User login
Novel Gene Risk Score Predicts Outcomes After RYGB Surgery
SAN DIEGO –
The findings suggested that the MyPhenome test (Phenomix Sciences) can help clinicians identify the patients most likely to benefit from bariatric procedures and at a greater risk for long-term weight regain after surgery.
“Patients with both a high genetic risk score and rare mutations in the leptin-melanocortin pathway (LMP) had significantly worse outcomes, maintaining only 4.9% total body weight loss [TBWL] over 15 years compared to up to 24.8% in other genetic groups,” Phenomix Sciences Co-founder Andres Acosta, MD, PhD, told GI & Hepatology News.
The study included details on the score’s development and predictive capability. It was presented at Digestive Disease Week® (DDW) 2025
‘More Precise Bariatric Care’
The researchers recently developed a machine learning-assisted gene risk score for calories to satiation (CTSGRS), which mainly involves genes in the LMP. To assess the role of the score with or without LMP gene variants on weight loss and weight recurrence after RYGB, they identified 707 patients with a history of bariatric procedures from the Mayo Clinic Biobank. Patients with duodenal switch, revisional procedures, or who used antiobesity medications or became pregnant during follow-up were excluded.
To make predictions for 442 of the patients, the team first collected anthropometric data up to 15 years after RYGB. Then they used a two-step approach: Assessing for monogenic variants in the LMP and defining participants as carriers (LMP+) or noncarriers (LMP-). Then they defined the gene risk score (CTSGRS+ or CTSGRS-).
The result was four groups: LMP+/CTSGRS+, LMP+/CTSGRS-, LMP-/CTSGRS+, and LMP-/CTSGRS-. Multiple regression analysis was used to analyze TBWL percentage (TBWL%) between the groups at different timepoints, adjusting for baseline weight, age, and gender.
At the 10-year follow-up, the LMP+/CTSGRS+ group demonstrated a significantly higher weight recurrence (regain) of TBW% compared to the other groups.
At 15 years post-RYGB, the mean TBWL% for LMP+/CTSGRS+ was -4.9 vs -20.3 for LMP+/CTSGRS-, -18.0 for LMP-/CTSGRS+, and -24.8 for LMP-/CTSGRS-.
Further analyses showed that the LMP+/CTSGRS+ group had significantly less weight loss than LMP+/CTSGRS- and LMP-/CTSGRS- groups.
Based on the findings, the authors wrote, “Genotyping patients could improve the implementation of individualized weight-loss interventions, enhance weight-loss outcomes, and/or may explain one of the etiological factors associated with weight recurrence after RYGB.”
Acosta noted, “We’re actively expanding our research to include more diverse populations by age, sex, and race. This includes ongoing analysis to understand whether certain demographic or physiological characteristics affect how the test performs, particularly in the context of bariatric surgery.”
The team also is investigating the benefits of phenotyping for obesity comorbidities such as heart disease and diabetes, he said, and exploring whether early interventions in high-risk patients can prevent long-term weight regain and improve outcomes.
In addition, Acosta said, the team recently launched “the first prospective, placebo-controlled clinical trial using the MyPhenome test to predict response to semaglutide.” That study is based on earlier findings showing that patients identified with a Hungry Gut phenotype lost nearly twice as much weight on semaglutide compared with those who tested negative.
Overall, he concluded, “These findings open the door to more precise bariatric care. When we understand a patient’s biological drivers of obesity, we can make better decisions about the right procedure, follow-up, and long-term support. This moves us away from a one-size-fits-all model to care rooted in each patient’s unique biology.”
Potentially Paradigm-Shifting
Onur Kutlu, MD, associate professor of surgery and director of the Metabolic Surgery and Metabolic Health Program at the Miller School of Medicine, University of Miami, in Miami, Florida, commented on the study for GI & Hepatology News. “By integrating polygenic risk scores into predictive models, the authors offer an innovative method for identifying patients at elevated risk for weight regain following RYGB.”
“Their findings support the hypothesis that genetic predisposition — particularly involving energy homeostasis pathways — may underlie differential postoperative trajectories,” he said. “This approach has the potential to shift the paradigm from reactive to proactive management of weight recurrence.”
Because current options for treat weight regain are “suboptimal,” he said, “prevention becomes paramount. Preoperative identification of high-risk individuals could inform surgical decision-making, enable earlier interventions, and facilitate personalized postoperative monitoring and support.”
“If validated in larger, prospective cohorts, genetic risk stratification could enhance the precision of bariatric care and improve long-term outcomes,” he added. “Future studies should aim to validate these genetic models across diverse populations and explore how integration of behavioral, psychological, and genetic data may further refine patient selection and care pathways.”
The study was funded by Mayo Clinic and Phenomix Sciences. Gila Therapeutics and Phenomix Sciences licensed Acosta’s research technologies from the University of Florida and Mayo Clinic. Acosta declared receiving consultant fees in the past 5 years from Rhythm Pharmaceuticals, Gila Therapeutics, Amgen, General Mills, BI, Currax, Nestle, Phenomix Sciences, Bausch Health, and RareDiseases, as well as funding support from the National Institutes of Health, Vivus Pharmaceuticals, Novo Nordisk, Apollo Endosurgery, Satiogen Pharmaceuticals, Spatz Medical, and Rhythm Pharmaceuticals. Kutlu declared having no conflicts of interest.
A version of this article appeared on Medscape.com.
SAN DIEGO –
The findings suggested that the MyPhenome test (Phenomix Sciences) can help clinicians identify the patients most likely to benefit from bariatric procedures and at a greater risk for long-term weight regain after surgery.
“Patients with both a high genetic risk score and rare mutations in the leptin-melanocortin pathway (LMP) had significantly worse outcomes, maintaining only 4.9% total body weight loss [TBWL] over 15 years compared to up to 24.8% in other genetic groups,” Phenomix Sciences Co-founder Andres Acosta, MD, PhD, told GI & Hepatology News.
The study included details on the score’s development and predictive capability. It was presented at Digestive Disease Week® (DDW) 2025
‘More Precise Bariatric Care’
The researchers recently developed a machine learning-assisted gene risk score for calories to satiation (CTSGRS), which mainly involves genes in the LMP. To assess the role of the score with or without LMP gene variants on weight loss and weight recurrence after RYGB, they identified 707 patients with a history of bariatric procedures from the Mayo Clinic Biobank. Patients with duodenal switch, revisional procedures, or who used antiobesity medications or became pregnant during follow-up were excluded.
To make predictions for 442 of the patients, the team first collected anthropometric data up to 15 years after RYGB. Then they used a two-step approach: Assessing for monogenic variants in the LMP and defining participants as carriers (LMP+) or noncarriers (LMP-). Then they defined the gene risk score (CTSGRS+ or CTSGRS-).
The result was four groups: LMP+/CTSGRS+, LMP+/CTSGRS-, LMP-/CTSGRS+, and LMP-/CTSGRS-. Multiple regression analysis was used to analyze TBWL percentage (TBWL%) between the groups at different timepoints, adjusting for baseline weight, age, and gender.
At the 10-year follow-up, the LMP+/CTSGRS+ group demonstrated a significantly higher weight recurrence (regain) of TBW% compared to the other groups.
At 15 years post-RYGB, the mean TBWL% for LMP+/CTSGRS+ was -4.9 vs -20.3 for LMP+/CTSGRS-, -18.0 for LMP-/CTSGRS+, and -24.8 for LMP-/CTSGRS-.
Further analyses showed that the LMP+/CTSGRS+ group had significantly less weight loss than LMP+/CTSGRS- and LMP-/CTSGRS- groups.
Based on the findings, the authors wrote, “Genotyping patients could improve the implementation of individualized weight-loss interventions, enhance weight-loss outcomes, and/or may explain one of the etiological factors associated with weight recurrence after RYGB.”
Acosta noted, “We’re actively expanding our research to include more diverse populations by age, sex, and race. This includes ongoing analysis to understand whether certain demographic or physiological characteristics affect how the test performs, particularly in the context of bariatric surgery.”
The team also is investigating the benefits of phenotyping for obesity comorbidities such as heart disease and diabetes, he said, and exploring whether early interventions in high-risk patients can prevent long-term weight regain and improve outcomes.
In addition, Acosta said, the team recently launched “the first prospective, placebo-controlled clinical trial using the MyPhenome test to predict response to semaglutide.” That study is based on earlier findings showing that patients identified with a Hungry Gut phenotype lost nearly twice as much weight on semaglutide compared with those who tested negative.
Overall, he concluded, “These findings open the door to more precise bariatric care. When we understand a patient’s biological drivers of obesity, we can make better decisions about the right procedure, follow-up, and long-term support. This moves us away from a one-size-fits-all model to care rooted in each patient’s unique biology.”
Potentially Paradigm-Shifting
Onur Kutlu, MD, associate professor of surgery and director of the Metabolic Surgery and Metabolic Health Program at the Miller School of Medicine, University of Miami, in Miami, Florida, commented on the study for GI & Hepatology News. “By integrating polygenic risk scores into predictive models, the authors offer an innovative method for identifying patients at elevated risk for weight regain following RYGB.”
“Their findings support the hypothesis that genetic predisposition — particularly involving energy homeostasis pathways — may underlie differential postoperative trajectories,” he said. “This approach has the potential to shift the paradigm from reactive to proactive management of weight recurrence.”
Because current options for treat weight regain are “suboptimal,” he said, “prevention becomes paramount. Preoperative identification of high-risk individuals could inform surgical decision-making, enable earlier interventions, and facilitate personalized postoperative monitoring and support.”
“If validated in larger, prospective cohorts, genetic risk stratification could enhance the precision of bariatric care and improve long-term outcomes,” he added. “Future studies should aim to validate these genetic models across diverse populations and explore how integration of behavioral, psychological, and genetic data may further refine patient selection and care pathways.”
The study was funded by Mayo Clinic and Phenomix Sciences. Gila Therapeutics and Phenomix Sciences licensed Acosta’s research technologies from the University of Florida and Mayo Clinic. Acosta declared receiving consultant fees in the past 5 years from Rhythm Pharmaceuticals, Gila Therapeutics, Amgen, General Mills, BI, Currax, Nestle, Phenomix Sciences, Bausch Health, and RareDiseases, as well as funding support from the National Institutes of Health, Vivus Pharmaceuticals, Novo Nordisk, Apollo Endosurgery, Satiogen Pharmaceuticals, Spatz Medical, and Rhythm Pharmaceuticals. Kutlu declared having no conflicts of interest.
A version of this article appeared on Medscape.com.
SAN DIEGO –
The findings suggested that the MyPhenome test (Phenomix Sciences) can help clinicians identify the patients most likely to benefit from bariatric procedures and at a greater risk for long-term weight regain after surgery.
“Patients with both a high genetic risk score and rare mutations in the leptin-melanocortin pathway (LMP) had significantly worse outcomes, maintaining only 4.9% total body weight loss [TBWL] over 15 years compared to up to 24.8% in other genetic groups,” Phenomix Sciences Co-founder Andres Acosta, MD, PhD, told GI & Hepatology News.
The study included details on the score’s development and predictive capability. It was presented at Digestive Disease Week® (DDW) 2025
‘More Precise Bariatric Care’
The researchers recently developed a machine learning-assisted gene risk score for calories to satiation (CTSGRS), which mainly involves genes in the LMP. To assess the role of the score with or without LMP gene variants on weight loss and weight recurrence after RYGB, they identified 707 patients with a history of bariatric procedures from the Mayo Clinic Biobank. Patients with duodenal switch, revisional procedures, or who used antiobesity medications or became pregnant during follow-up were excluded.
To make predictions for 442 of the patients, the team first collected anthropometric data up to 15 years after RYGB. Then they used a two-step approach: Assessing for monogenic variants in the LMP and defining participants as carriers (LMP+) or noncarriers (LMP-). Then they defined the gene risk score (CTSGRS+ or CTSGRS-).
The result was four groups: LMP+/CTSGRS+, LMP+/CTSGRS-, LMP-/CTSGRS+, and LMP-/CTSGRS-. Multiple regression analysis was used to analyze TBWL percentage (TBWL%) between the groups at different timepoints, adjusting for baseline weight, age, and gender.
At the 10-year follow-up, the LMP+/CTSGRS+ group demonstrated a significantly higher weight recurrence (regain) of TBW% compared to the other groups.
At 15 years post-RYGB, the mean TBWL% for LMP+/CTSGRS+ was -4.9 vs -20.3 for LMP+/CTSGRS-, -18.0 for LMP-/CTSGRS+, and -24.8 for LMP-/CTSGRS-.
Further analyses showed that the LMP+/CTSGRS+ group had significantly less weight loss than LMP+/CTSGRS- and LMP-/CTSGRS- groups.
Based on the findings, the authors wrote, “Genotyping patients could improve the implementation of individualized weight-loss interventions, enhance weight-loss outcomes, and/or may explain one of the etiological factors associated with weight recurrence after RYGB.”
Acosta noted, “We’re actively expanding our research to include more diverse populations by age, sex, and race. This includes ongoing analysis to understand whether certain demographic or physiological characteristics affect how the test performs, particularly in the context of bariatric surgery.”
The team also is investigating the benefits of phenotyping for obesity comorbidities such as heart disease and diabetes, he said, and exploring whether early interventions in high-risk patients can prevent long-term weight regain and improve outcomes.
In addition, Acosta said, the team recently launched “the first prospective, placebo-controlled clinical trial using the MyPhenome test to predict response to semaglutide.” That study is based on earlier findings showing that patients identified with a Hungry Gut phenotype lost nearly twice as much weight on semaglutide compared with those who tested negative.
Overall, he concluded, “These findings open the door to more precise bariatric care. When we understand a patient’s biological drivers of obesity, we can make better decisions about the right procedure, follow-up, and long-term support. This moves us away from a one-size-fits-all model to care rooted in each patient’s unique biology.”
Potentially Paradigm-Shifting
Onur Kutlu, MD, associate professor of surgery and director of the Metabolic Surgery and Metabolic Health Program at the Miller School of Medicine, University of Miami, in Miami, Florida, commented on the study for GI & Hepatology News. “By integrating polygenic risk scores into predictive models, the authors offer an innovative method for identifying patients at elevated risk for weight regain following RYGB.”
“Their findings support the hypothesis that genetic predisposition — particularly involving energy homeostasis pathways — may underlie differential postoperative trajectories,” he said. “This approach has the potential to shift the paradigm from reactive to proactive management of weight recurrence.”
Because current options for treat weight regain are “suboptimal,” he said, “prevention becomes paramount. Preoperative identification of high-risk individuals could inform surgical decision-making, enable earlier interventions, and facilitate personalized postoperative monitoring and support.”
“If validated in larger, prospective cohorts, genetic risk stratification could enhance the precision of bariatric care and improve long-term outcomes,” he added. “Future studies should aim to validate these genetic models across diverse populations and explore how integration of behavioral, psychological, and genetic data may further refine patient selection and care pathways.”
The study was funded by Mayo Clinic and Phenomix Sciences. Gila Therapeutics and Phenomix Sciences licensed Acosta’s research technologies from the University of Florida and Mayo Clinic. Acosta declared receiving consultant fees in the past 5 years from Rhythm Pharmaceuticals, Gila Therapeutics, Amgen, General Mills, BI, Currax, Nestle, Phenomix Sciences, Bausch Health, and RareDiseases, as well as funding support from the National Institutes of Health, Vivus Pharmaceuticals, Novo Nordisk, Apollo Endosurgery, Satiogen Pharmaceuticals, Spatz Medical, and Rhythm Pharmaceuticals. Kutlu declared having no conflicts of interest.
A version of this article appeared on Medscape.com.
FROM DDW 2025
Walnuts Cut Gut Permeability in Obesity
, a small study showed.
“Less than 10% of adults are meeting their fiber needs each day, and walnuts are a source of dietary fiber, which helps nourish the gut microbiota,” study coauthor Hannah Holscher, PhD, RD, associate professor of nutrition at the University of Illinois at Urbana-Champaign, told GI & Hepatology News.
Holscher and her colleagues previously conducted a study on the effects of walnut consumption on the human intestinal microbiota “and found interesting results,” she said. Among 18 healthy men and women with a mean age of 53 years, “walnuts enriched intestinal microorganisms, including Roseburia that provide important gut-health promoting attributes, like short-chain fatty acid production. We also saw lower proinflammatory secondary bile acid concentrations in individuals that ate walnuts.”
The current study, presented at NUTRITION 2025 in Orlando, Florida, found similar benefits among 30 adults with obesity but without diabetes or gastrointestinal disease.
Walnut Halves, Walnut Oil, Corn Oil — Compared
The researchers aimed to determine the impact of walnut consumption on the gut microbiome, serum and fecal bile acid profiles, systemic inflammation, and oral glucose tolerance to a mixed-meal challenge.
Participants were enrolled in a randomized, controlled, crossover, complete feeding trial with three 3-week conditions, each identical except for walnut halves (WH), walnut oil (WO), or corn oil (CO) in the diet. A 3-week washout separated each condition.
“This was a fully controlled dietary feeding intervention,” Holscher said. “We provided their breakfast, lunch, snacks and dinners — all of their foods and beverages during the three dietary intervention periods that lasted for 3 weeks each. Their base diet consisted of typical American foods that you would find in a grocery store in central Illinois.”
Fecal samples were collected on days 18-20. On day 20, participants underwent a 6-hour mixed-meal tolerance test (75 g glucose + treatment) with a fasting blood draw followed by blood sampling every 30 minutes.
The fecal microbiome and microbiota were assessed using metagenomic and amplicon sequencing, respectively. Fecal microbial metabolites were quantified using gas chromatography-mass spectrometry.
Blood glucose, insulin, and inflammatory biomarkers (interleukin-6, tumor necrosis factor-alpha, C-reactive protein, and lipopolysaccharide-binding protein) were quantified. Fecal and circulating bile acids were measured via liquid chromatography tandem mass spectrometry.
Gut permeability was assessed by quantifying 24-hour urinary excretion of orally ingested sucralose and erythritol on day 21.
Linear mixed-effects models and repeated measures ANOVA were used for the statistical analysis.
The team found that Roseburia spp were greatest following WH (3.9%) vs WO (1.6) and CO (1.9); Lachnospiraceae UCG-001 and UCG-004 were also greatest with WH vs WO and CO.
WH fecal isobutyrate concentrations (5.41 µmol/g) were lower than WO (7.17 µmol/g) and CO (7.77). Similarly, fecal isovalerate concentrations were lowest with WH (7.84 µmol/g) vs WO (10.3µmol/g) and CO (11.6 µmol/g).
In contrast, indoles were highest in WH (36.8 µmol/g) vs WO (6.78 µmol/g) and CO (8.67µmol/g).
No differences in glucose concentrations were seen among groups. The 2-hour area under the curve (AUC) for insulin was lower with WH (469 µIU/mL/min) and WO (494) vs CO (604 µIU/mL/min).
The 4-hour AUC for glycolithocholic acid was lower with WH vs WO and CO. Furthermore, sucralose recovery was lowest following WH (10.5) vs WO (14.3) and CO (14.6).
“Our current efforts are focused on understanding connections between plasma bile acids and glycemic control (ie, blood glucose and insulin concentrations),” Holscher said. “We are also interested in studying individualized or personalized responses, since people had different magnitudes of responses.”
In addition, she said, “as the gut microbiome is one of the factors that can underpin the physiological response to the diet, we are interested in determining if there are microbial signatures that are predictive of glycemic control.”
Because the research is still in the early stages, at this point, Holscher simply encourages people to eat a variety of fruits, vegetables, whole grains, legumes and nuts to meet their daily fiber recommendations and support their gut microbiome.
This study was funded by a USDA NIFA grant. No competing interests were reported.
A version of this article appeared on Medscape.com .
, a small study showed.
“Less than 10% of adults are meeting their fiber needs each day, and walnuts are a source of dietary fiber, which helps nourish the gut microbiota,” study coauthor Hannah Holscher, PhD, RD, associate professor of nutrition at the University of Illinois at Urbana-Champaign, told GI & Hepatology News.
Holscher and her colleagues previously conducted a study on the effects of walnut consumption on the human intestinal microbiota “and found interesting results,” she said. Among 18 healthy men and women with a mean age of 53 years, “walnuts enriched intestinal microorganisms, including Roseburia that provide important gut-health promoting attributes, like short-chain fatty acid production. We also saw lower proinflammatory secondary bile acid concentrations in individuals that ate walnuts.”
The current study, presented at NUTRITION 2025 in Orlando, Florida, found similar benefits among 30 adults with obesity but without diabetes or gastrointestinal disease.
Walnut Halves, Walnut Oil, Corn Oil — Compared
The researchers aimed to determine the impact of walnut consumption on the gut microbiome, serum and fecal bile acid profiles, systemic inflammation, and oral glucose tolerance to a mixed-meal challenge.
Participants were enrolled in a randomized, controlled, crossover, complete feeding trial with three 3-week conditions, each identical except for walnut halves (WH), walnut oil (WO), or corn oil (CO) in the diet. A 3-week washout separated each condition.
“This was a fully controlled dietary feeding intervention,” Holscher said. “We provided their breakfast, lunch, snacks and dinners — all of their foods and beverages during the three dietary intervention periods that lasted for 3 weeks each. Their base diet consisted of typical American foods that you would find in a grocery store in central Illinois.”
Fecal samples were collected on days 18-20. On day 20, participants underwent a 6-hour mixed-meal tolerance test (75 g glucose + treatment) with a fasting blood draw followed by blood sampling every 30 minutes.
The fecal microbiome and microbiota were assessed using metagenomic and amplicon sequencing, respectively. Fecal microbial metabolites were quantified using gas chromatography-mass spectrometry.
Blood glucose, insulin, and inflammatory biomarkers (interleukin-6, tumor necrosis factor-alpha, C-reactive protein, and lipopolysaccharide-binding protein) were quantified. Fecal and circulating bile acids were measured via liquid chromatography tandem mass spectrometry.
Gut permeability was assessed by quantifying 24-hour urinary excretion of orally ingested sucralose and erythritol on day 21.
Linear mixed-effects models and repeated measures ANOVA were used for the statistical analysis.
The team found that Roseburia spp were greatest following WH (3.9%) vs WO (1.6) and CO (1.9); Lachnospiraceae UCG-001 and UCG-004 were also greatest with WH vs WO and CO.
WH fecal isobutyrate concentrations (5.41 µmol/g) were lower than WO (7.17 µmol/g) and CO (7.77). Similarly, fecal isovalerate concentrations were lowest with WH (7.84 µmol/g) vs WO (10.3µmol/g) and CO (11.6 µmol/g).
In contrast, indoles were highest in WH (36.8 µmol/g) vs WO (6.78 µmol/g) and CO (8.67µmol/g).
No differences in glucose concentrations were seen among groups. The 2-hour area under the curve (AUC) for insulin was lower with WH (469 µIU/mL/min) and WO (494) vs CO (604 µIU/mL/min).
The 4-hour AUC for glycolithocholic acid was lower with WH vs WO and CO. Furthermore, sucralose recovery was lowest following WH (10.5) vs WO (14.3) and CO (14.6).
“Our current efforts are focused on understanding connections between plasma bile acids and glycemic control (ie, blood glucose and insulin concentrations),” Holscher said. “We are also interested in studying individualized or personalized responses, since people had different magnitudes of responses.”
In addition, she said, “as the gut microbiome is one of the factors that can underpin the physiological response to the diet, we are interested in determining if there are microbial signatures that are predictive of glycemic control.”
Because the research is still in the early stages, at this point, Holscher simply encourages people to eat a variety of fruits, vegetables, whole grains, legumes and nuts to meet their daily fiber recommendations and support their gut microbiome.
This study was funded by a USDA NIFA grant. No competing interests were reported.
A version of this article appeared on Medscape.com .
, a small study showed.
“Less than 10% of adults are meeting their fiber needs each day, and walnuts are a source of dietary fiber, which helps nourish the gut microbiota,” study coauthor Hannah Holscher, PhD, RD, associate professor of nutrition at the University of Illinois at Urbana-Champaign, told GI & Hepatology News.
Holscher and her colleagues previously conducted a study on the effects of walnut consumption on the human intestinal microbiota “and found interesting results,” she said. Among 18 healthy men and women with a mean age of 53 years, “walnuts enriched intestinal microorganisms, including Roseburia that provide important gut-health promoting attributes, like short-chain fatty acid production. We also saw lower proinflammatory secondary bile acid concentrations in individuals that ate walnuts.”
The current study, presented at NUTRITION 2025 in Orlando, Florida, found similar benefits among 30 adults with obesity but without diabetes or gastrointestinal disease.
Walnut Halves, Walnut Oil, Corn Oil — Compared
The researchers aimed to determine the impact of walnut consumption on the gut microbiome, serum and fecal bile acid profiles, systemic inflammation, and oral glucose tolerance to a mixed-meal challenge.
Participants were enrolled in a randomized, controlled, crossover, complete feeding trial with three 3-week conditions, each identical except for walnut halves (WH), walnut oil (WO), or corn oil (CO) in the diet. A 3-week washout separated each condition.
“This was a fully controlled dietary feeding intervention,” Holscher said. “We provided their breakfast, lunch, snacks and dinners — all of their foods and beverages during the three dietary intervention periods that lasted for 3 weeks each. Their base diet consisted of typical American foods that you would find in a grocery store in central Illinois.”
Fecal samples were collected on days 18-20. On day 20, participants underwent a 6-hour mixed-meal tolerance test (75 g glucose + treatment) with a fasting blood draw followed by blood sampling every 30 minutes.
The fecal microbiome and microbiota were assessed using metagenomic and amplicon sequencing, respectively. Fecal microbial metabolites were quantified using gas chromatography-mass spectrometry.
Blood glucose, insulin, and inflammatory biomarkers (interleukin-6, tumor necrosis factor-alpha, C-reactive protein, and lipopolysaccharide-binding protein) were quantified. Fecal and circulating bile acids were measured via liquid chromatography tandem mass spectrometry.
Gut permeability was assessed by quantifying 24-hour urinary excretion of orally ingested sucralose and erythritol on day 21.
Linear mixed-effects models and repeated measures ANOVA were used for the statistical analysis.
The team found that Roseburia spp were greatest following WH (3.9%) vs WO (1.6) and CO (1.9); Lachnospiraceae UCG-001 and UCG-004 were also greatest with WH vs WO and CO.
WH fecal isobutyrate concentrations (5.41 µmol/g) were lower than WO (7.17 µmol/g) and CO (7.77). Similarly, fecal isovalerate concentrations were lowest with WH (7.84 µmol/g) vs WO (10.3µmol/g) and CO (11.6 µmol/g).
In contrast, indoles were highest in WH (36.8 µmol/g) vs WO (6.78 µmol/g) and CO (8.67µmol/g).
No differences in glucose concentrations were seen among groups. The 2-hour area under the curve (AUC) for insulin was lower with WH (469 µIU/mL/min) and WO (494) vs CO (604 µIU/mL/min).
The 4-hour AUC for glycolithocholic acid was lower with WH vs WO and CO. Furthermore, sucralose recovery was lowest following WH (10.5) vs WO (14.3) and CO (14.6).
“Our current efforts are focused on understanding connections between plasma bile acids and glycemic control (ie, blood glucose and insulin concentrations),” Holscher said. “We are also interested in studying individualized or personalized responses, since people had different magnitudes of responses.”
In addition, she said, “as the gut microbiome is one of the factors that can underpin the physiological response to the diet, we are interested in determining if there are microbial signatures that are predictive of glycemic control.”
Because the research is still in the early stages, at this point, Holscher simply encourages people to eat a variety of fruits, vegetables, whole grains, legumes and nuts to meet their daily fiber recommendations and support their gut microbiome.
This study was funded by a USDA NIFA grant. No competing interests were reported.
A version of this article appeared on Medscape.com .
Can Popular Weight-Loss Drugs Protect Against Obesity-Related Cancers?
Can Popular Weight-Loss Drugs Protect Against Obesity-Related Cancers?
New data suggest that glucagon-like peptide 1 (GLP-1) receptor agonists, used to treat diabetes and obesity, may also help guard against obesity-related cancers.
In a large observational study, new GLP-1 agonist users with obesity and diabetes had a significantly lower risk for 14 obesity-related cancers than similar individuals who received dipeptidyl peptidase-4 (DPP-4) inhibitors, which are weight-neutral.
This study provides a “reassuring safety signal” showing that GLP-1 drugs are linked to a modest drop in obesity-related cancer risk, and not a higher risk for these cancers, said lead investigator Lucas Mavromatis, medical student at NYU Grossman School of Medicine in New York City, during a press conference at American Society of Clinical Oncology (ASCO) 2025 annual meeting.
However, there were some nuances to the findings. The protective effect of GLP-1 agonists was only significant for colon and rectal cancers and for women, Mavromatis reported. And although GLP-1 users had an 8% lower risk of dying from any cause, the survival benefit was also only significant for women.
Still, the overall “message to patients is GLP-1 receptor treatments remain a strong option for patients with diabetes and obesity and may have an additional, small favorable benefit in cancer,” Mavromatis explained at the press briefing.
'Intriguing Hypothesis'
Obesity is linked to an increased risk of developing more than a dozen cancer types, including esophageal, colon, rectal, stomach, liver, gallbladder, pancreatic, kidney, postmenopausal breast, ovarian, endometrial and thyroid, as well as multiple myeloma and meningiomas.
About 12% of Americans have been prescribed a GLP-1 medication to treat diabetes and/or obesity. However, little is known about how these drugs affect cancer risk.
To investigate, Mavromatis and colleagues used the Optum healthcare database to identify 170,030 adults with obesity and type 2 diabetes from 43 health systems in the United States.
Between 2013 and 2023, half started a GLP-1 agonist and half started a DPP-4 inhibitor, with propensity score matching used to balance characteristics of the two cohorts.
Participants were a mean age of 56.8 years, with an average body mass index of 38.5; more than 70% were White individuals and more than 14% were Black individuals.
During a mean follow-up of 3.9 years, 2501 new obesity-related cancers were identified in the GLP-1 group and 2671 in the DPP-4 group — representing a 7% overall reduced risk for any obesity-related cancer in the GLP-1 group (hazard ratio [HR], 0.93).
When analyzing each of the 14 obesity-related cancers separately, the protective link between GLP-1 use and cancer was primarily driven by colon and rectal cancers. GLP-1 users had a 16% lower risk for colon cancer (HR, 0.84) and a 28% lower risk for rectal cancer (HR, 0.72).
“No other cancers had statistically significant associations with GLP-1 use,” Mavromatis told briefing attendees. But “importantly, no cancers had statistically significant adverse associations with GLP-1 use,” he added.
Experts have expressed some concern about a possible link between GLP-1 use and pancreatic cancer given that pancreatitis is a known side effect of GLP-1 use. However, “this is not borne out by epidemiological data,” Mavromatis said.
“Additionally, we were not able to specifically assess medullary thyroid cancer, which is on the warning label for several GLP-1 medications, but we did see a reassuring lack of association between GLP-1 use and thyroid cancer as a whole,” he added.
During follow-up, there were 2783 deaths in the GLP-1 group and 2961 deaths in the DPP-4 group — translating to an 8% lower risk for death due to any cause among GLP-1 users (HR, 0.92; P = .001).
Mavromatis and colleagues observed sex differences as well. Women taking a GLP-1 had an 8% lower risk for obesity-related cancers (HR, 0.92; P = .01) and a 20% lower risk for death from any cause (HR, 0.80; P < .001) compared with women taking a DPP-4 inhibitor.
Among men, researchers found no statistically significant difference between GLP-1 and DPP-4 use for obesity-related cancer risk (HR, 0.95; P = .29) or all-cause mortality (HR, 1.04; P = .34).
Overall, Mavromatis said, it’s important to note that the absolute risk reduction seen in the study is “small and the number of patients that would need to be given one of these medications to prevent an obesity-related cancer, based on our data, would be very large.”
Mavromatis also noted that the length of follow-up was short, and the study assessed primarily older and weaker GLP-1 agonists compared with newer agents on the market. Therefore, longer-term studies with newer GLP-1s are needed to confirm the effects seen as well as safety.
In a statement, ASCO President Robin Zon, MD, said this trial raises the “intriguing hypothesis” that the increasingly popular GLP-1 medications might offer some benefit in reducing the risk of developing cancer.
Zon said she sees many patients with obesity, and given the clear link between cancer and obesity, defining the clinical role of GLP-1 medications in cancer prevention is “important.”
This study “leads us in the direction” of a potential protective effect of GLP-1s on cancer, but “there are a lot of questions that are generated by this particular study, especially as we move forward and we think about prevention of cancers,” Zon told the briefing.
This study was funded by the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health. Mavromatis reported no relevant disclosures. Zon reported stock or ownership interests in Oncolytics Biotech, TG Therapeutics, Select Sector SPDR Health Care, AstraZeneca, CRISPR, McKesson, and Berkshire Hathaway.
A version of this article first appeared on Medscape.com.
New data suggest that glucagon-like peptide 1 (GLP-1) receptor agonists, used to treat diabetes and obesity, may also help guard against obesity-related cancers.
In a large observational study, new GLP-1 agonist users with obesity and diabetes had a significantly lower risk for 14 obesity-related cancers than similar individuals who received dipeptidyl peptidase-4 (DPP-4) inhibitors, which are weight-neutral.
This study provides a “reassuring safety signal” showing that GLP-1 drugs are linked to a modest drop in obesity-related cancer risk, and not a higher risk for these cancers, said lead investigator Lucas Mavromatis, medical student at NYU Grossman School of Medicine in New York City, during a press conference at American Society of Clinical Oncology (ASCO) 2025 annual meeting.
However, there were some nuances to the findings. The protective effect of GLP-1 agonists was only significant for colon and rectal cancers and for women, Mavromatis reported. And although GLP-1 users had an 8% lower risk of dying from any cause, the survival benefit was also only significant for women.
Still, the overall “message to patients is GLP-1 receptor treatments remain a strong option for patients with diabetes and obesity and may have an additional, small favorable benefit in cancer,” Mavromatis explained at the press briefing.
'Intriguing Hypothesis'
Obesity is linked to an increased risk of developing more than a dozen cancer types, including esophageal, colon, rectal, stomach, liver, gallbladder, pancreatic, kidney, postmenopausal breast, ovarian, endometrial and thyroid, as well as multiple myeloma and meningiomas.
About 12% of Americans have been prescribed a GLP-1 medication to treat diabetes and/or obesity. However, little is known about how these drugs affect cancer risk.
To investigate, Mavromatis and colleagues used the Optum healthcare database to identify 170,030 adults with obesity and type 2 diabetes from 43 health systems in the United States.
Between 2013 and 2023, half started a GLP-1 agonist and half started a DPP-4 inhibitor, with propensity score matching used to balance characteristics of the two cohorts.
Participants were a mean age of 56.8 years, with an average body mass index of 38.5; more than 70% were White individuals and more than 14% were Black individuals.
During a mean follow-up of 3.9 years, 2501 new obesity-related cancers were identified in the GLP-1 group and 2671 in the DPP-4 group — representing a 7% overall reduced risk for any obesity-related cancer in the GLP-1 group (hazard ratio [HR], 0.93).
When analyzing each of the 14 obesity-related cancers separately, the protective link between GLP-1 use and cancer was primarily driven by colon and rectal cancers. GLP-1 users had a 16% lower risk for colon cancer (HR, 0.84) and a 28% lower risk for rectal cancer (HR, 0.72).
“No other cancers had statistically significant associations with GLP-1 use,” Mavromatis told briefing attendees. But “importantly, no cancers had statistically significant adverse associations with GLP-1 use,” he added.
Experts have expressed some concern about a possible link between GLP-1 use and pancreatic cancer given that pancreatitis is a known side effect of GLP-1 use. However, “this is not borne out by epidemiological data,” Mavromatis said.
“Additionally, we were not able to specifically assess medullary thyroid cancer, which is on the warning label for several GLP-1 medications, but we did see a reassuring lack of association between GLP-1 use and thyroid cancer as a whole,” he added.
During follow-up, there were 2783 deaths in the GLP-1 group and 2961 deaths in the DPP-4 group — translating to an 8% lower risk for death due to any cause among GLP-1 users (HR, 0.92; P = .001).
Mavromatis and colleagues observed sex differences as well. Women taking a GLP-1 had an 8% lower risk for obesity-related cancers (HR, 0.92; P = .01) and a 20% lower risk for death from any cause (HR, 0.80; P < .001) compared with women taking a DPP-4 inhibitor.
Among men, researchers found no statistically significant difference between GLP-1 and DPP-4 use for obesity-related cancer risk (HR, 0.95; P = .29) or all-cause mortality (HR, 1.04; P = .34).
Overall, Mavromatis said, it’s important to note that the absolute risk reduction seen in the study is “small and the number of patients that would need to be given one of these medications to prevent an obesity-related cancer, based on our data, would be very large.”
Mavromatis also noted that the length of follow-up was short, and the study assessed primarily older and weaker GLP-1 agonists compared with newer agents on the market. Therefore, longer-term studies with newer GLP-1s are needed to confirm the effects seen as well as safety.
In a statement, ASCO President Robin Zon, MD, said this trial raises the “intriguing hypothesis” that the increasingly popular GLP-1 medications might offer some benefit in reducing the risk of developing cancer.
Zon said she sees many patients with obesity, and given the clear link between cancer and obesity, defining the clinical role of GLP-1 medications in cancer prevention is “important.”
This study “leads us in the direction” of a potential protective effect of GLP-1s on cancer, but “there are a lot of questions that are generated by this particular study, especially as we move forward and we think about prevention of cancers,” Zon told the briefing.
This study was funded by the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health. Mavromatis reported no relevant disclosures. Zon reported stock or ownership interests in Oncolytics Biotech, TG Therapeutics, Select Sector SPDR Health Care, AstraZeneca, CRISPR, McKesson, and Berkshire Hathaway.
A version of this article first appeared on Medscape.com.
New data suggest that glucagon-like peptide 1 (GLP-1) receptor agonists, used to treat diabetes and obesity, may also help guard against obesity-related cancers.
In a large observational study, new GLP-1 agonist users with obesity and diabetes had a significantly lower risk for 14 obesity-related cancers than similar individuals who received dipeptidyl peptidase-4 (DPP-4) inhibitors, which are weight-neutral.
This study provides a “reassuring safety signal” showing that GLP-1 drugs are linked to a modest drop in obesity-related cancer risk, and not a higher risk for these cancers, said lead investigator Lucas Mavromatis, medical student at NYU Grossman School of Medicine in New York City, during a press conference at American Society of Clinical Oncology (ASCO) 2025 annual meeting.
However, there were some nuances to the findings. The protective effect of GLP-1 agonists was only significant for colon and rectal cancers and for women, Mavromatis reported. And although GLP-1 users had an 8% lower risk of dying from any cause, the survival benefit was also only significant for women.
Still, the overall “message to patients is GLP-1 receptor treatments remain a strong option for patients with diabetes and obesity and may have an additional, small favorable benefit in cancer,” Mavromatis explained at the press briefing.
'Intriguing Hypothesis'
Obesity is linked to an increased risk of developing more than a dozen cancer types, including esophageal, colon, rectal, stomach, liver, gallbladder, pancreatic, kidney, postmenopausal breast, ovarian, endometrial and thyroid, as well as multiple myeloma and meningiomas.
About 12% of Americans have been prescribed a GLP-1 medication to treat diabetes and/or obesity. However, little is known about how these drugs affect cancer risk.
To investigate, Mavromatis and colleagues used the Optum healthcare database to identify 170,030 adults with obesity and type 2 diabetes from 43 health systems in the United States.
Between 2013 and 2023, half started a GLP-1 agonist and half started a DPP-4 inhibitor, with propensity score matching used to balance characteristics of the two cohorts.
Participants were a mean age of 56.8 years, with an average body mass index of 38.5; more than 70% were White individuals and more than 14% were Black individuals.
During a mean follow-up of 3.9 years, 2501 new obesity-related cancers were identified in the GLP-1 group and 2671 in the DPP-4 group — representing a 7% overall reduced risk for any obesity-related cancer in the GLP-1 group (hazard ratio [HR], 0.93).
When analyzing each of the 14 obesity-related cancers separately, the protective link between GLP-1 use and cancer was primarily driven by colon and rectal cancers. GLP-1 users had a 16% lower risk for colon cancer (HR, 0.84) and a 28% lower risk for rectal cancer (HR, 0.72).
“No other cancers had statistically significant associations with GLP-1 use,” Mavromatis told briefing attendees. But “importantly, no cancers had statistically significant adverse associations with GLP-1 use,” he added.
Experts have expressed some concern about a possible link between GLP-1 use and pancreatic cancer given that pancreatitis is a known side effect of GLP-1 use. However, “this is not borne out by epidemiological data,” Mavromatis said.
“Additionally, we were not able to specifically assess medullary thyroid cancer, which is on the warning label for several GLP-1 medications, but we did see a reassuring lack of association between GLP-1 use and thyroid cancer as a whole,” he added.
During follow-up, there were 2783 deaths in the GLP-1 group and 2961 deaths in the DPP-4 group — translating to an 8% lower risk for death due to any cause among GLP-1 users (HR, 0.92; P = .001).
Mavromatis and colleagues observed sex differences as well. Women taking a GLP-1 had an 8% lower risk for obesity-related cancers (HR, 0.92; P = .01) and a 20% lower risk for death from any cause (HR, 0.80; P < .001) compared with women taking a DPP-4 inhibitor.
Among men, researchers found no statistically significant difference between GLP-1 and DPP-4 use for obesity-related cancer risk (HR, 0.95; P = .29) or all-cause mortality (HR, 1.04; P = .34).
Overall, Mavromatis said, it’s important to note that the absolute risk reduction seen in the study is “small and the number of patients that would need to be given one of these medications to prevent an obesity-related cancer, based on our data, would be very large.”
Mavromatis also noted that the length of follow-up was short, and the study assessed primarily older and weaker GLP-1 agonists compared with newer agents on the market. Therefore, longer-term studies with newer GLP-1s are needed to confirm the effects seen as well as safety.
In a statement, ASCO President Robin Zon, MD, said this trial raises the “intriguing hypothesis” that the increasingly popular GLP-1 medications might offer some benefit in reducing the risk of developing cancer.
Zon said she sees many patients with obesity, and given the clear link between cancer and obesity, defining the clinical role of GLP-1 medications in cancer prevention is “important.”
This study “leads us in the direction” of a potential protective effect of GLP-1s on cancer, but “there are a lot of questions that are generated by this particular study, especially as we move forward and we think about prevention of cancers,” Zon told the briefing.
This study was funded by the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health. Mavromatis reported no relevant disclosures. Zon reported stock or ownership interests in Oncolytics Biotech, TG Therapeutics, Select Sector SPDR Health Care, AstraZeneca, CRISPR, McKesson, and Berkshire Hathaway.
A version of this article first appeared on Medscape.com.
Can Popular Weight-Loss Drugs Protect Against Obesity-Related Cancers?
Can Popular Weight-Loss Drugs Protect Against Obesity-Related Cancers?
GLP-1s Treat and Even Reverse Some Forms of Liver Disease
In the past two decades, the global prevalence of metabolic dysfunction–associated steatohepatitis (MASH) has increased dramatically as a result of the obesity epidemic. Researchers project that by 2040, rates of MASH will increase by 55%. Prior to that most liver diseases were caused by alcohol use and hepatitis C, a viral infection that primarily affects the liver.
MASH, a preventable form of liver disease previously called nonalcoholic fatty liver disease, is caused by a buildup of visceral fat cells that accumulate on top of the internal organs, in this case the liver, and keep it from functioning properly. The liver’s primary role is to filter blood, nutrients, and bile used for digestion, as well as to remove toxins from the body. Excess fat cells blanket the liver and keep it from working at full capacity.
Fat cells are also metabolically active and can cause a chronic state of inflammation in the part of the body where they reside. Over time, these fat cells can cause cirrhosis of the liver, or permanent scarring. Once patients reach this stage, the only option is a liver transplant.
New Research on GLP-1 Agonists and MASH
Until recently, the lone treatment for early-stage MASH was weight loss to reduce the number of fat cells that surround the internal organs. But new research has shown that glucagon-like peptide 1 (GLP-1) agonists can reduce and even reverse the condition. In a study published in April, researchers were able to show that semaglutide resolved fatty liver and inflammation in over 60% of cases and decreased scar tissue in just over a third of patients.
“These findings suggest that semaglutide may prevent fatty liver disease from progressing to cirrhosis and can indeed reverse the course of the disease,” said Arun J. Sanyal, MD, study author and director of the Stravitz-Sanyal Institute for Liver Disease and Metabolic Health at Virginia Commonwealth University in Richmond, Virginia.
Another study published last year had a similar finding, showing that GLP-1 agonists were associated with less progression of the disease and reduced mortality in patients with MASH and diabetes. Another large-scale observational study found that GLP-1s reduced the risk for hepatic failure, which occurs when the liver is unable to perform basic functions, as well as liver cancer, both of which are downstream consequences of MASH.
How GLP-1s Improve Liver Function
“These medications reduce fat burden, which results in fat loss everywhere, including around the liver,” said Ziyad Al-Aly, MD, an assistant professor in the Division of General Medicine & Geriatrics at Washington University School of Medicine in St. Louis. “When fat cells are reduced in size and volume, the normal liver cells have more room to grow and function.”
These medications also seem to work on reducing the inflammation and oxidative stress caused by metabolic disease, which allows for a better environment for the liver to function.
“Fat is not an inert tissue, it’s metabolically active, causing a slow burn to all the cells surrounding it,” said Al-Aly. These medications keep the disease from progressing and reduce scarring, which improves the damage that’s already been done, he said.
Changing How Liver Disease Is Diagnosed
Physicians need to be vigilant in the way that they screen for the condition, said Charu Sawhney, DO, MPH, of Harbor Health in Round Rock, Texas. She said that if liver enzymes appear even slightly elevated, there still could be a reason to utilize GLP-1s to prevent later-stage MASH.
“Normal levels for liver enzymes in some patients can be lower than what labs show,” said Sawhney. This is especially true if a patient has other metabolic risk factors such as diabetes, obesity, or high cholesterol.
If liver enzymes continue to go up even after diet and lifestyle changes, patients might require liver imaging, specifically a wave-based ultrasound called elastography, which measures the elasticity or stiffness of tissues on the liver and can judge if certain portions of it have scarred or hardened. When liver cells change texture and become harder, the scan can estimate levels of fibrosis and, therefore, the stage of MASH that a patient is in.
Additionally, the severity of fatty liver disease depends on other factors besides weight and can sometimes be surprising.
“How bad fatty liver disease is in a patient isn’t always related to how much weight someone has gained,” said Carolynn Francavilla, MD, a nationally recognized obesity physician who owns and operates Green Mountain Partners for Health and Colorado Weight Care, both in Denver.
It’s important for physicians to realize that some patients with fatty liver disease might not have obesity as would be expected. For these patients, adipose tissue seems to accumulate on the liver before it does on other parts of the body. This could be related to the quality of our food system, including the use of sugar substitutes like high fructose corn syrup, which research has shown is even harder on the liver. There might also be a genetic propensity toward fat storage around the organs.
A New Way to Treat MASH
, said Francavilla. Right now, there’s not an official approval from the US Food and Drug Administration (FDA) for prescribing GLP-1s in patients with MASH, but Francavilla hopes that it’s forthcoming.
“It will be really exciting to have these medications as a treatment option because right now there’s only one medication, and it’s for people who have pretty advanced fatty liver disease,” said Francavilla. This medication, called resmetirom, is approved by the FDA to target a protein in the liver to reduce fat and inflammation and scarring. But GLP-1s can be used much earlier to prevent the condition.
“With so many cases of MASH happening so much younger, it’s a disease that physicians really need to take seriously,” said Sawhney.
A version of this article appeared on Medscape.com.
In the past two decades, the global prevalence of metabolic dysfunction–associated steatohepatitis (MASH) has increased dramatically as a result of the obesity epidemic. Researchers project that by 2040, rates of MASH will increase by 55%. Prior to that most liver diseases were caused by alcohol use and hepatitis C, a viral infection that primarily affects the liver.
MASH, a preventable form of liver disease previously called nonalcoholic fatty liver disease, is caused by a buildup of visceral fat cells that accumulate on top of the internal organs, in this case the liver, and keep it from functioning properly. The liver’s primary role is to filter blood, nutrients, and bile used for digestion, as well as to remove toxins from the body. Excess fat cells blanket the liver and keep it from working at full capacity.
Fat cells are also metabolically active and can cause a chronic state of inflammation in the part of the body where they reside. Over time, these fat cells can cause cirrhosis of the liver, or permanent scarring. Once patients reach this stage, the only option is a liver transplant.
New Research on GLP-1 Agonists and MASH
Until recently, the lone treatment for early-stage MASH was weight loss to reduce the number of fat cells that surround the internal organs. But new research has shown that glucagon-like peptide 1 (GLP-1) agonists can reduce and even reverse the condition. In a study published in April, researchers were able to show that semaglutide resolved fatty liver and inflammation in over 60% of cases and decreased scar tissue in just over a third of patients.
“These findings suggest that semaglutide may prevent fatty liver disease from progressing to cirrhosis and can indeed reverse the course of the disease,” said Arun J. Sanyal, MD, study author and director of the Stravitz-Sanyal Institute for Liver Disease and Metabolic Health at Virginia Commonwealth University in Richmond, Virginia.
Another study published last year had a similar finding, showing that GLP-1 agonists were associated with less progression of the disease and reduced mortality in patients with MASH and diabetes. Another large-scale observational study found that GLP-1s reduced the risk for hepatic failure, which occurs when the liver is unable to perform basic functions, as well as liver cancer, both of which are downstream consequences of MASH.
How GLP-1s Improve Liver Function
“These medications reduce fat burden, which results in fat loss everywhere, including around the liver,” said Ziyad Al-Aly, MD, an assistant professor in the Division of General Medicine & Geriatrics at Washington University School of Medicine in St. Louis. “When fat cells are reduced in size and volume, the normal liver cells have more room to grow and function.”
These medications also seem to work on reducing the inflammation and oxidative stress caused by metabolic disease, which allows for a better environment for the liver to function.
“Fat is not an inert tissue, it’s metabolically active, causing a slow burn to all the cells surrounding it,” said Al-Aly. These medications keep the disease from progressing and reduce scarring, which improves the damage that’s already been done, he said.
Changing How Liver Disease Is Diagnosed
Physicians need to be vigilant in the way that they screen for the condition, said Charu Sawhney, DO, MPH, of Harbor Health in Round Rock, Texas. She said that if liver enzymes appear even slightly elevated, there still could be a reason to utilize GLP-1s to prevent later-stage MASH.
“Normal levels for liver enzymes in some patients can be lower than what labs show,” said Sawhney. This is especially true if a patient has other metabolic risk factors such as diabetes, obesity, or high cholesterol.
If liver enzymes continue to go up even after diet and lifestyle changes, patients might require liver imaging, specifically a wave-based ultrasound called elastography, which measures the elasticity or stiffness of tissues on the liver and can judge if certain portions of it have scarred or hardened. When liver cells change texture and become harder, the scan can estimate levels of fibrosis and, therefore, the stage of MASH that a patient is in.
Additionally, the severity of fatty liver disease depends on other factors besides weight and can sometimes be surprising.
“How bad fatty liver disease is in a patient isn’t always related to how much weight someone has gained,” said Carolynn Francavilla, MD, a nationally recognized obesity physician who owns and operates Green Mountain Partners for Health and Colorado Weight Care, both in Denver.
It’s important for physicians to realize that some patients with fatty liver disease might not have obesity as would be expected. For these patients, adipose tissue seems to accumulate on the liver before it does on other parts of the body. This could be related to the quality of our food system, including the use of sugar substitutes like high fructose corn syrup, which research has shown is even harder on the liver. There might also be a genetic propensity toward fat storage around the organs.
A New Way to Treat MASH
, said Francavilla. Right now, there’s not an official approval from the US Food and Drug Administration (FDA) for prescribing GLP-1s in patients with MASH, but Francavilla hopes that it’s forthcoming.
“It will be really exciting to have these medications as a treatment option because right now there’s only one medication, and it’s for people who have pretty advanced fatty liver disease,” said Francavilla. This medication, called resmetirom, is approved by the FDA to target a protein in the liver to reduce fat and inflammation and scarring. But GLP-1s can be used much earlier to prevent the condition.
“With so many cases of MASH happening so much younger, it’s a disease that physicians really need to take seriously,” said Sawhney.
A version of this article appeared on Medscape.com.
In the past two decades, the global prevalence of metabolic dysfunction–associated steatohepatitis (MASH) has increased dramatically as a result of the obesity epidemic. Researchers project that by 2040, rates of MASH will increase by 55%. Prior to that most liver diseases were caused by alcohol use and hepatitis C, a viral infection that primarily affects the liver.
MASH, a preventable form of liver disease previously called nonalcoholic fatty liver disease, is caused by a buildup of visceral fat cells that accumulate on top of the internal organs, in this case the liver, and keep it from functioning properly. The liver’s primary role is to filter blood, nutrients, and bile used for digestion, as well as to remove toxins from the body. Excess fat cells blanket the liver and keep it from working at full capacity.
Fat cells are also metabolically active and can cause a chronic state of inflammation in the part of the body where they reside. Over time, these fat cells can cause cirrhosis of the liver, or permanent scarring. Once patients reach this stage, the only option is a liver transplant.
New Research on GLP-1 Agonists and MASH
Until recently, the lone treatment for early-stage MASH was weight loss to reduce the number of fat cells that surround the internal organs. But new research has shown that glucagon-like peptide 1 (GLP-1) agonists can reduce and even reverse the condition. In a study published in April, researchers were able to show that semaglutide resolved fatty liver and inflammation in over 60% of cases and decreased scar tissue in just over a third of patients.
“These findings suggest that semaglutide may prevent fatty liver disease from progressing to cirrhosis and can indeed reverse the course of the disease,” said Arun J. Sanyal, MD, study author and director of the Stravitz-Sanyal Institute for Liver Disease and Metabolic Health at Virginia Commonwealth University in Richmond, Virginia.
Another study published last year had a similar finding, showing that GLP-1 agonists were associated with less progression of the disease and reduced mortality in patients with MASH and diabetes. Another large-scale observational study found that GLP-1s reduced the risk for hepatic failure, which occurs when the liver is unable to perform basic functions, as well as liver cancer, both of which are downstream consequences of MASH.
How GLP-1s Improve Liver Function
“These medications reduce fat burden, which results in fat loss everywhere, including around the liver,” said Ziyad Al-Aly, MD, an assistant professor in the Division of General Medicine & Geriatrics at Washington University School of Medicine in St. Louis. “When fat cells are reduced in size and volume, the normal liver cells have more room to grow and function.”
These medications also seem to work on reducing the inflammation and oxidative stress caused by metabolic disease, which allows for a better environment for the liver to function.
“Fat is not an inert tissue, it’s metabolically active, causing a slow burn to all the cells surrounding it,” said Al-Aly. These medications keep the disease from progressing and reduce scarring, which improves the damage that’s already been done, he said.
Changing How Liver Disease Is Diagnosed
Physicians need to be vigilant in the way that they screen for the condition, said Charu Sawhney, DO, MPH, of Harbor Health in Round Rock, Texas. She said that if liver enzymes appear even slightly elevated, there still could be a reason to utilize GLP-1s to prevent later-stage MASH.
“Normal levels for liver enzymes in some patients can be lower than what labs show,” said Sawhney. This is especially true if a patient has other metabolic risk factors such as diabetes, obesity, or high cholesterol.
If liver enzymes continue to go up even after diet and lifestyle changes, patients might require liver imaging, specifically a wave-based ultrasound called elastography, which measures the elasticity or stiffness of tissues on the liver and can judge if certain portions of it have scarred or hardened. When liver cells change texture and become harder, the scan can estimate levels of fibrosis and, therefore, the stage of MASH that a patient is in.
Additionally, the severity of fatty liver disease depends on other factors besides weight and can sometimes be surprising.
“How bad fatty liver disease is in a patient isn’t always related to how much weight someone has gained,” said Carolynn Francavilla, MD, a nationally recognized obesity physician who owns and operates Green Mountain Partners for Health and Colorado Weight Care, both in Denver.
It’s important for physicians to realize that some patients with fatty liver disease might not have obesity as would be expected. For these patients, adipose tissue seems to accumulate on the liver before it does on other parts of the body. This could be related to the quality of our food system, including the use of sugar substitutes like high fructose corn syrup, which research has shown is even harder on the liver. There might also be a genetic propensity toward fat storage around the organs.
A New Way to Treat MASH
, said Francavilla. Right now, there’s not an official approval from the US Food and Drug Administration (FDA) for prescribing GLP-1s in patients with MASH, but Francavilla hopes that it’s forthcoming.
“It will be really exciting to have these medications as a treatment option because right now there’s only one medication, and it’s for people who have pretty advanced fatty liver disease,” said Francavilla. This medication, called resmetirom, is approved by the FDA to target a protein in the liver to reduce fat and inflammation and scarring. But GLP-1s can be used much earlier to prevent the condition.
“With so many cases of MASH happening so much younger, it’s a disease that physicians really need to take seriously,” said Sawhney.
A version of this article appeared on Medscape.com.
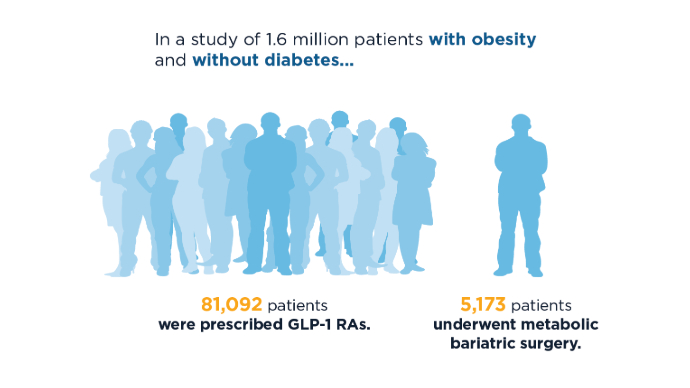
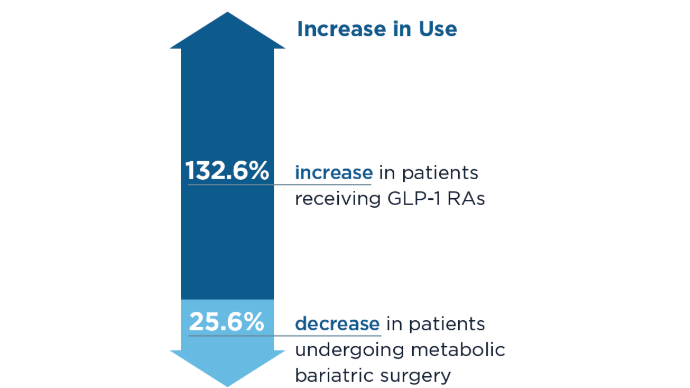
Do GLP-1s Lower CRC Risk in Patients With Obesity and T2D?
SAN DIEGO — new research showed.
CRC risk was also lower for patients taking GLP-1s than the general population.
“Our findings show we might need to evaluate these therapies beyond their glycemic or weight loss [effects],” said first author Omar Al Ta’ani, MD, of the Allegheny Health Network, Pittsburgh.
This supports future prospective studies examining GLP-1s for CRC reduction, added Ta’ani, who presented the results at Digestive Disease Week (DDW) 2025.
Patients with type 2 diabetes and obesity are known to have a higher risk for CRC, stemming from metabolic risk factors. Whereas prior studies suggested that GLP-1s decrease the risk for CRC compared with other antidiabetic medications, studies looking at the risk for CRC associated with bariatric surgery have had more mixed results, Ta’ani said.
For the comparison, Ta’ani and colleagues conducted a retrospective analysis of the TriNetX database, identifying patients with type 2 diabetes and obesity (body mass index [BMI] > 30) enrolled in the database between 2005 and 2019.
Overall, the study included 94,098 GLP-1 users and 24,969 patients who underwent bariatric surgery. Those with a prior history of CRC were excluded.
Using propensity score matching, patients treated with GLP-1s were matched 1:1 with patients who had bariatric surgery based on wide-ranging factors including age, race, gender, demographics, diseases, medications, personal and family history, and hemoglobin A1c.
After the propensity matching, each group included 21,022 patients. About 64% in each group were women; their median age was 53 years and about 65% were White.
Overall, the results showed that patients on GLP-1s had a significantly lower CRC risk compared with those who had bariatric surgery (adjusted hazard ratio [aHR], 0.29; P < .0001). The lower risk was also observed among those with high obesity (defined as BMI > 35) compared with those who had surgery (aHR, 0.39; P < .0001).
The results were consistent across genders; however, the differences between GLP-1s and bariatric surgery were not observed in the 18- to 45-year-old age group (BMI > 30, P = .0809; BMI > 35, P = .2318).
Compared with the general population, patients on GLP-1s also had a reduced risk for CRC (aHR, 0.28; P < .0001); however, the difference was not observed between the bariatric surgery group and the general population (aHR, 1.11; P = .3).
Among patients with type 2 diabetes with CRC and a BMI > 30, the 5-year mortality rate was lower in the GLP-1 group vs the bariatric surgery group (aHR, 0.42; P < .001).
Speculating on the mechanisms of GLP-1s that could result in a greater reduction in CRC risk, Ta’ani explained that the key pathways linking type 2 diabetes, obesity, and CRC include hyperinsulinemia, chronic inflammation, and impaired immune surveillance.
Studies have shown that GLP-1s may be more effective in addressing the collective pathways, he said. They “may improve insulin resistance and lower systemic inflammation.”
Furthermore, GLP1s “inhibit tumor pathways like Wnt/beta-catenin and PI3K/Akt/mTOR signaling, which promote apoptosis and reduce tumor cell proliferation,” he added.
Bariatric Surgery Findings Questioned
Meanwhile, “bariatric surgery’s impact on CRC remains mixed,” said Ta’ani.
Commenting on the study, Vance L. Albaugh, MD, an assistant professor of metabolic surgery at the Metamor Institute, Pennington Biomedical Research Center, Baton Rouge, Louisiana, noted that prior studies, including a recent meta-analysis, suggest a potential benefit of bariatric surgery in cancer prevention.
“I think the [current study] is interesting, but it’s been pretty [well-reported] that bariatric surgery does decrease cancer incidence, so I find it questionable that this study shows the opposite of what’s in the literature,” Albaugh, an obesity medicine specialist and bariatric surgeon, said in an interview.
Ta’ani acknowledged the study’s important limitations, including that with a retrospective design, causality cannot be firmly established.
And, as noted by an audience member in the session’s Q&A, the study ended in 2019, which was before GLP-1s had taken off as anti-obesity drugs and before US Food and Drug Administration approvals for weight loss.
Participants were matched based on BMI, however, Ta’ani pointed out.
Albaugh agreed that the study ending in 2019 was a notable limitation. However, the relatively long study period — extending from 2005 to 2019 — was a strength.
“It’s nice to have a very long period to capture people who are diagnosed, because it takes a long time to develop CRC,” he said. “To evaluate effects [of more recent drug regimens], you would not be able to have the follow-up they had.”
Other study limitations included the need to adjust for ranges of obesity severity, said Albaugh. “The risk of colorectal cancer is probably much different for someone with a BMI of 60 vs a BMI of 30.”
Ultimately, a key question the study results raise is whether GLP-1 drugs have protective effects above and beyond that of weight loss, he said.
“I think that’s a very exciting question and that’s what I think the researchers’ next work should really focus on.”
Ta’ani had no disclosures to report. Albaugh reported that he had consulted for Novo Nordisk.
A version of this article appeared on Medscape.com.
SAN DIEGO — new research showed.
CRC risk was also lower for patients taking GLP-1s than the general population.
“Our findings show we might need to evaluate these therapies beyond their glycemic or weight loss [effects],” said first author Omar Al Ta’ani, MD, of the Allegheny Health Network, Pittsburgh.
This supports future prospective studies examining GLP-1s for CRC reduction, added Ta’ani, who presented the results at Digestive Disease Week (DDW) 2025.
Patients with type 2 diabetes and obesity are known to have a higher risk for CRC, stemming from metabolic risk factors. Whereas prior studies suggested that GLP-1s decrease the risk for CRC compared with other antidiabetic medications, studies looking at the risk for CRC associated with bariatric surgery have had more mixed results, Ta’ani said.
For the comparison, Ta’ani and colleagues conducted a retrospective analysis of the TriNetX database, identifying patients with type 2 diabetes and obesity (body mass index [BMI] > 30) enrolled in the database between 2005 and 2019.
Overall, the study included 94,098 GLP-1 users and 24,969 patients who underwent bariatric surgery. Those with a prior history of CRC were excluded.
Using propensity score matching, patients treated with GLP-1s were matched 1:1 with patients who had bariatric surgery based on wide-ranging factors including age, race, gender, demographics, diseases, medications, personal and family history, and hemoglobin A1c.
After the propensity matching, each group included 21,022 patients. About 64% in each group were women; their median age was 53 years and about 65% were White.
Overall, the results showed that patients on GLP-1s had a significantly lower CRC risk compared with those who had bariatric surgery (adjusted hazard ratio [aHR], 0.29; P < .0001). The lower risk was also observed among those with high obesity (defined as BMI > 35) compared with those who had surgery (aHR, 0.39; P < .0001).
The results were consistent across genders; however, the differences between GLP-1s and bariatric surgery were not observed in the 18- to 45-year-old age group (BMI > 30, P = .0809; BMI > 35, P = .2318).
Compared with the general population, patients on GLP-1s also had a reduced risk for CRC (aHR, 0.28; P < .0001); however, the difference was not observed between the bariatric surgery group and the general population (aHR, 1.11; P = .3).
Among patients with type 2 diabetes with CRC and a BMI > 30, the 5-year mortality rate was lower in the GLP-1 group vs the bariatric surgery group (aHR, 0.42; P < .001).
Speculating on the mechanisms of GLP-1s that could result in a greater reduction in CRC risk, Ta’ani explained that the key pathways linking type 2 diabetes, obesity, and CRC include hyperinsulinemia, chronic inflammation, and impaired immune surveillance.
Studies have shown that GLP-1s may be more effective in addressing the collective pathways, he said. They “may improve insulin resistance and lower systemic inflammation.”
Furthermore, GLP1s “inhibit tumor pathways like Wnt/beta-catenin and PI3K/Akt/mTOR signaling, which promote apoptosis and reduce tumor cell proliferation,” he added.
Bariatric Surgery Findings Questioned
Meanwhile, “bariatric surgery’s impact on CRC remains mixed,” said Ta’ani.
Commenting on the study, Vance L. Albaugh, MD, an assistant professor of metabolic surgery at the Metamor Institute, Pennington Biomedical Research Center, Baton Rouge, Louisiana, noted that prior studies, including a recent meta-analysis, suggest a potential benefit of bariatric surgery in cancer prevention.
“I think the [current study] is interesting, but it’s been pretty [well-reported] that bariatric surgery does decrease cancer incidence, so I find it questionable that this study shows the opposite of what’s in the literature,” Albaugh, an obesity medicine specialist and bariatric surgeon, said in an interview.
Ta’ani acknowledged the study’s important limitations, including that with a retrospective design, causality cannot be firmly established.
And, as noted by an audience member in the session’s Q&A, the study ended in 2019, which was before GLP-1s had taken off as anti-obesity drugs and before US Food and Drug Administration approvals for weight loss.
Participants were matched based on BMI, however, Ta’ani pointed out.
Albaugh agreed that the study ending in 2019 was a notable limitation. However, the relatively long study period — extending from 2005 to 2019 — was a strength.
“It’s nice to have a very long period to capture people who are diagnosed, because it takes a long time to develop CRC,” he said. “To evaluate effects [of more recent drug regimens], you would not be able to have the follow-up they had.”
Other study limitations included the need to adjust for ranges of obesity severity, said Albaugh. “The risk of colorectal cancer is probably much different for someone with a BMI of 60 vs a BMI of 30.”
Ultimately, a key question the study results raise is whether GLP-1 drugs have protective effects above and beyond that of weight loss, he said.
“I think that’s a very exciting question and that’s what I think the researchers’ next work should really focus on.”
Ta’ani had no disclosures to report. Albaugh reported that he had consulted for Novo Nordisk.
A version of this article appeared on Medscape.com.
SAN DIEGO — new research showed.
CRC risk was also lower for patients taking GLP-1s than the general population.
“Our findings show we might need to evaluate these therapies beyond their glycemic or weight loss [effects],” said first author Omar Al Ta’ani, MD, of the Allegheny Health Network, Pittsburgh.
This supports future prospective studies examining GLP-1s for CRC reduction, added Ta’ani, who presented the results at Digestive Disease Week (DDW) 2025.
Patients with type 2 diabetes and obesity are known to have a higher risk for CRC, stemming from metabolic risk factors. Whereas prior studies suggested that GLP-1s decrease the risk for CRC compared with other antidiabetic medications, studies looking at the risk for CRC associated with bariatric surgery have had more mixed results, Ta’ani said.
For the comparison, Ta’ani and colleagues conducted a retrospective analysis of the TriNetX database, identifying patients with type 2 diabetes and obesity (body mass index [BMI] > 30) enrolled in the database between 2005 and 2019.
Overall, the study included 94,098 GLP-1 users and 24,969 patients who underwent bariatric surgery. Those with a prior history of CRC were excluded.
Using propensity score matching, patients treated with GLP-1s were matched 1:1 with patients who had bariatric surgery based on wide-ranging factors including age, race, gender, demographics, diseases, medications, personal and family history, and hemoglobin A1c.
After the propensity matching, each group included 21,022 patients. About 64% in each group were women; their median age was 53 years and about 65% were White.
Overall, the results showed that patients on GLP-1s had a significantly lower CRC risk compared with those who had bariatric surgery (adjusted hazard ratio [aHR], 0.29; P < .0001). The lower risk was also observed among those with high obesity (defined as BMI > 35) compared with those who had surgery (aHR, 0.39; P < .0001).
The results were consistent across genders; however, the differences between GLP-1s and bariatric surgery were not observed in the 18- to 45-year-old age group (BMI > 30, P = .0809; BMI > 35, P = .2318).
Compared with the general population, patients on GLP-1s also had a reduced risk for CRC (aHR, 0.28; P < .0001); however, the difference was not observed between the bariatric surgery group and the general population (aHR, 1.11; P = .3).
Among patients with type 2 diabetes with CRC and a BMI > 30, the 5-year mortality rate was lower in the GLP-1 group vs the bariatric surgery group (aHR, 0.42; P < .001).
Speculating on the mechanisms of GLP-1s that could result in a greater reduction in CRC risk, Ta’ani explained that the key pathways linking type 2 diabetes, obesity, and CRC include hyperinsulinemia, chronic inflammation, and impaired immune surveillance.
Studies have shown that GLP-1s may be more effective in addressing the collective pathways, he said. They “may improve insulin resistance and lower systemic inflammation.”
Furthermore, GLP1s “inhibit tumor pathways like Wnt/beta-catenin and PI3K/Akt/mTOR signaling, which promote apoptosis and reduce tumor cell proliferation,” he added.
Bariatric Surgery Findings Questioned
Meanwhile, “bariatric surgery’s impact on CRC remains mixed,” said Ta’ani.
Commenting on the study, Vance L. Albaugh, MD, an assistant professor of metabolic surgery at the Metamor Institute, Pennington Biomedical Research Center, Baton Rouge, Louisiana, noted that prior studies, including a recent meta-analysis, suggest a potential benefit of bariatric surgery in cancer prevention.
“I think the [current study] is interesting, but it’s been pretty [well-reported] that bariatric surgery does decrease cancer incidence, so I find it questionable that this study shows the opposite of what’s in the literature,” Albaugh, an obesity medicine specialist and bariatric surgeon, said in an interview.
Ta’ani acknowledged the study’s important limitations, including that with a retrospective design, causality cannot be firmly established.
And, as noted by an audience member in the session’s Q&A, the study ended in 2019, which was before GLP-1s had taken off as anti-obesity drugs and before US Food and Drug Administration approvals for weight loss.
Participants were matched based on BMI, however, Ta’ani pointed out.
Albaugh agreed that the study ending in 2019 was a notable limitation. However, the relatively long study period — extending from 2005 to 2019 — was a strength.
“It’s nice to have a very long period to capture people who are diagnosed, because it takes a long time to develop CRC,” he said. “To evaluate effects [of more recent drug regimens], you would not be able to have the follow-up they had.”
Other study limitations included the need to adjust for ranges of obesity severity, said Albaugh. “The risk of colorectal cancer is probably much different for someone with a BMI of 60 vs a BMI of 30.”
Ultimately, a key question the study results raise is whether GLP-1 drugs have protective effects above and beyond that of weight loss, he said.
“I think that’s a very exciting question and that’s what I think the researchers’ next work should really focus on.”
Ta’ani had no disclosures to report. Albaugh reported that he had consulted for Novo Nordisk.
A version of this article appeared on Medscape.com.
FROM DDW 2025
Obesity Management in the Era of GLP-1 RAs: The Role of GLP-1 RAs
Obesity Management in the Era of GLP-1 RAs: The Role of GLP-1 RAs
Click to view more from Gastroenterology Data Trends 2025.
- Lin K, Mehrotra A, Tsai TC. Metabolic Bariatric Surgery in the Era of GLP-1 Receptor Agonists for Obesity Management. JAMA Netw Open. 2024;7(10):e2441380. doi:10.1001/jamanetworkopen.2024.41380
- Camilleri M, El-Omar EM. Ten reasons gastroenterologists and hepatologists should be treating obesity. Gut. 2023;72(6):1033-1038. doi:10.1136/gutjnl-2023-329639
- Camilleri M. Definite benefits of GLP-1 receptor agonists: what is the risk of gastroparesis and lung aspiration? Gut. 2024. doi:10.1136/gutjnl-2024-333036
- Camilleri M, Carlson P, Dilmaghani S. Letter to the Editor. Prevalence and variations in gastric emptying delay in response to GLP-1 receptor agonist liraglutide. Obesity (Silver Spring). 2024;32(2):232-233. doi:10.1002/oby.23941
- Camilleri, M. Incretin impact on gastric function in obesity: physiology, and pharmacological, surgical and endoscopic treatments. J Physiol. 2024.doi:10.1113/JP287535
- Kindel TL, Wang AY, Wadhwa A, et al; American Gastroenterological Association; American Society for Metabolic and Bariatric Surgery; American Society of Anesthesiologists; International Society of Perioperative Care of Patients with Obesity;
Society of American Gastrointestinal and Endoscopic Surgeons. Multisociety Clinical Practice Guidance for the Safe Use of Glucagon-like Peptide-1 Receptor Agonists in the Perioperative Period. Clin Gastroenterol Hepatol. 2024:S1542-3565(24)00910-8. doi:10.1016/j.cgh.2024.10.003
Click to view more from Gastroenterology Data Trends 2025.
Click to view more from Gastroenterology Data Trends 2025.
- Lin K, Mehrotra A, Tsai TC. Metabolic Bariatric Surgery in the Era of GLP-1 Receptor Agonists for Obesity Management. JAMA Netw Open. 2024;7(10):e2441380. doi:10.1001/jamanetworkopen.2024.41380
- Camilleri M, El-Omar EM. Ten reasons gastroenterologists and hepatologists should be treating obesity. Gut. 2023;72(6):1033-1038. doi:10.1136/gutjnl-2023-329639
- Camilleri M. Definite benefits of GLP-1 receptor agonists: what is the risk of gastroparesis and lung aspiration? Gut. 2024. doi:10.1136/gutjnl-2024-333036
- Camilleri M, Carlson P, Dilmaghani S. Letter to the Editor. Prevalence and variations in gastric emptying delay in response to GLP-1 receptor agonist liraglutide. Obesity (Silver Spring). 2024;32(2):232-233. doi:10.1002/oby.23941
- Camilleri, M. Incretin impact on gastric function in obesity: physiology, and pharmacological, surgical and endoscopic treatments. J Physiol. 2024.doi:10.1113/JP287535
- Kindel TL, Wang AY, Wadhwa A, et al; American Gastroenterological Association; American Society for Metabolic and Bariatric Surgery; American Society of Anesthesiologists; International Society of Perioperative Care of Patients with Obesity;
Society of American Gastrointestinal and Endoscopic Surgeons. Multisociety Clinical Practice Guidance for the Safe Use of Glucagon-like Peptide-1 Receptor Agonists in the Perioperative Period. Clin Gastroenterol Hepatol. 2024:S1542-3565(24)00910-8. doi:10.1016/j.cgh.2024.10.003
- Lin K, Mehrotra A, Tsai TC. Metabolic Bariatric Surgery in the Era of GLP-1 Receptor Agonists for Obesity Management. JAMA Netw Open. 2024;7(10):e2441380. doi:10.1001/jamanetworkopen.2024.41380
- Camilleri M, El-Omar EM. Ten reasons gastroenterologists and hepatologists should be treating obesity. Gut. 2023;72(6):1033-1038. doi:10.1136/gutjnl-2023-329639
- Camilleri M. Definite benefits of GLP-1 receptor agonists: what is the risk of gastroparesis and lung aspiration? Gut. 2024. doi:10.1136/gutjnl-2024-333036
- Camilleri M, Carlson P, Dilmaghani S. Letter to the Editor. Prevalence and variations in gastric emptying delay in response to GLP-1 receptor agonist liraglutide. Obesity (Silver Spring). 2024;32(2):232-233. doi:10.1002/oby.23941
- Camilleri, M. Incretin impact on gastric function in obesity: physiology, and pharmacological, surgical and endoscopic treatments. J Physiol. 2024.doi:10.1113/JP287535
- Kindel TL, Wang AY, Wadhwa A, et al; American Gastroenterological Association; American Society for Metabolic and Bariatric Surgery; American Society of Anesthesiologists; International Society of Perioperative Care of Patients with Obesity;
Society of American Gastrointestinal and Endoscopic Surgeons. Multisociety Clinical Practice Guidance for the Safe Use of Glucagon-like Peptide-1 Receptor Agonists in the Perioperative Period. Clin Gastroenterol Hepatol. 2024:S1542-3565(24)00910-8. doi:10.1016/j.cgh.2024.10.003
Obesity Management in the Era of GLP-1 RAs: The Role of GLP-1 RAs
Obesity Management in the Era of GLP-1 RAs: The Role of GLP-1 RAs
Intermittent Fasting Outperforms Daily Calorie Cutting for Weight Loss
a randomized study found.
A 4:3 IMF program produced modestly superior weight loss than DCR of 2.89 kg over 12 months in the context of a guidelines-based, high-intensity, comprehensive behavioral weight loss program, according to Danielle M. Ostendorf, PhD, MS, co–lead author and an assistant professor at the University of Tennessee, Knoxville, and Victoria Catenacci, MD, study principal investigator, co–lead author, and an associate professor located at the University of Colorado Anschutz Medical Campus, Aurora.
The study, published in Annals of Internal Medicine, found that objectively measured percentage caloric restriction was greater in the 4:3 IMF group, whereas there was no between-group difference in change in total moderate to vigorous physical activity, suggesting that differences in weight loss may have been caused by greater adherence to 4:3 IMF. The 4:3 IMF program was well tolerated and attrition was lower in this group: 19% for IMF group vs 30% for DCR group.
The authors noted that alternative patterns for restricting dietary energy intake are gaining attention owing to the difficulty of adhering to a reduced-calorie diet daily, with most adults who lose weight through DCR showing significant weight regain a year later.
According to Ostendorf and Catenacci, fasting strategies “come in two different flavors and oftentimes get confused in the lay press and by patients and researchers. And there is a difference between IMF and time-restricted eating (TRE),” they said in an interview. “TRE involves limiting the daily window of food intake to 8-10 hours or less on most days of the week — for example, 16:8 or 14:10 strategies. TRE is done every day, consistently and involves eating in the predefined window, and fasting outside of that window.”
IMF is a more periodic and significant fast and involves cycling between complete or near-complete (> 75%) energy restriction on fast days and ad libitum energy intake on nonfast days.
An appealing feature of IMF is that dieters do not have to focus on counting calories and restricting intake every day as they do with DCR, the authors wrote. Furthermore, the periodic nature of fasting is simpler and may mitigate the constant hunger associated with DCR.
Some said the diet was dreadful, but many said it was the easiest diet they had ever been on. “But it did take time for people to adjust to this strategy,” Catenacci said. “It was reassuring to see no evidence of increased binge-eating behaviors.”
Although objectively measured adherence to the targeted energy deficit (percentage caloric restriction from baseline) was below the target of 34.3% in both groups, the 4:3 IMF group showed greater percentage caloric restriction over 12 months. This suggests that, on average, the 4:3 IMF group may be more sustainable over a year than the DCR group. However, weight loss varied in both groups. Future studies should evaluate biological and behavioral predictors of response to both 4:3 IMF and DCR groups in order to personalize recommendations for weight loss.
Study Details
The investigators randomized 165 patients at the University of Colorado Anschutz Medical Campus, with a mean age of 42 years (18-60), a mean baseline weight of 97.4 kg, and a mean baseline body mass index (BMI) of 34.1 to IMF (n = 84) or DCR (n = 81). Of these, 74% were women and 86% were White individuals, and 125 (76%) completed the trial.
The 4:3 IMF group restricted energy intake by 80% on 3 nonconsecutive fast days per week, with ad libitum intake on the other 4 days (4:3 IMF). The 80% calorie reduction fasting corresponded to about 400-600 kcals/d for women and 500-700 kcals/d for men.
“Participants were only required to count calories on their fast days, which is part of the appeal,” Ostendorf said. Although permitted to eat what they wanted on nonfast days, participants were encouraged to make healthy food choices and consume healthy portion sizes.
For its part, the DCR group reduced daily energy intake by 34% to match the weekly energy deficit of 4:3 IMF.
Both groups participated in a high-intensity comprehensive weight loss program with group-based behavioral support and a recommended increase in moderate-intensity physical activity to 300 min/wk.
On the primary endpoint, the 4:3 IMF group showed a weight loss of 7.7 kg (95% CI, –9.6 to –5.9 kg) compared with 4.8 kg (95% CI, –6.8 to –2.8 kg, P =.040) in the DCR group at 12 months. The percentage change in body weight from baseline was –7.6% (95% CI, –9.5% to –5.7%) in the 4:3 IMF group and –5% (95% CI, –6.9% to –3.1%) in the DCR group.
At 12 months, 58% (n = 50) of participants in the 4:3 IMF group achieved weight loss of at least 5% vs 47% (n = 27) of those in the DCR group. In addition, 38% (n = 26) of participants in the 4:3 IMF group achieved weight loss of at least 10% at 12 months vs 16% (n = 9) of those in the DCR group. Changes in body composition, BMI, and waist circumference also tended to favor the 4:3 IMF group.
On other 12-month measures, point estimates of change in systolic blood pressure, total and low-density lipoprotein cholesterol levels, triglyceride level, homeostasis model assessment of insulin resistance, fasting glucose level, and hemoglobin A1c level favored 4:3 IMF. Point estimates of change in diastolic blood pressure and high-density lipoprotein cholesterol level favored DCR.
Currently lacking, the authors said, are data on safety in children and older adults, and adults affected by a long list of conditions: Diabetes, cardiovascular disease, kidney disease (stage 4 or 5), cancer, and eating disorders. Also, people of normal weight or only mild overweight, and pregnant or lactating women. “There have been concerns about IMF causing eating disorders, so we did not include people with eating disorders in our study,” Ostendorf and Catenacci said.
Offering an outside perspective on the findings, James O. Hill, PhD, director of the Nutrition Obesity Research Center and a professor at the University of Alabama at Birmingham believes IMF is a viable option for people trying to lose weight and has prescribed this approach for some in his practice. “But there is no one strategy that works for everyone,” he said in an interview. “I recommend IMF as a science-based strategy that can be effective for some people, and I think it should be on the list of science-based tools that people can consider using.” But as it won’t work for everyone, “we need to consider both metabolic success and behavioral success. In other words, would it be more effective if people could do it and how easy or hard is it for people to do?”
Audra Wilson, MS, RD, a bariatric dietitian at Northwestern Medicine Delnor Hospital in Geneva, Illinois, who was not involved in the study, expressed more reservations. “We do not specifically recommend intermittent fasting at Northwestern Medicine. There is no set protocol for this diet, and it can vary in ways that can limit nutrition to the point where we are not meeting needs on a regular basis,” she said in an interview.
Moreover, this study did not specify exact nutritional recommendations for participants but merely reduced overall caloric intake. “Although intermittent fasting may be helpful to some, in my nearly 10 years of experience I have not seen it be effective for many and especially not long term,” Wilson added.
Concerningly, IMF can foster disordered eating patterns of restriction followed by binging. “Although a balanced diet is more difficult to achieve, guidance from professionals like dietitians can give patients the tools to achieve balance, meet all nutrient needs, achieve satiety, and maybe most importantly, have a better relationship with food,” she said.
As for the influence of metabolic factors that may be associated with better weight loss, Ostendorf said, “be on the lookout for future publications in this area. We are analyzing data around changes in energy expenditure and changes in hunger-related hormones, among others.” A colleague is collecting biological samples to study genetics in this context. “However, in general, it appeared that the difference in weight loss was due to a greater caloric deficit in the 4:3 IMF group.”
Ostendorf and Catenacci are currently conducting a pilot study testing 4:3 IMF in breast cancer survivors. “We think this is a promising strategy for weight loss in breast cancer survivors who struggle with overweight/obesity in addition to their cancer diagnosis,” Ostendorf said.
This study was funded by the National Institute of Diabetes and Digestive and Kidney Diseases. Ostendorf, Catenacci, Hill, and Wilson disclosed no relevant financial conflicts of interest.
A version of this article appeared on Medscape.com.
a randomized study found.
A 4:3 IMF program produced modestly superior weight loss than DCR of 2.89 kg over 12 months in the context of a guidelines-based, high-intensity, comprehensive behavioral weight loss program, according to Danielle M. Ostendorf, PhD, MS, co–lead author and an assistant professor at the University of Tennessee, Knoxville, and Victoria Catenacci, MD, study principal investigator, co–lead author, and an associate professor located at the University of Colorado Anschutz Medical Campus, Aurora.
The study, published in Annals of Internal Medicine, found that objectively measured percentage caloric restriction was greater in the 4:3 IMF group, whereas there was no between-group difference in change in total moderate to vigorous physical activity, suggesting that differences in weight loss may have been caused by greater adherence to 4:3 IMF. The 4:3 IMF program was well tolerated and attrition was lower in this group: 19% for IMF group vs 30% for DCR group.
The authors noted that alternative patterns for restricting dietary energy intake are gaining attention owing to the difficulty of adhering to a reduced-calorie diet daily, with most adults who lose weight through DCR showing significant weight regain a year later.
According to Ostendorf and Catenacci, fasting strategies “come in two different flavors and oftentimes get confused in the lay press and by patients and researchers. And there is a difference between IMF and time-restricted eating (TRE),” they said in an interview. “TRE involves limiting the daily window of food intake to 8-10 hours or less on most days of the week — for example, 16:8 or 14:10 strategies. TRE is done every day, consistently and involves eating in the predefined window, and fasting outside of that window.”
IMF is a more periodic and significant fast and involves cycling between complete or near-complete (> 75%) energy restriction on fast days and ad libitum energy intake on nonfast days.
An appealing feature of IMF is that dieters do not have to focus on counting calories and restricting intake every day as they do with DCR, the authors wrote. Furthermore, the periodic nature of fasting is simpler and may mitigate the constant hunger associated with DCR.
Some said the diet was dreadful, but many said it was the easiest diet they had ever been on. “But it did take time for people to adjust to this strategy,” Catenacci said. “It was reassuring to see no evidence of increased binge-eating behaviors.”
Although objectively measured adherence to the targeted energy deficit (percentage caloric restriction from baseline) was below the target of 34.3% in both groups, the 4:3 IMF group showed greater percentage caloric restriction over 12 months. This suggests that, on average, the 4:3 IMF group may be more sustainable over a year than the DCR group. However, weight loss varied in both groups. Future studies should evaluate biological and behavioral predictors of response to both 4:3 IMF and DCR groups in order to personalize recommendations for weight loss.
Study Details
The investigators randomized 165 patients at the University of Colorado Anschutz Medical Campus, with a mean age of 42 years (18-60), a mean baseline weight of 97.4 kg, and a mean baseline body mass index (BMI) of 34.1 to IMF (n = 84) or DCR (n = 81). Of these, 74% were women and 86% were White individuals, and 125 (76%) completed the trial.
The 4:3 IMF group restricted energy intake by 80% on 3 nonconsecutive fast days per week, with ad libitum intake on the other 4 days (4:3 IMF). The 80% calorie reduction fasting corresponded to about 400-600 kcals/d for women and 500-700 kcals/d for men.
“Participants were only required to count calories on their fast days, which is part of the appeal,” Ostendorf said. Although permitted to eat what they wanted on nonfast days, participants were encouraged to make healthy food choices and consume healthy portion sizes.
For its part, the DCR group reduced daily energy intake by 34% to match the weekly energy deficit of 4:3 IMF.
Both groups participated in a high-intensity comprehensive weight loss program with group-based behavioral support and a recommended increase in moderate-intensity physical activity to 300 min/wk.
On the primary endpoint, the 4:3 IMF group showed a weight loss of 7.7 kg (95% CI, –9.6 to –5.9 kg) compared with 4.8 kg (95% CI, –6.8 to –2.8 kg, P =.040) in the DCR group at 12 months. The percentage change in body weight from baseline was –7.6% (95% CI, –9.5% to –5.7%) in the 4:3 IMF group and –5% (95% CI, –6.9% to –3.1%) in the DCR group.
At 12 months, 58% (n = 50) of participants in the 4:3 IMF group achieved weight loss of at least 5% vs 47% (n = 27) of those in the DCR group. In addition, 38% (n = 26) of participants in the 4:3 IMF group achieved weight loss of at least 10% at 12 months vs 16% (n = 9) of those in the DCR group. Changes in body composition, BMI, and waist circumference also tended to favor the 4:3 IMF group.
On other 12-month measures, point estimates of change in systolic blood pressure, total and low-density lipoprotein cholesterol levels, triglyceride level, homeostasis model assessment of insulin resistance, fasting glucose level, and hemoglobin A1c level favored 4:3 IMF. Point estimates of change in diastolic blood pressure and high-density lipoprotein cholesterol level favored DCR.
Currently lacking, the authors said, are data on safety in children and older adults, and adults affected by a long list of conditions: Diabetes, cardiovascular disease, kidney disease (stage 4 or 5), cancer, and eating disorders. Also, people of normal weight or only mild overweight, and pregnant or lactating women. “There have been concerns about IMF causing eating disorders, so we did not include people with eating disorders in our study,” Ostendorf and Catenacci said.
Offering an outside perspective on the findings, James O. Hill, PhD, director of the Nutrition Obesity Research Center and a professor at the University of Alabama at Birmingham believes IMF is a viable option for people trying to lose weight and has prescribed this approach for some in his practice. “But there is no one strategy that works for everyone,” he said in an interview. “I recommend IMF as a science-based strategy that can be effective for some people, and I think it should be on the list of science-based tools that people can consider using.” But as it won’t work for everyone, “we need to consider both metabolic success and behavioral success. In other words, would it be more effective if people could do it and how easy or hard is it for people to do?”
Audra Wilson, MS, RD, a bariatric dietitian at Northwestern Medicine Delnor Hospital in Geneva, Illinois, who was not involved in the study, expressed more reservations. “We do not specifically recommend intermittent fasting at Northwestern Medicine. There is no set protocol for this diet, and it can vary in ways that can limit nutrition to the point where we are not meeting needs on a regular basis,” she said in an interview.
Moreover, this study did not specify exact nutritional recommendations for participants but merely reduced overall caloric intake. “Although intermittent fasting may be helpful to some, in my nearly 10 years of experience I have not seen it be effective for many and especially not long term,” Wilson added.
Concerningly, IMF can foster disordered eating patterns of restriction followed by binging. “Although a balanced diet is more difficult to achieve, guidance from professionals like dietitians can give patients the tools to achieve balance, meet all nutrient needs, achieve satiety, and maybe most importantly, have a better relationship with food,” she said.
As for the influence of metabolic factors that may be associated with better weight loss, Ostendorf said, “be on the lookout for future publications in this area. We are analyzing data around changes in energy expenditure and changes in hunger-related hormones, among others.” A colleague is collecting biological samples to study genetics in this context. “However, in general, it appeared that the difference in weight loss was due to a greater caloric deficit in the 4:3 IMF group.”
Ostendorf and Catenacci are currently conducting a pilot study testing 4:3 IMF in breast cancer survivors. “We think this is a promising strategy for weight loss in breast cancer survivors who struggle with overweight/obesity in addition to their cancer diagnosis,” Ostendorf said.
This study was funded by the National Institute of Diabetes and Digestive and Kidney Diseases. Ostendorf, Catenacci, Hill, and Wilson disclosed no relevant financial conflicts of interest.
A version of this article appeared on Medscape.com.
a randomized study found.
A 4:3 IMF program produced modestly superior weight loss than DCR of 2.89 kg over 12 months in the context of a guidelines-based, high-intensity, comprehensive behavioral weight loss program, according to Danielle M. Ostendorf, PhD, MS, co–lead author and an assistant professor at the University of Tennessee, Knoxville, and Victoria Catenacci, MD, study principal investigator, co–lead author, and an associate professor located at the University of Colorado Anschutz Medical Campus, Aurora.
The study, published in Annals of Internal Medicine, found that objectively measured percentage caloric restriction was greater in the 4:3 IMF group, whereas there was no between-group difference in change in total moderate to vigorous physical activity, suggesting that differences in weight loss may have been caused by greater adherence to 4:3 IMF. The 4:3 IMF program was well tolerated and attrition was lower in this group: 19% for IMF group vs 30% for DCR group.
The authors noted that alternative patterns for restricting dietary energy intake are gaining attention owing to the difficulty of adhering to a reduced-calorie diet daily, with most adults who lose weight through DCR showing significant weight regain a year later.
According to Ostendorf and Catenacci, fasting strategies “come in two different flavors and oftentimes get confused in the lay press and by patients and researchers. And there is a difference between IMF and time-restricted eating (TRE),” they said in an interview. “TRE involves limiting the daily window of food intake to 8-10 hours or less on most days of the week — for example, 16:8 or 14:10 strategies. TRE is done every day, consistently and involves eating in the predefined window, and fasting outside of that window.”
IMF is a more periodic and significant fast and involves cycling between complete or near-complete (> 75%) energy restriction on fast days and ad libitum energy intake on nonfast days.
An appealing feature of IMF is that dieters do not have to focus on counting calories and restricting intake every day as they do with DCR, the authors wrote. Furthermore, the periodic nature of fasting is simpler and may mitigate the constant hunger associated with DCR.
Some said the diet was dreadful, but many said it was the easiest diet they had ever been on. “But it did take time for people to adjust to this strategy,” Catenacci said. “It was reassuring to see no evidence of increased binge-eating behaviors.”
Although objectively measured adherence to the targeted energy deficit (percentage caloric restriction from baseline) was below the target of 34.3% in both groups, the 4:3 IMF group showed greater percentage caloric restriction over 12 months. This suggests that, on average, the 4:3 IMF group may be more sustainable over a year than the DCR group. However, weight loss varied in both groups. Future studies should evaluate biological and behavioral predictors of response to both 4:3 IMF and DCR groups in order to personalize recommendations for weight loss.
Study Details
The investigators randomized 165 patients at the University of Colorado Anschutz Medical Campus, with a mean age of 42 years (18-60), a mean baseline weight of 97.4 kg, and a mean baseline body mass index (BMI) of 34.1 to IMF (n = 84) or DCR (n = 81). Of these, 74% were women and 86% were White individuals, and 125 (76%) completed the trial.
The 4:3 IMF group restricted energy intake by 80% on 3 nonconsecutive fast days per week, with ad libitum intake on the other 4 days (4:3 IMF). The 80% calorie reduction fasting corresponded to about 400-600 kcals/d for women and 500-700 kcals/d for men.
“Participants were only required to count calories on their fast days, which is part of the appeal,” Ostendorf said. Although permitted to eat what they wanted on nonfast days, participants were encouraged to make healthy food choices and consume healthy portion sizes.
For its part, the DCR group reduced daily energy intake by 34% to match the weekly energy deficit of 4:3 IMF.
Both groups participated in a high-intensity comprehensive weight loss program with group-based behavioral support and a recommended increase in moderate-intensity physical activity to 300 min/wk.
On the primary endpoint, the 4:3 IMF group showed a weight loss of 7.7 kg (95% CI, –9.6 to –5.9 kg) compared with 4.8 kg (95% CI, –6.8 to –2.8 kg, P =.040) in the DCR group at 12 months. The percentage change in body weight from baseline was –7.6% (95% CI, –9.5% to –5.7%) in the 4:3 IMF group and –5% (95% CI, –6.9% to –3.1%) in the DCR group.
At 12 months, 58% (n = 50) of participants in the 4:3 IMF group achieved weight loss of at least 5% vs 47% (n = 27) of those in the DCR group. In addition, 38% (n = 26) of participants in the 4:3 IMF group achieved weight loss of at least 10% at 12 months vs 16% (n = 9) of those in the DCR group. Changes in body composition, BMI, and waist circumference also tended to favor the 4:3 IMF group.
On other 12-month measures, point estimates of change in systolic blood pressure, total and low-density lipoprotein cholesterol levels, triglyceride level, homeostasis model assessment of insulin resistance, fasting glucose level, and hemoglobin A1c level favored 4:3 IMF. Point estimates of change in diastolic blood pressure and high-density lipoprotein cholesterol level favored DCR.
Currently lacking, the authors said, are data on safety in children and older adults, and adults affected by a long list of conditions: Diabetes, cardiovascular disease, kidney disease (stage 4 or 5), cancer, and eating disorders. Also, people of normal weight or only mild overweight, and pregnant or lactating women. “There have been concerns about IMF causing eating disorders, so we did not include people with eating disorders in our study,” Ostendorf and Catenacci said.
Offering an outside perspective on the findings, James O. Hill, PhD, director of the Nutrition Obesity Research Center and a professor at the University of Alabama at Birmingham believes IMF is a viable option for people trying to lose weight and has prescribed this approach for some in his practice. “But there is no one strategy that works for everyone,” he said in an interview. “I recommend IMF as a science-based strategy that can be effective for some people, and I think it should be on the list of science-based tools that people can consider using.” But as it won’t work for everyone, “we need to consider both metabolic success and behavioral success. In other words, would it be more effective if people could do it and how easy or hard is it for people to do?”
Audra Wilson, MS, RD, a bariatric dietitian at Northwestern Medicine Delnor Hospital in Geneva, Illinois, who was not involved in the study, expressed more reservations. “We do not specifically recommend intermittent fasting at Northwestern Medicine. There is no set protocol for this diet, and it can vary in ways that can limit nutrition to the point where we are not meeting needs on a regular basis,” she said in an interview.
Moreover, this study did not specify exact nutritional recommendations for participants but merely reduced overall caloric intake. “Although intermittent fasting may be helpful to some, in my nearly 10 years of experience I have not seen it be effective for many and especially not long term,” Wilson added.
Concerningly, IMF can foster disordered eating patterns of restriction followed by binging. “Although a balanced diet is more difficult to achieve, guidance from professionals like dietitians can give patients the tools to achieve balance, meet all nutrient needs, achieve satiety, and maybe most importantly, have a better relationship with food,” she said.
As for the influence of metabolic factors that may be associated with better weight loss, Ostendorf said, “be on the lookout for future publications in this area. We are analyzing data around changes in energy expenditure and changes in hunger-related hormones, among others.” A colleague is collecting biological samples to study genetics in this context. “However, in general, it appeared that the difference in weight loss was due to a greater caloric deficit in the 4:3 IMF group.”
Ostendorf and Catenacci are currently conducting a pilot study testing 4:3 IMF in breast cancer survivors. “We think this is a promising strategy for weight loss in breast cancer survivors who struggle with overweight/obesity in addition to their cancer diagnosis,” Ostendorf said.
This study was funded by the National Institute of Diabetes and Digestive and Kidney Diseases. Ostendorf, Catenacci, Hill, and Wilson disclosed no relevant financial conflicts of interest.
A version of this article appeared on Medscape.com.
FROM ANNALS OF INTERNAL MEDICINE
Safety Profile of GLP-1s ‘Reassuring’ in Upper Endoscopy
according to a meta-analysis of more than 80,000 patients.
Safety profiles, however, were comparable across groups, suggesting that prolonged fasting may be a sufficient management strategy, instead of withholding GLP-1RAs, lead author Antonio Facciorusso, MD, PhD, of the University of Foggia, Italy, and colleagues reported.
“The impact of GLP-1RAs on slowing gastric motility has raised concerns in patients undergoing endoscopic procedures, particularly upper endoscopies,” the investigators wrote in Clinical Gastroenterology and Hepatology. “This is due to the perceived risk of aspiration of retained gastric contents in sedated patients and the decreased visibility of the gastric mucosa, which can reduce the diagnostic yield of the examination.”
The American Society of Anesthesiologists (ASA) recommends withholding GLP-1RAs before procedures or surgery, whereas AGA suggests an individualized approach, citing limited supporting data.
A previous meta-analysis reported that GLP-1RAs mildly delayed gastric emptying, but clinical relevance remained unclear.
The present meta-analysis aimed to clarify this uncertainty by analyzing 13 retrospective studies that involved 84,065 patients undergoing upper endoscopy. Outcomes were compared among GLP-1RA users vs non-users, including rates of retained gastric contents, aborted procedures, and adverse events.
Patients on GLP-1RAs had significantly higher rates of retained gastric contents than non-users (odds ratio [OR], 5.56), a finding that held steady (OR, 4.20) after adjusting for age, sex, diabetes, body mass index, and other therapies.
GLP-1RAs were also associated with an increased likelihood of aborted procedures (OR, 5.13; 1% vs. 0.3%) and a higher need for repeat endoscopies (OR, 2.19; 1% vs 2%); however, Facciorusso and colleagues noted that these events, in absolute terms, were relatively uncommon.
“The rate of aborted and repeat procedures in the included studies was low,” the investigators wrote. “This meant that only for every 110 patients undergoing upper endoscopy while in GLP-1RA therapy would we observe an aborted procedure and only for every 120 patients would we need to repeat the procedure.”
The overall safety profile of GLP-1RAs in the context of upper endoscopy remained largely reassuring, they added. Specifically, rates of bronchial aspiration were not significantly different between users and non-users. What’s more, no single study reported a statistically significant increase in major complications, including pulmonary adverse events, among GLP-1RA users.
According to Facciorusso and colleagues, these findings suggest that retained gastric contents do not appear to substantially heighten the risk of serious harm, though further prospective studies are needed.
“Our comprehensive analysis indicates that, while the use of GLP-1RA results in higher rates of [retained gastric contents], the actual clinical impact appears to be limited,” they wrote. “Therefore, there is no strong evidence to support the routine discontinuation of the drug before upper endoscopy procedures.”
Instead, they supported the AGA task force’s recommendation for an individualized approach, and not withholding GLP-1RAs unnecessarily, calling this “the best compromise.”
“Prolonging the duration of fasting for solids could represent the optimal approach in these patients, although this strategy requires further evaluation,” the investigators concluded.
The investigators disclosed no conflicts of interest.
according to a meta-analysis of more than 80,000 patients.
Safety profiles, however, were comparable across groups, suggesting that prolonged fasting may be a sufficient management strategy, instead of withholding GLP-1RAs, lead author Antonio Facciorusso, MD, PhD, of the University of Foggia, Italy, and colleagues reported.
“The impact of GLP-1RAs on slowing gastric motility has raised concerns in patients undergoing endoscopic procedures, particularly upper endoscopies,” the investigators wrote in Clinical Gastroenterology and Hepatology. “This is due to the perceived risk of aspiration of retained gastric contents in sedated patients and the decreased visibility of the gastric mucosa, which can reduce the diagnostic yield of the examination.”
The American Society of Anesthesiologists (ASA) recommends withholding GLP-1RAs before procedures or surgery, whereas AGA suggests an individualized approach, citing limited supporting data.
A previous meta-analysis reported that GLP-1RAs mildly delayed gastric emptying, but clinical relevance remained unclear.
The present meta-analysis aimed to clarify this uncertainty by analyzing 13 retrospective studies that involved 84,065 patients undergoing upper endoscopy. Outcomes were compared among GLP-1RA users vs non-users, including rates of retained gastric contents, aborted procedures, and adverse events.
Patients on GLP-1RAs had significantly higher rates of retained gastric contents than non-users (odds ratio [OR], 5.56), a finding that held steady (OR, 4.20) after adjusting for age, sex, diabetes, body mass index, and other therapies.
GLP-1RAs were also associated with an increased likelihood of aborted procedures (OR, 5.13; 1% vs. 0.3%) and a higher need for repeat endoscopies (OR, 2.19; 1% vs 2%); however, Facciorusso and colleagues noted that these events, in absolute terms, were relatively uncommon.
“The rate of aborted and repeat procedures in the included studies was low,” the investigators wrote. “This meant that only for every 110 patients undergoing upper endoscopy while in GLP-1RA therapy would we observe an aborted procedure and only for every 120 patients would we need to repeat the procedure.”
The overall safety profile of GLP-1RAs in the context of upper endoscopy remained largely reassuring, they added. Specifically, rates of bronchial aspiration were not significantly different between users and non-users. What’s more, no single study reported a statistically significant increase in major complications, including pulmonary adverse events, among GLP-1RA users.
According to Facciorusso and colleagues, these findings suggest that retained gastric contents do not appear to substantially heighten the risk of serious harm, though further prospective studies are needed.
“Our comprehensive analysis indicates that, while the use of GLP-1RA results in higher rates of [retained gastric contents], the actual clinical impact appears to be limited,” they wrote. “Therefore, there is no strong evidence to support the routine discontinuation of the drug before upper endoscopy procedures.”
Instead, they supported the AGA task force’s recommendation for an individualized approach, and not withholding GLP-1RAs unnecessarily, calling this “the best compromise.”
“Prolonging the duration of fasting for solids could represent the optimal approach in these patients, although this strategy requires further evaluation,” the investigators concluded.
The investigators disclosed no conflicts of interest.
according to a meta-analysis of more than 80,000 patients.
Safety profiles, however, were comparable across groups, suggesting that prolonged fasting may be a sufficient management strategy, instead of withholding GLP-1RAs, lead author Antonio Facciorusso, MD, PhD, of the University of Foggia, Italy, and colleagues reported.
“The impact of GLP-1RAs on slowing gastric motility has raised concerns in patients undergoing endoscopic procedures, particularly upper endoscopies,” the investigators wrote in Clinical Gastroenterology and Hepatology. “This is due to the perceived risk of aspiration of retained gastric contents in sedated patients and the decreased visibility of the gastric mucosa, which can reduce the diagnostic yield of the examination.”
The American Society of Anesthesiologists (ASA) recommends withholding GLP-1RAs before procedures or surgery, whereas AGA suggests an individualized approach, citing limited supporting data.
A previous meta-analysis reported that GLP-1RAs mildly delayed gastric emptying, but clinical relevance remained unclear.
The present meta-analysis aimed to clarify this uncertainty by analyzing 13 retrospective studies that involved 84,065 patients undergoing upper endoscopy. Outcomes were compared among GLP-1RA users vs non-users, including rates of retained gastric contents, aborted procedures, and adverse events.
Patients on GLP-1RAs had significantly higher rates of retained gastric contents than non-users (odds ratio [OR], 5.56), a finding that held steady (OR, 4.20) after adjusting for age, sex, diabetes, body mass index, and other therapies.
GLP-1RAs were also associated with an increased likelihood of aborted procedures (OR, 5.13; 1% vs. 0.3%) and a higher need for repeat endoscopies (OR, 2.19; 1% vs 2%); however, Facciorusso and colleagues noted that these events, in absolute terms, were relatively uncommon.
“The rate of aborted and repeat procedures in the included studies was low,” the investigators wrote. “This meant that only for every 110 patients undergoing upper endoscopy while in GLP-1RA therapy would we observe an aborted procedure and only for every 120 patients would we need to repeat the procedure.”
The overall safety profile of GLP-1RAs in the context of upper endoscopy remained largely reassuring, they added. Specifically, rates of bronchial aspiration were not significantly different between users and non-users. What’s more, no single study reported a statistically significant increase in major complications, including pulmonary adverse events, among GLP-1RA users.
According to Facciorusso and colleagues, these findings suggest that retained gastric contents do not appear to substantially heighten the risk of serious harm, though further prospective studies are needed.
“Our comprehensive analysis indicates that, while the use of GLP-1RA results in higher rates of [retained gastric contents], the actual clinical impact appears to be limited,” they wrote. “Therefore, there is no strong evidence to support the routine discontinuation of the drug before upper endoscopy procedures.”
Instead, they supported the AGA task force’s recommendation for an individualized approach, and not withholding GLP-1RAs unnecessarily, calling this “the best compromise.”
“Prolonging the duration of fasting for solids could represent the optimal approach in these patients, although this strategy requires further evaluation,” the investigators concluded.
The investigators disclosed no conflicts of interest.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Bariatric Surgery: Nutrition’s Role in Patient Outcomes
, according to an updated clinical practice statement from the Obesity Medicine Association (OMA).
The update offers guidance on how to manage metabolic and bariatric surgery patients’ nutrition, from preoperative nutritional assessments through identification and treatment of the most common nutritional problems associated with bariatric procedures.
“The main takeaway really is that obesity is a complex and chronic disease. It requires the same model of care as diabetes or other chronic conditions,” said Rutuja Patel, DO, senior author and an obesity medicine specialist at Northwestern Medicine Regional Medical Group in Winfield, Illinois.
The development of an interdisciplinary team of medical providers with evidence-based nutrition knowledge and consistent information improves the quality of nutrition care provided to bariatric surgery patients, the authors wrote.
“Collaborative multidisciplinary care that takes into consideration the whole patient in a biopsychosocial way and uses multiple modalities — including medical, behavioral, nutritional, and others — leads to the best outcomes in these complex patients,” Patel said.
The updated statement, published online in Obesity Pillars, offers a variety of tools and checklists to aid clinicians, especially those who may not have access to a multidisciplinary team or dietitian knowledgeable about bariatric nutrition.
It is a follow-up to the OMA’s 2022 clinical practice statement, which provided an overview of bariatric surgery, gastrointestinal hormones, and the microbiome in patients with obesity.
Presurgical Guidance
The new guidance lays out the various components of preoperative nutrition screenings, among which is a medication review to determine if the patient is taking drugs that may affect weight and calorie intake. These include antihypertensives, diabetes agents, hormonal contraceptives, antidepressants, migraine medications, and antipsychotics.
In taking a patient’s history, clinicians should ask about major events associated with weight changes, such as medication changes, illness, pregnancy, divorce, stressful employment, food insecurity, and periods of disordered eating.
The fundamental approach to conducting a nutrition assessment is an understanding of the role that various sections of the gastrointestinal tract play in micronutrient absorption, the authors wrote. As an educational tool, the update includes a diagram that indicates the areas of the stomach, duodenum, jejunum, and ileum that may be altered by bariatric surgery and how they factor into micronutrient absorption.
“It makes it easier to see why certain surgical procedures are more likely to cause certain deficiencies,” Patel said.
Postsurgery Patient Management
Post surgery, clinicians should monitor patients for other problems that could affect nutrient absorption, including food intolerances, drug-nutrient interactions, and increased gastrointestinal transit time.
Patel and coauthors discussed the pros and cons of multivitamin mineral supplement formulations as well as specific vitamin and mineral recommendations for patients undergoing certain metabolic or bariatric surgery procedures. They included three supplemental cases in the appendix to illustrate supplementation recommendations and long-term maintenance suggestions.
“It’s important to remember that most of these deficiencies present without many clinical symptoms, so it becomes essential to screen for them and repeat as needed,” Patel said.
The update also tackles postoperative nutritional assessments and diet progression. No evidence supports following one postsurgical diet progression protocol over another, but they generally proceed from a clear liquid diet to foods with normal textures, the authors noted. Clinicians should adapt them according to the procedure type, they added.
In addition, clinicians must troubleshoot any nutrition-related concerns, including constipation, dehydration, nausea, heartburn, and fatigue, for up to a year after surgery, they wrote.
Metabolic and bariatric surgery patients should be evaluated annually at a minimum, if not more frequently, to gauge nutritional health, the authors wrote. Treating obesity as a disease involves more than weight loss — instead, it’s about improving the quality of life of patients through procedures, medications, and lifestyle modifications, they added.
Track New Developments
With ongoing changes in the field of metabolic and bariatric surgery, it’s helpful for clinicians to remain updated about new approaches across various disciplines linked to obesity management and treatment, said Christina Poa-Li, MD, a surgeon at Huntington Health Medical Center, affiliated with Cedars-Sinai Health System, in Pasadena, California, who was not involved in developing the updated practice statement.
“For example, the rapidly growing prescription of anti-obesity medications and their use in both preoperative and postoperative surgical patients drastically affect their nutrition,” she said. “Providers of various backgrounds and specialties will benefit from the most updated guidance on evaluating patient nutrition.”
Clinicians should consider expanding their patient population to include those with metabolic dysfunction–associated steatohepatitis or metabolic dysfunction–associated steatotic liver disease, Poa-Li said.
“These patient subpopulations may not have been considered for bariatric surgery or even referral to a bariatric surgeon for consultation previously,” she said. “It is important to increase awareness among clinicians of the potential benefits for metabolic and bariatric surgery for these patients.”
The report didn’t receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. Patel and Poa-Li reported no relevant disclosures.
A version of this article appeared on Medscape.com.
, according to an updated clinical practice statement from the Obesity Medicine Association (OMA).
The update offers guidance on how to manage metabolic and bariatric surgery patients’ nutrition, from preoperative nutritional assessments through identification and treatment of the most common nutritional problems associated with bariatric procedures.
“The main takeaway really is that obesity is a complex and chronic disease. It requires the same model of care as diabetes or other chronic conditions,” said Rutuja Patel, DO, senior author and an obesity medicine specialist at Northwestern Medicine Regional Medical Group in Winfield, Illinois.
The development of an interdisciplinary team of medical providers with evidence-based nutrition knowledge and consistent information improves the quality of nutrition care provided to bariatric surgery patients, the authors wrote.
“Collaborative multidisciplinary care that takes into consideration the whole patient in a biopsychosocial way and uses multiple modalities — including medical, behavioral, nutritional, and others — leads to the best outcomes in these complex patients,” Patel said.
The updated statement, published online in Obesity Pillars, offers a variety of tools and checklists to aid clinicians, especially those who may not have access to a multidisciplinary team or dietitian knowledgeable about bariatric nutrition.
It is a follow-up to the OMA’s 2022 clinical practice statement, which provided an overview of bariatric surgery, gastrointestinal hormones, and the microbiome in patients with obesity.
Presurgical Guidance
The new guidance lays out the various components of preoperative nutrition screenings, among which is a medication review to determine if the patient is taking drugs that may affect weight and calorie intake. These include antihypertensives, diabetes agents, hormonal contraceptives, antidepressants, migraine medications, and antipsychotics.
In taking a patient’s history, clinicians should ask about major events associated with weight changes, such as medication changes, illness, pregnancy, divorce, stressful employment, food insecurity, and periods of disordered eating.
The fundamental approach to conducting a nutrition assessment is an understanding of the role that various sections of the gastrointestinal tract play in micronutrient absorption, the authors wrote. As an educational tool, the update includes a diagram that indicates the areas of the stomach, duodenum, jejunum, and ileum that may be altered by bariatric surgery and how they factor into micronutrient absorption.
“It makes it easier to see why certain surgical procedures are more likely to cause certain deficiencies,” Patel said.
Postsurgery Patient Management
Post surgery, clinicians should monitor patients for other problems that could affect nutrient absorption, including food intolerances, drug-nutrient interactions, and increased gastrointestinal transit time.
Patel and coauthors discussed the pros and cons of multivitamin mineral supplement formulations as well as specific vitamin and mineral recommendations for patients undergoing certain metabolic or bariatric surgery procedures. They included three supplemental cases in the appendix to illustrate supplementation recommendations and long-term maintenance suggestions.
“It’s important to remember that most of these deficiencies present without many clinical symptoms, so it becomes essential to screen for them and repeat as needed,” Patel said.
The update also tackles postoperative nutritional assessments and diet progression. No evidence supports following one postsurgical diet progression protocol over another, but they generally proceed from a clear liquid diet to foods with normal textures, the authors noted. Clinicians should adapt them according to the procedure type, they added.
In addition, clinicians must troubleshoot any nutrition-related concerns, including constipation, dehydration, nausea, heartburn, and fatigue, for up to a year after surgery, they wrote.
Metabolic and bariatric surgery patients should be evaluated annually at a minimum, if not more frequently, to gauge nutritional health, the authors wrote. Treating obesity as a disease involves more than weight loss — instead, it’s about improving the quality of life of patients through procedures, medications, and lifestyle modifications, they added.
Track New Developments
With ongoing changes in the field of metabolic and bariatric surgery, it’s helpful for clinicians to remain updated about new approaches across various disciplines linked to obesity management and treatment, said Christina Poa-Li, MD, a surgeon at Huntington Health Medical Center, affiliated with Cedars-Sinai Health System, in Pasadena, California, who was not involved in developing the updated practice statement.
“For example, the rapidly growing prescription of anti-obesity medications and their use in both preoperative and postoperative surgical patients drastically affect their nutrition,” she said. “Providers of various backgrounds and specialties will benefit from the most updated guidance on evaluating patient nutrition.”
Clinicians should consider expanding their patient population to include those with metabolic dysfunction–associated steatohepatitis or metabolic dysfunction–associated steatotic liver disease, Poa-Li said.
“These patient subpopulations may not have been considered for bariatric surgery or even referral to a bariatric surgeon for consultation previously,” she said. “It is important to increase awareness among clinicians of the potential benefits for metabolic and bariatric surgery for these patients.”
The report didn’t receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. Patel and Poa-Li reported no relevant disclosures.
A version of this article appeared on Medscape.com.
, according to an updated clinical practice statement from the Obesity Medicine Association (OMA).
The update offers guidance on how to manage metabolic and bariatric surgery patients’ nutrition, from preoperative nutritional assessments through identification and treatment of the most common nutritional problems associated with bariatric procedures.
“The main takeaway really is that obesity is a complex and chronic disease. It requires the same model of care as diabetes or other chronic conditions,” said Rutuja Patel, DO, senior author and an obesity medicine specialist at Northwestern Medicine Regional Medical Group in Winfield, Illinois.
The development of an interdisciplinary team of medical providers with evidence-based nutrition knowledge and consistent information improves the quality of nutrition care provided to bariatric surgery patients, the authors wrote.
“Collaborative multidisciplinary care that takes into consideration the whole patient in a biopsychosocial way and uses multiple modalities — including medical, behavioral, nutritional, and others — leads to the best outcomes in these complex patients,” Patel said.
The updated statement, published online in Obesity Pillars, offers a variety of tools and checklists to aid clinicians, especially those who may not have access to a multidisciplinary team or dietitian knowledgeable about bariatric nutrition.
It is a follow-up to the OMA’s 2022 clinical practice statement, which provided an overview of bariatric surgery, gastrointestinal hormones, and the microbiome in patients with obesity.
Presurgical Guidance
The new guidance lays out the various components of preoperative nutrition screenings, among which is a medication review to determine if the patient is taking drugs that may affect weight and calorie intake. These include antihypertensives, diabetes agents, hormonal contraceptives, antidepressants, migraine medications, and antipsychotics.
In taking a patient’s history, clinicians should ask about major events associated with weight changes, such as medication changes, illness, pregnancy, divorce, stressful employment, food insecurity, and periods of disordered eating.
The fundamental approach to conducting a nutrition assessment is an understanding of the role that various sections of the gastrointestinal tract play in micronutrient absorption, the authors wrote. As an educational tool, the update includes a diagram that indicates the areas of the stomach, duodenum, jejunum, and ileum that may be altered by bariatric surgery and how they factor into micronutrient absorption.
“It makes it easier to see why certain surgical procedures are more likely to cause certain deficiencies,” Patel said.
Postsurgery Patient Management
Post surgery, clinicians should monitor patients for other problems that could affect nutrient absorption, including food intolerances, drug-nutrient interactions, and increased gastrointestinal transit time.
Patel and coauthors discussed the pros and cons of multivitamin mineral supplement formulations as well as specific vitamin and mineral recommendations for patients undergoing certain metabolic or bariatric surgery procedures. They included three supplemental cases in the appendix to illustrate supplementation recommendations and long-term maintenance suggestions.
“It’s important to remember that most of these deficiencies present without many clinical symptoms, so it becomes essential to screen for them and repeat as needed,” Patel said.
The update also tackles postoperative nutritional assessments and diet progression. No evidence supports following one postsurgical diet progression protocol over another, but they generally proceed from a clear liquid diet to foods with normal textures, the authors noted. Clinicians should adapt them according to the procedure type, they added.
In addition, clinicians must troubleshoot any nutrition-related concerns, including constipation, dehydration, nausea, heartburn, and fatigue, for up to a year after surgery, they wrote.
Metabolic and bariatric surgery patients should be evaluated annually at a minimum, if not more frequently, to gauge nutritional health, the authors wrote. Treating obesity as a disease involves more than weight loss — instead, it’s about improving the quality of life of patients through procedures, medications, and lifestyle modifications, they added.
Track New Developments
With ongoing changes in the field of metabolic and bariatric surgery, it’s helpful for clinicians to remain updated about new approaches across various disciplines linked to obesity management and treatment, said Christina Poa-Li, MD, a surgeon at Huntington Health Medical Center, affiliated with Cedars-Sinai Health System, in Pasadena, California, who was not involved in developing the updated practice statement.
“For example, the rapidly growing prescription of anti-obesity medications and their use in both preoperative and postoperative surgical patients drastically affect their nutrition,” she said. “Providers of various backgrounds and specialties will benefit from the most updated guidance on evaluating patient nutrition.”
Clinicians should consider expanding their patient population to include those with metabolic dysfunction–associated steatohepatitis or metabolic dysfunction–associated steatotic liver disease, Poa-Li said.
“These patient subpopulations may not have been considered for bariatric surgery or even referral to a bariatric surgeon for consultation previously,” she said. “It is important to increase awareness among clinicians of the potential benefits for metabolic and bariatric surgery for these patients.”
The report didn’t receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. Patel and Poa-Li reported no relevant disclosures.
A version of this article appeared on Medscape.com.
Bariatric Surgery Lowers Risk for Long-Term Liver Complications in MASH-Related Cirrhosis
according to a recent study by Cleveland Clinic researchers.
Compared with patients who received medical therapy alone, those who underwent bariatric surgery had a 72% lower risk of developing serious complications of liver disease and an 80% lower risk for progression to decompensated cirrhosis.
The results could have major implications for patients with metabolic dysfunction–associated steatohepatitis (MASH), particularly given that about 20% of patients with MASH progress to cirrhosis, the researchers said.
“Currently, lifestyle intervention is the only therapeutic recommendation for compensated MASH-related cirrhosis,” said Steven Nissen, MD, the senior author and chief academic officer of the Miller Family Heart, Vascular, and Thoracic Institute, Cleveland Clinic, Ohio.
“However, lifestyle changes alone rarely provide the weight loss and metabolic changes needed to reduce the risk of liver complications in this patient population,” he said. “This study shows that bariatric surgery is an effective treatment that can influence the trajectory of cirrhosis progression in select patients.”
The study was published online in Nature Medicine.
Significantly Reduced Risks
As part of the Surgical Procedures Eliminate Compensated Cirrhosis in Advancing Long-Term (SPECCIAL) observational study, Nissen and colleagues compared the long-term effects of metabolic surgery and medical treatment in patients with obesity and compensated, biopsy-proven MASH-related cirrhosis. They looked for six major adverse liver outcomes (MALO): ascites, variceal hemorrhage, hepatic encephalopathy, hepatocellular carcinoma, liver transplantation, and all-cause mortality.
Among nearly 37,000 patients who underwent liver biopsy at the Cleveland Clinic Health System between 1995 and 2020, the research team identified 168 patients (69.6% women) with MASH-related cirrhosis, Child-Pugh class A, and model for end-stage liver disease scores ≤ 10. Among those, 62 underwent metabolic surgery (37 Roux-en-Y gastric bypass and 25 sleeve gastrectomy) and 106 had nonsurgical treatment.
After overlap weighting, the groups had balanced baseline characteristics, including mean body mass index of 42.7, Fibrosis-4 score of 2.1, albumin of 4.1 g/dL, bilirubin of 0.6 mg/dL, and Elixhauser comorbidity index of 9. In each group, 84.5% had type 2 diabetes and 79.1% had Ishak fibrosis stage 6.
Overall, the 15-year cumulative incidence of MALO was 20.9% in the surgical group and 46.4% in the nonsurgical group (adjusted hazard ratio [aHR], 0.28; P = .003), with MALO occurring in 10 surgical patients and 42 nonsurgical patients.
Assuming a causal effect, the number needed to treat with metabolic surgery to avoid one incidence of MALO over a 15-year timespan was 4.5.
The 15-year incidence of MALO was similar between surgical methods, with 20.1% for Roux-en-Y gastric bypass and 19.9% for sleeve gastrectomy.
In addition, the 15-year cumulative incidence of progression to decompensated cirrhosis was 15.6% in the surgical group and 30.7% in the nonsurgical group (aHR, 0.2; P = .01), with decompensation occurring in four surgical patients and 33 nonsurgical patients.
At 15 years, patients in the surgical group lost 31.6 kg or about 26.6% of their weight, and those in the nonsurgical group lost 10.7 kg or 9.8%.
Among patients with type 2 diabetes at baseline, metabolic surgery was associated with a reduction in hemoglobin A1c levels, as well as diabetes remission for some patients.
Potential to Fill an Unmet Need
Previous studies have indicated that bariatric surgery can effectively treat noncirrhotic MASH and lead to histologic resolution of MASH. In a 2021 study, Nissen and colleagues found bariatric surgery was associated with a lower risk for MALO and major adverse cardiovascular events in patients with biopsy-proven MASH without cirrhosis. Now, Nissen said, the SPECCIAL study indicates surgery may be a good option for MASH-related cirrhosis as well.
The study authors also noted that similar findings are theoretically possible from medical therapies, given the emergence of a new generation of anti-obesity medications. In this study, 16.8% of the surgical group and 14.3% of the nonsurgical group took semaglutide or tirzepatide at some point during the follow-up period.
“Patients with MASH-related cirrhosis have extremely limited treatment options,” said Sobia Laique, MD, study coauthor and a transplant hepatologist at the Cleveland Clinic who specializes in MASH-related cirrhosis. She cofounded the Cleveland Clinic’s task force on metabolic dysfunction–associated steatotic liver disease (MASLD) to improve screening, management, and patient outcomes for MASLD and related comorbidities.
“No therapeutic interventions have demonstrated efficacy in mitigating the risk of severe liver complications for these patients,” she said. “This underscores a critical unmet need for the development of effective therapies specifically targeting patients with compensated MASH-related cirrhosis.”
No funding was reported for this study. Several authors reported receiving grant funding, consultant fees, and advisory roles for various pharmaceutical companies.
A version of this article appeared on Medscape.com.
according to a recent study by Cleveland Clinic researchers.
Compared with patients who received medical therapy alone, those who underwent bariatric surgery had a 72% lower risk of developing serious complications of liver disease and an 80% lower risk for progression to decompensated cirrhosis.
The results could have major implications for patients with metabolic dysfunction–associated steatohepatitis (MASH), particularly given that about 20% of patients with MASH progress to cirrhosis, the researchers said.
“Currently, lifestyle intervention is the only therapeutic recommendation for compensated MASH-related cirrhosis,” said Steven Nissen, MD, the senior author and chief academic officer of the Miller Family Heart, Vascular, and Thoracic Institute, Cleveland Clinic, Ohio.
“However, lifestyle changes alone rarely provide the weight loss and metabolic changes needed to reduce the risk of liver complications in this patient population,” he said. “This study shows that bariatric surgery is an effective treatment that can influence the trajectory of cirrhosis progression in select patients.”
The study was published online in Nature Medicine.
Significantly Reduced Risks
As part of the Surgical Procedures Eliminate Compensated Cirrhosis in Advancing Long-Term (SPECCIAL) observational study, Nissen and colleagues compared the long-term effects of metabolic surgery and medical treatment in patients with obesity and compensated, biopsy-proven MASH-related cirrhosis. They looked for six major adverse liver outcomes (MALO): ascites, variceal hemorrhage, hepatic encephalopathy, hepatocellular carcinoma, liver transplantation, and all-cause mortality.
Among nearly 37,000 patients who underwent liver biopsy at the Cleveland Clinic Health System between 1995 and 2020, the research team identified 168 patients (69.6% women) with MASH-related cirrhosis, Child-Pugh class A, and model for end-stage liver disease scores ≤ 10. Among those, 62 underwent metabolic surgery (37 Roux-en-Y gastric bypass and 25 sleeve gastrectomy) and 106 had nonsurgical treatment.
After overlap weighting, the groups had balanced baseline characteristics, including mean body mass index of 42.7, Fibrosis-4 score of 2.1, albumin of 4.1 g/dL, bilirubin of 0.6 mg/dL, and Elixhauser comorbidity index of 9. In each group, 84.5% had type 2 diabetes and 79.1% had Ishak fibrosis stage 6.
Overall, the 15-year cumulative incidence of MALO was 20.9% in the surgical group and 46.4% in the nonsurgical group (adjusted hazard ratio [aHR], 0.28; P = .003), with MALO occurring in 10 surgical patients and 42 nonsurgical patients.
Assuming a causal effect, the number needed to treat with metabolic surgery to avoid one incidence of MALO over a 15-year timespan was 4.5.
The 15-year incidence of MALO was similar between surgical methods, with 20.1% for Roux-en-Y gastric bypass and 19.9% for sleeve gastrectomy.
In addition, the 15-year cumulative incidence of progression to decompensated cirrhosis was 15.6% in the surgical group and 30.7% in the nonsurgical group (aHR, 0.2; P = .01), with decompensation occurring in four surgical patients and 33 nonsurgical patients.
At 15 years, patients in the surgical group lost 31.6 kg or about 26.6% of their weight, and those in the nonsurgical group lost 10.7 kg or 9.8%.
Among patients with type 2 diabetes at baseline, metabolic surgery was associated with a reduction in hemoglobin A1c levels, as well as diabetes remission for some patients.
Potential to Fill an Unmet Need
Previous studies have indicated that bariatric surgery can effectively treat noncirrhotic MASH and lead to histologic resolution of MASH. In a 2021 study, Nissen and colleagues found bariatric surgery was associated with a lower risk for MALO and major adverse cardiovascular events in patients with biopsy-proven MASH without cirrhosis. Now, Nissen said, the SPECCIAL study indicates surgery may be a good option for MASH-related cirrhosis as well.
The study authors also noted that similar findings are theoretically possible from medical therapies, given the emergence of a new generation of anti-obesity medications. In this study, 16.8% of the surgical group and 14.3% of the nonsurgical group took semaglutide or tirzepatide at some point during the follow-up period.
“Patients with MASH-related cirrhosis have extremely limited treatment options,” said Sobia Laique, MD, study coauthor and a transplant hepatologist at the Cleveland Clinic who specializes in MASH-related cirrhosis. She cofounded the Cleveland Clinic’s task force on metabolic dysfunction–associated steatotic liver disease (MASLD) to improve screening, management, and patient outcomes for MASLD and related comorbidities.
“No therapeutic interventions have demonstrated efficacy in mitigating the risk of severe liver complications for these patients,” she said. “This underscores a critical unmet need for the development of effective therapies specifically targeting patients with compensated MASH-related cirrhosis.”
No funding was reported for this study. Several authors reported receiving grant funding, consultant fees, and advisory roles for various pharmaceutical companies.
A version of this article appeared on Medscape.com.
according to a recent study by Cleveland Clinic researchers.
Compared with patients who received medical therapy alone, those who underwent bariatric surgery had a 72% lower risk of developing serious complications of liver disease and an 80% lower risk for progression to decompensated cirrhosis.
The results could have major implications for patients with metabolic dysfunction–associated steatohepatitis (MASH), particularly given that about 20% of patients with MASH progress to cirrhosis, the researchers said.
“Currently, lifestyle intervention is the only therapeutic recommendation for compensated MASH-related cirrhosis,” said Steven Nissen, MD, the senior author and chief academic officer of the Miller Family Heart, Vascular, and Thoracic Institute, Cleveland Clinic, Ohio.
“However, lifestyle changes alone rarely provide the weight loss and metabolic changes needed to reduce the risk of liver complications in this patient population,” he said. “This study shows that bariatric surgery is an effective treatment that can influence the trajectory of cirrhosis progression in select patients.”
The study was published online in Nature Medicine.
Significantly Reduced Risks
As part of the Surgical Procedures Eliminate Compensated Cirrhosis in Advancing Long-Term (SPECCIAL) observational study, Nissen and colleagues compared the long-term effects of metabolic surgery and medical treatment in patients with obesity and compensated, biopsy-proven MASH-related cirrhosis. They looked for six major adverse liver outcomes (MALO): ascites, variceal hemorrhage, hepatic encephalopathy, hepatocellular carcinoma, liver transplantation, and all-cause mortality.
Among nearly 37,000 patients who underwent liver biopsy at the Cleveland Clinic Health System between 1995 and 2020, the research team identified 168 patients (69.6% women) with MASH-related cirrhosis, Child-Pugh class A, and model for end-stage liver disease scores ≤ 10. Among those, 62 underwent metabolic surgery (37 Roux-en-Y gastric bypass and 25 sleeve gastrectomy) and 106 had nonsurgical treatment.
After overlap weighting, the groups had balanced baseline characteristics, including mean body mass index of 42.7, Fibrosis-4 score of 2.1, albumin of 4.1 g/dL, bilirubin of 0.6 mg/dL, and Elixhauser comorbidity index of 9. In each group, 84.5% had type 2 diabetes and 79.1% had Ishak fibrosis stage 6.
Overall, the 15-year cumulative incidence of MALO was 20.9% in the surgical group and 46.4% in the nonsurgical group (adjusted hazard ratio [aHR], 0.28; P = .003), with MALO occurring in 10 surgical patients and 42 nonsurgical patients.
Assuming a causal effect, the number needed to treat with metabolic surgery to avoid one incidence of MALO over a 15-year timespan was 4.5.
The 15-year incidence of MALO was similar between surgical methods, with 20.1% for Roux-en-Y gastric bypass and 19.9% for sleeve gastrectomy.
In addition, the 15-year cumulative incidence of progression to decompensated cirrhosis was 15.6% in the surgical group and 30.7% in the nonsurgical group (aHR, 0.2; P = .01), with decompensation occurring in four surgical patients and 33 nonsurgical patients.
At 15 years, patients in the surgical group lost 31.6 kg or about 26.6% of their weight, and those in the nonsurgical group lost 10.7 kg or 9.8%.
Among patients with type 2 diabetes at baseline, metabolic surgery was associated with a reduction in hemoglobin A1c levels, as well as diabetes remission for some patients.
Potential to Fill an Unmet Need
Previous studies have indicated that bariatric surgery can effectively treat noncirrhotic MASH and lead to histologic resolution of MASH. In a 2021 study, Nissen and colleagues found bariatric surgery was associated with a lower risk for MALO and major adverse cardiovascular events in patients with biopsy-proven MASH without cirrhosis. Now, Nissen said, the SPECCIAL study indicates surgery may be a good option for MASH-related cirrhosis as well.
The study authors also noted that similar findings are theoretically possible from medical therapies, given the emergence of a new generation of anti-obesity medications. In this study, 16.8% of the surgical group and 14.3% of the nonsurgical group took semaglutide or tirzepatide at some point during the follow-up period.
“Patients with MASH-related cirrhosis have extremely limited treatment options,” said Sobia Laique, MD, study coauthor and a transplant hepatologist at the Cleveland Clinic who specializes in MASH-related cirrhosis. She cofounded the Cleveland Clinic’s task force on metabolic dysfunction–associated steatotic liver disease (MASLD) to improve screening, management, and patient outcomes for MASLD and related comorbidities.
“No therapeutic interventions have demonstrated efficacy in mitigating the risk of severe liver complications for these patients,” she said. “This underscores a critical unmet need for the development of effective therapies specifically targeting patients with compensated MASH-related cirrhosis.”
No funding was reported for this study. Several authors reported receiving grant funding, consultant fees, and advisory roles for various pharmaceutical companies.
A version of this article appeared on Medscape.com.
FROM NATURE MEDICINE