User login
Blue to Slate Gray Discoloration of the Proximal Fingernails
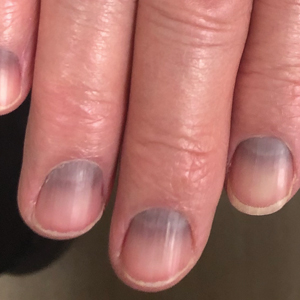
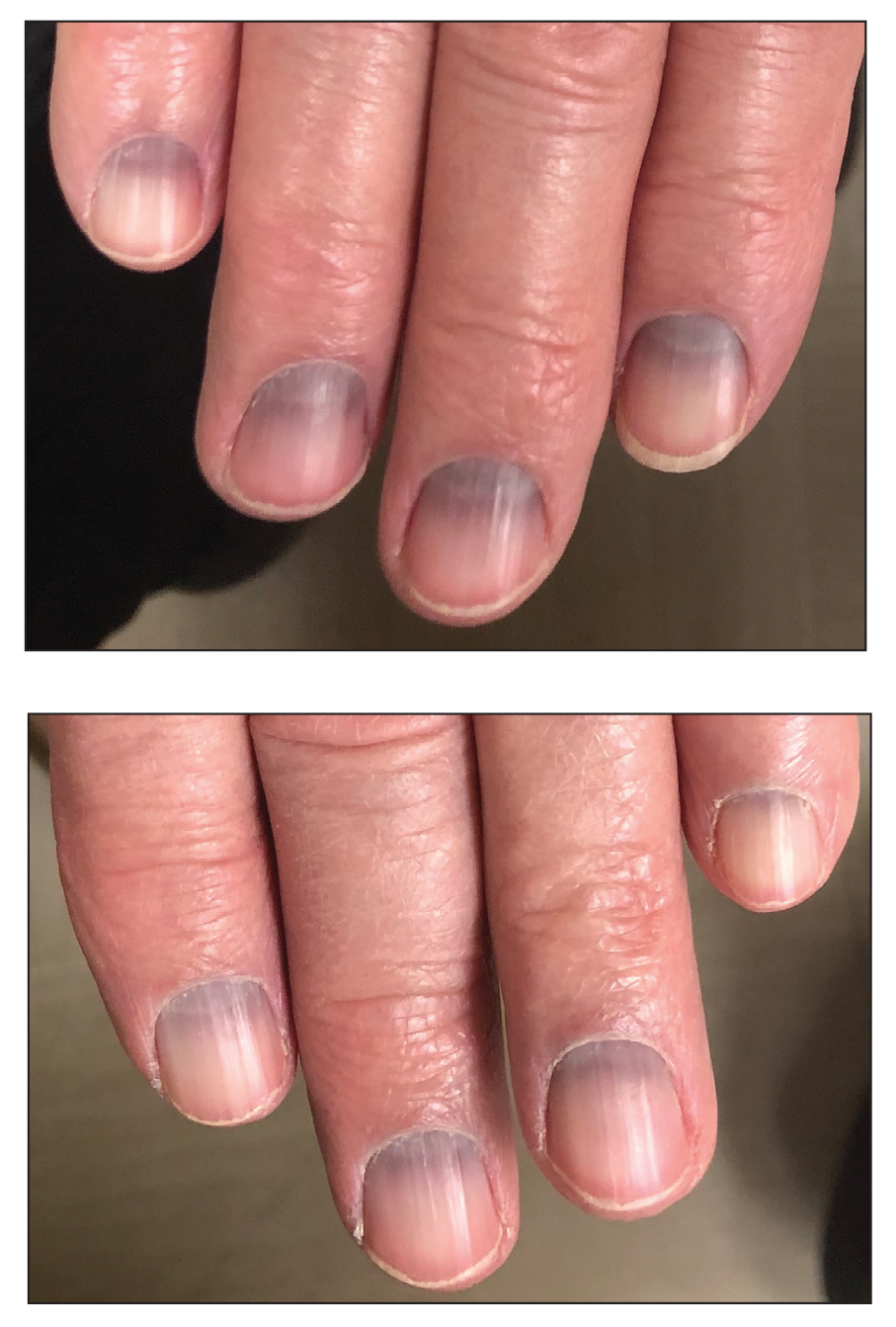
The Diagnosis: Argyria-Induced Azure Lunulae
Argyria is an acquired condition resulting from excessive exogenous exposure to silver with subsequent gastrointestinal absorption and pigmentary tissue deposition. Upon further questioning, our patient disclosed a lifetime history of colloidal silver use, both as a topical antiseptic agent and intraorally for aphthous ulcers. Silver has a predilection for granular deposition in stromal tissues and basement membranes with sparing of the epidermis, manifesting as progressive, permanent, blue to slate gray discoloration of sunexposed skin, mucous membranes, and nail beds.1 The patient was advised to discontinue use of colloidal silver to avoid development of further pigmentary changes. The appearance of his nails remained unchanged in the months following initial presentation, as expected, since argyria pigmentation is not anticipated to reverse upon colloidal silver cessation.
Nail involvement may be an early presentation of generalized argyria or may be found in isolation, as seen in our patient. Early recognition and patient education are essential to minimize cumulative silver deposition. Although dyspigmentation may impact psychosocial well-being secondary to aesthetic concerns, there is limited research supporting adverse systemic effects of argyria confined to the nail beds. Similarly, the majority of generalized cases are not associated with systemic complications; however, potential toxicities, as described in isolated case reports without conclusive causal relationships, include nyctalopia, renal or hepatic toxicity, pulmonary fibrosis, and neuropsychiatric events.1-6 Successful treatment of cutaneous argyria has been reported with the 1064-nm Q-switched Nd:YAG laser; however, there have been no reported treatments for nail bed involvement.7 Due to the absence of systemic symptoms, additional mucocutaneous dyspigmentation, or cosmetic concerns regarding nail bed lunulae discoloration in our patient, no further intervention was pursued, except for continued colloidal silver cessation.
The differential diagnosis of blue-gray nail bed dyspigmentation is broad and includes cyanosis secondary to cardiopulmonary disease, drug-induced dyspigmentation, Wilson disease, argyria, chrysiasis, hereditary acrolabial telangiectasia, and pseudomonal infection or chloronychia.1,8,9 Etiologic insight may be provided from a thorough review of prescription and over-the-counter medications as well as careful attention to the distribution of dyspigmentation. Medications commonly associated with bluish nail bed dyspigmentation include antimalarials, amiodarone, minocycline, clofazimine, chlorpromazine/phenothiazines, and various chemotherapeutic drugs; our patient was not taking any of these.1,9
Cyanotic nail bed dyspigmentation secondary to cardiopulmonary disease likely manifests with more diffuse nail bed dyspigmentation and is not confined solely to the lunulae. Only drug-induced dyspigmentation, classically due to phenolphthalein-containing laxatives; Wilson disease; and argyria have a tendency to spare the distal nail bed, which is a presentation termed azure lunulae.8 The toenails typically are spared in argyria, while toenail involvement is variable in Wilson disease, and additional systemic symptoms—including hepatic, ophthalmologic, and neuropsychiatric—as well as potential family history would be expected.8 Phenolphthalein is no longer available in over-the-counter laxatives, as it was formally banned by the US Food and Drug Administration in 1999 due to concerns of carcinogenicity.10
Hereditary acrolabial telangiectasia is a familial condition with autosomal-dominant inheritance that can manifest similarly to argyria with blue-gray discoloration of the proximal nail bed; however, this condition also would demonstrate involvement of the vermilion border and nipple areolae, often with associated telangiectasia and migraine headaches.11
Chloronychia (also known as green nail syndrome) is an infection of the nail bed with Pseudomonas aeruginosa that more commonly presents with greenblack discoloration with variable involvement of the fingernails and toenails. Chloronychia, often with associated onycholysis, typically is found in individuals with repeated exposure to water, soaps, and detergents.12 Our patient’s long-standing and unwavering nail bed appearance, involvement of all fingernail lunulae, lack of additional symptoms, and disclosed use of over-the-counter colloidal silver supported a clinical diagnosis of argyriainduced azure lunulae.
Argyria-induced azure lunulae secondary to colloidal silver exposure is an uncommon yet clinically significant cause of nail bed dyspigmentation. Prompt identification and cessation of the offending agent can prevent progression of mucocutaneous dyspigmentation and avoid potential long-term sequelae from systemic deposition.
- Mota L, Dinis-Oliveira RJ. Clinical and forensic aspects of the different subtypes of argyria. J Clin Med. 2021;10:2086. doi:10.3390/ jcm10102086
- Osin´ska J, Poborc-Godlewska J, Kiec´-Swierczyn´ska M, et al. 6 cases of argyria among workers engaged in silverplating radio subunits. Med Pr. 1982;33:361-364.
- Mayr M, Kim MJ, Wanner D, et al. Argyria and decreased kidney function: are silver compounds toxic to the kidney? Am J Kidney Dis. 2009;53:890-894. doi:10.1053/j.ajkd.2008.08.028
- Trop M, Novak M, Rodl S, et al. Silver-coated dressing acticoat caused raised liver enzymes and argyria-like symptoms in burn patient. J Trauma. 2006;60:648-652. doi:10.1097/01.ta.0000208126 .22089.b6
- Mirsattari SM, Hammond RR, Sharpe MD, et al. Myoclonic status epilepticus following repeated oral ingestion of colloidal silver. Neurology. 2004;62:1408-1410. doi:10.1212/01.wnl.0000120671.73335.ec
- Barrie HJ, Harding HE. Argyro-siderosis of the lungs in silver finishers. Br J Ind Med. 1947;4:225-229. doi:10.1136/oem.4.4.225
- Griffith RD, Simmons BJ, Bray FN, et al. 1064 nm Q-switched Nd:YAG laser for the treatment of argyria: a systematic review. J Eur Acad Dermatol Venereol. 2015;29:2100-2103. doi:10.111 1/jdv.13117
- Rubin AI, Jellinek NJ, Daniel CR III, et al, eds. Scher and Daniel’s Nails: Diagnosis, Surgery, Therapy. 4th ed. Springer; 2018.
- Slater K, Sommariva E, Kartono F. A case study of argyria of the nails secondary to colloidal silver ingestion [published online October 28, 2022]. Cureus. 2022;14:E30818. doi:10.7759/cureus.30818
- Hubbard WK. Laxative drug products for over-the-counter human use. Fed Register. 1999;64:4535-4540. Accessed January 5, 2024. https://www.govinfo.gov/content/pkg/FR-1999-01-29/html/99-1938.htm
- Millns JL, Dicken CH. Hereditary acrolabial telangiectasia. a report of familial blue lips, nails, and nipples. Arch Dermatol. 1979;115:474-478. doi:10.1001/archderm.115.4.474
- Chiriac A, Brzezinski P, Foia L, et al. Chloronychia: green nail syndrome caused by Pseudomonas aeruginosa in elderly persons [published online January 14, 2015]. Clin Interv Aging. 2015;10:265-267. doi:10.2147/CIA.S75525
The Diagnosis: Argyria-Induced Azure Lunulae
Argyria is an acquired condition resulting from excessive exogenous exposure to silver with subsequent gastrointestinal absorption and pigmentary tissue deposition. Upon further questioning, our patient disclosed a lifetime history of colloidal silver use, both as a topical antiseptic agent and intraorally for aphthous ulcers. Silver has a predilection for granular deposition in stromal tissues and basement membranes with sparing of the epidermis, manifesting as progressive, permanent, blue to slate gray discoloration of sunexposed skin, mucous membranes, and nail beds.1 The patient was advised to discontinue use of colloidal silver to avoid development of further pigmentary changes. The appearance of his nails remained unchanged in the months following initial presentation, as expected, since argyria pigmentation is not anticipated to reverse upon colloidal silver cessation.
Nail involvement may be an early presentation of generalized argyria or may be found in isolation, as seen in our patient. Early recognition and patient education are essential to minimize cumulative silver deposition. Although dyspigmentation may impact psychosocial well-being secondary to aesthetic concerns, there is limited research supporting adverse systemic effects of argyria confined to the nail beds. Similarly, the majority of generalized cases are not associated with systemic complications; however, potential toxicities, as described in isolated case reports without conclusive causal relationships, include nyctalopia, renal or hepatic toxicity, pulmonary fibrosis, and neuropsychiatric events.1-6 Successful treatment of cutaneous argyria has been reported with the 1064-nm Q-switched Nd:YAG laser; however, there have been no reported treatments for nail bed involvement.7 Due to the absence of systemic symptoms, additional mucocutaneous dyspigmentation, or cosmetic concerns regarding nail bed lunulae discoloration in our patient, no further intervention was pursued, except for continued colloidal silver cessation.
The differential diagnosis of blue-gray nail bed dyspigmentation is broad and includes cyanosis secondary to cardiopulmonary disease, drug-induced dyspigmentation, Wilson disease, argyria, chrysiasis, hereditary acrolabial telangiectasia, and pseudomonal infection or chloronychia.1,8,9 Etiologic insight may be provided from a thorough review of prescription and over-the-counter medications as well as careful attention to the distribution of dyspigmentation. Medications commonly associated with bluish nail bed dyspigmentation include antimalarials, amiodarone, minocycline, clofazimine, chlorpromazine/phenothiazines, and various chemotherapeutic drugs; our patient was not taking any of these.1,9
Cyanotic nail bed dyspigmentation secondary to cardiopulmonary disease likely manifests with more diffuse nail bed dyspigmentation and is not confined solely to the lunulae. Only drug-induced dyspigmentation, classically due to phenolphthalein-containing laxatives; Wilson disease; and argyria have a tendency to spare the distal nail bed, which is a presentation termed azure lunulae.8 The toenails typically are spared in argyria, while toenail involvement is variable in Wilson disease, and additional systemic symptoms—including hepatic, ophthalmologic, and neuropsychiatric—as well as potential family history would be expected.8 Phenolphthalein is no longer available in over-the-counter laxatives, as it was formally banned by the US Food and Drug Administration in 1999 due to concerns of carcinogenicity.10
Hereditary acrolabial telangiectasia is a familial condition with autosomal-dominant inheritance that can manifest similarly to argyria with blue-gray discoloration of the proximal nail bed; however, this condition also would demonstrate involvement of the vermilion border and nipple areolae, often with associated telangiectasia and migraine headaches.11
Chloronychia (also known as green nail syndrome) is an infection of the nail bed with Pseudomonas aeruginosa that more commonly presents with greenblack discoloration with variable involvement of the fingernails and toenails. Chloronychia, often with associated onycholysis, typically is found in individuals with repeated exposure to water, soaps, and detergents.12 Our patient’s long-standing and unwavering nail bed appearance, involvement of all fingernail lunulae, lack of additional symptoms, and disclosed use of over-the-counter colloidal silver supported a clinical diagnosis of argyriainduced azure lunulae.
Argyria-induced azure lunulae secondary to colloidal silver exposure is an uncommon yet clinically significant cause of nail bed dyspigmentation. Prompt identification and cessation of the offending agent can prevent progression of mucocutaneous dyspigmentation and avoid potential long-term sequelae from systemic deposition.
The Diagnosis: Argyria-Induced Azure Lunulae
Argyria is an acquired condition resulting from excessive exogenous exposure to silver with subsequent gastrointestinal absorption and pigmentary tissue deposition. Upon further questioning, our patient disclosed a lifetime history of colloidal silver use, both as a topical antiseptic agent and intraorally for aphthous ulcers. Silver has a predilection for granular deposition in stromal tissues and basement membranes with sparing of the epidermis, manifesting as progressive, permanent, blue to slate gray discoloration of sunexposed skin, mucous membranes, and nail beds.1 The patient was advised to discontinue use of colloidal silver to avoid development of further pigmentary changes. The appearance of his nails remained unchanged in the months following initial presentation, as expected, since argyria pigmentation is not anticipated to reverse upon colloidal silver cessation.
Nail involvement may be an early presentation of generalized argyria or may be found in isolation, as seen in our patient. Early recognition and patient education are essential to minimize cumulative silver deposition. Although dyspigmentation may impact psychosocial well-being secondary to aesthetic concerns, there is limited research supporting adverse systemic effects of argyria confined to the nail beds. Similarly, the majority of generalized cases are not associated with systemic complications; however, potential toxicities, as described in isolated case reports without conclusive causal relationships, include nyctalopia, renal or hepatic toxicity, pulmonary fibrosis, and neuropsychiatric events.1-6 Successful treatment of cutaneous argyria has been reported with the 1064-nm Q-switched Nd:YAG laser; however, there have been no reported treatments for nail bed involvement.7 Due to the absence of systemic symptoms, additional mucocutaneous dyspigmentation, or cosmetic concerns regarding nail bed lunulae discoloration in our patient, no further intervention was pursued, except for continued colloidal silver cessation.
The differential diagnosis of blue-gray nail bed dyspigmentation is broad and includes cyanosis secondary to cardiopulmonary disease, drug-induced dyspigmentation, Wilson disease, argyria, chrysiasis, hereditary acrolabial telangiectasia, and pseudomonal infection or chloronychia.1,8,9 Etiologic insight may be provided from a thorough review of prescription and over-the-counter medications as well as careful attention to the distribution of dyspigmentation. Medications commonly associated with bluish nail bed dyspigmentation include antimalarials, amiodarone, minocycline, clofazimine, chlorpromazine/phenothiazines, and various chemotherapeutic drugs; our patient was not taking any of these.1,9
Cyanotic nail bed dyspigmentation secondary to cardiopulmonary disease likely manifests with more diffuse nail bed dyspigmentation and is not confined solely to the lunulae. Only drug-induced dyspigmentation, classically due to phenolphthalein-containing laxatives; Wilson disease; and argyria have a tendency to spare the distal nail bed, which is a presentation termed azure lunulae.8 The toenails typically are spared in argyria, while toenail involvement is variable in Wilson disease, and additional systemic symptoms—including hepatic, ophthalmologic, and neuropsychiatric—as well as potential family history would be expected.8 Phenolphthalein is no longer available in over-the-counter laxatives, as it was formally banned by the US Food and Drug Administration in 1999 due to concerns of carcinogenicity.10
Hereditary acrolabial telangiectasia is a familial condition with autosomal-dominant inheritance that can manifest similarly to argyria with blue-gray discoloration of the proximal nail bed; however, this condition also would demonstrate involvement of the vermilion border and nipple areolae, often with associated telangiectasia and migraine headaches.11
Chloronychia (also known as green nail syndrome) is an infection of the nail bed with Pseudomonas aeruginosa that more commonly presents with greenblack discoloration with variable involvement of the fingernails and toenails. Chloronychia, often with associated onycholysis, typically is found in individuals with repeated exposure to water, soaps, and detergents.12 Our patient’s long-standing and unwavering nail bed appearance, involvement of all fingernail lunulae, lack of additional symptoms, and disclosed use of over-the-counter colloidal silver supported a clinical diagnosis of argyriainduced azure lunulae.
Argyria-induced azure lunulae secondary to colloidal silver exposure is an uncommon yet clinically significant cause of nail bed dyspigmentation. Prompt identification and cessation of the offending agent can prevent progression of mucocutaneous dyspigmentation and avoid potential long-term sequelae from systemic deposition.
- Mota L, Dinis-Oliveira RJ. Clinical and forensic aspects of the different subtypes of argyria. J Clin Med. 2021;10:2086. doi:10.3390/ jcm10102086
- Osin´ska J, Poborc-Godlewska J, Kiec´-Swierczyn´ska M, et al. 6 cases of argyria among workers engaged in silverplating radio subunits. Med Pr. 1982;33:361-364.
- Mayr M, Kim MJ, Wanner D, et al. Argyria and decreased kidney function: are silver compounds toxic to the kidney? Am J Kidney Dis. 2009;53:890-894. doi:10.1053/j.ajkd.2008.08.028
- Trop M, Novak M, Rodl S, et al. Silver-coated dressing acticoat caused raised liver enzymes and argyria-like symptoms in burn patient. J Trauma. 2006;60:648-652. doi:10.1097/01.ta.0000208126 .22089.b6
- Mirsattari SM, Hammond RR, Sharpe MD, et al. Myoclonic status epilepticus following repeated oral ingestion of colloidal silver. Neurology. 2004;62:1408-1410. doi:10.1212/01.wnl.0000120671.73335.ec
- Barrie HJ, Harding HE. Argyro-siderosis of the lungs in silver finishers. Br J Ind Med. 1947;4:225-229. doi:10.1136/oem.4.4.225
- Griffith RD, Simmons BJ, Bray FN, et al. 1064 nm Q-switched Nd:YAG laser for the treatment of argyria: a systematic review. J Eur Acad Dermatol Venereol. 2015;29:2100-2103. doi:10.111 1/jdv.13117
- Rubin AI, Jellinek NJ, Daniel CR III, et al, eds. Scher and Daniel’s Nails: Diagnosis, Surgery, Therapy. 4th ed. Springer; 2018.
- Slater K, Sommariva E, Kartono F. A case study of argyria of the nails secondary to colloidal silver ingestion [published online October 28, 2022]. Cureus. 2022;14:E30818. doi:10.7759/cureus.30818
- Hubbard WK. Laxative drug products for over-the-counter human use. Fed Register. 1999;64:4535-4540. Accessed January 5, 2024. https://www.govinfo.gov/content/pkg/FR-1999-01-29/html/99-1938.htm
- Millns JL, Dicken CH. Hereditary acrolabial telangiectasia. a report of familial blue lips, nails, and nipples. Arch Dermatol. 1979;115:474-478. doi:10.1001/archderm.115.4.474
- Chiriac A, Brzezinski P, Foia L, et al. Chloronychia: green nail syndrome caused by Pseudomonas aeruginosa in elderly persons [published online January 14, 2015]. Clin Interv Aging. 2015;10:265-267. doi:10.2147/CIA.S75525
- Mota L, Dinis-Oliveira RJ. Clinical and forensic aspects of the different subtypes of argyria. J Clin Med. 2021;10:2086. doi:10.3390/ jcm10102086
- Osin´ska J, Poborc-Godlewska J, Kiec´-Swierczyn´ska M, et al. 6 cases of argyria among workers engaged in silverplating radio subunits. Med Pr. 1982;33:361-364.
- Mayr M, Kim MJ, Wanner D, et al. Argyria and decreased kidney function: are silver compounds toxic to the kidney? Am J Kidney Dis. 2009;53:890-894. doi:10.1053/j.ajkd.2008.08.028
- Trop M, Novak M, Rodl S, et al. Silver-coated dressing acticoat caused raised liver enzymes and argyria-like symptoms in burn patient. J Trauma. 2006;60:648-652. doi:10.1097/01.ta.0000208126 .22089.b6
- Mirsattari SM, Hammond RR, Sharpe MD, et al. Myoclonic status epilepticus following repeated oral ingestion of colloidal silver. Neurology. 2004;62:1408-1410. doi:10.1212/01.wnl.0000120671.73335.ec
- Barrie HJ, Harding HE. Argyro-siderosis of the lungs in silver finishers. Br J Ind Med. 1947;4:225-229. doi:10.1136/oem.4.4.225
- Griffith RD, Simmons BJ, Bray FN, et al. 1064 nm Q-switched Nd:YAG laser for the treatment of argyria: a systematic review. J Eur Acad Dermatol Venereol. 2015;29:2100-2103. doi:10.111 1/jdv.13117
- Rubin AI, Jellinek NJ, Daniel CR III, et al, eds. Scher and Daniel’s Nails: Diagnosis, Surgery, Therapy. 4th ed. Springer; 2018.
- Slater K, Sommariva E, Kartono F. A case study of argyria of the nails secondary to colloidal silver ingestion [published online October 28, 2022]. Cureus. 2022;14:E30818. doi:10.7759/cureus.30818
- Hubbard WK. Laxative drug products for over-the-counter human use. Fed Register. 1999;64:4535-4540. Accessed January 5, 2024. https://www.govinfo.gov/content/pkg/FR-1999-01-29/html/99-1938.htm
- Millns JL, Dicken CH. Hereditary acrolabial telangiectasia. a report of familial blue lips, nails, and nipples. Arch Dermatol. 1979;115:474-478. doi:10.1001/archderm.115.4.474
- Chiriac A, Brzezinski P, Foia L, et al. Chloronychia: green nail syndrome caused by Pseudomonas aeruginosa in elderly persons [published online January 14, 2015]. Clin Interv Aging. 2015;10:265-267. doi:10.2147/CIA.S75525
An 88-year-old man presented with asymptomatic and unchanging discoloration of the proximal fingernails of both hands of 50 years’ duration. Physical examination revealed blue to slate gray, subungual pigmentary changes of the fingernails of both hands sparing the nail bed distal to the lunulae. There was no overlying plate dystrophy, toenail involvement, or additional mucocutaneous abnormalities. His medical history was notable for heart failure, obstructive sleep apnea, and type 2 diabetes mellitus. He had no history of hepatic, ophthalmologic, or neurologic dysfunction.
Guarding against nonmelanoma skin cancer in solid organ transplant recipients
The incidence of posttransplant malignancy among solid organ transplant recipients (SOTRs) is 10%; skin cancer, primarily nonmelanoma skin cancer (NMSC), constitutes 49.5% of all malignancies in this population.1 The etiology of the increased risk of cutaneous malignancy in SOTR is multifactorial:
- The skin of SOTRs is photosensitive, compared to that of immunocompetent patients, thus predisposing SOTRs to carcinogenic damage resulting from exposure to UV light.2
- Immunosuppression plays a key role in increasing the risk of cutaneous malignancy by inhibiting the ability of the immune system to recognize and destroy tumor cells.3
- Human papillomavirus (HPV) can play a role in carcinogenesis by promoting molecular pathways to proliferation and survival of nascent tumor cells4;Times New Romanβ-HPV strains are disseminated ubiquitously in the skin of immunosuppressed patients.5
- Some medications administered after transplantation can be directly carcinogenic.
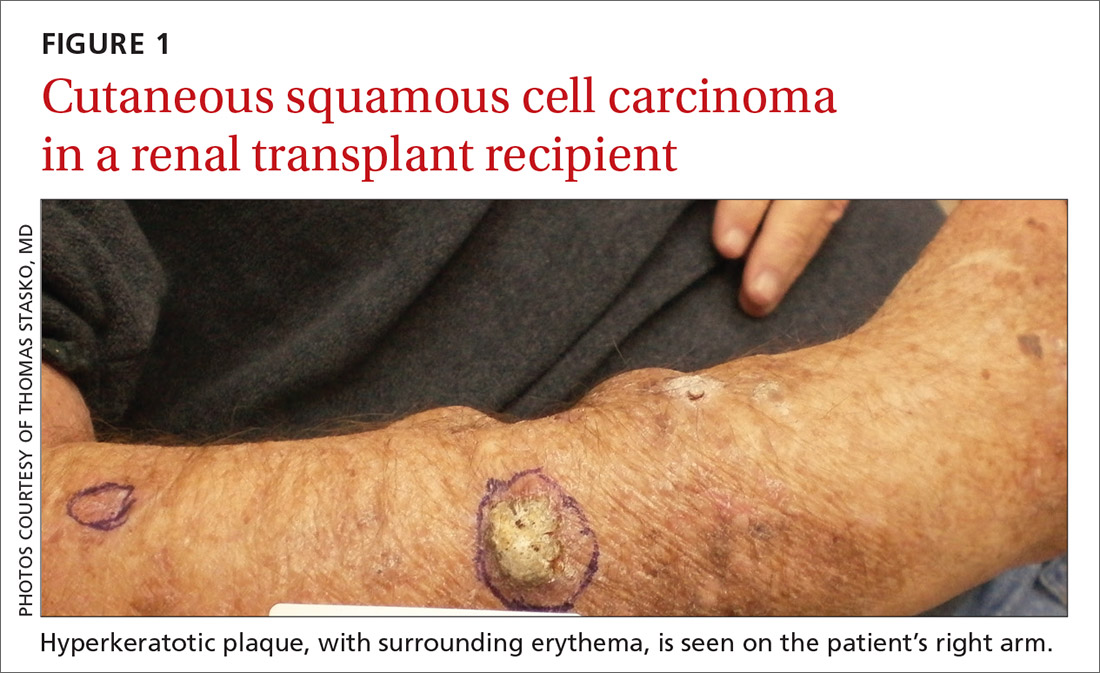
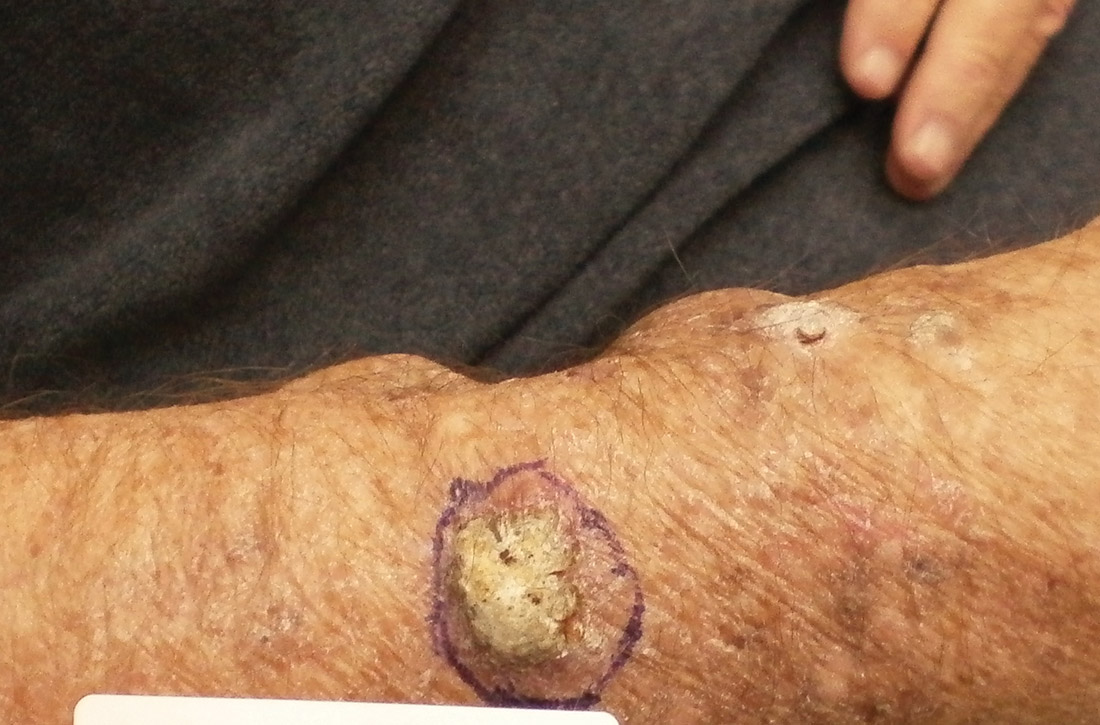
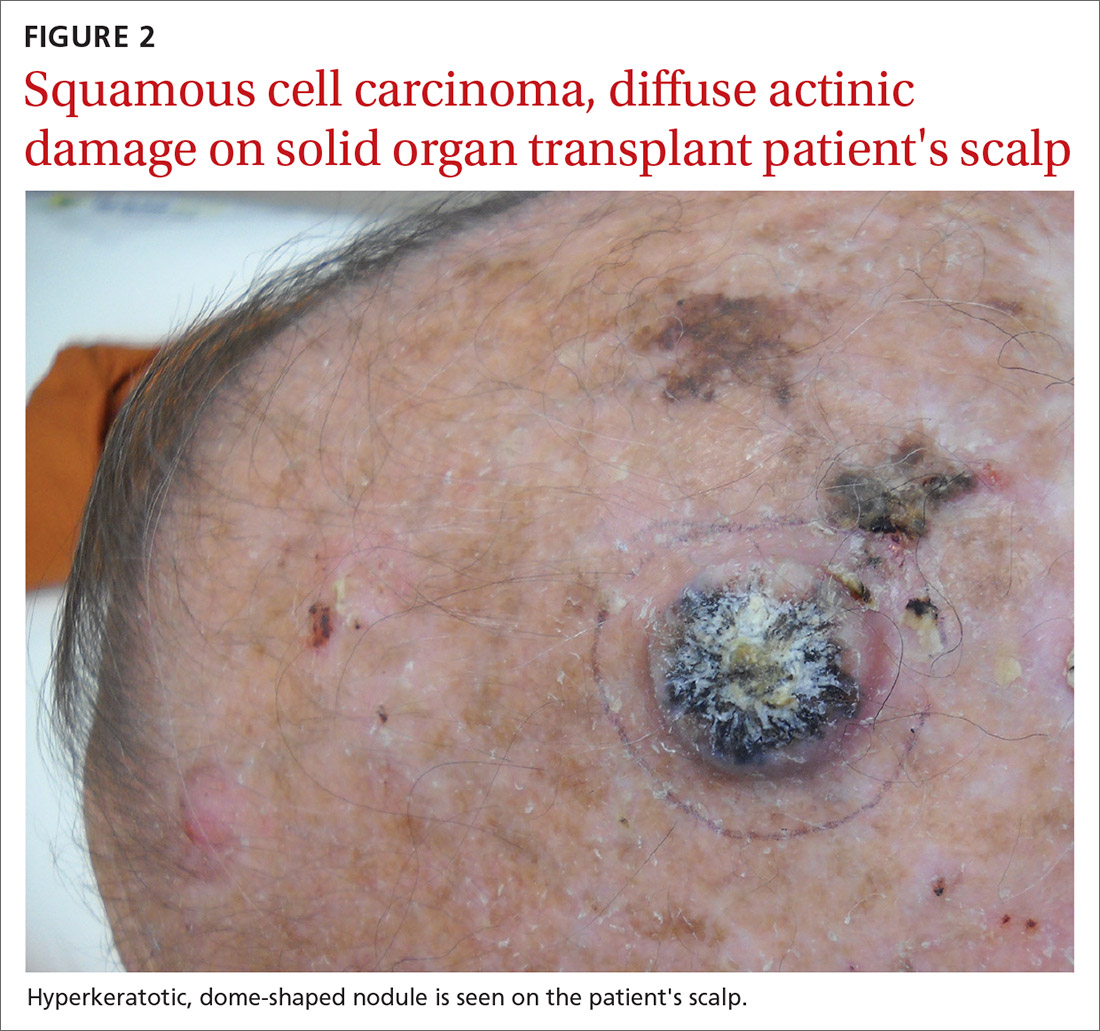
NMSC in SOTRs also differs qualitatively from NMSC in immunocompetent patients. Cutaneous squamous cell carcinoma (cSCC) (FIGUREs 1 and 2) is the most common skin cancer among SOTRs, whereas basal cell carcinoma (BCC) is the most common skin cancer in the general population.3 cSCC in the SOTR population tends to be more aggressive, with more rapid local invasion and an increased rate of both in-transit and distant metastases, leading to an increase in morbidity and mortality. Mortality of metastatic cSCC among SOTRs is approximately 50%, compared to 20% in an otherwise healthy population.3,6-8
The problem is relevant to primary care
Screening. Because there is a demonstrated reduction in morbidity and mortality associated with early detection and treatment of NMSC, regular screening of skin is important in the SOTR population.9 A study in Ontario, Canada, from 1994 to 2012 and comprising 10,183 SOTRs, found that adherence to an annual skin check regimen for ≥ 75% of the observation period was associated with a 34% reduction in cutaneous BCC- and cSCC-related morbidity or death (adjusted hazard ratio = 0.66; 95% CI, 0.48-0.92).10 Although routine follow-up with a dermatologist is recommended for SOTRs,9,11-15 only 2.1% of patients in the Canadian study were fully adherent with annual skin examination, and 55% never visited a dermatologist.10 Consequently, primary care physicians can play a key role in skin cancer screening for SOTRs.
Education regarding the importance of protection from the sun is also an essential part of primary care. A 2018 study of SOTRs in Turkey demonstrated that16
- 46% expressed a lack of knowledge of the hazards of sun exposure
- 44% did not recall ever receiving medical advice regarding sun protection
- 89% did not wear sun-protective clothing
- 86% did not use sunscreen daily.
Multiple studies have demonstrated the positive effect that preventive education and attendance at a dermatology or skin cancer screening clinic can have on sun-protective behaviors among SOTRs.9,16-18 In the Turkish study, 100% of patients who reported using sunscreen daily had been undergoing regular dermatologic examination.16
In this article, we review current management guidelines regarding the prevention and treatment of NMSC in SOTRs.
Recommendations for prevention
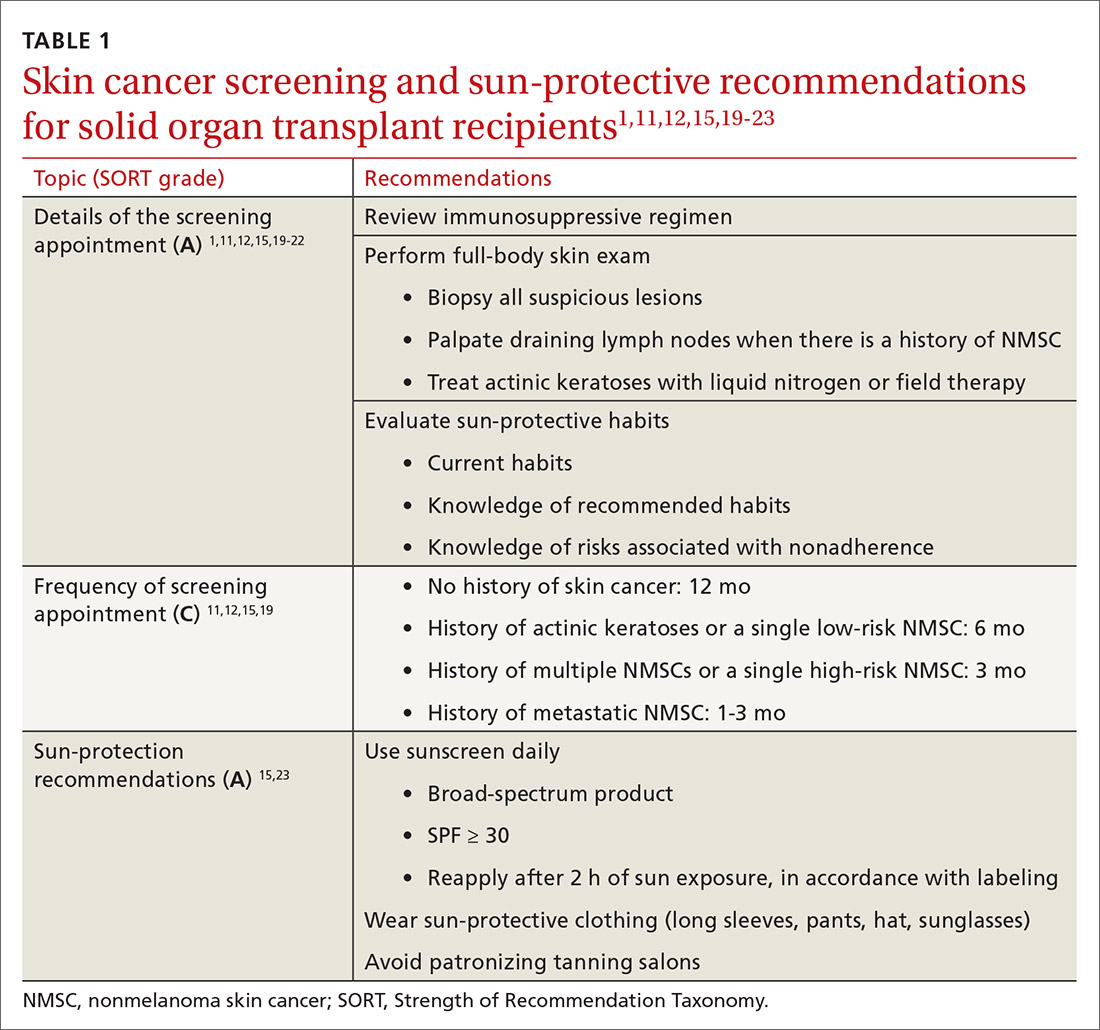
Screening skin exams (TABLE 11,11,12,15,19-23). Although definitive guidelines do not exist regarding the frequency of a screening skin exam for SOTRs, multiple frequency-determining algorithms have been proposed.11,12,15,19 The recommended frequency of a skin exam is based on history of skin cancer; for SOTRs, the most common recommendation19 is a full-body skin examination as follows:
- annually—when there is no history of skin cancer
- every 6 months—when there is a history of actinic keratoses (AKs; precancerous lesions that carry a risk of transforming into cSCC) or a single low-risk NMSC
- every 3 months—when there is a history of multiple NMSCs or a single high-risk NMSC
- every 1 to 3 months—when there is a history of metastatic disease.
Continue to: Other risk factors...
Other risk factors for NMSC to consider in SOTRs when determining an appropriate follow-up regimen include any of the following1,20,21,24-26:
- male gender, fair skin, history of childhood sunburn, history of smoking
- lung or heart transplantation, history of episodes of transplant rejection, age ≥ 50 years at transplantation
- immunosuppression with calcineurin inhibitors, compared to mammalian target of rapamycin (mTOR) inhibitors
- immunosuppression with cyclosporine, compared to tacrolimus
- an immunosuppressive regimen with > 1 immunosuppressant or an increased degree of immunosuppression
- antithymocyte globulin within the first year posttransplantation.
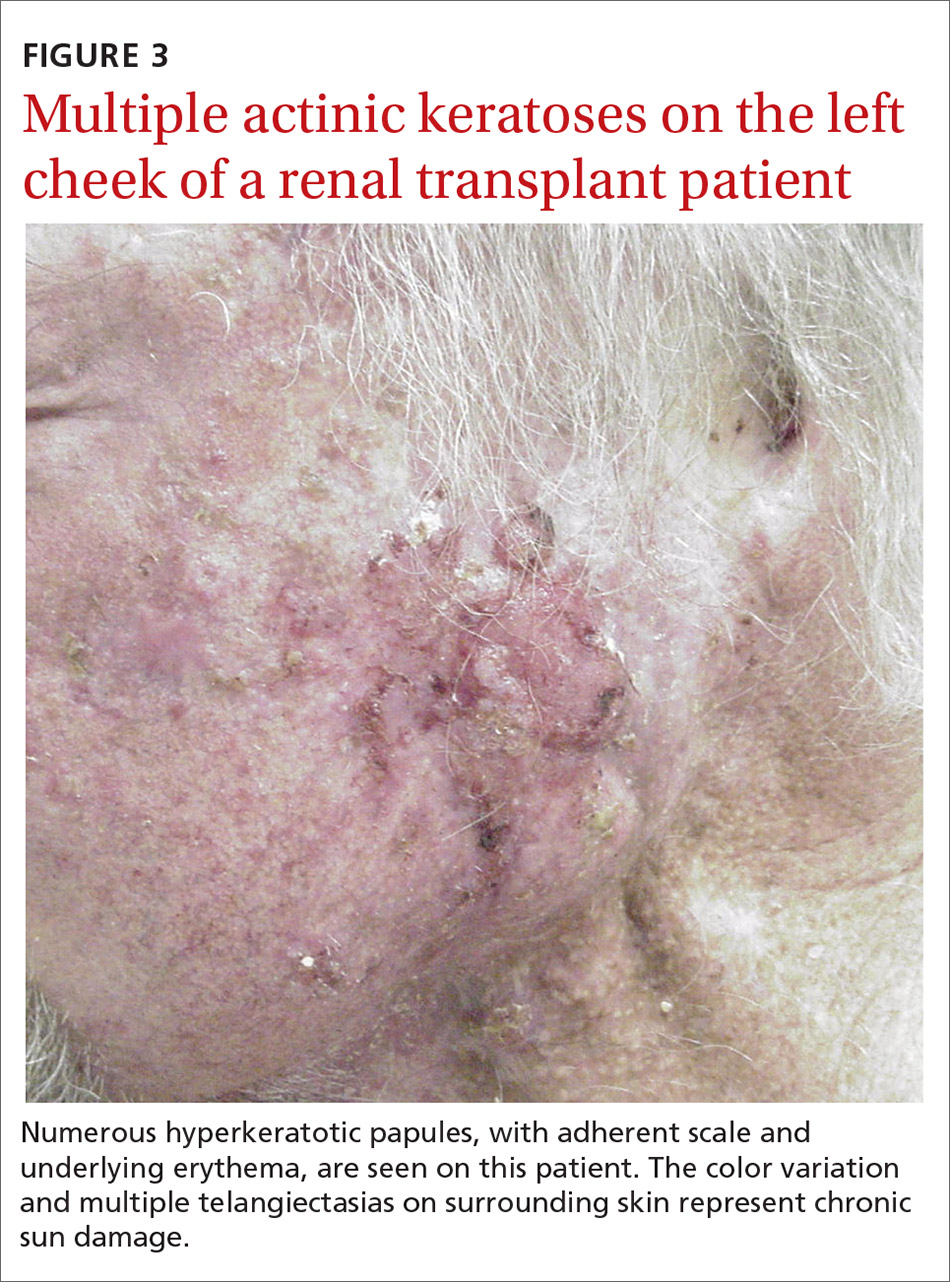
Because the intensity of immunosuppression and individual immunosuppressants used affect the risk of NMSC, conduct a thorough medication review with SOTRs at all visits. Ask about new, changing, or symptomatic (pruritic, painful, bleeding) skin lesions, and perform a full-body skin exam. Palpate draining lymph nodes if the patient has a history of NMSC.15 AKs (FIGURE 3) should be treated aggressively with liquid nitrogen or field therapy. Lesions suspicious for NMSC should be biopsied and sent for histologic evaluation.22 Shave, punch, and excisional biopsies are all adequate techniques; however, because all cSCCs in SOTRs are considered high risk for aggressive features, biopsy should extend at least into the reticular dermis to allow evaluation for invasive disease.22
Sun-protective measures (TABLE 11,11,12,15,19-23). Inquire about patients’ habits related to protection from the sun, their knowledge of recommended sun-protective measures, and risks associated with nonadherence. Recommended sun-protective measures include
- daily broad-spectrum sunscreen (SPF ≥ 30), reapplied every 2 hours of sun exposure, in accordance with labeling instructions23
- sun-protective clothing (pants, long sleeves, hat, sunglasses)23
- avoidance of tanning salons.15
SOTRs who adequately adhere to sun-protective measures might need vitamin D supplementation because sunscreen and sun-protective clothing inhibit cutaneous synthesis of vitamin D.15
Recommendations for treatment
Consider chemoprophylactic therapy for SOTRs who have had multiple prior cutaneous malignancies or multiple AKs.
Continue to: Topical chemoprophylaxis
Topical chemoprophylaxis
Topical medications used for cSCC chemoprophylaxis include 5-fluorouracil (5-FU), photodynamic therapy (PDT), imiquimod, ingenol mebutate, topical retinoids, and diclofenac.27 (See TABLE 2.27-40) Of these, the latter 3 are used less commonly because of the small packaging size of ingenol mebutate and the relative lack of efficacy data for topical retinoids and diclofenac.27 Imiquimod is often avoided when treating large surface areas because of the risk of systemic adverse effects associated with cytokine release.27
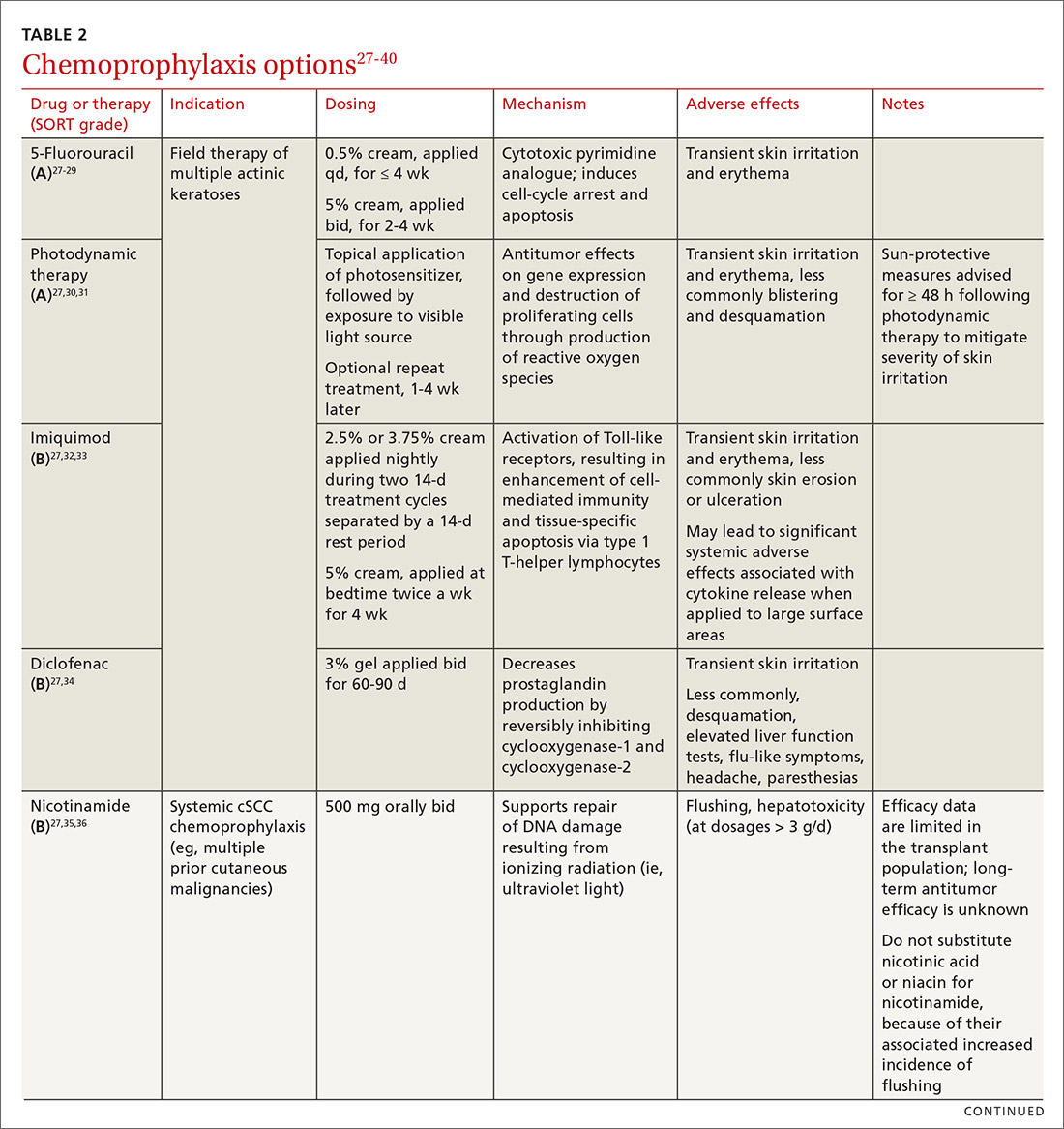
5-FU is US Food and Drug Administration (FDA)-approved for the treatment of AKs, and is used off-label for treating cSCC in situ (Bowen disease). It is the most commonly used topical therapy for field disease.27-29 5-FU is typically applied once or twice daily for 3 to 4 weeks. Common adverse effects include transient skin irritation and erythema.27
PDT involves topical application of a photosensitizer, such as 5-aminolevulinic acid or methyl aminolevulinate, followed by exposure to a visible light source, leading to antitumor effects on gene expression and destruction of proliferating cells through production of reactive oxygen species.30,31 Evidence is sufficient to support routine use of PDT for AKs and Bowen disease.30 A mild sunburn-like reaction is common following PDT, with transient erythema and discomfort typically lasting 1 to 2 weeks but not typically necessitating analgesic therapy.27
Imiquimod is a ligand that binds to and activates Toll-like receptor 7, leading to enhancement of the cell-mediated antitumor immune response and resultant tissue-specific apoptosis coordinated by type 1 T-helper lymphocytes.32 Topical imiquimod cream is FDA approved for field treatment of AKs at 2.5%, 3.75%, and 5% concentrations; efficacy has been demonstrated in the SOTR population.33,41 Multiple studies in immunocompetent patients have suggested that imiquimod might be slightly less efficacious than 5-FU.42-44
The tolerability of field treatment with imiquimod has been called into question.27 However, in a 2019 study comparing adverse reactions among 513 immunocompetent patients with field disease who were treated with either 5-FU 5% cream; imiquimod 5% cream; PDT with methyl aminolevulinate; or ingenol mebutate 0.015% gel, a similar or smaller percentage of patients treated with imiquimod reported moderate-to-severe itching, moderate-to-severe pain, and any adverse events, compared to patients treated with the other options.44
Continue to: Diclofenac
Diclofenac is a nonsteroidal anti-inflammatory drug that reversibly inhibits the enzymes cyclooxygenase-1 and cyclooxygenase-2, resulting in a decrease in the formation of inflammatory prostaglandins, which have been observed in chronically sun-damaged skin, AKs, and cSCC.34,45 Diclofenac 3% gel, applied topically twice daily for 60 to 90 days has been approved by the FDA for treatment of AKs, in conjunction with sun avoidance.34 Topical diclofenac has been demonstrated to be efficacious in treating AKs in the SOTR population46,47; however, multiple meta-analyses using data from immunocompetent patients have demonstrated that topical diclofenac is inferior to other treatment options, particularly 5-FU, at achieving complete clearance of AKs.43,48,49 Diclofenac might be a useful option when patient adherence is expected to be difficult because of adverse effects of therapy: Multiple studies have suggested that diclofenac might be more tolerable than other options.43,48,50
Systemic chemoprophylaxis
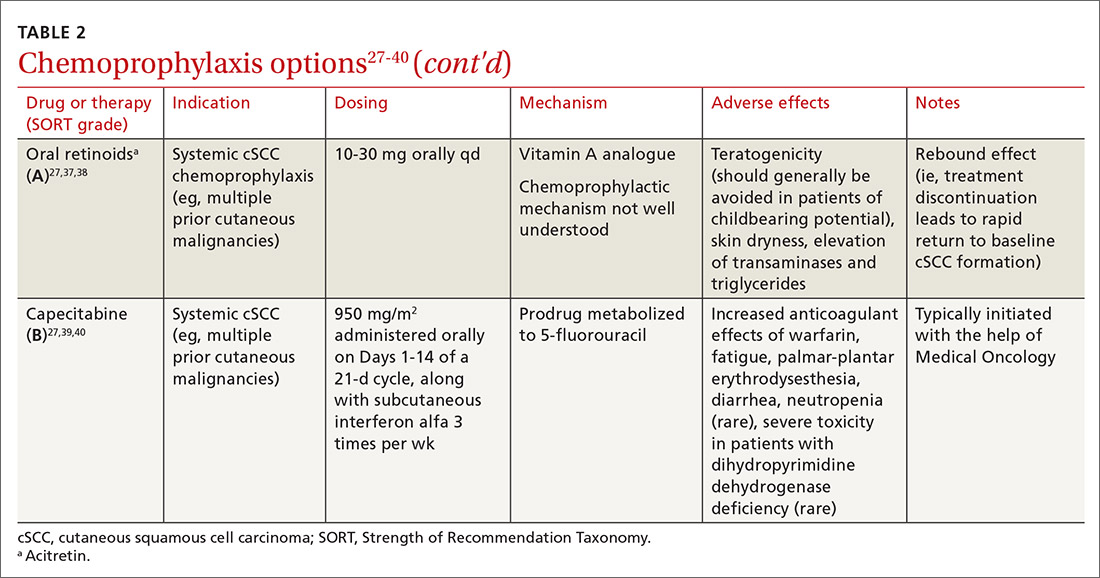
Systemic therapies that have been used for chemoprophylaxis against cutaneous malignancy include nicotinamide, oral retinoids, capecitabine, and HPV vaccination. (See TABLE 2.27-40)
Nicotinamide, the amide form of vitamin B3, protects against cutaneous malignancy by aiding repair of DNA damaged by ionizing radiation, such as UV light.27 Efficacy has been demonstrated in reducing development of new AKs and cSCC in immunocompetent patients with a history of more than 2 keratinocyte carcinomas within a 5-year span.27,35 Nicotinamide is especially relevant to the SOTR population because it reduces the level of cutaneous immunity suppression induced by UV radiation without altering patients’ baseline immunity.27,36
There are insufficient long-term follow-up data in the literature to assess the sustainability of the antitumor effects of nicotinamide; studies specific to the SOTR population have been underpowered for assessing its impact on formation of cSCC.27,35Patients taking nicotinamide should be informed of the risk of liver failure at dosages > 3 g/d (antitumor efficacy has been demonstrated at 500 mg twice daily) and advised to avoid purchasing over-the-counter nicotinic acid or niacin as a substitute for nicotinamide, because of the increased incidence of flushing associated with their use.27
Oral retinoids. Systemic retinoids—in particular, acitretin—are efficacious in reducing the risk of cSCC in SOTRs.27,37,38 The primary drawback to cSCC prophylaxis with oral retinoids is a rebound effect, in which treatment discontinuation leads to a rapid return to baseline cSCC formation.27
Continue to: Pregnancy must be avoided...
Pregnancy must be avoided while taking an oral retinoid. Because acitretin can persist in the body for years after discontinuation, its use should generally be avoided in patients of childbearing potential. An FDA black box warning states that patients of childbearing potential must be counseled to use 2 forms of birth control to avoid pregnancy for ≥ 3 years after cessation of oral acitretin. Prior to initiation of oral retinoid therapy, the following baseline laboratory tests should be obtained: complete blood count, creatinine, lipid panel, and liver function tests. For patients with a history of chronic kidney disease or renal transplantation, the lipid panel, liver function tests, and creatinine assay should be repeated with each dosage adjustment and every 3 months once goal-dosing is achieved.27
Capecitabine is typically initiated with the help of Medical Oncology.27,40 A prodrug metabolized by dihydropyrimidine dehydrogenase to 5-FU, capecitabine interacts with warfarin, leading to a significant increase in prothrombin time.39 Other adverse effects associated with oral capecitabine include fatigue, palmar-plantar erythrodysesthesia, diarrhea, and, rarely, neutropenia. Although dihydropyrimidine dehydrogenase deficiency is rare, treatment with capecitabine in patients who have this enzyme deficiency might lead to severe toxicity or death.27
HPV vaccination. HPV might play a role in the development of cutaneous malignancy, especially in immunosuppressed patients.4,5 The utility of HPV vaccination in the prevention of NMSC has yet to be determined, but vaccination has been shown, in case reports, to be helpful in immunocompetent patients.51,52 The immunogenicity of HPV vaccination in the SOTR population is uncertain, and the most common HPV types found in SOTRs are not specifically covered by available HPV vaccines.19
The role of immunosuppression reduction and immunosuppressive replacement
Both the degree of immunosuppression and the individual agents used can affect a patient’s risk of NMSC. Immunosuppression reduction should be considered if skin cancer poses a major risk to the patient’s health and if that risk outweighs the risk of graft rejection associated with immunosuppression reduction.27 In a cohort of 180 kidney and liver SOTRs who developed de novo carcinoma (excluding NMSC) after transplantation, neither reduction of immunosuppression nor introduction of an mTOR inhibitor affected graft survival or oncologic treatment tolerance.53 Because mTOR inhibitors have a protective effect against development of NMSC, they are the preferred choice of immunosuppressive agent from a dermatologic perspective.1,27,54-57 Decisions regarding changes in immunosuppression are generally made by, or in collaboration with, the patient’s transplant physician.
Recommendations: Treating cSCC
Risk should guide strategy
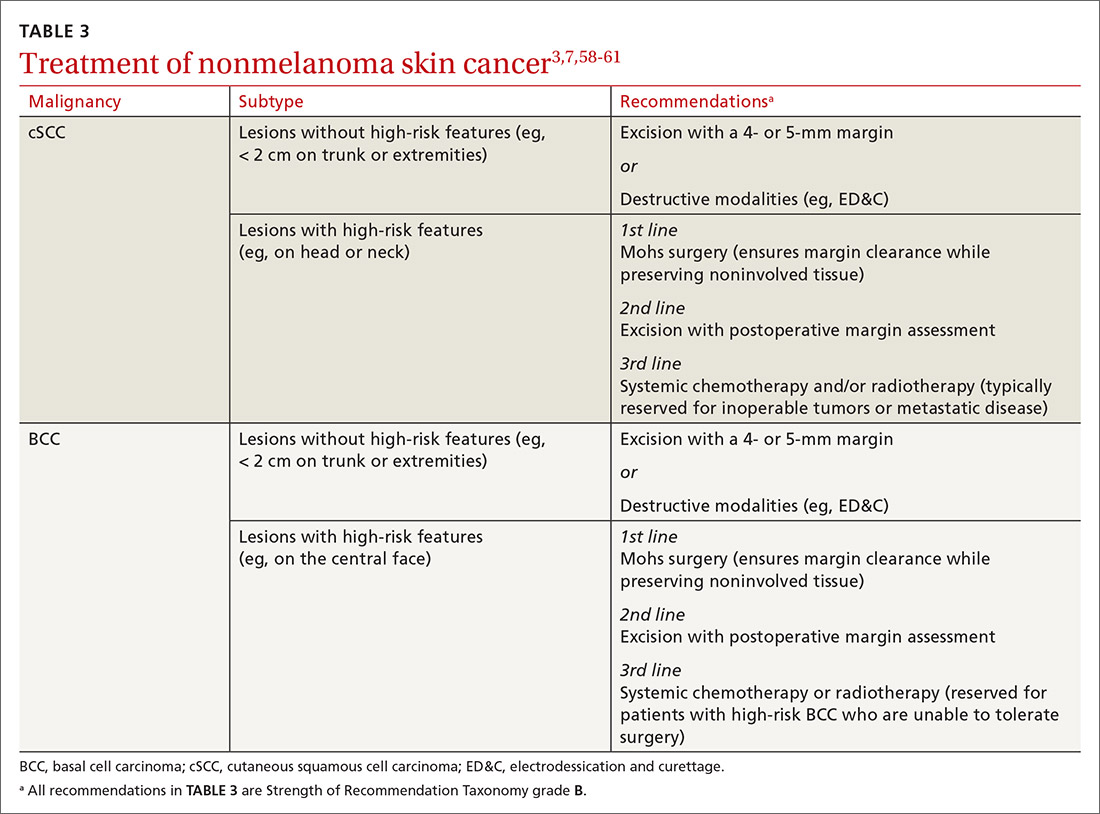
Small lesions of the trunk and extremities without high-risk features can be treated with a destructive method (eg, electrodessication and curettage). However, lesions of the head and neck and those found to have features consistent with an increased risk of recurrence or metastasis should be treated aggressively.3,58,59
Continue to: Risk factors for invasive growth...
Risk factors for invasive growth, recurrence, or metastasis of cSCC in SOTRs are multiple lesions or satellite lesions, indistinct clinical borders, rapid growth, ulceration, and recurrence after treatment.60 The risk of invasive growth, recurrence, and metastasis of cSCC also increases with size and location of the lesion, according to this framework60:
- any size in scar tissue, areas of chronic inflammation, and fields of prior radiation therapy
- ≥ 0.6 cm on hands, feet, genitalia, and mask areas of the face (central face, eyelids, eyebrows, nose, lips, chin, mandible, and temporal, preauricular, postauricular, and periorbital areas)
- > 1 cm on cheeks, forehead, neck, and scalp
- > 2 cm on the trunk and extremities.
In addition, specific findings on histologic analysis portend increased risk of invasive growth, recurrence, or metastasis:
- poor differentiation
- deep extension of the tumor into subcutaneous fat
- perineural invasion or inflammation
- perivascular or intravascular invasion.
Treatment modalities
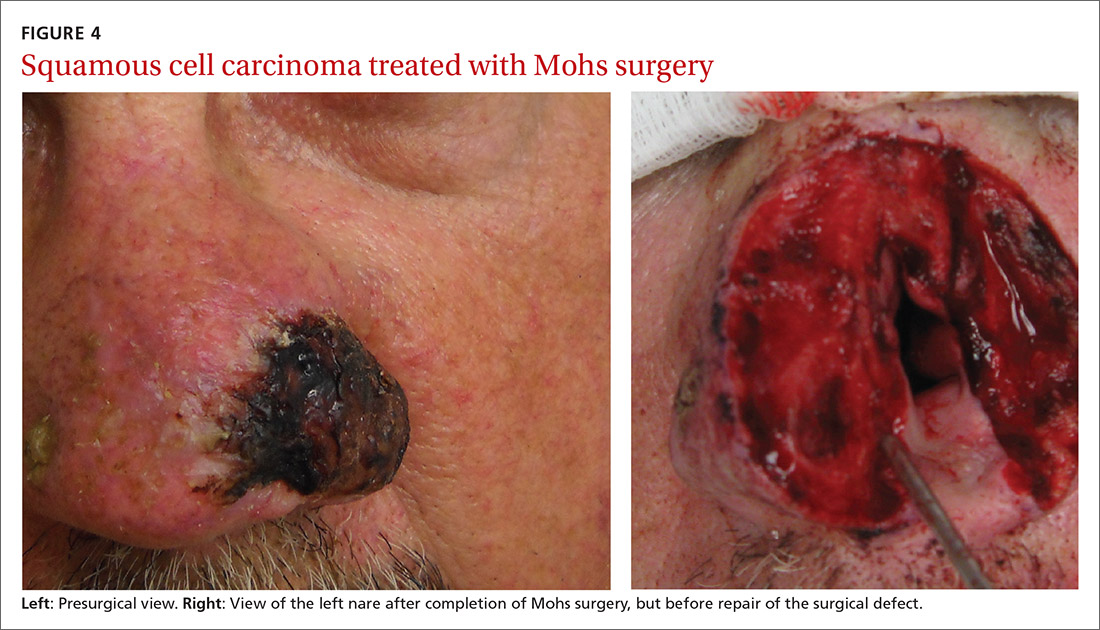
Mohs surgery is preferred to ensure margin clearance while preserving noninvolved tissue3,7 (FIGURE 4). If Mohs surgery is not possible, the lesion should be excised with 3- to 10-mm margins.3,60 Based on current literature, the roles of nodal staging, sentinel lymph node biopsy, and adjuvant therapy are not well defined, but it is likely that these interventions will play a pivotal role in the management of advanced cSCC in SOTRs in the future.3
Nonsurgical therapeutic options for primary or adjuvant treatment of cSCC include systemic chemotherapy, radiotherapy, and programmed cell death protein 1 inhibitors. (For more on treatment modalities, see TABLE 3.3,7,58-61)
Recommendations: Treating BCC
BCC in SOTRs is treated similarly (TABLE 33,7,58-61) to how it is treated in the immunocompetent population—except that SOTRs require closer follow-up than nontransplant patients because they are at higher risk of recurrence and new NMSCs.3 Standard management after biopsy is either3,61:
- Mohs surgery to ensure margin control (for most BCCs on the head and neck and those with clinical or histologic risk factors for recurrence or aggressive behavior)
- excision with a 4- or 5-mm margin or a destructive modality (for BCCs on the trunk and extremities without risk factors for recurrence).
Radiotherapy is an alternative for patients with high-risk BCCs who are unable to tolerate surgery.3
CORRESPONDENCE
Lindsey Collins, MD, Department of Dermatology, University of Oklahoma Health Sciences Center, 619 NE 13th Steet, Oklahoma City, OK 73104; Lindsey-Collins@ouhsc.edu
1. Bhat M, Mara K, Dierkhising R, et al. Immunosuppression, race, and donor-related risk factors affect de novo cancer incidence across solid organ transplant recipients. Mayo Clin Proc. 2018;93:1236-1246. doi: 10.1016/j.mayocp.2018.04.025
2. Togsverd-Bo K, Philipsen PA, Haedersdal M, et al. Organ transplant recipients express enhanced skin autofluorescence and pigmentation at skin cancer sites. J Photochem Photobiol B. 2018;188:1-5. doi: 10.1016/j.jphotobiol.2018.08.008
3. Kearney L, Hogan D, Conlon P, et al. High-risk cutaneous malignancies and immunosuppression: challenges for the reconstructive surgeon in the renal transplant population. J Plast Reconstr Aesthet Surg. 2017;70:922-930. doi: 10.1016/j.bjps.2017.03.005
4. Borgogna C, Olivero C, Lanfredini S, et al. β-HPV infection correlates with early stages of carcinogenesis in skin tumors and patient-derived xenografts from a kidney transplant recipient cohort. Front Microbiol. 2018;9:117. doi: 10.3389/fmicb.2018.00117
5. Nunes EM, Talpe-Nunes V, Sichero L. Epidemiology and biology of cutaneous human papillomavirus. Clinics (Sao Paulo). 2018;73(suppl 1):e489s. doi: 10.6061/clinics/2018/e489s
6. Pini AM, Koch S, L, et al. Eruptive keratoacanthoma following topical imiquimod for in situ squamous cell carcinoma of the skin in a renal transplant recipient. J Am Acad Dermatol. 2008;59(suppl 5):S116-S117. doi: 10.1016/j.jaad.2008.06.018
7. Ilyas M, Zhang N, Sharma A. Residual squamous cell carcinoma after shave biopsy in solid organ transplant recipients. Dermatol Surg. 2018;44:370-374. doi: 10.1097/DSS.0000000000001340
8. Brunner M, Veness MJ, Ch‘ng S, et al. Distant metastases from cutaneous squamous cell carcinoma—analysis of AJCC stage IV. Head Neck. 2013;35:72-75. doi: 10.1002/hed.22913
9. Hartman RI, Green AC, Gordon LG; . Sun protection among organ transplant recipients after participation in a skin cancer research study. JAMA Dermatol. 2018;154:842-844. doi: 10.1001/jamadermatol.2018.1164
10. Chan A-W, Fung K, Austin PC, et al. Improved keratinocyte carcinoma outcomes with annual dermatology assessment after solid organ transplantation: population-based cohort study. Am J Transplant. 2018;19:522-531. doi: 10.1111/ajt.14966
11. Hofbauer GF, Anliker M, Arnold A, et al. Swiss clinical practice guidelines for skin cancer in organ transplant recipients. Swiss Med Wkly. 2009;139:407-415. doi: https://doi.org/10.4414/smw.2014.14026
12. Ulrich C, Kanitakis J, Stockfleth E, et al. Skin cancer in organ transplant recipients—where do we stand today? Am J Transplant. 2008;8:2192-2198. doi: 10.1111/j.1600-6143.2008.02386.x
13. Chen SC, Pennie ML, Kolm P, et al. Diagnosing and managing cutaneous pigmented lesions: primary care physicians versus dermatologists. J Gen Intern Med. 2006;21:678-682. doi: 10.1111/j.1525-1497.2006.00462.x
14. Ismail F, Mitchell L, Casabonne D, et al. Specialist dermatology clinics for organ transplant recipients significantly improve compliance with photoprotection and levels of skin cancer awareness. Br J Dermatol. 2006;155:916-925. doi: 10.1111/j.1365-2133.2006.07454.x
15. O‘Reilly Zwald F, Brown M. Skin cancer in solid organ transplant recipients: advances in therapy and management: part II. Management of skin cancer in solid organ transplant recipients. J Am Acad Dermatol. 2011;65:263-279. doi: 10.1016/j.jaad.2010.11.063
16. Vural A, A, Kirnap M, et al. Skin cancer risk awareness and sun-protective behavior among solid-organ transplant recipients. Exp Clin Transplant. 2018;16(suppl 1):203-207. doi: 10.6002/ect.TOND-TDTD2017.P65
17. Papier K, Gordon LG, Khosrotehrani K, et al. Increase in preventive behaviour by organ transplant recipients after sun protection information in a skin cancer surveillance clinic. Br J Dermatol. 2018;179:1195-1196. doi: 10.1111/bjd.16836
18. Wu SZ, Jiang P, DeCaro JE, et al. A qualitative systematic review of the efficacy of sun protection education in organ transplant recipients. J Am Acad Dermatol. 2016;75:1238-1244.e5. doi: 10.1016/j.jaad.2016.06.031
19. Blomberg M, He SY, Harwood C, et al; . Research gaps in the management and prevention of cutaneous squamous cell carcinoma in organ transplant recipients. Br J Dermatol. 2017;177:1225-1233. doi: 10.1111/bjd.15950
20. Urwin HR, Jones PW, Harden PN, et al. Predicting risk of nonmelanoma skin cancer and premalignant skin lesions in renal transplant recipients. Transplantation. 2009;87:1667-1671. doi: 10.1097/TP.0b013e3181a5ce2e
21. Infusino SD, Loi C, Ravaioli GM, et al. Cutaneous complications of immunosuppression in 812 transplant recipients: a 40-year single center experience. G Ital Dermatol Venereol. 2020;155:662-668. doi: 10.23736/S0392-0488.18.06091-1
22. Naldi L, Venturuzzo A, Invernizzi P. Dermatological complications after solid organ transplantation. Clin Rev Allergy Immunol. 2018;54:185-212. doi: 10.1007/s12016-017-8657-9
23. Sunscreen FAQs. American Academy of Dermatology Web site. Accessed February 25, 2021. www.aad.org/media/stats/prevention-and-care/sunscreen-faqs
24. Vos M, Plasmeijer EI, van Bemmel BC, et al. Azathioprine to mycophenolate mofetil transition and risk of squamous cell carcinoma after lung transplantation. J Heart Lung Transplant. 2018;37:853-859. doi: 10.1016/j.healun.2018.03.012
25. Puza CJ, Myers SA, Cardones AR, et al. The impact of transplant rejection on cutaneous squamous cell carcinoma in renal transplant recipients. Clin Exp Dermatol. 2018;44:265-269. doi: 10.1111/ced.13699
26. Abikhair Burgo M, Roudiani N, Chen J, et al. Ruxolitinib inhibits cyclosporine-induced proliferation of cutaneous squamous cell carcinoma. JCI Insight. 2018;3:e120750. doi: 10.1172/jci.insight.120750
27. Que SKT, Zwald FO, Schmults CD. Cutaneous squamous cell carcinoma: management of advanced and high-stage tumors. J Am Acad Dermatol. 2018;78:249-261. doi: 10.1016/j.jaad.2017.08.058
28. Askew DA, Mickan SM, Soyer HP, et al. Effectiveness of 5-fluorouracil treatment for actinic keratosis—a systematic review of randomized controlled trials. Int J Dermatol. 2009;48:453-463. doi: 10.1111/j.1365-4632.2009.04045.x
29. Salim A, Leman JA, McColl JH, et al. Randomized comparison of photodynamic therapy with topical 5-fluorouracil in Bowen‘s disease. Br J Dermatol. 2003;148:539-543. doi: 10.1046/j.1365-2133.2003.05033.x
30. Morton CA. A synthesis of the world‘s guidelines on photodynamic therapy for non-melanoma skin cancer. G Ital Dermatol Venereol. 2018;153:783-792. doi: 10.23736/S0392-0488.18.05896-0
31. Joly F, Deret S, Gamboa B, et al. Photodynamic therapy corrects abnormal cancer-associated gene expression observed in actinic keratosis lesions and induces a remodeling effect in photodamaged skin. J Dermatol Sci. 2018;S0923-1811(17)30775-2. doi: 10.1016/j.jdermsci.2018.05.002
32. Patel GK, Goodwin R, Chawla M, et al. Imiquimod 5% cream monotherapy for cutaneous squamous cell carcinoma in situ (Bowen‘s disease): a randomized, double-blind, placebo-controlled trial. J Am Acad Dermatol. 2006;54:1025-1032. doi: 10.1016/j.jaad.2006.01.055
33. Imiquimod. Wolters Kluwer Clinical Drug Information, Inc.; 2019. Accessed August 6, 2019. http://online.lexi.com/lco/action/doc/retrieve/docid/patch_f/7077?cesid=aRo1Yh9sd0Q&searchUrl=%2Flco%2Faction%2Fsearch%3Fq%3Dimiquimod%26t%3Dname%26va%3Dimiquimod
34. Diclofenac. Wolters Kluwer Clinical Drug Information, Inc.; 2019. Accessed August 6th, 2019. http://online.lexi.com/lco/action/doc/retrieve/docid/patch_f/1772965?cesid=5vTk7J3Vmvc&searchUrl=%2Flco%2Faction%2Fsearch%3Fq%3Ddiclofenac%26t%3Dname%26va%3Ddiclofenac
35. Chen AC, Martin AJ, Choy B, et al. A phase 3 randomized trial of nicotinamide for skin-cancer chemoprevention. N Engl J Med. 2015;373:1618-1626. doi: 10.1056/NEJMoa1506197
36. Yiasemides E, Sivapirabu G, Halliday GM, et al. Oral nicotinamide protects against ultraviolet radiation-induced immunosuppression in humans. Carcinogenesis. 2009;30:101-105. doi: 10.1093/carcin/bgn248
37. Otley CC, Stasko T, Tope WD, et al. Chemoprevention of nonmelanoma skin cancer with systemic retinoids: practical dosing and management of adverse effects. Dermatol Surg. 2006;32:562-568. doi: 10.1111/j.1524-4725.2006.32115.x
38. McKenna DB, Murphy GM. Skin cancer chemoprophylaxis in renal transplant recipients: 5 years of experience using low-dose acitretin. Br J Dermatol. 1999;140:656-660. doi: 10.1046/j.1365-2133.1999.02765.x
39. Capecitabine. Wolters Kluwer Clinical Drug Information, Inc.; 2019. Accessed February 13, 2019. http://online.lexi.com/lco/action/doc/retrieve/docid/patch_f/6519?cesid=7WMsK72X7T7&searchUrl=%2Flco%2Faction%2Fsearch%3Fq%3Dcapecitabine%26t%3Dname%26va%3Dcapecitabine
40. Wollina U, Hansel G, Koch A, et al. Oral capecitabine plus subcutaneous interferon alpha in advanced squamous cell carcinoma of the skin. J Cancer Res Clin Oncol. 2005;131:300-304. doi: 10.1007/s00432-004-0656-6
41. Zavattaro E, Veronese F, Landucci G, et al. Efficacy of topical imiquimod 3.75% in the treatment of actinic keratosis of the scalp in immunosuppressed patients: our case series. J Dermatol Treat. 2020;31:285-289. doi: 10.1080/09546634.2019.1590524
42. Neugebauer R, Su KA, Zhu Z, et al. Comparative effectiveness of treatment of actinic keratosis with topical fluorouracil and imiquimod in the prevention of keratinocyte carcinoma: a cohort study. J Am Acad Dermatol. 2019;80:998-1005. doi: 10.1016/j.jaad.2018.11.024
43. Gupta AK, Paquet M. Network meta-analysis of the outcome ‚participant complete clearance in nonimmunosuppressed participants of eight interventions for actinic keratosis: a follow-up on a Cochrane review. Br J Dermatol. 2013;169:250-259. doi: 10.1111/bjd.12343
44. Jansen MHE, Kessels JPHM, Nelemans PJ, et al. Randomized trial of four treatment approaches for actinic keratosis. N Engl J Med. 2019;380:935-946. doi: 10.1056/NEJMoa1811850
45. Bangash HK, Colegio OR. Management of non-melanoma skin cancer in immunocompromised solid organ transplant recipients. Curr Treat Options Oncol. 2012;13:354-376. doi: 10.1007/s11864-012-0195-3
46. Ulrich C, Johannsen A, J, et al. Results of a randomized, placebo-controlled safety and efficacy study of topical diclofenac 3% gel in organ transplant patients with multiple actinic keratoses. Eur J Dermatol. 2010;20:482-488. doi: 10.1684/ejd.2010.1010
47. Ulrich C, Hackethal M, Ulrich M, et al. Treatment of multiple actinic keratoses with topical diclofenac 3% gel in organ transplant recipients: a series of six cases. Br J Dermatol. 2007;156(suppl 3):40-42. doi: 10.1111/j.1365-2133.2007.07864.x
48. Wu Y, Tang N, Cai L, et al. Relative efficacy of 5-fluorouracil compared with other treatments among patients with actinic keratosis: a network meta-analysis. Dermatol Ther. 2019;32:e12822. doi: 10.1111/dth.12822
49. Stockfleth E, Kerl H, Zwingers T, et al. Low-dose 5-fluorouracil in combination with salicylic acid as a new lesion-directed option to treat topically actinic keratoses: histological and clinical study results. Br J Dermatol. 2011;165:1101-1108. doi: 10.1111/j.1365-2133.2011.10387.x
50. Smith SR, Morhenn VB, Piacquadio DJ. Bilateral comparison of the efficacy and tolerability of 3% diclofenac sodium gel and 5% 5-fluorouracil cream in the treatment of actinic keratoses of the face and scalp. J Drug Dermatol. 2006;5:156-159.
51. Nichols AJ, Allen AH, Shareef S, et al. Association of human papillomavirus vaccine with the development of keratinocyte carcinomas. JAMA Dermatol. 2017;153:571-574. doi: 10.1001/jamadermatol.2016.5703
52. Nichols AJ, Gonzalez A, Clark ES, et al. Combined systemic and intratumoral administration of human papillomavirus vaccine to treat multiple cutaneous basaloid squamous cell carcinomas. JAMA Dermatol. 2018;154:927-930. doi: 10.1001/jamadermatol.2018.1748
53. Rousseau B, Guillemin A, Duvoux C, et al. Optimal oncologic management and mTOR inhibitor introduction are safe and improve survival in kidney and liver allograft recipients with de novo carcinoma. Int J Cancer. 2019;144:886-896. doi: 10.1002/ijc.31769
54. Mathew T, Kreis H, Friend P. Two-year incidence of malignancy in sirolimus-treated renal transplant recipients: results from five multicenter studies. Clin Transplant. 2004;18:446-449. doi: 10.1111/j.1399-0012.2004.00188.x
55. Euvrard S, Morelon E, Rostaing L, et al; Sirolimus and secondary skin-cancer prevention in kidney transplantation. N Engl J Med. 2012;367:329-339. doi: 10.1056/NEJMoa1204166
56. Hoogendijk-van den Akker JM, Harden PN, Hoitsma AJ, et al. Two-year randomized controlled prospective trial converting treatment of stable renal transplant recipients with cutaneous invasive squamous cell carcinomas to sirolimus. J Clin Oncol. 2013;31:1317-1323. doi: 10.1200/JCO.2012.45.6376
57. Karia PS, Azzi JR, Heher EC, et al. Association of sirolimus use with risk for skin cancer in a mixed-organ cohort of solid-organ transplant recipients with a history of cancer. JAMA Dermatol. 2016;152:533-540. doi: 10.1001/jamadermatol.2015.5548
58. Stratigos A, Garbe C, Lebbe C, et al; . Diagnosis and treatment of invasive squamous cell carcinoma of the skin: European consensus-based interdisciplinary guideline. Eur J Cancer. 2015;51:1989-2007. doi: 10.1016/j.ejca.2015.06.110
59. Goldman G. The current status of curettage and electrodesiccation. Dermatol Clin. 2002;20:569-578, ix. doi: 10.1016/s0733-8635(02)00022-0
60. Stasko T, Brown MD, Carucci JA, et al; ; . Guidelines for the management of squamous cell carcinoma in organ transplant recipients. Derm Surg. 2004;30:642-650. doi: 10.1111/j.1524-4725.2004.30150.x
61. Telfer NR, Colver GB, Morton CA; . Guidelines for the management of basal cell carcinoma. Br J Dermatol. 2008;159:35-48. doi: 10.1111/j.1365-2133.2008.08666.x
The incidence of posttransplant malignancy among solid organ transplant recipients (SOTRs) is 10%; skin cancer, primarily nonmelanoma skin cancer (NMSC), constitutes 49.5% of all malignancies in this population.1 The etiology of the increased risk of cutaneous malignancy in SOTR is multifactorial:
- The skin of SOTRs is photosensitive, compared to that of immunocompetent patients, thus predisposing SOTRs to carcinogenic damage resulting from exposure to UV light.2
- Immunosuppression plays a key role in increasing the risk of cutaneous malignancy by inhibiting the ability of the immune system to recognize and destroy tumor cells.3
- Human papillomavirus (HPV) can play a role in carcinogenesis by promoting molecular pathways to proliferation and survival of nascent tumor cells4;Times New Romanβ-HPV strains are disseminated ubiquitously in the skin of immunosuppressed patients.5
- Some medications administered after transplantation can be directly carcinogenic.

NMSC in SOTRs also differs qualitatively from NMSC in immunocompetent patients. Cutaneous squamous cell carcinoma (cSCC) (FIGUREs 1 and 2) is the most common skin cancer among SOTRs, whereas basal cell carcinoma (BCC) is the most common skin cancer in the general population.3 cSCC in the SOTR population tends to be more aggressive, with more rapid local invasion and an increased rate of both in-transit and distant metastases, leading to an increase in morbidity and mortality. Mortality of metastatic cSCC among SOTRs is approximately 50%, compared to 20% in an otherwise healthy population.3,6-8

The problem is relevant to primary care
Screening. Because there is a demonstrated reduction in morbidity and mortality associated with early detection and treatment of NMSC, regular screening of skin is important in the SOTR population.9 A study in Ontario, Canada, from 1994 to 2012 and comprising 10,183 SOTRs, found that adherence to an annual skin check regimen for ≥ 75% of the observation period was associated with a 34% reduction in cutaneous BCC- and cSCC-related morbidity or death (adjusted hazard ratio = 0.66; 95% CI, 0.48-0.92).10 Although routine follow-up with a dermatologist is recommended for SOTRs,9,11-15 only 2.1% of patients in the Canadian study were fully adherent with annual skin examination, and 55% never visited a dermatologist.10 Consequently, primary care physicians can play a key role in skin cancer screening for SOTRs.
Education regarding the importance of protection from the sun is also an essential part of primary care. A 2018 study of SOTRs in Turkey demonstrated that16
- 46% expressed a lack of knowledge of the hazards of sun exposure
- 44% did not recall ever receiving medical advice regarding sun protection
- 89% did not wear sun-protective clothing
- 86% did not use sunscreen daily.
Multiple studies have demonstrated the positive effect that preventive education and attendance at a dermatology or skin cancer screening clinic can have on sun-protective behaviors among SOTRs.9,16-18 In the Turkish study, 100% of patients who reported using sunscreen daily had been undergoing regular dermatologic examination.16
In this article, we review current management guidelines regarding the prevention and treatment of NMSC in SOTRs.
Recommendations for prevention
Screening skin exams (TABLE 11,11,12,15,19-23). Although definitive guidelines do not exist regarding the frequency of a screening skin exam for SOTRs, multiple frequency-determining algorithms have been proposed.11,12,15,19 The recommended frequency of a skin exam is based on history of skin cancer; for SOTRs, the most common recommendation19 is a full-body skin examination as follows:
- annually—when there is no history of skin cancer
- every 6 months—when there is a history of actinic keratoses (AKs; precancerous lesions that carry a risk of transforming into cSCC) or a single low-risk NMSC
- every 3 months—when there is a history of multiple NMSCs or a single high-risk NMSC
- every 1 to 3 months—when there is a history of metastatic disease.
Continue to: Other risk factors...
Other risk factors for NMSC to consider in SOTRs when determining an appropriate follow-up regimen include any of the following1,20,21,24-26:
- male gender, fair skin, history of childhood sunburn, history of smoking
- lung or heart transplantation, history of episodes of transplant rejection, age ≥ 50 years at transplantation
- immunosuppression with calcineurin inhibitors, compared to mammalian target of rapamycin (mTOR) inhibitors
- immunosuppression with cyclosporine, compared to tacrolimus
- an immunosuppressive regimen with > 1 immunosuppressant or an increased degree of immunosuppression
- antithymocyte globulin within the first year posttransplantation.
Because the intensity of immunosuppression and individual immunosuppressants used affect the risk of NMSC, conduct a thorough medication review with SOTRs at all visits. Ask about new, changing, or symptomatic (pruritic, painful, bleeding) skin lesions, and perform a full-body skin exam. Palpate draining lymph nodes if the patient has a history of NMSC.15 AKs (FIGURE 3) should be treated aggressively with liquid nitrogen or field therapy. Lesions suspicious for NMSC should be biopsied and sent for histologic evaluation.22 Shave, punch, and excisional biopsies are all adequate techniques; however, because all cSCCs in SOTRs are considered high risk for aggressive features, biopsy should extend at least into the reticular dermis to allow evaluation for invasive disease.22

Sun-protective measures (TABLE 11,11,12,15,19-23). Inquire about patients’ habits related to protection from the sun, their knowledge of recommended sun-protective measures, and risks associated with nonadherence. Recommended sun-protective measures include
- daily broad-spectrum sunscreen (SPF ≥ 30), reapplied every 2 hours of sun exposure, in accordance with labeling instructions23
- sun-protective clothing (pants, long sleeves, hat, sunglasses)23
- avoidance of tanning salons.15

SOTRs who adequately adhere to sun-protective measures might need vitamin D supplementation because sunscreen and sun-protective clothing inhibit cutaneous synthesis of vitamin D.15
Recommendations for treatment
Consider chemoprophylactic therapy for SOTRs who have had multiple prior cutaneous malignancies or multiple AKs.
Continue to: Topical chemoprophylaxis
Topical chemoprophylaxis
Topical medications used for cSCC chemoprophylaxis include 5-fluorouracil (5-FU), photodynamic therapy (PDT), imiquimod, ingenol mebutate, topical retinoids, and diclofenac.27 (See TABLE 2.27-40) Of these, the latter 3 are used less commonly because of the small packaging size of ingenol mebutate and the relative lack of efficacy data for topical retinoids and diclofenac.27 Imiquimod is often avoided when treating large surface areas because of the risk of systemic adverse effects associated with cytokine release.27

5-FU is US Food and Drug Administration (FDA)-approved for the treatment of AKs, and is used off-label for treating cSCC in situ (Bowen disease). It is the most commonly used topical therapy for field disease.27-29 5-FU is typically applied once or twice daily for 3 to 4 weeks. Common adverse effects include transient skin irritation and erythema.27

PDT involves topical application of a photosensitizer, such as 5-aminolevulinic acid or methyl aminolevulinate, followed by exposure to a visible light source, leading to antitumor effects on gene expression and destruction of proliferating cells through production of reactive oxygen species.30,31 Evidence is sufficient to support routine use of PDT for AKs and Bowen disease.30 A mild sunburn-like reaction is common following PDT, with transient erythema and discomfort typically lasting 1 to 2 weeks but not typically necessitating analgesic therapy.27
Imiquimod is a ligand that binds to and activates Toll-like receptor 7, leading to enhancement of the cell-mediated antitumor immune response and resultant tissue-specific apoptosis coordinated by type 1 T-helper lymphocytes.32 Topical imiquimod cream is FDA approved for field treatment of AKs at 2.5%, 3.75%, and 5% concentrations; efficacy has been demonstrated in the SOTR population.33,41 Multiple studies in immunocompetent patients have suggested that imiquimod might be slightly less efficacious than 5-FU.42-44
The tolerability of field treatment with imiquimod has been called into question.27 However, in a 2019 study comparing adverse reactions among 513 immunocompetent patients with field disease who were treated with either 5-FU 5% cream; imiquimod 5% cream; PDT with methyl aminolevulinate; or ingenol mebutate 0.015% gel, a similar or smaller percentage of patients treated with imiquimod reported moderate-to-severe itching, moderate-to-severe pain, and any adverse events, compared to patients treated with the other options.44
Continue to: Diclofenac
Diclofenac is a nonsteroidal anti-inflammatory drug that reversibly inhibits the enzymes cyclooxygenase-1 and cyclooxygenase-2, resulting in a decrease in the formation of inflammatory prostaglandins, which have been observed in chronically sun-damaged skin, AKs, and cSCC.34,45 Diclofenac 3% gel, applied topically twice daily for 60 to 90 days has been approved by the FDA for treatment of AKs, in conjunction with sun avoidance.34 Topical diclofenac has been demonstrated to be efficacious in treating AKs in the SOTR population46,47; however, multiple meta-analyses using data from immunocompetent patients have demonstrated that topical diclofenac is inferior to other treatment options, particularly 5-FU, at achieving complete clearance of AKs.43,48,49 Diclofenac might be a useful option when patient adherence is expected to be difficult because of adverse effects of therapy: Multiple studies have suggested that diclofenac might be more tolerable than other options.43,48,50
Systemic chemoprophylaxis
Systemic therapies that have been used for chemoprophylaxis against cutaneous malignancy include nicotinamide, oral retinoids, capecitabine, and HPV vaccination. (See TABLE 2.27-40)
Nicotinamide, the amide form of vitamin B3, protects against cutaneous malignancy by aiding repair of DNA damaged by ionizing radiation, such as UV light.27 Efficacy has been demonstrated in reducing development of new AKs and cSCC in immunocompetent patients with a history of more than 2 keratinocyte carcinomas within a 5-year span.27,35 Nicotinamide is especially relevant to the SOTR population because it reduces the level of cutaneous immunity suppression induced by UV radiation without altering patients’ baseline immunity.27,36
There are insufficient long-term follow-up data in the literature to assess the sustainability of the antitumor effects of nicotinamide; studies specific to the SOTR population have been underpowered for assessing its impact on formation of cSCC.27,35Patients taking nicotinamide should be informed of the risk of liver failure at dosages > 3 g/d (antitumor efficacy has been demonstrated at 500 mg twice daily) and advised to avoid purchasing over-the-counter nicotinic acid or niacin as a substitute for nicotinamide, because of the increased incidence of flushing associated with their use.27
Oral retinoids. Systemic retinoids—in particular, acitretin—are efficacious in reducing the risk of cSCC in SOTRs.27,37,38 The primary drawback to cSCC prophylaxis with oral retinoids is a rebound effect, in which treatment discontinuation leads to a rapid return to baseline cSCC formation.27
Continue to: Pregnancy must be avoided...
Pregnancy must be avoided while taking an oral retinoid. Because acitretin can persist in the body for years after discontinuation, its use should generally be avoided in patients of childbearing potential. An FDA black box warning states that patients of childbearing potential must be counseled to use 2 forms of birth control to avoid pregnancy for ≥ 3 years after cessation of oral acitretin. Prior to initiation of oral retinoid therapy, the following baseline laboratory tests should be obtained: complete blood count, creatinine, lipid panel, and liver function tests. For patients with a history of chronic kidney disease or renal transplantation, the lipid panel, liver function tests, and creatinine assay should be repeated with each dosage adjustment and every 3 months once goal-dosing is achieved.27
Capecitabine is typically initiated with the help of Medical Oncology.27,40 A prodrug metabolized by dihydropyrimidine dehydrogenase to 5-FU, capecitabine interacts with warfarin, leading to a significant increase in prothrombin time.39 Other adverse effects associated with oral capecitabine include fatigue, palmar-plantar erythrodysesthesia, diarrhea, and, rarely, neutropenia. Although dihydropyrimidine dehydrogenase deficiency is rare, treatment with capecitabine in patients who have this enzyme deficiency might lead to severe toxicity or death.27
HPV vaccination. HPV might play a role in the development of cutaneous malignancy, especially in immunosuppressed patients.4,5 The utility of HPV vaccination in the prevention of NMSC has yet to be determined, but vaccination has been shown, in case reports, to be helpful in immunocompetent patients.51,52 The immunogenicity of HPV vaccination in the SOTR population is uncertain, and the most common HPV types found in SOTRs are not specifically covered by available HPV vaccines.19
The role of immunosuppression reduction and immunosuppressive replacement
Both the degree of immunosuppression and the individual agents used can affect a patient’s risk of NMSC. Immunosuppression reduction should be considered if skin cancer poses a major risk to the patient’s health and if that risk outweighs the risk of graft rejection associated with immunosuppression reduction.27 In a cohort of 180 kidney and liver SOTRs who developed de novo carcinoma (excluding NMSC) after transplantation, neither reduction of immunosuppression nor introduction of an mTOR inhibitor affected graft survival or oncologic treatment tolerance.53 Because mTOR inhibitors have a protective effect against development of NMSC, they are the preferred choice of immunosuppressive agent from a dermatologic perspective.1,27,54-57 Decisions regarding changes in immunosuppression are generally made by, or in collaboration with, the patient’s transplant physician.
Recommendations: Treating cSCC
Risk should guide strategy
Small lesions of the trunk and extremities without high-risk features can be treated with a destructive method (eg, electrodessication and curettage). However, lesions of the head and neck and those found to have features consistent with an increased risk of recurrence or metastasis should be treated aggressively.3,58,59
Continue to: Risk factors for invasive growth...
Risk factors for invasive growth, recurrence, or metastasis of cSCC in SOTRs are multiple lesions or satellite lesions, indistinct clinical borders, rapid growth, ulceration, and recurrence after treatment.60 The risk of invasive growth, recurrence, and metastasis of cSCC also increases with size and location of the lesion, according to this framework60:
- any size in scar tissue, areas of chronic inflammation, and fields of prior radiation therapy
- ≥ 0.6 cm on hands, feet, genitalia, and mask areas of the face (central face, eyelids, eyebrows, nose, lips, chin, mandible, and temporal, preauricular, postauricular, and periorbital areas)
- > 1 cm on cheeks, forehead, neck, and scalp
- > 2 cm on the trunk and extremities.
In addition, specific findings on histologic analysis portend increased risk of invasive growth, recurrence, or metastasis:
- poor differentiation
- deep extension of the tumor into subcutaneous fat
- perineural invasion or inflammation
- perivascular or intravascular invasion.
Treatment modalities
Mohs surgery is preferred to ensure margin clearance while preserving noninvolved tissue3,7 (FIGURE 4). If Mohs surgery is not possible, the lesion should be excised with 3- to 10-mm margins.3,60 Based on current literature, the roles of nodal staging, sentinel lymph node biopsy, and adjuvant therapy are not well defined, but it is likely that these interventions will play a pivotal role in the management of advanced cSCC in SOTRs in the future.3

Nonsurgical therapeutic options for primary or adjuvant treatment of cSCC include systemic chemotherapy, radiotherapy, and programmed cell death protein 1 inhibitors. (For more on treatment modalities, see TABLE 3.3,7,58-61)

Recommendations: Treating BCC
BCC in SOTRs is treated similarly (TABLE 33,7,58-61) to how it is treated in the immunocompetent population—except that SOTRs require closer follow-up than nontransplant patients because they are at higher risk of recurrence and new NMSCs.3 Standard management after biopsy is either3,61:
- Mohs surgery to ensure margin control (for most BCCs on the head and neck and those with clinical or histologic risk factors for recurrence or aggressive behavior)
- excision with a 4- or 5-mm margin or a destructive modality (for BCCs on the trunk and extremities without risk factors for recurrence).
Radiotherapy is an alternative for patients with high-risk BCCs who are unable to tolerate surgery.3
CORRESPONDENCE
Lindsey Collins, MD, Department of Dermatology, University of Oklahoma Health Sciences Center, 619 NE 13th Steet, Oklahoma City, OK 73104; Lindsey-Collins@ouhsc.edu
The incidence of posttransplant malignancy among solid organ transplant recipients (SOTRs) is 10%; skin cancer, primarily nonmelanoma skin cancer (NMSC), constitutes 49.5% of all malignancies in this population.1 The etiology of the increased risk of cutaneous malignancy in SOTR is multifactorial:
- The skin of SOTRs is photosensitive, compared to that of immunocompetent patients, thus predisposing SOTRs to carcinogenic damage resulting from exposure to UV light.2
- Immunosuppression plays a key role in increasing the risk of cutaneous malignancy by inhibiting the ability of the immune system to recognize and destroy tumor cells.3
- Human papillomavirus (HPV) can play a role in carcinogenesis by promoting molecular pathways to proliferation and survival of nascent tumor cells4;Times New Romanβ-HPV strains are disseminated ubiquitously in the skin of immunosuppressed patients.5
- Some medications administered after transplantation can be directly carcinogenic.

NMSC in SOTRs also differs qualitatively from NMSC in immunocompetent patients. Cutaneous squamous cell carcinoma (cSCC) (FIGUREs 1 and 2) is the most common skin cancer among SOTRs, whereas basal cell carcinoma (BCC) is the most common skin cancer in the general population.3 cSCC in the SOTR population tends to be more aggressive, with more rapid local invasion and an increased rate of both in-transit and distant metastases, leading to an increase in morbidity and mortality. Mortality of metastatic cSCC among SOTRs is approximately 50%, compared to 20% in an otherwise healthy population.3,6-8

The problem is relevant to primary care
Screening. Because there is a demonstrated reduction in morbidity and mortality associated with early detection and treatment of NMSC, regular screening of skin is important in the SOTR population.9 A study in Ontario, Canada, from 1994 to 2012 and comprising 10,183 SOTRs, found that adherence to an annual skin check regimen for ≥ 75% of the observation period was associated with a 34% reduction in cutaneous BCC- and cSCC-related morbidity or death (adjusted hazard ratio = 0.66; 95% CI, 0.48-0.92).10 Although routine follow-up with a dermatologist is recommended for SOTRs,9,11-15 only 2.1% of patients in the Canadian study were fully adherent with annual skin examination, and 55% never visited a dermatologist.10 Consequently, primary care physicians can play a key role in skin cancer screening for SOTRs.
Education regarding the importance of protection from the sun is also an essential part of primary care. A 2018 study of SOTRs in Turkey demonstrated that16
- 46% expressed a lack of knowledge of the hazards of sun exposure
- 44% did not recall ever receiving medical advice regarding sun protection
- 89% did not wear sun-protective clothing
- 86% did not use sunscreen daily.
Multiple studies have demonstrated the positive effect that preventive education and attendance at a dermatology or skin cancer screening clinic can have on sun-protective behaviors among SOTRs.9,16-18 In the Turkish study, 100% of patients who reported using sunscreen daily had been undergoing regular dermatologic examination.16
In this article, we review current management guidelines regarding the prevention and treatment of NMSC in SOTRs.
Recommendations for prevention
Screening skin exams (TABLE 11,11,12,15,19-23). Although definitive guidelines do not exist regarding the frequency of a screening skin exam for SOTRs, multiple frequency-determining algorithms have been proposed.11,12,15,19 The recommended frequency of a skin exam is based on history of skin cancer; for SOTRs, the most common recommendation19 is a full-body skin examination as follows:
- annually—when there is no history of skin cancer
- every 6 months—when there is a history of actinic keratoses (AKs; precancerous lesions that carry a risk of transforming into cSCC) or a single low-risk NMSC
- every 3 months—when there is a history of multiple NMSCs or a single high-risk NMSC
- every 1 to 3 months—when there is a history of metastatic disease.
Continue to: Other risk factors...
Other risk factors for NMSC to consider in SOTRs when determining an appropriate follow-up regimen include any of the following1,20,21,24-26:
- male gender, fair skin, history of childhood sunburn, history of smoking
- lung or heart transplantation, history of episodes of transplant rejection, age ≥ 50 years at transplantation
- immunosuppression with calcineurin inhibitors, compared to mammalian target of rapamycin (mTOR) inhibitors
- immunosuppression with cyclosporine, compared to tacrolimus
- an immunosuppressive regimen with > 1 immunosuppressant or an increased degree of immunosuppression
- antithymocyte globulin within the first year posttransplantation.
Because the intensity of immunosuppression and individual immunosuppressants used affect the risk of NMSC, conduct a thorough medication review with SOTRs at all visits. Ask about new, changing, or symptomatic (pruritic, painful, bleeding) skin lesions, and perform a full-body skin exam. Palpate draining lymph nodes if the patient has a history of NMSC.15 AKs (FIGURE 3) should be treated aggressively with liquid nitrogen or field therapy. Lesions suspicious for NMSC should be biopsied and sent for histologic evaluation.22 Shave, punch, and excisional biopsies are all adequate techniques; however, because all cSCCs in SOTRs are considered high risk for aggressive features, biopsy should extend at least into the reticular dermis to allow evaluation for invasive disease.22

Sun-protective measures (TABLE 11,11,12,15,19-23). Inquire about patients’ habits related to protection from the sun, their knowledge of recommended sun-protective measures, and risks associated with nonadherence. Recommended sun-protective measures include
- daily broad-spectrum sunscreen (SPF ≥ 30), reapplied every 2 hours of sun exposure, in accordance with labeling instructions23
- sun-protective clothing (pants, long sleeves, hat, sunglasses)23
- avoidance of tanning salons.15

SOTRs who adequately adhere to sun-protective measures might need vitamin D supplementation because sunscreen and sun-protective clothing inhibit cutaneous synthesis of vitamin D.15
Recommendations for treatment
Consider chemoprophylactic therapy for SOTRs who have had multiple prior cutaneous malignancies or multiple AKs.
Continue to: Topical chemoprophylaxis
Topical chemoprophylaxis
Topical medications used for cSCC chemoprophylaxis include 5-fluorouracil (5-FU), photodynamic therapy (PDT), imiquimod, ingenol mebutate, topical retinoids, and diclofenac.27 (See TABLE 2.27-40) Of these, the latter 3 are used less commonly because of the small packaging size of ingenol mebutate and the relative lack of efficacy data for topical retinoids and diclofenac.27 Imiquimod is often avoided when treating large surface areas because of the risk of systemic adverse effects associated with cytokine release.27

5-FU is US Food and Drug Administration (FDA)-approved for the treatment of AKs, and is used off-label for treating cSCC in situ (Bowen disease). It is the most commonly used topical therapy for field disease.27-29 5-FU is typically applied once or twice daily for 3 to 4 weeks. Common adverse effects include transient skin irritation and erythema.27

PDT involves topical application of a photosensitizer, such as 5-aminolevulinic acid or methyl aminolevulinate, followed by exposure to a visible light source, leading to antitumor effects on gene expression and destruction of proliferating cells through production of reactive oxygen species.30,31 Evidence is sufficient to support routine use of PDT for AKs and Bowen disease.30 A mild sunburn-like reaction is common following PDT, with transient erythema and discomfort typically lasting 1 to 2 weeks but not typically necessitating analgesic therapy.27
Imiquimod is a ligand that binds to and activates Toll-like receptor 7, leading to enhancement of the cell-mediated antitumor immune response and resultant tissue-specific apoptosis coordinated by type 1 T-helper lymphocytes.32 Topical imiquimod cream is FDA approved for field treatment of AKs at 2.5%, 3.75%, and 5% concentrations; efficacy has been demonstrated in the SOTR population.33,41 Multiple studies in immunocompetent patients have suggested that imiquimod might be slightly less efficacious than 5-FU.42-44
The tolerability of field treatment with imiquimod has been called into question.27 However, in a 2019 study comparing adverse reactions among 513 immunocompetent patients with field disease who were treated with either 5-FU 5% cream; imiquimod 5% cream; PDT with methyl aminolevulinate; or ingenol mebutate 0.015% gel, a similar or smaller percentage of patients treated with imiquimod reported moderate-to-severe itching, moderate-to-severe pain, and any adverse events, compared to patients treated with the other options.44
Continue to: Diclofenac
Diclofenac is a nonsteroidal anti-inflammatory drug that reversibly inhibits the enzymes cyclooxygenase-1 and cyclooxygenase-2, resulting in a decrease in the formation of inflammatory prostaglandins, which have been observed in chronically sun-damaged skin, AKs, and cSCC.34,45 Diclofenac 3% gel, applied topically twice daily for 60 to 90 days has been approved by the FDA for treatment of AKs, in conjunction with sun avoidance.34 Topical diclofenac has been demonstrated to be efficacious in treating AKs in the SOTR population46,47; however, multiple meta-analyses using data from immunocompetent patients have demonstrated that topical diclofenac is inferior to other treatment options, particularly 5-FU, at achieving complete clearance of AKs.43,48,49 Diclofenac might be a useful option when patient adherence is expected to be difficult because of adverse effects of therapy: Multiple studies have suggested that diclofenac might be more tolerable than other options.43,48,50
Systemic chemoprophylaxis
Systemic therapies that have been used for chemoprophylaxis against cutaneous malignancy include nicotinamide, oral retinoids, capecitabine, and HPV vaccination. (See TABLE 2.27-40)
Nicotinamide, the amide form of vitamin B3, protects against cutaneous malignancy by aiding repair of DNA damaged by ionizing radiation, such as UV light.27 Efficacy has been demonstrated in reducing development of new AKs and cSCC in immunocompetent patients with a history of more than 2 keratinocyte carcinomas within a 5-year span.27,35 Nicotinamide is especially relevant to the SOTR population because it reduces the level of cutaneous immunity suppression induced by UV radiation without altering patients’ baseline immunity.27,36
There are insufficient long-term follow-up data in the literature to assess the sustainability of the antitumor effects of nicotinamide; studies specific to the SOTR population have been underpowered for assessing its impact on formation of cSCC.27,35Patients taking nicotinamide should be informed of the risk of liver failure at dosages > 3 g/d (antitumor efficacy has been demonstrated at 500 mg twice daily) and advised to avoid purchasing over-the-counter nicotinic acid or niacin as a substitute for nicotinamide, because of the increased incidence of flushing associated with their use.27
Oral retinoids. Systemic retinoids—in particular, acitretin—are efficacious in reducing the risk of cSCC in SOTRs.27,37,38 The primary drawback to cSCC prophylaxis with oral retinoids is a rebound effect, in which treatment discontinuation leads to a rapid return to baseline cSCC formation.27
Continue to: Pregnancy must be avoided...
Pregnancy must be avoided while taking an oral retinoid. Because acitretin can persist in the body for years after discontinuation, its use should generally be avoided in patients of childbearing potential. An FDA black box warning states that patients of childbearing potential must be counseled to use 2 forms of birth control to avoid pregnancy for ≥ 3 years after cessation of oral acitretin. Prior to initiation of oral retinoid therapy, the following baseline laboratory tests should be obtained: complete blood count, creatinine, lipid panel, and liver function tests. For patients with a history of chronic kidney disease or renal transplantation, the lipid panel, liver function tests, and creatinine assay should be repeated with each dosage adjustment and every 3 months once goal-dosing is achieved.27
Capecitabine is typically initiated with the help of Medical Oncology.27,40 A prodrug metabolized by dihydropyrimidine dehydrogenase to 5-FU, capecitabine interacts with warfarin, leading to a significant increase in prothrombin time.39 Other adverse effects associated with oral capecitabine include fatigue, palmar-plantar erythrodysesthesia, diarrhea, and, rarely, neutropenia. Although dihydropyrimidine dehydrogenase deficiency is rare, treatment with capecitabine in patients who have this enzyme deficiency might lead to severe toxicity or death.27
HPV vaccination. HPV might play a role in the development of cutaneous malignancy, especially in immunosuppressed patients.4,5 The utility of HPV vaccination in the prevention of NMSC has yet to be determined, but vaccination has been shown, in case reports, to be helpful in immunocompetent patients.51,52 The immunogenicity of HPV vaccination in the SOTR population is uncertain, and the most common HPV types found in SOTRs are not specifically covered by available HPV vaccines.19
The role of immunosuppression reduction and immunosuppressive replacement
Both the degree of immunosuppression and the individual agents used can affect a patient’s risk of NMSC. Immunosuppression reduction should be considered if skin cancer poses a major risk to the patient’s health and if that risk outweighs the risk of graft rejection associated with immunosuppression reduction.27 In a cohort of 180 kidney and liver SOTRs who developed de novo carcinoma (excluding NMSC) after transplantation, neither reduction of immunosuppression nor introduction of an mTOR inhibitor affected graft survival or oncologic treatment tolerance.53 Because mTOR inhibitors have a protective effect against development of NMSC, they are the preferred choice of immunosuppressive agent from a dermatologic perspective.1,27,54-57 Decisions regarding changes in immunosuppression are generally made by, or in collaboration with, the patient’s transplant physician.
Recommendations: Treating cSCC
Risk should guide strategy
Small lesions of the trunk and extremities without high-risk features can be treated with a destructive method (eg, electrodessication and curettage). However, lesions of the head and neck and those found to have features consistent with an increased risk of recurrence or metastasis should be treated aggressively.3,58,59
Continue to: Risk factors for invasive growth...
Risk factors for invasive growth, recurrence, or metastasis of cSCC in SOTRs are multiple lesions or satellite lesions, indistinct clinical borders, rapid growth, ulceration, and recurrence after treatment.60 The risk of invasive growth, recurrence, and metastasis of cSCC also increases with size and location of the lesion, according to this framework60:
- any size in scar tissue, areas of chronic inflammation, and fields of prior radiation therapy
- ≥ 0.6 cm on hands, feet, genitalia, and mask areas of the face (central face, eyelids, eyebrows, nose, lips, chin, mandible, and temporal, preauricular, postauricular, and periorbital areas)
- > 1 cm on cheeks, forehead, neck, and scalp
- > 2 cm on the trunk and extremities.
In addition, specific findings on histologic analysis portend increased risk of invasive growth, recurrence, or metastasis:
- poor differentiation
- deep extension of the tumor into subcutaneous fat
- perineural invasion or inflammation
- perivascular or intravascular invasion.
Treatment modalities
Mohs surgery is preferred to ensure margin clearance while preserving noninvolved tissue3,7 (FIGURE 4). If Mohs surgery is not possible, the lesion should be excised with 3- to 10-mm margins.3,60 Based on current literature, the roles of nodal staging, sentinel lymph node biopsy, and adjuvant therapy are not well defined, but it is likely that these interventions will play a pivotal role in the management of advanced cSCC in SOTRs in the future.3

Nonsurgical therapeutic options for primary or adjuvant treatment of cSCC include systemic chemotherapy, radiotherapy, and programmed cell death protein 1 inhibitors. (For more on treatment modalities, see TABLE 3.3,7,58-61)

Recommendations: Treating BCC
BCC in SOTRs is treated similarly (TABLE 33,7,58-61) to how it is treated in the immunocompetent population—except that SOTRs require closer follow-up than nontransplant patients because they are at higher risk of recurrence and new NMSCs.3 Standard management after biopsy is either3,61:
- Mohs surgery to ensure margin control (for most BCCs on the head and neck and those with clinical or histologic risk factors for recurrence or aggressive behavior)
- excision with a 4- or 5-mm margin or a destructive modality (for BCCs on the trunk and extremities without risk factors for recurrence).
Radiotherapy is an alternative for patients with high-risk BCCs who are unable to tolerate surgery.3
CORRESPONDENCE
Lindsey Collins, MD, Department of Dermatology, University of Oklahoma Health Sciences Center, 619 NE 13th Steet, Oklahoma City, OK 73104; Lindsey-Collins@ouhsc.edu
1. Bhat M, Mara K, Dierkhising R, et al. Immunosuppression, race, and donor-related risk factors affect de novo cancer incidence across solid organ transplant recipients. Mayo Clin Proc. 2018;93:1236-1246. doi: 10.1016/j.mayocp.2018.04.025
2. Togsverd-Bo K, Philipsen PA, Haedersdal M, et al. Organ transplant recipients express enhanced skin autofluorescence and pigmentation at skin cancer sites. J Photochem Photobiol B. 2018;188:1-5. doi: 10.1016/j.jphotobiol.2018.08.008
3. Kearney L, Hogan D, Conlon P, et al. High-risk cutaneous malignancies and immunosuppression: challenges for the reconstructive surgeon in the renal transplant population. J Plast Reconstr Aesthet Surg. 2017;70:922-930. doi: 10.1016/j.bjps.2017.03.005
4. Borgogna C, Olivero C, Lanfredini S, et al. β-HPV infection correlates with early stages of carcinogenesis in skin tumors and patient-derived xenografts from a kidney transplant recipient cohort. Front Microbiol. 2018;9:117. doi: 10.3389/fmicb.2018.00117
5. Nunes EM, Talpe-Nunes V, Sichero L. Epidemiology and biology of cutaneous human papillomavirus. Clinics (Sao Paulo). 2018;73(suppl 1):e489s. doi: 10.6061/clinics/2018/e489s
6. Pini AM, Koch S, L, et al. Eruptive keratoacanthoma following topical imiquimod for in situ squamous cell carcinoma of the skin in a renal transplant recipient. J Am Acad Dermatol. 2008;59(suppl 5):S116-S117. doi: 10.1016/j.jaad.2008.06.018
7. Ilyas M, Zhang N, Sharma A. Residual squamous cell carcinoma after shave biopsy in solid organ transplant recipients. Dermatol Surg. 2018;44:370-374. doi: 10.1097/DSS.0000000000001340
8. Brunner M, Veness MJ, Ch‘ng S, et al. Distant metastases from cutaneous squamous cell carcinoma—analysis of AJCC stage IV. Head Neck. 2013;35:72-75. doi: 10.1002/hed.22913
9. Hartman RI, Green AC, Gordon LG; . Sun protection among organ transplant recipients after participation in a skin cancer research study. JAMA Dermatol. 2018;154:842-844. doi: 10.1001/jamadermatol.2018.1164
10. Chan A-W, Fung K, Austin PC, et al. Improved keratinocyte carcinoma outcomes with annual dermatology assessment after solid organ transplantation: population-based cohort study. Am J Transplant. 2018;19:522-531. doi: 10.1111/ajt.14966
11. Hofbauer GF, Anliker M, Arnold A, et al. Swiss clinical practice guidelines for skin cancer in organ transplant recipients. Swiss Med Wkly. 2009;139:407-415. doi: https://doi.org/10.4414/smw.2014.14026
12. Ulrich C, Kanitakis J, Stockfleth E, et al. Skin cancer in organ transplant recipients—where do we stand today? Am J Transplant. 2008;8:2192-2198. doi: 10.1111/j.1600-6143.2008.02386.x
13. Chen SC, Pennie ML, Kolm P, et al. Diagnosing and managing cutaneous pigmented lesions: primary care physicians versus dermatologists. J Gen Intern Med. 2006;21:678-682. doi: 10.1111/j.1525-1497.2006.00462.x
14. Ismail F, Mitchell L, Casabonne D, et al. Specialist dermatology clinics for organ transplant recipients significantly improve compliance with photoprotection and levels of skin cancer awareness. Br J Dermatol. 2006;155:916-925. doi: 10.1111/j.1365-2133.2006.07454.x
15. O‘Reilly Zwald F, Brown M. Skin cancer in solid organ transplant recipients: advances in therapy and management: part II. Management of skin cancer in solid organ transplant recipients. J Am Acad Dermatol. 2011;65:263-279. doi: 10.1016/j.jaad.2010.11.063
16. Vural A, A, Kirnap M, et al. Skin cancer risk awareness and sun-protective behavior among solid-organ transplant recipients. Exp Clin Transplant. 2018;16(suppl 1):203-207. doi: 10.6002/ect.TOND-TDTD2017.P65
17. Papier K, Gordon LG, Khosrotehrani K, et al. Increase in preventive behaviour by organ transplant recipients after sun protection information in a skin cancer surveillance clinic. Br J Dermatol. 2018;179:1195-1196. doi: 10.1111/bjd.16836
18. Wu SZ, Jiang P, DeCaro JE, et al. A qualitative systematic review of the efficacy of sun protection education in organ transplant recipients. J Am Acad Dermatol. 2016;75:1238-1244.e5. doi: 10.1016/j.jaad.2016.06.031
19. Blomberg M, He SY, Harwood C, et al; . Research gaps in the management and prevention of cutaneous squamous cell carcinoma in organ transplant recipients. Br J Dermatol. 2017;177:1225-1233. doi: 10.1111/bjd.15950
20. Urwin HR, Jones PW, Harden PN, et al. Predicting risk of nonmelanoma skin cancer and premalignant skin lesions in renal transplant recipients. Transplantation. 2009;87:1667-1671. doi: 10.1097/TP.0b013e3181a5ce2e
21. Infusino SD, Loi C, Ravaioli GM, et al. Cutaneous complications of immunosuppression in 812 transplant recipients: a 40-year single center experience. G Ital Dermatol Venereol. 2020;155:662-668. doi: 10.23736/S0392-0488.18.06091-1
22. Naldi L, Venturuzzo A, Invernizzi P. Dermatological complications after solid organ transplantation. Clin Rev Allergy Immunol. 2018;54:185-212. doi: 10.1007/s12016-017-8657-9
23. Sunscreen FAQs. American Academy of Dermatology Web site. Accessed February 25, 2021. www.aad.org/media/stats/prevention-and-care/sunscreen-faqs
24. Vos M, Plasmeijer EI, van Bemmel BC, et al. Azathioprine to mycophenolate mofetil transition and risk of squamous cell carcinoma after lung transplantation. J Heart Lung Transplant. 2018;37:853-859. doi: 10.1016/j.healun.2018.03.012
25. Puza CJ, Myers SA, Cardones AR, et al. The impact of transplant rejection on cutaneous squamous cell carcinoma in renal transplant recipients. Clin Exp Dermatol. 2018;44:265-269. doi: 10.1111/ced.13699
26. Abikhair Burgo M, Roudiani N, Chen J, et al. Ruxolitinib inhibits cyclosporine-induced proliferation of cutaneous squamous cell carcinoma. JCI Insight. 2018;3:e120750. doi: 10.1172/jci.insight.120750
27. Que SKT, Zwald FO, Schmults CD. Cutaneous squamous cell carcinoma: management of advanced and high-stage tumors. J Am Acad Dermatol. 2018;78:249-261. doi: 10.1016/j.jaad.2017.08.058
28. Askew DA, Mickan SM, Soyer HP, et al. Effectiveness of 5-fluorouracil treatment for actinic keratosis—a systematic review of randomized controlled trials. Int J Dermatol. 2009;48:453-463. doi: 10.1111/j.1365-4632.2009.04045.x
29. Salim A, Leman JA, McColl JH, et al. Randomized comparison of photodynamic therapy with topical 5-fluorouracil in Bowen‘s disease. Br J Dermatol. 2003;148:539-543. doi: 10.1046/j.1365-2133.2003.05033.x
30. Morton CA. A synthesis of the world‘s guidelines on photodynamic therapy for non-melanoma skin cancer. G Ital Dermatol Venereol. 2018;153:783-792. doi: 10.23736/S0392-0488.18.05896-0
31. Joly F, Deret S, Gamboa B, et al. Photodynamic therapy corrects abnormal cancer-associated gene expression observed in actinic keratosis lesions and induces a remodeling effect in photodamaged skin. J Dermatol Sci. 2018;S0923-1811(17)30775-2. doi: 10.1016/j.jdermsci.2018.05.002
32. Patel GK, Goodwin R, Chawla M, et al. Imiquimod 5% cream monotherapy for cutaneous squamous cell carcinoma in situ (Bowen‘s disease): a randomized, double-blind, placebo-controlled trial. J Am Acad Dermatol. 2006;54:1025-1032. doi: 10.1016/j.jaad.2006.01.055
33. Imiquimod. Wolters Kluwer Clinical Drug Information, Inc.; 2019. Accessed August 6, 2019. http://online.lexi.com/lco/action/doc/retrieve/docid/patch_f/7077?cesid=aRo1Yh9sd0Q&searchUrl=%2Flco%2Faction%2Fsearch%3Fq%3Dimiquimod%26t%3Dname%26va%3Dimiquimod
34. Diclofenac. Wolters Kluwer Clinical Drug Information, Inc.; 2019. Accessed August 6th, 2019. http://online.lexi.com/lco/action/doc/retrieve/docid/patch_f/1772965?cesid=5vTk7J3Vmvc&searchUrl=%2Flco%2Faction%2Fsearch%3Fq%3Ddiclofenac%26t%3Dname%26va%3Ddiclofenac
35. Chen AC, Martin AJ, Choy B, et al. A phase 3 randomized trial of nicotinamide for skin-cancer chemoprevention. N Engl J Med. 2015;373:1618-1626. doi: 10.1056/NEJMoa1506197
36. Yiasemides E, Sivapirabu G, Halliday GM, et al. Oral nicotinamide protects against ultraviolet radiation-induced immunosuppression in humans. Carcinogenesis. 2009;30:101-105. doi: 10.1093/carcin/bgn248
37. Otley CC, Stasko T, Tope WD, et al. Chemoprevention of nonmelanoma skin cancer with systemic retinoids: practical dosing and management of adverse effects. Dermatol Surg. 2006;32:562-568. doi: 10.1111/j.1524-4725.2006.32115.x
38. McKenna DB, Murphy GM. Skin cancer chemoprophylaxis in renal transplant recipients: 5 years of experience using low-dose acitretin. Br J Dermatol. 1999;140:656-660. doi: 10.1046/j.1365-2133.1999.02765.x
39. Capecitabine. Wolters Kluwer Clinical Drug Information, Inc.; 2019. Accessed February 13, 2019. http://online.lexi.com/lco/action/doc/retrieve/docid/patch_f/6519?cesid=7WMsK72X7T7&searchUrl=%2Flco%2Faction%2Fsearch%3Fq%3Dcapecitabine%26t%3Dname%26va%3Dcapecitabine
40. Wollina U, Hansel G, Koch A, et al. Oral capecitabine plus subcutaneous interferon alpha in advanced squamous cell carcinoma of the skin. J Cancer Res Clin Oncol. 2005;131:300-304. doi: 10.1007/s00432-004-0656-6
41. Zavattaro E, Veronese F, Landucci G, et al. Efficacy of topical imiquimod 3.75% in the treatment of actinic keratosis of the scalp in immunosuppressed patients: our case series. J Dermatol Treat. 2020;31:285-289. doi: 10.1080/09546634.2019.1590524
42. Neugebauer R, Su KA, Zhu Z, et al. Comparative effectiveness of treatment of actinic keratosis with topical fluorouracil and imiquimod in the prevention of keratinocyte carcinoma: a cohort study. J Am Acad Dermatol. 2019;80:998-1005. doi: 10.1016/j.jaad.2018.11.024
43. Gupta AK, Paquet M. Network meta-analysis of the outcome ‚participant complete clearance in nonimmunosuppressed participants of eight interventions for actinic keratosis: a follow-up on a Cochrane review. Br J Dermatol. 2013;169:250-259. doi: 10.1111/bjd.12343
44. Jansen MHE, Kessels JPHM, Nelemans PJ, et al. Randomized trial of four treatment approaches for actinic keratosis. N Engl J Med. 2019;380:935-946. doi: 10.1056/NEJMoa1811850
45. Bangash HK, Colegio OR. Management of non-melanoma skin cancer in immunocompromised solid organ transplant recipients. Curr Treat Options Oncol. 2012;13:354-376. doi: 10.1007/s11864-012-0195-3
46. Ulrich C, Johannsen A, J, et al. Results of a randomized, placebo-controlled safety and efficacy study of topical diclofenac 3% gel in organ transplant patients with multiple actinic keratoses. Eur J Dermatol. 2010;20:482-488. doi: 10.1684/ejd.2010.1010
47. Ulrich C, Hackethal M, Ulrich M, et al. Treatment of multiple actinic keratoses with topical diclofenac 3% gel in organ transplant recipients: a series of six cases. Br J Dermatol. 2007;156(suppl 3):40-42. doi: 10.1111/j.1365-2133.2007.07864.x
48. Wu Y, Tang N, Cai L, et al. Relative efficacy of 5-fluorouracil compared with other treatments among patients with actinic keratosis: a network meta-analysis. Dermatol Ther. 2019;32:e12822. doi: 10.1111/dth.12822
49. Stockfleth E, Kerl H, Zwingers T, et al. Low-dose 5-fluorouracil in combination with salicylic acid as a new lesion-directed option to treat topically actinic keratoses: histological and clinical study results. Br J Dermatol. 2011;165:1101-1108. doi: 10.1111/j.1365-2133.2011.10387.x
50. Smith SR, Morhenn VB, Piacquadio DJ. Bilateral comparison of the efficacy and tolerability of 3% diclofenac sodium gel and 5% 5-fluorouracil cream in the treatment of actinic keratoses of the face and scalp. J Drug Dermatol. 2006;5:156-159.
51. Nichols AJ, Allen AH, Shareef S, et al. Association of human papillomavirus vaccine with the development of keratinocyte carcinomas. JAMA Dermatol. 2017;153:571-574. doi: 10.1001/jamadermatol.2016.5703
52. Nichols AJ, Gonzalez A, Clark ES, et al. Combined systemic and intratumoral administration of human papillomavirus vaccine to treat multiple cutaneous basaloid squamous cell carcinomas. JAMA Dermatol. 2018;154:927-930. doi: 10.1001/jamadermatol.2018.1748
53. Rousseau B, Guillemin A, Duvoux C, et al. Optimal oncologic management and mTOR inhibitor introduction are safe and improve survival in kidney and liver allograft recipients with de novo carcinoma. Int J Cancer. 2019;144:886-896. doi: 10.1002/ijc.31769
54. Mathew T, Kreis H, Friend P. Two-year incidence of malignancy in sirolimus-treated renal transplant recipients: results from five multicenter studies. Clin Transplant. 2004;18:446-449. doi: 10.1111/j.1399-0012.2004.00188.x
55. Euvrard S, Morelon E, Rostaing L, et al; Sirolimus and secondary skin-cancer prevention in kidney transplantation. N Engl J Med. 2012;367:329-339. doi: 10.1056/NEJMoa1204166
56. Hoogendijk-van den Akker JM, Harden PN, Hoitsma AJ, et al. Two-year randomized controlled prospective trial converting treatment of stable renal transplant recipients with cutaneous invasive squamous cell carcinomas to sirolimus. J Clin Oncol. 2013;31:1317-1323. doi: 10.1200/JCO.2012.45.6376
57. Karia PS, Azzi JR, Heher EC, et al. Association of sirolimus use with risk for skin cancer in a mixed-organ cohort of solid-organ transplant recipients with a history of cancer. JAMA Dermatol. 2016;152:533-540. doi: 10.1001/jamadermatol.2015.5548
58. Stratigos A, Garbe C, Lebbe C, et al; . Diagnosis and treatment of invasive squamous cell carcinoma of the skin: European consensus-based interdisciplinary guideline. Eur J Cancer. 2015;51:1989-2007. doi: 10.1016/j.ejca.2015.06.110
59. Goldman G. The current status of curettage and electrodesiccation. Dermatol Clin. 2002;20:569-578, ix. doi: 10.1016/s0733-8635(02)00022-0
60. Stasko T, Brown MD, Carucci JA, et al; ; . Guidelines for the management of squamous cell carcinoma in organ transplant recipients. Derm Surg. 2004;30:642-650. doi: 10.1111/j.1524-4725.2004.30150.x
61. Telfer NR, Colver GB, Morton CA; . Guidelines for the management of basal cell carcinoma. Br J Dermatol. 2008;159:35-48. doi: 10.1111/j.1365-2133.2008.08666.x
1. Bhat M, Mara K, Dierkhising R, et al. Immunosuppression, race, and donor-related risk factors affect de novo cancer incidence across solid organ transplant recipients. Mayo Clin Proc. 2018;93:1236-1246. doi: 10.1016/j.mayocp.2018.04.025
2. Togsverd-Bo K, Philipsen PA, Haedersdal M, et al. Organ transplant recipients express enhanced skin autofluorescence and pigmentation at skin cancer sites. J Photochem Photobiol B. 2018;188:1-5. doi: 10.1016/j.jphotobiol.2018.08.008
3. Kearney L, Hogan D, Conlon P, et al. High-risk cutaneous malignancies and immunosuppression: challenges for the reconstructive surgeon in the renal transplant population. J Plast Reconstr Aesthet Surg. 2017;70:922-930. doi: 10.1016/j.bjps.2017.03.005
4. Borgogna C, Olivero C, Lanfredini S, et al. β-HPV infection correlates with early stages of carcinogenesis in skin tumors and patient-derived xenografts from a kidney transplant recipient cohort. Front Microbiol. 2018;9:117. doi: 10.3389/fmicb.2018.00117
5. Nunes EM, Talpe-Nunes V, Sichero L. Epidemiology and biology of cutaneous human papillomavirus. Clinics (Sao Paulo). 2018;73(suppl 1):e489s. doi: 10.6061/clinics/2018/e489s
6. Pini AM, Koch S, L, et al. Eruptive keratoacanthoma following topical imiquimod for in situ squamous cell carcinoma of the skin in a renal transplant recipient. J Am Acad Dermatol. 2008;59(suppl 5):S116-S117. doi: 10.1016/j.jaad.2008.06.018
7. Ilyas M, Zhang N, Sharma A. Residual squamous cell carcinoma after shave biopsy in solid organ transplant recipients. Dermatol Surg. 2018;44:370-374. doi: 10.1097/DSS.0000000000001340
8. Brunner M, Veness MJ, Ch‘ng S, et al. Distant metastases from cutaneous squamous cell carcinoma—analysis of AJCC stage IV. Head Neck. 2013;35:72-75. doi: 10.1002/hed.22913
9. Hartman RI, Green AC, Gordon LG; . Sun protection among organ transplant recipients after participation in a skin cancer research study. JAMA Dermatol. 2018;154:842-844. doi: 10.1001/jamadermatol.2018.1164
10. Chan A-W, Fung K, Austin PC, et al. Improved keratinocyte carcinoma outcomes with annual dermatology assessment after solid organ transplantation: population-based cohort study. Am J Transplant. 2018;19:522-531. doi: 10.1111/ajt.14966
11. Hofbauer GF, Anliker M, Arnold A, et al. Swiss clinical practice guidelines for skin cancer in organ transplant recipients. Swiss Med Wkly. 2009;139:407-415. doi: https://doi.org/10.4414/smw.2014.14026
12. Ulrich C, Kanitakis J, Stockfleth E, et al. Skin cancer in organ transplant recipients—where do we stand today? Am J Transplant. 2008;8:2192-2198. doi: 10.1111/j.1600-6143.2008.02386.x
13. Chen SC, Pennie ML, Kolm P, et al. Diagnosing and managing cutaneous pigmented lesions: primary care physicians versus dermatologists. J Gen Intern Med. 2006;21:678-682. doi: 10.1111/j.1525-1497.2006.00462.x
14. Ismail F, Mitchell L, Casabonne D, et al. Specialist dermatology clinics for organ transplant recipients significantly improve compliance with photoprotection and levels of skin cancer awareness. Br J Dermatol. 2006;155:916-925. doi: 10.1111/j.1365-2133.2006.07454.x
15. O‘Reilly Zwald F, Brown M. Skin cancer in solid organ transplant recipients: advances in therapy and management: part II. Management of skin cancer in solid organ transplant recipients. J Am Acad Dermatol. 2011;65:263-279. doi: 10.1016/j.jaad.2010.11.063
16. Vural A, A, Kirnap M, et al. Skin cancer risk awareness and sun-protective behavior among solid-organ transplant recipients. Exp Clin Transplant. 2018;16(suppl 1):203-207. doi: 10.6002/ect.TOND-TDTD2017.P65
17. Papier K, Gordon LG, Khosrotehrani K, et al. Increase in preventive behaviour by organ transplant recipients after sun protection information in a skin cancer surveillance clinic. Br J Dermatol. 2018;179:1195-1196. doi: 10.1111/bjd.16836
18. Wu SZ, Jiang P, DeCaro JE, et al. A qualitative systematic review of the efficacy of sun protection education in organ transplant recipients. J Am Acad Dermatol. 2016;75:1238-1244.e5. doi: 10.1016/j.jaad.2016.06.031
19. Blomberg M, He SY, Harwood C, et al; . Research gaps in the management and prevention of cutaneous squamous cell carcinoma in organ transplant recipients. Br J Dermatol. 2017;177:1225-1233. doi: 10.1111/bjd.15950
20. Urwin HR, Jones PW, Harden PN, et al. Predicting risk of nonmelanoma skin cancer and premalignant skin lesions in renal transplant recipients. Transplantation. 2009;87:1667-1671. doi: 10.1097/TP.0b013e3181a5ce2e
21. Infusino SD, Loi C, Ravaioli GM, et al. Cutaneous complications of immunosuppression in 812 transplant recipients: a 40-year single center experience. G Ital Dermatol Venereol. 2020;155:662-668. doi: 10.23736/S0392-0488.18.06091-1
22. Naldi L, Venturuzzo A, Invernizzi P. Dermatological complications after solid organ transplantation. Clin Rev Allergy Immunol. 2018;54:185-212. doi: 10.1007/s12016-017-8657-9
23. Sunscreen FAQs. American Academy of Dermatology Web site. Accessed February 25, 2021. www.aad.org/media/stats/prevention-and-care/sunscreen-faqs
24. Vos M, Plasmeijer EI, van Bemmel BC, et al. Azathioprine to mycophenolate mofetil transition and risk of squamous cell carcinoma after lung transplantation. J Heart Lung Transplant. 2018;37:853-859. doi: 10.1016/j.healun.2018.03.012
25. Puza CJ, Myers SA, Cardones AR, et al. The impact of transplant rejection on cutaneous squamous cell carcinoma in renal transplant recipients. Clin Exp Dermatol. 2018;44:265-269. doi: 10.1111/ced.13699
26. Abikhair Burgo M, Roudiani N, Chen J, et al. Ruxolitinib inhibits cyclosporine-induced proliferation of cutaneous squamous cell carcinoma. JCI Insight. 2018;3:e120750. doi: 10.1172/jci.insight.120750
27. Que SKT, Zwald FO, Schmults CD. Cutaneous squamous cell carcinoma: management of advanced and high-stage tumors. J Am Acad Dermatol. 2018;78:249-261. doi: 10.1016/j.jaad.2017.08.058
28. Askew DA, Mickan SM, Soyer HP, et al. Effectiveness of 5-fluorouracil treatment for actinic keratosis—a systematic review of randomized controlled trials. Int J Dermatol. 2009;48:453-463. doi: 10.1111/j.1365-4632.2009.04045.x
29. Salim A, Leman JA, McColl JH, et al. Randomized comparison of photodynamic therapy with topical 5-fluorouracil in Bowen‘s disease. Br J Dermatol. 2003;148:539-543. doi: 10.1046/j.1365-2133.2003.05033.x
30. Morton CA. A synthesis of the world‘s guidelines on photodynamic therapy for non-melanoma skin cancer. G Ital Dermatol Venereol. 2018;153:783-792. doi: 10.23736/S0392-0488.18.05896-0
31. Joly F, Deret S, Gamboa B, et al. Photodynamic therapy corrects abnormal cancer-associated gene expression observed in actinic keratosis lesions and induces a remodeling effect in photodamaged skin. J Dermatol Sci. 2018;S0923-1811(17)30775-2. doi: 10.1016/j.jdermsci.2018.05.002
32. Patel GK, Goodwin R, Chawla M, et al. Imiquimod 5% cream monotherapy for cutaneous squamous cell carcinoma in situ (Bowen‘s disease): a randomized, double-blind, placebo-controlled trial. J Am Acad Dermatol. 2006;54:1025-1032. doi: 10.1016/j.jaad.2006.01.055
33. Imiquimod. Wolters Kluwer Clinical Drug Information, Inc.; 2019. Accessed August 6, 2019. http://online.lexi.com/lco/action/doc/retrieve/docid/patch_f/7077?cesid=aRo1Yh9sd0Q&searchUrl=%2Flco%2Faction%2Fsearch%3Fq%3Dimiquimod%26t%3Dname%26va%3Dimiquimod
34. Diclofenac. Wolters Kluwer Clinical Drug Information, Inc.; 2019. Accessed August 6th, 2019. http://online.lexi.com/lco/action/doc/retrieve/docid/patch_f/1772965?cesid=5vTk7J3Vmvc&searchUrl=%2Flco%2Faction%2Fsearch%3Fq%3Ddiclofenac%26t%3Dname%26va%3Ddiclofenac
35. Chen AC, Martin AJ, Choy B, et al. A phase 3 randomized trial of nicotinamide for skin-cancer chemoprevention. N Engl J Med. 2015;373:1618-1626. doi: 10.1056/NEJMoa1506197
36. Yiasemides E, Sivapirabu G, Halliday GM, et al. Oral nicotinamide protects against ultraviolet radiation-induced immunosuppression in humans. Carcinogenesis. 2009;30:101-105. doi: 10.1093/carcin/bgn248
37. Otley CC, Stasko T, Tope WD, et al. Chemoprevention of nonmelanoma skin cancer with systemic retinoids: practical dosing and management of adverse effects. Dermatol Surg. 2006;32:562-568. doi: 10.1111/j.1524-4725.2006.32115.x
38. McKenna DB, Murphy GM. Skin cancer chemoprophylaxis in renal transplant recipients: 5 years of experience using low-dose acitretin. Br J Dermatol. 1999;140:656-660. doi: 10.1046/j.1365-2133.1999.02765.x
39. Capecitabine. Wolters Kluwer Clinical Drug Information, Inc.; 2019. Accessed February 13, 2019. http://online.lexi.com/lco/action/doc/retrieve/docid/patch_f/6519?cesid=7WMsK72X7T7&searchUrl=%2Flco%2Faction%2Fsearch%3Fq%3Dcapecitabine%26t%3Dname%26va%3Dcapecitabine
40. Wollina U, Hansel G, Koch A, et al. Oral capecitabine plus subcutaneous interferon alpha in advanced squamous cell carcinoma of the skin. J Cancer Res Clin Oncol. 2005;131:300-304. doi: 10.1007/s00432-004-0656-6
41. Zavattaro E, Veronese F, Landucci G, et al. Efficacy of topical imiquimod 3.75% in the treatment of actinic keratosis of the scalp in immunosuppressed patients: our case series. J Dermatol Treat. 2020;31:285-289. doi: 10.1080/09546634.2019.1590524
42. Neugebauer R, Su KA, Zhu Z, et al. Comparative effectiveness of treatment of actinic keratosis with topical fluorouracil and imiquimod in the prevention of keratinocyte carcinoma: a cohort study. J Am Acad Dermatol. 2019;80:998-1005. doi: 10.1016/j.jaad.2018.11.024
43. Gupta AK, Paquet M. Network meta-analysis of the outcome ‚participant complete clearance in nonimmunosuppressed participants of eight interventions for actinic keratosis: a follow-up on a Cochrane review. Br J Dermatol. 2013;169:250-259. doi: 10.1111/bjd.12343
44. Jansen MHE, Kessels JPHM, Nelemans PJ, et al. Randomized trial of four treatment approaches for actinic keratosis. N Engl J Med. 2019;380:935-946. doi: 10.1056/NEJMoa1811850
45. Bangash HK, Colegio OR. Management of non-melanoma skin cancer in immunocompromised solid organ transplant recipients. Curr Treat Options Oncol. 2012;13:354-376. doi: 10.1007/s11864-012-0195-3
46. Ulrich C, Johannsen A, J, et al. Results of a randomized, placebo-controlled safety and efficacy study of topical diclofenac 3% gel in organ transplant patients with multiple actinic keratoses. Eur J Dermatol. 2010;20:482-488. doi: 10.1684/ejd.2010.1010
47. Ulrich C, Hackethal M, Ulrich M, et al. Treatment of multiple actinic keratoses with topical diclofenac 3% gel in organ transplant recipients: a series of six cases. Br J Dermatol. 2007;156(suppl 3):40-42. doi: 10.1111/j.1365-2133.2007.07864.x
48. Wu Y, Tang N, Cai L, et al. Relative efficacy of 5-fluorouracil compared with other treatments among patients with actinic keratosis: a network meta-analysis. Dermatol Ther. 2019;32:e12822. doi: 10.1111/dth.12822
49. Stockfleth E, Kerl H, Zwingers T, et al. Low-dose 5-fluorouracil in combination with salicylic acid as a new lesion-directed option to treat topically actinic keratoses: histological and clinical study results. Br J Dermatol. 2011;165:1101-1108. doi: 10.1111/j.1365-2133.2011.10387.x
50. Smith SR, Morhenn VB, Piacquadio DJ. Bilateral comparison of the efficacy and tolerability of 3% diclofenac sodium gel and 5% 5-fluorouracil cream in the treatment of actinic keratoses of the face and scalp. J Drug Dermatol. 2006;5:156-159.
51. Nichols AJ, Allen AH, Shareef S, et al. Association of human papillomavirus vaccine with the development of keratinocyte carcinomas. JAMA Dermatol. 2017;153:571-574. doi: 10.1001/jamadermatol.2016.5703
52. Nichols AJ, Gonzalez A, Clark ES, et al. Combined systemic and intratumoral administration of human papillomavirus vaccine to treat multiple cutaneous basaloid squamous cell carcinomas. JAMA Dermatol. 2018;154:927-930. doi: 10.1001/jamadermatol.2018.1748
53. Rousseau B, Guillemin A, Duvoux C, et al. Optimal oncologic management and mTOR inhibitor introduction are safe and improve survival in kidney and liver allograft recipients with de novo carcinoma. Int J Cancer. 2019;144:886-896. doi: 10.1002/ijc.31769
54. Mathew T, Kreis H, Friend P. Two-year incidence of malignancy in sirolimus-treated renal transplant recipients: results from five multicenter studies. Clin Transplant. 2004;18:446-449. doi: 10.1111/j.1399-0012.2004.00188.x
55. Euvrard S, Morelon E, Rostaing L, et al; Sirolimus and secondary skin-cancer prevention in kidney transplantation. N Engl J Med. 2012;367:329-339. doi: 10.1056/NEJMoa1204166
56. Hoogendijk-van den Akker JM, Harden PN, Hoitsma AJ, et al. Two-year randomized controlled prospective trial converting treatment of stable renal transplant recipients with cutaneous invasive squamous cell carcinomas to sirolimus. J Clin Oncol. 2013;31:1317-1323. doi: 10.1200/JCO.2012.45.6376
57. Karia PS, Azzi JR, Heher EC, et al. Association of sirolimus use with risk for skin cancer in a mixed-organ cohort of solid-organ transplant recipients with a history of cancer. JAMA Dermatol. 2016;152:533-540. doi: 10.1001/jamadermatol.2015.5548
58. Stratigos A, Garbe C, Lebbe C, et al; . Diagnosis and treatment of invasive squamous cell carcinoma of the skin: European consensus-based interdisciplinary guideline. Eur J Cancer. 2015;51:1989-2007. doi: 10.1016/j.ejca.2015.06.110
59. Goldman G. The current status of curettage and electrodesiccation. Dermatol Clin. 2002;20:569-578, ix. doi: 10.1016/s0733-8635(02)00022-0
60. Stasko T, Brown MD, Carucci JA, et al; ; . Guidelines for the management of squamous cell carcinoma in organ transplant recipients. Derm Surg. 2004;30:642-650. doi: 10.1111/j.1524-4725.2004.30150.x
61. Telfer NR, Colver GB, Morton CA; . Guidelines for the management of basal cell carcinoma. Br J Dermatol. 2008;159:35-48. doi: 10.1111/j.1365-2133.2008.08666.x
PRACTICE RECOMMENDATIONS
› Conduct a full-body skin examination at least once annually for solid organ transplant recipients. C
› Encourage daily use of broad-spectrum SPF ≥ 30 sunscreen and sun-protective clothing (long sleeves, pants, wide-brimmed hats) for these patients. A
› Consider chemoprophylactic agents for patients at especially high risk of nonmelanoma skin cancer. A
› Treat nonmelanoma skin cancer in a solid organ transplant recipient aggressively because of their increased risk of recurrence, local invasion, and metastasis. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series