User login
Phase 3 study of new levodopa/carbidopa delivery system meets all efficacy endpoints
BOSTON – presented at the 2023 annual meeting of the American Academy of Neurology.
When compared with optimized oral immediate-release medication, the delivery system, called ND0612 (NeuroDerm, Rehovot, Israel), improved ON time without troublesome dyskinesias while improving symptoms according to ratings from both patients and clinicians, according to Alberto J. Espay, MD, professor of neurology and director of the Gardner Family Center for Parkinson’s Disease and Movement Disorders, University of Cincinnati.
The new delivery system addresses the challenge of reducing the variability in levodopa plasma concentrations, a major factor in motor fluctuations and diminishing benefit from orally administered drug, according to Dr. Espay. He said that continuous infusion strategies have long been sought as a method to preserve levodopa efficacy.
BouNDless findings
There were two phases to this multinational trial, called BouNDless. In the first, an open-label run-in phase, 381 patients with Parkinson’s disease were dose titrated for optimization of oral immediate-release levodopa and carbidopa. They were then optimized for the same drugs delivered with ND0612. The study was conducted over 12 weeks; 122 patients left the study after this phase due to adverse events, lack of efficacy, or withdrawal of consent.
In the second phase, the 259 remaining patients were randomized to the continuous infusion arm or to immediate release oral therapy. In this double-blind, double-dummy phase, those randomized to the ND0612 infusion also received oral placebos. Those randomized to oral therapy received a placebo infusion. Efficacy and safety were assessed at the end of 12 weeks.
At the end of phase 1, the ON time increased by about 3 hours when levodopa-carbidopa dosing was optimized on either delivery method. Dr. Espay attributed the improvement to the value of optimized dosing even in patients with relatively advanced disease.
However, for the purposes of the double-blind comparison, this improvement in ON time provided a new baseline for comparison of the two delivery methods. This is important for interpreting the primary result, which was a 1.72-hour difference in ON time at the end of the study. The difference was created when ON time was maintained with ND0612 continuous drug delivery but eroded in the group randomized to oral immediate-release treatment.
Several secondary endpoints supported the greater efficacy of continuous subcutaneous delivery. These included lower OFF time (0.50 vs. 1.90 hours), less accumulation of disability on the United Parkinson’s Disease Rating Scale part II-M-EDL (-0.30 vs. +2.75 points), and greater improvement on the Patient Global Impression of Change (+0.31 vs. +0.70 points), and the Clinical Global Impression of change (+0.31 vs. +0.77 points). The differences were highly statistically significant (all P < .0001).
The patients participating in the double-blind phase of the study were similar with a mean age of 63.5 years in both groups and time since Parkinson’s disease diagnosis (> 9 years). The median ON time without troublesome dyskinesias was about 12 hours at baseline in both groups and the median OFF time was about 3.5 hours.
The higher rate of treatment-related adverse events in the ND0612 group (67.2% vs. 52.7%) was largely explained by the greater rate of infusion site reactions (57.0% vs. 42.7%). The rates of severe reactions in the two groups were the same (0.8%), but both mild (43.8% vs. 36.6%) and moderate (12.5% vs. 5.3%) reactions occurred more commonly in the group receiving active therapy.
“Infusion reactions are the Achilles heel of all subcutaneous therapies,” acknowledged Dr. Espay, who expects other infusion systems in development to share this risk. He suggested that the clinical impact can be attenuated to some degree by rotating infusion sites.
BeyoND extension study
Data from an open-label extension (OLE) of the phase 2b BeyoND trial were also presented at the AAN meeting and generated generally similar results. Largely a safety study, there was no active control in the initial BeyoND or the BeyoND OLE. In BeyoND, the improvement in ON time from baseline was even greater than that seen in BouNDless, but, again, the optimization of dosing in the BouNDless run-in established a greater baseline of disease control.
In the OLE of BeyoND, presented by Aaron Ellenbogen, DO, a neurologist in Farmington, Mich., one of the notable findings was the retention of patients. After 2 years of follow-up, 82% completed at least 2 years of follow-up and 66.7% have now remained on treatment for at least 3 years. Dr. Ellenbogen maintains that this retention rate provides compelling evidence of a favorable benefit-to-risk ratio.
Fulfilling an unmet need
The favorable efficacy data from this trial represent “a big advance,” according to Ihtsham Ul Haq, MD, chief, movement disorders division, University of Miami, who was reached for comment. He noted that continuous infusion delivery has been anticipated for some time, and he expects these types of systems to fulfill an unmet need.
“This will be a useful option in a carefully selected group of patients,” said Dr. Haq, who considers the types of improvement in ON time to be highly clinically meaningful.
However, he cautioned that the nodules created by injection site reactions might limit the utility of this treatment option in at least some patients. Wearing the external device might also be a limiting factor for some patients.
In complex Parkinson’s disease, a stage that can be reached fairly rapidly in some patients but might take 15 years or more in others, all of the options involve a careful benefit-to-risk calculation, according to Dr. Haq. Deep brain stimulation is among the most effective options, but continuous infusion might appeal to some patients for delaying this procedure or as an alternative.
“We need multiple options for these types of patients, and it appears that continuous infusion will be one of them,” Dr. Haq said.
Dr. Espay has financial relationships with Acadia, Acorda, Amneal, AskBio, Bexion, Kyowa Kirin, Neuroderm, Neurocrine, and Sunovion. Dr. Ellenbogen has financial relationships with Allergan, Acorda, Supernus, and Teva. Dr. Haq reports no potential conflicts of interest.
BOSTON – presented at the 2023 annual meeting of the American Academy of Neurology.
When compared with optimized oral immediate-release medication, the delivery system, called ND0612 (NeuroDerm, Rehovot, Israel), improved ON time without troublesome dyskinesias while improving symptoms according to ratings from both patients and clinicians, according to Alberto J. Espay, MD, professor of neurology and director of the Gardner Family Center for Parkinson’s Disease and Movement Disorders, University of Cincinnati.
The new delivery system addresses the challenge of reducing the variability in levodopa plasma concentrations, a major factor in motor fluctuations and diminishing benefit from orally administered drug, according to Dr. Espay. He said that continuous infusion strategies have long been sought as a method to preserve levodopa efficacy.
BouNDless findings
There were two phases to this multinational trial, called BouNDless. In the first, an open-label run-in phase, 381 patients with Parkinson’s disease were dose titrated for optimization of oral immediate-release levodopa and carbidopa. They were then optimized for the same drugs delivered with ND0612. The study was conducted over 12 weeks; 122 patients left the study after this phase due to adverse events, lack of efficacy, or withdrawal of consent.
In the second phase, the 259 remaining patients were randomized to the continuous infusion arm or to immediate release oral therapy. In this double-blind, double-dummy phase, those randomized to the ND0612 infusion also received oral placebos. Those randomized to oral therapy received a placebo infusion. Efficacy and safety were assessed at the end of 12 weeks.
At the end of phase 1, the ON time increased by about 3 hours when levodopa-carbidopa dosing was optimized on either delivery method. Dr. Espay attributed the improvement to the value of optimized dosing even in patients with relatively advanced disease.
However, for the purposes of the double-blind comparison, this improvement in ON time provided a new baseline for comparison of the two delivery methods. This is important for interpreting the primary result, which was a 1.72-hour difference in ON time at the end of the study. The difference was created when ON time was maintained with ND0612 continuous drug delivery but eroded in the group randomized to oral immediate-release treatment.
Several secondary endpoints supported the greater efficacy of continuous subcutaneous delivery. These included lower OFF time (0.50 vs. 1.90 hours), less accumulation of disability on the United Parkinson’s Disease Rating Scale part II-M-EDL (-0.30 vs. +2.75 points), and greater improvement on the Patient Global Impression of Change (+0.31 vs. +0.70 points), and the Clinical Global Impression of change (+0.31 vs. +0.77 points). The differences were highly statistically significant (all P < .0001).
The patients participating in the double-blind phase of the study were similar with a mean age of 63.5 years in both groups and time since Parkinson’s disease diagnosis (> 9 years). The median ON time without troublesome dyskinesias was about 12 hours at baseline in both groups and the median OFF time was about 3.5 hours.
The higher rate of treatment-related adverse events in the ND0612 group (67.2% vs. 52.7%) was largely explained by the greater rate of infusion site reactions (57.0% vs. 42.7%). The rates of severe reactions in the two groups were the same (0.8%), but both mild (43.8% vs. 36.6%) and moderate (12.5% vs. 5.3%) reactions occurred more commonly in the group receiving active therapy.
“Infusion reactions are the Achilles heel of all subcutaneous therapies,” acknowledged Dr. Espay, who expects other infusion systems in development to share this risk. He suggested that the clinical impact can be attenuated to some degree by rotating infusion sites.
BeyoND extension study
Data from an open-label extension (OLE) of the phase 2b BeyoND trial were also presented at the AAN meeting and generated generally similar results. Largely a safety study, there was no active control in the initial BeyoND or the BeyoND OLE. In BeyoND, the improvement in ON time from baseline was even greater than that seen in BouNDless, but, again, the optimization of dosing in the BouNDless run-in established a greater baseline of disease control.
In the OLE of BeyoND, presented by Aaron Ellenbogen, DO, a neurologist in Farmington, Mich., one of the notable findings was the retention of patients. After 2 years of follow-up, 82% completed at least 2 years of follow-up and 66.7% have now remained on treatment for at least 3 years. Dr. Ellenbogen maintains that this retention rate provides compelling evidence of a favorable benefit-to-risk ratio.
Fulfilling an unmet need
The favorable efficacy data from this trial represent “a big advance,” according to Ihtsham Ul Haq, MD, chief, movement disorders division, University of Miami, who was reached for comment. He noted that continuous infusion delivery has been anticipated for some time, and he expects these types of systems to fulfill an unmet need.
“This will be a useful option in a carefully selected group of patients,” said Dr. Haq, who considers the types of improvement in ON time to be highly clinically meaningful.
However, he cautioned that the nodules created by injection site reactions might limit the utility of this treatment option in at least some patients. Wearing the external device might also be a limiting factor for some patients.
In complex Parkinson’s disease, a stage that can be reached fairly rapidly in some patients but might take 15 years or more in others, all of the options involve a careful benefit-to-risk calculation, according to Dr. Haq. Deep brain stimulation is among the most effective options, but continuous infusion might appeal to some patients for delaying this procedure or as an alternative.
“We need multiple options for these types of patients, and it appears that continuous infusion will be one of them,” Dr. Haq said.
Dr. Espay has financial relationships with Acadia, Acorda, Amneal, AskBio, Bexion, Kyowa Kirin, Neuroderm, Neurocrine, and Sunovion. Dr. Ellenbogen has financial relationships with Allergan, Acorda, Supernus, and Teva. Dr. Haq reports no potential conflicts of interest.
BOSTON – presented at the 2023 annual meeting of the American Academy of Neurology.
When compared with optimized oral immediate-release medication, the delivery system, called ND0612 (NeuroDerm, Rehovot, Israel), improved ON time without troublesome dyskinesias while improving symptoms according to ratings from both patients and clinicians, according to Alberto J. Espay, MD, professor of neurology and director of the Gardner Family Center for Parkinson’s Disease and Movement Disorders, University of Cincinnati.
The new delivery system addresses the challenge of reducing the variability in levodopa plasma concentrations, a major factor in motor fluctuations and diminishing benefit from orally administered drug, according to Dr. Espay. He said that continuous infusion strategies have long been sought as a method to preserve levodopa efficacy.
BouNDless findings
There were two phases to this multinational trial, called BouNDless. In the first, an open-label run-in phase, 381 patients with Parkinson’s disease were dose titrated for optimization of oral immediate-release levodopa and carbidopa. They were then optimized for the same drugs delivered with ND0612. The study was conducted over 12 weeks; 122 patients left the study after this phase due to adverse events, lack of efficacy, or withdrawal of consent.
In the second phase, the 259 remaining patients were randomized to the continuous infusion arm or to immediate release oral therapy. In this double-blind, double-dummy phase, those randomized to the ND0612 infusion also received oral placebos. Those randomized to oral therapy received a placebo infusion. Efficacy and safety were assessed at the end of 12 weeks.
At the end of phase 1, the ON time increased by about 3 hours when levodopa-carbidopa dosing was optimized on either delivery method. Dr. Espay attributed the improvement to the value of optimized dosing even in patients with relatively advanced disease.
However, for the purposes of the double-blind comparison, this improvement in ON time provided a new baseline for comparison of the two delivery methods. This is important for interpreting the primary result, which was a 1.72-hour difference in ON time at the end of the study. The difference was created when ON time was maintained with ND0612 continuous drug delivery but eroded in the group randomized to oral immediate-release treatment.
Several secondary endpoints supported the greater efficacy of continuous subcutaneous delivery. These included lower OFF time (0.50 vs. 1.90 hours), less accumulation of disability on the United Parkinson’s Disease Rating Scale part II-M-EDL (-0.30 vs. +2.75 points), and greater improvement on the Patient Global Impression of Change (+0.31 vs. +0.70 points), and the Clinical Global Impression of change (+0.31 vs. +0.77 points). The differences were highly statistically significant (all P < .0001).
The patients participating in the double-blind phase of the study were similar with a mean age of 63.5 years in both groups and time since Parkinson’s disease diagnosis (> 9 years). The median ON time without troublesome dyskinesias was about 12 hours at baseline in both groups and the median OFF time was about 3.5 hours.
The higher rate of treatment-related adverse events in the ND0612 group (67.2% vs. 52.7%) was largely explained by the greater rate of infusion site reactions (57.0% vs. 42.7%). The rates of severe reactions in the two groups were the same (0.8%), but both mild (43.8% vs. 36.6%) and moderate (12.5% vs. 5.3%) reactions occurred more commonly in the group receiving active therapy.
“Infusion reactions are the Achilles heel of all subcutaneous therapies,” acknowledged Dr. Espay, who expects other infusion systems in development to share this risk. He suggested that the clinical impact can be attenuated to some degree by rotating infusion sites.
BeyoND extension study
Data from an open-label extension (OLE) of the phase 2b BeyoND trial were also presented at the AAN meeting and generated generally similar results. Largely a safety study, there was no active control in the initial BeyoND or the BeyoND OLE. In BeyoND, the improvement in ON time from baseline was even greater than that seen in BouNDless, but, again, the optimization of dosing in the BouNDless run-in established a greater baseline of disease control.
In the OLE of BeyoND, presented by Aaron Ellenbogen, DO, a neurologist in Farmington, Mich., one of the notable findings was the retention of patients. After 2 years of follow-up, 82% completed at least 2 years of follow-up and 66.7% have now remained on treatment for at least 3 years. Dr. Ellenbogen maintains that this retention rate provides compelling evidence of a favorable benefit-to-risk ratio.
Fulfilling an unmet need
The favorable efficacy data from this trial represent “a big advance,” according to Ihtsham Ul Haq, MD, chief, movement disorders division, University of Miami, who was reached for comment. He noted that continuous infusion delivery has been anticipated for some time, and he expects these types of systems to fulfill an unmet need.
“This will be a useful option in a carefully selected group of patients,” said Dr. Haq, who considers the types of improvement in ON time to be highly clinically meaningful.
However, he cautioned that the nodules created by injection site reactions might limit the utility of this treatment option in at least some patients. Wearing the external device might also be a limiting factor for some patients.
In complex Parkinson’s disease, a stage that can be reached fairly rapidly in some patients but might take 15 years or more in others, all of the options involve a careful benefit-to-risk calculation, according to Dr. Haq. Deep brain stimulation is among the most effective options, but continuous infusion might appeal to some patients for delaying this procedure or as an alternative.
“We need multiple options for these types of patients, and it appears that continuous infusion will be one of them,” Dr. Haq said.
Dr. Espay has financial relationships with Acadia, Acorda, Amneal, AskBio, Bexion, Kyowa Kirin, Neuroderm, Neurocrine, and Sunovion. Dr. Ellenbogen has financial relationships with Allergan, Acorda, Supernus, and Teva. Dr. Haq reports no potential conflicts of interest.
FROM AAN 2023
New assay hailed as a game changer for early Parkinson’s diagnosis
, and provides information on molecular subtypes, new research indicates.
“Identifying an effective biomarker for Parkinson’s disease pathology could have profound implications for the way we treat the condition, potentially making it possible to diagnose people earlier, identify the best treatments for different subsets of patients, and speed up clinical trials,” the study’s co-lead author Andrew Siderowf, MD, of the University of Pennsylvania, Philadelphia, said in a news release.
“Our findings suggest that the αSyn-SAA technique is highly accurate at detecting the biomarker for Parkinson’s disease regardless of the clinical features, making it possible to accurately diagnose the disease in patients at early stages,” added co-lead author Luis Concha-Marambio, PhD, director of research and development at Amprion, San Diego, Calif.
The study was published online in The Lancet Neurology.
‘New era’ in Parkinson’s disease
The researchers assessed the usefulness of αSyn-SAA in a cross-sectional analysis of 1,123 participants in the Parkinson’s Progression Markers Initiative (PPMI) cohort from 33 participating academic neurology outpatient practices in 12 countries.
The cohort included individuals with sporadic Parkinson’s disease from LRRK2 or GBA variants, healthy controls, individuals with clinical syndromes prodromal to Parkinson’s disease (rapid eye movement sleep behavior disorder [RBD] or hyposmia), and nonmanifesting carriers of LRRK2 and GBA variants. Cerebrospinal fluid (CSF) samples from each participant were analyzed using αSyn-SAA.
Overall, αSyn-SAA differentiated Parkinson’s disease from healthy controls with 87.7% sensitivity and 96.3% specificity.
Sensitivity of the assay varied across subgroups based on genetic and clinical features. Among genetic Parkinson’s disease subgroups, sensitivity was highest for GBA Parkinson’s disease (95.9%), followed by sporadic Parkinson’s disease (93.3%), and lowest for LRRK2 Parkinson’s disease (67.5%). Among clinical features, hyposmia was the most robust predictor of a positive assay result.
Among all Parkinson’s disease cases with hyposmia, the sensitivity of the assay was 97.2%, compared with 63.0% for Parkinson’s disease without olfactory dysfunction. Combining genetic and clinical features, the sensitivity of positive αSyn-SAA in sporadic Parkinson’s disease with olfactory deficit was 98.6%, compared with 78.3% in sporadic Parkinson’s disease without hyposmia. Most prodromal participants (86%) with RBD and hyposmia had positive αSyn-SAA results, indicating they had α-synuclein aggregates despite not yet being diagnosed with Parkinson’s disease.
Among those recruited based on their loss of smell, 89% (16 of 18 participants) had positive αSyn-SAA results. Similarly, in those with RBD, positive αSyn-SAA results were present in 85% of cases (28 of 33). No other clinical features were associated with a positive αSyn-SAA result.
In participants who carried LRRK2 or GBA variants but had no Parkinson’s disease diagnosis or prodromal symptoms (nonmanifesting carriers), 9% (14 of 159) and 7% (11 of 151), respectively, had positive αSyn-SAA results.
To date, this is the largest analysis of α-Syn-SAA for the biochemical diagnosis of Parkinson’s disease, the researchers said.
The results show that the assay classifies people with Parkinson’s disease with “high sensitivity and specificity, provides information about molecular heterogeneity, and detects prodromal individuals before diagnosis,” they wrote.
“These findings suggest a crucial role for the α-synuclein SAA in therapeutic development, both to identify pathologically defined subgroups of people with Parkinson’s disease and to establish biomarker-defined at-risk cohorts,” they added.
Amprion has commercialized the assay (SYNTap test), which can be ordered online.
‘Seminal development’
The authors of an accompanying editorial noted the study “lays the foundation for a biological diagnosis” of Parkinson’s disease. “We have entered a new era of biomarker and treatment development for Parkinson’s disease. The possibility of detecting a misfolded α-synuclein, the pathological hallmark of Parkinson’s disease, by employing an SSA, is a seminal development,” wrote Daniela Berg, MD, PhD, and Christine Klein, MD, with University Hospital Schleswig-Holstein, Germany.
“However, to fully leverage the enormous potential of the α-synuclein seed amplification, the test would have to be performed in blood rather than the CSF, a less invasive approach that has proven to be viable,” they added.
“Although the blood-based method needs to be further elaborated for scalability, α-synuclein SAA is a game changer in Parkinson’s disease diagnostics, research, and treatment trials,” they concluded.
The study was funded by The Michael J. Fox Foundation for Parkinson’s Research and a consortium of more than 40 private and philanthropic partners. Dr. Siderowf has declared consulting for Merck and Parkinson Study Group, and receiving honoraria from Bial. A full list of author disclosures is available with the original article. Dr. Berg and Dr. Klein have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, and provides information on molecular subtypes, new research indicates.
“Identifying an effective biomarker for Parkinson’s disease pathology could have profound implications for the way we treat the condition, potentially making it possible to diagnose people earlier, identify the best treatments for different subsets of patients, and speed up clinical trials,” the study’s co-lead author Andrew Siderowf, MD, of the University of Pennsylvania, Philadelphia, said in a news release.
“Our findings suggest that the αSyn-SAA technique is highly accurate at detecting the biomarker for Parkinson’s disease regardless of the clinical features, making it possible to accurately diagnose the disease in patients at early stages,” added co-lead author Luis Concha-Marambio, PhD, director of research and development at Amprion, San Diego, Calif.
The study was published online in The Lancet Neurology.
‘New era’ in Parkinson’s disease
The researchers assessed the usefulness of αSyn-SAA in a cross-sectional analysis of 1,123 participants in the Parkinson’s Progression Markers Initiative (PPMI) cohort from 33 participating academic neurology outpatient practices in 12 countries.
The cohort included individuals with sporadic Parkinson’s disease from LRRK2 or GBA variants, healthy controls, individuals with clinical syndromes prodromal to Parkinson’s disease (rapid eye movement sleep behavior disorder [RBD] or hyposmia), and nonmanifesting carriers of LRRK2 and GBA variants. Cerebrospinal fluid (CSF) samples from each participant were analyzed using αSyn-SAA.
Overall, αSyn-SAA differentiated Parkinson’s disease from healthy controls with 87.7% sensitivity and 96.3% specificity.
Sensitivity of the assay varied across subgroups based on genetic and clinical features. Among genetic Parkinson’s disease subgroups, sensitivity was highest for GBA Parkinson’s disease (95.9%), followed by sporadic Parkinson’s disease (93.3%), and lowest for LRRK2 Parkinson’s disease (67.5%). Among clinical features, hyposmia was the most robust predictor of a positive assay result.
Among all Parkinson’s disease cases with hyposmia, the sensitivity of the assay was 97.2%, compared with 63.0% for Parkinson’s disease without olfactory dysfunction. Combining genetic and clinical features, the sensitivity of positive αSyn-SAA in sporadic Parkinson’s disease with olfactory deficit was 98.6%, compared with 78.3% in sporadic Parkinson’s disease without hyposmia. Most prodromal participants (86%) with RBD and hyposmia had positive αSyn-SAA results, indicating they had α-synuclein aggregates despite not yet being diagnosed with Parkinson’s disease.
Among those recruited based on their loss of smell, 89% (16 of 18 participants) had positive αSyn-SAA results. Similarly, in those with RBD, positive αSyn-SAA results were present in 85% of cases (28 of 33). No other clinical features were associated with a positive αSyn-SAA result.
In participants who carried LRRK2 or GBA variants but had no Parkinson’s disease diagnosis or prodromal symptoms (nonmanifesting carriers), 9% (14 of 159) and 7% (11 of 151), respectively, had positive αSyn-SAA results.
To date, this is the largest analysis of α-Syn-SAA for the biochemical diagnosis of Parkinson’s disease, the researchers said.
The results show that the assay classifies people with Parkinson’s disease with “high sensitivity and specificity, provides information about molecular heterogeneity, and detects prodromal individuals before diagnosis,” they wrote.
“These findings suggest a crucial role for the α-synuclein SAA in therapeutic development, both to identify pathologically defined subgroups of people with Parkinson’s disease and to establish biomarker-defined at-risk cohorts,” they added.
Amprion has commercialized the assay (SYNTap test), which can be ordered online.
‘Seminal development’
The authors of an accompanying editorial noted the study “lays the foundation for a biological diagnosis” of Parkinson’s disease. “We have entered a new era of biomarker and treatment development for Parkinson’s disease. The possibility of detecting a misfolded α-synuclein, the pathological hallmark of Parkinson’s disease, by employing an SSA, is a seminal development,” wrote Daniela Berg, MD, PhD, and Christine Klein, MD, with University Hospital Schleswig-Holstein, Germany.
“However, to fully leverage the enormous potential of the α-synuclein seed amplification, the test would have to be performed in blood rather than the CSF, a less invasive approach that has proven to be viable,” they added.
“Although the blood-based method needs to be further elaborated for scalability, α-synuclein SAA is a game changer in Parkinson’s disease diagnostics, research, and treatment trials,” they concluded.
The study was funded by The Michael J. Fox Foundation for Parkinson’s Research and a consortium of more than 40 private and philanthropic partners. Dr. Siderowf has declared consulting for Merck and Parkinson Study Group, and receiving honoraria from Bial. A full list of author disclosures is available with the original article. Dr. Berg and Dr. Klein have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, and provides information on molecular subtypes, new research indicates.
“Identifying an effective biomarker for Parkinson’s disease pathology could have profound implications for the way we treat the condition, potentially making it possible to diagnose people earlier, identify the best treatments for different subsets of patients, and speed up clinical trials,” the study’s co-lead author Andrew Siderowf, MD, of the University of Pennsylvania, Philadelphia, said in a news release.
“Our findings suggest that the αSyn-SAA technique is highly accurate at detecting the biomarker for Parkinson’s disease regardless of the clinical features, making it possible to accurately diagnose the disease in patients at early stages,” added co-lead author Luis Concha-Marambio, PhD, director of research and development at Amprion, San Diego, Calif.
The study was published online in The Lancet Neurology.
‘New era’ in Parkinson’s disease
The researchers assessed the usefulness of αSyn-SAA in a cross-sectional analysis of 1,123 participants in the Parkinson’s Progression Markers Initiative (PPMI) cohort from 33 participating academic neurology outpatient practices in 12 countries.
The cohort included individuals with sporadic Parkinson’s disease from LRRK2 or GBA variants, healthy controls, individuals with clinical syndromes prodromal to Parkinson’s disease (rapid eye movement sleep behavior disorder [RBD] or hyposmia), and nonmanifesting carriers of LRRK2 and GBA variants. Cerebrospinal fluid (CSF) samples from each participant were analyzed using αSyn-SAA.
Overall, αSyn-SAA differentiated Parkinson’s disease from healthy controls with 87.7% sensitivity and 96.3% specificity.
Sensitivity of the assay varied across subgroups based on genetic and clinical features. Among genetic Parkinson’s disease subgroups, sensitivity was highest for GBA Parkinson’s disease (95.9%), followed by sporadic Parkinson’s disease (93.3%), and lowest for LRRK2 Parkinson’s disease (67.5%). Among clinical features, hyposmia was the most robust predictor of a positive assay result.
Among all Parkinson’s disease cases with hyposmia, the sensitivity of the assay was 97.2%, compared with 63.0% for Parkinson’s disease without olfactory dysfunction. Combining genetic and clinical features, the sensitivity of positive αSyn-SAA in sporadic Parkinson’s disease with olfactory deficit was 98.6%, compared with 78.3% in sporadic Parkinson’s disease without hyposmia. Most prodromal participants (86%) with RBD and hyposmia had positive αSyn-SAA results, indicating they had α-synuclein aggregates despite not yet being diagnosed with Parkinson’s disease.
Among those recruited based on their loss of smell, 89% (16 of 18 participants) had positive αSyn-SAA results. Similarly, in those with RBD, positive αSyn-SAA results were present in 85% of cases (28 of 33). No other clinical features were associated with a positive αSyn-SAA result.
In participants who carried LRRK2 or GBA variants but had no Parkinson’s disease diagnosis or prodromal symptoms (nonmanifesting carriers), 9% (14 of 159) and 7% (11 of 151), respectively, had positive αSyn-SAA results.
To date, this is the largest analysis of α-Syn-SAA for the biochemical diagnosis of Parkinson’s disease, the researchers said.
The results show that the assay classifies people with Parkinson’s disease with “high sensitivity and specificity, provides information about molecular heterogeneity, and detects prodromal individuals before diagnosis,” they wrote.
“These findings suggest a crucial role for the α-synuclein SAA in therapeutic development, both to identify pathologically defined subgroups of people with Parkinson’s disease and to establish biomarker-defined at-risk cohorts,” they added.
Amprion has commercialized the assay (SYNTap test), which can be ordered online.
‘Seminal development’
The authors of an accompanying editorial noted the study “lays the foundation for a biological diagnosis” of Parkinson’s disease. “We have entered a new era of biomarker and treatment development for Parkinson’s disease. The possibility of detecting a misfolded α-synuclein, the pathological hallmark of Parkinson’s disease, by employing an SSA, is a seminal development,” wrote Daniela Berg, MD, PhD, and Christine Klein, MD, with University Hospital Schleswig-Holstein, Germany.
“However, to fully leverage the enormous potential of the α-synuclein seed amplification, the test would have to be performed in blood rather than the CSF, a less invasive approach that has proven to be viable,” they added.
“Although the blood-based method needs to be further elaborated for scalability, α-synuclein SAA is a game changer in Parkinson’s disease diagnostics, research, and treatment trials,” they concluded.
The study was funded by The Michael J. Fox Foundation for Parkinson’s Research and a consortium of more than 40 private and philanthropic partners. Dr. Siderowf has declared consulting for Merck and Parkinson Study Group, and receiving honoraria from Bial. A full list of author disclosures is available with the original article. Dr. Berg and Dr. Klein have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE LANCET NEUROLOGY
Mississippi–Ohio River valley linked to higher risk of Parkinson’s disease
according to findings from a study that was released ahead of its scheduled presentation at the annual meeting of the American Academy of Neurology.
The association was attributed to concentrations of particulate matter (PM) 2.5 in the Mississippi–Ohio River valley, which was on average higher than in other areas, but that didn’t entirely explain the increase in Parkinson’s disease in that region, Brittany Krzyzanowski, PhD, a postdoctoral research fellow in the neuroepidemiology research program of the department of neurology at Barrow Neurological Institute, Dignity Health St. Joseph’s Hospital and Medical Center, Phoenix, said in an interview.
“This study revealed Parkinson’s disease hot spots in the Mississippi–Ohio River valley, a region that has some of the highest levels of air pollution in the nation,” she said, “but we also still find a relationship between air pollution and Parkinson’s risk in the regions in the western half of the United States where Parkinson’s disease and air pollution levels are relatively low.”
Dr. Krzyzanowski and colleagues evaluated 22,546,965 Medicare beneficiaries in 2009, using a multimethod approach that included geospatial analytical techniques to categorize their exposure to PM2.5 based on age, sex, race, smoking status, and health care usage. The researchers also performed individual-level case-control analysis to assess PM2.5 results at the county level. The Medicare beneficiaries were grouped according to average exposure, with the lowest group having an average annual exposure of 5 mcg/m3 and the group with the highest exposure having an average annual exposure of 19 mcg/m3.
In total, researchers identified 83,674 Medicare beneficiaries with incident Parkinson’s disease, with 434 new cases per 100,000 people in the highest exposure group, compared with 359 new cases per 100,000 people in the lowest-exposure group. The relative risk for Parkinson’s disease increased in the highest quartile of PM2.5 by 25%, compared with the lowest quartile after adjusting for factors such as age, smoking status, and health care usage (95% confidence interval, 20%–29%).
The results showed the nationwide average annual PM2.5 was associated with incident Parkinson’s disease, and the Rocky Mountain region carried a strong association between PM2.5 and Parkson’s disease with a 16% increase in risk per level of exposure to PM2.5. While the Mississippi-Ohio River valley was also associated with Parkinson’s disease, there was a weaker association between PM2.5 and Parkinson’s disease, which the researchers attributed to a “ceiling effect” of PM2.5 between approximately 12-19 mcg/m3.
Dr. Krzyzanowski said that use of a large-population-based dataset and high-resolution location data were major strengths of the study. “Having this level of information leaves less room for uncertainty in our measures and analyses,” she said. “Our study also leveraged innovative geographic information systems which allowed us to refine local patterns of disease by using population behavior and demographic information (such as smoking and age) to ensure that we could provide the most accurate map representation available to date.”
A focus on air pollution
Existing research in examining the etiology of Parkinson’s mainly focused on exposure to pesticides,* Dr. Krzyzanowski explained, and “consists of studies using relatively small populations and low-resolution air pollution data.” Genetics is another possible cause, she noted, but only explains some Parkinson’s disease cases.
“Our work suggests that we should also be looking at air pollution as a contributor in the development of Parkinson’s disease,” she said.
Ray Dorsey, MD, professor of neurology at the University of Rochester (N.Y.), who was not involved with the study, said that evidence is mounting that “air pollution may be an important causal factor in Parkinson’s and especially Alzheimer’s disease.”
“This study by a well-regarded group of researchers adds epidemiological evidence for that association,” he said. Another strength is that the study was conducted in the United States, as many epidemiological studies evaluating air pollution and Parkinson’s disease have been performed outside the country because of “a dearth of reliable data sources.”
“This study, along with others, suggest that some of the important environmental toxicants tied to brain disease may be inhaled,” Dr. Dorsey said. “The nose may be the front door to the brain.”
Dr. Krzyzanowski said the next step in their research is further examination of different types of air pollution. “Air pollution contains a variety of toxic components which vary from region to region. Understanding the different components in air pollution and how they interact with climate, temperature, and topography could help explain the regional differences we observed.”
One potential limitation in the study is a lag between air pollution exposure and development of Parkinson’s disease, Dr. Dorsey noted.
“Here, it looks like (but I am not certain) that the investigators looked at current air pollution levels and new cases of Parkinson’s. Ideally, for incident cases of Parkinson’s disease, we would want to know historical data on exposure to air pollution,” he said.
Future studies should include prospective evaluation of adults as well as babies and children who have been exposed to both high and low levels of air pollution. That kind of study “would be incredibly valuable for determining the role of an important environmental toxicant in many brain diseases, including stroke, Alzheimer’s, and Parkinson’s,” he said.
Dr. Krzyzanowski and Dr. Dorsey reported no relevant financial disclosures. This study was supported by grants from the Department of Defense, the National Institute of Environmental Health Sciences, and The Michael J. Fox Foundation for Parkinson’s Research.
*Correction, 4/14/23: An earlier version of this article mischaracterized the disease that was the subject of this research.
according to findings from a study that was released ahead of its scheduled presentation at the annual meeting of the American Academy of Neurology.
The association was attributed to concentrations of particulate matter (PM) 2.5 in the Mississippi–Ohio River valley, which was on average higher than in other areas, but that didn’t entirely explain the increase in Parkinson’s disease in that region, Brittany Krzyzanowski, PhD, a postdoctoral research fellow in the neuroepidemiology research program of the department of neurology at Barrow Neurological Institute, Dignity Health St. Joseph’s Hospital and Medical Center, Phoenix, said in an interview.
“This study revealed Parkinson’s disease hot spots in the Mississippi–Ohio River valley, a region that has some of the highest levels of air pollution in the nation,” she said, “but we also still find a relationship between air pollution and Parkinson’s risk in the regions in the western half of the United States where Parkinson’s disease and air pollution levels are relatively low.”
Dr. Krzyzanowski and colleagues evaluated 22,546,965 Medicare beneficiaries in 2009, using a multimethod approach that included geospatial analytical techniques to categorize their exposure to PM2.5 based on age, sex, race, smoking status, and health care usage. The researchers also performed individual-level case-control analysis to assess PM2.5 results at the county level. The Medicare beneficiaries were grouped according to average exposure, with the lowest group having an average annual exposure of 5 mcg/m3 and the group with the highest exposure having an average annual exposure of 19 mcg/m3.
In total, researchers identified 83,674 Medicare beneficiaries with incident Parkinson’s disease, with 434 new cases per 100,000 people in the highest exposure group, compared with 359 new cases per 100,000 people in the lowest-exposure group. The relative risk for Parkinson’s disease increased in the highest quartile of PM2.5 by 25%, compared with the lowest quartile after adjusting for factors such as age, smoking status, and health care usage (95% confidence interval, 20%–29%).
The results showed the nationwide average annual PM2.5 was associated with incident Parkinson’s disease, and the Rocky Mountain region carried a strong association between PM2.5 and Parkson’s disease with a 16% increase in risk per level of exposure to PM2.5. While the Mississippi-Ohio River valley was also associated with Parkinson’s disease, there was a weaker association between PM2.5 and Parkinson’s disease, which the researchers attributed to a “ceiling effect” of PM2.5 between approximately 12-19 mcg/m3.
Dr. Krzyzanowski said that use of a large-population-based dataset and high-resolution location data were major strengths of the study. “Having this level of information leaves less room for uncertainty in our measures and analyses,” she said. “Our study also leveraged innovative geographic information systems which allowed us to refine local patterns of disease by using population behavior and demographic information (such as smoking and age) to ensure that we could provide the most accurate map representation available to date.”
A focus on air pollution
Existing research in examining the etiology of Parkinson’s mainly focused on exposure to pesticides,* Dr. Krzyzanowski explained, and “consists of studies using relatively small populations and low-resolution air pollution data.” Genetics is another possible cause, she noted, but only explains some Parkinson’s disease cases.
“Our work suggests that we should also be looking at air pollution as a contributor in the development of Parkinson’s disease,” she said.
Ray Dorsey, MD, professor of neurology at the University of Rochester (N.Y.), who was not involved with the study, said that evidence is mounting that “air pollution may be an important causal factor in Parkinson’s and especially Alzheimer’s disease.”
“This study by a well-regarded group of researchers adds epidemiological evidence for that association,” he said. Another strength is that the study was conducted in the United States, as many epidemiological studies evaluating air pollution and Parkinson’s disease have been performed outside the country because of “a dearth of reliable data sources.”
“This study, along with others, suggest that some of the important environmental toxicants tied to brain disease may be inhaled,” Dr. Dorsey said. “The nose may be the front door to the brain.”
Dr. Krzyzanowski said the next step in their research is further examination of different types of air pollution. “Air pollution contains a variety of toxic components which vary from region to region. Understanding the different components in air pollution and how they interact with climate, temperature, and topography could help explain the regional differences we observed.”
One potential limitation in the study is a lag between air pollution exposure and development of Parkinson’s disease, Dr. Dorsey noted.
“Here, it looks like (but I am not certain) that the investigators looked at current air pollution levels and new cases of Parkinson’s. Ideally, for incident cases of Parkinson’s disease, we would want to know historical data on exposure to air pollution,” he said.
Future studies should include prospective evaluation of adults as well as babies and children who have been exposed to both high and low levels of air pollution. That kind of study “would be incredibly valuable for determining the role of an important environmental toxicant in many brain diseases, including stroke, Alzheimer’s, and Parkinson’s,” he said.
Dr. Krzyzanowski and Dr. Dorsey reported no relevant financial disclosures. This study was supported by grants from the Department of Defense, the National Institute of Environmental Health Sciences, and The Michael J. Fox Foundation for Parkinson’s Research.
*Correction, 4/14/23: An earlier version of this article mischaracterized the disease that was the subject of this research.
according to findings from a study that was released ahead of its scheduled presentation at the annual meeting of the American Academy of Neurology.
The association was attributed to concentrations of particulate matter (PM) 2.5 in the Mississippi–Ohio River valley, which was on average higher than in other areas, but that didn’t entirely explain the increase in Parkinson’s disease in that region, Brittany Krzyzanowski, PhD, a postdoctoral research fellow in the neuroepidemiology research program of the department of neurology at Barrow Neurological Institute, Dignity Health St. Joseph’s Hospital and Medical Center, Phoenix, said in an interview.
“This study revealed Parkinson’s disease hot spots in the Mississippi–Ohio River valley, a region that has some of the highest levels of air pollution in the nation,” she said, “but we also still find a relationship between air pollution and Parkinson’s risk in the regions in the western half of the United States where Parkinson’s disease and air pollution levels are relatively low.”
Dr. Krzyzanowski and colleagues evaluated 22,546,965 Medicare beneficiaries in 2009, using a multimethod approach that included geospatial analytical techniques to categorize their exposure to PM2.5 based on age, sex, race, smoking status, and health care usage. The researchers also performed individual-level case-control analysis to assess PM2.5 results at the county level. The Medicare beneficiaries were grouped according to average exposure, with the lowest group having an average annual exposure of 5 mcg/m3 and the group with the highest exposure having an average annual exposure of 19 mcg/m3.
In total, researchers identified 83,674 Medicare beneficiaries with incident Parkinson’s disease, with 434 new cases per 100,000 people in the highest exposure group, compared with 359 new cases per 100,000 people in the lowest-exposure group. The relative risk for Parkinson’s disease increased in the highest quartile of PM2.5 by 25%, compared with the lowest quartile after adjusting for factors such as age, smoking status, and health care usage (95% confidence interval, 20%–29%).
The results showed the nationwide average annual PM2.5 was associated with incident Parkinson’s disease, and the Rocky Mountain region carried a strong association between PM2.5 and Parkson’s disease with a 16% increase in risk per level of exposure to PM2.5. While the Mississippi-Ohio River valley was also associated with Parkinson’s disease, there was a weaker association between PM2.5 and Parkinson’s disease, which the researchers attributed to a “ceiling effect” of PM2.5 between approximately 12-19 mcg/m3.
Dr. Krzyzanowski said that use of a large-population-based dataset and high-resolution location data were major strengths of the study. “Having this level of information leaves less room for uncertainty in our measures and analyses,” she said. “Our study also leveraged innovative geographic information systems which allowed us to refine local patterns of disease by using population behavior and demographic information (such as smoking and age) to ensure that we could provide the most accurate map representation available to date.”
A focus on air pollution
Existing research in examining the etiology of Parkinson’s mainly focused on exposure to pesticides,* Dr. Krzyzanowski explained, and “consists of studies using relatively small populations and low-resolution air pollution data.” Genetics is another possible cause, she noted, but only explains some Parkinson’s disease cases.
“Our work suggests that we should also be looking at air pollution as a contributor in the development of Parkinson’s disease,” she said.
Ray Dorsey, MD, professor of neurology at the University of Rochester (N.Y.), who was not involved with the study, said that evidence is mounting that “air pollution may be an important causal factor in Parkinson’s and especially Alzheimer’s disease.”
“This study by a well-regarded group of researchers adds epidemiological evidence for that association,” he said. Another strength is that the study was conducted in the United States, as many epidemiological studies evaluating air pollution and Parkinson’s disease have been performed outside the country because of “a dearth of reliable data sources.”
“This study, along with others, suggest that some of the important environmental toxicants tied to brain disease may be inhaled,” Dr. Dorsey said. “The nose may be the front door to the brain.”
Dr. Krzyzanowski said the next step in their research is further examination of different types of air pollution. “Air pollution contains a variety of toxic components which vary from region to region. Understanding the different components in air pollution and how they interact with climate, temperature, and topography could help explain the regional differences we observed.”
One potential limitation in the study is a lag between air pollution exposure and development of Parkinson’s disease, Dr. Dorsey noted.
“Here, it looks like (but I am not certain) that the investigators looked at current air pollution levels and new cases of Parkinson’s. Ideally, for incident cases of Parkinson’s disease, we would want to know historical data on exposure to air pollution,” he said.
Future studies should include prospective evaluation of adults as well as babies and children who have been exposed to both high and low levels of air pollution. That kind of study “would be incredibly valuable for determining the role of an important environmental toxicant in many brain diseases, including stroke, Alzheimer’s, and Parkinson’s,” he said.
Dr. Krzyzanowski and Dr. Dorsey reported no relevant financial disclosures. This study was supported by grants from the Department of Defense, the National Institute of Environmental Health Sciences, and The Michael J. Fox Foundation for Parkinson’s Research.
*Correction, 4/14/23: An earlier version of this article mischaracterized the disease that was the subject of this research.
FROM AAN 2023
Picking up the premotor symptoms of Parkinson’s
This transcript has been edited for clarity.
Matthew F. Watto, MD: Welcome back to The Curbsiders. We had a great discussion on Parkinson’s Disease for Primary Care with Dr. Albert Hung. Paul, this was something that really made me nervous. I didn’t have a lot of comfort with it. But he taught us a lot of tips about how to recognize Parkinson’s.
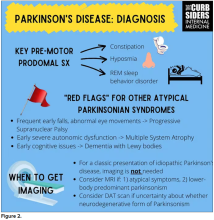
I hadn’t been as aware of the premotor symptoms: constipation, hyposmia (loss of sense of smell), and rapid eye movement sleep behavior disorder. If patients have those early on and they aren’t explained by other things (especially the REM sleep behavior disorder), you should really key in because those patients are at risk of developing Parkinson’s years down the line. Those symptoms could present first, which just kind of blew my mind.
What tips do you have about how to recognize Parkinson’s? Do you want to talk about the physical exam?
Paul N. Williams, MD: You know I love the physical exam stuff, so I’m happy to talk about that.
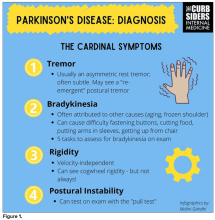
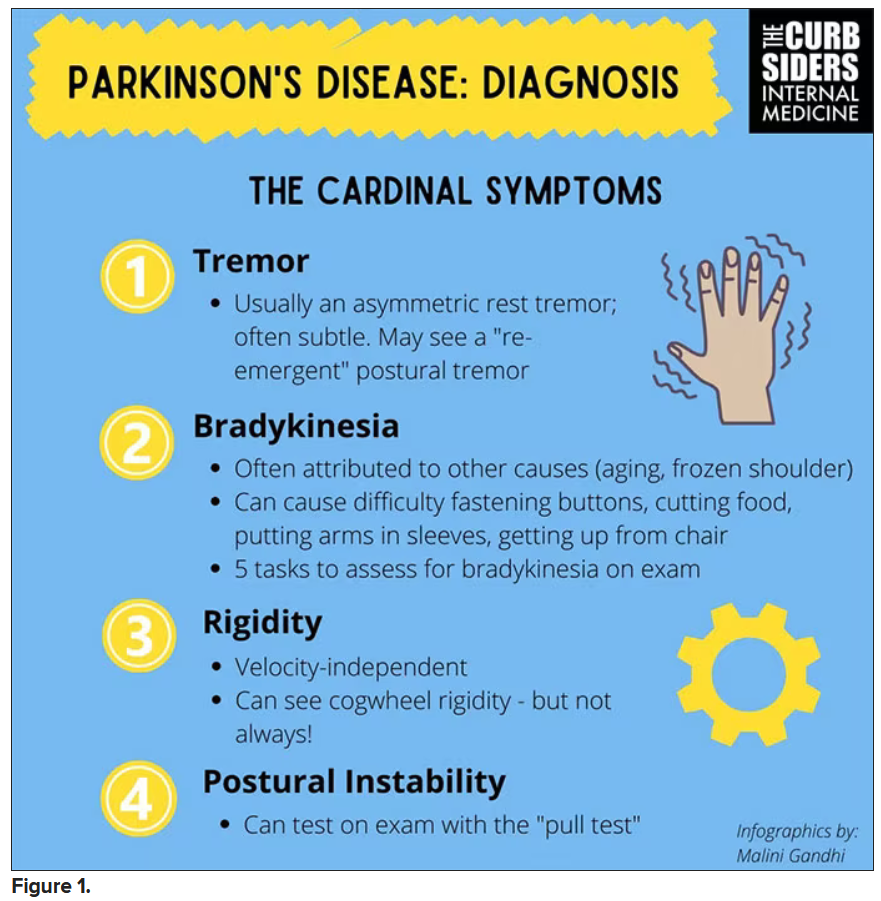
You were deeply upset that cogwheel rigidity was not pathognomonic for Parkinson’s, but you made the point – and our guest agreed – that asymmetry tends to be the key here. And I really appreciated the point about reemergent tremor. This is this idea of a resting tremor. If someone has more parkinsonian features, you might see an intention tremor with essential tremor. If they reach out, it might seem steady at first, but if they hold long enough, then the tremor may kind of reemerge. I thought that was a neat distinction.
And this idea of cogwheel rigidity is a combination of some of the cardinal features of Parkinson’s – it’s a little bit of tremor and a little bit of rigidity too. There’s a baseline increase in tone, and then the tremor is superimposed on top of that. When you’re feeling cogwheeling, that’s actually what you’re feeling on examination. Parkinson’s, with all of its physical exam findings has always fascinated me.
Dr. Watto: He also told us about some red flags.
With classic idiopathic parkinsonism, there’s asymmetric involvement of the tremor. So red flags include a symmetric tremor, which might be something other than idiopathic parkinsonism. He also mentioned that one of the reasons you may want to get imaging (which is not always necessary if someone has a classic presentation), is if you see lower body–predominant symptoms of parkinsonism. These patients have rigidity or slowness of movement in their legs, but their upper bodies are not affected. They don’t have masked facies or the tremor in their hands. You might get an MRI in that case because that could be presentation of vascular dementia or vascular disease in the brain or even normal pressure hydrocephalus, which is a treatable condition. That would be one reason to get imaging.
What if the patient was exposed to a drug like a dopamine antagonist? They will get better in a couple of days, right?
Dr. Williams: This was a really fascinating point because we typically think if a patient’s symptoms are related to a drug exposure – in this case, drug-induced parkinsonism – we can just stop the medication and the symptoms will disappear in a couple of days as the drug leaves the system. But as it turns out, it might take much longer. A mistake that Dr Hung often sees is that the clinician stops the possibly offending agent, but when they don’t see an immediate relief of symptoms, they assume the drug wasn’t causing them. You really have to give the patient a fair shot off the medication to experience recovery because those symptoms can last weeks or even months after the drug is discontinued.
Dr. Watto: Dr Hung looks at the patient’s problem list and asks whether is there any reason this patient might have been exposed to one of these medications?
We’re not going to get too much into specific Parkinson’s treatment, but I was glad to hear that exercise actually improves mobility and may even have some neuroprotective effects. He mentioned ongoing trials looking at that. We always love an excuse to tell patients that they should be moving around more and being physically active.
Dr. Williams: That was one of the more shocking things I learned, that exercise might actually be good for you. That will deeply inform my practice. Many of the treatments that we use for Parkinson’s only address symptoms. They don’t address progression or fix anything, but exercise can help with that.
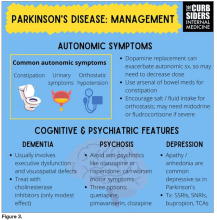
Dr. Watto: Paul, the last question I wanted to ask you is about our role in primary care. Patients with Parkinson’s have autonomic symptoms. They have neurocognitive symptoms. What is our role in that as primary care physicians?
Dr. Williams: Myriad symptoms can accompany Parkinson’s, and we have experience with most of them. We should all feel fairly comfortable dealing with constipation, which can be a very bothersome symptom. And we can use our full arsenal for symptoms such as depression, anxiety, and even apathy – the anhedonia, which apparently can be the predominant feature. We do have the tools to address these problems.
This might be a situation where we might reach for bupropion or a tricyclic antidepressant, which might not be your initial choice for a patient with a possibly annoying mood disorder. But for someone with Parkinson’s disease, this actually may be very helpful. We know how to manage a lot of the symptoms that come along with Parkinson’s that are not just the motor symptoms, and we should take ownership of those things.
Dr. Watto: You can hear the rest of this podcast here. This has been another episode of The Curbsiders bringing you a little knowledge food for your brain hole. Until next time, I’ve been Dr Matthew Frank Watto.
Dr. Williams: And I’m Dr Paul Nelson Williams.
Dr. Watto is a clinical assistant professor, department of medicine, at the University of Pennsylvania, Philadelphia. Dr. Williams is Associate Professor of Clinical Medicine, Department of General Internal Medicine, at Temple University, Philadelphia. Neither Dr. Watto nor Dr. Williams reported any relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Matthew F. Watto, MD: Welcome back to The Curbsiders. We had a great discussion on Parkinson’s Disease for Primary Care with Dr. Albert Hung. Paul, this was something that really made me nervous. I didn’t have a lot of comfort with it. But he taught us a lot of tips about how to recognize Parkinson’s.
I hadn’t been as aware of the premotor symptoms: constipation, hyposmia (loss of sense of smell), and rapid eye movement sleep behavior disorder. If patients have those early on and they aren’t explained by other things (especially the REM sleep behavior disorder), you should really key in because those patients are at risk of developing Parkinson’s years down the line. Those symptoms could present first, which just kind of blew my mind.
What tips do you have about how to recognize Parkinson’s? Do you want to talk about the physical exam?
Paul N. Williams, MD: You know I love the physical exam stuff, so I’m happy to talk about that.
You were deeply upset that cogwheel rigidity was not pathognomonic for Parkinson’s, but you made the point – and our guest agreed – that asymmetry tends to be the key here. And I really appreciated the point about reemergent tremor. This is this idea of a resting tremor. If someone has more parkinsonian features, you might see an intention tremor with essential tremor. If they reach out, it might seem steady at first, but if they hold long enough, then the tremor may kind of reemerge. I thought that was a neat distinction.
And this idea of cogwheel rigidity is a combination of some of the cardinal features of Parkinson’s – it’s a little bit of tremor and a little bit of rigidity too. There’s a baseline increase in tone, and then the tremor is superimposed on top of that. When you’re feeling cogwheeling, that’s actually what you’re feeling on examination. Parkinson’s, with all of its physical exam findings has always fascinated me.
Dr. Watto: He also told us about some red flags.
With classic idiopathic parkinsonism, there’s asymmetric involvement of the tremor. So red flags include a symmetric tremor, which might be something other than idiopathic parkinsonism. He also mentioned that one of the reasons you may want to get imaging (which is not always necessary if someone has a classic presentation), is if you see lower body–predominant symptoms of parkinsonism. These patients have rigidity or slowness of movement in their legs, but their upper bodies are not affected. They don’t have masked facies or the tremor in their hands. You might get an MRI in that case because that could be presentation of vascular dementia or vascular disease in the brain or even normal pressure hydrocephalus, which is a treatable condition. That would be one reason to get imaging.
What if the patient was exposed to a drug like a dopamine antagonist? They will get better in a couple of days, right?
Dr. Williams: This was a really fascinating point because we typically think if a patient’s symptoms are related to a drug exposure – in this case, drug-induced parkinsonism – we can just stop the medication and the symptoms will disappear in a couple of days as the drug leaves the system. But as it turns out, it might take much longer. A mistake that Dr Hung often sees is that the clinician stops the possibly offending agent, but when they don’t see an immediate relief of symptoms, they assume the drug wasn’t causing them. You really have to give the patient a fair shot off the medication to experience recovery because those symptoms can last weeks or even months after the drug is discontinued.
Dr. Watto: Dr Hung looks at the patient’s problem list and asks whether is there any reason this patient might have been exposed to one of these medications?
We’re not going to get too much into specific Parkinson’s treatment, but I was glad to hear that exercise actually improves mobility and may even have some neuroprotective effects. He mentioned ongoing trials looking at that. We always love an excuse to tell patients that they should be moving around more and being physically active.
Dr. Williams: That was one of the more shocking things I learned, that exercise might actually be good for you. That will deeply inform my practice. Many of the treatments that we use for Parkinson’s only address symptoms. They don’t address progression or fix anything, but exercise can help with that.
Dr. Watto: Paul, the last question I wanted to ask you is about our role in primary care. Patients with Parkinson’s have autonomic symptoms. They have neurocognitive symptoms. What is our role in that as primary care physicians?
Dr. Williams: Myriad symptoms can accompany Parkinson’s, and we have experience with most of them. We should all feel fairly comfortable dealing with constipation, which can be a very bothersome symptom. And we can use our full arsenal for symptoms such as depression, anxiety, and even apathy – the anhedonia, which apparently can be the predominant feature. We do have the tools to address these problems.
This might be a situation where we might reach for bupropion or a tricyclic antidepressant, which might not be your initial choice for a patient with a possibly annoying mood disorder. But for someone with Parkinson’s disease, this actually may be very helpful. We know how to manage a lot of the symptoms that come along with Parkinson’s that are not just the motor symptoms, and we should take ownership of those things.
Dr. Watto: You can hear the rest of this podcast here. This has been another episode of The Curbsiders bringing you a little knowledge food for your brain hole. Until next time, I’ve been Dr Matthew Frank Watto.
Dr. Williams: And I’m Dr Paul Nelson Williams.
Dr. Watto is a clinical assistant professor, department of medicine, at the University of Pennsylvania, Philadelphia. Dr. Williams is Associate Professor of Clinical Medicine, Department of General Internal Medicine, at Temple University, Philadelphia. Neither Dr. Watto nor Dr. Williams reported any relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Matthew F. Watto, MD: Welcome back to The Curbsiders. We had a great discussion on Parkinson’s Disease for Primary Care with Dr. Albert Hung. Paul, this was something that really made me nervous. I didn’t have a lot of comfort with it. But he taught us a lot of tips about how to recognize Parkinson’s.
I hadn’t been as aware of the premotor symptoms: constipation, hyposmia (loss of sense of smell), and rapid eye movement sleep behavior disorder. If patients have those early on and they aren’t explained by other things (especially the REM sleep behavior disorder), you should really key in because those patients are at risk of developing Parkinson’s years down the line. Those symptoms could present first, which just kind of blew my mind.
What tips do you have about how to recognize Parkinson’s? Do you want to talk about the physical exam?
Paul N. Williams, MD: You know I love the physical exam stuff, so I’m happy to talk about that.
You were deeply upset that cogwheel rigidity was not pathognomonic for Parkinson’s, but you made the point – and our guest agreed – that asymmetry tends to be the key here. And I really appreciated the point about reemergent tremor. This is this idea of a resting tremor. If someone has more parkinsonian features, you might see an intention tremor with essential tremor. If they reach out, it might seem steady at first, but if they hold long enough, then the tremor may kind of reemerge. I thought that was a neat distinction.
And this idea of cogwheel rigidity is a combination of some of the cardinal features of Parkinson’s – it’s a little bit of tremor and a little bit of rigidity too. There’s a baseline increase in tone, and then the tremor is superimposed on top of that. When you’re feeling cogwheeling, that’s actually what you’re feeling on examination. Parkinson’s, with all of its physical exam findings has always fascinated me.
Dr. Watto: He also told us about some red flags.
With classic idiopathic parkinsonism, there’s asymmetric involvement of the tremor. So red flags include a symmetric tremor, which might be something other than idiopathic parkinsonism. He also mentioned that one of the reasons you may want to get imaging (which is not always necessary if someone has a classic presentation), is if you see lower body–predominant symptoms of parkinsonism. These patients have rigidity or slowness of movement in their legs, but their upper bodies are not affected. They don’t have masked facies or the tremor in their hands. You might get an MRI in that case because that could be presentation of vascular dementia or vascular disease in the brain or even normal pressure hydrocephalus, which is a treatable condition. That would be one reason to get imaging.
What if the patient was exposed to a drug like a dopamine antagonist? They will get better in a couple of days, right?
Dr. Williams: This was a really fascinating point because we typically think if a patient’s symptoms are related to a drug exposure – in this case, drug-induced parkinsonism – we can just stop the medication and the symptoms will disappear in a couple of days as the drug leaves the system. But as it turns out, it might take much longer. A mistake that Dr Hung often sees is that the clinician stops the possibly offending agent, but when they don’t see an immediate relief of symptoms, they assume the drug wasn’t causing them. You really have to give the patient a fair shot off the medication to experience recovery because those symptoms can last weeks or even months after the drug is discontinued.
Dr. Watto: Dr Hung looks at the patient’s problem list and asks whether is there any reason this patient might have been exposed to one of these medications?
We’re not going to get too much into specific Parkinson’s treatment, but I was glad to hear that exercise actually improves mobility and may even have some neuroprotective effects. He mentioned ongoing trials looking at that. We always love an excuse to tell patients that they should be moving around more and being physically active.
Dr. Williams: That was one of the more shocking things I learned, that exercise might actually be good for you. That will deeply inform my practice. Many of the treatments that we use for Parkinson’s only address symptoms. They don’t address progression or fix anything, but exercise can help with that.
Dr. Watto: Paul, the last question I wanted to ask you is about our role in primary care. Patients with Parkinson’s have autonomic symptoms. They have neurocognitive symptoms. What is our role in that as primary care physicians?
Dr. Williams: Myriad symptoms can accompany Parkinson’s, and we have experience with most of them. We should all feel fairly comfortable dealing with constipation, which can be a very bothersome symptom. And we can use our full arsenal for symptoms such as depression, anxiety, and even apathy – the anhedonia, which apparently can be the predominant feature. We do have the tools to address these problems.
This might be a situation where we might reach for bupropion or a tricyclic antidepressant, which might not be your initial choice for a patient with a possibly annoying mood disorder. But for someone with Parkinson’s disease, this actually may be very helpful. We know how to manage a lot of the symptoms that come along with Parkinson’s that are not just the motor symptoms, and we should take ownership of those things.
Dr. Watto: You can hear the rest of this podcast here. This has been another episode of The Curbsiders bringing you a little knowledge food for your brain hole. Until next time, I’ve been Dr Matthew Frank Watto.
Dr. Williams: And I’m Dr Paul Nelson Williams.
Dr. Watto is a clinical assistant professor, department of medicine, at the University of Pennsylvania, Philadelphia. Dr. Williams is Associate Professor of Clinical Medicine, Department of General Internal Medicine, at Temple University, Philadelphia. Neither Dr. Watto nor Dr. Williams reported any relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
Exercise tied to reduced Parkinson’s motor symptoms and increased well-being
A systematic review of 156 clinical trials involving 8,000 patients with Parkinson’s disease showed dancing and aquatic exercise, in particular, were most likely to improve motor symptoms, while swimming, endurance training, and mind-body training were most likely to benefit quality of life.
“For most types of exercise we studied, we observed positive effects on both the severity of motor signs and quality of life. These results highlight the importance of exercise in general, as they suggest people with Parkinson’s disease can benefit from a variety of exercises,” said study investigator Moritz Ernst, MSc, deputy head of the working group on evidence-based medicine at the University Hospital Cologne (Germany).
“Clinicians and people with Parkinson’s disease may have several options of exercise programs to choose from when establishing an individual training routine,” he added, emphasizing that overall those with Parkinson’s disease should seek professional advice, including assessment of motor and nonmotor symptoms, to develop a training agenda based on their individual needs.
The study was published online in the Cochrane Database of Systematic Reviews.
May I have this dance?
The investigators analyzed data from randomized, controlled trials comparing different types of exercise and no exercise and the subsequent effect on Parkinson’s disease symptoms. Exercise included dance, strength-resistance training, mind-body training such as tai chi and yoga, water-based training, resistance training, gait/balance/functional training, and endurance training.
The average age of study participants ranged from 60 to 74 years, and most of the studies included patients with mild to moderate Parkinson’s disease. The mean length of the various interventions was 12 weeks.
When the researchers examined the effect of exercise on motor symptoms, they found that dance (P = .88), aqua-based training (P = .69), and gait/balance/functional training (P = .67) were most likely to reduce symptom severity.
Aqua-based training (P = .95), endurance training (P = .77), and mind-body training (P = .75) were most were most likely to benefit quality of life, although the investigators caution that these findings were at risk of bias because quality of life was self-reported.
The investigators noted other study limitations including the fact that most of the studies included in the review had small sample sizes and their study only included patients with mild to moderate versus severe Parkinson’s disease.
The authors said that future research should include larger samples, report intent-to-treat analyses, and involve participants with more advanced forms of Parkinson’s disease who may also have cognitive difficulties.
Prescribe exercise
“We should be giving our patients, no matter where they are in their disease stage, a ‘prescription’ to exercise,” said Mitra Afshari, MD, MPH. Dr. Afshari was not involved in the study but leads her own research on Parkinson’s disease and exercise as the site principal investigator on the National Institutes of Health–funded SPARX3 Study in Parkinson’s Disease and Exercise at Rush University in Chicago. She said that, based on her experience caring for patients with Parkinson’s disease at all disease stages, “patients who have been physically active their whole lives and can maintain that activity despite their diagnosis fare the best.”
However, she added, those who initiate physical exercise after diagnosis can also do very well and reap benefits, including improved motor symptoms.
The study was funded by University Hospital of Cologne, Faculty of Medicine and University Hospital, University of Cologne, and the German Ministry of Education and Research. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A systematic review of 156 clinical trials involving 8,000 patients with Parkinson’s disease showed dancing and aquatic exercise, in particular, were most likely to improve motor symptoms, while swimming, endurance training, and mind-body training were most likely to benefit quality of life.
“For most types of exercise we studied, we observed positive effects on both the severity of motor signs and quality of life. These results highlight the importance of exercise in general, as they suggest people with Parkinson’s disease can benefit from a variety of exercises,” said study investigator Moritz Ernst, MSc, deputy head of the working group on evidence-based medicine at the University Hospital Cologne (Germany).
“Clinicians and people with Parkinson’s disease may have several options of exercise programs to choose from when establishing an individual training routine,” he added, emphasizing that overall those with Parkinson’s disease should seek professional advice, including assessment of motor and nonmotor symptoms, to develop a training agenda based on their individual needs.
The study was published online in the Cochrane Database of Systematic Reviews.
May I have this dance?
The investigators analyzed data from randomized, controlled trials comparing different types of exercise and no exercise and the subsequent effect on Parkinson’s disease symptoms. Exercise included dance, strength-resistance training, mind-body training such as tai chi and yoga, water-based training, resistance training, gait/balance/functional training, and endurance training.
The average age of study participants ranged from 60 to 74 years, and most of the studies included patients with mild to moderate Parkinson’s disease. The mean length of the various interventions was 12 weeks.
When the researchers examined the effect of exercise on motor symptoms, they found that dance (P = .88), aqua-based training (P = .69), and gait/balance/functional training (P = .67) were most likely to reduce symptom severity.
Aqua-based training (P = .95), endurance training (P = .77), and mind-body training (P = .75) were most were most likely to benefit quality of life, although the investigators caution that these findings were at risk of bias because quality of life was self-reported.
The investigators noted other study limitations including the fact that most of the studies included in the review had small sample sizes and their study only included patients with mild to moderate versus severe Parkinson’s disease.
The authors said that future research should include larger samples, report intent-to-treat analyses, and involve participants with more advanced forms of Parkinson’s disease who may also have cognitive difficulties.
Prescribe exercise
“We should be giving our patients, no matter where they are in their disease stage, a ‘prescription’ to exercise,” said Mitra Afshari, MD, MPH. Dr. Afshari was not involved in the study but leads her own research on Parkinson’s disease and exercise as the site principal investigator on the National Institutes of Health–funded SPARX3 Study in Parkinson’s Disease and Exercise at Rush University in Chicago. She said that, based on her experience caring for patients with Parkinson’s disease at all disease stages, “patients who have been physically active their whole lives and can maintain that activity despite their diagnosis fare the best.”
However, she added, those who initiate physical exercise after diagnosis can also do very well and reap benefits, including improved motor symptoms.
The study was funded by University Hospital of Cologne, Faculty of Medicine and University Hospital, University of Cologne, and the German Ministry of Education and Research. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A systematic review of 156 clinical trials involving 8,000 patients with Parkinson’s disease showed dancing and aquatic exercise, in particular, were most likely to improve motor symptoms, while swimming, endurance training, and mind-body training were most likely to benefit quality of life.
“For most types of exercise we studied, we observed positive effects on both the severity of motor signs and quality of life. These results highlight the importance of exercise in general, as they suggest people with Parkinson’s disease can benefit from a variety of exercises,” said study investigator Moritz Ernst, MSc, deputy head of the working group on evidence-based medicine at the University Hospital Cologne (Germany).
“Clinicians and people with Parkinson’s disease may have several options of exercise programs to choose from when establishing an individual training routine,” he added, emphasizing that overall those with Parkinson’s disease should seek professional advice, including assessment of motor and nonmotor symptoms, to develop a training agenda based on their individual needs.
The study was published online in the Cochrane Database of Systematic Reviews.
May I have this dance?
The investigators analyzed data from randomized, controlled trials comparing different types of exercise and no exercise and the subsequent effect on Parkinson’s disease symptoms. Exercise included dance, strength-resistance training, mind-body training such as tai chi and yoga, water-based training, resistance training, gait/balance/functional training, and endurance training.
The average age of study participants ranged from 60 to 74 years, and most of the studies included patients with mild to moderate Parkinson’s disease. The mean length of the various interventions was 12 weeks.
When the researchers examined the effect of exercise on motor symptoms, they found that dance (P = .88), aqua-based training (P = .69), and gait/balance/functional training (P = .67) were most likely to reduce symptom severity.
Aqua-based training (P = .95), endurance training (P = .77), and mind-body training (P = .75) were most were most likely to benefit quality of life, although the investigators caution that these findings were at risk of bias because quality of life was self-reported.
The investigators noted other study limitations including the fact that most of the studies included in the review had small sample sizes and their study only included patients with mild to moderate versus severe Parkinson’s disease.
The authors said that future research should include larger samples, report intent-to-treat analyses, and involve participants with more advanced forms of Parkinson’s disease who may also have cognitive difficulties.
Prescribe exercise
“We should be giving our patients, no matter where they are in their disease stage, a ‘prescription’ to exercise,” said Mitra Afshari, MD, MPH. Dr. Afshari was not involved in the study but leads her own research on Parkinson’s disease and exercise as the site principal investigator on the National Institutes of Health–funded SPARX3 Study in Parkinson’s Disease and Exercise at Rush University in Chicago. She said that, based on her experience caring for patients with Parkinson’s disease at all disease stages, “patients who have been physically active their whole lives and can maintain that activity despite their diagnosis fare the best.”
However, she added, those who initiate physical exercise after diagnosis can also do very well and reap benefits, including improved motor symptoms.
The study was funded by University Hospital of Cologne, Faculty of Medicine and University Hospital, University of Cologne, and the German Ministry of Education and Research. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE COCHRANE DATABASE OF SYSTEMATIC REVIEWS
What’s driving the "world’s fastest-growing brain disease"?
An international team of researchers reviewed previous research and cited data that suggest the chemical trichloroethylene (TCE) is associated with as much as a 500% increased risk for Parkinson’s disease (PD).
Lead investigator Ray Dorsey, MD, professor of neurology, University of Rochester, N.Y., called PD “the world’s fastest-growing brain disease,” and told this news organization that it “may be largely preventable.”
“Countless people have died over generations from cancer and other disease linked to TCE [and] Parkinson’s may be the latest,” he said. “Banning these chemicals, containing contaminated sites, and protecting homes, schools, and buildings at risk may all create a world where Parkinson’s is increasingly rare, not common.”
The paper was published online in the Journal of Parkinson’s Disease.
Invisible, ubiquitous
TCE was first synthesized in a lab in 1864, with commercial production beginning in 1920, the researchers noted.
“Because of its unique properties, TCE has had countless industrial, commercial, military, and medical applications,” including producing refrigerants, cleaning electronics, and degreasing engine parts.
In addition, it’s been used in dry cleaning, although a similar chemical (perchloroethylene [PCE]) is currently more widely used for that purpose. Nevertheless, the authors noted, in anaerobic conditions, perchloroethylene often transforms into TCE “and their toxicity may be similar.”
Consumer products in which TCE is found include typewriter correction fluid, paint removers, gun cleaners, and aerosol cleaning products. Up until the 1970s, it was used to decaffeinate coffee.
TCE exposure isn’t confined to those who work with it. It also pollutes outdoor air, taints groundwater, and contaminates indoor air. It’s present in a substantial amount of groundwater in the United States and it “evaporates from underlying soil and groundwater and enters homes, workplaces, or schools, often undetected,” the researchers noted.
“Exposure can come via occupation or the environment and is often largely unknown at the time it occurs,” Dr. Dorsey said.
He noted that the rapid increase in PD incidence cannot be explained by genetic factors alone, which affect only about 15% of patients with PD, nor can it be explained by aging alone. “Certain pesticides ... are likely causes but would not explain the high prevalence of PD in urban areas, as is the case in the U.S.” Rather, “other factors” are involved, and “TCE is likely one such factor.”
Yet, “despite widespread contamination and increasing industrial, commercial, and military use, clinical investigations of TCE and PD have been limited.”
To fill this knowledge gap, Dr. Dorsey and his coauthors of the book, “Ending Parkinson’s Disease: A Prescription for Action,” took a deep dive into studies focusing on the potential association of TCE and PD and presented seven cases to illustrate the association.
“Like many genetic mutations (e.g., Parkin) and other environmental toxicants ... TCE damages the energy-producing parts of cells, i.e., the mitochondria,” said Dr. Dorsey.
TCE and PCE “likely mediate their toxicity through a common metabolite.” Because both are lipophilic, they “readily distribute in the brain and body tissues and appear to cause mitochondrial dysfunction at high doses,” the researchers hypothesized.
Dopaminergic neurons are particularly sensitive to mitochondrial neurotoxicants, so this might “partially explain the link to PD.”
Animal studies have shown that TCE “caused selective loss of dopaminergic neurons.” Moreover, PD-related neuropathology was found in the substantia nigra of rodents exposed to TCE over time. In addition, studies as early as 1960 were showing an association between TCE and parkinsonism.
The authors describe TCE as “ubiquitous” in the 1970s, with 10 million Americans working with the chemical or other organic solvents daily. The review details an extensive list of industries and occupations in which TCE exposure continues to occur.
People working with TCE might inhale it or touch it; but “millions more encounter the chemical unknowingly through outdoor air, contaminated groundwater, and indoor air pollution.”
They noted that TCE contaminates up to one-third of U.S. drinking water, has polluted the groundwater in more than 20 different countries on five continents, and is found in half of the 1,300 most toxic “Superfund” sites that are “part of a federal clean-up program, including 15 in California’s Silicon Valley, where TCE was used to clean electronics.”
Although the U.S. military stopped using TCE, numerous sites have been contaminated, including Marine Corps Base Camp Lejeune in North Carolina, where TCE and PCE were found in drinking water at 280 times the recommended safety standards.
The researchers highlighted seven cases of individuals who developed PD after likely exposure to TCE, including NBA basketball player Brian Grant, who developed symptoms of PD in 2006 at the age of 34.
Mr. Grant and his family had lived in Camp Lejeune when he was a child, during which time he drank, bathed, and swam in contaminated water, “unaware of its toxicity.” His father also died of esophageal cancer, “which is linked to TCE,” the authors of the study wrote. Mr. Grant has created a foundation to inspire and support patients with PD.
All of the individuals either grew up in or spent time in an area where they were extensively exposed to TCE, PCE, or other chemicals, or experienced occupational exposure.
The authors acknowledged that the role of TCE in PD, as illustrated by the cases, is “far from definitive.” For example, exposure to TCE is often combined with exposure to other toxins, or with unmeasured genetic risk factors.
They highlighted the need for more research and called for cleaning and containing contaminated sites, monitoring TCE levels, and publicly communicating risk and a ban on TCE.
Recall bias?
Commenting for this news organization, Rebecca Gilbert, MD, PhD, chief scientific officer, American Parkinson Disease Association (APDA), noted that the authors “are very frank about the limitations of this approach [illustrative cases] as proof of causation between PD and TCE exposure.”
Another limitation is that TCE exposure is very common, “as argued in the paper.” But “most people with exposure do not develop PD,” Dr. Gilbert pointed out. “By probing the TCE exposure of those who already have PD, there is a danger of recall bias.”
Dr. Gilbert, associate professor of neurology at NYU Langone Health, who was not involved with the study, acknowledged that the authors “present their work as hypothesis and clearly state that more work is needed to understand the connection between TCE and PD.”
In the meantime, however, there are “well-established health risks of TCE exposure, including development of various cancers,” she said. Therefore, the authors’ goals appear to be educating the public about known health risks, working to clean up known sites of contamination, and advocating to ban future use of TCE.
These goals “do not need to wait for [proof of] firm causation between TCE and PD,” she stated.
Dr. Dorsey reported he has received honoraria for speaking at the American Academy of Neurology and at multiple other societies and foundations and has received compensation for consulting services from pharmaceutical companies, foundations, medical education companies, and medical publications; he owns stock in several companies. The other authors’ disclosures can be found in the original paper. Dr. Gilbert is employed by the American Parkinson Disease Association and Bellevue Hospital Center in New York City.
A version of this article first appeared on Medscape.com.
An international team of researchers reviewed previous research and cited data that suggest the chemical trichloroethylene (TCE) is associated with as much as a 500% increased risk for Parkinson’s disease (PD).
Lead investigator Ray Dorsey, MD, professor of neurology, University of Rochester, N.Y., called PD “the world’s fastest-growing brain disease,” and told this news organization that it “may be largely preventable.”
“Countless people have died over generations from cancer and other disease linked to TCE [and] Parkinson’s may be the latest,” he said. “Banning these chemicals, containing contaminated sites, and protecting homes, schools, and buildings at risk may all create a world where Parkinson’s is increasingly rare, not common.”
The paper was published online in the Journal of Parkinson’s Disease.
Invisible, ubiquitous
TCE was first synthesized in a lab in 1864, with commercial production beginning in 1920, the researchers noted.
“Because of its unique properties, TCE has had countless industrial, commercial, military, and medical applications,” including producing refrigerants, cleaning electronics, and degreasing engine parts.
In addition, it’s been used in dry cleaning, although a similar chemical (perchloroethylene [PCE]) is currently more widely used for that purpose. Nevertheless, the authors noted, in anaerobic conditions, perchloroethylene often transforms into TCE “and their toxicity may be similar.”
Consumer products in which TCE is found include typewriter correction fluid, paint removers, gun cleaners, and aerosol cleaning products. Up until the 1970s, it was used to decaffeinate coffee.
TCE exposure isn’t confined to those who work with it. It also pollutes outdoor air, taints groundwater, and contaminates indoor air. It’s present in a substantial amount of groundwater in the United States and it “evaporates from underlying soil and groundwater and enters homes, workplaces, or schools, often undetected,” the researchers noted.
“Exposure can come via occupation or the environment and is often largely unknown at the time it occurs,” Dr. Dorsey said.
He noted that the rapid increase in PD incidence cannot be explained by genetic factors alone, which affect only about 15% of patients with PD, nor can it be explained by aging alone. “Certain pesticides ... are likely causes but would not explain the high prevalence of PD in urban areas, as is the case in the U.S.” Rather, “other factors” are involved, and “TCE is likely one such factor.”
Yet, “despite widespread contamination and increasing industrial, commercial, and military use, clinical investigations of TCE and PD have been limited.”
To fill this knowledge gap, Dr. Dorsey and his coauthors of the book, “Ending Parkinson’s Disease: A Prescription for Action,” took a deep dive into studies focusing on the potential association of TCE and PD and presented seven cases to illustrate the association.
“Like many genetic mutations (e.g., Parkin) and other environmental toxicants ... TCE damages the energy-producing parts of cells, i.e., the mitochondria,” said Dr. Dorsey.
TCE and PCE “likely mediate their toxicity through a common metabolite.” Because both are lipophilic, they “readily distribute in the brain and body tissues and appear to cause mitochondrial dysfunction at high doses,” the researchers hypothesized.
Dopaminergic neurons are particularly sensitive to mitochondrial neurotoxicants, so this might “partially explain the link to PD.”
Animal studies have shown that TCE “caused selective loss of dopaminergic neurons.” Moreover, PD-related neuropathology was found in the substantia nigra of rodents exposed to TCE over time. In addition, studies as early as 1960 were showing an association between TCE and parkinsonism.
The authors describe TCE as “ubiquitous” in the 1970s, with 10 million Americans working with the chemical or other organic solvents daily. The review details an extensive list of industries and occupations in which TCE exposure continues to occur.
People working with TCE might inhale it or touch it; but “millions more encounter the chemical unknowingly through outdoor air, contaminated groundwater, and indoor air pollution.”
They noted that TCE contaminates up to one-third of U.S. drinking water, has polluted the groundwater in more than 20 different countries on five continents, and is found in half of the 1,300 most toxic “Superfund” sites that are “part of a federal clean-up program, including 15 in California’s Silicon Valley, where TCE was used to clean electronics.”
Although the U.S. military stopped using TCE, numerous sites have been contaminated, including Marine Corps Base Camp Lejeune in North Carolina, where TCE and PCE were found in drinking water at 280 times the recommended safety standards.
The researchers highlighted seven cases of individuals who developed PD after likely exposure to TCE, including NBA basketball player Brian Grant, who developed symptoms of PD in 2006 at the age of 34.
Mr. Grant and his family had lived in Camp Lejeune when he was a child, during which time he drank, bathed, and swam in contaminated water, “unaware of its toxicity.” His father also died of esophageal cancer, “which is linked to TCE,” the authors of the study wrote. Mr. Grant has created a foundation to inspire and support patients with PD.
All of the individuals either grew up in or spent time in an area where they were extensively exposed to TCE, PCE, or other chemicals, or experienced occupational exposure.
The authors acknowledged that the role of TCE in PD, as illustrated by the cases, is “far from definitive.” For example, exposure to TCE is often combined with exposure to other toxins, or with unmeasured genetic risk factors.
They highlighted the need for more research and called for cleaning and containing contaminated sites, monitoring TCE levels, and publicly communicating risk and a ban on TCE.
Recall bias?
Commenting for this news organization, Rebecca Gilbert, MD, PhD, chief scientific officer, American Parkinson Disease Association (APDA), noted that the authors “are very frank about the limitations of this approach [illustrative cases] as proof of causation between PD and TCE exposure.”
Another limitation is that TCE exposure is very common, “as argued in the paper.” But “most people with exposure do not develop PD,” Dr. Gilbert pointed out. “By probing the TCE exposure of those who already have PD, there is a danger of recall bias.”
Dr. Gilbert, associate professor of neurology at NYU Langone Health, who was not involved with the study, acknowledged that the authors “present their work as hypothesis and clearly state that more work is needed to understand the connection between TCE and PD.”
In the meantime, however, there are “well-established health risks of TCE exposure, including development of various cancers,” she said. Therefore, the authors’ goals appear to be educating the public about known health risks, working to clean up known sites of contamination, and advocating to ban future use of TCE.
These goals “do not need to wait for [proof of] firm causation between TCE and PD,” she stated.
Dr. Dorsey reported he has received honoraria for speaking at the American Academy of Neurology and at multiple other societies and foundations and has received compensation for consulting services from pharmaceutical companies, foundations, medical education companies, and medical publications; he owns stock in several companies. The other authors’ disclosures can be found in the original paper. Dr. Gilbert is employed by the American Parkinson Disease Association and Bellevue Hospital Center in New York City.
A version of this article first appeared on Medscape.com.
An international team of researchers reviewed previous research and cited data that suggest the chemical trichloroethylene (TCE) is associated with as much as a 500% increased risk for Parkinson’s disease (PD).
Lead investigator Ray Dorsey, MD, professor of neurology, University of Rochester, N.Y., called PD “the world’s fastest-growing brain disease,” and told this news organization that it “may be largely preventable.”
“Countless people have died over generations from cancer and other disease linked to TCE [and] Parkinson’s may be the latest,” he said. “Banning these chemicals, containing contaminated sites, and protecting homes, schools, and buildings at risk may all create a world where Parkinson’s is increasingly rare, not common.”
The paper was published online in the Journal of Parkinson’s Disease.
Invisible, ubiquitous
TCE was first synthesized in a lab in 1864, with commercial production beginning in 1920, the researchers noted.
“Because of its unique properties, TCE has had countless industrial, commercial, military, and medical applications,” including producing refrigerants, cleaning electronics, and degreasing engine parts.
In addition, it’s been used in dry cleaning, although a similar chemical (perchloroethylene [PCE]) is currently more widely used for that purpose. Nevertheless, the authors noted, in anaerobic conditions, perchloroethylene often transforms into TCE “and their toxicity may be similar.”
Consumer products in which TCE is found include typewriter correction fluid, paint removers, gun cleaners, and aerosol cleaning products. Up until the 1970s, it was used to decaffeinate coffee.
TCE exposure isn’t confined to those who work with it. It also pollutes outdoor air, taints groundwater, and contaminates indoor air. It’s present in a substantial amount of groundwater in the United States and it “evaporates from underlying soil and groundwater and enters homes, workplaces, or schools, often undetected,” the researchers noted.
“Exposure can come via occupation or the environment and is often largely unknown at the time it occurs,” Dr. Dorsey said.
He noted that the rapid increase in PD incidence cannot be explained by genetic factors alone, which affect only about 15% of patients with PD, nor can it be explained by aging alone. “Certain pesticides ... are likely causes but would not explain the high prevalence of PD in urban areas, as is the case in the U.S.” Rather, “other factors” are involved, and “TCE is likely one such factor.”
Yet, “despite widespread contamination and increasing industrial, commercial, and military use, clinical investigations of TCE and PD have been limited.”
To fill this knowledge gap, Dr. Dorsey and his coauthors of the book, “Ending Parkinson’s Disease: A Prescription for Action,” took a deep dive into studies focusing on the potential association of TCE and PD and presented seven cases to illustrate the association.
“Like many genetic mutations (e.g., Parkin) and other environmental toxicants ... TCE damages the energy-producing parts of cells, i.e., the mitochondria,” said Dr. Dorsey.
TCE and PCE “likely mediate their toxicity through a common metabolite.” Because both are lipophilic, they “readily distribute in the brain and body tissues and appear to cause mitochondrial dysfunction at high doses,” the researchers hypothesized.
Dopaminergic neurons are particularly sensitive to mitochondrial neurotoxicants, so this might “partially explain the link to PD.”
Animal studies have shown that TCE “caused selective loss of dopaminergic neurons.” Moreover, PD-related neuropathology was found in the substantia nigra of rodents exposed to TCE over time. In addition, studies as early as 1960 were showing an association between TCE and parkinsonism.
The authors describe TCE as “ubiquitous” in the 1970s, with 10 million Americans working with the chemical or other organic solvents daily. The review details an extensive list of industries and occupations in which TCE exposure continues to occur.
People working with TCE might inhale it or touch it; but “millions more encounter the chemical unknowingly through outdoor air, contaminated groundwater, and indoor air pollution.”
They noted that TCE contaminates up to one-third of U.S. drinking water, has polluted the groundwater in more than 20 different countries on five continents, and is found in half of the 1,300 most toxic “Superfund” sites that are “part of a federal clean-up program, including 15 in California’s Silicon Valley, where TCE was used to clean electronics.”
Although the U.S. military stopped using TCE, numerous sites have been contaminated, including Marine Corps Base Camp Lejeune in North Carolina, where TCE and PCE were found in drinking water at 280 times the recommended safety standards.
The researchers highlighted seven cases of individuals who developed PD after likely exposure to TCE, including NBA basketball player Brian Grant, who developed symptoms of PD in 2006 at the age of 34.
Mr. Grant and his family had lived in Camp Lejeune when he was a child, during which time he drank, bathed, and swam in contaminated water, “unaware of its toxicity.” His father also died of esophageal cancer, “which is linked to TCE,” the authors of the study wrote. Mr. Grant has created a foundation to inspire and support patients with PD.
All of the individuals either grew up in or spent time in an area where they were extensively exposed to TCE, PCE, or other chemicals, or experienced occupational exposure.
The authors acknowledged that the role of TCE in PD, as illustrated by the cases, is “far from definitive.” For example, exposure to TCE is often combined with exposure to other toxins, or with unmeasured genetic risk factors.
They highlighted the need for more research and called for cleaning and containing contaminated sites, monitoring TCE levels, and publicly communicating risk and a ban on TCE.
Recall bias?
Commenting for this news organization, Rebecca Gilbert, MD, PhD, chief scientific officer, American Parkinson Disease Association (APDA), noted that the authors “are very frank about the limitations of this approach [illustrative cases] as proof of causation between PD and TCE exposure.”
Another limitation is that TCE exposure is very common, “as argued in the paper.” But “most people with exposure do not develop PD,” Dr. Gilbert pointed out. “By probing the TCE exposure of those who already have PD, there is a danger of recall bias.”
Dr. Gilbert, associate professor of neurology at NYU Langone Health, who was not involved with the study, acknowledged that the authors “present their work as hypothesis and clearly state that more work is needed to understand the connection between TCE and PD.”
In the meantime, however, there are “well-established health risks of TCE exposure, including development of various cancers,” she said. Therefore, the authors’ goals appear to be educating the public about known health risks, working to clean up known sites of contamination, and advocating to ban future use of TCE.
These goals “do not need to wait for [proof of] firm causation between TCE and PD,” she stated.
Dr. Dorsey reported he has received honoraria for speaking at the American Academy of Neurology and at multiple other societies and foundations and has received compensation for consulting services from pharmaceutical companies, foundations, medical education companies, and medical publications; he owns stock in several companies. The other authors’ disclosures can be found in the original paper. Dr. Gilbert is employed by the American Parkinson Disease Association and Bellevue Hospital Center in New York City.
A version of this article first appeared on Medscape.com.
FROM JOURNAL OF PARKINSON’S DISEASE
Focused ultrasound ablation reduces dyskinesia in Parkinson’s disease
, new research shows.
The technique requires no sedation or brain implants. Surgeons use MRI to identify the globus pallidus internus, a part of the basal ganglia involved in movement disorders, and a focused ultrasound beam to heat and destroy the tissue.
Investigators performed the procedure with a device called Exablate Neuro, which was first approved by the Food and Drug Administration in 2016 to treat essential tremor.
On the basis of the results of a multicenter, randomized, sham-controlled trial, the agency expanded the indication in 2021 to include unilateral pallidotomy to treat advanced Parkinson’s disease for patients with mobility, rigidity, or dyskinesia symptoms.
“In some patients with Parkinson’s disease, you get dyskinesias, and ablation of the globus pallidus significantly reduces those dyskinesias and motor impairment,” said lead investigator Vibhor Krishna, MD, associate professor of neurosurgery at the University of North Carolina at Chapel Hill. “It could be used to treat patients when other surgical procedures can’t be applied.”
The study was published online in the New England Journal of Medicine.
Strong response
For the study, 94 patients with advanced Parkinson’s disease who had dyskinesias or motor fluctuations and motor impairment in the off-medication state wore transducer helmets while lying in an MRI scanner. Patients were awake during the entire procedure.
The treatment group received unilateral FUSA on the side of the brain with the greatest motor impairment. The device initially delivered target temperatures of 40°-45° C. Ablative temperatures were gradually increased following evaluations to test for improvement of motor symptoms. The maximum temperature used was 54.3° C.
Patients in the control group underwent an identical procedure with the sonication energy disabled.
The primary outcome was a response to therapy at 3 months, defined as a decrease of at least three points from baseline either in the score on the Movement Disorders Society–Unified Parkinson’s Disease Rating Scale (MDS-UPDRS), part III, while off medication or in the score on the Unified Dyskinesia Rating Scale (UDRS) while on medication.
At 3 months, 69% of the treatment group reported a response, compared with 32% of the control group (P = .003).
When researchers analyzed MDS-UPDRS scores, they found that 29% of the treatment group and 27% of the control group showed improvement. For UDRS scores, 12% of the treatment group demonstrated improvement. In the control group, there was no improvement on this score. Improvements in both scores were reported in 28% of the treatment group and 5% of the control group.
Among those who reported a response at 3 months, 77% continued to show a response at 12 months.
‘Unforgiving’ area of the brain
While the response rate was a promising sign of this finding, it was not what interested Dr. Krishna the most. “The most surprising finding of this trial is how safe focused ultrasound pallidotomy is in treating patients with Parkinson’s disease,” he said.
The globus pallidus internus is an area of the brain that Dr. Krishna calls “unforgiving.”
“One side is motor fibers, and any problem with that can paralyze the patient, and just below that is the optic tract, and any problem there, you would lose vision,” Dr. Krishna said. “It is a very tough neighborhood to be in.”
By using MRI-guided ultrasound, surgeons can change the target and temperature of the ultrasound beam during the procedure to allow more precise treatment.
Pallidotomy-related adverse events in the treatment group included dysarthria, gait disturbance, loss of taste, visual disturbance, and facial weakness. All were mild to moderate, Dr. Krishna said.
More study is needed
Dyskinesia is a challenge in the management of Parkinson’s disease. Patients need antiparkinsonian medications to slow deterioration of motor function, but those medications can cause the involuntary movements that are a hallmark of dyskinesia.
The most common treatment for this complication, deep-brain stimulation (DBS), has its own drawbacks. It’s an open procedure, and there is a low-level risk for intracranial bleeding and infection. In addition, the electrode implants require ongoing maintenance and adjustment.
But the findings of this study show that, for patients who aren’t candidates for other therapies, such as DBS and ablative radiofrequency, FUSA may be an alternative, wrote Anette Schrag, PhD, professor of clinical neurosciences at University College London, in an accompanying commentary.
“The results confirm that it is effective in reducing motor complications of Parkinson’s disease, at least in the short term,” Dr. Schrag wrote. However, more long-term studies are needed, she added.
One-third of patients in the treatment group had no response to the treatment, and investigators aren’t sure why. Dr. Krishna noted that the benefits of the procedure waned in about a quarter of patients within a year of treatment.
Investigators plan to probe these questions in future trials.
“The results of this trial are promising,” Dr. Schrag wrote, “but given the nonreversible nature of the intervention and the progressive nature of the disease, it will be important to establish whether improvements in motor complications are maintained over longer periods and whether treatment results in improved overall functioning and quality of life for patients.”
The study was funded by Insightec. Disclosure forms for Dr. Krishna and Dr. Schrag are provided on the journal’s website.
A version of this article originally appeared on Medscape.com.
, new research shows.
The technique requires no sedation or brain implants. Surgeons use MRI to identify the globus pallidus internus, a part of the basal ganglia involved in movement disorders, and a focused ultrasound beam to heat and destroy the tissue.
Investigators performed the procedure with a device called Exablate Neuro, which was first approved by the Food and Drug Administration in 2016 to treat essential tremor.
On the basis of the results of a multicenter, randomized, sham-controlled trial, the agency expanded the indication in 2021 to include unilateral pallidotomy to treat advanced Parkinson’s disease for patients with mobility, rigidity, or dyskinesia symptoms.
“In some patients with Parkinson’s disease, you get dyskinesias, and ablation of the globus pallidus significantly reduces those dyskinesias and motor impairment,” said lead investigator Vibhor Krishna, MD, associate professor of neurosurgery at the University of North Carolina at Chapel Hill. “It could be used to treat patients when other surgical procedures can’t be applied.”
The study was published online in the New England Journal of Medicine.
Strong response
For the study, 94 patients with advanced Parkinson’s disease who had dyskinesias or motor fluctuations and motor impairment in the off-medication state wore transducer helmets while lying in an MRI scanner. Patients were awake during the entire procedure.
The treatment group received unilateral FUSA on the side of the brain with the greatest motor impairment. The device initially delivered target temperatures of 40°-45° C. Ablative temperatures were gradually increased following evaluations to test for improvement of motor symptoms. The maximum temperature used was 54.3° C.
Patients in the control group underwent an identical procedure with the sonication energy disabled.
The primary outcome was a response to therapy at 3 months, defined as a decrease of at least three points from baseline either in the score on the Movement Disorders Society–Unified Parkinson’s Disease Rating Scale (MDS-UPDRS), part III, while off medication or in the score on the Unified Dyskinesia Rating Scale (UDRS) while on medication.
At 3 months, 69% of the treatment group reported a response, compared with 32% of the control group (P = .003).
When researchers analyzed MDS-UPDRS scores, they found that 29% of the treatment group and 27% of the control group showed improvement. For UDRS scores, 12% of the treatment group demonstrated improvement. In the control group, there was no improvement on this score. Improvements in both scores were reported in 28% of the treatment group and 5% of the control group.
Among those who reported a response at 3 months, 77% continued to show a response at 12 months.
‘Unforgiving’ area of the brain
While the response rate was a promising sign of this finding, it was not what interested Dr. Krishna the most. “The most surprising finding of this trial is how safe focused ultrasound pallidotomy is in treating patients with Parkinson’s disease,” he said.
The globus pallidus internus is an area of the brain that Dr. Krishna calls “unforgiving.”
“One side is motor fibers, and any problem with that can paralyze the patient, and just below that is the optic tract, and any problem there, you would lose vision,” Dr. Krishna said. “It is a very tough neighborhood to be in.”
By using MRI-guided ultrasound, surgeons can change the target and temperature of the ultrasound beam during the procedure to allow more precise treatment.
Pallidotomy-related adverse events in the treatment group included dysarthria, gait disturbance, loss of taste, visual disturbance, and facial weakness. All were mild to moderate, Dr. Krishna said.
More study is needed
Dyskinesia is a challenge in the management of Parkinson’s disease. Patients need antiparkinsonian medications to slow deterioration of motor function, but those medications can cause the involuntary movements that are a hallmark of dyskinesia.
The most common treatment for this complication, deep-brain stimulation (DBS), has its own drawbacks. It’s an open procedure, and there is a low-level risk for intracranial bleeding and infection. In addition, the electrode implants require ongoing maintenance and adjustment.
But the findings of this study show that, for patients who aren’t candidates for other therapies, such as DBS and ablative radiofrequency, FUSA may be an alternative, wrote Anette Schrag, PhD, professor of clinical neurosciences at University College London, in an accompanying commentary.
“The results confirm that it is effective in reducing motor complications of Parkinson’s disease, at least in the short term,” Dr. Schrag wrote. However, more long-term studies are needed, she added.
One-third of patients in the treatment group had no response to the treatment, and investigators aren’t sure why. Dr. Krishna noted that the benefits of the procedure waned in about a quarter of patients within a year of treatment.
Investigators plan to probe these questions in future trials.
“The results of this trial are promising,” Dr. Schrag wrote, “but given the nonreversible nature of the intervention and the progressive nature of the disease, it will be important to establish whether improvements in motor complications are maintained over longer periods and whether treatment results in improved overall functioning and quality of life for patients.”
The study was funded by Insightec. Disclosure forms for Dr. Krishna and Dr. Schrag are provided on the journal’s website.
A version of this article originally appeared on Medscape.com.
, new research shows.
The technique requires no sedation or brain implants. Surgeons use MRI to identify the globus pallidus internus, a part of the basal ganglia involved in movement disorders, and a focused ultrasound beam to heat and destroy the tissue.
Investigators performed the procedure with a device called Exablate Neuro, which was first approved by the Food and Drug Administration in 2016 to treat essential tremor.
On the basis of the results of a multicenter, randomized, sham-controlled trial, the agency expanded the indication in 2021 to include unilateral pallidotomy to treat advanced Parkinson’s disease for patients with mobility, rigidity, or dyskinesia symptoms.
“In some patients with Parkinson’s disease, you get dyskinesias, and ablation of the globus pallidus significantly reduces those dyskinesias and motor impairment,” said lead investigator Vibhor Krishna, MD, associate professor of neurosurgery at the University of North Carolina at Chapel Hill. “It could be used to treat patients when other surgical procedures can’t be applied.”
The study was published online in the New England Journal of Medicine.
Strong response
For the study, 94 patients with advanced Parkinson’s disease who had dyskinesias or motor fluctuations and motor impairment in the off-medication state wore transducer helmets while lying in an MRI scanner. Patients were awake during the entire procedure.
The treatment group received unilateral FUSA on the side of the brain with the greatest motor impairment. The device initially delivered target temperatures of 40°-45° C. Ablative temperatures were gradually increased following evaluations to test for improvement of motor symptoms. The maximum temperature used was 54.3° C.
Patients in the control group underwent an identical procedure with the sonication energy disabled.
The primary outcome was a response to therapy at 3 months, defined as a decrease of at least three points from baseline either in the score on the Movement Disorders Society–Unified Parkinson’s Disease Rating Scale (MDS-UPDRS), part III, while off medication or in the score on the Unified Dyskinesia Rating Scale (UDRS) while on medication.
At 3 months, 69% of the treatment group reported a response, compared with 32% of the control group (P = .003).
When researchers analyzed MDS-UPDRS scores, they found that 29% of the treatment group and 27% of the control group showed improvement. For UDRS scores, 12% of the treatment group demonstrated improvement. In the control group, there was no improvement on this score. Improvements in both scores were reported in 28% of the treatment group and 5% of the control group.
Among those who reported a response at 3 months, 77% continued to show a response at 12 months.
‘Unforgiving’ area of the brain
While the response rate was a promising sign of this finding, it was not what interested Dr. Krishna the most. “The most surprising finding of this trial is how safe focused ultrasound pallidotomy is in treating patients with Parkinson’s disease,” he said.
The globus pallidus internus is an area of the brain that Dr. Krishna calls “unforgiving.”
“One side is motor fibers, and any problem with that can paralyze the patient, and just below that is the optic tract, and any problem there, you would lose vision,” Dr. Krishna said. “It is a very tough neighborhood to be in.”
By using MRI-guided ultrasound, surgeons can change the target and temperature of the ultrasound beam during the procedure to allow more precise treatment.
Pallidotomy-related adverse events in the treatment group included dysarthria, gait disturbance, loss of taste, visual disturbance, and facial weakness. All were mild to moderate, Dr. Krishna said.
More study is needed
Dyskinesia is a challenge in the management of Parkinson’s disease. Patients need antiparkinsonian medications to slow deterioration of motor function, but those medications can cause the involuntary movements that are a hallmark of dyskinesia.
The most common treatment for this complication, deep-brain stimulation (DBS), has its own drawbacks. It’s an open procedure, and there is a low-level risk for intracranial bleeding and infection. In addition, the electrode implants require ongoing maintenance and adjustment.
But the findings of this study show that, for patients who aren’t candidates for other therapies, such as DBS and ablative radiofrequency, FUSA may be an alternative, wrote Anette Schrag, PhD, professor of clinical neurosciences at University College London, in an accompanying commentary.
“The results confirm that it is effective in reducing motor complications of Parkinson’s disease, at least in the short term,” Dr. Schrag wrote. However, more long-term studies are needed, she added.
One-third of patients in the treatment group had no response to the treatment, and investigators aren’t sure why. Dr. Krishna noted that the benefits of the procedure waned in about a quarter of patients within a year of treatment.
Investigators plan to probe these questions in future trials.
“The results of this trial are promising,” Dr. Schrag wrote, “but given the nonreversible nature of the intervention and the progressive nature of the disease, it will be important to establish whether improvements in motor complications are maintained over longer periods and whether treatment results in improved overall functioning and quality of life for patients.”
The study was funded by Insightec. Disclosure forms for Dr. Krishna and Dr. Schrag are provided on the journal’s website.
A version of this article originally appeared on Medscape.com.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Childhood nightmares a prelude to cognitive problems, Parkinson’s?
new research shows.
Compared with children who never had distressing dreams between ages 7 and 11 years, those who had persistent distressing dreams were 76% more likely to develop cognitive impairment and roughly seven times more likely to develop PD by age 50 years.
It’s been shown previously that sleep problems in adulthood, including distressing dreams, can precede the onset of neurodegenerative diseases such as Alzheimer’s disease (AD) or PD by several years, and in some cases decades, study investigator Abidemi Otaiku, BMBS, University of Birmingham (England), told this news organization.
However, no studies have investigated whether distressing dreams during childhood might also be associated with increased risk for cognitive decline or PD.
“As such, these findings provide evidence for the first time that certain sleep problems in childhood (having regular distressing dreams) could be an early indicator of increased dementia and PD risk,” Dr. Otaiku said.
He noted that the findings build on previous studies which showed that regular nightmares in childhood could be an early indicator for psychiatric problems in adolescence, such as borderline personality disorder, attention-deficit/hyperactivity disorder, and psychosis.
The study was published online February 26 in The Lancet journal eClinicalMedicine.
Statistically significant
The prospective, longitudinal analysis used data from the 1958 British Birth Cohort Study, a prospective birth cohort which included all people born in Britain during a single week in 1958.
At age 7 years (in 1965) and 11 years (in 1969), mothers were asked to report whether their child experienced “bad dreams or night terrors” in the past 3 months, and cognitive impairment and PD were determined at age 50 (2008).
Among a total of 6,991 children (51% girls), 78.2% never had distressing dreams, 17.9% had transient distressing dreams (either at ages 7 or 11 years), and 3.8% had persistent distressing dreams (at both ages 7 and 11 years).
By age 50, 262 participants had developed cognitive impairment, and five had been diagnosed with PD.
After adjusting for all covariates, having more regular distressing dreams during childhood was “linearly and statistically significantly” associated with higher risk of developing cognitive impairment or PD by age 50 years (P = .037). This was the case in both boys and girls.
Compared with children who never had bad dreams, peers who had persistent distressing dreams (at ages 7 and 11 years) had an 85% increased risk for cognitive impairment or PD by age 50 (adjusted odds ratio, 1.85; 95% confidence interval, 1.10-3.11; P = .019).
The associations remained when incident cognitive impairment and incident PD were analyzed separately.
Compared with children who never had distressing dreams, children who had persistent distressing dreams were 76% more likely to develop cognitive impairment by age 50 years (aOR, 1.76; 95% CI, 1.03-2.99; P = .037), and were about seven times more likely to be diagnosed with PD by age 50 years (aOR, 7.35; 95% CI, 1.03-52.73; P = .047).
The linear association was statistically significant for PD (P = .050) and had a trend toward statistical significance for cognitive impairment (P = .074).
Mechanism unclear
“Early-life nightmares might be causally associated with cognitive impairment and PD, noncausally associated with cognitive impairment and PD, or both. At this stage it remains unclear which of the three options is correct. Therefore, further research on mechanisms is needed,” Dr. Otaiku told this news organization.
“One plausible noncausal explanation is that there are shared genetic factors which predispose individuals to having frequent nightmares in childhood, and to developing neurodegenerative diseases such as AD or PD in adulthood,” he added.
It’s also plausible that having regular nightmares throughout childhood could be a causal risk factor for cognitive impairment and PD by causing chronic sleep disruption, he noted.
“Chronic sleep disruption due to nightmares might lead to impaired glymphatic clearance during sleep – and thus greater accumulation of pathological proteins in the brain, such as amyloid-beta and alpha-synuclein,” Dr. Otaiku said.
Disrupted sleep throughout childhood might also impair normal brain development, which could make children’s brains less resilient to neuropathologic damage, he said.
Clinical implications?
There are established treatments for childhood nightmares, including nonpharmacologic approaches.
“For children who have regular nightmares that lead to impaired daytime functioning, it may well be a good idea for them to see a sleep physician to discuss whether treatment may be needed,” Dr. Otaiku said.
But should doctors treat children with persistent nightmares for the purpose of preventing neurodegenerative diseases in adulthood or psychiatric problems in adolescence?
“It’s an interesting possibility. However, more research is needed to confirm these epidemiological associations and to determine whether or not nightmares are a causal risk factor for these conditions,” Dr. Otaiku concluded.
The study received no external funding. Dr. Otaiku reports no relevant disclosures.
A version of this article first appeared on Medscape.com.
new research shows.
Compared with children who never had distressing dreams between ages 7 and 11 years, those who had persistent distressing dreams were 76% more likely to develop cognitive impairment and roughly seven times more likely to develop PD by age 50 years.
It’s been shown previously that sleep problems in adulthood, including distressing dreams, can precede the onset of neurodegenerative diseases such as Alzheimer’s disease (AD) or PD by several years, and in some cases decades, study investigator Abidemi Otaiku, BMBS, University of Birmingham (England), told this news organization.
However, no studies have investigated whether distressing dreams during childhood might also be associated with increased risk for cognitive decline or PD.
“As such, these findings provide evidence for the first time that certain sleep problems in childhood (having regular distressing dreams) could be an early indicator of increased dementia and PD risk,” Dr. Otaiku said.
He noted that the findings build on previous studies which showed that regular nightmares in childhood could be an early indicator for psychiatric problems in adolescence, such as borderline personality disorder, attention-deficit/hyperactivity disorder, and psychosis.
The study was published online February 26 in The Lancet journal eClinicalMedicine.
Statistically significant
The prospective, longitudinal analysis used data from the 1958 British Birth Cohort Study, a prospective birth cohort which included all people born in Britain during a single week in 1958.
At age 7 years (in 1965) and 11 years (in 1969), mothers were asked to report whether their child experienced “bad dreams or night terrors” in the past 3 months, and cognitive impairment and PD were determined at age 50 (2008).
Among a total of 6,991 children (51% girls), 78.2% never had distressing dreams, 17.9% had transient distressing dreams (either at ages 7 or 11 years), and 3.8% had persistent distressing dreams (at both ages 7 and 11 years).
By age 50, 262 participants had developed cognitive impairment, and five had been diagnosed with PD.
After adjusting for all covariates, having more regular distressing dreams during childhood was “linearly and statistically significantly” associated with higher risk of developing cognitive impairment or PD by age 50 years (P = .037). This was the case in both boys and girls.
Compared with children who never had bad dreams, peers who had persistent distressing dreams (at ages 7 and 11 years) had an 85% increased risk for cognitive impairment or PD by age 50 (adjusted odds ratio, 1.85; 95% confidence interval, 1.10-3.11; P = .019).
The associations remained when incident cognitive impairment and incident PD were analyzed separately.
Compared with children who never had distressing dreams, children who had persistent distressing dreams were 76% more likely to develop cognitive impairment by age 50 years (aOR, 1.76; 95% CI, 1.03-2.99; P = .037), and were about seven times more likely to be diagnosed with PD by age 50 years (aOR, 7.35; 95% CI, 1.03-52.73; P = .047).
The linear association was statistically significant for PD (P = .050) and had a trend toward statistical significance for cognitive impairment (P = .074).
Mechanism unclear
“Early-life nightmares might be causally associated with cognitive impairment and PD, noncausally associated with cognitive impairment and PD, or both. At this stage it remains unclear which of the three options is correct. Therefore, further research on mechanisms is needed,” Dr. Otaiku told this news organization.
“One plausible noncausal explanation is that there are shared genetic factors which predispose individuals to having frequent nightmares in childhood, and to developing neurodegenerative diseases such as AD or PD in adulthood,” he added.
It’s also plausible that having regular nightmares throughout childhood could be a causal risk factor for cognitive impairment and PD by causing chronic sleep disruption, he noted.
“Chronic sleep disruption due to nightmares might lead to impaired glymphatic clearance during sleep – and thus greater accumulation of pathological proteins in the brain, such as amyloid-beta and alpha-synuclein,” Dr. Otaiku said.
Disrupted sleep throughout childhood might also impair normal brain development, which could make children’s brains less resilient to neuropathologic damage, he said.
Clinical implications?
There are established treatments for childhood nightmares, including nonpharmacologic approaches.
“For children who have regular nightmares that lead to impaired daytime functioning, it may well be a good idea for them to see a sleep physician to discuss whether treatment may be needed,” Dr. Otaiku said.
But should doctors treat children with persistent nightmares for the purpose of preventing neurodegenerative diseases in adulthood or psychiatric problems in adolescence?
“It’s an interesting possibility. However, more research is needed to confirm these epidemiological associations and to determine whether or not nightmares are a causal risk factor for these conditions,” Dr. Otaiku concluded.
The study received no external funding. Dr. Otaiku reports no relevant disclosures.
A version of this article first appeared on Medscape.com.
new research shows.
Compared with children who never had distressing dreams between ages 7 and 11 years, those who had persistent distressing dreams were 76% more likely to develop cognitive impairment and roughly seven times more likely to develop PD by age 50 years.
It’s been shown previously that sleep problems in adulthood, including distressing dreams, can precede the onset of neurodegenerative diseases such as Alzheimer’s disease (AD) or PD by several years, and in some cases decades, study investigator Abidemi Otaiku, BMBS, University of Birmingham (England), told this news organization.
However, no studies have investigated whether distressing dreams during childhood might also be associated with increased risk for cognitive decline or PD.
“As such, these findings provide evidence for the first time that certain sleep problems in childhood (having regular distressing dreams) could be an early indicator of increased dementia and PD risk,” Dr. Otaiku said.
He noted that the findings build on previous studies which showed that regular nightmares in childhood could be an early indicator for psychiatric problems in adolescence, such as borderline personality disorder, attention-deficit/hyperactivity disorder, and psychosis.
The study was published online February 26 in The Lancet journal eClinicalMedicine.
Statistically significant
The prospective, longitudinal analysis used data from the 1958 British Birth Cohort Study, a prospective birth cohort which included all people born in Britain during a single week in 1958.
At age 7 years (in 1965) and 11 years (in 1969), mothers were asked to report whether their child experienced “bad dreams or night terrors” in the past 3 months, and cognitive impairment and PD were determined at age 50 (2008).
Among a total of 6,991 children (51% girls), 78.2% never had distressing dreams, 17.9% had transient distressing dreams (either at ages 7 or 11 years), and 3.8% had persistent distressing dreams (at both ages 7 and 11 years).
By age 50, 262 participants had developed cognitive impairment, and five had been diagnosed with PD.
After adjusting for all covariates, having more regular distressing dreams during childhood was “linearly and statistically significantly” associated with higher risk of developing cognitive impairment or PD by age 50 years (P = .037). This was the case in both boys and girls.
Compared with children who never had bad dreams, peers who had persistent distressing dreams (at ages 7 and 11 years) had an 85% increased risk for cognitive impairment or PD by age 50 (adjusted odds ratio, 1.85; 95% confidence interval, 1.10-3.11; P = .019).
The associations remained when incident cognitive impairment and incident PD were analyzed separately.
Compared with children who never had distressing dreams, children who had persistent distressing dreams were 76% more likely to develop cognitive impairment by age 50 years (aOR, 1.76; 95% CI, 1.03-2.99; P = .037), and were about seven times more likely to be diagnosed with PD by age 50 years (aOR, 7.35; 95% CI, 1.03-52.73; P = .047).
The linear association was statistically significant for PD (P = .050) and had a trend toward statistical significance for cognitive impairment (P = .074).
Mechanism unclear
“Early-life nightmares might be causally associated with cognitive impairment and PD, noncausally associated with cognitive impairment and PD, or both. At this stage it remains unclear which of the three options is correct. Therefore, further research on mechanisms is needed,” Dr. Otaiku told this news organization.
“One plausible noncausal explanation is that there are shared genetic factors which predispose individuals to having frequent nightmares in childhood, and to developing neurodegenerative diseases such as AD or PD in adulthood,” he added.
It’s also plausible that having regular nightmares throughout childhood could be a causal risk factor for cognitive impairment and PD by causing chronic sleep disruption, he noted.
“Chronic sleep disruption due to nightmares might lead to impaired glymphatic clearance during sleep – and thus greater accumulation of pathological proteins in the brain, such as amyloid-beta and alpha-synuclein,” Dr. Otaiku said.
Disrupted sleep throughout childhood might also impair normal brain development, which could make children’s brains less resilient to neuropathologic damage, he said.
Clinical implications?
There are established treatments for childhood nightmares, including nonpharmacologic approaches.
“For children who have regular nightmares that lead to impaired daytime functioning, it may well be a good idea for them to see a sleep physician to discuss whether treatment may be needed,” Dr. Otaiku said.
But should doctors treat children with persistent nightmares for the purpose of preventing neurodegenerative diseases in adulthood or psychiatric problems in adolescence?
“It’s an interesting possibility. However, more research is needed to confirm these epidemiological associations and to determine whether or not nightmares are a causal risk factor for these conditions,” Dr. Otaiku concluded.
The study received no external funding. Dr. Otaiku reports no relevant disclosures.
A version of this article first appeared on Medscape.com.
FROM ECLINICALMEDICINE
Childhood trauma tied to increased Parkinson’s disease severity
new research shows.
Results of the first study to evaluate the relationship between childhood trauma and PD investigators found that the relationship appears to be dose dependent. Patients with PD who reported more than one ACE all experienced a statistically significant decrease in QOL, and for each additional ACE, there was significant worsening of motor symptoms.
This study supports a recent-call to-action paper in JAMA Neurology encouraging adoption of “trauma-informed neurology,” study investigator Indu Subramanian, MD, clinical professor, department of neurology, University of California, Los Angeles, said in an interview.
“We need to start asking about ACEs in everyone. It should be part of our medical intake,” said Dr. Subramanian, who is also the director of the Southwest Parkinson’s Disease Research, Education, and Clinical Center, West Los Angeles Veterans Affairs Medical Center.
The study was published online in Neurology: Clinical Practice.
Hard on the mind and body
A robust body of literature has clearly established a connection between ACEs, which include physical and emotional abuse, neglect, and household dysfunction, and negative physical health outcomes across the lifespan. These include stroke, dementia, diabetes, cancer, cardiovascular disease, autoimmune disorders, hypertension, and premature death as well as psychosocial health outcomes such as anxiety, depression, substance use, and suicide.
However, until now, the effects of childhood trauma have not been evaluated in a PD population.
As part of the MVP study, 712 adults with PD responded to an online survey asking about childhood trauma.
As anticipated, patients with the least reported childhood trauma reported the highest current QOL and lowest patient-reported motor and nonmotor symptom burden compared with peers with higher reported childhood trauma, the researchers reported.
PD symptom burden increased and QOL decreased as the number of ACEs increased.
Patients with ACE scores of 4 or higher reported greater PD symptom severity for 45% of the variables assessed, including apathy, muscle pain, daytime sleepiness, restless leg syndrome, depression, fatigue, comprehension, and anxiety (P < .05), compared with peers with trauma scores of 0.
Limitations of the study included the cross-sectional nature, which prevents making any causal determinations. Also, the ACE questionnaire, because it is self-reported and a retrospective collection of data, introduces the risk for recall bias. In addition, 65% of respondents were women, and racial and ethnic minority groups were not well represented.
Looking ahead, Dr. Subramanian and coauthors believe future research should “attempt to include more diverse populations, attempt improve the response rate of these sensitive questions and, most importantly, determine whether the adverse outcomes associated with childhood trauma can be mitigated with lifestyle modification, psychosocial support, and intervention in adulthood.”
“As a trauma-informed approach, something sorely lacking yet needed in the field of movement disorders, clinicians can proactively screen for ACEs while being mindful to avoid retraumatization,” they suggested. “They can begin to identify how ACEs may physiologically contribute to PD symptom and focus on targeting appropriate interventions that may improve outcomes.”
Life experiences matter
In a comment, Michael S. Okun, MD, medical advisor, Parkinson’s Foundation, and director of the Norman Fixel Institute for Neurological Diseases, University of Florida Health, Gainesville, said that “the idea that childhood trauma could be associated with a mild increase in severity of Parkinson’s symptoms such as apathy, pain, sleepiness and depression is fascinating.”
“We should however temper our enthusiasm for the results of this study because they were obtained through a direct patient survey, and not collected from large well characterized medical database,” Dr. Okun said.
He added” “If the data on childhood trauma and Parkinson’s can be replicated, we must ask why this could be?
“For Parkinson clinicians this as a reminder of how important obtaining a complete life history can be when strategizing on a plan to reduce motor and nonmotor Parkinson symptoms. Life experiences matter and can impact symptoms,” Dr. Okun said.
The MVP study was initiated with support of the National Center for Complementary and Integrative Health. The ongoing data collection has been supported by a donation from Sondra and Bill Fondren. Dr. Subramanian and Dr. Okun disclosed no potential conflicts of interest.
A version of this article first appeared on Medscape.com.
new research shows.
Results of the first study to evaluate the relationship between childhood trauma and PD investigators found that the relationship appears to be dose dependent. Patients with PD who reported more than one ACE all experienced a statistically significant decrease in QOL, and for each additional ACE, there was significant worsening of motor symptoms.
This study supports a recent-call to-action paper in JAMA Neurology encouraging adoption of “trauma-informed neurology,” study investigator Indu Subramanian, MD, clinical professor, department of neurology, University of California, Los Angeles, said in an interview.
“We need to start asking about ACEs in everyone. It should be part of our medical intake,” said Dr. Subramanian, who is also the director of the Southwest Parkinson’s Disease Research, Education, and Clinical Center, West Los Angeles Veterans Affairs Medical Center.
The study was published online in Neurology: Clinical Practice.
Hard on the mind and body
A robust body of literature has clearly established a connection between ACEs, which include physical and emotional abuse, neglect, and household dysfunction, and negative physical health outcomes across the lifespan. These include stroke, dementia, diabetes, cancer, cardiovascular disease, autoimmune disorders, hypertension, and premature death as well as psychosocial health outcomes such as anxiety, depression, substance use, and suicide.
However, until now, the effects of childhood trauma have not been evaluated in a PD population.
As part of the MVP study, 712 adults with PD responded to an online survey asking about childhood trauma.
As anticipated, patients with the least reported childhood trauma reported the highest current QOL and lowest patient-reported motor and nonmotor symptom burden compared with peers with higher reported childhood trauma, the researchers reported.
PD symptom burden increased and QOL decreased as the number of ACEs increased.
Patients with ACE scores of 4 or higher reported greater PD symptom severity for 45% of the variables assessed, including apathy, muscle pain, daytime sleepiness, restless leg syndrome, depression, fatigue, comprehension, and anxiety (P < .05), compared with peers with trauma scores of 0.
Limitations of the study included the cross-sectional nature, which prevents making any causal determinations. Also, the ACE questionnaire, because it is self-reported and a retrospective collection of data, introduces the risk for recall bias. In addition, 65% of respondents were women, and racial and ethnic minority groups were not well represented.
Looking ahead, Dr. Subramanian and coauthors believe future research should “attempt to include more diverse populations, attempt improve the response rate of these sensitive questions and, most importantly, determine whether the adverse outcomes associated with childhood trauma can be mitigated with lifestyle modification, psychosocial support, and intervention in adulthood.”
“As a trauma-informed approach, something sorely lacking yet needed in the field of movement disorders, clinicians can proactively screen for ACEs while being mindful to avoid retraumatization,” they suggested. “They can begin to identify how ACEs may physiologically contribute to PD symptom and focus on targeting appropriate interventions that may improve outcomes.”
Life experiences matter
In a comment, Michael S. Okun, MD, medical advisor, Parkinson’s Foundation, and director of the Norman Fixel Institute for Neurological Diseases, University of Florida Health, Gainesville, said that “the idea that childhood trauma could be associated with a mild increase in severity of Parkinson’s symptoms such as apathy, pain, sleepiness and depression is fascinating.”
“We should however temper our enthusiasm for the results of this study because they were obtained through a direct patient survey, and not collected from large well characterized medical database,” Dr. Okun said.
He added” “If the data on childhood trauma and Parkinson’s can be replicated, we must ask why this could be?
“For Parkinson clinicians this as a reminder of how important obtaining a complete life history can be when strategizing on a plan to reduce motor and nonmotor Parkinson symptoms. Life experiences matter and can impact symptoms,” Dr. Okun said.
The MVP study was initiated with support of the National Center for Complementary and Integrative Health. The ongoing data collection has been supported by a donation from Sondra and Bill Fondren. Dr. Subramanian and Dr. Okun disclosed no potential conflicts of interest.
A version of this article first appeared on Medscape.com.
new research shows.
Results of the first study to evaluate the relationship between childhood trauma and PD investigators found that the relationship appears to be dose dependent. Patients with PD who reported more than one ACE all experienced a statistically significant decrease in QOL, and for each additional ACE, there was significant worsening of motor symptoms.
This study supports a recent-call to-action paper in JAMA Neurology encouraging adoption of “trauma-informed neurology,” study investigator Indu Subramanian, MD, clinical professor, department of neurology, University of California, Los Angeles, said in an interview.
“We need to start asking about ACEs in everyone. It should be part of our medical intake,” said Dr. Subramanian, who is also the director of the Southwest Parkinson’s Disease Research, Education, and Clinical Center, West Los Angeles Veterans Affairs Medical Center.
The study was published online in Neurology: Clinical Practice.
Hard on the mind and body
A robust body of literature has clearly established a connection between ACEs, which include physical and emotional abuse, neglect, and household dysfunction, and negative physical health outcomes across the lifespan. These include stroke, dementia, diabetes, cancer, cardiovascular disease, autoimmune disorders, hypertension, and premature death as well as psychosocial health outcomes such as anxiety, depression, substance use, and suicide.
However, until now, the effects of childhood trauma have not been evaluated in a PD population.
As part of the MVP study, 712 adults with PD responded to an online survey asking about childhood trauma.
As anticipated, patients with the least reported childhood trauma reported the highest current QOL and lowest patient-reported motor and nonmotor symptom burden compared with peers with higher reported childhood trauma, the researchers reported.
PD symptom burden increased and QOL decreased as the number of ACEs increased.
Patients with ACE scores of 4 or higher reported greater PD symptom severity for 45% of the variables assessed, including apathy, muscle pain, daytime sleepiness, restless leg syndrome, depression, fatigue, comprehension, and anxiety (P < .05), compared with peers with trauma scores of 0.
Limitations of the study included the cross-sectional nature, which prevents making any causal determinations. Also, the ACE questionnaire, because it is self-reported and a retrospective collection of data, introduces the risk for recall bias. In addition, 65% of respondents were women, and racial and ethnic minority groups were not well represented.
Looking ahead, Dr. Subramanian and coauthors believe future research should “attempt to include more diverse populations, attempt improve the response rate of these sensitive questions and, most importantly, determine whether the adverse outcomes associated with childhood trauma can be mitigated with lifestyle modification, psychosocial support, and intervention in adulthood.”
“As a trauma-informed approach, something sorely lacking yet needed in the field of movement disorders, clinicians can proactively screen for ACEs while being mindful to avoid retraumatization,” they suggested. “They can begin to identify how ACEs may physiologically contribute to PD symptom and focus on targeting appropriate interventions that may improve outcomes.”
Life experiences matter
In a comment, Michael S. Okun, MD, medical advisor, Parkinson’s Foundation, and director of the Norman Fixel Institute for Neurological Diseases, University of Florida Health, Gainesville, said that “the idea that childhood trauma could be associated with a mild increase in severity of Parkinson’s symptoms such as apathy, pain, sleepiness and depression is fascinating.”
“We should however temper our enthusiasm for the results of this study because they were obtained through a direct patient survey, and not collected from large well characterized medical database,” Dr. Okun said.
He added” “If the data on childhood trauma and Parkinson’s can be replicated, we must ask why this could be?
“For Parkinson clinicians this as a reminder of how important obtaining a complete life history can be when strategizing on a plan to reduce motor and nonmotor Parkinson symptoms. Life experiences matter and can impact symptoms,” Dr. Okun said.
The MVP study was initiated with support of the National Center for Complementary and Integrative Health. The ongoing data collection has been supported by a donation from Sondra and Bill Fondren. Dr. Subramanian and Dr. Okun disclosed no potential conflicts of interest.
A version of this article first appeared on Medscape.com.
FROM NEUROLOGY: CLINICAL PRACTICE
What’s new in brain health?
This transcript has been edited for clarity.
Dear colleagues, I am Christoph Diener from the medical faculty of the University of Duisburg-Essen in Germany.
Treatment of tension-type headache
I would like to start with headache. You are all aware that we have several new studies regarding the prevention of migraine, but very few studies involving nondrug treatments for tension-type headache.
A working group in Göttingen, Germany, conducted a study in people with frequent episodic and chronic tension-type headache. The first of the four randomized groups received traditional Chinese acupuncture for 3 months. The second group received physical therapy and exercise for 1 hour per week for 12 weeks. The third group received a combination of acupuncture and exercise. The last was a control group that received only standard care.
The outcome parameters of tension-type headache were evaluated after 6 months and again after 12 months. Previously, these same researchers published that the intensity but not the frequency of tension-type headache was reduced by active therapy.
In Cephalalgia, they published the outcome for the endpoints of depression, anxiety, and quality of life. Acupuncture, exercise, and the combination of the two improved depression, anxiety, and quality of life. This shows that nonmedical treatment is effective in people with frequent episodic and chronic tension-type headache.
Headache after COVID-19
The next study was published in Headache and discusses headache after COVID-19. In this review of published studies, more than 50% of people with COVID-19 develop headache. It is more frequent in young patients and people with preexisting primary headaches, such as migraine and tension-type headache. Prognosis is usually good, but some patients develop new, daily persistent headache, which is a major problem because treatment is unclear. We desperately need studies investigating how to treat this new, daily persistent headache after COVID-19.
SSRIs during COVID-19 infection
The next study also focuses on COVID-19. We have conflicting results from several studies suggesting that selective serotonin reuptake inhibitors might be effective in people with mild COVID-19 infection. This hypothesis was tested in a study in Brazil and was published in JAMA, The study included 1,288 outpatients with mild COVID-19 who either received 50 mg of fluvoxamine twice daily for 10 days or placebo. There was no benefit of the treatment for any outcome.
Preventing dementia with antihypertensive treatment
The next study was published in the European Heart Journal and addresses the question of whether effective antihypertensive treatment in elderly persons can prevent dementia. This is a meta-analysis of five placebo-controlled trials with more than 28,000 patients. The meta-analysis clearly shows that treating hypertension in elderly patients does prevent dementia. The benefit is higher if the blood pressure is lowered by a larger amount which also stays true for elderly patients. There is no negative impact of lowering blood pressure in this population.
Antiplatelet therapy
The next study was published in Stroke and reexamines whether resumption of antiplatelet therapy should be early or late in people who had an intracerebral hemorrhage while on antiplatelet therapy. In the Taiwanese Health Registry, this was studied in 1,584 patients. The researchers divided participants into groups based on whether antiplatelet therapy was resumed within 30 days or after 30 days. In 1 year, the rate of recurrent intracerebral hemorrhage was 3.2%. There was no difference whether antiplatelet therapy was resumed early or late.
Regular exercise in Parkinson’s disease
The final study is a review of nonmedical therapy. This meta-analysis of 19 randomized trials looked at the benefit of regular exercise in patients with Parkinson’s disease and depression. The analysis clearly showed that rigorous and moderate exercise improved depression in patients with Parkinson’s disease. This is very important because exercise improves not only the symptoms of Parkinson’s disease but also comorbid depression while presenting no serious adverse events or side effects.
Dr. Diener is a professor in the department of neurology at Stroke Center–Headache Center, University Duisburg-Essen, Germany. He disclosed ties with Abbott, Addex Pharma, Alder, Allergan, Almirall, Amgen, Autonomic Technology, AstraZeneca, Bayer Vital, Berlin Chemie, Bristol-Myers Squibb, Boehringer Ingelheim, Chordate, CoAxia, Corimmun, Covidien, Coherex, CoLucid, Daiichi Sankyo, D-Pharm, Electrocore, Fresenius, GlaxoSmithKline, Grunenthal, Janssen-Cilag, Labrys Biologics Lilly, La Roche, Lundbeck, 3M Medica, MSD, Medtronic, Menarini, MindFrame, Minster, Neuroscore, Neurobiological Technologies, Novartis, Novo Nordisk, Johnson & Johnson, Knoll, Paion, Parke-Davis, Pierre Fabre, Pfizer Inc, Schaper and Brummer, Sanofi-Aventis, Schering-Plough, Servier, Solvay, St. Jude, Talecris, Thrombogenics, WebMD Global, Weber and Weber, Wyeth, and Yamanouchi. Dr. Diener has served as editor of Aktuelle Neurologie, Arzneimitteltherapie, Kopfschmerz News, Stroke News, and the Treatment Guidelines of the German Neurological Society; as co-editor of Cephalalgia; and on the editorial board of The Lancet Neurology, Stroke, European Neurology, and Cerebrovascular Disorders. The department of neurology in Essen is supported by the German Research Council, the German Ministry of Education and Research, European Union, National Institutes of Health, Bertelsmann Foundation, and Heinz Nixdorf Foundation. Dr. Diener has no ownership interest and does not own stocks in any pharmaceutical company. A version of this article originally appeared on Medscape.com.
This transcript has been edited for clarity.
Dear colleagues, I am Christoph Diener from the medical faculty of the University of Duisburg-Essen in Germany.
Treatment of tension-type headache
I would like to start with headache. You are all aware that we have several new studies regarding the prevention of migraine, but very few studies involving nondrug treatments for tension-type headache.
A working group in Göttingen, Germany, conducted a study in people with frequent episodic and chronic tension-type headache. The first of the four randomized groups received traditional Chinese acupuncture for 3 months. The second group received physical therapy and exercise for 1 hour per week for 12 weeks. The third group received a combination of acupuncture and exercise. The last was a control group that received only standard care.
The outcome parameters of tension-type headache were evaluated after 6 months and again after 12 months. Previously, these same researchers published that the intensity but not the frequency of tension-type headache was reduced by active therapy.
In Cephalalgia, they published the outcome for the endpoints of depression, anxiety, and quality of life. Acupuncture, exercise, and the combination of the two improved depression, anxiety, and quality of life. This shows that nonmedical treatment is effective in people with frequent episodic and chronic tension-type headache.
Headache after COVID-19
The next study was published in Headache and discusses headache after COVID-19. In this review of published studies, more than 50% of people with COVID-19 develop headache. It is more frequent in young patients and people with preexisting primary headaches, such as migraine and tension-type headache. Prognosis is usually good, but some patients develop new, daily persistent headache, which is a major problem because treatment is unclear. We desperately need studies investigating how to treat this new, daily persistent headache after COVID-19.
SSRIs during COVID-19 infection
The next study also focuses on COVID-19. We have conflicting results from several studies suggesting that selective serotonin reuptake inhibitors might be effective in people with mild COVID-19 infection. This hypothesis was tested in a study in Brazil and was published in JAMA, The study included 1,288 outpatients with mild COVID-19 who either received 50 mg of fluvoxamine twice daily for 10 days or placebo. There was no benefit of the treatment for any outcome.
Preventing dementia with antihypertensive treatment
The next study was published in the European Heart Journal and addresses the question of whether effective antihypertensive treatment in elderly persons can prevent dementia. This is a meta-analysis of five placebo-controlled trials with more than 28,000 patients. The meta-analysis clearly shows that treating hypertension in elderly patients does prevent dementia. The benefit is higher if the blood pressure is lowered by a larger amount which also stays true for elderly patients. There is no negative impact of lowering blood pressure in this population.
Antiplatelet therapy
The next study was published in Stroke and reexamines whether resumption of antiplatelet therapy should be early or late in people who had an intracerebral hemorrhage while on antiplatelet therapy. In the Taiwanese Health Registry, this was studied in 1,584 patients. The researchers divided participants into groups based on whether antiplatelet therapy was resumed within 30 days or after 30 days. In 1 year, the rate of recurrent intracerebral hemorrhage was 3.2%. There was no difference whether antiplatelet therapy was resumed early or late.
Regular exercise in Parkinson’s disease
The final study is a review of nonmedical therapy. This meta-analysis of 19 randomized trials looked at the benefit of regular exercise in patients with Parkinson’s disease and depression. The analysis clearly showed that rigorous and moderate exercise improved depression in patients with Parkinson’s disease. This is very important because exercise improves not only the symptoms of Parkinson’s disease but also comorbid depression while presenting no serious adverse events or side effects.
Dr. Diener is a professor in the department of neurology at Stroke Center–Headache Center, University Duisburg-Essen, Germany. He disclosed ties with Abbott, Addex Pharma, Alder, Allergan, Almirall, Amgen, Autonomic Technology, AstraZeneca, Bayer Vital, Berlin Chemie, Bristol-Myers Squibb, Boehringer Ingelheim, Chordate, CoAxia, Corimmun, Covidien, Coherex, CoLucid, Daiichi Sankyo, D-Pharm, Electrocore, Fresenius, GlaxoSmithKline, Grunenthal, Janssen-Cilag, Labrys Biologics Lilly, La Roche, Lundbeck, 3M Medica, MSD, Medtronic, Menarini, MindFrame, Minster, Neuroscore, Neurobiological Technologies, Novartis, Novo Nordisk, Johnson & Johnson, Knoll, Paion, Parke-Davis, Pierre Fabre, Pfizer Inc, Schaper and Brummer, Sanofi-Aventis, Schering-Plough, Servier, Solvay, St. Jude, Talecris, Thrombogenics, WebMD Global, Weber and Weber, Wyeth, and Yamanouchi. Dr. Diener has served as editor of Aktuelle Neurologie, Arzneimitteltherapie, Kopfschmerz News, Stroke News, and the Treatment Guidelines of the German Neurological Society; as co-editor of Cephalalgia; and on the editorial board of The Lancet Neurology, Stroke, European Neurology, and Cerebrovascular Disorders. The department of neurology in Essen is supported by the German Research Council, the German Ministry of Education and Research, European Union, National Institutes of Health, Bertelsmann Foundation, and Heinz Nixdorf Foundation. Dr. Diener has no ownership interest and does not own stocks in any pharmaceutical company. A version of this article originally appeared on Medscape.com.
This transcript has been edited for clarity.
Dear colleagues, I am Christoph Diener from the medical faculty of the University of Duisburg-Essen in Germany.
Treatment of tension-type headache
I would like to start with headache. You are all aware that we have several new studies regarding the prevention of migraine, but very few studies involving nondrug treatments for tension-type headache.
A working group in Göttingen, Germany, conducted a study in people with frequent episodic and chronic tension-type headache. The first of the four randomized groups received traditional Chinese acupuncture for 3 months. The second group received physical therapy and exercise for 1 hour per week for 12 weeks. The third group received a combination of acupuncture and exercise. The last was a control group that received only standard care.
The outcome parameters of tension-type headache were evaluated after 6 months and again after 12 months. Previously, these same researchers published that the intensity but not the frequency of tension-type headache was reduced by active therapy.
In Cephalalgia, they published the outcome for the endpoints of depression, anxiety, and quality of life. Acupuncture, exercise, and the combination of the two improved depression, anxiety, and quality of life. This shows that nonmedical treatment is effective in people with frequent episodic and chronic tension-type headache.
Headache after COVID-19
The next study was published in Headache and discusses headache after COVID-19. In this review of published studies, more than 50% of people with COVID-19 develop headache. It is more frequent in young patients and people with preexisting primary headaches, such as migraine and tension-type headache. Prognosis is usually good, but some patients develop new, daily persistent headache, which is a major problem because treatment is unclear. We desperately need studies investigating how to treat this new, daily persistent headache after COVID-19.
SSRIs during COVID-19 infection
The next study also focuses on COVID-19. We have conflicting results from several studies suggesting that selective serotonin reuptake inhibitors might be effective in people with mild COVID-19 infection. This hypothesis was tested in a study in Brazil and was published in JAMA, The study included 1,288 outpatients with mild COVID-19 who either received 50 mg of fluvoxamine twice daily for 10 days or placebo. There was no benefit of the treatment for any outcome.
Preventing dementia with antihypertensive treatment
The next study was published in the European Heart Journal and addresses the question of whether effective antihypertensive treatment in elderly persons can prevent dementia. This is a meta-analysis of five placebo-controlled trials with more than 28,000 patients. The meta-analysis clearly shows that treating hypertension in elderly patients does prevent dementia. The benefit is higher if the blood pressure is lowered by a larger amount which also stays true for elderly patients. There is no negative impact of lowering blood pressure in this population.
Antiplatelet therapy
The next study was published in Stroke and reexamines whether resumption of antiplatelet therapy should be early or late in people who had an intracerebral hemorrhage while on antiplatelet therapy. In the Taiwanese Health Registry, this was studied in 1,584 patients. The researchers divided participants into groups based on whether antiplatelet therapy was resumed within 30 days or after 30 days. In 1 year, the rate of recurrent intracerebral hemorrhage was 3.2%. There was no difference whether antiplatelet therapy was resumed early or late.
Regular exercise in Parkinson’s disease
The final study is a review of nonmedical therapy. This meta-analysis of 19 randomized trials looked at the benefit of regular exercise in patients with Parkinson’s disease and depression. The analysis clearly showed that rigorous and moderate exercise improved depression in patients with Parkinson’s disease. This is very important because exercise improves not only the symptoms of Parkinson’s disease but also comorbid depression while presenting no serious adverse events or side effects.
Dr. Diener is a professor in the department of neurology at Stroke Center–Headache Center, University Duisburg-Essen, Germany. He disclosed ties with Abbott, Addex Pharma, Alder, Allergan, Almirall, Amgen, Autonomic Technology, AstraZeneca, Bayer Vital, Berlin Chemie, Bristol-Myers Squibb, Boehringer Ingelheim, Chordate, CoAxia, Corimmun, Covidien, Coherex, CoLucid, Daiichi Sankyo, D-Pharm, Electrocore, Fresenius, GlaxoSmithKline, Grunenthal, Janssen-Cilag, Labrys Biologics Lilly, La Roche, Lundbeck, 3M Medica, MSD, Medtronic, Menarini, MindFrame, Minster, Neuroscore, Neurobiological Technologies, Novartis, Novo Nordisk, Johnson & Johnson, Knoll, Paion, Parke-Davis, Pierre Fabre, Pfizer Inc, Schaper and Brummer, Sanofi-Aventis, Schering-Plough, Servier, Solvay, St. Jude, Talecris, Thrombogenics, WebMD Global, Weber and Weber, Wyeth, and Yamanouchi. Dr. Diener has served as editor of Aktuelle Neurologie, Arzneimitteltherapie, Kopfschmerz News, Stroke News, and the Treatment Guidelines of the German Neurological Society; as co-editor of Cephalalgia; and on the editorial board of The Lancet Neurology, Stroke, European Neurology, and Cerebrovascular Disorders. The department of neurology in Essen is supported by the German Research Council, the German Ministry of Education and Research, European Union, National Institutes of Health, Bertelsmann Foundation, and Heinz Nixdorf Foundation. Dr. Diener has no ownership interest and does not own stocks in any pharmaceutical company. A version of this article originally appeared on Medscape.com.