User login
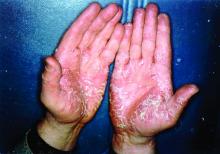
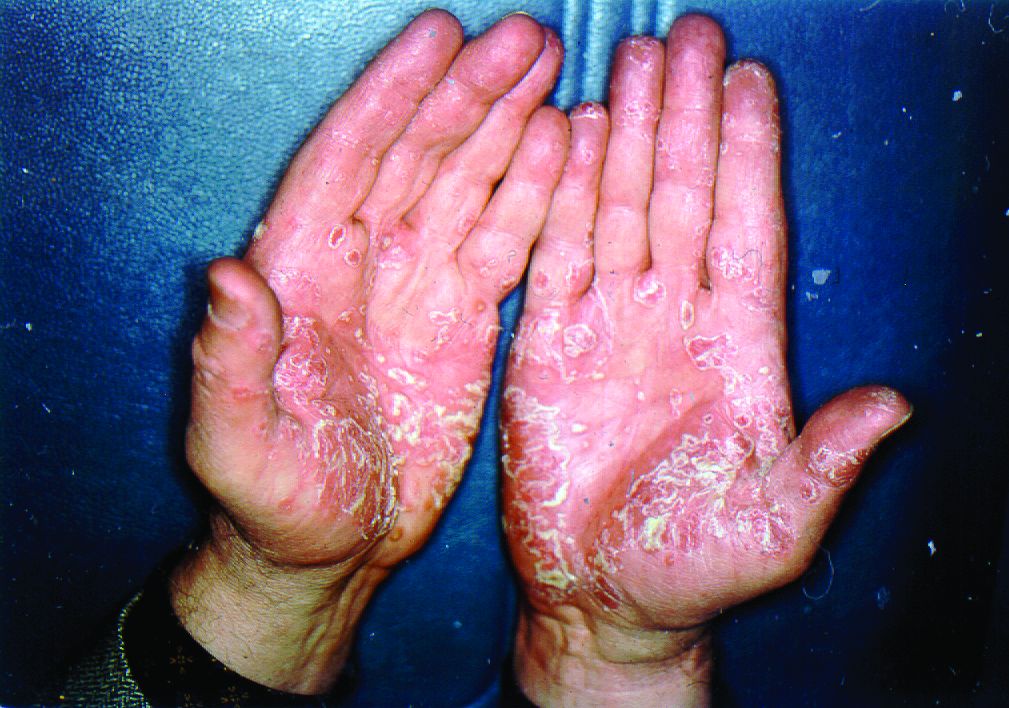
Phase 3 trials show halobetasol/tazarotene lotion works for psoriasis
KAUAI, HAWAII – in two phase 3 randomized, double-blind, multicenter clinical trials, Linda Stein Gold, MD, reported at the Hawaii Dermatology Seminar provided by the Global Academy for Medical Education/Skin Disease Education Foundation.
The fixed combination of halobetasol 0.01%/tazarotene 0.045% lotion takes advantage of an observation made 20 years ago: When tazarotene is combined with a potent topical corticosteroid, therapeutic efficacy is amplified synergistically while the problematic local side effects of each agent are diminished, explained Dr. Stein Gold, director of dermatology research at the Henry Ford Health System, Detroit.
“Tazarotene: Great for acne, but think of it again for psoriasis,” Dr. Stein Gold said. “It makes sense. Tazarotene improves differentiation of the skin; it decreases inflammation; it decreases proliferation – it does all the good things that we want to do for psoriasis.
“It’s got a little bit of baggage, though,” she continued. “It’s pregnancy Category X, so you have to make sure a woman who is or may become pregnant is not using it. And there are some side effects. It can be tough to use. When you use it in psoriasis you can get local irritation up to 30% of the time.”
The two parallel phase 3 randomized trials plus a separate phase 2 study, all of which Dr. Stein Gold was involved in, showed that the efficacy of the investigational halobetasol/tazarotene fixed combination was greater than either component alone, side effects were minimized, and efficacy remained durable 4 weeks after the 8-week treatment course ended.
The not-yet-published phase 3 trials included 418 patients with moderate to severe psoriasis randomized 2:1 to once-daily application of halobetasol/tazarotene or its vehicle for 8 weeks. Treatment success, defined as at least a two-grade improvement from baseline in Investigator’s Global Assessment score plus a score of clear or almost clear, was documented at 8 weeks in 35.8% of the halobetasol/tazarotene group in one study and 45.3% in the other, compared with 7% and 12.5% of controls, respectively.
In addition, after 8 weeks, affected body surface area was reduced by a mean of 32.8% in one study and by 42.5% in the other. There was also at least a two-grade improvement in plaque erythema at the target lesion site in 42.2% and 49.6% of halobetasol/tazarotene–treated patients in the two trials. A two-grade improvement in plaque elevation was noted in 59.3% and 59.7% of patients, while for plaque scaling, the figures were 59.4% and 62.9%.
“What we found in the two sister studies was statistically significant success in getting those plaques from moderate/severe all the way down to clear/almost clear,” Dr. Stein Gold said.
The most frequently reported treatment-emergent adverse events included contact dermatitis in 7.4% of the active treatment group and application site pain in 2.6%. Most side effects were mild or moderate in nature.
The phase 2 study, which included 212 psoriasis patients, looked specifically at maintenance of efficacy after end of treatment. Here, halobetasol/tazarotene showed durability of therapeutic benefit: 4 weeks after completing the 8-week course of once-daily halobetasol/tazarotene, 38.2% of patients still met the criteria for treatment success. The minimal skin atrophy that arose during treatment largely resolved during the subsequent 4 weeks off treatment.
The clinical trials were supported by Valeant. Dr. Stein Gold reported receiving research grants from and serving as a consultant to, paid speaker for, and scientific advisory board member for Valeant and numerous other pharmaceutical companies active in dermatologic drug development.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
KAUAI, HAWAII – in two phase 3 randomized, double-blind, multicenter clinical trials, Linda Stein Gold, MD, reported at the Hawaii Dermatology Seminar provided by the Global Academy for Medical Education/Skin Disease Education Foundation.
The fixed combination of halobetasol 0.01%/tazarotene 0.045% lotion takes advantage of an observation made 20 years ago: When tazarotene is combined with a potent topical corticosteroid, therapeutic efficacy is amplified synergistically while the problematic local side effects of each agent are diminished, explained Dr. Stein Gold, director of dermatology research at the Henry Ford Health System, Detroit.
“Tazarotene: Great for acne, but think of it again for psoriasis,” Dr. Stein Gold said. “It makes sense. Tazarotene improves differentiation of the skin; it decreases inflammation; it decreases proliferation – it does all the good things that we want to do for psoriasis.
“It’s got a little bit of baggage, though,” she continued. “It’s pregnancy Category X, so you have to make sure a woman who is or may become pregnant is not using it. And there are some side effects. It can be tough to use. When you use it in psoriasis you can get local irritation up to 30% of the time.”
The two parallel phase 3 randomized trials plus a separate phase 2 study, all of which Dr. Stein Gold was involved in, showed that the efficacy of the investigational halobetasol/tazarotene fixed combination was greater than either component alone, side effects were minimized, and efficacy remained durable 4 weeks after the 8-week treatment course ended.
The not-yet-published phase 3 trials included 418 patients with moderate to severe psoriasis randomized 2:1 to once-daily application of halobetasol/tazarotene or its vehicle for 8 weeks. Treatment success, defined as at least a two-grade improvement from baseline in Investigator’s Global Assessment score plus a score of clear or almost clear, was documented at 8 weeks in 35.8% of the halobetasol/tazarotene group in one study and 45.3% in the other, compared with 7% and 12.5% of controls, respectively.
In addition, after 8 weeks, affected body surface area was reduced by a mean of 32.8% in one study and by 42.5% in the other. There was also at least a two-grade improvement in plaque erythema at the target lesion site in 42.2% and 49.6% of halobetasol/tazarotene–treated patients in the two trials. A two-grade improvement in plaque elevation was noted in 59.3% and 59.7% of patients, while for plaque scaling, the figures were 59.4% and 62.9%.
“What we found in the two sister studies was statistically significant success in getting those plaques from moderate/severe all the way down to clear/almost clear,” Dr. Stein Gold said.
The most frequently reported treatment-emergent adverse events included contact dermatitis in 7.4% of the active treatment group and application site pain in 2.6%. Most side effects were mild or moderate in nature.
The phase 2 study, which included 212 psoriasis patients, looked specifically at maintenance of efficacy after end of treatment. Here, halobetasol/tazarotene showed durability of therapeutic benefit: 4 weeks after completing the 8-week course of once-daily halobetasol/tazarotene, 38.2% of patients still met the criteria for treatment success. The minimal skin atrophy that arose during treatment largely resolved during the subsequent 4 weeks off treatment.
The clinical trials were supported by Valeant. Dr. Stein Gold reported receiving research grants from and serving as a consultant to, paid speaker for, and scientific advisory board member for Valeant and numerous other pharmaceutical companies active in dermatologic drug development.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
KAUAI, HAWAII – in two phase 3 randomized, double-blind, multicenter clinical trials, Linda Stein Gold, MD, reported at the Hawaii Dermatology Seminar provided by the Global Academy for Medical Education/Skin Disease Education Foundation.
The fixed combination of halobetasol 0.01%/tazarotene 0.045% lotion takes advantage of an observation made 20 years ago: When tazarotene is combined with a potent topical corticosteroid, therapeutic efficacy is amplified synergistically while the problematic local side effects of each agent are diminished, explained Dr. Stein Gold, director of dermatology research at the Henry Ford Health System, Detroit.
“Tazarotene: Great for acne, but think of it again for psoriasis,” Dr. Stein Gold said. “It makes sense. Tazarotene improves differentiation of the skin; it decreases inflammation; it decreases proliferation – it does all the good things that we want to do for psoriasis.
“It’s got a little bit of baggage, though,” she continued. “It’s pregnancy Category X, so you have to make sure a woman who is or may become pregnant is not using it. And there are some side effects. It can be tough to use. When you use it in psoriasis you can get local irritation up to 30% of the time.”
The two parallel phase 3 randomized trials plus a separate phase 2 study, all of which Dr. Stein Gold was involved in, showed that the efficacy of the investigational halobetasol/tazarotene fixed combination was greater than either component alone, side effects were minimized, and efficacy remained durable 4 weeks after the 8-week treatment course ended.
The not-yet-published phase 3 trials included 418 patients with moderate to severe psoriasis randomized 2:1 to once-daily application of halobetasol/tazarotene or its vehicle for 8 weeks. Treatment success, defined as at least a two-grade improvement from baseline in Investigator’s Global Assessment score plus a score of clear or almost clear, was documented at 8 weeks in 35.8% of the halobetasol/tazarotene group in one study and 45.3% in the other, compared with 7% and 12.5% of controls, respectively.
In addition, after 8 weeks, affected body surface area was reduced by a mean of 32.8% in one study and by 42.5% in the other. There was also at least a two-grade improvement in plaque erythema at the target lesion site in 42.2% and 49.6% of halobetasol/tazarotene–treated patients in the two trials. A two-grade improvement in plaque elevation was noted in 59.3% and 59.7% of patients, while for plaque scaling, the figures were 59.4% and 62.9%.
“What we found in the two sister studies was statistically significant success in getting those plaques from moderate/severe all the way down to clear/almost clear,” Dr. Stein Gold said.
The most frequently reported treatment-emergent adverse events included contact dermatitis in 7.4% of the active treatment group and application site pain in 2.6%. Most side effects were mild or moderate in nature.
The phase 2 study, which included 212 psoriasis patients, looked specifically at maintenance of efficacy after end of treatment. Here, halobetasol/tazarotene showed durability of therapeutic benefit: 4 weeks after completing the 8-week course of once-daily halobetasol/tazarotene, 38.2% of patients still met the criteria for treatment success. The minimal skin atrophy that arose during treatment largely resolved during the subsequent 4 weeks off treatment.
The clinical trials were supported by Valeant. Dr. Stein Gold reported receiving research grants from and serving as a consultant to, paid speaker for, and scientific advisory board member for Valeant and numerous other pharmaceutical companies active in dermatologic drug development.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
EXPERT ANALYSIS FROM SDEF HAWAII DERMATOLOGY SEMINAR
Online psoriasis consultations shown equivalent to office visits
SAN DIEGO – Online consultations between dermatologists, patients with psoriasis, and the patients’ primary care physicians were as effective as in-person consultations in successfully treating the disease in a multicenter, randomized study of 296 patients.
“Innovative telehealth delivery models that emphasize collaboration, quality, and efficiency can be transformative to improving patient-centered outcomes in chronic disease,” April W. Armstrong, MD, said at the annual meeting of the American Academy of Dermatology. The online model she tested fostered “increased patient engagement” and provided “comprehensive specialist support,” said Dr. Armstrong, director of the psoriasis program at the University of Southern California, Los Angeles.
To objectively assess whether online consultations are as effective as in-person examinations, Dr. Armstrong and her associates at three U.S. centers randomized adult psoriasis patients from across the disease spectrum to receive 1 year of dermatology care either in person or online. Patients enrolled in the online arm received training in taking digital images of their skin lesions and uploading the data for remote access by their dermatologist and primary care physician. The frequency of in-person and online consultations was left to the discretion of each patient and his or her physician.
Among the 148 patients randomized to each arm, 17 in the online group and 13 in the in-person group withdrew from the study or were lost to follow-up. The researchers analyzed the results on an intention-to-treat basis.
They assessed three parameters of treatment efficacy that they measured at baseline and then every 3 months out to 1 year: Psoriasis Area and Severity Index, body surface area score, and patient global self-assessment. A comparison of changes between the two treatment arms after 1 year for the first two measures met the study’s prespecified definition of equivalence, Dr. Armstrong reported. The third measure, a patient’s global self-assessment, showed lower patient-assessed disease severity after 1 year among the patients managed online, compared with those managed in person.
The incidence of adverse events and serious adverse events was similar in the two treatment arms.
Dr. Armstrong had no relevant financial disclosures.
Source: Armstrong A et al. AAD 2018, abstract 6730..
SAN DIEGO – Online consultations between dermatologists, patients with psoriasis, and the patients’ primary care physicians were as effective as in-person consultations in successfully treating the disease in a multicenter, randomized study of 296 patients.
“Innovative telehealth delivery models that emphasize collaboration, quality, and efficiency can be transformative to improving patient-centered outcomes in chronic disease,” April W. Armstrong, MD, said at the annual meeting of the American Academy of Dermatology. The online model she tested fostered “increased patient engagement” and provided “comprehensive specialist support,” said Dr. Armstrong, director of the psoriasis program at the University of Southern California, Los Angeles.
To objectively assess whether online consultations are as effective as in-person examinations, Dr. Armstrong and her associates at three U.S. centers randomized adult psoriasis patients from across the disease spectrum to receive 1 year of dermatology care either in person or online. Patients enrolled in the online arm received training in taking digital images of their skin lesions and uploading the data for remote access by their dermatologist and primary care physician. The frequency of in-person and online consultations was left to the discretion of each patient and his or her physician.
Among the 148 patients randomized to each arm, 17 in the online group and 13 in the in-person group withdrew from the study or were lost to follow-up. The researchers analyzed the results on an intention-to-treat basis.
They assessed three parameters of treatment efficacy that they measured at baseline and then every 3 months out to 1 year: Psoriasis Area and Severity Index, body surface area score, and patient global self-assessment. A comparison of changes between the two treatment arms after 1 year for the first two measures met the study’s prespecified definition of equivalence, Dr. Armstrong reported. The third measure, a patient’s global self-assessment, showed lower patient-assessed disease severity after 1 year among the patients managed online, compared with those managed in person.
The incidence of adverse events and serious adverse events was similar in the two treatment arms.
Dr. Armstrong had no relevant financial disclosures.
Source: Armstrong A et al. AAD 2018, abstract 6730..
SAN DIEGO – Online consultations between dermatologists, patients with psoriasis, and the patients’ primary care physicians were as effective as in-person consultations in successfully treating the disease in a multicenter, randomized study of 296 patients.
“Innovative telehealth delivery models that emphasize collaboration, quality, and efficiency can be transformative to improving patient-centered outcomes in chronic disease,” April W. Armstrong, MD, said at the annual meeting of the American Academy of Dermatology. The online model she tested fostered “increased patient engagement” and provided “comprehensive specialist support,” said Dr. Armstrong, director of the psoriasis program at the University of Southern California, Los Angeles.
To objectively assess whether online consultations are as effective as in-person examinations, Dr. Armstrong and her associates at three U.S. centers randomized adult psoriasis patients from across the disease spectrum to receive 1 year of dermatology care either in person or online. Patients enrolled in the online arm received training in taking digital images of their skin lesions and uploading the data for remote access by their dermatologist and primary care physician. The frequency of in-person and online consultations was left to the discretion of each patient and his or her physician.
Among the 148 patients randomized to each arm, 17 in the online group and 13 in the in-person group withdrew from the study or were lost to follow-up. The researchers analyzed the results on an intention-to-treat basis.
They assessed three parameters of treatment efficacy that they measured at baseline and then every 3 months out to 1 year: Psoriasis Area and Severity Index, body surface area score, and patient global self-assessment. A comparison of changes between the two treatment arms after 1 year for the first two measures met the study’s prespecified definition of equivalence, Dr. Armstrong reported. The third measure, a patient’s global self-assessment, showed lower patient-assessed disease severity after 1 year among the patients managed online, compared with those managed in person.
The incidence of adverse events and serious adverse events was similar in the two treatment arms.
Dr. Armstrong had no relevant financial disclosures.
Source: Armstrong A et al. AAD 2018, abstract 6730..
REPORTING FROM AAD 18
Key clinical point: Online physician telemonitoring of psoriasis patients was equivalent to in-person management.
Major finding: After 1 year, changes in the Psoriasis Area and Severity Index in the two arms met the prespecified definition of equivalence.
Study details: A multicenter, randomized trial with 296 psoriasis patients in which outcomes were compared for online monitoring and in-person examinations.
Disclosures: Dr. Armstrong had no relevant financial disclosures.
Source: Armstrong A et al. AAD 18, abstract 6730.
VIDEO: PPACMAN aims to advance the combined rheum-derm clinic approach in the community
SAN DIEGO – A new endeavor that aims to promote the concept of the combined clinic approach to caring for psoriatic patients is now underway.
PPACMAN (Psoriasis and Psoriatic Arthritis Clinics Multicenter Advancement Network) is made up of dermatologists and rheumatologists who play a key role in the management of psoriatic disease and are interested in combined clinics, with the mission “to nucleate psoriatic disease combined clinics and centers to advance a multilevel approach to psoriatic patients, increase disease awareness, and accelerate management,” according to Joseph Merola, MD, codirector of the center for skin and related musculoskeletal diseases at Brigham and Women’s Hospital, Boston.
There are now about 12 centers in North America with formal rheumatology-dermatology clinics for patients with psoriasis and psoriatic arthritis, including the one at Brigham and Women’s, where Dr. Merola and his colleagues have seen the “myriad benefits that come with having a combined clinic,” he said in a video interview at the annual meeting of the American Academy of Dermatology. The idea behind starting PPACMAN was to help form new clinics at academic centers but, also, “to start to catalyze local-regional partnerships in the community so we could get dermatologists and rheumatologists in the community to start interacting, communicating, [and] sharing patients,” he explained.
“The group is really very much focused on this mission of getting combined ... treatment models out there,” added Dr. Merola, president and chair of the board of PPACMAN, which is a 501c3 nonprofit organization.
In the interview, he discusses other benefits of the combined clinic model and other elements of the PPACMAN mission, including education and the potential for shared EMR templates.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
SAN DIEGO – A new endeavor that aims to promote the concept of the combined clinic approach to caring for psoriatic patients is now underway.
PPACMAN (Psoriasis and Psoriatic Arthritis Clinics Multicenter Advancement Network) is made up of dermatologists and rheumatologists who play a key role in the management of psoriatic disease and are interested in combined clinics, with the mission “to nucleate psoriatic disease combined clinics and centers to advance a multilevel approach to psoriatic patients, increase disease awareness, and accelerate management,” according to Joseph Merola, MD, codirector of the center for skin and related musculoskeletal diseases at Brigham and Women’s Hospital, Boston.
There are now about 12 centers in North America with formal rheumatology-dermatology clinics for patients with psoriasis and psoriatic arthritis, including the one at Brigham and Women’s, where Dr. Merola and his colleagues have seen the “myriad benefits that come with having a combined clinic,” he said in a video interview at the annual meeting of the American Academy of Dermatology. The idea behind starting PPACMAN was to help form new clinics at academic centers but, also, “to start to catalyze local-regional partnerships in the community so we could get dermatologists and rheumatologists in the community to start interacting, communicating, [and] sharing patients,” he explained.
“The group is really very much focused on this mission of getting combined ... treatment models out there,” added Dr. Merola, president and chair of the board of PPACMAN, which is a 501c3 nonprofit organization.
In the interview, he discusses other benefits of the combined clinic model and other elements of the PPACMAN mission, including education and the potential for shared EMR templates.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
SAN DIEGO – A new endeavor that aims to promote the concept of the combined clinic approach to caring for psoriatic patients is now underway.
PPACMAN (Psoriasis and Psoriatic Arthritis Clinics Multicenter Advancement Network) is made up of dermatologists and rheumatologists who play a key role in the management of psoriatic disease and are interested in combined clinics, with the mission “to nucleate psoriatic disease combined clinics and centers to advance a multilevel approach to psoriatic patients, increase disease awareness, and accelerate management,” according to Joseph Merola, MD, codirector of the center for skin and related musculoskeletal diseases at Brigham and Women’s Hospital, Boston.
There are now about 12 centers in North America with formal rheumatology-dermatology clinics for patients with psoriasis and psoriatic arthritis, including the one at Brigham and Women’s, where Dr. Merola and his colleagues have seen the “myriad benefits that come with having a combined clinic,” he said in a video interview at the annual meeting of the American Academy of Dermatology. The idea behind starting PPACMAN was to help form new clinics at academic centers but, also, “to start to catalyze local-regional partnerships in the community so we could get dermatologists and rheumatologists in the community to start interacting, communicating, [and] sharing patients,” he explained.
“The group is really very much focused on this mission of getting combined ... treatment models out there,” added Dr. Merola, president and chair of the board of PPACMAN, which is a 501c3 nonprofit organization.
In the interview, he discusses other benefits of the combined clinic model and other elements of the PPACMAN mission, including education and the potential for shared EMR templates.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
REPORTING FROM AAD 18
Could guselkumab be a disease-modifying agent in plaque psoriasis?
SAN DIEGO – Could some of the monoclonal antibodies posting striking results in psoriasis trials be doing more than quelling symptoms?
At least some researchers think so, as evidenced by a brief discussion during AAD 2018 of the durable responses some guselkumab-treated patients achieved in the VOYAGE 2 trial.
“Isn’t this amazing?” asked Kristian Reich, MD, after listening to several late-breaking, solidly positive trials of monoclonal antibodies for plaque psoriasis. “I think it’s fantastic that we now have drugs that clear 50% or more of a patient’s psoriasis. We should not be taking this for granted.”
VOYAGE 2 was an active-comparator, placebo-controlled study that pitted guselkumab against adalimumab (Humira) and placebo in a crossover design. It enrolled about 900 patients with moderate to severe plaque psoriasis.
Patients were randomized to 28 weeks of treatment in three arms: guselkumab 100 mg (weeks 0 and 4, then every 8 weeks); placebo for 16 weeks, then guselkumab 100 mg at weeks 16 and 20; or adalimumab (80 mg at week 0, then 40 mg at week 1, and every 2 weeks through week 23).
At 28 weeks, a total of 375 Psoriasis Area and Severity Index (PASI) 90 treatment responders in the guselkumab arm were rerandomized to either stay on guselkumab (n = 193) or withdraw to placebo (n = 182) until they lost whatever response they had gained at that point.
Although PASI 90 responses were much better maintained in the guselkumab group that stayed on therapy, they did not fade quickly. And although 89% of the maintenance group maintained their PASI 90 response at 48 weeks, 37% of those in the withdrawal group had still maintained that 90% improvement over baseline by 48 weeks.
“Is this drug opening the door to disease modification? Is it doing something that allows disease control even if we stop the therapy? This is what we see happening when we stop the drug in PASI 90 responders. Yes, the disease is coming back, but the median time to recurrence is more than 3 months.”
The cytokine profiles of these patients appear to support this idea, Dr. Reich contended.
“In the first 28 weeks, when they were all receiving the drug, their IL-23, IL-17A, and IL-17F levels were all going down rapidly. But this is the interesting part. In some patients who maintained their PASI response after withdrawal, those cytokines continued to be suppressed. They rose in patients who lost response. We need to do more tests to understand what’s going on here, but I do think the door is opening to what I would call disease modification.”
“I admit, I did at one time believe this story about disease modification,” said Dr. Papp, founder and president of Probity Medical Research in Waterloo, Ont. “But now I think we are simply seeing a pharmacokinetic effect. How can you reconcile what is clearly a pharmacologic and mechanistic perspective with this suggestion that you’re modifying disease?”
Session moderator Hensin Tsao, MD, suggested that the answer might lie in some unknown in-between territory.
“We do see about 10%-20% of patients in whom drug-free remission is not explained by pharmacokinetics. In some patients, the drug is long gone, and they are still clear of disease – and we don’t know how to talk about those patients yet. But we do need to study them because, for those people, clearly it is not a [pharmacokinetic] issue.”
Dr. Reich disclosed financial relationships with numerous pharmaceutical companies, including Janssen, which manufactures guselkumab. Dr. Papp also disclosed multiple relationships with drug manufacturers.
SOURCE: Gordon K et al. AAD 2018 Abstract 6748.
SAN DIEGO – Could some of the monoclonal antibodies posting striking results in psoriasis trials be doing more than quelling symptoms?
At least some researchers think so, as evidenced by a brief discussion during AAD 2018 of the durable responses some guselkumab-treated patients achieved in the VOYAGE 2 trial.
“Isn’t this amazing?” asked Kristian Reich, MD, after listening to several late-breaking, solidly positive trials of monoclonal antibodies for plaque psoriasis. “I think it’s fantastic that we now have drugs that clear 50% or more of a patient’s psoriasis. We should not be taking this for granted.”
VOYAGE 2 was an active-comparator, placebo-controlled study that pitted guselkumab against adalimumab (Humira) and placebo in a crossover design. It enrolled about 900 patients with moderate to severe plaque psoriasis.
Patients were randomized to 28 weeks of treatment in three arms: guselkumab 100 mg (weeks 0 and 4, then every 8 weeks); placebo for 16 weeks, then guselkumab 100 mg at weeks 16 and 20; or adalimumab (80 mg at week 0, then 40 mg at week 1, and every 2 weeks through week 23).
At 28 weeks, a total of 375 Psoriasis Area and Severity Index (PASI) 90 treatment responders in the guselkumab arm were rerandomized to either stay on guselkumab (n = 193) or withdraw to placebo (n = 182) until they lost whatever response they had gained at that point.
Although PASI 90 responses were much better maintained in the guselkumab group that stayed on therapy, they did not fade quickly. And although 89% of the maintenance group maintained their PASI 90 response at 48 weeks, 37% of those in the withdrawal group had still maintained that 90% improvement over baseline by 48 weeks.
“Is this drug opening the door to disease modification? Is it doing something that allows disease control even if we stop the therapy? This is what we see happening when we stop the drug in PASI 90 responders. Yes, the disease is coming back, but the median time to recurrence is more than 3 months.”
The cytokine profiles of these patients appear to support this idea, Dr. Reich contended.
“In the first 28 weeks, when they were all receiving the drug, their IL-23, IL-17A, and IL-17F levels were all going down rapidly. But this is the interesting part. In some patients who maintained their PASI response after withdrawal, those cytokines continued to be suppressed. They rose in patients who lost response. We need to do more tests to understand what’s going on here, but I do think the door is opening to what I would call disease modification.”
“I admit, I did at one time believe this story about disease modification,” said Dr. Papp, founder and president of Probity Medical Research in Waterloo, Ont. “But now I think we are simply seeing a pharmacokinetic effect. How can you reconcile what is clearly a pharmacologic and mechanistic perspective with this suggestion that you’re modifying disease?”
Session moderator Hensin Tsao, MD, suggested that the answer might lie in some unknown in-between territory.
“We do see about 10%-20% of patients in whom drug-free remission is not explained by pharmacokinetics. In some patients, the drug is long gone, and they are still clear of disease – and we don’t know how to talk about those patients yet. But we do need to study them because, for those people, clearly it is not a [pharmacokinetic] issue.”
Dr. Reich disclosed financial relationships with numerous pharmaceutical companies, including Janssen, which manufactures guselkumab. Dr. Papp also disclosed multiple relationships with drug manufacturers.
SOURCE: Gordon K et al. AAD 2018 Abstract 6748.
SAN DIEGO – Could some of the monoclonal antibodies posting striking results in psoriasis trials be doing more than quelling symptoms?
At least some researchers think so, as evidenced by a brief discussion during AAD 2018 of the durable responses some guselkumab-treated patients achieved in the VOYAGE 2 trial.
“Isn’t this amazing?” asked Kristian Reich, MD, after listening to several late-breaking, solidly positive trials of monoclonal antibodies for plaque psoriasis. “I think it’s fantastic that we now have drugs that clear 50% or more of a patient’s psoriasis. We should not be taking this for granted.”
VOYAGE 2 was an active-comparator, placebo-controlled study that pitted guselkumab against adalimumab (Humira) and placebo in a crossover design. It enrolled about 900 patients with moderate to severe plaque psoriasis.
Patients were randomized to 28 weeks of treatment in three arms: guselkumab 100 mg (weeks 0 and 4, then every 8 weeks); placebo for 16 weeks, then guselkumab 100 mg at weeks 16 and 20; or adalimumab (80 mg at week 0, then 40 mg at week 1, and every 2 weeks through week 23).
At 28 weeks, a total of 375 Psoriasis Area and Severity Index (PASI) 90 treatment responders in the guselkumab arm were rerandomized to either stay on guselkumab (n = 193) or withdraw to placebo (n = 182) until they lost whatever response they had gained at that point.
Although PASI 90 responses were much better maintained in the guselkumab group that stayed on therapy, they did not fade quickly. And although 89% of the maintenance group maintained their PASI 90 response at 48 weeks, 37% of those in the withdrawal group had still maintained that 90% improvement over baseline by 48 weeks.
“Is this drug opening the door to disease modification? Is it doing something that allows disease control even if we stop the therapy? This is what we see happening when we stop the drug in PASI 90 responders. Yes, the disease is coming back, but the median time to recurrence is more than 3 months.”
The cytokine profiles of these patients appear to support this idea, Dr. Reich contended.
“In the first 28 weeks, when they were all receiving the drug, their IL-23, IL-17A, and IL-17F levels were all going down rapidly. But this is the interesting part. In some patients who maintained their PASI response after withdrawal, those cytokines continued to be suppressed. They rose in patients who lost response. We need to do more tests to understand what’s going on here, but I do think the door is opening to what I would call disease modification.”
“I admit, I did at one time believe this story about disease modification,” said Dr. Papp, founder and president of Probity Medical Research in Waterloo, Ont. “But now I think we are simply seeing a pharmacokinetic effect. How can you reconcile what is clearly a pharmacologic and mechanistic perspective with this suggestion that you’re modifying disease?”
Session moderator Hensin Tsao, MD, suggested that the answer might lie in some unknown in-between territory.
“We do see about 10%-20% of patients in whom drug-free remission is not explained by pharmacokinetics. In some patients, the drug is long gone, and they are still clear of disease – and we don’t know how to talk about those patients yet. But we do need to study them because, for those people, clearly it is not a [pharmacokinetic] issue.”
Dr. Reich disclosed financial relationships with numerous pharmaceutical companies, including Janssen, which manufactures guselkumab. Dr. Papp also disclosed multiple relationships with drug manufacturers.
SOURCE: Gordon K et al. AAD 2018 Abstract 6748.
REPORTING FROM AAD 2018
Key clinical point: Guselkumab shows some signs of having a disease-modifying effect in moderate to severe psoriasis after 28 weeks of treatment.
Major finding: A total of 37% of patients who withdrew from guselkumab at 28 weeks had still maintained PASI 90 improvement over baseline at 48 weeks.
Study details: An analysis of randomization to drug continuation vs. withdrawal in 375 patients with PASI 90 response to guselkumab in the VOYAGE 2 trial.
Disclosures: Dr. Reich disclosed financial relationships with numerous pharmaceutical companies, including Janssen, which manufactures guselkumab. Dr. Papp also disclosed multiple relationships with drug manufacturers.
Source: Gordon K et al. AAD 2018 Abstract 6748.
Ustekinumab quells aortic inflammation in patients with severe psoriasis
SAN DIEGO – A 12-week course of ustekinumab significantly reduced inflammation in the aorta – an effect on par with the benefit of statins – in patients with moderate to severe plaque psoriasis.
Whether or not the reduction in aortic inflammation will translate into a reduction in cardiovascular risk remains to be determined, but investigators are very encouraged by the results of the Vascular Inflammation in Psoriasis-Ustekinumab (VIP-U) study, Joel M. Gelfand, MD, said at the annual meeting of the American Academy of Dermatology.
For the past decade, researchers have worked on the assumption that the chronic systemic inflammation of severe psoriasis overlaps with similar inflammatory pathways that drive atherosclerosis, plaques that rupture, and cardiovascular events.
Recently, they have employed fluorodeoxyglucose positron emission tomography (FDG-PET) to confirm some of this. Studies in 2011, 2015, and 2017 confirm that patients with severe plaque psoriasis can develop diffuse vascular inflammation with increased noncalcified coronary artery plaques. These more fragile plaques are the type that are prone to rupture and cause cardiovascular events, Dr. Gelfand said.
“In these studies, the more imaging signal we saw in the aorta, the higher the risk of a future cardiovascular event. In fact, we determined that patients with severe psoriasis can have increased aortic inflammation equivalent to a decade of aging. As your PASI [Psoriasis Area and Severity Index] score goes up, so does the amount of aortic inflammation. The anatomic consequence is that people develop a high risk of atherosclerotic plaques, and a higher rate of noncalcified coronary plaques that are more likely to lead to these events.”
The VIP-U study was conceived to examine whether calming psoriatic inflammation with ustekinumab (Stelara), an anti interleukin-12 and -23 antibody, could also improve vascular inflammation as measured by FDG-PET. The small study comprised 43 patients, half of whom received placebo and half of whom received two injections of ustekinumab: 45 or 90 mg depending on weight, at baseline and at week 4. There was an imaging assessment at week 12, after which the placebo patients crossed over to open-label ustekinumab every 2 weeks. Everyone was followed out to 64 weeks.
Dr. Gelfand reported the 12-week data; the 64-week data will be forthcoming later this year, he said.
The patients were typical for such a study, with a mean age of about 43 and a mean disease duration of 18 years. The mean body surface area was about 25%, and the mean PASI was 20. Most had been on prior therapy, including phototherapy, oral agents, and biologics.
Unsurprisingly, ustekinumab was significantly more effective than placebo in treating the psoriasis. At week 12, 10% of the placebo patients had achieved a PASI 75, compared with 77% of the ustekinumab patients. A Physicians Global Assessment (PGA) score of clear or almost clear occurred in 10% of those taking placebo and 64% of those taking the biologic.
However, the drug was also highly effective in reducing total aortic inflammation, Dr. Gelfand said. “In just 12 weeks, we saw a 6.6% reduction in total aortic inflammation among those taking ustekinumab, but a 12.1% increase in inflammation among those taking placebo. When you compare the delta, you see a highly statistically significant improvement in aortic inflammation in ustekinumab-treated patients, with the effect size similar to statin therapy.”
The benefit may be a class-associated one. Two recent similar studies of adalimumab, a tumor necrosis factor–alpha inhibitor, failed to find any improvement in vascular inflammation, compared with placebo.
“We need more information about the longer-term effects of ustekinumab treatment, as well as cardiometabolic biomarkers, and these are currently underway,” Dr. Gelfand said. “We also need additional trials to determine if this effect is due to the inhibition of IL-12, IL-23, or a combination. Cardiovascular studies will be necessary to fully determine the clinical implications of our findings.”
To refer a patient into the VIP studies, call 215-662-SKIN or email SkinVIP@upenn.edu.
The National Institutes of Health and University of Pennsylvania sponsored the study. Dr. Gelfand reported no financial disclosures.
SOURCE: Gelfand J et al. AAD 2018 Abstract 6645
SAN DIEGO – A 12-week course of ustekinumab significantly reduced inflammation in the aorta – an effect on par with the benefit of statins – in patients with moderate to severe plaque psoriasis.
Whether or not the reduction in aortic inflammation will translate into a reduction in cardiovascular risk remains to be determined, but investigators are very encouraged by the results of the Vascular Inflammation in Psoriasis-Ustekinumab (VIP-U) study, Joel M. Gelfand, MD, said at the annual meeting of the American Academy of Dermatology.
For the past decade, researchers have worked on the assumption that the chronic systemic inflammation of severe psoriasis overlaps with similar inflammatory pathways that drive atherosclerosis, plaques that rupture, and cardiovascular events.
Recently, they have employed fluorodeoxyglucose positron emission tomography (FDG-PET) to confirm some of this. Studies in 2011, 2015, and 2017 confirm that patients with severe plaque psoriasis can develop diffuse vascular inflammation with increased noncalcified coronary artery plaques. These more fragile plaques are the type that are prone to rupture and cause cardiovascular events, Dr. Gelfand said.
“In these studies, the more imaging signal we saw in the aorta, the higher the risk of a future cardiovascular event. In fact, we determined that patients with severe psoriasis can have increased aortic inflammation equivalent to a decade of aging. As your PASI [Psoriasis Area and Severity Index] score goes up, so does the amount of aortic inflammation. The anatomic consequence is that people develop a high risk of atherosclerotic plaques, and a higher rate of noncalcified coronary plaques that are more likely to lead to these events.”
The VIP-U study was conceived to examine whether calming psoriatic inflammation with ustekinumab (Stelara), an anti interleukin-12 and -23 antibody, could also improve vascular inflammation as measured by FDG-PET. The small study comprised 43 patients, half of whom received placebo and half of whom received two injections of ustekinumab: 45 or 90 mg depending on weight, at baseline and at week 4. There was an imaging assessment at week 12, after which the placebo patients crossed over to open-label ustekinumab every 2 weeks. Everyone was followed out to 64 weeks.
Dr. Gelfand reported the 12-week data; the 64-week data will be forthcoming later this year, he said.
The patients were typical for such a study, with a mean age of about 43 and a mean disease duration of 18 years. The mean body surface area was about 25%, and the mean PASI was 20. Most had been on prior therapy, including phototherapy, oral agents, and biologics.
Unsurprisingly, ustekinumab was significantly more effective than placebo in treating the psoriasis. At week 12, 10% of the placebo patients had achieved a PASI 75, compared with 77% of the ustekinumab patients. A Physicians Global Assessment (PGA) score of clear or almost clear occurred in 10% of those taking placebo and 64% of those taking the biologic.
However, the drug was also highly effective in reducing total aortic inflammation, Dr. Gelfand said. “In just 12 weeks, we saw a 6.6% reduction in total aortic inflammation among those taking ustekinumab, but a 12.1% increase in inflammation among those taking placebo. When you compare the delta, you see a highly statistically significant improvement in aortic inflammation in ustekinumab-treated patients, with the effect size similar to statin therapy.”
The benefit may be a class-associated one. Two recent similar studies of adalimumab, a tumor necrosis factor–alpha inhibitor, failed to find any improvement in vascular inflammation, compared with placebo.
“We need more information about the longer-term effects of ustekinumab treatment, as well as cardiometabolic biomarkers, and these are currently underway,” Dr. Gelfand said. “We also need additional trials to determine if this effect is due to the inhibition of IL-12, IL-23, or a combination. Cardiovascular studies will be necessary to fully determine the clinical implications of our findings.”
To refer a patient into the VIP studies, call 215-662-SKIN or email SkinVIP@upenn.edu.
The National Institutes of Health and University of Pennsylvania sponsored the study. Dr. Gelfand reported no financial disclosures.
SOURCE: Gelfand J et al. AAD 2018 Abstract 6645
SAN DIEGO – A 12-week course of ustekinumab significantly reduced inflammation in the aorta – an effect on par with the benefit of statins – in patients with moderate to severe plaque psoriasis.
Whether or not the reduction in aortic inflammation will translate into a reduction in cardiovascular risk remains to be determined, but investigators are very encouraged by the results of the Vascular Inflammation in Psoriasis-Ustekinumab (VIP-U) study, Joel M. Gelfand, MD, said at the annual meeting of the American Academy of Dermatology.
For the past decade, researchers have worked on the assumption that the chronic systemic inflammation of severe psoriasis overlaps with similar inflammatory pathways that drive atherosclerosis, plaques that rupture, and cardiovascular events.
Recently, they have employed fluorodeoxyglucose positron emission tomography (FDG-PET) to confirm some of this. Studies in 2011, 2015, and 2017 confirm that patients with severe plaque psoriasis can develop diffuse vascular inflammation with increased noncalcified coronary artery plaques. These more fragile plaques are the type that are prone to rupture and cause cardiovascular events, Dr. Gelfand said.
“In these studies, the more imaging signal we saw in the aorta, the higher the risk of a future cardiovascular event. In fact, we determined that patients with severe psoriasis can have increased aortic inflammation equivalent to a decade of aging. As your PASI [Psoriasis Area and Severity Index] score goes up, so does the amount of aortic inflammation. The anatomic consequence is that people develop a high risk of atherosclerotic plaques, and a higher rate of noncalcified coronary plaques that are more likely to lead to these events.”
The VIP-U study was conceived to examine whether calming psoriatic inflammation with ustekinumab (Stelara), an anti interleukin-12 and -23 antibody, could also improve vascular inflammation as measured by FDG-PET. The small study comprised 43 patients, half of whom received placebo and half of whom received two injections of ustekinumab: 45 or 90 mg depending on weight, at baseline and at week 4. There was an imaging assessment at week 12, after which the placebo patients crossed over to open-label ustekinumab every 2 weeks. Everyone was followed out to 64 weeks.
Dr. Gelfand reported the 12-week data; the 64-week data will be forthcoming later this year, he said.
The patients were typical for such a study, with a mean age of about 43 and a mean disease duration of 18 years. The mean body surface area was about 25%, and the mean PASI was 20. Most had been on prior therapy, including phototherapy, oral agents, and biologics.
Unsurprisingly, ustekinumab was significantly more effective than placebo in treating the psoriasis. At week 12, 10% of the placebo patients had achieved a PASI 75, compared with 77% of the ustekinumab patients. A Physicians Global Assessment (PGA) score of clear or almost clear occurred in 10% of those taking placebo and 64% of those taking the biologic.
However, the drug was also highly effective in reducing total aortic inflammation, Dr. Gelfand said. “In just 12 weeks, we saw a 6.6% reduction in total aortic inflammation among those taking ustekinumab, but a 12.1% increase in inflammation among those taking placebo. When you compare the delta, you see a highly statistically significant improvement in aortic inflammation in ustekinumab-treated patients, with the effect size similar to statin therapy.”
The benefit may be a class-associated one. Two recent similar studies of adalimumab, a tumor necrosis factor–alpha inhibitor, failed to find any improvement in vascular inflammation, compared with placebo.
“We need more information about the longer-term effects of ustekinumab treatment, as well as cardiometabolic biomarkers, and these are currently underway,” Dr. Gelfand said. “We also need additional trials to determine if this effect is due to the inhibition of IL-12, IL-23, or a combination. Cardiovascular studies will be necessary to fully determine the clinical implications of our findings.”
To refer a patient into the VIP studies, call 215-662-SKIN or email SkinVIP@upenn.edu.
The National Institutes of Health and University of Pennsylvania sponsored the study. Dr. Gelfand reported no financial disclosures.
SOURCE: Gelfand J et al. AAD 2018 Abstract 6645
REPORTING FROM AAD 2018
Key clinical point:
Major finding: Aortic inflammation decreased by 6.6% in those taking ustekinumab, but increased by 12.1% in those taking placebo.
Study details: The randomized trial comprised 43 patients.
Disclosures: The National Institutes of Health and University of Pennsylvania sponsored the study. Dr. Gelfand made no financial disclosures.
Source: Gelfand J et al. AAD 2018 Abstract 6645.
VIDEO: With new therapies available, it’s the ‘decade of eczema,’ researcher says
SAN DIEGO – A long dry spell in the development of new atopic dermatitis (AD) medications came to an end in 2016 with the approval of a topical treatment, and last year brought the first biologic for AD to the market. With more targets and potential treatments being studied, “it’s the decade of eczema,” according to a leading researcher.
And I think we’ll have many more approvals in the next few years,” said Emma Guttman, MD, PhD, said in a video interview at the annual meeting of the American Academy of Dermatology, where she was presenting a talk on the translational revolution in atopic dermatitis.
In the interview, she also discussed research showing that children with AD don’t have the same distribution of lesions as adults, and a study of young children, which found that during an early stage of the disease, when compared with adults, they showed much higher increases in Th17 similar to that seen in psoriasis. It will be interesting to see if “some drugs that work for psoriasis may work in children,” said Dr. Guttman, professor of dermatology and director of the laboratory of inflammatory skin diseases at the Icahn School of Medicine at Mount Sinai, New York.
Dr. Guttman disclosed research support, consulting, or lecture fees from Regeneron, Sanofi, Pfizer, and other companies developing AD treatments.
SAN DIEGO – A long dry spell in the development of new atopic dermatitis (AD) medications came to an end in 2016 with the approval of a topical treatment, and last year brought the first biologic for AD to the market. With more targets and potential treatments being studied, “it’s the decade of eczema,” according to a leading researcher.
And I think we’ll have many more approvals in the next few years,” said Emma Guttman, MD, PhD, said in a video interview at the annual meeting of the American Academy of Dermatology, where she was presenting a talk on the translational revolution in atopic dermatitis.
In the interview, she also discussed research showing that children with AD don’t have the same distribution of lesions as adults, and a study of young children, which found that during an early stage of the disease, when compared with adults, they showed much higher increases in Th17 similar to that seen in psoriasis. It will be interesting to see if “some drugs that work for psoriasis may work in children,” said Dr. Guttman, professor of dermatology and director of the laboratory of inflammatory skin diseases at the Icahn School of Medicine at Mount Sinai, New York.
Dr. Guttman disclosed research support, consulting, or lecture fees from Regeneron, Sanofi, Pfizer, and other companies developing AD treatments.
SAN DIEGO – A long dry spell in the development of new atopic dermatitis (AD) medications came to an end in 2016 with the approval of a topical treatment, and last year brought the first biologic for AD to the market. With more targets and potential treatments being studied, “it’s the decade of eczema,” according to a leading researcher.
And I think we’ll have many more approvals in the next few years,” said Emma Guttman, MD, PhD, said in a video interview at the annual meeting of the American Academy of Dermatology, where she was presenting a talk on the translational revolution in atopic dermatitis.
In the interview, she also discussed research showing that children with AD don’t have the same distribution of lesions as adults, and a study of young children, which found that during an early stage of the disease, when compared with adults, they showed much higher increases in Th17 similar to that seen in psoriasis. It will be interesting to see if “some drugs that work for psoriasis may work in children,” said Dr. Guttman, professor of dermatology and director of the laboratory of inflammatory skin diseases at the Icahn School of Medicine at Mount Sinai, New York.
Dr. Guttman disclosed research support, consulting, or lecture fees from Regeneron, Sanofi, Pfizer, and other companies developing AD treatments.
REPORTING FROM AAD 18
Biologics have best chance of achieving PASI 90 in psoriasis
The biologics ixekizumab, secukinumab, brodalumab, guselkumab, certolizumab pegol, and ustekinumab provide the best chances for achieving Psoriasis Area and Severity Index (PASI) 90 when compared with placebo in patients with moderate to severe psoriasis, according to Emilie Sbidian, MD, and her associates.
Dr. Sbidian of Henri Mondor Hospital, Créteil, France, and her colleagues conducted a network meta-analysis of 109 randomized, controlled trials that collectively had a total of 39,882 participants. The results showed that all of the interventions appeared superior to placebo in terms of reaching PASI 90.
The investigators also found that there was no significant difference between the three anti–IL-17 agents (brodalumab, ixekizumab, and secukinumab) and the two anti–IL-23 (tildrakizumab and guselkumab) monoclonal antibodies in terms of reaching PASI 90. However, all of the anti–IL-17 drugs (brodalumab, ixekizumab, and secukinumab) and guselkumab (an anti–IL-23) were significantly more effective than three anti–TNF-alpha agents (infliximab, adalimumab, and etanercept).
Additionally, in the network meta-analysis, anti–IL-17 drugs were the best for achieving PASI 90, compared with placebo (RR, 30.81), followed by anti–IL-12/23 (RR, 23.16), anti–IL-23 (RR, 16.53), and anti–TNF-alpha (RR, 11.58). At the individual drug level, results showed ixekizumab was the best treatment for attaining PASI 90 when compared with placebo (RR, 32.45), followed by secukinumab (RR, 26.55), brodalumab (RR, 25.45), guselkumab (RR, 21.03), certolizumab pegol (RR, 24.58), and ustekinumab (RR, 19.91). The investigators found that “there was no significant difference between all of the interventions and the placebo regarding the risk of serious adverse effects.”
“Our main results do not reflect the way patients are managed in ‘real life,’ ” the researchers concluded. “Currently, biological treatments have been positioned as third-line therapies by regulatory bodies, with mandatory reimbursement criteria that patients must meet before being considered for these treatments (moderate to severe disease after failure, intolerance or contraindication to conventional systemic agents).”
SOURCE: Sbidian E et al. Cochrane Database Syst Rev. 2017 Dec 22. doi: 10.1002/14651858.CD011535.pub2.
The biologics ixekizumab, secukinumab, brodalumab, guselkumab, certolizumab pegol, and ustekinumab provide the best chances for achieving Psoriasis Area and Severity Index (PASI) 90 when compared with placebo in patients with moderate to severe psoriasis, according to Emilie Sbidian, MD, and her associates.
Dr. Sbidian of Henri Mondor Hospital, Créteil, France, and her colleagues conducted a network meta-analysis of 109 randomized, controlled trials that collectively had a total of 39,882 participants. The results showed that all of the interventions appeared superior to placebo in terms of reaching PASI 90.
The investigators also found that there was no significant difference between the three anti–IL-17 agents (brodalumab, ixekizumab, and secukinumab) and the two anti–IL-23 (tildrakizumab and guselkumab) monoclonal antibodies in terms of reaching PASI 90. However, all of the anti–IL-17 drugs (brodalumab, ixekizumab, and secukinumab) and guselkumab (an anti–IL-23) were significantly more effective than three anti–TNF-alpha agents (infliximab, adalimumab, and etanercept).
Additionally, in the network meta-analysis, anti–IL-17 drugs were the best for achieving PASI 90, compared with placebo (RR, 30.81), followed by anti–IL-12/23 (RR, 23.16), anti–IL-23 (RR, 16.53), and anti–TNF-alpha (RR, 11.58). At the individual drug level, results showed ixekizumab was the best treatment for attaining PASI 90 when compared with placebo (RR, 32.45), followed by secukinumab (RR, 26.55), brodalumab (RR, 25.45), guselkumab (RR, 21.03), certolizumab pegol (RR, 24.58), and ustekinumab (RR, 19.91). The investigators found that “there was no significant difference between all of the interventions and the placebo regarding the risk of serious adverse effects.”
“Our main results do not reflect the way patients are managed in ‘real life,’ ” the researchers concluded. “Currently, biological treatments have been positioned as third-line therapies by regulatory bodies, with mandatory reimbursement criteria that patients must meet before being considered for these treatments (moderate to severe disease after failure, intolerance or contraindication to conventional systemic agents).”
SOURCE: Sbidian E et al. Cochrane Database Syst Rev. 2017 Dec 22. doi: 10.1002/14651858.CD011535.pub2.
The biologics ixekizumab, secukinumab, brodalumab, guselkumab, certolizumab pegol, and ustekinumab provide the best chances for achieving Psoriasis Area and Severity Index (PASI) 90 when compared with placebo in patients with moderate to severe psoriasis, according to Emilie Sbidian, MD, and her associates.
Dr. Sbidian of Henri Mondor Hospital, Créteil, France, and her colleagues conducted a network meta-analysis of 109 randomized, controlled trials that collectively had a total of 39,882 participants. The results showed that all of the interventions appeared superior to placebo in terms of reaching PASI 90.
The investigators also found that there was no significant difference between the three anti–IL-17 agents (brodalumab, ixekizumab, and secukinumab) and the two anti–IL-23 (tildrakizumab and guselkumab) monoclonal antibodies in terms of reaching PASI 90. However, all of the anti–IL-17 drugs (brodalumab, ixekizumab, and secukinumab) and guselkumab (an anti–IL-23) were significantly more effective than three anti–TNF-alpha agents (infliximab, adalimumab, and etanercept).
Additionally, in the network meta-analysis, anti–IL-17 drugs were the best for achieving PASI 90, compared with placebo (RR, 30.81), followed by anti–IL-12/23 (RR, 23.16), anti–IL-23 (RR, 16.53), and anti–TNF-alpha (RR, 11.58). At the individual drug level, results showed ixekizumab was the best treatment for attaining PASI 90 when compared with placebo (RR, 32.45), followed by secukinumab (RR, 26.55), brodalumab (RR, 25.45), guselkumab (RR, 21.03), certolizumab pegol (RR, 24.58), and ustekinumab (RR, 19.91). The investigators found that “there was no significant difference between all of the interventions and the placebo regarding the risk of serious adverse effects.”
“Our main results do not reflect the way patients are managed in ‘real life,’ ” the researchers concluded. “Currently, biological treatments have been positioned as third-line therapies by regulatory bodies, with mandatory reimbursement criteria that patients must meet before being considered for these treatments (moderate to severe disease after failure, intolerance or contraindication to conventional systemic agents).”
SOURCE: Sbidian E et al. Cochrane Database Syst Rev. 2017 Dec 22. doi: 10.1002/14651858.CD011535.pub2.
FROM COCHRANE DATABASE OF SYSTEMATIC REVIEWS
Risankizumab outpaced ustekinumab for complete clearance of plaque psoriasis
SAN DIEGO – Risankizumab outperformed ustekinumab in two phase 3 trials investigating the IL-23 blocker for moderate to severe plaque psoriasis.
In two year-long studies, 56% and 59% of those taking risankizumab and 21% and 30% of those taking ustekinumab achieved completely clear skin, Kenneth B. Gordon, MD, said at the annual meeting of the American Academy of Dermatology.
“One of the things we are striving for now is complete skin clearance,” said Dr. Gordon, chair of the dermatology department the Medical College of Wisconsin, Milwaukee. “In the past, people have said that it wasn’t important to reach that, yet here we are, getting more than 50% of patients to that point.”
Risankizumab is an investigational monoclonal antibody that selectively blocks IL-23, a key inflammatory protein. The drug is also in phase 3 trials for Crohn's disease, and being investigated for psoriatic arthritis. AbbVie, which is developing risankizumab, plans future trials for treating ulcerative colitis.
Dr. Gordon reported the results of UltIMMa-1 and UltIMMa-2, identical three-armed studies that randomized a total of 797 patients with moderate to severe plaque psoriasis to risankizumab 150 mg, ustekinumab 45 mg or 90 mg (based on weight), or to a crossover group that took placebo for the first 16 weeks of the study and then were switched to risankizumab 150 mg for the remainder of the study. Study drugs were delivered at weeks 0, 4, 16, 28, and 40.
The coprimary endpoints were at least a 90% improvement in the Psoriasis Area Severity Index score (PASI 90) at week 16 and a score of 0 or 1 on the Static Physicians’ Assessment scale (sPGA 0/1) at week 16, compared with placebo. Key secondary endpoints compared risankizumab with ustekinumab: PASI 90, sPGA score of clear (sPGA 0), sPGA 0/1, and Dermatology Quality of Life (DLQI) score of 0/1 at week 16, and PASI 90, PASI 100 and sPGA 0 at week 52.
In both trials, patients were 48 years old on average; about 20% had severe plaque psoriasis. The mean PASI score was about 20 at trial entry. Prior therapy included biologics in 30%-43%, depending on the trial, and TNF-alpha inhibitors in about 25%.
Patient retention in the study was good, Dr. Gordon noted, with 95% of risankizumab patients still taking the drug at 52 weeks. Patients also stayed on ustekinumab, with 94% of UltIMMa-1 patients and 91% of UltIMMa-2 patients still taking the drug at 52 weeks.
At week 16, risankizumab was clearly superior to placebo in both endpoints. In both studies, 75% of actively treated patients achieved PASI 90, compared to 5% of those taking placebo. In UltIMMa-1, a clear or almost clear sPGA was seen in 88% of risankizumab patients as compared to 8% of those taking placebo. In UltIMMa-2, these numbers were 84% and 5%, respectively.
In the secondary comparison of the two active drugs, risankizumab significantly outperformed ustekinumab on PASI90 at 16 weeks in UltIMMa-1 (75% vs. 42%) and in UltIMMa-2 (75% vs. 47%). The PASI90 outcomes similarly favored risankizumab at 52 weeks in UltIMMa-1 (82% vs. 44%) and in UltIMMa-2 (81% vs. 50%).
As compared with ustekinumab, risankizumab aced the secondary endpoint of complete skin clearance in UltIMMa-1 and (36% vs. 12%) and UltIMMa-2 (51% vs. 24%). The results similarly favored risankizumab at 52 weeks in both trials (56% vs. 21% and 59% vs. 30%).
Another secondary endpoint looked at how the crossover group fared. At week 51, the PASI90 for this group was 78% in UltIMMa-1 and 85% in UltIMMa-2; the PASI100 at 52 weeks for these patients was 55% and 67%.
A responder time curve demonstrated just how quickly the crossover patients made up for lost time after switching to risankizumab. Although these patients made no progress toward disease clearance during their placebo period, they quickly caught up with the primary risankizumab group. At 16 weeks, 5% in this group had a PASI 90; by week 28, 51% did; and by week 52, PASI 90 topped out at 78%.
“The time course seen in this trial is very important,” Dr. Gordon said. “By 8 weeks, almost 44% [of the primary risankizumab group] was already at PASI90. They reached an extremely high level of response that was very consistent over 1 year. In the ustekinumab group, we saw some saw-toothing of response, indicating that people were losing effectiveness at the end of the dosing period. With risankizumab, we did not see that, indicating that the once every 12 weeks dosing period is effective.”
The DLQI 0/1 outcome occurred at 16 and 52 weeks in significantly more patients taking risankizumab in both studies. By week 52 in UltIMMa-1, 75% of patients on risankizumab had achieved a DLQ1 0/1, compared with 47% of the ustekinumab group. In UltIMMa-2, these numbers were 71% and 44%, with the crossover group posting scores similar to the primary risankizumab group in both studies (62% and 68%).
Risankizumab proved safe and well tolerated, Dr. Gordon said. Less than 1% of patients discontinued the medication due to an adverse event. In both the UltIMMa-1 and UltIMMa-2 trials, the most frequently reported treatment-emergent adverse event in the risankizumab groups was upper respiratory tract infection. In UltIMMa-1, one patient receiving risankizumab presented with latent tuberculosis and was treated with rifampicin. There were no new cases of tuberculosis.
The serious adverse event rate hovered between 2%-3% in both trials. The rate of serious infection was 1%. The rate of malignancy was 0.3%, but fell to 0 when nonmelanoma skin cancer was excluded. There were no major cardiovascular events.
"Not only do these data show significant rates of clear skin, but because we know the burden of psoriasis extends beyond the skin, we are encouraged by the patient-reported improvement in quality of life after one year of treatment," he said. "Given the significant impact of psoriasis, it is important to continue to investigate additional treatment options."
AbbVie sponsored the trials. Dr. Gordon is a consultant for the company.
SOURCE: Gordon et al. AAD, Abstract 6495
SAN DIEGO – Risankizumab outperformed ustekinumab in two phase 3 trials investigating the IL-23 blocker for moderate to severe plaque psoriasis.
In two year-long studies, 56% and 59% of those taking risankizumab and 21% and 30% of those taking ustekinumab achieved completely clear skin, Kenneth B. Gordon, MD, said at the annual meeting of the American Academy of Dermatology.
“One of the things we are striving for now is complete skin clearance,” said Dr. Gordon, chair of the dermatology department the Medical College of Wisconsin, Milwaukee. “In the past, people have said that it wasn’t important to reach that, yet here we are, getting more than 50% of patients to that point.”
Risankizumab is an investigational monoclonal antibody that selectively blocks IL-23, a key inflammatory protein. The drug is also in phase 3 trials for Crohn's disease, and being investigated for psoriatic arthritis. AbbVie, which is developing risankizumab, plans future trials for treating ulcerative colitis.
Dr. Gordon reported the results of UltIMMa-1 and UltIMMa-2, identical three-armed studies that randomized a total of 797 patients with moderate to severe plaque psoriasis to risankizumab 150 mg, ustekinumab 45 mg or 90 mg (based on weight), or to a crossover group that took placebo for the first 16 weeks of the study and then were switched to risankizumab 150 mg for the remainder of the study. Study drugs were delivered at weeks 0, 4, 16, 28, and 40.
The coprimary endpoints were at least a 90% improvement in the Psoriasis Area Severity Index score (PASI 90) at week 16 and a score of 0 or 1 on the Static Physicians’ Assessment scale (sPGA 0/1) at week 16, compared with placebo. Key secondary endpoints compared risankizumab with ustekinumab: PASI 90, sPGA score of clear (sPGA 0), sPGA 0/1, and Dermatology Quality of Life (DLQI) score of 0/1 at week 16, and PASI 90, PASI 100 and sPGA 0 at week 52.
In both trials, patients were 48 years old on average; about 20% had severe plaque psoriasis. The mean PASI score was about 20 at trial entry. Prior therapy included biologics in 30%-43%, depending on the trial, and TNF-alpha inhibitors in about 25%.
Patient retention in the study was good, Dr. Gordon noted, with 95% of risankizumab patients still taking the drug at 52 weeks. Patients also stayed on ustekinumab, with 94% of UltIMMa-1 patients and 91% of UltIMMa-2 patients still taking the drug at 52 weeks.
At week 16, risankizumab was clearly superior to placebo in both endpoints. In both studies, 75% of actively treated patients achieved PASI 90, compared to 5% of those taking placebo. In UltIMMa-1, a clear or almost clear sPGA was seen in 88% of risankizumab patients as compared to 8% of those taking placebo. In UltIMMa-2, these numbers were 84% and 5%, respectively.
In the secondary comparison of the two active drugs, risankizumab significantly outperformed ustekinumab on PASI90 at 16 weeks in UltIMMa-1 (75% vs. 42%) and in UltIMMa-2 (75% vs. 47%). The PASI90 outcomes similarly favored risankizumab at 52 weeks in UltIMMa-1 (82% vs. 44%) and in UltIMMa-2 (81% vs. 50%).
As compared with ustekinumab, risankizumab aced the secondary endpoint of complete skin clearance in UltIMMa-1 and (36% vs. 12%) and UltIMMa-2 (51% vs. 24%). The results similarly favored risankizumab at 52 weeks in both trials (56% vs. 21% and 59% vs. 30%).
Another secondary endpoint looked at how the crossover group fared. At week 51, the PASI90 for this group was 78% in UltIMMa-1 and 85% in UltIMMa-2; the PASI100 at 52 weeks for these patients was 55% and 67%.
A responder time curve demonstrated just how quickly the crossover patients made up for lost time after switching to risankizumab. Although these patients made no progress toward disease clearance during their placebo period, they quickly caught up with the primary risankizumab group. At 16 weeks, 5% in this group had a PASI 90; by week 28, 51% did; and by week 52, PASI 90 topped out at 78%.
“The time course seen in this trial is very important,” Dr. Gordon said. “By 8 weeks, almost 44% [of the primary risankizumab group] was already at PASI90. They reached an extremely high level of response that was very consistent over 1 year. In the ustekinumab group, we saw some saw-toothing of response, indicating that people were losing effectiveness at the end of the dosing period. With risankizumab, we did not see that, indicating that the once every 12 weeks dosing period is effective.”
The DLQI 0/1 outcome occurred at 16 and 52 weeks in significantly more patients taking risankizumab in both studies. By week 52 in UltIMMa-1, 75% of patients on risankizumab had achieved a DLQ1 0/1, compared with 47% of the ustekinumab group. In UltIMMa-2, these numbers were 71% and 44%, with the crossover group posting scores similar to the primary risankizumab group in both studies (62% and 68%).
Risankizumab proved safe and well tolerated, Dr. Gordon said. Less than 1% of patients discontinued the medication due to an adverse event. In both the UltIMMa-1 and UltIMMa-2 trials, the most frequently reported treatment-emergent adverse event in the risankizumab groups was upper respiratory tract infection. In UltIMMa-1, one patient receiving risankizumab presented with latent tuberculosis and was treated with rifampicin. There were no new cases of tuberculosis.
The serious adverse event rate hovered between 2%-3% in both trials. The rate of serious infection was 1%. The rate of malignancy was 0.3%, but fell to 0 when nonmelanoma skin cancer was excluded. There were no major cardiovascular events.
"Not only do these data show significant rates of clear skin, but because we know the burden of psoriasis extends beyond the skin, we are encouraged by the patient-reported improvement in quality of life after one year of treatment," he said. "Given the significant impact of psoriasis, it is important to continue to investigate additional treatment options."
AbbVie sponsored the trials. Dr. Gordon is a consultant for the company.
SOURCE: Gordon et al. AAD, Abstract 6495
SAN DIEGO – Risankizumab outperformed ustekinumab in two phase 3 trials investigating the IL-23 blocker for moderate to severe plaque psoriasis.
In two year-long studies, 56% and 59% of those taking risankizumab and 21% and 30% of those taking ustekinumab achieved completely clear skin, Kenneth B. Gordon, MD, said at the annual meeting of the American Academy of Dermatology.
“One of the things we are striving for now is complete skin clearance,” said Dr. Gordon, chair of the dermatology department the Medical College of Wisconsin, Milwaukee. “In the past, people have said that it wasn’t important to reach that, yet here we are, getting more than 50% of patients to that point.”
Risankizumab is an investigational monoclonal antibody that selectively blocks IL-23, a key inflammatory protein. The drug is also in phase 3 trials for Crohn's disease, and being investigated for psoriatic arthritis. AbbVie, which is developing risankizumab, plans future trials for treating ulcerative colitis.
Dr. Gordon reported the results of UltIMMa-1 and UltIMMa-2, identical three-armed studies that randomized a total of 797 patients with moderate to severe plaque psoriasis to risankizumab 150 mg, ustekinumab 45 mg or 90 mg (based on weight), or to a crossover group that took placebo for the first 16 weeks of the study and then were switched to risankizumab 150 mg for the remainder of the study. Study drugs were delivered at weeks 0, 4, 16, 28, and 40.
The coprimary endpoints were at least a 90% improvement in the Psoriasis Area Severity Index score (PASI 90) at week 16 and a score of 0 or 1 on the Static Physicians’ Assessment scale (sPGA 0/1) at week 16, compared with placebo. Key secondary endpoints compared risankizumab with ustekinumab: PASI 90, sPGA score of clear (sPGA 0), sPGA 0/1, and Dermatology Quality of Life (DLQI) score of 0/1 at week 16, and PASI 90, PASI 100 and sPGA 0 at week 52.
In both trials, patients were 48 years old on average; about 20% had severe plaque psoriasis. The mean PASI score was about 20 at trial entry. Prior therapy included biologics in 30%-43%, depending on the trial, and TNF-alpha inhibitors in about 25%.
Patient retention in the study was good, Dr. Gordon noted, with 95% of risankizumab patients still taking the drug at 52 weeks. Patients also stayed on ustekinumab, with 94% of UltIMMa-1 patients and 91% of UltIMMa-2 patients still taking the drug at 52 weeks.
At week 16, risankizumab was clearly superior to placebo in both endpoints. In both studies, 75% of actively treated patients achieved PASI 90, compared to 5% of those taking placebo. In UltIMMa-1, a clear or almost clear sPGA was seen in 88% of risankizumab patients as compared to 8% of those taking placebo. In UltIMMa-2, these numbers were 84% and 5%, respectively.
In the secondary comparison of the two active drugs, risankizumab significantly outperformed ustekinumab on PASI90 at 16 weeks in UltIMMa-1 (75% vs. 42%) and in UltIMMa-2 (75% vs. 47%). The PASI90 outcomes similarly favored risankizumab at 52 weeks in UltIMMa-1 (82% vs. 44%) and in UltIMMa-2 (81% vs. 50%).
As compared with ustekinumab, risankizumab aced the secondary endpoint of complete skin clearance in UltIMMa-1 and (36% vs. 12%) and UltIMMa-2 (51% vs. 24%). The results similarly favored risankizumab at 52 weeks in both trials (56% vs. 21% and 59% vs. 30%).
Another secondary endpoint looked at how the crossover group fared. At week 51, the PASI90 for this group was 78% in UltIMMa-1 and 85% in UltIMMa-2; the PASI100 at 52 weeks for these patients was 55% and 67%.
A responder time curve demonstrated just how quickly the crossover patients made up for lost time after switching to risankizumab. Although these patients made no progress toward disease clearance during their placebo period, they quickly caught up with the primary risankizumab group. At 16 weeks, 5% in this group had a PASI 90; by week 28, 51% did; and by week 52, PASI 90 topped out at 78%.
“The time course seen in this trial is very important,” Dr. Gordon said. “By 8 weeks, almost 44% [of the primary risankizumab group] was already at PASI90. They reached an extremely high level of response that was very consistent over 1 year. In the ustekinumab group, we saw some saw-toothing of response, indicating that people were losing effectiveness at the end of the dosing period. With risankizumab, we did not see that, indicating that the once every 12 weeks dosing period is effective.”
The DLQI 0/1 outcome occurred at 16 and 52 weeks in significantly more patients taking risankizumab in both studies. By week 52 in UltIMMa-1, 75% of patients on risankizumab had achieved a DLQ1 0/1, compared with 47% of the ustekinumab group. In UltIMMa-2, these numbers were 71% and 44%, with the crossover group posting scores similar to the primary risankizumab group in both studies (62% and 68%).
Risankizumab proved safe and well tolerated, Dr. Gordon said. Less than 1% of patients discontinued the medication due to an adverse event. In both the UltIMMa-1 and UltIMMa-2 trials, the most frequently reported treatment-emergent adverse event in the risankizumab groups was upper respiratory tract infection. In UltIMMa-1, one patient receiving risankizumab presented with latent tuberculosis and was treated with rifampicin. There were no new cases of tuberculosis.
The serious adverse event rate hovered between 2%-3% in both trials. The rate of serious infection was 1%. The rate of malignancy was 0.3%, but fell to 0 when nonmelanoma skin cancer was excluded. There were no major cardiovascular events.
"Not only do these data show significant rates of clear skin, but because we know the burden of psoriasis extends beyond the skin, we are encouraged by the patient-reported improvement in quality of life after one year of treatment," he said. "Given the significant impact of psoriasis, it is important to continue to investigate additional treatment options."
AbbVie sponsored the trials. Dr. Gordon is a consultant for the company.
SOURCE: Gordon et al. AAD, Abstract 6495
REPORTING FROM AAD 18
Key clinical point: Risankizumab outperformed placebo and the active comparator ustekinumab.
Major finding: In the two studies, 56% and 59% of those taking risankizumab had clear skin as compared to 21% and 30% of those taking ustekinumab.
Study details: The twin placebo-crossover active comparator trials randomized 797 patients.
Disclosures: AbbVie sponsored the studies. Dr. Gordon is a consultant for the company.
Source: Gordon et al. AAD abstract 6495
Biologics gaining traction in children with moderate to severe psoriasis
SAN DIEGO – Systemic therapies are increasingly being used for children with moderate to severe psoriasis; methotrexate is still the mainstay of systemic treatment, but biologics appear to achieve superior results with fewer side effects, Amy S. Paller, MD, said at the annual meeting of the American Academy of Dermatology.
Etanercept was approved in 2016 for children ages 6 and up, and ustekinumab was approved for use in patients aged 12 years or older in October 2017. Ongoing trials are examining adalimumab, apremilast, ustekinumab, and ixekizumab for use in adolescents and younger children. Trials are also being planned for other therapies that inhibit the Th17/IL-23 pathway, said Dr. Paller, the Walter J. Hamlin Professor and chair of dermatology at Northwestern University Feinberg School of Medicine, Chicago.

Further, the study found that biologic agents, primarily etanercept, were used by 27%, acitretin by nearly 15%, cyclosporine by about 8%, and fumaric acid esters by 5%. More than 1 medication was used by 19%, according to the study results.
Adverse events affected the ability to tolerate therapy, and methotrexate and biologic agents were taken for a mean duration that was 2-fold greater than the mean duration for cyclosporine or fumaric acid esters. “A prospective registry is needed to track the long-term risks of systemic agents for pediatric psoriasis,” the authors concluded.
Dr. Paller reported that, in her practice, "we're still primarily using methotrexate. It takes time to see an effect with methotrexate, and you have to let people know this up front.” She pointed to a 2015 single-site prospective study of 25 children that found just 40% achieved Psoriasis Area and Severity Index 50 at 12 weeks, with that number rising to 80% by 36 weeks. (J Derm Treat 2015; 26: 406-12)
Dr. Paller recommends baseline and annual TB testing, updated vaccinations and pregnancy counseling for all patients taking immunosuppressant therapies.
"I don't use a lot of retinoids for plaque psoriasis in kids," Dr. Paller said, "but for pustular psoriasis, I use (them) quite a bit. The beauty of retinoids is that they are not immunosuppressants, and you can start and stop them without loss of efficacy. There are many potential side effects, primarily skin and mucosal dryness."
Cyclosporine "has the greatest potential toxicity, which leaves it lower on the therapeutic ladder," Dr. Paller said. "But it has a pretty good safety record. The nice thing we can say is that (cyclosporine has) been around a long time. We have decades of experience in children, and we're using a low dose."
Benefits of biologics include convenience, infrequent dosing, and, potentially, fewer lab tests, Dr. Paller said. She added that there's no consensus about whether lab tests beyond annual TB tests are a good idea for patients on biologics.
Long-term risks are unclear, however, and drug holidays could spell trouble for efficacy when kids return to the medications.
Dr. Paller noted that biologics can cost tens of thousands of dollars for several weeks of treatment, and insurers may not cover them.
A 2014 meta-analysis of 48 randomized, controlled trials of 16,696 adult patients with psoriasis put biologics as the most effective therapies, with infliximab at the top (risk difference 76%), followed by adalimumab (RD 61%) and ustekinumab (RD 63%).
“These biologics are more effective than etanercept and all conventional treatments. Head-to-head trials indicate the superiority of adalimumab and infliximab over methotrexate (MTX), the superiority of ustekinumab over etanercept …” the meta-analysis concluded. (Br J Dermatol. 2014 Feb;170(2):274-303)
Dr. Paller disclosed that she is an investigator for Abbvie; Celgene; Eli Lilly, Janssen, Leo Foundation; Novartis. She is a consultant with honorarium for Amgen; Celgene; Eli Lilly; and Novartis.
SOURCE: Paller, A. et al, Session F025 Update on systemic therapies and emerging treatments
SAN DIEGO – Systemic therapies are increasingly being used for children with moderate to severe psoriasis; methotrexate is still the mainstay of systemic treatment, but biologics appear to achieve superior results with fewer side effects, Amy S. Paller, MD, said at the annual meeting of the American Academy of Dermatology.
Etanercept was approved in 2016 for children ages 6 and up, and ustekinumab was approved for use in patients aged 12 years or older in October 2017. Ongoing trials are examining adalimumab, apremilast, ustekinumab, and ixekizumab for use in adolescents and younger children. Trials are also being planned for other therapies that inhibit the Th17/IL-23 pathway, said Dr. Paller, the Walter J. Hamlin Professor and chair of dermatology at Northwestern University Feinberg School of Medicine, Chicago.

Further, the study found that biologic agents, primarily etanercept, were used by 27%, acitretin by nearly 15%, cyclosporine by about 8%, and fumaric acid esters by 5%. More than 1 medication was used by 19%, according to the study results.
Adverse events affected the ability to tolerate therapy, and methotrexate and biologic agents were taken for a mean duration that was 2-fold greater than the mean duration for cyclosporine or fumaric acid esters. “A prospective registry is needed to track the long-term risks of systemic agents for pediatric psoriasis,” the authors concluded.
Dr. Paller reported that, in her practice, "we're still primarily using methotrexate. It takes time to see an effect with methotrexate, and you have to let people know this up front.” She pointed to a 2015 single-site prospective study of 25 children that found just 40% achieved Psoriasis Area and Severity Index 50 at 12 weeks, with that number rising to 80% by 36 weeks. (J Derm Treat 2015; 26: 406-12)
Dr. Paller recommends baseline and annual TB testing, updated vaccinations and pregnancy counseling for all patients taking immunosuppressant therapies.
"I don't use a lot of retinoids for plaque psoriasis in kids," Dr. Paller said, "but for pustular psoriasis, I use (them) quite a bit. The beauty of retinoids is that they are not immunosuppressants, and you can start and stop them without loss of efficacy. There are many potential side effects, primarily skin and mucosal dryness."
Cyclosporine "has the greatest potential toxicity, which leaves it lower on the therapeutic ladder," Dr. Paller said. "But it has a pretty good safety record. The nice thing we can say is that (cyclosporine has) been around a long time. We have decades of experience in children, and we're using a low dose."
Benefits of biologics include convenience, infrequent dosing, and, potentially, fewer lab tests, Dr. Paller said. She added that there's no consensus about whether lab tests beyond annual TB tests are a good idea for patients on biologics.
Long-term risks are unclear, however, and drug holidays could spell trouble for efficacy when kids return to the medications.
Dr. Paller noted that biologics can cost tens of thousands of dollars for several weeks of treatment, and insurers may not cover them.
A 2014 meta-analysis of 48 randomized, controlled trials of 16,696 adult patients with psoriasis put biologics as the most effective therapies, with infliximab at the top (risk difference 76%), followed by adalimumab (RD 61%) and ustekinumab (RD 63%).
“These biologics are more effective than etanercept and all conventional treatments. Head-to-head trials indicate the superiority of adalimumab and infliximab over methotrexate (MTX), the superiority of ustekinumab over etanercept …” the meta-analysis concluded. (Br J Dermatol. 2014 Feb;170(2):274-303)
Dr. Paller disclosed that she is an investigator for Abbvie; Celgene; Eli Lilly, Janssen, Leo Foundation; Novartis. She is a consultant with honorarium for Amgen; Celgene; Eli Lilly; and Novartis.
SOURCE: Paller, A. et al, Session F025 Update on systemic therapies and emerging treatments
SAN DIEGO – Systemic therapies are increasingly being used for children with moderate to severe psoriasis; methotrexate is still the mainstay of systemic treatment, but biologics appear to achieve superior results with fewer side effects, Amy S. Paller, MD, said at the annual meeting of the American Academy of Dermatology.
Etanercept was approved in 2016 for children ages 6 and up, and ustekinumab was approved for use in patients aged 12 years or older in October 2017. Ongoing trials are examining adalimumab, apremilast, ustekinumab, and ixekizumab for use in adolescents and younger children. Trials are also being planned for other therapies that inhibit the Th17/IL-23 pathway, said Dr. Paller, the Walter J. Hamlin Professor and chair of dermatology at Northwestern University Feinberg School of Medicine, Chicago.

Further, the study found that biologic agents, primarily etanercept, were used by 27%, acitretin by nearly 15%, cyclosporine by about 8%, and fumaric acid esters by 5%. More than 1 medication was used by 19%, according to the study results.
Adverse events affected the ability to tolerate therapy, and methotrexate and biologic agents were taken for a mean duration that was 2-fold greater than the mean duration for cyclosporine or fumaric acid esters. “A prospective registry is needed to track the long-term risks of systemic agents for pediatric psoriasis,” the authors concluded.
Dr. Paller reported that, in her practice, "we're still primarily using methotrexate. It takes time to see an effect with methotrexate, and you have to let people know this up front.” She pointed to a 2015 single-site prospective study of 25 children that found just 40% achieved Psoriasis Area and Severity Index 50 at 12 weeks, with that number rising to 80% by 36 weeks. (J Derm Treat 2015; 26: 406-12)
Dr. Paller recommends baseline and annual TB testing, updated vaccinations and pregnancy counseling for all patients taking immunosuppressant therapies.
"I don't use a lot of retinoids for plaque psoriasis in kids," Dr. Paller said, "but for pustular psoriasis, I use (them) quite a bit. The beauty of retinoids is that they are not immunosuppressants, and you can start and stop them without loss of efficacy. There are many potential side effects, primarily skin and mucosal dryness."
Cyclosporine "has the greatest potential toxicity, which leaves it lower on the therapeutic ladder," Dr. Paller said. "But it has a pretty good safety record. The nice thing we can say is that (cyclosporine has) been around a long time. We have decades of experience in children, and we're using a low dose."
Benefits of biologics include convenience, infrequent dosing, and, potentially, fewer lab tests, Dr. Paller said. She added that there's no consensus about whether lab tests beyond annual TB tests are a good idea for patients on biologics.
Long-term risks are unclear, however, and drug holidays could spell trouble for efficacy when kids return to the medications.
Dr. Paller noted that biologics can cost tens of thousands of dollars for several weeks of treatment, and insurers may not cover them.
A 2014 meta-analysis of 48 randomized, controlled trials of 16,696 adult patients with psoriasis put biologics as the most effective therapies, with infliximab at the top (risk difference 76%), followed by adalimumab (RD 61%) and ustekinumab (RD 63%).
“These biologics are more effective than etanercept and all conventional treatments. Head-to-head trials indicate the superiority of adalimumab and infliximab over methotrexate (MTX), the superiority of ustekinumab over etanercept …” the meta-analysis concluded. (Br J Dermatol. 2014 Feb;170(2):274-303)
Dr. Paller disclosed that she is an investigator for Abbvie; Celgene; Eli Lilly, Janssen, Leo Foundation; Novartis. She is a consultant with honorarium for Amgen; Celgene; Eli Lilly; and Novartis.
SOURCE: Paller, A. et al, Session F025 Update on systemic therapies and emerging treatments
EXPERT ANALYSIS AT AAD 18
Here comes bimekizumab, the newest IL-17 inhibitor
KAUAI, HAWAII –
And that’s not all: Much the same was true in patients with psoriatic arthritis in the parallel phase 2b BE ACTIVE study and for ankylosing spondylitis in the BE AGILE study. BE ABLE was a double-blind, 12-week, multicenter, five-arm, placebo-controlled, dose-ranging study of 250 psoriasis patients randomized to various doses of subcutaneous bimekizumab or placebo every 4 weeks. The primary outcome – the PASI 90 response rate at 12 weeks, rather than the lower-bar PASI 75 endpoint more typically used in clinical trials – was 79% at the optimal dose. The PASI 100 rate – complete clearance of disease – at 12 weeks was 60%, Craig L. Leonardi, MD, said at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
lthough Dr. Leonardi is a distinguished psoriasis clinical trialist, he wasn’t involved in the BE ABLE study. Only top line results have been announced to date, although publication of the BE ABLE, BE AGILE, and BE ACTIVE results and presentations at major medical meetings are pending.
Bimekizumab is a humanized IgG1 monoclonal antibody which uniquely neutralizes both IL-17A and IL-17F. In contrast, secukinumab (Cosentyx) and ixekizumab (Taltz) specifically inhibit IL-17A, while brodalumab (Siliq) is a pan-IL-17 receptor antagonist, inhibiting the IL-17 A, A/F, E, F, and C receptors. The impressive clinical outcomes of the three BE phase 2b studies validate the notion that IL-17F is an important cytokine in tissue inflammation across a range of dermatologic and rheumatologic diseases.
A long-term extension of BE ABLE is ongoing. In addition, a phase 3 randomized trial of bimekizumab versus adalimumab (Humira) versus placebo in 450 psoriasis patients is now recruiting, as is a separate phase 3 placebo-controlled head to head comparison of bimekizumab versus ustekinumab (Stelara).
The BE ACTIVE study included 206 psoriatic arthritis patients. At the top dose of bimekizumab, the week 12 rate of at least a 50% improvement in joint symptoms, or ACR 50 response, was 46%, compared with 7% in placebo-treated controls. Patients with concomitant psoriasis over at least 3% of their body surface area demonstrated a 65% PASI 90 response rate at 12 weeks.
BE AGILE included 303 patients with ankylosing spondylitis. Forty-seven percent of those randomized to the top-performing dose of bimekizumab had at least a 40% improvement in their Ankylosing Spondylitis Activity Score, or ASAS 40, at week 12, as did 13% in the placebo arm.
Bimekizumab is being developed by UCB, which is planning additional studies advancing the biologic in all three diseases studied in the phase 2b trials.
Dr. Leonardi reported receiving research grants from well over a dozen pharmaceutical companies and serving as a consultant to UCB and others.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
KAUAI, HAWAII –
And that’s not all: Much the same was true in patients with psoriatic arthritis in the parallel phase 2b BE ACTIVE study and for ankylosing spondylitis in the BE AGILE study. BE ABLE was a double-blind, 12-week, multicenter, five-arm, placebo-controlled, dose-ranging study of 250 psoriasis patients randomized to various doses of subcutaneous bimekizumab or placebo every 4 weeks. The primary outcome – the PASI 90 response rate at 12 weeks, rather than the lower-bar PASI 75 endpoint more typically used in clinical trials – was 79% at the optimal dose. The PASI 100 rate – complete clearance of disease – at 12 weeks was 60%, Craig L. Leonardi, MD, said at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
lthough Dr. Leonardi is a distinguished psoriasis clinical trialist, he wasn’t involved in the BE ABLE study. Only top line results have been announced to date, although publication of the BE ABLE, BE AGILE, and BE ACTIVE results and presentations at major medical meetings are pending.
Bimekizumab is a humanized IgG1 monoclonal antibody which uniquely neutralizes both IL-17A and IL-17F. In contrast, secukinumab (Cosentyx) and ixekizumab (Taltz) specifically inhibit IL-17A, while brodalumab (Siliq) is a pan-IL-17 receptor antagonist, inhibiting the IL-17 A, A/F, E, F, and C receptors. The impressive clinical outcomes of the three BE phase 2b studies validate the notion that IL-17F is an important cytokine in tissue inflammation across a range of dermatologic and rheumatologic diseases.
A long-term extension of BE ABLE is ongoing. In addition, a phase 3 randomized trial of bimekizumab versus adalimumab (Humira) versus placebo in 450 psoriasis patients is now recruiting, as is a separate phase 3 placebo-controlled head to head comparison of bimekizumab versus ustekinumab (Stelara).
The BE ACTIVE study included 206 psoriatic arthritis patients. At the top dose of bimekizumab, the week 12 rate of at least a 50% improvement in joint symptoms, or ACR 50 response, was 46%, compared with 7% in placebo-treated controls. Patients with concomitant psoriasis over at least 3% of their body surface area demonstrated a 65% PASI 90 response rate at 12 weeks.
BE AGILE included 303 patients with ankylosing spondylitis. Forty-seven percent of those randomized to the top-performing dose of bimekizumab had at least a 40% improvement in their Ankylosing Spondylitis Activity Score, or ASAS 40, at week 12, as did 13% in the placebo arm.
Bimekizumab is being developed by UCB, which is planning additional studies advancing the biologic in all three diseases studied in the phase 2b trials.
Dr. Leonardi reported receiving research grants from well over a dozen pharmaceutical companies and serving as a consultant to UCB and others.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
KAUAI, HAWAII –
And that’s not all: Much the same was true in patients with psoriatic arthritis in the parallel phase 2b BE ACTIVE study and for ankylosing spondylitis in the BE AGILE study. BE ABLE was a double-blind, 12-week, multicenter, five-arm, placebo-controlled, dose-ranging study of 250 psoriasis patients randomized to various doses of subcutaneous bimekizumab or placebo every 4 weeks. The primary outcome – the PASI 90 response rate at 12 weeks, rather than the lower-bar PASI 75 endpoint more typically used in clinical trials – was 79% at the optimal dose. The PASI 100 rate – complete clearance of disease – at 12 weeks was 60%, Craig L. Leonardi, MD, said at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
lthough Dr. Leonardi is a distinguished psoriasis clinical trialist, he wasn’t involved in the BE ABLE study. Only top line results have been announced to date, although publication of the BE ABLE, BE AGILE, and BE ACTIVE results and presentations at major medical meetings are pending.
Bimekizumab is a humanized IgG1 monoclonal antibody which uniquely neutralizes both IL-17A and IL-17F. In contrast, secukinumab (Cosentyx) and ixekizumab (Taltz) specifically inhibit IL-17A, while brodalumab (Siliq) is a pan-IL-17 receptor antagonist, inhibiting the IL-17 A, A/F, E, F, and C receptors. The impressive clinical outcomes of the three BE phase 2b studies validate the notion that IL-17F is an important cytokine in tissue inflammation across a range of dermatologic and rheumatologic diseases.
A long-term extension of BE ABLE is ongoing. In addition, a phase 3 randomized trial of bimekizumab versus adalimumab (Humira) versus placebo in 450 psoriasis patients is now recruiting, as is a separate phase 3 placebo-controlled head to head comparison of bimekizumab versus ustekinumab (Stelara).
The BE ACTIVE study included 206 psoriatic arthritis patients. At the top dose of bimekizumab, the week 12 rate of at least a 50% improvement in joint symptoms, or ACR 50 response, was 46%, compared with 7% in placebo-treated controls. Patients with concomitant psoriasis over at least 3% of their body surface area demonstrated a 65% PASI 90 response rate at 12 weeks.
BE AGILE included 303 patients with ankylosing spondylitis. Forty-seven percent of those randomized to the top-performing dose of bimekizumab had at least a 40% improvement in their Ankylosing Spondylitis Activity Score, or ASAS 40, at week 12, as did 13% in the placebo arm.
Bimekizumab is being developed by UCB, which is planning additional studies advancing the biologic in all three diseases studied in the phase 2b trials.
Dr. Leonardi reported receiving research grants from well over a dozen pharmaceutical companies and serving as a consultant to UCB and others.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
EXPERT ANALYSIS FROM SDEF HAWAII DERMATOLOGY SEMINAR