User login
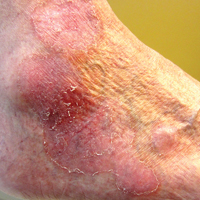
Recalcitrant Solitary Erythematous Scaly Patch on the Foot
The Diagnosis: Pagetoid Reticulosis
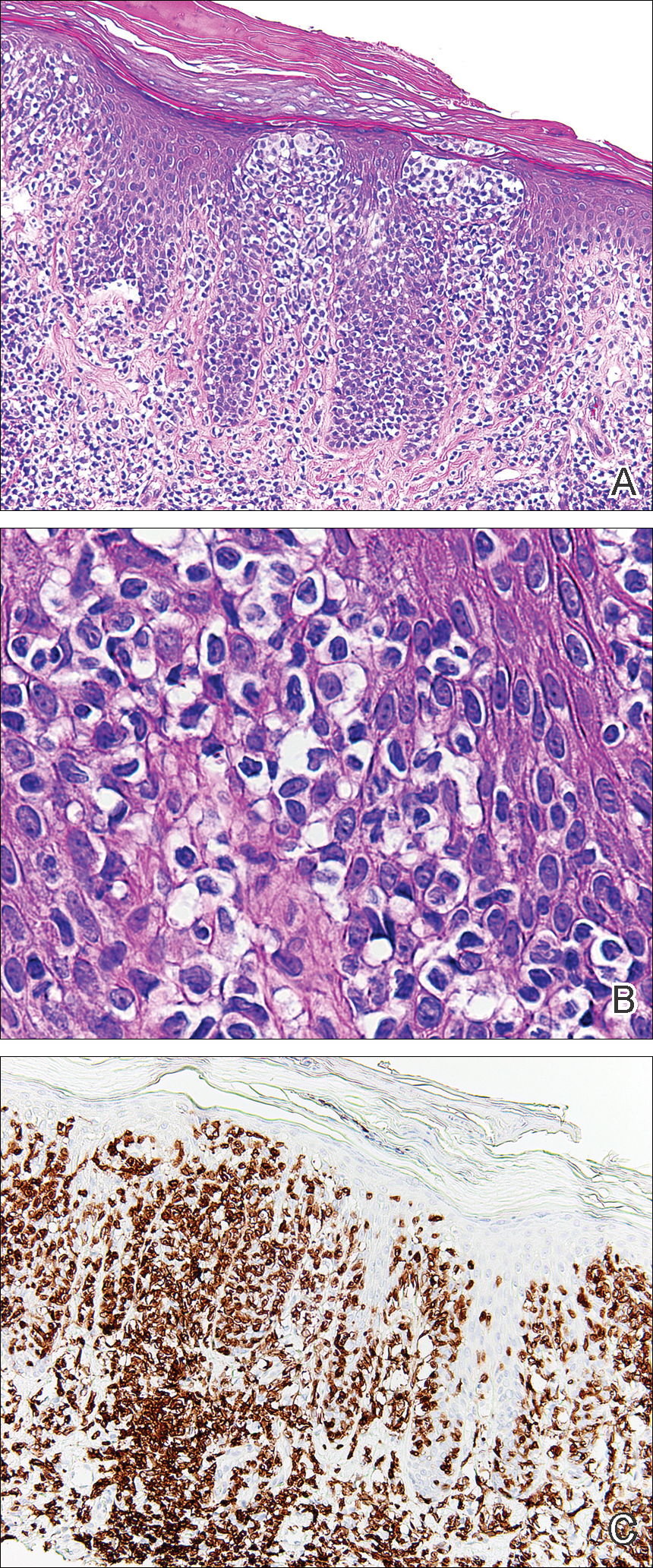
Histopathologic examination demonstrated a dense infiltrate and psoriasiform pattern epidermal hyperplasia (Figure, A). There was conspicuous epidermotropism of moderately enlarged, hyperchromatic lymphocytes. Intraepidermal lymphocytes were slightly larger, darker, and more convoluted than those in the subjacent dermis (Figure, B). These cells exhibited CD3+ T-cell differentiation with an abnormal CD4-CD7-CD8- phenotype (Figure, C). The histopathologic finding of atypical epidermotropic T-cell infiltrate was compatible with a rare variant of mycosis fungoides known as pagetoid reticulosis (PR). After discussing the diagnosis and treatment options, the patient elected to begin with a conservative approach to therapy. We prescribed fluocinonide ointment 0.05% twice daily under occlusion. At 1 month follow-up, the patient experienced marked improvement of the erythema and scaling of the lesion.
Pagetoid reticulosis is a primary cutaneous T-cell lymphoma that has been categorized as an indolent localized variant of mycosis fungoides. This rare skin disorder was originally described by Woringer and Kolopp in 19391 and was further renamed in 1973 by Braun-Falco et al.2 At that time the term pagetoid reticulosis was introduced due to similarities in histopathologic findings seen in Paget disease of the nipple. Two variants of the disease have been described since then: the localized type and the disseminated type. The localized type, also known as Woringer-Kolopp disease (WKD), typically presents as a persistent, sharply localized, scaly patch that slowly expands over several years. The lesion is classically located on the extensor surface of the hand or foot and often is asymptomatic. Due to the benign presentation, WKD can easily be confused with much more common diseases, such as psoriasis or fungal infections, resulting in a substantial delay in the diagnosis. The patient will often report a medical history notable for frequent office visits and numerous failed therapies. Even though it is exceedingly uncommon, these findings should prompt the practitioner to add WKD to their differential. The disseminated type of PR (also known as Ketron-Goodman disease) is characterized by diffuse cutaneous involvement, carries a much more progressive course, and often leads to a poor outcome.3 The histopathologic features of WKD and Ketron-Goodman disease are identical, and the 2 types are distinguished on clinical grounds alone.
Histopathologic features of PR are unique and often distinct in comparison to mycosis fungoides. Pagetoid reticulosis often is described as epidermal hyperplasia with parakeratosis, prominent acanthosis, and excessive epidermotropism of atypical lymphocytes scattered throughout the epidermis.3 The distinct pattern of epidermotropism seen in PR is the characteristic finding. Review of immunocytochemistry from reported cases has shown that CD marker expression of neoplastic T cells in PR can be variable in nature.4 Although it is known that immunophenotyping can be useful in diagnosing and distinguishing PR from other types of primary cutaneous T-cell lymphoma, the clinical significance of the observed phenotypic variation remains a mystery. As of now, it appears to be prognostically irrelevant.5
There are numerous therapeutic options available for PR. Depending on the size and extent of the disease, surgical excision and radiotherapy may be an option and are the most effective.6 For patients who are not good candidates or opt out of these options, there are various pharmacotherapies that also have proven to work. Traditional therapies include topical corticosteroids, corticosteroid injections, and phototherapy. However, more recent trials with retinoids, such as alitretinoin or bexarotene, appear to offer a promising therapeutic approach.7
Pagetoid reticulosis is a true malignant lymphoma of T-cell lineage, but it typically carries an excellent prognosis. Rare cases have been reported to progress to disseminated lymphoma.8 Therefore, long-term follow-up for a patient diagnosed with PR is recommended.
- Woringer FR, Kolopp P. Lésion érythémato-squameuse polycyclique de l'avant-bras évoluantdepuis 6 ans chez un garçonnet de 13 ans. Ann Dermatol Venereol. 1939;10:945-948.
- Braun-Falco O, Marghescu S, Wolff HH. Pagetoid reticulosis--Woringer-Kolopp's disease [in German]. Hautarzt. 1973;24:11-21.
- Haghighi B, Smoller BR, Leboit PE, et al. Pagetoid reticulosis (Woringer-Kolopp disease): an immunophenotypic, molecular, and clinicopathologic study. Mod Pathol. 2000;13:502-510.
- Willemze R, Jaffe ES, Burg G, et al. WHO-EORTC classification for cutaneous lymphomas. Blood. 2005;105:3768-3785.
- Mourtzinos N, Puri PK, Wang G, et al. CD4/CD8 double negative pagetoid reticulosis: a case report and literature review. J Cutan Pathol. 2010;37:491-496.
- Lee J, Viakhireva N, Cesca C, et al. Clinicopathologic features and treatment outcomes in Woringer-Kolopp disease. J Am Acad Dermatol. 2008;59:706-712.
- Schmitz L, Bierhoff E, Dirschka T. Alitretinoin: an effective treatment option for pagetoid reticulosis. J Dtsch Dermatol Ges. 2013;11:1194-1195.
- Ioannides G, Engel MF, Rywlin AM. Woringer-Kolopp disease (pagetoid reticulosis). Am J Dermatopathol. 1983;5:153-158.
The Diagnosis: Pagetoid Reticulosis
Histopathologic examination demonstrated a dense infiltrate and psoriasiform pattern epidermal hyperplasia (Figure, A). There was conspicuous epidermotropism of moderately enlarged, hyperchromatic lymphocytes. Intraepidermal lymphocytes were slightly larger, darker, and more convoluted than those in the subjacent dermis (Figure, B). These cells exhibited CD3+ T-cell differentiation with an abnormal CD4-CD7-CD8- phenotype (Figure, C). The histopathologic finding of atypical epidermotropic T-cell infiltrate was compatible with a rare variant of mycosis fungoides known as pagetoid reticulosis (PR). After discussing the diagnosis and treatment options, the patient elected to begin with a conservative approach to therapy. We prescribed fluocinonide ointment 0.05% twice daily under occlusion. At 1 month follow-up, the patient experienced marked improvement of the erythema and scaling of the lesion.

Pagetoid reticulosis is a primary cutaneous T-cell lymphoma that has been categorized as an indolent localized variant of mycosis fungoides. This rare skin disorder was originally described by Woringer and Kolopp in 19391 and was further renamed in 1973 by Braun-Falco et al.2 At that time the term pagetoid reticulosis was introduced due to similarities in histopathologic findings seen in Paget disease of the nipple. Two variants of the disease have been described since then: the localized type and the disseminated type. The localized type, also known as Woringer-Kolopp disease (WKD), typically presents as a persistent, sharply localized, scaly patch that slowly expands over several years. The lesion is classically located on the extensor surface of the hand or foot and often is asymptomatic. Due to the benign presentation, WKD can easily be confused with much more common diseases, such as psoriasis or fungal infections, resulting in a substantial delay in the diagnosis. The patient will often report a medical history notable for frequent office visits and numerous failed therapies. Even though it is exceedingly uncommon, these findings should prompt the practitioner to add WKD to their differential. The disseminated type of PR (also known as Ketron-Goodman disease) is characterized by diffuse cutaneous involvement, carries a much more progressive course, and often leads to a poor outcome.3 The histopathologic features of WKD and Ketron-Goodman disease are identical, and the 2 types are distinguished on clinical grounds alone.
Histopathologic features of PR are unique and often distinct in comparison to mycosis fungoides. Pagetoid reticulosis often is described as epidermal hyperplasia with parakeratosis, prominent acanthosis, and excessive epidermotropism of atypical lymphocytes scattered throughout the epidermis.3 The distinct pattern of epidermotropism seen in PR is the characteristic finding. Review of immunocytochemistry from reported cases has shown that CD marker expression of neoplastic T cells in PR can be variable in nature.4 Although it is known that immunophenotyping can be useful in diagnosing and distinguishing PR from other types of primary cutaneous T-cell lymphoma, the clinical significance of the observed phenotypic variation remains a mystery. As of now, it appears to be prognostically irrelevant.5
There are numerous therapeutic options available for PR. Depending on the size and extent of the disease, surgical excision and radiotherapy may be an option and are the most effective.6 For patients who are not good candidates or opt out of these options, there are various pharmacotherapies that also have proven to work. Traditional therapies include topical corticosteroids, corticosteroid injections, and phototherapy. However, more recent trials with retinoids, such as alitretinoin or bexarotene, appear to offer a promising therapeutic approach.7
Pagetoid reticulosis is a true malignant lymphoma of T-cell lineage, but it typically carries an excellent prognosis. Rare cases have been reported to progress to disseminated lymphoma.8 Therefore, long-term follow-up for a patient diagnosed with PR is recommended.
The Diagnosis: Pagetoid Reticulosis
Histopathologic examination demonstrated a dense infiltrate and psoriasiform pattern epidermal hyperplasia (Figure, A). There was conspicuous epidermotropism of moderately enlarged, hyperchromatic lymphocytes. Intraepidermal lymphocytes were slightly larger, darker, and more convoluted than those in the subjacent dermis (Figure, B). These cells exhibited CD3+ T-cell differentiation with an abnormal CD4-CD7-CD8- phenotype (Figure, C). The histopathologic finding of atypical epidermotropic T-cell infiltrate was compatible with a rare variant of mycosis fungoides known as pagetoid reticulosis (PR). After discussing the diagnosis and treatment options, the patient elected to begin with a conservative approach to therapy. We prescribed fluocinonide ointment 0.05% twice daily under occlusion. At 1 month follow-up, the patient experienced marked improvement of the erythema and scaling of the lesion.

Pagetoid reticulosis is a primary cutaneous T-cell lymphoma that has been categorized as an indolent localized variant of mycosis fungoides. This rare skin disorder was originally described by Woringer and Kolopp in 19391 and was further renamed in 1973 by Braun-Falco et al.2 At that time the term pagetoid reticulosis was introduced due to similarities in histopathologic findings seen in Paget disease of the nipple. Two variants of the disease have been described since then: the localized type and the disseminated type. The localized type, also known as Woringer-Kolopp disease (WKD), typically presents as a persistent, sharply localized, scaly patch that slowly expands over several years. The lesion is classically located on the extensor surface of the hand or foot and often is asymptomatic. Due to the benign presentation, WKD can easily be confused with much more common diseases, such as psoriasis or fungal infections, resulting in a substantial delay in the diagnosis. The patient will often report a medical history notable for frequent office visits and numerous failed therapies. Even though it is exceedingly uncommon, these findings should prompt the practitioner to add WKD to their differential. The disseminated type of PR (also known as Ketron-Goodman disease) is characterized by diffuse cutaneous involvement, carries a much more progressive course, and often leads to a poor outcome.3 The histopathologic features of WKD and Ketron-Goodman disease are identical, and the 2 types are distinguished on clinical grounds alone.
Histopathologic features of PR are unique and often distinct in comparison to mycosis fungoides. Pagetoid reticulosis often is described as epidermal hyperplasia with parakeratosis, prominent acanthosis, and excessive epidermotropism of atypical lymphocytes scattered throughout the epidermis.3 The distinct pattern of epidermotropism seen in PR is the characteristic finding. Review of immunocytochemistry from reported cases has shown that CD marker expression of neoplastic T cells in PR can be variable in nature.4 Although it is known that immunophenotyping can be useful in diagnosing and distinguishing PR from other types of primary cutaneous T-cell lymphoma, the clinical significance of the observed phenotypic variation remains a mystery. As of now, it appears to be prognostically irrelevant.5
There are numerous therapeutic options available for PR. Depending on the size and extent of the disease, surgical excision and radiotherapy may be an option and are the most effective.6 For patients who are not good candidates or opt out of these options, there are various pharmacotherapies that also have proven to work. Traditional therapies include topical corticosteroids, corticosteroid injections, and phototherapy. However, more recent trials with retinoids, such as alitretinoin or bexarotene, appear to offer a promising therapeutic approach.7
Pagetoid reticulosis is a true malignant lymphoma of T-cell lineage, but it typically carries an excellent prognosis. Rare cases have been reported to progress to disseminated lymphoma.8 Therefore, long-term follow-up for a patient diagnosed with PR is recommended.
- Woringer FR, Kolopp P. Lésion érythémato-squameuse polycyclique de l'avant-bras évoluantdepuis 6 ans chez un garçonnet de 13 ans. Ann Dermatol Venereol. 1939;10:945-948.
- Braun-Falco O, Marghescu S, Wolff HH. Pagetoid reticulosis--Woringer-Kolopp's disease [in German]. Hautarzt. 1973;24:11-21.
- Haghighi B, Smoller BR, Leboit PE, et al. Pagetoid reticulosis (Woringer-Kolopp disease): an immunophenotypic, molecular, and clinicopathologic study. Mod Pathol. 2000;13:502-510.
- Willemze R, Jaffe ES, Burg G, et al. WHO-EORTC classification for cutaneous lymphomas. Blood. 2005;105:3768-3785.
- Mourtzinos N, Puri PK, Wang G, et al. CD4/CD8 double negative pagetoid reticulosis: a case report and literature review. J Cutan Pathol. 2010;37:491-496.
- Lee J, Viakhireva N, Cesca C, et al. Clinicopathologic features and treatment outcomes in Woringer-Kolopp disease. J Am Acad Dermatol. 2008;59:706-712.
- Schmitz L, Bierhoff E, Dirschka T. Alitretinoin: an effective treatment option for pagetoid reticulosis. J Dtsch Dermatol Ges. 2013;11:1194-1195.
- Ioannides G, Engel MF, Rywlin AM. Woringer-Kolopp disease (pagetoid reticulosis). Am J Dermatopathol. 1983;5:153-158.
- Woringer FR, Kolopp P. Lésion érythémato-squameuse polycyclique de l'avant-bras évoluantdepuis 6 ans chez un garçonnet de 13 ans. Ann Dermatol Venereol. 1939;10:945-948.
- Braun-Falco O, Marghescu S, Wolff HH. Pagetoid reticulosis--Woringer-Kolopp's disease [in German]. Hautarzt. 1973;24:11-21.
- Haghighi B, Smoller BR, Leboit PE, et al. Pagetoid reticulosis (Woringer-Kolopp disease): an immunophenotypic, molecular, and clinicopathologic study. Mod Pathol. 2000;13:502-510.
- Willemze R, Jaffe ES, Burg G, et al. WHO-EORTC classification for cutaneous lymphomas. Blood. 2005;105:3768-3785.
- Mourtzinos N, Puri PK, Wang G, et al. CD4/CD8 double negative pagetoid reticulosis: a case report and literature review. J Cutan Pathol. 2010;37:491-496.
- Lee J, Viakhireva N, Cesca C, et al. Clinicopathologic features and treatment outcomes in Woringer-Kolopp disease. J Am Acad Dermatol. 2008;59:706-712.
- Schmitz L, Bierhoff E, Dirschka T. Alitretinoin: an effective treatment option for pagetoid reticulosis. J Dtsch Dermatol Ges. 2013;11:1194-1195.
- Ioannides G, Engel MF, Rywlin AM. Woringer-Kolopp disease (pagetoid reticulosis). Am J Dermatopathol. 1983;5:153-158.
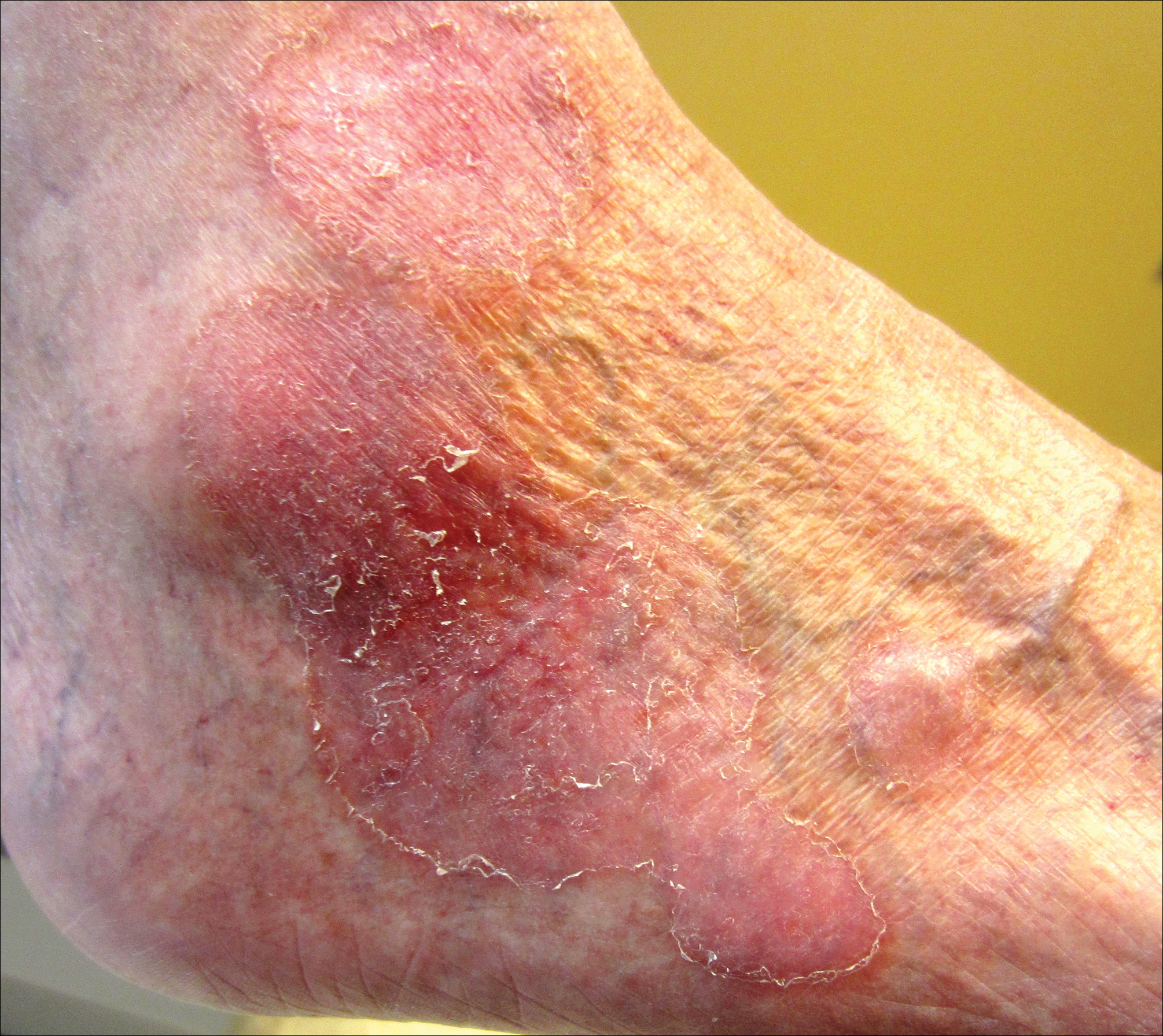
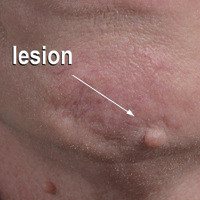
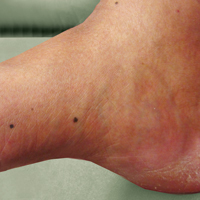
An 80-year-old man with a history of malignant melanoma and squamous cell carcinoma presented to the dermatology clinic with a chronic rash of 20 years' duration on the right ankle that extended to the instep of the right foot. His medical history was notable for hypertension and hyperlipidemia. Family history was unremarkable. The patient described the rash as red and scaly but denied associated pain or pruritus. Over the last 2 to 3 years he had tried treating the affected area with petroleum jelly, topical and oral antifungals, and mild topical steroids with minimal improvement. Complete review of systems was performed and was negative other than some mild constipation. Physical examination revealed an erythematous scaly patch on the dorsal aspect of the right ankle. Potassium hydroxide preparation and fungal culture swab yielded negative results, and a shave biopsy was performed.
Skin Cancer Mortality in Patients With Skin of Color
Skin cancers in patients with skin of color are less prevalent but have a higher morbidity and mortality compared to white patients. Challenges to early detection, including clinical differences in presentation, low public awareness, lower index of suspicion among health care providers, and access to specialty care, likely contribute to observed differences in prognosis between skin of color and white populations.
Skin cancer is the most common malignancy in the United States, accounting for approximately 40% of all neoplasms in white patients but only 1% to 4% in Asian American and black patients.1,2 Largely due to the photoprotective effects of increased constitutive epidermal melanin, melanoma is approximately 10 to 20 times less frequent in black patients and 3 to 7 times less common in Hispanics than age-matched whites.1 Nonmelanoma skin cancers including squamous cell carcinoma (SCC) and basal cell carcinoma also are less prevalent in darker skin types.3,4
In the United States, Hispanic, American Indian
Similar to melanoma, the mortality from SCC is disproportionately increased in skin of color populations, ranging from 18% to 29% in black patients.3,10,11 There is a paucity of population-based studies in the United States looking at mortality rates of nonmelanoma skin cancers and their trends over time, but a 1993 study suggests that mortality rates are declining less consistently in black patients than white patients.11
Factors that may contribute to higher mortality rates in patients with skin of color include a greater propensity for inherently aggressive skin cancers (eg, higher risk of SCC) and delays in diagnosis (eg, late-stage diagnosis of melanoma).1,4 For melanoma, increased mortality has been attributed to a predominance of acral lentiginous melanomas, which are more frequently diagnosed at more advanced stages than other melanoma subtypes.6,12,13 Black patients, Hispanics, Asians, and Pacific Islanders are all more likely to present with thicker tumors and metastases on initial presentation than their white counterparts (P<.001).2,8,9,12-14 The higher risk of death from SCC results from the predominance of lesions on non–sun-exposed areas, particularly the legs and anogenital areas, and within sites of chronic scarring or inflammation.4 Unlike sun-induced SCC, the most commonly observed type of SCC in lighter skin types, SCCs that develop in association with chronic inflammatory or ulcerative processes are aggressive and invasive, and they metastasize to distant sites in 20% to 40% of cases (versus 1%–4% in sun-induced SCC).1,3,4 For all skin cancers, poor access to medical care, patients’ unawareness of their skin cancer risk, lack of adequate skin examinations, and prevalence of lesions on uncommon sites that may be inconspicuous or overlooked have all been suggested to delay diagnosis.1,15,16 Given that more advanced disease is associated with worse outcomes, the implications of this delay are enormous and remain a cause for concern.
The alarming skin cancer mortality rates in patients with skin of color are a call to action for the medical community. The consistent use of full-body skin examinations including close inspection of mucosal, acral, and genital areas for all patients independent of skin type and racial/ethnic background is paramount. Advancing skin cancer education in skin of color populations, such as through distribution of patient-directed educational materials produced by organizations such as the American Academy of Dermatology, Skin Cancer Foundation, and Skin of Color Society, is an important step toward increased public awareness.16 Use of social and traditional media outlets as well as community-directed health outreach campaigns also are important strategies to change the common misconception that darker-skinned individuals do not get skin cancer. We hope that with a multipronged approach, disparities in skin cancer mortality will steadily be eliminated.
- Gloster HM Jr, Neal K. Skin cancer in skin of color. J Am Acad Dermatol. 2006;55:741-760; quiz 761-764.
- Cormier JN, Xing Y, Ding M, et al. Ethnic differences among patients with cutaneous melanoma. Arch Intern Med. 2006;166:1907-1914.
- Mora RG, Perniciaro C. Cancer of the skin in blacks: I. a review of 163 black patients with cutaneous squamous cell carcinoma. J Am Acad Dermatol. 1981;5:535-543.
- Halder RM, Bridgeman-Shah S. Skin cancer in African Americans. Cancer. 1995;75:667-673.
- Howlader N, Noone AM, Krapcho M, et al. SEER Cancer Statistics Review, 1975-2013. Bethesda, MD: National Cancer Institute; April 2016. http://seer.cancer.gov/csr/1975_2013/. Updated September 12, 2016. Accessed April 7, 2017.
- Bellows CF, Belafsky P, Fortgang IS, et al. Melanoma in African-Americans: trends in biological behavior and clinical characteristics over two decades. J Surg Oncol. 2001;78:10-16.
- Chen L, Jin S. Trends in mortality rates of cutaneous melanoma in East Asian populations. Peer J. 2014;4:e2809.
- Cress RD, Holly EA. Incidence of cutaneous melanoma among non-Hispanic whites, Hispanics, Asians, and blacks: an analysis of California Cancer Registry data. Cancer Causes Control. 1997;8:246-252.
- Johnson DS, Yamane S, Morita S, et al. Malignant melanoma in non-Caucasians: experience from Hawaii. Surg Clin N Am. 2003;83:275-282.
- Fleming ID, Barnawell JR, Burlison PE, et al. Skin cancer in black patients. Cancer. 1975;35:600-605.
- Weinstock MA. Nonmelanoma skin cancer mortality in the United States, 1969 through 1988. Arch Dermatol. 1993;129:1286-1290.
- Byrd KM, Wilson DC, Hoyler SS. Advanced presentation of melanoma in African Americans. J Am Acad Dermatol. 2004;50:142-143.
- Hu S, Parmet Y, Allen G, et al. Disparity in melanoma: a trend analysis of melanoma incidence and stage at diagnosis among whites, Hispanics, and blacks in Florida. Arch Dermatol. 2009;145:1369-1374.
- Black WC, Goldhahn RT, Wiggins C. Melanoma within a southwestern Hispanic population. Arch Dermatol. 1987;123:1331-1334.
- Harvey VM, Oldfield CW, Chen JT, et al. Melanoma disparities among US Hispanics: use of the social ecological model to contextualize reasons for inequitable outcomes and frame a research agenda [published online August 29, 2016]. J Skin Cancer. 2016;2016:4635740.
- Robinson JK, Joshi KM, Ortiz S, et al. Melanoma knowledge, perception, and awareness in ethnic minorities in Chicago: recommendations regarding education. Psychooncology. 2011;20:313-320.
Skin cancers in patients with skin of color are less prevalent but have a higher morbidity and mortality compared to white patients. Challenges to early detection, including clinical differences in presentation, low public awareness, lower index of suspicion among health care providers, and access to specialty care, likely contribute to observed differences in prognosis between skin of color and white populations.
Skin cancer is the most common malignancy in the United States, accounting for approximately 40% of all neoplasms in white patients but only 1% to 4% in Asian American and black patients.1,2 Largely due to the photoprotective effects of increased constitutive epidermal melanin, melanoma is approximately 10 to 20 times less frequent in black patients and 3 to 7 times less common in Hispanics than age-matched whites.1 Nonmelanoma skin cancers including squamous cell carcinoma (SCC) and basal cell carcinoma also are less prevalent in darker skin types.3,4
In the United States, Hispanic, American Indian
Similar to melanoma, the mortality from SCC is disproportionately increased in skin of color populations, ranging from 18% to 29% in black patients.3,10,11 There is a paucity of population-based studies in the United States looking at mortality rates of nonmelanoma skin cancers and their trends over time, but a 1993 study suggests that mortality rates are declining less consistently in black patients than white patients.11
Factors that may contribute to higher mortality rates in patients with skin of color include a greater propensity for inherently aggressive skin cancers (eg, higher risk of SCC) and delays in diagnosis (eg, late-stage diagnosis of melanoma).1,4 For melanoma, increased mortality has been attributed to a predominance of acral lentiginous melanomas, which are more frequently diagnosed at more advanced stages than other melanoma subtypes.6,12,13 Black patients, Hispanics, Asians, and Pacific Islanders are all more likely to present with thicker tumors and metastases on initial presentation than their white counterparts (P<.001).2,8,9,12-14 The higher risk of death from SCC results from the predominance of lesions on non–sun-exposed areas, particularly the legs and anogenital areas, and within sites of chronic scarring or inflammation.4 Unlike sun-induced SCC, the most commonly observed type of SCC in lighter skin types, SCCs that develop in association with chronic inflammatory or ulcerative processes are aggressive and invasive, and they metastasize to distant sites in 20% to 40% of cases (versus 1%–4% in sun-induced SCC).1,3,4 For all skin cancers, poor access to medical care, patients’ unawareness of their skin cancer risk, lack of adequate skin examinations, and prevalence of lesions on uncommon sites that may be inconspicuous or overlooked have all been suggested to delay diagnosis.1,15,16 Given that more advanced disease is associated with worse outcomes, the implications of this delay are enormous and remain a cause for concern.
The alarming skin cancer mortality rates in patients with skin of color are a call to action for the medical community. The consistent use of full-body skin examinations including close inspection of mucosal, acral, and genital areas for all patients independent of skin type and racial/ethnic background is paramount. Advancing skin cancer education in skin of color populations, such as through distribution of patient-directed educational materials produced by organizations such as the American Academy of Dermatology, Skin Cancer Foundation, and Skin of Color Society, is an important step toward increased public awareness.16 Use of social and traditional media outlets as well as community-directed health outreach campaigns also are important strategies to change the common misconception that darker-skinned individuals do not get skin cancer. We hope that with a multipronged approach, disparities in skin cancer mortality will steadily be eliminated.
Skin cancers in patients with skin of color are less prevalent but have a higher morbidity and mortality compared to white patients. Challenges to early detection, including clinical differences in presentation, low public awareness, lower index of suspicion among health care providers, and access to specialty care, likely contribute to observed differences in prognosis between skin of color and white populations.
Skin cancer is the most common malignancy in the United States, accounting for approximately 40% of all neoplasms in white patients but only 1% to 4% in Asian American and black patients.1,2 Largely due to the photoprotective effects of increased constitutive epidermal melanin, melanoma is approximately 10 to 20 times less frequent in black patients and 3 to 7 times less common in Hispanics than age-matched whites.1 Nonmelanoma skin cancers including squamous cell carcinoma (SCC) and basal cell carcinoma also are less prevalent in darker skin types.3,4
In the United States, Hispanic, American Indian
Similar to melanoma, the mortality from SCC is disproportionately increased in skin of color populations, ranging from 18% to 29% in black patients.3,10,11 There is a paucity of population-based studies in the United States looking at mortality rates of nonmelanoma skin cancers and their trends over time, but a 1993 study suggests that mortality rates are declining less consistently in black patients than white patients.11
Factors that may contribute to higher mortality rates in patients with skin of color include a greater propensity for inherently aggressive skin cancers (eg, higher risk of SCC) and delays in diagnosis (eg, late-stage diagnosis of melanoma).1,4 For melanoma, increased mortality has been attributed to a predominance of acral lentiginous melanomas, which are more frequently diagnosed at more advanced stages than other melanoma subtypes.6,12,13 Black patients, Hispanics, Asians, and Pacific Islanders are all more likely to present with thicker tumors and metastases on initial presentation than their white counterparts (P<.001).2,8,9,12-14 The higher risk of death from SCC results from the predominance of lesions on non–sun-exposed areas, particularly the legs and anogenital areas, and within sites of chronic scarring or inflammation.4 Unlike sun-induced SCC, the most commonly observed type of SCC in lighter skin types, SCCs that develop in association with chronic inflammatory or ulcerative processes are aggressive and invasive, and they metastasize to distant sites in 20% to 40% of cases (versus 1%–4% in sun-induced SCC).1,3,4 For all skin cancers, poor access to medical care, patients’ unawareness of their skin cancer risk, lack of adequate skin examinations, and prevalence of lesions on uncommon sites that may be inconspicuous or overlooked have all been suggested to delay diagnosis.1,15,16 Given that more advanced disease is associated with worse outcomes, the implications of this delay are enormous and remain a cause for concern.
The alarming skin cancer mortality rates in patients with skin of color are a call to action for the medical community. The consistent use of full-body skin examinations including close inspection of mucosal, acral, and genital areas for all patients independent of skin type and racial/ethnic background is paramount. Advancing skin cancer education in skin of color populations, such as through distribution of patient-directed educational materials produced by organizations such as the American Academy of Dermatology, Skin Cancer Foundation, and Skin of Color Society, is an important step toward increased public awareness.16 Use of social and traditional media outlets as well as community-directed health outreach campaigns also are important strategies to change the common misconception that darker-skinned individuals do not get skin cancer. We hope that with a multipronged approach, disparities in skin cancer mortality will steadily be eliminated.
- Gloster HM Jr, Neal K. Skin cancer in skin of color. J Am Acad Dermatol. 2006;55:741-760; quiz 761-764.
- Cormier JN, Xing Y, Ding M, et al. Ethnic differences among patients with cutaneous melanoma. Arch Intern Med. 2006;166:1907-1914.
- Mora RG, Perniciaro C. Cancer of the skin in blacks: I. a review of 163 black patients with cutaneous squamous cell carcinoma. J Am Acad Dermatol. 1981;5:535-543.
- Halder RM, Bridgeman-Shah S. Skin cancer in African Americans. Cancer. 1995;75:667-673.
- Howlader N, Noone AM, Krapcho M, et al. SEER Cancer Statistics Review, 1975-2013. Bethesda, MD: National Cancer Institute; April 2016. http://seer.cancer.gov/csr/1975_2013/. Updated September 12, 2016. Accessed April 7, 2017.
- Bellows CF, Belafsky P, Fortgang IS, et al. Melanoma in African-Americans: trends in biological behavior and clinical characteristics over two decades. J Surg Oncol. 2001;78:10-16.
- Chen L, Jin S. Trends in mortality rates of cutaneous melanoma in East Asian populations. Peer J. 2014;4:e2809.
- Cress RD, Holly EA. Incidence of cutaneous melanoma among non-Hispanic whites, Hispanics, Asians, and blacks: an analysis of California Cancer Registry data. Cancer Causes Control. 1997;8:246-252.
- Johnson DS, Yamane S, Morita S, et al. Malignant melanoma in non-Caucasians: experience from Hawaii. Surg Clin N Am. 2003;83:275-282.
- Fleming ID, Barnawell JR, Burlison PE, et al. Skin cancer in black patients. Cancer. 1975;35:600-605.
- Weinstock MA. Nonmelanoma skin cancer mortality in the United States, 1969 through 1988. Arch Dermatol. 1993;129:1286-1290.
- Byrd KM, Wilson DC, Hoyler SS. Advanced presentation of melanoma in African Americans. J Am Acad Dermatol. 2004;50:142-143.
- Hu S, Parmet Y, Allen G, et al. Disparity in melanoma: a trend analysis of melanoma incidence and stage at diagnosis among whites, Hispanics, and blacks in Florida. Arch Dermatol. 2009;145:1369-1374.
- Black WC, Goldhahn RT, Wiggins C. Melanoma within a southwestern Hispanic population. Arch Dermatol. 1987;123:1331-1334.
- Harvey VM, Oldfield CW, Chen JT, et al. Melanoma disparities among US Hispanics: use of the social ecological model to contextualize reasons for inequitable outcomes and frame a research agenda [published online August 29, 2016]. J Skin Cancer. 2016;2016:4635740.
- Robinson JK, Joshi KM, Ortiz S, et al. Melanoma knowledge, perception, and awareness in ethnic minorities in Chicago: recommendations regarding education. Psychooncology. 2011;20:313-320.
- Gloster HM Jr, Neal K. Skin cancer in skin of color. J Am Acad Dermatol. 2006;55:741-760; quiz 761-764.
- Cormier JN, Xing Y, Ding M, et al. Ethnic differences among patients with cutaneous melanoma. Arch Intern Med. 2006;166:1907-1914.
- Mora RG, Perniciaro C. Cancer of the skin in blacks: I. a review of 163 black patients with cutaneous squamous cell carcinoma. J Am Acad Dermatol. 1981;5:535-543.
- Halder RM, Bridgeman-Shah S. Skin cancer in African Americans. Cancer. 1995;75:667-673.
- Howlader N, Noone AM, Krapcho M, et al. SEER Cancer Statistics Review, 1975-2013. Bethesda, MD: National Cancer Institute; April 2016. http://seer.cancer.gov/csr/1975_2013/. Updated September 12, 2016. Accessed April 7, 2017.
- Bellows CF, Belafsky P, Fortgang IS, et al. Melanoma in African-Americans: trends in biological behavior and clinical characteristics over two decades. J Surg Oncol. 2001;78:10-16.
- Chen L, Jin S. Trends in mortality rates of cutaneous melanoma in East Asian populations. Peer J. 2014;4:e2809.
- Cress RD, Holly EA. Incidence of cutaneous melanoma among non-Hispanic whites, Hispanics, Asians, and blacks: an analysis of California Cancer Registry data. Cancer Causes Control. 1997;8:246-252.
- Johnson DS, Yamane S, Morita S, et al. Malignant melanoma in non-Caucasians: experience from Hawaii. Surg Clin N Am. 2003;83:275-282.
- Fleming ID, Barnawell JR, Burlison PE, et al. Skin cancer in black patients. Cancer. 1975;35:600-605.
- Weinstock MA. Nonmelanoma skin cancer mortality in the United States, 1969 through 1988. Arch Dermatol. 1993;129:1286-1290.
- Byrd KM, Wilson DC, Hoyler SS. Advanced presentation of melanoma in African Americans. J Am Acad Dermatol. 2004;50:142-143.
- Hu S, Parmet Y, Allen G, et al. Disparity in melanoma: a trend analysis of melanoma incidence and stage at diagnosis among whites, Hispanics, and blacks in Florida. Arch Dermatol. 2009;145:1369-1374.
- Black WC, Goldhahn RT, Wiggins C. Melanoma within a southwestern Hispanic population. Arch Dermatol. 1987;123:1331-1334.
- Harvey VM, Oldfield CW, Chen JT, et al. Melanoma disparities among US Hispanics: use of the social ecological model to contextualize reasons for inequitable outcomes and frame a research agenda [published online August 29, 2016]. J Skin Cancer. 2016;2016:4635740.
- Robinson JK, Joshi KM, Ortiz S, et al. Melanoma knowledge, perception, and awareness in ethnic minorities in Chicago: recommendations regarding education. Psychooncology. 2011;20:313-320.
Approach to Management of Giant Basal Cell Carcinomas
Nonmelanoma skin cancer is the most common malignancy in the United States, with basal cell carcinoma (BCC) being the major histological subtype and accounting for approximately 80% of all skin cancers.1-3 The age-adjusted incidence of BCC in the United States between 2004 and 2006 was estimated at 1019 cases per 100,000 in women and 1488 cases per 100,000 in men, and an estimated 2.8 million new cases are diagnosed in the United States each year.3,4 Rates have been shown to increase with advancing age and are higher in males than females at all ages.3 Exposure to solar UVB radiation generally is considered to be the greatest risk factor for development of BCC.3,5,6 Severe or frequent sunburn and recreational exposure to sun in childhood (from birth to 19 years of age), particularly in individuals who tend to burn rather than tan, have been shown to substantially increase the risk for developing BCC as an adult.7 Additional risk factors include light skin color, red or blonde hair color, presence of a large number of moles on the extremities, and a family history of melanoma or painful/blistering sunburn reactions.3,7 Exposure to certain toxins, immunosuppression, and several genetic cancer syndromes also have been linked to BCC.5
Eighty percent of BCC cases involve the head and neck, with the trunk, arms, and legs being the next most common sites.5 Basal cell carcinoma can be classified by histologic subtype including nodular, superficial, nodulocystic, morpheic, metatypical, pigmented, and ulcerative, as well as other rarer forms.8 Elder9 recommended that it may be most clinically practical to divide BCC into subtypes that are known to have low (eg, nodular, nodulocystic) or relatively high risk for local recurrence (eg, infiltrating, morpheic, and metatypical).9,10 The most common histologic subtype is nodular BCC, with an incidence of 40% to 60%, which typically presents as a red to white pearly nodule or papule with a rolled border; overlying telangiectasia; and occasionally crusting, ulceration, or a cyst.5,11,12
Basal cell carcinoma generally is a slow-growing and highly curable form of skin cancer.5,13,14 Compared to either squamous cell carcinoma or melanoma, BCC is generally easier to treat and carries a more favorable prognosis with a lower incidence of recurrence and metastasis.15 Malignancy in BCC is due to local growth and destruction of the primary tumor rather than metastasis, which is quite rare (estimated to occur in 0.0028% to 0.55% of cases) but carries a poor prognosis.5,11,16 Basal cell carcinoma grows continuously along the path of least resistance, showing an affinity for the dermis, fascial planes, nerve sheaths, blood vessels, and lymphatic vessels. It is through these pathways that certain locally aggressive tumors can achieve great depths and distant spread. Tumors also are known to spread along embryonic fascial planes, which allows cells to extend in a direction perpendicular to the skin surface and achieve greater depths.13 Metastasis has been found to occur more frequently in white men, arising from large tumors larger than 7.5 cm on the head and neck with spread to local lymph nodes. The median survival rate in this group, even in patients receiving adjuvant chemotherapy or radiation, is 10 months but is lower in patients with larger tumors and those who neglect to seek medical care.16 Although mortality is low, its high and increasing prevalence makes BCC an important and costly health problem in the United States.2,17
Case Report
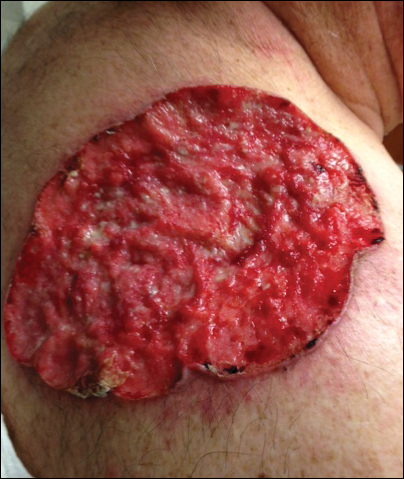
A 60-year-old white man with a history of diabetes mellitus presented to the dermatology clinic with concerns about a nonhealing sore on the right upper back that had been present for more than 10 years and had gradually increased in size. The patient reported he did not have health insurance and thus did not seek medical care. Despite the size and location of the lesion, he was able to maintain an active lifestyle and worked as a janitor without difficulty until shortly before presentation when the lesion began to ooze and bleed, requiring him to change the dressing multiple times each day. The patient had no systemic symptoms and described himself as an otherwise healthy man.
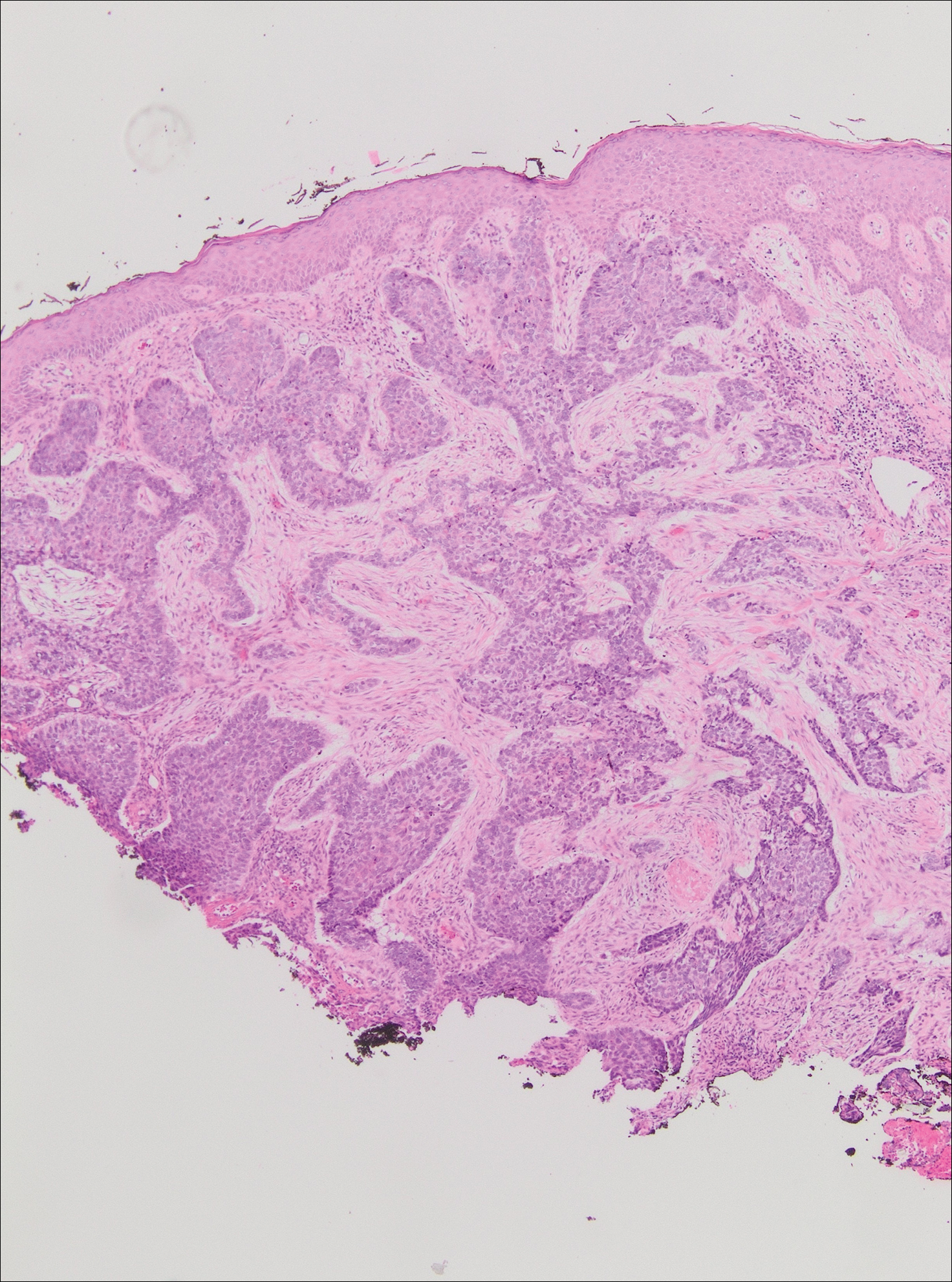
On evaluation, the patient was noted to have a 20×15-cm ulcerated tumor on the right side of the upper back and shoulder with no satellite lesions (Figure 1). There were no palpable lymph nodes or satellite lesions and the rest of the physical examination was unremarkable. An 8-mm shave biopsy was collected on the day of presentation and sent for pathology to evaluate for suspected malignancy. On histology, BCC was present with islands of tumor cells extending from the epidermis into the dermis (Figure 2). These nests of cells displayed classic peripheral palisading of hyperchromatic, ovoid-shaped, basaloid nuclei at the periphery. Clefting around islands of tumor cells in the dermis also was apparent. Several foci suggested squamous differentiation, but the bulk of the lesion suggested a conventional nodular BCC.
The patient was referred to a surgical oncologist who recommended a wide surgical excision (SE) and delayed split-thickness skin graft (STSG) due to the size and location of the lesion. Eighteen days after receiving the diagnosis of BCC, the patient was taken to the operating room and underwent wide en bloc resection of the soft tissue tumor. Upon lifting the specimen off the underlying muscles, it was found to be penetrating into portions of the trapezius, deltoid, paraspinal, supraspinalis, and infraspinatus muscles. As such, the ulcerated tumor was removed as well as portions of the underlying musculature measuring 21×18 cm. The wound was left open until final pathology on margin clearance was available. It was covered with a wound vac to encourage granulation in anticipation of a planned delayed STSG. There were no complications, and the patient returned to the recovery unit in good condition where the dressing was replaced with a large wound vac system.
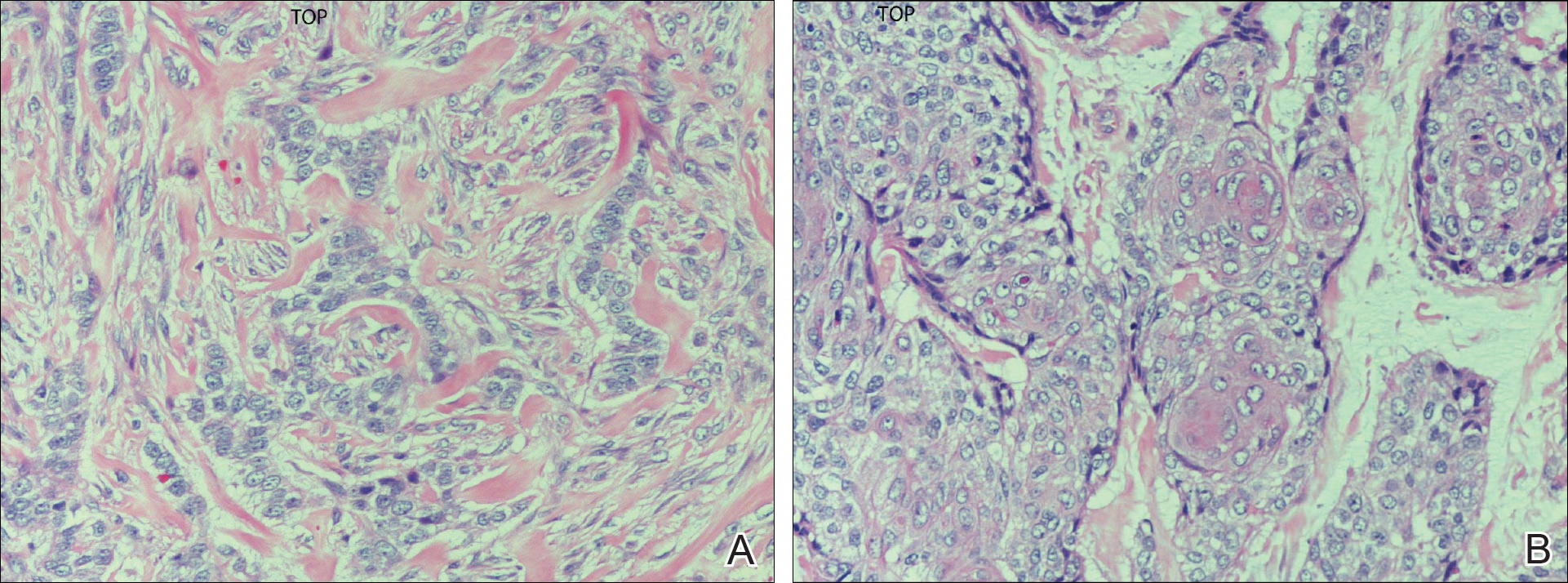
Final histologic examination showed negative deep and peripheral margins. More extensive examination of histology of the excised tumor was found to have characteristics consistent with metatypical and morpheic-type BCC. In addition to islands of tumor cells noted in the dermis on original biopsy, this sample also revealed basaloid cells arranged in thin elongated trabeculae invading deeper into the reticular dermis without peripheral palisading, suggestive of the morpheic variant (Figure 3A).8,9,10 Other areas were found to have focal squamous differentiation with keratin pearls and intercellular bridges (Figure 3B). These findings support the diagnosis of a completely excised BCC of the metatypical (referred to by some authorities as basosquamous)8,9 type.
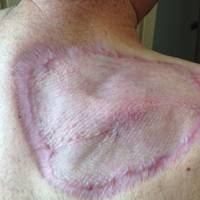
The patient was seen for postoperative evaluations at 2 and 3 weeks. Each time granulation was noted to be proceeding well without signs of infection, and the wound vac was left in place. One month after the initial SE, the patient returned for the planned STSG. The skin graft was harvested from the right lateral thigh and was meshed and transferred to the recipient site on the right upper back, sewn circumferentially to the wound edges. Occlusive petrolatum gauze was placed over the graft followed by the wound vac for coverage until the graft matured.
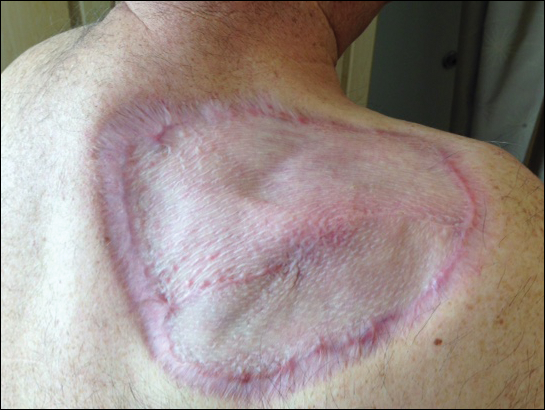
The patient returned for follow-up approximately 7 months after his initial visit to the clinic. He reported feeling well, and his only concern was mild soreness of the scapular muscles while playing golf. The site of tumor excision showed 100% take of the STSG with no nodules in or around the site to suggest recurrence (Figure 4). The patient denied experiencing any constitutional symptoms and had no palpable lymph nodes or physical examination findings suggestive of metastatic disease or new tumor development at other sites. At 36 months after his initial clinic visit, he remained free of recurrence.
Comment
Typical BCC lesions are indolent and small, occurring primarily on the head and neck.5,11,12,17 We report the case of a locally advanced, extremely large and penetrating lesion located on the trunk. This relatively unique case provides for an interesting comparison between available treatments for BCC as well as several of the generally accepted principles of management previously described in the literature.
Treatment Considerations
The approach to management of BCC considers factors related to the tumor and those related to the patient and practitioner. Telfer et al6 recommended that tumors be categorized as relatively low or high risk based on prognostic factors including size, site, histologic subtype and growth pattern; definition of margins; and presence or absence of prior treatment. Characteristics of high-risk tumors include size greater than 2.5 to 3 cm in diameter; location on the midface, nose, or ears; aggressive histologic subtype including morpheic, infiltrating, and metatypical; deep extension; perineural invasion; neglected or long-standing lesions; incomplete SE or Mohs micrographic surgery (MMS); and recurrence of tumor after prior treatment.13,14,18 Although rare, tumors of the metatypical subtype are particularly important to identify, as they are known to be more aggressive and prone to spread than other forms of BCC.19,20 The clinical appearance of metatypical BCCs often is identical to lower-risk subtypes, reinforcing the importance of careful histologic examination of an adequately deep biopsy, given that metatypical features often are present only in the deep tissue planes.19
The practitioner also must consider patient-related factors such as age, general health, immunocompromised states, coexisting medical conditions, and current medications. The skills, experience, and recommendations of the physician also are expected to influence treatment selection.6,21
Surgical Versus Nonsurgical Treatment Approaches
Treatment of large, locally advanced, primary BCCs can be divided into surgical and nonsurgical approaches.5,6 Surgical approaches include MMS and SE. Mohs micrographic surgery, electrodesiccation and curettage, and cryosurgery may achieve high cure rates in lesions that are low risk but generally are not recommended for use with recurrent or high-risk large and aggressive tumors.5,6 Nonsurgical approaches include radiotherapy; chemotherapy; and vismodegib, an oral inhibitor of the hedgehog pathway involved in the development of many BCCs.5,6,22 Topical photodynamic therapy with 5-aminolevulinic acid, topical imiquimod (immune-response modulator) and 5-fluorouracil, and intralesional interferon are other nonsurgical options that are primarily effective for small superficial BCCs. These modalities are not indicated for high-risk tumors.5,6,23
For small tumors, MMS is regarded by most practitioners as the gold standard due to the high cure rate and cosmetic results it provides.5,6,18,24 This procedure allows for precise mapping of tumor location on frozen sections and, unlike surgical excision, examination of close to 100% of the deep and peripheral margins.18 Excision and evaluation of thin horizontal sections for tumor extension also allows for a greater degree of tissue conservation than other modalities.6,25 Mohs micrographic surgery is particularly useful for tumors of the midface, aggressive histologic subtype (eg, morpheic, infiltrating, basosquamous, micronodular), deep invasion, and perineural spread.6,8,18,25 In a large review of 3 studies including a total of 7670 patients with primary BCC treated by MMS, Rowe et al26 reported a 5-year recurrence rate of 1.0%, which was 8.7 times less than the weighted average of all non-MMS modalities. Similarly, in a large prospective review by Leibovitch et al,18 the 5-year recurrence rate of BCC treated with MMS was 1.4% in primary cases and 4.0% in previously recurrent cases.18 They reported that the main predictors of recurrence included longer tumor duration, more levels of excision required to obtain clear margins, notable subclinical extension, and prior recurrence. Interestingly, tumor and postexcision defect size did not predict recurrence.18 Margin-controlled excision with MMS was associated with higher success rates than modalities based on clinical margins without histologic control (eg, surgical excision, electrocautery, curettage) and potentially incomplete excision.12,18
Although MMS has been demonstrated to have a high success rate, it has relative disadvantages. Tumors that are multicentric or have indistinct borders are more difficult to treat with MMS, and cure rates with MMS have been shown to decrease with increasing tumor diameter.13,25 For example, reported cure rates are greater than 99% for MMS in BCCs less than 2 cm in diameter compared to 98.6% for those between 2 and 3 cm, and only 90.5% for those greater than 3 cm.27 Mohs micrographic surgery requires a highly trained surgeon and can be extremely time consuming and labor intensive, particularly with large and locally aggressive tumors.6,25 Tumors that involve fat and cartilage require modifications to standardized processing techniques, and deep wounds involving muscle and bone create technical challenges in maintaining orientation.25 In the past, MMS was more expensive than other treatment modalities; however, cost analyses have demonstrated a near-equal cost of MMS compared to surgical excision with permanent section control and lower cost as compared to radiation therapy for selected cases.28
Surgical excision also is considered a highly effective treatment of primary BCC and is the most commonly used treatment modality for BCC.5,18,29 In this procedure, the peripheral and deep margins of excised tissue can be examined by a pathologist.6 Telfer et al6 recommended SE as the preferable treatment of choice for both large and small tumors in low-risk sites (ie, those that do not include the face) with nodular histology, tumors with morpheic histology in low-risk sites, and small (<2 cm) superficial tumors in high-risk sites. It is recommended that the size of surgical margins correlate with the likelihood of the presence of subclinical tumor extensions. Larger and morpheic-type BCCs require wider margins to achieve complete excision. In these cases, a 3-mm margin yields only a 66% cure rate, while 5-mm margins yield an 82% cure rate and 13- to 15-mm margins yield cure rates higher than 95%.6,29,30 In a series examining recurrence rates of primary BCC, Rowe et al26 reviewed 10 studies (2606 patients treated by SE) and calculated a 5-year recurrence rate of 10.1%. Silverman et al31 reviewed 5-year recurrence rates in 588 cases of BCC treated with SE. They concluded that BCC on the neck, trunk, arms, and legs of any size may be effectively treated with this modality, with 1 case of recurrence among 187 cases (0.5% recurrence rate). Multivariate analysis identified 2 independent risk factors for recurrence: anatomic site (head) and patient sex (male). Analysis of BCCs on the head distinct from other body sites demonstrated a moderately significant trend (P=.196) of increasing diameter with increasing recurrence rates. Age at treatment, duration of lesion, and length of treatment were not significantly associated with an increased risk of recurrence.31 Similarly, a review of 1417 cases of BCC by Dubin and Kopf21 demonstrated an increased risk with tumors located on the head and larger lesions.
RELATED ARTICLE: Basal Cell Carcinoma: Analysis of Factors Associated With Incomplete Excision
Radiotherapy (RT) is a commonly employed nonsurgical approach to management. Its use has been declining in recent years due to relative disadvantages and side effects. Similar to MMS, it can be extremely effective for carefully selected patients.11,31 Radiotherapy is most effective for use with aggressive, rapidly growing BCC subtypes that are more sensitive to radiation, as replicating cells undergo mitotic death when radiation is applied.15 Radiotherapy is considered a viable option for patients who are not candidates for surgery, tumors in locations difficult to access for SE, and for rare unresectable tumors as a primary therapy.5,11 In a randomized comparison between RT and SE approaches to the treatment of primary BCCs on the face, RT was found to be inferior to SE both in efficacy (4-year recurrence rate, 7.5% vs 0.7%) and cosmesis (rate of good results, 69% vs 87%).32
The major disadvantages of RT as compared to other treatment modalities such as MMS or SE are the lack of control at margins and compromised inferior cosmetic outcomes. Hair loss, hyperpigmentation or hypopigmentation, telangiectasia, keloids, cutaneous necrosis, and RT-induced dermatitis have been reported as side effects of RT.6,11,32-34 Other disadvantages of RT include the inconvenience of multiple visits to the hospital for treatment, and high cost as compared to other modalities such as MMS.35 Finally, use of RT even for relatively benign disease has been linked to an increased risk for both squamous cell carcinoma, BCC, and sarcomas.15,36
Vismodegib is an oral drug approved by the US Food and Drug Administration in 2012 for the treatment of locally advanced BCC. It is a first-in-class small-molecule systemic inhibitor of the intracellular hedgehog signaling pathway, which has been implicated in the growth and development of several types of cancer, including BCC.36-38 Most patients with BCC carry loss-of-function mutations that affect PTCH1 and result in unregulated reactivation of the hedgehog pathway and uncontrolled cell growth.38-40 Vismodegib is a small molecule that selectively deactivates the hedgehog pathway. It currently is indicated for the treatment of metastatic BCC or patients with locally advanced BCCs who are not candidates for SE or RT.38-41 An open-label nonrandomized phase 2 study by Sekulic et al42 evaluated the effectiveness of vismodegib for treatment of metastatic or inoperable BCCs. In 33 patients with metastatic BCCs, the response rate was 30% (10/33) with a 9.5-month median progression-free survival. All responses were partial, with 73% (24/33) showing tumor shrinkage. In 63 patients with locally advanced BCCs, the response rate was 43% (27/63). Most patients demonstrated visible reductions in tumor size and improvement in appearance, but 13 patients (21%) in this group were noted to have a complete response (ie, absence of residual BCC on biopsy). Both cohorts had a median response time of 7.6 months.42
Conclusion
Our patient presented with an extremely large and ulcerating lesion on the upper back that met the criteria for classification as a high-risk tumor. In light of the tumor location and size as well as the involvement of deep tissues and muscles, we elected to pursue SE for management. This modality proved to be extremely effective, and the patient continues to be free of residual or recurrent BCC more than 36 months after surgery. Two large systematic reviews lend support to this management approach and report excellent outcomes. In a review article by Rubin et al,5 SE was shown to provide cure rates greater than 99% for BCC lesions of any size on the neck, trunk, and extremities. Moreover, Thissen et al43 performed a systematic meta-analysis of 18 studies reporting recurrence rates of primary BCC after treatment with various modalities and concluded that when surgery is not contraindicated, SE is the treatment of choice for nodular and superficial BCC. Both groups agree in their recommendations that MMS should be used for BCCs in cosmetically compromised zones (eg, midface), sites where tissue sparing is essential, aggressive growth patterns (eg, perineural invasion, morpheaform histology), and when high risk of recurrence is unacceptable.5,43 In contrast, MMS is not recommended for tumors of large diameter or with indistinct borders due to decreased cure rates.13,25,27 Vismodegib is an interesting new option in development for management of metastatic and aggressive nonresectable BCCs. It was not an option in our patient. Although consideration for use of vismodegib as a neoadjuvant treatment to shrink the tumor prior to surgery is reasonable, the decision to proceed directly with SE proved to be the superior option for our patient.
- Basal and squamous cell skin cancers. American Cancer Society website. www.cancer.org/acs/groups/cid/documents/webcontent/003139-pdf.pdf. Updated April 14, 2016. Accessed April 26, 2016.
- Rogers HW, Weinstock MA, Harris AR, et al. Incidence estimate of nonmelanoma skin cancer in the United States, 2006. Arch Dermatol. 2010;146:283-287.
- Wu S, Han J, Li W, et al. Basal cell carcinoma incidence and associated risk factors in US women and men. Am J Epidemiol. 2013;178:890-897.
- Skin cancer facts & statistics. Skin Cancer Foundation website. www.skincancer.org/skin-cancer-information/skin-cancer-facts. Updated March 18, 2016. Accessed April 26, 2016.
- Rubin AI, Chen EH, Ratner D. Basal cell carcinoma. N Engl J Med. 2005;353:2262-2269.
- Telfer NR, Colver GB, Bowers PW. Guidelines for the management of basal cell carcinoma. British Association of Dermatologists. Br J Dermatol. 1999;141:415-423.
- Gallagher RP, Hill GB, Bajdik CD, et al. Sunlight exposure, pigmentary factors, and risk of nonmelanocytic skin cancer: I. basal cell carcinoma. Arch Dermatol. 1995;131:157-163.
- McKee PH, Calonje J, Lazar A, et al, eds. Pathology of the Skin with Clinical Correlations. 4th ed. Vol 2. Philadelphia, PA: Elsevier Mosby; 2011.
- Elder DE. Basal cell carcinoma. In: Elder DE, Elenitsas R, Johnson Jr BL, et al, eds. Lever’s Histopathology of the Skin. 10th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2009:826-832.
- Bastiaens MT, Hoefnagel JJ, Buijn JA, et al. Differences in age, site distribution, and sex between superficial basal cell carcinomas indicate different types of tumors. J Invest Dermatol. 1998;110:880-884.
- Kuijpers DI, Thissen MM, Neumann MA. Basal cell carcinoma: treatment options and prognosis, a scientific approach to a common malignancy. Am J Clin Dermatol. 2002;3:247-259.
- Leibovitch I, Huilgol SC, Selva D, et al. Basal cell carcinoma treated with Mohs surgery in Australia I: experience over 10 years. J Am Acad Dermatol. 2005;53:445-451.
- Walling H, Fosko S, Geraminejad P, et al. Aggressive basal cell carcinoma: presentation, pathogenesis, and management. Cancer Metastasis Rev. 2004;23:389-402.
- Veness M, Richards S. Role of modern radiotherapy in treating skin cancer. Australas J Dermatol. 2003;44:159-168.
- Wysong A, Aasi SZ, Tang JY. Update on metastatic basal cell carcinoma: a summary of published cases from 1981 through 2011. JAMA Dermatol. 2013;149:615-616.
- Bolognia J, Jorizzo J, Rapini R, eds. Dermatology. Vol 2. Philadelphia, PA: Mosby; 2003.
- Swanson NA. Mohs surgery: technique, indications, applications, and the future. Arch Dermatol. 1983;119:761-773.
- Leibovitch I, Huilgol SC, Selva D, et al. Basal cell carcinoma treated with Mohs surgery in Australia II: outcome at 5-year follow-up. J Am Acad Dermatol. 2005;53:452-457.
- De Stefano A, Dispenza F, Petrucci AG, et al. Features of biopsy in diagnosis of metatypical basal cell carcinoma (basosquamous carcinoma) of head and neck. Otolaryngol Pol. 2012;66:419-423.
- Tarallo M, Cigna E, Frati R, et al. Metatypical basal cell carcinoma: a clinical review. J Exp Clin Cancer Res. 2008;27:65.
- Dubin N, Kopf AW. Multivariate risk score for recurrence of cutaneous basal cell carcinomas. Arch Dermatol. 1983;119:373-377.
- Rodriguez DA. Basal cell carcinoma: a primer on diagnosis and treatment. Practical Dermatology. 2014;11:36-38.
- Kirby JS, Miller CJ. Intralesional chemotherapy for nonmelanoma skin cancer: a practical review. J Am Acad Dermatol. 2010;63:689-702.
- Rowe DE. Comparison of treatment modalities for basal cell carcinoma. Clin Dermatol. 1995;13:617-620.
- Shriner DL, McCoy DK, Goldberg DJ, et al. Mohs micrographic surgery. J Am Acad Dermatol. 1998;39:79-97.
- Rowe DE, Carroll RJ, Day CL Jr. Mohs surgery is the treatment of choice for recurrent (previously treated) basal cell carcinoma. J Dermatol Surg Oncol. 1989;15:424-431.
- Mohs FE. Chemosurgery: Microscopically Controlled Surgery for Skin Cancer. Springfield, IL: Charles C. Thomas; 1978.
- Cook J, Zitelli JA. Mohs micrographic surgery: a cost analysis. J Am Acad Dermatol. 1996;39(5 pt 1):698-703.
- Breuninger H, Dietz K. Prediction of subclinical tumor infiltration in basal cell carcinoma. J Dermatol Surg Oncol. 1991;17:574-578.
- Wolf DJ, Zitelli JA. Surgical margins for basal cell carcinoma. Arch Dermatol. 1987;123:340-344.
- Silverman MK, Kopf AW, Bart RS, et al. Recurrence rates of treated basal cell carcinomas, part 3: surgical excision. J Dermatol Surg Oncol. 1992;18:471-476.
- Avril MF, Auperin A, Margulis A, et al. Basal cell carcinoma of the face: surgery or radiotherapy? results of a randomized study. Br J Cancer. 1997;76:100-106.
- Caccialanza M, Piccinno R, Beretta M, et al. Results and side effects of dermatologic radiotherapy: a retrospective study of irradiated cutaneous epithelial neoplasms. J Am Acad Dermatol. 1999;41:589-594.
- Silverman MK, Kopf AW, Gladstein AH, et al. Recurrence rates of treated basal cell carcinomas, part 4: x-ray therapy. J Dermatol Surg Oncol. 1992;18:549-554.
- Rowe DE, Carroll RJ, Day CL Jr. Long-term recurrence rates in previously untreated (primary) basal cell carcinoma: implications for patient follow-up. J Dermatol Surg Oncol. 1989;15:315-328.
- Beswick SJ, Garrido MC, Fryer AA, et al. Multiple basal cell carcinomas and malignant melanoma following radiotherapy for ankylosing spondylitis. Clin Exp Dermatol. 2000;25:381-383.
- Motley RJ. The treatment of basal cell carcinoma. J Dermatolog Treat. 1995;6:121-125.
- Dlugosz A, Agrawal S, Kirkpatrick P. Vismodegib. Nat Rev Drug Discov. 2012;11:437-438.
- Fellner C. Vismodegib (Erivedge) for advanced basal cell carcinoma. P T. 2012;37:670-682.
- Harms KL, Dlugosz AA. Harnessing hedgehog for the treatment of basal cell carcinoma. JAMA Dermatol. 2013;149:607-608.
- Rudin CM. Vismodegib. Clin Cancer Res. 2012;18:3218-3222.
- Sekulic A, Migden M, Oro A, et al. Efficacy and safety of vismodegib in advanced basal-cell carcinoma. N Engl J Med. 2012;366:2171-2179.
- Thissen MM, Neumann MA, Schouten LJ. A systematic review of treatment modalities for primary basal cell carcinomas. Arch Dermatol. 1999;135:1177-1183.
Nonmelanoma skin cancer is the most common malignancy in the United States, with basal cell carcinoma (BCC) being the major histological subtype and accounting for approximately 80% of all skin cancers.1-3 The age-adjusted incidence of BCC in the United States between 2004 and 2006 was estimated at 1019 cases per 100,000 in women and 1488 cases per 100,000 in men, and an estimated 2.8 million new cases are diagnosed in the United States each year.3,4 Rates have been shown to increase with advancing age and are higher in males than females at all ages.3 Exposure to solar UVB radiation generally is considered to be the greatest risk factor for development of BCC.3,5,6 Severe or frequent sunburn and recreational exposure to sun in childhood (from birth to 19 years of age), particularly in individuals who tend to burn rather than tan, have been shown to substantially increase the risk for developing BCC as an adult.7 Additional risk factors include light skin color, red or blonde hair color, presence of a large number of moles on the extremities, and a family history of melanoma or painful/blistering sunburn reactions.3,7 Exposure to certain toxins, immunosuppression, and several genetic cancer syndromes also have been linked to BCC.5
Eighty percent of BCC cases involve the head and neck, with the trunk, arms, and legs being the next most common sites.5 Basal cell carcinoma can be classified by histologic subtype including nodular, superficial, nodulocystic, morpheic, metatypical, pigmented, and ulcerative, as well as other rarer forms.8 Elder9 recommended that it may be most clinically practical to divide BCC into subtypes that are known to have low (eg, nodular, nodulocystic) or relatively high risk for local recurrence (eg, infiltrating, morpheic, and metatypical).9,10 The most common histologic subtype is nodular BCC, with an incidence of 40% to 60%, which typically presents as a red to white pearly nodule or papule with a rolled border; overlying telangiectasia; and occasionally crusting, ulceration, or a cyst.5,11,12
Basal cell carcinoma generally is a slow-growing and highly curable form of skin cancer.5,13,14 Compared to either squamous cell carcinoma or melanoma, BCC is generally easier to treat and carries a more favorable prognosis with a lower incidence of recurrence and metastasis.15 Malignancy in BCC is due to local growth and destruction of the primary tumor rather than metastasis, which is quite rare (estimated to occur in 0.0028% to 0.55% of cases) but carries a poor prognosis.5,11,16 Basal cell carcinoma grows continuously along the path of least resistance, showing an affinity for the dermis, fascial planes, nerve sheaths, blood vessels, and lymphatic vessels. It is through these pathways that certain locally aggressive tumors can achieve great depths and distant spread. Tumors also are known to spread along embryonic fascial planes, which allows cells to extend in a direction perpendicular to the skin surface and achieve greater depths.13 Metastasis has been found to occur more frequently in white men, arising from large tumors larger than 7.5 cm on the head and neck with spread to local lymph nodes. The median survival rate in this group, even in patients receiving adjuvant chemotherapy or radiation, is 10 months but is lower in patients with larger tumors and those who neglect to seek medical care.16 Although mortality is low, its high and increasing prevalence makes BCC an important and costly health problem in the United States.2,17
Case Report
A 60-year-old white man with a history of diabetes mellitus presented to the dermatology clinic with concerns about a nonhealing sore on the right upper back that had been present for more than 10 years and had gradually increased in size. The patient reported he did not have health insurance and thus did not seek medical care. Despite the size and location of the lesion, he was able to maintain an active lifestyle and worked as a janitor without difficulty until shortly before presentation when the lesion began to ooze and bleed, requiring him to change the dressing multiple times each day. The patient had no systemic symptoms and described himself as an otherwise healthy man.
On evaluation, the patient was noted to have a 20×15-cm ulcerated tumor on the right side of the upper back and shoulder with no satellite lesions (Figure 1). There were no palpable lymph nodes or satellite lesions and the rest of the physical examination was unremarkable. An 8-mm shave biopsy was collected on the day of presentation and sent for pathology to evaluate for suspected malignancy. On histology, BCC was present with islands of tumor cells extending from the epidermis into the dermis (Figure 2). These nests of cells displayed classic peripheral palisading of hyperchromatic, ovoid-shaped, basaloid nuclei at the periphery. Clefting around islands of tumor cells in the dermis also was apparent. Several foci suggested squamous differentiation, but the bulk of the lesion suggested a conventional nodular BCC.


The patient was referred to a surgical oncologist who recommended a wide surgical excision (SE) and delayed split-thickness skin graft (STSG) due to the size and location of the lesion. Eighteen days after receiving the diagnosis of BCC, the patient was taken to the operating room and underwent wide en bloc resection of the soft tissue tumor. Upon lifting the specimen off the underlying muscles, it was found to be penetrating into portions of the trapezius, deltoid, paraspinal, supraspinalis, and infraspinatus muscles. As such, the ulcerated tumor was removed as well as portions of the underlying musculature measuring 21×18 cm. The wound was left open until final pathology on margin clearance was available. It was covered with a wound vac to encourage granulation in anticipation of a planned delayed STSG. There were no complications, and the patient returned to the recovery unit in good condition where the dressing was replaced with a large wound vac system.
Final histologic examination showed negative deep and peripheral margins. More extensive examination of histology of the excised tumor was found to have characteristics consistent with metatypical and morpheic-type BCC. In addition to islands of tumor cells noted in the dermis on original biopsy, this sample also revealed basaloid cells arranged in thin elongated trabeculae invading deeper into the reticular dermis without peripheral palisading, suggestive of the morpheic variant (Figure 3A).8,9,10 Other areas were found to have focal squamous differentiation with keratin pearls and intercellular bridges (Figure 3B). These findings support the diagnosis of a completely excised BCC of the metatypical (referred to by some authorities as basosquamous)8,9 type.

The patient was seen for postoperative evaluations at 2 and 3 weeks. Each time granulation was noted to be proceeding well without signs of infection, and the wound vac was left in place. One month after the initial SE, the patient returned for the planned STSG. The skin graft was harvested from the right lateral thigh and was meshed and transferred to the recipient site on the right upper back, sewn circumferentially to the wound edges. Occlusive petrolatum gauze was placed over the graft followed by the wound vac for coverage until the graft matured.
The patient returned for follow-up approximately 7 months after his initial visit to the clinic. He reported feeling well, and his only concern was mild soreness of the scapular muscles while playing golf. The site of tumor excision showed 100% take of the STSG with no nodules in or around the site to suggest recurrence (Figure 4). The patient denied experiencing any constitutional symptoms and had no palpable lymph nodes or physical examination findings suggestive of metastatic disease or new tumor development at other sites. At 36 months after his initial clinic visit, he remained free of recurrence.

Comment
Typical BCC lesions are indolent and small, occurring primarily on the head and neck.5,11,12,17 We report the case of a locally advanced, extremely large and penetrating lesion located on the trunk. This relatively unique case provides for an interesting comparison between available treatments for BCC as well as several of the generally accepted principles of management previously described in the literature.
Treatment Considerations
The approach to management of BCC considers factors related to the tumor and those related to the patient and practitioner. Telfer et al6 recommended that tumors be categorized as relatively low or high risk based on prognostic factors including size, site, histologic subtype and growth pattern; definition of margins; and presence or absence of prior treatment. Characteristics of high-risk tumors include size greater than 2.5 to 3 cm in diameter; location on the midface, nose, or ears; aggressive histologic subtype including morpheic, infiltrating, and metatypical; deep extension; perineural invasion; neglected or long-standing lesions; incomplete SE or Mohs micrographic surgery (MMS); and recurrence of tumor after prior treatment.13,14,18 Although rare, tumors of the metatypical subtype are particularly important to identify, as they are known to be more aggressive and prone to spread than other forms of BCC.19,20 The clinical appearance of metatypical BCCs often is identical to lower-risk subtypes, reinforcing the importance of careful histologic examination of an adequately deep biopsy, given that metatypical features often are present only in the deep tissue planes.19
The practitioner also must consider patient-related factors such as age, general health, immunocompromised states, coexisting medical conditions, and current medications. The skills, experience, and recommendations of the physician also are expected to influence treatment selection.6,21
Surgical Versus Nonsurgical Treatment Approaches
Treatment of large, locally advanced, primary BCCs can be divided into surgical and nonsurgical approaches.5,6 Surgical approaches include MMS and SE. Mohs micrographic surgery, electrodesiccation and curettage, and cryosurgery may achieve high cure rates in lesions that are low risk but generally are not recommended for use with recurrent or high-risk large and aggressive tumors.5,6 Nonsurgical approaches include radiotherapy; chemotherapy; and vismodegib, an oral inhibitor of the hedgehog pathway involved in the development of many BCCs.5,6,22 Topical photodynamic therapy with 5-aminolevulinic acid, topical imiquimod (immune-response modulator) and 5-fluorouracil, and intralesional interferon are other nonsurgical options that are primarily effective for small superficial BCCs. These modalities are not indicated for high-risk tumors.5,6,23
For small tumors, MMS is regarded by most practitioners as the gold standard due to the high cure rate and cosmetic results it provides.5,6,18,24 This procedure allows for precise mapping of tumor location on frozen sections and, unlike surgical excision, examination of close to 100% of the deep and peripheral margins.18 Excision and evaluation of thin horizontal sections for tumor extension also allows for a greater degree of tissue conservation than other modalities.6,25 Mohs micrographic surgery is particularly useful for tumors of the midface, aggressive histologic subtype (eg, morpheic, infiltrating, basosquamous, micronodular), deep invasion, and perineural spread.6,8,18,25 In a large review of 3 studies including a total of 7670 patients with primary BCC treated by MMS, Rowe et al26 reported a 5-year recurrence rate of 1.0%, which was 8.7 times less than the weighted average of all non-MMS modalities. Similarly, in a large prospective review by Leibovitch et al,18 the 5-year recurrence rate of BCC treated with MMS was 1.4% in primary cases and 4.0% in previously recurrent cases.18 They reported that the main predictors of recurrence included longer tumor duration, more levels of excision required to obtain clear margins, notable subclinical extension, and prior recurrence. Interestingly, tumor and postexcision defect size did not predict recurrence.18 Margin-controlled excision with MMS was associated with higher success rates than modalities based on clinical margins without histologic control (eg, surgical excision, electrocautery, curettage) and potentially incomplete excision.12,18
Although MMS has been demonstrated to have a high success rate, it has relative disadvantages. Tumors that are multicentric or have indistinct borders are more difficult to treat with MMS, and cure rates with MMS have been shown to decrease with increasing tumor diameter.13,25 For example, reported cure rates are greater than 99% for MMS in BCCs less than 2 cm in diameter compared to 98.6% for those between 2 and 3 cm, and only 90.5% for those greater than 3 cm.27 Mohs micrographic surgery requires a highly trained surgeon and can be extremely time consuming and labor intensive, particularly with large and locally aggressive tumors.6,25 Tumors that involve fat and cartilage require modifications to standardized processing techniques, and deep wounds involving muscle and bone create technical challenges in maintaining orientation.25 In the past, MMS was more expensive than other treatment modalities; however, cost analyses have demonstrated a near-equal cost of MMS compared to surgical excision with permanent section control and lower cost as compared to radiation therapy for selected cases.28
Surgical excision also is considered a highly effective treatment of primary BCC and is the most commonly used treatment modality for BCC.5,18,29 In this procedure, the peripheral and deep margins of excised tissue can be examined by a pathologist.6 Telfer et al6 recommended SE as the preferable treatment of choice for both large and small tumors in low-risk sites (ie, those that do not include the face) with nodular histology, tumors with morpheic histology in low-risk sites, and small (<2 cm) superficial tumors in high-risk sites. It is recommended that the size of surgical margins correlate with the likelihood of the presence of subclinical tumor extensions. Larger and morpheic-type BCCs require wider margins to achieve complete excision. In these cases, a 3-mm margin yields only a 66% cure rate, while 5-mm margins yield an 82% cure rate and 13- to 15-mm margins yield cure rates higher than 95%.6,29,30 In a series examining recurrence rates of primary BCC, Rowe et al26 reviewed 10 studies (2606 patients treated by SE) and calculated a 5-year recurrence rate of 10.1%. Silverman et al31 reviewed 5-year recurrence rates in 588 cases of BCC treated with SE. They concluded that BCC on the neck, trunk, arms, and legs of any size may be effectively treated with this modality, with 1 case of recurrence among 187 cases (0.5% recurrence rate). Multivariate analysis identified 2 independent risk factors for recurrence: anatomic site (head) and patient sex (male). Analysis of BCCs on the head distinct from other body sites demonstrated a moderately significant trend (P=.196) of increasing diameter with increasing recurrence rates. Age at treatment, duration of lesion, and length of treatment were not significantly associated with an increased risk of recurrence.31 Similarly, a review of 1417 cases of BCC by Dubin and Kopf21 demonstrated an increased risk with tumors located on the head and larger lesions.
RELATED ARTICLE: Basal Cell Carcinoma: Analysis of Factors Associated With Incomplete Excision
Radiotherapy (RT) is a commonly employed nonsurgical approach to management. Its use has been declining in recent years due to relative disadvantages and side effects. Similar to MMS, it can be extremely effective for carefully selected patients.11,31 Radiotherapy is most effective for use with aggressive, rapidly growing BCC subtypes that are more sensitive to radiation, as replicating cells undergo mitotic death when radiation is applied.15 Radiotherapy is considered a viable option for patients who are not candidates for surgery, tumors in locations difficult to access for SE, and for rare unresectable tumors as a primary therapy.5,11 In a randomized comparison between RT and SE approaches to the treatment of primary BCCs on the face, RT was found to be inferior to SE both in efficacy (4-year recurrence rate, 7.5% vs 0.7%) and cosmesis (rate of good results, 69% vs 87%).32
The major disadvantages of RT as compared to other treatment modalities such as MMS or SE are the lack of control at margins and compromised inferior cosmetic outcomes. Hair loss, hyperpigmentation or hypopigmentation, telangiectasia, keloids, cutaneous necrosis, and RT-induced dermatitis have been reported as side effects of RT.6,11,32-34 Other disadvantages of RT include the inconvenience of multiple visits to the hospital for treatment, and high cost as compared to other modalities such as MMS.35 Finally, use of RT even for relatively benign disease has been linked to an increased risk for both squamous cell carcinoma, BCC, and sarcomas.15,36
Vismodegib is an oral drug approved by the US Food and Drug Administration in 2012 for the treatment of locally advanced BCC. It is a first-in-class small-molecule systemic inhibitor of the intracellular hedgehog signaling pathway, which has been implicated in the growth and development of several types of cancer, including BCC.36-38 Most patients with BCC carry loss-of-function mutations that affect PTCH1 and result in unregulated reactivation of the hedgehog pathway and uncontrolled cell growth.38-40 Vismodegib is a small molecule that selectively deactivates the hedgehog pathway. It currently is indicated for the treatment of metastatic BCC or patients with locally advanced BCCs who are not candidates for SE or RT.38-41 An open-label nonrandomized phase 2 study by Sekulic et al42 evaluated the effectiveness of vismodegib for treatment of metastatic or inoperable BCCs. In 33 patients with metastatic BCCs, the response rate was 30% (10/33) with a 9.5-month median progression-free survival. All responses were partial, with 73% (24/33) showing tumor shrinkage. In 63 patients with locally advanced BCCs, the response rate was 43% (27/63). Most patients demonstrated visible reductions in tumor size and improvement in appearance, but 13 patients (21%) in this group were noted to have a complete response (ie, absence of residual BCC on biopsy). Both cohorts had a median response time of 7.6 months.42
Conclusion
Our patient presented with an extremely large and ulcerating lesion on the upper back that met the criteria for classification as a high-risk tumor. In light of the tumor location and size as well as the involvement of deep tissues and muscles, we elected to pursue SE for management. This modality proved to be extremely effective, and the patient continues to be free of residual or recurrent BCC more than 36 months after surgery. Two large systematic reviews lend support to this management approach and report excellent outcomes. In a review article by Rubin et al,5 SE was shown to provide cure rates greater than 99% for BCC lesions of any size on the neck, trunk, and extremities. Moreover, Thissen et al43 performed a systematic meta-analysis of 18 studies reporting recurrence rates of primary BCC after treatment with various modalities and concluded that when surgery is not contraindicated, SE is the treatment of choice for nodular and superficial BCC. Both groups agree in their recommendations that MMS should be used for BCCs in cosmetically compromised zones (eg, midface), sites where tissue sparing is essential, aggressive growth patterns (eg, perineural invasion, morpheaform histology), and when high risk of recurrence is unacceptable.5,43 In contrast, MMS is not recommended for tumors of large diameter or with indistinct borders due to decreased cure rates.13,25,27 Vismodegib is an interesting new option in development for management of metastatic and aggressive nonresectable BCCs. It was not an option in our patient. Although consideration for use of vismodegib as a neoadjuvant treatment to shrink the tumor prior to surgery is reasonable, the decision to proceed directly with SE proved to be the superior option for our patient.
Nonmelanoma skin cancer is the most common malignancy in the United States, with basal cell carcinoma (BCC) being the major histological subtype and accounting for approximately 80% of all skin cancers.1-3 The age-adjusted incidence of BCC in the United States between 2004 and 2006 was estimated at 1019 cases per 100,000 in women and 1488 cases per 100,000 in men, and an estimated 2.8 million new cases are diagnosed in the United States each year.3,4 Rates have been shown to increase with advancing age and are higher in males than females at all ages.3 Exposure to solar UVB radiation generally is considered to be the greatest risk factor for development of BCC.3,5,6 Severe or frequent sunburn and recreational exposure to sun in childhood (from birth to 19 years of age), particularly in individuals who tend to burn rather than tan, have been shown to substantially increase the risk for developing BCC as an adult.7 Additional risk factors include light skin color, red or blonde hair color, presence of a large number of moles on the extremities, and a family history of melanoma or painful/blistering sunburn reactions.3,7 Exposure to certain toxins, immunosuppression, and several genetic cancer syndromes also have been linked to BCC.5
Eighty percent of BCC cases involve the head and neck, with the trunk, arms, and legs being the next most common sites.5 Basal cell carcinoma can be classified by histologic subtype including nodular, superficial, nodulocystic, morpheic, metatypical, pigmented, and ulcerative, as well as other rarer forms.8 Elder9 recommended that it may be most clinically practical to divide BCC into subtypes that are known to have low (eg, nodular, nodulocystic) or relatively high risk for local recurrence (eg, infiltrating, morpheic, and metatypical).9,10 The most common histologic subtype is nodular BCC, with an incidence of 40% to 60%, which typically presents as a red to white pearly nodule or papule with a rolled border; overlying telangiectasia; and occasionally crusting, ulceration, or a cyst.5,11,12
Basal cell carcinoma generally is a slow-growing and highly curable form of skin cancer.5,13,14 Compared to either squamous cell carcinoma or melanoma, BCC is generally easier to treat and carries a more favorable prognosis with a lower incidence of recurrence and metastasis.15 Malignancy in BCC is due to local growth and destruction of the primary tumor rather than metastasis, which is quite rare (estimated to occur in 0.0028% to 0.55% of cases) but carries a poor prognosis.5,11,16 Basal cell carcinoma grows continuously along the path of least resistance, showing an affinity for the dermis, fascial planes, nerve sheaths, blood vessels, and lymphatic vessels. It is through these pathways that certain locally aggressive tumors can achieve great depths and distant spread. Tumors also are known to spread along embryonic fascial planes, which allows cells to extend in a direction perpendicular to the skin surface and achieve greater depths.13 Metastasis has been found to occur more frequently in white men, arising from large tumors larger than 7.5 cm on the head and neck with spread to local lymph nodes. The median survival rate in this group, even in patients receiving adjuvant chemotherapy or radiation, is 10 months but is lower in patients with larger tumors and those who neglect to seek medical care.16 Although mortality is low, its high and increasing prevalence makes BCC an important and costly health problem in the United States.2,17
Case Report
A 60-year-old white man with a history of diabetes mellitus presented to the dermatology clinic with concerns about a nonhealing sore on the right upper back that had been present for more than 10 years and had gradually increased in size. The patient reported he did not have health insurance and thus did not seek medical care. Despite the size and location of the lesion, he was able to maintain an active lifestyle and worked as a janitor without difficulty until shortly before presentation when the lesion began to ooze and bleed, requiring him to change the dressing multiple times each day. The patient had no systemic symptoms and described himself as an otherwise healthy man.
On evaluation, the patient was noted to have a 20×15-cm ulcerated tumor on the right side of the upper back and shoulder with no satellite lesions (Figure 1). There were no palpable lymph nodes or satellite lesions and the rest of the physical examination was unremarkable. An 8-mm shave biopsy was collected on the day of presentation and sent for pathology to evaluate for suspected malignancy. On histology, BCC was present with islands of tumor cells extending from the epidermis into the dermis (Figure 2). These nests of cells displayed classic peripheral palisading of hyperchromatic, ovoid-shaped, basaloid nuclei at the periphery. Clefting around islands of tumor cells in the dermis also was apparent. Several foci suggested squamous differentiation, but the bulk of the lesion suggested a conventional nodular BCC.


The patient was referred to a surgical oncologist who recommended a wide surgical excision (SE) and delayed split-thickness skin graft (STSG) due to the size and location of the lesion. Eighteen days after receiving the diagnosis of BCC, the patient was taken to the operating room and underwent wide en bloc resection of the soft tissue tumor. Upon lifting the specimen off the underlying muscles, it was found to be penetrating into portions of the trapezius, deltoid, paraspinal, supraspinalis, and infraspinatus muscles. As such, the ulcerated tumor was removed as well as portions of the underlying musculature measuring 21×18 cm. The wound was left open until final pathology on margin clearance was available. It was covered with a wound vac to encourage granulation in anticipation of a planned delayed STSG. There were no complications, and the patient returned to the recovery unit in good condition where the dressing was replaced with a large wound vac system.
Final histologic examination showed negative deep and peripheral margins. More extensive examination of histology of the excised tumor was found to have characteristics consistent with metatypical and morpheic-type BCC. In addition to islands of tumor cells noted in the dermis on original biopsy, this sample also revealed basaloid cells arranged in thin elongated trabeculae invading deeper into the reticular dermis without peripheral palisading, suggestive of the morpheic variant (Figure 3A).8,9,10 Other areas were found to have focal squamous differentiation with keratin pearls and intercellular bridges (Figure 3B). These findings support the diagnosis of a completely excised BCC of the metatypical (referred to by some authorities as basosquamous)8,9 type.

The patient was seen for postoperative evaluations at 2 and 3 weeks. Each time granulation was noted to be proceeding well without signs of infection, and the wound vac was left in place. One month after the initial SE, the patient returned for the planned STSG. The skin graft was harvested from the right lateral thigh and was meshed and transferred to the recipient site on the right upper back, sewn circumferentially to the wound edges. Occlusive petrolatum gauze was placed over the graft followed by the wound vac for coverage until the graft matured.
The patient returned for follow-up approximately 7 months after his initial visit to the clinic. He reported feeling well, and his only concern was mild soreness of the scapular muscles while playing golf. The site of tumor excision showed 100% take of the STSG with no nodules in or around the site to suggest recurrence (Figure 4). The patient denied experiencing any constitutional symptoms and had no palpable lymph nodes or physical examination findings suggestive of metastatic disease or new tumor development at other sites. At 36 months after his initial clinic visit, he remained free of recurrence.

Comment
Typical BCC lesions are indolent and small, occurring primarily on the head and neck.5,11,12,17 We report the case of a locally advanced, extremely large and penetrating lesion located on the trunk. This relatively unique case provides for an interesting comparison between available treatments for BCC as well as several of the generally accepted principles of management previously described in the literature.
Treatment Considerations
The approach to management of BCC considers factors related to the tumor and those related to the patient and practitioner. Telfer et al6 recommended that tumors be categorized as relatively low or high risk based on prognostic factors including size, site, histologic subtype and growth pattern; definition of margins; and presence or absence of prior treatment. Characteristics of high-risk tumors include size greater than 2.5 to 3 cm in diameter; location on the midface, nose, or ears; aggressive histologic subtype including morpheic, infiltrating, and metatypical; deep extension; perineural invasion; neglected or long-standing lesions; incomplete SE or Mohs micrographic surgery (MMS); and recurrence of tumor after prior treatment.13,14,18 Although rare, tumors of the metatypical subtype are particularly important to identify, as they are known to be more aggressive and prone to spread than other forms of BCC.19,20 The clinical appearance of metatypical BCCs often is identical to lower-risk subtypes, reinforcing the importance of careful histologic examination of an adequately deep biopsy, given that metatypical features often are present only in the deep tissue planes.19
The practitioner also must consider patient-related factors such as age, general health, immunocompromised states, coexisting medical conditions, and current medications. The skills, experience, and recommendations of the physician also are expected to influence treatment selection.6,21
Surgical Versus Nonsurgical Treatment Approaches
Treatment of large, locally advanced, primary BCCs can be divided into surgical and nonsurgical approaches.5,6 Surgical approaches include MMS and SE. Mohs micrographic surgery, electrodesiccation and curettage, and cryosurgery may achieve high cure rates in lesions that are low risk but generally are not recommended for use with recurrent or high-risk large and aggressive tumors.5,6 Nonsurgical approaches include radiotherapy; chemotherapy; and vismodegib, an oral inhibitor of the hedgehog pathway involved in the development of many BCCs.5,6,22 Topical photodynamic therapy with 5-aminolevulinic acid, topical imiquimod (immune-response modulator) and 5-fluorouracil, and intralesional interferon are other nonsurgical options that are primarily effective for small superficial BCCs. These modalities are not indicated for high-risk tumors.5,6,23
For small tumors, MMS is regarded by most practitioners as the gold standard due to the high cure rate and cosmetic results it provides.5,6,18,24 This procedure allows for precise mapping of tumor location on frozen sections and, unlike surgical excision, examination of close to 100% of the deep and peripheral margins.18 Excision and evaluation of thin horizontal sections for tumor extension also allows for a greater degree of tissue conservation than other modalities.6,25 Mohs micrographic surgery is particularly useful for tumors of the midface, aggressive histologic subtype (eg, morpheic, infiltrating, basosquamous, micronodular), deep invasion, and perineural spread.6,8,18,25 In a large review of 3 studies including a total of 7670 patients with primary BCC treated by MMS, Rowe et al26 reported a 5-year recurrence rate of 1.0%, which was 8.7 times less than the weighted average of all non-MMS modalities. Similarly, in a large prospective review by Leibovitch et al,18 the 5-year recurrence rate of BCC treated with MMS was 1.4% in primary cases and 4.0% in previously recurrent cases.18 They reported that the main predictors of recurrence included longer tumor duration, more levels of excision required to obtain clear margins, notable subclinical extension, and prior recurrence. Interestingly, tumor and postexcision defect size did not predict recurrence.18 Margin-controlled excision with MMS was associated with higher success rates than modalities based on clinical margins without histologic control (eg, surgical excision, electrocautery, curettage) and potentially incomplete excision.12,18
Although MMS has been demonstrated to have a high success rate, it has relative disadvantages. Tumors that are multicentric or have indistinct borders are more difficult to treat with MMS, and cure rates with MMS have been shown to decrease with increasing tumor diameter.13,25 For example, reported cure rates are greater than 99% for MMS in BCCs less than 2 cm in diameter compared to 98.6% for those between 2 and 3 cm, and only 90.5% for those greater than 3 cm.27 Mohs micrographic surgery requires a highly trained surgeon and can be extremely time consuming and labor intensive, particularly with large and locally aggressive tumors.6,25 Tumors that involve fat and cartilage require modifications to standardized processing techniques, and deep wounds involving muscle and bone create technical challenges in maintaining orientation.25 In the past, MMS was more expensive than other treatment modalities; however, cost analyses have demonstrated a near-equal cost of MMS compared to surgical excision with permanent section control and lower cost as compared to radiation therapy for selected cases.28
Surgical excision also is considered a highly effective treatment of primary BCC and is the most commonly used treatment modality for BCC.5,18,29 In this procedure, the peripheral and deep margins of excised tissue can be examined by a pathologist.6 Telfer et al6 recommended SE as the preferable treatment of choice for both large and small tumors in low-risk sites (ie, those that do not include the face) with nodular histology, tumors with morpheic histology in low-risk sites, and small (<2 cm) superficial tumors in high-risk sites. It is recommended that the size of surgical margins correlate with the likelihood of the presence of subclinical tumor extensions. Larger and morpheic-type BCCs require wider margins to achieve complete excision. In these cases, a 3-mm margin yields only a 66% cure rate, while 5-mm margins yield an 82% cure rate and 13- to 15-mm margins yield cure rates higher than 95%.6,29,30 In a series examining recurrence rates of primary BCC, Rowe et al26 reviewed 10 studies (2606 patients treated by SE) and calculated a 5-year recurrence rate of 10.1%. Silverman et al31 reviewed 5-year recurrence rates in 588 cases of BCC treated with SE. They concluded that BCC on the neck, trunk, arms, and legs of any size may be effectively treated with this modality, with 1 case of recurrence among 187 cases (0.5% recurrence rate). Multivariate analysis identified 2 independent risk factors for recurrence: anatomic site (head) and patient sex (male). Analysis of BCCs on the head distinct from other body sites demonstrated a moderately significant trend (P=.196) of increasing diameter with increasing recurrence rates. Age at treatment, duration of lesion, and length of treatment were not significantly associated with an increased risk of recurrence.31 Similarly, a review of 1417 cases of BCC by Dubin and Kopf21 demonstrated an increased risk with tumors located on the head and larger lesions.
RELATED ARTICLE: Basal Cell Carcinoma: Analysis of Factors Associated With Incomplete Excision
Radiotherapy (RT) is a commonly employed nonsurgical approach to management. Its use has been declining in recent years due to relative disadvantages and side effects. Similar to MMS, it can be extremely effective for carefully selected patients.11,31 Radiotherapy is most effective for use with aggressive, rapidly growing BCC subtypes that are more sensitive to radiation, as replicating cells undergo mitotic death when radiation is applied.15 Radiotherapy is considered a viable option for patients who are not candidates for surgery, tumors in locations difficult to access for SE, and for rare unresectable tumors as a primary therapy.5,11 In a randomized comparison between RT and SE approaches to the treatment of primary BCCs on the face, RT was found to be inferior to SE both in efficacy (4-year recurrence rate, 7.5% vs 0.7%) and cosmesis (rate of good results, 69% vs 87%).32
The major disadvantages of RT as compared to other treatment modalities such as MMS or SE are the lack of control at margins and compromised inferior cosmetic outcomes. Hair loss, hyperpigmentation or hypopigmentation, telangiectasia, keloids, cutaneous necrosis, and RT-induced dermatitis have been reported as side effects of RT.6,11,32-34 Other disadvantages of RT include the inconvenience of multiple visits to the hospital for treatment, and high cost as compared to other modalities such as MMS.35 Finally, use of RT even for relatively benign disease has been linked to an increased risk for both squamous cell carcinoma, BCC, and sarcomas.15,36
Vismodegib is an oral drug approved by the US Food and Drug Administration in 2012 for the treatment of locally advanced BCC. It is a first-in-class small-molecule systemic inhibitor of the intracellular hedgehog signaling pathway, which has been implicated in the growth and development of several types of cancer, including BCC.36-38 Most patients with BCC carry loss-of-function mutations that affect PTCH1 and result in unregulated reactivation of the hedgehog pathway and uncontrolled cell growth.38-40 Vismodegib is a small molecule that selectively deactivates the hedgehog pathway. It currently is indicated for the treatment of metastatic BCC or patients with locally advanced BCCs who are not candidates for SE or RT.38-41 An open-label nonrandomized phase 2 study by Sekulic et al42 evaluated the effectiveness of vismodegib for treatment of metastatic or inoperable BCCs. In 33 patients with metastatic BCCs, the response rate was 30% (10/33) with a 9.5-month median progression-free survival. All responses were partial, with 73% (24/33) showing tumor shrinkage. In 63 patients with locally advanced BCCs, the response rate was 43% (27/63). Most patients demonstrated visible reductions in tumor size and improvement in appearance, but 13 patients (21%) in this group were noted to have a complete response (ie, absence of residual BCC on biopsy). Both cohorts had a median response time of 7.6 months.42
Conclusion
Our patient presented with an extremely large and ulcerating lesion on the upper back that met the criteria for classification as a high-risk tumor. In light of the tumor location and size as well as the involvement of deep tissues and muscles, we elected to pursue SE for management. This modality proved to be extremely effective, and the patient continues to be free of residual or recurrent BCC more than 36 months after surgery. Two large systematic reviews lend support to this management approach and report excellent outcomes. In a review article by Rubin et al,5 SE was shown to provide cure rates greater than 99% for BCC lesions of any size on the neck, trunk, and extremities. Moreover, Thissen et al43 performed a systematic meta-analysis of 18 studies reporting recurrence rates of primary BCC after treatment with various modalities and concluded that when surgery is not contraindicated, SE is the treatment of choice for nodular and superficial BCC. Both groups agree in their recommendations that MMS should be used for BCCs in cosmetically compromised zones (eg, midface), sites where tissue sparing is essential, aggressive growth patterns (eg, perineural invasion, morpheaform histology), and when high risk of recurrence is unacceptable.5,43 In contrast, MMS is not recommended for tumors of large diameter or with indistinct borders due to decreased cure rates.13,25,27 Vismodegib is an interesting new option in development for management of metastatic and aggressive nonresectable BCCs. It was not an option in our patient. Although consideration for use of vismodegib as a neoadjuvant treatment to shrink the tumor prior to surgery is reasonable, the decision to proceed directly with SE proved to be the superior option for our patient.
- Basal and squamous cell skin cancers. American Cancer Society website. www.cancer.org/acs/groups/cid/documents/webcontent/003139-pdf.pdf. Updated April 14, 2016. Accessed April 26, 2016.
- Rogers HW, Weinstock MA, Harris AR, et al. Incidence estimate of nonmelanoma skin cancer in the United States, 2006. Arch Dermatol. 2010;146:283-287.
- Wu S, Han J, Li W, et al. Basal cell carcinoma incidence and associated risk factors in US women and men. Am J Epidemiol. 2013;178:890-897.
- Skin cancer facts & statistics. Skin Cancer Foundation website. www.skincancer.org/skin-cancer-information/skin-cancer-facts. Updated March 18, 2016. Accessed April 26, 2016.
- Rubin AI, Chen EH, Ratner D. Basal cell carcinoma. N Engl J Med. 2005;353:2262-2269.
- Telfer NR, Colver GB, Bowers PW. Guidelines for the management of basal cell carcinoma. British Association of Dermatologists. Br J Dermatol. 1999;141:415-423.
- Gallagher RP, Hill GB, Bajdik CD, et al. Sunlight exposure, pigmentary factors, and risk of nonmelanocytic skin cancer: I. basal cell carcinoma. Arch Dermatol. 1995;131:157-163.
- McKee PH, Calonje J, Lazar A, et al, eds. Pathology of the Skin with Clinical Correlations. 4th ed. Vol 2. Philadelphia, PA: Elsevier Mosby; 2011.
- Elder DE. Basal cell carcinoma. In: Elder DE, Elenitsas R, Johnson Jr BL, et al, eds. Lever’s Histopathology of the Skin. 10th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2009:826-832.
- Bastiaens MT, Hoefnagel JJ, Buijn JA, et al. Differences in age, site distribution, and sex between superficial basal cell carcinomas indicate different types of tumors. J Invest Dermatol. 1998;110:880-884.
- Kuijpers DI, Thissen MM, Neumann MA. Basal cell carcinoma: treatment options and prognosis, a scientific approach to a common malignancy. Am J Clin Dermatol. 2002;3:247-259.
- Leibovitch I, Huilgol SC, Selva D, et al. Basal cell carcinoma treated with Mohs surgery in Australia I: experience over 10 years. J Am Acad Dermatol. 2005;53:445-451.
- Walling H, Fosko S, Geraminejad P, et al. Aggressive basal cell carcinoma: presentation, pathogenesis, and management. Cancer Metastasis Rev. 2004;23:389-402.
- Veness M, Richards S. Role of modern radiotherapy in treating skin cancer. Australas J Dermatol. 2003;44:159-168.
- Wysong A, Aasi SZ, Tang JY. Update on metastatic basal cell carcinoma: a summary of published cases from 1981 through 2011. JAMA Dermatol. 2013;149:615-616.
- Bolognia J, Jorizzo J, Rapini R, eds. Dermatology. Vol 2. Philadelphia, PA: Mosby; 2003.
- Swanson NA. Mohs surgery: technique, indications, applications, and the future. Arch Dermatol. 1983;119:761-773.
- Leibovitch I, Huilgol SC, Selva D, et al. Basal cell carcinoma treated with Mohs surgery in Australia II: outcome at 5-year follow-up. J Am Acad Dermatol. 2005;53:452-457.
- De Stefano A, Dispenza F, Petrucci AG, et al. Features of biopsy in diagnosis of metatypical basal cell carcinoma (basosquamous carcinoma) of head and neck. Otolaryngol Pol. 2012;66:419-423.
- Tarallo M, Cigna E, Frati R, et al. Metatypical basal cell carcinoma: a clinical review. J Exp Clin Cancer Res. 2008;27:65.
- Dubin N, Kopf AW. Multivariate risk score for recurrence of cutaneous basal cell carcinomas. Arch Dermatol. 1983;119:373-377.
- Rodriguez DA. Basal cell carcinoma: a primer on diagnosis and treatment. Practical Dermatology. 2014;11:36-38.
- Kirby JS, Miller CJ. Intralesional chemotherapy for nonmelanoma skin cancer: a practical review. J Am Acad Dermatol. 2010;63:689-702.
- Rowe DE. Comparison of treatment modalities for basal cell carcinoma. Clin Dermatol. 1995;13:617-620.
- Shriner DL, McCoy DK, Goldberg DJ, et al. Mohs micrographic surgery. J Am Acad Dermatol. 1998;39:79-97.
- Rowe DE, Carroll RJ, Day CL Jr. Mohs surgery is the treatment of choice for recurrent (previously treated) basal cell carcinoma. J Dermatol Surg Oncol. 1989;15:424-431.
- Mohs FE. Chemosurgery: Microscopically Controlled Surgery for Skin Cancer. Springfield, IL: Charles C. Thomas; 1978.
- Cook J, Zitelli JA. Mohs micrographic surgery: a cost analysis. J Am Acad Dermatol. 1996;39(5 pt 1):698-703.
- Breuninger H, Dietz K. Prediction of subclinical tumor infiltration in basal cell carcinoma. J Dermatol Surg Oncol. 1991;17:574-578.
- Wolf DJ, Zitelli JA. Surgical margins for basal cell carcinoma. Arch Dermatol. 1987;123:340-344.
- Silverman MK, Kopf AW, Bart RS, et al. Recurrence rates of treated basal cell carcinomas, part 3: surgical excision. J Dermatol Surg Oncol. 1992;18:471-476.
- Avril MF, Auperin A, Margulis A, et al. Basal cell carcinoma of the face: surgery or radiotherapy? results of a randomized study. Br J Cancer. 1997;76:100-106.
- Caccialanza M, Piccinno R, Beretta M, et al. Results and side effects of dermatologic radiotherapy: a retrospective study of irradiated cutaneous epithelial neoplasms. J Am Acad Dermatol. 1999;41:589-594.
- Silverman MK, Kopf AW, Gladstein AH, et al. Recurrence rates of treated basal cell carcinomas, part 4: x-ray therapy. J Dermatol Surg Oncol. 1992;18:549-554.
- Rowe DE, Carroll RJ, Day CL Jr. Long-term recurrence rates in previously untreated (primary) basal cell carcinoma: implications for patient follow-up. J Dermatol Surg Oncol. 1989;15:315-328.
- Beswick SJ, Garrido MC, Fryer AA, et al. Multiple basal cell carcinomas and malignant melanoma following radiotherapy for ankylosing spondylitis. Clin Exp Dermatol. 2000;25:381-383.
- Motley RJ. The treatment of basal cell carcinoma. J Dermatolog Treat. 1995;6:121-125.
- Dlugosz A, Agrawal S, Kirkpatrick P. Vismodegib. Nat Rev Drug Discov. 2012;11:437-438.
- Fellner C. Vismodegib (Erivedge) for advanced basal cell carcinoma. P T. 2012;37:670-682.
- Harms KL, Dlugosz AA. Harnessing hedgehog for the treatment of basal cell carcinoma. JAMA Dermatol. 2013;149:607-608.
- Rudin CM. Vismodegib. Clin Cancer Res. 2012;18:3218-3222.
- Sekulic A, Migden M, Oro A, et al. Efficacy and safety of vismodegib in advanced basal-cell carcinoma. N Engl J Med. 2012;366:2171-2179.
- Thissen MM, Neumann MA, Schouten LJ. A systematic review of treatment modalities for primary basal cell carcinomas. Arch Dermatol. 1999;135:1177-1183.
- Basal and squamous cell skin cancers. American Cancer Society website. www.cancer.org/acs/groups/cid/documents/webcontent/003139-pdf.pdf. Updated April 14, 2016. Accessed April 26, 2016.
- Rogers HW, Weinstock MA, Harris AR, et al. Incidence estimate of nonmelanoma skin cancer in the United States, 2006. Arch Dermatol. 2010;146:283-287.
- Wu S, Han J, Li W, et al. Basal cell carcinoma incidence and associated risk factors in US women and men. Am J Epidemiol. 2013;178:890-897.
- Skin cancer facts & statistics. Skin Cancer Foundation website. www.skincancer.org/skin-cancer-information/skin-cancer-facts. Updated March 18, 2016. Accessed April 26, 2016.
- Rubin AI, Chen EH, Ratner D. Basal cell carcinoma. N Engl J Med. 2005;353:2262-2269.
- Telfer NR, Colver GB, Bowers PW. Guidelines for the management of basal cell carcinoma. British Association of Dermatologists. Br J Dermatol. 1999;141:415-423.
- Gallagher RP, Hill GB, Bajdik CD, et al. Sunlight exposure, pigmentary factors, and risk of nonmelanocytic skin cancer: I. basal cell carcinoma. Arch Dermatol. 1995;131:157-163.
- McKee PH, Calonje J, Lazar A, et al, eds. Pathology of the Skin with Clinical Correlations. 4th ed. Vol 2. Philadelphia, PA: Elsevier Mosby; 2011.
- Elder DE. Basal cell carcinoma. In: Elder DE, Elenitsas R, Johnson Jr BL, et al, eds. Lever’s Histopathology of the Skin. 10th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2009:826-832.
- Bastiaens MT, Hoefnagel JJ, Buijn JA, et al. Differences in age, site distribution, and sex between superficial basal cell carcinomas indicate different types of tumors. J Invest Dermatol. 1998;110:880-884.
- Kuijpers DI, Thissen MM, Neumann MA. Basal cell carcinoma: treatment options and prognosis, a scientific approach to a common malignancy. Am J Clin Dermatol. 2002;3:247-259.
- Leibovitch I, Huilgol SC, Selva D, et al. Basal cell carcinoma treated with Mohs surgery in Australia I: experience over 10 years. J Am Acad Dermatol. 2005;53:445-451.
- Walling H, Fosko S, Geraminejad P, et al. Aggressive basal cell carcinoma: presentation, pathogenesis, and management. Cancer Metastasis Rev. 2004;23:389-402.
- Veness M, Richards S. Role of modern radiotherapy in treating skin cancer. Australas J Dermatol. 2003;44:159-168.
- Wysong A, Aasi SZ, Tang JY. Update on metastatic basal cell carcinoma: a summary of published cases from 1981 through 2011. JAMA Dermatol. 2013;149:615-616.
- Bolognia J, Jorizzo J, Rapini R, eds. Dermatology. Vol 2. Philadelphia, PA: Mosby; 2003.
- Swanson NA. Mohs surgery: technique, indications, applications, and the future. Arch Dermatol. 1983;119:761-773.
- Leibovitch I, Huilgol SC, Selva D, et al. Basal cell carcinoma treated with Mohs surgery in Australia II: outcome at 5-year follow-up. J Am Acad Dermatol. 2005;53:452-457.
- De Stefano A, Dispenza F, Petrucci AG, et al. Features of biopsy in diagnosis of metatypical basal cell carcinoma (basosquamous carcinoma) of head and neck. Otolaryngol Pol. 2012;66:419-423.
- Tarallo M, Cigna E, Frati R, et al. Metatypical basal cell carcinoma: a clinical review. J Exp Clin Cancer Res. 2008;27:65.
- Dubin N, Kopf AW. Multivariate risk score for recurrence of cutaneous basal cell carcinomas. Arch Dermatol. 1983;119:373-377.
- Rodriguez DA. Basal cell carcinoma: a primer on diagnosis and treatment. Practical Dermatology. 2014;11:36-38.
- Kirby JS, Miller CJ. Intralesional chemotherapy for nonmelanoma skin cancer: a practical review. J Am Acad Dermatol. 2010;63:689-702.
- Rowe DE. Comparison of treatment modalities for basal cell carcinoma. Clin Dermatol. 1995;13:617-620.
- Shriner DL, McCoy DK, Goldberg DJ, et al. Mohs micrographic surgery. J Am Acad Dermatol. 1998;39:79-97.
- Rowe DE, Carroll RJ, Day CL Jr. Mohs surgery is the treatment of choice for recurrent (previously treated) basal cell carcinoma. J Dermatol Surg Oncol. 1989;15:424-431.
- Mohs FE. Chemosurgery: Microscopically Controlled Surgery for Skin Cancer. Springfield, IL: Charles C. Thomas; 1978.
- Cook J, Zitelli JA. Mohs micrographic surgery: a cost analysis. J Am Acad Dermatol. 1996;39(5 pt 1):698-703.
- Breuninger H, Dietz K. Prediction of subclinical tumor infiltration in basal cell carcinoma. J Dermatol Surg Oncol. 1991;17:574-578.
- Wolf DJ, Zitelli JA. Surgical margins for basal cell carcinoma. Arch Dermatol. 1987;123:340-344.
- Silverman MK, Kopf AW, Bart RS, et al. Recurrence rates of treated basal cell carcinomas, part 3: surgical excision. J Dermatol Surg Oncol. 1992;18:471-476.
- Avril MF, Auperin A, Margulis A, et al. Basal cell carcinoma of the face: surgery or radiotherapy? results of a randomized study. Br J Cancer. 1997;76:100-106.
- Caccialanza M, Piccinno R, Beretta M, et al. Results and side effects of dermatologic radiotherapy: a retrospective study of irradiated cutaneous epithelial neoplasms. J Am Acad Dermatol. 1999;41:589-594.
- Silverman MK, Kopf AW, Gladstein AH, et al. Recurrence rates of treated basal cell carcinomas, part 4: x-ray therapy. J Dermatol Surg Oncol. 1992;18:549-554.
- Rowe DE, Carroll RJ, Day CL Jr. Long-term recurrence rates in previously untreated (primary) basal cell carcinoma: implications for patient follow-up. J Dermatol Surg Oncol. 1989;15:315-328.
- Beswick SJ, Garrido MC, Fryer AA, et al. Multiple basal cell carcinomas and malignant melanoma following radiotherapy for ankylosing spondylitis. Clin Exp Dermatol. 2000;25:381-383.
- Motley RJ. The treatment of basal cell carcinoma. J Dermatolog Treat. 1995;6:121-125.
- Dlugosz A, Agrawal S, Kirkpatrick P. Vismodegib. Nat Rev Drug Discov. 2012;11:437-438.
- Fellner C. Vismodegib (Erivedge) for advanced basal cell carcinoma. P T. 2012;37:670-682.
- Harms KL, Dlugosz AA. Harnessing hedgehog for the treatment of basal cell carcinoma. JAMA Dermatol. 2013;149:607-608.
- Rudin CM. Vismodegib. Clin Cancer Res. 2012;18:3218-3222.
- Sekulic A, Migden M, Oro A, et al. Efficacy and safety of vismodegib in advanced basal-cell carcinoma. N Engl J Med. 2012;366:2171-2179.
- Thissen MM, Neumann MA, Schouten LJ. A systematic review of treatment modalities for primary basal cell carcinomas. Arch Dermatol. 1999;135:1177-1183.
Practice Points
- Unusually large basal cell carcinomas (BCCs) present a therapeutic challenge.
- A number of therapeutic options exist. Wide excision with margin control and complex reconstruction remains an excellent treatment option for BCC.
Handheld Reflectance Confocal Microscopy to Aid in the Management of Complex Facial Lentigo Maligna
Lentigo maligna (LM) and LM melanoma (LMM) represent diagnostic and therapeutic challenges due to their heterogeneous nature and location on cosmetically sensitive areas. Newer ancillary technologies such as reflectance confocal microscopy (RCM) have helped improve diagnosis and management of these challenging lesions.1,2
Reflectance confocal microscopy is a noninvasive laser system that provides real-time imaging of the epidermis and dermis with cellular resolution and improves diagnostic accuracy of melanocytic lesions.2,3 Normal melanocytes appear as round bright structures on RCM that are similar in size to surrounding keratinocytes located in the basal layer and regularly distributed around the dermal papillae (junctional nevi) or form regular dense nests in the dermis (intradermal nevi).4,5 In LM/LMM, there may be widespread infiltration of atypical melanocytes invading hair follicles; large, round, pagetoid melanocytes (larger than surrounding keratinocytes); sheets of large atypical cells at the dermoepidermal junction (DEJ); loss of contour in the dermal papillae; and atypical melanocytes invading the dermal papillae.2 Indeed, RCM has good correlation with the degree of histologic atypia and is useful to distinguish between benign nevi, atypical nevi, and melanoma.6 By combining lateral mosaics with vertical stacks, RCM allows 3-dimensional approximation of tumor margins and monitoring of nonsurgical therapies.7,8 The advent of handheld RCM (HRCM) has allowed assessment of large lesions as well as those presenting in difficult locations.9 Furthermore, the generation of videomosaics overcomes the limited field of view of traditional RCM and allows for accurate assessment of large lesions.10
Traditional and handheld RCM have been used to diagnose and map primary LM.1,2,11 Guitera et al2 developed an algorithm using traditional RCM to distinguish benign facial macules and LM. In their training set, they found that when their score resulted in 2 or more points, the sensitivity and specificity to diagnose LM was 85% and 76%, respectively, with an odds ratio of 18.6 for LM. They later applied the algorithm in a test set of 44 benign facial macules and 29 LM and obtained an odds ratio of 60.7 for LM, with sensitivity and specificity rates of 93% and 82%, respectively.2 This algorithm also was tested by Menge et al11 using the HRCM. They found 100% sensitivity and 71% specificity for LM when evaluating 63 equivocal facial lesions. Although these results suggest that RCM can accurately distinguish LM from benign lesions in the primary setting, few reports have studied the impact of HRCM in the recurrent setting and its impact in monitoring treatment of LM.12,13
Herein, we present 5 cases in which HRCM was used to manage complex facial LM/LMM, highlighting its versatility and potential for use in the clinical setting (eTable).
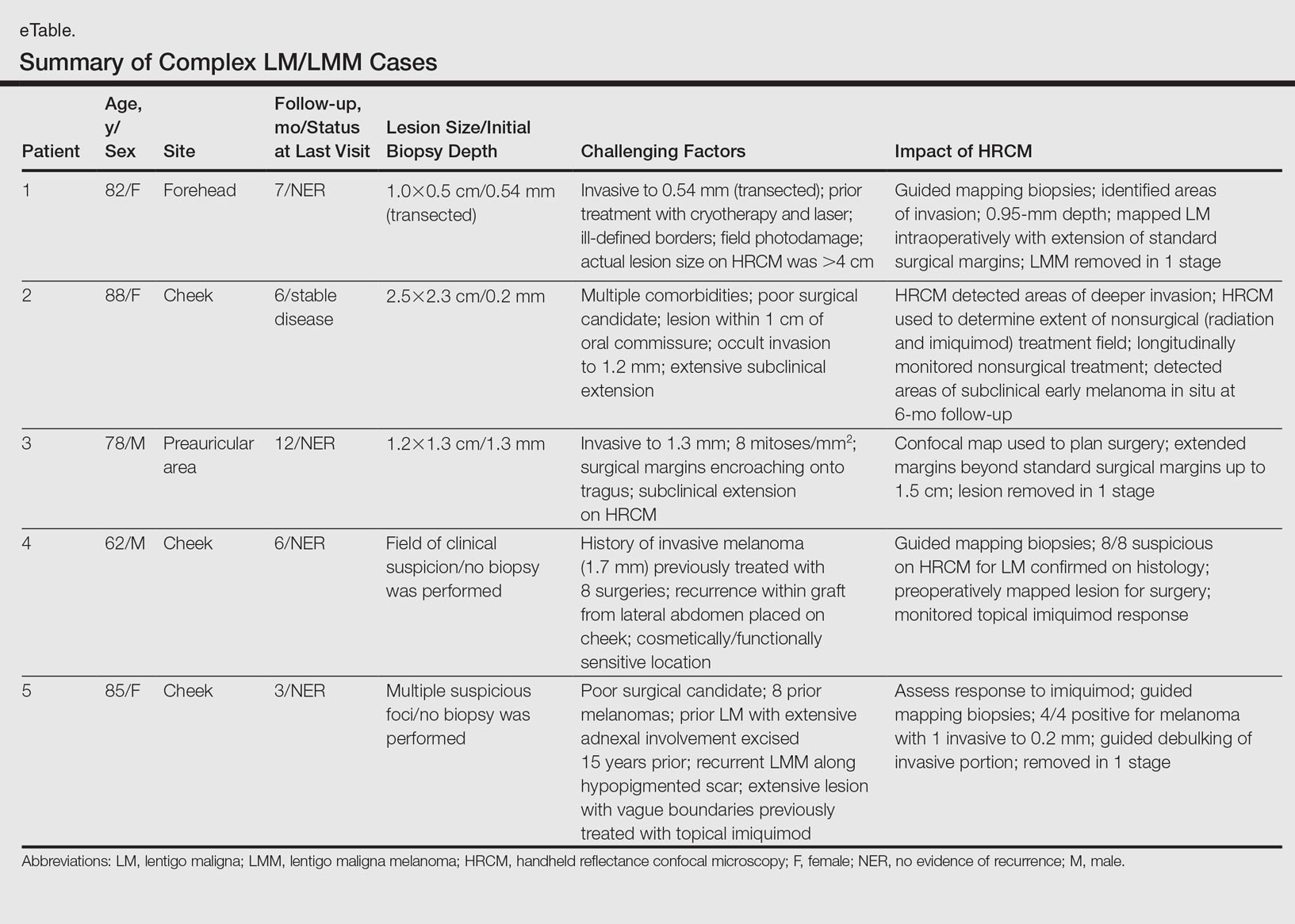
Case Series
Following institutional review board approval, cases of facial LM/LMM presenting for assessment and treatment from January 2014 to December 2015 were retrospectively reviewed. Initially, the clinical margins of the lesions were determined using Wood lamp and/or dermoscopy. Using HRCM, vertical stacks were taken at the 12-, 3-, 6-, and 9-o'clock positions, and videos were captured along the peripheral margins at the DEJ. To create videomosaics, HRCM video frames were extracted and later stitched using a computer algorithm written in a fourth-generation programming language based on prior studies.10,14 An example HRCM video that was captured and turned into a videomosaic accompanies this article online (http://bit.ly/2oDYS6k). Additional stacks were taken in suspicious areas. We considered an area positive for LM under HRCM when the LM score developed by Guitera et al2 was 2 or more. The algorithm scoring includes 2 major criteria--nonedged papillae and round large pagetoid cells--which score 2 points, and 4 minor criteria, including 3 positive criteria--atypical cells at the DEJ, follicular invasion, nucleated cells in the papillae--which each score 1 point, and 1 negative criterion--broadened honeycomb pattern--which scores -1 point.2
RELATED VIDEO: RCM Videomosaic of Melanoma In Situ
Patient 1
An 82-year-old woman was referred to us for management of an LMM on the left side of the forehead (Figure 1A). Handheld RCM from the biopsy site showed large atypical cells in the epidermis, DEJ, and papillary dermis. Superiorly, HRCM showed large dendritic processes but did not reveal LM features in 3 additional clinically worrisome areas. Biopsies showed LMM at the prior biopsy site, LM superiorly, and actinic keratosis in the remaining 3 areas, supporting the HRCM findings. Due to upstaging, the patient was referred for head and neck surgery. To aid in resection, HRCM was performed intraoperatively in a multidisciplinary approach (Figure 1B). Due to the large size of the lesion, surgical margins were taken right outside the HRCM border. Pathology showed LMM extending focally into the margins that were reexcised, achieving clearance.

Patient 2
An 88-year-old woman presented with a slightly pigmented, 2.5×2.3-cm LMM on the left cheek. Because of her age and comorbidities (eg, osteoporosis, deep vein thrombosis in both lower legs requiring anticoagulation therapy, presence of an inferior vena cava filter, bilateral lymphedema of the legs, irritable bowel syndrome, hyperparathyroidism), she was treated with imiquimod cream 5% achieving partial response. The lesion was subsequently excised showing LMM extending to the margins. Not wanting to undergo further surgery, she opted for radiation therapy. Handheld RCM was performed to guide the radiation field, showing pagetoid cells within 1 cm of the scar and clear margins beyond 2 cm. She underwent radiation therapy followed by treatment with imiquimod. On 6-month follow-up, no clinical lesion was apparent, but HRCM showed atypical cells. Biopsies revealed an atypical intraepidermal melanocytic proliferation, but due to patient's comorbidities, close observation was decided.
Patient 3
A 78-year-old man presented with an LMM on the right preauricular area. Handheld RCM demonstrated pleomorphic pagetoid cells along and beyond the clinical margins. Wide excision with sentinel lymph node biopsy was planned, and to aid surgery a confocal map was created (Figure 2). Margins were clear at 1 cm, except inferiorly where they extended to 1.5 cm. Using this preoperative HRCM map, all intraoperative sections were clear. Final pathology confirmed clear margins throughout.
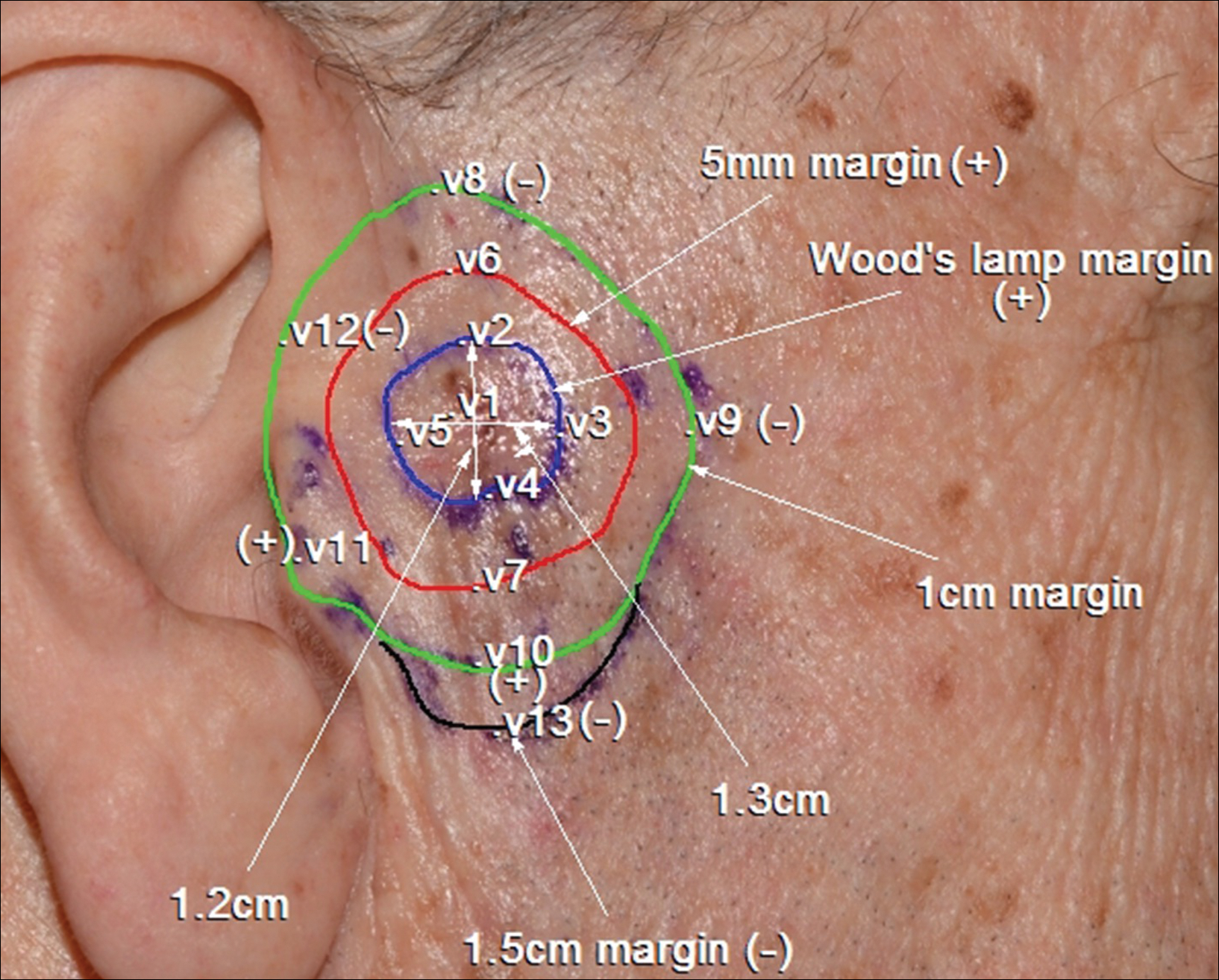
Patient 4
A 62-year-old man presented with hyperpigmentation and bleeding on the left cheek where an LMM was previously removed 8 times over 18 years. Handheld RCM showed pleomorphic cells along the graft border and interestingly within the graft. Ten biopsies were taken, 8 at sites with confocal features that were worrisome for LM (Figures 3A and 3B) and 2 at clinically suspicious sites. The former revealed melanomas (2 that were invasive to 0.3 mm), and the latter revealed solar lentigines. The patient underwent staged excision guided by HRCM (Figure 3C), achieving clear histologic margins except for a focus in the helix. This area was RCM positive but was intentionally not resected due to reconstructive difficulties; imiquimod was indicated in this area.
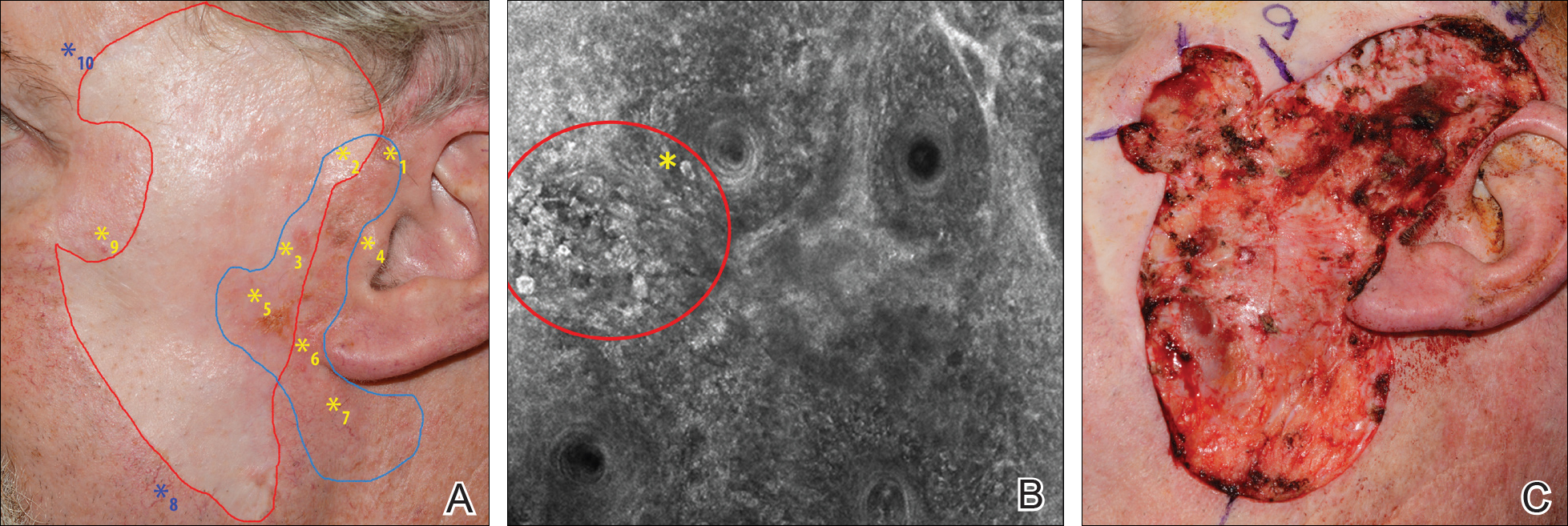
Patient 5
An 85-year-old woman with 6 prior melanomas over 15 years presented with ill-defined light brown patches on the left cheek at the site where an LM was previously excised 15 years prior. Biopsies showed LM, and due to the patient's age, health, and personal preference to avoid extensive surgery, treatment with imiquimod cream 5% was decided. Over a period of 6 to 12 months, she developed multiple erythematous macules with 2 faintly pigmented areas. Handheld RCM demonstrated atypical cells within the papillae in previously biopsied sites that were rebiopsied, revealing LMM (Breslow depth, 0.2 mm). Staged excision achieved clear margins, but after 8 months HRCM showed LM features. Histology confirmed the diagnosis and imiquimod was reapplied.
Comment
Diagnosis and choice of treatment modality for cases of facial LM is a challenge, and there are a number of factors that may create even more of a clinical dilemma. Surgical excision is the treatment of choice for LM/LMM, and better results are achieved when using histologically controlled surgical procedures such as Mohs micrographic surgery, staged excision, or the "spaghetti technique."15-17 However, advanced patient age, multiple comorbidities (eg, coronary artery disease, deep vein thrombosis, other conditions requiring anticoagulation therapy), large lesion size in functionally or aesthetically sensitive areas, and indiscriminate borders on photodamaged skin may make surgical excision complicated or not feasible. Additionally, prior treatments to the affected area may further obscure clinical borders, complicating the diagnosis of recurrence/persistence when observed with the naked eye, dermoscopy, or Wood lamp. Because RCM can detect small amounts of melanin and has cellular resolution, it has been suggested as a great diagnostic tool to be combined with dermoscopy when evaluating lightly pigmented/amelanotic facial lesions arising on sun-damaged skin.18,19 In this case series, we highlighted these difficulties and showed how HRCM can be useful in a variety of scenarios, both pretreatment and posttreatment in complex LM/LMM cases.
Pretreatment Evaluation
Blind mapping biopsies of LM are prone to sample bias and depend greatly on biopsy technique; however, HRCM can guide mapping biopsies by detecting features of LM in vivo with high sensitivity.11 Due to the cosmetically sensitive nature of the lesions, many physicians are discouraged to do multiple mapping biopsies, making it difficult to assess the breadth of the lesion and occult invasion. Multiple studies have shown that occult invasion was not apparent until complete lesion excision was done.15,20,21 Agarwal-Antal et al20 reported 92 cases of LM, of which 16% (15/92) had unsuspected invasion on final excisional pathology. A long-standing disadvantage of treating LM with nonsurgical modalities has been the inability to detect occult invasion or multifocal invasion within the lesion. As described in patients 1, 4, and 5 in the current case series, utilizing real-time video imaging of the DEJ at the margins and within the lesion has allowed for the detection of deep atypical melanocytes suspicious for perifollicular infiltration and invasion. Knowing the depth of invasion before treatment is essential for not only counseling the patient about disease risk but also for choosing an appropriate treatment modality. Therefore, prospective studies evaluating the performance of RCM to identify invasion are crucial to improve sampling error and avoid unnecessary biopsies.
Surgical Treatment
Although surgery is the first-line treatment option for facial LM, it is not without associated morbidity, and LM is known to have histological subclinical extension, which makes margin assessment difficult. Wide surgical margins on the face are not always possible and become further complicated when trying to maintain adequate functional and cosmetic outcomes. Additionally, the margin for surgical clearance may not be straightforward for facial lesions. Hazan et al15 showed the mean total surgical margins required for excision of LM and LMM was 7.1 and 10.3 mm, respectively; of the 91 tumors initially diagnosed as LM on biopsy, 16% (15/91) had unsuspected invasion. Guitera et al2 reported that the presence of atypical cells within the dermal papillae might be a sign of invasion, which occasionally is not detected histologically due to sampling bias. Handheld RCM offers the advantage of a rapid real-time assessment in areas that may not have been amenable to previous iterations of the device, and it also provides a larger field of view that would be time consuming if performed using conventional RCM. Compared to prior RCM devices that were not handheld, the use of the HRCM does not need to attach a ring to the skin and is less bulky, permitting its use at the bedside of the patient or even intraoperatively.13 In our experience, HRCM has helped to better characterize subclinical spread of LM during the initial consultation and better counsel patients about the extent of the lesion. Handheld RCM also has been used to guide the spaghetti technique in patients with LM/LMM with good correlation between HRCM and histology.22 In our case series, HRCM was used in complex LM/LMM to delineate surgical margins, though in some cases the histologic margins were too close or affected, suggesting HRCM underestimation. Lentigo maligna margin assessment with RCM uses an algorithm that evaluates confocal features in the center of the lesion.1,2 Therefore, further studies using HRCM should evaluate minor confocal features in the margins as potential markers of positivity to accurately delineate surgical margins.
Nonsurgical Treatment Options
For patients unable or unwilling to pursue surgical treatment, therapies such as imiquimod or radiation have been suggested.23,24 However, the lack of histological confirmation and possibility for invasive spread has limited these modalities. Lentigo malignas treated with radiation have a 5% recurrence rate, with a median follow-up time of 3 years.23 Recurrence often can be difficult to detect clinically, as it may manifest as an amelanotic lesion, or postradiation changes can hinder detection. Handheld RCM allows for a cellular-level observation of the irradiated field and can identify radiation-induced changes in LM lesions, including superficial necrosis, apoptotic cells, dilated vessels, and increased inflammatory cells.25 Handheld RCM has previously been used to assess LM treated with radiation and, as in patient 2, can help define the radiation field and detect treatment failure or recurrence.12,25
Similarly, as described in patient 5, HRCM was utilized to monitor treatment with imiquimod. Many reports use imiquimod for treatment of LM, but application and response vary greatly. Reflectance confocal microscopy has been shown to be useful in monitoring LM treated with imiquimod,8 which is important because clinical findings such as inflammation and erythema do not correlate well with response to therapy. Thus, RCM is an appealing noninvasive modality to monitor response to treatment and assess the need for longer treatment duration. Moreover, similar to postradiation changes, treatment with imiquimod may cause an alteration of the clinically apparent pigment. Therefore, it is difficult to assess treatment success by clinical inspection alone. The use of RCM before, during, and after treatment provides a longitudinal assessment of the lesion and has augmented dermatologists' ability to determine treatment success or failure; however, prospective studies evaluating the usefulness of HRCM in the recurrent setting are needed to validate these results.
Limitations
Limitations of this technology include the time needed to image large areas; technology cost; and associated learning curve, which may take from 6 months to 1 year based on our experience. Others have reported the training required for accurate RCM interpretation to be less than that of dermoscopy.26 It has been shown that key RCM diagnostic criteria for lesions including melanoma and basal cell carcinoma are reproducibly recognized among RCM users and that diagnostic accuracy increases with experience.27 These limitations can be overcome with advances in videomosaicing that may streamline the imaging as well as an eventual decrease in cost with greater user adoption and the development of training platforms that enable a faster learning of RCM.28
Conclusion
The use of HRCM can help in the diagnosis and management of facial LMs. Handheld RCM provides longitudinal assessment of LM/LMM that may help determine treatment success or failure and has proven to be useful in detecting the presence of recurrence/persistence in cases that were clinically poorly evident. Moreover, HRCM is a notable ancillary tool, as it can be performed at the bedside of the patient or even intraoperatively and provides a faster approach than conventional RCM in cases where large areas need to be mapped.
In summary, HRCM may eventually be a useful screening tool to guide scouting biopsies to diagnose de novo LM; guide surgical and nonsurgical therapies; and evaluate the presence of recurrence/persistence, especially in large, complex, amelanotic or poorly pigmented lesions. A more standardized use of HRCM in mapping surgical and nonsurgical approaches needs to be evaluated in further studies to provide a fast and reliable complement to histology in such complex cases; therefore, larger studies need to be performed to validate this technique in such complex cases.
- Guitera P, Moloney FJ, Menzies SW, et al. Improving management and patient care in lentigo maligna by mapping with in vivo confocal microscopy. JAMA Dermatol. 2013;149:692-698.
- Guitera P, Pellacani G, Crotty KA, et al. The impact of in vivo reflectance confocal microscopy on the diagnostic accuracy of lentigo maligna and equivocal pigmented and nonpigmented macules of the face. J Invest Dermatol. 2010;130:2080-2091.
- Pellacani G, Guitera P, Longo C, et al. The impact of in vivo reflectance confocal microscopy for the diagnostic accuracy of melanoma and equivocal melanocytic lesions. J Invest Dermatol. 2007;127:2759-2765.
- Segura S, Puig S, Carrera C, et al. Development of a two-step method for the diagnosis of melanoma by reflectance confocal microscopy. J Am Acad Dermatol. 2009;61:216-229.
- Hofmann-Wellenhof R, Pellacani G, Malvehy J, et al. Reflectance Confocal Microscopy for Skin Diseases. New York, NY: Springer; 2012.
- Pellacani G, Farnetani F, Gonzalez S, et al. In vivo confocal microscopy for detection and grading of dysplastic nevi: a pilot study. J Am Acad Dermatol. 2012;66:E109-E121.
- Nadiminti H, Scope A, Marghoob AA, et al. Use of reflectance confocal microscopy to monitor response of lentigo maligna to nonsurgical treatment. Dermatol Surg. 2010;36:177-184.
- Alarcon I, Carrera C, Alos L, et al. In vivo reflectance confocal microscopy to monitor the response of lentigo maligna to imiquimod. J Am Acad Dermatol. 2014;71:49-55.
- Fraga-Braghiroli NA, Stephens A, Grossman D, et al. Use of handheld reflectance confocal microscopy for in vivo diagnosis of solitary facial papules: a case series. J Eur Acad Dermatol Venereol. 2014;28:933-942.
- Kose K, Cordova M, Duffy M, et al. Video-mosaicing of reflectance confocal images for examination of extended areas of skin in vivo. Br J Dermatol. 2014;171:1239-1241.
- Menge TD, Hibler BP, Cordova MA, et al. Concordance of handheld reflectance confocal microscopy (RCM) with histopathology in the diagnosis of lentigo maligna (LM): a prospective study [published online January 27, 2016]. J Am Acad Dermatol. 2016;74:1114-1120.
- Hibler BP, Connolly KL, Cordova M, et al. Radiation therapy for synchronous basal cell carcinoma and lentigo maligna of the nose: response assessment by clinical examination and reflectance confocal microscopy. Pract Radiat Oncol. 2015;5:E543-E547.
- Hibler BP, Cordova M, Wong RJ, et al. Intraoperative real-time reflectance confocal microscopy for guiding surgical margins of lentigo maligna melanoma. Dermatol Surg. 2015;41:980-983.
- Kose K, Gou M, Yelamos O, et al. Video-mosaicking of in vivo reflectance confocal microscopy images for noninvasive examination of skin lesions [published February 6, 2017]. Proceedings of SPIE Photonics West. doi:10.1117/12.2253085.
- Hazan C, Dusza SW, Delgado R, et al. Staged excision for lentigo maligna and lentigo maligna melanoma: a retrospective analysis of 117 cases. J Am Acad Dermatol. 2008;58:142-148.
- Etzkorn JR, Sobanko JF, Elenitsas R, et al. Low recurrence rates for in situ and invasive melanomas using Mohs micrographic surgery with melanoma antigen recognized by T cells 1 (MART-1) immunostaining: tissue processing methodology to optimize pathologic staging and margin assessment. J Am Acad Dermatol. 2015;72:840-850.
- Gaudy-Marqueste C, Perchenet AS, Tasei AM, et al. The "spaghetti technique": an alternative to Mohs surgery or staged surgery for problematic lentiginous melanoma (lentigo maligna and acral lentiginous melanoma). J Am Acad Dermatol. 2011;64:113-118.
- Guitera P, Menzies SW, Argenziano G, et al. Dermoscopy and in vivo confocal microscopy are complementary techniques for diagnosis of difficult amelanotic and light-coloured skin lesions [published online October 12, 2016]. Br J Dermatol. 2016;175:1311-1319.
- Borsari S, Pampena R, Lallas A, et al. Clinical indications for use of reflectance confocal microscopy for skin cancer diagnosis. JAMA Dermatol. 2016;152:1093-1098.
- Agarwal-Antal N, Bowen GM, Gerwels JW. Histologic evaluation of lentigo maligna with permanent sections: implications regarding current guidelines. J Am Acad Dermatol. 2002;47:743-748.
- Gardner KH, Hill DE, Wright AC, et al. Upstaging from melanoma in situ to invasive melanoma on the head and neck after complete surgical resection. Dermatol Surg. 2015;41:1122-1125.
- Champin J, Perrot JL, Cinotti E, et al. In vivo reflectance confocal microscopy to optimize the spaghetti technique for defining surgical margins of lentigo maligna. Dermatolog Surg. 2014;40:247-256.
- Fogarty GB, Hong A, Scolyer RA, et al. Radiotherapy for lentigo maligna: a literature review and recommendations for treatment. Br J Dermatol. 2014;170:52-58.
- Swetter SM, Chen FW, Kim DD, et al. Imiquimod 5% cream as primary or adjuvant therapy for melanoma in situ, lentigo maligna type. J Am Acad Dermatol. 2015;72:1047-1053.
- Richtig E, Arzberger E, Hofmann-Wellenhof R, et al. Assessment of changes in lentigo maligna during radiotherapy by in-vivo reflectance confocal microscopy--a pilot study. Br J Dermatol. 2015;172:81-87.
- Gerger A, Koller S, Kern T, et al. Diagnostic applicability of in vivo confocal laser scanning microscopy in melanocytic skin tumors. J Invest Dermatol. 2005;124:493-498.
- Farnetani F, Scope A, Braun RP, et al. Skin cancer diagnosis with reflectance confocal microscopy: reproducibility of feature recognition and accuracy of diagnosis. JAMA Dermatol. 2015;151:1075-1080.
- Rajadhyaksha M, Marghoob A, Rossi A, et al. Reflectance confocal microscopy of skin in vivo: from bench to bedside [published online October 27, 2016]. Lasers Surg Med. 2017;49:7-19.
Lentigo maligna (LM) and LM melanoma (LMM) represent diagnostic and therapeutic challenges due to their heterogeneous nature and location on cosmetically sensitive areas. Newer ancillary technologies such as reflectance confocal microscopy (RCM) have helped improve diagnosis and management of these challenging lesions.1,2
Reflectance confocal microscopy is a noninvasive laser system that provides real-time imaging of the epidermis and dermis with cellular resolution and improves diagnostic accuracy of melanocytic lesions.2,3 Normal melanocytes appear as round bright structures on RCM that are similar in size to surrounding keratinocytes located in the basal layer and regularly distributed around the dermal papillae (junctional nevi) or form regular dense nests in the dermis (intradermal nevi).4,5 In LM/LMM, there may be widespread infiltration of atypical melanocytes invading hair follicles; large, round, pagetoid melanocytes (larger than surrounding keratinocytes); sheets of large atypical cells at the dermoepidermal junction (DEJ); loss of contour in the dermal papillae; and atypical melanocytes invading the dermal papillae.2 Indeed, RCM has good correlation with the degree of histologic atypia and is useful to distinguish between benign nevi, atypical nevi, and melanoma.6 By combining lateral mosaics with vertical stacks, RCM allows 3-dimensional approximation of tumor margins and monitoring of nonsurgical therapies.7,8 The advent of handheld RCM (HRCM) has allowed assessment of large lesions as well as those presenting in difficult locations.9 Furthermore, the generation of videomosaics overcomes the limited field of view of traditional RCM and allows for accurate assessment of large lesions.10
Traditional and handheld RCM have been used to diagnose and map primary LM.1,2,11 Guitera et al2 developed an algorithm using traditional RCM to distinguish benign facial macules and LM. In their training set, they found that when their score resulted in 2 or more points, the sensitivity and specificity to diagnose LM was 85% and 76%, respectively, with an odds ratio of 18.6 for LM. They later applied the algorithm in a test set of 44 benign facial macules and 29 LM and obtained an odds ratio of 60.7 for LM, with sensitivity and specificity rates of 93% and 82%, respectively.2 This algorithm also was tested by Menge et al11 using the HRCM. They found 100% sensitivity and 71% specificity for LM when evaluating 63 equivocal facial lesions. Although these results suggest that RCM can accurately distinguish LM from benign lesions in the primary setting, few reports have studied the impact of HRCM in the recurrent setting and its impact in monitoring treatment of LM.12,13
Herein, we present 5 cases in which HRCM was used to manage complex facial LM/LMM, highlighting its versatility and potential for use in the clinical setting (eTable).

Case Series
Following institutional review board approval, cases of facial LM/LMM presenting for assessment and treatment from January 2014 to December 2015 were retrospectively reviewed. Initially, the clinical margins of the lesions were determined using Wood lamp and/or dermoscopy. Using HRCM, vertical stacks were taken at the 12-, 3-, 6-, and 9-o'clock positions, and videos were captured along the peripheral margins at the DEJ. To create videomosaics, HRCM video frames were extracted and later stitched using a computer algorithm written in a fourth-generation programming language based on prior studies.10,14 An example HRCM video that was captured and turned into a videomosaic accompanies this article online (http://bit.ly/2oDYS6k). Additional stacks were taken in suspicious areas. We considered an area positive for LM under HRCM when the LM score developed by Guitera et al2 was 2 or more. The algorithm scoring includes 2 major criteria--nonedged papillae and round large pagetoid cells--which score 2 points, and 4 minor criteria, including 3 positive criteria--atypical cells at the DEJ, follicular invasion, nucleated cells in the papillae--which each score 1 point, and 1 negative criterion--broadened honeycomb pattern--which scores -1 point.2
RELATED VIDEO: RCM Videomosaic of Melanoma In Situ
Patient 1
An 82-year-old woman was referred to us for management of an LMM on the left side of the forehead (Figure 1A). Handheld RCM from the biopsy site showed large atypical cells in the epidermis, DEJ, and papillary dermis. Superiorly, HRCM showed large dendritic processes but did not reveal LM features in 3 additional clinically worrisome areas. Biopsies showed LMM at the prior biopsy site, LM superiorly, and actinic keratosis in the remaining 3 areas, supporting the HRCM findings. Due to upstaging, the patient was referred for head and neck surgery. To aid in resection, HRCM was performed intraoperatively in a multidisciplinary approach (Figure 1B). Due to the large size of the lesion, surgical margins were taken right outside the HRCM border. Pathology showed LMM extending focally into the margins that were reexcised, achieving clearance.

Patient 2
An 88-year-old woman presented with a slightly pigmented, 2.5×2.3-cm LMM on the left cheek. Because of her age and comorbidities (eg, osteoporosis, deep vein thrombosis in both lower legs requiring anticoagulation therapy, presence of an inferior vena cava filter, bilateral lymphedema of the legs, irritable bowel syndrome, hyperparathyroidism), she was treated with imiquimod cream 5% achieving partial response. The lesion was subsequently excised showing LMM extending to the margins. Not wanting to undergo further surgery, she opted for radiation therapy. Handheld RCM was performed to guide the radiation field, showing pagetoid cells within 1 cm of the scar and clear margins beyond 2 cm. She underwent radiation therapy followed by treatment with imiquimod. On 6-month follow-up, no clinical lesion was apparent, but HRCM showed atypical cells. Biopsies revealed an atypical intraepidermal melanocytic proliferation, but due to patient's comorbidities, close observation was decided.
Patient 3
A 78-year-old man presented with an LMM on the right preauricular area. Handheld RCM demonstrated pleomorphic pagetoid cells along and beyond the clinical margins. Wide excision with sentinel lymph node biopsy was planned, and to aid surgery a confocal map was created (Figure 2). Margins were clear at 1 cm, except inferiorly where they extended to 1.5 cm. Using this preoperative HRCM map, all intraoperative sections were clear. Final pathology confirmed clear margins throughout.

Patient 4
A 62-year-old man presented with hyperpigmentation and bleeding on the left cheek where an LMM was previously removed 8 times over 18 years. Handheld RCM showed pleomorphic cells along the graft border and interestingly within the graft. Ten biopsies were taken, 8 at sites with confocal features that were worrisome for LM (Figures 3A and 3B) and 2 at clinically suspicious sites. The former revealed melanomas (2 that were invasive to 0.3 mm), and the latter revealed solar lentigines. The patient underwent staged excision guided by HRCM (Figure 3C), achieving clear histologic margins except for a focus in the helix. This area was RCM positive but was intentionally not resected due to reconstructive difficulties; imiquimod was indicated in this area.

Patient 5
An 85-year-old woman with 6 prior melanomas over 15 years presented with ill-defined light brown patches on the left cheek at the site where an LM was previously excised 15 years prior. Biopsies showed LM, and due to the patient's age, health, and personal preference to avoid extensive surgery, treatment with imiquimod cream 5% was decided. Over a period of 6 to 12 months, she developed multiple erythematous macules with 2 faintly pigmented areas. Handheld RCM demonstrated atypical cells within the papillae in previously biopsied sites that were rebiopsied, revealing LMM (Breslow depth, 0.2 mm). Staged excision achieved clear margins, but after 8 months HRCM showed LM features. Histology confirmed the diagnosis and imiquimod was reapplied.
Comment
Diagnosis and choice of treatment modality for cases of facial LM is a challenge, and there are a number of factors that may create even more of a clinical dilemma. Surgical excision is the treatment of choice for LM/LMM, and better results are achieved when using histologically controlled surgical procedures such as Mohs micrographic surgery, staged excision, or the "spaghetti technique."15-17 However, advanced patient age, multiple comorbidities (eg, coronary artery disease, deep vein thrombosis, other conditions requiring anticoagulation therapy), large lesion size in functionally or aesthetically sensitive areas, and indiscriminate borders on photodamaged skin may make surgical excision complicated or not feasible. Additionally, prior treatments to the affected area may further obscure clinical borders, complicating the diagnosis of recurrence/persistence when observed with the naked eye, dermoscopy, or Wood lamp. Because RCM can detect small amounts of melanin and has cellular resolution, it has been suggested as a great diagnostic tool to be combined with dermoscopy when evaluating lightly pigmented/amelanotic facial lesions arising on sun-damaged skin.18,19 In this case series, we highlighted these difficulties and showed how HRCM can be useful in a variety of scenarios, both pretreatment and posttreatment in complex LM/LMM cases.
Pretreatment Evaluation
Blind mapping biopsies of LM are prone to sample bias and depend greatly on biopsy technique; however, HRCM can guide mapping biopsies by detecting features of LM in vivo with high sensitivity.11 Due to the cosmetically sensitive nature of the lesions, many physicians are discouraged to do multiple mapping biopsies, making it difficult to assess the breadth of the lesion and occult invasion. Multiple studies have shown that occult invasion was not apparent until complete lesion excision was done.15,20,21 Agarwal-Antal et al20 reported 92 cases of LM, of which 16% (15/92) had unsuspected invasion on final excisional pathology. A long-standing disadvantage of treating LM with nonsurgical modalities has been the inability to detect occult invasion or multifocal invasion within the lesion. As described in patients 1, 4, and 5 in the current case series, utilizing real-time video imaging of the DEJ at the margins and within the lesion has allowed for the detection of deep atypical melanocytes suspicious for perifollicular infiltration and invasion. Knowing the depth of invasion before treatment is essential for not only counseling the patient about disease risk but also for choosing an appropriate treatment modality. Therefore, prospective studies evaluating the performance of RCM to identify invasion are crucial to improve sampling error and avoid unnecessary biopsies.
Surgical Treatment
Although surgery is the first-line treatment option for facial LM, it is not without associated morbidity, and LM is known to have histological subclinical extension, which makes margin assessment difficult. Wide surgical margins on the face are not always possible and become further complicated when trying to maintain adequate functional and cosmetic outcomes. Additionally, the margin for surgical clearance may not be straightforward for facial lesions. Hazan et al15 showed the mean total surgical margins required for excision of LM and LMM was 7.1 and 10.3 mm, respectively; of the 91 tumors initially diagnosed as LM on biopsy, 16% (15/91) had unsuspected invasion. Guitera et al2 reported that the presence of atypical cells within the dermal papillae might be a sign of invasion, which occasionally is not detected histologically due to sampling bias. Handheld RCM offers the advantage of a rapid real-time assessment in areas that may not have been amenable to previous iterations of the device, and it also provides a larger field of view that would be time consuming if performed using conventional RCM. Compared to prior RCM devices that were not handheld, the use of the HRCM does not need to attach a ring to the skin and is less bulky, permitting its use at the bedside of the patient or even intraoperatively.13 In our experience, HRCM has helped to better characterize subclinical spread of LM during the initial consultation and better counsel patients about the extent of the lesion. Handheld RCM also has been used to guide the spaghetti technique in patients with LM/LMM with good correlation between HRCM and histology.22 In our case series, HRCM was used in complex LM/LMM to delineate surgical margins, though in some cases the histologic margins were too close or affected, suggesting HRCM underestimation. Lentigo maligna margin assessment with RCM uses an algorithm that evaluates confocal features in the center of the lesion.1,2 Therefore, further studies using HRCM should evaluate minor confocal features in the margins as potential markers of positivity to accurately delineate surgical margins.
Nonsurgical Treatment Options
For patients unable or unwilling to pursue surgical treatment, therapies such as imiquimod or radiation have been suggested.23,24 However, the lack of histological confirmation and possibility for invasive spread has limited these modalities. Lentigo malignas treated with radiation have a 5% recurrence rate, with a median follow-up time of 3 years.23 Recurrence often can be difficult to detect clinically, as it may manifest as an amelanotic lesion, or postradiation changes can hinder detection. Handheld RCM allows for a cellular-level observation of the irradiated field and can identify radiation-induced changes in LM lesions, including superficial necrosis, apoptotic cells, dilated vessels, and increased inflammatory cells.25 Handheld RCM has previously been used to assess LM treated with radiation and, as in patient 2, can help define the radiation field and detect treatment failure or recurrence.12,25
Similarly, as described in patient 5, HRCM was utilized to monitor treatment with imiquimod. Many reports use imiquimod for treatment of LM, but application and response vary greatly. Reflectance confocal microscopy has been shown to be useful in monitoring LM treated with imiquimod,8 which is important because clinical findings such as inflammation and erythema do not correlate well with response to therapy. Thus, RCM is an appealing noninvasive modality to monitor response to treatment and assess the need for longer treatment duration. Moreover, similar to postradiation changes, treatment with imiquimod may cause an alteration of the clinically apparent pigment. Therefore, it is difficult to assess treatment success by clinical inspection alone. The use of RCM before, during, and after treatment provides a longitudinal assessment of the lesion and has augmented dermatologists' ability to determine treatment success or failure; however, prospective studies evaluating the usefulness of HRCM in the recurrent setting are needed to validate these results.
Limitations
Limitations of this technology include the time needed to image large areas; technology cost; and associated learning curve, which may take from 6 months to 1 year based on our experience. Others have reported the training required for accurate RCM interpretation to be less than that of dermoscopy.26 It has been shown that key RCM diagnostic criteria for lesions including melanoma and basal cell carcinoma are reproducibly recognized among RCM users and that diagnostic accuracy increases with experience.27 These limitations can be overcome with advances in videomosaicing that may streamline the imaging as well as an eventual decrease in cost with greater user adoption and the development of training platforms that enable a faster learning of RCM.28
Conclusion
The use of HRCM can help in the diagnosis and management of facial LMs. Handheld RCM provides longitudinal assessment of LM/LMM that may help determine treatment success or failure and has proven to be useful in detecting the presence of recurrence/persistence in cases that were clinically poorly evident. Moreover, HRCM is a notable ancillary tool, as it can be performed at the bedside of the patient or even intraoperatively and provides a faster approach than conventional RCM in cases where large areas need to be mapped.
In summary, HRCM may eventually be a useful screening tool to guide scouting biopsies to diagnose de novo LM; guide surgical and nonsurgical therapies; and evaluate the presence of recurrence/persistence, especially in large, complex, amelanotic or poorly pigmented lesions. A more standardized use of HRCM in mapping surgical and nonsurgical approaches needs to be evaluated in further studies to provide a fast and reliable complement to histology in such complex cases; therefore, larger studies need to be performed to validate this technique in such complex cases.
Lentigo maligna (LM) and LM melanoma (LMM) represent diagnostic and therapeutic challenges due to their heterogeneous nature and location on cosmetically sensitive areas. Newer ancillary technologies such as reflectance confocal microscopy (RCM) have helped improve diagnosis and management of these challenging lesions.1,2
Reflectance confocal microscopy is a noninvasive laser system that provides real-time imaging of the epidermis and dermis with cellular resolution and improves diagnostic accuracy of melanocytic lesions.2,3 Normal melanocytes appear as round bright structures on RCM that are similar in size to surrounding keratinocytes located in the basal layer and regularly distributed around the dermal papillae (junctional nevi) or form regular dense nests in the dermis (intradermal nevi).4,5 In LM/LMM, there may be widespread infiltration of atypical melanocytes invading hair follicles; large, round, pagetoid melanocytes (larger than surrounding keratinocytes); sheets of large atypical cells at the dermoepidermal junction (DEJ); loss of contour in the dermal papillae; and atypical melanocytes invading the dermal papillae.2 Indeed, RCM has good correlation with the degree of histologic atypia and is useful to distinguish between benign nevi, atypical nevi, and melanoma.6 By combining lateral mosaics with vertical stacks, RCM allows 3-dimensional approximation of tumor margins and monitoring of nonsurgical therapies.7,8 The advent of handheld RCM (HRCM) has allowed assessment of large lesions as well as those presenting in difficult locations.9 Furthermore, the generation of videomosaics overcomes the limited field of view of traditional RCM and allows for accurate assessment of large lesions.10
Traditional and handheld RCM have been used to diagnose and map primary LM.1,2,11 Guitera et al2 developed an algorithm using traditional RCM to distinguish benign facial macules and LM. In their training set, they found that when their score resulted in 2 or more points, the sensitivity and specificity to diagnose LM was 85% and 76%, respectively, with an odds ratio of 18.6 for LM. They later applied the algorithm in a test set of 44 benign facial macules and 29 LM and obtained an odds ratio of 60.7 for LM, with sensitivity and specificity rates of 93% and 82%, respectively.2 This algorithm also was tested by Menge et al11 using the HRCM. They found 100% sensitivity and 71% specificity for LM when evaluating 63 equivocal facial lesions. Although these results suggest that RCM can accurately distinguish LM from benign lesions in the primary setting, few reports have studied the impact of HRCM in the recurrent setting and its impact in monitoring treatment of LM.12,13
Herein, we present 5 cases in which HRCM was used to manage complex facial LM/LMM, highlighting its versatility and potential for use in the clinical setting (eTable).

Case Series
Following institutional review board approval, cases of facial LM/LMM presenting for assessment and treatment from January 2014 to December 2015 were retrospectively reviewed. Initially, the clinical margins of the lesions were determined using Wood lamp and/or dermoscopy. Using HRCM, vertical stacks were taken at the 12-, 3-, 6-, and 9-o'clock positions, and videos were captured along the peripheral margins at the DEJ. To create videomosaics, HRCM video frames were extracted and later stitched using a computer algorithm written in a fourth-generation programming language based on prior studies.10,14 An example HRCM video that was captured and turned into a videomosaic accompanies this article online (http://bit.ly/2oDYS6k). Additional stacks were taken in suspicious areas. We considered an area positive for LM under HRCM when the LM score developed by Guitera et al2 was 2 or more. The algorithm scoring includes 2 major criteria--nonedged papillae and round large pagetoid cells--which score 2 points, and 4 minor criteria, including 3 positive criteria--atypical cells at the DEJ, follicular invasion, nucleated cells in the papillae--which each score 1 point, and 1 negative criterion--broadened honeycomb pattern--which scores -1 point.2
RELATED VIDEO: RCM Videomosaic of Melanoma In Situ
Patient 1
An 82-year-old woman was referred to us for management of an LMM on the left side of the forehead (Figure 1A). Handheld RCM from the biopsy site showed large atypical cells in the epidermis, DEJ, and papillary dermis. Superiorly, HRCM showed large dendritic processes but did not reveal LM features in 3 additional clinically worrisome areas. Biopsies showed LMM at the prior biopsy site, LM superiorly, and actinic keratosis in the remaining 3 areas, supporting the HRCM findings. Due to upstaging, the patient was referred for head and neck surgery. To aid in resection, HRCM was performed intraoperatively in a multidisciplinary approach (Figure 1B). Due to the large size of the lesion, surgical margins were taken right outside the HRCM border. Pathology showed LMM extending focally into the margins that were reexcised, achieving clearance.

Patient 2
An 88-year-old woman presented with a slightly pigmented, 2.5×2.3-cm LMM on the left cheek. Because of her age and comorbidities (eg, osteoporosis, deep vein thrombosis in both lower legs requiring anticoagulation therapy, presence of an inferior vena cava filter, bilateral lymphedema of the legs, irritable bowel syndrome, hyperparathyroidism), she was treated with imiquimod cream 5% achieving partial response. The lesion was subsequently excised showing LMM extending to the margins. Not wanting to undergo further surgery, she opted for radiation therapy. Handheld RCM was performed to guide the radiation field, showing pagetoid cells within 1 cm of the scar and clear margins beyond 2 cm. She underwent radiation therapy followed by treatment with imiquimod. On 6-month follow-up, no clinical lesion was apparent, but HRCM showed atypical cells. Biopsies revealed an atypical intraepidermal melanocytic proliferation, but due to patient's comorbidities, close observation was decided.
Patient 3
A 78-year-old man presented with an LMM on the right preauricular area. Handheld RCM demonstrated pleomorphic pagetoid cells along and beyond the clinical margins. Wide excision with sentinel lymph node biopsy was planned, and to aid surgery a confocal map was created (Figure 2). Margins were clear at 1 cm, except inferiorly where they extended to 1.5 cm. Using this preoperative HRCM map, all intraoperative sections were clear. Final pathology confirmed clear margins throughout.

Patient 4
A 62-year-old man presented with hyperpigmentation and bleeding on the left cheek where an LMM was previously removed 8 times over 18 years. Handheld RCM showed pleomorphic cells along the graft border and interestingly within the graft. Ten biopsies were taken, 8 at sites with confocal features that were worrisome for LM (Figures 3A and 3B) and 2 at clinically suspicious sites. The former revealed melanomas (2 that were invasive to 0.3 mm), and the latter revealed solar lentigines. The patient underwent staged excision guided by HRCM (Figure 3C), achieving clear histologic margins except for a focus in the helix. This area was RCM positive but was intentionally not resected due to reconstructive difficulties; imiquimod was indicated in this area.

Patient 5
An 85-year-old woman with 6 prior melanomas over 15 years presented with ill-defined light brown patches on the left cheek at the site where an LM was previously excised 15 years prior. Biopsies showed LM, and due to the patient's age, health, and personal preference to avoid extensive surgery, treatment with imiquimod cream 5% was decided. Over a period of 6 to 12 months, she developed multiple erythematous macules with 2 faintly pigmented areas. Handheld RCM demonstrated atypical cells within the papillae in previously biopsied sites that were rebiopsied, revealing LMM (Breslow depth, 0.2 mm). Staged excision achieved clear margins, but after 8 months HRCM showed LM features. Histology confirmed the diagnosis and imiquimod was reapplied.
Comment
Diagnosis and choice of treatment modality for cases of facial LM is a challenge, and there are a number of factors that may create even more of a clinical dilemma. Surgical excision is the treatment of choice for LM/LMM, and better results are achieved when using histologically controlled surgical procedures such as Mohs micrographic surgery, staged excision, or the "spaghetti technique."15-17 However, advanced patient age, multiple comorbidities (eg, coronary artery disease, deep vein thrombosis, other conditions requiring anticoagulation therapy), large lesion size in functionally or aesthetically sensitive areas, and indiscriminate borders on photodamaged skin may make surgical excision complicated or not feasible. Additionally, prior treatments to the affected area may further obscure clinical borders, complicating the diagnosis of recurrence/persistence when observed with the naked eye, dermoscopy, or Wood lamp. Because RCM can detect small amounts of melanin and has cellular resolution, it has been suggested as a great diagnostic tool to be combined with dermoscopy when evaluating lightly pigmented/amelanotic facial lesions arising on sun-damaged skin.18,19 In this case series, we highlighted these difficulties and showed how HRCM can be useful in a variety of scenarios, both pretreatment and posttreatment in complex LM/LMM cases.
Pretreatment Evaluation
Blind mapping biopsies of LM are prone to sample bias and depend greatly on biopsy technique; however, HRCM can guide mapping biopsies by detecting features of LM in vivo with high sensitivity.11 Due to the cosmetically sensitive nature of the lesions, many physicians are discouraged to do multiple mapping biopsies, making it difficult to assess the breadth of the lesion and occult invasion. Multiple studies have shown that occult invasion was not apparent until complete lesion excision was done.15,20,21 Agarwal-Antal et al20 reported 92 cases of LM, of which 16% (15/92) had unsuspected invasion on final excisional pathology. A long-standing disadvantage of treating LM with nonsurgical modalities has been the inability to detect occult invasion or multifocal invasion within the lesion. As described in patients 1, 4, and 5 in the current case series, utilizing real-time video imaging of the DEJ at the margins and within the lesion has allowed for the detection of deep atypical melanocytes suspicious for perifollicular infiltration and invasion. Knowing the depth of invasion before treatment is essential for not only counseling the patient about disease risk but also for choosing an appropriate treatment modality. Therefore, prospective studies evaluating the performance of RCM to identify invasion are crucial to improve sampling error and avoid unnecessary biopsies.
Surgical Treatment
Although surgery is the first-line treatment option for facial LM, it is not without associated morbidity, and LM is known to have histological subclinical extension, which makes margin assessment difficult. Wide surgical margins on the face are not always possible and become further complicated when trying to maintain adequate functional and cosmetic outcomes. Additionally, the margin for surgical clearance may not be straightforward for facial lesions. Hazan et al15 showed the mean total surgical margins required for excision of LM and LMM was 7.1 and 10.3 mm, respectively; of the 91 tumors initially diagnosed as LM on biopsy, 16% (15/91) had unsuspected invasion. Guitera et al2 reported that the presence of atypical cells within the dermal papillae might be a sign of invasion, which occasionally is not detected histologically due to sampling bias. Handheld RCM offers the advantage of a rapid real-time assessment in areas that may not have been amenable to previous iterations of the device, and it also provides a larger field of view that would be time consuming if performed using conventional RCM. Compared to prior RCM devices that were not handheld, the use of the HRCM does not need to attach a ring to the skin and is less bulky, permitting its use at the bedside of the patient or even intraoperatively.13 In our experience, HRCM has helped to better characterize subclinical spread of LM during the initial consultation and better counsel patients about the extent of the lesion. Handheld RCM also has been used to guide the spaghetti technique in patients with LM/LMM with good correlation between HRCM and histology.22 In our case series, HRCM was used in complex LM/LMM to delineate surgical margins, though in some cases the histologic margins were too close or affected, suggesting HRCM underestimation. Lentigo maligna margin assessment with RCM uses an algorithm that evaluates confocal features in the center of the lesion.1,2 Therefore, further studies using HRCM should evaluate minor confocal features in the margins as potential markers of positivity to accurately delineate surgical margins.
Nonsurgical Treatment Options
For patients unable or unwilling to pursue surgical treatment, therapies such as imiquimod or radiation have been suggested.23,24 However, the lack of histological confirmation and possibility for invasive spread has limited these modalities. Lentigo malignas treated with radiation have a 5% recurrence rate, with a median follow-up time of 3 years.23 Recurrence often can be difficult to detect clinically, as it may manifest as an amelanotic lesion, or postradiation changes can hinder detection. Handheld RCM allows for a cellular-level observation of the irradiated field and can identify radiation-induced changes in LM lesions, including superficial necrosis, apoptotic cells, dilated vessels, and increased inflammatory cells.25 Handheld RCM has previously been used to assess LM treated with radiation and, as in patient 2, can help define the radiation field and detect treatment failure or recurrence.12,25
Similarly, as described in patient 5, HRCM was utilized to monitor treatment with imiquimod. Many reports use imiquimod for treatment of LM, but application and response vary greatly. Reflectance confocal microscopy has been shown to be useful in monitoring LM treated with imiquimod,8 which is important because clinical findings such as inflammation and erythema do not correlate well with response to therapy. Thus, RCM is an appealing noninvasive modality to monitor response to treatment and assess the need for longer treatment duration. Moreover, similar to postradiation changes, treatment with imiquimod may cause an alteration of the clinically apparent pigment. Therefore, it is difficult to assess treatment success by clinical inspection alone. The use of RCM before, during, and after treatment provides a longitudinal assessment of the lesion and has augmented dermatologists' ability to determine treatment success or failure; however, prospective studies evaluating the usefulness of HRCM in the recurrent setting are needed to validate these results.
Limitations
Limitations of this technology include the time needed to image large areas; technology cost; and associated learning curve, which may take from 6 months to 1 year based on our experience. Others have reported the training required for accurate RCM interpretation to be less than that of dermoscopy.26 It has been shown that key RCM diagnostic criteria for lesions including melanoma and basal cell carcinoma are reproducibly recognized among RCM users and that diagnostic accuracy increases with experience.27 These limitations can be overcome with advances in videomosaicing that may streamline the imaging as well as an eventual decrease in cost with greater user adoption and the development of training platforms that enable a faster learning of RCM.28
Conclusion
The use of HRCM can help in the diagnosis and management of facial LMs. Handheld RCM provides longitudinal assessment of LM/LMM that may help determine treatment success or failure and has proven to be useful in detecting the presence of recurrence/persistence in cases that were clinically poorly evident. Moreover, HRCM is a notable ancillary tool, as it can be performed at the bedside of the patient or even intraoperatively and provides a faster approach than conventional RCM in cases where large areas need to be mapped.
In summary, HRCM may eventually be a useful screening tool to guide scouting biopsies to diagnose de novo LM; guide surgical and nonsurgical therapies; and evaluate the presence of recurrence/persistence, especially in large, complex, amelanotic or poorly pigmented lesions. A more standardized use of HRCM in mapping surgical and nonsurgical approaches needs to be evaluated in further studies to provide a fast and reliable complement to histology in such complex cases; therefore, larger studies need to be performed to validate this technique in such complex cases.
- Guitera P, Moloney FJ, Menzies SW, et al. Improving management and patient care in lentigo maligna by mapping with in vivo confocal microscopy. JAMA Dermatol. 2013;149:692-698.
- Guitera P, Pellacani G, Crotty KA, et al. The impact of in vivo reflectance confocal microscopy on the diagnostic accuracy of lentigo maligna and equivocal pigmented and nonpigmented macules of the face. J Invest Dermatol. 2010;130:2080-2091.
- Pellacani G, Guitera P, Longo C, et al. The impact of in vivo reflectance confocal microscopy for the diagnostic accuracy of melanoma and equivocal melanocytic lesions. J Invest Dermatol. 2007;127:2759-2765.
- Segura S, Puig S, Carrera C, et al. Development of a two-step method for the diagnosis of melanoma by reflectance confocal microscopy. J Am Acad Dermatol. 2009;61:216-229.
- Hofmann-Wellenhof R, Pellacani G, Malvehy J, et al. Reflectance Confocal Microscopy for Skin Diseases. New York, NY: Springer; 2012.
- Pellacani G, Farnetani F, Gonzalez S, et al. In vivo confocal microscopy for detection and grading of dysplastic nevi: a pilot study. J Am Acad Dermatol. 2012;66:E109-E121.
- Nadiminti H, Scope A, Marghoob AA, et al. Use of reflectance confocal microscopy to monitor response of lentigo maligna to nonsurgical treatment. Dermatol Surg. 2010;36:177-184.
- Alarcon I, Carrera C, Alos L, et al. In vivo reflectance confocal microscopy to monitor the response of lentigo maligna to imiquimod. J Am Acad Dermatol. 2014;71:49-55.
- Fraga-Braghiroli NA, Stephens A, Grossman D, et al. Use of handheld reflectance confocal microscopy for in vivo diagnosis of solitary facial papules: a case series. J Eur Acad Dermatol Venereol. 2014;28:933-942.
- Kose K, Cordova M, Duffy M, et al. Video-mosaicing of reflectance confocal images for examination of extended areas of skin in vivo. Br J Dermatol. 2014;171:1239-1241.
- Menge TD, Hibler BP, Cordova MA, et al. Concordance of handheld reflectance confocal microscopy (RCM) with histopathology in the diagnosis of lentigo maligna (LM): a prospective study [published online January 27, 2016]. J Am Acad Dermatol. 2016;74:1114-1120.
- Hibler BP, Connolly KL, Cordova M, et al. Radiation therapy for synchronous basal cell carcinoma and lentigo maligna of the nose: response assessment by clinical examination and reflectance confocal microscopy. Pract Radiat Oncol. 2015;5:E543-E547.
- Hibler BP, Cordova M, Wong RJ, et al. Intraoperative real-time reflectance confocal microscopy for guiding surgical margins of lentigo maligna melanoma. Dermatol Surg. 2015;41:980-983.
- Kose K, Gou M, Yelamos O, et al. Video-mosaicking of in vivo reflectance confocal microscopy images for noninvasive examination of skin lesions [published February 6, 2017]. Proceedings of SPIE Photonics West. doi:10.1117/12.2253085.
- Hazan C, Dusza SW, Delgado R, et al. Staged excision for lentigo maligna and lentigo maligna melanoma: a retrospective analysis of 117 cases. J Am Acad Dermatol. 2008;58:142-148.
- Etzkorn JR, Sobanko JF, Elenitsas R, et al. Low recurrence rates for in situ and invasive melanomas using Mohs micrographic surgery with melanoma antigen recognized by T cells 1 (MART-1) immunostaining: tissue processing methodology to optimize pathologic staging and margin assessment. J Am Acad Dermatol. 2015;72:840-850.
- Gaudy-Marqueste C, Perchenet AS, Tasei AM, et al. The "spaghetti technique": an alternative to Mohs surgery or staged surgery for problematic lentiginous melanoma (lentigo maligna and acral lentiginous melanoma). J Am Acad Dermatol. 2011;64:113-118.
- Guitera P, Menzies SW, Argenziano G, et al. Dermoscopy and in vivo confocal microscopy are complementary techniques for diagnosis of difficult amelanotic and light-coloured skin lesions [published online October 12, 2016]. Br J Dermatol. 2016;175:1311-1319.
- Borsari S, Pampena R, Lallas A, et al. Clinical indications for use of reflectance confocal microscopy for skin cancer diagnosis. JAMA Dermatol. 2016;152:1093-1098.
- Agarwal-Antal N, Bowen GM, Gerwels JW. Histologic evaluation of lentigo maligna with permanent sections: implications regarding current guidelines. J Am Acad Dermatol. 2002;47:743-748.
- Gardner KH, Hill DE, Wright AC, et al. Upstaging from melanoma in situ to invasive melanoma on the head and neck after complete surgical resection. Dermatol Surg. 2015;41:1122-1125.
- Champin J, Perrot JL, Cinotti E, et al. In vivo reflectance confocal microscopy to optimize the spaghetti technique for defining surgical margins of lentigo maligna. Dermatolog Surg. 2014;40:247-256.
- Fogarty GB, Hong A, Scolyer RA, et al. Radiotherapy for lentigo maligna: a literature review and recommendations for treatment. Br J Dermatol. 2014;170:52-58.
- Swetter SM, Chen FW, Kim DD, et al. Imiquimod 5% cream as primary or adjuvant therapy for melanoma in situ, lentigo maligna type. J Am Acad Dermatol. 2015;72:1047-1053.
- Richtig E, Arzberger E, Hofmann-Wellenhof R, et al. Assessment of changes in lentigo maligna during radiotherapy by in-vivo reflectance confocal microscopy--a pilot study. Br J Dermatol. 2015;172:81-87.
- Gerger A, Koller S, Kern T, et al. Diagnostic applicability of in vivo confocal laser scanning microscopy in melanocytic skin tumors. J Invest Dermatol. 2005;124:493-498.
- Farnetani F, Scope A, Braun RP, et al. Skin cancer diagnosis with reflectance confocal microscopy: reproducibility of feature recognition and accuracy of diagnosis. JAMA Dermatol. 2015;151:1075-1080.
- Rajadhyaksha M, Marghoob A, Rossi A, et al. Reflectance confocal microscopy of skin in vivo: from bench to bedside [published online October 27, 2016]. Lasers Surg Med. 2017;49:7-19.
- Guitera P, Moloney FJ, Menzies SW, et al. Improving management and patient care in lentigo maligna by mapping with in vivo confocal microscopy. JAMA Dermatol. 2013;149:692-698.
- Guitera P, Pellacani G, Crotty KA, et al. The impact of in vivo reflectance confocal microscopy on the diagnostic accuracy of lentigo maligna and equivocal pigmented and nonpigmented macules of the face. J Invest Dermatol. 2010;130:2080-2091.
- Pellacani G, Guitera P, Longo C, et al. The impact of in vivo reflectance confocal microscopy for the diagnostic accuracy of melanoma and equivocal melanocytic lesions. J Invest Dermatol. 2007;127:2759-2765.
- Segura S, Puig S, Carrera C, et al. Development of a two-step method for the diagnosis of melanoma by reflectance confocal microscopy. J Am Acad Dermatol. 2009;61:216-229.
- Hofmann-Wellenhof R, Pellacani G, Malvehy J, et al. Reflectance Confocal Microscopy for Skin Diseases. New York, NY: Springer; 2012.
- Pellacani G, Farnetani F, Gonzalez S, et al. In vivo confocal microscopy for detection and grading of dysplastic nevi: a pilot study. J Am Acad Dermatol. 2012;66:E109-E121.
- Nadiminti H, Scope A, Marghoob AA, et al. Use of reflectance confocal microscopy to monitor response of lentigo maligna to nonsurgical treatment. Dermatol Surg. 2010;36:177-184.
- Alarcon I, Carrera C, Alos L, et al. In vivo reflectance confocal microscopy to monitor the response of lentigo maligna to imiquimod. J Am Acad Dermatol. 2014;71:49-55.
- Fraga-Braghiroli NA, Stephens A, Grossman D, et al. Use of handheld reflectance confocal microscopy for in vivo diagnosis of solitary facial papules: a case series. J Eur Acad Dermatol Venereol. 2014;28:933-942.
- Kose K, Cordova M, Duffy M, et al. Video-mosaicing of reflectance confocal images for examination of extended areas of skin in vivo. Br J Dermatol. 2014;171:1239-1241.
- Menge TD, Hibler BP, Cordova MA, et al. Concordance of handheld reflectance confocal microscopy (RCM) with histopathology in the diagnosis of lentigo maligna (LM): a prospective study [published online January 27, 2016]. J Am Acad Dermatol. 2016;74:1114-1120.
- Hibler BP, Connolly KL, Cordova M, et al. Radiation therapy for synchronous basal cell carcinoma and lentigo maligna of the nose: response assessment by clinical examination and reflectance confocal microscopy. Pract Radiat Oncol. 2015;5:E543-E547.
- Hibler BP, Cordova M, Wong RJ, et al. Intraoperative real-time reflectance confocal microscopy for guiding surgical margins of lentigo maligna melanoma. Dermatol Surg. 2015;41:980-983.
- Kose K, Gou M, Yelamos O, et al. Video-mosaicking of in vivo reflectance confocal microscopy images for noninvasive examination of skin lesions [published February 6, 2017]. Proceedings of SPIE Photonics West. doi:10.1117/12.2253085.
- Hazan C, Dusza SW, Delgado R, et al. Staged excision for lentigo maligna and lentigo maligna melanoma: a retrospective analysis of 117 cases. J Am Acad Dermatol. 2008;58:142-148.
- Etzkorn JR, Sobanko JF, Elenitsas R, et al. Low recurrence rates for in situ and invasive melanomas using Mohs micrographic surgery with melanoma antigen recognized by T cells 1 (MART-1) immunostaining: tissue processing methodology to optimize pathologic staging and margin assessment. J Am Acad Dermatol. 2015;72:840-850.
- Gaudy-Marqueste C, Perchenet AS, Tasei AM, et al. The "spaghetti technique": an alternative to Mohs surgery or staged surgery for problematic lentiginous melanoma (lentigo maligna and acral lentiginous melanoma). J Am Acad Dermatol. 2011;64:113-118.
- Guitera P, Menzies SW, Argenziano G, et al. Dermoscopy and in vivo confocal microscopy are complementary techniques for diagnosis of difficult amelanotic and light-coloured skin lesions [published online October 12, 2016]. Br J Dermatol. 2016;175:1311-1319.
- Borsari S, Pampena R, Lallas A, et al. Clinical indications for use of reflectance confocal microscopy for skin cancer diagnosis. JAMA Dermatol. 2016;152:1093-1098.
- Agarwal-Antal N, Bowen GM, Gerwels JW. Histologic evaluation of lentigo maligna with permanent sections: implications regarding current guidelines. J Am Acad Dermatol. 2002;47:743-748.
- Gardner KH, Hill DE, Wright AC, et al. Upstaging from melanoma in situ to invasive melanoma on the head and neck after complete surgical resection. Dermatol Surg. 2015;41:1122-1125.
- Champin J, Perrot JL, Cinotti E, et al. In vivo reflectance confocal microscopy to optimize the spaghetti technique for defining surgical margins of lentigo maligna. Dermatolog Surg. 2014;40:247-256.
- Fogarty GB, Hong A, Scolyer RA, et al. Radiotherapy for lentigo maligna: a literature review and recommendations for treatment. Br J Dermatol. 2014;170:52-58.
- Swetter SM, Chen FW, Kim DD, et al. Imiquimod 5% cream as primary or adjuvant therapy for melanoma in situ, lentigo maligna type. J Am Acad Dermatol. 2015;72:1047-1053.
- Richtig E, Arzberger E, Hofmann-Wellenhof R, et al. Assessment of changes in lentigo maligna during radiotherapy by in-vivo reflectance confocal microscopy--a pilot study. Br J Dermatol. 2015;172:81-87.
- Gerger A, Koller S, Kern T, et al. Diagnostic applicability of in vivo confocal laser scanning microscopy in melanocytic skin tumors. J Invest Dermatol. 2005;124:493-498.
- Farnetani F, Scope A, Braun RP, et al. Skin cancer diagnosis with reflectance confocal microscopy: reproducibility of feature recognition and accuracy of diagnosis. JAMA Dermatol. 2015;151:1075-1080.
- Rajadhyaksha M, Marghoob A, Rossi A, et al. Reflectance confocal microscopy of skin in vivo: from bench to bedside [published online October 27, 2016]. Lasers Surg Med. 2017;49:7-19.
Practice Points
- Diagnosis and management of lentigo maligna (LM) and LM melanoma (LMM) is challenging due to their ill-defined margins and location mainly on the head and neck.
- Handheld reflectance confocal microscopy (RCM) has high diagnostic accuracy for LM/LMM and can be used in curved locations to assess large lesions.
- Handheld RCM can be a versatile tool in pretreatment decision-making, intraoperative surgical mapping, and posttreatment monitoring of both surgical and nonsurgical therapies for complex facial LM/LMM.
5-Fluorouracil failed four separate measures of photoaging
PORTLAND – A standard course of topical 5-fluorouracil (5-FU) does not noticeably improve visual signs of facial photoaging, such as forehead lines and crow’s feet, according to the results of a blinded, controlled study of 281 elderly white men.
Four validated photonumeric measures revealed no statistically significant differences between the intervention and vehicle control arms at 6, 12, or 18 months’ follow-up, Kaveri Korgavkar, MD, said at the annual meeting of the Society for Investigative Dermatology. “This might be a true lack of impact, or current scales may not be sensitive enough to capture aspects of aging that are improved by 5-fluorouracil,” commented Dr. Korgavkar, who presented the findings on behalf of the VAKCCT (the Veterans Affairs Keratinocyte Carcinoma Chemoprevention Trial) work group.
The treatment and control groups resembled each other demographically and clinically at baseline. Participants averaged 71.5 years of age (standard deviation, 0.57 years), 97% were male, 99% were white, and all had clinically meaningful histories of sun damage with at least two keratinocyte carcinomas in the previous 2 years, including at least one lesion on the face or ears. Previously, the VAKCCT investigators reported positive results for 5-FU as a chemopreventive – for example, it was associated with about a 60% reduction in actinic keratoses, compared with placebo, and the effects persisted for up to 3 years.
However, none of the four photonumeric scales of photoaging uncovered significant differences between the treatment and control groups at 6, 12, or 18 months’ follow-up, Dr. Korgavkar reported. That finding belies the results of two other previous studies, but they were small and uncontrolled, she added. One study of 19 patients reported statistically significant improvements over time in wrinkling, hyperpigmentation, lentigines, and sallowness based on the Griffith’s scale, while a second prospective study of 32 patients reported significant improvements in visual signs of photoaging on the forearms, with a corresponding rise in levels of procollagen 1 and a decrease in dermal elastosis at 1 month.
Existing scales might more effectively capture some aspects of photoaging – such as wrinkles or crow’s feet – than others, Dr. Korgavkar said in an interview. Therefore, she and her associates are working to construct more sensitive and comprehensive visual scales of photoaging, she said.
The VAKCCT was sponsored by the VA Office of Research and Development. Dr. Korgavkar had no conflicts of interest.
PORTLAND – A standard course of topical 5-fluorouracil (5-FU) does not noticeably improve visual signs of facial photoaging, such as forehead lines and crow’s feet, according to the results of a blinded, controlled study of 281 elderly white men.
Four validated photonumeric measures revealed no statistically significant differences between the intervention and vehicle control arms at 6, 12, or 18 months’ follow-up, Kaveri Korgavkar, MD, said at the annual meeting of the Society for Investigative Dermatology. “This might be a true lack of impact, or current scales may not be sensitive enough to capture aspects of aging that are improved by 5-fluorouracil,” commented Dr. Korgavkar, who presented the findings on behalf of the VAKCCT (the Veterans Affairs Keratinocyte Carcinoma Chemoprevention Trial) work group.
The treatment and control groups resembled each other demographically and clinically at baseline. Participants averaged 71.5 years of age (standard deviation, 0.57 years), 97% were male, 99% were white, and all had clinically meaningful histories of sun damage with at least two keratinocyte carcinomas in the previous 2 years, including at least one lesion on the face or ears. Previously, the VAKCCT investigators reported positive results for 5-FU as a chemopreventive – for example, it was associated with about a 60% reduction in actinic keratoses, compared with placebo, and the effects persisted for up to 3 years.
However, none of the four photonumeric scales of photoaging uncovered significant differences between the treatment and control groups at 6, 12, or 18 months’ follow-up, Dr. Korgavkar reported. That finding belies the results of two other previous studies, but they were small and uncontrolled, she added. One study of 19 patients reported statistically significant improvements over time in wrinkling, hyperpigmentation, lentigines, and sallowness based on the Griffith’s scale, while a second prospective study of 32 patients reported significant improvements in visual signs of photoaging on the forearms, with a corresponding rise in levels of procollagen 1 and a decrease in dermal elastosis at 1 month.
Existing scales might more effectively capture some aspects of photoaging – such as wrinkles or crow’s feet – than others, Dr. Korgavkar said in an interview. Therefore, she and her associates are working to construct more sensitive and comprehensive visual scales of photoaging, she said.
The VAKCCT was sponsored by the VA Office of Research and Development. Dr. Korgavkar had no conflicts of interest.
PORTLAND – A standard course of topical 5-fluorouracil (5-FU) does not noticeably improve visual signs of facial photoaging, such as forehead lines and crow’s feet, according to the results of a blinded, controlled study of 281 elderly white men.
Four validated photonumeric measures revealed no statistically significant differences between the intervention and vehicle control arms at 6, 12, or 18 months’ follow-up, Kaveri Korgavkar, MD, said at the annual meeting of the Society for Investigative Dermatology. “This might be a true lack of impact, or current scales may not be sensitive enough to capture aspects of aging that are improved by 5-fluorouracil,” commented Dr. Korgavkar, who presented the findings on behalf of the VAKCCT (the Veterans Affairs Keratinocyte Carcinoma Chemoprevention Trial) work group.
The treatment and control groups resembled each other demographically and clinically at baseline. Participants averaged 71.5 years of age (standard deviation, 0.57 years), 97% were male, 99% were white, and all had clinically meaningful histories of sun damage with at least two keratinocyte carcinomas in the previous 2 years, including at least one lesion on the face or ears. Previously, the VAKCCT investigators reported positive results for 5-FU as a chemopreventive – for example, it was associated with about a 60% reduction in actinic keratoses, compared with placebo, and the effects persisted for up to 3 years.
However, none of the four photonumeric scales of photoaging uncovered significant differences between the treatment and control groups at 6, 12, or 18 months’ follow-up, Dr. Korgavkar reported. That finding belies the results of two other previous studies, but they were small and uncontrolled, she added. One study of 19 patients reported statistically significant improvements over time in wrinkling, hyperpigmentation, lentigines, and sallowness based on the Griffith’s scale, while a second prospective study of 32 patients reported significant improvements in visual signs of photoaging on the forearms, with a corresponding rise in levels of procollagen 1 and a decrease in dermal elastosis at 1 month.
Existing scales might more effectively capture some aspects of photoaging – such as wrinkles or crow’s feet – than others, Dr. Korgavkar said in an interview. Therefore, she and her associates are working to construct more sensitive and comprehensive visual scales of photoaging, she said.
The VAKCCT was sponsored by the VA Office of Research and Development. Dr. Korgavkar had no conflicts of interest.
AT SID 2017
Key clinical point: A standard topical course of 5-fluorouracil did not noticeably improve visual signs of photoaging, such as forehead lines and crow’s feet.
Major finding: Four validated photonumeric measures of photoaging revealed no statistically significant differences between the intervention and the vehicle control at 6, 12, or 18 months’ follow-up.
Data source: An analysis of data from 281 participants in the Veterans Affairs Keratinocyte Carcinoma Chemoprevention trial (VAKCCT).
Disclosures: The VAKCCT was sponsored by the VA Office of Research and Development. Dr. Korgavkar had no conflicts of interest.
Reflectance confocal microscopy offers one-stop solution for BCC
For selected patients with basal cell carcinoma, one-stop shopping – diagnosing, subtyping, and excising the lesion all in one visit – using reflectance confocal microscopy was found noninferior to the standard approach of obtaining a punch biopsy to diagnose and subtype the lesion in one visit and performing surgical excision in a separate visit.
Those were the findings of an open-label, randomized, noninferiority trial in the Netherlands comparing the two approaches in 95 adults with suspected basal cell carcinoma (BCC), investigators reported.
In addition to reducing the number of visits and the total time required for treatment, this new approach uses noninvasive reflectance confocal microscopy in the place of punch biopsy, which patients will likely prefer, said Daniel J. Kadouch, MD, of the department of dermatology, Academic Medical Center, Amsterdam, and his associates (British J Derm. 2017 Apr 9. doi: 10.1111/bjd.15559).
The study excluded patients who had lesions in a high-risk location of the face, lesions larger than 20 mm, recurrent lesions, and macroscopic ulcerating lesions, as well as patients who had basal cell nevus syndrome. Another 22 patients who were found to have non-BCC lesions (1 melanoma, 2 squamous cell carcinomas, 5 cases of Bowen’s disease, and 11 nonmalignant lesions) also were excluded, leaving 40 patients with BCC in the one-stop shopping group and 33 in the standard of care (control) group.
The primary outcome – the proportion of patients with tumor-free margins on the final pathology report after surgical excision – was 100% (40 of 40) in the one-stop shopping group and 94% (31 of 33) in the control group, which demonstrates the noninferiority of the new, less invasive approach, Dr. Kadouch and his associates said.
The mean total treatment time was 2 hours and 23 minutes for the one-stop shopping group. The total treatment time could not be determined for the control group because their surgical times weren’t recorded.
Adverse events included four postoperative wound infections in the one-stop shopping group, all of which were successfully treated with oral antibiotics, and one case of excessive postoperative bleeding in the control group, which required 3 days of hospitalization.
This study was limited in that it excluded patients with large lesions and those with BCC on high-risk areas of the face, which reduces the generalizability of the findings. In addition, a follow-up time of at least 1 year would be needed to detect signs of BCC recurrence in the study participants, the investigators said.
For selected patients with basal cell carcinoma, one-stop shopping – diagnosing, subtyping, and excising the lesion all in one visit – using reflectance confocal microscopy was found noninferior to the standard approach of obtaining a punch biopsy to diagnose and subtype the lesion in one visit and performing surgical excision in a separate visit.
Those were the findings of an open-label, randomized, noninferiority trial in the Netherlands comparing the two approaches in 95 adults with suspected basal cell carcinoma (BCC), investigators reported.
In addition to reducing the number of visits and the total time required for treatment, this new approach uses noninvasive reflectance confocal microscopy in the place of punch biopsy, which patients will likely prefer, said Daniel J. Kadouch, MD, of the department of dermatology, Academic Medical Center, Amsterdam, and his associates (British J Derm. 2017 Apr 9. doi: 10.1111/bjd.15559).
The study excluded patients who had lesions in a high-risk location of the face, lesions larger than 20 mm, recurrent lesions, and macroscopic ulcerating lesions, as well as patients who had basal cell nevus syndrome. Another 22 patients who were found to have non-BCC lesions (1 melanoma, 2 squamous cell carcinomas, 5 cases of Bowen’s disease, and 11 nonmalignant lesions) also were excluded, leaving 40 patients with BCC in the one-stop shopping group and 33 in the standard of care (control) group.
The primary outcome – the proportion of patients with tumor-free margins on the final pathology report after surgical excision – was 100% (40 of 40) in the one-stop shopping group and 94% (31 of 33) in the control group, which demonstrates the noninferiority of the new, less invasive approach, Dr. Kadouch and his associates said.
The mean total treatment time was 2 hours and 23 minutes for the one-stop shopping group. The total treatment time could not be determined for the control group because their surgical times weren’t recorded.
Adverse events included four postoperative wound infections in the one-stop shopping group, all of which were successfully treated with oral antibiotics, and one case of excessive postoperative bleeding in the control group, which required 3 days of hospitalization.
This study was limited in that it excluded patients with large lesions and those with BCC on high-risk areas of the face, which reduces the generalizability of the findings. In addition, a follow-up time of at least 1 year would be needed to detect signs of BCC recurrence in the study participants, the investigators said.
For selected patients with basal cell carcinoma, one-stop shopping – diagnosing, subtyping, and excising the lesion all in one visit – using reflectance confocal microscopy was found noninferior to the standard approach of obtaining a punch biopsy to diagnose and subtype the lesion in one visit and performing surgical excision in a separate visit.
Those were the findings of an open-label, randomized, noninferiority trial in the Netherlands comparing the two approaches in 95 adults with suspected basal cell carcinoma (BCC), investigators reported.
In addition to reducing the number of visits and the total time required for treatment, this new approach uses noninvasive reflectance confocal microscopy in the place of punch biopsy, which patients will likely prefer, said Daniel J. Kadouch, MD, of the department of dermatology, Academic Medical Center, Amsterdam, and his associates (British J Derm. 2017 Apr 9. doi: 10.1111/bjd.15559).
The study excluded patients who had lesions in a high-risk location of the face, lesions larger than 20 mm, recurrent lesions, and macroscopic ulcerating lesions, as well as patients who had basal cell nevus syndrome. Another 22 patients who were found to have non-BCC lesions (1 melanoma, 2 squamous cell carcinomas, 5 cases of Bowen’s disease, and 11 nonmalignant lesions) also were excluded, leaving 40 patients with BCC in the one-stop shopping group and 33 in the standard of care (control) group.
The primary outcome – the proportion of patients with tumor-free margins on the final pathology report after surgical excision – was 100% (40 of 40) in the one-stop shopping group and 94% (31 of 33) in the control group, which demonstrates the noninferiority of the new, less invasive approach, Dr. Kadouch and his associates said.
The mean total treatment time was 2 hours and 23 minutes for the one-stop shopping group. The total treatment time could not be determined for the control group because their surgical times weren’t recorded.
Adverse events included four postoperative wound infections in the one-stop shopping group, all of which were successfully treated with oral antibiotics, and one case of excessive postoperative bleeding in the control group, which required 3 days of hospitalization.
This study was limited in that it excluded patients with large lesions and those with BCC on high-risk areas of the face, which reduces the generalizability of the findings. In addition, a follow-up time of at least 1 year would be needed to detect signs of BCC recurrence in the study participants, the investigators said.
FROM THE BRITISH JOURNAL OF DERMATOLOGY
Key clinical point: For selected patients with BCC, one-stop shopping – diagnosing, subtyping, and excising the lesion all in one visit – using reflectance confocal microscopy was found noninferior to the standard approach using punch biopsy.
Major finding: The percentage of patients with tumor-free margins after surgical excision was 100% (40 of 40) in the one-stop-shopping group and 94% (31 of 33) in the control group.
Data source: An open-label, randomized, controlled, noninferiority trial involving 95 adults.
Disclosures: The study received no outside funding. Dr. Kadouch and his associates reported having no relevant financial disclosures.
Flesh-Colored Nodule With Underlying Sclerotic Plaque
The Diagnosis: Collision Tumor
Excisional biopsy and histopathological examination demonstrated a collision tumor composed of a benign intradermal melanocytic nevus, tumor of follicular infundibulum, and an underlying sclerosing epithelial neoplasm, with a differential diagnosis of desmoplastic trichoepithelioma, morpheaform basal cell carcinoma, and microcystic adnexal carcinoma (Figure).
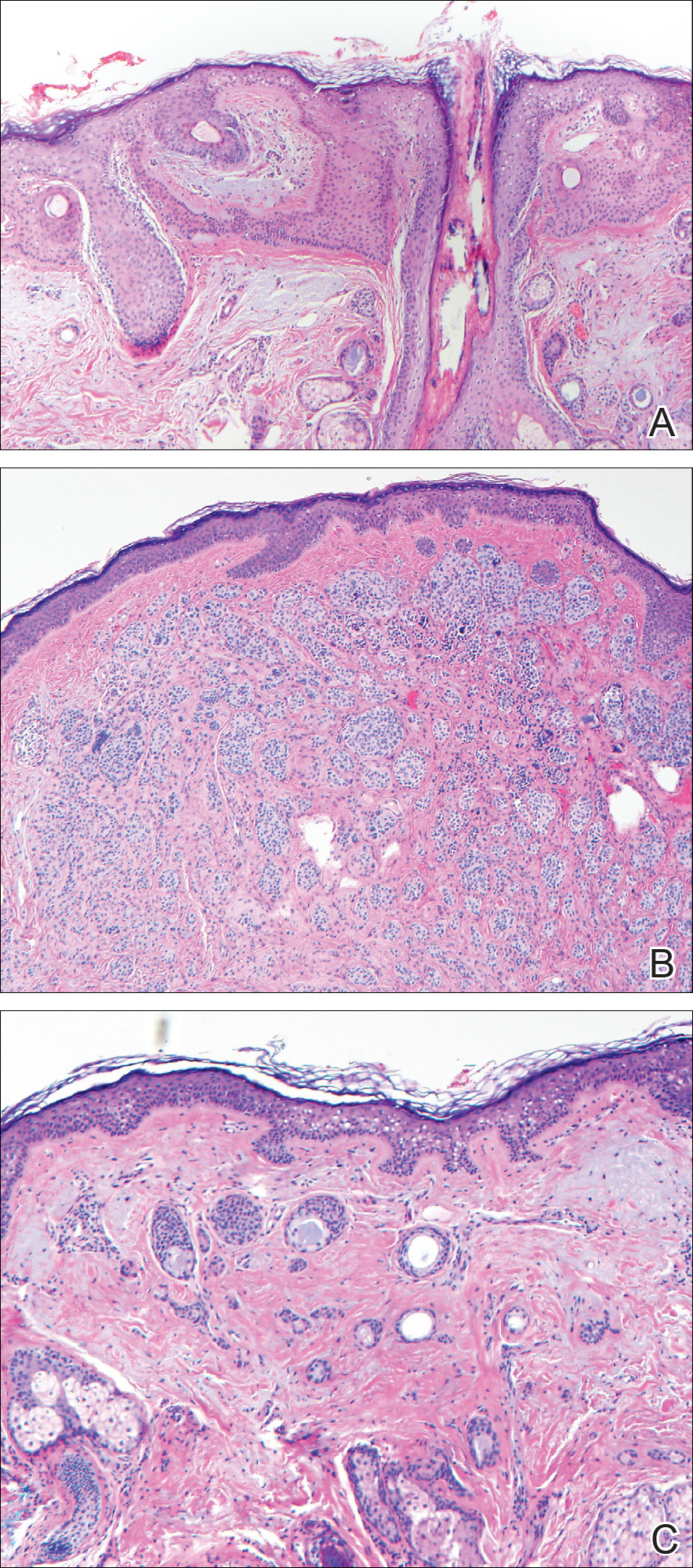
Common acquired melanocytic nevus presents clinically as a macule, papule, or nodule with smooth regular borders. The pigmented variant displays an evenly distributed pigment on the lesion. Intradermal melanocytic nevus often presents as a flesh-colored nodule, as in our case. Histopathologically, benign intradermal nevus typically is composed of a proliferation of melanocytes that exhibit dispersion as they go deeper in the dermis and maturation that manifests as melanocytes becoming smaller and more spindled in the deeper portions of the lesion.1 These 2 characteristics plus the bland cytology seen in the present case confirm the benign characteristic of this lesion (Figure, B).
In addition to the benign intradermal melanocytic nevus, an adjacent tumor of follicular infundibulum was noted. Tumor of follicular infundibulum is a rare adnexal tumor. It occurs frequently on the head and neck and shows some female predominance.2,3 Multiple lesions and eruptive lesions are rare forms that also have been reported.4 Histopathologically, the tumor demonstrates an epithelial plate that is present in the papillary dermis and is connected to the epidermis at multiple points with attachment to the follicular outer root sheath. Peripheral palisading is characteristically present above an eosinophilic basement membrane (Figure, A). Rare reports have documented sebaceous and eccrine differentiation.5,6
Tumor of follicular infundibulum has been reported to be associated with other tumors. Organoid nevus (nevus sebaceous), trichilemmal tumor, and fibroma have been reported to occur as a collision tumor with tumor of follicular infundibulum. An association with Cowden disease also has been described.7 Biopsies that represent partial samples should be interpreted cautiously, as step sections can reveal basal cell carcinoma.
The term sclerosing epithelial neoplasm describes tumors that share a paisley tielike epithelial pattern and sclerotic stroma. Small specimens often require clinicopathologic correlation (Figure, C). The differential diagnosis includes morpheaform basal cell carcinoma, desmoplastic trichoepithelioma, and microcystic adnexal carcinoma. A panel of stains using Ber-EP4, PHLDA1, cytokeratin 15, and cytokeratin 19 has been proposed to help differentiate these entities.8 CD34 and cytokeratin 20 also have been used with varying success in small specimens.9,10
- Ferringer T, Peckham S, Ko CJ, et al. Melanocytic neoplasms. In: Elston DM, Ferringer T, eds. Dermatopathology. 2nd ed. Philadelphia, PA: Elsevier Saunders; 2014:105-109.
- Headington JT. Tumors of the hair follicle. Am J Pathol. 1976;85:480-505.
- Davis DA, Cohen PR. Hair follicle nevus: case report and review of the literature. Pediatr Dermatol. 1996;13:135-138.
- Ikeda S, Kawada J, Yaguchi H, et al. A case of unilateral, systematized linear hair follicle nevi associated with epidermal nevus-like lesions. Dermatology. 2003;206:172-174.
- Mehregan AH. Hair follicle tumors of the skin. J Cutan Pathol. 1985;12:189-195.
- Mahalingam M, Bhawan J, Finn R, et al. Tumor of the follicular infundibulum with sebaceous differentiation. J Cutan Pathol. 2001;28:314-317.
- Cribier B, Grosshans E. Tumor of the follicular infundibulum: a clinicopathologic study. J Am Acad Dermatol. 1995;33:979-984.
- Sellheyer K, Nelson P, Kutzner H, et al. The immunohistochemical differential diagnosis of microcystic adnexal carcinoma, desmoplastic trichoepithelioma and morpheaform basal cell carcinoma using BerEP4 and stem cell markers. J Cutan Pathol. 2013;40:363-370.
- Abesamis-Cubillan E, El-Shabrawi-Caelen L, LeBoit PE. Merkel cells and sclerosing epithelial neoplasms. Am J Dermatopathol. 2000;22:311-315.
- Smith KJ, Williams J, Corbett D, et al. Microcystic adnexal carcinoma: an immunohistochemical study including markers of proliferation and apoptosis. Am J Surg Pathol. 2001;25:464-471.
The Diagnosis: Collision Tumor
Excisional biopsy and histopathological examination demonstrated a collision tumor composed of a benign intradermal melanocytic nevus, tumor of follicular infundibulum, and an underlying sclerosing epithelial neoplasm, with a differential diagnosis of desmoplastic trichoepithelioma, morpheaform basal cell carcinoma, and microcystic adnexal carcinoma (Figure).

Common acquired melanocytic nevus presents clinically as a macule, papule, or nodule with smooth regular borders. The pigmented variant displays an evenly distributed pigment on the lesion. Intradermal melanocytic nevus often presents as a flesh-colored nodule, as in our case. Histopathologically, benign intradermal nevus typically is composed of a proliferation of melanocytes that exhibit dispersion as they go deeper in the dermis and maturation that manifests as melanocytes becoming smaller and more spindled in the deeper portions of the lesion.1 These 2 characteristics plus the bland cytology seen in the present case confirm the benign characteristic of this lesion (Figure, B).
In addition to the benign intradermal melanocytic nevus, an adjacent tumor of follicular infundibulum was noted. Tumor of follicular infundibulum is a rare adnexal tumor. It occurs frequently on the head and neck and shows some female predominance.2,3 Multiple lesions and eruptive lesions are rare forms that also have been reported.4 Histopathologically, the tumor demonstrates an epithelial plate that is present in the papillary dermis and is connected to the epidermis at multiple points with attachment to the follicular outer root sheath. Peripheral palisading is characteristically present above an eosinophilic basement membrane (Figure, A). Rare reports have documented sebaceous and eccrine differentiation.5,6
Tumor of follicular infundibulum has been reported to be associated with other tumors. Organoid nevus (nevus sebaceous), trichilemmal tumor, and fibroma have been reported to occur as a collision tumor with tumor of follicular infundibulum. An association with Cowden disease also has been described.7 Biopsies that represent partial samples should be interpreted cautiously, as step sections can reveal basal cell carcinoma.
The term sclerosing epithelial neoplasm describes tumors that share a paisley tielike epithelial pattern and sclerotic stroma. Small specimens often require clinicopathologic correlation (Figure, C). The differential diagnosis includes morpheaform basal cell carcinoma, desmoplastic trichoepithelioma, and microcystic adnexal carcinoma. A panel of stains using Ber-EP4, PHLDA1, cytokeratin 15, and cytokeratin 19 has been proposed to help differentiate these entities.8 CD34 and cytokeratin 20 also have been used with varying success in small specimens.9,10
The Diagnosis: Collision Tumor
Excisional biopsy and histopathological examination demonstrated a collision tumor composed of a benign intradermal melanocytic nevus, tumor of follicular infundibulum, and an underlying sclerosing epithelial neoplasm, with a differential diagnosis of desmoplastic trichoepithelioma, morpheaform basal cell carcinoma, and microcystic adnexal carcinoma (Figure).

Common acquired melanocytic nevus presents clinically as a macule, papule, or nodule with smooth regular borders. The pigmented variant displays an evenly distributed pigment on the lesion. Intradermal melanocytic nevus often presents as a flesh-colored nodule, as in our case. Histopathologically, benign intradermal nevus typically is composed of a proliferation of melanocytes that exhibit dispersion as they go deeper in the dermis and maturation that manifests as melanocytes becoming smaller and more spindled in the deeper portions of the lesion.1 These 2 characteristics plus the bland cytology seen in the present case confirm the benign characteristic of this lesion (Figure, B).
In addition to the benign intradermal melanocytic nevus, an adjacent tumor of follicular infundibulum was noted. Tumor of follicular infundibulum is a rare adnexal tumor. It occurs frequently on the head and neck and shows some female predominance.2,3 Multiple lesions and eruptive lesions are rare forms that also have been reported.4 Histopathologically, the tumor demonstrates an epithelial plate that is present in the papillary dermis and is connected to the epidermis at multiple points with attachment to the follicular outer root sheath. Peripheral palisading is characteristically present above an eosinophilic basement membrane (Figure, A). Rare reports have documented sebaceous and eccrine differentiation.5,6
Tumor of follicular infundibulum has been reported to be associated with other tumors. Organoid nevus (nevus sebaceous), trichilemmal tumor, and fibroma have been reported to occur as a collision tumor with tumor of follicular infundibulum. An association with Cowden disease also has been described.7 Biopsies that represent partial samples should be interpreted cautiously, as step sections can reveal basal cell carcinoma.
The term sclerosing epithelial neoplasm describes tumors that share a paisley tielike epithelial pattern and sclerotic stroma. Small specimens often require clinicopathologic correlation (Figure, C). The differential diagnosis includes morpheaform basal cell carcinoma, desmoplastic trichoepithelioma, and microcystic adnexal carcinoma. A panel of stains using Ber-EP4, PHLDA1, cytokeratin 15, and cytokeratin 19 has been proposed to help differentiate these entities.8 CD34 and cytokeratin 20 also have been used with varying success in small specimens.9,10
- Ferringer T, Peckham S, Ko CJ, et al. Melanocytic neoplasms. In: Elston DM, Ferringer T, eds. Dermatopathology. 2nd ed. Philadelphia, PA: Elsevier Saunders; 2014:105-109.
- Headington JT. Tumors of the hair follicle. Am J Pathol. 1976;85:480-505.
- Davis DA, Cohen PR. Hair follicle nevus: case report and review of the literature. Pediatr Dermatol. 1996;13:135-138.
- Ikeda S, Kawada J, Yaguchi H, et al. A case of unilateral, systematized linear hair follicle nevi associated with epidermal nevus-like lesions. Dermatology. 2003;206:172-174.
- Mehregan AH. Hair follicle tumors of the skin. J Cutan Pathol. 1985;12:189-195.
- Mahalingam M, Bhawan J, Finn R, et al. Tumor of the follicular infundibulum with sebaceous differentiation. J Cutan Pathol. 2001;28:314-317.
- Cribier B, Grosshans E. Tumor of the follicular infundibulum: a clinicopathologic study. J Am Acad Dermatol. 1995;33:979-984.
- Sellheyer K, Nelson P, Kutzner H, et al. The immunohistochemical differential diagnosis of microcystic adnexal carcinoma, desmoplastic trichoepithelioma and morpheaform basal cell carcinoma using BerEP4 and stem cell markers. J Cutan Pathol. 2013;40:363-370.
- Abesamis-Cubillan E, El-Shabrawi-Caelen L, LeBoit PE. Merkel cells and sclerosing epithelial neoplasms. Am J Dermatopathol. 2000;22:311-315.
- Smith KJ, Williams J, Corbett D, et al. Microcystic adnexal carcinoma: an immunohistochemical study including markers of proliferation and apoptosis. Am J Surg Pathol. 2001;25:464-471.
- Ferringer T, Peckham S, Ko CJ, et al. Melanocytic neoplasms. In: Elston DM, Ferringer T, eds. Dermatopathology. 2nd ed. Philadelphia, PA: Elsevier Saunders; 2014:105-109.
- Headington JT. Tumors of the hair follicle. Am J Pathol. 1976;85:480-505.
- Davis DA, Cohen PR. Hair follicle nevus: case report and review of the literature. Pediatr Dermatol. 1996;13:135-138.
- Ikeda S, Kawada J, Yaguchi H, et al. A case of unilateral, systematized linear hair follicle nevi associated with epidermal nevus-like lesions. Dermatology. 2003;206:172-174.
- Mehregan AH. Hair follicle tumors of the skin. J Cutan Pathol. 1985;12:189-195.
- Mahalingam M, Bhawan J, Finn R, et al. Tumor of the follicular infundibulum with sebaceous differentiation. J Cutan Pathol. 2001;28:314-317.
- Cribier B, Grosshans E. Tumor of the follicular infundibulum: a clinicopathologic study. J Am Acad Dermatol. 1995;33:979-984.
- Sellheyer K, Nelson P, Kutzner H, et al. The immunohistochemical differential diagnosis of microcystic adnexal carcinoma, desmoplastic trichoepithelioma and morpheaform basal cell carcinoma using BerEP4 and stem cell markers. J Cutan Pathol. 2013;40:363-370.
- Abesamis-Cubillan E, El-Shabrawi-Caelen L, LeBoit PE. Merkel cells and sclerosing epithelial neoplasms. Am J Dermatopathol. 2000;22:311-315.
- Smith KJ, Williams J, Corbett D, et al. Microcystic adnexal carcinoma: an immunohistochemical study including markers of proliferation and apoptosis. Am J Surg Pathol. 2001;25:464-471.
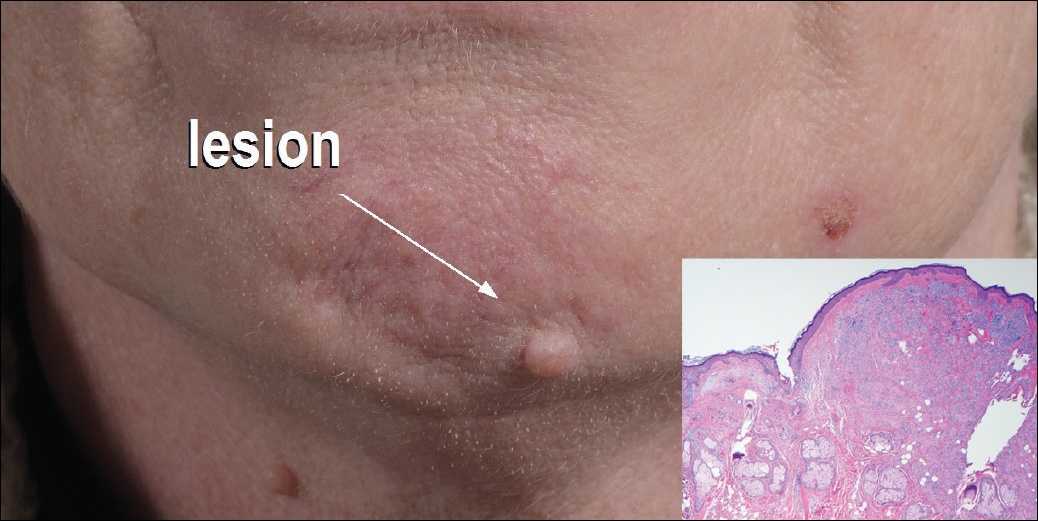
A 54-year-old man presented with a flesh-colored lesion on the chin. The nodule measured 0.6 cm in diameter. There was an underlying sclerotic plaque with indistinct borders.
Avelumab produces durable responses in Merkel cell carcinoma
Avelumab (Bavencio) is the first drug to receive approval from the Food and Drug Administration for Merkel cell carcinoma, and new findings show that it elicited durable responses in this hard-to-treat population.
The majority of responses were durable beyond 1 year, with an objective response rate of 33%.
“Merkel cell carcinoma is rare, aggressive skin cancer with a poor prognosis,” said lead author Howard L. Kaufman, MD, a surgical oncologist at the Rutgers Cancer Institute of New Jersey in New Brunswick, who discussed the findings during a presscast held at the annual meeting of the American Association for Cancer Research.
Even though Merkel cell carcinoma is a chemosensitive disease, long-term survival beyond 6 months has not been reported with chemotherapy, explained Dr. Kaufman.
In this phase II trial, Dr. Kaufman and his colleagues assessed the use of avelumab, a fully human anti–PD-L1 monoclonal antibody, in 88 patients with metastatic Merkel cell carcinoma that had progressed after treatment with chemotherapy.
All patients received 10 mg/kg avelumab as an intravenous infusion over one hour every 2 weeks. The primary endpoint was the best objective response and secondary endpoints included progression-free and overall survival.
At a median follow-up of 10.4 months, the response rate was 31.8% (28 responses), and this included 8 complete responses and 20 partial responses.
The estimated proportion of patients with duration of response of 6 months or longer was 92%, and the 6-month progression-free survival rate was 40%.
“The purpose of the presentation now is to report on longer 1-year follow-up data,” said Dr. Kaufman.
In the updated results, the objective response rate was 33.0% (95% confidence interval, 23.3%-43.8%) as two more patients moved into a total response. There were now a total of 10 (11.4%) complete responses and 19 (21.6%) partial responses.
The 6-month durable response rate was 30.6% (95% CI, 20.9%-40.3%), and the median duration of response has not yet been reached (range, 2.8–23.3-plus months; 95% CI, 18.0–not estimable). Responses were ongoing in 21 patients at the time of this analysis.
The estimated proportion of patients with a duration of response lasting 1 year or longer was 74% (95% CI, 53%-87%), and estimated 1-year progression-free survival was 30% (95% CI, 21%-41%). The 1-year overall survival rate was 52% (95% CI, 41%-62%) and median overall survival was 12.9 months (95% CI, 7.5–not estimable).
The maturing survival data suggest that there may be a long-term benefit for a proportion of patients.
“The findings of long-term responses and well-tolerated safety profile suggest that avelumab could be an important new agent for patients with Merkel cell carcinoma who have failed prior chemotherapy,” said Dr. Kaufman. “Given these results, it will be interesting to determine whether response rates could be increased by giving avelumab prior to chemotherapy or in combination with other treatments.”
Avelumab (Bavencio) is the first drug to receive approval from the Food and Drug Administration for Merkel cell carcinoma, and new findings show that it elicited durable responses in this hard-to-treat population.
The majority of responses were durable beyond 1 year, with an objective response rate of 33%.
“Merkel cell carcinoma is rare, aggressive skin cancer with a poor prognosis,” said lead author Howard L. Kaufman, MD, a surgical oncologist at the Rutgers Cancer Institute of New Jersey in New Brunswick, who discussed the findings during a presscast held at the annual meeting of the American Association for Cancer Research.
Even though Merkel cell carcinoma is a chemosensitive disease, long-term survival beyond 6 months has not been reported with chemotherapy, explained Dr. Kaufman.
In this phase II trial, Dr. Kaufman and his colleagues assessed the use of avelumab, a fully human anti–PD-L1 monoclonal antibody, in 88 patients with metastatic Merkel cell carcinoma that had progressed after treatment with chemotherapy.
All patients received 10 mg/kg avelumab as an intravenous infusion over one hour every 2 weeks. The primary endpoint was the best objective response and secondary endpoints included progression-free and overall survival.
At a median follow-up of 10.4 months, the response rate was 31.8% (28 responses), and this included 8 complete responses and 20 partial responses.
The estimated proportion of patients with duration of response of 6 months or longer was 92%, and the 6-month progression-free survival rate was 40%.
“The purpose of the presentation now is to report on longer 1-year follow-up data,” said Dr. Kaufman.
In the updated results, the objective response rate was 33.0% (95% confidence interval, 23.3%-43.8%) as two more patients moved into a total response. There were now a total of 10 (11.4%) complete responses and 19 (21.6%) partial responses.
The 6-month durable response rate was 30.6% (95% CI, 20.9%-40.3%), and the median duration of response has not yet been reached (range, 2.8–23.3-plus months; 95% CI, 18.0–not estimable). Responses were ongoing in 21 patients at the time of this analysis.
The estimated proportion of patients with a duration of response lasting 1 year or longer was 74% (95% CI, 53%-87%), and estimated 1-year progression-free survival was 30% (95% CI, 21%-41%). The 1-year overall survival rate was 52% (95% CI, 41%-62%) and median overall survival was 12.9 months (95% CI, 7.5–not estimable).
The maturing survival data suggest that there may be a long-term benefit for a proportion of patients.
“The findings of long-term responses and well-tolerated safety profile suggest that avelumab could be an important new agent for patients with Merkel cell carcinoma who have failed prior chemotherapy,” said Dr. Kaufman. “Given these results, it will be interesting to determine whether response rates could be increased by giving avelumab prior to chemotherapy or in combination with other treatments.”
Avelumab (Bavencio) is the first drug to receive approval from the Food and Drug Administration for Merkel cell carcinoma, and new findings show that it elicited durable responses in this hard-to-treat population.
The majority of responses were durable beyond 1 year, with an objective response rate of 33%.
“Merkel cell carcinoma is rare, aggressive skin cancer with a poor prognosis,” said lead author Howard L. Kaufman, MD, a surgical oncologist at the Rutgers Cancer Institute of New Jersey in New Brunswick, who discussed the findings during a presscast held at the annual meeting of the American Association for Cancer Research.
Even though Merkel cell carcinoma is a chemosensitive disease, long-term survival beyond 6 months has not been reported with chemotherapy, explained Dr. Kaufman.
In this phase II trial, Dr. Kaufman and his colleagues assessed the use of avelumab, a fully human anti–PD-L1 monoclonal antibody, in 88 patients with metastatic Merkel cell carcinoma that had progressed after treatment with chemotherapy.
All patients received 10 mg/kg avelumab as an intravenous infusion over one hour every 2 weeks. The primary endpoint was the best objective response and secondary endpoints included progression-free and overall survival.
At a median follow-up of 10.4 months, the response rate was 31.8% (28 responses), and this included 8 complete responses and 20 partial responses.
The estimated proportion of patients with duration of response of 6 months or longer was 92%, and the 6-month progression-free survival rate was 40%.
“The purpose of the presentation now is to report on longer 1-year follow-up data,” said Dr. Kaufman.
In the updated results, the objective response rate was 33.0% (95% confidence interval, 23.3%-43.8%) as two more patients moved into a total response. There were now a total of 10 (11.4%) complete responses and 19 (21.6%) partial responses.
The 6-month durable response rate was 30.6% (95% CI, 20.9%-40.3%), and the median duration of response has not yet been reached (range, 2.8–23.3-plus months; 95% CI, 18.0–not estimable). Responses were ongoing in 21 patients at the time of this analysis.
The estimated proportion of patients with a duration of response lasting 1 year or longer was 74% (95% CI, 53%-87%), and estimated 1-year progression-free survival was 30% (95% CI, 21%-41%). The 1-year overall survival rate was 52% (95% CI, 41%-62%) and median overall survival was 12.9 months (95% CI, 7.5–not estimable).
The maturing survival data suggest that there may be a long-term benefit for a proportion of patients.
“The findings of long-term responses and well-tolerated safety profile suggest that avelumab could be an important new agent for patients with Merkel cell carcinoma who have failed prior chemotherapy,” said Dr. Kaufman. “Given these results, it will be interesting to determine whether response rates could be increased by giving avelumab prior to chemotherapy or in combination with other treatments.”
Key clinical point: Treatment with avelumab resulted in an objective response rate of 33% in patients with progressive Merkel cell carcinoma.
Major finding: The estimated proportion of patients with a duration of response lasting 1 year or longer was 74% and estimated 1-year progression-free survival was 30%.
Data source: Updated results from a phase II study that included 88 patients with progressive Merkel cell carcinoma.
Disclosures: This study was funded by EMD Serono. Dr. Kaufman has served on advisory boards for Amgen, Celldex, Compass Therapeutics, EMD Serono, Merck, Prometheus, and Turnstone Biologics.
Eruptive Melanocytic Nevi During Azathioprine Therapy for Antisynthetase Syndrome
Case Report
A 50-year-old man with a history of antisynthetase syndrome (positive for anti–Jo-1 polymyositis with interstitial lung disease) and sarcoidosis presented for evaluation of numerous new moles. The lesions had developed on the trunk, arms, legs, hands, and feet approximately 3 weeks after starting azathioprine 100 mg once daily for pulmonary and muscular involvement of antisynthetase syndrome. He denied any preceding cutaneous inflammation or sunburns. He had no personal or family history of skin cancer, and no family members had multiple nevi. Physical examination revealed 30 to 40 benign-appearing, 2- to 5-mm, hyperpigmented macules scattered on the medial aspect of the right foot (Figure 1A), left palm (Figure 1B), back, abdomen, chest, arms, and legs. A larger, somewhat asymmetric, irregularly bordered, and irregularly pigmented macule was noted on the left side of the upper back. A punch biopsy of the lesion revealed a benign, mildly atypical lentiginous compound nevus (Figure 2). Pathology confirmed that the lesions represented eruptive melanocytic nevi (EMN). The patient continued azathioprine therapy and was followed with regular full-body skin examinations. Mycophenolate mofetil was suggested as an alternative therapy, if clinically appropriate, though this change has not been made by the patient’s rheumatologists.
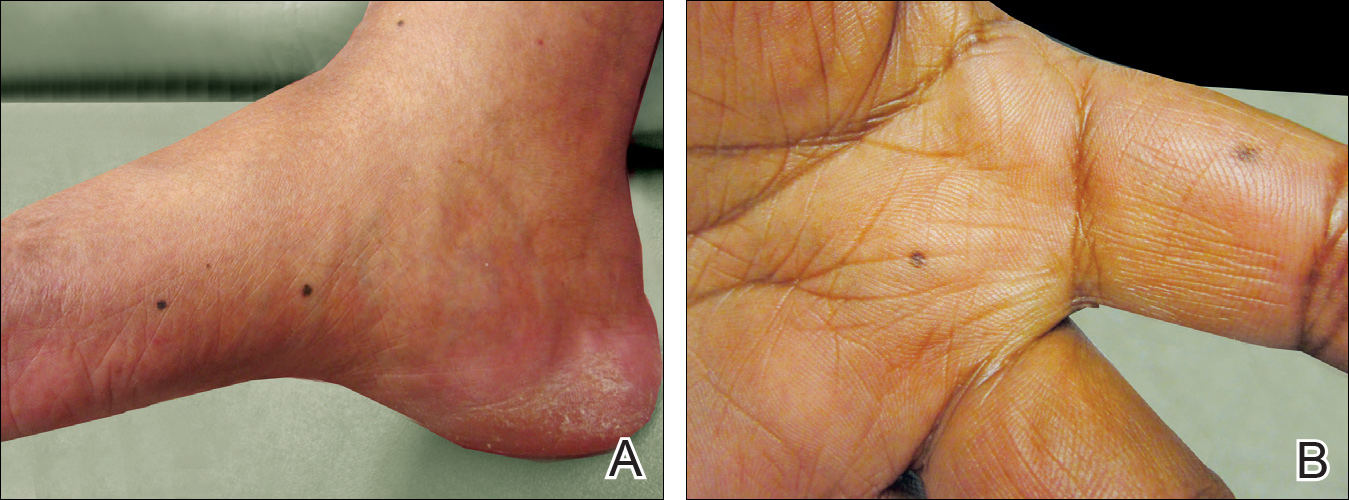
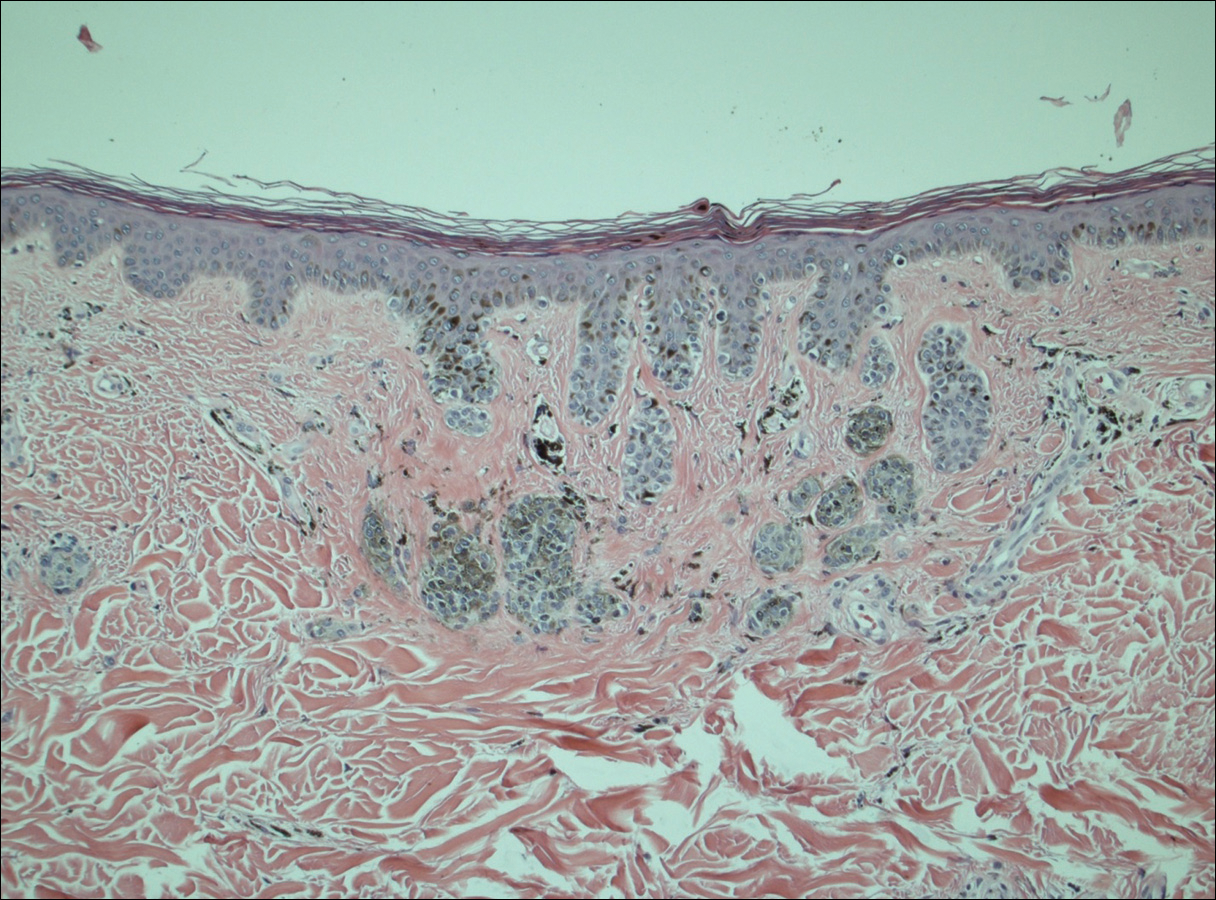
Comment
A PubMed search of articles indexed for MEDLINE using the search terms eruptive melanocytic nevi and azathioprine revealed 14 cases of EMN in the setting of azathioprine therapy, either during azathioprine monotherapy or in combination with other immunosuppressants, including systemic corticosteroids, biologics, and cyclosporine (Table).1-5 The majority of these cases occurred in renal transplant patients,1 with 3 additional cases reported in the setting of Crohn disease,2,3,5 and another in a patient with myasthenia gravis.4 Patients ranged in age from 8 to 42 years (mean age, 22 years), with lesions developing a few months to up to 7 years after starting therapy. When specified, the reported lesions typically were small, ranging from 1 to 3 mm in size, and developed rapidly over a couple of months with a predilection for the palms, soles, and trunk. Although dysplastic nevi were described in only 2 patients, melanomas were not detected.
Various hypotheses have sought to explain the largely unknown etiology of EMN. Bovenschen et al3 suggested that immunocompromised patients have diminished immune surveillance in the skin, which allows for unchecked proliferation of melanocytes. Specifically, immune suppression may induce melanocyte-stimulating hormone or melanoma growth stimulatory activity, with composition-specific growth in skin at the palms and soles.3,4 The preferential growth on the palms and soles suggests that those regions may have special sensitivity to melanocyte-stimulating hormone.4 Woodhouse and Maytin6 postulated that the increased density of eccrine sweat glands in the palms and soles as well as the absence of pilosebaceous units and apocrine glands and plentiful Pacinian and Meissner corpuscles may allow for a unique response to circulating melanocytic growth factors. Another hypothesis suggests the presence of genetic factors that allow subclinical nests of nevus cells to form, which become clinical eruptions following chemotherapy or immunosuppressive therapy.3 Azathioprine also has been suggested to induce various transcription factors that play a critical role in differentiation and proliferation of melanocytic stem cells, which leads to the formation of nevi.4 Our case and others similar to it implore that further studies be done to determine the molecular mechanism driving this phenomenon and whether a specific genetic predisposition exists that lowers the threshold for rapid proliferation of melanocytes given an immunosuppressed status.2
The risk for melanoma development in cases of EMN is unknown. Although our review of the literature did not reveal any melanomas reported in cases attributed to azathioprine, a theoretical risk exists given the established associations between melanoma and immunosuppression as well as increased numbers of nevi.6 Accordingly, these patients should be followed with regular skin examinations and biopsies of atypical-appearing lesions as indicated.2,3,5 Braun et al4 also suggested the discontinuance of azathioprine and switch to mycophenolic acid, which has not been noted to cause such eruptions; this drug was recommended in our case.
- Alaibac M, Piaserico S, Rossi CR, et al. Eruptive melanocytic nevi in patients with renal allografts: report of 10 cases with dermoscopic findings. J Am Acad Dermatol. 2003;49:1020-1022.
- Belloni FA, Piaserico S, Zattra E, et al. Dermoscopic features of eruptive melanocytic naevi in an adult patient receiving immunosuppressive therapy for Crohn’s disease. Melanoma Res. 2005;15:223-224.
- Bovenschen HJ, Tjioe M, Vermaat H, et al. Induction of eruptive benign melanocytic naevi by immune suppressive agents, including biologicals. Br J Dermatol. 2006;154:880-884.
- Braun SA, Helbig D, Frank J, et al. Eruptive melanocytic nevi during azathioprine therapy in myasthenia gravis [in German]. Hautarzt. 2012;63:756-759.
- Wonders J, De Boer N, Van Weyenberg S. Spot diagnosis: eruptive melanocytic naevi during azathioprine therapy in Crohn’s disease [published online March 6, 2012]. J Crohns Colitis. 2012;6:636.
- Woodhouse J, Maytin EV. Eruptive nevi of the palms and soles. J Am Acad Dermatol. 2005;52(5 suppl 1):S96-S100.
Case Report
A 50-year-old man with a history of antisynthetase syndrome (positive for anti–Jo-1 polymyositis with interstitial lung disease) and sarcoidosis presented for evaluation of numerous new moles. The lesions had developed on the trunk, arms, legs, hands, and feet approximately 3 weeks after starting azathioprine 100 mg once daily for pulmonary and muscular involvement of antisynthetase syndrome. He denied any preceding cutaneous inflammation or sunburns. He had no personal or family history of skin cancer, and no family members had multiple nevi. Physical examination revealed 30 to 40 benign-appearing, 2- to 5-mm, hyperpigmented macules scattered on the medial aspect of the right foot (Figure 1A), left palm (Figure 1B), back, abdomen, chest, arms, and legs. A larger, somewhat asymmetric, irregularly bordered, and irregularly pigmented macule was noted on the left side of the upper back. A punch biopsy of the lesion revealed a benign, mildly atypical lentiginous compound nevus (Figure 2). Pathology confirmed that the lesions represented eruptive melanocytic nevi (EMN). The patient continued azathioprine therapy and was followed with regular full-body skin examinations. Mycophenolate mofetil was suggested as an alternative therapy, if clinically appropriate, though this change has not been made by the patient’s rheumatologists.


Comment
A PubMed search of articles indexed for MEDLINE using the search terms eruptive melanocytic nevi and azathioprine revealed 14 cases of EMN in the setting of azathioprine therapy, either during azathioprine monotherapy or in combination with other immunosuppressants, including systemic corticosteroids, biologics, and cyclosporine (Table).1-5 The majority of these cases occurred in renal transplant patients,1 with 3 additional cases reported in the setting of Crohn disease,2,3,5 and another in a patient with myasthenia gravis.4 Patients ranged in age from 8 to 42 years (mean age, 22 years), with lesions developing a few months to up to 7 years after starting therapy. When specified, the reported lesions typically were small, ranging from 1 to 3 mm in size, and developed rapidly over a couple of months with a predilection for the palms, soles, and trunk. Although dysplastic nevi were described in only 2 patients, melanomas were not detected.

Various hypotheses have sought to explain the largely unknown etiology of EMN. Bovenschen et al3 suggested that immunocompromised patients have diminished immune surveillance in the skin, which allows for unchecked proliferation of melanocytes. Specifically, immune suppression may induce melanocyte-stimulating hormone or melanoma growth stimulatory activity, with composition-specific growth in skin at the palms and soles.3,4 The preferential growth on the palms and soles suggests that those regions may have special sensitivity to melanocyte-stimulating hormone.4 Woodhouse and Maytin6 postulated that the increased density of eccrine sweat glands in the palms and soles as well as the absence of pilosebaceous units and apocrine glands and plentiful Pacinian and Meissner corpuscles may allow for a unique response to circulating melanocytic growth factors. Another hypothesis suggests the presence of genetic factors that allow subclinical nests of nevus cells to form, which become clinical eruptions following chemotherapy or immunosuppressive therapy.3 Azathioprine also has been suggested to induce various transcription factors that play a critical role in differentiation and proliferation of melanocytic stem cells, which leads to the formation of nevi.4 Our case and others similar to it implore that further studies be done to determine the molecular mechanism driving this phenomenon and whether a specific genetic predisposition exists that lowers the threshold for rapid proliferation of melanocytes given an immunosuppressed status.2
The risk for melanoma development in cases of EMN is unknown. Although our review of the literature did not reveal any melanomas reported in cases attributed to azathioprine, a theoretical risk exists given the established associations between melanoma and immunosuppression as well as increased numbers of nevi.6 Accordingly, these patients should be followed with regular skin examinations and biopsies of atypical-appearing lesions as indicated.2,3,5 Braun et al4 also suggested the discontinuance of azathioprine and switch to mycophenolic acid, which has not been noted to cause such eruptions; this drug was recommended in our case.
Case Report
A 50-year-old man with a history of antisynthetase syndrome (positive for anti–Jo-1 polymyositis with interstitial lung disease) and sarcoidosis presented for evaluation of numerous new moles. The lesions had developed on the trunk, arms, legs, hands, and feet approximately 3 weeks after starting azathioprine 100 mg once daily for pulmonary and muscular involvement of antisynthetase syndrome. He denied any preceding cutaneous inflammation or sunburns. He had no personal or family history of skin cancer, and no family members had multiple nevi. Physical examination revealed 30 to 40 benign-appearing, 2- to 5-mm, hyperpigmented macules scattered on the medial aspect of the right foot (Figure 1A), left palm (Figure 1B), back, abdomen, chest, arms, and legs. A larger, somewhat asymmetric, irregularly bordered, and irregularly pigmented macule was noted on the left side of the upper back. A punch biopsy of the lesion revealed a benign, mildly atypical lentiginous compound nevus (Figure 2). Pathology confirmed that the lesions represented eruptive melanocytic nevi (EMN). The patient continued azathioprine therapy and was followed with regular full-body skin examinations. Mycophenolate mofetil was suggested as an alternative therapy, if clinically appropriate, though this change has not been made by the patient’s rheumatologists.


Comment
A PubMed search of articles indexed for MEDLINE using the search terms eruptive melanocytic nevi and azathioprine revealed 14 cases of EMN in the setting of azathioprine therapy, either during azathioprine monotherapy or in combination with other immunosuppressants, including systemic corticosteroids, biologics, and cyclosporine (Table).1-5 The majority of these cases occurred in renal transplant patients,1 with 3 additional cases reported in the setting of Crohn disease,2,3,5 and another in a patient with myasthenia gravis.4 Patients ranged in age from 8 to 42 years (mean age, 22 years), with lesions developing a few months to up to 7 years after starting therapy. When specified, the reported lesions typically were small, ranging from 1 to 3 mm in size, and developed rapidly over a couple of months with a predilection for the palms, soles, and trunk. Although dysplastic nevi were described in only 2 patients, melanomas were not detected.

Various hypotheses have sought to explain the largely unknown etiology of EMN. Bovenschen et al3 suggested that immunocompromised patients have diminished immune surveillance in the skin, which allows for unchecked proliferation of melanocytes. Specifically, immune suppression may induce melanocyte-stimulating hormone or melanoma growth stimulatory activity, with composition-specific growth in skin at the palms and soles.3,4 The preferential growth on the palms and soles suggests that those regions may have special sensitivity to melanocyte-stimulating hormone.4 Woodhouse and Maytin6 postulated that the increased density of eccrine sweat glands in the palms and soles as well as the absence of pilosebaceous units and apocrine glands and plentiful Pacinian and Meissner corpuscles may allow for a unique response to circulating melanocytic growth factors. Another hypothesis suggests the presence of genetic factors that allow subclinical nests of nevus cells to form, which become clinical eruptions following chemotherapy or immunosuppressive therapy.3 Azathioprine also has been suggested to induce various transcription factors that play a critical role in differentiation and proliferation of melanocytic stem cells, which leads to the formation of nevi.4 Our case and others similar to it implore that further studies be done to determine the molecular mechanism driving this phenomenon and whether a specific genetic predisposition exists that lowers the threshold for rapid proliferation of melanocytes given an immunosuppressed status.2
The risk for melanoma development in cases of EMN is unknown. Although our review of the literature did not reveal any melanomas reported in cases attributed to azathioprine, a theoretical risk exists given the established associations between melanoma and immunosuppression as well as increased numbers of nevi.6 Accordingly, these patients should be followed with regular skin examinations and biopsies of atypical-appearing lesions as indicated.2,3,5 Braun et al4 also suggested the discontinuance of azathioprine and switch to mycophenolic acid, which has not been noted to cause such eruptions; this drug was recommended in our case.
- Alaibac M, Piaserico S, Rossi CR, et al. Eruptive melanocytic nevi in patients with renal allografts: report of 10 cases with dermoscopic findings. J Am Acad Dermatol. 2003;49:1020-1022.
- Belloni FA, Piaserico S, Zattra E, et al. Dermoscopic features of eruptive melanocytic naevi in an adult patient receiving immunosuppressive therapy for Crohn’s disease. Melanoma Res. 2005;15:223-224.
- Bovenschen HJ, Tjioe M, Vermaat H, et al. Induction of eruptive benign melanocytic naevi by immune suppressive agents, including biologicals. Br J Dermatol. 2006;154:880-884.
- Braun SA, Helbig D, Frank J, et al. Eruptive melanocytic nevi during azathioprine therapy in myasthenia gravis [in German]. Hautarzt. 2012;63:756-759.
- Wonders J, De Boer N, Van Weyenberg S. Spot diagnosis: eruptive melanocytic naevi during azathioprine therapy in Crohn’s disease [published online March 6, 2012]. J Crohns Colitis. 2012;6:636.
- Woodhouse J, Maytin EV. Eruptive nevi of the palms and soles. J Am Acad Dermatol. 2005;52(5 suppl 1):S96-S100.
- Alaibac M, Piaserico S, Rossi CR, et al. Eruptive melanocytic nevi in patients with renal allografts: report of 10 cases with dermoscopic findings. J Am Acad Dermatol. 2003;49:1020-1022.
- Belloni FA, Piaserico S, Zattra E, et al. Dermoscopic features of eruptive melanocytic naevi in an adult patient receiving immunosuppressive therapy for Crohn’s disease. Melanoma Res. 2005;15:223-224.
- Bovenschen HJ, Tjioe M, Vermaat H, et al. Induction of eruptive benign melanocytic naevi by immune suppressive agents, including biologicals. Br J Dermatol. 2006;154:880-884.
- Braun SA, Helbig D, Frank J, et al. Eruptive melanocytic nevi during azathioprine therapy in myasthenia gravis [in German]. Hautarzt. 2012;63:756-759.
- Wonders J, De Boer N, Van Weyenberg S. Spot diagnosis: eruptive melanocytic naevi during azathioprine therapy in Crohn’s disease [published online March 6, 2012]. J Crohns Colitis. 2012;6:636.
- Woodhouse J, Maytin EV. Eruptive nevi of the palms and soles. J Am Acad Dermatol. 2005;52(5 suppl 1):S96-S100.
Practice Points
- A theoretical risk exists in the setting of eruptive melanocytic nevi (EMN) given the established associations between melanoma and immunosuppression as well as increased numbers of nevi.
- Follow patients with EMN with regular skin examinations and biopsies of atypical-appearing lesions given the increased risk for melanoma in this population.
FDA approves first treatment for metastatic Merkel cell carcinoma
The Food and Drug Administration has granted accelerated approval to avelumab for the treatment of metastatic Merkel cell carcinoma (MCC) in adult and pediatric patients aged 12 years and older.
Avelumab, a programmed death-ligand 1 (PD-L1)–blocking human IgG1 lambda monoclonal antibody, is the first FDA-approved treatment for metastatic MCC.
Approval was based on a 33% overall response rate in a single arm trial (JAVELIN Merkel 200 trial) of 88 patients with metastatic MCC who had been previously treated with at least one prior chemotherapy regimen, the FDA said in a written statement.
The response duration among that 33% ranged from 2.8 to 23.3+ months, and 86% of responses were durable for 6 months or more. “Responses were observed in patients regardless of PD-L1 tumor expression or presence of Merkel cell polyomavirus,” the FDA said.
There were safety data in 1,738 patients, who received 10 mg/kg of avelumab every 2 weeks. Immune-mediated adverse reactions (pneumonitis, colitis, hepatitis, adrenal insufficiency, hypo- and hyperthyroidism, diabetes mellitus, and nephritis) and life-threatening infusion reactions were the most common, serious adverse events associated with avelumab. Of the 88 patients in the JAVELIN Merkel 200 trial, the most common adverse reactions were fatigue, musculoskeletal pain, diarrhea, nausea, infusion-related reaction, rash, decreased appetite, and peripheral edema. Serious adverse reactions that occurred in more than one patient in the trial were acute kidney injury, anemia, abdominal pain, ileus, asthenia, and cellulitis, the FDA said.
The recommended dose of avelumab is 10 mg/kg administered in an intravenous infusion over 60 minutes every 2 weeks. Labeling includes the recommendation that all patients should be premedicated with an antihistamine and acetaminophen before each of the first four infusions.
“As a condition of accelerated approval, an additional study is required to confirm the clinical benefit of avelumab for this indication,” according to the FDA.
The drug is being marketed as Bavencio by EMD Serono.
The Food and Drug Administration has granted accelerated approval to avelumab for the treatment of metastatic Merkel cell carcinoma (MCC) in adult and pediatric patients aged 12 years and older.
Avelumab, a programmed death-ligand 1 (PD-L1)–blocking human IgG1 lambda monoclonal antibody, is the first FDA-approved treatment for metastatic MCC.
Approval was based on a 33% overall response rate in a single arm trial (JAVELIN Merkel 200 trial) of 88 patients with metastatic MCC who had been previously treated with at least one prior chemotherapy regimen, the FDA said in a written statement.
The response duration among that 33% ranged from 2.8 to 23.3+ months, and 86% of responses were durable for 6 months or more. “Responses were observed in patients regardless of PD-L1 tumor expression or presence of Merkel cell polyomavirus,” the FDA said.
There were safety data in 1,738 patients, who received 10 mg/kg of avelumab every 2 weeks. Immune-mediated adverse reactions (pneumonitis, colitis, hepatitis, adrenal insufficiency, hypo- and hyperthyroidism, diabetes mellitus, and nephritis) and life-threatening infusion reactions were the most common, serious adverse events associated with avelumab. Of the 88 patients in the JAVELIN Merkel 200 trial, the most common adverse reactions were fatigue, musculoskeletal pain, diarrhea, nausea, infusion-related reaction, rash, decreased appetite, and peripheral edema. Serious adverse reactions that occurred in more than one patient in the trial were acute kidney injury, anemia, abdominal pain, ileus, asthenia, and cellulitis, the FDA said.
The recommended dose of avelumab is 10 mg/kg administered in an intravenous infusion over 60 minutes every 2 weeks. Labeling includes the recommendation that all patients should be premedicated with an antihistamine and acetaminophen before each of the first four infusions.
“As a condition of accelerated approval, an additional study is required to confirm the clinical benefit of avelumab for this indication,” according to the FDA.
The drug is being marketed as Bavencio by EMD Serono.
The Food and Drug Administration has granted accelerated approval to avelumab for the treatment of metastatic Merkel cell carcinoma (MCC) in adult and pediatric patients aged 12 years and older.
Avelumab, a programmed death-ligand 1 (PD-L1)–blocking human IgG1 lambda monoclonal antibody, is the first FDA-approved treatment for metastatic MCC.
Approval was based on a 33% overall response rate in a single arm trial (JAVELIN Merkel 200 trial) of 88 patients with metastatic MCC who had been previously treated with at least one prior chemotherapy regimen, the FDA said in a written statement.
The response duration among that 33% ranged from 2.8 to 23.3+ months, and 86% of responses were durable for 6 months or more. “Responses were observed in patients regardless of PD-L1 tumor expression or presence of Merkel cell polyomavirus,” the FDA said.
There were safety data in 1,738 patients, who received 10 mg/kg of avelumab every 2 weeks. Immune-mediated adverse reactions (pneumonitis, colitis, hepatitis, adrenal insufficiency, hypo- and hyperthyroidism, diabetes mellitus, and nephritis) and life-threatening infusion reactions were the most common, serious adverse events associated with avelumab. Of the 88 patients in the JAVELIN Merkel 200 trial, the most common adverse reactions were fatigue, musculoskeletal pain, diarrhea, nausea, infusion-related reaction, rash, decreased appetite, and peripheral edema. Serious adverse reactions that occurred in more than one patient in the trial were acute kidney injury, anemia, abdominal pain, ileus, asthenia, and cellulitis, the FDA said.
The recommended dose of avelumab is 10 mg/kg administered in an intravenous infusion over 60 minutes every 2 weeks. Labeling includes the recommendation that all patients should be premedicated with an antihistamine and acetaminophen before each of the first four infusions.
“As a condition of accelerated approval, an additional study is required to confirm the clinical benefit of avelumab for this indication,” according to the FDA.
The drug is being marketed as Bavencio by EMD Serono.