User login
Stroke-smoking link is dose dependent in young men
In men younger than 50 years, even just a reduction in the number of cigarettes smoked may decrease the risk of ischemic stroke, according to a population-based, case-control study.
The odds ratio for a stroke was 1.21 for men who smoked fewer than 11 cigarettes per day, compared with nonsmokers, and 5.24 for those who smoked 40 or more per day, reported Janina Markidan and her coinvestigators in Stroke.
A prior study showed a similar relationship in young women, but the researchers decided to conduct a follow-up study in men in order to eliminate hormonal confounders (Stroke. 2008 Sep;39[9]:2439-43).
Ms. Markidan and her colleagues used data from the Stroke Prevention in Young Men Study, which recruited 615 men who had experienced a stroke in the previous three years, and compared these men with 530 age-, ethnicity-, and geography-matched controls.
There were some statistically significant differences in the two populations: Cases had lower levels of education and had greater incidences of hypertension, diabetes, myocardial infarction, angina, and obesity (all P < .05).
Current smokers were identified as those who had smoked more than 100 cigarettes in their lifetime and who had smoked a cigarette in the 30 days preceding the stroke. Never smokers were those who had smoked fewer than 100 cigarettes in their lifetime or who had never smoked five packs.
Compared with never smokers, current smokers had an odds ratio for stroke of 1.88 (95% confidence interval, 1.44-2.44). When the researchers stratified smokers by the number of cigarettes smoked, the stroke risk appeared to be dose dependent in the fully adjusted models: The OR for 1-10 cigarettes/day was 1.21 (95% CI, 0.83-1.77), 1.64 for 11-20 cigarettes/day (95% CI, 1.10-2.43), 3.51 for 21-39 cigarettes/day (95% CI, 1.65-7.45), and 5.24 for 40 or more cigarettes/day (95% CI, 1.90-14.42).
The study cannot prove causation and did not include smoking of nontobacco products, alcohol consumption, or physical activity.
“While complete cessation of smoking is the goal, even reducing the number of cigarettes smoked may have beneficial health effects,” wrote Ms. Markidan, a medical student at the University of Maryland, Baltimore, and her colleagues.
SOURCE: Markidan J et al. Stroke. 2018 May;49(5):1276-8.
In men younger than 50 years, even just a reduction in the number of cigarettes smoked may decrease the risk of ischemic stroke, according to a population-based, case-control study.
The odds ratio for a stroke was 1.21 for men who smoked fewer than 11 cigarettes per day, compared with nonsmokers, and 5.24 for those who smoked 40 or more per day, reported Janina Markidan and her coinvestigators in Stroke.
A prior study showed a similar relationship in young women, but the researchers decided to conduct a follow-up study in men in order to eliminate hormonal confounders (Stroke. 2008 Sep;39[9]:2439-43).
Ms. Markidan and her colleagues used data from the Stroke Prevention in Young Men Study, which recruited 615 men who had experienced a stroke in the previous three years, and compared these men with 530 age-, ethnicity-, and geography-matched controls.
There were some statistically significant differences in the two populations: Cases had lower levels of education and had greater incidences of hypertension, diabetes, myocardial infarction, angina, and obesity (all P < .05).
Current smokers were identified as those who had smoked more than 100 cigarettes in their lifetime and who had smoked a cigarette in the 30 days preceding the stroke. Never smokers were those who had smoked fewer than 100 cigarettes in their lifetime or who had never smoked five packs.
Compared with never smokers, current smokers had an odds ratio for stroke of 1.88 (95% confidence interval, 1.44-2.44). When the researchers stratified smokers by the number of cigarettes smoked, the stroke risk appeared to be dose dependent in the fully adjusted models: The OR for 1-10 cigarettes/day was 1.21 (95% CI, 0.83-1.77), 1.64 for 11-20 cigarettes/day (95% CI, 1.10-2.43), 3.51 for 21-39 cigarettes/day (95% CI, 1.65-7.45), and 5.24 for 40 or more cigarettes/day (95% CI, 1.90-14.42).
The study cannot prove causation and did not include smoking of nontobacco products, alcohol consumption, or physical activity.
“While complete cessation of smoking is the goal, even reducing the number of cigarettes smoked may have beneficial health effects,” wrote Ms. Markidan, a medical student at the University of Maryland, Baltimore, and her colleagues.
SOURCE: Markidan J et al. Stroke. 2018 May;49(5):1276-8.
In men younger than 50 years, even just a reduction in the number of cigarettes smoked may decrease the risk of ischemic stroke, according to a population-based, case-control study.
The odds ratio for a stroke was 1.21 for men who smoked fewer than 11 cigarettes per day, compared with nonsmokers, and 5.24 for those who smoked 40 or more per day, reported Janina Markidan and her coinvestigators in Stroke.
A prior study showed a similar relationship in young women, but the researchers decided to conduct a follow-up study in men in order to eliminate hormonal confounders (Stroke. 2008 Sep;39[9]:2439-43).
Ms. Markidan and her colleagues used data from the Stroke Prevention in Young Men Study, which recruited 615 men who had experienced a stroke in the previous three years, and compared these men with 530 age-, ethnicity-, and geography-matched controls.
There were some statistically significant differences in the two populations: Cases had lower levels of education and had greater incidences of hypertension, diabetes, myocardial infarction, angina, and obesity (all P < .05).
Current smokers were identified as those who had smoked more than 100 cigarettes in their lifetime and who had smoked a cigarette in the 30 days preceding the stroke. Never smokers were those who had smoked fewer than 100 cigarettes in their lifetime or who had never smoked five packs.
Compared with never smokers, current smokers had an odds ratio for stroke of 1.88 (95% confidence interval, 1.44-2.44). When the researchers stratified smokers by the number of cigarettes smoked, the stroke risk appeared to be dose dependent in the fully adjusted models: The OR for 1-10 cigarettes/day was 1.21 (95% CI, 0.83-1.77), 1.64 for 11-20 cigarettes/day (95% CI, 1.10-2.43), 3.51 for 21-39 cigarettes/day (95% CI, 1.65-7.45), and 5.24 for 40 or more cigarettes/day (95% CI, 1.90-14.42).
The study cannot prove causation and did not include smoking of nontobacco products, alcohol consumption, or physical activity.
“While complete cessation of smoking is the goal, even reducing the number of cigarettes smoked may have beneficial health effects,” wrote Ms. Markidan, a medical student at the University of Maryland, Baltimore, and her colleagues.
SOURCE: Markidan J et al. Stroke. 2018 May;49(5):1276-8.
FROM STROKE
Key clinical point:
Major finding: The odds ratio for stroke ranged from 1.21 to 5.24 with increased smoking.
Study details: Case-controlled study of 1,145 stroke patients and controls.
Disclosures: The study was funded by the Department of Veterans Affairs, the Centers for Disease Control and Prevention, and the National Institutes of Health. The authors declared no relevant financial relationships.
Source: Markidan J et al. Stroke. 2018 May;49(5):1276-8.
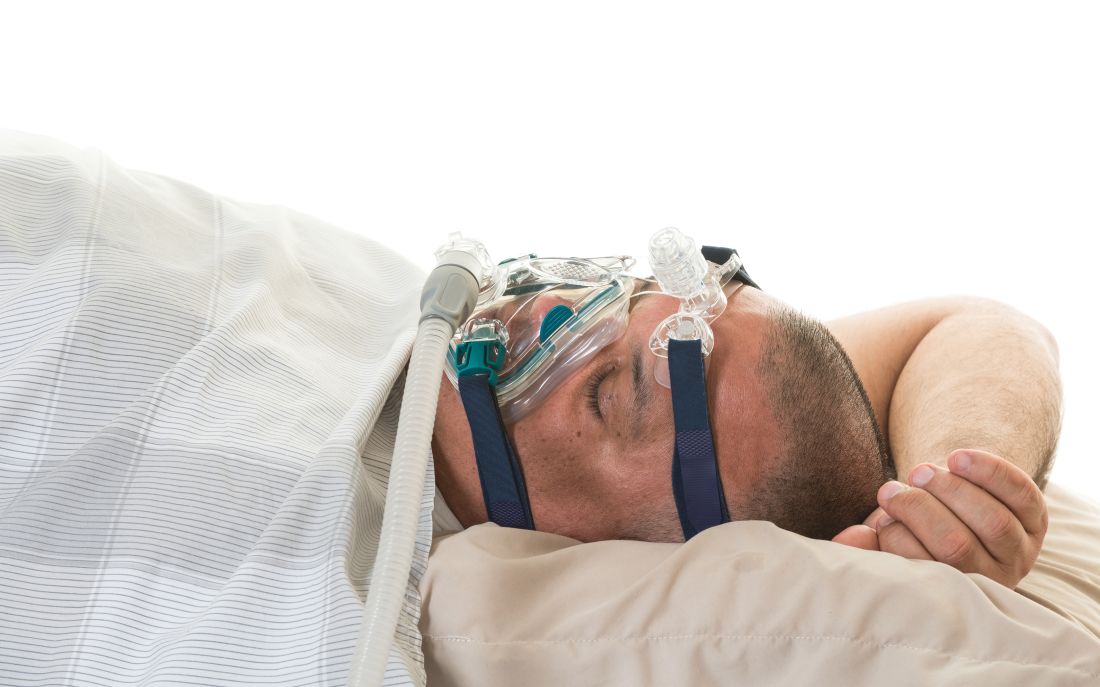
Stroke patients benefited from CPAP
the results of a randomized study suggest.
Obstructive sleep apnea is present in 50%-80% of patients with stroke, previous studies show, and its presence is associated with impaired function and cognition, delirium, and longer rehabilitation time, among other negative impacts, wrote Anupama Gupta, PhD, and her coauthors from the All India Institute of Medical Sciences, New Delhi, in the Journal of Clinical Sleep Medicine. Although multiple trials have shown a positive effect of CPAP on stroke recovery, relatively few investigations have looked specifically at whether the intervention prevents subsequent vascular events.
Patients’ clinical stroke outcomes were categorized in accordance with the Modified Rankin Scale (mRS), which is most widely used to assess disability and dependence outcomes among patients with stroke.
Significantly more patients who were treated with CPAP experienced an improvement in their mRS score by at least 1 point, when assessed at both 6 and 12 months following entrance into the study. Specifically, 53% (16) of patients in the CPAP group had an improvement of at least 1 point in their mRS score at 12 months, compared with 27% (11) of patients who did not use CPAP (P = .03).
“These differences are statistically significant, as well as clinically meaningful and relevant,” Dr. Gupta and her colleagues said in their report.
This finding was consistent with what researchers have seen in some earlier studies of stroke patients who used CPAP, the researchers wrote.
Additionally, CPAP-treated patients had fewer subsequent vascular events, compared with those who did not use CPAP, though the difference did not reach statistical significance. There was only one new vascular event (3.33%) in the CPAP group at 12-month follow-up, versus six events (15%) in the non-CPAP group (P = .23).
Nevertheless, the results provide more evidence for the potential benefit of CPAP in stroke patients with obstructive sleep apnea, the researchers noted.
“Our results indicate that new vascular events may be better prevented – and significantly more patients may make good stroke recovery – with CPAP treatment as compared to only best medical treatment,” Dr. Gupta and her colleagues wrote.
Before the study started, investigators determined that they would have needed 80 patients per arm for a power of 80%. A total of 679 patients were screened, but only 116 reported for polysomnography testing, and of those, 83 had at least moderate obstructive sleep apnea.
Due to a lack of CPAP devices, only 70 of those 83 patients made it all the way to randomization, investigators reported.
Dr. Gupta and her coauthors reported no conflicts of interest related to the study.
SOURCE: Gupta A et al. J Clin Sleep Med. 2018 Mar 30. pii:jc-17-00230.
the results of a randomized study suggest.
Obstructive sleep apnea is present in 50%-80% of patients with stroke, previous studies show, and its presence is associated with impaired function and cognition, delirium, and longer rehabilitation time, among other negative impacts, wrote Anupama Gupta, PhD, and her coauthors from the All India Institute of Medical Sciences, New Delhi, in the Journal of Clinical Sleep Medicine. Although multiple trials have shown a positive effect of CPAP on stroke recovery, relatively few investigations have looked specifically at whether the intervention prevents subsequent vascular events.
Patients’ clinical stroke outcomes were categorized in accordance with the Modified Rankin Scale (mRS), which is most widely used to assess disability and dependence outcomes among patients with stroke.
Significantly more patients who were treated with CPAP experienced an improvement in their mRS score by at least 1 point, when assessed at both 6 and 12 months following entrance into the study. Specifically, 53% (16) of patients in the CPAP group had an improvement of at least 1 point in their mRS score at 12 months, compared with 27% (11) of patients who did not use CPAP (P = .03).
“These differences are statistically significant, as well as clinically meaningful and relevant,” Dr. Gupta and her colleagues said in their report.
This finding was consistent with what researchers have seen in some earlier studies of stroke patients who used CPAP, the researchers wrote.
Additionally, CPAP-treated patients had fewer subsequent vascular events, compared with those who did not use CPAP, though the difference did not reach statistical significance. There was only one new vascular event (3.33%) in the CPAP group at 12-month follow-up, versus six events (15%) in the non-CPAP group (P = .23).
Nevertheless, the results provide more evidence for the potential benefit of CPAP in stroke patients with obstructive sleep apnea, the researchers noted.
“Our results indicate that new vascular events may be better prevented – and significantly more patients may make good stroke recovery – with CPAP treatment as compared to only best medical treatment,” Dr. Gupta and her colleagues wrote.
Before the study started, investigators determined that they would have needed 80 patients per arm for a power of 80%. A total of 679 patients were screened, but only 116 reported for polysomnography testing, and of those, 83 had at least moderate obstructive sleep apnea.
Due to a lack of CPAP devices, only 70 of those 83 patients made it all the way to randomization, investigators reported.
Dr. Gupta and her coauthors reported no conflicts of interest related to the study.
SOURCE: Gupta A et al. J Clin Sleep Med. 2018 Mar 30. pii:jc-17-00230.
the results of a randomized study suggest.
Obstructive sleep apnea is present in 50%-80% of patients with stroke, previous studies show, and its presence is associated with impaired function and cognition, delirium, and longer rehabilitation time, among other negative impacts, wrote Anupama Gupta, PhD, and her coauthors from the All India Institute of Medical Sciences, New Delhi, in the Journal of Clinical Sleep Medicine. Although multiple trials have shown a positive effect of CPAP on stroke recovery, relatively few investigations have looked specifically at whether the intervention prevents subsequent vascular events.
Patients’ clinical stroke outcomes were categorized in accordance with the Modified Rankin Scale (mRS), which is most widely used to assess disability and dependence outcomes among patients with stroke.
Significantly more patients who were treated with CPAP experienced an improvement in their mRS score by at least 1 point, when assessed at both 6 and 12 months following entrance into the study. Specifically, 53% (16) of patients in the CPAP group had an improvement of at least 1 point in their mRS score at 12 months, compared with 27% (11) of patients who did not use CPAP (P = .03).
“These differences are statistically significant, as well as clinically meaningful and relevant,” Dr. Gupta and her colleagues said in their report.
This finding was consistent with what researchers have seen in some earlier studies of stroke patients who used CPAP, the researchers wrote.
Additionally, CPAP-treated patients had fewer subsequent vascular events, compared with those who did not use CPAP, though the difference did not reach statistical significance. There was only one new vascular event (3.33%) in the CPAP group at 12-month follow-up, versus six events (15%) in the non-CPAP group (P = .23).
Nevertheless, the results provide more evidence for the potential benefit of CPAP in stroke patients with obstructive sleep apnea, the researchers noted.
“Our results indicate that new vascular events may be better prevented – and significantly more patients may make good stroke recovery – with CPAP treatment as compared to only best medical treatment,” Dr. Gupta and her colleagues wrote.
Before the study started, investigators determined that they would have needed 80 patients per arm for a power of 80%. A total of 679 patients were screened, but only 116 reported for polysomnography testing, and of those, 83 had at least moderate obstructive sleep apnea.
Due to a lack of CPAP devices, only 70 of those 83 patients made it all the way to randomization, investigators reported.
Dr. Gupta and her coauthors reported no conflicts of interest related to the study.
SOURCE: Gupta A et al. J Clin Sleep Med. 2018 Mar 30. pii:jc-17-00230.
FROM THE JOURNAL OF CLINICAL SLEEP MEDICINE
Key clinical point: Continuous positive airway pressure (CPAP) treatment may prevent vascular events in patients with stroke who have OSA.
Major finding: There was one vascular event (3.33%) at 12 months for CPAP-treated patients, versus six events (15%) in non-CPAP patients, though the difference was not significant (P = .23).
Study details: A single-blind, randomized, controlled trial including 70 patients with imaging-confirmed first arterial stroke and OSA.
Disclosures: The authors reported no conflicts of interest related to the study.
Source: Gupta A et al. J Clin Sleep Med. 2018 Mar 30. pii:jc-17-00230.
Impaired kidney function no problem for dabigatran reversal
ORLANDO – Idarucizumab, the reversal agent for the anticoagulant dabigatran, appeared as effective in quickly reversing dabigatran’s effects in patients with severe renal dysfunction as in patients with normally working kidneys, in a post hoc analysis of data collected in the drug’s pivotal trial.
A standard dose of idarucizumab “works just as well in patients with bad kidney function as it does in patients with preserved kidney function,” John W. Eikelboom, MD, said at the annual meeting of the American College of Cardiology. “The time to cessation of bleeding and the degree of normal hemostasis achieved was consistent” across the entire range of renal function examined, from severe renal dysfunction, with a creatinine clearance rate of less than 30 mL/min, to normal function, with an estimated rate of 80 mL/min or greater.
The ability of idarucizumab (Praxbind), conditionally approved by the Food and Drug Administration in 2015 and then fully approved in April 2018, to work in patients with impaired renal function has been an open question and concern because dabigatran (Pradaxa) is excreted renally, so it builds to unusually high levels in patients with poor kidney function. “Plasma dabigatran levels might be sky high, so a standard dose of idarucizumab might not work. That’s been a fear of clinicians,” explained Dr. Eikelboom, a hematologist at McMaster University in Hamilton, Ont.
To examine whether idarucizumab’s activity varied by renal function he used data from the patients enrolled in the RE-VERSE AD (Reversal Effects of Idarucizumab on Active Dabigatran) study, the pivotal dataset that led to idarucizumab’s U.S. approval. The new, post hoc analysis divided patients into four subgroups based on their kidney function, and focused on the 489 patients for whom renal data were available out of the 503 patients in the study (N Engl J Med. 2017 Aug 3;377[5]:431-41). The subgroups included 91 patients with severe dysfunction with a creatinine clearance rate of less than 30 mL/min; 127 with moderate dysfunction and a clearance rate of 30-49 mL/min; 163 with mild dysfunction and a clearance rate of 50-79 mL/min; and 108 with normal function and a creatinine clearance of at least 80 mL/min.
Patients in the subgroup with severe renal dysfunction had the worst clinical profile overall, and as predicted, had a markedly elevated average plasma level of dabigatran, 231 ng/mL, nearly five times higher than the 47-ng/mL average level in patients with normal renal function.
The ability of a single, standard dose of idarucizumab to reverse the anticoagulant effects of dabigatran were essentially identical across the four strata of renal activity, with 98% of patients in both the severely impaired subgroup and the normal subgroup having 100% reversal within 4 hours of treatment, Dr. Eikelboom reported. Every patient included in the analysis had more than 50% reversal.
The study followed patients to 12-24 hours after they received idarucizumab, and 55% of patients with severe renal dysfunction showed a plasma dabigatran level that crept back toward a clinically meaningful level and so might need a second idarucizumab dose. In contrast, this happened in 8% of patients with normal renal function.
In patients with severe renal dysfunction given idarucizumab, “be alert for a recurrent bleed,” which could require a second dose of idarucizumab, Dr. Eikelboom suggested.
SOURCE: Eikelboom JW et al. ACC 18, Abstract 1231M-11.
ORLANDO – Idarucizumab, the reversal agent for the anticoagulant dabigatran, appeared as effective in quickly reversing dabigatran’s effects in patients with severe renal dysfunction as in patients with normally working kidneys, in a post hoc analysis of data collected in the drug’s pivotal trial.
A standard dose of idarucizumab “works just as well in patients with bad kidney function as it does in patients with preserved kidney function,” John W. Eikelboom, MD, said at the annual meeting of the American College of Cardiology. “The time to cessation of bleeding and the degree of normal hemostasis achieved was consistent” across the entire range of renal function examined, from severe renal dysfunction, with a creatinine clearance rate of less than 30 mL/min, to normal function, with an estimated rate of 80 mL/min or greater.
The ability of idarucizumab (Praxbind), conditionally approved by the Food and Drug Administration in 2015 and then fully approved in April 2018, to work in patients with impaired renal function has been an open question and concern because dabigatran (Pradaxa) is excreted renally, so it builds to unusually high levels in patients with poor kidney function. “Plasma dabigatran levels might be sky high, so a standard dose of idarucizumab might not work. That’s been a fear of clinicians,” explained Dr. Eikelboom, a hematologist at McMaster University in Hamilton, Ont.
To examine whether idarucizumab’s activity varied by renal function he used data from the patients enrolled in the RE-VERSE AD (Reversal Effects of Idarucizumab on Active Dabigatran) study, the pivotal dataset that led to idarucizumab’s U.S. approval. The new, post hoc analysis divided patients into four subgroups based on their kidney function, and focused on the 489 patients for whom renal data were available out of the 503 patients in the study (N Engl J Med. 2017 Aug 3;377[5]:431-41). The subgroups included 91 patients with severe dysfunction with a creatinine clearance rate of less than 30 mL/min; 127 with moderate dysfunction and a clearance rate of 30-49 mL/min; 163 with mild dysfunction and a clearance rate of 50-79 mL/min; and 108 with normal function and a creatinine clearance of at least 80 mL/min.
Patients in the subgroup with severe renal dysfunction had the worst clinical profile overall, and as predicted, had a markedly elevated average plasma level of dabigatran, 231 ng/mL, nearly five times higher than the 47-ng/mL average level in patients with normal renal function.
The ability of a single, standard dose of idarucizumab to reverse the anticoagulant effects of dabigatran were essentially identical across the four strata of renal activity, with 98% of patients in both the severely impaired subgroup and the normal subgroup having 100% reversal within 4 hours of treatment, Dr. Eikelboom reported. Every patient included in the analysis had more than 50% reversal.
The study followed patients to 12-24 hours after they received idarucizumab, and 55% of patients with severe renal dysfunction showed a plasma dabigatran level that crept back toward a clinically meaningful level and so might need a second idarucizumab dose. In contrast, this happened in 8% of patients with normal renal function.
In patients with severe renal dysfunction given idarucizumab, “be alert for a recurrent bleed,” which could require a second dose of idarucizumab, Dr. Eikelboom suggested.
SOURCE: Eikelboom JW et al. ACC 18, Abstract 1231M-11.
ORLANDO – Idarucizumab, the reversal agent for the anticoagulant dabigatran, appeared as effective in quickly reversing dabigatran’s effects in patients with severe renal dysfunction as in patients with normally working kidneys, in a post hoc analysis of data collected in the drug’s pivotal trial.
A standard dose of idarucizumab “works just as well in patients with bad kidney function as it does in patients with preserved kidney function,” John W. Eikelboom, MD, said at the annual meeting of the American College of Cardiology. “The time to cessation of bleeding and the degree of normal hemostasis achieved was consistent” across the entire range of renal function examined, from severe renal dysfunction, with a creatinine clearance rate of less than 30 mL/min, to normal function, with an estimated rate of 80 mL/min or greater.
The ability of idarucizumab (Praxbind), conditionally approved by the Food and Drug Administration in 2015 and then fully approved in April 2018, to work in patients with impaired renal function has been an open question and concern because dabigatran (Pradaxa) is excreted renally, so it builds to unusually high levels in patients with poor kidney function. “Plasma dabigatran levels might be sky high, so a standard dose of idarucizumab might not work. That’s been a fear of clinicians,” explained Dr. Eikelboom, a hematologist at McMaster University in Hamilton, Ont.
To examine whether idarucizumab’s activity varied by renal function he used data from the patients enrolled in the RE-VERSE AD (Reversal Effects of Idarucizumab on Active Dabigatran) study, the pivotal dataset that led to idarucizumab’s U.S. approval. The new, post hoc analysis divided patients into four subgroups based on their kidney function, and focused on the 489 patients for whom renal data were available out of the 503 patients in the study (N Engl J Med. 2017 Aug 3;377[5]:431-41). The subgroups included 91 patients with severe dysfunction with a creatinine clearance rate of less than 30 mL/min; 127 with moderate dysfunction and a clearance rate of 30-49 mL/min; 163 with mild dysfunction and a clearance rate of 50-79 mL/min; and 108 with normal function and a creatinine clearance of at least 80 mL/min.
Patients in the subgroup with severe renal dysfunction had the worst clinical profile overall, and as predicted, had a markedly elevated average plasma level of dabigatran, 231 ng/mL, nearly five times higher than the 47-ng/mL average level in patients with normal renal function.
The ability of a single, standard dose of idarucizumab to reverse the anticoagulant effects of dabigatran were essentially identical across the four strata of renal activity, with 98% of patients in both the severely impaired subgroup and the normal subgroup having 100% reversal within 4 hours of treatment, Dr. Eikelboom reported. Every patient included in the analysis had more than 50% reversal.
The study followed patients to 12-24 hours after they received idarucizumab, and 55% of patients with severe renal dysfunction showed a plasma dabigatran level that crept back toward a clinically meaningful level and so might need a second idarucizumab dose. In contrast, this happened in 8% of patients with normal renal function.
In patients with severe renal dysfunction given idarucizumab, “be alert for a recurrent bleed,” which could require a second dose of idarucizumab, Dr. Eikelboom suggested.
SOURCE: Eikelboom JW et al. ACC 18, Abstract 1231M-11.
REPORTING FROM ACC 18
Key clinical point: Renal function had no impact on idarucizumab’s efficacy for dabigatran reversal.
Major finding: Complete dabigatran reversal occurred in 98% of patients with severe renal dysfunction who received idarucizumab.
Study details: Post hoc analysis of data from RE-VERSE AD, idarucizumab’s pivotal trial with 503 patients.
Disclosures: RE-VERSE AD was funded by Boehringer Ingelheim, the company that markets idarucizumab (Praxbind) and dabigatran (Pradaxa). Dr. Eikelboom has been a consultant to and has received research support from Boehringer Ingelheim, as well as from Bayer, Bristol-Myers Squibb, Daiichi-Sankyo, Janssen, and Pfizer.
Source: Eikelboom JW et al. ACC 18, Abstract 1231M-11.
Clopidogrel flunks platelet reactivity control test in TAVI
WASHINGTON – For antithrombotic therapy after transcatheter aortic valve implantation (TAVI), ticagrelor plus aspirin may be a better strategy than clopidogrel plus aspirin even though the latter combination is guideline recommended, according to a late-breaking, randomized study presented at CRT 2018 sponsored by the Cardiovascular Research Institute at Washington Hospital Center.
Unlike the ticagrelor regimen, which did deliver the goal antiplatelet effect for all 3 months of the study, “clopidogrel did not achieve adequate platelet inhibition before or after TAVI in most patients,” reported Victor A. Jimenez Diaz, MD, a cardiologist at University Hospital, Vigo, Spain.
The current American Heart Association/American College of Cardiology guidelines label the clopidogrel/aspirin combination for the first 6 months after TAVI “reasonable,” but Dr. Jimenez Diaz said that the value of this combination over other antiplatelet strategies has not been supported by a randomized clinical trial. The known variability in response to clopidogrel is among the reasons such data are needed.
Thrombotic and hemorrhagic complications are frequent after TAVI, making choice of antithrombotic treatment an important consideration for improving outcomes, according to Dr. Jimenez Diaz. The aim of the REAC TAVI trial was to evaluate whether ticagrelor provides a more consistent antiplatelet effect than clopidogrel for TAVI patients, which was undertaken at seven participating centers in Spain.
A total of 65 candidates for TAVI were enrolled in this study. The key exclusion criterion was chronic oral anticoagulation therapy. In a baseline assessment, patients in the study, all of whom were on 75 mg clopidogrel plus aspirin, were evaluated for high on-treatment platelet reactivity (HTPR), defined as a score of at least 208 platelet reaction units (PRU) on a standard assay.
The 46 (71%) patients found to have HTPR were randomized to 90 mg ticagrelor twice daily plus aspirin or to remain on the clopidogrel/aspirin combination. Unlike those with HTPR, in whom the mean PRU was 274 units, all of the patients without HTPR, who had a mean PRU of 134 units, remained on the baseline dual antiplatelet therapy. The study was open label.
The primary endpoint was adequate platelet antiaggregation, defined as absence of HTPR (less than 208 PRU), which was greater in the ticagrelor-treated group than the clopidogrel-treated group at 6 hours (91% vs. 4%), 5 days (100% vs. 10%), and 3 months (100% vs. 21%). In the patients without HTPR, the proportion with adequate platelet antiaggregation at these three time points were 73%, 64%, and 78%, respectively.
“The net difference in the randomized arms over the course of the study was 79%,” reported Dr. Jimenez Diaz, emphasizing that the study verified the hypothesis that ticagrelor would provide a more consistent antiplatelet effect than clopidogrel.
Although in-hospital bleeding complications were numerically higher in the clopidogrel-treated group (25% vs. 4%), this difference did not reach significance, and there were no significant differences in bleeding complications at any other time points or overall. There were two deaths in the clopidogrel-treated group, two deaths in the group without baseline HTPR, but no deaths in the ticagrelor-treated group.
While acknowledging that this study was small and not powered to show a difference in clinical events, Dr. Jimenez Diaz said it is important to emphasize that two-thirds of patients had HTPR at baseline. The high rate of HTPR among TAVI patients on clopidogrel and aspirin at baseline was identified as an important message from this study. However, a study is now needed to determine whether a ticagrelor strategy improves clinical outcomes when compared with a clopidogrel strategy.
A panel of experts at the CRT late-breaker session where these results were presented offered mixed reactions. While Jeffrey Popma, MD, director of interventional cardiology at Beth Israel Deaconess Hospital, Boston, called the results both “intriguing” and “provocative,” Ron Waksman, MD, associate director of the division of cardiology at the Medstar Health Institute, Washington, offered a note of caution, commenting that this application of ticagrelor “is off label, and then you would have to be concerned about the bleeding risk.”
However, all agreed that the optimal antithrombotic therapy for TAVI remains poorly defined and that randomized trials are needed to explore this issue.
This investigator-initiated study had no commercial sponsor. Dr. Jimenez Diaz reported no relevant financial relationships.
SOURCE: Jimenez Diaz VA. CRT 2018, Abstract LBT-10.
WASHINGTON – For antithrombotic therapy after transcatheter aortic valve implantation (TAVI), ticagrelor plus aspirin may be a better strategy than clopidogrel plus aspirin even though the latter combination is guideline recommended, according to a late-breaking, randomized study presented at CRT 2018 sponsored by the Cardiovascular Research Institute at Washington Hospital Center.
Unlike the ticagrelor regimen, which did deliver the goal antiplatelet effect for all 3 months of the study, “clopidogrel did not achieve adequate platelet inhibition before or after TAVI in most patients,” reported Victor A. Jimenez Diaz, MD, a cardiologist at University Hospital, Vigo, Spain.
The current American Heart Association/American College of Cardiology guidelines label the clopidogrel/aspirin combination for the first 6 months after TAVI “reasonable,” but Dr. Jimenez Diaz said that the value of this combination over other antiplatelet strategies has not been supported by a randomized clinical trial. The known variability in response to clopidogrel is among the reasons such data are needed.
Thrombotic and hemorrhagic complications are frequent after TAVI, making choice of antithrombotic treatment an important consideration for improving outcomes, according to Dr. Jimenez Diaz. The aim of the REAC TAVI trial was to evaluate whether ticagrelor provides a more consistent antiplatelet effect than clopidogrel for TAVI patients, which was undertaken at seven participating centers in Spain.
A total of 65 candidates for TAVI were enrolled in this study. The key exclusion criterion was chronic oral anticoagulation therapy. In a baseline assessment, patients in the study, all of whom were on 75 mg clopidogrel plus aspirin, were evaluated for high on-treatment platelet reactivity (HTPR), defined as a score of at least 208 platelet reaction units (PRU) on a standard assay.
The 46 (71%) patients found to have HTPR were randomized to 90 mg ticagrelor twice daily plus aspirin or to remain on the clopidogrel/aspirin combination. Unlike those with HTPR, in whom the mean PRU was 274 units, all of the patients without HTPR, who had a mean PRU of 134 units, remained on the baseline dual antiplatelet therapy. The study was open label.
The primary endpoint was adequate platelet antiaggregation, defined as absence of HTPR (less than 208 PRU), which was greater in the ticagrelor-treated group than the clopidogrel-treated group at 6 hours (91% vs. 4%), 5 days (100% vs. 10%), and 3 months (100% vs. 21%). In the patients without HTPR, the proportion with adequate platelet antiaggregation at these three time points were 73%, 64%, and 78%, respectively.
“The net difference in the randomized arms over the course of the study was 79%,” reported Dr. Jimenez Diaz, emphasizing that the study verified the hypothesis that ticagrelor would provide a more consistent antiplatelet effect than clopidogrel.
Although in-hospital bleeding complications were numerically higher in the clopidogrel-treated group (25% vs. 4%), this difference did not reach significance, and there were no significant differences in bleeding complications at any other time points or overall. There were two deaths in the clopidogrel-treated group, two deaths in the group without baseline HTPR, but no deaths in the ticagrelor-treated group.
While acknowledging that this study was small and not powered to show a difference in clinical events, Dr. Jimenez Diaz said it is important to emphasize that two-thirds of patients had HTPR at baseline. The high rate of HTPR among TAVI patients on clopidogrel and aspirin at baseline was identified as an important message from this study. However, a study is now needed to determine whether a ticagrelor strategy improves clinical outcomes when compared with a clopidogrel strategy.
A panel of experts at the CRT late-breaker session where these results were presented offered mixed reactions. While Jeffrey Popma, MD, director of interventional cardiology at Beth Israel Deaconess Hospital, Boston, called the results both “intriguing” and “provocative,” Ron Waksman, MD, associate director of the division of cardiology at the Medstar Health Institute, Washington, offered a note of caution, commenting that this application of ticagrelor “is off label, and then you would have to be concerned about the bleeding risk.”
However, all agreed that the optimal antithrombotic therapy for TAVI remains poorly defined and that randomized trials are needed to explore this issue.
This investigator-initiated study had no commercial sponsor. Dr. Jimenez Diaz reported no relevant financial relationships.
SOURCE: Jimenez Diaz VA. CRT 2018, Abstract LBT-10.
WASHINGTON – For antithrombotic therapy after transcatheter aortic valve implantation (TAVI), ticagrelor plus aspirin may be a better strategy than clopidogrel plus aspirin even though the latter combination is guideline recommended, according to a late-breaking, randomized study presented at CRT 2018 sponsored by the Cardiovascular Research Institute at Washington Hospital Center.
Unlike the ticagrelor regimen, which did deliver the goal antiplatelet effect for all 3 months of the study, “clopidogrel did not achieve adequate platelet inhibition before or after TAVI in most patients,” reported Victor A. Jimenez Diaz, MD, a cardiologist at University Hospital, Vigo, Spain.
The current American Heart Association/American College of Cardiology guidelines label the clopidogrel/aspirin combination for the first 6 months after TAVI “reasonable,” but Dr. Jimenez Diaz said that the value of this combination over other antiplatelet strategies has not been supported by a randomized clinical trial. The known variability in response to clopidogrel is among the reasons such data are needed.
Thrombotic and hemorrhagic complications are frequent after TAVI, making choice of antithrombotic treatment an important consideration for improving outcomes, according to Dr. Jimenez Diaz. The aim of the REAC TAVI trial was to evaluate whether ticagrelor provides a more consistent antiplatelet effect than clopidogrel for TAVI patients, which was undertaken at seven participating centers in Spain.
A total of 65 candidates for TAVI were enrolled in this study. The key exclusion criterion was chronic oral anticoagulation therapy. In a baseline assessment, patients in the study, all of whom were on 75 mg clopidogrel plus aspirin, were evaluated for high on-treatment platelet reactivity (HTPR), defined as a score of at least 208 platelet reaction units (PRU) on a standard assay.
The 46 (71%) patients found to have HTPR were randomized to 90 mg ticagrelor twice daily plus aspirin or to remain on the clopidogrel/aspirin combination. Unlike those with HTPR, in whom the mean PRU was 274 units, all of the patients without HTPR, who had a mean PRU of 134 units, remained on the baseline dual antiplatelet therapy. The study was open label.
The primary endpoint was adequate platelet antiaggregation, defined as absence of HTPR (less than 208 PRU), which was greater in the ticagrelor-treated group than the clopidogrel-treated group at 6 hours (91% vs. 4%), 5 days (100% vs. 10%), and 3 months (100% vs. 21%). In the patients without HTPR, the proportion with adequate platelet antiaggregation at these three time points were 73%, 64%, and 78%, respectively.
“The net difference in the randomized arms over the course of the study was 79%,” reported Dr. Jimenez Diaz, emphasizing that the study verified the hypothesis that ticagrelor would provide a more consistent antiplatelet effect than clopidogrel.
Although in-hospital bleeding complications were numerically higher in the clopidogrel-treated group (25% vs. 4%), this difference did not reach significance, and there were no significant differences in bleeding complications at any other time points or overall. There were two deaths in the clopidogrel-treated group, two deaths in the group without baseline HTPR, but no deaths in the ticagrelor-treated group.
While acknowledging that this study was small and not powered to show a difference in clinical events, Dr. Jimenez Diaz said it is important to emphasize that two-thirds of patients had HTPR at baseline. The high rate of HTPR among TAVI patients on clopidogrel and aspirin at baseline was identified as an important message from this study. However, a study is now needed to determine whether a ticagrelor strategy improves clinical outcomes when compared with a clopidogrel strategy.
A panel of experts at the CRT late-breaker session where these results were presented offered mixed reactions. While Jeffrey Popma, MD, director of interventional cardiology at Beth Israel Deaconess Hospital, Boston, called the results both “intriguing” and “provocative,” Ron Waksman, MD, associate director of the division of cardiology at the Medstar Health Institute, Washington, offered a note of caution, commenting that this application of ticagrelor “is off label, and then you would have to be concerned about the bleeding risk.”
However, all agreed that the optimal antithrombotic therapy for TAVI remains poorly defined and that randomized trials are needed to explore this issue.
This investigator-initiated study had no commercial sponsor. Dr. Jimenez Diaz reported no relevant financial relationships.
SOURCE: Jimenez Diaz VA. CRT 2018, Abstract LBT-10.
REPORTING FROM CRT 2018
Key clinical point: For platelet reactivity after transcatheter aortic valve implantation (TAVI), ticagrelor is more effective than clopidogrel.
Major finding:
Study details: A multicenter, randomized trial with 65 patients.
Disclosures: This investigator-initiated study had no commercial sponsor. Dr. Jimenez Diaz reported no relevant financial relationships.
Source: Jimenez Diaz VA. CRT 2018, Abstract LBT-10.
Apixaban prevails in study of 163,000 DOAC users
ORLANDO – Apixaban outperformed both rivaroxaban and dabigatran in a retrospective, observational study of real-world prescribing of direct oral anticoagulants in nearly 163,000 U.S. patients with nonvalvular atrial fibrillation, Steven B. Deitelzweig, MD, reported at the annual meeting of the American College of Cardiology.
This ongoing study, known as ARISTOPHANES (Anticoagulants for Reduction in Stroke: Observational Pooled Analysis on Health Outcomes and Experience of Patients), is the largest real-world analysis of direct oral anticoagulants (DOACs) to date. Unlike most of the previous observational studies of DOACs, which used a single insurance claims database, ARISTOPHANES pools data from Medicare and four large U.S. commercial insurance claims databases that collectively cover more than 180 million Americans.
This was a study of real-world prescribing. Unlike in randomized trials, where everyone is on a standard-dose DOAC, lower-dose therapy was common. It was prescribed for 21% of patients on apixaban, 15% on dabigatran, and 24% on rivaroxaban.
ARISTOPHANES results
The biggest difference in outcome was between patients on apixaban and those on dabigatran. The incidence of stroke/systemic embolism in 27,096 patients on apixaban (Eliquis) was 1.01% in 360 days, for a statistically significant 31% reduction in risk relative to the 1.42% incidence rate in 27,096 extensively matched patients on dabigatran (Pradaxa). The 360-day incidence of major bleeding was 2.7% in the apixaban group and 3.3% in those on dabigatran, for a 23% relative risk reduction. In the apixaban/rivaroxaban comparison, which included 62,619 patients in each group, the incidence of stroke/systemic embolism was 1.21% with apixaban and 1.42% with rivaroxaban, for a 27% relative risk reduction. Major bleeding occurred in 3.1% of the apixaban group and 5.3% of those on rivaroxaban, for a 46% reduction in risk favoring apixaban.
In a comparison of 27,538 patients on dabigatran and an equal number of rivaroxaban, the 360-day cumulative incidence of stroke/systemic embolism was 1.40% with dabigatran versus 1.23% with rivaroxaban, while the major bleeding rate was 3.28% in the dabigatran group and 4.76% with rivaroxaban.
Dr. Deitelzweig was quick to acknowledge the major limitation of ARISTOPHANES.
“Only associations can be drawn from a nonrandomized, retrospective, observational study, not conclusions regarding causality, even though the cohorts were matched using propensity scoring,” he emphasized.
The critique
Session cochair Jeanne E. Poole, MD, professor of medicine and director of the clinical cardiac electrophysiology program at the University of Washington, Seattle, commented, “The problem, of course, with using these large databases is that you may not be able to find out important information, such as whether rivaroxaban was being taken appropriately with meals, which is frequently not the case. If it wasn’t, that decreases absorption and efficacy by 40%. That’s a limitation.”
Audience member James A. Reiffel, MD, rose to add that, in his view, another significant limitation of all real-world, observational analyses using claims data is that it’s not possible to know why physicians selected a given drug for a given patient. He used as an example a patient with atrial fibrillation and gastroesophageal reflux disease (GERD).
“People with GERD may be less likely to get dabigatran, and if they’re less likely to get dabigatran with GERD, maybe they’re less likely to get a GI bleed. I don’t know,” said Dr. Reiffel, professor of clinical medicine and director of the electrocardiography laboratory at Columbia University Medical Center, New York.
“We have to take all the real-world analyses with a little grain of salt,” he added.
Dr. Deitelzweig replied, “This study is not meant to be the be-all and end-all.”
That being said, he noted that although randomized trials have shown that DOACs are at least as effective and safe as warfarin for stroke prevention in atrial fibrillation, there have been no randomized, head-to-head clinical trials comparing them, nor are any such studies likely to be done for the foreseeable future. Yet physicians and their patients are hungry for comparative effectiveness data, even if it doesn’t rise to the status of level I evidence.
ARISTOPHANES is sponsored by Bristol-Myers Squibb and Pfizer. Dr. Deitelzweig reported serving as a consultant to Pfizer and receiving research grants from Bristol-Myers Squibb and Portola.
SOURCE: Deitelzweig SB. ACC 2018.
ORLANDO – Apixaban outperformed both rivaroxaban and dabigatran in a retrospective, observational study of real-world prescribing of direct oral anticoagulants in nearly 163,000 U.S. patients with nonvalvular atrial fibrillation, Steven B. Deitelzweig, MD, reported at the annual meeting of the American College of Cardiology.
This ongoing study, known as ARISTOPHANES (Anticoagulants for Reduction in Stroke: Observational Pooled Analysis on Health Outcomes and Experience of Patients), is the largest real-world analysis of direct oral anticoagulants (DOACs) to date. Unlike most of the previous observational studies of DOACs, which used a single insurance claims database, ARISTOPHANES pools data from Medicare and four large U.S. commercial insurance claims databases that collectively cover more than 180 million Americans.
This was a study of real-world prescribing. Unlike in randomized trials, where everyone is on a standard-dose DOAC, lower-dose therapy was common. It was prescribed for 21% of patients on apixaban, 15% on dabigatran, and 24% on rivaroxaban.
ARISTOPHANES results
The biggest difference in outcome was between patients on apixaban and those on dabigatran. The incidence of stroke/systemic embolism in 27,096 patients on apixaban (Eliquis) was 1.01% in 360 days, for a statistically significant 31% reduction in risk relative to the 1.42% incidence rate in 27,096 extensively matched patients on dabigatran (Pradaxa). The 360-day incidence of major bleeding was 2.7% in the apixaban group and 3.3% in those on dabigatran, for a 23% relative risk reduction. In the apixaban/rivaroxaban comparison, which included 62,619 patients in each group, the incidence of stroke/systemic embolism was 1.21% with apixaban and 1.42% with rivaroxaban, for a 27% relative risk reduction. Major bleeding occurred in 3.1% of the apixaban group and 5.3% of those on rivaroxaban, for a 46% reduction in risk favoring apixaban.
In a comparison of 27,538 patients on dabigatran and an equal number of rivaroxaban, the 360-day cumulative incidence of stroke/systemic embolism was 1.40% with dabigatran versus 1.23% with rivaroxaban, while the major bleeding rate was 3.28% in the dabigatran group and 4.76% with rivaroxaban.
Dr. Deitelzweig was quick to acknowledge the major limitation of ARISTOPHANES.
“Only associations can be drawn from a nonrandomized, retrospective, observational study, not conclusions regarding causality, even though the cohorts were matched using propensity scoring,” he emphasized.
The critique
Session cochair Jeanne E. Poole, MD, professor of medicine and director of the clinical cardiac electrophysiology program at the University of Washington, Seattle, commented, “The problem, of course, with using these large databases is that you may not be able to find out important information, such as whether rivaroxaban was being taken appropriately with meals, which is frequently not the case. If it wasn’t, that decreases absorption and efficacy by 40%. That’s a limitation.”
Audience member James A. Reiffel, MD, rose to add that, in his view, another significant limitation of all real-world, observational analyses using claims data is that it’s not possible to know why physicians selected a given drug for a given patient. He used as an example a patient with atrial fibrillation and gastroesophageal reflux disease (GERD).
“People with GERD may be less likely to get dabigatran, and if they’re less likely to get dabigatran with GERD, maybe they’re less likely to get a GI bleed. I don’t know,” said Dr. Reiffel, professor of clinical medicine and director of the electrocardiography laboratory at Columbia University Medical Center, New York.
“We have to take all the real-world analyses with a little grain of salt,” he added.
Dr. Deitelzweig replied, “This study is not meant to be the be-all and end-all.”
That being said, he noted that although randomized trials have shown that DOACs are at least as effective and safe as warfarin for stroke prevention in atrial fibrillation, there have been no randomized, head-to-head clinical trials comparing them, nor are any such studies likely to be done for the foreseeable future. Yet physicians and their patients are hungry for comparative effectiveness data, even if it doesn’t rise to the status of level I evidence.
ARISTOPHANES is sponsored by Bristol-Myers Squibb and Pfizer. Dr. Deitelzweig reported serving as a consultant to Pfizer and receiving research grants from Bristol-Myers Squibb and Portola.
SOURCE: Deitelzweig SB. ACC 2018.
ORLANDO – Apixaban outperformed both rivaroxaban and dabigatran in a retrospective, observational study of real-world prescribing of direct oral anticoagulants in nearly 163,000 U.S. patients with nonvalvular atrial fibrillation, Steven B. Deitelzweig, MD, reported at the annual meeting of the American College of Cardiology.
This ongoing study, known as ARISTOPHANES (Anticoagulants for Reduction in Stroke: Observational Pooled Analysis on Health Outcomes and Experience of Patients), is the largest real-world analysis of direct oral anticoagulants (DOACs) to date. Unlike most of the previous observational studies of DOACs, which used a single insurance claims database, ARISTOPHANES pools data from Medicare and four large U.S. commercial insurance claims databases that collectively cover more than 180 million Americans.
This was a study of real-world prescribing. Unlike in randomized trials, where everyone is on a standard-dose DOAC, lower-dose therapy was common. It was prescribed for 21% of patients on apixaban, 15% on dabigatran, and 24% on rivaroxaban.
ARISTOPHANES results
The biggest difference in outcome was between patients on apixaban and those on dabigatran. The incidence of stroke/systemic embolism in 27,096 patients on apixaban (Eliquis) was 1.01% in 360 days, for a statistically significant 31% reduction in risk relative to the 1.42% incidence rate in 27,096 extensively matched patients on dabigatran (Pradaxa). The 360-day incidence of major bleeding was 2.7% in the apixaban group and 3.3% in those on dabigatran, for a 23% relative risk reduction. In the apixaban/rivaroxaban comparison, which included 62,619 patients in each group, the incidence of stroke/systemic embolism was 1.21% with apixaban and 1.42% with rivaroxaban, for a 27% relative risk reduction. Major bleeding occurred in 3.1% of the apixaban group and 5.3% of those on rivaroxaban, for a 46% reduction in risk favoring apixaban.
In a comparison of 27,538 patients on dabigatran and an equal number of rivaroxaban, the 360-day cumulative incidence of stroke/systemic embolism was 1.40% with dabigatran versus 1.23% with rivaroxaban, while the major bleeding rate was 3.28% in the dabigatran group and 4.76% with rivaroxaban.
Dr. Deitelzweig was quick to acknowledge the major limitation of ARISTOPHANES.
“Only associations can be drawn from a nonrandomized, retrospective, observational study, not conclusions regarding causality, even though the cohorts were matched using propensity scoring,” he emphasized.
The critique
Session cochair Jeanne E. Poole, MD, professor of medicine and director of the clinical cardiac electrophysiology program at the University of Washington, Seattle, commented, “The problem, of course, with using these large databases is that you may not be able to find out important information, such as whether rivaroxaban was being taken appropriately with meals, which is frequently not the case. If it wasn’t, that decreases absorption and efficacy by 40%. That’s a limitation.”
Audience member James A. Reiffel, MD, rose to add that, in his view, another significant limitation of all real-world, observational analyses using claims data is that it’s not possible to know why physicians selected a given drug for a given patient. He used as an example a patient with atrial fibrillation and gastroesophageal reflux disease (GERD).
“People with GERD may be less likely to get dabigatran, and if they’re less likely to get dabigatran with GERD, maybe they’re less likely to get a GI bleed. I don’t know,” said Dr. Reiffel, professor of clinical medicine and director of the electrocardiography laboratory at Columbia University Medical Center, New York.
“We have to take all the real-world analyses with a little grain of salt,” he added.
Dr. Deitelzweig replied, “This study is not meant to be the be-all and end-all.”
That being said, he noted that although randomized trials have shown that DOACs are at least as effective and safe as warfarin for stroke prevention in atrial fibrillation, there have been no randomized, head-to-head clinical trials comparing them, nor are any such studies likely to be done for the foreseeable future. Yet physicians and their patients are hungry for comparative effectiveness data, even if it doesn’t rise to the status of level I evidence.
ARISTOPHANES is sponsored by Bristol-Myers Squibb and Pfizer. Dr. Deitelzweig reported serving as a consultant to Pfizer and receiving research grants from Bristol-Myers Squibb and Portola.
SOURCE: Deitelzweig SB. ACC 2018.
REPORTING FROM ACC 2018
Key clinical point:
Major finding: The risk of stroke/systemic embolism in apixaban-treated patients was 31% lower than in those on dabigatran and 27% lower than with rivaroxaban.
Study details: This retrospective, observational study based upon claims data included 162,707 propensity score-matched patients with atrial fibrillation on a direct oral anticoagulant for stroke prevention.
Disclosures: The ongoing ARISTOPHANES study is sponsored by Bristol-Myers Squibb and Pfizer. The presenter reported serving as a consultant to Pfizer and receiving research grants from Bristol-Myers Squibb and Portola.
Source: Deitelzweig SB. ACC 2018.
Common infections are potent risk factor for MI, stroke
ORLANDO – , according to a “big data” registry study from the United Kingdom.
“Our data show infection was just as much a risk factor or more compared with the traditional atherosclerotic cardiovascular disease risk factors,” Paul Carter, MD, said at the annual meeting of the American College of Cardiology.
Dr. Carter of Aston Medical School in Birmingham, England, presented a retrospective analysis from the ACALM (Algorithm for Comorbidities Associated with Length of stay and Mortality) study of administrative data on all of the more than 1.22 million patients admitted to seven U.K. hospitals in 2000-2013. His analysis included all 34,027 adults aged 40 years or older admitted with a urinary tract or respiratory infection on their index hospitalization who had no history of ischemic heart disease or ischemic stroke.
These patients, with a mean age of 73 years, 59% of whom were women, were compared with an equal number of age- and gender-matched adults whose index hospitalization was for reasons other than ischemic heart disease, stroke, urinary tract infection (UTI), or respiratory infection – the two most common infections resulting in hospitalization in the United Kingdom.
Patients with a respiratory infection or UTI had a 9.9% incidence of new-onset ischemic heart disease and a 4.1% rate of ischemic stroke during follow-up starting upon discharge from their index hospitalization, significantly higher than the 5.9% and 1.5% rates in controls. In a multivariate logistic regression analysis adjusted for demographics, standard cardiovascular risk factors, and the top 10 causes of mortality in the United Kingdom, patients with respiratory infection or UTI as their admitting diagnosis had a 1.36-fold increased likelihood of developing ischemic heart disease post discharge and a 2.5-fold greater risk of ischemic stroke than matched controls.
Moreover, mortality following diagnosis of ischemic heart disease was 75.2% in patients whose index hospitalization was for infection, compared with 51.1% in controls who developed ischemic heart disease without a history of hospitalization for infection, for an adjusted 2.98-fold increased likelihood of death. Similarly, mortality after an ischemic stroke was 59.8% in patients with a prior severe infection, compared with 30.8% in controls, which translated to an adjusted 3.1-fold increased risk of death post stroke in patients with a prior hospitalization for infection.
In the multivariate analysis, hospitalization for infection was a stronger risk factor for subsequent ischemic stroke than was atrial fibrillation, heart failure, type 1 or type 2 diabetes, hypertension, or hyperlipidemia. The risk of ischemic heart disease in patients with an infectious disease hospitalization was similar to the risks associated with most of those recognized risk factors.
Two possible mechanisms by which infection might predispose to subsequent ischemic heart disease and stroke are via a direct effect whereby pathogens such as Chlamydia pneumoniae are taken up into arterial plaques, where they cause a local inflammatory response, or an indirect effect in which systemic inflammation primes the atherosclerotic plaque through distribution of inflammatory cytokines, according to Dr. Carter.
He said the ACALM findings are particularly intriguing when considered in the context of the 2017 results of the landmark CANTOS trial, in which canakinumab (Ilaris), a targeted anti-inflammatory agent that inhibits the interleukin-1 beta innate immunity pathway, reduced recurrent ischemic events in post-MI patients who had high systemic inflammation as evidenced by their elevated C-reactive protein level but a normal-range LDL cholesterol (N Engl J Med. 2017 Aug 27. doi: 10.1056/NEJMoa1707914).
“If atherosclerosis is an inflammatory condition, this begs the question of whether other inflammatory conditions, like infection, which induces a large systemic inflammatory response, might drive atherosclerosis,” Dr. Carter commented.
“It’s now very well understood that inflammatory mediators, cells, and processes are involved in every step from the initial endothelial dysfunction that leads to uptake of LDL, inflammatory cells, and monocytes all the way through to plaque progression and rupture, where Th1 cytokines have been implicated in causing that rupture, and ultimately in patient presentation at the hospital,” he added.
He sees the ACALM findings as hypothesis generating, serving to help lay the groundwork for future clinical trials of vaccine or anti-inflammatory antibiotic therapies.
Dr. Carter reported having no financial conflicts related to his presentation.
SOURCE: Carter P. ACC 2018, Abstract 1325M-0.
ORLANDO – , according to a “big data” registry study from the United Kingdom.
“Our data show infection was just as much a risk factor or more compared with the traditional atherosclerotic cardiovascular disease risk factors,” Paul Carter, MD, said at the annual meeting of the American College of Cardiology.
Dr. Carter of Aston Medical School in Birmingham, England, presented a retrospective analysis from the ACALM (Algorithm for Comorbidities Associated with Length of stay and Mortality) study of administrative data on all of the more than 1.22 million patients admitted to seven U.K. hospitals in 2000-2013. His analysis included all 34,027 adults aged 40 years or older admitted with a urinary tract or respiratory infection on their index hospitalization who had no history of ischemic heart disease or ischemic stroke.
These patients, with a mean age of 73 years, 59% of whom were women, were compared with an equal number of age- and gender-matched adults whose index hospitalization was for reasons other than ischemic heart disease, stroke, urinary tract infection (UTI), or respiratory infection – the two most common infections resulting in hospitalization in the United Kingdom.
Patients with a respiratory infection or UTI had a 9.9% incidence of new-onset ischemic heart disease and a 4.1% rate of ischemic stroke during follow-up starting upon discharge from their index hospitalization, significantly higher than the 5.9% and 1.5% rates in controls. In a multivariate logistic regression analysis adjusted for demographics, standard cardiovascular risk factors, and the top 10 causes of mortality in the United Kingdom, patients with respiratory infection or UTI as their admitting diagnosis had a 1.36-fold increased likelihood of developing ischemic heart disease post discharge and a 2.5-fold greater risk of ischemic stroke than matched controls.
Moreover, mortality following diagnosis of ischemic heart disease was 75.2% in patients whose index hospitalization was for infection, compared with 51.1% in controls who developed ischemic heart disease without a history of hospitalization for infection, for an adjusted 2.98-fold increased likelihood of death. Similarly, mortality after an ischemic stroke was 59.8% in patients with a prior severe infection, compared with 30.8% in controls, which translated to an adjusted 3.1-fold increased risk of death post stroke in patients with a prior hospitalization for infection.
In the multivariate analysis, hospitalization for infection was a stronger risk factor for subsequent ischemic stroke than was atrial fibrillation, heart failure, type 1 or type 2 diabetes, hypertension, or hyperlipidemia. The risk of ischemic heart disease in patients with an infectious disease hospitalization was similar to the risks associated with most of those recognized risk factors.
Two possible mechanisms by which infection might predispose to subsequent ischemic heart disease and stroke are via a direct effect whereby pathogens such as Chlamydia pneumoniae are taken up into arterial plaques, where they cause a local inflammatory response, or an indirect effect in which systemic inflammation primes the atherosclerotic plaque through distribution of inflammatory cytokines, according to Dr. Carter.
He said the ACALM findings are particularly intriguing when considered in the context of the 2017 results of the landmark CANTOS trial, in which canakinumab (Ilaris), a targeted anti-inflammatory agent that inhibits the interleukin-1 beta innate immunity pathway, reduced recurrent ischemic events in post-MI patients who had high systemic inflammation as evidenced by their elevated C-reactive protein level but a normal-range LDL cholesterol (N Engl J Med. 2017 Aug 27. doi: 10.1056/NEJMoa1707914).
“If atherosclerosis is an inflammatory condition, this begs the question of whether other inflammatory conditions, like infection, which induces a large systemic inflammatory response, might drive atherosclerosis,” Dr. Carter commented.
“It’s now very well understood that inflammatory mediators, cells, and processes are involved in every step from the initial endothelial dysfunction that leads to uptake of LDL, inflammatory cells, and monocytes all the way through to plaque progression and rupture, where Th1 cytokines have been implicated in causing that rupture, and ultimately in patient presentation at the hospital,” he added.
He sees the ACALM findings as hypothesis generating, serving to help lay the groundwork for future clinical trials of vaccine or anti-inflammatory antibiotic therapies.
Dr. Carter reported having no financial conflicts related to his presentation.
SOURCE: Carter P. ACC 2018, Abstract 1325M-0.
ORLANDO – , according to a “big data” registry study from the United Kingdom.
“Our data show infection was just as much a risk factor or more compared with the traditional atherosclerotic cardiovascular disease risk factors,” Paul Carter, MD, said at the annual meeting of the American College of Cardiology.
Dr. Carter of Aston Medical School in Birmingham, England, presented a retrospective analysis from the ACALM (Algorithm for Comorbidities Associated with Length of stay and Mortality) study of administrative data on all of the more than 1.22 million patients admitted to seven U.K. hospitals in 2000-2013. His analysis included all 34,027 adults aged 40 years or older admitted with a urinary tract or respiratory infection on their index hospitalization who had no history of ischemic heart disease or ischemic stroke.
These patients, with a mean age of 73 years, 59% of whom were women, were compared with an equal number of age- and gender-matched adults whose index hospitalization was for reasons other than ischemic heart disease, stroke, urinary tract infection (UTI), or respiratory infection – the two most common infections resulting in hospitalization in the United Kingdom.
Patients with a respiratory infection or UTI had a 9.9% incidence of new-onset ischemic heart disease and a 4.1% rate of ischemic stroke during follow-up starting upon discharge from their index hospitalization, significantly higher than the 5.9% and 1.5% rates in controls. In a multivariate logistic regression analysis adjusted for demographics, standard cardiovascular risk factors, and the top 10 causes of mortality in the United Kingdom, patients with respiratory infection or UTI as their admitting diagnosis had a 1.36-fold increased likelihood of developing ischemic heart disease post discharge and a 2.5-fold greater risk of ischemic stroke than matched controls.
Moreover, mortality following diagnosis of ischemic heart disease was 75.2% in patients whose index hospitalization was for infection, compared with 51.1% in controls who developed ischemic heart disease without a history of hospitalization for infection, for an adjusted 2.98-fold increased likelihood of death. Similarly, mortality after an ischemic stroke was 59.8% in patients with a prior severe infection, compared with 30.8% in controls, which translated to an adjusted 3.1-fold increased risk of death post stroke in patients with a prior hospitalization for infection.
In the multivariate analysis, hospitalization for infection was a stronger risk factor for subsequent ischemic stroke than was atrial fibrillation, heart failure, type 1 or type 2 diabetes, hypertension, or hyperlipidemia. The risk of ischemic heart disease in patients with an infectious disease hospitalization was similar to the risks associated with most of those recognized risk factors.
Two possible mechanisms by which infection might predispose to subsequent ischemic heart disease and stroke are via a direct effect whereby pathogens such as Chlamydia pneumoniae are taken up into arterial plaques, where they cause a local inflammatory response, or an indirect effect in which systemic inflammation primes the atherosclerotic plaque through distribution of inflammatory cytokines, according to Dr. Carter.
He said the ACALM findings are particularly intriguing when considered in the context of the 2017 results of the landmark CANTOS trial, in which canakinumab (Ilaris), a targeted anti-inflammatory agent that inhibits the interleukin-1 beta innate immunity pathway, reduced recurrent ischemic events in post-MI patients who had high systemic inflammation as evidenced by their elevated C-reactive protein level but a normal-range LDL cholesterol (N Engl J Med. 2017 Aug 27. doi: 10.1056/NEJMoa1707914).
“If atherosclerosis is an inflammatory condition, this begs the question of whether other inflammatory conditions, like infection, which induces a large systemic inflammatory response, might drive atherosclerosis,” Dr. Carter commented.
“It’s now very well understood that inflammatory mediators, cells, and processes are involved in every step from the initial endothelial dysfunction that leads to uptake of LDL, inflammatory cells, and monocytes all the way through to plaque progression and rupture, where Th1 cytokines have been implicated in causing that rupture, and ultimately in patient presentation at the hospital,” he added.
He sees the ACALM findings as hypothesis generating, serving to help lay the groundwork for future clinical trials of vaccine or anti-inflammatory antibiotic therapies.
Dr. Carter reported having no financial conflicts related to his presentation.
SOURCE: Carter P. ACC 2018, Abstract 1325M-0.
REPORTING FROM ACC 2018
Key clinical point: Once patients have been hospitalized for a respiratory infection or UTI, their postdischarge risk of ischemic stroke is 2.5-fold greater than in those without such a history.
Major finding: Patients with a history of hospitalization for UTI or a respiratory infection who later develop ischemic heart disease or stroke have a threefold higher mortality risk than those without such a hospitalization.
Study details: This was a retrospective study of more than 68,000 subjects in the U.K. ACALM registry study.
Disclosures: The study presenter reported having no financial conflicts of interest.
Source: Carter P. ACC 2018, Abstract 1325M-0.
Post-ACS death lowered in ODYSSEY Outcomes
ORLANDO – should set the stage for broader use of the PCSK9-inhibitor in certain high-risk patients, Gabriel Steg, MD, said in a video interview at the annual meeting of the American College of Cardiology.
The findings from the 3-year trial are threefold: First, the trial met its primary goal, significantly lower major adverse cardiovascular events and 15% lower mortality in alirocumab-treated patients compared with those on placebo; second, the effect was greater in patients who started with an LDL level above 100 mg/dL; and third, alirocumab was remarkably safe, said Dr. Steg, director of the coronary care unit of Bichat Hospital in Paris.
“We now have good reason to target a lower LDL range of at least less than 50 mg/dL, and possibly even lower, using PCSK9 inhibitors to get the benefits we’re seeing in the trial applied to broader groups of patients,” he added.
SOURCE: Steg G ACC 18.
ORLANDO – should set the stage for broader use of the PCSK9-inhibitor in certain high-risk patients, Gabriel Steg, MD, said in a video interview at the annual meeting of the American College of Cardiology.
The findings from the 3-year trial are threefold: First, the trial met its primary goal, significantly lower major adverse cardiovascular events and 15% lower mortality in alirocumab-treated patients compared with those on placebo; second, the effect was greater in patients who started with an LDL level above 100 mg/dL; and third, alirocumab was remarkably safe, said Dr. Steg, director of the coronary care unit of Bichat Hospital in Paris.
“We now have good reason to target a lower LDL range of at least less than 50 mg/dL, and possibly even lower, using PCSK9 inhibitors to get the benefits we’re seeing in the trial applied to broader groups of patients,” he added.
SOURCE: Steg G ACC 18.
ORLANDO – should set the stage for broader use of the PCSK9-inhibitor in certain high-risk patients, Gabriel Steg, MD, said in a video interview at the annual meeting of the American College of Cardiology.
The findings from the 3-year trial are threefold: First, the trial met its primary goal, significantly lower major adverse cardiovascular events and 15% lower mortality in alirocumab-treated patients compared with those on placebo; second, the effect was greater in patients who started with an LDL level above 100 mg/dL; and third, alirocumab was remarkably safe, said Dr. Steg, director of the coronary care unit of Bichat Hospital in Paris.
“We now have good reason to target a lower LDL range of at least less than 50 mg/dL, and possibly even lower, using PCSK9 inhibitors to get the benefits we’re seeing in the trial applied to broader groups of patients,” he added.
SOURCE: Steg G ACC 18.
REPORTING FROM ACC 18
Adding hypertension in pregnancy doesn’t refine ASCVD risk prediction tool
ANAHEIM, CALIF. – The first-ever study to examine the clinical utility of incorporating a history of hypertensive disorders of pregnancy into the American College of Cardiology/American Heart Association Atherosclerotic Cardiovascular Disease Risk Calculator in an effort to better delineate risk in women failed to show any added benefit.
But that doesn’t mean such a history is without value in daily clinical practice, Jennifer J. Stuart, ScD, asserted at the American Heart Association scientific sessions.
“While we did not demonstrate that hypertensive disorders of pregnancy improved cardiovascular risk discrimination in women, we do still believe – and I still believe – that hypertensive disorders of pregnancy remain an important risk marker for cardiovascular disease in women,” said Dr. Stuart of the Harvard School of Public Health, Boston.
“In this latest analysis we’ve demonstrated over a fourfold increased risk of chronic hypertension in these women in the first 5 years after delivery. So we do see increased risk very soon after delivery, and it persists for decades. And hypertensive disorders of pregnancy as a risk marker does offer practical advantages: ease of ascertainment, low cost, and availability earlier in life,” the researcher noted.
Her study hypothesis was that adding hypertensive disorders of pregnancy – that is, gestational hypertension and preeclampsia – and parity to the current ACC/AHA ASCVD Risk Calculator would enhance the tool’s predictive tool’s power in women.
“We wanted to know if a history of hypertensive disorders of pregnancy can be leveraged to capture women currently being missed in screening,” she explained.
Her expectation that this would be the case was based on solid evidence that hypertensive disorders of pregnancy, which occur in 10%-15% of all pregnancies, are associated with subsequent increased risk of cardiovascular events. For example, a meta-analysis of published studies involving nearly 3.5 million women, including almost 200,000 with a history of preeclampsia, showed that preeclampsia was associated with a 2.16-fold increased risk of ischemic heart disease during 11.7 years of follow-up (BMJ. 2007 Nov 10;335[7627]:974). This meta-analysis was among the evidence that persuaded the AHA in 2011 to formally add hypertensive disorders of pregnancy to the list of risk factors for cardiovascular disease in women (Circulation. 2011 Mar 22;123[11]:1243-62).
Dr. Stuart also incorporated parity in her investigational revised ASCVD risk model, because parity has been shown to be an independent risk factor of cardiovascular disease in a relationship described by a J-shaped curve.
She put the souped-up risk prediction model to the test by separately applying it and the current version to data from the longitudinal prospective Nurses’ Health Study II. For this analysis, nearly 68,000 female registered nurses were followed for new diseases every 2 years from 1989, when they were aged 25-42, through 2013. She and her coinvestigators applied the two 10-year risk-prediction models to the overall cohort and again separately to women at aged 40-49 and 50-59.
The bottom line: This might be because the nurses comprise a relatively low-risk population. Also, even though hypertensive disorders of pregnancy are associated with 10-year cardiovascular disease risk independent of the established risk factors, there may be enough overlap that the standard risk factors sufficiently capture the risk.
“It may be that the information on history of hypertensive disorders of pregnancy should be ascertained in the 20s and 30s, rather than waiting until age 40 or later, when we generally apply cardiovascular risk scores in practice. That [earlier assessment] may be a really important and valuable opportunity to identify these women at high risk and utilize primary prevention,” according to Dr. Stuart.
What’s next? “I’m certainly interested in educating these women about their increased risk. This is not something that’s done consistently across the country and across practices, and we really believe this is an important message for women to get when they deliver these pregnancies,” she added.
Dr. Stuart reported having no financial conflicts of interest regarding her study.
SOURCE: Stuart JJ. AHA Scientific Sessions.
ANAHEIM, CALIF. – The first-ever study to examine the clinical utility of incorporating a history of hypertensive disorders of pregnancy into the American College of Cardiology/American Heart Association Atherosclerotic Cardiovascular Disease Risk Calculator in an effort to better delineate risk in women failed to show any added benefit.
But that doesn’t mean such a history is without value in daily clinical practice, Jennifer J. Stuart, ScD, asserted at the American Heart Association scientific sessions.
“While we did not demonstrate that hypertensive disorders of pregnancy improved cardiovascular risk discrimination in women, we do still believe – and I still believe – that hypertensive disorders of pregnancy remain an important risk marker for cardiovascular disease in women,” said Dr. Stuart of the Harvard School of Public Health, Boston.
“In this latest analysis we’ve demonstrated over a fourfold increased risk of chronic hypertension in these women in the first 5 years after delivery. So we do see increased risk very soon after delivery, and it persists for decades. And hypertensive disorders of pregnancy as a risk marker does offer practical advantages: ease of ascertainment, low cost, and availability earlier in life,” the researcher noted.
Her study hypothesis was that adding hypertensive disorders of pregnancy – that is, gestational hypertension and preeclampsia – and parity to the current ACC/AHA ASCVD Risk Calculator would enhance the tool’s predictive tool’s power in women.
“We wanted to know if a history of hypertensive disorders of pregnancy can be leveraged to capture women currently being missed in screening,” she explained.
Her expectation that this would be the case was based on solid evidence that hypertensive disorders of pregnancy, which occur in 10%-15% of all pregnancies, are associated with subsequent increased risk of cardiovascular events. For example, a meta-analysis of published studies involving nearly 3.5 million women, including almost 200,000 with a history of preeclampsia, showed that preeclampsia was associated with a 2.16-fold increased risk of ischemic heart disease during 11.7 years of follow-up (BMJ. 2007 Nov 10;335[7627]:974). This meta-analysis was among the evidence that persuaded the AHA in 2011 to formally add hypertensive disorders of pregnancy to the list of risk factors for cardiovascular disease in women (Circulation. 2011 Mar 22;123[11]:1243-62).
Dr. Stuart also incorporated parity in her investigational revised ASCVD risk model, because parity has been shown to be an independent risk factor of cardiovascular disease in a relationship described by a J-shaped curve.
She put the souped-up risk prediction model to the test by separately applying it and the current version to data from the longitudinal prospective Nurses’ Health Study II. For this analysis, nearly 68,000 female registered nurses were followed for new diseases every 2 years from 1989, when they were aged 25-42, through 2013. She and her coinvestigators applied the two 10-year risk-prediction models to the overall cohort and again separately to women at aged 40-49 and 50-59.
The bottom line: This might be because the nurses comprise a relatively low-risk population. Also, even though hypertensive disorders of pregnancy are associated with 10-year cardiovascular disease risk independent of the established risk factors, there may be enough overlap that the standard risk factors sufficiently capture the risk.
“It may be that the information on history of hypertensive disorders of pregnancy should be ascertained in the 20s and 30s, rather than waiting until age 40 or later, when we generally apply cardiovascular risk scores in practice. That [earlier assessment] may be a really important and valuable opportunity to identify these women at high risk and utilize primary prevention,” according to Dr. Stuart.
What’s next? “I’m certainly interested in educating these women about their increased risk. This is not something that’s done consistently across the country and across practices, and we really believe this is an important message for women to get when they deliver these pregnancies,” she added.
Dr. Stuart reported having no financial conflicts of interest regarding her study.
SOURCE: Stuart JJ. AHA Scientific Sessions.
ANAHEIM, CALIF. – The first-ever study to examine the clinical utility of incorporating a history of hypertensive disorders of pregnancy into the American College of Cardiology/American Heart Association Atherosclerotic Cardiovascular Disease Risk Calculator in an effort to better delineate risk in women failed to show any added benefit.
But that doesn’t mean such a history is without value in daily clinical practice, Jennifer J. Stuart, ScD, asserted at the American Heart Association scientific sessions.
“While we did not demonstrate that hypertensive disorders of pregnancy improved cardiovascular risk discrimination in women, we do still believe – and I still believe – that hypertensive disorders of pregnancy remain an important risk marker for cardiovascular disease in women,” said Dr. Stuart of the Harvard School of Public Health, Boston.
“In this latest analysis we’ve demonstrated over a fourfold increased risk of chronic hypertension in these women in the first 5 years after delivery. So we do see increased risk very soon after delivery, and it persists for decades. And hypertensive disorders of pregnancy as a risk marker does offer practical advantages: ease of ascertainment, low cost, and availability earlier in life,” the researcher noted.
Her study hypothesis was that adding hypertensive disorders of pregnancy – that is, gestational hypertension and preeclampsia – and parity to the current ACC/AHA ASCVD Risk Calculator would enhance the tool’s predictive tool’s power in women.
“We wanted to know if a history of hypertensive disorders of pregnancy can be leveraged to capture women currently being missed in screening,” she explained.
Her expectation that this would be the case was based on solid evidence that hypertensive disorders of pregnancy, which occur in 10%-15% of all pregnancies, are associated with subsequent increased risk of cardiovascular events. For example, a meta-analysis of published studies involving nearly 3.5 million women, including almost 200,000 with a history of preeclampsia, showed that preeclampsia was associated with a 2.16-fold increased risk of ischemic heart disease during 11.7 years of follow-up (BMJ. 2007 Nov 10;335[7627]:974). This meta-analysis was among the evidence that persuaded the AHA in 2011 to formally add hypertensive disorders of pregnancy to the list of risk factors for cardiovascular disease in women (Circulation. 2011 Mar 22;123[11]:1243-62).
Dr. Stuart also incorporated parity in her investigational revised ASCVD risk model, because parity has been shown to be an independent risk factor of cardiovascular disease in a relationship described by a J-shaped curve.
She put the souped-up risk prediction model to the test by separately applying it and the current version to data from the longitudinal prospective Nurses’ Health Study II. For this analysis, nearly 68,000 female registered nurses were followed for new diseases every 2 years from 1989, when they were aged 25-42, through 2013. She and her coinvestigators applied the two 10-year risk-prediction models to the overall cohort and again separately to women at aged 40-49 and 50-59.
The bottom line: This might be because the nurses comprise a relatively low-risk population. Also, even though hypertensive disorders of pregnancy are associated with 10-year cardiovascular disease risk independent of the established risk factors, there may be enough overlap that the standard risk factors sufficiently capture the risk.
“It may be that the information on history of hypertensive disorders of pregnancy should be ascertained in the 20s and 30s, rather than waiting until age 40 or later, when we generally apply cardiovascular risk scores in practice. That [earlier assessment] may be a really important and valuable opportunity to identify these women at high risk and utilize primary prevention,” according to Dr. Stuart.
What’s next? “I’m certainly interested in educating these women about their increased risk. This is not something that’s done consistently across the country and across practices, and we really believe this is an important message for women to get when they deliver these pregnancies,” she added.
Dr. Stuart reported having no financial conflicts of interest regarding her study.
SOURCE: Stuart JJ. AHA Scientific Sessions.
REPORTING FROM THE AHA SCIENTIFIC SESSIONS
Key clinical point: The ACC/AHA Atherosclerotic Cardiovascular Disease Risk Calculator doesn’t do a better job of predicting 10-year risk in women when a history of hypertensive disorders of pregnancy is added into the formula.
Major finding: Incorporating a woman’s history of hypertensive disorders of pregnancy into the Atherosclerotic Cardiovascular Disease Risk Calculator doesn’t improve the tool’s accuracy in predicting 10-year risk.
Study details: An analysis of data from the longitudinal, prospective Nurses’ Health Study II.
Disclosures: The study presenter reported having no financial conflicts of interest.
Source: Stuart JJ. AHA Scientific Sessions.
How to lower occupational radiation exposure in the cath lab
Adding some lead shielding in cardiac catheterization labs can cut the amount of radiation that nurse circulators and scrub technologists are exposed to, according to a single-center study of more than 750 patients.
“The simple and relatively inexpensive approach of providing staff members with a dedicated accessory lead shield during cardiac catheterization was associated with a nearly two-thirds reduction in radiation exposure among both nurses and technologists” according to Ryan Madder, MD, and his associates. “The present study is the first, to our knowledge, to observe a similar benefit among nonphysician staff members, an observation that may have important implications for occupational safety in the cardiac catheterization laboratory.”
The report was published in JACC: Cardiovascular Interventions.
SHIELD (Combining Robotic-Stenting and Proactive Shielding Techniques in the Catheterization Laboratory to Achieve Lowest Possible Radiation Exposure to Physicians and Staff) was a single-center, prospective, observational designed study to research radiation exposure levels among physicians and other staff members in cardiac catheterization labs. Dr. Madder, a cardiologist at Spectrum Health in Grand Rapids, Mich., and his colleagues collected radiation exposure information for staff members such as nurse circulators and scrub technologists during August 2015–February 2016 and measured radiation exposure in 764 consecutive cases. Radiation exposure was monitored for these staff members by dosimeters worn on either the left anterior side of the glasses or thyroid collar, and a body dosimeter underneath the lead apparel on the V-neck of their scrub shirt.
The radiation protection used in the study followed standard institutional operating procedures, which includes two shields between the patient and physician, a ceiling mounted upper body shield with a patient contour cutout, and a lower body shield attached to the side of the operating table, extending from the table to the floor. Staff members wore traditional lead garments, consisting of a lead skirt, apron, and thyroid collar. Additional radiation absorbing disposable pads were used as desired by the staff members.
The study was split into two phases to evaluate the effectiveness of accessory shields with phase 1 (401 cases) utilizing traditional protective measures. Phase 2 (363 cases) used the same material as phase I, but a dedicated accessory lead shield was used. For nurse circulators, this shield was placed between the patient and the intravenous medication pole. For scrub technologists, the shield was placed at the foot of the patient’s bed.
Radiation exposure was higher in phase 1 than phase 2 for all workers. In phase I, technologists faced an effective dose normalized to the dose-area product (EDAP) of 2.4 mcSv/(mGy x cm2) x 10-5 per case. This dropped significantly in phase 2 to an EDAP of 0.9per case, a 62.5% lower dose per case (P less than .0001).
Nurses experienced a similar decrease in exposure per case from phase 1 (EDAP 1.1) and phase 2 (EDAP 0.4). This accounted for a 63.6% lower dose per case (P less than .0001).
The contrast delivery system used at the lab did not allow technologists to maximize their distance from radiation sources, so it is unclear whether the results of this study can be extrapolated to centers where technologists do not perform contrast injections.
Dr. Madder and his colleagues pointed out that this research only begins to scratch the surface of the occupational hazards that catheterization laboratory personnel face.“The use of accessory lead shields, which might be effective to reduce staff radiation exposure, will not likely reduce other occupational hazards of working in the catheterization laboratory related to wearing heavy lead garments, including the risks for orthopedic injuries and experiencing chronic work-related pain.”
Corindus Vascular Robotics partially funded this study and has provided research support to Dr. Madder. All other researchers have no financial conflicts to report.
SOURCE: Madder R. JACC: Cardiovasc Interven. 2018 Jan 22;11[2]:206-12.
While concerns about occupational radiation exposure to cardiologists has prompted equipment, shielding, and behavioral modifications to reduce radiation exposure in cardiologists, exposure to staff members such as nurses and technologists has not received this same attention.
Considering that the shield should also absorb approximately 98% of the scatter radiation, the only real issue about its effectiveness is whether technologists and nurses can remain behind the wall while still being able to assist during a procedure.
All cardiology labs should be equipped with these shields to provide adequate radiation protection to those who work there. The work of Madder et al. should provide confidence to nurses and technologists that their occupational radiation exposure can be expected to be low, as well as their cancer risk.
Kenneth A. Fetterly, PhD , a radiologist, and Malcolm R. Bell, MD , a cardiologist and director of the ischemic heart disease program at the Mayo Clinic in Rochester, Minn., made these comments in an editorial ( JACC: Cardiovasc Interven. 2018 Jan 22;11[2]:213-4 ).
While concerns about occupational radiation exposure to cardiologists has prompted equipment, shielding, and behavioral modifications to reduce radiation exposure in cardiologists, exposure to staff members such as nurses and technologists has not received this same attention.
Considering that the shield should also absorb approximately 98% of the scatter radiation, the only real issue about its effectiveness is whether technologists and nurses can remain behind the wall while still being able to assist during a procedure.
All cardiology labs should be equipped with these shields to provide adequate radiation protection to those who work there. The work of Madder et al. should provide confidence to nurses and technologists that their occupational radiation exposure can be expected to be low, as well as their cancer risk.
Kenneth A. Fetterly, PhD , a radiologist, and Malcolm R. Bell, MD , a cardiologist and director of the ischemic heart disease program at the Mayo Clinic in Rochester, Minn., made these comments in an editorial ( JACC: Cardiovasc Interven. 2018 Jan 22;11[2]:213-4 ).
While concerns about occupational radiation exposure to cardiologists has prompted equipment, shielding, and behavioral modifications to reduce radiation exposure in cardiologists, exposure to staff members such as nurses and technologists has not received this same attention.
Considering that the shield should also absorb approximately 98% of the scatter radiation, the only real issue about its effectiveness is whether technologists and nurses can remain behind the wall while still being able to assist during a procedure.
All cardiology labs should be equipped with these shields to provide adequate radiation protection to those who work there. The work of Madder et al. should provide confidence to nurses and technologists that their occupational radiation exposure can be expected to be low, as well as their cancer risk.
Kenneth A. Fetterly, PhD , a radiologist, and Malcolm R. Bell, MD , a cardiologist and director of the ischemic heart disease program at the Mayo Clinic in Rochester, Minn., made these comments in an editorial ( JACC: Cardiovasc Interven. 2018 Jan 22;11[2]:213-4 ).
Adding some lead shielding in cardiac catheterization labs can cut the amount of radiation that nurse circulators and scrub technologists are exposed to, according to a single-center study of more than 750 patients.
“The simple and relatively inexpensive approach of providing staff members with a dedicated accessory lead shield during cardiac catheterization was associated with a nearly two-thirds reduction in radiation exposure among both nurses and technologists” according to Ryan Madder, MD, and his associates. “The present study is the first, to our knowledge, to observe a similar benefit among nonphysician staff members, an observation that may have important implications for occupational safety in the cardiac catheterization laboratory.”
The report was published in JACC: Cardiovascular Interventions.
SHIELD (Combining Robotic-Stenting and Proactive Shielding Techniques in the Catheterization Laboratory to Achieve Lowest Possible Radiation Exposure to Physicians and Staff) was a single-center, prospective, observational designed study to research radiation exposure levels among physicians and other staff members in cardiac catheterization labs. Dr. Madder, a cardiologist at Spectrum Health in Grand Rapids, Mich., and his colleagues collected radiation exposure information for staff members such as nurse circulators and scrub technologists during August 2015–February 2016 and measured radiation exposure in 764 consecutive cases. Radiation exposure was monitored for these staff members by dosimeters worn on either the left anterior side of the glasses or thyroid collar, and a body dosimeter underneath the lead apparel on the V-neck of their scrub shirt.
The radiation protection used in the study followed standard institutional operating procedures, which includes two shields between the patient and physician, a ceiling mounted upper body shield with a patient contour cutout, and a lower body shield attached to the side of the operating table, extending from the table to the floor. Staff members wore traditional lead garments, consisting of a lead skirt, apron, and thyroid collar. Additional radiation absorbing disposable pads were used as desired by the staff members.
The study was split into two phases to evaluate the effectiveness of accessory shields with phase 1 (401 cases) utilizing traditional protective measures. Phase 2 (363 cases) used the same material as phase I, but a dedicated accessory lead shield was used. For nurse circulators, this shield was placed between the patient and the intravenous medication pole. For scrub technologists, the shield was placed at the foot of the patient’s bed.
Radiation exposure was higher in phase 1 than phase 2 for all workers. In phase I, technologists faced an effective dose normalized to the dose-area product (EDAP) of 2.4 mcSv/(mGy x cm2) x 10-5 per case. This dropped significantly in phase 2 to an EDAP of 0.9per case, a 62.5% lower dose per case (P less than .0001).
Nurses experienced a similar decrease in exposure per case from phase 1 (EDAP 1.1) and phase 2 (EDAP 0.4). This accounted for a 63.6% lower dose per case (P less than .0001).
The contrast delivery system used at the lab did not allow technologists to maximize their distance from radiation sources, so it is unclear whether the results of this study can be extrapolated to centers where technologists do not perform contrast injections.
Dr. Madder and his colleagues pointed out that this research only begins to scratch the surface of the occupational hazards that catheterization laboratory personnel face.“The use of accessory lead shields, which might be effective to reduce staff radiation exposure, will not likely reduce other occupational hazards of working in the catheterization laboratory related to wearing heavy lead garments, including the risks for orthopedic injuries and experiencing chronic work-related pain.”
Corindus Vascular Robotics partially funded this study and has provided research support to Dr. Madder. All other researchers have no financial conflicts to report.
SOURCE: Madder R. JACC: Cardiovasc Interven. 2018 Jan 22;11[2]:206-12.
Adding some lead shielding in cardiac catheterization labs can cut the amount of radiation that nurse circulators and scrub technologists are exposed to, according to a single-center study of more than 750 patients.
“The simple and relatively inexpensive approach of providing staff members with a dedicated accessory lead shield during cardiac catheterization was associated with a nearly two-thirds reduction in radiation exposure among both nurses and technologists” according to Ryan Madder, MD, and his associates. “The present study is the first, to our knowledge, to observe a similar benefit among nonphysician staff members, an observation that may have important implications for occupational safety in the cardiac catheterization laboratory.”
The report was published in JACC: Cardiovascular Interventions.
SHIELD (Combining Robotic-Stenting and Proactive Shielding Techniques in the Catheterization Laboratory to Achieve Lowest Possible Radiation Exposure to Physicians and Staff) was a single-center, prospective, observational designed study to research radiation exposure levels among physicians and other staff members in cardiac catheterization labs. Dr. Madder, a cardiologist at Spectrum Health in Grand Rapids, Mich., and his colleagues collected radiation exposure information for staff members such as nurse circulators and scrub technologists during August 2015–February 2016 and measured radiation exposure in 764 consecutive cases. Radiation exposure was monitored for these staff members by dosimeters worn on either the left anterior side of the glasses or thyroid collar, and a body dosimeter underneath the lead apparel on the V-neck of their scrub shirt.
The radiation protection used in the study followed standard institutional operating procedures, which includes two shields between the patient and physician, a ceiling mounted upper body shield with a patient contour cutout, and a lower body shield attached to the side of the operating table, extending from the table to the floor. Staff members wore traditional lead garments, consisting of a lead skirt, apron, and thyroid collar. Additional radiation absorbing disposable pads were used as desired by the staff members.
The study was split into two phases to evaluate the effectiveness of accessory shields with phase 1 (401 cases) utilizing traditional protective measures. Phase 2 (363 cases) used the same material as phase I, but a dedicated accessory lead shield was used. For nurse circulators, this shield was placed between the patient and the intravenous medication pole. For scrub technologists, the shield was placed at the foot of the patient’s bed.
Radiation exposure was higher in phase 1 than phase 2 for all workers. In phase I, technologists faced an effective dose normalized to the dose-area product (EDAP) of 2.4 mcSv/(mGy x cm2) x 10-5 per case. This dropped significantly in phase 2 to an EDAP of 0.9per case, a 62.5% lower dose per case (P less than .0001).
Nurses experienced a similar decrease in exposure per case from phase 1 (EDAP 1.1) and phase 2 (EDAP 0.4). This accounted for a 63.6% lower dose per case (P less than .0001).
The contrast delivery system used at the lab did not allow technologists to maximize their distance from radiation sources, so it is unclear whether the results of this study can be extrapolated to centers where technologists do not perform contrast injections.
Dr. Madder and his colleagues pointed out that this research only begins to scratch the surface of the occupational hazards that catheterization laboratory personnel face.“The use of accessory lead shields, which might be effective to reduce staff radiation exposure, will not likely reduce other occupational hazards of working in the catheterization laboratory related to wearing heavy lead garments, including the risks for orthopedic injuries and experiencing chronic work-related pain.”
Corindus Vascular Robotics partially funded this study and has provided research support to Dr. Madder. All other researchers have no financial conflicts to report.
SOURCE: Madder R. JACC: Cardiovasc Interven. 2018 Jan 22;11[2]:206-12.
FROM JACC: CARDIOVASCULAR INTERVENTIONS
Key clinical point: Accessory lead shielding reduces radiation exposure for nurses and technologists.
Major finding: Accessory lead shielding reduced radiation exposure by over two-thirds.
Study details: A single-center, prospective, observational study of 764 cases.
Disclosures: Corindus Vascular Robotics partially funded this study and has provided research support to Dr. Madder. All other researchers have no financial conflicts to report.
Source: Madder R. JACC: Cardiovasc Interven. 2018 Jan 22;11[2]:206-12.
Viremic suppression linked to decreased MACE rate in patients with HCV-cirrhosis
Hepatitis C viremic suppression was associated with a lower rate of cardiovascular events in patients with compensated HCV-related cirrhosis, compared with control patients who did not achieve a sustained virological response. In addition, predictive factors for major adverse cardiovascular events (MACEs) in compensated HCV-related cirrhosis were Asian ethnic origin, hypertension, smoking, and low serum albumin, according to a report in American Heart Journal.
A total of 878 patients with HCV-related cirrhosis were enrolled at 35 French centers. Upon enrollment, all patients received HCV treatment and were followed for MACEs, including stroke, myocardial infarction, ischemic heart disease, heart failure, peripheral arterial disease, cardiac arrest, and cardiovascular-related death, according to Patrice Cacoub, MD, of Sorbonne Universités, Paris, and his colleagues.
Five-year survival for patients presenting with a MACE was 60% vs. 88% in patients who did not have an event.
“[Our] results strengthen the systemic nature of HCV infection, a chronic disease in which cardiovascular risk must be carefully assessed. The decreased rate of MACEs after [sustained virological response] in this population should be taken into account to enable wider access to new [direct-acting antivirals]. Further studies are warranted to evaluate whether a similar benefit can be obtained in less severe patients, such as noncirrhotic HCV-infected patients,” the researchers concluded.
The authors reported having no conflicts of interest.
SOURCE: Cacoub, P et al. Am Heart J. 2018;198:4-17.
Hepatitis C viremic suppression was associated with a lower rate of cardiovascular events in patients with compensated HCV-related cirrhosis, compared with control patients who did not achieve a sustained virological response. In addition, predictive factors for major adverse cardiovascular events (MACEs) in compensated HCV-related cirrhosis were Asian ethnic origin, hypertension, smoking, and low serum albumin, according to a report in American Heart Journal.
A total of 878 patients with HCV-related cirrhosis were enrolled at 35 French centers. Upon enrollment, all patients received HCV treatment and were followed for MACEs, including stroke, myocardial infarction, ischemic heart disease, heart failure, peripheral arterial disease, cardiac arrest, and cardiovascular-related death, according to Patrice Cacoub, MD, of Sorbonne Universités, Paris, and his colleagues.
Five-year survival for patients presenting with a MACE was 60% vs. 88% in patients who did not have an event.
“[Our] results strengthen the systemic nature of HCV infection, a chronic disease in which cardiovascular risk must be carefully assessed. The decreased rate of MACEs after [sustained virological response] in this population should be taken into account to enable wider access to new [direct-acting antivirals]. Further studies are warranted to evaluate whether a similar benefit can be obtained in less severe patients, such as noncirrhotic HCV-infected patients,” the researchers concluded.
The authors reported having no conflicts of interest.
SOURCE: Cacoub, P et al. Am Heart J. 2018;198:4-17.
Hepatitis C viremic suppression was associated with a lower rate of cardiovascular events in patients with compensated HCV-related cirrhosis, compared with control patients who did not achieve a sustained virological response. In addition, predictive factors for major adverse cardiovascular events (MACEs) in compensated HCV-related cirrhosis were Asian ethnic origin, hypertension, smoking, and low serum albumin, according to a report in American Heart Journal.
A total of 878 patients with HCV-related cirrhosis were enrolled at 35 French centers. Upon enrollment, all patients received HCV treatment and were followed for MACEs, including stroke, myocardial infarction, ischemic heart disease, heart failure, peripheral arterial disease, cardiac arrest, and cardiovascular-related death, according to Patrice Cacoub, MD, of Sorbonne Universités, Paris, and his colleagues.
Five-year survival for patients presenting with a MACE was 60% vs. 88% in patients who did not have an event.
“[Our] results strengthen the systemic nature of HCV infection, a chronic disease in which cardiovascular risk must be carefully assessed. The decreased rate of MACEs after [sustained virological response] in this population should be taken into account to enable wider access to new [direct-acting antivirals]. Further studies are warranted to evaluate whether a similar benefit can be obtained in less severe patients, such as noncirrhotic HCV-infected patients,” the researchers concluded.
The authors reported having no conflicts of interest.
SOURCE: Cacoub, P et al. Am Heart J. 2018;198:4-17.
FROM AMERICAN HEART JOURNAL
Key clinical point: Predictive factors for MACE in compensated HCV-related cirrhosis were Asian ethnic origin, hypertension, smoking, and low serum albumin.
Major finding: Achieving viremic suppression was associated with a lower rate of cardiovascular events in patients with compensated HCV-related cirrhosis.
Study details: A study at 35 French centers of 878 patients with HCV-related cirrhosis.
Disclosures: The authors reported having no conflicts of interest.
Source: Cacoub, P et al. Am Heart J. 2018;198:4-17.