User login
An Ethical Analysis of Treatment of an Active-Duty Service Member With Limited Follow-up
For active-duty service members, dermatologic conditions are among the most common presenting concerns, comprising 15% to 75% of wartime outpatient visits.1 In general, there are unique considerations when caring for active-duty service members, including meeting designated active-duty retention and hierarchical standards.2 We present a hypothetical case: An active-duty military patient presents to a new dermatologist for cosmetic enhancement of facial skin dyspigmentation. The patient will be leaving soon for deployment and will not be able to follow up for 9 months. How should the dermatologist treat a patient who cannot follow up for so long?
The therapeutic modalities offered can be impacted by forthcoming deployments3 that may result in delayed time to administer repeat treatments or follow-up. The patient may have high expectations for a single appointment for a condition that requires prolonged treatment courses. Because there often is no reliable mechanism for patients to obtain refills during deployment, any medications prescribed would need to be provided in advance for the entire deployment duration, which often is 6 to 9 months. Additionally, treatment monitoring or modifications are severely limited, especially in the context of treatment nonresponse or adverse reactions. Considering the unique limitations of this patient population, both military and civilian physicians are faced with a need to maximize beneficence and autonomy while balancing nonmaleficence and justice.
One possible option is to decline to treat until the patient can follow up after returning from deployment. However, denying a request for an active treatable indication for which the patient desires treatment compromises patient autonomy and beneficence. Further, treatment should be provided to patients equitably to maintain justice. Although there may be a role for discussing active monitoring with nonintervention with the patient, denying treatment can negatively impact their physical and mental health and may be harmful. However, the patient should know and fully understand the risks and benefits of nonintervention with limited follow-up, including suboptimal outcomes or adverse events.
Another possibility for the management of this case may be conducting a one-time laser or light-based therapy or a one-time superficial- to medium-depth chemical peel before the patient leaves on deployment. Often, a series of laser- or light-based treatments is required to maximize outcomes for dyspigmentation. Without follow-up and with possible deployment to an environment with high UV exposure, the patient may experience disease exacerbation or other adverse effects. Treatment of those adverse effects may be delayed, as further intervention is not possible during deployment. Lower initial laser settings may be safer but may not be highly effective initially. More rigorous treatment upon return from deployment may be considered. Similar to laser therapies, chemical peels usually require several treatments for optimal outcomes. Without follow-up and with potential deployment to remote environments, there is a risk for adverse events that outweighs the minimal benefit of a single treatment. Therefore, either intervention may violate the principle of nonmaleficence.
A more reasonable approach may be initiating topical therapy and following up via telemedicine evaluation. Topical therapy often is the least-invasive approach and carries a reduced risk for adverse effects. Triple-combination therapy with topical retinoids, hydroquinone, and topical steroids is a common first-line approach.4 Because this approach is patient dependent, therapy can be more easily modulated or halted in the context of undesired results. Additionally, if internet connectivity is available, an asynchronous telemedicine approach could be utilized during deployment to monitor and advise changes as necessary, provided the regulatory framework allows for it.5
Although there is no uniformly correct approach in a scenario of limited patient follow-up, the last solution may be most ethically favorable: to begin therapy with milder and safer therapies (topical) and defer higher-intensity regimens until the patient returns from deployment. This allows some treatment initiation to preserve justice, beneficence, and patient autonomy. Associated virtual follow-up via telemedicine also allows avoidance of nonmaleficence in this context.
- Hwang J, Kakimoto C. Teledermatology in the US military: a historic foundation for current and future applications. Cutis. 2018;101:335;337;345.
- Dodd JG, Grant-Kels JM. Ethical concerns in caring for active duty service members who may be seeking dermatologic care outside the military soon. Int J Womens Dermatol. 2020;6:445-447. doi:10.1016/j.ijwd.2020.07.001
- Burke KR, Larrymore DC, Cho S. Treatment consideration for US military members with skin disease. Cutis. 2019;103:329-332.
- Desai SR. Hyperpigmentation therapy: a review. J Clin Aesthet Dermatol. 2014;7:13-17.
- Hwang JS, Lappan CM, Sperling LC, et al. Utilization of telemedicine in the U.S. military in a deployed setting. Mil Med. 2014;179:1347-1353. doi:10.7205/MILMED-D-14-00115
For active-duty service members, dermatologic conditions are among the most common presenting concerns, comprising 15% to 75% of wartime outpatient visits.1 In general, there are unique considerations when caring for active-duty service members, including meeting designated active-duty retention and hierarchical standards.2 We present a hypothetical case: An active-duty military patient presents to a new dermatologist for cosmetic enhancement of facial skin dyspigmentation. The patient will be leaving soon for deployment and will not be able to follow up for 9 months. How should the dermatologist treat a patient who cannot follow up for so long?
The therapeutic modalities offered can be impacted by forthcoming deployments3 that may result in delayed time to administer repeat treatments or follow-up. The patient may have high expectations for a single appointment for a condition that requires prolonged treatment courses. Because there often is no reliable mechanism for patients to obtain refills during deployment, any medications prescribed would need to be provided in advance for the entire deployment duration, which often is 6 to 9 months. Additionally, treatment monitoring or modifications are severely limited, especially in the context of treatment nonresponse or adverse reactions. Considering the unique limitations of this patient population, both military and civilian physicians are faced with a need to maximize beneficence and autonomy while balancing nonmaleficence and justice.
One possible option is to decline to treat until the patient can follow up after returning from deployment. However, denying a request for an active treatable indication for which the patient desires treatment compromises patient autonomy and beneficence. Further, treatment should be provided to patients equitably to maintain justice. Although there may be a role for discussing active monitoring with nonintervention with the patient, denying treatment can negatively impact their physical and mental health and may be harmful. However, the patient should know and fully understand the risks and benefits of nonintervention with limited follow-up, including suboptimal outcomes or adverse events.
Another possibility for the management of this case may be conducting a one-time laser or light-based therapy or a one-time superficial- to medium-depth chemical peel before the patient leaves on deployment. Often, a series of laser- or light-based treatments is required to maximize outcomes for dyspigmentation. Without follow-up and with possible deployment to an environment with high UV exposure, the patient may experience disease exacerbation or other adverse effects. Treatment of those adverse effects may be delayed, as further intervention is not possible during deployment. Lower initial laser settings may be safer but may not be highly effective initially. More rigorous treatment upon return from deployment may be considered. Similar to laser therapies, chemical peels usually require several treatments for optimal outcomes. Without follow-up and with potential deployment to remote environments, there is a risk for adverse events that outweighs the minimal benefit of a single treatment. Therefore, either intervention may violate the principle of nonmaleficence.
A more reasonable approach may be initiating topical therapy and following up via telemedicine evaluation. Topical therapy often is the least-invasive approach and carries a reduced risk for adverse effects. Triple-combination therapy with topical retinoids, hydroquinone, and topical steroids is a common first-line approach.4 Because this approach is patient dependent, therapy can be more easily modulated or halted in the context of undesired results. Additionally, if internet connectivity is available, an asynchronous telemedicine approach could be utilized during deployment to monitor and advise changes as necessary, provided the regulatory framework allows for it.5
Although there is no uniformly correct approach in a scenario of limited patient follow-up, the last solution may be most ethically favorable: to begin therapy with milder and safer therapies (topical) and defer higher-intensity regimens until the patient returns from deployment. This allows some treatment initiation to preserve justice, beneficence, and patient autonomy. Associated virtual follow-up via telemedicine also allows avoidance of nonmaleficence in this context.
For active-duty service members, dermatologic conditions are among the most common presenting concerns, comprising 15% to 75% of wartime outpatient visits.1 In general, there are unique considerations when caring for active-duty service members, including meeting designated active-duty retention and hierarchical standards.2 We present a hypothetical case: An active-duty military patient presents to a new dermatologist for cosmetic enhancement of facial skin dyspigmentation. The patient will be leaving soon for deployment and will not be able to follow up for 9 months. How should the dermatologist treat a patient who cannot follow up for so long?
The therapeutic modalities offered can be impacted by forthcoming deployments3 that may result in delayed time to administer repeat treatments or follow-up. The patient may have high expectations for a single appointment for a condition that requires prolonged treatment courses. Because there often is no reliable mechanism for patients to obtain refills during deployment, any medications prescribed would need to be provided in advance for the entire deployment duration, which often is 6 to 9 months. Additionally, treatment monitoring or modifications are severely limited, especially in the context of treatment nonresponse or adverse reactions. Considering the unique limitations of this patient population, both military and civilian physicians are faced with a need to maximize beneficence and autonomy while balancing nonmaleficence and justice.
One possible option is to decline to treat until the patient can follow up after returning from deployment. However, denying a request for an active treatable indication for which the patient desires treatment compromises patient autonomy and beneficence. Further, treatment should be provided to patients equitably to maintain justice. Although there may be a role for discussing active monitoring with nonintervention with the patient, denying treatment can negatively impact their physical and mental health and may be harmful. However, the patient should know and fully understand the risks and benefits of nonintervention with limited follow-up, including suboptimal outcomes or adverse events.
Another possibility for the management of this case may be conducting a one-time laser or light-based therapy or a one-time superficial- to medium-depth chemical peel before the patient leaves on deployment. Often, a series of laser- or light-based treatments is required to maximize outcomes for dyspigmentation. Without follow-up and with possible deployment to an environment with high UV exposure, the patient may experience disease exacerbation or other adverse effects. Treatment of those adverse effects may be delayed, as further intervention is not possible during deployment. Lower initial laser settings may be safer but may not be highly effective initially. More rigorous treatment upon return from deployment may be considered. Similar to laser therapies, chemical peels usually require several treatments for optimal outcomes. Without follow-up and with potential deployment to remote environments, there is a risk for adverse events that outweighs the minimal benefit of a single treatment. Therefore, either intervention may violate the principle of nonmaleficence.
A more reasonable approach may be initiating topical therapy and following up via telemedicine evaluation. Topical therapy often is the least-invasive approach and carries a reduced risk for adverse effects. Triple-combination therapy with topical retinoids, hydroquinone, and topical steroids is a common first-line approach.4 Because this approach is patient dependent, therapy can be more easily modulated or halted in the context of undesired results. Additionally, if internet connectivity is available, an asynchronous telemedicine approach could be utilized during deployment to monitor and advise changes as necessary, provided the regulatory framework allows for it.5
Although there is no uniformly correct approach in a scenario of limited patient follow-up, the last solution may be most ethically favorable: to begin therapy with milder and safer therapies (topical) and defer higher-intensity regimens until the patient returns from deployment. This allows some treatment initiation to preserve justice, beneficence, and patient autonomy. Associated virtual follow-up via telemedicine also allows avoidance of nonmaleficence in this context.
- Hwang J, Kakimoto C. Teledermatology in the US military: a historic foundation for current and future applications. Cutis. 2018;101:335;337;345.
- Dodd JG, Grant-Kels JM. Ethical concerns in caring for active duty service members who may be seeking dermatologic care outside the military soon. Int J Womens Dermatol. 2020;6:445-447. doi:10.1016/j.ijwd.2020.07.001
- Burke KR, Larrymore DC, Cho S. Treatment consideration for US military members with skin disease. Cutis. 2019;103:329-332.
- Desai SR. Hyperpigmentation therapy: a review. J Clin Aesthet Dermatol. 2014;7:13-17.
- Hwang JS, Lappan CM, Sperling LC, et al. Utilization of telemedicine in the U.S. military in a deployed setting. Mil Med. 2014;179:1347-1353. doi:10.7205/MILMED-D-14-00115
- Hwang J, Kakimoto C. Teledermatology in the US military: a historic foundation for current and future applications. Cutis. 2018;101:335;337;345.
- Dodd JG, Grant-Kels JM. Ethical concerns in caring for active duty service members who may be seeking dermatologic care outside the military soon. Int J Womens Dermatol. 2020;6:445-447. doi:10.1016/j.ijwd.2020.07.001
- Burke KR, Larrymore DC, Cho S. Treatment consideration for US military members with skin disease. Cutis. 2019;103:329-332.
- Desai SR. Hyperpigmentation therapy: a review. J Clin Aesthet Dermatol. 2014;7:13-17.
- Hwang JS, Lappan CM, Sperling LC, et al. Utilization of telemedicine in the U.S. military in a deployed setting. Mil Med. 2014;179:1347-1353. doi:10.7205/MILMED-D-14-00115
PRACTICE POINTS
- Dermatologic conditions are among the most common concerns reported by active-duty service members.
- The unique considerations of deployments are important for dermatologists to consider in the treatment of skin disease.
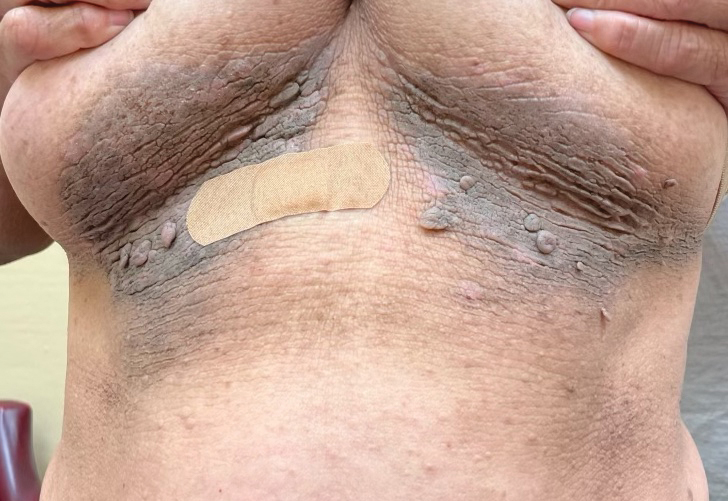
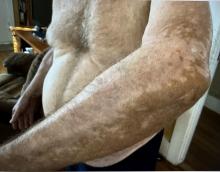
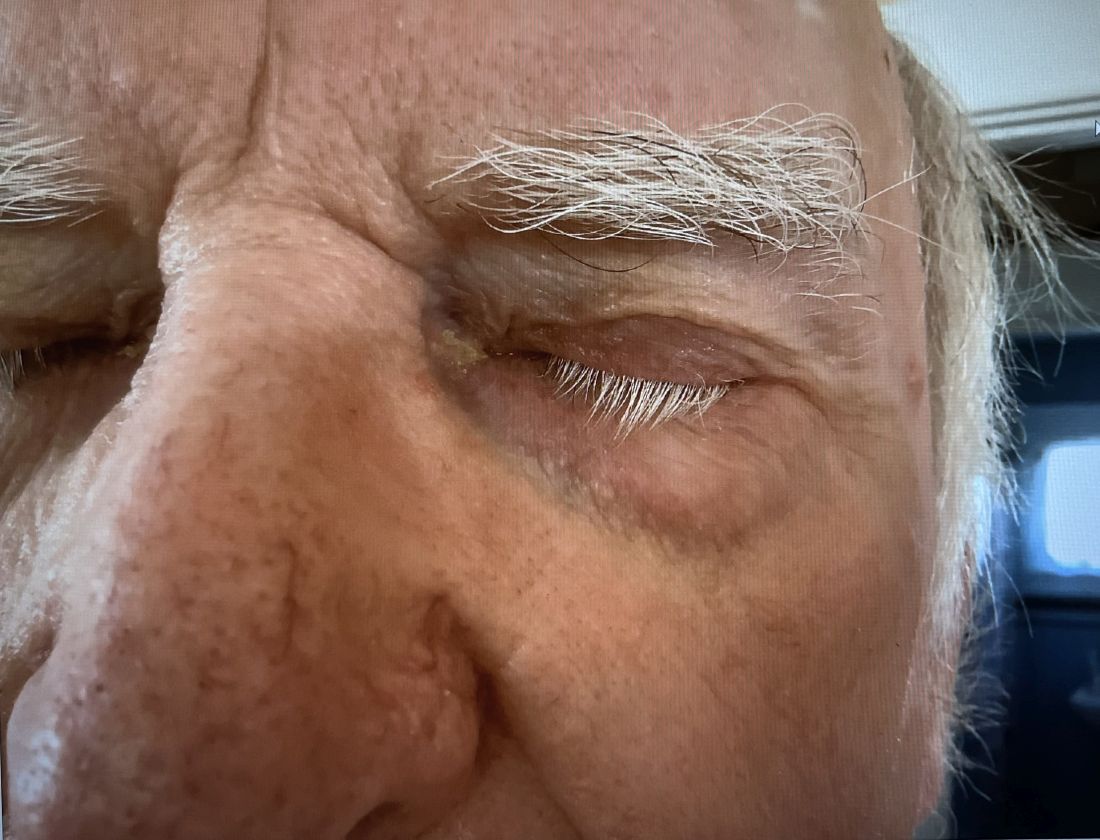
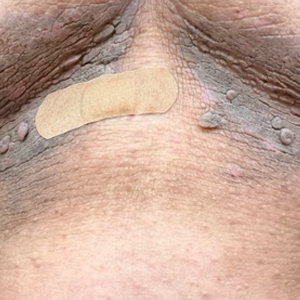
A 74-year-old White male presented with a 1-year history of depigmented patches on the hands, arms, and face, as well as white eyelashes and eyebrows
This patient showed no evidence of recurrence in the scar where the melanoma was excised, and had no enlarged lymph nodes on palpation. His complete blood count and liver function tests were normal. A positron emission tomography (PET) scan was ordered by Dr. Nasser that revealed hypermetabolic right paratracheal, right hilar, and subcarinal lymph nodes, highly suspicious for malignant lymph nodes. The patient was referred to oncology for metastatic melanoma treatment and has been doing well on ipilimumab and nivolumab.
Vitiligo is an autoimmune condition characterized by the progressive destruction of melanocytes resulting in hypopigmentation or depigmentation of the skin. Vitiligo has been associated with cutaneous melanoma. Melanoma-associated leukoderma occurs in a portion of patients with melanoma and is correlated with a favorable prognosis. Additionally, leukoderma has been described as a side effect of melanoma treatment itself. However, cases such as this one have also been reported of vitiligo-like depigmentation presenting prior to the diagnosis of metastatic melanoma.
Melanoma, like vitiligo, is considered highly immunogenic, and cytotoxic T lymphocytes (CTLs) can recognize antigens in melanoma. Furthermore, studies have shown a vitiligo-like halo around melanoma tumors, likely caused by T-cell recruitment, and this may lead to tumor destruction, but rarely total clearance. It seems that the CTL infiltrate in both diseases is similar, but regulatory T cells are decreased in vitiligo, whereas they are present in melanomas and may contribute to the immunosuppressive tumor microenvironment found at the margin of these lesions.
Leukoderma is also associated with melanoma immunotherapy which may be described as drug-induced leukoderma. Additionally, the frequency of recognition of melanoma cells by CTLs leading to hypopigmentation appears to be higher in those with metastatic disease. High immune infiltrate with CTLs and interferon-gamma (IFN-gamma) expression by type 1 T helper cells is associated with favorable prognosis. Immunotherapy with checkpoint inhibitors has shown promise in treatment augmentation for melanoma, but not all patients fully respond to therapy. Nonetheless, development of leukoderma with these treatments has been significantly associated with good therapeutic response. Depigmentation of hair and retinal epithelium has also been reported. However, drug-induced leukoderma and vitiligo seem to have clinical and biological differences, including family history of disease and serum chemokine levels. Vaccines are in production to aid in the treatment of melanoma, but researchers must first identify the appropriate antigen(s) to include.
Conversely, vitiligo-like depigmentation has been reported as a harbinger of metastatic melanoma. Patients with previous excision of primary melanoma have presented months or years later with depigmentation and, upon further evaluation, have been diagnosed with metastatic melanoma. The prevalence of depigmentation in melanoma patients is about 3%-6%, and is estimated to be 7-10 times more common in those with melanoma than in the general population. In most cases, hypopigmentation follows the diagnosis of melanoma, with an average of 4.8 years after the initial diagnosis and 1-2 years after lymph node or distant metastases. It is unclear whether hypopigmentation occurs before or after the growth of metastatic lesions, but this clinical finding in a patient with previous melanoma may serve as an important clue to conduct further investigation for metastasis.
This case and the photos were submitted by Lucas Shapiro, BS, of Nova Southeastern University College of Osteopathic Medicine, Fort Lauderdale, Florida, and Natalie Y. Nasser, MD, Kaiser Permanente Riverside Medical Center; Riverside, California. The column was edited by Donna Bilu Martin, MD.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Florida More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to dermnews@mdedge.com.
References
Cerci FB et al. Cutis. 2017 Jun;99(6):E1-E2. PMID: 28686764.
Cho EA et al. Ann Dermatol. 2009 May;21(2):178-181.
Failla CM et al. Int J Mol Sci. 2019 Nov 15;20(22):5731.
This patient showed no evidence of recurrence in the scar where the melanoma was excised, and had no enlarged lymph nodes on palpation. His complete blood count and liver function tests were normal. A positron emission tomography (PET) scan was ordered by Dr. Nasser that revealed hypermetabolic right paratracheal, right hilar, and subcarinal lymph nodes, highly suspicious for malignant lymph nodes. The patient was referred to oncology for metastatic melanoma treatment and has been doing well on ipilimumab and nivolumab.
Vitiligo is an autoimmune condition characterized by the progressive destruction of melanocytes resulting in hypopigmentation or depigmentation of the skin. Vitiligo has been associated with cutaneous melanoma. Melanoma-associated leukoderma occurs in a portion of patients with melanoma and is correlated with a favorable prognosis. Additionally, leukoderma has been described as a side effect of melanoma treatment itself. However, cases such as this one have also been reported of vitiligo-like depigmentation presenting prior to the diagnosis of metastatic melanoma.
Melanoma, like vitiligo, is considered highly immunogenic, and cytotoxic T lymphocytes (CTLs) can recognize antigens in melanoma. Furthermore, studies have shown a vitiligo-like halo around melanoma tumors, likely caused by T-cell recruitment, and this may lead to tumor destruction, but rarely total clearance. It seems that the CTL infiltrate in both diseases is similar, but regulatory T cells are decreased in vitiligo, whereas they are present in melanomas and may contribute to the immunosuppressive tumor microenvironment found at the margin of these lesions.
Leukoderma is also associated with melanoma immunotherapy which may be described as drug-induced leukoderma. Additionally, the frequency of recognition of melanoma cells by CTLs leading to hypopigmentation appears to be higher in those with metastatic disease. High immune infiltrate with CTLs and interferon-gamma (IFN-gamma) expression by type 1 T helper cells is associated with favorable prognosis. Immunotherapy with checkpoint inhibitors has shown promise in treatment augmentation for melanoma, but not all patients fully respond to therapy. Nonetheless, development of leukoderma with these treatments has been significantly associated with good therapeutic response. Depigmentation of hair and retinal epithelium has also been reported. However, drug-induced leukoderma and vitiligo seem to have clinical and biological differences, including family history of disease and serum chemokine levels. Vaccines are in production to aid in the treatment of melanoma, but researchers must first identify the appropriate antigen(s) to include.
Conversely, vitiligo-like depigmentation has been reported as a harbinger of metastatic melanoma. Patients with previous excision of primary melanoma have presented months or years later with depigmentation and, upon further evaluation, have been diagnosed with metastatic melanoma. The prevalence of depigmentation in melanoma patients is about 3%-6%, and is estimated to be 7-10 times more common in those with melanoma than in the general population. In most cases, hypopigmentation follows the diagnosis of melanoma, with an average of 4.8 years after the initial diagnosis and 1-2 years after lymph node or distant metastases. It is unclear whether hypopigmentation occurs before or after the growth of metastatic lesions, but this clinical finding in a patient with previous melanoma may serve as an important clue to conduct further investigation for metastasis.
This case and the photos were submitted by Lucas Shapiro, BS, of Nova Southeastern University College of Osteopathic Medicine, Fort Lauderdale, Florida, and Natalie Y. Nasser, MD, Kaiser Permanente Riverside Medical Center; Riverside, California. The column was edited by Donna Bilu Martin, MD.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Florida More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to dermnews@mdedge.com.
References
Cerci FB et al. Cutis. 2017 Jun;99(6):E1-E2. PMID: 28686764.
Cho EA et al. Ann Dermatol. 2009 May;21(2):178-181.
Failla CM et al. Int J Mol Sci. 2019 Nov 15;20(22):5731.
This patient showed no evidence of recurrence in the scar where the melanoma was excised, and had no enlarged lymph nodes on palpation. His complete blood count and liver function tests were normal. A positron emission tomography (PET) scan was ordered by Dr. Nasser that revealed hypermetabolic right paratracheal, right hilar, and subcarinal lymph nodes, highly suspicious for malignant lymph nodes. The patient was referred to oncology for metastatic melanoma treatment and has been doing well on ipilimumab and nivolumab.
Vitiligo is an autoimmune condition characterized by the progressive destruction of melanocytes resulting in hypopigmentation or depigmentation of the skin. Vitiligo has been associated with cutaneous melanoma. Melanoma-associated leukoderma occurs in a portion of patients with melanoma and is correlated with a favorable prognosis. Additionally, leukoderma has been described as a side effect of melanoma treatment itself. However, cases such as this one have also been reported of vitiligo-like depigmentation presenting prior to the diagnosis of metastatic melanoma.
Melanoma, like vitiligo, is considered highly immunogenic, and cytotoxic T lymphocytes (CTLs) can recognize antigens in melanoma. Furthermore, studies have shown a vitiligo-like halo around melanoma tumors, likely caused by T-cell recruitment, and this may lead to tumor destruction, but rarely total clearance. It seems that the CTL infiltrate in both diseases is similar, but regulatory T cells are decreased in vitiligo, whereas they are present in melanomas and may contribute to the immunosuppressive tumor microenvironment found at the margin of these lesions.
Leukoderma is also associated with melanoma immunotherapy which may be described as drug-induced leukoderma. Additionally, the frequency of recognition of melanoma cells by CTLs leading to hypopigmentation appears to be higher in those with metastatic disease. High immune infiltrate with CTLs and interferon-gamma (IFN-gamma) expression by type 1 T helper cells is associated with favorable prognosis. Immunotherapy with checkpoint inhibitors has shown promise in treatment augmentation for melanoma, but not all patients fully respond to therapy. Nonetheless, development of leukoderma with these treatments has been significantly associated with good therapeutic response. Depigmentation of hair and retinal epithelium has also been reported. However, drug-induced leukoderma and vitiligo seem to have clinical and biological differences, including family history of disease and serum chemokine levels. Vaccines are in production to aid in the treatment of melanoma, but researchers must first identify the appropriate antigen(s) to include.
Conversely, vitiligo-like depigmentation has been reported as a harbinger of metastatic melanoma. Patients with previous excision of primary melanoma have presented months or years later with depigmentation and, upon further evaluation, have been diagnosed with metastatic melanoma. The prevalence of depigmentation in melanoma patients is about 3%-6%, and is estimated to be 7-10 times more common in those with melanoma than in the general population. In most cases, hypopigmentation follows the diagnosis of melanoma, with an average of 4.8 years after the initial diagnosis and 1-2 years after lymph node or distant metastases. It is unclear whether hypopigmentation occurs before or after the growth of metastatic lesions, but this clinical finding in a patient with previous melanoma may serve as an important clue to conduct further investigation for metastasis.
This case and the photos were submitted by Lucas Shapiro, BS, of Nova Southeastern University College of Osteopathic Medicine, Fort Lauderdale, Florida, and Natalie Y. Nasser, MD, Kaiser Permanente Riverside Medical Center; Riverside, California. The column was edited by Donna Bilu Martin, MD.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Florida More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to dermnews@mdedge.com.
References
Cerci FB et al. Cutis. 2017 Jun;99(6):E1-E2. PMID: 28686764.
Cho EA et al. Ann Dermatol. 2009 May;21(2):178-181.
Failla CM et al. Int J Mol Sci. 2019 Nov 15;20(22):5731.
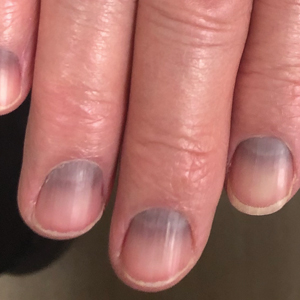
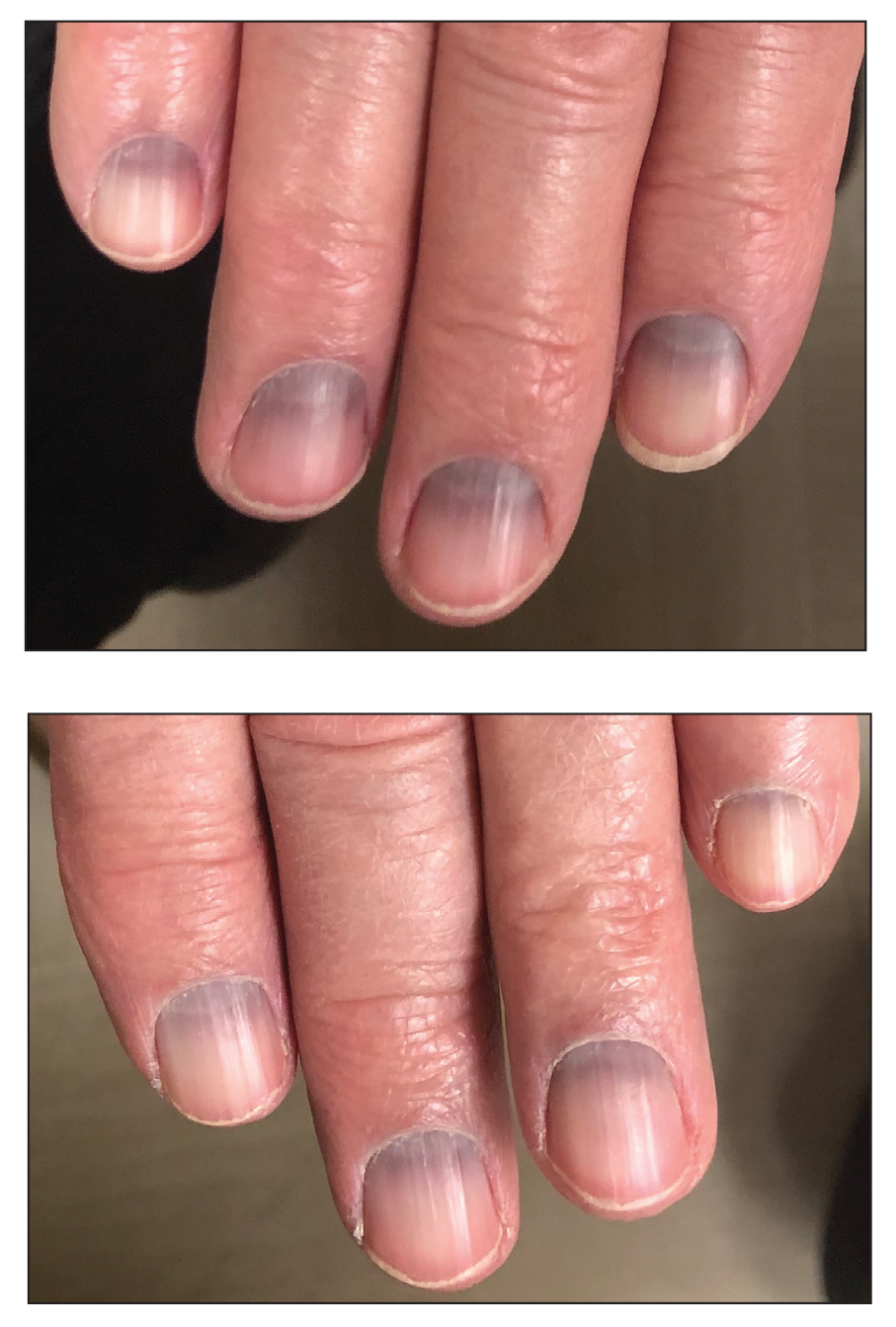
Blue to Slate Gray Discoloration of the Proximal Fingernails
The Diagnosis: Argyria-Induced Azure Lunulae
Argyria is an acquired condition resulting from excessive exogenous exposure to silver with subsequent gastrointestinal absorption and pigmentary tissue deposition. Upon further questioning, our patient disclosed a lifetime history of colloidal silver use, both as a topical antiseptic agent and intraorally for aphthous ulcers. Silver has a predilection for granular deposition in stromal tissues and basement membranes with sparing of the epidermis, manifesting as progressive, permanent, blue to slate gray discoloration of sunexposed skin, mucous membranes, and nail beds.1 The patient was advised to discontinue use of colloidal silver to avoid development of further pigmentary changes. The appearance of his nails remained unchanged in the months following initial presentation, as expected, since argyria pigmentation is not anticipated to reverse upon colloidal silver cessation.
Nail involvement may be an early presentation of generalized argyria or may be found in isolation, as seen in our patient. Early recognition and patient education are essential to minimize cumulative silver deposition. Although dyspigmentation may impact psychosocial well-being secondary to aesthetic concerns, there is limited research supporting adverse systemic effects of argyria confined to the nail beds. Similarly, the majority of generalized cases are not associated with systemic complications; however, potential toxicities, as described in isolated case reports without conclusive causal relationships, include nyctalopia, renal or hepatic toxicity, pulmonary fibrosis, and neuropsychiatric events.1-6 Successful treatment of cutaneous argyria has been reported with the 1064-nm Q-switched Nd:YAG laser; however, there have been no reported treatments for nail bed involvement.7 Due to the absence of systemic symptoms, additional mucocutaneous dyspigmentation, or cosmetic concerns regarding nail bed lunulae discoloration in our patient, no further intervention was pursued, except for continued colloidal silver cessation.
The differential diagnosis of blue-gray nail bed dyspigmentation is broad and includes cyanosis secondary to cardiopulmonary disease, drug-induced dyspigmentation, Wilson disease, argyria, chrysiasis, hereditary acrolabial telangiectasia, and pseudomonal infection or chloronychia.1,8,9 Etiologic insight may be provided from a thorough review of prescription and over-the-counter medications as well as careful attention to the distribution of dyspigmentation. Medications commonly associated with bluish nail bed dyspigmentation include antimalarials, amiodarone, minocycline, clofazimine, chlorpromazine/phenothiazines, and various chemotherapeutic drugs; our patient was not taking any of these.1,9
Cyanotic nail bed dyspigmentation secondary to cardiopulmonary disease likely manifests with more diffuse nail bed dyspigmentation and is not confined solely to the lunulae. Only drug-induced dyspigmentation, classically due to phenolphthalein-containing laxatives; Wilson disease; and argyria have a tendency to spare the distal nail bed, which is a presentation termed azure lunulae.8 The toenails typically are spared in argyria, while toenail involvement is variable in Wilson disease, and additional systemic symptoms—including hepatic, ophthalmologic, and neuropsychiatric—as well as potential family history would be expected.8 Phenolphthalein is no longer available in over-the-counter laxatives, as it was formally banned by the US Food and Drug Administration in 1999 due to concerns of carcinogenicity.10
Hereditary acrolabial telangiectasia is a familial condition with autosomal-dominant inheritance that can manifest similarly to argyria with blue-gray discoloration of the proximal nail bed; however, this condition also would demonstrate involvement of the vermilion border and nipple areolae, often with associated telangiectasia and migraine headaches.11
Chloronychia (also known as green nail syndrome) is an infection of the nail bed with Pseudomonas aeruginosa that more commonly presents with greenblack discoloration with variable involvement of the fingernails and toenails. Chloronychia, often with associated onycholysis, typically is found in individuals with repeated exposure to water, soaps, and detergents.12 Our patient’s long-standing and unwavering nail bed appearance, involvement of all fingernail lunulae, lack of additional symptoms, and disclosed use of over-the-counter colloidal silver supported a clinical diagnosis of argyriainduced azure lunulae.
Argyria-induced azure lunulae secondary to colloidal silver exposure is an uncommon yet clinically significant cause of nail bed dyspigmentation. Prompt identification and cessation of the offending agent can prevent progression of mucocutaneous dyspigmentation and avoid potential long-term sequelae from systemic deposition.
- Mota L, Dinis-Oliveira RJ. Clinical and forensic aspects of the different subtypes of argyria. J Clin Med. 2021;10:2086. doi:10.3390/ jcm10102086
- Osin´ska J, Poborc-Godlewska J, Kiec´-Swierczyn´ska M, et al. 6 cases of argyria among workers engaged in silverplating radio subunits. Med Pr. 1982;33:361-364.
- Mayr M, Kim MJ, Wanner D, et al. Argyria and decreased kidney function: are silver compounds toxic to the kidney? Am J Kidney Dis. 2009;53:890-894. doi:10.1053/j.ajkd.2008.08.028
- Trop M, Novak M, Rodl S, et al. Silver-coated dressing acticoat caused raised liver enzymes and argyria-like symptoms in burn patient. J Trauma. 2006;60:648-652. doi:10.1097/01.ta.0000208126 .22089.b6
- Mirsattari SM, Hammond RR, Sharpe MD, et al. Myoclonic status epilepticus following repeated oral ingestion of colloidal silver. Neurology. 2004;62:1408-1410. doi:10.1212/01.wnl.0000120671.73335.ec
- Barrie HJ, Harding HE. Argyro-siderosis of the lungs in silver finishers. Br J Ind Med. 1947;4:225-229. doi:10.1136/oem.4.4.225
- Griffith RD, Simmons BJ, Bray FN, et al. 1064 nm Q-switched Nd:YAG laser for the treatment of argyria: a systematic review. J Eur Acad Dermatol Venereol. 2015;29:2100-2103. doi:10.111 1/jdv.13117
- Rubin AI, Jellinek NJ, Daniel CR III, et al, eds. Scher and Daniel’s Nails: Diagnosis, Surgery, Therapy. 4th ed. Springer; 2018.
- Slater K, Sommariva E, Kartono F. A case study of argyria of the nails secondary to colloidal silver ingestion [published online October 28, 2022]. Cureus. 2022;14:E30818. doi:10.7759/cureus.30818
- Hubbard WK. Laxative drug products for over-the-counter human use. Fed Register. 1999;64:4535-4540. Accessed January 5, 2024. https://www.govinfo.gov/content/pkg/FR-1999-01-29/html/99-1938.htm
- Millns JL, Dicken CH. Hereditary acrolabial telangiectasia. a report of familial blue lips, nails, and nipples. Arch Dermatol. 1979;115:474-478. doi:10.1001/archderm.115.4.474
- Chiriac A, Brzezinski P, Foia L, et al. Chloronychia: green nail syndrome caused by Pseudomonas aeruginosa in elderly persons [published online January 14, 2015]. Clin Interv Aging. 2015;10:265-267. doi:10.2147/CIA.S75525
The Diagnosis: Argyria-Induced Azure Lunulae
Argyria is an acquired condition resulting from excessive exogenous exposure to silver with subsequent gastrointestinal absorption and pigmentary tissue deposition. Upon further questioning, our patient disclosed a lifetime history of colloidal silver use, both as a topical antiseptic agent and intraorally for aphthous ulcers. Silver has a predilection for granular deposition in stromal tissues and basement membranes with sparing of the epidermis, manifesting as progressive, permanent, blue to slate gray discoloration of sunexposed skin, mucous membranes, and nail beds.1 The patient was advised to discontinue use of colloidal silver to avoid development of further pigmentary changes. The appearance of his nails remained unchanged in the months following initial presentation, as expected, since argyria pigmentation is not anticipated to reverse upon colloidal silver cessation.
Nail involvement may be an early presentation of generalized argyria or may be found in isolation, as seen in our patient. Early recognition and patient education are essential to minimize cumulative silver deposition. Although dyspigmentation may impact psychosocial well-being secondary to aesthetic concerns, there is limited research supporting adverse systemic effects of argyria confined to the nail beds. Similarly, the majority of generalized cases are not associated with systemic complications; however, potential toxicities, as described in isolated case reports without conclusive causal relationships, include nyctalopia, renal or hepatic toxicity, pulmonary fibrosis, and neuropsychiatric events.1-6 Successful treatment of cutaneous argyria has been reported with the 1064-nm Q-switched Nd:YAG laser; however, there have been no reported treatments for nail bed involvement.7 Due to the absence of systemic symptoms, additional mucocutaneous dyspigmentation, or cosmetic concerns regarding nail bed lunulae discoloration in our patient, no further intervention was pursued, except for continued colloidal silver cessation.
The differential diagnosis of blue-gray nail bed dyspigmentation is broad and includes cyanosis secondary to cardiopulmonary disease, drug-induced dyspigmentation, Wilson disease, argyria, chrysiasis, hereditary acrolabial telangiectasia, and pseudomonal infection or chloronychia.1,8,9 Etiologic insight may be provided from a thorough review of prescription and over-the-counter medications as well as careful attention to the distribution of dyspigmentation. Medications commonly associated with bluish nail bed dyspigmentation include antimalarials, amiodarone, minocycline, clofazimine, chlorpromazine/phenothiazines, and various chemotherapeutic drugs; our patient was not taking any of these.1,9
Cyanotic nail bed dyspigmentation secondary to cardiopulmonary disease likely manifests with more diffuse nail bed dyspigmentation and is not confined solely to the lunulae. Only drug-induced dyspigmentation, classically due to phenolphthalein-containing laxatives; Wilson disease; and argyria have a tendency to spare the distal nail bed, which is a presentation termed azure lunulae.8 The toenails typically are spared in argyria, while toenail involvement is variable in Wilson disease, and additional systemic symptoms—including hepatic, ophthalmologic, and neuropsychiatric—as well as potential family history would be expected.8 Phenolphthalein is no longer available in over-the-counter laxatives, as it was formally banned by the US Food and Drug Administration in 1999 due to concerns of carcinogenicity.10
Hereditary acrolabial telangiectasia is a familial condition with autosomal-dominant inheritance that can manifest similarly to argyria with blue-gray discoloration of the proximal nail bed; however, this condition also would demonstrate involvement of the vermilion border and nipple areolae, often with associated telangiectasia and migraine headaches.11
Chloronychia (also known as green nail syndrome) is an infection of the nail bed with Pseudomonas aeruginosa that more commonly presents with greenblack discoloration with variable involvement of the fingernails and toenails. Chloronychia, often with associated onycholysis, typically is found in individuals with repeated exposure to water, soaps, and detergents.12 Our patient’s long-standing and unwavering nail bed appearance, involvement of all fingernail lunulae, lack of additional symptoms, and disclosed use of over-the-counter colloidal silver supported a clinical diagnosis of argyriainduced azure lunulae.
Argyria-induced azure lunulae secondary to colloidal silver exposure is an uncommon yet clinically significant cause of nail bed dyspigmentation. Prompt identification and cessation of the offending agent can prevent progression of mucocutaneous dyspigmentation and avoid potential long-term sequelae from systemic deposition.
The Diagnosis: Argyria-Induced Azure Lunulae
Argyria is an acquired condition resulting from excessive exogenous exposure to silver with subsequent gastrointestinal absorption and pigmentary tissue deposition. Upon further questioning, our patient disclosed a lifetime history of colloidal silver use, both as a topical antiseptic agent and intraorally for aphthous ulcers. Silver has a predilection for granular deposition in stromal tissues and basement membranes with sparing of the epidermis, manifesting as progressive, permanent, blue to slate gray discoloration of sunexposed skin, mucous membranes, and nail beds.1 The patient was advised to discontinue use of colloidal silver to avoid development of further pigmentary changes. The appearance of his nails remained unchanged in the months following initial presentation, as expected, since argyria pigmentation is not anticipated to reverse upon colloidal silver cessation.
Nail involvement may be an early presentation of generalized argyria or may be found in isolation, as seen in our patient. Early recognition and patient education are essential to minimize cumulative silver deposition. Although dyspigmentation may impact psychosocial well-being secondary to aesthetic concerns, there is limited research supporting adverse systemic effects of argyria confined to the nail beds. Similarly, the majority of generalized cases are not associated with systemic complications; however, potential toxicities, as described in isolated case reports without conclusive causal relationships, include nyctalopia, renal or hepatic toxicity, pulmonary fibrosis, and neuropsychiatric events.1-6 Successful treatment of cutaneous argyria has been reported with the 1064-nm Q-switched Nd:YAG laser; however, there have been no reported treatments for nail bed involvement.7 Due to the absence of systemic symptoms, additional mucocutaneous dyspigmentation, or cosmetic concerns regarding nail bed lunulae discoloration in our patient, no further intervention was pursued, except for continued colloidal silver cessation.
The differential diagnosis of blue-gray nail bed dyspigmentation is broad and includes cyanosis secondary to cardiopulmonary disease, drug-induced dyspigmentation, Wilson disease, argyria, chrysiasis, hereditary acrolabial telangiectasia, and pseudomonal infection or chloronychia.1,8,9 Etiologic insight may be provided from a thorough review of prescription and over-the-counter medications as well as careful attention to the distribution of dyspigmentation. Medications commonly associated with bluish nail bed dyspigmentation include antimalarials, amiodarone, minocycline, clofazimine, chlorpromazine/phenothiazines, and various chemotherapeutic drugs; our patient was not taking any of these.1,9
Cyanotic nail bed dyspigmentation secondary to cardiopulmonary disease likely manifests with more diffuse nail bed dyspigmentation and is not confined solely to the lunulae. Only drug-induced dyspigmentation, classically due to phenolphthalein-containing laxatives; Wilson disease; and argyria have a tendency to spare the distal nail bed, which is a presentation termed azure lunulae.8 The toenails typically are spared in argyria, while toenail involvement is variable in Wilson disease, and additional systemic symptoms—including hepatic, ophthalmologic, and neuropsychiatric—as well as potential family history would be expected.8 Phenolphthalein is no longer available in over-the-counter laxatives, as it was formally banned by the US Food and Drug Administration in 1999 due to concerns of carcinogenicity.10
Hereditary acrolabial telangiectasia is a familial condition with autosomal-dominant inheritance that can manifest similarly to argyria with blue-gray discoloration of the proximal nail bed; however, this condition also would demonstrate involvement of the vermilion border and nipple areolae, often with associated telangiectasia and migraine headaches.11
Chloronychia (also known as green nail syndrome) is an infection of the nail bed with Pseudomonas aeruginosa that more commonly presents with greenblack discoloration with variable involvement of the fingernails and toenails. Chloronychia, often with associated onycholysis, typically is found in individuals with repeated exposure to water, soaps, and detergents.12 Our patient’s long-standing and unwavering nail bed appearance, involvement of all fingernail lunulae, lack of additional symptoms, and disclosed use of over-the-counter colloidal silver supported a clinical diagnosis of argyriainduced azure lunulae.
Argyria-induced azure lunulae secondary to colloidal silver exposure is an uncommon yet clinically significant cause of nail bed dyspigmentation. Prompt identification and cessation of the offending agent can prevent progression of mucocutaneous dyspigmentation and avoid potential long-term sequelae from systemic deposition.
- Mota L, Dinis-Oliveira RJ. Clinical and forensic aspects of the different subtypes of argyria. J Clin Med. 2021;10:2086. doi:10.3390/ jcm10102086
- Osin´ska J, Poborc-Godlewska J, Kiec´-Swierczyn´ska M, et al. 6 cases of argyria among workers engaged in silverplating radio subunits. Med Pr. 1982;33:361-364.
- Mayr M, Kim MJ, Wanner D, et al. Argyria and decreased kidney function: are silver compounds toxic to the kidney? Am J Kidney Dis. 2009;53:890-894. doi:10.1053/j.ajkd.2008.08.028
- Trop M, Novak M, Rodl S, et al. Silver-coated dressing acticoat caused raised liver enzymes and argyria-like symptoms in burn patient. J Trauma. 2006;60:648-652. doi:10.1097/01.ta.0000208126 .22089.b6
- Mirsattari SM, Hammond RR, Sharpe MD, et al. Myoclonic status epilepticus following repeated oral ingestion of colloidal silver. Neurology. 2004;62:1408-1410. doi:10.1212/01.wnl.0000120671.73335.ec
- Barrie HJ, Harding HE. Argyro-siderosis of the lungs in silver finishers. Br J Ind Med. 1947;4:225-229. doi:10.1136/oem.4.4.225
- Griffith RD, Simmons BJ, Bray FN, et al. 1064 nm Q-switched Nd:YAG laser for the treatment of argyria: a systematic review. J Eur Acad Dermatol Venereol. 2015;29:2100-2103. doi:10.111 1/jdv.13117
- Rubin AI, Jellinek NJ, Daniel CR III, et al, eds. Scher and Daniel’s Nails: Diagnosis, Surgery, Therapy. 4th ed. Springer; 2018.
- Slater K, Sommariva E, Kartono F. A case study of argyria of the nails secondary to colloidal silver ingestion [published online October 28, 2022]. Cureus. 2022;14:E30818. doi:10.7759/cureus.30818
- Hubbard WK. Laxative drug products for over-the-counter human use. Fed Register. 1999;64:4535-4540. Accessed January 5, 2024. https://www.govinfo.gov/content/pkg/FR-1999-01-29/html/99-1938.htm
- Millns JL, Dicken CH. Hereditary acrolabial telangiectasia. a report of familial blue lips, nails, and nipples. Arch Dermatol. 1979;115:474-478. doi:10.1001/archderm.115.4.474
- Chiriac A, Brzezinski P, Foia L, et al. Chloronychia: green nail syndrome caused by Pseudomonas aeruginosa in elderly persons [published online January 14, 2015]. Clin Interv Aging. 2015;10:265-267. doi:10.2147/CIA.S75525
- Mota L, Dinis-Oliveira RJ. Clinical and forensic aspects of the different subtypes of argyria. J Clin Med. 2021;10:2086. doi:10.3390/ jcm10102086
- Osin´ska J, Poborc-Godlewska J, Kiec´-Swierczyn´ska M, et al. 6 cases of argyria among workers engaged in silverplating radio subunits. Med Pr. 1982;33:361-364.
- Mayr M, Kim MJ, Wanner D, et al. Argyria and decreased kidney function: are silver compounds toxic to the kidney? Am J Kidney Dis. 2009;53:890-894. doi:10.1053/j.ajkd.2008.08.028
- Trop M, Novak M, Rodl S, et al. Silver-coated dressing acticoat caused raised liver enzymes and argyria-like symptoms in burn patient. J Trauma. 2006;60:648-652. doi:10.1097/01.ta.0000208126 .22089.b6
- Mirsattari SM, Hammond RR, Sharpe MD, et al. Myoclonic status epilepticus following repeated oral ingestion of colloidal silver. Neurology. 2004;62:1408-1410. doi:10.1212/01.wnl.0000120671.73335.ec
- Barrie HJ, Harding HE. Argyro-siderosis of the lungs in silver finishers. Br J Ind Med. 1947;4:225-229. doi:10.1136/oem.4.4.225
- Griffith RD, Simmons BJ, Bray FN, et al. 1064 nm Q-switched Nd:YAG laser for the treatment of argyria: a systematic review. J Eur Acad Dermatol Venereol. 2015;29:2100-2103. doi:10.111 1/jdv.13117
- Rubin AI, Jellinek NJ, Daniel CR III, et al, eds. Scher and Daniel’s Nails: Diagnosis, Surgery, Therapy. 4th ed. Springer; 2018.
- Slater K, Sommariva E, Kartono F. A case study of argyria of the nails secondary to colloidal silver ingestion [published online October 28, 2022]. Cureus. 2022;14:E30818. doi:10.7759/cureus.30818
- Hubbard WK. Laxative drug products for over-the-counter human use. Fed Register. 1999;64:4535-4540. Accessed January 5, 2024. https://www.govinfo.gov/content/pkg/FR-1999-01-29/html/99-1938.htm
- Millns JL, Dicken CH. Hereditary acrolabial telangiectasia. a report of familial blue lips, nails, and nipples. Arch Dermatol. 1979;115:474-478. doi:10.1001/archderm.115.4.474
- Chiriac A, Brzezinski P, Foia L, et al. Chloronychia: green nail syndrome caused by Pseudomonas aeruginosa in elderly persons [published online January 14, 2015]. Clin Interv Aging. 2015;10:265-267. doi:10.2147/CIA.S75525
An 88-year-old man presented with asymptomatic and unchanging discoloration of the proximal fingernails of both hands of 50 years’ duration. Physical examination revealed blue to slate gray, subungual pigmentary changes of the fingernails of both hands sparing the nail bed distal to the lunulae. There was no overlying plate dystrophy, toenail involvement, or additional mucocutaneous abnormalities. His medical history was notable for heart failure, obstructive sleep apnea, and type 2 diabetes mellitus. He had no history of hepatic, ophthalmologic, or neurologic dysfunction.
Botanical Briefs: Neem Oil (Azadirachta indica)
Commonly known as neem or nimba, Azadirachta indica traditionally has been used as an oil or poultice to lighten skin pigment and reduce joint inflammation. Neem is a drought-resistant evergreen tree with thin serrated leaves, white fragrant flowers, and olivelike fruit (Figure 1). This plant is indigenous to India but also is readily found within tropical and semitropical environments throughout the Middle East, Southeast Asia, North Africa, and Australia.

Traditional Uses
For more than 4000 years, neem leaves, bark, fruit, and seeds have been used in food, insecticide, and herbal medicine cross-culturally in Indian Ayurvedic medicine and across Southeast Asia, particularly in Cambodia, Laos, Thailand, Myanmar, and Vietnam.1-3 Because of its many essential nutrients—oleic acid, palmitic acid, stearic acid, linoleic acid, behenic acid, arachidic acid, and palmitoleic acid—and readily available nature, some ethnic groups include neem in their diet.4 Neem commonly is used as a seasoning in soups and rice, eaten as a cooked vegetable, infused into teas and tonics, and pickled with other spices.5
All parts of the neem tree—both externally and internally—have been utilized in traditional medicine for the treatment of various diseases and ailments. The flowers have been used to treat eye diseases and dyspepsia, the fruit has been employed as an anthelmintic, the seeds and leaves have been used for malaria treatment and insecticide, the stem bark has been used for the treatment of diarrhea, and the root bark has been used for skin diseases and inflammation.6 Neem oil is a yellow-brown bitter substance that often is utilized to treat skin diseases such as psoriasis, eczema, fungal infections, and abscesses.
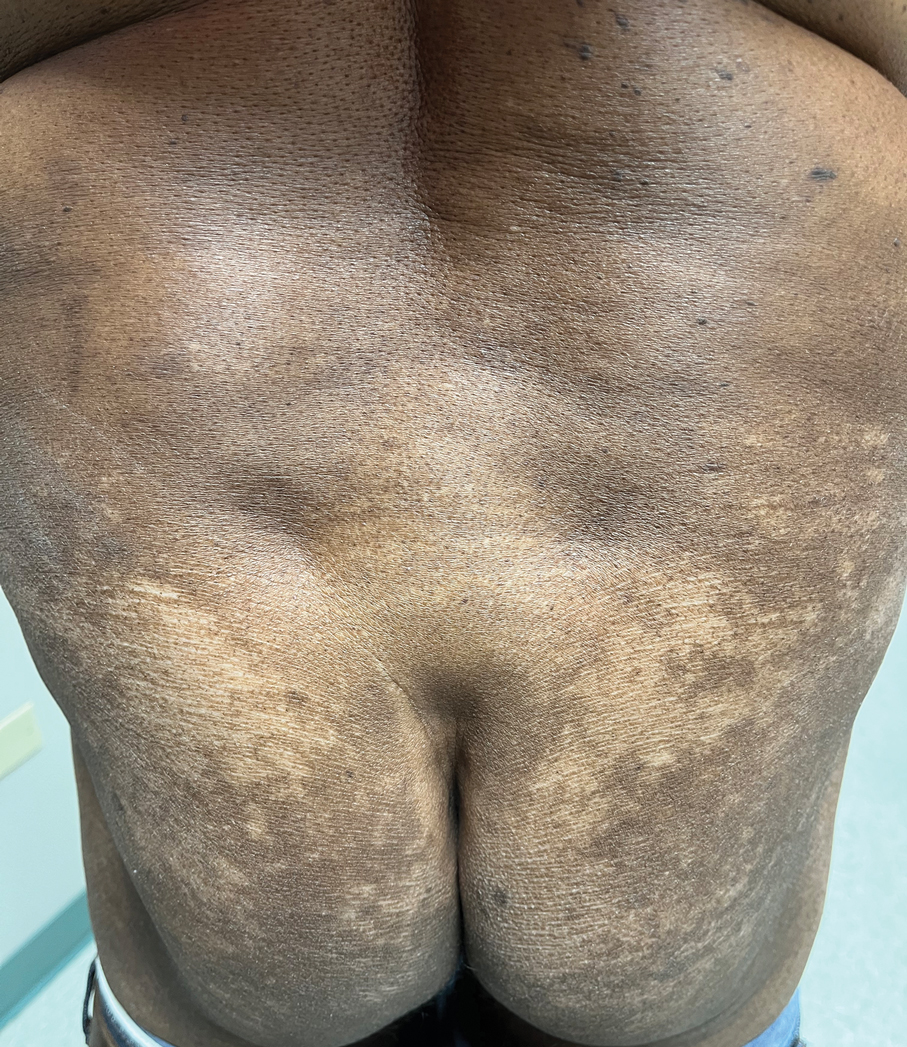
Case Report—A 77-year-old man presented with a diffuse rash across the lower back. He reported that he had been using topical neem oil to alleviate lower back pain and arthritis for the last 6 months with noted relief and improvement of back pain. After roughly 3 to 4 months of using neem oil, he noted a rash on the lower back, bilateral flanks, and buttocks (Figure 2). The rash was asymptomatic, and he denied any pruritus, scaling, pain, or burning. The patient was referred to dermatology and received a diagnosis of chemical leukoderma secondary to contact with A indica. The patient was advised to stop using the topical neem oil, and the rash was simply monitored, as it was asymptomatic.
Bioactivity
Research has elucidated multiple bioactivity mechanisms of neem, including melanogenesis-inhibitory activity, toxicity against pests, antimalarial activity, and antioxidant activity.1,7-9 Literature on the diverse phytochemical components of A indica indicate high levels of limonoids, flavonoids, and triterpenoids that are responsible for much of its antioxidant, anti-inflammatory, and insecticide properties.1,10
Melanogenesis-Inhibitory Activity—To date, neem has been added to a number of cosmetic products used in Ayurvedic medicine. One study of isolated compounds in A indica showed superior inhibitory activities against melanogenesis with minimal toxicity to cells (86.5%–105.1% cell viability). Western blot analysis of samples extracted and isolated from neem root and bark showed melanogenesis-inhibitory activities in B16 melanoma cells through the inhibition of microphthalmia-associated transcription factor expression and decreased expression of tyrosinase, as well as tyrosinase-related proteins 1 and 2, which are largely responsible for melanin synthesis.11 In another study, A indica flowers and their extracted constituents—6-deacetylnimbin and kaempferide—suggest melanogenesis-inhibitory activities in B16 melanoma cells with little to no toxicity to the cells (81.0%–111.7% cell viability).1 In an evaluationof A indica seed extracts, some of the isolated limonoids and diterpenoids exhibited a marked melanogenesis-inhibitory effect (74%–91% reduction of melanin content) with no toxicity to the cell.5 All of these studies indicate that active compounds in neem root, bark, flowers, and seeds may be potential skin-lightening agents.
Toxicity Against Pests—Neem seeds have phytochemicals that convey some insecticidal properties. The seeds often are ground into a powder, combined with water, and sprayed onto crops to act as an insecticide. As a natural method of nonpesticidal management, A indica acts as an antifeedant, insect repellent, and egg-laying deterrent that protects crops from damage. Studies of A indica have noted effective nonpesticidal management against arthropod pests such as armyworm, termites, and the oriental fruit fly.7,12,13
Antimalarial Activity—One study indicated that nimbolide, a limonoid from the neem plant, demonstrated antimalarial activity against Plasmodium falciparum. In separate cultures of asexual parasites and mature gametocytes, parasite numbers were less than 50% of the number in control cultures (8.0% vs 8.5% parasitemia, respectively).14 Thus, the lower parasite numbers indicated by this study highlight the antimalarial utility of nimbolide and neem oil.
Antioxidant and Anti-inflammatory Activity—Neem bark has been reported to have considerable antioxidant activity due to its high phenolic content.1,15 One study showed that azadirachtin and nimbolide in neem exhibited concentration-dependent antiradical scavenging activity and antioxidant properties.16
The anti-inflammatory potential for neem may occur via the inhibition of the nuclear factor-κB signaling pathway, which is linked to cancer, inflammation, and apoptosis.17 It also has been observed that nimbidin within neem extracts—such as leaves, bark, and seed extract—suppresses the function of macrophages and neutrophils relevant to inflammation.16 Another study indicated neem’s anti-inflammatory activity due to the regulation of proinflammatory enzymes such as cyclooxygenase and lipoxygenase.18
Safety, Toxicity, and Risks
Ingestion—Although neem is safe to use in the general population, neem oil poisoning has been reported, particularly in young children. Ingesting large quantities of neem has resulted in vomiting, hepatic toxicity, metabolic acidosis, late neurologic sequelae, and encephalopathy in young children.19 The diagnosis of neem oil poisoning is based on patient history, clinical examination, and imaging findings. Poisoning can manifest as drowsiness, tachypnea, and generalized seizures.20
Topical Application—Topical use of neem appears to be safe if the substance is diluted with other ingredients. However, direct application to the skin is not advised, as it may cause leukoderma and could induce allergic contact dermatitis and other allergic reactions.4
Final Thoughts
The use of neem extract for disease prevention and treatment has been prevalent around the world since ancient times. Neem has been documented to possess melanogenesis-inhibitory activity, toxicity against pests, antimalarial activity, and antioxidant activity by means of tyrosinase inhibition, phytochemical production, limonoid expression, and nuclear factor-κB regulation, respectively. However, topical use of neem may trigger a cutaneous response, highlighting the importance of considering a diagnosis of neem oil–induced chemical leukoderma when patients present with a hypopigmented rash and relevant history.
- Kitdamrongtham W, Ishii K, Ebina K, et al. Limonoids and flavonoids from the flowers of Azadirachta indica var. siamensis, and their melanogenesis-inhibitory and cytotoxic activities. Chem Biodivers. 2014;11:73-84. doi:10.1002/cbdv.201300266
- Singh A, Srivastava PS, Lakshmikumaran M. Comparison of AFLP and SAMPL markers for assessment of intra-population genetic variation in Azadirachta indica A. Juss. Plant Sci. 2002;162:17-25. doi:10.1016/S0168-9452(01)00503-9
- Pandey G, Verma K, Singh M. Evaluation of phytochemical, antibacterial and free radical scavenging properties of Azadirachta Indica (neem) leaves. Int J Pharm Pharmaceut Sci. 2014;6:444-447.
- Romita P, Calogiuri G, Bellino M, et al. Allergic contact dermatitis caused by neem oil: an underrated allergen. Contact Dermatitis. 2019;81:133-134. doi:10.1111/cod. 13256
- Akihisa T, Noto T, Takahashi A, et al. Melanogenesis inhibitory, anti-inflammatory, and chemopreventive effects of limonoids from the seeds of Azadirachta indica A. Juss. (neem). J Oleo Sci. 2009;58:581-594.
- Subapriya R, Nagini S. Medicinal properties of neem leaves: a review. Curr Med Chem Anticancer Agents. 2005;5:149-156. doi:10.2174/1568011053174828
- Areekul S, Sinchaisri P, Tigvatananon S. Effect of Thai plant extracts on the Oriental fruit fly. I: toxicity test. Agriculture and Natural Resources. 1987;21:395-407.
- Rochanakij S, Thebtaranonth Y, Yenjai C, et al. Nimbolide, a constituent of Azadirachta indica, inhibits Plasmodium falciparum in culture. Southeast Asian J Trop Med Public Health. 1985;16:66-72.
- Sithisarn P, Supabphol R, Gritsanapan W. Antioxidant activity of Siamese neem tree (VP1209). J Ethnopharmacol. 2005;99:109-112. doi:10.1016/j.jep.2005.02.008
- Yin F, Lei XX, Cheng L, et al. Isolation and structure identification of the compounds from the seeds and leaves of Azadirachta indica A. Juss. J China Pharmaceut University. 2005;36:10-12.
- Su S, Cheng J, Zhang C, et al. Melanogenesis-inhibitory activities of limonoids and tricyclic diterpenoids from Azadirachta indica. Bioorganic Chemistry. 2020;100:103941. doi:j.bioorg.2020.103941
- Tulashie SK, Adjei F, Abraham J, et al. Potential of neem extracts as natural insecticide against fall armyworm (Spodoptera frugiperda (JE Smith)(Lepidoptera: Noctuidae). Case Stud Chem Environ Eng. 2021;4:100130. doi:10.1016/j.cscee.2021.100130
- Yashroy RC, Gupta PK. Neem-seed oil inhibits growth of termite surface-tunnels. Indian J Toxicol. 2000;7:49-50.
- Udeinya JI, Shu EN, Quakyi I, et al. An antimalarial neem leaf extract has both schizonticidal and gametocytocidal activities. Am J Therapeutics. 2008;15:108-110. doi:10.1097/MJT.0b013e31804c6d1d
- Bindurani R, Kumar K. Evaluation of antioxidant activity of hydro distilled extracts of leaf, heart wood and flower of Azadirachta indica. Int J Pharm Sci Rev Res. 2013;20:222.
- Alzohairy MA. Therapeutics role of Azadirachta indica (Neem) and their active constituents in diseases prevention and treatment [published online March 1, 2016]. Evid Based Complement Alternat Med. doi:10.1155/2016/7382506
- Schumacher M, Cerella C, Reuter S, et al. Anti-inflammatory, pro-apoptotic, and anti-proliferative effects of a methanolic neem (Azadirachta indica) leaf extract are mediated via modulation of the nuclear factor-κB pathway. Genes Nutr. 2011;6:149-160. doi:10.1007/s12263-010-0194-6
- Kaur G, Sarwar Alam M, Athar M. Nimbidin suppresses functions of macrophages and neutrophils: relevance to its anti-inflammatory mechanisms. Phytotherapy Res. 2004;18:419-424. doi:10.1002/ptr.1474
- Dhongade RK, Kavade SG, Damle RS. Neem oil poisoning. Indian Pediatr. 2008;45:56-57.
- Bhaskar MV, Pramod SJ, Jeevika MU, et al. MR imaging findings of neem oil poisoning. Am J Neuroradiol. 2010;31:E60-E61. doi:10.3174/ajnr.A2146
Commonly known as neem or nimba, Azadirachta indica traditionally has been used as an oil or poultice to lighten skin pigment and reduce joint inflammation. Neem is a drought-resistant evergreen tree with thin serrated leaves, white fragrant flowers, and olivelike fruit (Figure 1). This plant is indigenous to India but also is readily found within tropical and semitropical environments throughout the Middle East, Southeast Asia, North Africa, and Australia.

Traditional Uses
For more than 4000 years, neem leaves, bark, fruit, and seeds have been used in food, insecticide, and herbal medicine cross-culturally in Indian Ayurvedic medicine and across Southeast Asia, particularly in Cambodia, Laos, Thailand, Myanmar, and Vietnam.1-3 Because of its many essential nutrients—oleic acid, palmitic acid, stearic acid, linoleic acid, behenic acid, arachidic acid, and palmitoleic acid—and readily available nature, some ethnic groups include neem in their diet.4 Neem commonly is used as a seasoning in soups and rice, eaten as a cooked vegetable, infused into teas and tonics, and pickled with other spices.5
All parts of the neem tree—both externally and internally—have been utilized in traditional medicine for the treatment of various diseases and ailments. The flowers have been used to treat eye diseases and dyspepsia, the fruit has been employed as an anthelmintic, the seeds and leaves have been used for malaria treatment and insecticide, the stem bark has been used for the treatment of diarrhea, and the root bark has been used for skin diseases and inflammation.6 Neem oil is a yellow-brown bitter substance that often is utilized to treat skin diseases such as psoriasis, eczema, fungal infections, and abscesses.
Case Report—A 77-year-old man presented with a diffuse rash across the lower back. He reported that he had been using topical neem oil to alleviate lower back pain and arthritis for the last 6 months with noted relief and improvement of back pain. After roughly 3 to 4 months of using neem oil, he noted a rash on the lower back, bilateral flanks, and buttocks (Figure 2). The rash was asymptomatic, and he denied any pruritus, scaling, pain, or burning. The patient was referred to dermatology and received a diagnosis of chemical leukoderma secondary to contact with A indica. The patient was advised to stop using the topical neem oil, and the rash was simply monitored, as it was asymptomatic.

Bioactivity
Research has elucidated multiple bioactivity mechanisms of neem, including melanogenesis-inhibitory activity, toxicity against pests, antimalarial activity, and antioxidant activity.1,7-9 Literature on the diverse phytochemical components of A indica indicate high levels of limonoids, flavonoids, and triterpenoids that are responsible for much of its antioxidant, anti-inflammatory, and insecticide properties.1,10
Melanogenesis-Inhibitory Activity—To date, neem has been added to a number of cosmetic products used in Ayurvedic medicine. One study of isolated compounds in A indica showed superior inhibitory activities against melanogenesis with minimal toxicity to cells (86.5%–105.1% cell viability). Western blot analysis of samples extracted and isolated from neem root and bark showed melanogenesis-inhibitory activities in B16 melanoma cells through the inhibition of microphthalmia-associated transcription factor expression and decreased expression of tyrosinase, as well as tyrosinase-related proteins 1 and 2, which are largely responsible for melanin synthesis.11 In another study, A indica flowers and their extracted constituents—6-deacetylnimbin and kaempferide—suggest melanogenesis-inhibitory activities in B16 melanoma cells with little to no toxicity to the cells (81.0%–111.7% cell viability).1 In an evaluationof A indica seed extracts, some of the isolated limonoids and diterpenoids exhibited a marked melanogenesis-inhibitory effect (74%–91% reduction of melanin content) with no toxicity to the cell.5 All of these studies indicate that active compounds in neem root, bark, flowers, and seeds may be potential skin-lightening agents.
Toxicity Against Pests—Neem seeds have phytochemicals that convey some insecticidal properties. The seeds often are ground into a powder, combined with water, and sprayed onto crops to act as an insecticide. As a natural method of nonpesticidal management, A indica acts as an antifeedant, insect repellent, and egg-laying deterrent that protects crops from damage. Studies of A indica have noted effective nonpesticidal management against arthropod pests such as armyworm, termites, and the oriental fruit fly.7,12,13
Antimalarial Activity—One study indicated that nimbolide, a limonoid from the neem plant, demonstrated antimalarial activity against Plasmodium falciparum. In separate cultures of asexual parasites and mature gametocytes, parasite numbers were less than 50% of the number in control cultures (8.0% vs 8.5% parasitemia, respectively).14 Thus, the lower parasite numbers indicated by this study highlight the antimalarial utility of nimbolide and neem oil.
Antioxidant and Anti-inflammatory Activity—Neem bark has been reported to have considerable antioxidant activity due to its high phenolic content.1,15 One study showed that azadirachtin and nimbolide in neem exhibited concentration-dependent antiradical scavenging activity and antioxidant properties.16
The anti-inflammatory potential for neem may occur via the inhibition of the nuclear factor-κB signaling pathway, which is linked to cancer, inflammation, and apoptosis.17 It also has been observed that nimbidin within neem extracts—such as leaves, bark, and seed extract—suppresses the function of macrophages and neutrophils relevant to inflammation.16 Another study indicated neem’s anti-inflammatory activity due to the regulation of proinflammatory enzymes such as cyclooxygenase and lipoxygenase.18
Safety, Toxicity, and Risks
Ingestion—Although neem is safe to use in the general population, neem oil poisoning has been reported, particularly in young children. Ingesting large quantities of neem has resulted in vomiting, hepatic toxicity, metabolic acidosis, late neurologic sequelae, and encephalopathy in young children.19 The diagnosis of neem oil poisoning is based on patient history, clinical examination, and imaging findings. Poisoning can manifest as drowsiness, tachypnea, and generalized seizures.20
Topical Application—Topical use of neem appears to be safe if the substance is diluted with other ingredients. However, direct application to the skin is not advised, as it may cause leukoderma and could induce allergic contact dermatitis and other allergic reactions.4
Final Thoughts
The use of neem extract for disease prevention and treatment has been prevalent around the world since ancient times. Neem has been documented to possess melanogenesis-inhibitory activity, toxicity against pests, antimalarial activity, and antioxidant activity by means of tyrosinase inhibition, phytochemical production, limonoid expression, and nuclear factor-κB regulation, respectively. However, topical use of neem may trigger a cutaneous response, highlighting the importance of considering a diagnosis of neem oil–induced chemical leukoderma when patients present with a hypopigmented rash and relevant history.
Commonly known as neem or nimba, Azadirachta indica traditionally has been used as an oil or poultice to lighten skin pigment and reduce joint inflammation. Neem is a drought-resistant evergreen tree with thin serrated leaves, white fragrant flowers, and olivelike fruit (Figure 1). This plant is indigenous to India but also is readily found within tropical and semitropical environments throughout the Middle East, Southeast Asia, North Africa, and Australia.

Traditional Uses
For more than 4000 years, neem leaves, bark, fruit, and seeds have been used in food, insecticide, and herbal medicine cross-culturally in Indian Ayurvedic medicine and across Southeast Asia, particularly in Cambodia, Laos, Thailand, Myanmar, and Vietnam.1-3 Because of its many essential nutrients—oleic acid, palmitic acid, stearic acid, linoleic acid, behenic acid, arachidic acid, and palmitoleic acid—and readily available nature, some ethnic groups include neem in their diet.4 Neem commonly is used as a seasoning in soups and rice, eaten as a cooked vegetable, infused into teas and tonics, and pickled with other spices.5
All parts of the neem tree—both externally and internally—have been utilized in traditional medicine for the treatment of various diseases and ailments. The flowers have been used to treat eye diseases and dyspepsia, the fruit has been employed as an anthelmintic, the seeds and leaves have been used for malaria treatment and insecticide, the stem bark has been used for the treatment of diarrhea, and the root bark has been used for skin diseases and inflammation.6 Neem oil is a yellow-brown bitter substance that often is utilized to treat skin diseases such as psoriasis, eczema, fungal infections, and abscesses.
Case Report—A 77-year-old man presented with a diffuse rash across the lower back. He reported that he had been using topical neem oil to alleviate lower back pain and arthritis for the last 6 months with noted relief and improvement of back pain. After roughly 3 to 4 months of using neem oil, he noted a rash on the lower back, bilateral flanks, and buttocks (Figure 2). The rash was asymptomatic, and he denied any pruritus, scaling, pain, or burning. The patient was referred to dermatology and received a diagnosis of chemical leukoderma secondary to contact with A indica. The patient was advised to stop using the topical neem oil, and the rash was simply monitored, as it was asymptomatic.

Bioactivity
Research has elucidated multiple bioactivity mechanisms of neem, including melanogenesis-inhibitory activity, toxicity against pests, antimalarial activity, and antioxidant activity.1,7-9 Literature on the diverse phytochemical components of A indica indicate high levels of limonoids, flavonoids, and triterpenoids that are responsible for much of its antioxidant, anti-inflammatory, and insecticide properties.1,10
Melanogenesis-Inhibitory Activity—To date, neem has been added to a number of cosmetic products used in Ayurvedic medicine. One study of isolated compounds in A indica showed superior inhibitory activities against melanogenesis with minimal toxicity to cells (86.5%–105.1% cell viability). Western blot analysis of samples extracted and isolated from neem root and bark showed melanogenesis-inhibitory activities in B16 melanoma cells through the inhibition of microphthalmia-associated transcription factor expression and decreased expression of tyrosinase, as well as tyrosinase-related proteins 1 and 2, which are largely responsible for melanin synthesis.11 In another study, A indica flowers and their extracted constituents—6-deacetylnimbin and kaempferide—suggest melanogenesis-inhibitory activities in B16 melanoma cells with little to no toxicity to the cells (81.0%–111.7% cell viability).1 In an evaluationof A indica seed extracts, some of the isolated limonoids and diterpenoids exhibited a marked melanogenesis-inhibitory effect (74%–91% reduction of melanin content) with no toxicity to the cell.5 All of these studies indicate that active compounds in neem root, bark, flowers, and seeds may be potential skin-lightening agents.
Toxicity Against Pests—Neem seeds have phytochemicals that convey some insecticidal properties. The seeds often are ground into a powder, combined with water, and sprayed onto crops to act as an insecticide. As a natural method of nonpesticidal management, A indica acts as an antifeedant, insect repellent, and egg-laying deterrent that protects crops from damage. Studies of A indica have noted effective nonpesticidal management against arthropod pests such as armyworm, termites, and the oriental fruit fly.7,12,13
Antimalarial Activity—One study indicated that nimbolide, a limonoid from the neem plant, demonstrated antimalarial activity against Plasmodium falciparum. In separate cultures of asexual parasites and mature gametocytes, parasite numbers were less than 50% of the number in control cultures (8.0% vs 8.5% parasitemia, respectively).14 Thus, the lower parasite numbers indicated by this study highlight the antimalarial utility of nimbolide and neem oil.
Antioxidant and Anti-inflammatory Activity—Neem bark has been reported to have considerable antioxidant activity due to its high phenolic content.1,15 One study showed that azadirachtin and nimbolide in neem exhibited concentration-dependent antiradical scavenging activity and antioxidant properties.16
The anti-inflammatory potential for neem may occur via the inhibition of the nuclear factor-κB signaling pathway, which is linked to cancer, inflammation, and apoptosis.17 It also has been observed that nimbidin within neem extracts—such as leaves, bark, and seed extract—suppresses the function of macrophages and neutrophils relevant to inflammation.16 Another study indicated neem’s anti-inflammatory activity due to the regulation of proinflammatory enzymes such as cyclooxygenase and lipoxygenase.18
Safety, Toxicity, and Risks
Ingestion—Although neem is safe to use in the general population, neem oil poisoning has been reported, particularly in young children. Ingesting large quantities of neem has resulted in vomiting, hepatic toxicity, metabolic acidosis, late neurologic sequelae, and encephalopathy in young children.19 The diagnosis of neem oil poisoning is based on patient history, clinical examination, and imaging findings. Poisoning can manifest as drowsiness, tachypnea, and generalized seizures.20
Topical Application—Topical use of neem appears to be safe if the substance is diluted with other ingredients. However, direct application to the skin is not advised, as it may cause leukoderma and could induce allergic contact dermatitis and other allergic reactions.4
Final Thoughts
The use of neem extract for disease prevention and treatment has been prevalent around the world since ancient times. Neem has been documented to possess melanogenesis-inhibitory activity, toxicity against pests, antimalarial activity, and antioxidant activity by means of tyrosinase inhibition, phytochemical production, limonoid expression, and nuclear factor-κB regulation, respectively. However, topical use of neem may trigger a cutaneous response, highlighting the importance of considering a diagnosis of neem oil–induced chemical leukoderma when patients present with a hypopigmented rash and relevant history.
- Kitdamrongtham W, Ishii K, Ebina K, et al. Limonoids and flavonoids from the flowers of Azadirachta indica var. siamensis, and their melanogenesis-inhibitory and cytotoxic activities. Chem Biodivers. 2014;11:73-84. doi:10.1002/cbdv.201300266
- Singh A, Srivastava PS, Lakshmikumaran M. Comparison of AFLP and SAMPL markers for assessment of intra-population genetic variation in Azadirachta indica A. Juss. Plant Sci. 2002;162:17-25. doi:10.1016/S0168-9452(01)00503-9
- Pandey G, Verma K, Singh M. Evaluation of phytochemical, antibacterial and free radical scavenging properties of Azadirachta Indica (neem) leaves. Int J Pharm Pharmaceut Sci. 2014;6:444-447.
- Romita P, Calogiuri G, Bellino M, et al. Allergic contact dermatitis caused by neem oil: an underrated allergen. Contact Dermatitis. 2019;81:133-134. doi:10.1111/cod. 13256
- Akihisa T, Noto T, Takahashi A, et al. Melanogenesis inhibitory, anti-inflammatory, and chemopreventive effects of limonoids from the seeds of Azadirachta indica A. Juss. (neem). J Oleo Sci. 2009;58:581-594.
- Subapriya R, Nagini S. Medicinal properties of neem leaves: a review. Curr Med Chem Anticancer Agents. 2005;5:149-156. doi:10.2174/1568011053174828
- Areekul S, Sinchaisri P, Tigvatananon S. Effect of Thai plant extracts on the Oriental fruit fly. I: toxicity test. Agriculture and Natural Resources. 1987;21:395-407.
- Rochanakij S, Thebtaranonth Y, Yenjai C, et al. Nimbolide, a constituent of Azadirachta indica, inhibits Plasmodium falciparum in culture. Southeast Asian J Trop Med Public Health. 1985;16:66-72.
- Sithisarn P, Supabphol R, Gritsanapan W. Antioxidant activity of Siamese neem tree (VP1209). J Ethnopharmacol. 2005;99:109-112. doi:10.1016/j.jep.2005.02.008
- Yin F, Lei XX, Cheng L, et al. Isolation and structure identification of the compounds from the seeds and leaves of Azadirachta indica A. Juss. J China Pharmaceut University. 2005;36:10-12.
- Su S, Cheng J, Zhang C, et al. Melanogenesis-inhibitory activities of limonoids and tricyclic diterpenoids from Azadirachta indica. Bioorganic Chemistry. 2020;100:103941. doi:j.bioorg.2020.103941
- Tulashie SK, Adjei F, Abraham J, et al. Potential of neem extracts as natural insecticide against fall armyworm (Spodoptera frugiperda (JE Smith)(Lepidoptera: Noctuidae). Case Stud Chem Environ Eng. 2021;4:100130. doi:10.1016/j.cscee.2021.100130
- Yashroy RC, Gupta PK. Neem-seed oil inhibits growth of termite surface-tunnels. Indian J Toxicol. 2000;7:49-50.
- Udeinya JI, Shu EN, Quakyi I, et al. An antimalarial neem leaf extract has both schizonticidal and gametocytocidal activities. Am J Therapeutics. 2008;15:108-110. doi:10.1097/MJT.0b013e31804c6d1d
- Bindurani R, Kumar K. Evaluation of antioxidant activity of hydro distilled extracts of leaf, heart wood and flower of Azadirachta indica. Int J Pharm Sci Rev Res. 2013;20:222.
- Alzohairy MA. Therapeutics role of Azadirachta indica (Neem) and their active constituents in diseases prevention and treatment [published online March 1, 2016]. Evid Based Complement Alternat Med. doi:10.1155/2016/7382506
- Schumacher M, Cerella C, Reuter S, et al. Anti-inflammatory, pro-apoptotic, and anti-proliferative effects of a methanolic neem (Azadirachta indica) leaf extract are mediated via modulation of the nuclear factor-κB pathway. Genes Nutr. 2011;6:149-160. doi:10.1007/s12263-010-0194-6
- Kaur G, Sarwar Alam M, Athar M. Nimbidin suppresses functions of macrophages and neutrophils: relevance to its anti-inflammatory mechanisms. Phytotherapy Res. 2004;18:419-424. doi:10.1002/ptr.1474
- Dhongade RK, Kavade SG, Damle RS. Neem oil poisoning. Indian Pediatr. 2008;45:56-57.
- Bhaskar MV, Pramod SJ, Jeevika MU, et al. MR imaging findings of neem oil poisoning. Am J Neuroradiol. 2010;31:E60-E61. doi:10.3174/ajnr.A2146
- Kitdamrongtham W, Ishii K, Ebina K, et al. Limonoids and flavonoids from the flowers of Azadirachta indica var. siamensis, and their melanogenesis-inhibitory and cytotoxic activities. Chem Biodivers. 2014;11:73-84. doi:10.1002/cbdv.201300266
- Singh A, Srivastava PS, Lakshmikumaran M. Comparison of AFLP and SAMPL markers for assessment of intra-population genetic variation in Azadirachta indica A. Juss. Plant Sci. 2002;162:17-25. doi:10.1016/S0168-9452(01)00503-9
- Pandey G, Verma K, Singh M. Evaluation of phytochemical, antibacterial and free radical scavenging properties of Azadirachta Indica (neem) leaves. Int J Pharm Pharmaceut Sci. 2014;6:444-447.
- Romita P, Calogiuri G, Bellino M, et al. Allergic contact dermatitis caused by neem oil: an underrated allergen. Contact Dermatitis. 2019;81:133-134. doi:10.1111/cod. 13256
- Akihisa T, Noto T, Takahashi A, et al. Melanogenesis inhibitory, anti-inflammatory, and chemopreventive effects of limonoids from the seeds of Azadirachta indica A. Juss. (neem). J Oleo Sci. 2009;58:581-594.
- Subapriya R, Nagini S. Medicinal properties of neem leaves: a review. Curr Med Chem Anticancer Agents. 2005;5:149-156. doi:10.2174/1568011053174828
- Areekul S, Sinchaisri P, Tigvatananon S. Effect of Thai plant extracts on the Oriental fruit fly. I: toxicity test. Agriculture and Natural Resources. 1987;21:395-407.
- Rochanakij S, Thebtaranonth Y, Yenjai C, et al. Nimbolide, a constituent of Azadirachta indica, inhibits Plasmodium falciparum in culture. Southeast Asian J Trop Med Public Health. 1985;16:66-72.
- Sithisarn P, Supabphol R, Gritsanapan W. Antioxidant activity of Siamese neem tree (VP1209). J Ethnopharmacol. 2005;99:109-112. doi:10.1016/j.jep.2005.02.008
- Yin F, Lei XX, Cheng L, et al. Isolation and structure identification of the compounds from the seeds and leaves of Azadirachta indica A. Juss. J China Pharmaceut University. 2005;36:10-12.
- Su S, Cheng J, Zhang C, et al. Melanogenesis-inhibitory activities of limonoids and tricyclic diterpenoids from Azadirachta indica. Bioorganic Chemistry. 2020;100:103941. doi:j.bioorg.2020.103941
- Tulashie SK, Adjei F, Abraham J, et al. Potential of neem extracts as natural insecticide against fall armyworm (Spodoptera frugiperda (JE Smith)(Lepidoptera: Noctuidae). Case Stud Chem Environ Eng. 2021;4:100130. doi:10.1016/j.cscee.2021.100130
- Yashroy RC, Gupta PK. Neem-seed oil inhibits growth of termite surface-tunnels. Indian J Toxicol. 2000;7:49-50.
- Udeinya JI, Shu EN, Quakyi I, et al. An antimalarial neem leaf extract has both schizonticidal and gametocytocidal activities. Am J Therapeutics. 2008;15:108-110. doi:10.1097/MJT.0b013e31804c6d1d
- Bindurani R, Kumar K. Evaluation of antioxidant activity of hydro distilled extracts of leaf, heart wood and flower of Azadirachta indica. Int J Pharm Sci Rev Res. 2013;20:222.
- Alzohairy MA. Therapeutics role of Azadirachta indica (Neem) and their active constituents in diseases prevention and treatment [published online March 1, 2016]. Evid Based Complement Alternat Med. doi:10.1155/2016/7382506
- Schumacher M, Cerella C, Reuter S, et al. Anti-inflammatory, pro-apoptotic, and anti-proliferative effects of a methanolic neem (Azadirachta indica) leaf extract are mediated via modulation of the nuclear factor-κB pathway. Genes Nutr. 2011;6:149-160. doi:10.1007/s12263-010-0194-6
- Kaur G, Sarwar Alam M, Athar M. Nimbidin suppresses functions of macrophages and neutrophils: relevance to its anti-inflammatory mechanisms. Phytotherapy Res. 2004;18:419-424. doi:10.1002/ptr.1474
- Dhongade RK, Kavade SG, Damle RS. Neem oil poisoning. Indian Pediatr. 2008;45:56-57.
- Bhaskar MV, Pramod SJ, Jeevika MU, et al. MR imaging findings of neem oil poisoning. Am J Neuroradiol. 2010;31:E60-E61. doi:10.3174/ajnr.A2146
Practice Points
- Neem is a traditional herb with various bioactivities, such as melanogenesis-inhibitory activity, toxicity against pests, antimalarial activity, and antioxidant activity.
- Neem should be used with caution as a remedy because of its skin-lightening properties, which are attributed to melanogenesis-inhibitory activity via tyrosinase inhibition.
- Chemical leukoderma should be included in the differential diagnosis when a patient presents with a hypopigmented rash after topical use of neem products.
Analysis of Online Diet Recommendations for Vitiligo
Internet platforms have become a common source of medical information for individuals with a broad range of skin conditions including vitiligo. The prevalence of vitiligo among US adults ranges from 0.76% to 1.11%, with approximately 40% of adult cases of vitiligo in the United States remaining undiagnosed.1 The vitiligo community has become more inquisitive of the relationship between diet and vitiligo, turning to online sources for suggestions on diet modifications that may be beneficial for their condition. Although there is an abundance of online information, few diets or foods have been medically recognized to definitively improve or worsen vitiligo symptoms. We reviewed the top online web pages accessible to the public regarding diet suggestions that affect vitiligo symptoms. We then compared these online results to published peer-reviewed scientific literature.
Methods
Two independent online searches were performed by Researcher 1 (Y.A.) and Researcher 2 (I.M.) using Google Advanced Search. The independent searches were performed by the reviewers in neighboring areas of Chicago, Illinois, using the same Internet browser (Google Chrome). The primary search terms were diet and vitiligo along with the optional additional terms dietary supplement(s), food(s), nutrition, herb(s), or vitamin(s). Our search included any web pages published or updated from January 1, 2010, to December 31, 2021, and originally scribed in the English language. The domains “.com,” “.org,” “.edu,” and “.cc” were included.
From this initial search, Researcher 1 identified 312 web pages and Researcher 2 identified 314 web pages. Each reviewer sorted their respective search results to identify the number of eligible records to be screened. Records were defined as unique web pages that met the search criteria. After removing duplicates, Researcher 1 screened 102 web pages and Researcher 2 screened 76 web pages. Of these records, web pages were excluded if they did not include any diet recommendations for vitiligo patients. Each reviewer independently created a list of eligible records, and the independent lists were then merged for a total of 58 web pages. Among these 58 web pages, there were 24 duplicate records and 3 records that were deemed ineligible for the study due to lack of subject matter relevance. A final total of 31 web pages were included in the data analysis (Figure). Of the 31 records selected, the reviewers jointly evaluated each web page and recorded the diet components that were recommended for individuals with vitiligo to either include or avoid (eTable).
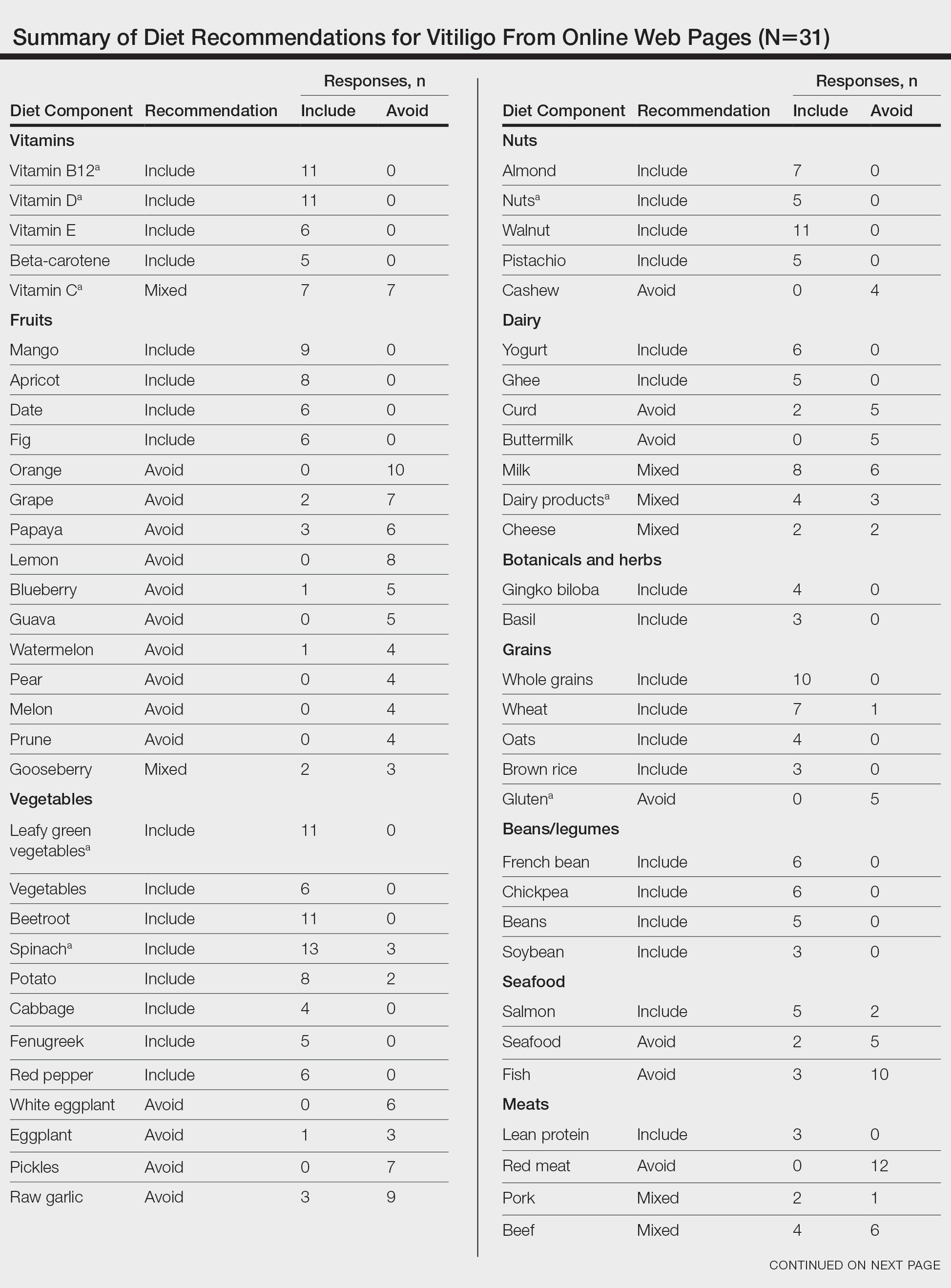
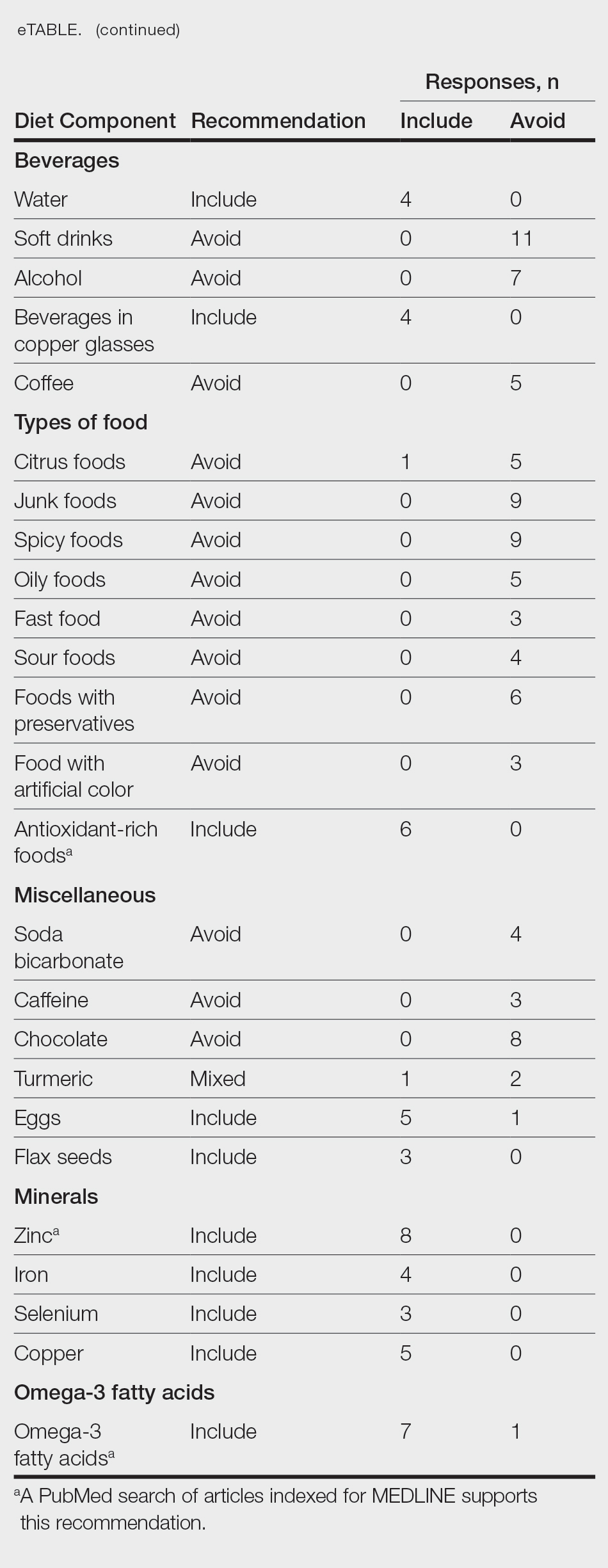
For comparison and support from published scientific literature, a search of PubMed articles indexed for MEDLINE was conducted using the terms diet and vitiligo. Relevant human clinical studies published in the English-language literature were reviewed for content regarding the relationship between diet and vitiligo.
Results
Our online search revealed an abundance of information regarding various dietary modifications suggested to aid in the management of vitiligo symptoms. Most web pages (27/31 [87%]) were not authored by medical professionals or dermatologists. There were 27 diet components mentioned 8 or more times within the 31 total web pages. These diet components were selected for further review via PubMed. Each item was searched on PubMed using the term “[respective diet component] and vitiligo” among all published literature in the English language. Our study focused on summarizing the data on dietary components for which we were able to gather scientific support. These data have been organized into the following categories: vitamins, fruits, omega-3 fatty acids, grains, minerals, vegetables, and nuts.
Vitamins—The online literature recommended inclusion of vitamin supplements, in particular vitamins D and B12, which aligned with published scientific literature.2,3 Eleven of 31 (35%) web pages recommended vitamin D in vitiligo. A 2010 study analyzing patients with vitiligo vulgaris (N=45) found that 68.9% of the cohort had insufficient (<30 ng/mL) 25-hydroxyvitamin D levels.2 A prospective study of 30 individuals found that the use of tacrolimus ointment plus oral vitamin D supplementation was found to be more successful in repigmentation than topical tacrolimus alone.3 Vitamin D dosage ranged from 1500 IU/d if the patient’s serum 25-hydroxyvitamin D levels were less than 20 ng/mL to 3000 IU/d if the serum levels were less than 10 ng/mL for 6 months.
Dairy products are a source of vitamin D.2,3 Of the web pages that mentioned dairy, a subtle majority (4/7 [57%]) recommended the inclusion of dairy products. Although many web pages did not specify whether oral vitamin D supplementation vs dietary food consumption is preferred, a 2013 controlled study of 16 vitiligo patients who received high doses of vitamin D supplementation with a low-calcium diet found that 4 patients showed 1% to 25% repigmentation, 5 patients showed 26% to 50% repigmentation, and 5 patients showed 51% to 75% repigmentation of the affected areas.4
Eleven of 31 (35%) web pages recommended inclusion of vitamin B12 supplementation in vitiligo. A 2-year study with 100 participants showed that supplementation with folic acid and vitamin B12 along with sun exposure yielded more effective repigmentation than either vitamins or sun exposure alone.5 An additional hypothesis suggested vitamin B12 may aid in repigmentation through its role in the homocysteine pathway. Although the theory is unproven, it is proposed that inhibition of homocysteine via vitamin B12 or folic acid supplementation may play a role in reducing melanocyte destruction and restoring melanin synthesis.6
There were mixed recommendations regarding vitamin C via supplementation and/or eating citrus fruits such as oranges. Although there are limited clinical studies on the use of vitamin C and the treatment of vitiligo, a 6-year prospective study from Madagascar consisting of approximately 300 participants with vitiligo who were treated with a combination of topical corticosteroids, oral vitamin C, and oral vitamin B12 supplementation showed excellent repigmentation (defined by repigmentation of more than 76% of the originally affected area) in 50 participants.7
Fruits—Most web pages had mixed recommendations on whether to include or avoid certain fruits. Interestingly, inclusion of mangoes and apricots in the diet were highly recommended (9/31 [29%] and 8/31 [26%], respectively) while fruits such as oranges, lemons, papayas, and grapes were discouraged (10/31 [32%], 8/31 [26%], 6/31 [19%], and 7/31 [23%], respectively). Although some web pages suggested that vitamin C–rich produce including citrus and berries may help to increase melanin formation, others strongly suggested avoiding these fruits. There is limited information on the effects of citrus on vitiligo, but a 2022 study indicated that 5-demethylnobiletin, a flavonoid found in sweet citrus fruits, may stimulate melanin synthesis, which can possibly be beneficial for vitiligo.8
Omega-3 Fatty Acids—Seven of 31 (23%) web pages recommended the inclusion of omega-3 fatty acids for their role as antioxidants to improve vitiligo symptoms. Research has indicated a strong association between vitiligo and oxidative stress.9 A 2007 controlled clinical trial that included 28 vitiligo patients demonstrated that oral antioxidant supplementation in combination with narrowband UVB phototherapy can significantly decrease vitiligo-associated oxidative stress (P<.05); 8 of 17 (47%) patients in the treatment group saw greater than 75% repigmentation after antioxidant treatment.10
Grains—Five of 31 (16%) web pages suggested avoiding gluten—a protein naturally found in some grains including wheat, barley, and rye—to improve vitiligo symptoms. A 2021 review suggested that a gluten-free diet may be effective in managing celiac disease, and it is hypothesized that vitiligo may be managed with similar dietary adjustments.11 Studies have shown that celiac disease and vitiligo—both autoimmune conditions—involve IL-2, IL-6, IL-7, and IL-21 in their disease pathways.12,13 Their shared immunogenic mechanism may account for similar management options.
Upon review, 2 case reports were identified that discussed a relationship between a gluten-free diet and vitiligo symptom improvement. In one report, a 9-year-old child diagnosed with both celiac disease and vitiligo saw intense repigmentation of the skin after adhering to a gluten-free diet for 1 year.14 Another case study reported a 22-year-old woman with vitiligo whose symptoms improved after 1 month of a gluten-free diet following 2 years of failed treatment with a topical steroid and phototherapy.15
Seven of 31 (23%) web pages suggested that individuals with vitiligo should include wheat in their diet. There is no published literature discussing the relationship between vitiligo and wheat. Of the 31 web pages reviewed, 10 (32%) suggested including whole grain. There is no relevant scientific evidence or hypotheses describing how whole grains may be beneficial in vitiligo.
Minerals—Eight of 31 (26%) web pages suggested including zinc in the diet to improve vitiligo symptoms. A 2020 study evaluated how different serum levels of zinc in vitiligo patients might be affiliated with interleukin activity. Fifty patients diagnosed with active vitiligo were tested for serum levels of zinc, IL-4, IL-6, and IL-17.16 The results showed that mean serum levels of zinc were lower in vitiligo patients compared with patients without vitiligo. The study concluded that zinc could possibly be used as a supplement to improve vitiligo, though the dosage needs to be further studied and confirmed.16
Vegetables—Eleven of 31 (35%) web pages recommended leafy green vegetables and 13 of 31 (42%) recommended spinach for patients with vitiligo. Spinach and other leafy green vegetables are known to be rich in antioxidants, which may have protective effects against reactive oxygen species that are thought to contribute to vitiligo progression.17,18
Nuts—Walnuts were recommended in 11 of 31 (35%) web pages. Nuts may be beneficial in reducing inflammation and providing protection against oxidative stress.9 However, there is no specific scientific literature that supports the inclusion of nuts in the diet to manage vitiligo symptoms.
Comment
With a growing amount of research suggesting that diet modifications may contribute to management of certain skin conditions, vitiligo patients often inquire about foods or supplements that may help improve their condition.19 Our review highlighted what information was available to the public regarding diet and vitiligo, with preliminary support of the following primary diet components: vitamin D, vitamin B12, zinc, and omega-3 fatty acids. Our review showed no support in the literature for the items that were recommended to avoid. It is important to note that 27 of 31 (87%) web pages from our online search were not authored by medical professionals or dermatologists. Additionally, many web pages suggested conflicting information, making it difficult to draw concrete conclusions about what diet modifications will be beneficial to the vitiligo community. Further controlled clinical trials are warranted due to the lack of formal studies that assess the relationship between diet and vitiligo.
- Gandhi K, Ezzedine K, Anastassopoulos KP, et al. Prevalence of vitiligo among adults in the United States. JAMA Dermatol. 2022;158:43-50. doi:10.1001/jamadermatol.2021.4724
- Silverberg JI, Silverberg AI, Malka E, et al. A pilot study assessing the role of 25 hydroxy vitamin D levels in patients with vitiligo vulgaris. J Am Acad Dermatol. 2010;62:937-941. doi:10.1016/j.jaad.2009.11.024
- Karagüzel G, Sakarya NP, Bahadır S, et al. Vitamin D status and the effects of oral vitamin D treatment in children with vitiligo: a prospective study. Clin Nutr ESPEN. 2016;15:28-31. doi:10.1016/j.clnesp.2016.05.006.
- Finamor DC, Sinigaglia-Coimbra R, Neves LC, et al. A pilot study assessing the effect of prolonged administration of high daily doses of vitamin D on the clinical course of vitiligo and psoriasis. Dermatoendocrinol. 2013;5:222-234. doi:10.4161/derm.24808
- Juhlin L, Olsson MJ. Improvement of vitiligo after oral treatment with vitamin B12 and folic acid and the importance of sun exposure. Acta Derm Venereol. 1997;77:460-462. doi:10.2340/000155555577460462
- Chen J, Zhuang T, Chen J, et al. Homocysteine induces melanocytes apoptosis via PERK-eIF2α-CHOP pathway in vitiligo. Clin Sci (Lond). 2020;134:1127-1141. doi:10.1042/CS20200218
- Sendrasoa FA, Ranaivo IM, Sata M, et al. Treatment responses in patients with vitiligo to very potent topical corticosteroids combined with vitamin therapy in Madagascar. Int J Dermatol. 2019;58:908-911. doi:10.1111/ijd.14510
- Wang HM, Qu LQ, Ng JPL, et al. Natural citrus flavanone 5-demethylnobiletin stimulates melanogenesis through the activation of cAMP/CREB pathway in B16F10 cells. Phytomedicine. 2022;98:153941. doi:10.1016/j.phymed.2022.153941
- Ros E. Health benefits of nut consumption. Nutrients. 2010;2:652-682.
- Dell’Anna ML, Mastrofrancesco A, Sala R, et al. Antioxidants and narrow band-UVB in the treatment of vitiligo: a double-blind placebo controlled trial. Clin Exp Dermatol. 2007;32:631-636.
- Gastrointestinal microbiome and gluten in celiac disease. Ann Med. 2021;53:1797-1805. doi:10.1080/07853890.2021.1990392
- Forabosco P, Neuhausen SL, Greco L, et al. Meta-analysis of genome-wide linkage studies in celiac disease. Hum Hered. 2009;68:223-230. doi:10.1159/000228920
- Akbulut UE, Çebi AH, Sag˘ E, et al. Interleukin-6 and interleukin-17 gene polymorphism association with celiac disease in children. Turk J Gastroenterol. 2017;28:471-475. doi:10.5152/tjg.2017.17092
- Rodríguez-García C, González-Hernández S, Pérez-Robayna N, et al. Repigmentation of vitiligo lesions in a child with celiac disease after a gluten-free diet. Pediatr Dermatol. 2011;28:209-210. doi:10.1111/j.1525-1470.2011.01388.x
- Khandalavala BN, Nirmalraj MC. Rapid partial repigmentation ofvitiligo in a young female adult with a gluten-free diet. Case Rep Dermatol. 2014;6:283-287.
- Sanad EM, El-Fallah AA, Al-Doori AR, et al. Serum zinc and inflammatory cytokines in vitiligo. J Clin Aesthet Dermatol. 2020;13:(12 suppl 1):S29-S33.
- Ames BN, Shigenaga MK, Hagen TM. Oxidants, antioxidants, and the degenerative diseases of aging. Proc Natl Acad Sci U S A. 1993;90:7915-7922. doi:10.1073/pnas.90.17.7915
- Xian D, Guo M, Xu J, et al. Current evidence to support the therapeutic potential of flavonoids in oxidative stress-related dermatoses. Redox Rep. 2021;26:134-146. doi:10.1080 /13510002.2021.1962094
- Katta R, Kramer MJ. Skin and diet: an update on the role of dietary change as a treatment strategy for skin disease. Skin Therapy Lett. 2018;23:1-5.
Internet platforms have become a common source of medical information for individuals with a broad range of skin conditions including vitiligo. The prevalence of vitiligo among US adults ranges from 0.76% to 1.11%, with approximately 40% of adult cases of vitiligo in the United States remaining undiagnosed.1 The vitiligo community has become more inquisitive of the relationship between diet and vitiligo, turning to online sources for suggestions on diet modifications that may be beneficial for their condition. Although there is an abundance of online information, few diets or foods have been medically recognized to definitively improve or worsen vitiligo symptoms. We reviewed the top online web pages accessible to the public regarding diet suggestions that affect vitiligo symptoms. We then compared these online results to published peer-reviewed scientific literature.
Methods
Two independent online searches were performed by Researcher 1 (Y.A.) and Researcher 2 (I.M.) using Google Advanced Search. The independent searches were performed by the reviewers in neighboring areas of Chicago, Illinois, using the same Internet browser (Google Chrome). The primary search terms were diet and vitiligo along with the optional additional terms dietary supplement(s), food(s), nutrition, herb(s), or vitamin(s). Our search included any web pages published or updated from January 1, 2010, to December 31, 2021, and originally scribed in the English language. The domains “.com,” “.org,” “.edu,” and “.cc” were included.

From this initial search, Researcher 1 identified 312 web pages and Researcher 2 identified 314 web pages. Each reviewer sorted their respective search results to identify the number of eligible records to be screened. Records were defined as unique web pages that met the search criteria. After removing duplicates, Researcher 1 screened 102 web pages and Researcher 2 screened 76 web pages. Of these records, web pages were excluded if they did not include any diet recommendations for vitiligo patients. Each reviewer independently created a list of eligible records, and the independent lists were then merged for a total of 58 web pages. Among these 58 web pages, there were 24 duplicate records and 3 records that were deemed ineligible for the study due to lack of subject matter relevance. A final total of 31 web pages were included in the data analysis (Figure). Of the 31 records selected, the reviewers jointly evaluated each web page and recorded the diet components that were recommended for individuals with vitiligo to either include or avoid (eTable).


For comparison and support from published scientific literature, a search of PubMed articles indexed for MEDLINE was conducted using the terms diet and vitiligo. Relevant human clinical studies published in the English-language literature were reviewed for content regarding the relationship between diet and vitiligo.
Results
Our online search revealed an abundance of information regarding various dietary modifications suggested to aid in the management of vitiligo symptoms. Most web pages (27/31 [87%]) were not authored by medical professionals or dermatologists. There were 27 diet components mentioned 8 or more times within the 31 total web pages. These diet components were selected for further review via PubMed. Each item was searched on PubMed using the term “[respective diet component] and vitiligo” among all published literature in the English language. Our study focused on summarizing the data on dietary components for which we were able to gather scientific support. These data have been organized into the following categories: vitamins, fruits, omega-3 fatty acids, grains, minerals, vegetables, and nuts.
Vitamins—The online literature recommended inclusion of vitamin supplements, in particular vitamins D and B12, which aligned with published scientific literature.2,3 Eleven of 31 (35%) web pages recommended vitamin D in vitiligo. A 2010 study analyzing patients with vitiligo vulgaris (N=45) found that 68.9% of the cohort had insufficient (<30 ng/mL) 25-hydroxyvitamin D levels.2 A prospective study of 30 individuals found that the use of tacrolimus ointment plus oral vitamin D supplementation was found to be more successful in repigmentation than topical tacrolimus alone.3 Vitamin D dosage ranged from 1500 IU/d if the patient’s serum 25-hydroxyvitamin D levels were less than 20 ng/mL to 3000 IU/d if the serum levels were less than 10 ng/mL for 6 months.
Dairy products are a source of vitamin D.2,3 Of the web pages that mentioned dairy, a subtle majority (4/7 [57%]) recommended the inclusion of dairy products. Although many web pages did not specify whether oral vitamin D supplementation vs dietary food consumption is preferred, a 2013 controlled study of 16 vitiligo patients who received high doses of vitamin D supplementation with a low-calcium diet found that 4 patients showed 1% to 25% repigmentation, 5 patients showed 26% to 50% repigmentation, and 5 patients showed 51% to 75% repigmentation of the affected areas.4
Eleven of 31 (35%) web pages recommended inclusion of vitamin B12 supplementation in vitiligo. A 2-year study with 100 participants showed that supplementation with folic acid and vitamin B12 along with sun exposure yielded more effective repigmentation than either vitamins or sun exposure alone.5 An additional hypothesis suggested vitamin B12 may aid in repigmentation through its role in the homocysteine pathway. Although the theory is unproven, it is proposed that inhibition of homocysteine via vitamin B12 or folic acid supplementation may play a role in reducing melanocyte destruction and restoring melanin synthesis.6
There were mixed recommendations regarding vitamin C via supplementation and/or eating citrus fruits such as oranges. Although there are limited clinical studies on the use of vitamin C and the treatment of vitiligo, a 6-year prospective study from Madagascar consisting of approximately 300 participants with vitiligo who were treated with a combination of topical corticosteroids, oral vitamin C, and oral vitamin B12 supplementation showed excellent repigmentation (defined by repigmentation of more than 76% of the originally affected area) in 50 participants.7
Fruits—Most web pages had mixed recommendations on whether to include or avoid certain fruits. Interestingly, inclusion of mangoes and apricots in the diet were highly recommended (9/31 [29%] and 8/31 [26%], respectively) while fruits such as oranges, lemons, papayas, and grapes were discouraged (10/31 [32%], 8/31 [26%], 6/31 [19%], and 7/31 [23%], respectively). Although some web pages suggested that vitamin C–rich produce including citrus and berries may help to increase melanin formation, others strongly suggested avoiding these fruits. There is limited information on the effects of citrus on vitiligo, but a 2022 study indicated that 5-demethylnobiletin, a flavonoid found in sweet citrus fruits, may stimulate melanin synthesis, which can possibly be beneficial for vitiligo.8
Omega-3 Fatty Acids—Seven of 31 (23%) web pages recommended the inclusion of omega-3 fatty acids for their role as antioxidants to improve vitiligo symptoms. Research has indicated a strong association between vitiligo and oxidative stress.9 A 2007 controlled clinical trial that included 28 vitiligo patients demonstrated that oral antioxidant supplementation in combination with narrowband UVB phototherapy can significantly decrease vitiligo-associated oxidative stress (P<.05); 8 of 17 (47%) patients in the treatment group saw greater than 75% repigmentation after antioxidant treatment.10
Grains—Five of 31 (16%) web pages suggested avoiding gluten—a protein naturally found in some grains including wheat, barley, and rye—to improve vitiligo symptoms. A 2021 review suggested that a gluten-free diet may be effective in managing celiac disease, and it is hypothesized that vitiligo may be managed with similar dietary adjustments.11 Studies have shown that celiac disease and vitiligo—both autoimmune conditions—involve IL-2, IL-6, IL-7, and IL-21 in their disease pathways.12,13 Their shared immunogenic mechanism may account for similar management options.
Upon review, 2 case reports were identified that discussed a relationship between a gluten-free diet and vitiligo symptom improvement. In one report, a 9-year-old child diagnosed with both celiac disease and vitiligo saw intense repigmentation of the skin after adhering to a gluten-free diet for 1 year.14 Another case study reported a 22-year-old woman with vitiligo whose symptoms improved after 1 month of a gluten-free diet following 2 years of failed treatment with a topical steroid and phototherapy.15
Seven of 31 (23%) web pages suggested that individuals with vitiligo should include wheat in their diet. There is no published literature discussing the relationship between vitiligo and wheat. Of the 31 web pages reviewed, 10 (32%) suggested including whole grain. There is no relevant scientific evidence or hypotheses describing how whole grains may be beneficial in vitiligo.
Minerals—Eight of 31 (26%) web pages suggested including zinc in the diet to improve vitiligo symptoms. A 2020 study evaluated how different serum levels of zinc in vitiligo patients might be affiliated with interleukin activity. Fifty patients diagnosed with active vitiligo were tested for serum levels of zinc, IL-4, IL-6, and IL-17.16 The results showed that mean serum levels of zinc were lower in vitiligo patients compared with patients without vitiligo. The study concluded that zinc could possibly be used as a supplement to improve vitiligo, though the dosage needs to be further studied and confirmed.16
Vegetables—Eleven of 31 (35%) web pages recommended leafy green vegetables and 13 of 31 (42%) recommended spinach for patients with vitiligo. Spinach and other leafy green vegetables are known to be rich in antioxidants, which may have protective effects against reactive oxygen species that are thought to contribute to vitiligo progression.17,18
Nuts—Walnuts were recommended in 11 of 31 (35%) web pages. Nuts may be beneficial in reducing inflammation and providing protection against oxidative stress.9 However, there is no specific scientific literature that supports the inclusion of nuts in the diet to manage vitiligo symptoms.
Comment
With a growing amount of research suggesting that diet modifications may contribute to management of certain skin conditions, vitiligo patients often inquire about foods or supplements that may help improve their condition.19 Our review highlighted what information was available to the public regarding diet and vitiligo, with preliminary support of the following primary diet components: vitamin D, vitamin B12, zinc, and omega-3 fatty acids. Our review showed no support in the literature for the items that were recommended to avoid. It is important to note that 27 of 31 (87%) web pages from our online search were not authored by medical professionals or dermatologists. Additionally, many web pages suggested conflicting information, making it difficult to draw concrete conclusions about what diet modifications will be beneficial to the vitiligo community. Further controlled clinical trials are warranted due to the lack of formal studies that assess the relationship between diet and vitiligo.
Internet platforms have become a common source of medical information for individuals with a broad range of skin conditions including vitiligo. The prevalence of vitiligo among US adults ranges from 0.76% to 1.11%, with approximately 40% of adult cases of vitiligo in the United States remaining undiagnosed.1 The vitiligo community has become more inquisitive of the relationship between diet and vitiligo, turning to online sources for suggestions on diet modifications that may be beneficial for their condition. Although there is an abundance of online information, few diets or foods have been medically recognized to definitively improve or worsen vitiligo symptoms. We reviewed the top online web pages accessible to the public regarding diet suggestions that affect vitiligo symptoms. We then compared these online results to published peer-reviewed scientific literature.
Methods
Two independent online searches were performed by Researcher 1 (Y.A.) and Researcher 2 (I.M.) using Google Advanced Search. The independent searches were performed by the reviewers in neighboring areas of Chicago, Illinois, using the same Internet browser (Google Chrome). The primary search terms were diet and vitiligo along with the optional additional terms dietary supplement(s), food(s), nutrition, herb(s), or vitamin(s). Our search included any web pages published or updated from January 1, 2010, to December 31, 2021, and originally scribed in the English language. The domains “.com,” “.org,” “.edu,” and “.cc” were included.

From this initial search, Researcher 1 identified 312 web pages and Researcher 2 identified 314 web pages. Each reviewer sorted their respective search results to identify the number of eligible records to be screened. Records were defined as unique web pages that met the search criteria. After removing duplicates, Researcher 1 screened 102 web pages and Researcher 2 screened 76 web pages. Of these records, web pages were excluded if they did not include any diet recommendations for vitiligo patients. Each reviewer independently created a list of eligible records, and the independent lists were then merged for a total of 58 web pages. Among these 58 web pages, there were 24 duplicate records and 3 records that were deemed ineligible for the study due to lack of subject matter relevance. A final total of 31 web pages were included in the data analysis (Figure). Of the 31 records selected, the reviewers jointly evaluated each web page and recorded the diet components that were recommended for individuals with vitiligo to either include or avoid (eTable).


For comparison and support from published scientific literature, a search of PubMed articles indexed for MEDLINE was conducted using the terms diet and vitiligo. Relevant human clinical studies published in the English-language literature were reviewed for content regarding the relationship between diet and vitiligo.
Results
Our online search revealed an abundance of information regarding various dietary modifications suggested to aid in the management of vitiligo symptoms. Most web pages (27/31 [87%]) were not authored by medical professionals or dermatologists. There were 27 diet components mentioned 8 or more times within the 31 total web pages. These diet components were selected for further review via PubMed. Each item was searched on PubMed using the term “[respective diet component] and vitiligo” among all published literature in the English language. Our study focused on summarizing the data on dietary components for which we were able to gather scientific support. These data have been organized into the following categories: vitamins, fruits, omega-3 fatty acids, grains, minerals, vegetables, and nuts.
Vitamins—The online literature recommended inclusion of vitamin supplements, in particular vitamins D and B12, which aligned with published scientific literature.2,3 Eleven of 31 (35%) web pages recommended vitamin D in vitiligo. A 2010 study analyzing patients with vitiligo vulgaris (N=45) found that 68.9% of the cohort had insufficient (<30 ng/mL) 25-hydroxyvitamin D levels.2 A prospective study of 30 individuals found that the use of tacrolimus ointment plus oral vitamin D supplementation was found to be more successful in repigmentation than topical tacrolimus alone.3 Vitamin D dosage ranged from 1500 IU/d if the patient’s serum 25-hydroxyvitamin D levels were less than 20 ng/mL to 3000 IU/d if the serum levels were less than 10 ng/mL for 6 months.
Dairy products are a source of vitamin D.2,3 Of the web pages that mentioned dairy, a subtle majority (4/7 [57%]) recommended the inclusion of dairy products. Although many web pages did not specify whether oral vitamin D supplementation vs dietary food consumption is preferred, a 2013 controlled study of 16 vitiligo patients who received high doses of vitamin D supplementation with a low-calcium diet found that 4 patients showed 1% to 25% repigmentation, 5 patients showed 26% to 50% repigmentation, and 5 patients showed 51% to 75% repigmentation of the affected areas.4
Eleven of 31 (35%) web pages recommended inclusion of vitamin B12 supplementation in vitiligo. A 2-year study with 100 participants showed that supplementation with folic acid and vitamin B12 along with sun exposure yielded more effective repigmentation than either vitamins or sun exposure alone.5 An additional hypothesis suggested vitamin B12 may aid in repigmentation through its role in the homocysteine pathway. Although the theory is unproven, it is proposed that inhibition of homocysteine via vitamin B12 or folic acid supplementation may play a role in reducing melanocyte destruction and restoring melanin synthesis.6
There were mixed recommendations regarding vitamin C via supplementation and/or eating citrus fruits such as oranges. Although there are limited clinical studies on the use of vitamin C and the treatment of vitiligo, a 6-year prospective study from Madagascar consisting of approximately 300 participants with vitiligo who were treated with a combination of topical corticosteroids, oral vitamin C, and oral vitamin B12 supplementation showed excellent repigmentation (defined by repigmentation of more than 76% of the originally affected area) in 50 participants.7
Fruits—Most web pages had mixed recommendations on whether to include or avoid certain fruits. Interestingly, inclusion of mangoes and apricots in the diet were highly recommended (9/31 [29%] and 8/31 [26%], respectively) while fruits such as oranges, lemons, papayas, and grapes were discouraged (10/31 [32%], 8/31 [26%], 6/31 [19%], and 7/31 [23%], respectively). Although some web pages suggested that vitamin C–rich produce including citrus and berries may help to increase melanin formation, others strongly suggested avoiding these fruits. There is limited information on the effects of citrus on vitiligo, but a 2022 study indicated that 5-demethylnobiletin, a flavonoid found in sweet citrus fruits, may stimulate melanin synthesis, which can possibly be beneficial for vitiligo.8
Omega-3 Fatty Acids—Seven of 31 (23%) web pages recommended the inclusion of omega-3 fatty acids for their role as antioxidants to improve vitiligo symptoms. Research has indicated a strong association between vitiligo and oxidative stress.9 A 2007 controlled clinical trial that included 28 vitiligo patients demonstrated that oral antioxidant supplementation in combination with narrowband UVB phototherapy can significantly decrease vitiligo-associated oxidative stress (P<.05); 8 of 17 (47%) patients in the treatment group saw greater than 75% repigmentation after antioxidant treatment.10
Grains—Five of 31 (16%) web pages suggested avoiding gluten—a protein naturally found in some grains including wheat, barley, and rye—to improve vitiligo symptoms. A 2021 review suggested that a gluten-free diet may be effective in managing celiac disease, and it is hypothesized that vitiligo may be managed with similar dietary adjustments.11 Studies have shown that celiac disease and vitiligo—both autoimmune conditions—involve IL-2, IL-6, IL-7, and IL-21 in their disease pathways.12,13 Their shared immunogenic mechanism may account for similar management options.
Upon review, 2 case reports were identified that discussed a relationship between a gluten-free diet and vitiligo symptom improvement. In one report, a 9-year-old child diagnosed with both celiac disease and vitiligo saw intense repigmentation of the skin after adhering to a gluten-free diet for 1 year.14 Another case study reported a 22-year-old woman with vitiligo whose symptoms improved after 1 month of a gluten-free diet following 2 years of failed treatment with a topical steroid and phototherapy.15
Seven of 31 (23%) web pages suggested that individuals with vitiligo should include wheat in their diet. There is no published literature discussing the relationship between vitiligo and wheat. Of the 31 web pages reviewed, 10 (32%) suggested including whole grain. There is no relevant scientific evidence or hypotheses describing how whole grains may be beneficial in vitiligo.
Minerals—Eight of 31 (26%) web pages suggested including zinc in the diet to improve vitiligo symptoms. A 2020 study evaluated how different serum levels of zinc in vitiligo patients might be affiliated with interleukin activity. Fifty patients diagnosed with active vitiligo were tested for serum levels of zinc, IL-4, IL-6, and IL-17.16 The results showed that mean serum levels of zinc were lower in vitiligo patients compared with patients without vitiligo. The study concluded that zinc could possibly be used as a supplement to improve vitiligo, though the dosage needs to be further studied and confirmed.16
Vegetables—Eleven of 31 (35%) web pages recommended leafy green vegetables and 13 of 31 (42%) recommended spinach for patients with vitiligo. Spinach and other leafy green vegetables are known to be rich in antioxidants, which may have protective effects against reactive oxygen species that are thought to contribute to vitiligo progression.17,18
Nuts—Walnuts were recommended in 11 of 31 (35%) web pages. Nuts may be beneficial in reducing inflammation and providing protection against oxidative stress.9 However, there is no specific scientific literature that supports the inclusion of nuts in the diet to manage vitiligo symptoms.
Comment
With a growing amount of research suggesting that diet modifications may contribute to management of certain skin conditions, vitiligo patients often inquire about foods or supplements that may help improve their condition.19 Our review highlighted what information was available to the public regarding diet and vitiligo, with preliminary support of the following primary diet components: vitamin D, vitamin B12, zinc, and omega-3 fatty acids. Our review showed no support in the literature for the items that were recommended to avoid. It is important to note that 27 of 31 (87%) web pages from our online search were not authored by medical professionals or dermatologists. Additionally, many web pages suggested conflicting information, making it difficult to draw concrete conclusions about what diet modifications will be beneficial to the vitiligo community. Further controlled clinical trials are warranted due to the lack of formal studies that assess the relationship between diet and vitiligo.
- Gandhi K, Ezzedine K, Anastassopoulos KP, et al. Prevalence of vitiligo among adults in the United States. JAMA Dermatol. 2022;158:43-50. doi:10.1001/jamadermatol.2021.4724
- Silverberg JI, Silverberg AI, Malka E, et al. A pilot study assessing the role of 25 hydroxy vitamin D levels in patients with vitiligo vulgaris. J Am Acad Dermatol. 2010;62:937-941. doi:10.1016/j.jaad.2009.11.024
- Karagüzel G, Sakarya NP, Bahadır S, et al. Vitamin D status and the effects of oral vitamin D treatment in children with vitiligo: a prospective study. Clin Nutr ESPEN. 2016;15:28-31. doi:10.1016/j.clnesp.2016.05.006.
- Finamor DC, Sinigaglia-Coimbra R, Neves LC, et al. A pilot study assessing the effect of prolonged administration of high daily doses of vitamin D on the clinical course of vitiligo and psoriasis. Dermatoendocrinol. 2013;5:222-234. doi:10.4161/derm.24808
- Juhlin L, Olsson MJ. Improvement of vitiligo after oral treatment with vitamin B12 and folic acid and the importance of sun exposure. Acta Derm Venereol. 1997;77:460-462. doi:10.2340/000155555577460462
- Chen J, Zhuang T, Chen J, et al. Homocysteine induces melanocytes apoptosis via PERK-eIF2α-CHOP pathway in vitiligo. Clin Sci (Lond). 2020;134:1127-1141. doi:10.1042/CS20200218
- Sendrasoa FA, Ranaivo IM, Sata M, et al. Treatment responses in patients with vitiligo to very potent topical corticosteroids combined with vitamin therapy in Madagascar. Int J Dermatol. 2019;58:908-911. doi:10.1111/ijd.14510
- Wang HM, Qu LQ, Ng JPL, et al. Natural citrus flavanone 5-demethylnobiletin stimulates melanogenesis through the activation of cAMP/CREB pathway in B16F10 cells. Phytomedicine. 2022;98:153941. doi:10.1016/j.phymed.2022.153941
- Ros E. Health benefits of nut consumption. Nutrients. 2010;2:652-682.
- Dell’Anna ML, Mastrofrancesco A, Sala R, et al. Antioxidants and narrow band-UVB in the treatment of vitiligo: a double-blind placebo controlled trial. Clin Exp Dermatol. 2007;32:631-636.
- Gastrointestinal microbiome and gluten in celiac disease. Ann Med. 2021;53:1797-1805. doi:10.1080/07853890.2021.1990392
- Forabosco P, Neuhausen SL, Greco L, et al. Meta-analysis of genome-wide linkage studies in celiac disease. Hum Hered. 2009;68:223-230. doi:10.1159/000228920
- Akbulut UE, Çebi AH, Sag˘ E, et al. Interleukin-6 and interleukin-17 gene polymorphism association with celiac disease in children. Turk J Gastroenterol. 2017;28:471-475. doi:10.5152/tjg.2017.17092
- Rodríguez-García C, González-Hernández S, Pérez-Robayna N, et al. Repigmentation of vitiligo lesions in a child with celiac disease after a gluten-free diet. Pediatr Dermatol. 2011;28:209-210. doi:10.1111/j.1525-1470.2011.01388.x
- Khandalavala BN, Nirmalraj MC. Rapid partial repigmentation ofvitiligo in a young female adult with a gluten-free diet. Case Rep Dermatol. 2014;6:283-287.
- Sanad EM, El-Fallah AA, Al-Doori AR, et al. Serum zinc and inflammatory cytokines in vitiligo. J Clin Aesthet Dermatol. 2020;13:(12 suppl 1):S29-S33.
- Ames BN, Shigenaga MK, Hagen TM. Oxidants, antioxidants, and the degenerative diseases of aging. Proc Natl Acad Sci U S A. 1993;90:7915-7922. doi:10.1073/pnas.90.17.7915
- Xian D, Guo M, Xu J, et al. Current evidence to support the therapeutic potential of flavonoids in oxidative stress-related dermatoses. Redox Rep. 2021;26:134-146. doi:10.1080 /13510002.2021.1962094
- Katta R, Kramer MJ. Skin and diet: an update on the role of dietary change as a treatment strategy for skin disease. Skin Therapy Lett. 2018;23:1-5.
- Gandhi K, Ezzedine K, Anastassopoulos KP, et al. Prevalence of vitiligo among adults in the United States. JAMA Dermatol. 2022;158:43-50. doi:10.1001/jamadermatol.2021.4724
- Silverberg JI, Silverberg AI, Malka E, et al. A pilot study assessing the role of 25 hydroxy vitamin D levels in patients with vitiligo vulgaris. J Am Acad Dermatol. 2010;62:937-941. doi:10.1016/j.jaad.2009.11.024
- Karagüzel G, Sakarya NP, Bahadır S, et al. Vitamin D status and the effects of oral vitamin D treatment in children with vitiligo: a prospective study. Clin Nutr ESPEN. 2016;15:28-31. doi:10.1016/j.clnesp.2016.05.006.
- Finamor DC, Sinigaglia-Coimbra R, Neves LC, et al. A pilot study assessing the effect of prolonged administration of high daily doses of vitamin D on the clinical course of vitiligo and psoriasis. Dermatoendocrinol. 2013;5:222-234. doi:10.4161/derm.24808
- Juhlin L, Olsson MJ. Improvement of vitiligo after oral treatment with vitamin B12 and folic acid and the importance of sun exposure. Acta Derm Venereol. 1997;77:460-462. doi:10.2340/000155555577460462
- Chen J, Zhuang T, Chen J, et al. Homocysteine induces melanocytes apoptosis via PERK-eIF2α-CHOP pathway in vitiligo. Clin Sci (Lond). 2020;134:1127-1141. doi:10.1042/CS20200218
- Sendrasoa FA, Ranaivo IM, Sata M, et al. Treatment responses in patients with vitiligo to very potent topical corticosteroids combined with vitamin therapy in Madagascar. Int J Dermatol. 2019;58:908-911. doi:10.1111/ijd.14510
- Wang HM, Qu LQ, Ng JPL, et al. Natural citrus flavanone 5-demethylnobiletin stimulates melanogenesis through the activation of cAMP/CREB pathway in B16F10 cells. Phytomedicine. 2022;98:153941. doi:10.1016/j.phymed.2022.153941
- Ros E. Health benefits of nut consumption. Nutrients. 2010;2:652-682.
- Dell’Anna ML, Mastrofrancesco A, Sala R, et al. Antioxidants and narrow band-UVB in the treatment of vitiligo: a double-blind placebo controlled trial. Clin Exp Dermatol. 2007;32:631-636.
- Gastrointestinal microbiome and gluten in celiac disease. Ann Med. 2021;53:1797-1805. doi:10.1080/07853890.2021.1990392
- Forabosco P, Neuhausen SL, Greco L, et al. Meta-analysis of genome-wide linkage studies in celiac disease. Hum Hered. 2009;68:223-230. doi:10.1159/000228920
- Akbulut UE, Çebi AH, Sag˘ E, et al. Interleukin-6 and interleukin-17 gene polymorphism association with celiac disease in children. Turk J Gastroenterol. 2017;28:471-475. doi:10.5152/tjg.2017.17092
- Rodríguez-García C, González-Hernández S, Pérez-Robayna N, et al. Repigmentation of vitiligo lesions in a child with celiac disease after a gluten-free diet. Pediatr Dermatol. 2011;28:209-210. doi:10.1111/j.1525-1470.2011.01388.x
- Khandalavala BN, Nirmalraj MC. Rapid partial repigmentation ofvitiligo in a young female adult with a gluten-free diet. Case Rep Dermatol. 2014;6:283-287.
- Sanad EM, El-Fallah AA, Al-Doori AR, et al. Serum zinc and inflammatory cytokines in vitiligo. J Clin Aesthet Dermatol. 2020;13:(12 suppl 1):S29-S33.
- Ames BN, Shigenaga MK, Hagen TM. Oxidants, antioxidants, and the degenerative diseases of aging. Proc Natl Acad Sci U S A. 1993;90:7915-7922. doi:10.1073/pnas.90.17.7915
- Xian D, Guo M, Xu J, et al. Current evidence to support the therapeutic potential of flavonoids in oxidative stress-related dermatoses. Redox Rep. 2021;26:134-146. doi:10.1080 /13510002.2021.1962094
- Katta R, Kramer MJ. Skin and diet: an update on the role of dietary change as a treatment strategy for skin disease. Skin Therapy Lett. 2018;23:1-5.
Practice Points
- There are numerous online dietary and supplement recommendations that claim to impact vitiligo but most are not authored by medical professionals or dermatologists.
- Scientific evidence supporting specific dietary and supplement recommendations for vitiligo is limited.
- Current preliminary data support the potential recommendation for dietary supplementation with vitamin D, vitamin B12, zinc, and omega-3 fatty acids.
US Dermatologic Drug Approvals Rose Between 2012 and 2022
TOPLINE:
METHODOLOGY:
- Only five new drugs for diseases treated mostly by dermatologists were approved by the FDA between 1999 and 2009.
- In a cross-sectional analysis to characterize the frequency and degree of innovation of dermatologic drugs approved more recently, researchers identified new and supplemental dermatologic drugs approved between January 1, 2012, and December 31, 2022, from FDA lists, Centers for Medicare & Medicaid Services CenterWatch, and peer-reviewed articles.
- They used five proxy measures to estimate each drug’s degree of innovation: FDA designation (first in class, advance in class, or addition to class), independent clinical usefulness ratings, and benefit ratings by health technology assessment organizations.
TAKEAWAY:
- The study authors identified 52 new drug applications and 26 supplemental new indications approved by the FDA for dermatologic indications between 2012 and 2022.
- Of the 52 new drugs, the researchers categorized 11 (21%) as first in class and 13 (25%) as first in indication.
- An analysis of benefit ratings available for 38 of the drugs showed that 15 (39%) were rated as being clinically useful or having high added therapeutic benefit.
- Of the 10 supplemental new indications with ratings by any organization, 3 (30%) were rated as clinically useful or having high added therapeutic benefit.
IN PRACTICE:
While innovative drug development in dermatology may have increased, “these findings also highlight opportunities to develop more truly innovative dermatologic agents, particularly for diseases with unmet therapeutic need,” the authors wrote.
SOURCE:
First author Samir Kamat, MD, of the Medical Education Department at Icahn School of Medicine at Mount Sinai, New York City, and corresponding author Ravi Gupta, MD, MSHP, of the Internal Medicine Division at Johns Hopkins University, Baltimore, Maryland, led the research. The study was published online as a research letter on December 20, 2023, in JAMA Dermatology.
LIMITATIONS:
They include the use of individual indications to assess clinical usefulness and benefit ratings. Many drugs, particularly supplemental indications, lacked such ratings. Reformulations of already marketed drugs or indications were not included.
DISCLOSURES:
Dr. Kamat and Dr. Gupta had no relevant disclosures. Three coauthors reported having received financial support outside of the submitted work.
A version of this article appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- Only five new drugs for diseases treated mostly by dermatologists were approved by the FDA between 1999 and 2009.
- In a cross-sectional analysis to characterize the frequency and degree of innovation of dermatologic drugs approved more recently, researchers identified new and supplemental dermatologic drugs approved between January 1, 2012, and December 31, 2022, from FDA lists, Centers for Medicare & Medicaid Services CenterWatch, and peer-reviewed articles.
- They used five proxy measures to estimate each drug’s degree of innovation: FDA designation (first in class, advance in class, or addition to class), independent clinical usefulness ratings, and benefit ratings by health technology assessment organizations.
TAKEAWAY:
- The study authors identified 52 new drug applications and 26 supplemental new indications approved by the FDA for dermatologic indications between 2012 and 2022.
- Of the 52 new drugs, the researchers categorized 11 (21%) as first in class and 13 (25%) as first in indication.
- An analysis of benefit ratings available for 38 of the drugs showed that 15 (39%) were rated as being clinically useful or having high added therapeutic benefit.
- Of the 10 supplemental new indications with ratings by any organization, 3 (30%) were rated as clinically useful or having high added therapeutic benefit.
IN PRACTICE:
While innovative drug development in dermatology may have increased, “these findings also highlight opportunities to develop more truly innovative dermatologic agents, particularly for diseases with unmet therapeutic need,” the authors wrote.
SOURCE:
First author Samir Kamat, MD, of the Medical Education Department at Icahn School of Medicine at Mount Sinai, New York City, and corresponding author Ravi Gupta, MD, MSHP, of the Internal Medicine Division at Johns Hopkins University, Baltimore, Maryland, led the research. The study was published online as a research letter on December 20, 2023, in JAMA Dermatology.
LIMITATIONS:
They include the use of individual indications to assess clinical usefulness and benefit ratings. Many drugs, particularly supplemental indications, lacked such ratings. Reformulations of already marketed drugs or indications were not included.
DISCLOSURES:
Dr. Kamat and Dr. Gupta had no relevant disclosures. Three coauthors reported having received financial support outside of the submitted work.
A version of this article appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- Only five new drugs for diseases treated mostly by dermatologists were approved by the FDA between 1999 and 2009.
- In a cross-sectional analysis to characterize the frequency and degree of innovation of dermatologic drugs approved more recently, researchers identified new and supplemental dermatologic drugs approved between January 1, 2012, and December 31, 2022, from FDA lists, Centers for Medicare & Medicaid Services CenterWatch, and peer-reviewed articles.
- They used five proxy measures to estimate each drug’s degree of innovation: FDA designation (first in class, advance in class, or addition to class), independent clinical usefulness ratings, and benefit ratings by health technology assessment organizations.
TAKEAWAY:
- The study authors identified 52 new drug applications and 26 supplemental new indications approved by the FDA for dermatologic indications between 2012 and 2022.
- Of the 52 new drugs, the researchers categorized 11 (21%) as first in class and 13 (25%) as first in indication.
- An analysis of benefit ratings available for 38 of the drugs showed that 15 (39%) were rated as being clinically useful or having high added therapeutic benefit.
- Of the 10 supplemental new indications with ratings by any organization, 3 (30%) were rated as clinically useful or having high added therapeutic benefit.
IN PRACTICE:
While innovative drug development in dermatology may have increased, “these findings also highlight opportunities to develop more truly innovative dermatologic agents, particularly for diseases with unmet therapeutic need,” the authors wrote.
SOURCE:
First author Samir Kamat, MD, of the Medical Education Department at Icahn School of Medicine at Mount Sinai, New York City, and corresponding author Ravi Gupta, MD, MSHP, of the Internal Medicine Division at Johns Hopkins University, Baltimore, Maryland, led the research. The study was published online as a research letter on December 20, 2023, in JAMA Dermatology.
LIMITATIONS:
They include the use of individual indications to assess clinical usefulness and benefit ratings. Many drugs, particularly supplemental indications, lacked such ratings. Reformulations of already marketed drugs or indications were not included.
DISCLOSURES:
Dr. Kamat and Dr. Gupta had no relevant disclosures. Three coauthors reported having received financial support outside of the submitted work.
A version of this article appeared on Medscape.com.
Nasal Tanning Sprays: Illuminating the Risks of a Popular TikTok Trend
Nasal tanning spray is a recent phenomenon that has been gaining popularity among consumers on TikTok and other social media platforms. The active ingredient in the tanning spray is melanotan II—a synthetic analog of α‒melanocyte-stimulating hormone,1,2 a naturally occurring hormone responsible for skin pigmentation. α‒Melanocyte-stimulating hormone is a derivative of the precursor proopiomelanocortin, an agonist on the melanocortin-1 receptor that promotes formation of eumelanin.1,3 Eumelanin then provides pigmentation to the skin.3 Apart from its use for tanning, melanotan II has been reported to increase sexual function and aid in weight loss.1
Melanotan II is not approved by the US Food and Drug Administration; however, injectable formulations can be obtained illegally on the Internet as well as at some tanning salons and beauty parlors.4 Although injectable forms of melanotan II have been used for years to artificially increase skin pigmentation, the newly hyped nasal tanning sprays are drawing the attention of consumers. The synthetic chemical spray is inhaled into the nasal mucosae, where it is readily absorbed into the bloodstream to act on melanocortin receptors throughout the body, thus enhancing skin pigmentation.2 Because melanotan II is not approved, there is no guarantee that the product purchased from those sources is pure; therefore, consumers risk inhaling or injecting contaminated chemicals.5
In a 2017 study, Kirk and Greenfield6 cited self-image as a common concern among participants who expressed a preference for appearing tanned.6 Societal influence and standards to which young adults, particularly young women, often are accustomed drive some to take steps to achieve tanned skin, which they view as more attractive and healthier than untanned skin.7,8
Social media consumption is a significant risk factor for developing or exacerbating body dissatisfaction among impressionable teenagers and young adults, who may be less risk averse and therefore choose to embrace trends such as nasal tanning sprays to enhance their appearance, without considering possible consequences. Most young adults, and even teens, are aware of the risks associated with tanning beds, which may propel them to seek out what they perceive as a less-risky tanning alternative such as a tanner delivered via a nasal route, but it is unlikely that this group is fully informed about the possible dangers of nasal tanning sprays.
It is crucial for dermatologists and other clinicians to provide awareness and education about the potential harm of nasal tanning sprays. Along with the general risks of using an unregulated substance, common adverse effects include acne, facial flushing, gastrointestinal tract upset, and sensitivity to sunlight (Table).1,9,10 Several case reports have linked melanotan II to cutaneous changes, including dysplastic nevi and even melanoma.1 Less common complications, such as renal infarction and priapism, also have been observed with melanotan II use.9,10

Even with the known risks involving tanning beds and skin cancer, an analysis by Kream et al11 in 2020 showed that 90% (441/488) of tanning-related videos on TikTok promoted a positive view of tanning. Of these TikTok videos involving pro-tanning trends, 3% (12/441) were specifically about melanotan II nasal spray, injection, or both, which has only become more popular since this study was published.11
Dermatologists should be aware of the impact that tanning trends, such as nasal tanning spray, can have on all patients and initiate discussions regarding the risks of using these products with patients as appropriate. Alternatives to nasal tanning sprays such as spray-on tans and self-tanning lotions are safer ways for patients to achieve a tanned look without the health risks associated with melanotan II.
- Habbema L, Halk AB, Neumann M, et al. Risks of unregulated use of alpha-melanocyte-stimulating hormone analogues: a review. Int J Dermatol. 2017;56:975-980. doi:10.1111/ijd.13585
- Why you should never use nasal tanning spray. Cleveland Clinic Health Essentials [Internet]. November 1, 2022. Accessed December 18, 2023. https://health.clevelandclinic.org/nasal-tanning-spray
- Hjuler KF, Lorentzen HF. Melanoma associated with the use of melanotan-II. Dermatology. 2014;228:34-36. doi:10.1159/000356389
- Evans-Brown M, Dawson RT, Chandler M, et al. Use of melanotan I and II in the general population. BMJ. 2009;338:b566. doi:10.116/bmj.b566
- Callaghan DJ III. A glimpse into the underground market of melanotan. Dermatol Online J. 2018;24:1-5. doi:10.5070/D3245040036
- Kirk L, Greenfield S. Knowledge and attitudes of UK university students in relation to ultraviolet radiation (UVR) exposure and their sun-related behaviours: a qualitative study. BMJ Open. 2017;7:e014388. doi:10.1136/bmjopen-2016-014388
- Hay JL, Geller AC, Schoenhammer M, et al. Tanning and beauty: mother and teenage daughters in discussion. J Health Psychol. 2016;21:1261-1270. doi:10.1177/1359105314551621
- Gillen MM, Markey CN. The role of body image and depression in tanning behaviors and attitudes. Behav Med. 2017;38:74-82.
- Peters B, Hadimeri H, Wahlberg R, et al. Melanotan II: a possible cause of renal infarction: review of the literature and case report. CEN Case Rep. 2020;9:159-161. doi:10.1007/s13730-020-00447-z
- Mallory CW, Lopategui DM, Cordon BH. Melanotan tanning injection: a rare cause of priapism. Sex Med. 2021;9:100298. doi:10.1016/j.esxm.2020.100298
- Kream E, Watchmaker JD, Dover JS. TikTok sheds light on tanning: tanning is still popular and emerging trends pose new risks. Dermatol Surg. 2022;48:1018-1021. doi:10.1097/DSS.0000000000003549
Nasal tanning spray is a recent phenomenon that has been gaining popularity among consumers on TikTok and other social media platforms. The active ingredient in the tanning spray is melanotan II—a synthetic analog of α‒melanocyte-stimulating hormone,1,2 a naturally occurring hormone responsible for skin pigmentation. α‒Melanocyte-stimulating hormone is a derivative of the precursor proopiomelanocortin, an agonist on the melanocortin-1 receptor that promotes formation of eumelanin.1,3 Eumelanin then provides pigmentation to the skin.3 Apart from its use for tanning, melanotan II has been reported to increase sexual function and aid in weight loss.1
Melanotan II is not approved by the US Food and Drug Administration; however, injectable formulations can be obtained illegally on the Internet as well as at some tanning salons and beauty parlors.4 Although injectable forms of melanotan II have been used for years to artificially increase skin pigmentation, the newly hyped nasal tanning sprays are drawing the attention of consumers. The synthetic chemical spray is inhaled into the nasal mucosae, where it is readily absorbed into the bloodstream to act on melanocortin receptors throughout the body, thus enhancing skin pigmentation.2 Because melanotan II is not approved, there is no guarantee that the product purchased from those sources is pure; therefore, consumers risk inhaling or injecting contaminated chemicals.5
In a 2017 study, Kirk and Greenfield6 cited self-image as a common concern among participants who expressed a preference for appearing tanned.6 Societal influence and standards to which young adults, particularly young women, often are accustomed drive some to take steps to achieve tanned skin, which they view as more attractive and healthier than untanned skin.7,8
Social media consumption is a significant risk factor for developing or exacerbating body dissatisfaction among impressionable teenagers and young adults, who may be less risk averse and therefore choose to embrace trends such as nasal tanning sprays to enhance their appearance, without considering possible consequences. Most young adults, and even teens, are aware of the risks associated with tanning beds, which may propel them to seek out what they perceive as a less-risky tanning alternative such as a tanner delivered via a nasal route, but it is unlikely that this group is fully informed about the possible dangers of nasal tanning sprays.
It is crucial for dermatologists and other clinicians to provide awareness and education about the potential harm of nasal tanning sprays. Along with the general risks of using an unregulated substance, common adverse effects include acne, facial flushing, gastrointestinal tract upset, and sensitivity to sunlight (Table).1,9,10 Several case reports have linked melanotan II to cutaneous changes, including dysplastic nevi and even melanoma.1 Less common complications, such as renal infarction and priapism, also have been observed with melanotan II use.9,10

Even with the known risks involving tanning beds and skin cancer, an analysis by Kream et al11 in 2020 showed that 90% (441/488) of tanning-related videos on TikTok promoted a positive view of tanning. Of these TikTok videos involving pro-tanning trends, 3% (12/441) were specifically about melanotan II nasal spray, injection, or both, which has only become more popular since this study was published.11
Dermatologists should be aware of the impact that tanning trends, such as nasal tanning spray, can have on all patients and initiate discussions regarding the risks of using these products with patients as appropriate. Alternatives to nasal tanning sprays such as spray-on tans and self-tanning lotions are safer ways for patients to achieve a tanned look without the health risks associated with melanotan II.
Nasal tanning spray is a recent phenomenon that has been gaining popularity among consumers on TikTok and other social media platforms. The active ingredient in the tanning spray is melanotan II—a synthetic analog of α‒melanocyte-stimulating hormone,1,2 a naturally occurring hormone responsible for skin pigmentation. α‒Melanocyte-stimulating hormone is a derivative of the precursor proopiomelanocortin, an agonist on the melanocortin-1 receptor that promotes formation of eumelanin.1,3 Eumelanin then provides pigmentation to the skin.3 Apart from its use for tanning, melanotan II has been reported to increase sexual function and aid in weight loss.1
Melanotan II is not approved by the US Food and Drug Administration; however, injectable formulations can be obtained illegally on the Internet as well as at some tanning salons and beauty parlors.4 Although injectable forms of melanotan II have been used for years to artificially increase skin pigmentation, the newly hyped nasal tanning sprays are drawing the attention of consumers. The synthetic chemical spray is inhaled into the nasal mucosae, where it is readily absorbed into the bloodstream to act on melanocortin receptors throughout the body, thus enhancing skin pigmentation.2 Because melanotan II is not approved, there is no guarantee that the product purchased from those sources is pure; therefore, consumers risk inhaling or injecting contaminated chemicals.5
In a 2017 study, Kirk and Greenfield6 cited self-image as a common concern among participants who expressed a preference for appearing tanned.6 Societal influence and standards to which young adults, particularly young women, often are accustomed drive some to take steps to achieve tanned skin, which they view as more attractive and healthier than untanned skin.7,8
Social media consumption is a significant risk factor for developing or exacerbating body dissatisfaction among impressionable teenagers and young adults, who may be less risk averse and therefore choose to embrace trends such as nasal tanning sprays to enhance their appearance, without considering possible consequences. Most young adults, and even teens, are aware of the risks associated with tanning beds, which may propel them to seek out what they perceive as a less-risky tanning alternative such as a tanner delivered via a nasal route, but it is unlikely that this group is fully informed about the possible dangers of nasal tanning sprays.
It is crucial for dermatologists and other clinicians to provide awareness and education about the potential harm of nasal tanning sprays. Along with the general risks of using an unregulated substance, common adverse effects include acne, facial flushing, gastrointestinal tract upset, and sensitivity to sunlight (Table).1,9,10 Several case reports have linked melanotan II to cutaneous changes, including dysplastic nevi and even melanoma.1 Less common complications, such as renal infarction and priapism, also have been observed with melanotan II use.9,10

Even with the known risks involving tanning beds and skin cancer, an analysis by Kream et al11 in 2020 showed that 90% (441/488) of tanning-related videos on TikTok promoted a positive view of tanning. Of these TikTok videos involving pro-tanning trends, 3% (12/441) were specifically about melanotan II nasal spray, injection, or both, which has only become more popular since this study was published.11
Dermatologists should be aware of the impact that tanning trends, such as nasal tanning spray, can have on all patients and initiate discussions regarding the risks of using these products with patients as appropriate. Alternatives to nasal tanning sprays such as spray-on tans and self-tanning lotions are safer ways for patients to achieve a tanned look without the health risks associated with melanotan II.
- Habbema L, Halk AB, Neumann M, et al. Risks of unregulated use of alpha-melanocyte-stimulating hormone analogues: a review. Int J Dermatol. 2017;56:975-980. doi:10.1111/ijd.13585
- Why you should never use nasal tanning spray. Cleveland Clinic Health Essentials [Internet]. November 1, 2022. Accessed December 18, 2023. https://health.clevelandclinic.org/nasal-tanning-spray
- Hjuler KF, Lorentzen HF. Melanoma associated with the use of melanotan-II. Dermatology. 2014;228:34-36. doi:10.1159/000356389
- Evans-Brown M, Dawson RT, Chandler M, et al. Use of melanotan I and II in the general population. BMJ. 2009;338:b566. doi:10.116/bmj.b566
- Callaghan DJ III. A glimpse into the underground market of melanotan. Dermatol Online J. 2018;24:1-5. doi:10.5070/D3245040036
- Kirk L, Greenfield S. Knowledge and attitudes of UK university students in relation to ultraviolet radiation (UVR) exposure and their sun-related behaviours: a qualitative study. BMJ Open. 2017;7:e014388. doi:10.1136/bmjopen-2016-014388
- Hay JL, Geller AC, Schoenhammer M, et al. Tanning and beauty: mother and teenage daughters in discussion. J Health Psychol. 2016;21:1261-1270. doi:10.1177/1359105314551621
- Gillen MM, Markey CN. The role of body image and depression in tanning behaviors and attitudes. Behav Med. 2017;38:74-82.
- Peters B, Hadimeri H, Wahlberg R, et al. Melanotan II: a possible cause of renal infarction: review of the literature and case report. CEN Case Rep. 2020;9:159-161. doi:10.1007/s13730-020-00447-z
- Mallory CW, Lopategui DM, Cordon BH. Melanotan tanning injection: a rare cause of priapism. Sex Med. 2021;9:100298. doi:10.1016/j.esxm.2020.100298
- Kream E, Watchmaker JD, Dover JS. TikTok sheds light on tanning: tanning is still popular and emerging trends pose new risks. Dermatol Surg. 2022;48:1018-1021. doi:10.1097/DSS.0000000000003549
- Habbema L, Halk AB, Neumann M, et al. Risks of unregulated use of alpha-melanocyte-stimulating hormone analogues: a review. Int J Dermatol. 2017;56:975-980. doi:10.1111/ijd.13585
- Why you should never use nasal tanning spray. Cleveland Clinic Health Essentials [Internet]. November 1, 2022. Accessed December 18, 2023. https://health.clevelandclinic.org/nasal-tanning-spray
- Hjuler KF, Lorentzen HF. Melanoma associated with the use of melanotan-II. Dermatology. 2014;228:34-36. doi:10.1159/000356389
- Evans-Brown M, Dawson RT, Chandler M, et al. Use of melanotan I and II in the general population. BMJ. 2009;338:b566. doi:10.116/bmj.b566
- Callaghan DJ III. A glimpse into the underground market of melanotan. Dermatol Online J. 2018;24:1-5. doi:10.5070/D3245040036
- Kirk L, Greenfield S. Knowledge and attitudes of UK university students in relation to ultraviolet radiation (UVR) exposure and their sun-related behaviours: a qualitative study. BMJ Open. 2017;7:e014388. doi:10.1136/bmjopen-2016-014388
- Hay JL, Geller AC, Schoenhammer M, et al. Tanning and beauty: mother and teenage daughters in discussion. J Health Psychol. 2016;21:1261-1270. doi:10.1177/1359105314551621
- Gillen MM, Markey CN. The role of body image and depression in tanning behaviors and attitudes. Behav Med. 2017;38:74-82.
- Peters B, Hadimeri H, Wahlberg R, et al. Melanotan II: a possible cause of renal infarction: review of the literature and case report. CEN Case Rep. 2020;9:159-161. doi:10.1007/s13730-020-00447-z
- Mallory CW, Lopategui DM, Cordon BH. Melanotan tanning injection: a rare cause of priapism. Sex Med. 2021;9:100298. doi:10.1016/j.esxm.2020.100298
- Kream E, Watchmaker JD, Dover JS. TikTok sheds light on tanning: tanning is still popular and emerging trends pose new risks. Dermatol Surg. 2022;48:1018-1021. doi:10.1097/DSS.0000000000003549
PRACTICE POINTS
- Although tanning beds are arguably the most common and dangerous method used by patients to tan their skin, dermatologists should be aware of the other means by which patients may artificially increase skin pigmentation and the risks imposed by undertaking such practices.
- We challenge dermatologists to note the influence of social media on tanning trends and consider creating a platform on these mediums to combat misinformation and promote sun safety and skin health.
- We encourage dermatologists to diligently stay informed about the popular societal trends related to the skin such as the use of nasal tanning products (eg, melanotan I and II) and be proactive in discussing their risks with patients as deemed appropriate.
GVHD raises vitiligo risk in transplant recipients
published online in JAMA Dermatology December 13.
In the cohort study, the greatest risk occurred with hematopoietic stem cell transplants (HSCTs) and in cases involving GVHD. Kidney and liver transplants carried slight increases in risk.
“The findings suggest that early detection and management of vitiligo lesions can be improved by estimating the likelihood of its development in transplant recipients and implementing a multidisciplinary approach for monitoring,” wrote the authors, from the departments of dermatology and biostatistics, at the Catholic University of Korea, Seoul.
Using claims data from South Korea’s National Health Insurance Service database, the investigators compared vitiligo incidence among 23,829 patients who had undergone solid organ transplantation (SOT) or HSCT between 2010 and 2017 versus that of 119,145 age- and sex-matched controls. At a mean observation time of 4.79 years in the transplant group (and 5.12 years for controls), the adjusted hazard ratio (AHR) for vitiligo among patients who had undergone any transplant was 1.73. AHRs for HSCT, liver transplants, and kidney transplants were 12.69, 1.63, and 1.50, respectively.
Patients who had undergone allogeneic HSCT (AHR, 14.43) or autologous transplants (AHR, 5.71), as well as those with and without GVHD (24.09 and 8.21, respectively) had significantly higher vitiligo risk than the control group.
Among those with GVHD, HSCT recipients (AHR, 16.42) and those with allogeneic grafts (AHR, 16.81) had a higher vitiligo risk than that of control patients.
In a subgroup that included 10,355 transplant recipients who underwent posttransplant health checkups, investigators found the highest vitiligo risk — AHR, 25.09 versus controls — among HSCT recipients with comorbid GVHD. However, patients who underwent SOT, autologous HSCT, or HSCT without GVHD showed no increased vitiligo risk in this analysis. “The results of health checkup data analysis may differ from the initial analysis due to additional adjustments for lifestyle factors and inclusion of only patients who underwent a health checkup,” the authors wrote.
Asked to comment on the results, George Han, MD, PhD, who was not involved with the study, told this news organization, “this is an interesting paper where the primary difference from previous studies is the new association between GVHD in hematopoietic stem cell transplant recipients and vitiligo.” Prior research had shown higher rates of vitiligo in HSCT recipients without making the GVHD distinction. Dr. Han is associate professor of dermatology in the Hofstra/Northwell Department of Dermatology, Hyde Park, New York.
Although GVHD may not be top-of-mind for dermatologists in daily practice, he said, the study enhances their understanding of vitiligo risk in HSCT recipients. “In some ways,” Dr. Han added, “the association makes sense, as the activated T cells from the graft attacking the skin in the HSCT recipient follow many of the mechanisms of vitiligo, including upregulating interferon gamma and the CXCR3/CXCL10 axis.”
Presently, he said, dermatologists worry more about solid organ recipients than about HSCT recipients because the long-term immunosuppression required by SOT increases the risk of squamous cell carcinoma (SCC). “However, the risk of skin cancers also seems to be elevated in HSCT recipients, and in this case the basal cell carcinoma (BCC):SCC ratio is not necessarily reversed as we see in solid organ transplant recipients. So the mechanisms are a bit less clear. Interestingly, acute and chronic GVHD have both been associated with increased risks of BCC and SCC/BCC, respectively.”
Overall, Dr. Han said, any transplant recipient should undergo yearly skin checks not only for skin cancers, but also for other skin conditions such as vitiligo. “It would be nice to see this codified into official guidelines, which can vary considerably but are overall more consistent in solid organ transplant recipients than in HSCT recipients. No such guidelines seem to be available for HSCTs.”
The study was funded by the Basic Research in Science & Engineering program through the National Research Foundation of Korea, which is funded by the country’s Ministry of Education. The study authors had no disclosures. Dr. Han reports no relevant financial interests.
published online in JAMA Dermatology December 13.
In the cohort study, the greatest risk occurred with hematopoietic stem cell transplants (HSCTs) and in cases involving GVHD. Kidney and liver transplants carried slight increases in risk.
“The findings suggest that early detection and management of vitiligo lesions can be improved by estimating the likelihood of its development in transplant recipients and implementing a multidisciplinary approach for monitoring,” wrote the authors, from the departments of dermatology and biostatistics, at the Catholic University of Korea, Seoul.
Using claims data from South Korea’s National Health Insurance Service database, the investigators compared vitiligo incidence among 23,829 patients who had undergone solid organ transplantation (SOT) or HSCT between 2010 and 2017 versus that of 119,145 age- and sex-matched controls. At a mean observation time of 4.79 years in the transplant group (and 5.12 years for controls), the adjusted hazard ratio (AHR) for vitiligo among patients who had undergone any transplant was 1.73. AHRs for HSCT, liver transplants, and kidney transplants were 12.69, 1.63, and 1.50, respectively.
Patients who had undergone allogeneic HSCT (AHR, 14.43) or autologous transplants (AHR, 5.71), as well as those with and without GVHD (24.09 and 8.21, respectively) had significantly higher vitiligo risk than the control group.
Among those with GVHD, HSCT recipients (AHR, 16.42) and those with allogeneic grafts (AHR, 16.81) had a higher vitiligo risk than that of control patients.
In a subgroup that included 10,355 transplant recipients who underwent posttransplant health checkups, investigators found the highest vitiligo risk — AHR, 25.09 versus controls — among HSCT recipients with comorbid GVHD. However, patients who underwent SOT, autologous HSCT, or HSCT without GVHD showed no increased vitiligo risk in this analysis. “The results of health checkup data analysis may differ from the initial analysis due to additional adjustments for lifestyle factors and inclusion of only patients who underwent a health checkup,” the authors wrote.
Asked to comment on the results, George Han, MD, PhD, who was not involved with the study, told this news organization, “this is an interesting paper where the primary difference from previous studies is the new association between GVHD in hematopoietic stem cell transplant recipients and vitiligo.” Prior research had shown higher rates of vitiligo in HSCT recipients without making the GVHD distinction. Dr. Han is associate professor of dermatology in the Hofstra/Northwell Department of Dermatology, Hyde Park, New York.
Although GVHD may not be top-of-mind for dermatologists in daily practice, he said, the study enhances their understanding of vitiligo risk in HSCT recipients. “In some ways,” Dr. Han added, “the association makes sense, as the activated T cells from the graft attacking the skin in the HSCT recipient follow many of the mechanisms of vitiligo, including upregulating interferon gamma and the CXCR3/CXCL10 axis.”
Presently, he said, dermatologists worry more about solid organ recipients than about HSCT recipients because the long-term immunosuppression required by SOT increases the risk of squamous cell carcinoma (SCC). “However, the risk of skin cancers also seems to be elevated in HSCT recipients, and in this case the basal cell carcinoma (BCC):SCC ratio is not necessarily reversed as we see in solid organ transplant recipients. So the mechanisms are a bit less clear. Interestingly, acute and chronic GVHD have both been associated with increased risks of BCC and SCC/BCC, respectively.”
Overall, Dr. Han said, any transplant recipient should undergo yearly skin checks not only for skin cancers, but also for other skin conditions such as vitiligo. “It would be nice to see this codified into official guidelines, which can vary considerably but are overall more consistent in solid organ transplant recipients than in HSCT recipients. No such guidelines seem to be available for HSCTs.”
The study was funded by the Basic Research in Science & Engineering program through the National Research Foundation of Korea, which is funded by the country’s Ministry of Education. The study authors had no disclosures. Dr. Han reports no relevant financial interests.
published online in JAMA Dermatology December 13.
In the cohort study, the greatest risk occurred with hematopoietic stem cell transplants (HSCTs) and in cases involving GVHD. Kidney and liver transplants carried slight increases in risk.
“The findings suggest that early detection and management of vitiligo lesions can be improved by estimating the likelihood of its development in transplant recipients and implementing a multidisciplinary approach for monitoring,” wrote the authors, from the departments of dermatology and biostatistics, at the Catholic University of Korea, Seoul.
Using claims data from South Korea’s National Health Insurance Service database, the investigators compared vitiligo incidence among 23,829 patients who had undergone solid organ transplantation (SOT) or HSCT between 2010 and 2017 versus that of 119,145 age- and sex-matched controls. At a mean observation time of 4.79 years in the transplant group (and 5.12 years for controls), the adjusted hazard ratio (AHR) for vitiligo among patients who had undergone any transplant was 1.73. AHRs for HSCT, liver transplants, and kidney transplants were 12.69, 1.63, and 1.50, respectively.
Patients who had undergone allogeneic HSCT (AHR, 14.43) or autologous transplants (AHR, 5.71), as well as those with and without GVHD (24.09 and 8.21, respectively) had significantly higher vitiligo risk than the control group.
Among those with GVHD, HSCT recipients (AHR, 16.42) and those with allogeneic grafts (AHR, 16.81) had a higher vitiligo risk than that of control patients.
In a subgroup that included 10,355 transplant recipients who underwent posttransplant health checkups, investigators found the highest vitiligo risk — AHR, 25.09 versus controls — among HSCT recipients with comorbid GVHD. However, patients who underwent SOT, autologous HSCT, or HSCT without GVHD showed no increased vitiligo risk in this analysis. “The results of health checkup data analysis may differ from the initial analysis due to additional adjustments for lifestyle factors and inclusion of only patients who underwent a health checkup,” the authors wrote.
Asked to comment on the results, George Han, MD, PhD, who was not involved with the study, told this news organization, “this is an interesting paper where the primary difference from previous studies is the new association between GVHD in hematopoietic stem cell transplant recipients and vitiligo.” Prior research had shown higher rates of vitiligo in HSCT recipients without making the GVHD distinction. Dr. Han is associate professor of dermatology in the Hofstra/Northwell Department of Dermatology, Hyde Park, New York.
Although GVHD may not be top-of-mind for dermatologists in daily practice, he said, the study enhances their understanding of vitiligo risk in HSCT recipients. “In some ways,” Dr. Han added, “the association makes sense, as the activated T cells from the graft attacking the skin in the HSCT recipient follow many of the mechanisms of vitiligo, including upregulating interferon gamma and the CXCR3/CXCL10 axis.”
Presently, he said, dermatologists worry more about solid organ recipients than about HSCT recipients because the long-term immunosuppression required by SOT increases the risk of squamous cell carcinoma (SCC). “However, the risk of skin cancers also seems to be elevated in HSCT recipients, and in this case the basal cell carcinoma (BCC):SCC ratio is not necessarily reversed as we see in solid organ transplant recipients. So the mechanisms are a bit less clear. Interestingly, acute and chronic GVHD have both been associated with increased risks of BCC and SCC/BCC, respectively.”
Overall, Dr. Han said, any transplant recipient should undergo yearly skin checks not only for skin cancers, but also for other skin conditions such as vitiligo. “It would be nice to see this codified into official guidelines, which can vary considerably but are overall more consistent in solid organ transplant recipients than in HSCT recipients. No such guidelines seem to be available for HSCTs.”
The study was funded by the Basic Research in Science & Engineering program through the National Research Foundation of Korea, which is funded by the country’s Ministry of Education. The study authors had no disclosures. Dr. Han reports no relevant financial interests.
FROM JAMA DERMATOLOGY
Dietary supplements may play a role in managing vitiligo
, Ammar Ahmed, MD, associate professor of dermatology at Dell Medical School at the University of Texas, Austin, said at the annual Integrative Dermatology Symposium.
Data on the use of dietary supplements for vitiligo are scarce and of limited quality, but existing studies and current understanding of the pathogenesis of vitiligo have convinced Dr. Ahmed to recommend oral Ginkgo biloba, vitamin C, vitamin E, and alpha-lipoic acid – as well as vitamin D if levels are insufficient – for patients receiving phototherapy, and outside of phototherapy when patients express interest, he said.
Melanocyte stress and subsequent autoimmune destruction appear to be “key pathways at play in vitiligo,” with melanocytes exhibiting increased susceptibility to physiologic stress, including a reduced capacity to manage exposure to reactive oxygen species. “It’s more theory than proven science, but if oxidative damage is one of the key factors [affecting] melanocytes, can we ... reverse the damage to those melanocytes with antioxidants?” he said. “I don’t know, but there’s certainly some emerging evidence that we may.”
There are no human data on the effectiveness of an antioxidant-rich diet for vitiligo, but given its theoretical basis of efficacy, it “seems reasonable to recommend,” said Dr. Ahmed. “When my patients ask me, I tell them to eat a colorful diet – with a lot of colorful fruits and vegetables.” In addition, he said, “we know that individuals with vitiligo, just as patients with psoriasis and other inflammatory disorders, appear to have a higher risk for insulin resistance and metabolic syndrome, even after accounting for confounders,” making a healthy diet all the more important.
Two case reports have described improvement with a gluten-free diet, but “that’s it,” he said. “My take is, unless stronger evidence exists, let your patients enjoy their bread.” No other specific diet has been shown to cause, exacerbate, or improve vitiligo, he noted.
Dr. Ahmed offered his views on the literature on this topic, highlighting studies that have caught his eye on antioxidants and other supplements in patients with vitiligo:
Vitamins C and E, and alpha-lipoic acid: In a randomized controlled trial of 35 patients with nonsegmental vitiligo conducted at the San Gallicano Dermatological Institute in Rome, those who received an antioxidant cocktail (alpha-lipoic acid, 100 mg; vitamin C, 100 mg; vitamin E, 40 mg; and polyunsaturated fatty acids) for 2 months before and during narrow-band ultraviolet-B (NB-UVB) therapy had significantly more repigmentation than that of patients who received NB-UVB alone. Forty-seven percent of those in the antioxidant group obtained greater than 75% repigmentation at 6 months vs. 18% in the control arm.
“This is a pretty high-quality trial. They even did in-vitro analysis showing that the antioxidant group had decreased measures of oxidative stress in the melanocytes,” Dr. Ahmed said. A handout he provided to patients receiving UVB therapy includes recommendations for vitamin C, vitamin E, and alpha-lipoic acid supplementation.
Another controlled prospective study of 130 patients with vitiligo, also conducted in Italy, utilized a different antioxidant cocktail in a tablet – Phyllanthus emblica (known as Indian gooseberry), vitamin E, and carotenoids – taken three times a day, in conjunction with standard topical therapy and phototherapy. At 6 months, a significantly higher number of patients receiving the cocktail had mild repigmentation and were less likely to have no repigmentation compared with patients who did not receive the antioxidants. “Nobody did really great, but the cocktail group did a little better,” he said. “So there’s promise.”
Vitamin D: In-vitro studies show that vitamin D may protect melanocytes against oxidative stress, and two small controlled trials showed improvement in vitiligo with vitamin D supplementation (1,500-5,000 IU daily) and no NB-UVB therapy. However, a recent, higher-quality 6-month trial that evaluated 5,000 IU/day of vitamin D in patients with generalized vitiligo showed no advantage over NB-UVB therapy alone. “I tell patients, if you’re insufficient, take vitamin D (supplements) to get your levels up,” Dr. Ahmed sad. “But if you’re already sufficient, I’m not confident there will be a significant benefit.”
Ginkgo biloba: A small double-blind controlled trial randomized 47 patients with limited and slow-spreading vitiligo to receive Ginkgo biloba extract 40 mg three times a day or placebo. At 6 months, 10 patients who received the extract had greater than 75% repigmentation compared with 2 patients in the placebo group. Patients receiving Ginkgo biloba, which has immunomodulatory and antioxidant properties, were also significantly more likely to have disease stabilization.
“I tend to recommend it to patients not doing phototherapy, as well as those receiving phototherapy, especially since the study showed benefit as a monotherapy,” Dr. Ahmed said in an interview after the meeting.
Phenylalanine: Various oral and/or topical formulations of this amino acid and precursor to tyrosine/melanin have been shown to have repigmentation effects when combined with UVA phototherapy or sunlight, but the studies are of limited quality and the oral dosages studied (50 mg/kg per day to 100 mg/kg per day) appear to be a bit high, Dr. Ahmed said at the meeting. “It can add up in cost, and I worry a little about side effects, so I don’t recommend it as much.”
Polypodium leucotomos (PL): This plant extract, from a fern native to Central America and parts of South America, is familiar as a photoprotective supplement, he said, and a few randomized controlled trials show that it may improve repigmentation outcomes, especially on the hands and neck, when combined with NB-UVB in patients with vitiligo.
One of these trials, published in 2021, showed greater than 50% repigmentation at 6 months in 48% of patients with generalized vitiligo who received oral PL (480 mg twice a day) and NB-UVB, versus 22% in patients receiving NB-UVB alone. PL may be “reasonable to consider, though it can get a little pricey,” he said.
Other supplements: Nigella sativa seed oil (black seed oil) and the Ayurvedic herb Picrorhiza kurroa (also known as kutki), have shown some promise and merit further study in vitiligo, Dr. Ahmed said. Data on vitamin B12 and folate are mixed, and there is no evidence of a helpful role of zinc for vitiligo, he noted at the meeting.
Overall, there is a “paucity of large, high-quality trials for [complementary] therapies for vitiligo,” Dr. Ahmed said. “We need big randomized controlled trials ... and we need stratification. The problem is a lot of these studies don’t stratify: Is the patient active or inactive, for instance? Do they have poliosis or not?” Also missing in many studies are data on safety and adverse events. “Is that because of an excellent safety profile or lack of scientific rigor? I don’t know.”
Future approaches to vitiligo management will likely integrate alternative/nutritional modalities with conventional medical treatments, newer targeted therapies, and surgery when necessary, he said. In the case of surgery, he referred to the June 2023 Food and Drug Administration approval of the RECELL Autologous Cell Harvesting Device for repigmentation of stable depigmented vitiligo lesions, an office-based grafting procedure.
The topical Janus kinase (JAK) inhibitor ruxolitinib (Opzelura) approved in 2022 for nonsegmental vitiligo, he said, produced “good, not great” results in two pivotal phase 3 trials . At 24 weeks, about 30% of patients on the treatment achieved at least a 75% improvement in the facial Vitiligo Area Scoring Index (F-VASI75), compared with about 10% of patients in the placebo groups.
Asked to comment on antioxidant pathways and the potential of complementary therapies for vitiligo, Jason Hawkes, MD, a dermatologist in Rocklin, Calif., who also spoke at the IDS meeting, said that oxidative stress is among the processes that may contribute to melanocyte degeneration seen in vitiligo.
The immunopathogenesis of vitiligo is “multilayered and complex,” he said. “While the T lymphocyte plays a central role in this disease, there are other genetic and biologic processes [including oxidative stress] that also contribute to the destruction of melanocytes.”
Reducing oxidative stress in the body and skin via supplements such as vitamin E, coenzyme Q10, and alpha-lipoic acid “may represent complementary treatments used for the treatment of vitiligo,” said Dr. Hawkes. And as more is learned about the pathogenic role of oxidative stress and its impact on diseases of pigmentation, “therapeutic targeting of the antioxidation-related signaling pathways in the skin may represent a novel treatment for vitiligo or other related conditions.”
Dr. Hawkes disclosed ties with AbbVie, Arcutis, Bristol-Myers Squibb, Boehringer Ingelheim, Janssen, LEO, Lilly, Novartis, Pfizer, Regeneron, Sanofi, Sun Pharma, and UCB. Dr. Hawkes disclosed serving as an investigator and advisory board member for Avita and an investigator for Pfizer.
, Ammar Ahmed, MD, associate professor of dermatology at Dell Medical School at the University of Texas, Austin, said at the annual Integrative Dermatology Symposium.
Data on the use of dietary supplements for vitiligo are scarce and of limited quality, but existing studies and current understanding of the pathogenesis of vitiligo have convinced Dr. Ahmed to recommend oral Ginkgo biloba, vitamin C, vitamin E, and alpha-lipoic acid – as well as vitamin D if levels are insufficient – for patients receiving phototherapy, and outside of phototherapy when patients express interest, he said.
Melanocyte stress and subsequent autoimmune destruction appear to be “key pathways at play in vitiligo,” with melanocytes exhibiting increased susceptibility to physiologic stress, including a reduced capacity to manage exposure to reactive oxygen species. “It’s more theory than proven science, but if oxidative damage is one of the key factors [affecting] melanocytes, can we ... reverse the damage to those melanocytes with antioxidants?” he said. “I don’t know, but there’s certainly some emerging evidence that we may.”
There are no human data on the effectiveness of an antioxidant-rich diet for vitiligo, but given its theoretical basis of efficacy, it “seems reasonable to recommend,” said Dr. Ahmed. “When my patients ask me, I tell them to eat a colorful diet – with a lot of colorful fruits and vegetables.” In addition, he said, “we know that individuals with vitiligo, just as patients with psoriasis and other inflammatory disorders, appear to have a higher risk for insulin resistance and metabolic syndrome, even after accounting for confounders,” making a healthy diet all the more important.
Two case reports have described improvement with a gluten-free diet, but “that’s it,” he said. “My take is, unless stronger evidence exists, let your patients enjoy their bread.” No other specific diet has been shown to cause, exacerbate, or improve vitiligo, he noted.
Dr. Ahmed offered his views on the literature on this topic, highlighting studies that have caught his eye on antioxidants and other supplements in patients with vitiligo:
Vitamins C and E, and alpha-lipoic acid: In a randomized controlled trial of 35 patients with nonsegmental vitiligo conducted at the San Gallicano Dermatological Institute in Rome, those who received an antioxidant cocktail (alpha-lipoic acid, 100 mg; vitamin C, 100 mg; vitamin E, 40 mg; and polyunsaturated fatty acids) for 2 months before and during narrow-band ultraviolet-B (NB-UVB) therapy had significantly more repigmentation than that of patients who received NB-UVB alone. Forty-seven percent of those in the antioxidant group obtained greater than 75% repigmentation at 6 months vs. 18% in the control arm.
“This is a pretty high-quality trial. They even did in-vitro analysis showing that the antioxidant group had decreased measures of oxidative stress in the melanocytes,” Dr. Ahmed said. A handout he provided to patients receiving UVB therapy includes recommendations for vitamin C, vitamin E, and alpha-lipoic acid supplementation.
Another controlled prospective study of 130 patients with vitiligo, also conducted in Italy, utilized a different antioxidant cocktail in a tablet – Phyllanthus emblica (known as Indian gooseberry), vitamin E, and carotenoids – taken three times a day, in conjunction with standard topical therapy and phototherapy. At 6 months, a significantly higher number of patients receiving the cocktail had mild repigmentation and were less likely to have no repigmentation compared with patients who did not receive the antioxidants. “Nobody did really great, but the cocktail group did a little better,” he said. “So there’s promise.”
Vitamin D: In-vitro studies show that vitamin D may protect melanocytes against oxidative stress, and two small controlled trials showed improvement in vitiligo with vitamin D supplementation (1,500-5,000 IU daily) and no NB-UVB therapy. However, a recent, higher-quality 6-month trial that evaluated 5,000 IU/day of vitamin D in patients with generalized vitiligo showed no advantage over NB-UVB therapy alone. “I tell patients, if you’re insufficient, take vitamin D (supplements) to get your levels up,” Dr. Ahmed sad. “But if you’re already sufficient, I’m not confident there will be a significant benefit.”
Ginkgo biloba: A small double-blind controlled trial randomized 47 patients with limited and slow-spreading vitiligo to receive Ginkgo biloba extract 40 mg three times a day or placebo. At 6 months, 10 patients who received the extract had greater than 75% repigmentation compared with 2 patients in the placebo group. Patients receiving Ginkgo biloba, which has immunomodulatory and antioxidant properties, were also significantly more likely to have disease stabilization.
“I tend to recommend it to patients not doing phototherapy, as well as those receiving phototherapy, especially since the study showed benefit as a monotherapy,” Dr. Ahmed said in an interview after the meeting.
Phenylalanine: Various oral and/or topical formulations of this amino acid and precursor to tyrosine/melanin have been shown to have repigmentation effects when combined with UVA phototherapy or sunlight, but the studies are of limited quality and the oral dosages studied (50 mg/kg per day to 100 mg/kg per day) appear to be a bit high, Dr. Ahmed said at the meeting. “It can add up in cost, and I worry a little about side effects, so I don’t recommend it as much.”
Polypodium leucotomos (PL): This plant extract, from a fern native to Central America and parts of South America, is familiar as a photoprotective supplement, he said, and a few randomized controlled trials show that it may improve repigmentation outcomes, especially on the hands and neck, when combined with NB-UVB in patients with vitiligo.
One of these trials, published in 2021, showed greater than 50% repigmentation at 6 months in 48% of patients with generalized vitiligo who received oral PL (480 mg twice a day) and NB-UVB, versus 22% in patients receiving NB-UVB alone. PL may be “reasonable to consider, though it can get a little pricey,” he said.
Other supplements: Nigella sativa seed oil (black seed oil) and the Ayurvedic herb Picrorhiza kurroa (also known as kutki), have shown some promise and merit further study in vitiligo, Dr. Ahmed said. Data on vitamin B12 and folate are mixed, and there is no evidence of a helpful role of zinc for vitiligo, he noted at the meeting.
Overall, there is a “paucity of large, high-quality trials for [complementary] therapies for vitiligo,” Dr. Ahmed said. “We need big randomized controlled trials ... and we need stratification. The problem is a lot of these studies don’t stratify: Is the patient active or inactive, for instance? Do they have poliosis or not?” Also missing in many studies are data on safety and adverse events. “Is that because of an excellent safety profile or lack of scientific rigor? I don’t know.”
Future approaches to vitiligo management will likely integrate alternative/nutritional modalities with conventional medical treatments, newer targeted therapies, and surgery when necessary, he said. In the case of surgery, he referred to the June 2023 Food and Drug Administration approval of the RECELL Autologous Cell Harvesting Device for repigmentation of stable depigmented vitiligo lesions, an office-based grafting procedure.
The topical Janus kinase (JAK) inhibitor ruxolitinib (Opzelura) approved in 2022 for nonsegmental vitiligo, he said, produced “good, not great” results in two pivotal phase 3 trials . At 24 weeks, about 30% of patients on the treatment achieved at least a 75% improvement in the facial Vitiligo Area Scoring Index (F-VASI75), compared with about 10% of patients in the placebo groups.
Asked to comment on antioxidant pathways and the potential of complementary therapies for vitiligo, Jason Hawkes, MD, a dermatologist in Rocklin, Calif., who also spoke at the IDS meeting, said that oxidative stress is among the processes that may contribute to melanocyte degeneration seen in vitiligo.
The immunopathogenesis of vitiligo is “multilayered and complex,” he said. “While the T lymphocyte plays a central role in this disease, there are other genetic and biologic processes [including oxidative stress] that also contribute to the destruction of melanocytes.”
Reducing oxidative stress in the body and skin via supplements such as vitamin E, coenzyme Q10, and alpha-lipoic acid “may represent complementary treatments used for the treatment of vitiligo,” said Dr. Hawkes. And as more is learned about the pathogenic role of oxidative stress and its impact on diseases of pigmentation, “therapeutic targeting of the antioxidation-related signaling pathways in the skin may represent a novel treatment for vitiligo or other related conditions.”
Dr. Hawkes disclosed ties with AbbVie, Arcutis, Bristol-Myers Squibb, Boehringer Ingelheim, Janssen, LEO, Lilly, Novartis, Pfizer, Regeneron, Sanofi, Sun Pharma, and UCB. Dr. Hawkes disclosed serving as an investigator and advisory board member for Avita and an investigator for Pfizer.
, Ammar Ahmed, MD, associate professor of dermatology at Dell Medical School at the University of Texas, Austin, said at the annual Integrative Dermatology Symposium.
Data on the use of dietary supplements for vitiligo are scarce and of limited quality, but existing studies and current understanding of the pathogenesis of vitiligo have convinced Dr. Ahmed to recommend oral Ginkgo biloba, vitamin C, vitamin E, and alpha-lipoic acid – as well as vitamin D if levels are insufficient – for patients receiving phototherapy, and outside of phototherapy when patients express interest, he said.
Melanocyte stress and subsequent autoimmune destruction appear to be “key pathways at play in vitiligo,” with melanocytes exhibiting increased susceptibility to physiologic stress, including a reduced capacity to manage exposure to reactive oxygen species. “It’s more theory than proven science, but if oxidative damage is one of the key factors [affecting] melanocytes, can we ... reverse the damage to those melanocytes with antioxidants?” he said. “I don’t know, but there’s certainly some emerging evidence that we may.”
There are no human data on the effectiveness of an antioxidant-rich diet for vitiligo, but given its theoretical basis of efficacy, it “seems reasonable to recommend,” said Dr. Ahmed. “When my patients ask me, I tell them to eat a colorful diet – with a lot of colorful fruits and vegetables.” In addition, he said, “we know that individuals with vitiligo, just as patients with psoriasis and other inflammatory disorders, appear to have a higher risk for insulin resistance and metabolic syndrome, even after accounting for confounders,” making a healthy diet all the more important.
Two case reports have described improvement with a gluten-free diet, but “that’s it,” he said. “My take is, unless stronger evidence exists, let your patients enjoy their bread.” No other specific diet has been shown to cause, exacerbate, or improve vitiligo, he noted.
Dr. Ahmed offered his views on the literature on this topic, highlighting studies that have caught his eye on antioxidants and other supplements in patients with vitiligo:
Vitamins C and E, and alpha-lipoic acid: In a randomized controlled trial of 35 patients with nonsegmental vitiligo conducted at the San Gallicano Dermatological Institute in Rome, those who received an antioxidant cocktail (alpha-lipoic acid, 100 mg; vitamin C, 100 mg; vitamin E, 40 mg; and polyunsaturated fatty acids) for 2 months before and during narrow-band ultraviolet-B (NB-UVB) therapy had significantly more repigmentation than that of patients who received NB-UVB alone. Forty-seven percent of those in the antioxidant group obtained greater than 75% repigmentation at 6 months vs. 18% in the control arm.
“This is a pretty high-quality trial. They even did in-vitro analysis showing that the antioxidant group had decreased measures of oxidative stress in the melanocytes,” Dr. Ahmed said. A handout he provided to patients receiving UVB therapy includes recommendations for vitamin C, vitamin E, and alpha-lipoic acid supplementation.
Another controlled prospective study of 130 patients with vitiligo, also conducted in Italy, utilized a different antioxidant cocktail in a tablet – Phyllanthus emblica (known as Indian gooseberry), vitamin E, and carotenoids – taken three times a day, in conjunction with standard topical therapy and phototherapy. At 6 months, a significantly higher number of patients receiving the cocktail had mild repigmentation and were less likely to have no repigmentation compared with patients who did not receive the antioxidants. “Nobody did really great, but the cocktail group did a little better,” he said. “So there’s promise.”
Vitamin D: In-vitro studies show that vitamin D may protect melanocytes against oxidative stress, and two small controlled trials showed improvement in vitiligo with vitamin D supplementation (1,500-5,000 IU daily) and no NB-UVB therapy. However, a recent, higher-quality 6-month trial that evaluated 5,000 IU/day of vitamin D in patients with generalized vitiligo showed no advantage over NB-UVB therapy alone. “I tell patients, if you’re insufficient, take vitamin D (supplements) to get your levels up,” Dr. Ahmed sad. “But if you’re already sufficient, I’m not confident there will be a significant benefit.”
Ginkgo biloba: A small double-blind controlled trial randomized 47 patients with limited and slow-spreading vitiligo to receive Ginkgo biloba extract 40 mg three times a day or placebo. At 6 months, 10 patients who received the extract had greater than 75% repigmentation compared with 2 patients in the placebo group. Patients receiving Ginkgo biloba, which has immunomodulatory and antioxidant properties, were also significantly more likely to have disease stabilization.
“I tend to recommend it to patients not doing phototherapy, as well as those receiving phototherapy, especially since the study showed benefit as a monotherapy,” Dr. Ahmed said in an interview after the meeting.
Phenylalanine: Various oral and/or topical formulations of this amino acid and precursor to tyrosine/melanin have been shown to have repigmentation effects when combined with UVA phototherapy or sunlight, but the studies are of limited quality and the oral dosages studied (50 mg/kg per day to 100 mg/kg per day) appear to be a bit high, Dr. Ahmed said at the meeting. “It can add up in cost, and I worry a little about side effects, so I don’t recommend it as much.”
Polypodium leucotomos (PL): This plant extract, from a fern native to Central America and parts of South America, is familiar as a photoprotective supplement, he said, and a few randomized controlled trials show that it may improve repigmentation outcomes, especially on the hands and neck, when combined with NB-UVB in patients with vitiligo.
One of these trials, published in 2021, showed greater than 50% repigmentation at 6 months in 48% of patients with generalized vitiligo who received oral PL (480 mg twice a day) and NB-UVB, versus 22% in patients receiving NB-UVB alone. PL may be “reasonable to consider, though it can get a little pricey,” he said.
Other supplements: Nigella sativa seed oil (black seed oil) and the Ayurvedic herb Picrorhiza kurroa (also known as kutki), have shown some promise and merit further study in vitiligo, Dr. Ahmed said. Data on vitamin B12 and folate are mixed, and there is no evidence of a helpful role of zinc for vitiligo, he noted at the meeting.
Overall, there is a “paucity of large, high-quality trials for [complementary] therapies for vitiligo,” Dr. Ahmed said. “We need big randomized controlled trials ... and we need stratification. The problem is a lot of these studies don’t stratify: Is the patient active or inactive, for instance? Do they have poliosis or not?” Also missing in many studies are data on safety and adverse events. “Is that because of an excellent safety profile or lack of scientific rigor? I don’t know.”
Future approaches to vitiligo management will likely integrate alternative/nutritional modalities with conventional medical treatments, newer targeted therapies, and surgery when necessary, he said. In the case of surgery, he referred to the June 2023 Food and Drug Administration approval of the RECELL Autologous Cell Harvesting Device for repigmentation of stable depigmented vitiligo lesions, an office-based grafting procedure.
The topical Janus kinase (JAK) inhibitor ruxolitinib (Opzelura) approved in 2022 for nonsegmental vitiligo, he said, produced “good, not great” results in two pivotal phase 3 trials . At 24 weeks, about 30% of patients on the treatment achieved at least a 75% improvement in the facial Vitiligo Area Scoring Index (F-VASI75), compared with about 10% of patients in the placebo groups.
Asked to comment on antioxidant pathways and the potential of complementary therapies for vitiligo, Jason Hawkes, MD, a dermatologist in Rocklin, Calif., who also spoke at the IDS meeting, said that oxidative stress is among the processes that may contribute to melanocyte degeneration seen in vitiligo.
The immunopathogenesis of vitiligo is “multilayered and complex,” he said. “While the T lymphocyte plays a central role in this disease, there are other genetic and biologic processes [including oxidative stress] that also contribute to the destruction of melanocytes.”
Reducing oxidative stress in the body and skin via supplements such as vitamin E, coenzyme Q10, and alpha-lipoic acid “may represent complementary treatments used for the treatment of vitiligo,” said Dr. Hawkes. And as more is learned about the pathogenic role of oxidative stress and its impact on diseases of pigmentation, “therapeutic targeting of the antioxidation-related signaling pathways in the skin may represent a novel treatment for vitiligo or other related conditions.”
Dr. Hawkes disclosed ties with AbbVie, Arcutis, Bristol-Myers Squibb, Boehringer Ingelheim, Janssen, LEO, Lilly, Novartis, Pfizer, Regeneron, Sanofi, Sun Pharma, and UCB. Dr. Hawkes disclosed serving as an investigator and advisory board member for Avita and an investigator for Pfizer.
FROM IDS 2023
Hyperpigmented Flexural Plaques, Hypohidrosis, and Hypotrichosis
The Diagnosis: Lelis Syndrome
Histopathology revealed spongiotic dermatitis with marked acanthosis and hyperkeratosis (Figure, A) with fungal colonization of the stratum corneum (Figure, B). Our patient was diagnosed with Lelis syndrome (also referred to as ectodermal dysplasia with acanthosis nigricans syndrome), a rare condition with hypotrichosis and hypohidrosis resulting from ectodermal dysplasia.1,2 The pruritic rash was diagnosed as chronic dermatitis due to fungal colonization in the setting of acanthosis nigricans. The fungal infection was treated with a 4-week course of oral fluconazole 200 mg/wk, ketoconazole cream 2% twice daily, and discontinuation of topical steroids, resulting in the thinning of the plaques on the neck and antecubital fossae as well as resolution of the pruritus. Following antifungal treatment, our patient was started on tazarotene cream 0.1% for acanthosis nigricans.
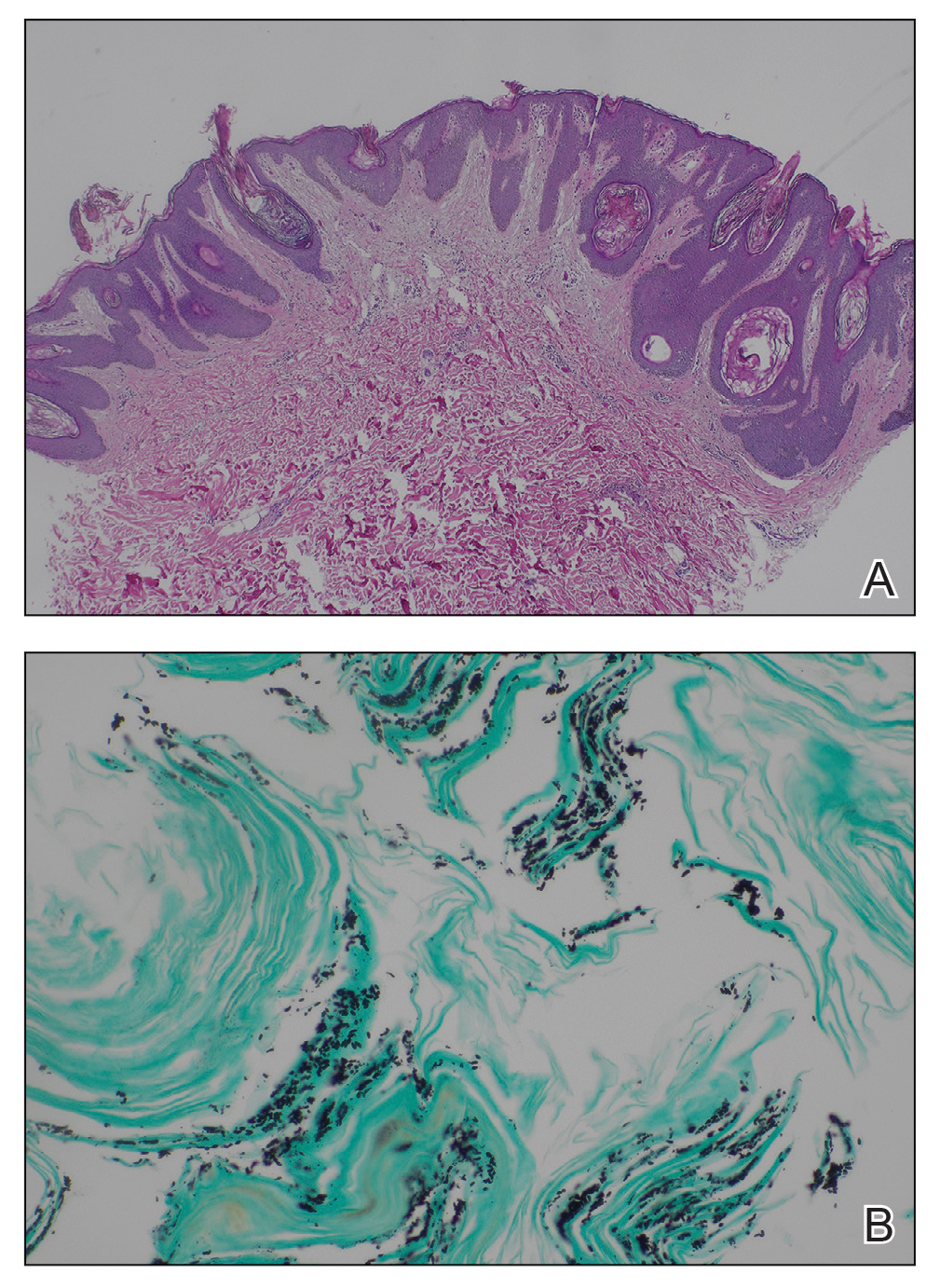
Ectodermal dysplasias are inherited disorders with abnormalities of the skin, hair, sweat glands, nails, teeth, and sometimes internal organs.3 Patients with Lelis syndrome may have other manifestations of ectodermal dysplasia in addition to hypohidrosis and hypotrichosis, including deafness and abnormal dentition,1,3 as seen in our patient. Intellectual disability has been described in many types of ectodermal dysplasia, including Lelis syndrome, but the association may be obscured by neurologic damage after repeat episodes of hyperthermia in infancy due to anhidrosis or hypohidrosis.4
When evaluating the differential diagnoses, the presence of hypotrichosis and hypohidrosis indicating ectodermal dysplasia is key. Confluent and reticulated papillomatosis presents with hyperkeratosis, papillomatosis, and focal acanthosis on histopathology. It can present on the neck and antecubital fossae; however, it is not associated with hypohidrosis and hypotrichosis.5 Although activating fibroblast growth factor receptor, FGFR, mutations have been implicated in the development of acanthosis nigricans in a variety of syndromes, these diagnoses are associated with abnormalities in skeletal development such as craniosynostosis and short stature; hypotrichosis and hypohidrosis are not seen.6,7 HAIR-AN (hyperandrogenism, insulin resistance, and acanthosis nigricans) syndrome typically presents in the prepubertal period with obesity and insulin resistance; acanthosis nigricans and alopecia can occur due to insulin resistance and hyperandrogenism, but concurrent clitoromegaly and hirsutism are common.6 Sudden onset of extensive acanthosis nigricans also is among the paraneoplastic dermatoses; it has been associated with multiple malignancies, but in these cases, hypotrichosis and hypohidrosis are not observed. Adenocarcinomas are the most common neoplasms associated with paraneoplastic acanthosis nigricans, which occurs through growth factor secretion by tumor cells stimulating hyperkeratosis and papillomatosis.6
Lelis syndrome is rare, and our case is unique because the patient had severe manifestations of acanthosis nigricans and hypotrichosis. Because the inheritance pattern and specific genetics of the condition have not been fully elucidated, the diagnosis primarily is clinical.1,8 Diagnosis may be complicated by the variety of other signs that can accompany acanthosis nigricans, hypohidrosis, and hypotrichosis.1,2 The condition also may alter or obscure presentation of other dermatologic conditions, as in our case.
Although there is no cure for Lelis syndrome, one case report described treatment with acitretin that resulted in marked improvement of the patient’s hyperkeratosis and acanthosis nigricans.9 Due to lack of health insurance coverage of acitretin, our patient was started on tazarotene cream 0.1% for acanthosis nigricans. General treatment of ectodermal dysplasia primarily consists of multidisciplinary symptom management, including careful monitoring of temperature and heat intolerance as well as provision of dental prosthetics.4,10 For ectodermal dysplasias caused by identified genetic mutations, prenatal interventions targeting gene pathways offer potentially curative treatment.10 However, for Lelis syndrome, along with many other disorders of ectodermal dysplasia, mitigation of signs and symptoms remains the primary treatment objective. Despite its rarity, increased awareness of Lelis syndrome is important to increase knowledge of ectodermal dysplasia syndromes and allow for the investigation of potential treatment options.
- Steiner CE, Cintra ML, Marques-de-Faria AP. Ectodermal dysplasia with acanthosis nigricans (Lelis syndrome). Am J Med Genet. 2002;113:381-384. doi:10.1002/ajmg.b.10787
- Lelis J. Autosomal recessive ectodermal dysplasia. Cutis. 1992; 49:435-437.
- Itin PH, Fistarol SK. Ectodermal dysplasias. Am J Med Genet C Semin Med Genet. 2004;131C:45-51. doi:10.1002/ajmg.c.30033
- Blüschke G, Nüsken KD, Schneider H. Prevalence and prevention of severe complications of hypohidrotic ectodermal dysplasia in infancy. Early Hum Dev. 2010;86:397-399. doi:10.1016/j .earlhumdev.2010.04.008
- Le C, Bedocs PM. Confluent and reticulated papillomatosis. StatPearls. StatPearls Publishing; 2022. http://www.ncbi.nlm.nih.gov/books/NBK459130/
- Das A, Datta D, Kassir M, et al. Acanthosis nigricans: a review. J Cosmet Dermatol. 2020;19:1857-1865. doi:10.1111/jocd.13544
- Torley D, Bellus GA, Munro CS. Genes, growth factors and acanthosis nigricans. Br J Dermatol. 2002;147:1096-1101. doi:10 .1046/j.1365-2133.2002.05150.x
- van Steensel MAM, van der Hout AH. Lelis syndrome may be a manifestation of hypohidrotic ectodermal dysplasia. Am J Med Genet A. 2009;149A:1612-1613. doi:10.1002/ajmg.a.32945
- Yoshimura AM, Neves Ferreira Velho PE, Ferreira Magalhães R, et al. Lelis’ syndrome: treatment with acitretin. Int J Dermatol. 2008;47: 1330-1331. doi:10.1111/j.1365-4632.2008.03874.x
- Schneider H. Ectodermal dysplasias: new perspectives on the treatment of so far immedicable genetic disorders. Front Genet. 2022;13:1000744. doi:10.3389/fgene.2022.1000744
The Diagnosis: Lelis Syndrome
Histopathology revealed spongiotic dermatitis with marked acanthosis and hyperkeratosis (Figure, A) with fungal colonization of the stratum corneum (Figure, B). Our patient was diagnosed with Lelis syndrome (also referred to as ectodermal dysplasia with acanthosis nigricans syndrome), a rare condition with hypotrichosis and hypohidrosis resulting from ectodermal dysplasia.1,2 The pruritic rash was diagnosed as chronic dermatitis due to fungal colonization in the setting of acanthosis nigricans. The fungal infection was treated with a 4-week course of oral fluconazole 200 mg/wk, ketoconazole cream 2% twice daily, and discontinuation of topical steroids, resulting in the thinning of the plaques on the neck and antecubital fossae as well as resolution of the pruritus. Following antifungal treatment, our patient was started on tazarotene cream 0.1% for acanthosis nigricans.

Ectodermal dysplasias are inherited disorders with abnormalities of the skin, hair, sweat glands, nails, teeth, and sometimes internal organs.3 Patients with Lelis syndrome may have other manifestations of ectodermal dysplasia in addition to hypohidrosis and hypotrichosis, including deafness and abnormal dentition,1,3 as seen in our patient. Intellectual disability has been described in many types of ectodermal dysplasia, including Lelis syndrome, but the association may be obscured by neurologic damage after repeat episodes of hyperthermia in infancy due to anhidrosis or hypohidrosis.4
When evaluating the differential diagnoses, the presence of hypotrichosis and hypohidrosis indicating ectodermal dysplasia is key. Confluent and reticulated papillomatosis presents with hyperkeratosis, papillomatosis, and focal acanthosis on histopathology. It can present on the neck and antecubital fossae; however, it is not associated with hypohidrosis and hypotrichosis.5 Although activating fibroblast growth factor receptor, FGFR, mutations have been implicated in the development of acanthosis nigricans in a variety of syndromes, these diagnoses are associated with abnormalities in skeletal development such as craniosynostosis and short stature; hypotrichosis and hypohidrosis are not seen.6,7 HAIR-AN (hyperandrogenism, insulin resistance, and acanthosis nigricans) syndrome typically presents in the prepubertal period with obesity and insulin resistance; acanthosis nigricans and alopecia can occur due to insulin resistance and hyperandrogenism, but concurrent clitoromegaly and hirsutism are common.6 Sudden onset of extensive acanthosis nigricans also is among the paraneoplastic dermatoses; it has been associated with multiple malignancies, but in these cases, hypotrichosis and hypohidrosis are not observed. Adenocarcinomas are the most common neoplasms associated with paraneoplastic acanthosis nigricans, which occurs through growth factor secretion by tumor cells stimulating hyperkeratosis and papillomatosis.6
Lelis syndrome is rare, and our case is unique because the patient had severe manifestations of acanthosis nigricans and hypotrichosis. Because the inheritance pattern and specific genetics of the condition have not been fully elucidated, the diagnosis primarily is clinical.1,8 Diagnosis may be complicated by the variety of other signs that can accompany acanthosis nigricans, hypohidrosis, and hypotrichosis.1,2 The condition also may alter or obscure presentation of other dermatologic conditions, as in our case.
Although there is no cure for Lelis syndrome, one case report described treatment with acitretin that resulted in marked improvement of the patient’s hyperkeratosis and acanthosis nigricans.9 Due to lack of health insurance coverage of acitretin, our patient was started on tazarotene cream 0.1% for acanthosis nigricans. General treatment of ectodermal dysplasia primarily consists of multidisciplinary symptom management, including careful monitoring of temperature and heat intolerance as well as provision of dental prosthetics.4,10 For ectodermal dysplasias caused by identified genetic mutations, prenatal interventions targeting gene pathways offer potentially curative treatment.10 However, for Lelis syndrome, along with many other disorders of ectodermal dysplasia, mitigation of signs and symptoms remains the primary treatment objective. Despite its rarity, increased awareness of Lelis syndrome is important to increase knowledge of ectodermal dysplasia syndromes and allow for the investigation of potential treatment options.
The Diagnosis: Lelis Syndrome
Histopathology revealed spongiotic dermatitis with marked acanthosis and hyperkeratosis (Figure, A) with fungal colonization of the stratum corneum (Figure, B). Our patient was diagnosed with Lelis syndrome (also referred to as ectodermal dysplasia with acanthosis nigricans syndrome), a rare condition with hypotrichosis and hypohidrosis resulting from ectodermal dysplasia.1,2 The pruritic rash was diagnosed as chronic dermatitis due to fungal colonization in the setting of acanthosis nigricans. The fungal infection was treated with a 4-week course of oral fluconazole 200 mg/wk, ketoconazole cream 2% twice daily, and discontinuation of topical steroids, resulting in the thinning of the plaques on the neck and antecubital fossae as well as resolution of the pruritus. Following antifungal treatment, our patient was started on tazarotene cream 0.1% for acanthosis nigricans.

Ectodermal dysplasias are inherited disorders with abnormalities of the skin, hair, sweat glands, nails, teeth, and sometimes internal organs.3 Patients with Lelis syndrome may have other manifestations of ectodermal dysplasia in addition to hypohidrosis and hypotrichosis, including deafness and abnormal dentition,1,3 as seen in our patient. Intellectual disability has been described in many types of ectodermal dysplasia, including Lelis syndrome, but the association may be obscured by neurologic damage after repeat episodes of hyperthermia in infancy due to anhidrosis or hypohidrosis.4
When evaluating the differential diagnoses, the presence of hypotrichosis and hypohidrosis indicating ectodermal dysplasia is key. Confluent and reticulated papillomatosis presents with hyperkeratosis, papillomatosis, and focal acanthosis on histopathology. It can present on the neck and antecubital fossae; however, it is not associated with hypohidrosis and hypotrichosis.5 Although activating fibroblast growth factor receptor, FGFR, mutations have been implicated in the development of acanthosis nigricans in a variety of syndromes, these diagnoses are associated with abnormalities in skeletal development such as craniosynostosis and short stature; hypotrichosis and hypohidrosis are not seen.6,7 HAIR-AN (hyperandrogenism, insulin resistance, and acanthosis nigricans) syndrome typically presents in the prepubertal period with obesity and insulin resistance; acanthosis nigricans and alopecia can occur due to insulin resistance and hyperandrogenism, but concurrent clitoromegaly and hirsutism are common.6 Sudden onset of extensive acanthosis nigricans also is among the paraneoplastic dermatoses; it has been associated with multiple malignancies, but in these cases, hypotrichosis and hypohidrosis are not observed. Adenocarcinomas are the most common neoplasms associated with paraneoplastic acanthosis nigricans, which occurs through growth factor secretion by tumor cells stimulating hyperkeratosis and papillomatosis.6
Lelis syndrome is rare, and our case is unique because the patient had severe manifestations of acanthosis nigricans and hypotrichosis. Because the inheritance pattern and specific genetics of the condition have not been fully elucidated, the diagnosis primarily is clinical.1,8 Diagnosis may be complicated by the variety of other signs that can accompany acanthosis nigricans, hypohidrosis, and hypotrichosis.1,2 The condition also may alter or obscure presentation of other dermatologic conditions, as in our case.
Although there is no cure for Lelis syndrome, one case report described treatment with acitretin that resulted in marked improvement of the patient’s hyperkeratosis and acanthosis nigricans.9 Due to lack of health insurance coverage of acitretin, our patient was started on tazarotene cream 0.1% for acanthosis nigricans. General treatment of ectodermal dysplasia primarily consists of multidisciplinary symptom management, including careful monitoring of temperature and heat intolerance as well as provision of dental prosthetics.4,10 For ectodermal dysplasias caused by identified genetic mutations, prenatal interventions targeting gene pathways offer potentially curative treatment.10 However, for Lelis syndrome, along with many other disorders of ectodermal dysplasia, mitigation of signs and symptoms remains the primary treatment objective. Despite its rarity, increased awareness of Lelis syndrome is important to increase knowledge of ectodermal dysplasia syndromes and allow for the investigation of potential treatment options.
- Steiner CE, Cintra ML, Marques-de-Faria AP. Ectodermal dysplasia with acanthosis nigricans (Lelis syndrome). Am J Med Genet. 2002;113:381-384. doi:10.1002/ajmg.b.10787
- Lelis J. Autosomal recessive ectodermal dysplasia. Cutis. 1992; 49:435-437.
- Itin PH, Fistarol SK. Ectodermal dysplasias. Am J Med Genet C Semin Med Genet. 2004;131C:45-51. doi:10.1002/ajmg.c.30033
- Blüschke G, Nüsken KD, Schneider H. Prevalence and prevention of severe complications of hypohidrotic ectodermal dysplasia in infancy. Early Hum Dev. 2010;86:397-399. doi:10.1016/j .earlhumdev.2010.04.008
- Le C, Bedocs PM. Confluent and reticulated papillomatosis. StatPearls. StatPearls Publishing; 2022. http://www.ncbi.nlm.nih.gov/books/NBK459130/
- Das A, Datta D, Kassir M, et al. Acanthosis nigricans: a review. J Cosmet Dermatol. 2020;19:1857-1865. doi:10.1111/jocd.13544
- Torley D, Bellus GA, Munro CS. Genes, growth factors and acanthosis nigricans. Br J Dermatol. 2002;147:1096-1101. doi:10 .1046/j.1365-2133.2002.05150.x
- van Steensel MAM, van der Hout AH. Lelis syndrome may be a manifestation of hypohidrotic ectodermal dysplasia. Am J Med Genet A. 2009;149A:1612-1613. doi:10.1002/ajmg.a.32945
- Yoshimura AM, Neves Ferreira Velho PE, Ferreira Magalhães R, et al. Lelis’ syndrome: treatment with acitretin. Int J Dermatol. 2008;47: 1330-1331. doi:10.1111/j.1365-4632.2008.03874.x
- Schneider H. Ectodermal dysplasias: new perspectives on the treatment of so far immedicable genetic disorders. Front Genet. 2022;13:1000744. doi:10.3389/fgene.2022.1000744
- Steiner CE, Cintra ML, Marques-de-Faria AP. Ectodermal dysplasia with acanthosis nigricans (Lelis syndrome). Am J Med Genet. 2002;113:381-384. doi:10.1002/ajmg.b.10787
- Lelis J. Autosomal recessive ectodermal dysplasia. Cutis. 1992; 49:435-437.
- Itin PH, Fistarol SK. Ectodermal dysplasias. Am J Med Genet C Semin Med Genet. 2004;131C:45-51. doi:10.1002/ajmg.c.30033
- Blüschke G, Nüsken KD, Schneider H. Prevalence and prevention of severe complications of hypohidrotic ectodermal dysplasia in infancy. Early Hum Dev. 2010;86:397-399. doi:10.1016/j .earlhumdev.2010.04.008
- Le C, Bedocs PM. Confluent and reticulated papillomatosis. StatPearls. StatPearls Publishing; 2022. http://www.ncbi.nlm.nih.gov/books/NBK459130/
- Das A, Datta D, Kassir M, et al. Acanthosis nigricans: a review. J Cosmet Dermatol. 2020;19:1857-1865. doi:10.1111/jocd.13544
- Torley D, Bellus GA, Munro CS. Genes, growth factors and acanthosis nigricans. Br J Dermatol. 2002;147:1096-1101. doi:10 .1046/j.1365-2133.2002.05150.x
- van Steensel MAM, van der Hout AH. Lelis syndrome may be a manifestation of hypohidrotic ectodermal dysplasia. Am J Med Genet A. 2009;149A:1612-1613. doi:10.1002/ajmg.a.32945
- Yoshimura AM, Neves Ferreira Velho PE, Ferreira Magalhães R, et al. Lelis’ syndrome: treatment with acitretin. Int J Dermatol. 2008;47: 1330-1331. doi:10.1111/j.1365-4632.2008.03874.x
- Schneider H. Ectodermal dysplasias: new perspectives on the treatment of so far immedicable genetic disorders. Front Genet. 2022;13:1000744. doi:10.3389/fgene.2022.1000744
A 61-year-old woman with a history of hypohidrosis and deafness presented with a pruritic rash on the neck and antecubital fossae of several years’ duration. Prior treatment with topical corticosteroids failed to resolve the rash. Physical examination revealed thick, velvety, hyperpigmented plaques on the inframammary folds, axillae, groin, posterior neck, and antecubital fossae with lichenification of the latter 2 areas. Many pedunculated papules were seen on the face, chest, shoulders, and trunk, as well as diffuse hair thinning, particularly of the frontal and vertex scalp. Eyebrows, eyelashes, and axillary hair were absent. Two 5-mm punch biopsies of the antecubital fossa and inframammary fold were obtained for histopathologic analysis.