User login
Largest meeting on cancer research canceled: AACR
The biggest cancer research meeting of the year has been canceled as a reaction to the novel coronavirus (COVID-19) outbreak, which has also led to many other medical conferences being canceled or postponed.
The annual meeting of the American Association for Cancer Research (AACR) was due to take place April 24-29 in San Diego, California. More than 24,000 delegates from 80 countries and more than 500 exhibitors were expected to attend.
There are plans to reschedule it for later this year.
This has been a “difficult decision,” said the AACR board of directors, but “we believe that the decision to postpone the meeting is absolutely the correct one to safeguard our meeting participants from further potential exposure to the coronavirus.”
The board goes on to explain that “this evidence-based decision was made after a thorough review and discussion of all factors impacting the annual meeting, including the US government’s enforcement of restrictions on international travelers to enter the US; the imposition of travel restrictions issued by US government agencies, cancer centers, academic institutions, and pharmaceutical and biotech companies; and the counsel of infectious disease experts. It is clear that all of these elements significantly affect the ability of delegates, speakers, presenters of proffered papers, and exhibitors to participate fully in the annual meeting.”
Other cancer conferences that were planned for March and that have been canceled include the following:
- European Breast Cancer Conference (EBCC), Barcelona, Spain, which was to have taken place March 18-20. This conference has been postponed and will now take place September 30 to October 2 at the same venue. Abstracts that have been accepted for the initial conference will remain in the program, and organizers will reopen abstract submissions in May.
- National Comprehensive Cancer Network (NCCN), Orlando, Florida, was scheduled for March 19-22. This conference has been postponed. No new dates have been provided, but the society notes that “NCCN staff is working as quickly as possible to notify all conference registrants about the postponement and further information regarding the refund process.”
- European Association of Urology (EAU), Amsterdam, the Netherlands, at which there is always new research presented on prostate, kidney, and bladder cancer, was due to take place March 20-24. This conference has been postponed to July 2020.
- Society of Gynecologic Oncology (SGO), in Toronto, Canada, which was scheduled for March 28-31. SGO is “exploring alternatives for delivering the science and education.”
Overall, the move to cancel medical conferences over the next few months is a good idea, commented F. Perry Wilson, MD, MSCE, associate professor of medicine and director of Yale’s Program of Applied Translational Research, in a Medscape Medical News commentary.
“There’s a pretty straightforward case here,” he argued. “Medical professionals are at higher risk for exposure to coronavirus because we come into contact with lots and lots of patients. Gathering a large group of medical professionals in a single place increases the risk for exposure further. Factor in airplane flights to and from the conferences, and the chance that infection is spread is significant.”
This article first appeared on Medscape.com.
The biggest cancer research meeting of the year has been canceled as a reaction to the novel coronavirus (COVID-19) outbreak, which has also led to many other medical conferences being canceled or postponed.
The annual meeting of the American Association for Cancer Research (AACR) was due to take place April 24-29 in San Diego, California. More than 24,000 delegates from 80 countries and more than 500 exhibitors were expected to attend.
There are plans to reschedule it for later this year.
This has been a “difficult decision,” said the AACR board of directors, but “we believe that the decision to postpone the meeting is absolutely the correct one to safeguard our meeting participants from further potential exposure to the coronavirus.”
The board goes on to explain that “this evidence-based decision was made after a thorough review and discussion of all factors impacting the annual meeting, including the US government’s enforcement of restrictions on international travelers to enter the US; the imposition of travel restrictions issued by US government agencies, cancer centers, academic institutions, and pharmaceutical and biotech companies; and the counsel of infectious disease experts. It is clear that all of these elements significantly affect the ability of delegates, speakers, presenters of proffered papers, and exhibitors to participate fully in the annual meeting.”
Other cancer conferences that were planned for March and that have been canceled include the following:
- European Breast Cancer Conference (EBCC), Barcelona, Spain, which was to have taken place March 18-20. This conference has been postponed and will now take place September 30 to October 2 at the same venue. Abstracts that have been accepted for the initial conference will remain in the program, and organizers will reopen abstract submissions in May.
- National Comprehensive Cancer Network (NCCN), Orlando, Florida, was scheduled for March 19-22. This conference has been postponed. No new dates have been provided, but the society notes that “NCCN staff is working as quickly as possible to notify all conference registrants about the postponement and further information regarding the refund process.”
- European Association of Urology (EAU), Amsterdam, the Netherlands, at which there is always new research presented on prostate, kidney, and bladder cancer, was due to take place March 20-24. This conference has been postponed to July 2020.
- Society of Gynecologic Oncology (SGO), in Toronto, Canada, which was scheduled for March 28-31. SGO is “exploring alternatives for delivering the science and education.”
Overall, the move to cancel medical conferences over the next few months is a good idea, commented F. Perry Wilson, MD, MSCE, associate professor of medicine and director of Yale’s Program of Applied Translational Research, in a Medscape Medical News commentary.
“There’s a pretty straightforward case here,” he argued. “Medical professionals are at higher risk for exposure to coronavirus because we come into contact with lots and lots of patients. Gathering a large group of medical professionals in a single place increases the risk for exposure further. Factor in airplane flights to and from the conferences, and the chance that infection is spread is significant.”
This article first appeared on Medscape.com.
The biggest cancer research meeting of the year has been canceled as a reaction to the novel coronavirus (COVID-19) outbreak, which has also led to many other medical conferences being canceled or postponed.
The annual meeting of the American Association for Cancer Research (AACR) was due to take place April 24-29 in San Diego, California. More than 24,000 delegates from 80 countries and more than 500 exhibitors were expected to attend.
There are plans to reschedule it for later this year.
This has been a “difficult decision,” said the AACR board of directors, but “we believe that the decision to postpone the meeting is absolutely the correct one to safeguard our meeting participants from further potential exposure to the coronavirus.”
The board goes on to explain that “this evidence-based decision was made after a thorough review and discussion of all factors impacting the annual meeting, including the US government’s enforcement of restrictions on international travelers to enter the US; the imposition of travel restrictions issued by US government agencies, cancer centers, academic institutions, and pharmaceutical and biotech companies; and the counsel of infectious disease experts. It is clear that all of these elements significantly affect the ability of delegates, speakers, presenters of proffered papers, and exhibitors to participate fully in the annual meeting.”
Other cancer conferences that were planned for March and that have been canceled include the following:
- European Breast Cancer Conference (EBCC), Barcelona, Spain, which was to have taken place March 18-20. This conference has been postponed and will now take place September 30 to October 2 at the same venue. Abstracts that have been accepted for the initial conference will remain in the program, and organizers will reopen abstract submissions in May.
- National Comprehensive Cancer Network (NCCN), Orlando, Florida, was scheduled for March 19-22. This conference has been postponed. No new dates have been provided, but the society notes that “NCCN staff is working as quickly as possible to notify all conference registrants about the postponement and further information regarding the refund process.”
- European Association of Urology (EAU), Amsterdam, the Netherlands, at which there is always new research presented on prostate, kidney, and bladder cancer, was due to take place March 20-24. This conference has been postponed to July 2020.
- Society of Gynecologic Oncology (SGO), in Toronto, Canada, which was scheduled for March 28-31. SGO is “exploring alternatives for delivering the science and education.”
Overall, the move to cancel medical conferences over the next few months is a good idea, commented F. Perry Wilson, MD, MSCE, associate professor of medicine and director of Yale’s Program of Applied Translational Research, in a Medscape Medical News commentary.
“There’s a pretty straightforward case here,” he argued. “Medical professionals are at higher risk for exposure to coronavirus because we come into contact with lots and lots of patients. Gathering a large group of medical professionals in a single place increases the risk for exposure further. Factor in airplane flights to and from the conferences, and the chance that infection is spread is significant.”
This article first appeared on Medscape.com.
Late effects in young cancer survivors underscore importance of high-risk screening
according to data from the Childhood Cancer Survivor Study.
At a median follow-up of 21 years, the SMR for all-cause mortality was 5.9 among survivors aged 15-20 years and 6.2 among diagnosis-matched children under 15 years, compared with expected rates at the same ages in the general population. For health-related causes – excluding primary cancer recurrence or progression but including late effects of cancer therapy – the SMRs were 4.8 in the older group and 6.8 in the younger group.
Eugene Suh, MD, of Loyola University Chicago Medical Center, Maywood, Ill., and colleagues reported these results in Lancet Oncology.
The difference between the older and younger survivors (n = 5,804 in each group) was most evident at least 20 years after cancer diagnosis, the authors noted.
For both groups, but more so for childhood cancer survivors, the risk of developing any chronic health condition and any grade 3-5 health condition was greater than for siblings of the same age who did not have cancer (hazard ratios, 4.2 for adolescents/young adults and 5.6 for childhood survivors). The same was true for grade 3-5 cardiac conditions (HRs, 4.3 and 5.6, respectively), endocrine conditions (HRs, 3.9 and 6.4, respectively), and musculoskeletal conditions (HRs, 6.5 and 8.0, respectively).
These findings, which confirm those of previous studies suggesting that younger children might be more vulnerable to the adverse effects of cancer treatment, “underscore that focused efforts are needed to ensure early-adolescent and young adult cancer survivors are receiving recommended risk-based care, with a focus on high-risk cancer screening, to reduce morbidity and premature mortality,” the researchers concluded, noting that “studies to date indicate that adherence to such high-risk screening is poor.”
In a related editorial, Päivi Lähteenmäki, MD, PhD, of University of Turku (Finland) and Turku University Hospital, wrote that these findings warrant long-term follow-up of adolescent and young adult cancer survivors. She also argued that the results “might not be fully generalizable to patients treated today who might be on different treatment regimens to those treated in previous decades” and that “[m]ore prospectively collected objective data focusing on survivors ... are needed.”
Accurate characterization of patients at high risk who would benefit from a tailored screening program is most important, and identifying underlying genetic or molecular factors that confer higher risk for late sequelae would be useful for “planning approaches to survivorship,” Dr. Lähteenmäki added.
This study was funded by the National Cancer Institute and American Lebanese-Syrian Associated Charities. Dr. Suh and Dr. Lähteenmäki reported having no competing interests.
SOURCES: Suh E et al. Lancet Oncology. 2020 Feb 14. doi: 10.1016/S1470-2045(19)30800-9;Lähteenmäki P. Lancet Oncol. 2020 Feb 14. doi: 10.106/S1470-2045(19)30858-7.
according to data from the Childhood Cancer Survivor Study.
At a median follow-up of 21 years, the SMR for all-cause mortality was 5.9 among survivors aged 15-20 years and 6.2 among diagnosis-matched children under 15 years, compared with expected rates at the same ages in the general population. For health-related causes – excluding primary cancer recurrence or progression but including late effects of cancer therapy – the SMRs were 4.8 in the older group and 6.8 in the younger group.
Eugene Suh, MD, of Loyola University Chicago Medical Center, Maywood, Ill., and colleagues reported these results in Lancet Oncology.
The difference between the older and younger survivors (n = 5,804 in each group) was most evident at least 20 years after cancer diagnosis, the authors noted.
For both groups, but more so for childhood cancer survivors, the risk of developing any chronic health condition and any grade 3-5 health condition was greater than for siblings of the same age who did not have cancer (hazard ratios, 4.2 for adolescents/young adults and 5.6 for childhood survivors). The same was true for grade 3-5 cardiac conditions (HRs, 4.3 and 5.6, respectively), endocrine conditions (HRs, 3.9 and 6.4, respectively), and musculoskeletal conditions (HRs, 6.5 and 8.0, respectively).
These findings, which confirm those of previous studies suggesting that younger children might be more vulnerable to the adverse effects of cancer treatment, “underscore that focused efforts are needed to ensure early-adolescent and young adult cancer survivors are receiving recommended risk-based care, with a focus on high-risk cancer screening, to reduce morbidity and premature mortality,” the researchers concluded, noting that “studies to date indicate that adherence to such high-risk screening is poor.”
In a related editorial, Päivi Lähteenmäki, MD, PhD, of University of Turku (Finland) and Turku University Hospital, wrote that these findings warrant long-term follow-up of adolescent and young adult cancer survivors. She also argued that the results “might not be fully generalizable to patients treated today who might be on different treatment regimens to those treated in previous decades” and that “[m]ore prospectively collected objective data focusing on survivors ... are needed.”
Accurate characterization of patients at high risk who would benefit from a tailored screening program is most important, and identifying underlying genetic or molecular factors that confer higher risk for late sequelae would be useful for “planning approaches to survivorship,” Dr. Lähteenmäki added.
This study was funded by the National Cancer Institute and American Lebanese-Syrian Associated Charities. Dr. Suh and Dr. Lähteenmäki reported having no competing interests.
SOURCES: Suh E et al. Lancet Oncology. 2020 Feb 14. doi: 10.1016/S1470-2045(19)30800-9;Lähteenmäki P. Lancet Oncol. 2020 Feb 14. doi: 10.106/S1470-2045(19)30858-7.
according to data from the Childhood Cancer Survivor Study.
At a median follow-up of 21 years, the SMR for all-cause mortality was 5.9 among survivors aged 15-20 years and 6.2 among diagnosis-matched children under 15 years, compared with expected rates at the same ages in the general population. For health-related causes – excluding primary cancer recurrence or progression but including late effects of cancer therapy – the SMRs were 4.8 in the older group and 6.8 in the younger group.
Eugene Suh, MD, of Loyola University Chicago Medical Center, Maywood, Ill., and colleagues reported these results in Lancet Oncology.
The difference between the older and younger survivors (n = 5,804 in each group) was most evident at least 20 years after cancer diagnosis, the authors noted.
For both groups, but more so for childhood cancer survivors, the risk of developing any chronic health condition and any grade 3-5 health condition was greater than for siblings of the same age who did not have cancer (hazard ratios, 4.2 for adolescents/young adults and 5.6 for childhood survivors). The same was true for grade 3-5 cardiac conditions (HRs, 4.3 and 5.6, respectively), endocrine conditions (HRs, 3.9 and 6.4, respectively), and musculoskeletal conditions (HRs, 6.5 and 8.0, respectively).
These findings, which confirm those of previous studies suggesting that younger children might be more vulnerable to the adverse effects of cancer treatment, “underscore that focused efforts are needed to ensure early-adolescent and young adult cancer survivors are receiving recommended risk-based care, with a focus on high-risk cancer screening, to reduce morbidity and premature mortality,” the researchers concluded, noting that “studies to date indicate that adherence to such high-risk screening is poor.”
In a related editorial, Päivi Lähteenmäki, MD, PhD, of University of Turku (Finland) and Turku University Hospital, wrote that these findings warrant long-term follow-up of adolescent and young adult cancer survivors. She also argued that the results “might not be fully generalizable to patients treated today who might be on different treatment regimens to those treated in previous decades” and that “[m]ore prospectively collected objective data focusing on survivors ... are needed.”
Accurate characterization of patients at high risk who would benefit from a tailored screening program is most important, and identifying underlying genetic or molecular factors that confer higher risk for late sequelae would be useful for “planning approaches to survivorship,” Dr. Lähteenmäki added.
This study was funded by the National Cancer Institute and American Lebanese-Syrian Associated Charities. Dr. Suh and Dr. Lähteenmäki reported having no competing interests.
SOURCES: Suh E et al. Lancet Oncology. 2020 Feb 14. doi: 10.1016/S1470-2045(19)30800-9;Lähteenmäki P. Lancet Oncol. 2020 Feb 14. doi: 10.106/S1470-2045(19)30858-7.
FROM LANCET ONCOLOGY
FDA: Cell phones still look safe
according to a review by the Food and Drug Administration.
The FDA reviewed the published literature from 2008 to 2018 and concluded that the data don’t support any quantifiable adverse health risks from RFR. However, the evidence is not without limitations.
The FDA’s evaluation included evidence from in vivo animal studies from Jan. 1, 2008, to Aug. 1, 2018, and epidemiologic studies in humans from Jan. 1, 2008, to May 8, 2018. Both kinds of evidence had limitations, but neither produced strong indications of any causal risks from cell phone use.
The FDA noted that in vivo animal studies are limited by variability of methods and RFR exposure, which make comparisons of results difficult. These studies are also impacted by the indirect effects of temperature increases (the only currently established biological effect of RFR) and stress experienced by the animals, which make teasing out the direct effects of RFR difficult.
The FDA noted that strong epidemiologic studies can provide more relevant and accurate information than in vivo studies, but epidemiologic studies are not without limitations. For example, most have participants track and self-report their cell phone use. There’s also no way to directly track certain factors of RFR exposure, such as frequency, duration, or intensity.
Even with those caveats in mind, the FDA wrote that, “based on the studies that are described in detail in this report, there is insufficient evidence to support a causal association between RFR exposure and tumorigenesis. There is a lack of clear dose-response relationship, a lack of consistent findings or specificity, and a lack of biological mechanistic plausibility.”
The full review is available on the FDA website.
according to a review by the Food and Drug Administration.
The FDA reviewed the published literature from 2008 to 2018 and concluded that the data don’t support any quantifiable adverse health risks from RFR. However, the evidence is not without limitations.
The FDA’s evaluation included evidence from in vivo animal studies from Jan. 1, 2008, to Aug. 1, 2018, and epidemiologic studies in humans from Jan. 1, 2008, to May 8, 2018. Both kinds of evidence had limitations, but neither produced strong indications of any causal risks from cell phone use.
The FDA noted that in vivo animal studies are limited by variability of methods and RFR exposure, which make comparisons of results difficult. These studies are also impacted by the indirect effects of temperature increases (the only currently established biological effect of RFR) and stress experienced by the animals, which make teasing out the direct effects of RFR difficult.
The FDA noted that strong epidemiologic studies can provide more relevant and accurate information than in vivo studies, but epidemiologic studies are not without limitations. For example, most have participants track and self-report their cell phone use. There’s also no way to directly track certain factors of RFR exposure, such as frequency, duration, or intensity.
Even with those caveats in mind, the FDA wrote that, “based on the studies that are described in detail in this report, there is insufficient evidence to support a causal association between RFR exposure and tumorigenesis. There is a lack of clear dose-response relationship, a lack of consistent findings or specificity, and a lack of biological mechanistic plausibility.”
The full review is available on the FDA website.
according to a review by the Food and Drug Administration.
The FDA reviewed the published literature from 2008 to 2018 and concluded that the data don’t support any quantifiable adverse health risks from RFR. However, the evidence is not without limitations.
The FDA’s evaluation included evidence from in vivo animal studies from Jan. 1, 2008, to Aug. 1, 2018, and epidemiologic studies in humans from Jan. 1, 2008, to May 8, 2018. Both kinds of evidence had limitations, but neither produced strong indications of any causal risks from cell phone use.
The FDA noted that in vivo animal studies are limited by variability of methods and RFR exposure, which make comparisons of results difficult. These studies are also impacted by the indirect effects of temperature increases (the only currently established biological effect of RFR) and stress experienced by the animals, which make teasing out the direct effects of RFR difficult.
The FDA noted that strong epidemiologic studies can provide more relevant and accurate information than in vivo studies, but epidemiologic studies are not without limitations. For example, most have participants track and self-report their cell phone use. There’s also no way to directly track certain factors of RFR exposure, such as frequency, duration, or intensity.
Even with those caveats in mind, the FDA wrote that, “based on the studies that are described in detail in this report, there is insufficient evidence to support a causal association between RFR exposure and tumorigenesis. There is a lack of clear dose-response relationship, a lack of consistent findings or specificity, and a lack of biological mechanistic plausibility.”
The full review is available on the FDA website.
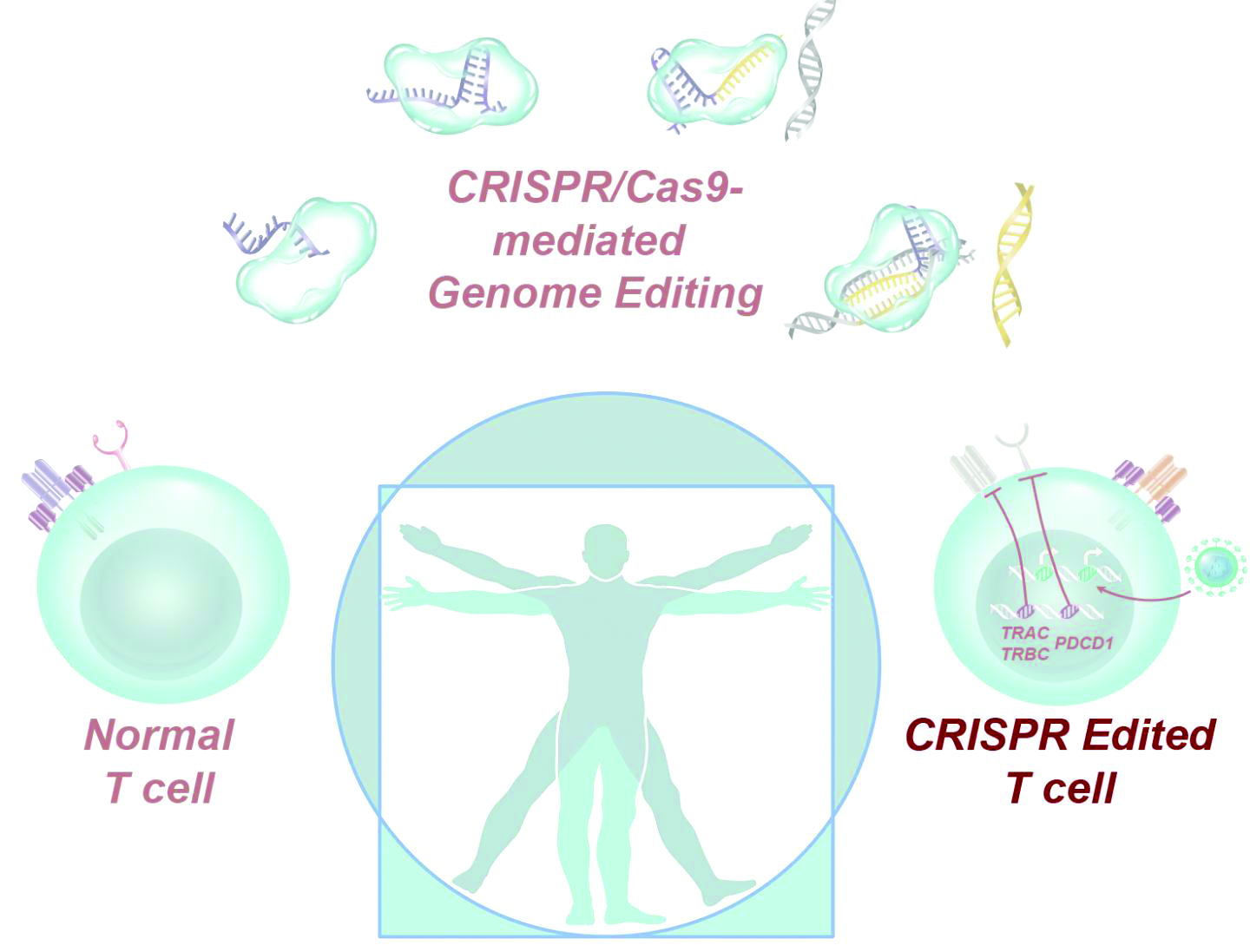
CRISPR-engineered T cells may be safe for cancer, but do they work?
according to a report in Science.
The results of no harm support this “promising” area of cancer immunotherapy, according to study investigator Edward A. Stadtmauer, MD, of the University of Pennsylvania in Philadelphia and colleagues.
However, there was no evidence of benefit in this trial. One patient transfused with CRISPR-engineered T cells has since died, and the other two have moved on to other treatments.
“The big question that remains unanswered by this study is whether gene-edited, engineered T cells are effective against advanced cancer,” Jennifer Hamilton, PhD, and Jennifer Doudna, PhD, both of the University of California, Berkeley, wrote in an accompanying editorial.
The study enrolled six patients with refractory cancer, and three of them received CRISPR-engineered T cells. Two patients had multiple myeloma, and one had metastatic sarcoma.
Dr. Stadtmauer and colleagues drew blood from the patients, isolated the T cells, and used CRISPR-Cas9 to modify the cells. The T cells were transfected with Cas9 protein complexed with single guide RNAs against TRAC and TRBC (genes encoding the T-cell receptor chains TCR-alpha and TCR-beta) as well as PDCD1 (a gene encoding programmed cell death protein 1). The T cells were then transduced with a lentiviral vector to express a transgenic NY-ESO-1 cancer-specific T-cell receptor.
The investigators expanded the cell lines and infused them back into the patients after administering lymphodepleting chemotherapy. The sarcoma patient initially had a 50% decrease in a large abdominal mass, but all three patients ultimately progressed.
The editorialists noted that gene disruption efficiencies in this study were “modest,” ranging from 15% to 45%, but the investigators used a protocol from 2016, when the study was given the go-ahead by the National Institutes of Health and the Food and Drug Administration. With current protocols, gene disruption efficiencies can exceed 90%, which means patients might do better in subsequent trials.
There was no more than mild toxicity in this trial, and most adverse events were attributed to the lymphodepleting chemotherapy.
There was concern about potential rejection of infused cells because of preexisting immune responses to Cas9, but it doesn’t seem “to be a barrier to the application of this promising technology,” the investigators said.
They noted that “the stable engraftment of our engineered T cells is remarkably different from previously reported trials ... where the half-life of the cells in blood was [about] 1 week. Biopsy specimens of bone marrow in the myeloma patients and tumor in the sarcoma patient demonstrated trafficking of the engineered T cells to the tumor in all three patients” beyond that point. The decay half-life of the transduced cells was 20.3 days, 121.8 days, and 293.5 days in these patients.
The editorialists said the details in the report are a model for other researchers to follow, but “as more gene-based therapies are demonstrated to be safe and effective, the barrier to clinical translation will become cell manufacturing and administration.”
This work was funded by the National Institutes of Health and others. Dr. Stadtmauer didn’t report any disclosures, but other investigators disclosed patent applications and commercialization efforts. Dr. Doudna disclosed that she is a cofounder or adviser for several companies developing gene-editing therapeutics.
SOURCE: Stadtmauer EA et al. Science. 2020 Feb 6. doi: 10.1126/science.aba7365.
according to a report in Science.
The results of no harm support this “promising” area of cancer immunotherapy, according to study investigator Edward A. Stadtmauer, MD, of the University of Pennsylvania in Philadelphia and colleagues.
However, there was no evidence of benefit in this trial. One patient transfused with CRISPR-engineered T cells has since died, and the other two have moved on to other treatments.
“The big question that remains unanswered by this study is whether gene-edited, engineered T cells are effective against advanced cancer,” Jennifer Hamilton, PhD, and Jennifer Doudna, PhD, both of the University of California, Berkeley, wrote in an accompanying editorial.
The study enrolled six patients with refractory cancer, and three of them received CRISPR-engineered T cells. Two patients had multiple myeloma, and one had metastatic sarcoma.
Dr. Stadtmauer and colleagues drew blood from the patients, isolated the T cells, and used CRISPR-Cas9 to modify the cells. The T cells were transfected with Cas9 protein complexed with single guide RNAs against TRAC and TRBC (genes encoding the T-cell receptor chains TCR-alpha and TCR-beta) as well as PDCD1 (a gene encoding programmed cell death protein 1). The T cells were then transduced with a lentiviral vector to express a transgenic NY-ESO-1 cancer-specific T-cell receptor.
The investigators expanded the cell lines and infused them back into the patients after administering lymphodepleting chemotherapy. The sarcoma patient initially had a 50% decrease in a large abdominal mass, but all three patients ultimately progressed.
The editorialists noted that gene disruption efficiencies in this study were “modest,” ranging from 15% to 45%, but the investigators used a protocol from 2016, when the study was given the go-ahead by the National Institutes of Health and the Food and Drug Administration. With current protocols, gene disruption efficiencies can exceed 90%, which means patients might do better in subsequent trials.
There was no more than mild toxicity in this trial, and most adverse events were attributed to the lymphodepleting chemotherapy.
There was concern about potential rejection of infused cells because of preexisting immune responses to Cas9, but it doesn’t seem “to be a barrier to the application of this promising technology,” the investigators said.
They noted that “the stable engraftment of our engineered T cells is remarkably different from previously reported trials ... where the half-life of the cells in blood was [about] 1 week. Biopsy specimens of bone marrow in the myeloma patients and tumor in the sarcoma patient demonstrated trafficking of the engineered T cells to the tumor in all three patients” beyond that point. The decay half-life of the transduced cells was 20.3 days, 121.8 days, and 293.5 days in these patients.
The editorialists said the details in the report are a model for other researchers to follow, but “as more gene-based therapies are demonstrated to be safe and effective, the barrier to clinical translation will become cell manufacturing and administration.”
This work was funded by the National Institutes of Health and others. Dr. Stadtmauer didn’t report any disclosures, but other investigators disclosed patent applications and commercialization efforts. Dr. Doudna disclosed that she is a cofounder or adviser for several companies developing gene-editing therapeutics.
SOURCE: Stadtmauer EA et al. Science. 2020 Feb 6. doi: 10.1126/science.aba7365.
according to a report in Science.
The results of no harm support this “promising” area of cancer immunotherapy, according to study investigator Edward A. Stadtmauer, MD, of the University of Pennsylvania in Philadelphia and colleagues.
However, there was no evidence of benefit in this trial. One patient transfused with CRISPR-engineered T cells has since died, and the other two have moved on to other treatments.
“The big question that remains unanswered by this study is whether gene-edited, engineered T cells are effective against advanced cancer,” Jennifer Hamilton, PhD, and Jennifer Doudna, PhD, both of the University of California, Berkeley, wrote in an accompanying editorial.
The study enrolled six patients with refractory cancer, and three of them received CRISPR-engineered T cells. Two patients had multiple myeloma, and one had metastatic sarcoma.
Dr. Stadtmauer and colleagues drew blood from the patients, isolated the T cells, and used CRISPR-Cas9 to modify the cells. The T cells were transfected with Cas9 protein complexed with single guide RNAs against TRAC and TRBC (genes encoding the T-cell receptor chains TCR-alpha and TCR-beta) as well as PDCD1 (a gene encoding programmed cell death protein 1). The T cells were then transduced with a lentiviral vector to express a transgenic NY-ESO-1 cancer-specific T-cell receptor.
The investigators expanded the cell lines and infused them back into the patients after administering lymphodepleting chemotherapy. The sarcoma patient initially had a 50% decrease in a large abdominal mass, but all three patients ultimately progressed.
The editorialists noted that gene disruption efficiencies in this study were “modest,” ranging from 15% to 45%, but the investigators used a protocol from 2016, when the study was given the go-ahead by the National Institutes of Health and the Food and Drug Administration. With current protocols, gene disruption efficiencies can exceed 90%, which means patients might do better in subsequent trials.
There was no more than mild toxicity in this trial, and most adverse events were attributed to the lymphodepleting chemotherapy.
There was concern about potential rejection of infused cells because of preexisting immune responses to Cas9, but it doesn’t seem “to be a barrier to the application of this promising technology,” the investigators said.
They noted that “the stable engraftment of our engineered T cells is remarkably different from previously reported trials ... where the half-life of the cells in blood was [about] 1 week. Biopsy specimens of bone marrow in the myeloma patients and tumor in the sarcoma patient demonstrated trafficking of the engineered T cells to the tumor in all three patients” beyond that point. The decay half-life of the transduced cells was 20.3 days, 121.8 days, and 293.5 days in these patients.
The editorialists said the details in the report are a model for other researchers to follow, but “as more gene-based therapies are demonstrated to be safe and effective, the barrier to clinical translation will become cell manufacturing and administration.”
This work was funded by the National Institutes of Health and others. Dr. Stadtmauer didn’t report any disclosures, but other investigators disclosed patent applications and commercialization efforts. Dr. Doudna disclosed that she is a cofounder or adviser for several companies developing gene-editing therapeutics.
SOURCE: Stadtmauer EA et al. Science. 2020 Feb 6. doi: 10.1126/science.aba7365.
FROM SCIENCE
Global project reveals cancer’s genomic playbook
A massive collaborative project spanning four continents and 744 research centers has revealed driver mutations in both protein-coding and noncoding regions of 38 cancer types.
The Pan-Cancer Analysis of Whole Genomes (PCAWG) is an integrative analysis of the whole-genome sequences from 2,658 donors across 38 common tumor types. The findings are expected to add exponentially to what’s currently known about the complex genetics of cancer, and they point to possible strategies for improving cancer prevention, diagnosis, and care.
Six articles summarizing the findings are presented in a series of papers in Nature, and 16 more appear in affiliated publications.
“It’s humbling that it was only 14 years ago that the genomics community sequenced its very first cancer exome, and it was able to identify mutations within the roughly 20,000 protein-coding genes in the human cell,” investigator Lincoln Stein, MD, PhD, of the Ontario Institute for Cancer Research in Toronto, said in a telephone briefing.
Exome sequencing, however, covers only protein-coding genomic regions, which constitute only about 1% of the entire genome, “so assembling an accurate portrait of the cancer genome using just the exome data is like trying to put together a 100,000-piece jigsaw puzzle when you’re missing 99% of the pieces and there’s no puzzle box with a completed picture to guide you,” Dr. Stein said.
Members of the PCAWG from centers in North America, Europe, Asia, and Australia screened 2,658 whole-cancer genomes and matched samples of noncancerous tissues from the same individuals, along with 1,188 transcriptomes cataloging the sequences and expression of RNA transcripts in a given tumor. The 6-year project netted more than 800 terabytes of genomic data, roughly equivalent to the digital holdings of the U.S. Library of Congress multiplied by 11.
The findings are summarized in papers focusing on cancer drivers, noncoding changes, mutational signatures, structural variants, cancer evolution over time, and RNA alterations.
Driver mutations
Investigators found that the average cancer genome contains four or five driver mutations located in both coding and noncoding regions. They also found, however, that in approximately 5% of cases no driver mutations could be identified.
A substantial proportion of tumors displayed “hallmarks of genomic catastrophes.” About 22% of tumors exhibited chromothripsis, a mutational process marked by hundreds or even thousands of clustered chromosomal rearrangements. About 18% showed chromoplexy, which is characterized by scattering and rearrangement of multiple strands of DNA from one or more chromosomes.
Analyzing driver point mutations and structural variants in noncoding regions, the investigators found the usual suspects – previously reported culprits – as well as novel candidates.
For example, they identified point mutations in the five prime region of the tumor suppressor gene TP53 and the three prime untranslated regions of NFKBIZ (a nuclear factor kappa B inhibitor) and TOB1 (an antiproliferative protein), focal deletion in BRD4 (a transcriptional and epigenetic regulator), and rearrangements in chromosomal loci in members of the AKR1C family of enzymes thought to play a role in disease progression.
In addition, investigators identified mutations in noncoding regions of TERT, a telomerase gene. These mutations result in ramped-up expression of telomerase, which in turn promotes uncontrollable division of tumor cells.
Mutational signatures
In a related line of research, PCAWG investigators identified new DNA mutational signatures ranging from single nucleotide polymorphisms to insertions and deletions, as well as to structural variants – rearrangements of large sections of the genome.
“The substantial size of our dataset, compared with previous analyses, enabled the discovery of new signatures, the separation of overlapping signatures, and the decomposition of signatures into components that may represent associated – but distinct – DNA damage, repair, and/or replication mechanisms. By estimating the contribution of each signature to the mutational catalogs of individual cancer genomes, we revealed associations of signatures to exogenous or endogenous exposures, as well as to defective DNA maintenance processes,” the investigators wrote.
They also acknowledged, however, that “many signatures are of unknown cause.”
Cancer evolution
One of the six main studies focused on the evolution of cancer over time. Instead of providing a “snapshot” of the genome as captured by sequencing tissue from a single biopsy, consortium investigators created full-length features of the “life history and evolution of mutational processes and driver mutation sequences.”
They found that early cancer development was marked by relatively few mutations in driver genes and by identifiable copy-number gains, including trisomy 7 in glioblastoma, and an abnormal mirroring of the arms (isochromosome) of chromosome 17 in medulloblastoma.
In 40% of the samples, however, there were significant changes in the mutational spectrum as the cancers grew, leading to a near quadrupling of driver genes and increased genomic instability in later-stage tumors.
“Copy-number alterations often occur in mitotic crises and lead to simultaneous gains of chromosomal segments,” the investigators wrote. “Timing analyses suggest that driver mutations often precede diagnosis by many years, if not decades. Together, these results determine the evolutionary trajectories of cancer and highlight opportunities for early cancer detection.”
Implications for cancer care
“When I used to treat patients with cancer, I was always completely amazed and puzzled by how two patients could have what looked like the same tumor. It would look the same under the microscope, have the same size, and the two patients would receive exactly the same treatment, but the two patients would have completely opposite outcomes; one would survive, and one would die. What this analysis … has done is really laid bare the reasons for that unpredictability in clinical outcomes,” Peter Campbell, MD, PhD, of the Wellcome Sanger Institute in Hinxton, England, said during the telebriefing.
“The most striking finding out of all of the suite of papers is just how different one person’s cancer genome is from another person’s. We see thousands of different combinations of mutations that can cause the cancer, and more than 80 different underlying processes generating the mutations in a cancer, and that leads to very different shapes and patterns in the genome that result,” he added.
On a positive note, the research shows that one or more driver mutations can be identified in about 95% of all cancer patients, and it elucidates the sequence of events leading to oncogenesis and tumor evolution, providing opportunities for earlier identification and potential interventions to prevent cancer, Dr. Campbell said.
The PCAWG was a collaborative multinational effort with multiple funding sources and many investigators.
SOURCE: Nature. 2020 Feb 5. https://www.nature.com/collections/pcawg/
A massive collaborative project spanning four continents and 744 research centers has revealed driver mutations in both protein-coding and noncoding regions of 38 cancer types.
The Pan-Cancer Analysis of Whole Genomes (PCAWG) is an integrative analysis of the whole-genome sequences from 2,658 donors across 38 common tumor types. The findings are expected to add exponentially to what’s currently known about the complex genetics of cancer, and they point to possible strategies for improving cancer prevention, diagnosis, and care.
Six articles summarizing the findings are presented in a series of papers in Nature, and 16 more appear in affiliated publications.
“It’s humbling that it was only 14 years ago that the genomics community sequenced its very first cancer exome, and it was able to identify mutations within the roughly 20,000 protein-coding genes in the human cell,” investigator Lincoln Stein, MD, PhD, of the Ontario Institute for Cancer Research in Toronto, said in a telephone briefing.
Exome sequencing, however, covers only protein-coding genomic regions, which constitute only about 1% of the entire genome, “so assembling an accurate portrait of the cancer genome using just the exome data is like trying to put together a 100,000-piece jigsaw puzzle when you’re missing 99% of the pieces and there’s no puzzle box with a completed picture to guide you,” Dr. Stein said.
Members of the PCAWG from centers in North America, Europe, Asia, and Australia screened 2,658 whole-cancer genomes and matched samples of noncancerous tissues from the same individuals, along with 1,188 transcriptomes cataloging the sequences and expression of RNA transcripts in a given tumor. The 6-year project netted more than 800 terabytes of genomic data, roughly equivalent to the digital holdings of the U.S. Library of Congress multiplied by 11.
The findings are summarized in papers focusing on cancer drivers, noncoding changes, mutational signatures, structural variants, cancer evolution over time, and RNA alterations.
Driver mutations
Investigators found that the average cancer genome contains four or five driver mutations located in both coding and noncoding regions. They also found, however, that in approximately 5% of cases no driver mutations could be identified.
A substantial proportion of tumors displayed “hallmarks of genomic catastrophes.” About 22% of tumors exhibited chromothripsis, a mutational process marked by hundreds or even thousands of clustered chromosomal rearrangements. About 18% showed chromoplexy, which is characterized by scattering and rearrangement of multiple strands of DNA from one or more chromosomes.
Analyzing driver point mutations and structural variants in noncoding regions, the investigators found the usual suspects – previously reported culprits – as well as novel candidates.
For example, they identified point mutations in the five prime region of the tumor suppressor gene TP53 and the three prime untranslated regions of NFKBIZ (a nuclear factor kappa B inhibitor) and TOB1 (an antiproliferative protein), focal deletion in BRD4 (a transcriptional and epigenetic regulator), and rearrangements in chromosomal loci in members of the AKR1C family of enzymes thought to play a role in disease progression.
In addition, investigators identified mutations in noncoding regions of TERT, a telomerase gene. These mutations result in ramped-up expression of telomerase, which in turn promotes uncontrollable division of tumor cells.
Mutational signatures
In a related line of research, PCAWG investigators identified new DNA mutational signatures ranging from single nucleotide polymorphisms to insertions and deletions, as well as to structural variants – rearrangements of large sections of the genome.
“The substantial size of our dataset, compared with previous analyses, enabled the discovery of new signatures, the separation of overlapping signatures, and the decomposition of signatures into components that may represent associated – but distinct – DNA damage, repair, and/or replication mechanisms. By estimating the contribution of each signature to the mutational catalogs of individual cancer genomes, we revealed associations of signatures to exogenous or endogenous exposures, as well as to defective DNA maintenance processes,” the investigators wrote.
They also acknowledged, however, that “many signatures are of unknown cause.”
Cancer evolution
One of the six main studies focused on the evolution of cancer over time. Instead of providing a “snapshot” of the genome as captured by sequencing tissue from a single biopsy, consortium investigators created full-length features of the “life history and evolution of mutational processes and driver mutation sequences.”
They found that early cancer development was marked by relatively few mutations in driver genes and by identifiable copy-number gains, including trisomy 7 in glioblastoma, and an abnormal mirroring of the arms (isochromosome) of chromosome 17 in medulloblastoma.
In 40% of the samples, however, there were significant changes in the mutational spectrum as the cancers grew, leading to a near quadrupling of driver genes and increased genomic instability in later-stage tumors.
“Copy-number alterations often occur in mitotic crises and lead to simultaneous gains of chromosomal segments,” the investigators wrote. “Timing analyses suggest that driver mutations often precede diagnosis by many years, if not decades. Together, these results determine the evolutionary trajectories of cancer and highlight opportunities for early cancer detection.”
Implications for cancer care
“When I used to treat patients with cancer, I was always completely amazed and puzzled by how two patients could have what looked like the same tumor. It would look the same under the microscope, have the same size, and the two patients would receive exactly the same treatment, but the two patients would have completely opposite outcomes; one would survive, and one would die. What this analysis … has done is really laid bare the reasons for that unpredictability in clinical outcomes,” Peter Campbell, MD, PhD, of the Wellcome Sanger Institute in Hinxton, England, said during the telebriefing.
“The most striking finding out of all of the suite of papers is just how different one person’s cancer genome is from another person’s. We see thousands of different combinations of mutations that can cause the cancer, and more than 80 different underlying processes generating the mutations in a cancer, and that leads to very different shapes and patterns in the genome that result,” he added.
On a positive note, the research shows that one or more driver mutations can be identified in about 95% of all cancer patients, and it elucidates the sequence of events leading to oncogenesis and tumor evolution, providing opportunities for earlier identification and potential interventions to prevent cancer, Dr. Campbell said.
The PCAWG was a collaborative multinational effort with multiple funding sources and many investigators.
SOURCE: Nature. 2020 Feb 5. https://www.nature.com/collections/pcawg/
A massive collaborative project spanning four continents and 744 research centers has revealed driver mutations in both protein-coding and noncoding regions of 38 cancer types.
The Pan-Cancer Analysis of Whole Genomes (PCAWG) is an integrative analysis of the whole-genome sequences from 2,658 donors across 38 common tumor types. The findings are expected to add exponentially to what’s currently known about the complex genetics of cancer, and they point to possible strategies for improving cancer prevention, diagnosis, and care.
Six articles summarizing the findings are presented in a series of papers in Nature, and 16 more appear in affiliated publications.
“It’s humbling that it was only 14 years ago that the genomics community sequenced its very first cancer exome, and it was able to identify mutations within the roughly 20,000 protein-coding genes in the human cell,” investigator Lincoln Stein, MD, PhD, of the Ontario Institute for Cancer Research in Toronto, said in a telephone briefing.
Exome sequencing, however, covers only protein-coding genomic regions, which constitute only about 1% of the entire genome, “so assembling an accurate portrait of the cancer genome using just the exome data is like trying to put together a 100,000-piece jigsaw puzzle when you’re missing 99% of the pieces and there’s no puzzle box with a completed picture to guide you,” Dr. Stein said.
Members of the PCAWG from centers in North America, Europe, Asia, and Australia screened 2,658 whole-cancer genomes and matched samples of noncancerous tissues from the same individuals, along with 1,188 transcriptomes cataloging the sequences and expression of RNA transcripts in a given tumor. The 6-year project netted more than 800 terabytes of genomic data, roughly equivalent to the digital holdings of the U.S. Library of Congress multiplied by 11.
The findings are summarized in papers focusing on cancer drivers, noncoding changes, mutational signatures, structural variants, cancer evolution over time, and RNA alterations.
Driver mutations
Investigators found that the average cancer genome contains four or five driver mutations located in both coding and noncoding regions. They also found, however, that in approximately 5% of cases no driver mutations could be identified.
A substantial proportion of tumors displayed “hallmarks of genomic catastrophes.” About 22% of tumors exhibited chromothripsis, a mutational process marked by hundreds or even thousands of clustered chromosomal rearrangements. About 18% showed chromoplexy, which is characterized by scattering and rearrangement of multiple strands of DNA from one or more chromosomes.
Analyzing driver point mutations and structural variants in noncoding regions, the investigators found the usual suspects – previously reported culprits – as well as novel candidates.
For example, they identified point mutations in the five prime region of the tumor suppressor gene TP53 and the three prime untranslated regions of NFKBIZ (a nuclear factor kappa B inhibitor) and TOB1 (an antiproliferative protein), focal deletion in BRD4 (a transcriptional and epigenetic regulator), and rearrangements in chromosomal loci in members of the AKR1C family of enzymes thought to play a role in disease progression.
In addition, investigators identified mutations in noncoding regions of TERT, a telomerase gene. These mutations result in ramped-up expression of telomerase, which in turn promotes uncontrollable division of tumor cells.
Mutational signatures
In a related line of research, PCAWG investigators identified new DNA mutational signatures ranging from single nucleotide polymorphisms to insertions and deletions, as well as to structural variants – rearrangements of large sections of the genome.
“The substantial size of our dataset, compared with previous analyses, enabled the discovery of new signatures, the separation of overlapping signatures, and the decomposition of signatures into components that may represent associated – but distinct – DNA damage, repair, and/or replication mechanisms. By estimating the contribution of each signature to the mutational catalogs of individual cancer genomes, we revealed associations of signatures to exogenous or endogenous exposures, as well as to defective DNA maintenance processes,” the investigators wrote.
They also acknowledged, however, that “many signatures are of unknown cause.”
Cancer evolution
One of the six main studies focused on the evolution of cancer over time. Instead of providing a “snapshot” of the genome as captured by sequencing tissue from a single biopsy, consortium investigators created full-length features of the “life history and evolution of mutational processes and driver mutation sequences.”
They found that early cancer development was marked by relatively few mutations in driver genes and by identifiable copy-number gains, including trisomy 7 in glioblastoma, and an abnormal mirroring of the arms (isochromosome) of chromosome 17 in medulloblastoma.
In 40% of the samples, however, there were significant changes in the mutational spectrum as the cancers grew, leading to a near quadrupling of driver genes and increased genomic instability in later-stage tumors.
“Copy-number alterations often occur in mitotic crises and lead to simultaneous gains of chromosomal segments,” the investigators wrote. “Timing analyses suggest that driver mutations often precede diagnosis by many years, if not decades. Together, these results determine the evolutionary trajectories of cancer and highlight opportunities for early cancer detection.”
Implications for cancer care
“When I used to treat patients with cancer, I was always completely amazed and puzzled by how two patients could have what looked like the same tumor. It would look the same under the microscope, have the same size, and the two patients would receive exactly the same treatment, but the two patients would have completely opposite outcomes; one would survive, and one would die. What this analysis … has done is really laid bare the reasons for that unpredictability in clinical outcomes,” Peter Campbell, MD, PhD, of the Wellcome Sanger Institute in Hinxton, England, said during the telebriefing.
“The most striking finding out of all of the suite of papers is just how different one person’s cancer genome is from another person’s. We see thousands of different combinations of mutations that can cause the cancer, and more than 80 different underlying processes generating the mutations in a cancer, and that leads to very different shapes and patterns in the genome that result,” he added.
On a positive note, the research shows that one or more driver mutations can be identified in about 95% of all cancer patients, and it elucidates the sequence of events leading to oncogenesis and tumor evolution, providing opportunities for earlier identification and potential interventions to prevent cancer, Dr. Campbell said.
The PCAWG was a collaborative multinational effort with multiple funding sources and many investigators.
SOURCE: Nature. 2020 Feb 5. https://www.nature.com/collections/pcawg/
FROM NATURE
Presentation of a Rare Malignancy: Leiomyosarcoma of the Prostate (FULL)
Prostatic leiomyosarcoma is an aggressive malignancy with a high risk of metastasis and a poor prognosis that poses unique diagnostic and treatment challenges.
Prostatic leiomyosarcoma is a rare tumor.1 This neoplasm is composed of highly aggressive prostatic smooth muscle cells that present with nonspecific signs and symptoms mimicking other forms of prostatic pathology. Of the primary prostatic sarcomas, leiomyosarcoma represents the most common subtype in adults and is found in 38% to 52% of newly diagnosed prostate sarcoma.1,2 The prognosis is poor, and no clear guidelines exist regarding the optimal treatment approach. We report a case of prostate leiomyosarcoma and describe the disease characteristics, diagnostic modalities, and treatment approach regarding these rare malignancies.
Case Presentation
A 72-year-old male presented with 6 months of progressive severe lower urinary tract symptoms (LUTS) secondary to bladder outlet obstruction. The patient was refractory to medical management with combination α-blocker and 5-α-reductase inhibitor therapy and continued to require multiple emergent bladder catheterizations. Workup with urinalysis, blood biochemistry, and prostate specific antigen (PSA) levels were persistently normal. He reported no hematuria, weight loss, or perineal pain. The patient reported no history of tobacco use, exposure to hazardous chemicals, and had no family history of genitourinary cancers. On rectal exam, the prostate was firm and nodular, with induration noted along the right upper lobe of the prostate.
The patient was referred for a urology consultation and subsequently underwent transurethral resection of the prostate (TURP) for suspected severe benign prostatic hypertrophy (BPH). A histopathologic examination demonstrated atypical cytology consistent with high- grade leiomyosarcoma. Immunohistochemical analysis revealed positive staining for vimentin, smooth muscle actin, desmin (partial), cytokeratin, smooth muscle myosin, muscle specific actin, and Ki-67 (50%-60% expression).
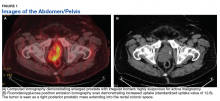
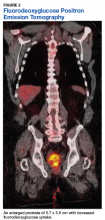
Fluorodeoxyglucose positron emission tomography (FDG-PET) scan revealed a 5.7 x 5.9 cm tumor with a maximum standardized uptake value (SUVmax) of 12.6 in the right posterior prostate, without evidence of metastatic disease (Figures 1A and 1B).
Discussion
Originating from prostatic interstitial cells, prostatic leiomyosarcoma is a rare tumor that accounts for < 0.1% of all primary prostatic malignancies.1 Since its first description in 1950 by Riba and colleagues, < 200 cases have been reported worldwide.2 Among the sarcomas of the prostate, it is the most common tumor, accounting for around 38% to 52% of prostate sarcoma presentations.1,2
Patients typically present between the ages of 41 and 78 years (mean age 61 years).2,3 Signs and symptoms at presentation may vary; however, the most common symptoms are related to lower urinary tract obstruction (89.4% of patients). These symptoms include urinary frequency, urgency, nocturia, and may mimic the presentation of BPH.
Symptoms commonly associated with other malignancies, including constitutional symptoms such as weight loss, tend to occur less frequently or may be absent. Perineal or rectal pain may only be present in 25.6% of patients. Hematuria, burning on ejaculation, and constitutional symptoms are a less common presentation (< 10% of patients).3,4 PSA levels typically do not rise and are found to be within normal limits. The lack of PSA elevation is related to the tumors nonepithelial origin and may contribute to a delay in diagnosis.2,4,5
Diagnosis
Diagnosis may be further eluded as digital rectal exam (DRE) findings tend to reveal nonspecific enlargement of the prostate, resembling that of BPH. DRE may show a hard and firm prostate with nodular induration at the base or over the lobes of the prostate.6 At this stage a urology consultation is useful, as diagnosis is most commonly achieved using transrectal ultrasound (TRUS) with ultrasound-guided needle biopsy or after a TURP procedure.3
Prostate sarcoma is associated with markedly enlarged prostate volume, irregular margins with invasion, or heterogenous hypoechoic lesions on TRUS.7 Transperineal biopsy, computed tomography (CT)-guided biopsy, or suprapubic prostatectomy have been less frequently employed for diagnosis in previously reported cases.8 Specialized imaging modalities, such as CT scan or bone scan, do not show any specific findings with regards to these tumors; their role is limited to evaluation of the local and distant metastasis and for follow-up assessments.9 Transabdominal ultrasound may assess hydronephrosis or enlarged prostate and its relation to nearby structures, although it has not been shown to be helpful in establishing a specific diagnosis.6
Histologically, prostatic leiomyosarcoma is a distinct subtype of prostatic sarcoma. Other subtypes include stromal tumors such as rhabdomyosarcoma, fibrosarcoma, and spindle cell sarcoma.2 The majority of leiomyosarcomas are high-grade lesions demonstrating neoplastic spindle cells with nuclear atypia, multifocal necrosis, and cystic degeneration. Low-grade leiomyosarcomas are very rare.10 Immunohistochemistry is characteristically positive for vimentin, smooth muscle actin, and desmin expression. Cytokeratin may be positive in up to 25% of cases, whereas S-100, CD34, CD117, and PSA are negative.2,3 These histopathological findings help to differentiate leiomyosarcoma from other prostatic tumors.
Tumor size may vary greatly, and measurements have been reported to range from 3 cm to 21 cm, frequently presenting with invasion of local structures.11 Advanced stage disease is commonly found at initial diagnosis and is thought to be due to the lack of early specific symptoms. Metastatic disease at presentation may be found in up to one-third of patients, with the lungs being the most common site of metastasis followed by the liver. Local extent and distant spread of disease may be determined by CT or magnetic resonance imaging (MRI) scans, which provide clear delineation of neoplastic and nonneoplastic tissues.
Treatment
Treatment regimens may include a multimodal approach of combination surgery, radiation, and chemotherapy. However, there are currently no standardized guidelines for treatment and the optimal therapy remains unknown.2,3,6 Surgery remains the mainstay of treatment, and patients with surgically resectable tumors are treated with curative intent. Surgeries performed include radical retropubic prostatectomy, radical cystoprostatectomy, suprapubic prostatectomy, and pelvic exenteration.2,5,8,12 These operations may be preceded or followed by radiation therapy and/or chemotherapy depending on extent of disease.
It has been reported that neo-adjuvant chemotherapy and/or radiotherapy can aid in decreasing tumor burden to facilitate a complete resection.2,8,13,14 Patients who are determined to not be candidates for surgery or whom have widespread disease may be offered systemic chemotherapy. Chemotherapy regimens vary, but common regimens include anthracyclines (doxorubicin or epirubicin), alkylating agents (cyclophosphamide, ifosfamide, dacarbazine), and/or vinca alkaloids (vinblastine or vincristine). Patients who do not receive surgical intervention rarely achieve a sustained remission.3,5,8
The long-term prognosis of prostatic leiomyosarcoma is poor due to the aggressive nature of the neoplasm and the high chance of disease recurrence or metastasis. Median survival is estimated at 17 months, and from 50% to 75% of patients die within 2 to 5 years of diagnosis.2,3 Prognosis may be improved in patients with localized disease at diagnosis who are candidates for complete surgical resection with negative margins.13 Adverse prognostic factors include metastatic disease at presentation and the presence of positive surgical margins after surgery.
Overall survival is very poor, and it is estimated that the 1-, 3-, and 5-year survival rates are 68%, 34%, and 26%, respectively.3 However, some studies estimate the 5-year survival to be anywhere from 0 to 60%.8,9 Due to the substantially high risk of death, prostatic leiomyosarcoma may be one of the most aggressive and poorly prognostic malignancies involving the prostate.
Conclusion
Prostatic leiomyosarcoma poses a unique diagnostic challenge, as clinical presentation alone may not always be suggestive of underlying malignancy. This challenge is further exacerbated by its aggressive nature, high risk of metastasis, and difficulties with unclear treatment. Proper history and physical examination, differential diagnosis, and a multidisciplinary approach to patient care are the foundation for early detection and promoting improved survival.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the US Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Miedler JD, MacLennan GT. Leiomyosarcoma of the prostate. J Urol. 2007;178(2):668.
2. Zazzara M, Divenuto L, Scarcia M, Cardo G, Maselli FP, Ludovico GM. Leiomyosarcoma of prostate: case report and literature review. Urol Case Rep. 2018;17:4-6.
3. Vandoros GP, Manolidis T, Karamouzis MV, et al. Leiomyosarcoma of the prostate: case report and review of 54 previously published cases. Sarcoma. 2008;2008:458709.
4. Talapatra K, Nemade B, Bhutani R, et al. Recurrent episodes of hematuria: a rare presentation of leiomyosarcoma of prostate. J Cancer Res Ther. 2006;2(4):212-214.
5. Cheville JC, Dundore PA, Nascimento AG, et al. Leiomyosarcoma of the prostate. Report of 23 cases. Cancer. 1995;76(8):1422-1427.
6. Venyo AK. A review of the literature on primary leiomyosarcoma of the prostate gland. Adv Urol. 2015;2015:485786.
7. Stilgenbauer R, Benedict M, Bamshad R, Viduetsky A. Sarcoma of the prostate: sonographic findings and pathologic correlation. J Ultrasound Med. 2007;26(12):1789-1793.
8. Sexton WJ, Lance RE, Reyes AO, Pisters PW, Tu SM, Pisters LL. Adult prostate sarcoma: the M.D. Anderson Cancer Center experience. J Urol. 2001;166(2):521-525.
9. Singh JP, Chakraborty D, Bera MK, Pal D. Leiomyosarcoma of prostate: a rare, aggressive tumor. J Cancer Res Ther. 2013;9(4):743-745.
10. Hansel DE, Herawi M, Montgomery E, Epstein JI. Spindle cell lesions of the adult prostate. Mod Pathol. 2007;20(1):148-158.
11. Punt SE, Eary JF, O'Sullivan J, Conrad EU. Fluorodeoxyglucose positron emission tomography in leiomyosarcoma: imaging characteristics. Nucl Med Commun. 2009;30(7):546-549.
12. Dotan ZA, Tal R, Golijanin D, et al. Adult genitourinary sarcoma: the 25-year Memorial Sloan-Kettering experience. J Urol. 2006;176(5):2033-2038.
13. Musser JE, Assel M, Mashni JW, Sjoberg DD, Russo P. Adult prostate sarcoma: the Memorial Sloan Kettering experience. Urology. 2014;84(3):624-628.
14. Janet NL, May AW, Akins RS. Sarcoma of the prostate: a single institutional review. Am J Clin Oncol. 2009;32:27-29
Prostatic leiomyosarcoma is an aggressive malignancy with a high risk of metastasis and a poor prognosis that poses unique diagnostic and treatment challenges.
Prostatic leiomyosarcoma is an aggressive malignancy with a high risk of metastasis and a poor prognosis that poses unique diagnostic and treatment challenges.
Prostatic leiomyosarcoma is a rare tumor.1 This neoplasm is composed of highly aggressive prostatic smooth muscle cells that present with nonspecific signs and symptoms mimicking other forms of prostatic pathology. Of the primary prostatic sarcomas, leiomyosarcoma represents the most common subtype in adults and is found in 38% to 52% of newly diagnosed prostate sarcoma.1,2 The prognosis is poor, and no clear guidelines exist regarding the optimal treatment approach. We report a case of prostate leiomyosarcoma and describe the disease characteristics, diagnostic modalities, and treatment approach regarding these rare malignancies.
Case Presentation
A 72-year-old male presented with 6 months of progressive severe lower urinary tract symptoms (LUTS) secondary to bladder outlet obstruction. The patient was refractory to medical management with combination α-blocker and 5-α-reductase inhibitor therapy and continued to require multiple emergent bladder catheterizations. Workup with urinalysis, blood biochemistry, and prostate specific antigen (PSA) levels were persistently normal. He reported no hematuria, weight loss, or perineal pain. The patient reported no history of tobacco use, exposure to hazardous chemicals, and had no family history of genitourinary cancers. On rectal exam, the prostate was firm and nodular, with induration noted along the right upper lobe of the prostate.
The patient was referred for a urology consultation and subsequently underwent transurethral resection of the prostate (TURP) for suspected severe benign prostatic hypertrophy (BPH). A histopathologic examination demonstrated atypical cytology consistent with high- grade leiomyosarcoma. Immunohistochemical analysis revealed positive staining for vimentin, smooth muscle actin, desmin (partial), cytokeratin, smooth muscle myosin, muscle specific actin, and Ki-67 (50%-60% expression).
Fluorodeoxyglucose positron emission tomography (FDG-PET) scan revealed a 5.7 x 5.9 cm tumor with a maximum standardized uptake value (SUVmax) of 12.6 in the right posterior prostate, without evidence of metastatic disease (Figures 1A and 1B).
Discussion
Originating from prostatic interstitial cells, prostatic leiomyosarcoma is a rare tumor that accounts for < 0.1% of all primary prostatic malignancies.1 Since its first description in 1950 by Riba and colleagues, < 200 cases have been reported worldwide.2 Among the sarcomas of the prostate, it is the most common tumor, accounting for around 38% to 52% of prostate sarcoma presentations.1,2
Patients typically present between the ages of 41 and 78 years (mean age 61 years).2,3 Signs and symptoms at presentation may vary; however, the most common symptoms are related to lower urinary tract obstruction (89.4% of patients). These symptoms include urinary frequency, urgency, nocturia, and may mimic the presentation of BPH.
Symptoms commonly associated with other malignancies, including constitutional symptoms such as weight loss, tend to occur less frequently or may be absent. Perineal or rectal pain may only be present in 25.6% of patients. Hematuria, burning on ejaculation, and constitutional symptoms are a less common presentation (< 10% of patients).3,4 PSA levels typically do not rise and are found to be within normal limits. The lack of PSA elevation is related to the tumors nonepithelial origin and may contribute to a delay in diagnosis.2,4,5
Diagnosis
Diagnosis may be further eluded as digital rectal exam (DRE) findings tend to reveal nonspecific enlargement of the prostate, resembling that of BPH. DRE may show a hard and firm prostate with nodular induration at the base or over the lobes of the prostate.6 At this stage a urology consultation is useful, as diagnosis is most commonly achieved using transrectal ultrasound (TRUS) with ultrasound-guided needle biopsy or after a TURP procedure.3
Prostate sarcoma is associated with markedly enlarged prostate volume, irregular margins with invasion, or heterogenous hypoechoic lesions on TRUS.7 Transperineal biopsy, computed tomography (CT)-guided biopsy, or suprapubic prostatectomy have been less frequently employed for diagnosis in previously reported cases.8 Specialized imaging modalities, such as CT scan or bone scan, do not show any specific findings with regards to these tumors; their role is limited to evaluation of the local and distant metastasis and for follow-up assessments.9 Transabdominal ultrasound may assess hydronephrosis or enlarged prostate and its relation to nearby structures, although it has not been shown to be helpful in establishing a specific diagnosis.6
Histologically, prostatic leiomyosarcoma is a distinct subtype of prostatic sarcoma. Other subtypes include stromal tumors such as rhabdomyosarcoma, fibrosarcoma, and spindle cell sarcoma.2 The majority of leiomyosarcomas are high-grade lesions demonstrating neoplastic spindle cells with nuclear atypia, multifocal necrosis, and cystic degeneration. Low-grade leiomyosarcomas are very rare.10 Immunohistochemistry is characteristically positive for vimentin, smooth muscle actin, and desmin expression. Cytokeratin may be positive in up to 25% of cases, whereas S-100, CD34, CD117, and PSA are negative.2,3 These histopathological findings help to differentiate leiomyosarcoma from other prostatic tumors.
Tumor size may vary greatly, and measurements have been reported to range from 3 cm to 21 cm, frequently presenting with invasion of local structures.11 Advanced stage disease is commonly found at initial diagnosis and is thought to be due to the lack of early specific symptoms. Metastatic disease at presentation may be found in up to one-third of patients, with the lungs being the most common site of metastasis followed by the liver. Local extent and distant spread of disease may be determined by CT or magnetic resonance imaging (MRI) scans, which provide clear delineation of neoplastic and nonneoplastic tissues.
Treatment
Treatment regimens may include a multimodal approach of combination surgery, radiation, and chemotherapy. However, there are currently no standardized guidelines for treatment and the optimal therapy remains unknown.2,3,6 Surgery remains the mainstay of treatment, and patients with surgically resectable tumors are treated with curative intent. Surgeries performed include radical retropubic prostatectomy, radical cystoprostatectomy, suprapubic prostatectomy, and pelvic exenteration.2,5,8,12 These operations may be preceded or followed by radiation therapy and/or chemotherapy depending on extent of disease.
It has been reported that neo-adjuvant chemotherapy and/or radiotherapy can aid in decreasing tumor burden to facilitate a complete resection.2,8,13,14 Patients who are determined to not be candidates for surgery or whom have widespread disease may be offered systemic chemotherapy. Chemotherapy regimens vary, but common regimens include anthracyclines (doxorubicin or epirubicin), alkylating agents (cyclophosphamide, ifosfamide, dacarbazine), and/or vinca alkaloids (vinblastine or vincristine). Patients who do not receive surgical intervention rarely achieve a sustained remission.3,5,8
The long-term prognosis of prostatic leiomyosarcoma is poor due to the aggressive nature of the neoplasm and the high chance of disease recurrence or metastasis. Median survival is estimated at 17 months, and from 50% to 75% of patients die within 2 to 5 years of diagnosis.2,3 Prognosis may be improved in patients with localized disease at diagnosis who are candidates for complete surgical resection with negative margins.13 Adverse prognostic factors include metastatic disease at presentation and the presence of positive surgical margins after surgery.
Overall survival is very poor, and it is estimated that the 1-, 3-, and 5-year survival rates are 68%, 34%, and 26%, respectively.3 However, some studies estimate the 5-year survival to be anywhere from 0 to 60%.8,9 Due to the substantially high risk of death, prostatic leiomyosarcoma may be one of the most aggressive and poorly prognostic malignancies involving the prostate.
Conclusion
Prostatic leiomyosarcoma poses a unique diagnostic challenge, as clinical presentation alone may not always be suggestive of underlying malignancy. This challenge is further exacerbated by its aggressive nature, high risk of metastasis, and difficulties with unclear treatment. Proper history and physical examination, differential diagnosis, and a multidisciplinary approach to patient care are the foundation for early detection and promoting improved survival.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the US Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Prostatic leiomyosarcoma is a rare tumor.1 This neoplasm is composed of highly aggressive prostatic smooth muscle cells that present with nonspecific signs and symptoms mimicking other forms of prostatic pathology. Of the primary prostatic sarcomas, leiomyosarcoma represents the most common subtype in adults and is found in 38% to 52% of newly diagnosed prostate sarcoma.1,2 The prognosis is poor, and no clear guidelines exist regarding the optimal treatment approach. We report a case of prostate leiomyosarcoma and describe the disease characteristics, diagnostic modalities, and treatment approach regarding these rare malignancies.
Case Presentation
A 72-year-old male presented with 6 months of progressive severe lower urinary tract symptoms (LUTS) secondary to bladder outlet obstruction. The patient was refractory to medical management with combination α-blocker and 5-α-reductase inhibitor therapy and continued to require multiple emergent bladder catheterizations. Workup with urinalysis, blood biochemistry, and prostate specific antigen (PSA) levels were persistently normal. He reported no hematuria, weight loss, or perineal pain. The patient reported no history of tobacco use, exposure to hazardous chemicals, and had no family history of genitourinary cancers. On rectal exam, the prostate was firm and nodular, with induration noted along the right upper lobe of the prostate.
The patient was referred for a urology consultation and subsequently underwent transurethral resection of the prostate (TURP) for suspected severe benign prostatic hypertrophy (BPH). A histopathologic examination demonstrated atypical cytology consistent with high- grade leiomyosarcoma. Immunohistochemical analysis revealed positive staining for vimentin, smooth muscle actin, desmin (partial), cytokeratin, smooth muscle myosin, muscle specific actin, and Ki-67 (50%-60% expression).
Fluorodeoxyglucose positron emission tomography (FDG-PET) scan revealed a 5.7 x 5.9 cm tumor with a maximum standardized uptake value (SUVmax) of 12.6 in the right posterior prostate, without evidence of metastatic disease (Figures 1A and 1B).
Discussion
Originating from prostatic interstitial cells, prostatic leiomyosarcoma is a rare tumor that accounts for < 0.1% of all primary prostatic malignancies.1 Since its first description in 1950 by Riba and colleagues, < 200 cases have been reported worldwide.2 Among the sarcomas of the prostate, it is the most common tumor, accounting for around 38% to 52% of prostate sarcoma presentations.1,2
Patients typically present between the ages of 41 and 78 years (mean age 61 years).2,3 Signs and symptoms at presentation may vary; however, the most common symptoms are related to lower urinary tract obstruction (89.4% of patients). These symptoms include urinary frequency, urgency, nocturia, and may mimic the presentation of BPH.
Symptoms commonly associated with other malignancies, including constitutional symptoms such as weight loss, tend to occur less frequently or may be absent. Perineal or rectal pain may only be present in 25.6% of patients. Hematuria, burning on ejaculation, and constitutional symptoms are a less common presentation (< 10% of patients).3,4 PSA levels typically do not rise and are found to be within normal limits. The lack of PSA elevation is related to the tumors nonepithelial origin and may contribute to a delay in diagnosis.2,4,5
Diagnosis
Diagnosis may be further eluded as digital rectal exam (DRE) findings tend to reveal nonspecific enlargement of the prostate, resembling that of BPH. DRE may show a hard and firm prostate with nodular induration at the base or over the lobes of the prostate.6 At this stage a urology consultation is useful, as diagnosis is most commonly achieved using transrectal ultrasound (TRUS) with ultrasound-guided needle biopsy or after a TURP procedure.3
Prostate sarcoma is associated with markedly enlarged prostate volume, irregular margins with invasion, or heterogenous hypoechoic lesions on TRUS.7 Transperineal biopsy, computed tomography (CT)-guided biopsy, or suprapubic prostatectomy have been less frequently employed for diagnosis in previously reported cases.8 Specialized imaging modalities, such as CT scan or bone scan, do not show any specific findings with regards to these tumors; their role is limited to evaluation of the local and distant metastasis and for follow-up assessments.9 Transabdominal ultrasound may assess hydronephrosis or enlarged prostate and its relation to nearby structures, although it has not been shown to be helpful in establishing a specific diagnosis.6
Histologically, prostatic leiomyosarcoma is a distinct subtype of prostatic sarcoma. Other subtypes include stromal tumors such as rhabdomyosarcoma, fibrosarcoma, and spindle cell sarcoma.2 The majority of leiomyosarcomas are high-grade lesions demonstrating neoplastic spindle cells with nuclear atypia, multifocal necrosis, and cystic degeneration. Low-grade leiomyosarcomas are very rare.10 Immunohistochemistry is characteristically positive for vimentin, smooth muscle actin, and desmin expression. Cytokeratin may be positive in up to 25% of cases, whereas S-100, CD34, CD117, and PSA are negative.2,3 These histopathological findings help to differentiate leiomyosarcoma from other prostatic tumors.
Tumor size may vary greatly, and measurements have been reported to range from 3 cm to 21 cm, frequently presenting with invasion of local structures.11 Advanced stage disease is commonly found at initial diagnosis and is thought to be due to the lack of early specific symptoms. Metastatic disease at presentation may be found in up to one-third of patients, with the lungs being the most common site of metastasis followed by the liver. Local extent and distant spread of disease may be determined by CT or magnetic resonance imaging (MRI) scans, which provide clear delineation of neoplastic and nonneoplastic tissues.
Treatment
Treatment regimens may include a multimodal approach of combination surgery, radiation, and chemotherapy. However, there are currently no standardized guidelines for treatment and the optimal therapy remains unknown.2,3,6 Surgery remains the mainstay of treatment, and patients with surgically resectable tumors are treated with curative intent. Surgeries performed include radical retropubic prostatectomy, radical cystoprostatectomy, suprapubic prostatectomy, and pelvic exenteration.2,5,8,12 These operations may be preceded or followed by radiation therapy and/or chemotherapy depending on extent of disease.
It has been reported that neo-adjuvant chemotherapy and/or radiotherapy can aid in decreasing tumor burden to facilitate a complete resection.2,8,13,14 Patients who are determined to not be candidates for surgery or whom have widespread disease may be offered systemic chemotherapy. Chemotherapy regimens vary, but common regimens include anthracyclines (doxorubicin or epirubicin), alkylating agents (cyclophosphamide, ifosfamide, dacarbazine), and/or vinca alkaloids (vinblastine or vincristine). Patients who do not receive surgical intervention rarely achieve a sustained remission.3,5,8
The long-term prognosis of prostatic leiomyosarcoma is poor due to the aggressive nature of the neoplasm and the high chance of disease recurrence or metastasis. Median survival is estimated at 17 months, and from 50% to 75% of patients die within 2 to 5 years of diagnosis.2,3 Prognosis may be improved in patients with localized disease at diagnosis who are candidates for complete surgical resection with negative margins.13 Adverse prognostic factors include metastatic disease at presentation and the presence of positive surgical margins after surgery.
Overall survival is very poor, and it is estimated that the 1-, 3-, and 5-year survival rates are 68%, 34%, and 26%, respectively.3 However, some studies estimate the 5-year survival to be anywhere from 0 to 60%.8,9 Due to the substantially high risk of death, prostatic leiomyosarcoma may be one of the most aggressive and poorly prognostic malignancies involving the prostate.
Conclusion
Prostatic leiomyosarcoma poses a unique diagnostic challenge, as clinical presentation alone may not always be suggestive of underlying malignancy. This challenge is further exacerbated by its aggressive nature, high risk of metastasis, and difficulties with unclear treatment. Proper history and physical examination, differential diagnosis, and a multidisciplinary approach to patient care are the foundation for early detection and promoting improved survival.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the US Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Miedler JD, MacLennan GT. Leiomyosarcoma of the prostate. J Urol. 2007;178(2):668.
2. Zazzara M, Divenuto L, Scarcia M, Cardo G, Maselli FP, Ludovico GM. Leiomyosarcoma of prostate: case report and literature review. Urol Case Rep. 2018;17:4-6.
3. Vandoros GP, Manolidis T, Karamouzis MV, et al. Leiomyosarcoma of the prostate: case report and review of 54 previously published cases. Sarcoma. 2008;2008:458709.
4. Talapatra K, Nemade B, Bhutani R, et al. Recurrent episodes of hematuria: a rare presentation of leiomyosarcoma of prostate. J Cancer Res Ther. 2006;2(4):212-214.
5. Cheville JC, Dundore PA, Nascimento AG, et al. Leiomyosarcoma of the prostate. Report of 23 cases. Cancer. 1995;76(8):1422-1427.
6. Venyo AK. A review of the literature on primary leiomyosarcoma of the prostate gland. Adv Urol. 2015;2015:485786.
7. Stilgenbauer R, Benedict M, Bamshad R, Viduetsky A. Sarcoma of the prostate: sonographic findings and pathologic correlation. J Ultrasound Med. 2007;26(12):1789-1793.
8. Sexton WJ, Lance RE, Reyes AO, Pisters PW, Tu SM, Pisters LL. Adult prostate sarcoma: the M.D. Anderson Cancer Center experience. J Urol. 2001;166(2):521-525.
9. Singh JP, Chakraborty D, Bera MK, Pal D. Leiomyosarcoma of prostate: a rare, aggressive tumor. J Cancer Res Ther. 2013;9(4):743-745.
10. Hansel DE, Herawi M, Montgomery E, Epstein JI. Spindle cell lesions of the adult prostate. Mod Pathol. 2007;20(1):148-158.
11. Punt SE, Eary JF, O'Sullivan J, Conrad EU. Fluorodeoxyglucose positron emission tomography in leiomyosarcoma: imaging characteristics. Nucl Med Commun. 2009;30(7):546-549.
12. Dotan ZA, Tal R, Golijanin D, et al. Adult genitourinary sarcoma: the 25-year Memorial Sloan-Kettering experience. J Urol. 2006;176(5):2033-2038.
13. Musser JE, Assel M, Mashni JW, Sjoberg DD, Russo P. Adult prostate sarcoma: the Memorial Sloan Kettering experience. Urology. 2014;84(3):624-628.
14. Janet NL, May AW, Akins RS. Sarcoma of the prostate: a single institutional review. Am J Clin Oncol. 2009;32:27-29
1. Miedler JD, MacLennan GT. Leiomyosarcoma of the prostate. J Urol. 2007;178(2):668.
2. Zazzara M, Divenuto L, Scarcia M, Cardo G, Maselli FP, Ludovico GM. Leiomyosarcoma of prostate: case report and literature review. Urol Case Rep. 2018;17:4-6.
3. Vandoros GP, Manolidis T, Karamouzis MV, et al. Leiomyosarcoma of the prostate: case report and review of 54 previously published cases. Sarcoma. 2008;2008:458709.
4. Talapatra K, Nemade B, Bhutani R, et al. Recurrent episodes of hematuria: a rare presentation of leiomyosarcoma of prostate. J Cancer Res Ther. 2006;2(4):212-214.
5. Cheville JC, Dundore PA, Nascimento AG, et al. Leiomyosarcoma of the prostate. Report of 23 cases. Cancer. 1995;76(8):1422-1427.
6. Venyo AK. A review of the literature on primary leiomyosarcoma of the prostate gland. Adv Urol. 2015;2015:485786.
7. Stilgenbauer R, Benedict M, Bamshad R, Viduetsky A. Sarcoma of the prostate: sonographic findings and pathologic correlation. J Ultrasound Med. 2007;26(12):1789-1793.
8. Sexton WJ, Lance RE, Reyes AO, Pisters PW, Tu SM, Pisters LL. Adult prostate sarcoma: the M.D. Anderson Cancer Center experience. J Urol. 2001;166(2):521-525.
9. Singh JP, Chakraborty D, Bera MK, Pal D. Leiomyosarcoma of prostate: a rare, aggressive tumor. J Cancer Res Ther. 2013;9(4):743-745.
10. Hansel DE, Herawi M, Montgomery E, Epstein JI. Spindle cell lesions of the adult prostate. Mod Pathol. 2007;20(1):148-158.
11. Punt SE, Eary JF, O'Sullivan J, Conrad EU. Fluorodeoxyglucose positron emission tomography in leiomyosarcoma: imaging characteristics. Nucl Med Commun. 2009;30(7):546-549.
12. Dotan ZA, Tal R, Golijanin D, et al. Adult genitourinary sarcoma: the 25-year Memorial Sloan-Kettering experience. J Urol. 2006;176(5):2033-2038.
13. Musser JE, Assel M, Mashni JW, Sjoberg DD, Russo P. Adult prostate sarcoma: the Memorial Sloan Kettering experience. Urology. 2014;84(3):624-628.
14. Janet NL, May AW, Akins RS. Sarcoma of the prostate: a single institutional review. Am J Clin Oncol. 2009;32:27-29
FDA approves first treatment for advanced epithelioid sarcoma
The Food and Drug Administration has granted accelerated approval to tazemetostat (Tazverik) for the treatment of adults and pediatric patients aged 16 years and older with metastatic or locally advanced epithelioid sarcoma not eligible for complete resection.
Approval was based on overall response rate in a trial enrolling 62 patients with metastatic or locally advanced epithelioid sarcoma. The overall response rate was 15%, with 1.6% of patients having a complete response and 13% having a partial response. Of the nine patients that had a response, six (67%) had a response lasting 6 months or longer, the FDA said in a press statement.
The most common side effects for patients taking tazemetostat were pain, fatigue, nausea, decreased appetite, vomiting, and constipation. Patients treated with tazemetostat are at increased risk of developing secondary malignancies, including T-cell lymphoblastic lymphoma, myelodysplastic syndrome, and acute myeloid leukemia.
“Epithelioid sarcoma accounts for less than 1% of all soft-tissue sarcomas,” said Richard Pazdur, MD, director of the FDA’s Oncology Center of Excellence and acting director of the Office of Oncologic Diseases in the Center for Drug Evaluation and Research. “Until today, there were no treatment options specifically for patients with epithelioid sarcoma. The approval of Tazverik provides a treatment option that specifically targets this disease.”
Tazemetostat must be dispensed with a patient medication guide that describes important information about the drug’s uses and risks, the FDA said.
The Food and Drug Administration has granted accelerated approval to tazemetostat (Tazverik) for the treatment of adults and pediatric patients aged 16 years and older with metastatic or locally advanced epithelioid sarcoma not eligible for complete resection.
Approval was based on overall response rate in a trial enrolling 62 patients with metastatic or locally advanced epithelioid sarcoma. The overall response rate was 15%, with 1.6% of patients having a complete response and 13% having a partial response. Of the nine patients that had a response, six (67%) had a response lasting 6 months or longer, the FDA said in a press statement.
The most common side effects for patients taking tazemetostat were pain, fatigue, nausea, decreased appetite, vomiting, and constipation. Patients treated with tazemetostat are at increased risk of developing secondary malignancies, including T-cell lymphoblastic lymphoma, myelodysplastic syndrome, and acute myeloid leukemia.
“Epithelioid sarcoma accounts for less than 1% of all soft-tissue sarcomas,” said Richard Pazdur, MD, director of the FDA’s Oncology Center of Excellence and acting director of the Office of Oncologic Diseases in the Center for Drug Evaluation and Research. “Until today, there were no treatment options specifically for patients with epithelioid sarcoma. The approval of Tazverik provides a treatment option that specifically targets this disease.”
Tazemetostat must be dispensed with a patient medication guide that describes important information about the drug’s uses and risks, the FDA said.
The Food and Drug Administration has granted accelerated approval to tazemetostat (Tazverik) for the treatment of adults and pediatric patients aged 16 years and older with metastatic or locally advanced epithelioid sarcoma not eligible for complete resection.
Approval was based on overall response rate in a trial enrolling 62 patients with metastatic or locally advanced epithelioid sarcoma. The overall response rate was 15%, with 1.6% of patients having a complete response and 13% having a partial response. Of the nine patients that had a response, six (67%) had a response lasting 6 months or longer, the FDA said in a press statement.
The most common side effects for patients taking tazemetostat were pain, fatigue, nausea, decreased appetite, vomiting, and constipation. Patients treated with tazemetostat are at increased risk of developing secondary malignancies, including T-cell lymphoblastic lymphoma, myelodysplastic syndrome, and acute myeloid leukemia.
“Epithelioid sarcoma accounts for less than 1% of all soft-tissue sarcomas,” said Richard Pazdur, MD, director of the FDA’s Oncology Center of Excellence and acting director of the Office of Oncologic Diseases in the Center for Drug Evaluation and Research. “Until today, there were no treatment options specifically for patients with epithelioid sarcoma. The approval of Tazverik provides a treatment option that specifically targets this disease.”
Tazemetostat must be dispensed with a patient medication guide that describes important information about the drug’s uses and risks, the FDA said.
T-VEC plus pembrolizumab yields promising response rate in phase 2 sarcoma study
The combination of oncolytic immunotherapy and an immune checkpoint inhibitor had promising activity in a phase 2 study including patients with previously treated advanced sarcoma, investigators report.
Responses were seen in about one-third of the patients (30%) at 24 weeks after starting treatment with talimogene laherparepvec (T-VEC) and pembrolizumab, according to the investigators, led by Ciara M. Kelly, MBBChBAO, MD, of the department of sarcoma oncology at Memorial Sloan Kettering Cancer Center, New York.
“To our knowledge, this is one of the highest ORRs [objective response rates] reported in an unselected sarcoma-specific study population evaluating the efficacy of combination immunotherapy,” Dr. Kelly and coauthors wrote in JAMA Oncology.
Two of the patients who responded to the combination had previously failed regimens that included an immune checkpoint inhibitor, suggesting T-VEC might be augmenting the efficacy of pembrolizumab, according to the authors.
Among responders, the mean number of prior treatments was one, while by contrast, most of the study cohort (60%) had three or more prior treatments. “This finding supports the rationale to enroll patients with sarcoma in immunotherapy trials earlier in their treatment course,” Dr. Kelly and colleagues wrote.
In studies of immunotherapy in Merkel cell carcinoma and other cancer types, higher response rates have been seen in patients who were naive to chemotherapy, as opposed to those who were refractory to it.
The present single-institution, phase 2 study by Dr. Kelly and colleagues included 20 adult patients with histologically confirmed locally advanced or metastatic sarcoma. The patients received a fixed dose of pembrolizumab administered intravenously, and had T-VEC injected into palpable lesions, on day 1 of 21-day cycles.
While the ORR was 30% at 24 weeks (7 of 35 patients), an additional patient had a response at 32 weeks, bringing the ORR to 35%, according to the report.
“Maximal response to therapy may take a prolonged period to achieve,” the investigators wrote, noting that the median time to response was 14.4 weeks.
Median duration of response was 56.1 weeks, suggesting the combination provided durable disease control, they added.
Safety was consistent with what was seen in a previous study of T-VEC and pembrolizumab in melanoma. In this sarcoma study, the incidence of grade 3 treatment-related adverse events was 20%, while in studies of conventional chemotherapy in this setting, the rate of those events has been “comparable but generally higher,” the investigators wrote.
Dr. Kelly and colleagues wrote that a randomized clinical trial is the “ideal next step” in the development of the regimen, adding that an expansion of the present phase 2 study is in progress.
The study was funded by Amgen and Merck, with additional grant support from Cycle for Survival and the National Institutes of Health/National Cancer Institute. Dr. Kelly reported disclosures related to Merck, Amgen, Agios Pharmaceuticals, and Exicure. Her coauthors reported disclosures with Regeneron, Novartis, Takeda, and Nektar, among others.
SOURCE: Kelly CM et al. JAMA Oncol. 2020 Jan 23. doi: 10.1001/jamaoncol.2019.6152.
The combination of oncolytic immunotherapy and an immune checkpoint inhibitor had promising activity in a phase 2 study including patients with previously treated advanced sarcoma, investigators report.
Responses were seen in about one-third of the patients (30%) at 24 weeks after starting treatment with talimogene laherparepvec (T-VEC) and pembrolizumab, according to the investigators, led by Ciara M. Kelly, MBBChBAO, MD, of the department of sarcoma oncology at Memorial Sloan Kettering Cancer Center, New York.
“To our knowledge, this is one of the highest ORRs [objective response rates] reported in an unselected sarcoma-specific study population evaluating the efficacy of combination immunotherapy,” Dr. Kelly and coauthors wrote in JAMA Oncology.
Two of the patients who responded to the combination had previously failed regimens that included an immune checkpoint inhibitor, suggesting T-VEC might be augmenting the efficacy of pembrolizumab, according to the authors.
Among responders, the mean number of prior treatments was one, while by contrast, most of the study cohort (60%) had three or more prior treatments. “This finding supports the rationale to enroll patients with sarcoma in immunotherapy trials earlier in their treatment course,” Dr. Kelly and colleagues wrote.
In studies of immunotherapy in Merkel cell carcinoma and other cancer types, higher response rates have been seen in patients who were naive to chemotherapy, as opposed to those who were refractory to it.
The present single-institution, phase 2 study by Dr. Kelly and colleagues included 20 adult patients with histologically confirmed locally advanced or metastatic sarcoma. The patients received a fixed dose of pembrolizumab administered intravenously, and had T-VEC injected into palpable lesions, on day 1 of 21-day cycles.
While the ORR was 30% at 24 weeks (7 of 35 patients), an additional patient had a response at 32 weeks, bringing the ORR to 35%, according to the report.
“Maximal response to therapy may take a prolonged period to achieve,” the investigators wrote, noting that the median time to response was 14.4 weeks.
Median duration of response was 56.1 weeks, suggesting the combination provided durable disease control, they added.
Safety was consistent with what was seen in a previous study of T-VEC and pembrolizumab in melanoma. In this sarcoma study, the incidence of grade 3 treatment-related adverse events was 20%, while in studies of conventional chemotherapy in this setting, the rate of those events has been “comparable but generally higher,” the investigators wrote.
Dr. Kelly and colleagues wrote that a randomized clinical trial is the “ideal next step” in the development of the regimen, adding that an expansion of the present phase 2 study is in progress.
The study was funded by Amgen and Merck, with additional grant support from Cycle for Survival and the National Institutes of Health/National Cancer Institute. Dr. Kelly reported disclosures related to Merck, Amgen, Agios Pharmaceuticals, and Exicure. Her coauthors reported disclosures with Regeneron, Novartis, Takeda, and Nektar, among others.
SOURCE: Kelly CM et al. JAMA Oncol. 2020 Jan 23. doi: 10.1001/jamaoncol.2019.6152.
The combination of oncolytic immunotherapy and an immune checkpoint inhibitor had promising activity in a phase 2 study including patients with previously treated advanced sarcoma, investigators report.
Responses were seen in about one-third of the patients (30%) at 24 weeks after starting treatment with talimogene laherparepvec (T-VEC) and pembrolizumab, according to the investigators, led by Ciara M. Kelly, MBBChBAO, MD, of the department of sarcoma oncology at Memorial Sloan Kettering Cancer Center, New York.
“To our knowledge, this is one of the highest ORRs [objective response rates] reported in an unselected sarcoma-specific study population evaluating the efficacy of combination immunotherapy,” Dr. Kelly and coauthors wrote in JAMA Oncology.
Two of the patients who responded to the combination had previously failed regimens that included an immune checkpoint inhibitor, suggesting T-VEC might be augmenting the efficacy of pembrolizumab, according to the authors.
Among responders, the mean number of prior treatments was one, while by contrast, most of the study cohort (60%) had three or more prior treatments. “This finding supports the rationale to enroll patients with sarcoma in immunotherapy trials earlier in their treatment course,” Dr. Kelly and colleagues wrote.
In studies of immunotherapy in Merkel cell carcinoma and other cancer types, higher response rates have been seen in patients who were naive to chemotherapy, as opposed to those who were refractory to it.
The present single-institution, phase 2 study by Dr. Kelly and colleagues included 20 adult patients with histologically confirmed locally advanced or metastatic sarcoma. The patients received a fixed dose of pembrolizumab administered intravenously, and had T-VEC injected into palpable lesions, on day 1 of 21-day cycles.
While the ORR was 30% at 24 weeks (7 of 35 patients), an additional patient had a response at 32 weeks, bringing the ORR to 35%, according to the report.
“Maximal response to therapy may take a prolonged period to achieve,” the investigators wrote, noting that the median time to response was 14.4 weeks.
Median duration of response was 56.1 weeks, suggesting the combination provided durable disease control, they added.
Safety was consistent with what was seen in a previous study of T-VEC and pembrolizumab in melanoma. In this sarcoma study, the incidence of grade 3 treatment-related adverse events was 20%, while in studies of conventional chemotherapy in this setting, the rate of those events has been “comparable but generally higher,” the investigators wrote.
Dr. Kelly and colleagues wrote that a randomized clinical trial is the “ideal next step” in the development of the regimen, adding that an expansion of the present phase 2 study is in progress.
The study was funded by Amgen and Merck, with additional grant support from Cycle for Survival and the National Institutes of Health/National Cancer Institute. Dr. Kelly reported disclosures related to Merck, Amgen, Agios Pharmaceuticals, and Exicure. Her coauthors reported disclosures with Regeneron, Novartis, Takeda, and Nektar, among others.
SOURCE: Kelly CM et al. JAMA Oncol. 2020 Jan 23. doi: 10.1001/jamaoncol.2019.6152.
FROM JAMA ONCOLOGY
FDA approves avapritinib for adults with GIST with PDGFRA mutation
The Food and Drug Administration has approved avapritinib (Ayvakit) for the treatment of adults with unresectable or metastatic gastrointestinal stromal tumors (GIST) with a platelet-derived growth factor receptor–alpha (PDGFRA) exon 18 mutation.
Approval was based on results from a clinical trial of 43 patients with PDGFRA exon 18 mutations, including 38 patients with a PDGFRA D842V mutation, who received 300 mg avapritinib once daily, the FDA said in a statement.
The overall response rate was 84% (7% with complete response, 77% with partial response); the response rate in patients with a D842V mutation was 89% (8% with complete response, 82% with partial response). Median response duration was not reached, but 61% of patients had a response lasting longer than 6 months.
The most common adverse events associated with avapritinib include edema, nausea, fatigue/asthenia, cognitive impairment, vomiting, decreased appetite, diarrhea, hair color changes, increased lacrimation, abdominal pain, constipation, rash, and dizziness. The drug also can cause intracranial hemorrhage and have effects on the central nervous system.
“GIST harboring a PDGFRA exon 18 mutation do not respond to standard therapies for GIST. However, today’s approval provides patients with the first drug specifically approved for GIST harboring this mutation,” Richard Pazdur, MD, director of the FDA’s Oncology Center of Excellence and acting director of the Office of Oncologic Diseases in the Center for Drug Evaluation and Research, said in the statement.
The Food and Drug Administration has approved avapritinib (Ayvakit) for the treatment of adults with unresectable or metastatic gastrointestinal stromal tumors (GIST) with a platelet-derived growth factor receptor–alpha (PDGFRA) exon 18 mutation.
Approval was based on results from a clinical trial of 43 patients with PDGFRA exon 18 mutations, including 38 patients with a PDGFRA D842V mutation, who received 300 mg avapritinib once daily, the FDA said in a statement.
The overall response rate was 84% (7% with complete response, 77% with partial response); the response rate in patients with a D842V mutation was 89% (8% with complete response, 82% with partial response). Median response duration was not reached, but 61% of patients had a response lasting longer than 6 months.
The most common adverse events associated with avapritinib include edema, nausea, fatigue/asthenia, cognitive impairment, vomiting, decreased appetite, diarrhea, hair color changes, increased lacrimation, abdominal pain, constipation, rash, and dizziness. The drug also can cause intracranial hemorrhage and have effects on the central nervous system.
“GIST harboring a PDGFRA exon 18 mutation do not respond to standard therapies for GIST. However, today’s approval provides patients with the first drug specifically approved for GIST harboring this mutation,” Richard Pazdur, MD, director of the FDA’s Oncology Center of Excellence and acting director of the Office of Oncologic Diseases in the Center for Drug Evaluation and Research, said in the statement.
The Food and Drug Administration has approved avapritinib (Ayvakit) for the treatment of adults with unresectable or metastatic gastrointestinal stromal tumors (GIST) with a platelet-derived growth factor receptor–alpha (PDGFRA) exon 18 mutation.
Approval was based on results from a clinical trial of 43 patients with PDGFRA exon 18 mutations, including 38 patients with a PDGFRA D842V mutation, who received 300 mg avapritinib once daily, the FDA said in a statement.
The overall response rate was 84% (7% with complete response, 77% with partial response); the response rate in patients with a D842V mutation was 89% (8% with complete response, 82% with partial response). Median response duration was not reached, but 61% of patients had a response lasting longer than 6 months.
The most common adverse events associated with avapritinib include edema, nausea, fatigue/asthenia, cognitive impairment, vomiting, decreased appetite, diarrhea, hair color changes, increased lacrimation, abdominal pain, constipation, rash, and dizziness. The drug also can cause intracranial hemorrhage and have effects on the central nervous system.
“GIST harboring a PDGFRA exon 18 mutation do not respond to standard therapies for GIST. However, today’s approval provides patients with the first drug specifically approved for GIST harboring this mutation,” Richard Pazdur, MD, director of the FDA’s Oncology Center of Excellence and acting director of the Office of Oncologic Diseases in the Center for Drug Evaluation and Research, said in the statement.
FDA Awards Grant To Study Temozolomide in Gist
A phase 2 study of temozolomide in gastrointestinal stromal tumors (GIST) received one of the 12 grants awarded in October by the US Food and Drug Administration (FDA) to enhance the development of medical products for patients with rare diseases. Jason Sicklick, MD, and the University of California San Diego in La Jolla will receive $1.5 million over 3 years to conduct the phase 2 study (NCT03556384). The objective of the study is to determine the efficacy at 6 months of temozolomide therapy in patients with the SDH-mutant/deficient subtype. Temozolomide is approved by the FDA to treat newly diagnosed glioblastoma multiforme and refractory anaplastic astrocytomas. It is not approved for the treatment of SDH-mutant/deficient GIST.
The FDA awarded the grants through the Orphan Products Clinical Trials Grants Program. Other orphan diseases receiving grants included glomerulopathy, gliomas, Fanconi anemia, sickle cell respiratory complications, Duchenne muscular dystrophy, HPV associated respiratory papillomatosis, refractory viral infections and T-cell immunodeficiency, oral cancer, retinoblastoma, cerebellar brain tumors, and acute myeloid leukemia.
A phase 2 study of temozolomide in gastrointestinal stromal tumors (GIST) received one of the 12 grants awarded in October by the US Food and Drug Administration (FDA) to enhance the development of medical products for patients with rare diseases. Jason Sicklick, MD, and the University of California San Diego in La Jolla will receive $1.5 million over 3 years to conduct the phase 2 study (NCT03556384). The objective of the study is to determine the efficacy at 6 months of temozolomide therapy in patients with the SDH-mutant/deficient subtype. Temozolomide is approved by the FDA to treat newly diagnosed glioblastoma multiforme and refractory anaplastic astrocytomas. It is not approved for the treatment of SDH-mutant/deficient GIST.
The FDA awarded the grants through the Orphan Products Clinical Trials Grants Program. Other orphan diseases receiving grants included glomerulopathy, gliomas, Fanconi anemia, sickle cell respiratory complications, Duchenne muscular dystrophy, HPV associated respiratory papillomatosis, refractory viral infections and T-cell immunodeficiency, oral cancer, retinoblastoma, cerebellar brain tumors, and acute myeloid leukemia.
A phase 2 study of temozolomide in gastrointestinal stromal tumors (GIST) received one of the 12 grants awarded in October by the US Food and Drug Administration (FDA) to enhance the development of medical products for patients with rare diseases. Jason Sicklick, MD, and the University of California San Diego in La Jolla will receive $1.5 million over 3 years to conduct the phase 2 study (NCT03556384). The objective of the study is to determine the efficacy at 6 months of temozolomide therapy in patients with the SDH-mutant/deficient subtype. Temozolomide is approved by the FDA to treat newly diagnosed glioblastoma multiforme and refractory anaplastic astrocytomas. It is not approved for the treatment of SDH-mutant/deficient GIST.
The FDA awarded the grants through the Orphan Products Clinical Trials Grants Program. Other orphan diseases receiving grants included glomerulopathy, gliomas, Fanconi anemia, sickle cell respiratory complications, Duchenne muscular dystrophy, HPV associated respiratory papillomatosis, refractory viral infections and T-cell immunodeficiency, oral cancer, retinoblastoma, cerebellar brain tumors, and acute myeloid leukemia.