User login
Cutaneous Manifestations of COVID-19
The pathogenesis of coronavirus disease 2019 (COVID-19), the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), is not yet completely understood. Thus far, it is known to affect multiple organ systems, including gastrointestinal, neurological, and cardiovascular, with typical clinical symptoms of COVID-19 including fever, cough, myalgia, headache, anosmia, and diarrhea.1 This multiorgan attack may be secondary to an exaggerated inflammatory reaction with vasculopathy and possibly a hypercoagulable state. Skin manifestations also are prevalent in COVID-19, and they often result in polymorphous presentations.2 This article aims to summarize cutaneous clinical signs of COVID-19 so that dermatologists can promptly identify and manage COVID-19 and prevent its spread.
Methods
A PubMed search of articles indexed for MEDLINE was conducted on June 30, 2020. The literature included observational studies, case reports, and literature reviews from January 1, 2020, to June 30, 2020. Search terms included COVID-19, SARS-CoV-2, and coronavirus used in combination with cutaneous, skin, and dermatology. All of the resulting articles were then reviewed for relevance to the cutaneous manifestations of COVID-19. Only confirmed cases of COVID-19 infection were included in this review; suspected unconfirmed cases were excluded. Further exclusion criteria included articles that discussed dermatology in the time of COVID-19 that did not explicitly address its cutaneous manifestations. The remaining literature was evaluated to provide dermatologists and patients with a concise resource for the cutaneous signs and symptoms of COVID-19. Data extracted from the literature included geographic region, number of patients with skin findings, status of COVID-19 infection and timeline, and cutaneous signs. If a cutaneous sign was not given a clear diagnosis in the literature, the senior authors (A.L. and J.J.) assigned it to its most similar classification to aid in ease of understanding and clarity for the readers.
Results
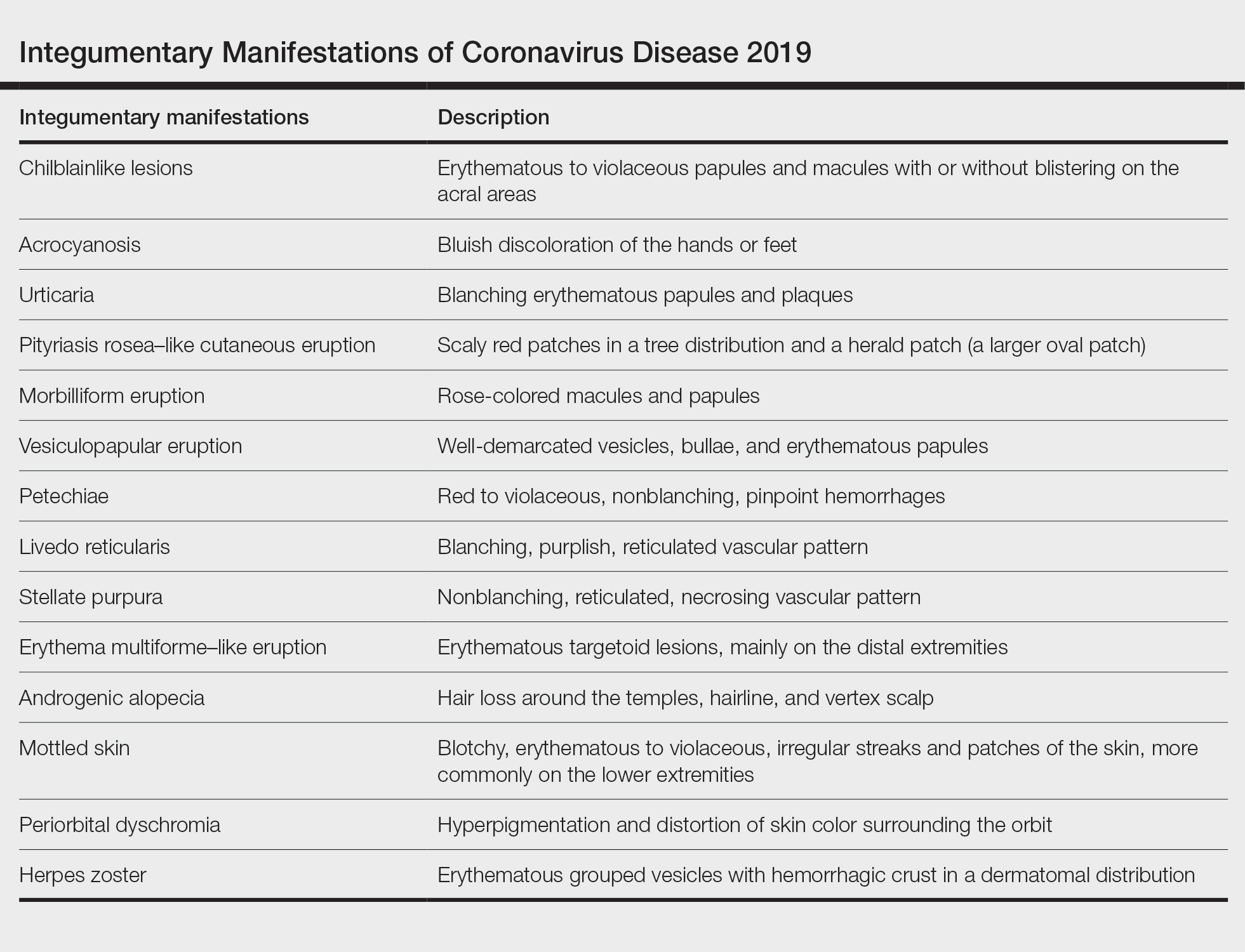
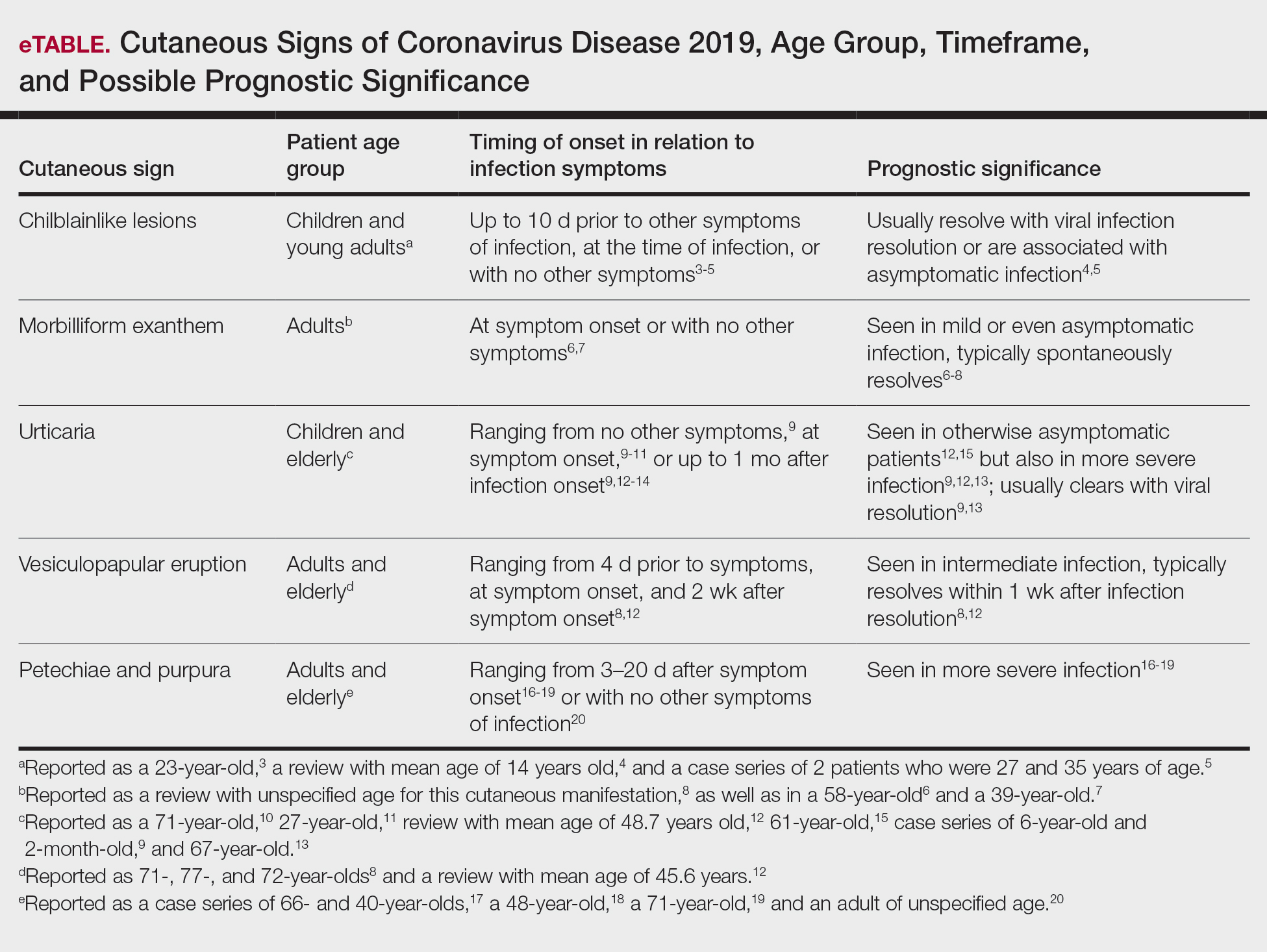
A search of the key terms resulted in 75 articles published in the specified date range. After excluding overtly irrelevant articles and dermatologic conditions in the time of COVID-19 without confirmed SARS-CoV-2 infection, 25 articles ultimately met inclusion criteria. Relevant references from the articles also were explored for cutaneous dermatologic manifestations of COVID-19. Cutaneous manifestations that were repeatedly reported included chilblainlike lesions; acrocyanosis; urticaria; pityriasis rosea–like cutaneous eruption; erythema multiforme–like, vesiculopapular, and morbilliform eruptions; petechiae; livedo reticularis; and purpuric livedo reticularis (dermatologists may label this stellate purpura). Fewer but nonetheless notable cases of androgenic alopecia, periorbital dyschromia, and herpes zoster exacerbations also were documented. The Table summarizes the reported integumentary findings. The eTable groups the common findings and describes patient age, time to onset of cutaneous sign, and any prognostic significance as seen in the literature.
Chilblainlike Lesions and Acrocyanosis
Chilblainlike lesions are edematous eruptions of the fingers and toes. They usually do not scar and are described as erythematous to violaceous papules and macules with possible bullae on the digits. Skin biopsies demonstrate a histopathologic pattern of vacuolar interface dermatitis with necrotic keratinocytes and a thickened basement membrane. Lymphocytic infiltrate presents in a perieccrine distribution, occasionally with plasma cells. The dermatopathologic findings mimic those of chilblain lupus but lack dermal edema.3
These eruptions have been reported in cases of COVID-19 that more frequently affect children and young adults. They usually resolve over the course of viral infection, averaging within 14 days. Chilblainlike eruptions often are associated with pruritus or pain. They commonly are asymmetrical and appear more often on the toes than the fingers.4 In cases of COVID-19 that lack systemic symptoms, chilblainlike lesions have been seen on the dorsal fingers as the first presenting sign of infection.5
Acral erythema and chilblainlike lesions frequently have been associated with milder infection. Another positive prognostic indicator is the manifestation of these signs in younger individuals.3
Morbilliform Exanthem
The morbilliform exanthem associated with COVID-19 also typically presents in patients with milder disease. It often affects the buttocks, lower abdomen, and thighs, but spares the palms, soles, and mucosae.4 This skin sign, which may start out as a generalized morbilliform exanthem, has been seen to morph into macular hemorrhagic purpura on the legs. These cutaneous lesions typically spontaneously resolve.8
In a case report by Najarian,6 a morbilliform exanthem was seen on the legs, arms, and trunk of a patient who was otherwise asymptomatic but tested positive for COVID-19. The morbilliform exanthem then became confluent on the trunk. Notably, the patient reported pain of the hands and feet.6
Another case report described a patient with edematous annular plaques on the palms, neck, and upper extremities who presented solely with fever.7 The biopsy specimen was nonspecific but indicated a viral exanthem. Histopathology showed perivascular lymphocytic infiltrate, dermal edema and vacuoles, spongiosis, dyskeratotic basilar keratinocytes, and few neutrophils without eosinophils.7
Eczematous Eruption
A confluent eczematous eruption in the flexural areas, the antecubital fossae, and axillary folds has been found in COVID-19 patients.21,22 An elderly patient with severe COVID-19 developed a squamous erythematous periumbilical patch 1 day after hospital admission. The cutaneous eruption rapidly progressed to digitate scaly plaques on the trunk, thighs, and flank. A biopsy specimen showed epidermal spongiosis, vesicles containing lymphocytes, and Langerhans cells. The upper dermis demonstrated a lymphohistiocytic infiltrate.23
Pityriasis Rosea–Like Eruption
In Iran, a COVID-19–infected patient developed an erythematous papulosquamous eruption with a herald patch and trailing scales 3 days after viral symptoms, resembling that of pityriasis rosea.24 Nests of Langerhans cells within the epidermis are seen in many viral exanthems, including cases of COVID-19 and pityriasis rosea.25
Urticaria
According to a number of case reports, urticarial lesions have been the first presenting sign of COVID-19 infection, most resolving with antihistamines.10,11 Some patients with more severe symptoms have had widespread urticaria. An urticarial exanthem appearing on the bilateral thighs and buttocks may be the initial sign of infection.12,15 Pruritic erythematous plaques over the face and acral areas is another initial sign. Interestingly, pediatric patients have reported nonpruritic urticaria.9
Urticaria also has been seen as a late dermatologic sign of viral infection. After battling relentless viral infection for 1 month, a pruritic, confluent, ill-defined eruption appeared along a patient’s trunk, back, and proximal extremities. Histopathologic examination concluded a perivascular lymphocytic infiltrate and dilated vessels in the dermis. The urticaria resolved a week later, and the patient’s nasopharyngeal swab finally came back negative.13
Vesiculopapular Eruption
Vesicles mimicking those of chickenpox have been reported. A study of 375 confirmed cases of COVID-19 by Galván Casas et al12 showed a 9% incidence of this vesicular eruption. A study by Sachdeva et al8 revealed vesicular eruptions in 25 of 72 patients. Pruritic papules and vesicles may resemble Grover disease. This cutaneous sign may be seen in the submammary folds, on the hips, or diffusely over the body.
Erythema Multiforme–Like Eruption
Targetoid lesions similar to those of erythema multiforme erupted in 2 of 27 patients with mild COVID-19 infection in a review by Wollina et al.4 In a study of 4 patients with erythema multiforme–like eruptions after COVID-19 symptoms resolved, 3 had palatal petechiae. Two of 4 patients had pseudovesicles in the center of the erythematous targetoid patches.26 Targetoid lesions on the extremities have been reported in pediatric patients with COVID-19 infections. These patients often present without any typical viral symptoms but rather just a febrile exanthem or exanthem alone. Thus, to minimize spread of the virus, it is vital to recognize COVID-19 infection early in patients with a viral exanthem during the time of high COVID-19 incidence.4
Livedo Reticularis
In the United States, a case series reported 2 patients with transient livedo reticularis throughout the course of COVID-19 infection. The cutaneous eruption resembled erythema ab igne, but there was no history of exposure to heat.16
Stellate Purpura
In severe COVID-19 infection, a reticulated nonblanching purpura on the buttocks has been reported to demonstrate pauci-inflammatory vascular thrombosis, complement membrane attack complex deposition, and endothelial injury on dermatopathology. Stellate purpura on palmoplantar surfaces also has shown arterial thrombosis in the deep dermis due to complement deposition.17
Petechiae and Purpura
A morbilliform exanthem may develop into significant petechiae in the popliteal fossae, buttocks, and thighs. A punch biopsy specimen demonstrates a perivascular lymphocytic infiltrate with erythrocyte extravasation and papillary dermal edema with dyskeratotic cells.18 Purpura of the lower extremities may develop, with histopathology showing fibrinoid necrosis of small vessel walls, neutrophilic infiltrate with karyorrhexis, and granular complement deposition.19
In Thailand, a patient was misdiagnosed with dengue after presenting with petechiae and low platelet count.20 Further progression of the viral illness resulted in respiratory symptoms. Subsequently, the patient tested positive for COVID-19. This case demonstrates that cutaneous signs of many sorts may be the first presenting signs of COVID-19, even prior to febrile symptoms.20
Androgenic Alopecia
Studies have shown that androgens are related in the pathogenesis of COVID-19. Coronavirus disease 2019 uses a cellular co-receptor, TMPRSS2, which is androgen regulated.27 In a study of 41 males with COVID-19, 29 had androgenic alopecia. However, this is only a correlation, and causation cannot be concluded here. It cannot be determined from this study whether androgenic alopecia is a risk factor, result of COVID-19, or confounder.28
Exaggerated Herpes Zoster
Shors29 reported a herpes zoster eruption in a patient who had symptoms of COVID-19 for 1 week. Further testing confirmed COVID-19 infection, and despite prompt treatment with valacyclovir, the eruption was slow to resolve. The patient then experienced severe postherpetic neuralgia for more than 4 weeks, even with treatment with gabapentin and lidocaine. It is hypothesized that because of the major inflammatory response caused by COVID-19, an exaggerated inflammation occurred in the dorsal root ganglion, resulting in relentless herpes zoster infection.29
Mottled Skin
Born at term, a 15-day-old neonate presented with sepsis and mottling of the skin. The patient did not have any typical COVID-19 symptoms, such as diarrhea or cough, but tested positive for COVID-19.30
Periorbital Dyschromia
Kalner and Vergilis31 reported 2 cases of periorbital dyschromia prior to any other COVID-19 infection symptoms. The discoloration improved with resolution of ensuing viral symptoms.31
Comment
Many dermatologic signs of COVID-19 have been identified. Their individual frequency and association with viral severity will become more apparent as more cases are reported. So far during this pandemic, common dermatologic manifestations have been polymorphic in clinical presentation.
Onset of Skin Manifestations
The timeline of skin signs and COVID-19 symptoms varies from the first reported sign to weeks after symptom resolution. In the Region of Murcia, Spain, Pérez-Suárez et al14 collected data on cutaneous signs of patients with COVID-19. Of the patients studied, 9 had tests confirming COVID-19 infection. Truncal urticaria, sacral ulcers, acrocyanosis, and erythema multiforme were all reported in patients more than 2 weeks after symptom onset. One case of tinea infection also was reported 4 days after fever and respiratory symptoms began.14
Presentation
Coronavirus disease 2019 has affected the skin of both the central thorax and peripheral locations. In a study of 72 patients with cutaneous signs of COVID-19 by Sachdeva et al,8 a truncal distribution was most common, but 14 patients reported acral site involvement. Sachdeva et al8 reported urticarial reactions in 7 of 72 patients with cutaneous signs. A painful acral cyanosis was seen in 11 of 72 patients. Livedo reticularis presented in 2 patients, and only 1 patient had petechiae. Cutaneous signs were the first indicators of viral infection in 9 of 72 patients; 52 patients presented with respiratory symptoms first. All of the reported cutaneous signs spontaneously resolved within 10 days.8
Recalcati32 reviewed 88 patients with COVID-19, and 18 had cutaneous signs at initial onset of viral infection or during hospitalization. The most common integumentary sign reported in this study was erythema, followed by diffuse urticaria, and then a vesicular eruption resembling varicella infection.32
Some less common phenomena have been identified in patients with COVID-19, including androgenic alopecia, exaggerated herpes zoster and postherpetic neuralgia, mottled skin, and periorbital dyschromia. Being aware of these complications may help in early treatment, diagnosis, and even prevention of viral spread.
Pathogenesis of Skin Manifestations
Few breakthroughs have been made in understanding the pathogenesis of skin manifestations of SARS-CoV-2. Acral ischemia may be a manifestation of COVID-19’s association with hypercoagulation. Increasing fibrinogen and prothrombin times lead to disseminated intravascular coagulation and microthrombi. These tiny blood clots then lodge in blood vessels and cause acral cyanosis and subsequent gangrene.2 The proposed mechanism behind this clinical manifestation in younger populations is the hypercoagulable state that COVID-19 creates. Conversely, acral erythema and chilblainlike lesions in older patients are thought to be from acral ischemia as a response to insufficient type 1 interferons. This pathophysiologic mechanism is indicative of a worse prognosis due to the large role that type 1 interferons play in antiviral responses. Coronavirus disease 2019 similarly triggers type 1 interferons; thus, their efficacy positively correlates with good disease prognosis.3
Similarly, the pathogenesis for livedo reticularis in patients with COVID-19 can only be hypothesized. Infected patients are in a hypercoagulable state, and in these cases, it was uncertain whether this was due to a disseminated intravascular coagulation, cold agglutinins, cryofibrinogens, or lupus anticoagulant.16
Nonetheless, it can be difficult to separate the primary event between vasculopathy or vasculitis in larger vessel pathology specimens. Some of the studies’ pathology reports discuss a granulocytic infiltrate and red blood cell extravasation, which represent small vessel vasculitis. However, the gangrene and necrosing livedo represent vasculopathy events. A final conclusion about the pathogenesis cannot be made without further clinical and histopathologic evaluation.
Histopathology
Biopsy specimens of reported morbilliform eruptions have demonstrated thrombosed vessels with evidence of necrosis and granulocytic infiltrate.25 Another biopsy specimen of a widespread erythematous exanthem demonstrated extravasated red blood cells and vessel wall damage similar to thrombophilic arteritis. Other reports of histopathology showed necrotic keratinocytes and lymphocytic satellitosis at the dermoepidermal junction, resembling Grover disease. These cases demonstrating necrosis suggest a strong cytokine reaction from the virus.25 A concern with these biopsy findings is that morbilliform eruptions generally show dilated vessels with lymphocytes, and these biopsy findings are consistent with a cutaneous small vessel vasculitis. Additionally, histopathologic evaluation of purpuric eruptions has shown erythrocyte extravasation and granulocytic infiltrate indicative of a cutaneous small vessel vasculitis.
Although most reported cases of cutaneous signs of COVID-19 do not have histopathologic reports, Yao et al33 conducted a dermatopathologic study that investigated the tissue in deceased patients who had COVID-19. This pathology showed hyaline thrombi within the small vessels of the skin, likely leading to the painful acral ischemia. Similarly, Yao et al33 reported autopsies finding hyaline thrombi within the small vessels of the lungs. More research should be done to explore this pathogenesis as part of prognostic factors and virulence.
Conclusion
Cutaneous signs may be the first reported symptom of COVID-19 infection, and dermatologists should be prepared to identify them. This review may be used as a guide for physicians to quickly identify potential infection as well as further understand the pathogenesis related to COVID-19. Future research is necessary to determine the dermatologic pathogenesis, infectivity, and prevalence of cutaneous manifestations of COVID-19. It also will be important to explore if vasculopathic lesions predict more severe multisystem disease.
- Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497-506.
- Criado PR, Abdalla BMZ, de Assis IC, et al. Are the cutaneous manifestations during or due to SARS-CoV-2 infection/COVID-19 frequent or not? revision of possible pathophysiologic mechanisms. Inflamm Res. 2020;69:745-756.
- Kolivras A, Dehavay F, Delplace D, et al. Coronavirus (COVID‐19) infection–induced chilblains: a case report with histopathological findings. JAAD Case Rep. 2020;6:489-492.
- Wollina U, Karadag˘ AS, Rowland-Payne C, et al. Cutaneous signs in COVID-19 patients: a review [published online May 10, 2020]. Dermatol Ther. 2020;33:E13549.
- Alramthan A, Aldaraji W. Two cases of COVID-19 presenting with a clinical picture resembling chilblains: first report from the Middle East. Clin Exp Dermatol. 2020;45:746-748.
- Najarian DJ. Morbilliform exanthem associated with COVID‐19. JAAD Case Rep. 2020;6:493-494.
- Amatore F, Macagno N, Mailhe M, et al. SARS-CoV-2 infection presenting as a febrile rash. J Eur Acad Dermatol Venereol. 2020;34:E304-E306.
- Sachdeva M, Gianotti R, Shah M, et al. Cutaneous manifestations of COVID-19: report of three cases and a review of literature. J Dermatol Sci. 2020;98:75-81.
- Morey-Olivé M, Espiau M, Mercadal-Hally M, et al. Cutaneous manifestations in the current pandemic of coronavirus infection disease (COVID 2019). An Pediatr (Engl Ed). 2020;92:374-375.
- van Damme C, Berlingin E, Saussez S, et al. Acute urticaria with pyrexia as the first manifestations of a COVID‐19 infection. J Eur Acad Dermatol Venereol. 2020;34:E300-E301.
- Henry D, Ackerman M, Sancelme E, et al. Urticarial eruption in COVID‐19 infection. J Eur Acad Dermatol Venereol. 2020;34:E244-E245.
- Galván Casas C, Català A, Carretero Hernández G, et al. Classification of the cutaneous manifestations of COVID-19: a rapid prospective nationwide consensus study in Spain with 375 cases. Br J Dermatol. 2020;183:71-77.
- Zengarini C, Orioni G, Cascavilla A, et al. Histological pattern in Covid-19-induced viral rash [published online May 2, 2020]. J Eur Acad Dermatol Venereol. doi:10.1111/jdv.16569.
- Pérez-Suárez B, Martínez-Menchón T, Cutillas-Marco E. Skin findings in the COVID-19 pandemic in the Region of Murcia [published online June 12, 2020]. Med Clin (Engl Ed). 2020;155:41-42.
- Quintana-Castanedo L, Feito-Rodríguez M, Valero-López I, et al. Urticarial exanthem as early diagnostic clue for COVID-19 infection [published online April 29, 2020]. JAAD Case Rep. 2020;6:498-499.
- Manalo IF, Smith MK, Cheeley J, et al. Reply to: “reply: a dermatologic manifestation of COVID-19: transient livedo reticularis” [published online May 7, 2020]. J Am Acad Dermatol. 2020;83:E157.
- Magro C, Mulvey JJ, Berlin D, et al. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: a report of five cases. Transl Res. 2020;220:1-13.
- Diaz-Guimaraens B, Dominguez-Santas M, Suarez-Valle A, et al. Petechial skin rash associated with severe acute respiratory syndrome coronavirus 2 infection. JAMA Dermatol. 2020;156:820-822.
- Dominguez-Santas M, Diaz-Guimaraens B, Garcia Abellas P, et al. Cutaneous small-vessel vasculitis associated with novel 2019 coronavirus SARS-CoV-2 infection (COVID-19) [published online July 2, 2020]. J Eur Acad Dermatol Venereol. 2020;34:E536-E537.
- Joob B, Wiwanitkit V. COVID-19 can present with a rash and be mistaken for dengue [published online March 22, 2020]. J Am Acad Dermatol. 2020;82:E177.
- Avellana Moreno R, Estella Villa LM, Avellana Moreno V, et al. Cutaneous manifestation of COVID‐19 in images: a case report [published online May 19, 2020]. J Eur Acad Dermatol Venereol. 2020;34:E307-E309.
- Mahé A, Birckel E, Krieger S, et al. A distinctive skin rash associated with coronavirus disease 2019 [published online June 8, 2020]? J Eur Acad Dermatol Venereol. 2020;34:E246-E247.
- Sanchez A, Sohier P, Benghanem S, et al. Digitate papulosquamous eruption associated with severe acute respiratory syndrome coronavirus 2 infection. JAMA Dermatol. 2020;156:819-820.
- Ehsani AH, Nasimi M, Bigdelo Z. Pityriasis rosea as a cutaneous manifestation of COVID‐19 infection [published online May 2, 2020]. J Eur Acad Dermatol Venereol. doi:10.1111/jdv.16579.
- Gianotti R, Veraldi S, Recalcati S, et al. Cutaneous clinico-pathological findings in three COVID-19-positive patients observed in the metropolitan area of Milan, Italy. Acta Derm Venereol. 2020;100:adv00124.
- Jimenez-Cauhe J, Ortega-Quijano D, Carretero-Barrio I, et al. Erythema multiforme-like eruption in patients with COVID-19 infection: clinical and histological findings [published online May 9, 2020]. Clin Exp Dermatol. doi:10.1111/ced.14281
- Hoffmann M, Kleine‐Weber H, Schroeder S, et al. SARS‐CoV‐2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor [published online March 5, 2020]. Cell. 2020;181:271‐280.e8.
- Goren A, Vaño‐Galván S, Wambier CG, et al. A preliminary observation: male pattern hair loss among hospitalized COVID‐19 patients in Spain—a potential clue to the role of androgens in COVID‐19 severity [published online April 23, 2020]. J Cosmet Dermatol. 2020;19:1545-1547.
- Shors AR. Herpes zoster and severe acute herpetic neuralgia as a complication of COVID-19 infection. JAAD Case Rep. 2020;6:656-657.
- Kamali Aghdam M, Jafari N, Eftekhari K. Novel coronavirus in a 15‐day‐old neonate with clinical signs of sepsis, a case report. Infect Dis (London). 2020;52:427‐429.
- Kalner S, Vergilis IJ. Periorbital erythema as a presenting sign of covid-19 [published online May 11, 2020]. JAAD Case Rep. 2020;6:996-998.
- Recalcati S. Cutaneous manifestations in COVID‐19: a first perspective. J Eur Acad Dermatol Venereol. 2020;34:E212-E213.
- Yao XH, Li TY, He ZC, et al. A pathological report of three COVID‐19 cases by minimally invasive autopsies [in Chinese]. Zhonghua Bing Li Xue Za Zhi. 2020;49:411-417.
The pathogenesis of coronavirus disease 2019 (COVID-19), the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), is not yet completely understood. Thus far, it is known to affect multiple organ systems, including gastrointestinal, neurological, and cardiovascular, with typical clinical symptoms of COVID-19 including fever, cough, myalgia, headache, anosmia, and diarrhea.1 This multiorgan attack may be secondary to an exaggerated inflammatory reaction with vasculopathy and possibly a hypercoagulable state. Skin manifestations also are prevalent in COVID-19, and they often result in polymorphous presentations.2 This article aims to summarize cutaneous clinical signs of COVID-19 so that dermatologists can promptly identify and manage COVID-19 and prevent its spread.
Methods
A PubMed search of articles indexed for MEDLINE was conducted on June 30, 2020. The literature included observational studies, case reports, and literature reviews from January 1, 2020, to June 30, 2020. Search terms included COVID-19, SARS-CoV-2, and coronavirus used in combination with cutaneous, skin, and dermatology. All of the resulting articles were then reviewed for relevance to the cutaneous manifestations of COVID-19. Only confirmed cases of COVID-19 infection were included in this review; suspected unconfirmed cases were excluded. Further exclusion criteria included articles that discussed dermatology in the time of COVID-19 that did not explicitly address its cutaneous manifestations. The remaining literature was evaluated to provide dermatologists and patients with a concise resource for the cutaneous signs and symptoms of COVID-19. Data extracted from the literature included geographic region, number of patients with skin findings, status of COVID-19 infection and timeline, and cutaneous signs. If a cutaneous sign was not given a clear diagnosis in the literature, the senior authors (A.L. and J.J.) assigned it to its most similar classification to aid in ease of understanding and clarity for the readers.
Results
A search of the key terms resulted in 75 articles published in the specified date range. After excluding overtly irrelevant articles and dermatologic conditions in the time of COVID-19 without confirmed SARS-CoV-2 infection, 25 articles ultimately met inclusion criteria. Relevant references from the articles also were explored for cutaneous dermatologic manifestations of COVID-19. Cutaneous manifestations that were repeatedly reported included chilblainlike lesions; acrocyanosis; urticaria; pityriasis rosea–like cutaneous eruption; erythema multiforme–like, vesiculopapular, and morbilliform eruptions; petechiae; livedo reticularis; and purpuric livedo reticularis (dermatologists may label this stellate purpura). Fewer but nonetheless notable cases of androgenic alopecia, periorbital dyschromia, and herpes zoster exacerbations also were documented. The Table summarizes the reported integumentary findings. The eTable groups the common findings and describes patient age, time to onset of cutaneous sign, and any prognostic significance as seen in the literature.
Chilblainlike Lesions and Acrocyanosis
Chilblainlike lesions are edematous eruptions of the fingers and toes. They usually do not scar and are described as erythematous to violaceous papules and macules with possible bullae on the digits. Skin biopsies demonstrate a histopathologic pattern of vacuolar interface dermatitis with necrotic keratinocytes and a thickened basement membrane. Lymphocytic infiltrate presents in a perieccrine distribution, occasionally with plasma cells. The dermatopathologic findings mimic those of chilblain lupus but lack dermal edema.3
These eruptions have been reported in cases of COVID-19 that more frequently affect children and young adults. They usually resolve over the course of viral infection, averaging within 14 days. Chilblainlike eruptions often are associated with pruritus or pain. They commonly are asymmetrical and appear more often on the toes than the fingers.4 In cases of COVID-19 that lack systemic symptoms, chilblainlike lesions have been seen on the dorsal fingers as the first presenting sign of infection.5
Acral erythema and chilblainlike lesions frequently have been associated with milder infection. Another positive prognostic indicator is the manifestation of these signs in younger individuals.3
Morbilliform Exanthem
The morbilliform exanthem associated with COVID-19 also typically presents in patients with milder disease. It often affects the buttocks, lower abdomen, and thighs, but spares the palms, soles, and mucosae.4 This skin sign, which may start out as a generalized morbilliform exanthem, has been seen to morph into macular hemorrhagic purpura on the legs. These cutaneous lesions typically spontaneously resolve.8
In a case report by Najarian,6 a morbilliform exanthem was seen on the legs, arms, and trunk of a patient who was otherwise asymptomatic but tested positive for COVID-19. The morbilliform exanthem then became confluent on the trunk. Notably, the patient reported pain of the hands and feet.6
Another case report described a patient with edematous annular plaques on the palms, neck, and upper extremities who presented solely with fever.7 The biopsy specimen was nonspecific but indicated a viral exanthem. Histopathology showed perivascular lymphocytic infiltrate, dermal edema and vacuoles, spongiosis, dyskeratotic basilar keratinocytes, and few neutrophils without eosinophils.7
Eczematous Eruption
A confluent eczematous eruption in the flexural areas, the antecubital fossae, and axillary folds has been found in COVID-19 patients.21,22 An elderly patient with severe COVID-19 developed a squamous erythematous periumbilical patch 1 day after hospital admission. The cutaneous eruption rapidly progressed to digitate scaly plaques on the trunk, thighs, and flank. A biopsy specimen showed epidermal spongiosis, vesicles containing lymphocytes, and Langerhans cells. The upper dermis demonstrated a lymphohistiocytic infiltrate.23
Pityriasis Rosea–Like Eruption
In Iran, a COVID-19–infected patient developed an erythematous papulosquamous eruption with a herald patch and trailing scales 3 days after viral symptoms, resembling that of pityriasis rosea.24 Nests of Langerhans cells within the epidermis are seen in many viral exanthems, including cases of COVID-19 and pityriasis rosea.25
Urticaria
According to a number of case reports, urticarial lesions have been the first presenting sign of COVID-19 infection, most resolving with antihistamines.10,11 Some patients with more severe symptoms have had widespread urticaria. An urticarial exanthem appearing on the bilateral thighs and buttocks may be the initial sign of infection.12,15 Pruritic erythematous plaques over the face and acral areas is another initial sign. Interestingly, pediatric patients have reported nonpruritic urticaria.9
Urticaria also has been seen as a late dermatologic sign of viral infection. After battling relentless viral infection for 1 month, a pruritic, confluent, ill-defined eruption appeared along a patient’s trunk, back, and proximal extremities. Histopathologic examination concluded a perivascular lymphocytic infiltrate and dilated vessels in the dermis. The urticaria resolved a week later, and the patient’s nasopharyngeal swab finally came back negative.13
Vesiculopapular Eruption
Vesicles mimicking those of chickenpox have been reported. A study of 375 confirmed cases of COVID-19 by Galván Casas et al12 showed a 9% incidence of this vesicular eruption. A study by Sachdeva et al8 revealed vesicular eruptions in 25 of 72 patients. Pruritic papules and vesicles may resemble Grover disease. This cutaneous sign may be seen in the submammary folds, on the hips, or diffusely over the body.
Erythema Multiforme–Like Eruption
Targetoid lesions similar to those of erythema multiforme erupted in 2 of 27 patients with mild COVID-19 infection in a review by Wollina et al.4 In a study of 4 patients with erythema multiforme–like eruptions after COVID-19 symptoms resolved, 3 had palatal petechiae. Two of 4 patients had pseudovesicles in the center of the erythematous targetoid patches.26 Targetoid lesions on the extremities have been reported in pediatric patients with COVID-19 infections. These patients often present without any typical viral symptoms but rather just a febrile exanthem or exanthem alone. Thus, to minimize spread of the virus, it is vital to recognize COVID-19 infection early in patients with a viral exanthem during the time of high COVID-19 incidence.4
Livedo Reticularis
In the United States, a case series reported 2 patients with transient livedo reticularis throughout the course of COVID-19 infection. The cutaneous eruption resembled erythema ab igne, but there was no history of exposure to heat.16
Stellate Purpura
In severe COVID-19 infection, a reticulated nonblanching purpura on the buttocks has been reported to demonstrate pauci-inflammatory vascular thrombosis, complement membrane attack complex deposition, and endothelial injury on dermatopathology. Stellate purpura on palmoplantar surfaces also has shown arterial thrombosis in the deep dermis due to complement deposition.17
Petechiae and Purpura
A morbilliform exanthem may develop into significant petechiae in the popliteal fossae, buttocks, and thighs. A punch biopsy specimen demonstrates a perivascular lymphocytic infiltrate with erythrocyte extravasation and papillary dermal edema with dyskeratotic cells.18 Purpura of the lower extremities may develop, with histopathology showing fibrinoid necrosis of small vessel walls, neutrophilic infiltrate with karyorrhexis, and granular complement deposition.19
In Thailand, a patient was misdiagnosed with dengue after presenting with petechiae and low platelet count.20 Further progression of the viral illness resulted in respiratory symptoms. Subsequently, the patient tested positive for COVID-19. This case demonstrates that cutaneous signs of many sorts may be the first presenting signs of COVID-19, even prior to febrile symptoms.20
Androgenic Alopecia
Studies have shown that androgens are related in the pathogenesis of COVID-19. Coronavirus disease 2019 uses a cellular co-receptor, TMPRSS2, which is androgen regulated.27 In a study of 41 males with COVID-19, 29 had androgenic alopecia. However, this is only a correlation, and causation cannot be concluded here. It cannot be determined from this study whether androgenic alopecia is a risk factor, result of COVID-19, or confounder.28
Exaggerated Herpes Zoster
Shors29 reported a herpes zoster eruption in a patient who had symptoms of COVID-19 for 1 week. Further testing confirmed COVID-19 infection, and despite prompt treatment with valacyclovir, the eruption was slow to resolve. The patient then experienced severe postherpetic neuralgia for more than 4 weeks, even with treatment with gabapentin and lidocaine. It is hypothesized that because of the major inflammatory response caused by COVID-19, an exaggerated inflammation occurred in the dorsal root ganglion, resulting in relentless herpes zoster infection.29
Mottled Skin
Born at term, a 15-day-old neonate presented with sepsis and mottling of the skin. The patient did not have any typical COVID-19 symptoms, such as diarrhea or cough, but tested positive for COVID-19.30
Periorbital Dyschromia
Kalner and Vergilis31 reported 2 cases of periorbital dyschromia prior to any other COVID-19 infection symptoms. The discoloration improved with resolution of ensuing viral symptoms.31
Comment
Many dermatologic signs of COVID-19 have been identified. Their individual frequency and association with viral severity will become more apparent as more cases are reported. So far during this pandemic, common dermatologic manifestations have been polymorphic in clinical presentation.
Onset of Skin Manifestations
The timeline of skin signs and COVID-19 symptoms varies from the first reported sign to weeks after symptom resolution. In the Region of Murcia, Spain, Pérez-Suárez et al14 collected data on cutaneous signs of patients with COVID-19. Of the patients studied, 9 had tests confirming COVID-19 infection. Truncal urticaria, sacral ulcers, acrocyanosis, and erythema multiforme were all reported in patients more than 2 weeks after symptom onset. One case of tinea infection also was reported 4 days after fever and respiratory symptoms began.14
Presentation
Coronavirus disease 2019 has affected the skin of both the central thorax and peripheral locations. In a study of 72 patients with cutaneous signs of COVID-19 by Sachdeva et al,8 a truncal distribution was most common, but 14 patients reported acral site involvement. Sachdeva et al8 reported urticarial reactions in 7 of 72 patients with cutaneous signs. A painful acral cyanosis was seen in 11 of 72 patients. Livedo reticularis presented in 2 patients, and only 1 patient had petechiae. Cutaneous signs were the first indicators of viral infection in 9 of 72 patients; 52 patients presented with respiratory symptoms first. All of the reported cutaneous signs spontaneously resolved within 10 days.8
Recalcati32 reviewed 88 patients with COVID-19, and 18 had cutaneous signs at initial onset of viral infection or during hospitalization. The most common integumentary sign reported in this study was erythema, followed by diffuse urticaria, and then a vesicular eruption resembling varicella infection.32
Some less common phenomena have been identified in patients with COVID-19, including androgenic alopecia, exaggerated herpes zoster and postherpetic neuralgia, mottled skin, and periorbital dyschromia. Being aware of these complications may help in early treatment, diagnosis, and even prevention of viral spread.
Pathogenesis of Skin Manifestations
Few breakthroughs have been made in understanding the pathogenesis of skin manifestations of SARS-CoV-2. Acral ischemia may be a manifestation of COVID-19’s association with hypercoagulation. Increasing fibrinogen and prothrombin times lead to disseminated intravascular coagulation and microthrombi. These tiny blood clots then lodge in blood vessels and cause acral cyanosis and subsequent gangrene.2 The proposed mechanism behind this clinical manifestation in younger populations is the hypercoagulable state that COVID-19 creates. Conversely, acral erythema and chilblainlike lesions in older patients are thought to be from acral ischemia as a response to insufficient type 1 interferons. This pathophysiologic mechanism is indicative of a worse prognosis due to the large role that type 1 interferons play in antiviral responses. Coronavirus disease 2019 similarly triggers type 1 interferons; thus, their efficacy positively correlates with good disease prognosis.3
Similarly, the pathogenesis for livedo reticularis in patients with COVID-19 can only be hypothesized. Infected patients are in a hypercoagulable state, and in these cases, it was uncertain whether this was due to a disseminated intravascular coagulation, cold agglutinins, cryofibrinogens, or lupus anticoagulant.16
Nonetheless, it can be difficult to separate the primary event between vasculopathy or vasculitis in larger vessel pathology specimens. Some of the studies’ pathology reports discuss a granulocytic infiltrate and red blood cell extravasation, which represent small vessel vasculitis. However, the gangrene and necrosing livedo represent vasculopathy events. A final conclusion about the pathogenesis cannot be made without further clinical and histopathologic evaluation.
Histopathology
Biopsy specimens of reported morbilliform eruptions have demonstrated thrombosed vessels with evidence of necrosis and granulocytic infiltrate.25 Another biopsy specimen of a widespread erythematous exanthem demonstrated extravasated red blood cells and vessel wall damage similar to thrombophilic arteritis. Other reports of histopathology showed necrotic keratinocytes and lymphocytic satellitosis at the dermoepidermal junction, resembling Grover disease. These cases demonstrating necrosis suggest a strong cytokine reaction from the virus.25 A concern with these biopsy findings is that morbilliform eruptions generally show dilated vessels with lymphocytes, and these biopsy findings are consistent with a cutaneous small vessel vasculitis. Additionally, histopathologic evaluation of purpuric eruptions has shown erythrocyte extravasation and granulocytic infiltrate indicative of a cutaneous small vessel vasculitis.
Although most reported cases of cutaneous signs of COVID-19 do not have histopathologic reports, Yao et al33 conducted a dermatopathologic study that investigated the tissue in deceased patients who had COVID-19. This pathology showed hyaline thrombi within the small vessels of the skin, likely leading to the painful acral ischemia. Similarly, Yao et al33 reported autopsies finding hyaline thrombi within the small vessels of the lungs. More research should be done to explore this pathogenesis as part of prognostic factors and virulence.
Conclusion
Cutaneous signs may be the first reported symptom of COVID-19 infection, and dermatologists should be prepared to identify them. This review may be used as a guide for physicians to quickly identify potential infection as well as further understand the pathogenesis related to COVID-19. Future research is necessary to determine the dermatologic pathogenesis, infectivity, and prevalence of cutaneous manifestations of COVID-19. It also will be important to explore if vasculopathic lesions predict more severe multisystem disease.
The pathogenesis of coronavirus disease 2019 (COVID-19), the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), is not yet completely understood. Thus far, it is known to affect multiple organ systems, including gastrointestinal, neurological, and cardiovascular, with typical clinical symptoms of COVID-19 including fever, cough, myalgia, headache, anosmia, and diarrhea.1 This multiorgan attack may be secondary to an exaggerated inflammatory reaction with vasculopathy and possibly a hypercoagulable state. Skin manifestations also are prevalent in COVID-19, and they often result in polymorphous presentations.2 This article aims to summarize cutaneous clinical signs of COVID-19 so that dermatologists can promptly identify and manage COVID-19 and prevent its spread.
Methods
A PubMed search of articles indexed for MEDLINE was conducted on June 30, 2020. The literature included observational studies, case reports, and literature reviews from January 1, 2020, to June 30, 2020. Search terms included COVID-19, SARS-CoV-2, and coronavirus used in combination with cutaneous, skin, and dermatology. All of the resulting articles were then reviewed for relevance to the cutaneous manifestations of COVID-19. Only confirmed cases of COVID-19 infection were included in this review; suspected unconfirmed cases were excluded. Further exclusion criteria included articles that discussed dermatology in the time of COVID-19 that did not explicitly address its cutaneous manifestations. The remaining literature was evaluated to provide dermatologists and patients with a concise resource for the cutaneous signs and symptoms of COVID-19. Data extracted from the literature included geographic region, number of patients with skin findings, status of COVID-19 infection and timeline, and cutaneous signs. If a cutaneous sign was not given a clear diagnosis in the literature, the senior authors (A.L. and J.J.) assigned it to its most similar classification to aid in ease of understanding and clarity for the readers.
Results
A search of the key terms resulted in 75 articles published in the specified date range. After excluding overtly irrelevant articles and dermatologic conditions in the time of COVID-19 without confirmed SARS-CoV-2 infection, 25 articles ultimately met inclusion criteria. Relevant references from the articles also were explored for cutaneous dermatologic manifestations of COVID-19. Cutaneous manifestations that were repeatedly reported included chilblainlike lesions; acrocyanosis; urticaria; pityriasis rosea–like cutaneous eruption; erythema multiforme–like, vesiculopapular, and morbilliform eruptions; petechiae; livedo reticularis; and purpuric livedo reticularis (dermatologists may label this stellate purpura). Fewer but nonetheless notable cases of androgenic alopecia, periorbital dyschromia, and herpes zoster exacerbations also were documented. The Table summarizes the reported integumentary findings. The eTable groups the common findings and describes patient age, time to onset of cutaneous sign, and any prognostic significance as seen in the literature.
Chilblainlike Lesions and Acrocyanosis
Chilblainlike lesions are edematous eruptions of the fingers and toes. They usually do not scar and are described as erythematous to violaceous papules and macules with possible bullae on the digits. Skin biopsies demonstrate a histopathologic pattern of vacuolar interface dermatitis with necrotic keratinocytes and a thickened basement membrane. Lymphocytic infiltrate presents in a perieccrine distribution, occasionally with plasma cells. The dermatopathologic findings mimic those of chilblain lupus but lack dermal edema.3
These eruptions have been reported in cases of COVID-19 that more frequently affect children and young adults. They usually resolve over the course of viral infection, averaging within 14 days. Chilblainlike eruptions often are associated with pruritus or pain. They commonly are asymmetrical and appear more often on the toes than the fingers.4 In cases of COVID-19 that lack systemic symptoms, chilblainlike lesions have been seen on the dorsal fingers as the first presenting sign of infection.5
Acral erythema and chilblainlike lesions frequently have been associated with milder infection. Another positive prognostic indicator is the manifestation of these signs in younger individuals.3
Morbilliform Exanthem
The morbilliform exanthem associated with COVID-19 also typically presents in patients with milder disease. It often affects the buttocks, lower abdomen, and thighs, but spares the palms, soles, and mucosae.4 This skin sign, which may start out as a generalized morbilliform exanthem, has been seen to morph into macular hemorrhagic purpura on the legs. These cutaneous lesions typically spontaneously resolve.8
In a case report by Najarian,6 a morbilliform exanthem was seen on the legs, arms, and trunk of a patient who was otherwise asymptomatic but tested positive for COVID-19. The morbilliform exanthem then became confluent on the trunk. Notably, the patient reported pain of the hands and feet.6
Another case report described a patient with edematous annular plaques on the palms, neck, and upper extremities who presented solely with fever.7 The biopsy specimen was nonspecific but indicated a viral exanthem. Histopathology showed perivascular lymphocytic infiltrate, dermal edema and vacuoles, spongiosis, dyskeratotic basilar keratinocytes, and few neutrophils without eosinophils.7
Eczematous Eruption
A confluent eczematous eruption in the flexural areas, the antecubital fossae, and axillary folds has been found in COVID-19 patients.21,22 An elderly patient with severe COVID-19 developed a squamous erythematous periumbilical patch 1 day after hospital admission. The cutaneous eruption rapidly progressed to digitate scaly plaques on the trunk, thighs, and flank. A biopsy specimen showed epidermal spongiosis, vesicles containing lymphocytes, and Langerhans cells. The upper dermis demonstrated a lymphohistiocytic infiltrate.23
Pityriasis Rosea–Like Eruption
In Iran, a COVID-19–infected patient developed an erythematous papulosquamous eruption with a herald patch and trailing scales 3 days after viral symptoms, resembling that of pityriasis rosea.24 Nests of Langerhans cells within the epidermis are seen in many viral exanthems, including cases of COVID-19 and pityriasis rosea.25
Urticaria
According to a number of case reports, urticarial lesions have been the first presenting sign of COVID-19 infection, most resolving with antihistamines.10,11 Some patients with more severe symptoms have had widespread urticaria. An urticarial exanthem appearing on the bilateral thighs and buttocks may be the initial sign of infection.12,15 Pruritic erythematous plaques over the face and acral areas is another initial sign. Interestingly, pediatric patients have reported nonpruritic urticaria.9
Urticaria also has been seen as a late dermatologic sign of viral infection. After battling relentless viral infection for 1 month, a pruritic, confluent, ill-defined eruption appeared along a patient’s trunk, back, and proximal extremities. Histopathologic examination concluded a perivascular lymphocytic infiltrate and dilated vessels in the dermis. The urticaria resolved a week later, and the patient’s nasopharyngeal swab finally came back negative.13
Vesiculopapular Eruption
Vesicles mimicking those of chickenpox have been reported. A study of 375 confirmed cases of COVID-19 by Galván Casas et al12 showed a 9% incidence of this vesicular eruption. A study by Sachdeva et al8 revealed vesicular eruptions in 25 of 72 patients. Pruritic papules and vesicles may resemble Grover disease. This cutaneous sign may be seen in the submammary folds, on the hips, or diffusely over the body.
Erythema Multiforme–Like Eruption
Targetoid lesions similar to those of erythema multiforme erupted in 2 of 27 patients with mild COVID-19 infection in a review by Wollina et al.4 In a study of 4 patients with erythema multiforme–like eruptions after COVID-19 symptoms resolved, 3 had palatal petechiae. Two of 4 patients had pseudovesicles in the center of the erythematous targetoid patches.26 Targetoid lesions on the extremities have been reported in pediatric patients with COVID-19 infections. These patients often present without any typical viral symptoms but rather just a febrile exanthem or exanthem alone. Thus, to minimize spread of the virus, it is vital to recognize COVID-19 infection early in patients with a viral exanthem during the time of high COVID-19 incidence.4
Livedo Reticularis
In the United States, a case series reported 2 patients with transient livedo reticularis throughout the course of COVID-19 infection. The cutaneous eruption resembled erythema ab igne, but there was no history of exposure to heat.16
Stellate Purpura
In severe COVID-19 infection, a reticulated nonblanching purpura on the buttocks has been reported to demonstrate pauci-inflammatory vascular thrombosis, complement membrane attack complex deposition, and endothelial injury on dermatopathology. Stellate purpura on palmoplantar surfaces also has shown arterial thrombosis in the deep dermis due to complement deposition.17
Petechiae and Purpura
A morbilliform exanthem may develop into significant petechiae in the popliteal fossae, buttocks, and thighs. A punch biopsy specimen demonstrates a perivascular lymphocytic infiltrate with erythrocyte extravasation and papillary dermal edema with dyskeratotic cells.18 Purpura of the lower extremities may develop, with histopathology showing fibrinoid necrosis of small vessel walls, neutrophilic infiltrate with karyorrhexis, and granular complement deposition.19
In Thailand, a patient was misdiagnosed with dengue after presenting with petechiae and low platelet count.20 Further progression of the viral illness resulted in respiratory symptoms. Subsequently, the patient tested positive for COVID-19. This case demonstrates that cutaneous signs of many sorts may be the first presenting signs of COVID-19, even prior to febrile symptoms.20
Androgenic Alopecia
Studies have shown that androgens are related in the pathogenesis of COVID-19. Coronavirus disease 2019 uses a cellular co-receptor, TMPRSS2, which is androgen regulated.27 In a study of 41 males with COVID-19, 29 had androgenic alopecia. However, this is only a correlation, and causation cannot be concluded here. It cannot be determined from this study whether androgenic alopecia is a risk factor, result of COVID-19, or confounder.28
Exaggerated Herpes Zoster
Shors29 reported a herpes zoster eruption in a patient who had symptoms of COVID-19 for 1 week. Further testing confirmed COVID-19 infection, and despite prompt treatment with valacyclovir, the eruption was slow to resolve. The patient then experienced severe postherpetic neuralgia for more than 4 weeks, even with treatment with gabapentin and lidocaine. It is hypothesized that because of the major inflammatory response caused by COVID-19, an exaggerated inflammation occurred in the dorsal root ganglion, resulting in relentless herpes zoster infection.29
Mottled Skin
Born at term, a 15-day-old neonate presented with sepsis and mottling of the skin. The patient did not have any typical COVID-19 symptoms, such as diarrhea or cough, but tested positive for COVID-19.30
Periorbital Dyschromia
Kalner and Vergilis31 reported 2 cases of periorbital dyschromia prior to any other COVID-19 infection symptoms. The discoloration improved with resolution of ensuing viral symptoms.31
Comment
Many dermatologic signs of COVID-19 have been identified. Their individual frequency and association with viral severity will become more apparent as more cases are reported. So far during this pandemic, common dermatologic manifestations have been polymorphic in clinical presentation.
Onset of Skin Manifestations
The timeline of skin signs and COVID-19 symptoms varies from the first reported sign to weeks after symptom resolution. In the Region of Murcia, Spain, Pérez-Suárez et al14 collected data on cutaneous signs of patients with COVID-19. Of the patients studied, 9 had tests confirming COVID-19 infection. Truncal urticaria, sacral ulcers, acrocyanosis, and erythema multiforme were all reported in patients more than 2 weeks after symptom onset. One case of tinea infection also was reported 4 days after fever and respiratory symptoms began.14
Presentation
Coronavirus disease 2019 has affected the skin of both the central thorax and peripheral locations. In a study of 72 patients with cutaneous signs of COVID-19 by Sachdeva et al,8 a truncal distribution was most common, but 14 patients reported acral site involvement. Sachdeva et al8 reported urticarial reactions in 7 of 72 patients with cutaneous signs. A painful acral cyanosis was seen in 11 of 72 patients. Livedo reticularis presented in 2 patients, and only 1 patient had petechiae. Cutaneous signs were the first indicators of viral infection in 9 of 72 patients; 52 patients presented with respiratory symptoms first. All of the reported cutaneous signs spontaneously resolved within 10 days.8
Recalcati32 reviewed 88 patients with COVID-19, and 18 had cutaneous signs at initial onset of viral infection or during hospitalization. The most common integumentary sign reported in this study was erythema, followed by diffuse urticaria, and then a vesicular eruption resembling varicella infection.32
Some less common phenomena have been identified in patients with COVID-19, including androgenic alopecia, exaggerated herpes zoster and postherpetic neuralgia, mottled skin, and periorbital dyschromia. Being aware of these complications may help in early treatment, diagnosis, and even prevention of viral spread.
Pathogenesis of Skin Manifestations
Few breakthroughs have been made in understanding the pathogenesis of skin manifestations of SARS-CoV-2. Acral ischemia may be a manifestation of COVID-19’s association with hypercoagulation. Increasing fibrinogen and prothrombin times lead to disseminated intravascular coagulation and microthrombi. These tiny blood clots then lodge in blood vessels and cause acral cyanosis and subsequent gangrene.2 The proposed mechanism behind this clinical manifestation in younger populations is the hypercoagulable state that COVID-19 creates. Conversely, acral erythema and chilblainlike lesions in older patients are thought to be from acral ischemia as a response to insufficient type 1 interferons. This pathophysiologic mechanism is indicative of a worse prognosis due to the large role that type 1 interferons play in antiviral responses. Coronavirus disease 2019 similarly triggers type 1 interferons; thus, their efficacy positively correlates with good disease prognosis.3
Similarly, the pathogenesis for livedo reticularis in patients with COVID-19 can only be hypothesized. Infected patients are in a hypercoagulable state, and in these cases, it was uncertain whether this was due to a disseminated intravascular coagulation, cold agglutinins, cryofibrinogens, or lupus anticoagulant.16
Nonetheless, it can be difficult to separate the primary event between vasculopathy or vasculitis in larger vessel pathology specimens. Some of the studies’ pathology reports discuss a granulocytic infiltrate and red blood cell extravasation, which represent small vessel vasculitis. However, the gangrene and necrosing livedo represent vasculopathy events. A final conclusion about the pathogenesis cannot be made without further clinical and histopathologic evaluation.
Histopathology
Biopsy specimens of reported morbilliform eruptions have demonstrated thrombosed vessels with evidence of necrosis and granulocytic infiltrate.25 Another biopsy specimen of a widespread erythematous exanthem demonstrated extravasated red blood cells and vessel wall damage similar to thrombophilic arteritis. Other reports of histopathology showed necrotic keratinocytes and lymphocytic satellitosis at the dermoepidermal junction, resembling Grover disease. These cases demonstrating necrosis suggest a strong cytokine reaction from the virus.25 A concern with these biopsy findings is that morbilliform eruptions generally show dilated vessels with lymphocytes, and these biopsy findings are consistent with a cutaneous small vessel vasculitis. Additionally, histopathologic evaluation of purpuric eruptions has shown erythrocyte extravasation and granulocytic infiltrate indicative of a cutaneous small vessel vasculitis.
Although most reported cases of cutaneous signs of COVID-19 do not have histopathologic reports, Yao et al33 conducted a dermatopathologic study that investigated the tissue in deceased patients who had COVID-19. This pathology showed hyaline thrombi within the small vessels of the skin, likely leading to the painful acral ischemia. Similarly, Yao et al33 reported autopsies finding hyaline thrombi within the small vessels of the lungs. More research should be done to explore this pathogenesis as part of prognostic factors and virulence.
Conclusion
Cutaneous signs may be the first reported symptom of COVID-19 infection, and dermatologists should be prepared to identify them. This review may be used as a guide for physicians to quickly identify potential infection as well as further understand the pathogenesis related to COVID-19. Future research is necessary to determine the dermatologic pathogenesis, infectivity, and prevalence of cutaneous manifestations of COVID-19. It also will be important to explore if vasculopathic lesions predict more severe multisystem disease.
- Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497-506.
- Criado PR, Abdalla BMZ, de Assis IC, et al. Are the cutaneous manifestations during or due to SARS-CoV-2 infection/COVID-19 frequent or not? revision of possible pathophysiologic mechanisms. Inflamm Res. 2020;69:745-756.
- Kolivras A, Dehavay F, Delplace D, et al. Coronavirus (COVID‐19) infection–induced chilblains: a case report with histopathological findings. JAAD Case Rep. 2020;6:489-492.
- Wollina U, Karadag˘ AS, Rowland-Payne C, et al. Cutaneous signs in COVID-19 patients: a review [published online May 10, 2020]. Dermatol Ther. 2020;33:E13549.
- Alramthan A, Aldaraji W. Two cases of COVID-19 presenting with a clinical picture resembling chilblains: first report from the Middle East. Clin Exp Dermatol. 2020;45:746-748.
- Najarian DJ. Morbilliform exanthem associated with COVID‐19. JAAD Case Rep. 2020;6:493-494.
- Amatore F, Macagno N, Mailhe M, et al. SARS-CoV-2 infection presenting as a febrile rash. J Eur Acad Dermatol Venereol. 2020;34:E304-E306.
- Sachdeva M, Gianotti R, Shah M, et al. Cutaneous manifestations of COVID-19: report of three cases and a review of literature. J Dermatol Sci. 2020;98:75-81.
- Morey-Olivé M, Espiau M, Mercadal-Hally M, et al. Cutaneous manifestations in the current pandemic of coronavirus infection disease (COVID 2019). An Pediatr (Engl Ed). 2020;92:374-375.
- van Damme C, Berlingin E, Saussez S, et al. Acute urticaria with pyrexia as the first manifestations of a COVID‐19 infection. J Eur Acad Dermatol Venereol. 2020;34:E300-E301.
- Henry D, Ackerman M, Sancelme E, et al. Urticarial eruption in COVID‐19 infection. J Eur Acad Dermatol Venereol. 2020;34:E244-E245.
- Galván Casas C, Català A, Carretero Hernández G, et al. Classification of the cutaneous manifestations of COVID-19: a rapid prospective nationwide consensus study in Spain with 375 cases. Br J Dermatol. 2020;183:71-77.
- Zengarini C, Orioni G, Cascavilla A, et al. Histological pattern in Covid-19-induced viral rash [published online May 2, 2020]. J Eur Acad Dermatol Venereol. doi:10.1111/jdv.16569.
- Pérez-Suárez B, Martínez-Menchón T, Cutillas-Marco E. Skin findings in the COVID-19 pandemic in the Region of Murcia [published online June 12, 2020]. Med Clin (Engl Ed). 2020;155:41-42.
- Quintana-Castanedo L, Feito-Rodríguez M, Valero-López I, et al. Urticarial exanthem as early diagnostic clue for COVID-19 infection [published online April 29, 2020]. JAAD Case Rep. 2020;6:498-499.
- Manalo IF, Smith MK, Cheeley J, et al. Reply to: “reply: a dermatologic manifestation of COVID-19: transient livedo reticularis” [published online May 7, 2020]. J Am Acad Dermatol. 2020;83:E157.
- Magro C, Mulvey JJ, Berlin D, et al. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: a report of five cases. Transl Res. 2020;220:1-13.
- Diaz-Guimaraens B, Dominguez-Santas M, Suarez-Valle A, et al. Petechial skin rash associated with severe acute respiratory syndrome coronavirus 2 infection. JAMA Dermatol. 2020;156:820-822.
- Dominguez-Santas M, Diaz-Guimaraens B, Garcia Abellas P, et al. Cutaneous small-vessel vasculitis associated with novel 2019 coronavirus SARS-CoV-2 infection (COVID-19) [published online July 2, 2020]. J Eur Acad Dermatol Venereol. 2020;34:E536-E537.
- Joob B, Wiwanitkit V. COVID-19 can present with a rash and be mistaken for dengue [published online March 22, 2020]. J Am Acad Dermatol. 2020;82:E177.
- Avellana Moreno R, Estella Villa LM, Avellana Moreno V, et al. Cutaneous manifestation of COVID‐19 in images: a case report [published online May 19, 2020]. J Eur Acad Dermatol Venereol. 2020;34:E307-E309.
- Mahé A, Birckel E, Krieger S, et al. A distinctive skin rash associated with coronavirus disease 2019 [published online June 8, 2020]? J Eur Acad Dermatol Venereol. 2020;34:E246-E247.
- Sanchez A, Sohier P, Benghanem S, et al. Digitate papulosquamous eruption associated with severe acute respiratory syndrome coronavirus 2 infection. JAMA Dermatol. 2020;156:819-820.
- Ehsani AH, Nasimi M, Bigdelo Z. Pityriasis rosea as a cutaneous manifestation of COVID‐19 infection [published online May 2, 2020]. J Eur Acad Dermatol Venereol. doi:10.1111/jdv.16579.
- Gianotti R, Veraldi S, Recalcati S, et al. Cutaneous clinico-pathological findings in three COVID-19-positive patients observed in the metropolitan area of Milan, Italy. Acta Derm Venereol. 2020;100:adv00124.
- Jimenez-Cauhe J, Ortega-Quijano D, Carretero-Barrio I, et al. Erythema multiforme-like eruption in patients with COVID-19 infection: clinical and histological findings [published online May 9, 2020]. Clin Exp Dermatol. doi:10.1111/ced.14281
- Hoffmann M, Kleine‐Weber H, Schroeder S, et al. SARS‐CoV‐2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor [published online March 5, 2020]. Cell. 2020;181:271‐280.e8.
- Goren A, Vaño‐Galván S, Wambier CG, et al. A preliminary observation: male pattern hair loss among hospitalized COVID‐19 patients in Spain—a potential clue to the role of androgens in COVID‐19 severity [published online April 23, 2020]. J Cosmet Dermatol. 2020;19:1545-1547.
- Shors AR. Herpes zoster and severe acute herpetic neuralgia as a complication of COVID-19 infection. JAAD Case Rep. 2020;6:656-657.
- Kamali Aghdam M, Jafari N, Eftekhari K. Novel coronavirus in a 15‐day‐old neonate with clinical signs of sepsis, a case report. Infect Dis (London). 2020;52:427‐429.
- Kalner S, Vergilis IJ. Periorbital erythema as a presenting sign of covid-19 [published online May 11, 2020]. JAAD Case Rep. 2020;6:996-998.
- Recalcati S. Cutaneous manifestations in COVID‐19: a first perspective. J Eur Acad Dermatol Venereol. 2020;34:E212-E213.
- Yao XH, Li TY, He ZC, et al. A pathological report of three COVID‐19 cases by minimally invasive autopsies [in Chinese]. Zhonghua Bing Li Xue Za Zhi. 2020;49:411-417.
- Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497-506.
- Criado PR, Abdalla BMZ, de Assis IC, et al. Are the cutaneous manifestations during or due to SARS-CoV-2 infection/COVID-19 frequent or not? revision of possible pathophysiologic mechanisms. Inflamm Res. 2020;69:745-756.
- Kolivras A, Dehavay F, Delplace D, et al. Coronavirus (COVID‐19) infection–induced chilblains: a case report with histopathological findings. JAAD Case Rep. 2020;6:489-492.
- Wollina U, Karadag˘ AS, Rowland-Payne C, et al. Cutaneous signs in COVID-19 patients: a review [published online May 10, 2020]. Dermatol Ther. 2020;33:E13549.
- Alramthan A, Aldaraji W. Two cases of COVID-19 presenting with a clinical picture resembling chilblains: first report from the Middle East. Clin Exp Dermatol. 2020;45:746-748.
- Najarian DJ. Morbilliform exanthem associated with COVID‐19. JAAD Case Rep. 2020;6:493-494.
- Amatore F, Macagno N, Mailhe M, et al. SARS-CoV-2 infection presenting as a febrile rash. J Eur Acad Dermatol Venereol. 2020;34:E304-E306.
- Sachdeva M, Gianotti R, Shah M, et al. Cutaneous manifestations of COVID-19: report of three cases and a review of literature. J Dermatol Sci. 2020;98:75-81.
- Morey-Olivé M, Espiau M, Mercadal-Hally M, et al. Cutaneous manifestations in the current pandemic of coronavirus infection disease (COVID 2019). An Pediatr (Engl Ed). 2020;92:374-375.
- van Damme C, Berlingin E, Saussez S, et al. Acute urticaria with pyrexia as the first manifestations of a COVID‐19 infection. J Eur Acad Dermatol Venereol. 2020;34:E300-E301.
- Henry D, Ackerman M, Sancelme E, et al. Urticarial eruption in COVID‐19 infection. J Eur Acad Dermatol Venereol. 2020;34:E244-E245.
- Galván Casas C, Català A, Carretero Hernández G, et al. Classification of the cutaneous manifestations of COVID-19: a rapid prospective nationwide consensus study in Spain with 375 cases. Br J Dermatol. 2020;183:71-77.
- Zengarini C, Orioni G, Cascavilla A, et al. Histological pattern in Covid-19-induced viral rash [published online May 2, 2020]. J Eur Acad Dermatol Venereol. doi:10.1111/jdv.16569.
- Pérez-Suárez B, Martínez-Menchón T, Cutillas-Marco E. Skin findings in the COVID-19 pandemic in the Region of Murcia [published online June 12, 2020]. Med Clin (Engl Ed). 2020;155:41-42.
- Quintana-Castanedo L, Feito-Rodríguez M, Valero-López I, et al. Urticarial exanthem as early diagnostic clue for COVID-19 infection [published online April 29, 2020]. JAAD Case Rep. 2020;6:498-499.
- Manalo IF, Smith MK, Cheeley J, et al. Reply to: “reply: a dermatologic manifestation of COVID-19: transient livedo reticularis” [published online May 7, 2020]. J Am Acad Dermatol. 2020;83:E157.
- Magro C, Mulvey JJ, Berlin D, et al. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: a report of five cases. Transl Res. 2020;220:1-13.
- Diaz-Guimaraens B, Dominguez-Santas M, Suarez-Valle A, et al. Petechial skin rash associated with severe acute respiratory syndrome coronavirus 2 infection. JAMA Dermatol. 2020;156:820-822.
- Dominguez-Santas M, Diaz-Guimaraens B, Garcia Abellas P, et al. Cutaneous small-vessel vasculitis associated with novel 2019 coronavirus SARS-CoV-2 infection (COVID-19) [published online July 2, 2020]. J Eur Acad Dermatol Venereol. 2020;34:E536-E537.
- Joob B, Wiwanitkit V. COVID-19 can present with a rash and be mistaken for dengue [published online March 22, 2020]. J Am Acad Dermatol. 2020;82:E177.
- Avellana Moreno R, Estella Villa LM, Avellana Moreno V, et al. Cutaneous manifestation of COVID‐19 in images: a case report [published online May 19, 2020]. J Eur Acad Dermatol Venereol. 2020;34:E307-E309.
- Mahé A, Birckel E, Krieger S, et al. A distinctive skin rash associated with coronavirus disease 2019 [published online June 8, 2020]? J Eur Acad Dermatol Venereol. 2020;34:E246-E247.
- Sanchez A, Sohier P, Benghanem S, et al. Digitate papulosquamous eruption associated with severe acute respiratory syndrome coronavirus 2 infection. JAMA Dermatol. 2020;156:819-820.
- Ehsani AH, Nasimi M, Bigdelo Z. Pityriasis rosea as a cutaneous manifestation of COVID‐19 infection [published online May 2, 2020]. J Eur Acad Dermatol Venereol. doi:10.1111/jdv.16579.
- Gianotti R, Veraldi S, Recalcati S, et al. Cutaneous clinico-pathological findings in three COVID-19-positive patients observed in the metropolitan area of Milan, Italy. Acta Derm Venereol. 2020;100:adv00124.
- Jimenez-Cauhe J, Ortega-Quijano D, Carretero-Barrio I, et al. Erythema multiforme-like eruption in patients with COVID-19 infection: clinical and histological findings [published online May 9, 2020]. Clin Exp Dermatol. doi:10.1111/ced.14281
- Hoffmann M, Kleine‐Weber H, Schroeder S, et al. SARS‐CoV‐2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor [published online March 5, 2020]. Cell. 2020;181:271‐280.e8.
- Goren A, Vaño‐Galván S, Wambier CG, et al. A preliminary observation: male pattern hair loss among hospitalized COVID‐19 patients in Spain—a potential clue to the role of androgens in COVID‐19 severity [published online April 23, 2020]. J Cosmet Dermatol. 2020;19:1545-1547.
- Shors AR. Herpes zoster and severe acute herpetic neuralgia as a complication of COVID-19 infection. JAAD Case Rep. 2020;6:656-657.
- Kamali Aghdam M, Jafari N, Eftekhari K. Novel coronavirus in a 15‐day‐old neonate with clinical signs of sepsis, a case report. Infect Dis (London). 2020;52:427‐429.
- Kalner S, Vergilis IJ. Periorbital erythema as a presenting sign of covid-19 [published online May 11, 2020]. JAAD Case Rep. 2020;6:996-998.
- Recalcati S. Cutaneous manifestations in COVID‐19: a first perspective. J Eur Acad Dermatol Venereol. 2020;34:E212-E213.
- Yao XH, Li TY, He ZC, et al. A pathological report of three COVID‐19 cases by minimally invasive autopsies [in Chinese]. Zhonghua Bing Li Xue Za Zhi. 2020;49:411-417.
Practice Points
- Coronavirus disease 2019 (COVID-19) is a worldwide pandemic that affects multiple organ systems via a pathogenesis that is still being elucidated.
- Understanding the various cutaneous manifestations of COVID-19 will aid in early detection and proper treatment, thus increasing patient satisfaction and outcomes.
Home Phototherapy During the COVID-19 Pandemic
Office-based phototherapy practices have closed or are operating below capacity because of the coronavirus disease 2019 (COVID-19) pandemic.1 Social distancing measures to reduce virus transmission are a significant driving factor.1-3 In the age of biologics, other options requiring fewer patient visits are available, such as UVB phototherapy. UV phototherapy is considered first line when more than 10% of the body surface area is affected.4 Although phototherapy often is performed in the office, it also may be delivered at home.2 Home-based phototherapy is safe, effective, and similar in cost to office-based phototherapy.4 Currently, there are limited COVID-19–specific guidelines for home-based phototherapy.
The risks and sequelae of COVID-19 are still being investigated, with cases varying by location. As such, local and national public health recommendations are evolving. Dermatologists must make individualized decisions about practice services, as local restrictions differ. As office-based phototherapy services may struggle to implement mitigation strategies, home-based phototherapy is an increasingly viable treatment option.1,4,5 Patient benefits of home therapy include improved treatment compliance; greater patient satisfaction; reduced travel/waiting time; and reduced long-term cost, including co-pays, depending on insurance coverage.2,4
We aim to provide recommendations on home-based phototherapy during the pandemic. Throughout the decision-making process, careful consideration of safety, risks, benefits, and treatment options for physicians, staff, and patients will be vital to the successful implementation of home-based phototherapy. Our recommendations are based on maximizing benefits and minimizing risks.
Considerations for Physicians
Physicians should take the following steps when assessing if home phototherapy is an option for each patient.1,2,4
• Determine patient eligibility for phototherapy treatment if currently not on phototherapy
• Carefully review patient and provider requirements for home phototherapy supplier
• Review patient history of treatment compliance
• Determine insurance coverage and consider exclusion criteria
• Review prior treatments
• Provide education on side effects
• Provide education on signs of adequate treatment response
• Indicate the type of UV light and unit on the prescription
• Consider whether the patient is in the maintenance or initiation phase when providing recommendations
• Work with the supplier if the light therapy unit is denied by submitting an appeal or prescribing a different unit
• Follow up with telemedicine to assess treatment effectiveness and monitor for adverse effects
Considerations for Patients
Counsel patients to weigh the risks and benefits of home phototherapy prescription and usage.1,2,4
• Evaluate cost
• Carefully review patient and provider requirements for home phototherapy supplier
• Ensure a complete understanding of treatment schedule
• Properly utilize protective equipment (eg, genital shields for men, eye shields for all)
• Avoid sharing phototherapy units with household members
• Disinfect and maintain units
• Maintain proper ventilation of spaces
• Maintain treatment log
• Attend follow-up
Treatment Alternatives
For patients with severe psoriasis, there are alternative treatments to office and home phototherapy. Biologics, immunosuppressive therapies, and other treatment options may be considered on a case-by-case basis.3,4,6 Currently, recommendations for the risk of COVID-19 with biologics or systemic immunosuppressive therapies remains inconsistent and should be carefully considered when providing alternative treatments.7-11
Final Thoughts
As restrictions are lifted according to local public health measures, prepandemic office phototherapy practices may resume operations. Home phototherapy is a practical and effective alternative for treatment of psoriasis when access to the office setting is limited.
- Lim HW, Feldman SR, Van Voorhees AS, et al. Recommendations for phototherapy during the COVID-19 pandemic. J Am Acad Dermatol. 2020;83:287-288.
Anderson KL, Feldman SR. A guide to prescribing home phototherapy for patients with psoriasis: the appropriate patient, the type of unit, the treatment regimen, and the potential obstacles. J Am Acad Dermatol. 2015;72:868.E1-878.E1. - Palmore TN, Smith BA. Coronavirus disease 2019 (COVID-19): infection control in health care and home settings. UpToDate. Updated January 7, 2021. Accessed January 25, 2021.https://www.uptodate.com/contents/coronavirus-disease-2019-covid-19-infection-control-in-health-care-and-home-settings
- Koek MB, Buskens E, van Weelden H, et al. Home versus outpatient ultraviolet B phototherapy for mild to severe psoriasis: pragmatic multicentre randomised controlled non-inferiority trial (PLUTO study). BMJ. 2009;338:b1542.
- Sadeghinia A, Daneshpazhooh M. Immunosuppressive drugs for patients with psoriasis during the COVID-19 pandemic era. a review [published online November 3, 2020]. Dermatol Ther. 2020:E14498. doi:10.1111/dth.14498
- Damiani G, Pacifico A, Bragazzi NL, et al. Biologics increase the risk of SARS-CoV-2 infection and hospitalization, but not ICU admission and death: real-life data from a large cohort during red-zone declaration. Dermatol Ther. 2020;33:E13475.
- Lebwohl M, Rivera-Oyola R, Murrell DF. Should biologics for psoriasis be interrupted in the era of COVID-19? J Am Acad Dermatol. 2020;82:1217-1218.
- Mehta P, Ciurtin C, Scully M, et al. JAK inhibitors in COVID-19: the need for vigilance regarding increased inherent thrombotic risk. Eur Respir J. 2020;56:2001919.
- Walz L, Cohen AJ, Rebaza AP, et al. JAK-inhibitor and type I interferon ability to produce favorable clinical outcomes in COVID-19 patients: a systematic review and meta-analysis. BMC Infect Dis. 2021;21:47.
- Carugno A, Gambini DM, Raponi F, et al. COVID-19 and biologics for psoriasis: a high-epidemic area experience-Bergamo, Lombardy, Italy. J Am Acad Dermatol. 2020;83:292-294.
- Gisondi P, Piaserico S, Naldi L, et al. Incidence rates of hospitalization and death from COVID-19 in patients with psoriasis receiving biological treatment: a Northern Italy experience [published online November 5, 2020]. J Allergy Clin Immunol. doi:10.1016/j.jaci.2020.10.032
Office-based phototherapy practices have closed or are operating below capacity because of the coronavirus disease 2019 (COVID-19) pandemic.1 Social distancing measures to reduce virus transmission are a significant driving factor.1-3 In the age of biologics, other options requiring fewer patient visits are available, such as UVB phototherapy. UV phototherapy is considered first line when more than 10% of the body surface area is affected.4 Although phototherapy often is performed in the office, it also may be delivered at home.2 Home-based phototherapy is safe, effective, and similar in cost to office-based phototherapy.4 Currently, there are limited COVID-19–specific guidelines for home-based phototherapy.
The risks and sequelae of COVID-19 are still being investigated, with cases varying by location. As such, local and national public health recommendations are evolving. Dermatologists must make individualized decisions about practice services, as local restrictions differ. As office-based phototherapy services may struggle to implement mitigation strategies, home-based phototherapy is an increasingly viable treatment option.1,4,5 Patient benefits of home therapy include improved treatment compliance; greater patient satisfaction; reduced travel/waiting time; and reduced long-term cost, including co-pays, depending on insurance coverage.2,4
We aim to provide recommendations on home-based phototherapy during the pandemic. Throughout the decision-making process, careful consideration of safety, risks, benefits, and treatment options for physicians, staff, and patients will be vital to the successful implementation of home-based phototherapy. Our recommendations are based on maximizing benefits and minimizing risks.
Considerations for Physicians
Physicians should take the following steps when assessing if home phototherapy is an option for each patient.1,2,4
• Determine patient eligibility for phototherapy treatment if currently not on phototherapy
• Carefully review patient and provider requirements for home phototherapy supplier
• Review patient history of treatment compliance
• Determine insurance coverage and consider exclusion criteria
• Review prior treatments
• Provide education on side effects
• Provide education on signs of adequate treatment response
• Indicate the type of UV light and unit on the prescription
• Consider whether the patient is in the maintenance or initiation phase when providing recommendations
• Work with the supplier if the light therapy unit is denied by submitting an appeal or prescribing a different unit
• Follow up with telemedicine to assess treatment effectiveness and monitor for adverse effects
Considerations for Patients
Counsel patients to weigh the risks and benefits of home phototherapy prescription and usage.1,2,4
• Evaluate cost
• Carefully review patient and provider requirements for home phototherapy supplier
• Ensure a complete understanding of treatment schedule
• Properly utilize protective equipment (eg, genital shields for men, eye shields for all)
• Avoid sharing phototherapy units with household members
• Disinfect and maintain units
• Maintain proper ventilation of spaces
• Maintain treatment log
• Attend follow-up
Treatment Alternatives
For patients with severe psoriasis, there are alternative treatments to office and home phototherapy. Biologics, immunosuppressive therapies, and other treatment options may be considered on a case-by-case basis.3,4,6 Currently, recommendations for the risk of COVID-19 with biologics or systemic immunosuppressive therapies remains inconsistent and should be carefully considered when providing alternative treatments.7-11
Final Thoughts
As restrictions are lifted according to local public health measures, prepandemic office phototherapy practices may resume operations. Home phototherapy is a practical and effective alternative for treatment of psoriasis when access to the office setting is limited.
Office-based phototherapy practices have closed or are operating below capacity because of the coronavirus disease 2019 (COVID-19) pandemic.1 Social distancing measures to reduce virus transmission are a significant driving factor.1-3 In the age of biologics, other options requiring fewer patient visits are available, such as UVB phototherapy. UV phototherapy is considered first line when more than 10% of the body surface area is affected.4 Although phototherapy often is performed in the office, it also may be delivered at home.2 Home-based phototherapy is safe, effective, and similar in cost to office-based phototherapy.4 Currently, there are limited COVID-19–specific guidelines for home-based phototherapy.
The risks and sequelae of COVID-19 are still being investigated, with cases varying by location. As such, local and national public health recommendations are evolving. Dermatologists must make individualized decisions about practice services, as local restrictions differ. As office-based phototherapy services may struggle to implement mitigation strategies, home-based phototherapy is an increasingly viable treatment option.1,4,5 Patient benefits of home therapy include improved treatment compliance; greater patient satisfaction; reduced travel/waiting time; and reduced long-term cost, including co-pays, depending on insurance coverage.2,4
We aim to provide recommendations on home-based phototherapy during the pandemic. Throughout the decision-making process, careful consideration of safety, risks, benefits, and treatment options for physicians, staff, and patients will be vital to the successful implementation of home-based phototherapy. Our recommendations are based on maximizing benefits and minimizing risks.
Considerations for Physicians
Physicians should take the following steps when assessing if home phototherapy is an option for each patient.1,2,4
• Determine patient eligibility for phototherapy treatment if currently not on phototherapy
• Carefully review patient and provider requirements for home phototherapy supplier
• Review patient history of treatment compliance
• Determine insurance coverage and consider exclusion criteria
• Review prior treatments
• Provide education on side effects
• Provide education on signs of adequate treatment response
• Indicate the type of UV light and unit on the prescription
• Consider whether the patient is in the maintenance or initiation phase when providing recommendations
• Work with the supplier if the light therapy unit is denied by submitting an appeal or prescribing a different unit
• Follow up with telemedicine to assess treatment effectiveness and monitor for adverse effects
Considerations for Patients
Counsel patients to weigh the risks and benefits of home phototherapy prescription and usage.1,2,4
• Evaluate cost
• Carefully review patient and provider requirements for home phototherapy supplier
• Ensure a complete understanding of treatment schedule
• Properly utilize protective equipment (eg, genital shields for men, eye shields for all)
• Avoid sharing phototherapy units with household members
• Disinfect and maintain units
• Maintain proper ventilation of spaces
• Maintain treatment log
• Attend follow-up
Treatment Alternatives
For patients with severe psoriasis, there are alternative treatments to office and home phototherapy. Biologics, immunosuppressive therapies, and other treatment options may be considered on a case-by-case basis.3,4,6 Currently, recommendations for the risk of COVID-19 with biologics or systemic immunosuppressive therapies remains inconsistent and should be carefully considered when providing alternative treatments.7-11
Final Thoughts
As restrictions are lifted according to local public health measures, prepandemic office phototherapy practices may resume operations. Home phototherapy is a practical and effective alternative for treatment of psoriasis when access to the office setting is limited.
- Lim HW, Feldman SR, Van Voorhees AS, et al. Recommendations for phototherapy during the COVID-19 pandemic. J Am Acad Dermatol. 2020;83:287-288.
Anderson KL, Feldman SR. A guide to prescribing home phototherapy for patients with psoriasis: the appropriate patient, the type of unit, the treatment regimen, and the potential obstacles. J Am Acad Dermatol. 2015;72:868.E1-878.E1. - Palmore TN, Smith BA. Coronavirus disease 2019 (COVID-19): infection control in health care and home settings. UpToDate. Updated January 7, 2021. Accessed January 25, 2021.https://www.uptodate.com/contents/coronavirus-disease-2019-covid-19-infection-control-in-health-care-and-home-settings
- Koek MB, Buskens E, van Weelden H, et al. Home versus outpatient ultraviolet B phototherapy for mild to severe psoriasis: pragmatic multicentre randomised controlled non-inferiority trial (PLUTO study). BMJ. 2009;338:b1542.
- Sadeghinia A, Daneshpazhooh M. Immunosuppressive drugs for patients with psoriasis during the COVID-19 pandemic era. a review [published online November 3, 2020]. Dermatol Ther. 2020:E14498. doi:10.1111/dth.14498
- Damiani G, Pacifico A, Bragazzi NL, et al. Biologics increase the risk of SARS-CoV-2 infection and hospitalization, but not ICU admission and death: real-life data from a large cohort during red-zone declaration. Dermatol Ther. 2020;33:E13475.
- Lebwohl M, Rivera-Oyola R, Murrell DF. Should biologics for psoriasis be interrupted in the era of COVID-19? J Am Acad Dermatol. 2020;82:1217-1218.
- Mehta P, Ciurtin C, Scully M, et al. JAK inhibitors in COVID-19: the need for vigilance regarding increased inherent thrombotic risk. Eur Respir J. 2020;56:2001919.
- Walz L, Cohen AJ, Rebaza AP, et al. JAK-inhibitor and type I interferon ability to produce favorable clinical outcomes in COVID-19 patients: a systematic review and meta-analysis. BMC Infect Dis. 2021;21:47.
- Carugno A, Gambini DM, Raponi F, et al. COVID-19 and biologics for psoriasis: a high-epidemic area experience-Bergamo, Lombardy, Italy. J Am Acad Dermatol. 2020;83:292-294.
- Gisondi P, Piaserico S, Naldi L, et al. Incidence rates of hospitalization and death from COVID-19 in patients with psoriasis receiving biological treatment: a Northern Italy experience [published online November 5, 2020]. J Allergy Clin Immunol. doi:10.1016/j.jaci.2020.10.032
- Lim HW, Feldman SR, Van Voorhees AS, et al. Recommendations for phototherapy during the COVID-19 pandemic. J Am Acad Dermatol. 2020;83:287-288.
Anderson KL, Feldman SR. A guide to prescribing home phototherapy for patients with psoriasis: the appropriate patient, the type of unit, the treatment regimen, and the potential obstacles. J Am Acad Dermatol. 2015;72:868.E1-878.E1. - Palmore TN, Smith BA. Coronavirus disease 2019 (COVID-19): infection control in health care and home settings. UpToDate. Updated January 7, 2021. Accessed January 25, 2021.https://www.uptodate.com/contents/coronavirus-disease-2019-covid-19-infection-control-in-health-care-and-home-settings
- Koek MB, Buskens E, van Weelden H, et al. Home versus outpatient ultraviolet B phototherapy for mild to severe psoriasis: pragmatic multicentre randomised controlled non-inferiority trial (PLUTO study). BMJ. 2009;338:b1542.
- Sadeghinia A, Daneshpazhooh M. Immunosuppressive drugs for patients with psoriasis during the COVID-19 pandemic era. a review [published online November 3, 2020]. Dermatol Ther. 2020:E14498. doi:10.1111/dth.14498
- Damiani G, Pacifico A, Bragazzi NL, et al. Biologics increase the risk of SARS-CoV-2 infection and hospitalization, but not ICU admission and death: real-life data from a large cohort during red-zone declaration. Dermatol Ther. 2020;33:E13475.
- Lebwohl M, Rivera-Oyola R, Murrell DF. Should biologics for psoriasis be interrupted in the era of COVID-19? J Am Acad Dermatol. 2020;82:1217-1218.
- Mehta P, Ciurtin C, Scully M, et al. JAK inhibitors in COVID-19: the need for vigilance regarding increased inherent thrombotic risk. Eur Respir J. 2020;56:2001919.
- Walz L, Cohen AJ, Rebaza AP, et al. JAK-inhibitor and type I interferon ability to produce favorable clinical outcomes in COVID-19 patients: a systematic review and meta-analysis. BMC Infect Dis. 2021;21:47.
- Carugno A, Gambini DM, Raponi F, et al. COVID-19 and biologics for psoriasis: a high-epidemic area experience-Bergamo, Lombardy, Italy. J Am Acad Dermatol. 2020;83:292-294.
- Gisondi P, Piaserico S, Naldi L, et al. Incidence rates of hospitalization and death from COVID-19 in patients with psoriasis receiving biological treatment: a Northern Italy experience [published online November 5, 2020]. J Allergy Clin Immunol. doi:10.1016/j.jaci.2020.10.032
Practice Points
- Home phototherapy is a safe and effective option for patients with psoriasis during the coronavirus disease 2019 (COVID-19) pandemic.
- Although a consensus has not been reached with systemic immunosuppressive therapies for patients with psoriasis and the risk of COVID-19, we continue to recommend caution and careful monitoring of clinical outcomes for patients.
Reimbursement for Teledermatology During the COVID-19 Public Health Emergency: Change Has Come, But Will It Stay?
The world of telemedicine—especially teledermatology—had been a sleepy underutilized afterthought for most physicians until we were faced with a global pandemic the likes of which none of us had seen in our lifetimes. And just like that, teledermatology went from an afterthought to part of the “new normal.” Although those of us already practicing telemedicine knew of potential pitfalls and concerns, this great social experiment of throwing everyone into unexplored territory led to a great deal of frustration with technology and workflows that were not optimized for dermatology visits. The process is still changing, and the technical aspects of conducting teledermatology visits will no doubt improve, but what about the bigger question of reimbursement? Without adequate payments and financial models, the long-term future of telemedicine is uncertain, so an understanding of the current and likely future landscape of telemedicine reimbursement is critical.
Waivers During the Public Health Emergency
The declaration of a public health emergency (PHE)allowed for significant flexibility by the Centers for Medicare & Medicaid Services (CMS) during the coronavirus disease 2019 (COVID-19) pandemic. Importantly, the CMS was permitted to act quickly to allow telehealth to flourish during the worst of the pandemic and throughout the declared PHE, which has been extended several times already. Currently, the PHE is set to expire on April 20, 2021, but may be extended again if the pandemic is ongoing. The most important of these waivers was probably the removal of both the originating site and geographic requirements for telehealth services.1 Prior to the COVID-19 PHE, a patient would have to travel to a doctor’s office, hospital, or skilled nursing facility to receive telehealth care (originating site requirement), and even then this was only allowed in defined rural areas of the country (geographic requirement). Both of these requirements were waived, allowing for any patient to receive telehealth services within their own homes. Concurrently, the requirement that patients must have an established relationship with the provider (ie, telehealth could not be used to provide care to new patients) also was waived.1
In the spirit of expanding access to care and providing reasonable reimbursement for medical services, other changes were made for which the CMS should be commended. In acknowledging that many Medicare/Medicaid beneficiaries may not have access to devices that permit real-time, 2-way audio/video communication, which previously were necessary to qualify for a telehealth encounter, the CMS decided to cover telephone visits and provide reimbursement at the level of an established visit.1 They also changed the billing structure to remove the place of service (POS) designation for telehealth (POS 02) and replace it with the normal physician’s office POS designation (usually POS 11), bringing back a telehealth modifier (modifier -95) in the process. The benefit of this change is solely to increase reimbursement for these services, as telehealth POS services generally are covered at lower facility rates, whereas POS 11 codes are reimbursed at the full level of a nonfacility physician’s office rate.
Finally, other waivers such as the Office of Civil Rights’ decision to waive HIPAA (Health Insurance Portability and Accountability Act) violations for telehealth platforms during the PHE allowed offices to take on telemedicine quickly without having to implement a new infrastructure.2 Numerous codes were added to the list of covered services for telehealth, but these generally are not relevant for dermatologists. The CMS also allowed physicians’ offices to waive the patient responsibility/co-pay during the COVID-19 PHE, which previously was not allowed due to concerns about the anti-kickback statute.1 These co-pay waivers were intended to remove another barrier to care for patients who were hesitant to participate in virtual visits. For the most part, the waiver of state licensing requirements is a bit less useful. As part of the CMS waiver, providers technically are allowed to see out-of-state Medicare/Medicaid beneficiaries, but state licensing laws are still in effect; thus, in the absence of a blanket state-level waiver (which some states enacted, modeled after the Uniform Emergency Volunteer Health Practitioner Act of 20063), providers still cannot see most out-of-state patients from a legal and malpractice coverage standpoint.
An important flexibility during the COVID-19 PHE is one that often is underrecognized. The CMS has been clear about the ability to provide direct supervision for advanced practice providers (APPs) and residents via telehealth during the PHE, which allows for incident-to billing for APPs at remote sites given that the supervising physician is immediately available via an interactive, 2-way, live audio/video telecommunications method. It also allows for direct supervision of APPs and residents using such technology. For dermatology, which does not have a primary care waiver, an attending must still directly supervise each patient and see the patient via a live audio/video modality but does not have to be on-site to do so. This is a very interesting concept that, if extended, could truly impact practice management for the long-term.
Response From Commercial Insurance Carriers
Tracking along with the CMS waivers and flexibilities during the PHE, most commercial carriers quickly adopted similar policies to cover telehealth services. It should be noted that for most commercial insurance carriers, the coverage was already broader than Medicare/Medicaid coverage for telehealth prior to the PHE, so in many ways it is an extension of that concept and acceptance of telemedicine as a whole. What is sometimes confusing, though, is that various policies and requirements around billing exist; for example, while most carriers emulated the POS requirements that the CMS adopted, some carriers still stuck with the telemedicine POS but paid full in-office visit rates for those codes. Some carriers adopted higher reimbursement rates for telephone visits, similar to the CMS, while others instructed providers to just bill for the established office visit codes and allowed for telephone-only visits to qualify for these billing codes. Some carriers also waived co-pays for telehealth visits for their members (whether related to COVID-19 or not). It is beyond the scope of this article to delve into the specifics, which may vary not only by carrier but by region and plan. However, it is important to stay on top of one’s insurance carriers to find out what their latest directives are for billing for telehealth.
Postpandemic Teledermatology
What about the future of teledermatology? Although many dermatologists have adopted telehealth services out of necessity during the COVID-19 PHE, the jury is still out on the long-term forecast for telemedicine in dermatology. Concerns about liability/malpractice and technology issues abound, and for many, the headaches of teledermatology—such as trying to focus on a blurry photograph of a nevus that the patient is concerned about—make it unappealing. Some of these issues will be addressed by better technology, but the reimbursement structure must continue for teledermatology to remain in widespread use.
Currently, the biggest question facing telehealth is whether the waivers for originating site and geographic requirements will be able to continue. The CMS itself does not have the statutory authority to make these changes permanent and was only allowed to act due to a waiver under section 1135 of the Social Security Act during a PHE. It would take an act of Congress to change the law to allow for this specific expansion of telehealth services. A number of federal bills, including S 2741 (Creating Opportunities Now for Necessary and Effective Care Technologies [CONNECT] for Health Act of 2019) and S 4796 (Fair Care Act of 2020) from the Senate, contain such provisions, but none have been passed at the time of writing. There does seem to be broad support of the concept of expanding telemedicine access, such as noted by New York State Governor Andrew Cuomo in his 2021 State of the State address,4 but it remains to be seen when action will come.
Some regulations, such as the HIPAA waiver and the ability to waive co-pays, are not slated to continue after the pandemic. The ability to supervise residents via telehealth (real-time audio/video) has been made permanent, but only in rural areas. Direct supervision of APPs via telehealth will continue through the end of the calendar year of the PHE or the end of 2021, whichever comes later, but it remains to be seen whether remote supervision will continue. The CMS has stated in its comments that it is looking at this issue closely and may establish certain guardrails to ensure quality of care is maintained.1 Telephone/audio-only visits also may come under further scrutiny, but research has supported the concept that patients who are more likely to gain access through audio-only modalities are older, Medicare/Medicaid (vs commercial), and Black (vs White) patients,5 so it would indeed introduce an unfair barrier to access if such coverage was rolled back.
Final Thoughts
Overall, we have made much progress in teledermatology. Once utilized by a small fraction of dermatologists, the vast majority of us turned to teledermatology to sustain our practices during the COVID-19 pandemic. Moving forward, there are 2 critical factors to consider: continued technological innovation and permanent coverage for telehealth reimbursement at in-office visit levels. With these challenges resolved, we can move forward and consider novel models that may be able to deliver dermatologic care to a broader patient population, thereby solving the critical issue of access to care for so many patients in need in our country.
- Medicare Program; CY 2021 Payment Policies Under the Physician Fee Schedule and Other Changes to Part B Payment Policies; Medicare Shared Savings Program Requirements; Medicaid Promoting Interoperability Program Requirements for Eligible Professionals; Quality Payment Program; Coverage of Opioid Use Disorder Services Furnished by Opioid Treatment Programs; Medicare Enrollment of Opioid Treatment Programs; Electronic Prescribing for Controlled Substances for a Covered Part D Drug; Payment for Office/Outpatient Evaluation and Management Services; Hospital IQR Program; Establish New Code Categories; Medicare Diabetes Prevention Program (MDPP) Expanded Model Emergency Policy; Coding and Payment for Virtual Check-in Services Interim Final Rule Policy; Coding and Payment for Personal Protective Equipment (PPE) Interim Final Rule Policy; Regulatory Revisions in Response to the Public Health Emergency (PHE) for COVID-19; and Finalization of Certain Provisions from the March 31st, May 8th and September 2nd Interim Final Rules in Response to the PHE for COVID-19. Fed Registr. 2020;85:84472-85377. To be codified at 42 CFR §400, 410, 414, 415, 423, 424, and 425. https://www.federalregister.gov/documents/2020/12/28/2020-26815/medicare-program-cy-2021-payment-policies-under-the-physician-fee-schedule-and-other-changes-to-part
- Office for Civil Rights. Notification of enforcement discretion for telehealth remote communications during the COVID-19 nationwide public health emergency. US Department of Health and Human Services website. Reviewed January 20, 2021. Accessed January 25, 2021. https://www.hhs.gov/hipaa/for-professionals/special-topics/emergency-preparedness/notification-enforcement-discretion-telehealth/index.html
- Hoffman DA. Increasing access to care: telehealth during COVID-19 [published online June 16, 2020]. J Law Biosci. doi:10.1093/jlb/lsaa043
- Governor Cuomo announces proposal to expand access to telehealth for all as part of 2021 State of the State. New York State website. Published January 10, 2021. Accessed January 25, 021. https://www.governor.ny.gov/news/governor-cuomo-announces-proposal-expand-access-telehealth-all-part-2021-state-state#:~:text=and%20Rural%20Communities-,Governor%20Andrew%20M.,2021%20State%20of%20the%20State.&text=New%20Yorkers%20have%20adapted%20throughout,into%20our%20existing%20healthcare%20system
- Gilson SF, Umscheid CA, Laiteerapong N, et al. Growth of ambulatory virtual visit and differential use by patient sociodemographics at one urban academic medical center during the COVID-19 pandemic: retrospective analysis. JMIR Med Inform. 2020;8:E24544.
The world of telemedicine—especially teledermatology—had been a sleepy underutilized afterthought for most physicians until we were faced with a global pandemic the likes of which none of us had seen in our lifetimes. And just like that, teledermatology went from an afterthought to part of the “new normal.” Although those of us already practicing telemedicine knew of potential pitfalls and concerns, this great social experiment of throwing everyone into unexplored territory led to a great deal of frustration with technology and workflows that were not optimized for dermatology visits. The process is still changing, and the technical aspects of conducting teledermatology visits will no doubt improve, but what about the bigger question of reimbursement? Without adequate payments and financial models, the long-term future of telemedicine is uncertain, so an understanding of the current and likely future landscape of telemedicine reimbursement is critical.
Waivers During the Public Health Emergency
The declaration of a public health emergency (PHE)allowed for significant flexibility by the Centers for Medicare & Medicaid Services (CMS) during the coronavirus disease 2019 (COVID-19) pandemic. Importantly, the CMS was permitted to act quickly to allow telehealth to flourish during the worst of the pandemic and throughout the declared PHE, which has been extended several times already. Currently, the PHE is set to expire on April 20, 2021, but may be extended again if the pandemic is ongoing. The most important of these waivers was probably the removal of both the originating site and geographic requirements for telehealth services.1 Prior to the COVID-19 PHE, a patient would have to travel to a doctor’s office, hospital, or skilled nursing facility to receive telehealth care (originating site requirement), and even then this was only allowed in defined rural areas of the country (geographic requirement). Both of these requirements were waived, allowing for any patient to receive telehealth services within their own homes. Concurrently, the requirement that patients must have an established relationship with the provider (ie, telehealth could not be used to provide care to new patients) also was waived.1
In the spirit of expanding access to care and providing reasonable reimbursement for medical services, other changes were made for which the CMS should be commended. In acknowledging that many Medicare/Medicaid beneficiaries may not have access to devices that permit real-time, 2-way audio/video communication, which previously were necessary to qualify for a telehealth encounter, the CMS decided to cover telephone visits and provide reimbursement at the level of an established visit.1 They also changed the billing structure to remove the place of service (POS) designation for telehealth (POS 02) and replace it with the normal physician’s office POS designation (usually POS 11), bringing back a telehealth modifier (modifier -95) in the process. The benefit of this change is solely to increase reimbursement for these services, as telehealth POS services generally are covered at lower facility rates, whereas POS 11 codes are reimbursed at the full level of a nonfacility physician’s office rate.
Finally, other waivers such as the Office of Civil Rights’ decision to waive HIPAA (Health Insurance Portability and Accountability Act) violations for telehealth platforms during the PHE allowed offices to take on telemedicine quickly without having to implement a new infrastructure.2 Numerous codes were added to the list of covered services for telehealth, but these generally are not relevant for dermatologists. The CMS also allowed physicians’ offices to waive the patient responsibility/co-pay during the COVID-19 PHE, which previously was not allowed due to concerns about the anti-kickback statute.1 These co-pay waivers were intended to remove another barrier to care for patients who were hesitant to participate in virtual visits. For the most part, the waiver of state licensing requirements is a bit less useful. As part of the CMS waiver, providers technically are allowed to see out-of-state Medicare/Medicaid beneficiaries, but state licensing laws are still in effect; thus, in the absence of a blanket state-level waiver (which some states enacted, modeled after the Uniform Emergency Volunteer Health Practitioner Act of 20063), providers still cannot see most out-of-state patients from a legal and malpractice coverage standpoint.
An important flexibility during the COVID-19 PHE is one that often is underrecognized. The CMS has been clear about the ability to provide direct supervision for advanced practice providers (APPs) and residents via telehealth during the PHE, which allows for incident-to billing for APPs at remote sites given that the supervising physician is immediately available via an interactive, 2-way, live audio/video telecommunications method. It also allows for direct supervision of APPs and residents using such technology. For dermatology, which does not have a primary care waiver, an attending must still directly supervise each patient and see the patient via a live audio/video modality but does not have to be on-site to do so. This is a very interesting concept that, if extended, could truly impact practice management for the long-term.
Response From Commercial Insurance Carriers
Tracking along with the CMS waivers and flexibilities during the PHE, most commercial carriers quickly adopted similar policies to cover telehealth services. It should be noted that for most commercial insurance carriers, the coverage was already broader than Medicare/Medicaid coverage for telehealth prior to the PHE, so in many ways it is an extension of that concept and acceptance of telemedicine as a whole. What is sometimes confusing, though, is that various policies and requirements around billing exist; for example, while most carriers emulated the POS requirements that the CMS adopted, some carriers still stuck with the telemedicine POS but paid full in-office visit rates for those codes. Some carriers adopted higher reimbursement rates for telephone visits, similar to the CMS, while others instructed providers to just bill for the established office visit codes and allowed for telephone-only visits to qualify for these billing codes. Some carriers also waived co-pays for telehealth visits for their members (whether related to COVID-19 or not). It is beyond the scope of this article to delve into the specifics, which may vary not only by carrier but by region and plan. However, it is important to stay on top of one’s insurance carriers to find out what their latest directives are for billing for telehealth.
Postpandemic Teledermatology
What about the future of teledermatology? Although many dermatologists have adopted telehealth services out of necessity during the COVID-19 PHE, the jury is still out on the long-term forecast for telemedicine in dermatology. Concerns about liability/malpractice and technology issues abound, and for many, the headaches of teledermatology—such as trying to focus on a blurry photograph of a nevus that the patient is concerned about—make it unappealing. Some of these issues will be addressed by better technology, but the reimbursement structure must continue for teledermatology to remain in widespread use.
Currently, the biggest question facing telehealth is whether the waivers for originating site and geographic requirements will be able to continue. The CMS itself does not have the statutory authority to make these changes permanent and was only allowed to act due to a waiver under section 1135 of the Social Security Act during a PHE. It would take an act of Congress to change the law to allow for this specific expansion of telehealth services. A number of federal bills, including S 2741 (Creating Opportunities Now for Necessary and Effective Care Technologies [CONNECT] for Health Act of 2019) and S 4796 (Fair Care Act of 2020) from the Senate, contain such provisions, but none have been passed at the time of writing. There does seem to be broad support of the concept of expanding telemedicine access, such as noted by New York State Governor Andrew Cuomo in his 2021 State of the State address,4 but it remains to be seen when action will come.
Some regulations, such as the HIPAA waiver and the ability to waive co-pays, are not slated to continue after the pandemic. The ability to supervise residents via telehealth (real-time audio/video) has been made permanent, but only in rural areas. Direct supervision of APPs via telehealth will continue through the end of the calendar year of the PHE or the end of 2021, whichever comes later, but it remains to be seen whether remote supervision will continue. The CMS has stated in its comments that it is looking at this issue closely and may establish certain guardrails to ensure quality of care is maintained.1 Telephone/audio-only visits also may come under further scrutiny, but research has supported the concept that patients who are more likely to gain access through audio-only modalities are older, Medicare/Medicaid (vs commercial), and Black (vs White) patients,5 so it would indeed introduce an unfair barrier to access if such coverage was rolled back.
Final Thoughts
Overall, we have made much progress in teledermatology. Once utilized by a small fraction of dermatologists, the vast majority of us turned to teledermatology to sustain our practices during the COVID-19 pandemic. Moving forward, there are 2 critical factors to consider: continued technological innovation and permanent coverage for telehealth reimbursement at in-office visit levels. With these challenges resolved, we can move forward and consider novel models that may be able to deliver dermatologic care to a broader patient population, thereby solving the critical issue of access to care for so many patients in need in our country.
The world of telemedicine—especially teledermatology—had been a sleepy underutilized afterthought for most physicians until we were faced with a global pandemic the likes of which none of us had seen in our lifetimes. And just like that, teledermatology went from an afterthought to part of the “new normal.” Although those of us already practicing telemedicine knew of potential pitfalls and concerns, this great social experiment of throwing everyone into unexplored territory led to a great deal of frustration with technology and workflows that were not optimized for dermatology visits. The process is still changing, and the technical aspects of conducting teledermatology visits will no doubt improve, but what about the bigger question of reimbursement? Without adequate payments and financial models, the long-term future of telemedicine is uncertain, so an understanding of the current and likely future landscape of telemedicine reimbursement is critical.
Waivers During the Public Health Emergency
The declaration of a public health emergency (PHE)allowed for significant flexibility by the Centers for Medicare & Medicaid Services (CMS) during the coronavirus disease 2019 (COVID-19) pandemic. Importantly, the CMS was permitted to act quickly to allow telehealth to flourish during the worst of the pandemic and throughout the declared PHE, which has been extended several times already. Currently, the PHE is set to expire on April 20, 2021, but may be extended again if the pandemic is ongoing. The most important of these waivers was probably the removal of both the originating site and geographic requirements for telehealth services.1 Prior to the COVID-19 PHE, a patient would have to travel to a doctor’s office, hospital, or skilled nursing facility to receive telehealth care (originating site requirement), and even then this was only allowed in defined rural areas of the country (geographic requirement). Both of these requirements were waived, allowing for any patient to receive telehealth services within their own homes. Concurrently, the requirement that patients must have an established relationship with the provider (ie, telehealth could not be used to provide care to new patients) also was waived.1
In the spirit of expanding access to care and providing reasonable reimbursement for medical services, other changes were made for which the CMS should be commended. In acknowledging that many Medicare/Medicaid beneficiaries may not have access to devices that permit real-time, 2-way audio/video communication, which previously were necessary to qualify for a telehealth encounter, the CMS decided to cover telephone visits and provide reimbursement at the level of an established visit.1 They also changed the billing structure to remove the place of service (POS) designation for telehealth (POS 02) and replace it with the normal physician’s office POS designation (usually POS 11), bringing back a telehealth modifier (modifier -95) in the process. The benefit of this change is solely to increase reimbursement for these services, as telehealth POS services generally are covered at lower facility rates, whereas POS 11 codes are reimbursed at the full level of a nonfacility physician’s office rate.
Finally, other waivers such as the Office of Civil Rights’ decision to waive HIPAA (Health Insurance Portability and Accountability Act) violations for telehealth platforms during the PHE allowed offices to take on telemedicine quickly without having to implement a new infrastructure.2 Numerous codes were added to the list of covered services for telehealth, but these generally are not relevant for dermatologists. The CMS also allowed physicians’ offices to waive the patient responsibility/co-pay during the COVID-19 PHE, which previously was not allowed due to concerns about the anti-kickback statute.1 These co-pay waivers were intended to remove another barrier to care for patients who were hesitant to participate in virtual visits. For the most part, the waiver of state licensing requirements is a bit less useful. As part of the CMS waiver, providers technically are allowed to see out-of-state Medicare/Medicaid beneficiaries, but state licensing laws are still in effect; thus, in the absence of a blanket state-level waiver (which some states enacted, modeled after the Uniform Emergency Volunteer Health Practitioner Act of 20063), providers still cannot see most out-of-state patients from a legal and malpractice coverage standpoint.
An important flexibility during the COVID-19 PHE is one that often is underrecognized. The CMS has been clear about the ability to provide direct supervision for advanced practice providers (APPs) and residents via telehealth during the PHE, which allows for incident-to billing for APPs at remote sites given that the supervising physician is immediately available via an interactive, 2-way, live audio/video telecommunications method. It also allows for direct supervision of APPs and residents using such technology. For dermatology, which does not have a primary care waiver, an attending must still directly supervise each patient and see the patient via a live audio/video modality but does not have to be on-site to do so. This is a very interesting concept that, if extended, could truly impact practice management for the long-term.
Response From Commercial Insurance Carriers
Tracking along with the CMS waivers and flexibilities during the PHE, most commercial carriers quickly adopted similar policies to cover telehealth services. It should be noted that for most commercial insurance carriers, the coverage was already broader than Medicare/Medicaid coverage for telehealth prior to the PHE, so in many ways it is an extension of that concept and acceptance of telemedicine as a whole. What is sometimes confusing, though, is that various policies and requirements around billing exist; for example, while most carriers emulated the POS requirements that the CMS adopted, some carriers still stuck with the telemedicine POS but paid full in-office visit rates for those codes. Some carriers adopted higher reimbursement rates for telephone visits, similar to the CMS, while others instructed providers to just bill for the established office visit codes and allowed for telephone-only visits to qualify for these billing codes. Some carriers also waived co-pays for telehealth visits for their members (whether related to COVID-19 or not). It is beyond the scope of this article to delve into the specifics, which may vary not only by carrier but by region and plan. However, it is important to stay on top of one’s insurance carriers to find out what their latest directives are for billing for telehealth.
Postpandemic Teledermatology
What about the future of teledermatology? Although many dermatologists have adopted telehealth services out of necessity during the COVID-19 PHE, the jury is still out on the long-term forecast for telemedicine in dermatology. Concerns about liability/malpractice and technology issues abound, and for many, the headaches of teledermatology—such as trying to focus on a blurry photograph of a nevus that the patient is concerned about—make it unappealing. Some of these issues will be addressed by better technology, but the reimbursement structure must continue for teledermatology to remain in widespread use.
Currently, the biggest question facing telehealth is whether the waivers for originating site and geographic requirements will be able to continue. The CMS itself does not have the statutory authority to make these changes permanent and was only allowed to act due to a waiver under section 1135 of the Social Security Act during a PHE. It would take an act of Congress to change the law to allow for this specific expansion of telehealth services. A number of federal bills, including S 2741 (Creating Opportunities Now for Necessary and Effective Care Technologies [CONNECT] for Health Act of 2019) and S 4796 (Fair Care Act of 2020) from the Senate, contain such provisions, but none have been passed at the time of writing. There does seem to be broad support of the concept of expanding telemedicine access, such as noted by New York State Governor Andrew Cuomo in his 2021 State of the State address,4 but it remains to be seen when action will come.
Some regulations, such as the HIPAA waiver and the ability to waive co-pays, are not slated to continue after the pandemic. The ability to supervise residents via telehealth (real-time audio/video) has been made permanent, but only in rural areas. Direct supervision of APPs via telehealth will continue through the end of the calendar year of the PHE or the end of 2021, whichever comes later, but it remains to be seen whether remote supervision will continue. The CMS has stated in its comments that it is looking at this issue closely and may establish certain guardrails to ensure quality of care is maintained.1 Telephone/audio-only visits also may come under further scrutiny, but research has supported the concept that patients who are more likely to gain access through audio-only modalities are older, Medicare/Medicaid (vs commercial), and Black (vs White) patients,5 so it would indeed introduce an unfair barrier to access if such coverage was rolled back.
Final Thoughts
Overall, we have made much progress in teledermatology. Once utilized by a small fraction of dermatologists, the vast majority of us turned to teledermatology to sustain our practices during the COVID-19 pandemic. Moving forward, there are 2 critical factors to consider: continued technological innovation and permanent coverage for telehealth reimbursement at in-office visit levels. With these challenges resolved, we can move forward and consider novel models that may be able to deliver dermatologic care to a broader patient population, thereby solving the critical issue of access to care for so many patients in need in our country.
- Medicare Program; CY 2021 Payment Policies Under the Physician Fee Schedule and Other Changes to Part B Payment Policies; Medicare Shared Savings Program Requirements; Medicaid Promoting Interoperability Program Requirements for Eligible Professionals; Quality Payment Program; Coverage of Opioid Use Disorder Services Furnished by Opioid Treatment Programs; Medicare Enrollment of Opioid Treatment Programs; Electronic Prescribing for Controlled Substances for a Covered Part D Drug; Payment for Office/Outpatient Evaluation and Management Services; Hospital IQR Program; Establish New Code Categories; Medicare Diabetes Prevention Program (MDPP) Expanded Model Emergency Policy; Coding and Payment for Virtual Check-in Services Interim Final Rule Policy; Coding and Payment for Personal Protective Equipment (PPE) Interim Final Rule Policy; Regulatory Revisions in Response to the Public Health Emergency (PHE) for COVID-19; and Finalization of Certain Provisions from the March 31st, May 8th and September 2nd Interim Final Rules in Response to the PHE for COVID-19. Fed Registr. 2020;85:84472-85377. To be codified at 42 CFR §400, 410, 414, 415, 423, 424, and 425. https://www.federalregister.gov/documents/2020/12/28/2020-26815/medicare-program-cy-2021-payment-policies-under-the-physician-fee-schedule-and-other-changes-to-part
- Office for Civil Rights. Notification of enforcement discretion for telehealth remote communications during the COVID-19 nationwide public health emergency. US Department of Health and Human Services website. Reviewed January 20, 2021. Accessed January 25, 2021. https://www.hhs.gov/hipaa/for-professionals/special-topics/emergency-preparedness/notification-enforcement-discretion-telehealth/index.html
- Hoffman DA. Increasing access to care: telehealth during COVID-19 [published online June 16, 2020]. J Law Biosci. doi:10.1093/jlb/lsaa043
- Governor Cuomo announces proposal to expand access to telehealth for all as part of 2021 State of the State. New York State website. Published January 10, 2021. Accessed January 25, 021. https://www.governor.ny.gov/news/governor-cuomo-announces-proposal-expand-access-telehealth-all-part-2021-state-state#:~:text=and%20Rural%20Communities-,Governor%20Andrew%20M.,2021%20State%20of%20the%20State.&text=New%20Yorkers%20have%20adapted%20throughout,into%20our%20existing%20healthcare%20system
- Gilson SF, Umscheid CA, Laiteerapong N, et al. Growth of ambulatory virtual visit and differential use by patient sociodemographics at one urban academic medical center during the COVID-19 pandemic: retrospective analysis. JMIR Med Inform. 2020;8:E24544.
- Medicare Program; CY 2021 Payment Policies Under the Physician Fee Schedule and Other Changes to Part B Payment Policies; Medicare Shared Savings Program Requirements; Medicaid Promoting Interoperability Program Requirements for Eligible Professionals; Quality Payment Program; Coverage of Opioid Use Disorder Services Furnished by Opioid Treatment Programs; Medicare Enrollment of Opioid Treatment Programs; Electronic Prescribing for Controlled Substances for a Covered Part D Drug; Payment for Office/Outpatient Evaluation and Management Services; Hospital IQR Program; Establish New Code Categories; Medicare Diabetes Prevention Program (MDPP) Expanded Model Emergency Policy; Coding and Payment for Virtual Check-in Services Interim Final Rule Policy; Coding and Payment for Personal Protective Equipment (PPE) Interim Final Rule Policy; Regulatory Revisions in Response to the Public Health Emergency (PHE) for COVID-19; and Finalization of Certain Provisions from the March 31st, May 8th and September 2nd Interim Final Rules in Response to the PHE for COVID-19. Fed Registr. 2020;85:84472-85377. To be codified at 42 CFR §400, 410, 414, 415, 423, 424, and 425. https://www.federalregister.gov/documents/2020/12/28/2020-26815/medicare-program-cy-2021-payment-policies-under-the-physician-fee-schedule-and-other-changes-to-part
- Office for Civil Rights. Notification of enforcement discretion for telehealth remote communications during the COVID-19 nationwide public health emergency. US Department of Health and Human Services website. Reviewed January 20, 2021. Accessed January 25, 2021. https://www.hhs.gov/hipaa/for-professionals/special-topics/emergency-preparedness/notification-enforcement-discretion-telehealth/index.html
- Hoffman DA. Increasing access to care: telehealth during COVID-19 [published online June 16, 2020]. J Law Biosci. doi:10.1093/jlb/lsaa043
- Governor Cuomo announces proposal to expand access to telehealth for all as part of 2021 State of the State. New York State website. Published January 10, 2021. Accessed January 25, 021. https://www.governor.ny.gov/news/governor-cuomo-announces-proposal-expand-access-telehealth-all-part-2021-state-state#:~:text=and%20Rural%20Communities-,Governor%20Andrew%20M.,2021%20State%20of%20the%20State.&text=New%20Yorkers%20have%20adapted%20throughout,into%20our%20existing%20healthcare%20system
- Gilson SF, Umscheid CA, Laiteerapong N, et al. Growth of ambulatory virtual visit and differential use by patient sociodemographics at one urban academic medical center during the COVID-19 pandemic: retrospective analysis. JMIR Med Inform. 2020;8:E24544.
Oral antibiotic treats most children with UTI
Oral antibiotic treatment for 7-10 days works for most feverish children with uncomplicated urinary tract infection (UTI), reported Tej K. Mattoo, MD, of Wayne State University, Detroit, and associates.
A good clinical assessment supported by laboratory results using a clean urine specimen is crucial to accurately diagnosing UTI in children, Dr. Mattoo and colleagues reported in a state-of-the-art review article in Pediatrics.
The authors set out to summarize the current literature on UTI in children with the goal of guiding clinical management. They provide a thorough summary of the research on a wide range of issues, including pathogenesis of acute pyelonephritis and renal scarring, risk factors for UTI and renal scarring, diagnosis and common errors in diagnosis, complications of UTI and post-UTI renal imaging, antibiotics, antimicrobial prophylaxis, surgical interventions, and prevention of recurrent UTIs.
What key steps make all the difference?
To help guide practicing physicians through this wealth of information, Dr. Mattoo noted in an interview that, although the review article offers “many takeaway messages,” there are several issues of crucial importance. Notably, urine collection in young children who are not yet toilet trained can present considerable challenges in achieving an accurate assessment. A contaminated urine specimen leads to unnecessary antibiotic treatment, and in some cases unwarranted hospitalization, intravenous lines, renal imaging, and follow-up investigations, Dr. Mattoo said.
Ureteral catheterization or suprapubic bladder aspiration are the preferred methods of specimen collection, especially in cases where specimens collected with a perineal bag are dipstick positive, the authors explained. Midstream collection (known as the Quick-Wee method) can also be used following stimulation of the suprapubic area with cold fluid-soaked gauze.
Also of considerable importance is distinguishing bladder infections from kidney infections whenever possible, Dr. Mattoo noted. The antibiotic treatment, complications, and follow-up plans can be different for each, he cautioned. The authors have provided a helpful table within the article to help make this differentiation.
Timing is crucial
Prescribing treatment with an antibiotic within 48 hours of fever onset is essential for the prevention of renal scarring, Dr. Mattoo advised. The key is to treat with the goal of avoiding long-term complications. Although there are some exceptions, most cases of UTI can be treated with oral antibiotics and do not require hospitalization.
Some children with first UTI need additional testing, such as renal imaging, to ensure that there are no underlying risk factors for UTI. These children, in particular, can be at an increased risk of recurrent UTI and renal scarring, Dr. Mattoo explained.
Antibiotic resistance is a major emerging problem in patients with UTI at all ages and we should use antibiotics only in patients who truly have a UTI that requires such treatment, he urged.
In an interview, Timothy Joos, MD, a Seattle internist and pediatrician in private practice, noted: “In the words of the British novelist Tom Holt, ‘There are few moments of clarity more profound than those that follow the emptying of an overcharged bladder. The world slows down, the focus sharpens, the brain comes back online. Huge nebulous difficulties prove on close calm examination to be merely cloud giants.’ Thank you to Drs. Mattoo, Nelson, and Shaikh for providing this clarity of current UTI diagnosis and management,” Dr. Joos said.
“It bears repeating that because of the rare prevalence of grade 4 to 5 vesicoureteral reflux in children with their first UTI, current guidelines recommend that a voiding cystourethrogram can be reserved for children with an abnormal ultrasound, atypical pathogen, complicated clinical course, or known renal scarring,” added Dr. Joos.
The authors had no relevant disclosures. Dr. Joos is a member of the Pediatric News editorial advisory board but had no other disclosures.
Oral antibiotic treatment for 7-10 days works for most feverish children with uncomplicated urinary tract infection (UTI), reported Tej K. Mattoo, MD, of Wayne State University, Detroit, and associates.
A good clinical assessment supported by laboratory results using a clean urine specimen is crucial to accurately diagnosing UTI in children, Dr. Mattoo and colleagues reported in a state-of-the-art review article in Pediatrics.
The authors set out to summarize the current literature on UTI in children with the goal of guiding clinical management. They provide a thorough summary of the research on a wide range of issues, including pathogenesis of acute pyelonephritis and renal scarring, risk factors for UTI and renal scarring, diagnosis and common errors in diagnosis, complications of UTI and post-UTI renal imaging, antibiotics, antimicrobial prophylaxis, surgical interventions, and prevention of recurrent UTIs.
What key steps make all the difference?
To help guide practicing physicians through this wealth of information, Dr. Mattoo noted in an interview that, although the review article offers “many takeaway messages,” there are several issues of crucial importance. Notably, urine collection in young children who are not yet toilet trained can present considerable challenges in achieving an accurate assessment. A contaminated urine specimen leads to unnecessary antibiotic treatment, and in some cases unwarranted hospitalization, intravenous lines, renal imaging, and follow-up investigations, Dr. Mattoo said.
Ureteral catheterization or suprapubic bladder aspiration are the preferred methods of specimen collection, especially in cases where specimens collected with a perineal bag are dipstick positive, the authors explained. Midstream collection (known as the Quick-Wee method) can also be used following stimulation of the suprapubic area with cold fluid-soaked gauze.
Also of considerable importance is distinguishing bladder infections from kidney infections whenever possible, Dr. Mattoo noted. The antibiotic treatment, complications, and follow-up plans can be different for each, he cautioned. The authors have provided a helpful table within the article to help make this differentiation.
Timing is crucial
Prescribing treatment with an antibiotic within 48 hours of fever onset is essential for the prevention of renal scarring, Dr. Mattoo advised. The key is to treat with the goal of avoiding long-term complications. Although there are some exceptions, most cases of UTI can be treated with oral antibiotics and do not require hospitalization.
Some children with first UTI need additional testing, such as renal imaging, to ensure that there are no underlying risk factors for UTI. These children, in particular, can be at an increased risk of recurrent UTI and renal scarring, Dr. Mattoo explained.
Antibiotic resistance is a major emerging problem in patients with UTI at all ages and we should use antibiotics only in patients who truly have a UTI that requires such treatment, he urged.
In an interview, Timothy Joos, MD, a Seattle internist and pediatrician in private practice, noted: “In the words of the British novelist Tom Holt, ‘There are few moments of clarity more profound than those that follow the emptying of an overcharged bladder. The world slows down, the focus sharpens, the brain comes back online. Huge nebulous difficulties prove on close calm examination to be merely cloud giants.’ Thank you to Drs. Mattoo, Nelson, and Shaikh for providing this clarity of current UTI diagnosis and management,” Dr. Joos said.
“It bears repeating that because of the rare prevalence of grade 4 to 5 vesicoureteral reflux in children with their first UTI, current guidelines recommend that a voiding cystourethrogram can be reserved for children with an abnormal ultrasound, atypical pathogen, complicated clinical course, or known renal scarring,” added Dr. Joos.
The authors had no relevant disclosures. Dr. Joos is a member of the Pediatric News editorial advisory board but had no other disclosures.
Oral antibiotic treatment for 7-10 days works for most feverish children with uncomplicated urinary tract infection (UTI), reported Tej K. Mattoo, MD, of Wayne State University, Detroit, and associates.
A good clinical assessment supported by laboratory results using a clean urine specimen is crucial to accurately diagnosing UTI in children, Dr. Mattoo and colleagues reported in a state-of-the-art review article in Pediatrics.
The authors set out to summarize the current literature on UTI in children with the goal of guiding clinical management. They provide a thorough summary of the research on a wide range of issues, including pathogenesis of acute pyelonephritis and renal scarring, risk factors for UTI and renal scarring, diagnosis and common errors in diagnosis, complications of UTI and post-UTI renal imaging, antibiotics, antimicrobial prophylaxis, surgical interventions, and prevention of recurrent UTIs.
What key steps make all the difference?
To help guide practicing physicians through this wealth of information, Dr. Mattoo noted in an interview that, although the review article offers “many takeaway messages,” there are several issues of crucial importance. Notably, urine collection in young children who are not yet toilet trained can present considerable challenges in achieving an accurate assessment. A contaminated urine specimen leads to unnecessary antibiotic treatment, and in some cases unwarranted hospitalization, intravenous lines, renal imaging, and follow-up investigations, Dr. Mattoo said.
Ureteral catheterization or suprapubic bladder aspiration are the preferred methods of specimen collection, especially in cases where specimens collected with a perineal bag are dipstick positive, the authors explained. Midstream collection (known as the Quick-Wee method) can also be used following stimulation of the suprapubic area with cold fluid-soaked gauze.
Also of considerable importance is distinguishing bladder infections from kidney infections whenever possible, Dr. Mattoo noted. The antibiotic treatment, complications, and follow-up plans can be different for each, he cautioned. The authors have provided a helpful table within the article to help make this differentiation.
Timing is crucial
Prescribing treatment with an antibiotic within 48 hours of fever onset is essential for the prevention of renal scarring, Dr. Mattoo advised. The key is to treat with the goal of avoiding long-term complications. Although there are some exceptions, most cases of UTI can be treated with oral antibiotics and do not require hospitalization.
Some children with first UTI need additional testing, such as renal imaging, to ensure that there are no underlying risk factors for UTI. These children, in particular, can be at an increased risk of recurrent UTI and renal scarring, Dr. Mattoo explained.
Antibiotic resistance is a major emerging problem in patients with UTI at all ages and we should use antibiotics only in patients who truly have a UTI that requires such treatment, he urged.
In an interview, Timothy Joos, MD, a Seattle internist and pediatrician in private practice, noted: “In the words of the British novelist Tom Holt, ‘There are few moments of clarity more profound than those that follow the emptying of an overcharged bladder. The world slows down, the focus sharpens, the brain comes back online. Huge nebulous difficulties prove on close calm examination to be merely cloud giants.’ Thank you to Drs. Mattoo, Nelson, and Shaikh for providing this clarity of current UTI diagnosis and management,” Dr. Joos said.
“It bears repeating that because of the rare prevalence of grade 4 to 5 vesicoureteral reflux in children with their first UTI, current guidelines recommend that a voiding cystourethrogram can be reserved for children with an abnormal ultrasound, atypical pathogen, complicated clinical course, or known renal scarring,” added Dr. Joos.
The authors had no relevant disclosures. Dr. Joos is a member of the Pediatric News editorial advisory board but had no other disclosures.
FROM PEDIATRICS
Influenza Plus COVID-19 Equals Greater Concern
Coinfection with COVID-19 and influenza was reported early in the pandemic. Although both infections on their own can cause severe complications and death, coinfection can double the odds of death when compared with COVID infection alone. Moreover, those odds can be raised by chronic medical conditions and environmental or occupational factors, such as congregate living settings, say physicians who report on the first 2 confirmed cases of COVID-19 and influenza coinfection among US Department of Defense personnel within the US Central Command area of responsibility.
In the first case, a 56-year-old contractor presented to a Role I clinic with anorexia, fever, chills, and headache, which had begun 3 days before. His initial vital signs were “unremarkable,” and he did not have symptoms of respiratory distress. An antigen test was positive for influenza type A. A COVID-19 test also was positive. He was placed on isolation and treated with oseltamivir, amlodipine, hydrochlorothiazide, and losartan. His condition did not warrant hospitalization. Of 3 close contacts, 1 tested positive and was isolated. Two remained asymptomatic during the 14-day quarantine. Ten days after onset, the patient returned to duty.
The second patient, a 34-year-old officer in the Army, was initially identified as a close contact of a confirmed COVID-19 case and placed in quarantine. He was asymptomatic but tested positive and was placed in isolation with precautions. As with the first patient, his vital signs were unremarkable. He continued to be asymptomatic, although he reported myalgias 2 days later. Since those are a classic sign of seasonal influenzas, he was tested and proved positive for type B influenza. He, too, was started on oseltamivir. By the end of the first week, he experienced loss of taste and smell, cough, and shortness of breath, but his vital signs remained normal. His symptoms improved through supportive care. All 6 of his close contacts remained asymptomatic. Ten days after his symptoms began, he also returned to duty.
Influenza-associated deaths among the US military have been relatively few, the authors say, most likely because of the good preexisting health status of the US military, prompt detection with rapid influenza diagnostic tests, several effective antiviral therapeutics, and a “robust, compulsory vaccination program.” Nonetheless, neither patient had received the 2020-2021 influenza vaccine, which underscores the importance of this intervention, the authors say.
Because both infections present with a wide variety of clinical manifestations and overlapping symptoms, providers should stay alert to the possibility of coinfection, especially among personnel who are higher risk. For instance, as a linguist who interacted daily with host nation partners, the civilian contractor had a high occupational exposure.
While the authors only discuss 2 cases, a Medical Surveillance Monthly Report editorial comment says their report “nevertheless supports the importance of implementing force health protection (FHP) measures to prevent, detect, and respond to the spread of both of these health threats.” It’s particularly important, the comment notes, in the current context of a drawdown in forces in many deployed locations, as further losses of personnel to illness may degrade the execution of critical missions.
Coinfection with COVID-19 and influenza was reported early in the pandemic. Although both infections on their own can cause severe complications and death, coinfection can double the odds of death when compared with COVID infection alone. Moreover, those odds can be raised by chronic medical conditions and environmental or occupational factors, such as congregate living settings, say physicians who report on the first 2 confirmed cases of COVID-19 and influenza coinfection among US Department of Defense personnel within the US Central Command area of responsibility.
In the first case, a 56-year-old contractor presented to a Role I clinic with anorexia, fever, chills, and headache, which had begun 3 days before. His initial vital signs were “unremarkable,” and he did not have symptoms of respiratory distress. An antigen test was positive for influenza type A. A COVID-19 test also was positive. He was placed on isolation and treated with oseltamivir, amlodipine, hydrochlorothiazide, and losartan. His condition did not warrant hospitalization. Of 3 close contacts, 1 tested positive and was isolated. Two remained asymptomatic during the 14-day quarantine. Ten days after onset, the patient returned to duty.
The second patient, a 34-year-old officer in the Army, was initially identified as a close contact of a confirmed COVID-19 case and placed in quarantine. He was asymptomatic but tested positive and was placed in isolation with precautions. As with the first patient, his vital signs were unremarkable. He continued to be asymptomatic, although he reported myalgias 2 days later. Since those are a classic sign of seasonal influenzas, he was tested and proved positive for type B influenza. He, too, was started on oseltamivir. By the end of the first week, he experienced loss of taste and smell, cough, and shortness of breath, but his vital signs remained normal. His symptoms improved through supportive care. All 6 of his close contacts remained asymptomatic. Ten days after his symptoms began, he also returned to duty.
Influenza-associated deaths among the US military have been relatively few, the authors say, most likely because of the good preexisting health status of the US military, prompt detection with rapid influenza diagnostic tests, several effective antiviral therapeutics, and a “robust, compulsory vaccination program.” Nonetheless, neither patient had received the 2020-2021 influenza vaccine, which underscores the importance of this intervention, the authors say.
Because both infections present with a wide variety of clinical manifestations and overlapping symptoms, providers should stay alert to the possibility of coinfection, especially among personnel who are higher risk. For instance, as a linguist who interacted daily with host nation partners, the civilian contractor had a high occupational exposure.
While the authors only discuss 2 cases, a Medical Surveillance Monthly Report editorial comment says their report “nevertheless supports the importance of implementing force health protection (FHP) measures to prevent, detect, and respond to the spread of both of these health threats.” It’s particularly important, the comment notes, in the current context of a drawdown in forces in many deployed locations, as further losses of personnel to illness may degrade the execution of critical missions.
Coinfection with COVID-19 and influenza was reported early in the pandemic. Although both infections on their own can cause severe complications and death, coinfection can double the odds of death when compared with COVID infection alone. Moreover, those odds can be raised by chronic medical conditions and environmental or occupational factors, such as congregate living settings, say physicians who report on the first 2 confirmed cases of COVID-19 and influenza coinfection among US Department of Defense personnel within the US Central Command area of responsibility.
In the first case, a 56-year-old contractor presented to a Role I clinic with anorexia, fever, chills, and headache, which had begun 3 days before. His initial vital signs were “unremarkable,” and he did not have symptoms of respiratory distress. An antigen test was positive for influenza type A. A COVID-19 test also was positive. He was placed on isolation and treated with oseltamivir, amlodipine, hydrochlorothiazide, and losartan. His condition did not warrant hospitalization. Of 3 close contacts, 1 tested positive and was isolated. Two remained asymptomatic during the 14-day quarantine. Ten days after onset, the patient returned to duty.
The second patient, a 34-year-old officer in the Army, was initially identified as a close contact of a confirmed COVID-19 case and placed in quarantine. He was asymptomatic but tested positive and was placed in isolation with precautions. As with the first patient, his vital signs were unremarkable. He continued to be asymptomatic, although he reported myalgias 2 days later. Since those are a classic sign of seasonal influenzas, he was tested and proved positive for type B influenza. He, too, was started on oseltamivir. By the end of the first week, he experienced loss of taste and smell, cough, and shortness of breath, but his vital signs remained normal. His symptoms improved through supportive care. All 6 of his close contacts remained asymptomatic. Ten days after his symptoms began, he also returned to duty.
Influenza-associated deaths among the US military have been relatively few, the authors say, most likely because of the good preexisting health status of the US military, prompt detection with rapid influenza diagnostic tests, several effective antiviral therapeutics, and a “robust, compulsory vaccination program.” Nonetheless, neither patient had received the 2020-2021 influenza vaccine, which underscores the importance of this intervention, the authors say.
Because both infections present with a wide variety of clinical manifestations and overlapping symptoms, providers should stay alert to the possibility of coinfection, especially among personnel who are higher risk. For instance, as a linguist who interacted daily with host nation partners, the civilian contractor had a high occupational exposure.
While the authors only discuss 2 cases, a Medical Surveillance Monthly Report editorial comment says their report “nevertheless supports the importance of implementing force health protection (FHP) measures to prevent, detect, and respond to the spread of both of these health threats.” It’s particularly important, the comment notes, in the current context of a drawdown in forces in many deployed locations, as further losses of personnel to illness may degrade the execution of critical missions.
New updates for Choosing Wisely in hospitalized patients with infection
Background: A new update to the Choosing Wisely Campaign was released September 2019.
Study design: Expert consensus recommendations from the American Society for Clinical Pathology.
Synopsis: Eleven of the 30 Choosing Wisely recommendations directly affect hospital medicine. Half of these recommendations are related to infectious diseases. Highlights include:
- Not routinely using broad respiratory viral testing and instead using more targeted approaches to respiratory pathogen tests (e.g., respiratory syncytial virus, influenza A/B, or group A pharyngitis) unless the results will lead to changes to or discontinuations of antimicrobial therapy or isolation.
- Not routinely testing for community gastrointestinal pathogens in patients that develop diarrhea 3 days after hospitalization and to primarily test for Clostridiodes difficile in these patients, unless they are immunocompromised or older adults.
- Not checking procalcitonin unless a specific evidence-based guideline is used for antibiotic stewardship, as it is often used incorrectly without benefit to the patient.
- Not ordering serology for Helicobacter pylori and instead ordering the stool antigen or breath test to test for active infection given higher sensitivity and specificity.
- Not repeating antibody tests for patients with history of hepatitis C and instead ordering a viral load if there is concern for reinfection.
Bottom line: Only order infectious disease tests that will guide changes in clinical management.
Citation: ASCP Effective Test Utilization Steering Committee. Thirty things patients and physicians should question. 2019 Sep 9. Choosingwisely.org.
Dr. Blount is clinical instructor of medicine, hospital medicine, at the Rocky Mountain Veterans Affairs Regional Medical Center, Aurora, Colo.
Background: A new update to the Choosing Wisely Campaign was released September 2019.
Study design: Expert consensus recommendations from the American Society for Clinical Pathology.
Synopsis: Eleven of the 30 Choosing Wisely recommendations directly affect hospital medicine. Half of these recommendations are related to infectious diseases. Highlights include:
- Not routinely using broad respiratory viral testing and instead using more targeted approaches to respiratory pathogen tests (e.g., respiratory syncytial virus, influenza A/B, or group A pharyngitis) unless the results will lead to changes to or discontinuations of antimicrobial therapy or isolation.
- Not routinely testing for community gastrointestinal pathogens in patients that develop diarrhea 3 days after hospitalization and to primarily test for Clostridiodes difficile in these patients, unless they are immunocompromised or older adults.
- Not checking procalcitonin unless a specific evidence-based guideline is used for antibiotic stewardship, as it is often used incorrectly without benefit to the patient.
- Not ordering serology for Helicobacter pylori and instead ordering the stool antigen or breath test to test for active infection given higher sensitivity and specificity.
- Not repeating antibody tests for patients with history of hepatitis C and instead ordering a viral load if there is concern for reinfection.
Bottom line: Only order infectious disease tests that will guide changes in clinical management.
Citation: ASCP Effective Test Utilization Steering Committee. Thirty things patients and physicians should question. 2019 Sep 9. Choosingwisely.org.
Dr. Blount is clinical instructor of medicine, hospital medicine, at the Rocky Mountain Veterans Affairs Regional Medical Center, Aurora, Colo.
Background: A new update to the Choosing Wisely Campaign was released September 2019.
Study design: Expert consensus recommendations from the American Society for Clinical Pathology.
Synopsis: Eleven of the 30 Choosing Wisely recommendations directly affect hospital medicine. Half of these recommendations are related to infectious diseases. Highlights include:
- Not routinely using broad respiratory viral testing and instead using more targeted approaches to respiratory pathogen tests (e.g., respiratory syncytial virus, influenza A/B, or group A pharyngitis) unless the results will lead to changes to or discontinuations of antimicrobial therapy or isolation.
- Not routinely testing for community gastrointestinal pathogens in patients that develop diarrhea 3 days after hospitalization and to primarily test for Clostridiodes difficile in these patients, unless they are immunocompromised or older adults.
- Not checking procalcitonin unless a specific evidence-based guideline is used for antibiotic stewardship, as it is often used incorrectly without benefit to the patient.
- Not ordering serology for Helicobacter pylori and instead ordering the stool antigen or breath test to test for active infection given higher sensitivity and specificity.
- Not repeating antibody tests for patients with history of hepatitis C and instead ordering a viral load if there is concern for reinfection.
Bottom line: Only order infectious disease tests that will guide changes in clinical management.
Citation: ASCP Effective Test Utilization Steering Committee. Thirty things patients and physicians should question. 2019 Sep 9. Choosingwisely.org.
Dr. Blount is clinical instructor of medicine, hospital medicine, at the Rocky Mountain Veterans Affairs Regional Medical Center, Aurora, Colo.
Maternal COVID antibodies cross placenta, detected in newborns
Antibodies against SARS-CoV-2 cross the placenta during pregnancy and are detectable in most newborns born to mothers who had COVID-19 during pregnancy, according to findings from a study presented Jan. 28 at the meeting sponsored by the Society for Maternal-Fetal Medicine.
“I think the most striking finding is that we noticed a high degree of neutralizing response to natural infection even among asymptomatic infection, but of course a higher degree was seen in those with symptomatic infection,” Naima Joseph, MD, MPH, of Emory University, Atlanta, said in an interview.
“Our data demonstrate maternal capacity to mount an appropriate and robust immune response,” and maternal protective immunity lasted at least 28 days after infection, Dr. Joseph said. “Also, we noted higher neonatal cord blood titers in moms with higher titers, which suggests a relationship, but we need to better understand how transplacental transfer occurs as well as establish neonatal correlates of protection in order to see if and how maternal immunity may also benefit neonates.”
The researchers analyzed the amount of IgG and IgM antibodies in maternal and cord blood samples prospectively collected at delivery from women who tested positive for COVID-19 at any time while pregnant. They used enzyme-linked immunosorbent assay to assess for antibodies for the receptor binding domain of the SARS-CoV-2 spike protein.
The 32 pairs of mothers and infants in the study were predominantly non-Hispanic Black (72%) and Hispanic (25%), and 84% used Medicaid as their payer. Most of the mothers (72%) had at least one comorbidity, most commonly obesity, hypertension, and asthma or pulmonary disease. Just over half the women (53%) were symptomatic while they were infected, and 88% were ill with COVID-19 during the third trimester. The average time from infection to delivery was 28 days.
All the mothers had IgG antibodies, 94% had IgM antibodies, and 94% had neutralizing antibodies against SARS-CoV-2. Among the cord blood samples, 91% had IgG antibodies, 9% had IgM antibodies, and 25% had neutralizing antibodies.
“It’s reassuring that, so far, the physiological response is exactly what we expected it to be,” Judette Louis, MD, MPH, an associate professor of ob.gyn. and the ob.gyn. department chair at the University of South Florida, Tampa, said in an interview. “It’s what we would expect, but it’s always helpful to have more data to support that. Otherwise, you’re extrapolating from what you know from other conditions,” said Dr. Louis, who moderated the oral abstracts session.
Symptomatic infection was associated with significantly higher IgG titers than asymptomatic infection (P = .03), but no correlation was seen for IgM or neutralizing antibodies. In addition, although mothers who delivered more than 28 days after their infection had higher IgG titers (P = .05), no differences existed in IgM or neutralizing response.
Infants’ cord blood titers were significantly lower than their corresponding maternal samples, independently of symptoms or latency from infection to delivery (P < .001), Dr. Joseph reported.
“Transplacental efficiency in other pathogens has been shown to be correlated with neonatal immunity when the ratio of cord to maternal blood is greater than 1,” Dr. Joseph said in her presentation. Their data showed “suboptimal efficiency” at a ratio of 0.81.
The study’s small sample size and lack of a control group were weaknesses, but a major strength was having a population at disproportionately higher risk for infection and severe morbidity than the general population.
Implications for maternal COVID-19 vaccination
Although the data are not yet available, Dr. Joseph said they have expanded their protocol to include vaccinated pregnant women.
“The key to developing an effective vaccine [for pregnant people] is in really characterizing adaptive immunity in pregnancy,” Dr. Joseph told SMFM attendees. “I think that these findings inform further vaccine development in demonstrating that maternal immunity is robust.”
The World Health Organization recently recommended withholding COVID-19 vaccines from pregnant people, but the SMFM and American College of Obstetricians and Gynecologists subsequently issued a joint statement reaffirming that the COVID-19 vaccines authorized by the FDA “should not be withheld from pregnant individuals who choose to receive the vaccine.”
“One of the questions people ask is whether in pregnancy you’re going to mount a good response to the vaccine the way you would outside of pregnancy,” Dr. Louis said. “If we can demonstrate that you do, that may provide the information that some mothers need to make their decisions.” Data such as those from Dr. Joseph’s study can also inform recommendations on timing of maternal vaccination.
“For instance, Dr. Joseph demonstrated that, 28 days out from the infection, you had more antibodies, so there may be a scenario where we say this vaccine may be more beneficial in the middle of the pregnancy for the purpose of forming those antibodies,” Dr. Louis said.
Consensus emerging from maternal antibodies data
The findings from Dr. Joseph’s study mirror those reported in a study published online Jan. 29 in JAMA Pediatrics. That study, led by Dustin D. Flannery, DO, MSCE, of Children’s Hospital of Philadelphia, also examined maternal and neonatal levels of IgG and IgM antibodies against the receptor binding domain of the SARS-CoV-2 spike protein. They also found a positive correlation between cord blood and maternal IgG concentrations (P < .001), but notably, the ratio of cord to maternal blood titers was greater than 1, unlike in Dr. Joseph’s study.
For their study, Dr. Flannery and colleagues obtained maternal and cord blood sera at the time of delivery from 1471 pairs of mothers and infants, independently of COVID status during pregnancy. The average maternal age was 32 years, and just over a quarter of the population (26%) were Black, non-Hispanic women. About half (51%) were White, 12% were Hispanic, and 7% were Asian.
About 6% of the women had either IgG or IgM antibodies at delivery, and 87% of infants born to those mothers had measurable IgG in their cord blood. No infants had IgM antibodies. As with the study presented at SMFM, the mothers’ infections included asymptomatic, mild, moderate, and severe cases, and the degree of severity of cases had no apparent effect on infant antibody concentrations. Most of the women who tested positive for COVID-19 (60%) were asymptomatic.
Among the 11 mothers who had antibodies but whose infants’ cord blood did not, 5 had only IgM antibodies, and 6 had significantly lower IgG concentrations than those seen in the other mothers.
In a commentary about the JAMA Pediatrics study, Flor Munoz, MD, of the Baylor College of Medicine, Houston, suggested that the findings are grounds for optimism about a maternal vaccination strategy to protect infants from COVID-19.
“However, the timing of maternal vaccination to protect the infant, as opposed to the mother alone, would necessitate an adequate interval from vaccination to delivery (of at least 4 weeks), while vaccination early in gestation and even late in the third trimester could still be protective for the mother,” Dr. Munoz wrote.
Given the interval between two-dose vaccination regimens and the fact that transplacental transfer begins at about the 17th week of gestation, “maternal vaccination starting in the early second trimester of gestation might be optimal to achieve the highest levels of antibodies in the newborn,” Dr. Munoz wrote. But questions remain, such as how effective the neonatal antibodies would be in protecting against COVID-19 and how long they last after birth.
No external funding was used in Dr. Joseph’s study. Dr. Joseph and Dr. Louis have disclosed no relevant financial relationships. The JAMA Pediatrics study was funded by the Children’s Hospital of Philadelphia. One coauthor received consultancy fees from Sanofi Pasteur, Lumen, Novavax, and Merck unrelated to the study. Dr. Munoz served on the data and safety monitoring boards of Moderna, Pfizer, Virometix, and Meissa Vaccines and has received grants from Novavax Research and Gilead Research.
A version of this article first appeared on Medscape.com.
Antibodies against SARS-CoV-2 cross the placenta during pregnancy and are detectable in most newborns born to mothers who had COVID-19 during pregnancy, according to findings from a study presented Jan. 28 at the meeting sponsored by the Society for Maternal-Fetal Medicine.
“I think the most striking finding is that we noticed a high degree of neutralizing response to natural infection even among asymptomatic infection, but of course a higher degree was seen in those with symptomatic infection,” Naima Joseph, MD, MPH, of Emory University, Atlanta, said in an interview.
“Our data demonstrate maternal capacity to mount an appropriate and robust immune response,” and maternal protective immunity lasted at least 28 days after infection, Dr. Joseph said. “Also, we noted higher neonatal cord blood titers in moms with higher titers, which suggests a relationship, but we need to better understand how transplacental transfer occurs as well as establish neonatal correlates of protection in order to see if and how maternal immunity may also benefit neonates.”
The researchers analyzed the amount of IgG and IgM antibodies in maternal and cord blood samples prospectively collected at delivery from women who tested positive for COVID-19 at any time while pregnant. They used enzyme-linked immunosorbent assay to assess for antibodies for the receptor binding domain of the SARS-CoV-2 spike protein.
The 32 pairs of mothers and infants in the study were predominantly non-Hispanic Black (72%) and Hispanic (25%), and 84% used Medicaid as their payer. Most of the mothers (72%) had at least one comorbidity, most commonly obesity, hypertension, and asthma or pulmonary disease. Just over half the women (53%) were symptomatic while they were infected, and 88% were ill with COVID-19 during the third trimester. The average time from infection to delivery was 28 days.
All the mothers had IgG antibodies, 94% had IgM antibodies, and 94% had neutralizing antibodies against SARS-CoV-2. Among the cord blood samples, 91% had IgG antibodies, 9% had IgM antibodies, and 25% had neutralizing antibodies.
“It’s reassuring that, so far, the physiological response is exactly what we expected it to be,” Judette Louis, MD, MPH, an associate professor of ob.gyn. and the ob.gyn. department chair at the University of South Florida, Tampa, said in an interview. “It’s what we would expect, but it’s always helpful to have more data to support that. Otherwise, you’re extrapolating from what you know from other conditions,” said Dr. Louis, who moderated the oral abstracts session.
Symptomatic infection was associated with significantly higher IgG titers than asymptomatic infection (P = .03), but no correlation was seen for IgM or neutralizing antibodies. In addition, although mothers who delivered more than 28 days after their infection had higher IgG titers (P = .05), no differences existed in IgM or neutralizing response.
Infants’ cord blood titers were significantly lower than their corresponding maternal samples, independently of symptoms or latency from infection to delivery (P < .001), Dr. Joseph reported.
“Transplacental efficiency in other pathogens has been shown to be correlated with neonatal immunity when the ratio of cord to maternal blood is greater than 1,” Dr. Joseph said in her presentation. Their data showed “suboptimal efficiency” at a ratio of 0.81.
The study’s small sample size and lack of a control group were weaknesses, but a major strength was having a population at disproportionately higher risk for infection and severe morbidity than the general population.
Implications for maternal COVID-19 vaccination
Although the data are not yet available, Dr. Joseph said they have expanded their protocol to include vaccinated pregnant women.
“The key to developing an effective vaccine [for pregnant people] is in really characterizing adaptive immunity in pregnancy,” Dr. Joseph told SMFM attendees. “I think that these findings inform further vaccine development in demonstrating that maternal immunity is robust.”
The World Health Organization recently recommended withholding COVID-19 vaccines from pregnant people, but the SMFM and American College of Obstetricians and Gynecologists subsequently issued a joint statement reaffirming that the COVID-19 vaccines authorized by the FDA “should not be withheld from pregnant individuals who choose to receive the vaccine.”
“One of the questions people ask is whether in pregnancy you’re going to mount a good response to the vaccine the way you would outside of pregnancy,” Dr. Louis said. “If we can demonstrate that you do, that may provide the information that some mothers need to make their decisions.” Data such as those from Dr. Joseph’s study can also inform recommendations on timing of maternal vaccination.
“For instance, Dr. Joseph demonstrated that, 28 days out from the infection, you had more antibodies, so there may be a scenario where we say this vaccine may be more beneficial in the middle of the pregnancy for the purpose of forming those antibodies,” Dr. Louis said.
Consensus emerging from maternal antibodies data
The findings from Dr. Joseph’s study mirror those reported in a study published online Jan. 29 in JAMA Pediatrics. That study, led by Dustin D. Flannery, DO, MSCE, of Children’s Hospital of Philadelphia, also examined maternal and neonatal levels of IgG and IgM antibodies against the receptor binding domain of the SARS-CoV-2 spike protein. They also found a positive correlation between cord blood and maternal IgG concentrations (P < .001), but notably, the ratio of cord to maternal blood titers was greater than 1, unlike in Dr. Joseph’s study.
For their study, Dr. Flannery and colleagues obtained maternal and cord blood sera at the time of delivery from 1471 pairs of mothers and infants, independently of COVID status during pregnancy. The average maternal age was 32 years, and just over a quarter of the population (26%) were Black, non-Hispanic women. About half (51%) were White, 12% were Hispanic, and 7% were Asian.
About 6% of the women had either IgG or IgM antibodies at delivery, and 87% of infants born to those mothers had measurable IgG in their cord blood. No infants had IgM antibodies. As with the study presented at SMFM, the mothers’ infections included asymptomatic, mild, moderate, and severe cases, and the degree of severity of cases had no apparent effect on infant antibody concentrations. Most of the women who tested positive for COVID-19 (60%) were asymptomatic.
Among the 11 mothers who had antibodies but whose infants’ cord blood did not, 5 had only IgM antibodies, and 6 had significantly lower IgG concentrations than those seen in the other mothers.
In a commentary about the JAMA Pediatrics study, Flor Munoz, MD, of the Baylor College of Medicine, Houston, suggested that the findings are grounds for optimism about a maternal vaccination strategy to protect infants from COVID-19.
“However, the timing of maternal vaccination to protect the infant, as opposed to the mother alone, would necessitate an adequate interval from vaccination to delivery (of at least 4 weeks), while vaccination early in gestation and even late in the third trimester could still be protective for the mother,” Dr. Munoz wrote.
Given the interval between two-dose vaccination regimens and the fact that transplacental transfer begins at about the 17th week of gestation, “maternal vaccination starting in the early second trimester of gestation might be optimal to achieve the highest levels of antibodies in the newborn,” Dr. Munoz wrote. But questions remain, such as how effective the neonatal antibodies would be in protecting against COVID-19 and how long they last after birth.
No external funding was used in Dr. Joseph’s study. Dr. Joseph and Dr. Louis have disclosed no relevant financial relationships. The JAMA Pediatrics study was funded by the Children’s Hospital of Philadelphia. One coauthor received consultancy fees from Sanofi Pasteur, Lumen, Novavax, and Merck unrelated to the study. Dr. Munoz served on the data and safety monitoring boards of Moderna, Pfizer, Virometix, and Meissa Vaccines and has received grants from Novavax Research and Gilead Research.
A version of this article first appeared on Medscape.com.
Antibodies against SARS-CoV-2 cross the placenta during pregnancy and are detectable in most newborns born to mothers who had COVID-19 during pregnancy, according to findings from a study presented Jan. 28 at the meeting sponsored by the Society for Maternal-Fetal Medicine.
“I think the most striking finding is that we noticed a high degree of neutralizing response to natural infection even among asymptomatic infection, but of course a higher degree was seen in those with symptomatic infection,” Naima Joseph, MD, MPH, of Emory University, Atlanta, said in an interview.
“Our data demonstrate maternal capacity to mount an appropriate and robust immune response,” and maternal protective immunity lasted at least 28 days after infection, Dr. Joseph said. “Also, we noted higher neonatal cord blood titers in moms with higher titers, which suggests a relationship, but we need to better understand how transplacental transfer occurs as well as establish neonatal correlates of protection in order to see if and how maternal immunity may also benefit neonates.”
The researchers analyzed the amount of IgG and IgM antibodies in maternal and cord blood samples prospectively collected at delivery from women who tested positive for COVID-19 at any time while pregnant. They used enzyme-linked immunosorbent assay to assess for antibodies for the receptor binding domain of the SARS-CoV-2 spike protein.
The 32 pairs of mothers and infants in the study were predominantly non-Hispanic Black (72%) and Hispanic (25%), and 84% used Medicaid as their payer. Most of the mothers (72%) had at least one comorbidity, most commonly obesity, hypertension, and asthma or pulmonary disease. Just over half the women (53%) were symptomatic while they were infected, and 88% were ill with COVID-19 during the third trimester. The average time from infection to delivery was 28 days.
All the mothers had IgG antibodies, 94% had IgM antibodies, and 94% had neutralizing antibodies against SARS-CoV-2. Among the cord blood samples, 91% had IgG antibodies, 9% had IgM antibodies, and 25% had neutralizing antibodies.
“It’s reassuring that, so far, the physiological response is exactly what we expected it to be,” Judette Louis, MD, MPH, an associate professor of ob.gyn. and the ob.gyn. department chair at the University of South Florida, Tampa, said in an interview. “It’s what we would expect, but it’s always helpful to have more data to support that. Otherwise, you’re extrapolating from what you know from other conditions,” said Dr. Louis, who moderated the oral abstracts session.
Symptomatic infection was associated with significantly higher IgG titers than asymptomatic infection (P = .03), but no correlation was seen for IgM or neutralizing antibodies. In addition, although mothers who delivered more than 28 days after their infection had higher IgG titers (P = .05), no differences existed in IgM or neutralizing response.
Infants’ cord blood titers were significantly lower than their corresponding maternal samples, independently of symptoms or latency from infection to delivery (P < .001), Dr. Joseph reported.
“Transplacental efficiency in other pathogens has been shown to be correlated with neonatal immunity when the ratio of cord to maternal blood is greater than 1,” Dr. Joseph said in her presentation. Their data showed “suboptimal efficiency” at a ratio of 0.81.
The study’s small sample size and lack of a control group were weaknesses, but a major strength was having a population at disproportionately higher risk for infection and severe morbidity than the general population.
Implications for maternal COVID-19 vaccination
Although the data are not yet available, Dr. Joseph said they have expanded their protocol to include vaccinated pregnant women.
“The key to developing an effective vaccine [for pregnant people] is in really characterizing adaptive immunity in pregnancy,” Dr. Joseph told SMFM attendees. “I think that these findings inform further vaccine development in demonstrating that maternal immunity is robust.”
The World Health Organization recently recommended withholding COVID-19 vaccines from pregnant people, but the SMFM and American College of Obstetricians and Gynecologists subsequently issued a joint statement reaffirming that the COVID-19 vaccines authorized by the FDA “should not be withheld from pregnant individuals who choose to receive the vaccine.”
“One of the questions people ask is whether in pregnancy you’re going to mount a good response to the vaccine the way you would outside of pregnancy,” Dr. Louis said. “If we can demonstrate that you do, that may provide the information that some mothers need to make their decisions.” Data such as those from Dr. Joseph’s study can also inform recommendations on timing of maternal vaccination.
“For instance, Dr. Joseph demonstrated that, 28 days out from the infection, you had more antibodies, so there may be a scenario where we say this vaccine may be more beneficial in the middle of the pregnancy for the purpose of forming those antibodies,” Dr. Louis said.
Consensus emerging from maternal antibodies data
The findings from Dr. Joseph’s study mirror those reported in a study published online Jan. 29 in JAMA Pediatrics. That study, led by Dustin D. Flannery, DO, MSCE, of Children’s Hospital of Philadelphia, also examined maternal and neonatal levels of IgG and IgM antibodies against the receptor binding domain of the SARS-CoV-2 spike protein. They also found a positive correlation between cord blood and maternal IgG concentrations (P < .001), but notably, the ratio of cord to maternal blood titers was greater than 1, unlike in Dr. Joseph’s study.
For their study, Dr. Flannery and colleagues obtained maternal and cord blood sera at the time of delivery from 1471 pairs of mothers and infants, independently of COVID status during pregnancy. The average maternal age was 32 years, and just over a quarter of the population (26%) were Black, non-Hispanic women. About half (51%) were White, 12% were Hispanic, and 7% were Asian.
About 6% of the women had either IgG or IgM antibodies at delivery, and 87% of infants born to those mothers had measurable IgG in their cord blood. No infants had IgM antibodies. As with the study presented at SMFM, the mothers’ infections included asymptomatic, mild, moderate, and severe cases, and the degree of severity of cases had no apparent effect on infant antibody concentrations. Most of the women who tested positive for COVID-19 (60%) were asymptomatic.
Among the 11 mothers who had antibodies but whose infants’ cord blood did not, 5 had only IgM antibodies, and 6 had significantly lower IgG concentrations than those seen in the other mothers.
In a commentary about the JAMA Pediatrics study, Flor Munoz, MD, of the Baylor College of Medicine, Houston, suggested that the findings are grounds for optimism about a maternal vaccination strategy to protect infants from COVID-19.
“However, the timing of maternal vaccination to protect the infant, as opposed to the mother alone, would necessitate an adequate interval from vaccination to delivery (of at least 4 weeks), while vaccination early in gestation and even late in the third trimester could still be protective for the mother,” Dr. Munoz wrote.
Given the interval between two-dose vaccination regimens and the fact that transplacental transfer begins at about the 17th week of gestation, “maternal vaccination starting in the early second trimester of gestation might be optimal to achieve the highest levels of antibodies in the newborn,” Dr. Munoz wrote. But questions remain, such as how effective the neonatal antibodies would be in protecting against COVID-19 and how long they last after birth.
No external funding was used in Dr. Joseph’s study. Dr. Joseph and Dr. Louis have disclosed no relevant financial relationships. The JAMA Pediatrics study was funded by the Children’s Hospital of Philadelphia. One coauthor received consultancy fees from Sanofi Pasteur, Lumen, Novavax, and Merck unrelated to the study. Dr. Munoz served on the data and safety monitoring boards of Moderna, Pfizer, Virometix, and Meissa Vaccines and has received grants from Novavax Research and Gilead Research.
A version of this article first appeared on Medscape.com.
Ceftolozane-tazobactam found effective in critically ill patients with Pseudomonas aeruginosa infections
, according to the results of a retrospective, observational study conducted in critically ill patients.
The multicenter, observational study assessed 95 patients who received C/T for P. aeruginosa serious infections, according to a report published online in the International Journal of Antimicrobial Agents.
C/T is a novel beta-lactam/ beta-lactamase inhibitor combination active against gram-negative bacteria including P. aeruginosa, “This paper presents the largest real-life experience published on C/T therapy for treating serious P. aeruginosa infections according to researchers Barbara Balandin, MD, of the Hospital Universitario Puerta de Hierro, Majadahonda, Spain, and colleagues.
The main infections treated were nosocomial pneumonia (56.2%), intra-abdominal infection (10.5%), tracheobronchitis (8.4%), and urinary tract infection (6.3%). Most infections were complicated with sepsis (49.5%) or septic shock (45.3%), and bacteremia (10.5%).
A total of 46 episodes were treated with high-dose C/T (3 g every 8 hours), and 38 episodes were treated with standard dosage (1.5 g every 8 hours). Almost half (44.2%) of the patients were treated with C/T monotherapy, and the remaining group received combination therapy with other antibiotics, according to the researchers.
The primary outcome of the study was to assess the efficacy and toxicity of C/T therapy. The secondary outcome was to evaluate the risk factors for all-cause 30-day mortality from the first day of therapy.
Favorable results
Most of the infections (93.7%) were severe and included the presence of sepsis (49.5%) or septic shock (45.3%). Bacteremia was observed in 15 (15.7%) patients. Bacteremia was secondary to nosocomial pneumonia in eight cases, catheter infection in five, urinary tract infection in one, and soft tissue infection in one. According to their susceptibility profiles, 46 (48.4%) of the strains were classified as extensively drug-resistant (XDR) P. aeruginosa and 35 (36.5%) were multidrug-resistant (MDR) P. aeruginosa.
Sixty-eight (71.6%) patients presented a favorable clinical response, which was defined as a resolution of presenting symptoms and signs of the infection by the end of therapy. An unfavorable clinical response was considered as persistence or worsening of the presenting symptoms and signs or death occurring during treatment with no other cause identified. Death associated with infection was defined as persistence of signs and symptoms of P. aeruginosa infection during C/T therapy with no other cause identified.
Microbiological eradication was documented in 42.1% (40/95) of the episodes. However, the global ICU mortality was still high, at 36.5%, with mortality mainly related to the severity of the infection.
Mortality was found to be significantly correlated with the Charlson Comorbidity Index (5.7 vs. 4.3; P = .04) and the need for life-supporting therapies such as vasopressors (66.6% vs. 46.9%; P = .03) and renal replacement therapy (46.6% vs. 18.1%; P = .002). In addition, mortality was significantly associated with a higher sequential organ failure assessment (SOFA) score during C/T therapy (SOFA1, SOFA 3, and SOFA 7; P < .001).
No significant differences in outcomes were correlated with demographic features, type and severity of infection, and dose of C/T. Also, there were no differences seen in outcomes between patients treated with C/T monotherapy and combined therapy (30.9% vs. 30.1%; P = .55).
“The lack of a positive effect from combined therapy suggests that C/T monotherapy may be sufficient for treating P. aeruginosa isolates that are susceptible to that agent,” the researchers suggested. “This study shows that C/T appears to be a suitable, effective, and safe drug for treating severe infections due to P. aeruginosa, highlighting nosocomial pneumonia caused by MDR/XDR P. aeruginosa in ICU patients with multiple comorbidities, such as immunosuppression, and needing life-sustaining therapies,” they concluded.
The authors reported that they had no outside funding source and had no conflicts of interest.
, according to the results of a retrospective, observational study conducted in critically ill patients.
The multicenter, observational study assessed 95 patients who received C/T for P. aeruginosa serious infections, according to a report published online in the International Journal of Antimicrobial Agents.
C/T is a novel beta-lactam/ beta-lactamase inhibitor combination active against gram-negative bacteria including P. aeruginosa, “This paper presents the largest real-life experience published on C/T therapy for treating serious P. aeruginosa infections according to researchers Barbara Balandin, MD, of the Hospital Universitario Puerta de Hierro, Majadahonda, Spain, and colleagues.
The main infections treated were nosocomial pneumonia (56.2%), intra-abdominal infection (10.5%), tracheobronchitis (8.4%), and urinary tract infection (6.3%). Most infections were complicated with sepsis (49.5%) or septic shock (45.3%), and bacteremia (10.5%).
A total of 46 episodes were treated with high-dose C/T (3 g every 8 hours), and 38 episodes were treated with standard dosage (1.5 g every 8 hours). Almost half (44.2%) of the patients were treated with C/T monotherapy, and the remaining group received combination therapy with other antibiotics, according to the researchers.
The primary outcome of the study was to assess the efficacy and toxicity of C/T therapy. The secondary outcome was to evaluate the risk factors for all-cause 30-day mortality from the first day of therapy.
Favorable results
Most of the infections (93.7%) were severe and included the presence of sepsis (49.5%) or septic shock (45.3%). Bacteremia was observed in 15 (15.7%) patients. Bacteremia was secondary to nosocomial pneumonia in eight cases, catheter infection in five, urinary tract infection in one, and soft tissue infection in one. According to their susceptibility profiles, 46 (48.4%) of the strains were classified as extensively drug-resistant (XDR) P. aeruginosa and 35 (36.5%) were multidrug-resistant (MDR) P. aeruginosa.
Sixty-eight (71.6%) patients presented a favorable clinical response, which was defined as a resolution of presenting symptoms and signs of the infection by the end of therapy. An unfavorable clinical response was considered as persistence or worsening of the presenting symptoms and signs or death occurring during treatment with no other cause identified. Death associated with infection was defined as persistence of signs and symptoms of P. aeruginosa infection during C/T therapy with no other cause identified.
Microbiological eradication was documented in 42.1% (40/95) of the episodes. However, the global ICU mortality was still high, at 36.5%, with mortality mainly related to the severity of the infection.
Mortality was found to be significantly correlated with the Charlson Comorbidity Index (5.7 vs. 4.3; P = .04) and the need for life-supporting therapies such as vasopressors (66.6% vs. 46.9%; P = .03) and renal replacement therapy (46.6% vs. 18.1%; P = .002). In addition, mortality was significantly associated with a higher sequential organ failure assessment (SOFA) score during C/T therapy (SOFA1, SOFA 3, and SOFA 7; P < .001).
No significant differences in outcomes were correlated with demographic features, type and severity of infection, and dose of C/T. Also, there were no differences seen in outcomes between patients treated with C/T monotherapy and combined therapy (30.9% vs. 30.1%; P = .55).
“The lack of a positive effect from combined therapy suggests that C/T monotherapy may be sufficient for treating P. aeruginosa isolates that are susceptible to that agent,” the researchers suggested. “This study shows that C/T appears to be a suitable, effective, and safe drug for treating severe infections due to P. aeruginosa, highlighting nosocomial pneumonia caused by MDR/XDR P. aeruginosa in ICU patients with multiple comorbidities, such as immunosuppression, and needing life-sustaining therapies,” they concluded.
The authors reported that they had no outside funding source and had no conflicts of interest.
, according to the results of a retrospective, observational study conducted in critically ill patients.
The multicenter, observational study assessed 95 patients who received C/T for P. aeruginosa serious infections, according to a report published online in the International Journal of Antimicrobial Agents.
C/T is a novel beta-lactam/ beta-lactamase inhibitor combination active against gram-negative bacteria including P. aeruginosa, “This paper presents the largest real-life experience published on C/T therapy for treating serious P. aeruginosa infections according to researchers Barbara Balandin, MD, of the Hospital Universitario Puerta de Hierro, Majadahonda, Spain, and colleagues.
The main infections treated were nosocomial pneumonia (56.2%), intra-abdominal infection (10.5%), tracheobronchitis (8.4%), and urinary tract infection (6.3%). Most infections were complicated with sepsis (49.5%) or septic shock (45.3%), and bacteremia (10.5%).
A total of 46 episodes were treated with high-dose C/T (3 g every 8 hours), and 38 episodes were treated with standard dosage (1.5 g every 8 hours). Almost half (44.2%) of the patients were treated with C/T monotherapy, and the remaining group received combination therapy with other antibiotics, according to the researchers.
The primary outcome of the study was to assess the efficacy and toxicity of C/T therapy. The secondary outcome was to evaluate the risk factors for all-cause 30-day mortality from the first day of therapy.
Favorable results
Most of the infections (93.7%) were severe and included the presence of sepsis (49.5%) or septic shock (45.3%). Bacteremia was observed in 15 (15.7%) patients. Bacteremia was secondary to nosocomial pneumonia in eight cases, catheter infection in five, urinary tract infection in one, and soft tissue infection in one. According to their susceptibility profiles, 46 (48.4%) of the strains were classified as extensively drug-resistant (XDR) P. aeruginosa and 35 (36.5%) were multidrug-resistant (MDR) P. aeruginosa.
Sixty-eight (71.6%) patients presented a favorable clinical response, which was defined as a resolution of presenting symptoms and signs of the infection by the end of therapy. An unfavorable clinical response was considered as persistence or worsening of the presenting symptoms and signs or death occurring during treatment with no other cause identified. Death associated with infection was defined as persistence of signs and symptoms of P. aeruginosa infection during C/T therapy with no other cause identified.
Microbiological eradication was documented in 42.1% (40/95) of the episodes. However, the global ICU mortality was still high, at 36.5%, with mortality mainly related to the severity of the infection.
Mortality was found to be significantly correlated with the Charlson Comorbidity Index (5.7 vs. 4.3; P = .04) and the need for life-supporting therapies such as vasopressors (66.6% vs. 46.9%; P = .03) and renal replacement therapy (46.6% vs. 18.1%; P = .002). In addition, mortality was significantly associated with a higher sequential organ failure assessment (SOFA) score during C/T therapy (SOFA1, SOFA 3, and SOFA 7; P < .001).
No significant differences in outcomes were correlated with demographic features, type and severity of infection, and dose of C/T. Also, there were no differences seen in outcomes between patients treated with C/T monotherapy and combined therapy (30.9% vs. 30.1%; P = .55).
“The lack of a positive effect from combined therapy suggests that C/T monotherapy may be sufficient for treating P. aeruginosa isolates that are susceptible to that agent,” the researchers suggested. “This study shows that C/T appears to be a suitable, effective, and safe drug for treating severe infections due to P. aeruginosa, highlighting nosocomial pneumonia caused by MDR/XDR P. aeruginosa in ICU patients with multiple comorbidities, such as immunosuppression, and needing life-sustaining therapies,” they concluded.
The authors reported that they had no outside funding source and had no conflicts of interest.
FROM THE INTERNATIONAL JOURNAL OF ANTIMICROBIAL AGENTS
Incidence of autoimmune hepatitis may be rising
The incidence of autoimmune hepatitis (AIH) may be rising, according to a prospective population-based study conducted in New Zealand.
From 2008 to 2016, the rising incidence of AIH led to a 40% increase in point prevalence, reported lead author Mehul Lamba, MD, of Christchurch (New Zealand) Hospital and colleagues.
The present study, which also assessed rates of primary biliary cholangitis (PBC) and primary sclerosing cholangitis (PSC), adds data to an area of inquiry historically characterized by limited and inconsistent results, the investigators wrote in Clinical Gastroenterology and Hepatology. They suggested that mixed findings from previous studies may be because of differences in population and environmental factors, but also varying diagnostic criteria.
“The epidemiological trends of these autoimmune liver diseases therefore remain incompletely understood,” wrote Dr. Lamba and colleagues.
Their study evaluated trends in autoimmune liver diseases over a 9-year time frame in Canterbury, New Zealand. According to the investigators, this region is well suited to an epidemiological investigation because it is a clearly defined geographic area with approximately 600,000 people, most of whom rely on one tertiary care center: Christchurch Hospital. The bulk of the data therefore came from this center, while a minority of cases were gathered from local private gastroenterology practices, “making complete case ascertainment possible.”
Incidence of AIH, PBC, and PSC was assessed at three time points: 2008-2010, 2011-2013, and 2014-2016. AIH had the highest overall incidence, at 1.93 cases per 100,000 people, followed by PSC (0.92) and PBC (0.51).
While the rates of PBC and PSC did not change significantly over time, the incidence of AIH rose from 1.37 cases per 100,000 people in the period from 2008-2010 to 2.39 per 100,000 in 2014-2016 (P = .04), which computes to an incidence rate ratio of 1.69 (95% confidence interval, 1.02-2.84). Point prevalence was also significantly higher in 2016, compared with 2008, at 27.5 per 100,000 versus 19.7 per 100,000 (P < .01). The investigators described a bimodal age of presentation, with the first peak among patients younger than 20 years, and a second, larger peak among individuals aged 50-69 years.
According to the investigators, these findings “are concordant with the results observed in the European cohort,” citing a Danish study spanning 1994-2012 and a Dutch study spanning 2000-2010. They noted that the Danish study also reported a bimodal distribution of age incidence, as did a Swedish study, and another study from New Zealand. The stable levels of PBC and PSC align with two recent retrospective studies conducted in the United States and, they added.
“We believe that the observed differential trends in the incidence of these autoimmune liver diseases truly reflects their contemporary epidemiology,” the investigators wrote. They went on to suggest that the findings did not stem from an increase in diagnostic scrutiny because the study period did not include any significant changes in gastroenterology service, coding, or diagnostic criteria in the region studied.
“The increased incidence of AIH parallels rising incidence and prevalence of other autoimmune disorders such as [inflammatory bowel disease], type 1 diabetes, and multiple sclerosis in New Zealand, and it is unclear whether these autoimmune conditions share a common local environmental trigger,” they wrote. “Environmental factors likely play a central role augmenting phenotypic expression in genetically predisposed individuals.”
While Dr. Lamba and colleagues proposed several possible factors, such as increased exposure to pharmaceuticals, definitive factors remain elusive, which the authors cited as one limitation of their study. Another limitation they cited is the possibility that other etiologies were mistakenly classified as “probable” AIH; however, the chances of that are small, and the proportion of probable versus definitive AIH noted in this study do reflect those seen in other epidemiological studies.
“The reason for observed differential change in incidence of these autoimmune liver diseases is unclear,” they wrote, “and future collaborative prospective epidemiological study would be required to assess this further.”
The investigators reported no conflicts of interest.
The incidence of autoimmune hepatitis (AIH) may be rising, according to a prospective population-based study conducted in New Zealand.
From 2008 to 2016, the rising incidence of AIH led to a 40% increase in point prevalence, reported lead author Mehul Lamba, MD, of Christchurch (New Zealand) Hospital and colleagues.
The present study, which also assessed rates of primary biliary cholangitis (PBC) and primary sclerosing cholangitis (PSC), adds data to an area of inquiry historically characterized by limited and inconsistent results, the investigators wrote in Clinical Gastroenterology and Hepatology. They suggested that mixed findings from previous studies may be because of differences in population and environmental factors, but also varying diagnostic criteria.
“The epidemiological trends of these autoimmune liver diseases therefore remain incompletely understood,” wrote Dr. Lamba and colleagues.
Their study evaluated trends in autoimmune liver diseases over a 9-year time frame in Canterbury, New Zealand. According to the investigators, this region is well suited to an epidemiological investigation because it is a clearly defined geographic area with approximately 600,000 people, most of whom rely on one tertiary care center: Christchurch Hospital. The bulk of the data therefore came from this center, while a minority of cases were gathered from local private gastroenterology practices, “making complete case ascertainment possible.”
Incidence of AIH, PBC, and PSC was assessed at three time points: 2008-2010, 2011-2013, and 2014-2016. AIH had the highest overall incidence, at 1.93 cases per 100,000 people, followed by PSC (0.92) and PBC (0.51).
While the rates of PBC and PSC did not change significantly over time, the incidence of AIH rose from 1.37 cases per 100,000 people in the period from 2008-2010 to 2.39 per 100,000 in 2014-2016 (P = .04), which computes to an incidence rate ratio of 1.69 (95% confidence interval, 1.02-2.84). Point prevalence was also significantly higher in 2016, compared with 2008, at 27.5 per 100,000 versus 19.7 per 100,000 (P < .01). The investigators described a bimodal age of presentation, with the first peak among patients younger than 20 years, and a second, larger peak among individuals aged 50-69 years.
According to the investigators, these findings “are concordant with the results observed in the European cohort,” citing a Danish study spanning 1994-2012 and a Dutch study spanning 2000-2010. They noted that the Danish study also reported a bimodal distribution of age incidence, as did a Swedish study, and another study from New Zealand. The stable levels of PBC and PSC align with two recent retrospective studies conducted in the United States and, they added.
“We believe that the observed differential trends in the incidence of these autoimmune liver diseases truly reflects their contemporary epidemiology,” the investigators wrote. They went on to suggest that the findings did not stem from an increase in diagnostic scrutiny because the study period did not include any significant changes in gastroenterology service, coding, or diagnostic criteria in the region studied.
“The increased incidence of AIH parallels rising incidence and prevalence of other autoimmune disorders such as [inflammatory bowel disease], type 1 diabetes, and multiple sclerosis in New Zealand, and it is unclear whether these autoimmune conditions share a common local environmental trigger,” they wrote. “Environmental factors likely play a central role augmenting phenotypic expression in genetically predisposed individuals.”
While Dr. Lamba and colleagues proposed several possible factors, such as increased exposure to pharmaceuticals, definitive factors remain elusive, which the authors cited as one limitation of their study. Another limitation they cited is the possibility that other etiologies were mistakenly classified as “probable” AIH; however, the chances of that are small, and the proportion of probable versus definitive AIH noted in this study do reflect those seen in other epidemiological studies.
“The reason for observed differential change in incidence of these autoimmune liver diseases is unclear,” they wrote, “and future collaborative prospective epidemiological study would be required to assess this further.”
The investigators reported no conflicts of interest.
The incidence of autoimmune hepatitis (AIH) may be rising, according to a prospective population-based study conducted in New Zealand.
From 2008 to 2016, the rising incidence of AIH led to a 40% increase in point prevalence, reported lead author Mehul Lamba, MD, of Christchurch (New Zealand) Hospital and colleagues.
The present study, which also assessed rates of primary biliary cholangitis (PBC) and primary sclerosing cholangitis (PSC), adds data to an area of inquiry historically characterized by limited and inconsistent results, the investigators wrote in Clinical Gastroenterology and Hepatology. They suggested that mixed findings from previous studies may be because of differences in population and environmental factors, but also varying diagnostic criteria.
“The epidemiological trends of these autoimmune liver diseases therefore remain incompletely understood,” wrote Dr. Lamba and colleagues.
Their study evaluated trends in autoimmune liver diseases over a 9-year time frame in Canterbury, New Zealand. According to the investigators, this region is well suited to an epidemiological investigation because it is a clearly defined geographic area with approximately 600,000 people, most of whom rely on one tertiary care center: Christchurch Hospital. The bulk of the data therefore came from this center, while a minority of cases were gathered from local private gastroenterology practices, “making complete case ascertainment possible.”
Incidence of AIH, PBC, and PSC was assessed at three time points: 2008-2010, 2011-2013, and 2014-2016. AIH had the highest overall incidence, at 1.93 cases per 100,000 people, followed by PSC (0.92) and PBC (0.51).
While the rates of PBC and PSC did not change significantly over time, the incidence of AIH rose from 1.37 cases per 100,000 people in the period from 2008-2010 to 2.39 per 100,000 in 2014-2016 (P = .04), which computes to an incidence rate ratio of 1.69 (95% confidence interval, 1.02-2.84). Point prevalence was also significantly higher in 2016, compared with 2008, at 27.5 per 100,000 versus 19.7 per 100,000 (P < .01). The investigators described a bimodal age of presentation, with the first peak among patients younger than 20 years, and a second, larger peak among individuals aged 50-69 years.
According to the investigators, these findings “are concordant with the results observed in the European cohort,” citing a Danish study spanning 1994-2012 and a Dutch study spanning 2000-2010. They noted that the Danish study also reported a bimodal distribution of age incidence, as did a Swedish study, and another study from New Zealand. The stable levels of PBC and PSC align with two recent retrospective studies conducted in the United States and, they added.
“We believe that the observed differential trends in the incidence of these autoimmune liver diseases truly reflects their contemporary epidemiology,” the investigators wrote. They went on to suggest that the findings did not stem from an increase in diagnostic scrutiny because the study period did not include any significant changes in gastroenterology service, coding, or diagnostic criteria in the region studied.
“The increased incidence of AIH parallels rising incidence and prevalence of other autoimmune disorders such as [inflammatory bowel disease], type 1 diabetes, and multiple sclerosis in New Zealand, and it is unclear whether these autoimmune conditions share a common local environmental trigger,” they wrote. “Environmental factors likely play a central role augmenting phenotypic expression in genetically predisposed individuals.”
While Dr. Lamba and colleagues proposed several possible factors, such as increased exposure to pharmaceuticals, definitive factors remain elusive, which the authors cited as one limitation of their study. Another limitation they cited is the possibility that other etiologies were mistakenly classified as “probable” AIH; however, the chances of that are small, and the proportion of probable versus definitive AIH noted in this study do reflect those seen in other epidemiological studies.
“The reason for observed differential change in incidence of these autoimmune liver diseases is unclear,” they wrote, “and future collaborative prospective epidemiological study would be required to assess this further.”
The investigators reported no conflicts of interest.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Expert highlights advances in DRESS
Mounting evidence suggests , Sarah Walsh, MD, said at the virtual annual congress of the European Academy of Dermatology and Venereology.
The standard dictum has been that diagnosis of this severe T-cell-mediated drug reaction requires more than a 2-week delay in symptom onset following initial drug intake. But this can steer physicians in the wrong direction and lead to stopping an innocent drug while the true culprit medication remains on board. This adversely affects patient prognosis, since a longer duration of drug exposure after symptom onset is associated with increased hospital length of stay and greater mortality risk, explained Dr. Walsh, clinical lead for dermatology at King’s College Hospital, London.
In addition to . These include clues provided by rash morphology and histopathology, HLA testing, and a novel scoring system to assess DRESS severity and the risk of potentially fatal cytomegalovirus reactivation.
Short-delay DRESS onset
In a retrospective study of 41 patients with a first episode of DRESS in three French dermatology departments, 14 (34%) had onset within 15 days or less of initial exposure to the causative drug. In 6 of 14 patients in the rapid-onset group the offending drug was an antibiotic, while in another 5 the culprit was iodinated contrast media. In the delayed-onset DRESS group, the chief sensitizers were allopurinol in 8 patients, lamotrigine in 6, carbamazepine in 4, and sulfasalazine in 2; of note, none of these 4 delayed-onset DRESS drugs were implicated in any cases of rapid-onset DRESS. There were no differences in the clinical manifestations of DRESS between the rapid- and delayed-onset groups.
Similarly, dermatologists at Government Medical College in Kerala, India, reported in a retrospective study of 100 consecutive patients with DRESS, the drug reaction emerged within 2 weeks after starting the culprit medication in 36% of cases. Indeed, 11 patients became symptomatic within 3-7 days after beginning the medication; in 10 of the 11 cases, the offending agent was an antibiotic, and in 1 patient it was terbinafine. In the 25 cases of DRESS that arose on day 8-14 of drug therapy, the culprit was phenytoin in 14, antibiotics in 6, and 1 each for clopidogrel, hydroxychloroquine, sodium valproate, lamotrigine, and vitamin D3.
Both groups of investigators concluded that a short time lag between starting a drug and development of symptoms of a drug reaction shouldn’t rule out DRESS as a possibility provided other criteria consistent with the diagnosis are present. Hallmarks of DRESS include an acute extensive rash, fever greater than 38 degrees C, enlarged lymph nodes at two or more sites, internal organ involvement, a low platelet count, elevated eosinophils, and abnormal lymphocyte levels.
Rash morphology and histology as prognostic indicators
Dr. Walsh was the lead investigator in a study that identified four distinct patterns of skin involvement in patients with DRESS. The most common type of rash in this single-center retrospective study of 27 consecutive patients was an urticated papular exanthem, present in 13 of the 27 patients. An erythema multiforme-like reaction was present in 8, exfoliative erythroderma in 3, and a morbilliform erythema in 3 others. The worst prognosis was in the subgroup with an erythema multiforme-like rash.
All 27 patients had hepatic involvement, which was severe in 9 cases. Six of the 9 with severe liver impairment had an erythema multiforme-like rash, compared with just 2 of the 18 with mild or moderate liver involvement; thus, an erythema multiforme-like skin eruption was associated with a fivefold increased likelihood of severe hepatic involvement.
“It is a clinical sign that we take seriously at presentation if atypical target lesions are present,” the dermatologist said.
Separately, Taiwanese investigators compared clinical and histopathologic features in a study of 32 patients with DRESS and 17 with maculopapular exanthem. Interface vacuolization, which was present in 29 of the 32 patients with DRESS, was far more prominent than in the comparator group. Moreover, severe dyskeratosis was significantly associated with more severe liver impairment in the DRESS group.
HLA testing
Testing for HLA haplotypes associated with severe drug reactions has a useful role as a screening tool prior to prescribing selected high-risk drugs, Dr. Walsh said. For example, it’s known that 6.8% of individuals of European ancestry carry HLA-A*32:01, an allele that was strongly associated with an increased rate of vancomycin-associated DRESS in a case-control study at Vanderbilt University, Nashville, Tenn. Indeed, 19 of 23 individuals with vancomycin-associated DRESS were HLA-A*32:01 positive, compared with none of 46 vancomycin-tolerant controls. Nineteen percent of HLA-A*32:01-positive patients developed DRESS during treatment with vancomycin, and the drug reaction occurred within 4 weeks.
The investigators noted that testing for HLA-A*32:01 is also useful in DRESS occurring in patients on vancomycin and multiple other drugs because the test’s high negative predictive value may safely allow continued therapy with this potent antibiotic for Gram-positive infections.
A DRESS prognostic scoring system
Japanese researchers have developed a scoring system for DRESS for use in monitoring severity of the drug reaction, predicting prognosis, and estimating the risk of developing cytomegalovirus disease and its potentially fatal complications. The scoring system incorporates patient factors, including age, duration of drug exposure after symptom onset; rash characteristics, such as percentage of body surface area involved and presence or absence of erythroderma; appetite loss; and laboratory values.
“It yields a prognostic score that can be used to determine treatment choices, such as immediate intervention with anti-CMV agents. It’s a very useful tool,” Dr. Walsh said.
She reported having no financial conflicts regarding her presentation.
Mounting evidence suggests , Sarah Walsh, MD, said at the virtual annual congress of the European Academy of Dermatology and Venereology.
The standard dictum has been that diagnosis of this severe T-cell-mediated drug reaction requires more than a 2-week delay in symptom onset following initial drug intake. But this can steer physicians in the wrong direction and lead to stopping an innocent drug while the true culprit medication remains on board. This adversely affects patient prognosis, since a longer duration of drug exposure after symptom onset is associated with increased hospital length of stay and greater mortality risk, explained Dr. Walsh, clinical lead for dermatology at King’s College Hospital, London.
In addition to . These include clues provided by rash morphology and histopathology, HLA testing, and a novel scoring system to assess DRESS severity and the risk of potentially fatal cytomegalovirus reactivation.
Short-delay DRESS onset
In a retrospective study of 41 patients with a first episode of DRESS in three French dermatology departments, 14 (34%) had onset within 15 days or less of initial exposure to the causative drug. In 6 of 14 patients in the rapid-onset group the offending drug was an antibiotic, while in another 5 the culprit was iodinated contrast media. In the delayed-onset DRESS group, the chief sensitizers were allopurinol in 8 patients, lamotrigine in 6, carbamazepine in 4, and sulfasalazine in 2; of note, none of these 4 delayed-onset DRESS drugs were implicated in any cases of rapid-onset DRESS. There were no differences in the clinical manifestations of DRESS between the rapid- and delayed-onset groups.
Similarly, dermatologists at Government Medical College in Kerala, India, reported in a retrospective study of 100 consecutive patients with DRESS, the drug reaction emerged within 2 weeks after starting the culprit medication in 36% of cases. Indeed, 11 patients became symptomatic within 3-7 days after beginning the medication; in 10 of the 11 cases, the offending agent was an antibiotic, and in 1 patient it was terbinafine. In the 25 cases of DRESS that arose on day 8-14 of drug therapy, the culprit was phenytoin in 14, antibiotics in 6, and 1 each for clopidogrel, hydroxychloroquine, sodium valproate, lamotrigine, and vitamin D3.
Both groups of investigators concluded that a short time lag between starting a drug and development of symptoms of a drug reaction shouldn’t rule out DRESS as a possibility provided other criteria consistent with the diagnosis are present. Hallmarks of DRESS include an acute extensive rash, fever greater than 38 degrees C, enlarged lymph nodes at two or more sites, internal organ involvement, a low platelet count, elevated eosinophils, and abnormal lymphocyte levels.
Rash morphology and histology as prognostic indicators
Dr. Walsh was the lead investigator in a study that identified four distinct patterns of skin involvement in patients with DRESS. The most common type of rash in this single-center retrospective study of 27 consecutive patients was an urticated papular exanthem, present in 13 of the 27 patients. An erythema multiforme-like reaction was present in 8, exfoliative erythroderma in 3, and a morbilliform erythema in 3 others. The worst prognosis was in the subgroup with an erythema multiforme-like rash.
All 27 patients had hepatic involvement, which was severe in 9 cases. Six of the 9 with severe liver impairment had an erythema multiforme-like rash, compared with just 2 of the 18 with mild or moderate liver involvement; thus, an erythema multiforme-like skin eruption was associated with a fivefold increased likelihood of severe hepatic involvement.
“It is a clinical sign that we take seriously at presentation if atypical target lesions are present,” the dermatologist said.
Separately, Taiwanese investigators compared clinical and histopathologic features in a study of 32 patients with DRESS and 17 with maculopapular exanthem. Interface vacuolization, which was present in 29 of the 32 patients with DRESS, was far more prominent than in the comparator group. Moreover, severe dyskeratosis was significantly associated with more severe liver impairment in the DRESS group.
HLA testing
Testing for HLA haplotypes associated with severe drug reactions has a useful role as a screening tool prior to prescribing selected high-risk drugs, Dr. Walsh said. For example, it’s known that 6.8% of individuals of European ancestry carry HLA-A*32:01, an allele that was strongly associated with an increased rate of vancomycin-associated DRESS in a case-control study at Vanderbilt University, Nashville, Tenn. Indeed, 19 of 23 individuals with vancomycin-associated DRESS were HLA-A*32:01 positive, compared with none of 46 vancomycin-tolerant controls. Nineteen percent of HLA-A*32:01-positive patients developed DRESS during treatment with vancomycin, and the drug reaction occurred within 4 weeks.
The investigators noted that testing for HLA-A*32:01 is also useful in DRESS occurring in patients on vancomycin and multiple other drugs because the test’s high negative predictive value may safely allow continued therapy with this potent antibiotic for Gram-positive infections.
A DRESS prognostic scoring system
Japanese researchers have developed a scoring system for DRESS for use in monitoring severity of the drug reaction, predicting prognosis, and estimating the risk of developing cytomegalovirus disease and its potentially fatal complications. The scoring system incorporates patient factors, including age, duration of drug exposure after symptom onset; rash characteristics, such as percentage of body surface area involved and presence or absence of erythroderma; appetite loss; and laboratory values.
“It yields a prognostic score that can be used to determine treatment choices, such as immediate intervention with anti-CMV agents. It’s a very useful tool,” Dr. Walsh said.
She reported having no financial conflicts regarding her presentation.
Mounting evidence suggests , Sarah Walsh, MD, said at the virtual annual congress of the European Academy of Dermatology and Venereology.
The standard dictum has been that diagnosis of this severe T-cell-mediated drug reaction requires more than a 2-week delay in symptom onset following initial drug intake. But this can steer physicians in the wrong direction and lead to stopping an innocent drug while the true culprit medication remains on board. This adversely affects patient prognosis, since a longer duration of drug exposure after symptom onset is associated with increased hospital length of stay and greater mortality risk, explained Dr. Walsh, clinical lead for dermatology at King’s College Hospital, London.
In addition to . These include clues provided by rash morphology and histopathology, HLA testing, and a novel scoring system to assess DRESS severity and the risk of potentially fatal cytomegalovirus reactivation.
Short-delay DRESS onset
In a retrospective study of 41 patients with a first episode of DRESS in three French dermatology departments, 14 (34%) had onset within 15 days or less of initial exposure to the causative drug. In 6 of 14 patients in the rapid-onset group the offending drug was an antibiotic, while in another 5 the culprit was iodinated contrast media. In the delayed-onset DRESS group, the chief sensitizers were allopurinol in 8 patients, lamotrigine in 6, carbamazepine in 4, and sulfasalazine in 2; of note, none of these 4 delayed-onset DRESS drugs were implicated in any cases of rapid-onset DRESS. There were no differences in the clinical manifestations of DRESS between the rapid- and delayed-onset groups.
Similarly, dermatologists at Government Medical College in Kerala, India, reported in a retrospective study of 100 consecutive patients with DRESS, the drug reaction emerged within 2 weeks after starting the culprit medication in 36% of cases. Indeed, 11 patients became symptomatic within 3-7 days after beginning the medication; in 10 of the 11 cases, the offending agent was an antibiotic, and in 1 patient it was terbinafine. In the 25 cases of DRESS that arose on day 8-14 of drug therapy, the culprit was phenytoin in 14, antibiotics in 6, and 1 each for clopidogrel, hydroxychloroquine, sodium valproate, lamotrigine, and vitamin D3.
Both groups of investigators concluded that a short time lag between starting a drug and development of symptoms of a drug reaction shouldn’t rule out DRESS as a possibility provided other criteria consistent with the diagnosis are present. Hallmarks of DRESS include an acute extensive rash, fever greater than 38 degrees C, enlarged lymph nodes at two or more sites, internal organ involvement, a low platelet count, elevated eosinophils, and abnormal lymphocyte levels.
Rash morphology and histology as prognostic indicators
Dr. Walsh was the lead investigator in a study that identified four distinct patterns of skin involvement in patients with DRESS. The most common type of rash in this single-center retrospective study of 27 consecutive patients was an urticated papular exanthem, present in 13 of the 27 patients. An erythema multiforme-like reaction was present in 8, exfoliative erythroderma in 3, and a morbilliform erythema in 3 others. The worst prognosis was in the subgroup with an erythema multiforme-like rash.
All 27 patients had hepatic involvement, which was severe in 9 cases. Six of the 9 with severe liver impairment had an erythema multiforme-like rash, compared with just 2 of the 18 with mild or moderate liver involvement; thus, an erythema multiforme-like skin eruption was associated with a fivefold increased likelihood of severe hepatic involvement.
“It is a clinical sign that we take seriously at presentation if atypical target lesions are present,” the dermatologist said.
Separately, Taiwanese investigators compared clinical and histopathologic features in a study of 32 patients with DRESS and 17 with maculopapular exanthem. Interface vacuolization, which was present in 29 of the 32 patients with DRESS, was far more prominent than in the comparator group. Moreover, severe dyskeratosis was significantly associated with more severe liver impairment in the DRESS group.
HLA testing
Testing for HLA haplotypes associated with severe drug reactions has a useful role as a screening tool prior to prescribing selected high-risk drugs, Dr. Walsh said. For example, it’s known that 6.8% of individuals of European ancestry carry HLA-A*32:01, an allele that was strongly associated with an increased rate of vancomycin-associated DRESS in a case-control study at Vanderbilt University, Nashville, Tenn. Indeed, 19 of 23 individuals with vancomycin-associated DRESS were HLA-A*32:01 positive, compared with none of 46 vancomycin-tolerant controls. Nineteen percent of HLA-A*32:01-positive patients developed DRESS during treatment with vancomycin, and the drug reaction occurred within 4 weeks.
The investigators noted that testing for HLA-A*32:01 is also useful in DRESS occurring in patients on vancomycin and multiple other drugs because the test’s high negative predictive value may safely allow continued therapy with this potent antibiotic for Gram-positive infections.
A DRESS prognostic scoring system
Japanese researchers have developed a scoring system for DRESS for use in monitoring severity of the drug reaction, predicting prognosis, and estimating the risk of developing cytomegalovirus disease and its potentially fatal complications. The scoring system incorporates patient factors, including age, duration of drug exposure after symptom onset; rash characteristics, such as percentage of body surface area involved and presence or absence of erythroderma; appetite loss; and laboratory values.
“It yields a prognostic score that can be used to determine treatment choices, such as immediate intervention with anti-CMV agents. It’s a very useful tool,” Dr. Walsh said.
She reported having no financial conflicts regarding her presentation.
FROM THE EADV CONGRESS