User login
Alarming gaps in gestational diabetes care
BY E. ALBERT REECE, MD, PhD, MBA
Much attention has been given in the media to the incidence of prediabetes in the general population. The Centers for Disease Control and Prevention estimates that approximately 86 million adults have prediabetes, and that the incidence of this condition is similar across racial and ethnic groups. Indeed, the seriousness of this public health concern prompted the Centers for Medicare & Medicaid Services to expand Medicare coverage for interventions for people with prediabetes, a move that was finalized in November 2016.
Despite a widespread focus on the need to prevent prediabetes from becoming type 2 diabetes, women diagnosed with gestational diabetes mellitus (GDM), which accounts for about 9% of women in the United States, may not be receiving critical advice and care.
The investigators analyzed data collected via the National Health and Nutrition Examination Survey from 2007-2012, and identified 284 women with a history of GDM. Only 67% of these women received diabetes screening, and approximately one-third of women included in the study had undiagnosed prediabetes and diabetes. The authors concluded that prediabetes in women who have had GDM may be underdiagnosed. They argued that women with GDM should be encouraged to have additional health visits and screenings to prevent the development of prediabetes or diabetes. Considering the fact that a number of studies have shown that GDM predisposes a woman to developing type 2 diabetes, the University of Illinois findings are alarming.
As ob.gyns., we have increasingly become a woman’s only health care practitioner. Although individuals may skip annual exams with a primary care physician, during which blood work is typically drawn, many women will see their ob.gyn. for regular check-ups. Therefore, we have a unique role to play in our patients’ lifelong health. This is especially important during pregnancy, when it may be easy to focus only on the mother’s health as it pertains to the health of the baby, rather than her health in pregnancy as it may affect her long-term well-being.
We have invited Robert Ratner, MD, the chief scientific and medical officer at the American Diabetes Association, to discuss the need to carefully follow up with patients who have had GDM and to educate them about their risk for developing type 2 diabetes later in life.
Dr. Reece, who specializes in maternal-fetal medicine, is vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. Dr. Reece said he had no relevant financial disclosures. He is the medical editor of this column. Contact him at obnews@frontlinemedcom.com.
Why postpartum GDM follow-up is so important
BY ROBERT E. RATNER, MD
Much of the attention paid to diagnosing gestational diabetes has focused on the fetus and on babies being born very large. However, it is important to appreciate that the original definitions of the condition were based entirely on the long-term outcomes of the mother.
John O’Sullivan, MD, and statistician Claire Mahan published diagnostic criteria in 1964 after performing 3-hour oral glucose tolerance tests (OGTTs) in more than 500 unselected women during their pregnancies, and then following these women and babies out as far as 23 years. Retrospectively, Dr. O’Sullivan and Ms. Mahan defined gestational diabetes mellitus (GDM) as glucose values exceeding two standard deviations above the mean on two out of four OGTT values.
They came to their conclusions after tracking the later development of diabetes outside of pregnancy. More than 20 years later, 70% of women with the higher OGTT values had developed type 2 diabetes, compared with approximately 10% of women who did not have higher values during pregnancy. The O’Sullivan criteria were established, essentially, based on their association with the development of diabetes after pregnancy. In addition to being a significant predictor of subsequent diabetes, a history of GDM also conferred a three- to fourfold increase in maternal mortality.
Fifty-some years later, these findings have been affirmed through additional research and are the crux of what drives the current recommendations for postpartum follow-up of women with a history of GDM.
Long-term maternal risks
Postpartum, the current recommendation from both the American Diabetes Association and the American College of Obstetricians and Gynecologists is that women with GDM be tested at 6-12 weeks after delivery to ensure that the diabetes has resolved.
This recommendation for initial postpartum testing carries with it a stipulation that’s different from subsequent postpartum testing. It says that postpartum testing at 6-12 weeks should be performed with either a fasting glucose test or a 2-hour OGTT. Since hemoglobin A1c may still be impacted by the rapid red blood cell turnover in pregnancy or blood loss at delivery, A1c testing lacks sensitivity for identifying diabetes during this window of time.
Initial postpartum testing also serves as a way to identify whether the diabetes during pregnancy was preexisting or purely secondary to the hormonal changes associated with the pregnancy.
If this first postpartum test shows diabetes, the patient most likely had preexisting diabetes, and therapy must be initiated immediately. In the case of a normal result, the patient remains at higher risk for the development of type 2 diabetes essentially for the rest of her life and should be tested at least every 3 years for the occurrence of the disease.
Much of the increased risk for different ethnic groups occurs within 5 years of the index pregnancy. This was shown in a systematic review led by Catherine Kim, MD; the review examined more than two dozen studies with follow-up of up to 28 years postpartum. The cumulative incidence of type 2 diabetes increased markedly in the first 5 years and then appeared to plateau after 10 years (Diabetes Care. 2002 Oct;25[10]:1862-8).
The best data on late-occurring diabetes following GDM comes from the multicenter National Institutes of Health–sponsored Diabetes Prevention Program (DPP) trial, which randomized more than 3,000 individuals with baseline impaired glucose tolerance – or prediabetes – to one of two interventions: metformin therapy or intensive lifestyle intervention, or to placebo.
Within this population, there were more than 1,700 women who had a previous live birth. Of these women, 350 reported a history of GDM at a mean of 12 years since the delivery of their first GDM pregnancy. The DPP gave us the opportunity, therefore, to look at a large group of women about 12 years away from their GDM pregnancy who had abnormal glucose levels but had not reached the level of type 2 diabetes, and compare them with women with similarly impaired glucose tolerance who did not have a history of GDM.
There were interesting similarities and differences. Women with a GDM history were on average 8 years younger than women without a GDM history, but they had comparable BMIs. In addition, within the placebo arm, we could observe the natural history of glucose intolerance in women with and without a history of GDM. Despite both groups entering the study with equivalent degrees of impaired glucose tolerance and similar BMI, women with a history of GDM had a 71% higher risk of developing diabetes during the 3-year intervention period than that of parous women without a history of GDM (J Clin Endocrinol Metab. 2008 Dec;93[12]:4774-9).
Clearly, there was something about the history of GDM that puts these women at greater risk for diabetes than women who had the same impaired glucose tolerance, but no GDM. The study demonstrated that GDM is an exceptionally strong predictor of the development of type 2 diabetes, even for those who manage to escape diabetes for the first 10 years.
Postpartum prevention
The DPP demonstrated, moreover, that intensive lifestyle therapy and metformin not only were both effective, but that they were equally effective, in delaying or preventing diabetes in women with impaired glucose tolerance and a history of GDM. Both reduced the risk by about 50% at 3 years. This was striking because in parous women without GDM, the reductions were 49% and 14%, respectively. Metformin thus appeared to be more effective in women with a history of GDM.
The effects of the interventions persisted over a 10-year follow up of the DPP population. In women with a history of GDM, the intensive lifestyle intervention and metformin reduced progression to diabetes by 35% and 40%, respectively, over 10 years (J Clin Endocrinol Metab. 2015 Apr;100[4]:1646-53).
Pregnancy presents a stress test for beta cell function, and gestational diabetes clearly is a harbinger of further deterioration in beta-cell function and metabolic abnormalities in the mother. Because of these risks and because early intervention makes a difference, surveillance is critically important. Most women see their ob.gyn. as their primary care physician in the 10 years following a pregnancy – the time when more than 50% of all cases of subsequent diabetes will occur – and many continue to see their ob.gyns. in the longer term, as their risk continues to linger.
Immediately after a pregnancy with GDM, ob.gyns. can counsel women not only about their risks of developing type 2 diabetes and the importance of screening, but also about the beneficial impact of lifestyle modification, caloric restriction and weight loss if necessary, and increased exercise. Mothers should also know that GDM is a family affair, and that lifestyle changes that are beneficial for the mother will be equally beneficial for the baby.
The Hyperglycemia and Adverse Pregnancy Outcome (HAPO) study taught us that there are continuous linear relationships between maternal glucose and adverse fetal outcomes like birth weight and percent body fat greater than the 90th percentile. Longitudinal studies of the Pima Indians showed us that offspring of women who had diabetes during pregnancy were more likely to be obese and more likely to develop diabetes than offspring of women who did not have diabetes during pregnancy. Even when GDM has been well treated and controlled, we should have heightened awareness to the potential risks in the fetus and the growing child and adolescent.
Patients who are found to have subsequent type 2 diabetes should know that aggressive therapy early on in the natural history of the disease reduces the risk of microvascular and macrovascular complications. And as the DPP has demonstrated, lifestyle interventions and metformin may also keep women who are found to have prediabetes outside of pregnancy from progressing on to diabetes.
Dr. Ratner is the chief scientific and medical officer for the American Diabetes Association. He reported having no financial disclosures relevant to this Master Class.
BY E. ALBERT REECE, MD, PhD, MBA
Much attention has been given in the media to the incidence of prediabetes in the general population. The Centers for Disease Control and Prevention estimates that approximately 86 million adults have prediabetes, and that the incidence of this condition is similar across racial and ethnic groups. Indeed, the seriousness of this public health concern prompted the Centers for Medicare & Medicaid Services to expand Medicare coverage for interventions for people with prediabetes, a move that was finalized in November 2016.
Despite a widespread focus on the need to prevent prediabetes from becoming type 2 diabetes, women diagnosed with gestational diabetes mellitus (GDM), which accounts for about 9% of women in the United States, may not be receiving critical advice and care.
The investigators analyzed data collected via the National Health and Nutrition Examination Survey from 2007-2012, and identified 284 women with a history of GDM. Only 67% of these women received diabetes screening, and approximately one-third of women included in the study had undiagnosed prediabetes and diabetes. The authors concluded that prediabetes in women who have had GDM may be underdiagnosed. They argued that women with GDM should be encouraged to have additional health visits and screenings to prevent the development of prediabetes or diabetes. Considering the fact that a number of studies have shown that GDM predisposes a woman to developing type 2 diabetes, the University of Illinois findings are alarming.
As ob.gyns., we have increasingly become a woman’s only health care practitioner. Although individuals may skip annual exams with a primary care physician, during which blood work is typically drawn, many women will see their ob.gyn. for regular check-ups. Therefore, we have a unique role to play in our patients’ lifelong health. This is especially important during pregnancy, when it may be easy to focus only on the mother’s health as it pertains to the health of the baby, rather than her health in pregnancy as it may affect her long-term well-being.
We have invited Robert Ratner, MD, the chief scientific and medical officer at the American Diabetes Association, to discuss the need to carefully follow up with patients who have had GDM and to educate them about their risk for developing type 2 diabetes later in life.
Dr. Reece, who specializes in maternal-fetal medicine, is vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. Dr. Reece said he had no relevant financial disclosures. He is the medical editor of this column. Contact him at obnews@frontlinemedcom.com.
Why postpartum GDM follow-up is so important
BY ROBERT E. RATNER, MD
Much of the attention paid to diagnosing gestational diabetes has focused on the fetus and on babies being born very large. However, it is important to appreciate that the original definitions of the condition were based entirely on the long-term outcomes of the mother.
John O’Sullivan, MD, and statistician Claire Mahan published diagnostic criteria in 1964 after performing 3-hour oral glucose tolerance tests (OGTTs) in more than 500 unselected women during their pregnancies, and then following these women and babies out as far as 23 years. Retrospectively, Dr. O’Sullivan and Ms. Mahan defined gestational diabetes mellitus (GDM) as glucose values exceeding two standard deviations above the mean on two out of four OGTT values.
They came to their conclusions after tracking the later development of diabetes outside of pregnancy. More than 20 years later, 70% of women with the higher OGTT values had developed type 2 diabetes, compared with approximately 10% of women who did not have higher values during pregnancy. The O’Sullivan criteria were established, essentially, based on their association with the development of diabetes after pregnancy. In addition to being a significant predictor of subsequent diabetes, a history of GDM also conferred a three- to fourfold increase in maternal mortality.
Fifty-some years later, these findings have been affirmed through additional research and are the crux of what drives the current recommendations for postpartum follow-up of women with a history of GDM.
Long-term maternal risks
Postpartum, the current recommendation from both the American Diabetes Association and the American College of Obstetricians and Gynecologists is that women with GDM be tested at 6-12 weeks after delivery to ensure that the diabetes has resolved.
This recommendation for initial postpartum testing carries with it a stipulation that’s different from subsequent postpartum testing. It says that postpartum testing at 6-12 weeks should be performed with either a fasting glucose test or a 2-hour OGTT. Since hemoglobin A1c may still be impacted by the rapid red blood cell turnover in pregnancy or blood loss at delivery, A1c testing lacks sensitivity for identifying diabetes during this window of time.
Initial postpartum testing also serves as a way to identify whether the diabetes during pregnancy was preexisting or purely secondary to the hormonal changes associated with the pregnancy.
If this first postpartum test shows diabetes, the patient most likely had preexisting diabetes, and therapy must be initiated immediately. In the case of a normal result, the patient remains at higher risk for the development of type 2 diabetes essentially for the rest of her life and should be tested at least every 3 years for the occurrence of the disease.
Much of the increased risk for different ethnic groups occurs within 5 years of the index pregnancy. This was shown in a systematic review led by Catherine Kim, MD; the review examined more than two dozen studies with follow-up of up to 28 years postpartum. The cumulative incidence of type 2 diabetes increased markedly in the first 5 years and then appeared to plateau after 10 years (Diabetes Care. 2002 Oct;25[10]:1862-8).
The best data on late-occurring diabetes following GDM comes from the multicenter National Institutes of Health–sponsored Diabetes Prevention Program (DPP) trial, which randomized more than 3,000 individuals with baseline impaired glucose tolerance – or prediabetes – to one of two interventions: metformin therapy or intensive lifestyle intervention, or to placebo.
Within this population, there were more than 1,700 women who had a previous live birth. Of these women, 350 reported a history of GDM at a mean of 12 years since the delivery of their first GDM pregnancy. The DPP gave us the opportunity, therefore, to look at a large group of women about 12 years away from their GDM pregnancy who had abnormal glucose levels but had not reached the level of type 2 diabetes, and compare them with women with similarly impaired glucose tolerance who did not have a history of GDM.
There were interesting similarities and differences. Women with a GDM history were on average 8 years younger than women without a GDM history, but they had comparable BMIs. In addition, within the placebo arm, we could observe the natural history of glucose intolerance in women with and without a history of GDM. Despite both groups entering the study with equivalent degrees of impaired glucose tolerance and similar BMI, women with a history of GDM had a 71% higher risk of developing diabetes during the 3-year intervention period than that of parous women without a history of GDM (J Clin Endocrinol Metab. 2008 Dec;93[12]:4774-9).
Clearly, there was something about the history of GDM that puts these women at greater risk for diabetes than women who had the same impaired glucose tolerance, but no GDM. The study demonstrated that GDM is an exceptionally strong predictor of the development of type 2 diabetes, even for those who manage to escape diabetes for the first 10 years.
Postpartum prevention
The DPP demonstrated, moreover, that intensive lifestyle therapy and metformin not only were both effective, but that they were equally effective, in delaying or preventing diabetes in women with impaired glucose tolerance and a history of GDM. Both reduced the risk by about 50% at 3 years. This was striking because in parous women without GDM, the reductions were 49% and 14%, respectively. Metformin thus appeared to be more effective in women with a history of GDM.
The effects of the interventions persisted over a 10-year follow up of the DPP population. In women with a history of GDM, the intensive lifestyle intervention and metformin reduced progression to diabetes by 35% and 40%, respectively, over 10 years (J Clin Endocrinol Metab. 2015 Apr;100[4]:1646-53).
Pregnancy presents a stress test for beta cell function, and gestational diabetes clearly is a harbinger of further deterioration in beta-cell function and metabolic abnormalities in the mother. Because of these risks and because early intervention makes a difference, surveillance is critically important. Most women see their ob.gyn. as their primary care physician in the 10 years following a pregnancy – the time when more than 50% of all cases of subsequent diabetes will occur – and many continue to see their ob.gyns. in the longer term, as their risk continues to linger.
Immediately after a pregnancy with GDM, ob.gyns. can counsel women not only about their risks of developing type 2 diabetes and the importance of screening, but also about the beneficial impact of lifestyle modification, caloric restriction and weight loss if necessary, and increased exercise. Mothers should also know that GDM is a family affair, and that lifestyle changes that are beneficial for the mother will be equally beneficial for the baby.
The Hyperglycemia and Adverse Pregnancy Outcome (HAPO) study taught us that there are continuous linear relationships between maternal glucose and adverse fetal outcomes like birth weight and percent body fat greater than the 90th percentile. Longitudinal studies of the Pima Indians showed us that offspring of women who had diabetes during pregnancy were more likely to be obese and more likely to develop diabetes than offspring of women who did not have diabetes during pregnancy. Even when GDM has been well treated and controlled, we should have heightened awareness to the potential risks in the fetus and the growing child and adolescent.
Patients who are found to have subsequent type 2 diabetes should know that aggressive therapy early on in the natural history of the disease reduces the risk of microvascular and macrovascular complications. And as the DPP has demonstrated, lifestyle interventions and metformin may also keep women who are found to have prediabetes outside of pregnancy from progressing on to diabetes.
Dr. Ratner is the chief scientific and medical officer for the American Diabetes Association. He reported having no financial disclosures relevant to this Master Class.
BY E. ALBERT REECE, MD, PhD, MBA
Much attention has been given in the media to the incidence of prediabetes in the general population. The Centers for Disease Control and Prevention estimates that approximately 86 million adults have prediabetes, and that the incidence of this condition is similar across racial and ethnic groups. Indeed, the seriousness of this public health concern prompted the Centers for Medicare & Medicaid Services to expand Medicare coverage for interventions for people with prediabetes, a move that was finalized in November 2016.
Despite a widespread focus on the need to prevent prediabetes from becoming type 2 diabetes, women diagnosed with gestational diabetes mellitus (GDM), which accounts for about 9% of women in the United States, may not be receiving critical advice and care.
The investigators analyzed data collected via the National Health and Nutrition Examination Survey from 2007-2012, and identified 284 women with a history of GDM. Only 67% of these women received diabetes screening, and approximately one-third of women included in the study had undiagnosed prediabetes and diabetes. The authors concluded that prediabetes in women who have had GDM may be underdiagnosed. They argued that women with GDM should be encouraged to have additional health visits and screenings to prevent the development of prediabetes or diabetes. Considering the fact that a number of studies have shown that GDM predisposes a woman to developing type 2 diabetes, the University of Illinois findings are alarming.
As ob.gyns., we have increasingly become a woman’s only health care practitioner. Although individuals may skip annual exams with a primary care physician, during which blood work is typically drawn, many women will see their ob.gyn. for regular check-ups. Therefore, we have a unique role to play in our patients’ lifelong health. This is especially important during pregnancy, when it may be easy to focus only on the mother’s health as it pertains to the health of the baby, rather than her health in pregnancy as it may affect her long-term well-being.
We have invited Robert Ratner, MD, the chief scientific and medical officer at the American Diabetes Association, to discuss the need to carefully follow up with patients who have had GDM and to educate them about their risk for developing type 2 diabetes later in life.
Dr. Reece, who specializes in maternal-fetal medicine, is vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. Dr. Reece said he had no relevant financial disclosures. He is the medical editor of this column. Contact him at obnews@frontlinemedcom.com.
Why postpartum GDM follow-up is so important
BY ROBERT E. RATNER, MD
Much of the attention paid to diagnosing gestational diabetes has focused on the fetus and on babies being born very large. However, it is important to appreciate that the original definitions of the condition were based entirely on the long-term outcomes of the mother.
John O’Sullivan, MD, and statistician Claire Mahan published diagnostic criteria in 1964 after performing 3-hour oral glucose tolerance tests (OGTTs) in more than 500 unselected women during their pregnancies, and then following these women and babies out as far as 23 years. Retrospectively, Dr. O’Sullivan and Ms. Mahan defined gestational diabetes mellitus (GDM) as glucose values exceeding two standard deviations above the mean on two out of four OGTT values.
They came to their conclusions after tracking the later development of diabetes outside of pregnancy. More than 20 years later, 70% of women with the higher OGTT values had developed type 2 diabetes, compared with approximately 10% of women who did not have higher values during pregnancy. The O’Sullivan criteria were established, essentially, based on their association with the development of diabetes after pregnancy. In addition to being a significant predictor of subsequent diabetes, a history of GDM also conferred a three- to fourfold increase in maternal mortality.
Fifty-some years later, these findings have been affirmed through additional research and are the crux of what drives the current recommendations for postpartum follow-up of women with a history of GDM.
Long-term maternal risks
Postpartum, the current recommendation from both the American Diabetes Association and the American College of Obstetricians and Gynecologists is that women with GDM be tested at 6-12 weeks after delivery to ensure that the diabetes has resolved.
This recommendation for initial postpartum testing carries with it a stipulation that’s different from subsequent postpartum testing. It says that postpartum testing at 6-12 weeks should be performed with either a fasting glucose test or a 2-hour OGTT. Since hemoglobin A1c may still be impacted by the rapid red blood cell turnover in pregnancy or blood loss at delivery, A1c testing lacks sensitivity for identifying diabetes during this window of time.
Initial postpartum testing also serves as a way to identify whether the diabetes during pregnancy was preexisting or purely secondary to the hormonal changes associated with the pregnancy.
If this first postpartum test shows diabetes, the patient most likely had preexisting diabetes, and therapy must be initiated immediately. In the case of a normal result, the patient remains at higher risk for the development of type 2 diabetes essentially for the rest of her life and should be tested at least every 3 years for the occurrence of the disease.
Much of the increased risk for different ethnic groups occurs within 5 years of the index pregnancy. This was shown in a systematic review led by Catherine Kim, MD; the review examined more than two dozen studies with follow-up of up to 28 years postpartum. The cumulative incidence of type 2 diabetes increased markedly in the first 5 years and then appeared to plateau after 10 years (Diabetes Care. 2002 Oct;25[10]:1862-8).
The best data on late-occurring diabetes following GDM comes from the multicenter National Institutes of Health–sponsored Diabetes Prevention Program (DPP) trial, which randomized more than 3,000 individuals with baseline impaired glucose tolerance – or prediabetes – to one of two interventions: metformin therapy or intensive lifestyle intervention, or to placebo.
Within this population, there were more than 1,700 women who had a previous live birth. Of these women, 350 reported a history of GDM at a mean of 12 years since the delivery of their first GDM pregnancy. The DPP gave us the opportunity, therefore, to look at a large group of women about 12 years away from their GDM pregnancy who had abnormal glucose levels but had not reached the level of type 2 diabetes, and compare them with women with similarly impaired glucose tolerance who did not have a history of GDM.
There were interesting similarities and differences. Women with a GDM history were on average 8 years younger than women without a GDM history, but they had comparable BMIs. In addition, within the placebo arm, we could observe the natural history of glucose intolerance in women with and without a history of GDM. Despite both groups entering the study with equivalent degrees of impaired glucose tolerance and similar BMI, women with a history of GDM had a 71% higher risk of developing diabetes during the 3-year intervention period than that of parous women without a history of GDM (J Clin Endocrinol Metab. 2008 Dec;93[12]:4774-9).
Clearly, there was something about the history of GDM that puts these women at greater risk for diabetes than women who had the same impaired glucose tolerance, but no GDM. The study demonstrated that GDM is an exceptionally strong predictor of the development of type 2 diabetes, even for those who manage to escape diabetes for the first 10 years.
Postpartum prevention
The DPP demonstrated, moreover, that intensive lifestyle therapy and metformin not only were both effective, but that they were equally effective, in delaying or preventing diabetes in women with impaired glucose tolerance and a history of GDM. Both reduced the risk by about 50% at 3 years. This was striking because in parous women without GDM, the reductions were 49% and 14%, respectively. Metformin thus appeared to be more effective in women with a history of GDM.
The effects of the interventions persisted over a 10-year follow up of the DPP population. In women with a history of GDM, the intensive lifestyle intervention and metformin reduced progression to diabetes by 35% and 40%, respectively, over 10 years (J Clin Endocrinol Metab. 2015 Apr;100[4]:1646-53).
Pregnancy presents a stress test for beta cell function, and gestational diabetes clearly is a harbinger of further deterioration in beta-cell function and metabolic abnormalities in the mother. Because of these risks and because early intervention makes a difference, surveillance is critically important. Most women see their ob.gyn. as their primary care physician in the 10 years following a pregnancy – the time when more than 50% of all cases of subsequent diabetes will occur – and many continue to see their ob.gyns. in the longer term, as their risk continues to linger.
Immediately after a pregnancy with GDM, ob.gyns. can counsel women not only about their risks of developing type 2 diabetes and the importance of screening, but also about the beneficial impact of lifestyle modification, caloric restriction and weight loss if necessary, and increased exercise. Mothers should also know that GDM is a family affair, and that lifestyle changes that are beneficial for the mother will be equally beneficial for the baby.
The Hyperglycemia and Adverse Pregnancy Outcome (HAPO) study taught us that there are continuous linear relationships between maternal glucose and adverse fetal outcomes like birth weight and percent body fat greater than the 90th percentile. Longitudinal studies of the Pima Indians showed us that offspring of women who had diabetes during pregnancy were more likely to be obese and more likely to develop diabetes than offspring of women who did not have diabetes during pregnancy. Even when GDM has been well treated and controlled, we should have heightened awareness to the potential risks in the fetus and the growing child and adolescent.
Patients who are found to have subsequent type 2 diabetes should know that aggressive therapy early on in the natural history of the disease reduces the risk of microvascular and macrovascular complications. And as the DPP has demonstrated, lifestyle interventions and metformin may also keep women who are found to have prediabetes outside of pregnancy from progressing on to diabetes.
Dr. Ratner is the chief scientific and medical officer for the American Diabetes Association. He reported having no financial disclosures relevant to this Master Class.
VIDEO: The surgical treatment of pelvic congestion
BY CHARLES E. MILLER, MD
Chronic pelvic pain is described as the presence of lower abdominal or pelvic pain for longer than 6 months. It is believed to affect approximately one in six women and 12%-15% of women of reproductive age. The diagnosis and treatment of chronic pelvic pain adds as much as a $2 billion burden to our health system annually.
It was first described clinically in the literature in 1857, while the existence of pelvic varicosities wasn’t documented for nearly another 100 years. Pelvic congestion syndrome (PCS) accounts for 30%-70% of cases presenting with chronic pelvic pain. PCS can be due to pelvic venous insufficiency, characterized by reflux into pelvic veins leading to pelvic varicosities or alternative venous pathways secondary to varicose veins of the leg.
Other etiologies of PCS include nutcracker syndrome (left renal vein compressed between the aorta and the superior mesenteric artery), May-Thurner syndrome (compression of the left common iliac vein by the right common iliac artery) or, less likely, tumor thrombosis of the inferior vena cava, portal vein thrombosis, renal cell carcinoma, left renal thrombosis, or left kidney arterial-venous fistula.
While there appears to be significant literature indicating a long-term success rate of greater than 80% in patients treated by percutaneous endovascular procedures (embolization, stenting), there is far less information on the postsurgical success of blocking the varicose gonadal vein. Nevertheless, our long-term results with gonadal vein clipping is virtually the same as that of our radiological colleagues.
It is a pleasure to welcome Courtney Steller, DO, to this edition of the Master Class in Gynecologic Surgery to discuss the diagnosis and treatment of PCS, with an emphasis on surgical correction.
Dr. Steller is a recent graduate of the AAGL/SRS Fellowship in Minimally Invasive Gynecologic Surgery at Advocate Lutheran General Hospital, Park Ridge, Ill. She is currently in private practice and is an associate at the Family Health Centers of San Diego, Calif.
Dr. Miller is clinical associate professor at the University of Illinois at Chicago, and past president of the AAGL and the International Society for Gynecologic Endoscopy. He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in private practice in Naperville and Schaumburg, Ill.; director of minimally invasive gynecologic surgery and the director of the AAGL/SRS Fellowship in Minimally Invasive Gynecologic Surgery at Advocate Lutheran General Hospital, Park Ridge, Ill.; and the medical editor of this column, Master Class. He reported having no financial disclosures relevant to this column. Email him at obnews@frontlinemedcom.com.
Pelvic congestion syndrome: A treatable cause of pain
BY COURTNEY STELLER, DO
Pelvic congestion syndrome is a poorly understood and underdiagnosed disease. Yet, over the last decade, the syndrome has become less controversial as the etiology has become better understood and as the diagnostic approach has become more specific. Through these advances, treatments have also become increasingly more successful.
This is an important shift, because the chronic pelvic pain experienced by patients with pelvic congestion significantly impacts their quality of life and well-being. As the pain persists, it can become exceedingly difficult to manage. Many patients we have ultimately treated for pelvic congestion syndrome have had years of various work-ups, significant diagnostic investigations, and trials of different treatments without having any cause of their pain identified or achieving any lasting symptom relief.
The pelvic pain in patients with pelvic congestion syndrome (PCS) can be noncyclical or cyclical. It is present most of the time but tends to get worse at the end of the day and after long periods of standing and/or sitting. The pain also may worsen with intercourse, largely afterward. The syndrome tends to occur in premenopausal and multiparous women, but it’s important to appreciate that this is not always the case; we have diagnosed and treated PCS in several young, nulliparous patients as well.
Features and diagnosis
PCS is a disorder of pelvic venous circulation that predominantly affects the ovarian veins. It is sometimes referred to as pelvic vein incompetence or pelvic vascular dysfunction. Just as veins in the legs can enlarge and become varicose, the ovarian veins – and sometimes the internal iliac veins – can become incompetent and unable to effectively return blood back to the heart.
Pregnancy may predispose patients to developing the abnormally dilated and refluxing veins that characterize PCS, as the increase in pelvic vein capacity and uterine compression can lead to significant stasis of blood in the pelvis and subsequent damage to the veins and the venous valves. There also is believed to be an estrogen component to the development of PCS, because estrogen is known to act as a vasodilator. Moreover, a congenital absence and incompetence of venous valves in some cases has been reported.
In a recent study looking at pelvic vein incompetence and symptoms of chronic pelvic pain, these women were reported to have a distinctive symptom profile, with the “most notable” features being the presence of dull pelvic pain that radiates to the upper thighs and is aggravated by prolonged standing and walking – symptoms that are similar to the leg symptoms experienced by patients with severe varicose veins (Eur J Obstet Gynecol Reprod Biol. 2016 Jan;196:21-5).
Other investigators have similarly described the pelvic pain related to PCS as a dull ache or heaviness sensation that is most severe at the end of the day and that is lessened with supine positioning (though not necessarily immediately) and often exacerbated with sexual intercourse, especially post coitus. These descriptions are in line with my experience with PCS. There is usually exquisite tenderness on pelvic exam, especially localized to the adnexa. Patients will often have varicose veins on their upper legs or labia.
Interestingly, it has been repeatedly shown that many women have dilated and incompetent pelvic veins without also having such pathognomonic pain. We therefore cannot treat women based solely on the finding of abnormal veins.
On the other hand we must determine which patients with chronic pelvic pain have PCS. The differential diagnosis for PCS includes endometriosis, adenomyosis chronic pelvic inflammatory disease, adhesive disease, adnexal masses, adnexal torsion, and several nongynecologic diseases including interstitial cystitis and irritable bowel syndrome.
Venography has become the gold standard for diagnosing pelvic congestion. The procedure involves catheterization of the ovarian veins through a femoral or jugular approach. In our experience, the common femoral vein is the more frequently used access point. Using a contrast injection, the interventional radiologist can assess the degree of venous dilation and reflux in the pelvis.

There currently is no consensus on a cutoff for vein diameter or on any validated measures for congestion. According to one report on PCS authored by interventional radiologists, the diagnosis of PCS is confirmed with the venographic findings of ovarian vein diameter greater than 6 mm, retrograde ovarian or pelvic venous flow, presence of several tortuous collateral pelvic venous pathways, and delayed or stagnant clearance on contrast (Semin Intervent Radiol. 2008 Dec;25[4]:361-8).
The criteria vary, however. A recent literature review on pelvic congestion syndrome by Chiara Borghi, MD, and Lucio Dell’Atti, MD, states that incompetent pelvic veins are defined as more than 5-10 mm in diameter (Arch Gynecol Obstet. 2016 Feb;293[2]:291-301).
To more accurately diagnose PCS, our patients undergo tilt-table venography. The patient is placed into a reverse-Trendelenburg upright or semi-upright position to potentially exacerbate any venous reflux or dilation.
Other methods of identifying and diagnosing pelvic congestion have included transabdominal and transvaginal ultrasound, CT, and MRI. While CT and MRI both offer an overview of the pelvic vasculature and are helpful for ruling out other causes of chronic pelvic pain, they have low specificity for pelvic varices, according to the Italian review.
Sonography performed in the supine position, on the other hand, appears to be increasingly viewed as an acceptable screening tool for determining which patients may ultimately benefit from venography. It is also important in evaluation to rule out other pathologies not yet excluded. However, it should not be used for diagnosis of PCS.
Treating PCS
There are two main approaches to treating PCS: venous ligation (a gynecologic surgical approach) and percutaneous transcatheter embolization (performed by interventional radiologists).
The literature and evidence base is still in its infancy, but is growing. In our experience, both approaches lead to good resolution of symptoms over time in the majority of patients, and appear superior to the medical therapies that have been proposed for treating PCS, such as progestins and gonadotropin-releasing hormone agonists. Success rates with medical therapy are more variable and appear to be more short lived.
A review published this year on the effectiveness of embolization of pelvic veins for reducing chronic pelvic pain showed that 75% of women undergoing embolization had symptomatic relief that generally increased over time and was sustained. The authors concluded that embolization appears to be effective for the majority of women, and is safe, although they also noted that the quality of the evidence is low (J Vasc Interv Radiol. 2016 Oct;27[10]:1478-86.e8). Their review was based almost entirely on prospective case series.
Dr. Borghi and Dr. Dell’Atti offered a similar assessment of embolization for PCS, stating in their review article that clinical success has been reported in 70%-85% of patients. They also report nearly equivalent success rates of up to 75% with treatment via surgical ligation of ovarian and/or pelvic vasculature. These findings are from mostly observational data and case series.
Decisions about which approach to take should be individualized. If there are no differences with respect to insurance coverage for the patient, then embolization may be the preferred approach because it is the most minimally invasive technique and can potentially be performed at the time of diagnostic venography, negating the need for a second procedure. A skilled interventional radiologist familiar with the disease and the treatment is necessary. Various embolic agents are utilized, including coils, glues, foams, and other agents that cause sclerosis of the abnormal veins.
In other cases, venous ligation is preferred, especially when an additional gynecologic surgery, such as a cystectomy or myomectomy, is required.
Surgical ligation of ovarian veins was initially performed via laparotomy using a traditional retroperitoneal approach. The surgical goal is to isolate the ovarian vein significantly above the pelvic brim and before the vein becomes substantially dilated. Laparotomy therefore requires a vertical mid-line incision to provide adequate access to the appropriate portion of the ovarian vessels, leading to potentially high morbidity and poor cosmesis.
More recently, gynecologic surgeons skilled in laparoscopy have successfully managed PCS transperitoneally. A few small series of bilateral laparoscopic transperitoneal ligation of ovarian veins have been reported, including one by Tigellio Gargiulo, MD, who clipped both veins in their upper third, near their distal ends at the inferior vena cava (right) and the renal vein (left) (J Am Assoc Gynecol Laparosc. 2003 Nov;10[4]:501-4).
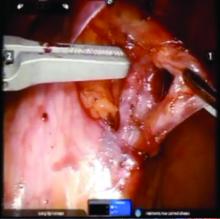
We prefer a robot-assisted laparoscopic approach for most of our patients. Not only does the improved dexterity help while working with sensitive vasculature, but more importantly we are able to use Firefly fluorescence.
The procedure generally is as follows. The uterine adnexa on the affected side is grasped and placed on tension so that the infundibulopelvic (IP) ligament can be visualized as it courses up and above the pelvic brim. The peritoneum immediately over the IP ligament is gently grasped and tented upward, and a small incision is made into the peritoneum, providing access into the retroperitoneum. The ureter should be visualized medial to this dissection.
The peritoneal tissue is then gently dissected off the ovarian vessels. Once the vessels are freed from the peritoneal tissue, the dilated ovarian vein is often clearly visualized. It is important to note that if no venous dilation is seen during laparoscopy, the procedure should not be aborted. Due to the Trendelenburg position that is utilized in gynecologic – and especially laparoscopic – surgery, the venous system sometimes appears falsely “normal” at this time.
Once the ovarian vessels have been isolated, the arteries must be separated from the veins. The adventitial tissue is dissected until the vessels are separated. Great care should be taken to ensure that all movements run parallel to the vessels and not perpendicular, therefore decreasing the risk of bleeding.
This process can be challenging. The surgeon is working with delicate vasculature. Often there are several branches from the vein that have formed due to the abnormal venous system. The best way to approach it is to identify planes and separate those planes in order to isolate individual vessels. If difficulties are still encountered, the surgeon should restart the dissection higher.
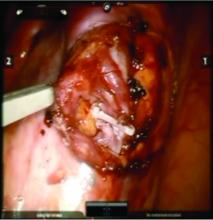
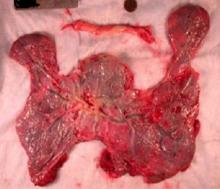
Once the dilated ovarian vein is isolated, one to two clips are placed.
Usually the artery is clearly distinct from the vein as it is smaller, more elastic, and can be seen pulsing. However, occasionally it is difficult to distinguish. In these cases, assistance with the da Vinci surgical system is useful: Indocyanine green (ICG) dye can be injected intravenously and visualized with a near-infrared light on the da Vinci platform. The dye is then seen glowing green as it first courses through the artery and then the vein.
For patients who have been found on venography to have bilateral disease, we perform the ligation procedure bilaterally. Once ligation is complete, the more competent collateral veins in the pelvis will assume more of the venous circulation.
In our experience, patients have ultimately noted substantial pain relief after these procedures, both with the endoscopic embolization and the surgical ligation. Patients are counseled that it can take several months to notice a relief in the pain.
In rare cases, pelvic congestion is related to extrinsic compression. For instance, the left renal vein can become compressed between the aorta and the superior mesenteric artery (the nutcracker syndrome), or the left common iliac vein can be compressed between the overlying right internal iliac artery and the underlying vertebral body (May-Thurner syndrome). Both of these conditions can lead to secondary PCS.
Such complex conditions are usually treated by vascular surgeons. May-Thurner syndrome is treated via stenting, while nutcracker syndrome can be treated with stenting or transposition of the renal vein to the distal vena cava.
Dr. Steller is an associate at the Family Health Centers of San Diego. She reported having no relevant financial disclosures.
BY CHARLES E. MILLER, MD
Chronic pelvic pain is described as the presence of lower abdominal or pelvic pain for longer than 6 months. It is believed to affect approximately one in six women and 12%-15% of women of reproductive age. The diagnosis and treatment of chronic pelvic pain adds as much as a $2 billion burden to our health system annually.
It was first described clinically in the literature in 1857, while the existence of pelvic varicosities wasn’t documented for nearly another 100 years. Pelvic congestion syndrome (PCS) accounts for 30%-70% of cases presenting with chronic pelvic pain. PCS can be due to pelvic venous insufficiency, characterized by reflux into pelvic veins leading to pelvic varicosities or alternative venous pathways secondary to varicose veins of the leg.
Other etiologies of PCS include nutcracker syndrome (left renal vein compressed between the aorta and the superior mesenteric artery), May-Thurner syndrome (compression of the left common iliac vein by the right common iliac artery) or, less likely, tumor thrombosis of the inferior vena cava, portal vein thrombosis, renal cell carcinoma, left renal thrombosis, or left kidney arterial-venous fistula.
While there appears to be significant literature indicating a long-term success rate of greater than 80% in patients treated by percutaneous endovascular procedures (embolization, stenting), there is far less information on the postsurgical success of blocking the varicose gonadal vein. Nevertheless, our long-term results with gonadal vein clipping is virtually the same as that of our radiological colleagues.
It is a pleasure to welcome Courtney Steller, DO, to this edition of the Master Class in Gynecologic Surgery to discuss the diagnosis and treatment of PCS, with an emphasis on surgical correction.
Dr. Steller is a recent graduate of the AAGL/SRS Fellowship in Minimally Invasive Gynecologic Surgery at Advocate Lutheran General Hospital, Park Ridge, Ill. She is currently in private practice and is an associate at the Family Health Centers of San Diego, Calif.
Dr. Miller is clinical associate professor at the University of Illinois at Chicago, and past president of the AAGL and the International Society for Gynecologic Endoscopy. He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in private practice in Naperville and Schaumburg, Ill.; director of minimally invasive gynecologic surgery and the director of the AAGL/SRS Fellowship in Minimally Invasive Gynecologic Surgery at Advocate Lutheran General Hospital, Park Ridge, Ill.; and the medical editor of this column, Master Class. He reported having no financial disclosures relevant to this column. Email him at obnews@frontlinemedcom.com.
Pelvic congestion syndrome: A treatable cause of pain
BY COURTNEY STELLER, DO
Pelvic congestion syndrome is a poorly understood and underdiagnosed disease. Yet, over the last decade, the syndrome has become less controversial as the etiology has become better understood and as the diagnostic approach has become more specific. Through these advances, treatments have also become increasingly more successful.
This is an important shift, because the chronic pelvic pain experienced by patients with pelvic congestion significantly impacts their quality of life and well-being. As the pain persists, it can become exceedingly difficult to manage. Many patients we have ultimately treated for pelvic congestion syndrome have had years of various work-ups, significant diagnostic investigations, and trials of different treatments without having any cause of their pain identified or achieving any lasting symptom relief.
The pelvic pain in patients with pelvic congestion syndrome (PCS) can be noncyclical or cyclical. It is present most of the time but tends to get worse at the end of the day and after long periods of standing and/or sitting. The pain also may worsen with intercourse, largely afterward. The syndrome tends to occur in premenopausal and multiparous women, but it’s important to appreciate that this is not always the case; we have diagnosed and treated PCS in several young, nulliparous patients as well.
Features and diagnosis
PCS is a disorder of pelvic venous circulation that predominantly affects the ovarian veins. It is sometimes referred to as pelvic vein incompetence or pelvic vascular dysfunction. Just as veins in the legs can enlarge and become varicose, the ovarian veins – and sometimes the internal iliac veins – can become incompetent and unable to effectively return blood back to the heart.
Pregnancy may predispose patients to developing the abnormally dilated and refluxing veins that characterize PCS, as the increase in pelvic vein capacity and uterine compression can lead to significant stasis of blood in the pelvis and subsequent damage to the veins and the venous valves. There also is believed to be an estrogen component to the development of PCS, because estrogen is known to act as a vasodilator. Moreover, a congenital absence and incompetence of venous valves in some cases has been reported.
In a recent study looking at pelvic vein incompetence and symptoms of chronic pelvic pain, these women were reported to have a distinctive symptom profile, with the “most notable” features being the presence of dull pelvic pain that radiates to the upper thighs and is aggravated by prolonged standing and walking – symptoms that are similar to the leg symptoms experienced by patients with severe varicose veins (Eur J Obstet Gynecol Reprod Biol. 2016 Jan;196:21-5).
Other investigators have similarly described the pelvic pain related to PCS as a dull ache or heaviness sensation that is most severe at the end of the day and that is lessened with supine positioning (though not necessarily immediately) and often exacerbated with sexual intercourse, especially post coitus. These descriptions are in line with my experience with PCS. There is usually exquisite tenderness on pelvic exam, especially localized to the adnexa. Patients will often have varicose veins on their upper legs or labia.
Interestingly, it has been repeatedly shown that many women have dilated and incompetent pelvic veins without also having such pathognomonic pain. We therefore cannot treat women based solely on the finding of abnormal veins.
On the other hand we must determine which patients with chronic pelvic pain have PCS. The differential diagnosis for PCS includes endometriosis, adenomyosis chronic pelvic inflammatory disease, adhesive disease, adnexal masses, adnexal torsion, and several nongynecologic diseases including interstitial cystitis and irritable bowel syndrome.
Venography has become the gold standard for diagnosing pelvic congestion. The procedure involves catheterization of the ovarian veins through a femoral or jugular approach. In our experience, the common femoral vein is the more frequently used access point. Using a contrast injection, the interventional radiologist can assess the degree of venous dilation and reflux in the pelvis.

There currently is no consensus on a cutoff for vein diameter or on any validated measures for congestion. According to one report on PCS authored by interventional radiologists, the diagnosis of PCS is confirmed with the venographic findings of ovarian vein diameter greater than 6 mm, retrograde ovarian or pelvic venous flow, presence of several tortuous collateral pelvic venous pathways, and delayed or stagnant clearance on contrast (Semin Intervent Radiol. 2008 Dec;25[4]:361-8).
The criteria vary, however. A recent literature review on pelvic congestion syndrome by Chiara Borghi, MD, and Lucio Dell’Atti, MD, states that incompetent pelvic veins are defined as more than 5-10 mm in diameter (Arch Gynecol Obstet. 2016 Feb;293[2]:291-301).
To more accurately diagnose PCS, our patients undergo tilt-table venography. The patient is placed into a reverse-Trendelenburg upright or semi-upright position to potentially exacerbate any venous reflux or dilation.
Other methods of identifying and diagnosing pelvic congestion have included transabdominal and transvaginal ultrasound, CT, and MRI. While CT and MRI both offer an overview of the pelvic vasculature and are helpful for ruling out other causes of chronic pelvic pain, they have low specificity for pelvic varices, according to the Italian review.
Sonography performed in the supine position, on the other hand, appears to be increasingly viewed as an acceptable screening tool for determining which patients may ultimately benefit from venography. It is also important in evaluation to rule out other pathologies not yet excluded. However, it should not be used for diagnosis of PCS.
Treating PCS
There are two main approaches to treating PCS: venous ligation (a gynecologic surgical approach) and percutaneous transcatheter embolization (performed by interventional radiologists).
The literature and evidence base is still in its infancy, but is growing. In our experience, both approaches lead to good resolution of symptoms over time in the majority of patients, and appear superior to the medical therapies that have been proposed for treating PCS, such as progestins and gonadotropin-releasing hormone agonists. Success rates with medical therapy are more variable and appear to be more short lived.
A review published this year on the effectiveness of embolization of pelvic veins for reducing chronic pelvic pain showed that 75% of women undergoing embolization had symptomatic relief that generally increased over time and was sustained. The authors concluded that embolization appears to be effective for the majority of women, and is safe, although they also noted that the quality of the evidence is low (J Vasc Interv Radiol. 2016 Oct;27[10]:1478-86.e8). Their review was based almost entirely on prospective case series.
Dr. Borghi and Dr. Dell’Atti offered a similar assessment of embolization for PCS, stating in their review article that clinical success has been reported in 70%-85% of patients. They also report nearly equivalent success rates of up to 75% with treatment via surgical ligation of ovarian and/or pelvic vasculature. These findings are from mostly observational data and case series.
Decisions about which approach to take should be individualized. If there are no differences with respect to insurance coverage for the patient, then embolization may be the preferred approach because it is the most minimally invasive technique and can potentially be performed at the time of diagnostic venography, negating the need for a second procedure. A skilled interventional radiologist familiar with the disease and the treatment is necessary. Various embolic agents are utilized, including coils, glues, foams, and other agents that cause sclerosis of the abnormal veins.
In other cases, venous ligation is preferred, especially when an additional gynecologic surgery, such as a cystectomy or myomectomy, is required.
Surgical ligation of ovarian veins was initially performed via laparotomy using a traditional retroperitoneal approach. The surgical goal is to isolate the ovarian vein significantly above the pelvic brim and before the vein becomes substantially dilated. Laparotomy therefore requires a vertical mid-line incision to provide adequate access to the appropriate portion of the ovarian vessels, leading to potentially high morbidity and poor cosmesis.
More recently, gynecologic surgeons skilled in laparoscopy have successfully managed PCS transperitoneally. A few small series of bilateral laparoscopic transperitoneal ligation of ovarian veins have been reported, including one by Tigellio Gargiulo, MD, who clipped both veins in their upper third, near their distal ends at the inferior vena cava (right) and the renal vein (left) (J Am Assoc Gynecol Laparosc. 2003 Nov;10[4]:501-4).
We prefer a robot-assisted laparoscopic approach for most of our patients. Not only does the improved dexterity help while working with sensitive vasculature, but more importantly we are able to use Firefly fluorescence.
The procedure generally is as follows. The uterine adnexa on the affected side is grasped and placed on tension so that the infundibulopelvic (IP) ligament can be visualized as it courses up and above the pelvic brim. The peritoneum immediately over the IP ligament is gently grasped and tented upward, and a small incision is made into the peritoneum, providing access into the retroperitoneum. The ureter should be visualized medial to this dissection.
The peritoneal tissue is then gently dissected off the ovarian vessels. Once the vessels are freed from the peritoneal tissue, the dilated ovarian vein is often clearly visualized. It is important to note that if no venous dilation is seen during laparoscopy, the procedure should not be aborted. Due to the Trendelenburg position that is utilized in gynecologic – and especially laparoscopic – surgery, the venous system sometimes appears falsely “normal” at this time.
Once the ovarian vessels have been isolated, the arteries must be separated from the veins. The adventitial tissue is dissected until the vessels are separated. Great care should be taken to ensure that all movements run parallel to the vessels and not perpendicular, therefore decreasing the risk of bleeding.
This process can be challenging. The surgeon is working with delicate vasculature. Often there are several branches from the vein that have formed due to the abnormal venous system. The best way to approach it is to identify planes and separate those planes in order to isolate individual vessels. If difficulties are still encountered, the surgeon should restart the dissection higher.
Once the dilated ovarian vein is isolated, one to two clips are placed.
Usually the artery is clearly distinct from the vein as it is smaller, more elastic, and can be seen pulsing. However, occasionally it is difficult to distinguish. In these cases, assistance with the da Vinci surgical system is useful: Indocyanine green (ICG) dye can be injected intravenously and visualized with a near-infrared light on the da Vinci platform. The dye is then seen glowing green as it first courses through the artery and then the vein.
For patients who have been found on venography to have bilateral disease, we perform the ligation procedure bilaterally. Once ligation is complete, the more competent collateral veins in the pelvis will assume more of the venous circulation.
In our experience, patients have ultimately noted substantial pain relief after these procedures, both with the endoscopic embolization and the surgical ligation. Patients are counseled that it can take several months to notice a relief in the pain.
In rare cases, pelvic congestion is related to extrinsic compression. For instance, the left renal vein can become compressed between the aorta and the superior mesenteric artery (the nutcracker syndrome), or the left common iliac vein can be compressed between the overlying right internal iliac artery and the underlying vertebral body (May-Thurner syndrome). Both of these conditions can lead to secondary PCS.
Such complex conditions are usually treated by vascular surgeons. May-Thurner syndrome is treated via stenting, while nutcracker syndrome can be treated with stenting or transposition of the renal vein to the distal vena cava.
Dr. Steller is an associate at the Family Health Centers of San Diego. She reported having no relevant financial disclosures.
BY CHARLES E. MILLER, MD
Chronic pelvic pain is described as the presence of lower abdominal or pelvic pain for longer than 6 months. It is believed to affect approximately one in six women and 12%-15% of women of reproductive age. The diagnosis and treatment of chronic pelvic pain adds as much as a $2 billion burden to our health system annually.
It was first described clinically in the literature in 1857, while the existence of pelvic varicosities wasn’t documented for nearly another 100 years. Pelvic congestion syndrome (PCS) accounts for 30%-70% of cases presenting with chronic pelvic pain. PCS can be due to pelvic venous insufficiency, characterized by reflux into pelvic veins leading to pelvic varicosities or alternative venous pathways secondary to varicose veins of the leg.
Other etiologies of PCS include nutcracker syndrome (left renal vein compressed between the aorta and the superior mesenteric artery), May-Thurner syndrome (compression of the left common iliac vein by the right common iliac artery) or, less likely, tumor thrombosis of the inferior vena cava, portal vein thrombosis, renal cell carcinoma, left renal thrombosis, or left kidney arterial-venous fistula.
While there appears to be significant literature indicating a long-term success rate of greater than 80% in patients treated by percutaneous endovascular procedures (embolization, stenting), there is far less information on the postsurgical success of blocking the varicose gonadal vein. Nevertheless, our long-term results with gonadal vein clipping is virtually the same as that of our radiological colleagues.
It is a pleasure to welcome Courtney Steller, DO, to this edition of the Master Class in Gynecologic Surgery to discuss the diagnosis and treatment of PCS, with an emphasis on surgical correction.
Dr. Steller is a recent graduate of the AAGL/SRS Fellowship in Minimally Invasive Gynecologic Surgery at Advocate Lutheran General Hospital, Park Ridge, Ill. She is currently in private practice and is an associate at the Family Health Centers of San Diego, Calif.
Dr. Miller is clinical associate professor at the University of Illinois at Chicago, and past president of the AAGL and the International Society for Gynecologic Endoscopy. He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in private practice in Naperville and Schaumburg, Ill.; director of minimally invasive gynecologic surgery and the director of the AAGL/SRS Fellowship in Minimally Invasive Gynecologic Surgery at Advocate Lutheran General Hospital, Park Ridge, Ill.; and the medical editor of this column, Master Class. He reported having no financial disclosures relevant to this column. Email him at obnews@frontlinemedcom.com.
Pelvic congestion syndrome: A treatable cause of pain
BY COURTNEY STELLER, DO
Pelvic congestion syndrome is a poorly understood and underdiagnosed disease. Yet, over the last decade, the syndrome has become less controversial as the etiology has become better understood and as the diagnostic approach has become more specific. Through these advances, treatments have also become increasingly more successful.
This is an important shift, because the chronic pelvic pain experienced by patients with pelvic congestion significantly impacts their quality of life and well-being. As the pain persists, it can become exceedingly difficult to manage. Many patients we have ultimately treated for pelvic congestion syndrome have had years of various work-ups, significant diagnostic investigations, and trials of different treatments without having any cause of their pain identified or achieving any lasting symptom relief.
The pelvic pain in patients with pelvic congestion syndrome (PCS) can be noncyclical or cyclical. It is present most of the time but tends to get worse at the end of the day and after long periods of standing and/or sitting. The pain also may worsen with intercourse, largely afterward. The syndrome tends to occur in premenopausal and multiparous women, but it’s important to appreciate that this is not always the case; we have diagnosed and treated PCS in several young, nulliparous patients as well.
Features and diagnosis
PCS is a disorder of pelvic venous circulation that predominantly affects the ovarian veins. It is sometimes referred to as pelvic vein incompetence or pelvic vascular dysfunction. Just as veins in the legs can enlarge and become varicose, the ovarian veins – and sometimes the internal iliac veins – can become incompetent and unable to effectively return blood back to the heart.
Pregnancy may predispose patients to developing the abnormally dilated and refluxing veins that characterize PCS, as the increase in pelvic vein capacity and uterine compression can lead to significant stasis of blood in the pelvis and subsequent damage to the veins and the venous valves. There also is believed to be an estrogen component to the development of PCS, because estrogen is known to act as a vasodilator. Moreover, a congenital absence and incompetence of venous valves in some cases has been reported.
In a recent study looking at pelvic vein incompetence and symptoms of chronic pelvic pain, these women were reported to have a distinctive symptom profile, with the “most notable” features being the presence of dull pelvic pain that radiates to the upper thighs and is aggravated by prolonged standing and walking – symptoms that are similar to the leg symptoms experienced by patients with severe varicose veins (Eur J Obstet Gynecol Reprod Biol. 2016 Jan;196:21-5).
Other investigators have similarly described the pelvic pain related to PCS as a dull ache or heaviness sensation that is most severe at the end of the day and that is lessened with supine positioning (though not necessarily immediately) and often exacerbated with sexual intercourse, especially post coitus. These descriptions are in line with my experience with PCS. There is usually exquisite tenderness on pelvic exam, especially localized to the adnexa. Patients will often have varicose veins on their upper legs or labia.
Interestingly, it has been repeatedly shown that many women have dilated and incompetent pelvic veins without also having such pathognomonic pain. We therefore cannot treat women based solely on the finding of abnormal veins.
On the other hand we must determine which patients with chronic pelvic pain have PCS. The differential diagnosis for PCS includes endometriosis, adenomyosis chronic pelvic inflammatory disease, adhesive disease, adnexal masses, adnexal torsion, and several nongynecologic diseases including interstitial cystitis and irritable bowel syndrome.
Venography has become the gold standard for diagnosing pelvic congestion. The procedure involves catheterization of the ovarian veins through a femoral or jugular approach. In our experience, the common femoral vein is the more frequently used access point. Using a contrast injection, the interventional radiologist can assess the degree of venous dilation and reflux in the pelvis.

There currently is no consensus on a cutoff for vein diameter or on any validated measures for congestion. According to one report on PCS authored by interventional radiologists, the diagnosis of PCS is confirmed with the venographic findings of ovarian vein diameter greater than 6 mm, retrograde ovarian or pelvic venous flow, presence of several tortuous collateral pelvic venous pathways, and delayed or stagnant clearance on contrast (Semin Intervent Radiol. 2008 Dec;25[4]:361-8).
The criteria vary, however. A recent literature review on pelvic congestion syndrome by Chiara Borghi, MD, and Lucio Dell’Atti, MD, states that incompetent pelvic veins are defined as more than 5-10 mm in diameter (Arch Gynecol Obstet. 2016 Feb;293[2]:291-301).
To more accurately diagnose PCS, our patients undergo tilt-table venography. The patient is placed into a reverse-Trendelenburg upright or semi-upright position to potentially exacerbate any venous reflux or dilation.
Other methods of identifying and diagnosing pelvic congestion have included transabdominal and transvaginal ultrasound, CT, and MRI. While CT and MRI both offer an overview of the pelvic vasculature and are helpful for ruling out other causes of chronic pelvic pain, they have low specificity for pelvic varices, according to the Italian review.
Sonography performed in the supine position, on the other hand, appears to be increasingly viewed as an acceptable screening tool for determining which patients may ultimately benefit from venography. It is also important in evaluation to rule out other pathologies not yet excluded. However, it should not be used for diagnosis of PCS.
Treating PCS
There are two main approaches to treating PCS: venous ligation (a gynecologic surgical approach) and percutaneous transcatheter embolization (performed by interventional radiologists).
The literature and evidence base is still in its infancy, but is growing. In our experience, both approaches lead to good resolution of symptoms over time in the majority of patients, and appear superior to the medical therapies that have been proposed for treating PCS, such as progestins and gonadotropin-releasing hormone agonists. Success rates with medical therapy are more variable and appear to be more short lived.
A review published this year on the effectiveness of embolization of pelvic veins for reducing chronic pelvic pain showed that 75% of women undergoing embolization had symptomatic relief that generally increased over time and was sustained. The authors concluded that embolization appears to be effective for the majority of women, and is safe, although they also noted that the quality of the evidence is low (J Vasc Interv Radiol. 2016 Oct;27[10]:1478-86.e8). Their review was based almost entirely on prospective case series.
Dr. Borghi and Dr. Dell’Atti offered a similar assessment of embolization for PCS, stating in their review article that clinical success has been reported in 70%-85% of patients. They also report nearly equivalent success rates of up to 75% with treatment via surgical ligation of ovarian and/or pelvic vasculature. These findings are from mostly observational data and case series.
Decisions about which approach to take should be individualized. If there are no differences with respect to insurance coverage for the patient, then embolization may be the preferred approach because it is the most minimally invasive technique and can potentially be performed at the time of diagnostic venography, negating the need for a second procedure. A skilled interventional radiologist familiar with the disease and the treatment is necessary. Various embolic agents are utilized, including coils, glues, foams, and other agents that cause sclerosis of the abnormal veins.
In other cases, venous ligation is preferred, especially when an additional gynecologic surgery, such as a cystectomy or myomectomy, is required.
Surgical ligation of ovarian veins was initially performed via laparotomy using a traditional retroperitoneal approach. The surgical goal is to isolate the ovarian vein significantly above the pelvic brim and before the vein becomes substantially dilated. Laparotomy therefore requires a vertical mid-line incision to provide adequate access to the appropriate portion of the ovarian vessels, leading to potentially high morbidity and poor cosmesis.
More recently, gynecologic surgeons skilled in laparoscopy have successfully managed PCS transperitoneally. A few small series of bilateral laparoscopic transperitoneal ligation of ovarian veins have been reported, including one by Tigellio Gargiulo, MD, who clipped both veins in their upper third, near their distal ends at the inferior vena cava (right) and the renal vein (left) (J Am Assoc Gynecol Laparosc. 2003 Nov;10[4]:501-4).
We prefer a robot-assisted laparoscopic approach for most of our patients. Not only does the improved dexterity help while working with sensitive vasculature, but more importantly we are able to use Firefly fluorescence.
The procedure generally is as follows. The uterine adnexa on the affected side is grasped and placed on tension so that the infundibulopelvic (IP) ligament can be visualized as it courses up and above the pelvic brim. The peritoneum immediately over the IP ligament is gently grasped and tented upward, and a small incision is made into the peritoneum, providing access into the retroperitoneum. The ureter should be visualized medial to this dissection.
The peritoneal tissue is then gently dissected off the ovarian vessels. Once the vessels are freed from the peritoneal tissue, the dilated ovarian vein is often clearly visualized. It is important to note that if no venous dilation is seen during laparoscopy, the procedure should not be aborted. Due to the Trendelenburg position that is utilized in gynecologic – and especially laparoscopic – surgery, the venous system sometimes appears falsely “normal” at this time.
Once the ovarian vessels have been isolated, the arteries must be separated from the veins. The adventitial tissue is dissected until the vessels are separated. Great care should be taken to ensure that all movements run parallel to the vessels and not perpendicular, therefore decreasing the risk of bleeding.
This process can be challenging. The surgeon is working with delicate vasculature. Often there are several branches from the vein that have formed due to the abnormal venous system. The best way to approach it is to identify planes and separate those planes in order to isolate individual vessels. If difficulties are still encountered, the surgeon should restart the dissection higher.
Once the dilated ovarian vein is isolated, one to two clips are placed.
Usually the artery is clearly distinct from the vein as it is smaller, more elastic, and can be seen pulsing. However, occasionally it is difficult to distinguish. In these cases, assistance with the da Vinci surgical system is useful: Indocyanine green (ICG) dye can be injected intravenously and visualized with a near-infrared light on the da Vinci platform. The dye is then seen glowing green as it first courses through the artery and then the vein.
For patients who have been found on venography to have bilateral disease, we perform the ligation procedure bilaterally. Once ligation is complete, the more competent collateral veins in the pelvis will assume more of the venous circulation.
In our experience, patients have ultimately noted substantial pain relief after these procedures, both with the endoscopic embolization and the surgical ligation. Patients are counseled that it can take several months to notice a relief in the pain.
In rare cases, pelvic congestion is related to extrinsic compression. For instance, the left renal vein can become compressed between the aorta and the superior mesenteric artery (the nutcracker syndrome), or the left common iliac vein can be compressed between the overlying right internal iliac artery and the underlying vertebral body (May-Thurner syndrome). Both of these conditions can lead to secondary PCS.
Such complex conditions are usually treated by vascular surgeons. May-Thurner syndrome is treated via stenting, while nutcracker syndrome can be treated with stenting or transposition of the renal vein to the distal vena cava.
Dr. Steller is an associate at the Family Health Centers of San Diego. She reported having no relevant financial disclosures.
The importance of studying the placenta
It makes logical, intellectual sense that the placenta, an organ that is so integrally involved in pregnancy, will be of such great importance to the well-being, sustenance, and growth and development of the fetus. After all, the placental compartment and fetal compartment have the same origin early in embryogenesis, and the placenta is the sole source of nutrients and oxygen for the fetus.
However, the placenta has been extraordinarily poorly understood. Much of medicine has regarded the placenta like the appendix – an organ that may be easily discarded. We know too little about its functions and its biology. We do not even know whether there is a minimum amount of placenta that’s necessary for fetal health.
Over the years, the National Institutes of Health (NIH) has placed an emphasis on certain key areas of study through efforts such as the Human Genome Project, the BRAIN Initiative, and the Cancer Moonshot. Such efforts involve sustained, fundamental research and usually lead to significant findings and subsequent application of the findings.
It is exciting to know that the NIH has launched its Human Placenta Project in an effort to better understand the biology of the placenta and to elucidate its functions. The technology that is employed will play an adjunctive role.
Fortunately, over the years various investigators have studied the placenta using ultrasound, color Doppler technology, and other techniques, and have reported important findings. The work of pathologist Carolyn M. Salafia, MD, and others has called attention to the importance of the shape and vasculature of the placenta, as well as blood flow.
To bring us up to date, as the NIH’s Human Placenta Project proceeds, I have asked Dr. Salafia to provide us with a review discussion of our current knowledge and its implications. Dr. Salafia specializes in reproductive and developmental pathology and reviews thousands of placentas each year through her work with various hospitals and as head of the Placental Modulation Laboratory at the Institute for Basic Research in Developmental Disabilities in Staten Island, N.Y.
Dr. Reece, who specializes in maternal-fetal medicine, is vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. Dr. Reece said he had no relevant financial disclosures. He is the medical editor of this column. Contact him at obnews@frontlinemedcom.com.
It makes logical, intellectual sense that the placenta, an organ that is so integrally involved in pregnancy, will be of such great importance to the well-being, sustenance, and growth and development of the fetus. After all, the placental compartment and fetal compartment have the same origin early in embryogenesis, and the placenta is the sole source of nutrients and oxygen for the fetus.
However, the placenta has been extraordinarily poorly understood. Much of medicine has regarded the placenta like the appendix – an organ that may be easily discarded. We know too little about its functions and its biology. We do not even know whether there is a minimum amount of placenta that’s necessary for fetal health.
Over the years, the National Institutes of Health (NIH) has placed an emphasis on certain key areas of study through efforts such as the Human Genome Project, the BRAIN Initiative, and the Cancer Moonshot. Such efforts involve sustained, fundamental research and usually lead to significant findings and subsequent application of the findings.
It is exciting to know that the NIH has launched its Human Placenta Project in an effort to better understand the biology of the placenta and to elucidate its functions. The technology that is employed will play an adjunctive role.
Fortunately, over the years various investigators have studied the placenta using ultrasound, color Doppler technology, and other techniques, and have reported important findings. The work of pathologist Carolyn M. Salafia, MD, and others has called attention to the importance of the shape and vasculature of the placenta, as well as blood flow.
To bring us up to date, as the NIH’s Human Placenta Project proceeds, I have asked Dr. Salafia to provide us with a review discussion of our current knowledge and its implications. Dr. Salafia specializes in reproductive and developmental pathology and reviews thousands of placentas each year through her work with various hospitals and as head of the Placental Modulation Laboratory at the Institute for Basic Research in Developmental Disabilities in Staten Island, N.Y.
Dr. Reece, who specializes in maternal-fetal medicine, is vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. Dr. Reece said he had no relevant financial disclosures. He is the medical editor of this column. Contact him at obnews@frontlinemedcom.com.
It makes logical, intellectual sense that the placenta, an organ that is so integrally involved in pregnancy, will be of such great importance to the well-being, sustenance, and growth and development of the fetus. After all, the placental compartment and fetal compartment have the same origin early in embryogenesis, and the placenta is the sole source of nutrients and oxygen for the fetus.
However, the placenta has been extraordinarily poorly understood. Much of medicine has regarded the placenta like the appendix – an organ that may be easily discarded. We know too little about its functions and its biology. We do not even know whether there is a minimum amount of placenta that’s necessary for fetal health.
Over the years, the National Institutes of Health (NIH) has placed an emphasis on certain key areas of study through efforts such as the Human Genome Project, the BRAIN Initiative, and the Cancer Moonshot. Such efforts involve sustained, fundamental research and usually lead to significant findings and subsequent application of the findings.
It is exciting to know that the NIH has launched its Human Placenta Project in an effort to better understand the biology of the placenta and to elucidate its functions. The technology that is employed will play an adjunctive role.
Fortunately, over the years various investigators have studied the placenta using ultrasound, color Doppler technology, and other techniques, and have reported important findings. The work of pathologist Carolyn M. Salafia, MD, and others has called attention to the importance of the shape and vasculature of the placenta, as well as blood flow.
To bring us up to date, as the NIH’s Human Placenta Project proceeds, I have asked Dr. Salafia to provide us with a review discussion of our current knowledge and its implications. Dr. Salafia specializes in reproductive and developmental pathology and reviews thousands of placentas each year through her work with various hospitals and as head of the Placental Modulation Laboratory at the Institute for Basic Research in Developmental Disabilities in Staten Island, N.Y.
Dr. Reece, who specializes in maternal-fetal medicine, is vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. Dr. Reece said he had no relevant financial disclosures. He is the medical editor of this column. Contact him at obnews@frontlinemedcom.com.
Why placental shape and vasculature matter
The intrauterine environment significantly influences not only fetal and infant health, but adult health risks as well. Yet current efforts in obstetrics to assess the environment and optimize fetal and long-term outcomes are based on diagnostics that focus on and measure fetal signs and symptoms. By and large, the current approach overlooks the placenta – the organ that serves as the principal regulator of fetal growth and health. If the fetus appears free of risk or complications, we assume the placenta must be “okay.”
Yet this isn’t always the case. By assuming the placenta is healthy and not observing and measuring its condition, we are too often too late to effectively alter fetal- and longer-term outcomes once fetal signs and symptoms appear.
Research in recent decades, and particularly in the past 10 years, has demonstrated that placental shape matters, that it’s linked to function, and that quantifying abnormalities in shape and growth can be a meaningful clinical tool for detecting and preventing disease early in pregnancy.
We now know, specifically, that abnormal shapes reflect alterations in placental vascular architecture that lead to reduced placental efficiency. We also now understand that placental weight or size may serve as a proxy for fetoplacental metabolism.
We have more research to do to further develop models, to collect more data, and to more fully understand the placental pathology that precedes detectable fetal and/or maternal disease. We also need to know whether the early detection of placental disease has sufficient positive predictive value to allow for safe and effective intervention.1
The National Institutes of Health is investing more than $40 million in its Human Placenta Project, which aims to develop new technologies to help researchers monitor the placenta in real time. Yet it is possible that the use of ultrasound and Doppler – technologies that we employ routinely and know are safe – may go a long way toward deepening our knowledge that will, in turn, hone our ability to identify early risks.
When I speak to fellow pathologists, my message is, “Let’s stop wasting data.” For ob.gyns., my message is twofold: First, appreciate the potential to predict and alter downstream fetal and/or maternal risks by observing and measuring the placenta. Second, be aware of the value of early in vivo placental images, as well as photographs, and more precise measures of delivered placentas.
Why shape matters
The “average” or “typical” placental shape is round or oval with a centrally inserted umbilical cord. In practice, we see a variety of surface shapes and cord insertion sites, with common variations such as bi- or multi-lobate shapes, or otherwise irregular shapes and cord insertions that are eccentric, marginal, or velamentous. Interestingly, many irregularly shaped placentas display symmetry and have regular, defined geometrical patterns, like snowflakes.2
We have long understood that the microscopic growth of the human placenta involves repeated vascular branching analogous to the roots of a tree. This vascular development, or “placental arborization,” reflects the health of the maternal environment and impacts fetal health.
It is only in recent years, however, that we’ve gained a much better understanding of the relationship of the vascular structure and the shape of the placenta, and an understanding of how early changes in the branching structure of the placenta’s vascular tree drive variation in mature placental shape.
By applying a well-accepted mathematical model for generating highly branched fractals (a model for random growth known in the mathematical physics world as diffusion limited aggregation, or DLA), we have reliably reproduced the variability in placental shapes and related these shapes to the structure of the underlying vascular tree.
When the model is run with unperturbed, random values of a branching growth parameter, we get round-oval fractal shapes. But when the growth parameter is perturbed at a single point in time – when a one-time, early change is introduced – arborization is negatively affected and we get irregular shapes.
The model’s output has explained and verified a clinically observed association between non-round, non-oval placental shapes and smaller newborn birth weight for given placental weight.
This association was evident in an analysis of data collected as part of the National Collaborative Perinatal Project (1959-1974), which included placental measures such as weight, shape, size, and thickness for more than 24,000 women. It also was apparent in an analysis of data and images collected as part of the Pregnancy, Infection, and Nutrition (PIN) Study, conducted in North Carolina.
One take-away from both of these studies has been that increased variability of placental shape is associated with lower placental functional efficiency. Moreover, in the University of North Carolina cohort, the impact of placental vascular pathology (either maternal uteroplacental or fetoplacental) on placental efficiency and function was shown to be dependent on shape. Only in the case of irregularly shaped chorionic plates did each of the two pathologies have a significant association with placental inefficiency.3
The realization that placental size (weight/mass/volume) may serve as a proxy for the fetoplacental basal metabolic rate came after it was shown that Kleiber’s law, which states that basal metabolic rate (BMR) is proportional to the body mass to the 3/4 power, can be applied to the newborn’s birth weight by substituting placental weight for BMR.
This fetal-placental version (placenta weight = .75 birth weight) of Kleiber’s law was validated through an analysis of the sets of placental measures and birth weights stored in the Collaborative Perinatal Project. It has implications for our ability to use ultrasound and Doppler measures to predict risk and to understand pathologic pregnancies, such as those complicated by diabetes or fetal growth restriction.
Research also has shed light on the timing of shape variants. We now know that abnormalities of placental surface shape result mainly from early influences – perturbations of placental growth that occur no later than mid-gestation – rather than from trophotropism (the placenta “grows where it can and does not grow where it can’t”) and passive uterine remodeling later in pregnancy, as has traditionally been believed.4
With respect to the umbilical cord, the location of cord insertion is independent of eventual disk shape, but is to a large degree determined by the end of the first trimester. In addition, cord insertion does influence and is correlated with chorionic vascular density and with disk thickness. Greater eccentricity of cord insertion appears to be linked to increased placental disk thickness, each of which is independently associated with reduced placental functional efficiency.5,6
We have worked with placentas from newborns in families with an older child diagnosed with autism and have found significant differences between these placentas and the placentas of low-risk newborns. In particular, we have measured a reduction in the number or chorionic surface vessel branch points of more than 40%.
Current implications
Irregularities in placental surface shape, disk thickness, and various descriptors of placental size may all be determined from ultrasound and Doppler imaging. We can also assess cord insertion and chorionic surface vessel distribution, track patterns and rates of placental growth, and use various placental measures to understand placental efficiency and to improve the specificity of placental histopathologic diagnoses.
At this point, our use of in vivo imaging of the placenta has mainly involved grayscale ultrasound, but with color or power Doppler and improved surface network tracing protocols, we could save the red and blue areas we visualize as a “shape” and assess the density of surface vessel branching, for instance, and the degree of uniformity in vessel distribution.
We currently have quantitative markers of placental shape and mathematical models to help us identify at-risk pregnancies. What we need are more data from early ultrasounds (from all pregnancies and not only complicated ones) and more comprehensive and precise models of placental growth and function. This will enable us to better identify preclinical fetoplacental pathophysiology and predict downstream risks.
In the meantime, the delivered placenta can be a valuable source of information – an extra dimension for looking back in time. With a paradigm shift toward more thorough pathologic analysis, the delivered placenta can provide unique insights into how placental growth evolved during the pregnancy.
Do not throw away the placenta, and do not just weigh it. Take a photograph, because even with a photograph we can assess vascular density, disk thickness, and other placental characteristics.
In the case of pregnancy complications or suboptimal outcomes, the knowledge we can gain from the delivered placenta can help the physician and patient to understand recurrence risks and to better target evaluation, monitoring, and management in the next pregnancy.
References
1. Am J Perinatol. 2016 Aug 4. doi: 10.1055/s-0036-1586508.
2. Placenta. 2008 Sep;29(9):790-7.
3. Placenta. 2010 Nov;31(11):958-62.
4. Placenta. 2012 Mar;33(3):164-70.
5. J Dev Orig Health Dis. 2011 Aug;2(4):205-11.
6. Placenta. 2009 Dec;30(12):1058-64.
Dr. Salafia leads the Placental Modulation Laboratory at New York State’s Institute for Basic Research in Developmental Disabilities, Staten Island, N.Y. She reported that she has no relevant financial disclosures.
The intrauterine environment significantly influences not only fetal and infant health, but adult health risks as well. Yet current efforts in obstetrics to assess the environment and optimize fetal and long-term outcomes are based on diagnostics that focus on and measure fetal signs and symptoms. By and large, the current approach overlooks the placenta – the organ that serves as the principal regulator of fetal growth and health. If the fetus appears free of risk or complications, we assume the placenta must be “okay.”
Yet this isn’t always the case. By assuming the placenta is healthy and not observing and measuring its condition, we are too often too late to effectively alter fetal- and longer-term outcomes once fetal signs and symptoms appear.
Research in recent decades, and particularly in the past 10 years, has demonstrated that placental shape matters, that it’s linked to function, and that quantifying abnormalities in shape and growth can be a meaningful clinical tool for detecting and preventing disease early in pregnancy.
We now know, specifically, that abnormal shapes reflect alterations in placental vascular architecture that lead to reduced placental efficiency. We also now understand that placental weight or size may serve as a proxy for fetoplacental metabolism.
We have more research to do to further develop models, to collect more data, and to more fully understand the placental pathology that precedes detectable fetal and/or maternal disease. We also need to know whether the early detection of placental disease has sufficient positive predictive value to allow for safe and effective intervention.1
The National Institutes of Health is investing more than $40 million in its Human Placenta Project, which aims to develop new technologies to help researchers monitor the placenta in real time. Yet it is possible that the use of ultrasound and Doppler – technologies that we employ routinely and know are safe – may go a long way toward deepening our knowledge that will, in turn, hone our ability to identify early risks.
When I speak to fellow pathologists, my message is, “Let’s stop wasting data.” For ob.gyns., my message is twofold: First, appreciate the potential to predict and alter downstream fetal and/or maternal risks by observing and measuring the placenta. Second, be aware of the value of early in vivo placental images, as well as photographs, and more precise measures of delivered placentas.
Why shape matters
The “average” or “typical” placental shape is round or oval with a centrally inserted umbilical cord. In practice, we see a variety of surface shapes and cord insertion sites, with common variations such as bi- or multi-lobate shapes, or otherwise irregular shapes and cord insertions that are eccentric, marginal, or velamentous. Interestingly, many irregularly shaped placentas display symmetry and have regular, defined geometrical patterns, like snowflakes.2
We have long understood that the microscopic growth of the human placenta involves repeated vascular branching analogous to the roots of a tree. This vascular development, or “placental arborization,” reflects the health of the maternal environment and impacts fetal health.
It is only in recent years, however, that we’ve gained a much better understanding of the relationship of the vascular structure and the shape of the placenta, and an understanding of how early changes in the branching structure of the placenta’s vascular tree drive variation in mature placental shape.
By applying a well-accepted mathematical model for generating highly branched fractals (a model for random growth known in the mathematical physics world as diffusion limited aggregation, or DLA), we have reliably reproduced the variability in placental shapes and related these shapes to the structure of the underlying vascular tree.
When the model is run with unperturbed, random values of a branching growth parameter, we get round-oval fractal shapes. But when the growth parameter is perturbed at a single point in time – when a one-time, early change is introduced – arborization is negatively affected and we get irregular shapes.
The model’s output has explained and verified a clinically observed association between non-round, non-oval placental shapes and smaller newborn birth weight for given placental weight.
This association was evident in an analysis of data collected as part of the National Collaborative Perinatal Project (1959-1974), which included placental measures such as weight, shape, size, and thickness for more than 24,000 women. It also was apparent in an analysis of data and images collected as part of the Pregnancy, Infection, and Nutrition (PIN) Study, conducted in North Carolina.
One take-away from both of these studies has been that increased variability of placental shape is associated with lower placental functional efficiency. Moreover, in the University of North Carolina cohort, the impact of placental vascular pathology (either maternal uteroplacental or fetoplacental) on placental efficiency and function was shown to be dependent on shape. Only in the case of irregularly shaped chorionic plates did each of the two pathologies have a significant association with placental inefficiency.3
The realization that placental size (weight/mass/volume) may serve as a proxy for the fetoplacental basal metabolic rate came after it was shown that Kleiber’s law, which states that basal metabolic rate (BMR) is proportional to the body mass to the 3/4 power, can be applied to the newborn’s birth weight by substituting placental weight for BMR.
This fetal-placental version (placenta weight = .75 birth weight) of Kleiber’s law was validated through an analysis of the sets of placental measures and birth weights stored in the Collaborative Perinatal Project. It has implications for our ability to use ultrasound and Doppler measures to predict risk and to understand pathologic pregnancies, such as those complicated by diabetes or fetal growth restriction.
Research also has shed light on the timing of shape variants. We now know that abnormalities of placental surface shape result mainly from early influences – perturbations of placental growth that occur no later than mid-gestation – rather than from trophotropism (the placenta “grows where it can and does not grow where it can’t”) and passive uterine remodeling later in pregnancy, as has traditionally been believed.4
With respect to the umbilical cord, the location of cord insertion is independent of eventual disk shape, but is to a large degree determined by the end of the first trimester. In addition, cord insertion does influence and is correlated with chorionic vascular density and with disk thickness. Greater eccentricity of cord insertion appears to be linked to increased placental disk thickness, each of which is independently associated with reduced placental functional efficiency.5,6
We have worked with placentas from newborns in families with an older child diagnosed with autism and have found significant differences between these placentas and the placentas of low-risk newborns. In particular, we have measured a reduction in the number or chorionic surface vessel branch points of more than 40%.
Current implications
Irregularities in placental surface shape, disk thickness, and various descriptors of placental size may all be determined from ultrasound and Doppler imaging. We can also assess cord insertion and chorionic surface vessel distribution, track patterns and rates of placental growth, and use various placental measures to understand placental efficiency and to improve the specificity of placental histopathologic diagnoses.
At this point, our use of in vivo imaging of the placenta has mainly involved grayscale ultrasound, but with color or power Doppler and improved surface network tracing protocols, we could save the red and blue areas we visualize as a “shape” and assess the density of surface vessel branching, for instance, and the degree of uniformity in vessel distribution.
We currently have quantitative markers of placental shape and mathematical models to help us identify at-risk pregnancies. What we need are more data from early ultrasounds (from all pregnancies and not only complicated ones) and more comprehensive and precise models of placental growth and function. This will enable us to better identify preclinical fetoplacental pathophysiology and predict downstream risks.
In the meantime, the delivered placenta can be a valuable source of information – an extra dimension for looking back in time. With a paradigm shift toward more thorough pathologic analysis, the delivered placenta can provide unique insights into how placental growth evolved during the pregnancy.
Do not throw away the placenta, and do not just weigh it. Take a photograph, because even with a photograph we can assess vascular density, disk thickness, and other placental characteristics.
In the case of pregnancy complications or suboptimal outcomes, the knowledge we can gain from the delivered placenta can help the physician and patient to understand recurrence risks and to better target evaluation, monitoring, and management in the next pregnancy.
References
1. Am J Perinatol. 2016 Aug 4. doi: 10.1055/s-0036-1586508.
2. Placenta. 2008 Sep;29(9):790-7.
3. Placenta. 2010 Nov;31(11):958-62.
4. Placenta. 2012 Mar;33(3):164-70.
5. J Dev Orig Health Dis. 2011 Aug;2(4):205-11.
6. Placenta. 2009 Dec;30(12):1058-64.
Dr. Salafia leads the Placental Modulation Laboratory at New York State’s Institute for Basic Research in Developmental Disabilities, Staten Island, N.Y. She reported that she has no relevant financial disclosures.
The intrauterine environment significantly influences not only fetal and infant health, but adult health risks as well. Yet current efforts in obstetrics to assess the environment and optimize fetal and long-term outcomes are based on diagnostics that focus on and measure fetal signs and symptoms. By and large, the current approach overlooks the placenta – the organ that serves as the principal regulator of fetal growth and health. If the fetus appears free of risk or complications, we assume the placenta must be “okay.”
Yet this isn’t always the case. By assuming the placenta is healthy and not observing and measuring its condition, we are too often too late to effectively alter fetal- and longer-term outcomes once fetal signs and symptoms appear.
Research in recent decades, and particularly in the past 10 years, has demonstrated that placental shape matters, that it’s linked to function, and that quantifying abnormalities in shape and growth can be a meaningful clinical tool for detecting and preventing disease early in pregnancy.
We now know, specifically, that abnormal shapes reflect alterations in placental vascular architecture that lead to reduced placental efficiency. We also now understand that placental weight or size may serve as a proxy for fetoplacental metabolism.
We have more research to do to further develop models, to collect more data, and to more fully understand the placental pathology that precedes detectable fetal and/or maternal disease. We also need to know whether the early detection of placental disease has sufficient positive predictive value to allow for safe and effective intervention.1
The National Institutes of Health is investing more than $40 million in its Human Placenta Project, which aims to develop new technologies to help researchers monitor the placenta in real time. Yet it is possible that the use of ultrasound and Doppler – technologies that we employ routinely and know are safe – may go a long way toward deepening our knowledge that will, in turn, hone our ability to identify early risks.
When I speak to fellow pathologists, my message is, “Let’s stop wasting data.” For ob.gyns., my message is twofold: First, appreciate the potential to predict and alter downstream fetal and/or maternal risks by observing and measuring the placenta. Second, be aware of the value of early in vivo placental images, as well as photographs, and more precise measures of delivered placentas.
Why shape matters
The “average” or “typical” placental shape is round or oval with a centrally inserted umbilical cord. In practice, we see a variety of surface shapes and cord insertion sites, with common variations such as bi- or multi-lobate shapes, or otherwise irregular shapes and cord insertions that are eccentric, marginal, or velamentous. Interestingly, many irregularly shaped placentas display symmetry and have regular, defined geometrical patterns, like snowflakes.2
We have long understood that the microscopic growth of the human placenta involves repeated vascular branching analogous to the roots of a tree. This vascular development, or “placental arborization,” reflects the health of the maternal environment and impacts fetal health.
It is only in recent years, however, that we’ve gained a much better understanding of the relationship of the vascular structure and the shape of the placenta, and an understanding of how early changes in the branching structure of the placenta’s vascular tree drive variation in mature placental shape.
By applying a well-accepted mathematical model for generating highly branched fractals (a model for random growth known in the mathematical physics world as diffusion limited aggregation, or DLA), we have reliably reproduced the variability in placental shapes and related these shapes to the structure of the underlying vascular tree.
When the model is run with unperturbed, random values of a branching growth parameter, we get round-oval fractal shapes. But when the growth parameter is perturbed at a single point in time – when a one-time, early change is introduced – arborization is negatively affected and we get irregular shapes.
The model’s output has explained and verified a clinically observed association between non-round, non-oval placental shapes and smaller newborn birth weight for given placental weight.
This association was evident in an analysis of data collected as part of the National Collaborative Perinatal Project (1959-1974), which included placental measures such as weight, shape, size, and thickness for more than 24,000 women. It also was apparent in an analysis of data and images collected as part of the Pregnancy, Infection, and Nutrition (PIN) Study, conducted in North Carolina.
One take-away from both of these studies has been that increased variability of placental shape is associated with lower placental functional efficiency. Moreover, in the University of North Carolina cohort, the impact of placental vascular pathology (either maternal uteroplacental or fetoplacental) on placental efficiency and function was shown to be dependent on shape. Only in the case of irregularly shaped chorionic plates did each of the two pathologies have a significant association with placental inefficiency.3
The realization that placental size (weight/mass/volume) may serve as a proxy for the fetoplacental basal metabolic rate came after it was shown that Kleiber’s law, which states that basal metabolic rate (BMR) is proportional to the body mass to the 3/4 power, can be applied to the newborn’s birth weight by substituting placental weight for BMR.
This fetal-placental version (placenta weight = .75 birth weight) of Kleiber’s law was validated through an analysis of the sets of placental measures and birth weights stored in the Collaborative Perinatal Project. It has implications for our ability to use ultrasound and Doppler measures to predict risk and to understand pathologic pregnancies, such as those complicated by diabetes or fetal growth restriction.
Research also has shed light on the timing of shape variants. We now know that abnormalities of placental surface shape result mainly from early influences – perturbations of placental growth that occur no later than mid-gestation – rather than from trophotropism (the placenta “grows where it can and does not grow where it can’t”) and passive uterine remodeling later in pregnancy, as has traditionally been believed.4
With respect to the umbilical cord, the location of cord insertion is independent of eventual disk shape, but is to a large degree determined by the end of the first trimester. In addition, cord insertion does influence and is correlated with chorionic vascular density and with disk thickness. Greater eccentricity of cord insertion appears to be linked to increased placental disk thickness, each of which is independently associated with reduced placental functional efficiency.5,6
We have worked with placentas from newborns in families with an older child diagnosed with autism and have found significant differences between these placentas and the placentas of low-risk newborns. In particular, we have measured a reduction in the number or chorionic surface vessel branch points of more than 40%.
Current implications
Irregularities in placental surface shape, disk thickness, and various descriptors of placental size may all be determined from ultrasound and Doppler imaging. We can also assess cord insertion and chorionic surface vessel distribution, track patterns and rates of placental growth, and use various placental measures to understand placental efficiency and to improve the specificity of placental histopathologic diagnoses.
At this point, our use of in vivo imaging of the placenta has mainly involved grayscale ultrasound, but with color or power Doppler and improved surface network tracing protocols, we could save the red and blue areas we visualize as a “shape” and assess the density of surface vessel branching, for instance, and the degree of uniformity in vessel distribution.
We currently have quantitative markers of placental shape and mathematical models to help us identify at-risk pregnancies. What we need are more data from early ultrasounds (from all pregnancies and not only complicated ones) and more comprehensive and precise models of placental growth and function. This will enable us to better identify preclinical fetoplacental pathophysiology and predict downstream risks.
In the meantime, the delivered placenta can be a valuable source of information – an extra dimension for looking back in time. With a paradigm shift toward more thorough pathologic analysis, the delivered placenta can provide unique insights into how placental growth evolved during the pregnancy.
Do not throw away the placenta, and do not just weigh it. Take a photograph, because even with a photograph we can assess vascular density, disk thickness, and other placental characteristics.
In the case of pregnancy complications or suboptimal outcomes, the knowledge we can gain from the delivered placenta can help the physician and patient to understand recurrence risks and to better target evaluation, monitoring, and management in the next pregnancy.
References
1. Am J Perinatol. 2016 Aug 4. doi: 10.1055/s-0036-1586508.
2. Placenta. 2008 Sep;29(9):790-7.
3. Placenta. 2010 Nov;31(11):958-62.
4. Placenta. 2012 Mar;33(3):164-70.
5. J Dev Orig Health Dis. 2011 Aug;2(4):205-11.
6. Placenta. 2009 Dec;30(12):1058-64.
Dr. Salafia leads the Placental Modulation Laboratory at New York State’s Institute for Basic Research in Developmental Disabilities, Staten Island, N.Y. She reported that she has no relevant financial disclosures.
Taking hysteroscopy to the office
Along with global endometrial ablation, diagnostic and minor operative hysteroscopy are excellent procedures to bring into your office environment. These operations are generally of short duration and provide little risk to the patient. Moreover, reimbursement exceeds that for the hospital setting. A constant revenue stream can be created after an initial moderate expenditure.
The key to a successful office procedure is patient comfort; this begins with minimizing pain and trauma. In our practice, we note decreased pain when performing vaginoscopy and hysteroscopy without the use of a speculum or tenaculum. This is well substantiated in literature by Professor Stefano Bettocchi, who immediately preceded me as president of the International Society for Gynecologic Endoscopy (ISGE).
In this issue of Master Class in Gynecologic Surgery, I have asked my partner, Aarathi Cholkeri-Singh, MD, to discuss vaginoscopy. Dr. Cholkeri-Singh is clinical assistant professor at the University of Illinois at Chicago, lecturer at Rosalind Franklin University of Medicine and Science, and associate director of minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill.
She also serves as codirector of the AAGL/Society of Reproductive Surgeons fellowship in minimally invasive gynecologic surgery and director of gynecologic surgical education at Advocate Lutheran, and is chair for a postgraduate course on hysteroscopy at the upcoming AAGL 45th Annual Global Congress. Among her publications is a recent review in the Journal of Minimally Invasive Gynecology on hysteroscopy for infertile women (doi:10.1016/j.jmig.2014.12.163).
Dr. Miller is clinical associate professor at the University of Illinois at Chicago, and past president of the AAGL and the International Society for Gynecologic Endoscopy. He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in private practice in Naperville and Schaumburg, Ill.; director of minimally invasive gynecologic surgery and the director of the AAGL/SRS fellowship in minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill.; and the medical editor of this column, Master Class. He reported having no financial disclosures relevant to this column. Email him at obnews@frontlinemedcom.com.
Along with global endometrial ablation, diagnostic and minor operative hysteroscopy are excellent procedures to bring into your office environment. These operations are generally of short duration and provide little risk to the patient. Moreover, reimbursement exceeds that for the hospital setting. A constant revenue stream can be created after an initial moderate expenditure.
The key to a successful office procedure is patient comfort; this begins with minimizing pain and trauma. In our practice, we note decreased pain when performing vaginoscopy and hysteroscopy without the use of a speculum or tenaculum. This is well substantiated in literature by Professor Stefano Bettocchi, who immediately preceded me as president of the International Society for Gynecologic Endoscopy (ISGE).
In this issue of Master Class in Gynecologic Surgery, I have asked my partner, Aarathi Cholkeri-Singh, MD, to discuss vaginoscopy. Dr. Cholkeri-Singh is clinical assistant professor at the University of Illinois at Chicago, lecturer at Rosalind Franklin University of Medicine and Science, and associate director of minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill.
She also serves as codirector of the AAGL/Society of Reproductive Surgeons fellowship in minimally invasive gynecologic surgery and director of gynecologic surgical education at Advocate Lutheran, and is chair for a postgraduate course on hysteroscopy at the upcoming AAGL 45th Annual Global Congress. Among her publications is a recent review in the Journal of Minimally Invasive Gynecology on hysteroscopy for infertile women (doi:10.1016/j.jmig.2014.12.163).
Dr. Miller is clinical associate professor at the University of Illinois at Chicago, and past president of the AAGL and the International Society for Gynecologic Endoscopy. He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in private practice in Naperville and Schaumburg, Ill.; director of minimally invasive gynecologic surgery and the director of the AAGL/SRS fellowship in minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill.; and the medical editor of this column, Master Class. He reported having no financial disclosures relevant to this column. Email him at obnews@frontlinemedcom.com.
Along with global endometrial ablation, diagnostic and minor operative hysteroscopy are excellent procedures to bring into your office environment. These operations are generally of short duration and provide little risk to the patient. Moreover, reimbursement exceeds that for the hospital setting. A constant revenue stream can be created after an initial moderate expenditure.
The key to a successful office procedure is patient comfort; this begins with minimizing pain and trauma. In our practice, we note decreased pain when performing vaginoscopy and hysteroscopy without the use of a speculum or tenaculum. This is well substantiated in literature by Professor Stefano Bettocchi, who immediately preceded me as president of the International Society for Gynecologic Endoscopy (ISGE).
In this issue of Master Class in Gynecologic Surgery, I have asked my partner, Aarathi Cholkeri-Singh, MD, to discuss vaginoscopy. Dr. Cholkeri-Singh is clinical assistant professor at the University of Illinois at Chicago, lecturer at Rosalind Franklin University of Medicine and Science, and associate director of minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill.
She also serves as codirector of the AAGL/Society of Reproductive Surgeons fellowship in minimally invasive gynecologic surgery and director of gynecologic surgical education at Advocate Lutheran, and is chair for a postgraduate course on hysteroscopy at the upcoming AAGL 45th Annual Global Congress. Among her publications is a recent review in the Journal of Minimally Invasive Gynecology on hysteroscopy for infertile women (doi:10.1016/j.jmig.2014.12.163).
Dr. Miller is clinical associate professor at the University of Illinois at Chicago, and past president of the AAGL and the International Society for Gynecologic Endoscopy. He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in private practice in Naperville and Schaumburg, Ill.; director of minimally invasive gynecologic surgery and the director of the AAGL/SRS fellowship in minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill.; and the medical editor of this column, Master Class. He reported having no financial disclosures relevant to this column. Email him at obnews@frontlinemedcom.com.
The benefits of integrating in-office hysteroscopy
The benefits of integrating hysteroscopy into office practice are compelling. Most importantly, patients appreciate the comfort and convenience of having hysteroscopic procedures done in a familiar setting. Patients can generally be in and out of the office in less than 30 minutes for a diagnostic procedure, and in less than 1-2 hours for an operative procedure.
Not only is an in-office approach patient centered and clinically valuable, but it is more efficient and economically favorable for the gynecologic surgeon. Physicians earn higher reimbursement for diagnostic hysteroscopies, as well as many therapeutic and operative hysteroscopies, when these procedures are done in the office rather than when they’re performed in a hospital or an outpatient center.
Transitioning to in-office hysteroscopy need not be daunting: The setup is relatively simple and does not require an operating suite, just a dedicated exam room. And the need for premedication and local anesthesia can be low, particularly when a vaginoscopic approach to hysteroscopy is employed. For most gynecologic surgeons, the necessary skills and comfort levels fall into place after only a few vaginoscopic procedures.
A vaginoscopic approach avoids the use of a vaginal speculum or cervical tenaculum, significantly decreasing discomfort or pain. Not using these instruments is the only difference between this and traditional hysteroscopy. It is a less invasive approach that is much more tolerable for patients. And for the surgeon, it can be easier and quicker and provides equally good visualization without any impairment in cervical passage.
Described in the literature as far back as the 1950s, vaginoscopy has its roots in the pediatric/adolescent population, where it was used for the removal of foreign bodies and evaluation of the vagina and external cervical os.
More recently, Stefano Bettocchi, MD, and Luigi Selvaggi, MD, in Italy were the first to describe a vaginoscopic approach to hysteroscopy for evaluating the endocervical canal and uterine cavity.
In a series of papers from 1997 to 2004, Dr. Bettocchi and Dr. Selvaggi documented their efforts to improve patient tolerance during diagnostic hysteroscopies. When they used both the speculum and tenaculum in 163 patients, with local anesthesia, 8% reported severe pain, 11% reported moderate pain, and 69% reported mild pain. Only 12% reported no discomfort. With speculum use only, and no anesthesia, in 308 patients, none reported severe pain, 2% reported moderate pain, 32% reported mild pain, and 66% reported no discomfort. When neither instrument was used (again, no anesthesia), patient discomfort was nearly eliminated: In 680 procedures, patients had a 96% no-discomfort rate (J Am Assoc Gynecol Laparosc. 1997 Feb;4[2]:255-8; Curr Opin Obstet Gynecol. 2003 Aug;15[4]:303-8; Obstet Gynecol Clin North Am. 2004 Sep;31[3]:641-54, xi).
Since then, research has affirmed the differences in patient tolerance and has shown that there is no significant difference between traditional and vaginoscopic hysteroscopy in the rate of procedure failure (0%-10%).
In my practice, in addition to vaginal or cervical examination and evaluation of the uterine cavity, I utilize a vaginoscopic approach to perform minor therapeutic and operative procedures such as biopsies, polypectomies, tubal occlusion using the Essure system, and removal of lost intrauterine devices. I can assess infertility, trauma, abnormal uterine bleeding, and mesh erosion, and provide pre- and postsurgical evaluations. In all of these cases, I use minimal premedication and only rarely need any local anesthetic and/or sedation.
Instrumentation and technique
There are a variety of hysteroscopes available on the market, from single-channel flexible diagnostic hysteroscopes that are 3 mm to 4 mm in diameter, to “see-and-treat” operative hysteroscopes that are rigid and have various diameters and camera lens angles.
A hysteroscope with a 5.5-mm outer diameter works well for a vaginoscopic approach that avoids cervical dilation. Accessory instrumentation includes semirigid 5 Fr 35-cm–long biopsy forceps, scissors, and alligator forceps.
In timing the procedure, our main goal is a thin uterine lining. This can be achieved by scheduling the procedure during the early proliferative phase of the menstrual cycle or by using a gonadotropin-releasing hormone agonist or a transdermal or transvaginal contraceptive medication.
By far the most important element of pain control and analgesia is the time spent with each patient to thoroughly discuss the experience of hysteroscopy and to set expectations about what she will hear, see, and feel. An unexpected experience can worsen anxiety, which in turn can worsen pain. If everything is as familiar and relaxed as possible, there will be little need for analgesia.
I tell patients in preprocedure counseling that the distention of the uterine walls usually causes some cramping, and that NSAIDs can minimize this cramping. In rare cases, when a patient is very worried about her pain tolerance, I will prescribe diazepam. However, many of my patients opt to do nothing other than take ibuprofen. On a case-by-case basis, you can determine with your patient what type and level of analgesia and preprocedure medication will be best.
Paracervical blocks are an option for some surgical patients, but I advise my patients to move forward without the block and assure them that it can be administered later if needed. Thus far, I’ve never proceeded with a paracervical block. There are other methods and sites for introducing local anesthesia, including intracervical, by injection or topical, or topical intracavitary techniques. Nevertheless, it is unclear from randomized controlled trials whether local anesthesia is effective. Trials of paracervical blocks similarly have had inconsistent outcomes.
I do commonly premedicate patients – mainly nulliparous patients and postmenopausal patients – with misoprostol, which softens the cervix and facilitates an easier entry of the hysteroscope into the cervix.
Published studies on misoprostol administration before hysteroscopy have had mixed results. A Cochrane review from 2015 concluded there is moderate-quality evidence in support of preoperative ripening with the agent, while another meta-analysis also published in 2015 concluded that data are poor and do not support its use. Recently, however, there appear to be more supportive studies demonstrating or suggesting that misoprostol is effective in reducing discomfort.
Patient discomfort is also minimized when there is little manipulation of the hysteroscope. Scopes that are angled (12, 25, or 30 degrees) allow optimal visualization with minimal movement; the scope can be brought to the midline of the uterine cavity and the light cord rotated to the 3:00 and 9:00 o’clock positions to enable visualization of the cornu. A 0-degree scope, on the other hand, must be manipulated quite a bit for the same degree of visualization, potentially increasing patient discomfort.
Prior to hysteroscopy, the cervix and vagina are cleaned with a small-diameter swab dipped in povidone-iodine or chlorhexidine gluconate in the case of allergies. One or two 1,000-cc bags of saline inserted into pressure bags are attached to Y-type tubing. (A diagnostic procedure rarely will require two bags.) I spread the labia initially while guiding the scope into the posterior fornix of the vagina. If the leakage of fluid causes inadequate distension of the vaginal walls, I will gently pinch the labia together with gauze.
I then gently pull back the scope and manipulate it posteriorly to visualize the external cervical os anteriorly. The hysteroscope may then be introduced through the cervical os, endocervical canal, and uterine cavity, with care taken so that the instrument does not rub against the cervix or the uterine tissue and cause trauma, pain, and bleeding. The uterus will progressively align with the cervix and vagina, thereby eliminating the need for a tenaculum to straighten the uterine axis.
Fluid monitoring is important, especially during operative hysteroscopy. In my practice, a nurse watches inflow and outflow amounts while I explain what I am doing and visualizing. Some patients like to be able to view the surgery, so I am always ready to tilt the screen accordingly.
The economics
How do you know if office hysteroscopy is right for you? Your own surgical skill and the skills of your staff, who must be trained to handle and sterilize equipment and to consistently assist you, are major factors, as is ensurance of a return on your investment.
One manufacturer contacted for this Master Class lists the price of a complete office tower (light source, camera, and monitor) at approximately $9,700 and the price of a rigid hysteroscope, sheath, and hand instruments at about $6,300. A complete setup for office hysteroscopy, including a standard operative (rigid) hysteroscope, should therefore cost between $15,000 and $17,000. Companies also offer leasing options for about $300-400/month.
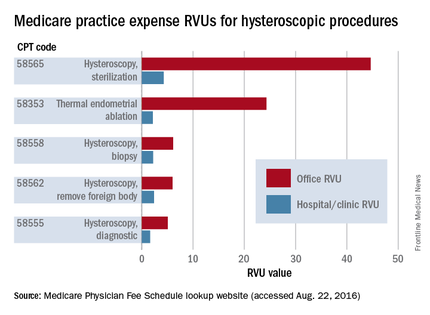
Flexible hysteroscopes cost about $6,000 more, which prompts many gynecologic surgeons to focus their investment on a rigid scope that can be used for both diagnostic and therapeutic procedures. Disposables cost $10 or less, and $40-50 or less, for each diagnostic and operative hysteroscopy, respectively.
A look at the Medicare Relative Value Units (RVUs) – a key component of the Medicare reimbursement system and a standard for many payers in determining compensation – shows higher reimbursement for quite a few hysteroscopic codes when these procedures are performed in the office.
Total RVUs have three components:
1. Physician work, including time and the technical skill and intensity of effort it takes to perform a service or procedure.
2. Practice expenses, such as rent, equipment and supplies, and nonphysician salaries.
3. Malpractice insurance premiums.
Each component is multiplied by a factor that accounts for geographic cost variations, and each total RVU is multiplied by a dollar amount known as the conversion factor.
Practice expense (PE) RVUs for services provided in a “facility” (e.g., hospital or outpatient clinic) are often lower than office-based PE RVUs for the same services. Hysteroscopy is no exception. The PE RVU value for diagnostic hysteroscopy performed in the office, for instance, is approximately 5 units, compared with 1.64 units for diagnostic hysteroscopy performed in a facility.
Information on hysteroscopic procedures, and their associated RVUs, on geographic practice cost indices and on pricing, can be accessed using Medicare’s Physician Fee Schedule lookup tool (www.cms.gov/apps/physician-fee-schedule/overview.aspx).
This tool is useful for calculating returns on investment. According to national payment amounts listed in August, a diagnostic hysteroscopy performed in the office will earn an average of $315.08 vs. $192.27 for each case performed in the hospital. If you perform 12 such procedures a year, that’s about $3,781 in the office, compared with $2,307 in the hospital.
This difference alone might not be worth an investment of $15,000 or more, but if you anticipate performing additional procedures with higher margins and higher reimbursement, such as 12 thermal endometrial ablations a year in combination with diagnostic hysteroscopy (which, according to the Medicare national fee schedule averages would earn $15,962 in the office vs. $4,971 in the hospital), or 12 Essure tubal occlusions ($22,595 vs. $5,263), the investment will look more favorable.
And if your patients are largely privately insured, your return on investment will occur much more quickly. In metropolitan Chicago, Blue Cross Blue Shield is reimbursing in-office diagnostic hysteroscopy at approximately $568, hysteroscopic ablations at $3,844, and Essure tubal occlusions at $3,885.
In addition to reimbursement levels, it’s important to consider the efficiencies of in-office hysteroscopy. You can perform an annual exam while the assistant sets up the room and greets each patient, for instance, or see another established patient while the assistant discharges your patient and turns the room over. Our patients, in turn, benefit from increased accessibility, with less time spent away from work or family, as well as more familiarity and comfort and reduced out-of-pocket expenses.
Dr. Cholkeri-Singh is clinical assistant professor at the University of Illinois in Chicago and is director of gynecologic surgical education and associate director of minimally invasive gynecology at Advocate Lutheran General Hospital. She is in private practice with Dr. Charles Miller and Dr. Kristen Sasaki at the Advanced Gynecologic Surgical Institute in Chicago. She is a consultant for DySIS Medical, Hologic, and Bayer HealthCare.
The benefits of integrating hysteroscopy into office practice are compelling. Most importantly, patients appreciate the comfort and convenience of having hysteroscopic procedures done in a familiar setting. Patients can generally be in and out of the office in less than 30 minutes for a diagnostic procedure, and in less than 1-2 hours for an operative procedure.
Not only is an in-office approach patient centered and clinically valuable, but it is more efficient and economically favorable for the gynecologic surgeon. Physicians earn higher reimbursement for diagnostic hysteroscopies, as well as many therapeutic and operative hysteroscopies, when these procedures are done in the office rather than when they’re performed in a hospital or an outpatient center.
Transitioning to in-office hysteroscopy need not be daunting: The setup is relatively simple and does not require an operating suite, just a dedicated exam room. And the need for premedication and local anesthesia can be low, particularly when a vaginoscopic approach to hysteroscopy is employed. For most gynecologic surgeons, the necessary skills and comfort levels fall into place after only a few vaginoscopic procedures.
A vaginoscopic approach avoids the use of a vaginal speculum or cervical tenaculum, significantly decreasing discomfort or pain. Not using these instruments is the only difference between this and traditional hysteroscopy. It is a less invasive approach that is much more tolerable for patients. And for the surgeon, it can be easier and quicker and provides equally good visualization without any impairment in cervical passage.
Described in the literature as far back as the 1950s, vaginoscopy has its roots in the pediatric/adolescent population, where it was used for the removal of foreign bodies and evaluation of the vagina and external cervical os.
More recently, Stefano Bettocchi, MD, and Luigi Selvaggi, MD, in Italy were the first to describe a vaginoscopic approach to hysteroscopy for evaluating the endocervical canal and uterine cavity.
In a series of papers from 1997 to 2004, Dr. Bettocchi and Dr. Selvaggi documented their efforts to improve patient tolerance during diagnostic hysteroscopies. When they used both the speculum and tenaculum in 163 patients, with local anesthesia, 8% reported severe pain, 11% reported moderate pain, and 69% reported mild pain. Only 12% reported no discomfort. With speculum use only, and no anesthesia, in 308 patients, none reported severe pain, 2% reported moderate pain, 32% reported mild pain, and 66% reported no discomfort. When neither instrument was used (again, no anesthesia), patient discomfort was nearly eliminated: In 680 procedures, patients had a 96% no-discomfort rate (J Am Assoc Gynecol Laparosc. 1997 Feb;4[2]:255-8; Curr Opin Obstet Gynecol. 2003 Aug;15[4]:303-8; Obstet Gynecol Clin North Am. 2004 Sep;31[3]:641-54, xi).
Since then, research has affirmed the differences in patient tolerance and has shown that there is no significant difference between traditional and vaginoscopic hysteroscopy in the rate of procedure failure (0%-10%).
In my practice, in addition to vaginal or cervical examination and evaluation of the uterine cavity, I utilize a vaginoscopic approach to perform minor therapeutic and operative procedures such as biopsies, polypectomies, tubal occlusion using the Essure system, and removal of lost intrauterine devices. I can assess infertility, trauma, abnormal uterine bleeding, and mesh erosion, and provide pre- and postsurgical evaluations. In all of these cases, I use minimal premedication and only rarely need any local anesthetic and/or sedation.
Instrumentation and technique
There are a variety of hysteroscopes available on the market, from single-channel flexible diagnostic hysteroscopes that are 3 mm to 4 mm in diameter, to “see-and-treat” operative hysteroscopes that are rigid and have various diameters and camera lens angles.
A hysteroscope with a 5.5-mm outer diameter works well for a vaginoscopic approach that avoids cervical dilation. Accessory instrumentation includes semirigid 5 Fr 35-cm–long biopsy forceps, scissors, and alligator forceps.
In timing the procedure, our main goal is a thin uterine lining. This can be achieved by scheduling the procedure during the early proliferative phase of the menstrual cycle or by using a gonadotropin-releasing hormone agonist or a transdermal or transvaginal contraceptive medication.
By far the most important element of pain control and analgesia is the time spent with each patient to thoroughly discuss the experience of hysteroscopy and to set expectations about what she will hear, see, and feel. An unexpected experience can worsen anxiety, which in turn can worsen pain. If everything is as familiar and relaxed as possible, there will be little need for analgesia.
I tell patients in preprocedure counseling that the distention of the uterine walls usually causes some cramping, and that NSAIDs can minimize this cramping. In rare cases, when a patient is very worried about her pain tolerance, I will prescribe diazepam. However, many of my patients opt to do nothing other than take ibuprofen. On a case-by-case basis, you can determine with your patient what type and level of analgesia and preprocedure medication will be best.
Paracervical blocks are an option for some surgical patients, but I advise my patients to move forward without the block and assure them that it can be administered later if needed. Thus far, I’ve never proceeded with a paracervical block. There are other methods and sites for introducing local anesthesia, including intracervical, by injection or topical, or topical intracavitary techniques. Nevertheless, it is unclear from randomized controlled trials whether local anesthesia is effective. Trials of paracervical blocks similarly have had inconsistent outcomes.
I do commonly premedicate patients – mainly nulliparous patients and postmenopausal patients – with misoprostol, which softens the cervix and facilitates an easier entry of the hysteroscope into the cervix.
Published studies on misoprostol administration before hysteroscopy have had mixed results. A Cochrane review from 2015 concluded there is moderate-quality evidence in support of preoperative ripening with the agent, while another meta-analysis also published in 2015 concluded that data are poor and do not support its use. Recently, however, there appear to be more supportive studies demonstrating or suggesting that misoprostol is effective in reducing discomfort.
Patient discomfort is also minimized when there is little manipulation of the hysteroscope. Scopes that are angled (12, 25, or 30 degrees) allow optimal visualization with minimal movement; the scope can be brought to the midline of the uterine cavity and the light cord rotated to the 3:00 and 9:00 o’clock positions to enable visualization of the cornu. A 0-degree scope, on the other hand, must be manipulated quite a bit for the same degree of visualization, potentially increasing patient discomfort.
Prior to hysteroscopy, the cervix and vagina are cleaned with a small-diameter swab dipped in povidone-iodine or chlorhexidine gluconate in the case of allergies. One or two 1,000-cc bags of saline inserted into pressure bags are attached to Y-type tubing. (A diagnostic procedure rarely will require two bags.) I spread the labia initially while guiding the scope into the posterior fornix of the vagina. If the leakage of fluid causes inadequate distension of the vaginal walls, I will gently pinch the labia together with gauze.
I then gently pull back the scope and manipulate it posteriorly to visualize the external cervical os anteriorly. The hysteroscope may then be introduced through the cervical os, endocervical canal, and uterine cavity, with care taken so that the instrument does not rub against the cervix or the uterine tissue and cause trauma, pain, and bleeding. The uterus will progressively align with the cervix and vagina, thereby eliminating the need for a tenaculum to straighten the uterine axis.
Fluid monitoring is important, especially during operative hysteroscopy. In my practice, a nurse watches inflow and outflow amounts while I explain what I am doing and visualizing. Some patients like to be able to view the surgery, so I am always ready to tilt the screen accordingly.
The economics
How do you know if office hysteroscopy is right for you? Your own surgical skill and the skills of your staff, who must be trained to handle and sterilize equipment and to consistently assist you, are major factors, as is ensurance of a return on your investment.
One manufacturer contacted for this Master Class lists the price of a complete office tower (light source, camera, and monitor) at approximately $9,700 and the price of a rigid hysteroscope, sheath, and hand instruments at about $6,300. A complete setup for office hysteroscopy, including a standard operative (rigid) hysteroscope, should therefore cost between $15,000 and $17,000. Companies also offer leasing options for about $300-400/month.

Flexible hysteroscopes cost about $6,000 more, which prompts many gynecologic surgeons to focus their investment on a rigid scope that can be used for both diagnostic and therapeutic procedures. Disposables cost $10 or less, and $40-50 or less, for each diagnostic and operative hysteroscopy, respectively.
A look at the Medicare Relative Value Units (RVUs) – a key component of the Medicare reimbursement system and a standard for many payers in determining compensation – shows higher reimbursement for quite a few hysteroscopic codes when these procedures are performed in the office.
Total RVUs have three components:
1. Physician work, including time and the technical skill and intensity of effort it takes to perform a service or procedure.
2. Practice expenses, such as rent, equipment and supplies, and nonphysician salaries.
3. Malpractice insurance premiums.
Each component is multiplied by a factor that accounts for geographic cost variations, and each total RVU is multiplied by a dollar amount known as the conversion factor.
Practice expense (PE) RVUs for services provided in a “facility” (e.g., hospital or outpatient clinic) are often lower than office-based PE RVUs for the same services. Hysteroscopy is no exception. The PE RVU value for diagnostic hysteroscopy performed in the office, for instance, is approximately 5 units, compared with 1.64 units for diagnostic hysteroscopy performed in a facility.
Information on hysteroscopic procedures, and their associated RVUs, on geographic practice cost indices and on pricing, can be accessed using Medicare’s Physician Fee Schedule lookup tool (www.cms.gov/apps/physician-fee-schedule/overview.aspx).
This tool is useful for calculating returns on investment. According to national payment amounts listed in August, a diagnostic hysteroscopy performed in the office will earn an average of $315.08 vs. $192.27 for each case performed in the hospital. If you perform 12 such procedures a year, that’s about $3,781 in the office, compared with $2,307 in the hospital.
This difference alone might not be worth an investment of $15,000 or more, but if you anticipate performing additional procedures with higher margins and higher reimbursement, such as 12 thermal endometrial ablations a year in combination with diagnostic hysteroscopy (which, according to the Medicare national fee schedule averages would earn $15,962 in the office vs. $4,971 in the hospital), or 12 Essure tubal occlusions ($22,595 vs. $5,263), the investment will look more favorable.
And if your patients are largely privately insured, your return on investment will occur much more quickly. In metropolitan Chicago, Blue Cross Blue Shield is reimbursing in-office diagnostic hysteroscopy at approximately $568, hysteroscopic ablations at $3,844, and Essure tubal occlusions at $3,885.
In addition to reimbursement levels, it’s important to consider the efficiencies of in-office hysteroscopy. You can perform an annual exam while the assistant sets up the room and greets each patient, for instance, or see another established patient while the assistant discharges your patient and turns the room over. Our patients, in turn, benefit from increased accessibility, with less time spent away from work or family, as well as more familiarity and comfort and reduced out-of-pocket expenses.
Dr. Cholkeri-Singh is clinical assistant professor at the University of Illinois in Chicago and is director of gynecologic surgical education and associate director of minimally invasive gynecology at Advocate Lutheran General Hospital. She is in private practice with Dr. Charles Miller and Dr. Kristen Sasaki at the Advanced Gynecologic Surgical Institute in Chicago. She is a consultant for DySIS Medical, Hologic, and Bayer HealthCare.
The benefits of integrating hysteroscopy into office practice are compelling. Most importantly, patients appreciate the comfort and convenience of having hysteroscopic procedures done in a familiar setting. Patients can generally be in and out of the office in less than 30 minutes for a diagnostic procedure, and in less than 1-2 hours for an operative procedure.
Not only is an in-office approach patient centered and clinically valuable, but it is more efficient and economically favorable for the gynecologic surgeon. Physicians earn higher reimbursement for diagnostic hysteroscopies, as well as many therapeutic and operative hysteroscopies, when these procedures are done in the office rather than when they’re performed in a hospital or an outpatient center.
Transitioning to in-office hysteroscopy need not be daunting: The setup is relatively simple and does not require an operating suite, just a dedicated exam room. And the need for premedication and local anesthesia can be low, particularly when a vaginoscopic approach to hysteroscopy is employed. For most gynecologic surgeons, the necessary skills and comfort levels fall into place after only a few vaginoscopic procedures.
A vaginoscopic approach avoids the use of a vaginal speculum or cervical tenaculum, significantly decreasing discomfort or pain. Not using these instruments is the only difference between this and traditional hysteroscopy. It is a less invasive approach that is much more tolerable for patients. And for the surgeon, it can be easier and quicker and provides equally good visualization without any impairment in cervical passage.
Described in the literature as far back as the 1950s, vaginoscopy has its roots in the pediatric/adolescent population, where it was used for the removal of foreign bodies and evaluation of the vagina and external cervical os.
More recently, Stefano Bettocchi, MD, and Luigi Selvaggi, MD, in Italy were the first to describe a vaginoscopic approach to hysteroscopy for evaluating the endocervical canal and uterine cavity.
In a series of papers from 1997 to 2004, Dr. Bettocchi and Dr. Selvaggi documented their efforts to improve patient tolerance during diagnostic hysteroscopies. When they used both the speculum and tenaculum in 163 patients, with local anesthesia, 8% reported severe pain, 11% reported moderate pain, and 69% reported mild pain. Only 12% reported no discomfort. With speculum use only, and no anesthesia, in 308 patients, none reported severe pain, 2% reported moderate pain, 32% reported mild pain, and 66% reported no discomfort. When neither instrument was used (again, no anesthesia), patient discomfort was nearly eliminated: In 680 procedures, patients had a 96% no-discomfort rate (J Am Assoc Gynecol Laparosc. 1997 Feb;4[2]:255-8; Curr Opin Obstet Gynecol. 2003 Aug;15[4]:303-8; Obstet Gynecol Clin North Am. 2004 Sep;31[3]:641-54, xi).
Since then, research has affirmed the differences in patient tolerance and has shown that there is no significant difference between traditional and vaginoscopic hysteroscopy in the rate of procedure failure (0%-10%).
In my practice, in addition to vaginal or cervical examination and evaluation of the uterine cavity, I utilize a vaginoscopic approach to perform minor therapeutic and operative procedures such as biopsies, polypectomies, tubal occlusion using the Essure system, and removal of lost intrauterine devices. I can assess infertility, trauma, abnormal uterine bleeding, and mesh erosion, and provide pre- and postsurgical evaluations. In all of these cases, I use minimal premedication and only rarely need any local anesthetic and/or sedation.
Instrumentation and technique
There are a variety of hysteroscopes available on the market, from single-channel flexible diagnostic hysteroscopes that are 3 mm to 4 mm in diameter, to “see-and-treat” operative hysteroscopes that are rigid and have various diameters and camera lens angles.
A hysteroscope with a 5.5-mm outer diameter works well for a vaginoscopic approach that avoids cervical dilation. Accessory instrumentation includes semirigid 5 Fr 35-cm–long biopsy forceps, scissors, and alligator forceps.
In timing the procedure, our main goal is a thin uterine lining. This can be achieved by scheduling the procedure during the early proliferative phase of the menstrual cycle or by using a gonadotropin-releasing hormone agonist or a transdermal or transvaginal contraceptive medication.
By far the most important element of pain control and analgesia is the time spent with each patient to thoroughly discuss the experience of hysteroscopy and to set expectations about what she will hear, see, and feel. An unexpected experience can worsen anxiety, which in turn can worsen pain. If everything is as familiar and relaxed as possible, there will be little need for analgesia.
I tell patients in preprocedure counseling that the distention of the uterine walls usually causes some cramping, and that NSAIDs can minimize this cramping. In rare cases, when a patient is very worried about her pain tolerance, I will prescribe diazepam. However, many of my patients opt to do nothing other than take ibuprofen. On a case-by-case basis, you can determine with your patient what type and level of analgesia and preprocedure medication will be best.
Paracervical blocks are an option for some surgical patients, but I advise my patients to move forward without the block and assure them that it can be administered later if needed. Thus far, I’ve never proceeded with a paracervical block. There are other methods and sites for introducing local anesthesia, including intracervical, by injection or topical, or topical intracavitary techniques. Nevertheless, it is unclear from randomized controlled trials whether local anesthesia is effective. Trials of paracervical blocks similarly have had inconsistent outcomes.
I do commonly premedicate patients – mainly nulliparous patients and postmenopausal patients – with misoprostol, which softens the cervix and facilitates an easier entry of the hysteroscope into the cervix.
Published studies on misoprostol administration before hysteroscopy have had mixed results. A Cochrane review from 2015 concluded there is moderate-quality evidence in support of preoperative ripening with the agent, while another meta-analysis also published in 2015 concluded that data are poor and do not support its use. Recently, however, there appear to be more supportive studies demonstrating or suggesting that misoprostol is effective in reducing discomfort.
Patient discomfort is also minimized when there is little manipulation of the hysteroscope. Scopes that are angled (12, 25, or 30 degrees) allow optimal visualization with minimal movement; the scope can be brought to the midline of the uterine cavity and the light cord rotated to the 3:00 and 9:00 o’clock positions to enable visualization of the cornu. A 0-degree scope, on the other hand, must be manipulated quite a bit for the same degree of visualization, potentially increasing patient discomfort.
Prior to hysteroscopy, the cervix and vagina are cleaned with a small-diameter swab dipped in povidone-iodine or chlorhexidine gluconate in the case of allergies. One or two 1,000-cc bags of saline inserted into pressure bags are attached to Y-type tubing. (A diagnostic procedure rarely will require two bags.) I spread the labia initially while guiding the scope into the posterior fornix of the vagina. If the leakage of fluid causes inadequate distension of the vaginal walls, I will gently pinch the labia together with gauze.
I then gently pull back the scope and manipulate it posteriorly to visualize the external cervical os anteriorly. The hysteroscope may then be introduced through the cervical os, endocervical canal, and uterine cavity, with care taken so that the instrument does not rub against the cervix or the uterine tissue and cause trauma, pain, and bleeding. The uterus will progressively align with the cervix and vagina, thereby eliminating the need for a tenaculum to straighten the uterine axis.
Fluid monitoring is important, especially during operative hysteroscopy. In my practice, a nurse watches inflow and outflow amounts while I explain what I am doing and visualizing. Some patients like to be able to view the surgery, so I am always ready to tilt the screen accordingly.
The economics
How do you know if office hysteroscopy is right for you? Your own surgical skill and the skills of your staff, who must be trained to handle and sterilize equipment and to consistently assist you, are major factors, as is ensurance of a return on your investment.
One manufacturer contacted for this Master Class lists the price of a complete office tower (light source, camera, and monitor) at approximately $9,700 and the price of a rigid hysteroscope, sheath, and hand instruments at about $6,300. A complete setup for office hysteroscopy, including a standard operative (rigid) hysteroscope, should therefore cost between $15,000 and $17,000. Companies also offer leasing options for about $300-400/month.

Flexible hysteroscopes cost about $6,000 more, which prompts many gynecologic surgeons to focus their investment on a rigid scope that can be used for both diagnostic and therapeutic procedures. Disposables cost $10 or less, and $40-50 or less, for each diagnostic and operative hysteroscopy, respectively.
A look at the Medicare Relative Value Units (RVUs) – a key component of the Medicare reimbursement system and a standard for many payers in determining compensation – shows higher reimbursement for quite a few hysteroscopic codes when these procedures are performed in the office.
Total RVUs have three components:
1. Physician work, including time and the technical skill and intensity of effort it takes to perform a service or procedure.
2. Practice expenses, such as rent, equipment and supplies, and nonphysician salaries.
3. Malpractice insurance premiums.
Each component is multiplied by a factor that accounts for geographic cost variations, and each total RVU is multiplied by a dollar amount known as the conversion factor.
Practice expense (PE) RVUs for services provided in a “facility” (e.g., hospital or outpatient clinic) are often lower than office-based PE RVUs for the same services. Hysteroscopy is no exception. The PE RVU value for diagnostic hysteroscopy performed in the office, for instance, is approximately 5 units, compared with 1.64 units for diagnostic hysteroscopy performed in a facility.
Information on hysteroscopic procedures, and their associated RVUs, on geographic practice cost indices and on pricing, can be accessed using Medicare’s Physician Fee Schedule lookup tool (www.cms.gov/apps/physician-fee-schedule/overview.aspx).
This tool is useful for calculating returns on investment. According to national payment amounts listed in August, a diagnostic hysteroscopy performed in the office will earn an average of $315.08 vs. $192.27 for each case performed in the hospital. If you perform 12 such procedures a year, that’s about $3,781 in the office, compared with $2,307 in the hospital.
This difference alone might not be worth an investment of $15,000 or more, but if you anticipate performing additional procedures with higher margins and higher reimbursement, such as 12 thermal endometrial ablations a year in combination with diagnostic hysteroscopy (which, according to the Medicare national fee schedule averages would earn $15,962 in the office vs. $4,971 in the hospital), or 12 Essure tubal occlusions ($22,595 vs. $5,263), the investment will look more favorable.
And if your patients are largely privately insured, your return on investment will occur much more quickly. In metropolitan Chicago, Blue Cross Blue Shield is reimbursing in-office diagnostic hysteroscopy at approximately $568, hysteroscopic ablations at $3,844, and Essure tubal occlusions at $3,885.
In addition to reimbursement levels, it’s important to consider the efficiencies of in-office hysteroscopy. You can perform an annual exam while the assistant sets up the room and greets each patient, for instance, or see another established patient while the assistant discharges your patient and turns the room over. Our patients, in turn, benefit from increased accessibility, with less time spent away from work or family, as well as more familiarity and comfort and reduced out-of-pocket expenses.
Dr. Cholkeri-Singh is clinical assistant professor at the University of Illinois in Chicago and is director of gynecologic surgical education and associate director of minimally invasive gynecology at Advocate Lutheran General Hospital. She is in private practice with Dr. Charles Miller and Dr. Kristen Sasaki at the Advanced Gynecologic Surgical Institute in Chicago. She is a consultant for DySIS Medical, Hologic, and Bayer HealthCare.
Obstetrics Moonshots: 50 years of discoveries
In 1961 before Congress, and in 1962 at Rice University, Houston, President John F. Kennedy called on America to land a man on the moon and bring him back safely, and to look beyond the moon as well, and pursue an ambitious space exploration program. He challenged the country to think and act boldly, telling Americans in his speech at Rice that “we choose to go the moon in this decade and do the other things, not because they are easy, but because they are hard.”
When Neil Armstrong and Buzz Aldrin set foot on the moon in 1969 – even before President Kennedy’s 10-year deadline had arrived – the country’s primary moonshot was realized. The President had inspired the nation, teams of engineers and others had collectively met daunting technological challenges, and space consequently was more open to us than ever before.
In looking at the field of obstetrics and how far it has come in the past 50 years, since the 1960s, it is similarly astonishing and inspiring to reflect on what extraordinary advances we have made. Who would have thought that the fetus would become such a visible and intimate patient – one who, like the mother, can be interrogated, monitored, and sometimes treated before birth? Who would have thought we would be utilizing genomic studies in a now well-established field of prenatal diagnosis, or that fetal therapy would become a field in and of itself?
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Our specialty has advanced through a series of moonshots that have been inspired and driven by technological advancement and by our continually bold goals and vision for the health and well-being of women and their offspring. We have taken on ambitious challenges, achieved many goals, and embraced advancements in practice only to then set new targets that previously were unimaginable.
Yet just as our country’s space exploration program has faced disappointments, so has our field. It is sobering, for instance, that we have made only incremental improvements in prematurity and infant mortality, and that the age-old maternal problem of preeclampsia is still with us. We also face new challenges, such as the rising rate of maternal obesity and diabetes, which threaten both maternal and fetal health.
President Kennedy spoke of having “examined where we are strong, and where we are not.” Such self-reflection and assessment is a critical underpinning of advancement in fields across all of science, medicine, and health care, and in our specialty, it is a process that has driven ambitious new research efforts to improve fetal and maternal health.
A step back to more in-depth fundamental research on the biomolecular mechanisms of premature labor and diabetes-associated birth defects, for instance, as well as new efforts to approach fetal surgery less invasively, are positioning us to both conquer our disappointments and achieve ambitious new moonshots.
The fetus as our patient
Fifty years ago, in 1966, a seminal paper in the Lancet reported that amniotic fluid cells could be cultured and were suitable for karyotyping (1[7434]:383-5). The tapping and examination of amniotic fluid had been reported on sporadically for many decades, for various clinical purposes, but by and large the fetal compartment was not invaded or directly examined. The fetus was instead the hopeful beneficiary of pregnancy care that focused on the mother. Fetal outcome was clouded in mystery, known only at birth.
With the Lancet report, prenatal detection of chromosomal disorders began to feel achievable, and the 1960s marked the beginning of a journey first through invasive methods of prenatal diagnosis and then through increasingly non-invasive approaches.
In 1970, just several years after the report on chromosome analysis of amniotic-fluid cells, another landmark paper in the New England Journal of Medicine described 162 amniocenteses performed between the 13th and 18th weeks of gestation and the detection of 10 cases of Down syndrome, as well as a few other cases of metabolic and other disorders (282[11]:596-9). This report provided an impetus for broader use of the procedure to detect neural tube defects, Down syndrome, and other abnormalities.
The adoption of amniocentesis for prenatal diagnosis still took some time, however. The procedure was used primarily early on to determine fetal lung maturity, and to predict the ability of the fetus to survive after delivery.
At the time, it was widely praised as an advanced method for evaluating the fetus. Yet, looking back, the early years of the procedure seem primitive. The procedure was done late in pregnancy and it was performed blindly, with the puncture site located either with external palpation of the uterus or with the assistance of static ultrasound. Patients who had scans would usually visit the radiologist, who would mark on the patient’s abdomen a suggested location for needle insertion. Upon the patient’s return, the obstetrician would then insert a needle into that spot, blindly and likely after the fetus had moved.
The development and adoption of real-time ultrasound was a revolutionary achievement. Ultrasound-guided amniocentesis was first described in 1972, 14 years after Ian Donald’s seminal paper introducing obstetric ultrasound was published in the Lancet (1958 Jun 7;1[7032]:1188-95).
As real-time ultrasound made its way into practice, it marked the true realization of a moonshot for obstetrics.
Not only could we simultaneously visualize the needle tip and place the needle safety, but we could see the real-time movement of the fetus, its activity, and the surrounding pockets of fluid. It was like looking up into the sky and seeing the stars for the first time. We could see fetal arrhythmia – not only hear it. With this window into the fetal compartment, we could visualize the fetal bowel migrating into the chest cavity due to a hole (hernia) in the diaphragm. We could visualize other malformations as well.
Chorionic villus sampling (CVS) was technically more difficult and took longer to evolve. For years, through the early 1980s, it was performed only at select centers throughout the country. Patients traveled for the procedure and faced relatively significant risks of complications.
By the end of the 1980s, however, with successive improvements in equipment and technique (including development of a transabdominal approach in addition to transvaginal) the procedure was deemed safe, effective, and acceptable for routine use. Fetoscopy, pioneered by John Hobbins, MD, and his colleagues at Yale University, New Haven, Conn., had also advanced and was being used to diagnose sickle cell anemia, Tay-Sachs disease, congenital fetal skin diseases, and other disorders.
With these advances and with our newfound ability to obtain and analyze a tissue sample earlier in pregnancy – even before a woman shared the news of her pregnancy, in some cases – it seemed that we had achieved our goals and may have even reached past the moon.
Yet there were other moonshots being pursued, including initiatives to make prenatal diagnosis less invasive. The discovery in 1997 of cell-free fetal DNA in maternal plasma and serum, for instance, was a pivotal development that opened the door for noninvasive prenatal testing.
This, and other advances in areas from biochemistry to ultrasound to genomic analysis, led to an array of prenatal diagnostic tools that today enable women and their physicians to assess the genetic, chromosomal, and biophysical aspects of their fetus considerably before the time of viability, and from both the maternal side and directly in the fetal compartment.
First-trimester screening is a current option, and we now have the ability to more selectively perform amniocentesis and CVS based on probability testing, and not solely on maternal age. Ultrasound technology now encompasses color Doppler, 3D and 4D imaging, and other techniques that can be used to assess the placenta, various structures inside the brain, and the heart, as well as blood flow through the ductus venosus.
Parents have called for and welcomed having the option of assessing the fetus in greater detail, and of having either assurance when anomalies are excluded or the opportunity to plan and make decisions when anomalies are detected.
Fetal surgery has been a natural extension of our unprecedented access to the fetus. Our ability to visualize malformations and their evolution led to animal studies that advanced our interest in arresting, correcting, or reversing fetal anomalies through in-utero interventions. In 1981, surgeons performed the first human open fetal surgery to correct congenital hydronephrosis.
Today, we can employ endoscopic laser ablation or laser coagulation to treat severe twin-to-twin syndrome, for instance, as well as other surgical techniques to repair defects such as congenital diaphragmatic hernia, lower urinary tract obstruction, and myelomeningocele. Such advances were unimaginable decades ago.
Old foes and new threats
Despite these advances in diagnosis and care, obstetrics faces unrealized moonshots – lingering challenges that, 50 years ago, we would have predicted would have been solved. Who would have thought that we would still have as high an infant mortality rate as we do, and that we would not be further along in solving the problem of prematurity? Our progress has been only incremental.
Fifty years ago, we lacked an understanding of the basic biology of preterm labor. Prematurity was viewed simply as term labor occurring too early, and many efforts were made over the years to halt the premature labor process through the use of various drugs and other therapeutics, with variable and minimally impactful levels of success.
In the last 25 years, and especially in the last decade, we have made greater efforts to better understand the biology of premature labor – to elucidate how and why it occurs – and we have come to understand that premature labor is very different physiologically from term labor.
Thanks to the work at the Perinatology Research Branch of the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), led by Roberto Romero, MD, attention has consequently shifted toward prediction, identification of women at highest risk, and prevention of the onset of premature labor among those deemed to be at highest risk.
Cervical length in the mid-trimester is now a well-verified predictor of preterm birth, and vaginal progesterone has been shown to benefit women without other known risk factors who are diagnosed with a shortened cervical length.
We have consequently seen the preterm birth rate decline a bit. In 2013, the last year for which we have complete data, the preterm birth rate dropped to 11.4%, down from a high of 12.8% in 2006, according to the Centers for Disease Control and Prevention.
Infant mortality similarly remains unacceptably high, due largely to the high preterm birth rate and to our failure to significantly alter the prevalence of birth defects. In 2010, according to the CDC, the infant mortality rate in the U.S. was 6.1 deaths per 1,000 live births (compared with 6.87 in 2005), and the United States ranked 26th in infant mortality among countries belonging to the Organisation for Economic Co-operation and Development, despite the fact that we spend a significant portion of our gross domestic product (17.5% in 2014) on health care.
Birth defects have taken over as a leading cause of infant mortality after early newborn life, and while we’ve made some advancements in understanding and diagnosing them, the majority of causes of birth defects are still unknown.
On the maternal side of obstetrical care, our progress has similarly been more modest than we have hoped for. Preeclampsia remains a problem, for instance. Despite decades of research into its pathogenesis, our advancements have been only incremental, and the condition – particularly its severe form – continues to be a vexing and high-risk problem.
Added to such age-old foes, moreover, are the growing threats of maternal obesity and diabetes, two closely related and often chronic conditions that affect not only the health of the mother but the in-utero environment and the health of the fetus. Today, more than one-third of all adults in the U.S., and 34% of women aged 20-39 years, are obese, and almost 10% of the U.S. population has diabetes.
Both conditions are on the rise, and obstetrics is confronting an epidemic of “diabesity” that would not necessarily have been predicted 50 years ago. It is particularly alarming given our growing knowledge of how obesity can be programmed in-utero and essentially passed on from generation to generation, of how diabetes can negatively affect perinatal outcomes, and of how the two conditions can have an additive effect on fetal complications.
Achieving new moonshots
Concerted efforts in the past several decades to step back and try to understand the basic biology and physiology of term labor and of premature labor have better positioned our specialty to achieve the moonshot of significantly reducing the incidence of preterm birth.
Establishment in the mid-1980s of the NICHD’s Perinatology Research Branch was a major development in this regard, helping to build and direct research efforts, including basic laboratory science, toward questions about what triggers and propagates labor. There has been notable progress in the past decade, in particular, and our specialty is now on the right path toward development of therapeutic interventions for preventing prematurity.
Additionally, the NICHD’s recently launched Human Placenta Project is building upon the branch-sponsored animal and cell culture model systems of the placenta to allow researchers, for the first time, to monitor human placental health in real time. By more fully understanding the role of the placenta in health and disease, we will be able to better evaluate pregnancy risks and improve pregnancy outcomes.
We also are learning through research in the University of Maryland Birth Defects Research Laboratory, which I am privileged to direct, and at other facilities, that maternal hyperglycemia is a teratogen, creating insults that can trigger a series of developmental fetal defects. By studying the biomolecular mechanisms of hyperglycemia-induced birth defects and developing “molecular maps,” we expect to be able to develop strategies for preventing or mitigating the development of such anomalies. I hope and expect that these future advancements, combined with reductions in prematurity, will significantly impact the infant mortality rate.
Fetal therapy and surgery will also continue to advance, with a much more minimally invasive approach taken in the next 50 years to addressing the fetal condition without putting the mother at increased risk. Just as surgery in other fields has moved from open laparotomy to minimally invasive techniques, I believe we will develop endoscopic or laparoscopic means of correcting the various problems in-utero, such as the repair of neural tube defects and diaphragmatic hernias. It already appears likely that a fetoscopic approach to treating myelomeningocele can reduce maternal morbidity while achieving infant neurological outcomes that are at least as good as outcomes achieved with open fetal surgery.
We’re in a much different position than we were 50 years ago in that we have two patients – the mother and the fetus – with whom we can closely work. We also have a relatively new and urgent obligation to place our attention not only on women’s reproductive health, but on the general gynecologic state. Ob.gyns. often are the only primary care physicians whom women see for routine care, and the quality of our attention to their weight and their diabetes risk factors will have far-reaching consequences, both for them and for their offspring.
As we have since the 1960s, we will continue to set new moonshots and meet new challenges, working with each other and with our patients to evaluate where we are strong and where we must improve. We will persistently harness the power of technology, choosing to do the things that “are hard,” while stepping back as needed to ask and address fundamental questions.
As a result, I can envision the next 50 years as a revolutionary time period for obstetrics – a time in which current problems and disorders are abated or eliminated through a combination of genomics, microbiomics, and other technological advances. Someday in the future, we will look back on some of our many achievements and marvel at how we have transformed the unimaginable to reality.
Dr. Reece, who specializes in maternal-fetal medicine, is vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. Dr. Reece said he had no relevant financial disclosures. He is the medical editor of this column. Contact him at obnews@frontlinemedcom.com.
Select advances through the years
1960s
1965: Siemens Corp. introduces first real-time ultrasound scanner.
1966: Lancet paper reports that amniotic fluid cells can be cultured and karyotyped.
1970s
1970: New England Journal of Medicine paper describes mid-trimester amniocenteses and detection of Down syndrome cases.
1972: Ultrasound-guided amniocentesis first described.
1973: Fetoscopy introduced.
1980s
1981: First human open fetal surgery to correct congenital hydronephrosis.
Early 1980s: Chorionic villus sampling introduced at select centers.
1985: Color Doppler incorporated into ultrasound.
1990s
1990: Embryoscopy first described.
Mid-1990s: 3D/4D ultrasound begins to assume major role in ob.gyn. imaging.1997: Discovery of cell-free fetal DNA in maternal plasma.
2000s
2003: MOMS (Management of Myelomeningocele Study) was launched.
2010s
2012: The American College of Obstetricians and Gynecologists and the Society for Maternal-Fetal Medicine support cell-free DNA screening for women at increased risk of fetal aneuploidy.
2013: Preterm birth rate drops to 11.4%
2014: Diabetes incidence marks a 4-fold increase since 1980.
In 1961 before Congress, and in 1962 at Rice University, Houston, President John F. Kennedy called on America to land a man on the moon and bring him back safely, and to look beyond the moon as well, and pursue an ambitious space exploration program. He challenged the country to think and act boldly, telling Americans in his speech at Rice that “we choose to go the moon in this decade and do the other things, not because they are easy, but because they are hard.”
When Neil Armstrong and Buzz Aldrin set foot on the moon in 1969 – even before President Kennedy’s 10-year deadline had arrived – the country’s primary moonshot was realized. The President had inspired the nation, teams of engineers and others had collectively met daunting technological challenges, and space consequently was more open to us than ever before.
In looking at the field of obstetrics and how far it has come in the past 50 years, since the 1960s, it is similarly astonishing and inspiring to reflect on what extraordinary advances we have made. Who would have thought that the fetus would become such a visible and intimate patient – one who, like the mother, can be interrogated, monitored, and sometimes treated before birth? Who would have thought we would be utilizing genomic studies in a now well-established field of prenatal diagnosis, or that fetal therapy would become a field in and of itself?
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Our specialty has advanced through a series of moonshots that have been inspired and driven by technological advancement and by our continually bold goals and vision for the health and well-being of women and their offspring. We have taken on ambitious challenges, achieved many goals, and embraced advancements in practice only to then set new targets that previously were unimaginable.
Yet just as our country’s space exploration program has faced disappointments, so has our field. It is sobering, for instance, that we have made only incremental improvements in prematurity and infant mortality, and that the age-old maternal problem of preeclampsia is still with us. We also face new challenges, such as the rising rate of maternal obesity and diabetes, which threaten both maternal and fetal health.
President Kennedy spoke of having “examined where we are strong, and where we are not.” Such self-reflection and assessment is a critical underpinning of advancement in fields across all of science, medicine, and health care, and in our specialty, it is a process that has driven ambitious new research efforts to improve fetal and maternal health.
A step back to more in-depth fundamental research on the biomolecular mechanisms of premature labor and diabetes-associated birth defects, for instance, as well as new efforts to approach fetal surgery less invasively, are positioning us to both conquer our disappointments and achieve ambitious new moonshots.
The fetus as our patient
Fifty years ago, in 1966, a seminal paper in the Lancet reported that amniotic fluid cells could be cultured and were suitable for karyotyping (1[7434]:383-5). The tapping and examination of amniotic fluid had been reported on sporadically for many decades, for various clinical purposes, but by and large the fetal compartment was not invaded or directly examined. The fetus was instead the hopeful beneficiary of pregnancy care that focused on the mother. Fetal outcome was clouded in mystery, known only at birth.
With the Lancet report, prenatal detection of chromosomal disorders began to feel achievable, and the 1960s marked the beginning of a journey first through invasive methods of prenatal diagnosis and then through increasingly non-invasive approaches.
In 1970, just several years after the report on chromosome analysis of amniotic-fluid cells, another landmark paper in the New England Journal of Medicine described 162 amniocenteses performed between the 13th and 18th weeks of gestation and the detection of 10 cases of Down syndrome, as well as a few other cases of metabolic and other disorders (282[11]:596-9). This report provided an impetus for broader use of the procedure to detect neural tube defects, Down syndrome, and other abnormalities.
The adoption of amniocentesis for prenatal diagnosis still took some time, however. The procedure was used primarily early on to determine fetal lung maturity, and to predict the ability of the fetus to survive after delivery.
At the time, it was widely praised as an advanced method for evaluating the fetus. Yet, looking back, the early years of the procedure seem primitive. The procedure was done late in pregnancy and it was performed blindly, with the puncture site located either with external palpation of the uterus or with the assistance of static ultrasound. Patients who had scans would usually visit the radiologist, who would mark on the patient’s abdomen a suggested location for needle insertion. Upon the patient’s return, the obstetrician would then insert a needle into that spot, blindly and likely after the fetus had moved.
The development and adoption of real-time ultrasound was a revolutionary achievement. Ultrasound-guided amniocentesis was first described in 1972, 14 years after Ian Donald’s seminal paper introducing obstetric ultrasound was published in the Lancet (1958 Jun 7;1[7032]:1188-95).
As real-time ultrasound made its way into practice, it marked the true realization of a moonshot for obstetrics.
Not only could we simultaneously visualize the needle tip and place the needle safety, but we could see the real-time movement of the fetus, its activity, and the surrounding pockets of fluid. It was like looking up into the sky and seeing the stars for the first time. We could see fetal arrhythmia – not only hear it. With this window into the fetal compartment, we could visualize the fetal bowel migrating into the chest cavity due to a hole (hernia) in the diaphragm. We could visualize other malformations as well.
Chorionic villus sampling (CVS) was technically more difficult and took longer to evolve. For years, through the early 1980s, it was performed only at select centers throughout the country. Patients traveled for the procedure and faced relatively significant risks of complications.
By the end of the 1980s, however, with successive improvements in equipment and technique (including development of a transabdominal approach in addition to transvaginal) the procedure was deemed safe, effective, and acceptable for routine use. Fetoscopy, pioneered by John Hobbins, MD, and his colleagues at Yale University, New Haven, Conn., had also advanced and was being used to diagnose sickle cell anemia, Tay-Sachs disease, congenital fetal skin diseases, and other disorders.
With these advances and with our newfound ability to obtain and analyze a tissue sample earlier in pregnancy – even before a woman shared the news of her pregnancy, in some cases – it seemed that we had achieved our goals and may have even reached past the moon.
Yet there were other moonshots being pursued, including initiatives to make prenatal diagnosis less invasive. The discovery in 1997 of cell-free fetal DNA in maternal plasma and serum, for instance, was a pivotal development that opened the door for noninvasive prenatal testing.
This, and other advances in areas from biochemistry to ultrasound to genomic analysis, led to an array of prenatal diagnostic tools that today enable women and their physicians to assess the genetic, chromosomal, and biophysical aspects of their fetus considerably before the time of viability, and from both the maternal side and directly in the fetal compartment.
First-trimester screening is a current option, and we now have the ability to more selectively perform amniocentesis and CVS based on probability testing, and not solely on maternal age. Ultrasound technology now encompasses color Doppler, 3D and 4D imaging, and other techniques that can be used to assess the placenta, various structures inside the brain, and the heart, as well as blood flow through the ductus venosus.
Parents have called for and welcomed having the option of assessing the fetus in greater detail, and of having either assurance when anomalies are excluded or the opportunity to plan and make decisions when anomalies are detected.
Fetal surgery has been a natural extension of our unprecedented access to the fetus. Our ability to visualize malformations and their evolution led to animal studies that advanced our interest in arresting, correcting, or reversing fetal anomalies through in-utero interventions. In 1981, surgeons performed the first human open fetal surgery to correct congenital hydronephrosis.
Today, we can employ endoscopic laser ablation or laser coagulation to treat severe twin-to-twin syndrome, for instance, as well as other surgical techniques to repair defects such as congenital diaphragmatic hernia, lower urinary tract obstruction, and myelomeningocele. Such advances were unimaginable decades ago.
Old foes and new threats
Despite these advances in diagnosis and care, obstetrics faces unrealized moonshots – lingering challenges that, 50 years ago, we would have predicted would have been solved. Who would have thought that we would still have as high an infant mortality rate as we do, and that we would not be further along in solving the problem of prematurity? Our progress has been only incremental.
Fifty years ago, we lacked an understanding of the basic biology of preterm labor. Prematurity was viewed simply as term labor occurring too early, and many efforts were made over the years to halt the premature labor process through the use of various drugs and other therapeutics, with variable and minimally impactful levels of success.
In the last 25 years, and especially in the last decade, we have made greater efforts to better understand the biology of premature labor – to elucidate how and why it occurs – and we have come to understand that premature labor is very different physiologically from term labor.
Thanks to the work at the Perinatology Research Branch of the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), led by Roberto Romero, MD, attention has consequently shifted toward prediction, identification of women at highest risk, and prevention of the onset of premature labor among those deemed to be at highest risk.
Cervical length in the mid-trimester is now a well-verified predictor of preterm birth, and vaginal progesterone has been shown to benefit women without other known risk factors who are diagnosed with a shortened cervical length.
We have consequently seen the preterm birth rate decline a bit. In 2013, the last year for which we have complete data, the preterm birth rate dropped to 11.4%, down from a high of 12.8% in 2006, according to the Centers for Disease Control and Prevention.
Infant mortality similarly remains unacceptably high, due largely to the high preterm birth rate and to our failure to significantly alter the prevalence of birth defects. In 2010, according to the CDC, the infant mortality rate in the U.S. was 6.1 deaths per 1,000 live births (compared with 6.87 in 2005), and the United States ranked 26th in infant mortality among countries belonging to the Organisation for Economic Co-operation and Development, despite the fact that we spend a significant portion of our gross domestic product (17.5% in 2014) on health care.
Birth defects have taken over as a leading cause of infant mortality after early newborn life, and while we’ve made some advancements in understanding and diagnosing them, the majority of causes of birth defects are still unknown.
On the maternal side of obstetrical care, our progress has similarly been more modest than we have hoped for. Preeclampsia remains a problem, for instance. Despite decades of research into its pathogenesis, our advancements have been only incremental, and the condition – particularly its severe form – continues to be a vexing and high-risk problem.
Added to such age-old foes, moreover, are the growing threats of maternal obesity and diabetes, two closely related and often chronic conditions that affect not only the health of the mother but the in-utero environment and the health of the fetus. Today, more than one-third of all adults in the U.S., and 34% of women aged 20-39 years, are obese, and almost 10% of the U.S. population has diabetes.
Both conditions are on the rise, and obstetrics is confronting an epidemic of “diabesity” that would not necessarily have been predicted 50 years ago. It is particularly alarming given our growing knowledge of how obesity can be programmed in-utero and essentially passed on from generation to generation, of how diabetes can negatively affect perinatal outcomes, and of how the two conditions can have an additive effect on fetal complications.
Achieving new moonshots
Concerted efforts in the past several decades to step back and try to understand the basic biology and physiology of term labor and of premature labor have better positioned our specialty to achieve the moonshot of significantly reducing the incidence of preterm birth.
Establishment in the mid-1980s of the NICHD’s Perinatology Research Branch was a major development in this regard, helping to build and direct research efforts, including basic laboratory science, toward questions about what triggers and propagates labor. There has been notable progress in the past decade, in particular, and our specialty is now on the right path toward development of therapeutic interventions for preventing prematurity.
Additionally, the NICHD’s recently launched Human Placenta Project is building upon the branch-sponsored animal and cell culture model systems of the placenta to allow researchers, for the first time, to monitor human placental health in real time. By more fully understanding the role of the placenta in health and disease, we will be able to better evaluate pregnancy risks and improve pregnancy outcomes.
We also are learning through research in the University of Maryland Birth Defects Research Laboratory, which I am privileged to direct, and at other facilities, that maternal hyperglycemia is a teratogen, creating insults that can trigger a series of developmental fetal defects. By studying the biomolecular mechanisms of hyperglycemia-induced birth defects and developing “molecular maps,” we expect to be able to develop strategies for preventing or mitigating the development of such anomalies. I hope and expect that these future advancements, combined with reductions in prematurity, will significantly impact the infant mortality rate.
Fetal therapy and surgery will also continue to advance, with a much more minimally invasive approach taken in the next 50 years to addressing the fetal condition without putting the mother at increased risk. Just as surgery in other fields has moved from open laparotomy to minimally invasive techniques, I believe we will develop endoscopic or laparoscopic means of correcting the various problems in-utero, such as the repair of neural tube defects and diaphragmatic hernias. It already appears likely that a fetoscopic approach to treating myelomeningocele can reduce maternal morbidity while achieving infant neurological outcomes that are at least as good as outcomes achieved with open fetal surgery.
We’re in a much different position than we were 50 years ago in that we have two patients – the mother and the fetus – with whom we can closely work. We also have a relatively new and urgent obligation to place our attention not only on women’s reproductive health, but on the general gynecologic state. Ob.gyns. often are the only primary care physicians whom women see for routine care, and the quality of our attention to their weight and their diabetes risk factors will have far-reaching consequences, both for them and for their offspring.
As we have since the 1960s, we will continue to set new moonshots and meet new challenges, working with each other and with our patients to evaluate where we are strong and where we must improve. We will persistently harness the power of technology, choosing to do the things that “are hard,” while stepping back as needed to ask and address fundamental questions.
As a result, I can envision the next 50 years as a revolutionary time period for obstetrics – a time in which current problems and disorders are abated or eliminated through a combination of genomics, microbiomics, and other technological advances. Someday in the future, we will look back on some of our many achievements and marvel at how we have transformed the unimaginable to reality.
Dr. Reece, who specializes in maternal-fetal medicine, is vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. Dr. Reece said he had no relevant financial disclosures. He is the medical editor of this column. Contact him at obnews@frontlinemedcom.com.
Select advances through the years
1960s
1965: Siemens Corp. introduces first real-time ultrasound scanner.
1966: Lancet paper reports that amniotic fluid cells can be cultured and karyotyped.
1970s
1970: New England Journal of Medicine paper describes mid-trimester amniocenteses and detection of Down syndrome cases.
1972: Ultrasound-guided amniocentesis first described.
1973: Fetoscopy introduced.
1980s
1981: First human open fetal surgery to correct congenital hydronephrosis.
Early 1980s: Chorionic villus sampling introduced at select centers.
1985: Color Doppler incorporated into ultrasound.
1990s
1990: Embryoscopy first described.
Mid-1990s: 3D/4D ultrasound begins to assume major role in ob.gyn. imaging.1997: Discovery of cell-free fetal DNA in maternal plasma.
2000s
2003: MOMS (Management of Myelomeningocele Study) was launched.
2010s
2012: The American College of Obstetricians and Gynecologists and the Society for Maternal-Fetal Medicine support cell-free DNA screening for women at increased risk of fetal aneuploidy.
2013: Preterm birth rate drops to 11.4%
2014: Diabetes incidence marks a 4-fold increase since 1980.
In 1961 before Congress, and in 1962 at Rice University, Houston, President John F. Kennedy called on America to land a man on the moon and bring him back safely, and to look beyond the moon as well, and pursue an ambitious space exploration program. He challenged the country to think and act boldly, telling Americans in his speech at Rice that “we choose to go the moon in this decade and do the other things, not because they are easy, but because they are hard.”
When Neil Armstrong and Buzz Aldrin set foot on the moon in 1969 – even before President Kennedy’s 10-year deadline had arrived – the country’s primary moonshot was realized. The President had inspired the nation, teams of engineers and others had collectively met daunting technological challenges, and space consequently was more open to us than ever before.
In looking at the field of obstetrics and how far it has come in the past 50 years, since the 1960s, it is similarly astonishing and inspiring to reflect on what extraordinary advances we have made. Who would have thought that the fetus would become such a visible and intimate patient – one who, like the mother, can be interrogated, monitored, and sometimes treated before birth? Who would have thought we would be utilizing genomic studies in a now well-established field of prenatal diagnosis, or that fetal therapy would become a field in and of itself?
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Our specialty has advanced through a series of moonshots that have been inspired and driven by technological advancement and by our continually bold goals and vision for the health and well-being of women and their offspring. We have taken on ambitious challenges, achieved many goals, and embraced advancements in practice only to then set new targets that previously were unimaginable.
Yet just as our country’s space exploration program has faced disappointments, so has our field. It is sobering, for instance, that we have made only incremental improvements in prematurity and infant mortality, and that the age-old maternal problem of preeclampsia is still with us. We also face new challenges, such as the rising rate of maternal obesity and diabetes, which threaten both maternal and fetal health.
President Kennedy spoke of having “examined where we are strong, and where we are not.” Such self-reflection and assessment is a critical underpinning of advancement in fields across all of science, medicine, and health care, and in our specialty, it is a process that has driven ambitious new research efforts to improve fetal and maternal health.
A step back to more in-depth fundamental research on the biomolecular mechanisms of premature labor and diabetes-associated birth defects, for instance, as well as new efforts to approach fetal surgery less invasively, are positioning us to both conquer our disappointments and achieve ambitious new moonshots.
The fetus as our patient
Fifty years ago, in 1966, a seminal paper in the Lancet reported that amniotic fluid cells could be cultured and were suitable for karyotyping (1[7434]:383-5). The tapping and examination of amniotic fluid had been reported on sporadically for many decades, for various clinical purposes, but by and large the fetal compartment was not invaded or directly examined. The fetus was instead the hopeful beneficiary of pregnancy care that focused on the mother. Fetal outcome was clouded in mystery, known only at birth.
With the Lancet report, prenatal detection of chromosomal disorders began to feel achievable, and the 1960s marked the beginning of a journey first through invasive methods of prenatal diagnosis and then through increasingly non-invasive approaches.
In 1970, just several years after the report on chromosome analysis of amniotic-fluid cells, another landmark paper in the New England Journal of Medicine described 162 amniocenteses performed between the 13th and 18th weeks of gestation and the detection of 10 cases of Down syndrome, as well as a few other cases of metabolic and other disorders (282[11]:596-9). This report provided an impetus for broader use of the procedure to detect neural tube defects, Down syndrome, and other abnormalities.
The adoption of amniocentesis for prenatal diagnosis still took some time, however. The procedure was used primarily early on to determine fetal lung maturity, and to predict the ability of the fetus to survive after delivery.
At the time, it was widely praised as an advanced method for evaluating the fetus. Yet, looking back, the early years of the procedure seem primitive. The procedure was done late in pregnancy and it was performed blindly, with the puncture site located either with external palpation of the uterus or with the assistance of static ultrasound. Patients who had scans would usually visit the radiologist, who would mark on the patient’s abdomen a suggested location for needle insertion. Upon the patient’s return, the obstetrician would then insert a needle into that spot, blindly and likely after the fetus had moved.
The development and adoption of real-time ultrasound was a revolutionary achievement. Ultrasound-guided amniocentesis was first described in 1972, 14 years after Ian Donald’s seminal paper introducing obstetric ultrasound was published in the Lancet (1958 Jun 7;1[7032]:1188-95).
As real-time ultrasound made its way into practice, it marked the true realization of a moonshot for obstetrics.
Not only could we simultaneously visualize the needle tip and place the needle safety, but we could see the real-time movement of the fetus, its activity, and the surrounding pockets of fluid. It was like looking up into the sky and seeing the stars for the first time. We could see fetal arrhythmia – not only hear it. With this window into the fetal compartment, we could visualize the fetal bowel migrating into the chest cavity due to a hole (hernia) in the diaphragm. We could visualize other malformations as well.
Chorionic villus sampling (CVS) was technically more difficult and took longer to evolve. For years, through the early 1980s, it was performed only at select centers throughout the country. Patients traveled for the procedure and faced relatively significant risks of complications.
By the end of the 1980s, however, with successive improvements in equipment and technique (including development of a transabdominal approach in addition to transvaginal) the procedure was deemed safe, effective, and acceptable for routine use. Fetoscopy, pioneered by John Hobbins, MD, and his colleagues at Yale University, New Haven, Conn., had also advanced and was being used to diagnose sickle cell anemia, Tay-Sachs disease, congenital fetal skin diseases, and other disorders.
With these advances and with our newfound ability to obtain and analyze a tissue sample earlier in pregnancy – even before a woman shared the news of her pregnancy, in some cases – it seemed that we had achieved our goals and may have even reached past the moon.
Yet there were other moonshots being pursued, including initiatives to make prenatal diagnosis less invasive. The discovery in 1997 of cell-free fetal DNA in maternal plasma and serum, for instance, was a pivotal development that opened the door for noninvasive prenatal testing.
This, and other advances in areas from biochemistry to ultrasound to genomic analysis, led to an array of prenatal diagnostic tools that today enable women and their physicians to assess the genetic, chromosomal, and biophysical aspects of their fetus considerably before the time of viability, and from both the maternal side and directly in the fetal compartment.
First-trimester screening is a current option, and we now have the ability to more selectively perform amniocentesis and CVS based on probability testing, and not solely on maternal age. Ultrasound technology now encompasses color Doppler, 3D and 4D imaging, and other techniques that can be used to assess the placenta, various structures inside the brain, and the heart, as well as blood flow through the ductus venosus.
Parents have called for and welcomed having the option of assessing the fetus in greater detail, and of having either assurance when anomalies are excluded or the opportunity to plan and make decisions when anomalies are detected.
Fetal surgery has been a natural extension of our unprecedented access to the fetus. Our ability to visualize malformations and their evolution led to animal studies that advanced our interest in arresting, correcting, or reversing fetal anomalies through in-utero interventions. In 1981, surgeons performed the first human open fetal surgery to correct congenital hydronephrosis.
Today, we can employ endoscopic laser ablation or laser coagulation to treat severe twin-to-twin syndrome, for instance, as well as other surgical techniques to repair defects such as congenital diaphragmatic hernia, lower urinary tract obstruction, and myelomeningocele. Such advances were unimaginable decades ago.
Old foes and new threats
Despite these advances in diagnosis and care, obstetrics faces unrealized moonshots – lingering challenges that, 50 years ago, we would have predicted would have been solved. Who would have thought that we would still have as high an infant mortality rate as we do, and that we would not be further along in solving the problem of prematurity? Our progress has been only incremental.
Fifty years ago, we lacked an understanding of the basic biology of preterm labor. Prematurity was viewed simply as term labor occurring too early, and many efforts were made over the years to halt the premature labor process through the use of various drugs and other therapeutics, with variable and minimally impactful levels of success.
In the last 25 years, and especially in the last decade, we have made greater efforts to better understand the biology of premature labor – to elucidate how and why it occurs – and we have come to understand that premature labor is very different physiologically from term labor.
Thanks to the work at the Perinatology Research Branch of the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), led by Roberto Romero, MD, attention has consequently shifted toward prediction, identification of women at highest risk, and prevention of the onset of premature labor among those deemed to be at highest risk.
Cervical length in the mid-trimester is now a well-verified predictor of preterm birth, and vaginal progesterone has been shown to benefit women without other known risk factors who are diagnosed with a shortened cervical length.
We have consequently seen the preterm birth rate decline a bit. In 2013, the last year for which we have complete data, the preterm birth rate dropped to 11.4%, down from a high of 12.8% in 2006, according to the Centers for Disease Control and Prevention.
Infant mortality similarly remains unacceptably high, due largely to the high preterm birth rate and to our failure to significantly alter the prevalence of birth defects. In 2010, according to the CDC, the infant mortality rate in the U.S. was 6.1 deaths per 1,000 live births (compared with 6.87 in 2005), and the United States ranked 26th in infant mortality among countries belonging to the Organisation for Economic Co-operation and Development, despite the fact that we spend a significant portion of our gross domestic product (17.5% in 2014) on health care.
Birth defects have taken over as a leading cause of infant mortality after early newborn life, and while we’ve made some advancements in understanding and diagnosing them, the majority of causes of birth defects are still unknown.
On the maternal side of obstetrical care, our progress has similarly been more modest than we have hoped for. Preeclampsia remains a problem, for instance. Despite decades of research into its pathogenesis, our advancements have been only incremental, and the condition – particularly its severe form – continues to be a vexing and high-risk problem.
Added to such age-old foes, moreover, are the growing threats of maternal obesity and diabetes, two closely related and often chronic conditions that affect not only the health of the mother but the in-utero environment and the health of the fetus. Today, more than one-third of all adults in the U.S., and 34% of women aged 20-39 years, are obese, and almost 10% of the U.S. population has diabetes.
Both conditions are on the rise, and obstetrics is confronting an epidemic of “diabesity” that would not necessarily have been predicted 50 years ago. It is particularly alarming given our growing knowledge of how obesity can be programmed in-utero and essentially passed on from generation to generation, of how diabetes can negatively affect perinatal outcomes, and of how the two conditions can have an additive effect on fetal complications.
Achieving new moonshots
Concerted efforts in the past several decades to step back and try to understand the basic biology and physiology of term labor and of premature labor have better positioned our specialty to achieve the moonshot of significantly reducing the incidence of preterm birth.
Establishment in the mid-1980s of the NICHD’s Perinatology Research Branch was a major development in this regard, helping to build and direct research efforts, including basic laboratory science, toward questions about what triggers and propagates labor. There has been notable progress in the past decade, in particular, and our specialty is now on the right path toward development of therapeutic interventions for preventing prematurity.
Additionally, the NICHD’s recently launched Human Placenta Project is building upon the branch-sponsored animal and cell culture model systems of the placenta to allow researchers, for the first time, to monitor human placental health in real time. By more fully understanding the role of the placenta in health and disease, we will be able to better evaluate pregnancy risks and improve pregnancy outcomes.
We also are learning through research in the University of Maryland Birth Defects Research Laboratory, which I am privileged to direct, and at other facilities, that maternal hyperglycemia is a teratogen, creating insults that can trigger a series of developmental fetal defects. By studying the biomolecular mechanisms of hyperglycemia-induced birth defects and developing “molecular maps,” we expect to be able to develop strategies for preventing or mitigating the development of such anomalies. I hope and expect that these future advancements, combined with reductions in prematurity, will significantly impact the infant mortality rate.
Fetal therapy and surgery will also continue to advance, with a much more minimally invasive approach taken in the next 50 years to addressing the fetal condition without putting the mother at increased risk. Just as surgery in other fields has moved from open laparotomy to minimally invasive techniques, I believe we will develop endoscopic or laparoscopic means of correcting the various problems in-utero, such as the repair of neural tube defects and diaphragmatic hernias. It already appears likely that a fetoscopic approach to treating myelomeningocele can reduce maternal morbidity while achieving infant neurological outcomes that are at least as good as outcomes achieved with open fetal surgery.
We’re in a much different position than we were 50 years ago in that we have two patients – the mother and the fetus – with whom we can closely work. We also have a relatively new and urgent obligation to place our attention not only on women’s reproductive health, but on the general gynecologic state. Ob.gyns. often are the only primary care physicians whom women see for routine care, and the quality of our attention to their weight and their diabetes risk factors will have far-reaching consequences, both for them and for their offspring.
As we have since the 1960s, we will continue to set new moonshots and meet new challenges, working with each other and with our patients to evaluate where we are strong and where we must improve. We will persistently harness the power of technology, choosing to do the things that “are hard,” while stepping back as needed to ask and address fundamental questions.
As a result, I can envision the next 50 years as a revolutionary time period for obstetrics – a time in which current problems and disorders are abated or eliminated through a combination of genomics, microbiomics, and other technological advances. Someday in the future, we will look back on some of our many achievements and marvel at how we have transformed the unimaginable to reality.
Dr. Reece, who specializes in maternal-fetal medicine, is vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. Dr. Reece said he had no relevant financial disclosures. He is the medical editor of this column. Contact him at obnews@frontlinemedcom.com.
Select advances through the years
1960s
1965: Siemens Corp. introduces first real-time ultrasound scanner.
1966: Lancet paper reports that amniotic fluid cells can be cultured and karyotyped.
1970s
1970: New England Journal of Medicine paper describes mid-trimester amniocenteses and detection of Down syndrome cases.
1972: Ultrasound-guided amniocentesis first described.
1973: Fetoscopy introduced.
1980s
1981: First human open fetal surgery to correct congenital hydronephrosis.
Early 1980s: Chorionic villus sampling introduced at select centers.
1985: Color Doppler incorporated into ultrasound.
1990s
1990: Embryoscopy first described.
Mid-1990s: 3D/4D ultrasound begins to assume major role in ob.gyn. imaging.1997: Discovery of cell-free fetal DNA in maternal plasma.
2000s
2003: MOMS (Management of Myelomeningocele Study) was launched.
2010s
2012: The American College of Obstetricians and Gynecologists and the Society for Maternal-Fetal Medicine support cell-free DNA screening for women at increased risk of fetal aneuploidy.
2013: Preterm birth rate drops to 11.4%
2014: Diabetes incidence marks a 4-fold increase since 1980.
Zika virus: The path to fetal infection
The question of how viruses can enter the intrauterine compartment and infect the fetus has long been a focus of research. It is of particular urgency today as the Zika virus spreads and causes perinatal infection that threatens the developing fetus with serious adverse outcomes such microcephaly and other brain anomalies, placental insufficiency, and fetal growth restriction.
We know that viruses can take a variety of routes to the fetal compartment, but we have also learned that the placenta has a robust level of inherent resistance to viruses. This resistance likely explains why we don’t see more viral infections in pregnancy.
Recent studies performed at our institution suggest that placental trophoblasts – the placenta’s primary line of defense – have inherent resistance to viruses such as Zika. It appears, therefore, that the Zika virus invades the intrauterine cavity by crossing the trophoblasts, perhaps earlier in pregnancy and prior to the development of full trophoblast resistance, by entering through breaks in this outer layer, or by utilizing alternative pathways to access the fetal compartment.
Further study of the placenta and its various cell types and mechanisms of viral defense will be critical for designing therapeutic strategies for preventing perinatal infections.
Various routes and affinities
Viruses have long been known to affect mothers and their unborn children. The rubella virus, for instance, posed a significant threat to the fetus until a vaccine program was introduced almost 50 years ago. Cytomegalovirus (CMV), on the other hand, continues be passed from mothers to their unborn children. While not as threatening as rubella once was, it can in some cases cause severe defects.
One might expect viruses to infect the placenta and then secondarily infect the fetus. While this may indeed occur, direct placental infection is not the only route by which viruses may enter the intrauterine compartment. Some viruses may be carried by macrophages or other immune cells through the placenta and into the fetal compartment, while others colonize the uterine cavity prior to conception, ready to proliferate during pregnancy.
In still other cases, viruses may be inadvertently introduced during medical procedures such as amniocentesis or transmitted through transvaginal ascending infection, most likely after rupture of the membranes. Viruses may also be transported through infected sperm (this appears to be one of the Zika virus’s modes of transportation), and as is the case with HIV and herpes simplex viruses, transmission sometimes occurs during delivery.
When we investigate whether or not the fetus is protected against particular viruses, we must therefore think about the multifaceted mechanisms by which viruses may be transmitted. With respect to the placenta specifically, we seek to understand how viruses enter the placenta, and how the placenta resists the propagation of some viruses while allowing other viruses to gain entry to the intrauterine compartment.
An additional consideration – one that is of utmost importance in the case of Zika – is whether viruses have any special affinity for particular fetal tissues. Some viruses, like CMV, infect multiple types of fetal tissue. The Zika virus, on the other hand, appears to target neuronal tissue in the fetus. In May, investigators of two studies reported that a strain of the Zika virus efficiently infected human cortical neural progenitor cells (Cell Stem Cell. 2016 May 5;18[5]:587-90), and that Zika infection of mice early in pregnancy resulted in infection of the placenta and of the fetal brain (Cell. 2016 May 19;165[5]:1081-91).
Interestingly, other flaviviruses such as the dengue and chikungunya viruses have not been associated with microcephaly or other congenital disorders. This suggests that the Zika virus employs unique mechanisms to infect or bypass the placental barrier and, in turn, to cause neuronal-focused damage.
Placental passage
The villous trophoblasts, cells that are bathed in maternal blood, form the placenta’s first line of defense. Viruses, including the Zika virus, must cross or somehow bypass this initial barrier before crossing the placental basement membrane and endothelial cells, if they are to potentially invade the intrauterine cavity and infect the fetal brain and other tissues.
Research has demonstrated that cells of various types of tissue may express certain proteins, such as AXL, MER and TYRO3. While not yet proven, these proteins may mediate the entry of viruses such as Zika, enabling them to cross the placental trophoblast layer. These proteins are indeed expressed in trophoblasts, especially in early pregnancy, but we do not yet know if the proteins actually aid Zika’s passage through the placenta.
Another mechanism that has been postulated in the case of Zika infection is antibody-dependent enhancement, a process by which a current infection is enhanced by prior infection with another virus from the same family. Some experts believe that pre-existing immunity to the dengue virus – another member of the flavivirus family that has been endemic in Brazil – may be enhancing the spread of Zika infection as antibodies against dengue cross-react with the Zika virus.
While antibody-dependent enhancement has been shown to occur and to advance infection in various body systems, it has not been proven to affect the placenta. Until we learn more, we must simply appreciate that the presence of antibodies from another member of a family of viruses does not necessarily confer resistance. Instead, it may enable new infections to advance.
One might view pregnancy as a time of immune compromise, but we have shown in our laboratories that trophoblasts in fact have inherent resistance to a number of viruses. In a recent study, we found that trophoblasts are refractory to direct infection with the Zika virus. We isolated trophoblast cells from healthy full-term human placentas, cultured these cells for several days, and infected them with the Zika virus. We then measured viral replication and compared the infectivity of these cells with the infectivity of human brain microvascular endothelial cells – nontrophoblast cells that served as a control.
Our findings were extremely interesting to us: The trophoblast cells appeared to be significantly more resistant to the Zika virus than the nontrophoblast cells.
We learned, moreover, that this resistance was mediated by a particular interferon released by the trophoblast cells – type III interferon IFN1 – and that this type III interferon appeared to protect not only the trophoblasts but the nontrophoblast cells as well. It acted in both an autocrine and a paracrine manner to protect cells from the Zika virus. When we blocked the antiviral signaling of this interferon, resistance to the virus was attenuated.
These findings suggest that while Zika appears able to cross through the placenta and infect the fetus, the mechanism does not involve direct infection of the trophoblasts, at least in the later stages of pregnancy. The virus must either evade the type III interferon antiviral signals generated by the trophoblasts or somehow bypass these cells to cross the placenta (Cell Host Microbe. 2016 May 11;19[5]:705-12).
Interestingly, the Cell study mentioned above, in which Zika infection of mice early in pregnancy infected placental cells and the brain, also showed reduced Zika presence in the mouse mononuclear trophoblasts and syncytiotrophoblasts, in areas of the placenta analogous to the human villi.
Some experts have suggested, based the study of other viruses, that the Zika virus is better able to infect the placenta when the infection occurs early in the first trimester or the second trimester. It is indeed possible – and makes intuitive sense – that first-trimester trophoblasts confer less resistance and a lower level of protection than the mature trophoblasts we studied. At this point, however, we cannot say with certainty whether or not the placenta is more or less permissive to Zika infection at different points in pregnancy.
Interestingly, investigators who prospectively followed a small cohort of pregnant women in Brazil with suspected Zika infection identified abnormalities in fetuses of women who were infected at various points of their pregnancies, even in the third trimester. Fetuses infected in the first trimester had findings suggestive of pathologic change during embryogenesis, but central nervous system abnormalities were seen in fetuses infected as late as 27 weeks of gestation, the investigators said (N Engl J Med. 2016 Mar 4. doi: 10.1056/NEJMoa1602412).
The interferon-conferred resistance demonstrated in our recent study is one of two mechanisms we’ve identified by which placental trophoblasts orchestrate resistance to viral infection. In earlier research, we found that resistance can be conferred to nontrophoblast cells by the delivery of micro-RNAs. These micro-RNAs (C19MC miRNAs) are uniquely expressed in the placenta and packaged within trophoblast-derived nanovesicles called exosomes. The nanovesicles can latch onto other cells in the vicinity of the trophoblasts, attenuating viral replication in these recipient cells.
This earlier in-vitro study involved a panel of diverse and unrelated viruses, including coxsackievirus B3, poliovirus, vesicular stomatitis virus, and human cytomegalovirus (Proc Natl Acad Sci U S A. 2013 Jul 16;110[29]:12048-53). It did not include the Zika virus, but our ongoing preliminary research suggests that the same mechanisms might be active against Zika.
Furthering research
Research at our institution and in other laboratories has shed light on various ways in which the fetus is protected from viruses, but we must learn more in order to understand how particular viruses, such as Zika, are able to reach the fetal compartment and cause particular birth defects.
We must further investigate the role and importance of antibody-dependent enhancement, and we must continue to study the placenta and its various cell types. Continuing efforts to better elucidate the placenta’s defense mechanisms and to identify cell types that are more or less resistant to the Zika virus – and understand their differences – may lead us to potential therapeutic strategies.
Dr. Sadovsky is scientific director of the Magee-Womens Research Institute, Elsie Hilliard Hillman Chair of Women’s Health Research, and professor of ob.gyn., reproductive sciences, microbiology, and molecular genetics at the University of Pittsburgh. Dr. Coyne is associate professor of microbiology and molecular genetics, and ob.gyn. and reproductive sciences, at the University of Pittsburgh.* Their research addressed in this Master Class was supported by grants from the National Institutes of Health, State of Pennsylvania Formula Research Funds, and Burroughs Wellcome Fund.
*Correction, 7/05/2016: An earlier version of this article misstated Dr. Coyne's academic title.
The question of how viruses can enter the intrauterine compartment and infect the fetus has long been a focus of research. It is of particular urgency today as the Zika virus spreads and causes perinatal infection that threatens the developing fetus with serious adverse outcomes such microcephaly and other brain anomalies, placental insufficiency, and fetal growth restriction.
We know that viruses can take a variety of routes to the fetal compartment, but we have also learned that the placenta has a robust level of inherent resistance to viruses. This resistance likely explains why we don’t see more viral infections in pregnancy.
Recent studies performed at our institution suggest that placental trophoblasts – the placenta’s primary line of defense – have inherent resistance to viruses such as Zika. It appears, therefore, that the Zika virus invades the intrauterine cavity by crossing the trophoblasts, perhaps earlier in pregnancy and prior to the development of full trophoblast resistance, by entering through breaks in this outer layer, or by utilizing alternative pathways to access the fetal compartment.
Further study of the placenta and its various cell types and mechanisms of viral defense will be critical for designing therapeutic strategies for preventing perinatal infections.
Various routes and affinities
Viruses have long been known to affect mothers and their unborn children. The rubella virus, for instance, posed a significant threat to the fetus until a vaccine program was introduced almost 50 years ago. Cytomegalovirus (CMV), on the other hand, continues be passed from mothers to their unborn children. While not as threatening as rubella once was, it can in some cases cause severe defects.
One might expect viruses to infect the placenta and then secondarily infect the fetus. While this may indeed occur, direct placental infection is not the only route by which viruses may enter the intrauterine compartment. Some viruses may be carried by macrophages or other immune cells through the placenta and into the fetal compartment, while others colonize the uterine cavity prior to conception, ready to proliferate during pregnancy.
In still other cases, viruses may be inadvertently introduced during medical procedures such as amniocentesis or transmitted through transvaginal ascending infection, most likely after rupture of the membranes. Viruses may also be transported through infected sperm (this appears to be one of the Zika virus’s modes of transportation), and as is the case with HIV and herpes simplex viruses, transmission sometimes occurs during delivery.
When we investigate whether or not the fetus is protected against particular viruses, we must therefore think about the multifaceted mechanisms by which viruses may be transmitted. With respect to the placenta specifically, we seek to understand how viruses enter the placenta, and how the placenta resists the propagation of some viruses while allowing other viruses to gain entry to the intrauterine compartment.
An additional consideration – one that is of utmost importance in the case of Zika – is whether viruses have any special affinity for particular fetal tissues. Some viruses, like CMV, infect multiple types of fetal tissue. The Zika virus, on the other hand, appears to target neuronal tissue in the fetus. In May, investigators of two studies reported that a strain of the Zika virus efficiently infected human cortical neural progenitor cells (Cell Stem Cell. 2016 May 5;18[5]:587-90), and that Zika infection of mice early in pregnancy resulted in infection of the placenta and of the fetal brain (Cell. 2016 May 19;165[5]:1081-91).
Interestingly, other flaviviruses such as the dengue and chikungunya viruses have not been associated with microcephaly or other congenital disorders. This suggests that the Zika virus employs unique mechanisms to infect or bypass the placental barrier and, in turn, to cause neuronal-focused damage.
Placental passage
The villous trophoblasts, cells that are bathed in maternal blood, form the placenta’s first line of defense. Viruses, including the Zika virus, must cross or somehow bypass this initial barrier before crossing the placental basement membrane and endothelial cells, if they are to potentially invade the intrauterine cavity and infect the fetal brain and other tissues.
Research has demonstrated that cells of various types of tissue may express certain proteins, such as AXL, MER and TYRO3. While not yet proven, these proteins may mediate the entry of viruses such as Zika, enabling them to cross the placental trophoblast layer. These proteins are indeed expressed in trophoblasts, especially in early pregnancy, but we do not yet know if the proteins actually aid Zika’s passage through the placenta.
Another mechanism that has been postulated in the case of Zika infection is antibody-dependent enhancement, a process by which a current infection is enhanced by prior infection with another virus from the same family. Some experts believe that pre-existing immunity to the dengue virus – another member of the flavivirus family that has been endemic in Brazil – may be enhancing the spread of Zika infection as antibodies against dengue cross-react with the Zika virus.
While antibody-dependent enhancement has been shown to occur and to advance infection in various body systems, it has not been proven to affect the placenta. Until we learn more, we must simply appreciate that the presence of antibodies from another member of a family of viruses does not necessarily confer resistance. Instead, it may enable new infections to advance.
One might view pregnancy as a time of immune compromise, but we have shown in our laboratories that trophoblasts in fact have inherent resistance to a number of viruses. In a recent study, we found that trophoblasts are refractory to direct infection with the Zika virus. We isolated trophoblast cells from healthy full-term human placentas, cultured these cells for several days, and infected them with the Zika virus. We then measured viral replication and compared the infectivity of these cells with the infectivity of human brain microvascular endothelial cells – nontrophoblast cells that served as a control.
Our findings were extremely interesting to us: The trophoblast cells appeared to be significantly more resistant to the Zika virus than the nontrophoblast cells.
We learned, moreover, that this resistance was mediated by a particular interferon released by the trophoblast cells – type III interferon IFN1 – and that this type III interferon appeared to protect not only the trophoblasts but the nontrophoblast cells as well. It acted in both an autocrine and a paracrine manner to protect cells from the Zika virus. When we blocked the antiviral signaling of this interferon, resistance to the virus was attenuated.
These findings suggest that while Zika appears able to cross through the placenta and infect the fetus, the mechanism does not involve direct infection of the trophoblasts, at least in the later stages of pregnancy. The virus must either evade the type III interferon antiviral signals generated by the trophoblasts or somehow bypass these cells to cross the placenta (Cell Host Microbe. 2016 May 11;19[5]:705-12).
Interestingly, the Cell study mentioned above, in which Zika infection of mice early in pregnancy infected placental cells and the brain, also showed reduced Zika presence in the mouse mononuclear trophoblasts and syncytiotrophoblasts, in areas of the placenta analogous to the human villi.
Some experts have suggested, based the study of other viruses, that the Zika virus is better able to infect the placenta when the infection occurs early in the first trimester or the second trimester. It is indeed possible – and makes intuitive sense – that first-trimester trophoblasts confer less resistance and a lower level of protection than the mature trophoblasts we studied. At this point, however, we cannot say with certainty whether or not the placenta is more or less permissive to Zika infection at different points in pregnancy.
Interestingly, investigators who prospectively followed a small cohort of pregnant women in Brazil with suspected Zika infection identified abnormalities in fetuses of women who were infected at various points of their pregnancies, even in the third trimester. Fetuses infected in the first trimester had findings suggestive of pathologic change during embryogenesis, but central nervous system abnormalities were seen in fetuses infected as late as 27 weeks of gestation, the investigators said (N Engl J Med. 2016 Mar 4. doi: 10.1056/NEJMoa1602412).
The interferon-conferred resistance demonstrated in our recent study is one of two mechanisms we’ve identified by which placental trophoblasts orchestrate resistance to viral infection. In earlier research, we found that resistance can be conferred to nontrophoblast cells by the delivery of micro-RNAs. These micro-RNAs (C19MC miRNAs) are uniquely expressed in the placenta and packaged within trophoblast-derived nanovesicles called exosomes. The nanovesicles can latch onto other cells in the vicinity of the trophoblasts, attenuating viral replication in these recipient cells.
This earlier in-vitro study involved a panel of diverse and unrelated viruses, including coxsackievirus B3, poliovirus, vesicular stomatitis virus, and human cytomegalovirus (Proc Natl Acad Sci U S A. 2013 Jul 16;110[29]:12048-53). It did not include the Zika virus, but our ongoing preliminary research suggests that the same mechanisms might be active against Zika.
Furthering research
Research at our institution and in other laboratories has shed light on various ways in which the fetus is protected from viruses, but we must learn more in order to understand how particular viruses, such as Zika, are able to reach the fetal compartment and cause particular birth defects.
We must further investigate the role and importance of antibody-dependent enhancement, and we must continue to study the placenta and its various cell types. Continuing efforts to better elucidate the placenta’s defense mechanisms and to identify cell types that are more or less resistant to the Zika virus – and understand their differences – may lead us to potential therapeutic strategies.
Dr. Sadovsky is scientific director of the Magee-Womens Research Institute, Elsie Hilliard Hillman Chair of Women’s Health Research, and professor of ob.gyn., reproductive sciences, microbiology, and molecular genetics at the University of Pittsburgh. Dr. Coyne is associate professor of microbiology and molecular genetics, and ob.gyn. and reproductive sciences, at the University of Pittsburgh.* Their research addressed in this Master Class was supported by grants from the National Institutes of Health, State of Pennsylvania Formula Research Funds, and Burroughs Wellcome Fund.
*Correction, 7/05/2016: An earlier version of this article misstated Dr. Coyne's academic title.
The question of how viruses can enter the intrauterine compartment and infect the fetus has long been a focus of research. It is of particular urgency today as the Zika virus spreads and causes perinatal infection that threatens the developing fetus with serious adverse outcomes such microcephaly and other brain anomalies, placental insufficiency, and fetal growth restriction.
We know that viruses can take a variety of routes to the fetal compartment, but we have also learned that the placenta has a robust level of inherent resistance to viruses. This resistance likely explains why we don’t see more viral infections in pregnancy.
Recent studies performed at our institution suggest that placental trophoblasts – the placenta’s primary line of defense – have inherent resistance to viruses such as Zika. It appears, therefore, that the Zika virus invades the intrauterine cavity by crossing the trophoblasts, perhaps earlier in pregnancy and prior to the development of full trophoblast resistance, by entering through breaks in this outer layer, or by utilizing alternative pathways to access the fetal compartment.
Further study of the placenta and its various cell types and mechanisms of viral defense will be critical for designing therapeutic strategies for preventing perinatal infections.
Various routes and affinities
Viruses have long been known to affect mothers and their unborn children. The rubella virus, for instance, posed a significant threat to the fetus until a vaccine program was introduced almost 50 years ago. Cytomegalovirus (CMV), on the other hand, continues be passed from mothers to their unborn children. While not as threatening as rubella once was, it can in some cases cause severe defects.
One might expect viruses to infect the placenta and then secondarily infect the fetus. While this may indeed occur, direct placental infection is not the only route by which viruses may enter the intrauterine compartment. Some viruses may be carried by macrophages or other immune cells through the placenta and into the fetal compartment, while others colonize the uterine cavity prior to conception, ready to proliferate during pregnancy.
In still other cases, viruses may be inadvertently introduced during medical procedures such as amniocentesis or transmitted through transvaginal ascending infection, most likely after rupture of the membranes. Viruses may also be transported through infected sperm (this appears to be one of the Zika virus’s modes of transportation), and as is the case with HIV and herpes simplex viruses, transmission sometimes occurs during delivery.
When we investigate whether or not the fetus is protected against particular viruses, we must therefore think about the multifaceted mechanisms by which viruses may be transmitted. With respect to the placenta specifically, we seek to understand how viruses enter the placenta, and how the placenta resists the propagation of some viruses while allowing other viruses to gain entry to the intrauterine compartment.
An additional consideration – one that is of utmost importance in the case of Zika – is whether viruses have any special affinity for particular fetal tissues. Some viruses, like CMV, infect multiple types of fetal tissue. The Zika virus, on the other hand, appears to target neuronal tissue in the fetus. In May, investigators of two studies reported that a strain of the Zika virus efficiently infected human cortical neural progenitor cells (Cell Stem Cell. 2016 May 5;18[5]:587-90), and that Zika infection of mice early in pregnancy resulted in infection of the placenta and of the fetal brain (Cell. 2016 May 19;165[5]:1081-91).
Interestingly, other flaviviruses such as the dengue and chikungunya viruses have not been associated with microcephaly or other congenital disorders. This suggests that the Zika virus employs unique mechanisms to infect or bypass the placental barrier and, in turn, to cause neuronal-focused damage.
Placental passage
The villous trophoblasts, cells that are bathed in maternal blood, form the placenta’s first line of defense. Viruses, including the Zika virus, must cross or somehow bypass this initial barrier before crossing the placental basement membrane and endothelial cells, if they are to potentially invade the intrauterine cavity and infect the fetal brain and other tissues.
Research has demonstrated that cells of various types of tissue may express certain proteins, such as AXL, MER and TYRO3. While not yet proven, these proteins may mediate the entry of viruses such as Zika, enabling them to cross the placental trophoblast layer. These proteins are indeed expressed in trophoblasts, especially in early pregnancy, but we do not yet know if the proteins actually aid Zika’s passage through the placenta.
Another mechanism that has been postulated in the case of Zika infection is antibody-dependent enhancement, a process by which a current infection is enhanced by prior infection with another virus from the same family. Some experts believe that pre-existing immunity to the dengue virus – another member of the flavivirus family that has been endemic in Brazil – may be enhancing the spread of Zika infection as antibodies against dengue cross-react with the Zika virus.
While antibody-dependent enhancement has been shown to occur and to advance infection in various body systems, it has not been proven to affect the placenta. Until we learn more, we must simply appreciate that the presence of antibodies from another member of a family of viruses does not necessarily confer resistance. Instead, it may enable new infections to advance.
One might view pregnancy as a time of immune compromise, but we have shown in our laboratories that trophoblasts in fact have inherent resistance to a number of viruses. In a recent study, we found that trophoblasts are refractory to direct infection with the Zika virus. We isolated trophoblast cells from healthy full-term human placentas, cultured these cells for several days, and infected them with the Zika virus. We then measured viral replication and compared the infectivity of these cells with the infectivity of human brain microvascular endothelial cells – nontrophoblast cells that served as a control.
Our findings were extremely interesting to us: The trophoblast cells appeared to be significantly more resistant to the Zika virus than the nontrophoblast cells.
We learned, moreover, that this resistance was mediated by a particular interferon released by the trophoblast cells – type III interferon IFN1 – and that this type III interferon appeared to protect not only the trophoblasts but the nontrophoblast cells as well. It acted in both an autocrine and a paracrine manner to protect cells from the Zika virus. When we blocked the antiviral signaling of this interferon, resistance to the virus was attenuated.
These findings suggest that while Zika appears able to cross through the placenta and infect the fetus, the mechanism does not involve direct infection of the trophoblasts, at least in the later stages of pregnancy. The virus must either evade the type III interferon antiviral signals generated by the trophoblasts or somehow bypass these cells to cross the placenta (Cell Host Microbe. 2016 May 11;19[5]:705-12).
Interestingly, the Cell study mentioned above, in which Zika infection of mice early in pregnancy infected placental cells and the brain, also showed reduced Zika presence in the mouse mononuclear trophoblasts and syncytiotrophoblasts, in areas of the placenta analogous to the human villi.
Some experts have suggested, based the study of other viruses, that the Zika virus is better able to infect the placenta when the infection occurs early in the first trimester or the second trimester. It is indeed possible – and makes intuitive sense – that first-trimester trophoblasts confer less resistance and a lower level of protection than the mature trophoblasts we studied. At this point, however, we cannot say with certainty whether or not the placenta is more or less permissive to Zika infection at different points in pregnancy.
Interestingly, investigators who prospectively followed a small cohort of pregnant women in Brazil with suspected Zika infection identified abnormalities in fetuses of women who were infected at various points of their pregnancies, even in the third trimester. Fetuses infected in the first trimester had findings suggestive of pathologic change during embryogenesis, but central nervous system abnormalities were seen in fetuses infected as late as 27 weeks of gestation, the investigators said (N Engl J Med. 2016 Mar 4. doi: 10.1056/NEJMoa1602412).
The interferon-conferred resistance demonstrated in our recent study is one of two mechanisms we’ve identified by which placental trophoblasts orchestrate resistance to viral infection. In earlier research, we found that resistance can be conferred to nontrophoblast cells by the delivery of micro-RNAs. These micro-RNAs (C19MC miRNAs) are uniquely expressed in the placenta and packaged within trophoblast-derived nanovesicles called exosomes. The nanovesicles can latch onto other cells in the vicinity of the trophoblasts, attenuating viral replication in these recipient cells.
This earlier in-vitro study involved a panel of diverse and unrelated viruses, including coxsackievirus B3, poliovirus, vesicular stomatitis virus, and human cytomegalovirus (Proc Natl Acad Sci U S A. 2013 Jul 16;110[29]:12048-53). It did not include the Zika virus, but our ongoing preliminary research suggests that the same mechanisms might be active against Zika.
Furthering research
Research at our institution and in other laboratories has shed light on various ways in which the fetus is protected from viruses, but we must learn more in order to understand how particular viruses, such as Zika, are able to reach the fetal compartment and cause particular birth defects.
We must further investigate the role and importance of antibody-dependent enhancement, and we must continue to study the placenta and its various cell types. Continuing efforts to better elucidate the placenta’s defense mechanisms and to identify cell types that are more or less resistant to the Zika virus – and understand their differences – may lead us to potential therapeutic strategies.
Dr. Sadovsky is scientific director of the Magee-Womens Research Institute, Elsie Hilliard Hillman Chair of Women’s Health Research, and professor of ob.gyn., reproductive sciences, microbiology, and molecular genetics at the University of Pittsburgh. Dr. Coyne is associate professor of microbiology and molecular genetics, and ob.gyn. and reproductive sciences, at the University of Pittsburgh.* Their research addressed in this Master Class was supported by grants from the National Institutes of Health, State of Pennsylvania Formula Research Funds, and Burroughs Wellcome Fund.
*Correction, 7/05/2016: An earlier version of this article misstated Dr. Coyne's academic title.
Zika virus challenges ob.gyn. practice
Viral illnesses in pregnancy are not unheard of. When a patient presents with symptoms, we often think of an influenza type of infection that will be cleared within a short period of time and with few negative consequences for the developing fetus. Other infections that can occur include TORCH – Toxoplasmosis, Other (syphilis, varicella-zoster, parvovirus B19), Rubella, Cytomegalovirus (CMV), and Herpes – infections, but these are also relatively common.
Rarely do we in the United States consider a gravida’s vulnerability to tropical infectious diseases such as dengue, chikungunya, and now Zika virus. With the popularity and ease of international travel, and the potential for women’s exposure to more exotic diseases, the practice of ob.gyn. must undergo a significant transition in perspective. It is vital for us to understand these illnesses because of their potency and reported injury to both the mother and baby, for several reasons.
First, there is the public health concern. As of June 16, 2016, the Pan American Health Organization of the World Health Organization, reported 39 countries and territories in the Americas with confirmed cases of Zika virus, with 21 of those countries having confirmed cases in pregnant women.
As of June 9, 2016, the Centers for Disease Control and Prevention reported that 234 pregnant women in the United States have laboratory evidence of possible Zika infection, along with 189 pregnant women living in U.S. territories. Since the current outbreak, which began in July 2015 in Brazil, seven countries – accounting for more than 1,600 cases – have reported babies with congenital syndrome associated with Zika virus, the majority of which have been in Brazil. With the Summer Olympics in Rio starting in August 2016, the potential spread of Zika virus is dizzying.
Second, there is the counseling and management concern. Without a treatment or vaccine available, ob.gyns. must stay current on the latest research and findings to inform their patients of the risks associated with travel to an area with confirmed, or areas at risk for developing, Zika virus transmission.
Third, there is a diagnostic concern. Women who have visited areas with Zika virus, or who have had intimate contact with someone who has traveled to these areas, must be diagnosed and then counseled immediately.
We have devoted this Master Class to a discussion of Zika virus and the work being conducted in the United States to understand this disease. We have invited Dr. Yoel Sadovsky, an expert on placental development and trophoblast function, and his colleague, Carolyn Coyne, Ph.D., a leading researcher on host-virus interactions, to address this important topic.
Dr. Reece, who specializes in maternal-fetal medicine, is vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. Dr. Reece said he had no relevant financial disclosures. He is the medical editor of this column. Contact him at obnews@frontlinemedcom.com.
Viral illnesses in pregnancy are not unheard of. When a patient presents with symptoms, we often think of an influenza type of infection that will be cleared within a short period of time and with few negative consequences for the developing fetus. Other infections that can occur include TORCH – Toxoplasmosis, Other (syphilis, varicella-zoster, parvovirus B19), Rubella, Cytomegalovirus (CMV), and Herpes – infections, but these are also relatively common.
Rarely do we in the United States consider a gravida’s vulnerability to tropical infectious diseases such as dengue, chikungunya, and now Zika virus. With the popularity and ease of international travel, and the potential for women’s exposure to more exotic diseases, the practice of ob.gyn. must undergo a significant transition in perspective. It is vital for us to understand these illnesses because of their potency and reported injury to both the mother and baby, for several reasons.
First, there is the public health concern. As of June 16, 2016, the Pan American Health Organization of the World Health Organization, reported 39 countries and territories in the Americas with confirmed cases of Zika virus, with 21 of those countries having confirmed cases in pregnant women.
As of June 9, 2016, the Centers for Disease Control and Prevention reported that 234 pregnant women in the United States have laboratory evidence of possible Zika infection, along with 189 pregnant women living in U.S. territories. Since the current outbreak, which began in July 2015 in Brazil, seven countries – accounting for more than 1,600 cases – have reported babies with congenital syndrome associated with Zika virus, the majority of which have been in Brazil. With the Summer Olympics in Rio starting in August 2016, the potential spread of Zika virus is dizzying.
Second, there is the counseling and management concern. Without a treatment or vaccine available, ob.gyns. must stay current on the latest research and findings to inform their patients of the risks associated with travel to an area with confirmed, or areas at risk for developing, Zika virus transmission.
Third, there is a diagnostic concern. Women who have visited areas with Zika virus, or who have had intimate contact with someone who has traveled to these areas, must be diagnosed and then counseled immediately.
We have devoted this Master Class to a discussion of Zika virus and the work being conducted in the United States to understand this disease. We have invited Dr. Yoel Sadovsky, an expert on placental development and trophoblast function, and his colleague, Carolyn Coyne, Ph.D., a leading researcher on host-virus interactions, to address this important topic.
Dr. Reece, who specializes in maternal-fetal medicine, is vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. Dr. Reece said he had no relevant financial disclosures. He is the medical editor of this column. Contact him at obnews@frontlinemedcom.com.
Viral illnesses in pregnancy are not unheard of. When a patient presents with symptoms, we often think of an influenza type of infection that will be cleared within a short period of time and with few negative consequences for the developing fetus. Other infections that can occur include TORCH – Toxoplasmosis, Other (syphilis, varicella-zoster, parvovirus B19), Rubella, Cytomegalovirus (CMV), and Herpes – infections, but these are also relatively common.
Rarely do we in the United States consider a gravida’s vulnerability to tropical infectious diseases such as dengue, chikungunya, and now Zika virus. With the popularity and ease of international travel, and the potential for women’s exposure to more exotic diseases, the practice of ob.gyn. must undergo a significant transition in perspective. It is vital for us to understand these illnesses because of their potency and reported injury to both the mother and baby, for several reasons.
First, there is the public health concern. As of June 16, 2016, the Pan American Health Organization of the World Health Organization, reported 39 countries and territories in the Americas with confirmed cases of Zika virus, with 21 of those countries having confirmed cases in pregnant women.
As of June 9, 2016, the Centers for Disease Control and Prevention reported that 234 pregnant women in the United States have laboratory evidence of possible Zika infection, along with 189 pregnant women living in U.S. territories. Since the current outbreak, which began in July 2015 in Brazil, seven countries – accounting for more than 1,600 cases – have reported babies with congenital syndrome associated with Zika virus, the majority of which have been in Brazil. With the Summer Olympics in Rio starting in August 2016, the potential spread of Zika virus is dizzying.
Second, there is the counseling and management concern. Without a treatment or vaccine available, ob.gyns. must stay current on the latest research and findings to inform their patients of the risks associated with travel to an area with confirmed, or areas at risk for developing, Zika virus transmission.
Third, there is a diagnostic concern. Women who have visited areas with Zika virus, or who have had intimate contact with someone who has traveled to these areas, must be diagnosed and then counseled immediately.
We have devoted this Master Class to a discussion of Zika virus and the work being conducted in the United States to understand this disease. We have invited Dr. Yoel Sadovsky, an expert on placental development and trophoblast function, and his colleague, Carolyn Coyne, Ph.D., a leading researcher on host-virus interactions, to address this important topic.
Dr. Reece, who specializes in maternal-fetal medicine, is vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. Dr. Reece said he had no relevant financial disclosures. He is the medical editor of this column. Contact him at obnews@frontlinemedcom.com.
50 years of gynecologic surgery: A large dose of ingenuity, a small dose of controversy
Over the past 50 years, there has been explosive change in gynecologic surgery. Ob.Gyn. News has been at the forefront of capturing and chronicling this paradigm shift in the treatment of the female patient.
Our beginnings
From antiquity, physicians and surgeons have struggled with pelvic prolapse, uterine fibroids, ovarian cysts, urinary incontinence, vesicovaginal fistulas, pelvic pain, and abnormal uterine bleeding. At the time of the first edition of Ob.Gyn. News, it had been less than a century since Thomas Edison invented the light bulb; just over 50 years since Hans Christian Jacobaeus first created air pneumoperitoneum using a trocar, followed by the Nitze cystoscope; about 40 years since Richard Zollikofer created a carbon dioxide pneumoperitoneum; 25 years since F.H. Powers and A.C. Barnes had first described laparoscopic tubal sterilization by cautery; and about 20 years since Raoul Palmer, considered the father of modern laparoscopy, had first described the technique – left upper quadrant entry, testing insufflation, Trendelenburg positioning, and simple laparoscopic instrumentation.
In the 1950s, Hans Frangenheim would bring monopolar electrosurgery to laparoscopy and Harold Hopkins would introduce fiber optics. It was not until 1967 that Patrick Steptoe would publish the first textbook on laparoscopy in the English language.
Although usage as a diagnostic tool and a method of sterilization increased popularity of laparoscopy in the 1960s and early 1970s, there were few advances. In fact, a review of early editions of Ob.Gyn. News during that time period shows that the majority of articles involving laparoscopy dealt with sterilization; including the introduction of clips for tubal sterilization by Jaroslav Hulka in 1972. This did not deter the efforts of Jordan Phillips, who along with Jacques Rioux, Louis Keith, Richard Soderstrom – four early laparoscopists – incorporated a new society, the American Association of Gynecologic Laparoscopists (the AAGL) in 1971.
Simultaneously, in 1979, James Daniell in the United States, Maurice Bruhart in France, and Yona Tadir in Israel were promoting efforts to couple the carbon dioxide laser to the laparoscope to treat pelvic adhesions and endometriosis. Later on, fiber lasers, KTP, Nd:YAG, and Argon lasers would be utilized in our field. Still, only a few extirpative procedures were being performed via a laparoscope route. This included linear salpingostomy for the treatment of ectopic pregnancy, championed by Professor Bruhart and H. Manhes in Europe, and Alan DeCherney in the United States.
During the 1980s, laparoscopic surgery was at its innovative best. Through the pioneering efforts of Professor Kurt Semm and his protégée, Liselotte Mettler, the gynecologic laparoscopist was introduced to endoloops, simple suturing techniques, and mechanical morcellation techniques.
Procedures such as salpingo-oophorectomy, appendectomy, and myomectomy could now be performed via the laparoscope. Dr. Camran Nezhat coupled the carbon dioxide laser, the laparoscope, and the television monitor, coining the term laparoscopy. Most importantly, the laparoscopic surgeon was liberated; he or she could remain upright and perform surgery with both hands. Through the 1980s and 1990s, Dr. Nezhat, Dr. Harry Reich, and other innovators pushed the envelope in increasing the ability to extirpate endometriosis, excise severe pelvic adhesions, and perform discoid and segmental bowel resection.
The day the earth stood still
Every gynecologic laparoscopic surgeon should remember Jan. 26, 1988, as that was the date that Dr. Harry Reich performed the first total laparoscopic hysterectomy. Now, little more than 25 years later, in many parts of the country, a laparoscopic approach to hysterectomy is indeed the most common route. Over the years, with the evolution of instrumentation, including new energy systems (ultrasonic, advanced bipolar) and the introduction of barbed sutures, hysterectomy can now be performed via minilaparoscopy, single-site laparoscopy, robot-assisted, and robotic single site, all of which have been featured in the Ob.Gyn. News’ Master Class in Gynecologic Surgery.
But hysteroscopy came first
Abulkasim utilized a mirror to reflect light into the vaginal vault in 1,000 A.D. In 1806, Philipp Bozzini originated the idea of illuminating body cavities by an external light source. Through a system of mirrors and tubes, candlelight could be reflected into the body. In 1869, D.C. Pantaleoni used a cystoscope developed by Antoine Desormeaux – who has been called the father of endoscopy – to treat endometrial polyps with silver nitrate.
Through the 50 years of Ob.Gyn. News and over the past 12 years of the Master Class in Gynecologic Surgery, our community has been consistently updated as to advances in hysteroscopy, not only to enhance treatment efficacy, but safety as well. This has included such advances as the continuous flow hysteroscope, the Hamou contact hysteroscope, and fluid management systems to enhance visualization.
In 1978, Robert Neuwirth introduced loops to perform hysteroscopic myomectomy. The loop resectoscope was quickly followed by the rollerball to perform endometrial ablation. In the late 1990s, hysteroscopic bipolar cutting loops were introduced. This enabled use of ionic distension media saline, instead of nonionic media, thus decreasing risks related to hyponatremia.
In 2003, Mark Emanuel introduced hysteroscopic morcellation systems, which enabled more gynecologists to perform operative hysteroscopy safely. Resected tissue is removed immediately to allow superior visualization. The flexible hysteroscope coupled with vaginoscopy has enabled hysteroscopy to be done with minimal to no anesthesia in an in-office setting.
With advances in hysteroscopy over the past 35 years, hysteroscopic procedures such as polypectomy, myomectomy, lysis of adhesions, transection of endometriosis, evacuation of retained products of conception, and endometrial ablation/resection have become routine.
And now, the controversy
Since its inception, laparoscopic surgery has not been without controversy. In 1933, Karl Fervers described explosion and flashes of light from a combination of high frequency electric current and oxygen distension gas while performing laparoscopic adhesiolysis with the coagulation probe of the ureterocystoscope.
In the early 1970s, Professor Kurt Semm’s pioneering effort was not rewarded by his department, in Kiel, Germany, which instead recommended he schedule a brain scan and psychological testing.
Nearly 20 years later, in a 1992 edition of Current Science, Professor Semm, along with Alan DeCherney, stated that “over 80% of gynecological operations can now be performed by laparoscopy.” Shortly thereafter, however, Dr. Roy Pitkin, who at the time was president of the American College of Obstetricians and Gynecologists, wrote an editorial in the Journal of Obstetrics and Gynecology – “Operative Laparoscopy: Surgical Advance or Technical Gimmick?” (Obstet Gynecol. 1992 Mar;79[3]:441-2).
Fortunately, 18 years later, with the continued advances in laparoscopic surgery making it less expensive, safer, and more accessible, Dr. Pitkin did retract his statement (Obstet Gynecol. 2010 May;115[5]:890-1).
Currently, the gynecologic community is embroiled in controversies involving the use of the robot to assist in the performance of laparoscopic surgery, the incorporation of synthetic mesh to enhance urogynecologic procedures, the placement of Essure micro-inserts to occlude fallopian tubes, and the use of electronic power morcellation at time of laparoscopic or robot-assisted hysterectomy, myomectomy, or sacrocolpopexy.
After reading the 2013 article by Dr. Jason Wright, published in JAMA, comparing laparoscopic hysterectomy to robotic hysterectomy, no one can deny that the rise in a minimally invasive route to hysterectomy has coincided with the advent of the robot (JAMA. 2013 Feb 20;309[7]:689-98). On the other hand, many detractors, including Dr. James Breeden (past ACOG president 2012-2013), find the higher cost of robotic surgery very problematic. In fact, many of these detractors cite the paucity of data showing a significant advantage to use of robotics.
While certainly cost, more than ever, must be a major consideration, remember that during the 1990s, there were multiple articles in Ob.Gyn. News raising concerns about the cost of laparoscopic hysterectomy. Interestingly, studies over the past decade by Warren and Jonsdottir show a cost savings when hysterectomy is done laparoscopically as opposed to its being done by laparotomy. Thus, it certainly can be anticipated that with more physician experience, improved instrumentation, and robotic industry competition, the overall cost will become more comparable to a laparoscopic route.
In 1995, Ulf Ulmsten first described the use of tension-free tape (TVT) to treat stress urinary incontinence. In 1998, the Food and Drug Administration approved the use of the TVT sling in the United States. Since then, transobturator tension-free vaginal tape (TVT-O) and single incision mini-slings have been introduced. All of these techniques have been shown to be successful and have been well adapted into the armamentarium of physicians treating stress urinary incontinence.
With the success of synthetic mesh for the treatment of stress urinary incontinence, its use was extended to pelvic prolapse. In 2002, the first mesh device with indications for the treatment of pelvic organ prolapse was approved by the FDA. While the erosion rate utilizing synthetic mesh for stress urinary incontinence has been noted to be 2%, rates up to 8.3% have been noted in patients treated for pelvic prolapse.
In 2008, the FDA issued a warning regarding the use of mesh for prolapse and incontinence repair secondary to the sequelae of mesh erosion. Subsequently, in 2011, the concern was limited to vaginal mesh to correct pelvic organ prolapse. Finally, on Jan. 4, 2016, the FDA issued an order to reclassify surgical mesh to repair pelvic organ prolapse from class II, which includes moderate-risk devices, to class III, which includes high-risk devices. Moreover, the FDA issued a second order to manufacturers to submit a premarket approval application to support the safety and effectiveness of synthetic mesh for transvaginal repair of pelvic organ prolapse.
Essure micro-inserts for permanent birth control received initial approval from the FDA in November 2002. Despite the fact that Essure can be easily placed, is highly effective, and has seemingly low complication rates, concerns have been raised by the Facebook group “Essure Problems” and Erin Brockovich, the focus of the 2000 biographical film starring Julia Roberts.
After more than 5,000 women filed grievances with the FDA between November 2002 and May 2015, based on unintended pregnancies, miscarriages, stillbirths, severe pain, and bleeding, the FDA announced in 2016 that it would require a boxed warning label for Essure. The FDA also called upon Bayer, which makes and markets Essure, to conduct surveillance to assess “risks of the device in a real-world environment.” The agency stated it will use the results to “determine what, if any, further actions related to Essure are needed to protect public health.”
While Jan. 26, 1988, is a very special date in minimally invasive gynecologic surgery, April 17, 2014, is a day of infamy for the gynecologic laparoscopist. For on this day, the FDA announced a warning regarding electronic power morcellation. Many hospitals and hospital systems throughout the country issued bans on electronic power morcellation, leading to needless open laparotomy procedures and thus, introducing prolonged recovery times and increased risk.
At a time when the recent introduction of barbed suture had made both closure of the vaginal cuff at time of hysterectomy and repair of the hysterotomy at myomectomy easier and faster, the gynecologic laparoscopist was taking a step backward. The FDA based this decision and a subsequent boxed warning – issued in November 2014 – on a small number of studies showing potential upstaging of leiomyosarcoma post electronic power morcellation. Interestingly, many of the morcellation procedures cited did not use power morcellation. Furthermore, a more comprehensive meta-analysis by Elizabeth A. Pritts and colleagues, showed a far lower risk than suggested by the FDA (Gynecol Surg. 2015;12[3]:165-77).
Recently, an article by William Parker and colleagues recommended that the FDA reverse its position (Obstet Gynecol. 2016 Jan;127[1]:18-22). Many believe that ultimately, the solution will be morcellation in a containment bag, which I and my colleagues have been performing in virtually every power morcellation procedure since May 2014. During this current power morcellation controversy, the Master Class in Gynecologic Surgery has continued to update its readers with three different articles related to the subject.
And in conclusion
Without a doubt, the past 50 years of gynecologic surgery has been a time of unparalleled innovation with occasional controversy thrown in. Ob.Gyn. News and more recently, the Master Class in Gynecologic Surgery, has had a major leadership role in bringing this profound ingenuity to the gynecology community by introducing this explosion of surgical creativity to its readers.
And what will the next 50 years bring? I believe we will continue to see tremendous advancements in minimally invasive gynecologic surgery. There will be a definite impact of costs on the marketplace. Thus, many of the minor minimally invasive procedures currently performed in the hospital or surgery center will be brought into office settings. In addition, secondary to reimbursement, the more complex cases will be carried out by fewer gynecologic surgeons who have undergone more intense training in pelvic surgery and who can perform these cases more efficiently and with fewer complications. Our ability to perform surgery and what type of procedures we do will not only be based on randomized, controlled trials, but big data collection as well.
Dr. Miller is clinical associate professor at the University of Illinois at Chicago, and past president of the AAGL and the International Society for Gynecologic Endoscopy (ISGE). He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in private practice in Naperville and Schaumburg, Ill.; director of minimally invasive gynecologic surgery and the director of the AAGL/Society of Reproductive Surgery fellowship in minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill.; and the medical editor of this column, Master Class. Dr. Miller is a consultant and is on the speakers bureau for Ethicon.
Over the past 50 years, there has been explosive change in gynecologic surgery. Ob.Gyn. News has been at the forefront of capturing and chronicling this paradigm shift in the treatment of the female patient.
Our beginnings
From antiquity, physicians and surgeons have struggled with pelvic prolapse, uterine fibroids, ovarian cysts, urinary incontinence, vesicovaginal fistulas, pelvic pain, and abnormal uterine bleeding. At the time of the first edition of Ob.Gyn. News, it had been less than a century since Thomas Edison invented the light bulb; just over 50 years since Hans Christian Jacobaeus first created air pneumoperitoneum using a trocar, followed by the Nitze cystoscope; about 40 years since Richard Zollikofer created a carbon dioxide pneumoperitoneum; 25 years since F.H. Powers and A.C. Barnes had first described laparoscopic tubal sterilization by cautery; and about 20 years since Raoul Palmer, considered the father of modern laparoscopy, had first described the technique – left upper quadrant entry, testing insufflation, Trendelenburg positioning, and simple laparoscopic instrumentation.
In the 1950s, Hans Frangenheim would bring monopolar electrosurgery to laparoscopy and Harold Hopkins would introduce fiber optics. It was not until 1967 that Patrick Steptoe would publish the first textbook on laparoscopy in the English language.
Although usage as a diagnostic tool and a method of sterilization increased popularity of laparoscopy in the 1960s and early 1970s, there were few advances. In fact, a review of early editions of Ob.Gyn. News during that time period shows that the majority of articles involving laparoscopy dealt with sterilization; including the introduction of clips for tubal sterilization by Jaroslav Hulka in 1972. This did not deter the efforts of Jordan Phillips, who along with Jacques Rioux, Louis Keith, Richard Soderstrom – four early laparoscopists – incorporated a new society, the American Association of Gynecologic Laparoscopists (the AAGL) in 1971.
Simultaneously, in 1979, James Daniell in the United States, Maurice Bruhart in France, and Yona Tadir in Israel were promoting efforts to couple the carbon dioxide laser to the laparoscope to treat pelvic adhesions and endometriosis. Later on, fiber lasers, KTP, Nd:YAG, and Argon lasers would be utilized in our field. Still, only a few extirpative procedures were being performed via a laparoscope route. This included linear salpingostomy for the treatment of ectopic pregnancy, championed by Professor Bruhart and H. Manhes in Europe, and Alan DeCherney in the United States.
During the 1980s, laparoscopic surgery was at its innovative best. Through the pioneering efforts of Professor Kurt Semm and his protégée, Liselotte Mettler, the gynecologic laparoscopist was introduced to endoloops, simple suturing techniques, and mechanical morcellation techniques.
Procedures such as salpingo-oophorectomy, appendectomy, and myomectomy could now be performed via the laparoscope. Dr. Camran Nezhat coupled the carbon dioxide laser, the laparoscope, and the television monitor, coining the term laparoscopy. Most importantly, the laparoscopic surgeon was liberated; he or she could remain upright and perform surgery with both hands. Through the 1980s and 1990s, Dr. Nezhat, Dr. Harry Reich, and other innovators pushed the envelope in increasing the ability to extirpate endometriosis, excise severe pelvic adhesions, and perform discoid and segmental bowel resection.
The day the earth stood still
Every gynecologic laparoscopic surgeon should remember Jan. 26, 1988, as that was the date that Dr. Harry Reich performed the first total laparoscopic hysterectomy. Now, little more than 25 years later, in many parts of the country, a laparoscopic approach to hysterectomy is indeed the most common route. Over the years, with the evolution of instrumentation, including new energy systems (ultrasonic, advanced bipolar) and the introduction of barbed sutures, hysterectomy can now be performed via minilaparoscopy, single-site laparoscopy, robot-assisted, and robotic single site, all of which have been featured in the Ob.Gyn. News’ Master Class in Gynecologic Surgery.
But hysteroscopy came first
Abulkasim utilized a mirror to reflect light into the vaginal vault in 1,000 A.D. In 1806, Philipp Bozzini originated the idea of illuminating body cavities by an external light source. Through a system of mirrors and tubes, candlelight could be reflected into the body. In 1869, D.C. Pantaleoni used a cystoscope developed by Antoine Desormeaux – who has been called the father of endoscopy – to treat endometrial polyps with silver nitrate.
Through the 50 years of Ob.Gyn. News and over the past 12 years of the Master Class in Gynecologic Surgery, our community has been consistently updated as to advances in hysteroscopy, not only to enhance treatment efficacy, but safety as well. This has included such advances as the continuous flow hysteroscope, the Hamou contact hysteroscope, and fluid management systems to enhance visualization.
In 1978, Robert Neuwirth introduced loops to perform hysteroscopic myomectomy. The loop resectoscope was quickly followed by the rollerball to perform endometrial ablation. In the late 1990s, hysteroscopic bipolar cutting loops were introduced. This enabled use of ionic distension media saline, instead of nonionic media, thus decreasing risks related to hyponatremia.
In 2003, Mark Emanuel introduced hysteroscopic morcellation systems, which enabled more gynecologists to perform operative hysteroscopy safely. Resected tissue is removed immediately to allow superior visualization. The flexible hysteroscope coupled with vaginoscopy has enabled hysteroscopy to be done with minimal to no anesthesia in an in-office setting.
With advances in hysteroscopy over the past 35 years, hysteroscopic procedures such as polypectomy, myomectomy, lysis of adhesions, transection of endometriosis, evacuation of retained products of conception, and endometrial ablation/resection have become routine.
And now, the controversy
Since its inception, laparoscopic surgery has not been without controversy. In 1933, Karl Fervers described explosion and flashes of light from a combination of high frequency electric current and oxygen distension gas while performing laparoscopic adhesiolysis with the coagulation probe of the ureterocystoscope.
In the early 1970s, Professor Kurt Semm’s pioneering effort was not rewarded by his department, in Kiel, Germany, which instead recommended he schedule a brain scan and psychological testing.
Nearly 20 years later, in a 1992 edition of Current Science, Professor Semm, along with Alan DeCherney, stated that “over 80% of gynecological operations can now be performed by laparoscopy.” Shortly thereafter, however, Dr. Roy Pitkin, who at the time was president of the American College of Obstetricians and Gynecologists, wrote an editorial in the Journal of Obstetrics and Gynecology – “Operative Laparoscopy: Surgical Advance or Technical Gimmick?” (Obstet Gynecol. 1992 Mar;79[3]:441-2).
Fortunately, 18 years later, with the continued advances in laparoscopic surgery making it less expensive, safer, and more accessible, Dr. Pitkin did retract his statement (Obstet Gynecol. 2010 May;115[5]:890-1).
Currently, the gynecologic community is embroiled in controversies involving the use of the robot to assist in the performance of laparoscopic surgery, the incorporation of synthetic mesh to enhance urogynecologic procedures, the placement of Essure micro-inserts to occlude fallopian tubes, and the use of electronic power morcellation at time of laparoscopic or robot-assisted hysterectomy, myomectomy, or sacrocolpopexy.
After reading the 2013 article by Dr. Jason Wright, published in JAMA, comparing laparoscopic hysterectomy to robotic hysterectomy, no one can deny that the rise in a minimally invasive route to hysterectomy has coincided with the advent of the robot (JAMA. 2013 Feb 20;309[7]:689-98). On the other hand, many detractors, including Dr. James Breeden (past ACOG president 2012-2013), find the higher cost of robotic surgery very problematic. In fact, many of these detractors cite the paucity of data showing a significant advantage to use of robotics.
While certainly cost, more than ever, must be a major consideration, remember that during the 1990s, there were multiple articles in Ob.Gyn. News raising concerns about the cost of laparoscopic hysterectomy. Interestingly, studies over the past decade by Warren and Jonsdottir show a cost savings when hysterectomy is done laparoscopically as opposed to its being done by laparotomy. Thus, it certainly can be anticipated that with more physician experience, improved instrumentation, and robotic industry competition, the overall cost will become more comparable to a laparoscopic route.
In 1995, Ulf Ulmsten first described the use of tension-free tape (TVT) to treat stress urinary incontinence. In 1998, the Food and Drug Administration approved the use of the TVT sling in the United States. Since then, transobturator tension-free vaginal tape (TVT-O) and single incision mini-slings have been introduced. All of these techniques have been shown to be successful and have been well adapted into the armamentarium of physicians treating stress urinary incontinence.
With the success of synthetic mesh for the treatment of stress urinary incontinence, its use was extended to pelvic prolapse. In 2002, the first mesh device with indications for the treatment of pelvic organ prolapse was approved by the FDA. While the erosion rate utilizing synthetic mesh for stress urinary incontinence has been noted to be 2%, rates up to 8.3% have been noted in patients treated for pelvic prolapse.
In 2008, the FDA issued a warning regarding the use of mesh for prolapse and incontinence repair secondary to the sequelae of mesh erosion. Subsequently, in 2011, the concern was limited to vaginal mesh to correct pelvic organ prolapse. Finally, on Jan. 4, 2016, the FDA issued an order to reclassify surgical mesh to repair pelvic organ prolapse from class II, which includes moderate-risk devices, to class III, which includes high-risk devices. Moreover, the FDA issued a second order to manufacturers to submit a premarket approval application to support the safety and effectiveness of synthetic mesh for transvaginal repair of pelvic organ prolapse.
Essure micro-inserts for permanent birth control received initial approval from the FDA in November 2002. Despite the fact that Essure can be easily placed, is highly effective, and has seemingly low complication rates, concerns have been raised by the Facebook group “Essure Problems” and Erin Brockovich, the focus of the 2000 biographical film starring Julia Roberts.
After more than 5,000 women filed grievances with the FDA between November 2002 and May 2015, based on unintended pregnancies, miscarriages, stillbirths, severe pain, and bleeding, the FDA announced in 2016 that it would require a boxed warning label for Essure. The FDA also called upon Bayer, which makes and markets Essure, to conduct surveillance to assess “risks of the device in a real-world environment.” The agency stated it will use the results to “determine what, if any, further actions related to Essure are needed to protect public health.”
While Jan. 26, 1988, is a very special date in minimally invasive gynecologic surgery, April 17, 2014, is a day of infamy for the gynecologic laparoscopist. For on this day, the FDA announced a warning regarding electronic power morcellation. Many hospitals and hospital systems throughout the country issued bans on electronic power morcellation, leading to needless open laparotomy procedures and thus, introducing prolonged recovery times and increased risk.
At a time when the recent introduction of barbed suture had made both closure of the vaginal cuff at time of hysterectomy and repair of the hysterotomy at myomectomy easier and faster, the gynecologic laparoscopist was taking a step backward. The FDA based this decision and a subsequent boxed warning – issued in November 2014 – on a small number of studies showing potential upstaging of leiomyosarcoma post electronic power morcellation. Interestingly, many of the morcellation procedures cited did not use power morcellation. Furthermore, a more comprehensive meta-analysis by Elizabeth A. Pritts and colleagues, showed a far lower risk than suggested by the FDA (Gynecol Surg. 2015;12[3]:165-77).
Recently, an article by William Parker and colleagues recommended that the FDA reverse its position (Obstet Gynecol. 2016 Jan;127[1]:18-22). Many believe that ultimately, the solution will be morcellation in a containment bag, which I and my colleagues have been performing in virtually every power morcellation procedure since May 2014. During this current power morcellation controversy, the Master Class in Gynecologic Surgery has continued to update its readers with three different articles related to the subject.
And in conclusion
Without a doubt, the past 50 years of gynecologic surgery has been a time of unparalleled innovation with occasional controversy thrown in. Ob.Gyn. News and more recently, the Master Class in Gynecologic Surgery, has had a major leadership role in bringing this profound ingenuity to the gynecology community by introducing this explosion of surgical creativity to its readers.
And what will the next 50 years bring? I believe we will continue to see tremendous advancements in minimally invasive gynecologic surgery. There will be a definite impact of costs on the marketplace. Thus, many of the minor minimally invasive procedures currently performed in the hospital or surgery center will be brought into office settings. In addition, secondary to reimbursement, the more complex cases will be carried out by fewer gynecologic surgeons who have undergone more intense training in pelvic surgery and who can perform these cases more efficiently and with fewer complications. Our ability to perform surgery and what type of procedures we do will not only be based on randomized, controlled trials, but big data collection as well.
Dr. Miller is clinical associate professor at the University of Illinois at Chicago, and past president of the AAGL and the International Society for Gynecologic Endoscopy (ISGE). He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in private practice in Naperville and Schaumburg, Ill.; director of minimally invasive gynecologic surgery and the director of the AAGL/Society of Reproductive Surgery fellowship in minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill.; and the medical editor of this column, Master Class. Dr. Miller is a consultant and is on the speakers bureau for Ethicon.
Over the past 50 years, there has been explosive change in gynecologic surgery. Ob.Gyn. News has been at the forefront of capturing and chronicling this paradigm shift in the treatment of the female patient.
Our beginnings
From antiquity, physicians and surgeons have struggled with pelvic prolapse, uterine fibroids, ovarian cysts, urinary incontinence, vesicovaginal fistulas, pelvic pain, and abnormal uterine bleeding. At the time of the first edition of Ob.Gyn. News, it had been less than a century since Thomas Edison invented the light bulb; just over 50 years since Hans Christian Jacobaeus first created air pneumoperitoneum using a trocar, followed by the Nitze cystoscope; about 40 years since Richard Zollikofer created a carbon dioxide pneumoperitoneum; 25 years since F.H. Powers and A.C. Barnes had first described laparoscopic tubal sterilization by cautery; and about 20 years since Raoul Palmer, considered the father of modern laparoscopy, had first described the technique – left upper quadrant entry, testing insufflation, Trendelenburg positioning, and simple laparoscopic instrumentation.
In the 1950s, Hans Frangenheim would bring monopolar electrosurgery to laparoscopy and Harold Hopkins would introduce fiber optics. It was not until 1967 that Patrick Steptoe would publish the first textbook on laparoscopy in the English language.
Although usage as a diagnostic tool and a method of sterilization increased popularity of laparoscopy in the 1960s and early 1970s, there were few advances. In fact, a review of early editions of Ob.Gyn. News during that time period shows that the majority of articles involving laparoscopy dealt with sterilization; including the introduction of clips for tubal sterilization by Jaroslav Hulka in 1972. This did not deter the efforts of Jordan Phillips, who along with Jacques Rioux, Louis Keith, Richard Soderstrom – four early laparoscopists – incorporated a new society, the American Association of Gynecologic Laparoscopists (the AAGL) in 1971.
Simultaneously, in 1979, James Daniell in the United States, Maurice Bruhart in France, and Yona Tadir in Israel were promoting efforts to couple the carbon dioxide laser to the laparoscope to treat pelvic adhesions and endometriosis. Later on, fiber lasers, KTP, Nd:YAG, and Argon lasers would be utilized in our field. Still, only a few extirpative procedures were being performed via a laparoscope route. This included linear salpingostomy for the treatment of ectopic pregnancy, championed by Professor Bruhart and H. Manhes in Europe, and Alan DeCherney in the United States.
During the 1980s, laparoscopic surgery was at its innovative best. Through the pioneering efforts of Professor Kurt Semm and his protégée, Liselotte Mettler, the gynecologic laparoscopist was introduced to endoloops, simple suturing techniques, and mechanical morcellation techniques.
Procedures such as salpingo-oophorectomy, appendectomy, and myomectomy could now be performed via the laparoscope. Dr. Camran Nezhat coupled the carbon dioxide laser, the laparoscope, and the television monitor, coining the term laparoscopy. Most importantly, the laparoscopic surgeon was liberated; he or she could remain upright and perform surgery with both hands. Through the 1980s and 1990s, Dr. Nezhat, Dr. Harry Reich, and other innovators pushed the envelope in increasing the ability to extirpate endometriosis, excise severe pelvic adhesions, and perform discoid and segmental bowel resection.
The day the earth stood still
Every gynecologic laparoscopic surgeon should remember Jan. 26, 1988, as that was the date that Dr. Harry Reich performed the first total laparoscopic hysterectomy. Now, little more than 25 years later, in many parts of the country, a laparoscopic approach to hysterectomy is indeed the most common route. Over the years, with the evolution of instrumentation, including new energy systems (ultrasonic, advanced bipolar) and the introduction of barbed sutures, hysterectomy can now be performed via minilaparoscopy, single-site laparoscopy, robot-assisted, and robotic single site, all of which have been featured in the Ob.Gyn. News’ Master Class in Gynecologic Surgery.
But hysteroscopy came first
Abulkasim utilized a mirror to reflect light into the vaginal vault in 1,000 A.D. In 1806, Philipp Bozzini originated the idea of illuminating body cavities by an external light source. Through a system of mirrors and tubes, candlelight could be reflected into the body. In 1869, D.C. Pantaleoni used a cystoscope developed by Antoine Desormeaux – who has been called the father of endoscopy – to treat endometrial polyps with silver nitrate.
Through the 50 years of Ob.Gyn. News and over the past 12 years of the Master Class in Gynecologic Surgery, our community has been consistently updated as to advances in hysteroscopy, not only to enhance treatment efficacy, but safety as well. This has included such advances as the continuous flow hysteroscope, the Hamou contact hysteroscope, and fluid management systems to enhance visualization.
In 1978, Robert Neuwirth introduced loops to perform hysteroscopic myomectomy. The loop resectoscope was quickly followed by the rollerball to perform endometrial ablation. In the late 1990s, hysteroscopic bipolar cutting loops were introduced. This enabled use of ionic distension media saline, instead of nonionic media, thus decreasing risks related to hyponatremia.
In 2003, Mark Emanuel introduced hysteroscopic morcellation systems, which enabled more gynecologists to perform operative hysteroscopy safely. Resected tissue is removed immediately to allow superior visualization. The flexible hysteroscope coupled with vaginoscopy has enabled hysteroscopy to be done with minimal to no anesthesia in an in-office setting.
With advances in hysteroscopy over the past 35 years, hysteroscopic procedures such as polypectomy, myomectomy, lysis of adhesions, transection of endometriosis, evacuation of retained products of conception, and endometrial ablation/resection have become routine.
And now, the controversy
Since its inception, laparoscopic surgery has not been without controversy. In 1933, Karl Fervers described explosion and flashes of light from a combination of high frequency electric current and oxygen distension gas while performing laparoscopic adhesiolysis with the coagulation probe of the ureterocystoscope.
In the early 1970s, Professor Kurt Semm’s pioneering effort was not rewarded by his department, in Kiel, Germany, which instead recommended he schedule a brain scan and psychological testing.
Nearly 20 years later, in a 1992 edition of Current Science, Professor Semm, along with Alan DeCherney, stated that “over 80% of gynecological operations can now be performed by laparoscopy.” Shortly thereafter, however, Dr. Roy Pitkin, who at the time was president of the American College of Obstetricians and Gynecologists, wrote an editorial in the Journal of Obstetrics and Gynecology – “Operative Laparoscopy: Surgical Advance or Technical Gimmick?” (Obstet Gynecol. 1992 Mar;79[3]:441-2).
Fortunately, 18 years later, with the continued advances in laparoscopic surgery making it less expensive, safer, and more accessible, Dr. Pitkin did retract his statement (Obstet Gynecol. 2010 May;115[5]:890-1).
Currently, the gynecologic community is embroiled in controversies involving the use of the robot to assist in the performance of laparoscopic surgery, the incorporation of synthetic mesh to enhance urogynecologic procedures, the placement of Essure micro-inserts to occlude fallopian tubes, and the use of electronic power morcellation at time of laparoscopic or robot-assisted hysterectomy, myomectomy, or sacrocolpopexy.
After reading the 2013 article by Dr. Jason Wright, published in JAMA, comparing laparoscopic hysterectomy to robotic hysterectomy, no one can deny that the rise in a minimally invasive route to hysterectomy has coincided with the advent of the robot (JAMA. 2013 Feb 20;309[7]:689-98). On the other hand, many detractors, including Dr. James Breeden (past ACOG president 2012-2013), find the higher cost of robotic surgery very problematic. In fact, many of these detractors cite the paucity of data showing a significant advantage to use of robotics.
While certainly cost, more than ever, must be a major consideration, remember that during the 1990s, there were multiple articles in Ob.Gyn. News raising concerns about the cost of laparoscopic hysterectomy. Interestingly, studies over the past decade by Warren and Jonsdottir show a cost savings when hysterectomy is done laparoscopically as opposed to its being done by laparotomy. Thus, it certainly can be anticipated that with more physician experience, improved instrumentation, and robotic industry competition, the overall cost will become more comparable to a laparoscopic route.
In 1995, Ulf Ulmsten first described the use of tension-free tape (TVT) to treat stress urinary incontinence. In 1998, the Food and Drug Administration approved the use of the TVT sling in the United States. Since then, transobturator tension-free vaginal tape (TVT-O) and single incision mini-slings have been introduced. All of these techniques have been shown to be successful and have been well adapted into the armamentarium of physicians treating stress urinary incontinence.
With the success of synthetic mesh for the treatment of stress urinary incontinence, its use was extended to pelvic prolapse. In 2002, the first mesh device with indications for the treatment of pelvic organ prolapse was approved by the FDA. While the erosion rate utilizing synthetic mesh for stress urinary incontinence has been noted to be 2%, rates up to 8.3% have been noted in patients treated for pelvic prolapse.
In 2008, the FDA issued a warning regarding the use of mesh for prolapse and incontinence repair secondary to the sequelae of mesh erosion. Subsequently, in 2011, the concern was limited to vaginal mesh to correct pelvic organ prolapse. Finally, on Jan. 4, 2016, the FDA issued an order to reclassify surgical mesh to repair pelvic organ prolapse from class II, which includes moderate-risk devices, to class III, which includes high-risk devices. Moreover, the FDA issued a second order to manufacturers to submit a premarket approval application to support the safety and effectiveness of synthetic mesh for transvaginal repair of pelvic organ prolapse.
Essure micro-inserts for permanent birth control received initial approval from the FDA in November 2002. Despite the fact that Essure can be easily placed, is highly effective, and has seemingly low complication rates, concerns have been raised by the Facebook group “Essure Problems” and Erin Brockovich, the focus of the 2000 biographical film starring Julia Roberts.
After more than 5,000 women filed grievances with the FDA between November 2002 and May 2015, based on unintended pregnancies, miscarriages, stillbirths, severe pain, and bleeding, the FDA announced in 2016 that it would require a boxed warning label for Essure. The FDA also called upon Bayer, which makes and markets Essure, to conduct surveillance to assess “risks of the device in a real-world environment.” The agency stated it will use the results to “determine what, if any, further actions related to Essure are needed to protect public health.”
While Jan. 26, 1988, is a very special date in minimally invasive gynecologic surgery, April 17, 2014, is a day of infamy for the gynecologic laparoscopist. For on this day, the FDA announced a warning regarding electronic power morcellation. Many hospitals and hospital systems throughout the country issued bans on electronic power morcellation, leading to needless open laparotomy procedures and thus, introducing prolonged recovery times and increased risk.
At a time when the recent introduction of barbed suture had made both closure of the vaginal cuff at time of hysterectomy and repair of the hysterotomy at myomectomy easier and faster, the gynecologic laparoscopist was taking a step backward. The FDA based this decision and a subsequent boxed warning – issued in November 2014 – on a small number of studies showing potential upstaging of leiomyosarcoma post electronic power morcellation. Interestingly, many of the morcellation procedures cited did not use power morcellation. Furthermore, a more comprehensive meta-analysis by Elizabeth A. Pritts and colleagues, showed a far lower risk than suggested by the FDA (Gynecol Surg. 2015;12[3]:165-77).
Recently, an article by William Parker and colleagues recommended that the FDA reverse its position (Obstet Gynecol. 2016 Jan;127[1]:18-22). Many believe that ultimately, the solution will be morcellation in a containment bag, which I and my colleagues have been performing in virtually every power morcellation procedure since May 2014. During this current power morcellation controversy, the Master Class in Gynecologic Surgery has continued to update its readers with three different articles related to the subject.
And in conclusion
Without a doubt, the past 50 years of gynecologic surgery has been a time of unparalleled innovation with occasional controversy thrown in. Ob.Gyn. News and more recently, the Master Class in Gynecologic Surgery, has had a major leadership role in bringing this profound ingenuity to the gynecology community by introducing this explosion of surgical creativity to its readers.
And what will the next 50 years bring? I believe we will continue to see tremendous advancements in minimally invasive gynecologic surgery. There will be a definite impact of costs on the marketplace. Thus, many of the minor minimally invasive procedures currently performed in the hospital or surgery center will be brought into office settings. In addition, secondary to reimbursement, the more complex cases will be carried out by fewer gynecologic surgeons who have undergone more intense training in pelvic surgery and who can perform these cases more efficiently and with fewer complications. Our ability to perform surgery and what type of procedures we do will not only be based on randomized, controlled trials, but big data collection as well.
Dr. Miller is clinical associate professor at the University of Illinois at Chicago, and past president of the AAGL and the International Society for Gynecologic Endoscopy (ISGE). He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in private practice in Naperville and Schaumburg, Ill.; director of minimally invasive gynecologic surgery and the director of the AAGL/Society of Reproductive Surgery fellowship in minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill.; and the medical editor of this column, Master Class. Dr. Miller is a consultant and is on the speakers bureau for Ethicon.