User login
Legal duty to nonpatients: Driving accidents
Question: Driver D strikes a pedestrian after losing control of his vehicle from insulin-induced hypoglycemia. Both Driver D and pedestrian were seriously injured. Driver D was recently diagnosed with diabetes, and his physician had started him on insulin, but did not warn of driving risks associated with hypoglycemia. The injured pedestrian is a total stranger to both Driver D and his doctor. Given these facts, which one of the following choices is correct?
A. Driver D can sue his doctor for failure to disclose hypoglycemic risk of insulin therapy under the doctrine of informed consent.
B. The pedestrian can sue Driver D for negligent driving.
C. The pedestrian may succeed in suing Driver D’s doctor for failure to warn of hypoglycemia.
D. The pedestrian’s lawsuit against Driver D’s doctor may fail in a jurisdiction that does not recognize a doctor’s legal duty to an unidentifiable, nonpatient third party.
E. All statements above are correct.
Answer: E. This legal duty grows out of the doctor-patient relationship, and is normally owed to the patient and to no one else. However, in limited circumstances, it may be extended to other individuals, so-called third parties, who may be total strangers. Injured nonpatient third parties from driving accidents have successfully sued doctors for failing to warn their patients that their medical conditions and/or medications can adversely affect driving ability.
Vizzoni v. Mulford-Dera is a New Jersey malpractice case that is currently before the state’s appellate court. The issue is whether Dr. Lerner, a psychiatrist, can be found negligent for the death of a bicyclist caused by the psychiatrist’s patient, Ms. Mulford-Dera, whose car struck and killed the cyclist. The decedent’s estate alleged that the physician should have warned the patient of the risks of driving while taking psychotropic medications. Dr. Lerner had been treating Ms. Mulford-Dera for psychological conditions, including major depression, panic disorder, and attention deficit disorder. As part of her treatment, Dr. Lerner prescribed several medications, allegedly without disclosing their potential adverse impact on driving. The trial court granted summary judgment and dismissed the case, ruling that the doctor owed no direct or indirect duty to the victim.
The case is currently on appeal. The AMA has filed an amicus brief in support of Dr. Lerner,1 pointing out that third-party claims had previously been rejected in New Jersey, where the injured victim is not readily identifiable. The brief emphasizes the folly of placing the physician or therapist in the untenable position of serving two potentially competing interests when a physician’s priority should be providing care to the patient. It referenced a similar case in Kansas, where a motorist who had fallen asleep at the wheel struck a bicyclist. The motorist was being treated by a neurologist for a sleep disorder.2 The Kansas Supreme Court held that there was no special relationship between the doctor and the cyclist that would impose a duty to warn the motorist about harming a third party.
Other jurisdictions have likewise rejected attempts at “derivative duties” in automobile accident cases. The Connecticut Supreme Court has ruled3 that doctors are immune from third party traffic accident lawsuits, as such litigation would detract from what’s best for the patient (“a physician’s desire to avoid lawsuits may result in far more restrictive advice than necessary for the patient’s well-being”). In that case, the defendant-gastroenterologist, Dr. Troncale, was treating a patient with hepatic encephalopathy and had not warned of the associated risk of driving. And an Illinois court dismissed a third party’s case against a hospital when one of its physicians fell asleep at the wheel after working excessive hours.4
In contrast, other jurisdictions have found a legal duty for physicians toward nonpatient victims. For example, in McKenzie v. Hawaii Permanente Medical Group,5 a car suddenly veered across five lanes of traffic, striking an 11-year-old girl and crushing her against a cement planter. The driver alleged that the prescription medication, Prazosin, caused him to lose control of the car, and that the treating physician was negligent, first in prescribing an inappropriate type and dose of medication, and second in failing to warn of potential side effects that could affect driving ability. The Hawaii Supreme Court emphasized that the risk of tort liability to an individual physician already discourages negligent prescribing; therefore, a physician does not have a duty to third parties where the alleged negligence involves prescribing decisions, i.e., whether to prescribe medication at all, which medication to prescribe, and what dosage to use. On the other hand, physicians have a duty to their patients to warn of potential adverse effects and this responsibility should therefore extend to third parties. Thus, liability would attach to injuries of innocent third parties as a result of failing to warn of a medication’s effects on driving—unless a reasonable person could be expected to be aware of this risk without the warning.
A foreseeable and unreasonable risk of harm is an important but not the only decisive factor in construing the existence of legal duty. Under some circumstances, the term “special relationship” has been employed based on a consideration of existing social values, customs, and policy considerations. In a Massachusetts case,6 a family physician had failed to warn his patient of the risk of diabetes drugs when operating a vehicle. Some 45 minutes after the patient’s discharge from the hospital, he developed hypoglycemia, losing consciousness and injuring a motorcyclist who then sued the doctor. The court invoked the “special relationship” rationale in ruling that the doctor owed a duty to the motorcyclist for public policy reasons.
Dr. Tan is professor of medicine and former adjunct professor of law at the University of Hawaii. This article is meant to be educational and does not constitute medical, ethical, or legal advice. For additional information, readers may contact the author at siang@hawaii.edu.
References
1. Vizzoni v. Mulford-Dera, In the Superior Court of New Jersey Appellate Division, Docket No. A-001255-18T3.
2. Calwell v. Hassan, 925 P.2d 422, 430 (Kan. 1996).
3. Jarmie v. Troncale, 50 A.3d 802 (Conn. 2012).
4. Brewster v. Rush-Presbyterian-St. Luke’s Med. Ctr., 836 N.E.2d 635 (Ill. Ct. App. 2005).
5. McKenzie v. Hawaii Permanente Medical Group, 47 P.3d 1209 (Haw. 2002).
6. Arsenault v. McConarty, 21 Mass. L. Rptr. 500 (2006).
Question: Driver D strikes a pedestrian after losing control of his vehicle from insulin-induced hypoglycemia. Both Driver D and pedestrian were seriously injured. Driver D was recently diagnosed with diabetes, and his physician had started him on insulin, but did not warn of driving risks associated with hypoglycemia. The injured pedestrian is a total stranger to both Driver D and his doctor. Given these facts, which one of the following choices is correct?
A. Driver D can sue his doctor for failure to disclose hypoglycemic risk of insulin therapy under the doctrine of informed consent.
B. The pedestrian can sue Driver D for negligent driving.
C. The pedestrian may succeed in suing Driver D’s doctor for failure to warn of hypoglycemia.
D. The pedestrian’s lawsuit against Driver D’s doctor may fail in a jurisdiction that does not recognize a doctor’s legal duty to an unidentifiable, nonpatient third party.
E. All statements above are correct.
Answer: E. This legal duty grows out of the doctor-patient relationship, and is normally owed to the patient and to no one else. However, in limited circumstances, it may be extended to other individuals, so-called third parties, who may be total strangers. Injured nonpatient third parties from driving accidents have successfully sued doctors for failing to warn their patients that their medical conditions and/or medications can adversely affect driving ability.
Vizzoni v. Mulford-Dera is a New Jersey malpractice case that is currently before the state’s appellate court. The issue is whether Dr. Lerner, a psychiatrist, can be found negligent for the death of a bicyclist caused by the psychiatrist’s patient, Ms. Mulford-Dera, whose car struck and killed the cyclist. The decedent’s estate alleged that the physician should have warned the patient of the risks of driving while taking psychotropic medications. Dr. Lerner had been treating Ms. Mulford-Dera for psychological conditions, including major depression, panic disorder, and attention deficit disorder. As part of her treatment, Dr. Lerner prescribed several medications, allegedly without disclosing their potential adverse impact on driving. The trial court granted summary judgment and dismissed the case, ruling that the doctor owed no direct or indirect duty to the victim.
The case is currently on appeal. The AMA has filed an amicus brief in support of Dr. Lerner,1 pointing out that third-party claims had previously been rejected in New Jersey, where the injured victim is not readily identifiable. The brief emphasizes the folly of placing the physician or therapist in the untenable position of serving two potentially competing interests when a physician’s priority should be providing care to the patient. It referenced a similar case in Kansas, where a motorist who had fallen asleep at the wheel struck a bicyclist. The motorist was being treated by a neurologist for a sleep disorder.2 The Kansas Supreme Court held that there was no special relationship between the doctor and the cyclist that would impose a duty to warn the motorist about harming a third party.
Other jurisdictions have likewise rejected attempts at “derivative duties” in automobile accident cases. The Connecticut Supreme Court has ruled3 that doctors are immune from third party traffic accident lawsuits, as such litigation would detract from what’s best for the patient (“a physician’s desire to avoid lawsuits may result in far more restrictive advice than necessary for the patient’s well-being”). In that case, the defendant-gastroenterologist, Dr. Troncale, was treating a patient with hepatic encephalopathy and had not warned of the associated risk of driving. And an Illinois court dismissed a third party’s case against a hospital when one of its physicians fell asleep at the wheel after working excessive hours.4
In contrast, other jurisdictions have found a legal duty for physicians toward nonpatient victims. For example, in McKenzie v. Hawaii Permanente Medical Group,5 a car suddenly veered across five lanes of traffic, striking an 11-year-old girl and crushing her against a cement planter. The driver alleged that the prescription medication, Prazosin, caused him to lose control of the car, and that the treating physician was negligent, first in prescribing an inappropriate type and dose of medication, and second in failing to warn of potential side effects that could affect driving ability. The Hawaii Supreme Court emphasized that the risk of tort liability to an individual physician already discourages negligent prescribing; therefore, a physician does not have a duty to third parties where the alleged negligence involves prescribing decisions, i.e., whether to prescribe medication at all, which medication to prescribe, and what dosage to use. On the other hand, physicians have a duty to their patients to warn of potential adverse effects and this responsibility should therefore extend to third parties. Thus, liability would attach to injuries of innocent third parties as a result of failing to warn of a medication’s effects on driving—unless a reasonable person could be expected to be aware of this risk without the warning.
A foreseeable and unreasonable risk of harm is an important but not the only decisive factor in construing the existence of legal duty. Under some circumstances, the term “special relationship” has been employed based on a consideration of existing social values, customs, and policy considerations. In a Massachusetts case,6 a family physician had failed to warn his patient of the risk of diabetes drugs when operating a vehicle. Some 45 minutes after the patient’s discharge from the hospital, he developed hypoglycemia, losing consciousness and injuring a motorcyclist who then sued the doctor. The court invoked the “special relationship” rationale in ruling that the doctor owed a duty to the motorcyclist for public policy reasons.
Dr. Tan is professor of medicine and former adjunct professor of law at the University of Hawaii. This article is meant to be educational and does not constitute medical, ethical, or legal advice. For additional information, readers may contact the author at siang@hawaii.edu.
References
1. Vizzoni v. Mulford-Dera, In the Superior Court of New Jersey Appellate Division, Docket No. A-001255-18T3.
2. Calwell v. Hassan, 925 P.2d 422, 430 (Kan. 1996).
3. Jarmie v. Troncale, 50 A.3d 802 (Conn. 2012).
4. Brewster v. Rush-Presbyterian-St. Luke’s Med. Ctr., 836 N.E.2d 635 (Ill. Ct. App. 2005).
5. McKenzie v. Hawaii Permanente Medical Group, 47 P.3d 1209 (Haw. 2002).
6. Arsenault v. McConarty, 21 Mass. L. Rptr. 500 (2006).
Question: Driver D strikes a pedestrian after losing control of his vehicle from insulin-induced hypoglycemia. Both Driver D and pedestrian were seriously injured. Driver D was recently diagnosed with diabetes, and his physician had started him on insulin, but did not warn of driving risks associated with hypoglycemia. The injured pedestrian is a total stranger to both Driver D and his doctor. Given these facts, which one of the following choices is correct?
A. Driver D can sue his doctor for failure to disclose hypoglycemic risk of insulin therapy under the doctrine of informed consent.
B. The pedestrian can sue Driver D for negligent driving.
C. The pedestrian may succeed in suing Driver D’s doctor for failure to warn of hypoglycemia.
D. The pedestrian’s lawsuit against Driver D’s doctor may fail in a jurisdiction that does not recognize a doctor’s legal duty to an unidentifiable, nonpatient third party.
E. All statements above are correct.
Answer: E. This legal duty grows out of the doctor-patient relationship, and is normally owed to the patient and to no one else. However, in limited circumstances, it may be extended to other individuals, so-called third parties, who may be total strangers. Injured nonpatient third parties from driving accidents have successfully sued doctors for failing to warn their patients that their medical conditions and/or medications can adversely affect driving ability.
Vizzoni v. Mulford-Dera is a New Jersey malpractice case that is currently before the state’s appellate court. The issue is whether Dr. Lerner, a psychiatrist, can be found negligent for the death of a bicyclist caused by the psychiatrist’s patient, Ms. Mulford-Dera, whose car struck and killed the cyclist. The decedent’s estate alleged that the physician should have warned the patient of the risks of driving while taking psychotropic medications. Dr. Lerner had been treating Ms. Mulford-Dera for psychological conditions, including major depression, panic disorder, and attention deficit disorder. As part of her treatment, Dr. Lerner prescribed several medications, allegedly without disclosing their potential adverse impact on driving. The trial court granted summary judgment and dismissed the case, ruling that the doctor owed no direct or indirect duty to the victim.
The case is currently on appeal. The AMA has filed an amicus brief in support of Dr. Lerner,1 pointing out that third-party claims had previously been rejected in New Jersey, where the injured victim is not readily identifiable. The brief emphasizes the folly of placing the physician or therapist in the untenable position of serving two potentially competing interests when a physician’s priority should be providing care to the patient. It referenced a similar case in Kansas, where a motorist who had fallen asleep at the wheel struck a bicyclist. The motorist was being treated by a neurologist for a sleep disorder.2 The Kansas Supreme Court held that there was no special relationship between the doctor and the cyclist that would impose a duty to warn the motorist about harming a third party.
Other jurisdictions have likewise rejected attempts at “derivative duties” in automobile accident cases. The Connecticut Supreme Court has ruled3 that doctors are immune from third party traffic accident lawsuits, as such litigation would detract from what’s best for the patient (“a physician’s desire to avoid lawsuits may result in far more restrictive advice than necessary for the patient’s well-being”). In that case, the defendant-gastroenterologist, Dr. Troncale, was treating a patient with hepatic encephalopathy and had not warned of the associated risk of driving. And an Illinois court dismissed a third party’s case against a hospital when one of its physicians fell asleep at the wheel after working excessive hours.4
In contrast, other jurisdictions have found a legal duty for physicians toward nonpatient victims. For example, in McKenzie v. Hawaii Permanente Medical Group,5 a car suddenly veered across five lanes of traffic, striking an 11-year-old girl and crushing her against a cement planter. The driver alleged that the prescription medication, Prazosin, caused him to lose control of the car, and that the treating physician was negligent, first in prescribing an inappropriate type and dose of medication, and second in failing to warn of potential side effects that could affect driving ability. The Hawaii Supreme Court emphasized that the risk of tort liability to an individual physician already discourages negligent prescribing; therefore, a physician does not have a duty to third parties where the alleged negligence involves prescribing decisions, i.e., whether to prescribe medication at all, which medication to prescribe, and what dosage to use. On the other hand, physicians have a duty to their patients to warn of potential adverse effects and this responsibility should therefore extend to third parties. Thus, liability would attach to injuries of innocent third parties as a result of failing to warn of a medication’s effects on driving—unless a reasonable person could be expected to be aware of this risk without the warning.
A foreseeable and unreasonable risk of harm is an important but not the only decisive factor in construing the existence of legal duty. Under some circumstances, the term “special relationship” has been employed based on a consideration of existing social values, customs, and policy considerations. In a Massachusetts case,6 a family physician had failed to warn his patient of the risk of diabetes drugs when operating a vehicle. Some 45 minutes after the patient’s discharge from the hospital, he developed hypoglycemia, losing consciousness and injuring a motorcyclist who then sued the doctor. The court invoked the “special relationship” rationale in ruling that the doctor owed a duty to the motorcyclist for public policy reasons.
Dr. Tan is professor of medicine and former adjunct professor of law at the University of Hawaii. This article is meant to be educational and does not constitute medical, ethical, or legal advice. For additional information, readers may contact the author at siang@hawaii.edu.
References
1. Vizzoni v. Mulford-Dera, In the Superior Court of New Jersey Appellate Division, Docket No. A-001255-18T3.
2. Calwell v. Hassan, 925 P.2d 422, 430 (Kan. 1996).
3. Jarmie v. Troncale, 50 A.3d 802 (Conn. 2012).
4. Brewster v. Rush-Presbyterian-St. Luke’s Med. Ctr., 836 N.E.2d 635 (Ill. Ct. App. 2005).
5. McKenzie v. Hawaii Permanente Medical Group, 47 P.3d 1209 (Haw. 2002).
6. Arsenault v. McConarty, 21 Mass. L. Rptr. 500 (2006).
Don’t Mix Off-label Use With Off-the-rack Pills
A pregnant woman in Wisconsin received prenatal care from a family practitioner. The patient had hypertension, so at about 38 weeks’ gestation, the decision was made to induce labor.
On May 15, 2012, the family practitioner used misoprostol to induce labor. The patient received 100 mcg vaginally at 12:24
At 1:28
Variable late decelerations occurred at 11:36
Although the family practitioner was present at the bedside at 12:40
The on-call physician accomplished a vacuum delivery at 1:30
The child now has severe cerebral palsy, with gross motor involvement in the arms and legs. He can communicate through augmentative communication devices but cannot actually speak. He will require full-time care for the rest of his life.
Continue to: The defense took the position...
The defense took the position that while the dosage of misoprostol was excessive, the drug was no longer active in the mother’s body, based on its half-life, when the fetal distress occurred.
VERDICT
Four days before trial, the case was settled for $9 million.
COMMENTARY
I suspect many of you have made a pot roast—and at least some of you have used the simple, tried-and-true method of putting the meat into the slow cooker with a packet of onion soup mix. It makes a tasty dinner with minimal effort. But onion soup packets are for making onion soup—not seasoning pot roast. Guess what? You just used that soup mix off-label!
As clinicians, we all use medications for clinical indications that haven’t been specifically authorized by the FDA—and we shouldn’t stop. Off-label prescribing is legal, common, and often supported by the standard of care.
But there is a risk: The pill or tablet prepared by the manufacturer is generally aimed at the intended on-label use, not off-label uses. In this case, misoprostol (brand name, Cytotec) is approved by the FDA for prevention and treatment of gastrointestinal ulcers and peptic ulcer disease. The package insert describes dosing as follows:
The recommended adult oral dose of Cytotec for reducing the risk of NSAID-induced gastric ulcers is 200 mcg four times daily with food. If this dose cannot be tolerated, a dose of 100 mcg can be used.1
Continue to: We should not be shocked...
We should not be shocked, then, that Cytotec is supplied as 100- and 200-mcg round white tablets. However, it is frequently used off label for cervical ripening during labor at a dose of “25 mcg inserted into the posterior vaginal fornix.”2
This brings us to the malpractice trap. While off-label use may be appropriate, off-label uses may not neatly “fit” with the substance prepared by the manufacturer. To be properly administered for cervical ripening, the available tablet of misoprostol must be cut with a pill cutter or razor prior to administration.3 Furthermore, dosage is more accurate if the tablet fragments are individually weighed after cutting.3
In this case, the discrepancy between the pill prepared by the manufacturer (100 mcg) and the dosage needed (25 mcg) appears to have caught the defendant family practitioner off guard. So the take-home message is: Use medications as supported by the standard of care—but when using a drug off label, do not assume the product supplied by the manufacturer is appropriate for use as is.
Another interesting aspect of this case is the defense strategy. Most clinicians are aware that the tort of negligence involves (1) duty, (2) breach, (3) causation, and (4) harm. However, it is more logically consistent to think of the elements in this way: (1) duty, (2) breach, (3) harm, and if harm has occurred, (4) examine causation (ie, the logical connection between breach and harm).
In malpractice cases, attorneys frequently focus on one of these specific elements. In this case, the physician’s duty of care and the harm stemming from cerebral palsy are clearly established. Thus, breach and causation take center stage.
Continue to: The defense lawyers...
The defense lawyers acknowledged there was a breach, noting the dosage was “excessive.” However, they argued that this error didn’t matter because the drug was no longer active in the patient’s body. In other words, there was no causal connection between the inappropriately high dose and the resultant uterine tachysystole and fetal distress. This is a difficult road for several reasons.
First, the chief danger of using misoprostol is uterine hyperstimulation and fetal distress. The defense would have to argue the hyperstimulation and fetal distress were coincidental and unrelated to the misoprostol—which carries a black box warning for these very adverse effects. The plaintiff’s attorney is sure to make a big deal out of the black box warning in front of the jury—noting any reasonable clinician practicing obstetrics should be aware of the risks that come with misoprostol’s use. You can almost hear the argument in summation: “It is so important, they drew a warning box around it.”
Furthermore, making the argument that the misoprostol was not in the mother’s system at the time the fetal distress started would entail dueling expert testimony about pharmacokinetics and bioavailability—concepts that are difficult for lay jurors to understand. Misoprostol has a half-life of about 20 to 40 min when administered orally and about 60 min when administered vaginally.4 We know the mother received the overdose of misoprostol at 12:24
The plaintiff’s team might counter with an expert’s explanation that misoprostol’s bioavailability is increased 2- to 3-fold with vaginal versus oral administration. It would also be observed that compared with oral administration, vaginal administration of misoprostol is associated with a slower increase in plasma concentrations but longer elevations (peaking about 1-2 hours after vaginal administration).5
At best, the defense expert would be able to argue that the serum level likely peaked 1 to 2 hours after administration (1:24-2:24
Continue to: Most jurors would...
Most jurors would take a skeptical view of the defendant’s argument that the negative outcome in this case was coincidental. Some might even be angered by it. This realization likely prompted the defense to settle this case for $9 million.
IN SUMMARY
Onion soup mix makes great soup, but it’s an even better seasoning for pot roast. Similarly, there are pharmacologic agents that are effective for conditions for which they are not formally indicated. Do not withhold judicious off-label use of medications when appropriate. However, be aware that off-label uses may require extra attention, and dosing and administration may not be consistent with the product you have on hand. Don’t hesitate to seek guidance from pharmacy colleagues when you have questions—they are an underutilized resource and are generally happy to share their expertise.
1. Cytotec [package insert]. New York, NY: Pfizer Inc; 2009.
2. Misoprostol: dosing considerations. PDR: Prescribers’ Digital Reference. www.pdr.net/drug-summary/Cytotec-misoprostol-1044#8. Accessed July 29, 2019.
3. Williams MC, Tsibris JC, Davis G, et al. Dose variation that is associated with approximated one-quarter tablet doses of misoprostol. Am J Obstet Gynecol. 2002;187(3):615-619.
4. Yount SM, Lassiter N. The pharmacology of prostaglandins for induction of labor. J Midwifery Womens Health. 2013;58(2):133-144; quiz 238-239.
5. Danielsson KG, Marions L, Rodriguez A, et al. Comparison between oral and vaginal administration of misoprostol on uterine contractility. Obstet Gynecol. 1999;93(2):275-280.
A pregnant woman in Wisconsin received prenatal care from a family practitioner. The patient had hypertension, so at about 38 weeks’ gestation, the decision was made to induce labor.
On May 15, 2012, the family practitioner used misoprostol to induce labor. The patient received 100 mcg vaginally at 12:24
At 1:28
Variable late decelerations occurred at 11:36
Although the family practitioner was present at the bedside at 12:40
The on-call physician accomplished a vacuum delivery at 1:30
The child now has severe cerebral palsy, with gross motor involvement in the arms and legs. He can communicate through augmentative communication devices but cannot actually speak. He will require full-time care for the rest of his life.
Continue to: The defense took the position...
The defense took the position that while the dosage of misoprostol was excessive, the drug was no longer active in the mother’s body, based on its half-life, when the fetal distress occurred.
VERDICT
Four days before trial, the case was settled for $9 million.
COMMENTARY
I suspect many of you have made a pot roast—and at least some of you have used the simple, tried-and-true method of putting the meat into the slow cooker with a packet of onion soup mix. It makes a tasty dinner with minimal effort. But onion soup packets are for making onion soup—not seasoning pot roast. Guess what? You just used that soup mix off-label!
As clinicians, we all use medications for clinical indications that haven’t been specifically authorized by the FDA—and we shouldn’t stop. Off-label prescribing is legal, common, and often supported by the standard of care.
But there is a risk: The pill or tablet prepared by the manufacturer is generally aimed at the intended on-label use, not off-label uses. In this case, misoprostol (brand name, Cytotec) is approved by the FDA for prevention and treatment of gastrointestinal ulcers and peptic ulcer disease. The package insert describes dosing as follows:
The recommended adult oral dose of Cytotec for reducing the risk of NSAID-induced gastric ulcers is 200 mcg four times daily with food. If this dose cannot be tolerated, a dose of 100 mcg can be used.1
Continue to: We should not be shocked...
We should not be shocked, then, that Cytotec is supplied as 100- and 200-mcg round white tablets. However, it is frequently used off label for cervical ripening during labor at a dose of “25 mcg inserted into the posterior vaginal fornix.”2
This brings us to the malpractice trap. While off-label use may be appropriate, off-label uses may not neatly “fit” with the substance prepared by the manufacturer. To be properly administered for cervical ripening, the available tablet of misoprostol must be cut with a pill cutter or razor prior to administration.3 Furthermore, dosage is more accurate if the tablet fragments are individually weighed after cutting.3
In this case, the discrepancy between the pill prepared by the manufacturer (100 mcg) and the dosage needed (25 mcg) appears to have caught the defendant family practitioner off guard. So the take-home message is: Use medications as supported by the standard of care—but when using a drug off label, do not assume the product supplied by the manufacturer is appropriate for use as is.
Another interesting aspect of this case is the defense strategy. Most clinicians are aware that the tort of negligence involves (1) duty, (2) breach, (3) causation, and (4) harm. However, it is more logically consistent to think of the elements in this way: (1) duty, (2) breach, (3) harm, and if harm has occurred, (4) examine causation (ie, the logical connection between breach and harm).
In malpractice cases, attorneys frequently focus on one of these specific elements. In this case, the physician’s duty of care and the harm stemming from cerebral palsy are clearly established. Thus, breach and causation take center stage.
Continue to: The defense lawyers...
The defense lawyers acknowledged there was a breach, noting the dosage was “excessive.” However, they argued that this error didn’t matter because the drug was no longer active in the patient’s body. In other words, there was no causal connection between the inappropriately high dose and the resultant uterine tachysystole and fetal distress. This is a difficult road for several reasons.
First, the chief danger of using misoprostol is uterine hyperstimulation and fetal distress. The defense would have to argue the hyperstimulation and fetal distress were coincidental and unrelated to the misoprostol—which carries a black box warning for these very adverse effects. The plaintiff’s attorney is sure to make a big deal out of the black box warning in front of the jury—noting any reasonable clinician practicing obstetrics should be aware of the risks that come with misoprostol’s use. You can almost hear the argument in summation: “It is so important, they drew a warning box around it.”
Furthermore, making the argument that the misoprostol was not in the mother’s system at the time the fetal distress started would entail dueling expert testimony about pharmacokinetics and bioavailability—concepts that are difficult for lay jurors to understand. Misoprostol has a half-life of about 20 to 40 min when administered orally and about 60 min when administered vaginally.4 We know the mother received the overdose of misoprostol at 12:24
The plaintiff’s team might counter with an expert’s explanation that misoprostol’s bioavailability is increased 2- to 3-fold with vaginal versus oral administration. It would also be observed that compared with oral administration, vaginal administration of misoprostol is associated with a slower increase in plasma concentrations but longer elevations (peaking about 1-2 hours after vaginal administration).5
At best, the defense expert would be able to argue that the serum level likely peaked 1 to 2 hours after administration (1:24-2:24
Continue to: Most jurors would...
Most jurors would take a skeptical view of the defendant’s argument that the negative outcome in this case was coincidental. Some might even be angered by it. This realization likely prompted the defense to settle this case for $9 million.
IN SUMMARY
Onion soup mix makes great soup, but it’s an even better seasoning for pot roast. Similarly, there are pharmacologic agents that are effective for conditions for which they are not formally indicated. Do not withhold judicious off-label use of medications when appropriate. However, be aware that off-label uses may require extra attention, and dosing and administration may not be consistent with the product you have on hand. Don’t hesitate to seek guidance from pharmacy colleagues when you have questions—they are an underutilized resource and are generally happy to share their expertise.
A pregnant woman in Wisconsin received prenatal care from a family practitioner. The patient had hypertension, so at about 38 weeks’ gestation, the decision was made to induce labor.
On May 15, 2012, the family practitioner used misoprostol to induce labor. The patient received 100 mcg vaginally at 12:24
At 1:28
Variable late decelerations occurred at 11:36
Although the family practitioner was present at the bedside at 12:40
The on-call physician accomplished a vacuum delivery at 1:30
The child now has severe cerebral palsy, with gross motor involvement in the arms and legs. He can communicate through augmentative communication devices but cannot actually speak. He will require full-time care for the rest of his life.
Continue to: The defense took the position...
The defense took the position that while the dosage of misoprostol was excessive, the drug was no longer active in the mother’s body, based on its half-life, when the fetal distress occurred.
VERDICT
Four days before trial, the case was settled for $9 million.
COMMENTARY
I suspect many of you have made a pot roast—and at least some of you have used the simple, tried-and-true method of putting the meat into the slow cooker with a packet of onion soup mix. It makes a tasty dinner with minimal effort. But onion soup packets are for making onion soup—not seasoning pot roast. Guess what? You just used that soup mix off-label!
As clinicians, we all use medications for clinical indications that haven’t been specifically authorized by the FDA—and we shouldn’t stop. Off-label prescribing is legal, common, and often supported by the standard of care.
But there is a risk: The pill or tablet prepared by the manufacturer is generally aimed at the intended on-label use, not off-label uses. In this case, misoprostol (brand name, Cytotec) is approved by the FDA for prevention and treatment of gastrointestinal ulcers and peptic ulcer disease. The package insert describes dosing as follows:
The recommended adult oral dose of Cytotec for reducing the risk of NSAID-induced gastric ulcers is 200 mcg four times daily with food. If this dose cannot be tolerated, a dose of 100 mcg can be used.1
Continue to: We should not be shocked...
We should not be shocked, then, that Cytotec is supplied as 100- and 200-mcg round white tablets. However, it is frequently used off label for cervical ripening during labor at a dose of “25 mcg inserted into the posterior vaginal fornix.”2
This brings us to the malpractice trap. While off-label use may be appropriate, off-label uses may not neatly “fit” with the substance prepared by the manufacturer. To be properly administered for cervical ripening, the available tablet of misoprostol must be cut with a pill cutter or razor prior to administration.3 Furthermore, dosage is more accurate if the tablet fragments are individually weighed after cutting.3
In this case, the discrepancy between the pill prepared by the manufacturer (100 mcg) and the dosage needed (25 mcg) appears to have caught the defendant family practitioner off guard. So the take-home message is: Use medications as supported by the standard of care—but when using a drug off label, do not assume the product supplied by the manufacturer is appropriate for use as is.
Another interesting aspect of this case is the defense strategy. Most clinicians are aware that the tort of negligence involves (1) duty, (2) breach, (3) causation, and (4) harm. However, it is more logically consistent to think of the elements in this way: (1) duty, (2) breach, (3) harm, and if harm has occurred, (4) examine causation (ie, the logical connection between breach and harm).
In malpractice cases, attorneys frequently focus on one of these specific elements. In this case, the physician’s duty of care and the harm stemming from cerebral palsy are clearly established. Thus, breach and causation take center stage.
Continue to: The defense lawyers...
The defense lawyers acknowledged there was a breach, noting the dosage was “excessive.” However, they argued that this error didn’t matter because the drug was no longer active in the patient’s body. In other words, there was no causal connection between the inappropriately high dose and the resultant uterine tachysystole and fetal distress. This is a difficult road for several reasons.
First, the chief danger of using misoprostol is uterine hyperstimulation and fetal distress. The defense would have to argue the hyperstimulation and fetal distress were coincidental and unrelated to the misoprostol—which carries a black box warning for these very adverse effects. The plaintiff’s attorney is sure to make a big deal out of the black box warning in front of the jury—noting any reasonable clinician practicing obstetrics should be aware of the risks that come with misoprostol’s use. You can almost hear the argument in summation: “It is so important, they drew a warning box around it.”
Furthermore, making the argument that the misoprostol was not in the mother’s system at the time the fetal distress started would entail dueling expert testimony about pharmacokinetics and bioavailability—concepts that are difficult for lay jurors to understand. Misoprostol has a half-life of about 20 to 40 min when administered orally and about 60 min when administered vaginally.4 We know the mother received the overdose of misoprostol at 12:24
The plaintiff’s team might counter with an expert’s explanation that misoprostol’s bioavailability is increased 2- to 3-fold with vaginal versus oral administration. It would also be observed that compared with oral administration, vaginal administration of misoprostol is associated with a slower increase in plasma concentrations but longer elevations (peaking about 1-2 hours after vaginal administration).5
At best, the defense expert would be able to argue that the serum level likely peaked 1 to 2 hours after administration (1:24-2:24
Continue to: Most jurors would...
Most jurors would take a skeptical view of the defendant’s argument that the negative outcome in this case was coincidental. Some might even be angered by it. This realization likely prompted the defense to settle this case for $9 million.
IN SUMMARY
Onion soup mix makes great soup, but it’s an even better seasoning for pot roast. Similarly, there are pharmacologic agents that are effective for conditions for which they are not formally indicated. Do not withhold judicious off-label use of medications when appropriate. However, be aware that off-label uses may require extra attention, and dosing and administration may not be consistent with the product you have on hand. Don’t hesitate to seek guidance from pharmacy colleagues when you have questions—they are an underutilized resource and are generally happy to share their expertise.
1. Cytotec [package insert]. New York, NY: Pfizer Inc; 2009.
2. Misoprostol: dosing considerations. PDR: Prescribers’ Digital Reference. www.pdr.net/drug-summary/Cytotec-misoprostol-1044#8. Accessed July 29, 2019.
3. Williams MC, Tsibris JC, Davis G, et al. Dose variation that is associated with approximated one-quarter tablet doses of misoprostol. Am J Obstet Gynecol. 2002;187(3):615-619.
4. Yount SM, Lassiter N. The pharmacology of prostaglandins for induction of labor. J Midwifery Womens Health. 2013;58(2):133-144; quiz 238-239.
5. Danielsson KG, Marions L, Rodriguez A, et al. Comparison between oral and vaginal administration of misoprostol on uterine contractility. Obstet Gynecol. 1999;93(2):275-280.
1. Cytotec [package insert]. New York, NY: Pfizer Inc; 2009.
2. Misoprostol: dosing considerations. PDR: Prescribers’ Digital Reference. www.pdr.net/drug-summary/Cytotec-misoprostol-1044#8. Accessed July 29, 2019.
3. Williams MC, Tsibris JC, Davis G, et al. Dose variation that is associated with approximated one-quarter tablet doses of misoprostol. Am J Obstet Gynecol. 2002;187(3):615-619.
4. Yount SM, Lassiter N. The pharmacology of prostaglandins for induction of labor. J Midwifery Womens Health. 2013;58(2):133-144; quiz 238-239.
5. Danielsson KG, Marions L, Rodriguez A, et al. Comparison between oral and vaginal administration of misoprostol on uterine contractility. Obstet Gynecol. 1999;93(2):275-280.
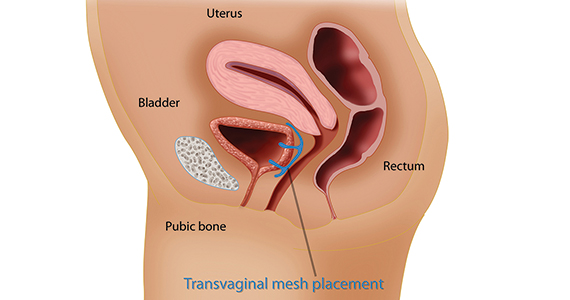
The mesh mess, enmeshed in controversy
CASE Complications with mesh placement for SUI
A 47-year-old woman (G4 P3013) presents 5 months posthysterectomy with evidence of urinary tract infection (UTI). Escherichia coli is isolated, and she responds to antibiotic therapy.
Her surgical history includes a mini-sling procedure using a needleless device and mesh placement in order to correct progressive worsening of loss of urine when coughing and sneezing. She also reported slight pelvic pain, dysuria, and urgency upon urination at that time. After subsequent development of pelvic organ prolapse (POP), she underwent the vaginal hysterectomy.
Following her UTI treatment, a host of problems occur for the patient, including pelvic pain and dyspareunia. Her male partner reports “feeling something during sex,” especially at the anterior vaginal wall. A plain radiograph of the abdomen identifies a 2 cm x 2 cm stone over the vaginal mesh. In consultation with female pelvic medicine and reconstructive surgery subspecialists, lithotripsy is performed, with the stone fragmented. The patient remains symptomatic, however.
The mesh is noted to be eroding through the vaginal wall. An attempt is made to excise the mesh, initially via transuretheral resection, then through a laparoscopic approach. Due to the mesh being embedded in the tissue, however, an open approach is undertaken. Extensive excision of the mesh and stone fragments is performed. Postoperatively, the patient reports “dry vagina,” with no other genitourinary complaints.
The patient sues. She sues the mesh manufacturer. She also seeks to sue the gynecologist who placed the sling and vaginal mesh (as she says she was not informed of “all the risks” of vaginal mesh placement. She is part of a class action lawsuit, along with thousands of other women.
WHAT’S THE VERDICT?
The device manufacturer settled out of court with the class action suit. (The gynecologist was never formally a defendant because the patient/plaintiff was advised to “drop the physician from the suit.”) The attorneys representing the class action received 40% of the award plus presented costs for the representation. The class as a whole received a little more than 50% of the negotiated award. The patient in this case received $60,000.
Medical background
Stress urinary incontinence (SUI) is a prevalent condition; it affects 35% of women.1 Overall, 80% of women aged 80 or younger will undergo some form of surgery for POP during their lifetime.2 The pathophysiology of SUI includes urethral hypermobility and intrinsic sphincter deficiency.3
Surgical correction for urinary incontinence: A timeline
Use of the gracilis muscle flap to surgically correct urinary incontinence was introduced in 1907. This technique has been replaced by today’s more common Burch procedure, which was first described in 1961. Surgical mesh use dates back to the 1950s, when it was primarily used for abdominal hernia repair. Tension-free tape was introduced in 1995.4-6
Continue to: In the late 1990s the US Food and Drug Administration...
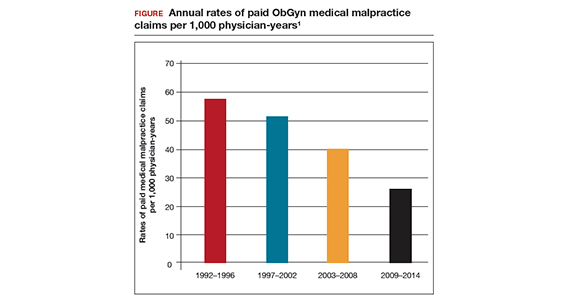
In the late 1990s the US Food and Drug Administration (FDA) permitted use of the first transvaginal meshes, which were designed to treat SUI—the midurethral sling. These mesh slings were so successful that similar meshes were developed to treat POP.7 Almost immediately there were problems with the new POP devices, and 3 years later Boston Scientific recalled its device.8 Nonetheless, the FDA cleared more than 150 devices using surgical mesh for urogynecologic indications (FIGURE).9

Mesh complications
Managing complications from intravesical mesh is a clinically challenging problem. Bladder perforation, stone formation, and penetration through the vagina can occur. Bladder-related complications can manifest as recurrent UTIs and obstructive urinary symptoms, especially in association with stone formation. From the gynecologic perspective, the more common complications with mesh utilization are pelvic pain, groin pain, dyspareunia, contracture and scarring of mesh, and narrowing of the vaginal canal.10 Mesh erosion problems will occur in an estimated 10% to 25% of transvaginal mesh POP implants.11
In 2008, a comparison of transvaginal mesh to native tissue repair (suture-based) or other (biologic) grafts was published.12 The bottom line: there is insufficient evidence to suggest that transvaginal mesh significantly improves outcomes for both posterior and apical defects.
Legal background
Mesh used for surgical purposes is a medical device, which legally is a product—a special product to be sure, but a product nonetheless. Products are subject to product liability rules. Mesh is also subject to an FDA regulatory system. We will briefly discuss products liability and the regulation of devices, both of which have played important roles in mesh-related injuries.
Products liability
As a general matter, defective products subject their manufacturer and seller to liability. There are several legal theories regarding product liability: negligence (in which the defect was caused through carelessness), breach of warranty or guarantee (in addition to express warranties, there are a number of implied warranties for products, including that it is fit for its intended purpose), and strict liability (there was a defect in the product, but it may not have been because of negligence). The product may be defective in the way it was designed, manufactured, or packaged, or it may be defective because adequate instructions and warning were not given to consumers.
Of course, not every product involved in an injury is defective—most automobile accidents, for example, are not the result of any defect in the automobile. In medicine, almost no product (device or pharmaceutical) is entirely safe. In some ways they are unavoidably unsafe and bound to cause some injuries. But when injuries are caused by a defect in the product (design or manufacturing defect or failure to warn), then there may be products liability. Most products liability cases arise under state law.
FDA’s device regulations
Both drugs and medical devices are subject to FDA review and ordinarily require some form of FDA clearance before they may be marketed. In the case of devices, the FDA has 3 classes, with an increase in risk to the user from Class I to III. Various levels of FDA review are required before marketing of the device is permitted, again with the intensity of review increasing from I to III as follows:
- Class I devices pose the least risk, have the least regulation, and are subject to general controls (ie, manufacturing and marketing practices).
- Class II devices pose slightly higher risks and are subject to special controls in addition to the criteria for Class I.
- Class III devices pose the most risk to patients and require premarket approval (scientific review and studies are required to ensure efficacy and safety).13
Continue to: There are a number of limits on manufacturer liability for defective devices...
There are a number of limits on manufacturer liability for defective devices. For Class III devices, the thorough FDA review of the safety of a device may limit the ability of an injured patient to sue based on the state product liability laws.14 For the most part, this “preemption” of state law has not played a major role in mesh litigation because they were initially classified as Class II devices which did not require or include a detailed FDA review.15
The duty to warn of the dangers and risk of medical devices means that manufacturers (or sellers) of devices are obligated to inform health care providers and other medical personnel of the risks. Unlike other manufacturers, device manufacturers do not have to directly warn consumers—because physicians deal directly with patients and prescribe the devices. Therefore, the health care providers, rather than the manufacturers, are obligated to inform the patient.16 This is known as the learned intermediary rule. Manufacturers may still be liable for failure to warn if they do not convey to health care providers proper warnings.
Manufacturers and sellers are not the only entities that may be subject to liability caused by medical devices. Hospitals or other entities that stock and care for devices are responsible for maintaining the safety and functionality of devices in their care.
Health care providers also may be responsible for injuries from medical devices. Generally, that liability is based on negligence. Negligence may relate to selecting an improper device, installing or using it incorrectly, or failing to give the patient adequate information (or informed consent) about the device and alternatives to it.17
A look at the mesh mess
There are a lot of distressing problems and professional disappointments in dissecting the “mesh mess,” including a failure of the FDA to regulate effectively, the extended sale and promotion of intrinsic sphincter deficiency mesh products, the improper use of mesh by physicians even after the risks were known, and, in some instances, the taking advantage of injured patients by attorneys and businesses.18 A lot of finger pointing also has occurred.19 We will recount some of the lowlights of this unfortunate tale.
Continue to: The FDA, in the 1990s, classified the first POP and SUI mesh...
The FDA, in the 1990s, classified the first POP and SUI mesh as Class II after deciding these products were “substantially equivalent” to older surgical meshes. This, of course, proved not to be the case.20 The FDA started receiving thousands of reports of adverse events and, in 2008, warned physicians to be vigilant for adverse events from the mesh. The FDA’s notification recommendations regarding mesh included the following13:
- Obtain specialized training for each mesh implantation technique, and be cognizant of risks.
- Be vigilant for potential adverse events from mesh, including erosion and infection.
- Be observant for complications associated with tools of transvaginal placement (ie, bowel, bladder, and vessel perforation).
- Inform patients that implantation of mesh is permanent and complications may require additional surgery for correction.
- Be aware that complications may affect quality of life—eg, pain with intercourse, scarring, and vaginal wall narrowing (POP repair).
- Provide patients with written copy of patient labeling from the surgical mesh manufacturer.
In 2011, the FDA issued a formal warning to providers that transvaginal mesh posed meaningful risks beyond nonmesh surgery. The FDA’s bulletin draws attention to how the mesh is placed more so than the material per se.19,21 Mesh was a Class II device for sacrocolpopexy or midurethral sling and, similarly, the transvaginal kit was also a Class II device. Overall, use of mesh midurethral slings has been well received as treatment for SUI. The FDA also accepted it for POP, however, but with increasingly strong warnings. The FDA’s 2011 communication stated, “This update is to inform you that serious complications associated with surgical mesh for transvaginal repair of POP are not rare….Furthermore, it is not clear that transvaginal POP repair with mesh is more effective than traditional non-mesh repair in all patients with POP and it may expose patients to greater risk.”7,13
In 2014 the FDA proposed reclassifying mesh to a Class III device, which would require that manufacturers obtain approval, based on safety and effectiveness, before selling mesh. Not until 2016 did the FDA actually reclassify the mess as Class III. Of course, during this time, mesh manufacturers were well aware of the substantial problems the products were causing.13
After serious problems with mesh became well known, and especially after FDA warnings, the use of mesh other than as indicated by the FDA was increasingly risky from a legal (as well as a health) standpoint. As long as mesh was still on the market, of course, it was available for use. But use of mesh for POP procedures without good indications in a way that was contrary to the FDA warnings might well be negligent.
Changes to informed consent
The FDA warnings also should have changed the informed consent for the use of mesh.22 Informed consent commonly consists of the following:
- informing the patient of the proposed procedure
- describing risks (and benefits) of the proposed process
- explaining reasonable alternatives
- noting the risks of taking no action.
Information that is material to a decision should be disclosed. If mesh were going to be used, after the problems of mesh were known and identified by the FDA (other than midurethral slings as treatment of SUI), the risks should have been clearly identified for patients, with alternatives outlined. The American College of Obstetricians and Gynecologists Committee on Ethics has 8 fundamental concepts with regard to informed consent that are worth keeping in mind23:
- Obtaining informed consent for medical treatment and research is an ethical requirement.
- The process expresses respect for the patient as a person.
- It protects patients against unwanted treatment and allows patients’ active involvement in medical planning and care.
- Communication is of paramount importance.
- Informed consent is a process and not a signature on a form.
- A commitment to informed consent and to provision of medical benefit to the patient are linked to provision of care.
- If obtaining informed consent is impossible, a designated surrogate should be identified representing the patient’s best interests.
- Knowledge on the part of the provider regarding state and federal requirements is necessary.
Continue to: Lawsuits line up...
Lawsuits line up
The widespread use of a product with a significant percentage of injuries and eventually with warnings about injuries from use sounds like the formula for a lot of lawsuits. This certainly has happened. A large number of suits—both class actions and individual actions—were filed as a result of mesh injuries.24 These suits were overwhelmingly against the manufacturer, although some included physicians.7 Device makers are more attractive defendants for several reasons. First, they have very deep pockets. In addition, jurors are generally much less sympathetic to large companies than to doctors. Large class actions meant that there were many different patients among the plaintiffs, and medical malpractice claims in most states have a number of trial difficulties not present in other product liability cases. Common defendants have included Johnson & Johnson, Boston Scientific, and Medtronic.
Some of the cases resulted in very large damage awards against manufacturers based on various kinds of product(s) liability. Many other cases were settled or tried with relatively small damages. There were, in addition, a number of instances in which the manufacturers were not liable. Of the 32 plaintiffs who have gone to trial thus far, 24 have obtained verdicts totaling $345 million ($14 million average). The cases that have settled have been for much less—perhaps $60,000 on average. A number of cases remain unresolved. To date, the estimate is that 100,000 women have received almost $8 billion from 7 device manufacturers to resolve claims.25
Some state attorneys general have gotten into the process as well. Attorneys general from California, Kentucky, Mississippi, and Washington have filed lawsuits against Johnson & Johnson, claiming that they deceived doctors and patients about the risks of their pelvic mesh. The states claim that marketing and instructional literature should have contained more information about the risks. Some physicians in these states have expressed concern that these lawsuit risks may do more harm than good because the suits conflate mesh used to treat incontinence with the more risky mesh for POP.26
The “ugly” of class action lawsuits
We have discussed both the sad (the injuries to patients) and the bad (the slow regulatory response and continuing injuries). (The ethics of the marketing by the manufacturers might also be raised as the bad.27) Next, let’s look briefly at the ugly.
Some of the patients affected by mesh injuries have been victimized a second time by medical “lenders” and some of their attorneys. Press reports describe patients with modest awards paying 40% in attorney fees (on the high side for personal injury settlements) plus extravagant costs—leaving modest amounts of actual recovery.25
Worse still, a process of “medical lending” has arisen in mesh cases.28 Medical lenders may contact mesh victims offering to pay up front for surgery to remove mesh, and then place a lien against the settlement for repayment at a much higher rate. They might pay the surgeon $2,500 for the surgery, but place a lien on the settlement amount for $60,000.29,30 In addition, there are allegations that lawyers may recruit the doctors to overstate the injuries or do unnecessary removal surgery because that will likely up the award.31 A quick Google search indicates dozens of offers of cash now for your mesh lawsuit (transvaginal and hernia repair).
The patient in our hypothetical case at the beginning had a fairly typical experience. She was a member of a class filing and received a modest settlement. The attorneys representing the class were allowed by the court to charge substantial attorneys’ fees and costs. The patient had the good sense to avoid medical lenders, although other members of the class did use medical lenders and are now filing complaints about the way they were treated by these lenders.
- Maintain surgical skills and be open to new technology. Medical practice requires constant updating and use of new and improved technology as it comes along. By definition, new technology often requires new skills and understanding. A significant portion of surgeons using mesh indicated that they had not read the instructions for use, or had done so only once.1 CME programs that include surgical education remain of particular value.
- Whether new technology or old, it is essential to keep up to date on all FDA bulletins pertinent to devices and pharmaceuticals that you use and prescribe. For example, in 2016 and 2018 the FDA warned that the use of a very old class of drugs (fluoroquinolones) should be limited. It advised "that the serious side effects associated with fluoroquinolones generally outweigh the benefits for patients with acute sinusitis, acute bronchitis, and uncomplicated urinary tract infections who have other treatment options. For patients with these conditions, fluoroquinolones should be reserved for those who do not have alternative treatment options."2 Continued, unnecessary prescriptions for fluoroquinolones would put a physician at some legal risk whether or not the physician had paid any attention to the warning.
- Informed consent is a very important legal and medical process. Take it seriously, and make sure the patient has the information necessary to make informed decisions about treatment. Document the process and the information provided. In some cases consider directing patients to appropriate literature or websites of the manufacturers.
- As to the use of mesh, if not following FDA advice, it is important to document the reason for this and to document the informed consent especially carefully.
- Follow patients after mesh placement for a minimum of 1 year and emphasize to patients they should convey signs and symptoms of complications from initial placement.3 High-risk patients should be of particular concern and be monitored very closely.
References
- Kirkpatrick G, Faber KD, Fromer DL. Transvaginal mesh placement and the instructions for use: a survey of North American urologists. J Urol. https://doi.org/10.1016/j.urpr.2018.05.004.
- FDA Drug Safety Communication: FDA advises restricting fluoroquinolone antibiotic use for certain uncomplicated infections; warns about disabling side effects that can occur together. July 26, 2016. https://www.fda.gov/Drugs/DrugSafety/ucm500143.htm. Accessed June 19, 2019.
- Karlovsky ME. How to avoid and deal with pelvic mesh litigation. Curr Urol Rep. 2016;17:55.
- Maral I, Ozkardeş H, Peşkircioğlu L, et al. Prevalence of stress urinary incontinence in both sexes at or after age 15 years: a cross-sectional study. J Urol. 2001;165:408-412.
- Olsen AL, Smith VJ, Bergstrom JO, et al. Epidemiology of surgically managed pelvic organ prolapse and urinary incontinence. Obstet Gynecol. 1997;89:501-506.
- Chang J, Lee D. Midurethral slings in the mesh litigation era. Transl Androl Urol. 2017;6(suppl 2): S68-S75.
- Mattingly R, ed. TeLinde's Operative Gynecology, 5th edition. Lippincott, William, and Wilkins: Philadelphia, PA; 1997.
- Burch J. Urethrovaginal fixation to Cooper's ligament for correction of stress incontinence, cystocele, and prolapse. Am J Obstet Gynecol. 1961;81:281-290.
- Ulmsten U, Falconer C, Johnson P, et al. A multicenter study of tension-free vaginal tape (TVT) for surgical treatment of stress urinary incontinence. Int Urogynecol J Pelvic Floor Dysfunct. 1998;9:210-213.
- Kuhlmann-Capek MJ, Kilic GS, Shah AB, et al. Enmeshed in controversy: use of vaginal mesh in the current medicolegal environment. Female Pelvic Med Reconstr Surg. 2015;21:241-243.
- Powell SF. Changing our minds: reforming the FDA medical device reclassification process. Food Drug Law J. 2018;73:177-209.
- US Food and Drug Administration. Surgical Mesh for Treatment of Women with Pelvic Organ Prolapse and Stress Urinary Incontinence. September 2011. https://www.thesenatorsfirm.com/documents/OBS.pdf. Accessed June 19, 2019.
- Maher C, Feiner B, Baessler K, et al. Surgical management of pelvic organ prolapse in women. Cochrane Database Syst Rev. 2013;(4):CD004014.
- Ganj FA, Ibeanu OA, Bedestani A, Nolan TE, Chesson RR. Complications of transvaginal monofilament polypropylene mesh in pelvic organ prolapse repair. Int Urogynecol J Pelvic Floor Dysfunct. 2009;20:919-925.
- Sung VW, Rogers RG, Schaffer JI, et al. Graft use in transvaginal pelvic organ prolapse repair: a systematic review. Obstet Gynecol. 2008;112:1131-1142.
- FDA public health notification: serious complications associated with transvaginal placement of surgical mesh in repair of pelvic organ prolapse and stress urinary incontinence. October 20, 2008. http://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/PublicHealthNotifications/ucm061976.htm. Accessed February 14, 2019.
- Riegel v. Medtronic, 552 U.S. 312 (2008).
- Whitney DW. Guide to preemption of state-law claims against Class III PMA medical devices. Food Drug Law J. 2010;65:113-139.
- Alam P, Iglesia CB. Informed consent for reconstructive pelvic surgery. Obstet Gynecol Clin North Am. 2016;43:131-139.
- Nosti PA, Iglesia CB. Medicolegal issues surrounding devices and mesh for surgical treatment of prolapse and incontinence. Clin Obstet Gynecol. 2013;56:221-228.
- Shepherd CG. Transvaginal mesh litigation: a new opportunity to resolve mass medical device failure claims. Tennessee Law Rev. 2012;80:3:477-94.
- Karlovsky ME. How to avoid and deal with pelvic mesh litigation. Curr Urol Rep. 2016;17:55.
- Cohn JA, Timbrook Brown E, Kowalik CG, et al. The mesh controversy. F1000Research website. https://f1000research.com/articles/5-2423/v1. Accessed June 17, 2019.
- Obstetrics and Gynecology Devices Panel Meeting, February 12, 2019. US Food and Drug Administration website. https://www.fda.gov/media/122867/download. Accessed June 19, 2019.
- Mucowski SJ, Jurnalov C, Phelps JY. Use of vaginal mesh in the face of recent FDA warnings and litigation. Am J Obstet Gynecol. 2010;203:103.e1-e4.
- American College of Obstetricians and Gynecologists Committee on Ethics. ACOG Committee Opinion No. 439: informed consent. Obstet Gynecol. 2009;114(2 pt 1):401-408.
- Souders CP, Eilber KS, McClelland L, et al. The truth behind transvaginal mesh litigation: devices, timelines, and provider characteristics. Female Pelvic Med Reconstr Surg. 2018;24:21-25.
- Goldstein M. As pelvic mesh settlements near $8 billion, women question lawyers' fees. New York Times. February 1, 2019. https://www.nytimes.com/2019/02/01/business/pelvic-mesh-settlements-lawyers.html. Accessed June 19, 2019.
- Johnson G. Surgeons fear pelvic mesh lawsuits will spook patients. Associated Press News. January 10, 2019. https://www.apnews.com/25777c3c33e3489283b1dc2ebdde6b55. Accessed June 19, 2019.
- Clarke RN. Medical device marketing and the ethics of vaginal mesh kit marketing. In The Innovation and Evolution of Medical Devices. New York, NY: Springer; 2019:103-123.
- Top 5 drug and medical device developments of 2018. Law 360. January 1, 2019. Accessed through LexisNexis.
- Frankel A, Dye J. The Lien Machine. New breed of investor profits by financing surgeries for desperate women patients. Reuters. August 18, 2015. https://www.reuters.com/investigates/special-report/usa-litigation-mesh/. Accessed June 19, 2019.
- Sullivan T. New report looks at intersection of "medical lending" and pelvic mesh lawsuits. Policy & Medicine. May 5, 2018. https://www.policymed.com/2015/08/medical-lending-and-pelvic-mesh-litigation.html. Accessed June 19, 2019.
- Goldstein M, Sliver-Greensberg J. How profiteers lure women into often-unneeded surgery. New York Times. April 14, 2018. https://www.nytimes.com/2018/04/14/business/vaginal-mesh-surgery-lawsuits-financing.html. Accessed June 19, 2019.
CASE Complications with mesh placement for SUI
A 47-year-old woman (G4 P3013) presents 5 months posthysterectomy with evidence of urinary tract infection (UTI). Escherichia coli is isolated, and she responds to antibiotic therapy.
Her surgical history includes a mini-sling procedure using a needleless device and mesh placement in order to correct progressive worsening of loss of urine when coughing and sneezing. She also reported slight pelvic pain, dysuria, and urgency upon urination at that time. After subsequent development of pelvic organ prolapse (POP), she underwent the vaginal hysterectomy.
Following her UTI treatment, a host of problems occur for the patient, including pelvic pain and dyspareunia. Her male partner reports “feeling something during sex,” especially at the anterior vaginal wall. A plain radiograph of the abdomen identifies a 2 cm x 2 cm stone over the vaginal mesh. In consultation with female pelvic medicine and reconstructive surgery subspecialists, lithotripsy is performed, with the stone fragmented. The patient remains symptomatic, however.
The mesh is noted to be eroding through the vaginal wall. An attempt is made to excise the mesh, initially via transuretheral resection, then through a laparoscopic approach. Due to the mesh being embedded in the tissue, however, an open approach is undertaken. Extensive excision of the mesh and stone fragments is performed. Postoperatively, the patient reports “dry vagina,” with no other genitourinary complaints.
The patient sues. She sues the mesh manufacturer. She also seeks to sue the gynecologist who placed the sling and vaginal mesh (as she says she was not informed of “all the risks” of vaginal mesh placement. She is part of a class action lawsuit, along with thousands of other women.
WHAT’S THE VERDICT?
The device manufacturer settled out of court with the class action suit. (The gynecologist was never formally a defendant because the patient/plaintiff was advised to “drop the physician from the suit.”) The attorneys representing the class action received 40% of the award plus presented costs for the representation. The class as a whole received a little more than 50% of the negotiated award. The patient in this case received $60,000.
Medical background
Stress urinary incontinence (SUI) is a prevalent condition; it affects 35% of women.1 Overall, 80% of women aged 80 or younger will undergo some form of surgery for POP during their lifetime.2 The pathophysiology of SUI includes urethral hypermobility and intrinsic sphincter deficiency.3
Surgical correction for urinary incontinence: A timeline
Use of the gracilis muscle flap to surgically correct urinary incontinence was introduced in 1907. This technique has been replaced by today’s more common Burch procedure, which was first described in 1961. Surgical mesh use dates back to the 1950s, when it was primarily used for abdominal hernia repair. Tension-free tape was introduced in 1995.4-6
Continue to: In the late 1990s the US Food and Drug Administration...
In the late 1990s the US Food and Drug Administration (FDA) permitted use of the first transvaginal meshes, which were designed to treat SUI—the midurethral sling. These mesh slings were so successful that similar meshes were developed to treat POP.7 Almost immediately there were problems with the new POP devices, and 3 years later Boston Scientific recalled its device.8 Nonetheless, the FDA cleared more than 150 devices using surgical mesh for urogynecologic indications (FIGURE).9

Mesh complications
Managing complications from intravesical mesh is a clinically challenging problem. Bladder perforation, stone formation, and penetration through the vagina can occur. Bladder-related complications can manifest as recurrent UTIs and obstructive urinary symptoms, especially in association with stone formation. From the gynecologic perspective, the more common complications with mesh utilization are pelvic pain, groin pain, dyspareunia, contracture and scarring of mesh, and narrowing of the vaginal canal.10 Mesh erosion problems will occur in an estimated 10% to 25% of transvaginal mesh POP implants.11
In 2008, a comparison of transvaginal mesh to native tissue repair (suture-based) or other (biologic) grafts was published.12 The bottom line: there is insufficient evidence to suggest that transvaginal mesh significantly improves outcomes for both posterior and apical defects.
Legal background
Mesh used for surgical purposes is a medical device, which legally is a product—a special product to be sure, but a product nonetheless. Products are subject to product liability rules. Mesh is also subject to an FDA regulatory system. We will briefly discuss products liability and the regulation of devices, both of which have played important roles in mesh-related injuries.
Products liability
As a general matter, defective products subject their manufacturer and seller to liability. There are several legal theories regarding product liability: negligence (in which the defect was caused through carelessness), breach of warranty or guarantee (in addition to express warranties, there are a number of implied warranties for products, including that it is fit for its intended purpose), and strict liability (there was a defect in the product, but it may not have been because of negligence). The product may be defective in the way it was designed, manufactured, or packaged, or it may be defective because adequate instructions and warning were not given to consumers.
Of course, not every product involved in an injury is defective—most automobile accidents, for example, are not the result of any defect in the automobile. In medicine, almost no product (device or pharmaceutical) is entirely safe. In some ways they are unavoidably unsafe and bound to cause some injuries. But when injuries are caused by a defect in the product (design or manufacturing defect or failure to warn), then there may be products liability. Most products liability cases arise under state law.
FDA’s device regulations
Both drugs and medical devices are subject to FDA review and ordinarily require some form of FDA clearance before they may be marketed. In the case of devices, the FDA has 3 classes, with an increase in risk to the user from Class I to III. Various levels of FDA review are required before marketing of the device is permitted, again with the intensity of review increasing from I to III as follows:
- Class I devices pose the least risk, have the least regulation, and are subject to general controls (ie, manufacturing and marketing practices).
- Class II devices pose slightly higher risks and are subject to special controls in addition to the criteria for Class I.
- Class III devices pose the most risk to patients and require premarket approval (scientific review and studies are required to ensure efficacy and safety).13
Continue to: There are a number of limits on manufacturer liability for defective devices...
There are a number of limits on manufacturer liability for defective devices. For Class III devices, the thorough FDA review of the safety of a device may limit the ability of an injured patient to sue based on the state product liability laws.14 For the most part, this “preemption” of state law has not played a major role in mesh litigation because they were initially classified as Class II devices which did not require or include a detailed FDA review.15
The duty to warn of the dangers and risk of medical devices means that manufacturers (or sellers) of devices are obligated to inform health care providers and other medical personnel of the risks. Unlike other manufacturers, device manufacturers do not have to directly warn consumers—because physicians deal directly with patients and prescribe the devices. Therefore, the health care providers, rather than the manufacturers, are obligated to inform the patient.16 This is known as the learned intermediary rule. Manufacturers may still be liable for failure to warn if they do not convey to health care providers proper warnings.
Manufacturers and sellers are not the only entities that may be subject to liability caused by medical devices. Hospitals or other entities that stock and care for devices are responsible for maintaining the safety and functionality of devices in their care.
Health care providers also may be responsible for injuries from medical devices. Generally, that liability is based on negligence. Negligence may relate to selecting an improper device, installing or using it incorrectly, or failing to give the patient adequate information (or informed consent) about the device and alternatives to it.17
A look at the mesh mess
There are a lot of distressing problems and professional disappointments in dissecting the “mesh mess,” including a failure of the FDA to regulate effectively, the extended sale and promotion of intrinsic sphincter deficiency mesh products, the improper use of mesh by physicians even after the risks were known, and, in some instances, the taking advantage of injured patients by attorneys and businesses.18 A lot of finger pointing also has occurred.19 We will recount some of the lowlights of this unfortunate tale.
Continue to: The FDA, in the 1990s, classified the first POP and SUI mesh...
The FDA, in the 1990s, classified the first POP and SUI mesh as Class II after deciding these products were “substantially equivalent” to older surgical meshes. This, of course, proved not to be the case.20 The FDA started receiving thousands of reports of adverse events and, in 2008, warned physicians to be vigilant for adverse events from the mesh. The FDA’s notification recommendations regarding mesh included the following13:
- Obtain specialized training for each mesh implantation technique, and be cognizant of risks.
- Be vigilant for potential adverse events from mesh, including erosion and infection.
- Be observant for complications associated with tools of transvaginal placement (ie, bowel, bladder, and vessel perforation).
- Inform patients that implantation of mesh is permanent and complications may require additional surgery for correction.
- Be aware that complications may affect quality of life—eg, pain with intercourse, scarring, and vaginal wall narrowing (POP repair).
- Provide patients with written copy of patient labeling from the surgical mesh manufacturer.
In 2011, the FDA issued a formal warning to providers that transvaginal mesh posed meaningful risks beyond nonmesh surgery. The FDA’s bulletin draws attention to how the mesh is placed more so than the material per se.19,21 Mesh was a Class II device for sacrocolpopexy or midurethral sling and, similarly, the transvaginal kit was also a Class II device. Overall, use of mesh midurethral slings has been well received as treatment for SUI. The FDA also accepted it for POP, however, but with increasingly strong warnings. The FDA’s 2011 communication stated, “This update is to inform you that serious complications associated with surgical mesh for transvaginal repair of POP are not rare….Furthermore, it is not clear that transvaginal POP repair with mesh is more effective than traditional non-mesh repair in all patients with POP and it may expose patients to greater risk.”7,13
In 2014 the FDA proposed reclassifying mesh to a Class III device, which would require that manufacturers obtain approval, based on safety and effectiveness, before selling mesh. Not until 2016 did the FDA actually reclassify the mess as Class III. Of course, during this time, mesh manufacturers were well aware of the substantial problems the products were causing.13
After serious problems with mesh became well known, and especially after FDA warnings, the use of mesh other than as indicated by the FDA was increasingly risky from a legal (as well as a health) standpoint. As long as mesh was still on the market, of course, it was available for use. But use of mesh for POP procedures without good indications in a way that was contrary to the FDA warnings might well be negligent.
Changes to informed consent
The FDA warnings also should have changed the informed consent for the use of mesh.22 Informed consent commonly consists of the following:
- informing the patient of the proposed procedure
- describing risks (and benefits) of the proposed process
- explaining reasonable alternatives
- noting the risks of taking no action.
Information that is material to a decision should be disclosed. If mesh were going to be used, after the problems of mesh were known and identified by the FDA (other than midurethral slings as treatment of SUI), the risks should have been clearly identified for patients, with alternatives outlined. The American College of Obstetricians and Gynecologists Committee on Ethics has 8 fundamental concepts with regard to informed consent that are worth keeping in mind23:
- Obtaining informed consent for medical treatment and research is an ethical requirement.
- The process expresses respect for the patient as a person.
- It protects patients against unwanted treatment and allows patients’ active involvement in medical planning and care.
- Communication is of paramount importance.
- Informed consent is a process and not a signature on a form.
- A commitment to informed consent and to provision of medical benefit to the patient are linked to provision of care.
- If obtaining informed consent is impossible, a designated surrogate should be identified representing the patient’s best interests.
- Knowledge on the part of the provider regarding state and federal requirements is necessary.
Continue to: Lawsuits line up...
Lawsuits line up
The widespread use of a product with a significant percentage of injuries and eventually with warnings about injuries from use sounds like the formula for a lot of lawsuits. This certainly has happened. A large number of suits—both class actions and individual actions—were filed as a result of mesh injuries.24 These suits were overwhelmingly against the manufacturer, although some included physicians.7 Device makers are more attractive defendants for several reasons. First, they have very deep pockets. In addition, jurors are generally much less sympathetic to large companies than to doctors. Large class actions meant that there were many different patients among the plaintiffs, and medical malpractice claims in most states have a number of trial difficulties not present in other product liability cases. Common defendants have included Johnson & Johnson, Boston Scientific, and Medtronic.
Some of the cases resulted in very large damage awards against manufacturers based on various kinds of product(s) liability. Many other cases were settled or tried with relatively small damages. There were, in addition, a number of instances in which the manufacturers were not liable. Of the 32 plaintiffs who have gone to trial thus far, 24 have obtained verdicts totaling $345 million ($14 million average). The cases that have settled have been for much less—perhaps $60,000 on average. A number of cases remain unresolved. To date, the estimate is that 100,000 women have received almost $8 billion from 7 device manufacturers to resolve claims.25
Some state attorneys general have gotten into the process as well. Attorneys general from California, Kentucky, Mississippi, and Washington have filed lawsuits against Johnson & Johnson, claiming that they deceived doctors and patients about the risks of their pelvic mesh. The states claim that marketing and instructional literature should have contained more information about the risks. Some physicians in these states have expressed concern that these lawsuit risks may do more harm than good because the suits conflate mesh used to treat incontinence with the more risky mesh for POP.26
The “ugly” of class action lawsuits
We have discussed both the sad (the injuries to patients) and the bad (the slow regulatory response and continuing injuries). (The ethics of the marketing by the manufacturers might also be raised as the bad.27) Next, let’s look briefly at the ugly.
Some of the patients affected by mesh injuries have been victimized a second time by medical “lenders” and some of their attorneys. Press reports describe patients with modest awards paying 40% in attorney fees (on the high side for personal injury settlements) plus extravagant costs—leaving modest amounts of actual recovery.25
Worse still, a process of “medical lending” has arisen in mesh cases.28 Medical lenders may contact mesh victims offering to pay up front for surgery to remove mesh, and then place a lien against the settlement for repayment at a much higher rate. They might pay the surgeon $2,500 for the surgery, but place a lien on the settlement amount for $60,000.29,30 In addition, there are allegations that lawyers may recruit the doctors to overstate the injuries or do unnecessary removal surgery because that will likely up the award.31 A quick Google search indicates dozens of offers of cash now for your mesh lawsuit (transvaginal and hernia repair).
The patient in our hypothetical case at the beginning had a fairly typical experience. She was a member of a class filing and received a modest settlement. The attorneys representing the class were allowed by the court to charge substantial attorneys’ fees and costs. The patient had the good sense to avoid medical lenders, although other members of the class did use medical lenders and are now filing complaints about the way they were treated by these lenders.
- Maintain surgical skills and be open to new technology. Medical practice requires constant updating and use of new and improved technology as it comes along. By definition, new technology often requires new skills and understanding. A significant portion of surgeons using mesh indicated that they had not read the instructions for use, or had done so only once.1 CME programs that include surgical education remain of particular value.
- Whether new technology or old, it is essential to keep up to date on all FDA bulletins pertinent to devices and pharmaceuticals that you use and prescribe. For example, in 2016 and 2018 the FDA warned that the use of a very old class of drugs (fluoroquinolones) should be limited. It advised "that the serious side effects associated with fluoroquinolones generally outweigh the benefits for patients with acute sinusitis, acute bronchitis, and uncomplicated urinary tract infections who have other treatment options. For patients with these conditions, fluoroquinolones should be reserved for those who do not have alternative treatment options."2 Continued, unnecessary prescriptions for fluoroquinolones would put a physician at some legal risk whether or not the physician had paid any attention to the warning.
- Informed consent is a very important legal and medical process. Take it seriously, and make sure the patient has the information necessary to make informed decisions about treatment. Document the process and the information provided. In some cases consider directing patients to appropriate literature or websites of the manufacturers.
- As to the use of mesh, if not following FDA advice, it is important to document the reason for this and to document the informed consent especially carefully.
- Follow patients after mesh placement for a minimum of 1 year and emphasize to patients they should convey signs and symptoms of complications from initial placement.3 High-risk patients should be of particular concern and be monitored very closely.
References
- Kirkpatrick G, Faber KD, Fromer DL. Transvaginal mesh placement and the instructions for use: a survey of North American urologists. J Urol. https://doi.org/10.1016/j.urpr.2018.05.004.
- FDA Drug Safety Communication: FDA advises restricting fluoroquinolone antibiotic use for certain uncomplicated infections; warns about disabling side effects that can occur together. July 26, 2016. https://www.fda.gov/Drugs/DrugSafety/ucm500143.htm. Accessed June 19, 2019.
- Karlovsky ME. How to avoid and deal with pelvic mesh litigation. Curr Urol Rep. 2016;17:55.
CASE Complications with mesh placement for SUI
A 47-year-old woman (G4 P3013) presents 5 months posthysterectomy with evidence of urinary tract infection (UTI). Escherichia coli is isolated, and she responds to antibiotic therapy.
Her surgical history includes a mini-sling procedure using a needleless device and mesh placement in order to correct progressive worsening of loss of urine when coughing and sneezing. She also reported slight pelvic pain, dysuria, and urgency upon urination at that time. After subsequent development of pelvic organ prolapse (POP), she underwent the vaginal hysterectomy.
Following her UTI treatment, a host of problems occur for the patient, including pelvic pain and dyspareunia. Her male partner reports “feeling something during sex,” especially at the anterior vaginal wall. A plain radiograph of the abdomen identifies a 2 cm x 2 cm stone over the vaginal mesh. In consultation with female pelvic medicine and reconstructive surgery subspecialists, lithotripsy is performed, with the stone fragmented. The patient remains symptomatic, however.
The mesh is noted to be eroding through the vaginal wall. An attempt is made to excise the mesh, initially via transuretheral resection, then through a laparoscopic approach. Due to the mesh being embedded in the tissue, however, an open approach is undertaken. Extensive excision of the mesh and stone fragments is performed. Postoperatively, the patient reports “dry vagina,” with no other genitourinary complaints.
The patient sues. She sues the mesh manufacturer. She also seeks to sue the gynecologist who placed the sling and vaginal mesh (as she says she was not informed of “all the risks” of vaginal mesh placement. She is part of a class action lawsuit, along with thousands of other women.
WHAT’S THE VERDICT?
The device manufacturer settled out of court with the class action suit. (The gynecologist was never formally a defendant because the patient/plaintiff was advised to “drop the physician from the suit.”) The attorneys representing the class action received 40% of the award plus presented costs for the representation. The class as a whole received a little more than 50% of the negotiated award. The patient in this case received $60,000.
Medical background
Stress urinary incontinence (SUI) is a prevalent condition; it affects 35% of women.1 Overall, 80% of women aged 80 or younger will undergo some form of surgery for POP during their lifetime.2 The pathophysiology of SUI includes urethral hypermobility and intrinsic sphincter deficiency.3
Surgical correction for urinary incontinence: A timeline
Use of the gracilis muscle flap to surgically correct urinary incontinence was introduced in 1907. This technique has been replaced by today’s more common Burch procedure, which was first described in 1961. Surgical mesh use dates back to the 1950s, when it was primarily used for abdominal hernia repair. Tension-free tape was introduced in 1995.4-6
Continue to: In the late 1990s the US Food and Drug Administration...
In the late 1990s the US Food and Drug Administration (FDA) permitted use of the first transvaginal meshes, which were designed to treat SUI—the midurethral sling. These mesh slings were so successful that similar meshes were developed to treat POP.7 Almost immediately there were problems with the new POP devices, and 3 years later Boston Scientific recalled its device.8 Nonetheless, the FDA cleared more than 150 devices using surgical mesh for urogynecologic indications (FIGURE).9

Mesh complications
Managing complications from intravesical mesh is a clinically challenging problem. Bladder perforation, stone formation, and penetration through the vagina can occur. Bladder-related complications can manifest as recurrent UTIs and obstructive urinary symptoms, especially in association with stone formation. From the gynecologic perspective, the more common complications with mesh utilization are pelvic pain, groin pain, dyspareunia, contracture and scarring of mesh, and narrowing of the vaginal canal.10 Mesh erosion problems will occur in an estimated 10% to 25% of transvaginal mesh POP implants.11
In 2008, a comparison of transvaginal mesh to native tissue repair (suture-based) or other (biologic) grafts was published.12 The bottom line: there is insufficient evidence to suggest that transvaginal mesh significantly improves outcomes for both posterior and apical defects.
Legal background
Mesh used for surgical purposes is a medical device, which legally is a product—a special product to be sure, but a product nonetheless. Products are subject to product liability rules. Mesh is also subject to an FDA regulatory system. We will briefly discuss products liability and the regulation of devices, both of which have played important roles in mesh-related injuries.
Products liability
As a general matter, defective products subject their manufacturer and seller to liability. There are several legal theories regarding product liability: negligence (in which the defect was caused through carelessness), breach of warranty or guarantee (in addition to express warranties, there are a number of implied warranties for products, including that it is fit for its intended purpose), and strict liability (there was a defect in the product, but it may not have been because of negligence). The product may be defective in the way it was designed, manufactured, or packaged, or it may be defective because adequate instructions and warning were not given to consumers.
Of course, not every product involved in an injury is defective—most automobile accidents, for example, are not the result of any defect in the automobile. In medicine, almost no product (device or pharmaceutical) is entirely safe. In some ways they are unavoidably unsafe and bound to cause some injuries. But when injuries are caused by a defect in the product (design or manufacturing defect or failure to warn), then there may be products liability. Most products liability cases arise under state law.
FDA’s device regulations
Both drugs and medical devices are subject to FDA review and ordinarily require some form of FDA clearance before they may be marketed. In the case of devices, the FDA has 3 classes, with an increase in risk to the user from Class I to III. Various levels of FDA review are required before marketing of the device is permitted, again with the intensity of review increasing from I to III as follows:
- Class I devices pose the least risk, have the least regulation, and are subject to general controls (ie, manufacturing and marketing practices).
- Class II devices pose slightly higher risks and are subject to special controls in addition to the criteria for Class I.
- Class III devices pose the most risk to patients and require premarket approval (scientific review and studies are required to ensure efficacy and safety).13
Continue to: There are a number of limits on manufacturer liability for defective devices...
There are a number of limits on manufacturer liability for defective devices. For Class III devices, the thorough FDA review of the safety of a device may limit the ability of an injured patient to sue based on the state product liability laws.14 For the most part, this “preemption” of state law has not played a major role in mesh litigation because they were initially classified as Class II devices which did not require or include a detailed FDA review.15
The duty to warn of the dangers and risk of medical devices means that manufacturers (or sellers) of devices are obligated to inform health care providers and other medical personnel of the risks. Unlike other manufacturers, device manufacturers do not have to directly warn consumers—because physicians deal directly with patients and prescribe the devices. Therefore, the health care providers, rather than the manufacturers, are obligated to inform the patient.16 This is known as the learned intermediary rule. Manufacturers may still be liable for failure to warn if they do not convey to health care providers proper warnings.
Manufacturers and sellers are not the only entities that may be subject to liability caused by medical devices. Hospitals or other entities that stock and care for devices are responsible for maintaining the safety and functionality of devices in their care.
Health care providers also may be responsible for injuries from medical devices. Generally, that liability is based on negligence. Negligence may relate to selecting an improper device, installing or using it incorrectly, or failing to give the patient adequate information (or informed consent) about the device and alternatives to it.17
A look at the mesh mess
There are a lot of distressing problems and professional disappointments in dissecting the “mesh mess,” including a failure of the FDA to regulate effectively, the extended sale and promotion of intrinsic sphincter deficiency mesh products, the improper use of mesh by physicians even after the risks were known, and, in some instances, the taking advantage of injured patients by attorneys and businesses.18 A lot of finger pointing also has occurred.19 We will recount some of the lowlights of this unfortunate tale.
Continue to: The FDA, in the 1990s, classified the first POP and SUI mesh...
The FDA, in the 1990s, classified the first POP and SUI mesh as Class II after deciding these products were “substantially equivalent” to older surgical meshes. This, of course, proved not to be the case.20 The FDA started receiving thousands of reports of adverse events and, in 2008, warned physicians to be vigilant for adverse events from the mesh. The FDA’s notification recommendations regarding mesh included the following13:
- Obtain specialized training for each mesh implantation technique, and be cognizant of risks.
- Be vigilant for potential adverse events from mesh, including erosion and infection.
- Be observant for complications associated with tools of transvaginal placement (ie, bowel, bladder, and vessel perforation).
- Inform patients that implantation of mesh is permanent and complications may require additional surgery for correction.
- Be aware that complications may affect quality of life—eg, pain with intercourse, scarring, and vaginal wall narrowing (POP repair).
- Provide patients with written copy of patient labeling from the surgical mesh manufacturer.
In 2011, the FDA issued a formal warning to providers that transvaginal mesh posed meaningful risks beyond nonmesh surgery. The FDA’s bulletin draws attention to how the mesh is placed more so than the material per se.19,21 Mesh was a Class II device for sacrocolpopexy or midurethral sling and, similarly, the transvaginal kit was also a Class II device. Overall, use of mesh midurethral slings has been well received as treatment for SUI. The FDA also accepted it for POP, however, but with increasingly strong warnings. The FDA’s 2011 communication stated, “This update is to inform you that serious complications associated with surgical mesh for transvaginal repair of POP are not rare….Furthermore, it is not clear that transvaginal POP repair with mesh is more effective than traditional non-mesh repair in all patients with POP and it may expose patients to greater risk.”7,13
In 2014 the FDA proposed reclassifying mesh to a Class III device, which would require that manufacturers obtain approval, based on safety and effectiveness, before selling mesh. Not until 2016 did the FDA actually reclassify the mess as Class III. Of course, during this time, mesh manufacturers were well aware of the substantial problems the products were causing.13
After serious problems with mesh became well known, and especially after FDA warnings, the use of mesh other than as indicated by the FDA was increasingly risky from a legal (as well as a health) standpoint. As long as mesh was still on the market, of course, it was available for use. But use of mesh for POP procedures without good indications in a way that was contrary to the FDA warnings might well be negligent.
Changes to informed consent
The FDA warnings also should have changed the informed consent for the use of mesh.22 Informed consent commonly consists of the following:
- informing the patient of the proposed procedure
- describing risks (and benefits) of the proposed process
- explaining reasonable alternatives
- noting the risks of taking no action.
Information that is material to a decision should be disclosed. If mesh were going to be used, after the problems of mesh were known and identified by the FDA (other than midurethral slings as treatment of SUI), the risks should have been clearly identified for patients, with alternatives outlined. The American College of Obstetricians and Gynecologists Committee on Ethics has 8 fundamental concepts with regard to informed consent that are worth keeping in mind23:
- Obtaining informed consent for medical treatment and research is an ethical requirement.
- The process expresses respect for the patient as a person.
- It protects patients against unwanted treatment and allows patients’ active involvement in medical planning and care.
- Communication is of paramount importance.
- Informed consent is a process and not a signature on a form.
- A commitment to informed consent and to provision of medical benefit to the patient are linked to provision of care.
- If obtaining informed consent is impossible, a designated surrogate should be identified representing the patient’s best interests.
- Knowledge on the part of the provider regarding state and federal requirements is necessary.
Continue to: Lawsuits line up...
Lawsuits line up
The widespread use of a product with a significant percentage of injuries and eventually with warnings about injuries from use sounds like the formula for a lot of lawsuits. This certainly has happened. A large number of suits—both class actions and individual actions—were filed as a result of mesh injuries.24 These suits were overwhelmingly against the manufacturer, although some included physicians.7 Device makers are more attractive defendants for several reasons. First, they have very deep pockets. In addition, jurors are generally much less sympathetic to large companies than to doctors. Large class actions meant that there were many different patients among the plaintiffs, and medical malpractice claims in most states have a number of trial difficulties not present in other product liability cases. Common defendants have included Johnson & Johnson, Boston Scientific, and Medtronic.
Some of the cases resulted in very large damage awards against manufacturers based on various kinds of product(s) liability. Many other cases were settled or tried with relatively small damages. There were, in addition, a number of instances in which the manufacturers were not liable. Of the 32 plaintiffs who have gone to trial thus far, 24 have obtained verdicts totaling $345 million ($14 million average). The cases that have settled have been for much less—perhaps $60,000 on average. A number of cases remain unresolved. To date, the estimate is that 100,000 women have received almost $8 billion from 7 device manufacturers to resolve claims.25
Some state attorneys general have gotten into the process as well. Attorneys general from California, Kentucky, Mississippi, and Washington have filed lawsuits against Johnson & Johnson, claiming that they deceived doctors and patients about the risks of their pelvic mesh. The states claim that marketing and instructional literature should have contained more information about the risks. Some physicians in these states have expressed concern that these lawsuit risks may do more harm than good because the suits conflate mesh used to treat incontinence with the more risky mesh for POP.26
The “ugly” of class action lawsuits
We have discussed both the sad (the injuries to patients) and the bad (the slow regulatory response and continuing injuries). (The ethics of the marketing by the manufacturers might also be raised as the bad.27) Next, let’s look briefly at the ugly.
Some of the patients affected by mesh injuries have been victimized a second time by medical “lenders” and some of their attorneys. Press reports describe patients with modest awards paying 40% in attorney fees (on the high side for personal injury settlements) plus extravagant costs—leaving modest amounts of actual recovery.25
Worse still, a process of “medical lending” has arisen in mesh cases.28 Medical lenders may contact mesh victims offering to pay up front for surgery to remove mesh, and then place a lien against the settlement for repayment at a much higher rate. They might pay the surgeon $2,500 for the surgery, but place a lien on the settlement amount for $60,000.29,30 In addition, there are allegations that lawyers may recruit the doctors to overstate the injuries or do unnecessary removal surgery because that will likely up the award.31 A quick Google search indicates dozens of offers of cash now for your mesh lawsuit (transvaginal and hernia repair).
The patient in our hypothetical case at the beginning had a fairly typical experience. She was a member of a class filing and received a modest settlement. The attorneys representing the class were allowed by the court to charge substantial attorneys’ fees and costs. The patient had the good sense to avoid medical lenders, although other members of the class did use medical lenders and are now filing complaints about the way they were treated by these lenders.
- Maintain surgical skills and be open to new technology. Medical practice requires constant updating and use of new and improved technology as it comes along. By definition, new technology often requires new skills and understanding. A significant portion of surgeons using mesh indicated that they had not read the instructions for use, or had done so only once.1 CME programs that include surgical education remain of particular value.
- Whether new technology or old, it is essential to keep up to date on all FDA bulletins pertinent to devices and pharmaceuticals that you use and prescribe. For example, in 2016 and 2018 the FDA warned that the use of a very old class of drugs (fluoroquinolones) should be limited. It advised "that the serious side effects associated with fluoroquinolones generally outweigh the benefits for patients with acute sinusitis, acute bronchitis, and uncomplicated urinary tract infections who have other treatment options. For patients with these conditions, fluoroquinolones should be reserved for those who do not have alternative treatment options."2 Continued, unnecessary prescriptions for fluoroquinolones would put a physician at some legal risk whether or not the physician had paid any attention to the warning.
- Informed consent is a very important legal and medical process. Take it seriously, and make sure the patient has the information necessary to make informed decisions about treatment. Document the process and the information provided. In some cases consider directing patients to appropriate literature or websites of the manufacturers.
- As to the use of mesh, if not following FDA advice, it is important to document the reason for this and to document the informed consent especially carefully.
- Follow patients after mesh placement for a minimum of 1 year and emphasize to patients they should convey signs and symptoms of complications from initial placement.3 High-risk patients should be of particular concern and be monitored very closely.
References
- Kirkpatrick G, Faber KD, Fromer DL. Transvaginal mesh placement and the instructions for use: a survey of North American urologists. J Urol. https://doi.org/10.1016/j.urpr.2018.05.004.
- FDA Drug Safety Communication: FDA advises restricting fluoroquinolone antibiotic use for certain uncomplicated infections; warns about disabling side effects that can occur together. July 26, 2016. https://www.fda.gov/Drugs/DrugSafety/ucm500143.htm. Accessed June 19, 2019.
- Karlovsky ME. How to avoid and deal with pelvic mesh litigation. Curr Urol Rep. 2016;17:55.
- Maral I, Ozkardeş H, Peşkircioğlu L, et al. Prevalence of stress urinary incontinence in both sexes at or after age 15 years: a cross-sectional study. J Urol. 2001;165:408-412.
- Olsen AL, Smith VJ, Bergstrom JO, et al. Epidemiology of surgically managed pelvic organ prolapse and urinary incontinence. Obstet Gynecol. 1997;89:501-506.
- Chang J, Lee D. Midurethral slings in the mesh litigation era. Transl Androl Urol. 2017;6(suppl 2): S68-S75.
- Mattingly R, ed. TeLinde's Operative Gynecology, 5th edition. Lippincott, William, and Wilkins: Philadelphia, PA; 1997.
- Burch J. Urethrovaginal fixation to Cooper's ligament for correction of stress incontinence, cystocele, and prolapse. Am J Obstet Gynecol. 1961;81:281-290.
- Ulmsten U, Falconer C, Johnson P, et al. A multicenter study of tension-free vaginal tape (TVT) for surgical treatment of stress urinary incontinence. Int Urogynecol J Pelvic Floor Dysfunct. 1998;9:210-213.
- Kuhlmann-Capek MJ, Kilic GS, Shah AB, et al. Enmeshed in controversy: use of vaginal mesh in the current medicolegal environment. Female Pelvic Med Reconstr Surg. 2015;21:241-243.
- Powell SF. Changing our minds: reforming the FDA medical device reclassification process. Food Drug Law J. 2018;73:177-209.
- US Food and Drug Administration. Surgical Mesh for Treatment of Women with Pelvic Organ Prolapse and Stress Urinary Incontinence. September 2011. https://www.thesenatorsfirm.com/documents/OBS.pdf. Accessed June 19, 2019.
- Maher C, Feiner B, Baessler K, et al. Surgical management of pelvic organ prolapse in women. Cochrane Database Syst Rev. 2013;(4):CD004014.
- Ganj FA, Ibeanu OA, Bedestani A, Nolan TE, Chesson RR. Complications of transvaginal monofilament polypropylene mesh in pelvic organ prolapse repair. Int Urogynecol J Pelvic Floor Dysfunct. 2009;20:919-925.
- Sung VW, Rogers RG, Schaffer JI, et al. Graft use in transvaginal pelvic organ prolapse repair: a systematic review. Obstet Gynecol. 2008;112:1131-1142.
- FDA public health notification: serious complications associated with transvaginal placement of surgical mesh in repair of pelvic organ prolapse and stress urinary incontinence. October 20, 2008. http://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/PublicHealthNotifications/ucm061976.htm. Accessed February 14, 2019.
- Riegel v. Medtronic, 552 U.S. 312 (2008).
- Whitney DW. Guide to preemption of state-law claims against Class III PMA medical devices. Food Drug Law J. 2010;65:113-139.
- Alam P, Iglesia CB. Informed consent for reconstructive pelvic surgery. Obstet Gynecol Clin North Am. 2016;43:131-139.
- Nosti PA, Iglesia CB. Medicolegal issues surrounding devices and mesh for surgical treatment of prolapse and incontinence. Clin Obstet Gynecol. 2013;56:221-228.
- Shepherd CG. Transvaginal mesh litigation: a new opportunity to resolve mass medical device failure claims. Tennessee Law Rev. 2012;80:3:477-94.
- Karlovsky ME. How to avoid and deal with pelvic mesh litigation. Curr Urol Rep. 2016;17:55.
- Cohn JA, Timbrook Brown E, Kowalik CG, et al. The mesh controversy. F1000Research website. https://f1000research.com/articles/5-2423/v1. Accessed June 17, 2019.
- Obstetrics and Gynecology Devices Panel Meeting, February 12, 2019. US Food and Drug Administration website. https://www.fda.gov/media/122867/download. Accessed June 19, 2019.
- Mucowski SJ, Jurnalov C, Phelps JY. Use of vaginal mesh in the face of recent FDA warnings and litigation. Am J Obstet Gynecol. 2010;203:103.e1-e4.
- American College of Obstetricians and Gynecologists Committee on Ethics. ACOG Committee Opinion No. 439: informed consent. Obstet Gynecol. 2009;114(2 pt 1):401-408.
- Souders CP, Eilber KS, McClelland L, et al. The truth behind transvaginal mesh litigation: devices, timelines, and provider characteristics. Female Pelvic Med Reconstr Surg. 2018;24:21-25.
- Goldstein M. As pelvic mesh settlements near $8 billion, women question lawyers' fees. New York Times. February 1, 2019. https://www.nytimes.com/2019/02/01/business/pelvic-mesh-settlements-lawyers.html. Accessed June 19, 2019.
- Johnson G. Surgeons fear pelvic mesh lawsuits will spook patients. Associated Press News. January 10, 2019. https://www.apnews.com/25777c3c33e3489283b1dc2ebdde6b55. Accessed June 19, 2019.
- Clarke RN. Medical device marketing and the ethics of vaginal mesh kit marketing. In The Innovation and Evolution of Medical Devices. New York, NY: Springer; 2019:103-123.
- Top 5 drug and medical device developments of 2018. Law 360. January 1, 2019. Accessed through LexisNexis.
- Frankel A, Dye J. The Lien Machine. New breed of investor profits by financing surgeries for desperate women patients. Reuters. August 18, 2015. https://www.reuters.com/investigates/special-report/usa-litigation-mesh/. Accessed June 19, 2019.
- Sullivan T. New report looks at intersection of "medical lending" and pelvic mesh lawsuits. Policy & Medicine. May 5, 2018. https://www.policymed.com/2015/08/medical-lending-and-pelvic-mesh-litigation.html. Accessed June 19, 2019.
- Goldstein M, Sliver-Greensberg J. How profiteers lure women into often-unneeded surgery. New York Times. April 14, 2018. https://www.nytimes.com/2018/04/14/business/vaginal-mesh-surgery-lawsuits-financing.html. Accessed June 19, 2019.
- Maral I, Ozkardeş H, Peşkircioğlu L, et al. Prevalence of stress urinary incontinence in both sexes at or after age 15 years: a cross-sectional study. J Urol. 2001;165:408-412.
- Olsen AL, Smith VJ, Bergstrom JO, et al. Epidemiology of surgically managed pelvic organ prolapse and urinary incontinence. Obstet Gynecol. 1997;89:501-506.
- Chang J, Lee D. Midurethral slings in the mesh litigation era. Transl Androl Urol. 2017;6(suppl 2): S68-S75.
- Mattingly R, ed. TeLinde's Operative Gynecology, 5th edition. Lippincott, William, and Wilkins: Philadelphia, PA; 1997.
- Burch J. Urethrovaginal fixation to Cooper's ligament for correction of stress incontinence, cystocele, and prolapse. Am J Obstet Gynecol. 1961;81:281-290.
- Ulmsten U, Falconer C, Johnson P, et al. A multicenter study of tension-free vaginal tape (TVT) for surgical treatment of stress urinary incontinence. Int Urogynecol J Pelvic Floor Dysfunct. 1998;9:210-213.
- Kuhlmann-Capek MJ, Kilic GS, Shah AB, et al. Enmeshed in controversy: use of vaginal mesh in the current medicolegal environment. Female Pelvic Med Reconstr Surg. 2015;21:241-243.
- Powell SF. Changing our minds: reforming the FDA medical device reclassification process. Food Drug Law J. 2018;73:177-209.
- US Food and Drug Administration. Surgical Mesh for Treatment of Women with Pelvic Organ Prolapse and Stress Urinary Incontinence. September 2011. https://www.thesenatorsfirm.com/documents/OBS.pdf. Accessed June 19, 2019.
- Maher C, Feiner B, Baessler K, et al. Surgical management of pelvic organ prolapse in women. Cochrane Database Syst Rev. 2013;(4):CD004014.
- Ganj FA, Ibeanu OA, Bedestani A, Nolan TE, Chesson RR. Complications of transvaginal monofilament polypropylene mesh in pelvic organ prolapse repair. Int Urogynecol J Pelvic Floor Dysfunct. 2009;20:919-925.
- Sung VW, Rogers RG, Schaffer JI, et al. Graft use in transvaginal pelvic organ prolapse repair: a systematic review. Obstet Gynecol. 2008;112:1131-1142.
- FDA public health notification: serious complications associated with transvaginal placement of surgical mesh in repair of pelvic organ prolapse and stress urinary incontinence. October 20, 2008. http://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/PublicHealthNotifications/ucm061976.htm. Accessed February 14, 2019.
- Riegel v. Medtronic, 552 U.S. 312 (2008).
- Whitney DW. Guide to preemption of state-law claims against Class III PMA medical devices. Food Drug Law J. 2010;65:113-139.
- Alam P, Iglesia CB. Informed consent for reconstructive pelvic surgery. Obstet Gynecol Clin North Am. 2016;43:131-139.
- Nosti PA, Iglesia CB. Medicolegal issues surrounding devices and mesh for surgical treatment of prolapse and incontinence. Clin Obstet Gynecol. 2013;56:221-228.
- Shepherd CG. Transvaginal mesh litigation: a new opportunity to resolve mass medical device failure claims. Tennessee Law Rev. 2012;80:3:477-94.
- Karlovsky ME. How to avoid and deal with pelvic mesh litigation. Curr Urol Rep. 2016;17:55.
- Cohn JA, Timbrook Brown E, Kowalik CG, et al. The mesh controversy. F1000Research website. https://f1000research.com/articles/5-2423/v1. Accessed June 17, 2019.
- Obstetrics and Gynecology Devices Panel Meeting, February 12, 2019. US Food and Drug Administration website. https://www.fda.gov/media/122867/download. Accessed June 19, 2019.
- Mucowski SJ, Jurnalov C, Phelps JY. Use of vaginal mesh in the face of recent FDA warnings and litigation. Am J Obstet Gynecol. 2010;203:103.e1-e4.
- American College of Obstetricians and Gynecologists Committee on Ethics. ACOG Committee Opinion No. 439: informed consent. Obstet Gynecol. 2009;114(2 pt 1):401-408.
- Souders CP, Eilber KS, McClelland L, et al. The truth behind transvaginal mesh litigation: devices, timelines, and provider characteristics. Female Pelvic Med Reconstr Surg. 2018;24:21-25.
- Goldstein M. As pelvic mesh settlements near $8 billion, women question lawyers' fees. New York Times. February 1, 2019. https://www.nytimes.com/2019/02/01/business/pelvic-mesh-settlements-lawyers.html. Accessed June 19, 2019.
- Johnson G. Surgeons fear pelvic mesh lawsuits will spook patients. Associated Press News. January 10, 2019. https://www.apnews.com/25777c3c33e3489283b1dc2ebdde6b55. Accessed June 19, 2019.
- Clarke RN. Medical device marketing and the ethics of vaginal mesh kit marketing. In The Innovation and Evolution of Medical Devices. New York, NY: Springer; 2019:103-123.
- Top 5 drug and medical device developments of 2018. Law 360. January 1, 2019. Accessed through LexisNexis.
- Frankel A, Dye J. The Lien Machine. New breed of investor profits by financing surgeries for desperate women patients. Reuters. August 18, 2015. https://www.reuters.com/investigates/special-report/usa-litigation-mesh/. Accessed June 19, 2019.
- Sullivan T. New report looks at intersection of "medical lending" and pelvic mesh lawsuits. Policy & Medicine. May 5, 2018. https://www.policymed.com/2015/08/medical-lending-and-pelvic-mesh-litigation.html. Accessed June 19, 2019.
- Goldstein M, Sliver-Greensberg J. How profiteers lure women into often-unneeded surgery. New York Times. April 14, 2018. https://www.nytimes.com/2018/04/14/business/vaginal-mesh-surgery-lawsuits-financing.html. Accessed June 19, 2019.
Question Marks Lead to Dollar Signs
An employee of a sawmill in Kentucky sustained paralyzing injuries when a large piece of milling equipment struck him in the back. His coworkers took him to the hospital in the back of a pickup truck.
At the time, some of the hospital’s nursing staff were on strike and had been replaced by temporary staff provided by US Nursing Corporation. A female nurse helped load the patient into a wheelchair for transfer into the hospital.
The patient was evaluated for a spinal injury; it was determined that he had sustained an L-3 burst fracture that impinged his spine. He was transferred to another hospital. However, due to the nature of his injuries, he is permanently paralyzed from the waist down.
The plaintiff presented a products liability claim against the machinery manufacturers, which was settled for $3.05 million. He later filed a medical malpractice complaint to include the nursing contractor and 3 individual nurses (1 from the contractor and 2 employed by the hospital). The complaint alleged that the nurses “failed to stabilize and immobilize” the patient when moving him from the pickup truck to the emergency department (ED), which worsened his injuries. A nurse employed by the contractor was identified as the nurse who had transferred him to the wheelchair.
The latter case was litigated for several years. On the eve of trial, the hospital settled for $2 million and the nursing contractor for $1.1 million. However, the hospital brought an indemnification claim against the nursing contractor to recover the $2 million settlement.
At the time of trial, there was a question regarding the identity of the nurse who had transferred the plaintiff from the pickup truck to the wheelchair. The US Nursing Corporation contract nurse contended she did not transfer the plaintiff to the wheelchair. Resolving the uncertainty, the jury concluded that the contract nurse was the nurse who had transferred the plaintiff.
VERDICT
At the conclusion of a 7-day trial, the jury awarded the plaintiff $2,823,522.
Continue to: COMMENTARY
COMMENTARY
Who doesn’t love a good mystery, right? Well, not everyone. Years ago, I was given a gift: a “host your own murder mystery party” game. I recently gave it away when I realized I was statistically more likely to be murdered than ever to host a “murder mystery party.” Love them or hate them, I think you will agree: Mysteries belong in novels or movies or board games. They have no place in your clinical practice.
In litigation, lawyers obsess over trivial details. I’ve attended enough malpractice depositions to see physicians, NPs, PAs, and nurses, with puzzled faces, answering seemingly nonsensical questions that appear to have no bearing on clinical matters. The clinicians respond half amused and half annoyed, through a litany of telephone logs, record access logs, chain-of-custody records, transfer center logs, recorded ambulance communications, time-stamped records, and recollections of who brought a specimen to the lab or what time someone was at the nurses’ station—all peripheral to practice. I understand the quizzical looks and sympathize with providers’ annoyance at having to answer seemingly inane questions. Yet these matters, collateral to practice, can take center stage in a legal case.
These issues form part of the puzzle: the who, what, where, when, why, and how of any case. For example
Who carried a specimen from the operating room (OR)? (Because it was sent from the OR, but the lab has no record of receiving it and knowing the identity of the runner is now key.)
What time did the attending call the hospital to alert the surgical team? (Because precise timing from surgeon’s knowledge to first incision is now at issue.)
Continue to: Where...
Where, specifically, was the culture taken from? (Because there were three wounds, and it turns out later two wounds were from a different source than the third.)
When did scrub tech A clock out of a surgery and scrub tech B clock in? (Because one of the surgical counts was wrong, and a surgical item was retained.)
Why did the patient leave against medical advice? (Because in the ED, he said he “needed to feed his cat.” This wasn’t recorded; the chart only states “patient left AMA.” During litigation, plaintiff claims he left because a nurse told him “it would be better to see your regular doctor.”)
How did a patient get a KFC value meal to eat in his hospital bed when strict oral intake was needed? (Because the hospital’s knowledge of the patient’s dietary intake is now at issue.)
I know—such a list of who, what, etc, can appear cutesy and cloying. Further, some of these trivial details are not recorded by clinicians, so why bring them up? I raise it because in your practice setting, you may be in a position to influence decision-making with regard to recording those minor details, which can become critically important later.
Continue to: In a medical malpractice case...
In a medical malpractice case, every tiny detail is potentially part of the puzzle. If a piece of the puzzle is missing, it becomes a mystery, and a mystery can become a problem. A plaintiff’s lawyer who sees question marks also sees dollar signs.
In this case, the presence of a “mystery nurse” likely kicked up enough dust to confuse the jury. Most clinicians are aware a malpractice plaintiff must prove 4 elements: (1) duty, (2) breach of duty, (3) causation, and (4) harm. The plaintiff must prove all elements by a preponderance of the evidence (ie, greater than 50% likely). Duty and damages are not at issue in this case; there was a clear patient relationship, and the plaintiff is clearly paralyzed. The plaintiff has the burden to prove elements (2) and (3): that there was a breach of the standard of care and that breach caused the plaintiff’s harm.
With respect to element (2), the plaintiff had the burden of showing that the act of putting him into the wheelchair was a breach of the standard of care. I think we’d all agree: The standard of care requires a registered nurse to recognize that a patient struck by a heavy object is at risk for spinal injury and spinal immobilization is required. The patient should have been removed from the vehicle with spinal immobilization techniques.
However, with respect to the causation element, the plaintiff would have been required to prove it was more probable than not (ie, 51% or greater) that the act of putting him into the wheelchair caused the paralysis. This is a stretch. The jury would have to believe it was at least 51% likely that the act of car-to-wheelchair transfer caused the injury—not the heavy mill equipment falling on him in the first place, not the efforts of his coworkers to move him from the scene, not the efforts of his coworkers to load him into the truck, not the bouncy ride in the back of a truck over to the hospital. The plaintiff was able to overcome a big causation hurdle because the identity of the nurse was not known.
The plaintiff would also generally have to show that the coworkers did not mislead the transferring nurse—that is, the statements made at the time of transfer would lead a reasonably skilled nurse to suspect spinal injury, halt transfer attempts, and see to it the patient’s spine was immobilized. Although doubtful, it is possible that in the split seconds when the car arrived at the ED, the initial communications were errant and a reasonable nurse would not have just cause to suspect spinal injury. However, we will never know. We don’t have testimony on what was said during transfer.
Continue to: So we don't know who...
So we don’t know who the nurse was. We don’t know what was said. We don’t know exactly how the plaintiff was transferred out of the vehicle. And those mysteries, to a jury, are suspicious.
IN SUMMARY
Any time a lawyer can draw a giant “?” on a whiteboard during summation, rest assured, someone is in trouble. That someone could be you. I’ve seen lots of question marks in my life; none carry a $1 million/$3 million malpractice policy. The presence of a mystery will transform a case that was defensible into one with unanswered questions. Those unanswered questions open the door to the suggestion or outright accusation of a cover-up. Do your best to document details and work within your system to encourage documentation. In short, don’t let the plaintiff host a mystery party at your expense.
An employee of a sawmill in Kentucky sustained paralyzing injuries when a large piece of milling equipment struck him in the back. His coworkers took him to the hospital in the back of a pickup truck.
At the time, some of the hospital’s nursing staff were on strike and had been replaced by temporary staff provided by US Nursing Corporation. A female nurse helped load the patient into a wheelchair for transfer into the hospital.
The patient was evaluated for a spinal injury; it was determined that he had sustained an L-3 burst fracture that impinged his spine. He was transferred to another hospital. However, due to the nature of his injuries, he is permanently paralyzed from the waist down.
The plaintiff presented a products liability claim against the machinery manufacturers, which was settled for $3.05 million. He later filed a medical malpractice complaint to include the nursing contractor and 3 individual nurses (1 from the contractor and 2 employed by the hospital). The complaint alleged that the nurses “failed to stabilize and immobilize” the patient when moving him from the pickup truck to the emergency department (ED), which worsened his injuries. A nurse employed by the contractor was identified as the nurse who had transferred him to the wheelchair.
The latter case was litigated for several years. On the eve of trial, the hospital settled for $2 million and the nursing contractor for $1.1 million. However, the hospital brought an indemnification claim against the nursing contractor to recover the $2 million settlement.
At the time of trial, there was a question regarding the identity of the nurse who had transferred the plaintiff from the pickup truck to the wheelchair. The US Nursing Corporation contract nurse contended she did not transfer the plaintiff to the wheelchair. Resolving the uncertainty, the jury concluded that the contract nurse was the nurse who had transferred the plaintiff.
VERDICT
At the conclusion of a 7-day trial, the jury awarded the plaintiff $2,823,522.
Continue to: COMMENTARY
COMMENTARY
Who doesn’t love a good mystery, right? Well, not everyone. Years ago, I was given a gift: a “host your own murder mystery party” game. I recently gave it away when I realized I was statistically more likely to be murdered than ever to host a “murder mystery party.” Love them or hate them, I think you will agree: Mysteries belong in novels or movies or board games. They have no place in your clinical practice.
In litigation, lawyers obsess over trivial details. I’ve attended enough malpractice depositions to see physicians, NPs, PAs, and nurses, with puzzled faces, answering seemingly nonsensical questions that appear to have no bearing on clinical matters. The clinicians respond half amused and half annoyed, through a litany of telephone logs, record access logs, chain-of-custody records, transfer center logs, recorded ambulance communications, time-stamped records, and recollections of who brought a specimen to the lab or what time someone was at the nurses’ station—all peripheral to practice. I understand the quizzical looks and sympathize with providers’ annoyance at having to answer seemingly inane questions. Yet these matters, collateral to practice, can take center stage in a legal case.
These issues form part of the puzzle: the who, what, where, when, why, and how of any case. For example
Who carried a specimen from the operating room (OR)? (Because it was sent from the OR, but the lab has no record of receiving it and knowing the identity of the runner is now key.)
What time did the attending call the hospital to alert the surgical team? (Because precise timing from surgeon’s knowledge to first incision is now at issue.)
Continue to: Where...
Where, specifically, was the culture taken from? (Because there were three wounds, and it turns out later two wounds were from a different source than the third.)
When did scrub tech A clock out of a surgery and scrub tech B clock in? (Because one of the surgical counts was wrong, and a surgical item was retained.)
Why did the patient leave against medical advice? (Because in the ED, he said he “needed to feed his cat.” This wasn’t recorded; the chart only states “patient left AMA.” During litigation, plaintiff claims he left because a nurse told him “it would be better to see your regular doctor.”)
How did a patient get a KFC value meal to eat in his hospital bed when strict oral intake was needed? (Because the hospital’s knowledge of the patient’s dietary intake is now at issue.)
I know—such a list of who, what, etc, can appear cutesy and cloying. Further, some of these trivial details are not recorded by clinicians, so why bring them up? I raise it because in your practice setting, you may be in a position to influence decision-making with regard to recording those minor details, which can become critically important later.
Continue to: In a medical malpractice case...
In a medical malpractice case, every tiny detail is potentially part of the puzzle. If a piece of the puzzle is missing, it becomes a mystery, and a mystery can become a problem. A plaintiff’s lawyer who sees question marks also sees dollar signs.
In this case, the presence of a “mystery nurse” likely kicked up enough dust to confuse the jury. Most clinicians are aware a malpractice plaintiff must prove 4 elements: (1) duty, (2) breach of duty, (3) causation, and (4) harm. The plaintiff must prove all elements by a preponderance of the evidence (ie, greater than 50% likely). Duty and damages are not at issue in this case; there was a clear patient relationship, and the plaintiff is clearly paralyzed. The plaintiff has the burden to prove elements (2) and (3): that there was a breach of the standard of care and that breach caused the plaintiff’s harm.
With respect to element (2), the plaintiff had the burden of showing that the act of putting him into the wheelchair was a breach of the standard of care. I think we’d all agree: The standard of care requires a registered nurse to recognize that a patient struck by a heavy object is at risk for spinal injury and spinal immobilization is required. The patient should have been removed from the vehicle with spinal immobilization techniques.
However, with respect to the causation element, the plaintiff would have been required to prove it was more probable than not (ie, 51% or greater) that the act of putting him into the wheelchair caused the paralysis. This is a stretch. The jury would have to believe it was at least 51% likely that the act of car-to-wheelchair transfer caused the injury—not the heavy mill equipment falling on him in the first place, not the efforts of his coworkers to move him from the scene, not the efforts of his coworkers to load him into the truck, not the bouncy ride in the back of a truck over to the hospital. The plaintiff was able to overcome a big causation hurdle because the identity of the nurse was not known.
The plaintiff would also generally have to show that the coworkers did not mislead the transferring nurse—that is, the statements made at the time of transfer would lead a reasonably skilled nurse to suspect spinal injury, halt transfer attempts, and see to it the patient’s spine was immobilized. Although doubtful, it is possible that in the split seconds when the car arrived at the ED, the initial communications were errant and a reasonable nurse would not have just cause to suspect spinal injury. However, we will never know. We don’t have testimony on what was said during transfer.
Continue to: So we don't know who...
So we don’t know who the nurse was. We don’t know what was said. We don’t know exactly how the plaintiff was transferred out of the vehicle. And those mysteries, to a jury, are suspicious.
IN SUMMARY
Any time a lawyer can draw a giant “?” on a whiteboard during summation, rest assured, someone is in trouble. That someone could be you. I’ve seen lots of question marks in my life; none carry a $1 million/$3 million malpractice policy. The presence of a mystery will transform a case that was defensible into one with unanswered questions. Those unanswered questions open the door to the suggestion or outright accusation of a cover-up. Do your best to document details and work within your system to encourage documentation. In short, don’t let the plaintiff host a mystery party at your expense.
An employee of a sawmill in Kentucky sustained paralyzing injuries when a large piece of milling equipment struck him in the back. His coworkers took him to the hospital in the back of a pickup truck.
At the time, some of the hospital’s nursing staff were on strike and had been replaced by temporary staff provided by US Nursing Corporation. A female nurse helped load the patient into a wheelchair for transfer into the hospital.
The patient was evaluated for a spinal injury; it was determined that he had sustained an L-3 burst fracture that impinged his spine. He was transferred to another hospital. However, due to the nature of his injuries, he is permanently paralyzed from the waist down.
The plaintiff presented a products liability claim against the machinery manufacturers, which was settled for $3.05 million. He later filed a medical malpractice complaint to include the nursing contractor and 3 individual nurses (1 from the contractor and 2 employed by the hospital). The complaint alleged that the nurses “failed to stabilize and immobilize” the patient when moving him from the pickup truck to the emergency department (ED), which worsened his injuries. A nurse employed by the contractor was identified as the nurse who had transferred him to the wheelchair.
The latter case was litigated for several years. On the eve of trial, the hospital settled for $2 million and the nursing contractor for $1.1 million. However, the hospital brought an indemnification claim against the nursing contractor to recover the $2 million settlement.
At the time of trial, there was a question regarding the identity of the nurse who had transferred the plaintiff from the pickup truck to the wheelchair. The US Nursing Corporation contract nurse contended she did not transfer the plaintiff to the wheelchair. Resolving the uncertainty, the jury concluded that the contract nurse was the nurse who had transferred the plaintiff.
VERDICT
At the conclusion of a 7-day trial, the jury awarded the plaintiff $2,823,522.
Continue to: COMMENTARY
COMMENTARY
Who doesn’t love a good mystery, right? Well, not everyone. Years ago, I was given a gift: a “host your own murder mystery party” game. I recently gave it away when I realized I was statistically more likely to be murdered than ever to host a “murder mystery party.” Love them or hate them, I think you will agree: Mysteries belong in novels or movies or board games. They have no place in your clinical practice.
In litigation, lawyers obsess over trivial details. I’ve attended enough malpractice depositions to see physicians, NPs, PAs, and nurses, with puzzled faces, answering seemingly nonsensical questions that appear to have no bearing on clinical matters. The clinicians respond half amused and half annoyed, through a litany of telephone logs, record access logs, chain-of-custody records, transfer center logs, recorded ambulance communications, time-stamped records, and recollections of who brought a specimen to the lab or what time someone was at the nurses’ station—all peripheral to practice. I understand the quizzical looks and sympathize with providers’ annoyance at having to answer seemingly inane questions. Yet these matters, collateral to practice, can take center stage in a legal case.
These issues form part of the puzzle: the who, what, where, when, why, and how of any case. For example
Who carried a specimen from the operating room (OR)? (Because it was sent from the OR, but the lab has no record of receiving it and knowing the identity of the runner is now key.)
What time did the attending call the hospital to alert the surgical team? (Because precise timing from surgeon’s knowledge to first incision is now at issue.)
Continue to: Where...
Where, specifically, was the culture taken from? (Because there were three wounds, and it turns out later two wounds were from a different source than the third.)
When did scrub tech A clock out of a surgery and scrub tech B clock in? (Because one of the surgical counts was wrong, and a surgical item was retained.)
Why did the patient leave against medical advice? (Because in the ED, he said he “needed to feed his cat.” This wasn’t recorded; the chart only states “patient left AMA.” During litigation, plaintiff claims he left because a nurse told him “it would be better to see your regular doctor.”)
How did a patient get a KFC value meal to eat in his hospital bed when strict oral intake was needed? (Because the hospital’s knowledge of the patient’s dietary intake is now at issue.)
I know—such a list of who, what, etc, can appear cutesy and cloying. Further, some of these trivial details are not recorded by clinicians, so why bring them up? I raise it because in your practice setting, you may be in a position to influence decision-making with regard to recording those minor details, which can become critically important later.
Continue to: In a medical malpractice case...
In a medical malpractice case, every tiny detail is potentially part of the puzzle. If a piece of the puzzle is missing, it becomes a mystery, and a mystery can become a problem. A plaintiff’s lawyer who sees question marks also sees dollar signs.
In this case, the presence of a “mystery nurse” likely kicked up enough dust to confuse the jury. Most clinicians are aware a malpractice plaintiff must prove 4 elements: (1) duty, (2) breach of duty, (3) causation, and (4) harm. The plaintiff must prove all elements by a preponderance of the evidence (ie, greater than 50% likely). Duty and damages are not at issue in this case; there was a clear patient relationship, and the plaintiff is clearly paralyzed. The plaintiff has the burden to prove elements (2) and (3): that there was a breach of the standard of care and that breach caused the plaintiff’s harm.
With respect to element (2), the plaintiff had the burden of showing that the act of putting him into the wheelchair was a breach of the standard of care. I think we’d all agree: The standard of care requires a registered nurse to recognize that a patient struck by a heavy object is at risk for spinal injury and spinal immobilization is required. The patient should have been removed from the vehicle with spinal immobilization techniques.
However, with respect to the causation element, the plaintiff would have been required to prove it was more probable than not (ie, 51% or greater) that the act of putting him into the wheelchair caused the paralysis. This is a stretch. The jury would have to believe it was at least 51% likely that the act of car-to-wheelchair transfer caused the injury—not the heavy mill equipment falling on him in the first place, not the efforts of his coworkers to move him from the scene, not the efforts of his coworkers to load him into the truck, not the bouncy ride in the back of a truck over to the hospital. The plaintiff was able to overcome a big causation hurdle because the identity of the nurse was not known.
The plaintiff would also generally have to show that the coworkers did not mislead the transferring nurse—that is, the statements made at the time of transfer would lead a reasonably skilled nurse to suspect spinal injury, halt transfer attempts, and see to it the patient’s spine was immobilized. Although doubtful, it is possible that in the split seconds when the car arrived at the ED, the initial communications were errant and a reasonable nurse would not have just cause to suspect spinal injury. However, we will never know. We don’t have testimony on what was said during transfer.
Continue to: So we don't know who...
So we don’t know who the nurse was. We don’t know what was said. We don’t know exactly how the plaintiff was transferred out of the vehicle. And those mysteries, to a jury, are suspicious.
IN SUMMARY
Any time a lawyer can draw a giant “?” on a whiteboard during summation, rest assured, someone is in trouble. That someone could be you. I’ve seen lots of question marks in my life; none carry a $1 million/$3 million malpractice policy. The presence of a mystery will transform a case that was defensible into one with unanswered questions. Those unanswered questions open the door to the suggestion or outright accusation of a cover-up. Do your best to document details and work within your system to encourage documentation. In short, don’t let the plaintiff host a mystery party at your expense.
Sometimes You Should Order Another Test
On August 7, 2012, a 44-year-old electrical engineer sustained a knee injury. He initially sought treatment at an emergency department (ED) in Indiana, where he lived, and was released with a splint on his leg.
On August 9, the patient presented to an Illinois medical clinic. He was seen by a physician who referred the patient to another physician at the clinic for evaluation for surgery. The procedure, to repair ruptured ligaments in the patient’s left knee, was scheduled for August 16.
After returning home, the patient called the first physician with complaints that his splinted left knee, calf, and leg felt hot. The physician sent approval for the patient to undergo Doppler imaging of his left leg at a hospital in Indiana. The Doppler was performed on August 10, and the results were sent to the referring physician. The imaging was negative for any abnormalities.
On the morning of August 13, the patient presented to the Illinois medical clinic for presurgical clearance. The examination was performed by an NP, and then the patient had a presurgical consultation with the surgeon. The patient allegedly reported to the NP and the surgeon that he had continuing pain, swelling, and heat sensation in his left leg. Relying on the Doppler performed a few days earlier, the surgeon told the patient that these symptoms were related to the knee trauma he had sustained. No additional Doppler imaging was ordered.
On August 16, the patient was anesthetized in preparation for surgery and soon thereafter suffered a pulmonary embolism (PE) when a deep vein thrombosis (DVT) in his left leg detached and traveled to his lung. He went into pulmonary arrest, coded, and was declared brain dead within hours of arriving for surgery.
The decedent left behind a wife and 2 daughters, ages 12 and 14. His wife, as the administrator of her husband’s estate, sued the NP and her employer. The 2 orthopedic physicians, a treating cardiologist, and their employer were named as respondents in discovery. The physicians’ employer was ultimately added as a defendant, along with the NP’s employer. Prior to trial, the 3 physicians and the NP were dismissed from the case. The matter proceeded against the 2 employing organizations.
The estate alleged that the NP and operating surgeon/physician, each as agents of their respective employers, failed to order a second Doppler image of the decedent’s left leg during presurgical clearance procedures and in the 4 days leading up to the surgery. The estate alleged that a second Doppler was needed because the decedent had complaints that were consistent with DVT—such as continuing pain, swelling, and heat sensation—in his left leg at the August 13 visit. The estate argued that the failure to order a second Doppler led to a failure to diagnose the DVT from which the decedent was suffering symptoms. The estate alleged that the earlier Doppler was performed too soon after the decedent’s injury to show a DVT, as DVTs do not develop immediately after trauma but grow and spread over time.
Continue to: While pain, swelling, and warmth...
While pain, swelling, and warmth/heat sensation are symptoms that accompany trauma, the estate asserted that these are also symptoms of a DVT and that the surgeon, as a reasonable orthopedic physician, should have tested the decedent for a DVT on August 13, on the morning of the surgery, or any day in between and that he also should have ordered a hematologic consult. The estate’s orthopedic surgery expert testified that the surgeon violated the standard of care by failing to appreciate the symptoms and risk factors the decedent was experiencing. The expert testified that the decedent had 4 of 5 high-risk factors for a DVT: Although there was no family history of DVT, the decedent had sustained leg trauma, he was older than 40, his leg was immobilized, and he was considered obese (BMI > 30).
The same orthopedist further opined that a Doppler performed on August 13 more likely than not would have shown the DVT, and the August 16 knee surgery would have been delayed until it was treated. He added that the failure to perform a second Doppler before surgery constituted negligence that caused the decedent’s death. The estate’s hematology expert testified that the decedent was a candidate for prophylactic anticoagulation on August 13 and, if a Doppler had been performed, the need for such medication would have been discovered.
The defense argued that the decedent’s signs and symptoms did not change following the Doppler on August 10 and, therefore, there was no reason for the surgeon to order a second Doppler prior to performing surgery. The defense further argued that the NP, in the scope of her practice, was not allowed to order a Doppler, knew that the surgeon would be seeing the decedent during the same visit, and could rely on the surgeon to order the necessary presurgical tests. The defense’s orthopedics expert testified that, unless there was an increase in signs and/or symptoms, the standard of care did not require the surgeon to order another Doppler. The expert further testified that the surgeon did not place the decedent on a presurgical anticoagulant because this would have increased his risk for bleeding. The defense’s hematology expert testified that there was no guarantee an anticoagulant would have prevented the PE because of the large size of the clot. He further stated that the decedent was not a candidate for prophylactic anticoagulants prior to surgery because the Doppler was negative for clotting, and there was no increase in his symptoms after the Doppler was performed.
The estate’s NP expert testified that, while performing the presurgical clearance, the defendant NP failed to obtain the Doppler history or a full description of the patient’s symptoms (which resulted from a DVT) and failed to order a Doppler. The defense’s NP expert testified that, based on the defendant NP’s testimony, she was not allowed to order a Doppler, and that, as an NP, she would have had a document in her credentials setting forth what she can and cannot recommend. Since such a document was not produced, this could not be determined, she opined.
VERDICT
After an 11-day trial and 2.5 hours of deliberation, the jury found in favor of the plaintiff estate. The jury found the NP’s employer not liable but the physicians’ employer responsible. Damages totaling $5,511,567 were awarded to the estate.
Continue to: COMMENTARY
COMMENTARY
If the Grim Reaper had an Employee of the Month plaque, DVT would proudly see its name etched thereon about 8 months of the year. Missed bleeding takes second place (muttering under its breath, promising to “up its game” next year). You may think the pathophysiology is boring. DVTs don’t care. They just kill.
While these comments may seem flip, the intention is not to minimize the threat posed by DVTs but rather to underscore it. DVT/PE is one of the most missed clinical entities giving rise to litigation; it is legally problematic because its development is often foreseeable. There is a clear setup (eg, surgery or immobilization) and a disease process that is easily understood by even lay people. Jurors understand the concept of a “clot”—if you aren’t moving much, you are apt to get a “clog” and if the clog is discovered and “dissolved” the threat goes away, but if it “breaks loose” it could kill. Jurors need not understand highbrow concepts such as the renin-angiotensin-aldosterone system or the hypothalamic pituitary adrenal axis; it is a clog. This is common sense; during deliberations, jurors will reason that if they “get it,” why couldn’t you?
To add insult to (endothelial) injury, DVT and PE are generally curable; most patients recover fully with proper treatment. Plaintiff’s counsel can trot out the tried-and-true argument: “If a simple, painless ultrasound test had been done, [the patient] would be having dinner with his family tonight.” Furthermore, affected patients are apt to be on the younger side, with a lengthy employment life ahead of them—potentially giving rise to substantial loss-of-earnings damages.
In the case presented here, we are told that the decedent complained of “continuing pain, swelling, and heat sensation” when he saw both an NP and a surgeon for presurgical clearance. We do not know if his leg was examined, but if it had been, the positive and negative findings likely would have been discussed in the case synopsis. It appears the NP and the surgeon saw the leg in the immobilizer and decided to rely on the previous negative Doppler.
First, let’s address the diagnosis of DVT: We should all realize that Homan’s sign sucks. You have permission to elicit Homan’s sign if you are in a museum for antiquated medicine (where other artifacts include AZT monotherapy, bite-and-swallow nifedipine for hypertensive urgency, and meperidine for sphincter of Oddi spasm). Everywhere else on the planet, Homan’s sign has always been bad and is certainly not the standard of care. If you are still attempting to elicit Homan’s sign—cut it out. It is the 1970s leisure suit in your closet: never was any good, never is going to be. Declutter your clinical test arsenal and KonMari Homan’s sign straight to the junk pile.
Continue to: A diagnostic tool...
A diagnostic tool that works better is the Wells’ Criteria, which operates on a points system (with 3-8 points indicating high probability of DVT, 1-2 points indicating moderate probability, and less than 1 point indicating low probability).1 Patients are assessed according to the following criteria:
- Paralysis, paresis, or recent orthopedic casting of lower extremity (1 pt)
- Recently bedridden (> 3 d) or major surgery within past 4 weeks (1 pt)
- Localized tenderness in deep vein system (1 pt)
- Swelling of entire leg (1 pt)
- Calf swelling 3 cm greater than other leg (measured 10 cm below the tibial tuberosity) (1 pt)
- Pitting edema greater in the symptomatic leg (1 pt)
- Collateral nonvaricose superficial veins (1 pt)
- Active cancer or cancer treated within 6 months (1 pt)
- Alternative diagnosis more likely than DVT (Baker cyst, cellulitis, muscle damage, superficial venous thrombosis, postphlebitic syndrome, inguinal lymphadenopathy, external venous compression) (–2 pts).1
Moving through the Wells’ score system, we don’t have enough clinical data input for this patient. We do know he had leg pain and possibly swelling (1 pt). His knee was immobilized (albeit without plaster) (1 pt). We don’t know how “bedridden” he was. I’ll argue we should not deduct for “an alternative diagnosis more likely” because the decedent injured his knee and we can expect knee pain and knee swelling, not symptoms and findings involving the entire leg. So this patient would score at least 1 point, possibly 2, and thus be stratified as “moderate probability.”
There was an initial suspicion of DVT in this patient, and a Doppler was ordered on August 10. The patient’s symptoms persisted. However, in light of the negative Doppler results, the continued symptoms were attributed to the knee derangement and not a DVT.
Which brings us to the first malpractice trap: reliance on a prior negative study to rule out a dynamic condition. For any condition that can evolve, do not hesitate to order a repeat test when needed. DVT is a dynamic process; given the right clinical setup (in this case, immobility and ongoing/increasing symptoms), a clinician should not be bashful about ordering a follow-up study. As providers, we recognize the static nature of certain studies and have no reservations about ordering serial complete blood counts or a repeat chest film. Yet we are more reluctant to order repeat studies for equally dynamic disease processes—even when they are required by the standard of care. Here, reliance on a 3-day-old Doppler was problematic. Don’t rely on an old study if the disease under suspicion evolves rapidly.
The second trap: Do not allow yourself to be scolded (or engage in self-scolding) if a correctly ordered test is negative. A clinical decision is correct if it is based on science and in the interest of safeguarding the patient. Don’t buy into the trap that your decision needs to be validated by a positive result. Here, the persisting or worsening leg pain with entire leg swelling warranted a new study—even if the result was expected to be negative.
Continue to: When your decision to order...
When your decision to order such a test is challenged, my favorite rhetorical defense is “Those are some pretty big dice to roll.” That is what you are doing if you skip a test that should be ordered. The bounceback kid with a prior negative lumbar puncture, who now appears toxic, needs a repeat tap. Why? Because you cannot afford to miss meningitis—that would be a risky roll of the dice.
One final point about this case: The NP argued “she was not allowed to order a Doppler” and her NP expert made the argument that “she would have had a document in her credentials setting forth what she can and cannot recommend.” The expert testified she could not find such a document and “could not determine” whether the defendant NP could “recommend” that test. I don’t fault the tactical decision to use this argument, in this case, when the surgeon also saw the patient on the same day. However, we should all recognize this will not normally work. A clinician cannot credibly argue she is not “credentialed” to recommend a course of action she can’t presently deliver.
Consider a clinician employed in an urgent care center without direct access to order CT. She evaluates an 80-year-old woman on warfarin who slipped and struck her head on a marble table. The standard of care requires a CT scan (likely several) to rule out an intracranial bleed. The urgent care clinician cannot send the patient away and later claim she was “not credentialed” to recommend CT imaging to rule out an intracranial bleed. As a matter of the standard of care, our hypothetical clinician would be duty bound to advise the patient of the risk for bleeding and then take steps to arrange for that care—even though she is not in a position to personally deliver it.
IN SUMMARY
Protect your patients from evolving cases by ordering updated tests. Do not be afraid of a negative result. Instead, fear the Reaper; keep him away from your patients. Let him get his “more cowbell” somewhere else.
1. Wells PS, Anderson DR, Bormanis J, et al. Value of assessment of pretest probability of deep-vein thrombosis in clinical management. Lancet. 1997;350(9094):1795-1798.
On August 7, 2012, a 44-year-old electrical engineer sustained a knee injury. He initially sought treatment at an emergency department (ED) in Indiana, where he lived, and was released with a splint on his leg.
On August 9, the patient presented to an Illinois medical clinic. He was seen by a physician who referred the patient to another physician at the clinic for evaluation for surgery. The procedure, to repair ruptured ligaments in the patient’s left knee, was scheduled for August 16.
After returning home, the patient called the first physician with complaints that his splinted left knee, calf, and leg felt hot. The physician sent approval for the patient to undergo Doppler imaging of his left leg at a hospital in Indiana. The Doppler was performed on August 10, and the results were sent to the referring physician. The imaging was negative for any abnormalities.
On the morning of August 13, the patient presented to the Illinois medical clinic for presurgical clearance. The examination was performed by an NP, and then the patient had a presurgical consultation with the surgeon. The patient allegedly reported to the NP and the surgeon that he had continuing pain, swelling, and heat sensation in his left leg. Relying on the Doppler performed a few days earlier, the surgeon told the patient that these symptoms were related to the knee trauma he had sustained. No additional Doppler imaging was ordered.
On August 16, the patient was anesthetized in preparation for surgery and soon thereafter suffered a pulmonary embolism (PE) when a deep vein thrombosis (DVT) in his left leg detached and traveled to his lung. He went into pulmonary arrest, coded, and was declared brain dead within hours of arriving for surgery.
The decedent left behind a wife and 2 daughters, ages 12 and 14. His wife, as the administrator of her husband’s estate, sued the NP and her employer. The 2 orthopedic physicians, a treating cardiologist, and their employer were named as respondents in discovery. The physicians’ employer was ultimately added as a defendant, along with the NP’s employer. Prior to trial, the 3 physicians and the NP were dismissed from the case. The matter proceeded against the 2 employing organizations.
The estate alleged that the NP and operating surgeon/physician, each as agents of their respective employers, failed to order a second Doppler image of the decedent’s left leg during presurgical clearance procedures and in the 4 days leading up to the surgery. The estate alleged that a second Doppler was needed because the decedent had complaints that were consistent with DVT—such as continuing pain, swelling, and heat sensation—in his left leg at the August 13 visit. The estate argued that the failure to order a second Doppler led to a failure to diagnose the DVT from which the decedent was suffering symptoms. The estate alleged that the earlier Doppler was performed too soon after the decedent’s injury to show a DVT, as DVTs do not develop immediately after trauma but grow and spread over time.
Continue to: While pain, swelling, and warmth...
While pain, swelling, and warmth/heat sensation are symptoms that accompany trauma, the estate asserted that these are also symptoms of a DVT and that the surgeon, as a reasonable orthopedic physician, should have tested the decedent for a DVT on August 13, on the morning of the surgery, or any day in between and that he also should have ordered a hematologic consult. The estate’s orthopedic surgery expert testified that the surgeon violated the standard of care by failing to appreciate the symptoms and risk factors the decedent was experiencing. The expert testified that the decedent had 4 of 5 high-risk factors for a DVT: Although there was no family history of DVT, the decedent had sustained leg trauma, he was older than 40, his leg was immobilized, and he was considered obese (BMI > 30).
The same orthopedist further opined that a Doppler performed on August 13 more likely than not would have shown the DVT, and the August 16 knee surgery would have been delayed until it was treated. He added that the failure to perform a second Doppler before surgery constituted negligence that caused the decedent’s death. The estate’s hematology expert testified that the decedent was a candidate for prophylactic anticoagulation on August 13 and, if a Doppler had been performed, the need for such medication would have been discovered.
The defense argued that the decedent’s signs and symptoms did not change following the Doppler on August 10 and, therefore, there was no reason for the surgeon to order a second Doppler prior to performing surgery. The defense further argued that the NP, in the scope of her practice, was not allowed to order a Doppler, knew that the surgeon would be seeing the decedent during the same visit, and could rely on the surgeon to order the necessary presurgical tests. The defense’s orthopedics expert testified that, unless there was an increase in signs and/or symptoms, the standard of care did not require the surgeon to order another Doppler. The expert further testified that the surgeon did not place the decedent on a presurgical anticoagulant because this would have increased his risk for bleeding. The defense’s hematology expert testified that there was no guarantee an anticoagulant would have prevented the PE because of the large size of the clot. He further stated that the decedent was not a candidate for prophylactic anticoagulants prior to surgery because the Doppler was negative for clotting, and there was no increase in his symptoms after the Doppler was performed.
The estate’s NP expert testified that, while performing the presurgical clearance, the defendant NP failed to obtain the Doppler history or a full description of the patient’s symptoms (which resulted from a DVT) and failed to order a Doppler. The defense’s NP expert testified that, based on the defendant NP’s testimony, she was not allowed to order a Doppler, and that, as an NP, she would have had a document in her credentials setting forth what she can and cannot recommend. Since such a document was not produced, this could not be determined, she opined.
VERDICT
After an 11-day trial and 2.5 hours of deliberation, the jury found in favor of the plaintiff estate. The jury found the NP’s employer not liable but the physicians’ employer responsible. Damages totaling $5,511,567 were awarded to the estate.
Continue to: COMMENTARY
COMMENTARY
If the Grim Reaper had an Employee of the Month plaque, DVT would proudly see its name etched thereon about 8 months of the year. Missed bleeding takes second place (muttering under its breath, promising to “up its game” next year). You may think the pathophysiology is boring. DVTs don’t care. They just kill.
While these comments may seem flip, the intention is not to minimize the threat posed by DVTs but rather to underscore it. DVT/PE is one of the most missed clinical entities giving rise to litigation; it is legally problematic because its development is often foreseeable. There is a clear setup (eg, surgery or immobilization) and a disease process that is easily understood by even lay people. Jurors understand the concept of a “clot”—if you aren’t moving much, you are apt to get a “clog” and if the clog is discovered and “dissolved” the threat goes away, but if it “breaks loose” it could kill. Jurors need not understand highbrow concepts such as the renin-angiotensin-aldosterone system or the hypothalamic pituitary adrenal axis; it is a clog. This is common sense; during deliberations, jurors will reason that if they “get it,” why couldn’t you?
To add insult to (endothelial) injury, DVT and PE are generally curable; most patients recover fully with proper treatment. Plaintiff’s counsel can trot out the tried-and-true argument: “If a simple, painless ultrasound test had been done, [the patient] would be having dinner with his family tonight.” Furthermore, affected patients are apt to be on the younger side, with a lengthy employment life ahead of them—potentially giving rise to substantial loss-of-earnings damages.
In the case presented here, we are told that the decedent complained of “continuing pain, swelling, and heat sensation” when he saw both an NP and a surgeon for presurgical clearance. We do not know if his leg was examined, but if it had been, the positive and negative findings likely would have been discussed in the case synopsis. It appears the NP and the surgeon saw the leg in the immobilizer and decided to rely on the previous negative Doppler.
First, let’s address the diagnosis of DVT: We should all realize that Homan’s sign sucks. You have permission to elicit Homan’s sign if you are in a museum for antiquated medicine (where other artifacts include AZT monotherapy, bite-and-swallow nifedipine for hypertensive urgency, and meperidine for sphincter of Oddi spasm). Everywhere else on the planet, Homan’s sign has always been bad and is certainly not the standard of care. If you are still attempting to elicit Homan’s sign—cut it out. It is the 1970s leisure suit in your closet: never was any good, never is going to be. Declutter your clinical test arsenal and KonMari Homan’s sign straight to the junk pile.
Continue to: A diagnostic tool...
A diagnostic tool that works better is the Wells’ Criteria, which operates on a points system (with 3-8 points indicating high probability of DVT, 1-2 points indicating moderate probability, and less than 1 point indicating low probability).1 Patients are assessed according to the following criteria:
- Paralysis, paresis, or recent orthopedic casting of lower extremity (1 pt)
- Recently bedridden (> 3 d) or major surgery within past 4 weeks (1 pt)
- Localized tenderness in deep vein system (1 pt)
- Swelling of entire leg (1 pt)
- Calf swelling 3 cm greater than other leg (measured 10 cm below the tibial tuberosity) (1 pt)
- Pitting edema greater in the symptomatic leg (1 pt)
- Collateral nonvaricose superficial veins (1 pt)
- Active cancer or cancer treated within 6 months (1 pt)
- Alternative diagnosis more likely than DVT (Baker cyst, cellulitis, muscle damage, superficial venous thrombosis, postphlebitic syndrome, inguinal lymphadenopathy, external venous compression) (–2 pts).1
Moving through the Wells’ score system, we don’t have enough clinical data input for this patient. We do know he had leg pain and possibly swelling (1 pt). His knee was immobilized (albeit without plaster) (1 pt). We don’t know how “bedridden” he was. I’ll argue we should not deduct for “an alternative diagnosis more likely” because the decedent injured his knee and we can expect knee pain and knee swelling, not symptoms and findings involving the entire leg. So this patient would score at least 1 point, possibly 2, and thus be stratified as “moderate probability.”
There was an initial suspicion of DVT in this patient, and a Doppler was ordered on August 10. The patient’s symptoms persisted. However, in light of the negative Doppler results, the continued symptoms were attributed to the knee derangement and not a DVT.
Which brings us to the first malpractice trap: reliance on a prior negative study to rule out a dynamic condition. For any condition that can evolve, do not hesitate to order a repeat test when needed. DVT is a dynamic process; given the right clinical setup (in this case, immobility and ongoing/increasing symptoms), a clinician should not be bashful about ordering a follow-up study. As providers, we recognize the static nature of certain studies and have no reservations about ordering serial complete blood counts or a repeat chest film. Yet we are more reluctant to order repeat studies for equally dynamic disease processes—even when they are required by the standard of care. Here, reliance on a 3-day-old Doppler was problematic. Don’t rely on an old study if the disease under suspicion evolves rapidly.
The second trap: Do not allow yourself to be scolded (or engage in self-scolding) if a correctly ordered test is negative. A clinical decision is correct if it is based on science and in the interest of safeguarding the patient. Don’t buy into the trap that your decision needs to be validated by a positive result. Here, the persisting or worsening leg pain with entire leg swelling warranted a new study—even if the result was expected to be negative.
Continue to: When your decision to order...
When your decision to order such a test is challenged, my favorite rhetorical defense is “Those are some pretty big dice to roll.” That is what you are doing if you skip a test that should be ordered. The bounceback kid with a prior negative lumbar puncture, who now appears toxic, needs a repeat tap. Why? Because you cannot afford to miss meningitis—that would be a risky roll of the dice.
One final point about this case: The NP argued “she was not allowed to order a Doppler” and her NP expert made the argument that “she would have had a document in her credentials setting forth what she can and cannot recommend.” The expert testified she could not find such a document and “could not determine” whether the defendant NP could “recommend” that test. I don’t fault the tactical decision to use this argument, in this case, when the surgeon also saw the patient on the same day. However, we should all recognize this will not normally work. A clinician cannot credibly argue she is not “credentialed” to recommend a course of action she can’t presently deliver.
Consider a clinician employed in an urgent care center without direct access to order CT. She evaluates an 80-year-old woman on warfarin who slipped and struck her head on a marble table. The standard of care requires a CT scan (likely several) to rule out an intracranial bleed. The urgent care clinician cannot send the patient away and later claim she was “not credentialed” to recommend CT imaging to rule out an intracranial bleed. As a matter of the standard of care, our hypothetical clinician would be duty bound to advise the patient of the risk for bleeding and then take steps to arrange for that care—even though she is not in a position to personally deliver it.
IN SUMMARY
Protect your patients from evolving cases by ordering updated tests. Do not be afraid of a negative result. Instead, fear the Reaper; keep him away from your patients. Let him get his “more cowbell” somewhere else.
On August 7, 2012, a 44-year-old electrical engineer sustained a knee injury. He initially sought treatment at an emergency department (ED) in Indiana, where he lived, and was released with a splint on his leg.
On August 9, the patient presented to an Illinois medical clinic. He was seen by a physician who referred the patient to another physician at the clinic for evaluation for surgery. The procedure, to repair ruptured ligaments in the patient’s left knee, was scheduled for August 16.
After returning home, the patient called the first physician with complaints that his splinted left knee, calf, and leg felt hot. The physician sent approval for the patient to undergo Doppler imaging of his left leg at a hospital in Indiana. The Doppler was performed on August 10, and the results were sent to the referring physician. The imaging was negative for any abnormalities.
On the morning of August 13, the patient presented to the Illinois medical clinic for presurgical clearance. The examination was performed by an NP, and then the patient had a presurgical consultation with the surgeon. The patient allegedly reported to the NP and the surgeon that he had continuing pain, swelling, and heat sensation in his left leg. Relying on the Doppler performed a few days earlier, the surgeon told the patient that these symptoms were related to the knee trauma he had sustained. No additional Doppler imaging was ordered.
On August 16, the patient was anesthetized in preparation for surgery and soon thereafter suffered a pulmonary embolism (PE) when a deep vein thrombosis (DVT) in his left leg detached and traveled to his lung. He went into pulmonary arrest, coded, and was declared brain dead within hours of arriving for surgery.
The decedent left behind a wife and 2 daughters, ages 12 and 14. His wife, as the administrator of her husband’s estate, sued the NP and her employer. The 2 orthopedic physicians, a treating cardiologist, and their employer were named as respondents in discovery. The physicians’ employer was ultimately added as a defendant, along with the NP’s employer. Prior to trial, the 3 physicians and the NP were dismissed from the case. The matter proceeded against the 2 employing organizations.
The estate alleged that the NP and operating surgeon/physician, each as agents of their respective employers, failed to order a second Doppler image of the decedent’s left leg during presurgical clearance procedures and in the 4 days leading up to the surgery. The estate alleged that a second Doppler was needed because the decedent had complaints that were consistent with DVT—such as continuing pain, swelling, and heat sensation—in his left leg at the August 13 visit. The estate argued that the failure to order a second Doppler led to a failure to diagnose the DVT from which the decedent was suffering symptoms. The estate alleged that the earlier Doppler was performed too soon after the decedent’s injury to show a DVT, as DVTs do not develop immediately after trauma but grow and spread over time.
Continue to: While pain, swelling, and warmth...
While pain, swelling, and warmth/heat sensation are symptoms that accompany trauma, the estate asserted that these are also symptoms of a DVT and that the surgeon, as a reasonable orthopedic physician, should have tested the decedent for a DVT on August 13, on the morning of the surgery, or any day in between and that he also should have ordered a hematologic consult. The estate’s orthopedic surgery expert testified that the surgeon violated the standard of care by failing to appreciate the symptoms and risk factors the decedent was experiencing. The expert testified that the decedent had 4 of 5 high-risk factors for a DVT: Although there was no family history of DVT, the decedent had sustained leg trauma, he was older than 40, his leg was immobilized, and he was considered obese (BMI > 30).
The same orthopedist further opined that a Doppler performed on August 13 more likely than not would have shown the DVT, and the August 16 knee surgery would have been delayed until it was treated. He added that the failure to perform a second Doppler before surgery constituted negligence that caused the decedent’s death. The estate’s hematology expert testified that the decedent was a candidate for prophylactic anticoagulation on August 13 and, if a Doppler had been performed, the need for such medication would have been discovered.
The defense argued that the decedent’s signs and symptoms did not change following the Doppler on August 10 and, therefore, there was no reason for the surgeon to order a second Doppler prior to performing surgery. The defense further argued that the NP, in the scope of her practice, was not allowed to order a Doppler, knew that the surgeon would be seeing the decedent during the same visit, and could rely on the surgeon to order the necessary presurgical tests. The defense’s orthopedics expert testified that, unless there was an increase in signs and/or symptoms, the standard of care did not require the surgeon to order another Doppler. The expert further testified that the surgeon did not place the decedent on a presurgical anticoagulant because this would have increased his risk for bleeding. The defense’s hematology expert testified that there was no guarantee an anticoagulant would have prevented the PE because of the large size of the clot. He further stated that the decedent was not a candidate for prophylactic anticoagulants prior to surgery because the Doppler was negative for clotting, and there was no increase in his symptoms after the Doppler was performed.
The estate’s NP expert testified that, while performing the presurgical clearance, the defendant NP failed to obtain the Doppler history or a full description of the patient’s symptoms (which resulted from a DVT) and failed to order a Doppler. The defense’s NP expert testified that, based on the defendant NP’s testimony, she was not allowed to order a Doppler, and that, as an NP, she would have had a document in her credentials setting forth what she can and cannot recommend. Since such a document was not produced, this could not be determined, she opined.
VERDICT
After an 11-day trial and 2.5 hours of deliberation, the jury found in favor of the plaintiff estate. The jury found the NP’s employer not liable but the physicians’ employer responsible. Damages totaling $5,511,567 were awarded to the estate.
Continue to: COMMENTARY
COMMENTARY
If the Grim Reaper had an Employee of the Month plaque, DVT would proudly see its name etched thereon about 8 months of the year. Missed bleeding takes second place (muttering under its breath, promising to “up its game” next year). You may think the pathophysiology is boring. DVTs don’t care. They just kill.
While these comments may seem flip, the intention is not to minimize the threat posed by DVTs but rather to underscore it. DVT/PE is one of the most missed clinical entities giving rise to litigation; it is legally problematic because its development is often foreseeable. There is a clear setup (eg, surgery or immobilization) and a disease process that is easily understood by even lay people. Jurors understand the concept of a “clot”—if you aren’t moving much, you are apt to get a “clog” and if the clog is discovered and “dissolved” the threat goes away, but if it “breaks loose” it could kill. Jurors need not understand highbrow concepts such as the renin-angiotensin-aldosterone system or the hypothalamic pituitary adrenal axis; it is a clog. This is common sense; during deliberations, jurors will reason that if they “get it,” why couldn’t you?
To add insult to (endothelial) injury, DVT and PE are generally curable; most patients recover fully with proper treatment. Plaintiff’s counsel can trot out the tried-and-true argument: “If a simple, painless ultrasound test had been done, [the patient] would be having dinner with his family tonight.” Furthermore, affected patients are apt to be on the younger side, with a lengthy employment life ahead of them—potentially giving rise to substantial loss-of-earnings damages.
In the case presented here, we are told that the decedent complained of “continuing pain, swelling, and heat sensation” when he saw both an NP and a surgeon for presurgical clearance. We do not know if his leg was examined, but if it had been, the positive and negative findings likely would have been discussed in the case synopsis. It appears the NP and the surgeon saw the leg in the immobilizer and decided to rely on the previous negative Doppler.
First, let’s address the diagnosis of DVT: We should all realize that Homan’s sign sucks. You have permission to elicit Homan’s sign if you are in a museum for antiquated medicine (where other artifacts include AZT monotherapy, bite-and-swallow nifedipine for hypertensive urgency, and meperidine for sphincter of Oddi spasm). Everywhere else on the planet, Homan’s sign has always been bad and is certainly not the standard of care. If you are still attempting to elicit Homan’s sign—cut it out. It is the 1970s leisure suit in your closet: never was any good, never is going to be. Declutter your clinical test arsenal and KonMari Homan’s sign straight to the junk pile.
Continue to: A diagnostic tool...
A diagnostic tool that works better is the Wells’ Criteria, which operates on a points system (with 3-8 points indicating high probability of DVT, 1-2 points indicating moderate probability, and less than 1 point indicating low probability).1 Patients are assessed according to the following criteria:
- Paralysis, paresis, or recent orthopedic casting of lower extremity (1 pt)
- Recently bedridden (> 3 d) or major surgery within past 4 weeks (1 pt)
- Localized tenderness in deep vein system (1 pt)
- Swelling of entire leg (1 pt)
- Calf swelling 3 cm greater than other leg (measured 10 cm below the tibial tuberosity) (1 pt)
- Pitting edema greater in the symptomatic leg (1 pt)
- Collateral nonvaricose superficial veins (1 pt)
- Active cancer or cancer treated within 6 months (1 pt)
- Alternative diagnosis more likely than DVT (Baker cyst, cellulitis, muscle damage, superficial venous thrombosis, postphlebitic syndrome, inguinal lymphadenopathy, external venous compression) (–2 pts).1
Moving through the Wells’ score system, we don’t have enough clinical data input for this patient. We do know he had leg pain and possibly swelling (1 pt). His knee was immobilized (albeit without plaster) (1 pt). We don’t know how “bedridden” he was. I’ll argue we should not deduct for “an alternative diagnosis more likely” because the decedent injured his knee and we can expect knee pain and knee swelling, not symptoms and findings involving the entire leg. So this patient would score at least 1 point, possibly 2, and thus be stratified as “moderate probability.”
There was an initial suspicion of DVT in this patient, and a Doppler was ordered on August 10. The patient’s symptoms persisted. However, in light of the negative Doppler results, the continued symptoms were attributed to the knee derangement and not a DVT.
Which brings us to the first malpractice trap: reliance on a prior negative study to rule out a dynamic condition. For any condition that can evolve, do not hesitate to order a repeat test when needed. DVT is a dynamic process; given the right clinical setup (in this case, immobility and ongoing/increasing symptoms), a clinician should not be bashful about ordering a follow-up study. As providers, we recognize the static nature of certain studies and have no reservations about ordering serial complete blood counts or a repeat chest film. Yet we are more reluctant to order repeat studies for equally dynamic disease processes—even when they are required by the standard of care. Here, reliance on a 3-day-old Doppler was problematic. Don’t rely on an old study if the disease under suspicion evolves rapidly.
The second trap: Do not allow yourself to be scolded (or engage in self-scolding) if a correctly ordered test is negative. A clinical decision is correct if it is based on science and in the interest of safeguarding the patient. Don’t buy into the trap that your decision needs to be validated by a positive result. Here, the persisting or worsening leg pain with entire leg swelling warranted a new study—even if the result was expected to be negative.
Continue to: When your decision to order...
When your decision to order such a test is challenged, my favorite rhetorical defense is “Those are some pretty big dice to roll.” That is what you are doing if you skip a test that should be ordered. The bounceback kid with a prior negative lumbar puncture, who now appears toxic, needs a repeat tap. Why? Because you cannot afford to miss meningitis—that would be a risky roll of the dice.
One final point about this case: The NP argued “she was not allowed to order a Doppler” and her NP expert made the argument that “she would have had a document in her credentials setting forth what she can and cannot recommend.” The expert testified she could not find such a document and “could not determine” whether the defendant NP could “recommend” that test. I don’t fault the tactical decision to use this argument, in this case, when the surgeon also saw the patient on the same day. However, we should all recognize this will not normally work. A clinician cannot credibly argue she is not “credentialed” to recommend a course of action she can’t presently deliver.
Consider a clinician employed in an urgent care center without direct access to order CT. She evaluates an 80-year-old woman on warfarin who slipped and struck her head on a marble table. The standard of care requires a CT scan (likely several) to rule out an intracranial bleed. The urgent care clinician cannot send the patient away and later claim she was “not credentialed” to recommend CT imaging to rule out an intracranial bleed. As a matter of the standard of care, our hypothetical clinician would be duty bound to advise the patient of the risk for bleeding and then take steps to arrange for that care—even though she is not in a position to personally deliver it.
IN SUMMARY
Protect your patients from evolving cases by ordering updated tests. Do not be afraid of a negative result. Instead, fear the Reaper; keep him away from your patients. Let him get his “more cowbell” somewhere else.
1. Wells PS, Anderson DR, Bormanis J, et al. Value of assessment of pretest probability of deep-vein thrombosis in clinical management. Lancet. 1997;350(9094):1795-1798.
1. Wells PS, Anderson DR, Bormanis J, et al. Value of assessment of pretest probability of deep-vein thrombosis in clinical management. Lancet. 1997;350(9094):1795-1798.
Good news for ObGyns: Medical liability claims resulting in payment are decreasing!
Medical professional liability claims (claims) are a major cause of worry and agony for physicians who are dedicated to optimizing the health of all their patients. Among physicians, those who practice neurosurgery, thoracic surgery, plastic surgery, and obstetrics and gynecology have the greatest rate of making a payment on a claim per year of practice.1 Physicians who practice psychiatry, pediatrics, pathology, and internal medicine have the lowest rate of making a payment on a claim. Among the physicians in high-risk specialties, greater than 90% will have a claim filed against them during their career.2 Although professional liability exposure reached a crisis during the 1980s and 1990s, recent data have shown a decrease in overall professional liability risk.
The good news: Paid claims per 1,000 ObGyns have decreased greatly
In a review of all paid claims reported to the National Practitioner Data Bank from 1992 to 2014, the annual rate of paid claims per 1,000 ObGyn physician-years was determined.1 For the time periods 1992–1996, 1997–2002, 2003–2008,and 2009–2014, the annual rate of paid claims per 1,000 ObGyn physician-years was 57.6, 51.5, 40.0, and 25.9, representing an astounding 55% decrease in paid claims from 1992 to 2014 (FIGURE).1
The majority of claims result in no payment
In a review of the experience of a nationwide professional liability insurer from 1991 to 2005, only 22% of claims resulted in a payment.2 In this study, for obstetrics and gynecology and gynecologic surgery, only 11% and 8% of claims, respectively, resulted in a payment.2 However, being named in a malpractice claim results in significant stress for a physician and requires a great deal of work and time to defend.
In another study using data from the Physician Insurer’s Association of America, among 10,915 claims closed from 2005 to 2014, 59.5% were dropped, withdrawn, or dismissed; 27.7% were settled; 2.5% were resolved using an alternative dispute resolution process; 1.8% were uncategorized; and 8.6% went to trial.3 Of the cases that went to trial, 87% resulted in a verdict for the physician and 13% resulted in a verdict for the plaintiff.3
Not as good news: Payments per claim and claims settling for a payment > $1 million are increasing
In the period 1992–1996, the average payment per paid claim in the field of obstetrics and gynecology was $387,186, rising to $447,034 in 2009–2014—a 16% increase.1 From 2004 to 2010, million dollar payments occurred in about 8% of cases of paid claims, but they represent 36% of the total of all paid claims.4 In the time periods 1992–1996 and 2009–2014, payments greater than $1 million occurred in 6% and 8% of paid claims, respectively.1
Claims settled for much more than $1 million are of great concern to physicians because the payment may exceed their policy limit, creating a complex legal problem that may take time to resolve. In some cases, where the award is greater than the insurance policy limit, aggressive plaintiff attorneys have obtained a lien on the defendant physician’s home pending settlement of the case. When a multimillion dollar payment is made to settle a professional liability claim, it can greatly influence physician practice and change hospital policies. Frequently, following a multimillion dollar payment a physician may decide to limit their practice to low-risk cases or retire from the practice of medicine.
Liability premiums are stable or decreasing
From 2014 to 2019, my ObGyn professional liability insurance premiums decreased by 18%. During the same time period, my colleagues who practice surgical gynecology (no obstetrics) had a premium decrease of 22%. Insurers use a complex algorithm to determine annual liability insurance premiums, and premiums for ObGyns may not have stabilized or decreased in all regions. Take this Instant Poll:
Create your own user feedback survey
Reform of the liability tort system
Litigation policies and practices that reduce liability risk reduce total medical liability losses. Policies that have helped to constrain medical liability risk include state constitutional amendments limiting payments for pain and suffering, caps on compensation to plaintiff attorneys, increased early resolution programs that compensate patients who experience an adverse event and no-fault conflict resolution programs.5 In 2003, Texas implemented a comprehensive package of tort reform laws. Experts believe the reforms decreased the financial burden of professional liability insurance6 and led to less defensive medical practices, reducing excessive use of imaging and laboratory tests.
Medical factors contributing to a decrease in claims
In 1999, the Institute of Medicine released the report, “To Err is Human,” which galvanized health care systems to deploy systems of care that reduce the rate of adverse patient outcomes.7 Over the past 20 years, health systems have implemented quality improvement programs in obstetrics and gynecology that have contributed to a reduction in the rate of adverse patient outcomes. This may have contributed to the decrease in the rate of paid claims.
In a quasi-experimental study performed in 13 health systems, 7 interventions were implemented with the goal of improving outcomes and reducing medical liability. The 7 interventions included8:
- an elective induction bundle focused on the safe use of oxytocin
- an augmentation bundle focused on early intervention for possible fetal metabolic acidosis
- an operative vaginal delivery bundle
- TeamSTEPPS teamwork training to improve the quality of communication
- best practices education with a focus on electronic fetal monitoring
- regular performance feedback to hospitals and clinicians
- implementation of a quality improvement collaboration to support implementation of the interventions.
During the two-year baseline period prior to the intervention there were 185,373 deliveries with 6.7 perinatal claims made per 10,000 deliveries and 1.3 claims paid per 10,000 deliveries. Following the intervention, the rate of claims made and claims paid per 10,000 deliveries decreased by 22% and 37%, respectively. In addition there was a marked decrease in claims over $1 million paid, greatly limiting total financial liability losses.
Experts with vast experience in obstetrics and obstetric liability litigation have identified 4 priority interventions that may improve outcomes and mitigate liability risk, including: 1) 24-hour in-house physician coverage of an obstetrics service, 2) a conservative approach to trial of labor after a prior cesarean delivery, 3) utilization of a comprehensive, standardized event note in cases of a shoulder dystocia, and 4) judicious use of oxytocin, misoprostol, and magnesium sulfate.9
Other health system interventions that may contribute to a reduction in claims include:
- systematic improvement in the quality of communication among physicians and nurses through the use of team training, preprocedure huddles, and time-out processes10
- rapid response systems to rescue hospital patients with worrisome vital signs11
- standardized responses to a worrisome category 2 or 3 fetal heart-rate tracing12
- rapid recognition, evaluation, and treatment of women with hemorrhage, severe hypertension, sepsis, and venous thromboembolism13
- identification and referral of high-risk patients to tertiary centers14
- closed loop communication of critical imaging and laboratory results15
- universal insurance coverage for health care including contraception, obstetrics, and pediatric care.
Medical liability risk is an important practice issue because it causes excessive use of imaging and laboratory tests and often traumatizes clinicians, which can result in burnout. In the 1980s and 1990s, medical liability litigation reached a crescendo and was a prominent concern among obstetrician-gynecologists. The good news is that, for ObGyns, liability risk has stabilized. Hopefully our resolute efforts to continuously improve the quality of care will result in a long-term reduction in medical liability risk.
- Schaffer AC, Jena AB, Seabury SA, et al. Rates and characteristics of paid malpractice claims among US physicians by specialty, 1992–2014. JAMA Intern Med. 2017;177:710-718.
- Jena AB, Seabury S, Lakdawalla D, et al. Malpractice risk according to physician specialty. N Engl J Med. 2011;365:629-636.
- Glaser LM, Alvi FA, Milad MP. Trends in malpractice claims for obstetric and gynecologic procedures, 2005 through 2014. Am J Obstet Gynecol. 2017;217:340.e1-e6.
- Bixenstine PJ, Shore AD, Mehtsun WT, et al. Catastrophic medical malpractice payouts in the United States. J Healthc Quality. 2014;36:43-53.
- Cardoso R, Zarin W, Nincic V, et al. Evaluative reports on medical malpractice policies in obstetrics: a rapid scoping review. Syst Rev. 2017;6:181.
- Stewart RM, Geoghegan K, Myers JG, et al. Malpractice risk and costs are significantly reduced after tort reform. J Am Coll Surg. 2011;212:463-467.
- Institute of Medicine Committee on Quality Health Care in America; Kohn LT, Corrigan JM, Donaldson MS, eds. To Err is Human: Building a Safer Health System. Washington, DC: National Academies Press; 2000.
- Riley W, Meredith LW, Price R, et al. Decreasing malpractice claims by reducing preventable perinatal harm. Health Serv Res. 2016;51(suppl 3):2453-2471.
- Clark SL, Belfort MA, Dildy GA, et al. Reducing obstetric litigation through alterations in practice patterns. Obstet Gynecol. 2008;112:1279-1283.
- Haynes AB, Weiser TG, Berry WR, et al; Safe Surgery Saves Lives Study Group. A surgical safety checklist to reduce morbidity and mortality in a global population. N Engl J Med. 2009;360:491-499.
- Patel S, Gillon SA, Jones DA. Rapid response systems: recognition and rescue of the deteriorating hospital patient. Br J Hosp Med (Lond). 2017;78:143-148.
- Clark SL, Hamilton EF, Garite TJ, et al. The limits of electronic fetal heart rate monitoring in the prevention of neonatal metabolic acidemia. Am J Obstet Gynecol. 2017;216:163.e1-163.e6.
- The Council on Patient Safety in Women’s Healthcare website. www.safehealthcareforeverywoman.org. Accessed April 12, 2019.
- Zahn CM, Remick A, Catalano A, et al. Levels of maternal care verification pilot: translating guidance into practice. Obstet Gynecol. 2018;132:1401-1406.
- Zuccotti G, Maloney FL, Feblowitz J, et al. Reducing risk with clinical decision support: a study of closed malpractice claims. Appl Clin Inform. 2014;5:746-756.
Medical professional liability claims (claims) are a major cause of worry and agony for physicians who are dedicated to optimizing the health of all their patients. Among physicians, those who practice neurosurgery, thoracic surgery, plastic surgery, and obstetrics and gynecology have the greatest rate of making a payment on a claim per year of practice.1 Physicians who practice psychiatry, pediatrics, pathology, and internal medicine have the lowest rate of making a payment on a claim. Among the physicians in high-risk specialties, greater than 90% will have a claim filed against them during their career.2 Although professional liability exposure reached a crisis during the 1980s and 1990s, recent data have shown a decrease in overall professional liability risk.
The good news: Paid claims per 1,000 ObGyns have decreased greatly
In a review of all paid claims reported to the National Practitioner Data Bank from 1992 to 2014, the annual rate of paid claims per 1,000 ObGyn physician-years was determined.1 For the time periods 1992–1996, 1997–2002, 2003–2008,and 2009–2014, the annual rate of paid claims per 1,000 ObGyn physician-years was 57.6, 51.5, 40.0, and 25.9, representing an astounding 55% decrease in paid claims from 1992 to 2014 (FIGURE).1
The majority of claims result in no payment
In a review of the experience of a nationwide professional liability insurer from 1991 to 2005, only 22% of claims resulted in a payment.2 In this study, for obstetrics and gynecology and gynecologic surgery, only 11% and 8% of claims, respectively, resulted in a payment.2 However, being named in a malpractice claim results in significant stress for a physician and requires a great deal of work and time to defend.
In another study using data from the Physician Insurer’s Association of America, among 10,915 claims closed from 2005 to 2014, 59.5% were dropped, withdrawn, or dismissed; 27.7% were settled; 2.5% were resolved using an alternative dispute resolution process; 1.8% were uncategorized; and 8.6% went to trial.3 Of the cases that went to trial, 87% resulted in a verdict for the physician and 13% resulted in a verdict for the plaintiff.3
Not as good news: Payments per claim and claims settling for a payment > $1 million are increasing
In the period 1992–1996, the average payment per paid claim in the field of obstetrics and gynecology was $387,186, rising to $447,034 in 2009–2014—a 16% increase.1 From 2004 to 2010, million dollar payments occurred in about 8% of cases of paid claims, but they represent 36% of the total of all paid claims.4 In the time periods 1992–1996 and 2009–2014, payments greater than $1 million occurred in 6% and 8% of paid claims, respectively.1
Claims settled for much more than $1 million are of great concern to physicians because the payment may exceed their policy limit, creating a complex legal problem that may take time to resolve. In some cases, where the award is greater than the insurance policy limit, aggressive plaintiff attorneys have obtained a lien on the defendant physician’s home pending settlement of the case. When a multimillion dollar payment is made to settle a professional liability claim, it can greatly influence physician practice and change hospital policies. Frequently, following a multimillion dollar payment a physician may decide to limit their practice to low-risk cases or retire from the practice of medicine.
Liability premiums are stable or decreasing
From 2014 to 2019, my ObGyn professional liability insurance premiums decreased by 18%. During the same time period, my colleagues who practice surgical gynecology (no obstetrics) had a premium decrease of 22%. Insurers use a complex algorithm to determine annual liability insurance premiums, and premiums for ObGyns may not have stabilized or decreased in all regions. Take this Instant Poll:
Create your own user feedback survey
Reform of the liability tort system
Litigation policies and practices that reduce liability risk reduce total medical liability losses. Policies that have helped to constrain medical liability risk include state constitutional amendments limiting payments for pain and suffering, caps on compensation to plaintiff attorneys, increased early resolution programs that compensate patients who experience an adverse event and no-fault conflict resolution programs.5 In 2003, Texas implemented a comprehensive package of tort reform laws. Experts believe the reforms decreased the financial burden of professional liability insurance6 and led to less defensive medical practices, reducing excessive use of imaging and laboratory tests.
Medical factors contributing to a decrease in claims
In 1999, the Institute of Medicine released the report, “To Err is Human,” which galvanized health care systems to deploy systems of care that reduce the rate of adverse patient outcomes.7 Over the past 20 years, health systems have implemented quality improvement programs in obstetrics and gynecology that have contributed to a reduction in the rate of adverse patient outcomes. This may have contributed to the decrease in the rate of paid claims.
In a quasi-experimental study performed in 13 health systems, 7 interventions were implemented with the goal of improving outcomes and reducing medical liability. The 7 interventions included8:
- an elective induction bundle focused on the safe use of oxytocin
- an augmentation bundle focused on early intervention for possible fetal metabolic acidosis
- an operative vaginal delivery bundle
- TeamSTEPPS teamwork training to improve the quality of communication
- best practices education with a focus on electronic fetal monitoring
- regular performance feedback to hospitals and clinicians
- implementation of a quality improvement collaboration to support implementation of the interventions.
During the two-year baseline period prior to the intervention there were 185,373 deliveries with 6.7 perinatal claims made per 10,000 deliveries and 1.3 claims paid per 10,000 deliveries. Following the intervention, the rate of claims made and claims paid per 10,000 deliveries decreased by 22% and 37%, respectively. In addition there was a marked decrease in claims over $1 million paid, greatly limiting total financial liability losses.
Experts with vast experience in obstetrics and obstetric liability litigation have identified 4 priority interventions that may improve outcomes and mitigate liability risk, including: 1) 24-hour in-house physician coverage of an obstetrics service, 2) a conservative approach to trial of labor after a prior cesarean delivery, 3) utilization of a comprehensive, standardized event note in cases of a shoulder dystocia, and 4) judicious use of oxytocin, misoprostol, and magnesium sulfate.9
Other health system interventions that may contribute to a reduction in claims include:
- systematic improvement in the quality of communication among physicians and nurses through the use of team training, preprocedure huddles, and time-out processes10
- rapid response systems to rescue hospital patients with worrisome vital signs11
- standardized responses to a worrisome category 2 or 3 fetal heart-rate tracing12
- rapid recognition, evaluation, and treatment of women with hemorrhage, severe hypertension, sepsis, and venous thromboembolism13
- identification and referral of high-risk patients to tertiary centers14
- closed loop communication of critical imaging and laboratory results15
- universal insurance coverage for health care including contraception, obstetrics, and pediatric care.
Medical liability risk is an important practice issue because it causes excessive use of imaging and laboratory tests and often traumatizes clinicians, which can result in burnout. In the 1980s and 1990s, medical liability litigation reached a crescendo and was a prominent concern among obstetrician-gynecologists. The good news is that, for ObGyns, liability risk has stabilized. Hopefully our resolute efforts to continuously improve the quality of care will result in a long-term reduction in medical liability risk.
Medical professional liability claims (claims) are a major cause of worry and agony for physicians who are dedicated to optimizing the health of all their patients. Among physicians, those who practice neurosurgery, thoracic surgery, plastic surgery, and obstetrics and gynecology have the greatest rate of making a payment on a claim per year of practice.1 Physicians who practice psychiatry, pediatrics, pathology, and internal medicine have the lowest rate of making a payment on a claim. Among the physicians in high-risk specialties, greater than 90% will have a claim filed against them during their career.2 Although professional liability exposure reached a crisis during the 1980s and 1990s, recent data have shown a decrease in overall professional liability risk.
The good news: Paid claims per 1,000 ObGyns have decreased greatly
In a review of all paid claims reported to the National Practitioner Data Bank from 1992 to 2014, the annual rate of paid claims per 1,000 ObGyn physician-years was determined.1 For the time periods 1992–1996, 1997–2002, 2003–2008,and 2009–2014, the annual rate of paid claims per 1,000 ObGyn physician-years was 57.6, 51.5, 40.0, and 25.9, representing an astounding 55% decrease in paid claims from 1992 to 2014 (FIGURE).1
The majority of claims result in no payment
In a review of the experience of a nationwide professional liability insurer from 1991 to 2005, only 22% of claims resulted in a payment.2 In this study, for obstetrics and gynecology and gynecologic surgery, only 11% and 8% of claims, respectively, resulted in a payment.2 However, being named in a malpractice claim results in significant stress for a physician and requires a great deal of work and time to defend.
In another study using data from the Physician Insurer’s Association of America, among 10,915 claims closed from 2005 to 2014, 59.5% were dropped, withdrawn, or dismissed; 27.7% were settled; 2.5% were resolved using an alternative dispute resolution process; 1.8% were uncategorized; and 8.6% went to trial.3 Of the cases that went to trial, 87% resulted in a verdict for the physician and 13% resulted in a verdict for the plaintiff.3
Not as good news: Payments per claim and claims settling for a payment > $1 million are increasing
In the period 1992–1996, the average payment per paid claim in the field of obstetrics and gynecology was $387,186, rising to $447,034 in 2009–2014—a 16% increase.1 From 2004 to 2010, million dollar payments occurred in about 8% of cases of paid claims, but they represent 36% of the total of all paid claims.4 In the time periods 1992–1996 and 2009–2014, payments greater than $1 million occurred in 6% and 8% of paid claims, respectively.1
Claims settled for much more than $1 million are of great concern to physicians because the payment may exceed their policy limit, creating a complex legal problem that may take time to resolve. In some cases, where the award is greater than the insurance policy limit, aggressive plaintiff attorneys have obtained a lien on the defendant physician’s home pending settlement of the case. When a multimillion dollar payment is made to settle a professional liability claim, it can greatly influence physician practice and change hospital policies. Frequently, following a multimillion dollar payment a physician may decide to limit their practice to low-risk cases or retire from the practice of medicine.
Liability premiums are stable or decreasing
From 2014 to 2019, my ObGyn professional liability insurance premiums decreased by 18%. During the same time period, my colleagues who practice surgical gynecology (no obstetrics) had a premium decrease of 22%. Insurers use a complex algorithm to determine annual liability insurance premiums, and premiums for ObGyns may not have stabilized or decreased in all regions. Take this Instant Poll:
Create your own user feedback survey
Reform of the liability tort system
Litigation policies and practices that reduce liability risk reduce total medical liability losses. Policies that have helped to constrain medical liability risk include state constitutional amendments limiting payments for pain and suffering, caps on compensation to plaintiff attorneys, increased early resolution programs that compensate patients who experience an adverse event and no-fault conflict resolution programs.5 In 2003, Texas implemented a comprehensive package of tort reform laws. Experts believe the reforms decreased the financial burden of professional liability insurance6 and led to less defensive medical practices, reducing excessive use of imaging and laboratory tests.
Medical factors contributing to a decrease in claims
In 1999, the Institute of Medicine released the report, “To Err is Human,” which galvanized health care systems to deploy systems of care that reduce the rate of adverse patient outcomes.7 Over the past 20 years, health systems have implemented quality improvement programs in obstetrics and gynecology that have contributed to a reduction in the rate of adverse patient outcomes. This may have contributed to the decrease in the rate of paid claims.
In a quasi-experimental study performed in 13 health systems, 7 interventions were implemented with the goal of improving outcomes and reducing medical liability. The 7 interventions included8:
- an elective induction bundle focused on the safe use of oxytocin
- an augmentation bundle focused on early intervention for possible fetal metabolic acidosis
- an operative vaginal delivery bundle
- TeamSTEPPS teamwork training to improve the quality of communication
- best practices education with a focus on electronic fetal monitoring
- regular performance feedback to hospitals and clinicians
- implementation of a quality improvement collaboration to support implementation of the interventions.
During the two-year baseline period prior to the intervention there were 185,373 deliveries with 6.7 perinatal claims made per 10,000 deliveries and 1.3 claims paid per 10,000 deliveries. Following the intervention, the rate of claims made and claims paid per 10,000 deliveries decreased by 22% and 37%, respectively. In addition there was a marked decrease in claims over $1 million paid, greatly limiting total financial liability losses.
Experts with vast experience in obstetrics and obstetric liability litigation have identified 4 priority interventions that may improve outcomes and mitigate liability risk, including: 1) 24-hour in-house physician coverage of an obstetrics service, 2) a conservative approach to trial of labor after a prior cesarean delivery, 3) utilization of a comprehensive, standardized event note in cases of a shoulder dystocia, and 4) judicious use of oxytocin, misoprostol, and magnesium sulfate.9
Other health system interventions that may contribute to a reduction in claims include:
- systematic improvement in the quality of communication among physicians and nurses through the use of team training, preprocedure huddles, and time-out processes10
- rapid response systems to rescue hospital patients with worrisome vital signs11
- standardized responses to a worrisome category 2 or 3 fetal heart-rate tracing12
- rapid recognition, evaluation, and treatment of women with hemorrhage, severe hypertension, sepsis, and venous thromboembolism13
- identification and referral of high-risk patients to tertiary centers14
- closed loop communication of critical imaging and laboratory results15
- universal insurance coverage for health care including contraception, obstetrics, and pediatric care.
Medical liability risk is an important practice issue because it causes excessive use of imaging and laboratory tests and often traumatizes clinicians, which can result in burnout. In the 1980s and 1990s, medical liability litigation reached a crescendo and was a prominent concern among obstetrician-gynecologists. The good news is that, for ObGyns, liability risk has stabilized. Hopefully our resolute efforts to continuously improve the quality of care will result in a long-term reduction in medical liability risk.
- Schaffer AC, Jena AB, Seabury SA, et al. Rates and characteristics of paid malpractice claims among US physicians by specialty, 1992–2014. JAMA Intern Med. 2017;177:710-718.
- Jena AB, Seabury S, Lakdawalla D, et al. Malpractice risk according to physician specialty. N Engl J Med. 2011;365:629-636.
- Glaser LM, Alvi FA, Milad MP. Trends in malpractice claims for obstetric and gynecologic procedures, 2005 through 2014. Am J Obstet Gynecol. 2017;217:340.e1-e6.
- Bixenstine PJ, Shore AD, Mehtsun WT, et al. Catastrophic medical malpractice payouts in the United States. J Healthc Quality. 2014;36:43-53.
- Cardoso R, Zarin W, Nincic V, et al. Evaluative reports on medical malpractice policies in obstetrics: a rapid scoping review. Syst Rev. 2017;6:181.
- Stewart RM, Geoghegan K, Myers JG, et al. Malpractice risk and costs are significantly reduced after tort reform. J Am Coll Surg. 2011;212:463-467.
- Institute of Medicine Committee on Quality Health Care in America; Kohn LT, Corrigan JM, Donaldson MS, eds. To Err is Human: Building a Safer Health System. Washington, DC: National Academies Press; 2000.
- Riley W, Meredith LW, Price R, et al. Decreasing malpractice claims by reducing preventable perinatal harm. Health Serv Res. 2016;51(suppl 3):2453-2471.
- Clark SL, Belfort MA, Dildy GA, et al. Reducing obstetric litigation through alterations in practice patterns. Obstet Gynecol. 2008;112:1279-1283.
- Haynes AB, Weiser TG, Berry WR, et al; Safe Surgery Saves Lives Study Group. A surgical safety checklist to reduce morbidity and mortality in a global population. N Engl J Med. 2009;360:491-499.
- Patel S, Gillon SA, Jones DA. Rapid response systems: recognition and rescue of the deteriorating hospital patient. Br J Hosp Med (Lond). 2017;78:143-148.
- Clark SL, Hamilton EF, Garite TJ, et al. The limits of electronic fetal heart rate monitoring in the prevention of neonatal metabolic acidemia. Am J Obstet Gynecol. 2017;216:163.e1-163.e6.
- The Council on Patient Safety in Women’s Healthcare website. www.safehealthcareforeverywoman.org. Accessed April 12, 2019.
- Zahn CM, Remick A, Catalano A, et al. Levels of maternal care verification pilot: translating guidance into practice. Obstet Gynecol. 2018;132:1401-1406.
- Zuccotti G, Maloney FL, Feblowitz J, et al. Reducing risk with clinical decision support: a study of closed malpractice claims. Appl Clin Inform. 2014;5:746-756.
- Schaffer AC, Jena AB, Seabury SA, et al. Rates and characteristics of paid malpractice claims among US physicians by specialty, 1992–2014. JAMA Intern Med. 2017;177:710-718.
- Jena AB, Seabury S, Lakdawalla D, et al. Malpractice risk according to physician specialty. N Engl J Med. 2011;365:629-636.
- Glaser LM, Alvi FA, Milad MP. Trends in malpractice claims for obstetric and gynecologic procedures, 2005 through 2014. Am J Obstet Gynecol. 2017;217:340.e1-e6.
- Bixenstine PJ, Shore AD, Mehtsun WT, et al. Catastrophic medical malpractice payouts in the United States. J Healthc Quality. 2014;36:43-53.
- Cardoso R, Zarin W, Nincic V, et al. Evaluative reports on medical malpractice policies in obstetrics: a rapid scoping review. Syst Rev. 2017;6:181.
- Stewart RM, Geoghegan K, Myers JG, et al. Malpractice risk and costs are significantly reduced after tort reform. J Am Coll Surg. 2011;212:463-467.
- Institute of Medicine Committee on Quality Health Care in America; Kohn LT, Corrigan JM, Donaldson MS, eds. To Err is Human: Building a Safer Health System. Washington, DC: National Academies Press; 2000.
- Riley W, Meredith LW, Price R, et al. Decreasing malpractice claims by reducing preventable perinatal harm. Health Serv Res. 2016;51(suppl 3):2453-2471.
- Clark SL, Belfort MA, Dildy GA, et al. Reducing obstetric litigation through alterations in practice patterns. Obstet Gynecol. 2008;112:1279-1283.
- Haynes AB, Weiser TG, Berry WR, et al; Safe Surgery Saves Lives Study Group. A surgical safety checklist to reduce morbidity and mortality in a global population. N Engl J Med. 2009;360:491-499.
- Patel S, Gillon SA, Jones DA. Rapid response systems: recognition and rescue of the deteriorating hospital patient. Br J Hosp Med (Lond). 2017;78:143-148.
- Clark SL, Hamilton EF, Garite TJ, et al. The limits of electronic fetal heart rate monitoring in the prevention of neonatal metabolic acidemia. Am J Obstet Gynecol. 2017;216:163.e1-163.e6.
- The Council on Patient Safety in Women’s Healthcare website. www.safehealthcareforeverywoman.org. Accessed April 12, 2019.
- Zahn CM, Remick A, Catalano A, et al. Levels of maternal care verification pilot: translating guidance into practice. Obstet Gynecol. 2018;132:1401-1406.
- Zuccotti G, Maloney FL, Feblowitz J, et al. Reducing risk with clinical decision support: a study of closed malpractice claims. Appl Clin Inform. 2014;5:746-756.
Good Notes Can Deter Litigation
At 11:15
While in the ED, the patient was examined and treated by a PA. At approximately 12:13
Given the lack of any positive pertinent findings, the PA irrigated the patient’s wounds and applied 1% lidocaine to all affected fingers so that pain would not mask any potential physical exam findings. He also used single-layer absorbable sutures to repair the injured digits. In addition, the PA tested the plaintiff for both distal interphalangeal (DIP) and proximal interphalangeal (PIP) flexion function and recorded normal results.
The PA discharged the patient from the ED at 5:56
The PA provided no further care or treatment to the patient following the visit to the hospital’s ED. However, the patient contended that he suffered an injury to the tendons of his right hand, which ultimately required several surgical procedures. He sued the hospital, the PA, the PA’s medical office, his supervising physician, and the physician who performed the later surgical procedures. The supervising physician and the surgeon were ultimately let out of the case by summary judgment motions. The hospital, which was named as a defendant under a respondeat superior theory, was also dismissed from the case when it was established that the PA was employed by his medical office and not by the hospital directly. The PA stipulated that he was within his course and scope of employment at the time he treated the plaintiff.
Continue to: Plaintiff's counsel contended...
Plaintiff’s counsel contended that the defendant PA was negligent in his examination and evaluation of the plaintiff’s digit lacerations and that he was negligent for failing to splint the plaintiff’s hand. Counsel also contended that the defendant was negligent for failing to refer the plaintiff to a hand surgeon (either directly or through the plaintiff’s primary care provider) and/or for failing to seek the assistance of his supervising physician, who was on site at the hospital’s ED and available for consultation.
Defense counsel argued that the defendant met the applicable standard of care at all times, in all aspects of his visit with the plaintiff in the early morning hours of September 1, 2014, and that there was nothing that he either did or did not do that was a substantial factor in causing the plaintiff’s alleged injuries and damages. The defendant claimed that upon his arrival at the patient’s bedside, the plaintiff verbally indicated to him that he could move his fingers (extension and flexion). He also claimed that he visualized the plaintiff moving his fingers while they were wrapped in the dressing that the plaintiff had placed on himself after the injury-producing event. However, the plaintiff disputed the defendant’s claim, denying ever being asked to extend and flex his fingers. The plaintiff also claimed that he never was able to make a full fist with his fingers on the night in question while in the ED, either by way of passive or active flexion.
Defense counsel noted that the defendant’s dictated ED note stated that the range of motion of all the plaintiff’s phalanges were normal, with no deficits, at all times while in the ED. The defendant testified about how he tested and evaluated the plaintiff’s DIP function. He also testified that he had the plaintiff lay his hand on the table, palm side up, and then laid his own hand across the plaintiff’s hand so as to isolate the DIP joint on each finger. He explained that he then had the plaintiff flex his fingers, which allowed him to determine whether there had been any kind of injury to the flexor digitorum profundus tendon (responsible for DIP function in the hand). The defendant claimed that he did the test for all the lacerated fingers and characterized them as active (as opposed to passive) flexion. Thus, he claimed that his physical exam findings were that the plaintiff had full range of motion (ROM) intact following the DIP function testing, which helped him conclude that the plaintiff did not have completely lacerated tendons as of that visit.
The defendant further explained that if the tendons were completely lacerated, the plaintiff would have had nonexistent DIP functioning on examination. The defendant testified that if he suspected a tendon laceration in a patient such as the plaintiff, his practice would be to notify his supervising physician in the ED and then either refer the patient to a primary care provider for an orthopedic hand surgeon referral or directly refer the patient to an orthopedic hand surgeon. He claimed that he took no such actions because there was no indication, from his perspective, that the plaintiff had suffered any tendon damage based on his physical exam findings, the plaintiff’s ability to make a fist, and the x-ray results.
Continue to: VERDICT
VERDICT
After a 5-day trial and 7 hours of deliberation, the jury found in favor of the defendants.
COMMENTARY
As human beings, we do a lot with our hands. They are vulnerable to injury, and misdiagnosis may result in life-altering debility. The impact is even greater when one’s livelihood requires fine dexterity. Thus, tendon lacerations are relatively common and must be managed properly.
In this case, we are told that the PA documented in his notes that the plaintiff had range of motion in all phalanges and no deficits. We are also told the defendant testified regarding his procedure for hand examination. But we are not told that his note included the details of his exam—and by inference, we have reason to suspect it did not.
You might think, “The jury found in favor of the defense, so why does this matter?” Because a well-documented chart may prevent liability.
If you wish to avoid lawsuits, it is helpful to understand how they originate: An aggrieved patient contacts a plaintiff’s lawyer, insists he or she has been wronged, and asks the lawyer to take the case. Often faced with the ticking clock of statute of limitations (the absolute deadline to file), plaintiff’s counsel will review whatever records are available (which may not be all of them), looking for perceived deficiencies of care. The case may also be reviewed by a medical professional (generally a physician) prior to filing; some states require an affidavit of merit—an attestation that there is just cause to bring the action.
Whether reviewed only by plaintiff’s counsel or with the aid of an expert, a well-documented medical record may prevent a case from being filed. Medical malpractice cases are a huge gamble for plaintiff firms: They are expensive, time consuming, difficult to litigate, document heavy, and technically complex—falling outside the experience of most lawyers. They are also less likely than other cases to be settled, thanks to National Practitioner Data Bank recording requirements and (in several states) automatic medical board inquiry for potential adverse action against a medical or nursing professional following settlement. Clinicians will often fight tooth and nail to avoid an adverse recording, hospital credentialing woes, and state investigation. A medical malpractice case can be a trap for both the clinician and the plaintiff’s attorney stuck with a bad case.
Continue to: In the early stages...
In the early stages of potential litigation, before a case is filed in court, do yourself a favor: Help plaintiff’s counsel realize it will be a losing case. You actually start the process much earlier, by conducting the proper exam and documenting lavishly. This is particularly important with specialty exams, such as the hand exam in this case.
Here, simply noting “positive ROM and distal CSM [circulation, sensation, and motion] intact” is inadequate. Why? Because it is a conclusion, not evidence of the specialty examination that was diligently performed. The mechanism of injury and initial presentation roused the clinician’s suspicions sufficiently to conduct a thorough hand examination—but the mechanics of the exam were not included, only conclusions. The trouble is, those conclusions may have been based on sound medical evidence or they may have been hastily and improvidently drawn. A plaintiff’s firm deciding whether to take this case doesn’t know but will bet on the latter.
The clinician testified he performed a detailed and thorough examination of the plaintiff’s hand. Had plaintiff’s counsel been confronted with the full details of the exam—which showed the defendant PA tested all the PIPs and DIPs by isolating each finger—early on, this case may never have been filed. Thus, conduct and document specialty exams fully. If you need a cheat sheet for exams you don’t do often, use one—that is still solid practice. If you don’t do many pelvic exams or mental status exams, make sure you aren’t missing anything. Practicing medicine is an open-book exam; if you need materials, use them.
Good documentation leads to good defense, and any good defense lawyer will recommend the Jerry Maguire rule: “Help me help you.” Solid records make a case easier to defend and win at all phases of litigation. Of course, this is not a universal cure that will prevent all lawsuits. But even if the case is filed, the strength of your records may have convinced stronger, more capable medical malpractice firms to turn it down. This is something of value: It is “you helping you” and potent proof that your human head weighs more than 8 lb.
IN SUMMARY
A well-documented chart may prevent liability by showcasing the strength of your care and preventing no-win lawsuits from being filed. Help the plaintiff’s attorney realize, early on, that he or she is facing a costly uphill battle. The key word is early, when the medical records are first reviewed—not 18 months later, when the attorney hears your testimony at deposition and realizes that he or she has invested time and sweat in a case only to learn that your care was fabulous. Showcase that fabulous care early and short circuit the whole process by detailing the substance of a key exam (not just conclusions) in the record. Detailed notes may spare you from a visit by a sheriff you don’t know holding papers you don’t want.
At 11:15
While in the ED, the patient was examined and treated by a PA. At approximately 12:13
Given the lack of any positive pertinent findings, the PA irrigated the patient’s wounds and applied 1% lidocaine to all affected fingers so that pain would not mask any potential physical exam findings. He also used single-layer absorbable sutures to repair the injured digits. In addition, the PA tested the plaintiff for both distal interphalangeal (DIP) and proximal interphalangeal (PIP) flexion function and recorded normal results.
The PA discharged the patient from the ED at 5:56
The PA provided no further care or treatment to the patient following the visit to the hospital’s ED. However, the patient contended that he suffered an injury to the tendons of his right hand, which ultimately required several surgical procedures. He sued the hospital, the PA, the PA’s medical office, his supervising physician, and the physician who performed the later surgical procedures. The supervising physician and the surgeon were ultimately let out of the case by summary judgment motions. The hospital, which was named as a defendant under a respondeat superior theory, was also dismissed from the case when it was established that the PA was employed by his medical office and not by the hospital directly. The PA stipulated that he was within his course and scope of employment at the time he treated the plaintiff.
Continue to: Plaintiff's counsel contended...
Plaintiff’s counsel contended that the defendant PA was negligent in his examination and evaluation of the plaintiff’s digit lacerations and that he was negligent for failing to splint the plaintiff’s hand. Counsel also contended that the defendant was negligent for failing to refer the plaintiff to a hand surgeon (either directly or through the plaintiff’s primary care provider) and/or for failing to seek the assistance of his supervising physician, who was on site at the hospital’s ED and available for consultation.
Defense counsel argued that the defendant met the applicable standard of care at all times, in all aspects of his visit with the plaintiff in the early morning hours of September 1, 2014, and that there was nothing that he either did or did not do that was a substantial factor in causing the plaintiff’s alleged injuries and damages. The defendant claimed that upon his arrival at the patient’s bedside, the plaintiff verbally indicated to him that he could move his fingers (extension and flexion). He also claimed that he visualized the plaintiff moving his fingers while they were wrapped in the dressing that the plaintiff had placed on himself after the injury-producing event. However, the plaintiff disputed the defendant’s claim, denying ever being asked to extend and flex his fingers. The plaintiff also claimed that he never was able to make a full fist with his fingers on the night in question while in the ED, either by way of passive or active flexion.
Defense counsel noted that the defendant’s dictated ED note stated that the range of motion of all the plaintiff’s phalanges were normal, with no deficits, at all times while in the ED. The defendant testified about how he tested and evaluated the plaintiff’s DIP function. He also testified that he had the plaintiff lay his hand on the table, palm side up, and then laid his own hand across the plaintiff’s hand so as to isolate the DIP joint on each finger. He explained that he then had the plaintiff flex his fingers, which allowed him to determine whether there had been any kind of injury to the flexor digitorum profundus tendon (responsible for DIP function in the hand). The defendant claimed that he did the test for all the lacerated fingers and characterized them as active (as opposed to passive) flexion. Thus, he claimed that his physical exam findings were that the plaintiff had full range of motion (ROM) intact following the DIP function testing, which helped him conclude that the plaintiff did not have completely lacerated tendons as of that visit.
The defendant further explained that if the tendons were completely lacerated, the plaintiff would have had nonexistent DIP functioning on examination. The defendant testified that if he suspected a tendon laceration in a patient such as the plaintiff, his practice would be to notify his supervising physician in the ED and then either refer the patient to a primary care provider for an orthopedic hand surgeon referral or directly refer the patient to an orthopedic hand surgeon. He claimed that he took no such actions because there was no indication, from his perspective, that the plaintiff had suffered any tendon damage based on his physical exam findings, the plaintiff’s ability to make a fist, and the x-ray results.
Continue to: VERDICT
VERDICT
After a 5-day trial and 7 hours of deliberation, the jury found in favor of the defendants.
COMMENTARY
As human beings, we do a lot with our hands. They are vulnerable to injury, and misdiagnosis may result in life-altering debility. The impact is even greater when one’s livelihood requires fine dexterity. Thus, tendon lacerations are relatively common and must be managed properly.
In this case, we are told that the PA documented in his notes that the plaintiff had range of motion in all phalanges and no deficits. We are also told the defendant testified regarding his procedure for hand examination. But we are not told that his note included the details of his exam—and by inference, we have reason to suspect it did not.
You might think, “The jury found in favor of the defense, so why does this matter?” Because a well-documented chart may prevent liability.
If you wish to avoid lawsuits, it is helpful to understand how they originate: An aggrieved patient contacts a plaintiff’s lawyer, insists he or she has been wronged, and asks the lawyer to take the case. Often faced with the ticking clock of statute of limitations (the absolute deadline to file), plaintiff’s counsel will review whatever records are available (which may not be all of them), looking for perceived deficiencies of care. The case may also be reviewed by a medical professional (generally a physician) prior to filing; some states require an affidavit of merit—an attestation that there is just cause to bring the action.
Whether reviewed only by plaintiff’s counsel or with the aid of an expert, a well-documented medical record may prevent a case from being filed. Medical malpractice cases are a huge gamble for plaintiff firms: They are expensive, time consuming, difficult to litigate, document heavy, and technically complex—falling outside the experience of most lawyers. They are also less likely than other cases to be settled, thanks to National Practitioner Data Bank recording requirements and (in several states) automatic medical board inquiry for potential adverse action against a medical or nursing professional following settlement. Clinicians will often fight tooth and nail to avoid an adverse recording, hospital credentialing woes, and state investigation. A medical malpractice case can be a trap for both the clinician and the plaintiff’s attorney stuck with a bad case.
Continue to: In the early stages...
In the early stages of potential litigation, before a case is filed in court, do yourself a favor: Help plaintiff’s counsel realize it will be a losing case. You actually start the process much earlier, by conducting the proper exam and documenting lavishly. This is particularly important with specialty exams, such as the hand exam in this case.
Here, simply noting “positive ROM and distal CSM [circulation, sensation, and motion] intact” is inadequate. Why? Because it is a conclusion, not evidence of the specialty examination that was diligently performed. The mechanism of injury and initial presentation roused the clinician’s suspicions sufficiently to conduct a thorough hand examination—but the mechanics of the exam were not included, only conclusions. The trouble is, those conclusions may have been based on sound medical evidence or they may have been hastily and improvidently drawn. A plaintiff’s firm deciding whether to take this case doesn’t know but will bet on the latter.
The clinician testified he performed a detailed and thorough examination of the plaintiff’s hand. Had plaintiff’s counsel been confronted with the full details of the exam—which showed the defendant PA tested all the PIPs and DIPs by isolating each finger—early on, this case may never have been filed. Thus, conduct and document specialty exams fully. If you need a cheat sheet for exams you don’t do often, use one—that is still solid practice. If you don’t do many pelvic exams or mental status exams, make sure you aren’t missing anything. Practicing medicine is an open-book exam; if you need materials, use them.
Good documentation leads to good defense, and any good defense lawyer will recommend the Jerry Maguire rule: “Help me help you.” Solid records make a case easier to defend and win at all phases of litigation. Of course, this is not a universal cure that will prevent all lawsuits. But even if the case is filed, the strength of your records may have convinced stronger, more capable medical malpractice firms to turn it down. This is something of value: It is “you helping you” and potent proof that your human head weighs more than 8 lb.
IN SUMMARY
A well-documented chart may prevent liability by showcasing the strength of your care and preventing no-win lawsuits from being filed. Help the plaintiff’s attorney realize, early on, that he or she is facing a costly uphill battle. The key word is early, when the medical records are first reviewed—not 18 months later, when the attorney hears your testimony at deposition and realizes that he or she has invested time and sweat in a case only to learn that your care was fabulous. Showcase that fabulous care early and short circuit the whole process by detailing the substance of a key exam (not just conclusions) in the record. Detailed notes may spare you from a visit by a sheriff you don’t know holding papers you don’t want.
At 11:15
While in the ED, the patient was examined and treated by a PA. At approximately 12:13
Given the lack of any positive pertinent findings, the PA irrigated the patient’s wounds and applied 1% lidocaine to all affected fingers so that pain would not mask any potential physical exam findings. He also used single-layer absorbable sutures to repair the injured digits. In addition, the PA tested the plaintiff for both distal interphalangeal (DIP) and proximal interphalangeal (PIP) flexion function and recorded normal results.
The PA discharged the patient from the ED at 5:56
The PA provided no further care or treatment to the patient following the visit to the hospital’s ED. However, the patient contended that he suffered an injury to the tendons of his right hand, which ultimately required several surgical procedures. He sued the hospital, the PA, the PA’s medical office, his supervising physician, and the physician who performed the later surgical procedures. The supervising physician and the surgeon were ultimately let out of the case by summary judgment motions. The hospital, which was named as a defendant under a respondeat superior theory, was also dismissed from the case when it was established that the PA was employed by his medical office and not by the hospital directly. The PA stipulated that he was within his course and scope of employment at the time he treated the plaintiff.
Continue to: Plaintiff's counsel contended...
Plaintiff’s counsel contended that the defendant PA was negligent in his examination and evaluation of the plaintiff’s digit lacerations and that he was negligent for failing to splint the plaintiff’s hand. Counsel also contended that the defendant was negligent for failing to refer the plaintiff to a hand surgeon (either directly or through the plaintiff’s primary care provider) and/or for failing to seek the assistance of his supervising physician, who was on site at the hospital’s ED and available for consultation.
Defense counsel argued that the defendant met the applicable standard of care at all times, in all aspects of his visit with the plaintiff in the early morning hours of September 1, 2014, and that there was nothing that he either did or did not do that was a substantial factor in causing the plaintiff’s alleged injuries and damages. The defendant claimed that upon his arrival at the patient’s bedside, the plaintiff verbally indicated to him that he could move his fingers (extension and flexion). He also claimed that he visualized the plaintiff moving his fingers while they were wrapped in the dressing that the plaintiff had placed on himself after the injury-producing event. However, the plaintiff disputed the defendant’s claim, denying ever being asked to extend and flex his fingers. The plaintiff also claimed that he never was able to make a full fist with his fingers on the night in question while in the ED, either by way of passive or active flexion.
Defense counsel noted that the defendant’s dictated ED note stated that the range of motion of all the plaintiff’s phalanges were normal, with no deficits, at all times while in the ED. The defendant testified about how he tested and evaluated the plaintiff’s DIP function. He also testified that he had the plaintiff lay his hand on the table, palm side up, and then laid his own hand across the plaintiff’s hand so as to isolate the DIP joint on each finger. He explained that he then had the plaintiff flex his fingers, which allowed him to determine whether there had been any kind of injury to the flexor digitorum profundus tendon (responsible for DIP function in the hand). The defendant claimed that he did the test for all the lacerated fingers and characterized them as active (as opposed to passive) flexion. Thus, he claimed that his physical exam findings were that the plaintiff had full range of motion (ROM) intact following the DIP function testing, which helped him conclude that the plaintiff did not have completely lacerated tendons as of that visit.
The defendant further explained that if the tendons were completely lacerated, the plaintiff would have had nonexistent DIP functioning on examination. The defendant testified that if he suspected a tendon laceration in a patient such as the plaintiff, his practice would be to notify his supervising physician in the ED and then either refer the patient to a primary care provider for an orthopedic hand surgeon referral or directly refer the patient to an orthopedic hand surgeon. He claimed that he took no such actions because there was no indication, from his perspective, that the plaintiff had suffered any tendon damage based on his physical exam findings, the plaintiff’s ability to make a fist, and the x-ray results.
Continue to: VERDICT
VERDICT
After a 5-day trial and 7 hours of deliberation, the jury found in favor of the defendants.
COMMENTARY
As human beings, we do a lot with our hands. They are vulnerable to injury, and misdiagnosis may result in life-altering debility. The impact is even greater when one’s livelihood requires fine dexterity. Thus, tendon lacerations are relatively common and must be managed properly.
In this case, we are told that the PA documented in his notes that the plaintiff had range of motion in all phalanges and no deficits. We are also told the defendant testified regarding his procedure for hand examination. But we are not told that his note included the details of his exam—and by inference, we have reason to suspect it did not.
You might think, “The jury found in favor of the defense, so why does this matter?” Because a well-documented chart may prevent liability.
If you wish to avoid lawsuits, it is helpful to understand how they originate: An aggrieved patient contacts a plaintiff’s lawyer, insists he or she has been wronged, and asks the lawyer to take the case. Often faced with the ticking clock of statute of limitations (the absolute deadline to file), plaintiff’s counsel will review whatever records are available (which may not be all of them), looking for perceived deficiencies of care. The case may also be reviewed by a medical professional (generally a physician) prior to filing; some states require an affidavit of merit—an attestation that there is just cause to bring the action.
Whether reviewed only by plaintiff’s counsel or with the aid of an expert, a well-documented medical record may prevent a case from being filed. Medical malpractice cases are a huge gamble for plaintiff firms: They are expensive, time consuming, difficult to litigate, document heavy, and technically complex—falling outside the experience of most lawyers. They are also less likely than other cases to be settled, thanks to National Practitioner Data Bank recording requirements and (in several states) automatic medical board inquiry for potential adverse action against a medical or nursing professional following settlement. Clinicians will often fight tooth and nail to avoid an adverse recording, hospital credentialing woes, and state investigation. A medical malpractice case can be a trap for both the clinician and the plaintiff’s attorney stuck with a bad case.
Continue to: In the early stages...
In the early stages of potential litigation, before a case is filed in court, do yourself a favor: Help plaintiff’s counsel realize it will be a losing case. You actually start the process much earlier, by conducting the proper exam and documenting lavishly. This is particularly important with specialty exams, such as the hand exam in this case.
Here, simply noting “positive ROM and distal CSM [circulation, sensation, and motion] intact” is inadequate. Why? Because it is a conclusion, not evidence of the specialty examination that was diligently performed. The mechanism of injury and initial presentation roused the clinician’s suspicions sufficiently to conduct a thorough hand examination—but the mechanics of the exam were not included, only conclusions. The trouble is, those conclusions may have been based on sound medical evidence or they may have been hastily and improvidently drawn. A plaintiff’s firm deciding whether to take this case doesn’t know but will bet on the latter.
The clinician testified he performed a detailed and thorough examination of the plaintiff’s hand. Had plaintiff’s counsel been confronted with the full details of the exam—which showed the defendant PA tested all the PIPs and DIPs by isolating each finger—early on, this case may never have been filed. Thus, conduct and document specialty exams fully. If you need a cheat sheet for exams you don’t do often, use one—that is still solid practice. If you don’t do many pelvic exams or mental status exams, make sure you aren’t missing anything. Practicing medicine is an open-book exam; if you need materials, use them.
Good documentation leads to good defense, and any good defense lawyer will recommend the Jerry Maguire rule: “Help me help you.” Solid records make a case easier to defend and win at all phases of litigation. Of course, this is not a universal cure that will prevent all lawsuits. But even if the case is filed, the strength of your records may have convinced stronger, more capable medical malpractice firms to turn it down. This is something of value: It is “you helping you” and potent proof that your human head weighs more than 8 lb.
IN SUMMARY
A well-documented chart may prevent liability by showcasing the strength of your care and preventing no-win lawsuits from being filed. Help the plaintiff’s attorney realize, early on, that he or she is facing a costly uphill battle. The key word is early, when the medical records are first reviewed—not 18 months later, when the attorney hears your testimony at deposition and realizes that he or she has invested time and sweat in a case only to learn that your care was fabulous. Showcase that fabulous care early and short circuit the whole process by detailing the substance of a key exam (not just conclusions) in the record. Detailed notes may spare you from a visit by a sheriff you don’t know holding papers you don’t want.