User login
COVID-19 in children: Weekly cases trending downward
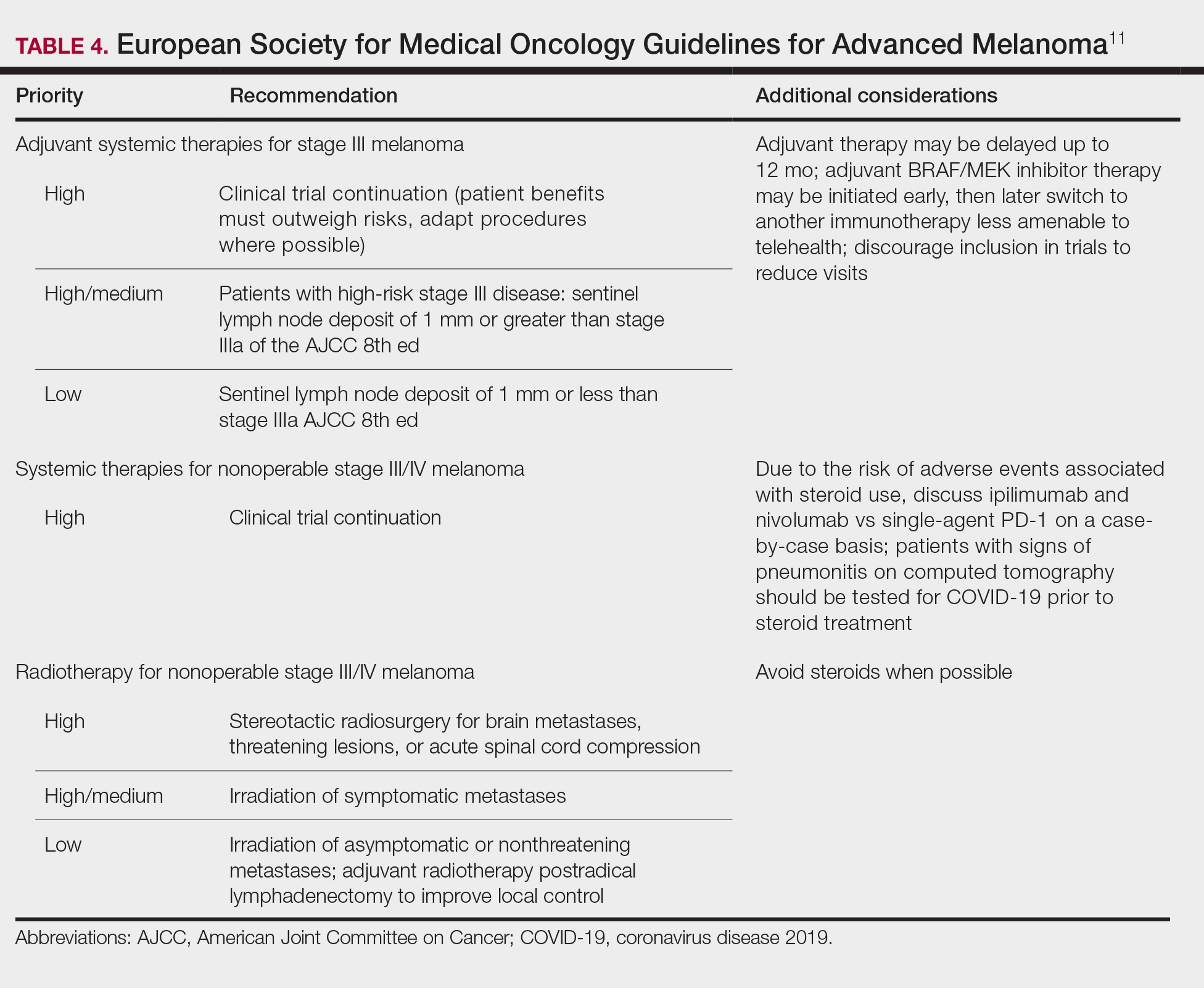
The United States added over 171,000 new COVID-19 cases in children during the week ending Jan. 7, but that figure is lower than 3 of the previous 4 weeks, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.
Despite an increase compared with the week ending Dec. 31, the most recent weekly total is down from the high of 182,000 cases reported for the week ending Dec. 17, based on data collected from the health department websites of 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam.
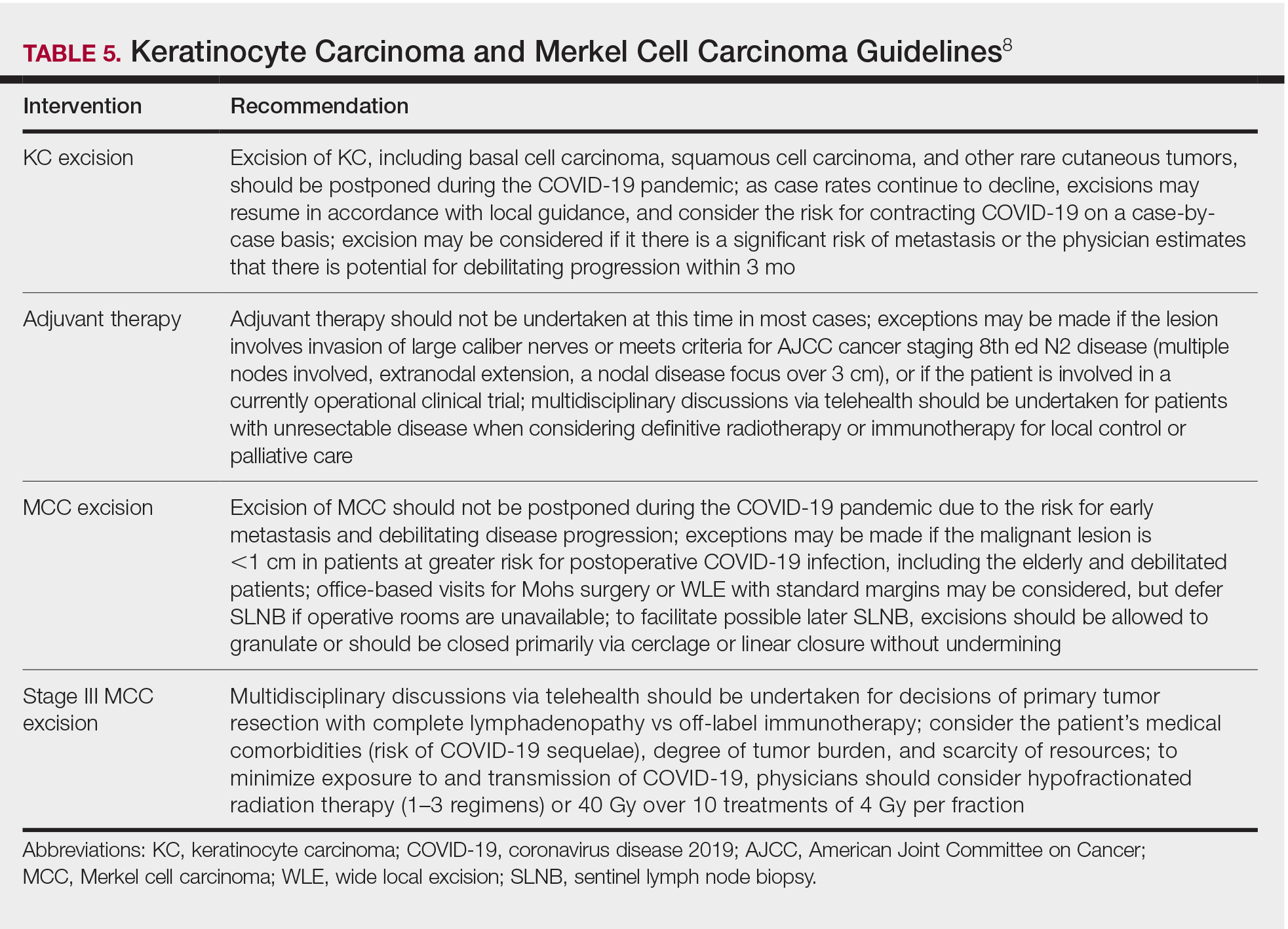
Those jurisdictions have recorded a total of almost 2.3 million COVID-19 cases in children since the beginning of the pandemic, which amounts to 12.5% of reported cases among all ages. The 171,000 child cases for the most recent week represented 12.9% of the more than 1.3 million cases nationwide, the AAP and CHA said in their latest weekly update.
The United States now has a rate of 3,055 COVID-19 cases per 100,000 children in the population, the report shows, with 31 states above that figure and 14 states reporting rates above 4,500 per 100,000 children.
Severe illness, however, continues to be rare among children. So far, children represent 1.8% of all hospitalizations in the jurisdictions reporting such data (24 states and New York City), and just 0.9% of infected children have been hospitalized. There have been 188 deaths among children in 42 states and New York City, which makes up just 0.06% of the total for all ages in those jurisdictions, the AAP and CHA reported.
There are 13 states that have reported no coronavirus-related deaths in children, while Texas (34), New York City (21), Arizona (17), and Illinois (11) are the only jurisdictions with 10 or more. Nevada has the highest proportion of child deaths to all deaths at 0.2%, with Arizona and Nebraska next at 0.18%, according to the AAP/CHA report.
The United States added over 171,000 new COVID-19 cases in children during the week ending Jan. 7, but that figure is lower than 3 of the previous 4 weeks, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.

Despite an increase compared with the week ending Dec. 31, the most recent weekly total is down from the high of 182,000 cases reported for the week ending Dec. 17, based on data collected from the health department websites of 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam.
Those jurisdictions have recorded a total of almost 2.3 million COVID-19 cases in children since the beginning of the pandemic, which amounts to 12.5% of reported cases among all ages. The 171,000 child cases for the most recent week represented 12.9% of the more than 1.3 million cases nationwide, the AAP and CHA said in their latest weekly update.
The United States now has a rate of 3,055 COVID-19 cases per 100,000 children in the population, the report shows, with 31 states above that figure and 14 states reporting rates above 4,500 per 100,000 children.
Severe illness, however, continues to be rare among children. So far, children represent 1.8% of all hospitalizations in the jurisdictions reporting such data (24 states and New York City), and just 0.9% of infected children have been hospitalized. There have been 188 deaths among children in 42 states and New York City, which makes up just 0.06% of the total for all ages in those jurisdictions, the AAP and CHA reported.
There are 13 states that have reported no coronavirus-related deaths in children, while Texas (34), New York City (21), Arizona (17), and Illinois (11) are the only jurisdictions with 10 or more. Nevada has the highest proportion of child deaths to all deaths at 0.2%, with Arizona and Nebraska next at 0.18%, according to the AAP/CHA report.
The United States added over 171,000 new COVID-19 cases in children during the week ending Jan. 7, but that figure is lower than 3 of the previous 4 weeks, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.

Despite an increase compared with the week ending Dec. 31, the most recent weekly total is down from the high of 182,000 cases reported for the week ending Dec. 17, based on data collected from the health department websites of 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam.
Those jurisdictions have recorded a total of almost 2.3 million COVID-19 cases in children since the beginning of the pandemic, which amounts to 12.5% of reported cases among all ages. The 171,000 child cases for the most recent week represented 12.9% of the more than 1.3 million cases nationwide, the AAP and CHA said in their latest weekly update.
The United States now has a rate of 3,055 COVID-19 cases per 100,000 children in the population, the report shows, with 31 states above that figure and 14 states reporting rates above 4,500 per 100,000 children.
Severe illness, however, continues to be rare among children. So far, children represent 1.8% of all hospitalizations in the jurisdictions reporting such data (24 states and New York City), and just 0.9% of infected children have been hospitalized. There have been 188 deaths among children in 42 states and New York City, which makes up just 0.06% of the total for all ages in those jurisdictions, the AAP and CHA reported.
There are 13 states that have reported no coronavirus-related deaths in children, while Texas (34), New York City (21), Arizona (17), and Illinois (11) are the only jurisdictions with 10 or more. Nevada has the highest proportion of child deaths to all deaths at 0.2%, with Arizona and Nebraska next at 0.18%, according to the AAP/CHA report.
Updated USPSTF HBV screening recommendation may be a ‘lost opportunity’
An update of the U.S. Preventive Services Task Force recommendation for hepatitis B screening shows little change from the 2014 version, but some wonder if it should have gone farther than a risk-based approach.
The recommendation, which was published in JAMA, reinforces that screening should be conducted among adolescents and adults who are at increased risk of hepatitis B virus (HBV) infection. The USPSTF named six categories of individuals at increased risk of infection: Persons born in countries with a 2% or higher prevalence of hepatitis B, such as Asia, Africa, the Pacific Islands, and some areas of South America; unvaccinated individuals born in the United States to parents from regions with a very high prevalence of HBV (≥8%); HIV-positive individuals; those who use injected drugs; men who have sex with men; and people who live with people who have HBV or who have HBV-infected sexual partners. It also recommended that pregnant women be screened for HBV infection during their first prenatal visit.
“I view the updated recommendations as an important document because it validates the importance of HBV screening, and the Grade B recommendation supports mandated insurance coverage for the screening test,” said Joseph Lim, MD, who is a professor of medicine at Yale University and director of the Yale Viral Hepatitis Program, both in New Haven, Conn.
Still, the recommendation could have gone further. Notably absent from the USPSTF document, yet featured in recommendations from the Centers for Disease Control and Prevention and the American Association for the Study of Liver Disease, are patients who have diabetes, are on immunosuppressive therapy, or have elevated liver enzymes or liver disease. Furthermore, a single-center study found that, among physicians administering immunosuppressive therapy, a setting in which HBV reactivation is a concern, there were low rates of screening for HBV infection, and the physicians did not reliably identify high-risk patients.
“This may also be viewed as a lost opportunity. Evidence suggests that risk factor–based screening is ineffective for the identification of chronic conditions such as hepatitis B. Risk factor–based screening is difficult to implement across health systems and exacerbates the burden on community-based organizations that are motivated to address viral hepatitis. It may further exacerbate labeling, stigma, and discrimination within already marginalized communities that are deemed to be at high risk,” said Dr. Lim.
A similar view was expressed by Avegail Flores, MD, medical director of liver transplantation at the Michael E. DeBakey Veterans Affairs Medical Center and assistant professor of medicine at Baylor College of Medicine, both in Houston. “This is a good launching point, and with further evidence provided, hopefully it will also bring in a broader conversation about other persons who are at risk but not included in these criteria.” Neither Dr. Lim nor Dr. Flores were involved in the study.
She noted that resistance to universal screening may be caused by the relatively low prevalence of hepatitis B infection in the United States. However, the CDC estimates that only about 61% of people infected with HBV are aware of it. “I don’t think we have done a good job screening those who are at risk,” said Dr. Flores.
Universal screening could help, but would have a low yield. Dr. Flores suggested expansion into other at-risk groups, such as Baby Boomers. With respect to other risk groups that could be stigmatized or discriminated against, Dr. Flores recalled her medical school days when some students went directly into underserved communities to provide information and screening services. “We have to think of creative ways of how to reach out to people, not just relying on the usual physician-patient relationship.”
The issue is especially timely because the World Health Organization has declared a target to reduce new hepatitis B infections by 90% by 2030, and that will require addressing gaps in diagnosis. “That’s why these recommendations are so consequential. We are at a critical juncture in terms of global hepatitis elimination efforts. There is a time sensitive need to have multistakeholder engagement in ensuring that all aspects of the care cascade are addressed. Because of the central role of screening and diagnosis, it’s of critical importance that organizations such as USPSTF are in alignment with other organizations that have already issued clear guidance on who should be screened. It is (my) hope that further examination of the evidence-base will further support broadening USPSTF guidance to include a larger group of at-risk individuals, or ideally a universal screening strategy,” said Dr. Lim.
The recommendation’s authors received travel reimbursement for their involvement, and one author reported receiving grants and personal fees from Healthwise. Dr. Flores has no relevant financial disclosures. Dr. Lim is a member of the American Association for the Study of Liver Disease’s Viral Hepatitis Elimination Task Force.
SOURCE: U.S. Preventive Services Task Force. JAMA. 2020 Dec 15. doi: 10.1001/jama.2020.22980.
Updated Jan. 20, 2021
An update of the U.S. Preventive Services Task Force recommendation for hepatitis B screening shows little change from the 2014 version, but some wonder if it should have gone farther than a risk-based approach.
The recommendation, which was published in JAMA, reinforces that screening should be conducted among adolescents and adults who are at increased risk of hepatitis B virus (HBV) infection. The USPSTF named six categories of individuals at increased risk of infection: Persons born in countries with a 2% or higher prevalence of hepatitis B, such as Asia, Africa, the Pacific Islands, and some areas of South America; unvaccinated individuals born in the United States to parents from regions with a very high prevalence of HBV (≥8%); HIV-positive individuals; those who use injected drugs; men who have sex with men; and people who live with people who have HBV or who have HBV-infected sexual partners. It also recommended that pregnant women be screened for HBV infection during their first prenatal visit.
“I view the updated recommendations as an important document because it validates the importance of HBV screening, and the Grade B recommendation supports mandated insurance coverage for the screening test,” said Joseph Lim, MD, who is a professor of medicine at Yale University and director of the Yale Viral Hepatitis Program, both in New Haven, Conn.
Still, the recommendation could have gone further. Notably absent from the USPSTF document, yet featured in recommendations from the Centers for Disease Control and Prevention and the American Association for the Study of Liver Disease, are patients who have diabetes, are on immunosuppressive therapy, or have elevated liver enzymes or liver disease. Furthermore, a single-center study found that, among physicians administering immunosuppressive therapy, a setting in which HBV reactivation is a concern, there were low rates of screening for HBV infection, and the physicians did not reliably identify high-risk patients.
“This may also be viewed as a lost opportunity. Evidence suggests that risk factor–based screening is ineffective for the identification of chronic conditions such as hepatitis B. Risk factor–based screening is difficult to implement across health systems and exacerbates the burden on community-based organizations that are motivated to address viral hepatitis. It may further exacerbate labeling, stigma, and discrimination within already marginalized communities that are deemed to be at high risk,” said Dr. Lim.
A similar view was expressed by Avegail Flores, MD, medical director of liver transplantation at the Michael E. DeBakey Veterans Affairs Medical Center and assistant professor of medicine at Baylor College of Medicine, both in Houston. “This is a good launching point, and with further evidence provided, hopefully it will also bring in a broader conversation about other persons who are at risk but not included in these criteria.” Neither Dr. Lim nor Dr. Flores were involved in the study.
She noted that resistance to universal screening may be caused by the relatively low prevalence of hepatitis B infection in the United States. However, the CDC estimates that only about 61% of people infected with HBV are aware of it. “I don’t think we have done a good job screening those who are at risk,” said Dr. Flores.
Universal screening could help, but would have a low yield. Dr. Flores suggested expansion into other at-risk groups, such as Baby Boomers. With respect to other risk groups that could be stigmatized or discriminated against, Dr. Flores recalled her medical school days when some students went directly into underserved communities to provide information and screening services. “We have to think of creative ways of how to reach out to people, not just relying on the usual physician-patient relationship.”
The issue is especially timely because the World Health Organization has declared a target to reduce new hepatitis B infections by 90% by 2030, and that will require addressing gaps in diagnosis. “That’s why these recommendations are so consequential. We are at a critical juncture in terms of global hepatitis elimination efforts. There is a time sensitive need to have multistakeholder engagement in ensuring that all aspects of the care cascade are addressed. Because of the central role of screening and diagnosis, it’s of critical importance that organizations such as USPSTF are in alignment with other organizations that have already issued clear guidance on who should be screened. It is (my) hope that further examination of the evidence-base will further support broadening USPSTF guidance to include a larger group of at-risk individuals, or ideally a universal screening strategy,” said Dr. Lim.
The recommendation’s authors received travel reimbursement for their involvement, and one author reported receiving grants and personal fees from Healthwise. Dr. Flores has no relevant financial disclosures. Dr. Lim is a member of the American Association for the Study of Liver Disease’s Viral Hepatitis Elimination Task Force.
SOURCE: U.S. Preventive Services Task Force. JAMA. 2020 Dec 15. doi: 10.1001/jama.2020.22980.
Updated Jan. 20, 2021
An update of the U.S. Preventive Services Task Force recommendation for hepatitis B screening shows little change from the 2014 version, but some wonder if it should have gone farther than a risk-based approach.
The recommendation, which was published in JAMA, reinforces that screening should be conducted among adolescents and adults who are at increased risk of hepatitis B virus (HBV) infection. The USPSTF named six categories of individuals at increased risk of infection: Persons born in countries with a 2% or higher prevalence of hepatitis B, such as Asia, Africa, the Pacific Islands, and some areas of South America; unvaccinated individuals born in the United States to parents from regions with a very high prevalence of HBV (≥8%); HIV-positive individuals; those who use injected drugs; men who have sex with men; and people who live with people who have HBV or who have HBV-infected sexual partners. It also recommended that pregnant women be screened for HBV infection during their first prenatal visit.
“I view the updated recommendations as an important document because it validates the importance of HBV screening, and the Grade B recommendation supports mandated insurance coverage for the screening test,” said Joseph Lim, MD, who is a professor of medicine at Yale University and director of the Yale Viral Hepatitis Program, both in New Haven, Conn.
Still, the recommendation could have gone further. Notably absent from the USPSTF document, yet featured in recommendations from the Centers for Disease Control and Prevention and the American Association for the Study of Liver Disease, are patients who have diabetes, are on immunosuppressive therapy, or have elevated liver enzymes or liver disease. Furthermore, a single-center study found that, among physicians administering immunosuppressive therapy, a setting in which HBV reactivation is a concern, there were low rates of screening for HBV infection, and the physicians did not reliably identify high-risk patients.
“This may also be viewed as a lost opportunity. Evidence suggests that risk factor–based screening is ineffective for the identification of chronic conditions such as hepatitis B. Risk factor–based screening is difficult to implement across health systems and exacerbates the burden on community-based organizations that are motivated to address viral hepatitis. It may further exacerbate labeling, stigma, and discrimination within already marginalized communities that are deemed to be at high risk,” said Dr. Lim.
A similar view was expressed by Avegail Flores, MD, medical director of liver transplantation at the Michael E. DeBakey Veterans Affairs Medical Center and assistant professor of medicine at Baylor College of Medicine, both in Houston. “This is a good launching point, and with further evidence provided, hopefully it will also bring in a broader conversation about other persons who are at risk but not included in these criteria.” Neither Dr. Lim nor Dr. Flores were involved in the study.
She noted that resistance to universal screening may be caused by the relatively low prevalence of hepatitis B infection in the United States. However, the CDC estimates that only about 61% of people infected with HBV are aware of it. “I don’t think we have done a good job screening those who are at risk,” said Dr. Flores.
Universal screening could help, but would have a low yield. Dr. Flores suggested expansion into other at-risk groups, such as Baby Boomers. With respect to other risk groups that could be stigmatized or discriminated against, Dr. Flores recalled her medical school days when some students went directly into underserved communities to provide information and screening services. “We have to think of creative ways of how to reach out to people, not just relying on the usual physician-patient relationship.”
The issue is especially timely because the World Health Organization has declared a target to reduce new hepatitis B infections by 90% by 2030, and that will require addressing gaps in diagnosis. “That’s why these recommendations are so consequential. We are at a critical juncture in terms of global hepatitis elimination efforts. There is a time sensitive need to have multistakeholder engagement in ensuring that all aspects of the care cascade are addressed. Because of the central role of screening and diagnosis, it’s of critical importance that organizations such as USPSTF are in alignment with other organizations that have already issued clear guidance on who should be screened. It is (my) hope that further examination of the evidence-base will further support broadening USPSTF guidance to include a larger group of at-risk individuals, or ideally a universal screening strategy,” said Dr. Lim.
The recommendation’s authors received travel reimbursement for their involvement, and one author reported receiving grants and personal fees from Healthwise. Dr. Flores has no relevant financial disclosures. Dr. Lim is a member of the American Association for the Study of Liver Disease’s Viral Hepatitis Elimination Task Force.
SOURCE: U.S. Preventive Services Task Force. JAMA. 2020 Dec 15. doi: 10.1001/jama.2020.22980.
Updated Jan. 20, 2021
FROM JAMA
Cloth masks provide inferior protection vs. medical masks, suggests evidence review
according to an evidence review published Jan. 11 in Annals of Family Medicine.
Nevertheless, cloth masks may provide some degree of protection, filtration studies indicate. If clinicians use cloth masks, they should take into account the fit, material, and number of layers, the review authors wrote.
And if cloth masks are used as a last resort, such as during shortages of personal protective equipment (PPE), additional measures may help, such as pairing cloth masks with plastic face shields.
“We recommend frequent cloth mask changes to reduce the risk of moisture retention and washing according to hospital laundry standards to decrease the risk of ineffective cleaning,” review author Ariel Kiyomi Daoud, a researcher at the University of Colorado at Denver, Aurora, and colleagues wrote.
The investigators identified and analyzed nine studies related to cloth masks’ ability to prevent respiratory viral infections among health care clinicians. The studies generally were not specific to SARS-CoV-2. They focused on four nonrandomized trials, three laboratory efficacy studies, one single-case experiment, and one randomized controlled trial.
Filtration and fit
“Seven publications addressed the filtration efficacy of commercial cloth masks or materials used to create homemade masks ... in a laboratory setting,” the researchers wrote. These studies found that cloth materials prevent some level of penetration, but generally have “lesser filtration efficiency and greater variability than medical masks” do.
One study found that the materials with the greatest filtration efficacy – vacuum bags and tea towels – had low airflow, which limits their use.
Two studies found that additional layers may increase the viral filtration efficacy of cloth masks.
Several studies that assessed mask fit and airflow found that cloth masks “have worse fit and a greater level of particle leakage, compared to medical masks,” the authors reported. Most studies did not examine cloth masks’ ability to protect wearers from respiratory droplets or contact, which the World Health Organization consider the primary means of SARS-CoV-2 spread, with aerosols playing a smaller role. “Thus, we must interpret these results with caution in the context of COVID-19,” the authors wrote. “For a primary care clinician without access to medical masks, our qualitative synthesis of the literature suggests that it is better to wear a cloth mask than no mask,” as long as other protective measures are considered along with cloth mask use.
Generally consistent guidance
Agencies and researchers have shared similar recommendations about the use of cloth masks in health care settings.
“Health care workers are at the frontline and they need to be protected,” said Abrar Ahmad Chughtai, MBBS, MPH, PhD, an epidemiologist at University of New South Wales, Sydney, in an interview. “Many studies show that respirators are more effective, compared to medical masks, and medical masks are more effective, compared to cloth masks. So ideally, all frontline health care workers should use respirators. If respirators are not available, then medical masks should be used. Cloth masks are not as effective as medical masks and ideally should not be used in health care settings.”
Dr. Chughtai has written about cloth masks for protection against SARS-CoV-2 and was an investigator for a 2015 randomized trial that compared medical masks and cloth masks in health care workers.
In that trial, which was considered in the review, greater rates of influenza-like illness occurred in the cloth mask arm, compared with the medical mask arm.
“Studies show that three or more layers of cloth may reduce the spread of droplets and aerosols from the wearers,” Dr. Chughtai said. “So, cloth masks may be used in community settings to prevent spread of infections from the sick, particularly asymptomatic, people.”
In addition, cloth masks “may be used by health care workers as a last resort, if no other option is available,” he said. In that case, they should have at least three layers, fit to the face, and be washed regularly.
Not considered PPE
According to routine infection prevention and control recommendations for health care personnel from the Centers for Disease Control and Prevention, face masks – often referred to as surgical masks or procedure masks – should be worn by workers “at all times while they are in the healthcare facility, including in break rooms or other spaces where they might encounter coworkers.”
Unlike cloth masks, face masks offer “protection for the wearer against exposure to splashes and sprays of infectious material from others,” as well as source control, the agency says. Health care personnel “should remove their respirator or face mask, perform hand hygiene, and put on their cloth mask when leaving the facility at the end of their shift,” according to the CDC.
“Cloth masks are NOT PPE and should not be worn for the care of patients with suspected or confirmed COVID-19 or other situations where use of a respirator or face mask is recommended,” the agency notes.
When respirators or face masks are unavailable, health care personnel “might use cloth masks as a last resort for care of patients with suspected or confirmed diagnosis for which face mask or respirator use is normally recommended,” according to CDC guidance.
In that scenario, cloth masks “should ideally be used in combination with a face shield that covers the entire front (that extends to the chin or below) and sides of the face,” the CDC says.
Limited data for comparisons
A Dec. 29, 2020, update in Annals of Internal Medicine about masks for prevention of respiratory virus infections highlighted two recent studies in the United States that reported on mask use in health care settings. A study of more than 16,000 health care workers and first responders found that those who used an N95 or surgical mask all of the time were less likely to have SARS-CoV-2 antibodies, compared with workers who did not wear masks all the time. The adjusted odds ratio with consistent N95 use was 0.83, and the aOR with consistent surgical mask use was 0.86.
In the second study, which included more than 20,000 asymptomatic health care workers, risk for infection was reduced with any mask use versus no mask use (OR, 0.58). An N95 mask was associated with decreased risk versus a surgical mask (OR, 0.76). The studies had methodological limitations, however, and “evidence for various comparisons about mask use in health care settings and risk for SARS-CoV-2 remains insufficient,” the authors of the update wrote.
The Annals of Family Medicine review authors had no relevant disclosures. Dr. Chughtai has tested filtration of 3M masks and worked with CleanSpace Technology to research fit testing of respirators, and the 2015 randomized trial was funded by an Australian Research Council Linkage Grant with 3M as a partner on the grant. The Dec. 29, 2020, update was of a review that originally was supported by grants from the Agency for Healthcare Research Quality.
SOURCE: Daoud AK et al. Ann Fam Med. 2020 Jan 11. doi: 10.1370/afm.2640.
according to an evidence review published Jan. 11 in Annals of Family Medicine.
Nevertheless, cloth masks may provide some degree of protection, filtration studies indicate. If clinicians use cloth masks, they should take into account the fit, material, and number of layers, the review authors wrote.
And if cloth masks are used as a last resort, such as during shortages of personal protective equipment (PPE), additional measures may help, such as pairing cloth masks with plastic face shields.
“We recommend frequent cloth mask changes to reduce the risk of moisture retention and washing according to hospital laundry standards to decrease the risk of ineffective cleaning,” review author Ariel Kiyomi Daoud, a researcher at the University of Colorado at Denver, Aurora, and colleagues wrote.
The investigators identified and analyzed nine studies related to cloth masks’ ability to prevent respiratory viral infections among health care clinicians. The studies generally were not specific to SARS-CoV-2. They focused on four nonrandomized trials, three laboratory efficacy studies, one single-case experiment, and one randomized controlled trial.
Filtration and fit
“Seven publications addressed the filtration efficacy of commercial cloth masks or materials used to create homemade masks ... in a laboratory setting,” the researchers wrote. These studies found that cloth materials prevent some level of penetration, but generally have “lesser filtration efficiency and greater variability than medical masks” do.
One study found that the materials with the greatest filtration efficacy – vacuum bags and tea towels – had low airflow, which limits their use.
Two studies found that additional layers may increase the viral filtration efficacy of cloth masks.
Several studies that assessed mask fit and airflow found that cloth masks “have worse fit and a greater level of particle leakage, compared to medical masks,” the authors reported. Most studies did not examine cloth masks’ ability to protect wearers from respiratory droplets or contact, which the World Health Organization consider the primary means of SARS-CoV-2 spread, with aerosols playing a smaller role. “Thus, we must interpret these results with caution in the context of COVID-19,” the authors wrote. “For a primary care clinician without access to medical masks, our qualitative synthesis of the literature suggests that it is better to wear a cloth mask than no mask,” as long as other protective measures are considered along with cloth mask use.
Generally consistent guidance
Agencies and researchers have shared similar recommendations about the use of cloth masks in health care settings.
“Health care workers are at the frontline and they need to be protected,” said Abrar Ahmad Chughtai, MBBS, MPH, PhD, an epidemiologist at University of New South Wales, Sydney, in an interview. “Many studies show that respirators are more effective, compared to medical masks, and medical masks are more effective, compared to cloth masks. So ideally, all frontline health care workers should use respirators. If respirators are not available, then medical masks should be used. Cloth masks are not as effective as medical masks and ideally should not be used in health care settings.”
Dr. Chughtai has written about cloth masks for protection against SARS-CoV-2 and was an investigator for a 2015 randomized trial that compared medical masks and cloth masks in health care workers.
In that trial, which was considered in the review, greater rates of influenza-like illness occurred in the cloth mask arm, compared with the medical mask arm.
“Studies show that three or more layers of cloth may reduce the spread of droplets and aerosols from the wearers,” Dr. Chughtai said. “So, cloth masks may be used in community settings to prevent spread of infections from the sick, particularly asymptomatic, people.”
In addition, cloth masks “may be used by health care workers as a last resort, if no other option is available,” he said. In that case, they should have at least three layers, fit to the face, and be washed regularly.
Not considered PPE
According to routine infection prevention and control recommendations for health care personnel from the Centers for Disease Control and Prevention, face masks – often referred to as surgical masks or procedure masks – should be worn by workers “at all times while they are in the healthcare facility, including in break rooms or other spaces where they might encounter coworkers.”
Unlike cloth masks, face masks offer “protection for the wearer against exposure to splashes and sprays of infectious material from others,” as well as source control, the agency says. Health care personnel “should remove their respirator or face mask, perform hand hygiene, and put on their cloth mask when leaving the facility at the end of their shift,” according to the CDC.
“Cloth masks are NOT PPE and should not be worn for the care of patients with suspected or confirmed COVID-19 or other situations where use of a respirator or face mask is recommended,” the agency notes.
When respirators or face masks are unavailable, health care personnel “might use cloth masks as a last resort for care of patients with suspected or confirmed diagnosis for which face mask or respirator use is normally recommended,” according to CDC guidance.
In that scenario, cloth masks “should ideally be used in combination with a face shield that covers the entire front (that extends to the chin or below) and sides of the face,” the CDC says.
Limited data for comparisons
A Dec. 29, 2020, update in Annals of Internal Medicine about masks for prevention of respiratory virus infections highlighted two recent studies in the United States that reported on mask use in health care settings. A study of more than 16,000 health care workers and first responders found that those who used an N95 or surgical mask all of the time were less likely to have SARS-CoV-2 antibodies, compared with workers who did not wear masks all the time. The adjusted odds ratio with consistent N95 use was 0.83, and the aOR with consistent surgical mask use was 0.86.
In the second study, which included more than 20,000 asymptomatic health care workers, risk for infection was reduced with any mask use versus no mask use (OR, 0.58). An N95 mask was associated with decreased risk versus a surgical mask (OR, 0.76). The studies had methodological limitations, however, and “evidence for various comparisons about mask use in health care settings and risk for SARS-CoV-2 remains insufficient,” the authors of the update wrote.
The Annals of Family Medicine review authors had no relevant disclosures. Dr. Chughtai has tested filtration of 3M masks and worked with CleanSpace Technology to research fit testing of respirators, and the 2015 randomized trial was funded by an Australian Research Council Linkage Grant with 3M as a partner on the grant. The Dec. 29, 2020, update was of a review that originally was supported by grants from the Agency for Healthcare Research Quality.
SOURCE: Daoud AK et al. Ann Fam Med. 2020 Jan 11. doi: 10.1370/afm.2640.
according to an evidence review published Jan. 11 in Annals of Family Medicine.
Nevertheless, cloth masks may provide some degree of protection, filtration studies indicate. If clinicians use cloth masks, they should take into account the fit, material, and number of layers, the review authors wrote.
And if cloth masks are used as a last resort, such as during shortages of personal protective equipment (PPE), additional measures may help, such as pairing cloth masks with plastic face shields.
“We recommend frequent cloth mask changes to reduce the risk of moisture retention and washing according to hospital laundry standards to decrease the risk of ineffective cleaning,” review author Ariel Kiyomi Daoud, a researcher at the University of Colorado at Denver, Aurora, and colleagues wrote.
The investigators identified and analyzed nine studies related to cloth masks’ ability to prevent respiratory viral infections among health care clinicians. The studies generally were not specific to SARS-CoV-2. They focused on four nonrandomized trials, three laboratory efficacy studies, one single-case experiment, and one randomized controlled trial.
Filtration and fit
“Seven publications addressed the filtration efficacy of commercial cloth masks or materials used to create homemade masks ... in a laboratory setting,” the researchers wrote. These studies found that cloth materials prevent some level of penetration, but generally have “lesser filtration efficiency and greater variability than medical masks” do.
One study found that the materials with the greatest filtration efficacy – vacuum bags and tea towels – had low airflow, which limits their use.
Two studies found that additional layers may increase the viral filtration efficacy of cloth masks.
Several studies that assessed mask fit and airflow found that cloth masks “have worse fit and a greater level of particle leakage, compared to medical masks,” the authors reported. Most studies did not examine cloth masks’ ability to protect wearers from respiratory droplets or contact, which the World Health Organization consider the primary means of SARS-CoV-2 spread, with aerosols playing a smaller role. “Thus, we must interpret these results with caution in the context of COVID-19,” the authors wrote. “For a primary care clinician without access to medical masks, our qualitative synthesis of the literature suggests that it is better to wear a cloth mask than no mask,” as long as other protective measures are considered along with cloth mask use.
Generally consistent guidance
Agencies and researchers have shared similar recommendations about the use of cloth masks in health care settings.
“Health care workers are at the frontline and they need to be protected,” said Abrar Ahmad Chughtai, MBBS, MPH, PhD, an epidemiologist at University of New South Wales, Sydney, in an interview. “Many studies show that respirators are more effective, compared to medical masks, and medical masks are more effective, compared to cloth masks. So ideally, all frontline health care workers should use respirators. If respirators are not available, then medical masks should be used. Cloth masks are not as effective as medical masks and ideally should not be used in health care settings.”
Dr. Chughtai has written about cloth masks for protection against SARS-CoV-2 and was an investigator for a 2015 randomized trial that compared medical masks and cloth masks in health care workers.
In that trial, which was considered in the review, greater rates of influenza-like illness occurred in the cloth mask arm, compared with the medical mask arm.
“Studies show that three or more layers of cloth may reduce the spread of droplets and aerosols from the wearers,” Dr. Chughtai said. “So, cloth masks may be used in community settings to prevent spread of infections from the sick, particularly asymptomatic, people.”
In addition, cloth masks “may be used by health care workers as a last resort, if no other option is available,” he said. In that case, they should have at least three layers, fit to the face, and be washed regularly.
Not considered PPE
According to routine infection prevention and control recommendations for health care personnel from the Centers for Disease Control and Prevention, face masks – often referred to as surgical masks or procedure masks – should be worn by workers “at all times while they are in the healthcare facility, including in break rooms or other spaces where they might encounter coworkers.”
Unlike cloth masks, face masks offer “protection for the wearer against exposure to splashes and sprays of infectious material from others,” as well as source control, the agency says. Health care personnel “should remove their respirator or face mask, perform hand hygiene, and put on their cloth mask when leaving the facility at the end of their shift,” according to the CDC.
“Cloth masks are NOT PPE and should not be worn for the care of patients with suspected or confirmed COVID-19 or other situations where use of a respirator or face mask is recommended,” the agency notes.
When respirators or face masks are unavailable, health care personnel “might use cloth masks as a last resort for care of patients with suspected or confirmed diagnosis for which face mask or respirator use is normally recommended,” according to CDC guidance.
In that scenario, cloth masks “should ideally be used in combination with a face shield that covers the entire front (that extends to the chin or below) and sides of the face,” the CDC says.
Limited data for comparisons
A Dec. 29, 2020, update in Annals of Internal Medicine about masks for prevention of respiratory virus infections highlighted two recent studies in the United States that reported on mask use in health care settings. A study of more than 16,000 health care workers and first responders found that those who used an N95 or surgical mask all of the time were less likely to have SARS-CoV-2 antibodies, compared with workers who did not wear masks all the time. The adjusted odds ratio with consistent N95 use was 0.83, and the aOR with consistent surgical mask use was 0.86.
In the second study, which included more than 20,000 asymptomatic health care workers, risk for infection was reduced with any mask use versus no mask use (OR, 0.58). An N95 mask was associated with decreased risk versus a surgical mask (OR, 0.76). The studies had methodological limitations, however, and “evidence for various comparisons about mask use in health care settings and risk for SARS-CoV-2 remains insufficient,” the authors of the update wrote.
The Annals of Family Medicine review authors had no relevant disclosures. Dr. Chughtai has tested filtration of 3M masks and worked with CleanSpace Technology to research fit testing of respirators, and the 2015 randomized trial was funded by an Australian Research Council Linkage Grant with 3M as a partner on the grant. The Dec. 29, 2020, update was of a review that originally was supported by grants from the Agency for Healthcare Research Quality.
SOURCE: Daoud AK et al. Ann Fam Med. 2020 Jan 11. doi: 10.1370/afm.2640.
FROM ANNALS OF FAMILY MEDICINE
Risk of HPV-related oropharyngeal cancer linked to number of oral sex partners
Having oral sex with more than 10 previous partners was associated with a 4.3 times’ greater likelihood of developing human papillomavirus (HPV)–related oropharyngeal cancer, according to new findings.
The study also found that having more partners in a shorter period (i.e., greater oral sex intensity) and starting oral sex at a younger age were associated with higher odds of having HPV-related cancer of the mouth and throat.
The new study, published online on Jan. 11 in Cancer, confirms previous findings and adds more nuance, say the researchers.
Previous studies have demonstrated that oral sex is a strong risk factor for HPV-related oropharyngeal cancer, which has increased in incidence in recent decades, particularly cancer of the base of the tongue and palatine and lingual tonsils.
“Our research adds more nuance in our understanding of how people acquire oral HPV infection and HPV-related oropharyngeal cancer,” said study author Gypsyamber D’Souza, PhD, professor of epidemiology at the Johns Hopkins Bloomberg School of Public Health, Baltimore. “It suggests that risk of infection is not only from the number of oral sexual partners but that the timing and type of partner also influence risk.”
The results of the study do not change the clinical care or screening of patients, Dr. D’Souza noted, but the study does add context for patients and providers in understanding, “Why did I get HPV-oropharyngeal cancer?” she said.
“We know that people who develop HPV-oropharyngeal cancer have a wide range of sexual histories, but we do not suggest sexual history be used for screening, as many patients have low-risk sexual histories,” she said. “By chance, it only takes one partner who is infected to acquire the infection, while others who have had many partners by chance do not get exposed, or who are exposed but clear the infection.”
Reinforces the need for vaccination
Approached for comment, Joseph Califano, MD, physician-in-chief at the Moores Cancer Center and director of the Head and Neck Cancer Center at the University of California, San Diego, noted that similar data have been published before. The novelty here is in the timing and intensity of oral sex. “It’s not new data, but it certainly reinforces what we knew,” he said in an interview.
These new data are not going to change monitoring, he suggested. “It’s not going to change how we screen, because we don’t do population-based screening for oropharyngeal cancer,” Dr. Califano said.
“It does underline the fact that vaccination is really the key to preventing HPV-mediated cancers,” he said.
He pointed out that some data show lower rates of high-risk oral HPV shedding by children who have been appropriately vaccinated.
“This paper really highlights the fact we need to get people vaccinated early, before sexual debut,” he said. “In this case, sexual debut doesn’t necessarily mean intercourse but oral sex, and that’s a different concept of when sex starts.”
These new data “reinforce the fact that early exposure is what we need to focus on,” he said.
Details of the new findings
The current study by Dr. D’Souza and colleagues included 163 patients with HPV-related oropharyngeal cancer who were enrolled in the Papillomavirus Role in Oral Cancer Viral Etiology (PROVE) study. These patients were compared with 345 matched control persons.
All participants completed a behavioral survey and provided a blood sample. For the patients with cancer, a tumor sample was obtained.
The majority of participants were male (85% and 82%), were aged 50-69 years, were currently married or living with a partner, and identified as heterosexual. Case patients were more likely to report a history of sexually transmitted infection than were control participants (P = .003).
Case patients were more likely to have ever performed oral sex compared to control persons (98.8% vs 90.4%; P < .001) and to have performed oral sex at the time of their sexual debut (33.3% of case patients vs 21.4% of control persons; P = .004; odds ratio [OR], 1.8).
Significantly more case patients than control persons reported starting oral sex before they were 18 years old (37.4% of cases vs. 22.6% of controls; P < .001; OR, 3.1), and they had a greater number of lifetime oral sex partners (44.8% of cases and 19.1% of controls reported having more than 10 partners; P < .001; OR, 4.3).
Intensity of oral sexual exposure, which the authors measured by number of partners per 10 years, was also significantly higher among cases than controls (30.8% vs 11.1%; P < .001; OR, 5.6).
After adjustment for confounders (such as the lifetime number of oral sex partners and tobacco use), ever performing oral sex (adjusted odds ratio [aOR], 4.4), early age of first oral sex encounter (20 years: aOR, 1.8), and oral sex intensity (aOR, 2.8) all remained significantly associated with increased odds of HPV-oropharyngeal cancer.
The type of sexual partner, such as partners who were older (OR, 1.7) and having a partner who engaged in extramarital sex (OR, 1.6), were also associated with increased odds of developing HPV-oropharyngeal cancer. In addition, seropositivity for antibodies to HPV16 E6 (OR, 286) and any HPV16 E protein (E1, E2, E6, E7; OR, 163) were also associated with increased odds of developing the disease.
The study was supported by the National Institute of Dental and Craniofacial Research and the National Institute on Deafness and Other Communication Disorders. Dr. D’Souza and Dr. Califano have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Having oral sex with more than 10 previous partners was associated with a 4.3 times’ greater likelihood of developing human papillomavirus (HPV)–related oropharyngeal cancer, according to new findings.
The study also found that having more partners in a shorter period (i.e., greater oral sex intensity) and starting oral sex at a younger age were associated with higher odds of having HPV-related cancer of the mouth and throat.
The new study, published online on Jan. 11 in Cancer, confirms previous findings and adds more nuance, say the researchers.
Previous studies have demonstrated that oral sex is a strong risk factor for HPV-related oropharyngeal cancer, which has increased in incidence in recent decades, particularly cancer of the base of the tongue and palatine and lingual tonsils.
“Our research adds more nuance in our understanding of how people acquire oral HPV infection and HPV-related oropharyngeal cancer,” said study author Gypsyamber D’Souza, PhD, professor of epidemiology at the Johns Hopkins Bloomberg School of Public Health, Baltimore. “It suggests that risk of infection is not only from the number of oral sexual partners but that the timing and type of partner also influence risk.”
The results of the study do not change the clinical care or screening of patients, Dr. D’Souza noted, but the study does add context for patients and providers in understanding, “Why did I get HPV-oropharyngeal cancer?” she said.
“We know that people who develop HPV-oropharyngeal cancer have a wide range of sexual histories, but we do not suggest sexual history be used for screening, as many patients have low-risk sexual histories,” she said. “By chance, it only takes one partner who is infected to acquire the infection, while others who have had many partners by chance do not get exposed, or who are exposed but clear the infection.”
Reinforces the need for vaccination
Approached for comment, Joseph Califano, MD, physician-in-chief at the Moores Cancer Center and director of the Head and Neck Cancer Center at the University of California, San Diego, noted that similar data have been published before. The novelty here is in the timing and intensity of oral sex. “It’s not new data, but it certainly reinforces what we knew,” he said in an interview.
These new data are not going to change monitoring, he suggested. “It’s not going to change how we screen, because we don’t do population-based screening for oropharyngeal cancer,” Dr. Califano said.
“It does underline the fact that vaccination is really the key to preventing HPV-mediated cancers,” he said.
He pointed out that some data show lower rates of high-risk oral HPV shedding by children who have been appropriately vaccinated.
“This paper really highlights the fact we need to get people vaccinated early, before sexual debut,” he said. “In this case, sexual debut doesn’t necessarily mean intercourse but oral sex, and that’s a different concept of when sex starts.”
These new data “reinforce the fact that early exposure is what we need to focus on,” he said.
Details of the new findings
The current study by Dr. D’Souza and colleagues included 163 patients with HPV-related oropharyngeal cancer who were enrolled in the Papillomavirus Role in Oral Cancer Viral Etiology (PROVE) study. These patients were compared with 345 matched control persons.
All participants completed a behavioral survey and provided a blood sample. For the patients with cancer, a tumor sample was obtained.
The majority of participants were male (85% and 82%), were aged 50-69 years, were currently married or living with a partner, and identified as heterosexual. Case patients were more likely to report a history of sexually transmitted infection than were control participants (P = .003).
Case patients were more likely to have ever performed oral sex compared to control persons (98.8% vs 90.4%; P < .001) and to have performed oral sex at the time of their sexual debut (33.3% of case patients vs 21.4% of control persons; P = .004; odds ratio [OR], 1.8).
Significantly more case patients than control persons reported starting oral sex before they were 18 years old (37.4% of cases vs. 22.6% of controls; P < .001; OR, 3.1), and they had a greater number of lifetime oral sex partners (44.8% of cases and 19.1% of controls reported having more than 10 partners; P < .001; OR, 4.3).
Intensity of oral sexual exposure, which the authors measured by number of partners per 10 years, was also significantly higher among cases than controls (30.8% vs 11.1%; P < .001; OR, 5.6).
After adjustment for confounders (such as the lifetime number of oral sex partners and tobacco use), ever performing oral sex (adjusted odds ratio [aOR], 4.4), early age of first oral sex encounter (20 years: aOR, 1.8), and oral sex intensity (aOR, 2.8) all remained significantly associated with increased odds of HPV-oropharyngeal cancer.
The type of sexual partner, such as partners who were older (OR, 1.7) and having a partner who engaged in extramarital sex (OR, 1.6), were also associated with increased odds of developing HPV-oropharyngeal cancer. In addition, seropositivity for antibodies to HPV16 E6 (OR, 286) and any HPV16 E protein (E1, E2, E6, E7; OR, 163) were also associated with increased odds of developing the disease.
The study was supported by the National Institute of Dental and Craniofacial Research and the National Institute on Deafness and Other Communication Disorders. Dr. D’Souza and Dr. Califano have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Having oral sex with more than 10 previous partners was associated with a 4.3 times’ greater likelihood of developing human papillomavirus (HPV)–related oropharyngeal cancer, according to new findings.
The study also found that having more partners in a shorter period (i.e., greater oral sex intensity) and starting oral sex at a younger age were associated with higher odds of having HPV-related cancer of the mouth and throat.
The new study, published online on Jan. 11 in Cancer, confirms previous findings and adds more nuance, say the researchers.
Previous studies have demonstrated that oral sex is a strong risk factor for HPV-related oropharyngeal cancer, which has increased in incidence in recent decades, particularly cancer of the base of the tongue and palatine and lingual tonsils.
“Our research adds more nuance in our understanding of how people acquire oral HPV infection and HPV-related oropharyngeal cancer,” said study author Gypsyamber D’Souza, PhD, professor of epidemiology at the Johns Hopkins Bloomberg School of Public Health, Baltimore. “It suggests that risk of infection is not only from the number of oral sexual partners but that the timing and type of partner also influence risk.”
The results of the study do not change the clinical care or screening of patients, Dr. D’Souza noted, but the study does add context for patients and providers in understanding, “Why did I get HPV-oropharyngeal cancer?” she said.
“We know that people who develop HPV-oropharyngeal cancer have a wide range of sexual histories, but we do not suggest sexual history be used for screening, as many patients have low-risk sexual histories,” she said. “By chance, it only takes one partner who is infected to acquire the infection, while others who have had many partners by chance do not get exposed, or who are exposed but clear the infection.”
Reinforces the need for vaccination
Approached for comment, Joseph Califano, MD, physician-in-chief at the Moores Cancer Center and director of the Head and Neck Cancer Center at the University of California, San Diego, noted that similar data have been published before. The novelty here is in the timing and intensity of oral sex. “It’s not new data, but it certainly reinforces what we knew,” he said in an interview.
These new data are not going to change monitoring, he suggested. “It’s not going to change how we screen, because we don’t do population-based screening for oropharyngeal cancer,” Dr. Califano said.
“It does underline the fact that vaccination is really the key to preventing HPV-mediated cancers,” he said.
He pointed out that some data show lower rates of high-risk oral HPV shedding by children who have been appropriately vaccinated.
“This paper really highlights the fact we need to get people vaccinated early, before sexual debut,” he said. “In this case, sexual debut doesn’t necessarily mean intercourse but oral sex, and that’s a different concept of when sex starts.”
These new data “reinforce the fact that early exposure is what we need to focus on,” he said.
Details of the new findings
The current study by Dr. D’Souza and colleagues included 163 patients with HPV-related oropharyngeal cancer who were enrolled in the Papillomavirus Role in Oral Cancer Viral Etiology (PROVE) study. These patients were compared with 345 matched control persons.
All participants completed a behavioral survey and provided a blood sample. For the patients with cancer, a tumor sample was obtained.
The majority of participants were male (85% and 82%), were aged 50-69 years, were currently married or living with a partner, and identified as heterosexual. Case patients were more likely to report a history of sexually transmitted infection than were control participants (P = .003).
Case patients were more likely to have ever performed oral sex compared to control persons (98.8% vs 90.4%; P < .001) and to have performed oral sex at the time of their sexual debut (33.3% of case patients vs 21.4% of control persons; P = .004; odds ratio [OR], 1.8).
Significantly more case patients than control persons reported starting oral sex before they were 18 years old (37.4% of cases vs. 22.6% of controls; P < .001; OR, 3.1), and they had a greater number of lifetime oral sex partners (44.8% of cases and 19.1% of controls reported having more than 10 partners; P < .001; OR, 4.3).
Intensity of oral sexual exposure, which the authors measured by number of partners per 10 years, was also significantly higher among cases than controls (30.8% vs 11.1%; P < .001; OR, 5.6).
After adjustment for confounders (such as the lifetime number of oral sex partners and tobacco use), ever performing oral sex (adjusted odds ratio [aOR], 4.4), early age of first oral sex encounter (20 years: aOR, 1.8), and oral sex intensity (aOR, 2.8) all remained significantly associated with increased odds of HPV-oropharyngeal cancer.
The type of sexual partner, such as partners who were older (OR, 1.7) and having a partner who engaged in extramarital sex (OR, 1.6), were also associated with increased odds of developing HPV-oropharyngeal cancer. In addition, seropositivity for antibodies to HPV16 E6 (OR, 286) and any HPV16 E protein (E1, E2, E6, E7; OR, 163) were also associated with increased odds of developing the disease.
The study was supported by the National Institute of Dental and Craniofacial Research and the National Institute on Deafness and Other Communication Disorders. Dr. D’Souza and Dr. Califano have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Long-Term Successful Treatment of Indolent Systemic Mastocytosis With Omalizumab
This case study suggests that omalizumab may help prevent anaphylaxis and reduce disease burden associated with systemic mastocytosis, but further studies and formal clinical trials are needed to confirm these findings.
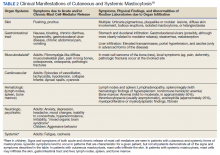
Mastocytosis is a rare disease that causes allergic and anaphylactic symptoms due to chronic or episodic, excessive mast cell degranulation as well as mast cell infiltration of the skin or other organs.1 Mast cells aid in innate immunity by generation of a vasodilatory and inflammatory response and are significant contributors to allergic reactions. Cutaneous mastocytosis is defined by isolated skin involvement. Systemic mastocytosis (SM) is characterized by mast cell infiltration of extracutaneous organs, most often bone marrow.2
Background
SM is divided into distinct subtypes (Table 1). Nonadvanced SM subtypes include indolent SM and smoldering SM. These are the most common forms and tend to have more slowly progressing courses without evidence of organ tissue dysfunction, a myelodysplastic syndrome, or of a myeloproliferative disorder.3 Advanced SM is less common and is associated with organ tissue dysfunction. It also may be associated with myeloproliferative, myelodysplastic, or lymphoproliferative hematologic neoplasms, and subtypes include aggressive SM, SM with an associated hematologic neoplasm, and mast cell leukemia (Table 2).4
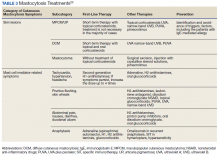
Treatment options approved by the US Food and Drug Administration (FDA) for advanced SM include disease-altering medications, such as tyrosine kinase inhibitors (eg, imatinib), but the approved treatment options for nonadvanced SM are generally aimed at managing only symptoms (Table 3). Although not approved by the FDA for the treatment of SM, omalizumab may aid in the prevention of anaphylaxis, the reduction of disease burden, and the improvement in quality of life for patients with SM.5 Omalizumab is a humanized monoclonal antibody against the Fc portion of immunoglobulin E (IgE). It is approved by the FDA for treatment of asthma as well as chronic idiopathic urticaria.6
Case Presentation
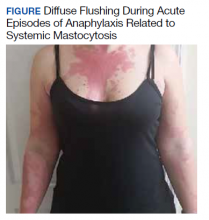
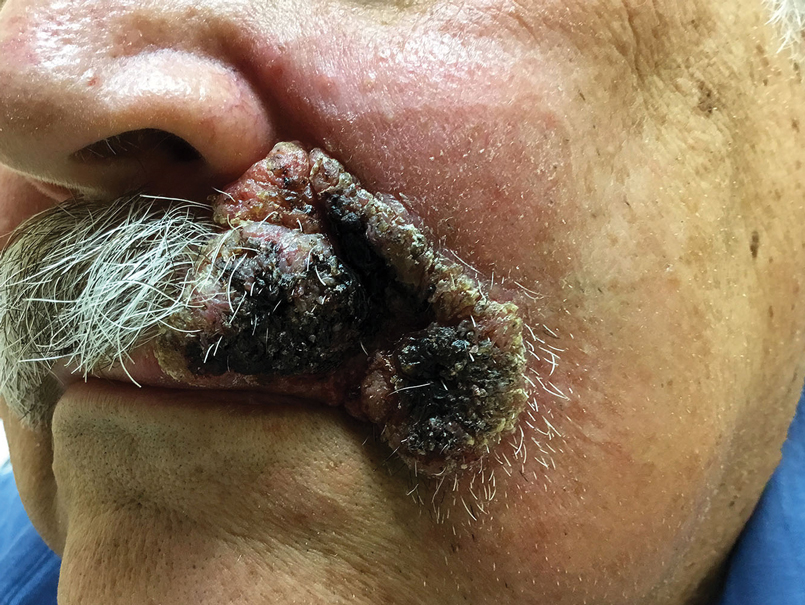
A 32-year-old female initially presented to Womack Army Medical Center at Fort Bragg, North Carolina, for evaluation due to recurrent episodes of anaphylaxis occurring 1 to 2 times per month as well as chronic skin rashes that progressed over the previous 5 years (Figure). She initially was diagnosed with idiopathic anaphylaxis and subsequently had multiple emergency department (ED) and clinic visits for vasovagal syncope, unexplained allergic reactions, dizziness, giddiness, and shortness of breath. More recently, she was diagnosed with idiopathic urticaria.
The patient reported at least 12 episodes in the previous year involving facial flushing that proceeded inferiorly, chest tightness, shortness of breath, labored breathing, crampy abdominal pain, and nausea without urticaria or significant pruritus. These bouts often were accompanied by mild facial angioedema, acute sinus pressure, vomiting, tachycardia, and lightheadedness. She reported experiencing brief losses of consciousness with at least 4 of these episodes. Home and ED blood pressure measurements revealed hypotension on several occasions with systolic readings in the 80s. She also developed nonpruritic freckles on her upper chest initially with subsequent increase in number and spread to involve her entire trunk, proximal extremities, and eventually distal extremities.
The patient had received intramuscular epinephrine several times, which led to rapid resolution of her symptoms. Intensive care unit admission for observation overnight was deemed necessary following one of her first episodes, but she did not require intubation or vasopressor support. Eventually, she began treating most episodes at home with diphenhydramine, ranitidine, and occasionally an epinephrine auto-injector, only presenting to the ED for severe dyspnea or loss of consciousness. Some episodes awoke her from sleeping but no triggers were identified (eg, foods, alcohol, supplements, medications, insect stings, latex exposure, exercise, strong emotions, or menstrual cycle).
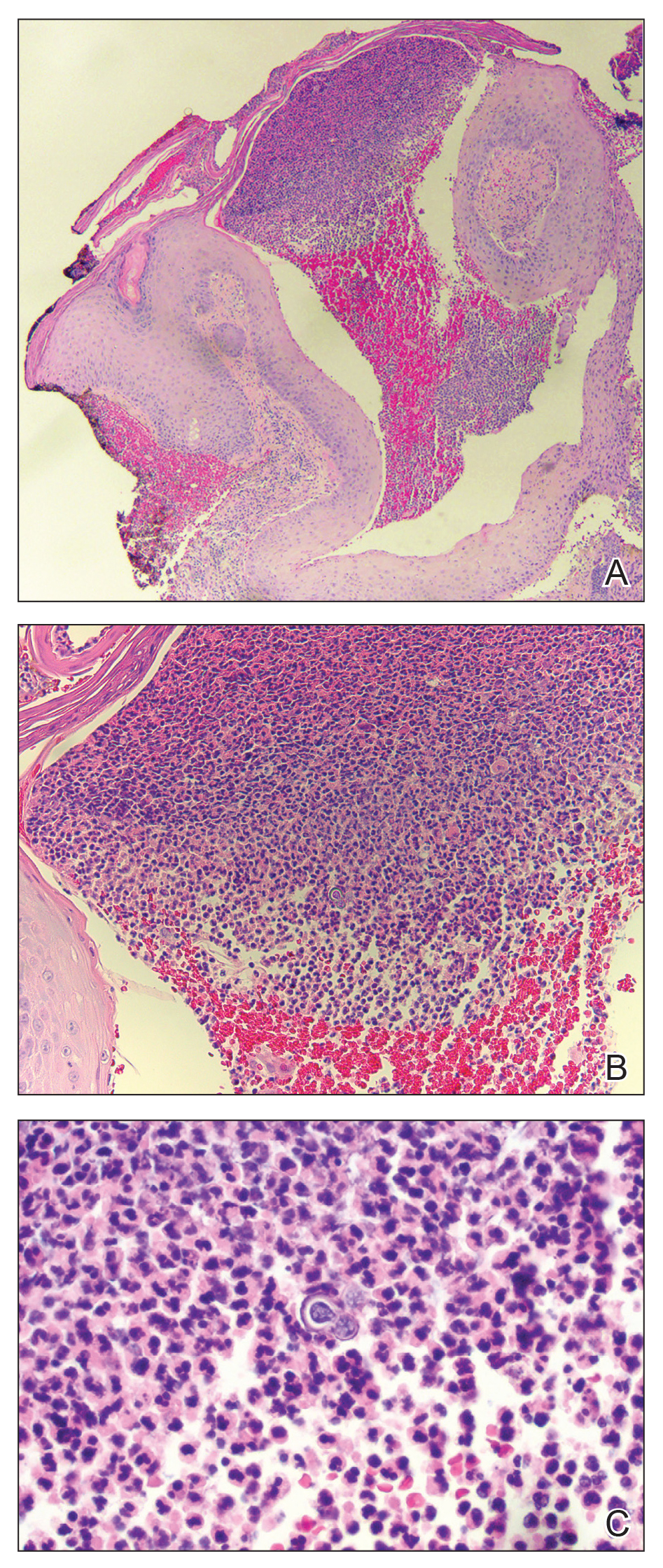
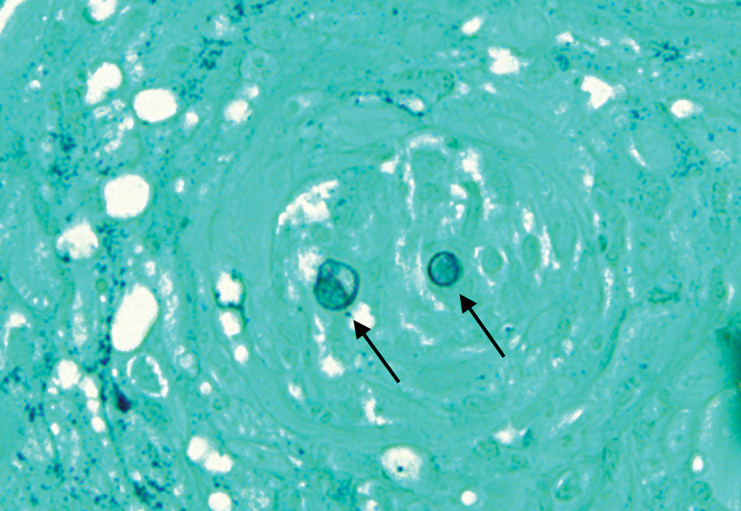
Examination revealed hyperpigmented macules and papules scattered on the trunk and extremities, with a positive Darier sign. Punch biopsy of one of the macules revealed focal basal cell hyperpigmentation and sheets of benign-appearing mast cells in the superficial dermis, highlighted by CD117 immunohistochemical stain. A serum tryptase level was obtained and found to be significantly elevated (134 mcg/L). The patient was diagnosed with maculopapular cutaneous mastocytosis (urticaria pigmentosa).
A bone marrow biopsy revealed multiple prominent infiltrates of monomorphic, spindled, CD117-positive, CD2-positive, and CD25-positive mast cells arranged interstitially and paratrabecularly, with associated reticulin fibrosis. Indolent SM was diagnosed according to the World Health Organization classification system with multifocal, dense aggregates of mast cells (> 25%) in the bone marrow and with persistently elevated serum tryptase levels (134, 134, 151, and 159 ng/mL) without laboratory evidence of an associated clonal myeloid disorder or findings consistent with infiltrating bone lesions on full body magnetic resonance imaging scan.4
Despite maximal antihistamine and antileukotriene therapy with ranitidine (150 mg twice daily), cetirizine (10 mg twice daily), montelukast (10 mg daily), and cromolyn sodium (200 mg daily), the patient continued to experience recurrent episodes of anaphylaxis requiring subcutaneous epinephrine and systemic corticosteroids. In May 2016, the patient began a trial of off-label therapy with omalizumab injections (300 mg subcutaneous every 4 weeks). She has continued on therapy for more than 4 years and experienced only 1 anaphylactic episode. She also has had significant improvement in cutaneous symptoms.
Discussion
Mast cell overactivation and degranulation in mastocytosis is largely driven by the IgE antibody, which plays a significant role in atopic conditions, immediate hypersensitivity reactions, and anaphylaxis, as well as in the immunologic response to parasitic infections. The severity of atopic disease seems to be associated with serum IgE levels in many patients.7 IgE binding to surface receptors on mast cells and eosinophils prompts the release of toxic mediators, incites inflammation, and induces allergic symptoms.8 Activation of mast cells is classically elicited by IgE binding to the high-affinity Fcε RI receptor, the expression of which correlates with IgE levels.9
The anti-IgE, recombinant, humanized immunoglobulin G monoclonal antibody, omalizumab, decreases mastocytic and eosinophilic symptoms by binding and inhibiting IgE. This diminishes free IgE levels, inhibits IgE binding to the Fcε RI receptor, and affects downregulation of this high-affinity receptor on mast cells and basophils.6 Omalizumab is currently FDA approved only for the treatment of moderate-to-severe, persistent, allergic asthma that is not controlled by inhaled corticosteroids in patients aged ≥ 6 years, and for chronic idiopathic urticaria not controlled by H1 antihistamine therapy in patients aged ≥ 12 years.10 However, it stands to reason that this therapy also should be effective in the treatment of other poorly controlled atopic conditions, especially mastocytosis, the symptoms of which are driven by excessive mast cell degranulation and tissue infiltration.
As early as 2007, preliminary data showed that treatment with omalizumab could decrease the frequency of episodes of anaphylaxis.11 A National Institutes of Health case report followed 2 patients, one for 5 months and the other for 24 months. Both patients experienced a decrease in frequency of anaphylaxis following initiation of omalizumab. In 2010, a second case report described the treatment of an Australian patient with recurrent idiopathic anaphylaxis also diagnosed with SM. After initiation of treatment with omalizumab, she, too, experienced decreased frequency of episodes of anaphylaxis over 14 months.12 A review of patients treated at the Mastocytosis Centre Odense University Hospital in Denmark was published in 2017. Of 13 patients with SM treated with omalizumab, 5 experienced what was considered a complete response to the medication, with 3 each experiencing major and partial responses.5 The median treatment time in these patients was 27 months. Each of these cases showed significant promise in the use of omalizumab to treat SM, informing the decision to attempt this treatment in our patient.
The potential positive effects of omalizumab in reducing symptom severity in patients with SM was further supported by a 2017 meta-analysis. This review included several individual case reports noting that omalizumab could decrease frequency of pulmonary and gastrointestinal manifestations of SM.13 A small randomized control trial of omalizumab for treatment of mild symptoms of SM found improvement in disease severity, although neither primary nor secondary endpoints reached statistical significance.14
This case demonstrates a substantial, long-term, clinical benefit and quality of life improvement with omalizumab therapy in a patient with indolent SM that was not adequately controlled by conventional therapies. This is evidenced by an impressive decline in the frequency of mastocytic anaphylactic episodes as well as diminished patient-endorsed cutaneous symptoms.
This case provides further evidence of the efficacy of this therapy in diminishing disease burden for patients with SM who are otherwise limited to treatments aimed at transient symptomatic relief without significant alteration of the underlying cause of symptoms. At the time this article was written, our patient had now 52 months of continuous treatment without any adverse reactions noted, suggesting the treatment's long-term efficacy. It also adds to a small but growing body of literature that supports the use of anti-IgE therapy as a treatment option for improved management of this distressing, life-altering illness. Even in the time that our patient has been receiving omalizumab for SM, another small case series of 2 patients has been published showing sustained treatment effect at 12 years of therapy.15 This adds further insight that omalizumab can offer long-term, safe treatment for this limiting condition.
Omalizumab therapy is not without risk, but for patients afflicted by unrestrained mastocytic disease, the benefits may outweigh the risks. The most common significant risk with this medication is anaphylaxis, occurring in 1 to 2 per 1,000 patients, usually within 2 hours of an injection.16 This may correlate to the underlying degree of atopy in patients receiving omalizumab, and the risk of anaphylaxis is relatively low compared with that of many other biologic medications.17 Additionally, early data from initial phases of clinical trials indicated a potentially elevated malignancy risk with omalizumab. However, subsequent pooled analysis of larger numbers of patients has decreased suspicion that a causal relationship exists.18
Conclusions
Omalizumab has proven value in the treatment of atopic conditions, such as asthma and idiopathic urticaria, for which it has been approved for use by the FDA. Its effectiveness in significantly decreasing free serum IgE levels, and inhibiting IgE activation of mast cells makes it a possible treatment option for patients with SM who are not sufficiently controlled with conventional therapy. The findings in this case suggest that omalizumab may be effective in the prevention of anaphylaxis and in the reduction of disease burden associated with SM. Further studies and formal clinical trials are needed to confirm these findings. Patients should be counseled appropriately concerning the risks, benefits, and off-label status of this treatment option.
1. Theoharides TC, Valent P, Akin C. Mast cells, mastocytosis, and related disorders. N Engl J Med. 2015;373(2):163-172. doi:10.1056/NEJMra1409760
2. Valent P, Sperr WR, Schwartz LB, Horny H-P. Diagnosis and classification of mast cell proliferative disorders: delineation from immunologic diseases and non-mast cell hematopoietic neoplasms. J Allergy Clin Immunol. 2004;114(1):3-11. doi:10.1016/j.jaci.2004.02.045
3. Valent P, Sotlar K, Sperr WR, et al. Refined diagnostic criteria and classification of mast cell leukemia (MCL) and myelomastocytic leukemia (MML): a consensus proposal. Ann Oncol. 2014;25(9):1691-1700. doi:10.1093/annonc/mdu047
4. Valent P, Akin C, Metcalfe DD. Mastocytosis: 2016 updated WHO classification and novel emerging treatment concepts. Blood. 2017;129(11):1420-1427. doi:10.1182/blood-2016-09-731893
5. Broesby-Olsen S, Vestergaard H, Mortz CG, et al. Omalizumab prevents anaphylaxis and improves symptoms in systemic mastocytosis: Efficacy and safety observations. 2018;73(1):230-238. doi:10.1111/all.13237
6. Kaplan AP, Giménez-Arnau AM, Saini SS.Mechanisms of action that contribute to efficacy of omalizumab in chronic spontaneous urticaria. Allergy. 2017;72(4):519-533. doi:10.1111/all.13083
7. Borish L, Chipps B, Deniz Y, Gujrathi S, Zheng B, Dolan C; TENOR Study Group. Total serum IgE levels in a large cohort of patients with severe or difficult-to-treat asthma. Ann Allergy Asthma Immunol. 2005;95(3):247-253. doi:10.1016/S1081-1206(10)61221-5
8. Corry DB, Kheradmand F. Induction and regulation of the IgE response. Nature. 1999;402(suppl 6760):18-23. doi:10.1038/35037014
9. MacGlashan D, McKenzie-White J, Chichester K, et al. In vitro regulation of FcRIα expression on human basophils by IgE antibody. Blood. 1998;91(5):1633-1643.
10. XOLAIR [package insert]. East Hanover, NJ: Novartis Pharmaceuticals Corporation. Revised 2019. Accessed November 11, 2020. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/103976s5234lbl.pdf
11. Carter MC, Robyn JA, Bressler PB, Walker JC, Shapiro GC, and Metcalfe DD. Omalizumab for the treatment of unprovoked anaphylaxis in patients with systemic mastocytosis. J Allergy Clin Immunol. 2007;119(6):1550-1551. doi:10.1016/j.jaci.2007.03.032
12. Douglass JA, Carroll K, Voskamp A, Bourke P, Wei A, O’Hehir RE. Omalizumab is effective in treating systemic mastocytosis in a nonatopic patient. Allergy. 2010; 65(7):926-927. doi:10.1111/j.1398-9995.2009.02259.x
13. Le M, Miedzybrodzki B, Olynych T, Chapdelaine H, Ben-Shoshan M. Natural history and treatment of cutaneous and systemic mastocytosis. Postgrad Med. 2017;129(8):896-901. doi:10.1080/00325481.2017.1364124
14. Distler M, Maul J-T, Steiner T, et al. Efficacy of omalizumab in mastocytosis: allusive indication obtained from a prospective, double-blind, multicenter study (XOLMA Study) [published online ahead of print January 20, 2020]. Dermatology. doi:10.1159/000504842
15. Constantine G, Bressler P, Petroni D, Metcalfe D, Carter M. Twelve-year follow-up of omalizumab for anaphylaxis in 2 patients with systemic mastocytosis. J Allergy Clin Immunol Pract. 2019;7(4)1314-1316. doi:10.1016/j.jaip.2018.07.041
16. Fanta CH. Asthma. N Engl J Med. 2009;360(10):1002-1014. doi:10.1056/NEJMra0804579
17. Baldo BA. Adverse events to monoclonal antibodies used for cancer therapy: focus on hypersensitivity responses. Oncoimmunology. 2013;2(10):e26333. doi:10.4161/onci.26333
18. Busse W, Buhl R, Fernandez Vidaurre C, et al. Omalizumab and the risk of malignancy: results from a pooled analysis. J Allergy Clin Immunol. 2012;129(4):983-989.e6. doi:10.1016/j.jaci.2012.01.033.
19. Castells M, Akin C. Mastocytosis (cutaneous and systemic): epidemiology, pathogenesis, and clinical manifestations. Accessed December 8, 2020. Updated June 12, 2018. https://www.uptodate.com/contents/mastocytosis-cutaneous-and-systemic-epidemiology-pathogenesis-and-clinical-manifestations
20. Czarny J, Lange M, Lugowska-Umer H, Nowicki R. Cutaneous mastocytosis treatment: strategies, limitations, and perspectives. Postepy Dermatol Alergol. 2018;35(6):541-545. doi:10.5114/ada.2018.77605
This case study suggests that omalizumab may help prevent anaphylaxis and reduce disease burden associated with systemic mastocytosis, but further studies and formal clinical trials are needed to confirm these findings.
This case study suggests that omalizumab may help prevent anaphylaxis and reduce disease burden associated with systemic mastocytosis, but further studies and formal clinical trials are needed to confirm these findings.
Mastocytosis is a rare disease that causes allergic and anaphylactic symptoms due to chronic or episodic, excessive mast cell degranulation as well as mast cell infiltration of the skin or other organs.1 Mast cells aid in innate immunity by generation of a vasodilatory and inflammatory response and are significant contributors to allergic reactions. Cutaneous mastocytosis is defined by isolated skin involvement. Systemic mastocytosis (SM) is characterized by mast cell infiltration of extracutaneous organs, most often bone marrow.2
Background
SM is divided into distinct subtypes (Table 1). Nonadvanced SM subtypes include indolent SM and smoldering SM. These are the most common forms and tend to have more slowly progressing courses without evidence of organ tissue dysfunction, a myelodysplastic syndrome, or of a myeloproliferative disorder.3 Advanced SM is less common and is associated with organ tissue dysfunction. It also may be associated with myeloproliferative, myelodysplastic, or lymphoproliferative hematologic neoplasms, and subtypes include aggressive SM, SM with an associated hematologic neoplasm, and mast cell leukemia (Table 2).4
Treatment options approved by the US Food and Drug Administration (FDA) for advanced SM include disease-altering medications, such as tyrosine kinase inhibitors (eg, imatinib), but the approved treatment options for nonadvanced SM are generally aimed at managing only symptoms (Table 3). Although not approved by the FDA for the treatment of SM, omalizumab may aid in the prevention of anaphylaxis, the reduction of disease burden, and the improvement in quality of life for patients with SM.5 Omalizumab is a humanized monoclonal antibody against the Fc portion of immunoglobulin E (IgE). It is approved by the FDA for treatment of asthma as well as chronic idiopathic urticaria.6
Case Presentation
A 32-year-old female initially presented to Womack Army Medical Center at Fort Bragg, North Carolina, for evaluation due to recurrent episodes of anaphylaxis occurring 1 to 2 times per month as well as chronic skin rashes that progressed over the previous 5 years (Figure). She initially was diagnosed with idiopathic anaphylaxis and subsequently had multiple emergency department (ED) and clinic visits for vasovagal syncope, unexplained allergic reactions, dizziness, giddiness, and shortness of breath. More recently, she was diagnosed with idiopathic urticaria.
The patient reported at least 12 episodes in the previous year involving facial flushing that proceeded inferiorly, chest tightness, shortness of breath, labored breathing, crampy abdominal pain, and nausea without urticaria or significant pruritus. These bouts often were accompanied by mild facial angioedema, acute sinus pressure, vomiting, tachycardia, and lightheadedness. She reported experiencing brief losses of consciousness with at least 4 of these episodes. Home and ED blood pressure measurements revealed hypotension on several occasions with systolic readings in the 80s. She also developed nonpruritic freckles on her upper chest initially with subsequent increase in number and spread to involve her entire trunk, proximal extremities, and eventually distal extremities.
The patient had received intramuscular epinephrine several times, which led to rapid resolution of her symptoms. Intensive care unit admission for observation overnight was deemed necessary following one of her first episodes, but she did not require intubation or vasopressor support. Eventually, she began treating most episodes at home with diphenhydramine, ranitidine, and occasionally an epinephrine auto-injector, only presenting to the ED for severe dyspnea or loss of consciousness. Some episodes awoke her from sleeping but no triggers were identified (eg, foods, alcohol, supplements, medications, insect stings, latex exposure, exercise, strong emotions, or menstrual cycle).
Examination revealed hyperpigmented macules and papules scattered on the trunk and extremities, with a positive Darier sign. Punch biopsy of one of the macules revealed focal basal cell hyperpigmentation and sheets of benign-appearing mast cells in the superficial dermis, highlighted by CD117 immunohistochemical stain. A serum tryptase level was obtained and found to be significantly elevated (134 mcg/L). The patient was diagnosed with maculopapular cutaneous mastocytosis (urticaria pigmentosa).
A bone marrow biopsy revealed multiple prominent infiltrates of monomorphic, spindled, CD117-positive, CD2-positive, and CD25-positive mast cells arranged interstitially and paratrabecularly, with associated reticulin fibrosis. Indolent SM was diagnosed according to the World Health Organization classification system with multifocal, dense aggregates of mast cells (> 25%) in the bone marrow and with persistently elevated serum tryptase levels (134, 134, 151, and 159 ng/mL) without laboratory evidence of an associated clonal myeloid disorder or findings consistent with infiltrating bone lesions on full body magnetic resonance imaging scan.4
Despite maximal antihistamine and antileukotriene therapy with ranitidine (150 mg twice daily), cetirizine (10 mg twice daily), montelukast (10 mg daily), and cromolyn sodium (200 mg daily), the patient continued to experience recurrent episodes of anaphylaxis requiring subcutaneous epinephrine and systemic corticosteroids. In May 2016, the patient began a trial of off-label therapy with omalizumab injections (300 mg subcutaneous every 4 weeks). She has continued on therapy for more than 4 years and experienced only 1 anaphylactic episode. She also has had significant improvement in cutaneous symptoms.
Discussion
Mast cell overactivation and degranulation in mastocytosis is largely driven by the IgE antibody, which plays a significant role in atopic conditions, immediate hypersensitivity reactions, and anaphylaxis, as well as in the immunologic response to parasitic infections. The severity of atopic disease seems to be associated with serum IgE levels in many patients.7 IgE binding to surface receptors on mast cells and eosinophils prompts the release of toxic mediators, incites inflammation, and induces allergic symptoms.8 Activation of mast cells is classically elicited by IgE binding to the high-affinity Fcε RI receptor, the expression of which correlates with IgE levels.9
The anti-IgE, recombinant, humanized immunoglobulin G monoclonal antibody, omalizumab, decreases mastocytic and eosinophilic symptoms by binding and inhibiting IgE. This diminishes free IgE levels, inhibits IgE binding to the Fcε RI receptor, and affects downregulation of this high-affinity receptor on mast cells and basophils.6 Omalizumab is currently FDA approved only for the treatment of moderate-to-severe, persistent, allergic asthma that is not controlled by inhaled corticosteroids in patients aged ≥ 6 years, and for chronic idiopathic urticaria not controlled by H1 antihistamine therapy in patients aged ≥ 12 years.10 However, it stands to reason that this therapy also should be effective in the treatment of other poorly controlled atopic conditions, especially mastocytosis, the symptoms of which are driven by excessive mast cell degranulation and tissue infiltration.
As early as 2007, preliminary data showed that treatment with omalizumab could decrease the frequency of episodes of anaphylaxis.11 A National Institutes of Health case report followed 2 patients, one for 5 months and the other for 24 months. Both patients experienced a decrease in frequency of anaphylaxis following initiation of omalizumab. In 2010, a second case report described the treatment of an Australian patient with recurrent idiopathic anaphylaxis also diagnosed with SM. After initiation of treatment with omalizumab, she, too, experienced decreased frequency of episodes of anaphylaxis over 14 months.12 A review of patients treated at the Mastocytosis Centre Odense University Hospital in Denmark was published in 2017. Of 13 patients with SM treated with omalizumab, 5 experienced what was considered a complete response to the medication, with 3 each experiencing major and partial responses.5 The median treatment time in these patients was 27 months. Each of these cases showed significant promise in the use of omalizumab to treat SM, informing the decision to attempt this treatment in our patient.
The potential positive effects of omalizumab in reducing symptom severity in patients with SM was further supported by a 2017 meta-analysis. This review included several individual case reports noting that omalizumab could decrease frequency of pulmonary and gastrointestinal manifestations of SM.13 A small randomized control trial of omalizumab for treatment of mild symptoms of SM found improvement in disease severity, although neither primary nor secondary endpoints reached statistical significance.14
This case demonstrates a substantial, long-term, clinical benefit and quality of life improvement with omalizumab therapy in a patient with indolent SM that was not adequately controlled by conventional therapies. This is evidenced by an impressive decline in the frequency of mastocytic anaphylactic episodes as well as diminished patient-endorsed cutaneous symptoms.
This case provides further evidence of the efficacy of this therapy in diminishing disease burden for patients with SM who are otherwise limited to treatments aimed at transient symptomatic relief without significant alteration of the underlying cause of symptoms. At the time this article was written, our patient had now 52 months of continuous treatment without any adverse reactions noted, suggesting the treatment's long-term efficacy. It also adds to a small but growing body of literature that supports the use of anti-IgE therapy as a treatment option for improved management of this distressing, life-altering illness. Even in the time that our patient has been receiving omalizumab for SM, another small case series of 2 patients has been published showing sustained treatment effect at 12 years of therapy.15 This adds further insight that omalizumab can offer long-term, safe treatment for this limiting condition.
Omalizumab therapy is not without risk, but for patients afflicted by unrestrained mastocytic disease, the benefits may outweigh the risks. The most common significant risk with this medication is anaphylaxis, occurring in 1 to 2 per 1,000 patients, usually within 2 hours of an injection.16 This may correlate to the underlying degree of atopy in patients receiving omalizumab, and the risk of anaphylaxis is relatively low compared with that of many other biologic medications.17 Additionally, early data from initial phases of clinical trials indicated a potentially elevated malignancy risk with omalizumab. However, subsequent pooled analysis of larger numbers of patients has decreased suspicion that a causal relationship exists.18
Conclusions
Omalizumab has proven value in the treatment of atopic conditions, such as asthma and idiopathic urticaria, for which it has been approved for use by the FDA. Its effectiveness in significantly decreasing free serum IgE levels, and inhibiting IgE activation of mast cells makes it a possible treatment option for patients with SM who are not sufficiently controlled with conventional therapy. The findings in this case suggest that omalizumab may be effective in the prevention of anaphylaxis and in the reduction of disease burden associated with SM. Further studies and formal clinical trials are needed to confirm these findings. Patients should be counseled appropriately concerning the risks, benefits, and off-label status of this treatment option.
Mastocytosis is a rare disease that causes allergic and anaphylactic symptoms due to chronic or episodic, excessive mast cell degranulation as well as mast cell infiltration of the skin or other organs.1 Mast cells aid in innate immunity by generation of a vasodilatory and inflammatory response and are significant contributors to allergic reactions. Cutaneous mastocytosis is defined by isolated skin involvement. Systemic mastocytosis (SM) is characterized by mast cell infiltration of extracutaneous organs, most often bone marrow.2
Background
SM is divided into distinct subtypes (Table 1). Nonadvanced SM subtypes include indolent SM and smoldering SM. These are the most common forms and tend to have more slowly progressing courses without evidence of organ tissue dysfunction, a myelodysplastic syndrome, or of a myeloproliferative disorder.3 Advanced SM is less common and is associated with organ tissue dysfunction. It also may be associated with myeloproliferative, myelodysplastic, or lymphoproliferative hematologic neoplasms, and subtypes include aggressive SM, SM with an associated hematologic neoplasm, and mast cell leukemia (Table 2).4
Treatment options approved by the US Food and Drug Administration (FDA) for advanced SM include disease-altering medications, such as tyrosine kinase inhibitors (eg, imatinib), but the approved treatment options for nonadvanced SM are generally aimed at managing only symptoms (Table 3). Although not approved by the FDA for the treatment of SM, omalizumab may aid in the prevention of anaphylaxis, the reduction of disease burden, and the improvement in quality of life for patients with SM.5 Omalizumab is a humanized monoclonal antibody against the Fc portion of immunoglobulin E (IgE). It is approved by the FDA for treatment of asthma as well as chronic idiopathic urticaria.6
Case Presentation
A 32-year-old female initially presented to Womack Army Medical Center at Fort Bragg, North Carolina, for evaluation due to recurrent episodes of anaphylaxis occurring 1 to 2 times per month as well as chronic skin rashes that progressed over the previous 5 years (Figure). She initially was diagnosed with idiopathic anaphylaxis and subsequently had multiple emergency department (ED) and clinic visits for vasovagal syncope, unexplained allergic reactions, dizziness, giddiness, and shortness of breath. More recently, she was diagnosed with idiopathic urticaria.
The patient reported at least 12 episodes in the previous year involving facial flushing that proceeded inferiorly, chest tightness, shortness of breath, labored breathing, crampy abdominal pain, and nausea without urticaria or significant pruritus. These bouts often were accompanied by mild facial angioedema, acute sinus pressure, vomiting, tachycardia, and lightheadedness. She reported experiencing brief losses of consciousness with at least 4 of these episodes. Home and ED blood pressure measurements revealed hypotension on several occasions with systolic readings in the 80s. She also developed nonpruritic freckles on her upper chest initially with subsequent increase in number and spread to involve her entire trunk, proximal extremities, and eventually distal extremities.
The patient had received intramuscular epinephrine several times, which led to rapid resolution of her symptoms. Intensive care unit admission for observation overnight was deemed necessary following one of her first episodes, but she did not require intubation or vasopressor support. Eventually, she began treating most episodes at home with diphenhydramine, ranitidine, and occasionally an epinephrine auto-injector, only presenting to the ED for severe dyspnea or loss of consciousness. Some episodes awoke her from sleeping but no triggers were identified (eg, foods, alcohol, supplements, medications, insect stings, latex exposure, exercise, strong emotions, or menstrual cycle).
Examination revealed hyperpigmented macules and papules scattered on the trunk and extremities, with a positive Darier sign. Punch biopsy of one of the macules revealed focal basal cell hyperpigmentation and sheets of benign-appearing mast cells in the superficial dermis, highlighted by CD117 immunohistochemical stain. A serum tryptase level was obtained and found to be significantly elevated (134 mcg/L). The patient was diagnosed with maculopapular cutaneous mastocytosis (urticaria pigmentosa).
A bone marrow biopsy revealed multiple prominent infiltrates of monomorphic, spindled, CD117-positive, CD2-positive, and CD25-positive mast cells arranged interstitially and paratrabecularly, with associated reticulin fibrosis. Indolent SM was diagnosed according to the World Health Organization classification system with multifocal, dense aggregates of mast cells (> 25%) in the bone marrow and with persistently elevated serum tryptase levels (134, 134, 151, and 159 ng/mL) without laboratory evidence of an associated clonal myeloid disorder or findings consistent with infiltrating bone lesions on full body magnetic resonance imaging scan.4
Despite maximal antihistamine and antileukotriene therapy with ranitidine (150 mg twice daily), cetirizine (10 mg twice daily), montelukast (10 mg daily), and cromolyn sodium (200 mg daily), the patient continued to experience recurrent episodes of anaphylaxis requiring subcutaneous epinephrine and systemic corticosteroids. In May 2016, the patient began a trial of off-label therapy with omalizumab injections (300 mg subcutaneous every 4 weeks). She has continued on therapy for more than 4 years and experienced only 1 anaphylactic episode. She also has had significant improvement in cutaneous symptoms.
Discussion
Mast cell overactivation and degranulation in mastocytosis is largely driven by the IgE antibody, which plays a significant role in atopic conditions, immediate hypersensitivity reactions, and anaphylaxis, as well as in the immunologic response to parasitic infections. The severity of atopic disease seems to be associated with serum IgE levels in many patients.7 IgE binding to surface receptors on mast cells and eosinophils prompts the release of toxic mediators, incites inflammation, and induces allergic symptoms.8 Activation of mast cells is classically elicited by IgE binding to the high-affinity Fcε RI receptor, the expression of which correlates with IgE levels.9
The anti-IgE, recombinant, humanized immunoglobulin G monoclonal antibody, omalizumab, decreases mastocytic and eosinophilic symptoms by binding and inhibiting IgE. This diminishes free IgE levels, inhibits IgE binding to the Fcε RI receptor, and affects downregulation of this high-affinity receptor on mast cells and basophils.6 Omalizumab is currently FDA approved only for the treatment of moderate-to-severe, persistent, allergic asthma that is not controlled by inhaled corticosteroids in patients aged ≥ 6 years, and for chronic idiopathic urticaria not controlled by H1 antihistamine therapy in patients aged ≥ 12 years.10 However, it stands to reason that this therapy also should be effective in the treatment of other poorly controlled atopic conditions, especially mastocytosis, the symptoms of which are driven by excessive mast cell degranulation and tissue infiltration.
As early as 2007, preliminary data showed that treatment with omalizumab could decrease the frequency of episodes of anaphylaxis.11 A National Institutes of Health case report followed 2 patients, one for 5 months and the other for 24 months. Both patients experienced a decrease in frequency of anaphylaxis following initiation of omalizumab. In 2010, a second case report described the treatment of an Australian patient with recurrent idiopathic anaphylaxis also diagnosed with SM. After initiation of treatment with omalizumab, she, too, experienced decreased frequency of episodes of anaphylaxis over 14 months.12 A review of patients treated at the Mastocytosis Centre Odense University Hospital in Denmark was published in 2017. Of 13 patients with SM treated with omalizumab, 5 experienced what was considered a complete response to the medication, with 3 each experiencing major and partial responses.5 The median treatment time in these patients was 27 months. Each of these cases showed significant promise in the use of omalizumab to treat SM, informing the decision to attempt this treatment in our patient.
The potential positive effects of omalizumab in reducing symptom severity in patients with SM was further supported by a 2017 meta-analysis. This review included several individual case reports noting that omalizumab could decrease frequency of pulmonary and gastrointestinal manifestations of SM.13 A small randomized control trial of omalizumab for treatment of mild symptoms of SM found improvement in disease severity, although neither primary nor secondary endpoints reached statistical significance.14
This case demonstrates a substantial, long-term, clinical benefit and quality of life improvement with omalizumab therapy in a patient with indolent SM that was not adequately controlled by conventional therapies. This is evidenced by an impressive decline in the frequency of mastocytic anaphylactic episodes as well as diminished patient-endorsed cutaneous symptoms.
This case provides further evidence of the efficacy of this therapy in diminishing disease burden for patients with SM who are otherwise limited to treatments aimed at transient symptomatic relief without significant alteration of the underlying cause of symptoms. At the time this article was written, our patient had now 52 months of continuous treatment without any adverse reactions noted, suggesting the treatment's long-term efficacy. It also adds to a small but growing body of literature that supports the use of anti-IgE therapy as a treatment option for improved management of this distressing, life-altering illness. Even in the time that our patient has been receiving omalizumab for SM, another small case series of 2 patients has been published showing sustained treatment effect at 12 years of therapy.15 This adds further insight that omalizumab can offer long-term, safe treatment for this limiting condition.
Omalizumab therapy is not without risk, but for patients afflicted by unrestrained mastocytic disease, the benefits may outweigh the risks. The most common significant risk with this medication is anaphylaxis, occurring in 1 to 2 per 1,000 patients, usually within 2 hours of an injection.16 This may correlate to the underlying degree of atopy in patients receiving omalizumab, and the risk of anaphylaxis is relatively low compared with that of many other biologic medications.17 Additionally, early data from initial phases of clinical trials indicated a potentially elevated malignancy risk with omalizumab. However, subsequent pooled analysis of larger numbers of patients has decreased suspicion that a causal relationship exists.18
Conclusions
Omalizumab has proven value in the treatment of atopic conditions, such as asthma and idiopathic urticaria, for which it has been approved for use by the FDA. Its effectiveness in significantly decreasing free serum IgE levels, and inhibiting IgE activation of mast cells makes it a possible treatment option for patients with SM who are not sufficiently controlled with conventional therapy. The findings in this case suggest that omalizumab may be effective in the prevention of anaphylaxis and in the reduction of disease burden associated with SM. Further studies and formal clinical trials are needed to confirm these findings. Patients should be counseled appropriately concerning the risks, benefits, and off-label status of this treatment option.
1. Theoharides TC, Valent P, Akin C. Mast cells, mastocytosis, and related disorders. N Engl J Med. 2015;373(2):163-172. doi:10.1056/NEJMra1409760
2. Valent P, Sperr WR, Schwartz LB, Horny H-P. Diagnosis and classification of mast cell proliferative disorders: delineation from immunologic diseases and non-mast cell hematopoietic neoplasms. J Allergy Clin Immunol. 2004;114(1):3-11. doi:10.1016/j.jaci.2004.02.045
3. Valent P, Sotlar K, Sperr WR, et al. Refined diagnostic criteria and classification of mast cell leukemia (MCL) and myelomastocytic leukemia (MML): a consensus proposal. Ann Oncol. 2014;25(9):1691-1700. doi:10.1093/annonc/mdu047
4. Valent P, Akin C, Metcalfe DD. Mastocytosis: 2016 updated WHO classification and novel emerging treatment concepts. Blood. 2017;129(11):1420-1427. doi:10.1182/blood-2016-09-731893
5. Broesby-Olsen S, Vestergaard H, Mortz CG, et al. Omalizumab prevents anaphylaxis and improves symptoms in systemic mastocytosis: Efficacy and safety observations. 2018;73(1):230-238. doi:10.1111/all.13237
6. Kaplan AP, Giménez-Arnau AM, Saini SS.Mechanisms of action that contribute to efficacy of omalizumab in chronic spontaneous urticaria. Allergy. 2017;72(4):519-533. doi:10.1111/all.13083
7. Borish L, Chipps B, Deniz Y, Gujrathi S, Zheng B, Dolan C; TENOR Study Group. Total serum IgE levels in a large cohort of patients with severe or difficult-to-treat asthma. Ann Allergy Asthma Immunol. 2005;95(3):247-253. doi:10.1016/S1081-1206(10)61221-5
8. Corry DB, Kheradmand F. Induction and regulation of the IgE response. Nature. 1999;402(suppl 6760):18-23. doi:10.1038/35037014
9. MacGlashan D, McKenzie-White J, Chichester K, et al. In vitro regulation of FcRIα expression on human basophils by IgE antibody. Blood. 1998;91(5):1633-1643.
10. XOLAIR [package insert]. East Hanover, NJ: Novartis Pharmaceuticals Corporation. Revised 2019. Accessed November 11, 2020. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/103976s5234lbl.pdf
11. Carter MC, Robyn JA, Bressler PB, Walker JC, Shapiro GC, and Metcalfe DD. Omalizumab for the treatment of unprovoked anaphylaxis in patients with systemic mastocytosis. J Allergy Clin Immunol. 2007;119(6):1550-1551. doi:10.1016/j.jaci.2007.03.032
12. Douglass JA, Carroll K, Voskamp A, Bourke P, Wei A, O’Hehir RE. Omalizumab is effective in treating systemic mastocytosis in a nonatopic patient. Allergy. 2010; 65(7):926-927. doi:10.1111/j.1398-9995.2009.02259.x
13. Le M, Miedzybrodzki B, Olynych T, Chapdelaine H, Ben-Shoshan M. Natural history and treatment of cutaneous and systemic mastocytosis. Postgrad Med. 2017;129(8):896-901. doi:10.1080/00325481.2017.1364124
14. Distler M, Maul J-T, Steiner T, et al. Efficacy of omalizumab in mastocytosis: allusive indication obtained from a prospective, double-blind, multicenter study (XOLMA Study) [published online ahead of print January 20, 2020]. Dermatology. doi:10.1159/000504842
15. Constantine G, Bressler P, Petroni D, Metcalfe D, Carter M. Twelve-year follow-up of omalizumab for anaphylaxis in 2 patients with systemic mastocytosis. J Allergy Clin Immunol Pract. 2019;7(4)1314-1316. doi:10.1016/j.jaip.2018.07.041
16. Fanta CH. Asthma. N Engl J Med. 2009;360(10):1002-1014. doi:10.1056/NEJMra0804579
17. Baldo BA. Adverse events to monoclonal antibodies used for cancer therapy: focus on hypersensitivity responses. Oncoimmunology. 2013;2(10):e26333. doi:10.4161/onci.26333
18. Busse W, Buhl R, Fernandez Vidaurre C, et al. Omalizumab and the risk of malignancy: results from a pooled analysis. J Allergy Clin Immunol. 2012;129(4):983-989.e6. doi:10.1016/j.jaci.2012.01.033.
19. Castells M, Akin C. Mastocytosis (cutaneous and systemic): epidemiology, pathogenesis, and clinical manifestations. Accessed December 8, 2020. Updated June 12, 2018. https://www.uptodate.com/contents/mastocytosis-cutaneous-and-systemic-epidemiology-pathogenesis-and-clinical-manifestations
20. Czarny J, Lange M, Lugowska-Umer H, Nowicki R. Cutaneous mastocytosis treatment: strategies, limitations, and perspectives. Postepy Dermatol Alergol. 2018;35(6):541-545. doi:10.5114/ada.2018.77605
1. Theoharides TC, Valent P, Akin C. Mast cells, mastocytosis, and related disorders. N Engl J Med. 2015;373(2):163-172. doi:10.1056/NEJMra1409760
2. Valent P, Sperr WR, Schwartz LB, Horny H-P. Diagnosis and classification of mast cell proliferative disorders: delineation from immunologic diseases and non-mast cell hematopoietic neoplasms. J Allergy Clin Immunol. 2004;114(1):3-11. doi:10.1016/j.jaci.2004.02.045
3. Valent P, Sotlar K, Sperr WR, et al. Refined diagnostic criteria and classification of mast cell leukemia (MCL) and myelomastocytic leukemia (MML): a consensus proposal. Ann Oncol. 2014;25(9):1691-1700. doi:10.1093/annonc/mdu047
4. Valent P, Akin C, Metcalfe DD. Mastocytosis: 2016 updated WHO classification and novel emerging treatment concepts. Blood. 2017;129(11):1420-1427. doi:10.1182/blood-2016-09-731893
5. Broesby-Olsen S, Vestergaard H, Mortz CG, et al. Omalizumab prevents anaphylaxis and improves symptoms in systemic mastocytosis: Efficacy and safety observations. 2018;73(1):230-238. doi:10.1111/all.13237
6. Kaplan AP, Giménez-Arnau AM, Saini SS.Mechanisms of action that contribute to efficacy of omalizumab in chronic spontaneous urticaria. Allergy. 2017;72(4):519-533. doi:10.1111/all.13083
7. Borish L, Chipps B, Deniz Y, Gujrathi S, Zheng B, Dolan C; TENOR Study Group. Total serum IgE levels in a large cohort of patients with severe or difficult-to-treat asthma. Ann Allergy Asthma Immunol. 2005;95(3):247-253. doi:10.1016/S1081-1206(10)61221-5
8. Corry DB, Kheradmand F. Induction and regulation of the IgE response. Nature. 1999;402(suppl 6760):18-23. doi:10.1038/35037014
9. MacGlashan D, McKenzie-White J, Chichester K, et al. In vitro regulation of FcRIα expression on human basophils by IgE antibody. Blood. 1998;91(5):1633-1643.
10. XOLAIR [package insert]. East Hanover, NJ: Novartis Pharmaceuticals Corporation. Revised 2019. Accessed November 11, 2020. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/103976s5234lbl.pdf
11. Carter MC, Robyn JA, Bressler PB, Walker JC, Shapiro GC, and Metcalfe DD. Omalizumab for the treatment of unprovoked anaphylaxis in patients with systemic mastocytosis. J Allergy Clin Immunol. 2007;119(6):1550-1551. doi:10.1016/j.jaci.2007.03.032
12. Douglass JA, Carroll K, Voskamp A, Bourke P, Wei A, O’Hehir RE. Omalizumab is effective in treating systemic mastocytosis in a nonatopic patient. Allergy. 2010; 65(7):926-927. doi:10.1111/j.1398-9995.2009.02259.x
13. Le M, Miedzybrodzki B, Olynych T, Chapdelaine H, Ben-Shoshan M. Natural history and treatment of cutaneous and systemic mastocytosis. Postgrad Med. 2017;129(8):896-901. doi:10.1080/00325481.2017.1364124
14. Distler M, Maul J-T, Steiner T, et al. Efficacy of omalizumab in mastocytosis: allusive indication obtained from a prospective, double-blind, multicenter study (XOLMA Study) [published online ahead of print January 20, 2020]. Dermatology. doi:10.1159/000504842
15. Constantine G, Bressler P, Petroni D, Metcalfe D, Carter M. Twelve-year follow-up of omalizumab for anaphylaxis in 2 patients with systemic mastocytosis. J Allergy Clin Immunol Pract. 2019;7(4)1314-1316. doi:10.1016/j.jaip.2018.07.041
16. Fanta CH. Asthma. N Engl J Med. 2009;360(10):1002-1014. doi:10.1056/NEJMra0804579
17. Baldo BA. Adverse events to monoclonal antibodies used for cancer therapy: focus on hypersensitivity responses. Oncoimmunology. 2013;2(10):e26333. doi:10.4161/onci.26333
18. Busse W, Buhl R, Fernandez Vidaurre C, et al. Omalizumab and the risk of malignancy: results from a pooled analysis. J Allergy Clin Immunol. 2012;129(4):983-989.e6. doi:10.1016/j.jaci.2012.01.033.
19. Castells M, Akin C. Mastocytosis (cutaneous and systemic): epidemiology, pathogenesis, and clinical manifestations. Accessed December 8, 2020. Updated June 12, 2018. https://www.uptodate.com/contents/mastocytosis-cutaneous-and-systemic-epidemiology-pathogenesis-and-clinical-manifestations
20. Czarny J, Lange M, Lugowska-Umer H, Nowicki R. Cutaneous mastocytosis treatment: strategies, limitations, and perspectives. Postepy Dermatol Alergol. 2018;35(6):541-545. doi:10.5114/ada.2018.77605
Expanding Verrucous Plaque on the Face
The Diagnosis: Blastomycosis
Histopathologic examination of 3 punch biopsies from the left side of the upper lip showed pseudoepitheliomatous hyperplasia with intraepidermal microabscesses and dermal suppurative granulomatous inflammation (Figure 1A). Stains were negative for periodic acid-Schiff, herpes simplex virus, and varicella-zoster virus. Direct and indirect immunofluorescence for skin autoantibodies were negative. Two separate tissue culture specimens showed no bacterial, fungal, or mycobacterial growth. Leishmania polymerase chain reaction and DNA sequencing were negative. An additional punch biopsy revealed yeast forms with broad-based budding and refractile walls (Figures 1B and 1C) that were highlighted with Grocott-Gomori methenamine-silver stain of the tissue (Figure 2). Chest radiography demonstrated no pulmonary involvement. In collaboration with an infectious disease specialist, the patient was started on itraconazole 200 mg twice daily for a total of 6 months.
Blastomycosis is a fungal infection caused by Blastomyces dermatitidis, a thermally dimorphic fungus endemic in the soils of the Ohio and Mississippi River valleys and southeastern United States.1 It most commonly manifests as a pulmonary infection following inhalation of spores that are transformed into thick-walled yeasts capable of evading the host's immune system. Unlike other deep fungal infections, blastomycosis occurs in both immunocompetent and immunocompromised hosts. Extrapulmonary disease after hematogenous dissemination from the lungs occurs in approximately 25% to 30% of patients, with the skin as the most common site of dissemination.2 Clinically, cutaneous blastomycosis typically starts as papules that evolve into crusted vegetative plaques, often with central clearing or ulceration. Primary cutaneous blastomycosis is rare and occurs due to direct inoculation after trauma to the skin via an infected animal bite, direct inoculation in laboratory settings, or due to injury during outdoor activities involving contact with soil.3 Given our patient's horticultural hobbies, lack of pulmonary symptoms, and negative radiologic examination, primary cutaneous blastomycosis infection due to direct inoculation from contaminated soil was a possibility; however, definite confirmation was difficult, as the primary pulmonary infection of blastomycosis can be asymptomatic and therefore often goes undetected.
Cutaneous blastomycosis can be mistaken for pemphigus vegetans, leishmaniasis, herpes vegetans, bacterial pyoderma, and other deep fungal infections that also display pseudoepitheliomatous hyperplasia with pyogranulomatous inflammation on histopathology. Direct visualization of the characteristic yeast forms in a histologic specimen or the growth of fungus in culture is essential for a definitive diagnosis. The yeasts are 8 to 15 µm in diameter with thick, double-contoured walls and characteristically display broad-based budding.4 This budding pattern aids in differentiating blastomycosis from other entities with a similar histopathologic appearance. Chromoblastomycosis would show brown, thick-walled fungal cells inside giant cells, while coccidioidomycosis displays large spherules containing endospores, and leishmaniasis demonstrates amastigotes (small oval organisms with a bar-shaped kinetoplast) highlighted with Giemsa staining. Pemphigus vegetans would show intercellular deposition of IgG on direct immunofluorescence. Blastomyces dermatitidis can be difficult to visualize with routine hematoxylin and eosin stains, and it is important to note that a negative result does not exclude the possibility of blastomycosis, as demonstrated in our case.4 Special stains including Grocott-Gomori methenamine-silver and periodic acid-Schiff can aid in examining tissue for the presence of fungal elements, which typically can be found within histiocytes or abscesses in the dermis. Culture is the most sensitive method for detecting and diagnosing blastomycosis. Growth typically is detected in 5 to 10 days but can take up to 30 days if few organisms are present in the specimen.1
Although spontaneous remission can occur, it is recommended that all patients with cutaneous blastomycosis be treated to avoid dissemination and recurrence. Itraconazole currently is the treatment of choice.5 Doses typically are 200 to 400 mg/d for 8 to 12 months.6 Itraconazole-related side effects experienced by our patient during his 6-month treatment course included leg edema, 20-lb weight gain, gastrointestinal upset, blurred vision, and a transient increase in blood pressure, all resolving once the medication was discontinued. Complete resolution of the lesion was noted at the completion of the treatment course. At a 6-month posttreatment follow-up, residual scarring and alopecia were noted in parts of the previously affected areas of the upper cutaneous lip and nasolabial fold.
- Saccente M, Woods GL. Clinical and laboratory update on blastomycosis. Clin Microbiol Rev. 2010;23:367-831.
- Chapman SW, Lin AC, Hendricks KA, et al. Endemic blastomycosis in Mississippi: epidemiological and clinical studies. Semin Respir Infect. 1997;12:219-228.
- Gray NA, Baddour LM. Cutaneous inoculation blastomycosis. Clin Infect Dis. 2002;34:E44-E49.
- Patel AJ, Gattuso P, Reddy VB. Diagnosis of blastomycosis in surgical pathology and cytopathology: correlation with microbiologic culture. Am J Surg Pathol. 2010;34:256-261.
- Chapman SW, Dismukes WE, Proia LA, et al. Clinical practice guidelines for the management of blastomycosis: 2008 update by the Infectious Diseases Society of America. Clin Infect Dis. 2008;46:1801-1812.
- Lomaestro, BM, Piatek MA. Update on drug interactions with azole antifungal agents. Ann Pharmacother. 1998;32:915-928.
The Diagnosis: Blastomycosis
Histopathologic examination of 3 punch biopsies from the left side of the upper lip showed pseudoepitheliomatous hyperplasia with intraepidermal microabscesses and dermal suppurative granulomatous inflammation (Figure 1A). Stains were negative for periodic acid-Schiff, herpes simplex virus, and varicella-zoster virus. Direct and indirect immunofluorescence for skin autoantibodies were negative. Two separate tissue culture specimens showed no bacterial, fungal, or mycobacterial growth. Leishmania polymerase chain reaction and DNA sequencing were negative. An additional punch biopsy revealed yeast forms with broad-based budding and refractile walls (Figures 1B and 1C) that were highlighted with Grocott-Gomori methenamine-silver stain of the tissue (Figure 2). Chest radiography demonstrated no pulmonary involvement. In collaboration with an infectious disease specialist, the patient was started on itraconazole 200 mg twice daily for a total of 6 months.
Blastomycosis is a fungal infection caused by Blastomyces dermatitidis, a thermally dimorphic fungus endemic in the soils of the Ohio and Mississippi River valleys and southeastern United States.1 It most commonly manifests as a pulmonary infection following inhalation of spores that are transformed into thick-walled yeasts capable of evading the host's immune system. Unlike other deep fungal infections, blastomycosis occurs in both immunocompetent and immunocompromised hosts. Extrapulmonary disease after hematogenous dissemination from the lungs occurs in approximately 25% to 30% of patients, with the skin as the most common site of dissemination.2 Clinically, cutaneous blastomycosis typically starts as papules that evolve into crusted vegetative plaques, often with central clearing or ulceration. Primary cutaneous blastomycosis is rare and occurs due to direct inoculation after trauma to the skin via an infected animal bite, direct inoculation in laboratory settings, or due to injury during outdoor activities involving contact with soil.3 Given our patient's horticultural hobbies, lack of pulmonary symptoms, and negative radiologic examination, primary cutaneous blastomycosis infection due to direct inoculation from contaminated soil was a possibility; however, definite confirmation was difficult, as the primary pulmonary infection of blastomycosis can be asymptomatic and therefore often goes undetected.
Cutaneous blastomycosis can be mistaken for pemphigus vegetans, leishmaniasis, herpes vegetans, bacterial pyoderma, and other deep fungal infections that also display pseudoepitheliomatous hyperplasia with pyogranulomatous inflammation on histopathology. Direct visualization of the characteristic yeast forms in a histologic specimen or the growth of fungus in culture is essential for a definitive diagnosis. The yeasts are 8 to 15 µm in diameter with thick, double-contoured walls and characteristically display broad-based budding.4 This budding pattern aids in differentiating blastomycosis from other entities with a similar histopathologic appearance. Chromoblastomycosis would show brown, thick-walled fungal cells inside giant cells, while coccidioidomycosis displays large spherules containing endospores, and leishmaniasis demonstrates amastigotes (small oval organisms with a bar-shaped kinetoplast) highlighted with Giemsa staining. Pemphigus vegetans would show intercellular deposition of IgG on direct immunofluorescence. Blastomyces dermatitidis can be difficult to visualize with routine hematoxylin and eosin stains, and it is important to note that a negative result does not exclude the possibility of blastomycosis, as demonstrated in our case.4 Special stains including Grocott-Gomori methenamine-silver and periodic acid-Schiff can aid in examining tissue for the presence of fungal elements, which typically can be found within histiocytes or abscesses in the dermis. Culture is the most sensitive method for detecting and diagnosing blastomycosis. Growth typically is detected in 5 to 10 days but can take up to 30 days if few organisms are present in the specimen.1
Although spontaneous remission can occur, it is recommended that all patients with cutaneous blastomycosis be treated to avoid dissemination and recurrence. Itraconazole currently is the treatment of choice.5 Doses typically are 200 to 400 mg/d for 8 to 12 months.6 Itraconazole-related side effects experienced by our patient during his 6-month treatment course included leg edema, 20-lb weight gain, gastrointestinal upset, blurred vision, and a transient increase in blood pressure, all resolving once the medication was discontinued. Complete resolution of the lesion was noted at the completion of the treatment course. At a 6-month posttreatment follow-up, residual scarring and alopecia were noted in parts of the previously affected areas of the upper cutaneous lip and nasolabial fold.
The Diagnosis: Blastomycosis
Histopathologic examination of 3 punch biopsies from the left side of the upper lip showed pseudoepitheliomatous hyperplasia with intraepidermal microabscesses and dermal suppurative granulomatous inflammation (Figure 1A). Stains were negative for periodic acid-Schiff, herpes simplex virus, and varicella-zoster virus. Direct and indirect immunofluorescence for skin autoantibodies were negative. Two separate tissue culture specimens showed no bacterial, fungal, or mycobacterial growth. Leishmania polymerase chain reaction and DNA sequencing were negative. An additional punch biopsy revealed yeast forms with broad-based budding and refractile walls (Figures 1B and 1C) that were highlighted with Grocott-Gomori methenamine-silver stain of the tissue (Figure 2). Chest radiography demonstrated no pulmonary involvement. In collaboration with an infectious disease specialist, the patient was started on itraconazole 200 mg twice daily for a total of 6 months.
Blastomycosis is a fungal infection caused by Blastomyces dermatitidis, a thermally dimorphic fungus endemic in the soils of the Ohio and Mississippi River valleys and southeastern United States.1 It most commonly manifests as a pulmonary infection following inhalation of spores that are transformed into thick-walled yeasts capable of evading the host's immune system. Unlike other deep fungal infections, blastomycosis occurs in both immunocompetent and immunocompromised hosts. Extrapulmonary disease after hematogenous dissemination from the lungs occurs in approximately 25% to 30% of patients, with the skin as the most common site of dissemination.2 Clinically, cutaneous blastomycosis typically starts as papules that evolve into crusted vegetative plaques, often with central clearing or ulceration. Primary cutaneous blastomycosis is rare and occurs due to direct inoculation after trauma to the skin via an infected animal bite, direct inoculation in laboratory settings, or due to injury during outdoor activities involving contact with soil.3 Given our patient's horticultural hobbies, lack of pulmonary symptoms, and negative radiologic examination, primary cutaneous blastomycosis infection due to direct inoculation from contaminated soil was a possibility; however, definite confirmation was difficult, as the primary pulmonary infection of blastomycosis can be asymptomatic and therefore often goes undetected.
Cutaneous blastomycosis can be mistaken for pemphigus vegetans, leishmaniasis, herpes vegetans, bacterial pyoderma, and other deep fungal infections that also display pseudoepitheliomatous hyperplasia with pyogranulomatous inflammation on histopathology. Direct visualization of the characteristic yeast forms in a histologic specimen or the growth of fungus in culture is essential for a definitive diagnosis. The yeasts are 8 to 15 µm in diameter with thick, double-contoured walls and characteristically display broad-based budding.4 This budding pattern aids in differentiating blastomycosis from other entities with a similar histopathologic appearance. Chromoblastomycosis would show brown, thick-walled fungal cells inside giant cells, while coccidioidomycosis displays large spherules containing endospores, and leishmaniasis demonstrates amastigotes (small oval organisms with a bar-shaped kinetoplast) highlighted with Giemsa staining. Pemphigus vegetans would show intercellular deposition of IgG on direct immunofluorescence. Blastomyces dermatitidis can be difficult to visualize with routine hematoxylin and eosin stains, and it is important to note that a negative result does not exclude the possibility of blastomycosis, as demonstrated in our case.4 Special stains including Grocott-Gomori methenamine-silver and periodic acid-Schiff can aid in examining tissue for the presence of fungal elements, which typically can be found within histiocytes or abscesses in the dermis. Culture is the most sensitive method for detecting and diagnosing blastomycosis. Growth typically is detected in 5 to 10 days but can take up to 30 days if few organisms are present in the specimen.1
Although spontaneous remission can occur, it is recommended that all patients with cutaneous blastomycosis be treated to avoid dissemination and recurrence. Itraconazole currently is the treatment of choice.5 Doses typically are 200 to 400 mg/d for 8 to 12 months.6 Itraconazole-related side effects experienced by our patient during his 6-month treatment course included leg edema, 20-lb weight gain, gastrointestinal upset, blurred vision, and a transient increase in blood pressure, all resolving once the medication was discontinued. Complete resolution of the lesion was noted at the completion of the treatment course. At a 6-month posttreatment follow-up, residual scarring and alopecia were noted in parts of the previously affected areas of the upper cutaneous lip and nasolabial fold.
- Saccente M, Woods GL. Clinical and laboratory update on blastomycosis. Clin Microbiol Rev. 2010;23:367-831.
- Chapman SW, Lin AC, Hendricks KA, et al. Endemic blastomycosis in Mississippi: epidemiological and clinical studies. Semin Respir Infect. 1997;12:219-228.
- Gray NA, Baddour LM. Cutaneous inoculation blastomycosis. Clin Infect Dis. 2002;34:E44-E49.
- Patel AJ, Gattuso P, Reddy VB. Diagnosis of blastomycosis in surgical pathology and cytopathology: correlation with microbiologic culture. Am J Surg Pathol. 2010;34:256-261.
- Chapman SW, Dismukes WE, Proia LA, et al. Clinical practice guidelines for the management of blastomycosis: 2008 update by the Infectious Diseases Society of America. Clin Infect Dis. 2008;46:1801-1812.
- Lomaestro, BM, Piatek MA. Update on drug interactions with azole antifungal agents. Ann Pharmacother. 1998;32:915-928.
- Saccente M, Woods GL. Clinical and laboratory update on blastomycosis. Clin Microbiol Rev. 2010;23:367-831.
- Chapman SW, Lin AC, Hendricks KA, et al. Endemic blastomycosis in Mississippi: epidemiological and clinical studies. Semin Respir Infect. 1997;12:219-228.
- Gray NA, Baddour LM. Cutaneous inoculation blastomycosis. Clin Infect Dis. 2002;34:E44-E49.
- Patel AJ, Gattuso P, Reddy VB. Diagnosis of blastomycosis in surgical pathology and cytopathology: correlation with microbiologic culture. Am J Surg Pathol. 2010;34:256-261.
- Chapman SW, Dismukes WE, Proia LA, et al. Clinical practice guidelines for the management of blastomycosis: 2008 update by the Infectious Diseases Society of America. Clin Infect Dis. 2008;46:1801-1812.
- Lomaestro, BM, Piatek MA. Update on drug interactions with azole antifungal agents. Ann Pharmacother. 1998;32:915-928.
A 69-year-old man presented with a slowly expanding, verrucous plaque on the left side of the upper cutaneous lip of 4 months’ duration. The lesion reportedly began as an abscess and had undergone incision and drainage followed by multiple courses of oral antibiotics that were unsuccessful prior to presentation to our clinic. The patient’s hobbies included gardening near his summer home in the mountains of western North Carolina, where he resided when the lesion appeared. Physical examination revealed an approximately 6×4-cm verrucous plaque with central ulceration on the left side of the upper cutaneous and vermilion lip extending to the nasolabial fold. A review of systems was negative for any systemic symptoms. Routine laboratory tests and computed tomography of the head and neck were normal.
No increase seen in children’s cumulative COVID-19 burden
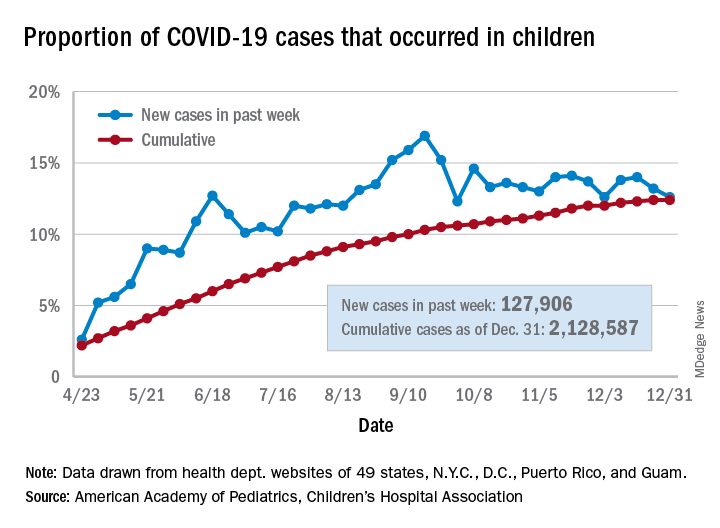
Children’s share of the cumulative COVID-19 burden remained at 12.4% for a second consecutive week, the AAP and CHA said in their weekly report. The last full week of 2020 also marked the second consecutive drop in new cases, although that may be holiday related.
There were almost 128,000 new cases of COVID-19 reported in children for the week, down from 179,000 cases the week before (Dec. 24) and down from the pandemic high of 182,000 reported 2 weeks earlier (Dec.17), based on data from 49 state health departments (excluding New York), along with the District of Columbia, New York City, Puerto Rico, and Guam.
Children’s proportion of new cases for the week, 12.6%, is at its lowest point since early October after dropping for the second week in a row. The cumulative rate of COVID-19 infection, however, is now 2,828 cases per 100,000 children, up from 2,658 the previous week, the AAP and CHA said.
State-level metrics show that North Dakota has the highest cumulative rate at 7,851 per 100,000 children and Hawaii the lowest at 828. Wyoming’s cumulative proportion of child cases, 20.3%, is the highest in the country, while Florida, which uses an age range of 0-14 years for children, is the lowest at 7.1%. California’s total of 268,000 cases is almost double the number of second-place Illinois (138,000), the AAP/CHA data show.
Cumulative child deaths from COVID-19 are up to 179 in the jurisdictions reporting such data (43 states and New York City). That represents just 0.6% of all coronavirus-related deaths and has changed little over the last several months – never rising higher than 0.7% or dropping below 0.6% since early July, according to the report.

Children’s share of the cumulative COVID-19 burden remained at 12.4% for a second consecutive week, the AAP and CHA said in their weekly report. The last full week of 2020 also marked the second consecutive drop in new cases, although that may be holiday related.
There were almost 128,000 new cases of COVID-19 reported in children for the week, down from 179,000 cases the week before (Dec. 24) and down from the pandemic high of 182,000 reported 2 weeks earlier (Dec.17), based on data from 49 state health departments (excluding New York), along with the District of Columbia, New York City, Puerto Rico, and Guam.
Children’s proportion of new cases for the week, 12.6%, is at its lowest point since early October after dropping for the second week in a row. The cumulative rate of COVID-19 infection, however, is now 2,828 cases per 100,000 children, up from 2,658 the previous week, the AAP and CHA said.
State-level metrics show that North Dakota has the highest cumulative rate at 7,851 per 100,000 children and Hawaii the lowest at 828. Wyoming’s cumulative proportion of child cases, 20.3%, is the highest in the country, while Florida, which uses an age range of 0-14 years for children, is the lowest at 7.1%. California’s total of 268,000 cases is almost double the number of second-place Illinois (138,000), the AAP/CHA data show.
Cumulative child deaths from COVID-19 are up to 179 in the jurisdictions reporting such data (43 states and New York City). That represents just 0.6% of all coronavirus-related deaths and has changed little over the last several months – never rising higher than 0.7% or dropping below 0.6% since early July, according to the report.

Children’s share of the cumulative COVID-19 burden remained at 12.4% for a second consecutive week, the AAP and CHA said in their weekly report. The last full week of 2020 also marked the second consecutive drop in new cases, although that may be holiday related.
There were almost 128,000 new cases of COVID-19 reported in children for the week, down from 179,000 cases the week before (Dec. 24) and down from the pandemic high of 182,000 reported 2 weeks earlier (Dec.17), based on data from 49 state health departments (excluding New York), along with the District of Columbia, New York City, Puerto Rico, and Guam.
Children’s proportion of new cases for the week, 12.6%, is at its lowest point since early October after dropping for the second week in a row. The cumulative rate of COVID-19 infection, however, is now 2,828 cases per 100,000 children, up from 2,658 the previous week, the AAP and CHA said.
State-level metrics show that North Dakota has the highest cumulative rate at 7,851 per 100,000 children and Hawaii the lowest at 828. Wyoming’s cumulative proportion of child cases, 20.3%, is the highest in the country, while Florida, which uses an age range of 0-14 years for children, is the lowest at 7.1%. California’s total of 268,000 cases is almost double the number of second-place Illinois (138,000), the AAP/CHA data show.
Cumulative child deaths from COVID-19 are up to 179 in the jurisdictions reporting such data (43 states and New York City). That represents just 0.6% of all coronavirus-related deaths and has changed little over the last several months – never rising higher than 0.7% or dropping below 0.6% since early July, according to the report.
COVID-19 vaccines: The rollout, the risks, and the reason to still wear a mask
REFERENCES
- Oliver SE, Gargano JW, Marin M; et al. The Advisory Committee on Immunization Practices’ interim recommendation for use of Pfizer-BioNTech COVID-19 vaccine—United States, December 2020. MMWR Morbid Mortal Wkly Rep. 2020;69:1922-1924. Accessed January 13, 2021. www.cdc.gov/mmwr/volumes/69/wr/mm6950e2.htm
- 2. Oliver SE, Gargano JW, Marin M; et al. The Advisory Committee on Immunization Practices’ interim recommendation for use of Moderna COVID-19 vaccine—United States, December 2020. MMWR Morbid Mortal Wkly Rep. 2021;69:1653-1656. Accessed January 13, 2021. www.cdc.gov/mmwr/volumes/69/wr/mm695152e1.htm
- CDC. COVID-19 vaccines: update on allergic reactions, contraindications, and precautions [webinar]. December 30, 2020. Accessed January 6, 2021. https://emergency.cdc.gov/coca/calls/2020/callinfo_123020.asp
- CDC. What clinicians need to know about the Pfizer-BioNTech and Moderna COVID-19 vaccines [webinar]. December 18, 2020. Accessed January 6, 2021. https://emergency.cdc.gov/coca/calls/2020/callinfo_121820.asp
- CDC COVID-19 Response Team; Food and Drug Administration. Allergic reactions including anaphylaxis after receipt of the first dose of Pfizer-BioNTech COVID-19 vaccine—United States, December 14-23, 2020. MMWR Morb Mortal Wkly Rep. ePub: January 6, 2021. Accessed January 13, 2021. www.cdc.gov/mmwr/volumes/70/wr/mm7002e1.htm
REFERENCES
- Oliver SE, Gargano JW, Marin M; et al. The Advisory Committee on Immunization Practices’ interim recommendation for use of Pfizer-BioNTech COVID-19 vaccine—United States, December 2020. MMWR Morbid Mortal Wkly Rep. 2020;69:1922-1924. Accessed January 13, 2021. www.cdc.gov/mmwr/volumes/69/wr/mm6950e2.htm
- 2. Oliver SE, Gargano JW, Marin M; et al. The Advisory Committee on Immunization Practices’ interim recommendation for use of Moderna COVID-19 vaccine—United States, December 2020. MMWR Morbid Mortal Wkly Rep. 2021;69:1653-1656. Accessed January 13, 2021. www.cdc.gov/mmwr/volumes/69/wr/mm695152e1.htm
- CDC. COVID-19 vaccines: update on allergic reactions, contraindications, and precautions [webinar]. December 30, 2020. Accessed January 6, 2021. https://emergency.cdc.gov/coca/calls/2020/callinfo_123020.asp
- CDC. What clinicians need to know about the Pfizer-BioNTech and Moderna COVID-19 vaccines [webinar]. December 18, 2020. Accessed January 6, 2021. https://emergency.cdc.gov/coca/calls/2020/callinfo_121820.asp
- CDC COVID-19 Response Team; Food and Drug Administration. Allergic reactions including anaphylaxis after receipt of the first dose of Pfizer-BioNTech COVID-19 vaccine—United States, December 14-23, 2020. MMWR Morb Mortal Wkly Rep. ePub: January 6, 2021. Accessed January 13, 2021. www.cdc.gov/mmwr/volumes/70/wr/mm7002e1.htm
REFERENCES
- Oliver SE, Gargano JW, Marin M; et al. The Advisory Committee on Immunization Practices’ interim recommendation for use of Pfizer-BioNTech COVID-19 vaccine—United States, December 2020. MMWR Morbid Mortal Wkly Rep. 2020;69:1922-1924. Accessed January 13, 2021. www.cdc.gov/mmwr/volumes/69/wr/mm6950e2.htm
- 2. Oliver SE, Gargano JW, Marin M; et al. The Advisory Committee on Immunization Practices’ interim recommendation for use of Moderna COVID-19 vaccine—United States, December 2020. MMWR Morbid Mortal Wkly Rep. 2021;69:1653-1656. Accessed January 13, 2021. www.cdc.gov/mmwr/volumes/69/wr/mm695152e1.htm
- CDC. COVID-19 vaccines: update on allergic reactions, contraindications, and precautions [webinar]. December 30, 2020. Accessed January 6, 2021. https://emergency.cdc.gov/coca/calls/2020/callinfo_123020.asp
- CDC. What clinicians need to know about the Pfizer-BioNTech and Moderna COVID-19 vaccines [webinar]. December 18, 2020. Accessed January 6, 2021. https://emergency.cdc.gov/coca/calls/2020/callinfo_121820.asp
- CDC COVID-19 Response Team; Food and Drug Administration. Allergic reactions including anaphylaxis after receipt of the first dose of Pfizer-BioNTech COVID-19 vaccine—United States, December 14-23, 2020. MMWR Morb Mortal Wkly Rep. ePub: January 6, 2021. Accessed January 13, 2021. www.cdc.gov/mmwr/volumes/70/wr/mm7002e1.htm
Skin Cancer Management During the COVID-19 Pandemic
The coronavirus disease 2019 (COVID-19) pandemic, caused by severe acute respiratory syndrome novel coronavirus 2 (SARS-CoV-2), has presented a unique challenge to providing essential care to patients. Increased demand for health care workers and medical supplies, in addition to the risk for COVID-19 infection and asymptomatic transmission of SARS-CoV-2 among health care workers and patients, prompted the delay of nonessential services during the surge of cases this summer.1 Key considerations for continuing operation included current and projected COVID-19 cases in the region, ability to implement telehealth, staffing availability, personal protective equipment availability, and office capacity.2 Providing care that is deemed essential often was determined by the urgency of the treatment or service.
The Centers for Medicare & Medicaid Services outlined a strategy to stratify patients, based on level of acuity, during the COVID-19 surge3:
- Low-acuity treatments or services: includes routine primary, specialty, or preventive care visits. They should be postponed; telehealth follow-ups should be considered.
- Intermediate-acuity treatments or services: includes pediatric and neonatal care, follow-up visits for existing conditions, and evaluation of new symptoms (including those consistent with COVID-19). These services should initially be evaluated using telehealth, then triaged to the appropriate site and level of care.
- High-acuity treatments or services: address symptoms consistent with COVID-19 or other severe disease, of which the lack of in-person evaluation would result in harm to the patient.
Employees in hospitals and health care clinics were classified as essential, but dermatologists were not given explicit direction regarding clinic operation. Many practices have restricted services, especially those in an area of higher COVID-19 prevalence. However, the challenge of determining day-to-day operation may have been left to the provider in most cases.4 As many states in the United States continue to relax restrictions, total cases and the rate of positivity of COVID-19 have been sharply rising again, after months of decline,5 which suggests increased transmission of SARS-CoV-2 and potential resurgence of the high case burden on our health care system. Furthermore, a lack of a widely distributed vaccine or herd immunity suggests we will need to take many of the same precautions as in the first surge.6
In general, patients with cancer have been found to be at greater risk for adverse outcomes and mortality after COVID-19.7 Therefore, resource rationing is particularly concerning for patients with skin cancer, including melanoma, Merkel cell carcinoma, mycosis fungoides, and keratinocyte carcinoma. Triaging patients based on level of acuity, type of skin cancer, disease burden, host immunosuppression, and risk for progression must be carefully considered in this population.2 Treatment and follow-up present additional challenges.
Guidelines provided by the National Comprehensive Cancer Network (NCCN) and the European Society for Medical Oncology (ESMO) elaborated on key considerations for the treatment of melanoma, keratinocyte carcinoma, and Merkel cell carcinoma during the COVID-19 pandemic.8-10 Guidelines from the NCCN concentrated on clear divisions between disease stages to determine provider response. Guidelines for melanoma patients proposed by the ESMO assign tiers by value-based priority in various treatment settings, which offered flexibility to providers as the COVID-19 landscape continued to change. Recommendations from the NCCN and ESMO are summarized in Tables 1 to 5.
Although these guidelines initially may have been proposed to delay treatment of lower-acuity tumors, such delay might not be feasible given the unknown duration of this pandemic and future disease waves. One review of several studies, which addressed the outcomes on melanoma survival following the surgical delay recommended by the NCCN, revealed contradictory evidence.12 Further, sufficiently powered studies will be needed to better understand the impact of delaying treatment during the summer COVID-19 surge on patients with skin cancer. Therefore, physicians must triage patients accordingly to manage and treat while also preventing disease spread.
Tips for Performing Dermatologic Surgery
Careful consideration should be made to protect both the patient and staff during office-based excisional surgery during the COVID-19 pandemic. To minimize the risk of transmission of SARS-CoV-2, patients and staff should (1) be screened for symptoms of COVID-19 at least 48 hours prior to entering the office via telephone screening questions, and (2) follow proper hygiene and contact procedures once entering the office. Consider obtaining a nasal polymerase chain reaction swab or saliva test 48 hours prior to the procedure if the patient is undergoing a head and neck procedure or there is risk for transmission.
Guidelines from the ESMO recommended that all patients undergoing surgery or therapy should be swabbed for SARS-CoV-2 before each treatment.11 Patients should wear a mask, remain 6-feet apart in the waiting room, and avoid touching objects until they enter the procedure room. Objects that the patient must touch, such as pens, should be cleaned immediately after such contact with either alcohol or soap and water for 20 seconds.
Office capacity should be reduced by allowing no more than 1 person to accompany the patient and ensuring the presence of only the minimum staff needed for the procedure. Staff who are deemed necessary should wear a mask continuously and gloves during patient contact.
Once in the procedure room, providers might be at elevated risk of contracting COVID-19 or transmitting SARS-CoV-2. A properly fitted N95 respirator and a face shield are recommended, especially for facial cases. N95 respirators can be reused by following the latest Centers for Disease Control and Prevention recommendations for reuse and decontamination techniques,13 which may include protecting the N95 respirator with a surgical mask and storing it in a paper bag when not in use. Consider testing asymptomatic patients in facial cases when they cannot wear a mask.
Steps should be taken to reduce in-person visits. Dissolving sutures can help avoid return visits. Follow-up visits and postprocedural questions should be managed by telehealth. However, patients with a high-risk underlying conditions (eg, posttransplantation, immunosuppressed) should continue to obtain regular skin checks because they are at higher risk for more aggressive malignancies, such as Merkel cell carcinoma.
Conclusion
The future trajectory of the COVID-19 pandemic is uncertain. Dermatologists should continue providing care for patients with skin cancer while mitigating the risk for COVID-19 infection and transmission of SARS-CoV-2. Guidelines provided by the NCCN and ESMO should help providers triage patients. Decisions should be made case by case, keeping in mind the availability of resources and practicing in compliance with local guidance.
- Moletta L, Pierobon ES, Capovilla G, et al. International guidelines and recommendations for surgery during COVID-19 pandemic: a systematic review. Int J Surg. 2020;79:180-188.
- Ueda M, Martins R, Hendrie PC, et al. Managing cancer care during the COVID-19 pandemic: agility and collaboration toward common goal. J Natl Compr Canc Netw. 2020:1-4.
- Center for Medicare & Medicaid Services. Non-emergent, elective medical services, and treatment recommendations. Published April 7, 2020. Accessed October 15, 2020. https://www.cms.gov/files/document/cms-non-emergent-elective-medical-recommendations.pdf
- Muddasani S, Housholder A, Fleischer AB. An assessment of United States dermatology practices during the COVID-19 outbreak. J Dermatolog Treat. 2020;31:436-438.
- Coronavirus Resource Center, Johns Hopkins University & Medicine. Rate of positive tests in the US and states over time. Updated December 11, 2020. Accessed December 11, 2020. https://coronavirus.jhu.edu/testing/individual-states
- Middleton J, Lopes H, Michelson K, et al. Planning for a second wave pandemic of COVID-19 and planning for winter: a statement from the Association of Schools of Public Health in the European Region. Int J Public Health. 2020;65:1525-1527.
- Liang W, Guan W, Chen R, et al. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol. 2020;21:335-337.
- National Comprehensive Cancer Network. Advisory statement for non-melanoma skin cancer care during the COVID-19 pandemic (version 4). Published May 22, 2020. Accessed December 11, 2020. https://www.nccn.org/covid-19/pdf/NCCN-NMSC.pdf
National Comprehensive Cancer Network. Short-term recommendations for cutaneous melanoma management during COVID-19 pandemic (version 3). Published May 6, 2020. Accessed December 11, 2020. www.nccn.org/covid-19/pdf/Melanoma.pdf - Conforti C, Giuffrida R, Di Meo N, et al. Management of advanced melanoma in the COVID-19 era. Dermatol Ther. 2020;33:e13444.
- ESMO [European Society for Medical Oncology]. Cancer patient management during the COVID-19 pandemic. Accessed Decemeber 11, 2020. https://www.esmo.org/guidelines/cancer-patient-management-during-the-covid-19-pandemic?hit=ehp
- Guhan S, Boland G, Tanabe K, et al. Surgical delay and mortality for primary cutaneous melanoma [published online July 22, 2020]. J Am Acad Dermatol. doi:10.1016/j.jaad.2020.07.078
- Centers for Disease Control and Prevention. Implementing filtering facepiece respirator (FFR) reuse, including reuse after decontamination, when there are known shortages of N95 respirators. Updated October 19, 2020. Accessed December 11, 2020. https://www.cdc.gov/coronavirus/2019-ncov/hcp/ppe-strategy/decontamination-reuse-respirators.html
The coronavirus disease 2019 (COVID-19) pandemic, caused by severe acute respiratory syndrome novel coronavirus 2 (SARS-CoV-2), has presented a unique challenge to providing essential care to patients. Increased demand for health care workers and medical supplies, in addition to the risk for COVID-19 infection and asymptomatic transmission of SARS-CoV-2 among health care workers and patients, prompted the delay of nonessential services during the surge of cases this summer.1 Key considerations for continuing operation included current and projected COVID-19 cases in the region, ability to implement telehealth, staffing availability, personal protective equipment availability, and office capacity.2 Providing care that is deemed essential often was determined by the urgency of the treatment or service.
The Centers for Medicare & Medicaid Services outlined a strategy to stratify patients, based on level of acuity, during the COVID-19 surge3:
- Low-acuity treatments or services: includes routine primary, specialty, or preventive care visits. They should be postponed; telehealth follow-ups should be considered.
- Intermediate-acuity treatments or services: includes pediatric and neonatal care, follow-up visits for existing conditions, and evaluation of new symptoms (including those consistent with COVID-19). These services should initially be evaluated using telehealth, then triaged to the appropriate site and level of care.
- High-acuity treatments or services: address symptoms consistent with COVID-19 or other severe disease, of which the lack of in-person evaluation would result in harm to the patient.
Employees in hospitals and health care clinics were classified as essential, but dermatologists were not given explicit direction regarding clinic operation. Many practices have restricted services, especially those in an area of higher COVID-19 prevalence. However, the challenge of determining day-to-day operation may have been left to the provider in most cases.4 As many states in the United States continue to relax restrictions, total cases and the rate of positivity of COVID-19 have been sharply rising again, after months of decline,5 which suggests increased transmission of SARS-CoV-2 and potential resurgence of the high case burden on our health care system. Furthermore, a lack of a widely distributed vaccine or herd immunity suggests we will need to take many of the same precautions as in the first surge.6
In general, patients with cancer have been found to be at greater risk for adverse outcomes and mortality after COVID-19.7 Therefore, resource rationing is particularly concerning for patients with skin cancer, including melanoma, Merkel cell carcinoma, mycosis fungoides, and keratinocyte carcinoma. Triaging patients based on level of acuity, type of skin cancer, disease burden, host immunosuppression, and risk for progression must be carefully considered in this population.2 Treatment and follow-up present additional challenges.
Guidelines provided by the National Comprehensive Cancer Network (NCCN) and the European Society for Medical Oncology (ESMO) elaborated on key considerations for the treatment of melanoma, keratinocyte carcinoma, and Merkel cell carcinoma during the COVID-19 pandemic.8-10 Guidelines from the NCCN concentrated on clear divisions between disease stages to determine provider response. Guidelines for melanoma patients proposed by the ESMO assign tiers by value-based priority in various treatment settings, which offered flexibility to providers as the COVID-19 landscape continued to change. Recommendations from the NCCN and ESMO are summarized in Tables 1 to 5.
Although these guidelines initially may have been proposed to delay treatment of lower-acuity tumors, such delay might not be feasible given the unknown duration of this pandemic and future disease waves. One review of several studies, which addressed the outcomes on melanoma survival following the surgical delay recommended by the NCCN, revealed contradictory evidence.12 Further, sufficiently powered studies will be needed to better understand the impact of delaying treatment during the summer COVID-19 surge on patients with skin cancer. Therefore, physicians must triage patients accordingly to manage and treat while also preventing disease spread.
Tips for Performing Dermatologic Surgery
Careful consideration should be made to protect both the patient and staff during office-based excisional surgery during the COVID-19 pandemic. To minimize the risk of transmission of SARS-CoV-2, patients and staff should (1) be screened for symptoms of COVID-19 at least 48 hours prior to entering the office via telephone screening questions, and (2) follow proper hygiene and contact procedures once entering the office. Consider obtaining a nasal polymerase chain reaction swab or saliva test 48 hours prior to the procedure if the patient is undergoing a head and neck procedure or there is risk for transmission.
Guidelines from the ESMO recommended that all patients undergoing surgery or therapy should be swabbed for SARS-CoV-2 before each treatment.11 Patients should wear a mask, remain 6-feet apart in the waiting room, and avoid touching objects until they enter the procedure room. Objects that the patient must touch, such as pens, should be cleaned immediately after such contact with either alcohol or soap and water for 20 seconds.
Office capacity should be reduced by allowing no more than 1 person to accompany the patient and ensuring the presence of only the minimum staff needed for the procedure. Staff who are deemed necessary should wear a mask continuously and gloves during patient contact.
Once in the procedure room, providers might be at elevated risk of contracting COVID-19 or transmitting SARS-CoV-2. A properly fitted N95 respirator and a face shield are recommended, especially for facial cases. N95 respirators can be reused by following the latest Centers for Disease Control and Prevention recommendations for reuse and decontamination techniques,13 which may include protecting the N95 respirator with a surgical mask and storing it in a paper bag when not in use. Consider testing asymptomatic patients in facial cases when they cannot wear a mask.
Steps should be taken to reduce in-person visits. Dissolving sutures can help avoid return visits. Follow-up visits and postprocedural questions should be managed by telehealth. However, patients with a high-risk underlying conditions (eg, posttransplantation, immunosuppressed) should continue to obtain regular skin checks because they are at higher risk for more aggressive malignancies, such as Merkel cell carcinoma.
Conclusion
The future trajectory of the COVID-19 pandemic is uncertain. Dermatologists should continue providing care for patients with skin cancer while mitigating the risk for COVID-19 infection and transmission of SARS-CoV-2. Guidelines provided by the NCCN and ESMO should help providers triage patients. Decisions should be made case by case, keeping in mind the availability of resources and practicing in compliance with local guidance.
The coronavirus disease 2019 (COVID-19) pandemic, caused by severe acute respiratory syndrome novel coronavirus 2 (SARS-CoV-2), has presented a unique challenge to providing essential care to patients. Increased demand for health care workers and medical supplies, in addition to the risk for COVID-19 infection and asymptomatic transmission of SARS-CoV-2 among health care workers and patients, prompted the delay of nonessential services during the surge of cases this summer.1 Key considerations for continuing operation included current and projected COVID-19 cases in the region, ability to implement telehealth, staffing availability, personal protective equipment availability, and office capacity.2 Providing care that is deemed essential often was determined by the urgency of the treatment or service.
The Centers for Medicare & Medicaid Services outlined a strategy to stratify patients, based on level of acuity, during the COVID-19 surge3:
- Low-acuity treatments or services: includes routine primary, specialty, or preventive care visits. They should be postponed; telehealth follow-ups should be considered.
- Intermediate-acuity treatments or services: includes pediatric and neonatal care, follow-up visits for existing conditions, and evaluation of new symptoms (including those consistent with COVID-19). These services should initially be evaluated using telehealth, then triaged to the appropriate site and level of care.
- High-acuity treatments or services: address symptoms consistent with COVID-19 or other severe disease, of which the lack of in-person evaluation would result in harm to the patient.
Employees in hospitals and health care clinics were classified as essential, but dermatologists were not given explicit direction regarding clinic operation. Many practices have restricted services, especially those in an area of higher COVID-19 prevalence. However, the challenge of determining day-to-day operation may have been left to the provider in most cases.4 As many states in the United States continue to relax restrictions, total cases and the rate of positivity of COVID-19 have been sharply rising again, after months of decline,5 which suggests increased transmission of SARS-CoV-2 and potential resurgence of the high case burden on our health care system. Furthermore, a lack of a widely distributed vaccine or herd immunity suggests we will need to take many of the same precautions as in the first surge.6
In general, patients with cancer have been found to be at greater risk for adverse outcomes and mortality after COVID-19.7 Therefore, resource rationing is particularly concerning for patients with skin cancer, including melanoma, Merkel cell carcinoma, mycosis fungoides, and keratinocyte carcinoma. Triaging patients based on level of acuity, type of skin cancer, disease burden, host immunosuppression, and risk for progression must be carefully considered in this population.2 Treatment and follow-up present additional challenges.
Guidelines provided by the National Comprehensive Cancer Network (NCCN) and the European Society for Medical Oncology (ESMO) elaborated on key considerations for the treatment of melanoma, keratinocyte carcinoma, and Merkel cell carcinoma during the COVID-19 pandemic.8-10 Guidelines from the NCCN concentrated on clear divisions between disease stages to determine provider response. Guidelines for melanoma patients proposed by the ESMO assign tiers by value-based priority in various treatment settings, which offered flexibility to providers as the COVID-19 landscape continued to change. Recommendations from the NCCN and ESMO are summarized in Tables 1 to 5.
Although these guidelines initially may have been proposed to delay treatment of lower-acuity tumors, such delay might not be feasible given the unknown duration of this pandemic and future disease waves. One review of several studies, which addressed the outcomes on melanoma survival following the surgical delay recommended by the NCCN, revealed contradictory evidence.12 Further, sufficiently powered studies will be needed to better understand the impact of delaying treatment during the summer COVID-19 surge on patients with skin cancer. Therefore, physicians must triage patients accordingly to manage and treat while also preventing disease spread.
Tips for Performing Dermatologic Surgery
Careful consideration should be made to protect both the patient and staff during office-based excisional surgery during the COVID-19 pandemic. To minimize the risk of transmission of SARS-CoV-2, patients and staff should (1) be screened for symptoms of COVID-19 at least 48 hours prior to entering the office via telephone screening questions, and (2) follow proper hygiene and contact procedures once entering the office. Consider obtaining a nasal polymerase chain reaction swab or saliva test 48 hours prior to the procedure if the patient is undergoing a head and neck procedure or there is risk for transmission.
Guidelines from the ESMO recommended that all patients undergoing surgery or therapy should be swabbed for SARS-CoV-2 before each treatment.11 Patients should wear a mask, remain 6-feet apart in the waiting room, and avoid touching objects until they enter the procedure room. Objects that the patient must touch, such as pens, should be cleaned immediately after such contact with either alcohol or soap and water for 20 seconds.
Office capacity should be reduced by allowing no more than 1 person to accompany the patient and ensuring the presence of only the minimum staff needed for the procedure. Staff who are deemed necessary should wear a mask continuously and gloves during patient contact.
Once in the procedure room, providers might be at elevated risk of contracting COVID-19 or transmitting SARS-CoV-2. A properly fitted N95 respirator and a face shield are recommended, especially for facial cases. N95 respirators can be reused by following the latest Centers for Disease Control and Prevention recommendations for reuse and decontamination techniques,13 which may include protecting the N95 respirator with a surgical mask and storing it in a paper bag when not in use. Consider testing asymptomatic patients in facial cases when they cannot wear a mask.
Steps should be taken to reduce in-person visits. Dissolving sutures can help avoid return visits. Follow-up visits and postprocedural questions should be managed by telehealth. However, patients with a high-risk underlying conditions (eg, posttransplantation, immunosuppressed) should continue to obtain regular skin checks because they are at higher risk for more aggressive malignancies, such as Merkel cell carcinoma.
Conclusion
The future trajectory of the COVID-19 pandemic is uncertain. Dermatologists should continue providing care for patients with skin cancer while mitigating the risk for COVID-19 infection and transmission of SARS-CoV-2. Guidelines provided by the NCCN and ESMO should help providers triage patients. Decisions should be made case by case, keeping in mind the availability of resources and practicing in compliance with local guidance.
- Moletta L, Pierobon ES, Capovilla G, et al. International guidelines and recommendations for surgery during COVID-19 pandemic: a systematic review. Int J Surg. 2020;79:180-188.
- Ueda M, Martins R, Hendrie PC, et al. Managing cancer care during the COVID-19 pandemic: agility and collaboration toward common goal. J Natl Compr Canc Netw. 2020:1-4.
- Center for Medicare & Medicaid Services. Non-emergent, elective medical services, and treatment recommendations. Published April 7, 2020. Accessed October 15, 2020. https://www.cms.gov/files/document/cms-non-emergent-elective-medical-recommendations.pdf
- Muddasani S, Housholder A, Fleischer AB. An assessment of United States dermatology practices during the COVID-19 outbreak. J Dermatolog Treat. 2020;31:436-438.
- Coronavirus Resource Center, Johns Hopkins University & Medicine. Rate of positive tests in the US and states over time. Updated December 11, 2020. Accessed December 11, 2020. https://coronavirus.jhu.edu/testing/individual-states
- Middleton J, Lopes H, Michelson K, et al. Planning for a second wave pandemic of COVID-19 and planning for winter: a statement from the Association of Schools of Public Health in the European Region. Int J Public Health. 2020;65:1525-1527.
- Liang W, Guan W, Chen R, et al. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol. 2020;21:335-337.
- National Comprehensive Cancer Network. Advisory statement for non-melanoma skin cancer care during the COVID-19 pandemic (version 4). Published May 22, 2020. Accessed December 11, 2020. https://www.nccn.org/covid-19/pdf/NCCN-NMSC.pdf
National Comprehensive Cancer Network. Short-term recommendations for cutaneous melanoma management during COVID-19 pandemic (version 3). Published May 6, 2020. Accessed December 11, 2020. www.nccn.org/covid-19/pdf/Melanoma.pdf - Conforti C, Giuffrida R, Di Meo N, et al. Management of advanced melanoma in the COVID-19 era. Dermatol Ther. 2020;33:e13444.
- ESMO [European Society for Medical Oncology]. Cancer patient management during the COVID-19 pandemic. Accessed Decemeber 11, 2020. https://www.esmo.org/guidelines/cancer-patient-management-during-the-covid-19-pandemic?hit=ehp
- Guhan S, Boland G, Tanabe K, et al. Surgical delay and mortality for primary cutaneous melanoma [published online July 22, 2020]. J Am Acad Dermatol. doi:10.1016/j.jaad.2020.07.078
- Centers for Disease Control and Prevention. Implementing filtering facepiece respirator (FFR) reuse, including reuse after decontamination, when there are known shortages of N95 respirators. Updated October 19, 2020. Accessed December 11, 2020. https://www.cdc.gov/coronavirus/2019-ncov/hcp/ppe-strategy/decontamination-reuse-respirators.html
- Moletta L, Pierobon ES, Capovilla G, et al. International guidelines and recommendations for surgery during COVID-19 pandemic: a systematic review. Int J Surg. 2020;79:180-188.
- Ueda M, Martins R, Hendrie PC, et al. Managing cancer care during the COVID-19 pandemic: agility and collaboration toward common goal. J Natl Compr Canc Netw. 2020:1-4.
- Center for Medicare & Medicaid Services. Non-emergent, elective medical services, and treatment recommendations. Published April 7, 2020. Accessed October 15, 2020. https://www.cms.gov/files/document/cms-non-emergent-elective-medical-recommendations.pdf
- Muddasani S, Housholder A, Fleischer AB. An assessment of United States dermatology practices during the COVID-19 outbreak. J Dermatolog Treat. 2020;31:436-438.
- Coronavirus Resource Center, Johns Hopkins University & Medicine. Rate of positive tests in the US and states over time. Updated December 11, 2020. Accessed December 11, 2020. https://coronavirus.jhu.edu/testing/individual-states
- Middleton J, Lopes H, Michelson K, et al. Planning for a second wave pandemic of COVID-19 and planning for winter: a statement from the Association of Schools of Public Health in the European Region. Int J Public Health. 2020;65:1525-1527.
- Liang W, Guan W, Chen R, et al. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol. 2020;21:335-337.
- National Comprehensive Cancer Network. Advisory statement for non-melanoma skin cancer care during the COVID-19 pandemic (version 4). Published May 22, 2020. Accessed December 11, 2020. https://www.nccn.org/covid-19/pdf/NCCN-NMSC.pdf
National Comprehensive Cancer Network. Short-term recommendations for cutaneous melanoma management during COVID-19 pandemic (version 3). Published May 6, 2020. Accessed December 11, 2020. www.nccn.org/covid-19/pdf/Melanoma.pdf - Conforti C, Giuffrida R, Di Meo N, et al. Management of advanced melanoma in the COVID-19 era. Dermatol Ther. 2020;33:e13444.
- ESMO [European Society for Medical Oncology]. Cancer patient management during the COVID-19 pandemic. Accessed Decemeber 11, 2020. https://www.esmo.org/guidelines/cancer-patient-management-during-the-covid-19-pandemic?hit=ehp
- Guhan S, Boland G, Tanabe K, et al. Surgical delay and mortality for primary cutaneous melanoma [published online July 22, 2020]. J Am Acad Dermatol. doi:10.1016/j.jaad.2020.07.078
- Centers for Disease Control and Prevention. Implementing filtering facepiece respirator (FFR) reuse, including reuse after decontamination, when there are known shortages of N95 respirators. Updated October 19, 2020. Accessed December 11, 2020. https://www.cdc.gov/coronavirus/2019-ncov/hcp/ppe-strategy/decontamination-reuse-respirators.html
Practice Points
- Consider the rate of cases and transmission in your area during a pandemic surge when triaging surgical and nonsurgical cases.
- If performing head and neck surgical procedures or cosmetic procedures in which the patient cannot wear a mask, consider testing them 24 to 48 hours before the procedure.
- Follow Centers for Disease Control and Prevention (CDC) guidelines concerning screening asymptomatic patients. Also, follow CDC guidelines on testing patients who have had prior infections.
- Ensure proper personal protective equipment for yourself and staff, including the use of properly fitting N95 respirators and face shields.
Many health plans now must cover full cost of expensive HIV prevention drugs
Ted Howard started taking Truvada a few years ago because he wanted to protect himself against HIV, the virus that causes AIDS. But the daily pill was so pricey he was seriously thinking about giving it up.
Under his insurance plan, the former flight attendant and customer service instructor owed $500 in copayments every month for the drug and an additional $250 every three months for lab work and clinic visits.
Luckily for Howard, his doctor at Las Vegas’ Huntridge Family Clinic, which specializes in LGBTQ care, enrolled him in a clinical trial that covered his medication and other costs in full.
“If I hadn’t been able to get into the trial, I wouldn’t have kept taking PrEP,” said Howard, 68, using the shorthand term for “preexposure prophylaxis.” Taken daily, these drugs — like Truvada — are more than 90% effective at preventing infection with HIV.
(some plans already began doing so last year).
Drugs in this category — Truvada, Descovy and, newly available, a generic version of Truvada — received an “A” recommendation by the U.S. Preventive Services Task Force. Under the Affordable Care Act, preventive services that receive an “A” or “B” rating by the task force, a group of medical experts in prevention and primary care, must be covered by most private health plans without making members share the cost, usually through copayments or deductibles. Only plans that are grandfathered under the health law are exempt.
The task force recommended PrEP for people at high risk of HIV infection, including men who have sex with men and injection drug users.
In the United States, more than 1 million people live with HIV, and nearly 40,000 new HIV cases are diagnosed every year. Yet fewer than 10% of people who could benefit from PrEP are taking it. One key reason is that out-of-pocket costs can exceed $1,000 annually, according to a study published in the American Journal of Public Health last year. Required periodic blood tests and doctor visits can add hundreds of dollars to the cost of the drug, and it’s not clear if insurers are required to pick up all those costs.
“Cost sharing has been a problem,” said Michael Crews, policy director at One Colorado, an advocacy group for the LGBTQ community. “It’s not just getting on PrEP and taking a pill. It’s the lab and clinical services. That’s a huge barrier for folks.”
Whether you’re shopping for a new plan during open enrollment or want to check out what your current plan covers, here are answers to questions you may have about the new preventive coverage requirement.
Q: How can people find out whether their health plan covers PrEP medications without charge?
The plan’s list of covered drugs, called a formulary, should spell out which drugs are covered, along with details about which drug tier they fall into. Drugs placed in higher tiers generally have higher cost sharing. That list should be online with the plan documents that give coverage details.
Sorting out coverage and cost sharing can be tricky. Both Truvada and Descovy can also be used to treat HIV, and if they are taken for that purpose, a plan may require members to pay some of the cost. But if the drugs are taken to prevent HIV infection, patients shouldn’t owe anything out-of-pocket, no matter which tier they are on.
In a recent analysis of online formularies for plans sold on the ACA marketplaces, Carl Schmid, executive director of the HIV + Hepatitis Policy Institute, found that many plans seemed out of compliance with the requirement to cover PrEP without cost sharing this year.
But representatives for Oscar and Kaiser Permanente, two insurers that were called out in the analysis for lack of compliance, said the drugs are covered without cost sharing in plans nationwide if they are taken to prevent HIV. Schmid later revised his analysis to reflect Oscar’s coverage.
Coverage and cost-sharing information needs to be transparent and easy to find, Schmid said.
“I acted like a shopper of insurance, just like any person would do,” he said. “Even when the information is correct, [it’s so] difficult to find [and there’s] no uniformity.”
It may be necessary to call the insurer directly to confirm coverage details if information on the website is unclear.
Q: Are all three drugs covered without cost sharing?
Health plans have to cover at least one of the drugs in this category — Descovy and the brand and generic versions of Truvada — without cost sharing. People may have to jump through some hoops to get approval for a specific drug, however. For example, Oscar plans sold in 18 states cover the three PrEP options without cost sharing. The generic version of Truvada doesn’t require prior authorization by the insurer. But if someone wants to take the name-brand drug, that person has to go through an approval process. Descovy, a newer drug, is available without cost sharing only if people are unable to use Truvada or its generic version because of clinical intolerance or other issues.
Q: What about the lab work and clinical visits that are necessary while taking PrEP? Are those services also covered without cost sharing?
That is the thousand-dollar question. People who are taking drugs to prevent HIV infection need to meet with a clinician and have blood work every three months to test for HIV, hepatitis B and sexually transmitted infections, and to check their kidney function.
The task force recommendation doesn’t specify whether these services must also be covered without cost sharing, and advocates say federal guidance is necessary to ensure they are free.
“If you’ve got a high-deductible plan and you’ve got to meet it before those services are covered, that’s going to add up,” said Amy Killelea, senior director of health systems and policy at the National Alliance of State & Territorial AIDS Directors. “We’re trying to emphasize that it’s integral to the intervention itself.”
A handful of states have programs that help people cover their out-of-pocket costs for lab and clinical visits, generally based on income.
There is precedent for including free ancillary care as part of a recommended preventive service. After consumers and advocates complained, the Centers for Medicare & Medicaid Services (CMS) clarified that under the ACA removing a polyp during a screening colonoscopy is considered an integral part of the procedure and patients shouldn’t be charged for it.
CMS officials declined to clarify whether PrEP services such as lab work and clinical visits are to be covered without cost sharing as part of the preventive service and noted that states generally enforce such insurance requirements. “CMS intends to contact state regulators, as appropriate, to discuss issuer’s compliance with the federal requirements and whether issuers need further guidance on which services associated with PrEP must be covered without cost sharing,” the agency said in a statement.
Q: What if someone runs into roadblocks getting a plan to cover PrEP or related services without cost sharing?
If an insurer charges for the medication or a follow-up visit, people may have to go through an appeals process to fight it.
“They’d have to appeal to the insurance company and then to the state if they don’t succeed,” said Nadeen Israel, vice president of policy and advocacy at the AIDS Foundation of Chicago. “Most people don’t know to do that.”
Q: Are uninsured people also protected by this new cost-sharing change for PrEP?
Unfortunately, no. The ACA requirement to cover recommended preventive services without charging patients applies only to private insurance plans. People without insurance don’t benefit. Gilead, which makes both Truvada and Descovy, has a patient assistance program for the uninsured.
Kaiser Health News is a nonprofit news service covering health issues. It is an editorially independent program of Kaiser Family Foundation, which is not affiliated with Kaiser Permanente.
Ted Howard started taking Truvada a few years ago because he wanted to protect himself against HIV, the virus that causes AIDS. But the daily pill was so pricey he was seriously thinking about giving it up.
Under his insurance plan, the former flight attendant and customer service instructor owed $500 in copayments every month for the drug and an additional $250 every three months for lab work and clinic visits.
Luckily for Howard, his doctor at Las Vegas’ Huntridge Family Clinic, which specializes in LGBTQ care, enrolled him in a clinical trial that covered his medication and other costs in full.
“If I hadn’t been able to get into the trial, I wouldn’t have kept taking PrEP,” said Howard, 68, using the shorthand term for “preexposure prophylaxis.” Taken daily, these drugs — like Truvada — are more than 90% effective at preventing infection with HIV.
(some plans already began doing so last year).
Drugs in this category — Truvada, Descovy and, newly available, a generic version of Truvada — received an “A” recommendation by the U.S. Preventive Services Task Force. Under the Affordable Care Act, preventive services that receive an “A” or “B” rating by the task force, a group of medical experts in prevention and primary care, must be covered by most private health plans without making members share the cost, usually through copayments or deductibles. Only plans that are grandfathered under the health law are exempt.
The task force recommended PrEP for people at high risk of HIV infection, including men who have sex with men and injection drug users.
In the United States, more than 1 million people live with HIV, and nearly 40,000 new HIV cases are diagnosed every year. Yet fewer than 10% of people who could benefit from PrEP are taking it. One key reason is that out-of-pocket costs can exceed $1,000 annually, according to a study published in the American Journal of Public Health last year. Required periodic blood tests and doctor visits can add hundreds of dollars to the cost of the drug, and it’s not clear if insurers are required to pick up all those costs.
“Cost sharing has been a problem,” said Michael Crews, policy director at One Colorado, an advocacy group for the LGBTQ community. “It’s not just getting on PrEP and taking a pill. It’s the lab and clinical services. That’s a huge barrier for folks.”
Whether you’re shopping for a new plan during open enrollment or want to check out what your current plan covers, here are answers to questions you may have about the new preventive coverage requirement.
Q: How can people find out whether their health plan covers PrEP medications without charge?
The plan’s list of covered drugs, called a formulary, should spell out which drugs are covered, along with details about which drug tier they fall into. Drugs placed in higher tiers generally have higher cost sharing. That list should be online with the plan documents that give coverage details.
Sorting out coverage and cost sharing can be tricky. Both Truvada and Descovy can also be used to treat HIV, and if they are taken for that purpose, a plan may require members to pay some of the cost. But if the drugs are taken to prevent HIV infection, patients shouldn’t owe anything out-of-pocket, no matter which tier they are on.
In a recent analysis of online formularies for plans sold on the ACA marketplaces, Carl Schmid, executive director of the HIV + Hepatitis Policy Institute, found that many plans seemed out of compliance with the requirement to cover PrEP without cost sharing this year.
But representatives for Oscar and Kaiser Permanente, two insurers that were called out in the analysis for lack of compliance, said the drugs are covered without cost sharing in plans nationwide if they are taken to prevent HIV. Schmid later revised his analysis to reflect Oscar’s coverage.
Coverage and cost-sharing information needs to be transparent and easy to find, Schmid said.
“I acted like a shopper of insurance, just like any person would do,” he said. “Even when the information is correct, [it’s so] difficult to find [and there’s] no uniformity.”
It may be necessary to call the insurer directly to confirm coverage details if information on the website is unclear.
Q: Are all three drugs covered without cost sharing?
Health plans have to cover at least one of the drugs in this category — Descovy and the brand and generic versions of Truvada — without cost sharing. People may have to jump through some hoops to get approval for a specific drug, however. For example, Oscar plans sold in 18 states cover the three PrEP options without cost sharing. The generic version of Truvada doesn’t require prior authorization by the insurer. But if someone wants to take the name-brand drug, that person has to go through an approval process. Descovy, a newer drug, is available without cost sharing only if people are unable to use Truvada or its generic version because of clinical intolerance or other issues.
Q: What about the lab work and clinical visits that are necessary while taking PrEP? Are those services also covered without cost sharing?
That is the thousand-dollar question. People who are taking drugs to prevent HIV infection need to meet with a clinician and have blood work every three months to test for HIV, hepatitis B and sexually transmitted infections, and to check their kidney function.
The task force recommendation doesn’t specify whether these services must also be covered without cost sharing, and advocates say federal guidance is necessary to ensure they are free.
“If you’ve got a high-deductible plan and you’ve got to meet it before those services are covered, that’s going to add up,” said Amy Killelea, senior director of health systems and policy at the National Alliance of State & Territorial AIDS Directors. “We’re trying to emphasize that it’s integral to the intervention itself.”
A handful of states have programs that help people cover their out-of-pocket costs for lab and clinical visits, generally based on income.
There is precedent for including free ancillary care as part of a recommended preventive service. After consumers and advocates complained, the Centers for Medicare & Medicaid Services (CMS) clarified that under the ACA removing a polyp during a screening colonoscopy is considered an integral part of the procedure and patients shouldn’t be charged for it.
CMS officials declined to clarify whether PrEP services such as lab work and clinical visits are to be covered without cost sharing as part of the preventive service and noted that states generally enforce such insurance requirements. “CMS intends to contact state regulators, as appropriate, to discuss issuer’s compliance with the federal requirements and whether issuers need further guidance on which services associated with PrEP must be covered without cost sharing,” the agency said in a statement.
Q: What if someone runs into roadblocks getting a plan to cover PrEP or related services without cost sharing?
If an insurer charges for the medication or a follow-up visit, people may have to go through an appeals process to fight it.
“They’d have to appeal to the insurance company and then to the state if they don’t succeed,” said Nadeen Israel, vice president of policy and advocacy at the AIDS Foundation of Chicago. “Most people don’t know to do that.”
Q: Are uninsured people also protected by this new cost-sharing change for PrEP?
Unfortunately, no. The ACA requirement to cover recommended preventive services without charging patients applies only to private insurance plans. People without insurance don’t benefit. Gilead, which makes both Truvada and Descovy, has a patient assistance program for the uninsured.
Kaiser Health News is a nonprofit news service covering health issues. It is an editorially independent program of Kaiser Family Foundation, which is not affiliated with Kaiser Permanente.
Ted Howard started taking Truvada a few years ago because he wanted to protect himself against HIV, the virus that causes AIDS. But the daily pill was so pricey he was seriously thinking about giving it up.
Under his insurance plan, the former flight attendant and customer service instructor owed $500 in copayments every month for the drug and an additional $250 every three months for lab work and clinic visits.
Luckily for Howard, his doctor at Las Vegas’ Huntridge Family Clinic, which specializes in LGBTQ care, enrolled him in a clinical trial that covered his medication and other costs in full.
“If I hadn’t been able to get into the trial, I wouldn’t have kept taking PrEP,” said Howard, 68, using the shorthand term for “preexposure prophylaxis.” Taken daily, these drugs — like Truvada — are more than 90% effective at preventing infection with HIV.
(some plans already began doing so last year).
Drugs in this category — Truvada, Descovy and, newly available, a generic version of Truvada — received an “A” recommendation by the U.S. Preventive Services Task Force. Under the Affordable Care Act, preventive services that receive an “A” or “B” rating by the task force, a group of medical experts in prevention and primary care, must be covered by most private health plans without making members share the cost, usually through copayments or deductibles. Only plans that are grandfathered under the health law are exempt.
The task force recommended PrEP for people at high risk of HIV infection, including men who have sex with men and injection drug users.
In the United States, more than 1 million people live with HIV, and nearly 40,000 new HIV cases are diagnosed every year. Yet fewer than 10% of people who could benefit from PrEP are taking it. One key reason is that out-of-pocket costs can exceed $1,000 annually, according to a study published in the American Journal of Public Health last year. Required periodic blood tests and doctor visits can add hundreds of dollars to the cost of the drug, and it’s not clear if insurers are required to pick up all those costs.
“Cost sharing has been a problem,” said Michael Crews, policy director at One Colorado, an advocacy group for the LGBTQ community. “It’s not just getting on PrEP and taking a pill. It’s the lab and clinical services. That’s a huge barrier for folks.”
Whether you’re shopping for a new plan during open enrollment or want to check out what your current plan covers, here are answers to questions you may have about the new preventive coverage requirement.
Q: How can people find out whether their health plan covers PrEP medications without charge?
The plan’s list of covered drugs, called a formulary, should spell out which drugs are covered, along with details about which drug tier they fall into. Drugs placed in higher tiers generally have higher cost sharing. That list should be online with the plan documents that give coverage details.
Sorting out coverage and cost sharing can be tricky. Both Truvada and Descovy can also be used to treat HIV, and if they are taken for that purpose, a plan may require members to pay some of the cost. But if the drugs are taken to prevent HIV infection, patients shouldn’t owe anything out-of-pocket, no matter which tier they are on.
In a recent analysis of online formularies for plans sold on the ACA marketplaces, Carl Schmid, executive director of the HIV + Hepatitis Policy Institute, found that many plans seemed out of compliance with the requirement to cover PrEP without cost sharing this year.
But representatives for Oscar and Kaiser Permanente, two insurers that were called out in the analysis for lack of compliance, said the drugs are covered without cost sharing in plans nationwide if they are taken to prevent HIV. Schmid later revised his analysis to reflect Oscar’s coverage.
Coverage and cost-sharing information needs to be transparent and easy to find, Schmid said.
“I acted like a shopper of insurance, just like any person would do,” he said. “Even when the information is correct, [it’s so] difficult to find [and there’s] no uniformity.”
It may be necessary to call the insurer directly to confirm coverage details if information on the website is unclear.
Q: Are all three drugs covered without cost sharing?
Health plans have to cover at least one of the drugs in this category — Descovy and the brand and generic versions of Truvada — without cost sharing. People may have to jump through some hoops to get approval for a specific drug, however. For example, Oscar plans sold in 18 states cover the three PrEP options without cost sharing. The generic version of Truvada doesn’t require prior authorization by the insurer. But if someone wants to take the name-brand drug, that person has to go through an approval process. Descovy, a newer drug, is available without cost sharing only if people are unable to use Truvada or its generic version because of clinical intolerance or other issues.
Q: What about the lab work and clinical visits that are necessary while taking PrEP? Are those services also covered without cost sharing?
That is the thousand-dollar question. People who are taking drugs to prevent HIV infection need to meet with a clinician and have blood work every three months to test for HIV, hepatitis B and sexually transmitted infections, and to check their kidney function.
The task force recommendation doesn’t specify whether these services must also be covered without cost sharing, and advocates say federal guidance is necessary to ensure they are free.
“If you’ve got a high-deductible plan and you’ve got to meet it before those services are covered, that’s going to add up,” said Amy Killelea, senior director of health systems and policy at the National Alliance of State & Territorial AIDS Directors. “We’re trying to emphasize that it’s integral to the intervention itself.”
A handful of states have programs that help people cover their out-of-pocket costs for lab and clinical visits, generally based on income.
There is precedent for including free ancillary care as part of a recommended preventive service. After consumers and advocates complained, the Centers for Medicare & Medicaid Services (CMS) clarified that under the ACA removing a polyp during a screening colonoscopy is considered an integral part of the procedure and patients shouldn’t be charged for it.
CMS officials declined to clarify whether PrEP services such as lab work and clinical visits are to be covered without cost sharing as part of the preventive service and noted that states generally enforce such insurance requirements. “CMS intends to contact state regulators, as appropriate, to discuss issuer’s compliance with the federal requirements and whether issuers need further guidance on which services associated with PrEP must be covered without cost sharing,” the agency said in a statement.
Q: What if someone runs into roadblocks getting a plan to cover PrEP or related services without cost sharing?
If an insurer charges for the medication or a follow-up visit, people may have to go through an appeals process to fight it.
“They’d have to appeal to the insurance company and then to the state if they don’t succeed,” said Nadeen Israel, vice president of policy and advocacy at the AIDS Foundation of Chicago. “Most people don’t know to do that.”
Q: Are uninsured people also protected by this new cost-sharing change for PrEP?
Unfortunately, no. The ACA requirement to cover recommended preventive services without charging patients applies only to private insurance plans. People without insurance don’t benefit. Gilead, which makes both Truvada and Descovy, has a patient assistance program for the uninsured.
Kaiser Health News is a nonprofit news service covering health issues. It is an editorially independent program of Kaiser Family Foundation, which is not affiliated with Kaiser Permanente.