User login
CDC: 20% of people in the U.S. are infected with an STD
Among the more than 320 million people in the United States, there was a prevalence estimate of 67.6 million sexually transmitted infections at the time of assessment in 2018, according to the results of an epidemiologic study using multiple data sources, including the National Health and Nutrition Examination Survey (NHANES).
In addition, almost half of the incident STIs occurred in the 15- to 24-year age bracket, according to a report published online in Sexually Transmitted Diseases. Researchers estimated the combined number of prevalent and incident infections of eight STIs in the United States in 2018: chlamydia, gonorrhea, trichomoniasis, syphilis, genital herpes (caused by herpes simplex virus type 2 [HSV-2]), human papillomavirus (HPV), sexually transmitted hepatitis B virus (HBV), and sexually transmitted HIV.
The estimated incidences of these STIs in this update, the first since 2008, were made using more recent data and improved estimation methods to provide updated STI prevalence and incidence estimates for 2018, both overall and by disease. “Having a combined estimate is crucial for policy purposes to illustrate the importance of STIs in the United States,” according to Kristen M. Kreisel, PhD, an epidemiologist at the Centers for Disease Control and Prevention, division of STD prevention, and colleagues.
The number of prevalent and incident infections were obtained by multiplying each STI’s updated per capita estimates by the 2018 full resident population estimates from the American Community Survey.
Detailed results
Chlamydia. The prevalence of chlamydia was estimated using 2015-2018 NHANES data, which was then used to create a modeled prevalence in 2018, according to the authors. There were an estimated 2.4 million prevalent urogenital chlamydial infections among persons aged 15-39 years in 2018; 1.1 and 1.3 million infections among men and women, respectively. Individuals aged 15-24 years comprised 56.7% and 75.8% of all infections in men and women respectively.
Gonorrhea. The prevalence of gonorrhea was estimated using ordinary differential equation based modeling. The number of prevalent urogenital gonococcal infections in 2018 among 15- to 39-year-olds was 209,000 overall; 50,000 in men and 155,000 in women. Of these, 113,000 (54.1%) occurred in 15- to 24-year-olds.
Trichomoniasis. The prevalence of trichomoniasis was estimated using 2015-2018 NHANES data, which was then used to create a modeled prevalence in 2018, according to the authors. The number of prevalent Trichomonas infections among 15- to 59-year-olds was 2.6 million, with 470,000 in men and 2.1 million in women. Persons aged 15-24 years comprised 15.6% of all prevalent infections, according to the authors.
Syphilis. The number of estimated prevalent syphilitic infections (all stages) among 14- to 49-year-old persons in 2018 was 156,000, with infections in men comprising 71.8% of all infections. Infections in both men and women aged 14-24 years accounted for about 25% of all infections, with 36,000 total prevalent syphilitic infections among 14- to 24-year-olds in 2018.
Genital herpes. The prevalence of genital herpes (caused by HSV-2) was estimated using 2015-2018 NHANES data, according to the authors. In persons aged 15-49 years in 2018, there were 18.6 million prevalent HSV-2 infections; 6.4 million among men and 12.2 million among women. Infections in 15- to 24-year-olds comprised 7.1% of all prevalent HSV-2 infections.
HPV. The prevalence of HPV was estimated using 2013-2016 NHANES data, which was assumed to reflect stable prevalence in 2018, according to the authors. Among 15- to 59-year-olds, the estimated number of persons, men, and women infected with one or more disease-associated HPV types in 2018 was 42.5, 23.4, and 19.2 million, respectively, with an estimated 9.0 million (21%) 15- to 24-year-olds infected,
HBV. NHANES 2013-2018 data were used to estimate the prevalence of sexually transmitted chronic HBV infections in 2018, according to the authors. The estimated number of infections among persons aged 15 years and older in 2018 was 103,000 (51,000 men and 52,000 women). There small sample size of individuals aged 15-24 years in the NHANES database made it impossible to obtain an accurate estimate for this group, according to the authors.
HIV. Data from the National HIV Surveillance System were used to estimate the prevalence and incidence of sexually transmitted HIV infections for persons aged 13 years and older in 2018. A total of 984,000 individuals aged 13 years and older were estimated to be living with sexually transmitted HIV at the end of 2018, according to the authors. Nearly 80% were men. In the 13- to 24-year-old age bracket, there were an estimated 45,400 living with sexually transmitted HIV.
Billions in costs
Commenting on the study by the CDC researchers, Raul Romaguera, acting director for CDC’s division of STD prevention, stated in a press release: “There are significant human and financial costs associated with these infections, and we know from other studies that cuts in STI prevention efforts result in higher costs down the road. Preventing STIs could save billions in medical costs, but more importantly, prevention would improve the health and lives of millions of people.”
“About 20% of the total U.S. population had an STI at a given point in 2018, while nearly half of all incident infections occurred in people aged 15-24 years. Focusing STI prevention efforts on the 15- to 24-year-old population may be key to lowering the STI burden in the U.S.,” the researchers concluded.
The authors reported that they had no disclosures.
Among the more than 320 million people in the United States, there was a prevalence estimate of 67.6 million sexually transmitted infections at the time of assessment in 2018, according to the results of an epidemiologic study using multiple data sources, including the National Health and Nutrition Examination Survey (NHANES).
In addition, almost half of the incident STIs occurred in the 15- to 24-year age bracket, according to a report published online in Sexually Transmitted Diseases. Researchers estimated the combined number of prevalent and incident infections of eight STIs in the United States in 2018: chlamydia, gonorrhea, trichomoniasis, syphilis, genital herpes (caused by herpes simplex virus type 2 [HSV-2]), human papillomavirus (HPV), sexually transmitted hepatitis B virus (HBV), and sexually transmitted HIV.
The estimated incidences of these STIs in this update, the first since 2008, were made using more recent data and improved estimation methods to provide updated STI prevalence and incidence estimates for 2018, both overall and by disease. “Having a combined estimate is crucial for policy purposes to illustrate the importance of STIs in the United States,” according to Kristen M. Kreisel, PhD, an epidemiologist at the Centers for Disease Control and Prevention, division of STD prevention, and colleagues.
The number of prevalent and incident infections were obtained by multiplying each STI’s updated per capita estimates by the 2018 full resident population estimates from the American Community Survey.
Detailed results
Chlamydia. The prevalence of chlamydia was estimated using 2015-2018 NHANES data, which was then used to create a modeled prevalence in 2018, according to the authors. There were an estimated 2.4 million prevalent urogenital chlamydial infections among persons aged 15-39 years in 2018; 1.1 and 1.3 million infections among men and women, respectively. Individuals aged 15-24 years comprised 56.7% and 75.8% of all infections in men and women respectively.
Gonorrhea. The prevalence of gonorrhea was estimated using ordinary differential equation based modeling. The number of prevalent urogenital gonococcal infections in 2018 among 15- to 39-year-olds was 209,000 overall; 50,000 in men and 155,000 in women. Of these, 113,000 (54.1%) occurred in 15- to 24-year-olds.
Trichomoniasis. The prevalence of trichomoniasis was estimated using 2015-2018 NHANES data, which was then used to create a modeled prevalence in 2018, according to the authors. The number of prevalent Trichomonas infections among 15- to 59-year-olds was 2.6 million, with 470,000 in men and 2.1 million in women. Persons aged 15-24 years comprised 15.6% of all prevalent infections, according to the authors.
Syphilis. The number of estimated prevalent syphilitic infections (all stages) among 14- to 49-year-old persons in 2018 was 156,000, with infections in men comprising 71.8% of all infections. Infections in both men and women aged 14-24 years accounted for about 25% of all infections, with 36,000 total prevalent syphilitic infections among 14- to 24-year-olds in 2018.
Genital herpes. The prevalence of genital herpes (caused by HSV-2) was estimated using 2015-2018 NHANES data, according to the authors. In persons aged 15-49 years in 2018, there were 18.6 million prevalent HSV-2 infections; 6.4 million among men and 12.2 million among women. Infections in 15- to 24-year-olds comprised 7.1% of all prevalent HSV-2 infections.
HPV. The prevalence of HPV was estimated using 2013-2016 NHANES data, which was assumed to reflect stable prevalence in 2018, according to the authors. Among 15- to 59-year-olds, the estimated number of persons, men, and women infected with one or more disease-associated HPV types in 2018 was 42.5, 23.4, and 19.2 million, respectively, with an estimated 9.0 million (21%) 15- to 24-year-olds infected,
HBV. NHANES 2013-2018 data were used to estimate the prevalence of sexually transmitted chronic HBV infections in 2018, according to the authors. The estimated number of infections among persons aged 15 years and older in 2018 was 103,000 (51,000 men and 52,000 women). There small sample size of individuals aged 15-24 years in the NHANES database made it impossible to obtain an accurate estimate for this group, according to the authors.
HIV. Data from the National HIV Surveillance System were used to estimate the prevalence and incidence of sexually transmitted HIV infections for persons aged 13 years and older in 2018. A total of 984,000 individuals aged 13 years and older were estimated to be living with sexually transmitted HIV at the end of 2018, according to the authors. Nearly 80% were men. In the 13- to 24-year-old age bracket, there were an estimated 45,400 living with sexually transmitted HIV.
Billions in costs
Commenting on the study by the CDC researchers, Raul Romaguera, acting director for CDC’s division of STD prevention, stated in a press release: “There are significant human and financial costs associated with these infections, and we know from other studies that cuts in STI prevention efforts result in higher costs down the road. Preventing STIs could save billions in medical costs, but more importantly, prevention would improve the health and lives of millions of people.”
“About 20% of the total U.S. population had an STI at a given point in 2018, while nearly half of all incident infections occurred in people aged 15-24 years. Focusing STI prevention efforts on the 15- to 24-year-old population may be key to lowering the STI burden in the U.S.,” the researchers concluded.
The authors reported that they had no disclosures.
Among the more than 320 million people in the United States, there was a prevalence estimate of 67.6 million sexually transmitted infections at the time of assessment in 2018, according to the results of an epidemiologic study using multiple data sources, including the National Health and Nutrition Examination Survey (NHANES).
In addition, almost half of the incident STIs occurred in the 15- to 24-year age bracket, according to a report published online in Sexually Transmitted Diseases. Researchers estimated the combined number of prevalent and incident infections of eight STIs in the United States in 2018: chlamydia, gonorrhea, trichomoniasis, syphilis, genital herpes (caused by herpes simplex virus type 2 [HSV-2]), human papillomavirus (HPV), sexually transmitted hepatitis B virus (HBV), and sexually transmitted HIV.
The estimated incidences of these STIs in this update, the first since 2008, were made using more recent data and improved estimation methods to provide updated STI prevalence and incidence estimates for 2018, both overall and by disease. “Having a combined estimate is crucial for policy purposes to illustrate the importance of STIs in the United States,” according to Kristen M. Kreisel, PhD, an epidemiologist at the Centers for Disease Control and Prevention, division of STD prevention, and colleagues.
The number of prevalent and incident infections were obtained by multiplying each STI’s updated per capita estimates by the 2018 full resident population estimates from the American Community Survey.
Detailed results
Chlamydia. The prevalence of chlamydia was estimated using 2015-2018 NHANES data, which was then used to create a modeled prevalence in 2018, according to the authors. There were an estimated 2.4 million prevalent urogenital chlamydial infections among persons aged 15-39 years in 2018; 1.1 and 1.3 million infections among men and women, respectively. Individuals aged 15-24 years comprised 56.7% and 75.8% of all infections in men and women respectively.
Gonorrhea. The prevalence of gonorrhea was estimated using ordinary differential equation based modeling. The number of prevalent urogenital gonococcal infections in 2018 among 15- to 39-year-olds was 209,000 overall; 50,000 in men and 155,000 in women. Of these, 113,000 (54.1%) occurred in 15- to 24-year-olds.
Trichomoniasis. The prevalence of trichomoniasis was estimated using 2015-2018 NHANES data, which was then used to create a modeled prevalence in 2018, according to the authors. The number of prevalent Trichomonas infections among 15- to 59-year-olds was 2.6 million, with 470,000 in men and 2.1 million in women. Persons aged 15-24 years comprised 15.6% of all prevalent infections, according to the authors.
Syphilis. The number of estimated prevalent syphilitic infections (all stages) among 14- to 49-year-old persons in 2018 was 156,000, with infections in men comprising 71.8% of all infections. Infections in both men and women aged 14-24 years accounted for about 25% of all infections, with 36,000 total prevalent syphilitic infections among 14- to 24-year-olds in 2018.
Genital herpes. The prevalence of genital herpes (caused by HSV-2) was estimated using 2015-2018 NHANES data, according to the authors. In persons aged 15-49 years in 2018, there were 18.6 million prevalent HSV-2 infections; 6.4 million among men and 12.2 million among women. Infections in 15- to 24-year-olds comprised 7.1% of all prevalent HSV-2 infections.
HPV. The prevalence of HPV was estimated using 2013-2016 NHANES data, which was assumed to reflect stable prevalence in 2018, according to the authors. Among 15- to 59-year-olds, the estimated number of persons, men, and women infected with one or more disease-associated HPV types in 2018 was 42.5, 23.4, and 19.2 million, respectively, with an estimated 9.0 million (21%) 15- to 24-year-olds infected,
HBV. NHANES 2013-2018 data were used to estimate the prevalence of sexually transmitted chronic HBV infections in 2018, according to the authors. The estimated number of infections among persons aged 15 years and older in 2018 was 103,000 (51,000 men and 52,000 women). There small sample size of individuals aged 15-24 years in the NHANES database made it impossible to obtain an accurate estimate for this group, according to the authors.
HIV. Data from the National HIV Surveillance System were used to estimate the prevalence and incidence of sexually transmitted HIV infections for persons aged 13 years and older in 2018. A total of 984,000 individuals aged 13 years and older were estimated to be living with sexually transmitted HIV at the end of 2018, according to the authors. Nearly 80% were men. In the 13- to 24-year-old age bracket, there were an estimated 45,400 living with sexually transmitted HIV.
Billions in costs
Commenting on the study by the CDC researchers, Raul Romaguera, acting director for CDC’s division of STD prevention, stated in a press release: “There are significant human and financial costs associated with these infections, and we know from other studies that cuts in STI prevention efforts result in higher costs down the road. Preventing STIs could save billions in medical costs, but more importantly, prevention would improve the health and lives of millions of people.”
“About 20% of the total U.S. population had an STI at a given point in 2018, while nearly half of all incident infections occurred in people aged 15-24 years. Focusing STI prevention efforts on the 15- to 24-year-old population may be key to lowering the STI burden in the U.S.,” the researchers concluded.
The authors reported that they had no disclosures.
FROM SEXUALLY TRANSMITTED DISEASES
Weekly COVID-19 cases in children dropped 22%
according to new data from the American Academy of Pediatrics and the Children’s Hospital Association.
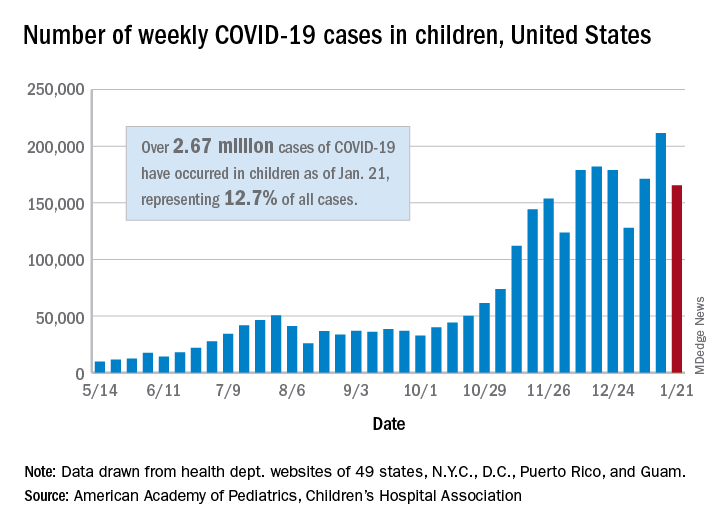
The 165,000 new cases reported during the week of Jan. 15-21 were down by almost 22% from the previous week’s 211,000, when the new-case count reached its highest point in the pandemic, the AAP and CHA said in their weekly COVID-19 report.
Cumulative cases in children now stand at just over 2.67 million, and children represent 12.7% of all COVID-19 cases reported by 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam. For the week of Jan. 15-21, children made up 14.8% of all new cases, the highest proportion since late September, the AAP/CHA data show.
The cumulative rate of infection among children is up to 3,556 per 100,000 nationally, with states ranging from 943 per 100,000 in Hawaii to 8,195 in North Dakota. California has the most reported cases at 383,000, while Vermont has the fewest at 1,820, the two organizations reported.
There were 14 more deaths among children in the last week, bringing the total to 205 in the 43 states (plus New York City and Guam) reporting such data. Children represent just 0.06% of all coronavirus-related deaths, and only 0.01% of all cases in children have resulted in death, the AAP and CHA said. There are still 10 states where no children have died from COVID-19.
Although severe illness appears to be rare in children, the AAP and CHA noted, “there is an urgent need to collect more data on longer-term impacts of the pandemic on children, including ways the virus may harm the long-term physical health of infected children, as well as its emotional and mental health effects.”
according to new data from the American Academy of Pediatrics and the Children’s Hospital Association.

The 165,000 new cases reported during the week of Jan. 15-21 were down by almost 22% from the previous week’s 211,000, when the new-case count reached its highest point in the pandemic, the AAP and CHA said in their weekly COVID-19 report.
Cumulative cases in children now stand at just over 2.67 million, and children represent 12.7% of all COVID-19 cases reported by 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam. For the week of Jan. 15-21, children made up 14.8% of all new cases, the highest proportion since late September, the AAP/CHA data show.
The cumulative rate of infection among children is up to 3,556 per 100,000 nationally, with states ranging from 943 per 100,000 in Hawaii to 8,195 in North Dakota. California has the most reported cases at 383,000, while Vermont has the fewest at 1,820, the two organizations reported.
There were 14 more deaths among children in the last week, bringing the total to 205 in the 43 states (plus New York City and Guam) reporting such data. Children represent just 0.06% of all coronavirus-related deaths, and only 0.01% of all cases in children have resulted in death, the AAP and CHA said. There are still 10 states where no children have died from COVID-19.
Although severe illness appears to be rare in children, the AAP and CHA noted, “there is an urgent need to collect more data on longer-term impacts of the pandemic on children, including ways the virus may harm the long-term physical health of infected children, as well as its emotional and mental health effects.”
according to new data from the American Academy of Pediatrics and the Children’s Hospital Association.

The 165,000 new cases reported during the week of Jan. 15-21 were down by almost 22% from the previous week’s 211,000, when the new-case count reached its highest point in the pandemic, the AAP and CHA said in their weekly COVID-19 report.
Cumulative cases in children now stand at just over 2.67 million, and children represent 12.7% of all COVID-19 cases reported by 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam. For the week of Jan. 15-21, children made up 14.8% of all new cases, the highest proportion since late September, the AAP/CHA data show.
The cumulative rate of infection among children is up to 3,556 per 100,000 nationally, with states ranging from 943 per 100,000 in Hawaii to 8,195 in North Dakota. California has the most reported cases at 383,000, while Vermont has the fewest at 1,820, the two organizations reported.
There were 14 more deaths among children in the last week, bringing the total to 205 in the 43 states (plus New York City and Guam) reporting such data. Children represent just 0.06% of all coronavirus-related deaths, and only 0.01% of all cases in children have resulted in death, the AAP and CHA said. There are still 10 states where no children have died from COVID-19.
Although severe illness appears to be rare in children, the AAP and CHA noted, “there is an urgent need to collect more data on longer-term impacts of the pandemic on children, including ways the virus may harm the long-term physical health of infected children, as well as its emotional and mental health effects.”
First monthly injectable HIV treatment approved by FDA
Cabenuva (cabotegravir and rilpivirine, a once-per-month injectable formulation) was approved by the Food and Drug Administration as a complete regimen for treatment of HIV-1 infection in adults. It is intended to replace current antiretroviral regimens in those patients who are virologically suppressed with no history of treatment failure and with no known or suspected resistance to either of the two component drugs.
Cabenuva is the first FDA-approved monthly injectable, complete regimen for HIV-infected adults, according to the agency’s announcement.
In addition, the FDA-approved Vocabria (cabotegravir, tablet formulation), a preparatory treatment intended to be taken in combination with oral rilpivirine (Edurant) for 1 month prior to starting treatment with Cabenuva to ensure the medications are well tolerated before switching to the extended-release injectable formulation. The FDA granted the approval of Cabenuva and Vocabria to ViiV Healthcare.
Cabotegravir is as an integrase strand transfer inhibitor that blocks HIV integrase by attaching to the active integrase site and inhibiting retroviral DNA integration, which is necessary in order for HIV to replicate. In contrast, rilpivirine acts as a diarylpyrimidine nonnucleoside reverse transcriptase inhibitor of HIV-1.
Approval of Cabenuva was based upon two randomized, open-label, controlled clinical trials in 1,182 HIV-infected adults who were virologically suppressed (HIV-1 RNA less than 50 copies/mL) before initiation of treatment with Cabenuva. The two pivotal phase three clinical studies were: Antiretroviral Therapy as Long-Acting Suppression (ATLAS; NCT02951052) and First Long-Acting Injectable Regimen (FLAIR; NCT02938520). Patients in both trials continued to show virologic suppression at the conclusion of each study, and no clinically relevant change from baseline in CD4+ cell counts was observed, according to the FDA announcement.
Adverse reactions with Cabenuva included injection-site reactions, fever, fatigue, headache, musculoskeletal pain, nausea, sleep disorders, dizziness, and rash. The FDA warned that Cabenuva should not be used if there is a known previous hypersensitivity reaction to cabotegravir or rilpivirine, or in patients who are not virally suppressed (HIV-1 RNA greater than 50 copies/mL).
Cabenuva and Vocabria were granted Fast Track and Priority Review designation by the FDA. Prescribing information for Cabenuva is available on the ViiV Healthcare website.
Cabenuva (cabotegravir and rilpivirine, a once-per-month injectable formulation) was approved by the Food and Drug Administration as a complete regimen for treatment of HIV-1 infection in adults. It is intended to replace current antiretroviral regimens in those patients who are virologically suppressed with no history of treatment failure and with no known or suspected resistance to either of the two component drugs.
Cabenuva is the first FDA-approved monthly injectable, complete regimen for HIV-infected adults, according to the agency’s announcement.
In addition, the FDA-approved Vocabria (cabotegravir, tablet formulation), a preparatory treatment intended to be taken in combination with oral rilpivirine (Edurant) for 1 month prior to starting treatment with Cabenuva to ensure the medications are well tolerated before switching to the extended-release injectable formulation. The FDA granted the approval of Cabenuva and Vocabria to ViiV Healthcare.
Cabotegravir is as an integrase strand transfer inhibitor that blocks HIV integrase by attaching to the active integrase site and inhibiting retroviral DNA integration, which is necessary in order for HIV to replicate. In contrast, rilpivirine acts as a diarylpyrimidine nonnucleoside reverse transcriptase inhibitor of HIV-1.
Approval of Cabenuva was based upon two randomized, open-label, controlled clinical trials in 1,182 HIV-infected adults who were virologically suppressed (HIV-1 RNA less than 50 copies/mL) before initiation of treatment with Cabenuva. The two pivotal phase three clinical studies were: Antiretroviral Therapy as Long-Acting Suppression (ATLAS; NCT02951052) and First Long-Acting Injectable Regimen (FLAIR; NCT02938520). Patients in both trials continued to show virologic suppression at the conclusion of each study, and no clinically relevant change from baseline in CD4+ cell counts was observed, according to the FDA announcement.
Adverse reactions with Cabenuva included injection-site reactions, fever, fatigue, headache, musculoskeletal pain, nausea, sleep disorders, dizziness, and rash. The FDA warned that Cabenuva should not be used if there is a known previous hypersensitivity reaction to cabotegravir or rilpivirine, or in patients who are not virally suppressed (HIV-1 RNA greater than 50 copies/mL).
Cabenuva and Vocabria were granted Fast Track and Priority Review designation by the FDA. Prescribing information for Cabenuva is available on the ViiV Healthcare website.
Cabenuva (cabotegravir and rilpivirine, a once-per-month injectable formulation) was approved by the Food and Drug Administration as a complete regimen for treatment of HIV-1 infection in adults. It is intended to replace current antiretroviral regimens in those patients who are virologically suppressed with no history of treatment failure and with no known or suspected resistance to either of the two component drugs.
Cabenuva is the first FDA-approved monthly injectable, complete regimen for HIV-infected adults, according to the agency’s announcement.
In addition, the FDA-approved Vocabria (cabotegravir, tablet formulation), a preparatory treatment intended to be taken in combination with oral rilpivirine (Edurant) for 1 month prior to starting treatment with Cabenuva to ensure the medications are well tolerated before switching to the extended-release injectable formulation. The FDA granted the approval of Cabenuva and Vocabria to ViiV Healthcare.
Cabotegravir is as an integrase strand transfer inhibitor that blocks HIV integrase by attaching to the active integrase site and inhibiting retroviral DNA integration, which is necessary in order for HIV to replicate. In contrast, rilpivirine acts as a diarylpyrimidine nonnucleoside reverse transcriptase inhibitor of HIV-1.
Approval of Cabenuva was based upon two randomized, open-label, controlled clinical trials in 1,182 HIV-infected adults who were virologically suppressed (HIV-1 RNA less than 50 copies/mL) before initiation of treatment with Cabenuva. The two pivotal phase three clinical studies were: Antiretroviral Therapy as Long-Acting Suppression (ATLAS; NCT02951052) and First Long-Acting Injectable Regimen (FLAIR; NCT02938520). Patients in both trials continued to show virologic suppression at the conclusion of each study, and no clinically relevant change from baseline in CD4+ cell counts was observed, according to the FDA announcement.
Adverse reactions with Cabenuva included injection-site reactions, fever, fatigue, headache, musculoskeletal pain, nausea, sleep disorders, dizziness, and rash. The FDA warned that Cabenuva should not be used if there is a known previous hypersensitivity reaction to cabotegravir or rilpivirine, or in patients who are not virally suppressed (HIV-1 RNA greater than 50 copies/mL).
Cabenuva and Vocabria were granted Fast Track and Priority Review designation by the FDA. Prescribing information for Cabenuva is available on the ViiV Healthcare website.
NEWS FROM THE FDA
Registry reveals H. pylori management mistakes
Many patients are receiving inadequate eradication therapy for Helicobacter pylori infection, according to analysis of a European registry.
In their analysis, published in the Journal of Clinical Gastroenterology, Olga P. Nyssen, BSc, PhD, of the Autonomous University of Madrid and colleagues discussed seven errors, which included prescribing a triple instead of quadruple regimen, prescribing therapy for too short of a duration, and prescribing a low dose of proton pump inhibitors (PPIs).
“[E]ven after more than 30 years of experience in H. pylori treatment, the ideal regimen to treat this infection remains undefined,” the investigators wrote. The European Registry on Helicobacter pylori management “represents a good mapping overview of the current situation regarding H. pylori management, allowing not only continuous assessment of the integration of clinical recommendations agreed on medical consensus, but also of the possible strategies for improvement.”
Patient data were drawn from registry-participating countries that each had more than 1,000 cases of H. pylori available; most came from Spain, followed by Russia, Italy, Slovenia, and Lithuania. Of these patients, data for 26,340 patients were analyzed, which ultimately represented 80% of the total registry from 2013 to 2019.
The first mistake discussed in the paper regarded use of less-effective triple therapies (typically PPI plus two antibiotics); one review showed that these regimens fail in 20%-40% of cases. Increasing antibiotic resistances have only worsened the success rate. According to this study, a triple regimen was given as first-line treatment in 46% of cases. Overall, frequency of triple-therapy prescriptions decreased from more than 50% in 2013 to about 40% in 2019. More significant improvements in this area were achieved in Spain, where use of triple therapies decreased from 24% in 2014 to 0% in 2019. According to the investigators, this finding serves as a “paradigmatic example of improvement with time.”
The authors pointed out that “overwhelming evidence” supports 14-day treatment; however, 69% of triple-therapy durations and 58% of quadruple therapy cases were for 7 or 10 days. Triple therapy at this duration showed only 81% cure rate, while it was 88% with 14 days, and quadruple therapy was only 80% effective at 7-10 days but 90% effective at 14 days.
“Fortunately,” the investigators wrote, “this mistake was progressively found less frequently and, at present, the prescription of 7-day standard triple therapy regimens has almost disappeared.”
The authors noted acid suppression via PPIs improves cure rates: In one meta-analysis, the cure rate of triple therapy regimens increased by 6%-10% with high doses of PPIs. However, the current study found that 48% of triple therapies included low-dose PPIs. This number decreased over time, the authors noted: from 67% in 2013 to 20% in 2019.
“From another perspective, the daily PPI dose has increased from a dose equivalent to 54 mg of omeprazole in 2013 to 104 mg in 2019,” they wrote.
The other four errors they discussed were failing to adequately consider penicillin allergies in prescription choices, failing to consider the importance of treatment compliance, repeating certain antibiotics after failures, and not checking eradication success after treatment.
Based on these findings, Dr. Nyssen and colleagues suggested that “penetration of recommendations in the participating European countries is still poor and delayed, even though some improvements from guidelines have been partially incorporated.”
According to Grigorios I. Leontiadis, MD, PhD, of McMaster University, Hamilton, Ont., who coauthored the 2017 American College of Gastroenterology H. pylori management guidelines and the Canadian Association of Gastroenterology “Toronto Consensus” in 2016, “This study is important and timely given the steadily increasing antibiotic resistance of H. pylori worldwide.”
Although Dr. Leontiadias described the results as “suboptimal,” he was partially reassured by the improvements over time, “especially following publication of the 2016 European clinical practice guidelines.” He also noted that some older clinical practice guidelines issued conditional recommendations, which could “justify the lower adherence seen in the early period of this study.”
“The unanswered question,” Dr. Leontiadias went on, “is whether the practice of gastroenterologists who volunteered to participate in this prospective registry is truly representative of how H. pylori is managed in Europe. Most likely it isn’t. Nonparticipating gastroenterologists and nongastroenterologist health care practitioners are probably less aware of and less adherent to clinical practice guidelines. This means that the actual situation in the real world is probably grimmer than what this study shows.”
William D. Chey, MD, Nostrant Collegiate Professor of Gastroenterology at the University of Michigan, Ann Arbor, considered the results “not entirely surprising, but nonetheless, noteworthy.”
Dr. Chey noted that the United States lacks a similar registry to compare real-world H. pylori management; even so, he suggested several findings that “bear reiteration” for clinicians in the United States.
“U.S. providers should consider regimens other than clarithromycin triple therapy when treating H. pylori infection,” Dr. Chey said. “Since U.S. providers do not have reliable data on H. pylori antimicrobial resistance, it is useful to ask about prior macrolide antibiotic exposure, and if a patient has received a macrolide for any reason, clarithromycin triple therapy should be avoided. Bismuth quadruple therapy remains a reliable first-line treatment option in the U.S. Another recently approved first-line treatment option is the combination of a proton pump inhibitor, rifabutin, and amoxicillin. Treatment regimens in the U.S. should be given for a minimum of 10 days and, preferably, for 14 days. Another point made by the article is that providers should be maximizing gastric acid suppression by using higher doses of proton pump inhibitors when treating H. pylori.”
Dr. Chey also noted an emerging treatment option that could soon be available. “Results from phase 3 trials in North America and Europe with the potassium-competitive acid blocker vonoprazan combined with amoxicillin, with and without clarithromycin, are expected in 2021 and may provide another novel first-line treatment option.”
Dr. Nyssen and colleagues disclosed relationships with Allergan, Mayoly, Janssen, and others. Dr. Chey is a consultant for Redhill, Phathom, and Takeda, which is developing vonoprazan. Dr. Leontiadias disclosed no conflicts of interest.
This article was updated 2/16/21.
Many patients are receiving inadequate eradication therapy for Helicobacter pylori infection, according to analysis of a European registry.
In their analysis, published in the Journal of Clinical Gastroenterology, Olga P. Nyssen, BSc, PhD, of the Autonomous University of Madrid and colleagues discussed seven errors, which included prescribing a triple instead of quadruple regimen, prescribing therapy for too short of a duration, and prescribing a low dose of proton pump inhibitors (PPIs).
“[E]ven after more than 30 years of experience in H. pylori treatment, the ideal regimen to treat this infection remains undefined,” the investigators wrote. The European Registry on Helicobacter pylori management “represents a good mapping overview of the current situation regarding H. pylori management, allowing not only continuous assessment of the integration of clinical recommendations agreed on medical consensus, but also of the possible strategies for improvement.”
Patient data were drawn from registry-participating countries that each had more than 1,000 cases of H. pylori available; most came from Spain, followed by Russia, Italy, Slovenia, and Lithuania. Of these patients, data for 26,340 patients were analyzed, which ultimately represented 80% of the total registry from 2013 to 2019.
The first mistake discussed in the paper regarded use of less-effective triple therapies (typically PPI plus two antibiotics); one review showed that these regimens fail in 20%-40% of cases. Increasing antibiotic resistances have only worsened the success rate. According to this study, a triple regimen was given as first-line treatment in 46% of cases. Overall, frequency of triple-therapy prescriptions decreased from more than 50% in 2013 to about 40% in 2019. More significant improvements in this area were achieved in Spain, where use of triple therapies decreased from 24% in 2014 to 0% in 2019. According to the investigators, this finding serves as a “paradigmatic example of improvement with time.”
The authors pointed out that “overwhelming evidence” supports 14-day treatment; however, 69% of triple-therapy durations and 58% of quadruple therapy cases were for 7 or 10 days. Triple therapy at this duration showed only 81% cure rate, while it was 88% with 14 days, and quadruple therapy was only 80% effective at 7-10 days but 90% effective at 14 days.
“Fortunately,” the investigators wrote, “this mistake was progressively found less frequently and, at present, the prescription of 7-day standard triple therapy regimens has almost disappeared.”
The authors noted acid suppression via PPIs improves cure rates: In one meta-analysis, the cure rate of triple therapy regimens increased by 6%-10% with high doses of PPIs. However, the current study found that 48% of triple therapies included low-dose PPIs. This number decreased over time, the authors noted: from 67% in 2013 to 20% in 2019.
“From another perspective, the daily PPI dose has increased from a dose equivalent to 54 mg of omeprazole in 2013 to 104 mg in 2019,” they wrote.
The other four errors they discussed were failing to adequately consider penicillin allergies in prescription choices, failing to consider the importance of treatment compliance, repeating certain antibiotics after failures, and not checking eradication success after treatment.
Based on these findings, Dr. Nyssen and colleagues suggested that “penetration of recommendations in the participating European countries is still poor and delayed, even though some improvements from guidelines have been partially incorporated.”
According to Grigorios I. Leontiadis, MD, PhD, of McMaster University, Hamilton, Ont., who coauthored the 2017 American College of Gastroenterology H. pylori management guidelines and the Canadian Association of Gastroenterology “Toronto Consensus” in 2016, “This study is important and timely given the steadily increasing antibiotic resistance of H. pylori worldwide.”
Although Dr. Leontiadias described the results as “suboptimal,” he was partially reassured by the improvements over time, “especially following publication of the 2016 European clinical practice guidelines.” He also noted that some older clinical practice guidelines issued conditional recommendations, which could “justify the lower adherence seen in the early period of this study.”
“The unanswered question,” Dr. Leontiadias went on, “is whether the practice of gastroenterologists who volunteered to participate in this prospective registry is truly representative of how H. pylori is managed in Europe. Most likely it isn’t. Nonparticipating gastroenterologists and nongastroenterologist health care practitioners are probably less aware of and less adherent to clinical practice guidelines. This means that the actual situation in the real world is probably grimmer than what this study shows.”
William D. Chey, MD, Nostrant Collegiate Professor of Gastroenterology at the University of Michigan, Ann Arbor, considered the results “not entirely surprising, but nonetheless, noteworthy.”
Dr. Chey noted that the United States lacks a similar registry to compare real-world H. pylori management; even so, he suggested several findings that “bear reiteration” for clinicians in the United States.
“U.S. providers should consider regimens other than clarithromycin triple therapy when treating H. pylori infection,” Dr. Chey said. “Since U.S. providers do not have reliable data on H. pylori antimicrobial resistance, it is useful to ask about prior macrolide antibiotic exposure, and if a patient has received a macrolide for any reason, clarithromycin triple therapy should be avoided. Bismuth quadruple therapy remains a reliable first-line treatment option in the U.S. Another recently approved first-line treatment option is the combination of a proton pump inhibitor, rifabutin, and amoxicillin. Treatment regimens in the U.S. should be given for a minimum of 10 days and, preferably, for 14 days. Another point made by the article is that providers should be maximizing gastric acid suppression by using higher doses of proton pump inhibitors when treating H. pylori.”
Dr. Chey also noted an emerging treatment option that could soon be available. “Results from phase 3 trials in North America and Europe with the potassium-competitive acid blocker vonoprazan combined with amoxicillin, with and without clarithromycin, are expected in 2021 and may provide another novel first-line treatment option.”
Dr. Nyssen and colleagues disclosed relationships with Allergan, Mayoly, Janssen, and others. Dr. Chey is a consultant for Redhill, Phathom, and Takeda, which is developing vonoprazan. Dr. Leontiadias disclosed no conflicts of interest.
This article was updated 2/16/21.
Many patients are receiving inadequate eradication therapy for Helicobacter pylori infection, according to analysis of a European registry.
In their analysis, published in the Journal of Clinical Gastroenterology, Olga P. Nyssen, BSc, PhD, of the Autonomous University of Madrid and colleagues discussed seven errors, which included prescribing a triple instead of quadruple regimen, prescribing therapy for too short of a duration, and prescribing a low dose of proton pump inhibitors (PPIs).
“[E]ven after more than 30 years of experience in H. pylori treatment, the ideal regimen to treat this infection remains undefined,” the investigators wrote. The European Registry on Helicobacter pylori management “represents a good mapping overview of the current situation regarding H. pylori management, allowing not only continuous assessment of the integration of clinical recommendations agreed on medical consensus, but also of the possible strategies for improvement.”
Patient data were drawn from registry-participating countries that each had more than 1,000 cases of H. pylori available; most came from Spain, followed by Russia, Italy, Slovenia, and Lithuania. Of these patients, data for 26,340 patients were analyzed, which ultimately represented 80% of the total registry from 2013 to 2019.
The first mistake discussed in the paper regarded use of less-effective triple therapies (typically PPI plus two antibiotics); one review showed that these regimens fail in 20%-40% of cases. Increasing antibiotic resistances have only worsened the success rate. According to this study, a triple regimen was given as first-line treatment in 46% of cases. Overall, frequency of triple-therapy prescriptions decreased from more than 50% in 2013 to about 40% in 2019. More significant improvements in this area were achieved in Spain, where use of triple therapies decreased from 24% in 2014 to 0% in 2019. According to the investigators, this finding serves as a “paradigmatic example of improvement with time.”
The authors pointed out that “overwhelming evidence” supports 14-day treatment; however, 69% of triple-therapy durations and 58% of quadruple therapy cases were for 7 or 10 days. Triple therapy at this duration showed only 81% cure rate, while it was 88% with 14 days, and quadruple therapy was only 80% effective at 7-10 days but 90% effective at 14 days.
“Fortunately,” the investigators wrote, “this mistake was progressively found less frequently and, at present, the prescription of 7-day standard triple therapy regimens has almost disappeared.”
The authors noted acid suppression via PPIs improves cure rates: In one meta-analysis, the cure rate of triple therapy regimens increased by 6%-10% with high doses of PPIs. However, the current study found that 48% of triple therapies included low-dose PPIs. This number decreased over time, the authors noted: from 67% in 2013 to 20% in 2019.
“From another perspective, the daily PPI dose has increased from a dose equivalent to 54 mg of omeprazole in 2013 to 104 mg in 2019,” they wrote.
The other four errors they discussed were failing to adequately consider penicillin allergies in prescription choices, failing to consider the importance of treatment compliance, repeating certain antibiotics after failures, and not checking eradication success after treatment.
Based on these findings, Dr. Nyssen and colleagues suggested that “penetration of recommendations in the participating European countries is still poor and delayed, even though some improvements from guidelines have been partially incorporated.”
According to Grigorios I. Leontiadis, MD, PhD, of McMaster University, Hamilton, Ont., who coauthored the 2017 American College of Gastroenterology H. pylori management guidelines and the Canadian Association of Gastroenterology “Toronto Consensus” in 2016, “This study is important and timely given the steadily increasing antibiotic resistance of H. pylori worldwide.”
Although Dr. Leontiadias described the results as “suboptimal,” he was partially reassured by the improvements over time, “especially following publication of the 2016 European clinical practice guidelines.” He also noted that some older clinical practice guidelines issued conditional recommendations, which could “justify the lower adherence seen in the early period of this study.”
“The unanswered question,” Dr. Leontiadias went on, “is whether the practice of gastroenterologists who volunteered to participate in this prospective registry is truly representative of how H. pylori is managed in Europe. Most likely it isn’t. Nonparticipating gastroenterologists and nongastroenterologist health care practitioners are probably less aware of and less adherent to clinical practice guidelines. This means that the actual situation in the real world is probably grimmer than what this study shows.”
William D. Chey, MD, Nostrant Collegiate Professor of Gastroenterology at the University of Michigan, Ann Arbor, considered the results “not entirely surprising, but nonetheless, noteworthy.”
Dr. Chey noted that the United States lacks a similar registry to compare real-world H. pylori management; even so, he suggested several findings that “bear reiteration” for clinicians in the United States.
“U.S. providers should consider regimens other than clarithromycin triple therapy when treating H. pylori infection,” Dr. Chey said. “Since U.S. providers do not have reliable data on H. pylori antimicrobial resistance, it is useful to ask about prior macrolide antibiotic exposure, and if a patient has received a macrolide for any reason, clarithromycin triple therapy should be avoided. Bismuth quadruple therapy remains a reliable first-line treatment option in the U.S. Another recently approved first-line treatment option is the combination of a proton pump inhibitor, rifabutin, and amoxicillin. Treatment regimens in the U.S. should be given for a minimum of 10 days and, preferably, for 14 days. Another point made by the article is that providers should be maximizing gastric acid suppression by using higher doses of proton pump inhibitors when treating H. pylori.”
Dr. Chey also noted an emerging treatment option that could soon be available. “Results from phase 3 trials in North America and Europe with the potassium-competitive acid blocker vonoprazan combined with amoxicillin, with and without clarithromycin, are expected in 2021 and may provide another novel first-line treatment option.”
Dr. Nyssen and colleagues disclosed relationships with Allergan, Mayoly, Janssen, and others. Dr. Chey is a consultant for Redhill, Phathom, and Takeda, which is developing vonoprazan. Dr. Leontiadias disclosed no conflicts of interest.
This article was updated 2/16/21.
FROM THE JOURNAL OF CLINICAL GASTROENTEROLOGY
Limiting antibiotic therapy after surgical drainage for native joint bacterial arthritis
Background: Currently the recommended duration of antibiotic therapy for native joint bacterial arthritis is 3-6 weeks based on expert opinion.
Study design: Prospective, unblinded, randomized, noninferiority.
Setting: Single center in Geneva.
Synopsis: In total, 154 patients were randomized to either 2 weeks or 4 weeks of antibiotic regimen selected in consultation with infectious disease specialists after surgical drainage of native joint bacterial arthritis.
The study population was 38% women with a median age of 51 years. Sites of infection were majority hand and wrist arthritis (64%). The most frequent pathogen was Staphylococcus aureus (31%) with no methicillin-resistant strains. There was a low incidence of patients with bacteremia (4%) and chronic immune compromise (10%). Antibiotic regimen varied with 13 different initial intravenous regimens and 11 different oral regimens.
The primary study outcome was rate of recurrent infection within 2 years, which was low with only one recurrence in the 2-week arm and two recurrences in the 4-week arm. This difference was well within the 10% noninferiority margin selected by the authors.
The study was underpowered for nonhand and nonwrist cases, limiting generalizability.
Bottom line: Consider a shorter duration of antibiotic therapy after surgical drainage for native joint bacterial arthritis of the hand and wrist in an otherwise healthy patient.
Citation: Gjika E et al. Two weeks versus four weeks of antibiotic therapy after surgical drainage for native joint bacterial arthritis: a prospective, randomized, non-inferiority trial. Ann Rheum Dis. 2019 Aug;78(8):1114-21.
Dr. Zarookian is a hospitalist at Maine Medical Center in Portland and Stephens Memorial Hospital in Norway, Maine.
Background: Currently the recommended duration of antibiotic therapy for native joint bacterial arthritis is 3-6 weeks based on expert opinion.
Study design: Prospective, unblinded, randomized, noninferiority.
Setting: Single center in Geneva.
Synopsis: In total, 154 patients were randomized to either 2 weeks or 4 weeks of antibiotic regimen selected in consultation with infectious disease specialists after surgical drainage of native joint bacterial arthritis.
The study population was 38% women with a median age of 51 years. Sites of infection were majority hand and wrist arthritis (64%). The most frequent pathogen was Staphylococcus aureus (31%) with no methicillin-resistant strains. There was a low incidence of patients with bacteremia (4%) and chronic immune compromise (10%). Antibiotic regimen varied with 13 different initial intravenous regimens and 11 different oral regimens.
The primary study outcome was rate of recurrent infection within 2 years, which was low with only one recurrence in the 2-week arm and two recurrences in the 4-week arm. This difference was well within the 10% noninferiority margin selected by the authors.
The study was underpowered for nonhand and nonwrist cases, limiting generalizability.
Bottom line: Consider a shorter duration of antibiotic therapy after surgical drainage for native joint bacterial arthritis of the hand and wrist in an otherwise healthy patient.
Citation: Gjika E et al. Two weeks versus four weeks of antibiotic therapy after surgical drainage for native joint bacterial arthritis: a prospective, randomized, non-inferiority trial. Ann Rheum Dis. 2019 Aug;78(8):1114-21.
Dr. Zarookian is a hospitalist at Maine Medical Center in Portland and Stephens Memorial Hospital in Norway, Maine.
Background: Currently the recommended duration of antibiotic therapy for native joint bacterial arthritis is 3-6 weeks based on expert opinion.
Study design: Prospective, unblinded, randomized, noninferiority.
Setting: Single center in Geneva.
Synopsis: In total, 154 patients were randomized to either 2 weeks or 4 weeks of antibiotic regimen selected in consultation with infectious disease specialists after surgical drainage of native joint bacterial arthritis.
The study population was 38% women with a median age of 51 years. Sites of infection were majority hand and wrist arthritis (64%). The most frequent pathogen was Staphylococcus aureus (31%) with no methicillin-resistant strains. There was a low incidence of patients with bacteremia (4%) and chronic immune compromise (10%). Antibiotic regimen varied with 13 different initial intravenous regimens and 11 different oral regimens.
The primary study outcome was rate of recurrent infection within 2 years, which was low with only one recurrence in the 2-week arm and two recurrences in the 4-week arm. This difference was well within the 10% noninferiority margin selected by the authors.
The study was underpowered for nonhand and nonwrist cases, limiting generalizability.
Bottom line: Consider a shorter duration of antibiotic therapy after surgical drainage for native joint bacterial arthritis of the hand and wrist in an otherwise healthy patient.
Citation: Gjika E et al. Two weeks versus four weeks of antibiotic therapy after surgical drainage for native joint bacterial arthritis: a prospective, randomized, non-inferiority trial. Ann Rheum Dis. 2019 Aug;78(8):1114-21.
Dr. Zarookian is a hospitalist at Maine Medical Center in Portland and Stephens Memorial Hospital in Norway, Maine.
Global Ebola vaccine stockpile established
The International Coordinating Group (ICG) on Vaccine Provision announced the establishment of a global Ebola vaccine stockpile initiative.
The ICG, which was established in 1997, is made up of the World Health Organization, the United Nations Children’s Fund, the International Federation of Red Cross and Red Crescent Societies, and Médecins Sans Frontières.
The stockpile was created in order to make the single-dose Ebola vaccine (rVSV∆G-ZEBOV-GP, live; trade name Everbo) rapidly available at the start of the next Ebola outbreak anywhere in the world. The vaccine was developed and is marketed by Merck Sharp & Dohme, with financial support from the United States.
The stockpile, which is maintained in Switzerland and managed by UNICEF, is designed to be readily deployed to other countries whenever there is an outbreak. The ICG will be the decision-making body for the vaccine’s allocation and release, as is also the case with previously created stockpiles of cholera, meningitis, and yellow fever vaccines.
“The decision to allocate the vaccine will be made within 48 hours of receiving a request from a country; vaccines will be made available together with ultra-cold chain packaging by the manufacturer for shipment to countries within 48 hours of the decision. The targeted overall delivery time from the stockpile to countries is 7 days,” according to the WHO press release.
Currently 6,890 doses are available for outbreak response, with further quantities to be delivered into the stockpile throughout 2021 and beyond. Initial use of the vaccine will be directed to health care and frontline workers. It is expected that it will take 2-3 years to reach the Strategic Advisory Group of Experts on Immunization–recommended level of 500,000 doses for the stockpile of Ebola vaccines.
The International Coordinating Group (ICG) on Vaccine Provision announced the establishment of a global Ebola vaccine stockpile initiative.
The ICG, which was established in 1997, is made up of the World Health Organization, the United Nations Children’s Fund, the International Federation of Red Cross and Red Crescent Societies, and Médecins Sans Frontières.
The stockpile was created in order to make the single-dose Ebola vaccine (rVSV∆G-ZEBOV-GP, live; trade name Everbo) rapidly available at the start of the next Ebola outbreak anywhere in the world. The vaccine was developed and is marketed by Merck Sharp & Dohme, with financial support from the United States.
The stockpile, which is maintained in Switzerland and managed by UNICEF, is designed to be readily deployed to other countries whenever there is an outbreak. The ICG will be the decision-making body for the vaccine’s allocation and release, as is also the case with previously created stockpiles of cholera, meningitis, and yellow fever vaccines.
“The decision to allocate the vaccine will be made within 48 hours of receiving a request from a country; vaccines will be made available together with ultra-cold chain packaging by the manufacturer for shipment to countries within 48 hours of the decision. The targeted overall delivery time from the stockpile to countries is 7 days,” according to the WHO press release.
Currently 6,890 doses are available for outbreak response, with further quantities to be delivered into the stockpile throughout 2021 and beyond. Initial use of the vaccine will be directed to health care and frontline workers. It is expected that it will take 2-3 years to reach the Strategic Advisory Group of Experts on Immunization–recommended level of 500,000 doses for the stockpile of Ebola vaccines.
The International Coordinating Group (ICG) on Vaccine Provision announced the establishment of a global Ebola vaccine stockpile initiative.
The ICG, which was established in 1997, is made up of the World Health Organization, the United Nations Children’s Fund, the International Federation of Red Cross and Red Crescent Societies, and Médecins Sans Frontières.
The stockpile was created in order to make the single-dose Ebola vaccine (rVSV∆G-ZEBOV-GP, live; trade name Everbo) rapidly available at the start of the next Ebola outbreak anywhere in the world. The vaccine was developed and is marketed by Merck Sharp & Dohme, with financial support from the United States.
The stockpile, which is maintained in Switzerland and managed by UNICEF, is designed to be readily deployed to other countries whenever there is an outbreak. The ICG will be the decision-making body for the vaccine’s allocation and release, as is also the case with previously created stockpiles of cholera, meningitis, and yellow fever vaccines.
“The decision to allocate the vaccine will be made within 48 hours of receiving a request from a country; vaccines will be made available together with ultra-cold chain packaging by the manufacturer for shipment to countries within 48 hours of the decision. The targeted overall delivery time from the stockpile to countries is 7 days,” according to the WHO press release.
Currently 6,890 doses are available for outbreak response, with further quantities to be delivered into the stockpile throughout 2021 and beyond. Initial use of the vaccine will be directed to health care and frontline workers. It is expected that it will take 2-3 years to reach the Strategic Advisory Group of Experts on Immunization–recommended level of 500,000 doses for the stockpile of Ebola vaccines.
Many EM docs have treated COVID-19 patients without proper PPE: Survey
Many emergency medicine (EM) physicians who responded to a Medscape survey said they have treated COVID-19 patients without appropriate personal protective equipment (PPE).
In the Medscape Emergency Medicine Physicians’ COVID-19 Experience Report, 21% of respondents said that that was sometimes the case; 7% said that it was often the case; and 1% said they always treat patients without appropriate PPE.
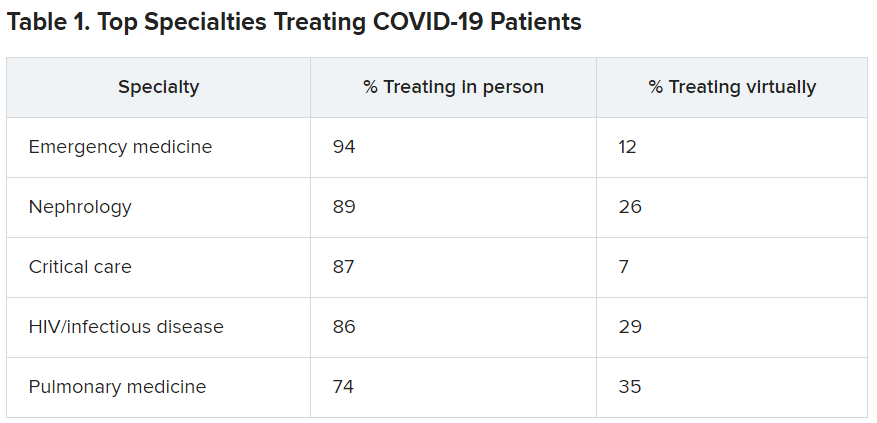
EM physicians were the physicians most likely to treat COVID-19 patients in person.
For comparison, among family medicine physicians, 58% said that they have treated COVID-19 patients in person, and 45% said they were treating them via telemedicine.
Data for the report were gathered from June 9 to July 20 as part of Medscape’s COVID-19 experience survey for all physicians. That survey drew more than 5,000 responses.
Nearly all (98%) of EM physicians who have treated COVID-19 patients said that they have done so since the beginning, when the World Health Organization declared a pandemic on March 11, 2020. For all U.S. physicians, the percentage was much higher than that – 73% said they had treated COVID-19 patients from the start.
EM physicians have often found themselves sacrificing their own safety for the sake of patients. More than half of EM physicians (54%) said that they had knowingly taken personal safety risks to treat a COVID-19 emergency, a percentage far higher than the 30% of all physicians who said they had done so.
Four percent of EM physicians have received a positive diagnosis of COVID-19 via testing. An additional 2% have been confirmed as having COVID on the basis of symptoms.
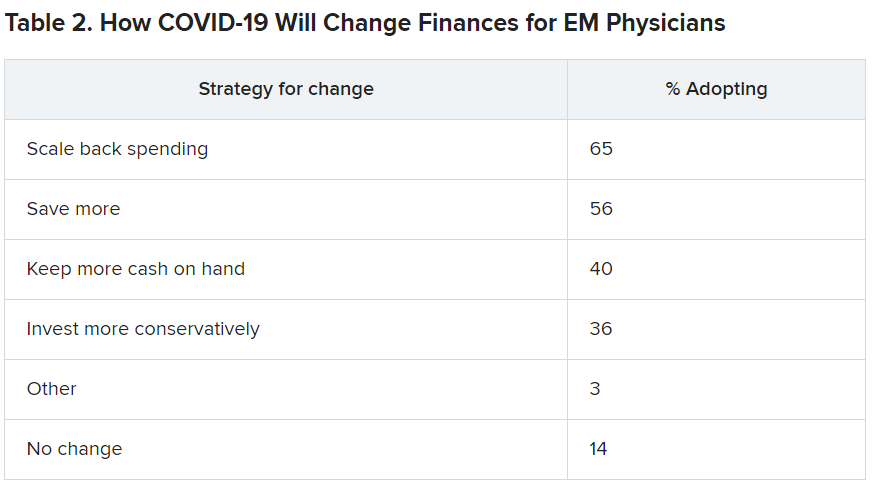
Steep income drops
Survey authors wrote that two-thirds of EM physicians have experienced income loss during the pandemic. Most (71%) saw their income drop by between 11% and 50%; 11% saw a decrease of more than 50%. Among other specialties, the percentages of those who have experienced a drop of more than 50% are far higher. Among ophthalmologists, 51% said they had experienced such a drop; among allergists, 46%; plastic surgeons, 46%; and otolaryngologists, 45%.
Asked whether their burnout levels have increased in the wake of COVID-19, 74% of EM physicians said burnout had intensified; 23% reported no change; and 3% said burnout had lessened.
Reports of loneliness have been widespread during the pandemic, owing to stay-at-home orders and social distancing. More EM physicians than physicians in general said feelings of loneliness had increased for them in the past year.
More than half of EM doctors (55%) said they are experiencing more loneliness in the pandemic, compared with 46% of all physicians who felt that way; 42% said those feelings have not changed; and 3% said they have been less lonely.
Grief and stress relief
Fewer than half (42%) of the respondents reported that their workplace offers clinician activities to help with grief and stress; 39% said their workplace didn’t offer such help; and 19% said they were unsure.
The percentages were nearly identical to the percentages of physicians overall who answered whether their workplace offered help for grief and stress.
Along with insecurity regarding physical and mental health, COVID-19 has introduced more questions about financial health. Here’s a look at how emergency physicians said they would change the way they save and spend.
Challenges to daily practice
By the time this survey was taken, a large percentage of patients had delayed or avoided urgent or routine medical care for reasons related to COVID-19, so survey authors asked whether EM physicians’ patient population had changed.
Survey authors wrote that “most EM physicians (82%) are seeing patients with non-COVID diseases, such as cardiovascular problems or diabetes, who otherwise probably would have sought treatment earlier.”
COVID-19 has also thrown a major obstacle into most EM physicians’ careers by preventing them from doing the job to the best of their ability. That loss is one of the three primary components of burnout.
More than two-thirds (67%) said COVID-19 has hampered their ability to be as good a doctor as they would like.
A version of this article first appeared on Medscape.com.
Many emergency medicine (EM) physicians who responded to a Medscape survey said they have treated COVID-19 patients without appropriate personal protective equipment (PPE).
In the Medscape Emergency Medicine Physicians’ COVID-19 Experience Report, 21% of respondents said that that was sometimes the case; 7% said that it was often the case; and 1% said they always treat patients without appropriate PPE.
EM physicians were the physicians most likely to treat COVID-19 patients in person.
For comparison, among family medicine physicians, 58% said that they have treated COVID-19 patients in person, and 45% said they were treating them via telemedicine.
Data for the report were gathered from June 9 to July 20 as part of Medscape’s COVID-19 experience survey for all physicians. That survey drew more than 5,000 responses.
Nearly all (98%) of EM physicians who have treated COVID-19 patients said that they have done so since the beginning, when the World Health Organization declared a pandemic on March 11, 2020. For all U.S. physicians, the percentage was much higher than that – 73% said they had treated COVID-19 patients from the start.
EM physicians have often found themselves sacrificing their own safety for the sake of patients. More than half of EM physicians (54%) said that they had knowingly taken personal safety risks to treat a COVID-19 emergency, a percentage far higher than the 30% of all physicians who said they had done so.
Four percent of EM physicians have received a positive diagnosis of COVID-19 via testing. An additional 2% have been confirmed as having COVID on the basis of symptoms.
Steep income drops
Survey authors wrote that two-thirds of EM physicians have experienced income loss during the pandemic. Most (71%) saw their income drop by between 11% and 50%; 11% saw a decrease of more than 50%. Among other specialties, the percentages of those who have experienced a drop of more than 50% are far higher. Among ophthalmologists, 51% said they had experienced such a drop; among allergists, 46%; plastic surgeons, 46%; and otolaryngologists, 45%.
Asked whether their burnout levels have increased in the wake of COVID-19, 74% of EM physicians said burnout had intensified; 23% reported no change; and 3% said burnout had lessened.
Reports of loneliness have been widespread during the pandemic, owing to stay-at-home orders and social distancing. More EM physicians than physicians in general said feelings of loneliness had increased for them in the past year.
More than half of EM doctors (55%) said they are experiencing more loneliness in the pandemic, compared with 46% of all physicians who felt that way; 42% said those feelings have not changed; and 3% said they have been less lonely.
Grief and stress relief
Fewer than half (42%) of the respondents reported that their workplace offers clinician activities to help with grief and stress; 39% said their workplace didn’t offer such help; and 19% said they were unsure.
The percentages were nearly identical to the percentages of physicians overall who answered whether their workplace offered help for grief and stress.
Along with insecurity regarding physical and mental health, COVID-19 has introduced more questions about financial health. Here’s a look at how emergency physicians said they would change the way they save and spend.
Challenges to daily practice
By the time this survey was taken, a large percentage of patients had delayed or avoided urgent or routine medical care for reasons related to COVID-19, so survey authors asked whether EM physicians’ patient population had changed.
Survey authors wrote that “most EM physicians (82%) are seeing patients with non-COVID diseases, such as cardiovascular problems or diabetes, who otherwise probably would have sought treatment earlier.”
COVID-19 has also thrown a major obstacle into most EM physicians’ careers by preventing them from doing the job to the best of their ability. That loss is one of the three primary components of burnout.
More than two-thirds (67%) said COVID-19 has hampered their ability to be as good a doctor as they would like.
A version of this article first appeared on Medscape.com.
Many emergency medicine (EM) physicians who responded to a Medscape survey said they have treated COVID-19 patients without appropriate personal protective equipment (PPE).
In the Medscape Emergency Medicine Physicians’ COVID-19 Experience Report, 21% of respondents said that that was sometimes the case; 7% said that it was often the case; and 1% said they always treat patients without appropriate PPE.
EM physicians were the physicians most likely to treat COVID-19 patients in person.
For comparison, among family medicine physicians, 58% said that they have treated COVID-19 patients in person, and 45% said they were treating them via telemedicine.
Data for the report were gathered from June 9 to July 20 as part of Medscape’s COVID-19 experience survey for all physicians. That survey drew more than 5,000 responses.
Nearly all (98%) of EM physicians who have treated COVID-19 patients said that they have done so since the beginning, when the World Health Organization declared a pandemic on March 11, 2020. For all U.S. physicians, the percentage was much higher than that – 73% said they had treated COVID-19 patients from the start.
EM physicians have often found themselves sacrificing their own safety for the sake of patients. More than half of EM physicians (54%) said that they had knowingly taken personal safety risks to treat a COVID-19 emergency, a percentage far higher than the 30% of all physicians who said they had done so.
Four percent of EM physicians have received a positive diagnosis of COVID-19 via testing. An additional 2% have been confirmed as having COVID on the basis of symptoms.
Steep income drops
Survey authors wrote that two-thirds of EM physicians have experienced income loss during the pandemic. Most (71%) saw their income drop by between 11% and 50%; 11% saw a decrease of more than 50%. Among other specialties, the percentages of those who have experienced a drop of more than 50% are far higher. Among ophthalmologists, 51% said they had experienced such a drop; among allergists, 46%; plastic surgeons, 46%; and otolaryngologists, 45%.
Asked whether their burnout levels have increased in the wake of COVID-19, 74% of EM physicians said burnout had intensified; 23% reported no change; and 3% said burnout had lessened.
Reports of loneliness have been widespread during the pandemic, owing to stay-at-home orders and social distancing. More EM physicians than physicians in general said feelings of loneliness had increased for them in the past year.
More than half of EM doctors (55%) said they are experiencing more loneliness in the pandemic, compared with 46% of all physicians who felt that way; 42% said those feelings have not changed; and 3% said they have been less lonely.
Grief and stress relief
Fewer than half (42%) of the respondents reported that their workplace offers clinician activities to help with grief and stress; 39% said their workplace didn’t offer such help; and 19% said they were unsure.
The percentages were nearly identical to the percentages of physicians overall who answered whether their workplace offered help for grief and stress.
Along with insecurity regarding physical and mental health, COVID-19 has introduced more questions about financial health. Here’s a look at how emergency physicians said they would change the way they save and spend.
Challenges to daily practice
By the time this survey was taken, a large percentage of patients had delayed or avoided urgent or routine medical care for reasons related to COVID-19, so survey authors asked whether EM physicians’ patient population had changed.
Survey authors wrote that “most EM physicians (82%) are seeing patients with non-COVID diseases, such as cardiovascular problems or diabetes, who otherwise probably would have sought treatment earlier.”
COVID-19 has also thrown a major obstacle into most EM physicians’ careers by preventing them from doing the job to the best of their ability. That loss is one of the three primary components of burnout.
More than two-thirds (67%) said COVID-19 has hampered their ability to be as good a doctor as they would like.
A version of this article first appeared on Medscape.com.
Further warning on SGLT2 inhibitor use and DKA risk in COVID-19
a new case series suggests.
Five patients with type 2 diabetes who were taking SGLT2 inhibitors presented in DKA despite having glucose levels below 300 mg/dL. The report was published online last month in AACE Clinical Case Reports by Rebecca J. Vitale, MD, and colleagues at Brigham and Women’s Hospital, Boston.
“A cluster of euglycemic DKA cases at our hospital during the first wave of the pandemic suggests that patients with diabetes taking SGLT2 inhibitors may be at enhanced risk for euDKA when they contract COVID-19,” senior author Naomi D.L. Fisher, MD, said in an interview.
Dr. Fisher, an endocrinologist, added: “This complication is preventable with the simple measure of holding the drug. We are hopeful that widespread patient and physician education will prevent future cases of euDKA as COVID-19 infections continue to surge.”
These cases underscore recommendations published early in the COVID-19 pandemic by an international panel, she noted.
“Patients who are acutely ill with nausea, vomiting, abdominal pain, or diarrhea, or who are experiencing loss of appetite with reduced food and fluid intake, should be advised to hold their SGLT2 inhibitor. This medication should not be resumed until patients are feeling better and eating and drinking normally.”
On the other hand, “If patients with asymptomatic or mild COVID-19 infection are otherwise well, and are eating and drinking normally, there is no evidence that SGLT2 inhibitors need to be stopped. These patients should monitor [themselves] closely for worsening symptoms, especially resulting in poor hydration and nutrition, which would be reason to discontinue their medication.”
Pay special attention to the elderly, those with complications
However, special consideration should be given to elderly patients and those with medical conditions known to increase the likelihood of severe infection, like heart failure and chronic obstructive pulmonary disease, Dr. Fisher added.
The SGLT2 inhibitor class of drugs causes significant urinary glucose excretion, and they are also diuretics. A decrease in available glucose and volume depletion are probably both important contributors to euDKA, she explained.
With COVID-19 infection the euDKA risk is compounded by several mechanisms. Most cases of euDKA are associated with an underlying state of starvation that can be triggered by vomiting, diarrhea, loss of appetite, and poor oral intake.
In addition – although not yet known for certain – SARS-CoV-2 may also be toxic to pancreatic beta cells and thus reduce insulin secretion. The maladaptive inflammatory response seen with COVID-19 may also contribute, she said.
The patients in the current case series were three men and two women seen between March and May 2020. They ranged in age from 52 to 79 years.
None had a prior history of DKA or any known diabetes complications. In all of them, antihyperglycemic medications, including SGLT2 inhibitors, were stopped on hospital admission. The patients were initially treated with intravenous insulin, and then subcutaneous insulin after the DKA diagnosis.
Three of the patients were discharged to rehabilitation facilities on hospital days 28-47 and one (age 53 years) was discharged home on day 11. The other patient also had hypertension and nonalcoholic steatohepatitis.
A version of this article first appeared on Medscape.com.
a new case series suggests.
Five patients with type 2 diabetes who were taking SGLT2 inhibitors presented in DKA despite having glucose levels below 300 mg/dL. The report was published online last month in AACE Clinical Case Reports by Rebecca J. Vitale, MD, and colleagues at Brigham and Women’s Hospital, Boston.
“A cluster of euglycemic DKA cases at our hospital during the first wave of the pandemic suggests that patients with diabetes taking SGLT2 inhibitors may be at enhanced risk for euDKA when they contract COVID-19,” senior author Naomi D.L. Fisher, MD, said in an interview.
Dr. Fisher, an endocrinologist, added: “This complication is preventable with the simple measure of holding the drug. We are hopeful that widespread patient and physician education will prevent future cases of euDKA as COVID-19 infections continue to surge.”
These cases underscore recommendations published early in the COVID-19 pandemic by an international panel, she noted.
“Patients who are acutely ill with nausea, vomiting, abdominal pain, or diarrhea, or who are experiencing loss of appetite with reduced food and fluid intake, should be advised to hold their SGLT2 inhibitor. This medication should not be resumed until patients are feeling better and eating and drinking normally.”
On the other hand, “If patients with asymptomatic or mild COVID-19 infection are otherwise well, and are eating and drinking normally, there is no evidence that SGLT2 inhibitors need to be stopped. These patients should monitor [themselves] closely for worsening symptoms, especially resulting in poor hydration and nutrition, which would be reason to discontinue their medication.”
Pay special attention to the elderly, those with complications
However, special consideration should be given to elderly patients and those with medical conditions known to increase the likelihood of severe infection, like heart failure and chronic obstructive pulmonary disease, Dr. Fisher added.
The SGLT2 inhibitor class of drugs causes significant urinary glucose excretion, and they are also diuretics. A decrease in available glucose and volume depletion are probably both important contributors to euDKA, she explained.
With COVID-19 infection the euDKA risk is compounded by several mechanisms. Most cases of euDKA are associated with an underlying state of starvation that can be triggered by vomiting, diarrhea, loss of appetite, and poor oral intake.
In addition – although not yet known for certain – SARS-CoV-2 may also be toxic to pancreatic beta cells and thus reduce insulin secretion. The maladaptive inflammatory response seen with COVID-19 may also contribute, she said.
The patients in the current case series were three men and two women seen between March and May 2020. They ranged in age from 52 to 79 years.
None had a prior history of DKA or any known diabetes complications. In all of them, antihyperglycemic medications, including SGLT2 inhibitors, were stopped on hospital admission. The patients were initially treated with intravenous insulin, and then subcutaneous insulin after the DKA diagnosis.
Three of the patients were discharged to rehabilitation facilities on hospital days 28-47 and one (age 53 years) was discharged home on day 11. The other patient also had hypertension and nonalcoholic steatohepatitis.
A version of this article first appeared on Medscape.com.
a new case series suggests.
Five patients with type 2 diabetes who were taking SGLT2 inhibitors presented in DKA despite having glucose levels below 300 mg/dL. The report was published online last month in AACE Clinical Case Reports by Rebecca J. Vitale, MD, and colleagues at Brigham and Women’s Hospital, Boston.
“A cluster of euglycemic DKA cases at our hospital during the first wave of the pandemic suggests that patients with diabetes taking SGLT2 inhibitors may be at enhanced risk for euDKA when they contract COVID-19,” senior author Naomi D.L. Fisher, MD, said in an interview.
Dr. Fisher, an endocrinologist, added: “This complication is preventable with the simple measure of holding the drug. We are hopeful that widespread patient and physician education will prevent future cases of euDKA as COVID-19 infections continue to surge.”
These cases underscore recommendations published early in the COVID-19 pandemic by an international panel, she noted.
“Patients who are acutely ill with nausea, vomiting, abdominal pain, or diarrhea, or who are experiencing loss of appetite with reduced food and fluid intake, should be advised to hold their SGLT2 inhibitor. This medication should not be resumed until patients are feeling better and eating and drinking normally.”
On the other hand, “If patients with asymptomatic or mild COVID-19 infection are otherwise well, and are eating and drinking normally, there is no evidence that SGLT2 inhibitors need to be stopped. These patients should monitor [themselves] closely for worsening symptoms, especially resulting in poor hydration and nutrition, which would be reason to discontinue their medication.”
Pay special attention to the elderly, those with complications
However, special consideration should be given to elderly patients and those with medical conditions known to increase the likelihood of severe infection, like heart failure and chronic obstructive pulmonary disease, Dr. Fisher added.
The SGLT2 inhibitor class of drugs causes significant urinary glucose excretion, and they are also diuretics. A decrease in available glucose and volume depletion are probably both important contributors to euDKA, she explained.
With COVID-19 infection the euDKA risk is compounded by several mechanisms. Most cases of euDKA are associated with an underlying state of starvation that can be triggered by vomiting, diarrhea, loss of appetite, and poor oral intake.
In addition – although not yet known for certain – SARS-CoV-2 may also be toxic to pancreatic beta cells and thus reduce insulin secretion. The maladaptive inflammatory response seen with COVID-19 may also contribute, she said.
The patients in the current case series were three men and two women seen between March and May 2020. They ranged in age from 52 to 79 years.
None had a prior history of DKA or any known diabetes complications. In all of them, antihyperglycemic medications, including SGLT2 inhibitors, were stopped on hospital admission. The patients were initially treated with intravenous insulin, and then subcutaneous insulin after the DKA diagnosis.
Three of the patients were discharged to rehabilitation facilities on hospital days 28-47 and one (age 53 years) was discharged home on day 11. The other patient also had hypertension and nonalcoholic steatohepatitis.
A version of this article first appeared on Medscape.com.
COVID-19 in children: Latest weekly increase is largest yet
according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.
There were 211,466 new cases reported in children during the week of Jan. 8-14, topping the previous high (Dec. 11-17) by almost 30,000. Those new cases bring the total for the pandemic to over 2.5 million children infected with the coronavirus, which represents 12.6% of all reported cases, the AAP and the CHA said Jan. 19 in their weekly COVID-19 report.
The rise in cases also brought an increase in the proportion reported among children. The week before (Jan. 1-7), cases in children were 12.9% of all cases reported, but the most recent week saw that number rise to 14.5% of all cases, the highest it’s been since early October, based on data collected from the health department websites of 49 states (excluding New York), the District of Columbia, New York City, Puerto Rio, and Guam.
The corresponding figures for severe illness continue to be low: Children represent 1.8% of all hospitalizations from COVID-19 in 24 states and New York City and 0.06% of all deaths in 43 states and New York City. Three deaths were reported for the week of Jan. 8-14, making for a total of 191 since the pandemic started, the AAP and CHA said in their report.
Among the states, California has the most overall cases at just over 350,000, Wyoming has the highest proportion of cases in children (20.3%), and North Dakota has the highest rate of infection (over 8,100 per 100,000 children). The infection rate for the nation is now above 3,300 per 100,000 children, and 11 states reported rates over 5,000, according to the AAP and the CHA.
according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.

There were 211,466 new cases reported in children during the week of Jan. 8-14, topping the previous high (Dec. 11-17) by almost 30,000. Those new cases bring the total for the pandemic to over 2.5 million children infected with the coronavirus, which represents 12.6% of all reported cases, the AAP and the CHA said Jan. 19 in their weekly COVID-19 report.
The rise in cases also brought an increase in the proportion reported among children. The week before (Jan. 1-7), cases in children were 12.9% of all cases reported, but the most recent week saw that number rise to 14.5% of all cases, the highest it’s been since early October, based on data collected from the health department websites of 49 states (excluding New York), the District of Columbia, New York City, Puerto Rio, and Guam.
The corresponding figures for severe illness continue to be low: Children represent 1.8% of all hospitalizations from COVID-19 in 24 states and New York City and 0.06% of all deaths in 43 states and New York City. Three deaths were reported for the week of Jan. 8-14, making for a total of 191 since the pandemic started, the AAP and CHA said in their report.
Among the states, California has the most overall cases at just over 350,000, Wyoming has the highest proportion of cases in children (20.3%), and North Dakota has the highest rate of infection (over 8,100 per 100,000 children). The infection rate for the nation is now above 3,300 per 100,000 children, and 11 states reported rates over 5,000, according to the AAP and the CHA.
according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.

There were 211,466 new cases reported in children during the week of Jan. 8-14, topping the previous high (Dec. 11-17) by almost 30,000. Those new cases bring the total for the pandemic to over 2.5 million children infected with the coronavirus, which represents 12.6% of all reported cases, the AAP and the CHA said Jan. 19 in their weekly COVID-19 report.
The rise in cases also brought an increase in the proportion reported among children. The week before (Jan. 1-7), cases in children were 12.9% of all cases reported, but the most recent week saw that number rise to 14.5% of all cases, the highest it’s been since early October, based on data collected from the health department websites of 49 states (excluding New York), the District of Columbia, New York City, Puerto Rio, and Guam.
The corresponding figures for severe illness continue to be low: Children represent 1.8% of all hospitalizations from COVID-19 in 24 states and New York City and 0.06% of all deaths in 43 states and New York City. Three deaths were reported for the week of Jan. 8-14, making for a total of 191 since the pandemic started, the AAP and CHA said in their report.
Among the states, California has the most overall cases at just over 350,000, Wyoming has the highest proportion of cases in children (20.3%), and North Dakota has the highest rate of infection (over 8,100 per 100,000 children). The infection rate for the nation is now above 3,300 per 100,000 children, and 11 states reported rates over 5,000, according to the AAP and the CHA.
Moderna needs more kids for COVID vaccine trials
according to the company CEO and a federal official.
The Moderna vaccine was authorized for use in December and is now being given to people 18 and over. But children would receive lower doses, so new clinical trials must be done, Moderna CEO Stephane Bancel said at the JPMorgan virtual Health Care Conference on Monday.
Clinical trials on children 11 and younger “will take much longer, because we have to age deescalate and start at a lower dose. So we should not anticipate clinical data in 2021, but more in 2022,” Ms. Bancel said, according to Business Insider.
Moderna’s clinical trials for 12- to 17-year-olds started 4 weeks ago, but the company is having trouble getting enough participants, said Moncef Slaoui, PhD, the scientific head of Operation Warp Speed, the U.S. government’s vaccine effort. That could delay Food and Drug Administration approval, he said.
“It’s really very important for all of us, for all the population in America, to realize that we can’t have that indication unless adolescents aged 12-18 decide to participate,” Dr. Slaoui said, according to USA Today.
He said the adolescent trials are getting only about 800 volunteers a month, but need at least 3,000 volunteers to complete the study, USA Today reported. Parents interested in having their child participate can check eligibility and sign at this website.
The Pfizer/BioNTech vaccine won authorization for use in 16- to 17-year-olds as well as adults.
The coronavirus doesn’t appear to have as serious complications for children as for adults.
“At this time, it appears that severe illness due to COVID-19 is rare among children,” the American Association of Pediatrics says. “However, there is an urgent need to collect more data on longer-term impacts of the pandemic on children, including ways the virus may harm the long-term physical health of infected children, as well as its emotional and mental health effects.”
The association says 179 children had died of COVID-related reasons in 43 states and New York City as of Dec. 31, 2020. That’s about 0.06% of total COVID deaths, it says.
But children do get sick. As of Jan. 7, 2021, nearly 2.3 million children had tested positive for COVID-19 since the start of the pandemic, the association says.
A version of this article first appeared on WebMD.com.
according to the company CEO and a federal official.
The Moderna vaccine was authorized for use in December and is now being given to people 18 and over. But children would receive lower doses, so new clinical trials must be done, Moderna CEO Stephane Bancel said at the JPMorgan virtual Health Care Conference on Monday.
Clinical trials on children 11 and younger “will take much longer, because we have to age deescalate and start at a lower dose. So we should not anticipate clinical data in 2021, but more in 2022,” Ms. Bancel said, according to Business Insider.
Moderna’s clinical trials for 12- to 17-year-olds started 4 weeks ago, but the company is having trouble getting enough participants, said Moncef Slaoui, PhD, the scientific head of Operation Warp Speed, the U.S. government’s vaccine effort. That could delay Food and Drug Administration approval, he said.
“It’s really very important for all of us, for all the population in America, to realize that we can’t have that indication unless adolescents aged 12-18 decide to participate,” Dr. Slaoui said, according to USA Today.
He said the adolescent trials are getting only about 800 volunteers a month, but need at least 3,000 volunteers to complete the study, USA Today reported. Parents interested in having their child participate can check eligibility and sign at this website.
The Pfizer/BioNTech vaccine won authorization for use in 16- to 17-year-olds as well as adults.
The coronavirus doesn’t appear to have as serious complications for children as for adults.
“At this time, it appears that severe illness due to COVID-19 is rare among children,” the American Association of Pediatrics says. “However, there is an urgent need to collect more data on longer-term impacts of the pandemic on children, including ways the virus may harm the long-term physical health of infected children, as well as its emotional and mental health effects.”
The association says 179 children had died of COVID-related reasons in 43 states and New York City as of Dec. 31, 2020. That’s about 0.06% of total COVID deaths, it says.
But children do get sick. As of Jan. 7, 2021, nearly 2.3 million children had tested positive for COVID-19 since the start of the pandemic, the association says.
A version of this article first appeared on WebMD.com.
according to the company CEO and a federal official.
The Moderna vaccine was authorized for use in December and is now being given to people 18 and over. But children would receive lower doses, so new clinical trials must be done, Moderna CEO Stephane Bancel said at the JPMorgan virtual Health Care Conference on Monday.
Clinical trials on children 11 and younger “will take much longer, because we have to age deescalate and start at a lower dose. So we should not anticipate clinical data in 2021, but more in 2022,” Ms. Bancel said, according to Business Insider.
Moderna’s clinical trials for 12- to 17-year-olds started 4 weeks ago, but the company is having trouble getting enough participants, said Moncef Slaoui, PhD, the scientific head of Operation Warp Speed, the U.S. government’s vaccine effort. That could delay Food and Drug Administration approval, he said.
“It’s really very important for all of us, for all the population in America, to realize that we can’t have that indication unless adolescents aged 12-18 decide to participate,” Dr. Slaoui said, according to USA Today.
He said the adolescent trials are getting only about 800 volunteers a month, but need at least 3,000 volunteers to complete the study, USA Today reported. Parents interested in having their child participate can check eligibility and sign at this website.
The Pfizer/BioNTech vaccine won authorization for use in 16- to 17-year-olds as well as adults.
The coronavirus doesn’t appear to have as serious complications for children as for adults.
“At this time, it appears that severe illness due to COVID-19 is rare among children,” the American Association of Pediatrics says. “However, there is an urgent need to collect more data on longer-term impacts of the pandemic on children, including ways the virus may harm the long-term physical health of infected children, as well as its emotional and mental health effects.”
The association says 179 children had died of COVID-related reasons in 43 states and New York City as of Dec. 31, 2020. That’s about 0.06% of total COVID deaths, it says.
But children do get sick. As of Jan. 7, 2021, nearly 2.3 million children had tested positive for COVID-19 since the start of the pandemic, the association says.
A version of this article first appeared on WebMD.com.