User login
Justices appear split over birth control mandate case
U.S. Supreme Court justices appear divided over whether the Trump administration acted properly when it expanded exemptions under the Affordable Care Act’s contraception mandate.
During oral arguments on May 6, the court expressed differing perspectives about the administration’s authority to allow for more exemptions under the health law’s birth control mandate and whether the expansions were reasonable. Justices heard the consolidated cases – Little Sisters of the Poor v. Pennsylvania and Trump v. Pennsylvania – by teleconference because of the COVID-19 pandemic. They are expected to make a decision by the summer.
Associate justice Ruth Bader Ginsburg, who participated in the telephone conference call from a hospital where she was recovering from a gallbladder condition, said the exemptions ignored the intent of Congress to provide women with comprehensive coverage through the ACA.
“The glaring feature of what the government has done in expanding this exemption is to toss to the winds entirely Congress’s instruction that women need and shall have seamless, no-cost, comprehensive coverage,” she said during oral arguments. “This leaves the women to hunt for other government programs that might cover them, and for those who are not covered by Medicaid or one of the other government programs, they can get contraceptive coverage only from paying out of their own pocket, which is exactly what Congress didn’t want to happen.”
Associate Justice Samuel Alito Jr., meanwhile, indicated that a lower court opinion that had blocked the exemptions from going forward conflicts with the Supreme Court’s ruling in a related case, Burwell v. Hobby Lobby.
“Explain to me why the Third Circuit’s analysis of the question of substantial burden is not squarely inconsistent with our reasoning in Hobby Lobby,” Associate Justice Alito said during oral arguments. “Hobby Lobby held that, if a person sincerely believes that it is immoral to perform an act that has the effect of enabling another person to commit an immoral act, a federal court does not have the right to say that this person is wrong on the question of moral complicity. That’s precisely the situation here. Reading the Third Circuit’s discussion of the substantial burden question, I wondered whether they had read that part of the Hobby Lobby decision.”
The dispute surrounding the ACA’s birth control mandate and the extent of exemptions afforded has gone on for a decade and has led to numerous legal challenges. The ACA initially required all employers to cover birth control for employees with no copayments, but exempted group health plans of religious employers. Those religious employers were primarily churches and other houses of worship. After a number of complaints and lawsuits, the Obama administration created a workaround for nonprofit religious employers not included in that exemption to opt out of the mandate. However, critics argued the process itself was a violation of their religious freedom.
The issue led to the case of Zubik v. Burwell, a legal challenge over the mandate exemption that went before the U.S. Supreme Court in March 2016. The issue was never resolved however, and in May 2016, the Supreme Court vacated the lower court rulings related to Zubik v. Burwell and remanded the case back to the four appeals courts that had originally ruled on the issue.
In 2018, the Trump administration announced new rules aimed at broadening exemptions to the ACA’s contraceptive mandate to entities that object to services covered by the mandate on the basis of “sincerely held religious beliefs.” A second rule allowed nonprofit organizations and small businesses that had nonreligious moral convictions against the mandate to opt out.
Thirteen states and the District of Columbia then sued the Trump administration over the rules, as well as Pennsylvania and New Jersey in a separate case. Little Sisters of the Poor, a religious nonprofit operating a home in Pittsburgh, intervened in the case as an aggrieved party. An appeal court temporarily barred the regulations from moving forward.
During oral arguments, Solicitor General for the Department of Justice Noel J. Francisco said the exemptions are lawful because they are authorized under a provision of the ACA as well as the Religious Freedom Restoration Act (RFRA).
“RFRA at the very least authorizes the religious exemption,” Mr. Francisco said during oral arguments.
Chief Deputy Attorney General for Pennsylvania Michael J. Fischer argued that the Trump administration’s moral and religious exemption rules rest on overly broad assertions of agency authority.
“First, the agencies twist a narrow delegation that allows the Health Resources and Services Administration to decide which preventive services insurers must cover under the Women’s Health Amendment into a grant of authority so broad it allows them to permit virtually any employer or college to opt out of providing contraceptive coverage entirely, including for reasons as amorphous as vaguely defined moral beliefs,” he said during oral arguments. “Second, the agencies claim that RFRA, a statute that limits government action, affirmatively authorizes them to permit employers to deny women their rights to contraceptive coverage even in the absence of a RFRA violation in the first place.”
U.S. Supreme Court justices appear divided over whether the Trump administration acted properly when it expanded exemptions under the Affordable Care Act’s contraception mandate.
During oral arguments on May 6, the court expressed differing perspectives about the administration’s authority to allow for more exemptions under the health law’s birth control mandate and whether the expansions were reasonable. Justices heard the consolidated cases – Little Sisters of the Poor v. Pennsylvania and Trump v. Pennsylvania – by teleconference because of the COVID-19 pandemic. They are expected to make a decision by the summer.
Associate justice Ruth Bader Ginsburg, who participated in the telephone conference call from a hospital where she was recovering from a gallbladder condition, said the exemptions ignored the intent of Congress to provide women with comprehensive coverage through the ACA.
“The glaring feature of what the government has done in expanding this exemption is to toss to the winds entirely Congress’s instruction that women need and shall have seamless, no-cost, comprehensive coverage,” she said during oral arguments. “This leaves the women to hunt for other government programs that might cover them, and for those who are not covered by Medicaid or one of the other government programs, they can get contraceptive coverage only from paying out of their own pocket, which is exactly what Congress didn’t want to happen.”
Associate Justice Samuel Alito Jr., meanwhile, indicated that a lower court opinion that had blocked the exemptions from going forward conflicts with the Supreme Court’s ruling in a related case, Burwell v. Hobby Lobby.
“Explain to me why the Third Circuit’s analysis of the question of substantial burden is not squarely inconsistent with our reasoning in Hobby Lobby,” Associate Justice Alito said during oral arguments. “Hobby Lobby held that, if a person sincerely believes that it is immoral to perform an act that has the effect of enabling another person to commit an immoral act, a federal court does not have the right to say that this person is wrong on the question of moral complicity. That’s precisely the situation here. Reading the Third Circuit’s discussion of the substantial burden question, I wondered whether they had read that part of the Hobby Lobby decision.”
The dispute surrounding the ACA’s birth control mandate and the extent of exemptions afforded has gone on for a decade and has led to numerous legal challenges. The ACA initially required all employers to cover birth control for employees with no copayments, but exempted group health plans of religious employers. Those religious employers were primarily churches and other houses of worship. After a number of complaints and lawsuits, the Obama administration created a workaround for nonprofit religious employers not included in that exemption to opt out of the mandate. However, critics argued the process itself was a violation of their religious freedom.
The issue led to the case of Zubik v. Burwell, a legal challenge over the mandate exemption that went before the U.S. Supreme Court in March 2016. The issue was never resolved however, and in May 2016, the Supreme Court vacated the lower court rulings related to Zubik v. Burwell and remanded the case back to the four appeals courts that had originally ruled on the issue.
In 2018, the Trump administration announced new rules aimed at broadening exemptions to the ACA’s contraceptive mandate to entities that object to services covered by the mandate on the basis of “sincerely held religious beliefs.” A second rule allowed nonprofit organizations and small businesses that had nonreligious moral convictions against the mandate to opt out.
Thirteen states and the District of Columbia then sued the Trump administration over the rules, as well as Pennsylvania and New Jersey in a separate case. Little Sisters of the Poor, a religious nonprofit operating a home in Pittsburgh, intervened in the case as an aggrieved party. An appeal court temporarily barred the regulations from moving forward.
During oral arguments, Solicitor General for the Department of Justice Noel J. Francisco said the exemptions are lawful because they are authorized under a provision of the ACA as well as the Religious Freedom Restoration Act (RFRA).
“RFRA at the very least authorizes the religious exemption,” Mr. Francisco said during oral arguments.
Chief Deputy Attorney General for Pennsylvania Michael J. Fischer argued that the Trump administration’s moral and religious exemption rules rest on overly broad assertions of agency authority.
“First, the agencies twist a narrow delegation that allows the Health Resources and Services Administration to decide which preventive services insurers must cover under the Women’s Health Amendment into a grant of authority so broad it allows them to permit virtually any employer or college to opt out of providing contraceptive coverage entirely, including for reasons as amorphous as vaguely defined moral beliefs,” he said during oral arguments. “Second, the agencies claim that RFRA, a statute that limits government action, affirmatively authorizes them to permit employers to deny women their rights to contraceptive coverage even in the absence of a RFRA violation in the first place.”
U.S. Supreme Court justices appear divided over whether the Trump administration acted properly when it expanded exemptions under the Affordable Care Act’s contraception mandate.
During oral arguments on May 6, the court expressed differing perspectives about the administration’s authority to allow for more exemptions under the health law’s birth control mandate and whether the expansions were reasonable. Justices heard the consolidated cases – Little Sisters of the Poor v. Pennsylvania and Trump v. Pennsylvania – by teleconference because of the COVID-19 pandemic. They are expected to make a decision by the summer.
Associate justice Ruth Bader Ginsburg, who participated in the telephone conference call from a hospital where she was recovering from a gallbladder condition, said the exemptions ignored the intent of Congress to provide women with comprehensive coverage through the ACA.
“The glaring feature of what the government has done in expanding this exemption is to toss to the winds entirely Congress’s instruction that women need and shall have seamless, no-cost, comprehensive coverage,” she said during oral arguments. “This leaves the women to hunt for other government programs that might cover them, and for those who are not covered by Medicaid or one of the other government programs, they can get contraceptive coverage only from paying out of their own pocket, which is exactly what Congress didn’t want to happen.”
Associate Justice Samuel Alito Jr., meanwhile, indicated that a lower court opinion that had blocked the exemptions from going forward conflicts with the Supreme Court’s ruling in a related case, Burwell v. Hobby Lobby.
“Explain to me why the Third Circuit’s analysis of the question of substantial burden is not squarely inconsistent with our reasoning in Hobby Lobby,” Associate Justice Alito said during oral arguments. “Hobby Lobby held that, if a person sincerely believes that it is immoral to perform an act that has the effect of enabling another person to commit an immoral act, a federal court does not have the right to say that this person is wrong on the question of moral complicity. That’s precisely the situation here. Reading the Third Circuit’s discussion of the substantial burden question, I wondered whether they had read that part of the Hobby Lobby decision.”
The dispute surrounding the ACA’s birth control mandate and the extent of exemptions afforded has gone on for a decade and has led to numerous legal challenges. The ACA initially required all employers to cover birth control for employees with no copayments, but exempted group health plans of religious employers. Those religious employers were primarily churches and other houses of worship. After a number of complaints and lawsuits, the Obama administration created a workaround for nonprofit religious employers not included in that exemption to opt out of the mandate. However, critics argued the process itself was a violation of their religious freedom.
The issue led to the case of Zubik v. Burwell, a legal challenge over the mandate exemption that went before the U.S. Supreme Court in March 2016. The issue was never resolved however, and in May 2016, the Supreme Court vacated the lower court rulings related to Zubik v. Burwell and remanded the case back to the four appeals courts that had originally ruled on the issue.
In 2018, the Trump administration announced new rules aimed at broadening exemptions to the ACA’s contraceptive mandate to entities that object to services covered by the mandate on the basis of “sincerely held religious beliefs.” A second rule allowed nonprofit organizations and small businesses that had nonreligious moral convictions against the mandate to opt out.
Thirteen states and the District of Columbia then sued the Trump administration over the rules, as well as Pennsylvania and New Jersey in a separate case. Little Sisters of the Poor, a religious nonprofit operating a home in Pittsburgh, intervened in the case as an aggrieved party. An appeal court temporarily barred the regulations from moving forward.
During oral arguments, Solicitor General for the Department of Justice Noel J. Francisco said the exemptions are lawful because they are authorized under a provision of the ACA as well as the Religious Freedom Restoration Act (RFRA).
“RFRA at the very least authorizes the religious exemption,” Mr. Francisco said during oral arguments.
Chief Deputy Attorney General for Pennsylvania Michael J. Fischer argued that the Trump administration’s moral and religious exemption rules rest on overly broad assertions of agency authority.
“First, the agencies twist a narrow delegation that allows the Health Resources and Services Administration to decide which preventive services insurers must cover under the Women’s Health Amendment into a grant of authority so broad it allows them to permit virtually any employer or college to opt out of providing contraceptive coverage entirely, including for reasons as amorphous as vaguely defined moral beliefs,” he said during oral arguments. “Second, the agencies claim that RFRA, a statute that limits government action, affirmatively authorizes them to permit employers to deny women their rights to contraceptive coverage even in the absence of a RFRA violation in the first place.”
Menstrual cup use with copper IUDs linked to higher expulsion rates
Citing menstrual cup use for menstrual hygiene as “increasingly popular,” researchers led by Jill Long, MD, MPH, studied women participating in a prospective contraceptive efficacy trial of two copper IUDs to evaluate the relationship between menstrual cup use and IUD expulsion over a period of 24 months. The findings were released ahead of the study’s scheduled presentation at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists. ACOG canceled the meeting and released abstracts for press coverage.
In the ongoing 3-year trial, which also was published in Obstetrics & Gynecology, 1,092 women were randomized to one of two copper IUDs. Dr. Long, project officer for the Contraceptive Clinical Trials Network, a project of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, Bethesda, Md. and colleagues conducted follow-up visits at 6 weeks after insertion in the first year, and then 3, 6, and 12 months after insertion. At the 9-month mark, the study counseling was amended to advise patients against concurrent use of the menstrual cup because of a higher risk of IUD expulsions noted in women using the cup.
Among the 1,092 women studied, 266 (24%) reported menstrual cup use. At 24 months after initiating enrollment, 43 cup users (17%) and 43 nonusers (5%) experienced expulsion (odds ratio, 3.81). Fourteen menstrual cup users with expulsion (30%) reported that the event occurred during menstrual cup removal. Dr. Long and colleagues found that, at year 1 of the study, expulsion rates among menstrual cup users and nonusers were 14% and 5%, respectively (P < .001). At the end of year 2, these rates rose to 23% and 7% (P < .001). The study won second place among abstracts in the category of current clinical and basic investigation.
“This outstanding abstract reflects an important study with results that should lead to changes in the way providers counsel patients about IUDs, namely that the risk of IUD expulsion is significantly higher in women who use menstrual cups than in those who use other menstrual hygiene products,” Eve Espey, MD, MPH, who was not affiliated with the study, said in an interview.
According to Dr. Espey, who chairs the department of obstetrics and gynecology at the University of New Mexico, Albuquerque, key strengths of the study include its prospective methodology and the relatively large number of patients with concurrent IUD and menstrual cup use.
“A limitation is the nonrandomized design for the current study’s aim, which would require randomizing women using the IUD to menstrual cup use versus nonuse,” said Dr. Espey, who is a member of the Ob.Gyn News editorial advisory board.* “Another limitation is that only copper IUDs were used, but it is plausible that this result would apply to other IUDs as well. The study is innovative and important in being the first prospective study to evaluate the association between menstrual cup use and IUD expulsion.”
Dr. Long and two coauthors reported having no financial disclosures, but the remaining three authors reported having numerous potential conflicts of interest. Dr. Espey reported having no financial disclosures.
SOURCE: Long J et al. Obstet Gynecol. 2020 May;135.1S. doi: 10.1097/01.AOG.0000662872.89062.83.
*The article was updated on 4/28/2020.
Citing menstrual cup use for menstrual hygiene as “increasingly popular,” researchers led by Jill Long, MD, MPH, studied women participating in a prospective contraceptive efficacy trial of two copper IUDs to evaluate the relationship between menstrual cup use and IUD expulsion over a period of 24 months. The findings were released ahead of the study’s scheduled presentation at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists. ACOG canceled the meeting and released abstracts for press coverage.
In the ongoing 3-year trial, which also was published in Obstetrics & Gynecology, 1,092 women were randomized to one of two copper IUDs. Dr. Long, project officer for the Contraceptive Clinical Trials Network, a project of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, Bethesda, Md. and colleagues conducted follow-up visits at 6 weeks after insertion in the first year, and then 3, 6, and 12 months after insertion. At the 9-month mark, the study counseling was amended to advise patients against concurrent use of the menstrual cup because of a higher risk of IUD expulsions noted in women using the cup.
Among the 1,092 women studied, 266 (24%) reported menstrual cup use. At 24 months after initiating enrollment, 43 cup users (17%) and 43 nonusers (5%) experienced expulsion (odds ratio, 3.81). Fourteen menstrual cup users with expulsion (30%) reported that the event occurred during menstrual cup removal. Dr. Long and colleagues found that, at year 1 of the study, expulsion rates among menstrual cup users and nonusers were 14% and 5%, respectively (P < .001). At the end of year 2, these rates rose to 23% and 7% (P < .001). The study won second place among abstracts in the category of current clinical and basic investigation.
“This outstanding abstract reflects an important study with results that should lead to changes in the way providers counsel patients about IUDs, namely that the risk of IUD expulsion is significantly higher in women who use menstrual cups than in those who use other menstrual hygiene products,” Eve Espey, MD, MPH, who was not affiliated with the study, said in an interview.
According to Dr. Espey, who chairs the department of obstetrics and gynecology at the University of New Mexico, Albuquerque, key strengths of the study include its prospective methodology and the relatively large number of patients with concurrent IUD and menstrual cup use.
“A limitation is the nonrandomized design for the current study’s aim, which would require randomizing women using the IUD to menstrual cup use versus nonuse,” said Dr. Espey, who is a member of the Ob.Gyn News editorial advisory board.* “Another limitation is that only copper IUDs were used, but it is plausible that this result would apply to other IUDs as well. The study is innovative and important in being the first prospective study to evaluate the association between menstrual cup use and IUD expulsion.”
Dr. Long and two coauthors reported having no financial disclosures, but the remaining three authors reported having numerous potential conflicts of interest. Dr. Espey reported having no financial disclosures.
SOURCE: Long J et al. Obstet Gynecol. 2020 May;135.1S. doi: 10.1097/01.AOG.0000662872.89062.83.
*The article was updated on 4/28/2020.
Citing menstrual cup use for menstrual hygiene as “increasingly popular,” researchers led by Jill Long, MD, MPH, studied women participating in a prospective contraceptive efficacy trial of two copper IUDs to evaluate the relationship between menstrual cup use and IUD expulsion over a period of 24 months. The findings were released ahead of the study’s scheduled presentation at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists. ACOG canceled the meeting and released abstracts for press coverage.
In the ongoing 3-year trial, which also was published in Obstetrics & Gynecology, 1,092 women were randomized to one of two copper IUDs. Dr. Long, project officer for the Contraceptive Clinical Trials Network, a project of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, Bethesda, Md. and colleagues conducted follow-up visits at 6 weeks after insertion in the first year, and then 3, 6, and 12 months after insertion. At the 9-month mark, the study counseling was amended to advise patients against concurrent use of the menstrual cup because of a higher risk of IUD expulsions noted in women using the cup.
Among the 1,092 women studied, 266 (24%) reported menstrual cup use. At 24 months after initiating enrollment, 43 cup users (17%) and 43 nonusers (5%) experienced expulsion (odds ratio, 3.81). Fourteen menstrual cup users with expulsion (30%) reported that the event occurred during menstrual cup removal. Dr. Long and colleagues found that, at year 1 of the study, expulsion rates among menstrual cup users and nonusers were 14% and 5%, respectively (P < .001). At the end of year 2, these rates rose to 23% and 7% (P < .001). The study won second place among abstracts in the category of current clinical and basic investigation.
“This outstanding abstract reflects an important study with results that should lead to changes in the way providers counsel patients about IUDs, namely that the risk of IUD expulsion is significantly higher in women who use menstrual cups than in those who use other menstrual hygiene products,” Eve Espey, MD, MPH, who was not affiliated with the study, said in an interview.
According to Dr. Espey, who chairs the department of obstetrics and gynecology at the University of New Mexico, Albuquerque, key strengths of the study include its prospective methodology and the relatively large number of patients with concurrent IUD and menstrual cup use.
“A limitation is the nonrandomized design for the current study’s aim, which would require randomizing women using the IUD to menstrual cup use versus nonuse,” said Dr. Espey, who is a member of the Ob.Gyn News editorial advisory board.* “Another limitation is that only copper IUDs were used, but it is plausible that this result would apply to other IUDs as well. The study is innovative and important in being the first prospective study to evaluate the association between menstrual cup use and IUD expulsion.”
Dr. Long and two coauthors reported having no financial disclosures, but the remaining three authors reported having numerous potential conflicts of interest. Dr. Espey reported having no financial disclosures.
SOURCE: Long J et al. Obstet Gynecol. 2020 May;135.1S. doi: 10.1097/01.AOG.0000662872.89062.83.
*The article was updated on 4/28/2020.
FROM ACOG 2020
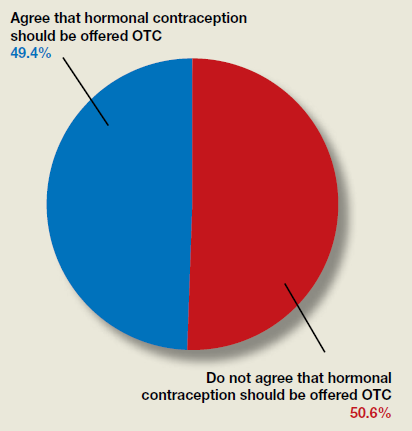
Do ObGyns think hormonal contraception should be offered over the counter?
In their advocacy column, “OTC hormonal contraception: An important goal in the fight for reproductive justice” (January 2020), Abby L. Schultz, MD, and Megan L. Evans, MD, MPH, discussed a recent committee opinion from the American College of Obstetricians and Gynecologists (ACOG) focused on improving contraception access by offering oral contraceptive pills, progesterone-only pills, the patch, vaginal rings, and depot medroxyprogesterone acetate over the counter (OTC). The authors agreed with ACOG’s stance and offered several reasons why.
OBG Management polled readers to see their thoughts on the question of whether or not hormonal contraception should be offered OTC.

In their advocacy column, “OTC hormonal contraception: An important goal in the fight for reproductive justice” (January 2020), Abby L. Schultz, MD, and Megan L. Evans, MD, MPH, discussed a recent committee opinion from the American College of Obstetricians and Gynecologists (ACOG) focused on improving contraception access by offering oral contraceptive pills, progesterone-only pills, the patch, vaginal rings, and depot medroxyprogesterone acetate over the counter (OTC). The authors agreed with ACOG’s stance and offered several reasons why.
OBG Management polled readers to see their thoughts on the question of whether or not hormonal contraception should be offered OTC.


In their advocacy column, “OTC hormonal contraception: An important goal in the fight for reproductive justice” (January 2020), Abby L. Schultz, MD, and Megan L. Evans, MD, MPH, discussed a recent committee opinion from the American College of Obstetricians and Gynecologists (ACOG) focused on improving contraception access by offering oral contraceptive pills, progesterone-only pills, the patch, vaginal rings, and depot medroxyprogesterone acetate over the counter (OTC). The authors agreed with ACOG’s stance and offered several reasons why.
OBG Management polled readers to see their thoughts on the question of whether or not hormonal contraception should be offered OTC.


iPLEDGE allows at-home pregnancy tests during pandemic
tests to comply with the requirements of the iPLEDGE program during the COVID-19 pandemic, according to an update program posted on the iPLEDGE website.
The program’s other requirements – the prescription window and two forms of birth control – remain unchanged.
The change follows recent guidance from the Department of Health & Human Services and the Food and Drug Administration regarding accommodations for medical care and drugs subject to Risk Evaluation and Mitigation Strategies (REMS) in the midst of a public health emergency that requires most people to remain in their homes except for essential services.
Allowing females to take at-home pregnancy tests and communicate the results to physician according to their preference is “a game changer for the middle of a pandemic, obviously,” Neil Goldberg, MD, a dermatologist in Westchester County, New York, said in an interview. “These are patients who don’t need to spend time outside just to get pregnancy tests done. It makes it a lot easier.”
Dr. Goldberg is frustrated, however, that the accommodations have not been more widely publicized; he discovered the change incidentally when speaking to an iPLEDGE program representative to request a waiver for a patient who had taken her pregnancy test too early. The program had denied a similar request for a 15-year-old patient of his the previous week, despite the patient being abstinent and having been in shelter-in-place for several weeks.
“The size of your notice [on the website] should be proportionate to how important it is,” Dr. Goldberg said, and the small red box on the site is easy to miss. By contrast, asking anyone to leave their homes to go to a lab for a pregnancy test in the midst of a global pandemic so they can continue their medication would be putting patients at risk, he added.
The iPLEDGE program is designed in part to ensure unplanned pregnancies do not occur in females while taking the teratogenic acne drug. But the rules are onerous and difficult even during normal times, pointed out Hilary Baldwin, MD, medical director of the Acne Treatment and Research Center in New York City and past president of the American Acne and Rosacea Society.
Male patients taking isotretinoin must visit their physician every month to get a new no-refills prescription, but females must get a pregnancy test at a Clinical Laboratory Improvement Amendments–certified lab, which must then provide physical results to the prescribing physician. The doctor enters the negative pregnancy test and the two forms of birth control the patient is taking in the iPLEDGE program site.
Then the patient must take an online test at home to acknowledge they understand what it means to not get pregnant and enter the two forms of birth control they are using – which must match what the doctor enters – before the pharmacy can dispense the drug. The entire process must occur within 7 days or else the patient has to wait 19 days before starting the process over.
“We run a very tight schedule for girls. And every month, we would worry that something would interfere, a snow storm or something else, and that they wouldn’t be able to complete their objectives within the 7-day period,” Dr Baldwin said in an interview. “It was always difficult, and now with us not being able to see the patient and the patient not wanting to go to the lab, this became completely impossible.”
Until this change, some patients may not have been able to get their prescription for severe nodulocystic acne, which can cause physical and psychological scarring, and “postponing treatment increases the likelihood of scarring,” Dr. Baldwin pointed out.

Dr. Goldberg’s patients now take a pregnancy test at home and send him a photo of the negative test that he then inserts into their EMR.
According to a March 17 statement from HHS, potential penalties for HIPAA violations are waived for good-faith use of “everyday communication technologies,” such as Skype or FaceTime, for telehealth treatment or diagnostics. The change was intended to allow telehealth services to continue healthcare for practices that had not previously had secure telehealth technology established.
Despite the changes for at-home pregnancy tests for females and in-person visits for all patients, the program has not altered the 7-day prescription window or the requirement to have two forms of birth control.
With reports of a global condom shortage, Dr Baldwin said she has more concerns about her adult patients being able to find a required barrier method of birth control than about her adolescent patients.
“This is a unique opportunity for us to trust our teenage patients because they can’t leave the house,” Dr. Baldwin said. “I’m actually more worried about my adult women on the drug who are bored and cooped up in a house with their significant other.”
Dr. Baldwin and Dr. Goldberg had no relevant disclosures. Dr. Goldberg is a Dermatology News board member.
tests to comply with the requirements of the iPLEDGE program during the COVID-19 pandemic, according to an update program posted on the iPLEDGE website.
The program’s other requirements – the prescription window and two forms of birth control – remain unchanged.
The change follows recent guidance from the Department of Health & Human Services and the Food and Drug Administration regarding accommodations for medical care and drugs subject to Risk Evaluation and Mitigation Strategies (REMS) in the midst of a public health emergency that requires most people to remain in their homes except for essential services.
Allowing females to take at-home pregnancy tests and communicate the results to physician according to their preference is “a game changer for the middle of a pandemic, obviously,” Neil Goldberg, MD, a dermatologist in Westchester County, New York, said in an interview. “These are patients who don’t need to spend time outside just to get pregnancy tests done. It makes it a lot easier.”
Dr. Goldberg is frustrated, however, that the accommodations have not been more widely publicized; he discovered the change incidentally when speaking to an iPLEDGE program representative to request a waiver for a patient who had taken her pregnancy test too early. The program had denied a similar request for a 15-year-old patient of his the previous week, despite the patient being abstinent and having been in shelter-in-place for several weeks.
“The size of your notice [on the website] should be proportionate to how important it is,” Dr. Goldberg said, and the small red box on the site is easy to miss. By contrast, asking anyone to leave their homes to go to a lab for a pregnancy test in the midst of a global pandemic so they can continue their medication would be putting patients at risk, he added.
The iPLEDGE program is designed in part to ensure unplanned pregnancies do not occur in females while taking the teratogenic acne drug. But the rules are onerous and difficult even during normal times, pointed out Hilary Baldwin, MD, medical director of the Acne Treatment and Research Center in New York City and past president of the American Acne and Rosacea Society.
Male patients taking isotretinoin must visit their physician every month to get a new no-refills prescription, but females must get a pregnancy test at a Clinical Laboratory Improvement Amendments–certified lab, which must then provide physical results to the prescribing physician. The doctor enters the negative pregnancy test and the two forms of birth control the patient is taking in the iPLEDGE program site.
Then the patient must take an online test at home to acknowledge they understand what it means to not get pregnant and enter the two forms of birth control they are using – which must match what the doctor enters – before the pharmacy can dispense the drug. The entire process must occur within 7 days or else the patient has to wait 19 days before starting the process over.
“We run a very tight schedule for girls. And every month, we would worry that something would interfere, a snow storm or something else, and that they wouldn’t be able to complete their objectives within the 7-day period,” Dr Baldwin said in an interview. “It was always difficult, and now with us not being able to see the patient and the patient not wanting to go to the lab, this became completely impossible.”
Until this change, some patients may not have been able to get their prescription for severe nodulocystic acne, which can cause physical and psychological scarring, and “postponing treatment increases the likelihood of scarring,” Dr. Baldwin pointed out.

Dr. Goldberg’s patients now take a pregnancy test at home and send him a photo of the negative test that he then inserts into their EMR.
According to a March 17 statement from HHS, potential penalties for HIPAA violations are waived for good-faith use of “everyday communication technologies,” such as Skype or FaceTime, for telehealth treatment or diagnostics. The change was intended to allow telehealth services to continue healthcare for practices that had not previously had secure telehealth technology established.
Despite the changes for at-home pregnancy tests for females and in-person visits for all patients, the program has not altered the 7-day prescription window or the requirement to have two forms of birth control.
With reports of a global condom shortage, Dr Baldwin said she has more concerns about her adult patients being able to find a required barrier method of birth control than about her adolescent patients.
“This is a unique opportunity for us to trust our teenage patients because they can’t leave the house,” Dr. Baldwin said. “I’m actually more worried about my adult women on the drug who are bored and cooped up in a house with their significant other.”
Dr. Baldwin and Dr. Goldberg had no relevant disclosures. Dr. Goldberg is a Dermatology News board member.
tests to comply with the requirements of the iPLEDGE program during the COVID-19 pandemic, according to an update program posted on the iPLEDGE website.
The program’s other requirements – the prescription window and two forms of birth control – remain unchanged.
The change follows recent guidance from the Department of Health & Human Services and the Food and Drug Administration regarding accommodations for medical care and drugs subject to Risk Evaluation and Mitigation Strategies (REMS) in the midst of a public health emergency that requires most people to remain in their homes except for essential services.
Allowing females to take at-home pregnancy tests and communicate the results to physician according to their preference is “a game changer for the middle of a pandemic, obviously,” Neil Goldberg, MD, a dermatologist in Westchester County, New York, said in an interview. “These are patients who don’t need to spend time outside just to get pregnancy tests done. It makes it a lot easier.”
Dr. Goldberg is frustrated, however, that the accommodations have not been more widely publicized; he discovered the change incidentally when speaking to an iPLEDGE program representative to request a waiver for a patient who had taken her pregnancy test too early. The program had denied a similar request for a 15-year-old patient of his the previous week, despite the patient being abstinent and having been in shelter-in-place for several weeks.
“The size of your notice [on the website] should be proportionate to how important it is,” Dr. Goldberg said, and the small red box on the site is easy to miss. By contrast, asking anyone to leave their homes to go to a lab for a pregnancy test in the midst of a global pandemic so they can continue their medication would be putting patients at risk, he added.
The iPLEDGE program is designed in part to ensure unplanned pregnancies do not occur in females while taking the teratogenic acne drug. But the rules are onerous and difficult even during normal times, pointed out Hilary Baldwin, MD, medical director of the Acne Treatment and Research Center in New York City and past president of the American Acne and Rosacea Society.
Male patients taking isotretinoin must visit their physician every month to get a new no-refills prescription, but females must get a pregnancy test at a Clinical Laboratory Improvement Amendments–certified lab, which must then provide physical results to the prescribing physician. The doctor enters the negative pregnancy test and the two forms of birth control the patient is taking in the iPLEDGE program site.
Then the patient must take an online test at home to acknowledge they understand what it means to not get pregnant and enter the two forms of birth control they are using – which must match what the doctor enters – before the pharmacy can dispense the drug. The entire process must occur within 7 days or else the patient has to wait 19 days before starting the process over.
“We run a very tight schedule for girls. And every month, we would worry that something would interfere, a snow storm or something else, and that they wouldn’t be able to complete their objectives within the 7-day period,” Dr Baldwin said in an interview. “It was always difficult, and now with us not being able to see the patient and the patient not wanting to go to the lab, this became completely impossible.”
Until this change, some patients may not have been able to get their prescription for severe nodulocystic acne, which can cause physical and psychological scarring, and “postponing treatment increases the likelihood of scarring,” Dr. Baldwin pointed out.

Dr. Goldberg’s patients now take a pregnancy test at home and send him a photo of the negative test that he then inserts into their EMR.
According to a March 17 statement from HHS, potential penalties for HIPAA violations are waived for good-faith use of “everyday communication technologies,” such as Skype or FaceTime, for telehealth treatment or diagnostics. The change was intended to allow telehealth services to continue healthcare for practices that had not previously had secure telehealth technology established.
Despite the changes for at-home pregnancy tests for females and in-person visits for all patients, the program has not altered the 7-day prescription window or the requirement to have two forms of birth control.
With reports of a global condom shortage, Dr Baldwin said she has more concerns about her adult patients being able to find a required barrier method of birth control than about her adolescent patients.
“This is a unique opportunity for us to trust our teenage patients because they can’t leave the house,” Dr. Baldwin said. “I’m actually more worried about my adult women on the drug who are bored and cooped up in a house with their significant other.”
Dr. Baldwin and Dr. Goldberg had no relevant disclosures. Dr. Goldberg is a Dermatology News board member.
High BMI does not complicate postpartum tubal ligation
GRAPEVINE, TEXAS – Higher body mass index is not associated with increased morbidity in women undergoing postpartum tubal ligation, according to a study of more than 1,000 patients.
John J. Byrne, MD, said at the Pregnancy Meeting. Dr. Byrne is affiliated with the department of obstetrics and gynecology at University of Texas Southwestern Medical Center in Dallas.
Physicians may recommend contraception within 6 weeks of delivery, but many patients do not attend postpartum visits. “One option for women who have completed childbearing is bilateral midsegment salpingectomy via minilaparotomy,” Dr. Byrne said at the Pregnancy Meeting, sponsored by the Society for Maternal-Fetal Medicine. “Offering this procedure immediately after delivery makes it available to women who face obstacles to follow-up care.”
The procedure entails the risk of anesthetic complications, bowel injury, and vascular injury. Subsequent pregnancy or ectopic pregnancy also may occur. Some centers will not perform the procedure if a patient’s size affects the surgeon’s ability to feel the relevant anatomy, Dr. Byrne said. “Although operative complications are presumed to be higher among obese women,” prior studies have not examined whether BMI affects rates of procedure completion, complication, or subsequent pregnancy, the researchers said.
To study this question, Dr. Byrne and colleagues examined data from women who requested postpartum sterilization following vaginal delivery at their center in 2018. The center uses the Parkland tubal ligation technique. The researchers assessed complication rates using a composite measure that included surgical complications (that is, blood transfusion, aborted procedure, or extension of incision), anesthetic complications, readmission, superficial or deep wound infection, venous thromboembolism, ileus or small bowel obstruction, incomplete transection, and subsequent pregnancy. The investigators used statistical tests to assess the relationship between BMI and morbidity.
In all, 1,014 patients underwent a postpartum tubal ligation; 17% had undergone prior abdominal surgery. The researchers classified patients’ BMI as normal (7% of the population), overweight (28%), class I obesity (38%), class II obesity (18%), or class III obesity (9%). A composite morbidity event occurred in 2%, and the proportion of patients with a complication did not significantly differ across BMI categories. No morbid events occurred in patients with normal BMI, which indicates “minimal risk” in this population, Dr. Byrne said. One incomplete transection occurred in a patient with class I obesity, and one subsequent pregnancy occurred in a patient with class II obesity. Estimated blood loss ranged from 9 mL in patients with normal BMI to 13 mL in patients with class III obesity, and length of surgery ranged from 32 minutes to 40 minutes. Neither difference is clinically significant, Dr. Byrne said.
“For the woman who desires permanent contraception, BMI should not impede her access to the procedure,” he noted.
The researchers had no relevant disclosures.
SOURCE: Byrne JJ et al. Am J Obstet Gynecol. 2020 Jan;222(1):S290, Abstract 442.
GRAPEVINE, TEXAS – Higher body mass index is not associated with increased morbidity in women undergoing postpartum tubal ligation, according to a study of more than 1,000 patients.
John J. Byrne, MD, said at the Pregnancy Meeting. Dr. Byrne is affiliated with the department of obstetrics and gynecology at University of Texas Southwestern Medical Center in Dallas.
Physicians may recommend contraception within 6 weeks of delivery, but many patients do not attend postpartum visits. “One option for women who have completed childbearing is bilateral midsegment salpingectomy via minilaparotomy,” Dr. Byrne said at the Pregnancy Meeting, sponsored by the Society for Maternal-Fetal Medicine. “Offering this procedure immediately after delivery makes it available to women who face obstacles to follow-up care.”
The procedure entails the risk of anesthetic complications, bowel injury, and vascular injury. Subsequent pregnancy or ectopic pregnancy also may occur. Some centers will not perform the procedure if a patient’s size affects the surgeon’s ability to feel the relevant anatomy, Dr. Byrne said. “Although operative complications are presumed to be higher among obese women,” prior studies have not examined whether BMI affects rates of procedure completion, complication, or subsequent pregnancy, the researchers said.
To study this question, Dr. Byrne and colleagues examined data from women who requested postpartum sterilization following vaginal delivery at their center in 2018. The center uses the Parkland tubal ligation technique. The researchers assessed complication rates using a composite measure that included surgical complications (that is, blood transfusion, aborted procedure, or extension of incision), anesthetic complications, readmission, superficial or deep wound infection, venous thromboembolism, ileus or small bowel obstruction, incomplete transection, and subsequent pregnancy. The investigators used statistical tests to assess the relationship between BMI and morbidity.
In all, 1,014 patients underwent a postpartum tubal ligation; 17% had undergone prior abdominal surgery. The researchers classified patients’ BMI as normal (7% of the population), overweight (28%), class I obesity (38%), class II obesity (18%), or class III obesity (9%). A composite morbidity event occurred in 2%, and the proportion of patients with a complication did not significantly differ across BMI categories. No morbid events occurred in patients with normal BMI, which indicates “minimal risk” in this population, Dr. Byrne said. One incomplete transection occurred in a patient with class I obesity, and one subsequent pregnancy occurred in a patient with class II obesity. Estimated blood loss ranged from 9 mL in patients with normal BMI to 13 mL in patients with class III obesity, and length of surgery ranged from 32 minutes to 40 minutes. Neither difference is clinically significant, Dr. Byrne said.
“For the woman who desires permanent contraception, BMI should not impede her access to the procedure,” he noted.
The researchers had no relevant disclosures.
SOURCE: Byrne JJ et al. Am J Obstet Gynecol. 2020 Jan;222(1):S290, Abstract 442.
GRAPEVINE, TEXAS – Higher body mass index is not associated with increased morbidity in women undergoing postpartum tubal ligation, according to a study of more than 1,000 patients.
John J. Byrne, MD, said at the Pregnancy Meeting. Dr. Byrne is affiliated with the department of obstetrics and gynecology at University of Texas Southwestern Medical Center in Dallas.
Physicians may recommend contraception within 6 weeks of delivery, but many patients do not attend postpartum visits. “One option for women who have completed childbearing is bilateral midsegment salpingectomy via minilaparotomy,” Dr. Byrne said at the Pregnancy Meeting, sponsored by the Society for Maternal-Fetal Medicine. “Offering this procedure immediately after delivery makes it available to women who face obstacles to follow-up care.”
The procedure entails the risk of anesthetic complications, bowel injury, and vascular injury. Subsequent pregnancy or ectopic pregnancy also may occur. Some centers will not perform the procedure if a patient’s size affects the surgeon’s ability to feel the relevant anatomy, Dr. Byrne said. “Although operative complications are presumed to be higher among obese women,” prior studies have not examined whether BMI affects rates of procedure completion, complication, or subsequent pregnancy, the researchers said.
To study this question, Dr. Byrne and colleagues examined data from women who requested postpartum sterilization following vaginal delivery at their center in 2018. The center uses the Parkland tubal ligation technique. The researchers assessed complication rates using a composite measure that included surgical complications (that is, blood transfusion, aborted procedure, or extension of incision), anesthetic complications, readmission, superficial or deep wound infection, venous thromboembolism, ileus or small bowel obstruction, incomplete transection, and subsequent pregnancy. The investigators used statistical tests to assess the relationship between BMI and morbidity.
In all, 1,014 patients underwent a postpartum tubal ligation; 17% had undergone prior abdominal surgery. The researchers classified patients’ BMI as normal (7% of the population), overweight (28%), class I obesity (38%), class II obesity (18%), or class III obesity (9%). A composite morbidity event occurred in 2%, and the proportion of patients with a complication did not significantly differ across BMI categories. No morbid events occurred in patients with normal BMI, which indicates “minimal risk” in this population, Dr. Byrne said. One incomplete transection occurred in a patient with class I obesity, and one subsequent pregnancy occurred in a patient with class II obesity. Estimated blood loss ranged from 9 mL in patients with normal BMI to 13 mL in patients with class III obesity, and length of surgery ranged from 32 minutes to 40 minutes. Neither difference is clinically significant, Dr. Byrne said.
“For the woman who desires permanent contraception, BMI should not impede her access to the procedure,” he noted.
The researchers had no relevant disclosures.
SOURCE: Byrne JJ et al. Am J Obstet Gynecol. 2020 Jan;222(1):S290, Abstract 442.
REPORTING FROM THE PREGNANCY MEETING
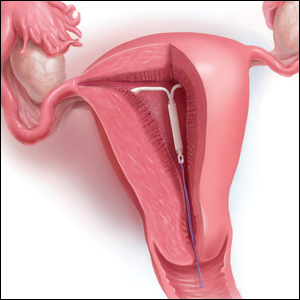
The IUD string check: Benefit or burden?
CASE A patient experiences unnessary inconvenience, distress, and cost following IUD placement
Ms. J had a levonorgestrel intrauterine device (IUD) placed at her postpartum visit. Her physician asked her to return for a string check in 4 to 6 weeks. She was dismayed at the prospect of re-presenting for care, as she is losing the Medicaid coverage that paid for her pregnancy care. One month later, she arranged for a babysitter so she could obtain the recommended string check. The physician told her the strings seemed longer than expected and ordered ultrasonography. Ms. J is distressed because of the mounting cost of care but is anxious to ensure that the IUD will prevent future pregnancy.
Should the routine IUD string check be reconsidered?
The string check dissension
Intrauterine devices offer reliable contraception with a high rate of satisfaction and a remarkably low rate of complications.1-3 With the increased uptake of IUDs, the value of “string checks” is being debated, with myriad responses from professional groups, manufacturers, and individual clinicians. For many practicing ObGyns, the question remains: Should patients be counseled about presenting for or doing their own IUD string checks?
Indeed, all IUD manufacturers recommend monthly self-examination to evaluate string presence.4-8 Manufacturers’ websites prominently display this information in material directed toward current or potential users, so many patients may be familiar already with this recommendation before their clinician visit. Yet, the Centers for Disease Control and Prevention state that no routine follow-up or monitoring is needed.9
In our case scenario, follow-up is clearly burdensome and ultimately costly. Instead, clinicians can advise patients to return with rare but important to recognize complications (such as perforation, expulsion, infection), adverse effects, or desire for change. While no data are available to support in-office or at-home string checks, data do show that women reliably present when intervention is needed.
Here, we explore 5 questions relevant to IUD string checks and discuss why it is time to rethink this practice habit.
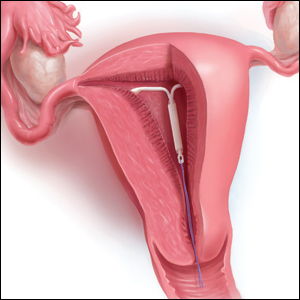
What is the purpose of a string check?
String checks serve as a surrogate for assessing an IUD’s position and function. A string check can be performed by a clinician, who observes the IUD strings on speculum exam or palpates the strings on bimanual exam, or by the patient doing a self-exam. A positive string check purportedly assures both the IUD user and the health care provider that an IUD remains in a fundal, intrauterine position, thus providing an ongoing reliable contraceptive effect.
However, string check reliability in detecting contraceptive effectiveness is uncertain. Strings that subjectively feel or appear longer than anticipated can lead to unnecessary additional evaluation and emotional distress: These are harms. By contrast, when an expulsion occurs, it often is a partial expulsion or displacement, with unclear effect on patient or physician perception of the strings on examination. One retrospective review identified women with a history of IUD placement and a positive pregnancy test; those with an intrauterine pregnancy (74%) frequently also had a malpositioned IUD (55%) and rarely identifiable string issues (16%).10 Before asking patients and clinicians to use resources for performing string evaluations, the association between this action and outcomes of interest must be elucidated.
If not for assessing risk of expulsion, IUD follow-up allows the clinician to evaluate for other complications or adverse effects and to address patient concerns. This practice often is performed when the patient is starting a new medication or medical intervention. However, a systematic review involving 4 studies of IUD follow-up visits or phone calls after contraceptive initiation generated limited data, with no notable impact on contraceptive continuation or indicated use.11
Most important, data show that patients present to their clinician when issues arise with IUD use. One prospective study of 280 women compared multiple follow-up visits with a single 6-week follow-up visit after IUD placement; 10 expulsions were identified, and 8 of these were noted at unscheduled visits when patients presented with symptoms.12 This study suggests that there is little benefit in scheduled follow-up or set self-checks.
Furthermore, in a study in Finland of more than 17,000 IUD users, the rare participants who became pregnant during IUD use promptly presented for care because of a change in menses, pain, or symptoms of pregnancy.13 While IUDs are touted as user independent, this overlooks the reality: Data show that device failure, although rare, is rapidly and appropriately addressed by the user.
Continue to: Does the risk of IUD expulsion warrant string checks?...
Does the risk of IUD expulsion warrant string checks?
The risk of IUD expulsion is estimated to be 1% at 1 month and 4% at 1 year, with a contraceptive failure rate of 0.4% at 1 year. The risk of expulsion does not differ by age group, including adolescents, or parity, but it is higher with use of the copper IUD (2% at 1 month, 6% at 1 year) and with prior expulsion (14%, limited by small numbers).1 Furthermore, risk of expulsion is higher with postplacental placement and second trimester abortion.14,15 Despite this risk, the contraceptive failure rate of all types of IUDs remains consistently lower than all other reversible methods besides the contraceptive implant.16
Furthermore, while IUD expulsion is rare, unnoticed expulsion is even more rare. In one study with more than 58,000 person-years of use, 132 pregnancies were noted, and 7 of these occurred in the setting of an unnoticed expulsion.13 Notably, a higher risk threshold is held for other medications. For example, statins are associated with a 3% risk of irreversible hepatic injury, yet serial liver function tests are not performed in patients without baseline liver dysfunction.17 A less than 0.1% risk of a non–life-threatening complication—unnoticed expulsion—does not warrant routine follow-up. Instead, the patient gauges the tolerability of that risk in making a follow-up plan, particularly given the varied individual preferences in patients’ management of the associated outcome of unintended pregnancy.
Are women interested in and able to perform their own string checks?
Recommendations to perform IUD string self-checks should consider whether women are willing and able to do so. In a study of 126 IUD users, 59% of women had attempted to check their IUD strings at home, and one-third were unable to do so successfully; all participants had visible strings on subsequent speculum exam.18 The women also were given the opportunity to perform a string self-check at the study visit. Overall, only 46% of participants found the exercise acceptable and were able to palpate the IUD strings.18 The authors aptly stated, “A universal recommendation for practice that is meant to identify a rare complication has no clinical utility if at least half of the women are unable to follow it.”
In which scenarios might a string check have clear utility?
The most important reason for follow-up after IUD placement or for patients to perform string self-checks is patient preference. At least anecdotally, some patients take comfort, particularly in the absence of menses, in palpating IUD strings regularly; these individuals should know that there is no necessity for but also no harm in this practice. In addition, patients may desire a string check or follow-up visit to discuss their new contraceptive’s goodness-of-fit.
While limited data show that routinely scheduling such visits does not improve contraceptive continuation, it is difficult to extrapolate these data to the select individuals who independently desire follow-up. (In addition, contraceptive continuance is hardly a metric of success, as clinicians and patients can agree that discontinuation in the setting of patient dissatisfaction is always appropriate.)
Clinicians should share with patients differing risks of IUD expulsion, and this may prompt more nuanced decisions about string checks and/or follow-up. Patients with postplacental or postabortion (second trimester) IUD placement or placement following prior expulsion may opt to perform string checks given the relatively higher risk of expulsion despite the maintained, absolutely low risk that such an event is unnoticed.
If a patient does present for a string check and strings are not visualized on exam, reasonable attempts should be made to identify the strings at that time. A cytobrush can be used to liberate and identify strings within the cervical canal. If the clinician cannot identify the strings or the patient is unable to tolerate such attempts, ultrasonography should be performed to localize the IUD. The ultrasound scan can be done in the office, if available, which is more cost-effective for women than a referral to radiology. If ultrasonography does not identify an intrauterine IUD, an x-ray is the next step to determine if the IUD has expulsed or perforated.
Continue to: Is a string check worth the cost?...
Is a string check worth the cost?
Health care providers may not be aware of the cost of care from the patient perspective. While the Affordable Care Act of 2010 mandates contraceptive coverage for women with insurance, a string check often is coded as a problem-based visit and thus may require a significant copay or out-of-pocket cost for high-deductible plans—without a proven benefit.19 Women who lack insurance coverage may forgo even necessary care due to the cost.20
The bottom line
The medical community and ObGyns specifically are familiar with a practice of patient self-examination falling by the wayside, as has been the case with breast self-examination.21 While counseling on string checks can complement conversations about risks and patients’ personal preferences regarding follow-up, no data support routine string checks in the clinic or at home. One of the great benefits of IUD use is its lack of barriers and resources for ongoing use. Physicians need not reintroduce burdens without benefits to those who desire this contraceptive method.
- Aoun J, Dines VA, Stovall DW, et al. Effects of age, parity, and device type on complications and discontinuation of intrauterine devices. Obstet Gynecol. 2014;123:585-592.
- Peipert JF, Zhao Q, Allsworth JE, et al. Continuation and satisfaction of reversible contraception. Obstet Gynecol. 2011;117:1105-1113.
- American College of Obstetricians and Gynecologists Committee on Gynecology Practice. Committee opinion No. 672. Clinical challenges of long-acting reversible contraceptive methods. Obstet Gynecol. 2016;128:e69-e77.
- Mirena website. Placement of Mirena. 2019. https://www.mirena-us.com/placement-of-mirena/. Accessed December 7, 2019.
- Kyleena website. Let’s get started. 2019. https://www.kyleena-us.com/lets-get-started/what-to-expect/. Accessed December 7, 2019.
- Skyla website. What to expect. 2019. https://www.skyla-us.com/getting-skyla/index.php. Accessed December 7, 2019.
- Liletta website. What should I expect after Liletta insertion? 2020. https://www.liletta.com/about/what-to-expect-afterinsertion. Accessed December 7, 2019.
- Paragard website. What to expect with Paragard. 2019. https://www.paragard.com/what-can-i-expect-with-paragard/. Accessed December 7, 2019.
- Curtis KM, Jatlaoui TC, Tepper NK, et al. US selected practice recommendations for contraceptive use, 2016. MMWR Recomm Rep. 2016;65(4):1-66. https://www.cdc.gov/mmwr/ volumes/65/rr/pdfs/rr6504.pdf. Accessed February 19, 2020.
- Moschos E, Twickler DM. Intrauterine devices in early pregnancy: findings on ultrasound and clinical outcomes. Am J Obstet Gynecol. 2011;204:427.e1-6.
- Steenland MW, Zapata LB, Brahmi D, et al. Appropriate follow up to detect potential adverse events after initiation of select contraceptive methods: a systematic review. Contraception 2013;87:611-624.
- Neuteboom K, de Kroon CD, Dersjant-Roorda M, et al. Follow-up visits after IUD-insertion: sense or nonsense? Contraception. 2003;68:101-104.
- Backman T, Rauramo I, Huhtala S, et al. Pregnancy during the use of levonorgestrel intrauterine system. Am J Obstet Gynecol. 2004;190:50-54.
- Whitaker AK, Chen BA. Society of Family Planning guidelines: postplacental insertion of intrauterine devices. Contraception. 2018;97:2-13.
- Roe AH, Bartz D. Society of Family Planning clinical recommendations: contraception after surgical abortion. Contraception. 2019;99:2-9.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins–Gynecology. Practice bulletin No. 186. Long-acting reversible contraception: implants and intrauterine devices. Obstet Gynecol. 2017;130:e251-e269.
- US Food and Drug Administration. FDA drug safety communication: important safety label changes to cholesterol-lowering statin drugs. 2016. https://www .fda.gov/drugs/drug-safety-and-availability/fda-drugsafety-communication-important-safety-label-changescholesterol-lowering-statin-drugs. Accessed January 9, 2020.
- Melo J, Tschann M, Soon R, et al. Women’s willingness and ability to feel the strings of their intrauterine device. Int J Gynaecol Obstet. 2017;137:309-313.
- Healthcare.gov website. Health benefits & coverage: birth control benefits. 2020. https://www.healthcare.gov/ coverage/birth-control-benefits/. Accessed January 6, 2020.
- NORC at the University of Chicago. Americans’ views of healthcare costs, coverage, and policy. 2018;1-15. https:// www.norc.org/PDFs/WHI%20Healthcare%20Costs%20 Coverage%20and%20Policy/WHI%20Healthcare%20 Costs%20Coverage%20and%20Policy%20Issue%20Brief.pdf. Accessed February 19, 2020.
- Kosters JP, Gotzsche PC. Regular self-examination or clinical examination for early detection of breast cancer. Cochrane Database Syst Rev. 2003. CD003373.
CASE A patient experiences unnessary inconvenience, distress, and cost following IUD placement
Ms. J had a levonorgestrel intrauterine device (IUD) placed at her postpartum visit. Her physician asked her to return for a string check in 4 to 6 weeks. She was dismayed at the prospect of re-presenting for care, as she is losing the Medicaid coverage that paid for her pregnancy care. One month later, she arranged for a babysitter so she could obtain the recommended string check. The physician told her the strings seemed longer than expected and ordered ultrasonography. Ms. J is distressed because of the mounting cost of care but is anxious to ensure that the IUD will prevent future pregnancy.
Should the routine IUD string check be reconsidered?
The string check dissension
Intrauterine devices offer reliable contraception with a high rate of satisfaction and a remarkably low rate of complications.1-3 With the increased uptake of IUDs, the value of “string checks” is being debated, with myriad responses from professional groups, manufacturers, and individual clinicians. For many practicing ObGyns, the question remains: Should patients be counseled about presenting for or doing their own IUD string checks?
Indeed, all IUD manufacturers recommend monthly self-examination to evaluate string presence.4-8 Manufacturers’ websites prominently display this information in material directed toward current or potential users, so many patients may be familiar already with this recommendation before their clinician visit. Yet, the Centers for Disease Control and Prevention state that no routine follow-up or monitoring is needed.9
In our case scenario, follow-up is clearly burdensome and ultimately costly. Instead, clinicians can advise patients to return with rare but important to recognize complications (such as perforation, expulsion, infection), adverse effects, or desire for change. While no data are available to support in-office or at-home string checks, data do show that women reliably present when intervention is needed.
Here, we explore 5 questions relevant to IUD string checks and discuss why it is time to rethink this practice habit.

What is the purpose of a string check?
String checks serve as a surrogate for assessing an IUD’s position and function. A string check can be performed by a clinician, who observes the IUD strings on speculum exam or palpates the strings on bimanual exam, or by the patient doing a self-exam. A positive string check purportedly assures both the IUD user and the health care provider that an IUD remains in a fundal, intrauterine position, thus providing an ongoing reliable contraceptive effect.
However, string check reliability in detecting contraceptive effectiveness is uncertain. Strings that subjectively feel or appear longer than anticipated can lead to unnecessary additional evaluation and emotional distress: These are harms. By contrast, when an expulsion occurs, it often is a partial expulsion or displacement, with unclear effect on patient or physician perception of the strings on examination. One retrospective review identified women with a history of IUD placement and a positive pregnancy test; those with an intrauterine pregnancy (74%) frequently also had a malpositioned IUD (55%) and rarely identifiable string issues (16%).10 Before asking patients and clinicians to use resources for performing string evaluations, the association between this action and outcomes of interest must be elucidated.
If not for assessing risk of expulsion, IUD follow-up allows the clinician to evaluate for other complications or adverse effects and to address patient concerns. This practice often is performed when the patient is starting a new medication or medical intervention. However, a systematic review involving 4 studies of IUD follow-up visits or phone calls after contraceptive initiation generated limited data, with no notable impact on contraceptive continuation or indicated use.11
Most important, data show that patients present to their clinician when issues arise with IUD use. One prospective study of 280 women compared multiple follow-up visits with a single 6-week follow-up visit after IUD placement; 10 expulsions were identified, and 8 of these were noted at unscheduled visits when patients presented with symptoms.12 This study suggests that there is little benefit in scheduled follow-up or set self-checks.
Furthermore, in a study in Finland of more than 17,000 IUD users, the rare participants who became pregnant during IUD use promptly presented for care because of a change in menses, pain, or symptoms of pregnancy.13 While IUDs are touted as user independent, this overlooks the reality: Data show that device failure, although rare, is rapidly and appropriately addressed by the user.
Continue to: Does the risk of IUD expulsion warrant string checks?...
Does the risk of IUD expulsion warrant string checks?
The risk of IUD expulsion is estimated to be 1% at 1 month and 4% at 1 year, with a contraceptive failure rate of 0.4% at 1 year. The risk of expulsion does not differ by age group, including adolescents, or parity, but it is higher with use of the copper IUD (2% at 1 month, 6% at 1 year) and with prior expulsion (14%, limited by small numbers).1 Furthermore, risk of expulsion is higher with postplacental placement and second trimester abortion.14,15 Despite this risk, the contraceptive failure rate of all types of IUDs remains consistently lower than all other reversible methods besides the contraceptive implant.16
Furthermore, while IUD expulsion is rare, unnoticed expulsion is even more rare. In one study with more than 58,000 person-years of use, 132 pregnancies were noted, and 7 of these occurred in the setting of an unnoticed expulsion.13 Notably, a higher risk threshold is held for other medications. For example, statins are associated with a 3% risk of irreversible hepatic injury, yet serial liver function tests are not performed in patients without baseline liver dysfunction.17 A less than 0.1% risk of a non–life-threatening complication—unnoticed expulsion—does not warrant routine follow-up. Instead, the patient gauges the tolerability of that risk in making a follow-up plan, particularly given the varied individual preferences in patients’ management of the associated outcome of unintended pregnancy.

Are women interested in and able to perform their own string checks?
Recommendations to perform IUD string self-checks should consider whether women are willing and able to do so. In a study of 126 IUD users, 59% of women had attempted to check their IUD strings at home, and one-third were unable to do so successfully; all participants had visible strings on subsequent speculum exam.18 The women also were given the opportunity to perform a string self-check at the study visit. Overall, only 46% of participants found the exercise acceptable and were able to palpate the IUD strings.18 The authors aptly stated, “A universal recommendation for practice that is meant to identify a rare complication has no clinical utility if at least half of the women are unable to follow it.”
In which scenarios might a string check have clear utility?
The most important reason for follow-up after IUD placement or for patients to perform string self-checks is patient preference. At least anecdotally, some patients take comfort, particularly in the absence of menses, in palpating IUD strings regularly; these individuals should know that there is no necessity for but also no harm in this practice. In addition, patients may desire a string check or follow-up visit to discuss their new contraceptive’s goodness-of-fit.
While limited data show that routinely scheduling such visits does not improve contraceptive continuation, it is difficult to extrapolate these data to the select individuals who independently desire follow-up. (In addition, contraceptive continuance is hardly a metric of success, as clinicians and patients can agree that discontinuation in the setting of patient dissatisfaction is always appropriate.)
Clinicians should share with patients differing risks of IUD expulsion, and this may prompt more nuanced decisions about string checks and/or follow-up. Patients with postplacental or postabortion (second trimester) IUD placement or placement following prior expulsion may opt to perform string checks given the relatively higher risk of expulsion despite the maintained, absolutely low risk that such an event is unnoticed.
If a patient does present for a string check and strings are not visualized on exam, reasonable attempts should be made to identify the strings at that time. A cytobrush can be used to liberate and identify strings within the cervical canal. If the clinician cannot identify the strings or the patient is unable to tolerate such attempts, ultrasonography should be performed to localize the IUD. The ultrasound scan can be done in the office, if available, which is more cost-effective for women than a referral to radiology. If ultrasonography does not identify an intrauterine IUD, an x-ray is the next step to determine if the IUD has expulsed or perforated.
Continue to: Is a string check worth the cost?...
Is a string check worth the cost?
Health care providers may not be aware of the cost of care from the patient perspective. While the Affordable Care Act of 2010 mandates contraceptive coverage for women with insurance, a string check often is coded as a problem-based visit and thus may require a significant copay or out-of-pocket cost for high-deductible plans—without a proven benefit.19 Women who lack insurance coverage may forgo even necessary care due to the cost.20
The bottom line
The medical community and ObGyns specifically are familiar with a practice of patient self-examination falling by the wayside, as has been the case with breast self-examination.21 While counseling on string checks can complement conversations about risks and patients’ personal preferences regarding follow-up, no data support routine string checks in the clinic or at home. One of the great benefits of IUD use is its lack of barriers and resources for ongoing use. Physicians need not reintroduce burdens without benefits to those who desire this contraceptive method.
CASE A patient experiences unnessary inconvenience, distress, and cost following IUD placement
Ms. J had a levonorgestrel intrauterine device (IUD) placed at her postpartum visit. Her physician asked her to return for a string check in 4 to 6 weeks. She was dismayed at the prospect of re-presenting for care, as she is losing the Medicaid coverage that paid for her pregnancy care. One month later, she arranged for a babysitter so she could obtain the recommended string check. The physician told her the strings seemed longer than expected and ordered ultrasonography. Ms. J is distressed because of the mounting cost of care but is anxious to ensure that the IUD will prevent future pregnancy.
Should the routine IUD string check be reconsidered?
The string check dissension
Intrauterine devices offer reliable contraception with a high rate of satisfaction and a remarkably low rate of complications.1-3 With the increased uptake of IUDs, the value of “string checks” is being debated, with myriad responses from professional groups, manufacturers, and individual clinicians. For many practicing ObGyns, the question remains: Should patients be counseled about presenting for or doing their own IUD string checks?
Indeed, all IUD manufacturers recommend monthly self-examination to evaluate string presence.4-8 Manufacturers’ websites prominently display this information in material directed toward current or potential users, so many patients may be familiar already with this recommendation before their clinician visit. Yet, the Centers for Disease Control and Prevention state that no routine follow-up or monitoring is needed.9
In our case scenario, follow-up is clearly burdensome and ultimately costly. Instead, clinicians can advise patients to return with rare but important to recognize complications (such as perforation, expulsion, infection), adverse effects, or desire for change. While no data are available to support in-office or at-home string checks, data do show that women reliably present when intervention is needed.
Here, we explore 5 questions relevant to IUD string checks and discuss why it is time to rethink this practice habit.

What is the purpose of a string check?
String checks serve as a surrogate for assessing an IUD’s position and function. A string check can be performed by a clinician, who observes the IUD strings on speculum exam or palpates the strings on bimanual exam, or by the patient doing a self-exam. A positive string check purportedly assures both the IUD user and the health care provider that an IUD remains in a fundal, intrauterine position, thus providing an ongoing reliable contraceptive effect.
However, string check reliability in detecting contraceptive effectiveness is uncertain. Strings that subjectively feel or appear longer than anticipated can lead to unnecessary additional evaluation and emotional distress: These are harms. By contrast, when an expulsion occurs, it often is a partial expulsion or displacement, with unclear effect on patient or physician perception of the strings on examination. One retrospective review identified women with a history of IUD placement and a positive pregnancy test; those with an intrauterine pregnancy (74%) frequently also had a malpositioned IUD (55%) and rarely identifiable string issues (16%).10 Before asking patients and clinicians to use resources for performing string evaluations, the association between this action and outcomes of interest must be elucidated.
If not for assessing risk of expulsion, IUD follow-up allows the clinician to evaluate for other complications or adverse effects and to address patient concerns. This practice often is performed when the patient is starting a new medication or medical intervention. However, a systematic review involving 4 studies of IUD follow-up visits or phone calls after contraceptive initiation generated limited data, with no notable impact on contraceptive continuation or indicated use.11
Most important, data show that patients present to their clinician when issues arise with IUD use. One prospective study of 280 women compared multiple follow-up visits with a single 6-week follow-up visit after IUD placement; 10 expulsions were identified, and 8 of these were noted at unscheduled visits when patients presented with symptoms.12 This study suggests that there is little benefit in scheduled follow-up or set self-checks.
Furthermore, in a study in Finland of more than 17,000 IUD users, the rare participants who became pregnant during IUD use promptly presented for care because of a change in menses, pain, or symptoms of pregnancy.13 While IUDs are touted as user independent, this overlooks the reality: Data show that device failure, although rare, is rapidly and appropriately addressed by the user.
Continue to: Does the risk of IUD expulsion warrant string checks?...
Does the risk of IUD expulsion warrant string checks?
The risk of IUD expulsion is estimated to be 1% at 1 month and 4% at 1 year, with a contraceptive failure rate of 0.4% at 1 year. The risk of expulsion does not differ by age group, including adolescents, or parity, but it is higher with use of the copper IUD (2% at 1 month, 6% at 1 year) and with prior expulsion (14%, limited by small numbers).1 Furthermore, risk of expulsion is higher with postplacental placement and second trimester abortion.14,15 Despite this risk, the contraceptive failure rate of all types of IUDs remains consistently lower than all other reversible methods besides the contraceptive implant.16
Furthermore, while IUD expulsion is rare, unnoticed expulsion is even more rare. In one study with more than 58,000 person-years of use, 132 pregnancies were noted, and 7 of these occurred in the setting of an unnoticed expulsion.13 Notably, a higher risk threshold is held for other medications. For example, statins are associated with a 3% risk of irreversible hepatic injury, yet serial liver function tests are not performed in patients without baseline liver dysfunction.17 A less than 0.1% risk of a non–life-threatening complication—unnoticed expulsion—does not warrant routine follow-up. Instead, the patient gauges the tolerability of that risk in making a follow-up plan, particularly given the varied individual preferences in patients’ management of the associated outcome of unintended pregnancy.

Are women interested in and able to perform their own string checks?
Recommendations to perform IUD string self-checks should consider whether women are willing and able to do so. In a study of 126 IUD users, 59% of women had attempted to check their IUD strings at home, and one-third were unable to do so successfully; all participants had visible strings on subsequent speculum exam.18 The women also were given the opportunity to perform a string self-check at the study visit. Overall, only 46% of participants found the exercise acceptable and were able to palpate the IUD strings.18 The authors aptly stated, “A universal recommendation for practice that is meant to identify a rare complication has no clinical utility if at least half of the women are unable to follow it.”
In which scenarios might a string check have clear utility?
The most important reason for follow-up after IUD placement or for patients to perform string self-checks is patient preference. At least anecdotally, some patients take comfort, particularly in the absence of menses, in palpating IUD strings regularly; these individuals should know that there is no necessity for but also no harm in this practice. In addition, patients may desire a string check or follow-up visit to discuss their new contraceptive’s goodness-of-fit.
While limited data show that routinely scheduling such visits does not improve contraceptive continuation, it is difficult to extrapolate these data to the select individuals who independently desire follow-up. (In addition, contraceptive continuance is hardly a metric of success, as clinicians and patients can agree that discontinuation in the setting of patient dissatisfaction is always appropriate.)
Clinicians should share with patients differing risks of IUD expulsion, and this may prompt more nuanced decisions about string checks and/or follow-up. Patients with postplacental or postabortion (second trimester) IUD placement or placement following prior expulsion may opt to perform string checks given the relatively higher risk of expulsion despite the maintained, absolutely low risk that such an event is unnoticed.
If a patient does present for a string check and strings are not visualized on exam, reasonable attempts should be made to identify the strings at that time. A cytobrush can be used to liberate and identify strings within the cervical canal. If the clinician cannot identify the strings or the patient is unable to tolerate such attempts, ultrasonography should be performed to localize the IUD. The ultrasound scan can be done in the office, if available, which is more cost-effective for women than a referral to radiology. If ultrasonography does not identify an intrauterine IUD, an x-ray is the next step to determine if the IUD has expulsed or perforated.
Continue to: Is a string check worth the cost?...
Is a string check worth the cost?
Health care providers may not be aware of the cost of care from the patient perspective. While the Affordable Care Act of 2010 mandates contraceptive coverage for women with insurance, a string check often is coded as a problem-based visit and thus may require a significant copay or out-of-pocket cost for high-deductible plans—without a proven benefit.19 Women who lack insurance coverage may forgo even necessary care due to the cost.20
The bottom line
The medical community and ObGyns specifically are familiar with a practice of patient self-examination falling by the wayside, as has been the case with breast self-examination.21 While counseling on string checks can complement conversations about risks and patients’ personal preferences regarding follow-up, no data support routine string checks in the clinic or at home. One of the great benefits of IUD use is its lack of barriers and resources for ongoing use. Physicians need not reintroduce burdens without benefits to those who desire this contraceptive method.
- Aoun J, Dines VA, Stovall DW, et al. Effects of age, parity, and device type on complications and discontinuation of intrauterine devices. Obstet Gynecol. 2014;123:585-592.
- Peipert JF, Zhao Q, Allsworth JE, et al. Continuation and satisfaction of reversible contraception. Obstet Gynecol. 2011;117:1105-1113.
- American College of Obstetricians and Gynecologists Committee on Gynecology Practice. Committee opinion No. 672. Clinical challenges of long-acting reversible contraceptive methods. Obstet Gynecol. 2016;128:e69-e77.
- Mirena website. Placement of Mirena. 2019. https://www.mirena-us.com/placement-of-mirena/. Accessed December 7, 2019.
- Kyleena website. Let’s get started. 2019. https://www.kyleena-us.com/lets-get-started/what-to-expect/. Accessed December 7, 2019.
- Skyla website. What to expect. 2019. https://www.skyla-us.com/getting-skyla/index.php. Accessed December 7, 2019.
- Liletta website. What should I expect after Liletta insertion? 2020. https://www.liletta.com/about/what-to-expect-afterinsertion. Accessed December 7, 2019.
- Paragard website. What to expect with Paragard. 2019. https://www.paragard.com/what-can-i-expect-with-paragard/. Accessed December 7, 2019.
- Curtis KM, Jatlaoui TC, Tepper NK, et al. US selected practice recommendations for contraceptive use, 2016. MMWR Recomm Rep. 2016;65(4):1-66. https://www.cdc.gov/mmwr/ volumes/65/rr/pdfs/rr6504.pdf. Accessed February 19, 2020.
- Moschos E, Twickler DM. Intrauterine devices in early pregnancy: findings on ultrasound and clinical outcomes. Am J Obstet Gynecol. 2011;204:427.e1-6.
- Steenland MW, Zapata LB, Brahmi D, et al. Appropriate follow up to detect potential adverse events after initiation of select contraceptive methods: a systematic review. Contraception 2013;87:611-624.
- Neuteboom K, de Kroon CD, Dersjant-Roorda M, et al. Follow-up visits after IUD-insertion: sense or nonsense? Contraception. 2003;68:101-104.
- Backman T, Rauramo I, Huhtala S, et al. Pregnancy during the use of levonorgestrel intrauterine system. Am J Obstet Gynecol. 2004;190:50-54.
- Whitaker AK, Chen BA. Society of Family Planning guidelines: postplacental insertion of intrauterine devices. Contraception. 2018;97:2-13.
- Roe AH, Bartz D. Society of Family Planning clinical recommendations: contraception after surgical abortion. Contraception. 2019;99:2-9.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins–Gynecology. Practice bulletin No. 186. Long-acting reversible contraception: implants and intrauterine devices. Obstet Gynecol. 2017;130:e251-e269.
- US Food and Drug Administration. FDA drug safety communication: important safety label changes to cholesterol-lowering statin drugs. 2016. https://www .fda.gov/drugs/drug-safety-and-availability/fda-drugsafety-communication-important-safety-label-changescholesterol-lowering-statin-drugs. Accessed January 9, 2020.
- Melo J, Tschann M, Soon R, et al. Women’s willingness and ability to feel the strings of their intrauterine device. Int J Gynaecol Obstet. 2017;137:309-313.
- Healthcare.gov website. Health benefits & coverage: birth control benefits. 2020. https://www.healthcare.gov/ coverage/birth-control-benefits/. Accessed January 6, 2020.
- NORC at the University of Chicago. Americans’ views of healthcare costs, coverage, and policy. 2018;1-15. https:// www.norc.org/PDFs/WHI%20Healthcare%20Costs%20 Coverage%20and%20Policy/WHI%20Healthcare%20 Costs%20Coverage%20and%20Policy%20Issue%20Brief.pdf. Accessed February 19, 2020.
- Kosters JP, Gotzsche PC. Regular self-examination or clinical examination for early detection of breast cancer. Cochrane Database Syst Rev. 2003. CD003373.
- Aoun J, Dines VA, Stovall DW, et al. Effects of age, parity, and device type on complications and discontinuation of intrauterine devices. Obstet Gynecol. 2014;123:585-592.
- Peipert JF, Zhao Q, Allsworth JE, et al. Continuation and satisfaction of reversible contraception. Obstet Gynecol. 2011;117:1105-1113.
- American College of Obstetricians and Gynecologists Committee on Gynecology Practice. Committee opinion No. 672. Clinical challenges of long-acting reversible contraceptive methods. Obstet Gynecol. 2016;128:e69-e77.
- Mirena website. Placement of Mirena. 2019. https://www.mirena-us.com/placement-of-mirena/. Accessed December 7, 2019.
- Kyleena website. Let’s get started. 2019. https://www.kyleena-us.com/lets-get-started/what-to-expect/. Accessed December 7, 2019.
- Skyla website. What to expect. 2019. https://www.skyla-us.com/getting-skyla/index.php. Accessed December 7, 2019.
- Liletta website. What should I expect after Liletta insertion? 2020. https://www.liletta.com/about/what-to-expect-afterinsertion. Accessed December 7, 2019.
- Paragard website. What to expect with Paragard. 2019. https://www.paragard.com/what-can-i-expect-with-paragard/. Accessed December 7, 2019.
- Curtis KM, Jatlaoui TC, Tepper NK, et al. US selected practice recommendations for contraceptive use, 2016. MMWR Recomm Rep. 2016;65(4):1-66. https://www.cdc.gov/mmwr/ volumes/65/rr/pdfs/rr6504.pdf. Accessed February 19, 2020.
- Moschos E, Twickler DM. Intrauterine devices in early pregnancy: findings on ultrasound and clinical outcomes. Am J Obstet Gynecol. 2011;204:427.e1-6.
- Steenland MW, Zapata LB, Brahmi D, et al. Appropriate follow up to detect potential adverse events after initiation of select contraceptive methods: a systematic review. Contraception 2013;87:611-624.
- Neuteboom K, de Kroon CD, Dersjant-Roorda M, et al. Follow-up visits after IUD-insertion: sense or nonsense? Contraception. 2003;68:101-104.
- Backman T, Rauramo I, Huhtala S, et al. Pregnancy during the use of levonorgestrel intrauterine system. Am J Obstet Gynecol. 2004;190:50-54.
- Whitaker AK, Chen BA. Society of Family Planning guidelines: postplacental insertion of intrauterine devices. Contraception. 2018;97:2-13.
- Roe AH, Bartz D. Society of Family Planning clinical recommendations: contraception after surgical abortion. Contraception. 2019;99:2-9.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins–Gynecology. Practice bulletin No. 186. Long-acting reversible contraception: implants and intrauterine devices. Obstet Gynecol. 2017;130:e251-e269.
- US Food and Drug Administration. FDA drug safety communication: important safety label changes to cholesterol-lowering statin drugs. 2016. https://www .fda.gov/drugs/drug-safety-and-availability/fda-drugsafety-communication-important-safety-label-changescholesterol-lowering-statin-drugs. Accessed January 9, 2020.
- Melo J, Tschann M, Soon R, et al. Women’s willingness and ability to feel the strings of their intrauterine device. Int J Gynaecol Obstet. 2017;137:309-313.
- Healthcare.gov website. Health benefits & coverage: birth control benefits. 2020. https://www.healthcare.gov/ coverage/birth-control-benefits/. Accessed January 6, 2020.
- NORC at the University of Chicago. Americans’ views of healthcare costs, coverage, and policy. 2018;1-15. https:// www.norc.org/PDFs/WHI%20Healthcare%20Costs%20 Coverage%20and%20Policy/WHI%20Healthcare%20 Costs%20Coverage%20and%20Policy%20Issue%20Brief.pdf. Accessed February 19, 2020.
- Kosters JP, Gotzsche PC. Regular self-examination or clinical examination for early detection of breast cancer. Cochrane Database Syst Rev. 2003. CD003373.
New guideline offers recommendations for reproductive health in patients with rheumatic diseases
A new guideline from the American College of Rheumatology offers the organization’s first clinical recommendations on how to manage reproductive health issues in patients with rheumatic and musculoskeletal diseases (RMDs).
“With the development of this guideline, the ACR recognizes the key role of clinical rheumatologists not only in managing disease activity but also in understanding the interactions of RMDs and their therapies in the context of reproductive health,” wrote Lisa R. Sammaritano, MD, of Weill Cornell Medicine and the Hospital for Special Surgery in New York, and coauthors. The guideline was published in Arthritis & Rheumatology.
To develop an evidence-based guideline on reproductive health in RMD patients, the researchers embarked on a systematic review of studies in areas like contraception, pregnancy and lactation, assisted reproductive technology (ART), fertility preservation, and hormone therapy. The guideline contains 12 ungraded good practice statements and 131 graded recommendations, all developed through the Grading of Recommendations Assessment, Development, and Evaluation methodology.
In counseling patients about these areas of care, the guideline says that rheumatologists and other clinicians “must collaborate with specialists in the fields of obstetrics-gynecology, maternal-fetal medicine, and reproductive endocrinology and infertility.”
“One thing this guideline does well is highlight the importance of involving maternal-fetal medicine colleagues,” Alison Cahill, MD, a professor in the department of women’s health at the University of Texas at Austin and a maternal-fetal medicine specialist within UT Health Austin’s Women’s Health Institute, said when asked for comment on the guideline. “We’re always very happy to see patients ahead of time who are planning pregnancy to be able to discuss what the care plan would look like. And specifically, to address medications, if required, for their rheumatologic care.
“As we learn more and more,” she added, “we’ve come to understand that most treatments and medications are actually safe or relatively safe to take in pregnancy. Certainly, the benefit of taking them outweighs any small or theoretic risks. On the flip side, the guideline does a nice job of highlighting the importance of good disease control, both at the time of conception and during pregnancy.”
Contraception
In regard to contraception, the guideline strongly recommends the use of effective contraceptives – with a conditional recommendation of IUDs or a subdermal progestin implant – in fertile women with a RMD who have neither systemic lupus erythematosus (SLE) nor positive antiphospholipid antibody (aPL). They also strongly recommend discussing the use of emergency contraception with all RMD patients.
For SLE patients, the guideline strongly recommends the use of effective contraceptives in those with stable or low disease activity who are not positive for aPL. They also strongly recommend progestin‐only or IUD contraceptives over combined estrogen‐progestin contraception. For aPL-positive patients, the guideline strongly recommends against combined estrogen‐progestin contraceptives and for levonorgestrel or copper IUDs or the progestin‐only pill.
Assisted reproductive technology
In regard to ART, the guideline strongly recommends proceeding as needed in aPL-negative women with uncomplicated, stable RMD who are on pregnancy‐compatible medications. They also strongly recommend deferring ART in any RMD patients with moderately or severely active disease.
For aPL-positive patients undergoing ART procedures, they strongly recommend prophylactic anticoagulation with heparin or low-molecular-weight heparin (LMWH) in women with obstetric antiphospholipid syndrome (APS) and therapeutic anticoagulation in women with thrombotic APS. In patients undergoing embryo and oocyte cryopreservation, they strongly recommend continuing immunosuppressive and biologic therapies – the exception being cyclophosphamide (CYC) – for anyone in stable condition.
Fertility preservation
In regard to fertility preservation in patients taking CYC, the guideline strongly suggests sperm cryopreservation as good practice prior to treatment. They also conditionally recommend monthly gonadotropin‐releasing hormone agonist cotherapy in premenopausal women with RMD.
Hormone therapy
In regard to menopause and hormone therapy, the guideline strongly suggests hormone therapy as good practice in postmenopausal women with RMD, without SLE or positive aPL, and who have severe vasomotor symptoms. Hormone therapy is conditionally recommended in patients with SLE, without positive aPL, and with no contraindications. For aPL-positive patients, they strongly recommend against hormone therapy in women with obstetric and/or thrombotic APS.
Pregnancy assessment and management
Among the many recommendations regarding pregnancy assessment and management, the guideline strongly suggests counseling women with RMD who are considering pregnancy to take into account the improved outcomes for pregnant women with low disease activity. They strongly recommend that women considering pregnancy should switch to pregnancy‐compatible medication and pause to assess its efficacy and tolerability before moving forward, along with strongly recommending that pregnant women with active disease initiate or continue a pregnancy‐compatible steroid‐sparing medication. They also recommend testing for anti‐Ro/SS-A and anti‐La/SS-B in women with SLE, Sjögren’s syndrome, systemic sclerosis, or rheumatoid arthritis, but only once and only before or early in the pregnancy.
For women with systemic sclerosis who develop scleroderma renal crisis during pregnancy, the authors strongly advise using ACE inhibitors or angiotensin receptor blockers “because the risk of maternal or fetal death with untreated disease is higher than the risk associated with use of these medications during pregnancy.”
Among women with SLE, the recommendations strongly call for testing either before or early in pregnancy for anticardiolipin antibody, anti–beta2-glycoprotein I, or positive lupus anticoagulant, as well as initiating or continuing hydroxychloroquine (HCQ) if possible. Starting in the first trimester, the authors also conditionally recommend that SLE patients take low-dose aspirin daily
For pregnant women who test positive for aPL but do not meet criteria for obstetric or thrombotic APS, the guideline conditionally recommends prophylactic treatment with low-dose aspirin daily to protect against preeclampsia. When obstetric APS criteria are met, the guideline strongly advises combined treatment with daily low-dose aspirin and prophylactic-dose heparin (or LMWH), as well as prophylactic-dose anticoagulation for 6-12 weeks post partum. When patients have thrombotic APS, this combination treatment should contain heparin dose at a therapeutic level throughout pregnancy and postpartum. However, the authors conditionally recommend against giving low-dose aspirin plus prophylactic-dose heparin to women without obstetric APS. For refractory obstetric APS, the guideline also contains recommendations that are conditionally against treatment with intravenous immunoglobulin or an increased LMWH dose and strongly against adding prednisone to prophylactic-dose heparin or LMWH and low-dose aspirin. In pregnant patients with primary APS, the authors conditionally advise adding HCQ to prophylactic-dose heparin or LMWH and low-dose aspirin therapy. However, women with aPL who do not meet APS criteria or have another indication for HCQ are conditionally advised against prophylactic treatment with the antimalarial.
For women with Anti-Ro/SS-A and/or anti-La/SS-B antibodies in pregnancy, there is conditional advice to use HCQ. When there is no history of an infant with complete heart block or neonatal lupus erythematosus among women with these antibodies, the guideline conditionally advises serial fetal echocardiography (less often than weekly) starting between 16 and 18 weeks and continuing through 26 weeks, but this should be weekly when there is a prior history. Treatment with oral dexamethasone 4 mg daily is conditionally advised when there is echocardiographic evidence of fetal first- or second-degree heart block, but dexamethasone is not recommended when complete heart block is present.
Finally, in regard to medication use, the authors strongly recommend that men who are planning to be fathers continue on HCQ, azathioprine, 6‐mercaptopurine, colchicine, or tumor necrosis factor inhibitors. Conditional treatment recommendations for men planning for pregnancy include methotrexate, mycophenolate mofetil/mycophenolic acid (MMF), leflunomide, sulfasalazine, calcineurin inhibitors, and NSAIDs. They also strongly recommend that this group of men discontinue CYC and thalidomide.
Pregnant women are strongly recommended to discontinue methotrexate, leflunomide (with cholestyramine washout if there are detectable serum levels of its metabolite prior to pregnancy or as soon as it is confirmed), MMF, CYC, and thalidomide within 3 months prior to conception, and they strongly recommend HCQ (in women with SLE), azathioprine/6‐mercaptopurine, colchicine, or sulfasalazine for use throughout pregnancy. They strongly recommend a combination of low‐dose aspirin and prophylactic‐dose heparin for pregnant women with obstetric APS, along with low‐dose aspirin and therapeutic‐dose heparin for women with thrombotic APS throughout pregnancy and postpartum. However, for women with SLE and those who test positive for aPL but do not meet criteria for obstetric or thrombotic APS, the authors conditionally recommend low-dose aspirin starting in the first trimester.
The guideline suggests that women with RMD should be encouraged to breastfeed if they are willing and able; they also suggest that disease control be maintained through lactation‐compatible medications and that the risks and benefits be reviewed on a patient-by-patient basis. Treatment with HCQ, colchicine, sulfasalazine, rituximab, and all tumor necrosis factor inhibitors are strongly recommended as being compatible with breastfeeding, and they strongly recommend against using CYC, leflunomide, MMF, and thalidomide while breastfeeding.
The authors acknowledged the limitations of their guideline, including the literature review being conducted on studies involving adults and an “inability to include recommendations for uncommon but important clinical situations,” including those involving transgender patients and hormonal therapies.
The authors reported numerous potential conflicts of interest, including receiving research support, consulting fees, speaking fees, and honoraria from various pharmaceutical companies.
SOURCE: Sammaritano LR et al. Arthritis Rheumatol. 2020 Feb 23. doi: 10.1002/art.41191.
A new guideline from the American College of Rheumatology offers the organization’s first clinical recommendations on how to manage reproductive health issues in patients with rheumatic and musculoskeletal diseases (RMDs).
“With the development of this guideline, the ACR recognizes the key role of clinical rheumatologists not only in managing disease activity but also in understanding the interactions of RMDs and their therapies in the context of reproductive health,” wrote Lisa R. Sammaritano, MD, of Weill Cornell Medicine and the Hospital for Special Surgery in New York, and coauthors. The guideline was published in Arthritis & Rheumatology.
To develop an evidence-based guideline on reproductive health in RMD patients, the researchers embarked on a systematic review of studies in areas like contraception, pregnancy and lactation, assisted reproductive technology (ART), fertility preservation, and hormone therapy. The guideline contains 12 ungraded good practice statements and 131 graded recommendations, all developed through the Grading of Recommendations Assessment, Development, and Evaluation methodology.
In counseling patients about these areas of care, the guideline says that rheumatologists and other clinicians “must collaborate with specialists in the fields of obstetrics-gynecology, maternal-fetal medicine, and reproductive endocrinology and infertility.”
“One thing this guideline does well is highlight the importance of involving maternal-fetal medicine colleagues,” Alison Cahill, MD, a professor in the department of women’s health at the University of Texas at Austin and a maternal-fetal medicine specialist within UT Health Austin’s Women’s Health Institute, said when asked for comment on the guideline. “We’re always very happy to see patients ahead of time who are planning pregnancy to be able to discuss what the care plan would look like. And specifically, to address medications, if required, for their rheumatologic care.
“As we learn more and more,” she added, “we’ve come to understand that most treatments and medications are actually safe or relatively safe to take in pregnancy. Certainly, the benefit of taking them outweighs any small or theoretic risks. On the flip side, the guideline does a nice job of highlighting the importance of good disease control, both at the time of conception and during pregnancy.”
Contraception
In regard to contraception, the guideline strongly recommends the use of effective contraceptives – with a conditional recommendation of IUDs or a subdermal progestin implant – in fertile women with a RMD who have neither systemic lupus erythematosus (SLE) nor positive antiphospholipid antibody (aPL). They also strongly recommend discussing the use of emergency contraception with all RMD patients.
For SLE patients, the guideline strongly recommends the use of effective contraceptives in those with stable or low disease activity who are not positive for aPL. They also strongly recommend progestin‐only or IUD contraceptives over combined estrogen‐progestin contraception. For aPL-positive patients, the guideline strongly recommends against combined estrogen‐progestin contraceptives and for levonorgestrel or copper IUDs or the progestin‐only pill.
Assisted reproductive technology
In regard to ART, the guideline strongly recommends proceeding as needed in aPL-negative women with uncomplicated, stable RMD who are on pregnancy‐compatible medications. They also strongly recommend deferring ART in any RMD patients with moderately or severely active disease.
For aPL-positive patients undergoing ART procedures, they strongly recommend prophylactic anticoagulation with heparin or low-molecular-weight heparin (LMWH) in women with obstetric antiphospholipid syndrome (APS) and therapeutic anticoagulation in women with thrombotic APS. In patients undergoing embryo and oocyte cryopreservation, they strongly recommend continuing immunosuppressive and biologic therapies – the exception being cyclophosphamide (CYC) – for anyone in stable condition.
Fertility preservation
In regard to fertility preservation in patients taking CYC, the guideline strongly suggests sperm cryopreservation as good practice prior to treatment. They also conditionally recommend monthly gonadotropin‐releasing hormone agonist cotherapy in premenopausal women with RMD.
Hormone therapy
In regard to menopause and hormone therapy, the guideline strongly suggests hormone therapy as good practice in postmenopausal women with RMD, without SLE or positive aPL, and who have severe vasomotor symptoms. Hormone therapy is conditionally recommended in patients with SLE, without positive aPL, and with no contraindications. For aPL-positive patients, they strongly recommend against hormone therapy in women with obstetric and/or thrombotic APS.
Pregnancy assessment and management
Among the many recommendations regarding pregnancy assessment and management, the guideline strongly suggests counseling women with RMD who are considering pregnancy to take into account the improved outcomes for pregnant women with low disease activity. They strongly recommend that women considering pregnancy should switch to pregnancy‐compatible medication and pause to assess its efficacy and tolerability before moving forward, along with strongly recommending that pregnant women with active disease initiate or continue a pregnancy‐compatible steroid‐sparing medication. They also recommend testing for anti‐Ro/SS-A and anti‐La/SS-B in women with SLE, Sjögren’s syndrome, systemic sclerosis, or rheumatoid arthritis, but only once and only before or early in the pregnancy.
For women with systemic sclerosis who develop scleroderma renal crisis during pregnancy, the authors strongly advise using ACE inhibitors or angiotensin receptor blockers “because the risk of maternal or fetal death with untreated disease is higher than the risk associated with use of these medications during pregnancy.”
Among women with SLE, the recommendations strongly call for testing either before or early in pregnancy for anticardiolipin antibody, anti–beta2-glycoprotein I, or positive lupus anticoagulant, as well as initiating or continuing hydroxychloroquine (HCQ) if possible. Starting in the first trimester, the authors also conditionally recommend that SLE patients take low-dose aspirin daily
For pregnant women who test positive for aPL but do not meet criteria for obstetric or thrombotic APS, the guideline conditionally recommends prophylactic treatment with low-dose aspirin daily to protect against preeclampsia. When obstetric APS criteria are met, the guideline strongly advises combined treatment with daily low-dose aspirin and prophylactic-dose heparin (or LMWH), as well as prophylactic-dose anticoagulation for 6-12 weeks post partum. When patients have thrombotic APS, this combination treatment should contain heparin dose at a therapeutic level throughout pregnancy and postpartum. However, the authors conditionally recommend against giving low-dose aspirin plus prophylactic-dose heparin to women without obstetric APS. For refractory obstetric APS, the guideline also contains recommendations that are conditionally against treatment with intravenous immunoglobulin or an increased LMWH dose and strongly against adding prednisone to prophylactic-dose heparin or LMWH and low-dose aspirin. In pregnant patients with primary APS, the authors conditionally advise adding HCQ to prophylactic-dose heparin or LMWH and low-dose aspirin therapy. However, women with aPL who do not meet APS criteria or have another indication for HCQ are conditionally advised against prophylactic treatment with the antimalarial.
For women with Anti-Ro/SS-A and/or anti-La/SS-B antibodies in pregnancy, there is conditional advice to use HCQ. When there is no history of an infant with complete heart block or neonatal lupus erythematosus among women with these antibodies, the guideline conditionally advises serial fetal echocardiography (less often than weekly) starting between 16 and 18 weeks and continuing through 26 weeks, but this should be weekly when there is a prior history. Treatment with oral dexamethasone 4 mg daily is conditionally advised when there is echocardiographic evidence of fetal first- or second-degree heart block, but dexamethasone is not recommended when complete heart block is present.
Finally, in regard to medication use, the authors strongly recommend that men who are planning to be fathers continue on HCQ, azathioprine, 6‐mercaptopurine, colchicine, or tumor necrosis factor inhibitors. Conditional treatment recommendations for men planning for pregnancy include methotrexate, mycophenolate mofetil/mycophenolic acid (MMF), leflunomide, sulfasalazine, calcineurin inhibitors, and NSAIDs. They also strongly recommend that this group of men discontinue CYC and thalidomide.
Pregnant women are strongly recommended to discontinue methotrexate, leflunomide (with cholestyramine washout if there are detectable serum levels of its metabolite prior to pregnancy or as soon as it is confirmed), MMF, CYC, and thalidomide within 3 months prior to conception, and they strongly recommend HCQ (in women with SLE), azathioprine/6‐mercaptopurine, colchicine, or sulfasalazine for use throughout pregnancy. They strongly recommend a combination of low‐dose aspirin and prophylactic‐dose heparin for pregnant women with obstetric APS, along with low‐dose aspirin and therapeutic‐dose heparin for women with thrombotic APS throughout pregnancy and postpartum. However, for women with SLE and those who test positive for aPL but do not meet criteria for obstetric or thrombotic APS, the authors conditionally recommend low-dose aspirin starting in the first trimester.
The guideline suggests that women with RMD should be encouraged to breastfeed if they are willing and able; they also suggest that disease control be maintained through lactation‐compatible medications and that the risks and benefits be reviewed on a patient-by-patient basis. Treatment with HCQ, colchicine, sulfasalazine, rituximab, and all tumor necrosis factor inhibitors are strongly recommended as being compatible with breastfeeding, and they strongly recommend against using CYC, leflunomide, MMF, and thalidomide while breastfeeding.
The authors acknowledged the limitations of their guideline, including the literature review being conducted on studies involving adults and an “inability to include recommendations for uncommon but important clinical situations,” including those involving transgender patients and hormonal therapies.
The authors reported numerous potential conflicts of interest, including receiving research support, consulting fees, speaking fees, and honoraria from various pharmaceutical companies.
SOURCE: Sammaritano LR et al. Arthritis Rheumatol. 2020 Feb 23. doi: 10.1002/art.41191.
A new guideline from the American College of Rheumatology offers the organization’s first clinical recommendations on how to manage reproductive health issues in patients with rheumatic and musculoskeletal diseases (RMDs).
“With the development of this guideline, the ACR recognizes the key role of clinical rheumatologists not only in managing disease activity but also in understanding the interactions of RMDs and their therapies in the context of reproductive health,” wrote Lisa R. Sammaritano, MD, of Weill Cornell Medicine and the Hospital for Special Surgery in New York, and coauthors. The guideline was published in Arthritis & Rheumatology.
To develop an evidence-based guideline on reproductive health in RMD patients, the researchers embarked on a systematic review of studies in areas like contraception, pregnancy and lactation, assisted reproductive technology (ART), fertility preservation, and hormone therapy. The guideline contains 12 ungraded good practice statements and 131 graded recommendations, all developed through the Grading of Recommendations Assessment, Development, and Evaluation methodology.
In counseling patients about these areas of care, the guideline says that rheumatologists and other clinicians “must collaborate with specialists in the fields of obstetrics-gynecology, maternal-fetal medicine, and reproductive endocrinology and infertility.”
“One thing this guideline does well is highlight the importance of involving maternal-fetal medicine colleagues,” Alison Cahill, MD, a professor in the department of women’s health at the University of Texas at Austin and a maternal-fetal medicine specialist within UT Health Austin’s Women’s Health Institute, said when asked for comment on the guideline. “We’re always very happy to see patients ahead of time who are planning pregnancy to be able to discuss what the care plan would look like. And specifically, to address medications, if required, for their rheumatologic care.
“As we learn more and more,” she added, “we’ve come to understand that most treatments and medications are actually safe or relatively safe to take in pregnancy. Certainly, the benefit of taking them outweighs any small or theoretic risks. On the flip side, the guideline does a nice job of highlighting the importance of good disease control, both at the time of conception and during pregnancy.”
Contraception
In regard to contraception, the guideline strongly recommends the use of effective contraceptives – with a conditional recommendation of IUDs or a subdermal progestin implant – in fertile women with a RMD who have neither systemic lupus erythematosus (SLE) nor positive antiphospholipid antibody (aPL). They also strongly recommend discussing the use of emergency contraception with all RMD patients.
For SLE patients, the guideline strongly recommends the use of effective contraceptives in those with stable or low disease activity who are not positive for aPL. They also strongly recommend progestin‐only or IUD contraceptives over combined estrogen‐progestin contraception. For aPL-positive patients, the guideline strongly recommends against combined estrogen‐progestin contraceptives and for levonorgestrel or copper IUDs or the progestin‐only pill.
Assisted reproductive technology
In regard to ART, the guideline strongly recommends proceeding as needed in aPL-negative women with uncomplicated, stable RMD who are on pregnancy‐compatible medications. They also strongly recommend deferring ART in any RMD patients with moderately or severely active disease.
For aPL-positive patients undergoing ART procedures, they strongly recommend prophylactic anticoagulation with heparin or low-molecular-weight heparin (LMWH) in women with obstetric antiphospholipid syndrome (APS) and therapeutic anticoagulation in women with thrombotic APS. In patients undergoing embryo and oocyte cryopreservation, they strongly recommend continuing immunosuppressive and biologic therapies – the exception being cyclophosphamide (CYC) – for anyone in stable condition.
Fertility preservation
In regard to fertility preservation in patients taking CYC, the guideline strongly suggests sperm cryopreservation as good practice prior to treatment. They also conditionally recommend monthly gonadotropin‐releasing hormone agonist cotherapy in premenopausal women with RMD.
Hormone therapy
In regard to menopause and hormone therapy, the guideline strongly suggests hormone therapy as good practice in postmenopausal women with RMD, without SLE or positive aPL, and who have severe vasomotor symptoms. Hormone therapy is conditionally recommended in patients with SLE, without positive aPL, and with no contraindications. For aPL-positive patients, they strongly recommend against hormone therapy in women with obstetric and/or thrombotic APS.
Pregnancy assessment and management
Among the many recommendations regarding pregnancy assessment and management, the guideline strongly suggests counseling women with RMD who are considering pregnancy to take into account the improved outcomes for pregnant women with low disease activity. They strongly recommend that women considering pregnancy should switch to pregnancy‐compatible medication and pause to assess its efficacy and tolerability before moving forward, along with strongly recommending that pregnant women with active disease initiate or continue a pregnancy‐compatible steroid‐sparing medication. They also recommend testing for anti‐Ro/SS-A and anti‐La/SS-B in women with SLE, Sjögren’s syndrome, systemic sclerosis, or rheumatoid arthritis, but only once and only before or early in the pregnancy.
For women with systemic sclerosis who develop scleroderma renal crisis during pregnancy, the authors strongly advise using ACE inhibitors or angiotensin receptor blockers “because the risk of maternal or fetal death with untreated disease is higher than the risk associated with use of these medications during pregnancy.”
Among women with SLE, the recommendations strongly call for testing either before or early in pregnancy for anticardiolipin antibody, anti–beta2-glycoprotein I, or positive lupus anticoagulant, as well as initiating or continuing hydroxychloroquine (HCQ) if possible. Starting in the first trimester, the authors also conditionally recommend that SLE patients take low-dose aspirin daily
For pregnant women who test positive for aPL but do not meet criteria for obstetric or thrombotic APS, the guideline conditionally recommends prophylactic treatment with low-dose aspirin daily to protect against preeclampsia. When obstetric APS criteria are met, the guideline strongly advises combined treatment with daily low-dose aspirin and prophylactic-dose heparin (or LMWH), as well as prophylactic-dose anticoagulation for 6-12 weeks post partum. When patients have thrombotic APS, this combination treatment should contain heparin dose at a therapeutic level throughout pregnancy and postpartum. However, the authors conditionally recommend against giving low-dose aspirin plus prophylactic-dose heparin to women without obstetric APS. For refractory obstetric APS, the guideline also contains recommendations that are conditionally against treatment with intravenous immunoglobulin or an increased LMWH dose and strongly against adding prednisone to prophylactic-dose heparin or LMWH and low-dose aspirin. In pregnant patients with primary APS, the authors conditionally advise adding HCQ to prophylactic-dose heparin or LMWH and low-dose aspirin therapy. However, women with aPL who do not meet APS criteria or have another indication for HCQ are conditionally advised against prophylactic treatment with the antimalarial.
For women with Anti-Ro/SS-A and/or anti-La/SS-B antibodies in pregnancy, there is conditional advice to use HCQ. When there is no history of an infant with complete heart block or neonatal lupus erythematosus among women with these antibodies, the guideline conditionally advises serial fetal echocardiography (less often than weekly) starting between 16 and 18 weeks and continuing through 26 weeks, but this should be weekly when there is a prior history. Treatment with oral dexamethasone 4 mg daily is conditionally advised when there is echocardiographic evidence of fetal first- or second-degree heart block, but dexamethasone is not recommended when complete heart block is present.
Finally, in regard to medication use, the authors strongly recommend that men who are planning to be fathers continue on HCQ, azathioprine, 6‐mercaptopurine, colchicine, or tumor necrosis factor inhibitors. Conditional treatment recommendations for men planning for pregnancy include methotrexate, mycophenolate mofetil/mycophenolic acid (MMF), leflunomide, sulfasalazine, calcineurin inhibitors, and NSAIDs. They also strongly recommend that this group of men discontinue CYC and thalidomide.
Pregnant women are strongly recommended to discontinue methotrexate, leflunomide (with cholestyramine washout if there are detectable serum levels of its metabolite prior to pregnancy or as soon as it is confirmed), MMF, CYC, and thalidomide within 3 months prior to conception, and they strongly recommend HCQ (in women with SLE), azathioprine/6‐mercaptopurine, colchicine, or sulfasalazine for use throughout pregnancy. They strongly recommend a combination of low‐dose aspirin and prophylactic‐dose heparin for pregnant women with obstetric APS, along with low‐dose aspirin and therapeutic‐dose heparin for women with thrombotic APS throughout pregnancy and postpartum. However, for women with SLE and those who test positive for aPL but do not meet criteria for obstetric or thrombotic APS, the authors conditionally recommend low-dose aspirin starting in the first trimester.
The guideline suggests that women with RMD should be encouraged to breastfeed if they are willing and able; they also suggest that disease control be maintained through lactation‐compatible medications and that the risks and benefits be reviewed on a patient-by-patient basis. Treatment with HCQ, colchicine, sulfasalazine, rituximab, and all tumor necrosis factor inhibitors are strongly recommended as being compatible with breastfeeding, and they strongly recommend against using CYC, leflunomide, MMF, and thalidomide while breastfeeding.
The authors acknowledged the limitations of their guideline, including the literature review being conducted on studies involving adults and an “inability to include recommendations for uncommon but important clinical situations,” including those involving transgender patients and hormonal therapies.
The authors reported numerous potential conflicts of interest, including receiving research support, consulting fees, speaking fees, and honoraria from various pharmaceutical companies.
SOURCE: Sammaritano LR et al. Arthritis Rheumatol. 2020 Feb 23. doi: 10.1002/art.41191.
FROM ARTHRITIS & RHEUMATOLOGY
Community-wide initiative ups teen LARC adoption sixfold
In Rochester, N.Y., a comprehensive community initiative that raised awareness about and delivered training in the use of long-acting reversible contraceptives (LARCs) significantly upped LARC adoption among sexually active female high schoolers.
Over the course of the 3-year project, LARC use rose from about 4% to 24% in this group, a statistically significant increase (P less than .0001). During the same time period, LARC use increased nationally, as well, but at a lower rate, rising from 2% to 5% for the same population, while New York state saw LARC use rise from 2% to 5%.
In New York City, where an unrelated LARC awareness campaign was conducted, LARC use went from 3% to 5% over the study period for sexually active female high school students. Comparing the trend in LARC use in Rochester to the secular trend in these control groups showed significantly higher uptake over time in Rochester (P less than .0001).
Through a series of lunch-and-learn talks given to adults who work with adolescents in community-based settings and in medical settings, the Greater Rochester LARC Initiative reached more than 1,300 individuals during July 2014-June 2017, C. Andrew Aligne, MD, MPH, of the University of Rochester (N.Y.), and coauthors reported in the American Journal of Obstetrics and Gynecology.
Of the 81 total talks delivered, 50 were in medical settings, reaching 703 attendees ranging from front-office personnel to primary care physicians, advanced practice clinicians, and nurses; the talks in community-based settings reached 662 attendees.
“We use the term ‘community detailing’ to describe the design of the intervention because it was an innovative hybrid of academic detailing and community health education,” explained Dr. Aligne and colleagues. This approach is a unique, feasible, and effective approach to unintended adolescent pregnancy programs. “The community detailing approach could be a useful complement to programs for preventing unintended adolescent pregnancy.”
The study’s primary outcome measure was LARC use among sexually active female high school students as identified by responses on the U.S. Centers for Disease Control and Statistics’ Youth Risk Behavior Survey (YRBS).
YRBS data were examined for the years 2013, 2015, and 2017, spanning the period before and after the LARC initiative was begun. A separate question about LARC use wasn’t included in the 2013 YRBS survey, so the investigators used a generous estimate that two-thirds of respondents who reported using the “other” contraceptive category for that year were using LARCs. That category was chosen by a total of 6% of respondents, and encompassed LARC use along with use of the patch, ring, diaphragm, and fertility awareness, explained Dr. Aligne and collaborators.
Addressing the problem of failure to use a condom with LARC use, Dr. Aligne and collaborators found overall low rates of dual-method use, but higher rates in Rochester than in the comparison groups. In Rochester, 78% of respondents reported that they also did not use condoms. This figure was lower than the 91% reported for the United States as a whole, and also was lower than the 93% reported in New York City and the 85% reported in New York state. No increase in sexually transmitted infections was seen in Rochester’s sexually active high school females during the study period.
“Our main finding of increased LARC use is consistent with the literature demonstrating that many sexually active young women, including adolescents, will choose LARC if they are given access not only to birth control itself, but also to accurate information about various contraceptive methods,” concluded Dr. Aligne and his associates.
A practical strength of the Greater Rochester LARC initiative was that it capitalized on existing resources, such as New York state’s preexisting program for free access to contraception and similar provisions in the Affordable Care Act. Also, local Title X clinics that were enrolled in New York’s free contraception initiative already had practitioners who were trained and able to provide same-day LARC insertion.
Pediatricians engaged in the initiative were able to receive free training from LARC manufacturers, as mandated by the Food and Drug Administration. Through collaboration with implant manufacturers, Rochester LARC Initiative staff were able to piggyback on training sessions to add education about contraception counseling and the importance of offering access to all contraception methods.
Taken as a whole, the LARC Initiative could be scaled up, wrote Dr. Aligne and his coauthors, a potential boon in the 21 states where qualifying individuals younger than 19 years of age are eligible for Medicaid reimbursement for family planning services. “Even though easy LARC access is far from universal, there are vast areas of the nation where cost need not be seen as an insurmountable barrier.” Dr. Aligne and coauthors also addressed the fraught history of reproductive justice in the United States, cautioning that universal LARC adoption was not – and should not be – the goal of such initiatives. “There is a history of reproductive coercion in the U.S. including forced sterilization of women of color; therefore, it is critical that LARC methods not be imposed on any particular group. On the other hand, LARC should not be withheld deliberately from adolescents who want it, as this is another form of injustice,” they wrote. “The goal should be to empower individuals to decide what is right for them in a context of social and reproductive justice.”
Using the nationally administered YRBS was a significant strength of the study, commented Dr. Aligne and his collaborators. “This allowed us to employ the study design of pre-post with a nonrandomized control group,” the investigators noted, adding that the “relatively rigorous” methodology reduced the risk of problems with internal validity, and also allowed comparisons between changes in Rochester and those at the state and national level.
However, the researchers acknowledged that the study was not a randomized trial, and there’s always the possibility of unknown confounders contributing to LARC uptake during the study period. Also, the YRBS is a self-report instrument and only includes those enrolled in school.
Dr. Aligne reported that his spouse received compensation for providing contraceptive implant insertion training, as did two coauthors. The LARC initiative was supported by a grant from the Greater Rochester Health Foundation.
SOURCE: Aligne CA et al. Am J Obstet Gynecol. 2020 Jan 22. doi: 10.1016/j.ajog.2020.01.029.
In Rochester, N.Y., a comprehensive community initiative that raised awareness about and delivered training in the use of long-acting reversible contraceptives (LARCs) significantly upped LARC adoption among sexually active female high schoolers.
Over the course of the 3-year project, LARC use rose from about 4% to 24% in this group, a statistically significant increase (P less than .0001). During the same time period, LARC use increased nationally, as well, but at a lower rate, rising from 2% to 5% for the same population, while New York state saw LARC use rise from 2% to 5%.
In New York City, where an unrelated LARC awareness campaign was conducted, LARC use went from 3% to 5% over the study period for sexually active female high school students. Comparing the trend in LARC use in Rochester to the secular trend in these control groups showed significantly higher uptake over time in Rochester (P less than .0001).
Through a series of lunch-and-learn talks given to adults who work with adolescents in community-based settings and in medical settings, the Greater Rochester LARC Initiative reached more than 1,300 individuals during July 2014-June 2017, C. Andrew Aligne, MD, MPH, of the University of Rochester (N.Y.), and coauthors reported in the American Journal of Obstetrics and Gynecology.
Of the 81 total talks delivered, 50 were in medical settings, reaching 703 attendees ranging from front-office personnel to primary care physicians, advanced practice clinicians, and nurses; the talks in community-based settings reached 662 attendees.
“We use the term ‘community detailing’ to describe the design of the intervention because it was an innovative hybrid of academic detailing and community health education,” explained Dr. Aligne and colleagues. This approach is a unique, feasible, and effective approach to unintended adolescent pregnancy programs. “The community detailing approach could be a useful complement to programs for preventing unintended adolescent pregnancy.”
The study’s primary outcome measure was LARC use among sexually active female high school students as identified by responses on the U.S. Centers for Disease Control and Statistics’ Youth Risk Behavior Survey (YRBS).
YRBS data were examined for the years 2013, 2015, and 2017, spanning the period before and after the LARC initiative was begun. A separate question about LARC use wasn’t included in the 2013 YRBS survey, so the investigators used a generous estimate that two-thirds of respondents who reported using the “other” contraceptive category for that year were using LARCs. That category was chosen by a total of 6% of respondents, and encompassed LARC use along with use of the patch, ring, diaphragm, and fertility awareness, explained Dr. Aligne and collaborators.
Addressing the problem of failure to use a condom with LARC use, Dr. Aligne and collaborators found overall low rates of dual-method use, but higher rates in Rochester than in the comparison groups. In Rochester, 78% of respondents reported that they also did not use condoms. This figure was lower than the 91% reported for the United States as a whole, and also was lower than the 93% reported in New York City and the 85% reported in New York state. No increase in sexually transmitted infections was seen in Rochester’s sexually active high school females during the study period.
“Our main finding of increased LARC use is consistent with the literature demonstrating that many sexually active young women, including adolescents, will choose LARC if they are given access not only to birth control itself, but also to accurate information about various contraceptive methods,” concluded Dr. Aligne and his associates.
A practical strength of the Greater Rochester LARC initiative was that it capitalized on existing resources, such as New York state’s preexisting program for free access to contraception and similar provisions in the Affordable Care Act. Also, local Title X clinics that were enrolled in New York’s free contraception initiative already had practitioners who were trained and able to provide same-day LARC insertion.
Pediatricians engaged in the initiative were able to receive free training from LARC manufacturers, as mandated by the Food and Drug Administration. Through collaboration with implant manufacturers, Rochester LARC Initiative staff were able to piggyback on training sessions to add education about contraception counseling and the importance of offering access to all contraception methods.
Taken as a whole, the LARC Initiative could be scaled up, wrote Dr. Aligne and his coauthors, a potential boon in the 21 states where qualifying individuals younger than 19 years of age are eligible for Medicaid reimbursement for family planning services. “Even though easy LARC access is far from universal, there are vast areas of the nation where cost need not be seen as an insurmountable barrier.” Dr. Aligne and coauthors also addressed the fraught history of reproductive justice in the United States, cautioning that universal LARC adoption was not – and should not be – the goal of such initiatives. “There is a history of reproductive coercion in the U.S. including forced sterilization of women of color; therefore, it is critical that LARC methods not be imposed on any particular group. On the other hand, LARC should not be withheld deliberately from adolescents who want it, as this is another form of injustice,” they wrote. “The goal should be to empower individuals to decide what is right for them in a context of social and reproductive justice.”
Using the nationally administered YRBS was a significant strength of the study, commented Dr. Aligne and his collaborators. “This allowed us to employ the study design of pre-post with a nonrandomized control group,” the investigators noted, adding that the “relatively rigorous” methodology reduced the risk of problems with internal validity, and also allowed comparisons between changes in Rochester and those at the state and national level.
However, the researchers acknowledged that the study was not a randomized trial, and there’s always the possibility of unknown confounders contributing to LARC uptake during the study period. Also, the YRBS is a self-report instrument and only includes those enrolled in school.
Dr. Aligne reported that his spouse received compensation for providing contraceptive implant insertion training, as did two coauthors. The LARC initiative was supported by a grant from the Greater Rochester Health Foundation.
SOURCE: Aligne CA et al. Am J Obstet Gynecol. 2020 Jan 22. doi: 10.1016/j.ajog.2020.01.029.
In Rochester, N.Y., a comprehensive community initiative that raised awareness about and delivered training in the use of long-acting reversible contraceptives (LARCs) significantly upped LARC adoption among sexually active female high schoolers.
Over the course of the 3-year project, LARC use rose from about 4% to 24% in this group, a statistically significant increase (P less than .0001). During the same time period, LARC use increased nationally, as well, but at a lower rate, rising from 2% to 5% for the same population, while New York state saw LARC use rise from 2% to 5%.
In New York City, where an unrelated LARC awareness campaign was conducted, LARC use went from 3% to 5% over the study period for sexually active female high school students. Comparing the trend in LARC use in Rochester to the secular trend in these control groups showed significantly higher uptake over time in Rochester (P less than .0001).
Through a series of lunch-and-learn talks given to adults who work with adolescents in community-based settings and in medical settings, the Greater Rochester LARC Initiative reached more than 1,300 individuals during July 2014-June 2017, C. Andrew Aligne, MD, MPH, of the University of Rochester (N.Y.), and coauthors reported in the American Journal of Obstetrics and Gynecology.
Of the 81 total talks delivered, 50 were in medical settings, reaching 703 attendees ranging from front-office personnel to primary care physicians, advanced practice clinicians, and nurses; the talks in community-based settings reached 662 attendees.
“We use the term ‘community detailing’ to describe the design of the intervention because it was an innovative hybrid of academic detailing and community health education,” explained Dr. Aligne and colleagues. This approach is a unique, feasible, and effective approach to unintended adolescent pregnancy programs. “The community detailing approach could be a useful complement to programs for preventing unintended adolescent pregnancy.”
The study’s primary outcome measure was LARC use among sexually active female high school students as identified by responses on the U.S. Centers for Disease Control and Statistics’ Youth Risk Behavior Survey (YRBS).
YRBS data were examined for the years 2013, 2015, and 2017, spanning the period before and after the LARC initiative was begun. A separate question about LARC use wasn’t included in the 2013 YRBS survey, so the investigators used a generous estimate that two-thirds of respondents who reported using the “other” contraceptive category for that year were using LARCs. That category was chosen by a total of 6% of respondents, and encompassed LARC use along with use of the patch, ring, diaphragm, and fertility awareness, explained Dr. Aligne and collaborators.
Addressing the problem of failure to use a condom with LARC use, Dr. Aligne and collaborators found overall low rates of dual-method use, but higher rates in Rochester than in the comparison groups. In Rochester, 78% of respondents reported that they also did not use condoms. This figure was lower than the 91% reported for the United States as a whole, and also was lower than the 93% reported in New York City and the 85% reported in New York state. No increase in sexually transmitted infections was seen in Rochester’s sexually active high school females during the study period.
“Our main finding of increased LARC use is consistent with the literature demonstrating that many sexually active young women, including adolescents, will choose LARC if they are given access not only to birth control itself, but also to accurate information about various contraceptive methods,” concluded Dr. Aligne and his associates.
A practical strength of the Greater Rochester LARC initiative was that it capitalized on existing resources, such as New York state’s preexisting program for free access to contraception and similar provisions in the Affordable Care Act. Also, local Title X clinics that were enrolled in New York’s free contraception initiative already had practitioners who were trained and able to provide same-day LARC insertion.
Pediatricians engaged in the initiative were able to receive free training from LARC manufacturers, as mandated by the Food and Drug Administration. Through collaboration with implant manufacturers, Rochester LARC Initiative staff were able to piggyback on training sessions to add education about contraception counseling and the importance of offering access to all contraception methods.
Taken as a whole, the LARC Initiative could be scaled up, wrote Dr. Aligne and his coauthors, a potential boon in the 21 states where qualifying individuals younger than 19 years of age are eligible for Medicaid reimbursement for family planning services. “Even though easy LARC access is far from universal, there are vast areas of the nation where cost need not be seen as an insurmountable barrier.” Dr. Aligne and coauthors also addressed the fraught history of reproductive justice in the United States, cautioning that universal LARC adoption was not – and should not be – the goal of such initiatives. “There is a history of reproductive coercion in the U.S. including forced sterilization of women of color; therefore, it is critical that LARC methods not be imposed on any particular group. On the other hand, LARC should not be withheld deliberately from adolescents who want it, as this is another form of injustice,” they wrote. “The goal should be to empower individuals to decide what is right for them in a context of social and reproductive justice.”
Using the nationally administered YRBS was a significant strength of the study, commented Dr. Aligne and his collaborators. “This allowed us to employ the study design of pre-post with a nonrandomized control group,” the investigators noted, adding that the “relatively rigorous” methodology reduced the risk of problems with internal validity, and also allowed comparisons between changes in Rochester and those at the state and national level.
However, the researchers acknowledged that the study was not a randomized trial, and there’s always the possibility of unknown confounders contributing to LARC uptake during the study period. Also, the YRBS is a self-report instrument and only includes those enrolled in school.
Dr. Aligne reported that his spouse received compensation for providing contraceptive implant insertion training, as did two coauthors. The LARC initiative was supported by a grant from the Greater Rochester Health Foundation.
SOURCE: Aligne CA et al. Am J Obstet Gynecol. 2020 Jan 22. doi: 10.1016/j.ajog.2020.01.029.
FROM AJOG
FDA approves weekly contraceptive patch Twirla
in women whose body mass index is less than 30 kg/m2 and for whom a combined hormonal contraceptive is appropriate.
Applied weekly to the abdomen, buttock, or upper torso (excluding the breasts), Twirla delivers a 30-mcg daily dose of ethinyl estradiol and 120-mcg daily dose of levonorgestrel.
“Twirla is an important addition to available hormonal contraceptive methods, allowing prescribers to now offer appropriate U.S. women a weekly transdermal option that delivers estrogen levels in line with labeled doses of many commonly prescribed oral contraceptives, David Portman, MD, an obstetrician/gynecologist in Columbus, Ohio, and a primary investigator of the SECURE trial, said in a news release issued by the company.
Twirla was evaluated in “a diverse population providing important data to prescribers and to women seeking contraception. It is vital to expand the full range of contraceptive methods and inform the choices that fit an individual’s family planning needs and lifestyle,” Dr. Portman added.
As part of approval, the FDA will require Agile Therapeutics to conduct a long-term, prospective, observational postmarketing study to assess risks for venous thromboembolism and arterial thromboembolism in new users of Twirla, compared with new users of other combined hormonal contraceptives.
Twirla is contraindicated in women at high risk for arterial or venous thrombotic disease, including women with a BMI equal to or greater than 30 kg/m2; women who have headaches with focal neurologic symptoms or migraine with aura; and women older than 35 years who have any migraine headache.
Twirla also should be avoided in women who have liver tumors, acute viral hepatitis, decompensated cirrhosis, liver disease, or undiagnosed abnormal uterine bleeding. It also should be avoided during pregnancy; in women who currently have or who have history of breast cancer or other estrogen- or progestin-sensitive cancer; in women who are hypersensitivity to any components of Twirla; and in women who use hepatitis C drug combinations containing ombitasvir/paraparesis/ritonavir, with or without dasabuvir.
Because cigarette smoking increases the risk for serious cardiovascular events from combined hormonal contraceptive use, Twirla also is contraindicated in women older than 35 who smoke.
Twirla will contain a boxed warning that will include these risks about cigarette smoking and the serious cardiovascular events, and it will stipulate that Twirla is contraindicated in women with a BMI greater than 30 kg/m2.
This article first appeared on Medscape.com.
in women whose body mass index is less than 30 kg/m2 and for whom a combined hormonal contraceptive is appropriate.
Applied weekly to the abdomen, buttock, or upper torso (excluding the breasts), Twirla delivers a 30-mcg daily dose of ethinyl estradiol and 120-mcg daily dose of levonorgestrel.
“Twirla is an important addition to available hormonal contraceptive methods, allowing prescribers to now offer appropriate U.S. women a weekly transdermal option that delivers estrogen levels in line with labeled doses of many commonly prescribed oral contraceptives, David Portman, MD, an obstetrician/gynecologist in Columbus, Ohio, and a primary investigator of the SECURE trial, said in a news release issued by the company.
Twirla was evaluated in “a diverse population providing important data to prescribers and to women seeking contraception. It is vital to expand the full range of contraceptive methods and inform the choices that fit an individual’s family planning needs and lifestyle,” Dr. Portman added.
As part of approval, the FDA will require Agile Therapeutics to conduct a long-term, prospective, observational postmarketing study to assess risks for venous thromboembolism and arterial thromboembolism in new users of Twirla, compared with new users of other combined hormonal contraceptives.
Twirla is contraindicated in women at high risk for arterial or venous thrombotic disease, including women with a BMI equal to or greater than 30 kg/m2; women who have headaches with focal neurologic symptoms or migraine with aura; and women older than 35 years who have any migraine headache.
Twirla also should be avoided in women who have liver tumors, acute viral hepatitis, decompensated cirrhosis, liver disease, or undiagnosed abnormal uterine bleeding. It also should be avoided during pregnancy; in women who currently have or who have history of breast cancer or other estrogen- or progestin-sensitive cancer; in women who are hypersensitivity to any components of Twirla; and in women who use hepatitis C drug combinations containing ombitasvir/paraparesis/ritonavir, with or without dasabuvir.
Because cigarette smoking increases the risk for serious cardiovascular events from combined hormonal contraceptive use, Twirla also is contraindicated in women older than 35 who smoke.
Twirla will contain a boxed warning that will include these risks about cigarette smoking and the serious cardiovascular events, and it will stipulate that Twirla is contraindicated in women with a BMI greater than 30 kg/m2.
This article first appeared on Medscape.com.
in women whose body mass index is less than 30 kg/m2 and for whom a combined hormonal contraceptive is appropriate.
Applied weekly to the abdomen, buttock, or upper torso (excluding the breasts), Twirla delivers a 30-mcg daily dose of ethinyl estradiol and 120-mcg daily dose of levonorgestrel.
“Twirla is an important addition to available hormonal contraceptive methods, allowing prescribers to now offer appropriate U.S. women a weekly transdermal option that delivers estrogen levels in line with labeled doses of many commonly prescribed oral contraceptives, David Portman, MD, an obstetrician/gynecologist in Columbus, Ohio, and a primary investigator of the SECURE trial, said in a news release issued by the company.
Twirla was evaluated in “a diverse population providing important data to prescribers and to women seeking contraception. It is vital to expand the full range of contraceptive methods and inform the choices that fit an individual’s family planning needs and lifestyle,” Dr. Portman added.
As part of approval, the FDA will require Agile Therapeutics to conduct a long-term, prospective, observational postmarketing study to assess risks for venous thromboembolism and arterial thromboembolism in new users of Twirla, compared with new users of other combined hormonal contraceptives.
Twirla is contraindicated in women at high risk for arterial or venous thrombotic disease, including women with a BMI equal to or greater than 30 kg/m2; women who have headaches with focal neurologic symptoms or migraine with aura; and women older than 35 years who have any migraine headache.
Twirla also should be avoided in women who have liver tumors, acute viral hepatitis, decompensated cirrhosis, liver disease, or undiagnosed abnormal uterine bleeding. It also should be avoided during pregnancy; in women who currently have or who have history of breast cancer or other estrogen- or progestin-sensitive cancer; in women who are hypersensitivity to any components of Twirla; and in women who use hepatitis C drug combinations containing ombitasvir/paraparesis/ritonavir, with or without dasabuvir.
Because cigarette smoking increases the risk for serious cardiovascular events from combined hormonal contraceptive use, Twirla also is contraindicated in women older than 35 who smoke.
Twirla will contain a boxed warning that will include these risks about cigarette smoking and the serious cardiovascular events, and it will stipulate that Twirla is contraindicated in women with a BMI greater than 30 kg/m2.
This article first appeared on Medscape.com.
What is optimal hormonal treatment for women with polycystic ovary syndrome?
Polycystic ovary syndrome (PCOS) is the triad of oligo-ovulation resulting in oligomenorrhea, hyperandrogenism and, often, an excess number of small antral follicles on high-resolution pelvic ultrasound. One meta-analysis reported that, in women of reproductive age, the prevalence of PCOS was 10% using the Rotterdam-European Society of Human Reproduction and Embryology/American Society for Reproductive Medicine (ESHRE/ASRM) criteria1 and 6% using the National Institutes of Health 1990 diagnostic criteria.2 (See “The PCOS trinity—3 findings in one syndrome: oligo-ovulation, hyperandrogenism, and a multifollicular ovary.”3)
PCOS is caused by abnormalities in 3 systems: reproductive, metabolic, and dermatologic. Reproductive abnormalities commonly observed in women with PCOS include4:
- an increase in pituitary secretion of luteinizing hormone (LH), resulting from both an increase in LH pulse amplitude and LH pulse frequency, suggesting a primary hypothalamic disorder
- an increase in ovarian secretion of androstenedione and testosterone due to stimulation by LH and possibly insulin
- oligo-ovulation with chronically low levels of progesterone that can result in endometrial hyperplasia
- ovulatory infertility.
Metabolic abnormalities commonly observed in women with PCOS include5,6:
- insulin resistance and hyperinsulinemia
- excess adipose tissue in the liver
- excess visceral fat
- elevated adipokines
- obesity
- an increased prevalence of glucose intolerance and frank diabetes.
Dermatologic abnormalities commonly observed in women with PCOS include7:
- facial hirsutism
- acne
- androgenetic alopecia.
Given that PCOS is caused by abnormalities in the reproductive, metabolic, and dermatologic systems, it is appropriate to consider multimodal hormonal therapy that addresses all 3 problems. In my practice, I believe that the best approach to the long-term hormonal treatment of PCOS for many women is to prescribe a combination of 3 medicines: a combination estrogen-progestin oral contraceptive (COC), an insulin sensitizer, and an antiandrogen.
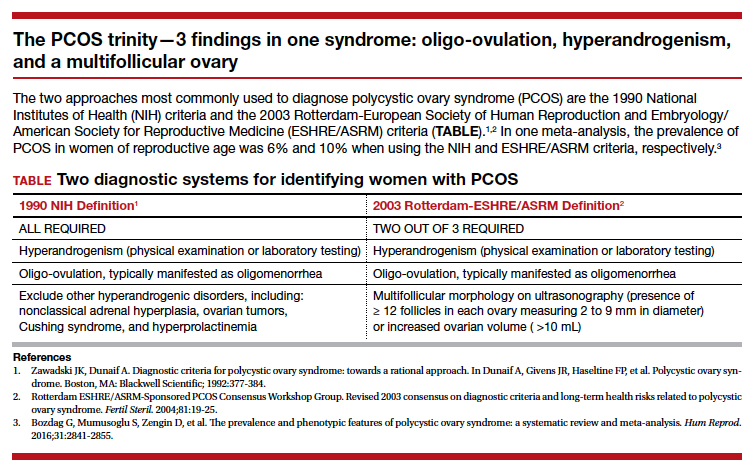
The COC reduces pituitary secretion of LH, decreases ovarian androgen production, and prevents the development of endometrial hyperplasia. When taken cyclically, the COC treatment also restores regular withdrawal uterine bleeding.
An insulin sensitizer, such as metformin or pioglitazone, helps to reduce insulin resistance, glucose intolerance, and hepatic adipose content, rebalancing central metabolism. It is important to include diet and exercise in the long-term treatment of PCOS, and I always encourage these lifestyle changes. However, my patients usually report that they have tried multiple times to restrict dietary caloric intake and increase exercise and have been unable to rebalance their metabolism with these interventions alone. Of note, in the women with PCOS and a body mass index >35 kg/m2, bariatric surgery, such as a sleeve gastrectomy, often results in marked improvement of their PCOS.8
The antiandrogen spironolactone provides effective treatment for the dermatologic problems of facial hirsutism and acne. Some COCs containing the progestins drospirenone, norgestimate, and norethindrone acetate are approved by the US Food and Drug Administration for the treatment of acne. A common approach I use in practice is to prescribe a COC, plus spironolactone 100 mg daily plus metformin extended-release 750 mg to 1,500 mg daily.
Continue to: Which COCs have low androgenicity?...
Which COCs have low androgenicity?
I believe that every COC is an effective treatment for PCOS, regardless of the androgenicity of the progestin in the contraceptive. However, some dermatologists believe that combination contraceptives containing progestins with low androgenicity, such as drospirenone, norgestimate, and desogestrel, are more likely to improve acne than contraceptives with an androgenic progestin such as levonorgestrel. In one study in which 2,147 women with acne were treated by one dermatologic practice, the percentage of women reporting that a birth control pill helped to improve their acne was 66% for pills containing drospirenone, 53% for pills containing norgestimate, 44% for pills containing desogestrel, 30% for pills containing norethindrone, and 25% for pills containing levonorgestrel. In the same study, the percent of women reporting that a birth control pill made their acne worse was 3% for pills containing drospirenone, 6% for pills containing norgestimate, 2% for pills containing desogestrel, 8% for pills containing norethindrone, and 10% for pills containing levonorgestrel.9 Given these findings, when treating a woman with PCOS, I generally prescribe a contraceptive that does not contain levonorgestrel.
Why is a spironolactone dose of 100 mg a good choice for PCOS treatment?
Spironolactone, an antiandrogen and inhibitor of 5-alpha-reductase, is commonly prescribed for the treatment of hirsutism and acne at doses ranging from 50 mg to 200 mg daily.10,11 In my clinical experience, spironolactone at a dose of 200 mg daily commonly causes irregular and bothersome uterine bleeding while spironolactone at a dose of 100 mg daily is seldom associated with irregular bleeding. I believe that spironolactone at a dose of 100 mg daily results in superior clinical efficacy than a 50-mg daily dose, although studies report that both doses are effective in the treatment of acne and hirsutism. Spironolactone should not be prescribed to women with renal failure because it can result in severe hyperkalemia. In a study of spironolactone safety in the treatment of acne, no adverse effects on the kidney, liver, or adrenal glands were reported over 8 years of use.12
What insulin sensitizers are useful in rebalancing the metabolic abnormalities observed with PCOS?
Diet and exercise are superb approaches to rebalancing metabolic abnormalities, but for many of my patients they are insufficient and treatment with an insulin sensitizer is warranted. The most commonly utilized insulin sensitizer for the treatment of PCOS is metformin because it is very inexpensive and has a low risk of serious adverse effects such as lactic acidosis. Metformin increases peripheral glucose uptake and reduces gastrointestinal glucose absorption. Insulin sensitizers also decrease visceral fat, a major source of adipokines. One major disadvantage of metformin is that at doses in the range of 1,500 mg to 2,250 mg it often causes gastrointestinal adverse effects such as borborygmi, nausea, abdominal discomfort, and loose stools.
Thiazolidinediones, including pioglitazone, have been reported to be effective in rebalancing central metabolism in women with PCOS. Pioglitazone carries a black box warning of an increased risk of congestive heart failure and nonfatal myocardial infarction. Pioglitazone is also associated with a risk of hepatotoxicity. However, at the pioglitazone dose commonly used in the treatment of PCOS (7.5 mg daily), these serious adverse effects are rare. In practice, I initiate metformin at a dose of 750 mg daily using the extended-release formulation. I increase the metformin dose to 1,500 mg daily if the patient has no bothersome gastrointestinal symptoms on the lower dose. If the patient cannot tolerate metformin treatment because of adverse effects, I will use pioglitazone 7.5 mg daily.
Continue to: Treatment of PCOS in women who are carriers of the Factor V Leiden mutation...
Treatment of PCOS in women who are carriers of the Factor V Leiden mutation
The Factor V Leiden allele is associated with an increased risk of venous thromboembolism. Estrogen-progestin contraception is contraindicated in women with the Factor V Leiden mutation. The prevalence of this mutation varies by race and ethnicity. It is present in about 5% of white, 2% of Hispanic, 1% of black, 1% of Native American, and 0.5% of Asian women. In women with PCOS who are known to be carriers of the mutation, dual therapy with metformin and spironolactone is highly effective.13-15 For these women I also offer a levonorgestrel IUD to provide contraception and reduce the risk of endometrial hyperplasia.
Combination triple medication treatment of PCOS
Optimal treatment of the reproductive, metabolic, and dermatologic problems associated with PCOS requires multimodal medications including an estrogen-progestin contraceptive, an antiandrogen, and an insulin sensitizer. In my practice, I initiate treatment of PCOS by offering patients 3 medications: a COC, spironolactone 100 mg daily, and metformin extended-release formulation 750 mg daily. Some patients elect dual medication therapy (COC plus spironolactone or COC plus metformin), but many patients select treatment with all 3 medications. Although triple medication treatment of PCOS has not been tested in large randomized clinical trials, small trials report that triple medication treatment produces optimal improvement in the reproductive, metabolic, and dermatologic problems associated with PCOS.16-18
- Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril. 2004;81:19-25.
- Zawadski JK, Dunaif A. Diagnostic criteria for polycystic ovary syndrome: towards a rational approach. In Dunaif A, Givens JR, Haseltine FP, et al. Polycystic ovary syndrome. Boston, MA: Blackwell Scientific; 1992:377-384.
- Bozdag G, Mumusoglu S, Zengin D, et al. The prevalence and phenotypic features of polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod. 2016;31:2841-2855.
- Baskind NE, Balen AH. Hypothalamic-pituitary, ovarian and adrenal contributions to polycystic ovary syndrome. Best Pract Res Clin Obstet Gynaecol. 2016;37:80-97.
- Gilbert EW, Tay CT, Hiam DS, et al. Comorbidities and complications of polycystic ovary syndrome: an overview of systematic reviews. Clin Endocrinol (Oxf). 2018;89:683-699.
- Harsha Varma S, Tirupati S, Pradeep TV, et al. Insulin resistance and hyperandrogenemia independently predict nonalcoholic fatty liver disease in women with polycystic ovary syndrome. Diabetes Metab Syndr. 2019;13:1065-1069.
- Housman E, Reynolds RV. Polycystic ovary syndrome: a review for dermatologists: Part I. Diagnosis and manifestations. J Am Acad Dermatol. 2014;71:847.e1-e10.
- Dilday J, Derickson M, Kuckelman J, et al. Sleeve gastrectomy for obesity in polycystic ovarian syndrome: a pilot study evaluating weight loss and fertility outcomes. Obes Surg. 2019;29:93-98.
- Lortscher D, Admani S, Satur N, et al. Hormonal contraceptives and acne: a retrospective analysis of 2147 patients. J Drugs Dermatol. 2016;15:670-674.
- Brown J, Farquhar C, Lee O, et al. Spironolactone versus placebo or in combination with steroids for hirsutism and/or acne. Cochrane Database Syst Rev. 2009;CD000194.
- Shaw JC. Low-dose adjunctive spironolactone in the treatment of acne in women: a retrospective analysis of 85 consecutively treated patients. J Am Acad Dermatol. 2000;43:498-502.
- Shaw JC, White LE. Long-term safety of spironolactone in acne: results of an 8-year follow-up study. J Cutan Med Surg. 2002;6:541-545.
- Ganie MA, Khurana ML, Nisar S, et al. Improved efficacy of low-dose spironolactone and metformin combination than either drug alone in the management of women with polycystic ovary syndrome (PCOS): a six-month, open-label randomized study. J Clin Endocrinol Metab. 2013;98:3599-3607.
- Mazza A, Fruci B, Guzzi P, et al. In PCOS patients the addition of low-dose spironolactone induces a more marked reduction of clinical and biochemical hyperandrogenism than metformin alone. Nutr Metab Cardiovascular Dis. 2014;24:132-139.
- Ganie MA, Khurana ML, Eunice M, et al. Comparison of efficacy of spironolactone with metformin in the management of polycystic ovary syndrome: an open-labeled study. J Clin Endocrinol Metab. 2004;89:2756-2762.
- Ibanez L, de Zegher F. Low-dose combination flutamide, metformin and an oral contraceptive for non-obese, young women with polycystic ovary syndrome. Hum Reprod. 2003;18:57-60.
- Ibanez L, de Zegher F. Flutamide-metformin plus an oral contraceptive (OC) for young women with polycystic ovary syndrome: switch from third- to fourth-generation OC reduces body adiposity. Hum Reprod. 2004;19:1725-1727.
Polycystic ovary syndrome (PCOS) is the triad of oligo-ovulation resulting in oligomenorrhea, hyperandrogenism and, often, an excess number of small antral follicles on high-resolution pelvic ultrasound. One meta-analysis reported that, in women of reproductive age, the prevalence of PCOS was 10% using the Rotterdam-European Society of Human Reproduction and Embryology/American Society for Reproductive Medicine (ESHRE/ASRM) criteria1 and 6% using the National Institutes of Health 1990 diagnostic criteria.2 (See “The PCOS trinity—3 findings in one syndrome: oligo-ovulation, hyperandrogenism, and a multifollicular ovary.”3)
PCOS is caused by abnormalities in 3 systems: reproductive, metabolic, and dermatologic. Reproductive abnormalities commonly observed in women with PCOS include4:
- an increase in pituitary secretion of luteinizing hormone (LH), resulting from both an increase in LH pulse amplitude and LH pulse frequency, suggesting a primary hypothalamic disorder
- an increase in ovarian secretion of androstenedione and testosterone due to stimulation by LH and possibly insulin
- oligo-ovulation with chronically low levels of progesterone that can result in endometrial hyperplasia
- ovulatory infertility.
Metabolic abnormalities commonly observed in women with PCOS include5,6:
- insulin resistance and hyperinsulinemia
- excess adipose tissue in the liver
- excess visceral fat
- elevated adipokines
- obesity
- an increased prevalence of glucose intolerance and frank diabetes.
Dermatologic abnormalities commonly observed in women with PCOS include7:
- facial hirsutism
- acne
- androgenetic alopecia.
Given that PCOS is caused by abnormalities in the reproductive, metabolic, and dermatologic systems, it is appropriate to consider multimodal hormonal therapy that addresses all 3 problems. In my practice, I believe that the best approach to the long-term hormonal treatment of PCOS for many women is to prescribe a combination of 3 medicines: a combination estrogen-progestin oral contraceptive (COC), an insulin sensitizer, and an antiandrogen.

The COC reduces pituitary secretion of LH, decreases ovarian androgen production, and prevents the development of endometrial hyperplasia. When taken cyclically, the COC treatment also restores regular withdrawal uterine bleeding.
An insulin sensitizer, such as metformin or pioglitazone, helps to reduce insulin resistance, glucose intolerance, and hepatic adipose content, rebalancing central metabolism. It is important to include diet and exercise in the long-term treatment of PCOS, and I always encourage these lifestyle changes. However, my patients usually report that they have tried multiple times to restrict dietary caloric intake and increase exercise and have been unable to rebalance their metabolism with these interventions alone. Of note, in the women with PCOS and a body mass index >35 kg/m2, bariatric surgery, such as a sleeve gastrectomy, often results in marked improvement of their PCOS.8
The antiandrogen spironolactone provides effective treatment for the dermatologic problems of facial hirsutism and acne. Some COCs containing the progestins drospirenone, norgestimate, and norethindrone acetate are approved by the US Food and Drug Administration for the treatment of acne. A common approach I use in practice is to prescribe a COC, plus spironolactone 100 mg daily plus metformin extended-release 750 mg to 1,500 mg daily.
Continue to: Which COCs have low androgenicity?...
Which COCs have low androgenicity?
I believe that every COC is an effective treatment for PCOS, regardless of the androgenicity of the progestin in the contraceptive. However, some dermatologists believe that combination contraceptives containing progestins with low androgenicity, such as drospirenone, norgestimate, and desogestrel, are more likely to improve acne than contraceptives with an androgenic progestin such as levonorgestrel. In one study in which 2,147 women with acne were treated by one dermatologic practice, the percentage of women reporting that a birth control pill helped to improve their acne was 66% for pills containing drospirenone, 53% for pills containing norgestimate, 44% for pills containing desogestrel, 30% for pills containing norethindrone, and 25% for pills containing levonorgestrel. In the same study, the percent of women reporting that a birth control pill made their acne worse was 3% for pills containing drospirenone, 6% for pills containing norgestimate, 2% for pills containing desogestrel, 8% for pills containing norethindrone, and 10% for pills containing levonorgestrel.9 Given these findings, when treating a woman with PCOS, I generally prescribe a contraceptive that does not contain levonorgestrel.

Why is a spironolactone dose of 100 mg a good choice for PCOS treatment?
Spironolactone, an antiandrogen and inhibitor of 5-alpha-reductase, is commonly prescribed for the treatment of hirsutism and acne at doses ranging from 50 mg to 200 mg daily.10,11 In my clinical experience, spironolactone at a dose of 200 mg daily commonly causes irregular and bothersome uterine bleeding while spironolactone at a dose of 100 mg daily is seldom associated with irregular bleeding. I believe that spironolactone at a dose of 100 mg daily results in superior clinical efficacy than a 50-mg daily dose, although studies report that both doses are effective in the treatment of acne and hirsutism. Spironolactone should not be prescribed to women with renal failure because it can result in severe hyperkalemia. In a study of spironolactone safety in the treatment of acne, no adverse effects on the kidney, liver, or adrenal glands were reported over 8 years of use.12
What insulin sensitizers are useful in rebalancing the metabolic abnormalities observed with PCOS?
Diet and exercise are superb approaches to rebalancing metabolic abnormalities, but for many of my patients they are insufficient and treatment with an insulin sensitizer is warranted. The most commonly utilized insulin sensitizer for the treatment of PCOS is metformin because it is very inexpensive and has a low risk of serious adverse effects such as lactic acidosis. Metformin increases peripheral glucose uptake and reduces gastrointestinal glucose absorption. Insulin sensitizers also decrease visceral fat, a major source of adipokines. One major disadvantage of metformin is that at doses in the range of 1,500 mg to 2,250 mg it often causes gastrointestinal adverse effects such as borborygmi, nausea, abdominal discomfort, and loose stools.
Thiazolidinediones, including pioglitazone, have been reported to be effective in rebalancing central metabolism in women with PCOS. Pioglitazone carries a black box warning of an increased risk of congestive heart failure and nonfatal myocardial infarction. Pioglitazone is also associated with a risk of hepatotoxicity. However, at the pioglitazone dose commonly used in the treatment of PCOS (7.5 mg daily), these serious adverse effects are rare. In practice, I initiate metformin at a dose of 750 mg daily using the extended-release formulation. I increase the metformin dose to 1,500 mg daily if the patient has no bothersome gastrointestinal symptoms on the lower dose. If the patient cannot tolerate metformin treatment because of adverse effects, I will use pioglitazone 7.5 mg daily.
Continue to: Treatment of PCOS in women who are carriers of the Factor V Leiden mutation...
Treatment of PCOS in women who are carriers of the Factor V Leiden mutation
The Factor V Leiden allele is associated with an increased risk of venous thromboembolism. Estrogen-progestin contraception is contraindicated in women with the Factor V Leiden mutation. The prevalence of this mutation varies by race and ethnicity. It is present in about 5% of white, 2% of Hispanic, 1% of black, 1% of Native American, and 0.5% of Asian women. In women with PCOS who are known to be carriers of the mutation, dual therapy with metformin and spironolactone is highly effective.13-15 For these women I also offer a levonorgestrel IUD to provide contraception and reduce the risk of endometrial hyperplasia.
Combination triple medication treatment of PCOS
Optimal treatment of the reproductive, metabolic, and dermatologic problems associated with PCOS requires multimodal medications including an estrogen-progestin contraceptive, an antiandrogen, and an insulin sensitizer. In my practice, I initiate treatment of PCOS by offering patients 3 medications: a COC, spironolactone 100 mg daily, and metformin extended-release formulation 750 mg daily. Some patients elect dual medication therapy (COC plus spironolactone or COC plus metformin), but many patients select treatment with all 3 medications. Although triple medication treatment of PCOS has not been tested in large randomized clinical trials, small trials report that triple medication treatment produces optimal improvement in the reproductive, metabolic, and dermatologic problems associated with PCOS.16-18
Polycystic ovary syndrome (PCOS) is the triad of oligo-ovulation resulting in oligomenorrhea, hyperandrogenism and, often, an excess number of small antral follicles on high-resolution pelvic ultrasound. One meta-analysis reported that, in women of reproductive age, the prevalence of PCOS was 10% using the Rotterdam-European Society of Human Reproduction and Embryology/American Society for Reproductive Medicine (ESHRE/ASRM) criteria1 and 6% using the National Institutes of Health 1990 diagnostic criteria.2 (See “The PCOS trinity—3 findings in one syndrome: oligo-ovulation, hyperandrogenism, and a multifollicular ovary.”3)
PCOS is caused by abnormalities in 3 systems: reproductive, metabolic, and dermatologic. Reproductive abnormalities commonly observed in women with PCOS include4:
- an increase in pituitary secretion of luteinizing hormone (LH), resulting from both an increase in LH pulse amplitude and LH pulse frequency, suggesting a primary hypothalamic disorder
- an increase in ovarian secretion of androstenedione and testosterone due to stimulation by LH and possibly insulin
- oligo-ovulation with chronically low levels of progesterone that can result in endometrial hyperplasia
- ovulatory infertility.
Metabolic abnormalities commonly observed in women with PCOS include5,6:
- insulin resistance and hyperinsulinemia
- excess adipose tissue in the liver
- excess visceral fat
- elevated adipokines
- obesity
- an increased prevalence of glucose intolerance and frank diabetes.
Dermatologic abnormalities commonly observed in women with PCOS include7:
- facial hirsutism
- acne
- androgenetic alopecia.
Given that PCOS is caused by abnormalities in the reproductive, metabolic, and dermatologic systems, it is appropriate to consider multimodal hormonal therapy that addresses all 3 problems. In my practice, I believe that the best approach to the long-term hormonal treatment of PCOS for many women is to prescribe a combination of 3 medicines: a combination estrogen-progestin oral contraceptive (COC), an insulin sensitizer, and an antiandrogen.

The COC reduces pituitary secretion of LH, decreases ovarian androgen production, and prevents the development of endometrial hyperplasia. When taken cyclically, the COC treatment also restores regular withdrawal uterine bleeding.
An insulin sensitizer, such as metformin or pioglitazone, helps to reduce insulin resistance, glucose intolerance, and hepatic adipose content, rebalancing central metabolism. It is important to include diet and exercise in the long-term treatment of PCOS, and I always encourage these lifestyle changes. However, my patients usually report that they have tried multiple times to restrict dietary caloric intake and increase exercise and have been unable to rebalance their metabolism with these interventions alone. Of note, in the women with PCOS and a body mass index >35 kg/m2, bariatric surgery, such as a sleeve gastrectomy, often results in marked improvement of their PCOS.8
The antiandrogen spironolactone provides effective treatment for the dermatologic problems of facial hirsutism and acne. Some COCs containing the progestins drospirenone, norgestimate, and norethindrone acetate are approved by the US Food and Drug Administration for the treatment of acne. A common approach I use in practice is to prescribe a COC, plus spironolactone 100 mg daily plus metformin extended-release 750 mg to 1,500 mg daily.
Continue to: Which COCs have low androgenicity?...
Which COCs have low androgenicity?
I believe that every COC is an effective treatment for PCOS, regardless of the androgenicity of the progestin in the contraceptive. However, some dermatologists believe that combination contraceptives containing progestins with low androgenicity, such as drospirenone, norgestimate, and desogestrel, are more likely to improve acne than contraceptives with an androgenic progestin such as levonorgestrel. In one study in which 2,147 women with acne were treated by one dermatologic practice, the percentage of women reporting that a birth control pill helped to improve their acne was 66% for pills containing drospirenone, 53% for pills containing norgestimate, 44% for pills containing desogestrel, 30% for pills containing norethindrone, and 25% for pills containing levonorgestrel. In the same study, the percent of women reporting that a birth control pill made their acne worse was 3% for pills containing drospirenone, 6% for pills containing norgestimate, 2% for pills containing desogestrel, 8% for pills containing norethindrone, and 10% for pills containing levonorgestrel.9 Given these findings, when treating a woman with PCOS, I generally prescribe a contraceptive that does not contain levonorgestrel.

Why is a spironolactone dose of 100 mg a good choice for PCOS treatment?
Spironolactone, an antiandrogen and inhibitor of 5-alpha-reductase, is commonly prescribed for the treatment of hirsutism and acne at doses ranging from 50 mg to 200 mg daily.10,11 In my clinical experience, spironolactone at a dose of 200 mg daily commonly causes irregular and bothersome uterine bleeding while spironolactone at a dose of 100 mg daily is seldom associated with irregular bleeding. I believe that spironolactone at a dose of 100 mg daily results in superior clinical efficacy than a 50-mg daily dose, although studies report that both doses are effective in the treatment of acne and hirsutism. Spironolactone should not be prescribed to women with renal failure because it can result in severe hyperkalemia. In a study of spironolactone safety in the treatment of acne, no adverse effects on the kidney, liver, or adrenal glands were reported over 8 years of use.12
What insulin sensitizers are useful in rebalancing the metabolic abnormalities observed with PCOS?
Diet and exercise are superb approaches to rebalancing metabolic abnormalities, but for many of my patients they are insufficient and treatment with an insulin sensitizer is warranted. The most commonly utilized insulin sensitizer for the treatment of PCOS is metformin because it is very inexpensive and has a low risk of serious adverse effects such as lactic acidosis. Metformin increases peripheral glucose uptake and reduces gastrointestinal glucose absorption. Insulin sensitizers also decrease visceral fat, a major source of adipokines. One major disadvantage of metformin is that at doses in the range of 1,500 mg to 2,250 mg it often causes gastrointestinal adverse effects such as borborygmi, nausea, abdominal discomfort, and loose stools.
Thiazolidinediones, including pioglitazone, have been reported to be effective in rebalancing central metabolism in women with PCOS. Pioglitazone carries a black box warning of an increased risk of congestive heart failure and nonfatal myocardial infarction. Pioglitazone is also associated with a risk of hepatotoxicity. However, at the pioglitazone dose commonly used in the treatment of PCOS (7.5 mg daily), these serious adverse effects are rare. In practice, I initiate metformin at a dose of 750 mg daily using the extended-release formulation. I increase the metformin dose to 1,500 mg daily if the patient has no bothersome gastrointestinal symptoms on the lower dose. If the patient cannot tolerate metformin treatment because of adverse effects, I will use pioglitazone 7.5 mg daily.
Continue to: Treatment of PCOS in women who are carriers of the Factor V Leiden mutation...
Treatment of PCOS in women who are carriers of the Factor V Leiden mutation
The Factor V Leiden allele is associated with an increased risk of venous thromboembolism. Estrogen-progestin contraception is contraindicated in women with the Factor V Leiden mutation. The prevalence of this mutation varies by race and ethnicity. It is present in about 5% of white, 2% of Hispanic, 1% of black, 1% of Native American, and 0.5% of Asian women. In women with PCOS who are known to be carriers of the mutation, dual therapy with metformin and spironolactone is highly effective.13-15 For these women I also offer a levonorgestrel IUD to provide contraception and reduce the risk of endometrial hyperplasia.
Combination triple medication treatment of PCOS
Optimal treatment of the reproductive, metabolic, and dermatologic problems associated with PCOS requires multimodal medications including an estrogen-progestin contraceptive, an antiandrogen, and an insulin sensitizer. In my practice, I initiate treatment of PCOS by offering patients 3 medications: a COC, spironolactone 100 mg daily, and metformin extended-release formulation 750 mg daily. Some patients elect dual medication therapy (COC plus spironolactone or COC plus metformin), but many patients select treatment with all 3 medications. Although triple medication treatment of PCOS has not been tested in large randomized clinical trials, small trials report that triple medication treatment produces optimal improvement in the reproductive, metabolic, and dermatologic problems associated with PCOS.16-18
- Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril. 2004;81:19-25.
- Zawadski JK, Dunaif A. Diagnostic criteria for polycystic ovary syndrome: towards a rational approach. In Dunaif A, Givens JR, Haseltine FP, et al. Polycystic ovary syndrome. Boston, MA: Blackwell Scientific; 1992:377-384.
- Bozdag G, Mumusoglu S, Zengin D, et al. The prevalence and phenotypic features of polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod. 2016;31:2841-2855.
- Baskind NE, Balen AH. Hypothalamic-pituitary, ovarian and adrenal contributions to polycystic ovary syndrome. Best Pract Res Clin Obstet Gynaecol. 2016;37:80-97.
- Gilbert EW, Tay CT, Hiam DS, et al. Comorbidities and complications of polycystic ovary syndrome: an overview of systematic reviews. Clin Endocrinol (Oxf). 2018;89:683-699.
- Harsha Varma S, Tirupati S, Pradeep TV, et al. Insulin resistance and hyperandrogenemia independently predict nonalcoholic fatty liver disease in women with polycystic ovary syndrome. Diabetes Metab Syndr. 2019;13:1065-1069.
- Housman E, Reynolds RV. Polycystic ovary syndrome: a review for dermatologists: Part I. Diagnosis and manifestations. J Am Acad Dermatol. 2014;71:847.e1-e10.
- Dilday J, Derickson M, Kuckelman J, et al. Sleeve gastrectomy for obesity in polycystic ovarian syndrome: a pilot study evaluating weight loss and fertility outcomes. Obes Surg. 2019;29:93-98.
- Lortscher D, Admani S, Satur N, et al. Hormonal contraceptives and acne: a retrospective analysis of 2147 patients. J Drugs Dermatol. 2016;15:670-674.
- Brown J, Farquhar C, Lee O, et al. Spironolactone versus placebo or in combination with steroids for hirsutism and/or acne. Cochrane Database Syst Rev. 2009;CD000194.
- Shaw JC. Low-dose adjunctive spironolactone in the treatment of acne in women: a retrospective analysis of 85 consecutively treated patients. J Am Acad Dermatol. 2000;43:498-502.
- Shaw JC, White LE. Long-term safety of spironolactone in acne: results of an 8-year follow-up study. J Cutan Med Surg. 2002;6:541-545.
- Ganie MA, Khurana ML, Nisar S, et al. Improved efficacy of low-dose spironolactone and metformin combination than either drug alone in the management of women with polycystic ovary syndrome (PCOS): a six-month, open-label randomized study. J Clin Endocrinol Metab. 2013;98:3599-3607.
- Mazza A, Fruci B, Guzzi P, et al. In PCOS patients the addition of low-dose spironolactone induces a more marked reduction of clinical and biochemical hyperandrogenism than metformin alone. Nutr Metab Cardiovascular Dis. 2014;24:132-139.
- Ganie MA, Khurana ML, Eunice M, et al. Comparison of efficacy of spironolactone with metformin in the management of polycystic ovary syndrome: an open-labeled study. J Clin Endocrinol Metab. 2004;89:2756-2762.
- Ibanez L, de Zegher F. Low-dose combination flutamide, metformin and an oral contraceptive for non-obese, young women with polycystic ovary syndrome. Hum Reprod. 2003;18:57-60.
- Ibanez L, de Zegher F. Flutamide-metformin plus an oral contraceptive (OC) for young women with polycystic ovary syndrome: switch from third- to fourth-generation OC reduces body adiposity. Hum Reprod. 2004;19:1725-1727.
- Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril. 2004;81:19-25.
- Zawadski JK, Dunaif A. Diagnostic criteria for polycystic ovary syndrome: towards a rational approach. In Dunaif A, Givens JR, Haseltine FP, et al. Polycystic ovary syndrome. Boston, MA: Blackwell Scientific; 1992:377-384.
- Bozdag G, Mumusoglu S, Zengin D, et al. The prevalence and phenotypic features of polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod. 2016;31:2841-2855.
- Baskind NE, Balen AH. Hypothalamic-pituitary, ovarian and adrenal contributions to polycystic ovary syndrome. Best Pract Res Clin Obstet Gynaecol. 2016;37:80-97.
- Gilbert EW, Tay CT, Hiam DS, et al. Comorbidities and complications of polycystic ovary syndrome: an overview of systematic reviews. Clin Endocrinol (Oxf). 2018;89:683-699.
- Harsha Varma S, Tirupati S, Pradeep TV, et al. Insulin resistance and hyperandrogenemia independently predict nonalcoholic fatty liver disease in women with polycystic ovary syndrome. Diabetes Metab Syndr. 2019;13:1065-1069.
- Housman E, Reynolds RV. Polycystic ovary syndrome: a review for dermatologists: Part I. Diagnosis and manifestations. J Am Acad Dermatol. 2014;71:847.e1-e10.
- Dilday J, Derickson M, Kuckelman J, et al. Sleeve gastrectomy for obesity in polycystic ovarian syndrome: a pilot study evaluating weight loss and fertility outcomes. Obes Surg. 2019;29:93-98.
- Lortscher D, Admani S, Satur N, et al. Hormonal contraceptives and acne: a retrospective analysis of 2147 patients. J Drugs Dermatol. 2016;15:670-674.
- Brown J, Farquhar C, Lee O, et al. Spironolactone versus placebo or in combination with steroids for hirsutism and/or acne. Cochrane Database Syst Rev. 2009;CD000194.
- Shaw JC. Low-dose adjunctive spironolactone in the treatment of acne in women: a retrospective analysis of 85 consecutively treated patients. J Am Acad Dermatol. 2000;43:498-502.
- Shaw JC, White LE. Long-term safety of spironolactone in acne: results of an 8-year follow-up study. J Cutan Med Surg. 2002;6:541-545.
- Ganie MA, Khurana ML, Nisar S, et al. Improved efficacy of low-dose spironolactone and metformin combination than either drug alone in the management of women with polycystic ovary syndrome (PCOS): a six-month, open-label randomized study. J Clin Endocrinol Metab. 2013;98:3599-3607.
- Mazza A, Fruci B, Guzzi P, et al. In PCOS patients the addition of low-dose spironolactone induces a more marked reduction of clinical and biochemical hyperandrogenism than metformin alone. Nutr Metab Cardiovascular Dis. 2014;24:132-139.
- Ganie MA, Khurana ML, Eunice M, et al. Comparison of efficacy of spironolactone with metformin in the management of polycystic ovary syndrome: an open-labeled study. J Clin Endocrinol Metab. 2004;89:2756-2762.
- Ibanez L, de Zegher F. Low-dose combination flutamide, metformin and an oral contraceptive for non-obese, young women with polycystic ovary syndrome. Hum Reprod. 2003;18:57-60.
- Ibanez L, de Zegher F. Flutamide-metformin plus an oral contraceptive (OC) for young women with polycystic ovary syndrome: switch from third- to fourth-generation OC reduces body adiposity. Hum Reprod. 2004;19:1725-1727.