User login
Navigating the Search for a Financial Adviser
As gastroenterologists, we spend innumerable years in medical training with an abrupt and significant increase in our earning potential upon beginning practice. The majority of us also carry a sizeable amount of student loan debt. This combination results in a unique situation that can make us hesitant about how best to set ourselves up financially while also making us vulnerable to potentially predatory financial practices.
Although your initial steps to achieve financial wellness and build wealth can be obtained on your own with some education, a financial adviser becomes indispensable when you have significant assets, a high income, complex finances, and/or are experiencing a major life change. Additionally, as there are so many avenues to invest and grow your capital, a financial adviser can assist in designing a portfolio to best accomplish specific monetary goals. Studies have demonstrated that those working with a financial adviser reduce their single-stock risk and have more significant increase in portfolio value, reducing the total cost associated with their investments’ management.1 Those working with a financial adviser will also net up to a 3% larger annual return, compared with a standard baseline investment plan.2,3
Based on this information, it may appear that working with a personal financial adviser would be a no-brainer. Unfortunately, there is a caveat: There is no legal regulation regarding who can use the title “financial adviser.” It is therefore crucial to be aware of common practices and terminology to best help you identify a reputable financial adviser and reduce your risk of excessive fees or financial loss. This is also a highly personal decision and your search should first begin with understanding why you are looking for an adviser, as this will determine the appropriate type of service to look for.
Types of Advisers
A certified financial planner (CFP) is an expert in estate planning, taxes, retirement saving, and financial planning who has a formal designation by the Certified Financial Planner Board of Standards Inc.4 They must undergo stringent licensing examinations following a 3-year course with required continuing education to maintain their credentials. CFPs are fiduciaries, meaning they must make financial decisions in your best interest, even if they may make less money with that product or investment strategy. In other words, they are beholden to give honest, impartial recommendations to their clients, and may face sanctions by the CFP Board if found to violate its Code of Ethics and Standards of Conduct, which includes failure to act in a fiduciary duty.5
CFPs evaluate your total financial picture, such as investments, insurance policies, and overall current financial position, to develop a comprehensive strategy that will successfully guide you to your financial goal. There are many individuals who may refer to themselves as financial planners without having the CFP designation; while they may offer similar services as above, they will not be required to act as a fiduciary. Hence, it is important to do your due diligence and verify they hold this certification via the CFP Board website: www.cfp.net/verify-a-cfp-professional.
An investment adviser is a legal term from the U.S. Securities and Exchange Commission (SEC) and the Financial Industry Regulatory Authority (FINRA) referring to an individual who provides recommendations and analyses for financial securities such as stock. Both of these agencies ensure investment advisers adhere to regulatory requirements designed to protect client investers. Similar to CFPs, they are held to a fiduciary standard, and their firm is required to register with the SEC or the state of practice based on the amount of assets under management.6
An individual investment adviser must also register with their state as an Investment Adviser Representative (IAR), the distinctive term referring to an individual as opposed to an investment advising firm. Investment advisers are required to pass the extensive Series 65, Uniform Investment Advisor Law Exam, or equivalent, by states requiring licensure.7 They can guide you on the selection of particular investments and portfolio management based on a discussion with you regarding your current financial standing and what fiscal ambitions you wish to achieve.
A financial adviser provides direction on a multitude of financially related topics such as investing, tax laws, and life insurance with the goal to help you reach specific financial objectives. However, this term is often used quite ubiquitously given the lack of formal regulation of the title. Essentially, those with varying types of educational background can give themselves the title of financial adviser.
If a financial adviser buys or sells financial securities such as stocks or bonds, then they must be registered as a licensed broker with the SEC and IAR and pass the Series 6 or Series 7 exam. Unlike CFPs and investment advisers, a financial adviser (if also a licensed broker) is not required to be a fiduciary, and instead works under the suitability standard.8 Suitability requires that financial recommendations made by the adviser are appropriate but not necessarily the best for the client. In fact, these recommendations do not even have to be the most suitable. This is where conflicts of interest can arise with the adviser recommending products and securities that best compensate them while not serving the best return on investment for you.
Making the search for a financial adviser more complex, an individual can be a combination of any of the above, pending the appropriate licensing. For example, a CFP can also be an asset manager and thus hold the title of a financial adviser and/or IAR. A financial adviser may also not directly manage your assets if they have a partnership with a third party or another licensed individual. Questions to ask of your potential financial adviser should therefore include the following:
- What licensure and related education do you have?
- What is your particular area of expertise?
- How long have you been in practice?
- How will you be managing my assets?
Financial Adviser Fee Schedules
Prior to working with a financial adviser, you must also inquire about their fee structure. There are two kinds of fee schedules used by financial advisers: fee-only and fee-based.
Fee-only advisers receive payment solely for the services they provide. They do not collect commissions from third parties providing the recommended products. There is variability in how this type of payment schedule is structured, encompassing flat fees, hourly rates, or the adviser charging a retainer. The Table below compares the types of fee-only structures and range of charges based on 2023 rates.9 Of note, fee-only advisers serve as fiduciaries.10
Fee-based financial advisers receive payment for services but may also receive commission on specific products they sell to you.9 Most, if not all, financial experts recommend avoiding advisers using commission-based charges given the potential conflict of interest: How can one be absolutely sure this recommended financial product is best for you, knowing your adviser has a financial stake in said item?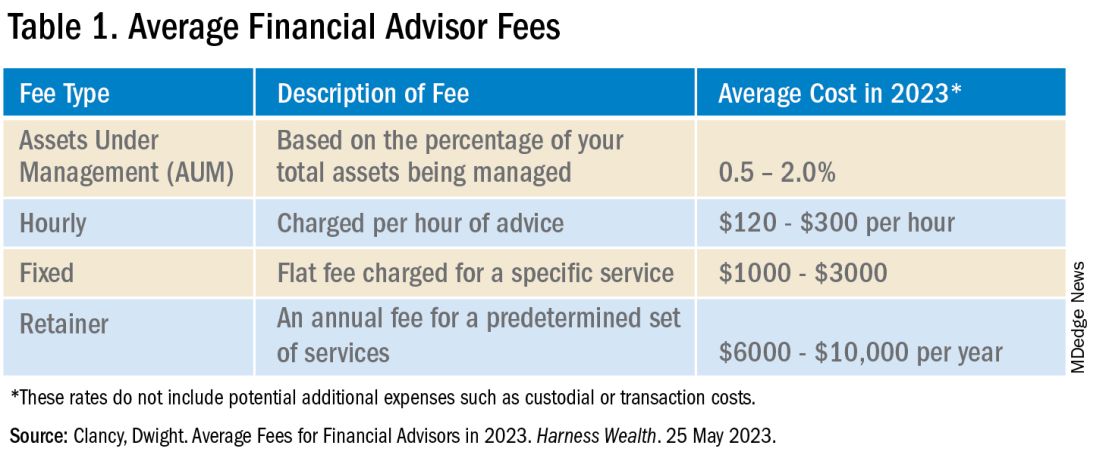
In addition to charging the fees above, your financial adviser, if they are actively managing your investment portfolio, will also charge an assets under management (AUM) fee. This is a percentage of the dollar amount within your portfolio. For example, if your adviser charges a 1% AUM rate for your account totaling $100,000, this equates to a $1,000 fee in that calendar year. AUM fees typically decrease as the size of your portfolio increases. As seen in the Table, there is a wide range of the average AUM rate (0.5%–2%); however, an AUM fee approaching 2% is unnecessarily high and consumes a significant portion of your portfolio. Thus, it is recommended to look for a money manager with an approximate 1% AUM fee.
Many of us delay or avoid working with a financial adviser due to the potential perceived risks of having poor portfolio management from an adviser not working in our best interest, along with the concern for excessive fees. In many ways, it is how we counsel our patients. While they can seek medical information on their own, their best care is under the guidance of an expert: a healthcare professional. That being said, personal finance is indeed personal, so I hope this guide helps facilitate your search and increase your financial wellness.
Dr. Luthra is a therapeutic endoscopist at Moffitt Cancer Center, Tampa, Florida, and the founder of The Scope of Finance, a financial wellness education and coaching company focused on physicians. Her interest in financial well-being is thanks to the teachings of her father, an entrepreneur and former Certified Financial Planner (CFP). She can be found on Instagram (thescopeoffinance) and X (@ScopeofFinance). She reports no financial disclosures relevant to this article.
References
1. Pagliaro CA and Utkus SP. Assessing the value of advice. Vanguard. 2019 Sept.
2. Kinniry Jr. FM et al. Putting a value on your value: Quantifying Vanguard Advisor’s Alpha. Vanguard. 2022 July.
3. Horan S. What Are the Benefits of Working with a Financial Advisor? – 2021 Study. Smart Asset. 2023 July 27.
4. Kagan J. Certified Financial PlannerTM(CFP): What It Is and How to Become One. Investopedia. 2023 Aug 3.
5. CFP Board. Our Commitment to Ethical Standards. CFP Board. 2024.
6. Staff of the Investment Adviser Regulation Office Division of Investment Management, U.S. Securities and Exchange Commission. Regulation of Investment Advisers by the U.S. Securities and Exchange Commission. 2013 Mar.
7. Hicks C. Investment Advisor vs. Financial Advisor: There is a Difference. US News & World Report. 2019 June 13.
8. Roberts K. Financial advisor vs. financial planner: What is the difference? Bankrate. 2023 Nov 21.
9. Clancy D. Average Fees for Financial Advisors in 2023. Harness Wealth. 2023 May 25.
10. Palmer B. Fee- vs. Commission-Based Advisor: What’s the Difference? Investopedia. 2023 June 20.
As gastroenterologists, we spend innumerable years in medical training with an abrupt and significant increase in our earning potential upon beginning practice. The majority of us also carry a sizeable amount of student loan debt. This combination results in a unique situation that can make us hesitant about how best to set ourselves up financially while also making us vulnerable to potentially predatory financial practices.
Although your initial steps to achieve financial wellness and build wealth can be obtained on your own with some education, a financial adviser becomes indispensable when you have significant assets, a high income, complex finances, and/or are experiencing a major life change. Additionally, as there are so many avenues to invest and grow your capital, a financial adviser can assist in designing a portfolio to best accomplish specific monetary goals. Studies have demonstrated that those working with a financial adviser reduce their single-stock risk and have more significant increase in portfolio value, reducing the total cost associated with their investments’ management.1 Those working with a financial adviser will also net up to a 3% larger annual return, compared with a standard baseline investment plan.2,3
Based on this information, it may appear that working with a personal financial adviser would be a no-brainer. Unfortunately, there is a caveat: There is no legal regulation regarding who can use the title “financial adviser.” It is therefore crucial to be aware of common practices and terminology to best help you identify a reputable financial adviser and reduce your risk of excessive fees or financial loss. This is also a highly personal decision and your search should first begin with understanding why you are looking for an adviser, as this will determine the appropriate type of service to look for.
Types of Advisers
A certified financial planner (CFP) is an expert in estate planning, taxes, retirement saving, and financial planning who has a formal designation by the Certified Financial Planner Board of Standards Inc.4 They must undergo stringent licensing examinations following a 3-year course with required continuing education to maintain their credentials. CFPs are fiduciaries, meaning they must make financial decisions in your best interest, even if they may make less money with that product or investment strategy. In other words, they are beholden to give honest, impartial recommendations to their clients, and may face sanctions by the CFP Board if found to violate its Code of Ethics and Standards of Conduct, which includes failure to act in a fiduciary duty.5
CFPs evaluate your total financial picture, such as investments, insurance policies, and overall current financial position, to develop a comprehensive strategy that will successfully guide you to your financial goal. There are many individuals who may refer to themselves as financial planners without having the CFP designation; while they may offer similar services as above, they will not be required to act as a fiduciary. Hence, it is important to do your due diligence and verify they hold this certification via the CFP Board website: www.cfp.net/verify-a-cfp-professional.
An investment adviser is a legal term from the U.S. Securities and Exchange Commission (SEC) and the Financial Industry Regulatory Authority (FINRA) referring to an individual who provides recommendations and analyses for financial securities such as stock. Both of these agencies ensure investment advisers adhere to regulatory requirements designed to protect client investers. Similar to CFPs, they are held to a fiduciary standard, and their firm is required to register with the SEC or the state of practice based on the amount of assets under management.6
An individual investment adviser must also register with their state as an Investment Adviser Representative (IAR), the distinctive term referring to an individual as opposed to an investment advising firm. Investment advisers are required to pass the extensive Series 65, Uniform Investment Advisor Law Exam, or equivalent, by states requiring licensure.7 They can guide you on the selection of particular investments and portfolio management based on a discussion with you regarding your current financial standing and what fiscal ambitions you wish to achieve.
A financial adviser provides direction on a multitude of financially related topics such as investing, tax laws, and life insurance with the goal to help you reach specific financial objectives. However, this term is often used quite ubiquitously given the lack of formal regulation of the title. Essentially, those with varying types of educational background can give themselves the title of financial adviser.
If a financial adviser buys or sells financial securities such as stocks or bonds, then they must be registered as a licensed broker with the SEC and IAR and pass the Series 6 or Series 7 exam. Unlike CFPs and investment advisers, a financial adviser (if also a licensed broker) is not required to be a fiduciary, and instead works under the suitability standard.8 Suitability requires that financial recommendations made by the adviser are appropriate but not necessarily the best for the client. In fact, these recommendations do not even have to be the most suitable. This is where conflicts of interest can arise with the adviser recommending products and securities that best compensate them while not serving the best return on investment for you.
Making the search for a financial adviser more complex, an individual can be a combination of any of the above, pending the appropriate licensing. For example, a CFP can also be an asset manager and thus hold the title of a financial adviser and/or IAR. A financial adviser may also not directly manage your assets if they have a partnership with a third party or another licensed individual. Questions to ask of your potential financial adviser should therefore include the following:
- What licensure and related education do you have?
- What is your particular area of expertise?
- How long have you been in practice?
- How will you be managing my assets?
Financial Adviser Fee Schedules
Prior to working with a financial adviser, you must also inquire about their fee structure. There are two kinds of fee schedules used by financial advisers: fee-only and fee-based.
Fee-only advisers receive payment solely for the services they provide. They do not collect commissions from third parties providing the recommended products. There is variability in how this type of payment schedule is structured, encompassing flat fees, hourly rates, or the adviser charging a retainer. The Table below compares the types of fee-only structures and range of charges based on 2023 rates.9 Of note, fee-only advisers serve as fiduciaries.10
Fee-based financial advisers receive payment for services but may also receive commission on specific products they sell to you.9 Most, if not all, financial experts recommend avoiding advisers using commission-based charges given the potential conflict of interest: How can one be absolutely sure this recommended financial product is best for you, knowing your adviser has a financial stake in said item?
In addition to charging the fees above, your financial adviser, if they are actively managing your investment portfolio, will also charge an assets under management (AUM) fee. This is a percentage of the dollar amount within your portfolio. For example, if your adviser charges a 1% AUM rate for your account totaling $100,000, this equates to a $1,000 fee in that calendar year. AUM fees typically decrease as the size of your portfolio increases. As seen in the Table, there is a wide range of the average AUM rate (0.5%–2%); however, an AUM fee approaching 2% is unnecessarily high and consumes a significant portion of your portfolio. Thus, it is recommended to look for a money manager with an approximate 1% AUM fee.
Many of us delay or avoid working with a financial adviser due to the potential perceived risks of having poor portfolio management from an adviser not working in our best interest, along with the concern for excessive fees. In many ways, it is how we counsel our patients. While they can seek medical information on their own, their best care is under the guidance of an expert: a healthcare professional. That being said, personal finance is indeed personal, so I hope this guide helps facilitate your search and increase your financial wellness.
Dr. Luthra is a therapeutic endoscopist at Moffitt Cancer Center, Tampa, Florida, and the founder of The Scope of Finance, a financial wellness education and coaching company focused on physicians. Her interest in financial well-being is thanks to the teachings of her father, an entrepreneur and former Certified Financial Planner (CFP). She can be found on Instagram (thescopeoffinance) and X (@ScopeofFinance). She reports no financial disclosures relevant to this article.
References
1. Pagliaro CA and Utkus SP. Assessing the value of advice. Vanguard. 2019 Sept.
2. Kinniry Jr. FM et al. Putting a value on your value: Quantifying Vanguard Advisor’s Alpha. Vanguard. 2022 July.
3. Horan S. What Are the Benefits of Working with a Financial Advisor? – 2021 Study. Smart Asset. 2023 July 27.
4. Kagan J. Certified Financial PlannerTM(CFP): What It Is and How to Become One. Investopedia. 2023 Aug 3.
5. CFP Board. Our Commitment to Ethical Standards. CFP Board. 2024.
6. Staff of the Investment Adviser Regulation Office Division of Investment Management, U.S. Securities and Exchange Commission. Regulation of Investment Advisers by the U.S. Securities and Exchange Commission. 2013 Mar.
7. Hicks C. Investment Advisor vs. Financial Advisor: There is a Difference. US News & World Report. 2019 June 13.
8. Roberts K. Financial advisor vs. financial planner: What is the difference? Bankrate. 2023 Nov 21.
9. Clancy D. Average Fees for Financial Advisors in 2023. Harness Wealth. 2023 May 25.
10. Palmer B. Fee- vs. Commission-Based Advisor: What’s the Difference? Investopedia. 2023 June 20.
As gastroenterologists, we spend innumerable years in medical training with an abrupt and significant increase in our earning potential upon beginning practice. The majority of us also carry a sizeable amount of student loan debt. This combination results in a unique situation that can make us hesitant about how best to set ourselves up financially while also making us vulnerable to potentially predatory financial practices.
Although your initial steps to achieve financial wellness and build wealth can be obtained on your own with some education, a financial adviser becomes indispensable when you have significant assets, a high income, complex finances, and/or are experiencing a major life change. Additionally, as there are so many avenues to invest and grow your capital, a financial adviser can assist in designing a portfolio to best accomplish specific monetary goals. Studies have demonstrated that those working with a financial adviser reduce their single-stock risk and have more significant increase in portfolio value, reducing the total cost associated with their investments’ management.1 Those working with a financial adviser will also net up to a 3% larger annual return, compared with a standard baseline investment plan.2,3
Based on this information, it may appear that working with a personal financial adviser would be a no-brainer. Unfortunately, there is a caveat: There is no legal regulation regarding who can use the title “financial adviser.” It is therefore crucial to be aware of common practices and terminology to best help you identify a reputable financial adviser and reduce your risk of excessive fees or financial loss. This is also a highly personal decision and your search should first begin with understanding why you are looking for an adviser, as this will determine the appropriate type of service to look for.
Types of Advisers
A certified financial planner (CFP) is an expert in estate planning, taxes, retirement saving, and financial planning who has a formal designation by the Certified Financial Planner Board of Standards Inc.4 They must undergo stringent licensing examinations following a 3-year course with required continuing education to maintain their credentials. CFPs are fiduciaries, meaning they must make financial decisions in your best interest, even if they may make less money with that product or investment strategy. In other words, they are beholden to give honest, impartial recommendations to their clients, and may face sanctions by the CFP Board if found to violate its Code of Ethics and Standards of Conduct, which includes failure to act in a fiduciary duty.5
CFPs evaluate your total financial picture, such as investments, insurance policies, and overall current financial position, to develop a comprehensive strategy that will successfully guide you to your financial goal. There are many individuals who may refer to themselves as financial planners without having the CFP designation; while they may offer similar services as above, they will not be required to act as a fiduciary. Hence, it is important to do your due diligence and verify they hold this certification via the CFP Board website: www.cfp.net/verify-a-cfp-professional.
An investment adviser is a legal term from the U.S. Securities and Exchange Commission (SEC) and the Financial Industry Regulatory Authority (FINRA) referring to an individual who provides recommendations and analyses for financial securities such as stock. Both of these agencies ensure investment advisers adhere to regulatory requirements designed to protect client investers. Similar to CFPs, they are held to a fiduciary standard, and their firm is required to register with the SEC or the state of practice based on the amount of assets under management.6
An individual investment adviser must also register with their state as an Investment Adviser Representative (IAR), the distinctive term referring to an individual as opposed to an investment advising firm. Investment advisers are required to pass the extensive Series 65, Uniform Investment Advisor Law Exam, or equivalent, by states requiring licensure.7 They can guide you on the selection of particular investments and portfolio management based on a discussion with you regarding your current financial standing and what fiscal ambitions you wish to achieve.
A financial adviser provides direction on a multitude of financially related topics such as investing, tax laws, and life insurance with the goal to help you reach specific financial objectives. However, this term is often used quite ubiquitously given the lack of formal regulation of the title. Essentially, those with varying types of educational background can give themselves the title of financial adviser.
If a financial adviser buys or sells financial securities such as stocks or bonds, then they must be registered as a licensed broker with the SEC and IAR and pass the Series 6 or Series 7 exam. Unlike CFPs and investment advisers, a financial adviser (if also a licensed broker) is not required to be a fiduciary, and instead works under the suitability standard.8 Suitability requires that financial recommendations made by the adviser are appropriate but not necessarily the best for the client. In fact, these recommendations do not even have to be the most suitable. This is where conflicts of interest can arise with the adviser recommending products and securities that best compensate them while not serving the best return on investment for you.
Making the search for a financial adviser more complex, an individual can be a combination of any of the above, pending the appropriate licensing. For example, a CFP can also be an asset manager and thus hold the title of a financial adviser and/or IAR. A financial adviser may also not directly manage your assets if they have a partnership with a third party or another licensed individual. Questions to ask of your potential financial adviser should therefore include the following:
- What licensure and related education do you have?
- What is your particular area of expertise?
- How long have you been in practice?
- How will you be managing my assets?
Financial Adviser Fee Schedules
Prior to working with a financial adviser, you must also inquire about their fee structure. There are two kinds of fee schedules used by financial advisers: fee-only and fee-based.
Fee-only advisers receive payment solely for the services they provide. They do not collect commissions from third parties providing the recommended products. There is variability in how this type of payment schedule is structured, encompassing flat fees, hourly rates, or the adviser charging a retainer. The Table below compares the types of fee-only structures and range of charges based on 2023 rates.9 Of note, fee-only advisers serve as fiduciaries.10
Fee-based financial advisers receive payment for services but may also receive commission on specific products they sell to you.9 Most, if not all, financial experts recommend avoiding advisers using commission-based charges given the potential conflict of interest: How can one be absolutely sure this recommended financial product is best for you, knowing your adviser has a financial stake in said item?
In addition to charging the fees above, your financial adviser, if they are actively managing your investment portfolio, will also charge an assets under management (AUM) fee. This is a percentage of the dollar amount within your portfolio. For example, if your adviser charges a 1% AUM rate for your account totaling $100,000, this equates to a $1,000 fee in that calendar year. AUM fees typically decrease as the size of your portfolio increases. As seen in the Table, there is a wide range of the average AUM rate (0.5%–2%); however, an AUM fee approaching 2% is unnecessarily high and consumes a significant portion of your portfolio. Thus, it is recommended to look for a money manager with an approximate 1% AUM fee.
Many of us delay or avoid working with a financial adviser due to the potential perceived risks of having poor portfolio management from an adviser not working in our best interest, along with the concern for excessive fees. In many ways, it is how we counsel our patients. While they can seek medical information on their own, their best care is under the guidance of an expert: a healthcare professional. That being said, personal finance is indeed personal, so I hope this guide helps facilitate your search and increase your financial wellness.
Dr. Luthra is a therapeutic endoscopist at Moffitt Cancer Center, Tampa, Florida, and the founder of The Scope of Finance, a financial wellness education and coaching company focused on physicians. Her interest in financial well-being is thanks to the teachings of her father, an entrepreneur and former Certified Financial Planner (CFP). She can be found on Instagram (thescopeoffinance) and X (@ScopeofFinance). She reports no financial disclosures relevant to this article.
References
1. Pagliaro CA and Utkus SP. Assessing the value of advice. Vanguard. 2019 Sept.
2. Kinniry Jr. FM et al. Putting a value on your value: Quantifying Vanguard Advisor’s Alpha. Vanguard. 2022 July.
3. Horan S. What Are the Benefits of Working with a Financial Advisor? – 2021 Study. Smart Asset. 2023 July 27.
4. Kagan J. Certified Financial PlannerTM(CFP): What It Is and How to Become One. Investopedia. 2023 Aug 3.
5. CFP Board. Our Commitment to Ethical Standards. CFP Board. 2024.
6. Staff of the Investment Adviser Regulation Office Division of Investment Management, U.S. Securities and Exchange Commission. Regulation of Investment Advisers by the U.S. Securities and Exchange Commission. 2013 Mar.
7. Hicks C. Investment Advisor vs. Financial Advisor: There is a Difference. US News & World Report. 2019 June 13.
8. Roberts K. Financial advisor vs. financial planner: What is the difference? Bankrate. 2023 Nov 21.
9. Clancy D. Average Fees for Financial Advisors in 2023. Harness Wealth. 2023 May 25.
10. Palmer B. Fee- vs. Commission-Based Advisor: What’s the Difference? Investopedia. 2023 June 20.
Achieving Promotion for Junior Faculty in Academic Medicine: An Interview With Experts
Academic medicine plays a crucial role at the crossroads of medical practice, education, and research, influencing the future landscape of healthcare. Many physicians aspire to pursue and sustain a career in academic medicine to contribute to the advancement of medical knowledge, enhance patient care, and influence the trajectory of the medical field. Opting for a career in academic medicine can offer benefits such as increased autonomy and scheduling flexibility, which can significantly improve the quality of life. In addition, engagement in scholarly activities and working in a dynamic environment with continuous learning opportunities can help mitigate burnout.
However, embarking on an academic career can be daunting for junior faculty members who face the challenge of providing clinical care while excelling in research and dedicating time to mentorship and teaching trainees. According to a report by the Association of American Medical Colleges, 38% of physicians leave academic medicine within a decade of obtaining a faculty position. Barriers to promotion and retention within academic medicine include ineffective mentorship, unclear or inconsistent promotion criteria, and disparities in gender/ethnic representation.
In this article, we interview two accomplished physicians in academic medicine who have attained the rank of professors.
Interview with Sophie Balzora, MD
Dr. Balzora is a professor of medicine at NYU Grossman School of Medicine and a practicing gastroenterologist specializing in the care of patients with inflammatory bowel disease at NYU Langone Health. She serves as the American College of Gastroenterology’s Diversity, Equity, and Inclusion Committee Chair, on the Advisory Board of ACG’s Leadership, Ethics, and Equity (LE&E) Center, and is president and cofounder of the Association of Black Gastroenterologists and Hepatologists (ABGH). Dr. Balzora was promoted to full professor 11 years after graduating from fellowship.
What would you identify as some of the most important factors that led to your success in achieving a promotion to professor of medicine?
Surround yourself with individuals whose professional and personal priorities align with yours. To achieve this, it is essential to gain an understanding of what is important to you, what you envision your success to look like, and establish a timeline to achieve it. The concept of personal success and how to best achieve it will absolutely change as you grow, and that is okay and expected. Connecting with those outside of your clinical interests, at other institutions, and even outside of the medical field, can help you achieve these goals and better shape how you see your career unfolding and how you want it to look.
Historically, the proportion of physicians who achieve professorship is lower among women compared with men. What do you believe are some of the barriers involved in this, and how would you counsel women who are interested in pursuing the rank of professor?
Systemic gender bias and discrimination, over-mentorship and under-sponsorship, inconsistent parental leave, and delayed parenthood are a few of the factors that contribute to the observed disparities in academic rank. Predictably, for women from underrepresented backgrounds in medicine, the chasm grows.
What has helped me most is to keep my eyes on the prize, and to recognize that the prize is different for everyone. It’s important not to make direct comparisons to any other individual, because they are not you. Harness what makes you different and drown out the naysayers — the “we’ve never seen this done before” camp, the “it’s too soon [for someone like you] to go up for promotion” folks. While these voices are sometimes well intentioned, they can distract you from your goals and ambitions because they are rooted in bias and adherence to traditional expectations. To do something new, and to change the game, requires going against the grain and utilizing your skills and talents to achieve what you want to achieve in a way that works for you.
What are some practical tips you have for junior gastroenterologists to track their promotion in academia?
- Keep your curriculum vitae (CV) up to date and formatted to your institutional guidelines. Ensure that you document your academic activities, even if it doesn’t seem important in the moment. When it’s time to submit that promotion portfolio, you want to be ready and organized.
- Remember: “No” is a full sentence, and saying it takes practice and time and confidence. It is a skill I still struggle to adopt at times, but it’s important to recognize the power of no, for it opens opportunities to say yes to other things.
- Lift as you climb — a critical part of changing the status quo is fostering the future of those underrepresented in medicine. A professional goal of mine that keeps me steady and passionate is to create supporting and enriching systemic and institutional changes that work to dismantle the obstacles perpetuating disparities in academic rank for women and those underrepresented in medicine. Discovering your “why” is a complex, difficult, and rewarding journey.
Interview with Mark Schattner, MD, AGAF
Dr. Schattner is a professor of clinical medicine at Weill Cornell College of Medicine and chief of the gastroenterology, hepatology, and nutrition service at Memorial Sloan Kettering Cancer Center, both in New York. He is a former president of the New York Society for Gastrointestinal Endoscopy and a fellow of the AGA and ASGE.
In your role as chief, you serve as a mentor for early career gastroenterologists for pursuing career promotion. What advice do you have for achieving this?
Promoting junior faculty is one of the prime responsibilities of a service chief. Generally, the early steps of promotion are straightforward, with criteria becoming more stringent as you progress. I think it is critical to understand the criteria used by promotion committees and to be aware of the various available tracks. I believe every meeting a junior faculty member has with their service chief should include, at the least, a brief check-in on where they are in the promotion process and plans (both short term and long term) to move forward. Successful promotion is facilitated when done upon a solid foundation of production and accomplishment. It is very challenging or even impossible when trying to piece together a package from discordant activities.
Most institutions require or encourage academic involvement at both national and international levels for career promotion. Do you have advice for junior faculty about how to achieve this type of recognition or experience?
The easiest place to start is with regional professional societies. Active involvement in these local societies fosters valuable networking and lays the groundwork for involvement at the national or international level. I would strongly encourage junior faculty to seek opportunities for a leadership position at any level in these societies and move up the ladder as their career matures. This is also a very good avenue to network and get invited to join collaborative research projects, which can be a fruitful means to enhance your academic productivity.
In your opinion, what factors are likely to hinder or delay an individual’s promotion?
I think it is crucial to consider the career track you are on. If you are very clinically productive and love to teach, that is completely appropriate, and most institutions will recognize the value of that and promote you along a clinical-educator tract. On the other hand, if you have a passion for research and can successfully lead research and compete for grants, then you would move along a traditional tenure track. It is also critical to think ahead, know the criteria on which you will be judged, and incorporate that into your practice early. Trying to scramble to enhance your CV in a short time just for promotion will likely prove ineffective.
Do you have advice for junior faculty who have families about how to manage career goals but also prioritize time with family?
There is no one-size-fits-all approach to this. I think this requires a lot of shared decision-making with your family. Compromise will undoubtedly be required. For example, I always chose to live in close proximity to my workplace, eliminating any commuting time. This choice really allowed me spend time with my family.
In conclusion, a career in academic medicine presents both opportunities and challenges. A successful academic career, and achieving promotion to the rank of professor of medicine, requires a combination of factors including understanding institution-specific criteria for promotion, proactive engagement at the regional and national level, and envisioning your career goals and creating a timeline to achieve them. There are challenges to promotion, including navigating systemic biases and balancing career goals with family commitments, which also requires consideration and open communication. Ultimately, we hope these insights provide valuable guidance and advice for junior faculty who are navigating this complex environment of academic medicine and are motivated toward achieving professional fulfillment and satisfaction in their careers.
Dr. Rolston is based in the Department of Gastroenterology, Hepatology, and Nutrition, Memorial Sloan Kettering Cancer Center, New York. She reports no conflicts in relation this article. Dr. Balzora and Dr. Schattner are based in the Division of Gastroenterology and Hepatology, New York University Langone Health, New York. Dr. Schattner is a consultant for Boston Scientific and Novo Nordisk. Dr. Balzora reports no conflicts in relation to this article.
References
Campbell KM. Mitigating the isolation of minoritized faculty in academic medicine. J Gen Intern Med. 2023 May. doi: 10.1007/s11606-022-07982-8.
Howard-Anderson JR et al. Strategies for developing a successful career in academic medicine. Am J Med Sci. 2024 Apr. doi: 10.1016/j.amjms.2023.12.010.
Murphy M et al. Women’s experiences of promotion and tenure in academic medicine and potential implications for gender disparities in career advancement: A qualitative analysis. JAMA Netw Open. 2021 Sep 1. doi: 10.1001/jamanetworkopen.2021.25843.
Sambunjak D et al. Mentoring in academic medicine: A systematic review. JAMA. 2006 Sep 6. doi: 10.1001/jama.296.9.1103.
Shen MR et al. Impact of mentoring on academic career success for women in medicine: A systematic review. Acad Med. 2022 Mar 1. doi: 10.1097/ACM.0000000000004563.
Academic medicine plays a crucial role at the crossroads of medical practice, education, and research, influencing the future landscape of healthcare. Many physicians aspire to pursue and sustain a career in academic medicine to contribute to the advancement of medical knowledge, enhance patient care, and influence the trajectory of the medical field. Opting for a career in academic medicine can offer benefits such as increased autonomy and scheduling flexibility, which can significantly improve the quality of life. In addition, engagement in scholarly activities and working in a dynamic environment with continuous learning opportunities can help mitigate burnout.
However, embarking on an academic career can be daunting for junior faculty members who face the challenge of providing clinical care while excelling in research and dedicating time to mentorship and teaching trainees. According to a report by the Association of American Medical Colleges, 38% of physicians leave academic medicine within a decade of obtaining a faculty position. Barriers to promotion and retention within academic medicine include ineffective mentorship, unclear or inconsistent promotion criteria, and disparities in gender/ethnic representation.
In this article, we interview two accomplished physicians in academic medicine who have attained the rank of professors.
Interview with Sophie Balzora, MD
Dr. Balzora is a professor of medicine at NYU Grossman School of Medicine and a practicing gastroenterologist specializing in the care of patients with inflammatory bowel disease at NYU Langone Health. She serves as the American College of Gastroenterology’s Diversity, Equity, and Inclusion Committee Chair, on the Advisory Board of ACG’s Leadership, Ethics, and Equity (LE&E) Center, and is president and cofounder of the Association of Black Gastroenterologists and Hepatologists (ABGH). Dr. Balzora was promoted to full professor 11 years after graduating from fellowship.
What would you identify as some of the most important factors that led to your success in achieving a promotion to professor of medicine?
Surround yourself with individuals whose professional and personal priorities align with yours. To achieve this, it is essential to gain an understanding of what is important to you, what you envision your success to look like, and establish a timeline to achieve it. The concept of personal success and how to best achieve it will absolutely change as you grow, and that is okay and expected. Connecting with those outside of your clinical interests, at other institutions, and even outside of the medical field, can help you achieve these goals and better shape how you see your career unfolding and how you want it to look.
Historically, the proportion of physicians who achieve professorship is lower among women compared with men. What do you believe are some of the barriers involved in this, and how would you counsel women who are interested in pursuing the rank of professor?
Systemic gender bias and discrimination, over-mentorship and under-sponsorship, inconsistent parental leave, and delayed parenthood are a few of the factors that contribute to the observed disparities in academic rank. Predictably, for women from underrepresented backgrounds in medicine, the chasm grows.
What has helped me most is to keep my eyes on the prize, and to recognize that the prize is different for everyone. It’s important not to make direct comparisons to any other individual, because they are not you. Harness what makes you different and drown out the naysayers — the “we’ve never seen this done before” camp, the “it’s too soon [for someone like you] to go up for promotion” folks. While these voices are sometimes well intentioned, they can distract you from your goals and ambitions because they are rooted in bias and adherence to traditional expectations. To do something new, and to change the game, requires going against the grain and utilizing your skills and talents to achieve what you want to achieve in a way that works for you.
What are some practical tips you have for junior gastroenterologists to track their promotion in academia?
- Keep your curriculum vitae (CV) up to date and formatted to your institutional guidelines. Ensure that you document your academic activities, even if it doesn’t seem important in the moment. When it’s time to submit that promotion portfolio, you want to be ready and organized.
- Remember: “No” is a full sentence, and saying it takes practice and time and confidence. It is a skill I still struggle to adopt at times, but it’s important to recognize the power of no, for it opens opportunities to say yes to other things.
- Lift as you climb — a critical part of changing the status quo is fostering the future of those underrepresented in medicine. A professional goal of mine that keeps me steady and passionate is to create supporting and enriching systemic and institutional changes that work to dismantle the obstacles perpetuating disparities in academic rank for women and those underrepresented in medicine. Discovering your “why” is a complex, difficult, and rewarding journey.
Interview with Mark Schattner, MD, AGAF
Dr. Schattner is a professor of clinical medicine at Weill Cornell College of Medicine and chief of the gastroenterology, hepatology, and nutrition service at Memorial Sloan Kettering Cancer Center, both in New York. He is a former president of the New York Society for Gastrointestinal Endoscopy and a fellow of the AGA and ASGE.
In your role as chief, you serve as a mentor for early career gastroenterologists for pursuing career promotion. What advice do you have for achieving this?
Promoting junior faculty is one of the prime responsibilities of a service chief. Generally, the early steps of promotion are straightforward, with criteria becoming more stringent as you progress. I think it is critical to understand the criteria used by promotion committees and to be aware of the various available tracks. I believe every meeting a junior faculty member has with their service chief should include, at the least, a brief check-in on where they are in the promotion process and plans (both short term and long term) to move forward. Successful promotion is facilitated when done upon a solid foundation of production and accomplishment. It is very challenging or even impossible when trying to piece together a package from discordant activities.
Most institutions require or encourage academic involvement at both national and international levels for career promotion. Do you have advice for junior faculty about how to achieve this type of recognition or experience?
The easiest place to start is with regional professional societies. Active involvement in these local societies fosters valuable networking and lays the groundwork for involvement at the national or international level. I would strongly encourage junior faculty to seek opportunities for a leadership position at any level in these societies and move up the ladder as their career matures. This is also a very good avenue to network and get invited to join collaborative research projects, which can be a fruitful means to enhance your academic productivity.
In your opinion, what factors are likely to hinder or delay an individual’s promotion?
I think it is crucial to consider the career track you are on. If you are very clinically productive and love to teach, that is completely appropriate, and most institutions will recognize the value of that and promote you along a clinical-educator tract. On the other hand, if you have a passion for research and can successfully lead research and compete for grants, then you would move along a traditional tenure track. It is also critical to think ahead, know the criteria on which you will be judged, and incorporate that into your practice early. Trying to scramble to enhance your CV in a short time just for promotion will likely prove ineffective.
Do you have advice for junior faculty who have families about how to manage career goals but also prioritize time with family?
There is no one-size-fits-all approach to this. I think this requires a lot of shared decision-making with your family. Compromise will undoubtedly be required. For example, I always chose to live in close proximity to my workplace, eliminating any commuting time. This choice really allowed me spend time with my family.
In conclusion, a career in academic medicine presents both opportunities and challenges. A successful academic career, and achieving promotion to the rank of professor of medicine, requires a combination of factors including understanding institution-specific criteria for promotion, proactive engagement at the regional and national level, and envisioning your career goals and creating a timeline to achieve them. There are challenges to promotion, including navigating systemic biases and balancing career goals with family commitments, which also requires consideration and open communication. Ultimately, we hope these insights provide valuable guidance and advice for junior faculty who are navigating this complex environment of academic medicine and are motivated toward achieving professional fulfillment and satisfaction in their careers.
Dr. Rolston is based in the Department of Gastroenterology, Hepatology, and Nutrition, Memorial Sloan Kettering Cancer Center, New York. She reports no conflicts in relation this article. Dr. Balzora and Dr. Schattner are based in the Division of Gastroenterology and Hepatology, New York University Langone Health, New York. Dr. Schattner is a consultant for Boston Scientific and Novo Nordisk. Dr. Balzora reports no conflicts in relation to this article.
References
Campbell KM. Mitigating the isolation of minoritized faculty in academic medicine. J Gen Intern Med. 2023 May. doi: 10.1007/s11606-022-07982-8.
Howard-Anderson JR et al. Strategies for developing a successful career in academic medicine. Am J Med Sci. 2024 Apr. doi: 10.1016/j.amjms.2023.12.010.
Murphy M et al. Women’s experiences of promotion and tenure in academic medicine and potential implications for gender disparities in career advancement: A qualitative analysis. JAMA Netw Open. 2021 Sep 1. doi: 10.1001/jamanetworkopen.2021.25843.
Sambunjak D et al. Mentoring in academic medicine: A systematic review. JAMA. 2006 Sep 6. doi: 10.1001/jama.296.9.1103.
Shen MR et al. Impact of mentoring on academic career success for women in medicine: A systematic review. Acad Med. 2022 Mar 1. doi: 10.1097/ACM.0000000000004563.
Academic medicine plays a crucial role at the crossroads of medical practice, education, and research, influencing the future landscape of healthcare. Many physicians aspire to pursue and sustain a career in academic medicine to contribute to the advancement of medical knowledge, enhance patient care, and influence the trajectory of the medical field. Opting for a career in academic medicine can offer benefits such as increased autonomy and scheduling flexibility, which can significantly improve the quality of life. In addition, engagement in scholarly activities and working in a dynamic environment with continuous learning opportunities can help mitigate burnout.
However, embarking on an academic career can be daunting for junior faculty members who face the challenge of providing clinical care while excelling in research and dedicating time to mentorship and teaching trainees. According to a report by the Association of American Medical Colleges, 38% of physicians leave academic medicine within a decade of obtaining a faculty position. Barriers to promotion and retention within academic medicine include ineffective mentorship, unclear or inconsistent promotion criteria, and disparities in gender/ethnic representation.
In this article, we interview two accomplished physicians in academic medicine who have attained the rank of professors.
Interview with Sophie Balzora, MD
Dr. Balzora is a professor of medicine at NYU Grossman School of Medicine and a practicing gastroenterologist specializing in the care of patients with inflammatory bowel disease at NYU Langone Health. She serves as the American College of Gastroenterology’s Diversity, Equity, and Inclusion Committee Chair, on the Advisory Board of ACG’s Leadership, Ethics, and Equity (LE&E) Center, and is president and cofounder of the Association of Black Gastroenterologists and Hepatologists (ABGH). Dr. Balzora was promoted to full professor 11 years after graduating from fellowship.
What would you identify as some of the most important factors that led to your success in achieving a promotion to professor of medicine?
Surround yourself with individuals whose professional and personal priorities align with yours. To achieve this, it is essential to gain an understanding of what is important to you, what you envision your success to look like, and establish a timeline to achieve it. The concept of personal success and how to best achieve it will absolutely change as you grow, and that is okay and expected. Connecting with those outside of your clinical interests, at other institutions, and even outside of the medical field, can help you achieve these goals and better shape how you see your career unfolding and how you want it to look.
Historically, the proportion of physicians who achieve professorship is lower among women compared with men. What do you believe are some of the barriers involved in this, and how would you counsel women who are interested in pursuing the rank of professor?
Systemic gender bias and discrimination, over-mentorship and under-sponsorship, inconsistent parental leave, and delayed parenthood are a few of the factors that contribute to the observed disparities in academic rank. Predictably, for women from underrepresented backgrounds in medicine, the chasm grows.
What has helped me most is to keep my eyes on the prize, and to recognize that the prize is different for everyone. It’s important not to make direct comparisons to any other individual, because they are not you. Harness what makes you different and drown out the naysayers — the “we’ve never seen this done before” camp, the “it’s too soon [for someone like you] to go up for promotion” folks. While these voices are sometimes well intentioned, they can distract you from your goals and ambitions because they are rooted in bias and adherence to traditional expectations. To do something new, and to change the game, requires going against the grain and utilizing your skills and talents to achieve what you want to achieve in a way that works for you.
What are some practical tips you have for junior gastroenterologists to track their promotion in academia?
- Keep your curriculum vitae (CV) up to date and formatted to your institutional guidelines. Ensure that you document your academic activities, even if it doesn’t seem important in the moment. When it’s time to submit that promotion portfolio, you want to be ready and organized.
- Remember: “No” is a full sentence, and saying it takes practice and time and confidence. It is a skill I still struggle to adopt at times, but it’s important to recognize the power of no, for it opens opportunities to say yes to other things.
- Lift as you climb — a critical part of changing the status quo is fostering the future of those underrepresented in medicine. A professional goal of mine that keeps me steady and passionate is to create supporting and enriching systemic and institutional changes that work to dismantle the obstacles perpetuating disparities in academic rank for women and those underrepresented in medicine. Discovering your “why” is a complex, difficult, and rewarding journey.
Interview with Mark Schattner, MD, AGAF
Dr. Schattner is a professor of clinical medicine at Weill Cornell College of Medicine and chief of the gastroenterology, hepatology, and nutrition service at Memorial Sloan Kettering Cancer Center, both in New York. He is a former president of the New York Society for Gastrointestinal Endoscopy and a fellow of the AGA and ASGE.
In your role as chief, you serve as a mentor for early career gastroenterologists for pursuing career promotion. What advice do you have for achieving this?
Promoting junior faculty is one of the prime responsibilities of a service chief. Generally, the early steps of promotion are straightforward, with criteria becoming more stringent as you progress. I think it is critical to understand the criteria used by promotion committees and to be aware of the various available tracks. I believe every meeting a junior faculty member has with their service chief should include, at the least, a brief check-in on where they are in the promotion process and plans (both short term and long term) to move forward. Successful promotion is facilitated when done upon a solid foundation of production and accomplishment. It is very challenging or even impossible when trying to piece together a package from discordant activities.
Most institutions require or encourage academic involvement at both national and international levels for career promotion. Do you have advice for junior faculty about how to achieve this type of recognition or experience?
The easiest place to start is with regional professional societies. Active involvement in these local societies fosters valuable networking and lays the groundwork for involvement at the national or international level. I would strongly encourage junior faculty to seek opportunities for a leadership position at any level in these societies and move up the ladder as their career matures. This is also a very good avenue to network and get invited to join collaborative research projects, which can be a fruitful means to enhance your academic productivity.
In your opinion, what factors are likely to hinder or delay an individual’s promotion?
I think it is crucial to consider the career track you are on. If you are very clinically productive and love to teach, that is completely appropriate, and most institutions will recognize the value of that and promote you along a clinical-educator tract. On the other hand, if you have a passion for research and can successfully lead research and compete for grants, then you would move along a traditional tenure track. It is also critical to think ahead, know the criteria on which you will be judged, and incorporate that into your practice early. Trying to scramble to enhance your CV in a short time just for promotion will likely prove ineffective.
Do you have advice for junior faculty who have families about how to manage career goals but also prioritize time with family?
There is no one-size-fits-all approach to this. I think this requires a lot of shared decision-making with your family. Compromise will undoubtedly be required. For example, I always chose to live in close proximity to my workplace, eliminating any commuting time. This choice really allowed me spend time with my family.
In conclusion, a career in academic medicine presents both opportunities and challenges. A successful academic career, and achieving promotion to the rank of professor of medicine, requires a combination of factors including understanding institution-specific criteria for promotion, proactive engagement at the regional and national level, and envisioning your career goals and creating a timeline to achieve them. There are challenges to promotion, including navigating systemic biases and balancing career goals with family commitments, which also requires consideration and open communication. Ultimately, we hope these insights provide valuable guidance and advice for junior faculty who are navigating this complex environment of academic medicine and are motivated toward achieving professional fulfillment and satisfaction in their careers.
Dr. Rolston is based in the Department of Gastroenterology, Hepatology, and Nutrition, Memorial Sloan Kettering Cancer Center, New York. She reports no conflicts in relation this article. Dr. Balzora and Dr. Schattner are based in the Division of Gastroenterology and Hepatology, New York University Langone Health, New York. Dr. Schattner is a consultant for Boston Scientific and Novo Nordisk. Dr. Balzora reports no conflicts in relation to this article.
References
Campbell KM. Mitigating the isolation of minoritized faculty in academic medicine. J Gen Intern Med. 2023 May. doi: 10.1007/s11606-022-07982-8.
Howard-Anderson JR et al. Strategies for developing a successful career in academic medicine. Am J Med Sci. 2024 Apr. doi: 10.1016/j.amjms.2023.12.010.
Murphy M et al. Women’s experiences of promotion and tenure in academic medicine and potential implications for gender disparities in career advancement: A qualitative analysis. JAMA Netw Open. 2021 Sep 1. doi: 10.1001/jamanetworkopen.2021.25843.
Sambunjak D et al. Mentoring in academic medicine: A systematic review. JAMA. 2006 Sep 6. doi: 10.1001/jama.296.9.1103.
Shen MR et al. Impact of mentoring on academic career success for women in medicine: A systematic review. Acad Med. 2022 Mar 1. doi: 10.1097/ACM.0000000000004563.
A Simplified Approach to Pelvic Floor Dysfunction
Pelvic floor dysfunction (PFD) represents a spectrum of symptoms involving sensory and emptying abnormalities of the bowel and bladder and pelvic organ prolapse. The pelvic floor refers to a group of muscles that spans the pelvic outlet, providing support to the pelvic organs and coordinating constrictor mechanisms to control urination and defecation. Symptoms reported by patients experiencing PFD include involuntary loss of stool or urine, incomplete emptying of the bowel and bladder, a sensation of fullness, bulging in the vagina, and sexual dysfunction.1
As such, symptoms related to PFD are very common concerns raised by patients to their gastroenterologists. Data from the National Health and Nutrition Examination Survey show that 23.7% of women over the age of 20 had at least one symptom of PFD.2 Unfortunately, patients experiencing pelvic floor dysfunction often are hesitant to seek care because of embarrassment or perception that limited treatment options exist for their symptoms.
Pelvic Floor Anatomy
Regions of the pelvis are often referred to by anatomic compartment: anterior (bladder and urethra), middle (vagina and uterus or prostate), and posterior (colon, rectum, and anal canal). Supporting these compartments is the levator ani, a muscle group that is used synonymously with the term “pelvic diaphragm.”
Continence of stool is provided by the anal sphincter muscles and the puborectalis muscle, which wraps around the posterior aspect of the anorectal canal. Damage to the musculature or sensory perception to this area may result in fecal incontinence. Defecation is a coordinated process during which the abdominal and rectal muscles contract, while the anal sphincter muscles and puborectalis simultaneously relax. A disturbance in neuromuscular coordination (dyssynergic defecation) or structural pathology such as pelvic organ prolapse may lead to obstructed defecation.
PFD is thought to be a result of one or more insults to the pelvic floor such as chronic straining, childbirth, iatrogenic injury, or systemic disease such as diabetes.3
Evaluation of PFD Symptoms
Patients presenting with suspected PFD necessitate a comprehensive interdisciplinary assessment. In addition to obtaining a medical, surgical, and obstetric history, details about symptoms and lifestyle should include toileting habits, diet, and physical activity. The Pelvic Floor Distress Inventory (PFDI-20) is a commonly used tool that can be employed in the clinical setting.4
A pelvic exam can reveal pelvic organ prolapse and other mucosal pathology. The Pelvic Organ Prolapse Quantification System (POP-Q) is a widely used classification system for describing pelvic organ prolapse.5 Protrusion of the rectal wall into the vagina is referred to as a rectocele, while prolapse of small bowel into the upper posterior wall of the vagina is called an enterocele. While the finding of a rectocele on exam is common in parous women and may not cause any symptoms, a larger rectocele may cause a sensation of incomplete evacuation of stool.
A digital rectal exam (DRE) should be performed to assess pelvic floor function and help identify structural abnormalities.
Initial Management
A stepwise approach to the management of PFD can allow many patients to be effectively treated without the need for surgical intervention. For patients reporting liquid stool consistency, the evaluation should pivot toward the workup and management of diarrhea, which can easily overwhelm continence mechanisms and cause fecal incontinence. Fiber supplementation to normalize stool consistency is considered first-line therapy for patients presenting with both fecal incontinence and obstructed defecation. Other tools for fecal incontinence include avoiding foods that trigger diarrhea and use of loperamide.6 For patients with obstructed defecation, a trial of laxatives can be followed by a prescription agent if needed, such as a secretagogue or prokinetic.7
Vaginal splinting is a technique that can be used in patients with rectocele, whereby a finger is inserted into the vagina and pressure is applied on the posterior vaginal wall toward the rectum. Reducing the rectocele can facilitate emptying stool from the rectum and prevent leakage of retained stool.8 Similarly, use of rectal irrigation enemas can also help clear retained stool.
Pelvic floor physical therapists examine the strength, coordination, and tone of the pelvic floor muscles. When hypertonic musculature is present, manual interventions may be performed including trigger point release, myofascial release, and dry needling.9 When hypotonic musculature or dyssynergia is present, strengthening and neuromuscular re-education are recommended. Biofeedback can be administered via surface electromyography and/or balloon training to improve rectal sensitivity. Proper defecation techniques, including positioning, breathing, and behavioral modifications, improve clinical outcomes.
Diagnostic Testing
For patients who do not improve with conservative management, further testing is recommended to characterize the underlying pathology. Typically, anorectal manometry (ARM) is performed in conjunction with the balloon expulsion test and imaging. Each modality has its strengths and limitations (see Table 1).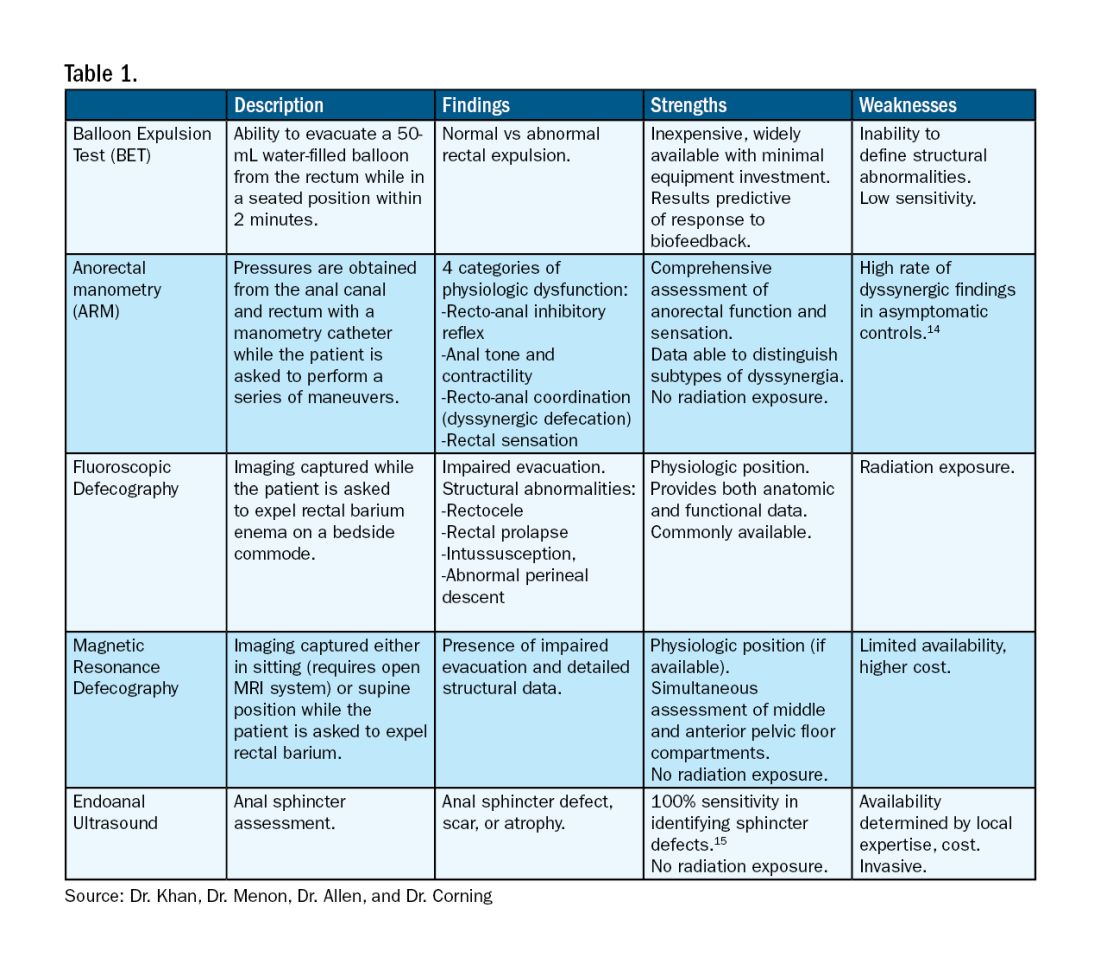
ARM allows for the assessment of rectal sensation and recto-anal pressures and coordination.10
Dynamic imaging, by barium defecography under fluoroscopy or MRI, captures anatomy at rest and with simulated defecation to identify pelvic organ prolapse, compartmental defects, and organ mobility.11 Endoanal ultrasonography is considered in patients experiencing fecal incontinence to evaluate the integrity of the anal sphincter muscles.
Minimally Invasive Procedures and Surgical Options for PFD
Functional abnormalities such as dyssynergia often coexist with structural abnormalities. Because structural abnormalities are commonly found in asymptomatic patients, noninvasive functional therapy, such as pelvic floor physical therapy and anorectal biofeedback, are preferred prior to surgical repair of a structural finding. For patients with fecal incontinence, sacral nerve stimulation (SNS) has emerged as a preferred therapy due to demonstrated efficacy in symptom improvement.12 Sphincteroplasty is reserved for those with acute sphincter injury or failure of SNS.
In patients with findings of intussusception, prolapse, or rectocele that have not responded to conservative therapy, referral for surgical repair may be considered. While the specific surgical approach will depend on many factors, the goal is typically excision and/or suspension of rectal tissue and reinforcement of the rectovaginal septum.
It is critical that we are equipped with the available knowledge and tools to provide these patients with optimal care.
Dr. Khan, Dr. Menon, Dr. Allen, and Dr. Corning are based at the University of Texas Medical Branch in Galveston, Texas. They report no conflicts of interest.
References
1. Grimes WR and Stratton M. Pelvic floor dysfunction. 2023 Jun 26. In: StatPearls [Internet]. Treasure Island (Fla.): StatPearls Publishing; 2024 Jan. PMID: 32644672.
2. Nygaard I et al. Prevalence of symptomatic pelvic floor disorders in US women. JAMA. 2008 Sep 17. doi: 10.1001/jama.300.11.1311.
3. Lawrence JM et al. Pelvic floor disorders, diabetes, and obesity in women: Findings from the Kaiser Permanente Continence Associated Risk Epidemiology Study. Diabetes Care. 2007 Oct. doi: 10.2337/dc07-0262.
4. Barber MD et al. Short forms of two condition-specific quality-of-life questionnaires for women with pelvic floor disorders (PFDI-20 and PFIQ-7). Am J Obstet Gynecol. 2005 Jul. doi: 10.1016/j.ajog.2004.12.025.
5. Persu C et al. Pelvic Organ Prolapse Quantification System (POP-Q) — A new era in pelvic prolapse staging. J Med Life. 2011 Jan-Mar. PMID: 21505577.
6. Wald A et al. ACG Clinical Guidelines: Management of benign anorectal disorders. Am J Gastroenterol. 2021 Oct 1. doi: 10.14309/ajg.0000000000001507.
7. Bharucha AE and Lacy BE. Mechanisms, evaluation, and management of chronic constipation. Gastroenterology. 2020 Apr. doi: 10.1053/j.gastro.2019.12.034.
8. Menees S and Chey WD. Fecal incontinence: Pathogenesis, diagnosis, and updated treatment strategies. Gastroenterol Clin North Am. 2022 Mar. doi: 10.1016/j.gtc.2021.10.005.
9. Wallace SL et al. Pelvic floor physical therapy in the treatment of pelvic floor dysfunction in women. Curr Opin Obstet Gynecol. 2019 Dec. doi: 10.1097/GCO.0000000000000584.
10. Carrington EV et al. The international anorectal physiology working group (IAPWG) recommendations: Standardized testing protocol and the London classification for disorders of anorectal function. Neurogastroenterol Motil. 2020 Jan. doi: 10.1111/nmo.13679.
11. El Sayed RF et al. Magnetic resonance imaging of pelvic floor dysfunction — Joint recommendations of the ESUR and ESGAR Pelvic Floor Working Group. Eur Radiol. 2017 May. doi: 10.1007/s00330-016-4471-7.
12. Thaha MA et al. Sacral nerve stimulation for faecal incontinence and constipation in adults. Cochrane Database Syst Rev. 2015 Aug 24. doi: 10.1002/14651858.CD004464.pub3.
13. Chiarioni G et al. Biofeedback benefits only patients with outlet dysfunction, not patients with isolated slow transit constipation. Gastroenterology. 2005 Jul. doi: 10.1053/j.gastro.2005.05.015.
14. Grossi U et al. Diagnostic accuracy study of anorectal manometry for diagnosis of dyssynergic defecation. Gut. 2016 Mar. doi: 10.1136/gutjnl-2014-308835.
15. Albuquerque A. Endoanal ultrasonography in fecal incontinence: Current and future perspectives. World J Gastrointest Endosc. 2015 Jun 10. doi: 10.4253/wjge.v7.i6.575.
Pelvic floor dysfunction (PFD) represents a spectrum of symptoms involving sensory and emptying abnormalities of the bowel and bladder and pelvic organ prolapse. The pelvic floor refers to a group of muscles that spans the pelvic outlet, providing support to the pelvic organs and coordinating constrictor mechanisms to control urination and defecation. Symptoms reported by patients experiencing PFD include involuntary loss of stool or urine, incomplete emptying of the bowel and bladder, a sensation of fullness, bulging in the vagina, and sexual dysfunction.1
As such, symptoms related to PFD are very common concerns raised by patients to their gastroenterologists. Data from the National Health and Nutrition Examination Survey show that 23.7% of women over the age of 20 had at least one symptom of PFD.2 Unfortunately, patients experiencing pelvic floor dysfunction often are hesitant to seek care because of embarrassment or perception that limited treatment options exist for their symptoms.
Pelvic Floor Anatomy
Regions of the pelvis are often referred to by anatomic compartment: anterior (bladder and urethra), middle (vagina and uterus or prostate), and posterior (colon, rectum, and anal canal). Supporting these compartments is the levator ani, a muscle group that is used synonymously with the term “pelvic diaphragm.”
Continence of stool is provided by the anal sphincter muscles and the puborectalis muscle, which wraps around the posterior aspect of the anorectal canal. Damage to the musculature or sensory perception to this area may result in fecal incontinence. Defecation is a coordinated process during which the abdominal and rectal muscles contract, while the anal sphincter muscles and puborectalis simultaneously relax. A disturbance in neuromuscular coordination (dyssynergic defecation) or structural pathology such as pelvic organ prolapse may lead to obstructed defecation.
PFD is thought to be a result of one or more insults to the pelvic floor such as chronic straining, childbirth, iatrogenic injury, or systemic disease such as diabetes.3
Evaluation of PFD Symptoms
Patients presenting with suspected PFD necessitate a comprehensive interdisciplinary assessment. In addition to obtaining a medical, surgical, and obstetric history, details about symptoms and lifestyle should include toileting habits, diet, and physical activity. The Pelvic Floor Distress Inventory (PFDI-20) is a commonly used tool that can be employed in the clinical setting.4
A pelvic exam can reveal pelvic organ prolapse and other mucosal pathology. The Pelvic Organ Prolapse Quantification System (POP-Q) is a widely used classification system for describing pelvic organ prolapse.5 Protrusion of the rectal wall into the vagina is referred to as a rectocele, while prolapse of small bowel into the upper posterior wall of the vagina is called an enterocele. While the finding of a rectocele on exam is common in parous women and may not cause any symptoms, a larger rectocele may cause a sensation of incomplete evacuation of stool.
A digital rectal exam (DRE) should be performed to assess pelvic floor function and help identify structural abnormalities.
Initial Management
A stepwise approach to the management of PFD can allow many patients to be effectively treated without the need for surgical intervention. For patients reporting liquid stool consistency, the evaluation should pivot toward the workup and management of diarrhea, which can easily overwhelm continence mechanisms and cause fecal incontinence. Fiber supplementation to normalize stool consistency is considered first-line therapy for patients presenting with both fecal incontinence and obstructed defecation. Other tools for fecal incontinence include avoiding foods that trigger diarrhea and use of loperamide.6 For patients with obstructed defecation, a trial of laxatives can be followed by a prescription agent if needed, such as a secretagogue or prokinetic.7
Vaginal splinting is a technique that can be used in patients with rectocele, whereby a finger is inserted into the vagina and pressure is applied on the posterior vaginal wall toward the rectum. Reducing the rectocele can facilitate emptying stool from the rectum and prevent leakage of retained stool.8 Similarly, use of rectal irrigation enemas can also help clear retained stool.
Pelvic floor physical therapists examine the strength, coordination, and tone of the pelvic floor muscles. When hypertonic musculature is present, manual interventions may be performed including trigger point release, myofascial release, and dry needling.9 When hypotonic musculature or dyssynergia is present, strengthening and neuromuscular re-education are recommended. Biofeedback can be administered via surface electromyography and/or balloon training to improve rectal sensitivity. Proper defecation techniques, including positioning, breathing, and behavioral modifications, improve clinical outcomes.
Diagnostic Testing
For patients who do not improve with conservative management, further testing is recommended to characterize the underlying pathology. Typically, anorectal manometry (ARM) is performed in conjunction with the balloon expulsion test and imaging. Each modality has its strengths and limitations (see Table 1).
ARM allows for the assessment of rectal sensation and recto-anal pressures and coordination.10
Dynamic imaging, by barium defecography under fluoroscopy or MRI, captures anatomy at rest and with simulated defecation to identify pelvic organ prolapse, compartmental defects, and organ mobility.11 Endoanal ultrasonography is considered in patients experiencing fecal incontinence to evaluate the integrity of the anal sphincter muscles.
Minimally Invasive Procedures and Surgical Options for PFD
Functional abnormalities such as dyssynergia often coexist with structural abnormalities. Because structural abnormalities are commonly found in asymptomatic patients, noninvasive functional therapy, such as pelvic floor physical therapy and anorectal biofeedback, are preferred prior to surgical repair of a structural finding. For patients with fecal incontinence, sacral nerve stimulation (SNS) has emerged as a preferred therapy due to demonstrated efficacy in symptom improvement.12 Sphincteroplasty is reserved for those with acute sphincter injury or failure of SNS.
In patients with findings of intussusception, prolapse, or rectocele that have not responded to conservative therapy, referral for surgical repair may be considered. While the specific surgical approach will depend on many factors, the goal is typically excision and/or suspension of rectal tissue and reinforcement of the rectovaginal septum.
It is critical that we are equipped with the available knowledge and tools to provide these patients with optimal care.
Dr. Khan, Dr. Menon, Dr. Allen, and Dr. Corning are based at the University of Texas Medical Branch in Galveston, Texas. They report no conflicts of interest.
References
1. Grimes WR and Stratton M. Pelvic floor dysfunction. 2023 Jun 26. In: StatPearls [Internet]. Treasure Island (Fla.): StatPearls Publishing; 2024 Jan. PMID: 32644672.
2. Nygaard I et al. Prevalence of symptomatic pelvic floor disorders in US women. JAMA. 2008 Sep 17. doi: 10.1001/jama.300.11.1311.
3. Lawrence JM et al. Pelvic floor disorders, diabetes, and obesity in women: Findings from the Kaiser Permanente Continence Associated Risk Epidemiology Study. Diabetes Care. 2007 Oct. doi: 10.2337/dc07-0262.
4. Barber MD et al. Short forms of two condition-specific quality-of-life questionnaires for women with pelvic floor disorders (PFDI-20 and PFIQ-7). Am J Obstet Gynecol. 2005 Jul. doi: 10.1016/j.ajog.2004.12.025.
5. Persu C et al. Pelvic Organ Prolapse Quantification System (POP-Q) — A new era in pelvic prolapse staging. J Med Life. 2011 Jan-Mar. PMID: 21505577.
6. Wald A et al. ACG Clinical Guidelines: Management of benign anorectal disorders. Am J Gastroenterol. 2021 Oct 1. doi: 10.14309/ajg.0000000000001507.
7. Bharucha AE and Lacy BE. Mechanisms, evaluation, and management of chronic constipation. Gastroenterology. 2020 Apr. doi: 10.1053/j.gastro.2019.12.034.
8. Menees S and Chey WD. Fecal incontinence: Pathogenesis, diagnosis, and updated treatment strategies. Gastroenterol Clin North Am. 2022 Mar. doi: 10.1016/j.gtc.2021.10.005.
9. Wallace SL et al. Pelvic floor physical therapy in the treatment of pelvic floor dysfunction in women. Curr Opin Obstet Gynecol. 2019 Dec. doi: 10.1097/GCO.0000000000000584.
10. Carrington EV et al. The international anorectal physiology working group (IAPWG) recommendations: Standardized testing protocol and the London classification for disorders of anorectal function. Neurogastroenterol Motil. 2020 Jan. doi: 10.1111/nmo.13679.
11. El Sayed RF et al. Magnetic resonance imaging of pelvic floor dysfunction — Joint recommendations of the ESUR and ESGAR Pelvic Floor Working Group. Eur Radiol. 2017 May. doi: 10.1007/s00330-016-4471-7.
12. Thaha MA et al. Sacral nerve stimulation for faecal incontinence and constipation in adults. Cochrane Database Syst Rev. 2015 Aug 24. doi: 10.1002/14651858.CD004464.pub3.
13. Chiarioni G et al. Biofeedback benefits only patients with outlet dysfunction, not patients with isolated slow transit constipation. Gastroenterology. 2005 Jul. doi: 10.1053/j.gastro.2005.05.015.
14. Grossi U et al. Diagnostic accuracy study of anorectal manometry for diagnosis of dyssynergic defecation. Gut. 2016 Mar. doi: 10.1136/gutjnl-2014-308835.
15. Albuquerque A. Endoanal ultrasonography in fecal incontinence: Current and future perspectives. World J Gastrointest Endosc. 2015 Jun 10. doi: 10.4253/wjge.v7.i6.575.
Pelvic floor dysfunction (PFD) represents a spectrum of symptoms involving sensory and emptying abnormalities of the bowel and bladder and pelvic organ prolapse. The pelvic floor refers to a group of muscles that spans the pelvic outlet, providing support to the pelvic organs and coordinating constrictor mechanisms to control urination and defecation. Symptoms reported by patients experiencing PFD include involuntary loss of stool or urine, incomplete emptying of the bowel and bladder, a sensation of fullness, bulging in the vagina, and sexual dysfunction.1
As such, symptoms related to PFD are very common concerns raised by patients to their gastroenterologists. Data from the National Health and Nutrition Examination Survey show that 23.7% of women over the age of 20 had at least one symptom of PFD.2 Unfortunately, patients experiencing pelvic floor dysfunction often are hesitant to seek care because of embarrassment or perception that limited treatment options exist for their symptoms.
Pelvic Floor Anatomy
Regions of the pelvis are often referred to by anatomic compartment: anterior (bladder and urethra), middle (vagina and uterus or prostate), and posterior (colon, rectum, and anal canal). Supporting these compartments is the levator ani, a muscle group that is used synonymously with the term “pelvic diaphragm.”
Continence of stool is provided by the anal sphincter muscles and the puborectalis muscle, which wraps around the posterior aspect of the anorectal canal. Damage to the musculature or sensory perception to this area may result in fecal incontinence. Defecation is a coordinated process during which the abdominal and rectal muscles contract, while the anal sphincter muscles and puborectalis simultaneously relax. A disturbance in neuromuscular coordination (dyssynergic defecation) or structural pathology such as pelvic organ prolapse may lead to obstructed defecation.
PFD is thought to be a result of one or more insults to the pelvic floor such as chronic straining, childbirth, iatrogenic injury, or systemic disease such as diabetes.3
Evaluation of PFD Symptoms
Patients presenting with suspected PFD necessitate a comprehensive interdisciplinary assessment. In addition to obtaining a medical, surgical, and obstetric history, details about symptoms and lifestyle should include toileting habits, diet, and physical activity. The Pelvic Floor Distress Inventory (PFDI-20) is a commonly used tool that can be employed in the clinical setting.4
A pelvic exam can reveal pelvic organ prolapse and other mucosal pathology. The Pelvic Organ Prolapse Quantification System (POP-Q) is a widely used classification system for describing pelvic organ prolapse.5 Protrusion of the rectal wall into the vagina is referred to as a rectocele, while prolapse of small bowel into the upper posterior wall of the vagina is called an enterocele. While the finding of a rectocele on exam is common in parous women and may not cause any symptoms, a larger rectocele may cause a sensation of incomplete evacuation of stool.
A digital rectal exam (DRE) should be performed to assess pelvic floor function and help identify structural abnormalities.
Initial Management
A stepwise approach to the management of PFD can allow many patients to be effectively treated without the need for surgical intervention. For patients reporting liquid stool consistency, the evaluation should pivot toward the workup and management of diarrhea, which can easily overwhelm continence mechanisms and cause fecal incontinence. Fiber supplementation to normalize stool consistency is considered first-line therapy for patients presenting with both fecal incontinence and obstructed defecation. Other tools for fecal incontinence include avoiding foods that trigger diarrhea and use of loperamide.6 For patients with obstructed defecation, a trial of laxatives can be followed by a prescription agent if needed, such as a secretagogue or prokinetic.7
Vaginal splinting is a technique that can be used in patients with rectocele, whereby a finger is inserted into the vagina and pressure is applied on the posterior vaginal wall toward the rectum. Reducing the rectocele can facilitate emptying stool from the rectum and prevent leakage of retained stool.8 Similarly, use of rectal irrigation enemas can also help clear retained stool.
Pelvic floor physical therapists examine the strength, coordination, and tone of the pelvic floor muscles. When hypertonic musculature is present, manual interventions may be performed including trigger point release, myofascial release, and dry needling.9 When hypotonic musculature or dyssynergia is present, strengthening and neuromuscular re-education are recommended. Biofeedback can be administered via surface electromyography and/or balloon training to improve rectal sensitivity. Proper defecation techniques, including positioning, breathing, and behavioral modifications, improve clinical outcomes.
Diagnostic Testing
For patients who do not improve with conservative management, further testing is recommended to characterize the underlying pathology. Typically, anorectal manometry (ARM) is performed in conjunction with the balloon expulsion test and imaging. Each modality has its strengths and limitations (see Table 1).
ARM allows for the assessment of rectal sensation and recto-anal pressures and coordination.10
Dynamic imaging, by barium defecography under fluoroscopy or MRI, captures anatomy at rest and with simulated defecation to identify pelvic organ prolapse, compartmental defects, and organ mobility.11 Endoanal ultrasonography is considered in patients experiencing fecal incontinence to evaluate the integrity of the anal sphincter muscles.
Minimally Invasive Procedures and Surgical Options for PFD
Functional abnormalities such as dyssynergia often coexist with structural abnormalities. Because structural abnormalities are commonly found in asymptomatic patients, noninvasive functional therapy, such as pelvic floor physical therapy and anorectal biofeedback, are preferred prior to surgical repair of a structural finding. For patients with fecal incontinence, sacral nerve stimulation (SNS) has emerged as a preferred therapy due to demonstrated efficacy in symptom improvement.12 Sphincteroplasty is reserved for those with acute sphincter injury or failure of SNS.
In patients with findings of intussusception, prolapse, or rectocele that have not responded to conservative therapy, referral for surgical repair may be considered. While the specific surgical approach will depend on many factors, the goal is typically excision and/or suspension of rectal tissue and reinforcement of the rectovaginal septum.
It is critical that we are equipped with the available knowledge and tools to provide these patients with optimal care.
Dr. Khan, Dr. Menon, Dr. Allen, and Dr. Corning are based at the University of Texas Medical Branch in Galveston, Texas. They report no conflicts of interest.
References
1. Grimes WR and Stratton M. Pelvic floor dysfunction. 2023 Jun 26. In: StatPearls [Internet]. Treasure Island (Fla.): StatPearls Publishing; 2024 Jan. PMID: 32644672.
2. Nygaard I et al. Prevalence of symptomatic pelvic floor disorders in US women. JAMA. 2008 Sep 17. doi: 10.1001/jama.300.11.1311.
3. Lawrence JM et al. Pelvic floor disorders, diabetes, and obesity in women: Findings from the Kaiser Permanente Continence Associated Risk Epidemiology Study. Diabetes Care. 2007 Oct. doi: 10.2337/dc07-0262.
4. Barber MD et al. Short forms of two condition-specific quality-of-life questionnaires for women with pelvic floor disorders (PFDI-20 and PFIQ-7). Am J Obstet Gynecol. 2005 Jul. doi: 10.1016/j.ajog.2004.12.025.
5. Persu C et al. Pelvic Organ Prolapse Quantification System (POP-Q) — A new era in pelvic prolapse staging. J Med Life. 2011 Jan-Mar. PMID: 21505577.
6. Wald A et al. ACG Clinical Guidelines: Management of benign anorectal disorders. Am J Gastroenterol. 2021 Oct 1. doi: 10.14309/ajg.0000000000001507.
7. Bharucha AE and Lacy BE. Mechanisms, evaluation, and management of chronic constipation. Gastroenterology. 2020 Apr. doi: 10.1053/j.gastro.2019.12.034.
8. Menees S and Chey WD. Fecal incontinence: Pathogenesis, diagnosis, and updated treatment strategies. Gastroenterol Clin North Am. 2022 Mar. doi: 10.1016/j.gtc.2021.10.005.
9. Wallace SL et al. Pelvic floor physical therapy in the treatment of pelvic floor dysfunction in women. Curr Opin Obstet Gynecol. 2019 Dec. doi: 10.1097/GCO.0000000000000584.
10. Carrington EV et al. The international anorectal physiology working group (IAPWG) recommendations: Standardized testing protocol and the London classification for disorders of anorectal function. Neurogastroenterol Motil. 2020 Jan. doi: 10.1111/nmo.13679.
11. El Sayed RF et al. Magnetic resonance imaging of pelvic floor dysfunction — Joint recommendations of the ESUR and ESGAR Pelvic Floor Working Group. Eur Radiol. 2017 May. doi: 10.1007/s00330-016-4471-7.
12. Thaha MA et al. Sacral nerve stimulation for faecal incontinence and constipation in adults. Cochrane Database Syst Rev. 2015 Aug 24. doi: 10.1002/14651858.CD004464.pub3.
13. Chiarioni G et al. Biofeedback benefits only patients with outlet dysfunction, not patients with isolated slow transit constipation. Gastroenterology. 2005 Jul. doi: 10.1053/j.gastro.2005.05.015.
14. Grossi U et al. Diagnostic accuracy study of anorectal manometry for diagnosis of dyssynergic defecation. Gut. 2016 Mar. doi: 10.1136/gutjnl-2014-308835.
15. Albuquerque A. Endoanal ultrasonography in fecal incontinence: Current and future perspectives. World J Gastrointest Endosc. 2015 Jun 10. doi: 10.4253/wjge.v7.i6.575.
Defining Your ‘Success’
Dear Friends,
The prevailing theme of this issue is “Success.” I have learned that “success” is personal and personalized. What “success” looked like 10, or even 5, years ago to me is very different from how I perceive it now; and I know it may be different 5 years from now. My definition of success should not look like another’s — that was the best advice I have gotten over the years and it has kept me constantly redefining what is important to me and placing value on where I want to allocate my time and efforts, at work and at home.
This issue of The New Gastroenterologist highlights topics from successful GIs within their own realms of expertise, offering insights on advancing in academic medicine, navigating financial wellness with a financial adviser, and becoming a future leader in GI.
In this issue’s clinically-focused articles, we spotlight two very nuanced and challenging topics. Dr. Sachin Srinivasan and Dr. Prateek Sharma review Barrett’s esophagus management for our “In Focus” section, with a particular emphasis on Barrett’s endoscopic therapy modalities for dysplasia and early neoplasia. Dr. Brooke Corning and team simplify their approach to pelvic floor dysfunction (PFD) in our “Short Clinical Reviews.” They suggest validated ways to assess patient history, pros and cons of various diagnostic tests, and stepwise management of PFD.
Navigating academic promotion can be overwhelming and may not be at the forefront with our early career GIs’ priorities. In our “Early Career” section, Dr. Vineet Rolston interviews two highly accomplished professors in academic medicine, Dr. Sophie Balzora and Dr. Mark Schattner, for their insights into the promotion process and recommendations for junior faculty.
Dr. Anjuli K. Luthra, a therapeutic endoscopist and founder of The Scope of Finance, emphasizes financial wellness for physicians. She breaks down the search for a financial adviser, including the different types, what to ask when searching for the right fit, and what to expect.
Lastly, this issue highlights an AGA program that invests in the development of leaders for the field — the Future Leaders Program (FLP). Dr. Parakkal Deepak and Dr. Edward L. Barnes, along with their mentor, Dr. Aasma Shaukat, describe their experience as a mentee-mentor triad of FLP and how this program has impacted their careers.
If you are interested in contributing or have ideas for future TNG topics, please contact me (tjudy@wustl.edu), or Danielle Kiefer (dkiefer@gastro.org), managing editor of TNG.
Until next time, I leave you with a historical fun fact because we would not be where we are now without appreciating where we were: Dr. C.G. Stockton was the first AGA president in 1897, a Professor of the Principles and Practice of Medicine and Clinical Medicine at the University of Buffalo in New York, and published on the relationship between GI/Hepatology and gout in the Journal of the American Medical Association the same year of his presidency.
Yours truly,
Judy A. Trieu, MD, MPH
Editor-in-Chief
Interventional Endoscopy, Division of Gastroenterology
Washington University in St. Louis
Dear Friends,
The prevailing theme of this issue is “Success.” I have learned that “success” is personal and personalized. What “success” looked like 10, or even 5, years ago to me is very different from how I perceive it now; and I know it may be different 5 years from now. My definition of success should not look like another’s — that was the best advice I have gotten over the years and it has kept me constantly redefining what is important to me and placing value on where I want to allocate my time and efforts, at work and at home.
This issue of The New Gastroenterologist highlights topics from successful GIs within their own realms of expertise, offering insights on advancing in academic medicine, navigating financial wellness with a financial adviser, and becoming a future leader in GI.
In this issue’s clinically-focused articles, we spotlight two very nuanced and challenging topics. Dr. Sachin Srinivasan and Dr. Prateek Sharma review Barrett’s esophagus management for our “In Focus” section, with a particular emphasis on Barrett’s endoscopic therapy modalities for dysplasia and early neoplasia. Dr. Brooke Corning and team simplify their approach to pelvic floor dysfunction (PFD) in our “Short Clinical Reviews.” They suggest validated ways to assess patient history, pros and cons of various diagnostic tests, and stepwise management of PFD.
Navigating academic promotion can be overwhelming and may not be at the forefront with our early career GIs’ priorities. In our “Early Career” section, Dr. Vineet Rolston interviews two highly accomplished professors in academic medicine, Dr. Sophie Balzora and Dr. Mark Schattner, for their insights into the promotion process and recommendations for junior faculty.
Dr. Anjuli K. Luthra, a therapeutic endoscopist and founder of The Scope of Finance, emphasizes financial wellness for physicians. She breaks down the search for a financial adviser, including the different types, what to ask when searching for the right fit, and what to expect.
Lastly, this issue highlights an AGA program that invests in the development of leaders for the field — the Future Leaders Program (FLP). Dr. Parakkal Deepak and Dr. Edward L. Barnes, along with their mentor, Dr. Aasma Shaukat, describe their experience as a mentee-mentor triad of FLP and how this program has impacted their careers.
If you are interested in contributing or have ideas for future TNG topics, please contact me (tjudy@wustl.edu), or Danielle Kiefer (dkiefer@gastro.org), managing editor of TNG.
Until next time, I leave you with a historical fun fact because we would not be where we are now without appreciating where we were: Dr. C.G. Stockton was the first AGA president in 1897, a Professor of the Principles and Practice of Medicine and Clinical Medicine at the University of Buffalo in New York, and published on the relationship between GI/Hepatology and gout in the Journal of the American Medical Association the same year of his presidency.
Yours truly,
Judy A. Trieu, MD, MPH
Editor-in-Chief
Interventional Endoscopy, Division of Gastroenterology
Washington University in St. Louis
Dear Friends,
The prevailing theme of this issue is “Success.” I have learned that “success” is personal and personalized. What “success” looked like 10, or even 5, years ago to me is very different from how I perceive it now; and I know it may be different 5 years from now. My definition of success should not look like another’s — that was the best advice I have gotten over the years and it has kept me constantly redefining what is important to me and placing value on where I want to allocate my time and efforts, at work and at home.
This issue of The New Gastroenterologist highlights topics from successful GIs within their own realms of expertise, offering insights on advancing in academic medicine, navigating financial wellness with a financial adviser, and becoming a future leader in GI.
In this issue’s clinically-focused articles, we spotlight two very nuanced and challenging topics. Dr. Sachin Srinivasan and Dr. Prateek Sharma review Barrett’s esophagus management for our “In Focus” section, with a particular emphasis on Barrett’s endoscopic therapy modalities for dysplasia and early neoplasia. Dr. Brooke Corning and team simplify their approach to pelvic floor dysfunction (PFD) in our “Short Clinical Reviews.” They suggest validated ways to assess patient history, pros and cons of various diagnostic tests, and stepwise management of PFD.
Navigating academic promotion can be overwhelming and may not be at the forefront with our early career GIs’ priorities. In our “Early Career” section, Dr. Vineet Rolston interviews two highly accomplished professors in academic medicine, Dr. Sophie Balzora and Dr. Mark Schattner, for their insights into the promotion process and recommendations for junior faculty.
Dr. Anjuli K. Luthra, a therapeutic endoscopist and founder of The Scope of Finance, emphasizes financial wellness for physicians. She breaks down the search for a financial adviser, including the different types, what to ask when searching for the right fit, and what to expect.
Lastly, this issue highlights an AGA program that invests in the development of leaders for the field — the Future Leaders Program (FLP). Dr. Parakkal Deepak and Dr. Edward L. Barnes, along with their mentor, Dr. Aasma Shaukat, describe their experience as a mentee-mentor triad of FLP and how this program has impacted their careers.
If you are interested in contributing or have ideas for future TNG topics, please contact me (tjudy@wustl.edu), or Danielle Kiefer (dkiefer@gastro.org), managing editor of TNG.
Until next time, I leave you with a historical fun fact because we would not be where we are now without appreciating where we were: Dr. C.G. Stockton was the first AGA president in 1897, a Professor of the Principles and Practice of Medicine and Clinical Medicine at the University of Buffalo in New York, and published on the relationship between GI/Hepatology and gout in the Journal of the American Medical Association the same year of his presidency.
Yours truly,
Judy A. Trieu, MD, MPH
Editor-in-Chief
Interventional Endoscopy, Division of Gastroenterology
Washington University in St. Louis
Working together
Dear Friends,
After 6 months in my first faculty position, I have come to appreciate the term “multidisciplinary approach” more than ever. Not only does this facilitate optimal patient care, but I have personally learned so much from experts in other fields. This theme resonates across this issue of The New Gastroenterologist, from treating complex gallbladder disease, to caring for sexual and gender minorities, and collaborating with the tech industry to advance patient care.
Our “In Focus” feature, written by Dr. Andrew Gilman and Dr. Todd Baron, is on endoscopic management of gallbladder disease. They review endoscopic treatment options in patients with benign gallbladder disease, with emphasis on working with surgical and interventional radiology colleagues, as well as relaying endoscopic tips and techniques to achieve success in these complicated procedures.
In the “Short Clinical Reviews” section, Dr. David Chiang and Dr. Victor Chedid highlight the gaps in research and clinical care and competency for sexual and gender minorities, particularly in patients with inflammatory bowel disease. They describe the creation of the Pride in IBD clinic at Mayo Clinic in Rochester, Minn., that creates a culturally sensitive space to care for this community.
As trainees transition to early faculty, becoming a mentor is a new role that can be very rewarding and daunting at the same time. Dr. Anna Lok, recipient of the AGA’s Distinguished Mentor Award, and Dr. Vincent Chen share invaluable experiences and advice on being a mentor from senior and early-career perspectives, respectively. Similarly in the transition to early faculty, Erin Anderson, CPA, answers five common financial questions that arise to better understand and manage a significant increase in salary.
Lastly, Dr. Shifa Umar describes her unique experience as part of the AGA’s annual Tech Summit Fellows Program, a cross-section of medicine, technology, and innovation.
If you are interested in contributing or have ideas for future TNG topics, please contact me (tjudy@wustl.edu), or Jillian Schweitzer (jschweitzer@gastro.org), managing editor of TNG.
Until next time, I leave you with a historical fun fact because we would not be where we are now without appreciating where we were: The concept of the clinicopathologic conference (CPC) was introduced by Dr. Walter B. Cannon as a medical student at Harvard Medical School.
Yours truly,
Judy A. Trieu, MD, MPH
Editor-in-Chief
Interventional Endoscopy, Division of Gastroenterology
Washington University in St. Louis
Dear Friends,
After 6 months in my first faculty position, I have come to appreciate the term “multidisciplinary approach” more than ever. Not only does this facilitate optimal patient care, but I have personally learned so much from experts in other fields. This theme resonates across this issue of The New Gastroenterologist, from treating complex gallbladder disease, to caring for sexual and gender minorities, and collaborating with the tech industry to advance patient care.
Our “In Focus” feature, written by Dr. Andrew Gilman and Dr. Todd Baron, is on endoscopic management of gallbladder disease. They review endoscopic treatment options in patients with benign gallbladder disease, with emphasis on working with surgical and interventional radiology colleagues, as well as relaying endoscopic tips and techniques to achieve success in these complicated procedures.
In the “Short Clinical Reviews” section, Dr. David Chiang and Dr. Victor Chedid highlight the gaps in research and clinical care and competency for sexual and gender minorities, particularly in patients with inflammatory bowel disease. They describe the creation of the Pride in IBD clinic at Mayo Clinic in Rochester, Minn., that creates a culturally sensitive space to care for this community.
As trainees transition to early faculty, becoming a mentor is a new role that can be very rewarding and daunting at the same time. Dr. Anna Lok, recipient of the AGA’s Distinguished Mentor Award, and Dr. Vincent Chen share invaluable experiences and advice on being a mentor from senior and early-career perspectives, respectively. Similarly in the transition to early faculty, Erin Anderson, CPA, answers five common financial questions that arise to better understand and manage a significant increase in salary.
Lastly, Dr. Shifa Umar describes her unique experience as part of the AGA’s annual Tech Summit Fellows Program, a cross-section of medicine, technology, and innovation.
If you are interested in contributing or have ideas for future TNG topics, please contact me (tjudy@wustl.edu), or Jillian Schweitzer (jschweitzer@gastro.org), managing editor of TNG.
Until next time, I leave you with a historical fun fact because we would not be where we are now without appreciating where we were: The concept of the clinicopathologic conference (CPC) was introduced by Dr. Walter B. Cannon as a medical student at Harvard Medical School.
Yours truly,
Judy A. Trieu, MD, MPH
Editor-in-Chief
Interventional Endoscopy, Division of Gastroenterology
Washington University in St. Louis
Dear Friends,
After 6 months in my first faculty position, I have come to appreciate the term “multidisciplinary approach” more than ever. Not only does this facilitate optimal patient care, but I have personally learned so much from experts in other fields. This theme resonates across this issue of The New Gastroenterologist, from treating complex gallbladder disease, to caring for sexual and gender minorities, and collaborating with the tech industry to advance patient care.
Our “In Focus” feature, written by Dr. Andrew Gilman and Dr. Todd Baron, is on endoscopic management of gallbladder disease. They review endoscopic treatment options in patients with benign gallbladder disease, with emphasis on working with surgical and interventional radiology colleagues, as well as relaying endoscopic tips and techniques to achieve success in these complicated procedures.
In the “Short Clinical Reviews” section, Dr. David Chiang and Dr. Victor Chedid highlight the gaps in research and clinical care and competency for sexual and gender minorities, particularly in patients with inflammatory bowel disease. They describe the creation of the Pride in IBD clinic at Mayo Clinic in Rochester, Minn., that creates a culturally sensitive space to care for this community.
As trainees transition to early faculty, becoming a mentor is a new role that can be very rewarding and daunting at the same time. Dr. Anna Lok, recipient of the AGA’s Distinguished Mentor Award, and Dr. Vincent Chen share invaluable experiences and advice on being a mentor from senior and early-career perspectives, respectively. Similarly in the transition to early faculty, Erin Anderson, CPA, answers five common financial questions that arise to better understand and manage a significant increase in salary.
Lastly, Dr. Shifa Umar describes her unique experience as part of the AGA’s annual Tech Summit Fellows Program, a cross-section of medicine, technology, and innovation.
If you are interested in contributing or have ideas for future TNG topics, please contact me (tjudy@wustl.edu), or Jillian Schweitzer (jschweitzer@gastro.org), managing editor of TNG.
Until next time, I leave you with a historical fun fact because we would not be where we are now without appreciating where we were: The concept of the clinicopathologic conference (CPC) was introduced by Dr. Walter B. Cannon as a medical student at Harvard Medical School.
Yours truly,
Judy A. Trieu, MD, MPH
Editor-in-Chief
Interventional Endoscopy, Division of Gastroenterology
Washington University in St. Louis
Endoscopic Management of Benign Gallbladder Disease
Introduction
The treatment of benign gallbladder disease has changed substantially in the past decade, but this represents only a snapshot in the evolutionary history of the management of this organ. What began as a problem managed exclusively by open cholecystectomy (CCY) transitioned into a race toward minimally invasive approaches in the 1980s, with advances from gastroenterology, surgery, and radiology.
The opening strides were made in 1980 with the first description of percutaneous cholecystostomy (PC) by Dr. R.W. Radder.1 Shortly thereafter, in 1984, Dr. Richard Kozarek first reported the feasibility of selective cystic duct cannulation during endoscopic retrograde cholangiopancreatography (ERCP).2 Subsequent stenting for the treatment of acute cholecystitis (endoscopic transpapillary gallbladder drainage, ET-GBD) was then reported by Tamada et. al. in 1991.3 Not to be outdone, the first laparoscopic cholecystectomy (LC) was completed by Dr. Med Erich Mühe of Germany in 1985.4 More recently, with the expansion of interventional endoscopic ultrasound (EUS), the first transmural EUS-guided gallbladder drainage (EUS-GBD) was described by Dr. Baron and Dr. Topazian in 2007.5
The subsequent advent of lumen apposing metal stents (LAMS) has cemented EUS-GBD in the toolbox of treatment for benign gallbladder disease. Results of a recent prospective multicenter trial, with a Food and Drug Administration–approved protocol and investigational device exemption, have been published, opening the door for the expansion of FDA approved indications for this device.6
Benign gallbladder disease encompasses both polyps (benign and premalignant) and cholecystitis (acute/chronic, calculous/acalculous), in addition to others. The four management techniques (LC, PC, ET-GBD, and EUS-GBD) have filled integral niches in the management of these patients. Even gallbladder polyps have not been able to escape the reach of endoscopic approaches with the recent description of LAMS-assisted polypectomy as part of a gallbladder preserving strategy.7,8 While EUS-GBD also has been used for biliary decompression in the presence of a patent cystic duct and absence of cholecystitis, .9 Both of these techniques have gained wide recognition and/or guideline support for their use from the American Society for Gastrointestinal Endoscopy (ASGE) and the European Society of Gastrointestinal Endoscopy (ESGE).10,11 In addition, there is now one FDA-approved stent device for treatment of acute cholecystitis in patients unfit for surgery.
Techniques & Tips
ET-GBD
- During ERCP, after successful cannulation of the bile duct, attempted wire cannulation of the cystic duct is performed.
A cholangiogram, which clearly delineates the insertion of the cystic duct into the main bile duct, can enhance cannulation success. Rotatable fluoroscopy can facilitate identification.
- After anatomy is clear, wire access is often best achieved using a sphincterotome or stone retrieval (occlusion) balloon.
The balloon, once inflated, can be pulled downward to establish traction on the main bile duct, which can straighten the approach.
- After superficial wire engagement into the cystic duct, the accessory used can be slowly advanced into the cystic duct to stabilize the catheter and then navigate the valves of Heister to reach the gallbladder lumen.
Use of a sphincterotome, which directs toward the patient’s right (most often direction of cystic duct takeoff), is helpful. Angled guidewires are preferable. We often use a 0.035-inch, 260-cm angled hydrophilic wire (GLIDEWIRE; Terumo, Somerset, NJ) to overcome this challenging portion of ET-GBD.
If despite the above maneuvers the guidewire has failed to enter the cystic duct, cholangioscopy can be used to identify the orifice and/or stabilize deep wire cannulation. This is often cumbersome, time consuming, does not always produce success, and requires additional expertise.
- If a stone is encountered that cannot be extracted or traversed by a guidewire, cholangioscopy with electrohydraulic lithotripsy can be pursued.
- After the guidewire has entered the gallbladder, a 5 French or 7 French plastic double pigtail stent is placed. Typical lengths are 9-15 cm.
Some authors prefer to use two side-by-side plastic stents.12 This has been shown retrospectively to enhance the long term clinical success of ET-GBD but with additional technical difficulty.
- This stent can remain in place indefinitely and need not be exchanged, though it should be removed just prior to CCY if pursued. Alternatively, the surgeon can be alerted to its presence and, if comfortable, it can be removed intraoperatively.
EUS-GBD
- Use of fluoroscopy is optional but can enhance technical success in selected situations.
- Conversion, or internalization, of PC is reasonable and can enhance patient quality of life.13
- If the gallbladder wall is not in close apposition to the duodenal (or gastric) wall, consider measuring the distance.
We preferentially use 10-mm diameter by 10-mm saddle length LAMS for EUS-GBD, unless the above distance warrants use of a 15-mm by 15-mm LAMS (AXIOS, Boston Scientific, Marlborough, MA). If the distance is greater than 15 mm, consider searching for an alternative site, using a traditional biliary fully covered self-expandable metal stent (FCSEMS) for longer length, or converting to ET-GBD. Smaller diameter (8 mm) with an 8-mm saddle length can be used as well. The optimal diameter is unknown and also dependent on whether transluminal endoscopic diagnosis or therapy is a consideration.
- If there is difficulty locating the gallbladder, it may be decompressed or small (particularly if PC or a partial CCY has already been performed).
If a cholecystostomy tube is in place, instillation of sterile water via the tube can sometimes improve the target for LAMS placement, though caution should be made to not over-distend the gallbladder. ERCP with placement of a nasobiliary tube into the gallbladder can also serve this purpose and has been previously described.14
The gallbladder can be punctured with a 19-gauge FNA needle to instill sterile water and distend the gallbladder with the added benefit of being able to pass a guidewire, which may enhance procedural safety in difficult cases. However, success of this technique is contingent on fluid remaining within the gallbladder and not transiting out via the cystic duct. Expedient exchange of the FNA needle for the LAMS device may be necessary.
- Attempt to confirm location within the duodenum prior to puncture, as gastric origins can pose unique ramifications (i.e. potential for partial gastric outlet obstruction, obstruction of LAMS with food debris, etc.).
It can be easy to mistake an unintentional pre-pyloric position for a position within the duodenum since the working channel is behind (proximal to) the echoprobe.
- Turning off Doppler flow prior to advancement of the cautery enhanced LAMS can reduce obscurement of views on entry into the gallbladder. Lack of certainty about entry or misdeployment after presumed entry herald the most challenging aspect of EUS-GBD.
Utilization of a previously placed guidewire or advancement of one preloaded into the LAMS can aid in both enhancing confidence in location and assist with salvage maneuvers, if needed.
- After successful deployment of the LAMS we routinely place a double pigtail plastic stent through it (typically 7 French by 4 cm) to maintain patency. This may also prevent bleeding from the LAMS flange abrading the wall of either lumen.
- We routinely exchange the LAMS for two double pigtail plastic stents (typically 7 French by 4 cm) 4 weeks after initial placement especially when there is a more than modest residual stone burden (data in press). These plastic stents can remain in place indefinitely.
This exchange can be deferred if the patient is not expected to survive until the one-year anniversary of LAMS deployment. After one year the LAMS plastic covering may degrade and pose additional problems.15
LAMS Misdeployment Salvage Tips
- Salvage techniques can vary from simple to complex.
- If a wire is in place, it can be used to balloon or catheter dilate the tract and place a FCSEMS traversing the gallbladder and duodenal/gastric lumens. A similar approach can be used if the LAMS deployed on only one side (gallbladder or duodenum/stomach) and the other flange is within the peritoneum.
- The most challenging scenario to salvage is if the LAMS is misdeployed or becomes dislodged and no wire is present. This is why the use of a guidewire, even if preloaded into the LAMS and placement is freehand, is essential for EUS-GBD. A potential technique is to balloon dilate the duodenal/gastric defect and drive the endoscope into the peritoneum to reconnect that lumen to the gallbladder defect or LAMS, depending on the site of misdeployment. Doing so requires a high degree of commitment and skill and should not be done casually.
- If uncertainty remains or if misdeployment has occurred and salvage attempts have failed, consider closure of the duodenal/gastric defect and conversion to ET-GBD.
This may both treat the initial procedural indication and assist with what is essentially a large bile leak, which might also require percutaneous therapy for non-surgical management.
- For endoscopists with limited experience at salvage techniques, it is reasonable for the threshold for conversion to be low, assuming experience with and confidence in ET-GBD is high.
- If salvage is successful but ambiguity remains, consider obtaining a cholangiogram via the LAMS to confirm positioning and absence of leak.
Adverse Events
Both ET-GBD and EUS-GBD should be performed by an endoscopist comfortable with their techniques and the management of their adverse events (AEs). Rates for EUS-GBD AEs in patients at high risk for LC were reported in one international multicenter registry to be 15.3% with a 30-day mortality of 9.2%, with a significant predictor of AE being endoscopist experience less than 25 procedures.16 A meta-analysis also found an overall AE rate of 18.31%, with rates for perforation and stent related AEs (i.e. migration, occlusion, pneumoperitoneum) being 6.71% and 8.16%, respectively.17 For this reason, we recommend that patients with cholecystitis who are deemed to be poor surgical candidates be transferred to a tertiary referral center with expertise in these approaches. Rates of AEs for ET-GBD are similar to that for standard ERCP, with reported ranges of 5%-10.3%.10
Comparisons Between Techniques
The decision on which technique to utilize for endoscopic management of cholecystitis or symptomatic cholelithiasis depends first and foremost on the expertise and comfort level of the endoscopist. Given the additional training that an advanced endoscopist needs to perform EUS-GBD, combined with the perhaps slightly higher AE rate and permanency of endoscopic cholecystostomy, it is reasonable to proceed with a trial of ET-GBD if confidence is insufficient. However, ET-GBD can certainly be more technically challenging and less effective than EUS-GBD, with lower reported technical and clinical success rates (technical 85.3% vs 93.0%, clinical 95.2% vs 97.3%).18 Despite this, the rate of recurrence of cholecystitis is similar between ET-GBD and EUS-GBD (4.6% vs 4.2%).19 As stated above in the Techniques & Tips section, some authors utilize two plastic stents for ET-GBD for this purpose, though with increased technical difficulty. It is important to remember that these numbers, when paired with AE rates, represent the achievements of expert endoscopists.
Discussion with your surgery team is important when deciding modality. If the patient is felt to be a potential candidate for CCY, and EUS-GBD is not being used as a destination therapy, the surgeon may prefer ET-GBD. EUS-GBD may enhance the difficulty of CCY, though at least one study demonstrated that this was no different than PC with similar rates of conversion from LC to open CCY.20 This conversation is most critical for patients who are potential liver transplant candidates. For patients where this is not a consideration there is some evidence to suggest equivalency between LC and EUS-GBD, though certainly EUS-GBD has not yet supplanted LC as the treatment of choice.21
While there may eventually be a shift towards EUS-GBD instead of LC in certain patient groups, what is clearer are the advantages of EUS-GBD over PC. One recent meta-analysis revealed that EUS-GBD has significantly favorable odds of overall adverse events (OR 0.43, 95% CI 0.18-1.00), shorter hospital stay (2.76 less days, 95% CI 0.31-5.20 less days), reinterventions (OR 0.15, 95% CI 0.02-0.98), and unplanned readmissions (OR 0.14, 95% CI 0.03-0.70) compared to PC.22 Beyond the data, though, are the emotional and psychological impacts an external drain can have on a patient.
Conclusion
When expertise is available, endoscopic treatment of benign gallbladder disease has a definite role but should be undertaken only by those with the experience and skill to safely do so. Decision to proceed, especially with EUS-GBD, should be accompanied by conversation and collaboration with surgical teams. If a patient is under consideration for PC instead of LC, it may be worthwhile to seek consultation with a local center with expertise in EUS-GBD or ET-GBD. The adoption of these techniques is part of the paradigm shift, seen broadly throughout medicine, towards minimally invasive interventions, particularly in advanced endoscopy.
Dr. Gilman (X @a_gilman) and Dr. Baron (X @EndoTx) are with the University of North Carolina, Chapel Hill, Division of Gastroenterology & Hepatology. Dr. Gilman has no relevant financial disclosures. Dr. Baron is a consultant and speaker for Ambu, Boston Scientific, Cook Endoscopy, Medtronic, Olympus America, and W.L. Gore.
References
1. Radder RW. Ultrasonically guided percutaneous catheter drainage for gallbladder empyema. Diagn Imaging. 1980;49:330-333.
2. Kozarek RA. Selective cannulation of the cystic duct at time of ERCP. J Clin Gastroenterol. 1984;6:37-40.
3. Tamada K et al. Efficacy of endoscopic retrograde cholecystoendoprosthesis (ERCCE) for cholecystitis. Endoscopy. 1991;23:2-3.
4. Reynolds W. The first laparoscopic cholecystectomy. JSLS. 2001;5:89-94.
5. Baron TH, Topazian MD. Endoscopic transduodenal drainage of the gallbladder: Implications for endoluminal treatment of gallbladder disease. Gastrointest Endosc. 2007 Apr;65(4):735-7. doi: 10.1016/j.gie.2006.07.041.
6. Irani SS et al. Endoscopic ultrasound-guided transluminal gallbladder drainage in patients with acute cholecystitis: A prospective multicenter trial. Ann Surg. 2023 Sep 1;278(3):e556-e562. doi: 10.1097/SLA.0000000000005784.
7. Shen Y et al. Endoscopic ultrasound-guided cholecystostomy for resection of gallbladder polyps with lumen-apposing metal stent. Medicine (Baltimore). 2020 Oct 23;99(43):e22903. doi: 10.1097/MD.0000000000022903.
8. Pang H et al. Endoscopic ultrasound-guided gallbladder endoscopic mucosal resection: A pilot porcine study. Minim Invasive Ther Allied Technol. 2023 Feb;32(1):24-32. doi: 10.1080/13645706.2022.2153228.
9. Imai H et al. EUS-guided gallbladder drainage for rescue treatment of malignant distal biliary obstruction after unsuccessful ERCP. Gastrointest Endosc. 2016 Jul;84(1):147-51. doi: 10.1016/j.gie.2015.12.024.
10. Saumoy M et al. Endoscopic therapies for gallbladder drainage. Gastrointest Endosc. 2021 Oct;94(4):671-84. doi: 10.1016/j.gie.2021.05.031.
11. Van der Merwe SW et al. Therapeutic endoscopic ultrasound: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy. 2022 Feb;54(2):185-205. doi: 10.1055/a-1717-1391.
12. Storm AC et al. Transpapillary gallbladder stent placement for long-term therapy of acute cholecystitis. Gastrointest Endosc. 2021 Oct;94(4):742-8 e1. doi: 10.1016/j.gie.2021.03.025.
13. James TW, Baron TH. Converting percutaneous gallbladder drainage to internal drainage using EUS-guided therapy: A review of current practices and procedures. Endosc Ultrasound. 2018 Mar-Apr;7(2):93-6. doi: 10.4103/eus.eus_110_17.
14. James TW, Baron TH. Transpapillary nasocystic tube placement to allow gallbladder distention for EUS-guided cholecystoduodenostomy. VideoGIE. 2019 Dec;4(12):561-2. doi: 10.1016/j.vgie.2019.08.009.
15. Gilman AJ, Baron TH. Delamination of a lumen-apposing metal stent with tissue ingrowth and stent-in-stent removal. Gastrointest Endosc. 2023 Sep;98(3):451-3. doi: 10.1016/j.gie.2023.04.2087.
16. Teoh AY et al. Outcomes of an international multicenter registry on EUS-guided gallbladder drainage in patients at high risk for cholecystectomy. Endosc Int Open. 2019 Aug;7(8):E964-E973. doi: 10.1055/a-0915-2098.
17. Kalva NR et al. Efficacy and safety of lumen apposing self-expandable metal stents for EUS guided cholecystostomy: A meta-analysis and systematic review. Can J Gastroenterol Hepatol. 2018;2018:7070961. doi: 10.1155/2018/7070961.
18. Khan MA et al. Efficacy and safety of endoscopic gallbladder drainage in acute cholecystitis: Is it better than percutaneous gallbladder drainage? Gastrointest Endosc. 2017 Jan;85(1):76-87 e3. doi: 10.1016/j.gie.2016.06.032.
19. Mohan BP et al. Endoscopic ultrasound-guided gallbladder drainage, transpapillary drainage, or percutaneous drainage in high risk acute cholecystitis patients: a systematic review and comparative meta-analysis. Endoscopy. 2020 Feb;52(2):96-106. doi: 10.1055/a-1020-3932.
20. Jang JW et al. Endoscopic ultrasound-guided transmural and percutaneous transhepatic gallbladder drainage are comparable for acute cholecystitis. Gastroenterology. 2012 Apr;142(4):805-11. doi: 10.1053/j.gastro.2011.12.051.
21. Teoh AYB et al. EUS-guided gallbladder drainage versus laparoscopic cholecystectomy for acute cholecystitis: a propensity score analysis with 1-year follow-up data. Gastrointest Endosc. 2021 Mar;93(3):577-83. doi: 10.1016/j.gie.2020.06.066.
22. Luk SW et al. Endoscopic ultrasound-guided gallbladder drainage versus percutaneous cholecystostomy for high risk surgical patients with acute cholecystitis: a systematic review and meta-analysis. Endoscopy. 2019 Aug;51(8):722-32. doi: 10.1055/a-0929-6603.
Introduction
The treatment of benign gallbladder disease has changed substantially in the past decade, but this represents only a snapshot in the evolutionary history of the management of this organ. What began as a problem managed exclusively by open cholecystectomy (CCY) transitioned into a race toward minimally invasive approaches in the 1980s, with advances from gastroenterology, surgery, and radiology.
The opening strides were made in 1980 with the first description of percutaneous cholecystostomy (PC) by Dr. R.W. Radder.1 Shortly thereafter, in 1984, Dr. Richard Kozarek first reported the feasibility of selective cystic duct cannulation during endoscopic retrograde cholangiopancreatography (ERCP).2 Subsequent stenting for the treatment of acute cholecystitis (endoscopic transpapillary gallbladder drainage, ET-GBD) was then reported by Tamada et. al. in 1991.3 Not to be outdone, the first laparoscopic cholecystectomy (LC) was completed by Dr. Med Erich Mühe of Germany in 1985.4 More recently, with the expansion of interventional endoscopic ultrasound (EUS), the first transmural EUS-guided gallbladder drainage (EUS-GBD) was described by Dr. Baron and Dr. Topazian in 2007.5
The subsequent advent of lumen apposing metal stents (LAMS) has cemented EUS-GBD in the toolbox of treatment for benign gallbladder disease. Results of a recent prospective multicenter trial, with a Food and Drug Administration–approved protocol and investigational device exemption, have been published, opening the door for the expansion of FDA approved indications for this device.6
Benign gallbladder disease encompasses both polyps (benign and premalignant) and cholecystitis (acute/chronic, calculous/acalculous), in addition to others. The four management techniques (LC, PC, ET-GBD, and EUS-GBD) have filled integral niches in the management of these patients. Even gallbladder polyps have not been able to escape the reach of endoscopic approaches with the recent description of LAMS-assisted polypectomy as part of a gallbladder preserving strategy.7,8 While EUS-GBD also has been used for biliary decompression in the presence of a patent cystic duct and absence of cholecystitis, .9 Both of these techniques have gained wide recognition and/or guideline support for their use from the American Society for Gastrointestinal Endoscopy (ASGE) and the European Society of Gastrointestinal Endoscopy (ESGE).10,11 In addition, there is now one FDA-approved stent device for treatment of acute cholecystitis in patients unfit for surgery.
Techniques & Tips
ET-GBD
- During ERCP, after successful cannulation of the bile duct, attempted wire cannulation of the cystic duct is performed.
A cholangiogram, which clearly delineates the insertion of the cystic duct into the main bile duct, can enhance cannulation success. Rotatable fluoroscopy can facilitate identification.
- After anatomy is clear, wire access is often best achieved using a sphincterotome or stone retrieval (occlusion) balloon.
The balloon, once inflated, can be pulled downward to establish traction on the main bile duct, which can straighten the approach.
- After superficial wire engagement into the cystic duct, the accessory used can be slowly advanced into the cystic duct to stabilize the catheter and then navigate the valves of Heister to reach the gallbladder lumen.
Use of a sphincterotome, which directs toward the patient’s right (most often direction of cystic duct takeoff), is helpful. Angled guidewires are preferable. We often use a 0.035-inch, 260-cm angled hydrophilic wire (GLIDEWIRE; Terumo, Somerset, NJ) to overcome this challenging portion of ET-GBD.
If despite the above maneuvers the guidewire has failed to enter the cystic duct, cholangioscopy can be used to identify the orifice and/or stabilize deep wire cannulation. This is often cumbersome, time consuming, does not always produce success, and requires additional expertise.
- If a stone is encountered that cannot be extracted or traversed by a guidewire, cholangioscopy with electrohydraulic lithotripsy can be pursued.
- After the guidewire has entered the gallbladder, a 5 French or 7 French plastic double pigtail stent is placed. Typical lengths are 9-15 cm.
Some authors prefer to use two side-by-side plastic stents.12 This has been shown retrospectively to enhance the long term clinical success of ET-GBD but with additional technical difficulty.
- This stent can remain in place indefinitely and need not be exchanged, though it should be removed just prior to CCY if pursued. Alternatively, the surgeon can be alerted to its presence and, if comfortable, it can be removed intraoperatively.
EUS-GBD
- Use of fluoroscopy is optional but can enhance technical success in selected situations.
- Conversion, or internalization, of PC is reasonable and can enhance patient quality of life.13
- If the gallbladder wall is not in close apposition to the duodenal (or gastric) wall, consider measuring the distance.
We preferentially use 10-mm diameter by 10-mm saddle length LAMS for EUS-GBD, unless the above distance warrants use of a 15-mm by 15-mm LAMS (AXIOS, Boston Scientific, Marlborough, MA). If the distance is greater than 15 mm, consider searching for an alternative site, using a traditional biliary fully covered self-expandable metal stent (FCSEMS) for longer length, or converting to ET-GBD. Smaller diameter (8 mm) with an 8-mm saddle length can be used as well. The optimal diameter is unknown and also dependent on whether transluminal endoscopic diagnosis or therapy is a consideration.
- If there is difficulty locating the gallbladder, it may be decompressed or small (particularly if PC or a partial CCY has already been performed).
If a cholecystostomy tube is in place, instillation of sterile water via the tube can sometimes improve the target for LAMS placement, though caution should be made to not over-distend the gallbladder. ERCP with placement of a nasobiliary tube into the gallbladder can also serve this purpose and has been previously described.14
The gallbladder can be punctured with a 19-gauge FNA needle to instill sterile water and distend the gallbladder with the added benefit of being able to pass a guidewire, which may enhance procedural safety in difficult cases. However, success of this technique is contingent on fluid remaining within the gallbladder and not transiting out via the cystic duct. Expedient exchange of the FNA needle for the LAMS device may be necessary.
- Attempt to confirm location within the duodenum prior to puncture, as gastric origins can pose unique ramifications (i.e. potential for partial gastric outlet obstruction, obstruction of LAMS with food debris, etc.).
It can be easy to mistake an unintentional pre-pyloric position for a position within the duodenum since the working channel is behind (proximal to) the echoprobe.
- Turning off Doppler flow prior to advancement of the cautery enhanced LAMS can reduce obscurement of views on entry into the gallbladder. Lack of certainty about entry or misdeployment after presumed entry herald the most challenging aspect of EUS-GBD.
Utilization of a previously placed guidewire or advancement of one preloaded into the LAMS can aid in both enhancing confidence in location and assist with salvage maneuvers, if needed.
- After successful deployment of the LAMS we routinely place a double pigtail plastic stent through it (typically 7 French by 4 cm) to maintain patency. This may also prevent bleeding from the LAMS flange abrading the wall of either lumen.
- We routinely exchange the LAMS for two double pigtail plastic stents (typically 7 French by 4 cm) 4 weeks after initial placement especially when there is a more than modest residual stone burden (data in press). These plastic stents can remain in place indefinitely.
This exchange can be deferred if the patient is not expected to survive until the one-year anniversary of LAMS deployment. After one year the LAMS plastic covering may degrade and pose additional problems.15
LAMS Misdeployment Salvage Tips
- Salvage techniques can vary from simple to complex.
- If a wire is in place, it can be used to balloon or catheter dilate the tract and place a FCSEMS traversing the gallbladder and duodenal/gastric lumens. A similar approach can be used if the LAMS deployed on only one side (gallbladder or duodenum/stomach) and the other flange is within the peritoneum.
- The most challenging scenario to salvage is if the LAMS is misdeployed or becomes dislodged and no wire is present. This is why the use of a guidewire, even if preloaded into the LAMS and placement is freehand, is essential for EUS-GBD. A potential technique is to balloon dilate the duodenal/gastric defect and drive the endoscope into the peritoneum to reconnect that lumen to the gallbladder defect or LAMS, depending on the site of misdeployment. Doing so requires a high degree of commitment and skill and should not be done casually.
- If uncertainty remains or if misdeployment has occurred and salvage attempts have failed, consider closure of the duodenal/gastric defect and conversion to ET-GBD.
This may both treat the initial procedural indication and assist with what is essentially a large bile leak, which might also require percutaneous therapy for non-surgical management.
- For endoscopists with limited experience at salvage techniques, it is reasonable for the threshold for conversion to be low, assuming experience with and confidence in ET-GBD is high.
- If salvage is successful but ambiguity remains, consider obtaining a cholangiogram via the LAMS to confirm positioning and absence of leak.
Adverse Events
Both ET-GBD and EUS-GBD should be performed by an endoscopist comfortable with their techniques and the management of their adverse events (AEs). Rates for EUS-GBD AEs in patients at high risk for LC were reported in one international multicenter registry to be 15.3% with a 30-day mortality of 9.2%, with a significant predictor of AE being endoscopist experience less than 25 procedures.16 A meta-analysis also found an overall AE rate of 18.31%, with rates for perforation and stent related AEs (i.e. migration, occlusion, pneumoperitoneum) being 6.71% and 8.16%, respectively.17 For this reason, we recommend that patients with cholecystitis who are deemed to be poor surgical candidates be transferred to a tertiary referral center with expertise in these approaches. Rates of AEs for ET-GBD are similar to that for standard ERCP, with reported ranges of 5%-10.3%.10
Comparisons Between Techniques
The decision on which technique to utilize for endoscopic management of cholecystitis or symptomatic cholelithiasis depends first and foremost on the expertise and comfort level of the endoscopist. Given the additional training that an advanced endoscopist needs to perform EUS-GBD, combined with the perhaps slightly higher AE rate and permanency of endoscopic cholecystostomy, it is reasonable to proceed with a trial of ET-GBD if confidence is insufficient. However, ET-GBD can certainly be more technically challenging and less effective than EUS-GBD, with lower reported technical and clinical success rates (technical 85.3% vs 93.0%, clinical 95.2% vs 97.3%).18 Despite this, the rate of recurrence of cholecystitis is similar between ET-GBD and EUS-GBD (4.6% vs 4.2%).19 As stated above in the Techniques & Tips section, some authors utilize two plastic stents for ET-GBD for this purpose, though with increased technical difficulty. It is important to remember that these numbers, when paired with AE rates, represent the achievements of expert endoscopists.
Discussion with your surgery team is important when deciding modality. If the patient is felt to be a potential candidate for CCY, and EUS-GBD is not being used as a destination therapy, the surgeon may prefer ET-GBD. EUS-GBD may enhance the difficulty of CCY, though at least one study demonstrated that this was no different than PC with similar rates of conversion from LC to open CCY.20 This conversation is most critical for patients who are potential liver transplant candidates. For patients where this is not a consideration there is some evidence to suggest equivalency between LC and EUS-GBD, though certainly EUS-GBD has not yet supplanted LC as the treatment of choice.21
While there may eventually be a shift towards EUS-GBD instead of LC in certain patient groups, what is clearer are the advantages of EUS-GBD over PC. One recent meta-analysis revealed that EUS-GBD has significantly favorable odds of overall adverse events (OR 0.43, 95% CI 0.18-1.00), shorter hospital stay (2.76 less days, 95% CI 0.31-5.20 less days), reinterventions (OR 0.15, 95% CI 0.02-0.98), and unplanned readmissions (OR 0.14, 95% CI 0.03-0.70) compared to PC.22 Beyond the data, though, are the emotional and psychological impacts an external drain can have on a patient.
Conclusion
When expertise is available, endoscopic treatment of benign gallbladder disease has a definite role but should be undertaken only by those with the experience and skill to safely do so. Decision to proceed, especially with EUS-GBD, should be accompanied by conversation and collaboration with surgical teams. If a patient is under consideration for PC instead of LC, it may be worthwhile to seek consultation with a local center with expertise in EUS-GBD or ET-GBD. The adoption of these techniques is part of the paradigm shift, seen broadly throughout medicine, towards minimally invasive interventions, particularly in advanced endoscopy.
Dr. Gilman (X @a_gilman) and Dr. Baron (X @EndoTx) are with the University of North Carolina, Chapel Hill, Division of Gastroenterology & Hepatology. Dr. Gilman has no relevant financial disclosures. Dr. Baron is a consultant and speaker for Ambu, Boston Scientific, Cook Endoscopy, Medtronic, Olympus America, and W.L. Gore.
References
1. Radder RW. Ultrasonically guided percutaneous catheter drainage for gallbladder empyema. Diagn Imaging. 1980;49:330-333.
2. Kozarek RA. Selective cannulation of the cystic duct at time of ERCP. J Clin Gastroenterol. 1984;6:37-40.
3. Tamada K et al. Efficacy of endoscopic retrograde cholecystoendoprosthesis (ERCCE) for cholecystitis. Endoscopy. 1991;23:2-3.
4. Reynolds W. The first laparoscopic cholecystectomy. JSLS. 2001;5:89-94.
5. Baron TH, Topazian MD. Endoscopic transduodenal drainage of the gallbladder: Implications for endoluminal treatment of gallbladder disease. Gastrointest Endosc. 2007 Apr;65(4):735-7. doi: 10.1016/j.gie.2006.07.041.
6. Irani SS et al. Endoscopic ultrasound-guided transluminal gallbladder drainage in patients with acute cholecystitis: A prospective multicenter trial. Ann Surg. 2023 Sep 1;278(3):e556-e562. doi: 10.1097/SLA.0000000000005784.
7. Shen Y et al. Endoscopic ultrasound-guided cholecystostomy for resection of gallbladder polyps with lumen-apposing metal stent. Medicine (Baltimore). 2020 Oct 23;99(43):e22903. doi: 10.1097/MD.0000000000022903.
8. Pang H et al. Endoscopic ultrasound-guided gallbladder endoscopic mucosal resection: A pilot porcine study. Minim Invasive Ther Allied Technol. 2023 Feb;32(1):24-32. doi: 10.1080/13645706.2022.2153228.
9. Imai H et al. EUS-guided gallbladder drainage for rescue treatment of malignant distal biliary obstruction after unsuccessful ERCP. Gastrointest Endosc. 2016 Jul;84(1):147-51. doi: 10.1016/j.gie.2015.12.024.
10. Saumoy M et al. Endoscopic therapies for gallbladder drainage. Gastrointest Endosc. 2021 Oct;94(4):671-84. doi: 10.1016/j.gie.2021.05.031.
11. Van der Merwe SW et al. Therapeutic endoscopic ultrasound: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy. 2022 Feb;54(2):185-205. doi: 10.1055/a-1717-1391.
12. Storm AC et al. Transpapillary gallbladder stent placement for long-term therapy of acute cholecystitis. Gastrointest Endosc. 2021 Oct;94(4):742-8 e1. doi: 10.1016/j.gie.2021.03.025.
13. James TW, Baron TH. Converting percutaneous gallbladder drainage to internal drainage using EUS-guided therapy: A review of current practices and procedures. Endosc Ultrasound. 2018 Mar-Apr;7(2):93-6. doi: 10.4103/eus.eus_110_17.
14. James TW, Baron TH. Transpapillary nasocystic tube placement to allow gallbladder distention for EUS-guided cholecystoduodenostomy. VideoGIE. 2019 Dec;4(12):561-2. doi: 10.1016/j.vgie.2019.08.009.
15. Gilman AJ, Baron TH. Delamination of a lumen-apposing metal stent with tissue ingrowth and stent-in-stent removal. Gastrointest Endosc. 2023 Sep;98(3):451-3. doi: 10.1016/j.gie.2023.04.2087.
16. Teoh AY et al. Outcomes of an international multicenter registry on EUS-guided gallbladder drainage in patients at high risk for cholecystectomy. Endosc Int Open. 2019 Aug;7(8):E964-E973. doi: 10.1055/a-0915-2098.
17. Kalva NR et al. Efficacy and safety of lumen apposing self-expandable metal stents for EUS guided cholecystostomy: A meta-analysis and systematic review. Can J Gastroenterol Hepatol. 2018;2018:7070961. doi: 10.1155/2018/7070961.
18. Khan MA et al. Efficacy and safety of endoscopic gallbladder drainage in acute cholecystitis: Is it better than percutaneous gallbladder drainage? Gastrointest Endosc. 2017 Jan;85(1):76-87 e3. doi: 10.1016/j.gie.2016.06.032.
19. Mohan BP et al. Endoscopic ultrasound-guided gallbladder drainage, transpapillary drainage, or percutaneous drainage in high risk acute cholecystitis patients: a systematic review and comparative meta-analysis. Endoscopy. 2020 Feb;52(2):96-106. doi: 10.1055/a-1020-3932.
20. Jang JW et al. Endoscopic ultrasound-guided transmural and percutaneous transhepatic gallbladder drainage are comparable for acute cholecystitis. Gastroenterology. 2012 Apr;142(4):805-11. doi: 10.1053/j.gastro.2011.12.051.
21. Teoh AYB et al. EUS-guided gallbladder drainage versus laparoscopic cholecystectomy for acute cholecystitis: a propensity score analysis with 1-year follow-up data. Gastrointest Endosc. 2021 Mar;93(3):577-83. doi: 10.1016/j.gie.2020.06.066.
22. Luk SW et al. Endoscopic ultrasound-guided gallbladder drainage versus percutaneous cholecystostomy for high risk surgical patients with acute cholecystitis: a systematic review and meta-analysis. Endoscopy. 2019 Aug;51(8):722-32. doi: 10.1055/a-0929-6603.
Introduction
The treatment of benign gallbladder disease has changed substantially in the past decade, but this represents only a snapshot in the evolutionary history of the management of this organ. What began as a problem managed exclusively by open cholecystectomy (CCY) transitioned into a race toward minimally invasive approaches in the 1980s, with advances from gastroenterology, surgery, and radiology.
The opening strides were made in 1980 with the first description of percutaneous cholecystostomy (PC) by Dr. R.W. Radder.1 Shortly thereafter, in 1984, Dr. Richard Kozarek first reported the feasibility of selective cystic duct cannulation during endoscopic retrograde cholangiopancreatography (ERCP).2 Subsequent stenting for the treatment of acute cholecystitis (endoscopic transpapillary gallbladder drainage, ET-GBD) was then reported by Tamada et. al. in 1991.3 Not to be outdone, the first laparoscopic cholecystectomy (LC) was completed by Dr. Med Erich Mühe of Germany in 1985.4 More recently, with the expansion of interventional endoscopic ultrasound (EUS), the first transmural EUS-guided gallbladder drainage (EUS-GBD) was described by Dr. Baron and Dr. Topazian in 2007.5
The subsequent advent of lumen apposing metal stents (LAMS) has cemented EUS-GBD in the toolbox of treatment for benign gallbladder disease. Results of a recent prospective multicenter trial, with a Food and Drug Administration–approved protocol and investigational device exemption, have been published, opening the door for the expansion of FDA approved indications for this device.6
Benign gallbladder disease encompasses both polyps (benign and premalignant) and cholecystitis (acute/chronic, calculous/acalculous), in addition to others. The four management techniques (LC, PC, ET-GBD, and EUS-GBD) have filled integral niches in the management of these patients. Even gallbladder polyps have not been able to escape the reach of endoscopic approaches with the recent description of LAMS-assisted polypectomy as part of a gallbladder preserving strategy.7,8 While EUS-GBD also has been used for biliary decompression in the presence of a patent cystic duct and absence of cholecystitis, .9 Both of these techniques have gained wide recognition and/or guideline support for their use from the American Society for Gastrointestinal Endoscopy (ASGE) and the European Society of Gastrointestinal Endoscopy (ESGE).10,11 In addition, there is now one FDA-approved stent device for treatment of acute cholecystitis in patients unfit for surgery.
Techniques & Tips
ET-GBD
- During ERCP, after successful cannulation of the bile duct, attempted wire cannulation of the cystic duct is performed.
A cholangiogram, which clearly delineates the insertion of the cystic duct into the main bile duct, can enhance cannulation success. Rotatable fluoroscopy can facilitate identification.
- After anatomy is clear, wire access is often best achieved using a sphincterotome or stone retrieval (occlusion) balloon.
The balloon, once inflated, can be pulled downward to establish traction on the main bile duct, which can straighten the approach.
- After superficial wire engagement into the cystic duct, the accessory used can be slowly advanced into the cystic duct to stabilize the catheter and then navigate the valves of Heister to reach the gallbladder lumen.
Use of a sphincterotome, which directs toward the patient’s right (most often direction of cystic duct takeoff), is helpful. Angled guidewires are preferable. We often use a 0.035-inch, 260-cm angled hydrophilic wire (GLIDEWIRE; Terumo, Somerset, NJ) to overcome this challenging portion of ET-GBD.
If despite the above maneuvers the guidewire has failed to enter the cystic duct, cholangioscopy can be used to identify the orifice and/or stabilize deep wire cannulation. This is often cumbersome, time consuming, does not always produce success, and requires additional expertise.
- If a stone is encountered that cannot be extracted or traversed by a guidewire, cholangioscopy with electrohydraulic lithotripsy can be pursued.
- After the guidewire has entered the gallbladder, a 5 French or 7 French plastic double pigtail stent is placed. Typical lengths are 9-15 cm.
Some authors prefer to use two side-by-side plastic stents.12 This has been shown retrospectively to enhance the long term clinical success of ET-GBD but with additional technical difficulty.
- This stent can remain in place indefinitely and need not be exchanged, though it should be removed just prior to CCY if pursued. Alternatively, the surgeon can be alerted to its presence and, if comfortable, it can be removed intraoperatively.
EUS-GBD
- Use of fluoroscopy is optional but can enhance technical success in selected situations.
- Conversion, or internalization, of PC is reasonable and can enhance patient quality of life.13
- If the gallbladder wall is not in close apposition to the duodenal (or gastric) wall, consider measuring the distance.
We preferentially use 10-mm diameter by 10-mm saddle length LAMS for EUS-GBD, unless the above distance warrants use of a 15-mm by 15-mm LAMS (AXIOS, Boston Scientific, Marlborough, MA). If the distance is greater than 15 mm, consider searching for an alternative site, using a traditional biliary fully covered self-expandable metal stent (FCSEMS) for longer length, or converting to ET-GBD. Smaller diameter (8 mm) with an 8-mm saddle length can be used as well. The optimal diameter is unknown and also dependent on whether transluminal endoscopic diagnosis or therapy is a consideration.
- If there is difficulty locating the gallbladder, it may be decompressed or small (particularly if PC or a partial CCY has already been performed).
If a cholecystostomy tube is in place, instillation of sterile water via the tube can sometimes improve the target for LAMS placement, though caution should be made to not over-distend the gallbladder. ERCP with placement of a nasobiliary tube into the gallbladder can also serve this purpose and has been previously described.14
The gallbladder can be punctured with a 19-gauge FNA needle to instill sterile water and distend the gallbladder with the added benefit of being able to pass a guidewire, which may enhance procedural safety in difficult cases. However, success of this technique is contingent on fluid remaining within the gallbladder and not transiting out via the cystic duct. Expedient exchange of the FNA needle for the LAMS device may be necessary.
- Attempt to confirm location within the duodenum prior to puncture, as gastric origins can pose unique ramifications (i.e. potential for partial gastric outlet obstruction, obstruction of LAMS with food debris, etc.).
It can be easy to mistake an unintentional pre-pyloric position for a position within the duodenum since the working channel is behind (proximal to) the echoprobe.
- Turning off Doppler flow prior to advancement of the cautery enhanced LAMS can reduce obscurement of views on entry into the gallbladder. Lack of certainty about entry or misdeployment after presumed entry herald the most challenging aspect of EUS-GBD.
Utilization of a previously placed guidewire or advancement of one preloaded into the LAMS can aid in both enhancing confidence in location and assist with salvage maneuvers, if needed.
- After successful deployment of the LAMS we routinely place a double pigtail plastic stent through it (typically 7 French by 4 cm) to maintain patency. This may also prevent bleeding from the LAMS flange abrading the wall of either lumen.
- We routinely exchange the LAMS for two double pigtail plastic stents (typically 7 French by 4 cm) 4 weeks after initial placement especially when there is a more than modest residual stone burden (data in press). These plastic stents can remain in place indefinitely.
This exchange can be deferred if the patient is not expected to survive until the one-year anniversary of LAMS deployment. After one year the LAMS plastic covering may degrade and pose additional problems.15
LAMS Misdeployment Salvage Tips
- Salvage techniques can vary from simple to complex.
- If a wire is in place, it can be used to balloon or catheter dilate the tract and place a FCSEMS traversing the gallbladder and duodenal/gastric lumens. A similar approach can be used if the LAMS deployed on only one side (gallbladder or duodenum/stomach) and the other flange is within the peritoneum.
- The most challenging scenario to salvage is if the LAMS is misdeployed or becomes dislodged and no wire is present. This is why the use of a guidewire, even if preloaded into the LAMS and placement is freehand, is essential for EUS-GBD. A potential technique is to balloon dilate the duodenal/gastric defect and drive the endoscope into the peritoneum to reconnect that lumen to the gallbladder defect or LAMS, depending on the site of misdeployment. Doing so requires a high degree of commitment and skill and should not be done casually.
- If uncertainty remains or if misdeployment has occurred and salvage attempts have failed, consider closure of the duodenal/gastric defect and conversion to ET-GBD.
This may both treat the initial procedural indication and assist with what is essentially a large bile leak, which might also require percutaneous therapy for non-surgical management.
- For endoscopists with limited experience at salvage techniques, it is reasonable for the threshold for conversion to be low, assuming experience with and confidence in ET-GBD is high.
- If salvage is successful but ambiguity remains, consider obtaining a cholangiogram via the LAMS to confirm positioning and absence of leak.
Adverse Events
Both ET-GBD and EUS-GBD should be performed by an endoscopist comfortable with their techniques and the management of their adverse events (AEs). Rates for EUS-GBD AEs in patients at high risk for LC were reported in one international multicenter registry to be 15.3% with a 30-day mortality of 9.2%, with a significant predictor of AE being endoscopist experience less than 25 procedures.16 A meta-analysis also found an overall AE rate of 18.31%, with rates for perforation and stent related AEs (i.e. migration, occlusion, pneumoperitoneum) being 6.71% and 8.16%, respectively.17 For this reason, we recommend that patients with cholecystitis who are deemed to be poor surgical candidates be transferred to a tertiary referral center with expertise in these approaches. Rates of AEs for ET-GBD are similar to that for standard ERCP, with reported ranges of 5%-10.3%.10
Comparisons Between Techniques
The decision on which technique to utilize for endoscopic management of cholecystitis or symptomatic cholelithiasis depends first and foremost on the expertise and comfort level of the endoscopist. Given the additional training that an advanced endoscopist needs to perform EUS-GBD, combined with the perhaps slightly higher AE rate and permanency of endoscopic cholecystostomy, it is reasonable to proceed with a trial of ET-GBD if confidence is insufficient. However, ET-GBD can certainly be more technically challenging and less effective than EUS-GBD, with lower reported technical and clinical success rates (technical 85.3% vs 93.0%, clinical 95.2% vs 97.3%).18 Despite this, the rate of recurrence of cholecystitis is similar between ET-GBD and EUS-GBD (4.6% vs 4.2%).19 As stated above in the Techniques & Tips section, some authors utilize two plastic stents for ET-GBD for this purpose, though with increased technical difficulty. It is important to remember that these numbers, when paired with AE rates, represent the achievements of expert endoscopists.
Discussion with your surgery team is important when deciding modality. If the patient is felt to be a potential candidate for CCY, and EUS-GBD is not being used as a destination therapy, the surgeon may prefer ET-GBD. EUS-GBD may enhance the difficulty of CCY, though at least one study demonstrated that this was no different than PC with similar rates of conversion from LC to open CCY.20 This conversation is most critical for patients who are potential liver transplant candidates. For patients where this is not a consideration there is some evidence to suggest equivalency between LC and EUS-GBD, though certainly EUS-GBD has not yet supplanted LC as the treatment of choice.21
While there may eventually be a shift towards EUS-GBD instead of LC in certain patient groups, what is clearer are the advantages of EUS-GBD over PC. One recent meta-analysis revealed that EUS-GBD has significantly favorable odds of overall adverse events (OR 0.43, 95% CI 0.18-1.00), shorter hospital stay (2.76 less days, 95% CI 0.31-5.20 less days), reinterventions (OR 0.15, 95% CI 0.02-0.98), and unplanned readmissions (OR 0.14, 95% CI 0.03-0.70) compared to PC.22 Beyond the data, though, are the emotional and psychological impacts an external drain can have on a patient.
Conclusion
When expertise is available, endoscopic treatment of benign gallbladder disease has a definite role but should be undertaken only by those with the experience and skill to safely do so. Decision to proceed, especially with EUS-GBD, should be accompanied by conversation and collaboration with surgical teams. If a patient is under consideration for PC instead of LC, it may be worthwhile to seek consultation with a local center with expertise in EUS-GBD or ET-GBD. The adoption of these techniques is part of the paradigm shift, seen broadly throughout medicine, towards minimally invasive interventions, particularly in advanced endoscopy.
Dr. Gilman (X @a_gilman) and Dr. Baron (X @EndoTx) are with the University of North Carolina, Chapel Hill, Division of Gastroenterology & Hepatology. Dr. Gilman has no relevant financial disclosures. Dr. Baron is a consultant and speaker for Ambu, Boston Scientific, Cook Endoscopy, Medtronic, Olympus America, and W.L. Gore.
References
1. Radder RW. Ultrasonically guided percutaneous catheter drainage for gallbladder empyema. Diagn Imaging. 1980;49:330-333.
2. Kozarek RA. Selective cannulation of the cystic duct at time of ERCP. J Clin Gastroenterol. 1984;6:37-40.
3. Tamada K et al. Efficacy of endoscopic retrograde cholecystoendoprosthesis (ERCCE) for cholecystitis. Endoscopy. 1991;23:2-3.
4. Reynolds W. The first laparoscopic cholecystectomy. JSLS. 2001;5:89-94.
5. Baron TH, Topazian MD. Endoscopic transduodenal drainage of the gallbladder: Implications for endoluminal treatment of gallbladder disease. Gastrointest Endosc. 2007 Apr;65(4):735-7. doi: 10.1016/j.gie.2006.07.041.
6. Irani SS et al. Endoscopic ultrasound-guided transluminal gallbladder drainage in patients with acute cholecystitis: A prospective multicenter trial. Ann Surg. 2023 Sep 1;278(3):e556-e562. doi: 10.1097/SLA.0000000000005784.
7. Shen Y et al. Endoscopic ultrasound-guided cholecystostomy for resection of gallbladder polyps with lumen-apposing metal stent. Medicine (Baltimore). 2020 Oct 23;99(43):e22903. doi: 10.1097/MD.0000000000022903.
8. Pang H et al. Endoscopic ultrasound-guided gallbladder endoscopic mucosal resection: A pilot porcine study. Minim Invasive Ther Allied Technol. 2023 Feb;32(1):24-32. doi: 10.1080/13645706.2022.2153228.
9. Imai H et al. EUS-guided gallbladder drainage for rescue treatment of malignant distal biliary obstruction after unsuccessful ERCP. Gastrointest Endosc. 2016 Jul;84(1):147-51. doi: 10.1016/j.gie.2015.12.024.
10. Saumoy M et al. Endoscopic therapies for gallbladder drainage. Gastrointest Endosc. 2021 Oct;94(4):671-84. doi: 10.1016/j.gie.2021.05.031.
11. Van der Merwe SW et al. Therapeutic endoscopic ultrasound: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy. 2022 Feb;54(2):185-205. doi: 10.1055/a-1717-1391.
12. Storm AC et al. Transpapillary gallbladder stent placement for long-term therapy of acute cholecystitis. Gastrointest Endosc. 2021 Oct;94(4):742-8 e1. doi: 10.1016/j.gie.2021.03.025.
13. James TW, Baron TH. Converting percutaneous gallbladder drainage to internal drainage using EUS-guided therapy: A review of current practices and procedures. Endosc Ultrasound. 2018 Mar-Apr;7(2):93-6. doi: 10.4103/eus.eus_110_17.
14. James TW, Baron TH. Transpapillary nasocystic tube placement to allow gallbladder distention for EUS-guided cholecystoduodenostomy. VideoGIE. 2019 Dec;4(12):561-2. doi: 10.1016/j.vgie.2019.08.009.
15. Gilman AJ, Baron TH. Delamination of a lumen-apposing metal stent with tissue ingrowth and stent-in-stent removal. Gastrointest Endosc. 2023 Sep;98(3):451-3. doi: 10.1016/j.gie.2023.04.2087.
16. Teoh AY et al. Outcomes of an international multicenter registry on EUS-guided gallbladder drainage in patients at high risk for cholecystectomy. Endosc Int Open. 2019 Aug;7(8):E964-E973. doi: 10.1055/a-0915-2098.
17. Kalva NR et al. Efficacy and safety of lumen apposing self-expandable metal stents for EUS guided cholecystostomy: A meta-analysis and systematic review. Can J Gastroenterol Hepatol. 2018;2018:7070961. doi: 10.1155/2018/7070961.
18. Khan MA et al. Efficacy and safety of endoscopic gallbladder drainage in acute cholecystitis: Is it better than percutaneous gallbladder drainage? Gastrointest Endosc. 2017 Jan;85(1):76-87 e3. doi: 10.1016/j.gie.2016.06.032.
19. Mohan BP et al. Endoscopic ultrasound-guided gallbladder drainage, transpapillary drainage, or percutaneous drainage in high risk acute cholecystitis patients: a systematic review and comparative meta-analysis. Endoscopy. 2020 Feb;52(2):96-106. doi: 10.1055/a-1020-3932.
20. Jang JW et al. Endoscopic ultrasound-guided transmural and percutaneous transhepatic gallbladder drainage are comparable for acute cholecystitis. Gastroenterology. 2012 Apr;142(4):805-11. doi: 10.1053/j.gastro.2011.12.051.
21. Teoh AYB et al. EUS-guided gallbladder drainage versus laparoscopic cholecystectomy for acute cholecystitis: a propensity score analysis with 1-year follow-up data. Gastrointest Endosc. 2021 Mar;93(3):577-83. doi: 10.1016/j.gie.2020.06.066.
22. Luk SW et al. Endoscopic ultrasound-guided gallbladder drainage versus percutaneous cholecystostomy for high risk surgical patients with acute cholecystitis: a systematic review and meta-analysis. Endoscopy. 2019 Aug;51(8):722-32. doi: 10.1055/a-0929-6603.
February 2024 – ICYMI
Gastroenterology
October 2023
El-Salhy M et al. Efficacy of Fecal Microbiota Transplantation for Patients With Irritable Bowel Syndrome at 3 Years After Transplantation. Gastroenterology. 2022 Oct;163(4):982-994.e14. doi: 10.1053/j.gastro.2022.06.020. Epub 2022 Jun 14. PMID: 35709830.
Bajaj JS and Nagy LE. Natural History of Alcohol-Associated Liver Disease: Understanding the Changing Landscape of Pathophysiology and Patient Care. Gastroenterology. 2022 Oct;163(4):840-851. doi: 10.1053/j.gastro.2022.05.031. Epub 2022 May 19. PMID: 35598629; PMCID: PMC9509416.
Lo CH et al. Association of Proton Pump Inhibitor Use With All-Cause and Cause-Specific Mortality. Gastroenterology. 2022 Oct;163(4):852-861.e2. doi: 10.1053/j.gastro.2022.06.067. Epub 2022 Jul 1. PMID: 35788344; PMCID: PMC9509450.
November 2023
Khoshiwal AM et al. The Tissue Systems Pathology Test Outperforms Pathology Review in Risk Stratifying Patients With Low-Grade Dysplasia. Gastroenterology. 2023 Nov;165(5):1168-1179.e6. doi: 10.1053/j.gastro.2023.07.029. Epub 2023 Aug 30. PMID: 37657759.
Chen YI et al. Endoscopic Ultrasound-Guided Biliary Drainage of First Intent With a Lumen-Apposing Metal Stent vs Endoscopic Retrograde Cholangiopancreatography in Malignant Distal Biliary Obstruction: A Multicenter Randomized Controlled Study (ELEMENT Trial). Gastroenterology. 2023 Nov;165(5):1249-1261.e5. doi: 10.1053/j.gastro.2023.07.024. Epub 2023 Aug 6. PMID: 37549753.
December 2023
Almario CV et al. Prevalence and Burden of Illness of Rome IV Irritable Bowel Syndrome in the United States: Results From a Nationwide Cross-Sectional Study. Gastroenterology. 2023 Dec;165(6):1475-1487. doi: 10.1053/j.gastro.2023.08.010. Epub 2023 Aug 16. PMID: 37595647.
Koopmann BDM et al. The Natural Disease Course of Pancreatic Cyst-Associated Neoplasia, Dysplasia, and Ductal Adenocarcinoma: Results of a Microsimulation Model. Gastroenterology. 2023 Dec;165(6):1522-1532. doi: 10.1053/j.gastro.2023.08.027. Epub 2023 Aug 24. PMID: 37633497.
Clinical Gastroenterology and Hepatology
October 2023
Jung DH et al. Comparison of a Polysaccharide Hemostatic Powder and Conventional Therapy for Peptic Ulcer Bleeding. Clin Gastroenterol Hepatol. 2023 Oct;21(11):2844-2253.e5. doi: 10.1016/j.cgh.2023.02.031. Epub 2023 Mar 10. PMID: 36906081.
Liang PS et al. Blood Test Increases Colorectal Cancer Screening in Persons Who Declined Colonoscopy and Fecal Immunochemical Test: A Randomized Controlled Trial. Clin Gastroenterol Hepatol. 2023 Oct;21(11):2951-2957.e2. doi: 10.1016/j.cgh.2023.03.036. Epub 2023 Apr 8. PMID: 37037262; PMCID: PMC10523873.
November 2023
Li YK et al. Risk of Postcolonoscopy Thromboembolic Events: A Real-World Cohort Study. Clin Gastroenterol Hepatol. 2023 Nov;21(12):3051-3059.e4. doi: 10.1016/j.cgh.2022.09.021. Epub 2022 Sep 24. PMID: 36167228.
Tome J et al. Bile Acid Sequestrants in Microscopic Colitis: Clinical Outcomes and Utility of Bile Acid Testing. Clin Gastroenterol Hepatol. 2023 Nov;21(12):3125-3131.e2. doi: 10.1016/j.cgh.2023.04.031. Epub 2023 May 10. PMID: 37172800.
Berry SK et al. A Randomized Parallel-group Study of Digital Gut-directed Hypnotherapy vs Muscle Relaxation for Irritable Bowel Syndrome. Clin Gastroenterol Hepatol. 2023 Nov;21(12):3152-3159.e2. doi: 10.1016/j.cgh.2023.06.015. Epub 2023 Jun 28. PMID: 37391055.
December 2023
Kanwal F et al. Risk Stratification Model for Hepatocellular Cancer in Patients With Cirrhosis. Clin Gastroenterol Hepatol. 2023 Dec;21(13):3296-3304.e3. doi: 10.1016/j.cgh.2023.04.019. Epub 2023 Apr 30. PMID: 37390101; PMCID: PMC10661677.
Forss A et al. Patients With Microscopic Colitis Are at Higher Risk of Major Adverse Cardiovascular Events: A Matched Cohort Study. Clin Gastroenterol Hepatol. 2023 Dec;21(13):3356-3364.e9. doi: 10.1016/j.cgh.2023.05.014. Epub 2023 May 26. PMID: 37245713.
Zheng T et al. A Randomized, Controlled Trial of Efficacy and Safety of Cannabidiol in Idiopathic and Diabetic Gastroparesis. Clin Gastroenterol Hepatol. 2023 Dec;21(13):3405-3414.e4. doi: 10.1016/j.cgh.2023.07.008. Epub 2023 Jul 22. PMID: 37482172.
Techniques and Innovations in Gastrointestinal Endoscopy
Rengarajan A and Aadam A. Peroral Endoscopic Myotomy (POEM) and Its Use in Esophageal Dysmotility. Tech Innov Gastrointest Endosc. 2023 Dec 16. doi: 10.1016/j.tige.2023.12.004.
Wang D et al. Sphincterotomy vs Sham Procedure for Pain Relief in Sphincter of Oddi Dysfunction: Systematic Review and Meta-analysis. Tech Innov Gastrointest Endosc. 2023 Nov 7. doi: 10.1016/j.tige.2023.10.003
Gastro Hep Advances
Gregory MH et al. Short Bowel Syndrome: Transition of Pediatric Patients to Adult Gastroenterology Care. Gastro Hep Advances. 2023 Sep 8. doi: 10.1016/j.gastha.2023.09.006.
Viser AC et al. Inflammatory Bowel Disease Patients in the Ambulatory Setting Commonly Screen Positive for Malnutrition. Gastro Hep Advances. 2023 Nov 16. doi: 10.1016/j.gastha.2023.11.007.
Gastroenterology
October 2023
El-Salhy M et al. Efficacy of Fecal Microbiota Transplantation for Patients With Irritable Bowel Syndrome at 3 Years After Transplantation. Gastroenterology. 2022 Oct;163(4):982-994.e14. doi: 10.1053/j.gastro.2022.06.020. Epub 2022 Jun 14. PMID: 35709830.
Bajaj JS and Nagy LE. Natural History of Alcohol-Associated Liver Disease: Understanding the Changing Landscape of Pathophysiology and Patient Care. Gastroenterology. 2022 Oct;163(4):840-851. doi: 10.1053/j.gastro.2022.05.031. Epub 2022 May 19. PMID: 35598629; PMCID: PMC9509416.
Lo CH et al. Association of Proton Pump Inhibitor Use With All-Cause and Cause-Specific Mortality. Gastroenterology. 2022 Oct;163(4):852-861.e2. doi: 10.1053/j.gastro.2022.06.067. Epub 2022 Jul 1. PMID: 35788344; PMCID: PMC9509450.
November 2023
Khoshiwal AM et al. The Tissue Systems Pathology Test Outperforms Pathology Review in Risk Stratifying Patients With Low-Grade Dysplasia. Gastroenterology. 2023 Nov;165(5):1168-1179.e6. doi: 10.1053/j.gastro.2023.07.029. Epub 2023 Aug 30. PMID: 37657759.
Chen YI et al. Endoscopic Ultrasound-Guided Biliary Drainage of First Intent With a Lumen-Apposing Metal Stent vs Endoscopic Retrograde Cholangiopancreatography in Malignant Distal Biliary Obstruction: A Multicenter Randomized Controlled Study (ELEMENT Trial). Gastroenterology. 2023 Nov;165(5):1249-1261.e5. doi: 10.1053/j.gastro.2023.07.024. Epub 2023 Aug 6. PMID: 37549753.
December 2023
Almario CV et al. Prevalence and Burden of Illness of Rome IV Irritable Bowel Syndrome in the United States: Results From a Nationwide Cross-Sectional Study. Gastroenterology. 2023 Dec;165(6):1475-1487. doi: 10.1053/j.gastro.2023.08.010. Epub 2023 Aug 16. PMID: 37595647.
Koopmann BDM et al. The Natural Disease Course of Pancreatic Cyst-Associated Neoplasia, Dysplasia, and Ductal Adenocarcinoma: Results of a Microsimulation Model. Gastroenterology. 2023 Dec;165(6):1522-1532. doi: 10.1053/j.gastro.2023.08.027. Epub 2023 Aug 24. PMID: 37633497.
Clinical Gastroenterology and Hepatology
October 2023
Jung DH et al. Comparison of a Polysaccharide Hemostatic Powder and Conventional Therapy for Peptic Ulcer Bleeding. Clin Gastroenterol Hepatol. 2023 Oct;21(11):2844-2253.e5. doi: 10.1016/j.cgh.2023.02.031. Epub 2023 Mar 10. PMID: 36906081.
Liang PS et al. Blood Test Increases Colorectal Cancer Screening in Persons Who Declined Colonoscopy and Fecal Immunochemical Test: A Randomized Controlled Trial. Clin Gastroenterol Hepatol. 2023 Oct;21(11):2951-2957.e2. doi: 10.1016/j.cgh.2023.03.036. Epub 2023 Apr 8. PMID: 37037262; PMCID: PMC10523873.
November 2023
Li YK et al. Risk of Postcolonoscopy Thromboembolic Events: A Real-World Cohort Study. Clin Gastroenterol Hepatol. 2023 Nov;21(12):3051-3059.e4. doi: 10.1016/j.cgh.2022.09.021. Epub 2022 Sep 24. PMID: 36167228.
Tome J et al. Bile Acid Sequestrants in Microscopic Colitis: Clinical Outcomes and Utility of Bile Acid Testing. Clin Gastroenterol Hepatol. 2023 Nov;21(12):3125-3131.e2. doi: 10.1016/j.cgh.2023.04.031. Epub 2023 May 10. PMID: 37172800.
Berry SK et al. A Randomized Parallel-group Study of Digital Gut-directed Hypnotherapy vs Muscle Relaxation for Irritable Bowel Syndrome. Clin Gastroenterol Hepatol. 2023 Nov;21(12):3152-3159.e2. doi: 10.1016/j.cgh.2023.06.015. Epub 2023 Jun 28. PMID: 37391055.
December 2023
Kanwal F et al. Risk Stratification Model for Hepatocellular Cancer in Patients With Cirrhosis. Clin Gastroenterol Hepatol. 2023 Dec;21(13):3296-3304.e3. doi: 10.1016/j.cgh.2023.04.019. Epub 2023 Apr 30. PMID: 37390101; PMCID: PMC10661677.
Forss A et al. Patients With Microscopic Colitis Are at Higher Risk of Major Adverse Cardiovascular Events: A Matched Cohort Study. Clin Gastroenterol Hepatol. 2023 Dec;21(13):3356-3364.e9. doi: 10.1016/j.cgh.2023.05.014. Epub 2023 May 26. PMID: 37245713.
Zheng T et al. A Randomized, Controlled Trial of Efficacy and Safety of Cannabidiol in Idiopathic and Diabetic Gastroparesis. Clin Gastroenterol Hepatol. 2023 Dec;21(13):3405-3414.e4. doi: 10.1016/j.cgh.2023.07.008. Epub 2023 Jul 22. PMID: 37482172.
Techniques and Innovations in Gastrointestinal Endoscopy
Rengarajan A and Aadam A. Peroral Endoscopic Myotomy (POEM) and Its Use in Esophageal Dysmotility. Tech Innov Gastrointest Endosc. 2023 Dec 16. doi: 10.1016/j.tige.2023.12.004.
Wang D et al. Sphincterotomy vs Sham Procedure for Pain Relief in Sphincter of Oddi Dysfunction: Systematic Review and Meta-analysis. Tech Innov Gastrointest Endosc. 2023 Nov 7. doi: 10.1016/j.tige.2023.10.003
Gastro Hep Advances
Gregory MH et al. Short Bowel Syndrome: Transition of Pediatric Patients to Adult Gastroenterology Care. Gastro Hep Advances. 2023 Sep 8. doi: 10.1016/j.gastha.2023.09.006.
Viser AC et al. Inflammatory Bowel Disease Patients in the Ambulatory Setting Commonly Screen Positive for Malnutrition. Gastro Hep Advances. 2023 Nov 16. doi: 10.1016/j.gastha.2023.11.007.
Gastroenterology
October 2023
El-Salhy M et al. Efficacy of Fecal Microbiota Transplantation for Patients With Irritable Bowel Syndrome at 3 Years After Transplantation. Gastroenterology. 2022 Oct;163(4):982-994.e14. doi: 10.1053/j.gastro.2022.06.020. Epub 2022 Jun 14. PMID: 35709830.
Bajaj JS and Nagy LE. Natural History of Alcohol-Associated Liver Disease: Understanding the Changing Landscape of Pathophysiology and Patient Care. Gastroenterology. 2022 Oct;163(4):840-851. doi: 10.1053/j.gastro.2022.05.031. Epub 2022 May 19. PMID: 35598629; PMCID: PMC9509416.
Lo CH et al. Association of Proton Pump Inhibitor Use With All-Cause and Cause-Specific Mortality. Gastroenterology. 2022 Oct;163(4):852-861.e2. doi: 10.1053/j.gastro.2022.06.067. Epub 2022 Jul 1. PMID: 35788344; PMCID: PMC9509450.
November 2023
Khoshiwal AM et al. The Tissue Systems Pathology Test Outperforms Pathology Review in Risk Stratifying Patients With Low-Grade Dysplasia. Gastroenterology. 2023 Nov;165(5):1168-1179.e6. doi: 10.1053/j.gastro.2023.07.029. Epub 2023 Aug 30. PMID: 37657759.
Chen YI et al. Endoscopic Ultrasound-Guided Biliary Drainage of First Intent With a Lumen-Apposing Metal Stent vs Endoscopic Retrograde Cholangiopancreatography in Malignant Distal Biliary Obstruction: A Multicenter Randomized Controlled Study (ELEMENT Trial). Gastroenterology. 2023 Nov;165(5):1249-1261.e5. doi: 10.1053/j.gastro.2023.07.024. Epub 2023 Aug 6. PMID: 37549753.
December 2023
Almario CV et al. Prevalence and Burden of Illness of Rome IV Irritable Bowel Syndrome in the United States: Results From a Nationwide Cross-Sectional Study. Gastroenterology. 2023 Dec;165(6):1475-1487. doi: 10.1053/j.gastro.2023.08.010. Epub 2023 Aug 16. PMID: 37595647.
Koopmann BDM et al. The Natural Disease Course of Pancreatic Cyst-Associated Neoplasia, Dysplasia, and Ductal Adenocarcinoma: Results of a Microsimulation Model. Gastroenterology. 2023 Dec;165(6):1522-1532. doi: 10.1053/j.gastro.2023.08.027. Epub 2023 Aug 24. PMID: 37633497.
Clinical Gastroenterology and Hepatology
October 2023
Jung DH et al. Comparison of a Polysaccharide Hemostatic Powder and Conventional Therapy for Peptic Ulcer Bleeding. Clin Gastroenterol Hepatol. 2023 Oct;21(11):2844-2253.e5. doi: 10.1016/j.cgh.2023.02.031. Epub 2023 Mar 10. PMID: 36906081.
Liang PS et al. Blood Test Increases Colorectal Cancer Screening in Persons Who Declined Colonoscopy and Fecal Immunochemical Test: A Randomized Controlled Trial. Clin Gastroenterol Hepatol. 2023 Oct;21(11):2951-2957.e2. doi: 10.1016/j.cgh.2023.03.036. Epub 2023 Apr 8. PMID: 37037262; PMCID: PMC10523873.
November 2023
Li YK et al. Risk of Postcolonoscopy Thromboembolic Events: A Real-World Cohort Study. Clin Gastroenterol Hepatol. 2023 Nov;21(12):3051-3059.e4. doi: 10.1016/j.cgh.2022.09.021. Epub 2022 Sep 24. PMID: 36167228.
Tome J et al. Bile Acid Sequestrants in Microscopic Colitis: Clinical Outcomes and Utility of Bile Acid Testing. Clin Gastroenterol Hepatol. 2023 Nov;21(12):3125-3131.e2. doi: 10.1016/j.cgh.2023.04.031. Epub 2023 May 10. PMID: 37172800.
Berry SK et al. A Randomized Parallel-group Study of Digital Gut-directed Hypnotherapy vs Muscle Relaxation for Irritable Bowel Syndrome. Clin Gastroenterol Hepatol. 2023 Nov;21(12):3152-3159.e2. doi: 10.1016/j.cgh.2023.06.015. Epub 2023 Jun 28. PMID: 37391055.
December 2023
Kanwal F et al. Risk Stratification Model for Hepatocellular Cancer in Patients With Cirrhosis. Clin Gastroenterol Hepatol. 2023 Dec;21(13):3296-3304.e3. doi: 10.1016/j.cgh.2023.04.019. Epub 2023 Apr 30. PMID: 37390101; PMCID: PMC10661677.
Forss A et al. Patients With Microscopic Colitis Are at Higher Risk of Major Adverse Cardiovascular Events: A Matched Cohort Study. Clin Gastroenterol Hepatol. 2023 Dec;21(13):3356-3364.e9. doi: 10.1016/j.cgh.2023.05.014. Epub 2023 May 26. PMID: 37245713.
Zheng T et al. A Randomized, Controlled Trial of Efficacy and Safety of Cannabidiol in Idiopathic and Diabetic Gastroparesis. Clin Gastroenterol Hepatol. 2023 Dec;21(13):3405-3414.e4. doi: 10.1016/j.cgh.2023.07.008. Epub 2023 Jul 22. PMID: 37482172.
Techniques and Innovations in Gastrointestinal Endoscopy
Rengarajan A and Aadam A. Peroral Endoscopic Myotomy (POEM) and Its Use in Esophageal Dysmotility. Tech Innov Gastrointest Endosc. 2023 Dec 16. doi: 10.1016/j.tige.2023.12.004.
Wang D et al. Sphincterotomy vs Sham Procedure for Pain Relief in Sphincter of Oddi Dysfunction: Systematic Review and Meta-analysis. Tech Innov Gastrointest Endosc. 2023 Nov 7. doi: 10.1016/j.tige.2023.10.003
Gastro Hep Advances
Gregory MH et al. Short Bowel Syndrome: Transition of Pediatric Patients to Adult Gastroenterology Care. Gastro Hep Advances. 2023 Sep 8. doi: 10.1016/j.gastha.2023.09.006.
Viser AC et al. Inflammatory Bowel Disease Patients in the Ambulatory Setting Commonly Screen Positive for Malnutrition. Gastro Hep Advances. 2023 Nov 16. doi: 10.1016/j.gastha.2023.11.007.
Elevate Your Career: AGA Women in GI Regional Workshops Await
As a woman in a dynamic and ever-changing profession, balancing life as a powerhouse physician or scientist is no easy feat.
Expanded to six workshops in 2024, AGA is pleased to offer regionally curated workshops with distinguished speakers at all experience levels to fuel your professional and personal growth. Participate in candid discussions regarding the distinct challenges you face as a woman navigating the 21st century healthcare environment. Derive inspiration from your community and cultivate meaningful connections that will carry you beyond the workshop.
Join us in-person or virtually, whatever fits into your busy schedule. We are also pleased to offer travel grants of up to $300 (per workshop) to help offset the costs of attending this program for one selected individual per region. The travel grant is to support travel and registration fees for early-career women. Additional details for the Maria Leo-Lieber Travel Award may be found in your confirmation email.
Ready to thrive? Register today to attend one of our first workshops or stay tuned for an additional workshop coming near you.
This program is supported by Janssen.
Midwest Regional Workshop
Saturday, Feb. 24, 2024
8 a.m.-3 p.m. CT
University of Chicago, Gleacher Center, Chicago, IL
Deadline to apply for a travel grant: Feb. 9, 2024 Deadline to register: Feb. 16, 2024
Click here to register.
Western Regional Workshop
Saturday, April 27, 2024
8 a.m.-3 p.m. PT
UCLA Luskin Conference Center, Los Angeles, CA
Meet fellow attendees at our pre-workshop networking event on Friday, Apr. 26 from 8 p.m. to 10:30 p.m.
Deadline to apply for a travel grant: April 12, 2024 Deadline to register: April 19, 2024
Click here to register.
As a woman in a dynamic and ever-changing profession, balancing life as a powerhouse physician or scientist is no easy feat.
Expanded to six workshops in 2024, AGA is pleased to offer regionally curated workshops with distinguished speakers at all experience levels to fuel your professional and personal growth. Participate in candid discussions regarding the distinct challenges you face as a woman navigating the 21st century healthcare environment. Derive inspiration from your community and cultivate meaningful connections that will carry you beyond the workshop.
Join us in-person or virtually, whatever fits into your busy schedule. We are also pleased to offer travel grants of up to $300 (per workshop) to help offset the costs of attending this program for one selected individual per region. The travel grant is to support travel and registration fees for early-career women. Additional details for the Maria Leo-Lieber Travel Award may be found in your confirmation email.
Ready to thrive? Register today to attend one of our first workshops or stay tuned for an additional workshop coming near you.
This program is supported by Janssen.
Midwest Regional Workshop
Saturday, Feb. 24, 2024
8 a.m.-3 p.m. CT
University of Chicago, Gleacher Center, Chicago, IL
Deadline to apply for a travel grant: Feb. 9, 2024 Deadline to register: Feb. 16, 2024
Click here to register.
Western Regional Workshop
Saturday, April 27, 2024
8 a.m.-3 p.m. PT
UCLA Luskin Conference Center, Los Angeles, CA
Meet fellow attendees at our pre-workshop networking event on Friday, Apr. 26 from 8 p.m. to 10:30 p.m.
Deadline to apply for a travel grant: April 12, 2024 Deadline to register: April 19, 2024
Click here to register.
As a woman in a dynamic and ever-changing profession, balancing life as a powerhouse physician or scientist is no easy feat.
Expanded to six workshops in 2024, AGA is pleased to offer regionally curated workshops with distinguished speakers at all experience levels to fuel your professional and personal growth. Participate in candid discussions regarding the distinct challenges you face as a woman navigating the 21st century healthcare environment. Derive inspiration from your community and cultivate meaningful connections that will carry you beyond the workshop.
Join us in-person or virtually, whatever fits into your busy schedule. We are also pleased to offer travel grants of up to $300 (per workshop) to help offset the costs of attending this program for one selected individual per region. The travel grant is to support travel and registration fees for early-career women. Additional details for the Maria Leo-Lieber Travel Award may be found in your confirmation email.
Ready to thrive? Register today to attend one of our first workshops or stay tuned for an additional workshop coming near you.
This program is supported by Janssen.
Midwest Regional Workshop
Saturday, Feb. 24, 2024
8 a.m.-3 p.m. CT
University of Chicago, Gleacher Center, Chicago, IL
Deadline to apply for a travel grant: Feb. 9, 2024 Deadline to register: Feb. 16, 2024
Click here to register.
Western Regional Workshop
Saturday, April 27, 2024
8 a.m.-3 p.m. PT
UCLA Luskin Conference Center, Los Angeles, CA
Meet fellow attendees at our pre-workshop networking event on Friday, Apr. 26 from 8 p.m. to 10:30 p.m.
Deadline to apply for a travel grant: April 12, 2024 Deadline to register: April 19, 2024
Click here to register.
AGA Tech Summit: Bridging the Gap Between Innovation, Industry, and Gastroenterologists
Medicine is transforming at a remarkable pace. It is therefore imperative for the future of the field that physicians understand innovation and collaborate with industry partners. Innovation can be defined as invention, adoption, and diffusion.1 During my training in gastroenterology and advanced fellowships, I learned about multiple endoscopic tools and techniques and became familiar with industry names that I frequently encountered in the endoscopy unit or clinic.
I was nominated to attend the AGA Tech Summit Fellows Program by my advanced endoscopy fellowship program director. A total of 22 fellows from around the United States at various stages of their training and interests in the field of gastroenterology and hepatology were selected for the program through an application process. The program included registration, travel, and accommodations to attend the AGA Tech Summit and Fellows Immersion Day at Medtronic.
The first event in the program was a visit to the Medtronic Santa Clara office, where our initial stop was at the research and development lab. We were introduced to design and biomedical engineers who reviewed with us the extensive testing that devices and endoscopy equipment undergo before coming to the market. These labs have a heavy focus on prototyping and experimentation and exist to promote in-house innovation and inventions.
During the day, we met physicians who shared their journeys on how they developed and advanced their careers in partnership with industry. Our visit also included a session with the business development and strategy manager at Medtronic, who discussed strategy and steps involved in product development — from the inception of an idea, institutional policies, and patents, to industry collaboration, and finally to successful commercialization. During medical school and training, we are focused on appropriately learning and applying medical knowledge to clinical care. The Medtronic Fellows Immersion Day experience offered a different perspective and showed other ways by which clinical knowledge and experience can be used to make an impact, in collaboration with industry and stakeholders. It also highlighted alternative career paths for medical professionals. The evening concluded with a meet and greet with the AGA Center for GI Innovation & Technology (CGIT) members and leadership.
The AGA Tech Summit was unlike any conference I have been to in my 13 years of training in medicine (which included mostly clinically focused scientific meetings). Sessions involved ergonomics, applications of artificial intelligence, advances in imaging, environmental endoscopy, the role of the FDA, and innovations around the world. The audience included but was not limited to industry executives, AGA CGIT leadership, physician innovators, gastroenterologists, venture capitalists, and others. Attendees represented the diversity of our field in terms of organizational structures and backgrounds. This resulted in an opportunity to hear and learn different perspectives about products, emerging technology, and the costs involved for physicians, industry, and patients.
The final session of the summit, the AGA Shark Tank, was perhaps the most intriguing one of all. The session showcased landscape-changing technology to AGA investors and venture capitalists. The participants presented their own pitches and faced the sharks (judges). The winner received additional funding, tailored guidance from the AGA CGIT committee, partnering opportunities with interested parties, and the opportunity to represent AGA Shark Tank at the Digestive Disease Week (DDW).
The AGA Tech Summit Fellows Program is a learning platform that not only helps you find your niche in the world of GI innovation but also equips you with resources and connections to make an impact. It is also a great way to infuse new ideas into your practice or research. As healthcare professionals, we must create a culture where innovation can flourish, and where staff and patients feel empowered to contribute to the innovation process and help make change happen — to me, the AGA Tech Summit is one such avenue.
Reference
1. Kelly CJ and Young AJ. Promoting innovation in healthcare. Future Healthc J. 2017 Jun. doi: 10.7861/futurehosp.4-2-121.
Dr. Umar is Assistant Professor of Medicine, Section of Gastroenterology and Hepatology, Baylor College of Medicine, Houston, Texas, and a staff physician at Michael E. DeBakey VA Medical Center, Houston. Dr. Umar has no relevant financial conflicts and is on X, formerly Twitter, @shifaumarMD.
Medicine is transforming at a remarkable pace. It is therefore imperative for the future of the field that physicians understand innovation and collaborate with industry partners. Innovation can be defined as invention, adoption, and diffusion.1 During my training in gastroenterology and advanced fellowships, I learned about multiple endoscopic tools and techniques and became familiar with industry names that I frequently encountered in the endoscopy unit or clinic.
I was nominated to attend the AGA Tech Summit Fellows Program by my advanced endoscopy fellowship program director. A total of 22 fellows from around the United States at various stages of their training and interests in the field of gastroenterology and hepatology were selected for the program through an application process. The program included registration, travel, and accommodations to attend the AGA Tech Summit and Fellows Immersion Day at Medtronic.
The first event in the program was a visit to the Medtronic Santa Clara office, where our initial stop was at the research and development lab. We were introduced to design and biomedical engineers who reviewed with us the extensive testing that devices and endoscopy equipment undergo before coming to the market. These labs have a heavy focus on prototyping and experimentation and exist to promote in-house innovation and inventions.
During the day, we met physicians who shared their journeys on how they developed and advanced their careers in partnership with industry. Our visit also included a session with the business development and strategy manager at Medtronic, who discussed strategy and steps involved in product development — from the inception of an idea, institutional policies, and patents, to industry collaboration, and finally to successful commercialization. During medical school and training, we are focused on appropriately learning and applying medical knowledge to clinical care. The Medtronic Fellows Immersion Day experience offered a different perspective and showed other ways by which clinical knowledge and experience can be used to make an impact, in collaboration with industry and stakeholders. It also highlighted alternative career paths for medical professionals. The evening concluded with a meet and greet with the AGA Center for GI Innovation & Technology (CGIT) members and leadership.
The AGA Tech Summit was unlike any conference I have been to in my 13 years of training in medicine (which included mostly clinically focused scientific meetings). Sessions involved ergonomics, applications of artificial intelligence, advances in imaging, environmental endoscopy, the role of the FDA, and innovations around the world. The audience included but was not limited to industry executives, AGA CGIT leadership, physician innovators, gastroenterologists, venture capitalists, and others. Attendees represented the diversity of our field in terms of organizational structures and backgrounds. This resulted in an opportunity to hear and learn different perspectives about products, emerging technology, and the costs involved for physicians, industry, and patients.
The final session of the summit, the AGA Shark Tank, was perhaps the most intriguing one of all. The session showcased landscape-changing technology to AGA investors and venture capitalists. The participants presented their own pitches and faced the sharks (judges). The winner received additional funding, tailored guidance from the AGA CGIT committee, partnering opportunities with interested parties, and the opportunity to represent AGA Shark Tank at the Digestive Disease Week (DDW).
The AGA Tech Summit Fellows Program is a learning platform that not only helps you find your niche in the world of GI innovation but also equips you with resources and connections to make an impact. It is also a great way to infuse new ideas into your practice or research. As healthcare professionals, we must create a culture where innovation can flourish, and where staff and patients feel empowered to contribute to the innovation process and help make change happen — to me, the AGA Tech Summit is one such avenue.
Reference
1. Kelly CJ and Young AJ. Promoting innovation in healthcare. Future Healthc J. 2017 Jun. doi: 10.7861/futurehosp.4-2-121.
Dr. Umar is Assistant Professor of Medicine, Section of Gastroenterology and Hepatology, Baylor College of Medicine, Houston, Texas, and a staff physician at Michael E. DeBakey VA Medical Center, Houston. Dr. Umar has no relevant financial conflicts and is on X, formerly Twitter, @shifaumarMD.
Medicine is transforming at a remarkable pace. It is therefore imperative for the future of the field that physicians understand innovation and collaborate with industry partners. Innovation can be defined as invention, adoption, and diffusion.1 During my training in gastroenterology and advanced fellowships, I learned about multiple endoscopic tools and techniques and became familiar with industry names that I frequently encountered in the endoscopy unit or clinic.
I was nominated to attend the AGA Tech Summit Fellows Program by my advanced endoscopy fellowship program director. A total of 22 fellows from around the United States at various stages of their training and interests in the field of gastroenterology and hepatology were selected for the program through an application process. The program included registration, travel, and accommodations to attend the AGA Tech Summit and Fellows Immersion Day at Medtronic.
The first event in the program was a visit to the Medtronic Santa Clara office, where our initial stop was at the research and development lab. We were introduced to design and biomedical engineers who reviewed with us the extensive testing that devices and endoscopy equipment undergo before coming to the market. These labs have a heavy focus on prototyping and experimentation and exist to promote in-house innovation and inventions.
During the day, we met physicians who shared their journeys on how they developed and advanced their careers in partnership with industry. Our visit also included a session with the business development and strategy manager at Medtronic, who discussed strategy and steps involved in product development — from the inception of an idea, institutional policies, and patents, to industry collaboration, and finally to successful commercialization. During medical school and training, we are focused on appropriately learning and applying medical knowledge to clinical care. The Medtronic Fellows Immersion Day experience offered a different perspective and showed other ways by which clinical knowledge and experience can be used to make an impact, in collaboration with industry and stakeholders. It also highlighted alternative career paths for medical professionals. The evening concluded with a meet and greet with the AGA Center for GI Innovation & Technology (CGIT) members and leadership.
The AGA Tech Summit was unlike any conference I have been to in my 13 years of training in medicine (which included mostly clinically focused scientific meetings). Sessions involved ergonomics, applications of artificial intelligence, advances in imaging, environmental endoscopy, the role of the FDA, and innovations around the world. The audience included but was not limited to industry executives, AGA CGIT leadership, physician innovators, gastroenterologists, venture capitalists, and others. Attendees represented the diversity of our field in terms of organizational structures and backgrounds. This resulted in an opportunity to hear and learn different perspectives about products, emerging technology, and the costs involved for physicians, industry, and patients.
The final session of the summit, the AGA Shark Tank, was perhaps the most intriguing one of all. The session showcased landscape-changing technology to AGA investors and venture capitalists. The participants presented their own pitches and faced the sharks (judges). The winner received additional funding, tailored guidance from the AGA CGIT committee, partnering opportunities with interested parties, and the opportunity to represent AGA Shark Tank at the Digestive Disease Week (DDW).
The AGA Tech Summit Fellows Program is a learning platform that not only helps you find your niche in the world of GI innovation but also equips you with resources and connections to make an impact. It is also a great way to infuse new ideas into your practice or research. As healthcare professionals, we must create a culture where innovation can flourish, and where staff and patients feel empowered to contribute to the innovation process and help make change happen — to me, the AGA Tech Summit is one such avenue.
Reference
1. Kelly CJ and Young AJ. Promoting innovation in healthcare. Future Healthc J. 2017 Jun. doi: 10.7861/futurehosp.4-2-121.
Dr. Umar is Assistant Professor of Medicine, Section of Gastroenterology and Hepatology, Baylor College of Medicine, Houston, Texas, and a staff physician at Michael E. DeBakey VA Medical Center, Houston. Dr. Umar has no relevant financial conflicts and is on X, formerly Twitter, @shifaumarMD.
2024 Gut Microbiota for Health World Summit Explores the Clinical Impacts of the Microbiome
Join global experts in-person or online as they gather for the 2024 Gut Microbiota for Health World Summit (GMFH) on March 23-24, 2024, in Washington, DC.
This meeting brings together an international and multidisciplinary community of GI clinicians, dietitians, and researchers to discuss personalized approaches to modifying the gut microbiome to improve health and treat disease.
This year’s program will explore:
- Better health through the gut microbiome.
- Big data and the gut microbiome.
- Human-derived to synthetic communities.
- Bringing new microbiome-based products to market.
Early-career faculty and trainees are encouraged to submit abstracts for presentation during the reception. Five $1,000 abstract prizes are available for top-scoring submissions.
Register here.
Join global experts in-person or online as they gather for the 2024 Gut Microbiota for Health World Summit (GMFH) on March 23-24, 2024, in Washington, DC.
This meeting brings together an international and multidisciplinary community of GI clinicians, dietitians, and researchers to discuss personalized approaches to modifying the gut microbiome to improve health and treat disease.
This year’s program will explore:
- Better health through the gut microbiome.
- Big data and the gut microbiome.
- Human-derived to synthetic communities.
- Bringing new microbiome-based products to market.
Early-career faculty and trainees are encouraged to submit abstracts for presentation during the reception. Five $1,000 abstract prizes are available for top-scoring submissions.
Register here.
Join global experts in-person or online as they gather for the 2024 Gut Microbiota for Health World Summit (GMFH) on March 23-24, 2024, in Washington, DC.
This meeting brings together an international and multidisciplinary community of GI clinicians, dietitians, and researchers to discuss personalized approaches to modifying the gut microbiome to improve health and treat disease.
This year’s program will explore:
- Better health through the gut microbiome.
- Big data and the gut microbiome.
- Human-derived to synthetic communities.
- Bringing new microbiome-based products to market.
Early-career faculty and trainees are encouraged to submit abstracts for presentation during the reception. Five $1,000 abstract prizes are available for top-scoring submissions.
Register here.

















