User login
Three Anomalies and a Complication: Ruptured Noncoronary Sinus of Valsalva Aneurysm, Atrial Septal Aneurysm, and Patent Foramen Ovale
A 53 year-old white male with a past medical history of hypertension, hyperlipidemia, and former tobacco use was referred to the Dayton VAMC in Ohio for symptoms that included shortness of breath and a recent abnormal stress test. The patient reported no history of known coronary artery disease (CAD), congestive heart failure, or other cardiovascular diseases. The patient also reported no recent fever, bacterial blood infection, syphilis infection, recreational drug use, or chest trauma.
A physical examination was remarkable for grade 3/6 continuous murmur at the 5th interspace to the left of the sternum and a loud “pistol shot” sound heard over the femoral artery. The patient had jugular venous distension and 2+ leg edema bilaterally. His vital signs were normal, and laboratory blood tests showed normal hemoglobin level and kidney function.
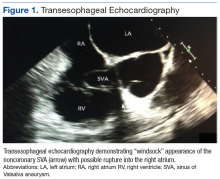
An electrocardiogram showed nonspecific ST segment changes and a transthoracic echocardiogram (TTE) revealed a high-velocity jet in the right atrium (RA) above the tricuspid valve concerning for sinus of Valsalva aneurysm (SVA).
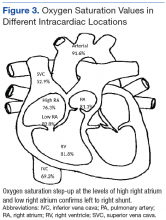
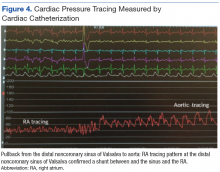
Right heart catheterization revealed elevated RA pressures with positive shunt study showing oxygen saturation step-up in the RA (Figure 3). Left heart hemodynamic measurement from an aortic approach to the distal part of the noncoronary cusp SVA revealed an RA pressure-tracing pattern consistent with rupture of the noncoronary SVA into the RA (Figure 4).
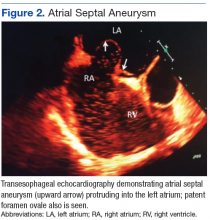
The primary diagnosis was of acute heart failure secondary to ruptured aneurysm of the noncoronary SVA into RA. The patient also received a secondary diagnosis of atrial septal aneurysm and PFO.
Treatment & Outcome
The patient was treated with aggressive diuresis and responded well to therapy. Considering the high mortality rate associated with a ruptured SVA, the patient was referred to a tertiary care center for surgical evaluation. He underwent repair of aorto-right atrial communication with a Cormatrix patch (Roswell, GA) from the aortic side and with primary closure from the right atrial side with resection of the windsock tract; coronary artery bypass graft x1 with right internal mammary artery to the right coronary artery; closure of the PFO with the Cormatrix patch.
The postoperative TEE confirmed preserved LV and RV function, no shunts, no aortic or tricuspid insufficiency. Biopsy of the tissue resected showed intimal fibroplasia. A TTE completed 1 year after surgery showed normal valvular function and without any structural abnormalities. The patient had improvement in symptoms and an uneventful year after surgical intervention followed by 24 session of cardiac rehabilitation.
Discussion
Sinus of Valsalva aneurysm is a dilation of the aortic wall between the aortic valve and the sinotubular junction that is caused by the lack of continuity between the middle layer of the aortic wall and the aortic valve.1 Cases of SVA are rare cardiac anomalies with prevalence of 1% in patients undergoing open-heart surgery.2 Between 65% and 85% of SVA cases originate from the right coronary sinus, 10% to 20% from the noncoronary sinus, and < 5% from the left coronary sinus.3
Sinus of Valsalva aneurysm is usually congenital, although cases associated with syphilis, bacterial endocarditis, trauma, Behçet disease, and aortic dissection have been reported. Structural defects associated with congenital SVAs include ventricular septal defect, bicuspid aortic valve, and aortic regurgitation. It is less commonly associated with pulmonary stenosis, coarctation of the aorta, patent ductus arteriosus, tricuspid regurgitation, and atrial septal defects.
The most common complication of the SVA is rupture into another cardiac chamber, frequently the right ventricle (60%) or RA (29%) and less frequently into left atrium (6%), left ventricle (4%), or pericardium (1%).1 Patients with ruptured SVA mainly develop dyspnea and chest pain, but cough, fatigue, peripheral edema, and continuous murmur have been reported.1
Atrial septal aneurysm is an uncommon finding in adults, with an incidence of 2.2 % in the general population, and it is often associated with atrial septal defect and PFO.1,4 Although ASA formation can be secondary to interatrial differences in pressures, it can be a primary malformation involving the region of the fossa ovalis or the entire atrial septum.4 Atrial septal aneurysm may be an isolated anomaly, but often is found in association with other structural cardiac anomalies, including SVA and PFO.4,5
Conclusion
Although coexistence of SVA and ASA has been reported previously, the case reported here, a ruptured noncoronary SVA that was associated with a large ASA and a PFO, has not been previously documented in the English literature. This patient’s anomalies are most likely congenital in origin. Progressive dyspnea and chest pain in the presence of a continuous loud murmur should raise the suspicion of ruptured sinus of Valsalva. Although no significant aortic regurgitation was noted on echocardiography, the pistol shot sound heard over the femoral artery was believed to be due to the rapid diastolic runoff into the RA through the ruptured SVA.
The significant increase in the RA pressure made the ASA and PFO more prominent. A TEE, left and right heart catheterizations with shunt study are vital for the diagnosis of SVA. If left untreated, SVA has an ominous prognosis. Surgical repair of ruptured SVA has an accepted risk and good prognosis with 10-year survival rate of 90%, whereas the mean survival of untreated ruptured SVA is about 4 years.6,7 Hence, the patient in this study was referred to a tertiary care center for surgical intervention.
1. Galicia-Tornell MM, Marín-Solís B, Mercado-Astorga O, Espinoza-Anguiano S, Martínez-Martínez M, Villalpando-Mendoza E. Sinus of Valsalva aneurysm with rupture. Case report and literature review. Cir Cir. 2009;77(6):441-445.
2. Takach TJ, Reul GJ, Duncan JM, et al. Sinus of Valsalva aneurysm or fistula: management and outcome. Ann Thorac Surg. 1999;68(5):1573-1577.
3. Meier JH, Seward JB, Miller FA Jr, Oh JK, Enriquez-Sarano M. Aneurysms in the left ventricular outflow tract: clinical presentation, causes, and echocardiographic features. J Am Soc Echocardiogr. 1998;11(7):729-745.
4. Mügge A, Daniel WG, Angermann C et al. Atrial septal aneurysm in adult patients: a multicenter study using transthoracic and transesophageal echocardiography. Circulation. 1995;91(11):2785-2792.
5. Silver MD, Dorsey JS. Aneurysms of the septum primum in adults. Arch Pathol Lab Med. 1978;102(2):62-65.
6. Wang ZJ, Zou CW, Li DC, et al. Surgical repair of sinus of Valsalva aneurysm in Asian patients. Ann Thorac Surg. 2007;84(1):156-160.
7. Yan F, Huo Q, Qiao J, Murat V, Ma SF. Surgery for sinus of valsalva aneurysm: 27-year experience with 100 patients. Asian Cardiovasc Thorac Ann. 2008;16(5):361-365.
A 53 year-old white male with a past medical history of hypertension, hyperlipidemia, and former tobacco use was referred to the Dayton VAMC in Ohio for symptoms that included shortness of breath and a recent abnormal stress test. The patient reported no history of known coronary artery disease (CAD), congestive heart failure, or other cardiovascular diseases. The patient also reported no recent fever, bacterial blood infection, syphilis infection, recreational drug use, or chest trauma.
A physical examination was remarkable for grade 3/6 continuous murmur at the 5th interspace to the left of the sternum and a loud “pistol shot” sound heard over the femoral artery. The patient had jugular venous distension and 2+ leg edema bilaterally. His vital signs were normal, and laboratory blood tests showed normal hemoglobin level and kidney function.
An electrocardiogram showed nonspecific ST segment changes and a transthoracic echocardiogram (TTE) revealed a high-velocity jet in the right atrium (RA) above the tricuspid valve concerning for sinus of Valsalva aneurysm (SVA).
Right heart catheterization revealed elevated RA pressures with positive shunt study showing oxygen saturation step-up in the RA (Figure 3). Left heart hemodynamic measurement from an aortic approach to the distal part of the noncoronary cusp SVA revealed an RA pressure-tracing pattern consistent with rupture of the noncoronary SVA into the RA (Figure 4).
The primary diagnosis was of acute heart failure secondary to ruptured aneurysm of the noncoronary SVA into RA. The patient also received a secondary diagnosis of atrial septal aneurysm and PFO.
Treatment & Outcome
The patient was treated with aggressive diuresis and responded well to therapy. Considering the high mortality rate associated with a ruptured SVA, the patient was referred to a tertiary care center for surgical evaluation. He underwent repair of aorto-right atrial communication with a Cormatrix patch (Roswell, GA) from the aortic side and with primary closure from the right atrial side with resection of the windsock tract; coronary artery bypass graft x1 with right internal mammary artery to the right coronary artery; closure of the PFO with the Cormatrix patch.
The postoperative TEE confirmed preserved LV and RV function, no shunts, no aortic or tricuspid insufficiency. Biopsy of the tissue resected showed intimal fibroplasia. A TTE completed 1 year after surgery showed normal valvular function and without any structural abnormalities. The patient had improvement in symptoms and an uneventful year after surgical intervention followed by 24 session of cardiac rehabilitation.
Discussion
Sinus of Valsalva aneurysm is a dilation of the aortic wall between the aortic valve and the sinotubular junction that is caused by the lack of continuity between the middle layer of the aortic wall and the aortic valve.1 Cases of SVA are rare cardiac anomalies with prevalence of 1% in patients undergoing open-heart surgery.2 Between 65% and 85% of SVA cases originate from the right coronary sinus, 10% to 20% from the noncoronary sinus, and < 5% from the left coronary sinus.3
Sinus of Valsalva aneurysm is usually congenital, although cases associated with syphilis, bacterial endocarditis, trauma, Behçet disease, and aortic dissection have been reported. Structural defects associated with congenital SVAs include ventricular septal defect, bicuspid aortic valve, and aortic regurgitation. It is less commonly associated with pulmonary stenosis, coarctation of the aorta, patent ductus arteriosus, tricuspid regurgitation, and atrial septal defects.
The most common complication of the SVA is rupture into another cardiac chamber, frequently the right ventricle (60%) or RA (29%) and less frequently into left atrium (6%), left ventricle (4%), or pericardium (1%).1 Patients with ruptured SVA mainly develop dyspnea and chest pain, but cough, fatigue, peripheral edema, and continuous murmur have been reported.1
Atrial septal aneurysm is an uncommon finding in adults, with an incidence of 2.2 % in the general population, and it is often associated with atrial septal defect and PFO.1,4 Although ASA formation can be secondary to interatrial differences in pressures, it can be a primary malformation involving the region of the fossa ovalis or the entire atrial septum.4 Atrial septal aneurysm may be an isolated anomaly, but often is found in association with other structural cardiac anomalies, including SVA and PFO.4,5
Conclusion
Although coexistence of SVA and ASA has been reported previously, the case reported here, a ruptured noncoronary SVA that was associated with a large ASA and a PFO, has not been previously documented in the English literature. This patient’s anomalies are most likely congenital in origin. Progressive dyspnea and chest pain in the presence of a continuous loud murmur should raise the suspicion of ruptured sinus of Valsalva. Although no significant aortic regurgitation was noted on echocardiography, the pistol shot sound heard over the femoral artery was believed to be due to the rapid diastolic runoff into the RA through the ruptured SVA.
The significant increase in the RA pressure made the ASA and PFO more prominent. A TEE, left and right heart catheterizations with shunt study are vital for the diagnosis of SVA. If left untreated, SVA has an ominous prognosis. Surgical repair of ruptured SVA has an accepted risk and good prognosis with 10-year survival rate of 90%, whereas the mean survival of untreated ruptured SVA is about 4 years.6,7 Hence, the patient in this study was referred to a tertiary care center for surgical intervention.
A 53 year-old white male with a past medical history of hypertension, hyperlipidemia, and former tobacco use was referred to the Dayton VAMC in Ohio for symptoms that included shortness of breath and a recent abnormal stress test. The patient reported no history of known coronary artery disease (CAD), congestive heart failure, or other cardiovascular diseases. The patient also reported no recent fever, bacterial blood infection, syphilis infection, recreational drug use, or chest trauma.
A physical examination was remarkable for grade 3/6 continuous murmur at the 5th interspace to the left of the sternum and a loud “pistol shot” sound heard over the femoral artery. The patient had jugular venous distension and 2+ leg edema bilaterally. His vital signs were normal, and laboratory blood tests showed normal hemoglobin level and kidney function.
An electrocardiogram showed nonspecific ST segment changes and a transthoracic echocardiogram (TTE) revealed a high-velocity jet in the right atrium (RA) above the tricuspid valve concerning for sinus of Valsalva aneurysm (SVA).
Right heart catheterization revealed elevated RA pressures with positive shunt study showing oxygen saturation step-up in the RA (Figure 3). Left heart hemodynamic measurement from an aortic approach to the distal part of the noncoronary cusp SVA revealed an RA pressure-tracing pattern consistent with rupture of the noncoronary SVA into the RA (Figure 4).
The primary diagnosis was of acute heart failure secondary to ruptured aneurysm of the noncoronary SVA into RA. The patient also received a secondary diagnosis of atrial septal aneurysm and PFO.
Treatment & Outcome
The patient was treated with aggressive diuresis and responded well to therapy. Considering the high mortality rate associated with a ruptured SVA, the patient was referred to a tertiary care center for surgical evaluation. He underwent repair of aorto-right atrial communication with a Cormatrix patch (Roswell, GA) from the aortic side and with primary closure from the right atrial side with resection of the windsock tract; coronary artery bypass graft x1 with right internal mammary artery to the right coronary artery; closure of the PFO with the Cormatrix patch.
The postoperative TEE confirmed preserved LV and RV function, no shunts, no aortic or tricuspid insufficiency. Biopsy of the tissue resected showed intimal fibroplasia. A TTE completed 1 year after surgery showed normal valvular function and without any structural abnormalities. The patient had improvement in symptoms and an uneventful year after surgical intervention followed by 24 session of cardiac rehabilitation.
Discussion
Sinus of Valsalva aneurysm is a dilation of the aortic wall between the aortic valve and the sinotubular junction that is caused by the lack of continuity between the middle layer of the aortic wall and the aortic valve.1 Cases of SVA are rare cardiac anomalies with prevalence of 1% in patients undergoing open-heart surgery.2 Between 65% and 85% of SVA cases originate from the right coronary sinus, 10% to 20% from the noncoronary sinus, and < 5% from the left coronary sinus.3
Sinus of Valsalva aneurysm is usually congenital, although cases associated with syphilis, bacterial endocarditis, trauma, Behçet disease, and aortic dissection have been reported. Structural defects associated with congenital SVAs include ventricular septal defect, bicuspid aortic valve, and aortic regurgitation. It is less commonly associated with pulmonary stenosis, coarctation of the aorta, patent ductus arteriosus, tricuspid regurgitation, and atrial septal defects.
The most common complication of the SVA is rupture into another cardiac chamber, frequently the right ventricle (60%) or RA (29%) and less frequently into left atrium (6%), left ventricle (4%), or pericardium (1%).1 Patients with ruptured SVA mainly develop dyspnea and chest pain, but cough, fatigue, peripheral edema, and continuous murmur have been reported.1
Atrial septal aneurysm is an uncommon finding in adults, with an incidence of 2.2 % in the general population, and it is often associated with atrial septal defect and PFO.1,4 Although ASA formation can be secondary to interatrial differences in pressures, it can be a primary malformation involving the region of the fossa ovalis or the entire atrial septum.4 Atrial septal aneurysm may be an isolated anomaly, but often is found in association with other structural cardiac anomalies, including SVA and PFO.4,5
Conclusion
Although coexistence of SVA and ASA has been reported previously, the case reported here, a ruptured noncoronary SVA that was associated with a large ASA and a PFO, has not been previously documented in the English literature. This patient’s anomalies are most likely congenital in origin. Progressive dyspnea and chest pain in the presence of a continuous loud murmur should raise the suspicion of ruptured sinus of Valsalva. Although no significant aortic regurgitation was noted on echocardiography, the pistol shot sound heard over the femoral artery was believed to be due to the rapid diastolic runoff into the RA through the ruptured SVA.
The significant increase in the RA pressure made the ASA and PFO more prominent. A TEE, left and right heart catheterizations with shunt study are vital for the diagnosis of SVA. If left untreated, SVA has an ominous prognosis. Surgical repair of ruptured SVA has an accepted risk and good prognosis with 10-year survival rate of 90%, whereas the mean survival of untreated ruptured SVA is about 4 years.6,7 Hence, the patient in this study was referred to a tertiary care center for surgical intervention.
1. Galicia-Tornell MM, Marín-Solís B, Mercado-Astorga O, Espinoza-Anguiano S, Martínez-Martínez M, Villalpando-Mendoza E. Sinus of Valsalva aneurysm with rupture. Case report and literature review. Cir Cir. 2009;77(6):441-445.
2. Takach TJ, Reul GJ, Duncan JM, et al. Sinus of Valsalva aneurysm or fistula: management and outcome. Ann Thorac Surg. 1999;68(5):1573-1577.
3. Meier JH, Seward JB, Miller FA Jr, Oh JK, Enriquez-Sarano M. Aneurysms in the left ventricular outflow tract: clinical presentation, causes, and echocardiographic features. J Am Soc Echocardiogr. 1998;11(7):729-745.
4. Mügge A, Daniel WG, Angermann C et al. Atrial septal aneurysm in adult patients: a multicenter study using transthoracic and transesophageal echocardiography. Circulation. 1995;91(11):2785-2792.
5. Silver MD, Dorsey JS. Aneurysms of the septum primum in adults. Arch Pathol Lab Med. 1978;102(2):62-65.
6. Wang ZJ, Zou CW, Li DC, et al. Surgical repair of sinus of Valsalva aneurysm in Asian patients. Ann Thorac Surg. 2007;84(1):156-160.
7. Yan F, Huo Q, Qiao J, Murat V, Ma SF. Surgery for sinus of valsalva aneurysm: 27-year experience with 100 patients. Asian Cardiovasc Thorac Ann. 2008;16(5):361-365.
1. Galicia-Tornell MM, Marín-Solís B, Mercado-Astorga O, Espinoza-Anguiano S, Martínez-Martínez M, Villalpando-Mendoza E. Sinus of Valsalva aneurysm with rupture. Case report and literature review. Cir Cir. 2009;77(6):441-445.
2. Takach TJ, Reul GJ, Duncan JM, et al. Sinus of Valsalva aneurysm or fistula: management and outcome. Ann Thorac Surg. 1999;68(5):1573-1577.
3. Meier JH, Seward JB, Miller FA Jr, Oh JK, Enriquez-Sarano M. Aneurysms in the left ventricular outflow tract: clinical presentation, causes, and echocardiographic features. J Am Soc Echocardiogr. 1998;11(7):729-745.
4. Mügge A, Daniel WG, Angermann C et al. Atrial septal aneurysm in adult patients: a multicenter study using transthoracic and transesophageal echocardiography. Circulation. 1995;91(11):2785-2792.
5. Silver MD, Dorsey JS. Aneurysms of the septum primum in adults. Arch Pathol Lab Med. 1978;102(2):62-65.
6. Wang ZJ, Zou CW, Li DC, et al. Surgical repair of sinus of Valsalva aneurysm in Asian patients. Ann Thorac Surg. 2007;84(1):156-160.
7. Yan F, Huo Q, Qiao J, Murat V, Ma SF. Surgery for sinus of valsalva aneurysm: 27-year experience with 100 patients. Asian Cardiovasc Thorac Ann. 2008;16(5):361-365.
Special Report II: Tackling Burnout
Last month, we introduced the epidemic of burnout and the adverse consequences for both our vascular surgery patients and ourselves. Today we will outline a framework for addressing these issues. The foundation of this framework is informed by the social and neurosciences.
From the perspective of the social scientist: Christina Maslach, the originator of the widely used Maslach Burnout Inventory, theorized that burnout arises from a chronic mismatch between people and their work setting in some or all of the following domains: Workload (too much, wrong kind); control (lack of autonomy, or insufficient control over resources); reward (insufficient financial or social rewards commensurate with achievements); community (loss of positive connection with others); fairness (lack of perceived fairness, inequity of work, pay, or promotion); and values (conflict of personal and organizational values). The reality of practicing medicine in today’s business milieu – of achieving service efficiencies by meeting performance targets – brings many of these mismatches into sharp focus.
From the perspective of the neuroscientist: Recent advances, including functional MRI, have demonstrated that the human brain is hard wired for compassion. Compassion is the deep feeling that arises when confronted with another’s suffering, coupled with a strong desire to alleviate that suffering. There are at least two neural pathways: one activated during empathy, having us experience another’s pain; and the other activated during compassion, resulting in our sense of reward. Thus, burnout is thought to occur when you know what your patient needs but you are unable to deliver it. Compassionate medical care is purposeful work, which promotes a sense of reward and mitigates burnout.
Because burnout affects all caregivers (anyone who touches the patient), a successful program addressing workforce well-being must be comprehensive and organization wide, similar to successful patient safety, CPI [continuous process improvement] and LEAN [Six Sigma] initiatives.
There are no shortcuts. Creating a culture of compassionate, collaborative care requires an understanding of the interrelationships between the individual provider, the unit or team, and organizational leadership.
1) The individual provider: There is evidence to support the use of programs that build personal resilience. A recently published meta-analysis by West and colleagues concluded that while no specific physician burnout intervention has been shown to be better than other types of interventions, mindfulness, stress management, and small-group discussions can be effective approaches to reducing burnout scores. Strategies to build individual resilience, such as mindfulness and meditation, are easy to teach but place the burden for success on the individual. No amount of resilience can withstand an unsupportive or toxic workplace environment, so both individual and organizational strategies in combination are necessary.
2) The unit or team: Scheduling time for open and honest discussion of social and emotional issues that arise in caring for patients helps nourish caregiver to caregiver compassion. The Schwartz Center for Compassionate Healthcare is a national nonprofit leading the movement to bring compassion to every patient-caregiver interaction. More than 425 health care organization are Schwartz Center members and conduct Schwartz Rounds™ to bring doctors, nurses, and other caregivers together to discuss the human side of health care. (www.theschwartzcenter.org). Team member to team member support is essential for navigating the stressors of practice. With having lunch in front of your computer being the norm, and the disappearance of traditional spaces for colleagues to connect (for example, nurses’ lounge, physician dining rooms), the opportunity for caregivers to have a safe place to escape, a place to have their own humanity reaffirmed, a place to offer support to their peers, has been eliminated.
3) Organizational Leadership: Making compassion a core value, articulating it, and establishing metrics whereby it can be measured, is a good start. The barriers to a culture of compassion are related to our systems of care. There are burgeoning administrative and documentation tasks to be performed, and productivity expectations that turn our clinics and hospitals into assembly lines. No, we cannot expect the EMR [electronic medical records] to be eliminated, but workforce well-being cannot be sustainable in the context of inadequate resources. A culture of compassionate collaborative care requires programs and policies that are implemented by the organization itself. Examples of organization-wide initiatives that support workforce well-being and provider engagement include: screening for caregiver burnout, establishing policies for managing adverse events with an eye toward the second victim, and most importantly, supporting systems that preserve work control autonomy of physicians and nurses in clinical settings. The business sector has long recognized that workplace stress is a function of how demanding a person’s job is and how much control that person has over his or her responsibilities. The business community has also recognized that the experience of the worker (provider) drives the experience of the customer (patient). In a study of hospital compassionate practices and HCAHPS [the Hospital Consumer Assessment of Healthcare Providers and Systems], McClelland and Vogus reported that how well a hospital compassionately supports it employees and rewards compassionate acts is significantly and positively is associated with that hospital’s ratings and likelihood of patients recommending it.
How does the Society of Vascular Surgery, or any professional medical/nursing society for that matter, fit into this model?
We propose that the SVS find ways to empower their members to be agents for culture change within their own health care organizations. How might this be done:
- Teach organizational leadership skills, starting with the SVS Board of Directors, the presidential line, and the chairs of committees. Offer leadership courses at the Annual Meeting.
- Develop a community of caregivers committed to creating a compassionate collaborative culture. The SVS is a founding member of the Schwartz Center Healthcare Society Leadership Council, and you, as members of the SVS benefit from reduced registration at the Annual Compassion in Action Healthcare Conference, June 24-27, 2017 in Boston. (http://compassioninactionconference.org) This conference is designed to be highly experiential, using a hands-on “how to do it” model.
- The SVS should make improving the overall wellness of its members a specific goal and find specific metrics to monitor our progress towards this goal. Members can be provided with the tools to identify, monitor, and measure burnout and compassion. Each committee and council of the SVS can reexamine their objectives through the lens of reducing burnout and improving the wellness of vascular surgeons.
- Provide members with evidence-based programs that build personal resilience. This will not be a successful initiative unless our surgeons recognize and acknowledge the symptoms of burnout, and are willing to admit vulnerability. Without doing so, it is difficult to reach out for help.
- Redesign postgraduate resident and fellowship education. Standardizing clinical care may reduce variation and promote efficiency. However, when processes such as time-limited appointment scheduling, EMR templates, and protocols that drive physician-patient interactions are embedded in Resident and Fellowship education, the result may well be inflexibility in practice, reduced face time with patients, and interactions that lack compassion; all leading to burnout. Graduate Medical Education leaders must develop programs that support the learner’s ability to connect with patients and families, cultivate and role-model skills and behaviors that strengthen compassionate interactions, and strive to develop clinical practice models that increase Resident and Fellow work control autonomy.
The SVS should work proactively to optimize workload, fairness, and reward on a larger scale for its members as it relates to the EMR, reimbursement, and systems coverage. While we may be relatively small in size, as leaders, we are perfectly poised to address these larger, global issues. Perhaps working within the current system (i.e., PAC and APM task force) and considering innovative solutions at a national leadership scale, the SVS can direct real change!
Changing culture is not easy, nor quick, nor does it have an easy-to-follow blueprint. The first step is recognizing the need. The second is taking a leadership role. The third is thinking deeply about implementation.
*The authors extend their thanks and appreciation for the guidance, resources and support of Michael Goldberg, MD, scholar in residence, Schwartz Center for Compassionate Care, Boston and clinical professor of orthopedics at Seattle Children’s Hospital.
REFERENCES
1. J Managerial Psychol. (2007) 22:309-28
2. Annu Rev Neurosci. (2012) 35:1-23
3. Medicine. (2016) 44:583-5
4. J Health Organization Manag. (2015) 29:973-87
5. De Zulueta P Developing compassionate leadership in health care: an integrative review. J Healthcare Leadership. (2016) 8:1-10
6. Dolan ED, Morh D, Lempa M et al. Using a single item to measure burnout in primary care staff: A psychometry evaluation. J Gen Intern Med. (2015) 30:582-7
7. Karasek RA Job demands, job decision latitude, and mental strain: implications for job design. Administrative Sciences Quarterly (1979) 24: 285-308
8. Lee VS, Miller T, Daniels C, et al. Creating the exceptional patient experience in one academic health system. Acad Med. (2016) 91:338-44
9. Linzer M, Levine R, Meltzer D, et al. 10 bold steps to prevent burnout in general internal medicine. J Gen Intern Med. (2013) 29:18-20
10. Lown BA, Manning CF The Schwartz Center Rounds: Evaluation of an interdisciplinary approach to enhancing patient-centered communication, teamwork, and provider support. Acad Med. (2010) 85:1073-81
11. Lown BA, Muncer SJ, Chadwick R Can compassionate healthcare be measured? The Schwartz Center Compassionate Care Scale. Patient Education and Counseling (2015) 98:1005-10
12. Lown BA, McIntosh S, Gaines ME, et. al. Integrating compassionate collaborative care (“the Triple C”) into health professional education to advance the triple aim of health care. Acad Med (2016) 91:1-7
13. Lown BA A social neuroscience-informed model for teaching and practicing compassion in health care. Medical Education (2016) 50: 332-342
14. Maslach C, Schaufeli WG, Leiter MP Job burnout. Annu Rev Psychol (2001) 52:397-422
15. McClelland LE, Vogus TJ Compassion practices and HCAHPS: Does rewarding and supporting workplace compassion influence patient perceptions? HSR: Health Serv Res. (2014) 49:1670-83
16. Shanafelt TD, Noseworthy JH Executive leadership and physician well-being: Nine organizational strategies to promote engagement and reduce burnout. Mayo Clin Proc. (2016) 6:1-18
17. Shanafelt TD, Dyrbye LN, West CP Addressing physician burnout: the way forward. JAMA (2017) 317:901-2
18. Singer T, Klimecki OM Empathy and compassion Curr Biol. (2014) 24: R875-8
19. West CP, Dyrbye LN, Satele DV et. al. Concurrent validity of single-item measures of emotional exhaustion and depersonalization in burnout assessment. J Gen Intern Med. (2012) 27:1445-52
20. West CP, Dyrbye LN, Erwin PJ, et al. Interventions to address and reduce physician burnout: a systematic review and meta-analysis. Lancet. (2016) 388:2272-81
21. Wuest TK, Goldberg MJ, Kelly JD Clinical faceoff: Physician burnout-Fact, fantasy, or the fourth component of the triple aim? Clin Orthop Relat Res. (2016) doi: 10.1007/5-11999-016-5193-5
Last month, we introduced the epidemic of burnout and the adverse consequences for both our vascular surgery patients and ourselves. Today we will outline a framework for addressing these issues. The foundation of this framework is informed by the social and neurosciences.
From the perspective of the social scientist: Christina Maslach, the originator of the widely used Maslach Burnout Inventory, theorized that burnout arises from a chronic mismatch between people and their work setting in some or all of the following domains: Workload (too much, wrong kind); control (lack of autonomy, or insufficient control over resources); reward (insufficient financial or social rewards commensurate with achievements); community (loss of positive connection with others); fairness (lack of perceived fairness, inequity of work, pay, or promotion); and values (conflict of personal and organizational values). The reality of practicing medicine in today’s business milieu – of achieving service efficiencies by meeting performance targets – brings many of these mismatches into sharp focus.
From the perspective of the neuroscientist: Recent advances, including functional MRI, have demonstrated that the human brain is hard wired for compassion. Compassion is the deep feeling that arises when confronted with another’s suffering, coupled with a strong desire to alleviate that suffering. There are at least two neural pathways: one activated during empathy, having us experience another’s pain; and the other activated during compassion, resulting in our sense of reward. Thus, burnout is thought to occur when you know what your patient needs but you are unable to deliver it. Compassionate medical care is purposeful work, which promotes a sense of reward and mitigates burnout.
Because burnout affects all caregivers (anyone who touches the patient), a successful program addressing workforce well-being must be comprehensive and organization wide, similar to successful patient safety, CPI [continuous process improvement] and LEAN [Six Sigma] initiatives.
There are no shortcuts. Creating a culture of compassionate, collaborative care requires an understanding of the interrelationships between the individual provider, the unit or team, and organizational leadership.
1) The individual provider: There is evidence to support the use of programs that build personal resilience. A recently published meta-analysis by West and colleagues concluded that while no specific physician burnout intervention has been shown to be better than other types of interventions, mindfulness, stress management, and small-group discussions can be effective approaches to reducing burnout scores. Strategies to build individual resilience, such as mindfulness and meditation, are easy to teach but place the burden for success on the individual. No amount of resilience can withstand an unsupportive or toxic workplace environment, so both individual and organizational strategies in combination are necessary.
2) The unit or team: Scheduling time for open and honest discussion of social and emotional issues that arise in caring for patients helps nourish caregiver to caregiver compassion. The Schwartz Center for Compassionate Healthcare is a national nonprofit leading the movement to bring compassion to every patient-caregiver interaction. More than 425 health care organization are Schwartz Center members and conduct Schwartz Rounds™ to bring doctors, nurses, and other caregivers together to discuss the human side of health care. (www.theschwartzcenter.org). Team member to team member support is essential for navigating the stressors of practice. With having lunch in front of your computer being the norm, and the disappearance of traditional spaces for colleagues to connect (for example, nurses’ lounge, physician dining rooms), the opportunity for caregivers to have a safe place to escape, a place to have their own humanity reaffirmed, a place to offer support to their peers, has been eliminated.
3) Organizational Leadership: Making compassion a core value, articulating it, and establishing metrics whereby it can be measured, is a good start. The barriers to a culture of compassion are related to our systems of care. There are burgeoning administrative and documentation tasks to be performed, and productivity expectations that turn our clinics and hospitals into assembly lines. No, we cannot expect the EMR [electronic medical records] to be eliminated, but workforce well-being cannot be sustainable in the context of inadequate resources. A culture of compassionate collaborative care requires programs and policies that are implemented by the organization itself. Examples of organization-wide initiatives that support workforce well-being and provider engagement include: screening for caregiver burnout, establishing policies for managing adverse events with an eye toward the second victim, and most importantly, supporting systems that preserve work control autonomy of physicians and nurses in clinical settings. The business sector has long recognized that workplace stress is a function of how demanding a person’s job is and how much control that person has over his or her responsibilities. The business community has also recognized that the experience of the worker (provider) drives the experience of the customer (patient). In a study of hospital compassionate practices and HCAHPS [the Hospital Consumer Assessment of Healthcare Providers and Systems], McClelland and Vogus reported that how well a hospital compassionately supports it employees and rewards compassionate acts is significantly and positively is associated with that hospital’s ratings and likelihood of patients recommending it.
How does the Society of Vascular Surgery, or any professional medical/nursing society for that matter, fit into this model?
We propose that the SVS find ways to empower their members to be agents for culture change within their own health care organizations. How might this be done:
- Teach organizational leadership skills, starting with the SVS Board of Directors, the presidential line, and the chairs of committees. Offer leadership courses at the Annual Meeting.
- Develop a community of caregivers committed to creating a compassionate collaborative culture. The SVS is a founding member of the Schwartz Center Healthcare Society Leadership Council, and you, as members of the SVS benefit from reduced registration at the Annual Compassion in Action Healthcare Conference, June 24-27, 2017 in Boston. (http://compassioninactionconference.org) This conference is designed to be highly experiential, using a hands-on “how to do it” model.
- The SVS should make improving the overall wellness of its members a specific goal and find specific metrics to monitor our progress towards this goal. Members can be provided with the tools to identify, monitor, and measure burnout and compassion. Each committee and council of the SVS can reexamine their objectives through the lens of reducing burnout and improving the wellness of vascular surgeons.
- Provide members with evidence-based programs that build personal resilience. This will not be a successful initiative unless our surgeons recognize and acknowledge the symptoms of burnout, and are willing to admit vulnerability. Without doing so, it is difficult to reach out for help.
- Redesign postgraduate resident and fellowship education. Standardizing clinical care may reduce variation and promote efficiency. However, when processes such as time-limited appointment scheduling, EMR templates, and protocols that drive physician-patient interactions are embedded in Resident and Fellowship education, the result may well be inflexibility in practice, reduced face time with patients, and interactions that lack compassion; all leading to burnout. Graduate Medical Education leaders must develop programs that support the learner’s ability to connect with patients and families, cultivate and role-model skills and behaviors that strengthen compassionate interactions, and strive to develop clinical practice models that increase Resident and Fellow work control autonomy.
The SVS should work proactively to optimize workload, fairness, and reward on a larger scale for its members as it relates to the EMR, reimbursement, and systems coverage. While we may be relatively small in size, as leaders, we are perfectly poised to address these larger, global issues. Perhaps working within the current system (i.e., PAC and APM task force) and considering innovative solutions at a national leadership scale, the SVS can direct real change!
Changing culture is not easy, nor quick, nor does it have an easy-to-follow blueprint. The first step is recognizing the need. The second is taking a leadership role. The third is thinking deeply about implementation.
*The authors extend their thanks and appreciation for the guidance, resources and support of Michael Goldberg, MD, scholar in residence, Schwartz Center for Compassionate Care, Boston and clinical professor of orthopedics at Seattle Children’s Hospital.
REFERENCES
1. J Managerial Psychol. (2007) 22:309-28
2. Annu Rev Neurosci. (2012) 35:1-23
3. Medicine. (2016) 44:583-5
4. J Health Organization Manag. (2015) 29:973-87
5. De Zulueta P Developing compassionate leadership in health care: an integrative review. J Healthcare Leadership. (2016) 8:1-10
6. Dolan ED, Morh D, Lempa M et al. Using a single item to measure burnout in primary care staff: A psychometry evaluation. J Gen Intern Med. (2015) 30:582-7
7. Karasek RA Job demands, job decision latitude, and mental strain: implications for job design. Administrative Sciences Quarterly (1979) 24: 285-308
8. Lee VS, Miller T, Daniels C, et al. Creating the exceptional patient experience in one academic health system. Acad Med. (2016) 91:338-44
9. Linzer M, Levine R, Meltzer D, et al. 10 bold steps to prevent burnout in general internal medicine. J Gen Intern Med. (2013) 29:18-20
10. Lown BA, Manning CF The Schwartz Center Rounds: Evaluation of an interdisciplinary approach to enhancing patient-centered communication, teamwork, and provider support. Acad Med. (2010) 85:1073-81
11. Lown BA, Muncer SJ, Chadwick R Can compassionate healthcare be measured? The Schwartz Center Compassionate Care Scale. Patient Education and Counseling (2015) 98:1005-10
12. Lown BA, McIntosh S, Gaines ME, et. al. Integrating compassionate collaborative care (“the Triple C”) into health professional education to advance the triple aim of health care. Acad Med (2016) 91:1-7
13. Lown BA A social neuroscience-informed model for teaching and practicing compassion in health care. Medical Education (2016) 50: 332-342
14. Maslach C, Schaufeli WG, Leiter MP Job burnout. Annu Rev Psychol (2001) 52:397-422
15. McClelland LE, Vogus TJ Compassion practices and HCAHPS: Does rewarding and supporting workplace compassion influence patient perceptions? HSR: Health Serv Res. (2014) 49:1670-83
16. Shanafelt TD, Noseworthy JH Executive leadership and physician well-being: Nine organizational strategies to promote engagement and reduce burnout. Mayo Clin Proc. (2016) 6:1-18
17. Shanafelt TD, Dyrbye LN, West CP Addressing physician burnout: the way forward. JAMA (2017) 317:901-2
18. Singer T, Klimecki OM Empathy and compassion Curr Biol. (2014) 24: R875-8
19. West CP, Dyrbye LN, Satele DV et. al. Concurrent validity of single-item measures of emotional exhaustion and depersonalization in burnout assessment. J Gen Intern Med. (2012) 27:1445-52
20. West CP, Dyrbye LN, Erwin PJ, et al. Interventions to address and reduce physician burnout: a systematic review and meta-analysis. Lancet. (2016) 388:2272-81
21. Wuest TK, Goldberg MJ, Kelly JD Clinical faceoff: Physician burnout-Fact, fantasy, or the fourth component of the triple aim? Clin Orthop Relat Res. (2016) doi: 10.1007/5-11999-016-5193-5
Last month, we introduced the epidemic of burnout and the adverse consequences for both our vascular surgery patients and ourselves. Today we will outline a framework for addressing these issues. The foundation of this framework is informed by the social and neurosciences.
From the perspective of the social scientist: Christina Maslach, the originator of the widely used Maslach Burnout Inventory, theorized that burnout arises from a chronic mismatch between people and their work setting in some or all of the following domains: Workload (too much, wrong kind); control (lack of autonomy, or insufficient control over resources); reward (insufficient financial or social rewards commensurate with achievements); community (loss of positive connection with others); fairness (lack of perceived fairness, inequity of work, pay, or promotion); and values (conflict of personal and organizational values). The reality of practicing medicine in today’s business milieu – of achieving service efficiencies by meeting performance targets – brings many of these mismatches into sharp focus.
From the perspective of the neuroscientist: Recent advances, including functional MRI, have demonstrated that the human brain is hard wired for compassion. Compassion is the deep feeling that arises when confronted with another’s suffering, coupled with a strong desire to alleviate that suffering. There are at least two neural pathways: one activated during empathy, having us experience another’s pain; and the other activated during compassion, resulting in our sense of reward. Thus, burnout is thought to occur when you know what your patient needs but you are unable to deliver it. Compassionate medical care is purposeful work, which promotes a sense of reward and mitigates burnout.
Because burnout affects all caregivers (anyone who touches the patient), a successful program addressing workforce well-being must be comprehensive and organization wide, similar to successful patient safety, CPI [continuous process improvement] and LEAN [Six Sigma] initiatives.
There are no shortcuts. Creating a culture of compassionate, collaborative care requires an understanding of the interrelationships between the individual provider, the unit or team, and organizational leadership.
1) The individual provider: There is evidence to support the use of programs that build personal resilience. A recently published meta-analysis by West and colleagues concluded that while no specific physician burnout intervention has been shown to be better than other types of interventions, mindfulness, stress management, and small-group discussions can be effective approaches to reducing burnout scores. Strategies to build individual resilience, such as mindfulness and meditation, are easy to teach but place the burden for success on the individual. No amount of resilience can withstand an unsupportive or toxic workplace environment, so both individual and organizational strategies in combination are necessary.
2) The unit or team: Scheduling time for open and honest discussion of social and emotional issues that arise in caring for patients helps nourish caregiver to caregiver compassion. The Schwartz Center for Compassionate Healthcare is a national nonprofit leading the movement to bring compassion to every patient-caregiver interaction. More than 425 health care organization are Schwartz Center members and conduct Schwartz Rounds™ to bring doctors, nurses, and other caregivers together to discuss the human side of health care. (www.theschwartzcenter.org). Team member to team member support is essential for navigating the stressors of practice. With having lunch in front of your computer being the norm, and the disappearance of traditional spaces for colleagues to connect (for example, nurses’ lounge, physician dining rooms), the opportunity for caregivers to have a safe place to escape, a place to have their own humanity reaffirmed, a place to offer support to their peers, has been eliminated.
3) Organizational Leadership: Making compassion a core value, articulating it, and establishing metrics whereby it can be measured, is a good start. The barriers to a culture of compassion are related to our systems of care. There are burgeoning administrative and documentation tasks to be performed, and productivity expectations that turn our clinics and hospitals into assembly lines. No, we cannot expect the EMR [electronic medical records] to be eliminated, but workforce well-being cannot be sustainable in the context of inadequate resources. A culture of compassionate collaborative care requires programs and policies that are implemented by the organization itself. Examples of organization-wide initiatives that support workforce well-being and provider engagement include: screening for caregiver burnout, establishing policies for managing adverse events with an eye toward the second victim, and most importantly, supporting systems that preserve work control autonomy of physicians and nurses in clinical settings. The business sector has long recognized that workplace stress is a function of how demanding a person’s job is and how much control that person has over his or her responsibilities. The business community has also recognized that the experience of the worker (provider) drives the experience of the customer (patient). In a study of hospital compassionate practices and HCAHPS [the Hospital Consumer Assessment of Healthcare Providers and Systems], McClelland and Vogus reported that how well a hospital compassionately supports it employees and rewards compassionate acts is significantly and positively is associated with that hospital’s ratings and likelihood of patients recommending it.
How does the Society of Vascular Surgery, or any professional medical/nursing society for that matter, fit into this model?
We propose that the SVS find ways to empower their members to be agents for culture change within their own health care organizations. How might this be done:
- Teach organizational leadership skills, starting with the SVS Board of Directors, the presidential line, and the chairs of committees. Offer leadership courses at the Annual Meeting.
- Develop a community of caregivers committed to creating a compassionate collaborative culture. The SVS is a founding member of the Schwartz Center Healthcare Society Leadership Council, and you, as members of the SVS benefit from reduced registration at the Annual Compassion in Action Healthcare Conference, June 24-27, 2017 in Boston. (http://compassioninactionconference.org) This conference is designed to be highly experiential, using a hands-on “how to do it” model.
- The SVS should make improving the overall wellness of its members a specific goal and find specific metrics to monitor our progress towards this goal. Members can be provided with the tools to identify, monitor, and measure burnout and compassion. Each committee and council of the SVS can reexamine their objectives through the lens of reducing burnout and improving the wellness of vascular surgeons.
- Provide members with evidence-based programs that build personal resilience. This will not be a successful initiative unless our surgeons recognize and acknowledge the symptoms of burnout, and are willing to admit vulnerability. Without doing so, it is difficult to reach out for help.
- Redesign postgraduate resident and fellowship education. Standardizing clinical care may reduce variation and promote efficiency. However, when processes such as time-limited appointment scheduling, EMR templates, and protocols that drive physician-patient interactions are embedded in Resident and Fellowship education, the result may well be inflexibility in practice, reduced face time with patients, and interactions that lack compassion; all leading to burnout. Graduate Medical Education leaders must develop programs that support the learner’s ability to connect with patients and families, cultivate and role-model skills and behaviors that strengthen compassionate interactions, and strive to develop clinical practice models that increase Resident and Fellow work control autonomy.
The SVS should work proactively to optimize workload, fairness, and reward on a larger scale for its members as it relates to the EMR, reimbursement, and systems coverage. While we may be relatively small in size, as leaders, we are perfectly poised to address these larger, global issues. Perhaps working within the current system (i.e., PAC and APM task force) and considering innovative solutions at a national leadership scale, the SVS can direct real change!
Changing culture is not easy, nor quick, nor does it have an easy-to-follow blueprint. The first step is recognizing the need. The second is taking a leadership role. The third is thinking deeply about implementation.
*The authors extend their thanks and appreciation for the guidance, resources and support of Michael Goldberg, MD, scholar in residence, Schwartz Center for Compassionate Care, Boston and clinical professor of orthopedics at Seattle Children’s Hospital.
REFERENCES
1. J Managerial Psychol. (2007) 22:309-28
2. Annu Rev Neurosci. (2012) 35:1-23
3. Medicine. (2016) 44:583-5
4. J Health Organization Manag. (2015) 29:973-87
5. De Zulueta P Developing compassionate leadership in health care: an integrative review. J Healthcare Leadership. (2016) 8:1-10
6. Dolan ED, Morh D, Lempa M et al. Using a single item to measure burnout in primary care staff: A psychometry evaluation. J Gen Intern Med. (2015) 30:582-7
7. Karasek RA Job demands, job decision latitude, and mental strain: implications for job design. Administrative Sciences Quarterly (1979) 24: 285-308
8. Lee VS, Miller T, Daniels C, et al. Creating the exceptional patient experience in one academic health system. Acad Med. (2016) 91:338-44
9. Linzer M, Levine R, Meltzer D, et al. 10 bold steps to prevent burnout in general internal medicine. J Gen Intern Med. (2013) 29:18-20
10. Lown BA, Manning CF The Schwartz Center Rounds: Evaluation of an interdisciplinary approach to enhancing patient-centered communication, teamwork, and provider support. Acad Med. (2010) 85:1073-81
11. Lown BA, Muncer SJ, Chadwick R Can compassionate healthcare be measured? The Schwartz Center Compassionate Care Scale. Patient Education and Counseling (2015) 98:1005-10
12. Lown BA, McIntosh S, Gaines ME, et. al. Integrating compassionate collaborative care (“the Triple C”) into health professional education to advance the triple aim of health care. Acad Med (2016) 91:1-7
13. Lown BA A social neuroscience-informed model for teaching and practicing compassion in health care. Medical Education (2016) 50: 332-342
14. Maslach C, Schaufeli WG, Leiter MP Job burnout. Annu Rev Psychol (2001) 52:397-422
15. McClelland LE, Vogus TJ Compassion practices and HCAHPS: Does rewarding and supporting workplace compassion influence patient perceptions? HSR: Health Serv Res. (2014) 49:1670-83
16. Shanafelt TD, Noseworthy JH Executive leadership and physician well-being: Nine organizational strategies to promote engagement and reduce burnout. Mayo Clin Proc. (2016) 6:1-18
17. Shanafelt TD, Dyrbye LN, West CP Addressing physician burnout: the way forward. JAMA (2017) 317:901-2
18. Singer T, Klimecki OM Empathy and compassion Curr Biol. (2014) 24: R875-8
19. West CP, Dyrbye LN, Satele DV et. al. Concurrent validity of single-item measures of emotional exhaustion and depersonalization in burnout assessment. J Gen Intern Med. (2012) 27:1445-52
20. West CP, Dyrbye LN, Erwin PJ, et al. Interventions to address and reduce physician burnout: a systematic review and meta-analysis. Lancet. (2016) 388:2272-81
21. Wuest TK, Goldberg MJ, Kelly JD Clinical faceoff: Physician burnout-Fact, fantasy, or the fourth component of the triple aim? Clin Orthop Relat Res. (2016) doi: 10.1007/5-11999-016-5193-5
VAM ’17 Will Be a ‘Spectacular Meeting’
Participants at the Vascular Annual Meeting (VAM) have lots more to look forward to than sunny skies, beaches and palm trees. A number of new program features are planned to add interest and value to the meeting, said Dr. Ron Dalman.
Dr. Dalman chairs the SVS Program Committee, which develops programming and content for VAM, the premiere meeting for vascular specialists.
The 2017 meeting will be May 31-June 3 in beautiful San Diego, with plenaries and exhibits set for June 1-3.
Changes for 2017 include:
• More and potentially longer sessions with collaborative specialty societies, such as the American Venous Forum, the Society for Vascular Ultrasound and the Society of Thoracic Surgeons. “These sessions provide a multi-disciplinary perspective on our common problems and showcase the SVS’ leadership role in vascular health and disease management,” said Dr. Dalman. Members provided positive feedback on last year’s partnership sessions, so this year, these program features will be significantly expanded.
• An educational review course highlighting some of the more frequently missed questions from the latest version of the Vascular Education Self-Assessment Program (VESAP3).
• Guideline summaries, organized by the SVS Document Oversight Committee and presented by the authorship group for each, on critical topics such as abdominal aortic aneurysms, aortic dissection, venous disease and more. These summaries will be incorporated into post-graduate programming. “It makes sense to cover current practice guidelines and consensus documents, as several high-profile efforts are being updated this year,” said Dr. Dalman. “We can give attendees an executive summary of current guidelines by their respective authors, and attendees will come away with unique insights into why the most impactful and significant changes were included in each respective document.”
• Sessions of potential interest to surgeons in community practice environments, marked in the schedule as such by the SVS Community Practice Committee.
“These improvements will increase the value of the Annual Meeting for all attendees,” Dr. Dalman said. “We’re emphasizing interactive education, not simply passive learning. It’s going to be very exciting – and different in both style and substance.”
A Californian himself, Dr. Dalman also is looking forward to showing off his state. “San Diego is a wonderful place to vacation and the meeting venue provides convenient access to the Gaslamp District, the waterfront and the world-famous beaches,” he said.
“We encourage our members to bring their families to San Diego and make a vacation out of it.”
With the programming additions, increased opportunities for participation, the educational activities planned plus the perfect location, he added, “This is going to be a spectacular meeting.”
Participants at the Vascular Annual Meeting (VAM) have lots more to look forward to than sunny skies, beaches and palm trees. A number of new program features are planned to add interest and value to the meeting, said Dr. Ron Dalman.
Dr. Dalman chairs the SVS Program Committee, which develops programming and content for VAM, the premiere meeting for vascular specialists.
The 2017 meeting will be May 31-June 3 in beautiful San Diego, with plenaries and exhibits set for June 1-3.
Changes for 2017 include:
• More and potentially longer sessions with collaborative specialty societies, such as the American Venous Forum, the Society for Vascular Ultrasound and the Society of Thoracic Surgeons. “These sessions provide a multi-disciplinary perspective on our common problems and showcase the SVS’ leadership role in vascular health and disease management,” said Dr. Dalman. Members provided positive feedback on last year’s partnership sessions, so this year, these program features will be significantly expanded.
• An educational review course highlighting some of the more frequently missed questions from the latest version of the Vascular Education Self-Assessment Program (VESAP3).
• Guideline summaries, organized by the SVS Document Oversight Committee and presented by the authorship group for each, on critical topics such as abdominal aortic aneurysms, aortic dissection, venous disease and more. These summaries will be incorporated into post-graduate programming. “It makes sense to cover current practice guidelines and consensus documents, as several high-profile efforts are being updated this year,” said Dr. Dalman. “We can give attendees an executive summary of current guidelines by their respective authors, and attendees will come away with unique insights into why the most impactful and significant changes were included in each respective document.”
• Sessions of potential interest to surgeons in community practice environments, marked in the schedule as such by the SVS Community Practice Committee.
“These improvements will increase the value of the Annual Meeting for all attendees,” Dr. Dalman said. “We’re emphasizing interactive education, not simply passive learning. It’s going to be very exciting – and different in both style and substance.”
A Californian himself, Dr. Dalman also is looking forward to showing off his state. “San Diego is a wonderful place to vacation and the meeting venue provides convenient access to the Gaslamp District, the waterfront and the world-famous beaches,” he said.
“We encourage our members to bring their families to San Diego and make a vacation out of it.”
With the programming additions, increased opportunities for participation, the educational activities planned plus the perfect location, he added, “This is going to be a spectacular meeting.”
Participants at the Vascular Annual Meeting (VAM) have lots more to look forward to than sunny skies, beaches and palm trees. A number of new program features are planned to add interest and value to the meeting, said Dr. Ron Dalman.
Dr. Dalman chairs the SVS Program Committee, which develops programming and content for VAM, the premiere meeting for vascular specialists.
The 2017 meeting will be May 31-June 3 in beautiful San Diego, with plenaries and exhibits set for June 1-3.
Changes for 2017 include:
• More and potentially longer sessions with collaborative specialty societies, such as the American Venous Forum, the Society for Vascular Ultrasound and the Society of Thoracic Surgeons. “These sessions provide a multi-disciplinary perspective on our common problems and showcase the SVS’ leadership role in vascular health and disease management,” said Dr. Dalman. Members provided positive feedback on last year’s partnership sessions, so this year, these program features will be significantly expanded.
• An educational review course highlighting some of the more frequently missed questions from the latest version of the Vascular Education Self-Assessment Program (VESAP3).
• Guideline summaries, organized by the SVS Document Oversight Committee and presented by the authorship group for each, on critical topics such as abdominal aortic aneurysms, aortic dissection, venous disease and more. These summaries will be incorporated into post-graduate programming. “It makes sense to cover current practice guidelines and consensus documents, as several high-profile efforts are being updated this year,” said Dr. Dalman. “We can give attendees an executive summary of current guidelines by their respective authors, and attendees will come away with unique insights into why the most impactful and significant changes were included in each respective document.”
• Sessions of potential interest to surgeons in community practice environments, marked in the schedule as such by the SVS Community Practice Committee.
“These improvements will increase the value of the Annual Meeting for all attendees,” Dr. Dalman said. “We’re emphasizing interactive education, not simply passive learning. It’s going to be very exciting – and different in both style and substance.”
A Californian himself, Dr. Dalman also is looking forward to showing off his state. “San Diego is a wonderful place to vacation and the meeting venue provides convenient access to the Gaslamp District, the waterfront and the world-famous beaches,” he said.
“We encourage our members to bring their families to San Diego and make a vacation out of it.”
With the programming additions, increased opportunities for participation, the educational activities planned plus the perfect location, he added, “This is going to be a spectacular meeting.”
Ready for post-acute care?
The definition of “hospitalist,” according to the SHM website, is a clinician “dedicated to delivering comprehensive medical care to hospitalized patients.” For years, the hospital setting was the specialties’ identifier. But as hospitalists’ scope has expanded, and post-acute care (PAC) in the United States has grown, more hospitalists are extending their roles into this space.
PAC today is more than the traditional nursing home, according to Manoj K. Mathew, MD, SFHM, national medical director of Agilon Health in Los Angeles.
Many of those expanded settings Dr. Mathew describes emerged as a result of the Affordable Care Act. Since its enactment in 2010, the ACA has heightened providers’ focus on the “Triple Aim” of improving the patient experience (including quality and satisfaction), improving the health of populations, and reducing the per capita cost of healthcare.1 Vishal Kuchaculla, MD, New England regional post-acute medical director of Knoxville,Tenn.-based TeamHealth, says new service lines also developed as Medicare clamped down on long-term inpatient hospital stays by giving financial impetus to discharge patients as soon as possible.
“Over the last few years, there’s been a major shift from fee-for-service to risk-based payment models,” Dr. Kuchaculla says. “The government’s financial incentives are driving outcomes to improve performance initiatives.”
“Today, LTACHs can be used as substitutes for short-term acute care,” says Sean R. Muldoon, MD, MPH, FCCP, chief medical officer of Kindred Healthcare in Louisville, Ky., and former chair of SHM’s Post-Acute Care Committee. “This means that a patient can be directly admitted from their home to an LTACH. In fact, many hospice and home-care patients are referred from physicians’ offices without a preceding hospitalization.”
Hospitalists can fill a need

More hospitalists are working in PACs for a number of reasons. Dr. Mathew says PAC facilities and services have “typically lacked the clinical structure and processes to obtain the results that patients and payors expect.
“These deficits needed to be quickly remedied as patients discharged from hospitals have increased acuity and higher disease burdens,” he adds. “Hospitalists were the natural choice to fill roles requiring their expertise and experience.”
Dr. Muldoon considers the expanded scope of practice into PACs an additional layer to hospital medicine’s value proposition to the healthcare system.
“As experts in the management of inpatient populations, it’s natural for hospitalists to expand to other facilities with inpatient-like populations,” he says, noting SNFs are the most popular choice, with IRFs and LTACHs also being common places to work. Few hospitalists work in home care or hospice.
PAC settings are designed to help patients who are transitioning from an inpatient setting back to their home or other setting.
“Many patients go home after a SNF stay, while others will move to a nursing home or other longer-term care setting for the first time,” says Tiffany Radcliff, PhD, a health economist in the department of health policy and management at Texas A&M University School of Public Health in College Station. “With this in mind, hospitalists working in PAC have the opportunity to address each patient’s ongoing care needs and prepare them for their next setting. Hospitalists can manage medication or other care regimen changes that resulted from an inpatient stay, reinforce discharge instructions to the patient and their caregivers, and identify any other issues with continuing care that need to be addressed before discharge to the next care setting.”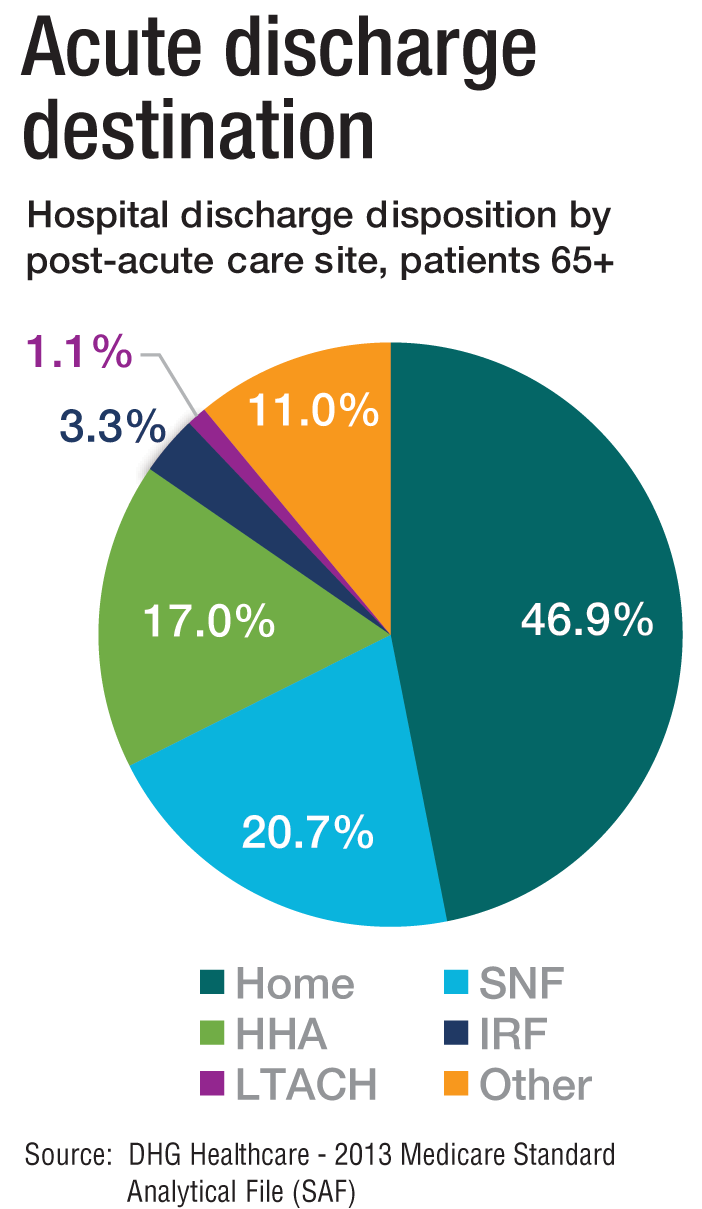
Transitioning Care
Even if a hospitalist is not employed at a PAC, it’s important that they know something about them.
“As patients are moved downstream earlier, hospitalists are being asked to help make a judgment regarding when and where an inpatient is transitioned,” Dr. Muldoon says. As organizations move toward becoming fully risk capable, it is necessary to develop referral networks of high-quality PAC providers to achieve the best clinical outcomes, reduce readmissions, and lower costs.2“Therefore, hospitalists should have a working knowledge of the different sites of service as well as some opinion on the suitability of available options in their community,” Dr. Muldoon says. “The hospitalist can also help to educate the hospitalized patient on what to expect at a PAC.”
If a patient is inappropriately prepared for the PAC setting, it could lead to incomplete management of their condition, which ultimately could lead to readmission.
“When hospitalists know how care is provided in a PAC setting, they are better able to ensure a smoother transition of care between settings,” says Tochi Iroku-Malize, MD, MPH, MBA, FAAFP, SFHM, chair of family medicine at Northwell Health in Long Island, N.Y. “This will ultimately prevent unnecessary readmissions.”
Further, the quality metrics that hospitals and thereby hospitalists are judged by no longer end at the hospital’s exit.
“The ownership of acute-care outcomes requires extending the accountability to outside of the institution’s four walls,” Dr. Mathew says. “The inpatient team needs to place great importance on the transition of care and the subsequent quality of that care when the patient is discharged.”
Robert W. Harrington Jr., MD, SFHM, chief medical officer of Plano, Texas–based Reliant Post-Acute Care Solutions and former SHM president, says the health system landscapes are pushing HM beyond the hospitals’ walls.
How PAC settings differ from hospitals
Practicing in PAC has some important nuances that hospitalists from short-term acute care need to get accustomed to, Dr. Muldoon says. Primarily, the diagnostic capabilities are much more limited, as is the presence of high-level staffing. Further, patients are less resilient to medication changes and interventions, so changes need to be done gradually.
“Hospitalists who try to practice acute-care medicine in a PAC setting may become frustrated by the length of time it takes to do a work-up, get a consultation, and respond to a patient’s change of condition,” Dr. Muldoon says. “Nonetheless, hospitalists can overcome this once recognizing this mind shift.”
According to Dr. Harrington, another challenge hospitalists may face is the inability of the hospital’s and PAC facility’s IT platforms to exchange electronic information.
“The major vendors on both sides need to figure out an interoperability strategy,” he says. “Currently, it often takes 1-3 days to receive a new patient’s discharge summary. The summary may consist of a stack of paper that takes significant time to sort through and requires the PAC facility to perform duplicate data entry. It’s a very highly inefficient process that opens up the doors to mistakes and errors of omission and commission that can result in bad patient outcomes.”
Arif Nazir, MD, CMD, FACP, AGSF, chief medical officer of Signature HealthCARE and president of SHC Medical Partners, both in Louisville, Ky., cites additional reasons the lack of seamless communication between a hospital and PAC facility is problematic. “I see physicians order laboratory tests and investigations that were already done in the hospital because they didn’t know they were already performed or never received the results,” he says. “Similarly, I see patients continue to take medications prescribed in the hospital long term even though they were only supposed to take them short term. I’ve also seen patients come to a PAC setting from a hospital without any formal understanding of their rehabilitative period and expectations for recovery.”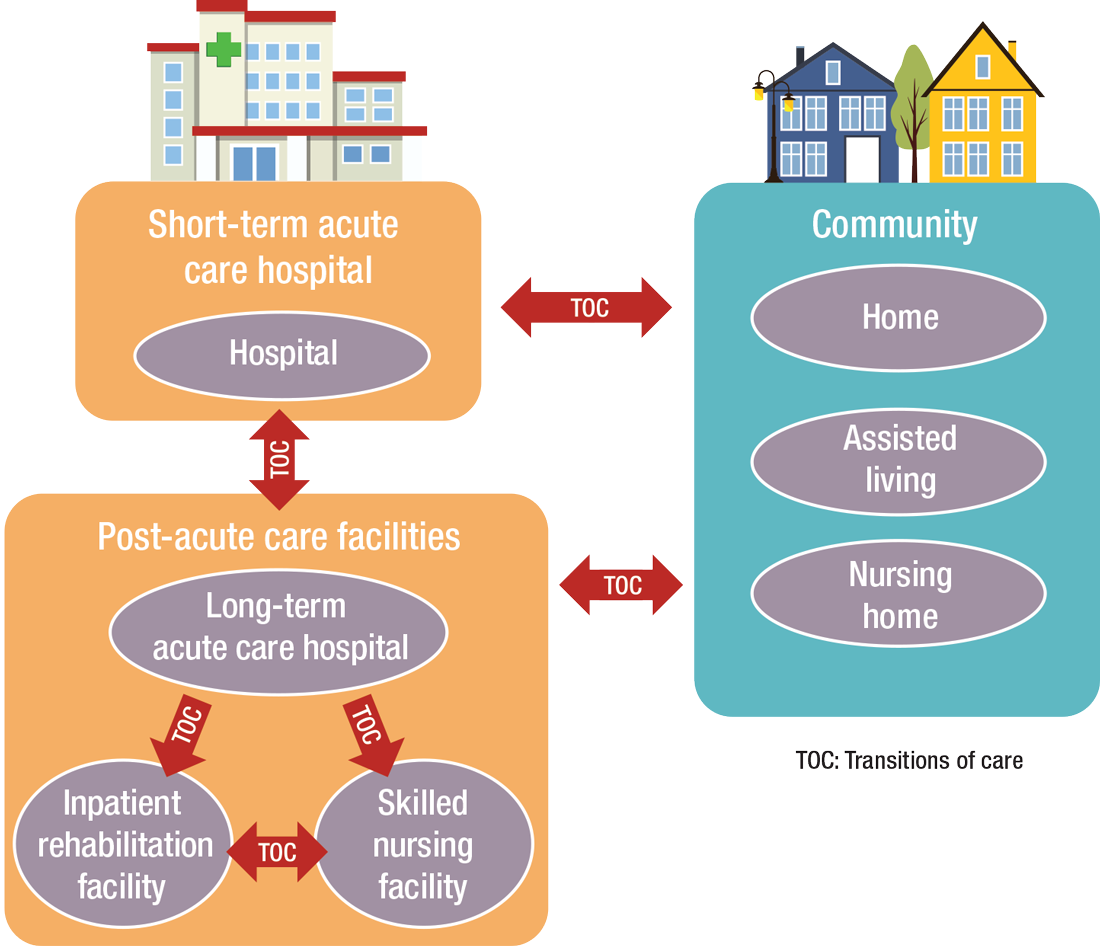
What’s ahead?
Looking to the future, Surafel Tsega, MD, clinical instructor at Mount Sinai Hospital in New York, says he thinks there will be a move toward greater collaboration among inpatient and PAC facilities, particularly in the discharge process, given that hospitals have an added incentive to ensure safe transitions because reimbursement from the Centers for Medicare & Medicaid Services is tied to readmissions and there are penalties for readmission. This involves more comprehensive planning regarding “warm handoffs” (e.g., real-time discussions with PAC providers about a patient’s hospital course and plan of care upon discharge), transferring of information, and so forth.
And while it can still be challenging to identify high-risk patients or determine the intensity and duration of their care, Dr. Mathew says risk-stratification tools and care pathways are continually being refined to maximize value with the limited resources available. In addition, with an increased emphasis on employing a team approach to care, there will be better integration of non-medical services to address the social determinants of health, which play significant roles in overall health and healing.
“Working with community-based organizations for this purpose will be a valuable tool for any of the population health–based initiatives,” he says.
Dr. Muldoon says he believes healthcare reform will increasingly view an inpatient admission as something to be avoided.
“If hospitalization can’t be avoided, then it should be shortened as much as possible,” he says. “This will shift inpatient care into LTACHs, SNFs, and IRFs. Hospitalists would be wise to follow patients into those settings as traditional inpatient census is reduced. This will take a few years, so hospitalists should start now in preparing for that downstream transition of individuals who were previously inpatients.”
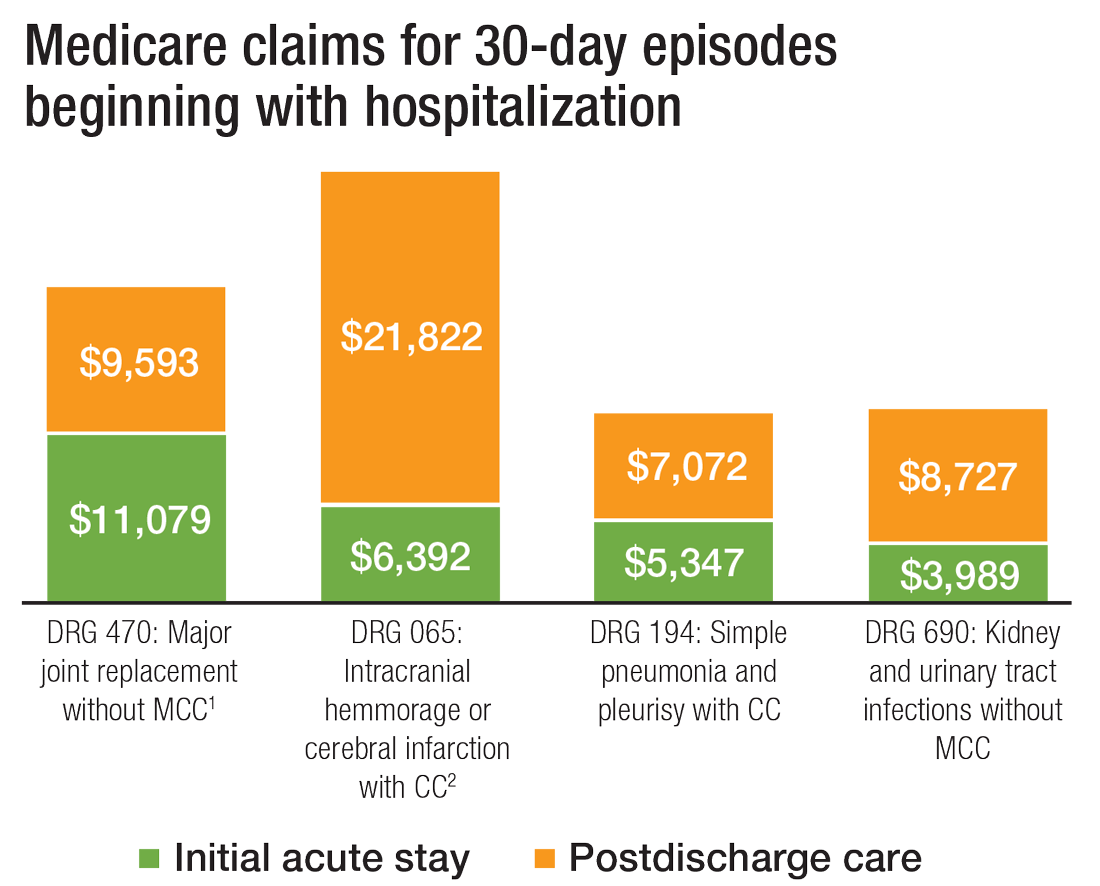
The cost of care, and other PAC facts and figures
The amount of money that Medicare spends on post-acute care (PAC) has been increasing. In 2012, 12.6% of Medicare beneficiaries used some form of PAC, costing $62 billion.2 That amounts to the Centers for Medicare & Medicaid Services spending close to 25% of Medicare beneficiary expenses on PAC, a 133% increase from 2001 to 2012. Among the different types, $30.4 billion was spent on skilled nursing facilities (SNFs), $18.6 billion on home health, and $13.1 billion on long-term acute care (LTAC) and acute-care rehabilitation.2
It’s also been reported that after short-term acute-care hospitalization, about one in five Medicare beneficiaries requires continued specialized treatment in one of the three typical Medicare PAC settings: inpatient rehabilitation facilities (IRFs), LTAC hospitals, and SNFs.3
What’s more, hospital readmission nearly doubles the cost of an episode, so the financial implications for organizations operating in risk-bearing arrangements are significant. In 2013, 2,213 hospitals were charged $280 million in readmission penalties.2
References
1. The role of post-acute care in new care delivery models. American Hospital Association website. Available at: http://www.aha.org/research/reports/tw/15dec-tw-postacute.pdf. Accessed Nov. 7, 2016.
2. Post-acute care integration: Today and in the future. DHG Healthcare website. Available at: http://www2.dhgllp.com/res_pubs/HCG-Post-Acute-Care-Integration.pdf. Accessed Nov. 7, 2016.
3. Overview: Post-acute care transitions toolkit. Society for Hospital Medicine website. Available at: http://www.hospitalmedicine.org/Web/Quality___Innovation/Implementation_Toolkit/pact/Overview_PACT.aspx?hkey=dea3da3c-8620-46db-a00f-89f07f021958. Accessed Nov. 10, 2016.
The definition of “hospitalist,” according to the SHM website, is a clinician “dedicated to delivering comprehensive medical care to hospitalized patients.” For years, the hospital setting was the specialties’ identifier. But as hospitalists’ scope has expanded, and post-acute care (PAC) in the United States has grown, more hospitalists are extending their roles into this space.
PAC today is more than the traditional nursing home, according to Manoj K. Mathew, MD, SFHM, national medical director of Agilon Health in Los Angeles.
Many of those expanded settings Dr. Mathew describes emerged as a result of the Affordable Care Act. Since its enactment in 2010, the ACA has heightened providers’ focus on the “Triple Aim” of improving the patient experience (including quality and satisfaction), improving the health of populations, and reducing the per capita cost of healthcare.1 Vishal Kuchaculla, MD, New England regional post-acute medical director of Knoxville,Tenn.-based TeamHealth, says new service lines also developed as Medicare clamped down on long-term inpatient hospital stays by giving financial impetus to discharge patients as soon as possible.
“Over the last few years, there’s been a major shift from fee-for-service to risk-based payment models,” Dr. Kuchaculla says. “The government’s financial incentives are driving outcomes to improve performance initiatives.”
“Today, LTACHs can be used as substitutes for short-term acute care,” says Sean R. Muldoon, MD, MPH, FCCP, chief medical officer of Kindred Healthcare in Louisville, Ky., and former chair of SHM’s Post-Acute Care Committee. “This means that a patient can be directly admitted from their home to an LTACH. In fact, many hospice and home-care patients are referred from physicians’ offices without a preceding hospitalization.”

Hospitalists can fill a need

More hospitalists are working in PACs for a number of reasons. Dr. Mathew says PAC facilities and services have “typically lacked the clinical structure and processes to obtain the results that patients and payors expect.
“These deficits needed to be quickly remedied as patients discharged from hospitals have increased acuity and higher disease burdens,” he adds. “Hospitalists were the natural choice to fill roles requiring their expertise and experience.”
Dr. Muldoon considers the expanded scope of practice into PACs an additional layer to hospital medicine’s value proposition to the healthcare system.
“As experts in the management of inpatient populations, it’s natural for hospitalists to expand to other facilities with inpatient-like populations,” he says, noting SNFs are the most popular choice, with IRFs and LTACHs also being common places to work. Few hospitalists work in home care or hospice.
PAC settings are designed to help patients who are transitioning from an inpatient setting back to their home or other setting.
“Many patients go home after a SNF stay, while others will move to a nursing home or other longer-term care setting for the first time,” says Tiffany Radcliff, PhD, a health economist in the department of health policy and management at Texas A&M University School of Public Health in College Station. “With this in mind, hospitalists working in PAC have the opportunity to address each patient’s ongoing care needs and prepare them for their next setting. Hospitalists can manage medication or other care regimen changes that resulted from an inpatient stay, reinforce discharge instructions to the patient and their caregivers, and identify any other issues with continuing care that need to be addressed before discharge to the next care setting.”
Transitioning Care
Even if a hospitalist is not employed at a PAC, it’s important that they know something about them.
“As patients are moved downstream earlier, hospitalists are being asked to help make a judgment regarding when and where an inpatient is transitioned,” Dr. Muldoon says. As organizations move toward becoming fully risk capable, it is necessary to develop referral networks of high-quality PAC providers to achieve the best clinical outcomes, reduce readmissions, and lower costs.2“Therefore, hospitalists should have a working knowledge of the different sites of service as well as some opinion on the suitability of available options in their community,” Dr. Muldoon says. “The hospitalist can also help to educate the hospitalized patient on what to expect at a PAC.”
If a patient is inappropriately prepared for the PAC setting, it could lead to incomplete management of their condition, which ultimately could lead to readmission.
“When hospitalists know how care is provided in a PAC setting, they are better able to ensure a smoother transition of care between settings,” says Tochi Iroku-Malize, MD, MPH, MBA, FAAFP, SFHM, chair of family medicine at Northwell Health in Long Island, N.Y. “This will ultimately prevent unnecessary readmissions.”
Further, the quality metrics that hospitals and thereby hospitalists are judged by no longer end at the hospital’s exit.
“The ownership of acute-care outcomes requires extending the accountability to outside of the institution’s four walls,” Dr. Mathew says. “The inpatient team needs to place great importance on the transition of care and the subsequent quality of that care when the patient is discharged.”
Robert W. Harrington Jr., MD, SFHM, chief medical officer of Plano, Texas–based Reliant Post-Acute Care Solutions and former SHM president, says the health system landscapes are pushing HM beyond the hospitals’ walls.
How PAC settings differ from hospitals
Practicing in PAC has some important nuances that hospitalists from short-term acute care need to get accustomed to, Dr. Muldoon says. Primarily, the diagnostic capabilities are much more limited, as is the presence of high-level staffing. Further, patients are less resilient to medication changes and interventions, so changes need to be done gradually.
“Hospitalists who try to practice acute-care medicine in a PAC setting may become frustrated by the length of time it takes to do a work-up, get a consultation, and respond to a patient’s change of condition,” Dr. Muldoon says. “Nonetheless, hospitalists can overcome this once recognizing this mind shift.”
According to Dr. Harrington, another challenge hospitalists may face is the inability of the hospital’s and PAC facility’s IT platforms to exchange electronic information.
“The major vendors on both sides need to figure out an interoperability strategy,” he says. “Currently, it often takes 1-3 days to receive a new patient’s discharge summary. The summary may consist of a stack of paper that takes significant time to sort through and requires the PAC facility to perform duplicate data entry. It’s a very highly inefficient process that opens up the doors to mistakes and errors of omission and commission that can result in bad patient outcomes.”
Arif Nazir, MD, CMD, FACP, AGSF, chief medical officer of Signature HealthCARE and president of SHC Medical Partners, both in Louisville, Ky., cites additional reasons the lack of seamless communication between a hospital and PAC facility is problematic. “I see physicians order laboratory tests and investigations that were already done in the hospital because they didn’t know they were already performed or never received the results,” he says. “Similarly, I see patients continue to take medications prescribed in the hospital long term even though they were only supposed to take them short term. I’ve also seen patients come to a PAC setting from a hospital without any formal understanding of their rehabilitative period and expectations for recovery.”
What’s ahead?
Looking to the future, Surafel Tsega, MD, clinical instructor at Mount Sinai Hospital in New York, says he thinks there will be a move toward greater collaboration among inpatient and PAC facilities, particularly in the discharge process, given that hospitals have an added incentive to ensure safe transitions because reimbursement from the Centers for Medicare & Medicaid Services is tied to readmissions and there are penalties for readmission. This involves more comprehensive planning regarding “warm handoffs” (e.g., real-time discussions with PAC providers about a patient’s hospital course and plan of care upon discharge), transferring of information, and so forth.
And while it can still be challenging to identify high-risk patients or determine the intensity and duration of their care, Dr. Mathew says risk-stratification tools and care pathways are continually being refined to maximize value with the limited resources available. In addition, with an increased emphasis on employing a team approach to care, there will be better integration of non-medical services to address the social determinants of health, which play significant roles in overall health and healing.
“Working with community-based organizations for this purpose will be a valuable tool for any of the population health–based initiatives,” he says.
Dr. Muldoon says he believes healthcare reform will increasingly view an inpatient admission as something to be avoided.
“If hospitalization can’t be avoided, then it should be shortened as much as possible,” he says. “This will shift inpatient care into LTACHs, SNFs, and IRFs. Hospitalists would be wise to follow patients into those settings as traditional inpatient census is reduced. This will take a few years, so hospitalists should start now in preparing for that downstream transition of individuals who were previously inpatients.”
The cost of care, and other PAC facts and figures
The amount of money that Medicare spends on post-acute care (PAC) has been increasing. In 2012, 12.6% of Medicare beneficiaries used some form of PAC, costing $62 billion.2 That amounts to the Centers for Medicare & Medicaid Services spending close to 25% of Medicare beneficiary expenses on PAC, a 133% increase from 2001 to 2012. Among the different types, $30.4 billion was spent on skilled nursing facilities (SNFs), $18.6 billion on home health, and $13.1 billion on long-term acute care (LTAC) and acute-care rehabilitation.2
It’s also been reported that after short-term acute-care hospitalization, about one in five Medicare beneficiaries requires continued specialized treatment in one of the three typical Medicare PAC settings: inpatient rehabilitation facilities (IRFs), LTAC hospitals, and SNFs.3
What’s more, hospital readmission nearly doubles the cost of an episode, so the financial implications for organizations operating in risk-bearing arrangements are significant. In 2013, 2,213 hospitals were charged $280 million in readmission penalties.2
References
1. The role of post-acute care in new care delivery models. American Hospital Association website. Available at: http://www.aha.org/research/reports/tw/15dec-tw-postacute.pdf. Accessed Nov. 7, 2016.
2. Post-acute care integration: Today and in the future. DHG Healthcare website. Available at: http://www2.dhgllp.com/res_pubs/HCG-Post-Acute-Care-Integration.pdf. Accessed Nov. 7, 2016.
3. Overview: Post-acute care transitions toolkit. Society for Hospital Medicine website. Available at: http://www.hospitalmedicine.org/Web/Quality___Innovation/Implementation_Toolkit/pact/Overview_PACT.aspx?hkey=dea3da3c-8620-46db-a00f-89f07f021958. Accessed Nov. 10, 2016.
The definition of “hospitalist,” according to the SHM website, is a clinician “dedicated to delivering comprehensive medical care to hospitalized patients.” For years, the hospital setting was the specialties’ identifier. But as hospitalists’ scope has expanded, and post-acute care (PAC) in the United States has grown, more hospitalists are extending their roles into this space.
PAC today is more than the traditional nursing home, according to Manoj K. Mathew, MD, SFHM, national medical director of Agilon Health in Los Angeles.
Many of those expanded settings Dr. Mathew describes emerged as a result of the Affordable Care Act. Since its enactment in 2010, the ACA has heightened providers’ focus on the “Triple Aim” of improving the patient experience (including quality and satisfaction), improving the health of populations, and reducing the per capita cost of healthcare.1 Vishal Kuchaculla, MD, New England regional post-acute medical director of Knoxville,Tenn.-based TeamHealth, says new service lines also developed as Medicare clamped down on long-term inpatient hospital stays by giving financial impetus to discharge patients as soon as possible.
“Over the last few years, there’s been a major shift from fee-for-service to risk-based payment models,” Dr. Kuchaculla says. “The government’s financial incentives are driving outcomes to improve performance initiatives.”
“Today, LTACHs can be used as substitutes for short-term acute care,” says Sean R. Muldoon, MD, MPH, FCCP, chief medical officer of Kindred Healthcare in Louisville, Ky., and former chair of SHM’s Post-Acute Care Committee. “This means that a patient can be directly admitted from their home to an LTACH. In fact, many hospice and home-care patients are referred from physicians’ offices without a preceding hospitalization.”

Hospitalists can fill a need

More hospitalists are working in PACs for a number of reasons. Dr. Mathew says PAC facilities and services have “typically lacked the clinical structure and processes to obtain the results that patients and payors expect.
“These deficits needed to be quickly remedied as patients discharged from hospitals have increased acuity and higher disease burdens,” he adds. “Hospitalists were the natural choice to fill roles requiring their expertise and experience.”
Dr. Muldoon considers the expanded scope of practice into PACs an additional layer to hospital medicine’s value proposition to the healthcare system.
“As experts in the management of inpatient populations, it’s natural for hospitalists to expand to other facilities with inpatient-like populations,” he says, noting SNFs are the most popular choice, with IRFs and LTACHs also being common places to work. Few hospitalists work in home care or hospice.
PAC settings are designed to help patients who are transitioning from an inpatient setting back to their home or other setting.
“Many patients go home after a SNF stay, while others will move to a nursing home or other longer-term care setting for the first time,” says Tiffany Radcliff, PhD, a health economist in the department of health policy and management at Texas A&M University School of Public Health in College Station. “With this in mind, hospitalists working in PAC have the opportunity to address each patient’s ongoing care needs and prepare them for their next setting. Hospitalists can manage medication or other care regimen changes that resulted from an inpatient stay, reinforce discharge instructions to the patient and their caregivers, and identify any other issues with continuing care that need to be addressed before discharge to the next care setting.”
Transitioning Care
Even if a hospitalist is not employed at a PAC, it’s important that they know something about them.
“As patients are moved downstream earlier, hospitalists are being asked to help make a judgment regarding when and where an inpatient is transitioned,” Dr. Muldoon says. As organizations move toward becoming fully risk capable, it is necessary to develop referral networks of high-quality PAC providers to achieve the best clinical outcomes, reduce readmissions, and lower costs.2“Therefore, hospitalists should have a working knowledge of the different sites of service as well as some opinion on the suitability of available options in their community,” Dr. Muldoon says. “The hospitalist can also help to educate the hospitalized patient on what to expect at a PAC.”
If a patient is inappropriately prepared for the PAC setting, it could lead to incomplete management of their condition, which ultimately could lead to readmission.
“When hospitalists know how care is provided in a PAC setting, they are better able to ensure a smoother transition of care between settings,” says Tochi Iroku-Malize, MD, MPH, MBA, FAAFP, SFHM, chair of family medicine at Northwell Health in Long Island, N.Y. “This will ultimately prevent unnecessary readmissions.”
Further, the quality metrics that hospitals and thereby hospitalists are judged by no longer end at the hospital’s exit.
“The ownership of acute-care outcomes requires extending the accountability to outside of the institution’s four walls,” Dr. Mathew says. “The inpatient team needs to place great importance on the transition of care and the subsequent quality of that care when the patient is discharged.”
Robert W. Harrington Jr., MD, SFHM, chief medical officer of Plano, Texas–based Reliant Post-Acute Care Solutions and former SHM president, says the health system landscapes are pushing HM beyond the hospitals’ walls.
How PAC settings differ from hospitals
Practicing in PAC has some important nuances that hospitalists from short-term acute care need to get accustomed to, Dr. Muldoon says. Primarily, the diagnostic capabilities are much more limited, as is the presence of high-level staffing. Further, patients are less resilient to medication changes and interventions, so changes need to be done gradually.
“Hospitalists who try to practice acute-care medicine in a PAC setting may become frustrated by the length of time it takes to do a work-up, get a consultation, and respond to a patient’s change of condition,” Dr. Muldoon says. “Nonetheless, hospitalists can overcome this once recognizing this mind shift.”
According to Dr. Harrington, another challenge hospitalists may face is the inability of the hospital’s and PAC facility’s IT platforms to exchange electronic information.
“The major vendors on both sides need to figure out an interoperability strategy,” he says. “Currently, it often takes 1-3 days to receive a new patient’s discharge summary. The summary may consist of a stack of paper that takes significant time to sort through and requires the PAC facility to perform duplicate data entry. It’s a very highly inefficient process that opens up the doors to mistakes and errors of omission and commission that can result in bad patient outcomes.”
Arif Nazir, MD, CMD, FACP, AGSF, chief medical officer of Signature HealthCARE and president of SHC Medical Partners, both in Louisville, Ky., cites additional reasons the lack of seamless communication between a hospital and PAC facility is problematic. “I see physicians order laboratory tests and investigations that were already done in the hospital because they didn’t know they were already performed or never received the results,” he says. “Similarly, I see patients continue to take medications prescribed in the hospital long term even though they were only supposed to take them short term. I’ve also seen patients come to a PAC setting from a hospital without any formal understanding of their rehabilitative period and expectations for recovery.”
What’s ahead?
Looking to the future, Surafel Tsega, MD, clinical instructor at Mount Sinai Hospital in New York, says he thinks there will be a move toward greater collaboration among inpatient and PAC facilities, particularly in the discharge process, given that hospitals have an added incentive to ensure safe transitions because reimbursement from the Centers for Medicare & Medicaid Services is tied to readmissions and there are penalties for readmission. This involves more comprehensive planning regarding “warm handoffs” (e.g., real-time discussions with PAC providers about a patient’s hospital course and plan of care upon discharge), transferring of information, and so forth.
And while it can still be challenging to identify high-risk patients or determine the intensity and duration of their care, Dr. Mathew says risk-stratification tools and care pathways are continually being refined to maximize value with the limited resources available. In addition, with an increased emphasis on employing a team approach to care, there will be better integration of non-medical services to address the social determinants of health, which play significant roles in overall health and healing.
“Working with community-based organizations for this purpose will be a valuable tool for any of the population health–based initiatives,” he says.
Dr. Muldoon says he believes healthcare reform will increasingly view an inpatient admission as something to be avoided.
“If hospitalization can’t be avoided, then it should be shortened as much as possible,” he says. “This will shift inpatient care into LTACHs, SNFs, and IRFs. Hospitalists would be wise to follow patients into those settings as traditional inpatient census is reduced. This will take a few years, so hospitalists should start now in preparing for that downstream transition of individuals who were previously inpatients.”
The cost of care, and other PAC facts and figures
The amount of money that Medicare spends on post-acute care (PAC) has been increasing. In 2012, 12.6% of Medicare beneficiaries used some form of PAC, costing $62 billion.2 That amounts to the Centers for Medicare & Medicaid Services spending close to 25% of Medicare beneficiary expenses on PAC, a 133% increase from 2001 to 2012. Among the different types, $30.4 billion was spent on skilled nursing facilities (SNFs), $18.6 billion on home health, and $13.1 billion on long-term acute care (LTAC) and acute-care rehabilitation.2
It’s also been reported that after short-term acute-care hospitalization, about one in five Medicare beneficiaries requires continued specialized treatment in one of the three typical Medicare PAC settings: inpatient rehabilitation facilities (IRFs), LTAC hospitals, and SNFs.3
What’s more, hospital readmission nearly doubles the cost of an episode, so the financial implications for organizations operating in risk-bearing arrangements are significant. In 2013, 2,213 hospitals were charged $280 million in readmission penalties.2
References
1. The role of post-acute care in new care delivery models. American Hospital Association website. Available at: http://www.aha.org/research/reports/tw/15dec-tw-postacute.pdf. Accessed Nov. 7, 2016.
2. Post-acute care integration: Today and in the future. DHG Healthcare website. Available at: http://www2.dhgllp.com/res_pubs/HCG-Post-Acute-Care-Integration.pdf. Accessed Nov. 7, 2016.
3. Overview: Post-acute care transitions toolkit. Society for Hospital Medicine website. Available at: http://www.hospitalmedicine.org/Web/Quality___Innovation/Implementation_Toolkit/pact/Overview_PACT.aspx?hkey=dea3da3c-8620-46db-a00f-89f07f021958. Accessed Nov. 10, 2016.
Transplantation palliative care: The time is ripe
Over 10 years ago, a challenge was made in a surgical publication for increased collaboration between the fields of transplantation and palliative care.1
Since that time not much progress has been made bringing these fields together in a consistent way that would mutually benefit patients and the specialties. However, other progress has been made, particularly in the field of palliative care, which could brighten the prospects and broaden the opportunities to accomplish collaboration between palliative care and transplantation.
Growth of palliative services
During the past decade there has been a robust proliferation of hospital-based palliative care programs in the United States. In all, 67% of U.S. hospitals with 50 or more beds report palliative care teams, up from 63% in 2011 and 53% in 2008.
Only a decade ago, critical care and palliative care were generally considered mutually exclusive. Evidence is trickling in to suggest that this is no longer the case. Although palliative care was not an integral part of critical care at that time, patients, families, and even practitioners began to demand these services. Cook and Rocker have eloquently advocated the rightful place of palliative care in the ICU.2
Studies in recent years have shown that the integration of palliative care into critical care decreases in length of ICU and hospital stay, decreases costs, enhances patient/family satisfaction, and promotes a more rapid consensus about goals of care, without increasing mortality. The ICU experience to date could be considered a reassuring precedent for transplantation palliative care.
Integration of palliative care with transplantation
Early palliative care intervention has been shown to improve symptom burden and depression scores in end-stage liver disease patients awaiting transplant. In addition, early palliative care consultation in conjunction with cancer treatment has been associated with increased survival in non–small-cell lung cancer patients. It has been demonstrated that early integration of palliative care in the surgical ICU alongside disease-directed curative care can be accomplished without change in mortality, while improving end-of-life practice in liver transplant patients.3
What palliative care can do for transplant patients
What does palliative care mean for the person (and family) awaiting transplantation? For the cirrhotic patient with cachexia, ascites, and encephalopathy, it means access to the services of a team trained in the management of these symptoms. Palliative care teams can also provide psychosocial and spiritual support for patients and families who are intimidated by the complex navigation of the health care system and the existential threat that end-stage organ failure presents to them. Skilled palliative care and services can be the difference between failing and extended life with a higher quality of life for these very sick patients
Resuscitation of a patient, whether through restoration of organ function or interdicting the progression of disease, begins with resuscitation of hope. Nothing achieves this more quickly than amelioration of burdensome symptoms for the patient and family.
The barriers for transplant surgeons and teams referring and incorporating palliative care services in their practices are multiple and profound. The unique dilemma facing the transplant team is to balance the treatment of the failing organ, the treatment of the patient (and family and friends), and the best use of the graft, a precious gift of society.
Palliative surgery has been defined as any invasive procedure in which the main intention is to mitigate physical symptoms in patients with noncurable disease without causing premature death. The very success of transplantation over the past 3 decades has obscured our memory of transplantation as a type of palliative surgery. It is a well-known axiom of reconstructive surgery that the reconstructed site should be compared to what was there, not to “normal.” Even in the current era of improved immunosuppression and posttransplant support services, one could hardly describe even a successful transplant patient’s experience as “normal.” These patients’ lives may be extended and/or enhanced but they need palliative care before, during, and after transplantation. The growing availability of trained palliative care clinicians and teams, the increased familiarity of palliative and end-of-life care to surgical residents and fellows, and quality metrics measuring palliative care outcomes will provide reassurance and guidance to address reservations about the convergence of the two seemingly opposite realities.
A modest proposal
We propose that palliative care be presented to the entire spectrum of transplantation care: on the ward, in the ICU, and after transplantation. More specific “triggers” for palliative care for referral of transplant patients should be identified. Wentlandt et al.4 have described a promising model for an ambulatory clinic, which provides early, integrated palliative care to patients awaiting and receiving organ transplantation. In addition, we propose an application for grant funding for a conference and eventual formation of a work group of transplant surgeons and team members, palliative care clinicians, and patient/families who have experienced one of the aspects of the transplant spectrum. We await the subspecialty certification in hospice and palliative medicine of a transplant surgeon. Outside of transplantation, every other surgical specialty in the United States has diplomates certified in hospice and palliative medicine. We await the benefits that will accrue from research about the merging of these fields.
1. Molmenti EP, Dunn GP: Transplantation and palliative care: The convergence of two seemingly opposite realities. Surg Clin North Am. 2005;85:373-82.
2. Cook D, Rocker G. Dying with dignity in the intensive care unit. N Engl J Med. 2014;370:2506-14.
3. Lamba S, Murphy P, McVicker S, Smith JH, and Mosenthal AC. Changing end-of-life care practice for liver transplant patients: structured palliative care intervention in the surgical intensive care unit. J Pain Symptom Manage. 2012; 44(4):508-19.
4. Wentlandt, K., Dall’Osto, A., Freeman, N., Le, L. W., Kaya, E., Ross, H., Singer, L. G., Abbey, S., Clarke, H. and Zimmermann, C. (2016), The Transplant Palliative Care Clinic: An early palliative care model for patients in a transplant program. Clin Transplant. 2016 Nov 4; doi: 10.1111/ctr.12838.
Dr. Azoulay is a transplantation specialist of Assistance Publique – Hôpitaux de Paris, and the University of Paris. Dr. Dunn is medical director of the Palliative Care Consultation Service at the University of Pittsburgh Medical Center Hamot, and vice-chair of the ACS Committee on Surgical Palliative Care.
Over 10 years ago, a challenge was made in a surgical publication for increased collaboration between the fields of transplantation and palliative care.1
Since that time not much progress has been made bringing these fields together in a consistent way that would mutually benefit patients and the specialties. However, other progress has been made, particularly in the field of palliative care, which could brighten the prospects and broaden the opportunities to accomplish collaboration between palliative care and transplantation.
Growth of palliative services
During the past decade there has been a robust proliferation of hospital-based palliative care programs in the United States. In all, 67% of U.S. hospitals with 50 or more beds report palliative care teams, up from 63% in 2011 and 53% in 2008.
Only a decade ago, critical care and palliative care were generally considered mutually exclusive. Evidence is trickling in to suggest that this is no longer the case. Although palliative care was not an integral part of critical care at that time, patients, families, and even practitioners began to demand these services. Cook and Rocker have eloquently advocated the rightful place of palliative care in the ICU.2
Studies in recent years have shown that the integration of palliative care into critical care decreases in length of ICU and hospital stay, decreases costs, enhances patient/family satisfaction, and promotes a more rapid consensus about goals of care, without increasing mortality. The ICU experience to date could be considered a reassuring precedent for transplantation palliative care.
Integration of palliative care with transplantation
Early palliative care intervention has been shown to improve symptom burden and depression scores in end-stage liver disease patients awaiting transplant. In addition, early palliative care consultation in conjunction with cancer treatment has been associated with increased survival in non–small-cell lung cancer patients. It has been demonstrated that early integration of palliative care in the surgical ICU alongside disease-directed curative care can be accomplished without change in mortality, while improving end-of-life practice in liver transplant patients.3
What palliative care can do for transplant patients
What does palliative care mean for the person (and family) awaiting transplantation? For the cirrhotic patient with cachexia, ascites, and encephalopathy, it means access to the services of a team trained in the management of these symptoms. Palliative care teams can also provide psychosocial and spiritual support for patients and families who are intimidated by the complex navigation of the health care system and the existential threat that end-stage organ failure presents to them. Skilled palliative care and services can be the difference between failing and extended life with a higher quality of life for these very sick patients
Resuscitation of a patient, whether through restoration of organ function or interdicting the progression of disease, begins with resuscitation of hope. Nothing achieves this more quickly than amelioration of burdensome symptoms for the patient and family.
The barriers for transplant surgeons and teams referring and incorporating palliative care services in their practices are multiple and profound. The unique dilemma facing the transplant team is to balance the treatment of the failing organ, the treatment of the patient (and family and friends), and the best use of the graft, a precious gift of society.
Palliative surgery has been defined as any invasive procedure in which the main intention is to mitigate physical symptoms in patients with noncurable disease without causing premature death. The very success of transplantation over the past 3 decades has obscured our memory of transplantation as a type of palliative surgery. It is a well-known axiom of reconstructive surgery that the reconstructed site should be compared to what was there, not to “normal.” Even in the current era of improved immunosuppression and posttransplant support services, one could hardly describe even a successful transplant patient’s experience as “normal.” These patients’ lives may be extended and/or enhanced but they need palliative care before, during, and after transplantation. The growing availability of trained palliative care clinicians and teams, the increased familiarity of palliative and end-of-life care to surgical residents and fellows, and quality metrics measuring palliative care outcomes will provide reassurance and guidance to address reservations about the convergence of the two seemingly opposite realities.
A modest proposal
We propose that palliative care be presented to the entire spectrum of transplantation care: on the ward, in the ICU, and after transplantation. More specific “triggers” for palliative care for referral of transplant patients should be identified. Wentlandt et al.4 have described a promising model for an ambulatory clinic, which provides early, integrated palliative care to patients awaiting and receiving organ transplantation. In addition, we propose an application for grant funding for a conference and eventual formation of a work group of transplant surgeons and team members, palliative care clinicians, and patient/families who have experienced one of the aspects of the transplant spectrum. We await the subspecialty certification in hospice and palliative medicine of a transplant surgeon. Outside of transplantation, every other surgical specialty in the United States has diplomates certified in hospice and palliative medicine. We await the benefits that will accrue from research about the merging of these fields.
1. Molmenti EP, Dunn GP: Transplantation and palliative care: The convergence of two seemingly opposite realities. Surg Clin North Am. 2005;85:373-82.
2. Cook D, Rocker G. Dying with dignity in the intensive care unit. N Engl J Med. 2014;370:2506-14.
3. Lamba S, Murphy P, McVicker S, Smith JH, and Mosenthal AC. Changing end-of-life care practice for liver transplant patients: structured palliative care intervention in the surgical intensive care unit. J Pain Symptom Manage. 2012; 44(4):508-19.
4. Wentlandt, K., Dall’Osto, A., Freeman, N., Le, L. W., Kaya, E., Ross, H., Singer, L. G., Abbey, S., Clarke, H. and Zimmermann, C. (2016), The Transplant Palliative Care Clinic: An early palliative care model for patients in a transplant program. Clin Transplant. 2016 Nov 4; doi: 10.1111/ctr.12838.
Dr. Azoulay is a transplantation specialist of Assistance Publique – Hôpitaux de Paris, and the University of Paris. Dr. Dunn is medical director of the Palliative Care Consultation Service at the University of Pittsburgh Medical Center Hamot, and vice-chair of the ACS Committee on Surgical Palliative Care.
Over 10 years ago, a challenge was made in a surgical publication for increased collaboration between the fields of transplantation and palliative care.1
Since that time not much progress has been made bringing these fields together in a consistent way that would mutually benefit patients and the specialties. However, other progress has been made, particularly in the field of palliative care, which could brighten the prospects and broaden the opportunities to accomplish collaboration between palliative care and transplantation.
Growth of palliative services
During the past decade there has been a robust proliferation of hospital-based palliative care programs in the United States. In all, 67% of U.S. hospitals with 50 or more beds report palliative care teams, up from 63% in 2011 and 53% in 2008.
Only a decade ago, critical care and palliative care were generally considered mutually exclusive. Evidence is trickling in to suggest that this is no longer the case. Although palliative care was not an integral part of critical care at that time, patients, families, and even practitioners began to demand these services. Cook and Rocker have eloquently advocated the rightful place of palliative care in the ICU.2
Studies in recent years have shown that the integration of palliative care into critical care decreases in length of ICU and hospital stay, decreases costs, enhances patient/family satisfaction, and promotes a more rapid consensus about goals of care, without increasing mortality. The ICU experience to date could be considered a reassuring precedent for transplantation palliative care.
Integration of palliative care with transplantation
Early palliative care intervention has been shown to improve symptom burden and depression scores in end-stage liver disease patients awaiting transplant. In addition, early palliative care consultation in conjunction with cancer treatment has been associated with increased survival in non–small-cell lung cancer patients. It has been demonstrated that early integration of palliative care in the surgical ICU alongside disease-directed curative care can be accomplished without change in mortality, while improving end-of-life practice in liver transplant patients.3
What palliative care can do for transplant patients
What does palliative care mean for the person (and family) awaiting transplantation? For the cirrhotic patient with cachexia, ascites, and encephalopathy, it means access to the services of a team trained in the management of these symptoms. Palliative care teams can also provide psychosocial and spiritual support for patients and families who are intimidated by the complex navigation of the health care system and the existential threat that end-stage organ failure presents to them. Skilled palliative care and services can be the difference between failing and extended life with a higher quality of life for these very sick patients
Resuscitation of a patient, whether through restoration of organ function or interdicting the progression of disease, begins with resuscitation of hope. Nothing achieves this more quickly than amelioration of burdensome symptoms for the patient and family.
The barriers for transplant surgeons and teams referring and incorporating palliative care services in their practices are multiple and profound. The unique dilemma facing the transplant team is to balance the treatment of the failing organ, the treatment of the patient (and family and friends), and the best use of the graft, a precious gift of society.
Palliative surgery has been defined as any invasive procedure in which the main intention is to mitigate physical symptoms in patients with noncurable disease without causing premature death. The very success of transplantation over the past 3 decades has obscured our memory of transplantation as a type of palliative surgery. It is a well-known axiom of reconstructive surgery that the reconstructed site should be compared to what was there, not to “normal.” Even in the current era of improved immunosuppression and posttransplant support services, one could hardly describe even a successful transplant patient’s experience as “normal.” These patients’ lives may be extended and/or enhanced but they need palliative care before, during, and after transplantation. The growing availability of trained palliative care clinicians and teams, the increased familiarity of palliative and end-of-life care to surgical residents and fellows, and quality metrics measuring palliative care outcomes will provide reassurance and guidance to address reservations about the convergence of the two seemingly opposite realities.
A modest proposal
We propose that palliative care be presented to the entire spectrum of transplantation care: on the ward, in the ICU, and after transplantation. More specific “triggers” for palliative care for referral of transplant patients should be identified. Wentlandt et al.4 have described a promising model for an ambulatory clinic, which provides early, integrated palliative care to patients awaiting and receiving organ transplantation. In addition, we propose an application for grant funding for a conference and eventual formation of a work group of transplant surgeons and team members, palliative care clinicians, and patient/families who have experienced one of the aspects of the transplant spectrum. We await the subspecialty certification in hospice and palliative medicine of a transplant surgeon. Outside of transplantation, every other surgical specialty in the United States has diplomates certified in hospice and palliative medicine. We await the benefits that will accrue from research about the merging of these fields.
1. Molmenti EP, Dunn GP: Transplantation and palliative care: The convergence of two seemingly opposite realities. Surg Clin North Am. 2005;85:373-82.
2. Cook D, Rocker G. Dying with dignity in the intensive care unit. N Engl J Med. 2014;370:2506-14.
3. Lamba S, Murphy P, McVicker S, Smith JH, and Mosenthal AC. Changing end-of-life care practice for liver transplant patients: structured palliative care intervention in the surgical intensive care unit. J Pain Symptom Manage. 2012; 44(4):508-19.
4. Wentlandt, K., Dall’Osto, A., Freeman, N., Le, L. W., Kaya, E., Ross, H., Singer, L. G., Abbey, S., Clarke, H. and Zimmermann, C. (2016), The Transplant Palliative Care Clinic: An early palliative care model for patients in a transplant program. Clin Transplant. 2016 Nov 4; doi: 10.1111/ctr.12838.
Dr. Azoulay is a transplantation specialist of Assistance Publique – Hôpitaux de Paris, and the University of Paris. Dr. Dunn is medical director of the Palliative Care Consultation Service at the University of Pittsburgh Medical Center Hamot, and vice-chair of the ACS Committee on Surgical Palliative Care.
Best Practices: Protecting Dry Vulnerable Skin with CeraVe® Healing Ointment
A supplement to Dermatology News. This advertising supplement is sponsored by Valeant Pharmaceuticals.
- Reinforcing the Skin Barrier
- NEA Seal of Acceptance
- A Preventative Approach to Dry, Cracked Skin
- CeraVe Ointment in the Clinical Setting
Faculty/Faculty Disclosure
Sheila Fallon Friedlander, MD
Professor of Clinical Dermatology & Pediatrics
Director, Pediatric Dermatology Fellowship Training Program
University of California at San Diego School of Medicine
Rady Children’s Hospital,
San Diego, California
Dr. Friedlander was compensated for her participation in the development of this article.
CeraVe is a registered trademark of Valeant Pharmaceuticals International, Inc. or its affiliates.
A supplement to Dermatology News. This advertising supplement is sponsored by Valeant Pharmaceuticals.
- Reinforcing the Skin Barrier
- NEA Seal of Acceptance
- A Preventative Approach to Dry, Cracked Skin
- CeraVe Ointment in the Clinical Setting
Faculty/Faculty Disclosure
Sheila Fallon Friedlander, MD
Professor of Clinical Dermatology & Pediatrics
Director, Pediatric Dermatology Fellowship Training Program
University of California at San Diego School of Medicine
Rady Children’s Hospital,
San Diego, California
Dr. Friedlander was compensated for her participation in the development of this article.
CeraVe is a registered trademark of Valeant Pharmaceuticals International, Inc. or its affiliates.
A supplement to Dermatology News. This advertising supplement is sponsored by Valeant Pharmaceuticals.
- Reinforcing the Skin Barrier
- NEA Seal of Acceptance
- A Preventative Approach to Dry, Cracked Skin
- CeraVe Ointment in the Clinical Setting
Faculty/Faculty Disclosure
Sheila Fallon Friedlander, MD
Professor of Clinical Dermatology & Pediatrics
Director, Pediatric Dermatology Fellowship Training Program
University of California at San Diego School of Medicine
Rady Children’s Hospital,
San Diego, California
Dr. Friedlander was compensated for her participation in the development of this article.
CeraVe is a registered trademark of Valeant Pharmaceuticals International, Inc. or its affiliates.
Negotiating the VUCA World Through Tiered Huddles
Negotiating the VUCA World Through Tiered Huddles
To see what is in front of one’s nose needs a constant struggle.
George Orwell (1946)1
In 2019, the Veterans Health Administration (VHA) initiated a process to become a high reliability organization (HRO).2 The COVID-19 pandemic has been described in medical literature as a volatile, uncertain, complex, and ambiguous (VUCA) event, underscoring the necessity of resilient communication strategies.3 Challenges posed by 2024 Hurricanes Helene and Milton further highlighted the need for resilient communication strategies within HRO implementation.
Central to the HRO journey within the VHA has been the development of tiered huddles, an evolution of the safety huddle concept.4 Emerging organically as an effective communication mechanism across multiple facilities between 2019 and 2020, tiered huddles were, in part, spurred by the onset of COVID-19. Tiered huddles represent a proactive approach to identifying and addressing organizational threats in their early stages, thereby preventing their escalation to a VUCA-laden crisis.5 When conditions evolve beyond the horizon of tractability, where challenges are easily identified and resolved, tiered huddles serve as a resilient mechanism to restore dynamic equilibrium within the organization.6,7
This article describes how tiered huddles were integrated within Veterans Integrated Service Network (VISN) 4 and explores why these huddles are essential, particularly in the context of VUCA events. What began as a local-level tactic has now gained widespread acceptance and continues to evolve across the VHA with full support from the US Department of Veterans Affairs (VA) Under Secretary for Health.8
The VHA is divided into 18 VISNs. Nine VA Medical Centers (VAMCs) and 46 outpatient clinics across Pennsylvania, Delaware, and parts of Ohio, New York, and New Jersey make up VISN 4. Disseminating vital information across VISN 4, in addition to the 17 other VISNs—including 170 VAMCs and 1193 clinics—presents a formidable challenge. As the largest integrated system in the US, the VHA is realigning its workforce to address organizational inefficiencies. An enterprise of this scale, shaped by recurrent organizational change, faces ongoing challenges in sustaining clear communication across all levels. These transitions create uncertainty for staff as roles and resources shift, underscoring the need for dependable vertical and horizontal information flow. Tiered huddles offer a steady means to support coordinated communication and strengthen the system’s ability to adapt.9
ERIE VA MEDICAL CENTER HRO JOURNEY
In 2019, John Gennaro, the Erie VAMC executive director, attended a presentation that showcased the Cleveland Clinic’s tiered huddle process, with an opportunity to observe its 5-tiered system.10 Erie VAMC already had a 3-tiered huddle system, but the Cleveland Clinic’s more robust model inspired Gennaro to propose a VISN 4 pilot program. Tiered huddles were perceived as innovative, yet not fully embraced within the VHA; nonetheless, VISN 4, much like several other VISNs, moved forward and established a VISN-level (Tier 4) huddle.8 It is important to note that there was a notional fifth-tier capability as VISN and program office leaders already participated in daily VHA-wide meetings under the auspices of the Hospital Operations Center (HOC).
Expanding the Tiered Huddle Process
The Erie VAMC huddle process begins with the unit level Managers and Frontline Staff (Tier 1), then moves to Service Chiefs and Managers (Tier 2). Tier 3 involves facility executive leadership team and service chiefs, clinical directors and top VAMC administrators (these configurations may vary depending on context). The sequencing and flow of information is bidirectional across levels, reflecting the importance of closed-loop communication to ensure staff at all levels understand that issues raised are followed up on and/or closed out (Figure 1).2
Tier 4 composition may vary among VISNs depending on size and unique mission requirements.8,11 The VISN 4 Tier 4 huddle includes the VISN director, 9 VAMC directors, and key network administrators and clinical experts. The Tier 5 huddle includes 18 VISN 4 directors with the VHA HOC (Figure 2). The tiered huddle process emphasizes team-based culture and psychological safety.12-15 Staff at all levels are encouraged to identify and transparently resolve issues, fostering a proactive and problem-solving environment across the organization. A more nuanced and detailed process across tier levels is depicted in the Table.
The vetting and distillation of information can present challenges as vital information ascends and spreads across organization levels. Visual management systems (VMS), whether a whiteboard or a digital platform, are key to facilitate decision-making related to what needs to be prioritized and disseminated at each tier level.2,8 At Tier 5, the HOC uses a digital VMS to provide a structured, user-friendly format for categorizing issues and topics and enhances clarity and accessibility (Figure 3). The Tier 5 VMS also facilitates tracking and reciprocal information exchange, helping to close the loop on emerging issues by monitoring their progression and resolution up and across tiers.2,8 The Tier 5 huddle process and technology supporting continue to evolve offering increasing sophistication in organizational situational awareness and responsiveness.
VUCA: A Lens for Health Care Challenges
First introduced by social scientists at the US Army War College in 1995, VUCA describes complex and unpredictable conditions often encountered in military operations.16,17 Prompted by the COVID-19 pandemic, the acronym VUCA gained recognition in health care, as leaders acknowledged the challenge of navigating rapidly changing environments. van Stralen, Byrum and Inozu, recognized authorities in high reliability, cited VUCA as the rationale for implementing HRO principles and practices. They argued that “HRO solves the problem of operations and performance in a volatile, uncertain, complex, ambiguous environment.” 18 To fully appreciate the VUCA environment and its relevance to health care, it is essential to unpack the 4 components of the acronym: volatile, uncertain, complex, and ambiguous.
Volatile refers to the speed and unpredictability of change. Health care systems are interactively complex and tightly coupled, meaning that changes in 1 part of the system can rapidly impact others.6,18,19 This high degree of interdependence amplifies volatility, especially when unexpected events occur. The rapid spread of COVID- 19 and the evolving nature of its transmission challenged health care systems’ ability to respond swiftly and effectively. Volatility also may emerge in acute medical situations, such as the rapid deterioration of a patient’s condition.
Uncertain captures the lack of predictability inherent in complex systems. In health care, uncertainty arises when there is insufficient information or when an excess of data make it difficult to discern meaningful patterns. COVID-19 and recent natural disasters have introduced profound uncertainty, as the disease’s behavior, transmission, and impact were initially unknown. Health care practitioners struggled to make decisions in real time, lacking clear guidance or precedent.3,20 While health care planning and established protocols are grounded in predictability, the COVID-19 pandemic revealed that as complexity increases, predictability diminishes. Moreover, complexity can complicate protocol selection, as situations may arise in which multiple protocols conflict or compete. The cognitive challenge of operating in this environment is analogous to what military strategists call the fog of war, where situational awareness is low and decision-makers must navigate without clarity.21 Tiered huddles, a core practice in HROs, mitigate uncertainty by fostering real-time communication and shared situational awareness among teams.20
Complex refers to the intricate interplay of multiple, interconnected factors within a system.22 In health care, this complexity is heightened by the sociotechnical nature of the field—where human, technology, and organizational elements all converge.19 Systems designed to prevent failures, such as redundancies and safety protocols, can themselves contribute to increased complexity. HRO practices such as tiered huddles are implemented to mitigate the risk of catastrophic failure by fostering collaborative sensemaking, enhanced situational awareness, and rapid problem-solving.5,20,23
Ambiguous refers to situations in which multiple interpretations, causes, or outcomes are possible. It explains how, despite following protocols, failure can still occur, or how individuals may reach different conclusions from the same data. Ambiguity does not offer binary solutions; instead, it presents a murky, multifaceted reality that requires thoughtful interpretation and adaptive responses. In these moments, leaders must act decisively, even in the absence of complete information, making trade-offs that balance immediate needs with long-term consequences.
MANAGING VUCA ENVIRONMENTS WITH TIERED HUDDLES
The tiered huddle process provides several key benefits that enable real-time issue resolution. These include the rapid dissemination of vital information, enhanced agility and resilience, and improved sensemaking within a VUCA environment. Additionally, tiered huddles prevent organizational drift by fostering heightened situational awareness. The tiered huddle process also supports leadership development, as unit-level leaders gain valuable insights into strategic decision-making through active participation. Each component is outlined in the following section.
Spread: The Challenge of Communicating
“The hallmark of a great organization is how quickly bad news travels upward,” argued Jay Forrester, the father of system dynamics.24 Unfortunately, steep power gradients and siloed organizational structures inhibit the flow of unfavorable information from frontline staff to senior leadership. This suppression is not necessarily intentional but is often a byproduct of organizational culture. Tiered huddles address the weakness of top-down communication models by promoting a reciprocal, bidirectional information exchange, with an emphasis on closed-loop communication. Open communication can foster a culture of trust and transparency, allowing leaders to make more informed decisions and respond quickly to emerging risks.
Enhancing Agility and Resilience
Tiered huddles contribute to a mindful infrastructure, an important aspect of maintaining organizational awareness and agility.21,25 A mindful infrastructure enables an organization to detect early warning signs of potential disruptions and respond to them before they escalate. In this sense, tiered huddles serve as a signal-sensing mechanism, providing the agility needed to adapt to changing circumstances and prevent patient harm. Tiered huddles facilitate self-organization, a concept from chaos theory known as autopoiesis. 26 This self-organizing capability allows teams to develop novel solutions in response to unforeseen challenges, exemplifying the adaptability and resilience needed in a VUCA environment. The diverse backgrounds of tiered huddle participants—both cognitively and culturally—enable a broader range of perspectives, which is critical for making sound decisions in complex and uncertain situations. “HROs cultivate diversity not just because it helps them notice more in complex environments, but also because it helps them adapt to the complexities they do spot,” argues Weick et al.27 This diversity of thought and experience enhances the organization’s ability to respond to complexity, much like firefighters continually adapt to the VUCA conditions they face.
Sensemaking and Sensitivity to Operations
Leaders at all levels must be attuned to what is happening both within and outside their organization. This continual sensing of the environment—looking for weak signals, threats, and opportunities—is important for HROs. This signal detection capability allows organizations to address problems in their nascent emerging state within a tractable horizon to successfully manage fluctuations. The horizon of tractability reflects a zone where weak signals and evolving issues can be identified, addressed, and resolved early before they evolve and cascade outside of safe operations. 7 Tiered huddles facilitate this process by creating a platform for team members to engage in respectful, collaborative dialogue. The diversity inherent in tiered huddles also supports sensemaking, a process of interpreting and understanding complex situations.27 In a VUCA environment, this multiperspective approach helps filter out noise and identify the most important signals. Tiered huddles can help overcome the phenomenon of dysfunctional momentum associated with cognitive lockup, fixation error, and tunnel vision, in which individuals or teams fixate on a particular solution, thus missing important alternative views.21,28 By fostering a common operating picture of the fluctuating environment, tiered huddles can enable more accurate decision-making and improve organizational resilience.
Avoiding Organizational Drift
One of the most significant contributions of tiered huddles is the ability to detect early signs of organizational drift, or subtle deviations from standard practices that can accumulate over time and lead to serious failures. By continuously monitoring for precursor conditions and weak signals, tiered huddles allow organizations to intervene early and prevent drift from becoming catastrophic.29,30 This vigilance is essential in health care, where complacency can lead to patient harm. Tiered huddles foster a culture of mindfulness and accountability, ensuring that staff stay engaged and alert to potential risks. This proactive approach is a safeguard against human error and the gradual erosion of safety standards.
Leadership Development
Tiered huddles serve as a powerful tool for leadership development. Effective leaders must be able to anticipate potential risks and foresee system failures. Involving future leaders in tiered huddles can facilitate the transfer of these critical skills. When emerging leaders at lower tiers participate in ascending-tier huddles, they gain a unique opportunity to engage in a structured, collaborative setting. This environment provides a safe space to develop and practice strategic skills, enhancing their ability to think proactively and manage complexity. By integrating future leaders into tiered huddles, organizations offer essential, hands-on experience in real-time decision making. This experiential learning is invaluable for preparing leaders to navigate the demands of a VUCA environment.
CONCLUSIONS
Since implementing the tiered huddle process, the Erie VAMC and VISN 4 have emerged as early adopters of VUCA, thus contributing to the expansion of this innovative communication approach across the VHA. Tiered huddles strengthen organizational resilience and agility, facilitate critical information flow to manage risk, and support the cultivation of future leaders. The Erie VAMC director and the VISN 4 network director regard the expansion of tiered huddles, including Tiers 4 and 5, as an adaptable model for the VHA. While tiered huddles have not yet been mandated across the VHA, a pilot at the Tier 5 HOC level was initiated on May 20, 2024. In a complex world in which VUCA events will continue to be inevitable, implementation of robust tiered huddles within complex health care systems provides the opportunity for improved responses and delivery of care.
- Orwell S, Angus I, eds. In Front of Your Nose, 1945-1950. Godine; 2000. Orwell G. The Collected Essays, Journalism, and Letters of George Orwell; vol 4.
- Murray JS, Baghdadi A, Dannenberg W, Crews P, Walsh ND. The role of high reliability organization foundational practices in building a culture of safety. Fed Pract. 2024;41:214-221. doi:10.12788/fp.0486
- Goldenhar LM, Brady PW, Sutcliffe KM, Muething SE. Huddling for high reliability and situation awareness. BMJ Qual Saf. 2013;22:899-906. doi:10.1136/bmjqs-2012-001467
- Pandit M. Critical factors for successful management of VUCA times. BMJ Lead. 2021;5:121-123. doi:10.1136/leader-2020-000305
- Mihaljevic T. Tiered daily huddles: the power of teamwork in managing large healthcare organisations. BMJ Qual Saf. 2020;29:1050-1052. doi:10.1136/bmjqs-2019-010575
- van Stralen D, Mercer TA. High-reliability organizing (HRO) in the COVID-19 liminal zone: characteristics of workers and local leaders. Neonatology Today. 2021;16:90-101. http://www.neonatologytoday.net /newsletters/nt-apr21.pdf
- Nemeth C, Wears R, Woods D, Hollnagel E, Cook R. Minding the gaps: creating resilience in health care. In: Henriksen K, Battles JB, Keyes MA, Grady ML, eds. Advances in Patient Safety: New Directions and Alternative Approaches. Vol 3: Performance and Tools. Agency for Healthcare Research and Quality; 2008.
- Merchant NB, O’Neal J, Montoya A, Cox GR, Murray JS. Creating a process for the implementation of tiered huddles in a Veterans Affairs medical center. Mil Med. 2023;188:901-906. doi:10.1093/milmed/usac073
- Starbuck WH, Farjoun M, eds. Organization at the Limit: Lessons From the Columbia Disaster. 1st ed. Wiley-Blackwell; 2005.
- Mihaljevic T. Tiered daily huddles: the power of teamwork in managing large healthcare organisations. BMJ Qual Saf. 2020;29:1050-1052. doi:10.1136/bmjqs-2019-010575
- Donnelly LF, Cherian SS, Chua KB, et al. The Daily Readiness Huddle: a process to rapidly identify issues and foster improvement through problem-solving accountability. Pediatr Radiol. 2017;47:22-30. doi:10.1007/s00247-016-3712-x
- Clark TR. The 4 Stages of Psychological Safety: Defining the Path to Inclusion and Innovation. Berrett-Koehler Publishers, Inc.; 2020.
- Edmondson AC. The Fearless Organization: Creating Psychological Safety in the Workplace for Learning, Innovation, and Growth. John Wiley & Sons; 2018.
- Edmondson AC. The Right Kind of Wrong: The Science of Failing Well. Simon Element/Simon Acumen; 2023.
- Murray JS, Kelly S, Hanover C. Promoting psychological safety in healthcare organizations. Mil Med. 2022;187:808 -810. doi:10.1093/milmed/usac041
- Barber HF. Developing strategic leadership: the US Army War College experience. J Manag Dev. 1992;11:4-12. doi:10.1108/02621719210018208
- US Army Heritage & Education Center. Who first originated the term VUCA (volatility, uncertainty, complexity and ambiguity)? Accessed November 5, 2025. https://usawc .libanswers.com/ahec/faq/84869
- van Stralen D, Byrum SL, Inozu B. High Reliability for a Highly Unreliable World: Preparing for Code Blue Through Daily Operations in Healthcare. CreateSpace Independent Publishing Platform; 2018.
- Perrow C. Normal Accidents: Living With High-Risk Technologies. Princeton University Press; 2000.
- Sculli G, Essen K. Soaring to Success: The Path to Developing High-Reliability Clinical Teams. HCPro; 2021. Accessed November 5, 2025. https://hcmarketplace.com /media/wysiwyg/CRM3_browse.pdf
- Barton MA, Sutcliffe KM, Vogus TJ, DeWitt T. Performing under uncertainty: contextualized engagement in wildland firefighting. J Contingencies Crisis Manag. 2015;23:74-83. doi:10.1111/1468-5973.12076
- Sutcliffe KM. Mindful organizing. In: Ramanujam R, Roberts KH, eds. Organizing for Reliability: A Guide for Research and Practice. Stanford University Press; 2018:61-89.
- Merchant NB, O’Neal J, Dealino-Perez C, Xiang J, Montoya A Jr, Murray JS. A high-reliability organization mindset. Am J Med Qual. 2022;37:504-510. doi:10.1097/jmq.0000000000000086
- Senge PM. The Fifth Discipline Fieldbook: Strategies and Tools for Building a Learning Organization. Crown Currency; 1994.
- Ramanujam R, Roberts KH, eds. Organizing for Reliability: A Guide for Research and Practice. Stanford University Press; 2018.
- Coveney PV. Self-organization and complexity: a new age for theory, computation and experiment. Philos Trans A Math Phys Eng Sci. 2003;361:1057-1079. doi:10.1098/rsta.2003.1191
- Weick KE, Sutcliffe KM. Managing the Unexpected: Sustained Performance in a Complex World. 3rd ed. Wiley; 2015.
- Barton M, Sutcliffe K. Overcoming dysfunctional momentum: organizational safety as a social achievement. Hum Relations. 2009;62:1327-1356. doi:10.1177/0018726709334491
- Dekker S. Drift Into Failure: From Hunting Broken Components to Understanding Complex Systems. Routledge; 2011.
- Price MR, Williams TC. When doing wrong feels so right: normalization of deviance. J Patient Saf. 2018;14:1-2. doi:10.1097/pts.0000000000000157
To see what is in front of one’s nose needs a constant struggle.
George Orwell (1946)1
In 2019, the Veterans Health Administration (VHA) initiated a process to become a high reliability organization (HRO).2 The COVID-19 pandemic has been described in medical literature as a volatile, uncertain, complex, and ambiguous (VUCA) event, underscoring the necessity of resilient communication strategies.3 Challenges posed by 2024 Hurricanes Helene and Milton further highlighted the need for resilient communication strategies within HRO implementation.
Central to the HRO journey within the VHA has been the development of tiered huddles, an evolution of the safety huddle concept.4 Emerging organically as an effective communication mechanism across multiple facilities between 2019 and 2020, tiered huddles were, in part, spurred by the onset of COVID-19. Tiered huddles represent a proactive approach to identifying and addressing organizational threats in their early stages, thereby preventing their escalation to a VUCA-laden crisis.5 When conditions evolve beyond the horizon of tractability, where challenges are easily identified and resolved, tiered huddles serve as a resilient mechanism to restore dynamic equilibrium within the organization.6,7
This article describes how tiered huddles were integrated within Veterans Integrated Service Network (VISN) 4 and explores why these huddles are essential, particularly in the context of VUCA events. What began as a local-level tactic has now gained widespread acceptance and continues to evolve across the VHA with full support from the US Department of Veterans Affairs (VA) Under Secretary for Health.8
The VHA is divided into 18 VISNs. Nine VA Medical Centers (VAMCs) and 46 outpatient clinics across Pennsylvania, Delaware, and parts of Ohio, New York, and New Jersey make up VISN 4. Disseminating vital information across VISN 4, in addition to the 17 other VISNs—including 170 VAMCs and 1193 clinics—presents a formidable challenge. As the largest integrated system in the US, the VHA is realigning its workforce to address organizational inefficiencies. An enterprise of this scale, shaped by recurrent organizational change, faces ongoing challenges in sustaining clear communication across all levels. These transitions create uncertainty for staff as roles and resources shift, underscoring the need for dependable vertical and horizontal information flow. Tiered huddles offer a steady means to support coordinated communication and strengthen the system’s ability to adapt.9
ERIE VA MEDICAL CENTER HRO JOURNEY
In 2019, John Gennaro, the Erie VAMC executive director, attended a presentation that showcased the Cleveland Clinic’s tiered huddle process, with an opportunity to observe its 5-tiered system.10 Erie VAMC already had a 3-tiered huddle system, but the Cleveland Clinic’s more robust model inspired Gennaro to propose a VISN 4 pilot program. Tiered huddles were perceived as innovative, yet not fully embraced within the VHA; nonetheless, VISN 4, much like several other VISNs, moved forward and established a VISN-level (Tier 4) huddle.8 It is important to note that there was a notional fifth-tier capability as VISN and program office leaders already participated in daily VHA-wide meetings under the auspices of the Hospital Operations Center (HOC).
Expanding the Tiered Huddle Process
The Erie VAMC huddle process begins with the unit level Managers and Frontline Staff (Tier 1), then moves to Service Chiefs and Managers (Tier 2). Tier 3 involves facility executive leadership team and service chiefs, clinical directors and top VAMC administrators (these configurations may vary depending on context). The sequencing and flow of information is bidirectional across levels, reflecting the importance of closed-loop communication to ensure staff at all levels understand that issues raised are followed up on and/or closed out (Figure 1).2

Tier 4 composition may vary among VISNs depending on size and unique mission requirements.8,11 The VISN 4 Tier 4 huddle includes the VISN director, 9 VAMC directors, and key network administrators and clinical experts. The Tier 5 huddle includes 18 VISN 4 directors with the VHA HOC (Figure 2). The tiered huddle process emphasizes team-based culture and psychological safety.12-15 Staff at all levels are encouraged to identify and transparently resolve issues, fostering a proactive and problem-solving environment across the organization. A more nuanced and detailed process across tier levels is depicted in the Table.


The vetting and distillation of information can present challenges as vital information ascends and spreads across organization levels. Visual management systems (VMS), whether a whiteboard or a digital platform, are key to facilitate decision-making related to what needs to be prioritized and disseminated at each tier level.2,8 At Tier 5, the HOC uses a digital VMS to provide a structured, user-friendly format for categorizing issues and topics and enhances clarity and accessibility (Figure 3). The Tier 5 VMS also facilitates tracking and reciprocal information exchange, helping to close the loop on emerging issues by monitoring their progression and resolution up and across tiers.2,8 The Tier 5 huddle process and technology supporting continue to evolve offering increasing sophistication in organizational situational awareness and responsiveness.

VUCA: A Lens for Health Care Challenges
First introduced by social scientists at the US Army War College in 1995, VUCA describes complex and unpredictable conditions often encountered in military operations.16,17 Prompted by the COVID-19 pandemic, the acronym VUCA gained recognition in health care, as leaders acknowledged the challenge of navigating rapidly changing environments. van Stralen, Byrum and Inozu, recognized authorities in high reliability, cited VUCA as the rationale for implementing HRO principles and practices. They argued that “HRO solves the problem of operations and performance in a volatile, uncertain, complex, ambiguous environment.” 18 To fully appreciate the VUCA environment and its relevance to health care, it is essential to unpack the 4 components of the acronym: volatile, uncertain, complex, and ambiguous.
Volatile refers to the speed and unpredictability of change. Health care systems are interactively complex and tightly coupled, meaning that changes in 1 part of the system can rapidly impact others.6,18,19 This high degree of interdependence amplifies volatility, especially when unexpected events occur. The rapid spread of COVID- 19 and the evolving nature of its transmission challenged health care systems’ ability to respond swiftly and effectively. Volatility also may emerge in acute medical situations, such as the rapid deterioration of a patient’s condition.
Uncertain captures the lack of predictability inherent in complex systems. In health care, uncertainty arises when there is insufficient information or when an excess of data make it difficult to discern meaningful patterns. COVID-19 and recent natural disasters have introduced profound uncertainty, as the disease’s behavior, transmission, and impact were initially unknown. Health care practitioners struggled to make decisions in real time, lacking clear guidance or precedent.3,20 While health care planning and established protocols are grounded in predictability, the COVID-19 pandemic revealed that as complexity increases, predictability diminishes. Moreover, complexity can complicate protocol selection, as situations may arise in which multiple protocols conflict or compete. The cognitive challenge of operating in this environment is analogous to what military strategists call the fog of war, where situational awareness is low and decision-makers must navigate without clarity.21 Tiered huddles, a core practice in HROs, mitigate uncertainty by fostering real-time communication and shared situational awareness among teams.20
Complex refers to the intricate interplay of multiple, interconnected factors within a system.22 In health care, this complexity is heightened by the sociotechnical nature of the field—where human, technology, and organizational elements all converge.19 Systems designed to prevent failures, such as redundancies and safety protocols, can themselves contribute to increased complexity. HRO practices such as tiered huddles are implemented to mitigate the risk of catastrophic failure by fostering collaborative sensemaking, enhanced situational awareness, and rapid problem-solving.5,20,23
Ambiguous refers to situations in which multiple interpretations, causes, or outcomes are possible. It explains how, despite following protocols, failure can still occur, or how individuals may reach different conclusions from the same data. Ambiguity does not offer binary solutions; instead, it presents a murky, multifaceted reality that requires thoughtful interpretation and adaptive responses. In these moments, leaders must act decisively, even in the absence of complete information, making trade-offs that balance immediate needs with long-term consequences.
MANAGING VUCA ENVIRONMENTS WITH TIERED HUDDLES
The tiered huddle process provides several key benefits that enable real-time issue resolution. These include the rapid dissemination of vital information, enhanced agility and resilience, and improved sensemaking within a VUCA environment. Additionally, tiered huddles prevent organizational drift by fostering heightened situational awareness. The tiered huddle process also supports leadership development, as unit-level leaders gain valuable insights into strategic decision-making through active participation. Each component is outlined in the following section.
Spread: The Challenge of Communicating
“The hallmark of a great organization is how quickly bad news travels upward,” argued Jay Forrester, the father of system dynamics.24 Unfortunately, steep power gradients and siloed organizational structures inhibit the flow of unfavorable information from frontline staff to senior leadership. This suppression is not necessarily intentional but is often a byproduct of organizational culture. Tiered huddles address the weakness of top-down communication models by promoting a reciprocal, bidirectional information exchange, with an emphasis on closed-loop communication. Open communication can foster a culture of trust and transparency, allowing leaders to make more informed decisions and respond quickly to emerging risks.
Enhancing Agility and Resilience
Tiered huddles contribute to a mindful infrastructure, an important aspect of maintaining organizational awareness and agility.21,25 A mindful infrastructure enables an organization to detect early warning signs of potential disruptions and respond to them before they escalate. In this sense, tiered huddles serve as a signal-sensing mechanism, providing the agility needed to adapt to changing circumstances and prevent patient harm. Tiered huddles facilitate self-organization, a concept from chaos theory known as autopoiesis. 26 This self-organizing capability allows teams to develop novel solutions in response to unforeseen challenges, exemplifying the adaptability and resilience needed in a VUCA environment. The diverse backgrounds of tiered huddle participants—both cognitively and culturally—enable a broader range of perspectives, which is critical for making sound decisions in complex and uncertain situations. “HROs cultivate diversity not just because it helps them notice more in complex environments, but also because it helps them adapt to the complexities they do spot,” argues Weick et al.27 This diversity of thought and experience enhances the organization’s ability to respond to complexity, much like firefighters continually adapt to the VUCA conditions they face.
Sensemaking and Sensitivity to Operations
Leaders at all levels must be attuned to what is happening both within and outside their organization. This continual sensing of the environment—looking for weak signals, threats, and opportunities—is important for HROs. This signal detection capability allows organizations to address problems in their nascent emerging state within a tractable horizon to successfully manage fluctuations. The horizon of tractability reflects a zone where weak signals and evolving issues can be identified, addressed, and resolved early before they evolve and cascade outside of safe operations. 7 Tiered huddles facilitate this process by creating a platform for team members to engage in respectful, collaborative dialogue. The diversity inherent in tiered huddles also supports sensemaking, a process of interpreting and understanding complex situations.27 In a VUCA environment, this multiperspective approach helps filter out noise and identify the most important signals. Tiered huddles can help overcome the phenomenon of dysfunctional momentum associated with cognitive lockup, fixation error, and tunnel vision, in which individuals or teams fixate on a particular solution, thus missing important alternative views.21,28 By fostering a common operating picture of the fluctuating environment, tiered huddles can enable more accurate decision-making and improve organizational resilience.
Avoiding Organizational Drift
One of the most significant contributions of tiered huddles is the ability to detect early signs of organizational drift, or subtle deviations from standard practices that can accumulate over time and lead to serious failures. By continuously monitoring for precursor conditions and weak signals, tiered huddles allow organizations to intervene early and prevent drift from becoming catastrophic.29,30 This vigilance is essential in health care, where complacency can lead to patient harm. Tiered huddles foster a culture of mindfulness and accountability, ensuring that staff stay engaged and alert to potential risks. This proactive approach is a safeguard against human error and the gradual erosion of safety standards.
Leadership Development
Tiered huddles serve as a powerful tool for leadership development. Effective leaders must be able to anticipate potential risks and foresee system failures. Involving future leaders in tiered huddles can facilitate the transfer of these critical skills. When emerging leaders at lower tiers participate in ascending-tier huddles, they gain a unique opportunity to engage in a structured, collaborative setting. This environment provides a safe space to develop and practice strategic skills, enhancing their ability to think proactively and manage complexity. By integrating future leaders into tiered huddles, organizations offer essential, hands-on experience in real-time decision making. This experiential learning is invaluable for preparing leaders to navigate the demands of a VUCA environment.
CONCLUSIONS
Since implementing the tiered huddle process, the Erie VAMC and VISN 4 have emerged as early adopters of VUCA, thus contributing to the expansion of this innovative communication approach across the VHA. Tiered huddles strengthen organizational resilience and agility, facilitate critical information flow to manage risk, and support the cultivation of future leaders. The Erie VAMC director and the VISN 4 network director regard the expansion of tiered huddles, including Tiers 4 and 5, as an adaptable model for the VHA. While tiered huddles have not yet been mandated across the VHA, a pilot at the Tier 5 HOC level was initiated on May 20, 2024. In a complex world in which VUCA events will continue to be inevitable, implementation of robust tiered huddles within complex health care systems provides the opportunity for improved responses and delivery of care.
To see what is in front of one’s nose needs a constant struggle.
George Orwell (1946)1
In 2019, the Veterans Health Administration (VHA) initiated a process to become a high reliability organization (HRO).2 The COVID-19 pandemic has been described in medical literature as a volatile, uncertain, complex, and ambiguous (VUCA) event, underscoring the necessity of resilient communication strategies.3 Challenges posed by 2024 Hurricanes Helene and Milton further highlighted the need for resilient communication strategies within HRO implementation.
Central to the HRO journey within the VHA has been the development of tiered huddles, an evolution of the safety huddle concept.4 Emerging organically as an effective communication mechanism across multiple facilities between 2019 and 2020, tiered huddles were, in part, spurred by the onset of COVID-19. Tiered huddles represent a proactive approach to identifying and addressing organizational threats in their early stages, thereby preventing their escalation to a VUCA-laden crisis.5 When conditions evolve beyond the horizon of tractability, where challenges are easily identified and resolved, tiered huddles serve as a resilient mechanism to restore dynamic equilibrium within the organization.6,7
This article describes how tiered huddles were integrated within Veterans Integrated Service Network (VISN) 4 and explores why these huddles are essential, particularly in the context of VUCA events. What began as a local-level tactic has now gained widespread acceptance and continues to evolve across the VHA with full support from the US Department of Veterans Affairs (VA) Under Secretary for Health.8
The VHA is divided into 18 VISNs. Nine VA Medical Centers (VAMCs) and 46 outpatient clinics across Pennsylvania, Delaware, and parts of Ohio, New York, and New Jersey make up VISN 4. Disseminating vital information across VISN 4, in addition to the 17 other VISNs—including 170 VAMCs and 1193 clinics—presents a formidable challenge. As the largest integrated system in the US, the VHA is realigning its workforce to address organizational inefficiencies. An enterprise of this scale, shaped by recurrent organizational change, faces ongoing challenges in sustaining clear communication across all levels. These transitions create uncertainty for staff as roles and resources shift, underscoring the need for dependable vertical and horizontal information flow. Tiered huddles offer a steady means to support coordinated communication and strengthen the system’s ability to adapt.9
ERIE VA MEDICAL CENTER HRO JOURNEY
In 2019, John Gennaro, the Erie VAMC executive director, attended a presentation that showcased the Cleveland Clinic’s tiered huddle process, with an opportunity to observe its 5-tiered system.10 Erie VAMC already had a 3-tiered huddle system, but the Cleveland Clinic’s more robust model inspired Gennaro to propose a VISN 4 pilot program. Tiered huddles were perceived as innovative, yet not fully embraced within the VHA; nonetheless, VISN 4, much like several other VISNs, moved forward and established a VISN-level (Tier 4) huddle.8 It is important to note that there was a notional fifth-tier capability as VISN and program office leaders already participated in daily VHA-wide meetings under the auspices of the Hospital Operations Center (HOC).
Expanding the Tiered Huddle Process
The Erie VAMC huddle process begins with the unit level Managers and Frontline Staff (Tier 1), then moves to Service Chiefs and Managers (Tier 2). Tier 3 involves facility executive leadership team and service chiefs, clinical directors and top VAMC administrators (these configurations may vary depending on context). The sequencing and flow of information is bidirectional across levels, reflecting the importance of closed-loop communication to ensure staff at all levels understand that issues raised are followed up on and/or closed out (Figure 1).2

Tier 4 composition may vary among VISNs depending on size and unique mission requirements.8,11 The VISN 4 Tier 4 huddle includes the VISN director, 9 VAMC directors, and key network administrators and clinical experts. The Tier 5 huddle includes 18 VISN 4 directors with the VHA HOC (Figure 2). The tiered huddle process emphasizes team-based culture and psychological safety.12-15 Staff at all levels are encouraged to identify and transparently resolve issues, fostering a proactive and problem-solving environment across the organization. A more nuanced and detailed process across tier levels is depicted in the Table.


The vetting and distillation of information can present challenges as vital information ascends and spreads across organization levels. Visual management systems (VMS), whether a whiteboard or a digital platform, are key to facilitate decision-making related to what needs to be prioritized and disseminated at each tier level.2,8 At Tier 5, the HOC uses a digital VMS to provide a structured, user-friendly format for categorizing issues and topics and enhances clarity and accessibility (Figure 3). The Tier 5 VMS also facilitates tracking and reciprocal information exchange, helping to close the loop on emerging issues by monitoring their progression and resolution up and across tiers.2,8 The Tier 5 huddle process and technology supporting continue to evolve offering increasing sophistication in organizational situational awareness and responsiveness.

VUCA: A Lens for Health Care Challenges
First introduced by social scientists at the US Army War College in 1995, VUCA describes complex and unpredictable conditions often encountered in military operations.16,17 Prompted by the COVID-19 pandemic, the acronym VUCA gained recognition in health care, as leaders acknowledged the challenge of navigating rapidly changing environments. van Stralen, Byrum and Inozu, recognized authorities in high reliability, cited VUCA as the rationale for implementing HRO principles and practices. They argued that “HRO solves the problem of operations and performance in a volatile, uncertain, complex, ambiguous environment.” 18 To fully appreciate the VUCA environment and its relevance to health care, it is essential to unpack the 4 components of the acronym: volatile, uncertain, complex, and ambiguous.
Volatile refers to the speed and unpredictability of change. Health care systems are interactively complex and tightly coupled, meaning that changes in 1 part of the system can rapidly impact others.6,18,19 This high degree of interdependence amplifies volatility, especially when unexpected events occur. The rapid spread of COVID- 19 and the evolving nature of its transmission challenged health care systems’ ability to respond swiftly and effectively. Volatility also may emerge in acute medical situations, such as the rapid deterioration of a patient’s condition.
Uncertain captures the lack of predictability inherent in complex systems. In health care, uncertainty arises when there is insufficient information or when an excess of data make it difficult to discern meaningful patterns. COVID-19 and recent natural disasters have introduced profound uncertainty, as the disease’s behavior, transmission, and impact were initially unknown. Health care practitioners struggled to make decisions in real time, lacking clear guidance or precedent.3,20 While health care planning and established protocols are grounded in predictability, the COVID-19 pandemic revealed that as complexity increases, predictability diminishes. Moreover, complexity can complicate protocol selection, as situations may arise in which multiple protocols conflict or compete. The cognitive challenge of operating in this environment is analogous to what military strategists call the fog of war, where situational awareness is low and decision-makers must navigate without clarity.21 Tiered huddles, a core practice in HROs, mitigate uncertainty by fostering real-time communication and shared situational awareness among teams.20
Complex refers to the intricate interplay of multiple, interconnected factors within a system.22 In health care, this complexity is heightened by the sociotechnical nature of the field—where human, technology, and organizational elements all converge.19 Systems designed to prevent failures, such as redundancies and safety protocols, can themselves contribute to increased complexity. HRO practices such as tiered huddles are implemented to mitigate the risk of catastrophic failure by fostering collaborative sensemaking, enhanced situational awareness, and rapid problem-solving.5,20,23
Ambiguous refers to situations in which multiple interpretations, causes, or outcomes are possible. It explains how, despite following protocols, failure can still occur, or how individuals may reach different conclusions from the same data. Ambiguity does not offer binary solutions; instead, it presents a murky, multifaceted reality that requires thoughtful interpretation and adaptive responses. In these moments, leaders must act decisively, even in the absence of complete information, making trade-offs that balance immediate needs with long-term consequences.
MANAGING VUCA ENVIRONMENTS WITH TIERED HUDDLES
The tiered huddle process provides several key benefits that enable real-time issue resolution. These include the rapid dissemination of vital information, enhanced agility and resilience, and improved sensemaking within a VUCA environment. Additionally, tiered huddles prevent organizational drift by fostering heightened situational awareness. The tiered huddle process also supports leadership development, as unit-level leaders gain valuable insights into strategic decision-making through active participation. Each component is outlined in the following section.
Spread: The Challenge of Communicating
“The hallmark of a great organization is how quickly bad news travels upward,” argued Jay Forrester, the father of system dynamics.24 Unfortunately, steep power gradients and siloed organizational structures inhibit the flow of unfavorable information from frontline staff to senior leadership. This suppression is not necessarily intentional but is often a byproduct of organizational culture. Tiered huddles address the weakness of top-down communication models by promoting a reciprocal, bidirectional information exchange, with an emphasis on closed-loop communication. Open communication can foster a culture of trust and transparency, allowing leaders to make more informed decisions and respond quickly to emerging risks.
Enhancing Agility and Resilience
Tiered huddles contribute to a mindful infrastructure, an important aspect of maintaining organizational awareness and agility.21,25 A mindful infrastructure enables an organization to detect early warning signs of potential disruptions and respond to them before they escalate. In this sense, tiered huddles serve as a signal-sensing mechanism, providing the agility needed to adapt to changing circumstances and prevent patient harm. Tiered huddles facilitate self-organization, a concept from chaos theory known as autopoiesis. 26 This self-organizing capability allows teams to develop novel solutions in response to unforeseen challenges, exemplifying the adaptability and resilience needed in a VUCA environment. The diverse backgrounds of tiered huddle participants—both cognitively and culturally—enable a broader range of perspectives, which is critical for making sound decisions in complex and uncertain situations. “HROs cultivate diversity not just because it helps them notice more in complex environments, but also because it helps them adapt to the complexities they do spot,” argues Weick et al.27 This diversity of thought and experience enhances the organization’s ability to respond to complexity, much like firefighters continually adapt to the VUCA conditions they face.
Sensemaking and Sensitivity to Operations
Leaders at all levels must be attuned to what is happening both within and outside their organization. This continual sensing of the environment—looking for weak signals, threats, and opportunities—is important for HROs. This signal detection capability allows organizations to address problems in their nascent emerging state within a tractable horizon to successfully manage fluctuations. The horizon of tractability reflects a zone where weak signals and evolving issues can be identified, addressed, and resolved early before they evolve and cascade outside of safe operations. 7 Tiered huddles facilitate this process by creating a platform for team members to engage in respectful, collaborative dialogue. The diversity inherent in tiered huddles also supports sensemaking, a process of interpreting and understanding complex situations.27 In a VUCA environment, this multiperspective approach helps filter out noise and identify the most important signals. Tiered huddles can help overcome the phenomenon of dysfunctional momentum associated with cognitive lockup, fixation error, and tunnel vision, in which individuals or teams fixate on a particular solution, thus missing important alternative views.21,28 By fostering a common operating picture of the fluctuating environment, tiered huddles can enable more accurate decision-making and improve organizational resilience.
Avoiding Organizational Drift
One of the most significant contributions of tiered huddles is the ability to detect early signs of organizational drift, or subtle deviations from standard practices that can accumulate over time and lead to serious failures. By continuously monitoring for precursor conditions and weak signals, tiered huddles allow organizations to intervene early and prevent drift from becoming catastrophic.29,30 This vigilance is essential in health care, where complacency can lead to patient harm. Tiered huddles foster a culture of mindfulness and accountability, ensuring that staff stay engaged and alert to potential risks. This proactive approach is a safeguard against human error and the gradual erosion of safety standards.
Leadership Development
Tiered huddles serve as a powerful tool for leadership development. Effective leaders must be able to anticipate potential risks and foresee system failures. Involving future leaders in tiered huddles can facilitate the transfer of these critical skills. When emerging leaders at lower tiers participate in ascending-tier huddles, they gain a unique opportunity to engage in a structured, collaborative setting. This environment provides a safe space to develop and practice strategic skills, enhancing their ability to think proactively and manage complexity. By integrating future leaders into tiered huddles, organizations offer essential, hands-on experience in real-time decision making. This experiential learning is invaluable for preparing leaders to navigate the demands of a VUCA environment.
CONCLUSIONS
Since implementing the tiered huddle process, the Erie VAMC and VISN 4 have emerged as early adopters of VUCA, thus contributing to the expansion of this innovative communication approach across the VHA. Tiered huddles strengthen organizational resilience and agility, facilitate critical information flow to manage risk, and support the cultivation of future leaders. The Erie VAMC director and the VISN 4 network director regard the expansion of tiered huddles, including Tiers 4 and 5, as an adaptable model for the VHA. While tiered huddles have not yet been mandated across the VHA, a pilot at the Tier 5 HOC level was initiated on May 20, 2024. In a complex world in which VUCA events will continue to be inevitable, implementation of robust tiered huddles within complex health care systems provides the opportunity for improved responses and delivery of care.
- Orwell S, Angus I, eds. In Front of Your Nose, 1945-1950. Godine; 2000. Orwell G. The Collected Essays, Journalism, and Letters of George Orwell; vol 4.
- Murray JS, Baghdadi A, Dannenberg W, Crews P, Walsh ND. The role of high reliability organization foundational practices in building a culture of safety. Fed Pract. 2024;41:214-221. doi:10.12788/fp.0486
- Goldenhar LM, Brady PW, Sutcliffe KM, Muething SE. Huddling for high reliability and situation awareness. BMJ Qual Saf. 2013;22:899-906. doi:10.1136/bmjqs-2012-001467
- Pandit M. Critical factors for successful management of VUCA times. BMJ Lead. 2021;5:121-123. doi:10.1136/leader-2020-000305
- Mihaljevic T. Tiered daily huddles: the power of teamwork in managing large healthcare organisations. BMJ Qual Saf. 2020;29:1050-1052. doi:10.1136/bmjqs-2019-010575
- van Stralen D, Mercer TA. High-reliability organizing (HRO) in the COVID-19 liminal zone: characteristics of workers and local leaders. Neonatology Today. 2021;16:90-101. http://www.neonatologytoday.net /newsletters/nt-apr21.pdf
- Nemeth C, Wears R, Woods D, Hollnagel E, Cook R. Minding the gaps: creating resilience in health care. In: Henriksen K, Battles JB, Keyes MA, Grady ML, eds. Advances in Patient Safety: New Directions and Alternative Approaches. Vol 3: Performance and Tools. Agency for Healthcare Research and Quality; 2008.
- Merchant NB, O’Neal J, Montoya A, Cox GR, Murray JS. Creating a process for the implementation of tiered huddles in a Veterans Affairs medical center. Mil Med. 2023;188:901-906. doi:10.1093/milmed/usac073
- Starbuck WH, Farjoun M, eds. Organization at the Limit: Lessons From the Columbia Disaster. 1st ed. Wiley-Blackwell; 2005.
- Mihaljevic T. Tiered daily huddles: the power of teamwork in managing large healthcare organisations. BMJ Qual Saf. 2020;29:1050-1052. doi:10.1136/bmjqs-2019-010575
- Donnelly LF, Cherian SS, Chua KB, et al. The Daily Readiness Huddle: a process to rapidly identify issues and foster improvement through problem-solving accountability. Pediatr Radiol. 2017;47:22-30. doi:10.1007/s00247-016-3712-x
- Clark TR. The 4 Stages of Psychological Safety: Defining the Path to Inclusion and Innovation. Berrett-Koehler Publishers, Inc.; 2020.
- Edmondson AC. The Fearless Organization: Creating Psychological Safety in the Workplace for Learning, Innovation, and Growth. John Wiley & Sons; 2018.
- Edmondson AC. The Right Kind of Wrong: The Science of Failing Well. Simon Element/Simon Acumen; 2023.
- Murray JS, Kelly S, Hanover C. Promoting psychological safety in healthcare organizations. Mil Med. 2022;187:808 -810. doi:10.1093/milmed/usac041
- Barber HF. Developing strategic leadership: the US Army War College experience. J Manag Dev. 1992;11:4-12. doi:10.1108/02621719210018208
- US Army Heritage & Education Center. Who first originated the term VUCA (volatility, uncertainty, complexity and ambiguity)? Accessed November 5, 2025. https://usawc .libanswers.com/ahec/faq/84869
- van Stralen D, Byrum SL, Inozu B. High Reliability for a Highly Unreliable World: Preparing for Code Blue Through Daily Operations in Healthcare. CreateSpace Independent Publishing Platform; 2018.
- Perrow C. Normal Accidents: Living With High-Risk Technologies. Princeton University Press; 2000.
- Sculli G, Essen K. Soaring to Success: The Path to Developing High-Reliability Clinical Teams. HCPro; 2021. Accessed November 5, 2025. https://hcmarketplace.com /media/wysiwyg/CRM3_browse.pdf
- Barton MA, Sutcliffe KM, Vogus TJ, DeWitt T. Performing under uncertainty: contextualized engagement in wildland firefighting. J Contingencies Crisis Manag. 2015;23:74-83. doi:10.1111/1468-5973.12076
- Sutcliffe KM. Mindful organizing. In: Ramanujam R, Roberts KH, eds. Organizing for Reliability: A Guide for Research and Practice. Stanford University Press; 2018:61-89.
- Merchant NB, O’Neal J, Dealino-Perez C, Xiang J, Montoya A Jr, Murray JS. A high-reliability organization mindset. Am J Med Qual. 2022;37:504-510. doi:10.1097/jmq.0000000000000086
- Senge PM. The Fifth Discipline Fieldbook: Strategies and Tools for Building a Learning Organization. Crown Currency; 1994.
- Ramanujam R, Roberts KH, eds. Organizing for Reliability: A Guide for Research and Practice. Stanford University Press; 2018.
- Coveney PV. Self-organization and complexity: a new age for theory, computation and experiment. Philos Trans A Math Phys Eng Sci. 2003;361:1057-1079. doi:10.1098/rsta.2003.1191
- Weick KE, Sutcliffe KM. Managing the Unexpected: Sustained Performance in a Complex World. 3rd ed. Wiley; 2015.
- Barton M, Sutcliffe K. Overcoming dysfunctional momentum: organizational safety as a social achievement. Hum Relations. 2009;62:1327-1356. doi:10.1177/0018726709334491
- Dekker S. Drift Into Failure: From Hunting Broken Components to Understanding Complex Systems. Routledge; 2011.
- Price MR, Williams TC. When doing wrong feels so right: normalization of deviance. J Patient Saf. 2018;14:1-2. doi:10.1097/pts.0000000000000157
- Orwell S, Angus I, eds. In Front of Your Nose, 1945-1950. Godine; 2000. Orwell G. The Collected Essays, Journalism, and Letters of George Orwell; vol 4.
- Murray JS, Baghdadi A, Dannenberg W, Crews P, Walsh ND. The role of high reliability organization foundational practices in building a culture of safety. Fed Pract. 2024;41:214-221. doi:10.12788/fp.0486
- Goldenhar LM, Brady PW, Sutcliffe KM, Muething SE. Huddling for high reliability and situation awareness. BMJ Qual Saf. 2013;22:899-906. doi:10.1136/bmjqs-2012-001467
- Pandit M. Critical factors for successful management of VUCA times. BMJ Lead. 2021;5:121-123. doi:10.1136/leader-2020-000305
- Mihaljevic T. Tiered daily huddles: the power of teamwork in managing large healthcare organisations. BMJ Qual Saf. 2020;29:1050-1052. doi:10.1136/bmjqs-2019-010575
- van Stralen D, Mercer TA. High-reliability organizing (HRO) in the COVID-19 liminal zone: characteristics of workers and local leaders. Neonatology Today. 2021;16:90-101. http://www.neonatologytoday.net /newsletters/nt-apr21.pdf
- Nemeth C, Wears R, Woods D, Hollnagel E, Cook R. Minding the gaps: creating resilience in health care. In: Henriksen K, Battles JB, Keyes MA, Grady ML, eds. Advances in Patient Safety: New Directions and Alternative Approaches. Vol 3: Performance and Tools. Agency for Healthcare Research and Quality; 2008.
- Merchant NB, O’Neal J, Montoya A, Cox GR, Murray JS. Creating a process for the implementation of tiered huddles in a Veterans Affairs medical center. Mil Med. 2023;188:901-906. doi:10.1093/milmed/usac073
- Starbuck WH, Farjoun M, eds. Organization at the Limit: Lessons From the Columbia Disaster. 1st ed. Wiley-Blackwell; 2005.
- Mihaljevic T. Tiered daily huddles: the power of teamwork in managing large healthcare organisations. BMJ Qual Saf. 2020;29:1050-1052. doi:10.1136/bmjqs-2019-010575
- Donnelly LF, Cherian SS, Chua KB, et al. The Daily Readiness Huddle: a process to rapidly identify issues and foster improvement through problem-solving accountability. Pediatr Radiol. 2017;47:22-30. doi:10.1007/s00247-016-3712-x
- Clark TR. The 4 Stages of Psychological Safety: Defining the Path to Inclusion and Innovation. Berrett-Koehler Publishers, Inc.; 2020.
- Edmondson AC. The Fearless Organization: Creating Psychological Safety in the Workplace for Learning, Innovation, and Growth. John Wiley & Sons; 2018.
- Edmondson AC. The Right Kind of Wrong: The Science of Failing Well. Simon Element/Simon Acumen; 2023.
- Murray JS, Kelly S, Hanover C. Promoting psychological safety in healthcare organizations. Mil Med. 2022;187:808 -810. doi:10.1093/milmed/usac041
- Barber HF. Developing strategic leadership: the US Army War College experience. J Manag Dev. 1992;11:4-12. doi:10.1108/02621719210018208
- US Army Heritage & Education Center. Who first originated the term VUCA (volatility, uncertainty, complexity and ambiguity)? Accessed November 5, 2025. https://usawc .libanswers.com/ahec/faq/84869
- van Stralen D, Byrum SL, Inozu B. High Reliability for a Highly Unreliable World: Preparing for Code Blue Through Daily Operations in Healthcare. CreateSpace Independent Publishing Platform; 2018.
- Perrow C. Normal Accidents: Living With High-Risk Technologies. Princeton University Press; 2000.
- Sculli G, Essen K. Soaring to Success: The Path to Developing High-Reliability Clinical Teams. HCPro; 2021. Accessed November 5, 2025. https://hcmarketplace.com /media/wysiwyg/CRM3_browse.pdf
- Barton MA, Sutcliffe KM, Vogus TJ, DeWitt T. Performing under uncertainty: contextualized engagement in wildland firefighting. J Contingencies Crisis Manag. 2015;23:74-83. doi:10.1111/1468-5973.12076
- Sutcliffe KM. Mindful organizing. In: Ramanujam R, Roberts KH, eds. Organizing for Reliability: A Guide for Research and Practice. Stanford University Press; 2018:61-89.
- Merchant NB, O’Neal J, Dealino-Perez C, Xiang J, Montoya A Jr, Murray JS. A high-reliability organization mindset. Am J Med Qual. 2022;37:504-510. doi:10.1097/jmq.0000000000000086
- Senge PM. The Fifth Discipline Fieldbook: Strategies and Tools for Building a Learning Organization. Crown Currency; 1994.
- Ramanujam R, Roberts KH, eds. Organizing for Reliability: A Guide for Research and Practice. Stanford University Press; 2018.
- Coveney PV. Self-organization and complexity: a new age for theory, computation and experiment. Philos Trans A Math Phys Eng Sci. 2003;361:1057-1079. doi:10.1098/rsta.2003.1191
- Weick KE, Sutcliffe KM. Managing the Unexpected: Sustained Performance in a Complex World. 3rd ed. Wiley; 2015.
- Barton M, Sutcliffe K. Overcoming dysfunctional momentum: organizational safety as a social achievement. Hum Relations. 2009;62:1327-1356. doi:10.1177/0018726709334491
- Dekker S. Drift Into Failure: From Hunting Broken Components to Understanding Complex Systems. Routledge; 2011.
- Price MR, Williams TC. When doing wrong feels so right: normalization of deviance. J Patient Saf. 2018;14:1-2. doi:10.1097/pts.0000000000000157
Negotiating the VUCA World Through Tiered Huddles
Negotiating the VUCA World Through Tiered Huddles
The Road Less Traveled: Why Rural Dermatology Could Be Your Path After Residency
The Road Less Traveled: Why Rural Dermatology Could Be Your Path After Residency
The myths persist: You will lack colleagues. Your practice will be thin. You must sacrifice academic engagement. In reality, rural practice offers variety, leadership opportunities, and the chance to influence the health of entire communities in profound ways. In this article, we aim to unpack what rural dermatology actually looks like as a potential career path for residents, with a focus on private-academic hybrid and hospital-based practice models.
Definitions of the term rural vary. For the US Census Bureau, it is synonymous with nonurban, and for the Office of Management and Budget, the term nonmetropolitan is preferred. The US Department of Agriculture’s Rural-Urban Commuting Area codes recognize a continuum of classifications from micropolitan to remote. In practice, the term rural covers a wide spectrum: the rolling farmlands of the Midwest, the mountains of Montana, the bayous of the South, the Native American reservations in New Mexico, and everything in between. It is not one uniform reality—rural America is diverse, resilient, and deeply connected.
Daily clinic flow may look familiar: a full schedule, a mix of new and established patients, and frequent simple procedures such as biopsies and corticosteroid injections. But the scope of practice is wider. You become the dermatologist for hundreds of miles in every direction, managing most conditions locally while referring select cases to subspecialty centers.
Case variety is striking. Neglected tumors, unusual inflammatory presentations, pediatric conditions, and occupational dermatoses/injuries appear alongside the routine. Each day requires flexibility, judgment, confidence, and the ability to think outside the box. You must consider how a patient’s seasonal work, such as ranching or farming, and/or their total commute time impacts the risk-benefit discussion around treatment recommendations.
Matthew P. Shaffer, MD (Salina, Kansas), who has practiced rural dermatology for more than 20 years, explained that the breadth of dermatologic cases in which he served as the expert was both exciting and intimidating, but it became clear that this was the right professional path for him (email communication, September 5, 2025). In small communities, your role extends beyond the clinic walls. You will see patients at the grocery store, the library, and school events. That continuity fosters loyalty and accountability in ways that are hard to quantify.
Many practice structures exist: independent clinics, multispecialty groups, hospital employment, and increasingly, hybrid partnerships with academic centers.
Academic institutions have recognized the importance of rural exposure, and many now collaborate with rural dermatologists. For example, Heartland Dermatology in Salina, Kansas, where 2 of the authors (B.R.L. and T.G.) practice, partners with St. Louis University in Missouri to provide a residency track and rotations in rural clinics.
Rural-based hospital systems can create similar structures. Monument Health Dermatology in Spearfish, South Dakota, is integrated into the fabric of the community’s larger rural health care model. The physician (M.E.L.) collaborates daily with primary care providers, surgeons, and oncologists through a shared electronic health record (sometimes even through telephone speed-dial given the close collegiality of small-town providers). Patients come from across 4 states, some driving 6 hours each way. Patients who once doubted whether dermatology was worth the trip will consistently return for follow-up care once trust is earned. The stability of hospital employment supports volunteer faculty positions and a free satellite clinic in partnership with a local Lakota Tribal health center. There is never a dull day: the providers see urgent add-ons daily, which keeps them on their toes but in exchange brings immense reward. This includes a recent case from rural Wyoming: a complex mixed infantile hemangioma on the mid face just entering the rapid proliferation phase. Propranolol was started immediately, as opposed to months later when it was too late—a common complication for the majority of rural patients by the time to get to a dermatologist.
Complex cases can overwhelm rural practices, and this is when the hub-and-spoke model is invaluable. Dermatologists embed in local communities as spokes, while subspecialty services such as pediatric dermatology, dermatopathology, or Mohs micrographic surgery remain centralized at hubs. The hubs can be but do not have to be academic institutions; for Heartland Dermatology in Kansas, private practices fulfill both hub and spoke roles. With that said, 10 states do not have academic dermatology programs.1 Mohs surgeons and pediatric dermatologists still can establish robust and successful independent rural subspecialty practices outside academic hubs. Christopher Gasbarre, DO (Spearfish, South Dakota), a board-certified, fellowship-trained Mohs surgeon in rural practice, advises residents to be confident in their abilities and to trust their training, noting that they often will be asked to manage complicated cases because of patient travel and cost constraints; however, clinicians should recognize their own limitations and those of nearby specialists and develop a referral network for cases that require multidisciplinary care (text communication, September 14, 2025).
The hub-and-spoke models—whether they entail an academic center as the hub with private practices as the spokes, or a network of private practices that include rural subspecialists—allows rural dermatologists to remain trusted local experts while ensuring that patients can access advanced care via a more streamlined referral process/network. The challenge is triage: what can be managed locally and what must patients travel for? As Dr. Shaffer explained, decisions about whether care is managed locally or referred to a hub often depend on the experience and comfort level of both the physician and the patient (email communication, September 5, 2025). Ultimately, continuity and trust are central. Patients rely on their local dermatologist to guide these decisions, and that guidance makes the model effective.
The idea that rural practice means being stuck in a small solo clinic is outdated. Multiple pathways exist, each with strengths and challenges. Independent private practice offers maximum autonomy and deep community integration, though financial and staffing risks are yours to manage. Hospital employment with outreach clinics provides stability, benefits, and collegiality, but bureaucracy can limit innovation and efficiency. Private equity platforms supply resources and rapid growth, but alignment with mission and autonomy must be weighed carefully. Hybrid joint ventures with hospitals combine private control and institutional support, but contracts can be complex. Locum tenens–to-permanent arrangements let you try rural life with minimal commitment, but continuity with patients may be sacrificed. A self-screener can clarify your path: How much autonomy do I want? Do I prefer predictability or variety? How important are procedures, teaching, or community roles? Answer these questions honestly and pair that insight with mentor guidance.
Launching a rural dermatology clinic is equal parts vision and structure. A focused 90-day plan can make the difference between a smooth opening and early frustration. Think in 4 domains: site selection, employment and licensing, credentialing and contracting, and operations. Even in a compressed timeline, dozens of small but crucial tasks may surface. There are resources—such as the Medical Group Management Association’s practice start-up checklist—that can provide a roadmap, ensuring no detail is overlooked as you transform a vision into a functioning clinic.2
Site Selection—First, determine whether you are opening a new standalone clinic, extending an existing practice, or creating a part-time satellite. Referral mapping with local primary care providers is essential, as is a scan of payer mix and dermatologist density in the region to ensure sustainability.
Employment and Licensing—Confirm state licensure and Drug Enforcement Administration registration and initiate hospital privileges early. These processes can stretch across the entire 90-day window, so starting immediately is critical.
Credentialing and Contracting—Applications with commercial and federal payers, along with Council for Affordable Quality Healthcare updates, often consume weeks if not months. If you plan to perform office microscopy or establish a dermatopathology laboratory, begin the Clinical Laboratory Improvement Amendments certification process in parallel.
Operations—Once the regulatory wheels are in motion, shift to building your practice infrastructure. Secure space, weigh lease vs purchase, and consider partnerships with local hospitals for shared clinic facilities. Recruit staff with dermatology-specific skills such as clinical photography and biopsy assistance. Implement an electronic health record, set up payroll and malpractice insurance, and establish supply chains for everything from liquid nitrogen to surgical trays. Decide whether revenue cycle management will be in-house or outsourced and finalize dermatopathology workflows including courier and transport agreements.
Compensation in rural dermatology mirrors that of other clinical settings: base salary with productivity bonuses, revenue pooling, or relative value unit structures. Financial planning is crucial. Develop a pro forma that models patient volume, expenses, and realistic growth. Risks exist, including payer mix, staffing, and competition, but the demand for care in underserved areas often offsets these, and communities may support practices with reduced overhead and strong loyalty. Hospital systems may add stipends for supervising advanced practitioners or outreach travel. Loan repayment programs, tax credits, and grants can further enhance packages. Consider checking with the state’s Office of Rural Health.
Career sustainability ultimately depends on more than finances. Geography, amenities, schedule flexibility, autonomy in medical decision-making, work-life balance, the value of being part of and serving a community, and other personal values will shape your “best-fit” practice model. Ask whether you can envision yourself thriving in the community you would be serving.
No one builds a rural dermatology practice alone. That is why one of the authors (M.E.L.) created the Rural Access to Dermatology Society (https://www.radsociety.org/), a nonprofit organization connecting dermatologists, residents, and medical students with a shared mission. The organization supports residents through scholarships, mentorship, and telementoring. Faculty can contribute through advocacy, residency track development, and outreach to uniquely underserved rural populations such as Native American reservations where access to dermatology care remains severely limited. Joining can be as simple as attending a webinar, finding a mentor, or volunteering at a free clinic. You do not need to launch your own clinic to get involved; you can begin by connecting with a network already laying the foundation.
Teledermatology and academic initiatives enhance rural care but do not replace in-person practice. Store-and-forward consultations extend reach but cannot match the continuity and trust of long-term patient relationships. Academic rural tracks prepare residents for unique challenges, but someone must staff the clinics. Private and hybrid models remain the backbone of rural access, where dermatologists take on the responsibility and the joy of being the local expert.
So here’s the invitation: bring one question to your mentor about rural practice and identify one rural site you could visit. The road less traveled in dermatology is closer than you think—and it might just be your path.
- Association of American Medical Colleges. ERAS Directory: Dermatology. Accessed December 11, 2025. https://systems.aamc.org/eras/erasstats/par/display.cfm?NAV_ROW=PAR&SPEC_CD=080
- Medical Group Management Association. Large group or organization practice startup checklist. Accessed December 11, 2025. https://www.mgma.com/member-tools/large-group-or-organization -practice-startup-checklist
The myths persist: You will lack colleagues. Your practice will be thin. You must sacrifice academic engagement. In reality, rural practice offers variety, leadership opportunities, and the chance to influence the health of entire communities in profound ways. In this article, we aim to unpack what rural dermatology actually looks like as a potential career path for residents, with a focus on private-academic hybrid and hospital-based practice models.
Definitions of the term rural vary. For the US Census Bureau, it is synonymous with nonurban, and for the Office of Management and Budget, the term nonmetropolitan is preferred. The US Department of Agriculture’s Rural-Urban Commuting Area codes recognize a continuum of classifications from micropolitan to remote. In practice, the term rural covers a wide spectrum: the rolling farmlands of the Midwest, the mountains of Montana, the bayous of the South, the Native American reservations in New Mexico, and everything in between. It is not one uniform reality—rural America is diverse, resilient, and deeply connected.
Daily clinic flow may look familiar: a full schedule, a mix of new and established patients, and frequent simple procedures such as biopsies and corticosteroid injections. But the scope of practice is wider. You become the dermatologist for hundreds of miles in every direction, managing most conditions locally while referring select cases to subspecialty centers.
Case variety is striking. Neglected tumors, unusual inflammatory presentations, pediatric conditions, and occupational dermatoses/injuries appear alongside the routine. Each day requires flexibility, judgment, confidence, and the ability to think outside the box. You must consider how a patient’s seasonal work, such as ranching or farming, and/or their total commute time impacts the risk-benefit discussion around treatment recommendations.
Matthew P. Shaffer, MD (Salina, Kansas), who has practiced rural dermatology for more than 20 years, explained that the breadth of dermatologic cases in which he served as the expert was both exciting and intimidating, but it became clear that this was the right professional path for him (email communication, September 5, 2025). In small communities, your role extends beyond the clinic walls. You will see patients at the grocery store, the library, and school events. That continuity fosters loyalty and accountability in ways that are hard to quantify.
Many practice structures exist: independent clinics, multispecialty groups, hospital employment, and increasingly, hybrid partnerships with academic centers.
Academic institutions have recognized the importance of rural exposure, and many now collaborate with rural dermatologists. For example, Heartland Dermatology in Salina, Kansas, where 2 of the authors (B.R.L. and T.G.) practice, partners with St. Louis University in Missouri to provide a residency track and rotations in rural clinics.
Rural-based hospital systems can create similar structures. Monument Health Dermatology in Spearfish, South Dakota, is integrated into the fabric of the community’s larger rural health care model. The physician (M.E.L.) collaborates daily with primary care providers, surgeons, and oncologists through a shared electronic health record (sometimes even through telephone speed-dial given the close collegiality of small-town providers). Patients come from across 4 states, some driving 6 hours each way. Patients who once doubted whether dermatology was worth the trip will consistently return for follow-up care once trust is earned. The stability of hospital employment supports volunteer faculty positions and a free satellite clinic in partnership with a local Lakota Tribal health center. There is never a dull day: the providers see urgent add-ons daily, which keeps them on their toes but in exchange brings immense reward. This includes a recent case from rural Wyoming: a complex mixed infantile hemangioma on the mid face just entering the rapid proliferation phase. Propranolol was started immediately, as opposed to months later when it was too late—a common complication for the majority of rural patients by the time to get to a dermatologist.
Complex cases can overwhelm rural practices, and this is when the hub-and-spoke model is invaluable. Dermatologists embed in local communities as spokes, while subspecialty services such as pediatric dermatology, dermatopathology, or Mohs micrographic surgery remain centralized at hubs. The hubs can be but do not have to be academic institutions; for Heartland Dermatology in Kansas, private practices fulfill both hub and spoke roles. With that said, 10 states do not have academic dermatology programs.1 Mohs surgeons and pediatric dermatologists still can establish robust and successful independent rural subspecialty practices outside academic hubs. Christopher Gasbarre, DO (Spearfish, South Dakota), a board-certified, fellowship-trained Mohs surgeon in rural practice, advises residents to be confident in their abilities and to trust their training, noting that they often will be asked to manage complicated cases because of patient travel and cost constraints; however, clinicians should recognize their own limitations and those of nearby specialists and develop a referral network for cases that require multidisciplinary care (text communication, September 14, 2025).
The hub-and-spoke models—whether they entail an academic center as the hub with private practices as the spokes, or a network of private practices that include rural subspecialists—allows rural dermatologists to remain trusted local experts while ensuring that patients can access advanced care via a more streamlined referral process/network. The challenge is triage: what can be managed locally and what must patients travel for? As Dr. Shaffer explained, decisions about whether care is managed locally or referred to a hub often depend on the experience and comfort level of both the physician and the patient (email communication, September 5, 2025). Ultimately, continuity and trust are central. Patients rely on their local dermatologist to guide these decisions, and that guidance makes the model effective.
The idea that rural practice means being stuck in a small solo clinic is outdated. Multiple pathways exist, each with strengths and challenges. Independent private practice offers maximum autonomy and deep community integration, though financial and staffing risks are yours to manage. Hospital employment with outreach clinics provides stability, benefits, and collegiality, but bureaucracy can limit innovation and efficiency. Private equity platforms supply resources and rapid growth, but alignment with mission and autonomy must be weighed carefully. Hybrid joint ventures with hospitals combine private control and institutional support, but contracts can be complex. Locum tenens–to-permanent arrangements let you try rural life with minimal commitment, but continuity with patients may be sacrificed. A self-screener can clarify your path: How much autonomy do I want? Do I prefer predictability or variety? How important are procedures, teaching, or community roles? Answer these questions honestly and pair that insight with mentor guidance.
Launching a rural dermatology clinic is equal parts vision and structure. A focused 90-day plan can make the difference between a smooth opening and early frustration. Think in 4 domains: site selection, employment and licensing, credentialing and contracting, and operations. Even in a compressed timeline, dozens of small but crucial tasks may surface. There are resources—such as the Medical Group Management Association’s practice start-up checklist—that can provide a roadmap, ensuring no detail is overlooked as you transform a vision into a functioning clinic.2
Site Selection—First, determine whether you are opening a new standalone clinic, extending an existing practice, or creating a part-time satellite. Referral mapping with local primary care providers is essential, as is a scan of payer mix and dermatologist density in the region to ensure sustainability.
Employment and Licensing—Confirm state licensure and Drug Enforcement Administration registration and initiate hospital privileges early. These processes can stretch across the entire 90-day window, so starting immediately is critical.
Credentialing and Contracting—Applications with commercial and federal payers, along with Council for Affordable Quality Healthcare updates, often consume weeks if not months. If you plan to perform office microscopy or establish a dermatopathology laboratory, begin the Clinical Laboratory Improvement Amendments certification process in parallel.
Operations—Once the regulatory wheels are in motion, shift to building your practice infrastructure. Secure space, weigh lease vs purchase, and consider partnerships with local hospitals for shared clinic facilities. Recruit staff with dermatology-specific skills such as clinical photography and biopsy assistance. Implement an electronic health record, set up payroll and malpractice insurance, and establish supply chains for everything from liquid nitrogen to surgical trays. Decide whether revenue cycle management will be in-house or outsourced and finalize dermatopathology workflows including courier and transport agreements.
Compensation in rural dermatology mirrors that of other clinical settings: base salary with productivity bonuses, revenue pooling, or relative value unit structures. Financial planning is crucial. Develop a pro forma that models patient volume, expenses, and realistic growth. Risks exist, including payer mix, staffing, and competition, but the demand for care in underserved areas often offsets these, and communities may support practices with reduced overhead and strong loyalty. Hospital systems may add stipends for supervising advanced practitioners or outreach travel. Loan repayment programs, tax credits, and grants can further enhance packages. Consider checking with the state’s Office of Rural Health.
Career sustainability ultimately depends on more than finances. Geography, amenities, schedule flexibility, autonomy in medical decision-making, work-life balance, the value of being part of and serving a community, and other personal values will shape your “best-fit” practice model. Ask whether you can envision yourself thriving in the community you would be serving.
No one builds a rural dermatology practice alone. That is why one of the authors (M.E.L.) created the Rural Access to Dermatology Society (https://www.radsociety.org/), a nonprofit organization connecting dermatologists, residents, and medical students with a shared mission. The organization supports residents through scholarships, mentorship, and telementoring. Faculty can contribute through advocacy, residency track development, and outreach to uniquely underserved rural populations such as Native American reservations where access to dermatology care remains severely limited. Joining can be as simple as attending a webinar, finding a mentor, or volunteering at a free clinic. You do not need to launch your own clinic to get involved; you can begin by connecting with a network already laying the foundation.
Teledermatology and academic initiatives enhance rural care but do not replace in-person practice. Store-and-forward consultations extend reach but cannot match the continuity and trust of long-term patient relationships. Academic rural tracks prepare residents for unique challenges, but someone must staff the clinics. Private and hybrid models remain the backbone of rural access, where dermatologists take on the responsibility and the joy of being the local expert.
So here’s the invitation: bring one question to your mentor about rural practice and identify one rural site you could visit. The road less traveled in dermatology is closer than you think—and it might just be your path.
The myths persist: You will lack colleagues. Your practice will be thin. You must sacrifice academic engagement. In reality, rural practice offers variety, leadership opportunities, and the chance to influence the health of entire communities in profound ways. In this article, we aim to unpack what rural dermatology actually looks like as a potential career path for residents, with a focus on private-academic hybrid and hospital-based practice models.
Definitions of the term rural vary. For the US Census Bureau, it is synonymous with nonurban, and for the Office of Management and Budget, the term nonmetropolitan is preferred. The US Department of Agriculture’s Rural-Urban Commuting Area codes recognize a continuum of classifications from micropolitan to remote. In practice, the term rural covers a wide spectrum: the rolling farmlands of the Midwest, the mountains of Montana, the bayous of the South, the Native American reservations in New Mexico, and everything in between. It is not one uniform reality—rural America is diverse, resilient, and deeply connected.
Daily clinic flow may look familiar: a full schedule, a mix of new and established patients, and frequent simple procedures such as biopsies and corticosteroid injections. But the scope of practice is wider. You become the dermatologist for hundreds of miles in every direction, managing most conditions locally while referring select cases to subspecialty centers.
Case variety is striking. Neglected tumors, unusual inflammatory presentations, pediatric conditions, and occupational dermatoses/injuries appear alongside the routine. Each day requires flexibility, judgment, confidence, and the ability to think outside the box. You must consider how a patient’s seasonal work, such as ranching or farming, and/or their total commute time impacts the risk-benefit discussion around treatment recommendations.
Matthew P. Shaffer, MD (Salina, Kansas), who has practiced rural dermatology for more than 20 years, explained that the breadth of dermatologic cases in which he served as the expert was both exciting and intimidating, but it became clear that this was the right professional path for him (email communication, September 5, 2025). In small communities, your role extends beyond the clinic walls. You will see patients at the grocery store, the library, and school events. That continuity fosters loyalty and accountability in ways that are hard to quantify.
Many practice structures exist: independent clinics, multispecialty groups, hospital employment, and increasingly, hybrid partnerships with academic centers.
Academic institutions have recognized the importance of rural exposure, and many now collaborate with rural dermatologists. For example, Heartland Dermatology in Salina, Kansas, where 2 of the authors (B.R.L. and T.G.) practice, partners with St. Louis University in Missouri to provide a residency track and rotations in rural clinics.
Rural-based hospital systems can create similar structures. Monument Health Dermatology in Spearfish, South Dakota, is integrated into the fabric of the community’s larger rural health care model. The physician (M.E.L.) collaborates daily with primary care providers, surgeons, and oncologists through a shared electronic health record (sometimes even through telephone speed-dial given the close collegiality of small-town providers). Patients come from across 4 states, some driving 6 hours each way. Patients who once doubted whether dermatology was worth the trip will consistently return for follow-up care once trust is earned. The stability of hospital employment supports volunteer faculty positions and a free satellite clinic in partnership with a local Lakota Tribal health center. There is never a dull day: the providers see urgent add-ons daily, which keeps them on their toes but in exchange brings immense reward. This includes a recent case from rural Wyoming: a complex mixed infantile hemangioma on the mid face just entering the rapid proliferation phase. Propranolol was started immediately, as opposed to months later when it was too late—a common complication for the majority of rural patients by the time to get to a dermatologist.
Complex cases can overwhelm rural practices, and this is when the hub-and-spoke model is invaluable. Dermatologists embed in local communities as spokes, while subspecialty services such as pediatric dermatology, dermatopathology, or Mohs micrographic surgery remain centralized at hubs. The hubs can be but do not have to be academic institutions; for Heartland Dermatology in Kansas, private practices fulfill both hub and spoke roles. With that said, 10 states do not have academic dermatology programs.1 Mohs surgeons and pediatric dermatologists still can establish robust and successful independent rural subspecialty practices outside academic hubs. Christopher Gasbarre, DO (Spearfish, South Dakota), a board-certified, fellowship-trained Mohs surgeon in rural practice, advises residents to be confident in their abilities and to trust their training, noting that they often will be asked to manage complicated cases because of patient travel and cost constraints; however, clinicians should recognize their own limitations and those of nearby specialists and develop a referral network for cases that require multidisciplinary care (text communication, September 14, 2025).
The hub-and-spoke models—whether they entail an academic center as the hub with private practices as the spokes, or a network of private practices that include rural subspecialists—allows rural dermatologists to remain trusted local experts while ensuring that patients can access advanced care via a more streamlined referral process/network. The challenge is triage: what can be managed locally and what must patients travel for? As Dr. Shaffer explained, decisions about whether care is managed locally or referred to a hub often depend on the experience and comfort level of both the physician and the patient (email communication, September 5, 2025). Ultimately, continuity and trust are central. Patients rely on their local dermatologist to guide these decisions, and that guidance makes the model effective.
The idea that rural practice means being stuck in a small solo clinic is outdated. Multiple pathways exist, each with strengths and challenges. Independent private practice offers maximum autonomy and deep community integration, though financial and staffing risks are yours to manage. Hospital employment with outreach clinics provides stability, benefits, and collegiality, but bureaucracy can limit innovation and efficiency. Private equity platforms supply resources and rapid growth, but alignment with mission and autonomy must be weighed carefully. Hybrid joint ventures with hospitals combine private control and institutional support, but contracts can be complex. Locum tenens–to-permanent arrangements let you try rural life with minimal commitment, but continuity with patients may be sacrificed. A self-screener can clarify your path: How much autonomy do I want? Do I prefer predictability or variety? How important are procedures, teaching, or community roles? Answer these questions honestly and pair that insight with mentor guidance.
Launching a rural dermatology clinic is equal parts vision and structure. A focused 90-day plan can make the difference between a smooth opening and early frustration. Think in 4 domains: site selection, employment and licensing, credentialing and contracting, and operations. Even in a compressed timeline, dozens of small but crucial tasks may surface. There are resources—such as the Medical Group Management Association’s practice start-up checklist—that can provide a roadmap, ensuring no detail is overlooked as you transform a vision into a functioning clinic.2
Site Selection—First, determine whether you are opening a new standalone clinic, extending an existing practice, or creating a part-time satellite. Referral mapping with local primary care providers is essential, as is a scan of payer mix and dermatologist density in the region to ensure sustainability.
Employment and Licensing—Confirm state licensure and Drug Enforcement Administration registration and initiate hospital privileges early. These processes can stretch across the entire 90-day window, so starting immediately is critical.
Credentialing and Contracting—Applications with commercial and federal payers, along with Council for Affordable Quality Healthcare updates, often consume weeks if not months. If you plan to perform office microscopy or establish a dermatopathology laboratory, begin the Clinical Laboratory Improvement Amendments certification process in parallel.
Operations—Once the regulatory wheels are in motion, shift to building your practice infrastructure. Secure space, weigh lease vs purchase, and consider partnerships with local hospitals for shared clinic facilities. Recruit staff with dermatology-specific skills such as clinical photography and biopsy assistance. Implement an electronic health record, set up payroll and malpractice insurance, and establish supply chains for everything from liquid nitrogen to surgical trays. Decide whether revenue cycle management will be in-house or outsourced and finalize dermatopathology workflows including courier and transport agreements.
Compensation in rural dermatology mirrors that of other clinical settings: base salary with productivity bonuses, revenue pooling, or relative value unit structures. Financial planning is crucial. Develop a pro forma that models patient volume, expenses, and realistic growth. Risks exist, including payer mix, staffing, and competition, but the demand for care in underserved areas often offsets these, and communities may support practices with reduced overhead and strong loyalty. Hospital systems may add stipends for supervising advanced practitioners or outreach travel. Loan repayment programs, tax credits, and grants can further enhance packages. Consider checking with the state’s Office of Rural Health.
Career sustainability ultimately depends on more than finances. Geography, amenities, schedule flexibility, autonomy in medical decision-making, work-life balance, the value of being part of and serving a community, and other personal values will shape your “best-fit” practice model. Ask whether you can envision yourself thriving in the community you would be serving.
No one builds a rural dermatology practice alone. That is why one of the authors (M.E.L.) created the Rural Access to Dermatology Society (https://www.radsociety.org/), a nonprofit organization connecting dermatologists, residents, and medical students with a shared mission. The organization supports residents through scholarships, mentorship, and telementoring. Faculty can contribute through advocacy, residency track development, and outreach to uniquely underserved rural populations such as Native American reservations where access to dermatology care remains severely limited. Joining can be as simple as attending a webinar, finding a mentor, or volunteering at a free clinic. You do not need to launch your own clinic to get involved; you can begin by connecting with a network already laying the foundation.
Teledermatology and academic initiatives enhance rural care but do not replace in-person practice. Store-and-forward consultations extend reach but cannot match the continuity and trust of long-term patient relationships. Academic rural tracks prepare residents for unique challenges, but someone must staff the clinics. Private and hybrid models remain the backbone of rural access, where dermatologists take on the responsibility and the joy of being the local expert.
So here’s the invitation: bring one question to your mentor about rural practice and identify one rural site you could visit. The road less traveled in dermatology is closer than you think—and it might just be your path.
- Association of American Medical Colleges. ERAS Directory: Dermatology. Accessed December 11, 2025. https://systems.aamc.org/eras/erasstats/par/display.cfm?NAV_ROW=PAR&SPEC_CD=080
- Medical Group Management Association. Large group or organization practice startup checklist. Accessed December 11, 2025. https://www.mgma.com/member-tools/large-group-or-organization -practice-startup-checklist
- Association of American Medical Colleges. ERAS Directory: Dermatology. Accessed December 11, 2025. https://systems.aamc.org/eras/erasstats/par/display.cfm?NAV_ROW=PAR&SPEC_CD=080
- Medical Group Management Association. Large group or organization practice startup checklist. Accessed December 11, 2025. https://www.mgma.com/member-tools/large-group-or-organization -practice-startup-checklist
The Road Less Traveled: Why Rural Dermatology Could Be Your Path After Residency
The Road Less Traveled: Why Rural Dermatology Could Be Your Path After Residency
Cobblestonelike Papules on the Neck
The Diagnosis: Fibroelastolytic Papulosis
Histopathology demonstrated decreased density and fragmentation of elastic fibers in the superficial reticular and papillary dermis consistent with an elastolytic disease process (Figure). Of note, elastolysis typically is visualized with Verhoeff-van Gieson stain but cannot be visualized well with standard hematoxylin and eosin staining. Additional staining with Congo red was negative for amyloid, and colloidal iron did not show any increase in dermal mucin, ruling out amyloidosis and scleromyxedema, respectively. Based on the histopathologic findings and the clinical history, a diagnosis of fibroelastolytic papulosis (FP) was made. Given the benign nature of the condition, the patient was prescribed a topical steroid (clobetasol 0.05%) for symptomatic relief.
Cutaneous conditions can arise from abnormalities in the elastin composition of connective tissue due to abnormal elastin formation or degradation (elastolysis).1 Fibroelastolytic papulosis is a distinct elastolytic disorder diagnosed histologically by a notable loss of elastic fibers localized to the papillary dermis.2 Fibroelastolytic papulosis is an acquired condition linked to exposure to UV radiation, abnormal elastogenesis, and hormonal factors that commonly involves the neck, supraclavicular area, and upper back.1-3 Predominantly affecting elderly women, FP is characterized by soft white papules that often coalesce into a cobblestonelike plaque.2 Because the condition rarely is seen in men, there is speculation that it may involve genetic, hereditary, and hormonal factors that have yet to be identified.1
Fibroelastolytic papulosis can be classified as either pseudoxanthoma elasticum–like papillary dermal elastolysis or white fibrous papulosis.2,3 White fibrous papulosis manifests with haphazardly arranged collagen fibers in the reticular and deep dermis with papillary dermal elastolysis and most commonly develops on the neck.3 Although our patient’s lesion was on the neck, the absence of thickened collagen bands on histology supported classification as the pseudoxanthoma elasticum– like papillary dermal elastolysis subtype.
Fibroelastolytic papulosis can be distinguished from other elastic abnormalities by its characteristic clinical appearance, demographic distribution, and associated histopathologic findings. The differential diagnosis of FP includes pseudoxanthoma elasticum (PXE), anetoderma, scleromyxedema, and lichen amyloidosis.
Pseudoxanthoma elasticum is a hereditary or acquired multisystem disease characterized by fragmentation and calcification of elastic fibers in the mid dermis.1,4 Its clinical presentation resembles that of FP, appearing as small, asymptomatic, yellowish or flesh-colored papules in a reticular pattern that progressively coalesce into larger plaques with a cobblestonelike appearance.1 Like FP, PXE commonly affects the flexural creases in women but in contrast may manifest earlier (ie, second or third decades of life). Additionally, the pathogenesis of PXE is not related to UV radiation exposure. The hereditary form develops due to a gene variation, whereas the acquired form may be due to conditions associated with physiologic and/or mechanical stress.1
Anetoderma, also known as macular atrophy, is another condition that demonstrates elastic tissue loss in the dermis on histopathology.1 Anetoderma commonly is seen in younger patients and can be differentiated from FP by the antecedent presence of an inflammatory process. Anetoderma is classified as primary or secondary. Primary anetoderma is associated with prothrombotic abnormalities, while secondary anetoderma is associated with systemic disease including but not limited to sarcoidosis, systemic lupus erythematous, and Graves disease.1
Neither lichen myxedematosus (LM) nor lichen amyloidosis (LA) are true elastolytic conditions. Lichen myxedematosus is considered in the differential diagnosis of FP due to the associated loss of elastin observed with disease progression. An idiopathic cutaneous mucinosis, LM is a localized form of scleromyxedema, which is characterized by small, firm, waxy papules; mucin deposition in the skin; fibroblast proliferation; and fibrosis. On histologic analysis, typical findings of LM include irregularly arranged fibroblasts, diffuse mucin deposition within the upper and mid reticular dermis, increased collagen deposition, and a decrease in elastin fibers.5
Lichen amyloidosis is a subtype of primary localized cutaneous amyloidosis, a rare condition characterized by the extracellular deposition of amyloid proteins in the skin and a lack of systemic involvement. Although it is not an elastolytic condition, LA is clinically similar to FP, often manifesting as multiple localized, pruritic, hyperpigmented papules that can coalesce into larger plaques; it tends to develop on the shins, calves, ankles, and thighs.6,7 The condition commonly manifests in the fifth and sixth decades of life; however, in contrast to FP, LA is more prevalent in men and individuals from Central and South American as well as Middle Eastern and non-Chinese Asian populations.8 Lichen amyloidosis is a keratin-derived amyloidosis with cytokeratin-based amyloid precursors that only deposit in the dermis.6 Histopathology reveals colloid bodies due to the presence of apoptotic basal keratinocytes. The etiology of LA is unknown, but on rare occasions it has been associated with multiple endocrine neoplasia 2A rearranged during transfection mutations.6
In summary, FP is an uncommonly diagnosed elastolytic condition that often is asymptomatic or associated with mild pruritus. Biopsy is warranted to help differentiate it from mimicker conditions that may be associated with systemic disease. Currently, there is no established therapy that provides successful treatment. Research suggests unsatisfactory results with the use of topical tretinoin or topical antioxidants.3 More recently, nonablative fractional resurfacing lasers have been evaluated as a possible therapeutic strategy of promise for elastic disorders.9
- Andrés-Ramos I, Alegría-Landa V, Gimeno I, et al. Cutaneous elastic tissue anomalies. Am J Dermatopathol. 2019;41:85-117. doi:10.1097/DAD.0000000000001275
- Valbuena V, Assaad D, Yeung J. Pseudoxanthoma elasticum-like papillary dermal elastolysis: a single case report. J Cutan Med Surg. 2017;21:345-347. doi:10.1177/1203475417699407
- Dokic Y, Tschen J. White fibrous papulosis of the axillae and neck. Cureus. 2020;12:E7635. doi:10.7759/cureus.7635
- Recio-Monescillo M, Torre-Castro J, Manzanas C, et al. Papillary dermal elastolysis histopathology mimicking folliculotropic mycosis fungoides. J Cutan Pathol. 2023;50:430-433. doi:10.1111/cup.14402
- Cokonis Georgakis CD, Falasca G, Georgakis A, et al. Scleromyxedema. Clin Dermatol. 2006;24:493-497. doi:10.1016/j.clindermatol.2006.07.011
- Weidner T, Illing T, Elsner P. Primary localized cutaneous amyloidosis: a systematic treatment review. Am J Clin Dermatol. 2017;18:629-642. doi:10.1007/s40257-017-0278-9
- Ladizinski B, Lee KC. Lichen amyloidosis. CMAJ. 2014;186:532. doi:10.1503/cmaj.130698
- Chen JF, Chen YF. Answer: can you identify this condition? Can Fam Physician. 2012;58:1234-1235.
- Foering K, Torbeck RL, Frank MP, et al. Treatment of pseudoxanthoma elasticum-like papillary dermal elastolysis with nonablative fractional resurfacing laser resulting in clinical and histologic improvement in elastin and collagen. J Cosmet Laser Ther. 2018;20:382-384. doi:10.1080/14764172.2017.1358457
The Diagnosis: Fibroelastolytic Papulosis
Histopathology demonstrated decreased density and fragmentation of elastic fibers in the superficial reticular and papillary dermis consistent with an elastolytic disease process (Figure). Of note, elastolysis typically is visualized with Verhoeff-van Gieson stain but cannot be visualized well with standard hematoxylin and eosin staining. Additional staining with Congo red was negative for amyloid, and colloidal iron did not show any increase in dermal mucin, ruling out amyloidosis and scleromyxedema, respectively. Based on the histopathologic findings and the clinical history, a diagnosis of fibroelastolytic papulosis (FP) was made. Given the benign nature of the condition, the patient was prescribed a topical steroid (clobetasol 0.05%) for symptomatic relief.

Cutaneous conditions can arise from abnormalities in the elastin composition of connective tissue due to abnormal elastin formation or degradation (elastolysis).1 Fibroelastolytic papulosis is a distinct elastolytic disorder diagnosed histologically by a notable loss of elastic fibers localized to the papillary dermis.2 Fibroelastolytic papulosis is an acquired condition linked to exposure to UV radiation, abnormal elastogenesis, and hormonal factors that commonly involves the neck, supraclavicular area, and upper back.1-3 Predominantly affecting elderly women, FP is characterized by soft white papules that often coalesce into a cobblestonelike plaque.2 Because the condition rarely is seen in men, there is speculation that it may involve genetic, hereditary, and hormonal factors that have yet to be identified.1
Fibroelastolytic papulosis can be classified as either pseudoxanthoma elasticum–like papillary dermal elastolysis or white fibrous papulosis.2,3 White fibrous papulosis manifests with haphazardly arranged collagen fibers in the reticular and deep dermis with papillary dermal elastolysis and most commonly develops on the neck.3 Although our patient’s lesion was on the neck, the absence of thickened collagen bands on histology supported classification as the pseudoxanthoma elasticum– like papillary dermal elastolysis subtype.
Fibroelastolytic papulosis can be distinguished from other elastic abnormalities by its characteristic clinical appearance, demographic distribution, and associated histopathologic findings. The differential diagnosis of FP includes pseudoxanthoma elasticum (PXE), anetoderma, scleromyxedema, and lichen amyloidosis.
Pseudoxanthoma elasticum is a hereditary or acquired multisystem disease characterized by fragmentation and calcification of elastic fibers in the mid dermis.1,4 Its clinical presentation resembles that of FP, appearing as small, asymptomatic, yellowish or flesh-colored papules in a reticular pattern that progressively coalesce into larger plaques with a cobblestonelike appearance.1 Like FP, PXE commonly affects the flexural creases in women but in contrast may manifest earlier (ie, second or third decades of life). Additionally, the pathogenesis of PXE is not related to UV radiation exposure. The hereditary form develops due to a gene variation, whereas the acquired form may be due to conditions associated with physiologic and/or mechanical stress.1
Anetoderma, also known as macular atrophy, is another condition that demonstrates elastic tissue loss in the dermis on histopathology.1 Anetoderma commonly is seen in younger patients and can be differentiated from FP by the antecedent presence of an inflammatory process. Anetoderma is classified as primary or secondary. Primary anetoderma is associated with prothrombotic abnormalities, while secondary anetoderma is associated with systemic disease including but not limited to sarcoidosis, systemic lupus erythematous, and Graves disease.1
Neither lichen myxedematosus (LM) nor lichen amyloidosis (LA) are true elastolytic conditions. Lichen myxedematosus is considered in the differential diagnosis of FP due to the associated loss of elastin observed with disease progression. An idiopathic cutaneous mucinosis, LM is a localized form of scleromyxedema, which is characterized by small, firm, waxy papules; mucin deposition in the skin; fibroblast proliferation; and fibrosis. On histologic analysis, typical findings of LM include irregularly arranged fibroblasts, diffuse mucin deposition within the upper and mid reticular dermis, increased collagen deposition, and a decrease in elastin fibers.5
Lichen amyloidosis is a subtype of primary localized cutaneous amyloidosis, a rare condition characterized by the extracellular deposition of amyloid proteins in the skin and a lack of systemic involvement. Although it is not an elastolytic condition, LA is clinically similar to FP, often manifesting as multiple localized, pruritic, hyperpigmented papules that can coalesce into larger plaques; it tends to develop on the shins, calves, ankles, and thighs.6,7 The condition commonly manifests in the fifth and sixth decades of life; however, in contrast to FP, LA is more prevalent in men and individuals from Central and South American as well as Middle Eastern and non-Chinese Asian populations.8 Lichen amyloidosis is a keratin-derived amyloidosis with cytokeratin-based amyloid precursors that only deposit in the dermis.6 Histopathology reveals colloid bodies due to the presence of apoptotic basal keratinocytes. The etiology of LA is unknown, but on rare occasions it has been associated with multiple endocrine neoplasia 2A rearranged during transfection mutations.6
In summary, FP is an uncommonly diagnosed elastolytic condition that often is asymptomatic or associated with mild pruritus. Biopsy is warranted to help differentiate it from mimicker conditions that may be associated with systemic disease. Currently, there is no established therapy that provides successful treatment. Research suggests unsatisfactory results with the use of topical tretinoin or topical antioxidants.3 More recently, nonablative fractional resurfacing lasers have been evaluated as a possible therapeutic strategy of promise for elastic disorders.9
The Diagnosis: Fibroelastolytic Papulosis
Histopathology demonstrated decreased density and fragmentation of elastic fibers in the superficial reticular and papillary dermis consistent with an elastolytic disease process (Figure). Of note, elastolysis typically is visualized with Verhoeff-van Gieson stain but cannot be visualized well with standard hematoxylin and eosin staining. Additional staining with Congo red was negative for amyloid, and colloidal iron did not show any increase in dermal mucin, ruling out amyloidosis and scleromyxedema, respectively. Based on the histopathologic findings and the clinical history, a diagnosis of fibroelastolytic papulosis (FP) was made. Given the benign nature of the condition, the patient was prescribed a topical steroid (clobetasol 0.05%) for symptomatic relief.

Cutaneous conditions can arise from abnormalities in the elastin composition of connective tissue due to abnormal elastin formation or degradation (elastolysis).1 Fibroelastolytic papulosis is a distinct elastolytic disorder diagnosed histologically by a notable loss of elastic fibers localized to the papillary dermis.2 Fibroelastolytic papulosis is an acquired condition linked to exposure to UV radiation, abnormal elastogenesis, and hormonal factors that commonly involves the neck, supraclavicular area, and upper back.1-3 Predominantly affecting elderly women, FP is characterized by soft white papules that often coalesce into a cobblestonelike plaque.2 Because the condition rarely is seen in men, there is speculation that it may involve genetic, hereditary, and hormonal factors that have yet to be identified.1
Fibroelastolytic papulosis can be classified as either pseudoxanthoma elasticum–like papillary dermal elastolysis or white fibrous papulosis.2,3 White fibrous papulosis manifests with haphazardly arranged collagen fibers in the reticular and deep dermis with papillary dermal elastolysis and most commonly develops on the neck.3 Although our patient’s lesion was on the neck, the absence of thickened collagen bands on histology supported classification as the pseudoxanthoma elasticum– like papillary dermal elastolysis subtype.
Fibroelastolytic papulosis can be distinguished from other elastic abnormalities by its characteristic clinical appearance, demographic distribution, and associated histopathologic findings. The differential diagnosis of FP includes pseudoxanthoma elasticum (PXE), anetoderma, scleromyxedema, and lichen amyloidosis.
Pseudoxanthoma elasticum is a hereditary or acquired multisystem disease characterized by fragmentation and calcification of elastic fibers in the mid dermis.1,4 Its clinical presentation resembles that of FP, appearing as small, asymptomatic, yellowish or flesh-colored papules in a reticular pattern that progressively coalesce into larger plaques with a cobblestonelike appearance.1 Like FP, PXE commonly affects the flexural creases in women but in contrast may manifest earlier (ie, second or third decades of life). Additionally, the pathogenesis of PXE is not related to UV radiation exposure. The hereditary form develops due to a gene variation, whereas the acquired form may be due to conditions associated with physiologic and/or mechanical stress.1
Anetoderma, also known as macular atrophy, is another condition that demonstrates elastic tissue loss in the dermis on histopathology.1 Anetoderma commonly is seen in younger patients and can be differentiated from FP by the antecedent presence of an inflammatory process. Anetoderma is classified as primary or secondary. Primary anetoderma is associated with prothrombotic abnormalities, while secondary anetoderma is associated with systemic disease including but not limited to sarcoidosis, systemic lupus erythematous, and Graves disease.1
Neither lichen myxedematosus (LM) nor lichen amyloidosis (LA) are true elastolytic conditions. Lichen myxedematosus is considered in the differential diagnosis of FP due to the associated loss of elastin observed with disease progression. An idiopathic cutaneous mucinosis, LM is a localized form of scleromyxedema, which is characterized by small, firm, waxy papules; mucin deposition in the skin; fibroblast proliferation; and fibrosis. On histologic analysis, typical findings of LM include irregularly arranged fibroblasts, diffuse mucin deposition within the upper and mid reticular dermis, increased collagen deposition, and a decrease in elastin fibers.5
Lichen amyloidosis is a subtype of primary localized cutaneous amyloidosis, a rare condition characterized by the extracellular deposition of amyloid proteins in the skin and a lack of systemic involvement. Although it is not an elastolytic condition, LA is clinically similar to FP, often manifesting as multiple localized, pruritic, hyperpigmented papules that can coalesce into larger plaques; it tends to develop on the shins, calves, ankles, and thighs.6,7 The condition commonly manifests in the fifth and sixth decades of life; however, in contrast to FP, LA is more prevalent in men and individuals from Central and South American as well as Middle Eastern and non-Chinese Asian populations.8 Lichen amyloidosis is a keratin-derived amyloidosis with cytokeratin-based amyloid precursors that only deposit in the dermis.6 Histopathology reveals colloid bodies due to the presence of apoptotic basal keratinocytes. The etiology of LA is unknown, but on rare occasions it has been associated with multiple endocrine neoplasia 2A rearranged during transfection mutations.6
In summary, FP is an uncommonly diagnosed elastolytic condition that often is asymptomatic or associated with mild pruritus. Biopsy is warranted to help differentiate it from mimicker conditions that may be associated with systemic disease. Currently, there is no established therapy that provides successful treatment. Research suggests unsatisfactory results with the use of topical tretinoin or topical antioxidants.3 More recently, nonablative fractional resurfacing lasers have been evaluated as a possible therapeutic strategy of promise for elastic disorders.9
- Andrés-Ramos I, Alegría-Landa V, Gimeno I, et al. Cutaneous elastic tissue anomalies. Am J Dermatopathol. 2019;41:85-117. doi:10.1097/DAD.0000000000001275
- Valbuena V, Assaad D, Yeung J. Pseudoxanthoma elasticum-like papillary dermal elastolysis: a single case report. J Cutan Med Surg. 2017;21:345-347. doi:10.1177/1203475417699407
- Dokic Y, Tschen J. White fibrous papulosis of the axillae and neck. Cureus. 2020;12:E7635. doi:10.7759/cureus.7635
- Recio-Monescillo M, Torre-Castro J, Manzanas C, et al. Papillary dermal elastolysis histopathology mimicking folliculotropic mycosis fungoides. J Cutan Pathol. 2023;50:430-433. doi:10.1111/cup.14402
- Cokonis Georgakis CD, Falasca G, Georgakis A, et al. Scleromyxedema. Clin Dermatol. 2006;24:493-497. doi:10.1016/j.clindermatol.2006.07.011
- Weidner T, Illing T, Elsner P. Primary localized cutaneous amyloidosis: a systematic treatment review. Am J Clin Dermatol. 2017;18:629-642. doi:10.1007/s40257-017-0278-9
- Ladizinski B, Lee KC. Lichen amyloidosis. CMAJ. 2014;186:532. doi:10.1503/cmaj.130698
- Chen JF, Chen YF. Answer: can you identify this condition? Can Fam Physician. 2012;58:1234-1235.
- Foering K, Torbeck RL, Frank MP, et al. Treatment of pseudoxanthoma elasticum-like papillary dermal elastolysis with nonablative fractional resurfacing laser resulting in clinical and histologic improvement in elastin and collagen. J Cosmet Laser Ther. 2018;20:382-384. doi:10.1080/14764172.2017.1358457
- Andrés-Ramos I, Alegría-Landa V, Gimeno I, et al. Cutaneous elastic tissue anomalies. Am J Dermatopathol. 2019;41:85-117. doi:10.1097/DAD.0000000000001275
- Valbuena V, Assaad D, Yeung J. Pseudoxanthoma elasticum-like papillary dermal elastolysis: a single case report. J Cutan Med Surg. 2017;21:345-347. doi:10.1177/1203475417699407
- Dokic Y, Tschen J. White fibrous papulosis of the axillae and neck. Cureus. 2020;12:E7635. doi:10.7759/cureus.7635
- Recio-Monescillo M, Torre-Castro J, Manzanas C, et al. Papillary dermal elastolysis histopathology mimicking folliculotropic mycosis fungoides. J Cutan Pathol. 2023;50:430-433. doi:10.1111/cup.14402
- Cokonis Georgakis CD, Falasca G, Georgakis A, et al. Scleromyxedema. Clin Dermatol. 2006;24:493-497. doi:10.1016/j.clindermatol.2006.07.011
- Weidner T, Illing T, Elsner P. Primary localized cutaneous amyloidosis: a systematic treatment review. Am J Clin Dermatol. 2017;18:629-642. doi:10.1007/s40257-017-0278-9
- Ladizinski B, Lee KC. Lichen amyloidosis. CMAJ. 2014;186:532. doi:10.1503/cmaj.130698
- Chen JF, Chen YF. Answer: can you identify this condition? Can Fam Physician. 2012;58:1234-1235.
- Foering K, Torbeck RL, Frank MP, et al. Treatment of pseudoxanthoma elasticum-like papillary dermal elastolysis with nonablative fractional resurfacing laser resulting in clinical and histologic improvement in elastin and collagen. J Cosmet Laser Ther. 2018;20:382-384. doi:10.1080/14764172.2017.1358457
A 76-year-old woman presented to the dermatology clinic for evaluation of a pruritic rash on the posterior lateral neck of several years’ duration. The rash had been slowly worsening and was intermittently symptomatic. Physical examination revealed monomorphous flesh-colored papules coalescing on the neck, yielding a cobblestonelike texture. The patient had been treated previously by dermatology with topical steroids, but symptoms persisted. A punch biopsy of the left lateral neck was performed.