User login
Reversible Cutaneous Side Effects of Vismodegib Treatment
To the Editor:
Vismodegib, a first-in-class inhibitor of the hedgehog signaling pathway, is useful in the treatment of advanced basal cell carcinomas (BCCs).1 Common side effects of vismodegib include alopecia (58%), muscle spasms (71%), and dysgeusia (71%).2 Some of these side effects have been hypothesized to be mechanism related.3,4 Keratoacanthomas have been reported to occur after vismodegib treatment of BCC.5 We report 3 cases illustrating reversible cutaneous side effects of vismodegib: alopecia, follicular dermatitis, and drug hypersensitivity reaction.
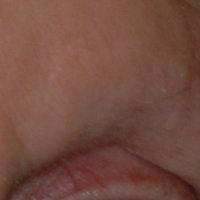
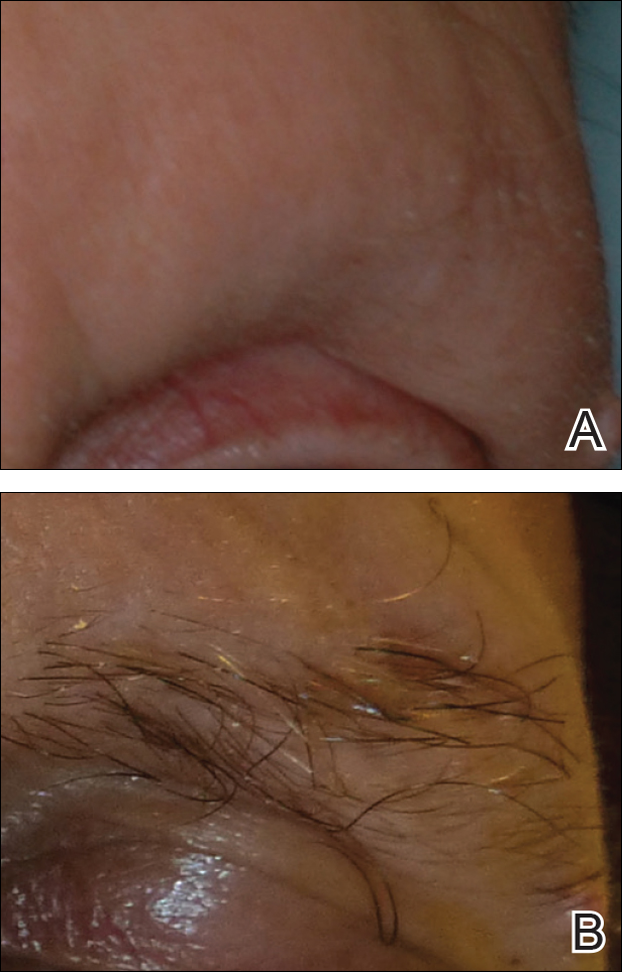
A 53-year-old man with a locally advanced BCC of the right medial canthus began experiencing progressive and diffuse hair loss on the beard area, parietal scalp, eyelashes, and eyebrows after 2 months of vismodegib treatment. At 12 months of treatment, he had complete loss of eyelashes and eyebrows (Figure, A). After vismodegib was discontinued due to disease progression, all of his hair began regrowing within several months, with complete hair regrowth observed at 20 months after the last dose (Figure, B).
A 55-year-old man with several locally advanced BCCs developed new-onset mildly pruritic, acneform lesions on the chest and back after 4 months of vismodegib treatment. Biopsy of the lesions showed a folliculocentric mixed dermal infiltrate. The patient did not have a history of follicular dermatitis. The dermatitis resolved several months after onset without treatment, despite continued vismodegib.
A 55-year-old man with locally advanced BCCs developed erythematous dermal plaques on the arms and chest after 2 months of vismodegib treatment. Lesions were asymptomatic. He was not using any other medications and did not have any contact allergen exposures. Punch biopsy showed superficial and deep perivascular dermatitis with occasional eosinophils, consistent with drug hypersensitivity. Although lesions spontaneously resolved without treatment after 1 month, he experienced a couple more bouts of these lesions over the next year. He continued vismodegib for 2 years without return of this eruption.
The average time frame for hair regrowth after vismodegib cessation has not been characterized and awaits future larger studies. The frequency of follicular dermatitis and drug eruption also has not been determined and may require careful observation by dermatologists in larger numbers of treated patients.
Because the hedgehog pathway is critical for normal hair follicle function, follicle-based toxicities of vismodegib including alopecia and folliculitis could be hypothesized to reflect effective blockade of the pathway.6 Currently, there are no data that these changes correlate with tumor response.
Although alopecia is a recognized side effect of vismodegib, regrowth has not been previously reported.1,2 Knowledge of the reversibility of alopecia as well as other toxicities has the potential to influence patient decision-making on drug initiation and adherence.
- Sekulic A, Migden MR, Oro AE, et al. Efficacy and safety of vismodegib in advanced basal-cell carcinoma. N Engl J Med. 2012;366:2171-2179.
- Chang AL, Solomon JA, Hainsworth JD, et al. Expanded access study of patients with advanced basal cell carcinoma treated with the Hedgehog pathway inhibitor, vismodegib. J Am Acad Dermatol. 2014;70:60-69.
- St-Jacques B, Dassule HR, Karavanova I, et al. Sonic hedgehog signaling is essential for hair development. Curr Biol. 1998;8:1058-1068.
- Hall JM, Bell ML, Finger TE. Disruption of sonic hedgehog signaling alters growth and patterning of lingual taste papillae. Dev Biol. 2003;255:263-277.
- Aasi S, Silkiss R, Tang JY, et al. New onset of keratoacanthomas after vismodegib treatment for locally advanced basal cell carcinomas: a report of 2 cases. JAMA Dermatol. 2013;149:242-243.
- Rittie L, Stoll SW, Kang S, et al. Hedgehog signaling maintains hair follicle stem cell phenotype in young and aged human skin. Aging Cell. 2009;8:738-751.
To the Editor:
Vismodegib, a first-in-class inhibitor of the hedgehog signaling pathway, is useful in the treatment of advanced basal cell carcinomas (BCCs).1 Common side effects of vismodegib include alopecia (58%), muscle spasms (71%), and dysgeusia (71%).2 Some of these side effects have been hypothesized to be mechanism related.3,4 Keratoacanthomas have been reported to occur after vismodegib treatment of BCC.5 We report 3 cases illustrating reversible cutaneous side effects of vismodegib: alopecia, follicular dermatitis, and drug hypersensitivity reaction.
A 53-year-old man with a locally advanced BCC of the right medial canthus began experiencing progressive and diffuse hair loss on the beard area, parietal scalp, eyelashes, and eyebrows after 2 months of vismodegib treatment. At 12 months of treatment, he had complete loss of eyelashes and eyebrows (Figure, A). After vismodegib was discontinued due to disease progression, all of his hair began regrowing within several months, with complete hair regrowth observed at 20 months after the last dose (Figure, B).

A 55-year-old man with several locally advanced BCCs developed new-onset mildly pruritic, acneform lesions on the chest and back after 4 months of vismodegib treatment. Biopsy of the lesions showed a folliculocentric mixed dermal infiltrate. The patient did not have a history of follicular dermatitis. The dermatitis resolved several months after onset without treatment, despite continued vismodegib.
A 55-year-old man with locally advanced BCCs developed erythematous dermal plaques on the arms and chest after 2 months of vismodegib treatment. Lesions were asymptomatic. He was not using any other medications and did not have any contact allergen exposures. Punch biopsy showed superficial and deep perivascular dermatitis with occasional eosinophils, consistent with drug hypersensitivity. Although lesions spontaneously resolved without treatment after 1 month, he experienced a couple more bouts of these lesions over the next year. He continued vismodegib for 2 years without return of this eruption.
The average time frame for hair regrowth after vismodegib cessation has not been characterized and awaits future larger studies. The frequency of follicular dermatitis and drug eruption also has not been determined and may require careful observation by dermatologists in larger numbers of treated patients.
Because the hedgehog pathway is critical for normal hair follicle function, follicle-based toxicities of vismodegib including alopecia and folliculitis could be hypothesized to reflect effective blockade of the pathway.6 Currently, there are no data that these changes correlate with tumor response.
Although alopecia is a recognized side effect of vismodegib, regrowth has not been previously reported.1,2 Knowledge of the reversibility of alopecia as well as other toxicities has the potential to influence patient decision-making on drug initiation and adherence.
To the Editor:
Vismodegib, a first-in-class inhibitor of the hedgehog signaling pathway, is useful in the treatment of advanced basal cell carcinomas (BCCs).1 Common side effects of vismodegib include alopecia (58%), muscle spasms (71%), and dysgeusia (71%).2 Some of these side effects have been hypothesized to be mechanism related.3,4 Keratoacanthomas have been reported to occur after vismodegib treatment of BCC.5 We report 3 cases illustrating reversible cutaneous side effects of vismodegib: alopecia, follicular dermatitis, and drug hypersensitivity reaction.
A 53-year-old man with a locally advanced BCC of the right medial canthus began experiencing progressive and diffuse hair loss on the beard area, parietal scalp, eyelashes, and eyebrows after 2 months of vismodegib treatment. At 12 months of treatment, he had complete loss of eyelashes and eyebrows (Figure, A). After vismodegib was discontinued due to disease progression, all of his hair began regrowing within several months, with complete hair regrowth observed at 20 months after the last dose (Figure, B).

A 55-year-old man with several locally advanced BCCs developed new-onset mildly pruritic, acneform lesions on the chest and back after 4 months of vismodegib treatment. Biopsy of the lesions showed a folliculocentric mixed dermal infiltrate. The patient did not have a history of follicular dermatitis. The dermatitis resolved several months after onset without treatment, despite continued vismodegib.
A 55-year-old man with locally advanced BCCs developed erythematous dermal plaques on the arms and chest after 2 months of vismodegib treatment. Lesions were asymptomatic. He was not using any other medications and did not have any contact allergen exposures. Punch biopsy showed superficial and deep perivascular dermatitis with occasional eosinophils, consistent with drug hypersensitivity. Although lesions spontaneously resolved without treatment after 1 month, he experienced a couple more bouts of these lesions over the next year. He continued vismodegib for 2 years without return of this eruption.
The average time frame for hair regrowth after vismodegib cessation has not been characterized and awaits future larger studies. The frequency of follicular dermatitis and drug eruption also has not been determined and may require careful observation by dermatologists in larger numbers of treated patients.
Because the hedgehog pathway is critical for normal hair follicle function, follicle-based toxicities of vismodegib including alopecia and folliculitis could be hypothesized to reflect effective blockade of the pathway.6 Currently, there are no data that these changes correlate with tumor response.
Although alopecia is a recognized side effect of vismodegib, regrowth has not been previously reported.1,2 Knowledge of the reversibility of alopecia as well as other toxicities has the potential to influence patient decision-making on drug initiation and adherence.
- Sekulic A, Migden MR, Oro AE, et al. Efficacy and safety of vismodegib in advanced basal-cell carcinoma. N Engl J Med. 2012;366:2171-2179.
- Chang AL, Solomon JA, Hainsworth JD, et al. Expanded access study of patients with advanced basal cell carcinoma treated with the Hedgehog pathway inhibitor, vismodegib. J Am Acad Dermatol. 2014;70:60-69.
- St-Jacques B, Dassule HR, Karavanova I, et al. Sonic hedgehog signaling is essential for hair development. Curr Biol. 1998;8:1058-1068.
- Hall JM, Bell ML, Finger TE. Disruption of sonic hedgehog signaling alters growth and patterning of lingual taste papillae. Dev Biol. 2003;255:263-277.
- Aasi S, Silkiss R, Tang JY, et al. New onset of keratoacanthomas after vismodegib treatment for locally advanced basal cell carcinomas: a report of 2 cases. JAMA Dermatol. 2013;149:242-243.
- Rittie L, Stoll SW, Kang S, et al. Hedgehog signaling maintains hair follicle stem cell phenotype in young and aged human skin. Aging Cell. 2009;8:738-751.
- Sekulic A, Migden MR, Oro AE, et al. Efficacy and safety of vismodegib in advanced basal-cell carcinoma. N Engl J Med. 2012;366:2171-2179.
- Chang AL, Solomon JA, Hainsworth JD, et al. Expanded access study of patients with advanced basal cell carcinoma treated with the Hedgehog pathway inhibitor, vismodegib. J Am Acad Dermatol. 2014;70:60-69.
- St-Jacques B, Dassule HR, Karavanova I, et al. Sonic hedgehog signaling is essential for hair development. Curr Biol. 1998;8:1058-1068.
- Hall JM, Bell ML, Finger TE. Disruption of sonic hedgehog signaling alters growth and patterning of lingual taste papillae. Dev Biol. 2003;255:263-277.
- Aasi S, Silkiss R, Tang JY, et al. New onset of keratoacanthomas after vismodegib treatment for locally advanced basal cell carcinomas: a report of 2 cases. JAMA Dermatol. 2013;149:242-243.
- Rittie L, Stoll SW, Kang S, et al. Hedgehog signaling maintains hair follicle stem cell phenotype in young and aged human skin. Aging Cell. 2009;8:738-751.
Practice Points
- Hair loss is a common late side effect of vismodegib usage and is reversible, but regrowth takes many months.
- Mild folliculitis that resolves spontaneously has been observed in patients using vismodegib.
- Dermal hypersensitivity has been observed in patients on vismodegib, though the exact frequency of this type of dermatitis is not known.
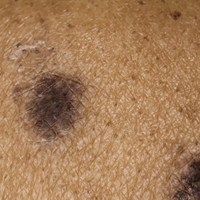
Racial differences in skin cancer risk after organ transplantation
Nonwhite organ transplant recipients (OTRs) are more likely to present with inflammatory or infectious conditions after transplantation, while white organ recipients more commonly present with malignant disease, new research suggests.
While the high incidence of skin cancers has been well described in patients who undergo solid organ transplants, little is known about the risk factors, incidence, locations, and types of skin disease that occur in nonwhite OTRs, wrote Christina Lee Chung, MD, from Drexel University, Philadelphia, and her coauthors in JAMA Dermatology.
In a retrospective review, the investigators examined the medical records of 412 organ transplant recipients treated at an academic referral center during 2011-2016, of whom 154 were white, 35 were Asian, 33 were Hispanic, and 190 were black (JAMA Dermatology. 2017 Mar 8. doi: 10.1001/jamadermatol.2017.0045).
Among the white patients, malignant or premalignant disease was the most common diagnostic category (67.8%), followed by inflammatory (20.7%) and infectious processes (11.6%). However, among nonwhite organ transplant recipients, inflammatory processes were present in 48.8% of patients, infectious processes in 37.5% and the remaining 13.7% presented with malignant or premalignant lesions.
Black and Hispanic patients were more likely to present with inflammatory or infectious disease; only 8.6% presented with malignant conditions and 16% presented with premalignant disease.
Among the Asian patient population, one-third presented with malignant or premalignant, one-third presented with infectious, and one-third presented with inflammatory conditions.
“Although early detection and treatment of cancer is vital, nonwhite OTRs would also benefit from addressing nonmalignant processes that are exacerbated by immunosuppression,” the authors wrote.
Overall, 389 skin cancers were diagnosed, with squamous cell carcinoma in situ (SCC) the most common type of skin cancer diagnosed in each racial or ethnic group. The mean time between transplant and first skin cancer lesion was 12.67 years in black patients, 6.5 years in Hispanic patients, 6.13 years among white patients, and 3.75 years in Asian patients.
The vast majority of skin cancers (95.1%) were found in white patients. While the majority of lesions in white and Asian patients were found in sun-exposed areas, the few skin cancers seen in black patients were more likely to be found in sun-protected areas, particularly the genitals.
Four of the six genital SCCs tested positive for high-risk human papillomavirus strains – in one Asian patient and three black patients – while the two SCCs found on lower extremities in Hispanic patients tested negative for HPV.
Researchers also looked at skin cancer awareness among the organ transplant recipients using data from initial visit questionnaires. They found that more than 17 of the 22 (77.3%) white organ transplant recipients surveyed were aware their skin cancer risk was increased, compared with 30 of the 44 (68.2%) nonwhite patients.
Similarly, 72.7% of white patients surveyed were aware that sunscreen decreased the risk of cancer, compared with 59.1% of nonwhite patients; 27.3% of white patients reported using a daily sunscreen, compared with 13.6% of nonwhite patients.
“Based on our findings, we suggest that optimal posttransplant dermatologic care be determined based on the race or ethnicity of the patients; however, regardless of skin type or race or ethnicity, a baseline full-skin assessment should be performed in all patients,” the authors wrote.
They proposed that skin cancer follow-up screenings should be given to Asian and Hispanic patients immediately after transplant, but that black organ transplant recipients could delay yearly screenings.
However, they said routine skin checks should begin earlier after transplantation for all nonwhite transplant recipients with a history of, or clinically evident HPV infection.
No conflicts of interest were declared.
Nonwhite organ transplant recipients (OTRs) are more likely to present with inflammatory or infectious conditions after transplantation, while white organ recipients more commonly present with malignant disease, new research suggests.
While the high incidence of skin cancers has been well described in patients who undergo solid organ transplants, little is known about the risk factors, incidence, locations, and types of skin disease that occur in nonwhite OTRs, wrote Christina Lee Chung, MD, from Drexel University, Philadelphia, and her coauthors in JAMA Dermatology.
In a retrospective review, the investigators examined the medical records of 412 organ transplant recipients treated at an academic referral center during 2011-2016, of whom 154 were white, 35 were Asian, 33 were Hispanic, and 190 were black (JAMA Dermatology. 2017 Mar 8. doi: 10.1001/jamadermatol.2017.0045).
Among the white patients, malignant or premalignant disease was the most common diagnostic category (67.8%), followed by inflammatory (20.7%) and infectious processes (11.6%). However, among nonwhite organ transplant recipients, inflammatory processes were present in 48.8% of patients, infectious processes in 37.5% and the remaining 13.7% presented with malignant or premalignant lesions.
Black and Hispanic patients were more likely to present with inflammatory or infectious disease; only 8.6% presented with malignant conditions and 16% presented with premalignant disease.
Among the Asian patient population, one-third presented with malignant or premalignant, one-third presented with infectious, and one-third presented with inflammatory conditions.
“Although early detection and treatment of cancer is vital, nonwhite OTRs would also benefit from addressing nonmalignant processes that are exacerbated by immunosuppression,” the authors wrote.
Overall, 389 skin cancers were diagnosed, with squamous cell carcinoma in situ (SCC) the most common type of skin cancer diagnosed in each racial or ethnic group. The mean time between transplant and first skin cancer lesion was 12.67 years in black patients, 6.5 years in Hispanic patients, 6.13 years among white patients, and 3.75 years in Asian patients.
The vast majority of skin cancers (95.1%) were found in white patients. While the majority of lesions in white and Asian patients were found in sun-exposed areas, the few skin cancers seen in black patients were more likely to be found in sun-protected areas, particularly the genitals.
Four of the six genital SCCs tested positive for high-risk human papillomavirus strains – in one Asian patient and three black patients – while the two SCCs found on lower extremities in Hispanic patients tested negative for HPV.
Researchers also looked at skin cancer awareness among the organ transplant recipients using data from initial visit questionnaires. They found that more than 17 of the 22 (77.3%) white organ transplant recipients surveyed were aware their skin cancer risk was increased, compared with 30 of the 44 (68.2%) nonwhite patients.
Similarly, 72.7% of white patients surveyed were aware that sunscreen decreased the risk of cancer, compared with 59.1% of nonwhite patients; 27.3% of white patients reported using a daily sunscreen, compared with 13.6% of nonwhite patients.
“Based on our findings, we suggest that optimal posttransplant dermatologic care be determined based on the race or ethnicity of the patients; however, regardless of skin type or race or ethnicity, a baseline full-skin assessment should be performed in all patients,” the authors wrote.
They proposed that skin cancer follow-up screenings should be given to Asian and Hispanic patients immediately after transplant, but that black organ transplant recipients could delay yearly screenings.
However, they said routine skin checks should begin earlier after transplantation for all nonwhite transplant recipients with a history of, or clinically evident HPV infection.
No conflicts of interest were declared.
Nonwhite organ transplant recipients (OTRs) are more likely to present with inflammatory or infectious conditions after transplantation, while white organ recipients more commonly present with malignant disease, new research suggests.
While the high incidence of skin cancers has been well described in patients who undergo solid organ transplants, little is known about the risk factors, incidence, locations, and types of skin disease that occur in nonwhite OTRs, wrote Christina Lee Chung, MD, from Drexel University, Philadelphia, and her coauthors in JAMA Dermatology.
In a retrospective review, the investigators examined the medical records of 412 organ transplant recipients treated at an academic referral center during 2011-2016, of whom 154 were white, 35 were Asian, 33 were Hispanic, and 190 were black (JAMA Dermatology. 2017 Mar 8. doi: 10.1001/jamadermatol.2017.0045).
Among the white patients, malignant or premalignant disease was the most common diagnostic category (67.8%), followed by inflammatory (20.7%) and infectious processes (11.6%). However, among nonwhite organ transplant recipients, inflammatory processes were present in 48.8% of patients, infectious processes in 37.5% and the remaining 13.7% presented with malignant or premalignant lesions.
Black and Hispanic patients were more likely to present with inflammatory or infectious disease; only 8.6% presented with malignant conditions and 16% presented with premalignant disease.
Among the Asian patient population, one-third presented with malignant or premalignant, one-third presented with infectious, and one-third presented with inflammatory conditions.
“Although early detection and treatment of cancer is vital, nonwhite OTRs would also benefit from addressing nonmalignant processes that are exacerbated by immunosuppression,” the authors wrote.
Overall, 389 skin cancers were diagnosed, with squamous cell carcinoma in situ (SCC) the most common type of skin cancer diagnosed in each racial or ethnic group. The mean time between transplant and first skin cancer lesion was 12.67 years in black patients, 6.5 years in Hispanic patients, 6.13 years among white patients, and 3.75 years in Asian patients.
The vast majority of skin cancers (95.1%) were found in white patients. While the majority of lesions in white and Asian patients were found in sun-exposed areas, the few skin cancers seen in black patients were more likely to be found in sun-protected areas, particularly the genitals.
Four of the six genital SCCs tested positive for high-risk human papillomavirus strains – in one Asian patient and three black patients – while the two SCCs found on lower extremities in Hispanic patients tested negative for HPV.
Researchers also looked at skin cancer awareness among the organ transplant recipients using data from initial visit questionnaires. They found that more than 17 of the 22 (77.3%) white organ transplant recipients surveyed were aware their skin cancer risk was increased, compared with 30 of the 44 (68.2%) nonwhite patients.
Similarly, 72.7% of white patients surveyed were aware that sunscreen decreased the risk of cancer, compared with 59.1% of nonwhite patients; 27.3% of white patients reported using a daily sunscreen, compared with 13.6% of nonwhite patients.
“Based on our findings, we suggest that optimal posttransplant dermatologic care be determined based on the race or ethnicity of the patients; however, regardless of skin type or race or ethnicity, a baseline full-skin assessment should be performed in all patients,” the authors wrote.
They proposed that skin cancer follow-up screenings should be given to Asian and Hispanic patients immediately after transplant, but that black organ transplant recipients could delay yearly screenings.
However, they said routine skin checks should begin earlier after transplantation for all nonwhite transplant recipients with a history of, or clinically evident HPV infection.
No conflicts of interest were declared.
FROM JAMA DERMATOLOGY
Key clinical point: Nonwhite organ transplant recipients are more likely than are white recipients to present with inflammatory or infectious conditions than with skin cancer after transplantation.
Major finding: Malignant or premalignant disease was seen in 67.8% of white organ transplant recipients but just 13.7% of nonwhite recipients.
Data source: A retrospective review of medical records from 412 organ transplant recipients.
Disclosures: No conflicts of interest were declared.
Update on Confocal Microscopy and Skin Cancer Imaging: Report from the AAD Meeting
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Imatinib Mesylate–Induced Lichenoid Drug Eruption
Imatinib mesylate is a tyrosine kinase inhibitor initially approved by the US Food and Drug Administration in 2001 for chronic myeloid leukemia (CML). The indications for imatinib have expanded since its initial approval. It is increasingly important that dermatologists recognize adverse cutaneous manifestations associated with imatinib and are aware of their management and outcomes to avoid unnecessarily discontinuing a potentially lifesaving medication.
Adverse cutaneous manifestations in response to imatinib are not infrequent, accounting for 7% to 21% of all side effects.1 The most frequent cutaneous manifestations of imatinib are dry skin, alopecia, facial edema, and photosensitivity rash, respectively.1 Other less common manifestations include exfoliative dermatitis, nail disorders, psoriasis, folliculitis, hypotrichosis, urticaria, petechiae, Stevens-Johnson syndrome, erythema multiforme, Sweet syndrome, and leukocytoclastic vasculitis.
We report a case of imatinib-induced lichenoid drug eruption (LDE), a rare cutaneous side effect of imatinib use, along with a review of the literature.
Case Report
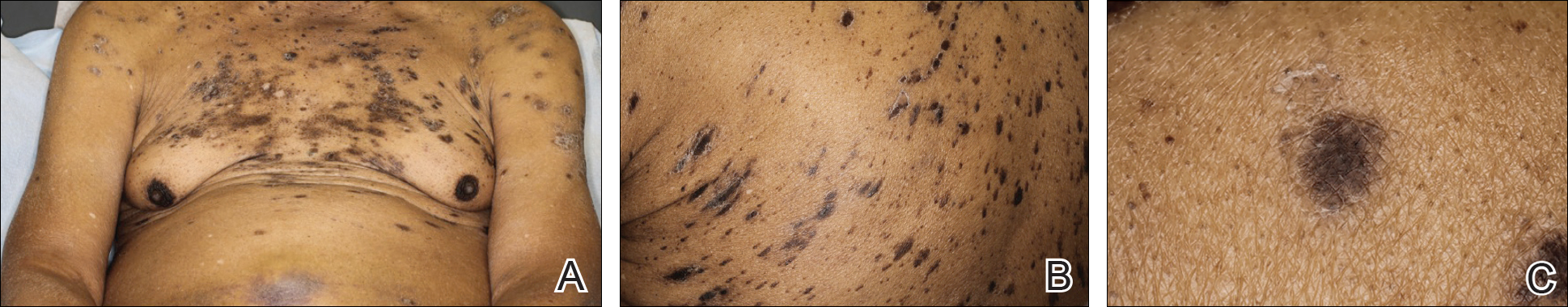
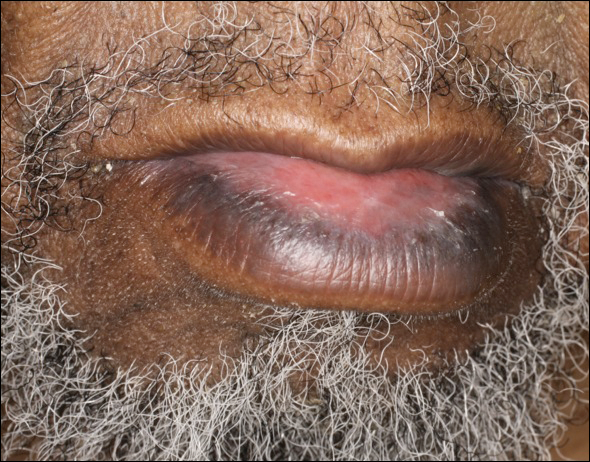
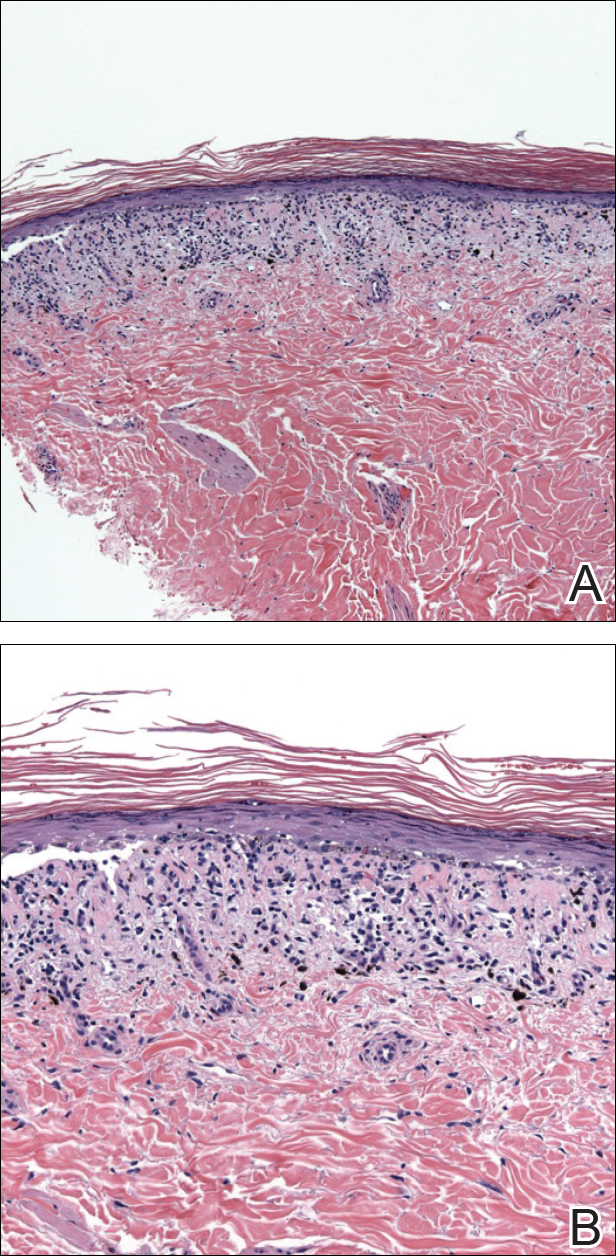
An 86-year-old man with a history of gastrointestinal stromal tumors (GISTs) and myelodysplastic syndrome presented with diffuse hyperpigmented skin lesions on the trunk, arms, legs, and lower lip of 2 weeks’ duration. He had been taking imatinib 400 mg once daily for 5 months for GIST. Although the oncologist stopped the medication 2 weeks prior, the lesions were persistent and gradually expanded to involve the trunk, arms, legs, and lower lip. He denied any pain or pruritus. Physical examination revealed multiple ill-defined, brown to violaceous, slightly scaly macules and patches on the trunk (Figures 1A and 1B), arms, and legs (Figure 1C), as well as violaceous to erythematous patches on the mucosal aspect of the lower lip (Figure 2). Two 4-mm punch biopsies were performed from the chest and back, which revealed an atrophic epidermis, lichenoid infiltration, and multiple melanophages in the upper dermis consistent with LDE (Figure 3). Direct immunofluorescence was negative. Therefore, based on the clinicopathologic correlation, the diagnosis of imatinib-induced LDE was made. He was treated with clobetasol ointment twice daily for 3 weeks with some improvement. His GIST was stable on follow-up computed tomography 3 months after presentation, and imatinib was resumed 1 month later with continued rash that was stable with topical corticosteroid treatment.
Comment
In addition to CML, imatinib has been approved for acute lymphoblastic leukemia, myelodysplastic syndromes, aggressive systemic mastocytosis, hypereosinophilic syndrome, chronic eosinophilic leukemia, dermatofibrosarcoma protuberans, and GIST. Moreover, off-label use of imatinib for various other tyrosine kinase–positive cancers and rheumatologic conditions have been documented.2,3 With the expanding use of imatinib, there will be more occasions for dermatologists to encounter cutaneous manifestations associated with its use.
According to a PubMed search of articles indexed for MEDLINE using the terms imatinib mesylate lichenoid drug, there have been few case reports of LDE associated with imatinib in the literature (eTable).4-24 Compared to classic LDE, imatinib-induced LDE has a few characteristic findings. Classic LDE frequently spares the oral mucosa and genitalia, but imatinib-induced LDE with manifestations on the oral mucosa and genitalia as well as cutaneous eruptions have been reported.4-9 In fact, the first known case of imatinib-induced LDE was an oral eruption in a patient with CML.4 In patients with oral involvement, lesions have been described as lacy reticular macules and violaceous papules, erosions, and ulcers.4,5,12 Interestingly, of those cases manifesting as concomitant oral and cutaneous LDE, the oral eruptions recurred more frequently, with 3 of 12 patients having recurrence of oral lesions after the cutaneous manifestations resolved.8,16 Genital manifestations of imatinib-induced LDE were much less common.9,11
To date, subsequent reports of imatinib-induced LDE have documented skin manifestations consistent with classic LDE occurring in a diffuse, bilateral, photodistributed pattern.10,15,16 One case presented with diffuse hyperpigmentation associated with LDE in a Japanese patient.20 The authors suggested this finding may be more prominent in patients with skin of color,20 which is consistent with the current case. Nail findings such as subungual hyperkeratosis and longitudinal ridging also have been reported.9,11
The latency period between initiation of imat-inib and onset of LDE generally ranges from 1 to 12 months, with onset most commonly occurring between 2 to 5 months or with dosage increase (eTable). Imatinib-induced LDE primarily has been documented with a 400-mg dose, with 1 case of a 600-mg dose and 1 case of an 800-mg dose, which suggests dose dependency. Furthermore, reports exist of several patients responding well to dose reduction with subsequent recurrence on dose reescalation.13,15
Historically, LDE resolves with discontinuation of the drug after a few weeks to months. When discontinuation of imatinib is unfavorable or patients report symptoms including severe pruritus or pain, treatment should be considered. Topical or oral corticosteroids can be used to treat imatinib-induced LDE, similar to lichen planus. When oral corticosteroids are contraindicated (eg, due to poor patient tolerance), oral acitretin at 25 to 35 mg once daily for 6 to 12 weeks has been reported as an alternative treatment.25
In the majority of cases of imatinib-induced LDE, it was undesirable to stop imatinib (eTable). Notably, in half the reported cases, imatinib was able to be continued and patients were treated symptomatically with either oral and/or topical steroids and/or acitretin with complete remission or tolerable recurrences. Dalmau et al9 reported 3 patients who responded poorly to topical and oral steroids and were subsequently treated with acitretin 25 mg once daily; 2 of 3 patients responded favorably to treatment and imatinib was able to be continued. In the current case imatinib initially helped, but because his rash was relatively asymptomatic, imatinib was restarted with control of rash with topical steroids. He developed some pancytopenia, which required intermittent stoppage of the imatinib.
Conclusion
We present a case of imatinib-induced cutaneous and oral LDE in a patient with GIST. Topical corticosteroids, oral acitretin, and oral steroids all may be reasonable treatment options if discontinuing imatinib is not possible in a symptomatic patient. If these therapies fail and the eruption is extensive or intolerable, dosage adjustment is another option to consider before discontinuation of imatinib.
- Scheinfeld N. Imatinib mesylate and dermatology part 2: a review of the cutaneous side effects of imatinib mesylate. J Drugs Dermatol. 2006;5:228-231.
- Kim H, Kim NH, Kang HJ, et al. Successful long-term use of imatinib mesylate in pediatric patients with sclerodermatous chronic GVHD. Pediatr Transplant. 2012;16:910-912.
- Prey S, Ezzedine K, Doussau A, et al. Imatinib mesylate in scleroderma-associated diffuse skin fibrosis: a phase II multicentre randomized double-blinded controlled trial. Br J Dermatol. 2012;167:1138-1144.
- Lim DS, Muir J. Oral lichenoid reaction to imatinib (STI 571, gleevec). Dermatology. 2002;205:169-171.
- Ena P, Chiarolini F, Siddi GM, et al. Oral lichenoid eruption secondary to imatinib (glivec). J Dermatolog Treat. 2004;15:253-255.
- Roux C, Boisseau-Garsaud AM, Saint-Cyr I, et al. Lichenoid cutaneous reaction to imatinib. Ann Dermatol Venereol. 2004;131:571-573.
- Prabhash K, Doval DC. Lichenoid eruption due to imat-inib. Indian J Dermatol Venereol Leprol. 2005;71:287-288.
- Pascual JC, Matarredona J, Miralles J, et al. Oral and cutaneous lichenoid reaction secondary to imatinib: report of two cases. Int J Dermatol. 2006;45:1471-1473.
- Dalmau J, Peramiquel L, Puig L, et al. Imatinib-associated lichenoid eruption: acitretin treatment allows maintained antineoplastic effect. Br J Dermatol. 2006;154:1213-1216.
- Chan CY, Browning J, Smith-Zagone MJ, et al. Cutaneous lichenoid dermatitis associated with imatinib mesylate. Dermatol Online J. 2007;13:29.
- Wahiduzzaman M, Pubalan M. Oral and cutaneous lichenoid reaction with nail changes secondary to imatinib: report of a case and literature review. Dermatol Online J. 2008;14:14.
- Basso FG, Boer CC, Correa ME, et al. Skin and oral lesions associated to imatinib mesylate therapy. Support Care Cancer. 2009;17:465-468.
- Kawakami T, Kawanabe T, Soma Y. Cutaneous lichenoid eruption caused by imatinib mesylate in a Japanese patient with chronic myeloid leukaemia. Acta Derm Venereol. 2009;89:325-326.
- Sendagorta E, Herranz P, Feito M, et al. Lichenoid drug eruption related to imatinib: report of a new case and review of the literature. Clin Exp Dermatol. 2009;34:E315-E316.
- Kuraishi N, Nagai Y, Hasegawa M, et al. Lichenoid drug eruption with palmoplantar hyperkeratosis due to imatinib mesylate: a case report and a review of the literature. Acta Derm Venereol. 2010;90:73-76.
- Brazzelli V, Muzio F, Manna G, et al. Photo-induced dermatitis and oral lichenoid reaction in a chronic myeloid leukemia patient treated with imatinib mesylate. Photodermatol Photoimmunol Photomed. 2012;28:2-5.
- Ghosh SK. Generalized lichenoid drug eruption associated with imatinib mesylate therapy. Indian J Dermatol. 2013;58:388-392.
- Lee J, Chung J, Jung M, et al. Lichenoid drug eruption after low-dose imatinib mesylate therapy. Ann Dermatol. 2013;25:500-502.
- Machaczka M, Gossart M. Multiple skin lesions caused by imatinib mesylate treatment of chronic myeloid leukemia. Pol Arch Med Wewn. 2013;123:251-252.
- Kagimoto Y, Mizuashi M, Kikuchi K, et al. Lichenoid drug eruption with hyperpigmentation caused by imatinib mesylate [published online June 20, 2013]. Int J Dermatol. 2014;53:E161-E162.
- Arshdeep, De D, Malhotra P, et al. Imatinib mesylate-induced severe lichenoid rash. Indian J Dermatol Venereol Leprol. 2014;80:93-95.
- Lau YM, Lam YK, Leung KH, et al. Trachyonychia in a patient with chronic myeloid leukaemia after imatinib mesylate. Hong Kong Med J. 2014;20:464.e2.
- Bhatia A, Kanish B, Chaudhary P. Lichenoid drug eruption due to imatinib mesylate. Int J Appl Basic Med Res. 2015;5:68-69.
- Luo JR, Xiang XJ, Xiong JP. Lichenoid drug eruption caused by imatinib mesylate in a Chinese patient with gastrointestinal stromal tumor. Int J Clin Pharmacol Ther. 2016;54:719-722.
- Laurberg G, Geiger JM, Hjorth N, et al. Treatment of lichen planus with acitretin. a double-blind, placebo-controlled study in 65 patients. J Am Acad Dermatol. 1991;24:434-437.
Imatinib mesylate is a tyrosine kinase inhibitor initially approved by the US Food and Drug Administration in 2001 for chronic myeloid leukemia (CML). The indications for imatinib have expanded since its initial approval. It is increasingly important that dermatologists recognize adverse cutaneous manifestations associated with imatinib and are aware of their management and outcomes to avoid unnecessarily discontinuing a potentially lifesaving medication.
Adverse cutaneous manifestations in response to imatinib are not infrequent, accounting for 7% to 21% of all side effects.1 The most frequent cutaneous manifestations of imatinib are dry skin, alopecia, facial edema, and photosensitivity rash, respectively.1 Other less common manifestations include exfoliative dermatitis, nail disorders, psoriasis, folliculitis, hypotrichosis, urticaria, petechiae, Stevens-Johnson syndrome, erythema multiforme, Sweet syndrome, and leukocytoclastic vasculitis.
We report a case of imatinib-induced lichenoid drug eruption (LDE), a rare cutaneous side effect of imatinib use, along with a review of the literature.
Case Report
An 86-year-old man with a history of gastrointestinal stromal tumors (GISTs) and myelodysplastic syndrome presented with diffuse hyperpigmented skin lesions on the trunk, arms, legs, and lower lip of 2 weeks’ duration. He had been taking imatinib 400 mg once daily for 5 months for GIST. Although the oncologist stopped the medication 2 weeks prior, the lesions were persistent and gradually expanded to involve the trunk, arms, legs, and lower lip. He denied any pain or pruritus. Physical examination revealed multiple ill-defined, brown to violaceous, slightly scaly macules and patches on the trunk (Figures 1A and 1B), arms, and legs (Figure 1C), as well as violaceous to erythematous patches on the mucosal aspect of the lower lip (Figure 2). Two 4-mm punch biopsies were performed from the chest and back, which revealed an atrophic epidermis, lichenoid infiltration, and multiple melanophages in the upper dermis consistent with LDE (Figure 3). Direct immunofluorescence was negative. Therefore, based on the clinicopathologic correlation, the diagnosis of imatinib-induced LDE was made. He was treated with clobetasol ointment twice daily for 3 weeks with some improvement. His GIST was stable on follow-up computed tomography 3 months after presentation, and imatinib was resumed 1 month later with continued rash that was stable with topical corticosteroid treatment.



Comment
In addition to CML, imatinib has been approved for acute lymphoblastic leukemia, myelodysplastic syndromes, aggressive systemic mastocytosis, hypereosinophilic syndrome, chronic eosinophilic leukemia, dermatofibrosarcoma protuberans, and GIST. Moreover, off-label use of imatinib for various other tyrosine kinase–positive cancers and rheumatologic conditions have been documented.2,3 With the expanding use of imatinib, there will be more occasions for dermatologists to encounter cutaneous manifestations associated with its use.
According to a PubMed search of articles indexed for MEDLINE using the terms imatinib mesylate lichenoid drug, there have been few case reports of LDE associated with imatinib in the literature (eTable).4-24 Compared to classic LDE, imatinib-induced LDE has a few characteristic findings. Classic LDE frequently spares the oral mucosa and genitalia, but imatinib-induced LDE with manifestations on the oral mucosa and genitalia as well as cutaneous eruptions have been reported.4-9 In fact, the first known case of imatinib-induced LDE was an oral eruption in a patient with CML.4 In patients with oral involvement, lesions have been described as lacy reticular macules and violaceous papules, erosions, and ulcers.4,5,12 Interestingly, of those cases manifesting as concomitant oral and cutaneous LDE, the oral eruptions recurred more frequently, with 3 of 12 patients having recurrence of oral lesions after the cutaneous manifestations resolved.8,16 Genital manifestations of imatinib-induced LDE were much less common.9,11
To date, subsequent reports of imatinib-induced LDE have documented skin manifestations consistent with classic LDE occurring in a diffuse, bilateral, photodistributed pattern.10,15,16 One case presented with diffuse hyperpigmentation associated with LDE in a Japanese patient.20 The authors suggested this finding may be more prominent in patients with skin of color,20 which is consistent with the current case. Nail findings such as subungual hyperkeratosis and longitudinal ridging also have been reported.9,11
The latency period between initiation of imat-inib and onset of LDE generally ranges from 1 to 12 months, with onset most commonly occurring between 2 to 5 months or with dosage increase (eTable). Imatinib-induced LDE primarily has been documented with a 400-mg dose, with 1 case of a 600-mg dose and 1 case of an 800-mg dose, which suggests dose dependency. Furthermore, reports exist of several patients responding well to dose reduction with subsequent recurrence on dose reescalation.13,15
Historically, LDE resolves with discontinuation of the drug after a few weeks to months. When discontinuation of imatinib is unfavorable or patients report symptoms including severe pruritus or pain, treatment should be considered. Topical or oral corticosteroids can be used to treat imatinib-induced LDE, similar to lichen planus. When oral corticosteroids are contraindicated (eg, due to poor patient tolerance), oral acitretin at 25 to 35 mg once daily for 6 to 12 weeks has been reported as an alternative treatment.25
In the majority of cases of imatinib-induced LDE, it was undesirable to stop imatinib (eTable). Notably, in half the reported cases, imatinib was able to be continued and patients were treated symptomatically with either oral and/or topical steroids and/or acitretin with complete remission or tolerable recurrences. Dalmau et al9 reported 3 patients who responded poorly to topical and oral steroids and were subsequently treated with acitretin 25 mg once daily; 2 of 3 patients responded favorably to treatment and imatinib was able to be continued. In the current case imatinib initially helped, but because his rash was relatively asymptomatic, imatinib was restarted with control of rash with topical steroids. He developed some pancytopenia, which required intermittent stoppage of the imatinib.
Conclusion
We present a case of imatinib-induced cutaneous and oral LDE in a patient with GIST. Topical corticosteroids, oral acitretin, and oral steroids all may be reasonable treatment options if discontinuing imatinib is not possible in a symptomatic patient. If these therapies fail and the eruption is extensive or intolerable, dosage adjustment is another option to consider before discontinuation of imatinib.
Imatinib mesylate is a tyrosine kinase inhibitor initially approved by the US Food and Drug Administration in 2001 for chronic myeloid leukemia (CML). The indications for imatinib have expanded since its initial approval. It is increasingly important that dermatologists recognize adverse cutaneous manifestations associated with imatinib and are aware of their management and outcomes to avoid unnecessarily discontinuing a potentially lifesaving medication.
Adverse cutaneous manifestations in response to imatinib are not infrequent, accounting for 7% to 21% of all side effects.1 The most frequent cutaneous manifestations of imatinib are dry skin, alopecia, facial edema, and photosensitivity rash, respectively.1 Other less common manifestations include exfoliative dermatitis, nail disorders, psoriasis, folliculitis, hypotrichosis, urticaria, petechiae, Stevens-Johnson syndrome, erythema multiforme, Sweet syndrome, and leukocytoclastic vasculitis.
We report a case of imatinib-induced lichenoid drug eruption (LDE), a rare cutaneous side effect of imatinib use, along with a review of the literature.
Case Report
An 86-year-old man with a history of gastrointestinal stromal tumors (GISTs) and myelodysplastic syndrome presented with diffuse hyperpigmented skin lesions on the trunk, arms, legs, and lower lip of 2 weeks’ duration. He had been taking imatinib 400 mg once daily for 5 months for GIST. Although the oncologist stopped the medication 2 weeks prior, the lesions were persistent and gradually expanded to involve the trunk, arms, legs, and lower lip. He denied any pain or pruritus. Physical examination revealed multiple ill-defined, brown to violaceous, slightly scaly macules and patches on the trunk (Figures 1A and 1B), arms, and legs (Figure 1C), as well as violaceous to erythematous patches on the mucosal aspect of the lower lip (Figure 2). Two 4-mm punch biopsies were performed from the chest and back, which revealed an atrophic epidermis, lichenoid infiltration, and multiple melanophages in the upper dermis consistent with LDE (Figure 3). Direct immunofluorescence was negative. Therefore, based on the clinicopathologic correlation, the diagnosis of imatinib-induced LDE was made. He was treated with clobetasol ointment twice daily for 3 weeks with some improvement. His GIST was stable on follow-up computed tomography 3 months after presentation, and imatinib was resumed 1 month later with continued rash that was stable with topical corticosteroid treatment.



Comment
In addition to CML, imatinib has been approved for acute lymphoblastic leukemia, myelodysplastic syndromes, aggressive systemic mastocytosis, hypereosinophilic syndrome, chronic eosinophilic leukemia, dermatofibrosarcoma protuberans, and GIST. Moreover, off-label use of imatinib for various other tyrosine kinase–positive cancers and rheumatologic conditions have been documented.2,3 With the expanding use of imatinib, there will be more occasions for dermatologists to encounter cutaneous manifestations associated with its use.
According to a PubMed search of articles indexed for MEDLINE using the terms imatinib mesylate lichenoid drug, there have been few case reports of LDE associated with imatinib in the literature (eTable).4-24 Compared to classic LDE, imatinib-induced LDE has a few characteristic findings. Classic LDE frequently spares the oral mucosa and genitalia, but imatinib-induced LDE with manifestations on the oral mucosa and genitalia as well as cutaneous eruptions have been reported.4-9 In fact, the first known case of imatinib-induced LDE was an oral eruption in a patient with CML.4 In patients with oral involvement, lesions have been described as lacy reticular macules and violaceous papules, erosions, and ulcers.4,5,12 Interestingly, of those cases manifesting as concomitant oral and cutaneous LDE, the oral eruptions recurred more frequently, with 3 of 12 patients having recurrence of oral lesions after the cutaneous manifestations resolved.8,16 Genital manifestations of imatinib-induced LDE were much less common.9,11
To date, subsequent reports of imatinib-induced LDE have documented skin manifestations consistent with classic LDE occurring in a diffuse, bilateral, photodistributed pattern.10,15,16 One case presented with diffuse hyperpigmentation associated with LDE in a Japanese patient.20 The authors suggested this finding may be more prominent in patients with skin of color,20 which is consistent with the current case. Nail findings such as subungual hyperkeratosis and longitudinal ridging also have been reported.9,11
The latency period between initiation of imat-inib and onset of LDE generally ranges from 1 to 12 months, with onset most commonly occurring between 2 to 5 months or with dosage increase (eTable). Imatinib-induced LDE primarily has been documented with a 400-mg dose, with 1 case of a 600-mg dose and 1 case of an 800-mg dose, which suggests dose dependency. Furthermore, reports exist of several patients responding well to dose reduction with subsequent recurrence on dose reescalation.13,15
Historically, LDE resolves with discontinuation of the drug after a few weeks to months. When discontinuation of imatinib is unfavorable or patients report symptoms including severe pruritus or pain, treatment should be considered. Topical or oral corticosteroids can be used to treat imatinib-induced LDE, similar to lichen planus. When oral corticosteroids are contraindicated (eg, due to poor patient tolerance), oral acitretin at 25 to 35 mg once daily for 6 to 12 weeks has been reported as an alternative treatment.25
In the majority of cases of imatinib-induced LDE, it was undesirable to stop imatinib (eTable). Notably, in half the reported cases, imatinib was able to be continued and patients were treated symptomatically with either oral and/or topical steroids and/or acitretin with complete remission or tolerable recurrences. Dalmau et al9 reported 3 patients who responded poorly to topical and oral steroids and were subsequently treated with acitretin 25 mg once daily; 2 of 3 patients responded favorably to treatment and imatinib was able to be continued. In the current case imatinib initially helped, but because his rash was relatively asymptomatic, imatinib was restarted with control of rash with topical steroids. He developed some pancytopenia, which required intermittent stoppage of the imatinib.
Conclusion
We present a case of imatinib-induced cutaneous and oral LDE in a patient with GIST. Topical corticosteroids, oral acitretin, and oral steroids all may be reasonable treatment options if discontinuing imatinib is not possible in a symptomatic patient. If these therapies fail and the eruption is extensive or intolerable, dosage adjustment is another option to consider before discontinuation of imatinib.
- Scheinfeld N. Imatinib mesylate and dermatology part 2: a review of the cutaneous side effects of imatinib mesylate. J Drugs Dermatol. 2006;5:228-231.
- Kim H, Kim NH, Kang HJ, et al. Successful long-term use of imatinib mesylate in pediatric patients with sclerodermatous chronic GVHD. Pediatr Transplant. 2012;16:910-912.
- Prey S, Ezzedine K, Doussau A, et al. Imatinib mesylate in scleroderma-associated diffuse skin fibrosis: a phase II multicentre randomized double-blinded controlled trial. Br J Dermatol. 2012;167:1138-1144.
- Lim DS, Muir J. Oral lichenoid reaction to imatinib (STI 571, gleevec). Dermatology. 2002;205:169-171.
- Ena P, Chiarolini F, Siddi GM, et al. Oral lichenoid eruption secondary to imatinib (glivec). J Dermatolog Treat. 2004;15:253-255.
- Roux C, Boisseau-Garsaud AM, Saint-Cyr I, et al. Lichenoid cutaneous reaction to imatinib. Ann Dermatol Venereol. 2004;131:571-573.
- Prabhash K, Doval DC. Lichenoid eruption due to imat-inib. Indian J Dermatol Venereol Leprol. 2005;71:287-288.
- Pascual JC, Matarredona J, Miralles J, et al. Oral and cutaneous lichenoid reaction secondary to imatinib: report of two cases. Int J Dermatol. 2006;45:1471-1473.
- Dalmau J, Peramiquel L, Puig L, et al. Imatinib-associated lichenoid eruption: acitretin treatment allows maintained antineoplastic effect. Br J Dermatol. 2006;154:1213-1216.
- Chan CY, Browning J, Smith-Zagone MJ, et al. Cutaneous lichenoid dermatitis associated with imatinib mesylate. Dermatol Online J. 2007;13:29.
- Wahiduzzaman M, Pubalan M. Oral and cutaneous lichenoid reaction with nail changes secondary to imatinib: report of a case and literature review. Dermatol Online J. 2008;14:14.
- Basso FG, Boer CC, Correa ME, et al. Skin and oral lesions associated to imatinib mesylate therapy. Support Care Cancer. 2009;17:465-468.
- Kawakami T, Kawanabe T, Soma Y. Cutaneous lichenoid eruption caused by imatinib mesylate in a Japanese patient with chronic myeloid leukaemia. Acta Derm Venereol. 2009;89:325-326.
- Sendagorta E, Herranz P, Feito M, et al. Lichenoid drug eruption related to imatinib: report of a new case and review of the literature. Clin Exp Dermatol. 2009;34:E315-E316.
- Kuraishi N, Nagai Y, Hasegawa M, et al. Lichenoid drug eruption with palmoplantar hyperkeratosis due to imatinib mesylate: a case report and a review of the literature. Acta Derm Venereol. 2010;90:73-76.
- Brazzelli V, Muzio F, Manna G, et al. Photo-induced dermatitis and oral lichenoid reaction in a chronic myeloid leukemia patient treated with imatinib mesylate. Photodermatol Photoimmunol Photomed. 2012;28:2-5.
- Ghosh SK. Generalized lichenoid drug eruption associated with imatinib mesylate therapy. Indian J Dermatol. 2013;58:388-392.
- Lee J, Chung J, Jung M, et al. Lichenoid drug eruption after low-dose imatinib mesylate therapy. Ann Dermatol. 2013;25:500-502.
- Machaczka M, Gossart M. Multiple skin lesions caused by imatinib mesylate treatment of chronic myeloid leukemia. Pol Arch Med Wewn. 2013;123:251-252.
- Kagimoto Y, Mizuashi M, Kikuchi K, et al. Lichenoid drug eruption with hyperpigmentation caused by imatinib mesylate [published online June 20, 2013]. Int J Dermatol. 2014;53:E161-E162.
- Arshdeep, De D, Malhotra P, et al. Imatinib mesylate-induced severe lichenoid rash. Indian J Dermatol Venereol Leprol. 2014;80:93-95.
- Lau YM, Lam YK, Leung KH, et al. Trachyonychia in a patient with chronic myeloid leukaemia after imatinib mesylate. Hong Kong Med J. 2014;20:464.e2.
- Bhatia A, Kanish B, Chaudhary P. Lichenoid drug eruption due to imatinib mesylate. Int J Appl Basic Med Res. 2015;5:68-69.
- Luo JR, Xiang XJ, Xiong JP. Lichenoid drug eruption caused by imatinib mesylate in a Chinese patient with gastrointestinal stromal tumor. Int J Clin Pharmacol Ther. 2016;54:719-722.
- Laurberg G, Geiger JM, Hjorth N, et al. Treatment of lichen planus with acitretin. a double-blind, placebo-controlled study in 65 patients. J Am Acad Dermatol. 1991;24:434-437.
- Scheinfeld N. Imatinib mesylate and dermatology part 2: a review of the cutaneous side effects of imatinib mesylate. J Drugs Dermatol. 2006;5:228-231.
- Kim H, Kim NH, Kang HJ, et al. Successful long-term use of imatinib mesylate in pediatric patients with sclerodermatous chronic GVHD. Pediatr Transplant. 2012;16:910-912.
- Prey S, Ezzedine K, Doussau A, et al. Imatinib mesylate in scleroderma-associated diffuse skin fibrosis: a phase II multicentre randomized double-blinded controlled trial. Br J Dermatol. 2012;167:1138-1144.
- Lim DS, Muir J. Oral lichenoid reaction to imatinib (STI 571, gleevec). Dermatology. 2002;205:169-171.
- Ena P, Chiarolini F, Siddi GM, et al. Oral lichenoid eruption secondary to imatinib (glivec). J Dermatolog Treat. 2004;15:253-255.
- Roux C, Boisseau-Garsaud AM, Saint-Cyr I, et al. Lichenoid cutaneous reaction to imatinib. Ann Dermatol Venereol. 2004;131:571-573.
- Prabhash K, Doval DC. Lichenoid eruption due to imat-inib. Indian J Dermatol Venereol Leprol. 2005;71:287-288.
- Pascual JC, Matarredona J, Miralles J, et al. Oral and cutaneous lichenoid reaction secondary to imatinib: report of two cases. Int J Dermatol. 2006;45:1471-1473.
- Dalmau J, Peramiquel L, Puig L, et al. Imatinib-associated lichenoid eruption: acitretin treatment allows maintained antineoplastic effect. Br J Dermatol. 2006;154:1213-1216.
- Chan CY, Browning J, Smith-Zagone MJ, et al. Cutaneous lichenoid dermatitis associated with imatinib mesylate. Dermatol Online J. 2007;13:29.
- Wahiduzzaman M, Pubalan M. Oral and cutaneous lichenoid reaction with nail changes secondary to imatinib: report of a case and literature review. Dermatol Online J. 2008;14:14.
- Basso FG, Boer CC, Correa ME, et al. Skin and oral lesions associated to imatinib mesylate therapy. Support Care Cancer. 2009;17:465-468.
- Kawakami T, Kawanabe T, Soma Y. Cutaneous lichenoid eruption caused by imatinib mesylate in a Japanese patient with chronic myeloid leukaemia. Acta Derm Venereol. 2009;89:325-326.
- Sendagorta E, Herranz P, Feito M, et al. Lichenoid drug eruption related to imatinib: report of a new case and review of the literature. Clin Exp Dermatol. 2009;34:E315-E316.
- Kuraishi N, Nagai Y, Hasegawa M, et al. Lichenoid drug eruption with palmoplantar hyperkeratosis due to imatinib mesylate: a case report and a review of the literature. Acta Derm Venereol. 2010;90:73-76.
- Brazzelli V, Muzio F, Manna G, et al. Photo-induced dermatitis and oral lichenoid reaction in a chronic myeloid leukemia patient treated with imatinib mesylate. Photodermatol Photoimmunol Photomed. 2012;28:2-5.
- Ghosh SK. Generalized lichenoid drug eruption associated with imatinib mesylate therapy. Indian J Dermatol. 2013;58:388-392.
- Lee J, Chung J, Jung M, et al. Lichenoid drug eruption after low-dose imatinib mesylate therapy. Ann Dermatol. 2013;25:500-502.
- Machaczka M, Gossart M. Multiple skin lesions caused by imatinib mesylate treatment of chronic myeloid leukemia. Pol Arch Med Wewn. 2013;123:251-252.
- Kagimoto Y, Mizuashi M, Kikuchi K, et al. Lichenoid drug eruption with hyperpigmentation caused by imatinib mesylate [published online June 20, 2013]. Int J Dermatol. 2014;53:E161-E162.
- Arshdeep, De D, Malhotra P, et al. Imatinib mesylate-induced severe lichenoid rash. Indian J Dermatol Venereol Leprol. 2014;80:93-95.
- Lau YM, Lam YK, Leung KH, et al. Trachyonychia in a patient with chronic myeloid leukaemia after imatinib mesylate. Hong Kong Med J. 2014;20:464.e2.
- Bhatia A, Kanish B, Chaudhary P. Lichenoid drug eruption due to imatinib mesylate. Int J Appl Basic Med Res. 2015;5:68-69.
- Luo JR, Xiang XJ, Xiong JP. Lichenoid drug eruption caused by imatinib mesylate in a Chinese patient with gastrointestinal stromal tumor. Int J Clin Pharmacol Ther. 2016;54:719-722.
- Laurberg G, Geiger JM, Hjorth N, et al. Treatment of lichen planus with acitretin. a double-blind, placebo-controlled study in 65 patients. J Am Acad Dermatol. 1991;24:434-437.
Practice Points
- Imatinib mesylate can cause cutaneous adverse reactions including dry skin, alopecia, facial edema, photosensitivity rash, and lichenoid drug eruption (LDE).
- Topical corticosteroids, oral acitretin, and oral steroids may be reasonable treatment options for imatinib-induced LDE if discontinuing imatinib is not possible in a symptomatic patient.
Late-Onset Bexarotene-Induced CD4 Lymphopenia in a Cutaneous T-cell Lymphoma Patient
Infections, autoimmune disease, bone marrow failure, medications, and total-body irradiation may induce CD4 lymphopenia, defined as a CD4 T-cell count below 300 cells/mL or less than 20% of total lymphocytes.1 Human immunodeficiency virus (HIV) is the most common cause of CD4 lymphopenia, with sepsis (bacterial and fungal) and postoperative states the most common causes in hospital settings.2 No underlying factors are found in 0.02% of CD4 lymphopenia cases, which are considered to be idiopathic.3,4 We report a patient with cutaneous T-cell lymphoma (CTCL) who developed profound CD4 lymphopenia in the setting of long-term bexarotene therapy.
Case Report
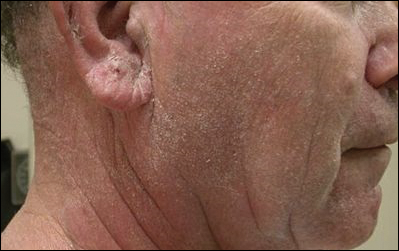
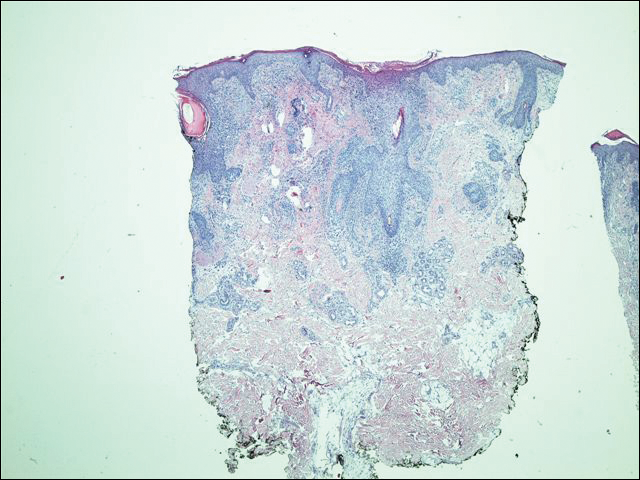
A 63-year-old man with hypertension presented to our dermatology clinic with pruritic scaly plaques on the scalp of 4 months’ duration that had progressed to full-body exfoliative erythroderma (Figure 1). He had diffuse palmoplantar keratoderma and lymphadenopathy. His only long-term medications were terazosin for benign prostatic hyperplasia and atenolol for hypertension; he reported no new medications. Laboratory evaluation revealed normal liver and kidney function. A complete blood cell count (CBC) revealed a white blood cell (WBC) count within reference range (8000/µL [reference range, 4500–11,000/µL]) but with increased eosinophils (12.9% [reference range, 2.7%]) and monocytes (11.8% [reference range, 4%]) and reduced lymphocytes (16.8% [reference range, 34%]). Flow cytometry showed a CD4:CD8 ratio of 1.18 to 1 (reference range, 0.8–4.2)(absolute CD4+ cells, 764/µL [reference range, 297–1551/µL]; absolute CD8+ cells, 654/µL [reference range, 100–1047/µL]). Skin biopsy revealed subacute spongiotic dermatitis with numerous eosinophils, exocytosis including folliculotropism, and rare atypical lymphocytes (Figure 2). Molecular studies showed T-cell receptor γ gene rearrangement. The patient did not have any other underlying conditions that would predispose him to lymphopenia. Based on these findings, a diagnosis of CTCL stage IIIA was made and agreed on by experts at the University of California, San Diego Dermatology Grand Rounds.

The patient was subsequently started on acitretin, topical corticosteroids, and hydroxyzine. However, the erythroderma progressed and he developed fever, chills, and malaise, and he was hospitalized 2 months later for intensive therapy and to rule out infection. He improved on daily wet wraps, topical steroids, oral antibiotics, and initiation of narrowband UVB therapy. He was discharged 1 week later. Acitretin was switched to bexarotene 3 months later due to peeling and cracking of the palmoplantar skin. The initial dose was 225 mg once daily, which was steadily increased over the next 4 months to a therapeutic dose of 600 mg once daily, which was much lower than the maximum dose of 400 mg/m2 daily (calculated at 750 mg/d in our patient). The patient achieved clinical remission 1 year after initiation of bexarotene in conjunction with narrowband UVB therapy. Serum eosinophils also normalized. Because there were no intolerable side effects, this dose was continued for 2 more years before it was slowly tapered to 375 mg once daily over a 1-year period. The new dose was maintained thereafter. Secondary hypertriglyceridemia and hypothyroidism, known side effects of bexarotene, developed 1 and 5 months after initiating therapy, respectively, and were treated with levothyroxine and fenofibrate. Blood counts were checked every 3 months and remained within reference range. Within the first few months of therapy, lymphocytes did trend down to 16.8%, but segmented neutrophils were normal at 59.4%. For the next 5 years the total WBC count and differential remained within reference range. T-cell subsets and flow cytometry data were not measured. No new medications were started during this period, and none of his existing medications had lymphopenia as a known side effect.
Five years after the initial diagnosis, the patient was still on bexarotene and was suspected to have pneumonia that was treated by his primary care provider with cefuroxime and azithromycin for 2 weeks with no improvement. He was then admitted to the hospital with shortness of breath, productive cough, night sweats, and dyspnea of 1 month’s duration. There was no associated weight loss or fever. Notably, the skin was clear. He was further treated for community-acquired pneumonia, first with vancomycin and ceftazidime, then with ciprofloxacin and sulfamethoxazole-trimethoprim, with no improvement. A CBC with differential was obtained on the patient’s first admission and revealed a WBC count of 3600/µL with decreased lymphocytes (8.6%), no eosinophilia, and anemia (hemoglobin, 10.5 g/dL [reference range, 33–37 g/dL]). T-cell subset studies revealed a CD4:CD8 ratio of 0.06 to 1 (absolute CD4+ cells, 6/µL; absolute CD8+ cells, 107/µL). The patient also had an elevated lactate dehydrogenase level of 1015 U/L (reference range, 100–200 U/L) and a normal comprehensive metabolic panel. A comprehensive workup, including urine and blood cultures, serum Cryptococcus and coccidioidomycosis IgG/IgM, histoplasmosis urine antigen, legionella, HIV, purified protein derivative (tuberculin), and aspergillosis galactomannan antigen panel, was negative. Blood tests for HIV and human T-lymphotropic virus also were negative. Bronchoscopy with cytology and sputum cultures for fungi, acid-fast bacteria, and viruses identified Pneumocystis jiroveci in the bronchial wash. Pneumocystis pneumonia was treated with intravenous clindamycin, primaquine, and leucovorin. The patient’s WBC count continued to drop over the next 2 weeks to a nadir of 1.7% with few lymphocytes noted on the differential. At that point, the bexarotene was stopped and was considered causative in inducing CD4 lymphopenia, resulting in opportunistic infection. The patient steadily improved and was discharged on sulfamethoxazole-trimethoprim prophyla
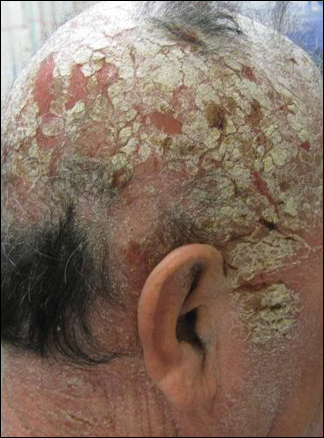
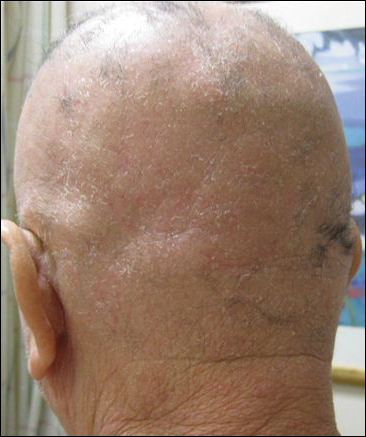
His CD4 count slowly improved over the next 18 months; however, his skin disease recurred and progressed to exfoliative erythroderma with marked scarring alopecia (Figure 3), facial swelling, extreme pruritus, and notable eosinophilia. Repeat computed tomography was negative for extracutaneous involvement. A repeat skin biopsy showed recurrent mycosis fungoides similar to the original biopsy (Figure 4). Topical steroids and narrowband UVB therapy were restarted. A bone marrow biopsy revealed no definitive lymphoma, but the peripheral blood showed occasional CD8+ “flower cells” and no CD4+ Sézary cells. Two repeat molecular studies failed to show the T-cell receptor gene rearrangement. Localized electron beam radiation therapy, lenalidomide, and clobetasol were tried without benefit. The patient was hospitalized 3 months later and was started on wet wraps as well as weekly infusions of the histone deacetylase inhibitor romidepsin (14 mg/m2 over a 4-hour period) on days 1, 8, and 15 of a 28-day cycle with rapid improvement. He experienced transient slight neutropenia with the first several treatments that quickly resolved. His skin was clear while on a regimen of triamcinolone, wet wraps, and intravenous romidepsin. He demonstrated visible improvement after 3 weekly infusions of romedepsin (Figure 5). His skin disease cleared after 9 infusions of romidepsin, and he currently remains in remission; however, he developed presumed bronchopneumonia after approximately 3 to 4 infusions. He then presented with severe headaches after his ninth infusion and was found to have cryptococcal meningitis. Romedepsin was stopped and he was treated with systemic antifungal therapy. His CTCL never recurred despite not restarting romidepsin.
Comment
The retinoids are chemically related to vitamin A. They regulate epithelial cell growth and are beneficial in inflammatory skin disorders and in patients with increased cell turnover as well as in skin cancer and precancer prevention/treatment.5 The first- and second-generation retinoids, isotretinoin and acitretin, respectively, cause anemia or leukopenia in less than 10% of patients; adverse effects are noted more commonly in doses greater than 1 mg/kg daily.6-8
Bexarotene is a third-generation retinoid drug that is more selective for retinoid X receptors. It was approved in 1991 for treatment of advanced CTCL (stages IIB–IVB) in adult patients who have failed at least 1 prior systemic therapy. Bexarotene is noted to promote cell cycle arrest and apoptosis in CTCL cell lines.9 However, one study suggested that for bexarotene, inhibition of proliferation is more important than causing apoptosis in CTCL cells, and this effect is achieved through triggering the p53/p73-dependent cell cycle inhibition pathway.10 Studies in patients with Sézary syndrome have shown that bexarotene changes the chemokine receptor expression in circulating malignant T cells, making them less likely to traffic to the skin (lower chemokine receptor type 4 expression),11 which may explain why some CTCL cases have shown improvement of skin disease on bexarotene despite progression of extradermal disease.12
Common side effects of bexarotene include hyperlipidemia and central hypothyroidism.13 In addition, dose-related myelosuppression with isolated leukopenia, particularly neutropenia, also has been reported (18% of patients at a dosage of 300 mg/m2/d and 43% of patients with a dosage greater than 300 mg/m2/d). Leukopenia generally occurs within the first 4 to 8 weeks of treatment, is relatively mild (WBC, 1000–2999/µL), and generally is reversible.13-15 One review of 66 mycosis fungoides patients treated with bexarotene described a patient who developed leukopenia 15 months after initiating bexarotene therapy.14 The manufacturer recommends that treatment with bexarotene be continued as long as the patient is receiving benefit from the treatment. One trial of 70 mycosis fungoides patient treated with bexarotene reported response rates of 48% on bexarotene monotherapy (n=54) and 69% on bexarotene plus an additional agent (n=16).15 The authors noted higher response rates in patients on 2 lipid-lowering agents. They concluded that bexarotene was a safe and effective agent for treatment of cutaneous T-cell lymphoma and recommended continued treatment with a lowered dose of bexarotene in those achieving complete responses for a period of 2 years. Although the recommended initial dose is 300 mg/m2/d, bexarotene can be increased to 400 mg/m2/d after 8 weeks if no response to treatment is appreciated.16 Our patient was on a maximum bexarotene dose of 600 mg once daily (280 mg/m2/d) for the first 2 years, and a maintenance dose of 300 mg once daily for the next 3 years. He was not on any medicines known to induce leukopenia and he was not given any known cytochrome P450 3A4 inhibitors that could increase the toxicity of bexarotene.
The patient’s CBC was checked routinely every 2 to 3 months after he was started on bexarotene. For 5 years, the CBC and differential remained within reference range; however, his CD4 counts were not followed during those 5 years. We attribute his CD4 lymphopenia and subsequent pneumocystis pneumonia to bexarotene. After our patient’s CD4 lymphopenia was discovered, he developed a precipitous drop in his WBC and lymphocyte counts while hospitalized that worsened over a 2-week period. At this point, the bexarotene was discontinued and his WBC count slowly recovered. We believe that one of the initial antibiotics prescribed by the patient’s primary care physician at initial onset of pneumonia symptoms as an outpatient could have acted synergistically with bexarotene to worsen lymphopenia. Specifically, ceftazidime, vancomycin, and ciprofloxacin have all been reported to cause leukopenia; however, it was neutropenia in these cases, not lymphopenia.17,18 Notwithstanding, the opportunistic pneumonia and therefore CD4 lymphopenia was present prior to any antibiotic use.
The CD4 lymphopenia was unlikely due to underlying infection(s) because an extensive workup was negative, except for the pneumocystis, which likely resulted from the lymphopenia. The CD4 lymphopenia also could be idiopathic, as it has been reported in 3 patients with mycosis fungoides.19 All 3 patients were erythrodermic at presentation and were noted to have numerous CD4+ lymphocytes in the cutaneous lesions but few circulating CD4+ T lymphocytes in the blood. The authors attributed the CD4 lymphopenia to cutaneous sequestration of CD4+ T lymphocytes.19 These cases contrast with our patient who was in clinical remission at the time of CD4 lymphopenia, which improved and normalized following discontinuation of bexarotene.
Conclusion
This case emphasizes the importance of monitoring for leukopenia, specifically CD4 lymphopenia, in patients on long-term bexarotene therapy. Routine CBC as well as T-cell subset counts should be performed during treatment. Rotation off bexarotene after several years of therapy should be considered, even in patients with continuous benefit from this systemic therapy.
- Smith DK, Neal JJ, Holmberg SD. Unexplained opportunistic infections and CD4+ T-lymphocytopenia without HIV infection. an investigation of cases in the United States. The Centers for Disease Control Idiopathic CD4+ T-lymphocytopenia Task Force. N Engl J Med. 1993;328:373-379.
- Castelino DJ, McNair P, Kay TW. Lymphocytopenia in a hospital population: what does it signify? Aust N Z J Med. 1997;27:170-174.
- Zonios DI, Falloon J, Bennett JE, et al. Idiopathic CD4+ lymphocytopenia: natural history and prognostic factors. Blood. 2008;112:287-294.
- Duncan RA, von Reyn CF, Alliegro GM, et al. Idiopathic CD4+ T-lymphocytopenia: four patients with opportunistic infections and no evidence of HIV infection. N Engl J Med. 1993;328:393-398.
- Bruno NP, Beacham BE, Burnett JW. Adverse effects of isotretinoin therapy. Cutis. 1984;33:484-486, 489.
- Strauss JS, Rapini RP, Shalita AR, et al. Isotretinoin therapy for acne: results of a multicenter dose-response study. J Am Acad Dermatol. 1984;10:490-496.
- Windhorst DB, Nigra T. General clinical toxicology of oral retinoids. J Am Acad Dermatol.1982;6:675-682.
- Glinnick SE. Leucopenia from accutane: in ten percent? Schoch Let. 1985;35:9.
- Wilcox RA. Cutaneous T-cell lymphoma: 2011 update on diagnosis, risk-stratification, and management. Am J Hematol. 2011;86:928-948.
- Nieto-Rementería N, Pérez-Yarza G, Boyano MD, et al. Bexarotene activates the p53/p73 pathway in human cutaneous T-cell lymphoma. Br J Dermatol. 2009;160:519-526.
- Richardson SK, Newton SB, Bach TL, et al. Bexarotene blunts malignant T-cell chemotaxis in Sézary syndrome: reduction of chemokine receptor 4-positive lymphocytes and decreased chemotaxis to thymus and activation-regulated chemokine. Am J Hematol. 2007;82:792-797.
- Bouwhuis SA, Davis MD, el-Azhary RA, et al. Bexarotene treatment of late-stage mycosis fungoides and Sézary syndrome: development of extracutaneous lymphoma in 6 patients. J Am Acad Dermatol. 2005;52:991-996.
- Targretin [package insert]. Bridgewater, NJ: Valeant Pharmaceuticals International, Inc; 2015.
- , , , et al. Bexarotene therapy for mycosis fungoides and Sézary syndrome. Br J Dermatol. 2009;160:1299-1307.
- , , et al. Optimizing bexarotene therapy for cutaneous T-cell lymphoma. J Am Acad Dermatol. 2002;47:672-684.
- Scarisbrick JJ, Morris S, Azurdia R, et al. U.K. consensus statement on safe clinical prescribing of bexarotene for patients with cutaneous T-cell lymphoma. Br J Dermatol. 2013;168:192-200.
- Black E, Lau TT, Ensom MH. Vancomycin-induced neutropenia: is it dose-or duration related? Ann Pharmacother. 2011;45:629-638.
- Choo PW, Gantz NM. Reversible leukopenia related to ciprofloxacin therapy. South Med J. 1990;83:597-598.
- Stevens SR, Griffiths TW, Cooper KD. Idiopathic CD4+ T lymphocytopenia in a patient with mycosis fungoides. J Am Acad Dermatol. 1995;32:1063-1064.
Infections, autoimmune disease, bone marrow failure, medications, and total-body irradiation may induce CD4 lymphopenia, defined as a CD4 T-cell count below 300 cells/mL or less than 20% of total lymphocytes.1 Human immunodeficiency virus (HIV) is the most common cause of CD4 lymphopenia, with sepsis (bacterial and fungal) and postoperative states the most common causes in hospital settings.2 No underlying factors are found in 0.02% of CD4 lymphopenia cases, which are considered to be idiopathic.3,4 We report a patient with cutaneous T-cell lymphoma (CTCL) who developed profound CD4 lymphopenia in the setting of long-term bexarotene therapy.
Case Report
A 63-year-old man with hypertension presented to our dermatology clinic with pruritic scaly plaques on the scalp of 4 months’ duration that had progressed to full-body exfoliative erythroderma (Figure 1). He had diffuse palmoplantar keratoderma and lymphadenopathy. His only long-term medications were terazosin for benign prostatic hyperplasia and atenolol for hypertension; he reported no new medications. Laboratory evaluation revealed normal liver and kidney function. A complete blood cell count (CBC) revealed a white blood cell (WBC) count within reference range (8000/µL [reference range, 4500–11,000/µL]) but with increased eosinophils (12.9% [reference range, 2.7%]) and monocytes (11.8% [reference range, 4%]) and reduced lymphocytes (16.8% [reference range, 34%]). Flow cytometry showed a CD4:CD8 ratio of 1.18 to 1 (reference range, 0.8–4.2)(absolute CD4+ cells, 764/µL [reference range, 297–1551/µL]; absolute CD8+ cells, 654/µL [reference range, 100–1047/µL]). Skin biopsy revealed subacute spongiotic dermatitis with numerous eosinophils, exocytosis including folliculotropism, and rare atypical lymphocytes (Figure 2). Molecular studies showed T-cell receptor γ gene rearrangement. The patient did not have any other underlying conditions that would predispose him to lymphopenia. Based on these findings, a diagnosis of CTCL stage IIIA was made and agreed on by experts at the University of California, San Diego Dermatology Grand Rounds.


The patient was subsequently started on acitretin, topical corticosteroids, and hydroxyzine. However, the erythroderma progressed and he developed fever, chills, and malaise, and he was hospitalized 2 months later for intensive therapy and to rule out infection. He improved on daily wet wraps, topical steroids, oral antibiotics, and initiation of narrowband UVB therapy. He was discharged 1 week later. Acitretin was switched to bexarotene 3 months later due to peeling and cracking of the palmoplantar skin. The initial dose was 225 mg once daily, which was steadily increased over the next 4 months to a therapeutic dose of 600 mg once daily, which was much lower than the maximum dose of 400 mg/m2 daily (calculated at 750 mg/d in our patient). The patient achieved clinical remission 1 year after initiation of bexarotene in conjunction with narrowband UVB therapy. Serum eosinophils also normalized. Because there were no intolerable side effects, this dose was continued for 2 more years before it was slowly tapered to 375 mg once daily over a 1-year period. The new dose was maintained thereafter. Secondary hypertriglyceridemia and hypothyroidism, known side effects of bexarotene, developed 1 and 5 months after initiating therapy, respectively, and were treated with levothyroxine and fenofibrate. Blood counts were checked every 3 months and remained within reference range. Within the first few months of therapy, lymphocytes did trend down to 16.8%, but segmented neutrophils were normal at 59.4%. For the next 5 years the total WBC count and differential remained within reference range. T-cell subsets and flow cytometry data were not measured. No new medications were started during this period, and none of his existing medications had lymphopenia as a known side effect.
Five years after the initial diagnosis, the patient was still on bexarotene and was suspected to have pneumonia that was treated by his primary care provider with cefuroxime and azithromycin for 2 weeks with no improvement. He was then admitted to the hospital with shortness of breath, productive cough, night sweats, and dyspnea of 1 month’s duration. There was no associated weight loss or fever. Notably, the skin was clear. He was further treated for community-acquired pneumonia, first with vancomycin and ceftazidime, then with ciprofloxacin and sulfamethoxazole-trimethoprim, with no improvement. A CBC with differential was obtained on the patient’s first admission and revealed a WBC count of 3600/µL with decreased lymphocytes (8.6%), no eosinophilia, and anemia (hemoglobin, 10.5 g/dL [reference range, 33–37 g/dL]). T-cell subset studies revealed a CD4:CD8 ratio of 0.06 to 1 (absolute CD4+ cells, 6/µL; absolute CD8+ cells, 107/µL). The patient also had an elevated lactate dehydrogenase level of 1015 U/L (reference range, 100–200 U/L) and a normal comprehensive metabolic panel. A comprehensive workup, including urine and blood cultures, serum Cryptococcus and coccidioidomycosis IgG/IgM, histoplasmosis urine antigen, legionella, HIV, purified protein derivative (tuberculin), and aspergillosis galactomannan antigen panel, was negative. Blood tests for HIV and human T-lymphotropic virus also were negative. Bronchoscopy with cytology and sputum cultures for fungi, acid-fast bacteria, and viruses identified Pneumocystis jiroveci in the bronchial wash. Pneumocystis pneumonia was treated with intravenous clindamycin, primaquine, and leucovorin. The patient’s WBC count continued to drop over the next 2 weeks to a nadir of 1.7% with few lymphocytes noted on the differential. At that point, the bexarotene was stopped and was considered causative in inducing CD4 lymphopenia, resulting in opportunistic infection. The patient steadily improved and was discharged on sulfamethoxazole-trimethoprim prophyla
His CD4 count slowly improved over the next 18 months; however, his skin disease recurred and progressed to exfoliative erythroderma with marked scarring alopecia (Figure 3), facial swelling, extreme pruritus, and notable eosinophilia. Repeat computed tomography was negative for extracutaneous involvement. A repeat skin biopsy showed recurrent mycosis fungoides similar to the original biopsy (Figure 4). Topical steroids and narrowband UVB therapy were restarted. A bone marrow biopsy revealed no definitive lymphoma, but the peripheral blood showed occasional CD8+ “flower cells” and no CD4+ Sézary cells. Two repeat molecular studies failed to show the T-cell receptor gene rearrangement. Localized electron beam radiation therapy, lenalidomide, and clobetasol were tried without benefit. The patient was hospitalized 3 months later and was started on wet wraps as well as weekly infusions of the histone deacetylase inhibitor romidepsin (14 mg/m2 over a 4-hour period) on days 1, 8, and 15 of a 28-day cycle with rapid improvement. He experienced transient slight neutropenia with the first several treatments that quickly resolved. His skin was clear while on a regimen of triamcinolone, wet wraps, and intravenous romidepsin. He demonstrated visible improvement after 3 weekly infusions of romedepsin (Figure 5). His skin disease cleared after 9 infusions of romidepsin, and he currently remains in remission; however, he developed presumed bronchopneumonia after approximately 3 to 4 infusions. He then presented with severe headaches after his ninth infusion and was found to have cryptococcal meningitis. Romedepsin was stopped and he was treated with systemic antifungal therapy. His CTCL never recurred despite not restarting romidepsin.



Comment
The retinoids are chemically related to vitamin A. They regulate epithelial cell growth and are beneficial in inflammatory skin disorders and in patients with increased cell turnover as well as in skin cancer and precancer prevention/treatment.5 The first- and second-generation retinoids, isotretinoin and acitretin, respectively, cause anemia or leukopenia in less than 10% of patients; adverse effects are noted more commonly in doses greater than 1 mg/kg daily.6-8
Bexarotene is a third-generation retinoid drug that is more selective for retinoid X receptors. It was approved in 1991 for treatment of advanced CTCL (stages IIB–IVB) in adult patients who have failed at least 1 prior systemic therapy. Bexarotene is noted to promote cell cycle arrest and apoptosis in CTCL cell lines.9 However, one study suggested that for bexarotene, inhibition of proliferation is more important than causing apoptosis in CTCL cells, and this effect is achieved through triggering the p53/p73-dependent cell cycle inhibition pathway.10 Studies in patients with Sézary syndrome have shown that bexarotene changes the chemokine receptor expression in circulating malignant T cells, making them less likely to traffic to the skin (lower chemokine receptor type 4 expression),11 which may explain why some CTCL cases have shown improvement of skin disease on bexarotene despite progression of extradermal disease.12
Common side effects of bexarotene include hyperlipidemia and central hypothyroidism.13 In addition, dose-related myelosuppression with isolated leukopenia, particularly neutropenia, also has been reported (18% of patients at a dosage of 300 mg/m2/d and 43% of patients with a dosage greater than 300 mg/m2/d). Leukopenia generally occurs within the first 4 to 8 weeks of treatment, is relatively mild (WBC, 1000–2999/µL), and generally is reversible.13-15 One review of 66 mycosis fungoides patients treated with bexarotene described a patient who developed leukopenia 15 months after initiating bexarotene therapy.14 The manufacturer recommends that treatment with bexarotene be continued as long as the patient is receiving benefit from the treatment. One trial of 70 mycosis fungoides patient treated with bexarotene reported response rates of 48% on bexarotene monotherapy (n=54) and 69% on bexarotene plus an additional agent (n=16).15 The authors noted higher response rates in patients on 2 lipid-lowering agents. They concluded that bexarotene was a safe and effective agent for treatment of cutaneous T-cell lymphoma and recommended continued treatment with a lowered dose of bexarotene in those achieving complete responses for a period of 2 years. Although the recommended initial dose is 300 mg/m2/d, bexarotene can be increased to 400 mg/m2/d after 8 weeks if no response to treatment is appreciated.16 Our patient was on a maximum bexarotene dose of 600 mg once daily (280 mg/m2/d) for the first 2 years, and a maintenance dose of 300 mg once daily for the next 3 years. He was not on any medicines known to induce leukopenia and he was not given any known cytochrome P450 3A4 inhibitors that could increase the toxicity of bexarotene.
The patient’s CBC was checked routinely every 2 to 3 months after he was started on bexarotene. For 5 years, the CBC and differential remained within reference range; however, his CD4 counts were not followed during those 5 years. We attribute his CD4 lymphopenia and subsequent pneumocystis pneumonia to bexarotene. After our patient’s CD4 lymphopenia was discovered, he developed a precipitous drop in his WBC and lymphocyte counts while hospitalized that worsened over a 2-week period. At this point, the bexarotene was discontinued and his WBC count slowly recovered. We believe that one of the initial antibiotics prescribed by the patient’s primary care physician at initial onset of pneumonia symptoms as an outpatient could have acted synergistically with bexarotene to worsen lymphopenia. Specifically, ceftazidime, vancomycin, and ciprofloxacin have all been reported to cause leukopenia; however, it was neutropenia in these cases, not lymphopenia.17,18 Notwithstanding, the opportunistic pneumonia and therefore CD4 lymphopenia was present prior to any antibiotic use.
The CD4 lymphopenia was unlikely due to underlying infection(s) because an extensive workup was negative, except for the pneumocystis, which likely resulted from the lymphopenia. The CD4 lymphopenia also could be idiopathic, as it has been reported in 3 patients with mycosis fungoides.19 All 3 patients were erythrodermic at presentation and were noted to have numerous CD4+ lymphocytes in the cutaneous lesions but few circulating CD4+ T lymphocytes in the blood. The authors attributed the CD4 lymphopenia to cutaneous sequestration of CD4+ T lymphocytes.19 These cases contrast with our patient who was in clinical remission at the time of CD4 lymphopenia, which improved and normalized following discontinuation of bexarotene.
Conclusion
This case emphasizes the importance of monitoring for leukopenia, specifically CD4 lymphopenia, in patients on long-term bexarotene therapy. Routine CBC as well as T-cell subset counts should be performed during treatment. Rotation off bexarotene after several years of therapy should be considered, even in patients with continuous benefit from this systemic therapy.
Infections, autoimmune disease, bone marrow failure, medications, and total-body irradiation may induce CD4 lymphopenia, defined as a CD4 T-cell count below 300 cells/mL or less than 20% of total lymphocytes.1 Human immunodeficiency virus (HIV) is the most common cause of CD4 lymphopenia, with sepsis (bacterial and fungal) and postoperative states the most common causes in hospital settings.2 No underlying factors are found in 0.02% of CD4 lymphopenia cases, which are considered to be idiopathic.3,4 We report a patient with cutaneous T-cell lymphoma (CTCL) who developed profound CD4 lymphopenia in the setting of long-term bexarotene therapy.
Case Report
A 63-year-old man with hypertension presented to our dermatology clinic with pruritic scaly plaques on the scalp of 4 months’ duration that had progressed to full-body exfoliative erythroderma (Figure 1). He had diffuse palmoplantar keratoderma and lymphadenopathy. His only long-term medications were terazosin for benign prostatic hyperplasia and atenolol for hypertension; he reported no new medications. Laboratory evaluation revealed normal liver and kidney function. A complete blood cell count (CBC) revealed a white blood cell (WBC) count within reference range (8000/µL [reference range, 4500–11,000/µL]) but with increased eosinophils (12.9% [reference range, 2.7%]) and monocytes (11.8% [reference range, 4%]) and reduced lymphocytes (16.8% [reference range, 34%]). Flow cytometry showed a CD4:CD8 ratio of 1.18 to 1 (reference range, 0.8–4.2)(absolute CD4+ cells, 764/µL [reference range, 297–1551/µL]; absolute CD8+ cells, 654/µL [reference range, 100–1047/µL]). Skin biopsy revealed subacute spongiotic dermatitis with numerous eosinophils, exocytosis including folliculotropism, and rare atypical lymphocytes (Figure 2). Molecular studies showed T-cell receptor γ gene rearrangement. The patient did not have any other underlying conditions that would predispose him to lymphopenia. Based on these findings, a diagnosis of CTCL stage IIIA was made and agreed on by experts at the University of California, San Diego Dermatology Grand Rounds.


The patient was subsequently started on acitretin, topical corticosteroids, and hydroxyzine. However, the erythroderma progressed and he developed fever, chills, and malaise, and he was hospitalized 2 months later for intensive therapy and to rule out infection. He improved on daily wet wraps, topical steroids, oral antibiotics, and initiation of narrowband UVB therapy. He was discharged 1 week later. Acitretin was switched to bexarotene 3 months later due to peeling and cracking of the palmoplantar skin. The initial dose was 225 mg once daily, which was steadily increased over the next 4 months to a therapeutic dose of 600 mg once daily, which was much lower than the maximum dose of 400 mg/m2 daily (calculated at 750 mg/d in our patient). The patient achieved clinical remission 1 year after initiation of bexarotene in conjunction with narrowband UVB therapy. Serum eosinophils also normalized. Because there were no intolerable side effects, this dose was continued for 2 more years before it was slowly tapered to 375 mg once daily over a 1-year period. The new dose was maintained thereafter. Secondary hypertriglyceridemia and hypothyroidism, known side effects of bexarotene, developed 1 and 5 months after initiating therapy, respectively, and were treated with levothyroxine and fenofibrate. Blood counts were checked every 3 months and remained within reference range. Within the first few months of therapy, lymphocytes did trend down to 16.8%, but segmented neutrophils were normal at 59.4%. For the next 5 years the total WBC count and differential remained within reference range. T-cell subsets and flow cytometry data were not measured. No new medications were started during this period, and none of his existing medications had lymphopenia as a known side effect.
Five years after the initial diagnosis, the patient was still on bexarotene and was suspected to have pneumonia that was treated by his primary care provider with cefuroxime and azithromycin for 2 weeks with no improvement. He was then admitted to the hospital with shortness of breath, productive cough, night sweats, and dyspnea of 1 month’s duration. There was no associated weight loss or fever. Notably, the skin was clear. He was further treated for community-acquired pneumonia, first with vancomycin and ceftazidime, then with ciprofloxacin and sulfamethoxazole-trimethoprim, with no improvement. A CBC with differential was obtained on the patient’s first admission and revealed a WBC count of 3600/µL with decreased lymphocytes (8.6%), no eosinophilia, and anemia (hemoglobin, 10.5 g/dL [reference range, 33–37 g/dL]). T-cell subset studies revealed a CD4:CD8 ratio of 0.06 to 1 (absolute CD4+ cells, 6/µL; absolute CD8+ cells, 107/µL). The patient also had an elevated lactate dehydrogenase level of 1015 U/L (reference range, 100–200 U/L) and a normal comprehensive metabolic panel. A comprehensive workup, including urine and blood cultures, serum Cryptococcus and coccidioidomycosis IgG/IgM, histoplasmosis urine antigen, legionella, HIV, purified protein derivative (tuberculin), and aspergillosis galactomannan antigen panel, was negative. Blood tests for HIV and human T-lymphotropic virus also were negative. Bronchoscopy with cytology and sputum cultures for fungi, acid-fast bacteria, and viruses identified Pneumocystis jiroveci in the bronchial wash. Pneumocystis pneumonia was treated with intravenous clindamycin, primaquine, and leucovorin. The patient’s WBC count continued to drop over the next 2 weeks to a nadir of 1.7% with few lymphocytes noted on the differential. At that point, the bexarotene was stopped and was considered causative in inducing CD4 lymphopenia, resulting in opportunistic infection. The patient steadily improved and was discharged on sulfamethoxazole-trimethoprim prophyla
His CD4 count slowly improved over the next 18 months; however, his skin disease recurred and progressed to exfoliative erythroderma with marked scarring alopecia (Figure 3), facial swelling, extreme pruritus, and notable eosinophilia. Repeat computed tomography was negative for extracutaneous involvement. A repeat skin biopsy showed recurrent mycosis fungoides similar to the original biopsy (Figure 4). Topical steroids and narrowband UVB therapy were restarted. A bone marrow biopsy revealed no definitive lymphoma, but the peripheral blood showed occasional CD8+ “flower cells” and no CD4+ Sézary cells. Two repeat molecular studies failed to show the T-cell receptor gene rearrangement. Localized electron beam radiation therapy, lenalidomide, and clobetasol were tried without benefit. The patient was hospitalized 3 months later and was started on wet wraps as well as weekly infusions of the histone deacetylase inhibitor romidepsin (14 mg/m2 over a 4-hour period) on days 1, 8, and 15 of a 28-day cycle with rapid improvement. He experienced transient slight neutropenia with the first several treatments that quickly resolved. His skin was clear while on a regimen of triamcinolone, wet wraps, and intravenous romidepsin. He demonstrated visible improvement after 3 weekly infusions of romedepsin (Figure 5). His skin disease cleared after 9 infusions of romidepsin, and he currently remains in remission; however, he developed presumed bronchopneumonia after approximately 3 to 4 infusions. He then presented with severe headaches after his ninth infusion and was found to have cryptococcal meningitis. Romedepsin was stopped and he was treated with systemic antifungal therapy. His CTCL never recurred despite not restarting romidepsin.



Comment
The retinoids are chemically related to vitamin A. They regulate epithelial cell growth and are beneficial in inflammatory skin disorders and in patients with increased cell turnover as well as in skin cancer and precancer prevention/treatment.5 The first- and second-generation retinoids, isotretinoin and acitretin, respectively, cause anemia or leukopenia in less than 10% of patients; adverse effects are noted more commonly in doses greater than 1 mg/kg daily.6-8
Bexarotene is a third-generation retinoid drug that is more selective for retinoid X receptors. It was approved in 1991 for treatment of advanced CTCL (stages IIB–IVB) in adult patients who have failed at least 1 prior systemic therapy. Bexarotene is noted to promote cell cycle arrest and apoptosis in CTCL cell lines.9 However, one study suggested that for bexarotene, inhibition of proliferation is more important than causing apoptosis in CTCL cells, and this effect is achieved through triggering the p53/p73-dependent cell cycle inhibition pathway.10 Studies in patients with Sézary syndrome have shown that bexarotene changes the chemokine receptor expression in circulating malignant T cells, making them less likely to traffic to the skin (lower chemokine receptor type 4 expression),11 which may explain why some CTCL cases have shown improvement of skin disease on bexarotene despite progression of extradermal disease.12
Common side effects of bexarotene include hyperlipidemia and central hypothyroidism.13 In addition, dose-related myelosuppression with isolated leukopenia, particularly neutropenia, also has been reported (18% of patients at a dosage of 300 mg/m2/d and 43% of patients with a dosage greater than 300 mg/m2/d). Leukopenia generally occurs within the first 4 to 8 weeks of treatment, is relatively mild (WBC, 1000–2999/µL), and generally is reversible.13-15 One review of 66 mycosis fungoides patients treated with bexarotene described a patient who developed leukopenia 15 months after initiating bexarotene therapy.14 The manufacturer recommends that treatment with bexarotene be continued as long as the patient is receiving benefit from the treatment. One trial of 70 mycosis fungoides patient treated with bexarotene reported response rates of 48% on bexarotene monotherapy (n=54) and 69% on bexarotene plus an additional agent (n=16).15 The authors noted higher response rates in patients on 2 lipid-lowering agents. They concluded that bexarotene was a safe and effective agent for treatment of cutaneous T-cell lymphoma and recommended continued treatment with a lowered dose of bexarotene in those achieving complete responses for a period of 2 years. Although the recommended initial dose is 300 mg/m2/d, bexarotene can be increased to 400 mg/m2/d after 8 weeks if no response to treatment is appreciated.16 Our patient was on a maximum bexarotene dose of 600 mg once daily (280 mg/m2/d) for the first 2 years, and a maintenance dose of 300 mg once daily for the next 3 years. He was not on any medicines known to induce leukopenia and he was not given any known cytochrome P450 3A4 inhibitors that could increase the toxicity of bexarotene.
The patient’s CBC was checked routinely every 2 to 3 months after he was started on bexarotene. For 5 years, the CBC and differential remained within reference range; however, his CD4 counts were not followed during those 5 years. We attribute his CD4 lymphopenia and subsequent pneumocystis pneumonia to bexarotene. After our patient’s CD4 lymphopenia was discovered, he developed a precipitous drop in his WBC and lymphocyte counts while hospitalized that worsened over a 2-week period. At this point, the bexarotene was discontinued and his WBC count slowly recovered. We believe that one of the initial antibiotics prescribed by the patient’s primary care physician at initial onset of pneumonia symptoms as an outpatient could have acted synergistically with bexarotene to worsen lymphopenia. Specifically, ceftazidime, vancomycin, and ciprofloxacin have all been reported to cause leukopenia; however, it was neutropenia in these cases, not lymphopenia.17,18 Notwithstanding, the opportunistic pneumonia and therefore CD4 lymphopenia was present prior to any antibiotic use.
The CD4 lymphopenia was unlikely due to underlying infection(s) because an extensive workup was negative, except for the pneumocystis, which likely resulted from the lymphopenia. The CD4 lymphopenia also could be idiopathic, as it has been reported in 3 patients with mycosis fungoides.19 All 3 patients were erythrodermic at presentation and were noted to have numerous CD4+ lymphocytes in the cutaneous lesions but few circulating CD4+ T lymphocytes in the blood. The authors attributed the CD4 lymphopenia to cutaneous sequestration of CD4+ T lymphocytes.19 These cases contrast with our patient who was in clinical remission at the time of CD4 lymphopenia, which improved and normalized following discontinuation of bexarotene.
Conclusion
This case emphasizes the importance of monitoring for leukopenia, specifically CD4 lymphopenia, in patients on long-term bexarotene therapy. Routine CBC as well as T-cell subset counts should be performed during treatment. Rotation off bexarotene after several years of therapy should be considered, even in patients with continuous benefit from this systemic therapy.
- Smith DK, Neal JJ, Holmberg SD. Unexplained opportunistic infections and CD4+ T-lymphocytopenia without HIV infection. an investigation of cases in the United States. The Centers for Disease Control Idiopathic CD4+ T-lymphocytopenia Task Force. N Engl J Med. 1993;328:373-379.
- Castelino DJ, McNair P, Kay TW. Lymphocytopenia in a hospital population: what does it signify? Aust N Z J Med. 1997;27:170-174.
- Zonios DI, Falloon J, Bennett JE, et al. Idiopathic CD4+ lymphocytopenia: natural history and prognostic factors. Blood. 2008;112:287-294.
- Duncan RA, von Reyn CF, Alliegro GM, et al. Idiopathic CD4+ T-lymphocytopenia: four patients with opportunistic infections and no evidence of HIV infection. N Engl J Med. 1993;328:393-398.
- Bruno NP, Beacham BE, Burnett JW. Adverse effects of isotretinoin therapy. Cutis. 1984;33:484-486, 489.
- Strauss JS, Rapini RP, Shalita AR, et al. Isotretinoin therapy for acne: results of a multicenter dose-response study. J Am Acad Dermatol. 1984;10:490-496.
- Windhorst DB, Nigra T. General clinical toxicology of oral retinoids. J Am Acad Dermatol.1982;6:675-682.
- Glinnick SE. Leucopenia from accutane: in ten percent? Schoch Let. 1985;35:9.
- Wilcox RA. Cutaneous T-cell lymphoma: 2011 update on diagnosis, risk-stratification, and management. Am J Hematol. 2011;86:928-948.
- Nieto-Rementería N, Pérez-Yarza G, Boyano MD, et al. Bexarotene activates the p53/p73 pathway in human cutaneous T-cell lymphoma. Br J Dermatol. 2009;160:519-526.
- Richardson SK, Newton SB, Bach TL, et al. Bexarotene blunts malignant T-cell chemotaxis in Sézary syndrome: reduction of chemokine receptor 4-positive lymphocytes and decreased chemotaxis to thymus and activation-regulated chemokine. Am J Hematol. 2007;82:792-797.
- Bouwhuis SA, Davis MD, el-Azhary RA, et al. Bexarotene treatment of late-stage mycosis fungoides and Sézary syndrome: development of extracutaneous lymphoma in 6 patients. J Am Acad Dermatol. 2005;52:991-996.
- Targretin [package insert]. Bridgewater, NJ: Valeant Pharmaceuticals International, Inc; 2015.
- , , , et al. Bexarotene therapy for mycosis fungoides and Sézary syndrome. Br J Dermatol. 2009;160:1299-1307.
- , , et al. Optimizing bexarotene therapy for cutaneous T-cell lymphoma. J Am Acad Dermatol. 2002;47:672-684.
- Scarisbrick JJ, Morris S, Azurdia R, et al. U.K. consensus statement on safe clinical prescribing of bexarotene for patients with cutaneous T-cell lymphoma. Br J Dermatol. 2013;168:192-200.
- Black E, Lau TT, Ensom MH. Vancomycin-induced neutropenia: is it dose-or duration related? Ann Pharmacother. 2011;45:629-638.
- Choo PW, Gantz NM. Reversible leukopenia related to ciprofloxacin therapy. South Med J. 1990;83:597-598.
- Stevens SR, Griffiths TW, Cooper KD. Idiopathic CD4+ T lymphocytopenia in a patient with mycosis fungoides. J Am Acad Dermatol. 1995;32:1063-1064.
- Smith DK, Neal JJ, Holmberg SD. Unexplained opportunistic infections and CD4+ T-lymphocytopenia without HIV infection. an investigation of cases in the United States. The Centers for Disease Control Idiopathic CD4+ T-lymphocytopenia Task Force. N Engl J Med. 1993;328:373-379.
- Castelino DJ, McNair P, Kay TW. Lymphocytopenia in a hospital population: what does it signify? Aust N Z J Med. 1997;27:170-174.
- Zonios DI, Falloon J, Bennett JE, et al. Idiopathic CD4+ lymphocytopenia: natural history and prognostic factors. Blood. 2008;112:287-294.
- Duncan RA, von Reyn CF, Alliegro GM, et al. Idiopathic CD4+ T-lymphocytopenia: four patients with opportunistic infections and no evidence of HIV infection. N Engl J Med. 1993;328:393-398.
- Bruno NP, Beacham BE, Burnett JW. Adverse effects of isotretinoin therapy. Cutis. 1984;33:484-486, 489.
- Strauss JS, Rapini RP, Shalita AR, et al. Isotretinoin therapy for acne: results of a multicenter dose-response study. J Am Acad Dermatol. 1984;10:490-496.
- Windhorst DB, Nigra T. General clinical toxicology of oral retinoids. J Am Acad Dermatol.1982;6:675-682.
- Glinnick SE. Leucopenia from accutane: in ten percent? Schoch Let. 1985;35:9.
- Wilcox RA. Cutaneous T-cell lymphoma: 2011 update on diagnosis, risk-stratification, and management. Am J Hematol. 2011;86:928-948.
- Nieto-Rementería N, Pérez-Yarza G, Boyano MD, et al. Bexarotene activates the p53/p73 pathway in human cutaneous T-cell lymphoma. Br J Dermatol. 2009;160:519-526.
- Richardson SK, Newton SB, Bach TL, et al. Bexarotene blunts malignant T-cell chemotaxis in Sézary syndrome: reduction of chemokine receptor 4-positive lymphocytes and decreased chemotaxis to thymus and activation-regulated chemokine. Am J Hematol. 2007;82:792-797.
- Bouwhuis SA, Davis MD, el-Azhary RA, et al. Bexarotene treatment of late-stage mycosis fungoides and Sézary syndrome: development of extracutaneous lymphoma in 6 patients. J Am Acad Dermatol. 2005;52:991-996.
- Targretin [package insert]. Bridgewater, NJ: Valeant Pharmaceuticals International, Inc; 2015.
- , , , et al. Bexarotene therapy for mycosis fungoides and Sézary syndrome. Br J Dermatol. 2009;160:1299-1307.
- , , et al. Optimizing bexarotene therapy for cutaneous T-cell lymphoma. J Am Acad Dermatol. 2002;47:672-684.
- Scarisbrick JJ, Morris S, Azurdia R, et al. U.K. consensus statement on safe clinical prescribing of bexarotene for patients with cutaneous T-cell lymphoma. Br J Dermatol. 2013;168:192-200.
- Black E, Lau TT, Ensom MH. Vancomycin-induced neutropenia: is it dose-or duration related? Ann Pharmacother. 2011;45:629-638.
- Choo PW, Gantz NM. Reversible leukopenia related to ciprofloxacin therapy. South Med J. 1990;83:597-598.
- Stevens SR, Griffiths TW, Cooper KD. Idiopathic CD4+ T lymphocytopenia in a patient with mycosis fungoides. J Am Acad Dermatol. 1995;32:1063-1064.
Practice Points
- Most adverse effects of bexarotene (eg, hypothyroidism, hyperlipidemia, leukopenia) occur within the first several months of therapy.
- Delayed-onset leukopenia, including CD4 lymphopenia, may occur several years after initiating bexarotene therapy, resulting in opportunistic infections.
- Long-term periodic monitoring of T lymphocyte counts at least twice yearly in addition to standard quarterly complete blood cell count with differential are recommended.
Online Patient-Reported Reviews of Mohs Micrographic Surgery: Qualitative Analysis of Positive and Negative Experiences
Mohs micrographic surgery (MMS) remains the gold standard for the removal of skin cancers in high-risk areas of the body while offering an excellent safety profile and sparing tissue.1 In the current health care environment, online patient reviews have grown in popularity and influence. More than 60% of consumers consult social media before making health care decisions.2 A recent analysis of online patient reviews of general dermatology practices demonstrated the perceived importance of physician empathy, thoroughness, and cognizance of cost in relation to patient-reported satisfaction.3 Because MMS is a well-recognized and unique outpatient-based surgical procedure, a review and analysis of online patient reviews specific to MMS can provide useful practice insights.
Materials and Methods
This study was conducted using an online platform (RealSelf [http://www.realself.com]) that connects patients and providers offering aesthetically oriented procedures; the site has 35 million unique visitors yearly.4 The community’s directory was used to identify and analyze all cumulative patient reviews from 2006 to December 20, 2015, using the search terms Mohs surgery or Mohs micrographic surgery. The study was exempt by the Northwestern University (Chicago, Illinois) institutional review board.
A standardized qualitative coding methodology was created and applied to all available comments regarding MMS. A broad list of positive and negative patient experiences was first created and agreed upon by all 3 investigators. Each individual comment was then attributed to 1 or more of these positive or negative themes. Of these comments, 10% were coded by 2 investigators (S.X. and Z.A.) to ensure internal validity; 1 investigator coded the remaining statements by patients (Z.A.). Patient-reported satisfaction ratings categorized as “worth it” or “not worth it” (as used by RealSelf to describe the patient-perceived value and utility of a given procedure) as well as cost of MMS were gathered. Cumulative patient ratings were collected for the procedure overall, physician’s bedside manner, answered questions, aftercare follow-up, time spent with patients, telephone/email responsiveness, staff professionalism/courtesy, payment process, and wait times. Patient-reported characteristics of MMS also were evaluated including physician specialty, lesion location, type of skin cancer, and type of closure. For lesion location, we graded whether the location represented a high-risk area as defined by the American Academy of Dermatology, American College of Mohs Surgery, and American Society for Dermatologic Surgery.5
Results
A total of 219 reviews related to MMS were collected as of December 20, 2015. Overall, MMS was considered “worth it” by 89% of patients (Table 1). Only 2% of patients described MMS as “not worth it.” There was a wide range reported for the cost of the procedure ($1–$100,000 [median, $1800]). Of those patients who reported their sex, females were 2.5-times more likely to post a review compared to males (51% vs 20%); however, 30% of reviewers did not report their sex. The mean (standard deviation) overall satisfaction rating was 4.8 (0.8). With regard to category-specific ratings (eg, bedside manner, aftercare follow-up, time spent with patients), the mean scores were all 4.7 or greater (Table 2).
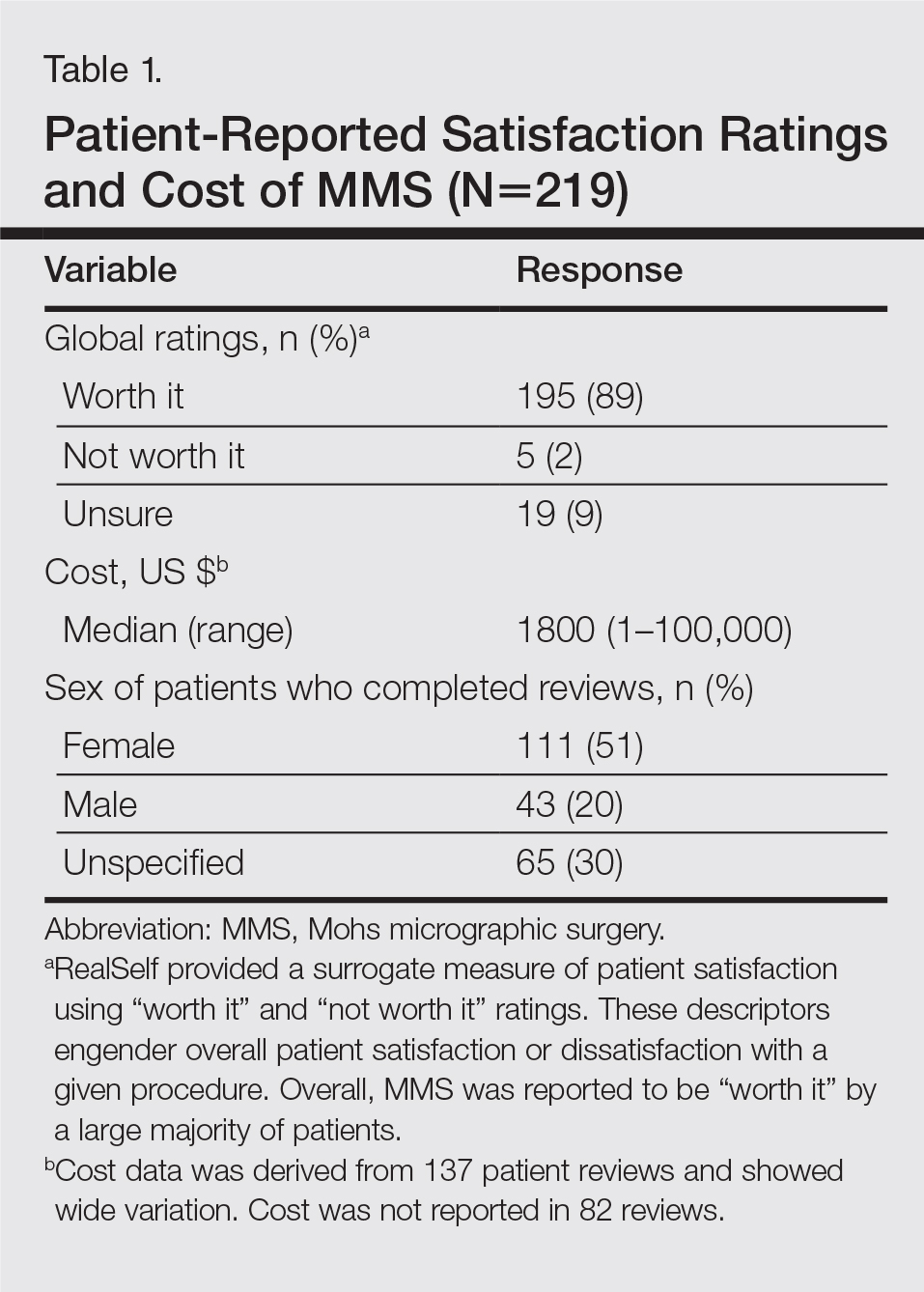

Regarding the surgical aspects of the procedure, the majority of patients reported that the excision of the lesion was performed by a dermatologist (62%). However, a notable portion of patients reported that the excision was performed by a plastic surgeon (21%). Physician specialty was not reported in 16% of the reviews. For the lesion closure, the patient-reported specialty of the physician was only slightly higher for dermatologists versus plastic surgeons (46% vs 44%)(Table 3).

The majority of patients who reported the location of the lesion treated with MMS identified a high-risk location (45%), a medium-risk location (18%), or an unspecified region of the face (15%), according to the appropriate-use criteria for MMS (Table 3).5 Patients did not specify the site of surgery 17% of the time. Only 5% of reported procedures were performed on low-risk areas.
Basal cell carcinomas were the most commonly reported lesions removed by MMS (38%), though 48% of reviews did not specify the type of tumor being treated (Table 3). A large majority (76%) did not specify the type of closure performed. When specified, secondary intention was used 10% of the time, followed by either a flap (6%) or skin graft (6%). Only 5% of patients reported an estimated size of the primary lesion in our study (data not shown).
The qualitative analysis demonstrated variance in themes for positive and negative characteristics (Table 4). Surgeon characteristics encompassed the 3 most commonly cited themes of positive remarks, including bedside manner (78%), communication skills (74%), and perceived expertise (58%). Specific to MMS, the tissue-sparing nature of the technique was cited by 14% of reviews as a positive theme. The most commonly cited themes of negative remarks were intraoperative and postoperative concerns, including postoperative disfigurement (16%), large scar (9%), healing time (9%), and procedural or postoperative pain (8%). A subtheme analysis of postoperative disfigurement revealed that eyelid or eyebrow distortion was the most common concern (29%), followed by redness and swelling (23%), an open wound (14%), and nostril/nose distortion (14%)(data not shown). Themes not commonly cited as either positive or negative included office environment, cost, and procedure time (data not shown).
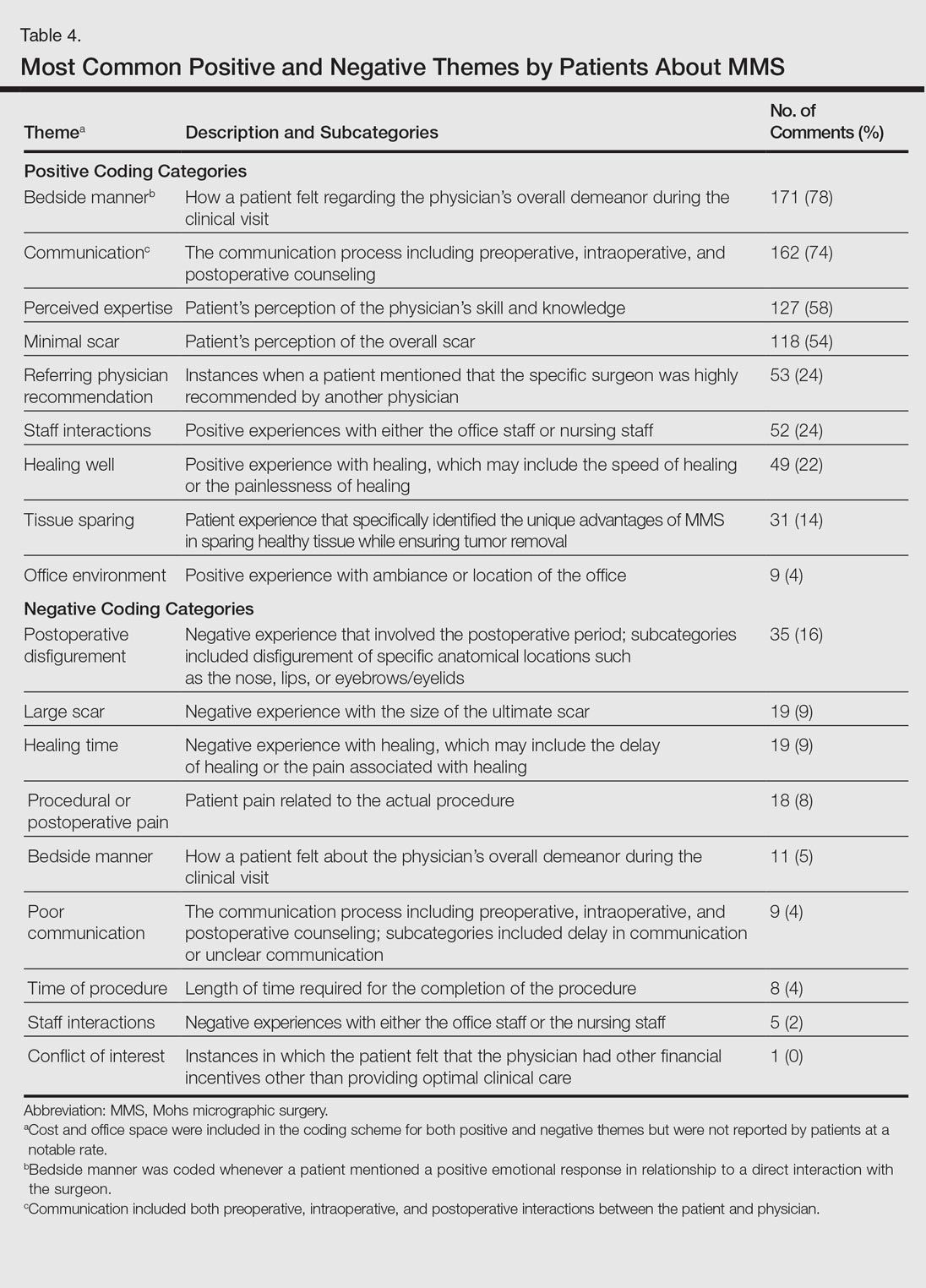
Comment
The overall satisfaction with MMS (89%) was one of the highest for any procedure on this online patient review site, albeit based on fewer reviews compared to other common aesthetic surgical procedures. In comparison, 78% of 13,500 reviewers rated breast augmentation as “worth it,” while 60% of 6800 reviewers rated rhinoplasty as “worth it” (as of December 2015). Overall, the online patient reviews evaluated in this study were consistent with a previously published structured data report on patient satisfaction with MMS.6
The results show a greater than expected proportion of both the MMS excision and closure being performed by plastic surgeons compared to dermatologists. In reality, the majority of MMS excisions are performed by dermatologists. Based on a survey of American College of Mohs Surgery (ACMS) members, only 6% of procedures were sent to other specialties for closure.7 Our results may reflect reporting bias or patients misconstruing true MMS with an excision and standard frozen sections, techniques that have lower cure rates. If so, there may be a need to educate patients regarding the specifics of MMS. Other possible explanations for the discrepancy between the online patient reviews and ACMS data include misinterpretation by patients on the exact definition of MMS or that a higher than expected number of procedures were performed by non-ACMS Mohs surgeons.
Our qualitative analysis revealed that patients most frequently commented on the interpersonal skills of their surgeons (eg, bedside manner, communication) as positive themes during MMS, similar to prior analyses of general dermatology practices.3 In comparison to a recent study assessing patient satisfaction with rhinoplasty on RealSelf, the final appearance of the nose represented the most common positive- and negative-cited theme.8 Mohs micrographic surgery procedures typically are done under local anesthesia, which may explain the greater importance of bedside manner and communication intraoperatively in comparison to final surgical outcomes for patient satisfaction. For negative themes, 3 of 4 most common concerns were directly related to the intraoperative and postoperative periods. Providers may be able to improve patient satisfaction by explaining the postoperative course, such as healing time and temporary physical restrictions, as well as possible sequelae in greater detail, which may be particularly pertinent for MMS involving the nose or near the eyes.
The global ratings for MMS are high, as shown in our data set of patient reviews; however, patient reviews are highly susceptible to reporting bias, recall bias, and missing information. Prior work using this online patient review website to investigate laser and light procedures also demonstrated the risk for imperfect information associated with patient reviews.9 Even so, the data does provide a glimpse into what is considered important to patients. Surgeon interpersonal skills and communication were the most frequently cited positive themes for MMS. The best surgical aspects of MMS focused on the unique tissue-sparing nature of the procedure and the removal of a cancerous lesion. Potential areas for improvement include a more thorough explanation of the intraoperative and postoperative process, specifically potential asymmetry related to the nose or the eyes, healing time, and scarring. These patient reviews underscore the importance of setting appropriate patient expectations. As patients become more connected and utilize online platforms to report their experiences, Mohs surgeons can take insights derived from online patient reviews for their own practice or geographic area to improve satisfaction and manage expectations.
- Alam M, Ibrahim O, Nodzenski M, et al. Adverse events associated with Mohs micrographic surgery: multicenter prospective cohort study of 20,821 cases at 23 centers. JAMA Dermatol. 2013;149:1378-1385.
- Fox S. The social life of health information. Pew Research Center website. http://www.pewresearch.org/fact-tank/2014/01/15/the-social-life-of-health-information/. Published January 15, 2014. Accessed February 11, 2017.
- Smith RJ, Lipoff JB. Evaluation of dermatology practice online reviews: lessons from qualitative analysis. JAMA Dermatol. 2016;152:153-157.
- Schlichte MJ, Karimkhani C, Jones T, et al. Patient use of social media to evaluate cosmetic treatments and procedures. Dermatol Online J. 2015;21. pii:13030/qt88z6r65x.
- American Academy of Dermatology; American College of Mohs Surgery; American Society for Dermatologic Surgery Association; American Society for Mohs Surgery; Ad Hoc Task Force, Connolly SM, Baker DR, Coldiron BM, et al. AAD/ACMS/ASDSA/ASMS 2012 appropriate use criteria for Mohs micrographic surgery: a report of the American Academy of Dermatology, American College of Mohs Surgery, American Society for Dermatologic Surgery Association, and the American Society for Mohs Surgery [published online September 7, 2012]. Dermatol Surg. 2012;38:1582-1603.
- Asgari MM, Bertenthal D, Sen S, et al. Patient satisfaction after treatment of nonmelanoma skin cancer. Derm Surg. 2009;35:1041-1049.
- Campbell RM, Perlis CS, Malik MK, et al. Characteristics of Mohs practices in the United States: a recall survey of ACMS surgeons. Dermatol Surg. 2007;33:1413-1418; discussion, 1418.
- Khansa I, Khansa L, Pearson GD. Patient satisfaction after rhinoplasty: a social media analysis. Aesthet Surg J. 2016;36:NP1-5.
- Xu S, Walter J, Bhatia A. Patient-reported online satisfaction for laser and light procedures: need for caution. Dermatol Surg. 2017;43:154-158.
Mohs micrographic surgery (MMS) remains the gold standard for the removal of skin cancers in high-risk areas of the body while offering an excellent safety profile and sparing tissue.1 In the current health care environment, online patient reviews have grown in popularity and influence. More than 60% of consumers consult social media before making health care decisions.2 A recent analysis of online patient reviews of general dermatology practices demonstrated the perceived importance of physician empathy, thoroughness, and cognizance of cost in relation to patient-reported satisfaction.3 Because MMS is a well-recognized and unique outpatient-based surgical procedure, a review and analysis of online patient reviews specific to MMS can provide useful practice insights.
Materials and Methods
This study was conducted using an online platform (RealSelf [http://www.realself.com]) that connects patients and providers offering aesthetically oriented procedures; the site has 35 million unique visitors yearly.4 The community’s directory was used to identify and analyze all cumulative patient reviews from 2006 to December 20, 2015, using the search terms Mohs surgery or Mohs micrographic surgery. The study was exempt by the Northwestern University (Chicago, Illinois) institutional review board.
A standardized qualitative coding methodology was created and applied to all available comments regarding MMS. A broad list of positive and negative patient experiences was first created and agreed upon by all 3 investigators. Each individual comment was then attributed to 1 or more of these positive or negative themes. Of these comments, 10% were coded by 2 investigators (S.X. and Z.A.) to ensure internal validity; 1 investigator coded the remaining statements by patients (Z.A.). Patient-reported satisfaction ratings categorized as “worth it” or “not worth it” (as used by RealSelf to describe the patient-perceived value and utility of a given procedure) as well as cost of MMS were gathered. Cumulative patient ratings were collected for the procedure overall, physician’s bedside manner, answered questions, aftercare follow-up, time spent with patients, telephone/email responsiveness, staff professionalism/courtesy, payment process, and wait times. Patient-reported characteristics of MMS also were evaluated including physician specialty, lesion location, type of skin cancer, and type of closure. For lesion location, we graded whether the location represented a high-risk area as defined by the American Academy of Dermatology, American College of Mohs Surgery, and American Society for Dermatologic Surgery.5
Results
A total of 219 reviews related to MMS were collected as of December 20, 2015. Overall, MMS was considered “worth it” by 89% of patients (Table 1). Only 2% of patients described MMS as “not worth it.” There was a wide range reported for the cost of the procedure ($1–$100,000 [median, $1800]). Of those patients who reported their sex, females were 2.5-times more likely to post a review compared to males (51% vs 20%); however, 30% of reviewers did not report their sex. The mean (standard deviation) overall satisfaction rating was 4.8 (0.8). With regard to category-specific ratings (eg, bedside manner, aftercare follow-up, time spent with patients), the mean scores were all 4.7 or greater (Table 2).


Regarding the surgical aspects of the procedure, the majority of patients reported that the excision of the lesion was performed by a dermatologist (62%). However, a notable portion of patients reported that the excision was performed by a plastic surgeon (21%). Physician specialty was not reported in 16% of the reviews. For the lesion closure, the patient-reported specialty of the physician was only slightly higher for dermatologists versus plastic surgeons (46% vs 44%)(Table 3).

The majority of patients who reported the location of the lesion treated with MMS identified a high-risk location (45%), a medium-risk location (18%), or an unspecified region of the face (15%), according to the appropriate-use criteria for MMS (Table 3).5 Patients did not specify the site of surgery 17% of the time. Only 5% of reported procedures were performed on low-risk areas.
Basal cell carcinomas were the most commonly reported lesions removed by MMS (38%), though 48% of reviews did not specify the type of tumor being treated (Table 3). A large majority (76%) did not specify the type of closure performed. When specified, secondary intention was used 10% of the time, followed by either a flap (6%) or skin graft (6%). Only 5% of patients reported an estimated size of the primary lesion in our study (data not shown).
The qualitative analysis demonstrated variance in themes for positive and negative characteristics (Table 4). Surgeon characteristics encompassed the 3 most commonly cited themes of positive remarks, including bedside manner (78%), communication skills (74%), and perceived expertise (58%). Specific to MMS, the tissue-sparing nature of the technique was cited by 14% of reviews as a positive theme. The most commonly cited themes of negative remarks were intraoperative and postoperative concerns, including postoperative disfigurement (16%), large scar (9%), healing time (9%), and procedural or postoperative pain (8%). A subtheme analysis of postoperative disfigurement revealed that eyelid or eyebrow distortion was the most common concern (29%), followed by redness and swelling (23%), an open wound (14%), and nostril/nose distortion (14%)(data not shown). Themes not commonly cited as either positive or negative included office environment, cost, and procedure time (data not shown).

Comment
The overall satisfaction with MMS (89%) was one of the highest for any procedure on this online patient review site, albeit based on fewer reviews compared to other common aesthetic surgical procedures. In comparison, 78% of 13,500 reviewers rated breast augmentation as “worth it,” while 60% of 6800 reviewers rated rhinoplasty as “worth it” (as of December 2015). Overall, the online patient reviews evaluated in this study were consistent with a previously published structured data report on patient satisfaction with MMS.6
The results show a greater than expected proportion of both the MMS excision and closure being performed by plastic surgeons compared to dermatologists. In reality, the majority of MMS excisions are performed by dermatologists. Based on a survey of American College of Mohs Surgery (ACMS) members, only 6% of procedures were sent to other specialties for closure.7 Our results may reflect reporting bias or patients misconstruing true MMS with an excision and standard frozen sections, techniques that have lower cure rates. If so, there may be a need to educate patients regarding the specifics of MMS. Other possible explanations for the discrepancy between the online patient reviews and ACMS data include misinterpretation by patients on the exact definition of MMS or that a higher than expected number of procedures were performed by non-ACMS Mohs surgeons.
Our qualitative analysis revealed that patients most frequently commented on the interpersonal skills of their surgeons (eg, bedside manner, communication) as positive themes during MMS, similar to prior analyses of general dermatology practices.3 In comparison to a recent study assessing patient satisfaction with rhinoplasty on RealSelf, the final appearance of the nose represented the most common positive- and negative-cited theme.8 Mohs micrographic surgery procedures typically are done under local anesthesia, which may explain the greater importance of bedside manner and communication intraoperatively in comparison to final surgical outcomes for patient satisfaction. For negative themes, 3 of 4 most common concerns were directly related to the intraoperative and postoperative periods. Providers may be able to improve patient satisfaction by explaining the postoperative course, such as healing time and temporary physical restrictions, as well as possible sequelae in greater detail, which may be particularly pertinent for MMS involving the nose or near the eyes.
The global ratings for MMS are high, as shown in our data set of patient reviews; however, patient reviews are highly susceptible to reporting bias, recall bias, and missing information. Prior work using this online patient review website to investigate laser and light procedures also demonstrated the risk for imperfect information associated with patient reviews.9 Even so, the data does provide a glimpse into what is considered important to patients. Surgeon interpersonal skills and communication were the most frequently cited positive themes for MMS. The best surgical aspects of MMS focused on the unique tissue-sparing nature of the procedure and the removal of a cancerous lesion. Potential areas for improvement include a more thorough explanation of the intraoperative and postoperative process, specifically potential asymmetry related to the nose or the eyes, healing time, and scarring. These patient reviews underscore the importance of setting appropriate patient expectations. As patients become more connected and utilize online platforms to report their experiences, Mohs surgeons can take insights derived from online patient reviews for their own practice or geographic area to improve satisfaction and manage expectations.
Mohs micrographic surgery (MMS) remains the gold standard for the removal of skin cancers in high-risk areas of the body while offering an excellent safety profile and sparing tissue.1 In the current health care environment, online patient reviews have grown in popularity and influence. More than 60% of consumers consult social media before making health care decisions.2 A recent analysis of online patient reviews of general dermatology practices demonstrated the perceived importance of physician empathy, thoroughness, and cognizance of cost in relation to patient-reported satisfaction.3 Because MMS is a well-recognized and unique outpatient-based surgical procedure, a review and analysis of online patient reviews specific to MMS can provide useful practice insights.
Materials and Methods
This study was conducted using an online platform (RealSelf [http://www.realself.com]) that connects patients and providers offering aesthetically oriented procedures; the site has 35 million unique visitors yearly.4 The community’s directory was used to identify and analyze all cumulative patient reviews from 2006 to December 20, 2015, using the search terms Mohs surgery or Mohs micrographic surgery. The study was exempt by the Northwestern University (Chicago, Illinois) institutional review board.
A standardized qualitative coding methodology was created and applied to all available comments regarding MMS. A broad list of positive and negative patient experiences was first created and agreed upon by all 3 investigators. Each individual comment was then attributed to 1 or more of these positive or negative themes. Of these comments, 10% were coded by 2 investigators (S.X. and Z.A.) to ensure internal validity; 1 investigator coded the remaining statements by patients (Z.A.). Patient-reported satisfaction ratings categorized as “worth it” or “not worth it” (as used by RealSelf to describe the patient-perceived value and utility of a given procedure) as well as cost of MMS were gathered. Cumulative patient ratings were collected for the procedure overall, physician’s bedside manner, answered questions, aftercare follow-up, time spent with patients, telephone/email responsiveness, staff professionalism/courtesy, payment process, and wait times. Patient-reported characteristics of MMS also were evaluated including physician specialty, lesion location, type of skin cancer, and type of closure. For lesion location, we graded whether the location represented a high-risk area as defined by the American Academy of Dermatology, American College of Mohs Surgery, and American Society for Dermatologic Surgery.5
Results
A total of 219 reviews related to MMS were collected as of December 20, 2015. Overall, MMS was considered “worth it” by 89% of patients (Table 1). Only 2% of patients described MMS as “not worth it.” There was a wide range reported for the cost of the procedure ($1–$100,000 [median, $1800]). Of those patients who reported their sex, females were 2.5-times more likely to post a review compared to males (51% vs 20%); however, 30% of reviewers did not report their sex. The mean (standard deviation) overall satisfaction rating was 4.8 (0.8). With regard to category-specific ratings (eg, bedside manner, aftercare follow-up, time spent with patients), the mean scores were all 4.7 or greater (Table 2).


Regarding the surgical aspects of the procedure, the majority of patients reported that the excision of the lesion was performed by a dermatologist (62%). However, a notable portion of patients reported that the excision was performed by a plastic surgeon (21%). Physician specialty was not reported in 16% of the reviews. For the lesion closure, the patient-reported specialty of the physician was only slightly higher for dermatologists versus plastic surgeons (46% vs 44%)(Table 3).

The majority of patients who reported the location of the lesion treated with MMS identified a high-risk location (45%), a medium-risk location (18%), or an unspecified region of the face (15%), according to the appropriate-use criteria for MMS (Table 3).5 Patients did not specify the site of surgery 17% of the time. Only 5% of reported procedures were performed on low-risk areas.
Basal cell carcinomas were the most commonly reported lesions removed by MMS (38%), though 48% of reviews did not specify the type of tumor being treated (Table 3). A large majority (76%) did not specify the type of closure performed. When specified, secondary intention was used 10% of the time, followed by either a flap (6%) or skin graft (6%). Only 5% of patients reported an estimated size of the primary lesion in our study (data not shown).
The qualitative analysis demonstrated variance in themes for positive and negative characteristics (Table 4). Surgeon characteristics encompassed the 3 most commonly cited themes of positive remarks, including bedside manner (78%), communication skills (74%), and perceived expertise (58%). Specific to MMS, the tissue-sparing nature of the technique was cited by 14% of reviews as a positive theme. The most commonly cited themes of negative remarks were intraoperative and postoperative concerns, including postoperative disfigurement (16%), large scar (9%), healing time (9%), and procedural or postoperative pain (8%). A subtheme analysis of postoperative disfigurement revealed that eyelid or eyebrow distortion was the most common concern (29%), followed by redness and swelling (23%), an open wound (14%), and nostril/nose distortion (14%)(data not shown). Themes not commonly cited as either positive or negative included office environment, cost, and procedure time (data not shown).

Comment
The overall satisfaction with MMS (89%) was one of the highest for any procedure on this online patient review site, albeit based on fewer reviews compared to other common aesthetic surgical procedures. In comparison, 78% of 13,500 reviewers rated breast augmentation as “worth it,” while 60% of 6800 reviewers rated rhinoplasty as “worth it” (as of December 2015). Overall, the online patient reviews evaluated in this study were consistent with a previously published structured data report on patient satisfaction with MMS.6
The results show a greater than expected proportion of both the MMS excision and closure being performed by plastic surgeons compared to dermatologists. In reality, the majority of MMS excisions are performed by dermatologists. Based on a survey of American College of Mohs Surgery (ACMS) members, only 6% of procedures were sent to other specialties for closure.7 Our results may reflect reporting bias or patients misconstruing true MMS with an excision and standard frozen sections, techniques that have lower cure rates. If so, there may be a need to educate patients regarding the specifics of MMS. Other possible explanations for the discrepancy between the online patient reviews and ACMS data include misinterpretation by patients on the exact definition of MMS or that a higher than expected number of procedures were performed by non-ACMS Mohs surgeons.
Our qualitative analysis revealed that patients most frequently commented on the interpersonal skills of their surgeons (eg, bedside manner, communication) as positive themes during MMS, similar to prior analyses of general dermatology practices.3 In comparison to a recent study assessing patient satisfaction with rhinoplasty on RealSelf, the final appearance of the nose represented the most common positive- and negative-cited theme.8 Mohs micrographic surgery procedures typically are done under local anesthesia, which may explain the greater importance of bedside manner and communication intraoperatively in comparison to final surgical outcomes for patient satisfaction. For negative themes, 3 of 4 most common concerns were directly related to the intraoperative and postoperative periods. Providers may be able to improve patient satisfaction by explaining the postoperative course, such as healing time and temporary physical restrictions, as well as possible sequelae in greater detail, which may be particularly pertinent for MMS involving the nose or near the eyes.
The global ratings for MMS are high, as shown in our data set of patient reviews; however, patient reviews are highly susceptible to reporting bias, recall bias, and missing information. Prior work using this online patient review website to investigate laser and light procedures also demonstrated the risk for imperfect information associated with patient reviews.9 Even so, the data does provide a glimpse into what is considered important to patients. Surgeon interpersonal skills and communication were the most frequently cited positive themes for MMS. The best surgical aspects of MMS focused on the unique tissue-sparing nature of the procedure and the removal of a cancerous lesion. Potential areas for improvement include a more thorough explanation of the intraoperative and postoperative process, specifically potential asymmetry related to the nose or the eyes, healing time, and scarring. These patient reviews underscore the importance of setting appropriate patient expectations. As patients become more connected and utilize online platforms to report their experiences, Mohs surgeons can take insights derived from online patient reviews for their own practice or geographic area to improve satisfaction and manage expectations.
- Alam M, Ibrahim O, Nodzenski M, et al. Adverse events associated with Mohs micrographic surgery: multicenter prospective cohort study of 20,821 cases at 23 centers. JAMA Dermatol. 2013;149:1378-1385.
- Fox S. The social life of health information. Pew Research Center website. http://www.pewresearch.org/fact-tank/2014/01/15/the-social-life-of-health-information/. Published January 15, 2014. Accessed February 11, 2017.
- Smith RJ, Lipoff JB. Evaluation of dermatology practice online reviews: lessons from qualitative analysis. JAMA Dermatol. 2016;152:153-157.
- Schlichte MJ, Karimkhani C, Jones T, et al. Patient use of social media to evaluate cosmetic treatments and procedures. Dermatol Online J. 2015;21. pii:13030/qt88z6r65x.
- American Academy of Dermatology; American College of Mohs Surgery; American Society for Dermatologic Surgery Association; American Society for Mohs Surgery; Ad Hoc Task Force, Connolly SM, Baker DR, Coldiron BM, et al. AAD/ACMS/ASDSA/ASMS 2012 appropriate use criteria for Mohs micrographic surgery: a report of the American Academy of Dermatology, American College of Mohs Surgery, American Society for Dermatologic Surgery Association, and the American Society for Mohs Surgery [published online September 7, 2012]. Dermatol Surg. 2012;38:1582-1603.
- Asgari MM, Bertenthal D, Sen S, et al. Patient satisfaction after treatment of nonmelanoma skin cancer. Derm Surg. 2009;35:1041-1049.
- Campbell RM, Perlis CS, Malik MK, et al. Characteristics of Mohs practices in the United States: a recall survey of ACMS surgeons. Dermatol Surg. 2007;33:1413-1418; discussion, 1418.
- Khansa I, Khansa L, Pearson GD. Patient satisfaction after rhinoplasty: a social media analysis. Aesthet Surg J. 2016;36:NP1-5.
- Xu S, Walter J, Bhatia A. Patient-reported online satisfaction for laser and light procedures: need for caution. Dermatol Surg. 2017;43:154-158.
- Alam M, Ibrahim O, Nodzenski M, et al. Adverse events associated with Mohs micrographic surgery: multicenter prospective cohort study of 20,821 cases at 23 centers. JAMA Dermatol. 2013;149:1378-1385.
- Fox S. The social life of health information. Pew Research Center website. http://www.pewresearch.org/fact-tank/2014/01/15/the-social-life-of-health-information/. Published January 15, 2014. Accessed February 11, 2017.
- Smith RJ, Lipoff JB. Evaluation of dermatology practice online reviews: lessons from qualitative analysis. JAMA Dermatol. 2016;152:153-157.
- Schlichte MJ, Karimkhani C, Jones T, et al. Patient use of social media to evaluate cosmetic treatments and procedures. Dermatol Online J. 2015;21. pii:13030/qt88z6r65x.
- American Academy of Dermatology; American College of Mohs Surgery; American Society for Dermatologic Surgery Association; American Society for Mohs Surgery; Ad Hoc Task Force, Connolly SM, Baker DR, Coldiron BM, et al. AAD/ACMS/ASDSA/ASMS 2012 appropriate use criteria for Mohs micrographic surgery: a report of the American Academy of Dermatology, American College of Mohs Surgery, American Society for Dermatologic Surgery Association, and the American Society for Mohs Surgery [published online September 7, 2012]. Dermatol Surg. 2012;38:1582-1603.
- Asgari MM, Bertenthal D, Sen S, et al. Patient satisfaction after treatment of nonmelanoma skin cancer. Derm Surg. 2009;35:1041-1049.
- Campbell RM, Perlis CS, Malik MK, et al. Characteristics of Mohs practices in the United States: a recall survey of ACMS surgeons. Dermatol Surg. 2007;33:1413-1418; discussion, 1418.
- Khansa I, Khansa L, Pearson GD. Patient satisfaction after rhinoplasty: a social media analysis. Aesthet Surg J. 2016;36:NP1-5.
- Xu S, Walter J, Bhatia A. Patient-reported online satisfaction for laser and light procedures: need for caution. Dermatol Surg. 2017;43:154-158.
Resident Pearl
Patients are posting reviews online now more than ever regarding their experiences with dermatologic surgical procedures. Mohs micrographic surgery is rated highly by patients but suspect to missing information and a higher than expected attribution of the procedure to plastic surgeons.
Laser resurfacing can effectively minimize post surgery scars
MIAMI – In his practice, Joel L. Cohen, MD, spends a good part of his day doing Mohs surgery, “with the goal of cancer removal, and after surgery, having the patient look good,” he said at the Orlando Dermatology Aesthetic and Clinical Conference.
“Having resurfacing in my practice has allowed me to treat not only wrinkles and etched lines, but also help skin cancer patients by blending and minimizing their skin cancer scars,” said Dr. Cohen, an aesthetic dermatologist and Mohs surgeon in private practice in Denver.
Resurfacing in his practice using a variety of lasers is very helpful, Dr. Cohen said. He published a study in November that compared pulse dye laser, CO2 ablative fractional lasers, or a combination of both for modification of scars following Mohs surgery (J Drugs Dermatol. 2016 Nov 1;15[11]:1315-9).
The prospective, multicenter study revealed that although both monotherapy approaches were safe and effective, the combination of pulse dye laser and fractional ablative laser offered some synergy that was preferred by patients.
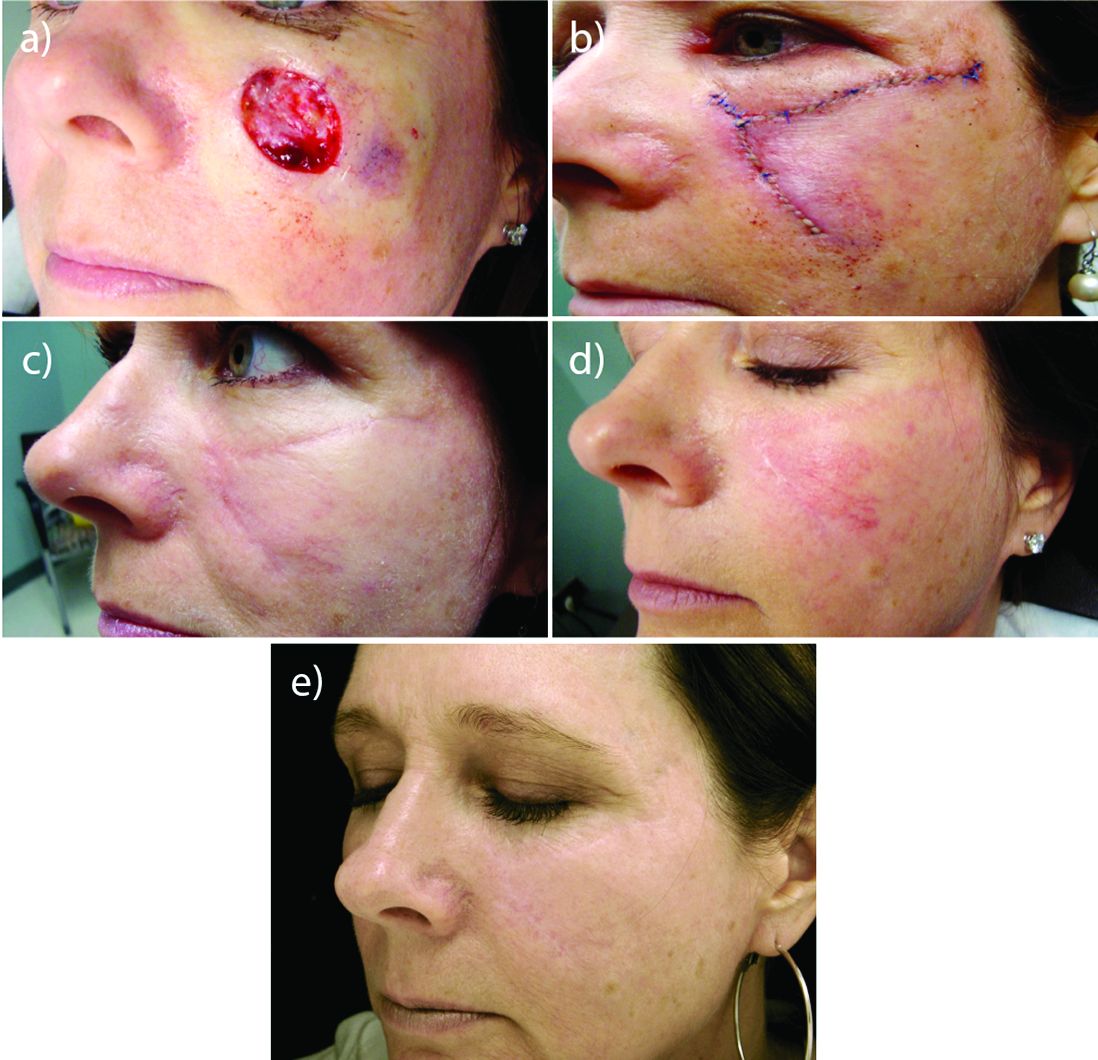
Perioral resurfacing possible
Beyond the world of treating scars, a typical cosmetic patient in Dr. Cohen’s practice presents with numerous lines around the perioral area. “When people think about rejuvenation of the lips, they only think of fillers. But fillers are not the only way to rejuvenate this area, and it is really about choosing the right tool for the right job – where resurfacing lasers are needed.”
Set realistic expectations
Setting the right expectations for people is extremely important, Dr. Cohen said. “You can educate the patient that if you’re putting the needle into the lines, you’re only treating the larger lines that you can get a 30-g needle into, but there are often a host of other lines in that area – many of which are too small to get a needle into.”
As a starting point, neuromodulators can have a role in trying to prevent or delay etched-in lines from forming around the mouth in the first place. “These are the lines between the musculature, the ones you see when you ask the patient to purse their lips,” Dr. Cohen said. He typically injects a medium dose of one of three neuromodulators – such as 6-10 U of onabotulinumtoxinA (Botox), 6-10 U of incobotulinumtoxinA (Xeomin) or 14-18 U of abobotulinumtoxinA (Dysport). “Then somewhere between week 8 and 10, there is an attenuation of the effect, and I often will see patients back then for additional treatment with a neuromodulator,” he added.
“For our every day patient complaining of lots of etched perioral lines, we have laser resurfacing,” Dr. Cohen noted. He is a bigger proponent of full-field erbium treatment versus fractional ablative laser resurfacing for these prominent upper cutaneous lip lines because the results are much more impressive with a single treatment. He added that dermatologists could do fractional treatment around the rest of the face, and reserve the erbium resurfacing to improve the appearance of lines around the mouth and prominent creping skin around the eyes.
Realistic postprocedure expectations are especially essential in the days after erbium laser resurfacing – as it is a tough downtime procedure for patients, often taking 7-9 days to re-epithelialize. “Having photos to show patients what they will look like is really helpful,” Dr. Cohen said. He suggested showing patients a chronologic set of photos of the downtime period as well as the results – so they realize improvement occurs slowly over time. “Getting people to understand they are gong to look terrible for 1.5-2 weeks is superimportant.”
“I like to have them back in the office for a postprocedure check a few days after the bigger laser resurfacing procedures are done, just to check on them,” Dr. Cohen said. “A lot of hand holding is often needed, as there is significantly more healing time with the full-field ablative resurfacing than there is with fractional. Full-field resurfacing patients will experience postprocedure erythema for a few weeks or even months,” Dr. Cohen said. A prescription of topical steroids, and sometimes some brimonidine topical gel (Mirvaso) as well can help reduce the redness.
Toxin injection then laser resurfacing
For some patients, injection of a neuromodulator a week or 2 before laser resurfacing treatment can decrease some of the movement and contraction of the muscle, “and hopefully give them better results,” Dr. Cohen said.
Timing is important. “You don’t want to use neuromodulators on the same day of treatment,” he advised. “The thinking is swelling could potentially cause the neuromodulators to spread to unwanted adjacent muscles.”
Safety first
Another tip for the postprocedure period is to supply patients with very specific written instructions. “I wish they would follow them. Patients don’t always listen to what we advise, demonstrate, and also have written down for them,” he commented. For example, one patient had resurfacing several weeks before leaving on an undisclosed kayaking trip. Despite instructions to use sunscreen, she said she wore a hat for sun protection and developed postinflammatory hyperpigmentation around the mouth that lasted for several months, Dr. Cohen said.*
With heavy resurfacing and ablative resurfacing in general, it is advised to always give patients an antiviral prophylaxis course such as valacyclovir, but it is unfortunate that not all patients will adhere to the recommended regimen, he added.
Another patient had an adverse reaction after resurfacing because she did not follow instructions to apply white petrolatum to her chest following laser resurfacing, Dr. Cohen said. She used Neosporin, “even though in all our paperwork we say never use Neosporin and just use the petrolatum. She had a big contact dermatitis reaction to the Neosporin.”
“So you really need to caution people about the importance of following instructions very carefully,” he emphasized.
Dr. Cohen is a consultant for Sciton and for companies that manufacture injectables, including Allergan, Galderma, and Merz.
Correction 2/24/17: An earlier version of this article mischaracterized the type of pigmentation disorder that the patient developed.
MIAMI – In his practice, Joel L. Cohen, MD, spends a good part of his day doing Mohs surgery, “with the goal of cancer removal, and after surgery, having the patient look good,” he said at the Orlando Dermatology Aesthetic and Clinical Conference.
“Having resurfacing in my practice has allowed me to treat not only wrinkles and etched lines, but also help skin cancer patients by blending and minimizing their skin cancer scars,” said Dr. Cohen, an aesthetic dermatologist and Mohs surgeon in private practice in Denver.
Resurfacing in his practice using a variety of lasers is very helpful, Dr. Cohen said. He published a study in November that compared pulse dye laser, CO2 ablative fractional lasers, or a combination of both for modification of scars following Mohs surgery (J Drugs Dermatol. 2016 Nov 1;15[11]:1315-9).
The prospective, multicenter study revealed that although both monotherapy approaches were safe and effective, the combination of pulse dye laser and fractional ablative laser offered some synergy that was preferred by patients.

Perioral resurfacing possible
Beyond the world of treating scars, a typical cosmetic patient in Dr. Cohen’s practice presents with numerous lines around the perioral area. “When people think about rejuvenation of the lips, they only think of fillers. But fillers are not the only way to rejuvenate this area, and it is really about choosing the right tool for the right job – where resurfacing lasers are needed.”

Set realistic expectations
Setting the right expectations for people is extremely important, Dr. Cohen said. “You can educate the patient that if you’re putting the needle into the lines, you’re only treating the larger lines that you can get a 30-g needle into, but there are often a host of other lines in that area – many of which are too small to get a needle into.”
As a starting point, neuromodulators can have a role in trying to prevent or delay etched-in lines from forming around the mouth in the first place. “These are the lines between the musculature, the ones you see when you ask the patient to purse their lips,” Dr. Cohen said. He typically injects a medium dose of one of three neuromodulators – such as 6-10 U of onabotulinumtoxinA (Botox), 6-10 U of incobotulinumtoxinA (Xeomin) or 14-18 U of abobotulinumtoxinA (Dysport). “Then somewhere between week 8 and 10, there is an attenuation of the effect, and I often will see patients back then for additional treatment with a neuromodulator,” he added.
“For our every day patient complaining of lots of etched perioral lines, we have laser resurfacing,” Dr. Cohen noted. He is a bigger proponent of full-field erbium treatment versus fractional ablative laser resurfacing for these prominent upper cutaneous lip lines because the results are much more impressive with a single treatment. He added that dermatologists could do fractional treatment around the rest of the face, and reserve the erbium resurfacing to improve the appearance of lines around the mouth and prominent creping skin around the eyes.
Realistic postprocedure expectations are especially essential in the days after erbium laser resurfacing – as it is a tough downtime procedure for patients, often taking 7-9 days to re-epithelialize. “Having photos to show patients what they will look like is really helpful,” Dr. Cohen said. He suggested showing patients a chronologic set of photos of the downtime period as well as the results – so they realize improvement occurs slowly over time. “Getting people to understand they are gong to look terrible for 1.5-2 weeks is superimportant.”
“I like to have them back in the office for a postprocedure check a few days after the bigger laser resurfacing procedures are done, just to check on them,” Dr. Cohen said. “A lot of hand holding is often needed, as there is significantly more healing time with the full-field ablative resurfacing than there is with fractional. Full-field resurfacing patients will experience postprocedure erythema for a few weeks or even months,” Dr. Cohen said. A prescription of topical steroids, and sometimes some brimonidine topical gel (Mirvaso) as well can help reduce the redness.
Toxin injection then laser resurfacing
For some patients, injection of a neuromodulator a week or 2 before laser resurfacing treatment can decrease some of the movement and contraction of the muscle, “and hopefully give them better results,” Dr. Cohen said.
Timing is important. “You don’t want to use neuromodulators on the same day of treatment,” he advised. “The thinking is swelling could potentially cause the neuromodulators to spread to unwanted adjacent muscles.”
Safety first
Another tip for the postprocedure period is to supply patients with very specific written instructions. “I wish they would follow them. Patients don’t always listen to what we advise, demonstrate, and also have written down for them,” he commented. For example, one patient had resurfacing several weeks before leaving on an undisclosed kayaking trip. Despite instructions to use sunscreen, she said she wore a hat for sun protection and developed postinflammatory hyperpigmentation around the mouth that lasted for several months, Dr. Cohen said.*
With heavy resurfacing and ablative resurfacing in general, it is advised to always give patients an antiviral prophylaxis course such as valacyclovir, but it is unfortunate that not all patients will adhere to the recommended regimen, he added.
Another patient had an adverse reaction after resurfacing because she did not follow instructions to apply white petrolatum to her chest following laser resurfacing, Dr. Cohen said. She used Neosporin, “even though in all our paperwork we say never use Neosporin and just use the petrolatum. She had a big contact dermatitis reaction to the Neosporin.”
“So you really need to caution people about the importance of following instructions very carefully,” he emphasized.
Dr. Cohen is a consultant for Sciton and for companies that manufacture injectables, including Allergan, Galderma, and Merz.
Correction 2/24/17: An earlier version of this article mischaracterized the type of pigmentation disorder that the patient developed.
MIAMI – In his practice, Joel L. Cohen, MD, spends a good part of his day doing Mohs surgery, “with the goal of cancer removal, and after surgery, having the patient look good,” he said at the Orlando Dermatology Aesthetic and Clinical Conference.
“Having resurfacing in my practice has allowed me to treat not only wrinkles and etched lines, but also help skin cancer patients by blending and minimizing their skin cancer scars,” said Dr. Cohen, an aesthetic dermatologist and Mohs surgeon in private practice in Denver.
Resurfacing in his practice using a variety of lasers is very helpful, Dr. Cohen said. He published a study in November that compared pulse dye laser, CO2 ablative fractional lasers, or a combination of both for modification of scars following Mohs surgery (J Drugs Dermatol. 2016 Nov 1;15[11]:1315-9).
The prospective, multicenter study revealed that although both monotherapy approaches were safe and effective, the combination of pulse dye laser and fractional ablative laser offered some synergy that was preferred by patients.

Perioral resurfacing possible
Beyond the world of treating scars, a typical cosmetic patient in Dr. Cohen’s practice presents with numerous lines around the perioral area. “When people think about rejuvenation of the lips, they only think of fillers. But fillers are not the only way to rejuvenate this area, and it is really about choosing the right tool for the right job – where resurfacing lasers are needed.”

Set realistic expectations
Setting the right expectations for people is extremely important, Dr. Cohen said. “You can educate the patient that if you’re putting the needle into the lines, you’re only treating the larger lines that you can get a 30-g needle into, but there are often a host of other lines in that area – many of which are too small to get a needle into.”
As a starting point, neuromodulators can have a role in trying to prevent or delay etched-in lines from forming around the mouth in the first place. “These are the lines between the musculature, the ones you see when you ask the patient to purse their lips,” Dr. Cohen said. He typically injects a medium dose of one of three neuromodulators – such as 6-10 U of onabotulinumtoxinA (Botox), 6-10 U of incobotulinumtoxinA (Xeomin) or 14-18 U of abobotulinumtoxinA (Dysport). “Then somewhere between week 8 and 10, there is an attenuation of the effect, and I often will see patients back then for additional treatment with a neuromodulator,” he added.
“For our every day patient complaining of lots of etched perioral lines, we have laser resurfacing,” Dr. Cohen noted. He is a bigger proponent of full-field erbium treatment versus fractional ablative laser resurfacing for these prominent upper cutaneous lip lines because the results are much more impressive with a single treatment. He added that dermatologists could do fractional treatment around the rest of the face, and reserve the erbium resurfacing to improve the appearance of lines around the mouth and prominent creping skin around the eyes.
Realistic postprocedure expectations are especially essential in the days after erbium laser resurfacing – as it is a tough downtime procedure for patients, often taking 7-9 days to re-epithelialize. “Having photos to show patients what they will look like is really helpful,” Dr. Cohen said. He suggested showing patients a chronologic set of photos of the downtime period as well as the results – so they realize improvement occurs slowly over time. “Getting people to understand they are gong to look terrible for 1.5-2 weeks is superimportant.”
“I like to have them back in the office for a postprocedure check a few days after the bigger laser resurfacing procedures are done, just to check on them,” Dr. Cohen said. “A lot of hand holding is often needed, as there is significantly more healing time with the full-field ablative resurfacing than there is with fractional. Full-field resurfacing patients will experience postprocedure erythema for a few weeks or even months,” Dr. Cohen said. A prescription of topical steroids, and sometimes some brimonidine topical gel (Mirvaso) as well can help reduce the redness.
Toxin injection then laser resurfacing
For some patients, injection of a neuromodulator a week or 2 before laser resurfacing treatment can decrease some of the movement and contraction of the muscle, “and hopefully give them better results,” Dr. Cohen said.
Timing is important. “You don’t want to use neuromodulators on the same day of treatment,” he advised. “The thinking is swelling could potentially cause the neuromodulators to spread to unwanted adjacent muscles.”
Safety first
Another tip for the postprocedure period is to supply patients with very specific written instructions. “I wish they would follow them. Patients don’t always listen to what we advise, demonstrate, and also have written down for them,” he commented. For example, one patient had resurfacing several weeks before leaving on an undisclosed kayaking trip. Despite instructions to use sunscreen, she said she wore a hat for sun protection and developed postinflammatory hyperpigmentation around the mouth that lasted for several months, Dr. Cohen said.*
With heavy resurfacing and ablative resurfacing in general, it is advised to always give patients an antiviral prophylaxis course such as valacyclovir, but it is unfortunate that not all patients will adhere to the recommended regimen, he added.
Another patient had an adverse reaction after resurfacing because she did not follow instructions to apply white petrolatum to her chest following laser resurfacing, Dr. Cohen said. She used Neosporin, “even though in all our paperwork we say never use Neosporin and just use the petrolatum. She had a big contact dermatitis reaction to the Neosporin.”
“So you really need to caution people about the importance of following instructions very carefully,” he emphasized.
Dr. Cohen is a consultant for Sciton and for companies that manufacture injectables, including Allergan, Galderma, and Merz.
Correction 2/24/17: An earlier version of this article mischaracterized the type of pigmentation disorder that the patient developed.
EXPERT ANALYSIS FROM THE ODAC CONFERENCE
Artificial intelligence, CNN, and diagnosing melanomas
I have a breakthrough article to share with you. It’s about a technology that detects skin cancer. Before I tell you about that, however, I need to teach you a few things. For example, do you know what AI is? How about machine learning? What about CNN? (This column is a nonpolitical arena, so, no, not that CNN).
AI stands for artificial intelligence. We are surrounded by it everywhere – computers, cars, and cell phones all use AI. AI describes a machine with the ability to problem solve, to create, to understand, to learn. These are characteristics we call “intelligence,” hence, artificial intelligence.
You and I intuitively know that a picture of a chair is a chair. This is true of an folding chair, a Barcelona chair, or a Ghost chair. This ability – to intuit – is a hallmark of humans. Computers don’t intuit, they learn. We don’t need to study 3 million chairs to identify chairs. (Nor could we study 3 million pictures of chairs, a feat that would take years.) Computers, in contrast, can review 3 million pictures of chairs. And learn. In minutes.
Not only do computers learn from millions of examples, they also layer learning. For example, one set of programs will look only for lines that appear to be legs of chairs. This information is then passed on to another layer of programming that can look for seats, then another for backs, then another and another until a final layer puts it together. Do these layers remind you of something we all learned in medical school? It is analogous to the mammalian visual cortex! In the brain, one layer of neurons talks with another. In machines, one layer of programs pushes information to another. We call these machine layers “neural networks.” A convoluted neural network or CNN, therefore, describes a complex network that is analogous to brain cortex. The implications are astounding.
Things get interesting when a CNN is given a complex task to learn and a massive observational data set to learn on. With recent advances in chips called GPUs, deeply nested program layers can accomplish difficult tasks like recognizing faces, understanding voices, and avoiding a bicyclist on a foggy day. Self-driving cars, airport security, and voice-activated assistants all rely on this “deep learning.” And they are getting smarter everyday.
So, now when I say a team at Stanford University has used a CNN and deep learning to diagnose melanoma from pictures, you’ll understand what I mean. And you’ll realize computers can do something heretofore unthinkable – make diagnoses as accurately as a doctor. That story should make you both a little giddy and afraid. But wait, there’s more! Read all about it next time.
Dr. Benabio is a partner physician and chief of service for the department of dermatology of the Southern California Permanente Group in San Diego. Dr. Benabio is @Dermdoc on Twitter. Write to him at dermnews@frontlinemedcom.com . He has no disclosures related to this column.
I have a breakthrough article to share with you. It’s about a technology that detects skin cancer. Before I tell you about that, however, I need to teach you a few things. For example, do you know what AI is? How about machine learning? What about CNN? (This column is a nonpolitical arena, so, no, not that CNN).
AI stands for artificial intelligence. We are surrounded by it everywhere – computers, cars, and cell phones all use AI. AI describes a machine with the ability to problem solve, to create, to understand, to learn. These are characteristics we call “intelligence,” hence, artificial intelligence.
You and I intuitively know that a picture of a chair is a chair. This is true of an folding chair, a Barcelona chair, or a Ghost chair. This ability – to intuit – is a hallmark of humans. Computers don’t intuit, they learn. We don’t need to study 3 million chairs to identify chairs. (Nor could we study 3 million pictures of chairs, a feat that would take years.) Computers, in contrast, can review 3 million pictures of chairs. And learn. In minutes.
Not only do computers learn from millions of examples, they also layer learning. For example, one set of programs will look only for lines that appear to be legs of chairs. This information is then passed on to another layer of programming that can look for seats, then another for backs, then another and another until a final layer puts it together. Do these layers remind you of something we all learned in medical school? It is analogous to the mammalian visual cortex! In the brain, one layer of neurons talks with another. In machines, one layer of programs pushes information to another. We call these machine layers “neural networks.” A convoluted neural network or CNN, therefore, describes a complex network that is analogous to brain cortex. The implications are astounding.
Things get interesting when a CNN is given a complex task to learn and a massive observational data set to learn on. With recent advances in chips called GPUs, deeply nested program layers can accomplish difficult tasks like recognizing faces, understanding voices, and avoiding a bicyclist on a foggy day. Self-driving cars, airport security, and voice-activated assistants all rely on this “deep learning.” And they are getting smarter everyday.
So, now when I say a team at Stanford University has used a CNN and deep learning to diagnose melanoma from pictures, you’ll understand what I mean. And you’ll realize computers can do something heretofore unthinkable – make diagnoses as accurately as a doctor. That story should make you both a little giddy and afraid. But wait, there’s more! Read all about it next time.
Dr. Benabio is a partner physician and chief of service for the department of dermatology of the Southern California Permanente Group in San Diego. Dr. Benabio is @Dermdoc on Twitter. Write to him at dermnews@frontlinemedcom.com . He has no disclosures related to this column.
I have a breakthrough article to share with you. It’s about a technology that detects skin cancer. Before I tell you about that, however, I need to teach you a few things. For example, do you know what AI is? How about machine learning? What about CNN? (This column is a nonpolitical arena, so, no, not that CNN).
AI stands for artificial intelligence. We are surrounded by it everywhere – computers, cars, and cell phones all use AI. AI describes a machine with the ability to problem solve, to create, to understand, to learn. These are characteristics we call “intelligence,” hence, artificial intelligence.
You and I intuitively know that a picture of a chair is a chair. This is true of an folding chair, a Barcelona chair, or a Ghost chair. This ability – to intuit – is a hallmark of humans. Computers don’t intuit, they learn. We don’t need to study 3 million chairs to identify chairs. (Nor could we study 3 million pictures of chairs, a feat that would take years.) Computers, in contrast, can review 3 million pictures of chairs. And learn. In minutes.
Not only do computers learn from millions of examples, they also layer learning. For example, one set of programs will look only for lines that appear to be legs of chairs. This information is then passed on to another layer of programming that can look for seats, then another for backs, then another and another until a final layer puts it together. Do these layers remind you of something we all learned in medical school? It is analogous to the mammalian visual cortex! In the brain, one layer of neurons talks with another. In machines, one layer of programs pushes information to another. We call these machine layers “neural networks.” A convoluted neural network or CNN, therefore, describes a complex network that is analogous to brain cortex. The implications are astounding.
Things get interesting when a CNN is given a complex task to learn and a massive observational data set to learn on. With recent advances in chips called GPUs, deeply nested program layers can accomplish difficult tasks like recognizing faces, understanding voices, and avoiding a bicyclist on a foggy day. Self-driving cars, airport security, and voice-activated assistants all rely on this “deep learning.” And they are getting smarter everyday.
So, now when I say a team at Stanford University has used a CNN and deep learning to diagnose melanoma from pictures, you’ll understand what I mean. And you’ll realize computers can do something heretofore unthinkable – make diagnoses as accurately as a doctor. That story should make you both a little giddy and afraid. But wait, there’s more! Read all about it next time.
Dr. Benabio is a partner physician and chief of service for the department of dermatology of the Southern California Permanente Group in San Diego. Dr. Benabio is @Dermdoc on Twitter. Write to him at dermnews@frontlinemedcom.com . He has no disclosures related to this column.
Verrucous Carcinoma of the Buccal Mucosa With Extension to the Cheek
To the Editor:
Verrucous carcinoma is an uncommon type of squamous cell carcinoma (SCC) and was first described by Ackerman1 in 1948. Rock and Fisher2 called this condition oral florid papillomatosis. The distinctive features of this tumor are low-grade malignancy, slow growth, local invasiveness, and rarely intraoral and extraoral metastasis. Extraorally, it can occur in any part of the body,3 a common site being the anogenital region. Depending on the area of occurrence, the condition also is known as Buschke-Lowenstein tumor4 or giant condyloma acuminatum (anogenital region) and carcinoma cuniculatum5 (plantar region). The exact etiology of the condition is unknown, though it is associated with human papillomavirus infection, traumatic scars, chronic infection, tobacco, and chemical carcinogens.3 We report a rare case of verrucous carcinoma originating from the buccal mucosa that subsequently spread to involve the lip and cheek as a large cauliflowerlike growth, which is an unusual presentation.
A 65-year-old man presented to the dermatology department with a painless growth inside the left side of the oral cavity that had developed 5 years prior as a growth on the left buccal mucosa. The lesion gradually increased in size to involve the left oral commissure including the upper and lower lips and the skin of the left cheek; it extended beyond the nasolabial fold in a cauliflowerlike pattern. The lesion was insidious in onset and was not associated with pain, itching, or bleeding. The patient chewed tobacco for the last 40 years, with no similar lesions on any part of the body. On physical examination a warty papilliform lesion was seen on the left buccal mucosa with extension to 2 cm of the upper and lower lip on the left side including the left oral commissure and the skin of the left cheek beyond the nasolabial fold where it appeared as a cauliflowerlike growth measuring 4×5 cm in size (Figure 1). No notable lymphadenopathy was present.
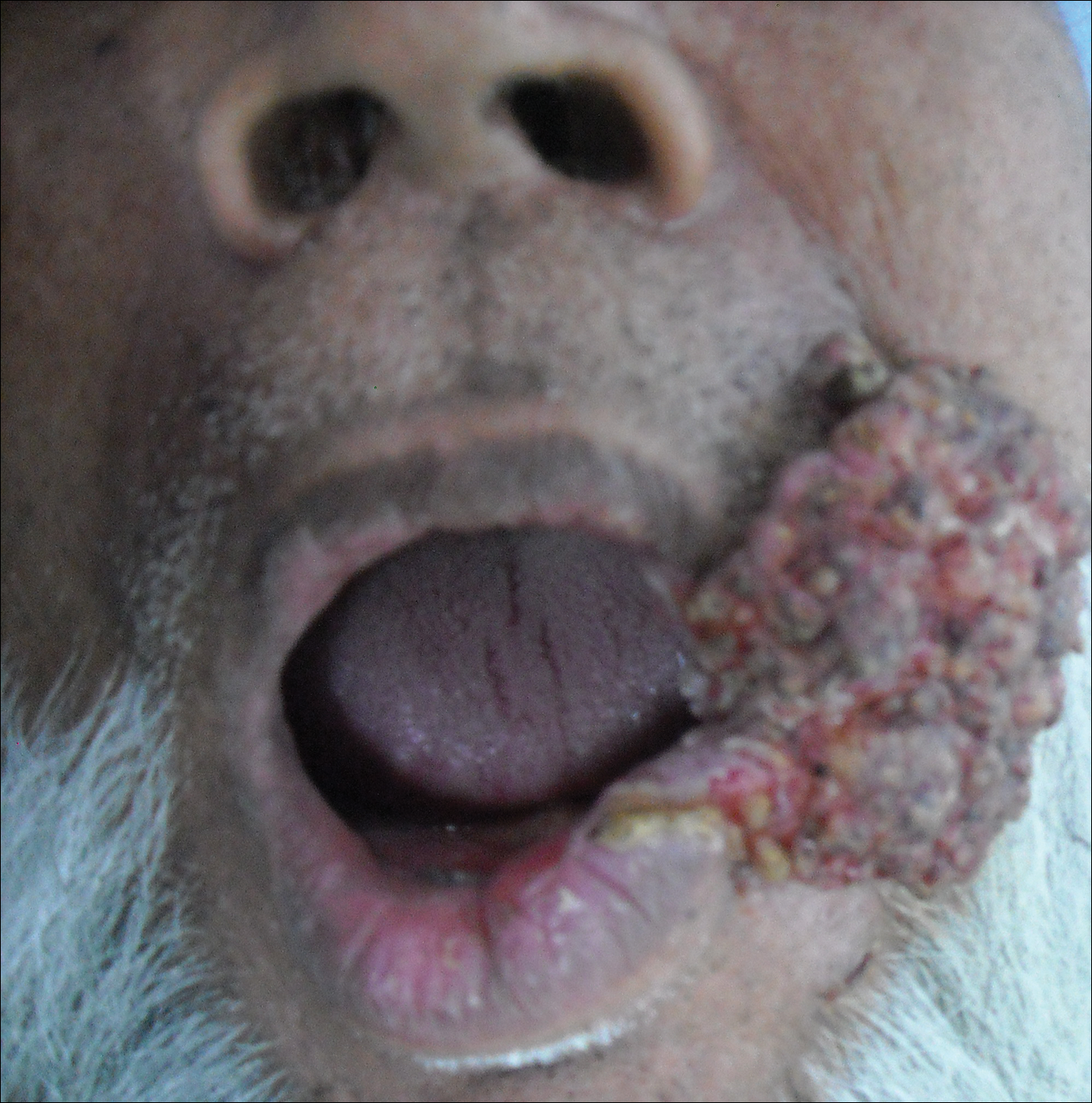
Digital radiographs of the skull (posteroanterior oblique view)(Figure 2) and mandible (left oblique view) showed a lobulated soft-tissue density lesion overlying the left half of the mandible (near the mandibular angle) with involvement of both the upper and lower lips on the left side. However, no obvious underlying bony erosion was noted.
Computed tomography revealed a large soft-tissue mass (41.3×35.3 mm)(Figure 3A) involving the left buccal mucosa with extension into overlying muscle, subcutaneous tissue, and skin. Externally, the lesion was exophytic, irregular, and polypoidal with surface ulceration. Medially, the lesion involved the left oral commissure and parts of the adjoining upper and lower lips. No underlying bony erosion was seen. An enlarged lymph node measuring 20×15 mm was noted in the left upper deep cervical group in the submandibular region (Figure 3B).
Our clinical differential diagnosis included verrucous carcinoma and hypertrophic variety of lupus vulgaris. A 1×2-cm diagnostic incisional biopsy was performed from the cauliflowerlike growth and ultrasound-guided fine-needle aspiration was done from the lymph node. Histopathology revealed a hyperplastic stratified squamous epithelium with upward extension of verrucous projections, which was largely superficial to the adjacent epithelium (Figure 4A). In addition to the surface verrucous projections, there was lesion extension into the subepithelial zone in the form of round club-shaped protrusions (Figure 4B). There was no loss of polarity in these downward proliferations. No horn pearl formation was present. Fine-needle aspiration revealed reactive lymphadenitis.

The final diagnosis of verrucous carcinoma was made and the patient was referred to the oncosurgery department for further management.
Verrucous carcinoma is a rare, low-grade, well-differentiated SCC of the skin or mucosa presenting with a verrucoid or cauliflowerlike appearance. It shows locally aggressive behavior and has low metastatic potential,6 a low degree of dysplasia, and a good prognosis. Because it is a tumor with predominantly horizontal growth, it tends to erode more than infiltrate. It does not present with remote metastasis.7 It has been known by several different names, usually related to anatomic sites (eg, Ackerman tumor, oral florid papillomatosis, carcinoma cuniculatum).
In the oral cavity, verrucous carcinoma constitutes 2% to 4.5% of all forms of SCC seen mainly in men older than 50 years and also is associated with a high incidence (37.7%) of a second primary tumor mainly in the oral mucosa (eg, tongue, lips, palate, salivary gland).8 Indudharan et al9 reported a case of verrucous carcinoma of the maxillary antrum in a young male patient, which also was a rare entity. Verrucous carcinoma is thought to predominantly affect elderly men. Walvekar et al10 reported a male to female ratio of 3.6 to 1 in patients with verrucous carcinoma, with a mean age of 53.9 years. According to Varshney et al,11 patients may range in age from the fourth to eighth decades of life, with a mean age of 60 years; 80% are male. The etiopathogenesis of verrucous carcinoma is related to the following carcinogens: biologic (eg, human papillomavirus), chemical (eg, smoking), and physical (eg, constant trauma).
Verrucous carcinoma should be considered in the differential diagnosis of slow-growing, locally spreading tumors. Oral tumors, especially in tobacco chewers, should raise suspicion of verrucous carcinoma, which will enable prompt management of the tumor.
- Ackerman LV. Verrucous carcinoma of the oral cavity. Surgery. 1948;23:670-678.
- Rock JA, Fisher ER. Florid papillomatosis of the oral cavity and larynx. Arch Otolaryngol. 1960;72:593-598.
- Pattee SF, Bordeaux J, Mahalingam M, et al. Verrucous carcinoma of the scalp. J Am Acad Dermatol. 2007;56:506-507.
- Buschke A, Lowenstein L. Uber carcinomahnliche condylomata acuminata despenis. Klin Wochenschr. 1925;4:1726-1728.
- Aird I, Johnson HD, Lennox B, et al. Epithelioma cuniculatum: a variety of squamous carcinoma peculiar to the foot. Br J Surg. 1954;42:245-250.
- Schwartz RA. Verrucous carcinoma of the skin and mucosa. J Am Acad Dermatol. 1995;32:1-21.
- Zanini M, Wulkan C, Paschoal FM, et al. Verrucous carcinoma: a clinical histopathologic variant of squamous cell carcinoma. An Bras Dermatol. 2004;79:619-621.
- Kalsotra P, Manhas M, Sood R. Verrucous carcinoma of hard palate. JK Science. 2000;2:52-54.
- Indudharan R, Das PK, Thida T. Verrucous carcinoma of maxillary antrum. Singapore Med J. 1996;37:559-561.
- Walvekar RR, Chaukar DA, Deshpande MS, et al. Verrucous carcinoma of the oral cavity: a clinical and pathological study of 101 cases. Oral Oncol. 2009;45:47-51.
- Varshney S, Singh J, Saxena RK, et al. Verrucous carcinoma of larynx. Indian J Otolaryngol Head Neck Surg. 2004;56:54-56.
To the Editor:
Verrucous carcinoma is an uncommon type of squamous cell carcinoma (SCC) and was first described by Ackerman1 in 1948. Rock and Fisher2 called this condition oral florid papillomatosis. The distinctive features of this tumor are low-grade malignancy, slow growth, local invasiveness, and rarely intraoral and extraoral metastasis. Extraorally, it can occur in any part of the body,3 a common site being the anogenital region. Depending on the area of occurrence, the condition also is known as Buschke-Lowenstein tumor4 or giant condyloma acuminatum (anogenital region) and carcinoma cuniculatum5 (plantar region). The exact etiology of the condition is unknown, though it is associated with human papillomavirus infection, traumatic scars, chronic infection, tobacco, and chemical carcinogens.3 We report a rare case of verrucous carcinoma originating from the buccal mucosa that subsequently spread to involve the lip and cheek as a large cauliflowerlike growth, which is an unusual presentation.
A 65-year-old man presented to the dermatology department with a painless growth inside the left side of the oral cavity that had developed 5 years prior as a growth on the left buccal mucosa. The lesion gradually increased in size to involve the left oral commissure including the upper and lower lips and the skin of the left cheek; it extended beyond the nasolabial fold in a cauliflowerlike pattern. The lesion was insidious in onset and was not associated with pain, itching, or bleeding. The patient chewed tobacco for the last 40 years, with no similar lesions on any part of the body. On physical examination a warty papilliform lesion was seen on the left buccal mucosa with extension to 2 cm of the upper and lower lip on the left side including the left oral commissure and the skin of the left cheek beyond the nasolabial fold where it appeared as a cauliflowerlike growth measuring 4×5 cm in size (Figure 1). No notable lymphadenopathy was present.

Digital radiographs of the skull (posteroanterior oblique view)(Figure 2) and mandible (left oblique view) showed a lobulated soft-tissue density lesion overlying the left half of the mandible (near the mandibular angle) with involvement of both the upper and lower lips on the left side. However, no obvious underlying bony erosion was noted.

Computed tomography revealed a large soft-tissue mass (41.3×35.3 mm)(Figure 3A) involving the left buccal mucosa with extension into overlying muscle, subcutaneous tissue, and skin. Externally, the lesion was exophytic, irregular, and polypoidal with surface ulceration. Medially, the lesion involved the left oral commissure and parts of the adjoining upper and lower lips. No underlying bony erosion was seen. An enlarged lymph node measuring 20×15 mm was noted in the left upper deep cervical group in the submandibular region (Figure 3B).

Our clinical differential diagnosis included verrucous carcinoma and hypertrophic variety of lupus vulgaris. A 1×2-cm diagnostic incisional biopsy was performed from the cauliflowerlike growth and ultrasound-guided fine-needle aspiration was done from the lymph node. Histopathology revealed a hyperplastic stratified squamous epithelium with upward extension of verrucous projections, which was largely superficial to the adjacent epithelium (Figure 4A). In addition to the surface verrucous projections, there was lesion extension into the subepithelial zone in the form of round club-shaped protrusions (Figure 4B). There was no loss of polarity in these downward proliferations. No horn pearl formation was present. Fine-needle aspiration revealed reactive lymphadenitis.

The final diagnosis of verrucous carcinoma was made and the patient was referred to the oncosurgery department for further management.
Verrucous carcinoma is a rare, low-grade, well-differentiated SCC of the skin or mucosa presenting with a verrucoid or cauliflowerlike appearance. It shows locally aggressive behavior and has low metastatic potential,6 a low degree of dysplasia, and a good prognosis. Because it is a tumor with predominantly horizontal growth, it tends to erode more than infiltrate. It does not present with remote metastasis.7 It has been known by several different names, usually related to anatomic sites (eg, Ackerman tumor, oral florid papillomatosis, carcinoma cuniculatum).
In the oral cavity, verrucous carcinoma constitutes 2% to 4.5% of all forms of SCC seen mainly in men older than 50 years and also is associated with a high incidence (37.7%) of a second primary tumor mainly in the oral mucosa (eg, tongue, lips, palate, salivary gland).8 Indudharan et al9 reported a case of verrucous carcinoma of the maxillary antrum in a young male patient, which also was a rare entity. Verrucous carcinoma is thought to predominantly affect elderly men. Walvekar et al10 reported a male to female ratio of 3.6 to 1 in patients with verrucous carcinoma, with a mean age of 53.9 years. According to Varshney et al,11 patients may range in age from the fourth to eighth decades of life, with a mean age of 60 years; 80% are male. The etiopathogenesis of verrucous carcinoma is related to the following carcinogens: biologic (eg, human papillomavirus), chemical (eg, smoking), and physical (eg, constant trauma).
Verrucous carcinoma should be considered in the differential diagnosis of slow-growing, locally spreading tumors. Oral tumors, especially in tobacco chewers, should raise suspicion of verrucous carcinoma, which will enable prompt management of the tumor.
To the Editor:
Verrucous carcinoma is an uncommon type of squamous cell carcinoma (SCC) and was first described by Ackerman1 in 1948. Rock and Fisher2 called this condition oral florid papillomatosis. The distinctive features of this tumor are low-grade malignancy, slow growth, local invasiveness, and rarely intraoral and extraoral metastasis. Extraorally, it can occur in any part of the body,3 a common site being the anogenital region. Depending on the area of occurrence, the condition also is known as Buschke-Lowenstein tumor4 or giant condyloma acuminatum (anogenital region) and carcinoma cuniculatum5 (plantar region). The exact etiology of the condition is unknown, though it is associated with human papillomavirus infection, traumatic scars, chronic infection, tobacco, and chemical carcinogens.3 We report a rare case of verrucous carcinoma originating from the buccal mucosa that subsequently spread to involve the lip and cheek as a large cauliflowerlike growth, which is an unusual presentation.
A 65-year-old man presented to the dermatology department with a painless growth inside the left side of the oral cavity that had developed 5 years prior as a growth on the left buccal mucosa. The lesion gradually increased in size to involve the left oral commissure including the upper and lower lips and the skin of the left cheek; it extended beyond the nasolabial fold in a cauliflowerlike pattern. The lesion was insidious in onset and was not associated with pain, itching, or bleeding. The patient chewed tobacco for the last 40 years, with no similar lesions on any part of the body. On physical examination a warty papilliform lesion was seen on the left buccal mucosa with extension to 2 cm of the upper and lower lip on the left side including the left oral commissure and the skin of the left cheek beyond the nasolabial fold where it appeared as a cauliflowerlike growth measuring 4×5 cm in size (Figure 1). No notable lymphadenopathy was present.

Digital radiographs of the skull (posteroanterior oblique view)(Figure 2) and mandible (left oblique view) showed a lobulated soft-tissue density lesion overlying the left half of the mandible (near the mandibular angle) with involvement of both the upper and lower lips on the left side. However, no obvious underlying bony erosion was noted.

Computed tomography revealed a large soft-tissue mass (41.3×35.3 mm)(Figure 3A) involving the left buccal mucosa with extension into overlying muscle, subcutaneous tissue, and skin. Externally, the lesion was exophytic, irregular, and polypoidal with surface ulceration. Medially, the lesion involved the left oral commissure and parts of the adjoining upper and lower lips. No underlying bony erosion was seen. An enlarged lymph node measuring 20×15 mm was noted in the left upper deep cervical group in the submandibular region (Figure 3B).

Our clinical differential diagnosis included verrucous carcinoma and hypertrophic variety of lupus vulgaris. A 1×2-cm diagnostic incisional biopsy was performed from the cauliflowerlike growth and ultrasound-guided fine-needle aspiration was done from the lymph node. Histopathology revealed a hyperplastic stratified squamous epithelium with upward extension of verrucous projections, which was largely superficial to the adjacent epithelium (Figure 4A). In addition to the surface verrucous projections, there was lesion extension into the subepithelial zone in the form of round club-shaped protrusions (Figure 4B). There was no loss of polarity in these downward proliferations. No horn pearl formation was present. Fine-needle aspiration revealed reactive lymphadenitis.

The final diagnosis of verrucous carcinoma was made and the patient was referred to the oncosurgery department for further management.
Verrucous carcinoma is a rare, low-grade, well-differentiated SCC of the skin or mucosa presenting with a verrucoid or cauliflowerlike appearance. It shows locally aggressive behavior and has low metastatic potential,6 a low degree of dysplasia, and a good prognosis. Because it is a tumor with predominantly horizontal growth, it tends to erode more than infiltrate. It does not present with remote metastasis.7 It has been known by several different names, usually related to anatomic sites (eg, Ackerman tumor, oral florid papillomatosis, carcinoma cuniculatum).
In the oral cavity, verrucous carcinoma constitutes 2% to 4.5% of all forms of SCC seen mainly in men older than 50 years and also is associated with a high incidence (37.7%) of a second primary tumor mainly in the oral mucosa (eg, tongue, lips, palate, salivary gland).8 Indudharan et al9 reported a case of verrucous carcinoma of the maxillary antrum in a young male patient, which also was a rare entity. Verrucous carcinoma is thought to predominantly affect elderly men. Walvekar et al10 reported a male to female ratio of 3.6 to 1 in patients with verrucous carcinoma, with a mean age of 53.9 years. According to Varshney et al,11 patients may range in age from the fourth to eighth decades of life, with a mean age of 60 years; 80% are male. The etiopathogenesis of verrucous carcinoma is related to the following carcinogens: biologic (eg, human papillomavirus), chemical (eg, smoking), and physical (eg, constant trauma).
Verrucous carcinoma should be considered in the differential diagnosis of slow-growing, locally spreading tumors. Oral tumors, especially in tobacco chewers, should raise suspicion of verrucous carcinoma, which will enable prompt management of the tumor.
- Ackerman LV. Verrucous carcinoma of the oral cavity. Surgery. 1948;23:670-678.
- Rock JA, Fisher ER. Florid papillomatosis of the oral cavity and larynx. Arch Otolaryngol. 1960;72:593-598.
- Pattee SF, Bordeaux J, Mahalingam M, et al. Verrucous carcinoma of the scalp. J Am Acad Dermatol. 2007;56:506-507.
- Buschke A, Lowenstein L. Uber carcinomahnliche condylomata acuminata despenis. Klin Wochenschr. 1925;4:1726-1728.
- Aird I, Johnson HD, Lennox B, et al. Epithelioma cuniculatum: a variety of squamous carcinoma peculiar to the foot. Br J Surg. 1954;42:245-250.
- Schwartz RA. Verrucous carcinoma of the skin and mucosa. J Am Acad Dermatol. 1995;32:1-21.
- Zanini M, Wulkan C, Paschoal FM, et al. Verrucous carcinoma: a clinical histopathologic variant of squamous cell carcinoma. An Bras Dermatol. 2004;79:619-621.
- Kalsotra P, Manhas M, Sood R. Verrucous carcinoma of hard palate. JK Science. 2000;2:52-54.
- Indudharan R, Das PK, Thida T. Verrucous carcinoma of maxillary antrum. Singapore Med J. 1996;37:559-561.
- Walvekar RR, Chaukar DA, Deshpande MS, et al. Verrucous carcinoma of the oral cavity: a clinical and pathological study of 101 cases. Oral Oncol. 2009;45:47-51.
- Varshney S, Singh J, Saxena RK, et al. Verrucous carcinoma of larynx. Indian J Otolaryngol Head Neck Surg. 2004;56:54-56.
- Ackerman LV. Verrucous carcinoma of the oral cavity. Surgery. 1948;23:670-678.
- Rock JA, Fisher ER. Florid papillomatosis of the oral cavity and larynx. Arch Otolaryngol. 1960;72:593-598.
- Pattee SF, Bordeaux J, Mahalingam M, et al. Verrucous carcinoma of the scalp. J Am Acad Dermatol. 2007;56:506-507.
- Buschke A, Lowenstein L. Uber carcinomahnliche condylomata acuminata despenis. Klin Wochenschr. 1925;4:1726-1728.
- Aird I, Johnson HD, Lennox B, et al. Epithelioma cuniculatum: a variety of squamous carcinoma peculiar to the foot. Br J Surg. 1954;42:245-250.
- Schwartz RA. Verrucous carcinoma of the skin and mucosa. J Am Acad Dermatol. 1995;32:1-21.
- Zanini M, Wulkan C, Paschoal FM, et al. Verrucous carcinoma: a clinical histopathologic variant of squamous cell carcinoma. An Bras Dermatol. 2004;79:619-621.
- Kalsotra P, Manhas M, Sood R. Verrucous carcinoma of hard palate. JK Science. 2000;2:52-54.
- Indudharan R, Das PK, Thida T. Verrucous carcinoma of maxillary antrum. Singapore Med J. 1996;37:559-561.
- Walvekar RR, Chaukar DA, Deshpande MS, et al. Verrucous carcinoma of the oral cavity: a clinical and pathological study of 101 cases. Oral Oncol. 2009;45:47-51.
- Varshney S, Singh J, Saxena RK, et al. Verrucous carcinoma of larynx. Indian J Otolaryngol Head Neck Surg. 2004;56:54-56.
Practice Points
- Verrucous carcinoma is a slow-growing tumor that often presents in advanced clinical stages because it is poorly understood and underrecognized, especially in developing countries.
- Good clinicopathological correlation is required in cases of verrucous carcinoma to avoid misdiagnosis and provide appropriate treatment.
- Case-specific management should be considered, as presentation of verrucous carcinoma varies.
- Radiography should be considered to assess for lymph node involvement.
Model: Quadrivalent vaccine could cost effectively cut MSM’s HPV-related cancers
Use of a quadrivalent HPV vaccine in men who have sex with men, administered in genitourinary specialty clinics, would cost effectively provide herd immunity against the ill effects of the virus, particularly anogenital warts and male HPV-related cancers, according to an English mathematical model.
Mark Jit, PhD, a professor of vaccine epidemiology at the London School of Hygiene & Tropical Medicine, and his colleagues considered in which settings HPV vaccination delivery would have the greatest effect size; the patterns of sexual behavior in MSM leading to the transmission of HPV 6, 11, 16, and 18; and the costs and quality adjusted life year (QALY) implications of disease outcomes (Clin Infect Dis. 2016 Dec 23. doi: 10.1093/cid/ciw845).
Clinic attendance rates were based on genitourinary clinic returns in England recorded in public health surveillance data recorded between 2009 and 2012, stratified according to diagnosed HIV-positive status. Also modeled were the effects of vaccination in MSM between the ages of 16 and 40 years, according to groups aged 16-25 years, 16-30 years, 16-35 years, and 16-40 years. Models for ages on either side of 16 or 40 years were not considered because of confidentiality constraints in the former and limited specialty clinic use in the latter.
Herd protection likely would be notable in the first year, because of the breadth of the age ranges modeled, Dr. Jit and his colleagues determined. Specifically, the models predicted a 35% decline in incidence rates of anogenital warts within 5 years of initiating the vaccine across all MSM men seen in specialty clinics. If only HIV-positive MSM across the age groups were vaccinated, the models predicted a 5-year decline of 15%.
Declines predicted in HPV-related cancers would happen more slowly, because progression from infection to malignancies tends to occur over years. For example, there would be a 55% reduction over 100 years for anal cancer if all 16- to 40-year-old MSM attending specialty clinics are offered vaccination. However, the reduction rate would drop to 40% in that same time period if only HIV-positive men across the age groups were vaccinated.
Using a cost-effectiveness threshold of 20,000 British pounds (about $24,500) per QALY, with no more than a 10% probability that the incremental cost-effectiveness ratio would exceed 30,000 pounds per QALY ($36,710), Dr. Jit and his colleagues determined that using a quadrivalent HPV vaccination in MSM between ages 16 and 40 years in the specialty clinic setting would be cost effective if delivery cost an average of 63 pounds ($77) per dose.
By offering vaccination only to HIV-positive MSM between 16 and 40 years, even if the quadrivalent vaccine were to cost as much as 96.50 pounds ($118), it would still be cost effective. A nonavalent vaccine at the same price would also be cost effective, because nearly all HPV-related cancers are linked to HPV 16 and 18. However, a bivalent vaccine was not shown by the models to be cost effective in such a limited program.
The investigators theorized that HIV infection is associated with the rate of HPV-related disease progression. To simplify computation, however, their models only considered the overall cost effectiveness of offering HPV vaccination to either HIV-positive MSM or to MSM regardless of current HIV status. That could mean “the cost effectiveness of MSM vaccination may be even better than reported,” the researchers noted.
This study was funded primarily by the National Institute for Health Research and Public Health England in the United Kingdom. The authors had no relevant disclosures.
Use of a quadrivalent HPV vaccine in men who have sex with men, administered in genitourinary specialty clinics, would cost effectively provide herd immunity against the ill effects of the virus, particularly anogenital warts and male HPV-related cancers, according to an English mathematical model.
Mark Jit, PhD, a professor of vaccine epidemiology at the London School of Hygiene & Tropical Medicine, and his colleagues considered in which settings HPV vaccination delivery would have the greatest effect size; the patterns of sexual behavior in MSM leading to the transmission of HPV 6, 11, 16, and 18; and the costs and quality adjusted life year (QALY) implications of disease outcomes (Clin Infect Dis. 2016 Dec 23. doi: 10.1093/cid/ciw845).
Clinic attendance rates were based on genitourinary clinic returns in England recorded in public health surveillance data recorded between 2009 and 2012, stratified according to diagnosed HIV-positive status. Also modeled were the effects of vaccination in MSM between the ages of 16 and 40 years, according to groups aged 16-25 years, 16-30 years, 16-35 years, and 16-40 years. Models for ages on either side of 16 or 40 years were not considered because of confidentiality constraints in the former and limited specialty clinic use in the latter.
Herd protection likely would be notable in the first year, because of the breadth of the age ranges modeled, Dr. Jit and his colleagues determined. Specifically, the models predicted a 35% decline in incidence rates of anogenital warts within 5 years of initiating the vaccine across all MSM men seen in specialty clinics. If only HIV-positive MSM across the age groups were vaccinated, the models predicted a 5-year decline of 15%.
Declines predicted in HPV-related cancers would happen more slowly, because progression from infection to malignancies tends to occur over years. For example, there would be a 55% reduction over 100 years for anal cancer if all 16- to 40-year-old MSM attending specialty clinics are offered vaccination. However, the reduction rate would drop to 40% in that same time period if only HIV-positive men across the age groups were vaccinated.
Using a cost-effectiveness threshold of 20,000 British pounds (about $24,500) per QALY, with no more than a 10% probability that the incremental cost-effectiveness ratio would exceed 30,000 pounds per QALY ($36,710), Dr. Jit and his colleagues determined that using a quadrivalent HPV vaccination in MSM between ages 16 and 40 years in the specialty clinic setting would be cost effective if delivery cost an average of 63 pounds ($77) per dose.
By offering vaccination only to HIV-positive MSM between 16 and 40 years, even if the quadrivalent vaccine were to cost as much as 96.50 pounds ($118), it would still be cost effective. A nonavalent vaccine at the same price would also be cost effective, because nearly all HPV-related cancers are linked to HPV 16 and 18. However, a bivalent vaccine was not shown by the models to be cost effective in such a limited program.
The investigators theorized that HIV infection is associated with the rate of HPV-related disease progression. To simplify computation, however, their models only considered the overall cost effectiveness of offering HPV vaccination to either HIV-positive MSM or to MSM regardless of current HIV status. That could mean “the cost effectiveness of MSM vaccination may be even better than reported,” the researchers noted.
This study was funded primarily by the National Institute for Health Research and Public Health England in the United Kingdom. The authors had no relevant disclosures.
Use of a quadrivalent HPV vaccine in men who have sex with men, administered in genitourinary specialty clinics, would cost effectively provide herd immunity against the ill effects of the virus, particularly anogenital warts and male HPV-related cancers, according to an English mathematical model.
Mark Jit, PhD, a professor of vaccine epidemiology at the London School of Hygiene & Tropical Medicine, and his colleagues considered in which settings HPV vaccination delivery would have the greatest effect size; the patterns of sexual behavior in MSM leading to the transmission of HPV 6, 11, 16, and 18; and the costs and quality adjusted life year (QALY) implications of disease outcomes (Clin Infect Dis. 2016 Dec 23. doi: 10.1093/cid/ciw845).
Clinic attendance rates were based on genitourinary clinic returns in England recorded in public health surveillance data recorded between 2009 and 2012, stratified according to diagnosed HIV-positive status. Also modeled were the effects of vaccination in MSM between the ages of 16 and 40 years, according to groups aged 16-25 years, 16-30 years, 16-35 years, and 16-40 years. Models for ages on either side of 16 or 40 years were not considered because of confidentiality constraints in the former and limited specialty clinic use in the latter.
Herd protection likely would be notable in the first year, because of the breadth of the age ranges modeled, Dr. Jit and his colleagues determined. Specifically, the models predicted a 35% decline in incidence rates of anogenital warts within 5 years of initiating the vaccine across all MSM men seen in specialty clinics. If only HIV-positive MSM across the age groups were vaccinated, the models predicted a 5-year decline of 15%.
Declines predicted in HPV-related cancers would happen more slowly, because progression from infection to malignancies tends to occur over years. For example, there would be a 55% reduction over 100 years for anal cancer if all 16- to 40-year-old MSM attending specialty clinics are offered vaccination. However, the reduction rate would drop to 40% in that same time period if only HIV-positive men across the age groups were vaccinated.
Using a cost-effectiveness threshold of 20,000 British pounds (about $24,500) per QALY, with no more than a 10% probability that the incremental cost-effectiveness ratio would exceed 30,000 pounds per QALY ($36,710), Dr. Jit and his colleagues determined that using a quadrivalent HPV vaccination in MSM between ages 16 and 40 years in the specialty clinic setting would be cost effective if delivery cost an average of 63 pounds ($77) per dose.
By offering vaccination only to HIV-positive MSM between 16 and 40 years, even if the quadrivalent vaccine were to cost as much as 96.50 pounds ($118), it would still be cost effective. A nonavalent vaccine at the same price would also be cost effective, because nearly all HPV-related cancers are linked to HPV 16 and 18. However, a bivalent vaccine was not shown by the models to be cost effective in such a limited program.
The investigators theorized that HIV infection is associated with the rate of HPV-related disease progression. To simplify computation, however, their models only considered the overall cost effectiveness of offering HPV vaccination to either HIV-positive MSM or to MSM regardless of current HIV status. That could mean “the cost effectiveness of MSM vaccination may be even better than reported,” the researchers noted.
This study was funded primarily by the National Institute for Health Research and Public Health England in the United Kingdom. The authors had no relevant disclosures.
Key clinical point:
Major finding: Substantial declines in HPV-related events in MSM were projected within 5 years of vaccination between ages 16 and 40 years in this cohort.
Data source: Mathematical modeling of HPV 6, 11, 16, and 18 sexual transmission in the MSM population of England.
Disclosures: This study was funded primarily by the National Institute for Health Research and Public Health England in the United Kingdom. The authors had no relevant disclosures.