User login
FDA to review dupilumab for treating chronic spontaneous urticaria
The that is inadequately controlled by current standard of care.
CSU is an inflammatory skin condition that causes sudden hives and angioedema, most often on the face, hands, and feet. However, the throat and upper airways also can be affected. CSU is generally treated with H1 antihistamines, but this strategy is insufficient for approximately 50% of patients, according to a press release from the manufacturer, Regeneron, announcing the FDA acceptance of the application on March 7.
Dupilumab (Dupixent), first approved in 2017 for treating atopic dermatitis in adults, is a fully human monoclonal antibody that inhibits the signaling of the interleukin (IL)-4 and IL-13 pathways.
The application for FDA approval for CSU is based on data from a pair of phase 3 trials in two different populations, LIBERTY-CUPID A and B.
The first study (LIBERTY-CUPID A) randomized 138 CSU patients aged 6 years and older who were uncontrolled on antihistamines to additional treatment with dupilumab or placebo over 24 weeks. The dupilumab-treated patients showed a 63% reduction in itch severity compared with a 35% reduction in patients who received the placebo, measured by changes in a 0-21 itch severity scale, according to data presented at the 2022 American Academy of Allergy, Asthma and Immunology (AAAAI) meeting.
Patients in the dupilumab group also showed a 65% reduction in the severity of urticaria activity (itch and hives) compared with 37% of those on placebo. Overall rates of adverse events were similar between groups; the most common were injection site reactions, according to the company.
The second study (LIBERTY-CUPID B) assessed efficacy and safety of dupilumab in 108 patients with CSU aged 12-80 years who were symptomatic despite standard-of-care treatment and were intolerant or incomplete responders to the anti-IgE antibody omalizumab (Xolair), approved for CSU. Last year, the company announced that this study had been halted after an interim analysis found that while there were positive numerical trends in reducing itch and hives, they “did not meet statistical significance.” In the March 7 press release, the company said that results from this study provide “additional supporting data” for the approval application.
The target date for the FDA’s decision is Oct. 22, 2023, according to Regeneron. Regeneron and Sanofi also are investigating dupilumab for treating chronic inducible urticaria triggered by cold in a phase 3 study.
The that is inadequately controlled by current standard of care.
CSU is an inflammatory skin condition that causes sudden hives and angioedema, most often on the face, hands, and feet. However, the throat and upper airways also can be affected. CSU is generally treated with H1 antihistamines, but this strategy is insufficient for approximately 50% of patients, according to a press release from the manufacturer, Regeneron, announcing the FDA acceptance of the application on March 7.
Dupilumab (Dupixent), first approved in 2017 for treating atopic dermatitis in adults, is a fully human monoclonal antibody that inhibits the signaling of the interleukin (IL)-4 and IL-13 pathways.
The application for FDA approval for CSU is based on data from a pair of phase 3 trials in two different populations, LIBERTY-CUPID A and B.
The first study (LIBERTY-CUPID A) randomized 138 CSU patients aged 6 years and older who were uncontrolled on antihistamines to additional treatment with dupilumab or placebo over 24 weeks. The dupilumab-treated patients showed a 63% reduction in itch severity compared with a 35% reduction in patients who received the placebo, measured by changes in a 0-21 itch severity scale, according to data presented at the 2022 American Academy of Allergy, Asthma and Immunology (AAAAI) meeting.
Patients in the dupilumab group also showed a 65% reduction in the severity of urticaria activity (itch and hives) compared with 37% of those on placebo. Overall rates of adverse events were similar between groups; the most common were injection site reactions, according to the company.
The second study (LIBERTY-CUPID B) assessed efficacy and safety of dupilumab in 108 patients with CSU aged 12-80 years who were symptomatic despite standard-of-care treatment and were intolerant or incomplete responders to the anti-IgE antibody omalizumab (Xolair), approved for CSU. Last year, the company announced that this study had been halted after an interim analysis found that while there were positive numerical trends in reducing itch and hives, they “did not meet statistical significance.” In the March 7 press release, the company said that results from this study provide “additional supporting data” for the approval application.
The target date for the FDA’s decision is Oct. 22, 2023, according to Regeneron. Regeneron and Sanofi also are investigating dupilumab for treating chronic inducible urticaria triggered by cold in a phase 3 study.
The that is inadequately controlled by current standard of care.
CSU is an inflammatory skin condition that causes sudden hives and angioedema, most often on the face, hands, and feet. However, the throat and upper airways also can be affected. CSU is generally treated with H1 antihistamines, but this strategy is insufficient for approximately 50% of patients, according to a press release from the manufacturer, Regeneron, announcing the FDA acceptance of the application on March 7.
Dupilumab (Dupixent), first approved in 2017 for treating atopic dermatitis in adults, is a fully human monoclonal antibody that inhibits the signaling of the interleukin (IL)-4 and IL-13 pathways.
The application for FDA approval for CSU is based on data from a pair of phase 3 trials in two different populations, LIBERTY-CUPID A and B.
The first study (LIBERTY-CUPID A) randomized 138 CSU patients aged 6 years and older who were uncontrolled on antihistamines to additional treatment with dupilumab or placebo over 24 weeks. The dupilumab-treated patients showed a 63% reduction in itch severity compared with a 35% reduction in patients who received the placebo, measured by changes in a 0-21 itch severity scale, according to data presented at the 2022 American Academy of Allergy, Asthma and Immunology (AAAAI) meeting.
Patients in the dupilumab group also showed a 65% reduction in the severity of urticaria activity (itch and hives) compared with 37% of those on placebo. Overall rates of adverse events were similar between groups; the most common were injection site reactions, according to the company.
The second study (LIBERTY-CUPID B) assessed efficacy and safety of dupilumab in 108 patients with CSU aged 12-80 years who were symptomatic despite standard-of-care treatment and were intolerant or incomplete responders to the anti-IgE antibody omalizumab (Xolair), approved for CSU. Last year, the company announced that this study had been halted after an interim analysis found that while there were positive numerical trends in reducing itch and hives, they “did not meet statistical significance.” In the March 7 press release, the company said that results from this study provide “additional supporting data” for the approval application.
The target date for the FDA’s decision is Oct. 22, 2023, according to Regeneron. Regeneron and Sanofi also are investigating dupilumab for treating chronic inducible urticaria triggered by cold in a phase 3 study.
FDA accepts application for topical molluscum treatment
If approved, berdazimer gel would be the first FDA-approved prescription product for molluscum contagiosum in the United States, according to the company, Novan. The active ingredient in berdazimer gel 10.3% is berdazimer sodium, a novel nitric oxide–releasing agent.
Molluscum contagiosum is a benign but contagious skin infection characterized by red papules on the face, trunk, limbs, and axillae that may persist for years if left untreated.
The treatment was evaluated in the B-SIMPLE4 study, a phase 3 clinical trial including 891 individuals with molluscum contagiosum aged 6 months and older, with 3-70 raised lesions The mean age of the patients was approximately 7 years (range, 0.9-47.5 years) and 85.5% were White (4.7% were Black, 21.2% were Hispanic, and 1.4% were Asian). Study participants were randomized to berdazimer gel 10.3% or a vehicle gel applied as a thin layer to all lesions once daily for 12 weeks.
The full results of the B-SIMPLE4 study were published in JAMA Dermatology in July 2022. After 12 weeks of treatment, 32.4% of patients in the berdazimer group met the primary outcome of complete clearance of all lesions, versus 19.7% of those on the vehicle (P < .001). The rates of adverse events were similar and low in both groups. The most common adverse events in both groups were application-site pain and erythema, and most cases were mild or moderate. A total of 4.1% of berdazimer patients and 0.7% of placebo patients experienced adverse events that prompted treatment discontinuation.
The Prescription Drug User Fee goal date for the approval of berdazimer 10.3% for molluscum contagiosum is set for Jan. 5, 2024, according to Novan.
If approved, berdazimer gel would be the first FDA-approved prescription product for molluscum contagiosum in the United States, according to the company, Novan. The active ingredient in berdazimer gel 10.3% is berdazimer sodium, a novel nitric oxide–releasing agent.
Molluscum contagiosum is a benign but contagious skin infection characterized by red papules on the face, trunk, limbs, and axillae that may persist for years if left untreated.
The treatment was evaluated in the B-SIMPLE4 study, a phase 3 clinical trial including 891 individuals with molluscum contagiosum aged 6 months and older, with 3-70 raised lesions The mean age of the patients was approximately 7 years (range, 0.9-47.5 years) and 85.5% were White (4.7% were Black, 21.2% were Hispanic, and 1.4% were Asian). Study participants were randomized to berdazimer gel 10.3% or a vehicle gel applied as a thin layer to all lesions once daily for 12 weeks.
The full results of the B-SIMPLE4 study were published in JAMA Dermatology in July 2022. After 12 weeks of treatment, 32.4% of patients in the berdazimer group met the primary outcome of complete clearance of all lesions, versus 19.7% of those on the vehicle (P < .001). The rates of adverse events were similar and low in both groups. The most common adverse events in both groups were application-site pain and erythema, and most cases were mild or moderate. A total of 4.1% of berdazimer patients and 0.7% of placebo patients experienced adverse events that prompted treatment discontinuation.
The Prescription Drug User Fee goal date for the approval of berdazimer 10.3% for molluscum contagiosum is set for Jan. 5, 2024, according to Novan.
If approved, berdazimer gel would be the first FDA-approved prescription product for molluscum contagiosum in the United States, according to the company, Novan. The active ingredient in berdazimer gel 10.3% is berdazimer sodium, a novel nitric oxide–releasing agent.
Molluscum contagiosum is a benign but contagious skin infection characterized by red papules on the face, trunk, limbs, and axillae that may persist for years if left untreated.
The treatment was evaluated in the B-SIMPLE4 study, a phase 3 clinical trial including 891 individuals with molluscum contagiosum aged 6 months and older, with 3-70 raised lesions The mean age of the patients was approximately 7 years (range, 0.9-47.5 years) and 85.5% were White (4.7% were Black, 21.2% were Hispanic, and 1.4% were Asian). Study participants were randomized to berdazimer gel 10.3% or a vehicle gel applied as a thin layer to all lesions once daily for 12 weeks.
The full results of the B-SIMPLE4 study were published in JAMA Dermatology in July 2022. After 12 weeks of treatment, 32.4% of patients in the berdazimer group met the primary outcome of complete clearance of all lesions, versus 19.7% of those on the vehicle (P < .001). The rates of adverse events were similar and low in both groups. The most common adverse events in both groups were application-site pain and erythema, and most cases were mild or moderate. A total of 4.1% of berdazimer patients and 0.7% of placebo patients experienced adverse events that prompted treatment discontinuation.
The Prescription Drug User Fee goal date for the approval of berdazimer 10.3% for molluscum contagiosum is set for Jan. 5, 2024, according to Novan.
Antibiotics and SJS/TEN: Study provides global prevalence
of SJS/TEN in connection with antibiotics.
“SJS/TEN is considered the most severe form of drug hypersensitivity reaction, and antibiotics are an important risk,” Erika Yue Lee, MD, and associates wrote in JAMA Dermatology.
Their analysis, which involved 38 studies published since 1987 with 2,917 patients from more than 20 countries, showed that 86% of all SJS/TEN cases were associated with a single drug, with the rest involving multiple drug triggers, infections, or other causes. More than a quarter (28%) of those patients had used an antibiotic, and the sulfonamides were the class most often triggering SJS/TEN, said Dr. Lee of the University of Toronto and associates.
Sulfonamides were responsible for 32% of the antibiotic-associated cases, which works out to 11% of all SJS/TEN cases included in the analysis. Penicillins were next with 22% of all antibiotic-associated cases, followed by the cephalosporins (11%), fluoroquinolones (4%), and macrolides (2%), the investigators reported.
A subgroup analysis conducted by age indicated that “there was no difference in the proportion of antibiotics associated with SJS/TEN between adult and pediatric groups,” they noted.
There were differences, however, among the various antibiotic classes. Sulfonamides represented 54% of antibiotic-triggered reactions in children, compared with 25% in adults, but adults were significantly more likely to have cephalosporin (23%) and fluoroquinolone (5%) involvement than were children (2% and 0, respectively). Macrolide-induced SJS/TEN was more common in children (18% vs. 1%), while the penicillin rate was 18% for both age groups, Dr. Lee and associates said.
A second subgroup analysis establishing the proportion of antibiotic-induced SJS/TEN by continent ranked Australia highest with 43%, but that was based on only one study of 42 patients. North America was slightly lower at 37%, but the analysis included 14 studies and 932 patients. Asia’s 16 studies and 1,298 patients were divided into three regions, with the lowest being the southeast at 16%, according to the researchers.
“Global sulfonamide antibiotic use has been decreasing since 2000 despite an ongoing upward trend of use in other antibiotic classes,” they wrote, but “antibiotics remain one of the most common culprit drugs for SJS/TEN in both adults and children worldwide.”
One of Dr. Lee’s associates has received personal fees from Janssen, AstraZeneca, UpToDate, Verve, BioCryst, Regeneron Pharmaceuticals, and Novavax and has served as codirector of IIID Pty Ltd, which holds a patent for HLA-B*57:01 testing and has a patent pending for detection of HLA-A*32:01 in connection with diagnosing drug reaction without any financial remuneration outside this study.
of SJS/TEN in connection with antibiotics.
“SJS/TEN is considered the most severe form of drug hypersensitivity reaction, and antibiotics are an important risk,” Erika Yue Lee, MD, and associates wrote in JAMA Dermatology.
Their analysis, which involved 38 studies published since 1987 with 2,917 patients from more than 20 countries, showed that 86% of all SJS/TEN cases were associated with a single drug, with the rest involving multiple drug triggers, infections, or other causes. More than a quarter (28%) of those patients had used an antibiotic, and the sulfonamides were the class most often triggering SJS/TEN, said Dr. Lee of the University of Toronto and associates.
Sulfonamides were responsible for 32% of the antibiotic-associated cases, which works out to 11% of all SJS/TEN cases included in the analysis. Penicillins were next with 22% of all antibiotic-associated cases, followed by the cephalosporins (11%), fluoroquinolones (4%), and macrolides (2%), the investigators reported.
A subgroup analysis conducted by age indicated that “there was no difference in the proportion of antibiotics associated with SJS/TEN between adult and pediatric groups,” they noted.
There were differences, however, among the various antibiotic classes. Sulfonamides represented 54% of antibiotic-triggered reactions in children, compared with 25% in adults, but adults were significantly more likely to have cephalosporin (23%) and fluoroquinolone (5%) involvement than were children (2% and 0, respectively). Macrolide-induced SJS/TEN was more common in children (18% vs. 1%), while the penicillin rate was 18% for both age groups, Dr. Lee and associates said.
A second subgroup analysis establishing the proportion of antibiotic-induced SJS/TEN by continent ranked Australia highest with 43%, but that was based on only one study of 42 patients. North America was slightly lower at 37%, but the analysis included 14 studies and 932 patients. Asia’s 16 studies and 1,298 patients were divided into three regions, with the lowest being the southeast at 16%, according to the researchers.
“Global sulfonamide antibiotic use has been decreasing since 2000 despite an ongoing upward trend of use in other antibiotic classes,” they wrote, but “antibiotics remain one of the most common culprit drugs for SJS/TEN in both adults and children worldwide.”
One of Dr. Lee’s associates has received personal fees from Janssen, AstraZeneca, UpToDate, Verve, BioCryst, Regeneron Pharmaceuticals, and Novavax and has served as codirector of IIID Pty Ltd, which holds a patent for HLA-B*57:01 testing and has a patent pending for detection of HLA-A*32:01 in connection with diagnosing drug reaction without any financial remuneration outside this study.
of SJS/TEN in connection with antibiotics.
“SJS/TEN is considered the most severe form of drug hypersensitivity reaction, and antibiotics are an important risk,” Erika Yue Lee, MD, and associates wrote in JAMA Dermatology.
Their analysis, which involved 38 studies published since 1987 with 2,917 patients from more than 20 countries, showed that 86% of all SJS/TEN cases were associated with a single drug, with the rest involving multiple drug triggers, infections, or other causes. More than a quarter (28%) of those patients had used an antibiotic, and the sulfonamides were the class most often triggering SJS/TEN, said Dr. Lee of the University of Toronto and associates.
Sulfonamides were responsible for 32% of the antibiotic-associated cases, which works out to 11% of all SJS/TEN cases included in the analysis. Penicillins were next with 22% of all antibiotic-associated cases, followed by the cephalosporins (11%), fluoroquinolones (4%), and macrolides (2%), the investigators reported.
A subgroup analysis conducted by age indicated that “there was no difference in the proportion of antibiotics associated with SJS/TEN between adult and pediatric groups,” they noted.
There were differences, however, among the various antibiotic classes. Sulfonamides represented 54% of antibiotic-triggered reactions in children, compared with 25% in adults, but adults were significantly more likely to have cephalosporin (23%) and fluoroquinolone (5%) involvement than were children (2% and 0, respectively). Macrolide-induced SJS/TEN was more common in children (18% vs. 1%), while the penicillin rate was 18% for both age groups, Dr. Lee and associates said.
A second subgroup analysis establishing the proportion of antibiotic-induced SJS/TEN by continent ranked Australia highest with 43%, but that was based on only one study of 42 patients. North America was slightly lower at 37%, but the analysis included 14 studies and 932 patients. Asia’s 16 studies and 1,298 patients were divided into three regions, with the lowest being the southeast at 16%, according to the researchers.
“Global sulfonamide antibiotic use has been decreasing since 2000 despite an ongoing upward trend of use in other antibiotic classes,” they wrote, but “antibiotics remain one of the most common culprit drugs for SJS/TEN in both adults and children worldwide.”
One of Dr. Lee’s associates has received personal fees from Janssen, AstraZeneca, UpToDate, Verve, BioCryst, Regeneron Pharmaceuticals, and Novavax and has served as codirector of IIID Pty Ltd, which holds a patent for HLA-B*57:01 testing and has a patent pending for detection of HLA-A*32:01 in connection with diagnosing drug reaction without any financial remuneration outside this study.
FROM JAMA DERMATOLOGY
Could ChatGPT write this column?
, but I am starting to think it is the real deal. Just how powerful is it? Well, ChatGPT might in fact be writing this column right now. It isn’t. No really, it’s me. But if not for the few cues (“super-buzzy”) that you’ll recognize as my writing voice, there might not be any way for you to know if I wrote this or not.
It’s perfectly OK if you’ve no clue what I’m talking about. ChatGPT is an AI chatbot that burst into public view just a couple months ago. Not your parent’s chatbot, this one is capable of answering questions in conversational language. It is jaw-droppingly good. Like Google, you can type in a question and it offers you answers. Rather than giving you a list of websites and a few Wikipedia blurbs, however, ChatGPT answers your question in human-like text. It can also create content on demand. For example, I asked it to write a Valentine poem to a dermatologist, and it gave me five stanzas starting with:
Oh gentle healer of skin so fair,
Not good enough to send to my wife. But not bad.
If you ask it again, it will create a whole new one for you. Amusing, yes? What if you asked ChatGPT to explain psoriasis, or any medical condition for that matter, to a patient? The replies are quite good. Some even better than what I’m currently using for my patients. It can also offer treatment recommendations, vacation advice, and plan, with recipes, a dinner party for six with one vegan and one gluten-free couple. If you are a programmer, it can write code. Ask it for a Wordpress plugin to add to your website and your eyes will widen as you see it magically appear before you. What if you find that you just don’t like your daughter’s new boyfriend? Yep, it will write the text or email for you to help with this discussion. I’ve saved that one.
I tried “What are treatments for bullous pemphigoid that has been refractory to topical steroid, oral prednisone, and oral tetracyclines?” It replied with five ideas, including the standard methotrexate and azathioprine but also IVIG, Rituxan, even other biologics. Write an op note? Appeal a denied prior authorization to a payer? Write a clinic note for a complete skin exam? Check, check, check. Are you starting to think it might be the real deal, too?
Before we sell the farm though, there are significant limitations. Despite how swotty ChatGPT seems, it is not smart. That is, “it” has no idea what “it” is saying. ChatGPT is an incredibly sophisticated algorithm that has learned the probability of what word comes next in a conversation. To do so, it read the Internet. Billions (trillions?) of words make it possible to predict what is the best answer to any question. But – it’s only as good as the Internet, so there’s that. My patient who used ChatGPT has dissecting cellulitis and asked what to do for scarring alopecia. Some of the answers were reasonable, but some, such as transplanting hairs into the scarred areas, would not likely be helpful. That is unless ChatGPT knows something I don’t.
Having wasted hours of time playing with this thing rather than writing my column, I asked ChatGPT to write an article about itself in the style of Christopher Hitchens. It was nothing like his incisive and eloquent prose, but it wrote 500 words in a few seconds ending with:
“The reality is that there is no substitute for human interaction and empathy in the field of dermatology. Dermatologists must be cautious in their adoption of ChatGPT and ensure that they are not sacrificing the quality of patient care in the pursuit of efficiency and convenience.”
I’m not sure I could have said it better myself.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at dermnews@mdedge.com.
, but I am starting to think it is the real deal. Just how powerful is it? Well, ChatGPT might in fact be writing this column right now. It isn’t. No really, it’s me. But if not for the few cues (“super-buzzy”) that you’ll recognize as my writing voice, there might not be any way for you to know if I wrote this or not.
It’s perfectly OK if you’ve no clue what I’m talking about. ChatGPT is an AI chatbot that burst into public view just a couple months ago. Not your parent’s chatbot, this one is capable of answering questions in conversational language. It is jaw-droppingly good. Like Google, you can type in a question and it offers you answers. Rather than giving you a list of websites and a few Wikipedia blurbs, however, ChatGPT answers your question in human-like text. It can also create content on demand. For example, I asked it to write a Valentine poem to a dermatologist, and it gave me five stanzas starting with:
Oh gentle healer of skin so fair,
Not good enough to send to my wife. But not bad.
If you ask it again, it will create a whole new one for you. Amusing, yes? What if you asked ChatGPT to explain psoriasis, or any medical condition for that matter, to a patient? The replies are quite good. Some even better than what I’m currently using for my patients. It can also offer treatment recommendations, vacation advice, and plan, with recipes, a dinner party for six with one vegan and one gluten-free couple. If you are a programmer, it can write code. Ask it for a Wordpress plugin to add to your website and your eyes will widen as you see it magically appear before you. What if you find that you just don’t like your daughter’s new boyfriend? Yep, it will write the text or email for you to help with this discussion. I’ve saved that one.
I tried “What are treatments for bullous pemphigoid that has been refractory to topical steroid, oral prednisone, and oral tetracyclines?” It replied with five ideas, including the standard methotrexate and azathioprine but also IVIG, Rituxan, even other biologics. Write an op note? Appeal a denied prior authorization to a payer? Write a clinic note for a complete skin exam? Check, check, check. Are you starting to think it might be the real deal, too?
Before we sell the farm though, there are significant limitations. Despite how swotty ChatGPT seems, it is not smart. That is, “it” has no idea what “it” is saying. ChatGPT is an incredibly sophisticated algorithm that has learned the probability of what word comes next in a conversation. To do so, it read the Internet. Billions (trillions?) of words make it possible to predict what is the best answer to any question. But – it’s only as good as the Internet, so there’s that. My patient who used ChatGPT has dissecting cellulitis and asked what to do for scarring alopecia. Some of the answers were reasonable, but some, such as transplanting hairs into the scarred areas, would not likely be helpful. That is unless ChatGPT knows something I don’t.
Having wasted hours of time playing with this thing rather than writing my column, I asked ChatGPT to write an article about itself in the style of Christopher Hitchens. It was nothing like his incisive and eloquent prose, but it wrote 500 words in a few seconds ending with:
“The reality is that there is no substitute for human interaction and empathy in the field of dermatology. Dermatologists must be cautious in their adoption of ChatGPT and ensure that they are not sacrificing the quality of patient care in the pursuit of efficiency and convenience.”
I’m not sure I could have said it better myself.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at dermnews@mdedge.com.
, but I am starting to think it is the real deal. Just how powerful is it? Well, ChatGPT might in fact be writing this column right now. It isn’t. No really, it’s me. But if not for the few cues (“super-buzzy”) that you’ll recognize as my writing voice, there might not be any way for you to know if I wrote this or not.
It’s perfectly OK if you’ve no clue what I’m talking about. ChatGPT is an AI chatbot that burst into public view just a couple months ago. Not your parent’s chatbot, this one is capable of answering questions in conversational language. It is jaw-droppingly good. Like Google, you can type in a question and it offers you answers. Rather than giving you a list of websites and a few Wikipedia blurbs, however, ChatGPT answers your question in human-like text. It can also create content on demand. For example, I asked it to write a Valentine poem to a dermatologist, and it gave me five stanzas starting with:
Oh gentle healer of skin so fair,
Not good enough to send to my wife. But not bad.
If you ask it again, it will create a whole new one for you. Amusing, yes? What if you asked ChatGPT to explain psoriasis, or any medical condition for that matter, to a patient? The replies are quite good. Some even better than what I’m currently using for my patients. It can also offer treatment recommendations, vacation advice, and plan, with recipes, a dinner party for six with one vegan and one gluten-free couple. If you are a programmer, it can write code. Ask it for a Wordpress plugin to add to your website and your eyes will widen as you see it magically appear before you. What if you find that you just don’t like your daughter’s new boyfriend? Yep, it will write the text or email for you to help with this discussion. I’ve saved that one.
I tried “What are treatments for bullous pemphigoid that has been refractory to topical steroid, oral prednisone, and oral tetracyclines?” It replied with five ideas, including the standard methotrexate and azathioprine but also IVIG, Rituxan, even other biologics. Write an op note? Appeal a denied prior authorization to a payer? Write a clinic note for a complete skin exam? Check, check, check. Are you starting to think it might be the real deal, too?
Before we sell the farm though, there are significant limitations. Despite how swotty ChatGPT seems, it is not smart. That is, “it” has no idea what “it” is saying. ChatGPT is an incredibly sophisticated algorithm that has learned the probability of what word comes next in a conversation. To do so, it read the Internet. Billions (trillions?) of words make it possible to predict what is the best answer to any question. But – it’s only as good as the Internet, so there’s that. My patient who used ChatGPT has dissecting cellulitis and asked what to do for scarring alopecia. Some of the answers were reasonable, but some, such as transplanting hairs into the scarred areas, would not likely be helpful. That is unless ChatGPT knows something I don’t.
Having wasted hours of time playing with this thing rather than writing my column, I asked ChatGPT to write an article about itself in the style of Christopher Hitchens. It was nothing like his incisive and eloquent prose, but it wrote 500 words in a few seconds ending with:
“The reality is that there is no substitute for human interaction and empathy in the field of dermatology. Dermatologists must be cautious in their adoption of ChatGPT and ensure that they are not sacrificing the quality of patient care in the pursuit of efficiency and convenience.”
I’m not sure I could have said it better myself.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at dermnews@mdedge.com.
Secukinumab shows benefit for hidradenitis suppurativa out to 52 weeks
results from two pivotal phase 3 clinical trials showed.
The findings build on week 16 data from two trials – SUNSHINE and SUNRISE – that investigated the efficacy, safety, and tolerability of the interleukin-17A inhibitor secukinumab (Cosentyx) versus placebo in the treatment of moderate to severe HS, and were presented at the 2022 annual congress of the European Academy of Dermatology and Venereology. In those studies, at 16 weeks, 42%-46% of patients achieved an HS Clinical Response (HiSCR) – the primary outcome measure in both trials. For the most recent analysis, which was published in The Lancet, investigators found that, at 52 weeks, 56.4% of patients in SUNSHINE and 65% of patients in SUNRISE who received secukinumab 300 mg every 2 weeks achieved a HiSCR, compared with 56.3% of patients in SUNSHINE and 62.2% of patients in SUNRISE who received secukinumab 300 mg every 4 weeks.
“This is great news for people with HS: it improves our knowledge about how to best treat patients today and leads us to new areas that will help us treat them even better in the future,” Alexa B. Kimball, MD, MPH, the lead investigator for both trials, said in an interview. “Dermatologists have been using biologics for decades. This data provides clinicians with information they can use to easily expand their HS management repertoire to include secukinumab.”
To date, the tumor necrosis factor inhibitor adalimumab is the only approved biologic therapy approved for the treatment of moderate-to-severe HS, in people aged 12 years and older.
The two trials were conducted in 40 countries, with SUNSHINE enrolling 541 patients, and SUNRISE enrolling 543. Patients in each study were randomized to one of three experimental arms: secukinumab 300 mg every 2 weeks after five weekly loading doses; secukinumab 300 mg every 4 weeks after five weekly loading doses; placebo dose every 2 weeks after five weekly placebo doses. The mean age was 37 years, about 55% were female, and about 76% were White (about 9% were Black and about 10% were Asian). Dr. Kimball, investigator at Beth Israel Deaconess Medical Center and professor of dermatology at Harvard Medical School, Boston, and coauthors observed that the group that received secukinumab every 4 weeks did not meet the primary endpoint in the SUNSHINE trial, but it was met in the SUNRISE trial. “Research and subgroup analyses are required and might improve our understanding of the effect of patient characteristics on treatment response and further refine the dosing recommendations for different populations,” they wrote.
In a pooled analysis, 55.2% of patients from SUNSHINE and SUNRISE who received secukinumab 300 mg every 2 weeks had a reduction in pain as measured by the Patient’s Global Assessment of Skin Pain Numeric Rating Scale, compared with 53% of patients from SUNSHINE and SUNRISE who received secukinumab 300 mg every 4 weeks. The most common adverse events up to week 16 in both trials were headache, nasopharyngitis, and hidradenitis; no deaths occurred.
“One limitation of most studies in HS is that the placebo-controlled period is short, so the data obtained after that time is harder to interpret,” Dr. Kimball said in an interview. “In my experience, optimizing treatment can take almost a year and I hope we will see longer controlled periods in future studies.” Another limitation of the studies she acknowledged was a modest imbalance with respect to disease severity between the treatment groups at baseline. “It was a little surprising that some imbalances in the characteristics of randomized subjects in different arms of the study impacted efficacy levels,” she said. “We’ll need to continue to identify how to match patients and dosing regimens to get the best results.”
According to a press release from Novartis, trial results have been submitted to regulatory authorities in Europe and the United States, and decisions are expected in 2023. If approved, secukinumab will be the first and only IL-17 inhibitor for the treatment of moderate to severe HS.
Adam Friedman, MD, professor and chair of dermatology at George Washington University, Washington, described HS as “an extraordinarily disabling, painful, deforming condition for which we only have one [Food and Drug Administration]–approved systemic therapy, requiring us to wear our ‘off-label bandit’ name tags proudly to tackle therapeutic challenges.
“Anecdotally,” he said, “we dabble with off-label biologics indicated for psoriasis in this setting, though limitations exist ranging from lack of large-scale clinical data to the recurring theme that psoriasis dosing typically doesn’t cut it, making access to said medications even more difficult. Investigators in this study addresses both gaps very effectively, and I for one welcome the implications and hopeful regulatory impact with open arms.”
The study was funded by Novartis. Dr. Kimball disclosed numerous conflicts of interest from various pharmaceutical companies. Dr. Friedman reported financial relationships with Sanova, Pfizer, Novartis, and other companies.
results from two pivotal phase 3 clinical trials showed.
The findings build on week 16 data from two trials – SUNSHINE and SUNRISE – that investigated the efficacy, safety, and tolerability of the interleukin-17A inhibitor secukinumab (Cosentyx) versus placebo in the treatment of moderate to severe HS, and were presented at the 2022 annual congress of the European Academy of Dermatology and Venereology. In those studies, at 16 weeks, 42%-46% of patients achieved an HS Clinical Response (HiSCR) – the primary outcome measure in both trials. For the most recent analysis, which was published in The Lancet, investigators found that, at 52 weeks, 56.4% of patients in SUNSHINE and 65% of patients in SUNRISE who received secukinumab 300 mg every 2 weeks achieved a HiSCR, compared with 56.3% of patients in SUNSHINE and 62.2% of patients in SUNRISE who received secukinumab 300 mg every 4 weeks.
“This is great news for people with HS: it improves our knowledge about how to best treat patients today and leads us to new areas that will help us treat them even better in the future,” Alexa B. Kimball, MD, MPH, the lead investigator for both trials, said in an interview. “Dermatologists have been using biologics for decades. This data provides clinicians with information they can use to easily expand their HS management repertoire to include secukinumab.”
To date, the tumor necrosis factor inhibitor adalimumab is the only approved biologic therapy approved for the treatment of moderate-to-severe HS, in people aged 12 years and older.
The two trials were conducted in 40 countries, with SUNSHINE enrolling 541 patients, and SUNRISE enrolling 543. Patients in each study were randomized to one of three experimental arms: secukinumab 300 mg every 2 weeks after five weekly loading doses; secukinumab 300 mg every 4 weeks after five weekly loading doses; placebo dose every 2 weeks after five weekly placebo doses. The mean age was 37 years, about 55% were female, and about 76% were White (about 9% were Black and about 10% were Asian). Dr. Kimball, investigator at Beth Israel Deaconess Medical Center and professor of dermatology at Harvard Medical School, Boston, and coauthors observed that the group that received secukinumab every 4 weeks did not meet the primary endpoint in the SUNSHINE trial, but it was met in the SUNRISE trial. “Research and subgroup analyses are required and might improve our understanding of the effect of patient characteristics on treatment response and further refine the dosing recommendations for different populations,” they wrote.
In a pooled analysis, 55.2% of patients from SUNSHINE and SUNRISE who received secukinumab 300 mg every 2 weeks had a reduction in pain as measured by the Patient’s Global Assessment of Skin Pain Numeric Rating Scale, compared with 53% of patients from SUNSHINE and SUNRISE who received secukinumab 300 mg every 4 weeks. The most common adverse events up to week 16 in both trials were headache, nasopharyngitis, and hidradenitis; no deaths occurred.
“One limitation of most studies in HS is that the placebo-controlled period is short, so the data obtained after that time is harder to interpret,” Dr. Kimball said in an interview. “In my experience, optimizing treatment can take almost a year and I hope we will see longer controlled periods in future studies.” Another limitation of the studies she acknowledged was a modest imbalance with respect to disease severity between the treatment groups at baseline. “It was a little surprising that some imbalances in the characteristics of randomized subjects in different arms of the study impacted efficacy levels,” she said. “We’ll need to continue to identify how to match patients and dosing regimens to get the best results.”
According to a press release from Novartis, trial results have been submitted to regulatory authorities in Europe and the United States, and decisions are expected in 2023. If approved, secukinumab will be the first and only IL-17 inhibitor for the treatment of moderate to severe HS.
Adam Friedman, MD, professor and chair of dermatology at George Washington University, Washington, described HS as “an extraordinarily disabling, painful, deforming condition for which we only have one [Food and Drug Administration]–approved systemic therapy, requiring us to wear our ‘off-label bandit’ name tags proudly to tackle therapeutic challenges.
“Anecdotally,” he said, “we dabble with off-label biologics indicated for psoriasis in this setting, though limitations exist ranging from lack of large-scale clinical data to the recurring theme that psoriasis dosing typically doesn’t cut it, making access to said medications even more difficult. Investigators in this study addresses both gaps very effectively, and I for one welcome the implications and hopeful regulatory impact with open arms.”
The study was funded by Novartis. Dr. Kimball disclosed numerous conflicts of interest from various pharmaceutical companies. Dr. Friedman reported financial relationships with Sanova, Pfizer, Novartis, and other companies.
results from two pivotal phase 3 clinical trials showed.
The findings build on week 16 data from two trials – SUNSHINE and SUNRISE – that investigated the efficacy, safety, and tolerability of the interleukin-17A inhibitor secukinumab (Cosentyx) versus placebo in the treatment of moderate to severe HS, and were presented at the 2022 annual congress of the European Academy of Dermatology and Venereology. In those studies, at 16 weeks, 42%-46% of patients achieved an HS Clinical Response (HiSCR) – the primary outcome measure in both trials. For the most recent analysis, which was published in The Lancet, investigators found that, at 52 weeks, 56.4% of patients in SUNSHINE and 65% of patients in SUNRISE who received secukinumab 300 mg every 2 weeks achieved a HiSCR, compared with 56.3% of patients in SUNSHINE and 62.2% of patients in SUNRISE who received secukinumab 300 mg every 4 weeks.
“This is great news for people with HS: it improves our knowledge about how to best treat patients today and leads us to new areas that will help us treat them even better in the future,” Alexa B. Kimball, MD, MPH, the lead investigator for both trials, said in an interview. “Dermatologists have been using biologics for decades. This data provides clinicians with information they can use to easily expand their HS management repertoire to include secukinumab.”
To date, the tumor necrosis factor inhibitor adalimumab is the only approved biologic therapy approved for the treatment of moderate-to-severe HS, in people aged 12 years and older.
The two trials were conducted in 40 countries, with SUNSHINE enrolling 541 patients, and SUNRISE enrolling 543. Patients in each study were randomized to one of three experimental arms: secukinumab 300 mg every 2 weeks after five weekly loading doses; secukinumab 300 mg every 4 weeks after five weekly loading doses; placebo dose every 2 weeks after five weekly placebo doses. The mean age was 37 years, about 55% were female, and about 76% were White (about 9% were Black and about 10% were Asian). Dr. Kimball, investigator at Beth Israel Deaconess Medical Center and professor of dermatology at Harvard Medical School, Boston, and coauthors observed that the group that received secukinumab every 4 weeks did not meet the primary endpoint in the SUNSHINE trial, but it was met in the SUNRISE trial. “Research and subgroup analyses are required and might improve our understanding of the effect of patient characteristics on treatment response and further refine the dosing recommendations for different populations,” they wrote.
In a pooled analysis, 55.2% of patients from SUNSHINE and SUNRISE who received secukinumab 300 mg every 2 weeks had a reduction in pain as measured by the Patient’s Global Assessment of Skin Pain Numeric Rating Scale, compared with 53% of patients from SUNSHINE and SUNRISE who received secukinumab 300 mg every 4 weeks. The most common adverse events up to week 16 in both trials were headache, nasopharyngitis, and hidradenitis; no deaths occurred.
“One limitation of most studies in HS is that the placebo-controlled period is short, so the data obtained after that time is harder to interpret,” Dr. Kimball said in an interview. “In my experience, optimizing treatment can take almost a year and I hope we will see longer controlled periods in future studies.” Another limitation of the studies she acknowledged was a modest imbalance with respect to disease severity between the treatment groups at baseline. “It was a little surprising that some imbalances in the characteristics of randomized subjects in different arms of the study impacted efficacy levels,” she said. “We’ll need to continue to identify how to match patients and dosing regimens to get the best results.”
According to a press release from Novartis, trial results have been submitted to regulatory authorities in Europe and the United States, and decisions are expected in 2023. If approved, secukinumab will be the first and only IL-17 inhibitor for the treatment of moderate to severe HS.
Adam Friedman, MD, professor and chair of dermatology at George Washington University, Washington, described HS as “an extraordinarily disabling, painful, deforming condition for which we only have one [Food and Drug Administration]–approved systemic therapy, requiring us to wear our ‘off-label bandit’ name tags proudly to tackle therapeutic challenges.
“Anecdotally,” he said, “we dabble with off-label biologics indicated for psoriasis in this setting, though limitations exist ranging from lack of large-scale clinical data to the recurring theme that psoriasis dosing typically doesn’t cut it, making access to said medications even more difficult. Investigators in this study addresses both gaps very effectively, and I for one welcome the implications and hopeful regulatory impact with open arms.”
The study was funded by Novartis. Dr. Kimball disclosed numerous conflicts of interest from various pharmaceutical companies. Dr. Friedman reported financial relationships with Sanova, Pfizer, Novartis, and other companies.
FROM THE LANCET
Notalgia paresthetica: Difelikefalin helps upper-back itch, but with side effects
from a randomized, double-blinded placebo-controlled trial suggest.
However, side effects were significant and caused 19% in the intervention group to discontinue the trial versus 6% in the placebo group.
Results of the study were published online in the New England Journal of Medicine.
There is currently no treatment approved by the U.S. Food and Drug Administration for the common condition, which typically causes itch in the hard-to-reach area between the shoulder blades or mid-back.
Drug reduced moderate to severe itch
Difelikefalin – a selective kappa-opioid receptor agonist – is FDA approved only as an injection for treating moderate to severe itch from chronic kidney disease in adults undergoing hemodialysis, and is marketed as Korsuva for that indication.
However, in a new trial, led by Brian S. Kim, MD, professor of dermatology and vice chair of research at the Icahn School of Medicine at Mount Sinai, New York, the drug gave moderate relief to patients with notalgia paresthetica who had moderate to severe itch.
Patients were randomly assigned 1:1 to receive oral difelikefalin 2 mg or a placebo twice daily for 8 weeks. The primary outcome was change in the weekly average of the daily 0-10 Worst Itch Numeric Rating Scale, for which 0 is “no itch” and 10 is “worst itch imaginable.”
Secondary clinical outcomes were itch-related quality-of-life and itch-related sleep measures.
The study included 126 patients; 62 received difelikefalin and 63 received placebo. One patient assigned to the difelikefalin group withdrew consent before the first dose.
The average baseline score on the Worst Itch scale was 7.6 (severe itch) in each group. Mean scores in the difelikefalin dropped by 4 points versus 2.4 points in the placebo group (95% confidence interval, −2.6 to −0.6; P = .001).
Difelikefalin did not help with sleep disturbance, compared with placebo, “except possibly in patients with impaired sleep at baseline,” the authors write. “Larger and longer trials are required to determine the effect and risks of difelikefalin treatment in this disorder.”
In a Mount Sinai press release, Dr. Kim, who is also director of the Lebwohl Center for Neuroinflammation and Sensation at Mount Sinai, called the team’s findings “encouraging.”
“The encouraging results achieved in this trial could reenergize the field and mark an important step toward improving symptoms of itch for patients with notalgia paresthetica,” he said.
Side effects ‘worrisome’
The main side effects reported included headaches, dizziness, constipation and increased urine output.
Shawn Kwatra, MD, director of the Johns Hopkins Itch Center, Baltimore, told this news organization that dizziness was “especially worrisome,” noting the average age of participants in the trial was 59-60 years. “We are very concerned about folks having falls or hip fractures,” he said.
“Things we use more commonly are topical steroids, topical capsaicin, the capsaicin patch, muscle strengthening, and gabapentin,” Dr. Kwatra said. “Off-label we use botulinum toxin (Botox) as well. I’m able to control” almost all of my notalgia paresthetica patients, he added.
In his view, for this type of drug, he said, “the right home for it is more for a generalized neuropathic pruritus or nociplastic itch vs. something very localized which is more amenable to topical therapies.”
He said that the associated central nervous system effects, such as dizziness and headache, “would limit therapeutic use to only the most severe cases in my mind.”
The trial was funded by Cara Therapeutics, manufacturer of difelikefalin.
Dr. Kim and coauthor Mark Lebwohl, MD, are paid consultants/advisers to Cara Therapeutics. Other coauthors also reported ties to Cara. Dr. Kwatra previously had done consulting work for Cara Therapeutics and is an advisory board member/consultant for AbbVie, Amgen, Arcutis Biotherapeutics, Aslan Pharmaceuticals, Castle Biosciences, Celldex Therapeutics, Galderma, Genzada Pharmaceuticals, Incyte Corporation, Johnson & Johnson, Leo Pharma, Novartis Pharmaceuticals Corporation, Pfizer, Regeneron Pharmaceuticals, and Sanofi and has served as an investigator for Galderma, Incyte, Pfizer, and Sanofi.
A version of this article first appeared on Medscape.com.
from a randomized, double-blinded placebo-controlled trial suggest.
However, side effects were significant and caused 19% in the intervention group to discontinue the trial versus 6% in the placebo group.
Results of the study were published online in the New England Journal of Medicine.
There is currently no treatment approved by the U.S. Food and Drug Administration for the common condition, which typically causes itch in the hard-to-reach area between the shoulder blades or mid-back.
Drug reduced moderate to severe itch
Difelikefalin – a selective kappa-opioid receptor agonist – is FDA approved only as an injection for treating moderate to severe itch from chronic kidney disease in adults undergoing hemodialysis, and is marketed as Korsuva for that indication.
However, in a new trial, led by Brian S. Kim, MD, professor of dermatology and vice chair of research at the Icahn School of Medicine at Mount Sinai, New York, the drug gave moderate relief to patients with notalgia paresthetica who had moderate to severe itch.
Patients were randomly assigned 1:1 to receive oral difelikefalin 2 mg or a placebo twice daily for 8 weeks. The primary outcome was change in the weekly average of the daily 0-10 Worst Itch Numeric Rating Scale, for which 0 is “no itch” and 10 is “worst itch imaginable.”
Secondary clinical outcomes were itch-related quality-of-life and itch-related sleep measures.
The study included 126 patients; 62 received difelikefalin and 63 received placebo. One patient assigned to the difelikefalin group withdrew consent before the first dose.
The average baseline score on the Worst Itch scale was 7.6 (severe itch) in each group. Mean scores in the difelikefalin dropped by 4 points versus 2.4 points in the placebo group (95% confidence interval, −2.6 to −0.6; P = .001).
Difelikefalin did not help with sleep disturbance, compared with placebo, “except possibly in patients with impaired sleep at baseline,” the authors write. “Larger and longer trials are required to determine the effect and risks of difelikefalin treatment in this disorder.”
In a Mount Sinai press release, Dr. Kim, who is also director of the Lebwohl Center for Neuroinflammation and Sensation at Mount Sinai, called the team’s findings “encouraging.”
“The encouraging results achieved in this trial could reenergize the field and mark an important step toward improving symptoms of itch for patients with notalgia paresthetica,” he said.
Side effects ‘worrisome’
The main side effects reported included headaches, dizziness, constipation and increased urine output.
Shawn Kwatra, MD, director of the Johns Hopkins Itch Center, Baltimore, told this news organization that dizziness was “especially worrisome,” noting the average age of participants in the trial was 59-60 years. “We are very concerned about folks having falls or hip fractures,” he said.
“Things we use more commonly are topical steroids, topical capsaicin, the capsaicin patch, muscle strengthening, and gabapentin,” Dr. Kwatra said. “Off-label we use botulinum toxin (Botox) as well. I’m able to control” almost all of my notalgia paresthetica patients, he added.
In his view, for this type of drug, he said, “the right home for it is more for a generalized neuropathic pruritus or nociplastic itch vs. something very localized which is more amenable to topical therapies.”
He said that the associated central nervous system effects, such as dizziness and headache, “would limit therapeutic use to only the most severe cases in my mind.”
The trial was funded by Cara Therapeutics, manufacturer of difelikefalin.
Dr. Kim and coauthor Mark Lebwohl, MD, are paid consultants/advisers to Cara Therapeutics. Other coauthors also reported ties to Cara. Dr. Kwatra previously had done consulting work for Cara Therapeutics and is an advisory board member/consultant for AbbVie, Amgen, Arcutis Biotherapeutics, Aslan Pharmaceuticals, Castle Biosciences, Celldex Therapeutics, Galderma, Genzada Pharmaceuticals, Incyte Corporation, Johnson & Johnson, Leo Pharma, Novartis Pharmaceuticals Corporation, Pfizer, Regeneron Pharmaceuticals, and Sanofi and has served as an investigator for Galderma, Incyte, Pfizer, and Sanofi.
A version of this article first appeared on Medscape.com.
from a randomized, double-blinded placebo-controlled trial suggest.
However, side effects were significant and caused 19% in the intervention group to discontinue the trial versus 6% in the placebo group.
Results of the study were published online in the New England Journal of Medicine.
There is currently no treatment approved by the U.S. Food and Drug Administration for the common condition, which typically causes itch in the hard-to-reach area between the shoulder blades or mid-back.
Drug reduced moderate to severe itch
Difelikefalin – a selective kappa-opioid receptor agonist – is FDA approved only as an injection for treating moderate to severe itch from chronic kidney disease in adults undergoing hemodialysis, and is marketed as Korsuva for that indication.
However, in a new trial, led by Brian S. Kim, MD, professor of dermatology and vice chair of research at the Icahn School of Medicine at Mount Sinai, New York, the drug gave moderate relief to patients with notalgia paresthetica who had moderate to severe itch.
Patients were randomly assigned 1:1 to receive oral difelikefalin 2 mg or a placebo twice daily for 8 weeks. The primary outcome was change in the weekly average of the daily 0-10 Worst Itch Numeric Rating Scale, for which 0 is “no itch” and 10 is “worst itch imaginable.”
Secondary clinical outcomes were itch-related quality-of-life and itch-related sleep measures.
The study included 126 patients; 62 received difelikefalin and 63 received placebo. One patient assigned to the difelikefalin group withdrew consent before the first dose.
The average baseline score on the Worst Itch scale was 7.6 (severe itch) in each group. Mean scores in the difelikefalin dropped by 4 points versus 2.4 points in the placebo group (95% confidence interval, −2.6 to −0.6; P = .001).
Difelikefalin did not help with sleep disturbance, compared with placebo, “except possibly in patients with impaired sleep at baseline,” the authors write. “Larger and longer trials are required to determine the effect and risks of difelikefalin treatment in this disorder.”
In a Mount Sinai press release, Dr. Kim, who is also director of the Lebwohl Center for Neuroinflammation and Sensation at Mount Sinai, called the team’s findings “encouraging.”
“The encouraging results achieved in this trial could reenergize the field and mark an important step toward improving symptoms of itch for patients with notalgia paresthetica,” he said.
Side effects ‘worrisome’
The main side effects reported included headaches, dizziness, constipation and increased urine output.
Shawn Kwatra, MD, director of the Johns Hopkins Itch Center, Baltimore, told this news organization that dizziness was “especially worrisome,” noting the average age of participants in the trial was 59-60 years. “We are very concerned about folks having falls or hip fractures,” he said.
“Things we use more commonly are topical steroids, topical capsaicin, the capsaicin patch, muscle strengthening, and gabapentin,” Dr. Kwatra said. “Off-label we use botulinum toxin (Botox) as well. I’m able to control” almost all of my notalgia paresthetica patients, he added.
In his view, for this type of drug, he said, “the right home for it is more for a generalized neuropathic pruritus or nociplastic itch vs. something very localized which is more amenable to topical therapies.”
He said that the associated central nervous system effects, such as dizziness and headache, “would limit therapeutic use to only the most severe cases in my mind.”
The trial was funded by Cara Therapeutics, manufacturer of difelikefalin.
Dr. Kim and coauthor Mark Lebwohl, MD, are paid consultants/advisers to Cara Therapeutics. Other coauthors also reported ties to Cara. Dr. Kwatra previously had done consulting work for Cara Therapeutics and is an advisory board member/consultant for AbbVie, Amgen, Arcutis Biotherapeutics, Aslan Pharmaceuticals, Castle Biosciences, Celldex Therapeutics, Galderma, Genzada Pharmaceuticals, Incyte Corporation, Johnson & Johnson, Leo Pharma, Novartis Pharmaceuticals Corporation, Pfizer, Regeneron Pharmaceuticals, and Sanofi and has served as an investigator for Galderma, Incyte, Pfizer, and Sanofi.
A version of this article first appeared on Medscape.com.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
A White male presented with a 1½-year history of a progressive hypoesthetic annular, hyperpigmented plaque on the upper arm
Paucibacillary tuberculoid leprosy is characterized by few anesthetic hypo- or hyperpigmented lesions and can be accompanied by palpable peripheral nerve enlargements.
Tuberculoid leprosy presents histologically with epithelioid histiocytes with lymphocytes and Langhans giant cells. Neurotropic granulomas are also characteristic of tuberculoid leprosy. Fite staining allows for the identification of the acid-fast bacilli of M. leprae, which in some cases are quite few in number. The standard mycobacterium stain, Ziehl-Neelsen, is a good option for M. tuberculosis, but because of the relative weak mycolic acid coat of M. leprae, the Fite stain is more appropriate for identifying M. leprae.
Clinically, other than the presence of fewer than five hypoesthetic lesions that are either hypopigmented or erythematous, tuberculoid leprosy often presents with additional peripheral nerve involvement that manifests as numbness and tingling in hands and feet.1 This patient denied any tingling, weakness, or numbness, outside of the anesthetic lesion on his posterior upper arm.
The patient, born in the United States, had a remote history of military travel to Iraq, Kuwait, and the Philippines, but had not traveled internationally within the last 15 years, apart from a cruise to the Bahamas. He denied any known contact with individuals with similar lesions. He denied a history of contact with armadillos, but acknowledged that they are native to where he resides in central Florida, and that he had seen them in his yard.
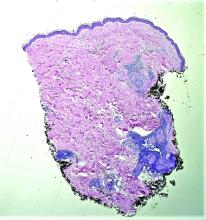
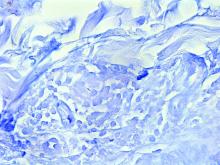
Histopathological examination revealed an unremarkable epidermis with a superficial and deep perivascular, periadnexal, and perineural lymphohistiocytic infiltrate. Fite stain revealed rare rod-shaped organisms (Figure 2). These findings are consistent with a diagnosis of paucibacillary, tuberculoid leprosy.
The patient’s travel history to highly endemic areas (Middle East), as well as possible environmental contact with armadillos – including contact with soil that the armadillos occupied – could explain plausible modes of transmission. Following consultation with our infectious disease department and the National Hansen’s Disease Program, our patient began a planned course of therapy with 18 months of minocycline, rifampin, and moxifloxacin.
Human-to-human transmission of HD has been well documented; however, zoonotic transmission – specifically via the nine-banded armadillo (Dasypus novemcinctus) – serves as another suggested means of transmission, especially in the Southeastern United States.2-6 Travel to highly-endemic areas increases the risk of contracting HD, which may take up to 20 years following contact with the bacteria to manifest clinically.
While central Florida was previously thought to be a nonendemic area of disease, the incidence of the disease in this region has increased in recent years.7 Human-to-human transmission, which remains a concern with immigration from highly-endemic regions, occurs via long-term contact with nasal droplets of an infected person.8,9
Many patients in regions with very few cases of leprosy deny travel to other endemic regions and contact with infected people. Thus, zoonotic transmission remains a legitimate concern in the Southeastern United States – accounting, at least in part, for many of the non–human-transmitted cases of leprosy.2,10 We encourage clinicians to maintain a high level of clinical suspicion for leprosy when evaluating patients presenting with hypoesthetic cutaneous lesions and to obtain a travel history and to ask about armadillo exposure.
This case and the photos were submitted by Ms. Smith, from the University of South Florida, Tampa; Dr. Hatch and Dr. Sarriera-Lazaro, from the department of dermatology and cutaneous surgery, University of South Florida; and Dr. Turner and Dr. Beachkofsky, from the department of pathology and laboratory medicine at the James A. Haley Veterans’ Hospital, Tampa. Dr. Bilu Martin edited this case. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to dermnews@mdedge.com.
References
1. Leprosy (Hansen’s Disease), in: “Goldman’s Cecil Medicine,” 24th ed. (Philadelphia: W.B. Saunders, 2012: pp. 1950-4.
2. Sharma R et al. Emerg Infect Dis. 2015 Dec;21(12):2127-34.
3. Lane JE et al. J Am Acad Dermatol. 2006 Oct;55(4):714-6.
4. Clark BM et al. Am J Trop Med Hyg. 2008 Jun;78(6):962-7.
5. Bruce S et al. J Am Acad Dermatol. 2000 Aug;43(2 Pt 1):223-8.
6. Loughry WJ et al. J Wildl Dis. 2009 Jan;45(1):144-52.
7. FDo H. Florida charts: Hansen’s Disease (Leprosy). Health FDo. 2019. https://www.flhealthcharts.gov/ChartsReports/rdPage.aspx?rdReport=NonVitalIndNoGrpCounts.DataViewer&cid=174.
8. Maymone MBC et al. J Am Acad Dermatol. 2020 Jul;83(1):1-14.
9. Scollard DM et al. Clin Microbiol Rev. 2006 Apr;19(2):338-81.
10. Domozych R et al. JAAD Case Rep. 2016 May 12;2(3):189-92.
Paucibacillary tuberculoid leprosy is characterized by few anesthetic hypo- or hyperpigmented lesions and can be accompanied by palpable peripheral nerve enlargements.
Tuberculoid leprosy presents histologically with epithelioid histiocytes with lymphocytes and Langhans giant cells. Neurotropic granulomas are also characteristic of tuberculoid leprosy. Fite staining allows for the identification of the acid-fast bacilli of M. leprae, which in some cases are quite few in number. The standard mycobacterium stain, Ziehl-Neelsen, is a good option for M. tuberculosis, but because of the relative weak mycolic acid coat of M. leprae, the Fite stain is more appropriate for identifying M. leprae.
Clinically, other than the presence of fewer than five hypoesthetic lesions that are either hypopigmented or erythematous, tuberculoid leprosy often presents with additional peripheral nerve involvement that manifests as numbness and tingling in hands and feet.1 This patient denied any tingling, weakness, or numbness, outside of the anesthetic lesion on his posterior upper arm.
The patient, born in the United States, had a remote history of military travel to Iraq, Kuwait, and the Philippines, but had not traveled internationally within the last 15 years, apart from a cruise to the Bahamas. He denied any known contact with individuals with similar lesions. He denied a history of contact with armadillos, but acknowledged that they are native to where he resides in central Florida, and that he had seen them in his yard.
Histopathological examination revealed an unremarkable epidermis with a superficial and deep perivascular, periadnexal, and perineural lymphohistiocytic infiltrate. Fite stain revealed rare rod-shaped organisms (Figure 2). These findings are consistent with a diagnosis of paucibacillary, tuberculoid leprosy.
The patient’s travel history to highly endemic areas (Middle East), as well as possible environmental contact with armadillos – including contact with soil that the armadillos occupied – could explain plausible modes of transmission. Following consultation with our infectious disease department and the National Hansen’s Disease Program, our patient began a planned course of therapy with 18 months of minocycline, rifampin, and moxifloxacin.
Human-to-human transmission of HD has been well documented; however, zoonotic transmission – specifically via the nine-banded armadillo (Dasypus novemcinctus) – serves as another suggested means of transmission, especially in the Southeastern United States.2-6 Travel to highly-endemic areas increases the risk of contracting HD, which may take up to 20 years following contact with the bacteria to manifest clinically.
While central Florida was previously thought to be a nonendemic area of disease, the incidence of the disease in this region has increased in recent years.7 Human-to-human transmission, which remains a concern with immigration from highly-endemic regions, occurs via long-term contact with nasal droplets of an infected person.8,9
Many patients in regions with very few cases of leprosy deny travel to other endemic regions and contact with infected people. Thus, zoonotic transmission remains a legitimate concern in the Southeastern United States – accounting, at least in part, for many of the non–human-transmitted cases of leprosy.2,10 We encourage clinicians to maintain a high level of clinical suspicion for leprosy when evaluating patients presenting with hypoesthetic cutaneous lesions and to obtain a travel history and to ask about armadillo exposure.
This case and the photos were submitted by Ms. Smith, from the University of South Florida, Tampa; Dr. Hatch and Dr. Sarriera-Lazaro, from the department of dermatology and cutaneous surgery, University of South Florida; and Dr. Turner and Dr. Beachkofsky, from the department of pathology and laboratory medicine at the James A. Haley Veterans’ Hospital, Tampa. Dr. Bilu Martin edited this case. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to dermnews@mdedge.com.
References
1. Leprosy (Hansen’s Disease), in: “Goldman’s Cecil Medicine,” 24th ed. (Philadelphia: W.B. Saunders, 2012: pp. 1950-4.
2. Sharma R et al. Emerg Infect Dis. 2015 Dec;21(12):2127-34.
3. Lane JE et al. J Am Acad Dermatol. 2006 Oct;55(4):714-6.
4. Clark BM et al. Am J Trop Med Hyg. 2008 Jun;78(6):962-7.
5. Bruce S et al. J Am Acad Dermatol. 2000 Aug;43(2 Pt 1):223-8.
6. Loughry WJ et al. J Wildl Dis. 2009 Jan;45(1):144-52.
7. FDo H. Florida charts: Hansen’s Disease (Leprosy). Health FDo. 2019. https://www.flhealthcharts.gov/ChartsReports/rdPage.aspx?rdReport=NonVitalIndNoGrpCounts.DataViewer&cid=174.
8. Maymone MBC et al. J Am Acad Dermatol. 2020 Jul;83(1):1-14.
9. Scollard DM et al. Clin Microbiol Rev. 2006 Apr;19(2):338-81.
10. Domozych R et al. JAAD Case Rep. 2016 May 12;2(3):189-92.
Paucibacillary tuberculoid leprosy is characterized by few anesthetic hypo- or hyperpigmented lesions and can be accompanied by palpable peripheral nerve enlargements.
Tuberculoid leprosy presents histologically with epithelioid histiocytes with lymphocytes and Langhans giant cells. Neurotropic granulomas are also characteristic of tuberculoid leprosy. Fite staining allows for the identification of the acid-fast bacilli of M. leprae, which in some cases are quite few in number. The standard mycobacterium stain, Ziehl-Neelsen, is a good option for M. tuberculosis, but because of the relative weak mycolic acid coat of M. leprae, the Fite stain is more appropriate for identifying M. leprae.
Clinically, other than the presence of fewer than five hypoesthetic lesions that are either hypopigmented or erythematous, tuberculoid leprosy often presents with additional peripheral nerve involvement that manifests as numbness and tingling in hands and feet.1 This patient denied any tingling, weakness, or numbness, outside of the anesthetic lesion on his posterior upper arm.
The patient, born in the United States, had a remote history of military travel to Iraq, Kuwait, and the Philippines, but had not traveled internationally within the last 15 years, apart from a cruise to the Bahamas. He denied any known contact with individuals with similar lesions. He denied a history of contact with armadillos, but acknowledged that they are native to where he resides in central Florida, and that he had seen them in his yard.
Histopathological examination revealed an unremarkable epidermis with a superficial and deep perivascular, periadnexal, and perineural lymphohistiocytic infiltrate. Fite stain revealed rare rod-shaped organisms (Figure 2). These findings are consistent with a diagnosis of paucibacillary, tuberculoid leprosy.
The patient’s travel history to highly endemic areas (Middle East), as well as possible environmental contact with armadillos – including contact with soil that the armadillos occupied – could explain plausible modes of transmission. Following consultation with our infectious disease department and the National Hansen’s Disease Program, our patient began a planned course of therapy with 18 months of minocycline, rifampin, and moxifloxacin.
Human-to-human transmission of HD has been well documented; however, zoonotic transmission – specifically via the nine-banded armadillo (Dasypus novemcinctus) – serves as another suggested means of transmission, especially in the Southeastern United States.2-6 Travel to highly-endemic areas increases the risk of contracting HD, which may take up to 20 years following contact with the bacteria to manifest clinically.
While central Florida was previously thought to be a nonendemic area of disease, the incidence of the disease in this region has increased in recent years.7 Human-to-human transmission, which remains a concern with immigration from highly-endemic regions, occurs via long-term contact with nasal droplets of an infected person.8,9
Many patients in regions with very few cases of leprosy deny travel to other endemic regions and contact with infected people. Thus, zoonotic transmission remains a legitimate concern in the Southeastern United States – accounting, at least in part, for many of the non–human-transmitted cases of leprosy.2,10 We encourage clinicians to maintain a high level of clinical suspicion for leprosy when evaluating patients presenting with hypoesthetic cutaneous lesions and to obtain a travel history and to ask about armadillo exposure.
This case and the photos were submitted by Ms. Smith, from the University of South Florida, Tampa; Dr. Hatch and Dr. Sarriera-Lazaro, from the department of dermatology and cutaneous surgery, University of South Florida; and Dr. Turner and Dr. Beachkofsky, from the department of pathology and laboratory medicine at the James A. Haley Veterans’ Hospital, Tampa. Dr. Bilu Martin edited this case. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to dermnews@mdedge.com.
References
1. Leprosy (Hansen’s Disease), in: “Goldman’s Cecil Medicine,” 24th ed. (Philadelphia: W.B. Saunders, 2012: pp. 1950-4.
2. Sharma R et al. Emerg Infect Dis. 2015 Dec;21(12):2127-34.
3. Lane JE et al. J Am Acad Dermatol. 2006 Oct;55(4):714-6.
4. Clark BM et al. Am J Trop Med Hyg. 2008 Jun;78(6):962-7.
5. Bruce S et al. J Am Acad Dermatol. 2000 Aug;43(2 Pt 1):223-8.
6. Loughry WJ et al. J Wildl Dis. 2009 Jan;45(1):144-52.
7. FDo H. Florida charts: Hansen’s Disease (Leprosy). Health FDo. 2019. https://www.flhealthcharts.gov/ChartsReports/rdPage.aspx?rdReport=NonVitalIndNoGrpCounts.DataViewer&cid=174.
8. Maymone MBC et al. J Am Acad Dermatol. 2020 Jul;83(1):1-14.
9. Scollard DM et al. Clin Microbiol Rev. 2006 Apr;19(2):338-81.
10. Domozych R et al. JAAD Case Rep. 2016 May 12;2(3):189-92.
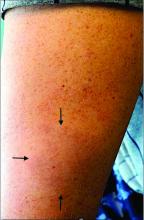
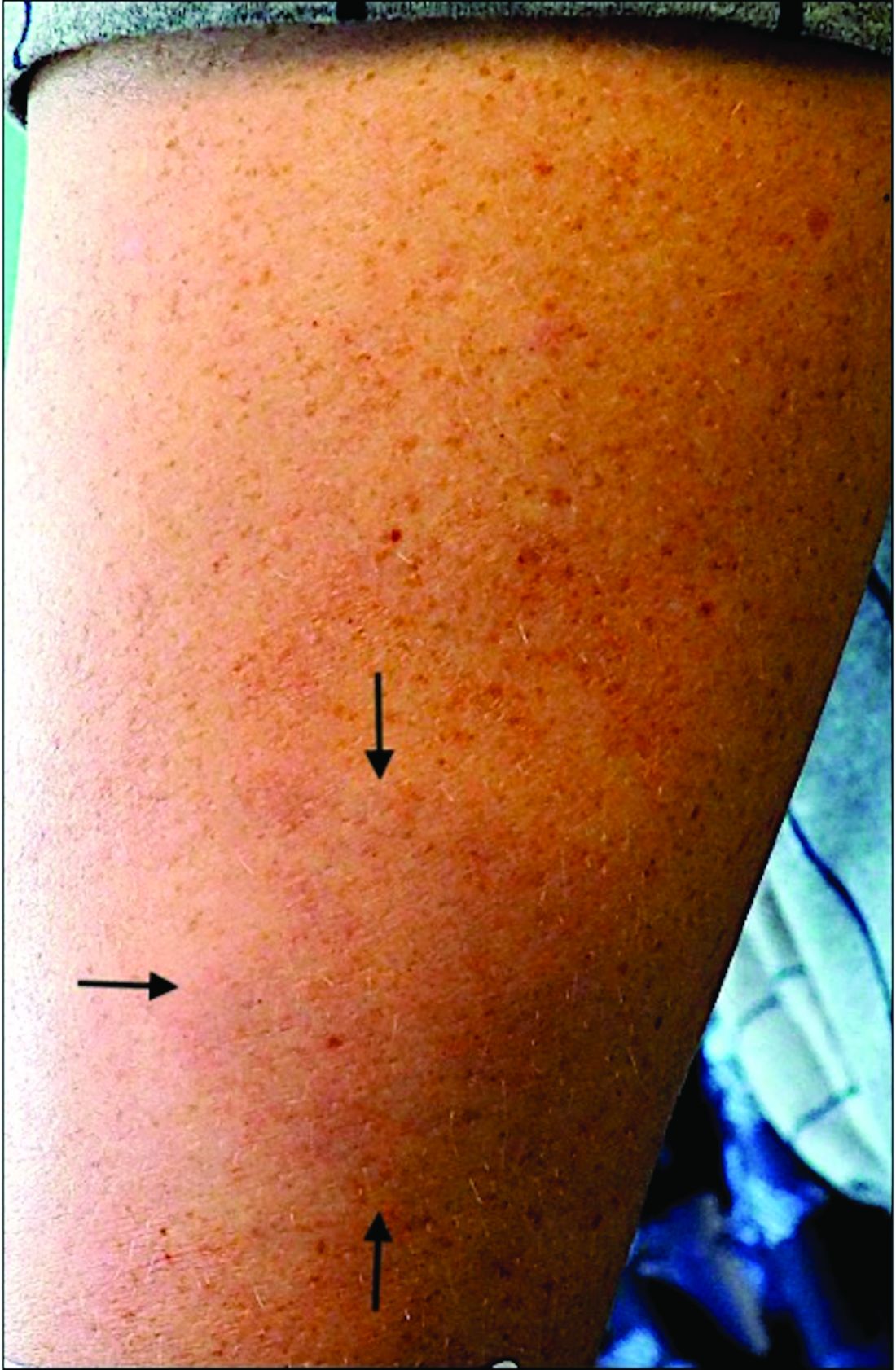
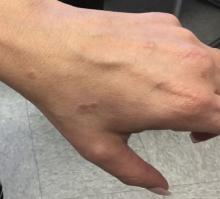
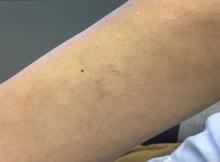
A 44-year-old White male presented with a 1½-year history of a progressive hypoesthetic annular, mildly hyperpigmented plaque on the left posterior upper arm.
He denied pruritus, pain, or systemic symptoms including weight loss, visual changes, cough, dyspnea, and abdominal pain. He also denied any paresthesia or weakness. On physical examination, there is a subtle, solitary 4-cm annular skin-colored thin plaque on the patient's left posterior upper arm (Figure 1).
Punch biopsy of the lesion was performed, and the histopathological findings are illustrated in Figure 2.
Developments in wound healing include different treatment options
ORLANDO – , Hadar Lev-Tov, MD, said at the ODAC Dermatology, Aesthetic & Surgery Conference.
At the meeting, Dr. Lev-Tov, associate professor of dermatology at the University of Miami, reviewed some of the latest developments in several conditions involving wound care.
Pyoderma gangrenosum (PG): In this condition, pustules or nodules become large ulcerations, and one-third of patients with PG have pathergy, exaggerated skin injury after a mild trauma such as a bump or a bruise.
“You want to look at the clues in the history because 20% of these patients had histories of PG elsewhere,” Dr. Lev-Tov said. “Ask them about other ulcers, maybe they had some wound dehiscence history.”
Criteria have been developed to help with the diagnosis of ulcerative PG, which includes one major criterion, a biopsy of the ulcer edge showing neutrophilic infiltrate, along with minor criteria, including exclusion of an infection, pathergy, and a history of inflammatory bowel disease or inflammatory arthritis.
“This is no longer a diagnosis of exclusion,” Dr. Lev-Tov said.
Cyclosporine and oral steroids have been found to work well, but it typically takes many months before healing occurs. Tacrolimus or topical steroids can work as well, but healing also takes a fairly long time with those medications, Dr. Lev-Tov said.
The tumor necrosis factor (TNF) blocker infliximab is another option. He had a patient who was referred to him who had been treated with cyclosporine for 3 years for PG on his feet, even though it had not been effective. Dr. Lev-Tov tried infliximab, and the wounds finally cleared, he said.
Apremilast, a phosphodiesterase 4 (PDE4)-inhibitor, is another option for treating PG, he said. “Anecdotally, I used apremilast on three patients with recurrent PG for long-term suppression, with success,” he noted.
Epidermal grafting using suction and heat is an approach that might deserve further exploration for PG, Dr. Lev-Tov suggested. With this procedure, described in an article in 2014, heat and suction are used to induce blistering to separate and remove the epidermis from the dermis at the dermal-epidermal junction, creating an epidermal graft is placed over the wound to promote healing. Patients with PG who are immunosuppressed but demonstrate pathergy do not tend to experience pathergy when epidermal skin grafting is performed, he said.
The heat-suction procedure is simple, painless, and scarless, but better controlled data on this approach are needed, he said.
Corona phlebectatica: This disease involving abnormally dilated veins near the ankle has received formal recognition as a sign of venous insufficiency, in a 2020 update of a classification system for describing patients with chronic venous disorders, Dr. Lev-Tov said.
“We knew about it for years, but now there’s some data that can actually predict the severity of disease,” and, he said, it is now a part of the diagnostic criteria for venous insufficiency .
Venous leg ulcers: These often painful sores on the inside of the leg typically take more than a month to heal. A systematic review of placebo-controlled studies of pentoxifylline as a treatment for venous leg ulcers, published in 2021, supports its use for healing venous leg ulcers, Dr. Lev-Tov said. “It improved the healing rate and increased what [the researchers] called ‘significant improvement,’ ” a category they created to account for the varying methods across the studies, he said.
Topical beta-blockers can improve epithelialization and fibroblast migration in wound healing, he said. A study on topical timolol for various wounds found that a 0.5% formulation of topical timolol, with one drop applied per square centimeter as frequently as possible, was effective in healing. But the healing process was prolonged – a median of 90 days, said Dr. Lev-Tov, one of the study authors.
“When you start this, I don’t want you to expect the wound to heal tomorrow,” he said. “You’ve got to educate your patient.”
Dr. Lev-Tov reports relevant financial relationships with Abbvie, Novartis, Pfizer and other companies.
ORLANDO – , Hadar Lev-Tov, MD, said at the ODAC Dermatology, Aesthetic & Surgery Conference.
At the meeting, Dr. Lev-Tov, associate professor of dermatology at the University of Miami, reviewed some of the latest developments in several conditions involving wound care.
Pyoderma gangrenosum (PG): In this condition, pustules or nodules become large ulcerations, and one-third of patients with PG have pathergy, exaggerated skin injury after a mild trauma such as a bump or a bruise.
“You want to look at the clues in the history because 20% of these patients had histories of PG elsewhere,” Dr. Lev-Tov said. “Ask them about other ulcers, maybe they had some wound dehiscence history.”
Criteria have been developed to help with the diagnosis of ulcerative PG, which includes one major criterion, a biopsy of the ulcer edge showing neutrophilic infiltrate, along with minor criteria, including exclusion of an infection, pathergy, and a history of inflammatory bowel disease or inflammatory arthritis.
“This is no longer a diagnosis of exclusion,” Dr. Lev-Tov said.
Cyclosporine and oral steroids have been found to work well, but it typically takes many months before healing occurs. Tacrolimus or topical steroids can work as well, but healing also takes a fairly long time with those medications, Dr. Lev-Tov said.
The tumor necrosis factor (TNF) blocker infliximab is another option. He had a patient who was referred to him who had been treated with cyclosporine for 3 years for PG on his feet, even though it had not been effective. Dr. Lev-Tov tried infliximab, and the wounds finally cleared, he said.
Apremilast, a phosphodiesterase 4 (PDE4)-inhibitor, is another option for treating PG, he said. “Anecdotally, I used apremilast on three patients with recurrent PG for long-term suppression, with success,” he noted.
Epidermal grafting using suction and heat is an approach that might deserve further exploration for PG, Dr. Lev-Tov suggested. With this procedure, described in an article in 2014, heat and suction are used to induce blistering to separate and remove the epidermis from the dermis at the dermal-epidermal junction, creating an epidermal graft is placed over the wound to promote healing. Patients with PG who are immunosuppressed but demonstrate pathergy do not tend to experience pathergy when epidermal skin grafting is performed, he said.
The heat-suction procedure is simple, painless, and scarless, but better controlled data on this approach are needed, he said.
Corona phlebectatica: This disease involving abnormally dilated veins near the ankle has received formal recognition as a sign of venous insufficiency, in a 2020 update of a classification system for describing patients with chronic venous disorders, Dr. Lev-Tov said.
“We knew about it for years, but now there’s some data that can actually predict the severity of disease,” and, he said, it is now a part of the diagnostic criteria for venous insufficiency .
Venous leg ulcers: These often painful sores on the inside of the leg typically take more than a month to heal. A systematic review of placebo-controlled studies of pentoxifylline as a treatment for venous leg ulcers, published in 2021, supports its use for healing venous leg ulcers, Dr. Lev-Tov said. “It improved the healing rate and increased what [the researchers] called ‘significant improvement,’ ” a category they created to account for the varying methods across the studies, he said.
Topical beta-blockers can improve epithelialization and fibroblast migration in wound healing, he said. A study on topical timolol for various wounds found that a 0.5% formulation of topical timolol, with one drop applied per square centimeter as frequently as possible, was effective in healing. But the healing process was prolonged – a median of 90 days, said Dr. Lev-Tov, one of the study authors.
“When you start this, I don’t want you to expect the wound to heal tomorrow,” he said. “You’ve got to educate your patient.”
Dr. Lev-Tov reports relevant financial relationships with Abbvie, Novartis, Pfizer and other companies.
ORLANDO – , Hadar Lev-Tov, MD, said at the ODAC Dermatology, Aesthetic & Surgery Conference.
At the meeting, Dr. Lev-Tov, associate professor of dermatology at the University of Miami, reviewed some of the latest developments in several conditions involving wound care.
Pyoderma gangrenosum (PG): In this condition, pustules or nodules become large ulcerations, and one-third of patients with PG have pathergy, exaggerated skin injury after a mild trauma such as a bump or a bruise.
“You want to look at the clues in the history because 20% of these patients had histories of PG elsewhere,” Dr. Lev-Tov said. “Ask them about other ulcers, maybe they had some wound dehiscence history.”
Criteria have been developed to help with the diagnosis of ulcerative PG, which includes one major criterion, a biopsy of the ulcer edge showing neutrophilic infiltrate, along with minor criteria, including exclusion of an infection, pathergy, and a history of inflammatory bowel disease or inflammatory arthritis.
“This is no longer a diagnosis of exclusion,” Dr. Lev-Tov said.
Cyclosporine and oral steroids have been found to work well, but it typically takes many months before healing occurs. Tacrolimus or topical steroids can work as well, but healing also takes a fairly long time with those medications, Dr. Lev-Tov said.
The tumor necrosis factor (TNF) blocker infliximab is another option. He had a patient who was referred to him who had been treated with cyclosporine for 3 years for PG on his feet, even though it had not been effective. Dr. Lev-Tov tried infliximab, and the wounds finally cleared, he said.
Apremilast, a phosphodiesterase 4 (PDE4)-inhibitor, is another option for treating PG, he said. “Anecdotally, I used apremilast on three patients with recurrent PG for long-term suppression, with success,” he noted.
Epidermal grafting using suction and heat is an approach that might deserve further exploration for PG, Dr. Lev-Tov suggested. With this procedure, described in an article in 2014, heat and suction are used to induce blistering to separate and remove the epidermis from the dermis at the dermal-epidermal junction, creating an epidermal graft is placed over the wound to promote healing. Patients with PG who are immunosuppressed but demonstrate pathergy do not tend to experience pathergy when epidermal skin grafting is performed, he said.
The heat-suction procedure is simple, painless, and scarless, but better controlled data on this approach are needed, he said.
Corona phlebectatica: This disease involving abnormally dilated veins near the ankle has received formal recognition as a sign of venous insufficiency, in a 2020 update of a classification system for describing patients with chronic venous disorders, Dr. Lev-Tov said.
“We knew about it for years, but now there’s some data that can actually predict the severity of disease,” and, he said, it is now a part of the diagnostic criteria for venous insufficiency .
Venous leg ulcers: These often painful sores on the inside of the leg typically take more than a month to heal. A systematic review of placebo-controlled studies of pentoxifylline as a treatment for venous leg ulcers, published in 2021, supports its use for healing venous leg ulcers, Dr. Lev-Tov said. “It improved the healing rate and increased what [the researchers] called ‘significant improvement,’ ” a category they created to account for the varying methods across the studies, he said.
Topical beta-blockers can improve epithelialization and fibroblast migration in wound healing, he said. A study on topical timolol for various wounds found that a 0.5% formulation of topical timolol, with one drop applied per square centimeter as frequently as possible, was effective in healing. But the healing process was prolonged – a median of 90 days, said Dr. Lev-Tov, one of the study authors.
“When you start this, I don’t want you to expect the wound to heal tomorrow,” he said. “You’ve got to educate your patient.”
Dr. Lev-Tov reports relevant financial relationships with Abbvie, Novartis, Pfizer and other companies.
AT ODAC 2023
Expert gives tips on less-discussed dermatologic diseases
ORLANDO – , according to Adam Friedman, MD, professor and chair of dermatology at George Washington University, Washington.
These semi-forsaken diseases are important not to miss and can “also be quite challenging when we think about their management,” he said at the ODAC Dermatology, Aesthetic & Surgical Conference.
Dr. Friedman, also director of the GW dermatology residency program, reviewed several of these diseases – along with tips for management – during a session at the meeting.
. It does not always have the classic ring pattern for which it is best known, he said. And in patients with darker skin tones, it is characterized by more of a brown or black color, rather than the pink-red color.
Dr. Friedman said that despite a kind of “Pavlovian response” linking GA with diabetes, this link might not be as strong as the field has come to believe, since the studies on which this belief was based included a patient population with narrow demographics. “Maybe GA and type 1 diabetes aren’t necessarily connected,” he said.
Dyslipidemia, on the other hand, has a strong connection with GA, he said. The disease is also linked to thyroid disease and is linked with malignancy, especially in older patients with generalized or atypical presentations of GA, he said.
Spontaneous resolution of the disease is seen within 2 years for 50% to 75% of patients, so “no treatment may be the best treatment,” but antimalarials can be effective, Dr. Friedman said. “I use antimalarials frequently in my practice,” he said. “The key is, they take time to work (4-5 months),” which should be explained to patients.
Antibiotics, he said, can be “somewhat effective,” but in the case of doxycycline at least, the disease can resolve within weeks but then may return when treatment is stopped.
There is some evidence to support using biologics and more recently, Janus kinase (JAK) inhibitors, off-label, to treat GA. Efficacy has been seen with the tumor necrosis factor (TNF) blocker infliximab and with the JAK inhibitor tofacitinib, he said.
Lichen planus (LP). This is another common disease that can go off-script with its presentation. The disease is often described with the “six P’s” indicating the following characteristics: pruritic, polygonal, planar or flat-topped, purple papules, and plaques. But LP “didn’t read the textbook,” Dr. Friedman said.
“The clinical presentation of lichen planus can be quite broad,” he said. “The P’s aren’t always followed as there are a variety of colors and configurations which can be witnessed.”
With LP, there is a clear association with dyslipidemia and diabetes, so “asking the right questions is going to be important” when talking to the patient. There is also a higher risk of autoimmune diseases, especially of the thyroid type, associated with LP, he said.
No treatment has been Food and Drug Administration approved for LP, but some are expected in the future, he said.
For now, he emphasized creativity in the management of patients with LP. “I love oral retinoids for this,” he said. Antimalarials and methotrexate are also options.
In one case Dr. Friedman saw, nothing seemed to work: light therapy for a year; metronidazole; isotretinoin; halobetasol/tazarotene lotion; and the TNF-blocker adalimumab either weren’t effective or resulted in complications in the patient.
Knowing the recent implication of the interleukin (IL)-17 pathway in the pathophysiology of LP, he then tried the anti-IL17 antibody secukinumab. “This patient had a pretty robust response to treatment,” Dr. Friedman said. “He was very excited. The problem, as always, is access, especially for off-label therapies.”
Tumid lupus erythematosus. This disease is characterized by erythematous, edematous, nonscarring plaques on sun-exposed sites. For treatment, Dr. Friedman said antimalarials can be up to 90% effective, sometimes with rapid resolution of the lesions.
“You want to dose below that 5 mg per kg of true body weight to limit the small potential for ocular toxicity over time,” he said. And, he emphasized, “always combine treatment with good sun-protective measures.”
Dr. Friedman reported financial relationships with Sanova, Pfizer, Novartis, and other companies.
ORLANDO – , according to Adam Friedman, MD, professor and chair of dermatology at George Washington University, Washington.
These semi-forsaken diseases are important not to miss and can “also be quite challenging when we think about their management,” he said at the ODAC Dermatology, Aesthetic & Surgical Conference.
Dr. Friedman, also director of the GW dermatology residency program, reviewed several of these diseases – along with tips for management – during a session at the meeting.
. It does not always have the classic ring pattern for which it is best known, he said. And in patients with darker skin tones, it is characterized by more of a brown or black color, rather than the pink-red color.
Dr. Friedman said that despite a kind of “Pavlovian response” linking GA with diabetes, this link might not be as strong as the field has come to believe, since the studies on which this belief was based included a patient population with narrow demographics. “Maybe GA and type 1 diabetes aren’t necessarily connected,” he said.
Dyslipidemia, on the other hand, has a strong connection with GA, he said. The disease is also linked to thyroid disease and is linked with malignancy, especially in older patients with generalized or atypical presentations of GA, he said.
Spontaneous resolution of the disease is seen within 2 years for 50% to 75% of patients, so “no treatment may be the best treatment,” but antimalarials can be effective, Dr. Friedman said. “I use antimalarials frequently in my practice,” he said. “The key is, they take time to work (4-5 months),” which should be explained to patients.
Antibiotics, he said, can be “somewhat effective,” but in the case of doxycycline at least, the disease can resolve within weeks but then may return when treatment is stopped.
There is some evidence to support using biologics and more recently, Janus kinase (JAK) inhibitors, off-label, to treat GA. Efficacy has been seen with the tumor necrosis factor (TNF) blocker infliximab and with the JAK inhibitor tofacitinib, he said.
Lichen planus (LP). This is another common disease that can go off-script with its presentation. The disease is often described with the “six P’s” indicating the following characteristics: pruritic, polygonal, planar or flat-topped, purple papules, and plaques. But LP “didn’t read the textbook,” Dr. Friedman said.
“The clinical presentation of lichen planus can be quite broad,” he said. “The P’s aren’t always followed as there are a variety of colors and configurations which can be witnessed.”
With LP, there is a clear association with dyslipidemia and diabetes, so “asking the right questions is going to be important” when talking to the patient. There is also a higher risk of autoimmune diseases, especially of the thyroid type, associated with LP, he said.
No treatment has been Food and Drug Administration approved for LP, but some are expected in the future, he said.
For now, he emphasized creativity in the management of patients with LP. “I love oral retinoids for this,” he said. Antimalarials and methotrexate are also options.
In one case Dr. Friedman saw, nothing seemed to work: light therapy for a year; metronidazole; isotretinoin; halobetasol/tazarotene lotion; and the TNF-blocker adalimumab either weren’t effective or resulted in complications in the patient.
Knowing the recent implication of the interleukin (IL)-17 pathway in the pathophysiology of LP, he then tried the anti-IL17 antibody secukinumab. “This patient had a pretty robust response to treatment,” Dr. Friedman said. “He was very excited. The problem, as always, is access, especially for off-label therapies.”
Tumid lupus erythematosus. This disease is characterized by erythematous, edematous, nonscarring plaques on sun-exposed sites. For treatment, Dr. Friedman said antimalarials can be up to 90% effective, sometimes with rapid resolution of the lesions.
“You want to dose below that 5 mg per kg of true body weight to limit the small potential for ocular toxicity over time,” he said. And, he emphasized, “always combine treatment with good sun-protective measures.”
Dr. Friedman reported financial relationships with Sanova, Pfizer, Novartis, and other companies.
ORLANDO – , according to Adam Friedman, MD, professor and chair of dermatology at George Washington University, Washington.
These semi-forsaken diseases are important not to miss and can “also be quite challenging when we think about their management,” he said at the ODAC Dermatology, Aesthetic & Surgical Conference.
Dr. Friedman, also director of the GW dermatology residency program, reviewed several of these diseases – along with tips for management – during a session at the meeting.
. It does not always have the classic ring pattern for which it is best known, he said. And in patients with darker skin tones, it is characterized by more of a brown or black color, rather than the pink-red color.
Dr. Friedman said that despite a kind of “Pavlovian response” linking GA with diabetes, this link might not be as strong as the field has come to believe, since the studies on which this belief was based included a patient population with narrow demographics. “Maybe GA and type 1 diabetes aren’t necessarily connected,” he said.
Dyslipidemia, on the other hand, has a strong connection with GA, he said. The disease is also linked to thyroid disease and is linked with malignancy, especially in older patients with generalized or atypical presentations of GA, he said.
Spontaneous resolution of the disease is seen within 2 years for 50% to 75% of patients, so “no treatment may be the best treatment,” but antimalarials can be effective, Dr. Friedman said. “I use antimalarials frequently in my practice,” he said. “The key is, they take time to work (4-5 months),” which should be explained to patients.
Antibiotics, he said, can be “somewhat effective,” but in the case of doxycycline at least, the disease can resolve within weeks but then may return when treatment is stopped.
There is some evidence to support using biologics and more recently, Janus kinase (JAK) inhibitors, off-label, to treat GA. Efficacy has been seen with the tumor necrosis factor (TNF) blocker infliximab and with the JAK inhibitor tofacitinib, he said.
Lichen planus (LP). This is another common disease that can go off-script with its presentation. The disease is often described with the “six P’s” indicating the following characteristics: pruritic, polygonal, planar or flat-topped, purple papules, and plaques. But LP “didn’t read the textbook,” Dr. Friedman said.
“The clinical presentation of lichen planus can be quite broad,” he said. “The P’s aren’t always followed as there are a variety of colors and configurations which can be witnessed.”
With LP, there is a clear association with dyslipidemia and diabetes, so “asking the right questions is going to be important” when talking to the patient. There is also a higher risk of autoimmune diseases, especially of the thyroid type, associated with LP, he said.
No treatment has been Food and Drug Administration approved for LP, but some are expected in the future, he said.
For now, he emphasized creativity in the management of patients with LP. “I love oral retinoids for this,” he said. Antimalarials and methotrexate are also options.
In one case Dr. Friedman saw, nothing seemed to work: light therapy for a year; metronidazole; isotretinoin; halobetasol/tazarotene lotion; and the TNF-blocker adalimumab either weren’t effective or resulted in complications in the patient.
Knowing the recent implication of the interleukin (IL)-17 pathway in the pathophysiology of LP, he then tried the anti-IL17 antibody secukinumab. “This patient had a pretty robust response to treatment,” Dr. Friedman said. “He was very excited. The problem, as always, is access, especially for off-label therapies.”
Tumid lupus erythematosus. This disease is characterized by erythematous, edematous, nonscarring plaques on sun-exposed sites. For treatment, Dr. Friedman said antimalarials can be up to 90% effective, sometimes with rapid resolution of the lesions.
“You want to dose below that 5 mg per kg of true body weight to limit the small potential for ocular toxicity over time,” he said. And, he emphasized, “always combine treatment with good sun-protective measures.”
Dr. Friedman reported financial relationships with Sanova, Pfizer, Novartis, and other companies.
AT ODAC 2023
A 50-year-old woman with no significant history presented with erythematous, annular plaques, and papules on the dorsal hands and arms
. The prevalence and incidence is approximately 0.1%-0.4%. Although the condition is benign, it may be associated with more serious conditions such as HIV and malignancy. GA affects women more frequently than men but can affect any age group, although it most commonly presents in those ages 30 years and younger. While the exact etiology is unknown, GA has been most strongly associated with diabetes mellitus, hyperlipidemia, and autoimmune diseases.
The disease presents as localized, annular erythematous plaques and papules on the dorsal hands and feet in approximately 75% of cases. However, eruptions may appear on the trunk and extremities and can be categorized into patchy, generalized, interstitial, subcutaneous, or perforating subtypes. The lesions are often asymptomatic and typically not associated with any other symptoms.
The pathogenesis of GA is still under investigation, but recent studies suggest that a Th1-mediated dysregulation of the JAK-STAT pathway may contribute to the disease. Other hypotheses include a delayed hypersensitivity reaction or cell mediated immune response. The mechanism may be multifaceted, and epidemiologic research suggests a genetic predisposition in White individuals, but these findings may be associated with socioeconomic factors and disparities in health care.
GA presents on histology with palisading histiocytes surrounding focal collagen necrobiosis with mucin deposition. Tissue samples also display leukocytic infiltration of the dermis featuring multinucleated giant cells. There are defining features of the different subtypes, but focal collagen necrosis, the presence of histiocytes, and mucin deposition are consistent findings across all presentations.
GA lesions commonly regress on their own, but they tend to recur and can be functionally and visually unappealing to patients. The most common treatments for GA include topical corticosteroids, intralesional corticosteroid injections, and other anti-inflammatory drugs. These interventions can be administered in a variety of ways as the inflammation caused by GA exists on a spectrum, and less severe cases can be managed with topical or intralesional treatment. Systemic therapy may be necessary for severe and recalcitrant cases. Other interventions that have shown promise in smaller studies include phototherapy, hydroxychloroquine, and TNF-alpha inhibitors.
This case and photo were submitted by Lucas Shapiro, BS, Nova Southeastern University College of Osteopathic Medicine, Tampa Bay Regional Campus, and Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to dermnews@mdedge.com.
References
Joshi TP and Duvic M. Am J Clin Dermatol. 2022 Jan;23(1):37-50. doi: 10.1007/s40257-021-00636-1.
Muse M et al. Dermatol Online J. 2021 Apr 15;27(4):13030/qt0m50398n.
Schmieder SJ et al. Granuloma Annulare. NIH National Center for Biotechnology Information [Updated 2022 Nov 7]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan. 7.
. The prevalence and incidence is approximately 0.1%-0.4%. Although the condition is benign, it may be associated with more serious conditions such as HIV and malignancy. GA affects women more frequently than men but can affect any age group, although it most commonly presents in those ages 30 years and younger. While the exact etiology is unknown, GA has been most strongly associated with diabetes mellitus, hyperlipidemia, and autoimmune diseases.
The disease presents as localized, annular erythematous plaques and papules on the dorsal hands and feet in approximately 75% of cases. However, eruptions may appear on the trunk and extremities and can be categorized into patchy, generalized, interstitial, subcutaneous, or perforating subtypes. The lesions are often asymptomatic and typically not associated with any other symptoms.
The pathogenesis of GA is still under investigation, but recent studies suggest that a Th1-mediated dysregulation of the JAK-STAT pathway may contribute to the disease. Other hypotheses include a delayed hypersensitivity reaction or cell mediated immune response. The mechanism may be multifaceted, and epidemiologic research suggests a genetic predisposition in White individuals, but these findings may be associated with socioeconomic factors and disparities in health care.
GA presents on histology with palisading histiocytes surrounding focal collagen necrobiosis with mucin deposition. Tissue samples also display leukocytic infiltration of the dermis featuring multinucleated giant cells. There are defining features of the different subtypes, but focal collagen necrosis, the presence of histiocytes, and mucin deposition are consistent findings across all presentations.
GA lesions commonly regress on their own, but they tend to recur and can be functionally and visually unappealing to patients. The most common treatments for GA include topical corticosteroids, intralesional corticosteroid injections, and other anti-inflammatory drugs. These interventions can be administered in a variety of ways as the inflammation caused by GA exists on a spectrum, and less severe cases can be managed with topical or intralesional treatment. Systemic therapy may be necessary for severe and recalcitrant cases. Other interventions that have shown promise in smaller studies include phototherapy, hydroxychloroquine, and TNF-alpha inhibitors.
This case and photo were submitted by Lucas Shapiro, BS, Nova Southeastern University College of Osteopathic Medicine, Tampa Bay Regional Campus, and Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to dermnews@mdedge.com.
References
Joshi TP and Duvic M. Am J Clin Dermatol. 2022 Jan;23(1):37-50. doi: 10.1007/s40257-021-00636-1.
Muse M et al. Dermatol Online J. 2021 Apr 15;27(4):13030/qt0m50398n.
Schmieder SJ et al. Granuloma Annulare. NIH National Center for Biotechnology Information [Updated 2022 Nov 7]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan. 7.
. The prevalence and incidence is approximately 0.1%-0.4%. Although the condition is benign, it may be associated with more serious conditions such as HIV and malignancy. GA affects women more frequently than men but can affect any age group, although it most commonly presents in those ages 30 years and younger. While the exact etiology is unknown, GA has been most strongly associated with diabetes mellitus, hyperlipidemia, and autoimmune diseases.
The disease presents as localized, annular erythematous plaques and papules on the dorsal hands and feet in approximately 75% of cases. However, eruptions may appear on the trunk and extremities and can be categorized into patchy, generalized, interstitial, subcutaneous, or perforating subtypes. The lesions are often asymptomatic and typically not associated with any other symptoms.
The pathogenesis of GA is still under investigation, but recent studies suggest that a Th1-mediated dysregulation of the JAK-STAT pathway may contribute to the disease. Other hypotheses include a delayed hypersensitivity reaction or cell mediated immune response. The mechanism may be multifaceted, and epidemiologic research suggests a genetic predisposition in White individuals, but these findings may be associated with socioeconomic factors and disparities in health care.
GA presents on histology with palisading histiocytes surrounding focal collagen necrobiosis with mucin deposition. Tissue samples also display leukocytic infiltration of the dermis featuring multinucleated giant cells. There are defining features of the different subtypes, but focal collagen necrosis, the presence of histiocytes, and mucin deposition are consistent findings across all presentations.
GA lesions commonly regress on their own, but they tend to recur and can be functionally and visually unappealing to patients. The most common treatments for GA include topical corticosteroids, intralesional corticosteroid injections, and other anti-inflammatory drugs. These interventions can be administered in a variety of ways as the inflammation caused by GA exists on a spectrum, and less severe cases can be managed with topical or intralesional treatment. Systemic therapy may be necessary for severe and recalcitrant cases. Other interventions that have shown promise in smaller studies include phototherapy, hydroxychloroquine, and TNF-alpha inhibitors.
This case and photo were submitted by Lucas Shapiro, BS, Nova Southeastern University College of Osteopathic Medicine, Tampa Bay Regional Campus, and Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to dermnews@mdedge.com.
References
Joshi TP and Duvic M. Am J Clin Dermatol. 2022 Jan;23(1):37-50. doi: 10.1007/s40257-021-00636-1.
Muse M et al. Dermatol Online J. 2021 Apr 15;27(4):13030/qt0m50398n.
Schmieder SJ et al. Granuloma Annulare. NIH National Center for Biotechnology Information [Updated 2022 Nov 7]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan. 7.