User login
Skin Cancer Screening and Prevention During the COVID-19 Pandemic
On March 11, 2020, the World Health Organization declared the outbreak of coronavirus disease 2019 (COVID-19) a pandemic, leading to an abrupt widespread shift to teledermatology, with postponement of nonessential in-office medical and surgical services, according to American Academy of Dermatology (AAD) recommendations.1 Perspectives have been offered regarding skin cancer management during the pandemic2; however, the current literature is lacking guidance on skin cancer screening and prevention during the COVID-19 era.
Preliminary data show a 34.3% reduction in skin cancer referrals from February to April 2020 compared to the same period in 2019. The authors also presented a subsequent reduction in the number of skin cancer diagnoses in March 2020 compared to March 2019.3 Although the COVID-19 public health emergency should be prioritized by all health care workers, the duty to maintain disease prevention remains.
We aim to provide recommendations for this urgent topic. Our goal is finding balance in preventing an increase in the incidence of and mortality from skin cancer that results from delayed detection, while conserving personalprotective equipment and minimizing exposure, by patients and clinical personnel, to the severe acute respiratory syndrome coronavirus 2. A primary benefit of skin cancer screening lies in the ability to detect melanoma, which is associated with higher mortality than the more common nonmelanoma skin cancers, basal and cutaneous squamous cell carcinomas. We place preeminence on screening directed toward detecting melanoma. The main screening method that dermatologists employ is the total-body skin examination (TBSE). Another widely encouraged and utilized component in skin cancer prevention is patient education, emphasizing avoidance of risk factors, undertaking protective factors, and providing clear instructions for performing the patient-led skin self-examination (SSE).
Teledermatology Essentials for Skin Cancer Screening
Arguably, dermatology possesses the most potential for successfully utilizing telemedicine. Teledermatology has become widely implemented across the United States, secondary to the implications of the current pandemic. A report by Perkins and colleagues4 provided a positive outlook in the preliminary transition to teledermatology beginning in March 2020, though reported time of use was relatively short (3 weeks). A May 2020 article in Dermatology News provided tips for implementing telemedicine for practices.5
We agree with the comprehensive screening algorithm for teledermatology presented by Perkins and colleagues4 (Figure 1A in their report) and recommend the following for the screening and prevention of skin cancer:
• Patients with any characteristics of increased risk, including a personal or family history of melanoma, large congenital nevi, many melanotic nevi, dysplastic nevi, and Fitzpatrick skin types I and II,6 should be prioritized for an in-person visit for TBSE.
• Immunosuppressed patients, particularly organ transplant recipients and those with a history of skin cancer, should be prioritized for an in-person visit for TBSE.
• Established patients evaluated and determined to be at average risk for skin cancer should be offered a teledermatology visit. Suspicious findings during these visits should be prioritized for an in-person visit, with subsequent biopsy and follow-up.
• New patients should be offered a teledermatology visit.
These recommendations must be reviewed alongside each patient’s risk for travel and being present in person as well as other factors that might place the patient at increased risk for COVID-19.
Total-body skin examination, a widely used tool in the dermatologist’s tool kit, presents minimal risk to patients while providing important data for each dermatology patient’s profile, ultimately directing patient care. The role of TBSE in skin cancer screening and prevention has been in discussion even prior to the current pandemic. The US Preventive Services Task Force (USPSTF) has not declared a role for TBSE in recent years; however, USPSTF recommendations are formulated using data from all forms of screening, not only dermatologist-led interventions. Accordingly, USPSTF recommendations target primary care. The AAD has released statements addressing the role of TBSE and skin cancer prevention in the past, when necessary, to provide clarity.7
There is no clear definition of SSE or guidelines on how to educate a patient to perform regular SSE; however, the AAD provides patients with resources on how to perform an SSE.8 Just as dermatologists would provide education, advice, and guidance by directing patients to the AAD website for the SSE during an in-person visit, we encourage dermatologists to continue this practice during all teledermatology visits.
The role of teledermatology in skin cancer screening and prevention is limited; dermatologists will not be able to adequately perform TBSE as it would be done at in-person visits. Furthermore, the true implications of teledermatology compared to in-person visits during the COVID-19 pandemic have yet to be realized and analyzed. It is nonetheless important to appreciate that teledermatology holds great promise of benefit in skin cancer prevention, especially in the form of patient education by dermatologists. Practices in the realm of screening and prevention by health care professionals should be continually addressed during the pandemic; it is important to consider the implications associated with delays in diagnosis and treatment.
Teledermatology Limitations and Recommendations for High-Quality Visits
A benefit of video consultation (VC) vs telephone visits is visual interaction—the crux of dermatology. A 2019 study investigated VC experiences among providers and patients in the primary care setting. Benefits of VC were reported to include convenience for working patients and patients with mobility or mental health problems, visual cues, building rapport, and improving communication.9
Despite these benefits, VC is not without limitations. Many technical factors create variability in the quality of teledermatology VCs for a melanocytic lesion, including patient environment and lighting, color distortion, video resolution, and Internet connection. We make the following recommendations:
• Environment: Locate or create a dedicated space for teledermatology visits that is well lit, private, and has minimal background noise. Place the device on a level surface, center yourself in the frame, and keep the camera at eye level.
• Lighting: Use neutral lighting, placing the light source in front of you but behind the camera of the device. Avoid placing light sources, such as a window, behind you.
• Video resolution: Regardless of the type of camera (eg, integrated webcam, external camera), close out all other running software programs to optimize bandwidth during the visit.
• Internet connection: Use a wired connection (via an Ethernet cable) instead of a Wi-Fi connection to greatly decrease the chance of losing the connection during the visit. It also is faster than Wi-Fi.
• Addressing specific lesions: Patients should keep the device in place, repositioning themselves to show the lesions rather than moving the device by hand.
• Video capacity: Test your device’s video capacity beforehand, which can be as simple as video-calling a family member or friend from your designated space. Feedback regarding video and audio quality will help fine-tune your setup.
• Instructions to the patient: Provide clear instructions to the patient when photographs of specific lesions are needed for further review. Specify what view(s) you need and whether size or bilateral comparison is needed. A web post by VisualDx10 provides advice to patients on taking high-quality photographs.
Final Thoughts
Teledermatology indubitably presents a learning curve for dermatologists and patients. As with other technological advances in society, we are optimistic that, first, the confidence level in teledermatology use will increase, and, second, evidence-based data will pave the way to enhance this experience. We realize the inherent limitation of accessibility to certain technologies, which is regrettably far from equitable. Patients need a personal device equipped with audio and video; access to a high-quality Internet connection; some degree of technological literacy; and a quiet private location.
We hope to learn from all experiences during the current pandemic. Future innovation in teledermatology and in telemedicine generally should aim to address technological inequities to allow for the delivery of quality care to as many patients as possible.
- American Academy of Dermatology. Everyday health and preparedness steps in clinic Updated April 4, 2020. Accessed December 17, 2020. https://assets.ctfassets.net/1ny4yoiyrqia/4LNCNjucOonbQx7aC970x/b56b540957ddad94dcc61949b8e3acc9/COVID-19_Preparedness_30Apr2020.pdf
- Geskin LJ, Trager MH, Aasi SZ, et al. Perspectives on the recommendations for skin cancer management during the COVID-19 pandemic. J Am Acad Dermatol. 2020;83:295-296.
- Earnshaw CH, Hunter HJA, McMullen E, et al. Reduction in skin cancer diagnosis, and overall cancer referrals, during the COVID-19 pandemic. Br J Dermatol. 2020;183:792-794.
- Perkins S, Cohen JM, Nelson CA, et al. Teledermatology in the era of COVID-19: experience of an academic department of dermatology. J Am Acad Dermatol. 2020;83:E43-E44.
- Marina F. COVID-19: telehealth at the forefront of the pandemic. Dermatology News. May 12, 2020. Accessed December 17, 2020. www.mdedge.com/dermatology/article/222089/coronavirus-updates/covid-19-telehealth-forefront-pandemic?channel=52
- Watts CG, Dieng M, Morton RL, et al. Clinical practice guidelines for identification, screening and follow-up of individuals at high risk of primary cutaneous melanoma: a systematic review. Br J Dermatol. 2015;172:33-47.
- Rosamilia LL. “Doctor, do I need a skin check?” Cutis. 2019;103:290-291.
- Detect skin cancer: how to perform a skin self-exam. American Academy of Dermatology. Accessed December 17, 2020. www.aad.org/public/diseases/skin-cancer/find/check-skin
- Donaghy E, Atherton H, Hammersley V, et al. Acceptability, benefits, and challenges of video consulting: a qualitative study in primary care. Br J Gen Pract. 2019;69:E586-E594.
- How to take the best photos for teledermatology. VisualDx. Accessed December 17, 2020. https://info.visualdx.com/l/11412/2020-03-31/6h4hdz
On March 11, 2020, the World Health Organization declared the outbreak of coronavirus disease 2019 (COVID-19) a pandemic, leading to an abrupt widespread shift to teledermatology, with postponement of nonessential in-office medical and surgical services, according to American Academy of Dermatology (AAD) recommendations.1 Perspectives have been offered regarding skin cancer management during the pandemic2; however, the current literature is lacking guidance on skin cancer screening and prevention during the COVID-19 era.
Preliminary data show a 34.3% reduction in skin cancer referrals from February to April 2020 compared to the same period in 2019. The authors also presented a subsequent reduction in the number of skin cancer diagnoses in March 2020 compared to March 2019.3 Although the COVID-19 public health emergency should be prioritized by all health care workers, the duty to maintain disease prevention remains.
We aim to provide recommendations for this urgent topic. Our goal is finding balance in preventing an increase in the incidence of and mortality from skin cancer that results from delayed detection, while conserving personalprotective equipment and minimizing exposure, by patients and clinical personnel, to the severe acute respiratory syndrome coronavirus 2. A primary benefit of skin cancer screening lies in the ability to detect melanoma, which is associated with higher mortality than the more common nonmelanoma skin cancers, basal and cutaneous squamous cell carcinomas. We place preeminence on screening directed toward detecting melanoma. The main screening method that dermatologists employ is the total-body skin examination (TBSE). Another widely encouraged and utilized component in skin cancer prevention is patient education, emphasizing avoidance of risk factors, undertaking protective factors, and providing clear instructions for performing the patient-led skin self-examination (SSE).
Teledermatology Essentials for Skin Cancer Screening
Arguably, dermatology possesses the most potential for successfully utilizing telemedicine. Teledermatology has become widely implemented across the United States, secondary to the implications of the current pandemic. A report by Perkins and colleagues4 provided a positive outlook in the preliminary transition to teledermatology beginning in March 2020, though reported time of use was relatively short (3 weeks). A May 2020 article in Dermatology News provided tips for implementing telemedicine for practices.5
We agree with the comprehensive screening algorithm for teledermatology presented by Perkins and colleagues4 (Figure 1A in their report) and recommend the following for the screening and prevention of skin cancer:
• Patients with any characteristics of increased risk, including a personal or family history of melanoma, large congenital nevi, many melanotic nevi, dysplastic nevi, and Fitzpatrick skin types I and II,6 should be prioritized for an in-person visit for TBSE.
• Immunosuppressed patients, particularly organ transplant recipients and those with a history of skin cancer, should be prioritized for an in-person visit for TBSE.
• Established patients evaluated and determined to be at average risk for skin cancer should be offered a teledermatology visit. Suspicious findings during these visits should be prioritized for an in-person visit, with subsequent biopsy and follow-up.
• New patients should be offered a teledermatology visit.
These recommendations must be reviewed alongside each patient’s risk for travel and being present in person as well as other factors that might place the patient at increased risk for COVID-19.
Total-body skin examination, a widely used tool in the dermatologist’s tool kit, presents minimal risk to patients while providing important data for each dermatology patient’s profile, ultimately directing patient care. The role of TBSE in skin cancer screening and prevention has been in discussion even prior to the current pandemic. The US Preventive Services Task Force (USPSTF) has not declared a role for TBSE in recent years; however, USPSTF recommendations are formulated using data from all forms of screening, not only dermatologist-led interventions. Accordingly, USPSTF recommendations target primary care. The AAD has released statements addressing the role of TBSE and skin cancer prevention in the past, when necessary, to provide clarity.7
There is no clear definition of SSE or guidelines on how to educate a patient to perform regular SSE; however, the AAD provides patients with resources on how to perform an SSE.8 Just as dermatologists would provide education, advice, and guidance by directing patients to the AAD website for the SSE during an in-person visit, we encourage dermatologists to continue this practice during all teledermatology visits.
The role of teledermatology in skin cancer screening and prevention is limited; dermatologists will not be able to adequately perform TBSE as it would be done at in-person visits. Furthermore, the true implications of teledermatology compared to in-person visits during the COVID-19 pandemic have yet to be realized and analyzed. It is nonetheless important to appreciate that teledermatology holds great promise of benefit in skin cancer prevention, especially in the form of patient education by dermatologists. Practices in the realm of screening and prevention by health care professionals should be continually addressed during the pandemic; it is important to consider the implications associated with delays in diagnosis and treatment.
Teledermatology Limitations and Recommendations for High-Quality Visits
A benefit of video consultation (VC) vs telephone visits is visual interaction—the crux of dermatology. A 2019 study investigated VC experiences among providers and patients in the primary care setting. Benefits of VC were reported to include convenience for working patients and patients with mobility or mental health problems, visual cues, building rapport, and improving communication.9
Despite these benefits, VC is not without limitations. Many technical factors create variability in the quality of teledermatology VCs for a melanocytic lesion, including patient environment and lighting, color distortion, video resolution, and Internet connection. We make the following recommendations:
• Environment: Locate or create a dedicated space for teledermatology visits that is well lit, private, and has minimal background noise. Place the device on a level surface, center yourself in the frame, and keep the camera at eye level.
• Lighting: Use neutral lighting, placing the light source in front of you but behind the camera of the device. Avoid placing light sources, such as a window, behind you.
• Video resolution: Regardless of the type of camera (eg, integrated webcam, external camera), close out all other running software programs to optimize bandwidth during the visit.
• Internet connection: Use a wired connection (via an Ethernet cable) instead of a Wi-Fi connection to greatly decrease the chance of losing the connection during the visit. It also is faster than Wi-Fi.
• Addressing specific lesions: Patients should keep the device in place, repositioning themselves to show the lesions rather than moving the device by hand.
• Video capacity: Test your device’s video capacity beforehand, which can be as simple as video-calling a family member or friend from your designated space. Feedback regarding video and audio quality will help fine-tune your setup.
• Instructions to the patient: Provide clear instructions to the patient when photographs of specific lesions are needed for further review. Specify what view(s) you need and whether size or bilateral comparison is needed. A web post by VisualDx10 provides advice to patients on taking high-quality photographs.
Final Thoughts
Teledermatology indubitably presents a learning curve for dermatologists and patients. As with other technological advances in society, we are optimistic that, first, the confidence level in teledermatology use will increase, and, second, evidence-based data will pave the way to enhance this experience. We realize the inherent limitation of accessibility to certain technologies, which is regrettably far from equitable. Patients need a personal device equipped with audio and video; access to a high-quality Internet connection; some degree of technological literacy; and a quiet private location.
We hope to learn from all experiences during the current pandemic. Future innovation in teledermatology and in telemedicine generally should aim to address technological inequities to allow for the delivery of quality care to as many patients as possible.
On March 11, 2020, the World Health Organization declared the outbreak of coronavirus disease 2019 (COVID-19) a pandemic, leading to an abrupt widespread shift to teledermatology, with postponement of nonessential in-office medical and surgical services, according to American Academy of Dermatology (AAD) recommendations.1 Perspectives have been offered regarding skin cancer management during the pandemic2; however, the current literature is lacking guidance on skin cancer screening and prevention during the COVID-19 era.
Preliminary data show a 34.3% reduction in skin cancer referrals from February to April 2020 compared to the same period in 2019. The authors also presented a subsequent reduction in the number of skin cancer diagnoses in March 2020 compared to March 2019.3 Although the COVID-19 public health emergency should be prioritized by all health care workers, the duty to maintain disease prevention remains.
We aim to provide recommendations for this urgent topic. Our goal is finding balance in preventing an increase in the incidence of and mortality from skin cancer that results from delayed detection, while conserving personalprotective equipment and minimizing exposure, by patients and clinical personnel, to the severe acute respiratory syndrome coronavirus 2. A primary benefit of skin cancer screening lies in the ability to detect melanoma, which is associated with higher mortality than the more common nonmelanoma skin cancers, basal and cutaneous squamous cell carcinomas. We place preeminence on screening directed toward detecting melanoma. The main screening method that dermatologists employ is the total-body skin examination (TBSE). Another widely encouraged and utilized component in skin cancer prevention is patient education, emphasizing avoidance of risk factors, undertaking protective factors, and providing clear instructions for performing the patient-led skin self-examination (SSE).
Teledermatology Essentials for Skin Cancer Screening
Arguably, dermatology possesses the most potential for successfully utilizing telemedicine. Teledermatology has become widely implemented across the United States, secondary to the implications of the current pandemic. A report by Perkins and colleagues4 provided a positive outlook in the preliminary transition to teledermatology beginning in March 2020, though reported time of use was relatively short (3 weeks). A May 2020 article in Dermatology News provided tips for implementing telemedicine for practices.5
We agree with the comprehensive screening algorithm for teledermatology presented by Perkins and colleagues4 (Figure 1A in their report) and recommend the following for the screening and prevention of skin cancer:
• Patients with any characteristics of increased risk, including a personal or family history of melanoma, large congenital nevi, many melanotic nevi, dysplastic nevi, and Fitzpatrick skin types I and II,6 should be prioritized for an in-person visit for TBSE.
• Immunosuppressed patients, particularly organ transplant recipients and those with a history of skin cancer, should be prioritized for an in-person visit for TBSE.
• Established patients evaluated and determined to be at average risk for skin cancer should be offered a teledermatology visit. Suspicious findings during these visits should be prioritized for an in-person visit, with subsequent biopsy and follow-up.
• New patients should be offered a teledermatology visit.
These recommendations must be reviewed alongside each patient’s risk for travel and being present in person as well as other factors that might place the patient at increased risk for COVID-19.
Total-body skin examination, a widely used tool in the dermatologist’s tool kit, presents minimal risk to patients while providing important data for each dermatology patient’s profile, ultimately directing patient care. The role of TBSE in skin cancer screening and prevention has been in discussion even prior to the current pandemic. The US Preventive Services Task Force (USPSTF) has not declared a role for TBSE in recent years; however, USPSTF recommendations are formulated using data from all forms of screening, not only dermatologist-led interventions. Accordingly, USPSTF recommendations target primary care. The AAD has released statements addressing the role of TBSE and skin cancer prevention in the past, when necessary, to provide clarity.7
There is no clear definition of SSE or guidelines on how to educate a patient to perform regular SSE; however, the AAD provides patients with resources on how to perform an SSE.8 Just as dermatologists would provide education, advice, and guidance by directing patients to the AAD website for the SSE during an in-person visit, we encourage dermatologists to continue this practice during all teledermatology visits.
The role of teledermatology in skin cancer screening and prevention is limited; dermatologists will not be able to adequately perform TBSE as it would be done at in-person visits. Furthermore, the true implications of teledermatology compared to in-person visits during the COVID-19 pandemic have yet to be realized and analyzed. It is nonetheless important to appreciate that teledermatology holds great promise of benefit in skin cancer prevention, especially in the form of patient education by dermatologists. Practices in the realm of screening and prevention by health care professionals should be continually addressed during the pandemic; it is important to consider the implications associated with delays in diagnosis and treatment.
Teledermatology Limitations and Recommendations for High-Quality Visits
A benefit of video consultation (VC) vs telephone visits is visual interaction—the crux of dermatology. A 2019 study investigated VC experiences among providers and patients in the primary care setting. Benefits of VC were reported to include convenience for working patients and patients with mobility or mental health problems, visual cues, building rapport, and improving communication.9
Despite these benefits, VC is not without limitations. Many technical factors create variability in the quality of teledermatology VCs for a melanocytic lesion, including patient environment and lighting, color distortion, video resolution, and Internet connection. We make the following recommendations:
• Environment: Locate or create a dedicated space for teledermatology visits that is well lit, private, and has minimal background noise. Place the device on a level surface, center yourself in the frame, and keep the camera at eye level.
• Lighting: Use neutral lighting, placing the light source in front of you but behind the camera of the device. Avoid placing light sources, such as a window, behind you.
• Video resolution: Regardless of the type of camera (eg, integrated webcam, external camera), close out all other running software programs to optimize bandwidth during the visit.
• Internet connection: Use a wired connection (via an Ethernet cable) instead of a Wi-Fi connection to greatly decrease the chance of losing the connection during the visit. It also is faster than Wi-Fi.
• Addressing specific lesions: Patients should keep the device in place, repositioning themselves to show the lesions rather than moving the device by hand.
• Video capacity: Test your device’s video capacity beforehand, which can be as simple as video-calling a family member or friend from your designated space. Feedback regarding video and audio quality will help fine-tune your setup.
• Instructions to the patient: Provide clear instructions to the patient when photographs of specific lesions are needed for further review. Specify what view(s) you need and whether size or bilateral comparison is needed. A web post by VisualDx10 provides advice to patients on taking high-quality photographs.
Final Thoughts
Teledermatology indubitably presents a learning curve for dermatologists and patients. As with other technological advances in society, we are optimistic that, first, the confidence level in teledermatology use will increase, and, second, evidence-based data will pave the way to enhance this experience. We realize the inherent limitation of accessibility to certain technologies, which is regrettably far from equitable. Patients need a personal device equipped with audio and video; access to a high-quality Internet connection; some degree of technological literacy; and a quiet private location.
We hope to learn from all experiences during the current pandemic. Future innovation in teledermatology and in telemedicine generally should aim to address technological inequities to allow for the delivery of quality care to as many patients as possible.
- American Academy of Dermatology. Everyday health and preparedness steps in clinic Updated April 4, 2020. Accessed December 17, 2020. https://assets.ctfassets.net/1ny4yoiyrqia/4LNCNjucOonbQx7aC970x/b56b540957ddad94dcc61949b8e3acc9/COVID-19_Preparedness_30Apr2020.pdf
- Geskin LJ, Trager MH, Aasi SZ, et al. Perspectives on the recommendations for skin cancer management during the COVID-19 pandemic. J Am Acad Dermatol. 2020;83:295-296.
- Earnshaw CH, Hunter HJA, McMullen E, et al. Reduction in skin cancer diagnosis, and overall cancer referrals, during the COVID-19 pandemic. Br J Dermatol. 2020;183:792-794.
- Perkins S, Cohen JM, Nelson CA, et al. Teledermatology in the era of COVID-19: experience of an academic department of dermatology. J Am Acad Dermatol. 2020;83:E43-E44.
- Marina F. COVID-19: telehealth at the forefront of the pandemic. Dermatology News. May 12, 2020. Accessed December 17, 2020. www.mdedge.com/dermatology/article/222089/coronavirus-updates/covid-19-telehealth-forefront-pandemic?channel=52
- Watts CG, Dieng M, Morton RL, et al. Clinical practice guidelines for identification, screening and follow-up of individuals at high risk of primary cutaneous melanoma: a systematic review. Br J Dermatol. 2015;172:33-47.
- Rosamilia LL. “Doctor, do I need a skin check?” Cutis. 2019;103:290-291.
- Detect skin cancer: how to perform a skin self-exam. American Academy of Dermatology. Accessed December 17, 2020. www.aad.org/public/diseases/skin-cancer/find/check-skin
- Donaghy E, Atherton H, Hammersley V, et al. Acceptability, benefits, and challenges of video consulting: a qualitative study in primary care. Br J Gen Pract. 2019;69:E586-E594.
- How to take the best photos for teledermatology. VisualDx. Accessed December 17, 2020. https://info.visualdx.com/l/11412/2020-03-31/6h4hdz
- American Academy of Dermatology. Everyday health and preparedness steps in clinic Updated April 4, 2020. Accessed December 17, 2020. https://assets.ctfassets.net/1ny4yoiyrqia/4LNCNjucOonbQx7aC970x/b56b540957ddad94dcc61949b8e3acc9/COVID-19_Preparedness_30Apr2020.pdf
- Geskin LJ, Trager MH, Aasi SZ, et al. Perspectives on the recommendations for skin cancer management during the COVID-19 pandemic. J Am Acad Dermatol. 2020;83:295-296.
- Earnshaw CH, Hunter HJA, McMullen E, et al. Reduction in skin cancer diagnosis, and overall cancer referrals, during the COVID-19 pandemic. Br J Dermatol. 2020;183:792-794.
- Perkins S, Cohen JM, Nelson CA, et al. Teledermatology in the era of COVID-19: experience of an academic department of dermatology. J Am Acad Dermatol. 2020;83:E43-E44.
- Marina F. COVID-19: telehealth at the forefront of the pandemic. Dermatology News. May 12, 2020. Accessed December 17, 2020. www.mdedge.com/dermatology/article/222089/coronavirus-updates/covid-19-telehealth-forefront-pandemic?channel=52
- Watts CG, Dieng M, Morton RL, et al. Clinical practice guidelines for identification, screening and follow-up of individuals at high risk of primary cutaneous melanoma: a systematic review. Br J Dermatol. 2015;172:33-47.
- Rosamilia LL. “Doctor, do I need a skin check?” Cutis. 2019;103:290-291.
- Detect skin cancer: how to perform a skin self-exam. American Academy of Dermatology. Accessed December 17, 2020. www.aad.org/public/diseases/skin-cancer/find/check-skin
- Donaghy E, Atherton H, Hammersley V, et al. Acceptability, benefits, and challenges of video consulting: a qualitative study in primary care. Br J Gen Pract. 2019;69:E586-E594.
- How to take the best photos for teledermatology. VisualDx. Accessed December 17, 2020. https://info.visualdx.com/l/11412/2020-03-31/6h4hdz
Practice Points
- It is important for dermatologists to maintain skin cancer screening and prevention efforts during the coronavirus disease 2019 pandemic.
- Patient populations at increased risk for skin cancer should be prioritized for in-person evaluations, but teledermatology should be considered for initial examination in new patients and patients at average risk for skin cancer.
- Teledermatology presents a learning curve for dermatologists and patients, but the confidence level will increase, and evidence-based data will pave the way to enhance this experience.
Bariatric surgery might reduce severity of COVID-19 infection
and the disease was less severe than among COVID patients with obesity who had not undergone the surgery, a new retrospective analysis shows.
The research was published in Surgery for Obesity and Related Diseases.
Because obesity is a well-known risk factor for poor COVID-19 outcomes, Ali Aminian, MD, Bariatric and Metabolic Institute, Cleveland Clinic, and colleagues decided to study whether weight-loss surgery had a bearing on outcomes of patients with COVID-19.
They matched 33 COVID-19 patients who had undergone metabolic surgery with 330 control patients with obesity who were infected with the virus during the first wave of the pandemic.
Surgery was associated with a 69% reduction in the risk of being hospitalized as a result of COVID-19. None of the surgery patients required intensive care, mechanical ventilation, or dialysis, and none died.
“Patients after bariatric surgery become significantly healthier and can fight the virus better,” said Dr. Aminian in a statement from his institution. “If confirmed by future studies, this can be added to the long list of health benefits of bariatric surgery.”
COVID-19 is a wake-up call for the consequences of obesity
Dr. Aminian said in an interview that COVID-19 is a “wake-up call to show the public and health care professionals that obesity is a major health problem and has multiple health consequences.”
More than 300 articles in the literature show that obesity is a major risk factor for poor outcomes following COVID-19 infection. Dr. Aminian said the pandemic has “improved public awareness about the consequences of obesity.”
Compared with last year at his institution, the intake of new patients “who would like to join a program to have surgery or have some tools to help them to lose weight is almost double,” he noted.
Furthermore, referrals to their unit from primary care physicians, as well as from endocrinologists and cardiologists, for bariatric surgery nearly doubled in recent months.
Although the unit had to stop all bariatric surgeries for around 6 weeks in April because of COVID-19, it has performed the same number of procedures this year as in 2019 and 2018.
Because of the recent surge in COVID cases in Ohio, bariatric procedures are once again on hold. “Elective operations that require hospital beds after surgery have been paused to provide beds for patients who have COVID-19,” he explained.
Small sample size, study should be repeated
For their study, Dr. Aminian and colleagues examined the records of 4,365 patients at the Cleveland Clinic Health System who tested positive for the virus between March 8 and July 22, 2020.
Of these, 1,003 had a body mass index of at least 35 mg/kg2; 482 had a BMI of at least 40. The team identified 33 patients who had previously undergone metabolic surgery, comprising 20 sleeve gastrectomies and 13 Roux-en-Y gastric bypasses.
The surgical patients were propensity matched in a 1:10 ratio with nonsurgical control patients with a BMI of at least 40. The patients were matched on the basis of age, sex, ethnicity, location, smoking status, and history of chronic obstructive pulmonary disease.
The mean BMI of surgical patients was 49.1 before their procedure. It fell to 37.2 by the time they tested positive for COVID-19. This compares with an average of 46.7 in the control group at the time they tested positive for the virus.
The team found that 18.2% of metabolic surgery patients were admitted to hospital versus 42.1% of control patients (P = .013).
Moreover, metabolic surgery patients did not require admission to the intensive care unit, nor did they require mechanical ventilation or dialysis, and none died. This compares with 13.0% (P = .021), 6.7% (P = .24), 1.5%, and 2.4%, respectively, of patients in the control group.
Multivariate analysis indicated that prior metabolic surgery was associated with lower hospital admission, at an odds ratio of 0.31 (P = .028), in comparison with control patients with obesity.
Acknowledging the limited sample size of their study, the team wrote: “As this study reflects findings early in the course of the pandemic, it will be of interest to repeat this study with larger data sets and later in the course of the pandemic.”
Continue as many aspects of obesity management as possible during pandemic
Dr. Aminian underlined that, for him, the take-home message from the study is that health care professionals should “ideally” continue all aspects of obesity management during the pandemic, including “medical management, behavioral therapy, lifestyle changes, and access to bariatric surgery.”
This is despite the fact that insurance coverage for bariatric surgery has “always been a challenge for many patients, since many insurance plans do not cover” bariatric procedures, he noted.
In July, the American Society for Metabolic and Bariatric Surgery issued a statement declaring that obesity surgery should not be considered an elective procedure and should be resumed as soon as it’s safe to do so during any resurgence of the COVID-19 pandemic.
The authors disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
and the disease was less severe than among COVID patients with obesity who had not undergone the surgery, a new retrospective analysis shows.
The research was published in Surgery for Obesity and Related Diseases.
Because obesity is a well-known risk factor for poor COVID-19 outcomes, Ali Aminian, MD, Bariatric and Metabolic Institute, Cleveland Clinic, and colleagues decided to study whether weight-loss surgery had a bearing on outcomes of patients with COVID-19.
They matched 33 COVID-19 patients who had undergone metabolic surgery with 330 control patients with obesity who were infected with the virus during the first wave of the pandemic.
Surgery was associated with a 69% reduction in the risk of being hospitalized as a result of COVID-19. None of the surgery patients required intensive care, mechanical ventilation, or dialysis, and none died.
“Patients after bariatric surgery become significantly healthier and can fight the virus better,” said Dr. Aminian in a statement from his institution. “If confirmed by future studies, this can be added to the long list of health benefits of bariatric surgery.”
COVID-19 is a wake-up call for the consequences of obesity
Dr. Aminian said in an interview that COVID-19 is a “wake-up call to show the public and health care professionals that obesity is a major health problem and has multiple health consequences.”
More than 300 articles in the literature show that obesity is a major risk factor for poor outcomes following COVID-19 infection. Dr. Aminian said the pandemic has “improved public awareness about the consequences of obesity.”
Compared with last year at his institution, the intake of new patients “who would like to join a program to have surgery or have some tools to help them to lose weight is almost double,” he noted.
Furthermore, referrals to their unit from primary care physicians, as well as from endocrinologists and cardiologists, for bariatric surgery nearly doubled in recent months.
Although the unit had to stop all bariatric surgeries for around 6 weeks in April because of COVID-19, it has performed the same number of procedures this year as in 2019 and 2018.
Because of the recent surge in COVID cases in Ohio, bariatric procedures are once again on hold. “Elective operations that require hospital beds after surgery have been paused to provide beds for patients who have COVID-19,” he explained.
Small sample size, study should be repeated
For their study, Dr. Aminian and colleagues examined the records of 4,365 patients at the Cleveland Clinic Health System who tested positive for the virus between March 8 and July 22, 2020.
Of these, 1,003 had a body mass index of at least 35 mg/kg2; 482 had a BMI of at least 40. The team identified 33 patients who had previously undergone metabolic surgery, comprising 20 sleeve gastrectomies and 13 Roux-en-Y gastric bypasses.
The surgical patients were propensity matched in a 1:10 ratio with nonsurgical control patients with a BMI of at least 40. The patients were matched on the basis of age, sex, ethnicity, location, smoking status, and history of chronic obstructive pulmonary disease.
The mean BMI of surgical patients was 49.1 before their procedure. It fell to 37.2 by the time they tested positive for COVID-19. This compares with an average of 46.7 in the control group at the time they tested positive for the virus.
The team found that 18.2% of metabolic surgery patients were admitted to hospital versus 42.1% of control patients (P = .013).
Moreover, metabolic surgery patients did not require admission to the intensive care unit, nor did they require mechanical ventilation or dialysis, and none died. This compares with 13.0% (P = .021), 6.7% (P = .24), 1.5%, and 2.4%, respectively, of patients in the control group.
Multivariate analysis indicated that prior metabolic surgery was associated with lower hospital admission, at an odds ratio of 0.31 (P = .028), in comparison with control patients with obesity.
Acknowledging the limited sample size of their study, the team wrote: “As this study reflects findings early in the course of the pandemic, it will be of interest to repeat this study with larger data sets and later in the course of the pandemic.”
Continue as many aspects of obesity management as possible during pandemic
Dr. Aminian underlined that, for him, the take-home message from the study is that health care professionals should “ideally” continue all aspects of obesity management during the pandemic, including “medical management, behavioral therapy, lifestyle changes, and access to bariatric surgery.”
This is despite the fact that insurance coverage for bariatric surgery has “always been a challenge for many patients, since many insurance plans do not cover” bariatric procedures, he noted.
In July, the American Society for Metabolic and Bariatric Surgery issued a statement declaring that obesity surgery should not be considered an elective procedure and should be resumed as soon as it’s safe to do so during any resurgence of the COVID-19 pandemic.
The authors disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
and the disease was less severe than among COVID patients with obesity who had not undergone the surgery, a new retrospective analysis shows.
The research was published in Surgery for Obesity and Related Diseases.
Because obesity is a well-known risk factor for poor COVID-19 outcomes, Ali Aminian, MD, Bariatric and Metabolic Institute, Cleveland Clinic, and colleagues decided to study whether weight-loss surgery had a bearing on outcomes of patients with COVID-19.
They matched 33 COVID-19 patients who had undergone metabolic surgery with 330 control patients with obesity who were infected with the virus during the first wave of the pandemic.
Surgery was associated with a 69% reduction in the risk of being hospitalized as a result of COVID-19. None of the surgery patients required intensive care, mechanical ventilation, or dialysis, and none died.
“Patients after bariatric surgery become significantly healthier and can fight the virus better,” said Dr. Aminian in a statement from his institution. “If confirmed by future studies, this can be added to the long list of health benefits of bariatric surgery.”
COVID-19 is a wake-up call for the consequences of obesity
Dr. Aminian said in an interview that COVID-19 is a “wake-up call to show the public and health care professionals that obesity is a major health problem and has multiple health consequences.”
More than 300 articles in the literature show that obesity is a major risk factor for poor outcomes following COVID-19 infection. Dr. Aminian said the pandemic has “improved public awareness about the consequences of obesity.”
Compared with last year at his institution, the intake of new patients “who would like to join a program to have surgery or have some tools to help them to lose weight is almost double,” he noted.
Furthermore, referrals to their unit from primary care physicians, as well as from endocrinologists and cardiologists, for bariatric surgery nearly doubled in recent months.
Although the unit had to stop all bariatric surgeries for around 6 weeks in April because of COVID-19, it has performed the same number of procedures this year as in 2019 and 2018.
Because of the recent surge in COVID cases in Ohio, bariatric procedures are once again on hold. “Elective operations that require hospital beds after surgery have been paused to provide beds for patients who have COVID-19,” he explained.
Small sample size, study should be repeated
For their study, Dr. Aminian and colleagues examined the records of 4,365 patients at the Cleveland Clinic Health System who tested positive for the virus between March 8 and July 22, 2020.
Of these, 1,003 had a body mass index of at least 35 mg/kg2; 482 had a BMI of at least 40. The team identified 33 patients who had previously undergone metabolic surgery, comprising 20 sleeve gastrectomies and 13 Roux-en-Y gastric bypasses.
The surgical patients were propensity matched in a 1:10 ratio with nonsurgical control patients with a BMI of at least 40. The patients were matched on the basis of age, sex, ethnicity, location, smoking status, and history of chronic obstructive pulmonary disease.
The mean BMI of surgical patients was 49.1 before their procedure. It fell to 37.2 by the time they tested positive for COVID-19. This compares with an average of 46.7 in the control group at the time they tested positive for the virus.
The team found that 18.2% of metabolic surgery patients were admitted to hospital versus 42.1% of control patients (P = .013).
Moreover, metabolic surgery patients did not require admission to the intensive care unit, nor did they require mechanical ventilation or dialysis, and none died. This compares with 13.0% (P = .021), 6.7% (P = .24), 1.5%, and 2.4%, respectively, of patients in the control group.
Multivariate analysis indicated that prior metabolic surgery was associated with lower hospital admission, at an odds ratio of 0.31 (P = .028), in comparison with control patients with obesity.
Acknowledging the limited sample size of their study, the team wrote: “As this study reflects findings early in the course of the pandemic, it will be of interest to repeat this study with larger data sets and later in the course of the pandemic.”
Continue as many aspects of obesity management as possible during pandemic
Dr. Aminian underlined that, for him, the take-home message from the study is that health care professionals should “ideally” continue all aspects of obesity management during the pandemic, including “medical management, behavioral therapy, lifestyle changes, and access to bariatric surgery.”
This is despite the fact that insurance coverage for bariatric surgery has “always been a challenge for many patients, since many insurance plans do not cover” bariatric procedures, he noted.
In July, the American Society for Metabolic and Bariatric Surgery issued a statement declaring that obesity surgery should not be considered an elective procedure and should be resumed as soon as it’s safe to do so during any resurgence of the COVID-19 pandemic.
The authors disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
New pediatric cases down as U.S. tops 2 million children with COVID-19
The United States exceeded 2 million reported cases of COVID-19 in children just 6 weeks after recording its 1 millionth case, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.

The total number of cases in children was 2,000,681 as of Dec. 24, which represents 12.4% of all cases reported by the health departments of 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam, the AAP and CHA stated Dec. 29.
The case count for just the latest week, 178,935, was actually down 1.7% from the 182,018 reported the week before, marking the second drop since the beginning of December. The first came during the week ending Dec. 3, when the number of cases dropped more than 19% from the previous week, based on data from the AAP/CHA report.
The cumulative national rate of coronavirus infection is now 2,658 cases per 100,000 children, and “13 states have reported more than 4,000 cases per 100,000,” the two groups said.
The highest rate for any state can be found in North Dakota, which has had 7,722 cases of COVID-19 per 100,000 children. Wyoming has the highest proportion of cases in children at 20.5%, and California has reported the most cases overall, 234,174, the report shows.
Data on testing, hospitalization, and mortality were not included in the Dec. 29 report because of the holiday but will be available in the next edition, scheduled for release on Jan. 5, 2021.
The United States exceeded 2 million reported cases of COVID-19 in children just 6 weeks after recording its 1 millionth case, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.

The total number of cases in children was 2,000,681 as of Dec. 24, which represents 12.4% of all cases reported by the health departments of 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam, the AAP and CHA stated Dec. 29.
The case count for just the latest week, 178,935, was actually down 1.7% from the 182,018 reported the week before, marking the second drop since the beginning of December. The first came during the week ending Dec. 3, when the number of cases dropped more than 19% from the previous week, based on data from the AAP/CHA report.
The cumulative national rate of coronavirus infection is now 2,658 cases per 100,000 children, and “13 states have reported more than 4,000 cases per 100,000,” the two groups said.
The highest rate for any state can be found in North Dakota, which has had 7,722 cases of COVID-19 per 100,000 children. Wyoming has the highest proportion of cases in children at 20.5%, and California has reported the most cases overall, 234,174, the report shows.
Data on testing, hospitalization, and mortality were not included in the Dec. 29 report because of the holiday but will be available in the next edition, scheduled for release on Jan. 5, 2021.
The United States exceeded 2 million reported cases of COVID-19 in children just 6 weeks after recording its 1 millionth case, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.

The total number of cases in children was 2,000,681 as of Dec. 24, which represents 12.4% of all cases reported by the health departments of 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam, the AAP and CHA stated Dec. 29.
The case count for just the latest week, 178,935, was actually down 1.7% from the 182,018 reported the week before, marking the second drop since the beginning of December. The first came during the week ending Dec. 3, when the number of cases dropped more than 19% from the previous week, based on data from the AAP/CHA report.
The cumulative national rate of coronavirus infection is now 2,658 cases per 100,000 children, and “13 states have reported more than 4,000 cases per 100,000,” the two groups said.
The highest rate for any state can be found in North Dakota, which has had 7,722 cases of COVID-19 per 100,000 children. Wyoming has the highest proportion of cases in children at 20.5%, and California has reported the most cases overall, 234,174, the report shows.
Data on testing, hospitalization, and mortality were not included in the Dec. 29 report because of the holiday but will be available in the next edition, scheduled for release on Jan. 5, 2021.
HPV vaccine appears effective for treating warts, particularly in children
The human papillomavirus (HPV) vaccine, recommended by the Centers for Disease Control and Prevention for the prevention of HPV-associated genital warts and neoplasia, appears to be an effective and perhaps underappreciated treatment of existing cutaneous warts, according to expert speaking at the annual Coastal Dermatology symposium, held virtually.
, but a recently published review provides strong evidence that this is a practical clinical strategy, according to Theodore Rosen, MD, professor of dermatology at Baylor College of Medicine, Houston.
“Clearly, if you have someone, particularly a youngster, and you’re having trouble getting rid of their warts and they are age 9 years or above – and they need the vaccine anyhow – that’s a win-win proposition,” Dr. Rosen said.
The current nonavalent HPV vaccine is approved for individuals from age 9 to age 45. Although the CDC recommends routine vaccination at age 11 or 12 years, it allows earlier vaccination within the label.
The recently published and updated evidence of a benefit from treatment comes from a systematic literature review. For the review, 63 articles were drawn from the PubMed and Cochrane databases. The studies yielded 4,439 patients with cutaneous warts at the time they received the HPV vaccine or who specifically received vaccine as a treatment strategy.
As has been suggested previously in the case series and in a limited number of prospective studies, the majority of warts, including cutaneous warts and anogenital warts, resolved following vaccine administration.
“Mostly these were common warts, plantar warts, and flat warts,” Dr. Rosen said, but the paper also reported successful treatment of recurrent respiratory papillomatosis, squamous cell carcinomas, and basal cell carcinomas.
Case reports and small studies associating HPV vaccine with successful resolution of warts are easy to find in the literature. For example, 60% of patients achieved a complete response and 30% a partial response to HPV vaccine in one small prospective study of 26 patients with genital warts. Following vaccination, no recurrences were observed after a median follow-up of more than 8 months.
In the review paper, most of the cases involved patients who received the quadrivalent HPV vaccine, Dr. Rosen noted. Only one received the updated nonavalent vaccine, which, in addition to protection against the 6, 11, 16, and 18 subtypes extends protection to subtypes 31, 33, 45, 52, and 58.
“You would expect the nonavalent vaccine to provide the same protection. It is the same vaccine. It just offers activity against more subtypes,” Dr. Rosen said at the meeting, jointly presented by the University of Louisville and Global Academy for Medical Education. He reported that he personally has used the nonavalent vaccine successfully to treat a cutaneous wart.
The nonavalent vaccine can be administered in just two doses for those who receive the first dose before age 15. In others, it is given in three doses at 1- to 2-month intervals, according to Dr. Rosen. He said the efficacy for preventing genital warts and most HPV-related neoplasia exceeds 90%, although it is lower for penile and anal cancer. The protection extends for at least 10 years, but he said that he believes that it is likely to be longer.
“The HPV vaccine is really, really safe,” Dr. Rosen said. Besides injection-site reactions, the most common adverse event is syncope. For this reason, patients are advised to stay seated for 30 minutes after administration.
There is some evidence for cross-immunity for HPV subtypes not covered by the vaccine, particularly among children, Dr. Rosen commented. Citing the review article, he said that, although almost all HPV-associated warts resolve in children when treated with the vaccine, response is somewhat lower in adolescents and further reduced in adults.
In an interview, the senior author of the recent literature review, Natasha A. Mesinkovska, MD, PhD, associate professor of dermatology, University of California, Irvine, agreed with Dr. Rosen about the value of HPV vaccine for patients not responding to conventional therapies for HPV-related cutaneous warts.
“I think HPV vaccine is an excellent option for those patients, even older ones at 45 years of age if cost is not an issue,” she said. She did offer a caveat. In a recent statement from the International Papillomavirus Society (IPVS) on a world shortage of HPV vaccine, it was estimated that supplies might be limited for the next 3-5 years.
Given this shortage, “obtaining them currently may prove to be difficult,” she cautioned.
This publication and Global Academy for Medical Education are owned by the same parent company.
The human papillomavirus (HPV) vaccine, recommended by the Centers for Disease Control and Prevention for the prevention of HPV-associated genital warts and neoplasia, appears to be an effective and perhaps underappreciated treatment of existing cutaneous warts, according to expert speaking at the annual Coastal Dermatology symposium, held virtually.
, but a recently published review provides strong evidence that this is a practical clinical strategy, according to Theodore Rosen, MD, professor of dermatology at Baylor College of Medicine, Houston.
“Clearly, if you have someone, particularly a youngster, and you’re having trouble getting rid of their warts and they are age 9 years or above – and they need the vaccine anyhow – that’s a win-win proposition,” Dr. Rosen said.
The current nonavalent HPV vaccine is approved for individuals from age 9 to age 45. Although the CDC recommends routine vaccination at age 11 or 12 years, it allows earlier vaccination within the label.
The recently published and updated evidence of a benefit from treatment comes from a systematic literature review. For the review, 63 articles were drawn from the PubMed and Cochrane databases. The studies yielded 4,439 patients with cutaneous warts at the time they received the HPV vaccine or who specifically received vaccine as a treatment strategy.
As has been suggested previously in the case series and in a limited number of prospective studies, the majority of warts, including cutaneous warts and anogenital warts, resolved following vaccine administration.
“Mostly these were common warts, plantar warts, and flat warts,” Dr. Rosen said, but the paper also reported successful treatment of recurrent respiratory papillomatosis, squamous cell carcinomas, and basal cell carcinomas.
Case reports and small studies associating HPV vaccine with successful resolution of warts are easy to find in the literature. For example, 60% of patients achieved a complete response and 30% a partial response to HPV vaccine in one small prospective study of 26 patients with genital warts. Following vaccination, no recurrences were observed after a median follow-up of more than 8 months.
In the review paper, most of the cases involved patients who received the quadrivalent HPV vaccine, Dr. Rosen noted. Only one received the updated nonavalent vaccine, which, in addition to protection against the 6, 11, 16, and 18 subtypes extends protection to subtypes 31, 33, 45, 52, and 58.
“You would expect the nonavalent vaccine to provide the same protection. It is the same vaccine. It just offers activity against more subtypes,” Dr. Rosen said at the meeting, jointly presented by the University of Louisville and Global Academy for Medical Education. He reported that he personally has used the nonavalent vaccine successfully to treat a cutaneous wart.
The nonavalent vaccine can be administered in just two doses for those who receive the first dose before age 15. In others, it is given in three doses at 1- to 2-month intervals, according to Dr. Rosen. He said the efficacy for preventing genital warts and most HPV-related neoplasia exceeds 90%, although it is lower for penile and anal cancer. The protection extends for at least 10 years, but he said that he believes that it is likely to be longer.
“The HPV vaccine is really, really safe,” Dr. Rosen said. Besides injection-site reactions, the most common adverse event is syncope. For this reason, patients are advised to stay seated for 30 minutes after administration.
There is some evidence for cross-immunity for HPV subtypes not covered by the vaccine, particularly among children, Dr. Rosen commented. Citing the review article, he said that, although almost all HPV-associated warts resolve in children when treated with the vaccine, response is somewhat lower in adolescents and further reduced in adults.
In an interview, the senior author of the recent literature review, Natasha A. Mesinkovska, MD, PhD, associate professor of dermatology, University of California, Irvine, agreed with Dr. Rosen about the value of HPV vaccine for patients not responding to conventional therapies for HPV-related cutaneous warts.
“I think HPV vaccine is an excellent option for those patients, even older ones at 45 years of age if cost is not an issue,” she said. She did offer a caveat. In a recent statement from the International Papillomavirus Society (IPVS) on a world shortage of HPV vaccine, it was estimated that supplies might be limited for the next 3-5 years.
Given this shortage, “obtaining them currently may prove to be difficult,” she cautioned.
This publication and Global Academy for Medical Education are owned by the same parent company.
The human papillomavirus (HPV) vaccine, recommended by the Centers for Disease Control and Prevention for the prevention of HPV-associated genital warts and neoplasia, appears to be an effective and perhaps underappreciated treatment of existing cutaneous warts, according to expert speaking at the annual Coastal Dermatology symposium, held virtually.
, but a recently published review provides strong evidence that this is a practical clinical strategy, according to Theodore Rosen, MD, professor of dermatology at Baylor College of Medicine, Houston.
“Clearly, if you have someone, particularly a youngster, and you’re having trouble getting rid of their warts and they are age 9 years or above – and they need the vaccine anyhow – that’s a win-win proposition,” Dr. Rosen said.
The current nonavalent HPV vaccine is approved for individuals from age 9 to age 45. Although the CDC recommends routine vaccination at age 11 or 12 years, it allows earlier vaccination within the label.
The recently published and updated evidence of a benefit from treatment comes from a systematic literature review. For the review, 63 articles were drawn from the PubMed and Cochrane databases. The studies yielded 4,439 patients with cutaneous warts at the time they received the HPV vaccine or who specifically received vaccine as a treatment strategy.
As has been suggested previously in the case series and in a limited number of prospective studies, the majority of warts, including cutaneous warts and anogenital warts, resolved following vaccine administration.
“Mostly these were common warts, plantar warts, and flat warts,” Dr. Rosen said, but the paper also reported successful treatment of recurrent respiratory papillomatosis, squamous cell carcinomas, and basal cell carcinomas.
Case reports and small studies associating HPV vaccine with successful resolution of warts are easy to find in the literature. For example, 60% of patients achieved a complete response and 30% a partial response to HPV vaccine in one small prospective study of 26 patients with genital warts. Following vaccination, no recurrences were observed after a median follow-up of more than 8 months.
In the review paper, most of the cases involved patients who received the quadrivalent HPV vaccine, Dr. Rosen noted. Only one received the updated nonavalent vaccine, which, in addition to protection against the 6, 11, 16, and 18 subtypes extends protection to subtypes 31, 33, 45, 52, and 58.
“You would expect the nonavalent vaccine to provide the same protection. It is the same vaccine. It just offers activity against more subtypes,” Dr. Rosen said at the meeting, jointly presented by the University of Louisville and Global Academy for Medical Education. He reported that he personally has used the nonavalent vaccine successfully to treat a cutaneous wart.
The nonavalent vaccine can be administered in just two doses for those who receive the first dose before age 15. In others, it is given in three doses at 1- to 2-month intervals, according to Dr. Rosen. He said the efficacy for preventing genital warts and most HPV-related neoplasia exceeds 90%, although it is lower for penile and anal cancer. The protection extends for at least 10 years, but he said that he believes that it is likely to be longer.
“The HPV vaccine is really, really safe,” Dr. Rosen said. Besides injection-site reactions, the most common adverse event is syncope. For this reason, patients are advised to stay seated for 30 minutes after administration.
There is some evidence for cross-immunity for HPV subtypes not covered by the vaccine, particularly among children, Dr. Rosen commented. Citing the review article, he said that, although almost all HPV-associated warts resolve in children when treated with the vaccine, response is somewhat lower in adolescents and further reduced in adults.
In an interview, the senior author of the recent literature review, Natasha A. Mesinkovska, MD, PhD, associate professor of dermatology, University of California, Irvine, agreed with Dr. Rosen about the value of HPV vaccine for patients not responding to conventional therapies for HPV-related cutaneous warts.
“I think HPV vaccine is an excellent option for those patients, even older ones at 45 years of age if cost is not an issue,” she said. She did offer a caveat. In a recent statement from the International Papillomavirus Society (IPVS) on a world shortage of HPV vaccine, it was estimated that supplies might be limited for the next 3-5 years.
Given this shortage, “obtaining them currently may prove to be difficult,” she cautioned.
This publication and Global Academy for Medical Education are owned by the same parent company.
FROM COASTAL DERM
Getting closer to a lifesaving RSV vaccine
Louis Bont, MD, PhD, provided an overview of the most recent developments in the complex respiratory syncytial virus (RSV) vaccine landscape at the annual meeting of the European Society for Paediatric Infectious Diseases, held virtually this year.
RSV imposes significant burden worldwide, with 33 million patients, 3 million hospitalizations, and at least 120,000 deaths, reported Dr. Bont of the Wilhelmina Children’s Hospital, University Medical Centre, Utrecht, the Netherlands. Of those deaths, more than 50% are in infants younger than 5 months, and “about 99% of the children dying from RSV live in low- and middle-income countries.”
“There are high-risk populations, such as children with prematurity, congenital heart disease, lung disease, and Down syndrome, but about 73% of all children who are hospitalized for RSV infection were previously healthy children,” Dr. Bont explained. “So, we need to find a solution for all children to prevent RSV infection.”
As observed by Nienke Scheltema in a Lancet Global Health article, population distributions of RSV infection mortality show that, regardless of whether children have comorbidities or they are previously healthy, most children die at a very young age, Dr. Bont explained. These data suggest “that a maternal vaccine or an antibody prophylaxis approach from birth onwards or during the first RSV season is the solution for the problem.”
The path to developing an RSV vaccine has now narrowed its focus onto a structural element of RSV, the prefusion F protein. This shift started with the discovery by Jason McLellan (Science, 2013 [two papers]) that there are two variants of the RSV F-fusion protein: the very stable postfusion conformation and the prefusion active conformation, a metastable protein that exists for a “fraction of a second,” Dr. Bont said.
“The interesting thing is that epitopes that are visible at the prefusion, metastable state … induce highly neutralizing antibodies, whereas epitopes at the postfusion conformation do not,” Dr. Bont explained. “So, by stabilizing the prefusion state, we start inducing neutralizing antibodies that will protect against severe RSV infection, and this is the basic concept of all the vaccine developments currently ongoing.”
These RSV vaccine developments fall into five approach types: live-attenuated or chimeric vaccines, vector-based vaccines, monoclonal antibodies, particle-based vaccines, and subunit or protein-based vaccines.
One breakthrough, which was presented at last year’s ESPID meeting, is the monoclonal antibody nirsevimab. In addition to being nine times more potent than the broadly used antibody palivizumab, it is also more stable; whereas many antibodies have a half-life of 3 weeks, nirsevimab has a half-life of 100 days. “The idea is that a single injection at the start of the RSV season protects children in the first RSV season of their life, a dangerous episode for them.” Dr. Bont explained. The originators, AstraZeneca and Sanofi Pasteur, have “the vision that every child on this planet should receive a single injection with this antibody in the first season,” he explained.
Studies of nanoparticle-based maternal vaccines have also revealed interesting results: Although a phase 3 trial investigating such vaccines didn’t achieve its primary endpoint, “interestingly, 15% of all RSV infections were mild, and only 2% were very severe and leading to hypoxemia,” Dr. Bont noted. “But if we look at vaccine efficacy, we see the opposite – the vaccine was not very efficacious to prevent mild disease, but very efficacious to prevent severe hypoxemia; actually, this is exactly what you would like to see in a vaccine.”
Investigations into live-attenuated and vector-based vaccines have been promising as well, Dr. Bont shared. Studies of live-attenuated vaccines suggest they have a future and that we can move onto their next phase of clinical development, and a study investigating adenoviral vector-based vaccines has demonstrated safety, efficacy, and immunogenicity, though it has also shown that we should anticipate some side effects when using them.
Simple subunit vaccines for RSV are also being explored – a study of DS-Cav1, a stabilized prefusion F subunit protein candidate vaccine, has shown that it has a superior functional profile, compared with previous pre-F subunit vaccines. However, it seemed to be more efficacious against strains of RSV A than strains of RSV B, the dominant strain.
Dr. Bont also discussed exciting work by Sesterhenn et al., in which they used a computer-based program to develop their own vaccine. Using their in-depth knowledge of the RSV prefusion F protein and a computer program, Sesterhenn et al. developed a trivalent vaccine, produced it, and showed – both in vitro and in monkeys – that such vaccines can work up to the level of preclinical in vivo experiments.
“We can now make vaccines behind our computer,” Dr. Bont declared. “And the system doesn’t only work for RSV vaccines, but also for other pathogens – as long as you have an in-depth molecular knowledge of the target epitope,” he added.
Joanne Wildenbeest, MD, PhD, at the Utrecht University, the Netherlands commented: “Lower respiratory tract infections due to RSV are among the leading causes of death worldwide in children under the age of 5, especially young infants. The recent advances in the development of a vaccine and passive immunization are important steps towards the goal to reduce childhood mortality due to RSV worldwide. Since RSV-related mortality is mainly seen in developing countries it is important that, once a vaccine has been approved, it will also be made easily available to these countries.”
Dr. Bont reported the following disclosures: ReSViNET (a nonprofit foundation); investigator-initiated studies with the Bill & Melinda Gates Foundation, AbbVie, MedImmune, and MeMed; participation with Pfizer, Regeneron, and Janssen; and consultancy with GlaxoSmithKline, Ablynx, Novavax, and Janssen.
Louis Bont, MD, PhD, provided an overview of the most recent developments in the complex respiratory syncytial virus (RSV) vaccine landscape at the annual meeting of the European Society for Paediatric Infectious Diseases, held virtually this year.
RSV imposes significant burden worldwide, with 33 million patients, 3 million hospitalizations, and at least 120,000 deaths, reported Dr. Bont of the Wilhelmina Children’s Hospital, University Medical Centre, Utrecht, the Netherlands. Of those deaths, more than 50% are in infants younger than 5 months, and “about 99% of the children dying from RSV live in low- and middle-income countries.”
“There are high-risk populations, such as children with prematurity, congenital heart disease, lung disease, and Down syndrome, but about 73% of all children who are hospitalized for RSV infection were previously healthy children,” Dr. Bont explained. “So, we need to find a solution for all children to prevent RSV infection.”
As observed by Nienke Scheltema in a Lancet Global Health article, population distributions of RSV infection mortality show that, regardless of whether children have comorbidities or they are previously healthy, most children die at a very young age, Dr. Bont explained. These data suggest “that a maternal vaccine or an antibody prophylaxis approach from birth onwards or during the first RSV season is the solution for the problem.”
The path to developing an RSV vaccine has now narrowed its focus onto a structural element of RSV, the prefusion F protein. This shift started with the discovery by Jason McLellan (Science, 2013 [two papers]) that there are two variants of the RSV F-fusion protein: the very stable postfusion conformation and the prefusion active conformation, a metastable protein that exists for a “fraction of a second,” Dr. Bont said.
“The interesting thing is that epitopes that are visible at the prefusion, metastable state … induce highly neutralizing antibodies, whereas epitopes at the postfusion conformation do not,” Dr. Bont explained. “So, by stabilizing the prefusion state, we start inducing neutralizing antibodies that will protect against severe RSV infection, and this is the basic concept of all the vaccine developments currently ongoing.”
These RSV vaccine developments fall into five approach types: live-attenuated or chimeric vaccines, vector-based vaccines, monoclonal antibodies, particle-based vaccines, and subunit or protein-based vaccines.
One breakthrough, which was presented at last year’s ESPID meeting, is the monoclonal antibody nirsevimab. In addition to being nine times more potent than the broadly used antibody palivizumab, it is also more stable; whereas many antibodies have a half-life of 3 weeks, nirsevimab has a half-life of 100 days. “The idea is that a single injection at the start of the RSV season protects children in the first RSV season of their life, a dangerous episode for them.” Dr. Bont explained. The originators, AstraZeneca and Sanofi Pasteur, have “the vision that every child on this planet should receive a single injection with this antibody in the first season,” he explained.
Studies of nanoparticle-based maternal vaccines have also revealed interesting results: Although a phase 3 trial investigating such vaccines didn’t achieve its primary endpoint, “interestingly, 15% of all RSV infections were mild, and only 2% were very severe and leading to hypoxemia,” Dr. Bont noted. “But if we look at vaccine efficacy, we see the opposite – the vaccine was not very efficacious to prevent mild disease, but very efficacious to prevent severe hypoxemia; actually, this is exactly what you would like to see in a vaccine.”
Investigations into live-attenuated and vector-based vaccines have been promising as well, Dr. Bont shared. Studies of live-attenuated vaccines suggest they have a future and that we can move onto their next phase of clinical development, and a study investigating adenoviral vector-based vaccines has demonstrated safety, efficacy, and immunogenicity, though it has also shown that we should anticipate some side effects when using them.
Simple subunit vaccines for RSV are also being explored – a study of DS-Cav1, a stabilized prefusion F subunit protein candidate vaccine, has shown that it has a superior functional profile, compared with previous pre-F subunit vaccines. However, it seemed to be more efficacious against strains of RSV A than strains of RSV B, the dominant strain.
Dr. Bont also discussed exciting work by Sesterhenn et al., in which they used a computer-based program to develop their own vaccine. Using their in-depth knowledge of the RSV prefusion F protein and a computer program, Sesterhenn et al. developed a trivalent vaccine, produced it, and showed – both in vitro and in monkeys – that such vaccines can work up to the level of preclinical in vivo experiments.
“We can now make vaccines behind our computer,” Dr. Bont declared. “And the system doesn’t only work for RSV vaccines, but also for other pathogens – as long as you have an in-depth molecular knowledge of the target epitope,” he added.
Joanne Wildenbeest, MD, PhD, at the Utrecht University, the Netherlands commented: “Lower respiratory tract infections due to RSV are among the leading causes of death worldwide in children under the age of 5, especially young infants. The recent advances in the development of a vaccine and passive immunization are important steps towards the goal to reduce childhood mortality due to RSV worldwide. Since RSV-related mortality is mainly seen in developing countries it is important that, once a vaccine has been approved, it will also be made easily available to these countries.”
Dr. Bont reported the following disclosures: ReSViNET (a nonprofit foundation); investigator-initiated studies with the Bill & Melinda Gates Foundation, AbbVie, MedImmune, and MeMed; participation with Pfizer, Regeneron, and Janssen; and consultancy with GlaxoSmithKline, Ablynx, Novavax, and Janssen.
Louis Bont, MD, PhD, provided an overview of the most recent developments in the complex respiratory syncytial virus (RSV) vaccine landscape at the annual meeting of the European Society for Paediatric Infectious Diseases, held virtually this year.
RSV imposes significant burden worldwide, with 33 million patients, 3 million hospitalizations, and at least 120,000 deaths, reported Dr. Bont of the Wilhelmina Children’s Hospital, University Medical Centre, Utrecht, the Netherlands. Of those deaths, more than 50% are in infants younger than 5 months, and “about 99% of the children dying from RSV live in low- and middle-income countries.”
“There are high-risk populations, such as children with prematurity, congenital heart disease, lung disease, and Down syndrome, but about 73% of all children who are hospitalized for RSV infection were previously healthy children,” Dr. Bont explained. “So, we need to find a solution for all children to prevent RSV infection.”
As observed by Nienke Scheltema in a Lancet Global Health article, population distributions of RSV infection mortality show that, regardless of whether children have comorbidities or they are previously healthy, most children die at a very young age, Dr. Bont explained. These data suggest “that a maternal vaccine or an antibody prophylaxis approach from birth onwards or during the first RSV season is the solution for the problem.”
The path to developing an RSV vaccine has now narrowed its focus onto a structural element of RSV, the prefusion F protein. This shift started with the discovery by Jason McLellan (Science, 2013 [two papers]) that there are two variants of the RSV F-fusion protein: the very stable postfusion conformation and the prefusion active conformation, a metastable protein that exists for a “fraction of a second,” Dr. Bont said.
“The interesting thing is that epitopes that are visible at the prefusion, metastable state … induce highly neutralizing antibodies, whereas epitopes at the postfusion conformation do not,” Dr. Bont explained. “So, by stabilizing the prefusion state, we start inducing neutralizing antibodies that will protect against severe RSV infection, and this is the basic concept of all the vaccine developments currently ongoing.”
These RSV vaccine developments fall into five approach types: live-attenuated or chimeric vaccines, vector-based vaccines, monoclonal antibodies, particle-based vaccines, and subunit or protein-based vaccines.
One breakthrough, which was presented at last year’s ESPID meeting, is the monoclonal antibody nirsevimab. In addition to being nine times more potent than the broadly used antibody palivizumab, it is also more stable; whereas many antibodies have a half-life of 3 weeks, nirsevimab has a half-life of 100 days. “The idea is that a single injection at the start of the RSV season protects children in the first RSV season of their life, a dangerous episode for them.” Dr. Bont explained. The originators, AstraZeneca and Sanofi Pasteur, have “the vision that every child on this planet should receive a single injection with this antibody in the first season,” he explained.
Studies of nanoparticle-based maternal vaccines have also revealed interesting results: Although a phase 3 trial investigating such vaccines didn’t achieve its primary endpoint, “interestingly, 15% of all RSV infections were mild, and only 2% were very severe and leading to hypoxemia,” Dr. Bont noted. “But if we look at vaccine efficacy, we see the opposite – the vaccine was not very efficacious to prevent mild disease, but very efficacious to prevent severe hypoxemia; actually, this is exactly what you would like to see in a vaccine.”
Investigations into live-attenuated and vector-based vaccines have been promising as well, Dr. Bont shared. Studies of live-attenuated vaccines suggest they have a future and that we can move onto their next phase of clinical development, and a study investigating adenoviral vector-based vaccines has demonstrated safety, efficacy, and immunogenicity, though it has also shown that we should anticipate some side effects when using them.
Simple subunit vaccines for RSV are also being explored – a study of DS-Cav1, a stabilized prefusion F subunit protein candidate vaccine, has shown that it has a superior functional profile, compared with previous pre-F subunit vaccines. However, it seemed to be more efficacious against strains of RSV A than strains of RSV B, the dominant strain.
Dr. Bont also discussed exciting work by Sesterhenn et al., in which they used a computer-based program to develop their own vaccine. Using their in-depth knowledge of the RSV prefusion F protein and a computer program, Sesterhenn et al. developed a trivalent vaccine, produced it, and showed – both in vitro and in monkeys – that such vaccines can work up to the level of preclinical in vivo experiments.
“We can now make vaccines behind our computer,” Dr. Bont declared. “And the system doesn’t only work for RSV vaccines, but also for other pathogens – as long as you have an in-depth molecular knowledge of the target epitope,” he added.
Joanne Wildenbeest, MD, PhD, at the Utrecht University, the Netherlands commented: “Lower respiratory tract infections due to RSV are among the leading causes of death worldwide in children under the age of 5, especially young infants. The recent advances in the development of a vaccine and passive immunization are important steps towards the goal to reduce childhood mortality due to RSV worldwide. Since RSV-related mortality is mainly seen in developing countries it is important that, once a vaccine has been approved, it will also be made easily available to these countries.”
Dr. Bont reported the following disclosures: ReSViNET (a nonprofit foundation); investigator-initiated studies with the Bill & Melinda Gates Foundation, AbbVie, MedImmune, and MeMed; participation with Pfizer, Regeneron, and Janssen; and consultancy with GlaxoSmithKline, Ablynx, Novavax, and Janssen.
FROM ESPID 2020
Analysis characterizes common wound microbes in epidermolysis bullosa
– in a retrospective analysis of over 700 wound cultures from 158 patients across the United States and Canada.
The findings from the EB Clinical Characterization and Outcomes Database speak to the value of surveillance cultures with routine testing for microbial resistance – including mupirocin resistance – and to the importance of antibiotic stewardship not only for oral antibiotics but for topicals as well, according to Laura E. Levin, MD, and Kimberly D. Morel, MD, of the departments of dermatology and pediatrics, Columbia University Irving Medical Center, New York, the lead and senior authors, respectively, of the paper recently published in Pediatric Dermatology.
Almost all of the 158 patients with at least one wound culture recorded in the database from the period of 2001-2018 had one or more positive culture results. Of 152 patients with positive cultures, 131 (86%) were positive for SA and 56 (37%) and 34 (22%) were positive for PA and GAS, respectively. Other bacteria isolated included Corynebacterium spp and Proteus spp. Nearly half (47%) of patients with SA-positive cultures had methicillin-resistant SA, and 68% had methicillin-susceptible SA. (Some patients grew both MSSA and MRSA at different points in time.)
Mupirocin-susceptibility testing was performed at only some of the 13 participating centers. Of 15 patients whose cultures had recorded SA mupirocin-susceptibility testing, 11 had cultures positive for mupirocin-susceptible SA and 6 (40%) had mupirocin-resistant SA isolates (2 patients grew both). Of these six patients, half had isolates that were also methicillin-resistant.
Mupirocin, a topical antibiotic, has been a cornerstone of decolonization regimens for MSSA and MRSA, but resistance has been demonstrated in other research as well and is not specific to EB, wrote Dr. Levin, Dr. Morel, and coauthors.
“Pediatric dermatologists often rely on topical antimicrobials in the treatment of patients’ open wounds to both prevent and treat infection, depending on the clinical scenario,” and surveillance cultures with routine testing for mupirocin resistance can help guide antibiotic choice and management strategies, Dr. Levin said in an interview.
More broadly, she added, “it’s helpful to know what bacteria are routinely colonizing wounds, not causing infection, versus those that are more likely to be associated with infection, chronic wounds, or the risk of developing skin cancer ... [to know] which wounds need to be treated more aggressively.”
A subset of patients with EB have been known to be at risk for squamous cell carcinoma, and research is implicating certain bacteria “as contributing to wound inflammation,” Dr. Morel said in an interview.
SCC was reported in 23 out of 717 patients in the database – but fewer than half of the patients with SCC had recorded wound cultures. The small numbers precluded the identification of microbes that may confer significant risk.
Correlating particular microbes with clinical features also will take more research. About half (57%) of the patients with recorded wound cultures had wounds with purulent exudate or other features of clinical infection. However, the presence or absence of clinical signs of infection was not temporally correlated with culture results in the database.
The 158 patients with recorded wound cultures had a mean age of 12.8 years and represented a range of EB subtypes.
PA was present in the wounds of patients as young as 1 month old, the authors noted. Investigators are “looking to further study PA and characterize clinical features ... to understand more about this microbe and its impact on patients with EB,” Dr. Morel said.
In the meantime, the analysis reaffirms the importance of antibiotic stewardship. Mupirocin is labeled to be used three times a day for a short period of time, but “tends to be prescribed and used less judiciously than intended,” Dr. Morel said. “It’s important [not to overuse it]. We have seen that patients’ culture results become sensitive to mupirocin again in the future when they avoid it for a period of time.”
The work was supported by the EB Research Partnership and EB Medical Research Foundation, as well as an NIH/NCATS grant. No investigator disclosures were listed.
SOURCE: Pediatr Dermatol. 2020 Nov 28. doi: 10.1111/pde.14444.
– in a retrospective analysis of over 700 wound cultures from 158 patients across the United States and Canada.
The findings from the EB Clinical Characterization and Outcomes Database speak to the value of surveillance cultures with routine testing for microbial resistance – including mupirocin resistance – and to the importance of antibiotic stewardship not only for oral antibiotics but for topicals as well, according to Laura E. Levin, MD, and Kimberly D. Morel, MD, of the departments of dermatology and pediatrics, Columbia University Irving Medical Center, New York, the lead and senior authors, respectively, of the paper recently published in Pediatric Dermatology.
Almost all of the 158 patients with at least one wound culture recorded in the database from the period of 2001-2018 had one or more positive culture results. Of 152 patients with positive cultures, 131 (86%) were positive for SA and 56 (37%) and 34 (22%) were positive for PA and GAS, respectively. Other bacteria isolated included Corynebacterium spp and Proteus spp. Nearly half (47%) of patients with SA-positive cultures had methicillin-resistant SA, and 68% had methicillin-susceptible SA. (Some patients grew both MSSA and MRSA at different points in time.)
Mupirocin-susceptibility testing was performed at only some of the 13 participating centers. Of 15 patients whose cultures had recorded SA mupirocin-susceptibility testing, 11 had cultures positive for mupirocin-susceptible SA and 6 (40%) had mupirocin-resistant SA isolates (2 patients grew both). Of these six patients, half had isolates that were also methicillin-resistant.
Mupirocin, a topical antibiotic, has been a cornerstone of decolonization regimens for MSSA and MRSA, but resistance has been demonstrated in other research as well and is not specific to EB, wrote Dr. Levin, Dr. Morel, and coauthors.
“Pediatric dermatologists often rely on topical antimicrobials in the treatment of patients’ open wounds to both prevent and treat infection, depending on the clinical scenario,” and surveillance cultures with routine testing for mupirocin resistance can help guide antibiotic choice and management strategies, Dr. Levin said in an interview.
More broadly, she added, “it’s helpful to know what bacteria are routinely colonizing wounds, not causing infection, versus those that are more likely to be associated with infection, chronic wounds, or the risk of developing skin cancer ... [to know] which wounds need to be treated more aggressively.”
A subset of patients with EB have been known to be at risk for squamous cell carcinoma, and research is implicating certain bacteria “as contributing to wound inflammation,” Dr. Morel said in an interview.
SCC was reported in 23 out of 717 patients in the database – but fewer than half of the patients with SCC had recorded wound cultures. The small numbers precluded the identification of microbes that may confer significant risk.
Correlating particular microbes with clinical features also will take more research. About half (57%) of the patients with recorded wound cultures had wounds with purulent exudate or other features of clinical infection. However, the presence or absence of clinical signs of infection was not temporally correlated with culture results in the database.
The 158 patients with recorded wound cultures had a mean age of 12.8 years and represented a range of EB subtypes.
PA was present in the wounds of patients as young as 1 month old, the authors noted. Investigators are “looking to further study PA and characterize clinical features ... to understand more about this microbe and its impact on patients with EB,” Dr. Morel said.
In the meantime, the analysis reaffirms the importance of antibiotic stewardship. Mupirocin is labeled to be used three times a day for a short period of time, but “tends to be prescribed and used less judiciously than intended,” Dr. Morel said. “It’s important [not to overuse it]. We have seen that patients’ culture results become sensitive to mupirocin again in the future when they avoid it for a period of time.”
The work was supported by the EB Research Partnership and EB Medical Research Foundation, as well as an NIH/NCATS grant. No investigator disclosures were listed.
SOURCE: Pediatr Dermatol. 2020 Nov 28. doi: 10.1111/pde.14444.
– in a retrospective analysis of over 700 wound cultures from 158 patients across the United States and Canada.
The findings from the EB Clinical Characterization and Outcomes Database speak to the value of surveillance cultures with routine testing for microbial resistance – including mupirocin resistance – and to the importance of antibiotic stewardship not only for oral antibiotics but for topicals as well, according to Laura E. Levin, MD, and Kimberly D. Morel, MD, of the departments of dermatology and pediatrics, Columbia University Irving Medical Center, New York, the lead and senior authors, respectively, of the paper recently published in Pediatric Dermatology.
Almost all of the 158 patients with at least one wound culture recorded in the database from the period of 2001-2018 had one or more positive culture results. Of 152 patients with positive cultures, 131 (86%) were positive for SA and 56 (37%) and 34 (22%) were positive for PA and GAS, respectively. Other bacteria isolated included Corynebacterium spp and Proteus spp. Nearly half (47%) of patients with SA-positive cultures had methicillin-resistant SA, and 68% had methicillin-susceptible SA. (Some patients grew both MSSA and MRSA at different points in time.)
Mupirocin-susceptibility testing was performed at only some of the 13 participating centers. Of 15 patients whose cultures had recorded SA mupirocin-susceptibility testing, 11 had cultures positive for mupirocin-susceptible SA and 6 (40%) had mupirocin-resistant SA isolates (2 patients grew both). Of these six patients, half had isolates that were also methicillin-resistant.
Mupirocin, a topical antibiotic, has been a cornerstone of decolonization regimens for MSSA and MRSA, but resistance has been demonstrated in other research as well and is not specific to EB, wrote Dr. Levin, Dr. Morel, and coauthors.
“Pediatric dermatologists often rely on topical antimicrobials in the treatment of patients’ open wounds to both prevent and treat infection, depending on the clinical scenario,” and surveillance cultures with routine testing for mupirocin resistance can help guide antibiotic choice and management strategies, Dr. Levin said in an interview.
More broadly, she added, “it’s helpful to know what bacteria are routinely colonizing wounds, not causing infection, versus those that are more likely to be associated with infection, chronic wounds, or the risk of developing skin cancer ... [to know] which wounds need to be treated more aggressively.”
A subset of patients with EB have been known to be at risk for squamous cell carcinoma, and research is implicating certain bacteria “as contributing to wound inflammation,” Dr. Morel said in an interview.
SCC was reported in 23 out of 717 patients in the database – but fewer than half of the patients with SCC had recorded wound cultures. The small numbers precluded the identification of microbes that may confer significant risk.
Correlating particular microbes with clinical features also will take more research. About half (57%) of the patients with recorded wound cultures had wounds with purulent exudate or other features of clinical infection. However, the presence or absence of clinical signs of infection was not temporally correlated with culture results in the database.
The 158 patients with recorded wound cultures had a mean age of 12.8 years and represented a range of EB subtypes.
PA was present in the wounds of patients as young as 1 month old, the authors noted. Investigators are “looking to further study PA and characterize clinical features ... to understand more about this microbe and its impact on patients with EB,” Dr. Morel said.
In the meantime, the analysis reaffirms the importance of antibiotic stewardship. Mupirocin is labeled to be used three times a day for a short period of time, but “tends to be prescribed and used less judiciously than intended,” Dr. Morel said. “It’s important [not to overuse it]. We have seen that patients’ culture results become sensitive to mupirocin again in the future when they avoid it for a period of time.”
The work was supported by the EB Research Partnership and EB Medical Research Foundation, as well as an NIH/NCATS grant. No investigator disclosures were listed.
SOURCE: Pediatr Dermatol. 2020 Nov 28. doi: 10.1111/pde.14444.
FROM PEDIATRIC DERMATOLOGY
Seeking new vaccines against whooping cough: The PERISCOPE project
Although there is an effective vaccine against Bordetella pertussis, whooping cough remains a leading cause of death. Cases are increasing, and scientists face challenges in developing new vaccines.
In a key research session at the start of the annual meeting of the European Society for Paediatric Infectious Diseases, held virtually this year, Dimitri Diavatopoulos, PhD, associate professor at the Radboud University Medical Centre Nijmegen, the Netherlands, summarized the pertussis vaccination problem and what the Pertussis Correlates of Protection Europe (PERISCOPE) project seeks to achieve. Dr. Diavatopoulos has a longstanding interest in pertussis and immunity and will soon take over as the scientific coordinator of PERISCOPE.
Pertussis is a highly contagious infectious disease that causes uncontrollable coughing. The disease begins with an atypical cough and rhinorrhea before entering a paroxysmal stage characterized by cyanosis, lymphocytosis, vomiting, and whoops. Generally, fever is absent and coughing increases at night. Finally, after weeks to months, the patient enters a convalescent stage. The World Health Organization estimates that there are 16 million pertussis cases annually and approximately 195,000 deaths in children. Most cases are caused by Bordetella pertussis and are preventable by vaccination.
In the United States, following the introduction of a national immunization program using a whole-cell vaccine in the 1950s, cases fell significantly. After a lag phase, the adoption of an acellular vaccine in the United States in 1997 and the Netherlands in 2005 – usually in combination with diphtheria and tetanus via DTaP – saw an increase in case numbers. Dr. Diavatopoulos stated that control is no longer as good, compared with other infectious diseases prevented by the MMR vaccine, such as mumps, measles, and rubella.
In the face of increasing numbers, how do we move to the next generation of vaccines to improve control? There are several barriers to licensure, including the following:
• Universal recommendation for pertussis prevention means that more than 90% of the population will have received DTaP (usually in combination with polio and Haemophilus influenzae B) and be protected for several years after vaccination.
• Because DTaP vaccines are only efficacious for a limited time, the problem is not immediately apparent.
• Pertussis epidemics are cyclical, occurring every 3-5 years. These peaks and troughs complicate the development of epidemiological studies.
What this means is that large-scale Phase III efficacy studies, in which disease is used as the endpoint, are not feasible. Also, formal correlates of protection have not been identified.
The PERISCOPE Project started in March 2016 and is designed to respond to some of these issues. Funding is made available by a public private consortium involving the Bill & Melinda Gates foundation, the European Union, and European Federation of Pharmaceutical Industries and Associations (EFPIA) partners, and in this case, GlaxoSmithKline and Sanofi Pasteur. In total, there are 22 partners in this project.
The strategic objectives of this partnership include the following:
• Foster expertise and increase capacity in Europe to evaluate new pertussis vaccines both in clinical and preclinical models.
• Identify early biomarkers of long-lasting protective immunity to pertussis in humans. (This step will accelerate and de-risk clinical development of next generation pertussis vaccines.)
• Investigate the impact of maternal vaccination on infant response to pertussis vaccination.
The problem is that there is no one single study design that addresses all questions about the pertussis vaccine. For example, in PERISCOPE, the results of preclinical studies using the baboon or mouse models and addressing disease and colonization endpoints or immunogenicity do not perfectly model human infection and disease.
By comparison, controlled human infection studies provide information on colonization but not disease endpoints. Such studies, however, do provide information on immunogenicity endpoints. Also available are booster vaccination studies and infant vaccination studies providing data on immunogenicity, as well as safety information.
Finally, there are patient studies, such as household contact studies where immunogenicity can be correlated to disease endpoints. From these studies, it will be seen that what is needed is integration of evidence from clinical and preclinical studies to support a new vaccine registration.
PERISCOPE addresses these issues by developing novel, functional antibody and cellular assays and employing cutting-edge methods to characterize innate immune responses and cell-mediated systemic and mucosal immunity. PERISCOPE combines two major industrial partners with public researchers from academic and public health institutes and small and medium-sized enterprises with expertise in clinical trials, vaccinology, immunology, molecular microbiology, challenge models, and bioinformatics.
Andrew Gorringe, PhD, from Public Health England and the Research and Development Institute at Porton Down, Wiltshire, England, said, “Vaccines have greatly reduced the incidence of pertussis, but it remains the most prevalent ‘vaccine preventable’ disease. This is an exciting period for pertussis vaccine research as we find new ways to understand the immunity that protects from both infection and disease. The PERISCOPE project provides a collaborative environment that combines expertise across Europe to provide a route to the development of new, more effective vaccines.”
GSK and Sanofi Pasteur have cofunded the PERISCOPE Project. Dr. Diavatopoulos made no other financial disclosures.
Although there is an effective vaccine against Bordetella pertussis, whooping cough remains a leading cause of death. Cases are increasing, and scientists face challenges in developing new vaccines.
In a key research session at the start of the annual meeting of the European Society for Paediatric Infectious Diseases, held virtually this year, Dimitri Diavatopoulos, PhD, associate professor at the Radboud University Medical Centre Nijmegen, the Netherlands, summarized the pertussis vaccination problem and what the Pertussis Correlates of Protection Europe (PERISCOPE) project seeks to achieve. Dr. Diavatopoulos has a longstanding interest in pertussis and immunity and will soon take over as the scientific coordinator of PERISCOPE.
Pertussis is a highly contagious infectious disease that causes uncontrollable coughing. The disease begins with an atypical cough and rhinorrhea before entering a paroxysmal stage characterized by cyanosis, lymphocytosis, vomiting, and whoops. Generally, fever is absent and coughing increases at night. Finally, after weeks to months, the patient enters a convalescent stage. The World Health Organization estimates that there are 16 million pertussis cases annually and approximately 195,000 deaths in children. Most cases are caused by Bordetella pertussis and are preventable by vaccination.
In the United States, following the introduction of a national immunization program using a whole-cell vaccine in the 1950s, cases fell significantly. After a lag phase, the adoption of an acellular vaccine in the United States in 1997 and the Netherlands in 2005 – usually in combination with diphtheria and tetanus via DTaP – saw an increase in case numbers. Dr. Diavatopoulos stated that control is no longer as good, compared with other infectious diseases prevented by the MMR vaccine, such as mumps, measles, and rubella.
In the face of increasing numbers, how do we move to the next generation of vaccines to improve control? There are several barriers to licensure, including the following:
• Universal recommendation for pertussis prevention means that more than 90% of the population will have received DTaP (usually in combination with polio and Haemophilus influenzae B) and be protected for several years after vaccination.
• Because DTaP vaccines are only efficacious for a limited time, the problem is not immediately apparent.
• Pertussis epidemics are cyclical, occurring every 3-5 years. These peaks and troughs complicate the development of epidemiological studies.
What this means is that large-scale Phase III efficacy studies, in which disease is used as the endpoint, are not feasible. Also, formal correlates of protection have not been identified.
The PERISCOPE Project started in March 2016 and is designed to respond to some of these issues. Funding is made available by a public private consortium involving the Bill & Melinda Gates foundation, the European Union, and European Federation of Pharmaceutical Industries and Associations (EFPIA) partners, and in this case, GlaxoSmithKline and Sanofi Pasteur. In total, there are 22 partners in this project.
The strategic objectives of this partnership include the following:
• Foster expertise and increase capacity in Europe to evaluate new pertussis vaccines both in clinical and preclinical models.
• Identify early biomarkers of long-lasting protective immunity to pertussis in humans. (This step will accelerate and de-risk clinical development of next generation pertussis vaccines.)
• Investigate the impact of maternal vaccination on infant response to pertussis vaccination.
The problem is that there is no one single study design that addresses all questions about the pertussis vaccine. For example, in PERISCOPE, the results of preclinical studies using the baboon or mouse models and addressing disease and colonization endpoints or immunogenicity do not perfectly model human infection and disease.
By comparison, controlled human infection studies provide information on colonization but not disease endpoints. Such studies, however, do provide information on immunogenicity endpoints. Also available are booster vaccination studies and infant vaccination studies providing data on immunogenicity, as well as safety information.
Finally, there are patient studies, such as household contact studies where immunogenicity can be correlated to disease endpoints. From these studies, it will be seen that what is needed is integration of evidence from clinical and preclinical studies to support a new vaccine registration.
PERISCOPE addresses these issues by developing novel, functional antibody and cellular assays and employing cutting-edge methods to characterize innate immune responses and cell-mediated systemic and mucosal immunity. PERISCOPE combines two major industrial partners with public researchers from academic and public health institutes and small and medium-sized enterprises with expertise in clinical trials, vaccinology, immunology, molecular microbiology, challenge models, and bioinformatics.
Andrew Gorringe, PhD, from Public Health England and the Research and Development Institute at Porton Down, Wiltshire, England, said, “Vaccines have greatly reduced the incidence of pertussis, but it remains the most prevalent ‘vaccine preventable’ disease. This is an exciting period for pertussis vaccine research as we find new ways to understand the immunity that protects from both infection and disease. The PERISCOPE project provides a collaborative environment that combines expertise across Europe to provide a route to the development of new, more effective vaccines.”
GSK and Sanofi Pasteur have cofunded the PERISCOPE Project. Dr. Diavatopoulos made no other financial disclosures.
Although there is an effective vaccine against Bordetella pertussis, whooping cough remains a leading cause of death. Cases are increasing, and scientists face challenges in developing new vaccines.
In a key research session at the start of the annual meeting of the European Society for Paediatric Infectious Diseases, held virtually this year, Dimitri Diavatopoulos, PhD, associate professor at the Radboud University Medical Centre Nijmegen, the Netherlands, summarized the pertussis vaccination problem and what the Pertussis Correlates of Protection Europe (PERISCOPE) project seeks to achieve. Dr. Diavatopoulos has a longstanding interest in pertussis and immunity and will soon take over as the scientific coordinator of PERISCOPE.
Pertussis is a highly contagious infectious disease that causes uncontrollable coughing. The disease begins with an atypical cough and rhinorrhea before entering a paroxysmal stage characterized by cyanosis, lymphocytosis, vomiting, and whoops. Generally, fever is absent and coughing increases at night. Finally, after weeks to months, the patient enters a convalescent stage. The World Health Organization estimates that there are 16 million pertussis cases annually and approximately 195,000 deaths in children. Most cases are caused by Bordetella pertussis and are preventable by vaccination.
In the United States, following the introduction of a national immunization program using a whole-cell vaccine in the 1950s, cases fell significantly. After a lag phase, the adoption of an acellular vaccine in the United States in 1997 and the Netherlands in 2005 – usually in combination with diphtheria and tetanus via DTaP – saw an increase in case numbers. Dr. Diavatopoulos stated that control is no longer as good, compared with other infectious diseases prevented by the MMR vaccine, such as mumps, measles, and rubella.
In the face of increasing numbers, how do we move to the next generation of vaccines to improve control? There are several barriers to licensure, including the following:
• Universal recommendation for pertussis prevention means that more than 90% of the population will have received DTaP (usually in combination with polio and Haemophilus influenzae B) and be protected for several years after vaccination.
• Because DTaP vaccines are only efficacious for a limited time, the problem is not immediately apparent.
• Pertussis epidemics are cyclical, occurring every 3-5 years. These peaks and troughs complicate the development of epidemiological studies.
What this means is that large-scale Phase III efficacy studies, in which disease is used as the endpoint, are not feasible. Also, formal correlates of protection have not been identified.
The PERISCOPE Project started in March 2016 and is designed to respond to some of these issues. Funding is made available by a public private consortium involving the Bill & Melinda Gates foundation, the European Union, and European Federation of Pharmaceutical Industries and Associations (EFPIA) partners, and in this case, GlaxoSmithKline and Sanofi Pasteur. In total, there are 22 partners in this project.
The strategic objectives of this partnership include the following:
• Foster expertise and increase capacity in Europe to evaluate new pertussis vaccines both in clinical and preclinical models.
• Identify early biomarkers of long-lasting protective immunity to pertussis in humans. (This step will accelerate and de-risk clinical development of next generation pertussis vaccines.)
• Investigate the impact of maternal vaccination on infant response to pertussis vaccination.
The problem is that there is no one single study design that addresses all questions about the pertussis vaccine. For example, in PERISCOPE, the results of preclinical studies using the baboon or mouse models and addressing disease and colonization endpoints or immunogenicity do not perfectly model human infection and disease.
By comparison, controlled human infection studies provide information on colonization but not disease endpoints. Such studies, however, do provide information on immunogenicity endpoints. Also available are booster vaccination studies and infant vaccination studies providing data on immunogenicity, as well as safety information.
Finally, there are patient studies, such as household contact studies where immunogenicity can be correlated to disease endpoints. From these studies, it will be seen that what is needed is integration of evidence from clinical and preclinical studies to support a new vaccine registration.
PERISCOPE addresses these issues by developing novel, functional antibody and cellular assays and employing cutting-edge methods to characterize innate immune responses and cell-mediated systemic and mucosal immunity. PERISCOPE combines two major industrial partners with public researchers from academic and public health institutes and small and medium-sized enterprises with expertise in clinical trials, vaccinology, immunology, molecular microbiology, challenge models, and bioinformatics.
Andrew Gorringe, PhD, from Public Health England and the Research and Development Institute at Porton Down, Wiltshire, England, said, “Vaccines have greatly reduced the incidence of pertussis, but it remains the most prevalent ‘vaccine preventable’ disease. This is an exciting period for pertussis vaccine research as we find new ways to understand the immunity that protects from both infection and disease. The PERISCOPE project provides a collaborative environment that combines expertise across Europe to provide a route to the development of new, more effective vaccines.”
GSK and Sanofi Pasteur have cofunded the PERISCOPE Project. Dr. Diavatopoulos made no other financial disclosures.
FROM ESPID 2020
Current PERISCOPE vaccine studies: Toward better pertussis prevention?
With increasing whooping cough numbers, developing an effective new vaccine against Bordetella pertussis is a priority. Results from the multifactorial PERISCOPE Project will help scientists and clinicians move forward.
Dominic Kelly, PhD, talked about vaccine-induced immunity and provided an overview of ongoing clinical trials in the PERISCOPE (Pertussis Correlates of Protection Europe) project in a key research session at the start of the annual meeting of the European Society for Paediatric Infectious Diseases, held virtually this year. Dr. Kelly, a pediatrician at the Children’s Hospital in Oxford and a member of the Oxford Vaccines Group, leads one of the studies in the project looking at infant vaccination.
Dr. Kelly began his presentation by showing a figure depicting where vaccine-induced immunity fits into the larger suite of clinical studies. These studies involve mouse models, human challenge models, and infection patients. A key theme is the use of a core group of immunoassays across all studies, with the hope that they will allow effective cross comparisons.
Dr. Kelly stated, “If we find a correlate of protection in the challenge model, we can then interpret the vaccine studies in the light of that because we are using standardized constant immunoassays.”
The assays being used depend in part on the specific study and the volume of blood available. They will generally include Bordetella-specific antibody and functional antibody assays, as well as interesting studies collecting mucosal samples from infants and adults to look at serological responses. Also under examination are a range of enzyme-linked immune absorbent spot, flow cytometry, and culture techniques looking at Memory B cells, T cells, and gene expression.
Complementing these assay studies, PERISCOPE includes a series of clinical investigations designed to throw light on three areas of interest, described below:
First, researchers hope to gain a better understanding regarding the effects of the original whole cell vaccine versus the current acellular variety. The former uses an inactivated version of the whole organism. Epidemiological studies, animal data, and experience in the field demonstrate that whole-cell vaccination results in a broad, long-lasting, and effective immune response.
By comparison, the acellular pertussis vaccine consists of between three and five protein components, which are purified from cultured Bordetella pertussis. While it is an effective vaccine, its effects are less durable; routine use in some countries is associated with cyclical outbreaks of increasing severity.
A second issue for researchers involved in the PERISCOPE project concerns the effects of maternal immunization. In the United Kingdom in 2012, for example, an increasing number of cases were noted 6-7 years after adoption of an acellular vaccine for routine vaccination in the 2nd-3rd trimester of pregnancy. Vaccination appears to effectively control neonatal disease, but whether this influences infant immune responses and long-term control of pertussis for a population is unknown.
Finally, the group is interested in the effects of an acellular booster across all age groups. While the effects may be short-lived, the booster is a potential strategy for controlling a population by repeated boosting of immunity. This is another area where using novel immunoassays may aid better understanding.
To find answers, the consortium has established four studies: the Gambia Pertussis study (GaPs) in Gambia and AWARE, the sister study to GaPs in the United Kingdom, addressing the acellular pertussis versus cellular pertussis question; the Pertussis Maternal Immunization Study in Finland (MIFI) addressing maternal immunization; and the Booster against Pertussis (BERT) study across three countries (U.K., the Netherlands, and Finland) looking at acellular booster across age groups.
Gambia pertussis study
GaPs is the largest single study in the project and is being run at the Medical Research Council–funded London School of Tropical Medicine center in Gambia. Beate Kampmann, MD, PhD, of Imperial College London, England, is the project lead. It is due to complete in 2022. GaPs seeks to enroll 600 mother/infant pairs and randomize the mothers to either an acellular pertussis booster in pregnancy or a tetanus toxoid control vaccine. Infants are subsequently randomized to an acellular or whole-cell pertussis schedule of primary immunization. The vaccine doses are being given at 2, 3, and 4 months. The primary endpoint is a serological finding being measured at 9 months of age, when the infant would usually receive yellow fever, measles, and rubella vaccination.
GaPs has a number of pathways. Within each of the four arms generated by the two randomizations, the maternal randomization and the infant randomization, there are five subgroups. They are designed to study time points in subgroups A and B after the first dose in more detail, looking at the innate immune responses using gene expression. It will enable researchers to study adaptive immune responses to T cells and B cells after the second dose of vaccine. By employing a range of subgroups, the team can explore the immune profile using the assays referred to above. Such information should provide new insights into the differences between acellular and whole-cell vaccines.
The AWARE study
AWARE is the sister study to GaPs and looks at the acellular/whole pertussis issue. Because many developed countries, such as the United Kingdom, have established maternal immunization programs, it is not possible to randomize mothers. Consequently, researchers have opted to recruit infants of mothers who have received an acellular vaccine in pregnancy and randomize them to either an acellular schedule of primary immunization or a whole-cell schedule.
The selected vaccine is ComVac5 from Bharat Biotech. This whole-cell vaccine differs from that used in Gambia. An early obstacle for AWARE has been seeking permission to import a non-conventional vaccine into Europe. It has delayed the anticipated end date to 2023. Participating infants will receive a two-dose schedule at 2 and 4 months of age per their randomization; then, both groups will go on to receive an acellular pertussis booster at 12 months. At all time points, the team will sample blood for cells and serum, as well as mucosal fluid from the nose. Because the mucosal surface is where the action is, this approach will likely generate new data around antibody responses.
The MIFI
The Pertussis Maternal Immunization Study in Finland is being run by Jussi Mertsola, of the University of Turku, Finland, and Qiushui He, of the National Public Health Institute, Turku. It is due to complete in late 2021. Where, in the United Kingdom, researchers are unable to randomize mothers because of the current guidelines, researchers in Finland do not have a maternal immunization program to consider. MIFI will randomize 80 mothers, 40 to immunization with acellular pertussis and 40 to a control group. Dr. Kelly stated that whole cell vaccines are not available for use in Finland. Participants will receive a two-dose schedule at 3 and 5 months. Blood samples will then be taken to compare the serological and cellular responses, which will help researchers understand the effects of maternal immunization. In addition, there will be sampling of mucosal fluid using a device that collects a standardized aliquot of fluid.
The BERT study
The final clinical element of PERISCOPE presented by Dr. Kelly was the Booster against Pertussis study. This study is near completion. It seeks to examine the use of an acellular booster across different age groups and three countries: the United Kingdom, the Netherlands, and Finland. The study is being coordinated by Guy Berbers, PhD, at the National Institute for Public Health and the Environment in the Netherlands.
BERT comprises four cohorts (A, B, C, D) of different ages: 7-10 years (36 participants), 11-15 years (36 participants), mid-adult (25 participants), and older age (25 participants). After receiving an acellular booster, participants will undergo intense sampling. Sampling will take place immediately after immunization at day 7 and look at adaptive effects, then again at day 28 and day 365.
Because some participants will have already received whole cell or acellular vaccination, this approach will allow researchers to look at the effects of priming (i.e., how long the B cell/T cell antibody responses last).
Involving different countries across Europe ensures wide applicability of results, but also allows researchers to compare the effects of very different immunization histories.
At the end of this ESPID session, Dimitri Diavatopoulos, PhD, assistant professor at the Radboud University Medical Centre Nijmegen, the Netherlands, commented that a future problem in studying pertussis vaccines and their potential clinical application is that most vaccination schedules now involve combination products. Obtaining a stand-alone vaccination may prove difficult, and there may be resistance if it complicates current vaccination programs.
Dr. Kelly acknowledged funding for the PERISCOPE project from GlaxoSmithKline and Pasteur Sanofi.
With increasing whooping cough numbers, developing an effective new vaccine against Bordetella pertussis is a priority. Results from the multifactorial PERISCOPE Project will help scientists and clinicians move forward.
Dominic Kelly, PhD, talked about vaccine-induced immunity and provided an overview of ongoing clinical trials in the PERISCOPE (Pertussis Correlates of Protection Europe) project in a key research session at the start of the annual meeting of the European Society for Paediatric Infectious Diseases, held virtually this year. Dr. Kelly, a pediatrician at the Children’s Hospital in Oxford and a member of the Oxford Vaccines Group, leads one of the studies in the project looking at infant vaccination.
Dr. Kelly began his presentation by showing a figure depicting where vaccine-induced immunity fits into the larger suite of clinical studies. These studies involve mouse models, human challenge models, and infection patients. A key theme is the use of a core group of immunoassays across all studies, with the hope that they will allow effective cross comparisons.
Dr. Kelly stated, “If we find a correlate of protection in the challenge model, we can then interpret the vaccine studies in the light of that because we are using standardized constant immunoassays.”
The assays being used depend in part on the specific study and the volume of blood available. They will generally include Bordetella-specific antibody and functional antibody assays, as well as interesting studies collecting mucosal samples from infants and adults to look at serological responses. Also under examination are a range of enzyme-linked immune absorbent spot, flow cytometry, and culture techniques looking at Memory B cells, T cells, and gene expression.
Complementing these assay studies, PERISCOPE includes a series of clinical investigations designed to throw light on three areas of interest, described below:
First, researchers hope to gain a better understanding regarding the effects of the original whole cell vaccine versus the current acellular variety. The former uses an inactivated version of the whole organism. Epidemiological studies, animal data, and experience in the field demonstrate that whole-cell vaccination results in a broad, long-lasting, and effective immune response.
By comparison, the acellular pertussis vaccine consists of between three and five protein components, which are purified from cultured Bordetella pertussis. While it is an effective vaccine, its effects are less durable; routine use in some countries is associated with cyclical outbreaks of increasing severity.
A second issue for researchers involved in the PERISCOPE project concerns the effects of maternal immunization. In the United Kingdom in 2012, for example, an increasing number of cases were noted 6-7 years after adoption of an acellular vaccine for routine vaccination in the 2nd-3rd trimester of pregnancy. Vaccination appears to effectively control neonatal disease, but whether this influences infant immune responses and long-term control of pertussis for a population is unknown.
Finally, the group is interested in the effects of an acellular booster across all age groups. While the effects may be short-lived, the booster is a potential strategy for controlling a population by repeated boosting of immunity. This is another area where using novel immunoassays may aid better understanding.
To find answers, the consortium has established four studies: the Gambia Pertussis study (GaPs) in Gambia and AWARE, the sister study to GaPs in the United Kingdom, addressing the acellular pertussis versus cellular pertussis question; the Pertussis Maternal Immunization Study in Finland (MIFI) addressing maternal immunization; and the Booster against Pertussis (BERT) study across three countries (U.K., the Netherlands, and Finland) looking at acellular booster across age groups.
Gambia pertussis study
GaPs is the largest single study in the project and is being run at the Medical Research Council–funded London School of Tropical Medicine center in Gambia. Beate Kampmann, MD, PhD, of Imperial College London, England, is the project lead. It is due to complete in 2022. GaPs seeks to enroll 600 mother/infant pairs and randomize the mothers to either an acellular pertussis booster in pregnancy or a tetanus toxoid control vaccine. Infants are subsequently randomized to an acellular or whole-cell pertussis schedule of primary immunization. The vaccine doses are being given at 2, 3, and 4 months. The primary endpoint is a serological finding being measured at 9 months of age, when the infant would usually receive yellow fever, measles, and rubella vaccination.
GaPs has a number of pathways. Within each of the four arms generated by the two randomizations, the maternal randomization and the infant randomization, there are five subgroups. They are designed to study time points in subgroups A and B after the first dose in more detail, looking at the innate immune responses using gene expression. It will enable researchers to study adaptive immune responses to T cells and B cells after the second dose of vaccine. By employing a range of subgroups, the team can explore the immune profile using the assays referred to above. Such information should provide new insights into the differences between acellular and whole-cell vaccines.
The AWARE study
AWARE is the sister study to GaPs and looks at the acellular/whole pertussis issue. Because many developed countries, such as the United Kingdom, have established maternal immunization programs, it is not possible to randomize mothers. Consequently, researchers have opted to recruit infants of mothers who have received an acellular vaccine in pregnancy and randomize them to either an acellular schedule of primary immunization or a whole-cell schedule.
The selected vaccine is ComVac5 from Bharat Biotech. This whole-cell vaccine differs from that used in Gambia. An early obstacle for AWARE has been seeking permission to import a non-conventional vaccine into Europe. It has delayed the anticipated end date to 2023. Participating infants will receive a two-dose schedule at 2 and 4 months of age per their randomization; then, both groups will go on to receive an acellular pertussis booster at 12 months. At all time points, the team will sample blood for cells and serum, as well as mucosal fluid from the nose. Because the mucosal surface is where the action is, this approach will likely generate new data around antibody responses.
The MIFI
The Pertussis Maternal Immunization Study in Finland is being run by Jussi Mertsola, of the University of Turku, Finland, and Qiushui He, of the National Public Health Institute, Turku. It is due to complete in late 2021. Where, in the United Kingdom, researchers are unable to randomize mothers because of the current guidelines, researchers in Finland do not have a maternal immunization program to consider. MIFI will randomize 80 mothers, 40 to immunization with acellular pertussis and 40 to a control group. Dr. Kelly stated that whole cell vaccines are not available for use in Finland. Participants will receive a two-dose schedule at 3 and 5 months. Blood samples will then be taken to compare the serological and cellular responses, which will help researchers understand the effects of maternal immunization. In addition, there will be sampling of mucosal fluid using a device that collects a standardized aliquot of fluid.
The BERT study
The final clinical element of PERISCOPE presented by Dr. Kelly was the Booster against Pertussis study. This study is near completion. It seeks to examine the use of an acellular booster across different age groups and three countries: the United Kingdom, the Netherlands, and Finland. The study is being coordinated by Guy Berbers, PhD, at the National Institute for Public Health and the Environment in the Netherlands.
BERT comprises four cohorts (A, B, C, D) of different ages: 7-10 years (36 participants), 11-15 years (36 participants), mid-adult (25 participants), and older age (25 participants). After receiving an acellular booster, participants will undergo intense sampling. Sampling will take place immediately after immunization at day 7 and look at adaptive effects, then again at day 28 and day 365.
Because some participants will have already received whole cell or acellular vaccination, this approach will allow researchers to look at the effects of priming (i.e., how long the B cell/T cell antibody responses last).
Involving different countries across Europe ensures wide applicability of results, but also allows researchers to compare the effects of very different immunization histories.
At the end of this ESPID session, Dimitri Diavatopoulos, PhD, assistant professor at the Radboud University Medical Centre Nijmegen, the Netherlands, commented that a future problem in studying pertussis vaccines and their potential clinical application is that most vaccination schedules now involve combination products. Obtaining a stand-alone vaccination may prove difficult, and there may be resistance if it complicates current vaccination programs.
Dr. Kelly acknowledged funding for the PERISCOPE project from GlaxoSmithKline and Pasteur Sanofi.
With increasing whooping cough numbers, developing an effective new vaccine against Bordetella pertussis is a priority. Results from the multifactorial PERISCOPE Project will help scientists and clinicians move forward.
Dominic Kelly, PhD, talked about vaccine-induced immunity and provided an overview of ongoing clinical trials in the PERISCOPE (Pertussis Correlates of Protection Europe) project in a key research session at the start of the annual meeting of the European Society for Paediatric Infectious Diseases, held virtually this year. Dr. Kelly, a pediatrician at the Children’s Hospital in Oxford and a member of the Oxford Vaccines Group, leads one of the studies in the project looking at infant vaccination.
Dr. Kelly began his presentation by showing a figure depicting where vaccine-induced immunity fits into the larger suite of clinical studies. These studies involve mouse models, human challenge models, and infection patients. A key theme is the use of a core group of immunoassays across all studies, with the hope that they will allow effective cross comparisons.
Dr. Kelly stated, “If we find a correlate of protection in the challenge model, we can then interpret the vaccine studies in the light of that because we are using standardized constant immunoassays.”
The assays being used depend in part on the specific study and the volume of blood available. They will generally include Bordetella-specific antibody and functional antibody assays, as well as interesting studies collecting mucosal samples from infants and adults to look at serological responses. Also under examination are a range of enzyme-linked immune absorbent spot, flow cytometry, and culture techniques looking at Memory B cells, T cells, and gene expression.
Complementing these assay studies, PERISCOPE includes a series of clinical investigations designed to throw light on three areas of interest, described below:
First, researchers hope to gain a better understanding regarding the effects of the original whole cell vaccine versus the current acellular variety. The former uses an inactivated version of the whole organism. Epidemiological studies, animal data, and experience in the field demonstrate that whole-cell vaccination results in a broad, long-lasting, and effective immune response.
By comparison, the acellular pertussis vaccine consists of between three and five protein components, which are purified from cultured Bordetella pertussis. While it is an effective vaccine, its effects are less durable; routine use in some countries is associated with cyclical outbreaks of increasing severity.
A second issue for researchers involved in the PERISCOPE project concerns the effects of maternal immunization. In the United Kingdom in 2012, for example, an increasing number of cases were noted 6-7 years after adoption of an acellular vaccine for routine vaccination in the 2nd-3rd trimester of pregnancy. Vaccination appears to effectively control neonatal disease, but whether this influences infant immune responses and long-term control of pertussis for a population is unknown.
Finally, the group is interested in the effects of an acellular booster across all age groups. While the effects may be short-lived, the booster is a potential strategy for controlling a population by repeated boosting of immunity. This is another area where using novel immunoassays may aid better understanding.
To find answers, the consortium has established four studies: the Gambia Pertussis study (GaPs) in Gambia and AWARE, the sister study to GaPs in the United Kingdom, addressing the acellular pertussis versus cellular pertussis question; the Pertussis Maternal Immunization Study in Finland (MIFI) addressing maternal immunization; and the Booster against Pertussis (BERT) study across three countries (U.K., the Netherlands, and Finland) looking at acellular booster across age groups.
Gambia pertussis study
GaPs is the largest single study in the project and is being run at the Medical Research Council–funded London School of Tropical Medicine center in Gambia. Beate Kampmann, MD, PhD, of Imperial College London, England, is the project lead. It is due to complete in 2022. GaPs seeks to enroll 600 mother/infant pairs and randomize the mothers to either an acellular pertussis booster in pregnancy or a tetanus toxoid control vaccine. Infants are subsequently randomized to an acellular or whole-cell pertussis schedule of primary immunization. The vaccine doses are being given at 2, 3, and 4 months. The primary endpoint is a serological finding being measured at 9 months of age, when the infant would usually receive yellow fever, measles, and rubella vaccination.
GaPs has a number of pathways. Within each of the four arms generated by the two randomizations, the maternal randomization and the infant randomization, there are five subgroups. They are designed to study time points in subgroups A and B after the first dose in more detail, looking at the innate immune responses using gene expression. It will enable researchers to study adaptive immune responses to T cells and B cells after the second dose of vaccine. By employing a range of subgroups, the team can explore the immune profile using the assays referred to above. Such information should provide new insights into the differences between acellular and whole-cell vaccines.
The AWARE study
AWARE is the sister study to GaPs and looks at the acellular/whole pertussis issue. Because many developed countries, such as the United Kingdom, have established maternal immunization programs, it is not possible to randomize mothers. Consequently, researchers have opted to recruit infants of mothers who have received an acellular vaccine in pregnancy and randomize them to either an acellular schedule of primary immunization or a whole-cell schedule.
The selected vaccine is ComVac5 from Bharat Biotech. This whole-cell vaccine differs from that used in Gambia. An early obstacle for AWARE has been seeking permission to import a non-conventional vaccine into Europe. It has delayed the anticipated end date to 2023. Participating infants will receive a two-dose schedule at 2 and 4 months of age per their randomization; then, both groups will go on to receive an acellular pertussis booster at 12 months. At all time points, the team will sample blood for cells and serum, as well as mucosal fluid from the nose. Because the mucosal surface is where the action is, this approach will likely generate new data around antibody responses.
The MIFI
The Pertussis Maternal Immunization Study in Finland is being run by Jussi Mertsola, of the University of Turku, Finland, and Qiushui He, of the National Public Health Institute, Turku. It is due to complete in late 2021. Where, in the United Kingdom, researchers are unable to randomize mothers because of the current guidelines, researchers in Finland do not have a maternal immunization program to consider. MIFI will randomize 80 mothers, 40 to immunization with acellular pertussis and 40 to a control group. Dr. Kelly stated that whole cell vaccines are not available for use in Finland. Participants will receive a two-dose schedule at 3 and 5 months. Blood samples will then be taken to compare the serological and cellular responses, which will help researchers understand the effects of maternal immunization. In addition, there will be sampling of mucosal fluid using a device that collects a standardized aliquot of fluid.
The BERT study
The final clinical element of PERISCOPE presented by Dr. Kelly was the Booster against Pertussis study. This study is near completion. It seeks to examine the use of an acellular booster across different age groups and three countries: the United Kingdom, the Netherlands, and Finland. The study is being coordinated by Guy Berbers, PhD, at the National Institute for Public Health and the Environment in the Netherlands.
BERT comprises four cohorts (A, B, C, D) of different ages: 7-10 years (36 participants), 11-15 years (36 participants), mid-adult (25 participants), and older age (25 participants). After receiving an acellular booster, participants will undergo intense sampling. Sampling will take place immediately after immunization at day 7 and look at adaptive effects, then again at day 28 and day 365.
Because some participants will have already received whole cell or acellular vaccination, this approach will allow researchers to look at the effects of priming (i.e., how long the B cell/T cell antibody responses last).
Involving different countries across Europe ensures wide applicability of results, but also allows researchers to compare the effects of very different immunization histories.
At the end of this ESPID session, Dimitri Diavatopoulos, PhD, assistant professor at the Radboud University Medical Centre Nijmegen, the Netherlands, commented that a future problem in studying pertussis vaccines and their potential clinical application is that most vaccination schedules now involve combination products. Obtaining a stand-alone vaccination may prove difficult, and there may be resistance if it complicates current vaccination programs.
Dr. Kelly acknowledged funding for the PERISCOPE project from GlaxoSmithKline and Pasteur Sanofi.
FROM ESPID 2020
COVID-19–induced drop in first measles vaccinations sparks resurgence concerns
Widespread use of the MMR vaccine is not only crucial for protecting the community against infectious outbreaks, but also serves as the overall pacesetter for preventive services, said Sara M. Bode, MD and colleagues at Nationwide Children’s Hospital in Columbus.
As part of a bivariate logistic regression analysis, Dr. Bode and colleagues sought to evaluate changes in measles vaccination rates across 12 clinic sites of the Nationwide Children’s Hospital pediatric primary care network in Columbus among 23,534 children aged 16 months. The study period targeted the time between April and May 2020, when clinic access and appointment attendance declined following the start of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic, until the June-to-August 2020 time period, when clinical care was allowed to return.
The need for the study was prompted by Centers for Disease Control and Prevention reporting on a state-specific precipitous decline in MMR vaccination rates shortly after the onset of COVID-19 in May 2020. Citing the results of one study, such reductions in vaccination have raised concerns over the possibility of a measles resurgence, noted Dr. Bode and associates.
MMR vaccination rates begin to drop with onset of COVID-19 pandemic.
From March 2017 to March 2020, the average rate of MMR vaccination in 16-month-olds was 72%. It subsequently decreased to 67% from April to May 2020, and then dropped further to 62% during the period June to August, 2020 (P = .001). Those without insurance were less likely to be vaccinated than were those carrying private insurance or Medicaid.
Among patients who had not attended a preventive care visit after 12 months of age, the proportion who received vaccines declined during the same time periods, from 10% before the pandemic to 6% at the start of the pandemic and 3% during the summer months of 2020.
“Given the baseline low vaccination rates even before the pandemic and the subsequent decline, we face a critical need to improve timely vaccination and provide catch-up opportunities” in areas with the highest incidence of COVID-19, observed Dr. Bode and colleagues.
Innovative approaches are needed to encourage families to seek preventive care.
In response, the researchers announced the implementation of new community-based vaccination approaches in Ohio, including pop-up vaccine clinics, mobile clinics, and school-based clinics to provide families, who are reluctant to visit health care facilities over COVID-19 related concerns, with safe alternatives. “We believe that it is critical to develop innovative approaches to have families return for preventive care,” they added.
In a separate interview, Herschel Lessin, MD, a private practice pediatrician in Poughkeepsie, N.Y., noted: “This study confirms the anecdotal experience of pediatricians around the country, and our greatest fear that the pandemic will interfere with herd immunity of children for vaccine-preventable illness. Although the study was of urban offices with a primarily Medicaid population, I believe the results to be very worrisome should they prove to be generalizable to the country, as a whole. The significant reduction of well-child visits due to COVID-19 (and fear of COVID-19) seriously impaired the vaccination status of a standard required vaccine in a large population. What is even more worrisome is that the rates continued to fall even after the initial closure of many offices and well into their reopening, despite concerted efforts to try to catch up these missed visits and immunizations.”
Measles is an intensely contagious illness that has not been eradicated, as evidenced by the enormous measles outbreak stemming from Disneyland in 2014-2015, and again with the possible exposure of hundreds to an infected Disneyland visitor last fall, where coverage rates were even higher than in this study, added Dr. Lessin. “This phenomenon, unless forcefully remedied, could easily result in large outbreaks of other vaccine-preventable illness besides COVID-19,” he cautioned.
Dr. Bode and colleagues as well as Dr. Lessin had no conflicts of interest and no relevant financial disclosures.
SOURCE: Bode SM et al. Pediatrics. 2021. doi: 10.1542/peds.2020-035576.
Widespread use of the MMR vaccine is not only crucial for protecting the community against infectious outbreaks, but also serves as the overall pacesetter for preventive services, said Sara M. Bode, MD and colleagues at Nationwide Children’s Hospital in Columbus.
As part of a bivariate logistic regression analysis, Dr. Bode and colleagues sought to evaluate changes in measles vaccination rates across 12 clinic sites of the Nationwide Children’s Hospital pediatric primary care network in Columbus among 23,534 children aged 16 months. The study period targeted the time between April and May 2020, when clinic access and appointment attendance declined following the start of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic, until the June-to-August 2020 time period, when clinical care was allowed to return.
The need for the study was prompted by Centers for Disease Control and Prevention reporting on a state-specific precipitous decline in MMR vaccination rates shortly after the onset of COVID-19 in May 2020. Citing the results of one study, such reductions in vaccination have raised concerns over the possibility of a measles resurgence, noted Dr. Bode and associates.
MMR vaccination rates begin to drop with onset of COVID-19 pandemic.
From March 2017 to March 2020, the average rate of MMR vaccination in 16-month-olds was 72%. It subsequently decreased to 67% from April to May 2020, and then dropped further to 62% during the period June to August, 2020 (P = .001). Those without insurance were less likely to be vaccinated than were those carrying private insurance or Medicaid.
Among patients who had not attended a preventive care visit after 12 months of age, the proportion who received vaccines declined during the same time periods, from 10% before the pandemic to 6% at the start of the pandemic and 3% during the summer months of 2020.
“Given the baseline low vaccination rates even before the pandemic and the subsequent decline, we face a critical need to improve timely vaccination and provide catch-up opportunities” in areas with the highest incidence of COVID-19, observed Dr. Bode and colleagues.
Innovative approaches are needed to encourage families to seek preventive care.
In response, the researchers announced the implementation of new community-based vaccination approaches in Ohio, including pop-up vaccine clinics, mobile clinics, and school-based clinics to provide families, who are reluctant to visit health care facilities over COVID-19 related concerns, with safe alternatives. “We believe that it is critical to develop innovative approaches to have families return for preventive care,” they added.
In a separate interview, Herschel Lessin, MD, a private practice pediatrician in Poughkeepsie, N.Y., noted: “This study confirms the anecdotal experience of pediatricians around the country, and our greatest fear that the pandemic will interfere with herd immunity of children for vaccine-preventable illness. Although the study was of urban offices with a primarily Medicaid population, I believe the results to be very worrisome should they prove to be generalizable to the country, as a whole. The significant reduction of well-child visits due to COVID-19 (and fear of COVID-19) seriously impaired the vaccination status of a standard required vaccine in a large population. What is even more worrisome is that the rates continued to fall even after the initial closure of many offices and well into their reopening, despite concerted efforts to try to catch up these missed visits and immunizations.”
Measles is an intensely contagious illness that has not been eradicated, as evidenced by the enormous measles outbreak stemming from Disneyland in 2014-2015, and again with the possible exposure of hundreds to an infected Disneyland visitor last fall, where coverage rates were even higher than in this study, added Dr. Lessin. “This phenomenon, unless forcefully remedied, could easily result in large outbreaks of other vaccine-preventable illness besides COVID-19,” he cautioned.
Dr. Bode and colleagues as well as Dr. Lessin had no conflicts of interest and no relevant financial disclosures.
SOURCE: Bode SM et al. Pediatrics. 2021. doi: 10.1542/peds.2020-035576.
Widespread use of the MMR vaccine is not only crucial for protecting the community against infectious outbreaks, but also serves as the overall pacesetter for preventive services, said Sara M. Bode, MD and colleagues at Nationwide Children’s Hospital in Columbus.
As part of a bivariate logistic regression analysis, Dr. Bode and colleagues sought to evaluate changes in measles vaccination rates across 12 clinic sites of the Nationwide Children’s Hospital pediatric primary care network in Columbus among 23,534 children aged 16 months. The study period targeted the time between April and May 2020, when clinic access and appointment attendance declined following the start of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic, until the June-to-August 2020 time period, when clinical care was allowed to return.
The need for the study was prompted by Centers for Disease Control and Prevention reporting on a state-specific precipitous decline in MMR vaccination rates shortly after the onset of COVID-19 in May 2020. Citing the results of one study, such reductions in vaccination have raised concerns over the possibility of a measles resurgence, noted Dr. Bode and associates.
MMR vaccination rates begin to drop with onset of COVID-19 pandemic.
From March 2017 to March 2020, the average rate of MMR vaccination in 16-month-olds was 72%. It subsequently decreased to 67% from April to May 2020, and then dropped further to 62% during the period June to August, 2020 (P = .001). Those without insurance were less likely to be vaccinated than were those carrying private insurance or Medicaid.
Among patients who had not attended a preventive care visit after 12 months of age, the proportion who received vaccines declined during the same time periods, from 10% before the pandemic to 6% at the start of the pandemic and 3% during the summer months of 2020.
“Given the baseline low vaccination rates even before the pandemic and the subsequent decline, we face a critical need to improve timely vaccination and provide catch-up opportunities” in areas with the highest incidence of COVID-19, observed Dr. Bode and colleagues.
Innovative approaches are needed to encourage families to seek preventive care.
In response, the researchers announced the implementation of new community-based vaccination approaches in Ohio, including pop-up vaccine clinics, mobile clinics, and school-based clinics to provide families, who are reluctant to visit health care facilities over COVID-19 related concerns, with safe alternatives. “We believe that it is critical to develop innovative approaches to have families return for preventive care,” they added.
In a separate interview, Herschel Lessin, MD, a private practice pediatrician in Poughkeepsie, N.Y., noted: “This study confirms the anecdotal experience of pediatricians around the country, and our greatest fear that the pandemic will interfere with herd immunity of children for vaccine-preventable illness. Although the study was of urban offices with a primarily Medicaid population, I believe the results to be very worrisome should they prove to be generalizable to the country, as a whole. The significant reduction of well-child visits due to COVID-19 (and fear of COVID-19) seriously impaired the vaccination status of a standard required vaccine in a large population. What is even more worrisome is that the rates continued to fall even after the initial closure of many offices and well into their reopening, despite concerted efforts to try to catch up these missed visits and immunizations.”
Measles is an intensely contagious illness that has not been eradicated, as evidenced by the enormous measles outbreak stemming from Disneyland in 2014-2015, and again with the possible exposure of hundreds to an infected Disneyland visitor last fall, where coverage rates were even higher than in this study, added Dr. Lessin. “This phenomenon, unless forcefully remedied, could easily result in large outbreaks of other vaccine-preventable illness besides COVID-19,” he cautioned.
Dr. Bode and colleagues as well as Dr. Lessin had no conflicts of interest and no relevant financial disclosures.
SOURCE: Bode SM et al. Pediatrics. 2021. doi: 10.1542/peds.2020-035576.
FROM PEDIATRICS
Latest rise in child COVID-19 cases is relatively small
For the seventh week out of the last eight, more new cases of COVID-19 in children were reported in the United States than any week before, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.
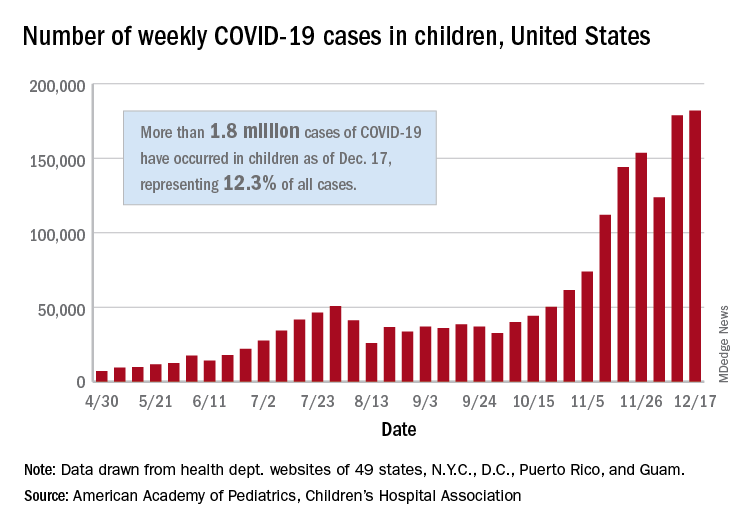
There were just over 182,000 new cases of COVID-19 in children during the week ending Dec. 17, topping the previous high of almost 179,000 set the previous week. – a stretch of 11 weeks that has produced only one decline, based on data from the latest AAP/CHA weekly report.
As of Dec. 17, there had been over 1.8 million cases of COVID-19 in children, which represents 12.3% of all U.S. cases. For the week, 14% of all cases occurred in children, which was up slightly from 13.8% the week before (Dec. 10). The overall rate of coronavirus infection is now 2,420 cases per 100,000 children in the population, the AAP and CHA said.
A total of 30 states are above that national rate, with North Dakota the highest at 7,515 cases per 100,000 children, followed by South Dakota (5,618), Wyoming (5,157), Wisconsin (5,106), and Tennessee (4,994). Wyoming has the highest proportion of cases occurring in children at 20.8%, but that is down from 23.4% in mid-November, based on data collected by the AAP and CHA from the health department websites of 49 states (New York does not provide age distributions), the District of Columbia, New York City, Puerto Rico, and Guam.
In the last 2 weeks, however, the largest percent increases in new cases came in states with low-to-average rates of cumulative child infection. California, Connecticut, Delaware, Maine, Maryland, New Hampshire, and Vermont all saw increases of over 35% from Dec. 3 to Dec. 17, while the smallest increases occurred in Hawaii, North Dakota, and Wyoming, the AAP and CHA reported.
For the seventh week out of the last eight, more new cases of COVID-19 in children were reported in the United States than any week before, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.

There were just over 182,000 new cases of COVID-19 in children during the week ending Dec. 17, topping the previous high of almost 179,000 set the previous week. – a stretch of 11 weeks that has produced only one decline, based on data from the latest AAP/CHA weekly report.
As of Dec. 17, there had been over 1.8 million cases of COVID-19 in children, which represents 12.3% of all U.S. cases. For the week, 14% of all cases occurred in children, which was up slightly from 13.8% the week before (Dec. 10). The overall rate of coronavirus infection is now 2,420 cases per 100,000 children in the population, the AAP and CHA said.
A total of 30 states are above that national rate, with North Dakota the highest at 7,515 cases per 100,000 children, followed by South Dakota (5,618), Wyoming (5,157), Wisconsin (5,106), and Tennessee (4,994). Wyoming has the highest proportion of cases occurring in children at 20.8%, but that is down from 23.4% in mid-November, based on data collected by the AAP and CHA from the health department websites of 49 states (New York does not provide age distributions), the District of Columbia, New York City, Puerto Rico, and Guam.
In the last 2 weeks, however, the largest percent increases in new cases came in states with low-to-average rates of cumulative child infection. California, Connecticut, Delaware, Maine, Maryland, New Hampshire, and Vermont all saw increases of over 35% from Dec. 3 to Dec. 17, while the smallest increases occurred in Hawaii, North Dakota, and Wyoming, the AAP and CHA reported.
For the seventh week out of the last eight, more new cases of COVID-19 in children were reported in the United States than any week before, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.

There were just over 182,000 new cases of COVID-19 in children during the week ending Dec. 17, topping the previous high of almost 179,000 set the previous week. – a stretch of 11 weeks that has produced only one decline, based on data from the latest AAP/CHA weekly report.
As of Dec. 17, there had been over 1.8 million cases of COVID-19 in children, which represents 12.3% of all U.S. cases. For the week, 14% of all cases occurred in children, which was up slightly from 13.8% the week before (Dec. 10). The overall rate of coronavirus infection is now 2,420 cases per 100,000 children in the population, the AAP and CHA said.
A total of 30 states are above that national rate, with North Dakota the highest at 7,515 cases per 100,000 children, followed by South Dakota (5,618), Wyoming (5,157), Wisconsin (5,106), and Tennessee (4,994). Wyoming has the highest proportion of cases occurring in children at 20.8%, but that is down from 23.4% in mid-November, based on data collected by the AAP and CHA from the health department websites of 49 states (New York does not provide age distributions), the District of Columbia, New York City, Puerto Rico, and Guam.
In the last 2 weeks, however, the largest percent increases in new cases came in states with low-to-average rates of cumulative child infection. California, Connecticut, Delaware, Maine, Maryland, New Hampshire, and Vermont all saw increases of over 35% from Dec. 3 to Dec. 17, while the smallest increases occurred in Hawaii, North Dakota, and Wyoming, the AAP and CHA reported.