User login
Moisturizers and skin barrier repair
There are dozens of skin care products that claim to repair the barrier that do not have the science or ingredient content to back them up.
Does a skin barrier repair moisturizer really repair?
First, let’s briefly review what the skin barrier is. The stratum corneum (SC), the most superficial layer of the epidermis, averages approximately 15-cell layers in thickness.1,2 The keratinocytes reside there in a pattern resembling a brick wall. The “mortar” is composed of the lipid contents extruded from the lamellar granules. This protective barrier functions to prevent transepidermal water loss (TEWL) and entry of allergens, irritants, and pathogens into deeper layers of the skin. This column will focus briefly on the structure and function of the skin barrier and the barrier repair technologies that use synthetic lipids such as myristoyl-palmitoyl and myristyl/palmityl-oxo-stearamide/arachamide MEA.
Structure of the skin barrier
SC keratinocytes are surrounded by lamella made from lipid bilayers. The lipids have hydrophilic heads and hydrophobic tails; the bilayer arises when the hydrophobic tails face the center and the hydrophilic heads face out of the bilayer. This formation yields a disc-shaped hydrophobic lamellar center. There are actually several of these lamellar layers between keratinocytes.
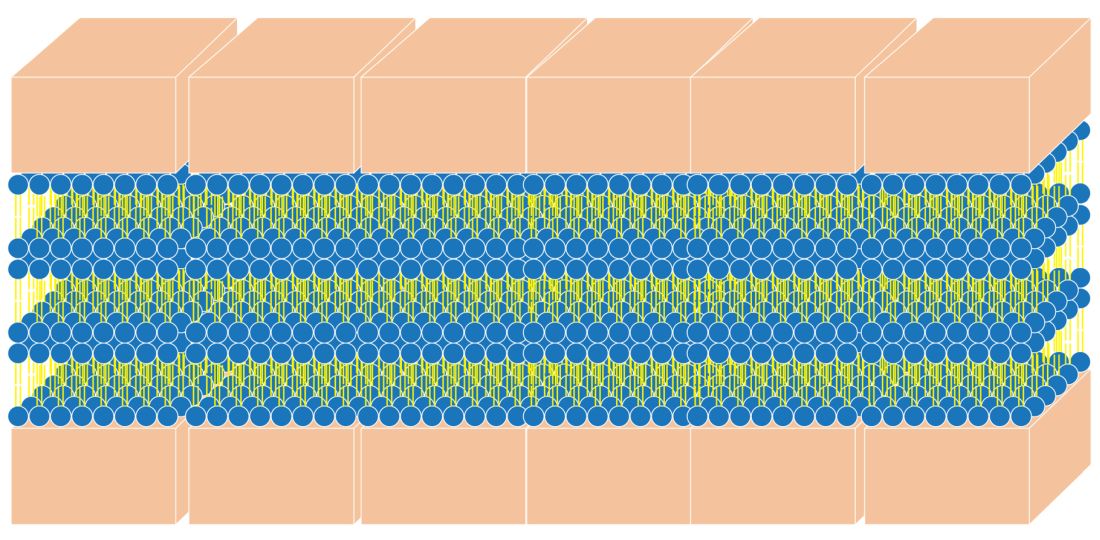
The naturally occurring primary lipids of the bilayer lamellae are made up of an equal ratio of ceramides, cholesterol, and free fatty acid. Arranged in a 1:1:1 ratio, they fit together like pieces of a puzzle to achieve skin barrier homeostasis. The shape and size of these puzzle pieces is critical. An incorrect shape results in a hole in the skin barrier resulting in dehydration, inflammation, and sensitivity.
Ceramides
Ceramides are a complex family of lipids (sphingolipids – a sphingoid base and a fatty acid) involved in cell, as well as barrier, homeostasis and water-holding capacity. In fact, they are known to play a crucial role in cell proliferation, differentiation, and apoptosis.3 There are at least 16 types of naturally occurring ceramides. For years, they have been included in barrier repair moisturizers. They are difficult to work with in moisturizers for several reasons:
- Ceramides are abundant in brain tissue and the ceramides used in moisturizers in the past were derived from bovine brain tissue. Prior to the emergence of bovine spongiform encephalopathy (mad cow disease), many ceramides in skin-care products were animal derived, which made them expensive and undesirable.
- Ceramides in skin care that are made from plant sources are referred to as phyto-derived ceramides. Although they share a similar structure with ceramides that occur in human skin, there are differences in chain length, hydroxylation pattern, and the degree of unsaturation that lead to structural diversity.4 The shape of ceramides is critical for a strong skin barrier because the lipids in the skin barrier must fit together like puzzle pieces to form a water-tight barrier. Natural sources of ceramides include rice, wheat, potato, konjac, and maize. Standardization of ceramide shape and structure makes using phyto-derived ceramides in skin care products challenging.
- Ceramides, because of their waxy consistency, require heat during the mixing process of skin care product manufacturing. This heat can make other ingredients inactive in the skin care formulation. (Ceramides are typically added early in the formulation process, and the heat-sensitive ones are added later.)
- Many forms of ceramides are unstable in the product manufacturing and bottling processes.
- Skin penetration of ceramides depends on the shape and size of ceramides.
Synthetic ceramides have been developed to make ceramides safe, affordable, and more easily formulated into moisturizers. These formulations synthesized in the lab are sometimes called pseudoceramides because they are structurally different compounds that mimic the activity of ceramides. They are developed to be less expensive to manufacture, safer than those derived from animals, and easier to formulate, and they can be made into the specific shape of the ceramide puzzle piece.
Ceramides in skin care
The naturally occurring intercellular lipids of the SC are composed of approximately equal proportions of ceramides, cholesterol, and fatty acids (referred to in this article as the “three barrier lipids” for simplicity).5-9 Alterations in any of these three barrier lipids or their regulatory enzymes result in impairments in the function of the epidermal barrier. Therefore, any synthetic ceramide must mimic the shape of natural ceramides, or the three barrier lipids in the moisturizer must mimic the shape of the entire bilayer lamella. Unfortunately, most barrier repair moisturizers do not meet these criteria and are not true barrier repair moisturizers.
How do you know if a moisturizer repairs the skin barrier?
Clinical tests such as measuring transepidermal water loss (TEWL) with a Tewameter are usually done to support the barrier repair claim. However, occlusive ingredients like oils can lower TEWL without affecting the barrier. In fact, we believe that sebum on the skin can make an impaired barrier and result in normal TEWL even when the barrier is impaired. So, just because a product improved TEWL does not necessarily mean that it repairs the barrier.
One way to test the ability of a moisturizer to repair the barrier is to look at a structural analysis of the moisturizer to see if it forms the requisite bilayer lamellar shape. An easy way to do this testing is to look for the cross pattern under a cross polarized microscope. The cross pattern is known as optical anisotropy. 8
The best barrier repair creams
Optimal barrier repair creams either feature a 1:1:1 ratio of epidermal lipids or form a cross structure when viewed with a cross-polarized microscope.8 There are several categories of barrier repair moisturizers that meet these criteria.
Barrier repair creams with a 1:1:1 ratio of lipids:
Peter Elias, MD, holds the patent on barrier repair moisturizer technology that has a 1:1:1 ratio. His well-established technology is used in a prescription barrier repair cream called EpiCeram® which is approved by the Food and Drug Administration to treat eczema. There are no other moisturizers that I know of that contain this 1:1:1 lipid ratio.
There is a barrier repair cream on the market that contains a 2:4:2 ratio of lipids based on a study that showed that this ratio is effective in older skin with an impaired barrier. It is unknown if this moisturizer forms a cross pattern.
Barrier repair creams that demonstrate a cross pattern:
Multilamellar emulsion (MLE) technology: This barrier repair technology, invented in South Korea, contains the synthetic pseudoceramide called myristyl/palmityl-oxo-stearamide/arachamide MEA (C34H67NO3/C36H71NO3/C38H75NO3), or the pseudoceramide myristoyl-palmitoyl-oxostearamide-arachamide MEA.
In a 2019 pilot study by Ye and colleagues, the investigators treated 33 older volunteers twice daily for 30 days with approximately 3 mL of an emollient containing MLE technology. In addition, 30 untreated older subjects and 11 young volunteers served as controls. The investigators found that the topically applied barrier repair emollient significantly improved barrier function, as well as stratum corneum hydration. Circulating levels of the important, age-related plasma cytokines interleukin-1 beta and IL-6 were found to have normalized, while tumor necrosis factor–alpha decreased markedly. The investigators suggested that repair of the skin barrier might diminish circulating proinflammatory cytokine levels (such as amyloid A) in aged humans, potentially mitigating the development of chronic inflammatory conditions.10
MLE technology has also been shown to improve childhood atopic dermatitis and prevent steroid atrophy.11,12 The consistent use of MLE technology in moisturizers has been shown to alleviate inflammatory factors in the blood and is believed to lessen systemic inflammation.10
Physiologic (PSL) lipid repair technology: This technology was invented by one of the South Korean researchers who helped develop MLE technology. It contains pseudoceramides, fatty acids, and cholesterol. The figure of the cross pattern above, as seen under the cross polarized microscope, is an image taken of this PSL lipid repair technology.
Conclusion
Do not believe that a moisturizer repairs the barrier just because it says so on the label. Three of the most popular body moisturizes used to treat eczema do not actually have the proper formula to repair the barrier. Unfortunately, there are dozens of skin care products that claim to repair the barrier that do not have the science or ingredient content to back them up. To restore the skin barrier to a healthy condition, it is imperative that the barrier repair moisturizers that you are recommending for patients have the correct 1:1:1 ratio of epidermal lipids or contain bilayer lamella that mimic the natural multilamellar layers and display the cross pattern under a cross-polarized microscope.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann has written two textbooks and a New York Times Best Sellers book for consumers. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Revance, Evolus, and Burt’s Bees. She is the CEO of Skin Type Solutions, a company that independently tests skin care products and makes recommendations to physicians on which skin care technologies are best. Write to her at dermnews@mdedge.com.
References
1. Christophers E and Kligman AM. J Invest Dermatol. 1964;42:407-9.
2. Blair C. Br J Dermatol. 1968;80(7):430-6.
3. Morita O et al. Food Chem Toxicol. 2009 Apr;47(4):681-6.
4. Tessema E N et al. Skin pharmacology and physiology. 2017;30(3):115-38.
5. Coderch L et al. Am J Clin Dermatol. 2003;4(2):107-29.
6. Man MQ et al. Arch Dermatol. 1993;129(6):728-38.
7. Man MQ M et al. J Invest Dermatol. 1996 May;106(5):1096-101.
8. Park BD et al. J Invest Dermatol. 2003;121(4):794-801.
9. Proksch E and Jensen J. Skin as an organ of protection, in “Fitzpatrick’s Dermatology in General Medicine,” 7th ed. New York: McGraw-Hill, 2008, pp. 383-95.
10. Ye L et al. J Eur Acad Dermatol Venereol. 2019;33(11):2197-201.
11. Lee EJ et al. Ann Dermatol. 2003;15(4):133-8.
12. Ahn SK et al. J Dermatol. 2006;33(2):80-90.
There are dozens of skin care products that claim to repair the barrier that do not have the science or ingredient content to back them up.
Does a skin barrier repair moisturizer really repair?
First, let’s briefly review what the skin barrier is. The stratum corneum (SC), the most superficial layer of the epidermis, averages approximately 15-cell layers in thickness.1,2 The keratinocytes reside there in a pattern resembling a brick wall. The “mortar” is composed of the lipid contents extruded from the lamellar granules. This protective barrier functions to prevent transepidermal water loss (TEWL) and entry of allergens, irritants, and pathogens into deeper layers of the skin. This column will focus briefly on the structure and function of the skin barrier and the barrier repair technologies that use synthetic lipids such as myristoyl-palmitoyl and myristyl/palmityl-oxo-stearamide/arachamide MEA.
Structure of the skin barrier
SC keratinocytes are surrounded by lamella made from lipid bilayers. The lipids have hydrophilic heads and hydrophobic tails; the bilayer arises when the hydrophobic tails face the center and the hydrophilic heads face out of the bilayer. This formation yields a disc-shaped hydrophobic lamellar center. There are actually several of these lamellar layers between keratinocytes.

The naturally occurring primary lipids of the bilayer lamellae are made up of an equal ratio of ceramides, cholesterol, and free fatty acid. Arranged in a 1:1:1 ratio, they fit together like pieces of a puzzle to achieve skin barrier homeostasis. The shape and size of these puzzle pieces is critical. An incorrect shape results in a hole in the skin barrier resulting in dehydration, inflammation, and sensitivity.
Ceramides
Ceramides are a complex family of lipids (sphingolipids – a sphingoid base and a fatty acid) involved in cell, as well as barrier, homeostasis and water-holding capacity. In fact, they are known to play a crucial role in cell proliferation, differentiation, and apoptosis.3 There are at least 16 types of naturally occurring ceramides. For years, they have been included in barrier repair moisturizers. They are difficult to work with in moisturizers for several reasons:
- Ceramides are abundant in brain tissue and the ceramides used in moisturizers in the past were derived from bovine brain tissue. Prior to the emergence of bovine spongiform encephalopathy (mad cow disease), many ceramides in skin-care products were animal derived, which made them expensive and undesirable.
- Ceramides in skin care that are made from plant sources are referred to as phyto-derived ceramides. Although they share a similar structure with ceramides that occur in human skin, there are differences in chain length, hydroxylation pattern, and the degree of unsaturation that lead to structural diversity.4 The shape of ceramides is critical for a strong skin barrier because the lipids in the skin barrier must fit together like puzzle pieces to form a water-tight barrier. Natural sources of ceramides include rice, wheat, potato, konjac, and maize. Standardization of ceramide shape and structure makes using phyto-derived ceramides in skin care products challenging.
- Ceramides, because of their waxy consistency, require heat during the mixing process of skin care product manufacturing. This heat can make other ingredients inactive in the skin care formulation. (Ceramides are typically added early in the formulation process, and the heat-sensitive ones are added later.)
- Many forms of ceramides are unstable in the product manufacturing and bottling processes.
- Skin penetration of ceramides depends on the shape and size of ceramides.
Synthetic ceramides have been developed to make ceramides safe, affordable, and more easily formulated into moisturizers. These formulations synthesized in the lab are sometimes called pseudoceramides because they are structurally different compounds that mimic the activity of ceramides. They are developed to be less expensive to manufacture, safer than those derived from animals, and easier to formulate, and they can be made into the specific shape of the ceramide puzzle piece.
Ceramides in skin care
The naturally occurring intercellular lipids of the SC are composed of approximately equal proportions of ceramides, cholesterol, and fatty acids (referred to in this article as the “three barrier lipids” for simplicity).5-9 Alterations in any of these three barrier lipids or their regulatory enzymes result in impairments in the function of the epidermal barrier. Therefore, any synthetic ceramide must mimic the shape of natural ceramides, or the three barrier lipids in the moisturizer must mimic the shape of the entire bilayer lamella. Unfortunately, most barrier repair moisturizers do not meet these criteria and are not true barrier repair moisturizers.
How do you know if a moisturizer repairs the skin barrier?
Clinical tests such as measuring transepidermal water loss (TEWL) with a Tewameter are usually done to support the barrier repair claim. However, occlusive ingredients like oils can lower TEWL without affecting the barrier. In fact, we believe that sebum on the skin can make an impaired barrier and result in normal TEWL even when the barrier is impaired. So, just because a product improved TEWL does not necessarily mean that it repairs the barrier.
One way to test the ability of a moisturizer to repair the barrier is to look at a structural analysis of the moisturizer to see if it forms the requisite bilayer lamellar shape. An easy way to do this testing is to look for the cross pattern under a cross polarized microscope. The cross pattern is known as optical anisotropy. 8
The best barrier repair creams
Optimal barrier repair creams either feature a 1:1:1 ratio of epidermal lipids or form a cross structure when viewed with a cross-polarized microscope.8 There are several categories of barrier repair moisturizers that meet these criteria.
Barrier repair creams with a 1:1:1 ratio of lipids:
Peter Elias, MD, holds the patent on barrier repair moisturizer technology that has a 1:1:1 ratio. His well-established technology is used in a prescription barrier repair cream called EpiCeram® which is approved by the Food and Drug Administration to treat eczema. There are no other moisturizers that I know of that contain this 1:1:1 lipid ratio.
There is a barrier repair cream on the market that contains a 2:4:2 ratio of lipids based on a study that showed that this ratio is effective in older skin with an impaired barrier. It is unknown if this moisturizer forms a cross pattern.
Barrier repair creams that demonstrate a cross pattern:
Multilamellar emulsion (MLE) technology: This barrier repair technology, invented in South Korea, contains the synthetic pseudoceramide called myristyl/palmityl-oxo-stearamide/arachamide MEA (C34H67NO3/C36H71NO3/C38H75NO3), or the pseudoceramide myristoyl-palmitoyl-oxostearamide-arachamide MEA.
In a 2019 pilot study by Ye and colleagues, the investigators treated 33 older volunteers twice daily for 30 days with approximately 3 mL of an emollient containing MLE technology. In addition, 30 untreated older subjects and 11 young volunteers served as controls. The investigators found that the topically applied barrier repair emollient significantly improved barrier function, as well as stratum corneum hydration. Circulating levels of the important, age-related plasma cytokines interleukin-1 beta and IL-6 were found to have normalized, while tumor necrosis factor–alpha decreased markedly. The investigators suggested that repair of the skin barrier might diminish circulating proinflammatory cytokine levels (such as amyloid A) in aged humans, potentially mitigating the development of chronic inflammatory conditions.10
MLE technology has also been shown to improve childhood atopic dermatitis and prevent steroid atrophy.11,12 The consistent use of MLE technology in moisturizers has been shown to alleviate inflammatory factors in the blood and is believed to lessen systemic inflammation.10
Physiologic (PSL) lipid repair technology: This technology was invented by one of the South Korean researchers who helped develop MLE technology. It contains pseudoceramides, fatty acids, and cholesterol. The figure of the cross pattern above, as seen under the cross polarized microscope, is an image taken of this PSL lipid repair technology.
Conclusion
Do not believe that a moisturizer repairs the barrier just because it says so on the label. Three of the most popular body moisturizes used to treat eczema do not actually have the proper formula to repair the barrier. Unfortunately, there are dozens of skin care products that claim to repair the barrier that do not have the science or ingredient content to back them up. To restore the skin barrier to a healthy condition, it is imperative that the barrier repair moisturizers that you are recommending for patients have the correct 1:1:1 ratio of epidermal lipids or contain bilayer lamella that mimic the natural multilamellar layers and display the cross pattern under a cross-polarized microscope.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann has written two textbooks and a New York Times Best Sellers book for consumers. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Revance, Evolus, and Burt’s Bees. She is the CEO of Skin Type Solutions, a company that independently tests skin care products and makes recommendations to physicians on which skin care technologies are best. Write to her at dermnews@mdedge.com.
References
1. Christophers E and Kligman AM. J Invest Dermatol. 1964;42:407-9.
2. Blair C. Br J Dermatol. 1968;80(7):430-6.
3. Morita O et al. Food Chem Toxicol. 2009 Apr;47(4):681-6.
4. Tessema E N et al. Skin pharmacology and physiology. 2017;30(3):115-38.
5. Coderch L et al. Am J Clin Dermatol. 2003;4(2):107-29.
6. Man MQ et al. Arch Dermatol. 1993;129(6):728-38.
7. Man MQ M et al. J Invest Dermatol. 1996 May;106(5):1096-101.
8. Park BD et al. J Invest Dermatol. 2003;121(4):794-801.
9. Proksch E and Jensen J. Skin as an organ of protection, in “Fitzpatrick’s Dermatology in General Medicine,” 7th ed. New York: McGraw-Hill, 2008, pp. 383-95.
10. Ye L et al. J Eur Acad Dermatol Venereol. 2019;33(11):2197-201.
11. Lee EJ et al. Ann Dermatol. 2003;15(4):133-8.
12. Ahn SK et al. J Dermatol. 2006;33(2):80-90.
There are dozens of skin care products that claim to repair the barrier that do not have the science or ingredient content to back them up.
Does a skin barrier repair moisturizer really repair?
First, let’s briefly review what the skin barrier is. The stratum corneum (SC), the most superficial layer of the epidermis, averages approximately 15-cell layers in thickness.1,2 The keratinocytes reside there in a pattern resembling a brick wall. The “mortar” is composed of the lipid contents extruded from the lamellar granules. This protective barrier functions to prevent transepidermal water loss (TEWL) and entry of allergens, irritants, and pathogens into deeper layers of the skin. This column will focus briefly on the structure and function of the skin barrier and the barrier repair technologies that use synthetic lipids such as myristoyl-palmitoyl and myristyl/palmityl-oxo-stearamide/arachamide MEA.
Structure of the skin barrier
SC keratinocytes are surrounded by lamella made from lipid bilayers. The lipids have hydrophilic heads and hydrophobic tails; the bilayer arises when the hydrophobic tails face the center and the hydrophilic heads face out of the bilayer. This formation yields a disc-shaped hydrophobic lamellar center. There are actually several of these lamellar layers between keratinocytes.

The naturally occurring primary lipids of the bilayer lamellae are made up of an equal ratio of ceramides, cholesterol, and free fatty acid. Arranged in a 1:1:1 ratio, they fit together like pieces of a puzzle to achieve skin barrier homeostasis. The shape and size of these puzzle pieces is critical. An incorrect shape results in a hole in the skin barrier resulting in dehydration, inflammation, and sensitivity.
Ceramides
Ceramides are a complex family of lipids (sphingolipids – a sphingoid base and a fatty acid) involved in cell, as well as barrier, homeostasis and water-holding capacity. In fact, they are known to play a crucial role in cell proliferation, differentiation, and apoptosis.3 There are at least 16 types of naturally occurring ceramides. For years, they have been included in barrier repair moisturizers. They are difficult to work with in moisturizers for several reasons:
- Ceramides are abundant in brain tissue and the ceramides used in moisturizers in the past were derived from bovine brain tissue. Prior to the emergence of bovine spongiform encephalopathy (mad cow disease), many ceramides in skin-care products were animal derived, which made them expensive and undesirable.
- Ceramides in skin care that are made from plant sources are referred to as phyto-derived ceramides. Although they share a similar structure with ceramides that occur in human skin, there are differences in chain length, hydroxylation pattern, and the degree of unsaturation that lead to structural diversity.4 The shape of ceramides is critical for a strong skin barrier because the lipids in the skin barrier must fit together like puzzle pieces to form a water-tight barrier. Natural sources of ceramides include rice, wheat, potato, konjac, and maize. Standardization of ceramide shape and structure makes using phyto-derived ceramides in skin care products challenging.
- Ceramides, because of their waxy consistency, require heat during the mixing process of skin care product manufacturing. This heat can make other ingredients inactive in the skin care formulation. (Ceramides are typically added early in the formulation process, and the heat-sensitive ones are added later.)
- Many forms of ceramides are unstable in the product manufacturing and bottling processes.
- Skin penetration of ceramides depends on the shape and size of ceramides.
Synthetic ceramides have been developed to make ceramides safe, affordable, and more easily formulated into moisturizers. These formulations synthesized in the lab are sometimes called pseudoceramides because they are structurally different compounds that mimic the activity of ceramides. They are developed to be less expensive to manufacture, safer than those derived from animals, and easier to formulate, and they can be made into the specific shape of the ceramide puzzle piece.
Ceramides in skin care
The naturally occurring intercellular lipids of the SC are composed of approximately equal proportions of ceramides, cholesterol, and fatty acids (referred to in this article as the “three barrier lipids” for simplicity).5-9 Alterations in any of these three barrier lipids or their regulatory enzymes result in impairments in the function of the epidermal barrier. Therefore, any synthetic ceramide must mimic the shape of natural ceramides, or the three barrier lipids in the moisturizer must mimic the shape of the entire bilayer lamella. Unfortunately, most barrier repair moisturizers do not meet these criteria and are not true barrier repair moisturizers.
How do you know if a moisturizer repairs the skin barrier?
Clinical tests such as measuring transepidermal water loss (TEWL) with a Tewameter are usually done to support the barrier repair claim. However, occlusive ingredients like oils can lower TEWL without affecting the barrier. In fact, we believe that sebum on the skin can make an impaired barrier and result in normal TEWL even when the barrier is impaired. So, just because a product improved TEWL does not necessarily mean that it repairs the barrier.
One way to test the ability of a moisturizer to repair the barrier is to look at a structural analysis of the moisturizer to see if it forms the requisite bilayer lamellar shape. An easy way to do this testing is to look for the cross pattern under a cross polarized microscope. The cross pattern is known as optical anisotropy. 8
The best barrier repair creams
Optimal barrier repair creams either feature a 1:1:1 ratio of epidermal lipids or form a cross structure when viewed with a cross-polarized microscope.8 There are several categories of barrier repair moisturizers that meet these criteria.
Barrier repair creams with a 1:1:1 ratio of lipids:
Peter Elias, MD, holds the patent on barrier repair moisturizer technology that has a 1:1:1 ratio. His well-established technology is used in a prescription barrier repair cream called EpiCeram® which is approved by the Food and Drug Administration to treat eczema. There are no other moisturizers that I know of that contain this 1:1:1 lipid ratio.
There is a barrier repair cream on the market that contains a 2:4:2 ratio of lipids based on a study that showed that this ratio is effective in older skin with an impaired barrier. It is unknown if this moisturizer forms a cross pattern.
Barrier repair creams that demonstrate a cross pattern:
Multilamellar emulsion (MLE) technology: This barrier repair technology, invented in South Korea, contains the synthetic pseudoceramide called myristyl/palmityl-oxo-stearamide/arachamide MEA (C34H67NO3/C36H71NO3/C38H75NO3), or the pseudoceramide myristoyl-palmitoyl-oxostearamide-arachamide MEA.
In a 2019 pilot study by Ye and colleagues, the investigators treated 33 older volunteers twice daily for 30 days with approximately 3 mL of an emollient containing MLE technology. In addition, 30 untreated older subjects and 11 young volunteers served as controls. The investigators found that the topically applied barrier repair emollient significantly improved barrier function, as well as stratum corneum hydration. Circulating levels of the important, age-related plasma cytokines interleukin-1 beta and IL-6 were found to have normalized, while tumor necrosis factor–alpha decreased markedly. The investigators suggested that repair of the skin barrier might diminish circulating proinflammatory cytokine levels (such as amyloid A) in aged humans, potentially mitigating the development of chronic inflammatory conditions.10
MLE technology has also been shown to improve childhood atopic dermatitis and prevent steroid atrophy.11,12 The consistent use of MLE technology in moisturizers has been shown to alleviate inflammatory factors in the blood and is believed to lessen systemic inflammation.10
Physiologic (PSL) lipid repair technology: This technology was invented by one of the South Korean researchers who helped develop MLE technology. It contains pseudoceramides, fatty acids, and cholesterol. The figure of the cross pattern above, as seen under the cross polarized microscope, is an image taken of this PSL lipid repair technology.
Conclusion
Do not believe that a moisturizer repairs the barrier just because it says so on the label. Three of the most popular body moisturizes used to treat eczema do not actually have the proper formula to repair the barrier. Unfortunately, there are dozens of skin care products that claim to repair the barrier that do not have the science or ingredient content to back them up. To restore the skin barrier to a healthy condition, it is imperative that the barrier repair moisturizers that you are recommending for patients have the correct 1:1:1 ratio of epidermal lipids or contain bilayer lamella that mimic the natural multilamellar layers and display the cross pattern under a cross-polarized microscope.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann has written two textbooks and a New York Times Best Sellers book for consumers. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Revance, Evolus, and Burt’s Bees. She is the CEO of Skin Type Solutions, a company that independently tests skin care products and makes recommendations to physicians on which skin care technologies are best. Write to her at dermnews@mdedge.com.
References
1. Christophers E and Kligman AM. J Invest Dermatol. 1964;42:407-9.
2. Blair C. Br J Dermatol. 1968;80(7):430-6.
3. Morita O et al. Food Chem Toxicol. 2009 Apr;47(4):681-6.
4. Tessema E N et al. Skin pharmacology and physiology. 2017;30(3):115-38.
5. Coderch L et al. Am J Clin Dermatol. 2003;4(2):107-29.
6. Man MQ et al. Arch Dermatol. 1993;129(6):728-38.
7. Man MQ M et al. J Invest Dermatol. 1996 May;106(5):1096-101.
8. Park BD et al. J Invest Dermatol. 2003;121(4):794-801.
9. Proksch E and Jensen J. Skin as an organ of protection, in “Fitzpatrick’s Dermatology in General Medicine,” 7th ed. New York: McGraw-Hill, 2008, pp. 383-95.
10. Ye L et al. J Eur Acad Dermatol Venereol. 2019;33(11):2197-201.
11. Lee EJ et al. Ann Dermatol. 2003;15(4):133-8.
12. Ahn SK et al. J Dermatol. 2006;33(2):80-90.
Does the use of frankincense make sense in dermatology?
The Boswellia serrata exudate or gum (known in India as “guggulu”) that forms an aromatic resin traditionally used as incense – and known as frankincense (especially when retrieved from Boswellia species found in Eritrea and Somalia but also from the Indian variety) – has been considered for thousands of years to possess therapeutic properties. It is used in Ayurvedic medicine, as well as in traditional medicine in China and the Middle East, particularly for its anti-inflammatory effects to treat chronic conditions.1-8 In fact, such essential oils have been used since 2800 BC to treat various inflammatory conditions, including skin sores and wounds, as well as in perfumes and incense.2,9 In the West, use of frankincense dates back to thousands of years as well, more often found in the form of incense for religious and cultural ceremonies.7 Over the past 2 decades, .3 This column focuses on some of the emerging data on this ancient botanical agent.
Chemical constituents
Terpenoids and essential oils are the primary components of frankincense and are known to impart anti-inflammatory and anticancer activity. The same is true for myrrh, which has been combined with frankincense in traditional Chinese medicine as a single medication for millennia, with the two acting synergistically and considered still to be a potent combination in conferring various biological benefits.7
In 2010, in a systematic review of the anti-inflammatory and anticancer activities of Boswellia species and their chemical ingredients, Efferth and Oesch found that frankincense blocks the production of leukotrienes, cyclooxygenase (COX) 1 and 2, as well as 5-lipoxygenase; and oxidative stress. It also contributes to regulation of immune cells from the innate and acquired immune systems and exerts anticancer activity by influencing signaling transduction responsible for cell cycle arrest, as well as inhibition of proliferation, angiogenesis, invasion, and metastasis. The investigators also reported on clinical trial results that have found efficacy of frankincense and its constituents in ameliorating symptoms of psoriasis and erythematous eczema, among other disorders.3
Anti-inflammatory activity
Li et al. completed a study in 2016 to identify the active ingredients responsible for the anti-inflammatory and analgesic effects of frankincense. They found that alpha-pinene, linalool, and 1-octanol were key contributors. These constituents were noted for suppressing COX-2 overexpression in mice, as well as nociceptive stimulus-induced inflammatory infiltrates.10
Noting the increasing popularity of frankincense essential oil in skin care, despite a paucity of data, in 2017, Han et al. evaluated the biological activities of the essential oil in pre-inflamed human dermal fibroblasts using 17 key protein biomarkers. Frankincense essential oil displayed significant antiproliferative activity and suppressed collagen III, interferon gamma-induced protein 10, and intracellular adhesion molecule 1. The investigators referred to the overall encouraging potential of frankincense essential oil to exert influence over inflammation and tissue remodeling in human skin and called for additional research into its mechanisms of action and active constituents.11
Anticancer activity
The main active ingredient in frankincense, boswellic acid, has been shown to promote apoptosis, suppress matrix metalloproteinase secretion, and hinder migration in metastatic melanoma cell lines in mice.6,12
In 2019, Hakkim et al. demonstrated that frankincense essential oil yielded substantial antimelanoma activity in vitro and in vivo and ameliorated hepatotoxicity caused by acetaminophen.13
There is one case report in the literature on the use of frankincense as a treatment for skin cancer. A 56-year-old man received frankincense oil multiple times a day for 4 months to treat a nodular basal cell carcinoma on one arm (which resolved) and an infiltrative BCC on the chest (some focal residual tumor remained).6,14 Topical frankincense or boswellic acid has been given a grade D recommendation for treating skin cancer, however, because of only one level-of-evidence-5 study.6
Antimicrobial activity
In 2012, de Rapper et al. collected samples of three essential oils of frankincense (Boswellia rivae, Boswellia neglecta, and Boswellia papyrifera) and two essential oil samples of myrrh and sweet myrrh from different regions of Ethiopia to study their anti-infective properties alone and in combination. The investigators observed synergistic and additive effects, particularly between B. papyrifera and Commiphora myrrha. While noting the long history of the combined use of frankincense and myrrh essential oils since 1500 BC, the investigators highlighted their study as the first antimicrobial work to verify the effectiveness of this combination, validating the use of this combination to thwart particular pathogens.15
Just 2 years ago, Ljaljević Grbić et al. evaluated the in vitro antimicrobial potential of the liquid and vapor phases of B. carteri and C. myrrha (frankincense and myrrh, respectively) essential oils, finding that frankincense demonstrated marked capacity to act as a natural antimicrobial agent.9
Transdermal delivery
In 2017, Zhu et al. showed that frankincense and myrrh essential oils promoted the permeability of the Chinese herb Chuanxiong and may facilitate drug elimination from the epidermis via dermal capillaries by dint of improved cutaneous blood flow, thereby augmenting transdermal drug delivery.16 The same team also showed that frankincense and myrrh essential oils, by fostering permeation by enhancing drug delivery across the stratum corneum, can also alter the structure of the stratum corneum.17
Conclusion
The use of frankincense in traditional medicine has a long and impressive track record. Recent research provides reason for optimism, and further investigating the possible incorporation of this botanical agent into modern dermatologic therapies appears warranted. Clearly, however, much more research is needed.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann has written two textbooks and a New York Times Best Sellers book for consumers. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Revance, Evolus, and Burt’s Bees. She is the CEO of Skin Type Solutions Inc., a company that independently tests skin care products and makes recommendations to physicians on which skin care technologies are best. Write to her at dermnews@mdedge.com.
References
1. Kimmatkar N et al. Phytomedicine. 2003 Jan;10(1):3-7.
2. Ammon HP. Wien Med Wochenschr. 2002;152(15-16):373-8.
3. Efferth T & Oesch F. Semin Cancer Biol. 2020 Feb 4;S1044-579X(20)30034-1.
4. Banno N et al. J Ethnopharmacol. 2006 Sep 19;107(2):249-53.
5. Poeckel D & Werz O. Curr Med Chem. 2006;13(28):3359-69.
6. Li JY, Kampp JT. Dermatol Surg. 2019 Jan;45(1):58-67.
7. Cao B et al. Molecules. 2019 Aug 24;24(17): 3076.
8. Mertens M et al. Flavour Fragr J. 2009;24:279-300.
9. Ljaljević Grbić M et al. J Ethnopharmacol. 2018 Jun 12;219:1-14.
10. Li XJ et al. J Ethnopharmacol. 2016 Feb 17;179:22-6.
11. Han X et al. Biochim Open. 2017 Feb 3;4:31-5.
12. Zhao W et al. Cancer Detect Prev. 2003;27:67-75.
13. Hakkim FL et al. Oncotarget. 2019 May 28;10(37):3472-90.
14. Fung K et al. OA Altern Med 2013;1:14.
15. de Rapper S et al. Lett Appl Microbiol. 2012 Apr;54(4):352-8.
16. Zhu XF et al. Zhongguo Zhong Yao Za Zhi. 2017 Feb;42(4):680-5.
17. Guan YM et al. Zhongguo Zhong Yao Za Zhi. 2017 Sep;42(17):3350-5.
The Boswellia serrata exudate or gum (known in India as “guggulu”) that forms an aromatic resin traditionally used as incense – and known as frankincense (especially when retrieved from Boswellia species found in Eritrea and Somalia but also from the Indian variety) – has been considered for thousands of years to possess therapeutic properties. It is used in Ayurvedic medicine, as well as in traditional medicine in China and the Middle East, particularly for its anti-inflammatory effects to treat chronic conditions.1-8 In fact, such essential oils have been used since 2800 BC to treat various inflammatory conditions, including skin sores and wounds, as well as in perfumes and incense.2,9 In the West, use of frankincense dates back to thousands of years as well, more often found in the form of incense for religious and cultural ceremonies.7 Over the past 2 decades, .3 This column focuses on some of the emerging data on this ancient botanical agent.
Chemical constituents
Terpenoids and essential oils are the primary components of frankincense and are known to impart anti-inflammatory and anticancer activity. The same is true for myrrh, which has been combined with frankincense in traditional Chinese medicine as a single medication for millennia, with the two acting synergistically and considered still to be a potent combination in conferring various biological benefits.7
In 2010, in a systematic review of the anti-inflammatory and anticancer activities of Boswellia species and their chemical ingredients, Efferth and Oesch found that frankincense blocks the production of leukotrienes, cyclooxygenase (COX) 1 and 2, as well as 5-lipoxygenase; and oxidative stress. It also contributes to regulation of immune cells from the innate and acquired immune systems and exerts anticancer activity by influencing signaling transduction responsible for cell cycle arrest, as well as inhibition of proliferation, angiogenesis, invasion, and metastasis. The investigators also reported on clinical trial results that have found efficacy of frankincense and its constituents in ameliorating symptoms of psoriasis and erythematous eczema, among other disorders.3
Anti-inflammatory activity
Li et al. completed a study in 2016 to identify the active ingredients responsible for the anti-inflammatory and analgesic effects of frankincense. They found that alpha-pinene, linalool, and 1-octanol were key contributors. These constituents were noted for suppressing COX-2 overexpression in mice, as well as nociceptive stimulus-induced inflammatory infiltrates.10
Noting the increasing popularity of frankincense essential oil in skin care, despite a paucity of data, in 2017, Han et al. evaluated the biological activities of the essential oil in pre-inflamed human dermal fibroblasts using 17 key protein biomarkers. Frankincense essential oil displayed significant antiproliferative activity and suppressed collagen III, interferon gamma-induced protein 10, and intracellular adhesion molecule 1. The investigators referred to the overall encouraging potential of frankincense essential oil to exert influence over inflammation and tissue remodeling in human skin and called for additional research into its mechanisms of action and active constituents.11
Anticancer activity
The main active ingredient in frankincense, boswellic acid, has been shown to promote apoptosis, suppress matrix metalloproteinase secretion, and hinder migration in metastatic melanoma cell lines in mice.6,12
In 2019, Hakkim et al. demonstrated that frankincense essential oil yielded substantial antimelanoma activity in vitro and in vivo and ameliorated hepatotoxicity caused by acetaminophen.13
There is one case report in the literature on the use of frankincense as a treatment for skin cancer. A 56-year-old man received frankincense oil multiple times a day for 4 months to treat a nodular basal cell carcinoma on one arm (which resolved) and an infiltrative BCC on the chest (some focal residual tumor remained).6,14 Topical frankincense or boswellic acid has been given a grade D recommendation for treating skin cancer, however, because of only one level-of-evidence-5 study.6
Antimicrobial activity
In 2012, de Rapper et al. collected samples of three essential oils of frankincense (Boswellia rivae, Boswellia neglecta, and Boswellia papyrifera) and two essential oil samples of myrrh and sweet myrrh from different regions of Ethiopia to study their anti-infective properties alone and in combination. The investigators observed synergistic and additive effects, particularly between B. papyrifera and Commiphora myrrha. While noting the long history of the combined use of frankincense and myrrh essential oils since 1500 BC, the investigators highlighted their study as the first antimicrobial work to verify the effectiveness of this combination, validating the use of this combination to thwart particular pathogens.15
Just 2 years ago, Ljaljević Grbić et al. evaluated the in vitro antimicrobial potential of the liquid and vapor phases of B. carteri and C. myrrha (frankincense and myrrh, respectively) essential oils, finding that frankincense demonstrated marked capacity to act as a natural antimicrobial agent.9
Transdermal delivery
In 2017, Zhu et al. showed that frankincense and myrrh essential oils promoted the permeability of the Chinese herb Chuanxiong and may facilitate drug elimination from the epidermis via dermal capillaries by dint of improved cutaneous blood flow, thereby augmenting transdermal drug delivery.16 The same team also showed that frankincense and myrrh essential oils, by fostering permeation by enhancing drug delivery across the stratum corneum, can also alter the structure of the stratum corneum.17
Conclusion
The use of frankincense in traditional medicine has a long and impressive track record. Recent research provides reason for optimism, and further investigating the possible incorporation of this botanical agent into modern dermatologic therapies appears warranted. Clearly, however, much more research is needed.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann has written two textbooks and a New York Times Best Sellers book for consumers. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Revance, Evolus, and Burt’s Bees. She is the CEO of Skin Type Solutions Inc., a company that independently tests skin care products and makes recommendations to physicians on which skin care technologies are best. Write to her at dermnews@mdedge.com.
References
1. Kimmatkar N et al. Phytomedicine. 2003 Jan;10(1):3-7.
2. Ammon HP. Wien Med Wochenschr. 2002;152(15-16):373-8.
3. Efferth T & Oesch F. Semin Cancer Biol. 2020 Feb 4;S1044-579X(20)30034-1.
4. Banno N et al. J Ethnopharmacol. 2006 Sep 19;107(2):249-53.
5. Poeckel D & Werz O. Curr Med Chem. 2006;13(28):3359-69.
6. Li JY, Kampp JT. Dermatol Surg. 2019 Jan;45(1):58-67.
7. Cao B et al. Molecules. 2019 Aug 24;24(17): 3076.
8. Mertens M et al. Flavour Fragr J. 2009;24:279-300.
9. Ljaljević Grbić M et al. J Ethnopharmacol. 2018 Jun 12;219:1-14.
10. Li XJ et al. J Ethnopharmacol. 2016 Feb 17;179:22-6.
11. Han X et al. Biochim Open. 2017 Feb 3;4:31-5.
12. Zhao W et al. Cancer Detect Prev. 2003;27:67-75.
13. Hakkim FL et al. Oncotarget. 2019 May 28;10(37):3472-90.
14. Fung K et al. OA Altern Med 2013;1:14.
15. de Rapper S et al. Lett Appl Microbiol. 2012 Apr;54(4):352-8.
16. Zhu XF et al. Zhongguo Zhong Yao Za Zhi. 2017 Feb;42(4):680-5.
17. Guan YM et al. Zhongguo Zhong Yao Za Zhi. 2017 Sep;42(17):3350-5.
The Boswellia serrata exudate or gum (known in India as “guggulu”) that forms an aromatic resin traditionally used as incense – and known as frankincense (especially when retrieved from Boswellia species found in Eritrea and Somalia but also from the Indian variety) – has been considered for thousands of years to possess therapeutic properties. It is used in Ayurvedic medicine, as well as in traditional medicine in China and the Middle East, particularly for its anti-inflammatory effects to treat chronic conditions.1-8 In fact, such essential oils have been used since 2800 BC to treat various inflammatory conditions, including skin sores and wounds, as well as in perfumes and incense.2,9 In the West, use of frankincense dates back to thousands of years as well, more often found in the form of incense for religious and cultural ceremonies.7 Over the past 2 decades, .3 This column focuses on some of the emerging data on this ancient botanical agent.
Chemical constituents
Terpenoids and essential oils are the primary components of frankincense and are known to impart anti-inflammatory and anticancer activity. The same is true for myrrh, which has been combined with frankincense in traditional Chinese medicine as a single medication for millennia, with the two acting synergistically and considered still to be a potent combination in conferring various biological benefits.7
In 2010, in a systematic review of the anti-inflammatory and anticancer activities of Boswellia species and their chemical ingredients, Efferth and Oesch found that frankincense blocks the production of leukotrienes, cyclooxygenase (COX) 1 and 2, as well as 5-lipoxygenase; and oxidative stress. It also contributes to regulation of immune cells from the innate and acquired immune systems and exerts anticancer activity by influencing signaling transduction responsible for cell cycle arrest, as well as inhibition of proliferation, angiogenesis, invasion, and metastasis. The investigators also reported on clinical trial results that have found efficacy of frankincense and its constituents in ameliorating symptoms of psoriasis and erythematous eczema, among other disorders.3
Anti-inflammatory activity
Li et al. completed a study in 2016 to identify the active ingredients responsible for the anti-inflammatory and analgesic effects of frankincense. They found that alpha-pinene, linalool, and 1-octanol were key contributors. These constituents were noted for suppressing COX-2 overexpression in mice, as well as nociceptive stimulus-induced inflammatory infiltrates.10
Noting the increasing popularity of frankincense essential oil in skin care, despite a paucity of data, in 2017, Han et al. evaluated the biological activities of the essential oil in pre-inflamed human dermal fibroblasts using 17 key protein biomarkers. Frankincense essential oil displayed significant antiproliferative activity and suppressed collagen III, interferon gamma-induced protein 10, and intracellular adhesion molecule 1. The investigators referred to the overall encouraging potential of frankincense essential oil to exert influence over inflammation and tissue remodeling in human skin and called for additional research into its mechanisms of action and active constituents.11
Anticancer activity
The main active ingredient in frankincense, boswellic acid, has been shown to promote apoptosis, suppress matrix metalloproteinase secretion, and hinder migration in metastatic melanoma cell lines in mice.6,12
In 2019, Hakkim et al. demonstrated that frankincense essential oil yielded substantial antimelanoma activity in vitro and in vivo and ameliorated hepatotoxicity caused by acetaminophen.13
There is one case report in the literature on the use of frankincense as a treatment for skin cancer. A 56-year-old man received frankincense oil multiple times a day for 4 months to treat a nodular basal cell carcinoma on one arm (which resolved) and an infiltrative BCC on the chest (some focal residual tumor remained).6,14 Topical frankincense or boswellic acid has been given a grade D recommendation for treating skin cancer, however, because of only one level-of-evidence-5 study.6
Antimicrobial activity
In 2012, de Rapper et al. collected samples of three essential oils of frankincense (Boswellia rivae, Boswellia neglecta, and Boswellia papyrifera) and two essential oil samples of myrrh and sweet myrrh from different regions of Ethiopia to study their anti-infective properties alone and in combination. The investigators observed synergistic and additive effects, particularly between B. papyrifera and Commiphora myrrha. While noting the long history of the combined use of frankincense and myrrh essential oils since 1500 BC, the investigators highlighted their study as the first antimicrobial work to verify the effectiveness of this combination, validating the use of this combination to thwart particular pathogens.15
Just 2 years ago, Ljaljević Grbić et al. evaluated the in vitro antimicrobial potential of the liquid and vapor phases of B. carteri and C. myrrha (frankincense and myrrh, respectively) essential oils, finding that frankincense demonstrated marked capacity to act as a natural antimicrobial agent.9
Transdermal delivery
In 2017, Zhu et al. showed that frankincense and myrrh essential oils promoted the permeability of the Chinese herb Chuanxiong and may facilitate drug elimination from the epidermis via dermal capillaries by dint of improved cutaneous blood flow, thereby augmenting transdermal drug delivery.16 The same team also showed that frankincense and myrrh essential oils, by fostering permeation by enhancing drug delivery across the stratum corneum, can also alter the structure of the stratum corneum.17
Conclusion
The use of frankincense in traditional medicine has a long and impressive track record. Recent research provides reason for optimism, and further investigating the possible incorporation of this botanical agent into modern dermatologic therapies appears warranted. Clearly, however, much more research is needed.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann has written two textbooks and a New York Times Best Sellers book for consumers. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Revance, Evolus, and Burt’s Bees. She is the CEO of Skin Type Solutions Inc., a company that independently tests skin care products and makes recommendations to physicians on which skin care technologies are best. Write to her at dermnews@mdedge.com.
References
1. Kimmatkar N et al. Phytomedicine. 2003 Jan;10(1):3-7.
2. Ammon HP. Wien Med Wochenschr. 2002;152(15-16):373-8.
3. Efferth T & Oesch F. Semin Cancer Biol. 2020 Feb 4;S1044-579X(20)30034-1.
4. Banno N et al. J Ethnopharmacol. 2006 Sep 19;107(2):249-53.
5. Poeckel D & Werz O. Curr Med Chem. 2006;13(28):3359-69.
6. Li JY, Kampp JT. Dermatol Surg. 2019 Jan;45(1):58-67.
7. Cao B et al. Molecules. 2019 Aug 24;24(17): 3076.
8. Mertens M et al. Flavour Fragr J. 2009;24:279-300.
9. Ljaljević Grbić M et al. J Ethnopharmacol. 2018 Jun 12;219:1-14.
10. Li XJ et al. J Ethnopharmacol. 2016 Feb 17;179:22-6.
11. Han X et al. Biochim Open. 2017 Feb 3;4:31-5.
12. Zhao W et al. Cancer Detect Prev. 2003;27:67-75.
13. Hakkim FL et al. Oncotarget. 2019 May 28;10(37):3472-90.
14. Fung K et al. OA Altern Med 2013;1:14.
15. de Rapper S et al. Lett Appl Microbiol. 2012 Apr;54(4):352-8.
16. Zhu XF et al. Zhongguo Zhong Yao Za Zhi. 2017 Feb;42(4):680-5.
17. Guan YM et al. Zhongguo Zhong Yao Za Zhi. 2017 Sep;42(17):3350-5.
The tryptophan photoproduct FICZ and its effects on the skin
The melatonin precursor tryptophan, an amino acid essential in the human diet, has been shown to display antioxidant effects.1 FICZ (also known as 6-formylindolo[3,2-b]carbazole) is a photoproduct of tryptophan that is engendered by exposure to UVB.2 This column discusses the beneficial and detrimental influence of FICZ in skin health.
Antioxidant activity
In 2005, Trommer and Neubert devised a skin lipid model system to screen 47 various compounds (drugs, plant extracts, other plant constituents, and polysaccharides) for topical antioxidative activity in response to UV-induced lipid peroxidation. Among the drugs evaluated, they observed that tryptophan exerted antioxidant effects.3
Wound healing potential
A murine study by Bandeira et al. in 2015 revealed that tryptophan-induced mitigation of the inflammatory response and indoleamine 2, 3-dioxygenase expression may have enhanced skin wound healing in mice who were repeatedly stressed.4
Antifibrotic activity
In 2018, Murai et al. endeavored to clarify the role of FICZ in regulating the expression of matrix metalloproteinases and tissue inhibitors of metalloproteinases in normal human dermal fibroblasts. They found that FICZ assists in imparting UV-mediated antifibrotic effects through the AHR/MEK/ERK signal pathway in normal human dermal fibroblasts and, thus, shows promise as a therapeutic option for scleroderma.5
Cutaneous leishmaniasis
In 2019, Rodrigues et al. conducted a quantitative analysis of the relative expression of 170 genes involved in various biological processes in the skin biopsies from patients with cutaneous leishmaniasis caused by infection with either Leishmania major or L. tropica. They identified tryptophan-2,3-deoxygenase as a restriction factor for the disorder.6
Photosensitizing activity
Park et al. showed that FICZ, a tryptophan photoproduct and endogenous high-affinity aryl hydrocarbon receptor (AhR) agonist, exhibits nanomolar photodynamic activity as a UVA photosensitizer in epidermal keratinocytes and, thus, is possibly operative in human skin.7 Syed and Mukhtar add that FICZ is effective at nanomolar concentrations and that future research may elucidate its applicability against UV-induced adverse effects and inflammatory skin conditions.8
FICZ, oxidative stress, and cancer promotion
FICZ is known to display detrimental, as well as beneficial, influences in skin. The tryptophan photoproduct, comparable to UVB, ligates AhR, generates reactive oxygen species, and strongly photosensitizes for UVA. As Furue et al. note, FICZ upregulates the expression of terminal differentiation molecules (i.e., filaggrin and loricrin via AhR), and its application has been shown to suppress cutaneous inflammation in a psoriasis and dermatitis mouse model.2
In 2016, Reid et al. reported that the protein photodamage brought about by endogenous photosensitizers such as tryptophan tyrosine residues can contribute to the deleterious impact of UVA on human skin.9
In 2018, Tanaka et al. showed that FICZ imparts a cascade of events tantamount in some cases to UVB, as it promoted the synthesis of proinflammatory cytokines such as interleukin (IL)-1 alpha, IL-1 beta, and IL-6 and boosted reactive oxygen species generation in human HaCaT keratinocytes in an AhR-dependent fashion. They concluded that observing FICZ activity contributes to the understanding of how UVB damages organisms.10
That same year, Murai et al. assessed the effects of FICZ on TGF-beta-mediated ACTA2 and collagen I expression in normal human dermal fibroblasts. They determined that it may act as a key chromophore and one approach to mitigating the effects of photoaging may be to downregulate FICZ signaling.11
A year earlier, Brem et al. showed that the combined effect of FICZ and UVA engendered significant protein damage in HaCaT human keratinocytes, with the oxidation yielded from the combination of FICZ and UVA blocking the removal of potentially mutagenic UVB-induced DNA photolesions by nucleotide excision repair. The researchers concluded that the development of FICZ may raise the risk of incurring skin cancer resulting from sun exposure via the promotion of photochemical impairment of the nucleotide excision repair proteome, which in turn inhibits the removal of UVB-induced DNA lesions.12
Conclusion
However, the tryptophan photoproduct FICZ, which results from UVB exposure, presents as a complicated substance, conferring healthy and harmful effects. Much more research is necessary to determine how best to harness and direct the useful activities of tryptophan and FICZ without incurring damaging effects. Nanotechnology may be one useful avenue of investigation for this purpose.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann has written two textbooks and a New York Times Best Sellers book for consumers. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Revance, Evolus, and Burt’s Bees. She is the CEO of Skin Type Solutions Inc., a company that independently tests skin care products and makes recommendations to physicians on which skin care technologies are best. Write to her at dermnews@mdedge.com.
References
1. Trommer H et al. J Pharm Pharmacol. 2003 Oct;55(10):1379-88.
2. Furue M et al. G Ital Dermatol Venereol. 2019 Feb;154(1):37-41.
3. Trommer H and Neubert RH. J Pharm Pharm Sci. 2005 Sep 15;8(3):494-506.
4. Bandeira LG et al. PLoS One. 2015 Jun 9:10(6):e0128439.
5. Murai M et al. J Dermatol Sci. 2018 Jul;91(1):97-103.
6. Rodrigues V et al. Front Cell Infect Microbiol. 2019 Oct 4;9:338. eCollection 2019.
7. Park SL et al. J Invest Dermatol. 2015 Jun;135(6):1649-58.
8. Syed DN and Mukhtar H. J Invest Dermatol. 2015 Jun;135(6):1478-81.
9. Reid LO et al. Biochemistry. 2016 Aug 30;55(34):4777-86.
10. Tanaka Y et al. Oxid Med Cell Longev. 2018 Nov 25;2018:9298052.
11. Murai M et al. J Dermatol Sci. 2018 Jan;89(1):19-26.
12. Brem R et al. Sci Rep. 2017 Jun 27;7(1):4310.
The melatonin precursor tryptophan, an amino acid essential in the human diet, has been shown to display antioxidant effects.1 FICZ (also known as 6-formylindolo[3,2-b]carbazole) is a photoproduct of tryptophan that is engendered by exposure to UVB.2 This column discusses the beneficial and detrimental influence of FICZ in skin health.
Antioxidant activity
In 2005, Trommer and Neubert devised a skin lipid model system to screen 47 various compounds (drugs, plant extracts, other plant constituents, and polysaccharides) for topical antioxidative activity in response to UV-induced lipid peroxidation. Among the drugs evaluated, they observed that tryptophan exerted antioxidant effects.3
Wound healing potential
A murine study by Bandeira et al. in 2015 revealed that tryptophan-induced mitigation of the inflammatory response and indoleamine 2, 3-dioxygenase expression may have enhanced skin wound healing in mice who were repeatedly stressed.4
Antifibrotic activity
In 2018, Murai et al. endeavored to clarify the role of FICZ in regulating the expression of matrix metalloproteinases and tissue inhibitors of metalloproteinases in normal human dermal fibroblasts. They found that FICZ assists in imparting UV-mediated antifibrotic effects through the AHR/MEK/ERK signal pathway in normal human dermal fibroblasts and, thus, shows promise as a therapeutic option for scleroderma.5
Cutaneous leishmaniasis
In 2019, Rodrigues et al. conducted a quantitative analysis of the relative expression of 170 genes involved in various biological processes in the skin biopsies from patients with cutaneous leishmaniasis caused by infection with either Leishmania major or L. tropica. They identified tryptophan-2,3-deoxygenase as a restriction factor for the disorder.6
Photosensitizing activity
Park et al. showed that FICZ, a tryptophan photoproduct and endogenous high-affinity aryl hydrocarbon receptor (AhR) agonist, exhibits nanomolar photodynamic activity as a UVA photosensitizer in epidermal keratinocytes and, thus, is possibly operative in human skin.7 Syed and Mukhtar add that FICZ is effective at nanomolar concentrations and that future research may elucidate its applicability against UV-induced adverse effects and inflammatory skin conditions.8
FICZ, oxidative stress, and cancer promotion
FICZ is known to display detrimental, as well as beneficial, influences in skin. The tryptophan photoproduct, comparable to UVB, ligates AhR, generates reactive oxygen species, and strongly photosensitizes for UVA. As Furue et al. note, FICZ upregulates the expression of terminal differentiation molecules (i.e., filaggrin and loricrin via AhR), and its application has been shown to suppress cutaneous inflammation in a psoriasis and dermatitis mouse model.2
In 2016, Reid et al. reported that the protein photodamage brought about by endogenous photosensitizers such as tryptophan tyrosine residues can contribute to the deleterious impact of UVA on human skin.9
In 2018, Tanaka et al. showed that FICZ imparts a cascade of events tantamount in some cases to UVB, as it promoted the synthesis of proinflammatory cytokines such as interleukin (IL)-1 alpha, IL-1 beta, and IL-6 and boosted reactive oxygen species generation in human HaCaT keratinocytes in an AhR-dependent fashion. They concluded that observing FICZ activity contributes to the understanding of how UVB damages organisms.10
That same year, Murai et al. assessed the effects of FICZ on TGF-beta-mediated ACTA2 and collagen I expression in normal human dermal fibroblasts. They determined that it may act as a key chromophore and one approach to mitigating the effects of photoaging may be to downregulate FICZ signaling.11
A year earlier, Brem et al. showed that the combined effect of FICZ and UVA engendered significant protein damage in HaCaT human keratinocytes, with the oxidation yielded from the combination of FICZ and UVA blocking the removal of potentially mutagenic UVB-induced DNA photolesions by nucleotide excision repair. The researchers concluded that the development of FICZ may raise the risk of incurring skin cancer resulting from sun exposure via the promotion of photochemical impairment of the nucleotide excision repair proteome, which in turn inhibits the removal of UVB-induced DNA lesions.12
Conclusion
However, the tryptophan photoproduct FICZ, which results from UVB exposure, presents as a complicated substance, conferring healthy and harmful effects. Much more research is necessary to determine how best to harness and direct the useful activities of tryptophan and FICZ without incurring damaging effects. Nanotechnology may be one useful avenue of investigation for this purpose.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann has written two textbooks and a New York Times Best Sellers book for consumers. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Revance, Evolus, and Burt’s Bees. She is the CEO of Skin Type Solutions Inc., a company that independently tests skin care products and makes recommendations to physicians on which skin care technologies are best. Write to her at dermnews@mdedge.com.
References
1. Trommer H et al. J Pharm Pharmacol. 2003 Oct;55(10):1379-88.
2. Furue M et al. G Ital Dermatol Venereol. 2019 Feb;154(1):37-41.
3. Trommer H and Neubert RH. J Pharm Pharm Sci. 2005 Sep 15;8(3):494-506.
4. Bandeira LG et al. PLoS One. 2015 Jun 9:10(6):e0128439.
5. Murai M et al. J Dermatol Sci. 2018 Jul;91(1):97-103.
6. Rodrigues V et al. Front Cell Infect Microbiol. 2019 Oct 4;9:338. eCollection 2019.
7. Park SL et al. J Invest Dermatol. 2015 Jun;135(6):1649-58.
8. Syed DN and Mukhtar H. J Invest Dermatol. 2015 Jun;135(6):1478-81.
9. Reid LO et al. Biochemistry. 2016 Aug 30;55(34):4777-86.
10. Tanaka Y et al. Oxid Med Cell Longev. 2018 Nov 25;2018:9298052.
11. Murai M et al. J Dermatol Sci. 2018 Jan;89(1):19-26.
12. Brem R et al. Sci Rep. 2017 Jun 27;7(1):4310.
The melatonin precursor tryptophan, an amino acid essential in the human diet, has been shown to display antioxidant effects.1 FICZ (also known as 6-formylindolo[3,2-b]carbazole) is a photoproduct of tryptophan that is engendered by exposure to UVB.2 This column discusses the beneficial and detrimental influence of FICZ in skin health.
Antioxidant activity
In 2005, Trommer and Neubert devised a skin lipid model system to screen 47 various compounds (drugs, plant extracts, other plant constituents, and polysaccharides) for topical antioxidative activity in response to UV-induced lipid peroxidation. Among the drugs evaluated, they observed that tryptophan exerted antioxidant effects.3
Wound healing potential
A murine study by Bandeira et al. in 2015 revealed that tryptophan-induced mitigation of the inflammatory response and indoleamine 2, 3-dioxygenase expression may have enhanced skin wound healing in mice who were repeatedly stressed.4
Antifibrotic activity
In 2018, Murai et al. endeavored to clarify the role of FICZ in regulating the expression of matrix metalloproteinases and tissue inhibitors of metalloproteinases in normal human dermal fibroblasts. They found that FICZ assists in imparting UV-mediated antifibrotic effects through the AHR/MEK/ERK signal pathway in normal human dermal fibroblasts and, thus, shows promise as a therapeutic option for scleroderma.5
Cutaneous leishmaniasis
In 2019, Rodrigues et al. conducted a quantitative analysis of the relative expression of 170 genes involved in various biological processes in the skin biopsies from patients with cutaneous leishmaniasis caused by infection with either Leishmania major or L. tropica. They identified tryptophan-2,3-deoxygenase as a restriction factor for the disorder.6
Photosensitizing activity
Park et al. showed that FICZ, a tryptophan photoproduct and endogenous high-affinity aryl hydrocarbon receptor (AhR) agonist, exhibits nanomolar photodynamic activity as a UVA photosensitizer in epidermal keratinocytes and, thus, is possibly operative in human skin.7 Syed and Mukhtar add that FICZ is effective at nanomolar concentrations and that future research may elucidate its applicability against UV-induced adverse effects and inflammatory skin conditions.8
FICZ, oxidative stress, and cancer promotion
FICZ is known to display detrimental, as well as beneficial, influences in skin. The tryptophan photoproduct, comparable to UVB, ligates AhR, generates reactive oxygen species, and strongly photosensitizes for UVA. As Furue et al. note, FICZ upregulates the expression of terminal differentiation molecules (i.e., filaggrin and loricrin via AhR), and its application has been shown to suppress cutaneous inflammation in a psoriasis and dermatitis mouse model.2
In 2016, Reid et al. reported that the protein photodamage brought about by endogenous photosensitizers such as tryptophan tyrosine residues can contribute to the deleterious impact of UVA on human skin.9
In 2018, Tanaka et al. showed that FICZ imparts a cascade of events tantamount in some cases to UVB, as it promoted the synthesis of proinflammatory cytokines such as interleukin (IL)-1 alpha, IL-1 beta, and IL-6 and boosted reactive oxygen species generation in human HaCaT keratinocytes in an AhR-dependent fashion. They concluded that observing FICZ activity contributes to the understanding of how UVB damages organisms.10
That same year, Murai et al. assessed the effects of FICZ on TGF-beta-mediated ACTA2 and collagen I expression in normal human dermal fibroblasts. They determined that it may act as a key chromophore and one approach to mitigating the effects of photoaging may be to downregulate FICZ signaling.11
A year earlier, Brem et al. showed that the combined effect of FICZ and UVA engendered significant protein damage in HaCaT human keratinocytes, with the oxidation yielded from the combination of FICZ and UVA blocking the removal of potentially mutagenic UVB-induced DNA photolesions by nucleotide excision repair. The researchers concluded that the development of FICZ may raise the risk of incurring skin cancer resulting from sun exposure via the promotion of photochemical impairment of the nucleotide excision repair proteome, which in turn inhibits the removal of UVB-induced DNA lesions.12
Conclusion
However, the tryptophan photoproduct FICZ, which results from UVB exposure, presents as a complicated substance, conferring healthy and harmful effects. Much more research is necessary to determine how best to harness and direct the useful activities of tryptophan and FICZ without incurring damaging effects. Nanotechnology may be one useful avenue of investigation for this purpose.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann has written two textbooks and a New York Times Best Sellers book for consumers. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Revance, Evolus, and Burt’s Bees. She is the CEO of Skin Type Solutions Inc., a company that independently tests skin care products and makes recommendations to physicians on which skin care technologies are best. Write to her at dermnews@mdedge.com.
References
1. Trommer H et al. J Pharm Pharmacol. 2003 Oct;55(10):1379-88.
2. Furue M et al. G Ital Dermatol Venereol. 2019 Feb;154(1):37-41.
3. Trommer H and Neubert RH. J Pharm Pharm Sci. 2005 Sep 15;8(3):494-506.
4. Bandeira LG et al. PLoS One. 2015 Jun 9:10(6):e0128439.
5. Murai M et al. J Dermatol Sci. 2018 Jul;91(1):97-103.
6. Rodrigues V et al. Front Cell Infect Microbiol. 2019 Oct 4;9:338. eCollection 2019.
7. Park SL et al. J Invest Dermatol. 2015 Jun;135(6):1649-58.
8. Syed DN and Mukhtar H. J Invest Dermatol. 2015 Jun;135(6):1478-81.
9. Reid LO et al. Biochemistry. 2016 Aug 30;55(34):4777-86.
10. Tanaka Y et al. Oxid Med Cell Longev. 2018 Nov 25;2018:9298052.
11. Murai M et al. J Dermatol Sci. 2018 Jan;89(1):19-26.
12. Brem R et al. Sci Rep. 2017 Jun 27;7(1):4310.
Vetiver: More than a pleasant aroma?
An important ingredient in the contemporary perfume and cosmetics industries, vetiver, is the only grass cultivated throughout the world to retain its essential oil, which contains sesquiterpene alcohols and hydrocarbons.1-3 Field and glasshouse studies have revealed that vetiver grass can tolerate extreme variations in climate well, including protracted drought, floods, submergence, temperature, and soils high in acidity, alkalinity, and various heavy metals. Its heartiness may explain its continuing or even increasing use in fragrances and other products pertinent to skin health as humanity strives to adapt to climate change.4 In a 2017 review of various commercial essential oils as antimicrobial therapy for cutaneous disorders, Orchard and van Vuuren identified vetiver as warranting particular attention for its capacity to confer broad benefits to the skin in addressing acne, cuts, eczema, oiliness, sores, wounds, and aging skin.5 The focus of this column will be the dermatologic potential of vetiver.
Chemical constituents
Vetiver is thought to be one of the most complex of the essential oils owing to the hundreds of sesquiterpene derivatives with large structural diversity that contribute to its composition. 3
In a 2012 analysis of the components of South Indian vetiver oils, Mallavarapu et al. found an abundance of sesquiterpenes and oxygenated sesquiterpenes with cedrane, bisabolane, eudesmane, eremophilane, and zizaane skeletons. The primary constituents identified in the four oils evaluated included eudesma-4,6-diene (delta-selinene) + beta-vetispirene (3.9%-6.1%), beta-vetivenene (0.9%-9.4%), 13-nor-trans-eudesma-4(15),7-dien-11-one + amorph-4-en-10-ol (5.0%-6.4%), trans-eudesma-4(15),7-dien-12-ol (vetiselinenol) + (E)-opposita-4(15),7(11)-dien-12-ol (3.7%-5.9%), eremophila-1 (10),11-dien-2alpha-ol (nootkatol) + ziza-6(13)-en-12-ol (khusimol) (16.1%-19.2%), and eremophila-1(10),7(11)-dien-2alpha-ol (isonootkatol) + (E)-eremophila-1(10),7(11)-12-ol (isovalencenol) (5.6%-6.9%).6
Antimicrobial activity
In 2012, Saikia et al. assessed the antimycobacterial activity of Vetiveria zizanioides against Mycobacterium tuberculosis H(37)Rv and H(37)Ra strains. Their results showed that ethanolic extracts and hexane fractions displayed robust antimycobacterial properties, buttressing the traditional medical uses of the plant, as well as consideration of this agent as a modern antituberculosis agent.7
Two years later, Dos Santos et al. showed that Vetiveria zizanioides roots grown in Brazil exhibited notable antimicrobial effects against various pathogenic organisms.8In 2017, Burger et al. showed that vetiver essential oil primarily contributes its scent to cosmetic formulations but also displayed antimicrobial activity against Gram-positive bacterial strains, as well as one strain of Candida glabrata. They suggest that vetiver should be considered for its antimicrobial capacity as an added bonus to cosmetic formulations.2
In a 2018 study to ascertain the antimicrobial activity of 247 essential oil combinations against five reference strains of wound pathogens, Orchard et al. found that 26 combinations exhibited extensive antimicrobial activity. Sandalwood and vetiver were found to contribute most to antimicrobial function when used in combination. The investigators concluded that such combinations warrant consideration for wound therapy.9
Antiacne activity
In 2018, Orchard et al. conducted another study of the efficacy of commercial essential oil combinations against the two pathogens responsible for acne, Propionibacterium acnes and Staphlyococcus epidermidis. They investigated 408 combinations, of which 167 exhibited notable antimicrobial activity. They observed that the combination with the lowest minimum inhibitory concentration value against P. acnes and S. epidermidis was vetiver and cinnamon bark.10 This usage points to the potential of vetiver use as an antiacne ingredient.
Safety
The Scientific Committee on Consumer Safety (SCCS) offered a final opinion on the safety of the fragrance ingredient acetylated vetiver oil in 2019, declaring its use with 1% alpha-tocopherol in cosmetic leave-on and rinse-off products safe at proposed concentration levels. They noted that acetylated vetiver oil has been used for several years without provoking contact allergies.11
Conclusion
Much more research is necessary to determine just what kind of a role this perfumery powerhouse can play in dermatology.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann has written two textbooks and a New York Times Best Sellers book for consumers. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Revance, Evolus, and Burt’s Bees. She is the CEO of Skin Type Solutions Inc., a company that independently tests skin care products and makes recommendations to physicians on which skin care technologies are best. Write to her at dermnews@mdedge.com.
References
1. Del Giudice L et al. Environ Microbiol. 2008 Oct;10(10):2824-41.
2. Burger P et al. Medicines (Basel). 2017 Jun 16;4(2):41.
3. Belhassen E et al. Chem Biodivers. 2014 Nov;11(11):1821–42.
4. Danh LT et al. Int J Phytoremediation. 2009 Oct-Dec;11(8):664–91.
5. Orchard A and van Vuuren S. Evid Based Complement Alternat Med. 2017;2017:4517971.
6. Mallavarapu GR et al. Nat Prod Commun. 2012 Feb;7(2):223–5.
7. Saikia D et al. Complement Ther Med. 2012 Dec;20(6):434–6.
8. Dos Santos DS et al. Acta Pharm. 2014 Dec;64(4):495-501.
9. Orchard A et al. Chem Biodivers. 2018 Dec;15(12):e1800405.
10. Orchard A et al. Int J Cosmet Sci. 2018 Mar 24. [Epub ahead of print].
11. SCCS members & External experts. Regul Toxicol Pharmacol. 2019 Oct;107:104389.
An important ingredient in the contemporary perfume and cosmetics industries, vetiver, is the only grass cultivated throughout the world to retain its essential oil, which contains sesquiterpene alcohols and hydrocarbons.1-3 Field and glasshouse studies have revealed that vetiver grass can tolerate extreme variations in climate well, including protracted drought, floods, submergence, temperature, and soils high in acidity, alkalinity, and various heavy metals. Its heartiness may explain its continuing or even increasing use in fragrances and other products pertinent to skin health as humanity strives to adapt to climate change.4 In a 2017 review of various commercial essential oils as antimicrobial therapy for cutaneous disorders, Orchard and van Vuuren identified vetiver as warranting particular attention for its capacity to confer broad benefits to the skin in addressing acne, cuts, eczema, oiliness, sores, wounds, and aging skin.5 The focus of this column will be the dermatologic potential of vetiver.
Chemical constituents
Vetiver is thought to be one of the most complex of the essential oils owing to the hundreds of sesquiterpene derivatives with large structural diversity that contribute to its composition. 3
In a 2012 analysis of the components of South Indian vetiver oils, Mallavarapu et al. found an abundance of sesquiterpenes and oxygenated sesquiterpenes with cedrane, bisabolane, eudesmane, eremophilane, and zizaane skeletons. The primary constituents identified in the four oils evaluated included eudesma-4,6-diene (delta-selinene) + beta-vetispirene (3.9%-6.1%), beta-vetivenene (0.9%-9.4%), 13-nor-trans-eudesma-4(15),7-dien-11-one + amorph-4-en-10-ol (5.0%-6.4%), trans-eudesma-4(15),7-dien-12-ol (vetiselinenol) + (E)-opposita-4(15),7(11)-dien-12-ol (3.7%-5.9%), eremophila-1 (10),11-dien-2alpha-ol (nootkatol) + ziza-6(13)-en-12-ol (khusimol) (16.1%-19.2%), and eremophila-1(10),7(11)-dien-2alpha-ol (isonootkatol) + (E)-eremophila-1(10),7(11)-12-ol (isovalencenol) (5.6%-6.9%).6
Antimicrobial activity
In 2012, Saikia et al. assessed the antimycobacterial activity of Vetiveria zizanioides against Mycobacterium tuberculosis H(37)Rv and H(37)Ra strains. Their results showed that ethanolic extracts and hexane fractions displayed robust antimycobacterial properties, buttressing the traditional medical uses of the plant, as well as consideration of this agent as a modern antituberculosis agent.7
Two years later, Dos Santos et al. showed that Vetiveria zizanioides roots grown in Brazil exhibited notable antimicrobial effects against various pathogenic organisms.8In 2017, Burger et al. showed that vetiver essential oil primarily contributes its scent to cosmetic formulations but also displayed antimicrobial activity against Gram-positive bacterial strains, as well as one strain of Candida glabrata. They suggest that vetiver should be considered for its antimicrobial capacity as an added bonus to cosmetic formulations.2
In a 2018 study to ascertain the antimicrobial activity of 247 essential oil combinations against five reference strains of wound pathogens, Orchard et al. found that 26 combinations exhibited extensive antimicrobial activity. Sandalwood and vetiver were found to contribute most to antimicrobial function when used in combination. The investigators concluded that such combinations warrant consideration for wound therapy.9
Antiacne activity
In 2018, Orchard et al. conducted another study of the efficacy of commercial essential oil combinations against the two pathogens responsible for acne, Propionibacterium acnes and Staphlyococcus epidermidis. They investigated 408 combinations, of which 167 exhibited notable antimicrobial activity. They observed that the combination with the lowest minimum inhibitory concentration value against P. acnes and S. epidermidis was vetiver and cinnamon bark.10 This usage points to the potential of vetiver use as an antiacne ingredient.
Safety
The Scientific Committee on Consumer Safety (SCCS) offered a final opinion on the safety of the fragrance ingredient acetylated vetiver oil in 2019, declaring its use with 1% alpha-tocopherol in cosmetic leave-on and rinse-off products safe at proposed concentration levels. They noted that acetylated vetiver oil has been used for several years without provoking contact allergies.11
Conclusion
Much more research is necessary to determine just what kind of a role this perfumery powerhouse can play in dermatology.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann has written two textbooks and a New York Times Best Sellers book for consumers. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Revance, Evolus, and Burt’s Bees. She is the CEO of Skin Type Solutions Inc., a company that independently tests skin care products and makes recommendations to physicians on which skin care technologies are best. Write to her at dermnews@mdedge.com.
References
1. Del Giudice L et al. Environ Microbiol. 2008 Oct;10(10):2824-41.
2. Burger P et al. Medicines (Basel). 2017 Jun 16;4(2):41.
3. Belhassen E et al. Chem Biodivers. 2014 Nov;11(11):1821–42.
4. Danh LT et al. Int J Phytoremediation. 2009 Oct-Dec;11(8):664–91.
5. Orchard A and van Vuuren S. Evid Based Complement Alternat Med. 2017;2017:4517971.
6. Mallavarapu GR et al. Nat Prod Commun. 2012 Feb;7(2):223–5.
7. Saikia D et al. Complement Ther Med. 2012 Dec;20(6):434–6.
8. Dos Santos DS et al. Acta Pharm. 2014 Dec;64(4):495-501.
9. Orchard A et al. Chem Biodivers. 2018 Dec;15(12):e1800405.
10. Orchard A et al. Int J Cosmet Sci. 2018 Mar 24. [Epub ahead of print].
11. SCCS members & External experts. Regul Toxicol Pharmacol. 2019 Oct;107:104389.
An important ingredient in the contemporary perfume and cosmetics industries, vetiver, is the only grass cultivated throughout the world to retain its essential oil, which contains sesquiterpene alcohols and hydrocarbons.1-3 Field and glasshouse studies have revealed that vetiver grass can tolerate extreme variations in climate well, including protracted drought, floods, submergence, temperature, and soils high in acidity, alkalinity, and various heavy metals. Its heartiness may explain its continuing or even increasing use in fragrances and other products pertinent to skin health as humanity strives to adapt to climate change.4 In a 2017 review of various commercial essential oils as antimicrobial therapy for cutaneous disorders, Orchard and van Vuuren identified vetiver as warranting particular attention for its capacity to confer broad benefits to the skin in addressing acne, cuts, eczema, oiliness, sores, wounds, and aging skin.5 The focus of this column will be the dermatologic potential of vetiver.
Chemical constituents
Vetiver is thought to be one of the most complex of the essential oils owing to the hundreds of sesquiterpene derivatives with large structural diversity that contribute to its composition. 3
In a 2012 analysis of the components of South Indian vetiver oils, Mallavarapu et al. found an abundance of sesquiterpenes and oxygenated sesquiterpenes with cedrane, bisabolane, eudesmane, eremophilane, and zizaane skeletons. The primary constituents identified in the four oils evaluated included eudesma-4,6-diene (delta-selinene) + beta-vetispirene (3.9%-6.1%), beta-vetivenene (0.9%-9.4%), 13-nor-trans-eudesma-4(15),7-dien-11-one + amorph-4-en-10-ol (5.0%-6.4%), trans-eudesma-4(15),7-dien-12-ol (vetiselinenol) + (E)-opposita-4(15),7(11)-dien-12-ol (3.7%-5.9%), eremophila-1 (10),11-dien-2alpha-ol (nootkatol) + ziza-6(13)-en-12-ol (khusimol) (16.1%-19.2%), and eremophila-1(10),7(11)-dien-2alpha-ol (isonootkatol) + (E)-eremophila-1(10),7(11)-12-ol (isovalencenol) (5.6%-6.9%).6
Antimicrobial activity
In 2012, Saikia et al. assessed the antimycobacterial activity of Vetiveria zizanioides against Mycobacterium tuberculosis H(37)Rv and H(37)Ra strains. Their results showed that ethanolic extracts and hexane fractions displayed robust antimycobacterial properties, buttressing the traditional medical uses of the plant, as well as consideration of this agent as a modern antituberculosis agent.7
Two years later, Dos Santos et al. showed that Vetiveria zizanioides roots grown in Brazil exhibited notable antimicrobial effects against various pathogenic organisms.8In 2017, Burger et al. showed that vetiver essential oil primarily contributes its scent to cosmetic formulations but also displayed antimicrobial activity against Gram-positive bacterial strains, as well as one strain of Candida glabrata. They suggest that vetiver should be considered for its antimicrobial capacity as an added bonus to cosmetic formulations.2
In a 2018 study to ascertain the antimicrobial activity of 247 essential oil combinations against five reference strains of wound pathogens, Orchard et al. found that 26 combinations exhibited extensive antimicrobial activity. Sandalwood and vetiver were found to contribute most to antimicrobial function when used in combination. The investigators concluded that such combinations warrant consideration for wound therapy.9
Antiacne activity
In 2018, Orchard et al. conducted another study of the efficacy of commercial essential oil combinations against the two pathogens responsible for acne, Propionibacterium acnes and Staphlyococcus epidermidis. They investigated 408 combinations, of which 167 exhibited notable antimicrobial activity. They observed that the combination with the lowest minimum inhibitory concentration value against P. acnes and S. epidermidis was vetiver and cinnamon bark.10 This usage points to the potential of vetiver use as an antiacne ingredient.
Safety
The Scientific Committee on Consumer Safety (SCCS) offered a final opinion on the safety of the fragrance ingredient acetylated vetiver oil in 2019, declaring its use with 1% alpha-tocopherol in cosmetic leave-on and rinse-off products safe at proposed concentration levels. They noted that acetylated vetiver oil has been used for several years without provoking contact allergies.11
Conclusion
Much more research is necessary to determine just what kind of a role this perfumery powerhouse can play in dermatology.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann has written two textbooks and a New York Times Best Sellers book for consumers. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Revance, Evolus, and Burt’s Bees. She is the CEO of Skin Type Solutions Inc., a company that independently tests skin care products and makes recommendations to physicians on which skin care technologies are best. Write to her at dermnews@mdedge.com.
References
1. Del Giudice L et al. Environ Microbiol. 2008 Oct;10(10):2824-41.
2. Burger P et al. Medicines (Basel). 2017 Jun 16;4(2):41.
3. Belhassen E et al. Chem Biodivers. 2014 Nov;11(11):1821–42.
4. Danh LT et al. Int J Phytoremediation. 2009 Oct-Dec;11(8):664–91.
5. Orchard A and van Vuuren S. Evid Based Complement Alternat Med. 2017;2017:4517971.
6. Mallavarapu GR et al. Nat Prod Commun. 2012 Feb;7(2):223–5.
7. Saikia D et al. Complement Ther Med. 2012 Dec;20(6):434–6.
8. Dos Santos DS et al. Acta Pharm. 2014 Dec;64(4):495-501.
9. Orchard A et al. Chem Biodivers. 2018 Dec;15(12):e1800405.
10. Orchard A et al. Int J Cosmet Sci. 2018 Mar 24. [Epub ahead of print].
11. SCCS members & External experts. Regul Toxicol Pharmacol. 2019 Oct;107:104389.
Exsanguinating the truth about dragon’s blood in cosmeceuticals
The use of dragon’s blood is renowned among various medical traditions around the world.1,2 It is known to confer anti-inflammatory, antioxidant, antitumor, antimicrobial, and wound healing benefits, among others. Dragon’s blood and its characteristic red sap has also been used in folk magic and as a coloring substance and varnish.1 In addition, dragon’s blood resin is one of the many botanical agents with roots in traditional medicine that are among the bioactive ingredients used in the booming contemporary Korean cosmeceutical agent market.3.
Many plants, only some have dermatologic properties
Essentially, the moniker “dragon’s blood” describes the deep red resin or sap that has been derived from multiple plant sources – primarily from the genera Daemonorops, Dracaena, Croton, and Pterocarpus – over multiple centuries.2,4 In traditional Chinese medicine (TCM), various plants have been used as dragon’s blood, including Butea monosperma, Liquidambar formosana, Daemonorops draco, and, more commonly now, Dracaena cochinchinensis.5
Chemical constituents and activity
Dragon’s blood represents the red exudate culled from 27 species of plants from four families. Among the six Dracaena plants (D. cochinchinensis, D. cambodiana, D. cinnabari, D. draco, D. loureiroi, and D. schizantha) from which dragon’s blood is derived, flavonoids and their oligomers are considered the main active constituents. Analgesic, anti-inflammatory, antibacterial, hypolipidemic, hypoglycemic, and cytotoxic activities have been associated with these botanicals.6
D. cochinchinensis is one source of the ethnomedicine “dragon’s blood” that has long been used in TCM. Contemporary studies have shown that the resin of D. cochinchinensis – key constituents of which include loureirin A, loureirin B, loureirin C, cochinchinenin, socotrin-4’-ol, 4’,7-dihydroxyflavan, 4-methylcholest-7-ene-3-ol, ethylparaben, resveratrol, and hydroxyphenol – exhibits antibacterial, anti-inflammatory, analgesic, antidiabetic, and antitumor activities. It has also been shown to support skin repair.4
In 2017, Wang et al. reported that flavonoids from artificially induced dragon’s blood of D. cambodiana showed antibacterial properties.7 The next year, Al Fatimi reported that the dragon’s blood derived from D. cinnabari is a key plant on Yemen’s Socotra Island, where it is used for its antifungal and antioxidant properties to treat various dermal, dental, eye, and gastrointestinal diseases in humans.8Croton lechleri (also one of the plants known as dragon’s blood), a medicinal plant found in the Amazon rainforest and characterized by its red sap, has been shown in preclinical studies to display anti-inflammatory, antioxidant, antimicrobial, antifungal, and antineoplastic activity. Pona et al. note that, while clinical studies of C. lechleri suggest wound healing and antiviral effects, the current use of this plant has limited cutaneous applications.9
Wound healing activity
In 1995, Pieters et al. performed an in vivo study on rats to assess the wound healing activity of dragon’s blood (Croton spp.) from South America. In comparing the effects with those of synthetic proanthocyanidins, the researchers verified the beneficial impact of dragon’s blood in stimulating wound contraction, crust formation, new collagen development, and epithelial layer regeneration. The dragon’s blood component 3’,4-O-dimethylcedrusin was also found to enhance healing by promoting fibroblast and collagen formation, though it was not as effective as crude dragon’s blood. The authors ascribed this effect to the proanthocyanidins in the plant.10
Late in 2003, Jones published a literature review on the evidence related to Croton lechleri (known in South America as “sangre de drago” or dragon’s blood) in support of various biological effects, particularly anti-inflammatory and wound healing capability. The results from multiple in vitro and in vivo investigations buttressed previous ethnomedical justifications for the use of dragon’s blood to treat herpes, insect bites, stomach ulcers, tumors, wounds, and diarrhea, as well as other conditions. Jones added that the sap of the plant has exhibited low toxicity and has been well tolerated in clinical studies.11
In 2012, Hu et al. investigated the impact of dragon’s blood powder with varying grain size on the transdermal absorption and adhesion of ZJHX paste, finding that, with decreasing grain size, penetration of dracorhodin increased, thus promoting transdermal permeability and adhesion.12
Lieu et al. assessed the wound healing potential of Resina Draconis, derived from D. cochinchinensis, which has long been used in traditional medicines by various cultures. In this 2013 evaluation, the investigators substantiated the traditional uses of this herb for wound healing, using excision and incision models in rats. Animals treated with D. cochinchinensis resin displayed significantly superior wound contraction and tensile strength as compared with controls, with histopathological results revealing better microvessel density and growth factor expression levels.13
In 2017, Jiang et al. showed that dracorhodin percolate, derived from dragon’s blood and used extensively to treat wound healing in TCM, accelerated wound healing in Wistar rats.14 A year later, they found that the use of dracorhodin perchlorate was effective in regulating fibroblast proliferation in vitro and in vivo to promote wound healing in rats. In addition, they noted that phosphorylated–extracellular signal-regulated kinase (ERK) in the wound tissue significantly increased with treatment of dracorhodin perchlorate ointment. The researchers called for clinical trials testing this compound in humans as the next step.15
In 2015, Namjoyan et al. conducted a randomized, double-blind, placebo-controlled clinical trial in 60 patients (between 14 and 65 years old) to assess the wound healing effect of a dragon’s blood cream on skin tag removal. Patients were visited every third day during this 3-week study, after which a significant difference in mean wound healing duration was identified. The investigators attributed the accelerated wound healing action to the phenolic constituents and alkaloid taspine in the resin. They also concluded that dragon’s blood warrants inclusion in the wound healing arsenal, while calling for studies in larger populations.16
Conclusion
The red resin extracts of multiple species of plants have and continue to be identified as “dragon’s blood.” This exudate has been used for various medical indications in traditional medicine for several centuries. Despite this lengthy history, modern research is hardly robust. Nevertheless, there are many credible reports of significant salutary activities associated with these resins and some evidence of cutaneous benefits. Much more research is necessary to determine how useful these ingredients are, despite their present use in a number of marketed cosmeceutical agents.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann has written two textbooks and a New York Times Best Sellers book for consumers. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Revance, Evolus, and Burt’s Bees. She is the CEO of Skin Type Solutions Inc., a company that independently tests skin care products and makes recommendations to physicians on which skin care technologies are best. Write to her at dermnews@mdedge.com.
References
1. Gupta D et al. J Ethnopharmacol. 2008 Feb 12;115(3):361-80.
2. Jura-Morawiec J & Tulik. Chemoecology. 2016;26:101-5.
3. Nguyen JK et al. J Cosmet Dermatol. 2020 Jul;19(7):155-69.
4. Fan JY et al. Molecules. 2014 Jul 22;19(7):10650-69.
5. Zhang W et al. Zhongguo Zhong Yao Za Zhi. 2016 Apr;41(7):1354-7.
6. Sun J et al. J Ethnopharmacol. 2019 Nov 15;244:112138.
7. Wang H et al. Fitoterapia. 2017 Sep;121:1-5.
8. Al-Fatimi M. Plants (Basel). 2018 Oct 26;7(4):91.
9. Pona A et al. Dermatol Ther. 2019 Mar;32(2):e12786.10. Pieters L et al. Phytomedicine. 1995 Jul;2(1):17-22.
11. Jones K. J Altern Complement Med. 2003 Dec;9(6):877-96.
12. Hu Q et al. Zhongguo Zhong Yao Za Zhi. 2012 Dec;37(23):3549-53.
13. Liu H et al. Evid Based Complement Alternat Med. 2013;2013:709865.
14. Jiang XW et al. Evid Based Complement Alternat Med. 2017:8950516.
15. Jiang X et al. J Pharmacol Sci. 2018 Feb;136(2):66-72.
16. Namjoyan F et al. J Tradit Complement Med. 2015 Jan 22;6(1):37-40.
The use of dragon’s blood is renowned among various medical traditions around the world.1,2 It is known to confer anti-inflammatory, antioxidant, antitumor, antimicrobial, and wound healing benefits, among others. Dragon’s blood and its characteristic red sap has also been used in folk magic and as a coloring substance and varnish.1 In addition, dragon’s blood resin is one of the many botanical agents with roots in traditional medicine that are among the bioactive ingredients used in the booming contemporary Korean cosmeceutical agent market.3.
Many plants, only some have dermatologic properties
Essentially, the moniker “dragon’s blood” describes the deep red resin or sap that has been derived from multiple plant sources – primarily from the genera Daemonorops, Dracaena, Croton, and Pterocarpus – over multiple centuries.2,4 In traditional Chinese medicine (TCM), various plants have been used as dragon’s blood, including Butea monosperma, Liquidambar formosana, Daemonorops draco, and, more commonly now, Dracaena cochinchinensis.5
Chemical constituents and activity
Dragon’s blood represents the red exudate culled from 27 species of plants from four families. Among the six Dracaena plants (D. cochinchinensis, D. cambodiana, D. cinnabari, D. draco, D. loureiroi, and D. schizantha) from which dragon’s blood is derived, flavonoids and their oligomers are considered the main active constituents. Analgesic, anti-inflammatory, antibacterial, hypolipidemic, hypoglycemic, and cytotoxic activities have been associated with these botanicals.6
D. cochinchinensis is one source of the ethnomedicine “dragon’s blood” that has long been used in TCM. Contemporary studies have shown that the resin of D. cochinchinensis – key constituents of which include loureirin A, loureirin B, loureirin C, cochinchinenin, socotrin-4’-ol, 4’,7-dihydroxyflavan, 4-methylcholest-7-ene-3-ol, ethylparaben, resveratrol, and hydroxyphenol – exhibits antibacterial, anti-inflammatory, analgesic, antidiabetic, and antitumor activities. It has also been shown to support skin repair.4
In 2017, Wang et al. reported that flavonoids from artificially induced dragon’s blood of D. cambodiana showed antibacterial properties.7 The next year, Al Fatimi reported that the dragon’s blood derived from D. cinnabari is a key plant on Yemen’s Socotra Island, where it is used for its antifungal and antioxidant properties to treat various dermal, dental, eye, and gastrointestinal diseases in humans.8Croton lechleri (also one of the plants known as dragon’s blood), a medicinal plant found in the Amazon rainforest and characterized by its red sap, has been shown in preclinical studies to display anti-inflammatory, antioxidant, antimicrobial, antifungal, and antineoplastic activity. Pona et al. note that, while clinical studies of C. lechleri suggest wound healing and antiviral effects, the current use of this plant has limited cutaneous applications.9
Wound healing activity
In 1995, Pieters et al. performed an in vivo study on rats to assess the wound healing activity of dragon’s blood (Croton spp.) from South America. In comparing the effects with those of synthetic proanthocyanidins, the researchers verified the beneficial impact of dragon’s blood in stimulating wound contraction, crust formation, new collagen development, and epithelial layer regeneration. The dragon’s blood component 3’,4-O-dimethylcedrusin was also found to enhance healing by promoting fibroblast and collagen formation, though it was not as effective as crude dragon’s blood. The authors ascribed this effect to the proanthocyanidins in the plant.10
Late in 2003, Jones published a literature review on the evidence related to Croton lechleri (known in South America as “sangre de drago” or dragon’s blood) in support of various biological effects, particularly anti-inflammatory and wound healing capability. The results from multiple in vitro and in vivo investigations buttressed previous ethnomedical justifications for the use of dragon’s blood to treat herpes, insect bites, stomach ulcers, tumors, wounds, and diarrhea, as well as other conditions. Jones added that the sap of the plant has exhibited low toxicity and has been well tolerated in clinical studies.11
In 2012, Hu et al. investigated the impact of dragon’s blood powder with varying grain size on the transdermal absorption and adhesion of ZJHX paste, finding that, with decreasing grain size, penetration of dracorhodin increased, thus promoting transdermal permeability and adhesion.12
Lieu et al. assessed the wound healing potential of Resina Draconis, derived from D. cochinchinensis, which has long been used in traditional medicines by various cultures. In this 2013 evaluation, the investigators substantiated the traditional uses of this herb for wound healing, using excision and incision models in rats. Animals treated with D. cochinchinensis resin displayed significantly superior wound contraction and tensile strength as compared with controls, with histopathological results revealing better microvessel density and growth factor expression levels.13
In 2017, Jiang et al. showed that dracorhodin percolate, derived from dragon’s blood and used extensively to treat wound healing in TCM, accelerated wound healing in Wistar rats.14 A year later, they found that the use of dracorhodin perchlorate was effective in regulating fibroblast proliferation in vitro and in vivo to promote wound healing in rats. In addition, they noted that phosphorylated–extracellular signal-regulated kinase (ERK) in the wound tissue significantly increased with treatment of dracorhodin perchlorate ointment. The researchers called for clinical trials testing this compound in humans as the next step.15
In 2015, Namjoyan et al. conducted a randomized, double-blind, placebo-controlled clinical trial in 60 patients (between 14 and 65 years old) to assess the wound healing effect of a dragon’s blood cream on skin tag removal. Patients were visited every third day during this 3-week study, after which a significant difference in mean wound healing duration was identified. The investigators attributed the accelerated wound healing action to the phenolic constituents and alkaloid taspine in the resin. They also concluded that dragon’s blood warrants inclusion in the wound healing arsenal, while calling for studies in larger populations.16
Conclusion
The red resin extracts of multiple species of plants have and continue to be identified as “dragon’s blood.” This exudate has been used for various medical indications in traditional medicine for several centuries. Despite this lengthy history, modern research is hardly robust. Nevertheless, there are many credible reports of significant salutary activities associated with these resins and some evidence of cutaneous benefits. Much more research is necessary to determine how useful these ingredients are, despite their present use in a number of marketed cosmeceutical agents.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann has written two textbooks and a New York Times Best Sellers book for consumers. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Revance, Evolus, and Burt’s Bees. She is the CEO of Skin Type Solutions Inc., a company that independently tests skin care products and makes recommendations to physicians on which skin care technologies are best. Write to her at dermnews@mdedge.com.
References
1. Gupta D et al. J Ethnopharmacol. 2008 Feb 12;115(3):361-80.
2. Jura-Morawiec J & Tulik. Chemoecology. 2016;26:101-5.
3. Nguyen JK et al. J Cosmet Dermatol. 2020 Jul;19(7):155-69.
4. Fan JY et al. Molecules. 2014 Jul 22;19(7):10650-69.
5. Zhang W et al. Zhongguo Zhong Yao Za Zhi. 2016 Apr;41(7):1354-7.
6. Sun J et al. J Ethnopharmacol. 2019 Nov 15;244:112138.
7. Wang H et al. Fitoterapia. 2017 Sep;121:1-5.
8. Al-Fatimi M. Plants (Basel). 2018 Oct 26;7(4):91.
9. Pona A et al. Dermatol Ther. 2019 Mar;32(2):e12786.10. Pieters L et al. Phytomedicine. 1995 Jul;2(1):17-22.
11. Jones K. J Altern Complement Med. 2003 Dec;9(6):877-96.
12. Hu Q et al. Zhongguo Zhong Yao Za Zhi. 2012 Dec;37(23):3549-53.
13. Liu H et al. Evid Based Complement Alternat Med. 2013;2013:709865.
14. Jiang XW et al. Evid Based Complement Alternat Med. 2017:8950516.
15. Jiang X et al. J Pharmacol Sci. 2018 Feb;136(2):66-72.
16. Namjoyan F et al. J Tradit Complement Med. 2015 Jan 22;6(1):37-40.
The use of dragon’s blood is renowned among various medical traditions around the world.1,2 It is known to confer anti-inflammatory, antioxidant, antitumor, antimicrobial, and wound healing benefits, among others. Dragon’s blood and its characteristic red sap has also been used in folk magic and as a coloring substance and varnish.1 In addition, dragon’s blood resin is one of the many botanical agents with roots in traditional medicine that are among the bioactive ingredients used in the booming contemporary Korean cosmeceutical agent market.3.
Many plants, only some have dermatologic properties
Essentially, the moniker “dragon’s blood” describes the deep red resin or sap that has been derived from multiple plant sources – primarily from the genera Daemonorops, Dracaena, Croton, and Pterocarpus – over multiple centuries.2,4 In traditional Chinese medicine (TCM), various plants have been used as dragon’s blood, including Butea monosperma, Liquidambar formosana, Daemonorops draco, and, more commonly now, Dracaena cochinchinensis.5
Chemical constituents and activity
Dragon’s blood represents the red exudate culled from 27 species of plants from four families. Among the six Dracaena plants (D. cochinchinensis, D. cambodiana, D. cinnabari, D. draco, D. loureiroi, and D. schizantha) from which dragon’s blood is derived, flavonoids and their oligomers are considered the main active constituents. Analgesic, anti-inflammatory, antibacterial, hypolipidemic, hypoglycemic, and cytotoxic activities have been associated with these botanicals.6
D. cochinchinensis is one source of the ethnomedicine “dragon’s blood” that has long been used in TCM. Contemporary studies have shown that the resin of D. cochinchinensis – key constituents of which include loureirin A, loureirin B, loureirin C, cochinchinenin, socotrin-4’-ol, 4’,7-dihydroxyflavan, 4-methylcholest-7-ene-3-ol, ethylparaben, resveratrol, and hydroxyphenol – exhibits antibacterial, anti-inflammatory, analgesic, antidiabetic, and antitumor activities. It has also been shown to support skin repair.4
In 2017, Wang et al. reported that flavonoids from artificially induced dragon’s blood of D. cambodiana showed antibacterial properties.7 The next year, Al Fatimi reported that the dragon’s blood derived from D. cinnabari is a key plant on Yemen’s Socotra Island, where it is used for its antifungal and antioxidant properties to treat various dermal, dental, eye, and gastrointestinal diseases in humans.8Croton lechleri (also one of the plants known as dragon’s blood), a medicinal plant found in the Amazon rainforest and characterized by its red sap, has been shown in preclinical studies to display anti-inflammatory, antioxidant, antimicrobial, antifungal, and antineoplastic activity. Pona et al. note that, while clinical studies of C. lechleri suggest wound healing and antiviral effects, the current use of this plant has limited cutaneous applications.9
Wound healing activity
In 1995, Pieters et al. performed an in vivo study on rats to assess the wound healing activity of dragon’s blood (Croton spp.) from South America. In comparing the effects with those of synthetic proanthocyanidins, the researchers verified the beneficial impact of dragon’s blood in stimulating wound contraction, crust formation, new collagen development, and epithelial layer regeneration. The dragon’s blood component 3’,4-O-dimethylcedrusin was also found to enhance healing by promoting fibroblast and collagen formation, though it was not as effective as crude dragon’s blood. The authors ascribed this effect to the proanthocyanidins in the plant.10
Late in 2003, Jones published a literature review on the evidence related to Croton lechleri (known in South America as “sangre de drago” or dragon’s blood) in support of various biological effects, particularly anti-inflammatory and wound healing capability. The results from multiple in vitro and in vivo investigations buttressed previous ethnomedical justifications for the use of dragon’s blood to treat herpes, insect bites, stomach ulcers, tumors, wounds, and diarrhea, as well as other conditions. Jones added that the sap of the plant has exhibited low toxicity and has been well tolerated in clinical studies.11
In 2012, Hu et al. investigated the impact of dragon’s blood powder with varying grain size on the transdermal absorption and adhesion of ZJHX paste, finding that, with decreasing grain size, penetration of dracorhodin increased, thus promoting transdermal permeability and adhesion.12
Lieu et al. assessed the wound healing potential of Resina Draconis, derived from D. cochinchinensis, which has long been used in traditional medicines by various cultures. In this 2013 evaluation, the investigators substantiated the traditional uses of this herb for wound healing, using excision and incision models in rats. Animals treated with D. cochinchinensis resin displayed significantly superior wound contraction and tensile strength as compared with controls, with histopathological results revealing better microvessel density and growth factor expression levels.13
In 2017, Jiang et al. showed that dracorhodin percolate, derived from dragon’s blood and used extensively to treat wound healing in TCM, accelerated wound healing in Wistar rats.14 A year later, they found that the use of dracorhodin perchlorate was effective in regulating fibroblast proliferation in vitro and in vivo to promote wound healing in rats. In addition, they noted that phosphorylated–extracellular signal-regulated kinase (ERK) in the wound tissue significantly increased with treatment of dracorhodin perchlorate ointment. The researchers called for clinical trials testing this compound in humans as the next step.15
In 2015, Namjoyan et al. conducted a randomized, double-blind, placebo-controlled clinical trial in 60 patients (between 14 and 65 years old) to assess the wound healing effect of a dragon’s blood cream on skin tag removal. Patients were visited every third day during this 3-week study, after which a significant difference in mean wound healing duration was identified. The investigators attributed the accelerated wound healing action to the phenolic constituents and alkaloid taspine in the resin. They also concluded that dragon’s blood warrants inclusion in the wound healing arsenal, while calling for studies in larger populations.16
Conclusion
The red resin extracts of multiple species of plants have and continue to be identified as “dragon’s blood.” This exudate has been used for various medical indications in traditional medicine for several centuries. Despite this lengthy history, modern research is hardly robust. Nevertheless, there are many credible reports of significant salutary activities associated with these resins and some evidence of cutaneous benefits. Much more research is necessary to determine how useful these ingredients are, despite their present use in a number of marketed cosmeceutical agents.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann has written two textbooks and a New York Times Best Sellers book for consumers. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Revance, Evolus, and Burt’s Bees. She is the CEO of Skin Type Solutions Inc., a company that independently tests skin care products and makes recommendations to physicians on which skin care technologies are best. Write to her at dermnews@mdedge.com.
References
1. Gupta D et al. J Ethnopharmacol. 2008 Feb 12;115(3):361-80.
2. Jura-Morawiec J & Tulik. Chemoecology. 2016;26:101-5.
3. Nguyen JK et al. J Cosmet Dermatol. 2020 Jul;19(7):155-69.
4. Fan JY et al. Molecules. 2014 Jul 22;19(7):10650-69.
5. Zhang W et al. Zhongguo Zhong Yao Za Zhi. 2016 Apr;41(7):1354-7.
6. Sun J et al. J Ethnopharmacol. 2019 Nov 15;244:112138.
7. Wang H et al. Fitoterapia. 2017 Sep;121:1-5.
8. Al-Fatimi M. Plants (Basel). 2018 Oct 26;7(4):91.
9. Pona A et al. Dermatol Ther. 2019 Mar;32(2):e12786.10. Pieters L et al. Phytomedicine. 1995 Jul;2(1):17-22.
11. Jones K. J Altern Complement Med. 2003 Dec;9(6):877-96.
12. Hu Q et al. Zhongguo Zhong Yao Za Zhi. 2012 Dec;37(23):3549-53.
13. Liu H et al. Evid Based Complement Alternat Med. 2013;2013:709865.
14. Jiang XW et al. Evid Based Complement Alternat Med. 2017:8950516.
15. Jiang X et al. J Pharmacol Sci. 2018 Feb;136(2):66-72.
16. Namjoyan F et al. J Tradit Complement Med. 2015 Jan 22;6(1):37-40.
Synthetic snake venom to the rescue? Potential uses in skin health and rejuvenation
1 This column discusses some of the emerging data in this novel area of medical and dermatologic research. For more detailed information, a review on the therapeutic potential of peptides in animal venom was published in 2003 (Nat Rev Drug Discov. 2003 Oct;2[10]:790-802).
The potential of peptides found in snake venom
Snake venom is known to contain carbohydrates, nucleosides, amino acids, and lipids, as well as enzymatic and nonenzymatic proteins and peptides, with proteins and peptides comprising the primary components.2
There are many different types of peptides in snake venom. The peptides and the small proteins found in snake venoms are known to confer a wide range of biologic activities, including antimicrobial, antihypertensive, analgesic, antitumor, and analgesic, in addition to several others. These peptides have been included in antiaging skin care products.3Pennington et al. have observed that venom-derived peptides appear to have potential as effective therapeutic agents in cosmetic formulations.4 In particular, Waglerin peptides appear to act with a Botox-like paralyzing effect and purportedly diminish skin wrinkles.5
Issues with efficacy of snake venom in skin care products
As with many skin care ingredients, what is seen in cell cultures or a laboratory setting may not translate to real life use. Shelf life, issues during manufacturing, interaction with other ingredients in the product, interactions with other products in the regimen, exposure to air and light, and difficulty of penetration can all affect efficacy. With snake venom in particular, stability and penetration make the efficacy in skin care products questionable.
The problem with many peptides in skin care products is that they are usually larger than 500 Dalton and, therefore, cannot penetrate into the skin. Bos et al. described the “500 Dalton rule” in 2000.6 Regardless of these issues, there are several publications looking at snake venom that will be discussed here.
Antimicrobial and wound healing activity
In 2011, Samy et al. found that phospholipase A2 purified from crotalid snake venom expressed antibacterial activity in vitro against various clinical human pathogens. The investigators synthesized peptides based on the sequence homology and ascertained that the synthetic peptides exhibited potent microbicidal properties against Gram-negative and Gram-positive (Staphylococcus aureus) bacteria with diminished toxicity against normal human cells. Subsequently, the investigators used a BALB/c mouse model to show that peptide-treated animals displayed accelerated healing of full-thickness skin wounds, with increased re-epithelialization, collagen production, and angiogenesis. They concluded that the protein/peptide complex developed from snake venoms was effective at fostering wound healing.7
In that same year, Samy et al. showed in vivo that the snake venom phospholipase A₂ (svPLA₂) proteins from Viperidae and Elapidae snakes activated innate immunity in the animals tested, providing protection against skin infection caused by S. aureus. In vitro experiments also revealed that svPLA₂ proteins dose dependently exerted bacteriostatic and bactericidal effects on S. aureus.8 In 2015, Al-Asmari et al. comparatively assessed the venoms of two cobras,four vipers, a standard antibiotic, and an antimycotic as antimicrobial agents. The methicillin resistant Staphylococcus aureus bacterium was the most susceptible, followed by Gram-positive S. aureus, Escherichia coli, Enterococcus faecalis, and Pseudomonas aeruginosa. While the antibiotic vancomycin was more effective against P. aeruginosa, the venoms more efficiently suppressed the resistant bacteria. The snake venoms had minimal effect on the fungus Candida albicans. The investigators concluded that the snake venoms exhibited antibacterial activity comparable to antibiotics and were more efficient in tackling resistant bacteria.9 In a review of animal venoms in 2017, Samy et al. reported that snake venom–derived synthetic peptide/snake cathelicidin exhibits robust antimicrobial and wound healing capacity, despite its instability and risk, and presents as a possible new treatment for S. aureus infections. They indicated that antimicrobial peptides derived from various animal venoms, including snakes, spiders, and scorpions, are in early experimental and preclinical development stages, and these cysteine-rich substances share hydrophobic alpha-helices or beta-sheets that yield lethal pores and membrane-impairing results on bacteria.10
New drugs and emerging indications
An ingredient that is said to mimic waglerin-1, a snake venom–derived peptide, is the main active ingredient in the Hanskin Syn-Ake Peptide Renewal Mask, a Korean product, which reportedly promotes facial muscle relaxation and wrinkle reduction, as the waglerin-1 provokes neuromuscular blockade via reversible antagonism of nicotinic acetylcholine receptors.2,4,5
Waheed et al. reported in 2017 that recent innovations in molecular research have led to scientific harnessing of the various proteins and peptides found in snake venoms to render them salutary, rather than toxic. Most of the drug development focuses on coagulopathy, hemostasis, and anticancer functions, but research continues in other areas.11 According to An et al., several studies have also been performed on the use of snake venom to treat atopic dermatitis.12
Conclusion
Snake venom is a substance known primarily for its extreme toxicity, but it seems to offer promise for having beneficial effects in medicine. Due to its size and instability, it is doubtful that snake venom will have utility as a topical application in the dermatologic arsenal. In spite of the lack of convincing evidence, a search on Amazon.com brings up dozens of various skin care products containing snake venom. Much more research is necessary, of course, to see if there are methods to facilitate entry of snake venom into the dermis and if this is even desirable.
Snake venom is, in fact, my favorite example of a skin care ingredient that is a waste of money in skin care products. Do you have any favorite “charlatan skincare ingredients”? If so, feel free to contact me, and I will write a column. As dermatologists, we have a responsibility to debunk skin care marketing claims not supported by scientific evidence. I am here to help.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann has written two textbooks and a New York Times Best Sellers book for consumers. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Revance, Evolus, and Burt’s Bees. She is the CEO of Skin Type Solutions Inc., a company that independently tests skin care products and makes recommendations to physicians on which skin care technologies are best. Write to her at dermnews@mdedge.com.
References
1. Nguyen JK et al. J Cosmet Dermatol. 2020 Jul;19(7):1555-69.
2. Munawar A et al. Snake venom peptides: tools of biodiscovery. Toxins (Basel). 2018 Nov 14;10(11):474.
3. Almeida JR et al. Curr Med Chem. 2017;24(30):3254-82.
4. Pennington MW et al. Bioorg Med Chem. 2018 Jun 1;26(10):2738-58.
5. Debono J et al. J Mol Evol. 2017 Jan;84(1):8-11.
6. Bos JD, Meinardi MM. Exp Dermatol. 2000 Jun;9(3):165-9.
7. Samy RP et al. Methods Mol Biol. 2011;716:245-65.
8. Samy RP et al. Curr Med Chem. 2011;18(33):5104-13.
9. Al-Asmari AK et al. Open Microbiol J. 2015 Jul;9:18-25.
10. Perumal Samy R et al. Biochem Pharmacol. 2017 Jun 15;134:127-38.
11. Waheed H et al. Curr Med Chem. 2017;24(17):1874-91.
12. An HJ et al. Br J Pharmacol. 2018 Dec;175(23):4310-24.
1 This column discusses some of the emerging data in this novel area of medical and dermatologic research. For more detailed information, a review on the therapeutic potential of peptides in animal venom was published in 2003 (Nat Rev Drug Discov. 2003 Oct;2[10]:790-802).
The potential of peptides found in snake venom
Snake venom is known to contain carbohydrates, nucleosides, amino acids, and lipids, as well as enzymatic and nonenzymatic proteins and peptides, with proteins and peptides comprising the primary components.2
There are many different types of peptides in snake venom. The peptides and the small proteins found in snake venoms are known to confer a wide range of biologic activities, including antimicrobial, antihypertensive, analgesic, antitumor, and analgesic, in addition to several others. These peptides have been included in antiaging skin care products.3Pennington et al. have observed that venom-derived peptides appear to have potential as effective therapeutic agents in cosmetic formulations.4 In particular, Waglerin peptides appear to act with a Botox-like paralyzing effect and purportedly diminish skin wrinkles.5
Issues with efficacy of snake venom in skin care products
As with many skin care ingredients, what is seen in cell cultures or a laboratory setting may not translate to real life use. Shelf life, issues during manufacturing, interaction with other ingredients in the product, interactions with other products in the regimen, exposure to air and light, and difficulty of penetration can all affect efficacy. With snake venom in particular, stability and penetration make the efficacy in skin care products questionable.
The problem with many peptides in skin care products is that they are usually larger than 500 Dalton and, therefore, cannot penetrate into the skin. Bos et al. described the “500 Dalton rule” in 2000.6 Regardless of these issues, there are several publications looking at snake venom that will be discussed here.
Antimicrobial and wound healing activity
In 2011, Samy et al. found that phospholipase A2 purified from crotalid snake venom expressed antibacterial activity in vitro against various clinical human pathogens. The investigators synthesized peptides based on the sequence homology and ascertained that the synthetic peptides exhibited potent microbicidal properties against Gram-negative and Gram-positive (Staphylococcus aureus) bacteria with diminished toxicity against normal human cells. Subsequently, the investigators used a BALB/c mouse model to show that peptide-treated animals displayed accelerated healing of full-thickness skin wounds, with increased re-epithelialization, collagen production, and angiogenesis. They concluded that the protein/peptide complex developed from snake venoms was effective at fostering wound healing.7
In that same year, Samy et al. showed in vivo that the snake venom phospholipase A₂ (svPLA₂) proteins from Viperidae and Elapidae snakes activated innate immunity in the animals tested, providing protection against skin infection caused by S. aureus. In vitro experiments also revealed that svPLA₂ proteins dose dependently exerted bacteriostatic and bactericidal effects on S. aureus.8 In 2015, Al-Asmari et al. comparatively assessed the venoms of two cobras,four vipers, a standard antibiotic, and an antimycotic as antimicrobial agents. The methicillin resistant Staphylococcus aureus bacterium was the most susceptible, followed by Gram-positive S. aureus, Escherichia coli, Enterococcus faecalis, and Pseudomonas aeruginosa. While the antibiotic vancomycin was more effective against P. aeruginosa, the venoms more efficiently suppressed the resistant bacteria. The snake venoms had minimal effect on the fungus Candida albicans. The investigators concluded that the snake venoms exhibited antibacterial activity comparable to antibiotics and were more efficient in tackling resistant bacteria.9 In a review of animal venoms in 2017, Samy et al. reported that snake venom–derived synthetic peptide/snake cathelicidin exhibits robust antimicrobial and wound healing capacity, despite its instability and risk, and presents as a possible new treatment for S. aureus infections. They indicated that antimicrobial peptides derived from various animal venoms, including snakes, spiders, and scorpions, are in early experimental and preclinical development stages, and these cysteine-rich substances share hydrophobic alpha-helices or beta-sheets that yield lethal pores and membrane-impairing results on bacteria.10
New drugs and emerging indications
An ingredient that is said to mimic waglerin-1, a snake venom–derived peptide, is the main active ingredient in the Hanskin Syn-Ake Peptide Renewal Mask, a Korean product, which reportedly promotes facial muscle relaxation and wrinkle reduction, as the waglerin-1 provokes neuromuscular blockade via reversible antagonism of nicotinic acetylcholine receptors.2,4,5
Waheed et al. reported in 2017 that recent innovations in molecular research have led to scientific harnessing of the various proteins and peptides found in snake venoms to render them salutary, rather than toxic. Most of the drug development focuses on coagulopathy, hemostasis, and anticancer functions, but research continues in other areas.11 According to An et al., several studies have also been performed on the use of snake venom to treat atopic dermatitis.12
Conclusion
Snake venom is a substance known primarily for its extreme toxicity, but it seems to offer promise for having beneficial effects in medicine. Due to its size and instability, it is doubtful that snake venom will have utility as a topical application in the dermatologic arsenal. In spite of the lack of convincing evidence, a search on Amazon.com brings up dozens of various skin care products containing snake venom. Much more research is necessary, of course, to see if there are methods to facilitate entry of snake venom into the dermis and if this is even desirable.
Snake venom is, in fact, my favorite example of a skin care ingredient that is a waste of money in skin care products. Do you have any favorite “charlatan skincare ingredients”? If so, feel free to contact me, and I will write a column. As dermatologists, we have a responsibility to debunk skin care marketing claims not supported by scientific evidence. I am here to help.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann has written two textbooks and a New York Times Best Sellers book for consumers. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Revance, Evolus, and Burt’s Bees. She is the CEO of Skin Type Solutions Inc., a company that independently tests skin care products and makes recommendations to physicians on which skin care technologies are best. Write to her at dermnews@mdedge.com.
References
1. Nguyen JK et al. J Cosmet Dermatol. 2020 Jul;19(7):1555-69.
2. Munawar A et al. Snake venom peptides: tools of biodiscovery. Toxins (Basel). 2018 Nov 14;10(11):474.
3. Almeida JR et al. Curr Med Chem. 2017;24(30):3254-82.
4. Pennington MW et al. Bioorg Med Chem. 2018 Jun 1;26(10):2738-58.
5. Debono J et al. J Mol Evol. 2017 Jan;84(1):8-11.
6. Bos JD, Meinardi MM. Exp Dermatol. 2000 Jun;9(3):165-9.
7. Samy RP et al. Methods Mol Biol. 2011;716:245-65.
8. Samy RP et al. Curr Med Chem. 2011;18(33):5104-13.
9. Al-Asmari AK et al. Open Microbiol J. 2015 Jul;9:18-25.
10. Perumal Samy R et al. Biochem Pharmacol. 2017 Jun 15;134:127-38.
11. Waheed H et al. Curr Med Chem. 2017;24(17):1874-91.
12. An HJ et al. Br J Pharmacol. 2018 Dec;175(23):4310-24.
1 This column discusses some of the emerging data in this novel area of medical and dermatologic research. For more detailed information, a review on the therapeutic potential of peptides in animal venom was published in 2003 (Nat Rev Drug Discov. 2003 Oct;2[10]:790-802).
The potential of peptides found in snake venom
Snake venom is known to contain carbohydrates, nucleosides, amino acids, and lipids, as well as enzymatic and nonenzymatic proteins and peptides, with proteins and peptides comprising the primary components.2
There are many different types of peptides in snake venom. The peptides and the small proteins found in snake venoms are known to confer a wide range of biologic activities, including antimicrobial, antihypertensive, analgesic, antitumor, and analgesic, in addition to several others. These peptides have been included in antiaging skin care products.3Pennington et al. have observed that venom-derived peptides appear to have potential as effective therapeutic agents in cosmetic formulations.4 In particular, Waglerin peptides appear to act with a Botox-like paralyzing effect and purportedly diminish skin wrinkles.5
Issues with efficacy of snake venom in skin care products
As with many skin care ingredients, what is seen in cell cultures or a laboratory setting may not translate to real life use. Shelf life, issues during manufacturing, interaction with other ingredients in the product, interactions with other products in the regimen, exposure to air and light, and difficulty of penetration can all affect efficacy. With snake venom in particular, stability and penetration make the efficacy in skin care products questionable.
The problem with many peptides in skin care products is that they are usually larger than 500 Dalton and, therefore, cannot penetrate into the skin. Bos et al. described the “500 Dalton rule” in 2000.6 Regardless of these issues, there are several publications looking at snake venom that will be discussed here.
Antimicrobial and wound healing activity
In 2011, Samy et al. found that phospholipase A2 purified from crotalid snake venom expressed antibacterial activity in vitro against various clinical human pathogens. The investigators synthesized peptides based on the sequence homology and ascertained that the synthetic peptides exhibited potent microbicidal properties against Gram-negative and Gram-positive (Staphylococcus aureus) bacteria with diminished toxicity against normal human cells. Subsequently, the investigators used a BALB/c mouse model to show that peptide-treated animals displayed accelerated healing of full-thickness skin wounds, with increased re-epithelialization, collagen production, and angiogenesis. They concluded that the protein/peptide complex developed from snake venoms was effective at fostering wound healing.7
In that same year, Samy et al. showed in vivo that the snake venom phospholipase A₂ (svPLA₂) proteins from Viperidae and Elapidae snakes activated innate immunity in the animals tested, providing protection against skin infection caused by S. aureus. In vitro experiments also revealed that svPLA₂ proteins dose dependently exerted bacteriostatic and bactericidal effects on S. aureus.8 In 2015, Al-Asmari et al. comparatively assessed the venoms of two cobras,four vipers, a standard antibiotic, and an antimycotic as antimicrobial agents. The methicillin resistant Staphylococcus aureus bacterium was the most susceptible, followed by Gram-positive S. aureus, Escherichia coli, Enterococcus faecalis, and Pseudomonas aeruginosa. While the antibiotic vancomycin was more effective against P. aeruginosa, the venoms more efficiently suppressed the resistant bacteria. The snake venoms had minimal effect on the fungus Candida albicans. The investigators concluded that the snake venoms exhibited antibacterial activity comparable to antibiotics and were more efficient in tackling resistant bacteria.9 In a review of animal venoms in 2017, Samy et al. reported that snake venom–derived synthetic peptide/snake cathelicidin exhibits robust antimicrobial and wound healing capacity, despite its instability and risk, and presents as a possible new treatment for S. aureus infections. They indicated that antimicrobial peptides derived from various animal venoms, including snakes, spiders, and scorpions, are in early experimental and preclinical development stages, and these cysteine-rich substances share hydrophobic alpha-helices or beta-sheets that yield lethal pores and membrane-impairing results on bacteria.10
New drugs and emerging indications
An ingredient that is said to mimic waglerin-1, a snake venom–derived peptide, is the main active ingredient in the Hanskin Syn-Ake Peptide Renewal Mask, a Korean product, which reportedly promotes facial muscle relaxation and wrinkle reduction, as the waglerin-1 provokes neuromuscular blockade via reversible antagonism of nicotinic acetylcholine receptors.2,4,5
Waheed et al. reported in 2017 that recent innovations in molecular research have led to scientific harnessing of the various proteins and peptides found in snake venoms to render them salutary, rather than toxic. Most of the drug development focuses on coagulopathy, hemostasis, and anticancer functions, but research continues in other areas.11 According to An et al., several studies have also been performed on the use of snake venom to treat atopic dermatitis.12
Conclusion
Snake venom is a substance known primarily for its extreme toxicity, but it seems to offer promise for having beneficial effects in medicine. Due to its size and instability, it is doubtful that snake venom will have utility as a topical application in the dermatologic arsenal. In spite of the lack of convincing evidence, a search on Amazon.com brings up dozens of various skin care products containing snake venom. Much more research is necessary, of course, to see if there are methods to facilitate entry of snake venom into the dermis and if this is even desirable.
Snake venom is, in fact, my favorite example of a skin care ingredient that is a waste of money in skin care products. Do you have any favorite “charlatan skincare ingredients”? If so, feel free to contact me, and I will write a column. As dermatologists, we have a responsibility to debunk skin care marketing claims not supported by scientific evidence. I am here to help.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann has written two textbooks and a New York Times Best Sellers book for consumers. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Revance, Evolus, and Burt’s Bees. She is the CEO of Skin Type Solutions Inc., a company that independently tests skin care products and makes recommendations to physicians on which skin care technologies are best. Write to her at dermnews@mdedge.com.
References
1. Nguyen JK et al. J Cosmet Dermatol. 2020 Jul;19(7):1555-69.
2. Munawar A et al. Snake venom peptides: tools of biodiscovery. Toxins (Basel). 2018 Nov 14;10(11):474.
3. Almeida JR et al. Curr Med Chem. 2017;24(30):3254-82.
4. Pennington MW et al. Bioorg Med Chem. 2018 Jun 1;26(10):2738-58.
5. Debono J et al. J Mol Evol. 2017 Jan;84(1):8-11.
6. Bos JD, Meinardi MM. Exp Dermatol. 2000 Jun;9(3):165-9.
7. Samy RP et al. Methods Mol Biol. 2011;716:245-65.
8. Samy RP et al. Curr Med Chem. 2011;18(33):5104-13.
9. Al-Asmari AK et al. Open Microbiol J. 2015 Jul;9:18-25.
10. Perumal Samy R et al. Biochem Pharmacol. 2017 Jun 15;134:127-38.
11. Waheed H et al. Curr Med Chem. 2017;24(17):1874-91.
12. An HJ et al. Br J Pharmacol. 2018 Dec;175(23):4310-24.
Cellular senescence, skin aging, and cosmeceuticals
I just completed the third edition of my Cosmetic Dermatology textbook (McGraw Hill), which will come out later this year. Although writing it is a huge effort, I really enjoy all the basic science. While I was working on the book, I was most surprised by the .
Right now, it is too early, and we don’t know enough yet, to have cosmeceuticals that affect cellular senescence and autophagy. But, it’s not too early to learn about this research, to avoid falling prey to any pseudoscience that invariably ends up affecting cosmeceuticals on the market. The following is a brief primer on cellular senescence, skin aging, and cosmeceuticals; it represents what we currently know.
Cell phases
Keratinocytes and fibroblasts go through five different phases: stem, proliferation, differentiation, senescence, and apoptosis. The difference between apoptotic cells and senescent cells is that apoptotic cells are not viable and are eliminated, while senescent cells, even though they have gone into cell cycle arrest, remain functional and are not eliminated from the skin.
What are senescent cells?
Senescent cells have lost the ability to proliferate but have not undergone apoptosis. Senescent human skin fibroblasts in cell culture lose the youthful spindlelike shape and become enlarged and flattened.1 Their lysosomes and mitochondria lose functionality.2 The presence of senescent cells is associated with increased aging and seems to speed aging.
Senescent cells and skin aging
Senescent cells are increased in the age-related phenotype3 because of an age-related decline of senescent cell removal systems, such as the immune system4 and the autophagy-lysosomal pathway.5 Senescent cells are deleterious because they develop into a senescence-associated secretory phenotype (SASP), which is believed to be one of the major causes of aging. SASP cells communicate with nearby cells using proinflammatory cytokines, which include catabolic modulators such as Matrix metalloproteinases. They are known to release growth factors, cytokines, chemokines, matrix-modeling enzymes, lipids, and extracellular vesicles. The last are lipid bilayer-lined vesicles that can transport functional RNA and microRNA and facilitate other modes of communication between cells.6
The SASP is likely a natural tumor suppressive mode employed by cells to prevent cells with cancerous mutations from undergoing replication;7 however, when it comes to aging, the deleterious effects of SASP outweigh the beneficial effects. For example, SASP contributes to a prolonged state of inflammation, known as “inflammaging,”8 which is detrimental to the skin’s appearance. Human fibroblasts that have assumed the SASP secrete proinflammatory cytokines and MMPs and release reactive oxygen species,9,10 resulting in degradation of the surrounding extracellular matrix (ECM). Loss of the ECM leads to fibroblast compaction and reduced DNA synthesis, all caused by SASPs.9
What causes cellular senescence?
Activation of the nuclear factor-erythroid 2-related transcription factor 2 (NRF2) induces cellular senescence via direct targeting of certain ECM genes. NRF2 is a key regulator of the skin’s antioxidant defense system, which controls the transcription of genes encoding reactive oxygen species–detoxifying enzymes and various other antioxidant proteins.11 Loss of mitochondrial autophagy also induces senescence, as do activation of the TP53 gene, inactivity of SIRT-1, and short telomeres.
Cellular senescence and skin aging
Timely clearance of senescent cells before they create too much damage postpones the onset and severity of age-related diseases and extends the life span of mice.12,6 Antiaging treatments should focus on decreasing the number of senescent cells and reverting senescent cells to the more juvenile forms: proliferating or differentiating cells as an approach to prevent skin aging.13 Restoration of the lysosomal-mitochondrial axis has been shown to revert SASP back to a juvenile status. Normalization of the lysosomal-mitochondrial axis is a prerequisite to reverse senescence.14
Cellular senescence, autophagy, the lysosomal-mitochondrial axis, and cosmeceuticals
Autophagy is the important process of organelles, like mitochondria,15 self-digesting their cytoplasmic material into lysosomes for degradation. Mitochondrial autophagy is very important in slowing the aging process because damaged mitochondria generate free radicals. As you can imagine, much research is focused on this area, but it is too early for any research to translate to efficacious cosmeceuticals.
Conclusion
To summarize, activation of sirtuin-1 (SIRT-1) has been shown to extend the lifespan of mammals, as does caloric restriction.16 This extension occurs because SIRT-1 decreases senescence and activates autophagy.
Although we do not yet know whether topical skincare products could affect senescence or autophagy, there are data to show that oral resveratrol16 and melatonin17 activate SIRT-1 and increase autophagy. I am closely watching this research and will let you know if there are any similar data on topical cosmeceuticals targeting senescence or autophagy. Stay tuned!
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann has written two textbooks and a New York Times Best Sellers book for consumers. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Revance, Evolus, and Burt’s Bees. She is the CEO of Skin Type Solutions Inc., a company that independently tests skin care products and makes recommendations to physicians on which skin care technologies are best. Write to her at dermnews@mdedge.com.
References
1. Papadopoulou A et al. Biogerontology. 2020 Dec;21(6):695-708.
2. López-Otin C et al. Cell. 2013 June 6;153, 1194–217.
3. Yoon J E et al. Theranostics. 2018 Sep 9;8(17):4620-32.
4. Rodier F, Campisi J. J Cell Biol. 2011 Feb 21;192(4):547-56.
5. Dutta D et al. Circ Res. 2012 Apr 13;110(8):1125-38.
6. Terlecki-Zaniewicz L et al. J Invest Dermatol. 2019 Dec;139(12):2425-36.e5.
7. Campisi J et al. Nat Rev Mol Cell Biol. 2007 Sep;8(9):729-40.
8. Franceschi C and Campisi J. J Gerontol A Biol Sci Med Sci. 2014 Jun;69 Suppl 1:S4-9.
9. Nelson G et al. Aging Cell. 2012 Apr;11(2):345-9.
10. Passos JF et al. PLoS Biol. 2007 May;5(5):e110.
11. Hiebert P et al. Dev Cell. 2018 Jul 16;46(2):145-61.e10.
12. Baker DJ et al. Nature. 2016 Feb 11:530(7589):184-9.
13. Mavrogonatou E et al. Matrix Biol. 2019 Jan;75-76:27-42.
14. Park JT et al. Ageing Res Rev. 2018 Nov;47:176-82.
15. Levine B and Kroemer G. Cell. 2019 Jan 10;176(1-2):11-42.
16. Morselli E et al. Cell Death Dis. 2010;1(1):e10.
17. Lee JH et al. Oncotarget. 2016 Mar 15;7(11):12075-88.
I just completed the third edition of my Cosmetic Dermatology textbook (McGraw Hill), which will come out later this year. Although writing it is a huge effort, I really enjoy all the basic science. While I was working on the book, I was most surprised by the .
Right now, it is too early, and we don’t know enough yet, to have cosmeceuticals that affect cellular senescence and autophagy. But, it’s not too early to learn about this research, to avoid falling prey to any pseudoscience that invariably ends up affecting cosmeceuticals on the market. The following is a brief primer on cellular senescence, skin aging, and cosmeceuticals; it represents what we currently know.
Cell phases
Keratinocytes and fibroblasts go through five different phases: stem, proliferation, differentiation, senescence, and apoptosis. The difference between apoptotic cells and senescent cells is that apoptotic cells are not viable and are eliminated, while senescent cells, even though they have gone into cell cycle arrest, remain functional and are not eliminated from the skin.
What are senescent cells?
Senescent cells have lost the ability to proliferate but have not undergone apoptosis. Senescent human skin fibroblasts in cell culture lose the youthful spindlelike shape and become enlarged and flattened.1 Their lysosomes and mitochondria lose functionality.2 The presence of senescent cells is associated with increased aging and seems to speed aging.

Senescent cells and skin aging
Senescent cells are increased in the age-related phenotype3 because of an age-related decline of senescent cell removal systems, such as the immune system4 and the autophagy-lysosomal pathway.5 Senescent cells are deleterious because they develop into a senescence-associated secretory phenotype (SASP), which is believed to be one of the major causes of aging. SASP cells communicate with nearby cells using proinflammatory cytokines, which include catabolic modulators such as Matrix metalloproteinases. They are known to release growth factors, cytokines, chemokines, matrix-modeling enzymes, lipids, and extracellular vesicles. The last are lipid bilayer-lined vesicles that can transport functional RNA and microRNA and facilitate other modes of communication between cells.6
The SASP is likely a natural tumor suppressive mode employed by cells to prevent cells with cancerous mutations from undergoing replication;7 however, when it comes to aging, the deleterious effects of SASP outweigh the beneficial effects. For example, SASP contributes to a prolonged state of inflammation, known as “inflammaging,”8 which is detrimental to the skin’s appearance. Human fibroblasts that have assumed the SASP secrete proinflammatory cytokines and MMPs and release reactive oxygen species,9,10 resulting in degradation of the surrounding extracellular matrix (ECM). Loss of the ECM leads to fibroblast compaction and reduced DNA synthesis, all caused by SASPs.9
What causes cellular senescence?
Activation of the nuclear factor-erythroid 2-related transcription factor 2 (NRF2) induces cellular senescence via direct targeting of certain ECM genes. NRF2 is a key regulator of the skin’s antioxidant defense system, which controls the transcription of genes encoding reactive oxygen species–detoxifying enzymes and various other antioxidant proteins.11 Loss of mitochondrial autophagy also induces senescence, as do activation of the TP53 gene, inactivity of SIRT-1, and short telomeres.
Cellular senescence and skin aging
Timely clearance of senescent cells before they create too much damage postpones the onset and severity of age-related diseases and extends the life span of mice.12,6 Antiaging treatments should focus on decreasing the number of senescent cells and reverting senescent cells to the more juvenile forms: proliferating or differentiating cells as an approach to prevent skin aging.13 Restoration of the lysosomal-mitochondrial axis has been shown to revert SASP back to a juvenile status. Normalization of the lysosomal-mitochondrial axis is a prerequisite to reverse senescence.14
Cellular senescence, autophagy, the lysosomal-mitochondrial axis, and cosmeceuticals
Autophagy is the important process of organelles, like mitochondria,15 self-digesting their cytoplasmic material into lysosomes for degradation. Mitochondrial autophagy is very important in slowing the aging process because damaged mitochondria generate free radicals. As you can imagine, much research is focused on this area, but it is too early for any research to translate to efficacious cosmeceuticals.
Conclusion
To summarize, activation of sirtuin-1 (SIRT-1) has been shown to extend the lifespan of mammals, as does caloric restriction.16 This extension occurs because SIRT-1 decreases senescence and activates autophagy.
Although we do not yet know whether topical skincare products could affect senescence or autophagy, there are data to show that oral resveratrol16 and melatonin17 activate SIRT-1 and increase autophagy. I am closely watching this research and will let you know if there are any similar data on topical cosmeceuticals targeting senescence or autophagy. Stay tuned!
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann has written two textbooks and a New York Times Best Sellers book for consumers. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Revance, Evolus, and Burt’s Bees. She is the CEO of Skin Type Solutions Inc., a company that independently tests skin care products and makes recommendations to physicians on which skin care technologies are best. Write to her at dermnews@mdedge.com.
References
1. Papadopoulou A et al. Biogerontology. 2020 Dec;21(6):695-708.
2. López-Otin C et al. Cell. 2013 June 6;153, 1194–217.
3. Yoon J E et al. Theranostics. 2018 Sep 9;8(17):4620-32.
4. Rodier F, Campisi J. J Cell Biol. 2011 Feb 21;192(4):547-56.
5. Dutta D et al. Circ Res. 2012 Apr 13;110(8):1125-38.
6. Terlecki-Zaniewicz L et al. J Invest Dermatol. 2019 Dec;139(12):2425-36.e5.
7. Campisi J et al. Nat Rev Mol Cell Biol. 2007 Sep;8(9):729-40.
8. Franceschi C and Campisi J. J Gerontol A Biol Sci Med Sci. 2014 Jun;69 Suppl 1:S4-9.
9. Nelson G et al. Aging Cell. 2012 Apr;11(2):345-9.
10. Passos JF et al. PLoS Biol. 2007 May;5(5):e110.
11. Hiebert P et al. Dev Cell. 2018 Jul 16;46(2):145-61.e10.
12. Baker DJ et al. Nature. 2016 Feb 11:530(7589):184-9.
13. Mavrogonatou E et al. Matrix Biol. 2019 Jan;75-76:27-42.
14. Park JT et al. Ageing Res Rev. 2018 Nov;47:176-82.
15. Levine B and Kroemer G. Cell. 2019 Jan 10;176(1-2):11-42.
16. Morselli E et al. Cell Death Dis. 2010;1(1):e10.
17. Lee JH et al. Oncotarget. 2016 Mar 15;7(11):12075-88.
I just completed the third edition of my Cosmetic Dermatology textbook (McGraw Hill), which will come out later this year. Although writing it is a huge effort, I really enjoy all the basic science. While I was working on the book, I was most surprised by the .
Right now, it is too early, and we don’t know enough yet, to have cosmeceuticals that affect cellular senescence and autophagy. But, it’s not too early to learn about this research, to avoid falling prey to any pseudoscience that invariably ends up affecting cosmeceuticals on the market. The following is a brief primer on cellular senescence, skin aging, and cosmeceuticals; it represents what we currently know.
Cell phases
Keratinocytes and fibroblasts go through five different phases: stem, proliferation, differentiation, senescence, and apoptosis. The difference between apoptotic cells and senescent cells is that apoptotic cells are not viable and are eliminated, while senescent cells, even though they have gone into cell cycle arrest, remain functional and are not eliminated from the skin.
What are senescent cells?
Senescent cells have lost the ability to proliferate but have not undergone apoptosis. Senescent human skin fibroblasts in cell culture lose the youthful spindlelike shape and become enlarged and flattened.1 Their lysosomes and mitochondria lose functionality.2 The presence of senescent cells is associated with increased aging and seems to speed aging.

Senescent cells and skin aging
Senescent cells are increased in the age-related phenotype3 because of an age-related decline of senescent cell removal systems, such as the immune system4 and the autophagy-lysosomal pathway.5 Senescent cells are deleterious because they develop into a senescence-associated secretory phenotype (SASP), which is believed to be one of the major causes of aging. SASP cells communicate with nearby cells using proinflammatory cytokines, which include catabolic modulators such as Matrix metalloproteinases. They are known to release growth factors, cytokines, chemokines, matrix-modeling enzymes, lipids, and extracellular vesicles. The last are lipid bilayer-lined vesicles that can transport functional RNA and microRNA and facilitate other modes of communication between cells.6
The SASP is likely a natural tumor suppressive mode employed by cells to prevent cells with cancerous mutations from undergoing replication;7 however, when it comes to aging, the deleterious effects of SASP outweigh the beneficial effects. For example, SASP contributes to a prolonged state of inflammation, known as “inflammaging,”8 which is detrimental to the skin’s appearance. Human fibroblasts that have assumed the SASP secrete proinflammatory cytokines and MMPs and release reactive oxygen species,9,10 resulting in degradation of the surrounding extracellular matrix (ECM). Loss of the ECM leads to fibroblast compaction and reduced DNA synthesis, all caused by SASPs.9
What causes cellular senescence?
Activation of the nuclear factor-erythroid 2-related transcription factor 2 (NRF2) induces cellular senescence via direct targeting of certain ECM genes. NRF2 is a key regulator of the skin’s antioxidant defense system, which controls the transcription of genes encoding reactive oxygen species–detoxifying enzymes and various other antioxidant proteins.11 Loss of mitochondrial autophagy also induces senescence, as do activation of the TP53 gene, inactivity of SIRT-1, and short telomeres.
Cellular senescence and skin aging
Timely clearance of senescent cells before they create too much damage postpones the onset and severity of age-related diseases and extends the life span of mice.12,6 Antiaging treatments should focus on decreasing the number of senescent cells and reverting senescent cells to the more juvenile forms: proliferating or differentiating cells as an approach to prevent skin aging.13 Restoration of the lysosomal-mitochondrial axis has been shown to revert SASP back to a juvenile status. Normalization of the lysosomal-mitochondrial axis is a prerequisite to reverse senescence.14
Cellular senescence, autophagy, the lysosomal-mitochondrial axis, and cosmeceuticals
Autophagy is the important process of organelles, like mitochondria,15 self-digesting their cytoplasmic material into lysosomes for degradation. Mitochondrial autophagy is very important in slowing the aging process because damaged mitochondria generate free radicals. As you can imagine, much research is focused on this area, but it is too early for any research to translate to efficacious cosmeceuticals.
Conclusion
To summarize, activation of sirtuin-1 (SIRT-1) has been shown to extend the lifespan of mammals, as does caloric restriction.16 This extension occurs because SIRT-1 decreases senescence and activates autophagy.
Although we do not yet know whether topical skincare products could affect senescence or autophagy, there are data to show that oral resveratrol16 and melatonin17 activate SIRT-1 and increase autophagy. I am closely watching this research and will let you know if there are any similar data on topical cosmeceuticals targeting senescence or autophagy. Stay tuned!
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann has written two textbooks and a New York Times Best Sellers book for consumers. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Revance, Evolus, and Burt’s Bees. She is the CEO of Skin Type Solutions Inc., a company that independently tests skin care products and makes recommendations to physicians on which skin care technologies are best. Write to her at dermnews@mdedge.com.
References
1. Papadopoulou A et al. Biogerontology. 2020 Dec;21(6):695-708.
2. López-Otin C et al. Cell. 2013 June 6;153, 1194–217.
3. Yoon J E et al. Theranostics. 2018 Sep 9;8(17):4620-32.
4. Rodier F, Campisi J. J Cell Biol. 2011 Feb 21;192(4):547-56.
5. Dutta D et al. Circ Res. 2012 Apr 13;110(8):1125-38.
6. Terlecki-Zaniewicz L et al. J Invest Dermatol. 2019 Dec;139(12):2425-36.e5.
7. Campisi J et al. Nat Rev Mol Cell Biol. 2007 Sep;8(9):729-40.
8. Franceschi C and Campisi J. J Gerontol A Biol Sci Med Sci. 2014 Jun;69 Suppl 1:S4-9.
9. Nelson G et al. Aging Cell. 2012 Apr;11(2):345-9.
10. Passos JF et al. PLoS Biol. 2007 May;5(5):e110.
11. Hiebert P et al. Dev Cell. 2018 Jul 16;46(2):145-61.e10.
12. Baker DJ et al. Nature. 2016 Feb 11:530(7589):184-9.
13. Mavrogonatou E et al. Matrix Biol. 2019 Jan;75-76:27-42.
14. Park JT et al. Ageing Res Rev. 2018 Nov;47:176-82.
15. Levine B and Kroemer G. Cell. 2019 Jan 10;176(1-2):11-42.
16. Morselli E et al. Cell Death Dis. 2010;1(1):e10.
17. Lee JH et al. Oncotarget. 2016 Mar 15;7(11):12075-88.
Seaweed and other marine-derived products in skin care, Part II: Cosmetic formulations, fucoidan, and salmon eggs
The use of bioactive ingredients culled from the marine environment has increased significantly in recent years for use in skin care because of the reputed antioxidant and anti-aging activity of these substances.1-3
In the last couple of decades, secondary metabolites with bioactive properties have been identified in seaweeds. Among these substances, phlorotannins have been isolated from brown seaweeds and demonstrated to exhibit anti-allergic, anti-inflammatory, antioxidant, anticancer, and antiwrinkling activity, as well as some capacity to promote hair growth.4 Sanjeewa et al. suggest that phlorotannins, or marine polyphenols, derived from brown seaweed are well suited for use in cosmeceutical formulations and appear to exhibit skin whitening and antiwrinkling properties in particular.4 This column will discuss recent findings regarding the use of marine ingredients in cosmetic formulations, with a particular focus on substances such as fucoidan, as well as emerging evidence regarding the benefits to human skin derived from salmon eggs.
Recent studies of marine products in cosmetic formulations
In 2017, Fabrowska et al. showed in two groups of 10 volunteers each (one ranging from 20 to 30 years old and one from 40 to 50 years old) that the freshwater alga Cladophora glomerate is an effective ingredient for use as a cosmetic agent intended to moisturize and firm the skin.5
The next year, Thu et al. reported on the preparation of a cream mask composed of Vietnamese seaweeds (Caulerpa lentillifera, Sargassum crassifolium, Ulva reticulata, and Kappaphycus alvarezii), which they found to be abundant in proteins, polysaccharides, carotenoids, and other vitamins and to have potent antibacterial, cell proliferation, moisture retention, and tyrosinase inhibitory properties. The authors added that the seaweed cream mask was safe, provoked no irritation, and appeared to be effective in delivering anti-aging and moisturizing benefits.6
In 2019, Jesumani et al., in reviewing the potential cutaneous benefits of bioactive substances in seaweed, noted a significant increase in the use of ingredients found in macroalgae or seaweed in cosmetic formulations, also noting the range of reputed bioactivity (i.e., antioxidant, antitumor, anti-inflammatory, antilipidemic, antimicrobial, and anti-allergic).7 Seaweeds are a significant source of vitamins A, B, C, D, and E, and green, red, and brown algae contain pigments that protect against UV irradiation.7,8
Also that year, Hameury et al. conducted an ex vivo assessment to predict the cutaneous anti-aging benefits of an aqueous gel containing 6.1% marine ingredients (amino acid-enriched giant kelp extract, trace element-enriched seawater, and dedifferentiated sea fennel cells) topically applied on human skin explants. The investigators found that 64 proteins were significantly regulated by the gel when marine ingredients were compared with untreated skin explants, with the ingredients shown to act on the epidermis and dermis. These proteins are involved in multiple functions including gene expression, inflammatory processes, dermal extracellular matrix production, and melanogenesis and keratinocyte proliferation, suggesting, according to the authors, that marine ingredients could play a role in preventing cutaneous aging and contributing to the health of the epidermis and dermis.9
Early in 2020, Poulose et al. reported on the first use of a photoprotective cosmetic cream combining nanomelanin and seaweed that exerts antioxidant, antibacterial, and wound healing activity.10
The skin-lightening potential of fucoidan
In 2017, Wang et al. investigated the antimelanogenic activity of fucoidan – a complex sulfated polysaccharide extracted from brown seaweed known to possess a broad array of biologic functions – on B16 murine melanoma cells. Their in vitro studies revealed that fucoidan suppresses B16 melanoma cell proliferation and cellular tyrosinase activity and has potential as a skin-whitening cosmeceutical agent.11
Two years later, Jesumani et al. investigated the polysaccharides extracted from the seaweed species Sargassum vachellianum, S. horneri, and S. hemiphyllum. Found to be abundant in fucose, all of the evaluated polysaccharides demonstrated dose-dependent antioxidant activity and effectiveness in hindering tyrosinase and elastase. The researchers concluded that all of the tested species display potential as key ingredients in cosmeceutical agents intended to treat wrinkles or lighten skin.12
More recently, a comparative study by the same team revealed that both fucoidan-rich polysaccharide extract and polyphenol-rich extract from the seaweed S. vachellianum delivered significant protective activity. Both protected the skin from UV harm: The fucoidan-rich extract showed superior free radical scavenging and antimicrobial activity, while the polyphenol extract performed better at absorbing UV radiation. The investigators suggested that both extracts could provide a balanced approach to skin protection when featured in skin care products.13
In addition, it is worth noting that a key monomeric component of red macroalgae (Rhodophyta), 3,6-anhydro-l-galactose, has been found in vitro to display skin-whitening activity.14
Salmon eggs
In a 2013 double-blind, randomized clinical trial with 66 patients, Lønne et al. reported that subjects treated topically with salmon egg extract experienced significant amelioration of photoaging, including wrinkles, pigmentation, erythema, and xerosis, yielding global skin appearance improvement.3,15
A pilot study by Mekas et al., which was reported 2 years later and included 75 patients, revealed that skin tone and evenness were improved by a topical exfoliative cream featuring hydrolyzed roe proteins, based on subjective and objective measures comparing 4% glycolic acid.3,16
In 2016, Yoshino et al. showed that human dermal fibroblasts incubated with salmon egg extract upregulated the expression of collagen type I genes and several oxidative genes.3,17 The topical application of hydrolyzed salmon roe proteins to human skin has also been demonstrated to eliminate cell-to-cell adhesions thus ameliorating the appearance of photodamaged skin.1,3,16
More recently, a comprehensive PubMed search on the bioactive ingredients used in Korean cosmeceuticals reported early in 2020 that there is increased interest in salmon eggs because they provide a copious supply of unsaturated fatty acids, proteins, vitamins, and minerals known to nurture cutaneous health.3,15
Conclusion
. Research into the numerous bioactive properties of these multitudinous species has ramped up in recent years and is yielding evidence regarding the efficacy and potential broader uses of such ingredients in cutaneous health care. As we build on our understanding of just how dynamic a source of treatment options may lie under the sea, we become increasingly aware, ironically, of the damage that human industrialization exerts on the planet, as well as these precious marine resources (including the possibly deleterious effects of chemical sunscreens like those that are now banned for sale in Hawai‘i). Humanity will need to become much better stewards of the Earth if we are to enhance our future opportunities and possibly harness the potent marine ingredients still available with the potential to enhance skin health and appearance.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann has written two textbooks and a New York Times Best Sellers book for consumers. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Revance, Evolus, and Burt’s Bees. She is the CEO of Skin Type Solutions Inc., a company that independently tests skin care products and makes recommendations to physicians on which skin care technologies are best. Write to her at dermnews@mdedge.com.
References
1. Kim SK. J Cosmet Dermatol. 2014;13(1):56-67.
2. Venkatesan J et al. Mar Drugs. 2017;15(5):1-18.
3. Nguyen JK et al. J Cosmet Dermatol 2020 Jul;19(7):1555-69.
4. Sanjeewa KKA et al. J Photochem Photobiol B. 2016 Sep;162:100-5.
5. Fabrowska J et al. Acta Pol Pharm. 2017 Mar;74(2):633-41.
6. Thu NTH et al. J Cosmet Sci. Nov/Dec 2018;69(6):447-62.
7. Jesumani V et al. Mar Drugs. 2019 Dec 6;17(12):688.
8. Kim MS et al. Photochem Photobiol. Jul-Aug 2013;89(4):911-8.
9. Hameury S et al. J Cosmet Dermatol. 2019 Feb;18(1):355-70.
10. Poulose N et al. J Photochem Photobiol B. 2020 Apr;205:111816.
11. Wang ZJ et al. Afr J Tradit Complement Altern Med. 2017 Jun 5;14(4);149-55.
12. Jesumani V et al. Int J Biol Macromol. 2019 Nov 1;140:216-24.
13. Jesumani V et al. PLoS One. 2020 Jan 7;15(1):e0227308.
14. Kim JH et al. Mar Drugs. 2017 Oct 20;15(10):321.
15. Lønne GK et al. Int J Cosmet Sci. 2013 Oct;35(5):515-22.
16. Mekas M et al. J Drugs Dermatol. 2015 Nov;14(11):1306-19.
17. Yoshino A et al. Clin Interv Aging. 2016;11:1159-68.
The use of bioactive ingredients culled from the marine environment has increased significantly in recent years for use in skin care because of the reputed antioxidant and anti-aging activity of these substances.1-3
In the last couple of decades, secondary metabolites with bioactive properties have been identified in seaweeds. Among these substances, phlorotannins have been isolated from brown seaweeds and demonstrated to exhibit anti-allergic, anti-inflammatory, antioxidant, anticancer, and antiwrinkling activity, as well as some capacity to promote hair growth.4 Sanjeewa et al. suggest that phlorotannins, or marine polyphenols, derived from brown seaweed are well suited for use in cosmeceutical formulations and appear to exhibit skin whitening and antiwrinkling properties in particular.4 This column will discuss recent findings regarding the use of marine ingredients in cosmetic formulations, with a particular focus on substances such as fucoidan, as well as emerging evidence regarding the benefits to human skin derived from salmon eggs.
Recent studies of marine products in cosmetic formulations
In 2017, Fabrowska et al. showed in two groups of 10 volunteers each (one ranging from 20 to 30 years old and one from 40 to 50 years old) that the freshwater alga Cladophora glomerate is an effective ingredient for use as a cosmetic agent intended to moisturize and firm the skin.5
The next year, Thu et al. reported on the preparation of a cream mask composed of Vietnamese seaweeds (Caulerpa lentillifera, Sargassum crassifolium, Ulva reticulata, and Kappaphycus alvarezii), which they found to be abundant in proteins, polysaccharides, carotenoids, and other vitamins and to have potent antibacterial, cell proliferation, moisture retention, and tyrosinase inhibitory properties. The authors added that the seaweed cream mask was safe, provoked no irritation, and appeared to be effective in delivering anti-aging and moisturizing benefits.6
In 2019, Jesumani et al., in reviewing the potential cutaneous benefits of bioactive substances in seaweed, noted a significant increase in the use of ingredients found in macroalgae or seaweed in cosmetic formulations, also noting the range of reputed bioactivity (i.e., antioxidant, antitumor, anti-inflammatory, antilipidemic, antimicrobial, and anti-allergic).7 Seaweeds are a significant source of vitamins A, B, C, D, and E, and green, red, and brown algae contain pigments that protect against UV irradiation.7,8
Also that year, Hameury et al. conducted an ex vivo assessment to predict the cutaneous anti-aging benefits of an aqueous gel containing 6.1% marine ingredients (amino acid-enriched giant kelp extract, trace element-enriched seawater, and dedifferentiated sea fennel cells) topically applied on human skin explants. The investigators found that 64 proteins were significantly regulated by the gel when marine ingredients were compared with untreated skin explants, with the ingredients shown to act on the epidermis and dermis. These proteins are involved in multiple functions including gene expression, inflammatory processes, dermal extracellular matrix production, and melanogenesis and keratinocyte proliferation, suggesting, according to the authors, that marine ingredients could play a role in preventing cutaneous aging and contributing to the health of the epidermis and dermis.9
Early in 2020, Poulose et al. reported on the first use of a photoprotective cosmetic cream combining nanomelanin and seaweed that exerts antioxidant, antibacterial, and wound healing activity.10
The skin-lightening potential of fucoidan
In 2017, Wang et al. investigated the antimelanogenic activity of fucoidan – a complex sulfated polysaccharide extracted from brown seaweed known to possess a broad array of biologic functions – on B16 murine melanoma cells. Their in vitro studies revealed that fucoidan suppresses B16 melanoma cell proliferation and cellular tyrosinase activity and has potential as a skin-whitening cosmeceutical agent.11
Two years later, Jesumani et al. investigated the polysaccharides extracted from the seaweed species Sargassum vachellianum, S. horneri, and S. hemiphyllum. Found to be abundant in fucose, all of the evaluated polysaccharides demonstrated dose-dependent antioxidant activity and effectiveness in hindering tyrosinase and elastase. The researchers concluded that all of the tested species display potential as key ingredients in cosmeceutical agents intended to treat wrinkles or lighten skin.12
More recently, a comparative study by the same team revealed that both fucoidan-rich polysaccharide extract and polyphenol-rich extract from the seaweed S. vachellianum delivered significant protective activity. Both protected the skin from UV harm: The fucoidan-rich extract showed superior free radical scavenging and antimicrobial activity, while the polyphenol extract performed better at absorbing UV radiation. The investigators suggested that both extracts could provide a balanced approach to skin protection when featured in skin care products.13
In addition, it is worth noting that a key monomeric component of red macroalgae (Rhodophyta), 3,6-anhydro-l-galactose, has been found in vitro to display skin-whitening activity.14
Salmon eggs
In a 2013 double-blind, randomized clinical trial with 66 patients, Lønne et al. reported that subjects treated topically with salmon egg extract experienced significant amelioration of photoaging, including wrinkles, pigmentation, erythema, and xerosis, yielding global skin appearance improvement.3,15
A pilot study by Mekas et al., which was reported 2 years later and included 75 patients, revealed that skin tone and evenness were improved by a topical exfoliative cream featuring hydrolyzed roe proteins, based on subjective and objective measures comparing 4% glycolic acid.3,16
In 2016, Yoshino et al. showed that human dermal fibroblasts incubated with salmon egg extract upregulated the expression of collagen type I genes and several oxidative genes.3,17 The topical application of hydrolyzed salmon roe proteins to human skin has also been demonstrated to eliminate cell-to-cell adhesions thus ameliorating the appearance of photodamaged skin.1,3,16
More recently, a comprehensive PubMed search on the bioactive ingredients used in Korean cosmeceuticals reported early in 2020 that there is increased interest in salmon eggs because they provide a copious supply of unsaturated fatty acids, proteins, vitamins, and minerals known to nurture cutaneous health.3,15
Conclusion
. Research into the numerous bioactive properties of these multitudinous species has ramped up in recent years and is yielding evidence regarding the efficacy and potential broader uses of such ingredients in cutaneous health care. As we build on our understanding of just how dynamic a source of treatment options may lie under the sea, we become increasingly aware, ironically, of the damage that human industrialization exerts on the planet, as well as these precious marine resources (including the possibly deleterious effects of chemical sunscreens like those that are now banned for sale in Hawai‘i). Humanity will need to become much better stewards of the Earth if we are to enhance our future opportunities and possibly harness the potent marine ingredients still available with the potential to enhance skin health and appearance.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann has written two textbooks and a New York Times Best Sellers book for consumers. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Revance, Evolus, and Burt’s Bees. She is the CEO of Skin Type Solutions Inc., a company that independently tests skin care products and makes recommendations to physicians on which skin care technologies are best. Write to her at dermnews@mdedge.com.
References
1. Kim SK. J Cosmet Dermatol. 2014;13(1):56-67.
2. Venkatesan J et al. Mar Drugs. 2017;15(5):1-18.
3. Nguyen JK et al. J Cosmet Dermatol 2020 Jul;19(7):1555-69.
4. Sanjeewa KKA et al. J Photochem Photobiol B. 2016 Sep;162:100-5.
5. Fabrowska J et al. Acta Pol Pharm. 2017 Mar;74(2):633-41.
6. Thu NTH et al. J Cosmet Sci. Nov/Dec 2018;69(6):447-62.
7. Jesumani V et al. Mar Drugs. 2019 Dec 6;17(12):688.
8. Kim MS et al. Photochem Photobiol. Jul-Aug 2013;89(4):911-8.
9. Hameury S et al. J Cosmet Dermatol. 2019 Feb;18(1):355-70.
10. Poulose N et al. J Photochem Photobiol B. 2020 Apr;205:111816.
11. Wang ZJ et al. Afr J Tradit Complement Altern Med. 2017 Jun 5;14(4);149-55.
12. Jesumani V et al. Int J Biol Macromol. 2019 Nov 1;140:216-24.
13. Jesumani V et al. PLoS One. 2020 Jan 7;15(1):e0227308.
14. Kim JH et al. Mar Drugs. 2017 Oct 20;15(10):321.
15. Lønne GK et al. Int J Cosmet Sci. 2013 Oct;35(5):515-22.
16. Mekas M et al. J Drugs Dermatol. 2015 Nov;14(11):1306-19.
17. Yoshino A et al. Clin Interv Aging. 2016;11:1159-68.
The use of bioactive ingredients culled from the marine environment has increased significantly in recent years for use in skin care because of the reputed antioxidant and anti-aging activity of these substances.1-3
In the last couple of decades, secondary metabolites with bioactive properties have been identified in seaweeds. Among these substances, phlorotannins have been isolated from brown seaweeds and demonstrated to exhibit anti-allergic, anti-inflammatory, antioxidant, anticancer, and antiwrinkling activity, as well as some capacity to promote hair growth.4 Sanjeewa et al. suggest that phlorotannins, or marine polyphenols, derived from brown seaweed are well suited for use in cosmeceutical formulations and appear to exhibit skin whitening and antiwrinkling properties in particular.4 This column will discuss recent findings regarding the use of marine ingredients in cosmetic formulations, with a particular focus on substances such as fucoidan, as well as emerging evidence regarding the benefits to human skin derived from salmon eggs.
Recent studies of marine products in cosmetic formulations
In 2017, Fabrowska et al. showed in two groups of 10 volunteers each (one ranging from 20 to 30 years old and one from 40 to 50 years old) that the freshwater alga Cladophora glomerate is an effective ingredient for use as a cosmetic agent intended to moisturize and firm the skin.5
The next year, Thu et al. reported on the preparation of a cream mask composed of Vietnamese seaweeds (Caulerpa lentillifera, Sargassum crassifolium, Ulva reticulata, and Kappaphycus alvarezii), which they found to be abundant in proteins, polysaccharides, carotenoids, and other vitamins and to have potent antibacterial, cell proliferation, moisture retention, and tyrosinase inhibitory properties. The authors added that the seaweed cream mask was safe, provoked no irritation, and appeared to be effective in delivering anti-aging and moisturizing benefits.6
In 2019, Jesumani et al., in reviewing the potential cutaneous benefits of bioactive substances in seaweed, noted a significant increase in the use of ingredients found in macroalgae or seaweed in cosmetic formulations, also noting the range of reputed bioactivity (i.e., antioxidant, antitumor, anti-inflammatory, antilipidemic, antimicrobial, and anti-allergic).7 Seaweeds are a significant source of vitamins A, B, C, D, and E, and green, red, and brown algae contain pigments that protect against UV irradiation.7,8
Also that year, Hameury et al. conducted an ex vivo assessment to predict the cutaneous anti-aging benefits of an aqueous gel containing 6.1% marine ingredients (amino acid-enriched giant kelp extract, trace element-enriched seawater, and dedifferentiated sea fennel cells) topically applied on human skin explants. The investigators found that 64 proteins were significantly regulated by the gel when marine ingredients were compared with untreated skin explants, with the ingredients shown to act on the epidermis and dermis. These proteins are involved in multiple functions including gene expression, inflammatory processes, dermal extracellular matrix production, and melanogenesis and keratinocyte proliferation, suggesting, according to the authors, that marine ingredients could play a role in preventing cutaneous aging and contributing to the health of the epidermis and dermis.9
Early in 2020, Poulose et al. reported on the first use of a photoprotective cosmetic cream combining nanomelanin and seaweed that exerts antioxidant, antibacterial, and wound healing activity.10
The skin-lightening potential of fucoidan
In 2017, Wang et al. investigated the antimelanogenic activity of fucoidan – a complex sulfated polysaccharide extracted from brown seaweed known to possess a broad array of biologic functions – on B16 murine melanoma cells. Their in vitro studies revealed that fucoidan suppresses B16 melanoma cell proliferation and cellular tyrosinase activity and has potential as a skin-whitening cosmeceutical agent.11
Two years later, Jesumani et al. investigated the polysaccharides extracted from the seaweed species Sargassum vachellianum, S. horneri, and S. hemiphyllum. Found to be abundant in fucose, all of the evaluated polysaccharides demonstrated dose-dependent antioxidant activity and effectiveness in hindering tyrosinase and elastase. The researchers concluded that all of the tested species display potential as key ingredients in cosmeceutical agents intended to treat wrinkles or lighten skin.12
More recently, a comparative study by the same team revealed that both fucoidan-rich polysaccharide extract and polyphenol-rich extract from the seaweed S. vachellianum delivered significant protective activity. Both protected the skin from UV harm: The fucoidan-rich extract showed superior free radical scavenging and antimicrobial activity, while the polyphenol extract performed better at absorbing UV radiation. The investigators suggested that both extracts could provide a balanced approach to skin protection when featured in skin care products.13
In addition, it is worth noting that a key monomeric component of red macroalgae (Rhodophyta), 3,6-anhydro-l-galactose, has been found in vitro to display skin-whitening activity.14
Salmon eggs
In a 2013 double-blind, randomized clinical trial with 66 patients, Lønne et al. reported that subjects treated topically with salmon egg extract experienced significant amelioration of photoaging, including wrinkles, pigmentation, erythema, and xerosis, yielding global skin appearance improvement.3,15
A pilot study by Mekas et al., which was reported 2 years later and included 75 patients, revealed that skin tone and evenness were improved by a topical exfoliative cream featuring hydrolyzed roe proteins, based on subjective and objective measures comparing 4% glycolic acid.3,16
In 2016, Yoshino et al. showed that human dermal fibroblasts incubated with salmon egg extract upregulated the expression of collagen type I genes and several oxidative genes.3,17 The topical application of hydrolyzed salmon roe proteins to human skin has also been demonstrated to eliminate cell-to-cell adhesions thus ameliorating the appearance of photodamaged skin.1,3,16
More recently, a comprehensive PubMed search on the bioactive ingredients used in Korean cosmeceuticals reported early in 2020 that there is increased interest in salmon eggs because they provide a copious supply of unsaturated fatty acids, proteins, vitamins, and minerals known to nurture cutaneous health.3,15
Conclusion
. Research into the numerous bioactive properties of these multitudinous species has ramped up in recent years and is yielding evidence regarding the efficacy and potential broader uses of such ingredients in cutaneous health care. As we build on our understanding of just how dynamic a source of treatment options may lie under the sea, we become increasingly aware, ironically, of the damage that human industrialization exerts on the planet, as well as these precious marine resources (including the possibly deleterious effects of chemical sunscreens like those that are now banned for sale in Hawai‘i). Humanity will need to become much better stewards of the Earth if we are to enhance our future opportunities and possibly harness the potent marine ingredients still available with the potential to enhance skin health and appearance.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann has written two textbooks and a New York Times Best Sellers book for consumers. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Revance, Evolus, and Burt’s Bees. She is the CEO of Skin Type Solutions Inc., a company that independently tests skin care products and makes recommendations to physicians on which skin care technologies are best. Write to her at dermnews@mdedge.com.
References
1. Kim SK. J Cosmet Dermatol. 2014;13(1):56-67.
2. Venkatesan J et al. Mar Drugs. 2017;15(5):1-18.
3. Nguyen JK et al. J Cosmet Dermatol 2020 Jul;19(7):1555-69.
4. Sanjeewa KKA et al. J Photochem Photobiol B. 2016 Sep;162:100-5.
5. Fabrowska J et al. Acta Pol Pharm. 2017 Mar;74(2):633-41.
6. Thu NTH et al. J Cosmet Sci. Nov/Dec 2018;69(6):447-62.
7. Jesumani V et al. Mar Drugs. 2019 Dec 6;17(12):688.
8. Kim MS et al. Photochem Photobiol. Jul-Aug 2013;89(4):911-8.
9. Hameury S et al. J Cosmet Dermatol. 2019 Feb;18(1):355-70.
10. Poulose N et al. J Photochem Photobiol B. 2020 Apr;205:111816.
11. Wang ZJ et al. Afr J Tradit Complement Altern Med. 2017 Jun 5;14(4);149-55.
12. Jesumani V et al. Int J Biol Macromol. 2019 Nov 1;140:216-24.
13. Jesumani V et al. PLoS One. 2020 Jan 7;15(1):e0227308.
14. Kim JH et al. Mar Drugs. 2017 Oct 20;15(10):321.
15. Lønne GK et al. Int J Cosmet Sci. 2013 Oct;35(5):515-22.
16. Mekas M et al. J Drugs Dermatol. 2015 Nov;14(11):1306-19.
17. Yoshino A et al. Clin Interv Aging. 2016;11:1159-68.
Seaweed and other marine-derived products in skin care, part 1: Current indications
Marine algae are relatively common raw sources for cosmeceutical products.1 The photoprotective compounds identified among marine algae range from mycosporinelike amino acids, sulfated polysaccharides, and carotenoids to polyphenols, all of which are noted for absorbing UV and conferring antioxidant, matrix metalloproteinase–suppressing, anti-aging, and immunomodulatory effects.2 Such biologic activities understandably account for the interest in harnessing their potential in the skin care realm. Indeed, marine ingredients have been steadily flowing into the market for skin care, and research has proliferated – so much so, in fact, that I’ll take two columns to cover some of the most recent research on various marine species and some of the indications or potential uses for these products in skin care.

Key activities and potential uses
Kim and associates note that carbohydrates are the primary components of marine algae, with copious amounts delivering a moisturizing and thickening effect when incorporated into cosmetic products. They add that marine carbohydrates are also known to impart antioxidant, antimelanogenic, and anti-aging activities.3
In 2017, Colantonio and Rivers reviewed the evidence supporting the use of seaweed, among other plants, for dermatologic purposes. The researchers considered four plants and algae (seaweed, witch hazel, bearberry, and mayapple) used in traditional First Nations approaches to skin disease. They found that seaweed shows promise for clinical use in treating acne and wrinkles and could deliver healthy benefits when included in biofunctional textiles.4
Atopic dermatitis
Found in the seaweed Fucus vesiculosus, fucoidan is known to impart anti-inflammatory, antioxidant, and antitumor activity.5 In a 2019 BALB/c mouse study, Tian and associates showed that fucoidan, which is rich in polysaccharides, significantly improved ear swelling and skin lesions and reduced inflammatory cell infiltration. Given the resolution of the 2,4-dinitrofluorobenzene–induced atopic dermatitis symptoms, the investigators suggested that fucoidan may have potential as an anti-AD agent.5
Also that year, Gil and associates studied the effects of Seaweed fulvescens, a chlorophyll-rich green alga (also called Maesaengi) known to have antioxidant properties, in a mouse model of Dermatophagoides farinae body-induced AD and in tumor necrosis factor–alpha and interferon-gamma–stimulated HaCaT keratinocytes. They observed that 200-mg/mouse treatment hindered AD symptom development, compared with controls, with enhanced dorsal skin lesions, diminished thickness and infiltration of inflammation, and decreased proinflammatory cytokines. In addition, the investigators reported the dose-dependent inhibition of proinflammatory cytokine synthesis in HaCaT keratinocytes. They concluded that Seaweed fulvescens shows promise as a therapeutic option for AD treatment.6
Alopecia
In 2017, Kang and associates studied the impact and mechanism of Undariopsis peterseniana, an edible brown alga, and determined that the extract promotes hair growth by activating the Wnt/beta-catenin and ERK pathways. Specifically, they found that U. peterseniana significantly enhanced hair-fiber length ex vivo and in vivo. They also concluded that the brown alga has potential to treat alopecia as it accelerated anagen initiation.7
Skin protection potential of Ishige okamurae
In 2015, Piao and associates demonstrated that diphlorethohydroxycarmalol (DPHC), a phlorotannin isolated from Ishige okamurae, protected human keratinocytes from UVB-induced matrix metalloproteinase (MMP) expression by inactivating ERK and JNK. MMPs are known to contribute to photoaging and tumor promotion.8
Early in 2020, Wang and associates demonstrated that DPHC, isolated from the marine brown alga I. okamurae, exerted protective effects against UVB-induced photodamage in vitro in human dermal fibroblasts and in vivo in zebrafish by suppressing collagenase and elastase production and the expression of matrix metalloproteinases. In vivo, the brown alga extract lowered cell death by decreasing lipid peroxidation and inflammatory response. The investigators concluded that DPHC warrants consideration as an ingredient in cosmeceutical formulations intended to protect against the effects of UVB radiation.9
The same team also reported on their study of the protective effects of DPHC against skin damage in human dermal fibroblasts caused by particulate matter. They found that DPHC dose-dependently exerted significant decreases in intracellular synthesis of reactive oxygen species. The seaweed product also stimulated collagen production and suppressed collagenase activity, as well as matrix metalloproteinases. The researchers concluded that DPHC may be an effective skin-protective ingredient against particulate matter for use in cosmeceutical products.10
Skin protection mouse studies using various marine species
The last 3 years alone have featured several studies in mice that may have significant implications in accelerating our understanding of how to harness the bioactive properties of multiple marine species.
In 2018, Wiraguna and associates studied the protective effects of 0.2% and 0.4% Caulerpa sp. (a genus of seaweed native to the Indo-Pacific region) extract gels on photoaging in the UVB-irradiated skin of Wistar mice, finding that topical applications of both concentrations of the seaweed extract protected mouse skin from UVB-induced photoaging, with treated mice revealed to have higher collagen expression and preserved collagen structure and decreased MMP-1 levels, compared with vehicle controls.11
The next year, Prasedya and associates showed that the brown macroalgae Sargassum cristafolium exerted photoprotective activity against UVA in mice. Mice pretreated with the seaweed before exposure displayed intact collagen formation and no increases in epidermal thickness, compared with controls.12
At the same time, Santos and associates demonstrated that mice fed a diet supplemented with the red seaweed Porphyra umbilicalis experienced significant decreases in the incidence of human papillomavirus type 16–induced premalignant dysplastic skin lesions.13
Also that year, Zhen and associates evaluated the protective effects of eckol, a phlorotannin isolated from brown seaweed, on human HaCaT keratinocytes against PM2.5-induced cell damage. They showed that eckol (30 mcm) reduced reactive oxygen species production and protected cells from apoptosis by hampering the MAPK signaling pathway.14Earlier that year, Kim and associates studied the viability of the microalga Nannochloropsis oceanica, considered most often as a possible biofuel, for potential photoprotective activity against UVB-irradiated human dermal fibroblasts. They determined that pigment extracts (violaxanthin was identified as the main pigment) were not cytotoxic to the fibroblasts and that treatment with the pigment extract upregulated collagen expression and significantly inhibited UVB-induced damage. Further study revealed that violaxanthin significantly mitigated UVB-induced G1 phase arrest, senescence-associated beta-galactosidase activation, and p16 and p21 up-regulation, among other functions, suggesting its consideration, according to the authors, as a possible antiphotoaging agent.15
Finally, early in 2020, Bellan and associates evaluated the antitumor characteristics of the sulfated heterorhamnan derived from the green seaweed Gayralia brasiliensis as seen on the biological activities in the B16-F10 murine melanoma cell line. The polysaccharidic fraction was found to be effective in reducing melanoma cell migration and invasion capacity.16
Conclusion
. Evidence suggests widespread potential across several species for dermatologic purposes. Indeed, data indicate that some species appear to be suited for treating AD, alopecia, and wrinkles and may possibly render effective photoprotection. More research is necessary, of course, to ascertain the extent to which such ingredients can adequately address cutaneous health and how truly effective the marine ingredients are in currently marketed products.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann has written two textbooks and a New York Times Best Sellers book for consumers. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Revance, Evolus, and Burt’s Bees. She is the CEO of Skin Type Solutions, a company that independently tests skin care products and makes recommendations to physicians on which skin care technologies are best. Write to her at dermnews@mdedge.com.
References
1. Fabrowska J et al. Acta Pol Pharm. 2017 Mar;74(2):633-41.
2. Pangestuti R et al. Mar Drugs. 2018 Oct 23;16(11):399.
3. Kim JH et al. Mar Drugs. 2018 Nov 21;16(11):459.
4. Colantonio S & Rivers JK. J Cutan Med Surg. Jul/Aug 2017;21(4):299-307.
5. Tian T et al. Int Immunopharmacol. 2019 Oct;75:105823.
6. Gil TY et al. Mediators Inflamm. 2019 Mar 17;2019:3760934.
7. Kang JI et al. Mar Drugs. 2017 May 5;15(5):130.
8. Piao MJ et al. Biomol Ther (Seoul). 2015 Nov;23(6):557-63.
9. Wang L et al. Food Chem Toxicol. 2020 Feb;136:110963.
10. Wang L et al. Molecules. 2020 Feb 26;25(5):1055.
11. Wiraguna AAGP et al. Dermatol Reports. 2018 Oct 1;10(2):7597.
12. Prasedya ES et al. Biomedicines. 2019 Sep 27;7(4):77.
13. Santos S et al. Mar Drugs. 2019 Oct 29;17(11):615.
14. Zhen AX et al. Mar Drugs. 2019 Jul 27;17(8):444.
15. Kim HM et al. Photochem Photobiol. 2019 Mar;95(2):595-604.
16. Bellan DL et al. Mar Biotechnol. 2020 Apr;22(2):194-206.
Marine algae are relatively common raw sources for cosmeceutical products.1 The photoprotective compounds identified among marine algae range from mycosporinelike amino acids, sulfated polysaccharides, and carotenoids to polyphenols, all of which are noted for absorbing UV and conferring antioxidant, matrix metalloproteinase–suppressing, anti-aging, and immunomodulatory effects.2 Such biologic activities understandably account for the interest in harnessing their potential in the skin care realm. Indeed, marine ingredients have been steadily flowing into the market for skin care, and research has proliferated – so much so, in fact, that I’ll take two columns to cover some of the most recent research on various marine species and some of the indications or potential uses for these products in skin care.

Key activities and potential uses
Kim and associates note that carbohydrates are the primary components of marine algae, with copious amounts delivering a moisturizing and thickening effect when incorporated into cosmetic products. They add that marine carbohydrates are also known to impart antioxidant, antimelanogenic, and anti-aging activities.3
In 2017, Colantonio and Rivers reviewed the evidence supporting the use of seaweed, among other plants, for dermatologic purposes. The researchers considered four plants and algae (seaweed, witch hazel, bearberry, and mayapple) used in traditional First Nations approaches to skin disease. They found that seaweed shows promise for clinical use in treating acne and wrinkles and could deliver healthy benefits when included in biofunctional textiles.4
Atopic dermatitis
Found in the seaweed Fucus vesiculosus, fucoidan is known to impart anti-inflammatory, antioxidant, and antitumor activity.5 In a 2019 BALB/c mouse study, Tian and associates showed that fucoidan, which is rich in polysaccharides, significantly improved ear swelling and skin lesions and reduced inflammatory cell infiltration. Given the resolution of the 2,4-dinitrofluorobenzene–induced atopic dermatitis symptoms, the investigators suggested that fucoidan may have potential as an anti-AD agent.5
Also that year, Gil and associates studied the effects of Seaweed fulvescens, a chlorophyll-rich green alga (also called Maesaengi) known to have antioxidant properties, in a mouse model of Dermatophagoides farinae body-induced AD and in tumor necrosis factor–alpha and interferon-gamma–stimulated HaCaT keratinocytes. They observed that 200-mg/mouse treatment hindered AD symptom development, compared with controls, with enhanced dorsal skin lesions, diminished thickness and infiltration of inflammation, and decreased proinflammatory cytokines. In addition, the investigators reported the dose-dependent inhibition of proinflammatory cytokine synthesis in HaCaT keratinocytes. They concluded that Seaweed fulvescens shows promise as a therapeutic option for AD treatment.6
Alopecia
In 2017, Kang and associates studied the impact and mechanism of Undariopsis peterseniana, an edible brown alga, and determined that the extract promotes hair growth by activating the Wnt/beta-catenin and ERK pathways. Specifically, they found that U. peterseniana significantly enhanced hair-fiber length ex vivo and in vivo. They also concluded that the brown alga has potential to treat alopecia as it accelerated anagen initiation.7
Skin protection potential of Ishige okamurae
In 2015, Piao and associates demonstrated that diphlorethohydroxycarmalol (DPHC), a phlorotannin isolated from Ishige okamurae, protected human keratinocytes from UVB-induced matrix metalloproteinase (MMP) expression by inactivating ERK and JNK. MMPs are known to contribute to photoaging and tumor promotion.8
Early in 2020, Wang and associates demonstrated that DPHC, isolated from the marine brown alga I. okamurae, exerted protective effects against UVB-induced photodamage in vitro in human dermal fibroblasts and in vivo in zebrafish by suppressing collagenase and elastase production and the expression of matrix metalloproteinases. In vivo, the brown alga extract lowered cell death by decreasing lipid peroxidation and inflammatory response. The investigators concluded that DPHC warrants consideration as an ingredient in cosmeceutical formulations intended to protect against the effects of UVB radiation.9
The same team also reported on their study of the protective effects of DPHC against skin damage in human dermal fibroblasts caused by particulate matter. They found that DPHC dose-dependently exerted significant decreases in intracellular synthesis of reactive oxygen species. The seaweed product also stimulated collagen production and suppressed collagenase activity, as well as matrix metalloproteinases. The researchers concluded that DPHC may be an effective skin-protective ingredient against particulate matter for use in cosmeceutical products.10
Skin protection mouse studies using various marine species
The last 3 years alone have featured several studies in mice that may have significant implications in accelerating our understanding of how to harness the bioactive properties of multiple marine species.
In 2018, Wiraguna and associates studied the protective effects of 0.2% and 0.4% Caulerpa sp. (a genus of seaweed native to the Indo-Pacific region) extract gels on photoaging in the UVB-irradiated skin of Wistar mice, finding that topical applications of both concentrations of the seaweed extract protected mouse skin from UVB-induced photoaging, with treated mice revealed to have higher collagen expression and preserved collagen structure and decreased MMP-1 levels, compared with vehicle controls.11
The next year, Prasedya and associates showed that the brown macroalgae Sargassum cristafolium exerted photoprotective activity against UVA in mice. Mice pretreated with the seaweed before exposure displayed intact collagen formation and no increases in epidermal thickness, compared with controls.12
At the same time, Santos and associates demonstrated that mice fed a diet supplemented with the red seaweed Porphyra umbilicalis experienced significant decreases in the incidence of human papillomavirus type 16–induced premalignant dysplastic skin lesions.13
Also that year, Zhen and associates evaluated the protective effects of eckol, a phlorotannin isolated from brown seaweed, on human HaCaT keratinocytes against PM2.5-induced cell damage. They showed that eckol (30 mcm) reduced reactive oxygen species production and protected cells from apoptosis by hampering the MAPK signaling pathway.14Earlier that year, Kim and associates studied the viability of the microalga Nannochloropsis oceanica, considered most often as a possible biofuel, for potential photoprotective activity against UVB-irradiated human dermal fibroblasts. They determined that pigment extracts (violaxanthin was identified as the main pigment) were not cytotoxic to the fibroblasts and that treatment with the pigment extract upregulated collagen expression and significantly inhibited UVB-induced damage. Further study revealed that violaxanthin significantly mitigated UVB-induced G1 phase arrest, senescence-associated beta-galactosidase activation, and p16 and p21 up-regulation, among other functions, suggesting its consideration, according to the authors, as a possible antiphotoaging agent.15
Finally, early in 2020, Bellan and associates evaluated the antitumor characteristics of the sulfated heterorhamnan derived from the green seaweed Gayralia brasiliensis as seen on the biological activities in the B16-F10 murine melanoma cell line. The polysaccharidic fraction was found to be effective in reducing melanoma cell migration and invasion capacity.16
Conclusion
. Evidence suggests widespread potential across several species for dermatologic purposes. Indeed, data indicate that some species appear to be suited for treating AD, alopecia, and wrinkles and may possibly render effective photoprotection. More research is necessary, of course, to ascertain the extent to which such ingredients can adequately address cutaneous health and how truly effective the marine ingredients are in currently marketed products.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann has written two textbooks and a New York Times Best Sellers book for consumers. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Revance, Evolus, and Burt’s Bees. She is the CEO of Skin Type Solutions, a company that independently tests skin care products and makes recommendations to physicians on which skin care technologies are best. Write to her at dermnews@mdedge.com.
References
1. Fabrowska J et al. Acta Pol Pharm. 2017 Mar;74(2):633-41.
2. Pangestuti R et al. Mar Drugs. 2018 Oct 23;16(11):399.
3. Kim JH et al. Mar Drugs. 2018 Nov 21;16(11):459.
4. Colantonio S & Rivers JK. J Cutan Med Surg. Jul/Aug 2017;21(4):299-307.
5. Tian T et al. Int Immunopharmacol. 2019 Oct;75:105823.
6. Gil TY et al. Mediators Inflamm. 2019 Mar 17;2019:3760934.
7. Kang JI et al. Mar Drugs. 2017 May 5;15(5):130.
8. Piao MJ et al. Biomol Ther (Seoul). 2015 Nov;23(6):557-63.
9. Wang L et al. Food Chem Toxicol. 2020 Feb;136:110963.
10. Wang L et al. Molecules. 2020 Feb 26;25(5):1055.
11. Wiraguna AAGP et al. Dermatol Reports. 2018 Oct 1;10(2):7597.
12. Prasedya ES et al. Biomedicines. 2019 Sep 27;7(4):77.
13. Santos S et al. Mar Drugs. 2019 Oct 29;17(11):615.
14. Zhen AX et al. Mar Drugs. 2019 Jul 27;17(8):444.
15. Kim HM et al. Photochem Photobiol. 2019 Mar;95(2):595-604.
16. Bellan DL et al. Mar Biotechnol. 2020 Apr;22(2):194-206.
Marine algae are relatively common raw sources for cosmeceutical products.1 The photoprotective compounds identified among marine algae range from mycosporinelike amino acids, sulfated polysaccharides, and carotenoids to polyphenols, all of which are noted for absorbing UV and conferring antioxidant, matrix metalloproteinase–suppressing, anti-aging, and immunomodulatory effects.2 Such biologic activities understandably account for the interest in harnessing their potential in the skin care realm. Indeed, marine ingredients have been steadily flowing into the market for skin care, and research has proliferated – so much so, in fact, that I’ll take two columns to cover some of the most recent research on various marine species and some of the indications or potential uses for these products in skin care.

Key activities and potential uses
Kim and associates note that carbohydrates are the primary components of marine algae, with copious amounts delivering a moisturizing and thickening effect when incorporated into cosmetic products. They add that marine carbohydrates are also known to impart antioxidant, antimelanogenic, and anti-aging activities.3
In 2017, Colantonio and Rivers reviewed the evidence supporting the use of seaweed, among other plants, for dermatologic purposes. The researchers considered four plants and algae (seaweed, witch hazel, bearberry, and mayapple) used in traditional First Nations approaches to skin disease. They found that seaweed shows promise for clinical use in treating acne and wrinkles and could deliver healthy benefits when included in biofunctional textiles.4
Atopic dermatitis
Found in the seaweed Fucus vesiculosus, fucoidan is known to impart anti-inflammatory, antioxidant, and antitumor activity.5 In a 2019 BALB/c mouse study, Tian and associates showed that fucoidan, which is rich in polysaccharides, significantly improved ear swelling and skin lesions and reduced inflammatory cell infiltration. Given the resolution of the 2,4-dinitrofluorobenzene–induced atopic dermatitis symptoms, the investigators suggested that fucoidan may have potential as an anti-AD agent.5
Also that year, Gil and associates studied the effects of Seaweed fulvescens, a chlorophyll-rich green alga (also called Maesaengi) known to have antioxidant properties, in a mouse model of Dermatophagoides farinae body-induced AD and in tumor necrosis factor–alpha and interferon-gamma–stimulated HaCaT keratinocytes. They observed that 200-mg/mouse treatment hindered AD symptom development, compared with controls, with enhanced dorsal skin lesions, diminished thickness and infiltration of inflammation, and decreased proinflammatory cytokines. In addition, the investigators reported the dose-dependent inhibition of proinflammatory cytokine synthesis in HaCaT keratinocytes. They concluded that Seaweed fulvescens shows promise as a therapeutic option for AD treatment.6
Alopecia
In 2017, Kang and associates studied the impact and mechanism of Undariopsis peterseniana, an edible brown alga, and determined that the extract promotes hair growth by activating the Wnt/beta-catenin and ERK pathways. Specifically, they found that U. peterseniana significantly enhanced hair-fiber length ex vivo and in vivo. They also concluded that the brown alga has potential to treat alopecia as it accelerated anagen initiation.7
Skin protection potential of Ishige okamurae
In 2015, Piao and associates demonstrated that diphlorethohydroxycarmalol (DPHC), a phlorotannin isolated from Ishige okamurae, protected human keratinocytes from UVB-induced matrix metalloproteinase (MMP) expression by inactivating ERK and JNK. MMPs are known to contribute to photoaging and tumor promotion.8
Early in 2020, Wang and associates demonstrated that DPHC, isolated from the marine brown alga I. okamurae, exerted protective effects against UVB-induced photodamage in vitro in human dermal fibroblasts and in vivo in zebrafish by suppressing collagenase and elastase production and the expression of matrix metalloproteinases. In vivo, the brown alga extract lowered cell death by decreasing lipid peroxidation and inflammatory response. The investigators concluded that DPHC warrants consideration as an ingredient in cosmeceutical formulations intended to protect against the effects of UVB radiation.9
The same team also reported on their study of the protective effects of DPHC against skin damage in human dermal fibroblasts caused by particulate matter. They found that DPHC dose-dependently exerted significant decreases in intracellular synthesis of reactive oxygen species. The seaweed product also stimulated collagen production and suppressed collagenase activity, as well as matrix metalloproteinases. The researchers concluded that DPHC may be an effective skin-protective ingredient against particulate matter for use in cosmeceutical products.10
Skin protection mouse studies using various marine species
The last 3 years alone have featured several studies in mice that may have significant implications in accelerating our understanding of how to harness the bioactive properties of multiple marine species.
In 2018, Wiraguna and associates studied the protective effects of 0.2% and 0.4% Caulerpa sp. (a genus of seaweed native to the Indo-Pacific region) extract gels on photoaging in the UVB-irradiated skin of Wistar mice, finding that topical applications of both concentrations of the seaweed extract protected mouse skin from UVB-induced photoaging, with treated mice revealed to have higher collagen expression and preserved collagen structure and decreased MMP-1 levels, compared with vehicle controls.11
The next year, Prasedya and associates showed that the brown macroalgae Sargassum cristafolium exerted photoprotective activity against UVA in mice. Mice pretreated with the seaweed before exposure displayed intact collagen formation and no increases in epidermal thickness, compared with controls.12
At the same time, Santos and associates demonstrated that mice fed a diet supplemented with the red seaweed Porphyra umbilicalis experienced significant decreases in the incidence of human papillomavirus type 16–induced premalignant dysplastic skin lesions.13
Also that year, Zhen and associates evaluated the protective effects of eckol, a phlorotannin isolated from brown seaweed, on human HaCaT keratinocytes against PM2.5-induced cell damage. They showed that eckol (30 mcm) reduced reactive oxygen species production and protected cells from apoptosis by hampering the MAPK signaling pathway.14Earlier that year, Kim and associates studied the viability of the microalga Nannochloropsis oceanica, considered most often as a possible biofuel, for potential photoprotective activity against UVB-irradiated human dermal fibroblasts. They determined that pigment extracts (violaxanthin was identified as the main pigment) were not cytotoxic to the fibroblasts and that treatment with the pigment extract upregulated collagen expression and significantly inhibited UVB-induced damage. Further study revealed that violaxanthin significantly mitigated UVB-induced G1 phase arrest, senescence-associated beta-galactosidase activation, and p16 and p21 up-regulation, among other functions, suggesting its consideration, according to the authors, as a possible antiphotoaging agent.15
Finally, early in 2020, Bellan and associates evaluated the antitumor characteristics of the sulfated heterorhamnan derived from the green seaweed Gayralia brasiliensis as seen on the biological activities in the B16-F10 murine melanoma cell line. The polysaccharidic fraction was found to be effective in reducing melanoma cell migration and invasion capacity.16
Conclusion
. Evidence suggests widespread potential across several species for dermatologic purposes. Indeed, data indicate that some species appear to be suited for treating AD, alopecia, and wrinkles and may possibly render effective photoprotection. More research is necessary, of course, to ascertain the extent to which such ingredients can adequately address cutaneous health and how truly effective the marine ingredients are in currently marketed products.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann has written two textbooks and a New York Times Best Sellers book for consumers. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Revance, Evolus, and Burt’s Bees. She is the CEO of Skin Type Solutions, a company that independently tests skin care products and makes recommendations to physicians on which skin care technologies are best. Write to her at dermnews@mdedge.com.
References
1. Fabrowska J et al. Acta Pol Pharm. 2017 Mar;74(2):633-41.
2. Pangestuti R et al. Mar Drugs. 2018 Oct 23;16(11):399.
3. Kim JH et al. Mar Drugs. 2018 Nov 21;16(11):459.
4. Colantonio S & Rivers JK. J Cutan Med Surg. Jul/Aug 2017;21(4):299-307.
5. Tian T et al. Int Immunopharmacol. 2019 Oct;75:105823.
6. Gil TY et al. Mediators Inflamm. 2019 Mar 17;2019:3760934.
7. Kang JI et al. Mar Drugs. 2017 May 5;15(5):130.
8. Piao MJ et al. Biomol Ther (Seoul). 2015 Nov;23(6):557-63.
9. Wang L et al. Food Chem Toxicol. 2020 Feb;136:110963.
10. Wang L et al. Molecules. 2020 Feb 26;25(5):1055.
11. Wiraguna AAGP et al. Dermatol Reports. 2018 Oct 1;10(2):7597.
12. Prasedya ES et al. Biomedicines. 2019 Sep 27;7(4):77.
13. Santos S et al. Mar Drugs. 2019 Oct 29;17(11):615.
14. Zhen AX et al. Mar Drugs. 2019 Jul 27;17(8):444.
15. Kim HM et al. Photochem Photobiol. 2019 Mar;95(2):595-604.
16. Bellan DL et al. Mar Biotechnol. 2020 Apr;22(2):194-206.
The cutaneous benefits of bee venom, Part II: Acupuncture, wound healing, and various potential indications
A wide range of products derived from bees, including honey, propolis, bee pollen, bee bread, royal jelly, beeswax, and bee venom, have been used since ancient times for medical purposes.1 Specifically, bee venom has been used in traditional medicine to treat multiple disorders, including arthritis, cancer, pain, rheumatism, and skin diseases.2,3 The primary active constituent of bee venom is melittin, an amphiphilic peptide containing 26 amino acid residues and known to impart anti-inflammatory, antibacterial, analgesic, and anticancer effects.4-7 Additional anti-inflammatory compounds found in bee venom include adolapin, apamin, and phospholipase A2; melittin and phospholipase A2 are also capable of delivering pro-inflammatory activity.8,9
The anti-aging, anti-inflammatory, and antibacterial properties of bee venom have been cited as justification for its use as a cosmetic ingredient.10 In experimental studies, antinociceptive and anti-inflammatory effects have been reported.11 Bee venom phospholipase A2 has also demonstrated notable success in vitro and in vivo in conferring immunomodulatory effects and is a key component in past and continuing use of bee venom therapy for immune-related disorders, such as arthritis.12
A recent review of the biomedical literature by Nguyen et al. reveals that bee venom is one of the key ingredients in the booming Korean cosmeceuticals industry.13 Kim et al. reviewed the therapeutic applications of bee venom in 2019, noting that anti-inflammatory, antiapoptotic, antifibrotic, antimicrobial, and anticancer properties have been cited in experimental and clinical reports, with cutaneous treatments ranging from acne, alopecia, and atopic dermatitis to melanoma, morphea, photoaging, psoriasis, vitiligo, wounds, and wrinkles.14 This column focuses on the use of bee venom in acupuncture and wound healing, as well as some other potential applications of this bee product used for millennia.
Acupuncture
Bee venom acupuncture entails the application of bee venom to the tips of acupuncture needles, which are then applied to acupoints on the skin. Cherniack and Govorushko state that several small studies in humans show that bee venom acupuncture has been used effectively to treat various musculoskeletal and neurological conditions.8
In 2016, Sur et al. explored the effects of bee venom acupuncture on atopic dermatitis in a mouse model with lesions induced by trimellitic anhydride. Bee venom treatment was found to significantly ease inflammation, lesion thickness, and lymph node weight. Suppression of T-cell proliferation and infiltration, Th1 and Th2 cytokine synthesis, and interleukin (IL)-4 and immunoglobulin E (IgE) production was also noted.15
A case report by Hwang and Kim in 2018 described the successful use of bee venom acupuncture in the treatment of a 64-year-old Korean woman with circumscribed morphea resulting from systemic sclerosis. Subcutaneous bee venom acupuncture along the margins resolved pruritus through 2 months of follow-up.11
Wound healing
A study by Hozzein et al. in 2018 on protecting functional macrophages from apoptosis and improving Nrf2, Ang-1, and Tie-2 signaling in diabetic wound healing in mice revealed that bee venom supports immune function, thus promoting healing from diabetic wounds.(16) Previously, this team had shown that bee venom facilitates wound healing in diabetic mice by inhibiting the activation of transcription factor-3 and inducible nitric oxide synthase-mediated stress.17
In early 2020, Nakashima et al. reported their results showing that bee venom-derived phospholipase A2 augmented poly(I:C)-induced activation in human keratinocytes, suggesting that it could play a role in wound healing promotion through enhanced TLR3 responses.18
Alopecia
A 2016 study on the effect of bee venom on alopecia in C57BL/6 mice by Park et al. showed that the bee toxin dose-dependently stimulated proliferation of several growth factors, including fibroblast growth factors 2 and 7, as compared with the control group. Bee venom also suppressed transition from the anagen to catagen phases, nurtured hair growth, and presented the potential as a strong 5α-reductase inhibitor.19
Anticancer and anti-arthritic activity
In 2007, Son et al. reported that the various peptides (melittin, apamin, adolapin, the mast-cell-degranulating peptide), enzymes (i.e., phospholipase A2), as well as biologically active amines (i.e., histamine and epinephrine) and nonpeptide components in bee venom are thought to account for multiple pharmaceutical properties that yield anti-arthritis, antinociceptive, and anticancer effects.2
In 2019, Lim et al. determined that bee venom and melittin inhibited the growth and migration of melanoma cells (B16F10, A375SM, and SK-MEL-28) by downregulating the PI3K/AKT/mTOR and MAPK signaling pathways. They concluded that melittin has the potential for use in preventing and treating malignant melanoma.4
Phototoxicity
Heo et al. conducted phototoxicity and skin sensitization studies of bee venom, as well as a bee venom from which they removed phospholipase A2, and determined that both were nonphototoxic substances and did not act as sensitizers.20
Han et al. assessed the skin safety of bee venom on tests in healthy male Hartley guinea pigs in 2017 and found that bee venom application engendered no toxic reactions, including any signs of cutaneous phototoxicity or skin photosensitization, and is likely safe for inclusion as a topical skin care ingredient.10
Antiwrinkle activity
Han et al. also evaluated the beneficial effects of bee venom serum on facial wrinkles in a small study on humans (22 South Korean women between 30 and 49 years old), finding clinical improvements as seen through reductions in wrinkle count, average wrinkle depth, and total wrinkle area. The authors, noting that this was the first clinical study to assess the results of using bee venom cosmetics on facial skin, also cited the relative safety of the product, which presents nominal irritation potential, and acknowledged its present use in the cosmetics industry.21
Conclusion
Bees play a critical role in the web of life as they pollinate approximately one-third of our food. Perhaps counterintuitively, given our awareness of the painful and potentially serious reactions to bee stings, bee venom has also been found to deliver multiple salutary effects. More research is necessary to ascertain the viability of using bee venom as a reliable treatment for the various cutaneous conditions for which it demonstrates potential benefits. Current evidence presents justification for further investigation.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann has written two textbooks and a New York Times Best Sellers book for consumers. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Revance, Evolus, and Burt’s Bees. She is the CEO of Skin Type Solutions Inc., a company that independently tests skin care products and makes recommendations to physicians on which skin care technologies are best. Write to her at dermnews@mdedge.com.
References
1. Kurek-Górecka A et al. Molecules. 2020 Jan 28;25(3):556.
2. Son DJ et al. Pharmacol Ther. 2007 Aug;115(2):246-70.
3. Lee G, Bae H. Molecules. 2016 May 11;21(5):616.
4. Lim HN et al. Molecules. 2019 Mar 7;24(5):929.
5. Gu H et al. Mol Med Rep. 2018 Oct;18(4):3711-8. 6. You CE et al. Ann Dermatol. 2016 Oct;28(5):593-9. 7. An HJ et al. Int J Mol Med. 2014 Nov;34(5):1341-8. 8. Cherniack EP, Govorushko S. Toxicon. 2018 Nov;154:74-8. 9. Cornara L et al. Front Pharmacol. 2017 Jun 28;8:412.
10. Han SM et al. J Cosmet Dermatol. 2017 Dec;16(4):e68-e75.
11. Hwang JH, Kim KH. Medicine (Baltimore). 2018 Dec;97(49):e13404. 12. Lee G, Bae H. Toxins (Basel). 2016 Feb 22;8(2):48. 13. Nguyen JK et al. J Cosmet Dermatol. 2020 Jul;19(7):1555-69.
14. Kim H et al. Toxins (Basel). 2019 Jun 27:11(7):374.
15. Sur B et al. BMC Complement Altern Med. 2016 Jan 29;16:38. 16. Hozzein WN et al. Mol Immunol. 2018 Nov;103:322-35. 17. Badr G et al. J Cell Physiol. 2016 Oct;231(10):2159-71. 18. Nakashima A et al. Int Immunol. 2020 May 30;32(6):371-83. 19. Park S et al. Biol Pharm Bull. 2016 Jun 1;39(6):1060-8.
20. Heo Y et al. Evid Based Complement Alternat Med. 2015;2015:157367. 21. Han SM et al. Clin Interv Aging. 2015 Oct 1;10:1587-92.
A wide range of products derived from bees, including honey, propolis, bee pollen, bee bread, royal jelly, beeswax, and bee venom, have been used since ancient times for medical purposes.1 Specifically, bee venom has been used in traditional medicine to treat multiple disorders, including arthritis, cancer, pain, rheumatism, and skin diseases.2,3 The primary active constituent of bee venom is melittin, an amphiphilic peptide containing 26 amino acid residues and known to impart anti-inflammatory, antibacterial, analgesic, and anticancer effects.4-7 Additional anti-inflammatory compounds found in bee venom include adolapin, apamin, and phospholipase A2; melittin and phospholipase A2 are also capable of delivering pro-inflammatory activity.8,9
The anti-aging, anti-inflammatory, and antibacterial properties of bee venom have been cited as justification for its use as a cosmetic ingredient.10 In experimental studies, antinociceptive and anti-inflammatory effects have been reported.11 Bee venom phospholipase A2 has also demonstrated notable success in vitro and in vivo in conferring immunomodulatory effects and is a key component in past and continuing use of bee venom therapy for immune-related disorders, such as arthritis.12
A recent review of the biomedical literature by Nguyen et al. reveals that bee venom is one of the key ingredients in the booming Korean cosmeceuticals industry.13 Kim et al. reviewed the therapeutic applications of bee venom in 2019, noting that anti-inflammatory, antiapoptotic, antifibrotic, antimicrobial, and anticancer properties have been cited in experimental and clinical reports, with cutaneous treatments ranging from acne, alopecia, and atopic dermatitis to melanoma, morphea, photoaging, psoriasis, vitiligo, wounds, and wrinkles.14 This column focuses on the use of bee venom in acupuncture and wound healing, as well as some other potential applications of this bee product used for millennia.
Acupuncture
Bee venom acupuncture entails the application of bee venom to the tips of acupuncture needles, which are then applied to acupoints on the skin. Cherniack and Govorushko state that several small studies in humans show that bee venom acupuncture has been used effectively to treat various musculoskeletal and neurological conditions.8
In 2016, Sur et al. explored the effects of bee venom acupuncture on atopic dermatitis in a mouse model with lesions induced by trimellitic anhydride. Bee venom treatment was found to significantly ease inflammation, lesion thickness, and lymph node weight. Suppression of T-cell proliferation and infiltration, Th1 and Th2 cytokine synthesis, and interleukin (IL)-4 and immunoglobulin E (IgE) production was also noted.15
A case report by Hwang and Kim in 2018 described the successful use of bee venom acupuncture in the treatment of a 64-year-old Korean woman with circumscribed morphea resulting from systemic sclerosis. Subcutaneous bee venom acupuncture along the margins resolved pruritus through 2 months of follow-up.11
Wound healing
A study by Hozzein et al. in 2018 on protecting functional macrophages from apoptosis and improving Nrf2, Ang-1, and Tie-2 signaling in diabetic wound healing in mice revealed that bee venom supports immune function, thus promoting healing from diabetic wounds.(16) Previously, this team had shown that bee venom facilitates wound healing in diabetic mice by inhibiting the activation of transcription factor-3 and inducible nitric oxide synthase-mediated stress.17
In early 2020, Nakashima et al. reported their results showing that bee venom-derived phospholipase A2 augmented poly(I:C)-induced activation in human keratinocytes, suggesting that it could play a role in wound healing promotion through enhanced TLR3 responses.18
Alopecia
A 2016 study on the effect of bee venom on alopecia in C57BL/6 mice by Park et al. showed that the bee toxin dose-dependently stimulated proliferation of several growth factors, including fibroblast growth factors 2 and 7, as compared with the control group. Bee venom also suppressed transition from the anagen to catagen phases, nurtured hair growth, and presented the potential as a strong 5α-reductase inhibitor.19
Anticancer and anti-arthritic activity
In 2007, Son et al. reported that the various peptides (melittin, apamin, adolapin, the mast-cell-degranulating peptide), enzymes (i.e., phospholipase A2), as well as biologically active amines (i.e., histamine and epinephrine) and nonpeptide components in bee venom are thought to account for multiple pharmaceutical properties that yield anti-arthritis, antinociceptive, and anticancer effects.2
In 2019, Lim et al. determined that bee venom and melittin inhibited the growth and migration of melanoma cells (B16F10, A375SM, and SK-MEL-28) by downregulating the PI3K/AKT/mTOR and MAPK signaling pathways. They concluded that melittin has the potential for use in preventing and treating malignant melanoma.4
Phototoxicity
Heo et al. conducted phototoxicity and skin sensitization studies of bee venom, as well as a bee venom from which they removed phospholipase A2, and determined that both were nonphototoxic substances and did not act as sensitizers.20
Han et al. assessed the skin safety of bee venom on tests in healthy male Hartley guinea pigs in 2017 and found that bee venom application engendered no toxic reactions, including any signs of cutaneous phototoxicity or skin photosensitization, and is likely safe for inclusion as a topical skin care ingredient.10
Antiwrinkle activity
Han et al. also evaluated the beneficial effects of bee venom serum on facial wrinkles in a small study on humans (22 South Korean women between 30 and 49 years old), finding clinical improvements as seen through reductions in wrinkle count, average wrinkle depth, and total wrinkle area. The authors, noting that this was the first clinical study to assess the results of using bee venom cosmetics on facial skin, also cited the relative safety of the product, which presents nominal irritation potential, and acknowledged its present use in the cosmetics industry.21
Conclusion
Bees play a critical role in the web of life as they pollinate approximately one-third of our food. Perhaps counterintuitively, given our awareness of the painful and potentially serious reactions to bee stings, bee venom has also been found to deliver multiple salutary effects. More research is necessary to ascertain the viability of using bee venom as a reliable treatment for the various cutaneous conditions for which it demonstrates potential benefits. Current evidence presents justification for further investigation.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann has written two textbooks and a New York Times Best Sellers book for consumers. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Revance, Evolus, and Burt’s Bees. She is the CEO of Skin Type Solutions Inc., a company that independently tests skin care products and makes recommendations to physicians on which skin care technologies are best. Write to her at dermnews@mdedge.com.
References
1. Kurek-Górecka A et al. Molecules. 2020 Jan 28;25(3):556.
2. Son DJ et al. Pharmacol Ther. 2007 Aug;115(2):246-70.
3. Lee G, Bae H. Molecules. 2016 May 11;21(5):616.
4. Lim HN et al. Molecules. 2019 Mar 7;24(5):929.
5. Gu H et al. Mol Med Rep. 2018 Oct;18(4):3711-8. 6. You CE et al. Ann Dermatol. 2016 Oct;28(5):593-9. 7. An HJ et al. Int J Mol Med. 2014 Nov;34(5):1341-8. 8. Cherniack EP, Govorushko S. Toxicon. 2018 Nov;154:74-8. 9. Cornara L et al. Front Pharmacol. 2017 Jun 28;8:412.
10. Han SM et al. J Cosmet Dermatol. 2017 Dec;16(4):e68-e75.
11. Hwang JH, Kim KH. Medicine (Baltimore). 2018 Dec;97(49):e13404. 12. Lee G, Bae H. Toxins (Basel). 2016 Feb 22;8(2):48. 13. Nguyen JK et al. J Cosmet Dermatol. 2020 Jul;19(7):1555-69.
14. Kim H et al. Toxins (Basel). 2019 Jun 27:11(7):374.
15. Sur B et al. BMC Complement Altern Med. 2016 Jan 29;16:38. 16. Hozzein WN et al. Mol Immunol. 2018 Nov;103:322-35. 17. Badr G et al. J Cell Physiol. 2016 Oct;231(10):2159-71. 18. Nakashima A et al. Int Immunol. 2020 May 30;32(6):371-83. 19. Park S et al. Biol Pharm Bull. 2016 Jun 1;39(6):1060-8.
20. Heo Y et al. Evid Based Complement Alternat Med. 2015;2015:157367. 21. Han SM et al. Clin Interv Aging. 2015 Oct 1;10:1587-92.
A wide range of products derived from bees, including honey, propolis, bee pollen, bee bread, royal jelly, beeswax, and bee venom, have been used since ancient times for medical purposes.1 Specifically, bee venom has been used in traditional medicine to treat multiple disorders, including arthritis, cancer, pain, rheumatism, and skin diseases.2,3 The primary active constituent of bee venom is melittin, an amphiphilic peptide containing 26 amino acid residues and known to impart anti-inflammatory, antibacterial, analgesic, and anticancer effects.4-7 Additional anti-inflammatory compounds found in bee venom include adolapin, apamin, and phospholipase A2; melittin and phospholipase A2 are also capable of delivering pro-inflammatory activity.8,9
The anti-aging, anti-inflammatory, and antibacterial properties of bee venom have been cited as justification for its use as a cosmetic ingredient.10 In experimental studies, antinociceptive and anti-inflammatory effects have been reported.11 Bee venom phospholipase A2 has also demonstrated notable success in vitro and in vivo in conferring immunomodulatory effects and is a key component in past and continuing use of bee venom therapy for immune-related disorders, such as arthritis.12
A recent review of the biomedical literature by Nguyen et al. reveals that bee venom is one of the key ingredients in the booming Korean cosmeceuticals industry.13 Kim et al. reviewed the therapeutic applications of bee venom in 2019, noting that anti-inflammatory, antiapoptotic, antifibrotic, antimicrobial, and anticancer properties have been cited in experimental and clinical reports, with cutaneous treatments ranging from acne, alopecia, and atopic dermatitis to melanoma, morphea, photoaging, psoriasis, vitiligo, wounds, and wrinkles.14 This column focuses on the use of bee venom in acupuncture and wound healing, as well as some other potential applications of this bee product used for millennia.
Acupuncture
Bee venom acupuncture entails the application of bee venom to the tips of acupuncture needles, which are then applied to acupoints on the skin. Cherniack and Govorushko state that several small studies in humans show that bee venom acupuncture has been used effectively to treat various musculoskeletal and neurological conditions.8
In 2016, Sur et al. explored the effects of bee venom acupuncture on atopic dermatitis in a mouse model with lesions induced by trimellitic anhydride. Bee venom treatment was found to significantly ease inflammation, lesion thickness, and lymph node weight. Suppression of T-cell proliferation and infiltration, Th1 and Th2 cytokine synthesis, and interleukin (IL)-4 and immunoglobulin E (IgE) production was also noted.15
A case report by Hwang and Kim in 2018 described the successful use of bee venom acupuncture in the treatment of a 64-year-old Korean woman with circumscribed morphea resulting from systemic sclerosis. Subcutaneous bee venom acupuncture along the margins resolved pruritus through 2 months of follow-up.11
Wound healing
A study by Hozzein et al. in 2018 on protecting functional macrophages from apoptosis and improving Nrf2, Ang-1, and Tie-2 signaling in diabetic wound healing in mice revealed that bee venom supports immune function, thus promoting healing from diabetic wounds.(16) Previously, this team had shown that bee venom facilitates wound healing in diabetic mice by inhibiting the activation of transcription factor-3 and inducible nitric oxide synthase-mediated stress.17
In early 2020, Nakashima et al. reported their results showing that bee venom-derived phospholipase A2 augmented poly(I:C)-induced activation in human keratinocytes, suggesting that it could play a role in wound healing promotion through enhanced TLR3 responses.18
Alopecia
A 2016 study on the effect of bee venom on alopecia in C57BL/6 mice by Park et al. showed that the bee toxin dose-dependently stimulated proliferation of several growth factors, including fibroblast growth factors 2 and 7, as compared with the control group. Bee venom also suppressed transition from the anagen to catagen phases, nurtured hair growth, and presented the potential as a strong 5α-reductase inhibitor.19
Anticancer and anti-arthritic activity
In 2007, Son et al. reported that the various peptides (melittin, apamin, adolapin, the mast-cell-degranulating peptide), enzymes (i.e., phospholipase A2), as well as biologically active amines (i.e., histamine and epinephrine) and nonpeptide components in bee venom are thought to account for multiple pharmaceutical properties that yield anti-arthritis, antinociceptive, and anticancer effects.2
In 2019, Lim et al. determined that bee venom and melittin inhibited the growth and migration of melanoma cells (B16F10, A375SM, and SK-MEL-28) by downregulating the PI3K/AKT/mTOR and MAPK signaling pathways. They concluded that melittin has the potential for use in preventing and treating malignant melanoma.4
Phototoxicity
Heo et al. conducted phototoxicity and skin sensitization studies of bee venom, as well as a bee venom from which they removed phospholipase A2, and determined that both were nonphototoxic substances and did not act as sensitizers.20
Han et al. assessed the skin safety of bee venom on tests in healthy male Hartley guinea pigs in 2017 and found that bee venom application engendered no toxic reactions, including any signs of cutaneous phototoxicity or skin photosensitization, and is likely safe for inclusion as a topical skin care ingredient.10
Antiwrinkle activity
Han et al. also evaluated the beneficial effects of bee venom serum on facial wrinkles in a small study on humans (22 South Korean women between 30 and 49 years old), finding clinical improvements as seen through reductions in wrinkle count, average wrinkle depth, and total wrinkle area. The authors, noting that this was the first clinical study to assess the results of using bee venom cosmetics on facial skin, also cited the relative safety of the product, which presents nominal irritation potential, and acknowledged its present use in the cosmetics industry.21
Conclusion
Bees play a critical role in the web of life as they pollinate approximately one-third of our food. Perhaps counterintuitively, given our awareness of the painful and potentially serious reactions to bee stings, bee venom has also been found to deliver multiple salutary effects. More research is necessary to ascertain the viability of using bee venom as a reliable treatment for the various cutaneous conditions for which it demonstrates potential benefits. Current evidence presents justification for further investigation.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann has written two textbooks and a New York Times Best Sellers book for consumers. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Revance, Evolus, and Burt’s Bees. She is the CEO of Skin Type Solutions Inc., a company that independently tests skin care products and makes recommendations to physicians on which skin care technologies are best. Write to her at dermnews@mdedge.com.
References
1. Kurek-Górecka A et al. Molecules. 2020 Jan 28;25(3):556.
2. Son DJ et al. Pharmacol Ther. 2007 Aug;115(2):246-70.
3. Lee G, Bae H. Molecules. 2016 May 11;21(5):616.
4. Lim HN et al. Molecules. 2019 Mar 7;24(5):929.
5. Gu H et al. Mol Med Rep. 2018 Oct;18(4):3711-8. 6. You CE et al. Ann Dermatol. 2016 Oct;28(5):593-9. 7. An HJ et al. Int J Mol Med. 2014 Nov;34(5):1341-8. 8. Cherniack EP, Govorushko S. Toxicon. 2018 Nov;154:74-8. 9. Cornara L et al. Front Pharmacol. 2017 Jun 28;8:412.
10. Han SM et al. J Cosmet Dermatol. 2017 Dec;16(4):e68-e75.
11. Hwang JH, Kim KH. Medicine (Baltimore). 2018 Dec;97(49):e13404. 12. Lee G, Bae H. Toxins (Basel). 2016 Feb 22;8(2):48. 13. Nguyen JK et al. J Cosmet Dermatol. 2020 Jul;19(7):1555-69.
14. Kim H et al. Toxins (Basel). 2019 Jun 27:11(7):374.
15. Sur B et al. BMC Complement Altern Med. 2016 Jan 29;16:38. 16. Hozzein WN et al. Mol Immunol. 2018 Nov;103:322-35. 17. Badr G et al. J Cell Physiol. 2016 Oct;231(10):2159-71. 18. Nakashima A et al. Int Immunol. 2020 May 30;32(6):371-83. 19. Park S et al. Biol Pharm Bull. 2016 Jun 1;39(6):1060-8.
20. Heo Y et al. Evid Based Complement Alternat Med. 2015;2015:157367. 21. Han SM et al. Clin Interv Aging. 2015 Oct 1;10:1587-92.