User login
Mupirocin plus chlorhexidine halved Mohs surgical-site infections
SYDNEY – All patients undergoing Mohs surgery should be treated with intranasal mupirocin and a chlorhexidine body wash for 5 days before surgery, without any requirement for a nasal swab positive for Staphylococcus aureus, according to Dr. Harvey Smith.
He presented data from a randomized, controlled trial investigating the prevention of surgical-site infection in 1,002 patients undergoing Mohs surgery who had a negative nasal swab result for S. aureus. Patients were randomized to intranasal mupirocin ointment twice daily and chlorhexidine body wash daily for the 5 days before surgery, or no intervention, said Dr. Smith, a dermatologist in group practice in Perth, Australia.
The results add to earlier studies by the same group. The first study – Staph 1 – showed that swab-positive nasal carriage of S. aureus was a greater risk factor for surgical-site infections in Mohs surgery than the Wright criteria, and that decolonization with intranasal mupirocin and chlorhexidine body wash for a few days before surgery reduced the risk of infection in these patients from 12% to 4%.
The second previous study – Staph 2 – showed that using mupirocin and chlorhexidine before surgery was actually superior to the recommended treatment of stat oral cephalexin in reducing the risk of surgical-site infection.
“So, our third paper has been wondering what to do about the silent majority: These are the two-thirds of patients on whom we operate who have a negative swab for S. aureus,” Dr. Harvey said.
A negative nasal swab was not significant, he said, because skin microbiome studies had already demonstrated that humans carry S. aureus in several places, particularly the feet and buttocks.
“What we’re basically saying is we don’t think you need to swab people, because they’ve got it somewhere,” Dr. Harvey said in an interview. “We don’t think risk stratification is useful anymore, because we’ve shown it’s a benefit to everybody.”
The strategy of treating all patients with mupirocin and chlorhexidine, regardless of nasal carriage, rather than using the broad-spectrum cephalexin, fits with the World Health Organization’s global action plan on antimicrobial resistance, Dr. Harvey explained.
While there had been cases of mupirocin resistance in the past, Dr. Harvey said these had been seen in places where the drug had previously been available over the counter, such as New Zealand. However, there was no evidence of resistance developing for such a short course of use as employed in this setting, he said.
An audience member asked about whether there were any side effects from the mupirocin or chlorhexidine. Dr. Harvey said the main potential adverse event from the treatment was the risk of chlorhexidine toxicity to the cornea. However, he said that patients were told not to get the wash near their eyes.
Apart from one or two patients with eczema who could not tolerate the full 5 days of the chlorhexidine, Dr. Harvey said they had now treated more than 4,000 patients with no other side effects observed.
The study was supported by the Australasian College of Dermatologists. No conflicts of interest were declared.
SYDNEY – All patients undergoing Mohs surgery should be treated with intranasal mupirocin and a chlorhexidine body wash for 5 days before surgery, without any requirement for a nasal swab positive for Staphylococcus aureus, according to Dr. Harvey Smith.
He presented data from a randomized, controlled trial investigating the prevention of surgical-site infection in 1,002 patients undergoing Mohs surgery who had a negative nasal swab result for S. aureus. Patients were randomized to intranasal mupirocin ointment twice daily and chlorhexidine body wash daily for the 5 days before surgery, or no intervention, said Dr. Smith, a dermatologist in group practice in Perth, Australia.
The results add to earlier studies by the same group. The first study – Staph 1 – showed that swab-positive nasal carriage of S. aureus was a greater risk factor for surgical-site infections in Mohs surgery than the Wright criteria, and that decolonization with intranasal mupirocin and chlorhexidine body wash for a few days before surgery reduced the risk of infection in these patients from 12% to 4%.
The second previous study – Staph 2 – showed that using mupirocin and chlorhexidine before surgery was actually superior to the recommended treatment of stat oral cephalexin in reducing the risk of surgical-site infection.
“So, our third paper has been wondering what to do about the silent majority: These are the two-thirds of patients on whom we operate who have a negative swab for S. aureus,” Dr. Harvey said.
A negative nasal swab was not significant, he said, because skin microbiome studies had already demonstrated that humans carry S. aureus in several places, particularly the feet and buttocks.
“What we’re basically saying is we don’t think you need to swab people, because they’ve got it somewhere,” Dr. Harvey said in an interview. “We don’t think risk stratification is useful anymore, because we’ve shown it’s a benefit to everybody.”
The strategy of treating all patients with mupirocin and chlorhexidine, regardless of nasal carriage, rather than using the broad-spectrum cephalexin, fits with the World Health Organization’s global action plan on antimicrobial resistance, Dr. Harvey explained.
While there had been cases of mupirocin resistance in the past, Dr. Harvey said these had been seen in places where the drug had previously been available over the counter, such as New Zealand. However, there was no evidence of resistance developing for such a short course of use as employed in this setting, he said.
An audience member asked about whether there were any side effects from the mupirocin or chlorhexidine. Dr. Harvey said the main potential adverse event from the treatment was the risk of chlorhexidine toxicity to the cornea. However, he said that patients were told not to get the wash near their eyes.
Apart from one or two patients with eczema who could not tolerate the full 5 days of the chlorhexidine, Dr. Harvey said they had now treated more than 4,000 patients with no other side effects observed.
The study was supported by the Australasian College of Dermatologists. No conflicts of interest were declared.
SYDNEY – All patients undergoing Mohs surgery should be treated with intranasal mupirocin and a chlorhexidine body wash for 5 days before surgery, without any requirement for a nasal swab positive for Staphylococcus aureus, according to Dr. Harvey Smith.
He presented data from a randomized, controlled trial investigating the prevention of surgical-site infection in 1,002 patients undergoing Mohs surgery who had a negative nasal swab result for S. aureus. Patients were randomized to intranasal mupirocin ointment twice daily and chlorhexidine body wash daily for the 5 days before surgery, or no intervention, said Dr. Smith, a dermatologist in group practice in Perth, Australia.
The results add to earlier studies by the same group. The first study – Staph 1 – showed that swab-positive nasal carriage of S. aureus was a greater risk factor for surgical-site infections in Mohs surgery than the Wright criteria, and that decolonization with intranasal mupirocin and chlorhexidine body wash for a few days before surgery reduced the risk of infection in these patients from 12% to 4%.
The second previous study – Staph 2 – showed that using mupirocin and chlorhexidine before surgery was actually superior to the recommended treatment of stat oral cephalexin in reducing the risk of surgical-site infection.
“So, our third paper has been wondering what to do about the silent majority: These are the two-thirds of patients on whom we operate who have a negative swab for S. aureus,” Dr. Harvey said.
A negative nasal swab was not significant, he said, because skin microbiome studies had already demonstrated that humans carry S. aureus in several places, particularly the feet and buttocks.
“What we’re basically saying is we don’t think you need to swab people, because they’ve got it somewhere,” Dr. Harvey said in an interview. “We don’t think risk stratification is useful anymore, because we’ve shown it’s a benefit to everybody.”
The strategy of treating all patients with mupirocin and chlorhexidine, regardless of nasal carriage, rather than using the broad-spectrum cephalexin, fits with the World Health Organization’s global action plan on antimicrobial resistance, Dr. Harvey explained.
While there had been cases of mupirocin resistance in the past, Dr. Harvey said these had been seen in places where the drug had previously been available over the counter, such as New Zealand. However, there was no evidence of resistance developing for such a short course of use as employed in this setting, he said.
An audience member asked about whether there were any side effects from the mupirocin or chlorhexidine. Dr. Harvey said the main potential adverse event from the treatment was the risk of chlorhexidine toxicity to the cornea. However, he said that patients were told not to get the wash near their eyes.
Apart from one or two patients with eczema who could not tolerate the full 5 days of the chlorhexidine, Dr. Harvey said they had now treated more than 4,000 patients with no other side effects observed.
The study was supported by the Australasian College of Dermatologists. No conflicts of interest were declared.
Key clinical point: Treat all patients undergoing Mohs surgery with intranasal mupirocin and a chlorhexidine body wash for 5 days before surgery, without the need for a nasal swab for Staphylococcus aureus.
Major finding: Treating patients undergoing Mohs surgery with intranasal mupirocin and a chlorhexidine body wash for 5 days before surgery halved the risk of surgical-site infections, even if the patients did not have a positive nasal swab for S. aureus.
Data source: A randomized, controlled trial in 1,002 patients with a negative nasal swab for S. aureus undergoing Mohs surgery.
Disclosures: The study was partly supported by the Australasian College of Dermatologists. No conflicts of interest were declared.
Skin cancer risk similar for liver and kidney transplant recipients
SYDNEY – The risk of developing nonmelanoma skin cancer among liver transplant recipients is similar to that among kidney transplant recipients, but the former tend to have more skin cancer risk factors at baseline, according to a longitudinal cohort study reported at the annual meeting of the Australasian College of Dermatologists.
Liver transplant recipients have been thought to be at a lower risk of developing nonmelanoma skin cancers than are other solid organ transplant recipients, said Ludi Ge, MD, of the department of dermatology at the University of Sydney and Royal Prince Alfred Hospital, Sydney. However, data from a longitudinal cohort study of 230 kidney or liver transplant patients suggest the risk of nonmelanoma skin cancer is similar – if not greater – among liver transplant recipients, compared with kidney transplant recipients.
Over a 5-year period, 47% of liver transplant recipients developed at least one nonmelanoma skin cancer, compared with 33% of renal transplant recipients, representing a 78% greater risk among liver transplant recipients. However, Dr. Ge said the confidence intervals were wide, and the difference lost statistical significance in the multivariate analysis.
The researchers also noted that the liver transplant recipients in the study tended to be older at baseline, with a history of more sun exposure and more previous skin cancers, and were more likely to have a high risk skin type that sunburns easily.
In an interview, Dr. Ge said the findings had implications for the screening and follow-up of liver transplant recipients.
“Previously, we always thought that liver transplant recipients were at lower risk, and, possibly, they’re not screened as much so not followed up as much,” she said. “I think they really should be thought ... as high risk as renal transplant patients and the heart and lung transplant patients.”
The study showed that, while the renal transplant patients developed fewer skin cancers, they developed 1.9 lesions per year on average, compared with liver transplant patients, who developed 1.4 lesions per year.
The majority of skin cancers in both groups were squamous cell carcinomas and basal cell carcinomas, with a small number of keratoacanthomas. There was a similar ratio of squamous cell carcinomas to basal cell carcinomas between the two groups of transplant recipients – 1.7:1 in renal transplant recipients and 1.6:1 in liver recipients – which differed from the previously reported ratios of about 3:1, Dr. Ge said at the meeting.
She noted that this may have been because not every squamous cell carcinoma in situ was biopsied because of the sheer number of tumors, so many were treated empirically and, therefore, not entered into the clinic database.
Dr. Ge also pointed out that the evidence for the 3:1 ratio was around 10 years old.
“I think there’s been quite a change in the immunosuppressants that are used by transplant physicians, so, more and more, we’re seeing the use of sirolimus and everolimus, which are antiangiogenic,” she said.
Dr. Ge also strongly recommended that dermatology clinics specifically manage organ transplant recipients and commented that this could revolutionize the management of these patients, who tend to get lost to follow-up in standard dermatology clinics. “They’re very difficult to look after, they develop innumerable skin cancers that can result in death, and you need to intervene quite early,” she said in the interview.
No conflicts of interest were declared.
SYDNEY – The risk of developing nonmelanoma skin cancer among liver transplant recipients is similar to that among kidney transplant recipients, but the former tend to have more skin cancer risk factors at baseline, according to a longitudinal cohort study reported at the annual meeting of the Australasian College of Dermatologists.
Liver transplant recipients have been thought to be at a lower risk of developing nonmelanoma skin cancers than are other solid organ transplant recipients, said Ludi Ge, MD, of the department of dermatology at the University of Sydney and Royal Prince Alfred Hospital, Sydney. However, data from a longitudinal cohort study of 230 kidney or liver transplant patients suggest the risk of nonmelanoma skin cancer is similar – if not greater – among liver transplant recipients, compared with kidney transplant recipients.
Over a 5-year period, 47% of liver transplant recipients developed at least one nonmelanoma skin cancer, compared with 33% of renal transplant recipients, representing a 78% greater risk among liver transplant recipients. However, Dr. Ge said the confidence intervals were wide, and the difference lost statistical significance in the multivariate analysis.
The researchers also noted that the liver transplant recipients in the study tended to be older at baseline, with a history of more sun exposure and more previous skin cancers, and were more likely to have a high risk skin type that sunburns easily.
In an interview, Dr. Ge said the findings had implications for the screening and follow-up of liver transplant recipients.
“Previously, we always thought that liver transplant recipients were at lower risk, and, possibly, they’re not screened as much so not followed up as much,” she said. “I think they really should be thought ... as high risk as renal transplant patients and the heart and lung transplant patients.”
The study showed that, while the renal transplant patients developed fewer skin cancers, they developed 1.9 lesions per year on average, compared with liver transplant patients, who developed 1.4 lesions per year.
The majority of skin cancers in both groups were squamous cell carcinomas and basal cell carcinomas, with a small number of keratoacanthomas. There was a similar ratio of squamous cell carcinomas to basal cell carcinomas between the two groups of transplant recipients – 1.7:1 in renal transplant recipients and 1.6:1 in liver recipients – which differed from the previously reported ratios of about 3:1, Dr. Ge said at the meeting.
She noted that this may have been because not every squamous cell carcinoma in situ was biopsied because of the sheer number of tumors, so many were treated empirically and, therefore, not entered into the clinic database.
Dr. Ge also pointed out that the evidence for the 3:1 ratio was around 10 years old.
“I think there’s been quite a change in the immunosuppressants that are used by transplant physicians, so, more and more, we’re seeing the use of sirolimus and everolimus, which are antiangiogenic,” she said.
Dr. Ge also strongly recommended that dermatology clinics specifically manage organ transplant recipients and commented that this could revolutionize the management of these patients, who tend to get lost to follow-up in standard dermatology clinics. “They’re very difficult to look after, they develop innumerable skin cancers that can result in death, and you need to intervene quite early,” she said in the interview.
No conflicts of interest were declared.
SYDNEY – The risk of developing nonmelanoma skin cancer among liver transplant recipients is similar to that among kidney transplant recipients, but the former tend to have more skin cancer risk factors at baseline, according to a longitudinal cohort study reported at the annual meeting of the Australasian College of Dermatologists.
Liver transplant recipients have been thought to be at a lower risk of developing nonmelanoma skin cancers than are other solid organ transplant recipients, said Ludi Ge, MD, of the department of dermatology at the University of Sydney and Royal Prince Alfred Hospital, Sydney. However, data from a longitudinal cohort study of 230 kidney or liver transplant patients suggest the risk of nonmelanoma skin cancer is similar – if not greater – among liver transplant recipients, compared with kidney transplant recipients.
Over a 5-year period, 47% of liver transplant recipients developed at least one nonmelanoma skin cancer, compared with 33% of renal transplant recipients, representing a 78% greater risk among liver transplant recipients. However, Dr. Ge said the confidence intervals were wide, and the difference lost statistical significance in the multivariate analysis.
The researchers also noted that the liver transplant recipients in the study tended to be older at baseline, with a history of more sun exposure and more previous skin cancers, and were more likely to have a high risk skin type that sunburns easily.
In an interview, Dr. Ge said the findings had implications for the screening and follow-up of liver transplant recipients.
“Previously, we always thought that liver transplant recipients were at lower risk, and, possibly, they’re not screened as much so not followed up as much,” she said. “I think they really should be thought ... as high risk as renal transplant patients and the heart and lung transplant patients.”
The study showed that, while the renal transplant patients developed fewer skin cancers, they developed 1.9 lesions per year on average, compared with liver transplant patients, who developed 1.4 lesions per year.
The majority of skin cancers in both groups were squamous cell carcinomas and basal cell carcinomas, with a small number of keratoacanthomas. There was a similar ratio of squamous cell carcinomas to basal cell carcinomas between the two groups of transplant recipients – 1.7:1 in renal transplant recipients and 1.6:1 in liver recipients – which differed from the previously reported ratios of about 3:1, Dr. Ge said at the meeting.
She noted that this may have been because not every squamous cell carcinoma in situ was biopsied because of the sheer number of tumors, so many were treated empirically and, therefore, not entered into the clinic database.
Dr. Ge also pointed out that the evidence for the 3:1 ratio was around 10 years old.
“I think there’s been quite a change in the immunosuppressants that are used by transplant physicians, so, more and more, we’re seeing the use of sirolimus and everolimus, which are antiangiogenic,” she said.
Dr. Ge also strongly recommended that dermatology clinics specifically manage organ transplant recipients and commented that this could revolutionize the management of these patients, who tend to get lost to follow-up in standard dermatology clinics. “They’re very difficult to look after, they develop innumerable skin cancers that can result in death, and you need to intervene quite early,” she said in the interview.
No conflicts of interest were declared.
AT ACDASM 2017
Key clinical point: Liver transplant recipients should be screened and followed for the development of nonmelanoma skin cancers as closely as are kidney transplant recipients.
Major finding: Over 5 years, 47% of liver transplant recipients developed at least one nonmelanoma skin cancer, compared with 33% of renal transplant recipients, a difference that was not statistically significant after a multivariate analysis was done.
Data source: A longitudinal cohort study of 230 kidney or liver transplant recipients attending a dermatology clinic affiliated with an organ transplant unit.
Disclosures: No conflicts of interest were disclosed.
Chemoprevention: Thinking outside the box
WAILEA, HAWAII – Nicotinamide is one of the rare proposed agents for skin cancer chemoprevention distinguished by dirt cheap cost combined with a highly reassuring safety profile plus evidence of efficacy – which, together, make it a reasonable option in high risk patients, according to Daniel M. Siegel, MD.
Other agents that fit into that category include the tropical rainforest fern Polypodium leucotomos and milk thistle, added Dr. Siegel, a dermatologist at the State University of New York, Brooklyn.
“That’s a really interesting one. I don’t know if, 5 years from now, we’ll all be taking low-dose rapamycin as an antiaging drug, but we might, especially if someone figures out the ideal dose,” he said at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Research Foundation.
Nicotinamide
In the case of nicotinamide, the efficacy is actually supported by published level 1 evidence in the form of a highly positive 1-year, double-blind, randomized, placebo-controlled phase III clinical trial.
“You can Google ‘nicotinamide’ and find it at places like Costco and Trader Joe’s for less than 6 cents per day. That makes for a really good risk/benefit ratio. A nickel a day: That’s a cheap one. That’s one where I’d say, ‘Why not?’ It seems to be safe,” Dr. Siegel said.
In the phase III ONTRAC trial, Australian investigators randomized 386 patients who averaged roughly eight nonmelanoma skin cancers in the past 5 years to either 500 mg of oral nicotinamide twice daily or matched placebo for 12 months. During the study period, the nicotinamide group had a statistically significant and clinically meaningful 23% reduction in new nonmelanoma skin cancers, compared with the control group. They also had 13% fewer actinic keratoses at 12 months than controls. And the side effect profile mirrored that of placebo (N Engl J Med. 2015 Oct 22;373[17]:1618-26).
“Nicotinamide is vitamin B3. It’s not niacin. It doesn’t cause flushing and other vasodilatory effects. It’s actually pretty innocuous,” Dr. Siegel said.
In laboratory studies, nicotinamide has been shown to enhance DNA repair following UV exposure, as well as curb UV-induced immunosuppression.
Polypodium leucotomos Samambaia
This plant, commonly known as calaguala in the Spanish-speaking tropics and samambaia in Brazil, has a centuries-long tradition of safe medicinal use. It is commercially available over-the-counter (OTC) as a standardized product called Heliocare, designed to avoid the guesswork involved in topical sunscreen application. Each capsule contains 240 mg of an extract of P. leucotomos. Dr. Siegel said he takes it daily when he’s in a sunny locale, such as Hawaii.
Milk thistle
This plant, known as Silybum marianum, has silymarin as its bioactive compound. Dermatologist Haines Ely, MD, of the University of California, Davis, has reported therapeutic success using it in porphyria cutanea tarda and other conditions. It has been shown to inhibit photocarcinogenesis in animal studies.
Dr. Siegel said that, while Dr. Ely has told him his preferred preparation is a German OTC product, milk thistle seeds can be found in health food stores, ground to a powder using a coffee bean grinder, and used as a food supplement. Like Polypodium leucotomos and nicotinamide, milk thistle is nontoxic.
Rapamycin
This macrolide compound is produced by the bacterium Streptomyces hygroscopicus. Rapamycin is an immunosuppressant used to coat coronary stents and prevent rejection of transplanted organs. It is an mechanistic target of rapamycin signaling pathway inhibitor being studied as a cancer prevention and antiaging agent.
Science magazine called the discovery that rapamycin increased the lifespan of mice one of the top scientific breakthroughs of 2009. Subsequent animal studies have established that the extended lifespan wasn’t solely the result of rapamycin’s antineoplastic effects but of across-the-board delayed onset of all the major age-related diseases. Thus, rapamycin could turn out to be a true antiaging agent, in Dr. Siegel’s view.
Studies in humans are underway. Researchers at Novartis have reported that a rapamycin-related compound curbed the typical decline in immune function that accompanies aging as reflected in a 20% enhancement in the response to influenza vaccine in elderly volunteers (Sci Transl Med. 2014 Dec 24;6[268]:268ra179).
Dr. Siegel reported serving as a consultant to Ferndale, which markets Heliocare. The SDEF and this news organization are owned by the same parent company.
WAILEA, HAWAII – Nicotinamide is one of the rare proposed agents for skin cancer chemoprevention distinguished by dirt cheap cost combined with a highly reassuring safety profile plus evidence of efficacy – which, together, make it a reasonable option in high risk patients, according to Daniel M. Siegel, MD.
Other agents that fit into that category include the tropical rainforest fern Polypodium leucotomos and milk thistle, added Dr. Siegel, a dermatologist at the State University of New York, Brooklyn.
“That’s a really interesting one. I don’t know if, 5 years from now, we’ll all be taking low-dose rapamycin as an antiaging drug, but we might, especially if someone figures out the ideal dose,” he said at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Research Foundation.
Nicotinamide
In the case of nicotinamide, the efficacy is actually supported by published level 1 evidence in the form of a highly positive 1-year, double-blind, randomized, placebo-controlled phase III clinical trial.
“You can Google ‘nicotinamide’ and find it at places like Costco and Trader Joe’s for less than 6 cents per day. That makes for a really good risk/benefit ratio. A nickel a day: That’s a cheap one. That’s one where I’d say, ‘Why not?’ It seems to be safe,” Dr. Siegel said.
In the phase III ONTRAC trial, Australian investigators randomized 386 patients who averaged roughly eight nonmelanoma skin cancers in the past 5 years to either 500 mg of oral nicotinamide twice daily or matched placebo for 12 months. During the study period, the nicotinamide group had a statistically significant and clinically meaningful 23% reduction in new nonmelanoma skin cancers, compared with the control group. They also had 13% fewer actinic keratoses at 12 months than controls. And the side effect profile mirrored that of placebo (N Engl J Med. 2015 Oct 22;373[17]:1618-26).
“Nicotinamide is vitamin B3. It’s not niacin. It doesn’t cause flushing and other vasodilatory effects. It’s actually pretty innocuous,” Dr. Siegel said.
In laboratory studies, nicotinamide has been shown to enhance DNA repair following UV exposure, as well as curb UV-induced immunosuppression.
Polypodium leucotomos Samambaia
This plant, commonly known as calaguala in the Spanish-speaking tropics and samambaia in Brazil, has a centuries-long tradition of safe medicinal use. It is commercially available over-the-counter (OTC) as a standardized product called Heliocare, designed to avoid the guesswork involved in topical sunscreen application. Each capsule contains 240 mg of an extract of P. leucotomos. Dr. Siegel said he takes it daily when he’s in a sunny locale, such as Hawaii.
Milk thistle
This plant, known as Silybum marianum, has silymarin as its bioactive compound. Dermatologist Haines Ely, MD, of the University of California, Davis, has reported therapeutic success using it in porphyria cutanea tarda and other conditions. It has been shown to inhibit photocarcinogenesis in animal studies.
Dr. Siegel said that, while Dr. Ely has told him his preferred preparation is a German OTC product, milk thistle seeds can be found in health food stores, ground to a powder using a coffee bean grinder, and used as a food supplement. Like Polypodium leucotomos and nicotinamide, milk thistle is nontoxic.
Rapamycin
This macrolide compound is produced by the bacterium Streptomyces hygroscopicus. Rapamycin is an immunosuppressant used to coat coronary stents and prevent rejection of transplanted organs. It is an mechanistic target of rapamycin signaling pathway inhibitor being studied as a cancer prevention and antiaging agent.
Science magazine called the discovery that rapamycin increased the lifespan of mice one of the top scientific breakthroughs of 2009. Subsequent animal studies have established that the extended lifespan wasn’t solely the result of rapamycin’s antineoplastic effects but of across-the-board delayed onset of all the major age-related diseases. Thus, rapamycin could turn out to be a true antiaging agent, in Dr. Siegel’s view.
Studies in humans are underway. Researchers at Novartis have reported that a rapamycin-related compound curbed the typical decline in immune function that accompanies aging as reflected in a 20% enhancement in the response to influenza vaccine in elderly volunteers (Sci Transl Med. 2014 Dec 24;6[268]:268ra179).
Dr. Siegel reported serving as a consultant to Ferndale, which markets Heliocare. The SDEF and this news organization are owned by the same parent company.
WAILEA, HAWAII – Nicotinamide is one of the rare proposed agents for skin cancer chemoprevention distinguished by dirt cheap cost combined with a highly reassuring safety profile plus evidence of efficacy – which, together, make it a reasonable option in high risk patients, according to Daniel M. Siegel, MD.
Other agents that fit into that category include the tropical rainforest fern Polypodium leucotomos and milk thistle, added Dr. Siegel, a dermatologist at the State University of New York, Brooklyn.
“That’s a really interesting one. I don’t know if, 5 years from now, we’ll all be taking low-dose rapamycin as an antiaging drug, but we might, especially if someone figures out the ideal dose,” he said at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Research Foundation.
Nicotinamide
In the case of nicotinamide, the efficacy is actually supported by published level 1 evidence in the form of a highly positive 1-year, double-blind, randomized, placebo-controlled phase III clinical trial.
“You can Google ‘nicotinamide’ and find it at places like Costco and Trader Joe’s for less than 6 cents per day. That makes for a really good risk/benefit ratio. A nickel a day: That’s a cheap one. That’s one where I’d say, ‘Why not?’ It seems to be safe,” Dr. Siegel said.
In the phase III ONTRAC trial, Australian investigators randomized 386 patients who averaged roughly eight nonmelanoma skin cancers in the past 5 years to either 500 mg of oral nicotinamide twice daily or matched placebo for 12 months. During the study period, the nicotinamide group had a statistically significant and clinically meaningful 23% reduction in new nonmelanoma skin cancers, compared with the control group. They also had 13% fewer actinic keratoses at 12 months than controls. And the side effect profile mirrored that of placebo (N Engl J Med. 2015 Oct 22;373[17]:1618-26).
“Nicotinamide is vitamin B3. It’s not niacin. It doesn’t cause flushing and other vasodilatory effects. It’s actually pretty innocuous,” Dr. Siegel said.
In laboratory studies, nicotinamide has been shown to enhance DNA repair following UV exposure, as well as curb UV-induced immunosuppression.
Polypodium leucotomos Samambaia
This plant, commonly known as calaguala in the Spanish-speaking tropics and samambaia in Brazil, has a centuries-long tradition of safe medicinal use. It is commercially available over-the-counter (OTC) as a standardized product called Heliocare, designed to avoid the guesswork involved in topical sunscreen application. Each capsule contains 240 mg of an extract of P. leucotomos. Dr. Siegel said he takes it daily when he’s in a sunny locale, such as Hawaii.
Milk thistle
This plant, known as Silybum marianum, has silymarin as its bioactive compound. Dermatologist Haines Ely, MD, of the University of California, Davis, has reported therapeutic success using it in porphyria cutanea tarda and other conditions. It has been shown to inhibit photocarcinogenesis in animal studies.
Dr. Siegel said that, while Dr. Ely has told him his preferred preparation is a German OTC product, milk thistle seeds can be found in health food stores, ground to a powder using a coffee bean grinder, and used as a food supplement. Like Polypodium leucotomos and nicotinamide, milk thistle is nontoxic.
Rapamycin
This macrolide compound is produced by the bacterium Streptomyces hygroscopicus. Rapamycin is an immunosuppressant used to coat coronary stents and prevent rejection of transplanted organs. It is an mechanistic target of rapamycin signaling pathway inhibitor being studied as a cancer prevention and antiaging agent.
Science magazine called the discovery that rapamycin increased the lifespan of mice one of the top scientific breakthroughs of 2009. Subsequent animal studies have established that the extended lifespan wasn’t solely the result of rapamycin’s antineoplastic effects but of across-the-board delayed onset of all the major age-related diseases. Thus, rapamycin could turn out to be a true antiaging agent, in Dr. Siegel’s view.
Studies in humans are underway. Researchers at Novartis have reported that a rapamycin-related compound curbed the typical decline in immune function that accompanies aging as reflected in a 20% enhancement in the response to influenza vaccine in elderly volunteers (Sci Transl Med. 2014 Dec 24;6[268]:268ra179).
Dr. Siegel reported serving as a consultant to Ferndale, which markets Heliocare. The SDEF and this news organization are owned by the same parent company.
EXPERT ANALYSIS FROM SDEF HAWAII DERMATOLOGY SEMINAR
Study links photosensitizing antihypertensives to SCC
PORTLAND, ORE. – Patients prescribed photosensitizing antihypertensive drugs had a 16% increase in risk of cutaneous squamous cell carcinoma (cSCC) in a large retrospective cohort study.
These drugs include alpha-2 receptor agonists and loop diuretics, potassium-sparing diuretics, thiazide diuretics, and combination diuretics, Katherine Levandoski said in an oral presentation at the annual meeting of the Society for Investigative Dermatology.
Furthermore, taking antihypertensive drugs of unknown photosensitizing potential conferred a 10% increase in risk of cSCC in the study, she added. Such medications include angiotensin–converting enzyme inhibitors, calcium channel blockers, and vasodilators, she said.
More than 50 million Americans take antihypertensive drugs, many of which are photosensitizing, noted Ms. Levandoski, a research assistant in the Patient Oriented Research on the Epidemiology of Skin Diseases Unit in the department of dermatology, Massachusetts General Hospital, and the department of population medicine, Harvard University, Boston. However, few studies have explored the oncogenic effects of exposure to these drugs, and those that have done so were subject to confounding, small sample sizes, missing data, lack of pathologic verification, and reliance on self-reported medication history, she added.
To help fill this knowledge gap, she and her associates studied 28,357 non-Hispanic whites diagnosed with hypertension and treated at Kaiser Permanente Northern California between 1997 and 2012. They limited the cohort to non-Hispanic whites because they represent the group with most cases of cSCC.
During follow-up, 3,010 patients were diagnosed with new-onset, pathologically verified cSCC, Ms. Levandoski said. Compared with nonusers of antihypertensives, users of photosensitizing antihypertensives had about a 16% increase in the rate of cSCC (hazard ratio, 1.16; 95% confidence interval, 1.06-1.27), even after accounting for age, sex, smoking, comorbidities, health care utilization, skin cancer history, length of health plan membership, and prior exposure to photosensitizing medications.
Strikingly, patients who used antihypertensives of unknown photosensitizing effect had a 10% increase in risk of incident cSCC (RR, 1.10; 95% CI, 1.02-1.19). Some antihypertensive drugs that are classified as unknown photosensitizers “may actually have photosensitizing properties,” Ms. Levandoski commented. Patients taking antihypertensives of known or unknown photosensitizing potential “should be educated on safe sun practices and may benefit from closer screening for cutaneous squamous cell carcinoma,” she added.
The risk of cSCC was not increased among users of nonphotosensitizing antihypertensives (HR, 0.99; 95% CI, 0.91-1.07), including alpha-blockers, beta-blockers, central agonists, and angiotensin receptor blockers, Ms. Levandoski reported.
Patients in the study cohort averaged aged 60 years (standard deviation, 10.6 years), and 56% were female. In all, 1,530 had never been prescribed antihypertensives, while about 17,000-19,000 had been prescribed unknown, known, or nonphotosensitizing antihypertensives.
The work was funded by the National Institutes of Health, a travel award from the Society for Investigative Dermatology, and a Massachusetts General Hospital Medical Student Award. Ms. Levandoski had no conflicts of interest.
PORTLAND, ORE. – Patients prescribed photosensitizing antihypertensive drugs had a 16% increase in risk of cutaneous squamous cell carcinoma (cSCC) in a large retrospective cohort study.
These drugs include alpha-2 receptor agonists and loop diuretics, potassium-sparing diuretics, thiazide diuretics, and combination diuretics, Katherine Levandoski said in an oral presentation at the annual meeting of the Society for Investigative Dermatology.
Furthermore, taking antihypertensive drugs of unknown photosensitizing potential conferred a 10% increase in risk of cSCC in the study, she added. Such medications include angiotensin–converting enzyme inhibitors, calcium channel blockers, and vasodilators, she said.
More than 50 million Americans take antihypertensive drugs, many of which are photosensitizing, noted Ms. Levandoski, a research assistant in the Patient Oriented Research on the Epidemiology of Skin Diseases Unit in the department of dermatology, Massachusetts General Hospital, and the department of population medicine, Harvard University, Boston. However, few studies have explored the oncogenic effects of exposure to these drugs, and those that have done so were subject to confounding, small sample sizes, missing data, lack of pathologic verification, and reliance on self-reported medication history, she added.
To help fill this knowledge gap, she and her associates studied 28,357 non-Hispanic whites diagnosed with hypertension and treated at Kaiser Permanente Northern California between 1997 and 2012. They limited the cohort to non-Hispanic whites because they represent the group with most cases of cSCC.
During follow-up, 3,010 patients were diagnosed with new-onset, pathologically verified cSCC, Ms. Levandoski said. Compared with nonusers of antihypertensives, users of photosensitizing antihypertensives had about a 16% increase in the rate of cSCC (hazard ratio, 1.16; 95% confidence interval, 1.06-1.27), even after accounting for age, sex, smoking, comorbidities, health care utilization, skin cancer history, length of health plan membership, and prior exposure to photosensitizing medications.
Strikingly, patients who used antihypertensives of unknown photosensitizing effect had a 10% increase in risk of incident cSCC (RR, 1.10; 95% CI, 1.02-1.19). Some antihypertensive drugs that are classified as unknown photosensitizers “may actually have photosensitizing properties,” Ms. Levandoski commented. Patients taking antihypertensives of known or unknown photosensitizing potential “should be educated on safe sun practices and may benefit from closer screening for cutaneous squamous cell carcinoma,” she added.
The risk of cSCC was not increased among users of nonphotosensitizing antihypertensives (HR, 0.99; 95% CI, 0.91-1.07), including alpha-blockers, beta-blockers, central agonists, and angiotensin receptor blockers, Ms. Levandoski reported.
Patients in the study cohort averaged aged 60 years (standard deviation, 10.6 years), and 56% were female. In all, 1,530 had never been prescribed antihypertensives, while about 17,000-19,000 had been prescribed unknown, known, or nonphotosensitizing antihypertensives.
The work was funded by the National Institutes of Health, a travel award from the Society for Investigative Dermatology, and a Massachusetts General Hospital Medical Student Award. Ms. Levandoski had no conflicts of interest.
PORTLAND, ORE. – Patients prescribed photosensitizing antihypertensive drugs had a 16% increase in risk of cutaneous squamous cell carcinoma (cSCC) in a large retrospective cohort study.
These drugs include alpha-2 receptor agonists and loop diuretics, potassium-sparing diuretics, thiazide diuretics, and combination diuretics, Katherine Levandoski said in an oral presentation at the annual meeting of the Society for Investigative Dermatology.
Furthermore, taking antihypertensive drugs of unknown photosensitizing potential conferred a 10% increase in risk of cSCC in the study, she added. Such medications include angiotensin–converting enzyme inhibitors, calcium channel blockers, and vasodilators, she said.
More than 50 million Americans take antihypertensive drugs, many of which are photosensitizing, noted Ms. Levandoski, a research assistant in the Patient Oriented Research on the Epidemiology of Skin Diseases Unit in the department of dermatology, Massachusetts General Hospital, and the department of population medicine, Harvard University, Boston. However, few studies have explored the oncogenic effects of exposure to these drugs, and those that have done so were subject to confounding, small sample sizes, missing data, lack of pathologic verification, and reliance on self-reported medication history, she added.
To help fill this knowledge gap, she and her associates studied 28,357 non-Hispanic whites diagnosed with hypertension and treated at Kaiser Permanente Northern California between 1997 and 2012. They limited the cohort to non-Hispanic whites because they represent the group with most cases of cSCC.
During follow-up, 3,010 patients were diagnosed with new-onset, pathologically verified cSCC, Ms. Levandoski said. Compared with nonusers of antihypertensives, users of photosensitizing antihypertensives had about a 16% increase in the rate of cSCC (hazard ratio, 1.16; 95% confidence interval, 1.06-1.27), even after accounting for age, sex, smoking, comorbidities, health care utilization, skin cancer history, length of health plan membership, and prior exposure to photosensitizing medications.
Strikingly, patients who used antihypertensives of unknown photosensitizing effect had a 10% increase in risk of incident cSCC (RR, 1.10; 95% CI, 1.02-1.19). Some antihypertensive drugs that are classified as unknown photosensitizers “may actually have photosensitizing properties,” Ms. Levandoski commented. Patients taking antihypertensives of known or unknown photosensitizing potential “should be educated on safe sun practices and may benefit from closer screening for cutaneous squamous cell carcinoma,” she added.
The risk of cSCC was not increased among users of nonphotosensitizing antihypertensives (HR, 0.99; 95% CI, 0.91-1.07), including alpha-blockers, beta-blockers, central agonists, and angiotensin receptor blockers, Ms. Levandoski reported.
Patients in the study cohort averaged aged 60 years (standard deviation, 10.6 years), and 56% were female. In all, 1,530 had never been prescribed antihypertensives, while about 17,000-19,000 had been prescribed unknown, known, or nonphotosensitizing antihypertensives.
The work was funded by the National Institutes of Health, a travel award from the Society for Investigative Dermatology, and a Massachusetts General Hospital Medical Student Award. Ms. Levandoski had no conflicts of interest.
AT SID 2017
Key clinical point: Consider skin cancer screening for patients who are taking antihypertensives of known or unknown photosensitizing potential.
Major finding: The risk of cutaneous squamous cell carcinoma associated with photosensitizing antihypertensives was about 16% .
Data source: A retrospective cohort study of 28,357 non-Hispanic whites with hypertension.
Disclosures: The work was funded by the National Institutes of Health, a travel award from the Society for Investigative Dermatology, and a Massachusetts General Hospital Medical Student Award. Ms. Levandoski had no conflicts of interest.
Chronic GVHD linked to fivefold increase in squamous cell skin carcinomas
PORTLAND – Chronic graft versus host disease (GVHD) was associated with a fivefold increase in risk of squamous cell carcinoma and a nearly twofold rise in the rate of basal cell carcinoma, based on a meta-analysis of eight studies.
Acute GVHD was not tied to an increase in secondary nonmelanoma skin cancers, Pooja H. Rambhia and her associates reported in a poster presented at the annual meeting of the Society for Investigative Dermatology. The findings highlight the need for multidisciplinary consults to distinguish malignancies from the cutaneous manifestations of chronic GVHD and for vigorous surveillance for skin cancer even years after hematopoietic stem cell transplantation.
GVHS has been linked to secondary nonmelanoma skin cancers in previous studies, but few have quantified the risk, according to the reviewers, who are from the department of dermatology and dermatopathology at the Cleveland Clinic Foundation. The increased risk may be related to the heavy immunosuppression needed to treat chronic GVHD.
For the meta-analysis, the researchers identified 1,411 studies recorded in academic databases and reviewed those that reported both cases of skin cancers and GVHD. Seven retrospective, and one prospective, studies published between 1997 and 2012 measured both variables in all patients.
The studies included more than 56,000 patients followed for up to 36 years after undergoing allogeneic or syngeneic transplantation, the reviewers reported. During follow-up, between 17% and 73% of patients developed chronic GVHD, and 29% to 67% developed acute GVHD. There were 98 cases of basal cell carcinoma, 49 cases of squamous cell carcinoma, and 34 cases of malignant melanoma. Chronic GVHD was significantly associated with both squamous cell carcinoma (risk ratio, 5.3; 95% confidence interval, 2.4-11.8; P less than .001) and basal cell carcinoma (RR, 2.0; 95% CI, 1.3-3.0; P = .002). In contrast, chronic GVHD showed a nonsignificant trend toward an inverse correlation with the risk of secondary melanoma. Acute GVHD was not linked with squamous cell carcinoma, basal cell carcinoma, or melanoma.
GVHD develops, up to half the time, after hematopoietic stem cell transplantation and often becomes chronic, the reviewers noted. Catching skin cancer early is crucial, and transplant patients should undergo regular skin checks with multidisciplinary consults to promptly, accurately distinguish malignancies from the cutaneous manifestations of GVHD, they added.
The researchers did not report external funding sources. They had no relevant financial conflicts of interest.
PORTLAND – Chronic graft versus host disease (GVHD) was associated with a fivefold increase in risk of squamous cell carcinoma and a nearly twofold rise in the rate of basal cell carcinoma, based on a meta-analysis of eight studies.
Acute GVHD was not tied to an increase in secondary nonmelanoma skin cancers, Pooja H. Rambhia and her associates reported in a poster presented at the annual meeting of the Society for Investigative Dermatology. The findings highlight the need for multidisciplinary consults to distinguish malignancies from the cutaneous manifestations of chronic GVHD and for vigorous surveillance for skin cancer even years after hematopoietic stem cell transplantation.
GVHS has been linked to secondary nonmelanoma skin cancers in previous studies, but few have quantified the risk, according to the reviewers, who are from the department of dermatology and dermatopathology at the Cleveland Clinic Foundation. The increased risk may be related to the heavy immunosuppression needed to treat chronic GVHD.
For the meta-analysis, the researchers identified 1,411 studies recorded in academic databases and reviewed those that reported both cases of skin cancers and GVHD. Seven retrospective, and one prospective, studies published between 1997 and 2012 measured both variables in all patients.
The studies included more than 56,000 patients followed for up to 36 years after undergoing allogeneic or syngeneic transplantation, the reviewers reported. During follow-up, between 17% and 73% of patients developed chronic GVHD, and 29% to 67% developed acute GVHD. There were 98 cases of basal cell carcinoma, 49 cases of squamous cell carcinoma, and 34 cases of malignant melanoma. Chronic GVHD was significantly associated with both squamous cell carcinoma (risk ratio, 5.3; 95% confidence interval, 2.4-11.8; P less than .001) and basal cell carcinoma (RR, 2.0; 95% CI, 1.3-3.0; P = .002). In contrast, chronic GVHD showed a nonsignificant trend toward an inverse correlation with the risk of secondary melanoma. Acute GVHD was not linked with squamous cell carcinoma, basal cell carcinoma, or melanoma.
GVHD develops, up to half the time, after hematopoietic stem cell transplantation and often becomes chronic, the reviewers noted. Catching skin cancer early is crucial, and transplant patients should undergo regular skin checks with multidisciplinary consults to promptly, accurately distinguish malignancies from the cutaneous manifestations of GVHD, they added.
The researchers did not report external funding sources. They had no relevant financial conflicts of interest.
PORTLAND – Chronic graft versus host disease (GVHD) was associated with a fivefold increase in risk of squamous cell carcinoma and a nearly twofold rise in the rate of basal cell carcinoma, based on a meta-analysis of eight studies.
Acute GVHD was not tied to an increase in secondary nonmelanoma skin cancers, Pooja H. Rambhia and her associates reported in a poster presented at the annual meeting of the Society for Investigative Dermatology. The findings highlight the need for multidisciplinary consults to distinguish malignancies from the cutaneous manifestations of chronic GVHD and for vigorous surveillance for skin cancer even years after hematopoietic stem cell transplantation.
GVHS has been linked to secondary nonmelanoma skin cancers in previous studies, but few have quantified the risk, according to the reviewers, who are from the department of dermatology and dermatopathology at the Cleveland Clinic Foundation. The increased risk may be related to the heavy immunosuppression needed to treat chronic GVHD.
For the meta-analysis, the researchers identified 1,411 studies recorded in academic databases and reviewed those that reported both cases of skin cancers and GVHD. Seven retrospective, and one prospective, studies published between 1997 and 2012 measured both variables in all patients.
The studies included more than 56,000 patients followed for up to 36 years after undergoing allogeneic or syngeneic transplantation, the reviewers reported. During follow-up, between 17% and 73% of patients developed chronic GVHD, and 29% to 67% developed acute GVHD. There were 98 cases of basal cell carcinoma, 49 cases of squamous cell carcinoma, and 34 cases of malignant melanoma. Chronic GVHD was significantly associated with both squamous cell carcinoma (risk ratio, 5.3; 95% confidence interval, 2.4-11.8; P less than .001) and basal cell carcinoma (RR, 2.0; 95% CI, 1.3-3.0; P = .002). In contrast, chronic GVHD showed a nonsignificant trend toward an inverse correlation with the risk of secondary melanoma. Acute GVHD was not linked with squamous cell carcinoma, basal cell carcinoma, or melanoma.
GVHD develops, up to half the time, after hematopoietic stem cell transplantation and often becomes chronic, the reviewers noted. Catching skin cancer early is crucial, and transplant patients should undergo regular skin checks with multidisciplinary consults to promptly, accurately distinguish malignancies from the cutaneous manifestations of GVHD, they added.
The researchers did not report external funding sources. They had no relevant financial conflicts of interest.
AT SID 2017
Key clinical point: Chronic graft versus host disease was associated with a significantly increased risk of squamous cell and basal cell carcinomas.
Major finding: Chronic GVHD was associated with a fivefold increase in squamous cell carcinoma (risk ratio, 5.3; 95% confidence interval, 2.4 to 11.8; P less than .001).
Data source: A meta-analysis of eight cohort studies of 56,000 patients who underwent hematopoietic stem cell transplantation.
Disclosures: The researchers did not report external funding sources. They had no conflicts of interest.
Tattoo artist survey finds almost half agree to tattoo skin with lesions
The importance of educating tattoo artists on identifying and being careful around skin with melanocytic nevi and other lesions was highlighted by the results of a survey of tattoo artists, according to a study from the University of Pittsburgh.
“While most of those surveyed reported deliberately avoiding nevi, a similar proportion reported either tattooing over them or simply deferring to the client’s preference,” wrote Westley S. Mori and his associates in the department of dermatology at the University of Pittsburgh, Pennsylvania. “This is concerning because few clients specifically ask tattoo artists to avoid skin lesions,” they added.
They surveyed 42 tattoo artists in July and August 2016 regarding their encounters with clients with skin lesions and their personal knowledge or experiences they may have had with skin cancer. Of those surveyed, 23 (55%) said they had declined to tattoo skin with a rash or lesion (JAMA Dermatology. 2017;153[4]:328-30).When asked about their reasoning for declining a client’s request, 21 (50%) of respondents said they did so because of a poor cosmetic outcome, while the next highest answer, a concern of potential skin cancer, was only cited by 12 (29%).
Most (74%) said there was no official store policy about tattooing over moles or other skin lesions. When asked about their approaches to tattooing skin with moles or other lesions, many said they choose to tattoo around the lesion (41%), tattoo over the lesion (19%), or defer to the client’s preferences (24%). However, with regards to deferring to a client, 29 artists (69%) reported never being asked to avoid a lesion.
Investigators noted that 12 respondents reported that they had identified a possible cancerous lesion on a client, followed by the same number of respondents reporting having recommended that a client see a dermatologist.
Tattoo artists who had seen a dermatologist for a skin examination were significantly more likely to refuse to tattoo a client with a lesion (P = .01) and recommend that the client see a dermatologist (P less than .001) when they had a lesion. Based on this response, the authors said that they believed that educating both clients and tattoo artists may be the best way to get tattoo artists to engage clients. “Our study highlights an opportunity for dermatologists to educate tattoo artists about skin cancer, particularly melanoma, to help reduce the incidence of skin cancers hidden in tattoos and to encourage appropriate referral to dermatologists for suspicious lesions on clients,” they concluded.
“When you perform a total body skin examination, it’s a little difficult to kind of tease out if a lesion looks suspicious or not if it’s surrounded by ink,” Mr. Mori, a medical student at the university, said in an interview. “Tattoos are becoming more and more common, especially among younger people, and incidence of melanoma has increased in younger populations as well. ... It is very concerning that skin cancers could be hidden in tattoos.”
In fact, Mr. Mori pointed out, there are opportunities for dermatologists to reach out to the tattoo artist community and start the communication process. “Tattoo artists have national conferences where they get together and discuss the state of the industry, and that represents one opportunity where dermatologists could talk about the effects of skin cancer,” he said.
The study was funded by the University of Pittsburgh. The authors reported no relevant financial disclosures.
ezimmerman@frontlinemedcom.com
On Twitter @eaztweets
The importance of educating tattoo artists on identifying and being careful around skin with melanocytic nevi and other lesions was highlighted by the results of a survey of tattoo artists, according to a study from the University of Pittsburgh.
“While most of those surveyed reported deliberately avoiding nevi, a similar proportion reported either tattooing over them or simply deferring to the client’s preference,” wrote Westley S. Mori and his associates in the department of dermatology at the University of Pittsburgh, Pennsylvania. “This is concerning because few clients specifically ask tattoo artists to avoid skin lesions,” they added.
They surveyed 42 tattoo artists in July and August 2016 regarding their encounters with clients with skin lesions and their personal knowledge or experiences they may have had with skin cancer. Of those surveyed, 23 (55%) said they had declined to tattoo skin with a rash or lesion (JAMA Dermatology. 2017;153[4]:328-30).When asked about their reasoning for declining a client’s request, 21 (50%) of respondents said they did so because of a poor cosmetic outcome, while the next highest answer, a concern of potential skin cancer, was only cited by 12 (29%).
Most (74%) said there was no official store policy about tattooing over moles or other skin lesions. When asked about their approaches to tattooing skin with moles or other lesions, many said they choose to tattoo around the lesion (41%), tattoo over the lesion (19%), or defer to the client’s preferences (24%). However, with regards to deferring to a client, 29 artists (69%) reported never being asked to avoid a lesion.
Investigators noted that 12 respondents reported that they had identified a possible cancerous lesion on a client, followed by the same number of respondents reporting having recommended that a client see a dermatologist.
Tattoo artists who had seen a dermatologist for a skin examination were significantly more likely to refuse to tattoo a client with a lesion (P = .01) and recommend that the client see a dermatologist (P less than .001) when they had a lesion. Based on this response, the authors said that they believed that educating both clients and tattoo artists may be the best way to get tattoo artists to engage clients. “Our study highlights an opportunity for dermatologists to educate tattoo artists about skin cancer, particularly melanoma, to help reduce the incidence of skin cancers hidden in tattoos and to encourage appropriate referral to dermatologists for suspicious lesions on clients,” they concluded.
“When you perform a total body skin examination, it’s a little difficult to kind of tease out if a lesion looks suspicious or not if it’s surrounded by ink,” Mr. Mori, a medical student at the university, said in an interview. “Tattoos are becoming more and more common, especially among younger people, and incidence of melanoma has increased in younger populations as well. ... It is very concerning that skin cancers could be hidden in tattoos.”
In fact, Mr. Mori pointed out, there are opportunities for dermatologists to reach out to the tattoo artist community and start the communication process. “Tattoo artists have national conferences where they get together and discuss the state of the industry, and that represents one opportunity where dermatologists could talk about the effects of skin cancer,” he said.
The study was funded by the University of Pittsburgh. The authors reported no relevant financial disclosures.
ezimmerman@frontlinemedcom.com
On Twitter @eaztweets
The importance of educating tattoo artists on identifying and being careful around skin with melanocytic nevi and other lesions was highlighted by the results of a survey of tattoo artists, according to a study from the University of Pittsburgh.
“While most of those surveyed reported deliberately avoiding nevi, a similar proportion reported either tattooing over them or simply deferring to the client’s preference,” wrote Westley S. Mori and his associates in the department of dermatology at the University of Pittsburgh, Pennsylvania. “This is concerning because few clients specifically ask tattoo artists to avoid skin lesions,” they added.
They surveyed 42 tattoo artists in July and August 2016 regarding their encounters with clients with skin lesions and their personal knowledge or experiences they may have had with skin cancer. Of those surveyed, 23 (55%) said they had declined to tattoo skin with a rash or lesion (JAMA Dermatology. 2017;153[4]:328-30).When asked about their reasoning for declining a client’s request, 21 (50%) of respondents said they did so because of a poor cosmetic outcome, while the next highest answer, a concern of potential skin cancer, was only cited by 12 (29%).
Most (74%) said there was no official store policy about tattooing over moles or other skin lesions. When asked about their approaches to tattooing skin with moles or other lesions, many said they choose to tattoo around the lesion (41%), tattoo over the lesion (19%), or defer to the client’s preferences (24%). However, with regards to deferring to a client, 29 artists (69%) reported never being asked to avoid a lesion.
Investigators noted that 12 respondents reported that they had identified a possible cancerous lesion on a client, followed by the same number of respondents reporting having recommended that a client see a dermatologist.
Tattoo artists who had seen a dermatologist for a skin examination were significantly more likely to refuse to tattoo a client with a lesion (P = .01) and recommend that the client see a dermatologist (P less than .001) when they had a lesion. Based on this response, the authors said that they believed that educating both clients and tattoo artists may be the best way to get tattoo artists to engage clients. “Our study highlights an opportunity for dermatologists to educate tattoo artists about skin cancer, particularly melanoma, to help reduce the incidence of skin cancers hidden in tattoos and to encourage appropriate referral to dermatologists for suspicious lesions on clients,” they concluded.
“When you perform a total body skin examination, it’s a little difficult to kind of tease out if a lesion looks suspicious or not if it’s surrounded by ink,” Mr. Mori, a medical student at the university, said in an interview. “Tattoos are becoming more and more common, especially among younger people, and incidence of melanoma has increased in younger populations as well. ... It is very concerning that skin cancers could be hidden in tattoos.”
In fact, Mr. Mori pointed out, there are opportunities for dermatologists to reach out to the tattoo artist community and start the communication process. “Tattoo artists have national conferences where they get together and discuss the state of the industry, and that represents one opportunity where dermatologists could talk about the effects of skin cancer,” he said.
The study was funded by the University of Pittsburgh. The authors reported no relevant financial disclosures.
ezimmerman@frontlinemedcom.com
On Twitter @eaztweets
Key clinical point: Dermatologists can educate tattoo artists about avoiding tattoos around moles and other skin lesions.
Major finding: Of 42 tattoo artists who were surveyed, 19 (45%) reported never declining a client’s request to tattoo skin with a lesion, and 31 (74%) reporting having no official store policy on tattooing over lesions.
Data source: An anonymous survey of 42 tattoo artists conducted in July and August 2016.
Disclosures: This study was funded by the University of Pittsburgh. Investigators reported no relevant disclosures.
Acquired Epidermodysplasia Verruciformis Occurring in a Renal Transplant Recipient
Acquired epidermodysplasia verruciformis (EDV) is a rare disorder occurring in patients with depressed cellular immunity, particularly individuals with human immunodeficiency virus (HIV). Rare cases of acquired EDV have been reported in stem cell or solid organ transplant recipients. Weakened cellular immunity predisposes the patient to human papillomavirus (HPV) infections, with 92% of renal transplant recipients developing warts within 5 years posttransplantation.1 Specific EDV-HPV subtypes have been isolated from lesions in several immunosuppressed individuals, with HPV-5 and HPV-8 being the most commonly isolated subtypes.2,3 Herein, we present the clinical findings of a renal transplant recipient who presented for evaluation of multiple skin lesions characteristic of EDV 5 years following transplantation and initiation of immunosuppressive therapy. Additionally, we review the current diagnostic findings, management, and treatment of acquired EDV.
A 44-year-old white woman presented for evaluation of several pruritic cutaneous lesions that had developed on the chest and neck of 1 month’s duration. The patient had been on the immunosuppressant medications cyclosporine and mycophenolate mofetil for more than 5 years following renal transplantation 7 years prior to the current presentation. She also was on low-dose prednisone for chronic systemic lupus erythematosus. Her family history was negative for any pertinent skin conditions.
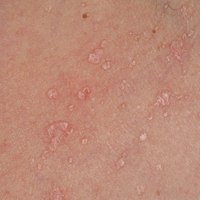
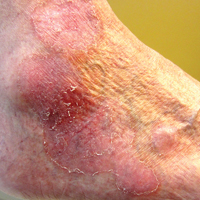
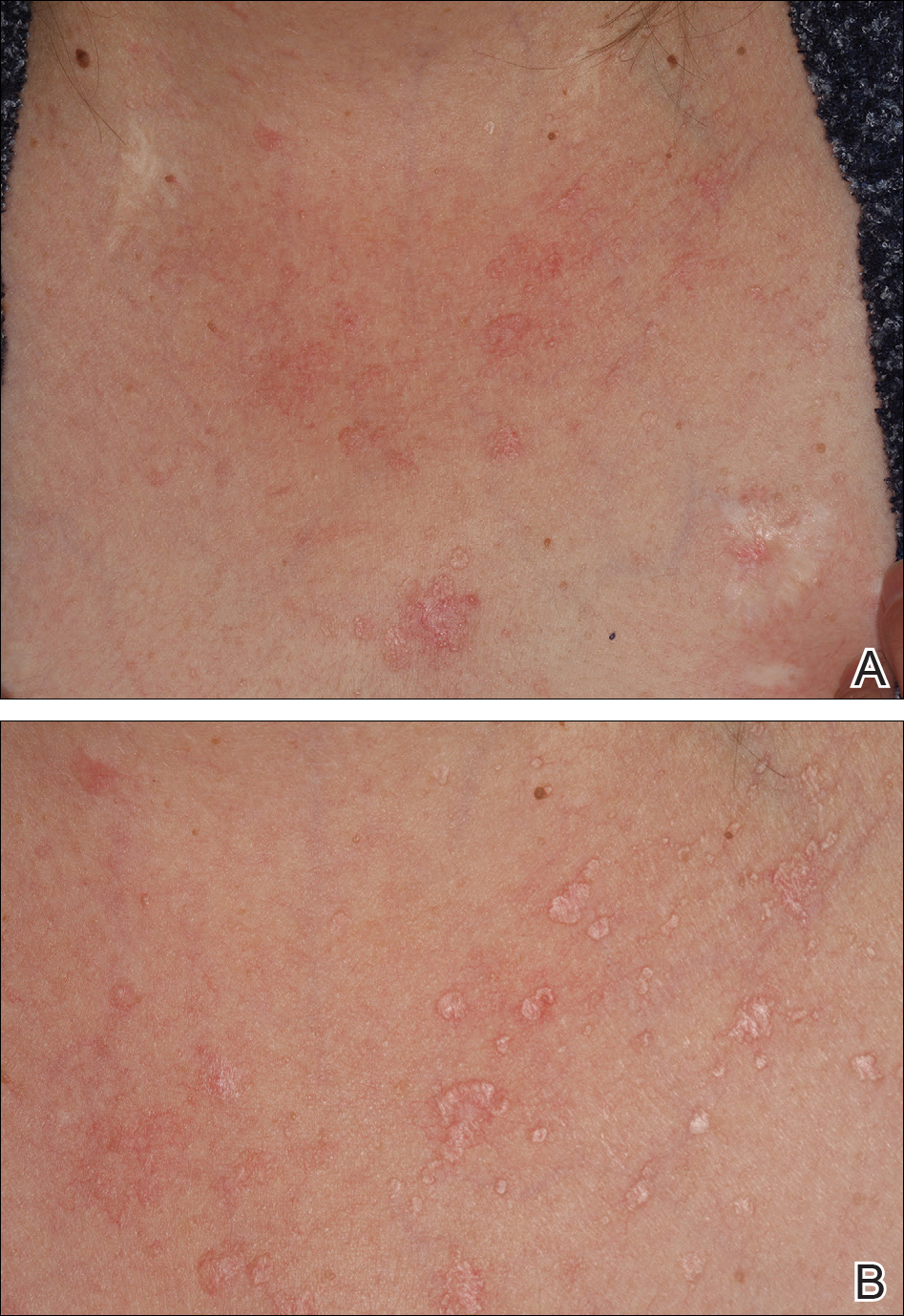
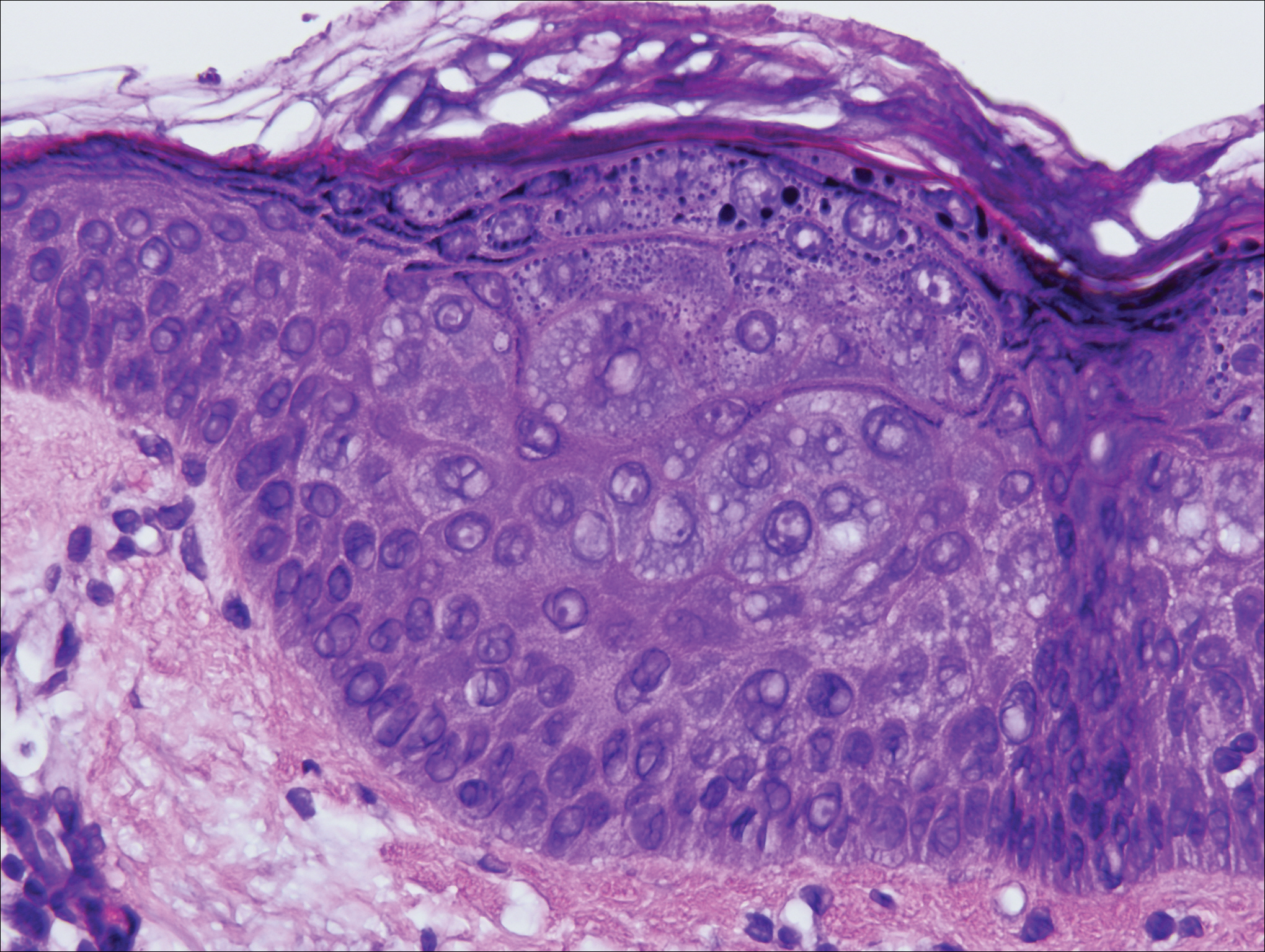
On physical examination the patient exhibited several grouped 0.5-cm, shiny, pink lichenoid macules located on the upper mid chest, anterior neck, and left leg clinically resembling the lesions of pityriasis versicolor (Figure 1). A shave biopsy was taken from one of the newest lesions on the left leg. Histopathology revealed viral epidermal cytopathic changes, blue cytoplasm, and coarse hypergranulosis characteristic of EDV (Figure 2). A diagnosis of acquired EDV was made based on the clinical and histopathologic findings.
The patient’s skin lesions became more widespread despite several different treatment regimens, including cryosurgery; tazarotene cream 0.05% nightly; imiquimod cream 5% once weekly; and intermittent short courses of 5-fluorouracil cream 5%, which provided the best response. At her most recent clinic visit 8 years after initial presentation, she continued to have more widespread lesions on the trunk, arms, and legs, but no evidence of malignant transformation.
Comment
Epidermodysplasia verruciformis was first recognized as an inherited condition, most commonly inherited in an autosomal-dominant fashion; however, X-linked recessive cases have been reported.4,5 Patients with the inherited forms of this condition are prone to recurrent HPV infections secondary to a missense mutation in the epidermodysplasia verruciformis 1 and 2 genes, EVER1 and EVER2, on the EV1 locus located on chromosome 17q25.6 Because of this mutation, the patient’s cellular immunity becomes weakened. Cellular presentation of the EDV-HPV antigen to T lymphocytes becomes impaired, thereby inhibiting the body’s ability to successfully clear itself of the virus.5,6 The most commonly isolated EDV-HPV subtypes are HPV-5 and HPV-8, but HPV types 9, 12, 14, 15, 17, 19, 20, 21, 22, 23, 24, 25, and 50 also have been associated with EDV.1,3,7
Patients who have suppressed cellular immunity, such as transplant recipients on long-term immunosuppressant medications and individuals with HIV, graft-vs-host disease, systemic lupus erythematosus, and hematologic malignancies, are susceptible to EDV, as well as patients with atopic dermatitis being treated with topical calcineurin inhibitors.2,3,8-15 These patients acquire depressed cellular immunity and become increasingly susceptible to infections with the EDV-HPV subtypes. When clinical and histopathologic findings are consistent with EDV, a diagnosis of acquired EDV is given, which was further confirmed in a study conducted by Harwood et al.16 They found immunocompromised patients carry more EDV-HPV subtypes in skin lesions analyzed by polymerase chain reaction than immunocompetent individuals.16 Additionally, there is a positive correlation between the length of immunosuppression and the development of HPV lesions, with a majority of patients developing lesions within 5 years following initial immunosuppression.1,7,10,17
Epidermodysplasia verruciformis commonly presents with multiple hypopigmented to red macules that may coalesce into patches with a fine scale, clinically resembling the lesions of pityriasis versicolor.2,3,8-15 Epidermodysplasia verruciformis also may present as multiple flesh-colored, flat-topped, verrucous papules that clinically resemble the lesions of verruca plana on sun-exposed areas such as the face, arms, and legs.9 The characteristic histopathologic findings are enlarged keratinocytes with perinuclear halos and blue-gray cytoplasm as well as hypergranulosis.18 Immunocompromised hosts infected with EDV-HPV histologically tend to display more severe dysplasia than immunocompetent individuals.19 The differential diagnosis includes pityriasis versicolor, squamous cell carcinoma (SCC), and verruca plana. Tissue cultures and potassium hydroxide scrapings for microorganisms should be negative.
The specific EDV-HPV strains 5, 8, and 41 carry the highest oncogenic potential, with more than 60% of inherited EDV patients developing SCC by the fourth and fifth decades of life.16 Unlike inherited EDV, the clinical course of acquired EDV is less well known; however, UV light is thought to act synergistically with the EDV-HPV in oncogenic transformation of the lesions, as most of the SCCs develop on sun-exposed areas, and darker-skinned patients seem to have a decreased risk for malignant transformation of EDV lesions.4,9,20,21 Preventative measures such as strict sun protection and annual surveillance of lesions can help to prevent oncogenic progression of the lesions; however, several single- and multiple-agent regimens have been used in the treatment of EDV with variable results. Topical imiquimod, 5-fluorouracil, tretinoin, and tazarotene have been used with variable success. Acitretin alone and in combination with interferon alfa-2a also has been used.22,23 Highly active antiretroviral therapy in patients with HIV has effectively decreased the number of lesions in a subset of patients.24 We (anecdotal) and others25 also have had success using photodynamic therapy. Squamous cell carcinoma arising in patients with EDV can be managed by excision or by Mohs micrographic surgery.
Conclusion
We report a rare case of acquired EDV in a solid organ transplant recipient. Epidermodysplasia verruciformis can be acquired in immunosuppressed patients such as ours, and these patients should be followed closely due to the potential for malignant transformation. More studies regarding the anticipated clinical course of skin lesions in patients with acquired EDV are needed to better predict the time frame for malignant transformation.
- Dyall-Smith D, Trowell H, Dyall-Smith ML. Benign human papillomavirus infection in renal transplant recipients. Int J Dermatol. 1991;30:785-789.
- Lutzner MA, Orth G, Dutronquay V, et al. Detection of human papillomavirus type 5 DNA in skin cancers of an immunosuppressed renal allograft recipient. Lancet. 1983;2:422-424.
- Lutzner M, Croissant O, Ducasse MF, et al. A potentially oncogenic human papillomavirus (HPV-5) found in two renal allograft recipients. J Invest Dermatol. 1980;75:353-356.
- Androphy EJ, Dvoretzky I, Lowy DR. X-linked inheritance of epidermodysplasia verruciformis. genetic and virologic studies of a kindred. Arch Dermatol. 1985;121:864-868.
- Lutzner MA. Epidermodysplasia verruciformis. an autosomal recessive disease characterized by viral warts and skin cancer. a model for viral oncogenesis. Bull Cancer. 1978;65:169-182.
- Ramoz N, Rueda LA, Bouadjar B, et al. Mutations in two adjacent novel genes are associated with epidermodysplasia verruciformis. Nat Genet. 2002;32:579-581.
- Rüdlinger R, Smith IW, Bunney MH, et al. Human papillomavirus infections in a group of renal transplant recipients. Br J Dermatol. 1986;115:681-692.
- Kawai K, Egawa N, Kiyono T, et al. Epidermodysplasia-verruciformis-like eruption associated with gamma-papillomavirus infection in a patient with adult T-cell leukemia. Dermatology. 2009;219:274-278.
- Barr BB, Benton EC, McLaren K, et al. Human papilloma virus infection and skin cancer in renal allograft recipients. Lancet. 1989;1:124-129.
- Tanigaki T, Kanda R, Sato K. Epidermodysplasia verruciformis (L-L, 1922) in a patient with systemic lupus erythematosus. Arch Dermatol Res. 1986;278:247-248.
- Holmes C, Chong AH, Tabrizi SN, et al. Epidermodysplasia verruciformis-like syndrome in association with systemic lupus erythematosus. Australas J Dermatol. 2009;50:44-47.
- Gross G, Ellinger K, Roussaki A, et al. Epidermodysplasia verruciformis in a patient with Hodgkin’s disease: characterization of a new papillomavirus type and interferon treatment. J Invest Dermatol. 1988;91:43-48.
- Fernandez KH, Rady P, Tyring S, et al. Acquired epidermodysplasia verruciformis in a child with atopic dermatitis [published online September 3, 2012]. Pediatr Dermatol. 2014;31:400-402.
- Hultgren TL, Srinivasan SK, DiMaio DJ. Epidermodysplasia verruciformis occurring in a patient with human immunodeficiency virus: a case report. Cutis. 2007;79:307-311.
- Kunishige JH, Hymes SR, Madkan V, et al. Epidermodysplasia verruciformis in the setting of graft-versus-host disease. J Am Acad Dermatol. 2007;57(5 suppl):S78-S80.
- Harwood CA, Surentheran T, McGregor JM, et al. Human papillomavirus infection and non-melanoma skin cancer in immunosuppressed and immunocompetent individuals. J Med Virol. 2000;61:289-297.
- Moloney FJ, Keane S, O’Kelly P, et al. The impact of skin disease following renal transplantation on quality of life. Br J Dermatol. 2005;153:574-578.
- Tanigaki T, Endo H. A case of epidermodysplasia verruciformis (Lewandowsky-Lutz, 1922) with skin cancer: histopathology of malignant cutaneous changes. Dermatologica. 1984;169:97-101.
- Morrison C, Eliezri Y, Magro C, et al. The histologic spectrum of epidermodysplasia verruciformis in transplant and AIDS patients. J Cutan Pathol. 2002;29:480-489.
- Majewski S, Jabło´nska S. Epidermodysplasia verruciformis as a model of human papillomavirus-induced genetic cancer of the skin. Arch Dermatol. 1995;131:1312-1318.
- Jacyk WK, De Villiers EM. Epidermodysplasia verruciformis in Africans. Int J Dermatol. 1993;32:806-810.
- Gubinelli E, Posteraro P, Cocuroccia B, et al. Epidermodysplasia verruciformis with multiple mucosal carcinomas treated with pegylated interferon alfa and acitretin. J Dermatolog Treat. 2003;14:184-188.
- Anadolu R, Oskay T, Erdem C, et al. Treatment of epidermodysplasia verruciformis with a combination of acitretin and interferon alfa-2a. J Am Acad Dermatol. 2001;45:296-299.
- Haas N, Fuchs PG, Hermes B, et al. Remission of epidermodysplasia verruciformis-like skin eruption after highly active antiretroviral therapy in a human immunodeficiency virus-positive patient. Br J Dermatol. 2001;145:669-670.
- Karrer S, Szeimies RM, Abels C, et al. Epidermo-dysplasia verruciformis treated using topical 5-aminolaevulinic acid photodynamic therapy. Br J Dermatol. 1999;140:935-938.
Acquired epidermodysplasia verruciformis (EDV) is a rare disorder occurring in patients with depressed cellular immunity, particularly individuals with human immunodeficiency virus (HIV). Rare cases of acquired EDV have been reported in stem cell or solid organ transplant recipients. Weakened cellular immunity predisposes the patient to human papillomavirus (HPV) infections, with 92% of renal transplant recipients developing warts within 5 years posttransplantation.1 Specific EDV-HPV subtypes have been isolated from lesions in several immunosuppressed individuals, with HPV-5 and HPV-8 being the most commonly isolated subtypes.2,3 Herein, we present the clinical findings of a renal transplant recipient who presented for evaluation of multiple skin lesions characteristic of EDV 5 years following transplantation and initiation of immunosuppressive therapy. Additionally, we review the current diagnostic findings, management, and treatment of acquired EDV.
A 44-year-old white woman presented for evaluation of several pruritic cutaneous lesions that had developed on the chest and neck of 1 month’s duration. The patient had been on the immunosuppressant medications cyclosporine and mycophenolate mofetil for more than 5 years following renal transplantation 7 years prior to the current presentation. She also was on low-dose prednisone for chronic systemic lupus erythematosus. Her family history was negative for any pertinent skin conditions.
On physical examination the patient exhibited several grouped 0.5-cm, shiny, pink lichenoid macules located on the upper mid chest, anterior neck, and left leg clinically resembling the lesions of pityriasis versicolor (Figure 1). A shave biopsy was taken from one of the newest lesions on the left leg. Histopathology revealed viral epidermal cytopathic changes, blue cytoplasm, and coarse hypergranulosis characteristic of EDV (Figure 2). A diagnosis of acquired EDV was made based on the clinical and histopathologic findings.


The patient’s skin lesions became more widespread despite several different treatment regimens, including cryosurgery; tazarotene cream 0.05% nightly; imiquimod cream 5% once weekly; and intermittent short courses of 5-fluorouracil cream 5%, which provided the best response. At her most recent clinic visit 8 years after initial presentation, she continued to have more widespread lesions on the trunk, arms, and legs, but no evidence of malignant transformation.
Comment
Epidermodysplasia verruciformis was first recognized as an inherited condition, most commonly inherited in an autosomal-dominant fashion; however, X-linked recessive cases have been reported.4,5 Patients with the inherited forms of this condition are prone to recurrent HPV infections secondary to a missense mutation in the epidermodysplasia verruciformis 1 and 2 genes, EVER1 and EVER2, on the EV1 locus located on chromosome 17q25.6 Because of this mutation, the patient’s cellular immunity becomes weakened. Cellular presentation of the EDV-HPV antigen to T lymphocytes becomes impaired, thereby inhibiting the body’s ability to successfully clear itself of the virus.5,6 The most commonly isolated EDV-HPV subtypes are HPV-5 and HPV-8, but HPV types 9, 12, 14, 15, 17, 19, 20, 21, 22, 23, 24, 25, and 50 also have been associated with EDV.1,3,7
Patients who have suppressed cellular immunity, such as transplant recipients on long-term immunosuppressant medications and individuals with HIV, graft-vs-host disease, systemic lupus erythematosus, and hematologic malignancies, are susceptible to EDV, as well as patients with atopic dermatitis being treated with topical calcineurin inhibitors.2,3,8-15 These patients acquire depressed cellular immunity and become increasingly susceptible to infections with the EDV-HPV subtypes. When clinical and histopathologic findings are consistent with EDV, a diagnosis of acquired EDV is given, which was further confirmed in a study conducted by Harwood et al.16 They found immunocompromised patients carry more EDV-HPV subtypes in skin lesions analyzed by polymerase chain reaction than immunocompetent individuals.16 Additionally, there is a positive correlation between the length of immunosuppression and the development of HPV lesions, with a majority of patients developing lesions within 5 years following initial immunosuppression.1,7,10,17
Epidermodysplasia verruciformis commonly presents with multiple hypopigmented to red macules that may coalesce into patches with a fine scale, clinically resembling the lesions of pityriasis versicolor.2,3,8-15 Epidermodysplasia verruciformis also may present as multiple flesh-colored, flat-topped, verrucous papules that clinically resemble the lesions of verruca plana on sun-exposed areas such as the face, arms, and legs.9 The characteristic histopathologic findings are enlarged keratinocytes with perinuclear halos and blue-gray cytoplasm as well as hypergranulosis.18 Immunocompromised hosts infected with EDV-HPV histologically tend to display more severe dysplasia than immunocompetent individuals.19 The differential diagnosis includes pityriasis versicolor, squamous cell carcinoma (SCC), and verruca plana. Tissue cultures and potassium hydroxide scrapings for microorganisms should be negative.
The specific EDV-HPV strains 5, 8, and 41 carry the highest oncogenic potential, with more than 60% of inherited EDV patients developing SCC by the fourth and fifth decades of life.16 Unlike inherited EDV, the clinical course of acquired EDV is less well known; however, UV light is thought to act synergistically with the EDV-HPV in oncogenic transformation of the lesions, as most of the SCCs develop on sun-exposed areas, and darker-skinned patients seem to have a decreased risk for malignant transformation of EDV lesions.4,9,20,21 Preventative measures such as strict sun protection and annual surveillance of lesions can help to prevent oncogenic progression of the lesions; however, several single- and multiple-agent regimens have been used in the treatment of EDV with variable results. Topical imiquimod, 5-fluorouracil, tretinoin, and tazarotene have been used with variable success. Acitretin alone and in combination with interferon alfa-2a also has been used.22,23 Highly active antiretroviral therapy in patients with HIV has effectively decreased the number of lesions in a subset of patients.24 We (anecdotal) and others25 also have had success using photodynamic therapy. Squamous cell carcinoma arising in patients with EDV can be managed by excision or by Mohs micrographic surgery.
Conclusion
We report a rare case of acquired EDV in a solid organ transplant recipient. Epidermodysplasia verruciformis can be acquired in immunosuppressed patients such as ours, and these patients should be followed closely due to the potential for malignant transformation. More studies regarding the anticipated clinical course of skin lesions in patients with acquired EDV are needed to better predict the time frame for malignant transformation.
Acquired epidermodysplasia verruciformis (EDV) is a rare disorder occurring in patients with depressed cellular immunity, particularly individuals with human immunodeficiency virus (HIV). Rare cases of acquired EDV have been reported in stem cell or solid organ transplant recipients. Weakened cellular immunity predisposes the patient to human papillomavirus (HPV) infections, with 92% of renal transplant recipients developing warts within 5 years posttransplantation.1 Specific EDV-HPV subtypes have been isolated from lesions in several immunosuppressed individuals, with HPV-5 and HPV-8 being the most commonly isolated subtypes.2,3 Herein, we present the clinical findings of a renal transplant recipient who presented for evaluation of multiple skin lesions characteristic of EDV 5 years following transplantation and initiation of immunosuppressive therapy. Additionally, we review the current diagnostic findings, management, and treatment of acquired EDV.
A 44-year-old white woman presented for evaluation of several pruritic cutaneous lesions that had developed on the chest and neck of 1 month’s duration. The patient had been on the immunosuppressant medications cyclosporine and mycophenolate mofetil for more than 5 years following renal transplantation 7 years prior to the current presentation. She also was on low-dose prednisone for chronic systemic lupus erythematosus. Her family history was negative for any pertinent skin conditions.
On physical examination the patient exhibited several grouped 0.5-cm, shiny, pink lichenoid macules located on the upper mid chest, anterior neck, and left leg clinically resembling the lesions of pityriasis versicolor (Figure 1). A shave biopsy was taken from one of the newest lesions on the left leg. Histopathology revealed viral epidermal cytopathic changes, blue cytoplasm, and coarse hypergranulosis characteristic of EDV (Figure 2). A diagnosis of acquired EDV was made based on the clinical and histopathologic findings.


The patient’s skin lesions became more widespread despite several different treatment regimens, including cryosurgery; tazarotene cream 0.05% nightly; imiquimod cream 5% once weekly; and intermittent short courses of 5-fluorouracil cream 5%, which provided the best response. At her most recent clinic visit 8 years after initial presentation, she continued to have more widespread lesions on the trunk, arms, and legs, but no evidence of malignant transformation.
Comment
Epidermodysplasia verruciformis was first recognized as an inherited condition, most commonly inherited in an autosomal-dominant fashion; however, X-linked recessive cases have been reported.4,5 Patients with the inherited forms of this condition are prone to recurrent HPV infections secondary to a missense mutation in the epidermodysplasia verruciformis 1 and 2 genes, EVER1 and EVER2, on the EV1 locus located on chromosome 17q25.6 Because of this mutation, the patient’s cellular immunity becomes weakened. Cellular presentation of the EDV-HPV antigen to T lymphocytes becomes impaired, thereby inhibiting the body’s ability to successfully clear itself of the virus.5,6 The most commonly isolated EDV-HPV subtypes are HPV-5 and HPV-8, but HPV types 9, 12, 14, 15, 17, 19, 20, 21, 22, 23, 24, 25, and 50 also have been associated with EDV.1,3,7
Patients who have suppressed cellular immunity, such as transplant recipients on long-term immunosuppressant medications and individuals with HIV, graft-vs-host disease, systemic lupus erythematosus, and hematologic malignancies, are susceptible to EDV, as well as patients with atopic dermatitis being treated with topical calcineurin inhibitors.2,3,8-15 These patients acquire depressed cellular immunity and become increasingly susceptible to infections with the EDV-HPV subtypes. When clinical and histopathologic findings are consistent with EDV, a diagnosis of acquired EDV is given, which was further confirmed in a study conducted by Harwood et al.16 They found immunocompromised patients carry more EDV-HPV subtypes in skin lesions analyzed by polymerase chain reaction than immunocompetent individuals.16 Additionally, there is a positive correlation between the length of immunosuppression and the development of HPV lesions, with a majority of patients developing lesions within 5 years following initial immunosuppression.1,7,10,17
Epidermodysplasia verruciformis commonly presents with multiple hypopigmented to red macules that may coalesce into patches with a fine scale, clinically resembling the lesions of pityriasis versicolor.2,3,8-15 Epidermodysplasia verruciformis also may present as multiple flesh-colored, flat-topped, verrucous papules that clinically resemble the lesions of verruca plana on sun-exposed areas such as the face, arms, and legs.9 The characteristic histopathologic findings are enlarged keratinocytes with perinuclear halos and blue-gray cytoplasm as well as hypergranulosis.18 Immunocompromised hosts infected with EDV-HPV histologically tend to display more severe dysplasia than immunocompetent individuals.19 The differential diagnosis includes pityriasis versicolor, squamous cell carcinoma (SCC), and verruca plana. Tissue cultures and potassium hydroxide scrapings for microorganisms should be negative.
The specific EDV-HPV strains 5, 8, and 41 carry the highest oncogenic potential, with more than 60% of inherited EDV patients developing SCC by the fourth and fifth decades of life.16 Unlike inherited EDV, the clinical course of acquired EDV is less well known; however, UV light is thought to act synergistically with the EDV-HPV in oncogenic transformation of the lesions, as most of the SCCs develop on sun-exposed areas, and darker-skinned patients seem to have a decreased risk for malignant transformation of EDV lesions.4,9,20,21 Preventative measures such as strict sun protection and annual surveillance of lesions can help to prevent oncogenic progression of the lesions; however, several single- and multiple-agent regimens have been used in the treatment of EDV with variable results. Topical imiquimod, 5-fluorouracil, tretinoin, and tazarotene have been used with variable success. Acitretin alone and in combination with interferon alfa-2a also has been used.22,23 Highly active antiretroviral therapy in patients with HIV has effectively decreased the number of lesions in a subset of patients.24 We (anecdotal) and others25 also have had success using photodynamic therapy. Squamous cell carcinoma arising in patients with EDV can be managed by excision or by Mohs micrographic surgery.
Conclusion
We report a rare case of acquired EDV in a solid organ transplant recipient. Epidermodysplasia verruciformis can be acquired in immunosuppressed patients such as ours, and these patients should be followed closely due to the potential for malignant transformation. More studies regarding the anticipated clinical course of skin lesions in patients with acquired EDV are needed to better predict the time frame for malignant transformation.
- Dyall-Smith D, Trowell H, Dyall-Smith ML. Benign human papillomavirus infection in renal transplant recipients. Int J Dermatol. 1991;30:785-789.
- Lutzner MA, Orth G, Dutronquay V, et al. Detection of human papillomavirus type 5 DNA in skin cancers of an immunosuppressed renal allograft recipient. Lancet. 1983;2:422-424.
- Lutzner M, Croissant O, Ducasse MF, et al. A potentially oncogenic human papillomavirus (HPV-5) found in two renal allograft recipients. J Invest Dermatol. 1980;75:353-356.
- Androphy EJ, Dvoretzky I, Lowy DR. X-linked inheritance of epidermodysplasia verruciformis. genetic and virologic studies of a kindred. Arch Dermatol. 1985;121:864-868.
- Lutzner MA. Epidermodysplasia verruciformis. an autosomal recessive disease characterized by viral warts and skin cancer. a model for viral oncogenesis. Bull Cancer. 1978;65:169-182.
- Ramoz N, Rueda LA, Bouadjar B, et al. Mutations in two adjacent novel genes are associated with epidermodysplasia verruciformis. Nat Genet. 2002;32:579-581.
- Rüdlinger R, Smith IW, Bunney MH, et al. Human papillomavirus infections in a group of renal transplant recipients. Br J Dermatol. 1986;115:681-692.
- Kawai K, Egawa N, Kiyono T, et al. Epidermodysplasia-verruciformis-like eruption associated with gamma-papillomavirus infection in a patient with adult T-cell leukemia. Dermatology. 2009;219:274-278.
- Barr BB, Benton EC, McLaren K, et al. Human papilloma virus infection and skin cancer in renal allograft recipients. Lancet. 1989;1:124-129.
- Tanigaki T, Kanda R, Sato K. Epidermodysplasia verruciformis (L-L, 1922) in a patient with systemic lupus erythematosus. Arch Dermatol Res. 1986;278:247-248.
- Holmes C, Chong AH, Tabrizi SN, et al. Epidermodysplasia verruciformis-like syndrome in association with systemic lupus erythematosus. Australas J Dermatol. 2009;50:44-47.
- Gross G, Ellinger K, Roussaki A, et al. Epidermodysplasia verruciformis in a patient with Hodgkin’s disease: characterization of a new papillomavirus type and interferon treatment. J Invest Dermatol. 1988;91:43-48.
- Fernandez KH, Rady P, Tyring S, et al. Acquired epidermodysplasia verruciformis in a child with atopic dermatitis [published online September 3, 2012]. Pediatr Dermatol. 2014;31:400-402.
- Hultgren TL, Srinivasan SK, DiMaio DJ. Epidermodysplasia verruciformis occurring in a patient with human immunodeficiency virus: a case report. Cutis. 2007;79:307-311.
- Kunishige JH, Hymes SR, Madkan V, et al. Epidermodysplasia verruciformis in the setting of graft-versus-host disease. J Am Acad Dermatol. 2007;57(5 suppl):S78-S80.
- Harwood CA, Surentheran T, McGregor JM, et al. Human papillomavirus infection and non-melanoma skin cancer in immunosuppressed and immunocompetent individuals. J Med Virol. 2000;61:289-297.
- Moloney FJ, Keane S, O’Kelly P, et al. The impact of skin disease following renal transplantation on quality of life. Br J Dermatol. 2005;153:574-578.
- Tanigaki T, Endo H. A case of epidermodysplasia verruciformis (Lewandowsky-Lutz, 1922) with skin cancer: histopathology of malignant cutaneous changes. Dermatologica. 1984;169:97-101.
- Morrison C, Eliezri Y, Magro C, et al. The histologic spectrum of epidermodysplasia verruciformis in transplant and AIDS patients. J Cutan Pathol. 2002;29:480-489.
- Majewski S, Jabło´nska S. Epidermodysplasia verruciformis as a model of human papillomavirus-induced genetic cancer of the skin. Arch Dermatol. 1995;131:1312-1318.
- Jacyk WK, De Villiers EM. Epidermodysplasia verruciformis in Africans. Int J Dermatol. 1993;32:806-810.
- Gubinelli E, Posteraro P, Cocuroccia B, et al. Epidermodysplasia verruciformis with multiple mucosal carcinomas treated with pegylated interferon alfa and acitretin. J Dermatolog Treat. 2003;14:184-188.
- Anadolu R, Oskay T, Erdem C, et al. Treatment of epidermodysplasia verruciformis with a combination of acitretin and interferon alfa-2a. J Am Acad Dermatol. 2001;45:296-299.
- Haas N, Fuchs PG, Hermes B, et al. Remission of epidermodysplasia verruciformis-like skin eruption after highly active antiretroviral therapy in a human immunodeficiency virus-positive patient. Br J Dermatol. 2001;145:669-670.
- Karrer S, Szeimies RM, Abels C, et al. Epidermo-dysplasia verruciformis treated using topical 5-aminolaevulinic acid photodynamic therapy. Br J Dermatol. 1999;140:935-938.
- Dyall-Smith D, Trowell H, Dyall-Smith ML. Benign human papillomavirus infection in renal transplant recipients. Int J Dermatol. 1991;30:785-789.
- Lutzner MA, Orth G, Dutronquay V, et al. Detection of human papillomavirus type 5 DNA in skin cancers of an immunosuppressed renal allograft recipient. Lancet. 1983;2:422-424.
- Lutzner M, Croissant O, Ducasse MF, et al. A potentially oncogenic human papillomavirus (HPV-5) found in two renal allograft recipients. J Invest Dermatol. 1980;75:353-356.
- Androphy EJ, Dvoretzky I, Lowy DR. X-linked inheritance of epidermodysplasia verruciformis. genetic and virologic studies of a kindred. Arch Dermatol. 1985;121:864-868.
- Lutzner MA. Epidermodysplasia verruciformis. an autosomal recessive disease characterized by viral warts and skin cancer. a model for viral oncogenesis. Bull Cancer. 1978;65:169-182.
- Ramoz N, Rueda LA, Bouadjar B, et al. Mutations in two adjacent novel genes are associated with epidermodysplasia verruciformis. Nat Genet. 2002;32:579-581.
- Rüdlinger R, Smith IW, Bunney MH, et al. Human papillomavirus infections in a group of renal transplant recipients. Br J Dermatol. 1986;115:681-692.
- Kawai K, Egawa N, Kiyono T, et al. Epidermodysplasia-verruciformis-like eruption associated with gamma-papillomavirus infection in a patient with adult T-cell leukemia. Dermatology. 2009;219:274-278.
- Barr BB, Benton EC, McLaren K, et al. Human papilloma virus infection and skin cancer in renal allograft recipients. Lancet. 1989;1:124-129.
- Tanigaki T, Kanda R, Sato K. Epidermodysplasia verruciformis (L-L, 1922) in a patient with systemic lupus erythematosus. Arch Dermatol Res. 1986;278:247-248.
- Holmes C, Chong AH, Tabrizi SN, et al. Epidermodysplasia verruciformis-like syndrome in association with systemic lupus erythematosus. Australas J Dermatol. 2009;50:44-47.
- Gross G, Ellinger K, Roussaki A, et al. Epidermodysplasia verruciformis in a patient with Hodgkin’s disease: characterization of a new papillomavirus type and interferon treatment. J Invest Dermatol. 1988;91:43-48.
- Fernandez KH, Rady P, Tyring S, et al. Acquired epidermodysplasia verruciformis in a child with atopic dermatitis [published online September 3, 2012]. Pediatr Dermatol. 2014;31:400-402.
- Hultgren TL, Srinivasan SK, DiMaio DJ. Epidermodysplasia verruciformis occurring in a patient with human immunodeficiency virus: a case report. Cutis. 2007;79:307-311.
- Kunishige JH, Hymes SR, Madkan V, et al. Epidermodysplasia verruciformis in the setting of graft-versus-host disease. J Am Acad Dermatol. 2007;57(5 suppl):S78-S80.
- Harwood CA, Surentheran T, McGregor JM, et al. Human papillomavirus infection and non-melanoma skin cancer in immunosuppressed and immunocompetent individuals. J Med Virol. 2000;61:289-297.
- Moloney FJ, Keane S, O’Kelly P, et al. The impact of skin disease following renal transplantation on quality of life. Br J Dermatol. 2005;153:574-578.
- Tanigaki T, Endo H. A case of epidermodysplasia verruciformis (Lewandowsky-Lutz, 1922) with skin cancer: histopathology of malignant cutaneous changes. Dermatologica. 1984;169:97-101.
- Morrison C, Eliezri Y, Magro C, et al. The histologic spectrum of epidermodysplasia verruciformis in transplant and AIDS patients. J Cutan Pathol. 2002;29:480-489.
- Majewski S, Jabło´nska S. Epidermodysplasia verruciformis as a model of human papillomavirus-induced genetic cancer of the skin. Arch Dermatol. 1995;131:1312-1318.
- Jacyk WK, De Villiers EM. Epidermodysplasia verruciformis in Africans. Int J Dermatol. 1993;32:806-810.
- Gubinelli E, Posteraro P, Cocuroccia B, et al. Epidermodysplasia verruciformis with multiple mucosal carcinomas treated with pegylated interferon alfa and acitretin. J Dermatolog Treat. 2003;14:184-188.
- Anadolu R, Oskay T, Erdem C, et al. Treatment of epidermodysplasia verruciformis with a combination of acitretin and interferon alfa-2a. J Am Acad Dermatol. 2001;45:296-299.
- Haas N, Fuchs PG, Hermes B, et al. Remission of epidermodysplasia verruciformis-like skin eruption after highly active antiretroviral therapy in a human immunodeficiency virus-positive patient. Br J Dermatol. 2001;145:669-670.
- Karrer S, Szeimies RM, Abels C, et al. Epidermo-dysplasia verruciformis treated using topical 5-aminolaevulinic acid photodynamic therapy. Br J Dermatol. 1999;140:935-938.
Practice Points
- Acquired epidermodysplasia verruciformis (EDV) is a rare complication of iatrogenic immuno-suppression in the setting of solid organ transplantation.
- Patients with EDV should be counseled to avoid exposure to UV radiation to reduce the risk formalignant transformation.
Cutaneous Metastasis of a Pulmonary Carcinoid Tumor
Case Report
A 72-year-old white man with a history of pancreatic adenocarcinoma presented for Mohs micrographic surgery of a basal cell carcinoma on the right helix. On the day of the surgery, the patient reported a new, rapidly growing, exquisitely painful lesion on the cheek of 3 to 4 weeks’ duration. Physical examination revealed a 0.8×0.8×0.8-cm, extremely tender, firm, pink papule on the right preauricular cheek. A horizontal deep shave excision was done and the histopathology was remarkable for neoplastic cells with necrosis in the dermis. We observed dermal cellular infiltrates in the form of sheets and nodules, some showing central necrosis (Figure 1). At higher magnification, a trabecular arrangement of cells was seen. These cells had a moderate amount of cytoplasm with eccentric nuclei and rare nucleoli (Figure 2). Mitotic figures were seen at higher magnification (Figure 3). Immunohistochemistry of the neoplastic cells exhibited similar positive staining for the neuroendocrine markers chromogranin A and synaptophysin (Figure 4). Staining of the neoplastic cells also was positive for thyroid transcription factor 1 (TTF-1) and cancer antigen 19-9. Villin and caudal type homeobox 2 stains were negative. These results were consistent with cutaneous metastasis from a known pulmonary carcinoid tumor.
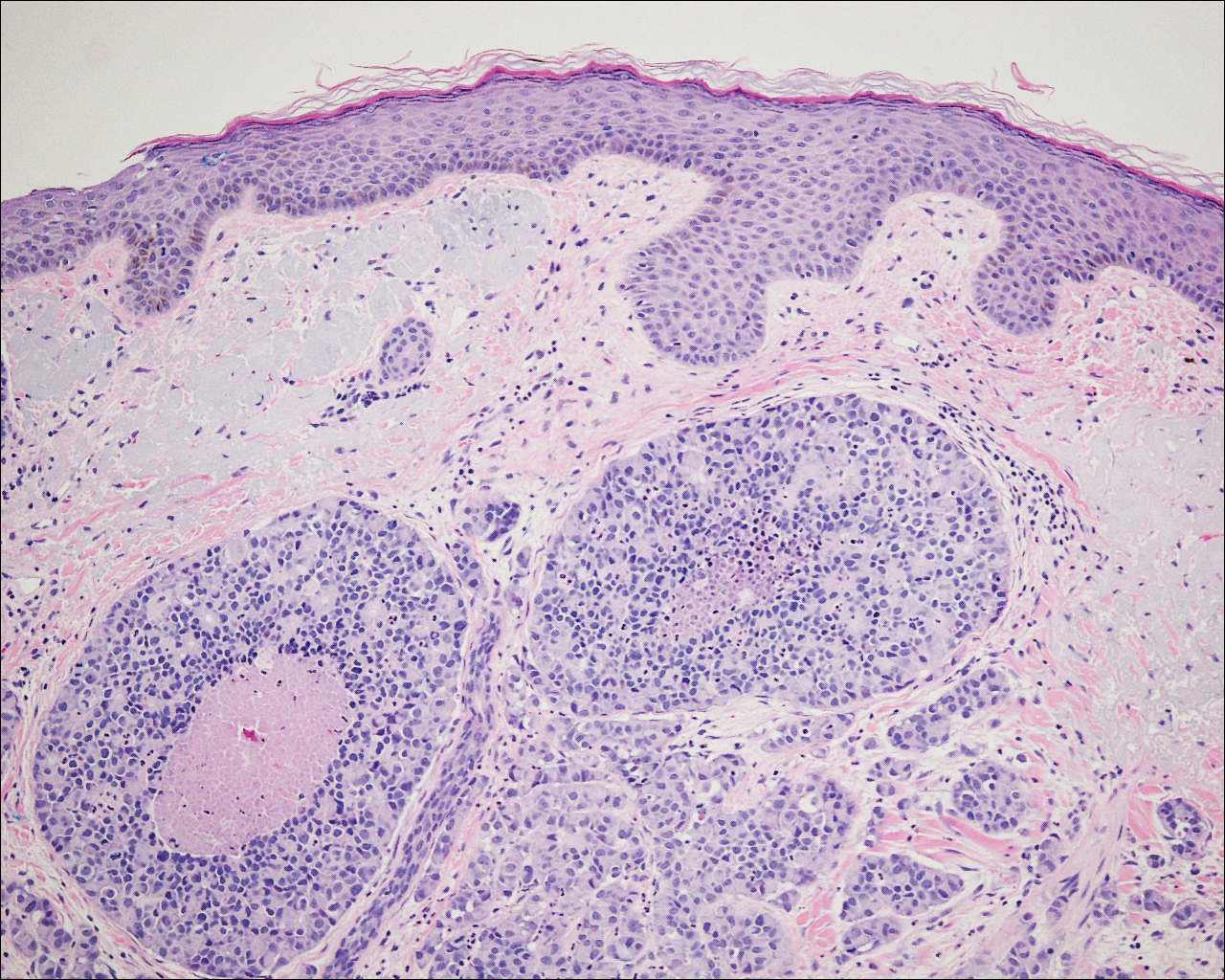
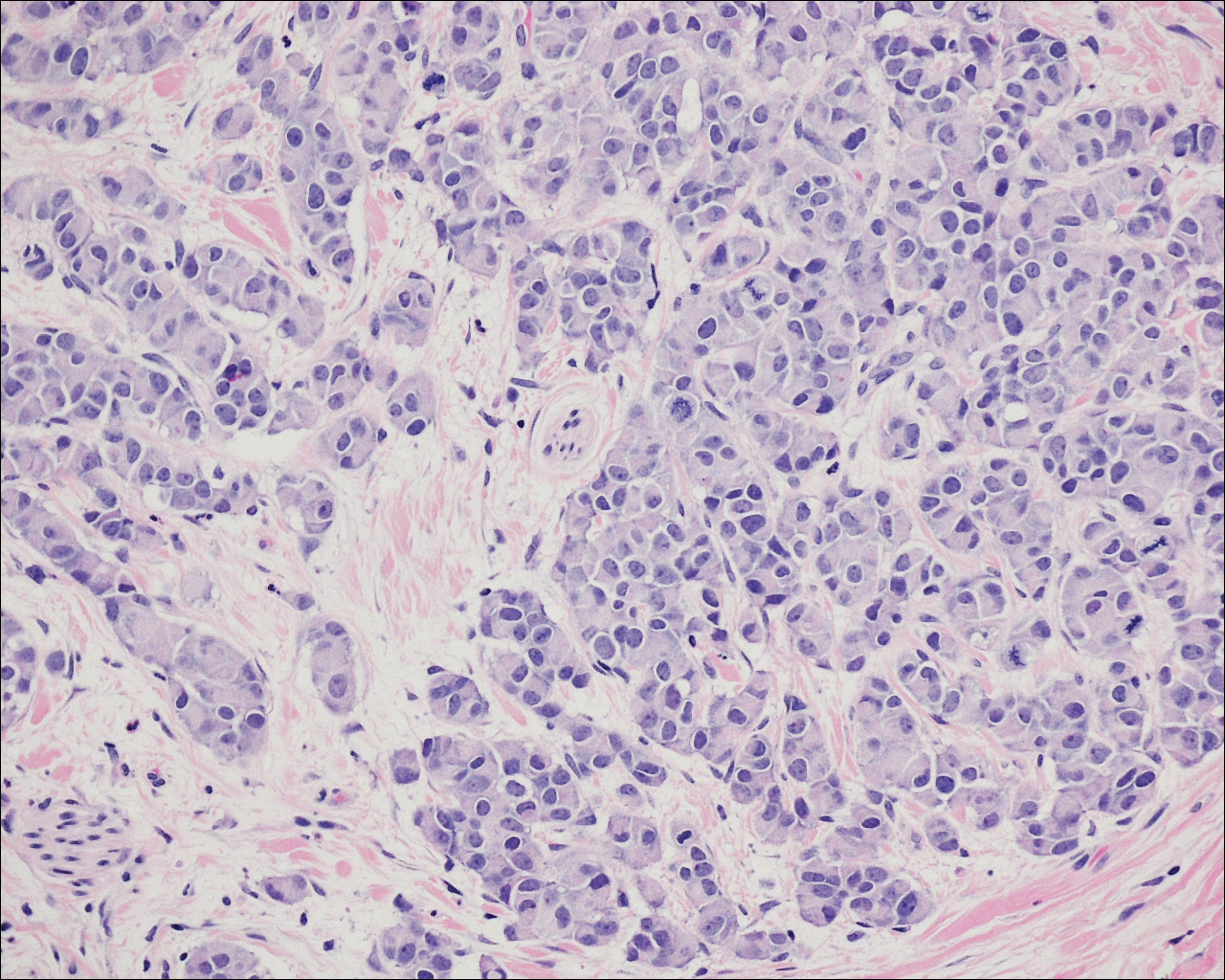
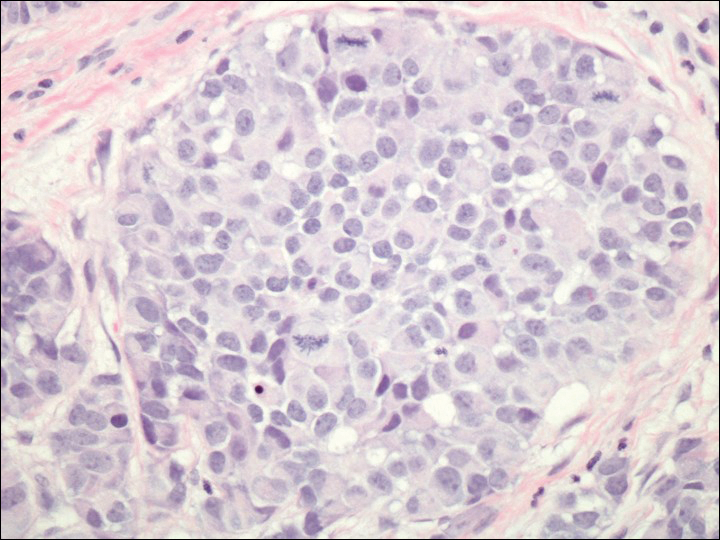
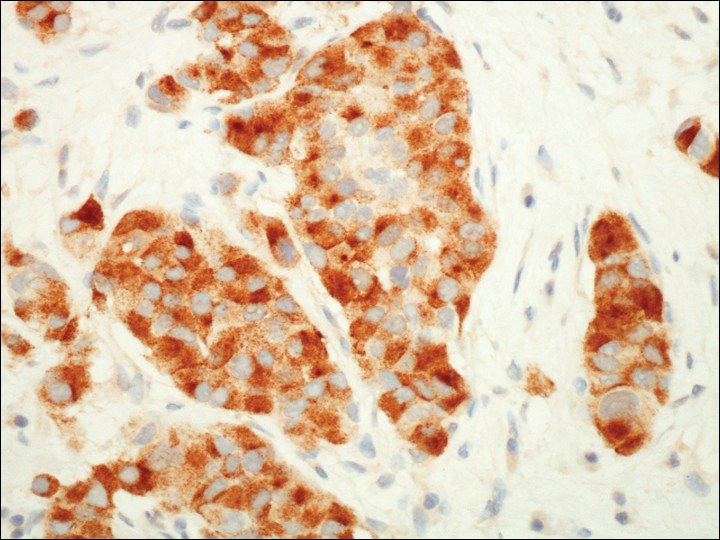
On further review of the patient’s medical history, it was discovered that he had undergone a Whipple procedure with adjuvant chemotherapy and radiation for pancreatic adenocarcinoma approximately 4 years prior to the current presentation. He was then followed by oncology, and 3 years later a chest computed tomography suggested possible disease progression with a new pulmonary metastasis. This pulmonary lesion was biopsied and immunologic staining was consistent with a primary neuroendocrine neoplasm of the lung, a new carcinoid tumor. The tissue was positive for cytokeratin (CK) 7,TTF-1, cancer antigen 19-9, CD56, synaptophysin, and chromogranin A, and was negative for villin and CK20. By the time he was seen in our clinic, several trials of chemotherapy had failed. Serial computed tomography subsequently demonstrated progression of the lung disease and he later developed malignant pleural effusions. Approximately 6 months after the cutaneous carcinoid metastasis was diagnosed, the patient died of respiratory failure.
Comment
Carcinoid tumors are uncommon neoplasms of neuroendocrine origin that generally arise in the gastrointestinal or bronchopulmonary tracts. Metastases from these primary neoplasms more commonly affect the regional lymph nodes or viscera, with rare reports of cutaneous metastases to the skin. The true incidence of carcinoid tumors with metastasis to the skin is unknown because it is limited to single case reports in the literature.
The clinical presentation of cutaneous carcinoid metastases has been reported most commonly as firm papules of varying sizes with no specific site predilection.1 The color of these lesions has ranged from erythematous to violaceous to brown.2 Several of the reported cases were noted to be extremely tender and painful, while other reports of lesions were noted to be asymptomatic or only mildly pruritic.3-7
Carcinoid syndrome is more common with neoplasms present within the gastrointestinal tract, but it also has been reported with large bronchial carcinoid tumors and with metastatic disease.8,9 Paroxysmal flushing is the most prominent cutaneous manifestation of this syndrome, occurring in 75% of patients.10,11 Other common symptoms include patchy cyanosis, telangiectasia, and pellagralike skin lesions.3 Carcinoid syndrome secondary to bronchial adenomas is thought to differ from gastrointestinal carcinoid neoplasms in that it has prolonged flushing (hours to days instead of minutes) and is characterized by marked anxiety, fever, disorientation, sweating, and lacrimation.8,9
Many cases of cutaneous carcinoid metastases have been accompanied by reports of exquisite tenderness,7 similar to our patient. The pathogenesis of the pain in these lesions is still unclear, but several hypotheses have been established. It has been postulated that perineural invasion by the tumor is responsible for the pain; however, this finding has been inconsistent, as neural involvement also has been present in nonpainful lesions.2,5,7,12 Another theory for the pain is that it is secondary to the release of vasoactive substances and peptide hormones from the carcinoid cells, such as kallikrein and serotonin. Lastly, local tissue necrosis and fibrosis also have been suggested as possible etiologies.7
The histology of cutaneous carcinoid metastases typically resembles the primary lesion and may demonstrate fascicles of spindle cells with focal areas of necrosis, mild atypia, and a relatively low mitotic rate.10 Other neoplasms such as Merkel cell carcinoma and carcinoidlike sebaceous carcinoma should be considered in the differential diagnosis. A primary malignant peripheral primitive neuroectodermal tumor or a primary cutaneous carcinoid tumor is less common but should be considered. Differing from carcinoid tumors, Merkel cell carcinomas usually have a higher mitotic rate and positive staining for CK20. The sebaceous neoplasms with a carcinoidlike pattern may appear histologically similar, requiring immunohistochemical evaluation with monoclonal antibodies such as D2-40.13 A diffuse granular cytoplasmic reaction to chromogranin A is characteristic of carcinoid tumors. Synaptophysin and TTF-1 also are positive in carcinoid tumors, with TTF-1 being highly specific for neuroendocrine tumors of the lung.10
Cutaneous metastases of internal malignancies are more common from carcinomas of the lungs, gastrointestinal tract, and breasts.5 Occasionally, the cutaneous metastasis will develop directly over the underlying malignancy. Our case of cutaneous metastasis of a carcinoid tumor presented as an exquisitely tender and painful papule on the cheek. The histology of the lesion was consistent with the known carcinoid tumor of the lung. Because these lesions are extremely uncommon, it is imperative to obtain an accurate clinical history and use the appropriate immunohistochemical panel to correctly diagnose these metastases.
- Blochin E, Stein JA, Wang NS. Atypical carcinoid metastasis to the skin. Am J Dermatopathol. 2010;32:735-739.
- Rodriguez G, Villamizar R. Carcinoid tumor with skin metastasis. Am J Dermatopathol. 1992;14:263-269.
- Archer CB, Rauch HJ, Allen MH, et al. Ultrastructural features of metastatic cutaneous carcinoid. J Cutan Pathol. 1984;11:485-490.
- Archer CB, Wells RS, MacDonald DM. Metastatic cutaneous carcinoid. J Am Acad Dermatol. 1985;13(2, pt 2):363-366.
- Krathen RA, Orengo IF, Rosen T. Cutaneous metastasis:a meta-analysis of data. South Med J. 2003;96:164-167.
- Oleksowicz L, Morris JC, Phelps RG, et al. Pulmonary carcinoid presenting as multiple subcutaneous nodules. Tumori. 1990;76:44-47.
- Zuetenhorst JM, van Velthuysen ML, Rutgers EJ, et al. Pathogenesis and treatment of pain caused by skin metastases in neuroendocrine tumours. Neth J Med. 2002;60:207-211.
- Melmon KL. Kinins: one of the many mediators of the carcinoid spectrum. Gastroenterology. 1968;55:545-548.
- Zuetenhorst JM, Taal BG. Metastatic carcinoid tumors: a clinical review. Oncologist. 2005;10:123-131.
- Sabir S, James WD, Schuchter LM. Cutaneous manifestations of cancer. Curr Opin Oncol. 1999;11:139-144.
- Braverman IM. Skin manifestations of internal malignancy. Clin Geriatr Med. 2002;18:1-19.
- Santi R, Massi D, Mazzoni F, et al. Skin metastasis from typical carcinoid tumor of the lung. J Cutan Pathol. 2008;35:418-422.
- Kazakov DV, Kutzner H, Rütten A, et al. Carcinoid-like pattern in sebaceous neoplasms. another distinctive, previously unrecognized pattern in extraocular sebaceous carcinoma and sebaceoma. Am J Dermatopathol. 2005;27:195-203.
Case Report
A 72-year-old white man with a history of pancreatic adenocarcinoma presented for Mohs micrographic surgery of a basal cell carcinoma on the right helix. On the day of the surgery, the patient reported a new, rapidly growing, exquisitely painful lesion on the cheek of 3 to 4 weeks’ duration. Physical examination revealed a 0.8×0.8×0.8-cm, extremely tender, firm, pink papule on the right preauricular cheek. A horizontal deep shave excision was done and the histopathology was remarkable for neoplastic cells with necrosis in the dermis. We observed dermal cellular infiltrates in the form of sheets and nodules, some showing central necrosis (Figure 1). At higher magnification, a trabecular arrangement of cells was seen. These cells had a moderate amount of cytoplasm with eccentric nuclei and rare nucleoli (Figure 2). Mitotic figures were seen at higher magnification (Figure 3). Immunohistochemistry of the neoplastic cells exhibited similar positive staining for the neuroendocrine markers chromogranin A and synaptophysin (Figure 4). Staining of the neoplastic cells also was positive for thyroid transcription factor 1 (TTF-1) and cancer antigen 19-9. Villin and caudal type homeobox 2 stains were negative. These results were consistent with cutaneous metastasis from a known pulmonary carcinoid tumor.




On further review of the patient’s medical history, it was discovered that he had undergone a Whipple procedure with adjuvant chemotherapy and radiation for pancreatic adenocarcinoma approximately 4 years prior to the current presentation. He was then followed by oncology, and 3 years later a chest computed tomography suggested possible disease progression with a new pulmonary metastasis. This pulmonary lesion was biopsied and immunologic staining was consistent with a primary neuroendocrine neoplasm of the lung, a new carcinoid tumor. The tissue was positive for cytokeratin (CK) 7,TTF-1, cancer antigen 19-9, CD56, synaptophysin, and chromogranin A, and was negative for villin and CK20. By the time he was seen in our clinic, several trials of chemotherapy had failed. Serial computed tomography subsequently demonstrated progression of the lung disease and he later developed malignant pleural effusions. Approximately 6 months after the cutaneous carcinoid metastasis was diagnosed, the patient died of respiratory failure.
Comment
Carcinoid tumors are uncommon neoplasms of neuroendocrine origin that generally arise in the gastrointestinal or bronchopulmonary tracts. Metastases from these primary neoplasms more commonly affect the regional lymph nodes or viscera, with rare reports of cutaneous metastases to the skin. The true incidence of carcinoid tumors with metastasis to the skin is unknown because it is limited to single case reports in the literature.
The clinical presentation of cutaneous carcinoid metastases has been reported most commonly as firm papules of varying sizes with no specific site predilection.1 The color of these lesions has ranged from erythematous to violaceous to brown.2 Several of the reported cases were noted to be extremely tender and painful, while other reports of lesions were noted to be asymptomatic or only mildly pruritic.3-7
Carcinoid syndrome is more common with neoplasms present within the gastrointestinal tract, but it also has been reported with large bronchial carcinoid tumors and with metastatic disease.8,9 Paroxysmal flushing is the most prominent cutaneous manifestation of this syndrome, occurring in 75% of patients.10,11 Other common symptoms include patchy cyanosis, telangiectasia, and pellagralike skin lesions.3 Carcinoid syndrome secondary to bronchial adenomas is thought to differ from gastrointestinal carcinoid neoplasms in that it has prolonged flushing (hours to days instead of minutes) and is characterized by marked anxiety, fever, disorientation, sweating, and lacrimation.8,9
Many cases of cutaneous carcinoid metastases have been accompanied by reports of exquisite tenderness,7 similar to our patient. The pathogenesis of the pain in these lesions is still unclear, but several hypotheses have been established. It has been postulated that perineural invasion by the tumor is responsible for the pain; however, this finding has been inconsistent, as neural involvement also has been present in nonpainful lesions.2,5,7,12 Another theory for the pain is that it is secondary to the release of vasoactive substances and peptide hormones from the carcinoid cells, such as kallikrein and serotonin. Lastly, local tissue necrosis and fibrosis also have been suggested as possible etiologies.7
The histology of cutaneous carcinoid metastases typically resembles the primary lesion and may demonstrate fascicles of spindle cells with focal areas of necrosis, mild atypia, and a relatively low mitotic rate.10 Other neoplasms such as Merkel cell carcinoma and carcinoidlike sebaceous carcinoma should be considered in the differential diagnosis. A primary malignant peripheral primitive neuroectodermal tumor or a primary cutaneous carcinoid tumor is less common but should be considered. Differing from carcinoid tumors, Merkel cell carcinomas usually have a higher mitotic rate and positive staining for CK20. The sebaceous neoplasms with a carcinoidlike pattern may appear histologically similar, requiring immunohistochemical evaluation with monoclonal antibodies such as D2-40.13 A diffuse granular cytoplasmic reaction to chromogranin A is characteristic of carcinoid tumors. Synaptophysin and TTF-1 also are positive in carcinoid tumors, with TTF-1 being highly specific for neuroendocrine tumors of the lung.10
Cutaneous metastases of internal malignancies are more common from carcinomas of the lungs, gastrointestinal tract, and breasts.5 Occasionally, the cutaneous metastasis will develop directly over the underlying malignancy. Our case of cutaneous metastasis of a carcinoid tumor presented as an exquisitely tender and painful papule on the cheek. The histology of the lesion was consistent with the known carcinoid tumor of the lung. Because these lesions are extremely uncommon, it is imperative to obtain an accurate clinical history and use the appropriate immunohistochemical panel to correctly diagnose these metastases.
Case Report
A 72-year-old white man with a history of pancreatic adenocarcinoma presented for Mohs micrographic surgery of a basal cell carcinoma on the right helix. On the day of the surgery, the patient reported a new, rapidly growing, exquisitely painful lesion on the cheek of 3 to 4 weeks’ duration. Physical examination revealed a 0.8×0.8×0.8-cm, extremely tender, firm, pink papule on the right preauricular cheek. A horizontal deep shave excision was done and the histopathology was remarkable for neoplastic cells with necrosis in the dermis. We observed dermal cellular infiltrates in the form of sheets and nodules, some showing central necrosis (Figure 1). At higher magnification, a trabecular arrangement of cells was seen. These cells had a moderate amount of cytoplasm with eccentric nuclei and rare nucleoli (Figure 2). Mitotic figures were seen at higher magnification (Figure 3). Immunohistochemistry of the neoplastic cells exhibited similar positive staining for the neuroendocrine markers chromogranin A and synaptophysin (Figure 4). Staining of the neoplastic cells also was positive for thyroid transcription factor 1 (TTF-1) and cancer antigen 19-9. Villin and caudal type homeobox 2 stains were negative. These results were consistent with cutaneous metastasis from a known pulmonary carcinoid tumor.




On further review of the patient’s medical history, it was discovered that he had undergone a Whipple procedure with adjuvant chemotherapy and radiation for pancreatic adenocarcinoma approximately 4 years prior to the current presentation. He was then followed by oncology, and 3 years later a chest computed tomography suggested possible disease progression with a new pulmonary metastasis. This pulmonary lesion was biopsied and immunologic staining was consistent with a primary neuroendocrine neoplasm of the lung, a new carcinoid tumor. The tissue was positive for cytokeratin (CK) 7,TTF-1, cancer antigen 19-9, CD56, synaptophysin, and chromogranin A, and was negative for villin and CK20. By the time he was seen in our clinic, several trials of chemotherapy had failed. Serial computed tomography subsequently demonstrated progression of the lung disease and he later developed malignant pleural effusions. Approximately 6 months after the cutaneous carcinoid metastasis was diagnosed, the patient died of respiratory failure.
Comment
Carcinoid tumors are uncommon neoplasms of neuroendocrine origin that generally arise in the gastrointestinal or bronchopulmonary tracts. Metastases from these primary neoplasms more commonly affect the regional lymph nodes or viscera, with rare reports of cutaneous metastases to the skin. The true incidence of carcinoid tumors with metastasis to the skin is unknown because it is limited to single case reports in the literature.
The clinical presentation of cutaneous carcinoid metastases has been reported most commonly as firm papules of varying sizes with no specific site predilection.1 The color of these lesions has ranged from erythematous to violaceous to brown.2 Several of the reported cases were noted to be extremely tender and painful, while other reports of lesions were noted to be asymptomatic or only mildly pruritic.3-7
Carcinoid syndrome is more common with neoplasms present within the gastrointestinal tract, but it also has been reported with large bronchial carcinoid tumors and with metastatic disease.8,9 Paroxysmal flushing is the most prominent cutaneous manifestation of this syndrome, occurring in 75% of patients.10,11 Other common symptoms include patchy cyanosis, telangiectasia, and pellagralike skin lesions.3 Carcinoid syndrome secondary to bronchial adenomas is thought to differ from gastrointestinal carcinoid neoplasms in that it has prolonged flushing (hours to days instead of minutes) and is characterized by marked anxiety, fever, disorientation, sweating, and lacrimation.8,9
Many cases of cutaneous carcinoid metastases have been accompanied by reports of exquisite tenderness,7 similar to our patient. The pathogenesis of the pain in these lesions is still unclear, but several hypotheses have been established. It has been postulated that perineural invasion by the tumor is responsible for the pain; however, this finding has been inconsistent, as neural involvement also has been present in nonpainful lesions.2,5,7,12 Another theory for the pain is that it is secondary to the release of vasoactive substances and peptide hormones from the carcinoid cells, such as kallikrein and serotonin. Lastly, local tissue necrosis and fibrosis also have been suggested as possible etiologies.7
The histology of cutaneous carcinoid metastases typically resembles the primary lesion and may demonstrate fascicles of spindle cells with focal areas of necrosis, mild atypia, and a relatively low mitotic rate.10 Other neoplasms such as Merkel cell carcinoma and carcinoidlike sebaceous carcinoma should be considered in the differential diagnosis. A primary malignant peripheral primitive neuroectodermal tumor or a primary cutaneous carcinoid tumor is less common but should be considered. Differing from carcinoid tumors, Merkel cell carcinomas usually have a higher mitotic rate and positive staining for CK20. The sebaceous neoplasms with a carcinoidlike pattern may appear histologically similar, requiring immunohistochemical evaluation with monoclonal antibodies such as D2-40.13 A diffuse granular cytoplasmic reaction to chromogranin A is characteristic of carcinoid tumors. Synaptophysin and TTF-1 also are positive in carcinoid tumors, with TTF-1 being highly specific for neuroendocrine tumors of the lung.10
Cutaneous metastases of internal malignancies are more common from carcinomas of the lungs, gastrointestinal tract, and breasts.5 Occasionally, the cutaneous metastasis will develop directly over the underlying malignancy. Our case of cutaneous metastasis of a carcinoid tumor presented as an exquisitely tender and painful papule on the cheek. The histology of the lesion was consistent with the known carcinoid tumor of the lung. Because these lesions are extremely uncommon, it is imperative to obtain an accurate clinical history and use the appropriate immunohistochemical panel to correctly diagnose these metastases.
- Blochin E, Stein JA, Wang NS. Atypical carcinoid metastasis to the skin. Am J Dermatopathol. 2010;32:735-739.
- Rodriguez G, Villamizar R. Carcinoid tumor with skin metastasis. Am J Dermatopathol. 1992;14:263-269.
- Archer CB, Rauch HJ, Allen MH, et al. Ultrastructural features of metastatic cutaneous carcinoid. J Cutan Pathol. 1984;11:485-490.
- Archer CB, Wells RS, MacDonald DM. Metastatic cutaneous carcinoid. J Am Acad Dermatol. 1985;13(2, pt 2):363-366.
- Krathen RA, Orengo IF, Rosen T. Cutaneous metastasis:a meta-analysis of data. South Med J. 2003;96:164-167.
- Oleksowicz L, Morris JC, Phelps RG, et al. Pulmonary carcinoid presenting as multiple subcutaneous nodules. Tumori. 1990;76:44-47.
- Zuetenhorst JM, van Velthuysen ML, Rutgers EJ, et al. Pathogenesis and treatment of pain caused by skin metastases in neuroendocrine tumours. Neth J Med. 2002;60:207-211.
- Melmon KL. Kinins: one of the many mediators of the carcinoid spectrum. Gastroenterology. 1968;55:545-548.
- Zuetenhorst JM, Taal BG. Metastatic carcinoid tumors: a clinical review. Oncologist. 2005;10:123-131.
- Sabir S, James WD, Schuchter LM. Cutaneous manifestations of cancer. Curr Opin Oncol. 1999;11:139-144.
- Braverman IM. Skin manifestations of internal malignancy. Clin Geriatr Med. 2002;18:1-19.
- Santi R, Massi D, Mazzoni F, et al. Skin metastasis from typical carcinoid tumor of the lung. J Cutan Pathol. 2008;35:418-422.
- Kazakov DV, Kutzner H, Rütten A, et al. Carcinoid-like pattern in sebaceous neoplasms. another distinctive, previously unrecognized pattern in extraocular sebaceous carcinoma and sebaceoma. Am J Dermatopathol. 2005;27:195-203.
- Blochin E, Stein JA, Wang NS. Atypical carcinoid metastasis to the skin. Am J Dermatopathol. 2010;32:735-739.
- Rodriguez G, Villamizar R. Carcinoid tumor with skin metastasis. Am J Dermatopathol. 1992;14:263-269.
- Archer CB, Rauch HJ, Allen MH, et al. Ultrastructural features of metastatic cutaneous carcinoid. J Cutan Pathol. 1984;11:485-490.
- Archer CB, Wells RS, MacDonald DM. Metastatic cutaneous carcinoid. J Am Acad Dermatol. 1985;13(2, pt 2):363-366.
- Krathen RA, Orengo IF, Rosen T. Cutaneous metastasis:a meta-analysis of data. South Med J. 2003;96:164-167.
- Oleksowicz L, Morris JC, Phelps RG, et al. Pulmonary carcinoid presenting as multiple subcutaneous nodules. Tumori. 1990;76:44-47.
- Zuetenhorst JM, van Velthuysen ML, Rutgers EJ, et al. Pathogenesis and treatment of pain caused by skin metastases in neuroendocrine tumours. Neth J Med. 2002;60:207-211.
- Melmon KL. Kinins: one of the many mediators of the carcinoid spectrum. Gastroenterology. 1968;55:545-548.
- Zuetenhorst JM, Taal BG. Metastatic carcinoid tumors: a clinical review. Oncologist. 2005;10:123-131.
- Sabir S, James WD, Schuchter LM. Cutaneous manifestations of cancer. Curr Opin Oncol. 1999;11:139-144.
- Braverman IM. Skin manifestations of internal malignancy. Clin Geriatr Med. 2002;18:1-19.
- Santi R, Massi D, Mazzoni F, et al. Skin metastasis from typical carcinoid tumor of the lung. J Cutan Pathol. 2008;35:418-422.
- Kazakov DV, Kutzner H, Rütten A, et al. Carcinoid-like pattern in sebaceous neoplasms. another distinctive, previously unrecognized pattern in extraocular sebaceous carcinoma and sebaceoma. Am J Dermatopathol. 2005;27:195-203.
Practice Points
- Cutaneous metastases of carcinoid tumors are extremely rare, and clinical presentation can vary. They can present as firm papules ranging in color from pink to brown, can be painful, and could occur at any site.
- It is imperative to obtain an accurate clinical history and use the appropriate immunohistochemical panel to correctly diagnose cutaneous metastases of carcinoid tumors.
- Neoplasms within the gastrointestinal tract commonly present with carcinoid syndrome, but it also has been observed with bronchial carcinoid tumors and with metastatic disease.
Merkel cell carcinoma most likely to recur within 2 years of diagnosis
PORTLAND, ORE. – The first 2 years after diagnosis are crucial when conducting surveillance for recurrence of Merkel cell carcinoma (MCC), Aubriana McEvoy said at the annual meeting of the Society for Investigative Dermatology.
Regardless of stage at diagnosis, the risk of recurrence peaked at about 1 year and leveled off by about year 2 in a retrospective cohort study, according to Ms. McEvoy, a medical student at the University of Washington, Seattle, who conducted the study with colleagues under the mentorship of Paul Nghiem, MD, PhD, professor and head of the division of dermatology. The study also inversely linked primary MCC stage with subsequent recurrence-free survival, highlighted the role of imaging for surveillance of patients who have advanced primary disease, and linked distant metastatic recurrence with significantly worse survival, compared with local or nodal recurrence.
“Patients with Merkel cell carcinoma always ask about recurrence,” Ms. McEvoy said. “Now, for the first time, we have the data to answer their questions.”
Surveillance of MCC is increasingly important, she said: The “treatment landscape is evolving quickly, and immunotherapies such as pembrolizumab can have a good response rate, especially in the setting of lower burden of disease.” But follow-up is costly on several fronts, making it crucial to aim for “enough” and not “too much” surveillance, she added.
“Imaging often costs thousands of dollars, and that’s only one piece of the pie. There’s also the cost of office visits, time spent by the patient and their family, and the emotional investment and uncertainty a patient goes through every time they have to come for a follow-up visit and scan,” Ms. McEvoy said.
Comprehensive, stage-specific guidelines can help clinicians and patients balance the benefits and costs of surveillance, but are lacking in MCC because no published study has characterized recurrence by stage, she said. To fill this gap, she and her associates analyzed 10 years of longitudinal MCC surveillance data on 468 patients who underwent pathologic staging and were followed at the Nghiem laboratory.
The risk of recurrence was highest within the first 2 years after diagnosis, regardless of whether patients had local (pathologic stage I–II) or nodal (stage III) MCC. However, the probability of recurrence-free survival correlated inversely with pathologic stage of primary MCC (P = .003). Median recurrence-free survival time was not reached by the 186 patients with local disease and small (2-cm maximum dimension) primary lesions, or by 135 patients with clinically occult nodal disease.
In contrast, median recurrence-free survival was about 6 years among 84 patients with local disease and lesions measuring more than 2 cm; was less than 2 years among 35 patients with clinically apparent, pathologically confirmed nodal disease or in-transit metastases; and was less than 1 year among patients with distant metastatic disease.
The researchers also investigated the risk of distant metastatic recurrence to confirm which patients need most intensive follow-up. Among 138 individuals with available data, 40% of stage I primary MCC patients developed a distant metastatic recurrence, as did 60% of patients with stage IIA or stage IIB primary MCC. And 80% of recurrences among patients with stage IIIA or stage IIIB primary disease were distant metastases. “I think it’s safe to say that stage III patients should receive appropriate, if not vigilant, surveillance,” Ms. McEvoy said. The site of recurrence also was significantly (P less than .001) tied to the risk of subsequent death from recurrent MCC; median survival time was not reached when recurrence was local or nodal, but was less than 2 years when it was distant or metastatic.
Early in 2018, the American Joint Committee on Cancer will update its MCC staging system to distinguish clinical versus pathologic staging. “This is important, because pathologic staging remains the gold standard, providing a much more in-depth view of the patient’s disease,” Ms. McEvoy commented. Ideally, clinicians would use more information to help predict the prognosis of MCC, including sex and immune and viral status, she noted. “But we hope these data provide information for more consistency across the country, so we can catch recurrences earlier, and avoid unnecessary visits and imaging scans for lower-risk patients.”
The study was supported by the National Institutes of Health, the Seattle Cancer Care Alliance, the University of Washington, and the Institute of Translational Health Sciences. Ms. McEvoy had no conflicts of interest.
PORTLAND, ORE. – The first 2 years after diagnosis are crucial when conducting surveillance for recurrence of Merkel cell carcinoma (MCC), Aubriana McEvoy said at the annual meeting of the Society for Investigative Dermatology.
Regardless of stage at diagnosis, the risk of recurrence peaked at about 1 year and leveled off by about year 2 in a retrospective cohort study, according to Ms. McEvoy, a medical student at the University of Washington, Seattle, who conducted the study with colleagues under the mentorship of Paul Nghiem, MD, PhD, professor and head of the division of dermatology. The study also inversely linked primary MCC stage with subsequent recurrence-free survival, highlighted the role of imaging for surveillance of patients who have advanced primary disease, and linked distant metastatic recurrence with significantly worse survival, compared with local or nodal recurrence.
“Patients with Merkel cell carcinoma always ask about recurrence,” Ms. McEvoy said. “Now, for the first time, we have the data to answer their questions.”
Surveillance of MCC is increasingly important, she said: The “treatment landscape is evolving quickly, and immunotherapies such as pembrolizumab can have a good response rate, especially in the setting of lower burden of disease.” But follow-up is costly on several fronts, making it crucial to aim for “enough” and not “too much” surveillance, she added.
“Imaging often costs thousands of dollars, and that’s only one piece of the pie. There’s also the cost of office visits, time spent by the patient and their family, and the emotional investment and uncertainty a patient goes through every time they have to come for a follow-up visit and scan,” Ms. McEvoy said.
Comprehensive, stage-specific guidelines can help clinicians and patients balance the benefits and costs of surveillance, but are lacking in MCC because no published study has characterized recurrence by stage, she said. To fill this gap, she and her associates analyzed 10 years of longitudinal MCC surveillance data on 468 patients who underwent pathologic staging and were followed at the Nghiem laboratory.
The risk of recurrence was highest within the first 2 years after diagnosis, regardless of whether patients had local (pathologic stage I–II) or nodal (stage III) MCC. However, the probability of recurrence-free survival correlated inversely with pathologic stage of primary MCC (P = .003). Median recurrence-free survival time was not reached by the 186 patients with local disease and small (2-cm maximum dimension) primary lesions, or by 135 patients with clinically occult nodal disease.
In contrast, median recurrence-free survival was about 6 years among 84 patients with local disease and lesions measuring more than 2 cm; was less than 2 years among 35 patients with clinically apparent, pathologically confirmed nodal disease or in-transit metastases; and was less than 1 year among patients with distant metastatic disease.
The researchers also investigated the risk of distant metastatic recurrence to confirm which patients need most intensive follow-up. Among 138 individuals with available data, 40% of stage I primary MCC patients developed a distant metastatic recurrence, as did 60% of patients with stage IIA or stage IIB primary MCC. And 80% of recurrences among patients with stage IIIA or stage IIIB primary disease were distant metastases. “I think it’s safe to say that stage III patients should receive appropriate, if not vigilant, surveillance,” Ms. McEvoy said. The site of recurrence also was significantly (P less than .001) tied to the risk of subsequent death from recurrent MCC; median survival time was not reached when recurrence was local or nodal, but was less than 2 years when it was distant or metastatic.
Early in 2018, the American Joint Committee on Cancer will update its MCC staging system to distinguish clinical versus pathologic staging. “This is important, because pathologic staging remains the gold standard, providing a much more in-depth view of the patient’s disease,” Ms. McEvoy commented. Ideally, clinicians would use more information to help predict the prognosis of MCC, including sex and immune and viral status, she noted. “But we hope these data provide information for more consistency across the country, so we can catch recurrences earlier, and avoid unnecessary visits and imaging scans for lower-risk patients.”
The study was supported by the National Institutes of Health, the Seattle Cancer Care Alliance, the University of Washington, and the Institute of Translational Health Sciences. Ms. McEvoy had no conflicts of interest.
PORTLAND, ORE. – The first 2 years after diagnosis are crucial when conducting surveillance for recurrence of Merkel cell carcinoma (MCC), Aubriana McEvoy said at the annual meeting of the Society for Investigative Dermatology.
Regardless of stage at diagnosis, the risk of recurrence peaked at about 1 year and leveled off by about year 2 in a retrospective cohort study, according to Ms. McEvoy, a medical student at the University of Washington, Seattle, who conducted the study with colleagues under the mentorship of Paul Nghiem, MD, PhD, professor and head of the division of dermatology. The study also inversely linked primary MCC stage with subsequent recurrence-free survival, highlighted the role of imaging for surveillance of patients who have advanced primary disease, and linked distant metastatic recurrence with significantly worse survival, compared with local or nodal recurrence.
“Patients with Merkel cell carcinoma always ask about recurrence,” Ms. McEvoy said. “Now, for the first time, we have the data to answer their questions.”
Surveillance of MCC is increasingly important, she said: The “treatment landscape is evolving quickly, and immunotherapies such as pembrolizumab can have a good response rate, especially in the setting of lower burden of disease.” But follow-up is costly on several fronts, making it crucial to aim for “enough” and not “too much” surveillance, she added.
“Imaging often costs thousands of dollars, and that’s only one piece of the pie. There’s also the cost of office visits, time spent by the patient and their family, and the emotional investment and uncertainty a patient goes through every time they have to come for a follow-up visit and scan,” Ms. McEvoy said.
Comprehensive, stage-specific guidelines can help clinicians and patients balance the benefits and costs of surveillance, but are lacking in MCC because no published study has characterized recurrence by stage, she said. To fill this gap, she and her associates analyzed 10 years of longitudinal MCC surveillance data on 468 patients who underwent pathologic staging and were followed at the Nghiem laboratory.
The risk of recurrence was highest within the first 2 years after diagnosis, regardless of whether patients had local (pathologic stage I–II) or nodal (stage III) MCC. However, the probability of recurrence-free survival correlated inversely with pathologic stage of primary MCC (P = .003). Median recurrence-free survival time was not reached by the 186 patients with local disease and small (2-cm maximum dimension) primary lesions, or by 135 patients with clinically occult nodal disease.
In contrast, median recurrence-free survival was about 6 years among 84 patients with local disease and lesions measuring more than 2 cm; was less than 2 years among 35 patients with clinically apparent, pathologically confirmed nodal disease or in-transit metastases; and was less than 1 year among patients with distant metastatic disease.
The researchers also investigated the risk of distant metastatic recurrence to confirm which patients need most intensive follow-up. Among 138 individuals with available data, 40% of stage I primary MCC patients developed a distant metastatic recurrence, as did 60% of patients with stage IIA or stage IIB primary MCC. And 80% of recurrences among patients with stage IIIA or stage IIIB primary disease were distant metastases. “I think it’s safe to say that stage III patients should receive appropriate, if not vigilant, surveillance,” Ms. McEvoy said. The site of recurrence also was significantly (P less than .001) tied to the risk of subsequent death from recurrent MCC; median survival time was not reached when recurrence was local or nodal, but was less than 2 years when it was distant or metastatic.
Early in 2018, the American Joint Committee on Cancer will update its MCC staging system to distinguish clinical versus pathologic staging. “This is important, because pathologic staging remains the gold standard, providing a much more in-depth view of the patient’s disease,” Ms. McEvoy commented. Ideally, clinicians would use more information to help predict the prognosis of MCC, including sex and immune and viral status, she noted. “But we hope these data provide information for more consistency across the country, so we can catch recurrences earlier, and avoid unnecessary visits and imaging scans for lower-risk patients.”
The study was supported by the National Institutes of Health, the Seattle Cancer Care Alliance, the University of Washington, and the Institute of Translational Health Sciences. Ms. McEvoy had no conflicts of interest.
AT SID 2017
Key clinical point:
Major finding: The risk of recurrence peaked about 1 year after diagnosis and leveled off at about year 2, regardless of whether patients had local (pathologic stage I–II) or nodal (stage III) disease.
Data source: A retrospective cohort study of 544 patients with Merkel cell carcinoma (468 with pathologic stage disease).
Disclosures: The study was supported by the National Institutes of Health, the Seattle Cancer Care Alliance, the University of Washington, and the Institute of Translational Health Sciences. Ms. McEvoy had no conflicts of interest.
Recalcitrant Solitary Erythematous Scaly Patch on the Foot
The Diagnosis: Pagetoid Reticulosis
Histopathologic examination demonstrated a dense infiltrate and psoriasiform pattern epidermal hyperplasia (Figure, A). There was conspicuous epidermotropism of moderately enlarged, hyperchromatic lymphocytes. Intraepidermal lymphocytes were slightly larger, darker, and more convoluted than those in the subjacent dermis (Figure, B). These cells exhibited CD3+ T-cell differentiation with an abnormal CD4-CD7-CD8- phenotype (Figure, C). The histopathologic finding of atypical epidermotropic T-cell infiltrate was compatible with a rare variant of mycosis fungoides known as pagetoid reticulosis (PR). After discussing the diagnosis and treatment options, the patient elected to begin with a conservative approach to therapy. We prescribed fluocinonide ointment 0.05% twice daily under occlusion. At 1 month follow-up, the patient experienced marked improvement of the erythema and scaling of the lesion.
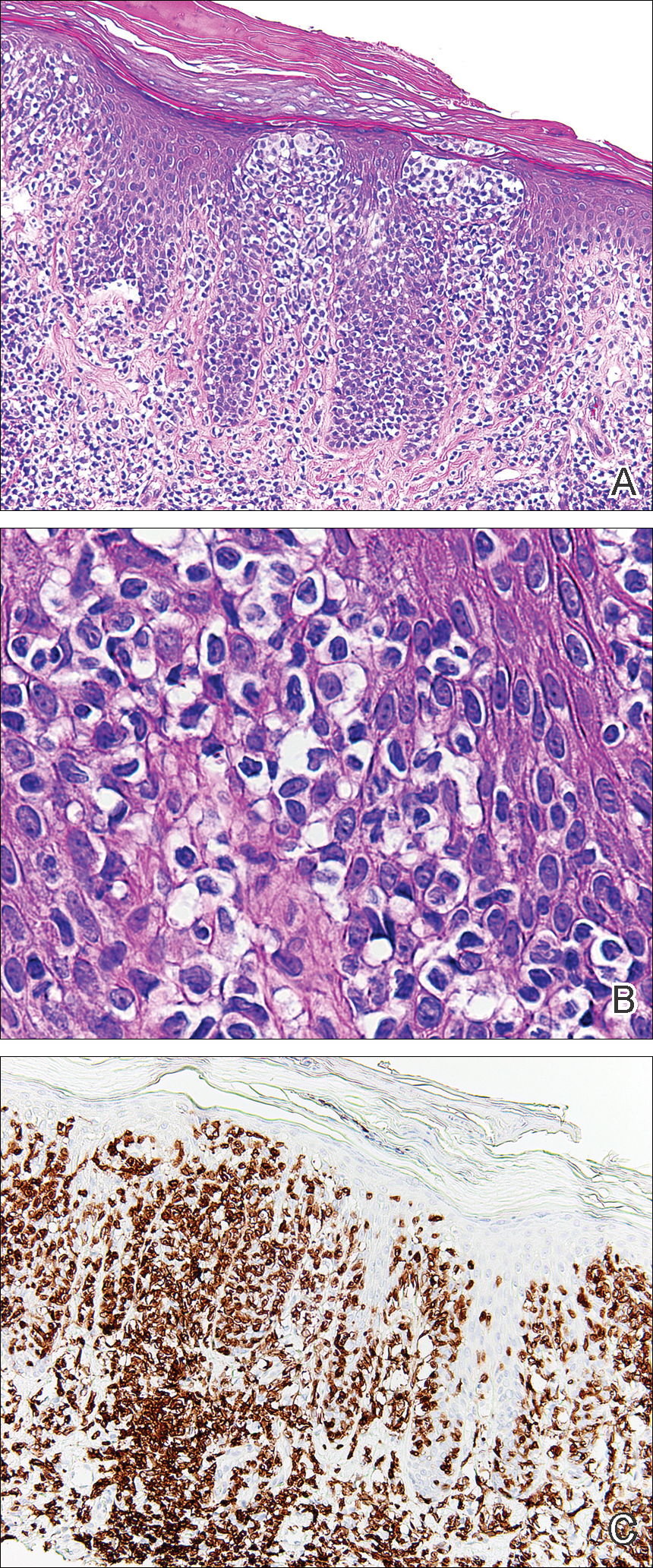
Pagetoid reticulosis is a primary cutaneous T-cell lymphoma that has been categorized as an indolent localized variant of mycosis fungoides. This rare skin disorder was originally described by Woringer and Kolopp in 19391 and was further renamed in 1973 by Braun-Falco et al.2 At that time the term pagetoid reticulosis was introduced due to similarities in histopathologic findings seen in Paget disease of the nipple. Two variants of the disease have been described since then: the localized type and the disseminated type. The localized type, also known as Woringer-Kolopp disease (WKD), typically presents as a persistent, sharply localized, scaly patch that slowly expands over several years. The lesion is classically located on the extensor surface of the hand or foot and often is asymptomatic. Due to the benign presentation, WKD can easily be confused with much more common diseases, such as psoriasis or fungal infections, resulting in a substantial delay in the diagnosis. The patient will often report a medical history notable for frequent office visits and numerous failed therapies. Even though it is exceedingly uncommon, these findings should prompt the practitioner to add WKD to their differential. The disseminated type of PR (also known as Ketron-Goodman disease) is characterized by diffuse cutaneous involvement, carries a much more progressive course, and often leads to a poor outcome.3 The histopathologic features of WKD and Ketron-Goodman disease are identical, and the 2 types are distinguished on clinical grounds alone.
Histopathologic features of PR are unique and often distinct in comparison to mycosis fungoides. Pagetoid reticulosis often is described as epidermal hyperplasia with parakeratosis, prominent acanthosis, and excessive epidermotropism of atypical lymphocytes scattered throughout the epidermis.3 The distinct pattern of epidermotropism seen in PR is the characteristic finding. Review of immunocytochemistry from reported cases has shown that CD marker expression of neoplastic T cells in PR can be variable in nature.4 Although it is known that immunophenotyping can be useful in diagnosing and distinguishing PR from other types of primary cutaneous T-cell lymphoma, the clinical significance of the observed phenotypic variation remains a mystery. As of now, it appears to be prognostically irrelevant.5
There are numerous therapeutic options available for PR. Depending on the size and extent of the disease, surgical excision and radiotherapy may be an option and are the most effective.6 For patients who are not good candidates or opt out of these options, there are various pharmacotherapies that also have proven to work. Traditional therapies include topical corticosteroids, corticosteroid injections, and phototherapy. However, more recent trials with retinoids, such as alitretinoin or bexarotene, appear to offer a promising therapeutic approach.7
Pagetoid reticulosis is a true malignant lymphoma of T-cell lineage, but it typically carries an excellent prognosis. Rare cases have been reported to progress to disseminated lymphoma.8 Therefore, long-term follow-up for a patient diagnosed with PR is recommended.
- Woringer FR, Kolopp P. Lésion érythémato-squameuse polycyclique de l'avant-bras évoluantdepuis 6 ans chez un garçonnet de 13 ans. Ann Dermatol Venereol. 1939;10:945-948.
- Braun-Falco O, Marghescu S, Wolff HH. Pagetoid reticulosis--Woringer-Kolopp's disease [in German]. Hautarzt. 1973;24:11-21.
- Haghighi B, Smoller BR, Leboit PE, et al. Pagetoid reticulosis (Woringer-Kolopp disease): an immunophenotypic, molecular, and clinicopathologic study. Mod Pathol. 2000;13:502-510.
- Willemze R, Jaffe ES, Burg G, et al. WHO-EORTC classification for cutaneous lymphomas. Blood. 2005;105:3768-3785.
- Mourtzinos N, Puri PK, Wang G, et al. CD4/CD8 double negative pagetoid reticulosis: a case report and literature review. J Cutan Pathol. 2010;37:491-496.
- Lee J, Viakhireva N, Cesca C, et al. Clinicopathologic features and treatment outcomes in Woringer-Kolopp disease. J Am Acad Dermatol. 2008;59:706-712.
- Schmitz L, Bierhoff E, Dirschka T. Alitretinoin: an effective treatment option for pagetoid reticulosis. J Dtsch Dermatol Ges. 2013;11:1194-1195.
- Ioannides G, Engel MF, Rywlin AM. Woringer-Kolopp disease (pagetoid reticulosis). Am J Dermatopathol. 1983;5:153-158.
The Diagnosis: Pagetoid Reticulosis
Histopathologic examination demonstrated a dense infiltrate and psoriasiform pattern epidermal hyperplasia (Figure, A). There was conspicuous epidermotropism of moderately enlarged, hyperchromatic lymphocytes. Intraepidermal lymphocytes were slightly larger, darker, and more convoluted than those in the subjacent dermis (Figure, B). These cells exhibited CD3+ T-cell differentiation with an abnormal CD4-CD7-CD8- phenotype (Figure, C). The histopathologic finding of atypical epidermotropic T-cell infiltrate was compatible with a rare variant of mycosis fungoides known as pagetoid reticulosis (PR). After discussing the diagnosis and treatment options, the patient elected to begin with a conservative approach to therapy. We prescribed fluocinonide ointment 0.05% twice daily under occlusion. At 1 month follow-up, the patient experienced marked improvement of the erythema and scaling of the lesion.

Pagetoid reticulosis is a primary cutaneous T-cell lymphoma that has been categorized as an indolent localized variant of mycosis fungoides. This rare skin disorder was originally described by Woringer and Kolopp in 19391 and was further renamed in 1973 by Braun-Falco et al.2 At that time the term pagetoid reticulosis was introduced due to similarities in histopathologic findings seen in Paget disease of the nipple. Two variants of the disease have been described since then: the localized type and the disseminated type. The localized type, also known as Woringer-Kolopp disease (WKD), typically presents as a persistent, sharply localized, scaly patch that slowly expands over several years. The lesion is classically located on the extensor surface of the hand or foot and often is asymptomatic. Due to the benign presentation, WKD can easily be confused with much more common diseases, such as psoriasis or fungal infections, resulting in a substantial delay in the diagnosis. The patient will often report a medical history notable for frequent office visits and numerous failed therapies. Even though it is exceedingly uncommon, these findings should prompt the practitioner to add WKD to their differential. The disseminated type of PR (also known as Ketron-Goodman disease) is characterized by diffuse cutaneous involvement, carries a much more progressive course, and often leads to a poor outcome.3 The histopathologic features of WKD and Ketron-Goodman disease are identical, and the 2 types are distinguished on clinical grounds alone.
Histopathologic features of PR are unique and often distinct in comparison to mycosis fungoides. Pagetoid reticulosis often is described as epidermal hyperplasia with parakeratosis, prominent acanthosis, and excessive epidermotropism of atypical lymphocytes scattered throughout the epidermis.3 The distinct pattern of epidermotropism seen in PR is the characteristic finding. Review of immunocytochemistry from reported cases has shown that CD marker expression of neoplastic T cells in PR can be variable in nature.4 Although it is known that immunophenotyping can be useful in diagnosing and distinguishing PR from other types of primary cutaneous T-cell lymphoma, the clinical significance of the observed phenotypic variation remains a mystery. As of now, it appears to be prognostically irrelevant.5
There are numerous therapeutic options available for PR. Depending on the size and extent of the disease, surgical excision and radiotherapy may be an option and are the most effective.6 For patients who are not good candidates or opt out of these options, there are various pharmacotherapies that also have proven to work. Traditional therapies include topical corticosteroids, corticosteroid injections, and phototherapy. However, more recent trials with retinoids, such as alitretinoin or bexarotene, appear to offer a promising therapeutic approach.7
Pagetoid reticulosis is a true malignant lymphoma of T-cell lineage, but it typically carries an excellent prognosis. Rare cases have been reported to progress to disseminated lymphoma.8 Therefore, long-term follow-up for a patient diagnosed with PR is recommended.
The Diagnosis: Pagetoid Reticulosis
Histopathologic examination demonstrated a dense infiltrate and psoriasiform pattern epidermal hyperplasia (Figure, A). There was conspicuous epidermotropism of moderately enlarged, hyperchromatic lymphocytes. Intraepidermal lymphocytes were slightly larger, darker, and more convoluted than those in the subjacent dermis (Figure, B). These cells exhibited CD3+ T-cell differentiation with an abnormal CD4-CD7-CD8- phenotype (Figure, C). The histopathologic finding of atypical epidermotropic T-cell infiltrate was compatible with a rare variant of mycosis fungoides known as pagetoid reticulosis (PR). After discussing the diagnosis and treatment options, the patient elected to begin with a conservative approach to therapy. We prescribed fluocinonide ointment 0.05% twice daily under occlusion. At 1 month follow-up, the patient experienced marked improvement of the erythema and scaling of the lesion.

Pagetoid reticulosis is a primary cutaneous T-cell lymphoma that has been categorized as an indolent localized variant of mycosis fungoides. This rare skin disorder was originally described by Woringer and Kolopp in 19391 and was further renamed in 1973 by Braun-Falco et al.2 At that time the term pagetoid reticulosis was introduced due to similarities in histopathologic findings seen in Paget disease of the nipple. Two variants of the disease have been described since then: the localized type and the disseminated type. The localized type, also known as Woringer-Kolopp disease (WKD), typically presents as a persistent, sharply localized, scaly patch that slowly expands over several years. The lesion is classically located on the extensor surface of the hand or foot and often is asymptomatic. Due to the benign presentation, WKD can easily be confused with much more common diseases, such as psoriasis or fungal infections, resulting in a substantial delay in the diagnosis. The patient will often report a medical history notable for frequent office visits and numerous failed therapies. Even though it is exceedingly uncommon, these findings should prompt the practitioner to add WKD to their differential. The disseminated type of PR (also known as Ketron-Goodman disease) is characterized by diffuse cutaneous involvement, carries a much more progressive course, and often leads to a poor outcome.3 The histopathologic features of WKD and Ketron-Goodman disease are identical, and the 2 types are distinguished on clinical grounds alone.
Histopathologic features of PR are unique and often distinct in comparison to mycosis fungoides. Pagetoid reticulosis often is described as epidermal hyperplasia with parakeratosis, prominent acanthosis, and excessive epidermotropism of atypical lymphocytes scattered throughout the epidermis.3 The distinct pattern of epidermotropism seen in PR is the characteristic finding. Review of immunocytochemistry from reported cases has shown that CD marker expression of neoplastic T cells in PR can be variable in nature.4 Although it is known that immunophenotyping can be useful in diagnosing and distinguishing PR from other types of primary cutaneous T-cell lymphoma, the clinical significance of the observed phenotypic variation remains a mystery. As of now, it appears to be prognostically irrelevant.5
There are numerous therapeutic options available for PR. Depending on the size and extent of the disease, surgical excision and radiotherapy may be an option and are the most effective.6 For patients who are not good candidates or opt out of these options, there are various pharmacotherapies that also have proven to work. Traditional therapies include topical corticosteroids, corticosteroid injections, and phototherapy. However, more recent trials with retinoids, such as alitretinoin or bexarotene, appear to offer a promising therapeutic approach.7
Pagetoid reticulosis is a true malignant lymphoma of T-cell lineage, but it typically carries an excellent prognosis. Rare cases have been reported to progress to disseminated lymphoma.8 Therefore, long-term follow-up for a patient diagnosed with PR is recommended.
- Woringer FR, Kolopp P. Lésion érythémato-squameuse polycyclique de l'avant-bras évoluantdepuis 6 ans chez un garçonnet de 13 ans. Ann Dermatol Venereol. 1939;10:945-948.
- Braun-Falco O, Marghescu S, Wolff HH. Pagetoid reticulosis--Woringer-Kolopp's disease [in German]. Hautarzt. 1973;24:11-21.
- Haghighi B, Smoller BR, Leboit PE, et al. Pagetoid reticulosis (Woringer-Kolopp disease): an immunophenotypic, molecular, and clinicopathologic study. Mod Pathol. 2000;13:502-510.
- Willemze R, Jaffe ES, Burg G, et al. WHO-EORTC classification for cutaneous lymphomas. Blood. 2005;105:3768-3785.
- Mourtzinos N, Puri PK, Wang G, et al. CD4/CD8 double negative pagetoid reticulosis: a case report and literature review. J Cutan Pathol. 2010;37:491-496.
- Lee J, Viakhireva N, Cesca C, et al. Clinicopathologic features and treatment outcomes in Woringer-Kolopp disease. J Am Acad Dermatol. 2008;59:706-712.
- Schmitz L, Bierhoff E, Dirschka T. Alitretinoin: an effective treatment option for pagetoid reticulosis. J Dtsch Dermatol Ges. 2013;11:1194-1195.
- Ioannides G, Engel MF, Rywlin AM. Woringer-Kolopp disease (pagetoid reticulosis). Am J Dermatopathol. 1983;5:153-158.
- Woringer FR, Kolopp P. Lésion érythémato-squameuse polycyclique de l'avant-bras évoluantdepuis 6 ans chez un garçonnet de 13 ans. Ann Dermatol Venereol. 1939;10:945-948.
- Braun-Falco O, Marghescu S, Wolff HH. Pagetoid reticulosis--Woringer-Kolopp's disease [in German]. Hautarzt. 1973;24:11-21.
- Haghighi B, Smoller BR, Leboit PE, et al. Pagetoid reticulosis (Woringer-Kolopp disease): an immunophenotypic, molecular, and clinicopathologic study. Mod Pathol. 2000;13:502-510.
- Willemze R, Jaffe ES, Burg G, et al. WHO-EORTC classification for cutaneous lymphomas. Blood. 2005;105:3768-3785.
- Mourtzinos N, Puri PK, Wang G, et al. CD4/CD8 double negative pagetoid reticulosis: a case report and literature review. J Cutan Pathol. 2010;37:491-496.
- Lee J, Viakhireva N, Cesca C, et al. Clinicopathologic features and treatment outcomes in Woringer-Kolopp disease. J Am Acad Dermatol. 2008;59:706-712.
- Schmitz L, Bierhoff E, Dirschka T. Alitretinoin: an effective treatment option for pagetoid reticulosis. J Dtsch Dermatol Ges. 2013;11:1194-1195.
- Ioannides G, Engel MF, Rywlin AM. Woringer-Kolopp disease (pagetoid reticulosis). Am J Dermatopathol. 1983;5:153-158.
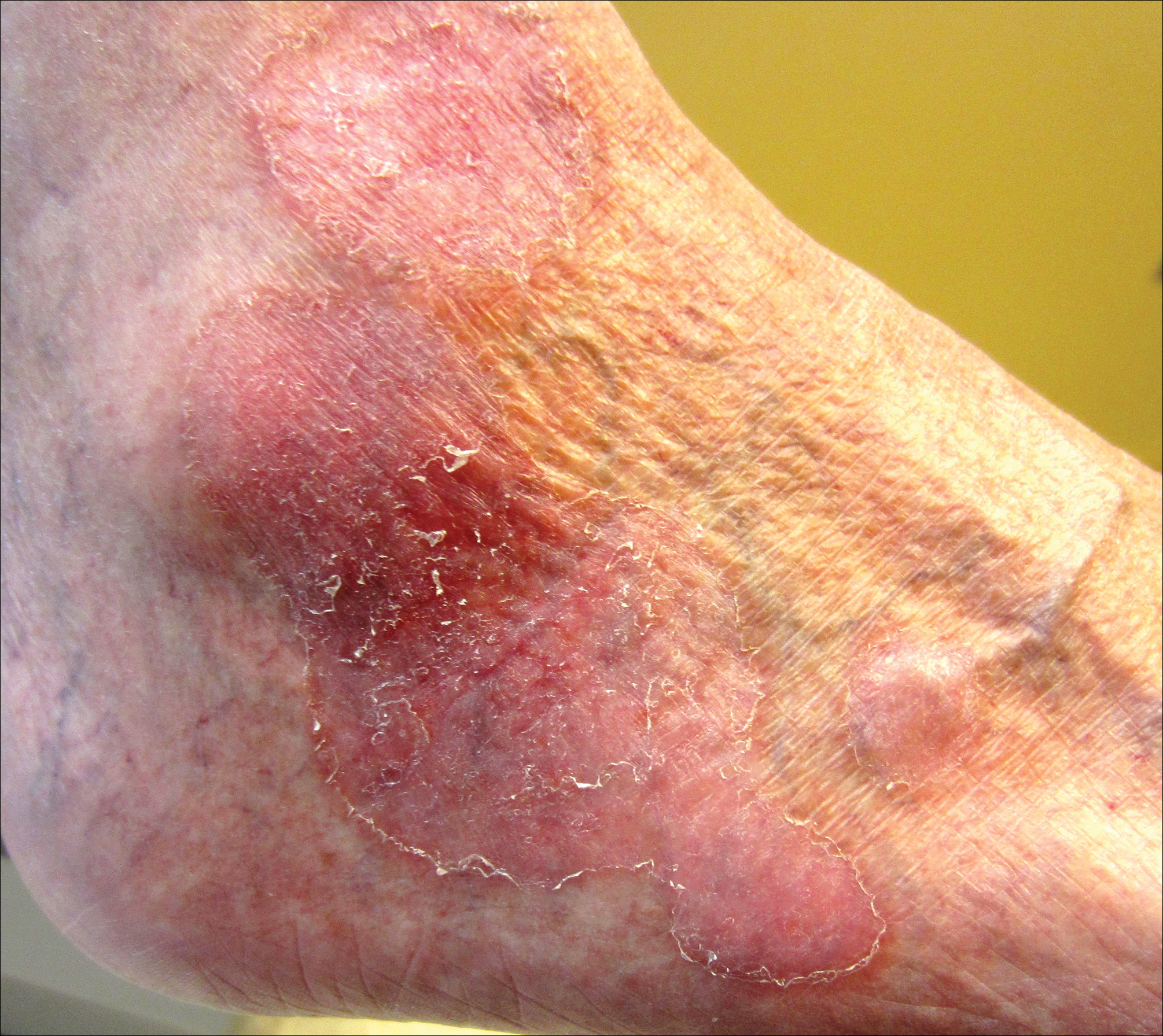
An 80-year-old man with a history of malignant melanoma and squamous cell carcinoma presented to the dermatology clinic with a chronic rash of 20 years' duration on the right ankle that extended to the instep of the right foot. His medical history was notable for hypertension and hyperlipidemia. Family history was unremarkable. The patient described the rash as red and scaly but denied associated pain or pruritus. Over the last 2 to 3 years he had tried treating the affected area with petroleum jelly, topical and oral antifungals, and mild topical steroids with minimal improvement. Complete review of systems was performed and was negative other than some mild constipation. Physical examination revealed an erythematous scaly patch on the dorsal aspect of the right ankle. Potassium hydroxide preparation and fungal culture swab yielded negative results, and a shave biopsy was performed.