological capabilities for the future.”
McIntyre has reported receiving grant support from the Stanley Medical Research Institute and the Canadian Institutes of Health Research/Global Alliance for Chronic Disease/Chinese National Natural Research Foundation, and speaker fees from Lundbeck, Janssen, Shire, Purdue, Pfizer, Otsuka, Allergan, Takeda, Neurocrine, Sunovion, Intra-Cellular, Alkermes, and Minerva, and is chief executive officer of Champignon. Disclosures for the other authors are listed in the article. Thase has reported consulting with and receiving research funding from many of the companies that manufacture/sell antidepressants and antipsychotics. He also has reported receiving royalties from the American Psychiatric Press Incorporated, Guilford Publications, Herald House, and W.W. Norton & Company.
A version of this article first appeared on Medscape.com.
A new monograph offers a far-reaching update on research and clinical management of bipolar disorders (BDs), including epidemiology, genetics, pathogenesis, psychosocial aspects, and current and investigational therapies.
“I regard this as a ‘global state-of-the-union’ type of paper designed to bring the world up to speed regarding where we’re at and where we’re going in terms of bipolar disorder, to present the changes on the scientific and clinical fronts, and to open up a global conversation about bipolar disorder,” lead author Roger S. McIntyre, MD, professor of psychiatry and pharmacology, University of Toronto, Ontario, Canada, told Medscape Medical News.
“The paper is oriented toward multidisciplinary care, with particular emphasis on primary care, as well as people in healthcare administration and policy, who want a snapshot of where we’re at,” said McIntyre, who is also the head of the Mood Disorders Psychopharmacology Unit and director of the Depression and Bipolar Support Alliance in Chicago, Illinois.
The article was published online December 5 in The Lancet.
Severe, complex
The authors call BPs “a complex group of severe and chronic disorders” that include both BP I and BP II disorders.
“These disorders continue to be the world’s leading causes of disability, morbidity, and mortality, which are significant and getting worse, with studies indicating that bipolar disorders are associated with a loss of roughly 10 to 20 potential years of life,” McIntyre said.
Cardiovascular disease is the most common cause of premature death in people with BD. The second is suicide, the authors state, noting that patients with BDs are roughly 20-30 times more likely to die by suicide compared with the general population. In addition, 30%-50% have a lifetime history of suicide attempts.
BP I is “defined by the presence of a syndromal manic episode,” while BP II is “defined by the presence of a syndromal hypomanic episode and a major depressive episode,” the authors state.
Unlike the DSM-IV-TR, the DSM-5 includes “persistently increased energy or activity, along with elevated, expansive, or irritable mood” in the diagnostic criteria for mania and hypomania, “so diagnosing mania on mood instability alone is no longer sufficient,” the authors note.
In addition, clinicians “should be aware that individuals with BDs presenting with depression will often manifest symptoms of anxiety, agitation, anger-irritability, and attentional disturbance-distractibility (the four A’s), all of which are highly suggestive of mixed features,” they write.
Depression is the “predominant index presentation of BD” and “differentiating BD from major depressive disorder (MDD) is the most common clinical challenge for most clinicians.”
Features suggesting a diagnosis of BD rather than MDD include earlier age of onset, phenomenology (e.g., hyperphagia, hypersomnia, psychosis), higher frequency of affective episodes, comorbidities (e.g., substance use disorders, anxiety disorders, binge eating disorders, and migraines), family history of psychopathology, nonresponse to antidepressants or induction of hypomania, mixed features, and comorbidities
The authors advise “routine and systematic screening for BDs in all patients presenting with depressive symptomatology” and recommend using the Mood Disorders Questionnaire and the Hypomania Checklist.
Additional differential diagnoses include psychiatric disorders involving impulsivity, affective instability, anxiety, cognitive disorganization, depression, and psychosis.
“Futuristic” technology
“Although the pathogenesis of BDs is unknown, approximately 70% of the risk for BDs is heritable,” the authors note. They review recent research into genetic loci associated with BDs, based on genome-wide association studies, and the role of genetics not only in BDs but also in overlapping neurologic and psychiatric conditions, insulin resistance, and endocannabinoid signaling.
Inflammatory disturbances may also be implicated, in part related to “lifestyle and environment exposures” common in BDs such as smoking, poor diet, physical inactivity, and trauma, they suggest.
An “exciting new technology” analyzing “pluripotent” stem cells might illuminate the pathogenesis of BDs and mechanism of action of treatments by shedding light on mitochondrial dysfunction, McIntyre said.
“This interest in stem cells might almost be seen as futuristic. It is currently being used in the laboratory to understand the biology of BD, and it may eventually lead to the development of new therapeutics,” he added.
“Exciting” treatments
“Our expansive list of treatments and soon-to-be new treatments is very exciting,” said McIntyre.
The authors highlight “ongoing controversy regarding the safe and appropriate use of antidepressants in BD,” cautioning against potential treatment-emergent hypomania and suggesting limited circumstances when antidepressants might be administered.
Lithium remains the “gold standard mood-stabilizing agent” and is “capable of reducing suicidality,” they note.
Nonpharmacologic interventions include patient self-management, compliance, and cognitive enhancement strategies, primary prevention for psychiatric and medical comorbidity, psychosocial treatments and lifestyle interventions during maintenance, as well as surveillance for suicidality during both acute and maintenance phases.
Novel potential treatments include coenzyme Q10, N-acetyl cysteine, statins, nonsteroidal anti-inflammatory drugs, omega-3 fatty acids, incretin-based therapies, insulin, nitrous oxide, ketamine, prebiotics, probiotics, antibiotics, and adjunctive bright light therapy.
The authors caution that these investigational agents “cannot be considered efficacious or safe” in the treatment of BDs at present.
Call to action
Commenting for Medscape Medical News, Michael Thase, MD, professor of psychiatry, Perelman School of Medicine, University of Pennsylvania, Philadelphia, said he is glad that this “stellar group of authors” with “worldwide psychiatric expertise” wrote the article and he hopes it “gets the readership it deserves.”
Thase, who was not an author, said, “One takeaway is that BDs together comprise one of the world’s great public health problems — probably within the top 10.”
Another “has to do with our ability to do more with the tools we have — ie, ensuring diagnosis, implementing treatment, engaging social support, and using proven therapies from both psychopharmacologic and psychosocial domains.”
McIntyre characterized the article as a “public health call to action, incorporating screening, interesting neurobiological insights, an extensive set of treatments, and cool technological capabilities for the future.”
McIntyre has reported receiving grant support from the Stanley Medical Research Institute and the Canadian Institutes of Health Research/Global Alliance for Chronic Disease/Chinese National Natural Research Foundation, and speaker fees from Lundbeck, Janssen, Shire, Purdue, Pfizer, Otsuka, Allergan, Takeda, Neurocrine, Sunovion, Intra-Cellular, Alkermes, and Minerva, and is chief executive officer of Champignon. Disclosures for the other authors are listed in the article. Thase has reported consulting with and receiving research funding from many of the companies that manufacture/sell antidepressants and antipsychotics. He also has reported receiving royalties from the American Psychiatric Press Incorporated, Guilford Publications, Herald House, and W.W. Norton & Company.
A version of this article first appeared on Medscape.com.
LDL cholesterol not the primary culprit in ASCVD?
Two new studies suggest that LDL cholesterol (LDL-C) may be not the main driver of atherosclerotic cardiovascular disease (ASCVD).
The findings instead implicate remnant cholesterol (remnant-C) and very-low-density lipoprotein (VLDL) cholesterol in the development of cardiovascular disease (CVD) and MI.
The PREDIMED study, conducted in Spain, examined the association of triglycerides and remnant-C with major cardiovascular events (MACE) in older individuals with high CVD risk. It found that levels of triglycerides and remnant-C were associated with MACE independently of other risk factors, but there was no similar association with LDL-C.
“These findings lead [clinicians] to consider in the clinical management of dyslipidemias a greater control of the lipid profiles as a whole, including remnant-cholesterol and/or triglycerides,” Montserrat Fitó Colomer, MD, PhD, of the Cardiovascular Risk and Nutrition Research Group, Hospital del Mar Medical Research Institute, Barcelona, said in an interview.
In a separate analysis, the Copenhagen General Population Study, which focused on 25,000 individuals who were not taking lipid-lowering therapy, looked at the role of VLDL cholesterol and triglycerides in driving MI risk from apolipoprotein B (apoB)–containing lipoproteins.
“Elevated VLDL cholesterol explained a larger fraction of risk than did elevated LDL cholesterol, or elevated VLDL triglycerides,” Børge G. Nordestgaard, MD, DMSc, professor, University of Copenhagen, said in an interview.
Both studies were published online Nov. 30 in the Journal of the American College of Cardiology.
But in an editorial accompanying both reports, John Burnett, MD, PhD, from the University of Western Australia, Perth, and colleagues cautioned that it would be “premature to discard LDL-C based on PREDIMED.”
The findings are “insufficient to offset the mountain of literally hundreds of studies that uphold the value of LDL-C in prediction and intervention of ASCVD,” Dr. Burnett and coauthors wrote.
Similarly, the editorialists cautioned that, although the findings from the study by Dr. Nordestgaard and colleagues indicate that VLDL cholesterol is the “new kid in town for prediction, LDL cholesterol retains predictive power.” Clinical cardiologists should not “shelve LDL cholesterol and embrace VLDL and remnant cholesterol as the new oracles of ASCVD risk.”
In a comment, Dr. Burnett said, “The take-home message for clinicians in both papers is that LDL-C is the main lipid measurement to guide clinical decisions; however, residual risk of atherosclerotic cardiovascular disease remains, even after LCL-C is treated.
“Assessment of residual ASCVD risk with nontraditional lipid biomarkers, including VLDL cholesterol and remnant cholesterol, as well as lipoprotein (a) and apoB, may improve prognostication and help guide preventive treatments,” he added.
“Affordable and inexpensive”
In their report, the PREDIMED study authors explained that atherogenic dyslipidemia is characterized by “an excess of serum triglycerides” contained in VLDL cholesterol, intermediate-density lipoproteins, and their remnants, all of which are called “triglyceride-rich lipoproteins (TRLs).”
TRLs and remnant-C “have the capacity to cross the arterial wall,” and may therefore play a causal role in atherosclerosis development, they wrote.
The main PREDIMED trial compared a low-fat diet with the Mediterranean diet for the primary prevention of CVD in high-risk participants. Those enrolled in the trial “had a high prevalence of diabetes, obesity, and metabolic syndrome, conditions that are associated with insulin resistance, hypertriglyceridemia, and atherogenic dyslipidemia,” the authors wrote. “Thus, this cohort of subjects at high cardiovascular risk was well suited to investigate the association of triglycerides and TRLs with cardiovascular outcomes.”
The researchers investigated the role of triglycerides and remnant-C in incident CVD among these high-risk individuals, particularly those with chronic cardiometabolic disorders (prediabetes, type 2 diabetes, and poorly controlled diabetes), overweight and obesity, metabolic syndrome, and renal failure.
Their 6,901 participants (42.6% male, mean age 67 years, mean BMI 30.0 kg/m2) had a diagnosis of type 2 diabetes or at least three CVD risk factors including current smoking, hypertension, elevated LDL-C levels, low HDL cholesterol levels, elevated body mass index, or family history of premature coronary heart disease.
The primary study endpoint was a composite of adverse cardiovascular events (MACE): MI, stroke, or cardiovascular death. Participants were followed for a mean of 4.8 years, during which there was a total of 263 MACE events.
Multivariable-adjusted analyses showed that levels of triglycerides and remnant-C were both associated with MACE independent of other risk factors (hazard ratio, 1.04; 95% confidence interval, 1.02-1.06; and HR, 1.21; 95% CI, 1.10-1.33 per 10 mg/dl, respectively, both P < .001). Non–HDL cholesterol was also associated with MACE (HR, 1.05; 95% CI, 1.01-1.10 per 10 mg/dl, P = .026).
In particular, elevated remnant-C (≥30 mg/dL), compared with lower concentrations, flagged subjects at a higher risk of MACE, even if their LDL-C levels were at target (defined as ≤ 100 mg/dL).
Levels of LDL-C and HDL cholesterol were not associated with MACE.
“The indirect calculation of remnant-C is an affordable and inexpensive method, which could provide valuable data for clinical management,” Dr. Fitó Colomer said.
“The results of this study suggest that, in individuals at high cardiovascular risk with well-controlled LDL-C, triglycerides and mainly remnant-C should be considered as a treatment target,” she proposed.
New oracles?
Evidence has pointed to triglyceride-rich remnants or VLDL cholesterol as contributing to atherosclerotic CVD, together with LDL-C, but it is “unclear which fraction of risk is explained by, respectively, cholesterol and triglycerides in VLDL,” write the authors of the Copenhagen population study.
Dr. Nordestgaard said their study was motivated by an awareness that “in clinical practice, the focus for lipid-related risk is almost solely on reduction of LDL-C for prevention of ASCVD,” so the current focus needs to be reevaluated because patients with low LDL-C but elevated VLDL cholesterol and plasma triglycerides “may not be offered adequate preventive lipid-lowering therapy in order to prevent future MI and ASCVD.”
His group therefore tested the hypothesis that VLDL cholesterol and triglycerides may each explain part of the MI risk from apoB-containing lipoproteins.
They used measurements of plasma apoB and cholesterol and triglyceride content of VLDL cholesterol, intermediate-density-lipoprotein cholesterol, and LDL-C in the study participants (N = 25,480, median age 61 years, 53% female), who were required to be free of MI and not receiving lipid-lowering therapy at baseline.
During a median 11-year follow-up period, 1,816 participants experienced an MI. They tended to be older, compared with those who did not experience an MI, and also more likely to be male, to smoke, and to have higher systolic blood pressure.
Each 39-mg/dL increase in lipid level was found to be associated with higher MI risk.
The researchers looked at MI-associated risk of specific subfractions of apoB-containing lipoproteins. “VLDL cholesterol explained half of the MI risk from elevated apoB-containing lipoproteins, and [intermediate-density-lipoprotein] and LDL-C together accounted for only 29% of the risk,” Dr. Nordestgaard said.
“If LDL cholesterol is adequately reduced, clinicians need to evaluate possible elevated triglyceride-rich lipoproteins, either as elevated plasma triglycerides, remnant cholesterol, or elevated VLDL cholesterol; and, if elevated, consideration should also be given to reduction of triglyceride-rich lipoproteins,” he advised.
The Copenhagen General Population study was funded by the Danish Heart Foundation and the Novo Nordisk Foundation. Dr. Nordestgaard disclosed consulting for AstraZeneca, Sanofi, Regeneron, Akcea, Amgen, Kowa, Denka Seiken, Amarin, Novartis, Novo Nordisk, and Silence Therapeutrics. PREDIMED was supported by grants from the Instituto de Salud Carlos III- FEDER, Fundació La Marató de TV3, and Agència de Gestió d’Ajuts Universitaris i de Recerca. Dr. Fitó Colomer disclosed no relevant financial relationships. Dr. Burnett disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Two new studies suggest that LDL cholesterol (LDL-C) may be not the main driver of atherosclerotic cardiovascular disease (ASCVD).
The findings instead implicate remnant cholesterol (remnant-C) and very-low-density lipoprotein (VLDL) cholesterol in the development of cardiovascular disease (CVD) and MI.
The PREDIMED study, conducted in Spain, examined the association of triglycerides and remnant-C with major cardiovascular events (MACE) in older individuals with high CVD risk. It found that levels of triglycerides and remnant-C were associated with MACE independently of other risk factors, but there was no similar association with LDL-C.
“These findings lead [clinicians] to consider in the clinical management of dyslipidemias a greater control of the lipid profiles as a whole, including remnant-cholesterol and/or triglycerides,” Montserrat Fitó Colomer, MD, PhD, of the Cardiovascular Risk and Nutrition Research Group, Hospital del Mar Medical Research Institute, Barcelona, said in an interview.
In a separate analysis, the Copenhagen General Population Study, which focused on 25,000 individuals who were not taking lipid-lowering therapy, looked at the role of VLDL cholesterol and triglycerides in driving MI risk from apolipoprotein B (apoB)–containing lipoproteins.
“Elevated VLDL cholesterol explained a larger fraction of risk than did elevated LDL cholesterol, or elevated VLDL triglycerides,” Børge G. Nordestgaard, MD, DMSc, professor, University of Copenhagen, said in an interview.
Both studies were published online Nov. 30 in the Journal of the American College of Cardiology.
But in an editorial accompanying both reports, John Burnett, MD, PhD, from the University of Western Australia, Perth, and colleagues cautioned that it would be “premature to discard LDL-C based on PREDIMED.”
The findings are “insufficient to offset the mountain of literally hundreds of studies that uphold the value of LDL-C in prediction and intervention of ASCVD,” Dr. Burnett and coauthors wrote.
Similarly, the editorialists cautioned that, although the findings from the study by Dr. Nordestgaard and colleagues indicate that VLDL cholesterol is the “new kid in town for prediction, LDL cholesterol retains predictive power.” Clinical cardiologists should not “shelve LDL cholesterol and embrace VLDL and remnant cholesterol as the new oracles of ASCVD risk.”
In a comment, Dr. Burnett said, “The take-home message for clinicians in both papers is that LDL-C is the main lipid measurement to guide clinical decisions; however, residual risk of atherosclerotic cardiovascular disease remains, even after LCL-C is treated.
“Assessment of residual ASCVD risk with nontraditional lipid biomarkers, including VLDL cholesterol and remnant cholesterol, as well as lipoprotein (a) and apoB, may improve prognostication and help guide preventive treatments,” he added.
“Affordable and inexpensive”
In their report, the PREDIMED study authors explained that atherogenic dyslipidemia is characterized by “an excess of serum triglycerides” contained in VLDL cholesterol, intermediate-density lipoproteins, and their remnants, all of which are called “triglyceride-rich lipoproteins (TRLs).”
TRLs and remnant-C “have the capacity to cross the arterial wall,” and may therefore play a causal role in atherosclerosis development, they wrote.
The main PREDIMED trial compared a low-fat diet with the Mediterranean diet for the primary prevention of CVD in high-risk participants. Those enrolled in the trial “had a high prevalence of diabetes, obesity, and metabolic syndrome, conditions that are associated with insulin resistance, hypertriglyceridemia, and atherogenic dyslipidemia,” the authors wrote. “Thus, this cohort of subjects at high cardiovascular risk was well suited to investigate the association of triglycerides and TRLs with cardiovascular outcomes.”
The researchers investigated the role of triglycerides and remnant-C in incident CVD among these high-risk individuals, particularly those with chronic cardiometabolic disorders (prediabetes, type 2 diabetes, and poorly controlled diabetes), overweight and obesity, metabolic syndrome, and renal failure.
Their 6,901 participants (42.6% male, mean age 67 years, mean BMI 30.0 kg/m2) had a diagnosis of type 2 diabetes or at least three CVD risk factors including current smoking, hypertension, elevated LDL-C levels, low HDL cholesterol levels, elevated body mass index, or family history of premature coronary heart disease.
The primary study endpoint was a composite of adverse cardiovascular events (MACE): MI, stroke, or cardiovascular death. Participants were followed for a mean of 4.8 years, during which there was a total of 263 MACE events.
Multivariable-adjusted analyses showed that levels of triglycerides and remnant-C were both associated with MACE independent of other risk factors (hazard ratio, 1.04; 95% confidence interval, 1.02-1.06; and HR, 1.21; 95% CI, 1.10-1.33 per 10 mg/dl, respectively, both P < .001). Non–HDL cholesterol was also associated with MACE (HR, 1.05; 95% CI, 1.01-1.10 per 10 mg/dl, P = .026).
In particular, elevated remnant-C (≥30 mg/dL), compared with lower concentrations, flagged subjects at a higher risk of MACE, even if their LDL-C levels were at target (defined as ≤ 100 mg/dL).
Levels of LDL-C and HDL cholesterol were not associated with MACE.
“The indirect calculation of remnant-C is an affordable and inexpensive method, which could provide valuable data for clinical management,” Dr. Fitó Colomer said.
“The results of this study suggest that, in individuals at high cardiovascular risk with well-controlled LDL-C, triglycerides and mainly remnant-C should be considered as a treatment target,” she proposed.
New oracles?
Evidence has pointed to triglyceride-rich remnants or VLDL cholesterol as contributing to atherosclerotic CVD, together with LDL-C, but it is “unclear which fraction of risk is explained by, respectively, cholesterol and triglycerides in VLDL,” write the authors of the Copenhagen population study.
Dr. Nordestgaard said their study was motivated by an awareness that “in clinical practice, the focus for lipid-related risk is almost solely on reduction of LDL-C for prevention of ASCVD,” so the current focus needs to be reevaluated because patients with low LDL-C but elevated VLDL cholesterol and plasma triglycerides “may not be offered adequate preventive lipid-lowering therapy in order to prevent future MI and ASCVD.”
His group therefore tested the hypothesis that VLDL cholesterol and triglycerides may each explain part of the MI risk from apoB-containing lipoproteins.
They used measurements of plasma apoB and cholesterol and triglyceride content of VLDL cholesterol, intermediate-density-lipoprotein cholesterol, and LDL-C in the study participants (N = 25,480, median age 61 years, 53% female), who were required to be free of MI and not receiving lipid-lowering therapy at baseline.
During a median 11-year follow-up period, 1,816 participants experienced an MI. They tended to be older, compared with those who did not experience an MI, and also more likely to be male, to smoke, and to have higher systolic blood pressure.
Each 39-mg/dL increase in lipid level was found to be associated with higher MI risk.
The researchers looked at MI-associated risk of specific subfractions of apoB-containing lipoproteins. “VLDL cholesterol explained half of the MI risk from elevated apoB-containing lipoproteins, and [intermediate-density-lipoprotein] and LDL-C together accounted for only 29% of the risk,” Dr. Nordestgaard said.
“If LDL cholesterol is adequately reduced, clinicians need to evaluate possible elevated triglyceride-rich lipoproteins, either as elevated plasma triglycerides, remnant cholesterol, or elevated VLDL cholesterol; and, if elevated, consideration should also be given to reduction of triglyceride-rich lipoproteins,” he advised.
The Copenhagen General Population study was funded by the Danish Heart Foundation and the Novo Nordisk Foundation. Dr. Nordestgaard disclosed consulting for AstraZeneca, Sanofi, Regeneron, Akcea, Amgen, Kowa, Denka Seiken, Amarin, Novartis, Novo Nordisk, and Silence Therapeutrics. PREDIMED was supported by grants from the Instituto de Salud Carlos III- FEDER, Fundació La Marató de TV3, and Agència de Gestió d’Ajuts Universitaris i de Recerca. Dr. Fitó Colomer disclosed no relevant financial relationships. Dr. Burnett disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Two new studies suggest that LDL cholesterol (LDL-C) may be not the main driver of atherosclerotic cardiovascular disease (ASCVD).
The findings instead implicate remnant cholesterol (remnant-C) and very-low-density lipoprotein (VLDL) cholesterol in the development of cardiovascular disease (CVD) and MI.
The PREDIMED study, conducted in Spain, examined the association of triglycerides and remnant-C with major cardiovascular events (MACE) in older individuals with high CVD risk. It found that levels of triglycerides and remnant-C were associated with MACE independently of other risk factors, but there was no similar association with LDL-C.
“These findings lead [clinicians] to consider in the clinical management of dyslipidemias a greater control of the lipid profiles as a whole, including remnant-cholesterol and/or triglycerides,” Montserrat Fitó Colomer, MD, PhD, of the Cardiovascular Risk and Nutrition Research Group, Hospital del Mar Medical Research Institute, Barcelona, said in an interview.
In a separate analysis, the Copenhagen General Population Study, which focused on 25,000 individuals who were not taking lipid-lowering therapy, looked at the role of VLDL cholesterol and triglycerides in driving MI risk from apolipoprotein B (apoB)–containing lipoproteins.
“Elevated VLDL cholesterol explained a larger fraction of risk than did elevated LDL cholesterol, or elevated VLDL triglycerides,” Børge G. Nordestgaard, MD, DMSc, professor, University of Copenhagen, said in an interview.
Both studies were published online Nov. 30 in the Journal of the American College of Cardiology.
But in an editorial accompanying both reports, John Burnett, MD, PhD, from the University of Western Australia, Perth, and colleagues cautioned that it would be “premature to discard LDL-C based on PREDIMED.”
The findings are “insufficient to offset the mountain of literally hundreds of studies that uphold the value of LDL-C in prediction and intervention of ASCVD,” Dr. Burnett and coauthors wrote.
Similarly, the editorialists cautioned that, although the findings from the study by Dr. Nordestgaard and colleagues indicate that VLDL cholesterol is the “new kid in town for prediction, LDL cholesterol retains predictive power.” Clinical cardiologists should not “shelve LDL cholesterol and embrace VLDL and remnant cholesterol as the new oracles of ASCVD risk.”
In a comment, Dr. Burnett said, “The take-home message for clinicians in both papers is that LDL-C is the main lipid measurement to guide clinical decisions; however, residual risk of atherosclerotic cardiovascular disease remains, even after LCL-C is treated.
“Assessment of residual ASCVD risk with nontraditional lipid biomarkers, including VLDL cholesterol and remnant cholesterol, as well as lipoprotein (a) and apoB, may improve prognostication and help guide preventive treatments,” he added.
“Affordable and inexpensive”
In their report, the PREDIMED study authors explained that atherogenic dyslipidemia is characterized by “an excess of serum triglycerides” contained in VLDL cholesterol, intermediate-density lipoproteins, and their remnants, all of which are called “triglyceride-rich lipoproteins (TRLs).”
TRLs and remnant-C “have the capacity to cross the arterial wall,” and may therefore play a causal role in atherosclerosis development, they wrote.
The main PREDIMED trial compared a low-fat diet with the Mediterranean diet for the primary prevention of CVD in high-risk participants. Those enrolled in the trial “had a high prevalence of diabetes, obesity, and metabolic syndrome, conditions that are associated with insulin resistance, hypertriglyceridemia, and atherogenic dyslipidemia,” the authors wrote. “Thus, this cohort of subjects at high cardiovascular risk was well suited to investigate the association of triglycerides and TRLs with cardiovascular outcomes.”
The researchers investigated the role of triglycerides and remnant-C in incident CVD among these high-risk individuals, particularly those with chronic cardiometabolic disorders (prediabetes, type 2 diabetes, and poorly controlled diabetes), overweight and obesity, metabolic syndrome, and renal failure.
Their 6,901 participants (42.6% male, mean age 67 years, mean BMI 30.0 kg/m2) had a diagnosis of type 2 diabetes or at least three CVD risk factors including current smoking, hypertension, elevated LDL-C levels, low HDL cholesterol levels, elevated body mass index, or family history of premature coronary heart disease.
The primary study endpoint was a composite of adverse cardiovascular events (MACE): MI, stroke, or cardiovascular death. Participants were followed for a mean of 4.8 years, during which there was a total of 263 MACE events.
Multivariable-adjusted analyses showed that levels of triglycerides and remnant-C were both associated with MACE independent of other risk factors (hazard ratio, 1.04; 95% confidence interval, 1.02-1.06; and HR, 1.21; 95% CI, 1.10-1.33 per 10 mg/dl, respectively, both P < .001). Non–HDL cholesterol was also associated with MACE (HR, 1.05; 95% CI, 1.01-1.10 per 10 mg/dl, P = .026).
In particular, elevated remnant-C (≥30 mg/dL), compared with lower concentrations, flagged subjects at a higher risk of MACE, even if their LDL-C levels were at target (defined as ≤ 100 mg/dL).
Levels of LDL-C and HDL cholesterol were not associated with MACE.
“The indirect calculation of remnant-C is an affordable and inexpensive method, which could provide valuable data for clinical management,” Dr. Fitó Colomer said.
“The results of this study suggest that, in individuals at high cardiovascular risk with well-controlled LDL-C, triglycerides and mainly remnant-C should be considered as a treatment target,” she proposed.
New oracles?
Evidence has pointed to triglyceride-rich remnants or VLDL cholesterol as contributing to atherosclerotic CVD, together with LDL-C, but it is “unclear which fraction of risk is explained by, respectively, cholesterol and triglycerides in VLDL,” write the authors of the Copenhagen population study.
Dr. Nordestgaard said their study was motivated by an awareness that “in clinical practice, the focus for lipid-related risk is almost solely on reduction of LDL-C for prevention of ASCVD,” so the current focus needs to be reevaluated because patients with low LDL-C but elevated VLDL cholesterol and plasma triglycerides “may not be offered adequate preventive lipid-lowering therapy in order to prevent future MI and ASCVD.”
His group therefore tested the hypothesis that VLDL cholesterol and triglycerides may each explain part of the MI risk from apoB-containing lipoproteins.
They used measurements of plasma apoB and cholesterol and triglyceride content of VLDL cholesterol, intermediate-density-lipoprotein cholesterol, and LDL-C in the study participants (N = 25,480, median age 61 years, 53% female), who were required to be free of MI and not receiving lipid-lowering therapy at baseline.
During a median 11-year follow-up period, 1,816 participants experienced an MI. They tended to be older, compared with those who did not experience an MI, and also more likely to be male, to smoke, and to have higher systolic blood pressure.
Each 39-mg/dL increase in lipid level was found to be associated with higher MI risk.
The researchers looked at MI-associated risk of specific subfractions of apoB-containing lipoproteins. “VLDL cholesterol explained half of the MI risk from elevated apoB-containing lipoproteins, and [intermediate-density-lipoprotein] and LDL-C together accounted for only 29% of the risk,” Dr. Nordestgaard said.
“If LDL cholesterol is adequately reduced, clinicians need to evaluate possible elevated triglyceride-rich lipoproteins, either as elevated plasma triglycerides, remnant cholesterol, or elevated VLDL cholesterol; and, if elevated, consideration should also be given to reduction of triglyceride-rich lipoproteins,” he advised.
The Copenhagen General Population study was funded by the Danish Heart Foundation and the Novo Nordisk Foundation. Dr. Nordestgaard disclosed consulting for AstraZeneca, Sanofi, Regeneron, Akcea, Amgen, Kowa, Denka Seiken, Amarin, Novartis, Novo Nordisk, and Silence Therapeutrics. PREDIMED was supported by grants from the Instituto de Salud Carlos III- FEDER, Fundació La Marató de TV3, and Agència de Gestió d’Ajuts Universitaris i de Recerca. Dr. Fitó Colomer disclosed no relevant financial relationships. Dr. Burnett disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Major depression linked to insulin resistance
Individuals experiencing a current episode of major depressive disorder (MDD) are significantly more likely to have insulin resistance (IR), research shows.
Investigators found patients with MDD were 51% more likely to have IR, compared with their counterparts without depressive disorder. In addition, in individuals experiencing current depression, IR was also associated with depression severity and depression chronicity.
“We learned two things from this study – first, that insulin resistance was associated with being in a depressive episode and with the severity of that episode,” Kathleen Watson, PhD, a postdoctoral research fellow in the department of psychiatry, Stanford (Calif.) University, told this news organization. “Second, we learned that we can estimate insulin resistance using a surrogate measure that is clinically accessible – the triglyceride/HDL ratio.”
The study was published online Dec. 2 in JAMA Psychiatry.
Targeted approach
Many studies have linked MDD and IR. However, said Dr. Watson, “We did not have much description of the nature of this relationship.” She added that her team wanted to gain a better understanding of how IR relates to depression characteristics, such as remission status, severity, and chronicity.
Characterizing these associations will “represent a critical step at better phenotyping, a prelude to longitudinal studies, and a more targeted approach to the treatment of MDD,” the authors note.
For the study, the researchers drew on data from the Netherlands Study of Depression and Anxiety, a longitudinal Dutch study of adults that “describes the course and consequences of depressive and anxiety disorders.”
The study included 1,269 study participants with current MDD (n = 536), remitted MDD (n = 394), and control participants without a history of MDD (n = 339).
In addition to investigating the association between MDD and IR, the researchers also wanted to understand “whether using different surrogate IR measures has consistent association with MDD.” IR was determined using two surrogate markers – the quantitative insulin sensitivity check index (QUICKI) and the triglyceride to high-density lipoprotein ratio. Participants in the bottom quartile of the QUICKI were categorized as IR, while all other participants were categorized as being “insulin sensitive.”
The second surrogate IR measure – the triglyceride-HDL ratio – is an index based on fasting blood sample measurements, in which the determination of IR was based on sex-specific cut points (female ratio, IR > 1.9; male ratio, IR > 2.8).
Depression was determined based on the Composite International Diagnostic Interview (version 2.1), while depression severity was based on the Inventory of Depression Symptomatology. “Chronicity” was defined as depression during the preceding 4 years and was measured using the life chart review.
State vs. trait
Insulin resistance was associated with current, but not with remitted, MDD (odds ratio, 1.51; 95% confidence interval, 1.08-2.12 and OR, 1.14; 95% CI, 0.79-1.64, respectively).
In a model adjusted for age, sex, education, partner status, smoking status, and alcohol consumption, IR, as assessed by both measures, was linked to depression severity – but only the triglyceride-HDL ratio yielded an association between IR and depression chronicity.
IR was not associated with depression severity or chronicity in remitted MDD on either measure.
The findings – specifically the association between current, but not remitted, MDD – suggest that “IR is a state, rather than a trait, biomarker of depression,” the authors note.
“There are many plausible mechanisms between IR and MDD,” said Dr. Watson. “Some hypotheses for the link include inflammations, alterations to the hypothalamic-pituitary-adrenal axis, and changes in health behavior.
“Understanding these nuances helped us to lay the foundation for future research, including asking whether IR can lead to the development of MDD,” she added.
Finally, and ways to target them with potential treatments or interventions.
Shared biological mechanisms?
Commenting on the study in an interview, Roger McIntyre, MD, professor of psychiatry and pharmacology, University of Toronto and head of the Mood Disorders Psychopharmacology Unit, said the results “suggest that a subpopulation of people with depression have what might be referred to as ‘metabolic syndrome type II’ – the depression is a consequence of abnormal metabolic processes.”
The results also suggest “maybe metabolic markers can be used as biomarkers of disease presence vs. absence,” said Dr. McIntyre, who is also the chairman and executive director of the Brain and Cognition Discovery Foundation, Toronto, and was not involved with the study.
Also commenting on the study, Andrea Fagiolini, MD, professor of psychiatry, University of Siena (Italy), said depression, metabolic, and inflammatory diseases “likely share some common biological mechanism, as they share risk factors such as unhealthy diet, unhealthy lifestyles, and frequent exposure to physical and psychological distress.”
It is “possible that treatment of depression improves IR; conversely, it is possible that lifestyle programs or medications that are able to improve IR may improve depressive symptoms,” suggested Dr. Fagiolini, who was not involved with the study. “It remains to be established which symptoms of depression are most involved in this correlation and whether their improvement precedes or follows the improvement in IR,” he noted.
The Netherlands Study of Depression and Anxiety is funded through the Geestkracht program of the Netherlands Organisation for Health Research and Development and is supported by several participating universities and mental health care organizations. Dr. Watson has disclosed no relevant financial relationships. The other authors’ disclosures are listed on the original paper. Dr. McIntyre reported research grant support from CIHR/GACD/Chinese National Natural Research Foundation and speaker/consultation fees from multiple pharmaceutical companies. Dr. McIntyre is also CEO of AltMed. Dr. Fagiolini has served or is currently serving as consultant or speaker for or is a research grant recipient from multiple pharmaceutical companies.
A version of this article originally appeared on Medscape.com.
Individuals experiencing a current episode of major depressive disorder (MDD) are significantly more likely to have insulin resistance (IR), research shows.
Investigators found patients with MDD were 51% more likely to have IR, compared with their counterparts without depressive disorder. In addition, in individuals experiencing current depression, IR was also associated with depression severity and depression chronicity.
“We learned two things from this study – first, that insulin resistance was associated with being in a depressive episode and with the severity of that episode,” Kathleen Watson, PhD, a postdoctoral research fellow in the department of psychiatry, Stanford (Calif.) University, told this news organization. “Second, we learned that we can estimate insulin resistance using a surrogate measure that is clinically accessible – the triglyceride/HDL ratio.”
The study was published online Dec. 2 in JAMA Psychiatry.
Targeted approach
Many studies have linked MDD and IR. However, said Dr. Watson, “We did not have much description of the nature of this relationship.” She added that her team wanted to gain a better understanding of how IR relates to depression characteristics, such as remission status, severity, and chronicity.
Characterizing these associations will “represent a critical step at better phenotyping, a prelude to longitudinal studies, and a more targeted approach to the treatment of MDD,” the authors note.
For the study, the researchers drew on data from the Netherlands Study of Depression and Anxiety, a longitudinal Dutch study of adults that “describes the course and consequences of depressive and anxiety disorders.”
The study included 1,269 study participants with current MDD (n = 536), remitted MDD (n = 394), and control participants without a history of MDD (n = 339).
In addition to investigating the association between MDD and IR, the researchers also wanted to understand “whether using different surrogate IR measures has consistent association with MDD.” IR was determined using two surrogate markers – the quantitative insulin sensitivity check index (QUICKI) and the triglyceride to high-density lipoprotein ratio. Participants in the bottom quartile of the QUICKI were categorized as IR, while all other participants were categorized as being “insulin sensitive.”
The second surrogate IR measure – the triglyceride-HDL ratio – is an index based on fasting blood sample measurements, in which the determination of IR was based on sex-specific cut points (female ratio, IR > 1.9; male ratio, IR > 2.8).
Depression was determined based on the Composite International Diagnostic Interview (version 2.1), while depression severity was based on the Inventory of Depression Symptomatology. “Chronicity” was defined as depression during the preceding 4 years and was measured using the life chart review.
State vs. trait
Insulin resistance was associated with current, but not with remitted, MDD (odds ratio, 1.51; 95% confidence interval, 1.08-2.12 and OR, 1.14; 95% CI, 0.79-1.64, respectively).
In a model adjusted for age, sex, education, partner status, smoking status, and alcohol consumption, IR, as assessed by both measures, was linked to depression severity – but only the triglyceride-HDL ratio yielded an association between IR and depression chronicity.
IR was not associated with depression severity or chronicity in remitted MDD on either measure.
The findings – specifically the association between current, but not remitted, MDD – suggest that “IR is a state, rather than a trait, biomarker of depression,” the authors note.
“There are many plausible mechanisms between IR and MDD,” said Dr. Watson. “Some hypotheses for the link include inflammations, alterations to the hypothalamic-pituitary-adrenal axis, and changes in health behavior.
“Understanding these nuances helped us to lay the foundation for future research, including asking whether IR can lead to the development of MDD,” she added.
Finally, and ways to target them with potential treatments or interventions.
Shared biological mechanisms?
Commenting on the study in an interview, Roger McIntyre, MD, professor of psychiatry and pharmacology, University of Toronto and head of the Mood Disorders Psychopharmacology Unit, said the results “suggest that a subpopulation of people with depression have what might be referred to as ‘metabolic syndrome type II’ – the depression is a consequence of abnormal metabolic processes.”
The results also suggest “maybe metabolic markers can be used as biomarkers of disease presence vs. absence,” said Dr. McIntyre, who is also the chairman and executive director of the Brain and Cognition Discovery Foundation, Toronto, and was not involved with the study.
Also commenting on the study, Andrea Fagiolini, MD, professor of psychiatry, University of Siena (Italy), said depression, metabolic, and inflammatory diseases “likely share some common biological mechanism, as they share risk factors such as unhealthy diet, unhealthy lifestyles, and frequent exposure to physical and psychological distress.”
It is “possible that treatment of depression improves IR; conversely, it is possible that lifestyle programs or medications that are able to improve IR may improve depressive symptoms,” suggested Dr. Fagiolini, who was not involved with the study. “It remains to be established which symptoms of depression are most involved in this correlation and whether their improvement precedes or follows the improvement in IR,” he noted.
The Netherlands Study of Depression and Anxiety is funded through the Geestkracht program of the Netherlands Organisation for Health Research and Development and is supported by several participating universities and mental health care organizations. Dr. Watson has disclosed no relevant financial relationships. The other authors’ disclosures are listed on the original paper. Dr. McIntyre reported research grant support from CIHR/GACD/Chinese National Natural Research Foundation and speaker/consultation fees from multiple pharmaceutical companies. Dr. McIntyre is also CEO of AltMed. Dr. Fagiolini has served or is currently serving as consultant or speaker for or is a research grant recipient from multiple pharmaceutical companies.
A version of this article originally appeared on Medscape.com.
Individuals experiencing a current episode of major depressive disorder (MDD) are significantly more likely to have insulin resistance (IR), research shows.
Investigators found patients with MDD were 51% more likely to have IR, compared with their counterparts without depressive disorder. In addition, in individuals experiencing current depression, IR was also associated with depression severity and depression chronicity.
“We learned two things from this study – first, that insulin resistance was associated with being in a depressive episode and with the severity of that episode,” Kathleen Watson, PhD, a postdoctoral research fellow in the department of psychiatry, Stanford (Calif.) University, told this news organization. “Second, we learned that we can estimate insulin resistance using a surrogate measure that is clinically accessible – the triglyceride/HDL ratio.”
The study was published online Dec. 2 in JAMA Psychiatry.
Targeted approach
Many studies have linked MDD and IR. However, said Dr. Watson, “We did not have much description of the nature of this relationship.” She added that her team wanted to gain a better understanding of how IR relates to depression characteristics, such as remission status, severity, and chronicity.
Characterizing these associations will “represent a critical step at better phenotyping, a prelude to longitudinal studies, and a more targeted approach to the treatment of MDD,” the authors note.
For the study, the researchers drew on data from the Netherlands Study of Depression and Anxiety, a longitudinal Dutch study of adults that “describes the course and consequences of depressive and anxiety disorders.”
The study included 1,269 study participants with current MDD (n = 536), remitted MDD (n = 394), and control participants without a history of MDD (n = 339).
In addition to investigating the association between MDD and IR, the researchers also wanted to understand “whether using different surrogate IR measures has consistent association with MDD.” IR was determined using two surrogate markers – the quantitative insulin sensitivity check index (QUICKI) and the triglyceride to high-density lipoprotein ratio. Participants in the bottom quartile of the QUICKI were categorized as IR, while all other participants were categorized as being “insulin sensitive.”
The second surrogate IR measure – the triglyceride-HDL ratio – is an index based on fasting blood sample measurements, in which the determination of IR was based on sex-specific cut points (female ratio, IR > 1.9; male ratio, IR > 2.8).
Depression was determined based on the Composite International Diagnostic Interview (version 2.1), while depression severity was based on the Inventory of Depression Symptomatology. “Chronicity” was defined as depression during the preceding 4 years and was measured using the life chart review.
State vs. trait
Insulin resistance was associated with current, but not with remitted, MDD (odds ratio, 1.51; 95% confidence interval, 1.08-2.12 and OR, 1.14; 95% CI, 0.79-1.64, respectively).
In a model adjusted for age, sex, education, partner status, smoking status, and alcohol consumption, IR, as assessed by both measures, was linked to depression severity – but only the triglyceride-HDL ratio yielded an association between IR and depression chronicity.
IR was not associated with depression severity or chronicity in remitted MDD on either measure.
The findings – specifically the association between current, but not remitted, MDD – suggest that “IR is a state, rather than a trait, biomarker of depression,” the authors note.
“There are many plausible mechanisms between IR and MDD,” said Dr. Watson. “Some hypotheses for the link include inflammations, alterations to the hypothalamic-pituitary-adrenal axis, and changes in health behavior.
“Understanding these nuances helped us to lay the foundation for future research, including asking whether IR can lead to the development of MDD,” she added.
Finally, and ways to target them with potential treatments or interventions.
Shared biological mechanisms?
Commenting on the study in an interview, Roger McIntyre, MD, professor of psychiatry and pharmacology, University of Toronto and head of the Mood Disorders Psychopharmacology Unit, said the results “suggest that a subpopulation of people with depression have what might be referred to as ‘metabolic syndrome type II’ – the depression is a consequence of abnormal metabolic processes.”
The results also suggest “maybe metabolic markers can be used as biomarkers of disease presence vs. absence,” said Dr. McIntyre, who is also the chairman and executive director of the Brain and Cognition Discovery Foundation, Toronto, and was not involved with the study.
Also commenting on the study, Andrea Fagiolini, MD, professor of psychiatry, University of Siena (Italy), said depression, metabolic, and inflammatory diseases “likely share some common biological mechanism, as they share risk factors such as unhealthy diet, unhealthy lifestyles, and frequent exposure to physical and psychological distress.”
It is “possible that treatment of depression improves IR; conversely, it is possible that lifestyle programs or medications that are able to improve IR may improve depressive symptoms,” suggested Dr. Fagiolini, who was not involved with the study. “It remains to be established which symptoms of depression are most involved in this correlation and whether their improvement precedes or follows the improvement in IR,” he noted.
The Netherlands Study of Depression and Anxiety is funded through the Geestkracht program of the Netherlands Organisation for Health Research and Development and is supported by several participating universities and mental health care organizations. Dr. Watson has disclosed no relevant financial relationships. The other authors’ disclosures are listed on the original paper. Dr. McIntyre reported research grant support from CIHR/GACD/Chinese National Natural Research Foundation and speaker/consultation fees from multiple pharmaceutical companies. Dr. McIntyre is also CEO of AltMed. Dr. Fagiolini has served or is currently serving as consultant or speaker for or is a research grant recipient from multiple pharmaceutical companies.
A version of this article originally appeared on Medscape.com.
Food preservative for early psychosis: Final word?
Adjunctive use of sodium benzoate (BZ), a common food preservative that has previously shown promise in the treatment of chronic refractory psychosis, appears to be ineffective in the early stages of the disorder, new research suggests.
Results of a randomized controlled trial show the agent was no more effective than placebo in reducing early psychosis symptoms, although it was safe and well tolerated.
“Both groups of patients improved over the 12 weeks of the study, [suggesting] that most people with early psychosis will get well with antipsychotic medication and psychosocial interventions and adding sodium benzoate to their treatment does not add any additional benefits,” the study’s lead author, James Scott, MBBS, PhD, head of mental health research, QIMR Berghofer Medical Research Institute, Herston, Australia, told Medscape Medical News.
The paper was published online November 10 in JAMA Network Open.
Positive outcomes in chronic disease
Despite treatment with antipsychotics, many patients with psychosis experience persistent impairment, the investigators note.
Most antipsychotics are dopaminergic in action, but it is now recognized that the pathophysiology underlying psychosis extends beyond dopaminergic dysregulation with hypofunction of the N-methyl-D-aspartate (NMDA) receptors also implicated but not addressed by standard antipsychotics, they add.
NMDA receptors consist of two main subunits – the glutamate and glycine-binding sites. D-amino acids (DAAs) are agonists of the glycine subunit and have shown promise as adjunctive therapies for the treatment of schizophrenia, the investigators note.
DAAs are subject to oxidation by the flavoenzyme D-amino acid oxidase (DAAO). The oxidation limits their bioavailability and can cause nephrotoxic side effects. The food preservative BZ, which is not related to the benzodiazepine class of medications, inhibits DAAO and therefore may make DAAs safer and more effective.
Scott noted that two previous trials of BZ – a 2013 study and a 2017 investigation – in chronic, treatment-refractory schizophrenia have “reported excellent outcomes with significant improvement in clinical symptoms.”
“We saw that sodium benzoate was a safe and well-tolerated agent, and we thought it was important to conduct a trial of this medication in people in the early stages of psychotic illness,” he said.
To investigate, the researchers randomly assigned 100 individuals who were experiencing early psychosis, which was defined as illness onset within the last 2 years, to receive either 500 mg of BZ twice daily or placebo for 12 weeks.
Participants (mean [SD] age 21.4 [4.1] years, 73% male) were required to be taking antipsychotic medications for at least 1 continuous month during the previous 2 years and to be free of comorbid physical illnesses requiring additional treatment or hospitalization.
Most participants (84%) had schizophrenia and the remainder had affective psychoses. Most participants (88%) lived independently.
The BZ and the placebo groups were similar with respect to baseline characteristics, except that the mean waist circumference was higher in the placebo group than in the BZ group.
The majority of patients were being treated with antipsychotics alone (83%), followed by antipsychotics in combination with mood stabilizers (13%) and a small number were taking mood stabilizers alone (4%). The most commonly used antipsychotics were olanzapine and aripiprazole.
Not recommended
Psychosis was confirmed using the Positive and Negative Syndrome Scale (PANSS), and the inclusion criteria was a baseline score of ≥ 55. Secondary outcomes were scores on the Hamilton Depression Rating Scale, the clinician-rated Global Assessment of Function, and the Assessment of Quality of Life Scale.
The researchers also measured concentrations of the amino acids oxidized by DAAO (D-alanine and L-alanine, D-serine and L-serine).
Although both groups experienced a reduction in total PANSS scores during the study, there were no significant differences in PANSS total score between the BZ and the placebo groups at 12 weeks (endpoint least-square mean difference [SE] −1.2 [2.4] t = −0.49, P = .63).
There were also no significant differences between the groups on all PANSS subscales as well as any of the secondary clinical measures (P < .007).
A total of 122 adverse events (AEs) overall were reported by 66 participants, but rates of AEs were comparable between the BZ and placebo groups at 55% vs. 46% respectively. There were 11 serious AEs reported by 10 participants. Only one of these was related to the study drug.
There were no statistically significant changes in amino acid concentrations between the two groups.
The authors note several limitations of the study, including the possibility that protective agents may need longer times than 12 weeks – possibly as long as 6 to 12 months – to show efficacy. Moreover, the dose of BZ needed to produce a response remains “uncertain.”
“ and should further investigate whether benzoate acts by altering amino acid levels or by reducing oxidative stress in people with schizophrenia,” the authors suggest.
Scott added that further research in patients with treatment-refractory schizophrenia is needed to determine whether BZ “has a role in this patient population.”
The authors conclude that at present, “the routine use of this agent as an adjunctive treatment for early psychosis is not recommended.”
No surprise?
Commenting on the study for Medscape Medical News, Kenji Hashimoto, PhD, Division of Clinical Neuroscience, Chiba University Center for Forensic Mental Health, Chiba, Japan, said that, although previous studies of BZ showed benefit in stable patients with chronic schizophrenia, “it is unlikely that [sodium] benzoate may have beneficial effects in the acute phase of psychosis.”
Hashimoto, who was not involved with the study, noted that BZ is a “weak DAAO inhibitor and that DAAO expression in the frontal cortex of human beings is very low.”
This project was supported by a John Cade Fellowship from the National Health and Medical Research Council and support from the Queensland Centre for Mental Health Research, which receives funding from the Queensland Health Department. Scott is supported by an NHMRC Practitioner Fellowship. The other authors’ disclosures are listed on the original article. Hashimoto has disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Adjunctive use of sodium benzoate (BZ), a common food preservative that has previously shown promise in the treatment of chronic refractory psychosis, appears to be ineffective in the early stages of the disorder, new research suggests.
Results of a randomized controlled trial show the agent was no more effective than placebo in reducing early psychosis symptoms, although it was safe and well tolerated.
“Both groups of patients improved over the 12 weeks of the study, [suggesting] that most people with early psychosis will get well with antipsychotic medication and psychosocial interventions and adding sodium benzoate to their treatment does not add any additional benefits,” the study’s lead author, James Scott, MBBS, PhD, head of mental health research, QIMR Berghofer Medical Research Institute, Herston, Australia, told Medscape Medical News.
The paper was published online November 10 in JAMA Network Open.
Positive outcomes in chronic disease
Despite treatment with antipsychotics, many patients with psychosis experience persistent impairment, the investigators note.
Most antipsychotics are dopaminergic in action, but it is now recognized that the pathophysiology underlying psychosis extends beyond dopaminergic dysregulation with hypofunction of the N-methyl-D-aspartate (NMDA) receptors also implicated but not addressed by standard antipsychotics, they add.
NMDA receptors consist of two main subunits – the glutamate and glycine-binding sites. D-amino acids (DAAs) are agonists of the glycine subunit and have shown promise as adjunctive therapies for the treatment of schizophrenia, the investigators note.
DAAs are subject to oxidation by the flavoenzyme D-amino acid oxidase (DAAO). The oxidation limits their bioavailability and can cause nephrotoxic side effects. The food preservative BZ, which is not related to the benzodiazepine class of medications, inhibits DAAO and therefore may make DAAs safer and more effective.
Scott noted that two previous trials of BZ – a 2013 study and a 2017 investigation – in chronic, treatment-refractory schizophrenia have “reported excellent outcomes with significant improvement in clinical symptoms.”
“We saw that sodium benzoate was a safe and well-tolerated agent, and we thought it was important to conduct a trial of this medication in people in the early stages of psychotic illness,” he said.
To investigate, the researchers randomly assigned 100 individuals who were experiencing early psychosis, which was defined as illness onset within the last 2 years, to receive either 500 mg of BZ twice daily or placebo for 12 weeks.
Participants (mean [SD] age 21.4 [4.1] years, 73% male) were required to be taking antipsychotic medications for at least 1 continuous month during the previous 2 years and to be free of comorbid physical illnesses requiring additional treatment or hospitalization.
Most participants (84%) had schizophrenia and the remainder had affective psychoses. Most participants (88%) lived independently.
The BZ and the placebo groups were similar with respect to baseline characteristics, except that the mean waist circumference was higher in the placebo group than in the BZ group.
The majority of patients were being treated with antipsychotics alone (83%), followed by antipsychotics in combination with mood stabilizers (13%) and a small number were taking mood stabilizers alone (4%). The most commonly used antipsychotics were olanzapine and aripiprazole.
Not recommended
Psychosis was confirmed using the Positive and Negative Syndrome Scale (PANSS), and the inclusion criteria was a baseline score of ≥ 55. Secondary outcomes were scores on the Hamilton Depression Rating Scale, the clinician-rated Global Assessment of Function, and the Assessment of Quality of Life Scale.
The researchers also measured concentrations of the amino acids oxidized by DAAO (D-alanine and L-alanine, D-serine and L-serine).
Although both groups experienced a reduction in total PANSS scores during the study, there were no significant differences in PANSS total score between the BZ and the placebo groups at 12 weeks (endpoint least-square mean difference [SE] −1.2 [2.4] t = −0.49, P = .63).
There were also no significant differences between the groups on all PANSS subscales as well as any of the secondary clinical measures (P < .007).
A total of 122 adverse events (AEs) overall were reported by 66 participants, but rates of AEs were comparable between the BZ and placebo groups at 55% vs. 46% respectively. There were 11 serious AEs reported by 10 participants. Only one of these was related to the study drug.
There were no statistically significant changes in amino acid concentrations between the two groups.
The authors note several limitations of the study, including the possibility that protective agents may need longer times than 12 weeks – possibly as long as 6 to 12 months – to show efficacy. Moreover, the dose of BZ needed to produce a response remains “uncertain.”
“ and should further investigate whether benzoate acts by altering amino acid levels or by reducing oxidative stress in people with schizophrenia,” the authors suggest.
Scott added that further research in patients with treatment-refractory schizophrenia is needed to determine whether BZ “has a role in this patient population.”
The authors conclude that at present, “the routine use of this agent as an adjunctive treatment for early psychosis is not recommended.”
No surprise?
Commenting on the study for Medscape Medical News, Kenji Hashimoto, PhD, Division of Clinical Neuroscience, Chiba University Center for Forensic Mental Health, Chiba, Japan, said that, although previous studies of BZ showed benefit in stable patients with chronic schizophrenia, “it is unlikely that [sodium] benzoate may have beneficial effects in the acute phase of psychosis.”
Hashimoto, who was not involved with the study, noted that BZ is a “weak DAAO inhibitor and that DAAO expression in the frontal cortex of human beings is very low.”
This project was supported by a John Cade Fellowship from the National Health and Medical Research Council and support from the Queensland Centre for Mental Health Research, which receives funding from the Queensland Health Department. Scott is supported by an NHMRC Practitioner Fellowship. The other authors’ disclosures are listed on the original article. Hashimoto has disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Adjunctive use of sodium benzoate (BZ), a common food preservative that has previously shown promise in the treatment of chronic refractory psychosis, appears to be ineffective in the early stages of the disorder, new research suggests.
Results of a randomized controlled trial show the agent was no more effective than placebo in reducing early psychosis symptoms, although it was safe and well tolerated.
“Both groups of patients improved over the 12 weeks of the study, [suggesting] that most people with early psychosis will get well with antipsychotic medication and psychosocial interventions and adding sodium benzoate to their treatment does not add any additional benefits,” the study’s lead author, James Scott, MBBS, PhD, head of mental health research, QIMR Berghofer Medical Research Institute, Herston, Australia, told Medscape Medical News.
The paper was published online November 10 in JAMA Network Open.
Positive outcomes in chronic disease
Despite treatment with antipsychotics, many patients with psychosis experience persistent impairment, the investigators note.
Most antipsychotics are dopaminergic in action, but it is now recognized that the pathophysiology underlying psychosis extends beyond dopaminergic dysregulation with hypofunction of the N-methyl-D-aspartate (NMDA) receptors also implicated but not addressed by standard antipsychotics, they add.
NMDA receptors consist of two main subunits – the glutamate and glycine-binding sites. D-amino acids (DAAs) are agonists of the glycine subunit and have shown promise as adjunctive therapies for the treatment of schizophrenia, the investigators note.
DAAs are subject to oxidation by the flavoenzyme D-amino acid oxidase (DAAO). The oxidation limits their bioavailability and can cause nephrotoxic side effects. The food preservative BZ, which is not related to the benzodiazepine class of medications, inhibits DAAO and therefore may make DAAs safer and more effective.
Scott noted that two previous trials of BZ – a 2013 study and a 2017 investigation – in chronic, treatment-refractory schizophrenia have “reported excellent outcomes with significant improvement in clinical symptoms.”
“We saw that sodium benzoate was a safe and well-tolerated agent, and we thought it was important to conduct a trial of this medication in people in the early stages of psychotic illness,” he said.
To investigate, the researchers randomly assigned 100 individuals who were experiencing early psychosis, which was defined as illness onset within the last 2 years, to receive either 500 mg of BZ twice daily or placebo for 12 weeks.
Participants (mean [SD] age 21.4 [4.1] years, 73% male) were required to be taking antipsychotic medications for at least 1 continuous month during the previous 2 years and to be free of comorbid physical illnesses requiring additional treatment or hospitalization.
Most participants (84%) had schizophrenia and the remainder had affective psychoses. Most participants (88%) lived independently.
The BZ and the placebo groups were similar with respect to baseline characteristics, except that the mean waist circumference was higher in the placebo group than in the BZ group.
The majority of patients were being treated with antipsychotics alone (83%), followed by antipsychotics in combination with mood stabilizers (13%) and a small number were taking mood stabilizers alone (4%). The most commonly used antipsychotics were olanzapine and aripiprazole.
Not recommended
Psychosis was confirmed using the Positive and Negative Syndrome Scale (PANSS), and the inclusion criteria was a baseline score of ≥ 55. Secondary outcomes were scores on the Hamilton Depression Rating Scale, the clinician-rated Global Assessment of Function, and the Assessment of Quality of Life Scale.
The researchers also measured concentrations of the amino acids oxidized by DAAO (D-alanine and L-alanine, D-serine and L-serine).
Although both groups experienced a reduction in total PANSS scores during the study, there were no significant differences in PANSS total score between the BZ and the placebo groups at 12 weeks (endpoint least-square mean difference [SE] −1.2 [2.4] t = −0.49, P = .63).
There were also no significant differences between the groups on all PANSS subscales as well as any of the secondary clinical measures (P < .007).
A total of 122 adverse events (AEs) overall were reported by 66 participants, but rates of AEs were comparable between the BZ and placebo groups at 55% vs. 46% respectively. There were 11 serious AEs reported by 10 participants. Only one of these was related to the study drug.
There were no statistically significant changes in amino acid concentrations between the two groups.
The authors note several limitations of the study, including the possibility that protective agents may need longer times than 12 weeks – possibly as long as 6 to 12 months – to show efficacy. Moreover, the dose of BZ needed to produce a response remains “uncertain.”
“ and should further investigate whether benzoate acts by altering amino acid levels or by reducing oxidative stress in people with schizophrenia,” the authors suggest.
Scott added that further research in patients with treatment-refractory schizophrenia is needed to determine whether BZ “has a role in this patient population.”
The authors conclude that at present, “the routine use of this agent as an adjunctive treatment for early psychosis is not recommended.”
No surprise?
Commenting on the study for Medscape Medical News, Kenji Hashimoto, PhD, Division of Clinical Neuroscience, Chiba University Center for Forensic Mental Health, Chiba, Japan, said that, although previous studies of BZ showed benefit in stable patients with chronic schizophrenia, “it is unlikely that [sodium] benzoate may have beneficial effects in the acute phase of psychosis.”
Hashimoto, who was not involved with the study, noted that BZ is a “weak DAAO inhibitor and that DAAO expression in the frontal cortex of human beings is very low.”
This project was supported by a John Cade Fellowship from the National Health and Medical Research Council and support from the Queensland Centre for Mental Health Research, which receives funding from the Queensland Health Department. Scott is supported by an NHMRC Practitioner Fellowship. The other authors’ disclosures are listed on the original article. Hashimoto has disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Mind menders: The future of psychedelic therapy in the United States
After a 50-year hiatus, psychedelic drugs are undergoing a research renaissance. Roland R. Griffiths, PhD, professor in the Departments of Psychiatry and Neuroscience and the Oliver Lee McCabe III, Professor in the Neuropsychopharmacology of Consciousness, and director of the Center for Psychedelic and Consciousness Research at Johns Hopkins University, Baltimore, discusses the status of these drugs in the United States and their potential to treat psychiatric disorders.
Classic psychedelics are compounds that bind to the 5-hydroxytryptamine 2A (5-HT2A) receptor and include the naturally occurring compounds psilocybin, N,N-dimethyltryptamine (DMT, a component of ayahuasca) and mescaline (peyote cactus), as well as the synthesized compound lysergic acid diethylamide (LSD).
Other drugs, such as ketamine, are sometimes referred to as “psychedelics” because they can produce subjective experiences that are similar to those of people who receive classic psychedelics. However, unlike classic psychedelics, the effects of ketamine tend to be short lived. Ketamine also has addictive potential and can be lethal in high doses, which is not the case with psilocybin.
Another compound sometimes referred to as a “psychedelic” is 3,4-methylenedioxymethamphetamine (MDMA), also known as “ecstasy.” The Food and Drug Administration granted breakthrough approval for the study of MDMA for posttraumatic stress disorder (PTSD). FDA-approved registration trials are ongoing. MDMA differs from classic psychedelics in risk profile and pharmacology. In particular, MDMA was widely abused as part of the “rave culture,” while classic psychedelic agents do not lend themselves to that type of misuse.
What is the current legal status of psychedelic agents in the United States? Can clinicians prescribe them, or are they available only in a research setting?
All classic psychedelics are considered to be “Schedule 1” which means they are illegal to possess and use except for research and only if approved by the FDA and under licensure of the Drug Enforcement Administration (DEA), so they are not available for clinical use.
In anticipation of the possibility that phase 3 research may support the efficacy and safety of psilocybin for one or more medical or mental health disorders, our team has reviewed available evidence regarding its abuse liability and concluded that, if psilocybin were approved as medication, it could possibly be included in the Schedule IV category, with additional FDA-mandated risk management provisions. However, this is not yet the case.
Which psychedelic agents are under investigation in the United States, and for which indications?
Psilocybin is under investigation in our center, as well as elsewhere in the United States. We have previously found it to be effective for smoking cessation, and we are conducting another study that is currently recruiting volunteers for this indication. We are also recruiting volunteers for studies on the use of psilocybin for major depression, Alzheimer’s disease, and anorexia nervosa. Further information about our studies can be found on the Web site for our center, the Center for Psychedelic and Consciousness Research.
Two companies – the Usona Institute and COMPASS Pathways – have received FDA Breakthrough Therapy Designation for their programs seeking approval of psilocybin as a treatment major depressive disorder and treatment-resistant depression (TRD), respectively. In addition, an international multicenter study currently underway, which includes US centers in Houston, Baltimore, New York, San Diego, and Atlanta, is investigating psilocybin for TRD.
A number of studies, including one conducted at our center, have investigated psilocybin for depression and anxiety in patients with cancer and found it effective.
Additional research showed that psilocybin alleviated symptoms of cancer-related anxiety and depression, both in the short-term and 5 years later.
LSD has been studied and found promising in the treatment of alcohol use disorder. Additional studies of LSD that are being conducted in Basel Switzerland and at the University of Chicago are examining its impact on mood in healthy volunteers.
Ayahuasca has been studied extensively for depression and anxiety and is also currently under investigation for PTSD. We found that its use in a naturalistic group setting was associated with unintended improvements in depression and anxiety.
Lastly, a lesser-known psychedelic agent is Salvinorin A, which our center has been studying, is the psychoactive constituent of the Salvia divinorum plant. While this is not a “classic” psychedelic compound, it is nevertheless the focus of much scientific interest because its effects are mediated at opioid receptors, rather than 5-HT2A receptors, and may prove to be a novel nonaddictive opioid that may ultimately be a promising treatment for pain and addiction.
What is the typical treatment regimen for psychedelic agents?
It is hard to speak of a “treatment regimen” in agents that are not used in clinical practice. Ongoing clinical trials with psilocybin generally involve one or two 6- to 8-hour sessions involving the oral administration of a moderately high dose under psychologically supported conditions.
Based on the current evidence base, which agents show the most promise?
Psilocybin is currently the most promising classic psychedelic undergoing clinical trials.
Do psychedelics have to be administered in a controlled setting in order to be effective?
Although many people have had meaningful experiences whether inside or outside of a controlled setting, there are serious potential risks associated with use of psilocybin and other classic psychedelics. The safety of psilocybin has been established in clinical studies in which participants have been carefully screened physically and psychologically, are psychologically prepared before their first session, and are psychologically supported during and after sessions. In vulnerable individuals, psilocybin has been associated with enduring psychiatric problems and sometimes persisting visual perceptual conditions. When taken in uncontrolled conditions, classic psychedelics can produce confusion and disorientation resulting in behavior dangerous to the participant and others – including life-threatening risk. Thus, for safety reasons, the optimal environment for using these agents is in a controlled setting.
Do results differ between patients who have used psychedelic agents previously and those who have not?
We have not found any difference between psychedelic-naive volunteers and those who have used psychedelics in the past.
Do you provide patient education prior to treatment initiation?
All of our study participants are thoroughly screened for medical concerns or mental health history such as psychosis, which would preclude their participation. They are educated about the effects of these agents and what they might expect and typically receive several hours of psychological preparation before the first session. They are also provided with psychological support after sessions. Additionally, we spend time developing trust and rapport prior to the first session.
How durable are the effects of psychedelic treatment?
Studies in patients and healthy participants suggest that the positive effects of psilocybin are long lasting, with most individuals reporting positive changes in moods, attitudes, and behavior that they attribute to psilocybin and which endure months or years after the session. The qualities of the acute session experience can vary widely ranging from experiences of transcendence or psychological insight to experiences of intense anxiety or fear.
An enduring shift in worldview and sense of self, as well as psychological insight, may increase psychological flexibility, thereby allowing individuals to subsequently avoid maladaptive patterns of behavior or thought and to make more healthy choices.
Our research has shown that the benefits of these experiences can last as long as 14 months, often longer, and that many participants characterize their psilocybin experience as among the most profound and personally meaningful experience of their lives.
Do participants experience any adverse effects? If so, how are they managed?
Sometimes, despite all the preparation, screening, and support we provide, some participants can have frightening experiences, such as fear and anxiety during the session. When that occurs, it is often shorted lived. The psychological preparation we provide before the session and the psychological support we provide during the session are important for managing such effects.
We provide support and encourage participants to stay with that experience, which may open to experiences of deep meaning or insight. A number of people report that these psychologically challenging states are a valuable part of the overall experience.
We conducted a survey of roughly 2,000 people who took high doses of psilocybin mushrooms and then had a challenging experience. About 10% reported they put themselves or others at risk of physical harm. Of more concern, of those whose experience occurred more than 1 year before, 8% sought treatment for enduring psychological symptoms. These findings underscore potential risks of psilocybin use but do not provide an estimate of the actual incidence of such effects.
Importantly, in our research at Johns Hopkins, we have not observed such effects in over 700 sessions that we have conducted with almost 400 participants, likely because we thoroughly screen and prepare participants and support them after they have completed the study. The potential for serious lasting harm represents a concern and points to the importance of adequate screening and aftercare.
What are the implications for future therapeutics?
We are living in exciting times, in terms of psychedelic research. The potential for a single treatment with a classic psychedelic to produce rapid and sustained therapeutic effects, possibly across a range of psychiatric conditions, is unprecedented in psychiatry. The effect appears to be an “inverse PTSD effect.”
In PTSD, a single exposure to a traumatic event can rewire the nervous system to the point that it produces enduring harm and toxicity. In the case of psychedelics, a single exposure appears to have enduring positive effects in worldview, mood, attitude, behavior, and overall life satisfaction. We can look forward to continued growth and expansion of this research including the refinement of protocols for a variety of therapeutic indications and to the development of a variety of new classic psychedelic compounds.
A version of this article originally appeared on Medscape.com.
After a 50-year hiatus, psychedelic drugs are undergoing a research renaissance. Roland R. Griffiths, PhD, professor in the Departments of Psychiatry and Neuroscience and the Oliver Lee McCabe III, Professor in the Neuropsychopharmacology of Consciousness, and director of the Center for Psychedelic and Consciousness Research at Johns Hopkins University, Baltimore, discusses the status of these drugs in the United States and their potential to treat psychiatric disorders.
Classic psychedelics are compounds that bind to the 5-hydroxytryptamine 2A (5-HT2A) receptor and include the naturally occurring compounds psilocybin, N,N-dimethyltryptamine (DMT, a component of ayahuasca) and mescaline (peyote cactus), as well as the synthesized compound lysergic acid diethylamide (LSD).
Other drugs, such as ketamine, are sometimes referred to as “psychedelics” because they can produce subjective experiences that are similar to those of people who receive classic psychedelics. However, unlike classic psychedelics, the effects of ketamine tend to be short lived. Ketamine also has addictive potential and can be lethal in high doses, which is not the case with psilocybin.
Another compound sometimes referred to as a “psychedelic” is 3,4-methylenedioxymethamphetamine (MDMA), also known as “ecstasy.” The Food and Drug Administration granted breakthrough approval for the study of MDMA for posttraumatic stress disorder (PTSD). FDA-approved registration trials are ongoing. MDMA differs from classic psychedelics in risk profile and pharmacology. In particular, MDMA was widely abused as part of the “rave culture,” while classic psychedelic agents do not lend themselves to that type of misuse.
What is the current legal status of psychedelic agents in the United States? Can clinicians prescribe them, or are they available only in a research setting?
All classic psychedelics are considered to be “Schedule 1” which means they are illegal to possess and use except for research and only if approved by the FDA and under licensure of the Drug Enforcement Administration (DEA), so they are not available for clinical use.
In anticipation of the possibility that phase 3 research may support the efficacy and safety of psilocybin for one or more medical or mental health disorders, our team has reviewed available evidence regarding its abuse liability and concluded that, if psilocybin were approved as medication, it could possibly be included in the Schedule IV category, with additional FDA-mandated risk management provisions. However, this is not yet the case.
Which psychedelic agents are under investigation in the United States, and for which indications?
Psilocybin is under investigation in our center, as well as elsewhere in the United States. We have previously found it to be effective for smoking cessation, and we are conducting another study that is currently recruiting volunteers for this indication. We are also recruiting volunteers for studies on the use of psilocybin for major depression, Alzheimer’s disease, and anorexia nervosa. Further information about our studies can be found on the Web site for our center, the Center for Psychedelic and Consciousness Research.
Two companies – the Usona Institute and COMPASS Pathways – have received FDA Breakthrough Therapy Designation for their programs seeking approval of psilocybin as a treatment major depressive disorder and treatment-resistant depression (TRD), respectively. In addition, an international multicenter study currently underway, which includes US centers in Houston, Baltimore, New York, San Diego, and Atlanta, is investigating psilocybin for TRD.
A number of studies, including one conducted at our center, have investigated psilocybin for depression and anxiety in patients with cancer and found it effective.
Additional research showed that psilocybin alleviated symptoms of cancer-related anxiety and depression, both in the short-term and 5 years later.
LSD has been studied and found promising in the treatment of alcohol use disorder. Additional studies of LSD that are being conducted in Basel Switzerland and at the University of Chicago are examining its impact on mood in healthy volunteers.
Ayahuasca has been studied extensively for depression and anxiety and is also currently under investigation for PTSD. We found that its use in a naturalistic group setting was associated with unintended improvements in depression and anxiety.
Lastly, a lesser-known psychedelic agent is Salvinorin A, which our center has been studying, is the psychoactive constituent of the Salvia divinorum plant. While this is not a “classic” psychedelic compound, it is nevertheless the focus of much scientific interest because its effects are mediated at opioid receptors, rather than 5-HT2A receptors, and may prove to be a novel nonaddictive opioid that may ultimately be a promising treatment for pain and addiction.
What is the typical treatment regimen for psychedelic agents?
It is hard to speak of a “treatment regimen” in agents that are not used in clinical practice. Ongoing clinical trials with psilocybin generally involve one or two 6- to 8-hour sessions involving the oral administration of a moderately high dose under psychologically supported conditions.
Based on the current evidence base, which agents show the most promise?
Psilocybin is currently the most promising classic psychedelic undergoing clinical trials.
Do psychedelics have to be administered in a controlled setting in order to be effective?
Although many people have had meaningful experiences whether inside or outside of a controlled setting, there are serious potential risks associated with use of psilocybin and other classic psychedelics. The safety of psilocybin has been established in clinical studies in which participants have been carefully screened physically and psychologically, are psychologically prepared before their first session, and are psychologically supported during and after sessions. In vulnerable individuals, psilocybin has been associated with enduring psychiatric problems and sometimes persisting visual perceptual conditions. When taken in uncontrolled conditions, classic psychedelics can produce confusion and disorientation resulting in behavior dangerous to the participant and others – including life-threatening risk. Thus, for safety reasons, the optimal environment for using these agents is in a controlled setting.
Do results differ between patients who have used psychedelic agents previously and those who have not?
We have not found any difference between psychedelic-naive volunteers and those who have used psychedelics in the past.
Do you provide patient education prior to treatment initiation?
All of our study participants are thoroughly screened for medical concerns or mental health history such as psychosis, which would preclude their participation. They are educated about the effects of these agents and what they might expect and typically receive several hours of psychological preparation before the first session. They are also provided with psychological support after sessions. Additionally, we spend time developing trust and rapport prior to the first session.
How durable are the effects of psychedelic treatment?
Studies in patients and healthy participants suggest that the positive effects of psilocybin are long lasting, with most individuals reporting positive changes in moods, attitudes, and behavior that they attribute to psilocybin and which endure months or years after the session. The qualities of the acute session experience can vary widely ranging from experiences of transcendence or psychological insight to experiences of intense anxiety or fear.
An enduring shift in worldview and sense of self, as well as psychological insight, may increase psychological flexibility, thereby allowing individuals to subsequently avoid maladaptive patterns of behavior or thought and to make more healthy choices.
Our research has shown that the benefits of these experiences can last as long as 14 months, often longer, and that many participants characterize their psilocybin experience as among the most profound and personally meaningful experience of their lives.
Do participants experience any adverse effects? If so, how are they managed?
Sometimes, despite all the preparation, screening, and support we provide, some participants can have frightening experiences, such as fear and anxiety during the session. When that occurs, it is often shorted lived. The psychological preparation we provide before the session and the psychological support we provide during the session are important for managing such effects.
We provide support and encourage participants to stay with that experience, which may open to experiences of deep meaning or insight. A number of people report that these psychologically challenging states are a valuable part of the overall experience.
We conducted a survey of roughly 2,000 people who took high doses of psilocybin mushrooms and then had a challenging experience. About 10% reported they put themselves or others at risk of physical harm. Of more concern, of those whose experience occurred more than 1 year before, 8% sought treatment for enduring psychological symptoms. These findings underscore potential risks of psilocybin use but do not provide an estimate of the actual incidence of such effects.
Importantly, in our research at Johns Hopkins, we have not observed such effects in over 700 sessions that we have conducted with almost 400 participants, likely because we thoroughly screen and prepare participants and support them after they have completed the study. The potential for serious lasting harm represents a concern and points to the importance of adequate screening and aftercare.
What are the implications for future therapeutics?
We are living in exciting times, in terms of psychedelic research. The potential for a single treatment with a classic psychedelic to produce rapid and sustained therapeutic effects, possibly across a range of psychiatric conditions, is unprecedented in psychiatry. The effect appears to be an “inverse PTSD effect.”
In PTSD, a single exposure to a traumatic event can rewire the nervous system to the point that it produces enduring harm and toxicity. In the case of psychedelics, a single exposure appears to have enduring positive effects in worldview, mood, attitude, behavior, and overall life satisfaction. We can look forward to continued growth and expansion of this research including the refinement of protocols for a variety of therapeutic indications and to the development of a variety of new classic psychedelic compounds.
A version of this article originally appeared on Medscape.com.
After a 50-year hiatus, psychedelic drugs are undergoing a research renaissance. Roland R. Griffiths, PhD, professor in the Departments of Psychiatry and Neuroscience and the Oliver Lee McCabe III, Professor in the Neuropsychopharmacology of Consciousness, and director of the Center for Psychedelic and Consciousness Research at Johns Hopkins University, Baltimore, discusses the status of these drugs in the United States and their potential to treat psychiatric disorders.
Classic psychedelics are compounds that bind to the 5-hydroxytryptamine 2A (5-HT2A) receptor and include the naturally occurring compounds psilocybin, N,N-dimethyltryptamine (DMT, a component of ayahuasca) and mescaline (peyote cactus), as well as the synthesized compound lysergic acid diethylamide (LSD).
Other drugs, such as ketamine, are sometimes referred to as “psychedelics” because they can produce subjective experiences that are similar to those of people who receive classic psychedelics. However, unlike classic psychedelics, the effects of ketamine tend to be short lived. Ketamine also has addictive potential and can be lethal in high doses, which is not the case with psilocybin.
Another compound sometimes referred to as a “psychedelic” is 3,4-methylenedioxymethamphetamine (MDMA), also known as “ecstasy.” The Food and Drug Administration granted breakthrough approval for the study of MDMA for posttraumatic stress disorder (PTSD). FDA-approved registration trials are ongoing. MDMA differs from classic psychedelics in risk profile and pharmacology. In particular, MDMA was widely abused as part of the “rave culture,” while classic psychedelic agents do not lend themselves to that type of misuse.
What is the current legal status of psychedelic agents in the United States? Can clinicians prescribe them, or are they available only in a research setting?
All classic psychedelics are considered to be “Schedule 1” which means they are illegal to possess and use except for research and only if approved by the FDA and under licensure of the Drug Enforcement Administration (DEA), so they are not available for clinical use.
In anticipation of the possibility that phase 3 research may support the efficacy and safety of psilocybin for one or more medical or mental health disorders, our team has reviewed available evidence regarding its abuse liability and concluded that, if psilocybin were approved as medication, it could possibly be included in the Schedule IV category, with additional FDA-mandated risk management provisions. However, this is not yet the case.
Which psychedelic agents are under investigation in the United States, and for which indications?
Psilocybin is under investigation in our center, as well as elsewhere in the United States. We have previously found it to be effective for smoking cessation, and we are conducting another study that is currently recruiting volunteers for this indication. We are also recruiting volunteers for studies on the use of psilocybin for major depression, Alzheimer’s disease, and anorexia nervosa. Further information about our studies can be found on the Web site for our center, the Center for Psychedelic and Consciousness Research.
Two companies – the Usona Institute and COMPASS Pathways – have received FDA Breakthrough Therapy Designation for their programs seeking approval of psilocybin as a treatment major depressive disorder and treatment-resistant depression (TRD), respectively. In addition, an international multicenter study currently underway, which includes US centers in Houston, Baltimore, New York, San Diego, and Atlanta, is investigating psilocybin for TRD.
A number of studies, including one conducted at our center, have investigated psilocybin for depression and anxiety in patients with cancer and found it effective.
Additional research showed that psilocybin alleviated symptoms of cancer-related anxiety and depression, both in the short-term and 5 years later.
LSD has been studied and found promising in the treatment of alcohol use disorder. Additional studies of LSD that are being conducted in Basel Switzerland and at the University of Chicago are examining its impact on mood in healthy volunteers.
Ayahuasca has been studied extensively for depression and anxiety and is also currently under investigation for PTSD. We found that its use in a naturalistic group setting was associated with unintended improvements in depression and anxiety.
Lastly, a lesser-known psychedelic agent is Salvinorin A, which our center has been studying, is the psychoactive constituent of the Salvia divinorum plant. While this is not a “classic” psychedelic compound, it is nevertheless the focus of much scientific interest because its effects are mediated at opioid receptors, rather than 5-HT2A receptors, and may prove to be a novel nonaddictive opioid that may ultimately be a promising treatment for pain and addiction.
What is the typical treatment regimen for psychedelic agents?
It is hard to speak of a “treatment regimen” in agents that are not used in clinical practice. Ongoing clinical trials with psilocybin generally involve one or two 6- to 8-hour sessions involving the oral administration of a moderately high dose under psychologically supported conditions.
Based on the current evidence base, which agents show the most promise?
Psilocybin is currently the most promising classic psychedelic undergoing clinical trials.
Do psychedelics have to be administered in a controlled setting in order to be effective?
Although many people have had meaningful experiences whether inside or outside of a controlled setting, there are serious potential risks associated with use of psilocybin and other classic psychedelics. The safety of psilocybin has been established in clinical studies in which participants have been carefully screened physically and psychologically, are psychologically prepared before their first session, and are psychologically supported during and after sessions. In vulnerable individuals, psilocybin has been associated with enduring psychiatric problems and sometimes persisting visual perceptual conditions. When taken in uncontrolled conditions, classic psychedelics can produce confusion and disorientation resulting in behavior dangerous to the participant and others – including life-threatening risk. Thus, for safety reasons, the optimal environment for using these agents is in a controlled setting.
Do results differ between patients who have used psychedelic agents previously and those who have not?
We have not found any difference between psychedelic-naive volunteers and those who have used psychedelics in the past.
Do you provide patient education prior to treatment initiation?
All of our study participants are thoroughly screened for medical concerns or mental health history such as psychosis, which would preclude their participation. They are educated about the effects of these agents and what they might expect and typically receive several hours of psychological preparation before the first session. They are also provided with psychological support after sessions. Additionally, we spend time developing trust and rapport prior to the first session.
How durable are the effects of psychedelic treatment?
Studies in patients and healthy participants suggest that the positive effects of psilocybin are long lasting, with most individuals reporting positive changes in moods, attitudes, and behavior that they attribute to psilocybin and which endure months or years after the session. The qualities of the acute session experience can vary widely ranging from experiences of transcendence or psychological insight to experiences of intense anxiety or fear.
An enduring shift in worldview and sense of self, as well as psychological insight, may increase psychological flexibility, thereby allowing individuals to subsequently avoid maladaptive patterns of behavior or thought and to make more healthy choices.
Our research has shown that the benefits of these experiences can last as long as 14 months, often longer, and that many participants characterize their psilocybin experience as among the most profound and personally meaningful experience of their lives.
Do participants experience any adverse effects? If so, how are they managed?
Sometimes, despite all the preparation, screening, and support we provide, some participants can have frightening experiences, such as fear and anxiety during the session. When that occurs, it is often shorted lived. The psychological preparation we provide before the session and the psychological support we provide during the session are important for managing such effects.
We provide support and encourage participants to stay with that experience, which may open to experiences of deep meaning or insight. A number of people report that these psychologically challenging states are a valuable part of the overall experience.
We conducted a survey of roughly 2,000 people who took high doses of psilocybin mushrooms and then had a challenging experience. About 10% reported they put themselves or others at risk of physical harm. Of more concern, of those whose experience occurred more than 1 year before, 8% sought treatment for enduring psychological symptoms. These findings underscore potential risks of psilocybin use but do not provide an estimate of the actual incidence of such effects.
Importantly, in our research at Johns Hopkins, we have not observed such effects in over 700 sessions that we have conducted with almost 400 participants, likely because we thoroughly screen and prepare participants and support them after they have completed the study. The potential for serious lasting harm represents a concern and points to the importance of adequate screening and aftercare.
What are the implications for future therapeutics?
We are living in exciting times, in terms of psychedelic research. The potential for a single treatment with a classic psychedelic to produce rapid and sustained therapeutic effects, possibly across a range of psychiatric conditions, is unprecedented in psychiatry. The effect appears to be an “inverse PTSD effect.”
In PTSD, a single exposure to a traumatic event can rewire the nervous system to the point that it produces enduring harm and toxicity. In the case of psychedelics, a single exposure appears to have enduring positive effects in worldview, mood, attitude, behavior, and overall life satisfaction. We can look forward to continued growth and expansion of this research including the refinement of protocols for a variety of therapeutic indications and to the development of a variety of new classic psychedelic compounds.
A version of this article originally appeared on Medscape.com.
Psilocybin delivers ‘remarkable’ relief in severe depression
Psilocybin, the psychedelic compound in “magic mushrooms,” rapidly improves symptoms and produces remission in as little as two sessions for patients with major depression, new research suggests.
Results of a small randomized trial showed that treatment with psilocybin was associated with a greater than 50% reduction in depressive symptoms in 67% of study participants. In addition, 71% showed improvement at 4-week follow-up, with more than 50% achieving remission.
“The finding that the majority of people whom we treated showed efficacy was quite a remarkable and gratifying finding and really sets the stage for psilocybin as a treatment for major depression,” senior investigator Roland Griffiths, PhD, Oliver Lee McCabe III Professor in the Neuropsychopharmacology of Consciousness, Johns Hopkins University, Baltimore, said in a statement.
“Perhaps the most exciting aspect of this as a new therapy is that psilocybin works as a therapeutic intervention with a single session or a few sessions, and then the effects are enduring. In contrast, most conventional treatments for depression ... are given chronically and also have chronic side effects,” added Dr. Griffiths, who is also director of the Johns Hopkins Center for Psychedelic and Consciousness Research.
The study was published online Nov.4 in JAMA Psychiatry.
Growing evidence base
As previously reported, psilocybin improves depressive symptoms for patients with cancer. However, these patients might be regarded as having a “reactive depression” to their life-threatening illness, said Dr. Griffiths.
“This study built on that previous research by asking the question, is psilocybin effective in patients who have major depressive disorder, [which is] a much larger population?” he said.
In addition, prior studies of psilocybin-assisted therapy had no control group, lead author Alan Davis, PhD, adjunct assistant professor in the psychedelic research unit, Johns Hopkins University, said in an interview.
The researchers created a control condition by randomly assigning 24 individuals (mean age, 39.8 years; 67% women) who were currently experiencing a moderate or severe major depressive episode to receive either immediate treatment (IT) (n = 13) or delayed treatment (DT) (n = 11).
Participants had longstanding depression, with a mean of 22.4 months in the current depressive episode. They were required to avoid using other antidepressants for 4 weeks prior to screening and up to 4 months following enrollment.
Patients were also required to be medically stable; have no personal/family history of psychotic or bipolar disorders; no past-year alcohol, substance, or nicotine use disorder; and no substantial lifetime or recent use of ketamine or classic hallucinogens.
Depression was measured using the Structured Clinical Interview for DSM-5 and the GRID-Hamilton Depression Rating Scale (GRID-HAMD). A baseline score of ≥17 was required for enrollment.
Participants received eight preparatory meetings with two session facilitators before the first psilocybin session and then 2-3 hours of follow-up meetings after the psilocybin sessions. In addition, they received 13 sessions of psychotherapy.
After completing these preparatory sessions, they underwent two psilocybin sessions, administered a mean of 1.6 weeks apart.
Participants in the DT group were assessed for depressive symptoms weekly for 8 weeks prior to entering the treatment protocol.
‘Surprising’ findings
Participants in the IT group exhibited significantly lower depression scores on the GRID-HAMD at 1 and 4 weeks after the second psilocybin session in comparison with patients in the DT group during the corresponding weeks.
Moreover, the effect sizes at weeks 5 and 8 were “large” (d = 2.2; 95% confidence interval, 1.4-3.0; and d = 2.6; 95% CI, 1.7-3.6, respectively).
An analysis of outcomes showed that, for all 24 participants, at 1 and at 4 weeks following the psilocybin intervention, 67% and 71% of participants, respectively, had a “clinically significant response” in depressive symptoms; 60% and 56%, respectively, met criteria for remission.
Within-subject T tests likewise revealed significant decreases in depression scores from baseline to 1- and 4-week follow-ups (P < .001; d = 3.6; 95% CI, 2.2-5.0; and P < .001; d = 3.6; 95% CI, 2.2-4.9, respectively).
Importantly, participants experienced no serious adverse effects.
Dr. Griffiths said he was “surprised” by the findings. “We knew that psilocybin would be effective in reactive depression of the type associated with illness, but we did not know that this would be the case in the large number of individuals who qualify for having [major depressive disorder].”
Dr. Davis said the finding “represents a large effect of this treatment among people with major depressive disorder – an approximately 4 times larger effect, compared to studies of antidepressant drugs.”
Dr. Davis noted that psychotherapy was an “essential” component of the study protocol. “ and that this treatment will always have a psychotherapy component and will not be Food and Drug Administration approved as a standalone medication.”
Tipping point
Collin Reiff, MD, clinical assistant professor in the department of psychiatry at New York University noted that because psychedelics are “still stigmatized,” the publication of this study in “one of the highest-impact journals in all of psychiatry suggests that research into psychedelics is now in the mainstream and that the academic psychiatry research community is paying close attention to what is happening.” He described this as a “tipping point.”
Dr. Reiff, who was not involved with the study, noted that research had been conducted on psychedelic compounds until the 1960s, “when they left the research lab and went mainstream, leading to the shutting down and subsequent dormancy of the research for the next 30-40 years.”
Psychedelic research is “undergoing a renaissance and no longer regarded with as much skepticism, but it is important to take our time doing this research so we do not repeat what happened in the 1960s,” said Dr. Reiff.
In an accompanying editorial, Charles F. Reynolds III, MD, endowed professor in geriatric psychiatry at the University of Pittsburgh Medical Center, Pennsylvania, questioned “for whom psychedelic-assisted psychotherapy is appropriate (or not), particularly in patients with depression who are suicidal of have a history of suicide attempt.”
Dr. Reynolds, who is also director of the Aging Institute of UPMC, who was not involved with the study, wrote that “personalizing the management of depression has to entail an understanding of the multiple contexts in which depression occurs, including genetic, developmental, psychosocial, cultural, medical, neurocognitive, and spiritual.”
The study was supported by a crowdsourcing campaign organized by Tim Ferris, as well as by grants from the Riverstyx Foundation. The Center for Psychedelic and Consciousness Research is funded by the Steven and Alexandra Cohen Foundation and receives support from Tim Ferriss, Matt Mullenweg, Craig Nerenberg, and Blake Mycoskie. It is also supported by grants from the National Institute on Drug Abuse. Dr. Davis received support from the NIDA. Dr. Griffiths was partially supported by a NIDA grant. Disclosures for the other authors are listed in the original article. Reiff reports owning stock in Compass Pathways. Dr. Reynolds reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Psilocybin, the psychedelic compound in “magic mushrooms,” rapidly improves symptoms and produces remission in as little as two sessions for patients with major depression, new research suggests.
Results of a small randomized trial showed that treatment with psilocybin was associated with a greater than 50% reduction in depressive symptoms in 67% of study participants. In addition, 71% showed improvement at 4-week follow-up, with more than 50% achieving remission.
“The finding that the majority of people whom we treated showed efficacy was quite a remarkable and gratifying finding and really sets the stage for psilocybin as a treatment for major depression,” senior investigator Roland Griffiths, PhD, Oliver Lee McCabe III Professor in the Neuropsychopharmacology of Consciousness, Johns Hopkins University, Baltimore, said in a statement.
“Perhaps the most exciting aspect of this as a new therapy is that psilocybin works as a therapeutic intervention with a single session or a few sessions, and then the effects are enduring. In contrast, most conventional treatments for depression ... are given chronically and also have chronic side effects,” added Dr. Griffiths, who is also director of the Johns Hopkins Center for Psychedelic and Consciousness Research.
The study was published online Nov.4 in JAMA Psychiatry.
Growing evidence base
As previously reported, psilocybin improves depressive symptoms for patients with cancer. However, these patients might be regarded as having a “reactive depression” to their life-threatening illness, said Dr. Griffiths.
“This study built on that previous research by asking the question, is psilocybin effective in patients who have major depressive disorder, [which is] a much larger population?” he said.
In addition, prior studies of psilocybin-assisted therapy had no control group, lead author Alan Davis, PhD, adjunct assistant professor in the psychedelic research unit, Johns Hopkins University, said in an interview.
The researchers created a control condition by randomly assigning 24 individuals (mean age, 39.8 years; 67% women) who were currently experiencing a moderate or severe major depressive episode to receive either immediate treatment (IT) (n = 13) or delayed treatment (DT) (n = 11).
Participants had longstanding depression, with a mean of 22.4 months in the current depressive episode. They were required to avoid using other antidepressants for 4 weeks prior to screening and up to 4 months following enrollment.
Patients were also required to be medically stable; have no personal/family history of psychotic or bipolar disorders; no past-year alcohol, substance, or nicotine use disorder; and no substantial lifetime or recent use of ketamine or classic hallucinogens.
Depression was measured using the Structured Clinical Interview for DSM-5 and the GRID-Hamilton Depression Rating Scale (GRID-HAMD). A baseline score of ≥17 was required for enrollment.
Participants received eight preparatory meetings with two session facilitators before the first psilocybin session and then 2-3 hours of follow-up meetings after the psilocybin sessions. In addition, they received 13 sessions of psychotherapy.
After completing these preparatory sessions, they underwent two psilocybin sessions, administered a mean of 1.6 weeks apart.
Participants in the DT group were assessed for depressive symptoms weekly for 8 weeks prior to entering the treatment protocol.
‘Surprising’ findings
Participants in the IT group exhibited significantly lower depression scores on the GRID-HAMD at 1 and 4 weeks after the second psilocybin session in comparison with patients in the DT group during the corresponding weeks.
Moreover, the effect sizes at weeks 5 and 8 were “large” (d = 2.2; 95% confidence interval, 1.4-3.0; and d = 2.6; 95% CI, 1.7-3.6, respectively).
An analysis of outcomes showed that, for all 24 participants, at 1 and at 4 weeks following the psilocybin intervention, 67% and 71% of participants, respectively, had a “clinically significant response” in depressive symptoms; 60% and 56%, respectively, met criteria for remission.
Within-subject T tests likewise revealed significant decreases in depression scores from baseline to 1- and 4-week follow-ups (P < .001; d = 3.6; 95% CI, 2.2-5.0; and P < .001; d = 3.6; 95% CI, 2.2-4.9, respectively).
Importantly, participants experienced no serious adverse effects.
Dr. Griffiths said he was “surprised” by the findings. “We knew that psilocybin would be effective in reactive depression of the type associated with illness, but we did not know that this would be the case in the large number of individuals who qualify for having [major depressive disorder].”
Dr. Davis said the finding “represents a large effect of this treatment among people with major depressive disorder – an approximately 4 times larger effect, compared to studies of antidepressant drugs.”
Dr. Davis noted that psychotherapy was an “essential” component of the study protocol. “ and that this treatment will always have a psychotherapy component and will not be Food and Drug Administration approved as a standalone medication.”
Tipping point
Collin Reiff, MD, clinical assistant professor in the department of psychiatry at New York University noted that because psychedelics are “still stigmatized,” the publication of this study in “one of the highest-impact journals in all of psychiatry suggests that research into psychedelics is now in the mainstream and that the academic psychiatry research community is paying close attention to what is happening.” He described this as a “tipping point.”
Dr. Reiff, who was not involved with the study, noted that research had been conducted on psychedelic compounds until the 1960s, “when they left the research lab and went mainstream, leading to the shutting down and subsequent dormancy of the research for the next 30-40 years.”
Psychedelic research is “undergoing a renaissance and no longer regarded with as much skepticism, but it is important to take our time doing this research so we do not repeat what happened in the 1960s,” said Dr. Reiff.
In an accompanying editorial, Charles F. Reynolds III, MD, endowed professor in geriatric psychiatry at the University of Pittsburgh Medical Center, Pennsylvania, questioned “for whom psychedelic-assisted psychotherapy is appropriate (or not), particularly in patients with depression who are suicidal of have a history of suicide attempt.”
Dr. Reynolds, who is also director of the Aging Institute of UPMC, who was not involved with the study, wrote that “personalizing the management of depression has to entail an understanding of the multiple contexts in which depression occurs, including genetic, developmental, psychosocial, cultural, medical, neurocognitive, and spiritual.”
The study was supported by a crowdsourcing campaign organized by Tim Ferris, as well as by grants from the Riverstyx Foundation. The Center for Psychedelic and Consciousness Research is funded by the Steven and Alexandra Cohen Foundation and receives support from Tim Ferriss, Matt Mullenweg, Craig Nerenberg, and Blake Mycoskie. It is also supported by grants from the National Institute on Drug Abuse. Dr. Davis received support from the NIDA. Dr. Griffiths was partially supported by a NIDA grant. Disclosures for the other authors are listed in the original article. Reiff reports owning stock in Compass Pathways. Dr. Reynolds reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Psilocybin, the psychedelic compound in “magic mushrooms,” rapidly improves symptoms and produces remission in as little as two sessions for patients with major depression, new research suggests.
Results of a small randomized trial showed that treatment with psilocybin was associated with a greater than 50% reduction in depressive symptoms in 67% of study participants. In addition, 71% showed improvement at 4-week follow-up, with more than 50% achieving remission.
“The finding that the majority of people whom we treated showed efficacy was quite a remarkable and gratifying finding and really sets the stage for psilocybin as a treatment for major depression,” senior investigator Roland Griffiths, PhD, Oliver Lee McCabe III Professor in the Neuropsychopharmacology of Consciousness, Johns Hopkins University, Baltimore, said in a statement.
“Perhaps the most exciting aspect of this as a new therapy is that psilocybin works as a therapeutic intervention with a single session or a few sessions, and then the effects are enduring. In contrast, most conventional treatments for depression ... are given chronically and also have chronic side effects,” added Dr. Griffiths, who is also director of the Johns Hopkins Center for Psychedelic and Consciousness Research.
The study was published online Nov.4 in JAMA Psychiatry.
Growing evidence base
As previously reported, psilocybin improves depressive symptoms for patients with cancer. However, these patients might be regarded as having a “reactive depression” to their life-threatening illness, said Dr. Griffiths.
“This study built on that previous research by asking the question, is psilocybin effective in patients who have major depressive disorder, [which is] a much larger population?” he said.
In addition, prior studies of psilocybin-assisted therapy had no control group, lead author Alan Davis, PhD, adjunct assistant professor in the psychedelic research unit, Johns Hopkins University, said in an interview.
The researchers created a control condition by randomly assigning 24 individuals (mean age, 39.8 years; 67% women) who were currently experiencing a moderate or severe major depressive episode to receive either immediate treatment (IT) (n = 13) or delayed treatment (DT) (n = 11).
Participants had longstanding depression, with a mean of 22.4 months in the current depressive episode. They were required to avoid using other antidepressants for 4 weeks prior to screening and up to 4 months following enrollment.
Patients were also required to be medically stable; have no personal/family history of psychotic or bipolar disorders; no past-year alcohol, substance, or nicotine use disorder; and no substantial lifetime or recent use of ketamine or classic hallucinogens.
Depression was measured using the Structured Clinical Interview for DSM-5 and the GRID-Hamilton Depression Rating Scale (GRID-HAMD). A baseline score of ≥17 was required for enrollment.
Participants received eight preparatory meetings with two session facilitators before the first psilocybin session and then 2-3 hours of follow-up meetings after the psilocybin sessions. In addition, they received 13 sessions of psychotherapy.
After completing these preparatory sessions, they underwent two psilocybin sessions, administered a mean of 1.6 weeks apart.
Participants in the DT group were assessed for depressive symptoms weekly for 8 weeks prior to entering the treatment protocol.
‘Surprising’ findings
Participants in the IT group exhibited significantly lower depression scores on the GRID-HAMD at 1 and 4 weeks after the second psilocybin session in comparison with patients in the DT group during the corresponding weeks.
Moreover, the effect sizes at weeks 5 and 8 were “large” (d = 2.2; 95% confidence interval, 1.4-3.0; and d = 2.6; 95% CI, 1.7-3.6, respectively).
An analysis of outcomes showed that, for all 24 participants, at 1 and at 4 weeks following the psilocybin intervention, 67% and 71% of participants, respectively, had a “clinically significant response” in depressive symptoms; 60% and 56%, respectively, met criteria for remission.
Within-subject T tests likewise revealed significant decreases in depression scores from baseline to 1- and 4-week follow-ups (P < .001; d = 3.6; 95% CI, 2.2-5.0; and P < .001; d = 3.6; 95% CI, 2.2-4.9, respectively).
Importantly, participants experienced no serious adverse effects.
Dr. Griffiths said he was “surprised” by the findings. “We knew that psilocybin would be effective in reactive depression of the type associated with illness, but we did not know that this would be the case in the large number of individuals who qualify for having [major depressive disorder].”
Dr. Davis said the finding “represents a large effect of this treatment among people with major depressive disorder – an approximately 4 times larger effect, compared to studies of antidepressant drugs.”
Dr. Davis noted that psychotherapy was an “essential” component of the study protocol. “ and that this treatment will always have a psychotherapy component and will not be Food and Drug Administration approved as a standalone medication.”
Tipping point
Collin Reiff, MD, clinical assistant professor in the department of psychiatry at New York University noted that because psychedelics are “still stigmatized,” the publication of this study in “one of the highest-impact journals in all of psychiatry suggests that research into psychedelics is now in the mainstream and that the academic psychiatry research community is paying close attention to what is happening.” He described this as a “tipping point.”
Dr. Reiff, who was not involved with the study, noted that research had been conducted on psychedelic compounds until the 1960s, “when they left the research lab and went mainstream, leading to the shutting down and subsequent dormancy of the research for the next 30-40 years.”
Psychedelic research is “undergoing a renaissance and no longer regarded with as much skepticism, but it is important to take our time doing this research so we do not repeat what happened in the 1960s,” said Dr. Reiff.
In an accompanying editorial, Charles F. Reynolds III, MD, endowed professor in geriatric psychiatry at the University of Pittsburgh Medical Center, Pennsylvania, questioned “for whom psychedelic-assisted psychotherapy is appropriate (or not), particularly in patients with depression who are suicidal of have a history of suicide attempt.”
Dr. Reynolds, who is also director of the Aging Institute of UPMC, who was not involved with the study, wrote that “personalizing the management of depression has to entail an understanding of the multiple contexts in which depression occurs, including genetic, developmental, psychosocial, cultural, medical, neurocognitive, and spiritual.”
The study was supported by a crowdsourcing campaign organized by Tim Ferris, as well as by grants from the Riverstyx Foundation. The Center for Psychedelic and Consciousness Research is funded by the Steven and Alexandra Cohen Foundation and receives support from Tim Ferriss, Matt Mullenweg, Craig Nerenberg, and Blake Mycoskie. It is also supported by grants from the National Institute on Drug Abuse. Dr. Davis received support from the NIDA. Dr. Griffiths was partially supported by a NIDA grant. Disclosures for the other authors are listed in the original article. Reiff reports owning stock in Compass Pathways. Dr. Reynolds reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Can an ‘unheard of’ approach up adherence to public health advice?
Using principles of psychoanalysis to craft public health messaging may be a novel and effective way of increasing adherence to public health advice during the COVID-19 pandemic, experts say.
In a letter published online Oct. 19 in The Lancet, coauthors Austin Ratner, MD, and Nisarg Gandhi, believe that, as expert communicators, psychoanalysts should be part of the public health care team to help battle the pandemic.
“The idea of using psychoanalysis in a public health setting is relatively unheard of,” Ratner, the author of a book titled “The Psychoanalyst’s Aversion to Proof,” told Medscape Medical News. Ratner earned his MD at John Hopkins School of Medicine but left medicine to become an author. Gandhi is a clinical research intern at Saint Barnabas Medical Center in Livingston, New Jersey.
Psychoanalysis postulates that defense mechanisms, such as denial, may play an important role in nonadherence to public health guidance regarding the pandemic, Ratner said.
including nonadherence to medical advice regarding COVID-19, as well as climate change and politics.
“By understanding that fear and anxiety underpin a lot of denial, the psychoanalytic viewpoint can help influence public health officials in recognizing the fear and anxiety, how to talk about the threat [of the pandemic], and what can be done about it,” he added.
“A new partnership”
“Psychoanalysts have historically resisted collaboration with disciplines such as social and experimental psychology,” Ratner said. This “insularity” results in “lost opportunities on the path for psychoanalysis to become part of the conversation regarding mass denial and mass nonadherence to medical advice.”
He noted that change is afoot in the psychoanalytic community. The American Psychoanalytic Association (APsaA) has begun to “empower constituents” who seek greater “integration with experimental science and greater involvement with public health.”
To that end, Ratner suggests a “new partnership” between three fields that have until now been disparate: experimental psychology, public health, and psychoanalysis.
Cognitive scientists have studied and documented denial, attributing it to “anxiety’s power to compromise rational thought,” but their approach has not focused on the psychoanalytic model of denial as a defense mechanism, Ratner observed.
Mark Smaller, PhD, past president of APsaA and board member of the International Psychoanalytical Association, elaborated.
“From a psychoanalytic perspective, I am interested in how a defense mechanism functions for individuals and groups,” Smaller told Medscape Medical News.
Denial as a defense mechanism often arises, whether in individuals or groups, from a sense of helplessness, explained Smaller, who is also the chair of the department of public advocacy at APsaA.
“People can only tolerate a certain amount of helplessness – in fact, I would suggest as an analyst that helplessness is the most difficult feeling for humans to come to terms with,” he said.
Helplessness can contribute to trauma and “I think we have a mass case of traumatic helplessness in our country right now because of the pandemic.”
Some people respond to a sense of helplessness with depression or hopelessness, while others “try to integrate the impact of the pandemic by focusing on things over which they have control, like wearing a mask, social distancing, and avoiding places with large numbers of people where the virus can be easily transmitted,” said Smaller.
However, “what seems to have occurred in our country is that, although many people have focused on what we do have control of, a large segment of our population are acting as if COVID-19 doesn’t exist, and we have leadership supporting this denial,” he added.
Is “denial” evidence-based?
Commenting for Medscape Medical News, Richard McAnulty, PhD, associate professor of psychology at the University of North Carolina at Charlotte expressed skepticism about the psychoanalytic view of denial, and its potential role in addressing the pandemic.
“A key criticism of psychoanalytic and psychodynamic viewpoints is that many – including the concept of a subconscious mind – are theoretical, not open to empirical research, and not measurable; and one of the most fundamental requirements in science is that all your constructs are measurable.”
For this reason, this approach is “limited in usefulness, although it might be an interesting source of speculation,” said McAnulty.
Ratner disagreed, noting that there is research corroborating the existence of an unconscious mind. Noted analyst Carl Jung, Ratner pointed out, conducted “some great experiments to prove some of the central tenets of psychoanalysis using word associations.”
Jung found that, if individuals were challenged with words that evoked painful associations, it took them longer to arrive at the answer to the test. They also made more mistakes.
Jung’s research “goes back to a core idea of psychoanalysis, which is that painful or difficult thoughts and feelings get distorted, pushed out of consciousness, forgotten, delayed, or suppressed,” Ratner said. These responses might account for “what we’re seeing the U.S. that people are resorting to irrational thinking without being aware of it.”
McAnulty suggested that the psychodynamic idea of denial as a defense mechanism is not relevant to mass nonadherence to pandemic-related medical advice.
Rather, the denial stems from “schemas and belief systems about the world, how people should operate and behave, and the role of government and the medical establishment,” he said.
“When certain recommendations are discrepant with the world view, it creates dissonance or a mismatch and the person will try to reconcile the mismatch,” McAnulty continued. “One way to do that is to say that these recommendations are invalid because they violate the individual’s political beliefs, world view, or religious ideas.”
Ultimately, “it depends on how we define denial,” said McAnulty. “If it means dismissing information that doesn’t fit an existing belief system, that’s denial, but the psychodynamic meaning of ‘denial’ is much deeper than that.”
Smaller, the past president of APsaA, emphasized the importance of empathy when addressing the public. “Psychoanalysts bring empathy to irrationality. Having a psychoanalyst as a team member can help public health officials to communicate better and craft the understanding of anxiety and fear into their message.”
Ratner said he is “not proposing a simplistic silver bullet as an answer to a very complex, multifaceted problem of nonadherence to medical advice.”
Instead, he is “proposing something that hasn’t happened yet, which is more research and more conversation, with psychoanalysis as part of the conversation, because the notion of denial is so relevant, despite how many other factors are involved.”
Ratner, Gandhi, Smaller, and McAnulty have disclosed no relevant financial relationships. Ratner is the author of The Psychoanalyst’s Aversion to Proof and the medical textbook Concepts in Medical Physiology.
This article first appeared on Medscape.com.
Using principles of psychoanalysis to craft public health messaging may be a novel and effective way of increasing adherence to public health advice during the COVID-19 pandemic, experts say.
In a letter published online Oct. 19 in The Lancet, coauthors Austin Ratner, MD, and Nisarg Gandhi, believe that, as expert communicators, psychoanalysts should be part of the public health care team to help battle the pandemic.
“The idea of using psychoanalysis in a public health setting is relatively unheard of,” Ratner, the author of a book titled “The Psychoanalyst’s Aversion to Proof,” told Medscape Medical News. Ratner earned his MD at John Hopkins School of Medicine but left medicine to become an author. Gandhi is a clinical research intern at Saint Barnabas Medical Center in Livingston, New Jersey.
Psychoanalysis postulates that defense mechanisms, such as denial, may play an important role in nonadherence to public health guidance regarding the pandemic, Ratner said.
including nonadherence to medical advice regarding COVID-19, as well as climate change and politics.
“By understanding that fear and anxiety underpin a lot of denial, the psychoanalytic viewpoint can help influence public health officials in recognizing the fear and anxiety, how to talk about the threat [of the pandemic], and what can be done about it,” he added.
“A new partnership”
“Psychoanalysts have historically resisted collaboration with disciplines such as social and experimental psychology,” Ratner said. This “insularity” results in “lost opportunities on the path for psychoanalysis to become part of the conversation regarding mass denial and mass nonadherence to medical advice.”
He noted that change is afoot in the psychoanalytic community. The American Psychoanalytic Association (APsaA) has begun to “empower constituents” who seek greater “integration with experimental science and greater involvement with public health.”
To that end, Ratner suggests a “new partnership” between three fields that have until now been disparate: experimental psychology, public health, and psychoanalysis.
Cognitive scientists have studied and documented denial, attributing it to “anxiety’s power to compromise rational thought,” but their approach has not focused on the psychoanalytic model of denial as a defense mechanism, Ratner observed.
Mark Smaller, PhD, past president of APsaA and board member of the International Psychoanalytical Association, elaborated.
“From a psychoanalytic perspective, I am interested in how a defense mechanism functions for individuals and groups,” Smaller told Medscape Medical News.
Denial as a defense mechanism often arises, whether in individuals or groups, from a sense of helplessness, explained Smaller, who is also the chair of the department of public advocacy at APsaA.
“People can only tolerate a certain amount of helplessness – in fact, I would suggest as an analyst that helplessness is the most difficult feeling for humans to come to terms with,” he said.
Helplessness can contribute to trauma and “I think we have a mass case of traumatic helplessness in our country right now because of the pandemic.”
Some people respond to a sense of helplessness with depression or hopelessness, while others “try to integrate the impact of the pandemic by focusing on things over which they have control, like wearing a mask, social distancing, and avoiding places with large numbers of people where the virus can be easily transmitted,” said Smaller.
However, “what seems to have occurred in our country is that, although many people have focused on what we do have control of, a large segment of our population are acting as if COVID-19 doesn’t exist, and we have leadership supporting this denial,” he added.
Is “denial” evidence-based?
Commenting for Medscape Medical News, Richard McAnulty, PhD, associate professor of psychology at the University of North Carolina at Charlotte expressed skepticism about the psychoanalytic view of denial, and its potential role in addressing the pandemic.
“A key criticism of psychoanalytic and psychodynamic viewpoints is that many – including the concept of a subconscious mind – are theoretical, not open to empirical research, and not measurable; and one of the most fundamental requirements in science is that all your constructs are measurable.”
For this reason, this approach is “limited in usefulness, although it might be an interesting source of speculation,” said McAnulty.
Ratner disagreed, noting that there is research corroborating the existence of an unconscious mind. Noted analyst Carl Jung, Ratner pointed out, conducted “some great experiments to prove some of the central tenets of psychoanalysis using word associations.”
Jung found that, if individuals were challenged with words that evoked painful associations, it took them longer to arrive at the answer to the test. They also made more mistakes.
Jung’s research “goes back to a core idea of psychoanalysis, which is that painful or difficult thoughts and feelings get distorted, pushed out of consciousness, forgotten, delayed, or suppressed,” Ratner said. These responses might account for “what we’re seeing the U.S. that people are resorting to irrational thinking without being aware of it.”
McAnulty suggested that the psychodynamic idea of denial as a defense mechanism is not relevant to mass nonadherence to pandemic-related medical advice.
Rather, the denial stems from “schemas and belief systems about the world, how people should operate and behave, and the role of government and the medical establishment,” he said.
“When certain recommendations are discrepant with the world view, it creates dissonance or a mismatch and the person will try to reconcile the mismatch,” McAnulty continued. “One way to do that is to say that these recommendations are invalid because they violate the individual’s political beliefs, world view, or religious ideas.”
Ultimately, “it depends on how we define denial,” said McAnulty. “If it means dismissing information that doesn’t fit an existing belief system, that’s denial, but the psychodynamic meaning of ‘denial’ is much deeper than that.”
Smaller, the past president of APsaA, emphasized the importance of empathy when addressing the public. “Psychoanalysts bring empathy to irrationality. Having a psychoanalyst as a team member can help public health officials to communicate better and craft the understanding of anxiety and fear into their message.”
Ratner said he is “not proposing a simplistic silver bullet as an answer to a very complex, multifaceted problem of nonadherence to medical advice.”
Instead, he is “proposing something that hasn’t happened yet, which is more research and more conversation, with psychoanalysis as part of the conversation, because the notion of denial is so relevant, despite how many other factors are involved.”
Ratner, Gandhi, Smaller, and McAnulty have disclosed no relevant financial relationships. Ratner is the author of The Psychoanalyst’s Aversion to Proof and the medical textbook Concepts in Medical Physiology.
This article first appeared on Medscape.com.
Using principles of psychoanalysis to craft public health messaging may be a novel and effective way of increasing adherence to public health advice during the COVID-19 pandemic, experts say.
In a letter published online Oct. 19 in The Lancet, coauthors Austin Ratner, MD, and Nisarg Gandhi, believe that, as expert communicators, psychoanalysts should be part of the public health care team to help battle the pandemic.
“The idea of using psychoanalysis in a public health setting is relatively unheard of,” Ratner, the author of a book titled “The Psychoanalyst’s Aversion to Proof,” told Medscape Medical News. Ratner earned his MD at John Hopkins School of Medicine but left medicine to become an author. Gandhi is a clinical research intern at Saint Barnabas Medical Center in Livingston, New Jersey.
Psychoanalysis postulates that defense mechanisms, such as denial, may play an important role in nonadherence to public health guidance regarding the pandemic, Ratner said.
including nonadherence to medical advice regarding COVID-19, as well as climate change and politics.
“By understanding that fear and anxiety underpin a lot of denial, the psychoanalytic viewpoint can help influence public health officials in recognizing the fear and anxiety, how to talk about the threat [of the pandemic], and what can be done about it,” he added.
“A new partnership”
“Psychoanalysts have historically resisted collaboration with disciplines such as social and experimental psychology,” Ratner said. This “insularity” results in “lost opportunities on the path for psychoanalysis to become part of the conversation regarding mass denial and mass nonadherence to medical advice.”
He noted that change is afoot in the psychoanalytic community. The American Psychoanalytic Association (APsaA) has begun to “empower constituents” who seek greater “integration with experimental science and greater involvement with public health.”
To that end, Ratner suggests a “new partnership” between three fields that have until now been disparate: experimental psychology, public health, and psychoanalysis.
Cognitive scientists have studied and documented denial, attributing it to “anxiety’s power to compromise rational thought,” but their approach has not focused on the psychoanalytic model of denial as a defense mechanism, Ratner observed.
Mark Smaller, PhD, past president of APsaA and board member of the International Psychoanalytical Association, elaborated.
“From a psychoanalytic perspective, I am interested in how a defense mechanism functions for individuals and groups,” Smaller told Medscape Medical News.
Denial as a defense mechanism often arises, whether in individuals or groups, from a sense of helplessness, explained Smaller, who is also the chair of the department of public advocacy at APsaA.
“People can only tolerate a certain amount of helplessness – in fact, I would suggest as an analyst that helplessness is the most difficult feeling for humans to come to terms with,” he said.
Helplessness can contribute to trauma and “I think we have a mass case of traumatic helplessness in our country right now because of the pandemic.”
Some people respond to a sense of helplessness with depression or hopelessness, while others “try to integrate the impact of the pandemic by focusing on things over which they have control, like wearing a mask, social distancing, and avoiding places with large numbers of people where the virus can be easily transmitted,” said Smaller.
However, “what seems to have occurred in our country is that, although many people have focused on what we do have control of, a large segment of our population are acting as if COVID-19 doesn’t exist, and we have leadership supporting this denial,” he added.
Is “denial” evidence-based?
Commenting for Medscape Medical News, Richard McAnulty, PhD, associate professor of psychology at the University of North Carolina at Charlotte expressed skepticism about the psychoanalytic view of denial, and its potential role in addressing the pandemic.
“A key criticism of psychoanalytic and psychodynamic viewpoints is that many – including the concept of a subconscious mind – are theoretical, not open to empirical research, and not measurable; and one of the most fundamental requirements in science is that all your constructs are measurable.”
For this reason, this approach is “limited in usefulness, although it might be an interesting source of speculation,” said McAnulty.
Ratner disagreed, noting that there is research corroborating the existence of an unconscious mind. Noted analyst Carl Jung, Ratner pointed out, conducted “some great experiments to prove some of the central tenets of psychoanalysis using word associations.”
Jung found that, if individuals were challenged with words that evoked painful associations, it took them longer to arrive at the answer to the test. They also made more mistakes.
Jung’s research “goes back to a core idea of psychoanalysis, which is that painful or difficult thoughts and feelings get distorted, pushed out of consciousness, forgotten, delayed, or suppressed,” Ratner said. These responses might account for “what we’re seeing the U.S. that people are resorting to irrational thinking without being aware of it.”
McAnulty suggested that the psychodynamic idea of denial as a defense mechanism is not relevant to mass nonadherence to pandemic-related medical advice.
Rather, the denial stems from “schemas and belief systems about the world, how people should operate and behave, and the role of government and the medical establishment,” he said.
“When certain recommendations are discrepant with the world view, it creates dissonance or a mismatch and the person will try to reconcile the mismatch,” McAnulty continued. “One way to do that is to say that these recommendations are invalid because they violate the individual’s political beliefs, world view, or religious ideas.”
Ultimately, “it depends on how we define denial,” said McAnulty. “If it means dismissing information that doesn’t fit an existing belief system, that’s denial, but the psychodynamic meaning of ‘denial’ is much deeper than that.”
Smaller, the past president of APsaA, emphasized the importance of empathy when addressing the public. “Psychoanalysts bring empathy to irrationality. Having a psychoanalyst as a team member can help public health officials to communicate better and craft the understanding of anxiety and fear into their message.”
Ratner said he is “not proposing a simplistic silver bullet as an answer to a very complex, multifaceted problem of nonadherence to medical advice.”
Instead, he is “proposing something that hasn’t happened yet, which is more research and more conversation, with psychoanalysis as part of the conversation, because the notion of denial is so relevant, despite how many other factors are involved.”
Ratner, Gandhi, Smaller, and McAnulty have disclosed no relevant financial relationships. Ratner is the author of The Psychoanalyst’s Aversion to Proof and the medical textbook Concepts in Medical Physiology.
This article first appeared on Medscape.com.