User login
The IUD string check: Benefit or burden?
CASE A patient experiences unnessary inconvenience, distress, and cost following IUD placement
Ms. J had a levonorgestrel intrauterine device (IUD) placed at her postpartum visit. Her physician asked her to return for a string check in 4 to 6 weeks. She was dismayed at the prospect of re-presenting for care, as she is losing the Medicaid coverage that paid for her pregnancy care. One month later, she arranged for a babysitter so she could obtain the recommended string check. The physician told her the strings seemed longer than expected and ordered ultrasonography. Ms. J is distressed because of the mounting cost of care but is anxious to ensure that the IUD will prevent future pregnancy.
Should the routine IUD string check be reconsidered?
The string check dissension
Intrauterine devices offer reliable contraception with a high rate of satisfaction and a remarkably low rate of complications.1-3 With the increased uptake of IUDs, the value of “string checks” is being debated, with myriad responses from professional groups, manufacturers, and individual clinicians. For many practicing ObGyns, the question remains: Should patients be counseled about presenting for or doing their own IUD string checks?
Indeed, all IUD manufacturers recommend monthly self-examination to evaluate string presence.4-8 Manufacturers’ websites prominently display this information in material directed toward current or potential users, so many patients may be familiar already with this recommendation before their clinician visit. Yet, the Centers for Disease Control and Prevention state that no routine follow-up or monitoring is needed.9
In our case scenario, follow-up is clearly burdensome and ultimately costly. Instead, clinicians can advise patients to return with rare but important to recognize complications (such as perforation, expulsion, infection), adverse effects, or desire for change. While no data are available to support in-office or at-home string checks, data do show that women reliably present when intervention is needed.
Here, we explore 5 questions relevant to IUD string checks and discuss why it is time to rethink this practice habit.
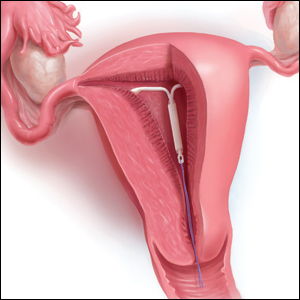
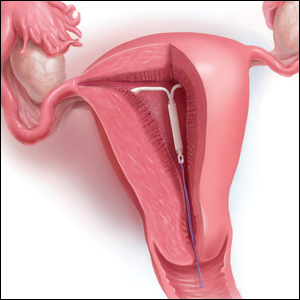
What is the purpose of a string check?
String checks serve as a surrogate for assessing an IUD’s position and function. A string check can be performed by a clinician, who observes the IUD strings on speculum exam or palpates the strings on bimanual exam, or by the patient doing a self-exam. A positive string check purportedly assures both the IUD user and the health care provider that an IUD remains in a fundal, intrauterine position, thus providing an ongoing reliable contraceptive effect.
However, string check reliability in detecting contraceptive effectiveness is uncertain. Strings that subjectively feel or appear longer than anticipated can lead to unnecessary additional evaluation and emotional distress: These are harms. By contrast, when an expulsion occurs, it often is a partial expulsion or displacement, with unclear effect on patient or physician perception of the strings on examination. One retrospective review identified women with a history of IUD placement and a positive pregnancy test; those with an intrauterine pregnancy (74%) frequently also had a malpositioned IUD (55%) and rarely identifiable string issues (16%).10 Before asking patients and clinicians to use resources for performing string evaluations, the association between this action and outcomes of interest must be elucidated.
If not for assessing risk of expulsion, IUD follow-up allows the clinician to evaluate for other complications or adverse effects and to address patient concerns. This practice often is performed when the patient is starting a new medication or medical intervention. However, a systematic review involving 4 studies of IUD follow-up visits or phone calls after contraceptive initiation generated limited data, with no notable impact on contraceptive continuation or indicated use.11
Most important, data show that patients present to their clinician when issues arise with IUD use. One prospective study of 280 women compared multiple follow-up visits with a single 6-week follow-up visit after IUD placement; 10 expulsions were identified, and 8 of these were noted at unscheduled visits when patients presented with symptoms.12 This study suggests that there is little benefit in scheduled follow-up or set self-checks.
Furthermore, in a study in Finland of more than 17,000 IUD users, the rare participants who became pregnant during IUD use promptly presented for care because of a change in menses, pain, or symptoms of pregnancy.13 While IUDs are touted as user independent, this overlooks the reality: Data show that device failure, although rare, is rapidly and appropriately addressed by the user.
Continue to: Does the risk of IUD expulsion warrant string checks?...
Does the risk of IUD expulsion warrant string checks?
The risk of IUD expulsion is estimated to be 1% at 1 month and 4% at 1 year, with a contraceptive failure rate of 0.4% at 1 year. The risk of expulsion does not differ by age group, including adolescents, or parity, but it is higher with use of the copper IUD (2% at 1 month, 6% at 1 year) and with prior expulsion (14%, limited by small numbers).1 Furthermore, risk of expulsion is higher with postplacental placement and second trimester abortion.14,15 Despite this risk, the contraceptive failure rate of all types of IUDs remains consistently lower than all other reversible methods besides the contraceptive implant.16
Furthermore, while IUD expulsion is rare, unnoticed expulsion is even more rare. In one study with more than 58,000 person-years of use, 132 pregnancies were noted, and 7 of these occurred in the setting of an unnoticed expulsion.13 Notably, a higher risk threshold is held for other medications. For example, statins are associated with a 3% risk of irreversible hepatic injury, yet serial liver function tests are not performed in patients without baseline liver dysfunction.17 A less than 0.1% risk of a non–life-threatening complication—unnoticed expulsion—does not warrant routine follow-up. Instead, the patient gauges the tolerability of that risk in making a follow-up plan, particularly given the varied individual preferences in patients’ management of the associated outcome of unintended pregnancy.
Are women interested in and able to perform their own string checks?
Recommendations to perform IUD string self-checks should consider whether women are willing and able to do so. In a study of 126 IUD users, 59% of women had attempted to check their IUD strings at home, and one-third were unable to do so successfully; all participants had visible strings on subsequent speculum exam.18 The women also were given the opportunity to perform a string self-check at the study visit. Overall, only 46% of participants found the exercise acceptable and were able to palpate the IUD strings.18 The authors aptly stated, “A universal recommendation for practice that is meant to identify a rare complication has no clinical utility if at least half of the women are unable to follow it.”
In which scenarios might a string check have clear utility?
The most important reason for follow-up after IUD placement or for patients to perform string self-checks is patient preference. At least anecdotally, some patients take comfort, particularly in the absence of menses, in palpating IUD strings regularly; these individuals should know that there is no necessity for but also no harm in this practice. In addition, patients may desire a string check or follow-up visit to discuss their new contraceptive’s goodness-of-fit.
While limited data show that routinely scheduling such visits does not improve contraceptive continuation, it is difficult to extrapolate these data to the select individuals who independently desire follow-up. (In addition, contraceptive continuance is hardly a metric of success, as clinicians and patients can agree that discontinuation in the setting of patient dissatisfaction is always appropriate.)
Clinicians should share with patients differing risks of IUD expulsion, and this may prompt more nuanced decisions about string checks and/or follow-up. Patients with postplacental or postabortion (second trimester) IUD placement or placement following prior expulsion may opt to perform string checks given the relatively higher risk of expulsion despite the maintained, absolutely low risk that such an event is unnoticed.
If a patient does present for a string check and strings are not visualized on exam, reasonable attempts should be made to identify the strings at that time. A cytobrush can be used to liberate and identify strings within the cervical canal. If the clinician cannot identify the strings or the patient is unable to tolerate such attempts, ultrasonography should be performed to localize the IUD. The ultrasound scan can be done in the office, if available, which is more cost-effective for women than a referral to radiology. If ultrasonography does not identify an intrauterine IUD, an x-ray is the next step to determine if the IUD has expulsed or perforated.
Continue to: Is a string check worth the cost?...
Is a string check worth the cost?
Health care providers may not be aware of the cost of care from the patient perspective. While the Affordable Care Act of 2010 mandates contraceptive coverage for women with insurance, a string check often is coded as a problem-based visit and thus may require a significant copay or out-of-pocket cost for high-deductible plans—without a proven benefit.19 Women who lack insurance coverage may forgo even necessary care due to the cost.20
The bottom line
The medical community and ObGyns specifically are familiar with a practice of patient self-examination falling by the wayside, as has been the case with breast self-examination.21 While counseling on string checks can complement conversations about risks and patients’ personal preferences regarding follow-up, no data support routine string checks in the clinic or at home. One of the great benefits of IUD use is its lack of barriers and resources for ongoing use. Physicians need not reintroduce burdens without benefits to those who desire this contraceptive method.
- Aoun J, Dines VA, Stovall DW, et al. Effects of age, parity, and device type on complications and discontinuation of intrauterine devices. Obstet Gynecol. 2014;123:585-592.
- Peipert JF, Zhao Q, Allsworth JE, et al. Continuation and satisfaction of reversible contraception. Obstet Gynecol. 2011;117:1105-1113.
- American College of Obstetricians and Gynecologists Committee on Gynecology Practice. Committee opinion No. 672. Clinical challenges of long-acting reversible contraceptive methods. Obstet Gynecol. 2016;128:e69-e77.
- Mirena website. Placement of Mirena. 2019. https://www.mirena-us.com/placement-of-mirena/. Accessed December 7, 2019.
- Kyleena website. Let’s get started. 2019. https://www.kyleena-us.com/lets-get-started/what-to-expect/. Accessed December 7, 2019.
- Skyla website. What to expect. 2019. https://www.skyla-us.com/getting-skyla/index.php. Accessed December 7, 2019.
- Liletta website. What should I expect after Liletta insertion? 2020. https://www.liletta.com/about/what-to-expect-afterinsertion. Accessed December 7, 2019.
- Paragard website. What to expect with Paragard. 2019. https://www.paragard.com/what-can-i-expect-with-paragard/. Accessed December 7, 2019.
- Curtis KM, Jatlaoui TC, Tepper NK, et al. US selected practice recommendations for contraceptive use, 2016. MMWR Recomm Rep. 2016;65(4):1-66. https://www.cdc.gov/mmwr/ volumes/65/rr/pdfs/rr6504.pdf. Accessed February 19, 2020.
- Moschos E, Twickler DM. Intrauterine devices in early pregnancy: findings on ultrasound and clinical outcomes. Am J Obstet Gynecol. 2011;204:427.e1-6.
- Steenland MW, Zapata LB, Brahmi D, et al. Appropriate follow up to detect potential adverse events after initiation of select contraceptive methods: a systematic review. Contraception 2013;87:611-624.
- Neuteboom K, de Kroon CD, Dersjant-Roorda M, et al. Follow-up visits after IUD-insertion: sense or nonsense? Contraception. 2003;68:101-104.
- Backman T, Rauramo I, Huhtala S, et al. Pregnancy during the use of levonorgestrel intrauterine system. Am J Obstet Gynecol. 2004;190:50-54.
- Whitaker AK, Chen BA. Society of Family Planning guidelines: postplacental insertion of intrauterine devices. Contraception. 2018;97:2-13.
- Roe AH, Bartz D. Society of Family Planning clinical recommendations: contraception after surgical abortion. Contraception. 2019;99:2-9.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins–Gynecology. Practice bulletin No. 186. Long-acting reversible contraception: implants and intrauterine devices. Obstet Gynecol. 2017;130:e251-e269.
- US Food and Drug Administration. FDA drug safety communication: important safety label changes to cholesterol-lowering statin drugs. 2016. https://www .fda.gov/drugs/drug-safety-and-availability/fda-drugsafety-communication-important-safety-label-changescholesterol-lowering-statin-drugs. Accessed January 9, 2020.
- Melo J, Tschann M, Soon R, et al. Women’s willingness and ability to feel the strings of their intrauterine device. Int J Gynaecol Obstet. 2017;137:309-313.
- Healthcare.gov website. Health benefits & coverage: birth control benefits. 2020. https://www.healthcare.gov/ coverage/birth-control-benefits/. Accessed January 6, 2020.
- NORC at the University of Chicago. Americans’ views of healthcare costs, coverage, and policy. 2018;1-15. https:// www.norc.org/PDFs/WHI%20Healthcare%20Costs%20 Coverage%20and%20Policy/WHI%20Healthcare%20 Costs%20Coverage%20and%20Policy%20Issue%20Brief.pdf. Accessed February 19, 2020.
- Kosters JP, Gotzsche PC. Regular self-examination or clinical examination for early detection of breast cancer. Cochrane Database Syst Rev. 2003. CD003373.
CASE A patient experiences unnessary inconvenience, distress, and cost following IUD placement
Ms. J had a levonorgestrel intrauterine device (IUD) placed at her postpartum visit. Her physician asked her to return for a string check in 4 to 6 weeks. She was dismayed at the prospect of re-presenting for care, as she is losing the Medicaid coverage that paid for her pregnancy care. One month later, she arranged for a babysitter so she could obtain the recommended string check. The physician told her the strings seemed longer than expected and ordered ultrasonography. Ms. J is distressed because of the mounting cost of care but is anxious to ensure that the IUD will prevent future pregnancy.
Should the routine IUD string check be reconsidered?
The string check dissension
Intrauterine devices offer reliable contraception with a high rate of satisfaction and a remarkably low rate of complications.1-3 With the increased uptake of IUDs, the value of “string checks” is being debated, with myriad responses from professional groups, manufacturers, and individual clinicians. For many practicing ObGyns, the question remains: Should patients be counseled about presenting for or doing their own IUD string checks?
Indeed, all IUD manufacturers recommend monthly self-examination to evaluate string presence.4-8 Manufacturers’ websites prominently display this information in material directed toward current or potential users, so many patients may be familiar already with this recommendation before their clinician visit. Yet, the Centers for Disease Control and Prevention state that no routine follow-up or monitoring is needed.9
In our case scenario, follow-up is clearly burdensome and ultimately costly. Instead, clinicians can advise patients to return with rare but important to recognize complications (such as perforation, expulsion, infection), adverse effects, or desire for change. While no data are available to support in-office or at-home string checks, data do show that women reliably present when intervention is needed.
Here, we explore 5 questions relevant to IUD string checks and discuss why it is time to rethink this practice habit.

What is the purpose of a string check?
String checks serve as a surrogate for assessing an IUD’s position and function. A string check can be performed by a clinician, who observes the IUD strings on speculum exam or palpates the strings on bimanual exam, or by the patient doing a self-exam. A positive string check purportedly assures both the IUD user and the health care provider that an IUD remains in a fundal, intrauterine position, thus providing an ongoing reliable contraceptive effect.
However, string check reliability in detecting contraceptive effectiveness is uncertain. Strings that subjectively feel or appear longer than anticipated can lead to unnecessary additional evaluation and emotional distress: These are harms. By contrast, when an expulsion occurs, it often is a partial expulsion or displacement, with unclear effect on patient or physician perception of the strings on examination. One retrospective review identified women with a history of IUD placement and a positive pregnancy test; those with an intrauterine pregnancy (74%) frequently also had a malpositioned IUD (55%) and rarely identifiable string issues (16%).10 Before asking patients and clinicians to use resources for performing string evaluations, the association between this action and outcomes of interest must be elucidated.
If not for assessing risk of expulsion, IUD follow-up allows the clinician to evaluate for other complications or adverse effects and to address patient concerns. This practice often is performed when the patient is starting a new medication or medical intervention. However, a systematic review involving 4 studies of IUD follow-up visits or phone calls after contraceptive initiation generated limited data, with no notable impact on contraceptive continuation or indicated use.11
Most important, data show that patients present to their clinician when issues arise with IUD use. One prospective study of 280 women compared multiple follow-up visits with a single 6-week follow-up visit after IUD placement; 10 expulsions were identified, and 8 of these were noted at unscheduled visits when patients presented with symptoms.12 This study suggests that there is little benefit in scheduled follow-up or set self-checks.
Furthermore, in a study in Finland of more than 17,000 IUD users, the rare participants who became pregnant during IUD use promptly presented for care because of a change in menses, pain, or symptoms of pregnancy.13 While IUDs are touted as user independent, this overlooks the reality: Data show that device failure, although rare, is rapidly and appropriately addressed by the user.
Continue to: Does the risk of IUD expulsion warrant string checks?...
Does the risk of IUD expulsion warrant string checks?
The risk of IUD expulsion is estimated to be 1% at 1 month and 4% at 1 year, with a contraceptive failure rate of 0.4% at 1 year. The risk of expulsion does not differ by age group, including adolescents, or parity, but it is higher with use of the copper IUD (2% at 1 month, 6% at 1 year) and with prior expulsion (14%, limited by small numbers).1 Furthermore, risk of expulsion is higher with postplacental placement and second trimester abortion.14,15 Despite this risk, the contraceptive failure rate of all types of IUDs remains consistently lower than all other reversible methods besides the contraceptive implant.16
Furthermore, while IUD expulsion is rare, unnoticed expulsion is even more rare. In one study with more than 58,000 person-years of use, 132 pregnancies were noted, and 7 of these occurred in the setting of an unnoticed expulsion.13 Notably, a higher risk threshold is held for other medications. For example, statins are associated with a 3% risk of irreversible hepatic injury, yet serial liver function tests are not performed in patients without baseline liver dysfunction.17 A less than 0.1% risk of a non–life-threatening complication—unnoticed expulsion—does not warrant routine follow-up. Instead, the patient gauges the tolerability of that risk in making a follow-up plan, particularly given the varied individual preferences in patients’ management of the associated outcome of unintended pregnancy.

Are women interested in and able to perform their own string checks?
Recommendations to perform IUD string self-checks should consider whether women are willing and able to do so. In a study of 126 IUD users, 59% of women had attempted to check their IUD strings at home, and one-third were unable to do so successfully; all participants had visible strings on subsequent speculum exam.18 The women also were given the opportunity to perform a string self-check at the study visit. Overall, only 46% of participants found the exercise acceptable and were able to palpate the IUD strings.18 The authors aptly stated, “A universal recommendation for practice that is meant to identify a rare complication has no clinical utility if at least half of the women are unable to follow it.”
In which scenarios might a string check have clear utility?
The most important reason for follow-up after IUD placement or for patients to perform string self-checks is patient preference. At least anecdotally, some patients take comfort, particularly in the absence of menses, in palpating IUD strings regularly; these individuals should know that there is no necessity for but also no harm in this practice. In addition, patients may desire a string check or follow-up visit to discuss their new contraceptive’s goodness-of-fit.
While limited data show that routinely scheduling such visits does not improve contraceptive continuation, it is difficult to extrapolate these data to the select individuals who independently desire follow-up. (In addition, contraceptive continuance is hardly a metric of success, as clinicians and patients can agree that discontinuation in the setting of patient dissatisfaction is always appropriate.)
Clinicians should share with patients differing risks of IUD expulsion, and this may prompt more nuanced decisions about string checks and/or follow-up. Patients with postplacental or postabortion (second trimester) IUD placement or placement following prior expulsion may opt to perform string checks given the relatively higher risk of expulsion despite the maintained, absolutely low risk that such an event is unnoticed.
If a patient does present for a string check and strings are not visualized on exam, reasonable attempts should be made to identify the strings at that time. A cytobrush can be used to liberate and identify strings within the cervical canal. If the clinician cannot identify the strings or the patient is unable to tolerate such attempts, ultrasonography should be performed to localize the IUD. The ultrasound scan can be done in the office, if available, which is more cost-effective for women than a referral to radiology. If ultrasonography does not identify an intrauterine IUD, an x-ray is the next step to determine if the IUD has expulsed or perforated.
Continue to: Is a string check worth the cost?...
Is a string check worth the cost?
Health care providers may not be aware of the cost of care from the patient perspective. While the Affordable Care Act of 2010 mandates contraceptive coverage for women with insurance, a string check often is coded as a problem-based visit and thus may require a significant copay or out-of-pocket cost for high-deductible plans—without a proven benefit.19 Women who lack insurance coverage may forgo even necessary care due to the cost.20
The bottom line
The medical community and ObGyns specifically are familiar with a practice of patient self-examination falling by the wayside, as has been the case with breast self-examination.21 While counseling on string checks can complement conversations about risks and patients’ personal preferences regarding follow-up, no data support routine string checks in the clinic or at home. One of the great benefits of IUD use is its lack of barriers and resources for ongoing use. Physicians need not reintroduce burdens without benefits to those who desire this contraceptive method.
CASE A patient experiences unnessary inconvenience, distress, and cost following IUD placement
Ms. J had a levonorgestrel intrauterine device (IUD) placed at her postpartum visit. Her physician asked her to return for a string check in 4 to 6 weeks. She was dismayed at the prospect of re-presenting for care, as she is losing the Medicaid coverage that paid for her pregnancy care. One month later, she arranged for a babysitter so she could obtain the recommended string check. The physician told her the strings seemed longer than expected and ordered ultrasonography. Ms. J is distressed because of the mounting cost of care but is anxious to ensure that the IUD will prevent future pregnancy.
Should the routine IUD string check be reconsidered?
The string check dissension
Intrauterine devices offer reliable contraception with a high rate of satisfaction and a remarkably low rate of complications.1-3 With the increased uptake of IUDs, the value of “string checks” is being debated, with myriad responses from professional groups, manufacturers, and individual clinicians. For many practicing ObGyns, the question remains: Should patients be counseled about presenting for or doing their own IUD string checks?
Indeed, all IUD manufacturers recommend monthly self-examination to evaluate string presence.4-8 Manufacturers’ websites prominently display this information in material directed toward current or potential users, so many patients may be familiar already with this recommendation before their clinician visit. Yet, the Centers for Disease Control and Prevention state that no routine follow-up or monitoring is needed.9
In our case scenario, follow-up is clearly burdensome and ultimately costly. Instead, clinicians can advise patients to return with rare but important to recognize complications (such as perforation, expulsion, infection), adverse effects, or desire for change. While no data are available to support in-office or at-home string checks, data do show that women reliably present when intervention is needed.
Here, we explore 5 questions relevant to IUD string checks and discuss why it is time to rethink this practice habit.

What is the purpose of a string check?
String checks serve as a surrogate for assessing an IUD’s position and function. A string check can be performed by a clinician, who observes the IUD strings on speculum exam or palpates the strings on bimanual exam, or by the patient doing a self-exam. A positive string check purportedly assures both the IUD user and the health care provider that an IUD remains in a fundal, intrauterine position, thus providing an ongoing reliable contraceptive effect.
However, string check reliability in detecting contraceptive effectiveness is uncertain. Strings that subjectively feel or appear longer than anticipated can lead to unnecessary additional evaluation and emotional distress: These are harms. By contrast, when an expulsion occurs, it often is a partial expulsion or displacement, with unclear effect on patient or physician perception of the strings on examination. One retrospective review identified women with a history of IUD placement and a positive pregnancy test; those with an intrauterine pregnancy (74%) frequently also had a malpositioned IUD (55%) and rarely identifiable string issues (16%).10 Before asking patients and clinicians to use resources for performing string evaluations, the association between this action and outcomes of interest must be elucidated.
If not for assessing risk of expulsion, IUD follow-up allows the clinician to evaluate for other complications or adverse effects and to address patient concerns. This practice often is performed when the patient is starting a new medication or medical intervention. However, a systematic review involving 4 studies of IUD follow-up visits or phone calls after contraceptive initiation generated limited data, with no notable impact on contraceptive continuation or indicated use.11
Most important, data show that patients present to their clinician when issues arise with IUD use. One prospective study of 280 women compared multiple follow-up visits with a single 6-week follow-up visit after IUD placement; 10 expulsions were identified, and 8 of these were noted at unscheduled visits when patients presented with symptoms.12 This study suggests that there is little benefit in scheduled follow-up or set self-checks.
Furthermore, in a study in Finland of more than 17,000 IUD users, the rare participants who became pregnant during IUD use promptly presented for care because of a change in menses, pain, or symptoms of pregnancy.13 While IUDs are touted as user independent, this overlooks the reality: Data show that device failure, although rare, is rapidly and appropriately addressed by the user.
Continue to: Does the risk of IUD expulsion warrant string checks?...
Does the risk of IUD expulsion warrant string checks?
The risk of IUD expulsion is estimated to be 1% at 1 month and 4% at 1 year, with a contraceptive failure rate of 0.4% at 1 year. The risk of expulsion does not differ by age group, including adolescents, or parity, but it is higher with use of the copper IUD (2% at 1 month, 6% at 1 year) and with prior expulsion (14%, limited by small numbers).1 Furthermore, risk of expulsion is higher with postplacental placement and second trimester abortion.14,15 Despite this risk, the contraceptive failure rate of all types of IUDs remains consistently lower than all other reversible methods besides the contraceptive implant.16
Furthermore, while IUD expulsion is rare, unnoticed expulsion is even more rare. In one study with more than 58,000 person-years of use, 132 pregnancies were noted, and 7 of these occurred in the setting of an unnoticed expulsion.13 Notably, a higher risk threshold is held for other medications. For example, statins are associated with a 3% risk of irreversible hepatic injury, yet serial liver function tests are not performed in patients without baseline liver dysfunction.17 A less than 0.1% risk of a non–life-threatening complication—unnoticed expulsion—does not warrant routine follow-up. Instead, the patient gauges the tolerability of that risk in making a follow-up plan, particularly given the varied individual preferences in patients’ management of the associated outcome of unintended pregnancy.

Are women interested in and able to perform their own string checks?
Recommendations to perform IUD string self-checks should consider whether women are willing and able to do so. In a study of 126 IUD users, 59% of women had attempted to check their IUD strings at home, and one-third were unable to do so successfully; all participants had visible strings on subsequent speculum exam.18 The women also were given the opportunity to perform a string self-check at the study visit. Overall, only 46% of participants found the exercise acceptable and were able to palpate the IUD strings.18 The authors aptly stated, “A universal recommendation for practice that is meant to identify a rare complication has no clinical utility if at least half of the women are unable to follow it.”
In which scenarios might a string check have clear utility?
The most important reason for follow-up after IUD placement or for patients to perform string self-checks is patient preference. At least anecdotally, some patients take comfort, particularly in the absence of menses, in palpating IUD strings regularly; these individuals should know that there is no necessity for but also no harm in this practice. In addition, patients may desire a string check or follow-up visit to discuss their new contraceptive’s goodness-of-fit.
While limited data show that routinely scheduling such visits does not improve contraceptive continuation, it is difficult to extrapolate these data to the select individuals who independently desire follow-up. (In addition, contraceptive continuance is hardly a metric of success, as clinicians and patients can agree that discontinuation in the setting of patient dissatisfaction is always appropriate.)
Clinicians should share with patients differing risks of IUD expulsion, and this may prompt more nuanced decisions about string checks and/or follow-up. Patients with postplacental or postabortion (second trimester) IUD placement or placement following prior expulsion may opt to perform string checks given the relatively higher risk of expulsion despite the maintained, absolutely low risk that such an event is unnoticed.
If a patient does present for a string check and strings are not visualized on exam, reasonable attempts should be made to identify the strings at that time. A cytobrush can be used to liberate and identify strings within the cervical canal. If the clinician cannot identify the strings or the patient is unable to tolerate such attempts, ultrasonography should be performed to localize the IUD. The ultrasound scan can be done in the office, if available, which is more cost-effective for women than a referral to radiology. If ultrasonography does not identify an intrauterine IUD, an x-ray is the next step to determine if the IUD has expulsed or perforated.
Continue to: Is a string check worth the cost?...
Is a string check worth the cost?
Health care providers may not be aware of the cost of care from the patient perspective. While the Affordable Care Act of 2010 mandates contraceptive coverage for women with insurance, a string check often is coded as a problem-based visit and thus may require a significant copay or out-of-pocket cost for high-deductible plans—without a proven benefit.19 Women who lack insurance coverage may forgo even necessary care due to the cost.20
The bottom line
The medical community and ObGyns specifically are familiar with a practice of patient self-examination falling by the wayside, as has been the case with breast self-examination.21 While counseling on string checks can complement conversations about risks and patients’ personal preferences regarding follow-up, no data support routine string checks in the clinic or at home. One of the great benefits of IUD use is its lack of barriers and resources for ongoing use. Physicians need not reintroduce burdens without benefits to those who desire this contraceptive method.
- Aoun J, Dines VA, Stovall DW, et al. Effects of age, parity, and device type on complications and discontinuation of intrauterine devices. Obstet Gynecol. 2014;123:585-592.
- Peipert JF, Zhao Q, Allsworth JE, et al. Continuation and satisfaction of reversible contraception. Obstet Gynecol. 2011;117:1105-1113.
- American College of Obstetricians and Gynecologists Committee on Gynecology Practice. Committee opinion No. 672. Clinical challenges of long-acting reversible contraceptive methods. Obstet Gynecol. 2016;128:e69-e77.
- Mirena website. Placement of Mirena. 2019. https://www.mirena-us.com/placement-of-mirena/. Accessed December 7, 2019.
- Kyleena website. Let’s get started. 2019. https://www.kyleena-us.com/lets-get-started/what-to-expect/. Accessed December 7, 2019.
- Skyla website. What to expect. 2019. https://www.skyla-us.com/getting-skyla/index.php. Accessed December 7, 2019.
- Liletta website. What should I expect after Liletta insertion? 2020. https://www.liletta.com/about/what-to-expect-afterinsertion. Accessed December 7, 2019.
- Paragard website. What to expect with Paragard. 2019. https://www.paragard.com/what-can-i-expect-with-paragard/. Accessed December 7, 2019.
- Curtis KM, Jatlaoui TC, Tepper NK, et al. US selected practice recommendations for contraceptive use, 2016. MMWR Recomm Rep. 2016;65(4):1-66. https://www.cdc.gov/mmwr/ volumes/65/rr/pdfs/rr6504.pdf. Accessed February 19, 2020.
- Moschos E, Twickler DM. Intrauterine devices in early pregnancy: findings on ultrasound and clinical outcomes. Am J Obstet Gynecol. 2011;204:427.e1-6.
- Steenland MW, Zapata LB, Brahmi D, et al. Appropriate follow up to detect potential adverse events after initiation of select contraceptive methods: a systematic review. Contraception 2013;87:611-624.
- Neuteboom K, de Kroon CD, Dersjant-Roorda M, et al. Follow-up visits after IUD-insertion: sense or nonsense? Contraception. 2003;68:101-104.
- Backman T, Rauramo I, Huhtala S, et al. Pregnancy during the use of levonorgestrel intrauterine system. Am J Obstet Gynecol. 2004;190:50-54.
- Whitaker AK, Chen BA. Society of Family Planning guidelines: postplacental insertion of intrauterine devices. Contraception. 2018;97:2-13.
- Roe AH, Bartz D. Society of Family Planning clinical recommendations: contraception after surgical abortion. Contraception. 2019;99:2-9.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins–Gynecology. Practice bulletin No. 186. Long-acting reversible contraception: implants and intrauterine devices. Obstet Gynecol. 2017;130:e251-e269.
- US Food and Drug Administration. FDA drug safety communication: important safety label changes to cholesterol-lowering statin drugs. 2016. https://www .fda.gov/drugs/drug-safety-and-availability/fda-drugsafety-communication-important-safety-label-changescholesterol-lowering-statin-drugs. Accessed January 9, 2020.
- Melo J, Tschann M, Soon R, et al. Women’s willingness and ability to feel the strings of their intrauterine device. Int J Gynaecol Obstet. 2017;137:309-313.
- Healthcare.gov website. Health benefits & coverage: birth control benefits. 2020. https://www.healthcare.gov/ coverage/birth-control-benefits/. Accessed January 6, 2020.
- NORC at the University of Chicago. Americans’ views of healthcare costs, coverage, and policy. 2018;1-15. https:// www.norc.org/PDFs/WHI%20Healthcare%20Costs%20 Coverage%20and%20Policy/WHI%20Healthcare%20 Costs%20Coverage%20and%20Policy%20Issue%20Brief.pdf. Accessed February 19, 2020.
- Kosters JP, Gotzsche PC. Regular self-examination or clinical examination for early detection of breast cancer. Cochrane Database Syst Rev. 2003. CD003373.
- Aoun J, Dines VA, Stovall DW, et al. Effects of age, parity, and device type on complications and discontinuation of intrauterine devices. Obstet Gynecol. 2014;123:585-592.
- Peipert JF, Zhao Q, Allsworth JE, et al. Continuation and satisfaction of reversible contraception. Obstet Gynecol. 2011;117:1105-1113.
- American College of Obstetricians and Gynecologists Committee on Gynecology Practice. Committee opinion No. 672. Clinical challenges of long-acting reversible contraceptive methods. Obstet Gynecol. 2016;128:e69-e77.
- Mirena website. Placement of Mirena. 2019. https://www.mirena-us.com/placement-of-mirena/. Accessed December 7, 2019.
- Kyleena website. Let’s get started. 2019. https://www.kyleena-us.com/lets-get-started/what-to-expect/. Accessed December 7, 2019.
- Skyla website. What to expect. 2019. https://www.skyla-us.com/getting-skyla/index.php. Accessed December 7, 2019.
- Liletta website. What should I expect after Liletta insertion? 2020. https://www.liletta.com/about/what-to-expect-afterinsertion. Accessed December 7, 2019.
- Paragard website. What to expect with Paragard. 2019. https://www.paragard.com/what-can-i-expect-with-paragard/. Accessed December 7, 2019.
- Curtis KM, Jatlaoui TC, Tepper NK, et al. US selected practice recommendations for contraceptive use, 2016. MMWR Recomm Rep. 2016;65(4):1-66. https://www.cdc.gov/mmwr/ volumes/65/rr/pdfs/rr6504.pdf. Accessed February 19, 2020.
- Moschos E, Twickler DM. Intrauterine devices in early pregnancy: findings on ultrasound and clinical outcomes. Am J Obstet Gynecol. 2011;204:427.e1-6.
- Steenland MW, Zapata LB, Brahmi D, et al. Appropriate follow up to detect potential adverse events after initiation of select contraceptive methods: a systematic review. Contraception 2013;87:611-624.
- Neuteboom K, de Kroon CD, Dersjant-Roorda M, et al. Follow-up visits after IUD-insertion: sense or nonsense? Contraception. 2003;68:101-104.
- Backman T, Rauramo I, Huhtala S, et al. Pregnancy during the use of levonorgestrel intrauterine system. Am J Obstet Gynecol. 2004;190:50-54.
- Whitaker AK, Chen BA. Society of Family Planning guidelines: postplacental insertion of intrauterine devices. Contraception. 2018;97:2-13.
- Roe AH, Bartz D. Society of Family Planning clinical recommendations: contraception after surgical abortion. Contraception. 2019;99:2-9.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins–Gynecology. Practice bulletin No. 186. Long-acting reversible contraception: implants and intrauterine devices. Obstet Gynecol. 2017;130:e251-e269.
- US Food and Drug Administration. FDA drug safety communication: important safety label changes to cholesterol-lowering statin drugs. 2016. https://www .fda.gov/drugs/drug-safety-and-availability/fda-drugsafety-communication-important-safety-label-changescholesterol-lowering-statin-drugs. Accessed January 9, 2020.
- Melo J, Tschann M, Soon R, et al. Women’s willingness and ability to feel the strings of their intrauterine device. Int J Gynaecol Obstet. 2017;137:309-313.
- Healthcare.gov website. Health benefits & coverage: birth control benefits. 2020. https://www.healthcare.gov/ coverage/birth-control-benefits/. Accessed January 6, 2020.
- NORC at the University of Chicago. Americans’ views of healthcare costs, coverage, and policy. 2018;1-15. https:// www.norc.org/PDFs/WHI%20Healthcare%20Costs%20 Coverage%20and%20Policy/WHI%20Healthcare%20 Costs%20Coverage%20and%20Policy%20Issue%20Brief.pdf. Accessed February 19, 2020.
- Kosters JP, Gotzsche PC. Regular self-examination or clinical examination for early detection of breast cancer. Cochrane Database Syst Rev. 2003. CD003373.