User login
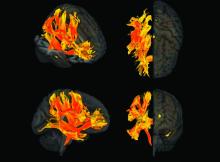
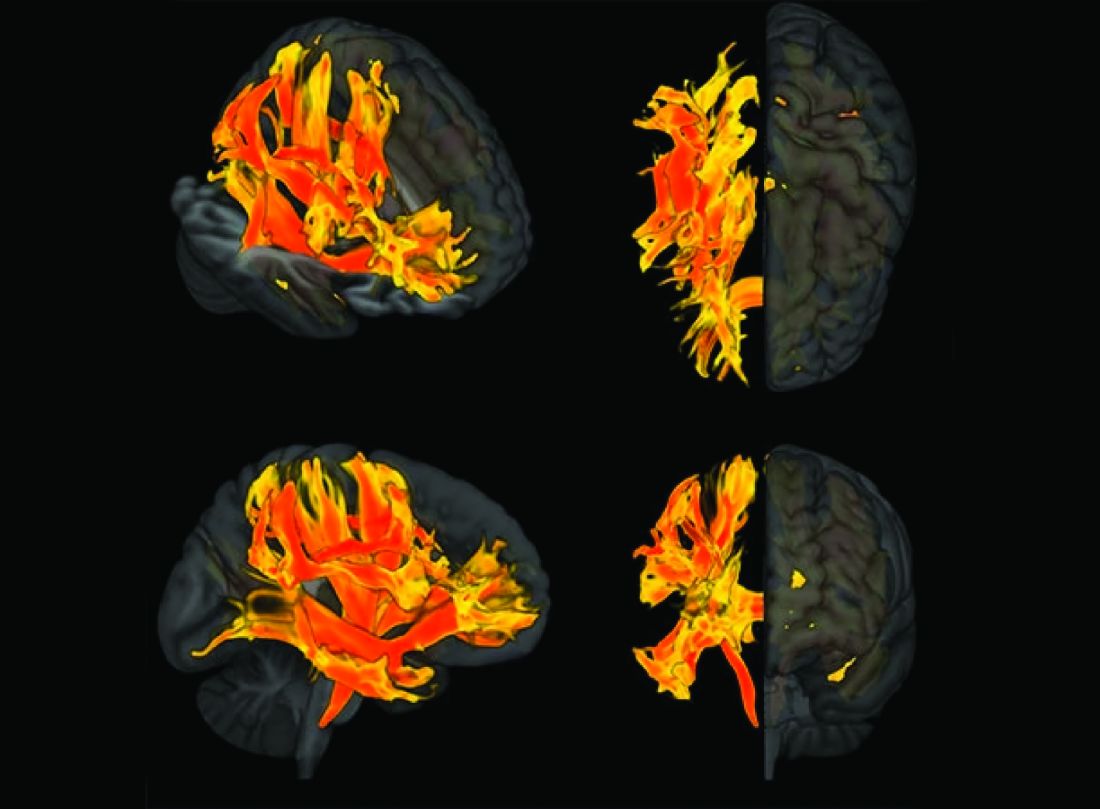
Arterial Stiffness May Predict Risk for Glaucoma
TOPLINE:
Arterial stiffness increases the risk for developing glaucoma, a new study found.
METHODOLOGY:
- To study the link between arterial stiffness and glaucoma, the researchers evaluated 4713 individuals (mean age, 66 years; 58% men) without the eye condition at baseline between April 2011 and November 2012.
- They assessed arterial stiffness by measuring aortic pulse wave velocity, estimated carotid-femoral pulse wave velocity, and aortic pulse pressure.
- The primary outcome was incident glaucoma, identified from prescriptions for eye drops or hospital records.
TAKEAWAY:
- Overall, 301 people in the study developed glaucoma over a mean follow-up period of 10.5 years.
- Incident glaucoma increased across all quartiles of arterial stiffness, with the highest risk observed in the fourth quartile for aortic pulse wave velocity (HR, 2.41; 95% CI, 1.36-4.26), estimated carotid-femoral pulse wave velocity (HR, 2.29; 95% CI, 1.27-4.13), and aortic pulse pressure (HR, 1.76; 95% CI, 1.10-2.82).
- The cumulative incidence of glaucoma rose with increases in arterial stiffness. This trend was statistically significant for both aortic and estimated pulse wave velocity (P < .0001) and aortic pulse pressure (P = .02).
IN PRACTICE:
“Arterial stiffness…which can be easily and accurately measured, could be used as a tool in clinical practice [as part of routine blood pressure measurement] to help identify people at risk of glaucoma and as a therapeutic target to prevent glaucoma progression,” the authors wrote.
SOURCE:
This study was led by Angela L. Beros, MPH, of the School of Population Health at the University of Auckland, Auckland, New Zealand, and published online in the American Journal of Ophthalmology.
LIMITATIONS:
The cohort study did not clinically assess for glaucoma, potentially leading to the inclusion of individuals with the condition. Not all participants with incident glaucoma, particularly those unaware of their diagnosis, may have been identified. Intraocular pressure and central corneal thickness, which are common risk factors for glaucoma, were not included in the multivariate analysis.
DISCLOSURES:
The study did not receive any funding. The authors declared no conflicts of interest.
A version of this article appeared on Medscape.com.
TOPLINE:
Arterial stiffness increases the risk for developing glaucoma, a new study found.
METHODOLOGY:
- To study the link between arterial stiffness and glaucoma, the researchers evaluated 4713 individuals (mean age, 66 years; 58% men) without the eye condition at baseline between April 2011 and November 2012.
- They assessed arterial stiffness by measuring aortic pulse wave velocity, estimated carotid-femoral pulse wave velocity, and aortic pulse pressure.
- The primary outcome was incident glaucoma, identified from prescriptions for eye drops or hospital records.
TAKEAWAY:
- Overall, 301 people in the study developed glaucoma over a mean follow-up period of 10.5 years.
- Incident glaucoma increased across all quartiles of arterial stiffness, with the highest risk observed in the fourth quartile for aortic pulse wave velocity (HR, 2.41; 95% CI, 1.36-4.26), estimated carotid-femoral pulse wave velocity (HR, 2.29; 95% CI, 1.27-4.13), and aortic pulse pressure (HR, 1.76; 95% CI, 1.10-2.82).
- The cumulative incidence of glaucoma rose with increases in arterial stiffness. This trend was statistically significant for both aortic and estimated pulse wave velocity (P < .0001) and aortic pulse pressure (P = .02).
IN PRACTICE:
“Arterial stiffness…which can be easily and accurately measured, could be used as a tool in clinical practice [as part of routine blood pressure measurement] to help identify people at risk of glaucoma and as a therapeutic target to prevent glaucoma progression,” the authors wrote.
SOURCE:
This study was led by Angela L. Beros, MPH, of the School of Population Health at the University of Auckland, Auckland, New Zealand, and published online in the American Journal of Ophthalmology.
LIMITATIONS:
The cohort study did not clinically assess for glaucoma, potentially leading to the inclusion of individuals with the condition. Not all participants with incident glaucoma, particularly those unaware of their diagnosis, may have been identified. Intraocular pressure and central corneal thickness, which are common risk factors for glaucoma, were not included in the multivariate analysis.
DISCLOSURES:
The study did not receive any funding. The authors declared no conflicts of interest.
A version of this article appeared on Medscape.com.
TOPLINE:
Arterial stiffness increases the risk for developing glaucoma, a new study found.
METHODOLOGY:
- To study the link between arterial stiffness and glaucoma, the researchers evaluated 4713 individuals (mean age, 66 years; 58% men) without the eye condition at baseline between April 2011 and November 2012.
- They assessed arterial stiffness by measuring aortic pulse wave velocity, estimated carotid-femoral pulse wave velocity, and aortic pulse pressure.
- The primary outcome was incident glaucoma, identified from prescriptions for eye drops or hospital records.
TAKEAWAY:
- Overall, 301 people in the study developed glaucoma over a mean follow-up period of 10.5 years.
- Incident glaucoma increased across all quartiles of arterial stiffness, with the highest risk observed in the fourth quartile for aortic pulse wave velocity (HR, 2.41; 95% CI, 1.36-4.26), estimated carotid-femoral pulse wave velocity (HR, 2.29; 95% CI, 1.27-4.13), and aortic pulse pressure (HR, 1.76; 95% CI, 1.10-2.82).
- The cumulative incidence of glaucoma rose with increases in arterial stiffness. This trend was statistically significant for both aortic and estimated pulse wave velocity (P < .0001) and aortic pulse pressure (P = .02).
IN PRACTICE:
“Arterial stiffness…which can be easily and accurately measured, could be used as a tool in clinical practice [as part of routine blood pressure measurement] to help identify people at risk of glaucoma and as a therapeutic target to prevent glaucoma progression,” the authors wrote.
SOURCE:
This study was led by Angela L. Beros, MPH, of the School of Population Health at the University of Auckland, Auckland, New Zealand, and published online in the American Journal of Ophthalmology.
LIMITATIONS:
The cohort study did not clinically assess for glaucoma, potentially leading to the inclusion of individuals with the condition. Not all participants with incident glaucoma, particularly those unaware of their diagnosis, may have been identified. Intraocular pressure and central corneal thickness, which are common risk factors for glaucoma, were not included in the multivariate analysis.
DISCLOSURES:
The study did not receive any funding. The authors declared no conflicts of interest.
A version of this article appeared on Medscape.com.
Home-based exercise benefits patients with PAD
TOPLINE:
Compared with supervised treadmill workouts at a gym, which is considered first-line therapy for walking impairment in lower extremity peripheral artery disease (PAD), exercising at home significantly improves 6-minute walking (6MW) distance, but not maximal treadmill walking distance, results of a new meta-analysis show.
METHODOLOGY:
- The analysis included five randomized clinical trials with a total of 719 participants, mean age 68.6 years, all led by researchers at Northwestern University, Chicago, that compared either supervised treadmill or home-based walking exercise with a nonexercise control group in people with PAD (defined as Ankle Brachial Index ≤ 0.90).
- All trials measured 6-minute walk (6MW) distance (walking as far as possible in 6 minutes), treadmill walking performance, and outcomes from the Walking Impairment Questionnaire (WIQ), which includes distance, walking speed, and stair-climbing domains, at baseline and at 6 months.
- Supervised treadmill exercise interventions included three individualized exercise sessions per week with an exercise physiologist at an exercise center, and home-based exercises involved walking near home 5 days per week, both for up to 50 minutes per session.
TAKEAWAY:
- After adjusting for study, age, sex, race, smoking, history of myocardial infarction, heart failure, and baseline 6MW distance, the study found both exercise interventions were better than nonexercise controls for 6MW distance.
- Compared with supervised treadmill exercise, home-based walking was associated with significantly improved mean 6MW distance (31.8 m vs. 55.6 m; adjusted between-group difference: −23.8 m; 95% confidence interval, −44.0 to −3.6; P = .021), and significantly improved WIQ walking speed score.
- However, home-based walking was associated with significantly less improvement in maximal treadmill walking distance, compared with supervised treadmill exercise (adjusted between-group difference: 132.5 m; 95% CI, 72.1-192.9; P < .001).
IN PRACTICE:
Home-based walking exercise “circumvents” barriers to accessing supervised exercise such as having to travel to a facility, said the authors, who noted the new data “demonstrated a large and consistent effect of home-based walking exercise on improved 6MW distance and also significantly improved the WIQ walking speed score, compared with supervised treadmill exercise.”
SOURCE:
The study was conducted by Neela D. Thangada, MD, Northwestern University, Chicago, and colleagues. It was published online in JAMA Network Open.
LIMITATIONS:
Data were combined from different randomized clinical trials that were led by one investigative team, and reported comparisons were not prespecified. Comparisons between supervised and home-based exercise lacked statistical power for the WIQ distance and stair-climbing measures.
DISCLOSURES:
The study was sponsored by the National Center for Research Resources and the National Heart, Lung, and Blood Institute of the National Institutes of Health. Dr. Thangada reports no relevant financial relationships. Disclosures for study coauthors can be found with the original article.
A version of this article first appeared on Medscape.com.
TOPLINE:
Compared with supervised treadmill workouts at a gym, which is considered first-line therapy for walking impairment in lower extremity peripheral artery disease (PAD), exercising at home significantly improves 6-minute walking (6MW) distance, but not maximal treadmill walking distance, results of a new meta-analysis show.
METHODOLOGY:
- The analysis included five randomized clinical trials with a total of 719 participants, mean age 68.6 years, all led by researchers at Northwestern University, Chicago, that compared either supervised treadmill or home-based walking exercise with a nonexercise control group in people with PAD (defined as Ankle Brachial Index ≤ 0.90).
- All trials measured 6-minute walk (6MW) distance (walking as far as possible in 6 minutes), treadmill walking performance, and outcomes from the Walking Impairment Questionnaire (WIQ), which includes distance, walking speed, and stair-climbing domains, at baseline and at 6 months.
- Supervised treadmill exercise interventions included three individualized exercise sessions per week with an exercise physiologist at an exercise center, and home-based exercises involved walking near home 5 days per week, both for up to 50 minutes per session.
TAKEAWAY:
- After adjusting for study, age, sex, race, smoking, history of myocardial infarction, heart failure, and baseline 6MW distance, the study found both exercise interventions were better than nonexercise controls for 6MW distance.
- Compared with supervised treadmill exercise, home-based walking was associated with significantly improved mean 6MW distance (31.8 m vs. 55.6 m; adjusted between-group difference: −23.8 m; 95% confidence interval, −44.0 to −3.6; P = .021), and significantly improved WIQ walking speed score.
- However, home-based walking was associated with significantly less improvement in maximal treadmill walking distance, compared with supervised treadmill exercise (adjusted between-group difference: 132.5 m; 95% CI, 72.1-192.9; P < .001).
IN PRACTICE:
Home-based walking exercise “circumvents” barriers to accessing supervised exercise such as having to travel to a facility, said the authors, who noted the new data “demonstrated a large and consistent effect of home-based walking exercise on improved 6MW distance and also significantly improved the WIQ walking speed score, compared with supervised treadmill exercise.”
SOURCE:
The study was conducted by Neela D. Thangada, MD, Northwestern University, Chicago, and colleagues. It was published online in JAMA Network Open.
LIMITATIONS:
Data were combined from different randomized clinical trials that were led by one investigative team, and reported comparisons were not prespecified. Comparisons between supervised and home-based exercise lacked statistical power for the WIQ distance and stair-climbing measures.
DISCLOSURES:
The study was sponsored by the National Center for Research Resources and the National Heart, Lung, and Blood Institute of the National Institutes of Health. Dr. Thangada reports no relevant financial relationships. Disclosures for study coauthors can be found with the original article.
A version of this article first appeared on Medscape.com.
TOPLINE:
Compared with supervised treadmill workouts at a gym, which is considered first-line therapy for walking impairment in lower extremity peripheral artery disease (PAD), exercising at home significantly improves 6-minute walking (6MW) distance, but not maximal treadmill walking distance, results of a new meta-analysis show.
METHODOLOGY:
- The analysis included five randomized clinical trials with a total of 719 participants, mean age 68.6 years, all led by researchers at Northwestern University, Chicago, that compared either supervised treadmill or home-based walking exercise with a nonexercise control group in people with PAD (defined as Ankle Brachial Index ≤ 0.90).
- All trials measured 6-minute walk (6MW) distance (walking as far as possible in 6 minutes), treadmill walking performance, and outcomes from the Walking Impairment Questionnaire (WIQ), which includes distance, walking speed, and stair-climbing domains, at baseline and at 6 months.
- Supervised treadmill exercise interventions included three individualized exercise sessions per week with an exercise physiologist at an exercise center, and home-based exercises involved walking near home 5 days per week, both for up to 50 minutes per session.
TAKEAWAY:
- After adjusting for study, age, sex, race, smoking, history of myocardial infarction, heart failure, and baseline 6MW distance, the study found both exercise interventions were better than nonexercise controls for 6MW distance.
- Compared with supervised treadmill exercise, home-based walking was associated with significantly improved mean 6MW distance (31.8 m vs. 55.6 m; adjusted between-group difference: −23.8 m; 95% confidence interval, −44.0 to −3.6; P = .021), and significantly improved WIQ walking speed score.
- However, home-based walking was associated with significantly less improvement in maximal treadmill walking distance, compared with supervised treadmill exercise (adjusted between-group difference: 132.5 m; 95% CI, 72.1-192.9; P < .001).
IN PRACTICE:
Home-based walking exercise “circumvents” barriers to accessing supervised exercise such as having to travel to a facility, said the authors, who noted the new data “demonstrated a large and consistent effect of home-based walking exercise on improved 6MW distance and also significantly improved the WIQ walking speed score, compared with supervised treadmill exercise.”
SOURCE:
The study was conducted by Neela D. Thangada, MD, Northwestern University, Chicago, and colleagues. It was published online in JAMA Network Open.
LIMITATIONS:
Data were combined from different randomized clinical trials that were led by one investigative team, and reported comparisons were not prespecified. Comparisons between supervised and home-based exercise lacked statistical power for the WIQ distance and stair-climbing measures.
DISCLOSURES:
The study was sponsored by the National Center for Research Resources and the National Heart, Lung, and Blood Institute of the National Institutes of Health. Dr. Thangada reports no relevant financial relationships. Disclosures for study coauthors can be found with the original article.
A version of this article first appeared on Medscape.com.
PET scan at diagnosis may help to predict aneurysm risk in patients with giant cell arteritis
PET scans may serve as both a diagnostic and prognostic tool in giant cell arteritis (GCA), according to a new study.
In over 100 patients with GCA who underwent 18F-fluorodeoxyglucose PET imaging, those with elevated FDG uptake at diagnosis were more likely to develop thoracic aortic aneurysms.
“PET-CT has an excellent diagnostic accuracy for the diagnosis of GCA, certainly if both extracranial and intracranial vessels were assessed. This study shows that performing PET imaging at diagnosis in patients with GCA may also help estimate the future risk for aortic aneurysm formation,” lead author Lien Moreel, MD, of the department of internal medicine at University Hospitals Leuven (Belgium), wrote in an email. “PET imaging at diagnosis can provide both diagnostic and prognostic information in one imaging tool in patients with GCA.”
Previous retrospective studies have found an association between FDG uptake at diagnosis and risk for aortic complications, but “prospective studies confirming these findings are lacking,” the investigators wrote. The study was published online in Annals of Internal Medicine.
In the study, Dr. Moreel and colleagues prospectively followed 106 individuals diagnosed with GCA who received FDG-PET within 3 days after starting glucocorticoids. Patients also had CT imaging at diagnosis and then CT imaging annually for up to 10 years.
A PET scan was considered positive with an FDG uptake of grade 2 or higher in any of seven vascular regions (thoracic and abdominal aorta, subclavian, axillary, carotid, iliac, and femoral arteries). Researchers also used the results to quantify a total vascular score (TVS). Out of the entire cohort, 75 patients had a positive PET scan result.
These patients had a larger increase in the diameter of the ascending aorta and the descending aorta, as well the volume of thoracic aorta after 5 years, compared with those who had a negative PET scan result. These changes were also associated with higher TVS at diagnosis. Of the 23 patients who developed an aortic aneurysm, 18 had a positive PET scan at diagnosis.
The risk of incident thoracic aortic aneurysms was calculated to be 10 times higher in patients with positive PET scans. Fourteen of the 15 patients (93%) with an incident thoracic aortic aneurysm had positive PET results.
Up to now, “we’ve had no way of predicting which patients might be at risk of this potentially serious complication,” Kenneth Warrington, MD, chair of the department of rheumatology and director of the Vasculitis Clinic at the Mayo Clinic in Rochester, Minn., said in an interview. He was not involved with the research.
He hopes that the findings will help inform clinicians on how patients with GCA should be evaluated and monitored. Although the American College of Rheumatology conditionally recommends noninvasive imaging in patients newly diagnosed with GCA, guidance for follow-up on these patients is less clear.
“There are no clear guidelines, but most clinicians who take care of patients with GCA do obtain imaging periodically,” he said. “There is a lot of variability in the practice in terms of which type of scan is used and how often it’s done.”
Although this study did not specifically look at the benefit of screening patients, “we think that follow-up of aortic dimensions seems to be warranted in GCA patients with a positive PET scan result, especially in those with high intensity and broad extent of vascular inflammation,” Dr. Moreel said. “However, the added value of screening and the interval required should be addressed in future studies.”
Applying this study’s protocol in practice in the United States might be difficult, Dr. Warrington noted, as it can be challenging logistically to get imaging done within 3 days of starting steroids. However, Dr. Moreel said it is possible to delay the start of glucocorticoids until the PET scan is performed in patients without visual symptoms or jaw claudication.
PET scans are also expensive, and it can be difficult to get insurance coverage in the United States. However, other imaging modalities could potentially be used in similar ways, Dr. Warrington said. “One could potentially extrapolate to say that if there is difficulty with accessing PET scan, we could use other modalities like CT or MRI basically to see whether the aorta is inflamed or not.”
Dr. Moreel disclosed no relevant financial relationships. Dr. Warrington has received compensation for consulting activities with Sanofi. Eli Lilly, Kiniksa, and Bristol-Myers Squibb have provided support to the Mayo Clinic for clinical trials related to GCA, of which Dr. Warrington served as subinvestigator.
A version of this article appeared on Medscape.com.
PET scans may serve as both a diagnostic and prognostic tool in giant cell arteritis (GCA), according to a new study.
In over 100 patients with GCA who underwent 18F-fluorodeoxyglucose PET imaging, those with elevated FDG uptake at diagnosis were more likely to develop thoracic aortic aneurysms.
“PET-CT has an excellent diagnostic accuracy for the diagnosis of GCA, certainly if both extracranial and intracranial vessels were assessed. This study shows that performing PET imaging at diagnosis in patients with GCA may also help estimate the future risk for aortic aneurysm formation,” lead author Lien Moreel, MD, of the department of internal medicine at University Hospitals Leuven (Belgium), wrote in an email. “PET imaging at diagnosis can provide both diagnostic and prognostic information in one imaging tool in patients with GCA.”
Previous retrospective studies have found an association between FDG uptake at diagnosis and risk for aortic complications, but “prospective studies confirming these findings are lacking,” the investigators wrote. The study was published online in Annals of Internal Medicine.
In the study, Dr. Moreel and colleagues prospectively followed 106 individuals diagnosed with GCA who received FDG-PET within 3 days after starting glucocorticoids. Patients also had CT imaging at diagnosis and then CT imaging annually for up to 10 years.
A PET scan was considered positive with an FDG uptake of grade 2 or higher in any of seven vascular regions (thoracic and abdominal aorta, subclavian, axillary, carotid, iliac, and femoral arteries). Researchers also used the results to quantify a total vascular score (TVS). Out of the entire cohort, 75 patients had a positive PET scan result.
These patients had a larger increase in the diameter of the ascending aorta and the descending aorta, as well the volume of thoracic aorta after 5 years, compared with those who had a negative PET scan result. These changes were also associated with higher TVS at diagnosis. Of the 23 patients who developed an aortic aneurysm, 18 had a positive PET scan at diagnosis.
The risk of incident thoracic aortic aneurysms was calculated to be 10 times higher in patients with positive PET scans. Fourteen of the 15 patients (93%) with an incident thoracic aortic aneurysm had positive PET results.
Up to now, “we’ve had no way of predicting which patients might be at risk of this potentially serious complication,” Kenneth Warrington, MD, chair of the department of rheumatology and director of the Vasculitis Clinic at the Mayo Clinic in Rochester, Minn., said in an interview. He was not involved with the research.
He hopes that the findings will help inform clinicians on how patients with GCA should be evaluated and monitored. Although the American College of Rheumatology conditionally recommends noninvasive imaging in patients newly diagnosed with GCA, guidance for follow-up on these patients is less clear.
“There are no clear guidelines, but most clinicians who take care of patients with GCA do obtain imaging periodically,” he said. “There is a lot of variability in the practice in terms of which type of scan is used and how often it’s done.”
Although this study did not specifically look at the benefit of screening patients, “we think that follow-up of aortic dimensions seems to be warranted in GCA patients with a positive PET scan result, especially in those with high intensity and broad extent of vascular inflammation,” Dr. Moreel said. “However, the added value of screening and the interval required should be addressed in future studies.”
Applying this study’s protocol in practice in the United States might be difficult, Dr. Warrington noted, as it can be challenging logistically to get imaging done within 3 days of starting steroids. However, Dr. Moreel said it is possible to delay the start of glucocorticoids until the PET scan is performed in patients without visual symptoms or jaw claudication.
PET scans are also expensive, and it can be difficult to get insurance coverage in the United States. However, other imaging modalities could potentially be used in similar ways, Dr. Warrington said. “One could potentially extrapolate to say that if there is difficulty with accessing PET scan, we could use other modalities like CT or MRI basically to see whether the aorta is inflamed or not.”
Dr. Moreel disclosed no relevant financial relationships. Dr. Warrington has received compensation for consulting activities with Sanofi. Eli Lilly, Kiniksa, and Bristol-Myers Squibb have provided support to the Mayo Clinic for clinical trials related to GCA, of which Dr. Warrington served as subinvestigator.
A version of this article appeared on Medscape.com.
PET scans may serve as both a diagnostic and prognostic tool in giant cell arteritis (GCA), according to a new study.
In over 100 patients with GCA who underwent 18F-fluorodeoxyglucose PET imaging, those with elevated FDG uptake at diagnosis were more likely to develop thoracic aortic aneurysms.
“PET-CT has an excellent diagnostic accuracy for the diagnosis of GCA, certainly if both extracranial and intracranial vessels were assessed. This study shows that performing PET imaging at diagnosis in patients with GCA may also help estimate the future risk for aortic aneurysm formation,” lead author Lien Moreel, MD, of the department of internal medicine at University Hospitals Leuven (Belgium), wrote in an email. “PET imaging at diagnosis can provide both diagnostic and prognostic information in one imaging tool in patients with GCA.”
Previous retrospective studies have found an association between FDG uptake at diagnosis and risk for aortic complications, but “prospective studies confirming these findings are lacking,” the investigators wrote. The study was published online in Annals of Internal Medicine.
In the study, Dr. Moreel and colleagues prospectively followed 106 individuals diagnosed with GCA who received FDG-PET within 3 days after starting glucocorticoids. Patients also had CT imaging at diagnosis and then CT imaging annually for up to 10 years.
A PET scan was considered positive with an FDG uptake of grade 2 or higher in any of seven vascular regions (thoracic and abdominal aorta, subclavian, axillary, carotid, iliac, and femoral arteries). Researchers also used the results to quantify a total vascular score (TVS). Out of the entire cohort, 75 patients had a positive PET scan result.
These patients had a larger increase in the diameter of the ascending aorta and the descending aorta, as well the volume of thoracic aorta after 5 years, compared with those who had a negative PET scan result. These changes were also associated with higher TVS at diagnosis. Of the 23 patients who developed an aortic aneurysm, 18 had a positive PET scan at diagnosis.
The risk of incident thoracic aortic aneurysms was calculated to be 10 times higher in patients with positive PET scans. Fourteen of the 15 patients (93%) with an incident thoracic aortic aneurysm had positive PET results.
Up to now, “we’ve had no way of predicting which patients might be at risk of this potentially serious complication,” Kenneth Warrington, MD, chair of the department of rheumatology and director of the Vasculitis Clinic at the Mayo Clinic in Rochester, Minn., said in an interview. He was not involved with the research.
He hopes that the findings will help inform clinicians on how patients with GCA should be evaluated and monitored. Although the American College of Rheumatology conditionally recommends noninvasive imaging in patients newly diagnosed with GCA, guidance for follow-up on these patients is less clear.
“There are no clear guidelines, but most clinicians who take care of patients with GCA do obtain imaging periodically,” he said. “There is a lot of variability in the practice in terms of which type of scan is used and how often it’s done.”
Although this study did not specifically look at the benefit of screening patients, “we think that follow-up of aortic dimensions seems to be warranted in GCA patients with a positive PET scan result, especially in those with high intensity and broad extent of vascular inflammation,” Dr. Moreel said. “However, the added value of screening and the interval required should be addressed in future studies.”
Applying this study’s protocol in practice in the United States might be difficult, Dr. Warrington noted, as it can be challenging logistically to get imaging done within 3 days of starting steroids. However, Dr. Moreel said it is possible to delay the start of glucocorticoids until the PET scan is performed in patients without visual symptoms or jaw claudication.
PET scans are also expensive, and it can be difficult to get insurance coverage in the United States. However, other imaging modalities could potentially be used in similar ways, Dr. Warrington said. “One could potentially extrapolate to say that if there is difficulty with accessing PET scan, we could use other modalities like CT or MRI basically to see whether the aorta is inflamed or not.”
Dr. Moreel disclosed no relevant financial relationships. Dr. Warrington has received compensation for consulting activities with Sanofi. Eli Lilly, Kiniksa, and Bristol-Myers Squibb have provided support to the Mayo Clinic for clinical trials related to GCA, of which Dr. Warrington served as subinvestigator.
A version of this article appeared on Medscape.com.
FROM ANNALS OF INTERNAL MEDICINE
PAD procedure overuse: A field in peril or ‘a few bad apples’?
On May 24, the news outlet ProPublica published a scathing investigation of Jeffery Dormu, DO, said to have performed hundreds of “medically unnecessary and invasive vascular procedures” in his Laurel, Md. office, putting patients’ limbs and lives at risk.
On July 15, The New York Times published a broader-based investigation of several vascular specialists said to have performed “risky” procedures on patients with peripheral artery disease (PAD) who subsequently had to have amputations, or died. The focus was mainly on Michigan-based interventional cardiologist Jihad Mustapha, MD.
This follows a 2019 analysis of Medicare claims data that identified outlier physicians with a high early intervention rate for patients newly diagnosed with claudication. According to the American Heart Association statistics, PAD affects approximately 8.5 million U.S. adults age 40 and older (some claim that’s an underestimate); most cases don’t require invasive treatment.
Responding to the Times’ revelations, Joseph L. Mills, MD, president of the Society for Vascular Surgery, wrote on the society’s website: “The overwhelming majority of vascular surgeons, and a vast majority of other specialists that receive some training and play a role in the care of vascular patients, including those trained in vascular medicine, interventional cardiology, and interventional radiology are providing high-quality, evidence-based care with safety and the best patient outcomes in mind.
“This is a complex issue that requires the examination not only of the events detailed in this story ... but of the underlying health care economic, legal and regulatory policies that created fertile soil for this behavior to germinate and take root.”
‘A few bad apples’
“I think it’s a case of a few bad apples,” Sunil V. Rao, MD, director of interventional cardiology at NYU Langone Health, New York, said in an interview. “In general, I think physicians who take care of patients with vascular issues are trying to do the right thing. I think all of us who take care of patients with vascular disease see patients who are very, very complex, and there are going to be some procedures that have complications.
“Without knowing the clinical details, it’s hard to know whether the procedures described in the articles were overuse or unnecessary, or exactly what led to the amputations,” he said. “All we know is that these physicians are outliers in terms of the number of procedures they were billing for.
“But although correlation is not causation, it certainly is cause for concern because you would expect that the use of procedures for specific indications would fall within a certain range,” he added.
Lifestyle changes first
PAD is often asymptomatic or mild, making it difficult to diagnose. Revascularization procedures usually are reserved for the 5%-8% of patients at risk for chronic limb-threatening ischemia (CLTI) or those in whom the cornerstones of PAD treatment – lifestyle changes and, if needed, medication – fail.
Revascularization options include balloon angioplasty or stent placement; atherectomy to remove plaques from the artery; or bypass surgery if a long portion of a leg artery is completely blocked. All carry a risk of long-term adverse outcomes, but the rates are highest for atherectomy.
Lifestyle changes include regular exercise, following a healthy diet, quitting smoking, and controlling diabetes and high blood pressure. When PAD continues or progresses despite these modifications, medications such as antiplatelet agents, antihypertensives, and/or lipid-lowering drugs may be prescribed.
‘Medically unnecessary’
According to the latest American Heart Association/American College of Cardiology guideline on managing patients with lower-extremity PAD, patients should be selected for revascularization based on symptom severity.
Factors to consider include a significant disability as assessed by the patient, and adequacy of response to medical and structured exercise therapy.
There’s the rub regarding the clinicians investigated in the Times and ProPublica. Many patients, apparently, were not encouraged to make lifestyle changes, nor did they receive medication. Instead, they were advised from the get-go to undergo invasive procedures, and often multiple times. Underuse of prevention and lifestyle counseling n the management of PAD has long been a concern.
Furthermore, in at least some cases, patients without any symptoms were encouraged to be screened for blockages that were then treated invasively, according to the Times.
Dr. Dormu, as highlighted in ProPublica, positioned his practice as “life and limb saving.” Yet, in investigative findings that led to a suspension of Dr. Dormu’s license to practice medicine in Maryland, peer reviewers expressed concern regarding his repeated use of invasive and medically unnecessary procedures, exposing patients to “potential risks such as bleeding, infection, blood vessel injuries which could acutely or chronically worsen the patient’s circulation, and limb loss.”
The peer reviewers concurred that Dr. Dormu failed to use conservative management techniques to address the patients’ vascular complaints before resorting to invasive procedures.
Dr. Mustapha is described in the Times as a “high-volume” atherectomy provider. From 2017 to 2021, about half of Medicare’s atherectomy payments – $1.4 billion – went to 200 high-volume providers, with Dr. Mustapha near the top of the list.
Some of Dr. Mustapha’s patients underwent multiple procedures said to help prevent leg amputation, but their legs were amputated anyway, possibly because of the multiple atherectomies, according to the Times.
Judith Lin, MD, MBA, who treated some of Dr. Mustapha’s former patients, was among those who complained about his practice to Michigan’s licensing board. Some of the patients she treated needed amputations; others needed to have leftover wires extracted from their legs.
In 2020, the board investigated Dr. Lin’s complaint and referred it to Michigan’s attorney general, who brought a disciplinary action against Dr. Mustapha. An expert hired by the state to review eight patient cases concluded that Dr. Mustapha’s practice “was characterized by overtreatment and poor documentation.” In some cases, the expert wrote, “unnecessary procedures hastened amputations.”
The statement issued by Dr. Mills, the president of SVS, noted that the society’s practice guideline proposes a threshold of at least 2 years of likely durability for an intervention performed for claudication.
“The growing frequency of multiple, repeated procedures [is] emblematic of poor patient selection and inadequate durability of the chosen procedure, leading to a vicious cycle of repetitive interventions that is not only costly, but also dangerous,” he wrote.
Financial incentives to blame?
In 2008, Medicare created incentives for physicians to perform vascular procedures in offices rather than hospitals, in an effort to reduce medical costs, according to both investigative articles. But the effort backfired.
Before the changes, an office provider inserting a stent could make about $1,700 from Medicare; deploying a balloon could bring in roughly $3,800. By 2011, the payments rose to about $6,400 and $4,800, respectively.
Office-based atherectomies soared when, in 2011, the Centers for Medicare & Medicaid Services started reimbursing $13,500 per procedure, as opposed to roughly $11,450 in a hospital. Atherectomies increased by 60% from 2011 to 2014, and Medicare’s overall costs for peripheral vascular treatments climbed by nearly half a billion dollars.
“The government is really to blame for setting these tremendously high reimbursement values without looking into whether these procedures are helping people or are just worthless procedures or, in fact, are hurting people,” Dipankar Mukherjee, MD, a vascular surgeon and chief of vascular surgery at Inova Fairfax (Va.) Hospital, said in ProPublica.
The result, noted Dr. Rao, is that “there can be perverse or nefarious incentives for doing these procedures. People are incentivized by reimbursement to do something that really falls in the area of clinical judgment and guidelines.”
Major incentives also come from device manufacturers, who often reward physicians who do the most vascular procedures with payments for consulting and other services, according to the Times. In addition, these companies lend money to help physicians or their clinics to finance the purchase of equipment used to perform the procedures.
“Vascular medicine now is the frontier of the Wild West,” Marty Makary, MD, MPH, a professor of surgery and health care quality researcher at Johns Hopkins University, Baltimore, told ProPublica. “People are flying blind walking into the clinics of these doctors with egregious practice patterns, and we know that their pattern is indefensible.”
Recognizing that the situation posed a threat to patients and also damaged the credibility of his specialty, Kim J. Hodgson, MD, a former SVS president, told attendees at the 2021 annual meeting of the SVS, “Somebody has to address what should never have been allowed to get to this level of threat to us and our patients in the first place. We can play whack-a-mole every time the bad actors surface until the cows come home, but that leaves a trail of harmed patients and wasted resources.”
Dr. Hodgson described atherectomy as “a procedure that many believe provides no demonstrable value whatsoever to the patient” and challenged those who disagree to prove it.
Multidisciplinary teams needed
Other experts believe there are times that revascularization procedures, including atherectomy, are appropriate. However, the majority of patients with PAD do not require a procedure, Soo Hyun (Esther) Kim, MD, MPH, director of the Center for Women’s Cardiovascular Health at Atrium Health Sanger Heart and Vascular Institute in Charlotte, N.C., said in an interview. In fact, “many patients do not even know they have leg artery blockages.”
Invasive procedures may well be appropriate for patients with severe PAD, especially those with CLTI, and disparities may be keeping those who truly need such interventions – or for whom they may be at least considered – from accessing them. If PAD is not diagnosed and treated in a timely way, Dr. Kim said, those individuals “do indeed lose their limbs.”
Multidisciplinary teams can help, Dr. Kim said. “Specialists from multiple different training backgrounds [can] take good care of patients with PAD,” she said. This is important when access to a particular type of specialist is limited, and because patients with PAD often have complex medical problems that can benefit from a team approach.
Transcatheter aortic valve replacement heart teams and complex coronary disease heart teams are two examples, Dr. Kim noted. “When a high-stakes procedure is being considered, the patient’s case is reviewed by multiple stakeholders to ensure appropriateness of the procedure and collaboratively evaluate risk.”
Dr. Rao also emphasized a team approach. “PAD does not belong to a single specialty,” he said. The revelations from the Times, ProPublica, and other sources “point to the fact that we all – cardiologists, vascular surgeons, interventional radiologists – should start thinking about how best to police ourselves and also account for the variation in clinical judgment.”
Use of a multidisciplinary team is a “guideline-recommended approach” for coronary artery revascularization, he said, “I think the same should apply for PAD.”
PAD is a sign of systemic atherosclerosis, Dr. Kim noted. “The treatment of PAD includes addressing leg pain and wounds with procedures, but the interventions that will keep people alive are the medications we use to prevent heart attack and stroke. Patients with PAD need to understand that treatment is much more than opening up a blockage in the leg.”
Dr. Rao and Dr. Kim disclosed no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
On May 24, the news outlet ProPublica published a scathing investigation of Jeffery Dormu, DO, said to have performed hundreds of “medically unnecessary and invasive vascular procedures” in his Laurel, Md. office, putting patients’ limbs and lives at risk.
On July 15, The New York Times published a broader-based investigation of several vascular specialists said to have performed “risky” procedures on patients with peripheral artery disease (PAD) who subsequently had to have amputations, or died. The focus was mainly on Michigan-based interventional cardiologist Jihad Mustapha, MD.
This follows a 2019 analysis of Medicare claims data that identified outlier physicians with a high early intervention rate for patients newly diagnosed with claudication. According to the American Heart Association statistics, PAD affects approximately 8.5 million U.S. adults age 40 and older (some claim that’s an underestimate); most cases don’t require invasive treatment.
Responding to the Times’ revelations, Joseph L. Mills, MD, president of the Society for Vascular Surgery, wrote on the society’s website: “The overwhelming majority of vascular surgeons, and a vast majority of other specialists that receive some training and play a role in the care of vascular patients, including those trained in vascular medicine, interventional cardiology, and interventional radiology are providing high-quality, evidence-based care with safety and the best patient outcomes in mind.
“This is a complex issue that requires the examination not only of the events detailed in this story ... but of the underlying health care economic, legal and regulatory policies that created fertile soil for this behavior to germinate and take root.”
‘A few bad apples’
“I think it’s a case of a few bad apples,” Sunil V. Rao, MD, director of interventional cardiology at NYU Langone Health, New York, said in an interview. “In general, I think physicians who take care of patients with vascular issues are trying to do the right thing. I think all of us who take care of patients with vascular disease see patients who are very, very complex, and there are going to be some procedures that have complications.
“Without knowing the clinical details, it’s hard to know whether the procedures described in the articles were overuse or unnecessary, or exactly what led to the amputations,” he said. “All we know is that these physicians are outliers in terms of the number of procedures they were billing for.
“But although correlation is not causation, it certainly is cause for concern because you would expect that the use of procedures for specific indications would fall within a certain range,” he added.
Lifestyle changes first
PAD is often asymptomatic or mild, making it difficult to diagnose. Revascularization procedures usually are reserved for the 5%-8% of patients at risk for chronic limb-threatening ischemia (CLTI) or those in whom the cornerstones of PAD treatment – lifestyle changes and, if needed, medication – fail.
Revascularization options include balloon angioplasty or stent placement; atherectomy to remove plaques from the artery; or bypass surgery if a long portion of a leg artery is completely blocked. All carry a risk of long-term adverse outcomes, but the rates are highest for atherectomy.
Lifestyle changes include regular exercise, following a healthy diet, quitting smoking, and controlling diabetes and high blood pressure. When PAD continues or progresses despite these modifications, medications such as antiplatelet agents, antihypertensives, and/or lipid-lowering drugs may be prescribed.
‘Medically unnecessary’
According to the latest American Heart Association/American College of Cardiology guideline on managing patients with lower-extremity PAD, patients should be selected for revascularization based on symptom severity.
Factors to consider include a significant disability as assessed by the patient, and adequacy of response to medical and structured exercise therapy.
There’s the rub regarding the clinicians investigated in the Times and ProPublica. Many patients, apparently, were not encouraged to make lifestyle changes, nor did they receive medication. Instead, they were advised from the get-go to undergo invasive procedures, and often multiple times. Underuse of prevention and lifestyle counseling n the management of PAD has long been a concern.
Furthermore, in at least some cases, patients without any symptoms were encouraged to be screened for blockages that were then treated invasively, according to the Times.
Dr. Dormu, as highlighted in ProPublica, positioned his practice as “life and limb saving.” Yet, in investigative findings that led to a suspension of Dr. Dormu’s license to practice medicine in Maryland, peer reviewers expressed concern regarding his repeated use of invasive and medically unnecessary procedures, exposing patients to “potential risks such as bleeding, infection, blood vessel injuries which could acutely or chronically worsen the patient’s circulation, and limb loss.”
The peer reviewers concurred that Dr. Dormu failed to use conservative management techniques to address the patients’ vascular complaints before resorting to invasive procedures.
Dr. Mustapha is described in the Times as a “high-volume” atherectomy provider. From 2017 to 2021, about half of Medicare’s atherectomy payments – $1.4 billion – went to 200 high-volume providers, with Dr. Mustapha near the top of the list.
Some of Dr. Mustapha’s patients underwent multiple procedures said to help prevent leg amputation, but their legs were amputated anyway, possibly because of the multiple atherectomies, according to the Times.
Judith Lin, MD, MBA, who treated some of Dr. Mustapha’s former patients, was among those who complained about his practice to Michigan’s licensing board. Some of the patients she treated needed amputations; others needed to have leftover wires extracted from their legs.
In 2020, the board investigated Dr. Lin’s complaint and referred it to Michigan’s attorney general, who brought a disciplinary action against Dr. Mustapha. An expert hired by the state to review eight patient cases concluded that Dr. Mustapha’s practice “was characterized by overtreatment and poor documentation.” In some cases, the expert wrote, “unnecessary procedures hastened amputations.”
The statement issued by Dr. Mills, the president of SVS, noted that the society’s practice guideline proposes a threshold of at least 2 years of likely durability for an intervention performed for claudication.
“The growing frequency of multiple, repeated procedures [is] emblematic of poor patient selection and inadequate durability of the chosen procedure, leading to a vicious cycle of repetitive interventions that is not only costly, but also dangerous,” he wrote.
Financial incentives to blame?
In 2008, Medicare created incentives for physicians to perform vascular procedures in offices rather than hospitals, in an effort to reduce medical costs, according to both investigative articles. But the effort backfired.
Before the changes, an office provider inserting a stent could make about $1,700 from Medicare; deploying a balloon could bring in roughly $3,800. By 2011, the payments rose to about $6,400 and $4,800, respectively.
Office-based atherectomies soared when, in 2011, the Centers for Medicare & Medicaid Services started reimbursing $13,500 per procedure, as opposed to roughly $11,450 in a hospital. Atherectomies increased by 60% from 2011 to 2014, and Medicare’s overall costs for peripheral vascular treatments climbed by nearly half a billion dollars.
“The government is really to blame for setting these tremendously high reimbursement values without looking into whether these procedures are helping people or are just worthless procedures or, in fact, are hurting people,” Dipankar Mukherjee, MD, a vascular surgeon and chief of vascular surgery at Inova Fairfax (Va.) Hospital, said in ProPublica.
The result, noted Dr. Rao, is that “there can be perverse or nefarious incentives for doing these procedures. People are incentivized by reimbursement to do something that really falls in the area of clinical judgment and guidelines.”
Major incentives also come from device manufacturers, who often reward physicians who do the most vascular procedures with payments for consulting and other services, according to the Times. In addition, these companies lend money to help physicians or their clinics to finance the purchase of equipment used to perform the procedures.
“Vascular medicine now is the frontier of the Wild West,” Marty Makary, MD, MPH, a professor of surgery and health care quality researcher at Johns Hopkins University, Baltimore, told ProPublica. “People are flying blind walking into the clinics of these doctors with egregious practice patterns, and we know that their pattern is indefensible.”
Recognizing that the situation posed a threat to patients and also damaged the credibility of his specialty, Kim J. Hodgson, MD, a former SVS president, told attendees at the 2021 annual meeting of the SVS, “Somebody has to address what should never have been allowed to get to this level of threat to us and our patients in the first place. We can play whack-a-mole every time the bad actors surface until the cows come home, but that leaves a trail of harmed patients and wasted resources.”
Dr. Hodgson described atherectomy as “a procedure that many believe provides no demonstrable value whatsoever to the patient” and challenged those who disagree to prove it.
Multidisciplinary teams needed
Other experts believe there are times that revascularization procedures, including atherectomy, are appropriate. However, the majority of patients with PAD do not require a procedure, Soo Hyun (Esther) Kim, MD, MPH, director of the Center for Women’s Cardiovascular Health at Atrium Health Sanger Heart and Vascular Institute in Charlotte, N.C., said in an interview. In fact, “many patients do not even know they have leg artery blockages.”
Invasive procedures may well be appropriate for patients with severe PAD, especially those with CLTI, and disparities may be keeping those who truly need such interventions – or for whom they may be at least considered – from accessing them. If PAD is not diagnosed and treated in a timely way, Dr. Kim said, those individuals “do indeed lose their limbs.”
Multidisciplinary teams can help, Dr. Kim said. “Specialists from multiple different training backgrounds [can] take good care of patients with PAD,” she said. This is important when access to a particular type of specialist is limited, and because patients with PAD often have complex medical problems that can benefit from a team approach.
Transcatheter aortic valve replacement heart teams and complex coronary disease heart teams are two examples, Dr. Kim noted. “When a high-stakes procedure is being considered, the patient’s case is reviewed by multiple stakeholders to ensure appropriateness of the procedure and collaboratively evaluate risk.”
Dr. Rao also emphasized a team approach. “PAD does not belong to a single specialty,” he said. The revelations from the Times, ProPublica, and other sources “point to the fact that we all – cardiologists, vascular surgeons, interventional radiologists – should start thinking about how best to police ourselves and also account for the variation in clinical judgment.”
Use of a multidisciplinary team is a “guideline-recommended approach” for coronary artery revascularization, he said, “I think the same should apply for PAD.”
PAD is a sign of systemic atherosclerosis, Dr. Kim noted. “The treatment of PAD includes addressing leg pain and wounds with procedures, but the interventions that will keep people alive are the medications we use to prevent heart attack and stroke. Patients with PAD need to understand that treatment is much more than opening up a blockage in the leg.”
Dr. Rao and Dr. Kim disclosed no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
On May 24, the news outlet ProPublica published a scathing investigation of Jeffery Dormu, DO, said to have performed hundreds of “medically unnecessary and invasive vascular procedures” in his Laurel, Md. office, putting patients’ limbs and lives at risk.
On July 15, The New York Times published a broader-based investigation of several vascular specialists said to have performed “risky” procedures on patients with peripheral artery disease (PAD) who subsequently had to have amputations, or died. The focus was mainly on Michigan-based interventional cardiologist Jihad Mustapha, MD.
This follows a 2019 analysis of Medicare claims data that identified outlier physicians with a high early intervention rate for patients newly diagnosed with claudication. According to the American Heart Association statistics, PAD affects approximately 8.5 million U.S. adults age 40 and older (some claim that’s an underestimate); most cases don’t require invasive treatment.
Responding to the Times’ revelations, Joseph L. Mills, MD, president of the Society for Vascular Surgery, wrote on the society’s website: “The overwhelming majority of vascular surgeons, and a vast majority of other specialists that receive some training and play a role in the care of vascular patients, including those trained in vascular medicine, interventional cardiology, and interventional radiology are providing high-quality, evidence-based care with safety and the best patient outcomes in mind.
“This is a complex issue that requires the examination not only of the events detailed in this story ... but of the underlying health care economic, legal and regulatory policies that created fertile soil for this behavior to germinate and take root.”
‘A few bad apples’
“I think it’s a case of a few bad apples,” Sunil V. Rao, MD, director of interventional cardiology at NYU Langone Health, New York, said in an interview. “In general, I think physicians who take care of patients with vascular issues are trying to do the right thing. I think all of us who take care of patients with vascular disease see patients who are very, very complex, and there are going to be some procedures that have complications.
“Without knowing the clinical details, it’s hard to know whether the procedures described in the articles were overuse or unnecessary, or exactly what led to the amputations,” he said. “All we know is that these physicians are outliers in terms of the number of procedures they were billing for.
“But although correlation is not causation, it certainly is cause for concern because you would expect that the use of procedures for specific indications would fall within a certain range,” he added.
Lifestyle changes first
PAD is often asymptomatic or mild, making it difficult to diagnose. Revascularization procedures usually are reserved for the 5%-8% of patients at risk for chronic limb-threatening ischemia (CLTI) or those in whom the cornerstones of PAD treatment – lifestyle changes and, if needed, medication – fail.
Revascularization options include balloon angioplasty or stent placement; atherectomy to remove plaques from the artery; or bypass surgery if a long portion of a leg artery is completely blocked. All carry a risk of long-term adverse outcomes, but the rates are highest for atherectomy.
Lifestyle changes include regular exercise, following a healthy diet, quitting smoking, and controlling diabetes and high blood pressure. When PAD continues or progresses despite these modifications, medications such as antiplatelet agents, antihypertensives, and/or lipid-lowering drugs may be prescribed.
‘Medically unnecessary’
According to the latest American Heart Association/American College of Cardiology guideline on managing patients with lower-extremity PAD, patients should be selected for revascularization based on symptom severity.
Factors to consider include a significant disability as assessed by the patient, and adequacy of response to medical and structured exercise therapy.
There’s the rub regarding the clinicians investigated in the Times and ProPublica. Many patients, apparently, were not encouraged to make lifestyle changes, nor did they receive medication. Instead, they were advised from the get-go to undergo invasive procedures, and often multiple times. Underuse of prevention and lifestyle counseling n the management of PAD has long been a concern.
Furthermore, in at least some cases, patients without any symptoms were encouraged to be screened for blockages that were then treated invasively, according to the Times.
Dr. Dormu, as highlighted in ProPublica, positioned his practice as “life and limb saving.” Yet, in investigative findings that led to a suspension of Dr. Dormu’s license to practice medicine in Maryland, peer reviewers expressed concern regarding his repeated use of invasive and medically unnecessary procedures, exposing patients to “potential risks such as bleeding, infection, blood vessel injuries which could acutely or chronically worsen the patient’s circulation, and limb loss.”
The peer reviewers concurred that Dr. Dormu failed to use conservative management techniques to address the patients’ vascular complaints before resorting to invasive procedures.
Dr. Mustapha is described in the Times as a “high-volume” atherectomy provider. From 2017 to 2021, about half of Medicare’s atherectomy payments – $1.4 billion – went to 200 high-volume providers, with Dr. Mustapha near the top of the list.
Some of Dr. Mustapha’s patients underwent multiple procedures said to help prevent leg amputation, but their legs were amputated anyway, possibly because of the multiple atherectomies, according to the Times.
Judith Lin, MD, MBA, who treated some of Dr. Mustapha’s former patients, was among those who complained about his practice to Michigan’s licensing board. Some of the patients she treated needed amputations; others needed to have leftover wires extracted from their legs.
In 2020, the board investigated Dr. Lin’s complaint and referred it to Michigan’s attorney general, who brought a disciplinary action against Dr. Mustapha. An expert hired by the state to review eight patient cases concluded that Dr. Mustapha’s practice “was characterized by overtreatment and poor documentation.” In some cases, the expert wrote, “unnecessary procedures hastened amputations.”
The statement issued by Dr. Mills, the president of SVS, noted that the society’s practice guideline proposes a threshold of at least 2 years of likely durability for an intervention performed for claudication.
“The growing frequency of multiple, repeated procedures [is] emblematic of poor patient selection and inadequate durability of the chosen procedure, leading to a vicious cycle of repetitive interventions that is not only costly, but also dangerous,” he wrote.
Financial incentives to blame?
In 2008, Medicare created incentives for physicians to perform vascular procedures in offices rather than hospitals, in an effort to reduce medical costs, according to both investigative articles. But the effort backfired.
Before the changes, an office provider inserting a stent could make about $1,700 from Medicare; deploying a balloon could bring in roughly $3,800. By 2011, the payments rose to about $6,400 and $4,800, respectively.
Office-based atherectomies soared when, in 2011, the Centers for Medicare & Medicaid Services started reimbursing $13,500 per procedure, as opposed to roughly $11,450 in a hospital. Atherectomies increased by 60% from 2011 to 2014, and Medicare’s overall costs for peripheral vascular treatments climbed by nearly half a billion dollars.
“The government is really to blame for setting these tremendously high reimbursement values without looking into whether these procedures are helping people or are just worthless procedures or, in fact, are hurting people,” Dipankar Mukherjee, MD, a vascular surgeon and chief of vascular surgery at Inova Fairfax (Va.) Hospital, said in ProPublica.
The result, noted Dr. Rao, is that “there can be perverse or nefarious incentives for doing these procedures. People are incentivized by reimbursement to do something that really falls in the area of clinical judgment and guidelines.”
Major incentives also come from device manufacturers, who often reward physicians who do the most vascular procedures with payments for consulting and other services, according to the Times. In addition, these companies lend money to help physicians or their clinics to finance the purchase of equipment used to perform the procedures.
“Vascular medicine now is the frontier of the Wild West,” Marty Makary, MD, MPH, a professor of surgery and health care quality researcher at Johns Hopkins University, Baltimore, told ProPublica. “People are flying blind walking into the clinics of these doctors with egregious practice patterns, and we know that their pattern is indefensible.”
Recognizing that the situation posed a threat to patients and also damaged the credibility of his specialty, Kim J. Hodgson, MD, a former SVS president, told attendees at the 2021 annual meeting of the SVS, “Somebody has to address what should never have been allowed to get to this level of threat to us and our patients in the first place. We can play whack-a-mole every time the bad actors surface until the cows come home, but that leaves a trail of harmed patients and wasted resources.”
Dr. Hodgson described atherectomy as “a procedure that many believe provides no demonstrable value whatsoever to the patient” and challenged those who disagree to prove it.
Multidisciplinary teams needed
Other experts believe there are times that revascularization procedures, including atherectomy, are appropriate. However, the majority of patients with PAD do not require a procedure, Soo Hyun (Esther) Kim, MD, MPH, director of the Center for Women’s Cardiovascular Health at Atrium Health Sanger Heart and Vascular Institute in Charlotte, N.C., said in an interview. In fact, “many patients do not even know they have leg artery blockages.”
Invasive procedures may well be appropriate for patients with severe PAD, especially those with CLTI, and disparities may be keeping those who truly need such interventions – or for whom they may be at least considered – from accessing them. If PAD is not diagnosed and treated in a timely way, Dr. Kim said, those individuals “do indeed lose their limbs.”
Multidisciplinary teams can help, Dr. Kim said. “Specialists from multiple different training backgrounds [can] take good care of patients with PAD,” she said. This is important when access to a particular type of specialist is limited, and because patients with PAD often have complex medical problems that can benefit from a team approach.
Transcatheter aortic valve replacement heart teams and complex coronary disease heart teams are two examples, Dr. Kim noted. “When a high-stakes procedure is being considered, the patient’s case is reviewed by multiple stakeholders to ensure appropriateness of the procedure and collaboratively evaluate risk.”
Dr. Rao also emphasized a team approach. “PAD does not belong to a single specialty,” he said. The revelations from the Times, ProPublica, and other sources “point to the fact that we all – cardiologists, vascular surgeons, interventional radiologists – should start thinking about how best to police ourselves and also account for the variation in clinical judgment.”
Use of a multidisciplinary team is a “guideline-recommended approach” for coronary artery revascularization, he said, “I think the same should apply for PAD.”
PAD is a sign of systemic atherosclerosis, Dr. Kim noted. “The treatment of PAD includes addressing leg pain and wounds with procedures, but the interventions that will keep people alive are the medications we use to prevent heart attack and stroke. Patients with PAD need to understand that treatment is much more than opening up a blockage in the leg.”
Dr. Rao and Dr. Kim disclosed no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
Marijuana linked to higher PAD risk
But death, intervention rates same
PHOENIX – although there was no greater risk of death from myocardial infarction or other cardiac causes or need for revascularization.
The researchers noted, however, that the study population was young, with an average age of 37.4 years, and that the study period, from 2016 to 2019, predates the legalization of recreational marijuana in a number of states.
Nonetheless, even in this young study population, marijuana users’ risk of developing peripheral artery disease (PAD) was 3.68 times greater (P < .001) than that of nonusers. PAD at a young age could precede worse outcomes later in life, the study authors said.
“Basically, marijuana users were at increased risk of being diagnosed with peripheral artery disease, but there was no increased risk for them requiring any intervention, such as a peripheral vascular intervention, nor were they at increased risk of death from what we found,” said Hirva Vyas, DO, an internal medicine resident at Hackensack University Medical Center in New Jersey, who presented the results at the Society for Cardiovascular Angiography & Interventions annual scientific sessions.
The study used data on 623,768 marijuana users from the National Inpatient Sample, a nationwide database of inpatient visits covered by all public and commercial payers, then extracted a diagnosis for PAD from all 30 million–plus patient encounters to compare PAD rates between marijuana users and nonusers. Marijuana users were more likely to be White and to have elective rather than emergency admissions (P < .001). The researchers used diagnostic codes to identify marijuana users and PAD patients.
Recreational marijuana is legal in 22 states and the District of Columbia, according to ProCon.org. Since 2019, the last year of the study, 11 states have legalized marijuana for recreational use. “It’s a data point that we studied at one point in time, only from 2016 to 2019,” Dr. Vyas said in an interview.
“As we’ve seen over the past 4-5 years, legalization has skyrocketed and recreational use has become more and more favorable not only among younger folks but older folks,” study coauthor Harsh Jain, MD, a second-year internal medicine resident at Montefiore Medical Center in New York, said in the interview. “It would be really refreshing to see how these data change as we look at endpoints from 2019 to 2023.”
Because of the young age of the study population, Dr. Jain said, these findings may not accurately represent the true cardiovascular risks of marijuana use, especially later in life.
“One of the biggest secondary endpoints that we wanted to study was the development of chronic conditions that lead to multiple rehospitalizations, the most significant one of which would be the development of heart failure,” Dr. Jain said. “However, it was difficult to stratify because, again, many of these patients were very young and so they did not carry the diagnosis for heart failure, so we couldn’t complete that subset analysis.”
The goal is to extend the study period out to 2023, Dr. Jain said. “We know that these are very crude and rudimentary data findings that we presented so far, but we’re hoping that the final paper gives us a chance to flesh out all the details of our study and also gives us a chance to expand going forward,” he said.
The findings are in line with other research into the effects of marijuana and cardiovascular disease, said Carl “Chip” Lavie, MD, medical director for cardiac rehabilitation and prevention at the John Ochsner Heart and Vascular Institute in New Orleans who’s published a number of studies on PAD and substance use, including marijuana.
“It is known that cannabis is associated with more vasoconstriction, has sympathomimetic effects, causes endothelial dysfunction and increased platelet aggregation, and is known to increase the risk of acute myocardial infarction, especially in the hour or so after use,” he said in written comments sent to this news organization.
“It is also well known to be a cause of thromboangiitis obliterans, which is in the PAD family,” he added. “Based on these mechanisms, one would expect an increased PAD and, especially, PAD events. The 3.7-fold increased risk is supportive of this increased PAD.”
One study strength, Dr. Lavie pointed out, is that it’s one of the few studies that found an association between marijuana and PAD, which hasn’t been studied as well as other cardiovascular endpoints. “However,” he said, “the limitation is this is just an inpatient sample, and it is all based on coding – e.g., a patient could have PAD and it may not have been coded.”
But death, intervention rates same
But death, intervention rates same
PHOENIX – although there was no greater risk of death from myocardial infarction or other cardiac causes or need for revascularization.
The researchers noted, however, that the study population was young, with an average age of 37.4 years, and that the study period, from 2016 to 2019, predates the legalization of recreational marijuana in a number of states.
Nonetheless, even in this young study population, marijuana users’ risk of developing peripheral artery disease (PAD) was 3.68 times greater (P < .001) than that of nonusers. PAD at a young age could precede worse outcomes later in life, the study authors said.
“Basically, marijuana users were at increased risk of being diagnosed with peripheral artery disease, but there was no increased risk for them requiring any intervention, such as a peripheral vascular intervention, nor were they at increased risk of death from what we found,” said Hirva Vyas, DO, an internal medicine resident at Hackensack University Medical Center in New Jersey, who presented the results at the Society for Cardiovascular Angiography & Interventions annual scientific sessions.
The study used data on 623,768 marijuana users from the National Inpatient Sample, a nationwide database of inpatient visits covered by all public and commercial payers, then extracted a diagnosis for PAD from all 30 million–plus patient encounters to compare PAD rates between marijuana users and nonusers. Marijuana users were more likely to be White and to have elective rather than emergency admissions (P < .001). The researchers used diagnostic codes to identify marijuana users and PAD patients.
Recreational marijuana is legal in 22 states and the District of Columbia, according to ProCon.org. Since 2019, the last year of the study, 11 states have legalized marijuana for recreational use. “It’s a data point that we studied at one point in time, only from 2016 to 2019,” Dr. Vyas said in an interview.
“As we’ve seen over the past 4-5 years, legalization has skyrocketed and recreational use has become more and more favorable not only among younger folks but older folks,” study coauthor Harsh Jain, MD, a second-year internal medicine resident at Montefiore Medical Center in New York, said in the interview. “It would be really refreshing to see how these data change as we look at endpoints from 2019 to 2023.”
Because of the young age of the study population, Dr. Jain said, these findings may not accurately represent the true cardiovascular risks of marijuana use, especially later in life.
“One of the biggest secondary endpoints that we wanted to study was the development of chronic conditions that lead to multiple rehospitalizations, the most significant one of which would be the development of heart failure,” Dr. Jain said. “However, it was difficult to stratify because, again, many of these patients were very young and so they did not carry the diagnosis for heart failure, so we couldn’t complete that subset analysis.”
The goal is to extend the study period out to 2023, Dr. Jain said. “We know that these are very crude and rudimentary data findings that we presented so far, but we’re hoping that the final paper gives us a chance to flesh out all the details of our study and also gives us a chance to expand going forward,” he said.
The findings are in line with other research into the effects of marijuana and cardiovascular disease, said Carl “Chip” Lavie, MD, medical director for cardiac rehabilitation and prevention at the John Ochsner Heart and Vascular Institute in New Orleans who’s published a number of studies on PAD and substance use, including marijuana.
“It is known that cannabis is associated with more vasoconstriction, has sympathomimetic effects, causes endothelial dysfunction and increased platelet aggregation, and is known to increase the risk of acute myocardial infarction, especially in the hour or so after use,” he said in written comments sent to this news organization.
“It is also well known to be a cause of thromboangiitis obliterans, which is in the PAD family,” he added. “Based on these mechanisms, one would expect an increased PAD and, especially, PAD events. The 3.7-fold increased risk is supportive of this increased PAD.”
One study strength, Dr. Lavie pointed out, is that it’s one of the few studies that found an association between marijuana and PAD, which hasn’t been studied as well as other cardiovascular endpoints. “However,” he said, “the limitation is this is just an inpatient sample, and it is all based on coding – e.g., a patient could have PAD and it may not have been coded.”
PHOENIX – although there was no greater risk of death from myocardial infarction or other cardiac causes or need for revascularization.
The researchers noted, however, that the study population was young, with an average age of 37.4 years, and that the study period, from 2016 to 2019, predates the legalization of recreational marijuana in a number of states.
Nonetheless, even in this young study population, marijuana users’ risk of developing peripheral artery disease (PAD) was 3.68 times greater (P < .001) than that of nonusers. PAD at a young age could precede worse outcomes later in life, the study authors said.
“Basically, marijuana users were at increased risk of being diagnosed with peripheral artery disease, but there was no increased risk for them requiring any intervention, such as a peripheral vascular intervention, nor were they at increased risk of death from what we found,” said Hirva Vyas, DO, an internal medicine resident at Hackensack University Medical Center in New Jersey, who presented the results at the Society for Cardiovascular Angiography & Interventions annual scientific sessions.
The study used data on 623,768 marijuana users from the National Inpatient Sample, a nationwide database of inpatient visits covered by all public and commercial payers, then extracted a diagnosis for PAD from all 30 million–plus patient encounters to compare PAD rates between marijuana users and nonusers. Marijuana users were more likely to be White and to have elective rather than emergency admissions (P < .001). The researchers used diagnostic codes to identify marijuana users and PAD patients.
Recreational marijuana is legal in 22 states and the District of Columbia, according to ProCon.org. Since 2019, the last year of the study, 11 states have legalized marijuana for recreational use. “It’s a data point that we studied at one point in time, only from 2016 to 2019,” Dr. Vyas said in an interview.
“As we’ve seen over the past 4-5 years, legalization has skyrocketed and recreational use has become more and more favorable not only among younger folks but older folks,” study coauthor Harsh Jain, MD, a second-year internal medicine resident at Montefiore Medical Center in New York, said in the interview. “It would be really refreshing to see how these data change as we look at endpoints from 2019 to 2023.”
Because of the young age of the study population, Dr. Jain said, these findings may not accurately represent the true cardiovascular risks of marijuana use, especially later in life.
“One of the biggest secondary endpoints that we wanted to study was the development of chronic conditions that lead to multiple rehospitalizations, the most significant one of which would be the development of heart failure,” Dr. Jain said. “However, it was difficult to stratify because, again, many of these patients were very young and so they did not carry the diagnosis for heart failure, so we couldn’t complete that subset analysis.”
The goal is to extend the study period out to 2023, Dr. Jain said. “We know that these are very crude and rudimentary data findings that we presented so far, but we’re hoping that the final paper gives us a chance to flesh out all the details of our study and also gives us a chance to expand going forward,” he said.
The findings are in line with other research into the effects of marijuana and cardiovascular disease, said Carl “Chip” Lavie, MD, medical director for cardiac rehabilitation and prevention at the John Ochsner Heart and Vascular Institute in New Orleans who’s published a number of studies on PAD and substance use, including marijuana.
“It is known that cannabis is associated with more vasoconstriction, has sympathomimetic effects, causes endothelial dysfunction and increased platelet aggregation, and is known to increase the risk of acute myocardial infarction, especially in the hour or so after use,” he said in written comments sent to this news organization.
“It is also well known to be a cause of thromboangiitis obliterans, which is in the PAD family,” he added. “Based on these mechanisms, one would expect an increased PAD and, especially, PAD events. The 3.7-fold increased risk is supportive of this increased PAD.”
One study strength, Dr. Lavie pointed out, is that it’s one of the few studies that found an association between marijuana and PAD, which hasn’t been studied as well as other cardiovascular endpoints. “However,” he said, “the limitation is this is just an inpatient sample, and it is all based on coding – e.g., a patient could have PAD and it may not have been coded.”
AT SCAI 2023
Endovascular approach best for below-knee limb-threatening ischemia?
in a new randomized trial.
In the Bypass Versus Angioplasty in Severe Ischaemia of the Leg (BASIL-2) trial, patients who received vein bypass as the first approach were more likely to require a major amputation or to die during follow-up than patients who were randomly assigned to the endovascular approach as first strategy.
“Our findings suggest that a best endovascular treatment first revascularization strategy is associated with a better amputation-free survival. This is mainly because the best endovascular treatment first revascularization strategy resulted in fewer deaths. Limb-related outcomes were similar between groups,” the authors stated.
“The BASIL-2 trial has produced a statistically robust and clinically meaningful result that is likely to have an influence on the management of chronic limb-threatening ischemia worldwide,” added the study’s chief investigator, Andrew Bradbury, MD, professor of vascular surgery at the University of Birmingham (England).
However, the results of the BASIL-2 trial conflict with those from two previous studies – BASIL-1 and BEST-CLI, which both suggested that a surgical approach for chronic limb-threatening ischemia may be most appropriate.
The BASIL-2 study was published online in The Lancet.
The authors explained that chronic limb-threatening ischemia, previously known as critical limb ischemia and severe ischemia of the leg, is the most severe form of peripheral arterial disease caused by atherosclerosis. Patients present with ischemic rest pain and tissue loss (ulceration, gangrene, or both) that usually affects the foot.
Mainly because of tobacco smoking and the growing prevalence of type 2 diabetes, chronic limb-threatening ischemia represents a growing burden on health care and social care services around the world.
Unless the blood supply to the affected limb is restored, patients with chronic limb-threatening ischemia are at high risk for amputation or death. Although it is universally agreed that – in addition to best medical therapy – virtually all patients with chronic limb-threatening ischemia should at least be considered for revascularization, there is continuing debate as to whether conducting vein bypass surgery, preferably using a vein taken from the patient’s own leg, or endovascular treatment (balloon angioplasty with or without stents) is preferable.
“BASIL-2 is the only randomized trial to specifically compare a vein bypass first with best endovascular treatment first revascularisation strategy in patients with chronic limb-threatening ischemia who required an infrapopliteal (with or without an additional more proximal infrainguinal) revascularization procedure to restore limb perfusion,” the authors noted.
For the trial, which was conducted at 41 vascular surgery units in the United Kingdom, Sweden, and Denmark, 345 patients with chronic limb-threatening ischemia who required an infrapopliteal revascularization procedure to restore limb perfusion were randomly assigned to receive either vein bypass or best endovascular treatment as their first revascularization procedure.
Most vein bypasses used the great saphenous vein and originated from the common or superficial femoral arteries. Most endovascular interventions comprised plain balloon angioplasty with selective use of plain or drug-eluting stents. Participants were followed up for a minimum of 2 years.
The primary outcome was amputation-free survival, defined as time to first major (above the ankle) amputation or death from any cause measured in the intention-to-treat population.
Results showed that major amputation or death occurred in 63% of patients in the vein bypass group and in 53% of those in the best endovascular treatment group (adjusted hazard ratio, 1.35; P = .037).
The results were driven by a higher death rate in the vein bypass group (53% vs. 45%; aHR, 1.37).
In both groups, the most common causes of morbidity and death, including death occurring within 30 days of first revascularization, were cardiovascular and respiratory events.
The authors noted that outcomes for the patients in the BASIL-2 trial were poor (median amputation-free survival was 3.8 years, and half the patients died within 5 years).
They pointed out that severe, multilevel atherosclerotic disease that causes chronic limb-threatening ischemia develops over many years, but at baseline in this study, around 20% of patients said they were still smoking, and around 70% of patients had diabetes, of whom around 50% required insulin. In addition, around 90% of the participants often had quite extensive tissue loss.
“These baseline data suggest that there might still be missed opportunities in public health and primary care to prevent chronic limb-threatening ischemia through medical therapy and lifestyle interventions and missed opportunities to refer patients to secondary care earlier once chronic limb-threatening ischemia begins to develop,” they suggested.
“Better prevention and timely referral are important: the BASIL-2 trial shows that, by the time patients present to vascular and endovascular surgeons and interventional radiologists with established chronic limb-threatening ischemia, their prognosis is often poor regardless of what form of revascularization they are offered,” they added.
Conflicting results
In an accompanying comment, Ankur Kalra, MD, Franciscan Health, Lafayette, Ind., and Ashish Kumar, MD, Cleveland Clinic Akron (Ohio) General, noted that atherosclerotic lower-extremity peripheral artery disease affects more than 230 million people worldwide, and prevalence is increasing. Chronic limb-threatening ischemia is a severe form of peripheral artery disease that affects 11% of patients with peripheral artery disease and is associated with significant cardiovascular morbidity and death.
Furthermore, amputation rates of 10%-40% during a 6-month follow-up of patients with chronic limb-threatening ischemia who were unable to undergo revascularization have been reported, highlighting the severity of atherosclerotic burden and the need for improved treatment strategies.
Dr. Kalra and Dr. Kumar pointed out that two previous randomized clinical trials compared surgical vein graft bypass with endovascular treatment for patients with chronic limb-threatening ischemia – the BASIL-1 trial, and the BEST-CLI trial.
In the BASIL-1 trial, vein bypass was associated with improved overall survival and amputation-free survival for patients who survived at least 2 years. The BEST-CLI trial also reported a lower risk of a composite of major adverse limb events or death among patients undergoing a surgery-first strategy, compared with endovascular therapy, mostly in patients with suitable single segment of great saphenous vein.
Dr. Kalra and Dr. Kumar said the findings of the BASIL-2 trial should be put in context with these previous studies, which report a positive or equivocal effect of surgery. The results of the BEST-CLI trial were driven by fewer major reinterventions and above-ankle amputations in the surgical group, whereas the results of the BASIL-2 trial were driven by fewer deaths in the best endovascular treatment group, “which potentially points towards a difference in the characteristics of the patients randomly assigned in the two trials.”
They concluded: “Considering the results of the BASIL-2 trial and the BEST-CLI trial, choice of intervention should be based on shared decision making between interventional cardiology, vascular surgery, and the patient, until more evidence is accrued.”
The BASIL-2 trial was funded by the U.K. National Institute of Health Research.
A version of this article first appeared on Medscape.com.
in a new randomized trial.
In the Bypass Versus Angioplasty in Severe Ischaemia of the Leg (BASIL-2) trial, patients who received vein bypass as the first approach were more likely to require a major amputation or to die during follow-up than patients who were randomly assigned to the endovascular approach as first strategy.
“Our findings suggest that a best endovascular treatment first revascularization strategy is associated with a better amputation-free survival. This is mainly because the best endovascular treatment first revascularization strategy resulted in fewer deaths. Limb-related outcomes were similar between groups,” the authors stated.
“The BASIL-2 trial has produced a statistically robust and clinically meaningful result that is likely to have an influence on the management of chronic limb-threatening ischemia worldwide,” added the study’s chief investigator, Andrew Bradbury, MD, professor of vascular surgery at the University of Birmingham (England).
However, the results of the BASIL-2 trial conflict with those from two previous studies – BASIL-1 and BEST-CLI, which both suggested that a surgical approach for chronic limb-threatening ischemia may be most appropriate.
The BASIL-2 study was published online in The Lancet.
The authors explained that chronic limb-threatening ischemia, previously known as critical limb ischemia and severe ischemia of the leg, is the most severe form of peripheral arterial disease caused by atherosclerosis. Patients present with ischemic rest pain and tissue loss (ulceration, gangrene, or both) that usually affects the foot.
Mainly because of tobacco smoking and the growing prevalence of type 2 diabetes, chronic limb-threatening ischemia represents a growing burden on health care and social care services around the world.
Unless the blood supply to the affected limb is restored, patients with chronic limb-threatening ischemia are at high risk for amputation or death. Although it is universally agreed that – in addition to best medical therapy – virtually all patients with chronic limb-threatening ischemia should at least be considered for revascularization, there is continuing debate as to whether conducting vein bypass surgery, preferably using a vein taken from the patient’s own leg, or endovascular treatment (balloon angioplasty with or without stents) is preferable.
“BASIL-2 is the only randomized trial to specifically compare a vein bypass first with best endovascular treatment first revascularisation strategy in patients with chronic limb-threatening ischemia who required an infrapopliteal (with or without an additional more proximal infrainguinal) revascularization procedure to restore limb perfusion,” the authors noted.
For the trial, which was conducted at 41 vascular surgery units in the United Kingdom, Sweden, and Denmark, 345 patients with chronic limb-threatening ischemia who required an infrapopliteal revascularization procedure to restore limb perfusion were randomly assigned to receive either vein bypass or best endovascular treatment as their first revascularization procedure.
Most vein bypasses used the great saphenous vein and originated from the common or superficial femoral arteries. Most endovascular interventions comprised plain balloon angioplasty with selective use of plain or drug-eluting stents. Participants were followed up for a minimum of 2 years.
The primary outcome was amputation-free survival, defined as time to first major (above the ankle) amputation or death from any cause measured in the intention-to-treat population.
Results showed that major amputation or death occurred in 63% of patients in the vein bypass group and in 53% of those in the best endovascular treatment group (adjusted hazard ratio, 1.35; P = .037).
The results were driven by a higher death rate in the vein bypass group (53% vs. 45%; aHR, 1.37).
In both groups, the most common causes of morbidity and death, including death occurring within 30 days of first revascularization, were cardiovascular and respiratory events.
The authors noted that outcomes for the patients in the BASIL-2 trial were poor (median amputation-free survival was 3.8 years, and half the patients died within 5 years).
They pointed out that severe, multilevel atherosclerotic disease that causes chronic limb-threatening ischemia develops over many years, but at baseline in this study, around 20% of patients said they were still smoking, and around 70% of patients had diabetes, of whom around 50% required insulin. In addition, around 90% of the participants often had quite extensive tissue loss.
“These baseline data suggest that there might still be missed opportunities in public health and primary care to prevent chronic limb-threatening ischemia through medical therapy and lifestyle interventions and missed opportunities to refer patients to secondary care earlier once chronic limb-threatening ischemia begins to develop,” they suggested.
“Better prevention and timely referral are important: the BASIL-2 trial shows that, by the time patients present to vascular and endovascular surgeons and interventional radiologists with established chronic limb-threatening ischemia, their prognosis is often poor regardless of what form of revascularization they are offered,” they added.
Conflicting results
In an accompanying comment, Ankur Kalra, MD, Franciscan Health, Lafayette, Ind., and Ashish Kumar, MD, Cleveland Clinic Akron (Ohio) General, noted that atherosclerotic lower-extremity peripheral artery disease affects more than 230 million people worldwide, and prevalence is increasing. Chronic limb-threatening ischemia is a severe form of peripheral artery disease that affects 11% of patients with peripheral artery disease and is associated with significant cardiovascular morbidity and death.
Furthermore, amputation rates of 10%-40% during a 6-month follow-up of patients with chronic limb-threatening ischemia who were unable to undergo revascularization have been reported, highlighting the severity of atherosclerotic burden and the need for improved treatment strategies.
Dr. Kalra and Dr. Kumar pointed out that two previous randomized clinical trials compared surgical vein graft bypass with endovascular treatment for patients with chronic limb-threatening ischemia – the BASIL-1 trial, and the BEST-CLI trial.
In the BASIL-1 trial, vein bypass was associated with improved overall survival and amputation-free survival for patients who survived at least 2 years. The BEST-CLI trial also reported a lower risk of a composite of major adverse limb events or death among patients undergoing a surgery-first strategy, compared with endovascular therapy, mostly in patients with suitable single segment of great saphenous vein.
Dr. Kalra and Dr. Kumar said the findings of the BASIL-2 trial should be put in context with these previous studies, which report a positive or equivocal effect of surgery. The results of the BEST-CLI trial were driven by fewer major reinterventions and above-ankle amputations in the surgical group, whereas the results of the BASIL-2 trial were driven by fewer deaths in the best endovascular treatment group, “which potentially points towards a difference in the characteristics of the patients randomly assigned in the two trials.”
They concluded: “Considering the results of the BASIL-2 trial and the BEST-CLI trial, choice of intervention should be based on shared decision making between interventional cardiology, vascular surgery, and the patient, until more evidence is accrued.”
The BASIL-2 trial was funded by the U.K. National Institute of Health Research.
A version of this article first appeared on Medscape.com.
in a new randomized trial.
In the Bypass Versus Angioplasty in Severe Ischaemia of the Leg (BASIL-2) trial, patients who received vein bypass as the first approach were more likely to require a major amputation or to die during follow-up than patients who were randomly assigned to the endovascular approach as first strategy.
“Our findings suggest that a best endovascular treatment first revascularization strategy is associated with a better amputation-free survival. This is mainly because the best endovascular treatment first revascularization strategy resulted in fewer deaths. Limb-related outcomes were similar between groups,” the authors stated.
“The BASIL-2 trial has produced a statistically robust and clinically meaningful result that is likely to have an influence on the management of chronic limb-threatening ischemia worldwide,” added the study’s chief investigator, Andrew Bradbury, MD, professor of vascular surgery at the University of Birmingham (England).
However, the results of the BASIL-2 trial conflict with those from two previous studies – BASIL-1 and BEST-CLI, which both suggested that a surgical approach for chronic limb-threatening ischemia may be most appropriate.
The BASIL-2 study was published online in The Lancet.
The authors explained that chronic limb-threatening ischemia, previously known as critical limb ischemia and severe ischemia of the leg, is the most severe form of peripheral arterial disease caused by atherosclerosis. Patients present with ischemic rest pain and tissue loss (ulceration, gangrene, or both) that usually affects the foot.
Mainly because of tobacco smoking and the growing prevalence of type 2 diabetes, chronic limb-threatening ischemia represents a growing burden on health care and social care services around the world.
Unless the blood supply to the affected limb is restored, patients with chronic limb-threatening ischemia are at high risk for amputation or death. Although it is universally agreed that – in addition to best medical therapy – virtually all patients with chronic limb-threatening ischemia should at least be considered for revascularization, there is continuing debate as to whether conducting vein bypass surgery, preferably using a vein taken from the patient’s own leg, or endovascular treatment (balloon angioplasty with or without stents) is preferable.
“BASIL-2 is the only randomized trial to specifically compare a vein bypass first with best endovascular treatment first revascularisation strategy in patients with chronic limb-threatening ischemia who required an infrapopliteal (with or without an additional more proximal infrainguinal) revascularization procedure to restore limb perfusion,” the authors noted.
For the trial, which was conducted at 41 vascular surgery units in the United Kingdom, Sweden, and Denmark, 345 patients with chronic limb-threatening ischemia who required an infrapopliteal revascularization procedure to restore limb perfusion were randomly assigned to receive either vein bypass or best endovascular treatment as their first revascularization procedure.
Most vein bypasses used the great saphenous vein and originated from the common or superficial femoral arteries. Most endovascular interventions comprised plain balloon angioplasty with selective use of plain or drug-eluting stents. Participants were followed up for a minimum of 2 years.
The primary outcome was amputation-free survival, defined as time to first major (above the ankle) amputation or death from any cause measured in the intention-to-treat population.
Results showed that major amputation or death occurred in 63% of patients in the vein bypass group and in 53% of those in the best endovascular treatment group (adjusted hazard ratio, 1.35; P = .037).
The results were driven by a higher death rate in the vein bypass group (53% vs. 45%; aHR, 1.37).
In both groups, the most common causes of morbidity and death, including death occurring within 30 days of first revascularization, were cardiovascular and respiratory events.
The authors noted that outcomes for the patients in the BASIL-2 trial were poor (median amputation-free survival was 3.8 years, and half the patients died within 5 years).
They pointed out that severe, multilevel atherosclerotic disease that causes chronic limb-threatening ischemia develops over many years, but at baseline in this study, around 20% of patients said they were still smoking, and around 70% of patients had diabetes, of whom around 50% required insulin. In addition, around 90% of the participants often had quite extensive tissue loss.
“These baseline data suggest that there might still be missed opportunities in public health and primary care to prevent chronic limb-threatening ischemia through medical therapy and lifestyle interventions and missed opportunities to refer patients to secondary care earlier once chronic limb-threatening ischemia begins to develop,” they suggested.
“Better prevention and timely referral are important: the BASIL-2 trial shows that, by the time patients present to vascular and endovascular surgeons and interventional radiologists with established chronic limb-threatening ischemia, their prognosis is often poor regardless of what form of revascularization they are offered,” they added.
Conflicting results
In an accompanying comment, Ankur Kalra, MD, Franciscan Health, Lafayette, Ind., and Ashish Kumar, MD, Cleveland Clinic Akron (Ohio) General, noted that atherosclerotic lower-extremity peripheral artery disease affects more than 230 million people worldwide, and prevalence is increasing. Chronic limb-threatening ischemia is a severe form of peripheral artery disease that affects 11% of patients with peripheral artery disease and is associated with significant cardiovascular morbidity and death.
Furthermore, amputation rates of 10%-40% during a 6-month follow-up of patients with chronic limb-threatening ischemia who were unable to undergo revascularization have been reported, highlighting the severity of atherosclerotic burden and the need for improved treatment strategies.
Dr. Kalra and Dr. Kumar pointed out that two previous randomized clinical trials compared surgical vein graft bypass with endovascular treatment for patients with chronic limb-threatening ischemia – the BASIL-1 trial, and the BEST-CLI trial.
In the BASIL-1 trial, vein bypass was associated with improved overall survival and amputation-free survival for patients who survived at least 2 years. The BEST-CLI trial also reported a lower risk of a composite of major adverse limb events or death among patients undergoing a surgery-first strategy, compared with endovascular therapy, mostly in patients with suitable single segment of great saphenous vein.
Dr. Kalra and Dr. Kumar said the findings of the BASIL-2 trial should be put in context with these previous studies, which report a positive or equivocal effect of surgery. The results of the BEST-CLI trial were driven by fewer major reinterventions and above-ankle amputations in the surgical group, whereas the results of the BASIL-2 trial were driven by fewer deaths in the best endovascular treatment group, “which potentially points towards a difference in the characteristics of the patients randomly assigned in the two trials.”
They concluded: “Considering the results of the BASIL-2 trial and the BEST-CLI trial, choice of intervention should be based on shared decision making between interventional cardiology, vascular surgery, and the patient, until more evidence is accrued.”
The BASIL-2 trial was funded by the U.K. National Institute of Health Research.
A version of this article first appeared on Medscape.com.
FROM THE LANCET
Specific brain damage links hypertension to cognitive impairment
Researchers have identified specific regions of the brain that appear to be damaged by high blood pressure. The finding may explain the link between hypertension and cognitive impairment.
They used genetic information from genome-wide association studies (GWASs) and MRI scans of the brain to study the relationship between hypertension, changes in brain structures, and cognitive impairment. Using Mendelian randomization techniques, they identified nine brain structures related to cognitive impairment that are affected by blood pressure.
“We knew before that raised blood pressure was related to changes in the brain, but our research has narrowed down the changes to those that appear to be potentially causally related to cognitive impairment,” senior author Tomasz Guzik, professor of cardiovascular medicine, at the University of Edinburgh and of the Jagiellonian University, Krakow, Poland, told this news organization.
“Our study confirms a potentially causal relationship between raised blood pressure and cognitive impairment, emphasizing the importance of preventing and treating hypertension,” Prof. Guzik noted.
“But it also identifies the brain culprits of this relationship,” he added.
In the future, it may be possible to assess these nine brain structures in people with high blood pressure to identify those at increased risk of developing cognitive impairment, he said. “These patients may need more intensive care for their blood pressure. We can also investigate these brain structures for potential signaling pathways and molecular changes to see if we can find new targets for treatment to prevent cognitive impairment.”
For this report, the investigators married together different research datasets to identify brain structures potentially responsible for the effects of blood pressure on cognitive function, using results from previous GWASs and observational data from 39,000 people in the UK Biobank registry for whom brain MRI data were available.
First, they mapped brain structures potentially influenced by blood pressure in midlife using MRI scans from people in the UK Biobank registry. Then they examined the relationship between blood pressure and cognitive function in the UK Biobank registry. Next, of the brain structures affected by blood pressure, they identified those that are causally linked to cognitive impairment.
This was possible thanks to genetic markers coding for increased blood pressure, brain structure imaging phenotypes, and those coding for cognitive impairment that could be used in Mendelian randomization studies.
“We looked at 3935 brain magnetic resonance imaging–derived phenotypes in the brain and cognitive function defined by fluid intelligence score to identify genetically predicted causal relationships,” Prof. Guzik said.
They identified 200 brain structures that were causally affected by systolic blood pressure. Of these, nine were also causally related to cognitive impairment. The results were validated in a second prospective cohort of patients with hypertension.
“Some of these structures, including putamen and the white matter regions spanning between the anterior corona radiata, anterior thalamic radiation, and anterior limb of the internal capsule, may represent the target brain regions at which systolic blood pressure acts on cognitive function,” the authors comment.
In an accompanying editorial, Ernesto Schiffrin, MD, and James Engert, PhD, McGill University, Montreal, say that further mechanistic studies of the effects of blood pressure on cognitive function are required to determine precise causal pathways and the roles of relevant brain regions.
“Eventually, biomarkers could be developed to inform antihypertensive trials. Whether clinical trials targeting the specific brain structures will be feasible or if specific antihypertensives could be found that target specific structures remains to be demonstrated,” they write.
“Thus, these new studies could lead to an understanding of the signaling pathways that explain how these structures relate vascular damage to cognitive impairment in hypertension, and contribute to the development of novel interventions to more successfully address the scourge of cognitive decline and dementia in the future,” the editorialists conclude.
The study was funded by the European Research Council, the British Heart Foundation, and the Italian Ministry of Health.
A version of this article first appeared on Medscape.com.
Researchers have identified specific regions of the brain that appear to be damaged by high blood pressure. The finding may explain the link between hypertension and cognitive impairment.
They used genetic information from genome-wide association studies (GWASs) and MRI scans of the brain to study the relationship between hypertension, changes in brain structures, and cognitive impairment. Using Mendelian randomization techniques, they identified nine brain structures related to cognitive impairment that are affected by blood pressure.
“We knew before that raised blood pressure was related to changes in the brain, but our research has narrowed down the changes to those that appear to be potentially causally related to cognitive impairment,” senior author Tomasz Guzik, professor of cardiovascular medicine, at the University of Edinburgh and of the Jagiellonian University, Krakow, Poland, told this news organization.
“Our study confirms a potentially causal relationship between raised blood pressure and cognitive impairment, emphasizing the importance of preventing and treating hypertension,” Prof. Guzik noted.
“But it also identifies the brain culprits of this relationship,” he added.
In the future, it may be possible to assess these nine brain structures in people with high blood pressure to identify those at increased risk of developing cognitive impairment, he said. “These patients may need more intensive care for their blood pressure. We can also investigate these brain structures for potential signaling pathways and molecular changes to see if we can find new targets for treatment to prevent cognitive impairment.”
For this report, the investigators married together different research datasets to identify brain structures potentially responsible for the effects of blood pressure on cognitive function, using results from previous GWASs and observational data from 39,000 people in the UK Biobank registry for whom brain MRI data were available.
First, they mapped brain structures potentially influenced by blood pressure in midlife using MRI scans from people in the UK Biobank registry. Then they examined the relationship between blood pressure and cognitive function in the UK Biobank registry. Next, of the brain structures affected by blood pressure, they identified those that are causally linked to cognitive impairment.
This was possible thanks to genetic markers coding for increased blood pressure, brain structure imaging phenotypes, and those coding for cognitive impairment that could be used in Mendelian randomization studies.
“We looked at 3935 brain magnetic resonance imaging–derived phenotypes in the brain and cognitive function defined by fluid intelligence score to identify genetically predicted causal relationships,” Prof. Guzik said.
They identified 200 brain structures that were causally affected by systolic blood pressure. Of these, nine were also causally related to cognitive impairment. The results were validated in a second prospective cohort of patients with hypertension.
“Some of these structures, including putamen and the white matter regions spanning between the anterior corona radiata, anterior thalamic radiation, and anterior limb of the internal capsule, may represent the target brain regions at which systolic blood pressure acts on cognitive function,” the authors comment.
In an accompanying editorial, Ernesto Schiffrin, MD, and James Engert, PhD, McGill University, Montreal, say that further mechanistic studies of the effects of blood pressure on cognitive function are required to determine precise causal pathways and the roles of relevant brain regions.
“Eventually, biomarkers could be developed to inform antihypertensive trials. Whether clinical trials targeting the specific brain structures will be feasible or if specific antihypertensives could be found that target specific structures remains to be demonstrated,” they write.
“Thus, these new studies could lead to an understanding of the signaling pathways that explain how these structures relate vascular damage to cognitive impairment in hypertension, and contribute to the development of novel interventions to more successfully address the scourge of cognitive decline and dementia in the future,” the editorialists conclude.
The study was funded by the European Research Council, the British Heart Foundation, and the Italian Ministry of Health.
A version of this article first appeared on Medscape.com.
Researchers have identified specific regions of the brain that appear to be damaged by high blood pressure. The finding may explain the link between hypertension and cognitive impairment.
They used genetic information from genome-wide association studies (GWASs) and MRI scans of the brain to study the relationship between hypertension, changes in brain structures, and cognitive impairment. Using Mendelian randomization techniques, they identified nine brain structures related to cognitive impairment that are affected by blood pressure.
“We knew before that raised blood pressure was related to changes in the brain, but our research has narrowed down the changes to those that appear to be potentially causally related to cognitive impairment,” senior author Tomasz Guzik, professor of cardiovascular medicine, at the University of Edinburgh and of the Jagiellonian University, Krakow, Poland, told this news organization.
“Our study confirms a potentially causal relationship between raised blood pressure and cognitive impairment, emphasizing the importance of preventing and treating hypertension,” Prof. Guzik noted.
“But it also identifies the brain culprits of this relationship,” he added.
In the future, it may be possible to assess these nine brain structures in people with high blood pressure to identify those at increased risk of developing cognitive impairment, he said. “These patients may need more intensive care for their blood pressure. We can also investigate these brain structures for potential signaling pathways and molecular changes to see if we can find new targets for treatment to prevent cognitive impairment.”
For this report, the investigators married together different research datasets to identify brain structures potentially responsible for the effects of blood pressure on cognitive function, using results from previous GWASs and observational data from 39,000 people in the UK Biobank registry for whom brain MRI data were available.
First, they mapped brain structures potentially influenced by blood pressure in midlife using MRI scans from people in the UK Biobank registry. Then they examined the relationship between blood pressure and cognitive function in the UK Biobank registry. Next, of the brain structures affected by blood pressure, they identified those that are causally linked to cognitive impairment.
This was possible thanks to genetic markers coding for increased blood pressure, brain structure imaging phenotypes, and those coding for cognitive impairment that could be used in Mendelian randomization studies.
“We looked at 3935 brain magnetic resonance imaging–derived phenotypes in the brain and cognitive function defined by fluid intelligence score to identify genetically predicted causal relationships,” Prof. Guzik said.
They identified 200 brain structures that were causally affected by systolic blood pressure. Of these, nine were also causally related to cognitive impairment. The results were validated in a second prospective cohort of patients with hypertension.
“Some of these structures, including putamen and the white matter regions spanning between the anterior corona radiata, anterior thalamic radiation, and anterior limb of the internal capsule, may represent the target brain regions at which systolic blood pressure acts on cognitive function,” the authors comment.
In an accompanying editorial, Ernesto Schiffrin, MD, and James Engert, PhD, McGill University, Montreal, say that further mechanistic studies of the effects of blood pressure on cognitive function are required to determine precise causal pathways and the roles of relevant brain regions.
“Eventually, biomarkers could be developed to inform antihypertensive trials. Whether clinical trials targeting the specific brain structures will be feasible or if specific antihypertensives could be found that target specific structures remains to be demonstrated,” they write.
“Thus, these new studies could lead to an understanding of the signaling pathways that explain how these structures relate vascular damage to cognitive impairment in hypertension, and contribute to the development of novel interventions to more successfully address the scourge of cognitive decline and dementia in the future,” the editorialists conclude.
The study was funded by the European Research Council, the British Heart Foundation, and the Italian Ministry of Health.
A version of this article first appeared on Medscape.com.
‘Keto-like’ diet linked to doubling of heart disease risk
Consumption of a low-carbohydrate, high-fat diet, dubbed a “keto-like” diet, was associated with an increase in LDL levels and a twofold increase in the risk for future cardiovascular events, in a new observational study.
“To our knowledge this is the first study to demonstrate an association between a carbohydrate-restricted dietary platform and greater risk of atherosclerotic cardiovascular disease,” said study investigator Iulia Iatan, MD, PhD, University of British Columbia, Vancouver.
“Hypercholesterolemia occurring during a low-carb, high-fat diet should not be assumed to be benign,” she concluded.
Dr. Iatan presented the study March 5 at the joint scientific sessions of the American College of Cardiology and the World Heart Federation.
The presentation received much media attention, with headlines implying a causal relationship with cardiac events based on these observational results. But lipid expert Steven Nissen, MD, of the Cleveland Clinic, warned against paying much attention to the headlines or to the study’s conclusions.
In an interview, Dr. Nissen pointed out that the LDL increase in the “keto-like” diet group was relatively small and “certainly not enough to produce a doubling in cardiovascular risk.
“The people who were on the ‘keto-like’ diet in this study were different than those who were on the standard diet,” he said. “Those on the ‘keto-like’ diet were on it for a reason – they were more overweight, they had a higher incidence of diabetes, so their risk profile was completely different. Even though the researchers tried to adjust for other cardiovascular risk factors, there will be unmeasured confounding in a study like this.”
He said he doesn’t think this study “answers any significant questions in a way that we want to have them answered. I’m not a big fan of this type of diet, but I don’t think it doubles the risk of adverse cardiovascular events, and I don’t think this study tells us one way or another.”
For the study, Dr. Iatan and colleagues defined a low-carbohydrate, high-fat diet as consisting of no more than 25% of total daily energy from carbohydrates and more than 45% of total daily calories from fat. This is somewhat higher in carbohydrates and lower in fat than a strict ketogenic diet but could be thought of as a ‘keto-like’ diet.
They analyzed data from the UK Biobank, a large-scale prospective database with health information from over half a million people living in the United Kingdom who were followed for at least 10 years.
On enrollment in the Biobank, participants completed a one-time, self-reported 24-hour diet questionnaire and, at the same time, had blood drawn to check their levels of cholesterol. The researchers identified 305 participants whose questionnaire responses indicated that they followed a low-carbohydrate, high-fat diet. These participants were matched by age and sex with 1,220 individuals who reported being on a standard diet.
Of the study population, 73% were women and the average age was 54 years. Those on a low carbohydrate/high fat diet had a higher average body mass index (27.7 vs. 26.7) and a higher incidence of diabetes (4.9% vs. 1.7%).
Results showed that compared with participants on a standard diet, those on the “keto-like” diet had significantly higher levels of both LDL cholesterol and apolipoprotein B (ApoB).
Levels of LDL were 3.80 mmol/L (147 mg/dL) in the keto-like group vs. 3.64 mmol/L (141 mg/dL) in the standard group (P = .004). Levels of ApoB were 1.09 g/L (109 mg/dL) in the keto-like group and 1.04 g/L (104 mg/dL) in the standard group (P < .001).
After an average of 11.8 years of follow-up, 9.8% of participants on the low-carbohydrate/high-fat diet vs. 4.3% in the standard diet group experienced one of the events included in the composite event endpoint: Angina, myocardial infarction, coronary artery disease, ischemic stroke, peripheral arterial disease, or coronary/carotid revascularization.
After adjustment for other risk factors for heart disease – diabetes, hypertension, obesity, and smoking – individuals on a low-carbohydrate, high-fat diet were found to have a twofold risk of having a cardiovascular event (HR, 2.18; P < .001).
‘Closer monitoring needed’
“Our results have shown, I think for the first time, that there is an association between this increasingly popular dietary pattern and high LDL cholesterol and an increased future risk of cardiovascular events,” senior author Liam Brunham, MD, of the University of British Columbia, said in an interview. “This is concerning as there are many people out there following this type of diet, and I think it suggests there is a need for closer monitoring of these people.”
He explained that while it would be expected for cholesterol levels to rise on a high-fat diet, “there has been a perception by some that this is not worrisome as it is reflecting certain metabolic changes. What we’ve shown in this study is that if your cholesterol does increase significantly on this diet then you should not assume that this is not a problem.
“For some people with diabetes this diet can help lower blood sugar and some people can lose weight on it,” he noted, “but what our data show is that there is a subgroup of people who experience high levels of LDL and ApoB and that seems to be driving the risk.”
He pointed out that overall the mean level of LDL was only slightly increased in the individuals on the low-carb/high-fat diet but severe high cholesterol (more than 5 mmol/L or 190 mg/dL) was about doubled in that group (10% vs. 5%). And these patients had a sixfold increase in risk of cardiovascular disease (P < .001).
“This suggests that there is a subgroup of people who are susceptible to this exacerbation of hypercholesterolemia in response to a low-carb/high-fat diet.”
Dr. Brunham said his advice would be that if people choose to follow this diet, they should have their cholesterol monitored, and manage their cardiovascular risk factors.
“I wouldn’t say it is not appropriate to follow this diet based on this study,” he added. “This is just an observational study. It is not definitive. But if people do want to follow this dietary pattern because they feel there would be some benefits, then they should be aware of the potential risks and take steps to mitigate those risks.”
Jury still out
Dr. Nissen said in his view “the jury was still out” on this type of diet. “I’m open to the possibility that, particularly in the short run, a ‘keto-like’ diet may help some people lose weight and that’s a good thing. But I do not generally recommend this type of diet.”
Rather, he advises patients to follow a Mediterranean diet, which has been proven to reduce cardiovascular events in a randomized study, the PREDIMED trial.
“We can’t make decisions on what type of diet to recommend to patients based on observational studies like this where there is a lot of subtlety missing. But when studies like this are reported, the mass media seize on it. That’s not the way the public needs to be educated,” Dr. Nissen said.
“We refer to this type of study as hypothesis-generating. It raises a hypothesis. It doesn’t answer the question. It is worth looking at the question of whether a ketogenic-like diet is harmful. We don’t know at present, and I don’t think we know any more after this study,” he added.
The authors of the study reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Consumption of a low-carbohydrate, high-fat diet, dubbed a “keto-like” diet, was associated with an increase in LDL levels and a twofold increase in the risk for future cardiovascular events, in a new observational study.
“To our knowledge this is the first study to demonstrate an association between a carbohydrate-restricted dietary platform and greater risk of atherosclerotic cardiovascular disease,” said study investigator Iulia Iatan, MD, PhD, University of British Columbia, Vancouver.
“Hypercholesterolemia occurring during a low-carb, high-fat diet should not be assumed to be benign,” she concluded.
Dr. Iatan presented the study March 5 at the joint scientific sessions of the American College of Cardiology and the World Heart Federation.
The presentation received much media attention, with headlines implying a causal relationship with cardiac events based on these observational results. But lipid expert Steven Nissen, MD, of the Cleveland Clinic, warned against paying much attention to the headlines or to the study’s conclusions.
In an interview, Dr. Nissen pointed out that the LDL increase in the “keto-like” diet group was relatively small and “certainly not enough to produce a doubling in cardiovascular risk.
“The people who were on the ‘keto-like’ diet in this study were different than those who were on the standard diet,” he said. “Those on the ‘keto-like’ diet were on it for a reason – they were more overweight, they had a higher incidence of diabetes, so their risk profile was completely different. Even though the researchers tried to adjust for other cardiovascular risk factors, there will be unmeasured confounding in a study like this.”
He said he doesn’t think this study “answers any significant questions in a way that we want to have them answered. I’m not a big fan of this type of diet, but I don’t think it doubles the risk of adverse cardiovascular events, and I don’t think this study tells us one way or another.”
For the study, Dr. Iatan and colleagues defined a low-carbohydrate, high-fat diet as consisting of no more than 25% of total daily energy from carbohydrates and more than 45% of total daily calories from fat. This is somewhat higher in carbohydrates and lower in fat than a strict ketogenic diet but could be thought of as a ‘keto-like’ diet.
They analyzed data from the UK Biobank, a large-scale prospective database with health information from over half a million people living in the United Kingdom who were followed for at least 10 years.
On enrollment in the Biobank, participants completed a one-time, self-reported 24-hour diet questionnaire and, at the same time, had blood drawn to check their levels of cholesterol. The researchers identified 305 participants whose questionnaire responses indicated that they followed a low-carbohydrate, high-fat diet. These participants were matched by age and sex with 1,220 individuals who reported being on a standard diet.
Of the study population, 73% were women and the average age was 54 years. Those on a low carbohydrate/high fat diet had a higher average body mass index (27.7 vs. 26.7) and a higher incidence of diabetes (4.9% vs. 1.7%).
Results showed that compared with participants on a standard diet, those on the “keto-like” diet had significantly higher levels of both LDL cholesterol and apolipoprotein B (ApoB).
Levels of LDL were 3.80 mmol/L (147 mg/dL) in the keto-like group vs. 3.64 mmol/L (141 mg/dL) in the standard group (P = .004). Levels of ApoB were 1.09 g/L (109 mg/dL) in the keto-like group and 1.04 g/L (104 mg/dL) in the standard group (P < .001).
After an average of 11.8 years of follow-up, 9.8% of participants on the low-carbohydrate/high-fat diet vs. 4.3% in the standard diet group experienced one of the events included in the composite event endpoint: Angina, myocardial infarction, coronary artery disease, ischemic stroke, peripheral arterial disease, or coronary/carotid revascularization.
After adjustment for other risk factors for heart disease – diabetes, hypertension, obesity, and smoking – individuals on a low-carbohydrate, high-fat diet were found to have a twofold risk of having a cardiovascular event (HR, 2.18; P < .001).
‘Closer monitoring needed’
“Our results have shown, I think for the first time, that there is an association between this increasingly popular dietary pattern and high LDL cholesterol and an increased future risk of cardiovascular events,” senior author Liam Brunham, MD, of the University of British Columbia, said in an interview. “This is concerning as there are many people out there following this type of diet, and I think it suggests there is a need for closer monitoring of these people.”
He explained that while it would be expected for cholesterol levels to rise on a high-fat diet, “there has been a perception by some that this is not worrisome as it is reflecting certain metabolic changes. What we’ve shown in this study is that if your cholesterol does increase significantly on this diet then you should not assume that this is not a problem.
“For some people with diabetes this diet can help lower blood sugar and some people can lose weight on it,” he noted, “but what our data show is that there is a subgroup of people who experience high levels of LDL and ApoB and that seems to be driving the risk.”
He pointed out that overall the mean level of LDL was only slightly increased in the individuals on the low-carb/high-fat diet but severe high cholesterol (more than 5 mmol/L or 190 mg/dL) was about doubled in that group (10% vs. 5%). And these patients had a sixfold increase in risk of cardiovascular disease (P < .001).
“This suggests that there is a subgroup of people who are susceptible to this exacerbation of hypercholesterolemia in response to a low-carb/high-fat diet.”
Dr. Brunham said his advice would be that if people choose to follow this diet, they should have their cholesterol monitored, and manage their cardiovascular risk factors.
“I wouldn’t say it is not appropriate to follow this diet based on this study,” he added. “This is just an observational study. It is not definitive. But if people do want to follow this dietary pattern because they feel there would be some benefits, then they should be aware of the potential risks and take steps to mitigate those risks.”
Jury still out
Dr. Nissen said in his view “the jury was still out” on this type of diet. “I’m open to the possibility that, particularly in the short run, a ‘keto-like’ diet may help some people lose weight and that’s a good thing. But I do not generally recommend this type of diet.”
Rather, he advises patients to follow a Mediterranean diet, which has been proven to reduce cardiovascular events in a randomized study, the PREDIMED trial.
“We can’t make decisions on what type of diet to recommend to patients based on observational studies like this where there is a lot of subtlety missing. But when studies like this are reported, the mass media seize on it. That’s not the way the public needs to be educated,” Dr. Nissen said.
“We refer to this type of study as hypothesis-generating. It raises a hypothesis. It doesn’t answer the question. It is worth looking at the question of whether a ketogenic-like diet is harmful. We don’t know at present, and I don’t think we know any more after this study,” he added.
The authors of the study reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Consumption of a low-carbohydrate, high-fat diet, dubbed a “keto-like” diet, was associated with an increase in LDL levels and a twofold increase in the risk for future cardiovascular events, in a new observational study.
“To our knowledge this is the first study to demonstrate an association between a carbohydrate-restricted dietary platform and greater risk of atherosclerotic cardiovascular disease,” said study investigator Iulia Iatan, MD, PhD, University of British Columbia, Vancouver.
“Hypercholesterolemia occurring during a low-carb, high-fat diet should not be assumed to be benign,” she concluded.
Dr. Iatan presented the study March 5 at the joint scientific sessions of the American College of Cardiology and the World Heart Federation.
The presentation received much media attention, with headlines implying a causal relationship with cardiac events based on these observational results. But lipid expert Steven Nissen, MD, of the Cleveland Clinic, warned against paying much attention to the headlines or to the study’s conclusions.
In an interview, Dr. Nissen pointed out that the LDL increase in the “keto-like” diet group was relatively small and “certainly not enough to produce a doubling in cardiovascular risk.
“The people who were on the ‘keto-like’ diet in this study were different than those who were on the standard diet,” he said. “Those on the ‘keto-like’ diet were on it for a reason – they were more overweight, they had a higher incidence of diabetes, so their risk profile was completely different. Even though the researchers tried to adjust for other cardiovascular risk factors, there will be unmeasured confounding in a study like this.”
He said he doesn’t think this study “answers any significant questions in a way that we want to have them answered. I’m not a big fan of this type of diet, but I don’t think it doubles the risk of adverse cardiovascular events, and I don’t think this study tells us one way or another.”
For the study, Dr. Iatan and colleagues defined a low-carbohydrate, high-fat diet as consisting of no more than 25% of total daily energy from carbohydrates and more than 45% of total daily calories from fat. This is somewhat higher in carbohydrates and lower in fat than a strict ketogenic diet but could be thought of as a ‘keto-like’ diet.
They analyzed data from the UK Biobank, a large-scale prospective database with health information from over half a million people living in the United Kingdom who were followed for at least 10 years.
On enrollment in the Biobank, participants completed a one-time, self-reported 24-hour diet questionnaire and, at the same time, had blood drawn to check their levels of cholesterol. The researchers identified 305 participants whose questionnaire responses indicated that they followed a low-carbohydrate, high-fat diet. These participants were matched by age and sex with 1,220 individuals who reported being on a standard diet.
Of the study population, 73% were women and the average age was 54 years. Those on a low carbohydrate/high fat diet had a higher average body mass index (27.7 vs. 26.7) and a higher incidence of diabetes (4.9% vs. 1.7%).
Results showed that compared with participants on a standard diet, those on the “keto-like” diet had significantly higher levels of both LDL cholesterol and apolipoprotein B (ApoB).
Levels of LDL were 3.80 mmol/L (147 mg/dL) in the keto-like group vs. 3.64 mmol/L (141 mg/dL) in the standard group (P = .004). Levels of ApoB were 1.09 g/L (109 mg/dL) in the keto-like group and 1.04 g/L (104 mg/dL) in the standard group (P < .001).
After an average of 11.8 years of follow-up, 9.8% of participants on the low-carbohydrate/high-fat diet vs. 4.3% in the standard diet group experienced one of the events included in the composite event endpoint: Angina, myocardial infarction, coronary artery disease, ischemic stroke, peripheral arterial disease, or coronary/carotid revascularization.
After adjustment for other risk factors for heart disease – diabetes, hypertension, obesity, and smoking – individuals on a low-carbohydrate, high-fat diet were found to have a twofold risk of having a cardiovascular event (HR, 2.18; P < .001).
‘Closer monitoring needed’
“Our results have shown, I think for the first time, that there is an association between this increasingly popular dietary pattern and high LDL cholesterol and an increased future risk of cardiovascular events,” senior author Liam Brunham, MD, of the University of British Columbia, said in an interview. “This is concerning as there are many people out there following this type of diet, and I think it suggests there is a need for closer monitoring of these people.”
He explained that while it would be expected for cholesterol levels to rise on a high-fat diet, “there has been a perception by some that this is not worrisome as it is reflecting certain metabolic changes. What we’ve shown in this study is that if your cholesterol does increase significantly on this diet then you should not assume that this is not a problem.
“For some people with diabetes this diet can help lower blood sugar and some people can lose weight on it,” he noted, “but what our data show is that there is a subgroup of people who experience high levels of LDL and ApoB and that seems to be driving the risk.”
He pointed out that overall the mean level of LDL was only slightly increased in the individuals on the low-carb/high-fat diet but severe high cholesterol (more than 5 mmol/L or 190 mg/dL) was about doubled in that group (10% vs. 5%). And these patients had a sixfold increase in risk of cardiovascular disease (P < .001).
“This suggests that there is a subgroup of people who are susceptible to this exacerbation of hypercholesterolemia in response to a low-carb/high-fat diet.”
Dr. Brunham said his advice would be that if people choose to follow this diet, they should have their cholesterol monitored, and manage their cardiovascular risk factors.
“I wouldn’t say it is not appropriate to follow this diet based on this study,” he added. “This is just an observational study. It is not definitive. But if people do want to follow this dietary pattern because they feel there would be some benefits, then they should be aware of the potential risks and take steps to mitigate those risks.”
Jury still out
Dr. Nissen said in his view “the jury was still out” on this type of diet. “I’m open to the possibility that, particularly in the short run, a ‘keto-like’ diet may help some people lose weight and that’s a good thing. But I do not generally recommend this type of diet.”
Rather, he advises patients to follow a Mediterranean diet, which has been proven to reduce cardiovascular events in a randomized study, the PREDIMED trial.
“We can’t make decisions on what type of diet to recommend to patients based on observational studies like this where there is a lot of subtlety missing. But when studies like this are reported, the mass media seize on it. That’s not the way the public needs to be educated,” Dr. Nissen said.
“We refer to this type of study as hypothesis-generating. It raises a hypothesis. It doesn’t answer the question. It is worth looking at the question of whether a ketogenic-like diet is harmful. We don’t know at present, and I don’t think we know any more after this study,” he added.
The authors of the study reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
FROM ACC 2023
New ACC, AHA, SCAI interventional cardiology training guidance
The American College of Cardiology, the American Heart Association, and the Society for Cardiovascular Angiography and Interventions (SCAI) have jointly issued new guidance outlining competency-based advanced training requirements for interventional cardiology trainees.
It’s the first document of its kind to define the training requirements for the full breadth of interventional cardiology for adults, including coronary interventions, peripheral vascular interventions (PVIs), and structural heart interventions (SHIs), the organizations say.
“With this groundbreaking document, the writing committee provides a roadmap for both program directors and interventional cardiology trainees to help them progress through important training milestones,” Theodore A. Bass, MD, chair of the statement writing committee, says in a news release.
“The document defines the required competencies for the full scope of interventional cardiology, providing trainees for the first time with the information to support training across all these areas,” Dr. Bass adds.
Minimum of 250 procedures
To gain the necessary experience in interventional cardiology, cardiovascular fellows are advised to complete the following:
- A 3-year general cardiovascular disease fellowship (successful completion consists of Level I competency in all aspects of cardiovascular medicine and Level II competency in diagnostic cardiac catheterization to pursue interventional cardiology training);
- A 1-year accredited interventional cardiology fellowship, the focus of which is coronary intervention with the opportunity to gain procedural experience in various aspects of PVI or SHI (Level III competency);
- An option for additional post-fellowship training based on the trainee’s career goals.
The goal of Level III training is to provide the interventional cardiology trainees with a “well-rounded, competency-based education,” including didactic instruction, clinical experience in the diagnosis and care of patients, and hands-on procedural experience, the writing group says.
Competency requirements are defined using the Accreditation Council for Graduate Medical Education’s six “essential” competency domains: medical knowledge; patient care and procedural skills; practice-based learning and improvement; systems-based practice; interpersonal and communication skills; and professionalism.
To support attaining these competencies, the writing committee recommends a minimum of 250 interventional cardiology procedures. Of these, 200 should be coronary procedures, with the remaining 50 specialized in coronary, PVI, or SHI, which allows the fellows to customize training on the basis of their career goals.
Adjunctive procedures related to physiologic assessment and intracoronary imaging are also required (25 of each). “These minimum numbers are meant to provide trainees with exposure to a variety and spectrum of complexity of clinical case material and give supervising faculty sufficient opportunity to evaluate trainees’ competency,” the writing group says.
In addition to their procedural skills, evaluation of interventional cardiology trainee proficiency should include regular assessment of a trainee’s ability to clinically diagnose and manage patients across the broad spectrum of diseases.
Assessment of trainees should involve multiple components, including direct observation by instructors, case logs, chart reviews (including adherence to guideline recommendations, appropriate use criteria, and patient outcomes), simulation training, and assessment of leadership skills.
Trainees must also acquire experience working as part of a multidisciplinary team to provide a holistic approach to patient care. The document also highlights the importance of leadership skills, mentorship and lifelong learning beyond initial training.
The 2023 ACC/AHA/SCAI Advanced Training Statement on Interventional Cardiology (Coronary, Peripheral Vascular, and Structural Heart Interventions) was published online in the Journal of the American College of Cardiology.
The statement was developed in collaboration with and endorsed by the American Association for Thoracic Surgery, the American Society of Echocardiography, the Heart Failure Society of America, the Heart Rhythm Society, the Society of Cardiovascular Anesthesiologists, the Society of Cardiovascular Computed Tomography, the Society for Cardiovascular Magnetic Resonance, the Society of Thoracic Surgeons, and the Society for Vascular Medicine.
A version of this article first appeared on Medscape.com.
The American College of Cardiology, the American Heart Association, and the Society for Cardiovascular Angiography and Interventions (SCAI) have jointly issued new guidance outlining competency-based advanced training requirements for interventional cardiology trainees.
It’s the first document of its kind to define the training requirements for the full breadth of interventional cardiology for adults, including coronary interventions, peripheral vascular interventions (PVIs), and structural heart interventions (SHIs), the organizations say.
“With this groundbreaking document, the writing committee provides a roadmap for both program directors and interventional cardiology trainees to help them progress through important training milestones,” Theodore A. Bass, MD, chair of the statement writing committee, says in a news release.
“The document defines the required competencies for the full scope of interventional cardiology, providing trainees for the first time with the information to support training across all these areas,” Dr. Bass adds.
Minimum of 250 procedures
To gain the necessary experience in interventional cardiology, cardiovascular fellows are advised to complete the following:
- A 3-year general cardiovascular disease fellowship (successful completion consists of Level I competency in all aspects of cardiovascular medicine and Level II competency in diagnostic cardiac catheterization to pursue interventional cardiology training);
- A 1-year accredited interventional cardiology fellowship, the focus of which is coronary intervention with the opportunity to gain procedural experience in various aspects of PVI or SHI (Level III competency);
- An option for additional post-fellowship training based on the trainee’s career goals.
The goal of Level III training is to provide the interventional cardiology trainees with a “well-rounded, competency-based education,” including didactic instruction, clinical experience in the diagnosis and care of patients, and hands-on procedural experience, the writing group says.
Competency requirements are defined using the Accreditation Council for Graduate Medical Education’s six “essential” competency domains: medical knowledge; patient care and procedural skills; practice-based learning and improvement; systems-based practice; interpersonal and communication skills; and professionalism.
To support attaining these competencies, the writing committee recommends a minimum of 250 interventional cardiology procedures. Of these, 200 should be coronary procedures, with the remaining 50 specialized in coronary, PVI, or SHI, which allows the fellows to customize training on the basis of their career goals.
Adjunctive procedures related to physiologic assessment and intracoronary imaging are also required (25 of each). “These minimum numbers are meant to provide trainees with exposure to a variety and spectrum of complexity of clinical case material and give supervising faculty sufficient opportunity to evaluate trainees’ competency,” the writing group says.
In addition to their procedural skills, evaluation of interventional cardiology trainee proficiency should include regular assessment of a trainee’s ability to clinically diagnose and manage patients across the broad spectrum of diseases.
Assessment of trainees should involve multiple components, including direct observation by instructors, case logs, chart reviews (including adherence to guideline recommendations, appropriate use criteria, and patient outcomes), simulation training, and assessment of leadership skills.
Trainees must also acquire experience working as part of a multidisciplinary team to provide a holistic approach to patient care. The document also highlights the importance of leadership skills, mentorship and lifelong learning beyond initial training.
The 2023 ACC/AHA/SCAI Advanced Training Statement on Interventional Cardiology (Coronary, Peripheral Vascular, and Structural Heart Interventions) was published online in the Journal of the American College of Cardiology.
The statement was developed in collaboration with and endorsed by the American Association for Thoracic Surgery, the American Society of Echocardiography, the Heart Failure Society of America, the Heart Rhythm Society, the Society of Cardiovascular Anesthesiologists, the Society of Cardiovascular Computed Tomography, the Society for Cardiovascular Magnetic Resonance, the Society of Thoracic Surgeons, and the Society for Vascular Medicine.
A version of this article first appeared on Medscape.com.
The American College of Cardiology, the American Heart Association, and the Society for Cardiovascular Angiography and Interventions (SCAI) have jointly issued new guidance outlining competency-based advanced training requirements for interventional cardiology trainees.
It’s the first document of its kind to define the training requirements for the full breadth of interventional cardiology for adults, including coronary interventions, peripheral vascular interventions (PVIs), and structural heart interventions (SHIs), the organizations say.
“With this groundbreaking document, the writing committee provides a roadmap for both program directors and interventional cardiology trainees to help them progress through important training milestones,” Theodore A. Bass, MD, chair of the statement writing committee, says in a news release.
“The document defines the required competencies for the full scope of interventional cardiology, providing trainees for the first time with the information to support training across all these areas,” Dr. Bass adds.
Minimum of 250 procedures
To gain the necessary experience in interventional cardiology, cardiovascular fellows are advised to complete the following:
- A 3-year general cardiovascular disease fellowship (successful completion consists of Level I competency in all aspects of cardiovascular medicine and Level II competency in diagnostic cardiac catheterization to pursue interventional cardiology training);
- A 1-year accredited interventional cardiology fellowship, the focus of which is coronary intervention with the opportunity to gain procedural experience in various aspects of PVI or SHI (Level III competency);
- An option for additional post-fellowship training based on the trainee’s career goals.
The goal of Level III training is to provide the interventional cardiology trainees with a “well-rounded, competency-based education,” including didactic instruction, clinical experience in the diagnosis and care of patients, and hands-on procedural experience, the writing group says.
Competency requirements are defined using the Accreditation Council for Graduate Medical Education’s six “essential” competency domains: medical knowledge; patient care and procedural skills; practice-based learning and improvement; systems-based practice; interpersonal and communication skills; and professionalism.
To support attaining these competencies, the writing committee recommends a minimum of 250 interventional cardiology procedures. Of these, 200 should be coronary procedures, with the remaining 50 specialized in coronary, PVI, or SHI, which allows the fellows to customize training on the basis of their career goals.
Adjunctive procedures related to physiologic assessment and intracoronary imaging are also required (25 of each). “These minimum numbers are meant to provide trainees with exposure to a variety and spectrum of complexity of clinical case material and give supervising faculty sufficient opportunity to evaluate trainees’ competency,” the writing group says.
In addition to their procedural skills, evaluation of interventional cardiology trainee proficiency should include regular assessment of a trainee’s ability to clinically diagnose and manage patients across the broad spectrum of diseases.
Assessment of trainees should involve multiple components, including direct observation by instructors, case logs, chart reviews (including adherence to guideline recommendations, appropriate use criteria, and patient outcomes), simulation training, and assessment of leadership skills.
Trainees must also acquire experience working as part of a multidisciplinary team to provide a holistic approach to patient care. The document also highlights the importance of leadership skills, mentorship and lifelong learning beyond initial training.
The 2023 ACC/AHA/SCAI Advanced Training Statement on Interventional Cardiology (Coronary, Peripheral Vascular, and Structural Heart Interventions) was published online in the Journal of the American College of Cardiology.
The statement was developed in collaboration with and endorsed by the American Association for Thoracic Surgery, the American Society of Echocardiography, the Heart Failure Society of America, the Heart Rhythm Society, the Society of Cardiovascular Anesthesiologists, the Society of Cardiovascular Computed Tomography, the Society for Cardiovascular Magnetic Resonance, the Society of Thoracic Surgeons, and the Society for Vascular Medicine.
A version of this article first appeared on Medscape.com.
Steak dinners, sales reps, and risky procedures: Inside the big business of clogged arteries
On June 14, 2017, just before noon, a doctor made an incision near a patient’s groin. Kari Kirk, a representative for the world’s largest medical device company, Medtronic, looked on and began texting her colleague a play-by-play.
“Fixing both legs from the ankles,” she wrote.
It was a fairly common procedure at the Robert J. Dole Veterans Affairs Medical Center in Wichita, Kansas, performed to treat blockages in the leg vessels.
Within reach were an array of Medtronic products: tubes with blades attached to shave hardened deposits off of artery walls; stents to widen blood vessels; balloons coated with therapeutic drugs.
Each time a doctor puts a foreign device in someone’s body, it carries a risk of complication, which can include clots or even require amputation. So medical experts, research and even Medtronic’s own device instructions urge doctors to use as few as are necessary.
“Just used 12 [drug-coated balloons]!!” Kirk texted her colleague.
“Does that mean I owe u $$,” he responded.
“Thats what I’m thinking!!!” she said. “And now 14 balloons?!”
“but only one stent so far??”
“So far!”
As the texting continued, her colleague replied, “U are going to want to start going to the VA all the time.”
The messages, recently unsealed in an ongoing whistleblower lawsuit, give a window into the way money and medicine mingle in the booming business of peripheral artery disease, a condition that afflicts 6.5 million Americans over age 40 and is caused when fatty plaque builds up in arteries, blocking blood flow to the legs.
Representatives from companies are often present during vascular procedures to guide doctors on how to use their complex devices. This kind of access has the potential to influence treatment plans, as companies and their representatives profit when more of their product is used.
The suit, filed in 2017 by a sales representative for a competing medical device firm, alleges an illegal kickback scheme between Medtronic and hospital employees. According to the complaint and documents released in the suit, between 2011 and 2018, VA health care workers received steakhouse dinners, Apple electronics, and NASCAR tickets, and in turn, Medtronic secured a lucrative contract with the hospital. Meanwhile, the company’s representatives allegedly “groomed and trained” physicians at the facility, who then deployed the company’s devices even when it was not medically indicated.
Independently from the whistleblower suit, internal investigators at the Wichita facility have also examined the treatment patterns of its vascular patients in recent years and found numerous cases where medical devices were used excessively. While it’s not uncommon to deploy several devices, a medical expert on the investigation team found that the VA doctors sometimes used more than 15 at a time – one used 33 – deviating from the standard of care.
“It is unconscionable – there can be no valid medically acceptable basis to cram so many devices into a human being,” wrote attorneys representing the whistleblower in legal filings from January 2023. “This is not medical treatment. This is abuse.”
Dr. Kim Hodgson, former president of the Society for Vascular Surgery and an expert retained by the plaintiff, said the findings of the internal review of patient data raise “a high level of concern regarding necessity of treatment provided,” according to case documents.
Medtronic declined to respond to ProPublica’s questions, citing the ongoing litigation. “These allegations are false and Medtronic is defending against these claims in court,” said Boua Xiong, a spokesperson for the company. Medtronic representative Kirk declined to respond to ProPublica’s request for comment.
The hospital investigation found that amputations increased sixfold in the same time frame as the procedures in question, according to internal emails, but made no conclusion about whether those two things were connected. ProPublica reached out to the VA to ask whether any patients had been harmed.
The VA is “conducting an extensive review of patient care” at the Kansas hospital, “including the number of devices used on patients – to make sure that Veterans were not harmed by any procedures,” press secretary Terrence Hayes said in a statement. So far, the VA’s investigation has found no “quality of care issues,” he said, and the investigation will continue “until every Veteran’s case has been reviewed.” (Read the full statement here.) Neither the department nor the hospital has taken formal action against the medical providers, Hayes said.
The medical group that had a contract with the VA for vascular interventions, Wichita Radiological Group, did not respond to ProPublica’s requests for comment, nor did the doctors named in the suit: Dr. Shaun Gonda, Dr. Bret Winblad, and Dr. Kermit Rust. It is unclear from the case documents which doctors conducted which procedures. Eric Barth, an attorney for the medical group, denied the allegations in recent legal filings, calling the claims “baseless” and the lawsuit a “witch hunt.”
The lawsuit comes amid growing concern about one of these procedures – atherectomies – after researchers and doctors have uncovered patterns of excessive and inappropriate use. Recent research has found that this procedure, a common but costly treatment to shave or laser plaque from blood vessels, is not more effective than cheaper alternatives and may even be associated with a higher risk of complications including amputation. In recent years, several doctors and clinics have been investigated for allegedly taking advantage of Medicare’s reimbursement rates, and one study found that many doctors are resorting to atherectomies in the earliest stages of peripheral artery disease, against best practices that urge noninvasive treatment.
“Atherectomy is important in certain settings. But it’s being used in a way that is entirely inappropriate and it’s largely driven by the incentive structure,” said Dr. Caitlin Hicks, the lead author of the study and an associate professor of surgery at Johns Hopkins University School of Medicine.
Although different payment structures govern the care of veterans, the whistleblower lawsuit alleges that outside physicians, paid hourly by the Dole VA, were motivated to conduct longer and more complex procedures that would earn them higher payment.
Under different circumstances, the patient in the procedure room on that summer day could have been done after 2 hours.
But, 150 minutes in, those Medtronic representatives were still texting. At that point, more than 15 of their vascular devices had been used, including stents, balloons, and those for atherectomy.
“Long case!” Kirk’s colleague texted. “Is it looking ok??”
“It is,” she said. “Thought we were done a few times! Now he’s going back in to cut again!”
A little while later, she texted: “....17!”
He texted back [with laughing emoticons].
Hospital leaders had been scrutinizing the use of these procedures at the Dole VA for years.
In 2017, shortly after Rick Ament was hired to lead the facility, he noticed something was amiss. While the longtime hospital administrator was poring over the finances, he was alarmed to discover that the relatively small Dole VA had one of the most expensive cardiac programs in the country. As Ament dug deeper, he realized vascular interventions were the reason.
“It just did not make sense that the acuity level of our patients would generate such extreme cost variances from the norm,” he testified in December, in a deposition for the whistleblower case. “It was so significant, we needed to get to the bottom of it.”
Ament, a second generation Air Force veteran, quietly assembled a task force to investigate why the facility had purchased so many medical devices for these procedures. After they examined inventory records, calculating the total number of medical devices and the cost of devices per patient, they grew concerned.
“We were more expensive than, I believe it was, the top 10 hospitals in the VA combined,” he said. “My feeling was that we either had very, very bad providers or we had product walking out the door.”
Ament enlisted experts from other VA hospitals to help his team investigate, including an administrative officer who could understand finances and a respected interventional radiologist who could examine records. The task force gathered a list of patients from 2016 to 2018, according to internal emails, and analyzed their medical charts.
According to internal VA documents released through the whistleblower suit, the review found a number of clinical failings: Evidence-based medicine had not been followed in the majority of cases reviewed. Procedures were over-aggressive, treating lesions that should have been left alone. And there was a total disregard for established best practices for treating peripheral artery disease.
One of the experts on the investigative team explained to Ament that while it was not uncommon for doctors to use a couple of devices in one intervention, the total number of devices in many of the procedures at his facility went into the double digits, sometimes five times the expected amount.
In one encounter, a doctor deployed 33 devices in one procedure – 3 atherectomy devices, 9 stents, and 21 balloons.
This use of devices was exorbitant, Ament came to understand. “I want to say the term ‘egregious’ was used,” he testified. “It was kind of like validation, but I really wish I was wrong.”
“Did it make you concerned for patient care?” a lawyer asked during the deposition.
“It did,” Ament replied.
A member of his task force pulled data for veterans who had leg amputations due to vascular disease. Over 5 years, the number of veterans who had amputations increased, from about 6 in 2013 to 38 in 2018, according to internal emails released in the suit. The VA did not respond to ProPublica’s questions about the rise in amputations or whether it was due to complications from the procedures.
Even though Ament testified in December 2022 that he became aware of the excessive use of devices during his investigation that began about 5 years ago, neither he nor the VA have publicly acknowledged these findings outside of the lawsuit. It is unclear whether VA representatives informed the patients whose records were reviewed about their findings. ProPublica reached out to more than half a dozen veteran community groups in the Wichita area and none were aware of the investigation nor the allegations of overuse of vascular procedures at the facility.
The VA says that if its ongoing review finds instances of substandard care, it will reach out to affected patients and inform them about possible complications and benefits they may be entitled to. The press secretary said the review will take several months. Ament declined to respond to ProPublica’s questions, citing the ongoing case.
In 2018, Ament turned over his findings to the criminal division of the VA’s Office of Inspector General. He also shut down interventional radiology procedures at the facility’s catheter lab.
Federal agents separately opened an investigation into the same unit in the facility, looking into allegations of kickbacks.
More than 40 pages of expense reports from Medtronic, revealed in the whistleblower case, show sales representatives treating Dole health care workers to hundreds of meals over several years – lunches at Dempsey’s Biscuit Co.; business meals at the Scotch & Sirloin steakhouse; dinner at Chester’s Chophouse & Wine Bar, price per attendee: $122.39.
Federal agents obtained the receipts.
“Robert J. Dole VAMC employees may have received improper gratuities, in the forms of paid lunches, dinners, etc., from sales representatives from Medtronic,” Nathen Howard, a special agent in the VA OIG, wrote in an investigation memo from February 2019.
This kind of relationship could violate VA policy, which forbids federal employees from receiving any gifts, including meals, from people who do business or seek to do business with a federal institution. For health care workers, violating this policy could have serious implications for patients. Numerous studies have shown that even modest industry-sponsored gifts, including meals, may influence prescribing or treatment behavior of health care professionals.
The agents opened their investigation into kickbacks at the Wichita facility in response to the whistleblower lawsuit, which was filed by Thomas Schroeder in 2017. The VA OIG would not confirm or deny whether it was continuing to investigate kickbacks at the facility. The VA did not directly answer ProPublica’s questions about kickbacks at the Dole VA, but it said that every employee must complete an annual ethics training, which covers gift rules.
In recent years, Medtronic has settled a handful of other cases that have alleged kickbacks between company representatives and health care professionals.
In 2018, Medtronic’s subsidiary Covidien paid $13 million to settle claims with the U.S. Department of Justice that it paid kickbacks to health care institutions that used its mechanical blood clot devices. In 2019, the same subsidiary paid $17 million to resolve allegations that it provided in-kind marketing support to doctors using its vein products. And in 2020, Medtronic paid more than $8 million to settle claims that representatives had paid kickbacks to a neurosurgeon, including scores of lavish meals at a restaurant that the doctor owned, to induce him to purchase the company’s medication pumps.
Schroeder’s lawsuit is not the first time Medtronic’s vascular devices were named in an alleged kickback scheme. In early 2015, Medtronic acquired Covidien, and shortly after the merger, its subsidiary ev3 Inc. agreed to pay $1.25 million to resolve allegations that it had paid doctors who were “high volume users” of its atherectomy devices to act as evangelists for the company, and had provided physicians with company shares to participate in clinical trials for their tools.
The whistleblower in this earlier case, a former sales representative for the company, also alleged that the subsidiary was gaming Medicare’s payment system. Hospitals were often hesitant to conduct atherectomy procedures because of the low reimbursement rates. According to the suit, sales representatives encouraged doctors to admit patients for longer stays to reap greater reimbursements and make a profit, even though such stays were often not medically indicated.
“Medical device makers that try to boost their profits by causing patients to be admitted for unnecessary and expensive inpatient hospital stays will be held accountable,” special agent Thomas O’Donnell, from the Office of Inspector General at the U.S. Department of Health and Human Services, said in a press release for the settlement. “Both patients and taxpayers deserve to have medical decisions made based on what is medically appropriate.”
Medtronic spokesperson Xiong said that in each case, the company “cooperated fully with the DOJ to resolve its concerns and, where wrongdoing was found, took appropriate remedial action.”
Seton Hall Law School professor Jacob Elberg, a former assistant U.S. attorney for the District of New Jersey who led its health care and government fraud unit, is concerned by the frequency of such settlements in the last 2 decades. “There are, at this point, real questions as to whether the sanctions imposed by DOJ are sufficient to deter wrongdoing and to lead to meaningful change, especially within the medical device industry.”
Although the Department of Justice has declined to intervene in the lawsuit involving the Dole VA at this time, the case is ongoing and further depositions with Medtronic sales representatives and a former VA employee are scheduled for this month.
VA employees and doctors named in the suit declined to comment or did not respond to ProPublica’s questions about the alleged kickbacks and whether sales representatives may have influenced veterans’ treatment plans. In interviews with federal investigators, according to released transcripts, several of the employees who were questioned denied receiving frequent meals from sales representatives, contradicting Medtronic’s expense reports.
Their statements also stand in contrast to Medtronic representative Kari Kirk’s final text messages during that procedure in June 2017, which ultimately lasted more than 3 hours.
“Now u done??” her colleague asked.
“Just finished,” she texted. “Running to get them lunch!”
This story was originally published on ProPublica. ProPublica is a nonprofit newsroom that investigates abuses of power. Sign up to receive their biggest stories as soon as they’re published.
On June 14, 2017, just before noon, a doctor made an incision near a patient’s groin. Kari Kirk, a representative for the world’s largest medical device company, Medtronic, looked on and began texting her colleague a play-by-play.
“Fixing both legs from the ankles,” she wrote.
It was a fairly common procedure at the Robert J. Dole Veterans Affairs Medical Center in Wichita, Kansas, performed to treat blockages in the leg vessels.
Within reach were an array of Medtronic products: tubes with blades attached to shave hardened deposits off of artery walls; stents to widen blood vessels; balloons coated with therapeutic drugs.
Each time a doctor puts a foreign device in someone’s body, it carries a risk of complication, which can include clots or even require amputation. So medical experts, research and even Medtronic’s own device instructions urge doctors to use as few as are necessary.
“Just used 12 [drug-coated balloons]!!” Kirk texted her colleague.
“Does that mean I owe u $$,” he responded.
“Thats what I’m thinking!!!” she said. “And now 14 balloons?!”
“but only one stent so far??”
“So far!”
As the texting continued, her colleague replied, “U are going to want to start going to the VA all the time.”
The messages, recently unsealed in an ongoing whistleblower lawsuit, give a window into the way money and medicine mingle in the booming business of peripheral artery disease, a condition that afflicts 6.5 million Americans over age 40 and is caused when fatty plaque builds up in arteries, blocking blood flow to the legs.
Representatives from companies are often present during vascular procedures to guide doctors on how to use their complex devices. This kind of access has the potential to influence treatment plans, as companies and their representatives profit when more of their product is used.
The suit, filed in 2017 by a sales representative for a competing medical device firm, alleges an illegal kickback scheme between Medtronic and hospital employees. According to the complaint and documents released in the suit, between 2011 and 2018, VA health care workers received steakhouse dinners, Apple electronics, and NASCAR tickets, and in turn, Medtronic secured a lucrative contract with the hospital. Meanwhile, the company’s representatives allegedly “groomed and trained” physicians at the facility, who then deployed the company’s devices even when it was not medically indicated.
Independently from the whistleblower suit, internal investigators at the Wichita facility have also examined the treatment patterns of its vascular patients in recent years and found numerous cases where medical devices were used excessively. While it’s not uncommon to deploy several devices, a medical expert on the investigation team found that the VA doctors sometimes used more than 15 at a time – one used 33 – deviating from the standard of care.
“It is unconscionable – there can be no valid medically acceptable basis to cram so many devices into a human being,” wrote attorneys representing the whistleblower in legal filings from January 2023. “This is not medical treatment. This is abuse.”
Dr. Kim Hodgson, former president of the Society for Vascular Surgery and an expert retained by the plaintiff, said the findings of the internal review of patient data raise “a high level of concern regarding necessity of treatment provided,” according to case documents.
Medtronic declined to respond to ProPublica’s questions, citing the ongoing litigation. “These allegations are false and Medtronic is defending against these claims in court,” said Boua Xiong, a spokesperson for the company. Medtronic representative Kirk declined to respond to ProPublica’s request for comment.
The hospital investigation found that amputations increased sixfold in the same time frame as the procedures in question, according to internal emails, but made no conclusion about whether those two things were connected. ProPublica reached out to the VA to ask whether any patients had been harmed.
The VA is “conducting an extensive review of patient care” at the Kansas hospital, “including the number of devices used on patients – to make sure that Veterans were not harmed by any procedures,” press secretary Terrence Hayes said in a statement. So far, the VA’s investigation has found no “quality of care issues,” he said, and the investigation will continue “until every Veteran’s case has been reviewed.” (Read the full statement here.) Neither the department nor the hospital has taken formal action against the medical providers, Hayes said.
The medical group that had a contract with the VA for vascular interventions, Wichita Radiological Group, did not respond to ProPublica’s requests for comment, nor did the doctors named in the suit: Dr. Shaun Gonda, Dr. Bret Winblad, and Dr. Kermit Rust. It is unclear from the case documents which doctors conducted which procedures. Eric Barth, an attorney for the medical group, denied the allegations in recent legal filings, calling the claims “baseless” and the lawsuit a “witch hunt.”
The lawsuit comes amid growing concern about one of these procedures – atherectomies – after researchers and doctors have uncovered patterns of excessive and inappropriate use. Recent research has found that this procedure, a common but costly treatment to shave or laser plaque from blood vessels, is not more effective than cheaper alternatives and may even be associated with a higher risk of complications including amputation. In recent years, several doctors and clinics have been investigated for allegedly taking advantage of Medicare’s reimbursement rates, and one study found that many doctors are resorting to atherectomies in the earliest stages of peripheral artery disease, against best practices that urge noninvasive treatment.
“Atherectomy is important in certain settings. But it’s being used in a way that is entirely inappropriate and it’s largely driven by the incentive structure,” said Dr. Caitlin Hicks, the lead author of the study and an associate professor of surgery at Johns Hopkins University School of Medicine.
Although different payment structures govern the care of veterans, the whistleblower lawsuit alleges that outside physicians, paid hourly by the Dole VA, were motivated to conduct longer and more complex procedures that would earn them higher payment.
Under different circumstances, the patient in the procedure room on that summer day could have been done after 2 hours.
But, 150 minutes in, those Medtronic representatives were still texting. At that point, more than 15 of their vascular devices had been used, including stents, balloons, and those for atherectomy.
“Long case!” Kirk’s colleague texted. “Is it looking ok??”
“It is,” she said. “Thought we were done a few times! Now he’s going back in to cut again!”
A little while later, she texted: “....17!”
He texted back [with laughing emoticons].
Hospital leaders had been scrutinizing the use of these procedures at the Dole VA for years.
In 2017, shortly after Rick Ament was hired to lead the facility, he noticed something was amiss. While the longtime hospital administrator was poring over the finances, he was alarmed to discover that the relatively small Dole VA had one of the most expensive cardiac programs in the country. As Ament dug deeper, he realized vascular interventions were the reason.
“It just did not make sense that the acuity level of our patients would generate such extreme cost variances from the norm,” he testified in December, in a deposition for the whistleblower case. “It was so significant, we needed to get to the bottom of it.”
Ament, a second generation Air Force veteran, quietly assembled a task force to investigate why the facility had purchased so many medical devices for these procedures. After they examined inventory records, calculating the total number of medical devices and the cost of devices per patient, they grew concerned.
“We were more expensive than, I believe it was, the top 10 hospitals in the VA combined,” he said. “My feeling was that we either had very, very bad providers or we had product walking out the door.”
Ament enlisted experts from other VA hospitals to help his team investigate, including an administrative officer who could understand finances and a respected interventional radiologist who could examine records. The task force gathered a list of patients from 2016 to 2018, according to internal emails, and analyzed their medical charts.
According to internal VA documents released through the whistleblower suit, the review found a number of clinical failings: Evidence-based medicine had not been followed in the majority of cases reviewed. Procedures were over-aggressive, treating lesions that should have been left alone. And there was a total disregard for established best practices for treating peripheral artery disease.
One of the experts on the investigative team explained to Ament that while it was not uncommon for doctors to use a couple of devices in one intervention, the total number of devices in many of the procedures at his facility went into the double digits, sometimes five times the expected amount.
In one encounter, a doctor deployed 33 devices in one procedure – 3 atherectomy devices, 9 stents, and 21 balloons.
This use of devices was exorbitant, Ament came to understand. “I want to say the term ‘egregious’ was used,” he testified. “It was kind of like validation, but I really wish I was wrong.”
“Did it make you concerned for patient care?” a lawyer asked during the deposition.
“It did,” Ament replied.
A member of his task force pulled data for veterans who had leg amputations due to vascular disease. Over 5 years, the number of veterans who had amputations increased, from about 6 in 2013 to 38 in 2018, according to internal emails released in the suit. The VA did not respond to ProPublica’s questions about the rise in amputations or whether it was due to complications from the procedures.
Even though Ament testified in December 2022 that he became aware of the excessive use of devices during his investigation that began about 5 years ago, neither he nor the VA have publicly acknowledged these findings outside of the lawsuit. It is unclear whether VA representatives informed the patients whose records were reviewed about their findings. ProPublica reached out to more than half a dozen veteran community groups in the Wichita area and none were aware of the investigation nor the allegations of overuse of vascular procedures at the facility.
The VA says that if its ongoing review finds instances of substandard care, it will reach out to affected patients and inform them about possible complications and benefits they may be entitled to. The press secretary said the review will take several months. Ament declined to respond to ProPublica’s questions, citing the ongoing case.
In 2018, Ament turned over his findings to the criminal division of the VA’s Office of Inspector General. He also shut down interventional radiology procedures at the facility’s catheter lab.
Federal agents separately opened an investigation into the same unit in the facility, looking into allegations of kickbacks.
More than 40 pages of expense reports from Medtronic, revealed in the whistleblower case, show sales representatives treating Dole health care workers to hundreds of meals over several years – lunches at Dempsey’s Biscuit Co.; business meals at the Scotch & Sirloin steakhouse; dinner at Chester’s Chophouse & Wine Bar, price per attendee: $122.39.
Federal agents obtained the receipts.
“Robert J. Dole VAMC employees may have received improper gratuities, in the forms of paid lunches, dinners, etc., from sales representatives from Medtronic,” Nathen Howard, a special agent in the VA OIG, wrote in an investigation memo from February 2019.
This kind of relationship could violate VA policy, which forbids federal employees from receiving any gifts, including meals, from people who do business or seek to do business with a federal institution. For health care workers, violating this policy could have serious implications for patients. Numerous studies have shown that even modest industry-sponsored gifts, including meals, may influence prescribing or treatment behavior of health care professionals.
The agents opened their investigation into kickbacks at the Wichita facility in response to the whistleblower lawsuit, which was filed by Thomas Schroeder in 2017. The VA OIG would not confirm or deny whether it was continuing to investigate kickbacks at the facility. The VA did not directly answer ProPublica’s questions about kickbacks at the Dole VA, but it said that every employee must complete an annual ethics training, which covers gift rules.
In recent years, Medtronic has settled a handful of other cases that have alleged kickbacks between company representatives and health care professionals.
In 2018, Medtronic’s subsidiary Covidien paid $13 million to settle claims with the U.S. Department of Justice that it paid kickbacks to health care institutions that used its mechanical blood clot devices. In 2019, the same subsidiary paid $17 million to resolve allegations that it provided in-kind marketing support to doctors using its vein products. And in 2020, Medtronic paid more than $8 million to settle claims that representatives had paid kickbacks to a neurosurgeon, including scores of lavish meals at a restaurant that the doctor owned, to induce him to purchase the company’s medication pumps.
Schroeder’s lawsuit is not the first time Medtronic’s vascular devices were named in an alleged kickback scheme. In early 2015, Medtronic acquired Covidien, and shortly after the merger, its subsidiary ev3 Inc. agreed to pay $1.25 million to resolve allegations that it had paid doctors who were “high volume users” of its atherectomy devices to act as evangelists for the company, and had provided physicians with company shares to participate in clinical trials for their tools.
The whistleblower in this earlier case, a former sales representative for the company, also alleged that the subsidiary was gaming Medicare’s payment system. Hospitals were often hesitant to conduct atherectomy procedures because of the low reimbursement rates. According to the suit, sales representatives encouraged doctors to admit patients for longer stays to reap greater reimbursements and make a profit, even though such stays were often not medically indicated.
“Medical device makers that try to boost their profits by causing patients to be admitted for unnecessary and expensive inpatient hospital stays will be held accountable,” special agent Thomas O’Donnell, from the Office of Inspector General at the U.S. Department of Health and Human Services, said in a press release for the settlement. “Both patients and taxpayers deserve to have medical decisions made based on what is medically appropriate.”
Medtronic spokesperson Xiong said that in each case, the company “cooperated fully with the DOJ to resolve its concerns and, where wrongdoing was found, took appropriate remedial action.”
Seton Hall Law School professor Jacob Elberg, a former assistant U.S. attorney for the District of New Jersey who led its health care and government fraud unit, is concerned by the frequency of such settlements in the last 2 decades. “There are, at this point, real questions as to whether the sanctions imposed by DOJ are sufficient to deter wrongdoing and to lead to meaningful change, especially within the medical device industry.”
Although the Department of Justice has declined to intervene in the lawsuit involving the Dole VA at this time, the case is ongoing and further depositions with Medtronic sales representatives and a former VA employee are scheduled for this month.
VA employees and doctors named in the suit declined to comment or did not respond to ProPublica’s questions about the alleged kickbacks and whether sales representatives may have influenced veterans’ treatment plans. In interviews with federal investigators, according to released transcripts, several of the employees who were questioned denied receiving frequent meals from sales representatives, contradicting Medtronic’s expense reports.
Their statements also stand in contrast to Medtronic representative Kari Kirk’s final text messages during that procedure in June 2017, which ultimately lasted more than 3 hours.
“Now u done??” her colleague asked.
“Just finished,” she texted. “Running to get them lunch!”
This story was originally published on ProPublica. ProPublica is a nonprofit newsroom that investigates abuses of power. Sign up to receive their biggest stories as soon as they’re published.
On June 14, 2017, just before noon, a doctor made an incision near a patient’s groin. Kari Kirk, a representative for the world’s largest medical device company, Medtronic, looked on and began texting her colleague a play-by-play.
“Fixing both legs from the ankles,” she wrote.
It was a fairly common procedure at the Robert J. Dole Veterans Affairs Medical Center in Wichita, Kansas, performed to treat blockages in the leg vessels.
Within reach were an array of Medtronic products: tubes with blades attached to shave hardened deposits off of artery walls; stents to widen blood vessels; balloons coated with therapeutic drugs.
Each time a doctor puts a foreign device in someone’s body, it carries a risk of complication, which can include clots or even require amputation. So medical experts, research and even Medtronic’s own device instructions urge doctors to use as few as are necessary.
“Just used 12 [drug-coated balloons]!!” Kirk texted her colleague.
“Does that mean I owe u $$,” he responded.
“Thats what I’m thinking!!!” she said. “And now 14 balloons?!”
“but only one stent so far??”
“So far!”
As the texting continued, her colleague replied, “U are going to want to start going to the VA all the time.”
The messages, recently unsealed in an ongoing whistleblower lawsuit, give a window into the way money and medicine mingle in the booming business of peripheral artery disease, a condition that afflicts 6.5 million Americans over age 40 and is caused when fatty plaque builds up in arteries, blocking blood flow to the legs.
Representatives from companies are often present during vascular procedures to guide doctors on how to use their complex devices. This kind of access has the potential to influence treatment plans, as companies and their representatives profit when more of their product is used.
The suit, filed in 2017 by a sales representative for a competing medical device firm, alleges an illegal kickback scheme between Medtronic and hospital employees. According to the complaint and documents released in the suit, between 2011 and 2018, VA health care workers received steakhouse dinners, Apple electronics, and NASCAR tickets, and in turn, Medtronic secured a lucrative contract with the hospital. Meanwhile, the company’s representatives allegedly “groomed and trained” physicians at the facility, who then deployed the company’s devices even when it was not medically indicated.
Independently from the whistleblower suit, internal investigators at the Wichita facility have also examined the treatment patterns of its vascular patients in recent years and found numerous cases where medical devices were used excessively. While it’s not uncommon to deploy several devices, a medical expert on the investigation team found that the VA doctors sometimes used more than 15 at a time – one used 33 – deviating from the standard of care.
“It is unconscionable – there can be no valid medically acceptable basis to cram so many devices into a human being,” wrote attorneys representing the whistleblower in legal filings from January 2023. “This is not medical treatment. This is abuse.”
Dr. Kim Hodgson, former president of the Society for Vascular Surgery and an expert retained by the plaintiff, said the findings of the internal review of patient data raise “a high level of concern regarding necessity of treatment provided,” according to case documents.
Medtronic declined to respond to ProPublica’s questions, citing the ongoing litigation. “These allegations are false and Medtronic is defending against these claims in court,” said Boua Xiong, a spokesperson for the company. Medtronic representative Kirk declined to respond to ProPublica’s request for comment.
The hospital investigation found that amputations increased sixfold in the same time frame as the procedures in question, according to internal emails, but made no conclusion about whether those two things were connected. ProPublica reached out to the VA to ask whether any patients had been harmed.
The VA is “conducting an extensive review of patient care” at the Kansas hospital, “including the number of devices used on patients – to make sure that Veterans were not harmed by any procedures,” press secretary Terrence Hayes said in a statement. So far, the VA’s investigation has found no “quality of care issues,” he said, and the investigation will continue “until every Veteran’s case has been reviewed.” (Read the full statement here.) Neither the department nor the hospital has taken formal action against the medical providers, Hayes said.
The medical group that had a contract with the VA for vascular interventions, Wichita Radiological Group, did not respond to ProPublica’s requests for comment, nor did the doctors named in the suit: Dr. Shaun Gonda, Dr. Bret Winblad, and Dr. Kermit Rust. It is unclear from the case documents which doctors conducted which procedures. Eric Barth, an attorney for the medical group, denied the allegations in recent legal filings, calling the claims “baseless” and the lawsuit a “witch hunt.”
The lawsuit comes amid growing concern about one of these procedures – atherectomies – after researchers and doctors have uncovered patterns of excessive and inappropriate use. Recent research has found that this procedure, a common but costly treatment to shave or laser plaque from blood vessels, is not more effective than cheaper alternatives and may even be associated with a higher risk of complications including amputation. In recent years, several doctors and clinics have been investigated for allegedly taking advantage of Medicare’s reimbursement rates, and one study found that many doctors are resorting to atherectomies in the earliest stages of peripheral artery disease, against best practices that urge noninvasive treatment.
“Atherectomy is important in certain settings. But it’s being used in a way that is entirely inappropriate and it’s largely driven by the incentive structure,” said Dr. Caitlin Hicks, the lead author of the study and an associate professor of surgery at Johns Hopkins University School of Medicine.
Although different payment structures govern the care of veterans, the whistleblower lawsuit alleges that outside physicians, paid hourly by the Dole VA, were motivated to conduct longer and more complex procedures that would earn them higher payment.
Under different circumstances, the patient in the procedure room on that summer day could have been done after 2 hours.
But, 150 minutes in, those Medtronic representatives were still texting. At that point, more than 15 of their vascular devices had been used, including stents, balloons, and those for atherectomy.
“Long case!” Kirk’s colleague texted. “Is it looking ok??”
“It is,” she said. “Thought we were done a few times! Now he’s going back in to cut again!”
A little while later, she texted: “....17!”
He texted back [with laughing emoticons].
Hospital leaders had been scrutinizing the use of these procedures at the Dole VA for years.
In 2017, shortly after Rick Ament was hired to lead the facility, he noticed something was amiss. While the longtime hospital administrator was poring over the finances, he was alarmed to discover that the relatively small Dole VA had one of the most expensive cardiac programs in the country. As Ament dug deeper, he realized vascular interventions were the reason.
“It just did not make sense that the acuity level of our patients would generate such extreme cost variances from the norm,” he testified in December, in a deposition for the whistleblower case. “It was so significant, we needed to get to the bottom of it.”
Ament, a second generation Air Force veteran, quietly assembled a task force to investigate why the facility had purchased so many medical devices for these procedures. After they examined inventory records, calculating the total number of medical devices and the cost of devices per patient, they grew concerned.
“We were more expensive than, I believe it was, the top 10 hospitals in the VA combined,” he said. “My feeling was that we either had very, very bad providers or we had product walking out the door.”
Ament enlisted experts from other VA hospitals to help his team investigate, including an administrative officer who could understand finances and a respected interventional radiologist who could examine records. The task force gathered a list of patients from 2016 to 2018, according to internal emails, and analyzed their medical charts.
According to internal VA documents released through the whistleblower suit, the review found a number of clinical failings: Evidence-based medicine had not been followed in the majority of cases reviewed. Procedures were over-aggressive, treating lesions that should have been left alone. And there was a total disregard for established best practices for treating peripheral artery disease.
One of the experts on the investigative team explained to Ament that while it was not uncommon for doctors to use a couple of devices in one intervention, the total number of devices in many of the procedures at his facility went into the double digits, sometimes five times the expected amount.
In one encounter, a doctor deployed 33 devices in one procedure – 3 atherectomy devices, 9 stents, and 21 balloons.
This use of devices was exorbitant, Ament came to understand. “I want to say the term ‘egregious’ was used,” he testified. “It was kind of like validation, but I really wish I was wrong.”
“Did it make you concerned for patient care?” a lawyer asked during the deposition.
“It did,” Ament replied.
A member of his task force pulled data for veterans who had leg amputations due to vascular disease. Over 5 years, the number of veterans who had amputations increased, from about 6 in 2013 to 38 in 2018, according to internal emails released in the suit. The VA did not respond to ProPublica’s questions about the rise in amputations or whether it was due to complications from the procedures.
Even though Ament testified in December 2022 that he became aware of the excessive use of devices during his investigation that began about 5 years ago, neither he nor the VA have publicly acknowledged these findings outside of the lawsuit. It is unclear whether VA representatives informed the patients whose records were reviewed about their findings. ProPublica reached out to more than half a dozen veteran community groups in the Wichita area and none were aware of the investigation nor the allegations of overuse of vascular procedures at the facility.
The VA says that if its ongoing review finds instances of substandard care, it will reach out to affected patients and inform them about possible complications and benefits they may be entitled to. The press secretary said the review will take several months. Ament declined to respond to ProPublica’s questions, citing the ongoing case.
In 2018, Ament turned over his findings to the criminal division of the VA’s Office of Inspector General. He also shut down interventional radiology procedures at the facility’s catheter lab.
Federal agents separately opened an investigation into the same unit in the facility, looking into allegations of kickbacks.
More than 40 pages of expense reports from Medtronic, revealed in the whistleblower case, show sales representatives treating Dole health care workers to hundreds of meals over several years – lunches at Dempsey’s Biscuit Co.; business meals at the Scotch & Sirloin steakhouse; dinner at Chester’s Chophouse & Wine Bar, price per attendee: $122.39.
Federal agents obtained the receipts.
“Robert J. Dole VAMC employees may have received improper gratuities, in the forms of paid lunches, dinners, etc., from sales representatives from Medtronic,” Nathen Howard, a special agent in the VA OIG, wrote in an investigation memo from February 2019.
This kind of relationship could violate VA policy, which forbids federal employees from receiving any gifts, including meals, from people who do business or seek to do business with a federal institution. For health care workers, violating this policy could have serious implications for patients. Numerous studies have shown that even modest industry-sponsored gifts, including meals, may influence prescribing or treatment behavior of health care professionals.
The agents opened their investigation into kickbacks at the Wichita facility in response to the whistleblower lawsuit, which was filed by Thomas Schroeder in 2017. The VA OIG would not confirm or deny whether it was continuing to investigate kickbacks at the facility. The VA did not directly answer ProPublica’s questions about kickbacks at the Dole VA, but it said that every employee must complete an annual ethics training, which covers gift rules.
In recent years, Medtronic has settled a handful of other cases that have alleged kickbacks between company representatives and health care professionals.
In 2018, Medtronic’s subsidiary Covidien paid $13 million to settle claims with the U.S. Department of Justice that it paid kickbacks to health care institutions that used its mechanical blood clot devices. In 2019, the same subsidiary paid $17 million to resolve allegations that it provided in-kind marketing support to doctors using its vein products. And in 2020, Medtronic paid more than $8 million to settle claims that representatives had paid kickbacks to a neurosurgeon, including scores of lavish meals at a restaurant that the doctor owned, to induce him to purchase the company’s medication pumps.
Schroeder’s lawsuit is not the first time Medtronic’s vascular devices were named in an alleged kickback scheme. In early 2015, Medtronic acquired Covidien, and shortly after the merger, its subsidiary ev3 Inc. agreed to pay $1.25 million to resolve allegations that it had paid doctors who were “high volume users” of its atherectomy devices to act as evangelists for the company, and had provided physicians with company shares to participate in clinical trials for their tools.
The whistleblower in this earlier case, a former sales representative for the company, also alleged that the subsidiary was gaming Medicare’s payment system. Hospitals were often hesitant to conduct atherectomy procedures because of the low reimbursement rates. According to the suit, sales representatives encouraged doctors to admit patients for longer stays to reap greater reimbursements and make a profit, even though such stays were often not medically indicated.
“Medical device makers that try to boost their profits by causing patients to be admitted for unnecessary and expensive inpatient hospital stays will be held accountable,” special agent Thomas O’Donnell, from the Office of Inspector General at the U.S. Department of Health and Human Services, said in a press release for the settlement. “Both patients and taxpayers deserve to have medical decisions made based on what is medically appropriate.”
Medtronic spokesperson Xiong said that in each case, the company “cooperated fully with the DOJ to resolve its concerns and, where wrongdoing was found, took appropriate remedial action.”
Seton Hall Law School professor Jacob Elberg, a former assistant U.S. attorney for the District of New Jersey who led its health care and government fraud unit, is concerned by the frequency of such settlements in the last 2 decades. “There are, at this point, real questions as to whether the sanctions imposed by DOJ are sufficient to deter wrongdoing and to lead to meaningful change, especially within the medical device industry.”
Although the Department of Justice has declined to intervene in the lawsuit involving the Dole VA at this time, the case is ongoing and further depositions with Medtronic sales representatives and a former VA employee are scheduled for this month.
VA employees and doctors named in the suit declined to comment or did not respond to ProPublica’s questions about the alleged kickbacks and whether sales representatives may have influenced veterans’ treatment plans. In interviews with federal investigators, according to released transcripts, several of the employees who were questioned denied receiving frequent meals from sales representatives, contradicting Medtronic’s expense reports.
Their statements also stand in contrast to Medtronic representative Kari Kirk’s final text messages during that procedure in June 2017, which ultimately lasted more than 3 hours.
“Now u done??” her colleague asked.
“Just finished,” she texted. “Running to get them lunch!”
This story was originally published on ProPublica. ProPublica is a nonprofit newsroom that investigates abuses of power. Sign up to receive their biggest stories as soon as they’re published.