User login
High-grade cervical dysplasia in pregnancy
Cervical intraepithelial neoplasia (CIN) describes a precancerous lesion of the squamous epithelium of the ectocervix. The cervical cancer screening paradigm in the United States begins with collection of cervical cytology with a Pap smear, frequently in conjunction with human papillomavirus testing. Abnormalities will frequently lead to colposcopy with directed biopsy, which can result in a diagnosis of CIN. There are different grades of severity within CIN, which aids in making treatment recommendations.
Pregnancy is a convenient time to capture women for cervical cancer screening, given the increased contact with health care providers. Routine guidelines should be followed for screening women who are pregnant, as collection of cervical cytology and human papillomavirus (HPV) cotesting is safe.
In women who have been found to have abnormal cytology, CIN or malignancy has been identified in up to 19% of cases (Am J Obstet Gynecol. 2004 Jul;191[1]:105-13). High-grade lesions identified in pregnant women create a unique management dilemma.
Terminology
The Bethesda system describes colposcopic abnormalities as CIN and divides premalignant lesions into grades from 1 to 3 with the highest grade representing more worrisome lesions. CIN2 has been found to have poor reproducibility and likely represents a mix of low- and high-grade lesions. In addition, there is concern that HPV-associated lesions of the lower anogenital tract have incongruent terminology among different specialties that may not accurately represent the current understanding of HPV pathogenesis.
In 2012, the Lower Anogenital Squamous Terminology (LAST) project of the College of American Pathologists and the American Society for Colposcopy and Cervical Pathology (ASCCP) advocated for consistent terminology across all lower anogenital tract lesions with HPV, including CIN (Int J Gynecol Pathol. 2013 Jan;32[1]:76-115).
With this new terminology, CIN1 is referred to as low-grade squamous intraepithelial lesion (LSIL). CIN2 is characterized by its p16 immunostaining; lesions that are p16 negative are considered LSIL, while those that are positive are considered HSIL (high-grade squamous intraepithelial lesion). While this staining is not universally performed, physicians will start seeing p16 staining results with increasing frequency on their cervical biopsies. CIN3 lesions are referred to as HSIL.
Given the current understanding of HPV-mediated disease, and a commitment to represent the most up-to-date information, the LAST project terminology of HSIL to represent previously identified CIN2 and CIN3 lesions will be used for the remainder of this text.
Diagnosis
There is little data on the natural history of HSIL diagnosed after colposcopy, as most women get some form of therapy. The information that is available suggests that in patients with untreated HSIL, the cumulative incidence of malignancy is as high as 30% at 30 years (Lancet Oncol. 2008 May;9[5]:425-34). Treatment recommendations for excision are aimed at addressing this alarming number; however, care must be individualized, especially in the setting of pregnancy.
If abnormal cervical cytology is obtained on routine screening, appropriate patients should be referred for colposcopic exam. Physicians performing colposcopy should be familiar with the physiologic effects of pregnancy that can obscure the exam, including the increased cervical mucus production, prominence of endocervical glands, and increased vascularity.
Colposcopic-directed ectocervical biopsies have been found to be safe in pregnancy, and these women should be provided the same care as those who are not pregnant (Obstet Gynecol. 1993 Jun;81[6]:915-8). Endocervical sampling and endometrial sampling should not be performed, however, and physicians should remain dedicated to checking pregnancy tests prior to colposcopy.
HSIL cytology should prompt a biopsy in pregnancy; a decision to skip the biopsy and perform an excisional procedure in this setting is not recommended regardless of patient or gestational age. If LSIL (CIN1) is noted on biopsy, reevaluation post partum should be strongly considered, unless a suspicious lesion was felt to be inadequately biopsied.
Management
Managing HSIL in pregnancy focuses on diagnosis and excluding malignancy, while treatment can be reserved for the postpartum period. When choosing a management option, consider individual patient factors such as colposcopic appearance of the lesion, gestational age, and access to health care.
If HSIL is noted on colposcopic-directed biopsy, consider one of several options. The most conservative approach is reevaluation with cytology and colposcopy 6 weeks post partum. This is an option for patients who do not have a colposcopic exam that was concerning for an invasive lesion, were able to be adequately biopsied, and will reliably return for follow-up. Many physicians feel more comfortable with repeat cytology and colposcopy in 3 months from the original biopsy. The most aggressive management would include an excisional procedure during pregnancy.
There are varying rates of regression of biopsy-proven HSIL in pregnancy ranging from 34% to 70% (Obstet Gynecol. 1999 Mar;93[3]:359-62; Acta Obstet Gynecol Scand. 2006;85[9]:1134-7; Reprod Sci. 2009 Nov;16[11]:1034-9). Out of more than 200 patients across these three studies, just two patients were diagnosed with an invasive lesion post partum. Given the low likelihood of progression during pregnancy and the high rate of regression, an excisional procedure should be considered only in cases where there is concern about invasive carcinoma.
In cases where an invasive lesion is suspected, consider an an excisional procedure. While there is some evidence that performing a laser excisional procedure early in pregnancy (18 weeks and earlier) can be safely done, that is not the most common management strategy in the United States (Tumori. 1998 Sep-Oct;84[5]:567-70; Int J Gynecol Cancer. 2007 Jan-Feb;17[1]:127-31). In this circumstance, referral to a gynecologic oncologist is warranted where consideration can be made for performing a cold knife conization. Physicians should be aware of the increased risk of bleeding with this procedure in pregnancy and the potential for preterm birth. There is little literature to guide counseling regarding these risks, and the decision to perform an excisional procedure should be made with a multidisciplinary team (Arch Gynecol Obstet. 2016 Jan 4. doi: 10.1007/s00404-015-3980-y).
The see-and-treat paradigm is not recommended in pregnancy. Those patients with poor follow-up should still undergo colposcopic-directed biopsies prior to any excisional procedure.
Treatment recommendations in pregnancy should be made on the basis of careful consideration of individual patient factors, with strong consideration of repeat testing with cytology and colposcopy prior to an excision procedure.
Dr. Sullivan is a fellow in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. Dr. Gehrig is professor and director of gynecologic oncology at the university. Dr. Sullivan and Dr. Gehrig reported having no relevant financial disclosures. Email them at obnews@frontlinemedcom.com.
Cervical intraepithelial neoplasia (CIN) describes a precancerous lesion of the squamous epithelium of the ectocervix. The cervical cancer screening paradigm in the United States begins with collection of cervical cytology with a Pap smear, frequently in conjunction with human papillomavirus testing. Abnormalities will frequently lead to colposcopy with directed biopsy, which can result in a diagnosis of CIN. There are different grades of severity within CIN, which aids in making treatment recommendations.
Pregnancy is a convenient time to capture women for cervical cancer screening, given the increased contact with health care providers. Routine guidelines should be followed for screening women who are pregnant, as collection of cervical cytology and human papillomavirus (HPV) cotesting is safe.
In women who have been found to have abnormal cytology, CIN or malignancy has been identified in up to 19% of cases (Am J Obstet Gynecol. 2004 Jul;191[1]:105-13). High-grade lesions identified in pregnant women create a unique management dilemma.
Terminology
The Bethesda system describes colposcopic abnormalities as CIN and divides premalignant lesions into grades from 1 to 3 with the highest grade representing more worrisome lesions. CIN2 has been found to have poor reproducibility and likely represents a mix of low- and high-grade lesions. In addition, there is concern that HPV-associated lesions of the lower anogenital tract have incongruent terminology among different specialties that may not accurately represent the current understanding of HPV pathogenesis.
In 2012, the Lower Anogenital Squamous Terminology (LAST) project of the College of American Pathologists and the American Society for Colposcopy and Cervical Pathology (ASCCP) advocated for consistent terminology across all lower anogenital tract lesions with HPV, including CIN (Int J Gynecol Pathol. 2013 Jan;32[1]:76-115).
With this new terminology, CIN1 is referred to as low-grade squamous intraepithelial lesion (LSIL). CIN2 is characterized by its p16 immunostaining; lesions that are p16 negative are considered LSIL, while those that are positive are considered HSIL (high-grade squamous intraepithelial lesion). While this staining is not universally performed, physicians will start seeing p16 staining results with increasing frequency on their cervical biopsies. CIN3 lesions are referred to as HSIL.
Given the current understanding of HPV-mediated disease, and a commitment to represent the most up-to-date information, the LAST project terminology of HSIL to represent previously identified CIN2 and CIN3 lesions will be used for the remainder of this text.
Diagnosis
There is little data on the natural history of HSIL diagnosed after colposcopy, as most women get some form of therapy. The information that is available suggests that in patients with untreated HSIL, the cumulative incidence of malignancy is as high as 30% at 30 years (Lancet Oncol. 2008 May;9[5]:425-34). Treatment recommendations for excision are aimed at addressing this alarming number; however, care must be individualized, especially in the setting of pregnancy.
If abnormal cervical cytology is obtained on routine screening, appropriate patients should be referred for colposcopic exam. Physicians performing colposcopy should be familiar with the physiologic effects of pregnancy that can obscure the exam, including the increased cervical mucus production, prominence of endocervical glands, and increased vascularity.
Colposcopic-directed ectocervical biopsies have been found to be safe in pregnancy, and these women should be provided the same care as those who are not pregnant (Obstet Gynecol. 1993 Jun;81[6]:915-8). Endocervical sampling and endometrial sampling should not be performed, however, and physicians should remain dedicated to checking pregnancy tests prior to colposcopy.
HSIL cytology should prompt a biopsy in pregnancy; a decision to skip the biopsy and perform an excisional procedure in this setting is not recommended regardless of patient or gestational age. If LSIL (CIN1) is noted on biopsy, reevaluation post partum should be strongly considered, unless a suspicious lesion was felt to be inadequately biopsied.
Management
Managing HSIL in pregnancy focuses on diagnosis and excluding malignancy, while treatment can be reserved for the postpartum period. When choosing a management option, consider individual patient factors such as colposcopic appearance of the lesion, gestational age, and access to health care.
If HSIL is noted on colposcopic-directed biopsy, consider one of several options. The most conservative approach is reevaluation with cytology and colposcopy 6 weeks post partum. This is an option for patients who do not have a colposcopic exam that was concerning for an invasive lesion, were able to be adequately biopsied, and will reliably return for follow-up. Many physicians feel more comfortable with repeat cytology and colposcopy in 3 months from the original biopsy. The most aggressive management would include an excisional procedure during pregnancy.
There are varying rates of regression of biopsy-proven HSIL in pregnancy ranging from 34% to 70% (Obstet Gynecol. 1999 Mar;93[3]:359-62; Acta Obstet Gynecol Scand. 2006;85[9]:1134-7; Reprod Sci. 2009 Nov;16[11]:1034-9). Out of more than 200 patients across these three studies, just two patients were diagnosed with an invasive lesion post partum. Given the low likelihood of progression during pregnancy and the high rate of regression, an excisional procedure should be considered only in cases where there is concern about invasive carcinoma.
In cases where an invasive lesion is suspected, consider an an excisional procedure. While there is some evidence that performing a laser excisional procedure early in pregnancy (18 weeks and earlier) can be safely done, that is not the most common management strategy in the United States (Tumori. 1998 Sep-Oct;84[5]:567-70; Int J Gynecol Cancer. 2007 Jan-Feb;17[1]:127-31). In this circumstance, referral to a gynecologic oncologist is warranted where consideration can be made for performing a cold knife conization. Physicians should be aware of the increased risk of bleeding with this procedure in pregnancy and the potential for preterm birth. There is little literature to guide counseling regarding these risks, and the decision to perform an excisional procedure should be made with a multidisciplinary team (Arch Gynecol Obstet. 2016 Jan 4. doi: 10.1007/s00404-015-3980-y).
The see-and-treat paradigm is not recommended in pregnancy. Those patients with poor follow-up should still undergo colposcopic-directed biopsies prior to any excisional procedure.
Treatment recommendations in pregnancy should be made on the basis of careful consideration of individual patient factors, with strong consideration of repeat testing with cytology and colposcopy prior to an excision procedure.
Dr. Sullivan is a fellow in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. Dr. Gehrig is professor and director of gynecologic oncology at the university. Dr. Sullivan and Dr. Gehrig reported having no relevant financial disclosures. Email them at obnews@frontlinemedcom.com.
Cervical intraepithelial neoplasia (CIN) describes a precancerous lesion of the squamous epithelium of the ectocervix. The cervical cancer screening paradigm in the United States begins with collection of cervical cytology with a Pap smear, frequently in conjunction with human papillomavirus testing. Abnormalities will frequently lead to colposcopy with directed biopsy, which can result in a diagnosis of CIN. There are different grades of severity within CIN, which aids in making treatment recommendations.
Pregnancy is a convenient time to capture women for cervical cancer screening, given the increased contact with health care providers. Routine guidelines should be followed for screening women who are pregnant, as collection of cervical cytology and human papillomavirus (HPV) cotesting is safe.
In women who have been found to have abnormal cytology, CIN or malignancy has been identified in up to 19% of cases (Am J Obstet Gynecol. 2004 Jul;191[1]:105-13). High-grade lesions identified in pregnant women create a unique management dilemma.
Terminology
The Bethesda system describes colposcopic abnormalities as CIN and divides premalignant lesions into grades from 1 to 3 with the highest grade representing more worrisome lesions. CIN2 has been found to have poor reproducibility and likely represents a mix of low- and high-grade lesions. In addition, there is concern that HPV-associated lesions of the lower anogenital tract have incongruent terminology among different specialties that may not accurately represent the current understanding of HPV pathogenesis.
In 2012, the Lower Anogenital Squamous Terminology (LAST) project of the College of American Pathologists and the American Society for Colposcopy and Cervical Pathology (ASCCP) advocated for consistent terminology across all lower anogenital tract lesions with HPV, including CIN (Int J Gynecol Pathol. 2013 Jan;32[1]:76-115).
With this new terminology, CIN1 is referred to as low-grade squamous intraepithelial lesion (LSIL). CIN2 is characterized by its p16 immunostaining; lesions that are p16 negative are considered LSIL, while those that are positive are considered HSIL (high-grade squamous intraepithelial lesion). While this staining is not universally performed, physicians will start seeing p16 staining results with increasing frequency on their cervical biopsies. CIN3 lesions are referred to as HSIL.
Given the current understanding of HPV-mediated disease, and a commitment to represent the most up-to-date information, the LAST project terminology of HSIL to represent previously identified CIN2 and CIN3 lesions will be used for the remainder of this text.
Diagnosis
There is little data on the natural history of HSIL diagnosed after colposcopy, as most women get some form of therapy. The information that is available suggests that in patients with untreated HSIL, the cumulative incidence of malignancy is as high as 30% at 30 years (Lancet Oncol. 2008 May;9[5]:425-34). Treatment recommendations for excision are aimed at addressing this alarming number; however, care must be individualized, especially in the setting of pregnancy.
If abnormal cervical cytology is obtained on routine screening, appropriate patients should be referred for colposcopic exam. Physicians performing colposcopy should be familiar with the physiologic effects of pregnancy that can obscure the exam, including the increased cervical mucus production, prominence of endocervical glands, and increased vascularity.
Colposcopic-directed ectocervical biopsies have been found to be safe in pregnancy, and these women should be provided the same care as those who are not pregnant (Obstet Gynecol. 1993 Jun;81[6]:915-8). Endocervical sampling and endometrial sampling should not be performed, however, and physicians should remain dedicated to checking pregnancy tests prior to colposcopy.
HSIL cytology should prompt a biopsy in pregnancy; a decision to skip the biopsy and perform an excisional procedure in this setting is not recommended regardless of patient or gestational age. If LSIL (CIN1) is noted on biopsy, reevaluation post partum should be strongly considered, unless a suspicious lesion was felt to be inadequately biopsied.
Management
Managing HSIL in pregnancy focuses on diagnosis and excluding malignancy, while treatment can be reserved for the postpartum period. When choosing a management option, consider individual patient factors such as colposcopic appearance of the lesion, gestational age, and access to health care.
If HSIL is noted on colposcopic-directed biopsy, consider one of several options. The most conservative approach is reevaluation with cytology and colposcopy 6 weeks post partum. This is an option for patients who do not have a colposcopic exam that was concerning for an invasive lesion, were able to be adequately biopsied, and will reliably return for follow-up. Many physicians feel more comfortable with repeat cytology and colposcopy in 3 months from the original biopsy. The most aggressive management would include an excisional procedure during pregnancy.
There are varying rates of regression of biopsy-proven HSIL in pregnancy ranging from 34% to 70% (Obstet Gynecol. 1999 Mar;93[3]:359-62; Acta Obstet Gynecol Scand. 2006;85[9]:1134-7; Reprod Sci. 2009 Nov;16[11]:1034-9). Out of more than 200 patients across these three studies, just two patients were diagnosed with an invasive lesion post partum. Given the low likelihood of progression during pregnancy and the high rate of regression, an excisional procedure should be considered only in cases where there is concern about invasive carcinoma.
In cases where an invasive lesion is suspected, consider an an excisional procedure. While there is some evidence that performing a laser excisional procedure early in pregnancy (18 weeks and earlier) can be safely done, that is not the most common management strategy in the United States (Tumori. 1998 Sep-Oct;84[5]:567-70; Int J Gynecol Cancer. 2007 Jan-Feb;17[1]:127-31). In this circumstance, referral to a gynecologic oncologist is warranted where consideration can be made for performing a cold knife conization. Physicians should be aware of the increased risk of bleeding with this procedure in pregnancy and the potential for preterm birth. There is little literature to guide counseling regarding these risks, and the decision to perform an excisional procedure should be made with a multidisciplinary team (Arch Gynecol Obstet. 2016 Jan 4. doi: 10.1007/s00404-015-3980-y).
The see-and-treat paradigm is not recommended in pregnancy. Those patients with poor follow-up should still undergo colposcopic-directed biopsies prior to any excisional procedure.
Treatment recommendations in pregnancy should be made on the basis of careful consideration of individual patient factors, with strong consideration of repeat testing with cytology and colposcopy prior to an excision procedure.
Dr. Sullivan is a fellow in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. Dr. Gehrig is professor and director of gynecologic oncology at the university. Dr. Sullivan and Dr. Gehrig reported having no relevant financial disclosures. Email them at obnews@frontlinemedcom.com.
A trip through the history of gynecologic oncology
The subspecialty of gynecologic oncology was formalized less than 50 years ago with the creation of the Society of Gynecologic Oncology and subspecialty training and board certification. The formation of the Gynecologic Oncology Group (GOG) – and the many clinical trials spearheaded by that group – has further advanced evidence-based treatments, resulting in improved survival outcomes, quality of life, and preventive strategies.
While it is not possible to provide a comprehensive and exhaustive review of all of the advances, we hope to highlight many of the notable advances in this article.Cervical cancer
Cervical cancer is the fourth most common cancer in women worldwide with 528,000 new cases in 2012. The majority of cervical cancer cases are caused by infection with human papillomavirus (HPV). While the standard therapies for cervical cancer have been long established (radical hysterectomy for stage I and radiation therapy for locally advanced disease), one of the most significant advances in the past 50 years was the addition of radiation-sensitizing chemotherapy (cisplatin) administered concurrently with radiation therapy.
In randomized trials in both early and advanced cervical cancer, the risk of death was reduced by 30%-50%. These studies changed the paradigm for the treatment of cervical cancer (N Engl J Med. 1999 Apr 15;340[15]:1137-43; N Engl J Med. 1999 Apr 15;340[15]:1144-53; J Clin Oncol. 2000 Apr;18[8]:1606-13).
Future studies evaluating biologic adjuncts or additional chemotherapy are currently underway or awaiting data maturation.
The American Society of Clinical Oncology (ASCO) highlighted the “Top 5 advances in 50 years of Modern Oncology” in 2014, and second on the list was the approval of the HPV vaccine to prevent cervical cancer. Vaccines have been developed that can protect against types 2, 4 or 9 of HPV. In a 2014 study, depending on vaccination coverage, the relative number of cervical cancer cases avoided was 34% in Africa, 27% for America, 26% for Asia, 21% for Europe, and worldwide was estimated at 27% (Vaccine. 2014 Feb 3;32[6]:733-9).
While the benefit from HPV vaccination has been proven, in the United States, only about a third of eligible girls and women have been vaccinated. Efforts should focus on expanding vaccination penetration to eligible girls, boys, women, and men.
Endometrial cancer
Endometrial cancer is the most common gynecologic malignancy in the United States with an estimated 54,870 cases and 10,170 deaths annually. Notable advances in the management of women with endometrial cancer have arisen because of a better understanding that there are two types of endometrial cancer – type I and type II.
The type I endometrial cancers tend to be associated with lower stage of disease at the time of diagnosis and fewer recurrences, while type II endometrial cancer is associated with worse outcomes.
Tailoring the surgical approaches and adjuvant therapy for women with endometrial cancer has led to improved outcomes. The GOG conducted a large prospective randomized trial of laparotomy versus laparoscopic surgical staging for women with clinical early-stage endometrial cancer (LAP2). Laparoscopy was associated with improved perioperative outcomes and was found to be noninferior to laparotomy with regards to survival outcomes (J Clin Oncol. 2012 Mar 1;30[7]:695-700). Therefore, minimally invasive surgery has become widely accepted for the surgical staging of women with endometrial cancer.
Appropriate surgical staging allows for tailoring of postoperative adjuvant therapy. The current evidence suggests that vaginal brachytherapy should be the adjuvant treatment of choice over whole pelvic radiation in women with early-stage endometrial cancer (Lancet. 2010 Mar 6;375[9717]:816-23). Studies are underway to evaluate the role of both adjuvant radiation and chemotherapy in women with early-stage type II endometrial cancer who are felt to be at high risk for recurrent disease, as well as how to improve on the therapeutic options for women with advanced or recurrent disease.
Ovarian cancer
Epithelial ovarian cancer is the most deadly gynecologic malignancy in the United States with 21,290 cases and 14,180 deaths in 2015. The concept of ovarian tumor debulking was first described by Dr. Joe Meigs in 1934, but did not gain traction until the mid-1970s when Dr. C. Thomas Griffiths published his work (Natl Cancer Inst Monogr. 1975 Oct;42:101-4).
While there are no randomized trials proving that surgical cytoreduction improves overall survival, most retrospective studies support this concept. In 2009, Chi et al. showed improved median survival in women with ovarian cancer based on the increased percentage of women who underwent optimal cytoreduction (Gynecol Oncol. 2009 Jul;114[1]:26-31). This has led to modifications of surgical techniques and surgical goals with an effort to maximally cytoreduce all of the visible disease.
While initial surgical debulking is the goal, there are circumstances when a different approach may be indicated. Vergote et al. conducted a prospective randomized trial of 670 women with advanced ovarian cancer. In this study, neoadjuvant chemotherapy followed by interval debulking was not inferior to primary debulking followed by chemotherapy with regards to progression-free survival and overall survival. However, initial surgery was associated with increased surgical complications and perioperative mortality as compared with interval surgery. Therefore, in women who are not felt to be candidates for optimal cytoreduction, neoadjuvant chemotherapy followed by interval surgery may be an appropriate treatment strategy (N Engl J Med. 2010 Sep 2;363[10]:943-53.).
There have been several notable advances and a series of randomized trials – predominately conducted by the GOG – that have resulted in improved overall survival and progression-free interval in women with ovarian cancer. However, none are as significant as the discovery of paclitaxel and platinum-based chemotherapy (cisplatin and carboplatin).
In 1962, samples of the Pacific Yew’s bark were collected and, 2 years later, the extracts from this bark were found to have cytotoxic activity. There were initial difficulties suspending the drug in solution; however, ultimately a formulation in ethanol, cremophor, and saline was found to be effective. In 1984, the National Cancer Institute began clinical trials of paclitaxel and it was found to be highly effective in ovarian cancer. In 1992, it was approved for the treatment of ovarian cancer.
Cisplatin was approved in 1978. Carboplatin entered clinical trials in 1982 and was approved for women with recurrent ovarian cancer in 1989.
There were a series of trials beginning in the late 1980s that established the role of platinum agents and led us to GOG 111. This trial evaluated cisplatin with either cyclophosphamide or paclitaxel. The paclitaxel combination was superior and in 2003 two trials were published that solidified carboplatin and paclitaxel as the cornerstone in the treatment of women with ovarian cancer (J Clin Oncol. 2003 Sep 1;21[17]:3194-200; J Natl Cancer Inst. 2003 Sep 3;95[17]:1320-9).
What has most recently been debated is the route and schedule for both paclitaxel and the platinum agents. In January 2006, the National Cancer Institute released a Clinical Announcement regarding the role of intraperitoneal (IP) chemotherapy for the treatment of women with optimally debulked ovarian cancer. Of the six trials included in the announcement, four trials showed a benefit for progression-free survival and five studies showed an improvement in overall survival. Armstrong et al (GOG 172) showed a 16-month improvement in overall survival with intravenous (IV) paclitaxel, IP cisplatin, and IP paclitaxel. IP chemotherapy has not been universally embraced by physicians and patients in part because of its toxicity, treatment schedule, and the fact that no IP regimen has been compared with the current standard of IV carboplatin and paclitaxel (N Engl J Med. 2006 Jan 5;354[1]:34-43).
While there have been improvements in 5-year survival over time, most women with advanced ovarian cancer will undergo additional chemotherapy in order to achieve subsequent remissions or maintain stability of disease. Other drugs that have Food and Drug Administration approval in the setting of recurrent ovarian cancer include topotecan, liposomal doxorubicin, gemcitabine, bevacizumab, altretamine, carboplatin, cisplatin, cyclophosphamide, and melphalan. Olaparib was recently approved as monotherapy in women with a germline BRCA-mutation who had received three or more prior lines of chemotherapy.
Minimally invasive surgery
Over the last 30 years, minimally invasive surgery (MIS) in gynecologic oncology, particularly for endometrial cancer, has gone from a niche procedure to the standard of care. The introduction of laparoscopy into gynecologic oncology started in the early 1990s. In a series of 59 women undergoing laparoscopy for endometrial cancer, Childers et al. demonstrated feasibility of the technique and low laparotomy conversion rates (Gynecol Oncol. 1993 Oct;51[1]:33-8.). The GOG trial, LAP2, supported the equivalent oncologic outcomes of MIS versus laparotomy for the treatment of endometrial cancer. While many surgeons and centers offered laparoscopic surgery, there were issues with the learning curve that limited its widespread use.
In 2005, the FDA approval of the robotic platform for gynecologic surgery resulted in at least a doubling of the proportion of endometrial cancer patients treated with MIS (Int J Med Robot. 2009 Dec;5[4]:392-7.). In 2012, the Society of Gynecologic Oncology published a consensus statement regarding robotic-assisted surgery in gynecologic oncology (Gynecol Oncol. 2012 Feb;124[2]:180-4.). This review highlights the advantages of the robotics platform with regards to expanding MIS to women with cervical and ovarian cancer; the improvements in outcomes in the obese woman with endometrial cancer; and that the learning curve for robotic surgery is shorter than for traditional laparoscopy. Issues requiring further research include cost analysis as the cost of the new technology decreases, and opportunities for improvement in patient and physician quality of life.
Sentinel node mapping
The rationale for sentinel node mapping is that if one or more sentinel lymph nodes is/are negative for malignancy, then the other regional lymph nodes will also be negative. This would thereby avoid the need for a complete lymph node dissection and its resultant complications, including chronic lymphedema. Much of the work pioneering this strategy has been in breast cancer and melanoma, but data are rapidly emerging for these techniques in gynecologic malignancies.
Candidates for sentinel lymph node biopsy for vulvar cancer include those with a lesion more than 1mm in depth, a tumor less than 4 cm in size, and no obvious metastatic disease on exam or preoperative imaging. Additionally, recommendations have been made regarding case volume in order to achieve limited numbers of false-negative results and to maintain competency. In the study by Van der Zee et al. of 403 patients (623 groins) who underwent sentinel node procedures, the false-negative rate was 0-2%. The overall survival rate was 97% at 3 years (J Clin Oncol. 2008 Feb 20;26[6]:884-9). However, a more recent data from the Gynecologic Oncology Group (GOG 173) showed a slightly higher false-negative rate of 8% (J Clin Oncol. 2012 Nov 1;30[31]:3786-91). Overall survival data are pending from this study.
While sentinel lymph node mapping for endometrial cancer has been feasible for many years and has been well described, the questioned role of completed lymphadenectomy for early-stage endometrial cancer has led to a resurgence of interest in these techniques. While blue dye and radiolabeled tracer methods have historically been the most popular mapping solutions, the advent of endoscopic near-infrared imaging, with its higher sensitivity and good depth penetration, has added options. Indocyanine green fluorescence can be easily detected during robotic surgery and as experience with these techniques increase, successful mapping and sensitivity will increase.
Genetics
While hereditary cancer syndromes have been recognized for many years, detecting the genetic mutations that may increase an individual’s risk of developing a malignancy were not elucidated until the early 1990s. In gynecologic oncology, the most commonly encountered syndromes involve mutations in BRCA1 and BRCA2 and hereditary non–polyposis colorectal cancer, which causes mutations in DNA mismatch-repair genes and increase the risk of endometrial and ovarian cancer.
The SGO recently published a statement on risk assessment for inherited gynecologic cancer predispositions. In this statement “the evaluation for the presence of a hereditary cancer syndrome enables physicians to provide individualized and quantified assessment of cancer risk, as well as options for tailored screening and preventions strategies that may reduce morbidity associated with the development of malignancy” (Gynecol Oncol. 2015 Jan;136[1]:3-7). Beyond risk-reducing salpingo-oophorectomy, therapeutic strategies targeting patients with germline mutations have been developed (PARP inhibitors in BRCA-mutated women with ovarian cancer).
In August 2015, ASCO released an updated policy statement on genetic and genomic testing for cancer susceptibility and highlighted five key areas: germ-line implications of somatic mutation profiling; multigene panel testing for cancer susceptibility; quality assurance in genetic testing; education for oncology professionals; and access to cancer genetic services.
Antiemetics
Rounding out ASCO’s “Top 5 advances in 50 years of Modern Oncology” was the improvement in patients’ quality of life from supportive therapies, in particular antinausea medications.
Several of the agents commonly used in gynecologic oncology rate high (cisplatin) to moderate (carboplatin, cyclophosphamide, doxorubicin, ifosfamide) with regards to emetogenicity. The advent of 5-HT3 receptor antagonists (for example, ondansetron) has significantly improved the quality of life of patients undergoing cytotoxic chemotherapy. In addition to improving quality of life, the decrease in nausea and vomiting can also decrease life-threatening complications such as dehydration and electrolyte imbalance. Both ASCO and the National Comprehensive Cancer Network both have guidelines for the management of nausea and vomiting in patients undergoing chemotherapy.
Throughout 2016, Ob.Gyn. News will celebrate its 50th anniversary with exclusive articles looking at the evolution of the specialty, including the history of contraception, changes in gynecologic surgery, and the transformation of the well-woman visit. Look for these articles and more special features in the pages of Ob.Gyn. News and online at obgynnews.com.
Dr. Gehrig is professor and director of gynecologic oncology at the University of North Carolina, Chapel Hill. Dr. Clarke-Pearson is the chair and the Robert A. Ross Distinguished Professor of Obstetrics and Gynecology, and a professor in the division of gynecologic oncology at UNC. They reported having no relevant financial disclosures.
The subspecialty of gynecologic oncology was formalized less than 50 years ago with the creation of the Society of Gynecologic Oncology and subspecialty training and board certification. The formation of the Gynecologic Oncology Group (GOG) – and the many clinical trials spearheaded by that group – has further advanced evidence-based treatments, resulting in improved survival outcomes, quality of life, and preventive strategies.
While it is not possible to provide a comprehensive and exhaustive review of all of the advances, we hope to highlight many of the notable advances in this article.Cervical cancer
Cervical cancer is the fourth most common cancer in women worldwide with 528,000 new cases in 2012. The majority of cervical cancer cases are caused by infection with human papillomavirus (HPV). While the standard therapies for cervical cancer have been long established (radical hysterectomy for stage I and radiation therapy for locally advanced disease), one of the most significant advances in the past 50 years was the addition of radiation-sensitizing chemotherapy (cisplatin) administered concurrently with radiation therapy.
In randomized trials in both early and advanced cervical cancer, the risk of death was reduced by 30%-50%. These studies changed the paradigm for the treatment of cervical cancer (N Engl J Med. 1999 Apr 15;340[15]:1137-43; N Engl J Med. 1999 Apr 15;340[15]:1144-53; J Clin Oncol. 2000 Apr;18[8]:1606-13).
Future studies evaluating biologic adjuncts or additional chemotherapy are currently underway or awaiting data maturation.
The American Society of Clinical Oncology (ASCO) highlighted the “Top 5 advances in 50 years of Modern Oncology” in 2014, and second on the list was the approval of the HPV vaccine to prevent cervical cancer. Vaccines have been developed that can protect against types 2, 4 or 9 of HPV. In a 2014 study, depending on vaccination coverage, the relative number of cervical cancer cases avoided was 34% in Africa, 27% for America, 26% for Asia, 21% for Europe, and worldwide was estimated at 27% (Vaccine. 2014 Feb 3;32[6]:733-9).
While the benefit from HPV vaccination has been proven, in the United States, only about a third of eligible girls and women have been vaccinated. Efforts should focus on expanding vaccination penetration to eligible girls, boys, women, and men.
Endometrial cancer
Endometrial cancer is the most common gynecologic malignancy in the United States with an estimated 54,870 cases and 10,170 deaths annually. Notable advances in the management of women with endometrial cancer have arisen because of a better understanding that there are two types of endometrial cancer – type I and type II.
The type I endometrial cancers tend to be associated with lower stage of disease at the time of diagnosis and fewer recurrences, while type II endometrial cancer is associated with worse outcomes.
Tailoring the surgical approaches and adjuvant therapy for women with endometrial cancer has led to improved outcomes. The GOG conducted a large prospective randomized trial of laparotomy versus laparoscopic surgical staging for women with clinical early-stage endometrial cancer (LAP2). Laparoscopy was associated with improved perioperative outcomes and was found to be noninferior to laparotomy with regards to survival outcomes (J Clin Oncol. 2012 Mar 1;30[7]:695-700). Therefore, minimally invasive surgery has become widely accepted for the surgical staging of women with endometrial cancer.
Appropriate surgical staging allows for tailoring of postoperative adjuvant therapy. The current evidence suggests that vaginal brachytherapy should be the adjuvant treatment of choice over whole pelvic radiation in women with early-stage endometrial cancer (Lancet. 2010 Mar 6;375[9717]:816-23). Studies are underway to evaluate the role of both adjuvant radiation and chemotherapy in women with early-stage type II endometrial cancer who are felt to be at high risk for recurrent disease, as well as how to improve on the therapeutic options for women with advanced or recurrent disease.
Ovarian cancer
Epithelial ovarian cancer is the most deadly gynecologic malignancy in the United States with 21,290 cases and 14,180 deaths in 2015. The concept of ovarian tumor debulking was first described by Dr. Joe Meigs in 1934, but did not gain traction until the mid-1970s when Dr. C. Thomas Griffiths published his work (Natl Cancer Inst Monogr. 1975 Oct;42:101-4).
While there are no randomized trials proving that surgical cytoreduction improves overall survival, most retrospective studies support this concept. In 2009, Chi et al. showed improved median survival in women with ovarian cancer based on the increased percentage of women who underwent optimal cytoreduction (Gynecol Oncol. 2009 Jul;114[1]:26-31). This has led to modifications of surgical techniques and surgical goals with an effort to maximally cytoreduce all of the visible disease.
While initial surgical debulking is the goal, there are circumstances when a different approach may be indicated. Vergote et al. conducted a prospective randomized trial of 670 women with advanced ovarian cancer. In this study, neoadjuvant chemotherapy followed by interval debulking was not inferior to primary debulking followed by chemotherapy with regards to progression-free survival and overall survival. However, initial surgery was associated with increased surgical complications and perioperative mortality as compared with interval surgery. Therefore, in women who are not felt to be candidates for optimal cytoreduction, neoadjuvant chemotherapy followed by interval surgery may be an appropriate treatment strategy (N Engl J Med. 2010 Sep 2;363[10]:943-53.).
There have been several notable advances and a series of randomized trials – predominately conducted by the GOG – that have resulted in improved overall survival and progression-free interval in women with ovarian cancer. However, none are as significant as the discovery of paclitaxel and platinum-based chemotherapy (cisplatin and carboplatin).
In 1962, samples of the Pacific Yew’s bark were collected and, 2 years later, the extracts from this bark were found to have cytotoxic activity. There were initial difficulties suspending the drug in solution; however, ultimately a formulation in ethanol, cremophor, and saline was found to be effective. In 1984, the National Cancer Institute began clinical trials of paclitaxel and it was found to be highly effective in ovarian cancer. In 1992, it was approved for the treatment of ovarian cancer.
Cisplatin was approved in 1978. Carboplatin entered clinical trials in 1982 and was approved for women with recurrent ovarian cancer in 1989.
There were a series of trials beginning in the late 1980s that established the role of platinum agents and led us to GOG 111. This trial evaluated cisplatin with either cyclophosphamide or paclitaxel. The paclitaxel combination was superior and in 2003 two trials were published that solidified carboplatin and paclitaxel as the cornerstone in the treatment of women with ovarian cancer (J Clin Oncol. 2003 Sep 1;21[17]:3194-200; J Natl Cancer Inst. 2003 Sep 3;95[17]:1320-9).
What has most recently been debated is the route and schedule for both paclitaxel and the platinum agents. In January 2006, the National Cancer Institute released a Clinical Announcement regarding the role of intraperitoneal (IP) chemotherapy for the treatment of women with optimally debulked ovarian cancer. Of the six trials included in the announcement, four trials showed a benefit for progression-free survival and five studies showed an improvement in overall survival. Armstrong et al (GOG 172) showed a 16-month improvement in overall survival with intravenous (IV) paclitaxel, IP cisplatin, and IP paclitaxel. IP chemotherapy has not been universally embraced by physicians and patients in part because of its toxicity, treatment schedule, and the fact that no IP regimen has been compared with the current standard of IV carboplatin and paclitaxel (N Engl J Med. 2006 Jan 5;354[1]:34-43).
While there have been improvements in 5-year survival over time, most women with advanced ovarian cancer will undergo additional chemotherapy in order to achieve subsequent remissions or maintain stability of disease. Other drugs that have Food and Drug Administration approval in the setting of recurrent ovarian cancer include topotecan, liposomal doxorubicin, gemcitabine, bevacizumab, altretamine, carboplatin, cisplatin, cyclophosphamide, and melphalan. Olaparib was recently approved as monotherapy in women with a germline BRCA-mutation who had received three or more prior lines of chemotherapy.
Minimally invasive surgery
Over the last 30 years, minimally invasive surgery (MIS) in gynecologic oncology, particularly for endometrial cancer, has gone from a niche procedure to the standard of care. The introduction of laparoscopy into gynecologic oncology started in the early 1990s. In a series of 59 women undergoing laparoscopy for endometrial cancer, Childers et al. demonstrated feasibility of the technique and low laparotomy conversion rates (Gynecol Oncol. 1993 Oct;51[1]:33-8.). The GOG trial, LAP2, supported the equivalent oncologic outcomes of MIS versus laparotomy for the treatment of endometrial cancer. While many surgeons and centers offered laparoscopic surgery, there were issues with the learning curve that limited its widespread use.
In 2005, the FDA approval of the robotic platform for gynecologic surgery resulted in at least a doubling of the proportion of endometrial cancer patients treated with MIS (Int J Med Robot. 2009 Dec;5[4]:392-7.). In 2012, the Society of Gynecologic Oncology published a consensus statement regarding robotic-assisted surgery in gynecologic oncology (Gynecol Oncol. 2012 Feb;124[2]:180-4.). This review highlights the advantages of the robotics platform with regards to expanding MIS to women with cervical and ovarian cancer; the improvements in outcomes in the obese woman with endometrial cancer; and that the learning curve for robotic surgery is shorter than for traditional laparoscopy. Issues requiring further research include cost analysis as the cost of the new technology decreases, and opportunities for improvement in patient and physician quality of life.
Sentinel node mapping
The rationale for sentinel node mapping is that if one or more sentinel lymph nodes is/are negative for malignancy, then the other regional lymph nodes will also be negative. This would thereby avoid the need for a complete lymph node dissection and its resultant complications, including chronic lymphedema. Much of the work pioneering this strategy has been in breast cancer and melanoma, but data are rapidly emerging for these techniques in gynecologic malignancies.
Candidates for sentinel lymph node biopsy for vulvar cancer include those with a lesion more than 1mm in depth, a tumor less than 4 cm in size, and no obvious metastatic disease on exam or preoperative imaging. Additionally, recommendations have been made regarding case volume in order to achieve limited numbers of false-negative results and to maintain competency. In the study by Van der Zee et al. of 403 patients (623 groins) who underwent sentinel node procedures, the false-negative rate was 0-2%. The overall survival rate was 97% at 3 years (J Clin Oncol. 2008 Feb 20;26[6]:884-9). However, a more recent data from the Gynecologic Oncology Group (GOG 173) showed a slightly higher false-negative rate of 8% (J Clin Oncol. 2012 Nov 1;30[31]:3786-91). Overall survival data are pending from this study.
While sentinel lymph node mapping for endometrial cancer has been feasible for many years and has been well described, the questioned role of completed lymphadenectomy for early-stage endometrial cancer has led to a resurgence of interest in these techniques. While blue dye and radiolabeled tracer methods have historically been the most popular mapping solutions, the advent of endoscopic near-infrared imaging, with its higher sensitivity and good depth penetration, has added options. Indocyanine green fluorescence can be easily detected during robotic surgery and as experience with these techniques increase, successful mapping and sensitivity will increase.
Genetics
While hereditary cancer syndromes have been recognized for many years, detecting the genetic mutations that may increase an individual’s risk of developing a malignancy were not elucidated until the early 1990s. In gynecologic oncology, the most commonly encountered syndromes involve mutations in BRCA1 and BRCA2 and hereditary non–polyposis colorectal cancer, which causes mutations in DNA mismatch-repair genes and increase the risk of endometrial and ovarian cancer.
The SGO recently published a statement on risk assessment for inherited gynecologic cancer predispositions. In this statement “the evaluation for the presence of a hereditary cancer syndrome enables physicians to provide individualized and quantified assessment of cancer risk, as well as options for tailored screening and preventions strategies that may reduce morbidity associated with the development of malignancy” (Gynecol Oncol. 2015 Jan;136[1]:3-7). Beyond risk-reducing salpingo-oophorectomy, therapeutic strategies targeting patients with germline mutations have been developed (PARP inhibitors in BRCA-mutated women with ovarian cancer).
In August 2015, ASCO released an updated policy statement on genetic and genomic testing for cancer susceptibility and highlighted five key areas: germ-line implications of somatic mutation profiling; multigene panel testing for cancer susceptibility; quality assurance in genetic testing; education for oncology professionals; and access to cancer genetic services.
Antiemetics
Rounding out ASCO’s “Top 5 advances in 50 years of Modern Oncology” was the improvement in patients’ quality of life from supportive therapies, in particular antinausea medications.
Several of the agents commonly used in gynecologic oncology rate high (cisplatin) to moderate (carboplatin, cyclophosphamide, doxorubicin, ifosfamide) with regards to emetogenicity. The advent of 5-HT3 receptor antagonists (for example, ondansetron) has significantly improved the quality of life of patients undergoing cytotoxic chemotherapy. In addition to improving quality of life, the decrease in nausea and vomiting can also decrease life-threatening complications such as dehydration and electrolyte imbalance. Both ASCO and the National Comprehensive Cancer Network both have guidelines for the management of nausea and vomiting in patients undergoing chemotherapy.
Throughout 2016, Ob.Gyn. News will celebrate its 50th anniversary with exclusive articles looking at the evolution of the specialty, including the history of contraception, changes in gynecologic surgery, and the transformation of the well-woman visit. Look for these articles and more special features in the pages of Ob.Gyn. News and online at obgynnews.com.
Dr. Gehrig is professor and director of gynecologic oncology at the University of North Carolina, Chapel Hill. Dr. Clarke-Pearson is the chair and the Robert A. Ross Distinguished Professor of Obstetrics and Gynecology, and a professor in the division of gynecologic oncology at UNC. They reported having no relevant financial disclosures.
The subspecialty of gynecologic oncology was formalized less than 50 years ago with the creation of the Society of Gynecologic Oncology and subspecialty training and board certification. The formation of the Gynecologic Oncology Group (GOG) – and the many clinical trials spearheaded by that group – has further advanced evidence-based treatments, resulting in improved survival outcomes, quality of life, and preventive strategies.
While it is not possible to provide a comprehensive and exhaustive review of all of the advances, we hope to highlight many of the notable advances in this article.Cervical cancer
Cervical cancer is the fourth most common cancer in women worldwide with 528,000 new cases in 2012. The majority of cervical cancer cases are caused by infection with human papillomavirus (HPV). While the standard therapies for cervical cancer have been long established (radical hysterectomy for stage I and radiation therapy for locally advanced disease), one of the most significant advances in the past 50 years was the addition of radiation-sensitizing chemotherapy (cisplatin) administered concurrently with radiation therapy.
In randomized trials in both early and advanced cervical cancer, the risk of death was reduced by 30%-50%. These studies changed the paradigm for the treatment of cervical cancer (N Engl J Med. 1999 Apr 15;340[15]:1137-43; N Engl J Med. 1999 Apr 15;340[15]:1144-53; J Clin Oncol. 2000 Apr;18[8]:1606-13).
Future studies evaluating biologic adjuncts or additional chemotherapy are currently underway or awaiting data maturation.
The American Society of Clinical Oncology (ASCO) highlighted the “Top 5 advances in 50 years of Modern Oncology” in 2014, and second on the list was the approval of the HPV vaccine to prevent cervical cancer. Vaccines have been developed that can protect against types 2, 4 or 9 of HPV. In a 2014 study, depending on vaccination coverage, the relative number of cervical cancer cases avoided was 34% in Africa, 27% for America, 26% for Asia, 21% for Europe, and worldwide was estimated at 27% (Vaccine. 2014 Feb 3;32[6]:733-9).
While the benefit from HPV vaccination has been proven, in the United States, only about a third of eligible girls and women have been vaccinated. Efforts should focus on expanding vaccination penetration to eligible girls, boys, women, and men.
Endometrial cancer
Endometrial cancer is the most common gynecologic malignancy in the United States with an estimated 54,870 cases and 10,170 deaths annually. Notable advances in the management of women with endometrial cancer have arisen because of a better understanding that there are two types of endometrial cancer – type I and type II.
The type I endometrial cancers tend to be associated with lower stage of disease at the time of diagnosis and fewer recurrences, while type II endometrial cancer is associated with worse outcomes.
Tailoring the surgical approaches and adjuvant therapy for women with endometrial cancer has led to improved outcomes. The GOG conducted a large prospective randomized trial of laparotomy versus laparoscopic surgical staging for women with clinical early-stage endometrial cancer (LAP2). Laparoscopy was associated with improved perioperative outcomes and was found to be noninferior to laparotomy with regards to survival outcomes (J Clin Oncol. 2012 Mar 1;30[7]:695-700). Therefore, minimally invasive surgery has become widely accepted for the surgical staging of women with endometrial cancer.
Appropriate surgical staging allows for tailoring of postoperative adjuvant therapy. The current evidence suggests that vaginal brachytherapy should be the adjuvant treatment of choice over whole pelvic radiation in women with early-stage endometrial cancer (Lancet. 2010 Mar 6;375[9717]:816-23). Studies are underway to evaluate the role of both adjuvant radiation and chemotherapy in women with early-stage type II endometrial cancer who are felt to be at high risk for recurrent disease, as well as how to improve on the therapeutic options for women with advanced or recurrent disease.
Ovarian cancer
Epithelial ovarian cancer is the most deadly gynecologic malignancy in the United States with 21,290 cases and 14,180 deaths in 2015. The concept of ovarian tumor debulking was first described by Dr. Joe Meigs in 1934, but did not gain traction until the mid-1970s when Dr. C. Thomas Griffiths published his work (Natl Cancer Inst Monogr. 1975 Oct;42:101-4).
While there are no randomized trials proving that surgical cytoreduction improves overall survival, most retrospective studies support this concept. In 2009, Chi et al. showed improved median survival in women with ovarian cancer based on the increased percentage of women who underwent optimal cytoreduction (Gynecol Oncol. 2009 Jul;114[1]:26-31). This has led to modifications of surgical techniques and surgical goals with an effort to maximally cytoreduce all of the visible disease.
While initial surgical debulking is the goal, there are circumstances when a different approach may be indicated. Vergote et al. conducted a prospective randomized trial of 670 women with advanced ovarian cancer. In this study, neoadjuvant chemotherapy followed by interval debulking was not inferior to primary debulking followed by chemotherapy with regards to progression-free survival and overall survival. However, initial surgery was associated with increased surgical complications and perioperative mortality as compared with interval surgery. Therefore, in women who are not felt to be candidates for optimal cytoreduction, neoadjuvant chemotherapy followed by interval surgery may be an appropriate treatment strategy (N Engl J Med. 2010 Sep 2;363[10]:943-53.).
There have been several notable advances and a series of randomized trials – predominately conducted by the GOG – that have resulted in improved overall survival and progression-free interval in women with ovarian cancer. However, none are as significant as the discovery of paclitaxel and platinum-based chemotherapy (cisplatin and carboplatin).
In 1962, samples of the Pacific Yew’s bark were collected and, 2 years later, the extracts from this bark were found to have cytotoxic activity. There were initial difficulties suspending the drug in solution; however, ultimately a formulation in ethanol, cremophor, and saline was found to be effective. In 1984, the National Cancer Institute began clinical trials of paclitaxel and it was found to be highly effective in ovarian cancer. In 1992, it was approved for the treatment of ovarian cancer.
Cisplatin was approved in 1978. Carboplatin entered clinical trials in 1982 and was approved for women with recurrent ovarian cancer in 1989.
There were a series of trials beginning in the late 1980s that established the role of platinum agents and led us to GOG 111. This trial evaluated cisplatin with either cyclophosphamide or paclitaxel. The paclitaxel combination was superior and in 2003 two trials were published that solidified carboplatin and paclitaxel as the cornerstone in the treatment of women with ovarian cancer (J Clin Oncol. 2003 Sep 1;21[17]:3194-200; J Natl Cancer Inst. 2003 Sep 3;95[17]:1320-9).
What has most recently been debated is the route and schedule for both paclitaxel and the platinum agents. In January 2006, the National Cancer Institute released a Clinical Announcement regarding the role of intraperitoneal (IP) chemotherapy for the treatment of women with optimally debulked ovarian cancer. Of the six trials included in the announcement, four trials showed a benefit for progression-free survival and five studies showed an improvement in overall survival. Armstrong et al (GOG 172) showed a 16-month improvement in overall survival with intravenous (IV) paclitaxel, IP cisplatin, and IP paclitaxel. IP chemotherapy has not been universally embraced by physicians and patients in part because of its toxicity, treatment schedule, and the fact that no IP regimen has been compared with the current standard of IV carboplatin and paclitaxel (N Engl J Med. 2006 Jan 5;354[1]:34-43).
While there have been improvements in 5-year survival over time, most women with advanced ovarian cancer will undergo additional chemotherapy in order to achieve subsequent remissions or maintain stability of disease. Other drugs that have Food and Drug Administration approval in the setting of recurrent ovarian cancer include topotecan, liposomal doxorubicin, gemcitabine, bevacizumab, altretamine, carboplatin, cisplatin, cyclophosphamide, and melphalan. Olaparib was recently approved as monotherapy in women with a germline BRCA-mutation who had received three or more prior lines of chemotherapy.
Minimally invasive surgery
Over the last 30 years, minimally invasive surgery (MIS) in gynecologic oncology, particularly for endometrial cancer, has gone from a niche procedure to the standard of care. The introduction of laparoscopy into gynecologic oncology started in the early 1990s. In a series of 59 women undergoing laparoscopy for endometrial cancer, Childers et al. demonstrated feasibility of the technique and low laparotomy conversion rates (Gynecol Oncol. 1993 Oct;51[1]:33-8.). The GOG trial, LAP2, supported the equivalent oncologic outcomes of MIS versus laparotomy for the treatment of endometrial cancer. While many surgeons and centers offered laparoscopic surgery, there were issues with the learning curve that limited its widespread use.
In 2005, the FDA approval of the robotic platform for gynecologic surgery resulted in at least a doubling of the proportion of endometrial cancer patients treated with MIS (Int J Med Robot. 2009 Dec;5[4]:392-7.). In 2012, the Society of Gynecologic Oncology published a consensus statement regarding robotic-assisted surgery in gynecologic oncology (Gynecol Oncol. 2012 Feb;124[2]:180-4.). This review highlights the advantages of the robotics platform with regards to expanding MIS to women with cervical and ovarian cancer; the improvements in outcomes in the obese woman with endometrial cancer; and that the learning curve for robotic surgery is shorter than for traditional laparoscopy. Issues requiring further research include cost analysis as the cost of the new technology decreases, and opportunities for improvement in patient and physician quality of life.
Sentinel node mapping
The rationale for sentinel node mapping is that if one or more sentinel lymph nodes is/are negative for malignancy, then the other regional lymph nodes will also be negative. This would thereby avoid the need for a complete lymph node dissection and its resultant complications, including chronic lymphedema. Much of the work pioneering this strategy has been in breast cancer and melanoma, but data are rapidly emerging for these techniques in gynecologic malignancies.
Candidates for sentinel lymph node biopsy for vulvar cancer include those with a lesion more than 1mm in depth, a tumor less than 4 cm in size, and no obvious metastatic disease on exam or preoperative imaging. Additionally, recommendations have been made regarding case volume in order to achieve limited numbers of false-negative results and to maintain competency. In the study by Van der Zee et al. of 403 patients (623 groins) who underwent sentinel node procedures, the false-negative rate was 0-2%. The overall survival rate was 97% at 3 years (J Clin Oncol. 2008 Feb 20;26[6]:884-9). However, a more recent data from the Gynecologic Oncology Group (GOG 173) showed a slightly higher false-negative rate of 8% (J Clin Oncol. 2012 Nov 1;30[31]:3786-91). Overall survival data are pending from this study.
While sentinel lymph node mapping for endometrial cancer has been feasible for many years and has been well described, the questioned role of completed lymphadenectomy for early-stage endometrial cancer has led to a resurgence of interest in these techniques. While blue dye and radiolabeled tracer methods have historically been the most popular mapping solutions, the advent of endoscopic near-infrared imaging, with its higher sensitivity and good depth penetration, has added options. Indocyanine green fluorescence can be easily detected during robotic surgery and as experience with these techniques increase, successful mapping and sensitivity will increase.
Genetics
While hereditary cancer syndromes have been recognized for many years, detecting the genetic mutations that may increase an individual’s risk of developing a malignancy were not elucidated until the early 1990s. In gynecologic oncology, the most commonly encountered syndromes involve mutations in BRCA1 and BRCA2 and hereditary non–polyposis colorectal cancer, which causes mutations in DNA mismatch-repair genes and increase the risk of endometrial and ovarian cancer.
The SGO recently published a statement on risk assessment for inherited gynecologic cancer predispositions. In this statement “the evaluation for the presence of a hereditary cancer syndrome enables physicians to provide individualized and quantified assessment of cancer risk, as well as options for tailored screening and preventions strategies that may reduce morbidity associated with the development of malignancy” (Gynecol Oncol. 2015 Jan;136[1]:3-7). Beyond risk-reducing salpingo-oophorectomy, therapeutic strategies targeting patients with germline mutations have been developed (PARP inhibitors in BRCA-mutated women with ovarian cancer).
In August 2015, ASCO released an updated policy statement on genetic and genomic testing for cancer susceptibility and highlighted five key areas: germ-line implications of somatic mutation profiling; multigene panel testing for cancer susceptibility; quality assurance in genetic testing; education for oncology professionals; and access to cancer genetic services.
Antiemetics
Rounding out ASCO’s “Top 5 advances in 50 years of Modern Oncology” was the improvement in patients’ quality of life from supportive therapies, in particular antinausea medications.
Several of the agents commonly used in gynecologic oncology rate high (cisplatin) to moderate (carboplatin, cyclophosphamide, doxorubicin, ifosfamide) with regards to emetogenicity. The advent of 5-HT3 receptor antagonists (for example, ondansetron) has significantly improved the quality of life of patients undergoing cytotoxic chemotherapy. In addition to improving quality of life, the decrease in nausea and vomiting can also decrease life-threatening complications such as dehydration and electrolyte imbalance. Both ASCO and the National Comprehensive Cancer Network both have guidelines for the management of nausea and vomiting in patients undergoing chemotherapy.
Throughout 2016, Ob.Gyn. News will celebrate its 50th anniversary with exclusive articles looking at the evolution of the specialty, including the history of contraception, changes in gynecologic surgery, and the transformation of the well-woman visit. Look for these articles and more special features in the pages of Ob.Gyn. News and online at obgynnews.com.
Dr. Gehrig is professor and director of gynecologic oncology at the University of North Carolina, Chapel Hill. Dr. Clarke-Pearson is the chair and the Robert A. Ross Distinguished Professor of Obstetrics and Gynecology, and a professor in the division of gynecologic oncology at UNC. They reported having no relevant financial disclosures.
Fertility preservation in early cervical cancer
Historically, the standard of care for women diagnosed with early cervical cancer has been radical hysterectomy. Thus, young women are not only being confronted with a cancer diagnosis, but may also be forced to cope with the loss of their fertility.
As many young women with cervical cancer were not accepting of this treatment, Dr. Daniel Dargent pioneered the vaginal radical trachelectomy as a fertility-preserving treatment option for early cervical cancer in 1994. There have now been more than 900 vaginal radical trachelectomies performed and they have been shown to have oncologic outcomes similar to those of traditional radical hysterectomy, while sparing a woman’s fertility (Int J Gynecol Cancer. 2013 Jul;23[6]:982-9).
Obstetric outcomes following vaginal radical trachelectomy are acceptable with 17% miscarriage rate in the first trimester (compared to 10%-20% in the general population) and 8% in the second trimester (compared to 1%-5% in the general population) (Am Fam Physician. 2007 Nov 1;76[9]:1341-6). Following vaginal radical trachelectomy, 64% of pregnancies deliver at term.
The usual criteria required to undergo radical trachelectomy include:
1) Reproductive age with desire for fertility.
2) Stage IA1 with LVSI (lymphovascular space invasion), IA2, or IB1 with tumor less than 2 cm.
3) Limited endocervical involvement via preoperative MRI.
4) Negative pelvic lymph nodes.
Preoperative PET scan can be used to evaluate nodal status, but suspicious lymph nodes should be evaluated on frozen section at the time of surgery. The presence of LVSI alone is not a contraindication to trachelectomy.
A key limitation of vaginal radical trachelectomy is the specialized training required to perform this technically challenging procedure. Few surgeons in the United States are trained to perform vaginal radical trachelectomy. In response to this limitation, surgeons began to attempt radical trachelectomy via laparotomy (Gynecol Oncol. 2006 Dec;103[3]:807-13). Oncologic outcomes following fertility-sparing abdominal radical trachelectomy have been reported to be equivalent to radical hysterectomy. Concerns regarding the abdominal approach to radical trachelectomy include higher rates of second trimester loss (19%) when compared to the vaginal approach (8%), higher rate of loss of fertility (30%), and risk of postoperative adhesions.
The advent of minimally invasive surgery, particularly robotic surgery, now offers surgeons the ability to perform a procedure technically similar to radical hysterectomy using a minimally invasive approach. Given the similarity of procedural steps of radical trachelectomy to radical hysterectomy using the robotic platform, this procedure is gaining acceptance in the United States with an associated improved surgeon learning curve (Gynecol Oncol. 2008 Nov;111[2]:255-60). In addition, the use of minimally invasive surgery should result in less adhesion formation facilitating natural fertility options postoperatively.
Obstetric and fertility outcomes are limited following minimally invasive radical trachelectomy via laparoscopy or robotic surgery given the novelty of this procedure. Emerging obstetric outcomes appear reassuring, but further data are needed to fully understand the effects of this procedure on pregnancy outcomes and the need for assisted reproductive techniques to achieve pregnancy.
The management of pregnancies following radical trachelectomy is also an area with limited data, which presents a clinical challenge to obstetricians. Many gynecologic oncologists perform a permanent cerclage at the time of trachelectomy and recommend delivery via scheduled cesarean at term for all subsequent pregnancies prior to labor (usually 37-38 weeks).
At our institution, we recommend the use of progesterone from 16 to 36 weeks despite no clear evidence on the role of progesterone in this setting. Maternal-fetal medicine consultation should be considered to either follow these patients during their pregnancies or to perform a single consultative visit to guide antepartum care.
Some have advocated for less radical surgery, such as simple trachelectomy or large cold knife conization, as the risk of parametrial extension in these patients is low (Gynecol Oncol. 2011 Dec;123[3]:557-60). More data are needed to determine if this is a safe approach. Further, the use of neoadjuvant chemotherapy followed by cold knife conization for fertility preservation in women with larger tumors has been proposed. This may be a feasible option in women with chemo-sensitive tumors, but progression on chemotherapy and increased recurrences have been reported with this approach (Gynecol Oncol. 2008 Dec;111[3]:438-43).
Women of reproductive age diagnosed with early cervical cancer now have multiple options for fertility preservation. Ongoing research regarding obstetric and fertility outcomes is needed; however, oncologic outcomes appear to be equivalent.
Dr. Clark is a fellow in the division of gynecologic oncology, department of obstetrics and gynecology, at the University of North Carolina, Chapel Hill. Dr. Boggess is an expert in robotic surgery in gynecologic oncology and is a professor in the division of gynecologic oncology at UNC–Chapel Hill. They reported having no financial disclosures relevant to this column. Email them at obnews@frontlinemedcom.com.
Historically, the standard of care for women diagnosed with early cervical cancer has been radical hysterectomy. Thus, young women are not only being confronted with a cancer diagnosis, but may also be forced to cope with the loss of their fertility.
As many young women with cervical cancer were not accepting of this treatment, Dr. Daniel Dargent pioneered the vaginal radical trachelectomy as a fertility-preserving treatment option for early cervical cancer in 1994. There have now been more than 900 vaginal radical trachelectomies performed and they have been shown to have oncologic outcomes similar to those of traditional radical hysterectomy, while sparing a woman’s fertility (Int J Gynecol Cancer. 2013 Jul;23[6]:982-9).
Obstetric outcomes following vaginal radical trachelectomy are acceptable with 17% miscarriage rate in the first trimester (compared to 10%-20% in the general population) and 8% in the second trimester (compared to 1%-5% in the general population) (Am Fam Physician. 2007 Nov 1;76[9]:1341-6). Following vaginal radical trachelectomy, 64% of pregnancies deliver at term.
The usual criteria required to undergo radical trachelectomy include:
1) Reproductive age with desire for fertility.
2) Stage IA1 with LVSI (lymphovascular space invasion), IA2, or IB1 with tumor less than 2 cm.
3) Limited endocervical involvement via preoperative MRI.
4) Negative pelvic lymph nodes.
Preoperative PET scan can be used to evaluate nodal status, but suspicious lymph nodes should be evaluated on frozen section at the time of surgery. The presence of LVSI alone is not a contraindication to trachelectomy.
A key limitation of vaginal radical trachelectomy is the specialized training required to perform this technically challenging procedure. Few surgeons in the United States are trained to perform vaginal radical trachelectomy. In response to this limitation, surgeons began to attempt radical trachelectomy via laparotomy (Gynecol Oncol. 2006 Dec;103[3]:807-13). Oncologic outcomes following fertility-sparing abdominal radical trachelectomy have been reported to be equivalent to radical hysterectomy. Concerns regarding the abdominal approach to radical trachelectomy include higher rates of second trimester loss (19%) when compared to the vaginal approach (8%), higher rate of loss of fertility (30%), and risk of postoperative adhesions.
The advent of minimally invasive surgery, particularly robotic surgery, now offers surgeons the ability to perform a procedure technically similar to radical hysterectomy using a minimally invasive approach. Given the similarity of procedural steps of radical trachelectomy to radical hysterectomy using the robotic platform, this procedure is gaining acceptance in the United States with an associated improved surgeon learning curve (Gynecol Oncol. 2008 Nov;111[2]:255-60). In addition, the use of minimally invasive surgery should result in less adhesion formation facilitating natural fertility options postoperatively.
Obstetric and fertility outcomes are limited following minimally invasive radical trachelectomy via laparoscopy or robotic surgery given the novelty of this procedure. Emerging obstetric outcomes appear reassuring, but further data are needed to fully understand the effects of this procedure on pregnancy outcomes and the need for assisted reproductive techniques to achieve pregnancy.
The management of pregnancies following radical trachelectomy is also an area with limited data, which presents a clinical challenge to obstetricians. Many gynecologic oncologists perform a permanent cerclage at the time of trachelectomy and recommend delivery via scheduled cesarean at term for all subsequent pregnancies prior to labor (usually 37-38 weeks).
At our institution, we recommend the use of progesterone from 16 to 36 weeks despite no clear evidence on the role of progesterone in this setting. Maternal-fetal medicine consultation should be considered to either follow these patients during their pregnancies or to perform a single consultative visit to guide antepartum care.
Some have advocated for less radical surgery, such as simple trachelectomy or large cold knife conization, as the risk of parametrial extension in these patients is low (Gynecol Oncol. 2011 Dec;123[3]:557-60). More data are needed to determine if this is a safe approach. Further, the use of neoadjuvant chemotherapy followed by cold knife conization for fertility preservation in women with larger tumors has been proposed. This may be a feasible option in women with chemo-sensitive tumors, but progression on chemotherapy and increased recurrences have been reported with this approach (Gynecol Oncol. 2008 Dec;111[3]:438-43).
Women of reproductive age diagnosed with early cervical cancer now have multiple options for fertility preservation. Ongoing research regarding obstetric and fertility outcomes is needed; however, oncologic outcomes appear to be equivalent.
Dr. Clark is a fellow in the division of gynecologic oncology, department of obstetrics and gynecology, at the University of North Carolina, Chapel Hill. Dr. Boggess is an expert in robotic surgery in gynecologic oncology and is a professor in the division of gynecologic oncology at UNC–Chapel Hill. They reported having no financial disclosures relevant to this column. Email them at obnews@frontlinemedcom.com.
Historically, the standard of care for women diagnosed with early cervical cancer has been radical hysterectomy. Thus, young women are not only being confronted with a cancer diagnosis, but may also be forced to cope with the loss of their fertility.
As many young women with cervical cancer were not accepting of this treatment, Dr. Daniel Dargent pioneered the vaginal radical trachelectomy as a fertility-preserving treatment option for early cervical cancer in 1994. There have now been more than 900 vaginal radical trachelectomies performed and they have been shown to have oncologic outcomes similar to those of traditional radical hysterectomy, while sparing a woman’s fertility (Int J Gynecol Cancer. 2013 Jul;23[6]:982-9).
Obstetric outcomes following vaginal radical trachelectomy are acceptable with 17% miscarriage rate in the first trimester (compared to 10%-20% in the general population) and 8% in the second trimester (compared to 1%-5% in the general population) (Am Fam Physician. 2007 Nov 1;76[9]:1341-6). Following vaginal radical trachelectomy, 64% of pregnancies deliver at term.
The usual criteria required to undergo radical trachelectomy include:
1) Reproductive age with desire for fertility.
2) Stage IA1 with LVSI (lymphovascular space invasion), IA2, or IB1 with tumor less than 2 cm.
3) Limited endocervical involvement via preoperative MRI.
4) Negative pelvic lymph nodes.
Preoperative PET scan can be used to evaluate nodal status, but suspicious lymph nodes should be evaluated on frozen section at the time of surgery. The presence of LVSI alone is not a contraindication to trachelectomy.
A key limitation of vaginal radical trachelectomy is the specialized training required to perform this technically challenging procedure. Few surgeons in the United States are trained to perform vaginal radical trachelectomy. In response to this limitation, surgeons began to attempt radical trachelectomy via laparotomy (Gynecol Oncol. 2006 Dec;103[3]:807-13). Oncologic outcomes following fertility-sparing abdominal radical trachelectomy have been reported to be equivalent to radical hysterectomy. Concerns regarding the abdominal approach to radical trachelectomy include higher rates of second trimester loss (19%) when compared to the vaginal approach (8%), higher rate of loss of fertility (30%), and risk of postoperative adhesions.
The advent of minimally invasive surgery, particularly robotic surgery, now offers surgeons the ability to perform a procedure technically similar to radical hysterectomy using a minimally invasive approach. Given the similarity of procedural steps of radical trachelectomy to radical hysterectomy using the robotic platform, this procedure is gaining acceptance in the United States with an associated improved surgeon learning curve (Gynecol Oncol. 2008 Nov;111[2]:255-60). In addition, the use of minimally invasive surgery should result in less adhesion formation facilitating natural fertility options postoperatively.
Obstetric and fertility outcomes are limited following minimally invasive radical trachelectomy via laparoscopy or robotic surgery given the novelty of this procedure. Emerging obstetric outcomes appear reassuring, but further data are needed to fully understand the effects of this procedure on pregnancy outcomes and the need for assisted reproductive techniques to achieve pregnancy.
The management of pregnancies following radical trachelectomy is also an area with limited data, which presents a clinical challenge to obstetricians. Many gynecologic oncologists perform a permanent cerclage at the time of trachelectomy and recommend delivery via scheduled cesarean at term for all subsequent pregnancies prior to labor (usually 37-38 weeks).
At our institution, we recommend the use of progesterone from 16 to 36 weeks despite no clear evidence on the role of progesterone in this setting. Maternal-fetal medicine consultation should be considered to either follow these patients during their pregnancies or to perform a single consultative visit to guide antepartum care.
Some have advocated for less radical surgery, such as simple trachelectomy or large cold knife conization, as the risk of parametrial extension in these patients is low (Gynecol Oncol. 2011 Dec;123[3]:557-60). More data are needed to determine if this is a safe approach. Further, the use of neoadjuvant chemotherapy followed by cold knife conization for fertility preservation in women with larger tumors has been proposed. This may be a feasible option in women with chemo-sensitive tumors, but progression on chemotherapy and increased recurrences have been reported with this approach (Gynecol Oncol. 2008 Dec;111[3]:438-43).
Women of reproductive age diagnosed with early cervical cancer now have multiple options for fertility preservation. Ongoing research regarding obstetric and fertility outcomes is needed; however, oncologic outcomes appear to be equivalent.
Dr. Clark is a fellow in the division of gynecologic oncology, department of obstetrics and gynecology, at the University of North Carolina, Chapel Hill. Dr. Boggess is an expert in robotic surgery in gynecologic oncology and is a professor in the division of gynecologic oncology at UNC–Chapel Hill. They reported having no financial disclosures relevant to this column. Email them at obnews@frontlinemedcom.com.
Managing menopause symptoms in gynecologic cancer survivors
Due to advancements in surgical treatment, chemotherapy, and radiation therapy, gynecologic cancer survival rates are continuing to improve and quality of life is evolving into an even more significant focus in cancer care.
Roughly 30%-40% of all women with a gynecologic malignancy will experience climacteric symptoms and menopause prior to the anticipated time of natural menopause (J Clin Oncol. 2009 Mar 10;27[8]:1214-9). Cessation of ovarian estrogen and progesterone production can result in short-term as well as long-term sequelae, including vasomotor symptoms, vaginal dryness, osteoporosis, and mood disturbances. Iatrogenic menopause after cancer treatment can be more sudden and severe when compared with the natural course of physiologic menopause. As a result, determination of safe, effective modalities for treating these symptoms is of particular importance for survivor quality of life.
Both combination and estrogen-only hormone replacement therapy (HT) provide greater improvement in these specific symptoms and overall quality of life than placebo as demonstrated in several observational and randomized control trials (Cochrane Database Syst Rev. 2009 Apr 15;[2]:CD004143).
Endometrial cancer
Endometrial cancer is the most common gynecologic malignancy, with approximately 54,000 new cases anticipated in the United States in 2015. Twenty-five percent of these new cases will be in premenopausal women, and with an ever-increasing obesity rate, this number may continue to climb.
Women with early-stage Type 1 endometrial cancer who have vasomotor symptoms after surgery may be offered a short course of estrogen-based HT at the lowest effective dose following hysterectomy/bilateral salpingo-oophorectomy and staging procedure (J Clin Oncol. 2006 Feb 1;24[4]:587-92). For women with genitourinary symptoms, vaginal moisturizers and/or low-dose vaginal estrogen are reasonable options. Unfortunately, there are no data to guide the use of estrogen replacement therapy in women with Type 2 endometrial cancers (Gynecol Oncol. 2011 Aug;122[2]:447-54).
Ovarian cancer
There is minimal data implicating a hormonal causation to ovarian carcinogenesis. Most women with epithelial ovarian cancer do not express tumor estrogen or progesterone receptors. Treatment will result in abrupt, iatrogenic menopause, raising the question of whether it is safe to use HT in patients with epithelial ovarian cancer.
Multiple studies have failed to demonstrate a difference in 5-year survival rates in women with epithelial cancer using HT for 2 years or less (JAMA. 2009 Jul 15;302[3]:298-305, Eur J Gynaecol Oncol. 2000;21[2]:192-6, Cancer. 1999 Sep 15;86[6]:1013-8). As such, symptomatic patients could be offered a course of HT; however, caution should be exercised in women with estrogen/progesterone–expressing tumors or nonepithelial tumors. As with endometrial cancer patients, the lowest effective doses should be prescribed.
Cervical cancer
Most cervical squamous and adenocarcinomas are not hormone dependent. For women with early-stage squamous cell carcinoma, ovarian conservation may be possible or oophoropexy may be offered. However, for many patients, bilateral salpingo-oophorectomy at the time of hysterectomy is more common, and the local effect of radiation therapy can result in vaginal atrophy with subsequent dyspareunia or ovarian failure from radiation scatter. Even for patients who undergo oophoropexy, radiation scatter may still result in ovarian failure. In a few observational studies, there are no data to infer that cervical cancer is hormonally related or that survival rates are decreased.
Currently, HT use in cervical cancer survivors is considered safe. Of note, for women with more advanced-stage cervical cancer and who received chemoradiation for primary treatment, combination therapy with estrogen and progesterone may be more appropriate if the uterus remains in situ. However, for women who have undergone hysterectomy, combination therapy with progesterone may not be warranted and estrogen alone (orally or vaginally) is acceptable (Gynecol Oncol. 2011 Aug;122[2]:447-54)
Nonhormonal therapies
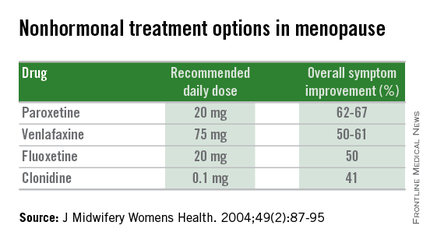
Women presenting with menopausal symptoms in whom estrogen therapy is contraindicated or not desired can also consider using nonhormonal therapies as an alternative. These include selective serotonin reuptake inhibitors (SSRIs) and alpha-2 adrenergic agonists, such as clonidine. Albeit not as effective as HT, these alternative therapies are reasonable options, particularly for management of vasomotor symptoms.
From a limited number of observational studies and a few randomized trials, short-term hormone replacement therapy does not present increased risk to survivors of gynecologic cancers. Additionally, patients have the added option of using nonhormonal therapies, which may provide some benefit. The decision to institute HT should occur after a thorough discussion of the potential to optimize symptom control and the theoretical risk of stimulating quiescent malignant disease.
Dr. Staley is a resident physician in the department of obstetrics and gynecology at the University of North Carolina at Chapel Hill. Dr. Gehrig is professor and director of gynecologic oncology at the university. They reported having no relevant financial disclosures.
Due to advancements in surgical treatment, chemotherapy, and radiation therapy, gynecologic cancer survival rates are continuing to improve and quality of life is evolving into an even more significant focus in cancer care.
Roughly 30%-40% of all women with a gynecologic malignancy will experience climacteric symptoms and menopause prior to the anticipated time of natural menopause (J Clin Oncol. 2009 Mar 10;27[8]:1214-9). Cessation of ovarian estrogen and progesterone production can result in short-term as well as long-term sequelae, including vasomotor symptoms, vaginal dryness, osteoporosis, and mood disturbances. Iatrogenic menopause after cancer treatment can be more sudden and severe when compared with the natural course of physiologic menopause. As a result, determination of safe, effective modalities for treating these symptoms is of particular importance for survivor quality of life.
Both combination and estrogen-only hormone replacement therapy (HT) provide greater improvement in these specific symptoms and overall quality of life than placebo as demonstrated in several observational and randomized control trials (Cochrane Database Syst Rev. 2009 Apr 15;[2]:CD004143).
Endometrial cancer
Endometrial cancer is the most common gynecologic malignancy, with approximately 54,000 new cases anticipated in the United States in 2015. Twenty-five percent of these new cases will be in premenopausal women, and with an ever-increasing obesity rate, this number may continue to climb.
Women with early-stage Type 1 endometrial cancer who have vasomotor symptoms after surgery may be offered a short course of estrogen-based HT at the lowest effective dose following hysterectomy/bilateral salpingo-oophorectomy and staging procedure (J Clin Oncol. 2006 Feb 1;24[4]:587-92). For women with genitourinary symptoms, vaginal moisturizers and/or low-dose vaginal estrogen are reasonable options. Unfortunately, there are no data to guide the use of estrogen replacement therapy in women with Type 2 endometrial cancers (Gynecol Oncol. 2011 Aug;122[2]:447-54).
Ovarian cancer
There is minimal data implicating a hormonal causation to ovarian carcinogenesis. Most women with epithelial ovarian cancer do not express tumor estrogen or progesterone receptors. Treatment will result in abrupt, iatrogenic menopause, raising the question of whether it is safe to use HT in patients with epithelial ovarian cancer.
Multiple studies have failed to demonstrate a difference in 5-year survival rates in women with epithelial cancer using HT for 2 years or less (JAMA. 2009 Jul 15;302[3]:298-305, Eur J Gynaecol Oncol. 2000;21[2]:192-6, Cancer. 1999 Sep 15;86[6]:1013-8). As such, symptomatic patients could be offered a course of HT; however, caution should be exercised in women with estrogen/progesterone–expressing tumors or nonepithelial tumors. As with endometrial cancer patients, the lowest effective doses should be prescribed.
Cervical cancer
Most cervical squamous and adenocarcinomas are not hormone dependent. For women with early-stage squamous cell carcinoma, ovarian conservation may be possible or oophoropexy may be offered. However, for many patients, bilateral salpingo-oophorectomy at the time of hysterectomy is more common, and the local effect of radiation therapy can result in vaginal atrophy with subsequent dyspareunia or ovarian failure from radiation scatter. Even for patients who undergo oophoropexy, radiation scatter may still result in ovarian failure. In a few observational studies, there are no data to infer that cervical cancer is hormonally related or that survival rates are decreased.
Currently, HT use in cervical cancer survivors is considered safe. Of note, for women with more advanced-stage cervical cancer and who received chemoradiation for primary treatment, combination therapy with estrogen and progesterone may be more appropriate if the uterus remains in situ. However, for women who have undergone hysterectomy, combination therapy with progesterone may not be warranted and estrogen alone (orally or vaginally) is acceptable (Gynecol Oncol. 2011 Aug;122[2]:447-54)
Nonhormonal therapies

Women presenting with menopausal symptoms in whom estrogen therapy is contraindicated or not desired can also consider using nonhormonal therapies as an alternative. These include selective serotonin reuptake inhibitors (SSRIs) and alpha-2 adrenergic agonists, such as clonidine. Albeit not as effective as HT, these alternative therapies are reasonable options, particularly for management of vasomotor symptoms.
From a limited number of observational studies and a few randomized trials, short-term hormone replacement therapy does not present increased risk to survivors of gynecologic cancers. Additionally, patients have the added option of using nonhormonal therapies, which may provide some benefit. The decision to institute HT should occur after a thorough discussion of the potential to optimize symptom control and the theoretical risk of stimulating quiescent malignant disease.
Dr. Staley is a resident physician in the department of obstetrics and gynecology at the University of North Carolina at Chapel Hill. Dr. Gehrig is professor and director of gynecologic oncology at the university. They reported having no relevant financial disclosures.
Due to advancements in surgical treatment, chemotherapy, and radiation therapy, gynecologic cancer survival rates are continuing to improve and quality of life is evolving into an even more significant focus in cancer care.
Roughly 30%-40% of all women with a gynecologic malignancy will experience climacteric symptoms and menopause prior to the anticipated time of natural menopause (J Clin Oncol. 2009 Mar 10;27[8]:1214-9). Cessation of ovarian estrogen and progesterone production can result in short-term as well as long-term sequelae, including vasomotor symptoms, vaginal dryness, osteoporosis, and mood disturbances. Iatrogenic menopause after cancer treatment can be more sudden and severe when compared with the natural course of physiologic menopause. As a result, determination of safe, effective modalities for treating these symptoms is of particular importance for survivor quality of life.
Both combination and estrogen-only hormone replacement therapy (HT) provide greater improvement in these specific symptoms and overall quality of life than placebo as demonstrated in several observational and randomized control trials (Cochrane Database Syst Rev. 2009 Apr 15;[2]:CD004143).
Endometrial cancer
Endometrial cancer is the most common gynecologic malignancy, with approximately 54,000 new cases anticipated in the United States in 2015. Twenty-five percent of these new cases will be in premenopausal women, and with an ever-increasing obesity rate, this number may continue to climb.
Women with early-stage Type 1 endometrial cancer who have vasomotor symptoms after surgery may be offered a short course of estrogen-based HT at the lowest effective dose following hysterectomy/bilateral salpingo-oophorectomy and staging procedure (J Clin Oncol. 2006 Feb 1;24[4]:587-92). For women with genitourinary symptoms, vaginal moisturizers and/or low-dose vaginal estrogen are reasonable options. Unfortunately, there are no data to guide the use of estrogen replacement therapy in women with Type 2 endometrial cancers (Gynecol Oncol. 2011 Aug;122[2]:447-54).
Ovarian cancer
There is minimal data implicating a hormonal causation to ovarian carcinogenesis. Most women with epithelial ovarian cancer do not express tumor estrogen or progesterone receptors. Treatment will result in abrupt, iatrogenic menopause, raising the question of whether it is safe to use HT in patients with epithelial ovarian cancer.
Multiple studies have failed to demonstrate a difference in 5-year survival rates in women with epithelial cancer using HT for 2 years or less (JAMA. 2009 Jul 15;302[3]:298-305, Eur J Gynaecol Oncol. 2000;21[2]:192-6, Cancer. 1999 Sep 15;86[6]:1013-8). As such, symptomatic patients could be offered a course of HT; however, caution should be exercised in women with estrogen/progesterone–expressing tumors or nonepithelial tumors. As with endometrial cancer patients, the lowest effective doses should be prescribed.
Cervical cancer
Most cervical squamous and adenocarcinomas are not hormone dependent. For women with early-stage squamous cell carcinoma, ovarian conservation may be possible or oophoropexy may be offered. However, for many patients, bilateral salpingo-oophorectomy at the time of hysterectomy is more common, and the local effect of radiation therapy can result in vaginal atrophy with subsequent dyspareunia or ovarian failure from radiation scatter. Even for patients who undergo oophoropexy, radiation scatter may still result in ovarian failure. In a few observational studies, there are no data to infer that cervical cancer is hormonally related or that survival rates are decreased.
Currently, HT use in cervical cancer survivors is considered safe. Of note, for women with more advanced-stage cervical cancer and who received chemoradiation for primary treatment, combination therapy with estrogen and progesterone may be more appropriate if the uterus remains in situ. However, for women who have undergone hysterectomy, combination therapy with progesterone may not be warranted and estrogen alone (orally or vaginally) is acceptable (Gynecol Oncol. 2011 Aug;122[2]:447-54)
Nonhormonal therapies

Women presenting with menopausal symptoms in whom estrogen therapy is contraindicated or not desired can also consider using nonhormonal therapies as an alternative. These include selective serotonin reuptake inhibitors (SSRIs) and alpha-2 adrenergic agonists, such as clonidine. Albeit not as effective as HT, these alternative therapies are reasonable options, particularly for management of vasomotor symptoms.
From a limited number of observational studies and a few randomized trials, short-term hormone replacement therapy does not present increased risk to survivors of gynecologic cancers. Additionally, patients have the added option of using nonhormonal therapies, which may provide some benefit. The decision to institute HT should occur after a thorough discussion of the potential to optimize symptom control and the theoretical risk of stimulating quiescent malignant disease.
Dr. Staley is a resident physician in the department of obstetrics and gynecology at the University of North Carolina at Chapel Hill. Dr. Gehrig is professor and director of gynecologic oncology at the university. They reported having no relevant financial disclosures.
Managing menopausal symptoms after risk-reducing salpingo-oophorectomy
Compared to the general population, women with mutations in the BRCA1 or BRCA2 genes have a significantly higher lifetime risk of ovarian and breast cancers (Science. 2003 Oct 24;302[5645]:643-6). Since the occurrence of ovarian and breast cancer in BRCA carriers is often prior to menopause, and because we have no screening test to detect early stage ovarian cancer, risk-reducing salpingo-oophorectomy has been recommended around age 40.
It has been shown that risk-reducing salpingo-oophorectomy significantly reduces ovarian cancer risk by 85%-95% in BRCA-affected women. Also, this surgery can reduce breast cancer risk by 53%-68% (N Engl J Med. 2002 May 23;346[21]:1609-15). The 2008 Practice Bulletin from the American College of Obstetricians and Gynecologists recommends that risk-reducing salpingo-oophorectomy should be performed in women with BRCA1 or BRCA2 mutations after the completion of childbearing or age 40 (Obstet Gynecol. 2008 Jan;111[1]:231-41).
Health implications
Nearly 60% of women who have a BRCA1 or BRCA2 mutation will elect to undergo risk-reducing salpingo-oophorectomy between the ages of 35 and 40 years (Open Med. 2007 Aug 13;1[2]:e92-8). As such, surgical menopause can result in hot flashes, vaginal dryness, sexual dysfunction, sleep disturbances, and cognitive changes, which may significantly impact a woman’s quality of life. In addition, increased risk of cardiovascular disease and osteoporosis following bilateral salpingo-oophorectomy may have a significant impact on a woman’s health.
Since these women undergo surgical menopause as opposed to natural menopause, they have an abrupt loss in hormones, and due to their younger age at the time of surgery, they may also have a longer exposure period to the detrimental effects of hypoestrogenism.
Symptom management
Various treatment options exist for relief of menopausal symptoms, including nonhormonal therapies and hormone replacement therapies (HT).
Nonhormonal therapies include serotonin receptor inhibitors (venlafaxine and paroxetine) and alpha-2 adrenergic agonists (clonidine), which are most appropriate for the treatment of vasomotor symptoms. Unfortunately, these options have proved to be as effective as HT. Also, women should be adequately counseled regarding the various side effects of these nonhormonal medications. Alternative approaches such as phytoestrogens are unproven and are still undergoing investigation. As such, HT remains the standard for treatment of menopausal symptoms, and many trials have confirmed that HT can effectively treat menopausal symptoms following risk-reducing salpingo-oophorectomy.
This then raises the question of safety regarding use of HT in this patient population; especially the possibility of increased risk of breast cancer. Interestingly, only 10%-25% of BRCA1 carriers will have estrogen receptor–positive breast cancer, while 65%-79% of BRCA2-associated breast cancers will be positive for the receptor (Clin Cancer Res. 2004 Mar 15;10[6]:2029-34).
Unfortunately, we do not have adequate trials or studies with sufficient long-term follow-up to validate whether HT increases the risk of breast cancer or recurrence. However, the PROSE Study Group did report on a prospective cohort of 462 women with BRCA1 or BRCA2 mutations. In this study, HT did not alter the reduction in breast cancer risk from risk-reducing salpingo-oophorectomy (J Clin Oncol. 2005 Nov 1;23[31]:7804-10). In addition to a paucity of data regarding systemic HT, there is little data in the BRCA-positive population to confirm the safety of local vaginal estrogen for treatment of vaginal atrophy (J Clin Oncol. 2004 Mar 15;22[6]:1045-54).
Understanding the options
Use of HT in women with BRCA1 and BRCA2 mutations requires further investigation. There should be shared decision making between the patient and provider when counseling on the management of menopausal symptoms following risk-reducing salpingo-oophorectomy. Most importantly, women should understand the options of nonhormonal therapies and their specific side effects. They should also understand the lack of significant data regarding use of systemic HT, and that if use is elected, there may be an increased risk of breast cancer.
Women who do elect to use systemic HT following risk-reducing salpingo-oophorectomy have options that can help reduce the risk of HT-associated breast cancer, including a shorter duration of systemic HT or concurrent hysterectomy to allow for estrogen-only HT, which has a decreased risk of breast cancer compared with combined therapies that include progestins. These women may also be considering prophylactic mastectomy, which would change the concerns regarding HT and an increased risk of breast cancer.
Increased awareness of these options among physicians and patients alike can help to decrease unsatisfactory symptoms and improve quality of life in women undergoing risk-reducing salpingo-oophorectomy.
Dr. Staley is a resident physician in the department of obstetrics and gynecology at the University of North Carolina at Chapel Hill. Dr. Gehrig is professor and director of gynecologic oncology at the university. They reported having no relevant financial disclosures. Email them at obnews@frontlinemedcom.com.
Compared to the general population, women with mutations in the BRCA1 or BRCA2 genes have a significantly higher lifetime risk of ovarian and breast cancers (Science. 2003 Oct 24;302[5645]:643-6). Since the occurrence of ovarian and breast cancer in BRCA carriers is often prior to menopause, and because we have no screening test to detect early stage ovarian cancer, risk-reducing salpingo-oophorectomy has been recommended around age 40.
It has been shown that risk-reducing salpingo-oophorectomy significantly reduces ovarian cancer risk by 85%-95% in BRCA-affected women. Also, this surgery can reduce breast cancer risk by 53%-68% (N Engl J Med. 2002 May 23;346[21]:1609-15). The 2008 Practice Bulletin from the American College of Obstetricians and Gynecologists recommends that risk-reducing salpingo-oophorectomy should be performed in women with BRCA1 or BRCA2 mutations after the completion of childbearing or age 40 (Obstet Gynecol. 2008 Jan;111[1]:231-41).
Health implications
Nearly 60% of women who have a BRCA1 or BRCA2 mutation will elect to undergo risk-reducing salpingo-oophorectomy between the ages of 35 and 40 years (Open Med. 2007 Aug 13;1[2]:e92-8). As such, surgical menopause can result in hot flashes, vaginal dryness, sexual dysfunction, sleep disturbances, and cognitive changes, which may significantly impact a woman’s quality of life. In addition, increased risk of cardiovascular disease and osteoporosis following bilateral salpingo-oophorectomy may have a significant impact on a woman’s health.
Since these women undergo surgical menopause as opposed to natural menopause, they have an abrupt loss in hormones, and due to their younger age at the time of surgery, they may also have a longer exposure period to the detrimental effects of hypoestrogenism.
Symptom management
Various treatment options exist for relief of menopausal symptoms, including nonhormonal therapies and hormone replacement therapies (HT).
Nonhormonal therapies include serotonin receptor inhibitors (venlafaxine and paroxetine) and alpha-2 adrenergic agonists (clonidine), which are most appropriate for the treatment of vasomotor symptoms. Unfortunately, these options have proved to be as effective as HT. Also, women should be adequately counseled regarding the various side effects of these nonhormonal medications. Alternative approaches such as phytoestrogens are unproven and are still undergoing investigation. As such, HT remains the standard for treatment of menopausal symptoms, and many trials have confirmed that HT can effectively treat menopausal symptoms following risk-reducing salpingo-oophorectomy.
This then raises the question of safety regarding use of HT in this patient population; especially the possibility of increased risk of breast cancer. Interestingly, only 10%-25% of BRCA1 carriers will have estrogen receptor–positive breast cancer, while 65%-79% of BRCA2-associated breast cancers will be positive for the receptor (Clin Cancer Res. 2004 Mar 15;10[6]:2029-34).
Unfortunately, we do not have adequate trials or studies with sufficient long-term follow-up to validate whether HT increases the risk of breast cancer or recurrence. However, the PROSE Study Group did report on a prospective cohort of 462 women with BRCA1 or BRCA2 mutations. In this study, HT did not alter the reduction in breast cancer risk from risk-reducing salpingo-oophorectomy (J Clin Oncol. 2005 Nov 1;23[31]:7804-10). In addition to a paucity of data regarding systemic HT, there is little data in the BRCA-positive population to confirm the safety of local vaginal estrogen for treatment of vaginal atrophy (J Clin Oncol. 2004 Mar 15;22[6]:1045-54).
Understanding the options
Use of HT in women with BRCA1 and BRCA2 mutations requires further investigation. There should be shared decision making between the patient and provider when counseling on the management of menopausal symptoms following risk-reducing salpingo-oophorectomy. Most importantly, women should understand the options of nonhormonal therapies and their specific side effects. They should also understand the lack of significant data regarding use of systemic HT, and that if use is elected, there may be an increased risk of breast cancer.
Women who do elect to use systemic HT following risk-reducing salpingo-oophorectomy have options that can help reduce the risk of HT-associated breast cancer, including a shorter duration of systemic HT or concurrent hysterectomy to allow for estrogen-only HT, which has a decreased risk of breast cancer compared with combined therapies that include progestins. These women may also be considering prophylactic mastectomy, which would change the concerns regarding HT and an increased risk of breast cancer.
Increased awareness of these options among physicians and patients alike can help to decrease unsatisfactory symptoms and improve quality of life in women undergoing risk-reducing salpingo-oophorectomy.
Dr. Staley is a resident physician in the department of obstetrics and gynecology at the University of North Carolina at Chapel Hill. Dr. Gehrig is professor and director of gynecologic oncology at the university. They reported having no relevant financial disclosures. Email them at obnews@frontlinemedcom.com.
Compared to the general population, women with mutations in the BRCA1 or BRCA2 genes have a significantly higher lifetime risk of ovarian and breast cancers (Science. 2003 Oct 24;302[5645]:643-6). Since the occurrence of ovarian and breast cancer in BRCA carriers is often prior to menopause, and because we have no screening test to detect early stage ovarian cancer, risk-reducing salpingo-oophorectomy has been recommended around age 40.
It has been shown that risk-reducing salpingo-oophorectomy significantly reduces ovarian cancer risk by 85%-95% in BRCA-affected women. Also, this surgery can reduce breast cancer risk by 53%-68% (N Engl J Med. 2002 May 23;346[21]:1609-15). The 2008 Practice Bulletin from the American College of Obstetricians and Gynecologists recommends that risk-reducing salpingo-oophorectomy should be performed in women with BRCA1 or BRCA2 mutations after the completion of childbearing or age 40 (Obstet Gynecol. 2008 Jan;111[1]:231-41).
Health implications
Nearly 60% of women who have a BRCA1 or BRCA2 mutation will elect to undergo risk-reducing salpingo-oophorectomy between the ages of 35 and 40 years (Open Med. 2007 Aug 13;1[2]:e92-8). As such, surgical menopause can result in hot flashes, vaginal dryness, sexual dysfunction, sleep disturbances, and cognitive changes, which may significantly impact a woman’s quality of life. In addition, increased risk of cardiovascular disease and osteoporosis following bilateral salpingo-oophorectomy may have a significant impact on a woman’s health.
Since these women undergo surgical menopause as opposed to natural menopause, they have an abrupt loss in hormones, and due to their younger age at the time of surgery, they may also have a longer exposure period to the detrimental effects of hypoestrogenism.
Symptom management
Various treatment options exist for relief of menopausal symptoms, including nonhormonal therapies and hormone replacement therapies (HT).
Nonhormonal therapies include serotonin receptor inhibitors (venlafaxine and paroxetine) and alpha-2 adrenergic agonists (clonidine), which are most appropriate for the treatment of vasomotor symptoms. Unfortunately, these options have proved to be as effective as HT. Also, women should be adequately counseled regarding the various side effects of these nonhormonal medications. Alternative approaches such as phytoestrogens are unproven and are still undergoing investigation. As such, HT remains the standard for treatment of menopausal symptoms, and many trials have confirmed that HT can effectively treat menopausal symptoms following risk-reducing salpingo-oophorectomy.
This then raises the question of safety regarding use of HT in this patient population; especially the possibility of increased risk of breast cancer. Interestingly, only 10%-25% of BRCA1 carriers will have estrogen receptor–positive breast cancer, while 65%-79% of BRCA2-associated breast cancers will be positive for the receptor (Clin Cancer Res. 2004 Mar 15;10[6]:2029-34).
Unfortunately, we do not have adequate trials or studies with sufficient long-term follow-up to validate whether HT increases the risk of breast cancer or recurrence. However, the PROSE Study Group did report on a prospective cohort of 462 women with BRCA1 or BRCA2 mutations. In this study, HT did not alter the reduction in breast cancer risk from risk-reducing salpingo-oophorectomy (J Clin Oncol. 2005 Nov 1;23[31]:7804-10). In addition to a paucity of data regarding systemic HT, there is little data in the BRCA-positive population to confirm the safety of local vaginal estrogen for treatment of vaginal atrophy (J Clin Oncol. 2004 Mar 15;22[6]:1045-54).
Understanding the options
Use of HT in women with BRCA1 and BRCA2 mutations requires further investigation. There should be shared decision making between the patient and provider when counseling on the management of menopausal symptoms following risk-reducing salpingo-oophorectomy. Most importantly, women should understand the options of nonhormonal therapies and their specific side effects. They should also understand the lack of significant data regarding use of systemic HT, and that if use is elected, there may be an increased risk of breast cancer.
Women who do elect to use systemic HT following risk-reducing salpingo-oophorectomy have options that can help reduce the risk of HT-associated breast cancer, including a shorter duration of systemic HT or concurrent hysterectomy to allow for estrogen-only HT, which has a decreased risk of breast cancer compared with combined therapies that include progestins. These women may also be considering prophylactic mastectomy, which would change the concerns regarding HT and an increased risk of breast cancer.
Increased awareness of these options among physicians and patients alike can help to decrease unsatisfactory symptoms and improve quality of life in women undergoing risk-reducing salpingo-oophorectomy.
Dr. Staley is a resident physician in the department of obstetrics and gynecology at the University of North Carolina at Chapel Hill. Dr. Gehrig is professor and director of gynecologic oncology at the university. They reported having no relevant financial disclosures. Email them at obnews@frontlinemedcom.com.
Vulvar intraepithelial neoplasia: Changing terms and therapy trends
Vulvar intraepithelial neoplasia is a premalignant lesion of the vulva frequently encountered by gynecologic providers. There has been an increase in the incidence of VIN in younger women in recent decades thought be to be secondary to human papillomavirus infection, cigarette smoking, and sexual behavior (J Reprod Med. 2000 Aug;45[8]:613-5).
Data from the Surveillance Epidemiology and End Results (SEER) database were significant for a 411% increase in the incidence of in situ carcinoma and a 20% increase in invasive vulvar carcinoma from 1973 to 2000 (Obstet Gynecol. 2006 May;107[5]:1018-22). In addition, younger age groups are seeing an increase of in situ disease until age 49. Vulvar cancer however, continues to be a disease of older age.
Terminology
Previously, the term vulvar intraepithelial neoplasia followed the cervical intraepithelial neoplasia (CIN) designation in the 1960s. Conventions for grading these lesions have changed over time. Most recently, in 2004, the International Society for the Study of Vulvar Disease (ISSVD), composed of dermatologists, pathologists, and gynecologists, agreed to change the classification of squamous VIN from the previous VIN 1-3 classification system. The committee described VIN in two forms, “usual type” and “differentiated type” (J Reprod Med 2005;50:807-10).
In making this transition, it was recognized that VIN 1 is not in fact an oncogenic lesion and is now solely referred to as condyloma acuminatum. Grade 2 and 3 are now collectively referred to as VIN. These changes made by the ISSVD reflect the current literature on grading of VIN. In addition to VIN 1 not having any progression to malignancy, it is a diagnosis that is difficult to reproduce and may, at times, reflect reactive changes or other dermatosis. VIN 2 and 3 are not discriminated from each other in a reproducible manner and clinically have no reason for individual distinction (J Low Genit Tract Dis. 2006 Jul;10[3]:161-9).
VIN, usual type is the most common intraepithelial lesion and is historically referred to as classic VIN or Bowen’s disease. This type is associated with HPV infection and includes the formerly described warty type, basaloid type, and mixed type. The carcinogenic subtypes of HPV, 16, 18, 31, and 33 are the most common HPV subtypes responsible. It should be noted, however, that diagnosis is morphological and not based on HPV testing. Usual type is also traditionally thought to be more closely associated with risk factors such as smoking and immunocompromised states.
VIN, differentiated type is not associated with the HPV virus and is frequently found in older women. This lesion is often associated with other dermatologic conditions such as lichen sclerosis and lichen simplex chronicus. Diagnosis is also made by histology with abnormal cells being confined to the parabasal and basal portion of the rete pegs. This type also finds genetic alterations that are seen in invasive squamous cell carcinoma (Appl Immunohistochem Mol Morphol 2001;9:150-63). Differentiated type is thought to be a precursor for HPV-negative keratinizing squamous cell carcinoma of the vulva (Am J Surg Pathol. 2000 Mar;24[3]:429-41).
As awareness of this distinct form of VIN increases and more is learned about the precursors of HPV-negative squamous cell carcinoma, physicians are encouraged to closely follow up hyperplastic lesions and lichen sclerosis with biopsies and excision. The diagnosis of differentiated VIN is rarely made at present; however, this distinction by the ISSVD may improve the ability of clinicians and pathologists to recognize this HPV-negative precursor before squamous cell carcinoma is present.
The Lower Anogenital Squamous Terminology project of the College of American Pathology and the American Society for Colposcopy and Cervical Pathology advocates for more consistent terminology across lower anogenital tract lesions. This terminology applies only to HPV-related lesions (usual type) and considers the VIN 1 or condyloma accuminatum to be a low-grade lesion (LSIL), and VIN 2-3 or usual type to be high-grade lesions (HSIL) (Int J Gynecol Pathol. 2013 Jan;32[1]:76-115).
Many clinicians and pathologists have not adopted this most recent terminology; however, there is evidence that the ISSVD classification is the most clinically relevant.
Diagnosis
The majority of patients with any VIN will present with complaints of vulvar pruritus. However, women can also present with pain, burning, or dysuria, or can have an asymptomatic lesion found on pelvic exam. There are no recommended screening strategies to diagnose early VIN. Cytologic testing is complicated by the keratinization of the vulva, making this an unreliable diagnostic assessment.
On physical exam, VIN can have a heterogeneous presentation including papules, plaques, color variations, or ulcer. Differentiated type is thought to have a more defined appearance that frequently develops in the setting of other vulvar dermatosis. These are distinct, solitary lesions that are commonly raised, can have an overlying scale, and have ill-defined borders. A distinct lesion with ulceration or erosion is concerning for invasion.
Diagnosis is ultimately made by biopsy. Physicians should have a low threshold to biopsy any suspicious lesions or those unresponsive to therapy. Colposcopy is a frequent adjunct to the physical exam. Acetic acid 3%-5% soaked gauze is allowed to rest on the vulva for several minutes prior to observation with a colposcope or hand-held magnifying glass. Colposcopic findings are usually those of focal “white” epithelium. Vascular changes seen on the cervix (punctuation and mosaicism) are rarely seen on the vulva.
The entire anogenital region shares the same susceptibility to the HPV virus, thus squamous intraepithelial lesions are frequently multifocal. Physicians should have a heightened awareness of other lesions, such as cervical, vaginal, or anal, when managing a patient with VIN (Gynecol Oncol. 1995 Feb;56[2]:276-9). Appropriate cervical screening should be strictly adhered to and a thorough exam done at the time of vulvar colposcopy or exam.
Treatment
The goals of treatment include preventing carcinoma and improving symptoms while maintaining function and preserving anatomy. Treatment options for both types of VIN include excision, ablation, or medical therapy pending an evaluation of concurrent risk factors.
Premalignant disease was traditionally treated surgically. While surgical excision is still the mainstay of therapy, less aggressive techniques and medical therapy are more readily utilized. The goal of surgical excision for VIN is both diagnostic and therapeutic. When an excision for high-grade dysplasia is done (formerly VIN 3), detection of occult carcinoma was found in up to 3.2% in one large review (Gynecol Oncol. 2005;97:645-51).
Using a wide local excision to completely remove lesions with a pathologically clear margin reduces a patient’s risk of recurrence for disease compared to those excisions with positive margins (Obstet Gynecol. 1998;92:962-6). It is therefore critical that physicians carefully counsel patients who desire conservative therapy for VIN.
With any treatment, however, patients and physicians should be aware of the risk of recurrence; for vulvectomy, partial vulvectomy, local excision, and laser ablation, recurrences were seen at rates of 19%, 18%, 22%, and 23%, respectively, in a review of 3,322 patients (Gynecol Oncol. 2005;97:645-51).
CO2 laser ablation has been used for single lesions as well as multifocal or confluent disease. Many physicians advocate for its use in patients with multifocal lesions as well as those with disease around the clitoris or anus, where excisional therapy is less desirable as laser therapy results in less scarring.
A 2015 Cochrane Database Review of medical therapy for high-grade dysplasia (usual-type VIN, VIN 2/3, or high-grade VIN) found that topical imiquimod can be used as a safe and effective option for high-grade VIN. Physicians should, however, be aware of unfavorable side effects that may require dose reductions. Cidofovir may be an alternative to imiquimod pending more evidence on long-term response and progression (Cochrane Database Syst Rev. 2015 Aug 18;8:CD007924). Topical 5-fluorouracil has fallen out of favor for VIN given its significant chemical desquamation, however response rates are thought to be favorable if tolerated.
As the use of VIN terminology solidifies and information emerges on medical therapy to treat VIN, it is critical that physicians remain current when counseling and providing treatment recommendations for vulvar intraepithelial neoplasia.
Dr. Gehrig is professor and director of gynecologic oncology at the University of North Carolina at Chapel Hill. Dr. Clarke-Pearson is the chair and the Robert A. Ross Distinguished Professor of Obstetrics and Gynecology and professor in the division of gynecologic oncology at the university. Dr. Sullivan is a fellow in the division of gynecologic oncology at the university. They reported having no relevant financial disclosures. Email them at obnews@frontlinemedcom.com.
Vulvar intraepithelial neoplasia is a premalignant lesion of the vulva frequently encountered by gynecologic providers. There has been an increase in the incidence of VIN in younger women in recent decades thought be to be secondary to human papillomavirus infection, cigarette smoking, and sexual behavior (J Reprod Med. 2000 Aug;45[8]:613-5).
Data from the Surveillance Epidemiology and End Results (SEER) database were significant for a 411% increase in the incidence of in situ carcinoma and a 20% increase in invasive vulvar carcinoma from 1973 to 2000 (Obstet Gynecol. 2006 May;107[5]:1018-22). In addition, younger age groups are seeing an increase of in situ disease until age 49. Vulvar cancer however, continues to be a disease of older age.
Terminology
Previously, the term vulvar intraepithelial neoplasia followed the cervical intraepithelial neoplasia (CIN) designation in the 1960s. Conventions for grading these lesions have changed over time. Most recently, in 2004, the International Society for the Study of Vulvar Disease (ISSVD), composed of dermatologists, pathologists, and gynecologists, agreed to change the classification of squamous VIN from the previous VIN 1-3 classification system. The committee described VIN in two forms, “usual type” and “differentiated type” (J Reprod Med 2005;50:807-10).
In making this transition, it was recognized that VIN 1 is not in fact an oncogenic lesion and is now solely referred to as condyloma acuminatum. Grade 2 and 3 are now collectively referred to as VIN. These changes made by the ISSVD reflect the current literature on grading of VIN. In addition to VIN 1 not having any progression to malignancy, it is a diagnosis that is difficult to reproduce and may, at times, reflect reactive changes or other dermatosis. VIN 2 and 3 are not discriminated from each other in a reproducible manner and clinically have no reason for individual distinction (J Low Genit Tract Dis. 2006 Jul;10[3]:161-9).
VIN, usual type is the most common intraepithelial lesion and is historically referred to as classic VIN or Bowen’s disease. This type is associated with HPV infection and includes the formerly described warty type, basaloid type, and mixed type. The carcinogenic subtypes of HPV, 16, 18, 31, and 33 are the most common HPV subtypes responsible. It should be noted, however, that diagnosis is morphological and not based on HPV testing. Usual type is also traditionally thought to be more closely associated with risk factors such as smoking and immunocompromised states.
VIN, differentiated type is not associated with the HPV virus and is frequently found in older women. This lesion is often associated with other dermatologic conditions such as lichen sclerosis and lichen simplex chronicus. Diagnosis is also made by histology with abnormal cells being confined to the parabasal and basal portion of the rete pegs. This type also finds genetic alterations that are seen in invasive squamous cell carcinoma (Appl Immunohistochem Mol Morphol 2001;9:150-63). Differentiated type is thought to be a precursor for HPV-negative keratinizing squamous cell carcinoma of the vulva (Am J Surg Pathol. 2000 Mar;24[3]:429-41).
As awareness of this distinct form of VIN increases and more is learned about the precursors of HPV-negative squamous cell carcinoma, physicians are encouraged to closely follow up hyperplastic lesions and lichen sclerosis with biopsies and excision. The diagnosis of differentiated VIN is rarely made at present; however, this distinction by the ISSVD may improve the ability of clinicians and pathologists to recognize this HPV-negative precursor before squamous cell carcinoma is present.
The Lower Anogenital Squamous Terminology project of the College of American Pathology and the American Society for Colposcopy and Cervical Pathology advocates for more consistent terminology across lower anogenital tract lesions. This terminology applies only to HPV-related lesions (usual type) and considers the VIN 1 or condyloma accuminatum to be a low-grade lesion (LSIL), and VIN 2-3 or usual type to be high-grade lesions (HSIL) (Int J Gynecol Pathol. 2013 Jan;32[1]:76-115).
Many clinicians and pathologists have not adopted this most recent terminology; however, there is evidence that the ISSVD classification is the most clinically relevant.
Diagnosis
The majority of patients with any VIN will present with complaints of vulvar pruritus. However, women can also present with pain, burning, or dysuria, or can have an asymptomatic lesion found on pelvic exam. There are no recommended screening strategies to diagnose early VIN. Cytologic testing is complicated by the keratinization of the vulva, making this an unreliable diagnostic assessment.
On physical exam, VIN can have a heterogeneous presentation including papules, plaques, color variations, or ulcer. Differentiated type is thought to have a more defined appearance that frequently develops in the setting of other vulvar dermatosis. These are distinct, solitary lesions that are commonly raised, can have an overlying scale, and have ill-defined borders. A distinct lesion with ulceration or erosion is concerning for invasion.
Diagnosis is ultimately made by biopsy. Physicians should have a low threshold to biopsy any suspicious lesions or those unresponsive to therapy. Colposcopy is a frequent adjunct to the physical exam. Acetic acid 3%-5% soaked gauze is allowed to rest on the vulva for several minutes prior to observation with a colposcope or hand-held magnifying glass. Colposcopic findings are usually those of focal “white” epithelium. Vascular changes seen on the cervix (punctuation and mosaicism) are rarely seen on the vulva.
The entire anogenital region shares the same susceptibility to the HPV virus, thus squamous intraepithelial lesions are frequently multifocal. Physicians should have a heightened awareness of other lesions, such as cervical, vaginal, or anal, when managing a patient with VIN (Gynecol Oncol. 1995 Feb;56[2]:276-9). Appropriate cervical screening should be strictly adhered to and a thorough exam done at the time of vulvar colposcopy or exam.
Treatment
The goals of treatment include preventing carcinoma and improving symptoms while maintaining function and preserving anatomy. Treatment options for both types of VIN include excision, ablation, or medical therapy pending an evaluation of concurrent risk factors.
Premalignant disease was traditionally treated surgically. While surgical excision is still the mainstay of therapy, less aggressive techniques and medical therapy are more readily utilized. The goal of surgical excision for VIN is both diagnostic and therapeutic. When an excision for high-grade dysplasia is done (formerly VIN 3), detection of occult carcinoma was found in up to 3.2% in one large review (Gynecol Oncol. 2005;97:645-51).
Using a wide local excision to completely remove lesions with a pathologically clear margin reduces a patient’s risk of recurrence for disease compared to those excisions with positive margins (Obstet Gynecol. 1998;92:962-6). It is therefore critical that physicians carefully counsel patients who desire conservative therapy for VIN.
With any treatment, however, patients and physicians should be aware of the risk of recurrence; for vulvectomy, partial vulvectomy, local excision, and laser ablation, recurrences were seen at rates of 19%, 18%, 22%, and 23%, respectively, in a review of 3,322 patients (Gynecol Oncol. 2005;97:645-51).
CO2 laser ablation has been used for single lesions as well as multifocal or confluent disease. Many physicians advocate for its use in patients with multifocal lesions as well as those with disease around the clitoris or anus, where excisional therapy is less desirable as laser therapy results in less scarring.
A 2015 Cochrane Database Review of medical therapy for high-grade dysplasia (usual-type VIN, VIN 2/3, or high-grade VIN) found that topical imiquimod can be used as a safe and effective option for high-grade VIN. Physicians should, however, be aware of unfavorable side effects that may require dose reductions. Cidofovir may be an alternative to imiquimod pending more evidence on long-term response and progression (Cochrane Database Syst Rev. 2015 Aug 18;8:CD007924). Topical 5-fluorouracil has fallen out of favor for VIN given its significant chemical desquamation, however response rates are thought to be favorable if tolerated.
As the use of VIN terminology solidifies and information emerges on medical therapy to treat VIN, it is critical that physicians remain current when counseling and providing treatment recommendations for vulvar intraepithelial neoplasia.
Dr. Gehrig is professor and director of gynecologic oncology at the University of North Carolina at Chapel Hill. Dr. Clarke-Pearson is the chair and the Robert A. Ross Distinguished Professor of Obstetrics and Gynecology and professor in the division of gynecologic oncology at the university. Dr. Sullivan is a fellow in the division of gynecologic oncology at the university. They reported having no relevant financial disclosures. Email them at obnews@frontlinemedcom.com.
Vulvar intraepithelial neoplasia is a premalignant lesion of the vulva frequently encountered by gynecologic providers. There has been an increase in the incidence of VIN in younger women in recent decades thought be to be secondary to human papillomavirus infection, cigarette smoking, and sexual behavior (J Reprod Med. 2000 Aug;45[8]:613-5).
Data from the Surveillance Epidemiology and End Results (SEER) database were significant for a 411% increase in the incidence of in situ carcinoma and a 20% increase in invasive vulvar carcinoma from 1973 to 2000 (Obstet Gynecol. 2006 May;107[5]:1018-22). In addition, younger age groups are seeing an increase of in situ disease until age 49. Vulvar cancer however, continues to be a disease of older age.
Terminology
Previously, the term vulvar intraepithelial neoplasia followed the cervical intraepithelial neoplasia (CIN) designation in the 1960s. Conventions for grading these lesions have changed over time. Most recently, in 2004, the International Society for the Study of Vulvar Disease (ISSVD), composed of dermatologists, pathologists, and gynecologists, agreed to change the classification of squamous VIN from the previous VIN 1-3 classification system. The committee described VIN in two forms, “usual type” and “differentiated type” (J Reprod Med 2005;50:807-10).
In making this transition, it was recognized that VIN 1 is not in fact an oncogenic lesion and is now solely referred to as condyloma acuminatum. Grade 2 and 3 are now collectively referred to as VIN. These changes made by the ISSVD reflect the current literature on grading of VIN. In addition to VIN 1 not having any progression to malignancy, it is a diagnosis that is difficult to reproduce and may, at times, reflect reactive changes or other dermatosis. VIN 2 and 3 are not discriminated from each other in a reproducible manner and clinically have no reason for individual distinction (J Low Genit Tract Dis. 2006 Jul;10[3]:161-9).
VIN, usual type is the most common intraepithelial lesion and is historically referred to as classic VIN or Bowen’s disease. This type is associated with HPV infection and includes the formerly described warty type, basaloid type, and mixed type. The carcinogenic subtypes of HPV, 16, 18, 31, and 33 are the most common HPV subtypes responsible. It should be noted, however, that diagnosis is morphological and not based on HPV testing. Usual type is also traditionally thought to be more closely associated with risk factors such as smoking and immunocompromised states.
VIN, differentiated type is not associated with the HPV virus and is frequently found in older women. This lesion is often associated with other dermatologic conditions such as lichen sclerosis and lichen simplex chronicus. Diagnosis is also made by histology with abnormal cells being confined to the parabasal and basal portion of the rete pegs. This type also finds genetic alterations that are seen in invasive squamous cell carcinoma (Appl Immunohistochem Mol Morphol 2001;9:150-63). Differentiated type is thought to be a precursor for HPV-negative keratinizing squamous cell carcinoma of the vulva (Am J Surg Pathol. 2000 Mar;24[3]:429-41).
As awareness of this distinct form of VIN increases and more is learned about the precursors of HPV-negative squamous cell carcinoma, physicians are encouraged to closely follow up hyperplastic lesions and lichen sclerosis with biopsies and excision. The diagnosis of differentiated VIN is rarely made at present; however, this distinction by the ISSVD may improve the ability of clinicians and pathologists to recognize this HPV-negative precursor before squamous cell carcinoma is present.
The Lower Anogenital Squamous Terminology project of the College of American Pathology and the American Society for Colposcopy and Cervical Pathology advocates for more consistent terminology across lower anogenital tract lesions. This terminology applies only to HPV-related lesions (usual type) and considers the VIN 1 or condyloma accuminatum to be a low-grade lesion (LSIL), and VIN 2-3 or usual type to be high-grade lesions (HSIL) (Int J Gynecol Pathol. 2013 Jan;32[1]:76-115).
Many clinicians and pathologists have not adopted this most recent terminology; however, there is evidence that the ISSVD classification is the most clinically relevant.
Diagnosis
The majority of patients with any VIN will present with complaints of vulvar pruritus. However, women can also present with pain, burning, or dysuria, or can have an asymptomatic lesion found on pelvic exam. There are no recommended screening strategies to diagnose early VIN. Cytologic testing is complicated by the keratinization of the vulva, making this an unreliable diagnostic assessment.
On physical exam, VIN can have a heterogeneous presentation including papules, plaques, color variations, or ulcer. Differentiated type is thought to have a more defined appearance that frequently develops in the setting of other vulvar dermatosis. These are distinct, solitary lesions that are commonly raised, can have an overlying scale, and have ill-defined borders. A distinct lesion with ulceration or erosion is concerning for invasion.
Diagnosis is ultimately made by biopsy. Physicians should have a low threshold to biopsy any suspicious lesions or those unresponsive to therapy. Colposcopy is a frequent adjunct to the physical exam. Acetic acid 3%-5% soaked gauze is allowed to rest on the vulva for several minutes prior to observation with a colposcope or hand-held magnifying glass. Colposcopic findings are usually those of focal “white” epithelium. Vascular changes seen on the cervix (punctuation and mosaicism) are rarely seen on the vulva.
The entire anogenital region shares the same susceptibility to the HPV virus, thus squamous intraepithelial lesions are frequently multifocal. Physicians should have a heightened awareness of other lesions, such as cervical, vaginal, or anal, when managing a patient with VIN (Gynecol Oncol. 1995 Feb;56[2]:276-9). Appropriate cervical screening should be strictly adhered to and a thorough exam done at the time of vulvar colposcopy or exam.
Treatment
The goals of treatment include preventing carcinoma and improving symptoms while maintaining function and preserving anatomy. Treatment options for both types of VIN include excision, ablation, or medical therapy pending an evaluation of concurrent risk factors.
Premalignant disease was traditionally treated surgically. While surgical excision is still the mainstay of therapy, less aggressive techniques and medical therapy are more readily utilized. The goal of surgical excision for VIN is both diagnostic and therapeutic. When an excision for high-grade dysplasia is done (formerly VIN 3), detection of occult carcinoma was found in up to 3.2% in one large review (Gynecol Oncol. 2005;97:645-51).
Using a wide local excision to completely remove lesions with a pathologically clear margin reduces a patient’s risk of recurrence for disease compared to those excisions with positive margins (Obstet Gynecol. 1998;92:962-6). It is therefore critical that physicians carefully counsel patients who desire conservative therapy for VIN.
With any treatment, however, patients and physicians should be aware of the risk of recurrence; for vulvectomy, partial vulvectomy, local excision, and laser ablation, recurrences were seen at rates of 19%, 18%, 22%, and 23%, respectively, in a review of 3,322 patients (Gynecol Oncol. 2005;97:645-51).
CO2 laser ablation has been used for single lesions as well as multifocal or confluent disease. Many physicians advocate for its use in patients with multifocal lesions as well as those with disease around the clitoris or anus, where excisional therapy is less desirable as laser therapy results in less scarring.
A 2015 Cochrane Database Review of medical therapy for high-grade dysplasia (usual-type VIN, VIN 2/3, or high-grade VIN) found that topical imiquimod can be used as a safe and effective option for high-grade VIN. Physicians should, however, be aware of unfavorable side effects that may require dose reductions. Cidofovir may be an alternative to imiquimod pending more evidence on long-term response and progression (Cochrane Database Syst Rev. 2015 Aug 18;8:CD007924). Topical 5-fluorouracil has fallen out of favor for VIN given its significant chemical desquamation, however response rates are thought to be favorable if tolerated.
As the use of VIN terminology solidifies and information emerges on medical therapy to treat VIN, it is critical that physicians remain current when counseling and providing treatment recommendations for vulvar intraepithelial neoplasia.
Dr. Gehrig is professor and director of gynecologic oncology at the University of North Carolina at Chapel Hill. Dr. Clarke-Pearson is the chair and the Robert A. Ross Distinguished Professor of Obstetrics and Gynecology and professor in the division of gynecologic oncology at the university. Dr. Sullivan is a fellow in the division of gynecologic oncology at the university. They reported having no relevant financial disclosures. Email them at obnews@frontlinemedcom.com.
Recognizing and treating vulvar cancer
Vulvar cancer is a rare gynecologic cancer comprising only 5% of gynecologic malignancies. Given the low incidence of disease, many primary providers and even obstetricians and gynecologists many never encounter a case. Increased awareness of vulvar cancer and vulvar dysplasia among patients and physicians may decrease diagnostic delays and expedite patient therapy.
Diagnosis
There is a well documented delay in diagnosis of vulvar cancer that is attributed to both the patient and the physician. Patients may feel uncomfortable or embarrassed telling their physicians about vulvar symptoms and providers may not recognize the risk for malignancy and provide alternative therapies prior to biopsy (J Reprod Med. 1999;44[9]:766-8.).
Risk factors for vulvar cancer include human papillomavirus (HPV) infection, a history of smoking, immunosuppression, and a history of an abnormal pap smear. Vulvar dystrophy, lichen sclerosis, and squamous intraepithelial lesions have also been suggested as precursor lesions of invasive cancers. The key to early diagnosis and treatment is immediate in-office biopsy.
When evaluating a patient with a vulvar lesion, the initial evaluation should include a thorough exam with a measurement of the lesion and evaluation of inguinal lymph nodes. Also, a detailed description of a lesion’s relationship to the midline (how many centimeters away) and other vital structures (clitoris, urethra, anus) is important.
An in-office biopsy can be done on initial presentation and should include the lesion in question and underlying stroma in an effort to delineate depth of invasion. While shave biopsies may be appropriate for some skin lesions, if there is any concern for malignancy, a punch biopsy is preferred.
Pathology
Squamous cell carcinoma is the most common histologic subtype (greater than 90%) followed by malignant melanoma. Malignant melanoma poses a diagnostic challenge as 25% may present with nonpigmented lesions. These lesions may arise from a junctional nevus and are more common in postmenopausal white women.
The measurement of tumor thickness is essential in evaluation of melanoma. A diagnosis of vulvar melanoma should be referred to a gynecologic oncologist for further evaluation and treatment. Frequently these patients require a multidisciplinary approach with other medical and surgical subspecialties consulting.
Adenocarcinoma of the vulva frequently arises within the Bartholin glands. Bartholin gland disease is typically a disease of young women. Any abscess or lesion in the bartholin gland in women older than 50 years should raise awareness of the possibility of malignancy. Providers should have a low threshold for biopsy of any Bartholin lesion in older women and for any Bartholin gland lesion or cyst that returns or persists after initial drainage.
Staging pearls
Vulvar cancer spreads by direct extension, lymphatic embolization and hematogenous spread. Lymphatic spread can occur early in the disease and portends a much worse prognosis. In 2009, the International Federation of Gynecology and Obstetrics (FIGO) revised the staging system. The most significant change was in stage III disease, which now includes any patient with lymph node involvement. This change emphasizes lymph node status as the single most important prognostic factor. The 5-year overall survival of patients with locally advanced tumors but negative regional lymph nodes (62%) has been found to be significantly better than those with positive nodal status (39%, P value less than.0001) (Gynecol Oncol. 2008;110[1]:83-6.).
In patients with stage IA disease, which includes lesions less than 2 cm in size with stromal invasion of less than 1 mm, the risk of lymph node metastasis is low. These patients do not require inguinal lymph node dissection. If lesions are greater than 2 cm and/or have greater than 1 mm depth of invasion, a lymph node dissection is indicated. Lymph node dissection is performed on the ipsilateral side of the lesion as long as the lesion is more than 2 cm from a midline structure. If the lesion is in the midline or within 2 cm of the midline, a bilateral inguinal lymph node dissection is recommended.
There has been a recent uptake of the sentinel inguinal lymph node biopsy technique after two large prospective studies (the GROINSS V trial and GOG 173) validated this methodology (Lancet Oncol. 2010 Jul;11[7]:646-52 and Gynecol Oncol. 2013 Feb;128[2]:155-9).
Treatment
Surgical management of stage I and II disease involves a wide radical excision of the tumor with a 1-cm circumferential margin. Tumors with a depth of invasion of less than 1 mm do not require lymphadenectomy (Gynecol Oncol. 1992 Mar;44[3]:240-4). Stage I/II disease with deeper than 1-mm invasion requires a 2-cm margin and either sentinel node evaluation or lymphadenectomy. Survival for women with adequate resection of primary squamous carcinoma with negative lymph node involvement is greater than 90%.
Patients with metastasis to the groin frequently receive bilateral groin and pelvic radiation; however, recommendations are individualized based on size and number of metastasis. Patients should expect to receive recommendations for therapy after pathologic review and multidisciplinary consultation; therapy should be individualized for each clinical situation.
This disease is one of the elderly, but it is important to remember that treatment recommendations should not be made according to age alone. A British study found that when women over the age of 80 received standard treatment, their recurrence rate was 25% compared with a 53% recurrence rate in those whose treatment was modified (Int J Gynecol Cancer. 2009;19[4]:752-5.). In patients with advanced disease, preoperative radiation, with or without chemotherapy, is frequently regarded as the treatment of choice and may eliminate the need for radical surgery.
While vulvar cancer is a rare gynecologic malignancy, it can be devastating for patients and families, especially at late stages. Early diagnosis and treatment is imperative for improved patient outcomes. An increased awareness among patients and physicians alike may allow for earlier diagnosis and treatment.
Dr. Sullivan is a fellow in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. Dr. Gehrig is professor and director of gynecologic oncology at the university. Dr. Sullivan and Dr. Gehrig reported having no relevant financial disclosures.
Vulvar cancer is a rare gynecologic cancer comprising only 5% of gynecologic malignancies. Given the low incidence of disease, many primary providers and even obstetricians and gynecologists many never encounter a case. Increased awareness of vulvar cancer and vulvar dysplasia among patients and physicians may decrease diagnostic delays and expedite patient therapy.
Diagnosis
There is a well documented delay in diagnosis of vulvar cancer that is attributed to both the patient and the physician. Patients may feel uncomfortable or embarrassed telling their physicians about vulvar symptoms and providers may not recognize the risk for malignancy and provide alternative therapies prior to biopsy (J Reprod Med. 1999;44[9]:766-8.).
Risk factors for vulvar cancer include human papillomavirus (HPV) infection, a history of smoking, immunosuppression, and a history of an abnormal pap smear. Vulvar dystrophy, lichen sclerosis, and squamous intraepithelial lesions have also been suggested as precursor lesions of invasive cancers. The key to early diagnosis and treatment is immediate in-office biopsy.
When evaluating a patient with a vulvar lesion, the initial evaluation should include a thorough exam with a measurement of the lesion and evaluation of inguinal lymph nodes. Also, a detailed description of a lesion’s relationship to the midline (how many centimeters away) and other vital structures (clitoris, urethra, anus) is important.
An in-office biopsy can be done on initial presentation and should include the lesion in question and underlying stroma in an effort to delineate depth of invasion. While shave biopsies may be appropriate for some skin lesions, if there is any concern for malignancy, a punch biopsy is preferred.
Pathology
Squamous cell carcinoma is the most common histologic subtype (greater than 90%) followed by malignant melanoma. Malignant melanoma poses a diagnostic challenge as 25% may present with nonpigmented lesions. These lesions may arise from a junctional nevus and are more common in postmenopausal white women.
The measurement of tumor thickness is essential in evaluation of melanoma. A diagnosis of vulvar melanoma should be referred to a gynecologic oncologist for further evaluation and treatment. Frequently these patients require a multidisciplinary approach with other medical and surgical subspecialties consulting.
Adenocarcinoma of the vulva frequently arises within the Bartholin glands. Bartholin gland disease is typically a disease of young women. Any abscess or lesion in the bartholin gland in women older than 50 years should raise awareness of the possibility of malignancy. Providers should have a low threshold for biopsy of any Bartholin lesion in older women and for any Bartholin gland lesion or cyst that returns or persists after initial drainage.
Staging pearls
Vulvar cancer spreads by direct extension, lymphatic embolization and hematogenous spread. Lymphatic spread can occur early in the disease and portends a much worse prognosis. In 2009, the International Federation of Gynecology and Obstetrics (FIGO) revised the staging system. The most significant change was in stage III disease, which now includes any patient with lymph node involvement. This change emphasizes lymph node status as the single most important prognostic factor. The 5-year overall survival of patients with locally advanced tumors but negative regional lymph nodes (62%) has been found to be significantly better than those with positive nodal status (39%, P value less than.0001) (Gynecol Oncol. 2008;110[1]:83-6.).
In patients with stage IA disease, which includes lesions less than 2 cm in size with stromal invasion of less than 1 mm, the risk of lymph node metastasis is low. These patients do not require inguinal lymph node dissection. If lesions are greater than 2 cm and/or have greater than 1 mm depth of invasion, a lymph node dissection is indicated. Lymph node dissection is performed on the ipsilateral side of the lesion as long as the lesion is more than 2 cm from a midline structure. If the lesion is in the midline or within 2 cm of the midline, a bilateral inguinal lymph node dissection is recommended.
There has been a recent uptake of the sentinel inguinal lymph node biopsy technique after two large prospective studies (the GROINSS V trial and GOG 173) validated this methodology (Lancet Oncol. 2010 Jul;11[7]:646-52 and Gynecol Oncol. 2013 Feb;128[2]:155-9).
Treatment
Surgical management of stage I and II disease involves a wide radical excision of the tumor with a 1-cm circumferential margin. Tumors with a depth of invasion of less than 1 mm do not require lymphadenectomy (Gynecol Oncol. 1992 Mar;44[3]:240-4). Stage I/II disease with deeper than 1-mm invasion requires a 2-cm margin and either sentinel node evaluation or lymphadenectomy. Survival for women with adequate resection of primary squamous carcinoma with negative lymph node involvement is greater than 90%.
Patients with metastasis to the groin frequently receive bilateral groin and pelvic radiation; however, recommendations are individualized based on size and number of metastasis. Patients should expect to receive recommendations for therapy after pathologic review and multidisciplinary consultation; therapy should be individualized for each clinical situation.
This disease is one of the elderly, but it is important to remember that treatment recommendations should not be made according to age alone. A British study found that when women over the age of 80 received standard treatment, their recurrence rate was 25% compared with a 53% recurrence rate in those whose treatment was modified (Int J Gynecol Cancer. 2009;19[4]:752-5.). In patients with advanced disease, preoperative radiation, with or without chemotherapy, is frequently regarded as the treatment of choice and may eliminate the need for radical surgery.
While vulvar cancer is a rare gynecologic malignancy, it can be devastating for patients and families, especially at late stages. Early diagnosis and treatment is imperative for improved patient outcomes. An increased awareness among patients and physicians alike may allow for earlier diagnosis and treatment.
Dr. Sullivan is a fellow in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. Dr. Gehrig is professor and director of gynecologic oncology at the university. Dr. Sullivan and Dr. Gehrig reported having no relevant financial disclosures.
Vulvar cancer is a rare gynecologic cancer comprising only 5% of gynecologic malignancies. Given the low incidence of disease, many primary providers and even obstetricians and gynecologists many never encounter a case. Increased awareness of vulvar cancer and vulvar dysplasia among patients and physicians may decrease diagnostic delays and expedite patient therapy.
Diagnosis
There is a well documented delay in diagnosis of vulvar cancer that is attributed to both the patient and the physician. Patients may feel uncomfortable or embarrassed telling their physicians about vulvar symptoms and providers may not recognize the risk for malignancy and provide alternative therapies prior to biopsy (J Reprod Med. 1999;44[9]:766-8.).
Risk factors for vulvar cancer include human papillomavirus (HPV) infection, a history of smoking, immunosuppression, and a history of an abnormal pap smear. Vulvar dystrophy, lichen sclerosis, and squamous intraepithelial lesions have also been suggested as precursor lesions of invasive cancers. The key to early diagnosis and treatment is immediate in-office biopsy.
When evaluating a patient with a vulvar lesion, the initial evaluation should include a thorough exam with a measurement of the lesion and evaluation of inguinal lymph nodes. Also, a detailed description of a lesion’s relationship to the midline (how many centimeters away) and other vital structures (clitoris, urethra, anus) is important.
An in-office biopsy can be done on initial presentation and should include the lesion in question and underlying stroma in an effort to delineate depth of invasion. While shave biopsies may be appropriate for some skin lesions, if there is any concern for malignancy, a punch biopsy is preferred.
Pathology
Squamous cell carcinoma is the most common histologic subtype (greater than 90%) followed by malignant melanoma. Malignant melanoma poses a diagnostic challenge as 25% may present with nonpigmented lesions. These lesions may arise from a junctional nevus and are more common in postmenopausal white women.
The measurement of tumor thickness is essential in evaluation of melanoma. A diagnosis of vulvar melanoma should be referred to a gynecologic oncologist for further evaluation and treatment. Frequently these patients require a multidisciplinary approach with other medical and surgical subspecialties consulting.
Adenocarcinoma of the vulva frequently arises within the Bartholin glands. Bartholin gland disease is typically a disease of young women. Any abscess or lesion in the bartholin gland in women older than 50 years should raise awareness of the possibility of malignancy. Providers should have a low threshold for biopsy of any Bartholin lesion in older women and for any Bartholin gland lesion or cyst that returns or persists after initial drainage.
Staging pearls
Vulvar cancer spreads by direct extension, lymphatic embolization and hematogenous spread. Lymphatic spread can occur early in the disease and portends a much worse prognosis. In 2009, the International Federation of Gynecology and Obstetrics (FIGO) revised the staging system. The most significant change was in stage III disease, which now includes any patient with lymph node involvement. This change emphasizes lymph node status as the single most important prognostic factor. The 5-year overall survival of patients with locally advanced tumors but negative regional lymph nodes (62%) has been found to be significantly better than those with positive nodal status (39%, P value less than.0001) (Gynecol Oncol. 2008;110[1]:83-6.).
In patients with stage IA disease, which includes lesions less than 2 cm in size with stromal invasion of less than 1 mm, the risk of lymph node metastasis is low. These patients do not require inguinal lymph node dissection. If lesions are greater than 2 cm and/or have greater than 1 mm depth of invasion, a lymph node dissection is indicated. Lymph node dissection is performed on the ipsilateral side of the lesion as long as the lesion is more than 2 cm from a midline structure. If the lesion is in the midline or within 2 cm of the midline, a bilateral inguinal lymph node dissection is recommended.
There has been a recent uptake of the sentinel inguinal lymph node biopsy technique after two large prospective studies (the GROINSS V trial and GOG 173) validated this methodology (Lancet Oncol. 2010 Jul;11[7]:646-52 and Gynecol Oncol. 2013 Feb;128[2]:155-9).
Treatment
Surgical management of stage I and II disease involves a wide radical excision of the tumor with a 1-cm circumferential margin. Tumors with a depth of invasion of less than 1 mm do not require lymphadenectomy (Gynecol Oncol. 1992 Mar;44[3]:240-4). Stage I/II disease with deeper than 1-mm invasion requires a 2-cm margin and either sentinel node evaluation or lymphadenectomy. Survival for women with adequate resection of primary squamous carcinoma with negative lymph node involvement is greater than 90%.
Patients with metastasis to the groin frequently receive bilateral groin and pelvic radiation; however, recommendations are individualized based on size and number of metastasis. Patients should expect to receive recommendations for therapy after pathologic review and multidisciplinary consultation; therapy should be individualized for each clinical situation.
This disease is one of the elderly, but it is important to remember that treatment recommendations should not be made according to age alone. A British study found that when women over the age of 80 received standard treatment, their recurrence rate was 25% compared with a 53% recurrence rate in those whose treatment was modified (Int J Gynecol Cancer. 2009;19[4]:752-5.). In patients with advanced disease, preoperative radiation, with or without chemotherapy, is frequently regarded as the treatment of choice and may eliminate the need for radical surgery.
While vulvar cancer is a rare gynecologic malignancy, it can be devastating for patients and families, especially at late stages. Early diagnosis and treatment is imperative for improved patient outcomes. An increased awareness among patients and physicians alike may allow for earlier diagnosis and treatment.
Dr. Sullivan is a fellow in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. Dr. Gehrig is professor and director of gynecologic oncology at the university. Dr. Sullivan and Dr. Gehrig reported having no relevant financial disclosures.
Selecting the right contraception method for cancer patients
Patient choice, contraceptive effectiveness, and medical eligibility all need to be incorporated into the contraceptive counseling for reproductive-age women who have cancer or are in remission. Based on these principles, women can minimize the risk of an unintended pregnancy, continue to receive necessary adjuvant or preventive therapy, and maintain high levels of contraception satisfaction.
The Centers for Disease Control and Prevention (CDC) has published medical eligibility criteria (MEC) to assist providers in selecting medically appropriate contraception for women with various health conditions, including cancer (MMWR Recomm. Rep. 2010;59(RR-4):1-6).
Certain classes of hormonal contraception are contraindicated in specific cancer types. It is important to note that the copper intrauterine device (ParaGard) is very effective (with a first-year failure rate of 0.8%) and has no cancer-related contraindications. Any contraceptive with estrogen or progesterone is relatively contraindicated in hormonally mediated cancers, including breast, endometrial, or other cancers that have estrogen (ER) or progesterone (PR) positive receptors. Combined hormonal contraception is contraindicated even in breast cancers that are ER/PR negative for the first 5 years, after which they are CDC MEC category 3 (risks likely outweigh the benefits).
Venous thromboembolism (VTE) is an important cancer-related morbidity. Active cancer increases the risk of VTE by fourfold, which is further increased if the patient is on chemotherapy (Arch. Intern. Med. 2000;160:809-15). Estrogen is known to increase thrombotic risk, and therefore it is contraindicated in any patient at risk for VTE or with a history of a VTE. There is some debate about the use of progestin-only contraceptives in those at risk of (or with a history of) VTE. The best evidence and CDC guidelines indicate that progestin-only methods can be used in patients with cancer or with a history of VTE. Importantly, no known association exists between emergency contraception and VTE (Obstet. Gynecol. 2010;115:1100-9).
Other cancer-specific problems that may impact contraception include thrombocytopenia, gastrointestinal side effects, and drug interactions. Thrombocytopenia may exacerbate or cause abnormal uterine bleeding. Therefore, menstrual suppression with continuous combined hormonal contraception or progestin-only methods, including the hormonal IUD and implant, may be ideal. Regarding gastrointestinal side effects, emesis and mucositis from cancer and treatment may reduce absorption of oral contraceptives, so alternatives should be considered. Antacids, analgesics, antifungals, anticonvulsants, and antiretrovirals are all known to affect hepatic metabolism and may affect oral contraceptive efficacy.
Given the possibility of chemotherapy-induced immunosuppression, there is a theoretical concern about the infectious risk of an indwelling foreign body such as an IUD or implant. The best evidence to date, however, does not support an increased risk, even in the setting of neutropenia. Chemotherapy also increases osteoporosis. Gynecologists should use caution with depot medroxyprogesterone acetate (DMPA), although there is no absolute contraindication, especially for shorter durations of use.
Many breast cancer patients are prescribed tamoxifen as adjuvant therapy, but the antiestrogenic effects of tamoxifen may not prevent pregnancy (Cancer Imaging 2008;8:135-45). Therefore, it is critical for reproductive-age women taking tamoxifen to be given effective contraception. Experts have not reached a consensus on the use of levonorgestrel intrauterine systems (LNG-IUS, Mirena, or Skyla) in the setting of breast cancer.
On the one hand, patients on long-term tamoxifen may benefit from the endometrial protective effect of an LNG-IUS (Lancet 2000;356:1711-7). It is uncertain if women with an LNG-IUS in place at the time of breast cancer diagnosis should have the device removed. Placing a LNG-IUS is contraindicated in all cases of active cancer, but if the patient has no evidence of disease for more than 5 years, the CDC lists the LNG-IUS as category 3. Expert consensus is that studies are needed with LNG-IUS use in women with breast cancer and that use of the LNG-IUS in this population should be made with careful consideration of the risks and benefits (Fertil. Steril. 2008;90:17-22; Contraception 2012;86:191-8).
Physicians should consider the contraceptive needs of women who are actively being or have recently been treated for cancer, as 17% of female cancers occur in women of reproductive age. The copper IUD is a highly effective option with very few contraindications. In patients with a history of non–hormonal related cancer (and without any history of VTE), all contraceptive options can be considered, including those containing estrogen. Estrogen-containing contraceptives should be avoided in those with a history of hormonally related cancers. Those not familiar with the wide array of options should consider referring early, and family planning specialists should consider medical eligibility while counseling women about the most effective contraceptive options.
Dr. Zerden is a family planning fellow in the department of obstetrics and gynecology at the University of North Carolina at Chapel Hill. He reported having no financial disclosures. E-mail Dr. Zerden at obnews@frontlinemedcom.com.
Patient choice, contraceptive effectiveness, and medical eligibility all need to be incorporated into the contraceptive counseling for reproductive-age women who have cancer or are in remission. Based on these principles, women can minimize the risk of an unintended pregnancy, continue to receive necessary adjuvant or preventive therapy, and maintain high levels of contraception satisfaction.
The Centers for Disease Control and Prevention (CDC) has published medical eligibility criteria (MEC) to assist providers in selecting medically appropriate contraception for women with various health conditions, including cancer (MMWR Recomm. Rep. 2010;59(RR-4):1-6).
Certain classes of hormonal contraception are contraindicated in specific cancer types. It is important to note that the copper intrauterine device (ParaGard) is very effective (with a first-year failure rate of 0.8%) and has no cancer-related contraindications. Any contraceptive with estrogen or progesterone is relatively contraindicated in hormonally mediated cancers, including breast, endometrial, or other cancers that have estrogen (ER) or progesterone (PR) positive receptors. Combined hormonal contraception is contraindicated even in breast cancers that are ER/PR negative for the first 5 years, after which they are CDC MEC category 3 (risks likely outweigh the benefits).
Venous thromboembolism (VTE) is an important cancer-related morbidity. Active cancer increases the risk of VTE by fourfold, which is further increased if the patient is on chemotherapy (Arch. Intern. Med. 2000;160:809-15). Estrogen is known to increase thrombotic risk, and therefore it is contraindicated in any patient at risk for VTE or with a history of a VTE. There is some debate about the use of progestin-only contraceptives in those at risk of (or with a history of) VTE. The best evidence and CDC guidelines indicate that progestin-only methods can be used in patients with cancer or with a history of VTE. Importantly, no known association exists between emergency contraception and VTE (Obstet. Gynecol. 2010;115:1100-9).
Other cancer-specific problems that may impact contraception include thrombocytopenia, gastrointestinal side effects, and drug interactions. Thrombocytopenia may exacerbate or cause abnormal uterine bleeding. Therefore, menstrual suppression with continuous combined hormonal contraception or progestin-only methods, including the hormonal IUD and implant, may be ideal. Regarding gastrointestinal side effects, emesis and mucositis from cancer and treatment may reduce absorption of oral contraceptives, so alternatives should be considered. Antacids, analgesics, antifungals, anticonvulsants, and antiretrovirals are all known to affect hepatic metabolism and may affect oral contraceptive efficacy.
Given the possibility of chemotherapy-induced immunosuppression, there is a theoretical concern about the infectious risk of an indwelling foreign body such as an IUD or implant. The best evidence to date, however, does not support an increased risk, even in the setting of neutropenia. Chemotherapy also increases osteoporosis. Gynecologists should use caution with depot medroxyprogesterone acetate (DMPA), although there is no absolute contraindication, especially for shorter durations of use.
Many breast cancer patients are prescribed tamoxifen as adjuvant therapy, but the antiestrogenic effects of tamoxifen may not prevent pregnancy (Cancer Imaging 2008;8:135-45). Therefore, it is critical for reproductive-age women taking tamoxifen to be given effective contraception. Experts have not reached a consensus on the use of levonorgestrel intrauterine systems (LNG-IUS, Mirena, or Skyla) in the setting of breast cancer.
On the one hand, patients on long-term tamoxifen may benefit from the endometrial protective effect of an LNG-IUS (Lancet 2000;356:1711-7). It is uncertain if women with an LNG-IUS in place at the time of breast cancer diagnosis should have the device removed. Placing a LNG-IUS is contraindicated in all cases of active cancer, but if the patient has no evidence of disease for more than 5 years, the CDC lists the LNG-IUS as category 3. Expert consensus is that studies are needed with LNG-IUS use in women with breast cancer and that use of the LNG-IUS in this population should be made with careful consideration of the risks and benefits (Fertil. Steril. 2008;90:17-22; Contraception 2012;86:191-8).
Physicians should consider the contraceptive needs of women who are actively being or have recently been treated for cancer, as 17% of female cancers occur in women of reproductive age. The copper IUD is a highly effective option with very few contraindications. In patients with a history of non–hormonal related cancer (and without any history of VTE), all contraceptive options can be considered, including those containing estrogen. Estrogen-containing contraceptives should be avoided in those with a history of hormonally related cancers. Those not familiar with the wide array of options should consider referring early, and family planning specialists should consider medical eligibility while counseling women about the most effective contraceptive options.
Dr. Zerden is a family planning fellow in the department of obstetrics and gynecology at the University of North Carolina at Chapel Hill. He reported having no financial disclosures. E-mail Dr. Zerden at obnews@frontlinemedcom.com.
Patient choice, contraceptive effectiveness, and medical eligibility all need to be incorporated into the contraceptive counseling for reproductive-age women who have cancer or are in remission. Based on these principles, women can minimize the risk of an unintended pregnancy, continue to receive necessary adjuvant or preventive therapy, and maintain high levels of contraception satisfaction.
The Centers for Disease Control and Prevention (CDC) has published medical eligibility criteria (MEC) to assist providers in selecting medically appropriate contraception for women with various health conditions, including cancer (MMWR Recomm. Rep. 2010;59(RR-4):1-6).
Certain classes of hormonal contraception are contraindicated in specific cancer types. It is important to note that the copper intrauterine device (ParaGard) is very effective (with a first-year failure rate of 0.8%) and has no cancer-related contraindications. Any contraceptive with estrogen or progesterone is relatively contraindicated in hormonally mediated cancers, including breast, endometrial, or other cancers that have estrogen (ER) or progesterone (PR) positive receptors. Combined hormonal contraception is contraindicated even in breast cancers that are ER/PR negative for the first 5 years, after which they are CDC MEC category 3 (risks likely outweigh the benefits).
Venous thromboembolism (VTE) is an important cancer-related morbidity. Active cancer increases the risk of VTE by fourfold, which is further increased if the patient is on chemotherapy (Arch. Intern. Med. 2000;160:809-15). Estrogen is known to increase thrombotic risk, and therefore it is contraindicated in any patient at risk for VTE or with a history of a VTE. There is some debate about the use of progestin-only contraceptives in those at risk of (or with a history of) VTE. The best evidence and CDC guidelines indicate that progestin-only methods can be used in patients with cancer or with a history of VTE. Importantly, no known association exists between emergency contraception and VTE (Obstet. Gynecol. 2010;115:1100-9).
Other cancer-specific problems that may impact contraception include thrombocytopenia, gastrointestinal side effects, and drug interactions. Thrombocytopenia may exacerbate or cause abnormal uterine bleeding. Therefore, menstrual suppression with continuous combined hormonal contraception or progestin-only methods, including the hormonal IUD and implant, may be ideal. Regarding gastrointestinal side effects, emesis and mucositis from cancer and treatment may reduce absorption of oral contraceptives, so alternatives should be considered. Antacids, analgesics, antifungals, anticonvulsants, and antiretrovirals are all known to affect hepatic metabolism and may affect oral contraceptive efficacy.
Given the possibility of chemotherapy-induced immunosuppression, there is a theoretical concern about the infectious risk of an indwelling foreign body such as an IUD or implant. The best evidence to date, however, does not support an increased risk, even in the setting of neutropenia. Chemotherapy also increases osteoporosis. Gynecologists should use caution with depot medroxyprogesterone acetate (DMPA), although there is no absolute contraindication, especially for shorter durations of use.
Many breast cancer patients are prescribed tamoxifen as adjuvant therapy, but the antiestrogenic effects of tamoxifen may not prevent pregnancy (Cancer Imaging 2008;8:135-45). Therefore, it is critical for reproductive-age women taking tamoxifen to be given effective contraception. Experts have not reached a consensus on the use of levonorgestrel intrauterine systems (LNG-IUS, Mirena, or Skyla) in the setting of breast cancer.
On the one hand, patients on long-term tamoxifen may benefit from the endometrial protective effect of an LNG-IUS (Lancet 2000;356:1711-7). It is uncertain if women with an LNG-IUS in place at the time of breast cancer diagnosis should have the device removed. Placing a LNG-IUS is contraindicated in all cases of active cancer, but if the patient has no evidence of disease for more than 5 years, the CDC lists the LNG-IUS as category 3. Expert consensus is that studies are needed with LNG-IUS use in women with breast cancer and that use of the LNG-IUS in this population should be made with careful consideration of the risks and benefits (Fertil. Steril. 2008;90:17-22; Contraception 2012;86:191-8).
Physicians should consider the contraceptive needs of women who are actively being or have recently been treated for cancer, as 17% of female cancers occur in women of reproductive age. The copper IUD is a highly effective option with very few contraindications. In patients with a history of non–hormonal related cancer (and without any history of VTE), all contraceptive options can be considered, including those containing estrogen. Estrogen-containing contraceptives should be avoided in those with a history of hormonally related cancers. Those not familiar with the wide array of options should consider referring early, and family planning specialists should consider medical eligibility while counseling women about the most effective contraceptive options.
Dr. Zerden is a family planning fellow in the department of obstetrics and gynecology at the University of North Carolina at Chapel Hill. He reported having no financial disclosures. E-mail Dr. Zerden at obnews@frontlinemedcom.com.
Addressing unmet contraception needs in patients with cancer
Approximately 740,000 women are diagnosed with cancer every year in the United States, and because of improved screening, diagnosis, and treatment, women of reproductive age have an 80%-90% 5-year survival rate. The most common cancers in reproductive age women include breast, thyroid, melanoma, colorectal, and cervical cancers. Fertility intention is a critical topic to discuss with reproductive-age cancer patients. Women with cancer often have unmet contraception needs during and following cancer treatment. Providing women with a desired, effective form of contraception that is appropriate with regard to the cancer is critical.
Multiple studies have demonstrated that pregnancy prevention is not adequately addressed in cancer patients. On the one hand, many patients believe they are no longer fertile because of a combination of the illness and the cancer treatment, and on the other hand, many providers may not be adequately trained to offer their patients the full range of contraceptive options (Am. J. Obstet. Gynecol. 2009;201:191.e1-4). One study demonstrated that discussions around fecundity and contraception are occurring about 50% of the time (J. Natl. Cancer. Inst. Monogr. 2005:98-100).
In response, the American Society for Reproductive Medicine has issued guidelines regarding fertility planning in cancer patients (Fertil. Steril. 2005;83:1622-8). While every patient’s circumstance is unique, recommendations are for patients to avoid pregnancy for at least 1 year beyond the completion of medical and surgical treatment of cancer. For those cancers that are hormone mediated, recommendations are to wait 2-5 years before attempting to conceive (J. Obstet. Gynaecol. Can. 2002;24:164-80; J. Gen. Intern. Med. 2009; 24: S401-6).
Unless patients are educated about and offered the most effective forms of contraception, they are at risk of unintended pregnancy, which may result in severe consequences, as patients may be on teratogenic medications or dealing with comorbid conditions originating from cancer and cancer treatment (Contraception 2012;86:191-8).
Cancer treatments have variable impact on subsequent fertility (with the obvious exception of surgical removal of gynecologic organs resulting in sterilization). With all nonsurgical cancer treatments, the potential for subsequent fertility depends on the chemotherapeutic agents, the duration of treatment, or use of pelvic radiation. As in patients without cancer, age is inversely related to subsequent fertility. Reviews of the literature have shown that fecundability decreases by 10%-50% post chemotherapy.
Clinicians caring for these women may find it challenging to assess future fertility. Some chemotherapies induce amenorrhea, but spontaneous return of menstruation and ovarian function is possible in younger women. Traditional diagnostic tests to assess fertility, including serum FSH (follicle stimulating hormone) and/or AMH (anti-Müllerian hormone), may help in predicting future fertility. These tests can be used both in patients who desire to pursue pregnancy and in those desiring to avoid pregnancy as menstrual status may not accurately predict fertility.
Contraception counseling should begin by informing women of the most effective forms of contraception (Obstet. Gynecol. 2011;118:184-96). It is important to consider the option of sterilization, especially when this desire predated the cancer diagnosis. In patients who are in a monogamous relationship with a male partner, vasectomy should be encouraged as a safe and effective alternative. When a woman is considering sterilization, she needs to be counseled as to the risk of regret, which is higher in younger women. Sterilization should not be performed if the consent or decision-making process is rushed by the cancer treatment.
As cancer screening, diagnosis, and treatment continue to improve, more reproductive-age women will be living longer with a need for effective contraception. In the next edition of Gynecologic Oncology Consult, I will review the safety and efficacy of specific contraceptive methods in patients with cancer.
Dr. Zerden is a family planning fellow in the department of obstetrics and gynecology at the University of North Carolina at Chapel Hill. His research interests include postpartum contraception, methods of female sterilization, and family planning health services integration. He reported having no financial disclosures.
Approximately 740,000 women are diagnosed with cancer every year in the United States, and because of improved screening, diagnosis, and treatment, women of reproductive age have an 80%-90% 5-year survival rate. The most common cancers in reproductive age women include breast, thyroid, melanoma, colorectal, and cervical cancers. Fertility intention is a critical topic to discuss with reproductive-age cancer patients. Women with cancer often have unmet contraception needs during and following cancer treatment. Providing women with a desired, effective form of contraception that is appropriate with regard to the cancer is critical.
Multiple studies have demonstrated that pregnancy prevention is not adequately addressed in cancer patients. On the one hand, many patients believe they are no longer fertile because of a combination of the illness and the cancer treatment, and on the other hand, many providers may not be adequately trained to offer their patients the full range of contraceptive options (Am. J. Obstet. Gynecol. 2009;201:191.e1-4). One study demonstrated that discussions around fecundity and contraception are occurring about 50% of the time (J. Natl. Cancer. Inst. Monogr. 2005:98-100).
In response, the American Society for Reproductive Medicine has issued guidelines regarding fertility planning in cancer patients (Fertil. Steril. 2005;83:1622-8). While every patient’s circumstance is unique, recommendations are for patients to avoid pregnancy for at least 1 year beyond the completion of medical and surgical treatment of cancer. For those cancers that are hormone mediated, recommendations are to wait 2-5 years before attempting to conceive (J. Obstet. Gynaecol. Can. 2002;24:164-80; J. Gen. Intern. Med. 2009; 24: S401-6).
Unless patients are educated about and offered the most effective forms of contraception, they are at risk of unintended pregnancy, which may result in severe consequences, as patients may be on teratogenic medications or dealing with comorbid conditions originating from cancer and cancer treatment (Contraception 2012;86:191-8).
Cancer treatments have variable impact on subsequent fertility (with the obvious exception of surgical removal of gynecologic organs resulting in sterilization). With all nonsurgical cancer treatments, the potential for subsequent fertility depends on the chemotherapeutic agents, the duration of treatment, or use of pelvic radiation. As in patients without cancer, age is inversely related to subsequent fertility. Reviews of the literature have shown that fecundability decreases by 10%-50% post chemotherapy.
Clinicians caring for these women may find it challenging to assess future fertility. Some chemotherapies induce amenorrhea, but spontaneous return of menstruation and ovarian function is possible in younger women. Traditional diagnostic tests to assess fertility, including serum FSH (follicle stimulating hormone) and/or AMH (anti-Müllerian hormone), may help in predicting future fertility. These tests can be used both in patients who desire to pursue pregnancy and in those desiring to avoid pregnancy as menstrual status may not accurately predict fertility.
Contraception counseling should begin by informing women of the most effective forms of contraception (Obstet. Gynecol. 2011;118:184-96). It is important to consider the option of sterilization, especially when this desire predated the cancer diagnosis. In patients who are in a monogamous relationship with a male partner, vasectomy should be encouraged as a safe and effective alternative. When a woman is considering sterilization, she needs to be counseled as to the risk of regret, which is higher in younger women. Sterilization should not be performed if the consent or decision-making process is rushed by the cancer treatment.
As cancer screening, diagnosis, and treatment continue to improve, more reproductive-age women will be living longer with a need for effective contraception. In the next edition of Gynecologic Oncology Consult, I will review the safety and efficacy of specific contraceptive methods in patients with cancer.
Dr. Zerden is a family planning fellow in the department of obstetrics and gynecology at the University of North Carolina at Chapel Hill. His research interests include postpartum contraception, methods of female sterilization, and family planning health services integration. He reported having no financial disclosures.
Approximately 740,000 women are diagnosed with cancer every year in the United States, and because of improved screening, diagnosis, and treatment, women of reproductive age have an 80%-90% 5-year survival rate. The most common cancers in reproductive age women include breast, thyroid, melanoma, colorectal, and cervical cancers. Fertility intention is a critical topic to discuss with reproductive-age cancer patients. Women with cancer often have unmet contraception needs during and following cancer treatment. Providing women with a desired, effective form of contraception that is appropriate with regard to the cancer is critical.
Multiple studies have demonstrated that pregnancy prevention is not adequately addressed in cancer patients. On the one hand, many patients believe they are no longer fertile because of a combination of the illness and the cancer treatment, and on the other hand, many providers may not be adequately trained to offer their patients the full range of contraceptive options (Am. J. Obstet. Gynecol. 2009;201:191.e1-4). One study demonstrated that discussions around fecundity and contraception are occurring about 50% of the time (J. Natl. Cancer. Inst. Monogr. 2005:98-100).
In response, the American Society for Reproductive Medicine has issued guidelines regarding fertility planning in cancer patients (Fertil. Steril. 2005;83:1622-8). While every patient’s circumstance is unique, recommendations are for patients to avoid pregnancy for at least 1 year beyond the completion of medical and surgical treatment of cancer. For those cancers that are hormone mediated, recommendations are to wait 2-5 years before attempting to conceive (J. Obstet. Gynaecol. Can. 2002;24:164-80; J. Gen. Intern. Med. 2009; 24: S401-6).
Unless patients are educated about and offered the most effective forms of contraception, they are at risk of unintended pregnancy, which may result in severe consequences, as patients may be on teratogenic medications or dealing with comorbid conditions originating from cancer and cancer treatment (Contraception 2012;86:191-8).
Cancer treatments have variable impact on subsequent fertility (with the obvious exception of surgical removal of gynecologic organs resulting in sterilization). With all nonsurgical cancer treatments, the potential for subsequent fertility depends on the chemotherapeutic agents, the duration of treatment, or use of pelvic radiation. As in patients without cancer, age is inversely related to subsequent fertility. Reviews of the literature have shown that fecundability decreases by 10%-50% post chemotherapy.
Clinicians caring for these women may find it challenging to assess future fertility. Some chemotherapies induce amenorrhea, but spontaneous return of menstruation and ovarian function is possible in younger women. Traditional diagnostic tests to assess fertility, including serum FSH (follicle stimulating hormone) and/or AMH (anti-Müllerian hormone), may help in predicting future fertility. These tests can be used both in patients who desire to pursue pregnancy and in those desiring to avoid pregnancy as menstrual status may not accurately predict fertility.
Contraception counseling should begin by informing women of the most effective forms of contraception (Obstet. Gynecol. 2011;118:184-96). It is important to consider the option of sterilization, especially when this desire predated the cancer diagnosis. In patients who are in a monogamous relationship with a male partner, vasectomy should be encouraged as a safe and effective alternative. When a woman is considering sterilization, she needs to be counseled as to the risk of regret, which is higher in younger women. Sterilization should not be performed if the consent or decision-making process is rushed by the cancer treatment.
As cancer screening, diagnosis, and treatment continue to improve, more reproductive-age women will be living longer with a need for effective contraception. In the next edition of Gynecologic Oncology Consult, I will review the safety and efficacy of specific contraceptive methods in patients with cancer.
Dr. Zerden is a family planning fellow in the department of obstetrics and gynecology at the University of North Carolina at Chapel Hill. His research interests include postpartum contraception, methods of female sterilization, and family planning health services integration. He reported having no financial disclosures.
Managing open wounds in ob.gyn.
Negative pressure wound therapy is a wound management system for chronic open subcutaneous or intra-abdominal wounds. Some popular commercial systems include V.A.C. therapy (KCI, San Antonio) and the Chariker-Jeter wound-sealing kit (Smith and Nephew, London). Within ob.gyn. and gynecologic oncology, they have use in the management of postoperative superficial wound dehiscence from routine surgery and in the management of the open abdomen.
The primary benefit of negative pressure wound therapy (NPWT) is the acceleration of wound healing. Postoperative superficial wound dehiscence can occur as a result of surgical factors such as wound infection and subcutaneous seroma/hematoma or systematic factors such as poor nutrition and wound ischemia.
Acceleration of wound healing results from the design of the NPWT systems. They consist of semipermeable dressings (foam), sealed with an adhesive sheet that is connected to a portable pump. By the application of –50 to –175 mm Hg of continuous or intermittent suction, the edges of the wound are drawn together, and this deforming process promotes tissue remodeling at the cellular level. Other potential benefits of negative pressure are increased blood flow, a decrease in mediators of inflammation, and an increase in collagen organization via changes in wound biochemistry.
An alternative to NPWT would be traditional gauze dressings, which can also be applied in the case of superficial wound dehiscence. These are changed up to three times a day, however, and this can result in significant patient discomfort, caregiver difficulties, and prolonged healing of weeks to months. In contrast, NPWT dressings are changed once every 2-3 days. They are also versatile and can be fit to traditionally shaped abdominal wounds, as well as difficult to dress vulvar and groin wounds (J. Obstet. Gynaecol. Can. 2011;33:1031-7).
In a series of 27 gynecologic oncology patients in whom NPWT was employed after primary wound–healing failure, there was a 96% reduction in the size of the wounds with a median number of therapy days of 32 (range, 3-88). The majority of these patients were also managed as outpatients without complication (Gynecol. Oncol. 2004;92:586-91).
There are some contraindications to NPWT that should be considered. The major, and perhaps most common, is an ongoing wound infection.
A wound that needs to be evaluated at least daily to assess the response to antibiotic therapy or need for debridement should not be managed with NPWT until the wound is deemed stable. There should be no devitalized tissue present in the wound upon application of the NPWT. If any necrotic tissue is present, then wound debridement is warranted until only well-vascularized tissue remains.
Another contraindication is the presence of malignant tissue in the wound. Negative pressure can promote this tissue growth and lead to chronic nonhealing. Other considerations would include adhesive allergies and fragile skin due to chronic steroid use or collagen vascular disorders, as NPWT can lead to skin necrosis.
Finally, the involvement of vital organs, such as exposed bowel, is a contraindication to the NPWT systems, as constant suction can promote fistula formation or hemorrhage. However, in the setting of an open abdomen after trauma surgery, there has been the development of intra-abdominal wound management systems that may be appropriate.
Although rare in obstetrics, gynecology, and gynecologic oncology, delayed abdominal closure may be necessary. This can occur after reoperation for bowel injury, in cases where bowel wall edema and increased intra-abdominal pressure preclude closure, or in cases of massive hemorrhage (for example, ruptured ectopic pregnancy) where patient instability necessitates rapid termination of the surgical case. These wounds can be managed with temporary abdominal closure techniques such as retention sutures, a Bogota bag, or loose packing (World. J. Surg. 2015; 39: 912-25).
The negative pressure systems developed for these instances are the V.A.C. abdominal dressing (KCI), Renasys NPWT (Smith and Nephew), and ABThera open abdomen negative pressure therapy (KCI). They consist of a perforated plastic sheet with foam attachments that is placed directly in the abdomen to cover the intestine. This is then covered with an adhesive dressing that is cut to accommodate the suction attachment for the negative pressure pump. This setup is easily applied and taken down, and therefore facilitates frequent abdominal washouts until true facial closure can be achieved.
There are many benefits to NPWT for the management of superficial and deep wound dehiscence in the ob.gyn. or gynecologic oncology patient. NPWT should be considered primarily with any surgical wound healing by secondary intention.
Dr. Doll is a third-year fellow in gynecologic oncology at the University of North Carolina at Chapel Hill. Dr. Gehrig is professor and director of gynecologic oncology at the university. The authors reported having no relevant financial disclosures.
Negative pressure wound therapy is a wound management system for chronic open subcutaneous or intra-abdominal wounds. Some popular commercial systems include V.A.C. therapy (KCI, San Antonio) and the Chariker-Jeter wound-sealing kit (Smith and Nephew, London). Within ob.gyn. and gynecologic oncology, they have use in the management of postoperative superficial wound dehiscence from routine surgery and in the management of the open abdomen.
The primary benefit of negative pressure wound therapy (NPWT) is the acceleration of wound healing. Postoperative superficial wound dehiscence can occur as a result of surgical factors such as wound infection and subcutaneous seroma/hematoma or systematic factors such as poor nutrition and wound ischemia.
Acceleration of wound healing results from the design of the NPWT systems. They consist of semipermeable dressings (foam), sealed with an adhesive sheet that is connected to a portable pump. By the application of –50 to –175 mm Hg of continuous or intermittent suction, the edges of the wound are drawn together, and this deforming process promotes tissue remodeling at the cellular level. Other potential benefits of negative pressure are increased blood flow, a decrease in mediators of inflammation, and an increase in collagen organization via changes in wound biochemistry.
An alternative to NPWT would be traditional gauze dressings, which can also be applied in the case of superficial wound dehiscence. These are changed up to three times a day, however, and this can result in significant patient discomfort, caregiver difficulties, and prolonged healing of weeks to months. In contrast, NPWT dressings are changed once every 2-3 days. They are also versatile and can be fit to traditionally shaped abdominal wounds, as well as difficult to dress vulvar and groin wounds (J. Obstet. Gynaecol. Can. 2011;33:1031-7).
In a series of 27 gynecologic oncology patients in whom NPWT was employed after primary wound–healing failure, there was a 96% reduction in the size of the wounds with a median number of therapy days of 32 (range, 3-88). The majority of these patients were also managed as outpatients without complication (Gynecol. Oncol. 2004;92:586-91).
There are some contraindications to NPWT that should be considered. The major, and perhaps most common, is an ongoing wound infection.
A wound that needs to be evaluated at least daily to assess the response to antibiotic therapy or need for debridement should not be managed with NPWT until the wound is deemed stable. There should be no devitalized tissue present in the wound upon application of the NPWT. If any necrotic tissue is present, then wound debridement is warranted until only well-vascularized tissue remains.
Another contraindication is the presence of malignant tissue in the wound. Negative pressure can promote this tissue growth and lead to chronic nonhealing. Other considerations would include adhesive allergies and fragile skin due to chronic steroid use or collagen vascular disorders, as NPWT can lead to skin necrosis.
Finally, the involvement of vital organs, such as exposed bowel, is a contraindication to the NPWT systems, as constant suction can promote fistula formation or hemorrhage. However, in the setting of an open abdomen after trauma surgery, there has been the development of intra-abdominal wound management systems that may be appropriate.
Although rare in obstetrics, gynecology, and gynecologic oncology, delayed abdominal closure may be necessary. This can occur after reoperation for bowel injury, in cases where bowel wall edema and increased intra-abdominal pressure preclude closure, or in cases of massive hemorrhage (for example, ruptured ectopic pregnancy) where patient instability necessitates rapid termination of the surgical case. These wounds can be managed with temporary abdominal closure techniques such as retention sutures, a Bogota bag, or loose packing (World. J. Surg. 2015; 39: 912-25).
The negative pressure systems developed for these instances are the V.A.C. abdominal dressing (KCI), Renasys NPWT (Smith and Nephew), and ABThera open abdomen negative pressure therapy (KCI). They consist of a perforated plastic sheet with foam attachments that is placed directly in the abdomen to cover the intestine. This is then covered with an adhesive dressing that is cut to accommodate the suction attachment for the negative pressure pump. This setup is easily applied and taken down, and therefore facilitates frequent abdominal washouts until true facial closure can be achieved.
There are many benefits to NPWT for the management of superficial and deep wound dehiscence in the ob.gyn. or gynecologic oncology patient. NPWT should be considered primarily with any surgical wound healing by secondary intention.
Dr. Doll is a third-year fellow in gynecologic oncology at the University of North Carolina at Chapel Hill. Dr. Gehrig is professor and director of gynecologic oncology at the university. The authors reported having no relevant financial disclosures.
Negative pressure wound therapy is a wound management system for chronic open subcutaneous or intra-abdominal wounds. Some popular commercial systems include V.A.C. therapy (KCI, San Antonio) and the Chariker-Jeter wound-sealing kit (Smith and Nephew, London). Within ob.gyn. and gynecologic oncology, they have use in the management of postoperative superficial wound dehiscence from routine surgery and in the management of the open abdomen.
The primary benefit of negative pressure wound therapy (NPWT) is the acceleration of wound healing. Postoperative superficial wound dehiscence can occur as a result of surgical factors such as wound infection and subcutaneous seroma/hematoma or systematic factors such as poor nutrition and wound ischemia.
Acceleration of wound healing results from the design of the NPWT systems. They consist of semipermeable dressings (foam), sealed with an adhesive sheet that is connected to a portable pump. By the application of –50 to –175 mm Hg of continuous or intermittent suction, the edges of the wound are drawn together, and this deforming process promotes tissue remodeling at the cellular level. Other potential benefits of negative pressure are increased blood flow, a decrease in mediators of inflammation, and an increase in collagen organization via changes in wound biochemistry.
An alternative to NPWT would be traditional gauze dressings, which can also be applied in the case of superficial wound dehiscence. These are changed up to three times a day, however, and this can result in significant patient discomfort, caregiver difficulties, and prolonged healing of weeks to months. In contrast, NPWT dressings are changed once every 2-3 days. They are also versatile and can be fit to traditionally shaped abdominal wounds, as well as difficult to dress vulvar and groin wounds (J. Obstet. Gynaecol. Can. 2011;33:1031-7).
In a series of 27 gynecologic oncology patients in whom NPWT was employed after primary wound–healing failure, there was a 96% reduction in the size of the wounds with a median number of therapy days of 32 (range, 3-88). The majority of these patients were also managed as outpatients without complication (Gynecol. Oncol. 2004;92:586-91).
There are some contraindications to NPWT that should be considered. The major, and perhaps most common, is an ongoing wound infection.
A wound that needs to be evaluated at least daily to assess the response to antibiotic therapy or need for debridement should not be managed with NPWT until the wound is deemed stable. There should be no devitalized tissue present in the wound upon application of the NPWT. If any necrotic tissue is present, then wound debridement is warranted until only well-vascularized tissue remains.
Another contraindication is the presence of malignant tissue in the wound. Negative pressure can promote this tissue growth and lead to chronic nonhealing. Other considerations would include adhesive allergies and fragile skin due to chronic steroid use or collagen vascular disorders, as NPWT can lead to skin necrosis.
Finally, the involvement of vital organs, such as exposed bowel, is a contraindication to the NPWT systems, as constant suction can promote fistula formation or hemorrhage. However, in the setting of an open abdomen after trauma surgery, there has been the development of intra-abdominal wound management systems that may be appropriate.
Although rare in obstetrics, gynecology, and gynecologic oncology, delayed abdominal closure may be necessary. This can occur after reoperation for bowel injury, in cases where bowel wall edema and increased intra-abdominal pressure preclude closure, or in cases of massive hemorrhage (for example, ruptured ectopic pregnancy) where patient instability necessitates rapid termination of the surgical case. These wounds can be managed with temporary abdominal closure techniques such as retention sutures, a Bogota bag, or loose packing (World. J. Surg. 2015; 39: 912-25).
The negative pressure systems developed for these instances are the V.A.C. abdominal dressing (KCI), Renasys NPWT (Smith and Nephew), and ABThera open abdomen negative pressure therapy (KCI). They consist of a perforated plastic sheet with foam attachments that is placed directly in the abdomen to cover the intestine. This is then covered with an adhesive dressing that is cut to accommodate the suction attachment for the negative pressure pump. This setup is easily applied and taken down, and therefore facilitates frequent abdominal washouts until true facial closure can be achieved.
There are many benefits to NPWT for the management of superficial and deep wound dehiscence in the ob.gyn. or gynecologic oncology patient. NPWT should be considered primarily with any surgical wound healing by secondary intention.
Dr. Doll is a third-year fellow in gynecologic oncology at the University of North Carolina at Chapel Hill. Dr. Gehrig is professor and director of gynecologic oncology at the university. The authors reported having no relevant financial disclosures.