User login
Make The Diagnosis - September 2018
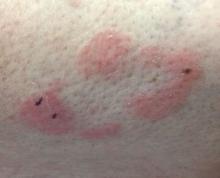
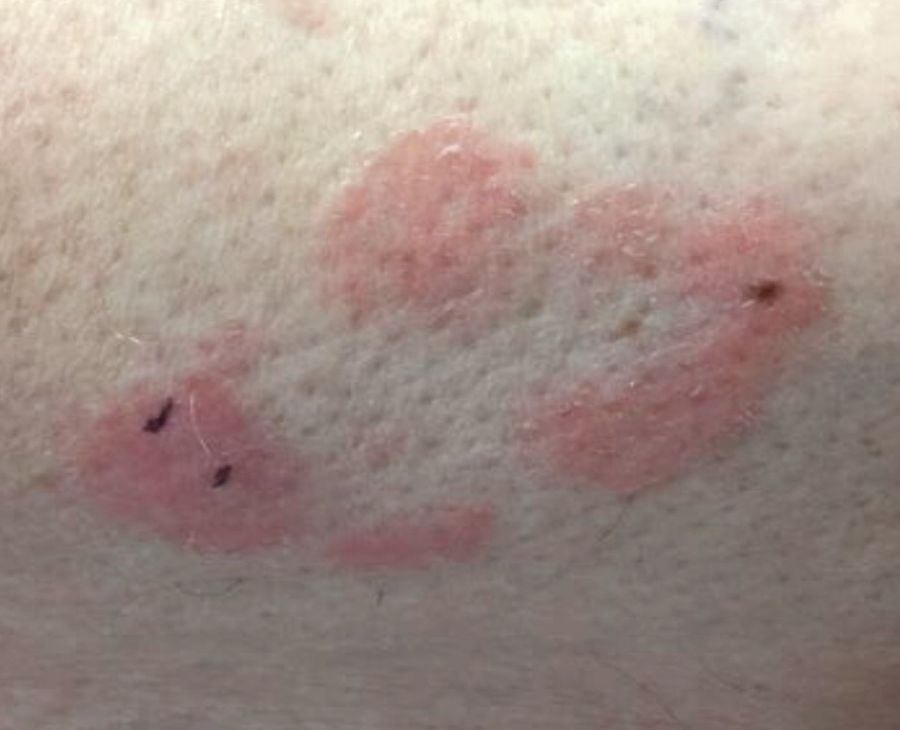
Some have postulated an infectious agent as the cause. Atopic dermatitis may confer an increased risk because of the chronic stimulation of T cells. Males are more commonly affected than females by a 2:1 ratio. A worse prognosis is associated with advanced age. Children and adolescents may be affected as well.
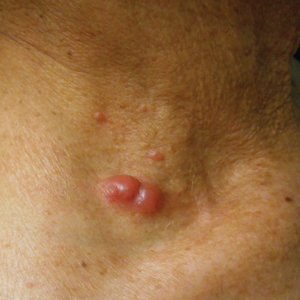
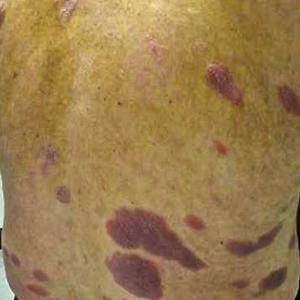
With mycosis fungoides, there are three main types of skin lesions: patch, plaque, and tumor. Patients will progress from patch to plaque to tumor stage in classic MF. Often, lesions begin as scaly, erythematous patches that resemble eczema. Because of the nonspecific nature of early lesions, the median duration from the onset of skin lesions to the diagnosis of MF is 4-6 years. Patch stage lesions may be pruritic or asymptomatic. Commonly, they present in non–sun-exposed areas, such as the buttocks. Annular, infiltrated, red-brown or violaceous plaques can develop, which represent malignant T-cell infiltration. Many patients never progress past the plaque stage. Tumor stage MF is more aggressive, with nodules that may undergo necrosis and ulceration.
The leukemic form of MF is Sézary syndrome. Patients present with pruritic erythroderma and lymphadenopathy. Nail dystrophy, scaling of palms and soles, and alopecia may be present. A peripheral blood smear reveals Sézary cells, which are large, hyperconvoluted lymphocytes. The count of Sézary cells is usually greater than 1000 cells/mm3.
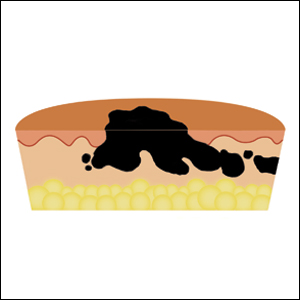
Histology of early lesions may not be diagnostic for CTCL. Often, biopsies will be read as eczematous or psoriasiform for years before the diagnosis of MF is made. Classically, epidermotropism (single-cell exocytosis of lymphocytes into the epidermis) is present. Advanced stages may show a dense infiltrate of lymphocytes in the dermis. Groups of lymphocytes in the epidermis form Pautrier’s microabscesses. Mycosis cells may exhibit cerebriform nuclei. Neoplastic cells in MF are CD3+, CD4+, CD45RO+, CD8–. Tissue can be sent for T-cell gene rearrangement polymerase chain reaction. The presence of monoclonal T-cell gene receptor rearrangements can aid in the diagnosis of MF.
Treatment includes topical steroids, mechlorethamine (nitrogen mustard) or bexarotene gel, PUVA therapy, and narrow-band UVB light for limited and/or patch disease. Localized radiotherapy can be used for more resistant lesions. Topical therapies are preferred in the early stages in MF. Systemic treatments for patients who do not respond to local therapy, or in more advanced disease include methotrexate, interferon-alpha, oral bexarotene, denileukin diftitox, and combination chemotherapy. Photopheresis is reserved for erythrodermic disease.
This case and photo were submitted by Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice in Aventura, Fla. More diagnostic cases are available at edermatologynews.com. To submit a case for possible publication, send an email to dermnews@mdedge.com.
Some have postulated an infectious agent as the cause. Atopic dermatitis may confer an increased risk because of the chronic stimulation of T cells. Males are more commonly affected than females by a 2:1 ratio. A worse prognosis is associated with advanced age. Children and adolescents may be affected as well.
With mycosis fungoides, there are three main types of skin lesions: patch, plaque, and tumor. Patients will progress from patch to plaque to tumor stage in classic MF. Often, lesions begin as scaly, erythematous patches that resemble eczema. Because of the nonspecific nature of early lesions, the median duration from the onset of skin lesions to the diagnosis of MF is 4-6 years. Patch stage lesions may be pruritic or asymptomatic. Commonly, they present in non–sun-exposed areas, such as the buttocks. Annular, infiltrated, red-brown or violaceous plaques can develop, which represent malignant T-cell infiltration. Many patients never progress past the plaque stage. Tumor stage MF is more aggressive, with nodules that may undergo necrosis and ulceration.
The leukemic form of MF is Sézary syndrome. Patients present with pruritic erythroderma and lymphadenopathy. Nail dystrophy, scaling of palms and soles, and alopecia may be present. A peripheral blood smear reveals Sézary cells, which are large, hyperconvoluted lymphocytes. The count of Sézary cells is usually greater than 1000 cells/mm3.
Histology of early lesions may not be diagnostic for CTCL. Often, biopsies will be read as eczematous or psoriasiform for years before the diagnosis of MF is made. Classically, epidermotropism (single-cell exocytosis of lymphocytes into the epidermis) is present. Advanced stages may show a dense infiltrate of lymphocytes in the dermis. Groups of lymphocytes in the epidermis form Pautrier’s microabscesses. Mycosis cells may exhibit cerebriform nuclei. Neoplastic cells in MF are CD3+, CD4+, CD45RO+, CD8–. Tissue can be sent for T-cell gene rearrangement polymerase chain reaction. The presence of monoclonal T-cell gene receptor rearrangements can aid in the diagnosis of MF.
Treatment includes topical steroids, mechlorethamine (nitrogen mustard) or bexarotene gel, PUVA therapy, and narrow-band UVB light for limited and/or patch disease. Localized radiotherapy can be used for more resistant lesions. Topical therapies are preferred in the early stages in MF. Systemic treatments for patients who do not respond to local therapy, or in more advanced disease include methotrexate, interferon-alpha, oral bexarotene, denileukin diftitox, and combination chemotherapy. Photopheresis is reserved for erythrodermic disease.
This case and photo were submitted by Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice in Aventura, Fla. More diagnostic cases are available at edermatologynews.com. To submit a case for possible publication, send an email to dermnews@mdedge.com.
Some have postulated an infectious agent as the cause. Atopic dermatitis may confer an increased risk because of the chronic stimulation of T cells. Males are more commonly affected than females by a 2:1 ratio. A worse prognosis is associated with advanced age. Children and adolescents may be affected as well.
With mycosis fungoides, there are three main types of skin lesions: patch, plaque, and tumor. Patients will progress from patch to plaque to tumor stage in classic MF. Often, lesions begin as scaly, erythematous patches that resemble eczema. Because of the nonspecific nature of early lesions, the median duration from the onset of skin lesions to the diagnosis of MF is 4-6 years. Patch stage lesions may be pruritic or asymptomatic. Commonly, they present in non–sun-exposed areas, such as the buttocks. Annular, infiltrated, red-brown or violaceous plaques can develop, which represent malignant T-cell infiltration. Many patients never progress past the plaque stage. Tumor stage MF is more aggressive, with nodules that may undergo necrosis and ulceration.
The leukemic form of MF is Sézary syndrome. Patients present with pruritic erythroderma and lymphadenopathy. Nail dystrophy, scaling of palms and soles, and alopecia may be present. A peripheral blood smear reveals Sézary cells, which are large, hyperconvoluted lymphocytes. The count of Sézary cells is usually greater than 1000 cells/mm3.
Histology of early lesions may not be diagnostic for CTCL. Often, biopsies will be read as eczematous or psoriasiform for years before the diagnosis of MF is made. Classically, epidermotropism (single-cell exocytosis of lymphocytes into the epidermis) is present. Advanced stages may show a dense infiltrate of lymphocytes in the dermis. Groups of lymphocytes in the epidermis form Pautrier’s microabscesses. Mycosis cells may exhibit cerebriform nuclei. Neoplastic cells in MF are CD3+, CD4+, CD45RO+, CD8–. Tissue can be sent for T-cell gene rearrangement polymerase chain reaction. The presence of monoclonal T-cell gene receptor rearrangements can aid in the diagnosis of MF.
Treatment includes topical steroids, mechlorethamine (nitrogen mustard) or bexarotene gel, PUVA therapy, and narrow-band UVB light for limited and/or patch disease. Localized radiotherapy can be used for more resistant lesions. Topical therapies are preferred in the early stages in MF. Systemic treatments for patients who do not respond to local therapy, or in more advanced disease include methotrexate, interferon-alpha, oral bexarotene, denileukin diftitox, and combination chemotherapy. Photopheresis is reserved for erythrodermic disease.
This case and photo were submitted by Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice in Aventura, Fla. More diagnostic cases are available at edermatologynews.com. To submit a case for possible publication, send an email to dermnews@mdedge.com.
Sonic hedgehog inhibitors have mixed efficacy for advanced BCC
For patients with locally advanced or metastatic basal cell carcinoma, Sonic hedgehog inhibitors (SSHi) are effective but are associated with primarily partial responses, and the two Food and Drug Administration–approved agents have significant toxicities, results of a systematic review and meta-analysis indicated.
Data on patients with metastatic or locally advanced basal cell carcinoma (BCC) treated with either vismodegib (Erivedge) or sonidegib (Odomzo) showed that the two agents had roughly similar overall response rates (ORR). Vismodegib, however, had a significantly higher rate of complete responses (CR) in patients with locally advanced disease, reported Pingxing Xie, MD, PhD, and Philippe Lefrançois, MD, PhD, from McGill University, Montreal.
“Common side effects of SHHi therapy tend to incapacitate patients, leading to high discontinuation rates. Over 25% of patients stopped treatment due to side effects. Most side effects are reversible after therapy cessation, except cases of persistent alopecia have been reported,” they wrote in the Journal of the American Academy of Dermatology.
The authors conducted the review to evaluate SHHi as a class and to get a better idea of the efficacy and safety of each agent in the class. They searched the literature to identify all studies using SHHi to treat BCC with vismodegib, sonidegib, itraconazole, or the investigational compound TAK-441, and identified 14 studies focused on vismodegib, 2 on sonidegib, and 1 each on itraconazole and TAK-441.
Of the 18 studies, data from 16 were pooled in fixed-effects linear models to analyze efficacy. The pooled ORR for all patients was 59.6%, “indicating that most patients receiving SHHi achieve at least a partial response.”
Combined ORR results showed a rate of 61.9% for vismodegib, 55.2% for sonidegib, 50% for itraconazole, and 20% for TAK-441, although data for the latter two agents were limited.
In studies looking at locally advanced and metastatic BCC separately, vismodegib was numerically better but statistically similar to sonidegib for locally advanced disease (ORR, 68.8% vs. 56.6%). However, vismodegib was significantly superior to sonidegib for patients with metastatic BCC (ORR, 39.7% vs. 14.7%; P = .007).
The pooled CR for all patients was 23.5%, and there were no CRs with either itraconazole or TAK-441. The combined CR rate for vismodegib was 28% (P = .012). The combined CR rate for sonidegib was just 8.9% and was not statistically significant, the investigators found.
In subgroup analyses for locally advanced BCC, the CR rate for vismodegib was 30.9% for vismodegib (P = .012), “meaning that many patients can expect cure.” In contrast, only 3% of patients treated for locally advanced disease had a CR with sonidegib. The difference between the drugs in this subpopulation was significant (P less than .0001).
Neither drug produced significant CRs in patients with metastatic melanoma, and the pooled clinical benefit rate (all patients with stable disease or better) was 94.9%, with rates similar among all four drugs.
Pooled prevalences of adverse events showed a 67.1% prevalence of muscle spasms, 54.1% prevalence of dysgeusia, and a 57.7% prevalence of alopecia. The proportions of side effects were similar between vismodegib and sonidegib, but sonidegib was associated with a higher prevalence of upper gastrointestinal tract distress than vismodegib.
The study was partially funded by the Canadian Dermatology Foundation. The authors reported having no conflicts of interest.
SOURCE: Xie P et al. J Am Acad Dermatol. 2018 Jul 9. doi: 10.1016/j.jaad.2018.07.004.
For patients with locally advanced or metastatic basal cell carcinoma, Sonic hedgehog inhibitors (SSHi) are effective but are associated with primarily partial responses, and the two Food and Drug Administration–approved agents have significant toxicities, results of a systematic review and meta-analysis indicated.
Data on patients with metastatic or locally advanced basal cell carcinoma (BCC) treated with either vismodegib (Erivedge) or sonidegib (Odomzo) showed that the two agents had roughly similar overall response rates (ORR). Vismodegib, however, had a significantly higher rate of complete responses (CR) in patients with locally advanced disease, reported Pingxing Xie, MD, PhD, and Philippe Lefrançois, MD, PhD, from McGill University, Montreal.
“Common side effects of SHHi therapy tend to incapacitate patients, leading to high discontinuation rates. Over 25% of patients stopped treatment due to side effects. Most side effects are reversible after therapy cessation, except cases of persistent alopecia have been reported,” they wrote in the Journal of the American Academy of Dermatology.
The authors conducted the review to evaluate SHHi as a class and to get a better idea of the efficacy and safety of each agent in the class. They searched the literature to identify all studies using SHHi to treat BCC with vismodegib, sonidegib, itraconazole, or the investigational compound TAK-441, and identified 14 studies focused on vismodegib, 2 on sonidegib, and 1 each on itraconazole and TAK-441.
Of the 18 studies, data from 16 were pooled in fixed-effects linear models to analyze efficacy. The pooled ORR for all patients was 59.6%, “indicating that most patients receiving SHHi achieve at least a partial response.”
Combined ORR results showed a rate of 61.9% for vismodegib, 55.2% for sonidegib, 50% for itraconazole, and 20% for TAK-441, although data for the latter two agents were limited.
In studies looking at locally advanced and metastatic BCC separately, vismodegib was numerically better but statistically similar to sonidegib for locally advanced disease (ORR, 68.8% vs. 56.6%). However, vismodegib was significantly superior to sonidegib for patients with metastatic BCC (ORR, 39.7% vs. 14.7%; P = .007).
The pooled CR for all patients was 23.5%, and there were no CRs with either itraconazole or TAK-441. The combined CR rate for vismodegib was 28% (P = .012). The combined CR rate for sonidegib was just 8.9% and was not statistically significant, the investigators found.
In subgroup analyses for locally advanced BCC, the CR rate for vismodegib was 30.9% for vismodegib (P = .012), “meaning that many patients can expect cure.” In contrast, only 3% of patients treated for locally advanced disease had a CR with sonidegib. The difference between the drugs in this subpopulation was significant (P less than .0001).
Neither drug produced significant CRs in patients with metastatic melanoma, and the pooled clinical benefit rate (all patients with stable disease or better) was 94.9%, with rates similar among all four drugs.
Pooled prevalences of adverse events showed a 67.1% prevalence of muscle spasms, 54.1% prevalence of dysgeusia, and a 57.7% prevalence of alopecia. The proportions of side effects were similar between vismodegib and sonidegib, but sonidegib was associated with a higher prevalence of upper gastrointestinal tract distress than vismodegib.
The study was partially funded by the Canadian Dermatology Foundation. The authors reported having no conflicts of interest.
SOURCE: Xie P et al. J Am Acad Dermatol. 2018 Jul 9. doi: 10.1016/j.jaad.2018.07.004.
For patients with locally advanced or metastatic basal cell carcinoma, Sonic hedgehog inhibitors (SSHi) are effective but are associated with primarily partial responses, and the two Food and Drug Administration–approved agents have significant toxicities, results of a systematic review and meta-analysis indicated.
Data on patients with metastatic or locally advanced basal cell carcinoma (BCC) treated with either vismodegib (Erivedge) or sonidegib (Odomzo) showed that the two agents had roughly similar overall response rates (ORR). Vismodegib, however, had a significantly higher rate of complete responses (CR) in patients with locally advanced disease, reported Pingxing Xie, MD, PhD, and Philippe Lefrançois, MD, PhD, from McGill University, Montreal.
“Common side effects of SHHi therapy tend to incapacitate patients, leading to high discontinuation rates. Over 25% of patients stopped treatment due to side effects. Most side effects are reversible after therapy cessation, except cases of persistent alopecia have been reported,” they wrote in the Journal of the American Academy of Dermatology.
The authors conducted the review to evaluate SHHi as a class and to get a better idea of the efficacy and safety of each agent in the class. They searched the literature to identify all studies using SHHi to treat BCC with vismodegib, sonidegib, itraconazole, or the investigational compound TAK-441, and identified 14 studies focused on vismodegib, 2 on sonidegib, and 1 each on itraconazole and TAK-441.
Of the 18 studies, data from 16 were pooled in fixed-effects linear models to analyze efficacy. The pooled ORR for all patients was 59.6%, “indicating that most patients receiving SHHi achieve at least a partial response.”
Combined ORR results showed a rate of 61.9% for vismodegib, 55.2% for sonidegib, 50% for itraconazole, and 20% for TAK-441, although data for the latter two agents were limited.
In studies looking at locally advanced and metastatic BCC separately, vismodegib was numerically better but statistically similar to sonidegib for locally advanced disease (ORR, 68.8% vs. 56.6%). However, vismodegib was significantly superior to sonidegib for patients with metastatic BCC (ORR, 39.7% vs. 14.7%; P = .007).
The pooled CR for all patients was 23.5%, and there were no CRs with either itraconazole or TAK-441. The combined CR rate for vismodegib was 28% (P = .012). The combined CR rate for sonidegib was just 8.9% and was not statistically significant, the investigators found.
In subgroup analyses for locally advanced BCC, the CR rate for vismodegib was 30.9% for vismodegib (P = .012), “meaning that many patients can expect cure.” In contrast, only 3% of patients treated for locally advanced disease had a CR with sonidegib. The difference between the drugs in this subpopulation was significant (P less than .0001).
Neither drug produced significant CRs in patients with metastatic melanoma, and the pooled clinical benefit rate (all patients with stable disease or better) was 94.9%, with rates similar among all four drugs.
Pooled prevalences of adverse events showed a 67.1% prevalence of muscle spasms, 54.1% prevalence of dysgeusia, and a 57.7% prevalence of alopecia. The proportions of side effects were similar between vismodegib and sonidegib, but sonidegib was associated with a higher prevalence of upper gastrointestinal tract distress than vismodegib.
The study was partially funded by the Canadian Dermatology Foundation. The authors reported having no conflicts of interest.
SOURCE: Xie P et al. J Am Acad Dermatol. 2018 Jul 9. doi: 10.1016/j.jaad.2018.07.004.
FROM THE JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY
Key clinical point: Sonic hedgehog inhibitors (SHHi) have mixed efficacy against metastatic or locally advanced basal cell carcinoma (BCC).
Major finding: Combined overall response rate results showed a rate of 61.9% for vismodegib, 55.2% for sonidegib, 50% for itraconazole, and 20% for TAK-441.
Study details: A systematic review and meta-analysis of studies looking at SHHi in patients with BCC.
Disclosures: The study was partially funded by the Canadian Dermatology Foundation. The authors reported having no conflicts of interest.
Source: Xie P et al. J Am Acad Dermatol. 2018 Jul 9. doi:10.1016/j.jaad.2018.07.004.
FDA approves biologic for mycosis fungoides, Sézary syndrome
The Food and Drug Administration has approved mogamulizumab-kpkc (Poteligeo) for the treatment of adults with relapsed or refractory mycosis fungoides (MF) or Sézary syndrome (SS) who have received at least one prior systemic therapy.
Mogamulizumab is a humanized monoclonal antibody directed against CC chemokine receptor 4 (CCR4). It is the first biologic agent targeting CCR4 to be approved for patients in the United States.
Mogamulizumab is expected to be commercially available in the fourth quarter of 2018.
The FDA previously granted mogamulizumab breakthrough therapy and orphan drug designations, as well as priority review.
The approval is supported by the phase 3 MAVORIC trial. Results from this trial were presented at the 10th Annual T-cell Lymphoma Forum in February 2018.
MAVORIC enrolled 372 adults with histologically confirmed MF or SS who had failed at least one systemic therapy. They were randomized to receive mogamulizumab at 1.0 mg/kg (weekly for the first 4-week cycle and then every 2 weeks) or vorinostat at 400 mg daily. Patients were treated until disease progression or unacceptable toxicity. Those receiving vorinostat could cross over to mogamulizumab if they progressed or experienced intolerable toxicity. Baseline characteristics were similar between the treatment arms. The study’s primary endpoint was progression-free survival. The median progression-free survival was 7.7 months with mogamulizumab and 3.1 months with vorinostat (hazard ratio, 0.53; P less than .0001).
The global overall response rate was 28% (52/189) in the mogamulizumab arm and 5% (9/186) in the vorinostat arm (P less than .0001). For patients with MF, the ORR was 21% with mogamulizumab and 7% with vorinostat; for patients with SS, the ORR was 37% and 2%, respectively. After crossover, the ORR in the mogamulizumab arm was 30% (41/136).
The median duration of response (DOR) was 14 months in the mogamulizumab arm and 9 months in the vorinostat arm. For MF patients, the median DOR was 13 months with mogamulizumab and 9 months with vorinostat; for SS patients, the median DOR was 17 months and 7 months, respectively.
The most common treatment-emergent adverse events (AEs), which occurred in at least 20% of patients in either arm (mogamulizumab and vorinostat, respectively), included the following:
- Infusion-related reactions (33.2% vs. 0.5%).
- Drug eruptions (23.9% vs. 0.5%).
- Diarrhea (23.4% vs. 61.8%).
- Nausea (15.2% vs. 42.5%).
- Thrombocytopenia (11.4% vs. 30.6%).
- Dysgeusia (3.3% vs. 28.0%).
- Increased blood creatinine (3.3% vs. 28.0%).
- Decreased appetite (7.6% vs. 24.7%).
There were no grade 4 AEs in the mogamulizumab arm. Grade 3 AEs in mogamulizumab recipients included drug eruptions (n = 8), infusion-related reactions (n = 3), fatigue (n = 3), decreased appetite (n = 2), nausea (n = 1), pyrexia (n = 1), and diarrhea (n = 1).
The drug is marketed by Kyowa Kirin.
The Food and Drug Administration has approved mogamulizumab-kpkc (Poteligeo) for the treatment of adults with relapsed or refractory mycosis fungoides (MF) or Sézary syndrome (SS) who have received at least one prior systemic therapy.
Mogamulizumab is a humanized monoclonal antibody directed against CC chemokine receptor 4 (CCR4). It is the first biologic agent targeting CCR4 to be approved for patients in the United States.
Mogamulizumab is expected to be commercially available in the fourth quarter of 2018.
The FDA previously granted mogamulizumab breakthrough therapy and orphan drug designations, as well as priority review.
The approval is supported by the phase 3 MAVORIC trial. Results from this trial were presented at the 10th Annual T-cell Lymphoma Forum in February 2018.
MAVORIC enrolled 372 adults with histologically confirmed MF or SS who had failed at least one systemic therapy. They were randomized to receive mogamulizumab at 1.0 mg/kg (weekly for the first 4-week cycle and then every 2 weeks) or vorinostat at 400 mg daily. Patients were treated until disease progression or unacceptable toxicity. Those receiving vorinostat could cross over to mogamulizumab if they progressed or experienced intolerable toxicity. Baseline characteristics were similar between the treatment arms. The study’s primary endpoint was progression-free survival. The median progression-free survival was 7.7 months with mogamulizumab and 3.1 months with vorinostat (hazard ratio, 0.53; P less than .0001).
The global overall response rate was 28% (52/189) in the mogamulizumab arm and 5% (9/186) in the vorinostat arm (P less than .0001). For patients with MF, the ORR was 21% with mogamulizumab and 7% with vorinostat; for patients with SS, the ORR was 37% and 2%, respectively. After crossover, the ORR in the mogamulizumab arm was 30% (41/136).
The median duration of response (DOR) was 14 months in the mogamulizumab arm and 9 months in the vorinostat arm. For MF patients, the median DOR was 13 months with mogamulizumab and 9 months with vorinostat; for SS patients, the median DOR was 17 months and 7 months, respectively.
The most common treatment-emergent adverse events (AEs), which occurred in at least 20% of patients in either arm (mogamulizumab and vorinostat, respectively), included the following:
- Infusion-related reactions (33.2% vs. 0.5%).
- Drug eruptions (23.9% vs. 0.5%).
- Diarrhea (23.4% vs. 61.8%).
- Nausea (15.2% vs. 42.5%).
- Thrombocytopenia (11.4% vs. 30.6%).
- Dysgeusia (3.3% vs. 28.0%).
- Increased blood creatinine (3.3% vs. 28.0%).
- Decreased appetite (7.6% vs. 24.7%).
There were no grade 4 AEs in the mogamulizumab arm. Grade 3 AEs in mogamulizumab recipients included drug eruptions (n = 8), infusion-related reactions (n = 3), fatigue (n = 3), decreased appetite (n = 2), nausea (n = 1), pyrexia (n = 1), and diarrhea (n = 1).
The drug is marketed by Kyowa Kirin.
The Food and Drug Administration has approved mogamulizumab-kpkc (Poteligeo) for the treatment of adults with relapsed or refractory mycosis fungoides (MF) or Sézary syndrome (SS) who have received at least one prior systemic therapy.
Mogamulizumab is a humanized monoclonal antibody directed against CC chemokine receptor 4 (CCR4). It is the first biologic agent targeting CCR4 to be approved for patients in the United States.
Mogamulizumab is expected to be commercially available in the fourth quarter of 2018.
The FDA previously granted mogamulizumab breakthrough therapy and orphan drug designations, as well as priority review.
The approval is supported by the phase 3 MAVORIC trial. Results from this trial were presented at the 10th Annual T-cell Lymphoma Forum in February 2018.
MAVORIC enrolled 372 adults with histologically confirmed MF or SS who had failed at least one systemic therapy. They were randomized to receive mogamulizumab at 1.0 mg/kg (weekly for the first 4-week cycle and then every 2 weeks) or vorinostat at 400 mg daily. Patients were treated until disease progression or unacceptable toxicity. Those receiving vorinostat could cross over to mogamulizumab if they progressed or experienced intolerable toxicity. Baseline characteristics were similar between the treatment arms. The study’s primary endpoint was progression-free survival. The median progression-free survival was 7.7 months with mogamulizumab and 3.1 months with vorinostat (hazard ratio, 0.53; P less than .0001).
The global overall response rate was 28% (52/189) in the mogamulizumab arm and 5% (9/186) in the vorinostat arm (P less than .0001). For patients with MF, the ORR was 21% with mogamulizumab and 7% with vorinostat; for patients with SS, the ORR was 37% and 2%, respectively. After crossover, the ORR in the mogamulizumab arm was 30% (41/136).
The median duration of response (DOR) was 14 months in the mogamulizumab arm and 9 months in the vorinostat arm. For MF patients, the median DOR was 13 months with mogamulizumab and 9 months with vorinostat; for SS patients, the median DOR was 17 months and 7 months, respectively.
The most common treatment-emergent adverse events (AEs), which occurred in at least 20% of patients in either arm (mogamulizumab and vorinostat, respectively), included the following:
- Infusion-related reactions (33.2% vs. 0.5%).
- Drug eruptions (23.9% vs. 0.5%).
- Diarrhea (23.4% vs. 61.8%).
- Nausea (15.2% vs. 42.5%).
- Thrombocytopenia (11.4% vs. 30.6%).
- Dysgeusia (3.3% vs. 28.0%).
- Increased blood creatinine (3.3% vs. 28.0%).
- Decreased appetite (7.6% vs. 24.7%).
There were no grade 4 AEs in the mogamulizumab arm. Grade 3 AEs in mogamulizumab recipients included drug eruptions (n = 8), infusion-related reactions (n = 3), fatigue (n = 3), decreased appetite (n = 2), nausea (n = 1), pyrexia (n = 1), and diarrhea (n = 1).
The drug is marketed by Kyowa Kirin.
Going Digital With Dermoscopy
Dermoscopic examination has been proven to increase diagnostic accuracy and decrease unnecessary biopsies of both melanoma and nonmelanoma skin cancers.1,2 Digital dermoscopy refers to acquiring and storing digital dermoscopic photographs via digital camera, smart image capture devices such as smartphones and tablets, or any other devices used for image acquisition. The stored images may then be used in a variety of ways, including sequential digital monitoring, teledermoscopy, and machine learning.
Sequential Digital Monitoring
Sequential digital dermoscopy imaging (SDDI) is the capture and storage of dermoscopic images of suspicious lesions that are then monitored over time for changes. Studies have shown that SDDI allows for early detection of melanomas and leads to a decrease in the number of unnecessary excisions.3,4 A meta-analysis of SDDI found that the chance of detecting melanoma increased with the length of monitoring, which suggests that continued follow-up, especially in high-risk groups, is crucial.4
Teledermoscopy
Teledermatology (telederm) is on the rise in the United States, with the number of programs and consultations increasing yearly. One study showed a 48% increase in telederm programs in the last 5 years.5 Studies have shown the addition of digital dermoscopic images improved the diagnostic accuracy in telederm skin cancer screenings versus clinical images alone.6,7
Telederm currently is practiced in 2 main models: live-interactive video consultation and storage of images for future consultation (store and forward). Medicare currently only reimburses live-interactive telederm for patients in nonmetropolitan areas and store-and-forward telederm pilot programs in Alaska and Hawaii; however, Medicaid does reimburse for store and forward in a handful of states.8 Similar to dermatoscope use during clinical examination, there currently is no additional reimbursement for teledermoscopy. Of note, a willingness-to-pay survey of 214 students from a southwestern university health center showed that participants were willing to pay an average (SD) of $55.27 ($39.11) out of pocket for a teledermoscopy/telederm evaluation, citing factors such as convenience.9
Direct-to-consumer telederm offers a new way for patients to receive care.10 Some dermatoscopes (eg, DermLite HÜD [3Gen], Molescope/Molescope II [Metaoptima Technology Inc]) currently are marketed directly to consumers along with telederm services to facilitate direct-to-patient teledermoscopy.11,12
Machine Learning
Big data and machine learning has been hailed as the future of medicine and dermatology alike.13 Machine learning is a type of artificial intelligence that uses computational algorithms (eg, neural networks) that allow computer programs to automatically improve their accuracy (learn) by analyzing large data sets. In dermatology, machine learning has been most notably used to train computers to identify images of skin cancer by way of large image databases.14-17 One algorithm, a convolutional neural network (CNN), made headlines in 2017 when it was able to identify dermoscopic and clinical images of skin cancer with comparable accuracy to a group of 21 dermatologists.14 In 2018, the International Skin Imaging Collaboration (ISIC) published results of a study of the diagnostic accuracy of 25 computer algorithms compared to 8 dermatologists using a set of 100 dermoscopic images of melanoma and benign nevi.15 Using the average sensitivity of the dermatologists (82%), the top fusion algorithm in the study had a sensitivity of 76% versus 59% for the dermatologists (P=.02). These results compared the mean sensitivity of the dermatologists, as some individual dermatologists outperformed the algorithm.15 More recently, another CNN was compared to 58 international dermatologists in the classification of a set of 100 dermoscopic images (20 melanoma and 80 melanocytic nevi).16 Using the mean sensitivity of the dermatologists (86.6%), the CNN had a specificity of 92.5% versus 71.3% for dermatologists (P<.01). In the second part of the study, the dermatologists were given some clinical information and close-up photographs of the lesions, which improved their average (SD) sensitivity and specificity to 88.9% (9.6%)(P=.19) and 75.7% (11.7%)(P<.05), respectively. When compared to the CNN at this higher sensitivity, the CNN still had a higher specificity than the dermatologists (82.5% vs 75.7% [P<.01]).16 However, in real-life clinical practice dermatologists perform better, not only because they can collect more in-person clinical information but also because humans gather more information during live examination than when they are interpreting close-up clinical and/or dermoscopic images. In a sense, we currently are limited to comparing data that is incommensurable.
Machine learning studies have other notable limitations, such as data sets that do not contain a full spectrum of skin lesions or less common lesions (eg, pigmented seborrheic keratoses, amelanotic melanomas) and variation in image databases used.15,16 For machine algorithms to improve, they require access to high-quality and ideally standardized digital dermoscopic image databases. The ISIC and other organizations currently have databases specifically for this purpose, but more images are needed.18 As additional practitioners incorporate digital dermoscopy in their clinical practice, the potential for larger databases and more accurate algorithms becomes a possibility.
Image Acquisition
Many devices are available for digital dermoscopic image acquisition, including dermatoscopes that attach to smartphones and/or digital cameras and all-in-one systems (eTable). The exact system employed will depend on the practitioner's requirements for price, portability, speed, image quality, and software. Digital single-lens reflex (DSLR) cameras boast the highest image quality, while video dermoscopy traditionally yields stored images with poor resolution.19 Macroscopic images obtained by other imaging devices, including spectral imaging devices and reflectance confocal microscopy, usually are yielded via video dermoscopy or a video camera to capture images; thus, stored images generally are not as high quality.
Smartphones are increasingly used for clinical imaging in dermatology.20 Although DSLR cameras still take the highest-quality images, current smartphone image quality is comparable to digital cameras.21,22 Computational photography uses computer processing power to enhance image quality and may bring smartphone image quality closer to DSLR cameras.22,23 Smartphones with newer dual-lens cameras have been reported to further improve image quality.21 Current smartphones have the option of enabling high-dynamic-range imaging, which combines multiple images taken with different exposures to create a single image with improved dynamic range of luminosity. It has been reported that high-dynamic-range imaging may even enhance dermoscopic features of more challenging hypopigmented skin cancers.24
Standardizing Imaging
There has been a concerted effort to standardize digital dermatologic image acquisition.25,26 Standardization promises to facilitate data analysis, improve collaboration, protect patient privacy, and improve patient care.13,26,27 At the forefront of image standardization is the ISIC organization, which recently published its Delphi consensus guidelines on standards for lesion imaging, including dermoscopy.26
The true holy grail of image standardization is the Digital Imaging and Communications in Medicine (DICOM) standard.26-28 The DICOM is a comprehensive imaging standard for storage, annotation, transfer, and display of images, and it is most notable for its use in radiology. The DICOM also could be applied to new imaging modalities in dermatology (eg, optical coherence tomography, reflectance confocal microscopy). Past efforts to develop a DICOM standard for dermatology were undertaken by a working group that has since disbanded.27 Work by the ISIC and many others will hopefully lead to adoption of the DICOM standard by dermatology at some point in the future.
Protected Health Information
The Health Insurance Portability and Accountability Act (HIPAA) requires protected health information (PHI) to be stored in a secure manner with limited access that sufficiently protects identifiable patient information. Although dermoscopic images generally are deidentified, they often are stored alongside clinical photographs and data that contains PHI in clinical practice.
Image storage can take 2 forms: (1) physical local storage on internal and external hard drives or (2) remote storage (eg, cloud-based storage). Encryption is essential regardless of the method of storage. It is required by law that loss of nonencrypted PHI be reported to all potentially affected patients, the US Department of Health & Human Services, and local/state media depending on the number of patients affected. Loss of PHI can result in fines of up to $1.5 million.29 On the contrary, loss of properly encrypted data would not be required to be reported.30
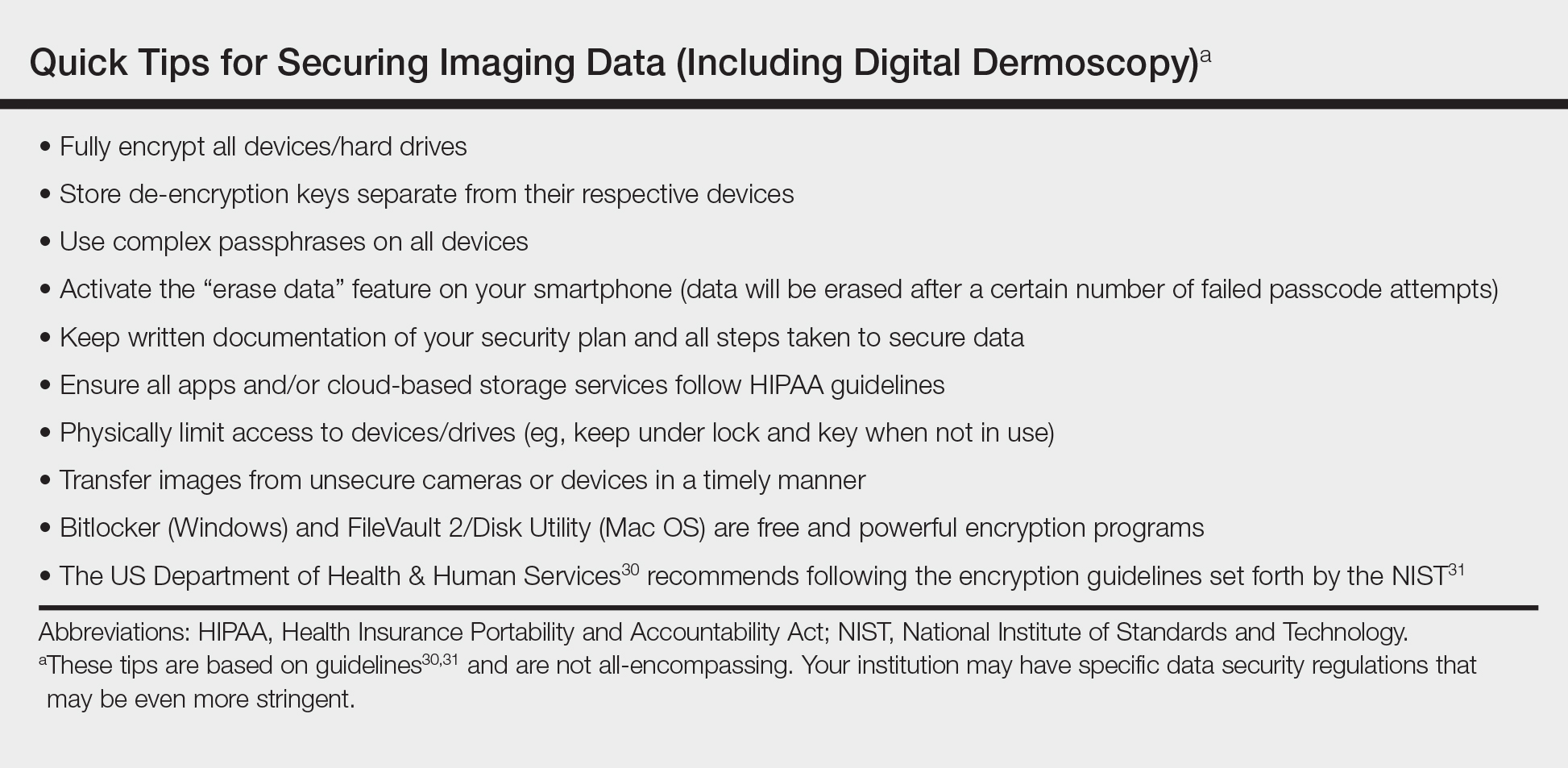
As smart image acquisition devices begin to dominate the clinical setting, practitioners need to be vigilant in securing patient PHI. There are multiple applications (apps) that allow for secure encrypted digital dermoscopic image acquisition and storage on smartphones. Additionally, it is important to secure smartphones with complex passcodes (eg, a mix of special characters, numbers, uppercase and lowercase letters). Most dermatoscope manufacturers have apps for image acquisition and storage that can be tied into other platforms or storage systems (eg, DermLite app [3Gen], Handyscope [FotoFinder Systems GmbH], VEOS app [Canfield Scientific, Inc]).28 Other options include syncing images with current electronic medical record technologies, transferring photographs to HIPAA-compliant cloud storage, or transferring photographs to an encrypted computer and/or external hard drive. Some tips for securing data based on HIPAA and other guidelines are listed in the Table.30,31
Conclusion
The expansion of teledermoscopy alongside direct-to-patient services may create additional incentives for clinicians to incorporate digital dermoscopy into their practice. As more practitioners adopt digital dermoscopy, machine learning driven by technological advancements and larger image data sets could influence the future practice of dermatology. With the rise in digital dermoscopy by way of smartphones, additional steps must be taken to ensure patients' PHI is safeguarded. Digital dermoscopy is a dynamic field that will likely see continued growth in the coming years.
- Vestergaard ME, Macaskill P, Holt PE, et al. Dermoscopy compared with naked eye examination for the diagnosis of primary melanoma: a meta-analysis of studies performed in a clinical setting. Br J Dermatol. 2008;159:669-676.
- Rosendahl C, Tschandl P, Cameron A, et al. Diagnostic accuracy of dermatoscopy for melanocytic and nonmelanocytic pigmented lesions. J Am Acad Dermatol. 2011;64:1068-1073.
- Salerni G, Lovatto L, Carrera C, et al. Melanomas detected in a follow-up program compared with melanomas referred to a melanoma unit. Arch Dermatol. 2011;147:549-555.
- Salerni G, Terán T, Puig S, et al. Meta-analysis of digital dermoscopy follow-up of melanocytic skin lesions: a study on behalf of the International Dermoscopy Society. J Eur Acad Dermatol Venereol. 2013;27:805-814.
- Yim KM, Armstrong AW, Oh DH, et al. Teledermatology in the United States: an update in a dynamic era [published online January 22, 2018]. Telemed J E Health. doi:10.1089/tmj.2017.0253.
- Ferrándiz L, Ojeda-Vila T, Corrales A, et al. Internet-based skin cancer screening using clinical images alone or in conjunction with dermoscopic images: a randomized teledermoscopy trial. J Am Acad Dermatol. 2017;76:676-682.
- Şenel E, Baba M, Durdu M. The contribution of teledermatoscopy to the diagnosis and management of non-melanocytic skin tumours. J Telemed Telecare. 2013;19:60-63.
- State telehealth laws and Medicaid program policies: a comprehensive scan of the 50 states and District of Columbia. Public Health Institute Center for Connected Health Policy website. http://www.cchpca.org/sites/default/files/resources/
50%20State%20FINAL%20April%202016.pdf. Published March 2016. Accessed July 2, 2018. - Raghu TS, Yiannias J, Sharma N, et al. Willingness to pay for teledermoscopy services at a university health center. J Patient Exp. 2018. doi:10.11772374373517748657.
- Fogel AL, Sarin KY. A survey of direct-to-consumer teledermatology services available to US patients: explosive growth, opportunities and controversy. J Telemed Telecare. 2017;23:19-25.
- MoleScope. MetaOptima Technology Inc website. https://molescope.com/product/. Accessed July 2, 2018.
- DermLite HÜD. 3Gen website. https://dermlite.com/products/dermlite-hud. Accessed July 2, 2018.
- Park AJ, Ko JM, Swerlick RA. Crowdsourcing dermatology: DataDerm, big data analytics, and machine learning technology. J Am Acad Dermatol. 2018;78:643-644.
- Esteva A, Kuprel B, Novoa RA, et al. Dermatologist-level classification of skin cancer with deep neural networks. Nature. 2017;542:115-118.
- Marchetti MA, Codella NCF, Dusza SW, et al; International Skin Imaging Collaboration. results of the 2016 International Skin Imaging Collaboration International Symposium on Biomedical Imaging challenge: comparison of the accuracy of computer algorithms to dermatologists for the diagnosis of melanoma from dermoscopic images. J Am Acad Dermatol. 2018;78:270-277.
- Haenssle HA, Fink C, Schneiderbauer R, et al. Man against machine: diagnostic performance of a deep learning convolutional neural network for dermoscopic melanoma recognition in comparison to 58 dermatologists [published online May 28, 2018]. doi:10.1093/annonc/mdy166.
- Prado G, Kovarik C. Cutting edge technology in dermatology: virtual reality and artificial intelligence. Cutis. 2018;101:236-237.
- Sultana NN, Puhan NB. Recent deep learning methods for melanoma detection: a review. In: Ghosh D, Giri D, Mohapatra R, et al, eds. Mathematics and Computing. Singapore: Springer Nature; 2018:118-132.
- Lake A, Jones B. Dermoscopy: to cross-polarize, or not to cross-polarize, that is the question. J Vis Commun Med. 2015;38:36-50.
- Abbott LM, Magnusson RS, Gibbs E, et al. Smartphone use in dermatology for clinical photography and consultation: current practice and the law [published online February 28, 2017]. Australas J Dermatol. 2018;59:101-107.
- Hauser W, Neveu B, Jourdain JB, et al. Image quality benchmark of computational bokeh. Electron Imaging. 2018;2018:1-10.
- Ignatov A, Kobyshev N, Timofte R, et al. DSLR-quality photos on mobile devices with deep convolutional networks. 2017 IEEE International Conference on Computer Vision (ICCV). Venice, Italy: IEEE; 2017:3297-3305.
- Greengard S. Computational photography comes into focus. Commun ACM. 2014;57:19-21.
- Braun RP, Marghoob A. High-dynamic-range dermoscopy imaging and diagnosis of hypopigmented skin cancers. JAMA Dermatol. 2015;151:456-457.
- Quigley EA, Tokay BA, Jewell ST, et al. Technology and technique standards for camera-acquired digital dermatologic images: a systematic review. JAMA Dermatol. 2015;151:883-890.
- Katragadda C, Finnane A, Soyer HP, et al. Technique standards for skin lesion imaging a delphi consensus statement. JAMA Dermatol. 2017;153:207-213.
- Caffery LJ, Clunie D, Curiel-Lewandrowski C, et al. Transforming dermatologic imaging for the digital era: metadata and standards [published online January 17, 2018]. J Digit Imaging. doi:10.1007/s10278-017-0045-8.
- Pagliarello C, Stanganelli I, Fabrizi G, et al. Digital dermoscopy monitoring: is it time to define a quality standard? Acta Derm Venereol. 2017;97:864-865.
- HITECH Act Enforcement Interim Final Rule. US Department of Health & Human Services website. https://www.hhs.gov/hipaa/for-professionals/special-topics/hitech-act-enforcement-interim-final-rule/index.html. Updated June 16, 2017. Accessed July 2, 2018.
- Guidance to render unsecured protected health information unusable, unreadable, or indecipherable to unauthorized individuals. US Department of Health & Human Services website. https://www.hhs.gov/hipaa/for-professionals/breach-notification/guidance/index.html. Updated July 26, 2013. Accessed July 2, 2018.
- Scarfone K, Souppaya M, Sexton M. Guide to Storage Encryption Technologies for End User Devices. Gaithersburg, MD: US Department of Commerce; 2007. NIST Special Publication 800-111.
Dermoscopic examination has been proven to increase diagnostic accuracy and decrease unnecessary biopsies of both melanoma and nonmelanoma skin cancers.1,2 Digital dermoscopy refers to acquiring and storing digital dermoscopic photographs via digital camera, smart image capture devices such as smartphones and tablets, or any other devices used for image acquisition. The stored images may then be used in a variety of ways, including sequential digital monitoring, teledermoscopy, and machine learning.
Sequential Digital Monitoring
Sequential digital dermoscopy imaging (SDDI) is the capture and storage of dermoscopic images of suspicious lesions that are then monitored over time for changes. Studies have shown that SDDI allows for early detection of melanomas and leads to a decrease in the number of unnecessary excisions.3,4 A meta-analysis of SDDI found that the chance of detecting melanoma increased with the length of monitoring, which suggests that continued follow-up, especially in high-risk groups, is crucial.4
Teledermoscopy
Teledermatology (telederm) is on the rise in the United States, with the number of programs and consultations increasing yearly. One study showed a 48% increase in telederm programs in the last 5 years.5 Studies have shown the addition of digital dermoscopic images improved the diagnostic accuracy in telederm skin cancer screenings versus clinical images alone.6,7
Telederm currently is practiced in 2 main models: live-interactive video consultation and storage of images for future consultation (store and forward). Medicare currently only reimburses live-interactive telederm for patients in nonmetropolitan areas and store-and-forward telederm pilot programs in Alaska and Hawaii; however, Medicaid does reimburse for store and forward in a handful of states.8 Similar to dermatoscope use during clinical examination, there currently is no additional reimbursement for teledermoscopy. Of note, a willingness-to-pay survey of 214 students from a southwestern university health center showed that participants were willing to pay an average (SD) of $55.27 ($39.11) out of pocket for a teledermoscopy/telederm evaluation, citing factors such as convenience.9
Direct-to-consumer telederm offers a new way for patients to receive care.10 Some dermatoscopes (eg, DermLite HÜD [3Gen], Molescope/Molescope II [Metaoptima Technology Inc]) currently are marketed directly to consumers along with telederm services to facilitate direct-to-patient teledermoscopy.11,12
Machine Learning
Big data and machine learning has been hailed as the future of medicine and dermatology alike.13 Machine learning is a type of artificial intelligence that uses computational algorithms (eg, neural networks) that allow computer programs to automatically improve their accuracy (learn) by analyzing large data sets. In dermatology, machine learning has been most notably used to train computers to identify images of skin cancer by way of large image databases.14-17 One algorithm, a convolutional neural network (CNN), made headlines in 2017 when it was able to identify dermoscopic and clinical images of skin cancer with comparable accuracy to a group of 21 dermatologists.14 In 2018, the International Skin Imaging Collaboration (ISIC) published results of a study of the diagnostic accuracy of 25 computer algorithms compared to 8 dermatologists using a set of 100 dermoscopic images of melanoma and benign nevi.15 Using the average sensitivity of the dermatologists (82%), the top fusion algorithm in the study had a sensitivity of 76% versus 59% for the dermatologists (P=.02). These results compared the mean sensitivity of the dermatologists, as some individual dermatologists outperformed the algorithm.15 More recently, another CNN was compared to 58 international dermatologists in the classification of a set of 100 dermoscopic images (20 melanoma and 80 melanocytic nevi).16 Using the mean sensitivity of the dermatologists (86.6%), the CNN had a specificity of 92.5% versus 71.3% for dermatologists (P<.01). In the second part of the study, the dermatologists were given some clinical information and close-up photographs of the lesions, which improved their average (SD) sensitivity and specificity to 88.9% (9.6%)(P=.19) and 75.7% (11.7%)(P<.05), respectively. When compared to the CNN at this higher sensitivity, the CNN still had a higher specificity than the dermatologists (82.5% vs 75.7% [P<.01]).16 However, in real-life clinical practice dermatologists perform better, not only because they can collect more in-person clinical information but also because humans gather more information during live examination than when they are interpreting close-up clinical and/or dermoscopic images. In a sense, we currently are limited to comparing data that is incommensurable.
Machine learning studies have other notable limitations, such as data sets that do not contain a full spectrum of skin lesions or less common lesions (eg, pigmented seborrheic keratoses, amelanotic melanomas) and variation in image databases used.15,16 For machine algorithms to improve, they require access to high-quality and ideally standardized digital dermoscopic image databases. The ISIC and other organizations currently have databases specifically for this purpose, but more images are needed.18 As additional practitioners incorporate digital dermoscopy in their clinical practice, the potential for larger databases and more accurate algorithms becomes a possibility.
Image Acquisition
Many devices are available for digital dermoscopic image acquisition, including dermatoscopes that attach to smartphones and/or digital cameras and all-in-one systems (eTable). The exact system employed will depend on the practitioner's requirements for price, portability, speed, image quality, and software. Digital single-lens reflex (DSLR) cameras boast the highest image quality, while video dermoscopy traditionally yields stored images with poor resolution.19 Macroscopic images obtained by other imaging devices, including spectral imaging devices and reflectance confocal microscopy, usually are yielded via video dermoscopy or a video camera to capture images; thus, stored images generally are not as high quality.
Smartphones are increasingly used for clinical imaging in dermatology.20 Although DSLR cameras still take the highest-quality images, current smartphone image quality is comparable to digital cameras.21,22 Computational photography uses computer processing power to enhance image quality and may bring smartphone image quality closer to DSLR cameras.22,23 Smartphones with newer dual-lens cameras have been reported to further improve image quality.21 Current smartphones have the option of enabling high-dynamic-range imaging, which combines multiple images taken with different exposures to create a single image with improved dynamic range of luminosity. It has been reported that high-dynamic-range imaging may even enhance dermoscopic features of more challenging hypopigmented skin cancers.24
Standardizing Imaging
There has been a concerted effort to standardize digital dermatologic image acquisition.25,26 Standardization promises to facilitate data analysis, improve collaboration, protect patient privacy, and improve patient care.13,26,27 At the forefront of image standardization is the ISIC organization, which recently published its Delphi consensus guidelines on standards for lesion imaging, including dermoscopy.26
The true holy grail of image standardization is the Digital Imaging and Communications in Medicine (DICOM) standard.26-28 The DICOM is a comprehensive imaging standard for storage, annotation, transfer, and display of images, and it is most notable for its use in radiology. The DICOM also could be applied to new imaging modalities in dermatology (eg, optical coherence tomography, reflectance confocal microscopy). Past efforts to develop a DICOM standard for dermatology were undertaken by a working group that has since disbanded.27 Work by the ISIC and many others will hopefully lead to adoption of the DICOM standard by dermatology at some point in the future.
Protected Health Information
The Health Insurance Portability and Accountability Act (HIPAA) requires protected health information (PHI) to be stored in a secure manner with limited access that sufficiently protects identifiable patient information. Although dermoscopic images generally are deidentified, they often are stored alongside clinical photographs and data that contains PHI in clinical practice.
Image storage can take 2 forms: (1) physical local storage on internal and external hard drives or (2) remote storage (eg, cloud-based storage). Encryption is essential regardless of the method of storage. It is required by law that loss of nonencrypted PHI be reported to all potentially affected patients, the US Department of Health & Human Services, and local/state media depending on the number of patients affected. Loss of PHI can result in fines of up to $1.5 million.29 On the contrary, loss of properly encrypted data would not be required to be reported.30
As smart image acquisition devices begin to dominate the clinical setting, practitioners need to be vigilant in securing patient PHI. There are multiple applications (apps) that allow for secure encrypted digital dermoscopic image acquisition and storage on smartphones. Additionally, it is important to secure smartphones with complex passcodes (eg, a mix of special characters, numbers, uppercase and lowercase letters). Most dermatoscope manufacturers have apps for image acquisition and storage that can be tied into other platforms or storage systems (eg, DermLite app [3Gen], Handyscope [FotoFinder Systems GmbH], VEOS app [Canfield Scientific, Inc]).28 Other options include syncing images with current electronic medical record technologies, transferring photographs to HIPAA-compliant cloud storage, or transferring photographs to an encrypted computer and/or external hard drive. Some tips for securing data based on HIPAA and other guidelines are listed in the Table.30,31
Conclusion
The expansion of teledermoscopy alongside direct-to-patient services may create additional incentives for clinicians to incorporate digital dermoscopy into their practice. As more practitioners adopt digital dermoscopy, machine learning driven by technological advancements and larger image data sets could influence the future practice of dermatology. With the rise in digital dermoscopy by way of smartphones, additional steps must be taken to ensure patients' PHI is safeguarded. Digital dermoscopy is a dynamic field that will likely see continued growth in the coming years.
Dermoscopic examination has been proven to increase diagnostic accuracy and decrease unnecessary biopsies of both melanoma and nonmelanoma skin cancers.1,2 Digital dermoscopy refers to acquiring and storing digital dermoscopic photographs via digital camera, smart image capture devices such as smartphones and tablets, or any other devices used for image acquisition. The stored images may then be used in a variety of ways, including sequential digital monitoring, teledermoscopy, and machine learning.
Sequential Digital Monitoring
Sequential digital dermoscopy imaging (SDDI) is the capture and storage of dermoscopic images of suspicious lesions that are then monitored over time for changes. Studies have shown that SDDI allows for early detection of melanomas and leads to a decrease in the number of unnecessary excisions.3,4 A meta-analysis of SDDI found that the chance of detecting melanoma increased with the length of monitoring, which suggests that continued follow-up, especially in high-risk groups, is crucial.4
Teledermoscopy
Teledermatology (telederm) is on the rise in the United States, with the number of programs and consultations increasing yearly. One study showed a 48% increase in telederm programs in the last 5 years.5 Studies have shown the addition of digital dermoscopic images improved the diagnostic accuracy in telederm skin cancer screenings versus clinical images alone.6,7
Telederm currently is practiced in 2 main models: live-interactive video consultation and storage of images for future consultation (store and forward). Medicare currently only reimburses live-interactive telederm for patients in nonmetropolitan areas and store-and-forward telederm pilot programs in Alaska and Hawaii; however, Medicaid does reimburse for store and forward in a handful of states.8 Similar to dermatoscope use during clinical examination, there currently is no additional reimbursement for teledermoscopy. Of note, a willingness-to-pay survey of 214 students from a southwestern university health center showed that participants were willing to pay an average (SD) of $55.27 ($39.11) out of pocket for a teledermoscopy/telederm evaluation, citing factors such as convenience.9
Direct-to-consumer telederm offers a new way for patients to receive care.10 Some dermatoscopes (eg, DermLite HÜD [3Gen], Molescope/Molescope II [Metaoptima Technology Inc]) currently are marketed directly to consumers along with telederm services to facilitate direct-to-patient teledermoscopy.11,12
Machine Learning
Big data and machine learning has been hailed as the future of medicine and dermatology alike.13 Machine learning is a type of artificial intelligence that uses computational algorithms (eg, neural networks) that allow computer programs to automatically improve their accuracy (learn) by analyzing large data sets. In dermatology, machine learning has been most notably used to train computers to identify images of skin cancer by way of large image databases.14-17 One algorithm, a convolutional neural network (CNN), made headlines in 2017 when it was able to identify dermoscopic and clinical images of skin cancer with comparable accuracy to a group of 21 dermatologists.14 In 2018, the International Skin Imaging Collaboration (ISIC) published results of a study of the diagnostic accuracy of 25 computer algorithms compared to 8 dermatologists using a set of 100 dermoscopic images of melanoma and benign nevi.15 Using the average sensitivity of the dermatologists (82%), the top fusion algorithm in the study had a sensitivity of 76% versus 59% for the dermatologists (P=.02). These results compared the mean sensitivity of the dermatologists, as some individual dermatologists outperformed the algorithm.15 More recently, another CNN was compared to 58 international dermatologists in the classification of a set of 100 dermoscopic images (20 melanoma and 80 melanocytic nevi).16 Using the mean sensitivity of the dermatologists (86.6%), the CNN had a specificity of 92.5% versus 71.3% for dermatologists (P<.01). In the second part of the study, the dermatologists were given some clinical information and close-up photographs of the lesions, which improved their average (SD) sensitivity and specificity to 88.9% (9.6%)(P=.19) and 75.7% (11.7%)(P<.05), respectively. When compared to the CNN at this higher sensitivity, the CNN still had a higher specificity than the dermatologists (82.5% vs 75.7% [P<.01]).16 However, in real-life clinical practice dermatologists perform better, not only because they can collect more in-person clinical information but also because humans gather more information during live examination than when they are interpreting close-up clinical and/or dermoscopic images. In a sense, we currently are limited to comparing data that is incommensurable.
Machine learning studies have other notable limitations, such as data sets that do not contain a full spectrum of skin lesions or less common lesions (eg, pigmented seborrheic keratoses, amelanotic melanomas) and variation in image databases used.15,16 For machine algorithms to improve, they require access to high-quality and ideally standardized digital dermoscopic image databases. The ISIC and other organizations currently have databases specifically for this purpose, but more images are needed.18 As additional practitioners incorporate digital dermoscopy in their clinical practice, the potential for larger databases and more accurate algorithms becomes a possibility.
Image Acquisition
Many devices are available for digital dermoscopic image acquisition, including dermatoscopes that attach to smartphones and/or digital cameras and all-in-one systems (eTable). The exact system employed will depend on the practitioner's requirements for price, portability, speed, image quality, and software. Digital single-lens reflex (DSLR) cameras boast the highest image quality, while video dermoscopy traditionally yields stored images with poor resolution.19 Macroscopic images obtained by other imaging devices, including spectral imaging devices and reflectance confocal microscopy, usually are yielded via video dermoscopy or a video camera to capture images; thus, stored images generally are not as high quality.
Smartphones are increasingly used for clinical imaging in dermatology.20 Although DSLR cameras still take the highest-quality images, current smartphone image quality is comparable to digital cameras.21,22 Computational photography uses computer processing power to enhance image quality and may bring smartphone image quality closer to DSLR cameras.22,23 Smartphones with newer dual-lens cameras have been reported to further improve image quality.21 Current smartphones have the option of enabling high-dynamic-range imaging, which combines multiple images taken with different exposures to create a single image with improved dynamic range of luminosity. It has been reported that high-dynamic-range imaging may even enhance dermoscopic features of more challenging hypopigmented skin cancers.24
Standardizing Imaging
There has been a concerted effort to standardize digital dermatologic image acquisition.25,26 Standardization promises to facilitate data analysis, improve collaboration, protect patient privacy, and improve patient care.13,26,27 At the forefront of image standardization is the ISIC organization, which recently published its Delphi consensus guidelines on standards for lesion imaging, including dermoscopy.26
The true holy grail of image standardization is the Digital Imaging and Communications in Medicine (DICOM) standard.26-28 The DICOM is a comprehensive imaging standard for storage, annotation, transfer, and display of images, and it is most notable for its use in radiology. The DICOM also could be applied to new imaging modalities in dermatology (eg, optical coherence tomography, reflectance confocal microscopy). Past efforts to develop a DICOM standard for dermatology were undertaken by a working group that has since disbanded.27 Work by the ISIC and many others will hopefully lead to adoption of the DICOM standard by dermatology at some point in the future.
Protected Health Information
The Health Insurance Portability and Accountability Act (HIPAA) requires protected health information (PHI) to be stored in a secure manner with limited access that sufficiently protects identifiable patient information. Although dermoscopic images generally are deidentified, they often are stored alongside clinical photographs and data that contains PHI in clinical practice.
Image storage can take 2 forms: (1) physical local storage on internal and external hard drives or (2) remote storage (eg, cloud-based storage). Encryption is essential regardless of the method of storage. It is required by law that loss of nonencrypted PHI be reported to all potentially affected patients, the US Department of Health & Human Services, and local/state media depending on the number of patients affected. Loss of PHI can result in fines of up to $1.5 million.29 On the contrary, loss of properly encrypted data would not be required to be reported.30
As smart image acquisition devices begin to dominate the clinical setting, practitioners need to be vigilant in securing patient PHI. There are multiple applications (apps) that allow for secure encrypted digital dermoscopic image acquisition and storage on smartphones. Additionally, it is important to secure smartphones with complex passcodes (eg, a mix of special characters, numbers, uppercase and lowercase letters). Most dermatoscope manufacturers have apps for image acquisition and storage that can be tied into other platforms or storage systems (eg, DermLite app [3Gen], Handyscope [FotoFinder Systems GmbH], VEOS app [Canfield Scientific, Inc]).28 Other options include syncing images with current electronic medical record technologies, transferring photographs to HIPAA-compliant cloud storage, or transferring photographs to an encrypted computer and/or external hard drive. Some tips for securing data based on HIPAA and other guidelines are listed in the Table.30,31
Conclusion
The expansion of teledermoscopy alongside direct-to-patient services may create additional incentives for clinicians to incorporate digital dermoscopy into their practice. As more practitioners adopt digital dermoscopy, machine learning driven by technological advancements and larger image data sets could influence the future practice of dermatology. With the rise in digital dermoscopy by way of smartphones, additional steps must be taken to ensure patients' PHI is safeguarded. Digital dermoscopy is a dynamic field that will likely see continued growth in the coming years.
- Vestergaard ME, Macaskill P, Holt PE, et al. Dermoscopy compared with naked eye examination for the diagnosis of primary melanoma: a meta-analysis of studies performed in a clinical setting. Br J Dermatol. 2008;159:669-676.
- Rosendahl C, Tschandl P, Cameron A, et al. Diagnostic accuracy of dermatoscopy for melanocytic and nonmelanocytic pigmented lesions. J Am Acad Dermatol. 2011;64:1068-1073.
- Salerni G, Lovatto L, Carrera C, et al. Melanomas detected in a follow-up program compared with melanomas referred to a melanoma unit. Arch Dermatol. 2011;147:549-555.
- Salerni G, Terán T, Puig S, et al. Meta-analysis of digital dermoscopy follow-up of melanocytic skin lesions: a study on behalf of the International Dermoscopy Society. J Eur Acad Dermatol Venereol. 2013;27:805-814.
- Yim KM, Armstrong AW, Oh DH, et al. Teledermatology in the United States: an update in a dynamic era [published online January 22, 2018]. Telemed J E Health. doi:10.1089/tmj.2017.0253.
- Ferrándiz L, Ojeda-Vila T, Corrales A, et al. Internet-based skin cancer screening using clinical images alone or in conjunction with dermoscopic images: a randomized teledermoscopy trial. J Am Acad Dermatol. 2017;76:676-682.
- Şenel E, Baba M, Durdu M. The contribution of teledermatoscopy to the diagnosis and management of non-melanocytic skin tumours. J Telemed Telecare. 2013;19:60-63.
- State telehealth laws and Medicaid program policies: a comprehensive scan of the 50 states and District of Columbia. Public Health Institute Center for Connected Health Policy website. http://www.cchpca.org/sites/default/files/resources/
50%20State%20FINAL%20April%202016.pdf. Published March 2016. Accessed July 2, 2018. - Raghu TS, Yiannias J, Sharma N, et al. Willingness to pay for teledermoscopy services at a university health center. J Patient Exp. 2018. doi:10.11772374373517748657.
- Fogel AL, Sarin KY. A survey of direct-to-consumer teledermatology services available to US patients: explosive growth, opportunities and controversy. J Telemed Telecare. 2017;23:19-25.
- MoleScope. MetaOptima Technology Inc website. https://molescope.com/product/. Accessed July 2, 2018.
- DermLite HÜD. 3Gen website. https://dermlite.com/products/dermlite-hud. Accessed July 2, 2018.
- Park AJ, Ko JM, Swerlick RA. Crowdsourcing dermatology: DataDerm, big data analytics, and machine learning technology. J Am Acad Dermatol. 2018;78:643-644.
- Esteva A, Kuprel B, Novoa RA, et al. Dermatologist-level classification of skin cancer with deep neural networks. Nature. 2017;542:115-118.
- Marchetti MA, Codella NCF, Dusza SW, et al; International Skin Imaging Collaboration. results of the 2016 International Skin Imaging Collaboration International Symposium on Biomedical Imaging challenge: comparison of the accuracy of computer algorithms to dermatologists for the diagnosis of melanoma from dermoscopic images. J Am Acad Dermatol. 2018;78:270-277.
- Haenssle HA, Fink C, Schneiderbauer R, et al. Man against machine: diagnostic performance of a deep learning convolutional neural network for dermoscopic melanoma recognition in comparison to 58 dermatologists [published online May 28, 2018]. doi:10.1093/annonc/mdy166.
- Prado G, Kovarik C. Cutting edge technology in dermatology: virtual reality and artificial intelligence. Cutis. 2018;101:236-237.
- Sultana NN, Puhan NB. Recent deep learning methods for melanoma detection: a review. In: Ghosh D, Giri D, Mohapatra R, et al, eds. Mathematics and Computing. Singapore: Springer Nature; 2018:118-132.
- Lake A, Jones B. Dermoscopy: to cross-polarize, or not to cross-polarize, that is the question. J Vis Commun Med. 2015;38:36-50.
- Abbott LM, Magnusson RS, Gibbs E, et al. Smartphone use in dermatology for clinical photography and consultation: current practice and the law [published online February 28, 2017]. Australas J Dermatol. 2018;59:101-107.
- Hauser W, Neveu B, Jourdain JB, et al. Image quality benchmark of computational bokeh. Electron Imaging. 2018;2018:1-10.
- Ignatov A, Kobyshev N, Timofte R, et al. DSLR-quality photos on mobile devices with deep convolutional networks. 2017 IEEE International Conference on Computer Vision (ICCV). Venice, Italy: IEEE; 2017:3297-3305.
- Greengard S. Computational photography comes into focus. Commun ACM. 2014;57:19-21.
- Braun RP, Marghoob A. High-dynamic-range dermoscopy imaging and diagnosis of hypopigmented skin cancers. JAMA Dermatol. 2015;151:456-457.
- Quigley EA, Tokay BA, Jewell ST, et al. Technology and technique standards for camera-acquired digital dermatologic images: a systematic review. JAMA Dermatol. 2015;151:883-890.
- Katragadda C, Finnane A, Soyer HP, et al. Technique standards for skin lesion imaging a delphi consensus statement. JAMA Dermatol. 2017;153:207-213.
- Caffery LJ, Clunie D, Curiel-Lewandrowski C, et al. Transforming dermatologic imaging for the digital era: metadata and standards [published online January 17, 2018]. J Digit Imaging. doi:10.1007/s10278-017-0045-8.
- Pagliarello C, Stanganelli I, Fabrizi G, et al. Digital dermoscopy monitoring: is it time to define a quality standard? Acta Derm Venereol. 2017;97:864-865.
- HITECH Act Enforcement Interim Final Rule. US Department of Health & Human Services website. https://www.hhs.gov/hipaa/for-professionals/special-topics/hitech-act-enforcement-interim-final-rule/index.html. Updated June 16, 2017. Accessed July 2, 2018.
- Guidance to render unsecured protected health information unusable, unreadable, or indecipherable to unauthorized individuals. US Department of Health & Human Services website. https://www.hhs.gov/hipaa/for-professionals/breach-notification/guidance/index.html. Updated July 26, 2013. Accessed July 2, 2018.
- Scarfone K, Souppaya M, Sexton M. Guide to Storage Encryption Technologies for End User Devices. Gaithersburg, MD: US Department of Commerce; 2007. NIST Special Publication 800-111.
- Vestergaard ME, Macaskill P, Holt PE, et al. Dermoscopy compared with naked eye examination for the diagnosis of primary melanoma: a meta-analysis of studies performed in a clinical setting. Br J Dermatol. 2008;159:669-676.
- Rosendahl C, Tschandl P, Cameron A, et al. Diagnostic accuracy of dermatoscopy for melanocytic and nonmelanocytic pigmented lesions. J Am Acad Dermatol. 2011;64:1068-1073.
- Salerni G, Lovatto L, Carrera C, et al. Melanomas detected in a follow-up program compared with melanomas referred to a melanoma unit. Arch Dermatol. 2011;147:549-555.
- Salerni G, Terán T, Puig S, et al. Meta-analysis of digital dermoscopy follow-up of melanocytic skin lesions: a study on behalf of the International Dermoscopy Society. J Eur Acad Dermatol Venereol. 2013;27:805-814.
- Yim KM, Armstrong AW, Oh DH, et al. Teledermatology in the United States: an update in a dynamic era [published online January 22, 2018]. Telemed J E Health. doi:10.1089/tmj.2017.0253.
- Ferrándiz L, Ojeda-Vila T, Corrales A, et al. Internet-based skin cancer screening using clinical images alone or in conjunction with dermoscopic images: a randomized teledermoscopy trial. J Am Acad Dermatol. 2017;76:676-682.
- Şenel E, Baba M, Durdu M. The contribution of teledermatoscopy to the diagnosis and management of non-melanocytic skin tumours. J Telemed Telecare. 2013;19:60-63.
- State telehealth laws and Medicaid program policies: a comprehensive scan of the 50 states and District of Columbia. Public Health Institute Center for Connected Health Policy website. http://www.cchpca.org/sites/default/files/resources/
50%20State%20FINAL%20April%202016.pdf. Published March 2016. Accessed July 2, 2018. - Raghu TS, Yiannias J, Sharma N, et al. Willingness to pay for teledermoscopy services at a university health center. J Patient Exp. 2018. doi:10.11772374373517748657.
- Fogel AL, Sarin KY. A survey of direct-to-consumer teledermatology services available to US patients: explosive growth, opportunities and controversy. J Telemed Telecare. 2017;23:19-25.
- MoleScope. MetaOptima Technology Inc website. https://molescope.com/product/. Accessed July 2, 2018.
- DermLite HÜD. 3Gen website. https://dermlite.com/products/dermlite-hud. Accessed July 2, 2018.
- Park AJ, Ko JM, Swerlick RA. Crowdsourcing dermatology: DataDerm, big data analytics, and machine learning technology. J Am Acad Dermatol. 2018;78:643-644.
- Esteva A, Kuprel B, Novoa RA, et al. Dermatologist-level classification of skin cancer with deep neural networks. Nature. 2017;542:115-118.
- Marchetti MA, Codella NCF, Dusza SW, et al; International Skin Imaging Collaboration. results of the 2016 International Skin Imaging Collaboration International Symposium on Biomedical Imaging challenge: comparison of the accuracy of computer algorithms to dermatologists for the diagnosis of melanoma from dermoscopic images. J Am Acad Dermatol. 2018;78:270-277.
- Haenssle HA, Fink C, Schneiderbauer R, et al. Man against machine: diagnostic performance of a deep learning convolutional neural network for dermoscopic melanoma recognition in comparison to 58 dermatologists [published online May 28, 2018]. doi:10.1093/annonc/mdy166.
- Prado G, Kovarik C. Cutting edge technology in dermatology: virtual reality and artificial intelligence. Cutis. 2018;101:236-237.
- Sultana NN, Puhan NB. Recent deep learning methods for melanoma detection: a review. In: Ghosh D, Giri D, Mohapatra R, et al, eds. Mathematics and Computing. Singapore: Springer Nature; 2018:118-132.
- Lake A, Jones B. Dermoscopy: to cross-polarize, or not to cross-polarize, that is the question. J Vis Commun Med. 2015;38:36-50.
- Abbott LM, Magnusson RS, Gibbs E, et al. Smartphone use in dermatology for clinical photography and consultation: current practice and the law [published online February 28, 2017]. Australas J Dermatol. 2018;59:101-107.
- Hauser W, Neveu B, Jourdain JB, et al. Image quality benchmark of computational bokeh. Electron Imaging. 2018;2018:1-10.
- Ignatov A, Kobyshev N, Timofte R, et al. DSLR-quality photos on mobile devices with deep convolutional networks. 2017 IEEE International Conference on Computer Vision (ICCV). Venice, Italy: IEEE; 2017:3297-3305.
- Greengard S. Computational photography comes into focus. Commun ACM. 2014;57:19-21.
- Braun RP, Marghoob A. High-dynamic-range dermoscopy imaging and diagnosis of hypopigmented skin cancers. JAMA Dermatol. 2015;151:456-457.
- Quigley EA, Tokay BA, Jewell ST, et al. Technology and technique standards for camera-acquired digital dermatologic images: a systematic review. JAMA Dermatol. 2015;151:883-890.
- Katragadda C, Finnane A, Soyer HP, et al. Technique standards for skin lesion imaging a delphi consensus statement. JAMA Dermatol. 2017;153:207-213.
- Caffery LJ, Clunie D, Curiel-Lewandrowski C, et al. Transforming dermatologic imaging for the digital era: metadata and standards [published online January 17, 2018]. J Digit Imaging. doi:10.1007/s10278-017-0045-8.
- Pagliarello C, Stanganelli I, Fabrizi G, et al. Digital dermoscopy monitoring: is it time to define a quality standard? Acta Derm Venereol. 2017;97:864-865.
- HITECH Act Enforcement Interim Final Rule. US Department of Health & Human Services website. https://www.hhs.gov/hipaa/for-professionals/special-topics/hitech-act-enforcement-interim-final-rule/index.html. Updated June 16, 2017. Accessed July 2, 2018.
- Guidance to render unsecured protected health information unusable, unreadable, or indecipherable to unauthorized individuals. US Department of Health & Human Services website. https://www.hhs.gov/hipaa/for-professionals/breach-notification/guidance/index.html. Updated July 26, 2013. Accessed July 2, 2018.
- Scarfone K, Souppaya M, Sexton M. Guide to Storage Encryption Technologies for End User Devices. Gaithersburg, MD: US Department of Commerce; 2007. NIST Special Publication 800-111.
SPOTme addresses unmet need for skin cancer screening
Almost half of the individuals diagnosed with melanoma in a free skin cancer screening program otherwise would not have gone to a doctor to have their skin examined, according to an analysis of the American Academy of Dermatology’s national skin cancer screening program, during 1986-2014.
The SPOTme program, a national skin cancer screening and education program conducted by volunteer dermatologists, was launched in 1985. More than 2 million free screenings have been provided by the program in a “predominantly high-risk population, rendering important clinical diagnoses for hundreds of thousands of participants,” according to first authors Jean-Phillip Okhovat, MD, of Beth Israel Deaconess Medical Center, and Derek Beaulieu, MD, of Tufts University, both in Boston, and their colleagues.
The analysis was published online in the Journal of the American Academy of Dermatology on July 26.
Their study analyzed data on almost 2 million people screened through the program from 1986-2014. About 62% were women; 90% were white, about 2% were black, and almost 4% were Hispanic. Almost 80% had no regular dermatologist, almost 73% had not been screened previously, almost 45% had never had a skin cancer check, and 9% were uninsured. Almost 31% reported a mole that had recently change in size, color, or shape; almost 34% said they had a family history of skin cancer, and about 14% said they had a personal history of skin cancer.
Participants were asked about demographics and risk factors, although some questions changed from year to year (for example, in 2009 and 2010, participants were asked about melanoma risk factors, and from 1992 through 2010, participants were asked about their access to dermatologic care).
During 1991-2014 (which did not include data for 1995, 1996, and 2000, which were not available), the screening program resulted in 20,628 clinical melanoma diagnoses, 156,087 clinical dysplastic nevi diagnoses, 32,893 clinical squamous cell carcinoma diagnoses, and 129,848 clinical basal cell carcinoma diagnoses.
Of those clinically diagnosed with melanoma during 1992-2010, 83% said they did not have a regular dermatologist, 77% said they had not been screened previously, and 47% said they would not have seen a doctor for a skin exam if the SPOTme program had not been available.
Of those screened in 2009 and 2010 , 72% were considered at high risk for melanoma (older than age 65 years, having a history of sunburns, a family history of skin cancer, and/or more than 50 moles or unusual moles).
Among the other findings was that from 1992 to 2010, about 12% of those with a clinical melanoma diagnosis were not insured, which increased over time, from almost 11% during 1992-1999 to almost 16% during 2007-2010.
The “consistently high rates” of multiple skin cancer risk factors among those newly screened in the study are consistent with previously reported data, “suggesting that there is an untapped pool of at-risk Americans who have yet to be screened for skin cancer,” the authors wrote. “While the SPOTme program cannot be expected to meet the demands of this larger at-risk population, increased publicity and educational campaigns led by the AAD and assistance to primary care physicians in triaging of patients who should be seen by dermatologists could decrease the number of Americans who need to be screened,” they added.
Limitations of the study included the inability to confirm the clinical diagnoses with histopathology, and no data from the providers were available.
The authors had no disclosures. SPOTme, part of the AAD’s SPOT Skin Cancer initiative, is supported by a grant from Bristol-Myers Squibb.
SOURCE: Okhovat JP et al. J Am Acad Dermatol. https://doi./org/10.1016.j.jaad.2018.05.1242.
Almost half of the individuals diagnosed with melanoma in a free skin cancer screening program otherwise would not have gone to a doctor to have their skin examined, according to an analysis of the American Academy of Dermatology’s national skin cancer screening program, during 1986-2014.
The SPOTme program, a national skin cancer screening and education program conducted by volunteer dermatologists, was launched in 1985. More than 2 million free screenings have been provided by the program in a “predominantly high-risk population, rendering important clinical diagnoses for hundreds of thousands of participants,” according to first authors Jean-Phillip Okhovat, MD, of Beth Israel Deaconess Medical Center, and Derek Beaulieu, MD, of Tufts University, both in Boston, and their colleagues.
The analysis was published online in the Journal of the American Academy of Dermatology on July 26.
Their study analyzed data on almost 2 million people screened through the program from 1986-2014. About 62% were women; 90% were white, about 2% were black, and almost 4% were Hispanic. Almost 80% had no regular dermatologist, almost 73% had not been screened previously, almost 45% had never had a skin cancer check, and 9% were uninsured. Almost 31% reported a mole that had recently change in size, color, or shape; almost 34% said they had a family history of skin cancer, and about 14% said they had a personal history of skin cancer.
Participants were asked about demographics and risk factors, although some questions changed from year to year (for example, in 2009 and 2010, participants were asked about melanoma risk factors, and from 1992 through 2010, participants were asked about their access to dermatologic care).
During 1991-2014 (which did not include data for 1995, 1996, and 2000, which were not available), the screening program resulted in 20,628 clinical melanoma diagnoses, 156,087 clinical dysplastic nevi diagnoses, 32,893 clinical squamous cell carcinoma diagnoses, and 129,848 clinical basal cell carcinoma diagnoses.
Of those clinically diagnosed with melanoma during 1992-2010, 83% said they did not have a regular dermatologist, 77% said they had not been screened previously, and 47% said they would not have seen a doctor for a skin exam if the SPOTme program had not been available.
Of those screened in 2009 and 2010 , 72% were considered at high risk for melanoma (older than age 65 years, having a history of sunburns, a family history of skin cancer, and/or more than 50 moles or unusual moles).
Among the other findings was that from 1992 to 2010, about 12% of those with a clinical melanoma diagnosis were not insured, which increased over time, from almost 11% during 1992-1999 to almost 16% during 2007-2010.
The “consistently high rates” of multiple skin cancer risk factors among those newly screened in the study are consistent with previously reported data, “suggesting that there is an untapped pool of at-risk Americans who have yet to be screened for skin cancer,” the authors wrote. “While the SPOTme program cannot be expected to meet the demands of this larger at-risk population, increased publicity and educational campaigns led by the AAD and assistance to primary care physicians in triaging of patients who should be seen by dermatologists could decrease the number of Americans who need to be screened,” they added.
Limitations of the study included the inability to confirm the clinical diagnoses with histopathology, and no data from the providers were available.
The authors had no disclosures. SPOTme, part of the AAD’s SPOT Skin Cancer initiative, is supported by a grant from Bristol-Myers Squibb.
SOURCE: Okhovat JP et al. J Am Acad Dermatol. https://doi./org/10.1016.j.jaad.2018.05.1242.
Almost half of the individuals diagnosed with melanoma in a free skin cancer screening program otherwise would not have gone to a doctor to have their skin examined, according to an analysis of the American Academy of Dermatology’s national skin cancer screening program, during 1986-2014.
The SPOTme program, a national skin cancer screening and education program conducted by volunteer dermatologists, was launched in 1985. More than 2 million free screenings have been provided by the program in a “predominantly high-risk population, rendering important clinical diagnoses for hundreds of thousands of participants,” according to first authors Jean-Phillip Okhovat, MD, of Beth Israel Deaconess Medical Center, and Derek Beaulieu, MD, of Tufts University, both in Boston, and their colleagues.
The analysis was published online in the Journal of the American Academy of Dermatology on July 26.
Their study analyzed data on almost 2 million people screened through the program from 1986-2014. About 62% were women; 90% were white, about 2% were black, and almost 4% were Hispanic. Almost 80% had no regular dermatologist, almost 73% had not been screened previously, almost 45% had never had a skin cancer check, and 9% were uninsured. Almost 31% reported a mole that had recently change in size, color, or shape; almost 34% said they had a family history of skin cancer, and about 14% said they had a personal history of skin cancer.
Participants were asked about demographics and risk factors, although some questions changed from year to year (for example, in 2009 and 2010, participants were asked about melanoma risk factors, and from 1992 through 2010, participants were asked about their access to dermatologic care).
During 1991-2014 (which did not include data for 1995, 1996, and 2000, which were not available), the screening program resulted in 20,628 clinical melanoma diagnoses, 156,087 clinical dysplastic nevi diagnoses, 32,893 clinical squamous cell carcinoma diagnoses, and 129,848 clinical basal cell carcinoma diagnoses.
Of those clinically diagnosed with melanoma during 1992-2010, 83% said they did not have a regular dermatologist, 77% said they had not been screened previously, and 47% said they would not have seen a doctor for a skin exam if the SPOTme program had not been available.
Of those screened in 2009 and 2010 , 72% were considered at high risk for melanoma (older than age 65 years, having a history of sunburns, a family history of skin cancer, and/or more than 50 moles or unusual moles).
Among the other findings was that from 1992 to 2010, about 12% of those with a clinical melanoma diagnosis were not insured, which increased over time, from almost 11% during 1992-1999 to almost 16% during 2007-2010.
The “consistently high rates” of multiple skin cancer risk factors among those newly screened in the study are consistent with previously reported data, “suggesting that there is an untapped pool of at-risk Americans who have yet to be screened for skin cancer,” the authors wrote. “While the SPOTme program cannot be expected to meet the demands of this larger at-risk population, increased publicity and educational campaigns led by the AAD and assistance to primary care physicians in triaging of patients who should be seen by dermatologists could decrease the number of Americans who need to be screened,” they added.
Limitations of the study included the inability to confirm the clinical diagnoses with histopathology, and no data from the providers were available.
The authors had no disclosures. SPOTme, part of the AAD’s SPOT Skin Cancer initiative, is supported by a grant from Bristol-Myers Squibb.
SOURCE: Okhovat JP et al. J Am Acad Dermatol. https://doi./org/10.1016.j.jaad.2018.05.1242.
FROM THE JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY
Key clinical point: Free skin cancer screening programs help meet an unmet need for people at high risk for skin cancer.
Major finding: Of those who received a clinical diagnosis of melanoma during 1992-2010, 47% said they would not have seen a doctor for a skin exam if the free program had not been available.
Study details: The study analyzed data on almost 2 million people screened through the free SPOTme skin cancer screening program during 1986-2014.
Disclosures: The authors had no disclosures. SPOTme, part of the AAD’s SPOT Skin Cancer initiative, is supported by a grant from Bristol-Myers Squibb.
Source: Okhovat JP et al. J Am Acad Dermatol. https://doi./org/10.1016.j.jaad.2018.05.1242.
Human T-Lymphotropic Virus 1 Associated With Adult T-Cell Leukemia/Lymphoma
Adult T-cell leukemia/lymphoma (ATLL) is an uncommon neoplasm of mature T lymphocytes associated with infection by human T-lymphotropic virus 1 (HTLV-1),1-3 which is increasing in incidence in areas of the United States with large immigrant populations.4 Human T-lymphotrophic virus 1 infection is asymptomatic in most patients and has been associated with ATLL as well as tropical spastic paraparesis.5 We present a case of rapid-onset ATLL in an 82-year-old Japanese man who had immigrated to the United States.
Case Report
An 82-year-old Japanese man who had immigrated to the United States presented with papules and nodules on the neck, trunk, and arms of 4 weeks’ duration. Minimal pruritus was associated with the lesions, which were otherwise asymptomatic. The patient reported that he was generally healthy, and a review of systems was negative.
Physical examination revealed numerous erythematous and violaceous papules and nodules on the right side of the neck (Figure 1A), chest, back, abdomen, groin, left arm (Figure 1B), and medial thighs. Bilateral axillary and inguinal lymphadenopathy also was noted.
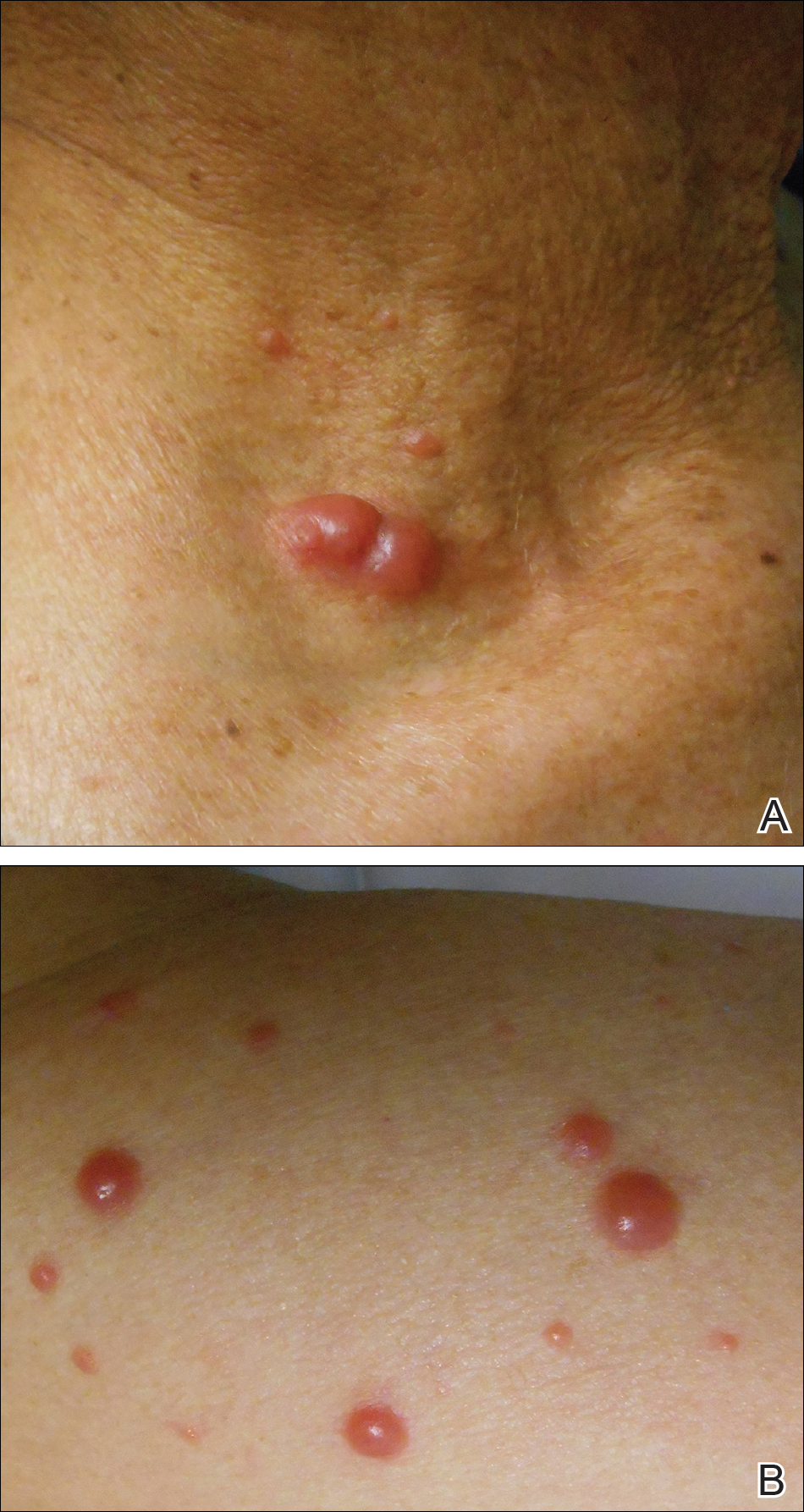
A biopsy from the abdomen revealed a dense, atypical, pandermal lymphoid infiltrate comprised of medium-sized lymphocytes with oval nuclei, fine chromatin, and pale cytoplasm (Figure 2). Mitotic figures and apoptotic cells also were observed. Immunostaining was strongly and diffusely positive for CD4 (Figure 3A), B-cell lymphoma 2 (Bcl-2)(Figure 3B), CD3, and programmed death 1, and was negative for CD8, CD10, CD20, CD30, and myeloperoxidase.
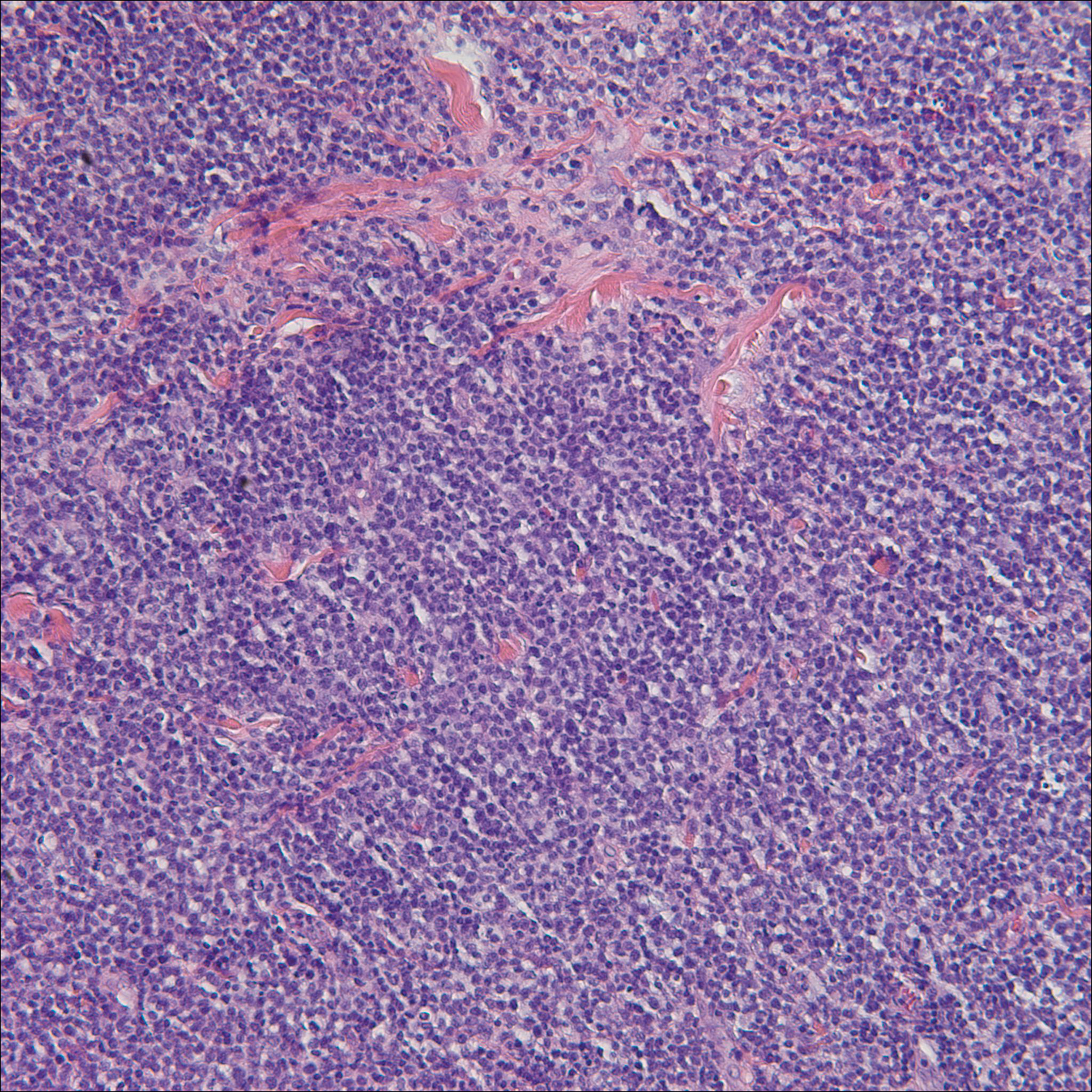
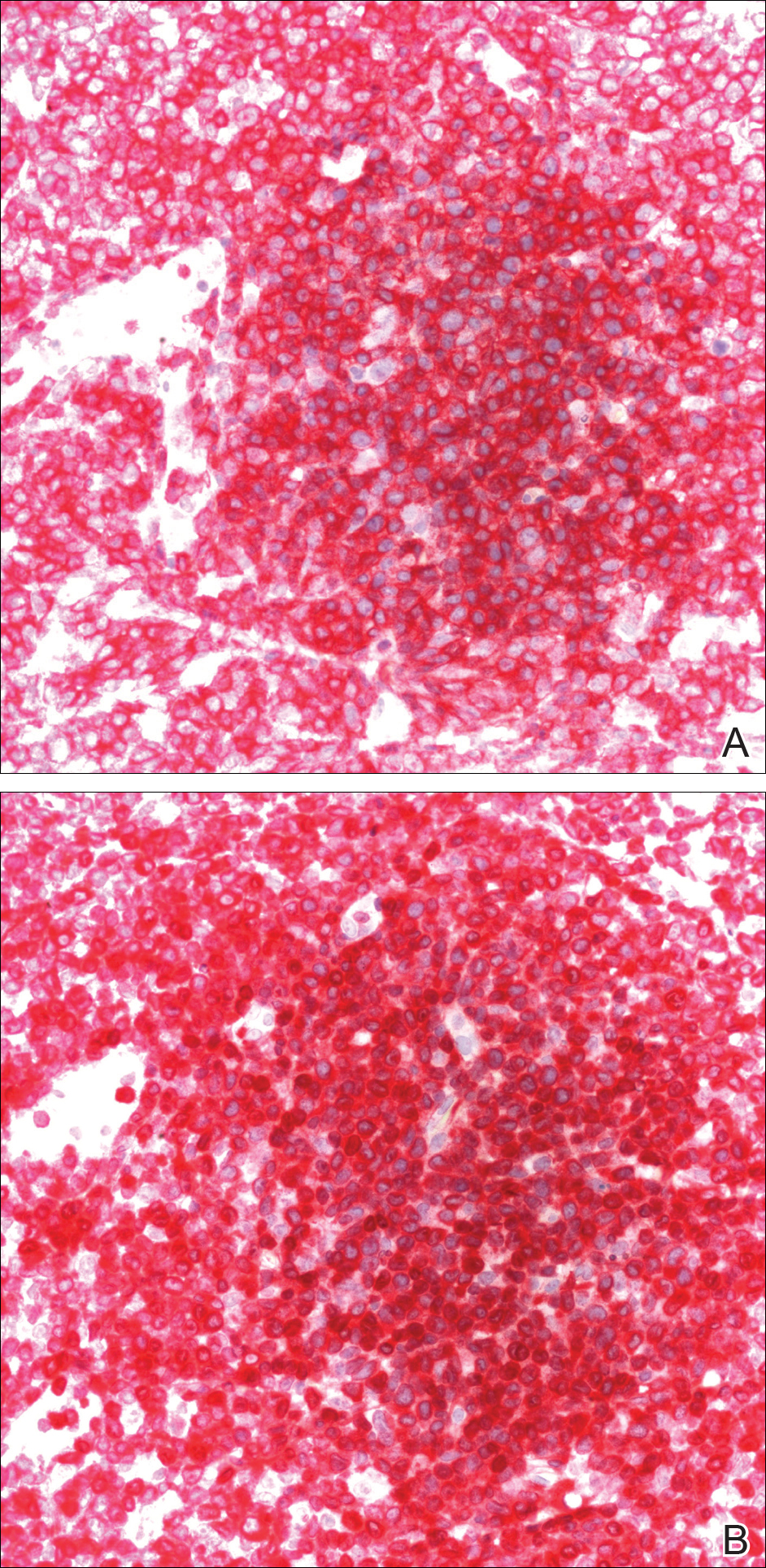
A bone marrow biopsy revealed an atypical T-cell population on flow cytometry. Western blot analysis for HTLV-1 antibodies was positive. Complete blood cell count and complete metabolic panel were within reference range.
Clinical and histopathologic findings fit the diagnosis of ATLL. The patient was referred to hematology/oncology, but the rapid progression of lesions continued, and the patient died within 4 months of initial presentation.
Comment
Etiology
First described in 1977, ATLL is an uncommon neoplasm of mature T cells.6 The etiology is associated with infection by the retrovirus HTLV-1, which is endemic in Southern Japan, the Caribbean, Central and West Africa, and Central and South America, with increasing incidence in areas of the United States with large immigrant populations.7 The incidence of ATLL among all registered lymphoma cases from 2003 to 2008 in Japan was 8.3% compared to 0.2% in the United States.7
Transmission of HTLV-1
Human T-lymphotropic virus 1 is a retrovirus most commonly found in CD4+T cells and can be transmitted through breast milk, sexual intercourse, and blood exposure (eg, blood transfusion), with breastfeeding and blood exposure being the most common.8-10 Human T-lymphotrophic virus 1 has been described as the causative agent for 3 entities: (1) ATLL, (2) a nervous system degenerative disorder known as HTLV-1–associated myelopathy or tropical spastic paraparesis, and (3) HTLV-1 uveitis.5,11 It is thought that 10 to 20 million individuals worldwide are infected with HTLV-1.12
The evolution from infection with HTLV-1 to ATLL is thought to involve multiple steps.13,14 Those who contract the virus later in life rarely, if ever, develop ATLL, suggesting that this progression requires considerable time to evolve to carcinogenesis. More than 90% of those infected with HTLV-1 remain asymptomatic, while only 2% to 3% of women and 6% to 7% of men develop ATLL with a median incubation period greater than 15 to 20 years.7
Subtypes
Adult T-cell leukemia/lymphoma has been divided into 4 clinical subtypes based on clinical presentation and prognosis.15 The acute type is more aggressive and has a poorer prognosis, while the chronic and smoldering types have a more indolent course. The smoldering variant largely has only cutaneous involvement with less than 1% of the peripheral leukocytes being atypical lymphocytes.16 A cutaneous subtype in which few to no leukemic cells are present also has been described and may overlap with the smoldering variant.The cutaneous variant has been further classified into 2 subtypes, tumoral and erythematopapular, with the tumoral subtype carrying a worse prognosis.17,18 Clinically, 39% to 57% of ATLL cases have skin involvement, with nearly one-third reporting skin manifestations as the first symptom.19,20 The cutaneous manifestations vary greatly and may include papules, plaques, nodules, tumors, erythematous patches, or erythroderma.4,21 In addition to skin manifestations, most patients with acute ATLL demonstrate leukemia, lymphadenopathy, organomegaly, and hypercalcemia.22
Histopathology
Histologically, both the smoldering and chronic forms of tumoral or erythematopapular ATLL demonstrate a cutaneous, dermal, or subcutaneous infiltrate of small- to medium-sized CD4+ T cells with histiocytes and admixed granulomas.4 Epidermotropism and Pautrier microabscesses often are limited or absent but can be seen.
Differential Diagnosis
The differential diagnosis includes other small- or medium-sized T-cell lymphomas. The chronic and smoldering types can be difficult to distinguish from mycosis fungoides.
Treatment
Treatment decisions should be made based on the subclassification and prognostic factors at the time of diagnosis. High doses of interferon alfa and zidovudine may show some benefit, but many cases require multiagent chemotherapy.22 The only possible curative treatment is allogeneic stem cell transplant. Mogamulizumab, an antichemokine receptor 4 monoclonal antibody, has demonstrated some ATLL antitumor activity.24
- Uchiyama T, Yodoi J, Sagawa K, et al. Adult T-cell leukemia: clinical and hematologic features of 16 cases. Blood. 1977;50:481-492.
- Poiesz BJ, Ruscetti FW, Gazdar AF, et al. Detection and isolation of type C retro-virus particles form fresh and cultured lymphocytes of a patient with cutaneous T-cell lymphoma. Proc Natl Acad Sci U S A. 1980;77:7415-7419.
- Hinuma Y, Gotoh Y, Sugamura K, et al. A retrovirus associated with human adult T-cell leukemia: in vitro activation. Gan. 1982;73:341-344.
- Marchetti MA, Pulitzer MP, Myskowski PL, et al. Cutaneous manifestations of human T-cell lymphotropic virus type-1-associated adult T-cell leukemia/lymphoma: a single-center, retrospective study. J Am Acad Dermatol. 2015;72:293-301.
- Gessain A, Barin F, Vernant JC, et al. Antibodies to human T-lymphotropic virus type-I in patients with tropical spastic paraparesis. Lancet. 1985;2:407-410.
- Takatsuki K, Uchiyama T, Sagawa K, et al. Adult T cell leukemia in Japan. In: Seno S, Takasu F, Irino S, eds. Topics in Hematology. Amsterdam, Netherlands: Excerpta Medica; 1977:73-77.
- Yoshida N, Chihara D. Incidence of adult T-cell leukemia/lymphoma in nonendemic areas. Curr Treat Options Oncol. 2015;16:7.
- Tajima K, Tominaga S, Suchi T, et al. Epidemiological analysis of the distribution of antibody to adult T-cell leukemia-virus-associated antigen: possible horizontal transmission of adult T-cell leukemia virus. Gan. 1982;73:893-901.
- Kajiyama W, Kashiwagi S, Ikematsu H, et al. Intrafamilial transmission of adult T cell leukemia virus. J Infect Dis. 1986;154:851-857.
- Ichimaru M, Ikeda S, Kinoshita K, et al. Mother-to-child transmission of HTLV-1. Cancer Detect Prev. 1991;15:177-181.
- Lyra-da-Silva JO, de Mello Gonzaga YB, de Melo Espíndola O, et al. Adult t-cell leukemia/lymphoma: a case report of primary cutaneous tumoral type. Dermatol Pract Concept. 2012;2:202a03.
- Edlich RF, Arnette JA, Williams FM. Global epidemic of human T-cell lymphotropic virus type-I (HTLV-I). J Emerg Med. 2000;18:109-119.
- Magalhaes M, Oliveira PD, Bittencourt AL, et al. Microsatellite alterations are also present in the less aggressive types of adult T-cell leukemia-lymphoma. PLoS Negl Trop Dis. 2015;9:e0003403.
- Okamoto T, Ohno Y, Tsugane S, et al. Multi-step carcinogenesis model for adult T-cell leukemia. Jpn J Cancer Res. 1989;80:191-195.
- Shimoyama M. Diagnostic criteria and classification of clinical subtypes of adult T-cell leukaemia-lymphoma. Br J Haematol. 1991;79:428-437.
- Takahashi K, Tanaka T, Fujita M, et al. Cutaneous-type adult T-cell leukemia lymphoma. a unique clinical feature with monoclonal T-cell proliferation detected by Southern blot analysis Arch Dermatol. 1988;124:399-404.
- Amano M, Kurokawa M, Ogata K, et al. New entity, definition and diagnostic criteria of cutaneous adult T-cell leukemia/lymphoma: human T-lymphotropic virus type 1 proviral DNA load can distinguish between cutaneous and smoldering types. J Dermatol. 2008;35:270-275.
- Johno M, Ohishi M, Kojo Y, et al. Cutaneous manifestations of adult T-cell leukemia lymphoma. Gann Monogr Cancer Res. 1992;39:33-42.
- Shimoyama M. Diagnostic criteria and classification of clinical subtypes of adult T-cell leukemia-lymphoma: a report from the Lymphoma Study Group (1984-87). Br J Haematol. 1991;79:428-437.
- Levine PH, Manns A, Jaffe ES, et al. The effect of ethnic differences on the pattern of HTLV-I-associated T-cell leukemia/lymphoma (HATL) in the United States. Int J Cancer. 1994;56:177-181.
- Pezeshkpoor F, Yazdanpanah MJ, Shirdel A. Specific cutaneous manifestations in adult T-cell leukemia/lymphoma. Int J Dermatol. 2008;47:359-362.
- Tsukasaki K, Hermine O, Bazarbachi A, et al. Definition, prognostic factors, treatment, and response criteria of adult T-cell leukemia-lymphoma: a proposal from an international consensus meeting. J Clin Oncol. 2009;27:453-459.
- Vose J, Armitage J, Weisenburger D; International T-Cell Lymphoma Project. International peripheral T-cell and natural killer/T-cell lymphoma study: pathology findings and clinical outcomes. J Clin Oncol. 2008;26:4124-4130.
- Ishida T, Joh T, Uike N, et al. Defucosylated anti-CCR4 monoclonal antibody (KW-0761) for relapsed adult T-cell leukemia-lymphoma: a multicenter phase II study. J Clin Oncol. 2012;30:837-842.
Adult T-cell leukemia/lymphoma (ATLL) is an uncommon neoplasm of mature T lymphocytes associated with infection by human T-lymphotropic virus 1 (HTLV-1),1-3 which is increasing in incidence in areas of the United States with large immigrant populations.4 Human T-lymphotrophic virus 1 infection is asymptomatic in most patients and has been associated with ATLL as well as tropical spastic paraparesis.5 We present a case of rapid-onset ATLL in an 82-year-old Japanese man who had immigrated to the United States.
Case Report
An 82-year-old Japanese man who had immigrated to the United States presented with papules and nodules on the neck, trunk, and arms of 4 weeks’ duration. Minimal pruritus was associated with the lesions, which were otherwise asymptomatic. The patient reported that he was generally healthy, and a review of systems was negative.
Physical examination revealed numerous erythematous and violaceous papules and nodules on the right side of the neck (Figure 1A), chest, back, abdomen, groin, left arm (Figure 1B), and medial thighs. Bilateral axillary and inguinal lymphadenopathy also was noted.

A biopsy from the abdomen revealed a dense, atypical, pandermal lymphoid infiltrate comprised of medium-sized lymphocytes with oval nuclei, fine chromatin, and pale cytoplasm (Figure 2). Mitotic figures and apoptotic cells also were observed. Immunostaining was strongly and diffusely positive for CD4 (Figure 3A), B-cell lymphoma 2 (Bcl-2)(Figure 3B), CD3, and programmed death 1, and was negative for CD8, CD10, CD20, CD30, and myeloperoxidase.


A bone marrow biopsy revealed an atypical T-cell population on flow cytometry. Western blot analysis for HTLV-1 antibodies was positive. Complete blood cell count and complete metabolic panel were within reference range.
Clinical and histopathologic findings fit the diagnosis of ATLL. The patient was referred to hematology/oncology, but the rapid progression of lesions continued, and the patient died within 4 months of initial presentation.
Comment
Etiology
First described in 1977, ATLL is an uncommon neoplasm of mature T cells.6 The etiology is associated with infection by the retrovirus HTLV-1, which is endemic in Southern Japan, the Caribbean, Central and West Africa, and Central and South America, with increasing incidence in areas of the United States with large immigrant populations.7 The incidence of ATLL among all registered lymphoma cases from 2003 to 2008 in Japan was 8.3% compared to 0.2% in the United States.7
Transmission of HTLV-1
Human T-lymphotropic virus 1 is a retrovirus most commonly found in CD4+T cells and can be transmitted through breast milk, sexual intercourse, and blood exposure (eg, blood transfusion), with breastfeeding and blood exposure being the most common.8-10 Human T-lymphotrophic virus 1 has been described as the causative agent for 3 entities: (1) ATLL, (2) a nervous system degenerative disorder known as HTLV-1–associated myelopathy or tropical spastic paraparesis, and (3) HTLV-1 uveitis.5,11 It is thought that 10 to 20 million individuals worldwide are infected with HTLV-1.12
The evolution from infection with HTLV-1 to ATLL is thought to involve multiple steps.13,14 Those who contract the virus later in life rarely, if ever, develop ATLL, suggesting that this progression requires considerable time to evolve to carcinogenesis. More than 90% of those infected with HTLV-1 remain asymptomatic, while only 2% to 3% of women and 6% to 7% of men develop ATLL with a median incubation period greater than 15 to 20 years.7
Subtypes
Adult T-cell leukemia/lymphoma has been divided into 4 clinical subtypes based on clinical presentation and prognosis.15 The acute type is more aggressive and has a poorer prognosis, while the chronic and smoldering types have a more indolent course. The smoldering variant largely has only cutaneous involvement with less than 1% of the peripheral leukocytes being atypical lymphocytes.16 A cutaneous subtype in which few to no leukemic cells are present also has been described and may overlap with the smoldering variant.The cutaneous variant has been further classified into 2 subtypes, tumoral and erythematopapular, with the tumoral subtype carrying a worse prognosis.17,18 Clinically, 39% to 57% of ATLL cases have skin involvement, with nearly one-third reporting skin manifestations as the first symptom.19,20 The cutaneous manifestations vary greatly and may include papules, plaques, nodules, tumors, erythematous patches, or erythroderma.4,21 In addition to skin manifestations, most patients with acute ATLL demonstrate leukemia, lymphadenopathy, organomegaly, and hypercalcemia.22
Histopathology
Histologically, both the smoldering and chronic forms of tumoral or erythematopapular ATLL demonstrate a cutaneous, dermal, or subcutaneous infiltrate of small- to medium-sized CD4+ T cells with histiocytes and admixed granulomas.4 Epidermotropism and Pautrier microabscesses often are limited or absent but can be seen.
Differential Diagnosis
The differential diagnosis includes other small- or medium-sized T-cell lymphomas. The chronic and smoldering types can be difficult to distinguish from mycosis fungoides.
Treatment
Treatment decisions should be made based on the subclassification and prognostic factors at the time of diagnosis. High doses of interferon alfa and zidovudine may show some benefit, but many cases require multiagent chemotherapy.22 The only possible curative treatment is allogeneic stem cell transplant. Mogamulizumab, an antichemokine receptor 4 monoclonal antibody, has demonstrated some ATLL antitumor activity.24
Adult T-cell leukemia/lymphoma (ATLL) is an uncommon neoplasm of mature T lymphocytes associated with infection by human T-lymphotropic virus 1 (HTLV-1),1-3 which is increasing in incidence in areas of the United States with large immigrant populations.4 Human T-lymphotrophic virus 1 infection is asymptomatic in most patients and has been associated with ATLL as well as tropical spastic paraparesis.5 We present a case of rapid-onset ATLL in an 82-year-old Japanese man who had immigrated to the United States.
Case Report
An 82-year-old Japanese man who had immigrated to the United States presented with papules and nodules on the neck, trunk, and arms of 4 weeks’ duration. Minimal pruritus was associated with the lesions, which were otherwise asymptomatic. The patient reported that he was generally healthy, and a review of systems was negative.
Physical examination revealed numerous erythematous and violaceous papules and nodules on the right side of the neck (Figure 1A), chest, back, abdomen, groin, left arm (Figure 1B), and medial thighs. Bilateral axillary and inguinal lymphadenopathy also was noted.

A biopsy from the abdomen revealed a dense, atypical, pandermal lymphoid infiltrate comprised of medium-sized lymphocytes with oval nuclei, fine chromatin, and pale cytoplasm (Figure 2). Mitotic figures and apoptotic cells also were observed. Immunostaining was strongly and diffusely positive for CD4 (Figure 3A), B-cell lymphoma 2 (Bcl-2)(Figure 3B), CD3, and programmed death 1, and was negative for CD8, CD10, CD20, CD30, and myeloperoxidase.


A bone marrow biopsy revealed an atypical T-cell population on flow cytometry. Western blot analysis for HTLV-1 antibodies was positive. Complete blood cell count and complete metabolic panel were within reference range.
Clinical and histopathologic findings fit the diagnosis of ATLL. The patient was referred to hematology/oncology, but the rapid progression of lesions continued, and the patient died within 4 months of initial presentation.
Comment
Etiology
First described in 1977, ATLL is an uncommon neoplasm of mature T cells.6 The etiology is associated with infection by the retrovirus HTLV-1, which is endemic in Southern Japan, the Caribbean, Central and West Africa, and Central and South America, with increasing incidence in areas of the United States with large immigrant populations.7 The incidence of ATLL among all registered lymphoma cases from 2003 to 2008 in Japan was 8.3% compared to 0.2% in the United States.7
Transmission of HTLV-1
Human T-lymphotropic virus 1 is a retrovirus most commonly found in CD4+T cells and can be transmitted through breast milk, sexual intercourse, and blood exposure (eg, blood transfusion), with breastfeeding and blood exposure being the most common.8-10 Human T-lymphotrophic virus 1 has been described as the causative agent for 3 entities: (1) ATLL, (2) a nervous system degenerative disorder known as HTLV-1–associated myelopathy or tropical spastic paraparesis, and (3) HTLV-1 uveitis.5,11 It is thought that 10 to 20 million individuals worldwide are infected with HTLV-1.12
The evolution from infection with HTLV-1 to ATLL is thought to involve multiple steps.13,14 Those who contract the virus later in life rarely, if ever, develop ATLL, suggesting that this progression requires considerable time to evolve to carcinogenesis. More than 90% of those infected with HTLV-1 remain asymptomatic, while only 2% to 3% of women and 6% to 7% of men develop ATLL with a median incubation period greater than 15 to 20 years.7
Subtypes
Adult T-cell leukemia/lymphoma has been divided into 4 clinical subtypes based on clinical presentation and prognosis.15 The acute type is more aggressive and has a poorer prognosis, while the chronic and smoldering types have a more indolent course. The smoldering variant largely has only cutaneous involvement with less than 1% of the peripheral leukocytes being atypical lymphocytes.16 A cutaneous subtype in which few to no leukemic cells are present also has been described and may overlap with the smoldering variant.The cutaneous variant has been further classified into 2 subtypes, tumoral and erythematopapular, with the tumoral subtype carrying a worse prognosis.17,18 Clinically, 39% to 57% of ATLL cases have skin involvement, with nearly one-third reporting skin manifestations as the first symptom.19,20 The cutaneous manifestations vary greatly and may include papules, plaques, nodules, tumors, erythematous patches, or erythroderma.4,21 In addition to skin manifestations, most patients with acute ATLL demonstrate leukemia, lymphadenopathy, organomegaly, and hypercalcemia.22
Histopathology
Histologically, both the smoldering and chronic forms of tumoral or erythematopapular ATLL demonstrate a cutaneous, dermal, or subcutaneous infiltrate of small- to medium-sized CD4+ T cells with histiocytes and admixed granulomas.4 Epidermotropism and Pautrier microabscesses often are limited or absent but can be seen.
Differential Diagnosis
The differential diagnosis includes other small- or medium-sized T-cell lymphomas. The chronic and smoldering types can be difficult to distinguish from mycosis fungoides.
Treatment
Treatment decisions should be made based on the subclassification and prognostic factors at the time of diagnosis. High doses of interferon alfa and zidovudine may show some benefit, but many cases require multiagent chemotherapy.22 The only possible curative treatment is allogeneic stem cell transplant. Mogamulizumab, an antichemokine receptor 4 monoclonal antibody, has demonstrated some ATLL antitumor activity.24
- Uchiyama T, Yodoi J, Sagawa K, et al. Adult T-cell leukemia: clinical and hematologic features of 16 cases. Blood. 1977;50:481-492.
- Poiesz BJ, Ruscetti FW, Gazdar AF, et al. Detection and isolation of type C retro-virus particles form fresh and cultured lymphocytes of a patient with cutaneous T-cell lymphoma. Proc Natl Acad Sci U S A. 1980;77:7415-7419.
- Hinuma Y, Gotoh Y, Sugamura K, et al. A retrovirus associated with human adult T-cell leukemia: in vitro activation. Gan. 1982;73:341-344.
- Marchetti MA, Pulitzer MP, Myskowski PL, et al. Cutaneous manifestations of human T-cell lymphotropic virus type-1-associated adult T-cell leukemia/lymphoma: a single-center, retrospective study. J Am Acad Dermatol. 2015;72:293-301.
- Gessain A, Barin F, Vernant JC, et al. Antibodies to human T-lymphotropic virus type-I in patients with tropical spastic paraparesis. Lancet. 1985;2:407-410.
- Takatsuki K, Uchiyama T, Sagawa K, et al. Adult T cell leukemia in Japan. In: Seno S, Takasu F, Irino S, eds. Topics in Hematology. Amsterdam, Netherlands: Excerpta Medica; 1977:73-77.
- Yoshida N, Chihara D. Incidence of adult T-cell leukemia/lymphoma in nonendemic areas. Curr Treat Options Oncol. 2015;16:7.
- Tajima K, Tominaga S, Suchi T, et al. Epidemiological analysis of the distribution of antibody to adult T-cell leukemia-virus-associated antigen: possible horizontal transmission of adult T-cell leukemia virus. Gan. 1982;73:893-901.
- Kajiyama W, Kashiwagi S, Ikematsu H, et al. Intrafamilial transmission of adult T cell leukemia virus. J Infect Dis. 1986;154:851-857.
- Ichimaru M, Ikeda S, Kinoshita K, et al. Mother-to-child transmission of HTLV-1. Cancer Detect Prev. 1991;15:177-181.
- Lyra-da-Silva JO, de Mello Gonzaga YB, de Melo Espíndola O, et al. Adult t-cell leukemia/lymphoma: a case report of primary cutaneous tumoral type. Dermatol Pract Concept. 2012;2:202a03.
- Edlich RF, Arnette JA, Williams FM. Global epidemic of human T-cell lymphotropic virus type-I (HTLV-I). J Emerg Med. 2000;18:109-119.
- Magalhaes M, Oliveira PD, Bittencourt AL, et al. Microsatellite alterations are also present in the less aggressive types of adult T-cell leukemia-lymphoma. PLoS Negl Trop Dis. 2015;9:e0003403.
- Okamoto T, Ohno Y, Tsugane S, et al. Multi-step carcinogenesis model for adult T-cell leukemia. Jpn J Cancer Res. 1989;80:191-195.
- Shimoyama M. Diagnostic criteria and classification of clinical subtypes of adult T-cell leukaemia-lymphoma. Br J Haematol. 1991;79:428-437.
- Takahashi K, Tanaka T, Fujita M, et al. Cutaneous-type adult T-cell leukemia lymphoma. a unique clinical feature with monoclonal T-cell proliferation detected by Southern blot analysis Arch Dermatol. 1988;124:399-404.
- Amano M, Kurokawa M, Ogata K, et al. New entity, definition and diagnostic criteria of cutaneous adult T-cell leukemia/lymphoma: human T-lymphotropic virus type 1 proviral DNA load can distinguish between cutaneous and smoldering types. J Dermatol. 2008;35:270-275.
- Johno M, Ohishi M, Kojo Y, et al. Cutaneous manifestations of adult T-cell leukemia lymphoma. Gann Monogr Cancer Res. 1992;39:33-42.
- Shimoyama M. Diagnostic criteria and classification of clinical subtypes of adult T-cell leukemia-lymphoma: a report from the Lymphoma Study Group (1984-87). Br J Haematol. 1991;79:428-437.
- Levine PH, Manns A, Jaffe ES, et al. The effect of ethnic differences on the pattern of HTLV-I-associated T-cell leukemia/lymphoma (HATL) in the United States. Int J Cancer. 1994;56:177-181.
- Pezeshkpoor F, Yazdanpanah MJ, Shirdel A. Specific cutaneous manifestations in adult T-cell leukemia/lymphoma. Int J Dermatol. 2008;47:359-362.
- Tsukasaki K, Hermine O, Bazarbachi A, et al. Definition, prognostic factors, treatment, and response criteria of adult T-cell leukemia-lymphoma: a proposal from an international consensus meeting. J Clin Oncol. 2009;27:453-459.
- Vose J, Armitage J, Weisenburger D; International T-Cell Lymphoma Project. International peripheral T-cell and natural killer/T-cell lymphoma study: pathology findings and clinical outcomes. J Clin Oncol. 2008;26:4124-4130.
- Ishida T, Joh T, Uike N, et al. Defucosylated anti-CCR4 monoclonal antibody (KW-0761) for relapsed adult T-cell leukemia-lymphoma: a multicenter phase II study. J Clin Oncol. 2012;30:837-842.
- Uchiyama T, Yodoi J, Sagawa K, et al. Adult T-cell leukemia: clinical and hematologic features of 16 cases. Blood. 1977;50:481-492.
- Poiesz BJ, Ruscetti FW, Gazdar AF, et al. Detection and isolation of type C retro-virus particles form fresh and cultured lymphocytes of a patient with cutaneous T-cell lymphoma. Proc Natl Acad Sci U S A. 1980;77:7415-7419.
- Hinuma Y, Gotoh Y, Sugamura K, et al. A retrovirus associated with human adult T-cell leukemia: in vitro activation. Gan. 1982;73:341-344.
- Marchetti MA, Pulitzer MP, Myskowski PL, et al. Cutaneous manifestations of human T-cell lymphotropic virus type-1-associated adult T-cell leukemia/lymphoma: a single-center, retrospective study. J Am Acad Dermatol. 2015;72:293-301.
- Gessain A, Barin F, Vernant JC, et al. Antibodies to human T-lymphotropic virus type-I in patients with tropical spastic paraparesis. Lancet. 1985;2:407-410.
- Takatsuki K, Uchiyama T, Sagawa K, et al. Adult T cell leukemia in Japan. In: Seno S, Takasu F, Irino S, eds. Topics in Hematology. Amsterdam, Netherlands: Excerpta Medica; 1977:73-77.
- Yoshida N, Chihara D. Incidence of adult T-cell leukemia/lymphoma in nonendemic areas. Curr Treat Options Oncol. 2015;16:7.
- Tajima K, Tominaga S, Suchi T, et al. Epidemiological analysis of the distribution of antibody to adult T-cell leukemia-virus-associated antigen: possible horizontal transmission of adult T-cell leukemia virus. Gan. 1982;73:893-901.
- Kajiyama W, Kashiwagi S, Ikematsu H, et al. Intrafamilial transmission of adult T cell leukemia virus. J Infect Dis. 1986;154:851-857.
- Ichimaru M, Ikeda S, Kinoshita K, et al. Mother-to-child transmission of HTLV-1. Cancer Detect Prev. 1991;15:177-181.
- Lyra-da-Silva JO, de Mello Gonzaga YB, de Melo Espíndola O, et al. Adult t-cell leukemia/lymphoma: a case report of primary cutaneous tumoral type. Dermatol Pract Concept. 2012;2:202a03.
- Edlich RF, Arnette JA, Williams FM. Global epidemic of human T-cell lymphotropic virus type-I (HTLV-I). J Emerg Med. 2000;18:109-119.
- Magalhaes M, Oliveira PD, Bittencourt AL, et al. Microsatellite alterations are also present in the less aggressive types of adult T-cell leukemia-lymphoma. PLoS Negl Trop Dis. 2015;9:e0003403.
- Okamoto T, Ohno Y, Tsugane S, et al. Multi-step carcinogenesis model for adult T-cell leukemia. Jpn J Cancer Res. 1989;80:191-195.
- Shimoyama M. Diagnostic criteria and classification of clinical subtypes of adult T-cell leukaemia-lymphoma. Br J Haematol. 1991;79:428-437.
- Takahashi K, Tanaka T, Fujita M, et al. Cutaneous-type adult T-cell leukemia lymphoma. a unique clinical feature with monoclonal T-cell proliferation detected by Southern blot analysis Arch Dermatol. 1988;124:399-404.
- Amano M, Kurokawa M, Ogata K, et al. New entity, definition and diagnostic criteria of cutaneous adult T-cell leukemia/lymphoma: human T-lymphotropic virus type 1 proviral DNA load can distinguish between cutaneous and smoldering types. J Dermatol. 2008;35:270-275.
- Johno M, Ohishi M, Kojo Y, et al. Cutaneous manifestations of adult T-cell leukemia lymphoma. Gann Monogr Cancer Res. 1992;39:33-42.
- Shimoyama M. Diagnostic criteria and classification of clinical subtypes of adult T-cell leukemia-lymphoma: a report from the Lymphoma Study Group (1984-87). Br J Haematol. 1991;79:428-437.
- Levine PH, Manns A, Jaffe ES, et al. The effect of ethnic differences on the pattern of HTLV-I-associated T-cell leukemia/lymphoma (HATL) in the United States. Int J Cancer. 1994;56:177-181.
- Pezeshkpoor F, Yazdanpanah MJ, Shirdel A. Specific cutaneous manifestations in adult T-cell leukemia/lymphoma. Int J Dermatol. 2008;47:359-362.
- Tsukasaki K, Hermine O, Bazarbachi A, et al. Definition, prognostic factors, treatment, and response criteria of adult T-cell leukemia-lymphoma: a proposal from an international consensus meeting. J Clin Oncol. 2009;27:453-459.
- Vose J, Armitage J, Weisenburger D; International T-Cell Lymphoma Project. International peripheral T-cell and natural killer/T-cell lymphoma study: pathology findings and clinical outcomes. J Clin Oncol. 2008;26:4124-4130.
- Ishida T, Joh T, Uike N, et al. Defucosylated anti-CCR4 monoclonal antibody (KW-0761) for relapsed adult T-cell leukemia-lymphoma: a multicenter phase II study. J Clin Oncol. 2012;30:837-842.
Practice Points
- Adult T-cell leukemia/lymphoma (ATLL) is an uncommon neoplasm of mature T lymphocytes associated with infection by human T-lymphotropic virus 1.
- In the United States, ATLL is increasing in incidence in areas with large immigrant populations.
- High suspicion and clinical features must be present to make the diagnosis of ATLL due to considerable histologic overlap with other cutaneous T-cell lymphomas.
Stress balls, hand-holding no help during dermatology procedures
according to a randomized trial of 135 patients at Northwestern University, Chicago.
In all three groups, anxiety levels were a little over 3 points on a 10-point Visual Analog Scale (VAS) before surgery and around 2 points during it. The 6-item State Trait Anxiety Inventory score was just under 9 in all three groups right after the procedure, meaning patients weren’t very anxious. Physiological measures did not change from before to after the procedure or between groups. Postoperative pain scores were all under 1 on a 10-point scale, and patients in all three groups were highly satisfied with their encounter, the researchers said in JAMA Dermatology.
“Many patients commented anecdotally on the calming effect of hand-holding or stress ball use,” so “it was surprising that the total data did not show these interventions to preferentially decrease anxiety or alleviate pain,” Arianna F. Yanes, a medical student at Northwestern University, and her coinvestigators said.
It could be that standard measures – giving patients an opportunity to ask questions, making sure they feel comfortable, and the like – are enough. However, “hand-holding and stress balls may still provide stress relief in patients who are particularly anxious before the procedure.” Perhaps patients would have preferred having their hand held by a loved one instead of a stranger, the investigators said.
Meanwhile, patients who researched their operation online beforehand had higher preoperative anxiety scores (3.84 vs. 2.62 points on the VAS; P = .04), but they could have been more anxious from the start.
The mean subject age was 66 years, and 62% were men.
The work was funded by Northwestern University and a grant from Merz. The investigators had no relevant disclosures.
SOURCE: Yanes AF et al. JAMA Dermatol. 2018 Jul 18. doi:10.1001/jamadermatol.2018.1783.
according to a randomized trial of 135 patients at Northwestern University, Chicago.
In all three groups, anxiety levels were a little over 3 points on a 10-point Visual Analog Scale (VAS) before surgery and around 2 points during it. The 6-item State Trait Anxiety Inventory score was just under 9 in all three groups right after the procedure, meaning patients weren’t very anxious. Physiological measures did not change from before to after the procedure or between groups. Postoperative pain scores were all under 1 on a 10-point scale, and patients in all three groups were highly satisfied with their encounter, the researchers said in JAMA Dermatology.
“Many patients commented anecdotally on the calming effect of hand-holding or stress ball use,” so “it was surprising that the total data did not show these interventions to preferentially decrease anxiety or alleviate pain,” Arianna F. Yanes, a medical student at Northwestern University, and her coinvestigators said.
It could be that standard measures – giving patients an opportunity to ask questions, making sure they feel comfortable, and the like – are enough. However, “hand-holding and stress balls may still provide stress relief in patients who are particularly anxious before the procedure.” Perhaps patients would have preferred having their hand held by a loved one instead of a stranger, the investigators said.
Meanwhile, patients who researched their operation online beforehand had higher preoperative anxiety scores (3.84 vs. 2.62 points on the VAS; P = .04), but they could have been more anxious from the start.
The mean subject age was 66 years, and 62% were men.
The work was funded by Northwestern University and a grant from Merz. The investigators had no relevant disclosures.
SOURCE: Yanes AF et al. JAMA Dermatol. 2018 Jul 18. doi:10.1001/jamadermatol.2018.1783.
according to a randomized trial of 135 patients at Northwestern University, Chicago.
In all three groups, anxiety levels were a little over 3 points on a 10-point Visual Analog Scale (VAS) before surgery and around 2 points during it. The 6-item State Trait Anxiety Inventory score was just under 9 in all three groups right after the procedure, meaning patients weren’t very anxious. Physiological measures did not change from before to after the procedure or between groups. Postoperative pain scores were all under 1 on a 10-point scale, and patients in all three groups were highly satisfied with their encounter, the researchers said in JAMA Dermatology.
“Many patients commented anecdotally on the calming effect of hand-holding or stress ball use,” so “it was surprising that the total data did not show these interventions to preferentially decrease anxiety or alleviate pain,” Arianna F. Yanes, a medical student at Northwestern University, and her coinvestigators said.
It could be that standard measures – giving patients an opportunity to ask questions, making sure they feel comfortable, and the like – are enough. However, “hand-holding and stress balls may still provide stress relief in patients who are particularly anxious before the procedure.” Perhaps patients would have preferred having their hand held by a loved one instead of a stranger, the investigators said.
Meanwhile, patients who researched their operation online beforehand had higher preoperative anxiety scores (3.84 vs. 2.62 points on the VAS; P = .04), but they could have been more anxious from the start.
The mean subject age was 66 years, and 62% were men.
The work was funded by Northwestern University and a grant from Merz. The investigators had no relevant disclosures.
SOURCE: Yanes AF et al. JAMA Dermatol. 2018 Jul 18. doi:10.1001/jamadermatol.2018.1783.
FROM JAMA DERMATOLOGY
Early BCC seen in teen kidney transplant patient
A 17-year-old girl seen in a Portuguese dermatology clinic was found to have a nodular basal cell carcinoma on the parietal region of her scalp. The nodule appeared 6 years after she had received a kidney transplant, according to João Borges-Costa, MD, PhD, who submitted the case report.
Since the transplant, the girl had been maintained on immunosuppressive medication of tacrolimus 1 mg twice daily, mycophenolate sodium 360 mg twice daily, and prednisolone 10 mg every other day. The 1-cm nodule was pigmented; dermatoscopy did not yield clarity about whether the lesion was melanocytic. An excisional biopsy with 0.5-cm margins was performed, and histology confirmed that the lesion was a nodular pigmented basal cell carcinoma that had been excised completely.
The case, said Dr. Borges-Costa, shows that skin cancers can develop earlier than the typical 12-18 years after pediatric transplantation. Most reported cases have been squamous cell cancers and melanomas, and often are associated with lack of appropriate sun protection behavior.
The patient, a Caucasian, was a sailor who used sunscreen but did not typically wear a hat while sailing, reported Dr. Borges-Costa, a dermatologist at the University of Lisbon. Her family history was significant for a grandparent with melanoma.
Dr. Borges noted that the parents and patient were given advice regarding the importance of the lifelong use of sun-protective clothing and headgear. “Education of pediatric organ recipients and their parents about sun protection is important because, as occurred with our patient, protective clothing and hats are frequently forgotten.”
Because of the ongoing potential for skin malignancies, early referral “after transplantation to specialized dermatology outpatient clinics, similar to what is now advocated for transplanted adults, could help in surveillance and improve adherence to sun-protective measures,” he added.
SOURCE: Borges-Costa J et al. Pediatr Dermatol. 2018. doi: 10.1111/pde.13537..
A 17-year-old girl seen in a Portuguese dermatology clinic was found to have a nodular basal cell carcinoma on the parietal region of her scalp. The nodule appeared 6 years after she had received a kidney transplant, according to João Borges-Costa, MD, PhD, who submitted the case report.
Since the transplant, the girl had been maintained on immunosuppressive medication of tacrolimus 1 mg twice daily, mycophenolate sodium 360 mg twice daily, and prednisolone 10 mg every other day. The 1-cm nodule was pigmented; dermatoscopy did not yield clarity about whether the lesion was melanocytic. An excisional biopsy with 0.5-cm margins was performed, and histology confirmed that the lesion was a nodular pigmented basal cell carcinoma that had been excised completely.
The case, said Dr. Borges-Costa, shows that skin cancers can develop earlier than the typical 12-18 years after pediatric transplantation. Most reported cases have been squamous cell cancers and melanomas, and often are associated with lack of appropriate sun protection behavior.
The patient, a Caucasian, was a sailor who used sunscreen but did not typically wear a hat while sailing, reported Dr. Borges-Costa, a dermatologist at the University of Lisbon. Her family history was significant for a grandparent with melanoma.
Dr. Borges noted that the parents and patient were given advice regarding the importance of the lifelong use of sun-protective clothing and headgear. “Education of pediatric organ recipients and their parents about sun protection is important because, as occurred with our patient, protective clothing and hats are frequently forgotten.”
Because of the ongoing potential for skin malignancies, early referral “after transplantation to specialized dermatology outpatient clinics, similar to what is now advocated for transplanted adults, could help in surveillance and improve adherence to sun-protective measures,” he added.
SOURCE: Borges-Costa J et al. Pediatr Dermatol. 2018. doi: 10.1111/pde.13537..
A 17-year-old girl seen in a Portuguese dermatology clinic was found to have a nodular basal cell carcinoma on the parietal region of her scalp. The nodule appeared 6 years after she had received a kidney transplant, according to João Borges-Costa, MD, PhD, who submitted the case report.
Since the transplant, the girl had been maintained on immunosuppressive medication of tacrolimus 1 mg twice daily, mycophenolate sodium 360 mg twice daily, and prednisolone 10 mg every other day. The 1-cm nodule was pigmented; dermatoscopy did not yield clarity about whether the lesion was melanocytic. An excisional biopsy with 0.5-cm margins was performed, and histology confirmed that the lesion was a nodular pigmented basal cell carcinoma that had been excised completely.
The case, said Dr. Borges-Costa, shows that skin cancers can develop earlier than the typical 12-18 years after pediatric transplantation. Most reported cases have been squamous cell cancers and melanomas, and often are associated with lack of appropriate sun protection behavior.
The patient, a Caucasian, was a sailor who used sunscreen but did not typically wear a hat while sailing, reported Dr. Borges-Costa, a dermatologist at the University of Lisbon. Her family history was significant for a grandparent with melanoma.
Dr. Borges noted that the parents and patient were given advice regarding the importance of the lifelong use of sun-protective clothing and headgear. “Education of pediatric organ recipients and their parents about sun protection is important because, as occurred with our patient, protective clothing and hats are frequently forgotten.”
Because of the ongoing potential for skin malignancies, early referral “after transplantation to specialized dermatology outpatient clinics, similar to what is now advocated for transplanted adults, could help in surveillance and improve adherence to sun-protective measures,” he added.
SOURCE: Borges-Costa J et al. Pediatr Dermatol. 2018. doi: 10.1111/pde.13537..
FROM PEDIATRIC DERMATOLOGY
Reflectance Confocal Microscopy as a First-Line Diagnostic Technique for Mycosis Fungoides
Case Report
A 60-year-old man with a history of Hodgkin lymphoma that had been treated with chemotherapy 6 years prior presented to our dermatology clinic with a persistent pruritic rash on the back, abdomen, and bilateral arms and legs. The eruption initially began as localized discrete lesions on the lower back 1 year prior to the current presentation; at that time a diagnosis of psoriasis was made at an outside dermatology clinic, and treatment with mometasone furoate cream was initiated. Despite the patient’s compliance with this treatment, the lesions did not resolve and began spreading to the arms, legs, chest, and abdomen. His current medications included lisinopril, escitalopram, aspirin, and omeprazole.
On presentation to our clinic, physical examination revealed round, scaly, pink plaques and tumors of variable sizes (3–10 cm) distributed asymmetrically on the chest, back, abdomen, arms, and legs (Figure 1). The lesions were grouped in well-defined areas encompassing approximately 30% of the body surface area. No lymphadenopathy was appreciated. In vivo reflectance confocal microscopy (RCM) performed on one of the lesions revealed disarray of the epidermis with small, weakly refractile, round to oval cells scattered within the spinous layer and dermoepidermal junction (Figure 2). Additionally, these weakly refractile, round to oval cells also were seen in vesiclelike dark spaces, and hyporefractile basal cells were appreciated surrounding the dermal papillae. Mycosis fungoides (MF) was diagnosed following correlation of the RCM findings with the clinical picture.
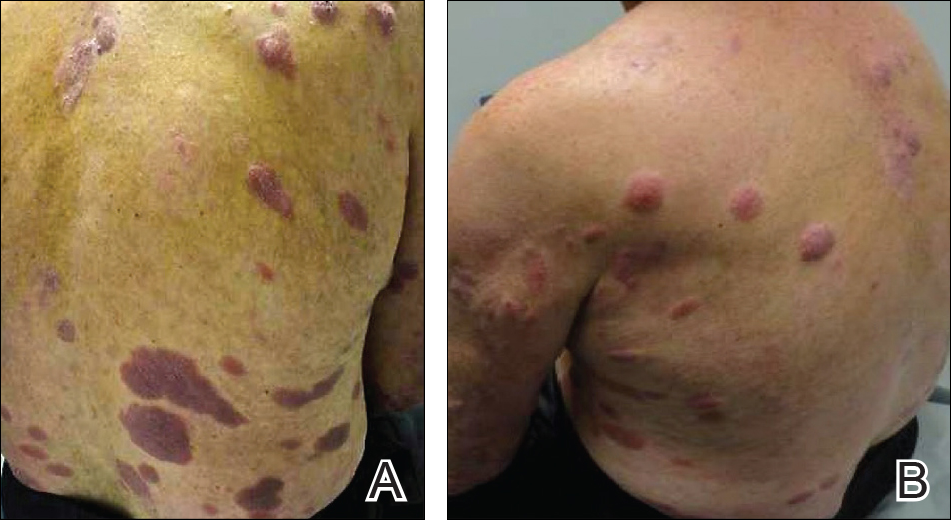
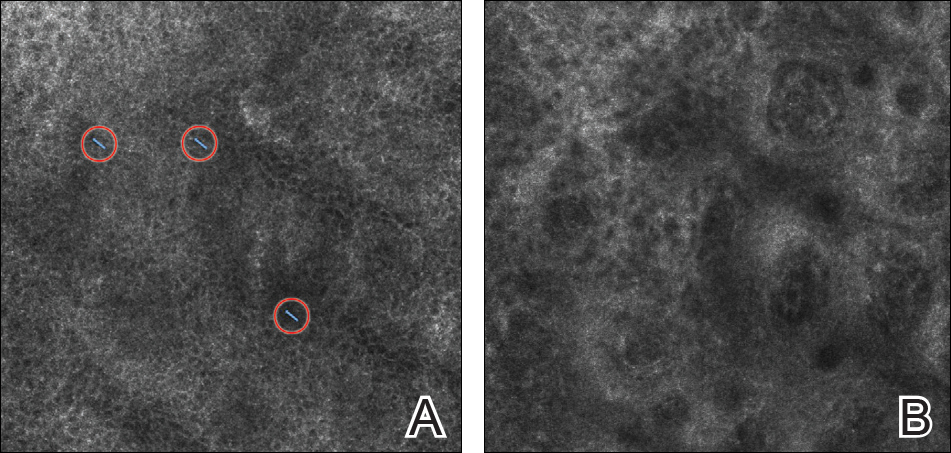
A biopsy was performed, with pathologic examination confirming the diagnosis of tumor-stage MF. Parakeratosis with epidermotropism of lymphocytes was noted along the basal layer and into the spinous layer of the epidermis (Figure 3). Underlying the epidermis there was a dense mononuclear infiltrate and conspicuous eosinophils extending to the deeper reticular dermis. The infiltrating cells had cerebriform nuclei and large pale cytoplasm. On immunostaining, the lymphocytes were positive for CD3 and CD4, and negative for CD5, CD7, and CD8. The patient was referred to the oncology department for disease management. Staging workup including computed tomography, flow cytometry, and T-cell receptor gene rearrangement were consistent with tumor-stage MF (T3N0M0B0).
![Atypical enlarged lymphocytes in the epidermis with hyperchromatic irregular nuclei of cells (inset) as well as a dense infiltrate in the dermis (A)(H&E, original magnifications ×10 and ×50 [inset])... Figure3](https://cdn.mdedge.com/files/s3fs-public/Image/July-2018/ct102001056_fig3.png)
Comment
Clinical Presentation of MF
Mycosis fungoides, a non-Hodgkin lymphoma of T-cell origin, is the most commonly diagnosed cutaneous lymphoma worldwide.1 It has an annual incidence of approximately 0.36 per 100,000 persons, and this number continues to rise.2,3 The median age of diagnosis is 55 to 60 years, and MF occurs twice as often in men versus women.4
The clinical presentation of MF varies and is classified by stages including patches, plaques, tumors, and erythroderma.5 Classically, MF is slowly progressive and begins as pruritic erythematous patches that have a predilection for non–sun-exposed areas of the skin. Over time, these patches may evolve into plaques and tumors. Early or patch-stage MF often presents as well-demarcated lesions of various sizes and shapes that tend to enlarge.6 These lesions may resemble eczema or psoriasis if there is scaling, such as in our patient. At the tumor stage, flat or dome-shaped nodules that may vary in color and are deeper than plaques begin to appear. Ulcerations, which were absent in our case, may often be seen.
Because of the diverse clinical manifestations of MF, which can mimic other common dermatoses, diagnosis often is challenging for clinicians. Furthermore, histology can yield nonspecific diagnostic results and may even resemble chronic inflammatory dermatoses.7 As a result, patients frequently are subjected to multiple skin biopsies to establish the diagnosis,8 and diagnosis may be delayed, with the median time from onset of skin symptoms to diagnosis being approximately 6 years.9
Reflectance Confocal Microscopy
In vivo RCM is a noninvasive technique that allows visualization of the skin at a cellular level and recently has been evaluated as a diagnostic tool for many skin conditions.10,11 Reflectance confocal microscopy findings have been well established for many cutaneous malignancies as well as inflammatory conditions such as psoriasis and atopic dermatitis.12,13 Specifically, 2 preliminary descriptive studies utilized RCM to visualize the characteristic features of MF in vivo.14,15 These studies reported the histopathologic correlation of RCM findings in biopsy-proven MF lesions. Consistent in all stages of MF is the presence of small, weakly refractile, round to oval cells within the spinous layer that correlate with atypical lymphocytes, in addition to hyporefractile basal cells surrounding the dermal papillae. Patch-stage MF lesions have more subtle epidermal findings compared to plaque-stage lesions, which tend to have more prominent vesiclelike dark spaces filled with collections of monomorphous, weakly refractile, round to oval cells corresponding with Pautrier microabscesses and evidence of spongiosis.14,15 The first descriptive study of RCM in the diagnosis of MF failed to identify features of tumor-stage MF that would distinguish it from patch- or plaque-stage disease. The investigators also stated that deep nodular collections of atypical lymphocytes seen on histopathology in tumor-stage MF were missed on RCM evaluation.14 Furthermore, the second descriptive study of RCM and MF, which included 2 patients with tumor-stage disease, also failed to differentiate tumor-stage MF from the patch or plaque stages.15
Because of these 2 descriptive studies, a pilot study was conducted to determine the applicability and reproducibility of RCM findings for MF diagnosis.16 Two blinded confocalists were asked to diagnose RCM images as MF when compared to either normal skin or a variety of lymphoproliferative disorders. Of 15 patients, the confocalists correctly diagnosed MF in 84% and 90% of cases, respectively. Additionally, they reported the specificity and sensitivity of the following RCM features in the diagnosis of MF: spongiosis, 88.9% and 94.7%; loss of demarcation, 88.9% and 94.7%; disarray of the epidermis, 77.8% and 89.5%; hyporefractile rings, 88.9% and 78.9%; junctional atypical lymphocytes, 100% and 73.7%; and vesiclelike structures (Pautrier microabscesses), 100% and 73.7%. Importantly, this study did not evaluate the specificity and sensitivity of MF diagnosis compared to other eczematous or inflammatory conditions that may share similar RCM findings; therefore, these results are not generalizable, and many of the RCM findings characteristically seen in MF are not specific to its diagnosis.16
One study assessed the diagnostic accuracy of RCM in evaluating erythematosquamous diseases including MF, psoriasis, contact dermatitis, discoid lupus, and subacute cutaneous lupus.17 In this study, 3 blinded confocalists achieved a 95.41% and 92.89% specificity and 89.13% and 63.33% sensitivity for psoriasis and MF, respectively. Typical features of psoriasis on RCM included parakeratosis, reduction or absence of the granular layer, papillomatosis, acanthosis with normal honeycomb pattern of the epidermis, and dilated vessels in the upper dermis. Features that were more specific to MF included epidermotropic atypical lymphocytes, interface dermatitis, pleomorphic tumor cells, and dendritic cells.17 However, atypical lymphocytes and interface dermatitis also may be seen in cutaneous lupus; therefore, additional studies are still needed to validate RCM’s utility in differentiating between erythematosquamous skin diseases, including psoriasis, cutaneous lupus, and MF. Currently, RCM findings must be interpreted in conjunction with the clinical and histologic picture.
Importantly, RCM also is limited when evaluating MF due to its limited depth of visualization, as it allows imaging only to the superficial papillary dermis. Furthermore, any infiltrative process such as epidermal hyperplasia, spongiosis, or scaling, which can be seen in MF, may further impair the imaging quality of the deeper dermis.
Conclusion
Despite its limitations, RCM has the potential to be advantageous in evaluating skin lesions suspicious for MF in real time and is a promising technology for a quick noninvasive bedside adjunct tool. Its utility in selecting the optimal site for biopsy for better yield of histopathologic results in suspected MF cases has been demonstrated.16 However, large-scale studies still are needed to evaluate RCM in the diagnosis of the wide diversity of MF lesions as well as its efficacy in selecting optimal biopsy sites.
- Lutzner M, Edelson R, Schein P, et al. Cutaneous T-cell lymphomas: the Sézary syndrome, mycosis fungoides, and related disorders. Ann Intern Med. 1975;83:534-552.
- Akinbami AA, Osikomaiya BI, John-Olabode SO, et al. Mycosis fungoides: case report and literature review. Clin Med Insights Case Rep. 2014;7:95-98.
- Criscione VD, Weinstock MA. Incidence of cutaneous T-cell lymphoma in the United States, 1973-2002. Arch Dermatol. 2007;143:854-959.
- Bradford PT, Devesa SS, Anderson WF, et al. Cutaneous lymphoma incidence patterns in the United States: a population-based study of 3884 cases. Blood. 2009;113:5064-5073.
- Willemze R, Jaffe ES, Burg G, et al. WHO-EORTC classification for cutaneous lymphomas. Blood. 2005;105:3768-3785.
- Nashan D, Faulhaber D, Stander S. Mycosis fungoides: a dermatological masquerader. Br J Dermatol. 2007;157:1-10.
- Santucci M, Biggeri A, Feller AC, et al. Efficacy of histologic criteria for diagnosing early mycosis fungoides: an EORTC cutaneous lymphoma study group investigation. European Organization for Research and Treatment of Cancer. Am J Surg Pathol. 2000;24:40-50.
- Glass LF, Keller KL, Messina JL, et al. Cutaneous T-cell lymphoma. Cancer Control. 1998;5:11-18.
- Hoppe RT, Wood GS, Abel EA. Mycosis fungoides and the Sézary syndrome: pathology, staging, and treatment. Curr Probl Cancer. 1990;14:293-371.
- Tannous ZS, Mihm MC, Flotte TJ, et al. In vivo examination of lentigo maligna and malignant melanoma in situ, lentigo maligna type by near-infrared reflectance confocal microscopy: comparison of in vivo confocal images with histologic sections. J Am Acad Dermatol. 2002;46:260-263.
- Gerger A, Koller S, Weger W, et al. Sensitivity and specificity of confocal laser-scanning microscopy for in vivo diagnosis of malignant skin tumors. Cancer. 2006;107:193-200.
- Branzan AL, Landthaler M, Szeimies RM. In vivo confocal scanning laser microscopy in dermatology [published online November 18, 2006]. Lasers Med Sci. 2007;22:73-82.
- González S. Confocal reflectance microscopy in dermatology: promise and reality of non-invasive diagnosis and monitoring. Actas Dermosifiliogr. 2009;100(suppl 2):59-69.
- Agero AL, Gill M, Ardigo M, et al. In vivo reflectance confocal microscopy of mycosis fungoides: a preliminary study [published online April 16, 2007]. J Am Acad Dermatol. 2007;57:435-441.
- Wi L, Dai H, Li Z, et al. Reflectance confocal microscopy for the characteristics of mycosis fungoides and correlation with histology: a pilot study [published online April 18, 2013]. Skin Res Technol. 2013;19:352-355.
- Lange-Asschenfeldt S, Babilli J, Beyer M, et al. Consistency and distribution of reflectance confocal microscopy features for diagnosis of cutaneous T cell lymphoma. J Biomed Opt. 2012;17:016001.
- Koller S, Gerger A, Ahlgrimm-Siess V. In vivo reflectance confocal microscopy of erythematosquamous skin diseases [published online March 6, 2009]. Exp Dermatol. 2009;18:536-540.
Case Report
A 60-year-old man with a history of Hodgkin lymphoma that had been treated with chemotherapy 6 years prior presented to our dermatology clinic with a persistent pruritic rash on the back, abdomen, and bilateral arms and legs. The eruption initially began as localized discrete lesions on the lower back 1 year prior to the current presentation; at that time a diagnosis of psoriasis was made at an outside dermatology clinic, and treatment with mometasone furoate cream was initiated. Despite the patient’s compliance with this treatment, the lesions did not resolve and began spreading to the arms, legs, chest, and abdomen. His current medications included lisinopril, escitalopram, aspirin, and omeprazole.
On presentation to our clinic, physical examination revealed round, scaly, pink plaques and tumors of variable sizes (3–10 cm) distributed asymmetrically on the chest, back, abdomen, arms, and legs (Figure 1). The lesions were grouped in well-defined areas encompassing approximately 30% of the body surface area. No lymphadenopathy was appreciated. In vivo reflectance confocal microscopy (RCM) performed on one of the lesions revealed disarray of the epidermis with small, weakly refractile, round to oval cells scattered within the spinous layer and dermoepidermal junction (Figure 2). Additionally, these weakly refractile, round to oval cells also were seen in vesiclelike dark spaces, and hyporefractile basal cells were appreciated surrounding the dermal papillae. Mycosis fungoides (MF) was diagnosed following correlation of the RCM findings with the clinical picture.


A biopsy was performed, with pathologic examination confirming the diagnosis of tumor-stage MF. Parakeratosis with epidermotropism of lymphocytes was noted along the basal layer and into the spinous layer of the epidermis (Figure 3). Underlying the epidermis there was a dense mononuclear infiltrate and conspicuous eosinophils extending to the deeper reticular dermis. The infiltrating cells had cerebriform nuclei and large pale cytoplasm. On immunostaining, the lymphocytes were positive for CD3 and CD4, and negative for CD5, CD7, and CD8. The patient was referred to the oncology department for disease management. Staging workup including computed tomography, flow cytometry, and T-cell receptor gene rearrangement were consistent with tumor-stage MF (T3N0M0B0).
![Atypical enlarged lymphocytes in the epidermis with hyperchromatic irregular nuclei of cells (inset) as well as a dense infiltrate in the dermis (A)(H&E, original magnifications ×10 and ×50 [inset])... Figure3](https://cdn.mdedge.com/files/s3fs-public/Image/July-2018/ct102001056_fig3.png)
Comment
Clinical Presentation of MF
Mycosis fungoides, a non-Hodgkin lymphoma of T-cell origin, is the most commonly diagnosed cutaneous lymphoma worldwide.1 It has an annual incidence of approximately 0.36 per 100,000 persons, and this number continues to rise.2,3 The median age of diagnosis is 55 to 60 years, and MF occurs twice as often in men versus women.4
The clinical presentation of MF varies and is classified by stages including patches, plaques, tumors, and erythroderma.5 Classically, MF is slowly progressive and begins as pruritic erythematous patches that have a predilection for non–sun-exposed areas of the skin. Over time, these patches may evolve into plaques and tumors. Early or patch-stage MF often presents as well-demarcated lesions of various sizes and shapes that tend to enlarge.6 These lesions may resemble eczema or psoriasis if there is scaling, such as in our patient. At the tumor stage, flat or dome-shaped nodules that may vary in color and are deeper than plaques begin to appear. Ulcerations, which were absent in our case, may often be seen.
Because of the diverse clinical manifestations of MF, which can mimic other common dermatoses, diagnosis often is challenging for clinicians. Furthermore, histology can yield nonspecific diagnostic results and may even resemble chronic inflammatory dermatoses.7 As a result, patients frequently are subjected to multiple skin biopsies to establish the diagnosis,8 and diagnosis may be delayed, with the median time from onset of skin symptoms to diagnosis being approximately 6 years.9
Reflectance Confocal Microscopy
In vivo RCM is a noninvasive technique that allows visualization of the skin at a cellular level and recently has been evaluated as a diagnostic tool for many skin conditions.10,11 Reflectance confocal microscopy findings have been well established for many cutaneous malignancies as well as inflammatory conditions such as psoriasis and atopic dermatitis.12,13 Specifically, 2 preliminary descriptive studies utilized RCM to visualize the characteristic features of MF in vivo.14,15 These studies reported the histopathologic correlation of RCM findings in biopsy-proven MF lesions. Consistent in all stages of MF is the presence of small, weakly refractile, round to oval cells within the spinous layer that correlate with atypical lymphocytes, in addition to hyporefractile basal cells surrounding the dermal papillae. Patch-stage MF lesions have more subtle epidermal findings compared to plaque-stage lesions, which tend to have more prominent vesiclelike dark spaces filled with collections of monomorphous, weakly refractile, round to oval cells corresponding with Pautrier microabscesses and evidence of spongiosis.14,15 The first descriptive study of RCM in the diagnosis of MF failed to identify features of tumor-stage MF that would distinguish it from patch- or plaque-stage disease. The investigators also stated that deep nodular collections of atypical lymphocytes seen on histopathology in tumor-stage MF were missed on RCM evaluation.14 Furthermore, the second descriptive study of RCM and MF, which included 2 patients with tumor-stage disease, also failed to differentiate tumor-stage MF from the patch or plaque stages.15
Because of these 2 descriptive studies, a pilot study was conducted to determine the applicability and reproducibility of RCM findings for MF diagnosis.16 Two blinded confocalists were asked to diagnose RCM images as MF when compared to either normal skin or a variety of lymphoproliferative disorders. Of 15 patients, the confocalists correctly diagnosed MF in 84% and 90% of cases, respectively. Additionally, they reported the specificity and sensitivity of the following RCM features in the diagnosis of MF: spongiosis, 88.9% and 94.7%; loss of demarcation, 88.9% and 94.7%; disarray of the epidermis, 77.8% and 89.5%; hyporefractile rings, 88.9% and 78.9%; junctional atypical lymphocytes, 100% and 73.7%; and vesiclelike structures (Pautrier microabscesses), 100% and 73.7%. Importantly, this study did not evaluate the specificity and sensitivity of MF diagnosis compared to other eczematous or inflammatory conditions that may share similar RCM findings; therefore, these results are not generalizable, and many of the RCM findings characteristically seen in MF are not specific to its diagnosis.16
One study assessed the diagnostic accuracy of RCM in evaluating erythematosquamous diseases including MF, psoriasis, contact dermatitis, discoid lupus, and subacute cutaneous lupus.17 In this study, 3 blinded confocalists achieved a 95.41% and 92.89% specificity and 89.13% and 63.33% sensitivity for psoriasis and MF, respectively. Typical features of psoriasis on RCM included parakeratosis, reduction or absence of the granular layer, papillomatosis, acanthosis with normal honeycomb pattern of the epidermis, and dilated vessels in the upper dermis. Features that were more specific to MF included epidermotropic atypical lymphocytes, interface dermatitis, pleomorphic tumor cells, and dendritic cells.17 However, atypical lymphocytes and interface dermatitis also may be seen in cutaneous lupus; therefore, additional studies are still needed to validate RCM’s utility in differentiating between erythematosquamous skin diseases, including psoriasis, cutaneous lupus, and MF. Currently, RCM findings must be interpreted in conjunction with the clinical and histologic picture.
Importantly, RCM also is limited when evaluating MF due to its limited depth of visualization, as it allows imaging only to the superficial papillary dermis. Furthermore, any infiltrative process such as epidermal hyperplasia, spongiosis, or scaling, which can be seen in MF, may further impair the imaging quality of the deeper dermis.
Conclusion
Despite its limitations, RCM has the potential to be advantageous in evaluating skin lesions suspicious for MF in real time and is a promising technology for a quick noninvasive bedside adjunct tool. Its utility in selecting the optimal site for biopsy for better yield of histopathologic results in suspected MF cases has been demonstrated.16 However, large-scale studies still are needed to evaluate RCM in the diagnosis of the wide diversity of MF lesions as well as its efficacy in selecting optimal biopsy sites.
Case Report
A 60-year-old man with a history of Hodgkin lymphoma that had been treated with chemotherapy 6 years prior presented to our dermatology clinic with a persistent pruritic rash on the back, abdomen, and bilateral arms and legs. The eruption initially began as localized discrete lesions on the lower back 1 year prior to the current presentation; at that time a diagnosis of psoriasis was made at an outside dermatology clinic, and treatment with mometasone furoate cream was initiated. Despite the patient’s compliance with this treatment, the lesions did not resolve and began spreading to the arms, legs, chest, and abdomen. His current medications included lisinopril, escitalopram, aspirin, and omeprazole.
On presentation to our clinic, physical examination revealed round, scaly, pink plaques and tumors of variable sizes (3–10 cm) distributed asymmetrically on the chest, back, abdomen, arms, and legs (Figure 1). The lesions were grouped in well-defined areas encompassing approximately 30% of the body surface area. No lymphadenopathy was appreciated. In vivo reflectance confocal microscopy (RCM) performed on one of the lesions revealed disarray of the epidermis with small, weakly refractile, round to oval cells scattered within the spinous layer and dermoepidermal junction (Figure 2). Additionally, these weakly refractile, round to oval cells also were seen in vesiclelike dark spaces, and hyporefractile basal cells were appreciated surrounding the dermal papillae. Mycosis fungoides (MF) was diagnosed following correlation of the RCM findings with the clinical picture.


A biopsy was performed, with pathologic examination confirming the diagnosis of tumor-stage MF. Parakeratosis with epidermotropism of lymphocytes was noted along the basal layer and into the spinous layer of the epidermis (Figure 3). Underlying the epidermis there was a dense mononuclear infiltrate and conspicuous eosinophils extending to the deeper reticular dermis. The infiltrating cells had cerebriform nuclei and large pale cytoplasm. On immunostaining, the lymphocytes were positive for CD3 and CD4, and negative for CD5, CD7, and CD8. The patient was referred to the oncology department for disease management. Staging workup including computed tomography, flow cytometry, and T-cell receptor gene rearrangement were consistent with tumor-stage MF (T3N0M0B0).
![Atypical enlarged lymphocytes in the epidermis with hyperchromatic irregular nuclei of cells (inset) as well as a dense infiltrate in the dermis (A)(H&E, original magnifications ×10 and ×50 [inset])... Figure3](https://cdn.mdedge.com/files/s3fs-public/Image/July-2018/ct102001056_fig3.png)
Comment
Clinical Presentation of MF
Mycosis fungoides, a non-Hodgkin lymphoma of T-cell origin, is the most commonly diagnosed cutaneous lymphoma worldwide.1 It has an annual incidence of approximately 0.36 per 100,000 persons, and this number continues to rise.2,3 The median age of diagnosis is 55 to 60 years, and MF occurs twice as often in men versus women.4
The clinical presentation of MF varies and is classified by stages including patches, plaques, tumors, and erythroderma.5 Classically, MF is slowly progressive and begins as pruritic erythematous patches that have a predilection for non–sun-exposed areas of the skin. Over time, these patches may evolve into plaques and tumors. Early or patch-stage MF often presents as well-demarcated lesions of various sizes and shapes that tend to enlarge.6 These lesions may resemble eczema or psoriasis if there is scaling, such as in our patient. At the tumor stage, flat or dome-shaped nodules that may vary in color and are deeper than plaques begin to appear. Ulcerations, which were absent in our case, may often be seen.
Because of the diverse clinical manifestations of MF, which can mimic other common dermatoses, diagnosis often is challenging for clinicians. Furthermore, histology can yield nonspecific diagnostic results and may even resemble chronic inflammatory dermatoses.7 As a result, patients frequently are subjected to multiple skin biopsies to establish the diagnosis,8 and diagnosis may be delayed, with the median time from onset of skin symptoms to diagnosis being approximately 6 years.9
Reflectance Confocal Microscopy
In vivo RCM is a noninvasive technique that allows visualization of the skin at a cellular level and recently has been evaluated as a diagnostic tool for many skin conditions.10,11 Reflectance confocal microscopy findings have been well established for many cutaneous malignancies as well as inflammatory conditions such as psoriasis and atopic dermatitis.12,13 Specifically, 2 preliminary descriptive studies utilized RCM to visualize the characteristic features of MF in vivo.14,15 These studies reported the histopathologic correlation of RCM findings in biopsy-proven MF lesions. Consistent in all stages of MF is the presence of small, weakly refractile, round to oval cells within the spinous layer that correlate with atypical lymphocytes, in addition to hyporefractile basal cells surrounding the dermal papillae. Patch-stage MF lesions have more subtle epidermal findings compared to plaque-stage lesions, which tend to have more prominent vesiclelike dark spaces filled with collections of monomorphous, weakly refractile, round to oval cells corresponding with Pautrier microabscesses and evidence of spongiosis.14,15 The first descriptive study of RCM in the diagnosis of MF failed to identify features of tumor-stage MF that would distinguish it from patch- or plaque-stage disease. The investigators also stated that deep nodular collections of atypical lymphocytes seen on histopathology in tumor-stage MF were missed on RCM evaluation.14 Furthermore, the second descriptive study of RCM and MF, which included 2 patients with tumor-stage disease, also failed to differentiate tumor-stage MF from the patch or plaque stages.15
Because of these 2 descriptive studies, a pilot study was conducted to determine the applicability and reproducibility of RCM findings for MF diagnosis.16 Two blinded confocalists were asked to diagnose RCM images as MF when compared to either normal skin or a variety of lymphoproliferative disorders. Of 15 patients, the confocalists correctly diagnosed MF in 84% and 90% of cases, respectively. Additionally, they reported the specificity and sensitivity of the following RCM features in the diagnosis of MF: spongiosis, 88.9% and 94.7%; loss of demarcation, 88.9% and 94.7%; disarray of the epidermis, 77.8% and 89.5%; hyporefractile rings, 88.9% and 78.9%; junctional atypical lymphocytes, 100% and 73.7%; and vesiclelike structures (Pautrier microabscesses), 100% and 73.7%. Importantly, this study did not evaluate the specificity and sensitivity of MF diagnosis compared to other eczematous or inflammatory conditions that may share similar RCM findings; therefore, these results are not generalizable, and many of the RCM findings characteristically seen in MF are not specific to its diagnosis.16
One study assessed the diagnostic accuracy of RCM in evaluating erythematosquamous diseases including MF, psoriasis, contact dermatitis, discoid lupus, and subacute cutaneous lupus.17 In this study, 3 blinded confocalists achieved a 95.41% and 92.89% specificity and 89.13% and 63.33% sensitivity for psoriasis and MF, respectively. Typical features of psoriasis on RCM included parakeratosis, reduction or absence of the granular layer, papillomatosis, acanthosis with normal honeycomb pattern of the epidermis, and dilated vessels in the upper dermis. Features that were more specific to MF included epidermotropic atypical lymphocytes, interface dermatitis, pleomorphic tumor cells, and dendritic cells.17 However, atypical lymphocytes and interface dermatitis also may be seen in cutaneous lupus; therefore, additional studies are still needed to validate RCM’s utility in differentiating between erythematosquamous skin diseases, including psoriasis, cutaneous lupus, and MF. Currently, RCM findings must be interpreted in conjunction with the clinical and histologic picture.
Importantly, RCM also is limited when evaluating MF due to its limited depth of visualization, as it allows imaging only to the superficial papillary dermis. Furthermore, any infiltrative process such as epidermal hyperplasia, spongiosis, or scaling, which can be seen in MF, may further impair the imaging quality of the deeper dermis.
Conclusion
Despite its limitations, RCM has the potential to be advantageous in evaluating skin lesions suspicious for MF in real time and is a promising technology for a quick noninvasive bedside adjunct tool. Its utility in selecting the optimal site for biopsy for better yield of histopathologic results in suspected MF cases has been demonstrated.16 However, large-scale studies still are needed to evaluate RCM in the diagnosis of the wide diversity of MF lesions as well as its efficacy in selecting optimal biopsy sites.
- Lutzner M, Edelson R, Schein P, et al. Cutaneous T-cell lymphomas: the Sézary syndrome, mycosis fungoides, and related disorders. Ann Intern Med. 1975;83:534-552.
- Akinbami AA, Osikomaiya BI, John-Olabode SO, et al. Mycosis fungoides: case report and literature review. Clin Med Insights Case Rep. 2014;7:95-98.
- Criscione VD, Weinstock MA. Incidence of cutaneous T-cell lymphoma in the United States, 1973-2002. Arch Dermatol. 2007;143:854-959.
- Bradford PT, Devesa SS, Anderson WF, et al. Cutaneous lymphoma incidence patterns in the United States: a population-based study of 3884 cases. Blood. 2009;113:5064-5073.
- Willemze R, Jaffe ES, Burg G, et al. WHO-EORTC classification for cutaneous lymphomas. Blood. 2005;105:3768-3785.
- Nashan D, Faulhaber D, Stander S. Mycosis fungoides: a dermatological masquerader. Br J Dermatol. 2007;157:1-10.
- Santucci M, Biggeri A, Feller AC, et al. Efficacy of histologic criteria for diagnosing early mycosis fungoides: an EORTC cutaneous lymphoma study group investigation. European Organization for Research and Treatment of Cancer. Am J Surg Pathol. 2000;24:40-50.
- Glass LF, Keller KL, Messina JL, et al. Cutaneous T-cell lymphoma. Cancer Control. 1998;5:11-18.
- Hoppe RT, Wood GS, Abel EA. Mycosis fungoides and the Sézary syndrome: pathology, staging, and treatment. Curr Probl Cancer. 1990;14:293-371.
- Tannous ZS, Mihm MC, Flotte TJ, et al. In vivo examination of lentigo maligna and malignant melanoma in situ, lentigo maligna type by near-infrared reflectance confocal microscopy: comparison of in vivo confocal images with histologic sections. J Am Acad Dermatol. 2002;46:260-263.
- Gerger A, Koller S, Weger W, et al. Sensitivity and specificity of confocal laser-scanning microscopy for in vivo diagnosis of malignant skin tumors. Cancer. 2006;107:193-200.
- Branzan AL, Landthaler M, Szeimies RM. In vivo confocal scanning laser microscopy in dermatology [published online November 18, 2006]. Lasers Med Sci. 2007;22:73-82.
- González S. Confocal reflectance microscopy in dermatology: promise and reality of non-invasive diagnosis and monitoring. Actas Dermosifiliogr. 2009;100(suppl 2):59-69.
- Agero AL, Gill M, Ardigo M, et al. In vivo reflectance confocal microscopy of mycosis fungoides: a preliminary study [published online April 16, 2007]. J Am Acad Dermatol. 2007;57:435-441.
- Wi L, Dai H, Li Z, et al. Reflectance confocal microscopy for the characteristics of mycosis fungoides and correlation with histology: a pilot study [published online April 18, 2013]. Skin Res Technol. 2013;19:352-355.
- Lange-Asschenfeldt S, Babilli J, Beyer M, et al. Consistency and distribution of reflectance confocal microscopy features for diagnosis of cutaneous T cell lymphoma. J Biomed Opt. 2012;17:016001.
- Koller S, Gerger A, Ahlgrimm-Siess V. In vivo reflectance confocal microscopy of erythematosquamous skin diseases [published online March 6, 2009]. Exp Dermatol. 2009;18:536-540.
- Lutzner M, Edelson R, Schein P, et al. Cutaneous T-cell lymphomas: the Sézary syndrome, mycosis fungoides, and related disorders. Ann Intern Med. 1975;83:534-552.
- Akinbami AA, Osikomaiya BI, John-Olabode SO, et al. Mycosis fungoides: case report and literature review. Clin Med Insights Case Rep. 2014;7:95-98.
- Criscione VD, Weinstock MA. Incidence of cutaneous T-cell lymphoma in the United States, 1973-2002. Arch Dermatol. 2007;143:854-959.
- Bradford PT, Devesa SS, Anderson WF, et al. Cutaneous lymphoma incidence patterns in the United States: a population-based study of 3884 cases. Blood. 2009;113:5064-5073.
- Willemze R, Jaffe ES, Burg G, et al. WHO-EORTC classification for cutaneous lymphomas. Blood. 2005;105:3768-3785.
- Nashan D, Faulhaber D, Stander S. Mycosis fungoides: a dermatological masquerader. Br J Dermatol. 2007;157:1-10.
- Santucci M, Biggeri A, Feller AC, et al. Efficacy of histologic criteria for diagnosing early mycosis fungoides: an EORTC cutaneous lymphoma study group investigation. European Organization for Research and Treatment of Cancer. Am J Surg Pathol. 2000;24:40-50.
- Glass LF, Keller KL, Messina JL, et al. Cutaneous T-cell lymphoma. Cancer Control. 1998;5:11-18.
- Hoppe RT, Wood GS, Abel EA. Mycosis fungoides and the Sézary syndrome: pathology, staging, and treatment. Curr Probl Cancer. 1990;14:293-371.
- Tannous ZS, Mihm MC, Flotte TJ, et al. In vivo examination of lentigo maligna and malignant melanoma in situ, lentigo maligna type by near-infrared reflectance confocal microscopy: comparison of in vivo confocal images with histologic sections. J Am Acad Dermatol. 2002;46:260-263.
- Gerger A, Koller S, Weger W, et al. Sensitivity and specificity of confocal laser-scanning microscopy for in vivo diagnosis of malignant skin tumors. Cancer. 2006;107:193-200.
- Branzan AL, Landthaler M, Szeimies RM. In vivo confocal scanning laser microscopy in dermatology [published online November 18, 2006]. Lasers Med Sci. 2007;22:73-82.
- González S. Confocal reflectance microscopy in dermatology: promise and reality of non-invasive diagnosis and monitoring. Actas Dermosifiliogr. 2009;100(suppl 2):59-69.
- Agero AL, Gill M, Ardigo M, et al. In vivo reflectance confocal microscopy of mycosis fungoides: a preliminary study [published online April 16, 2007]. J Am Acad Dermatol. 2007;57:435-441.
- Wi L, Dai H, Li Z, et al. Reflectance confocal microscopy for the characteristics of mycosis fungoides and correlation with histology: a pilot study [published online April 18, 2013]. Skin Res Technol. 2013;19:352-355.
- Lange-Asschenfeldt S, Babilli J, Beyer M, et al. Consistency and distribution of reflectance confocal microscopy features for diagnosis of cutaneous T cell lymphoma. J Biomed Opt. 2012;17:016001.
- Koller S, Gerger A, Ahlgrimm-Siess V. In vivo reflectance confocal microscopy of erythematosquamous skin diseases [published online March 6, 2009]. Exp Dermatol. 2009;18:536-540.
Practice Points
- Mycosis fungoides (MF) can be a challenging diagnosis to establish and often requires multiple biopsies.
- Reflectance confocal microscopy (RCM) may be helpful as a bedside noninvasive diagnostic technique.
- In suspected MF cases, RCM may assist in selecting the optimal biopsy site for better yield of histopathologic results.
Clinical Pearl: Mohs Cantaloupe Analogy for the Dermatology Resident
Practice Gap
Mohs micrographic surgery (MMS) is a highly curative tissue-sparing skin cancer treatment1 and is a required component of dermatology residency training. According to the Accreditation Council for Graduate Medical Education, residents must have exposure “either through direct observation or as an assistant in Mohs micrographic surgery, and reconstruction of these defects, to include flaps and grafts.”2 The MMS technique allows for complete circumferential peripheral and deep margin assessment of excised specimens; however, the conformation of a 3-dimensional gross tissue specimen into a 2-dimensional specimen as represented on a microscope slide is challenging to conceptualize.
Behavioral science research has shown that analogies and metaphors help integrate topics into a memorable format and produce deeper comprehension.3 As such, analogies can aid in the visualization of these complex spatial concepts. The MMS tissue-processing technique has been compared to flattening a pie pan.4 More recently, a peanut butter cup analogy was described as a visualization tool for explaining the various steps of MMS to patients.5 Although these analogies may help elucidate certain aspects of the MMS technique, none adequately account for the multilayered anatomy of the skin.
The Technique
To address this need, we developed the cantaloupe analogy, which provides visual representation of the 3 basic skin layers: (1) the rind represents the epidermis; (2) the flesh represents the dermis, and (3) the seed cavity represents the subcutaneous layer (Figures 1 and 2).

In MMS tissue processing, the peripheral margin of the ovoid excised skin specimen is pressed down into the same plane as the deepest layer through a process called relaxation.4 The cantaloupe represents the dome shape of the relaxed tissue, which is then serially sectioned in horizontal layers from deep to superficial (Figure 2). The first slice represents the deepest subcutaneous layer and most peripheral dermal and epidermal layers of the specimen (Figure 3). Using the cantaloupe analogy, subsequent stages (if warranted) would be guided by the location of the residual skin cancer. If the skin cancer is in the epidermis (rind) or dermis (flesh), then a skin specimen from the perimeter of the defect would be indicated. Residual skin cancer extending into the subcutaneous layer (seed cavity) would require a deeper resection.
Practice Implications
The cantaloupe provides a simple analogy to conceptualize the transition from the multilayered 3-dimensional skin tissue specimen to the 2-dimensional histologic slide specimen. Use of this cantaloupe analogy will aid dermatology residents and others interested in gaining a clearer understanding of MMS.
- Semkova K, Mallipeddi R, Robson A, et al. Mohs micrographic surgery concordance between Mohs surgeons and dermatopathologists. Dermatol Surg. 2013;39:1648-1652.
- ACGME program requirements for graduate medical education in dermatology. Accreditation Council for Graduate Medical Education website. https://www.acgme.org/Portals/0/PFAssets/ProgramRequirements/080_dermatology_2017-07-01.pdf. Updated July 1, 2017. Accessed June 6, 2018.
- Wolfe CR. Plant a tree in cyberspace: metaphor and analogy as design elements in Web-based learning environments. CyberPsychol Behav. 2001;4:67-76.
- Beck B, Peters SR. Frozen section techniques used in Mohs micrographic surgery. In: Peters SR, ed. A Practical Guide to Frozen Section Technique. New York, NY: Springer; 2010:151-170.
- Lee E, Wolverton JE, Somani AK. A simple, effective analogy to elucidate the Mohs micrographic surgery procedure—the peanut butter cup. JAMA Dermatol. 2017;153:743-744.
Practice Gap
Mohs micrographic surgery (MMS) is a highly curative tissue-sparing skin cancer treatment1 and is a required component of dermatology residency training. According to the Accreditation Council for Graduate Medical Education, residents must have exposure “either through direct observation or as an assistant in Mohs micrographic surgery, and reconstruction of these defects, to include flaps and grafts.”2 The MMS technique allows for complete circumferential peripheral and deep margin assessment of excised specimens; however, the conformation of a 3-dimensional gross tissue specimen into a 2-dimensional specimen as represented on a microscope slide is challenging to conceptualize.
Behavioral science research has shown that analogies and metaphors help integrate topics into a memorable format and produce deeper comprehension.3 As such, analogies can aid in the visualization of these complex spatial concepts. The MMS tissue-processing technique has been compared to flattening a pie pan.4 More recently, a peanut butter cup analogy was described as a visualization tool for explaining the various steps of MMS to patients.5 Although these analogies may help elucidate certain aspects of the MMS technique, none adequately account for the multilayered anatomy of the skin.
The Technique
To address this need, we developed the cantaloupe analogy, which provides visual representation of the 3 basic skin layers: (1) the rind represents the epidermis; (2) the flesh represents the dermis, and (3) the seed cavity represents the subcutaneous layer (Figures 1 and 2).


In MMS tissue processing, the peripheral margin of the ovoid excised skin specimen is pressed down into the same plane as the deepest layer through a process called relaxation.4 The cantaloupe represents the dome shape of the relaxed tissue, which is then serially sectioned in horizontal layers from deep to superficial (Figure 2). The first slice represents the deepest subcutaneous layer and most peripheral dermal and epidermal layers of the specimen (Figure 3). Using the cantaloupe analogy, subsequent stages (if warranted) would be guided by the location of the residual skin cancer. If the skin cancer is in the epidermis (rind) or dermis (flesh), then a skin specimen from the perimeter of the defect would be indicated. Residual skin cancer extending into the subcutaneous layer (seed cavity) would require a deeper resection.

Practice Implications
The cantaloupe provides a simple analogy to conceptualize the transition from the multilayered 3-dimensional skin tissue specimen to the 2-dimensional histologic slide specimen. Use of this cantaloupe analogy will aid dermatology residents and others interested in gaining a clearer understanding of MMS.
Practice Gap
Mohs micrographic surgery (MMS) is a highly curative tissue-sparing skin cancer treatment1 and is a required component of dermatology residency training. According to the Accreditation Council for Graduate Medical Education, residents must have exposure “either through direct observation or as an assistant in Mohs micrographic surgery, and reconstruction of these defects, to include flaps and grafts.”2 The MMS technique allows for complete circumferential peripheral and deep margin assessment of excised specimens; however, the conformation of a 3-dimensional gross tissue specimen into a 2-dimensional specimen as represented on a microscope slide is challenging to conceptualize.
Behavioral science research has shown that analogies and metaphors help integrate topics into a memorable format and produce deeper comprehension.3 As such, analogies can aid in the visualization of these complex spatial concepts. The MMS tissue-processing technique has been compared to flattening a pie pan.4 More recently, a peanut butter cup analogy was described as a visualization tool for explaining the various steps of MMS to patients.5 Although these analogies may help elucidate certain aspects of the MMS technique, none adequately account for the multilayered anatomy of the skin.
The Technique
To address this need, we developed the cantaloupe analogy, which provides visual representation of the 3 basic skin layers: (1) the rind represents the epidermis; (2) the flesh represents the dermis, and (3) the seed cavity represents the subcutaneous layer (Figures 1 and 2).


In MMS tissue processing, the peripheral margin of the ovoid excised skin specimen is pressed down into the same plane as the deepest layer through a process called relaxation.4 The cantaloupe represents the dome shape of the relaxed tissue, which is then serially sectioned in horizontal layers from deep to superficial (Figure 2). The first slice represents the deepest subcutaneous layer and most peripheral dermal and epidermal layers of the specimen (Figure 3). Using the cantaloupe analogy, subsequent stages (if warranted) would be guided by the location of the residual skin cancer. If the skin cancer is in the epidermis (rind) or dermis (flesh), then a skin specimen from the perimeter of the defect would be indicated. Residual skin cancer extending into the subcutaneous layer (seed cavity) would require a deeper resection.

Practice Implications
The cantaloupe provides a simple analogy to conceptualize the transition from the multilayered 3-dimensional skin tissue specimen to the 2-dimensional histologic slide specimen. Use of this cantaloupe analogy will aid dermatology residents and others interested in gaining a clearer understanding of MMS.
- Semkova K, Mallipeddi R, Robson A, et al. Mohs micrographic surgery concordance between Mohs surgeons and dermatopathologists. Dermatol Surg. 2013;39:1648-1652.
- ACGME program requirements for graduate medical education in dermatology. Accreditation Council for Graduate Medical Education website. https://www.acgme.org/Portals/0/PFAssets/ProgramRequirements/080_dermatology_2017-07-01.pdf. Updated July 1, 2017. Accessed June 6, 2018.
- Wolfe CR. Plant a tree in cyberspace: metaphor and analogy as design elements in Web-based learning environments. CyberPsychol Behav. 2001;4:67-76.
- Beck B, Peters SR. Frozen section techniques used in Mohs micrographic surgery. In: Peters SR, ed. A Practical Guide to Frozen Section Technique. New York, NY: Springer; 2010:151-170.
- Lee E, Wolverton JE, Somani AK. A simple, effective analogy to elucidate the Mohs micrographic surgery procedure—the peanut butter cup. JAMA Dermatol. 2017;153:743-744.
- Semkova K, Mallipeddi R, Robson A, et al. Mohs micrographic surgery concordance between Mohs surgeons and dermatopathologists. Dermatol Surg. 2013;39:1648-1652.
- ACGME program requirements for graduate medical education in dermatology. Accreditation Council for Graduate Medical Education website. https://www.acgme.org/Portals/0/PFAssets/ProgramRequirements/080_dermatology_2017-07-01.pdf. Updated July 1, 2017. Accessed June 6, 2018.
- Wolfe CR. Plant a tree in cyberspace: metaphor and analogy as design elements in Web-based learning environments. CyberPsychol Behav. 2001;4:67-76.
- Beck B, Peters SR. Frozen section techniques used in Mohs micrographic surgery. In: Peters SR, ed. A Practical Guide to Frozen Section Technique. New York, NY: Springer; 2010:151-170.
- Lee E, Wolverton JE, Somani AK. A simple, effective analogy to elucidate the Mohs micrographic surgery procedure—the peanut butter cup. JAMA Dermatol. 2017;153:743-744.