User login
I just completed the third edition of my Cosmetic Dermatology textbook (McGraw Hill), which will come out later this year. Although writing it is a huge effort, I really enjoy all the basic science. While I was working on the book, I was most surprised by the .
Right now, it is too early, and we don’t know enough yet, to have cosmeceuticals that affect cellular senescence and autophagy. But, it’s not too early to learn about this research, to avoid falling prey to any pseudoscience that invariably ends up affecting cosmeceuticals on the market. The following is a brief primer on cellular senescence, skin aging, and cosmeceuticals; it represents what we currently know.
Cell phases
Keratinocytes and fibroblasts go through five different phases: stem, proliferation, differentiation, senescence, and apoptosis. The difference between apoptotic cells and senescent cells is that apoptotic cells are not viable and are eliminated, while senescent cells, even though they have gone into cell cycle arrest, remain functional and are not eliminated from the skin.
What are senescent cells?
Senescent cells have lost the ability to proliferate but have not undergone apoptosis. Senescent human skin fibroblasts in cell culture lose the youthful spindlelike shape and become enlarged and flattened.1 Their lysosomes and mitochondria lose functionality.2 The presence of senescent cells is associated with increased aging and seems to speed aging.
Senescent cells and skin aging
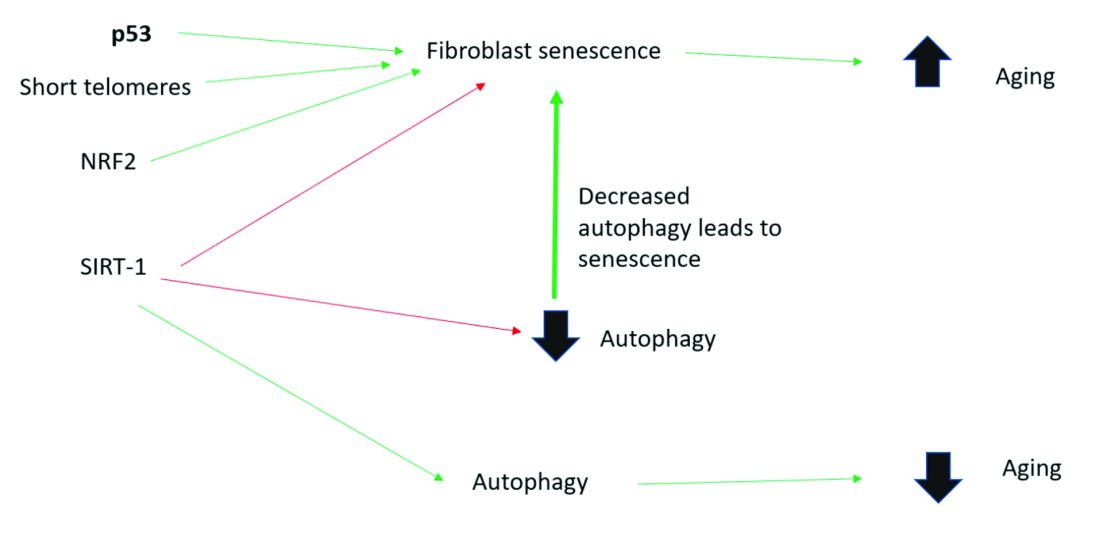
Senescent cells are increased in the age-related phenotype3 because of an age-related decline of senescent cell removal systems, such as the immune system4 and the autophagy-lysosomal pathway.5 Senescent cells are deleterious because they develop into a senescence-associated secretory phenotype (SASP), which is believed to be one of the major causes of aging. SASP cells communicate with nearby cells using proinflammatory cytokines, which include catabolic modulators such as Matrix metalloproteinases. They are known to release growth factors, cytokines, chemokines, matrix-modeling enzymes, lipids, and extracellular vesicles. The last are lipid bilayer-lined vesicles that can transport functional RNA and microRNA and facilitate other modes of communication between cells.6
The SASP is likely a natural tumor suppressive mode employed by cells to prevent cells with cancerous mutations from undergoing replication;7 however, when it comes to aging, the deleterious effects of SASP outweigh the beneficial effects. For example, SASP contributes to a prolonged state of inflammation, known as “inflammaging,”8 which is detrimental to the skin’s appearance. Human fibroblasts that have assumed the SASP secrete proinflammatory cytokines and MMPs and release reactive oxygen species,9,10 resulting in degradation of the surrounding extracellular matrix (ECM). Loss of the ECM leads to fibroblast compaction and reduced DNA synthesis, all caused by SASPs.9
What causes cellular senescence?
Activation of the nuclear factor-erythroid 2-related transcription factor 2 (NRF2) induces cellular senescence via direct targeting of certain ECM genes. NRF2 is a key regulator of the skin’s antioxidant defense system, which controls the transcription of genes encoding reactive oxygen species–detoxifying enzymes and various other antioxidant proteins.11 Loss of mitochondrial autophagy also induces senescence, as do activation of the TP53 gene, inactivity of SIRT-1, and short telomeres.
Cellular senescence and skin aging
Timely clearance of senescent cells before they create too much damage postpones the onset and severity of age-related diseases and extends the life span of mice.12,6 Antiaging treatments should focus on decreasing the number of senescent cells and reverting senescent cells to the more juvenile forms: proliferating or differentiating cells as an approach to prevent skin aging.13 Restoration of the lysosomal-mitochondrial axis has been shown to revert SASP back to a juvenile status. Normalization of the lysosomal-mitochondrial axis is a prerequisite to reverse senescence.14
Cellular senescence, autophagy, the lysosomal-mitochondrial axis, and cosmeceuticals
Autophagy is the important process of organelles, like mitochondria,15 self-digesting their cytoplasmic material into lysosomes for degradation. Mitochondrial autophagy is very important in slowing the aging process because damaged mitochondria generate free radicals. As you can imagine, much research is focused on this area, but it is too early for any research to translate to efficacious cosmeceuticals.
Conclusion
To summarize, activation of sirtuin-1 (SIRT-1) has been shown to extend the lifespan of mammals, as does caloric restriction.16 This extension occurs because SIRT-1 decreases senescence and activates autophagy.
Although we do not yet know whether topical skincare products could affect senescence or autophagy, there are data to show that oral resveratrol16 and melatonin17 activate SIRT-1 and increase autophagy. I am closely watching this research and will let you know if there are any similar data on topical cosmeceuticals targeting senescence or autophagy. Stay tuned!
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann has written two textbooks and a New York Times Best Sellers book for consumers. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Revance, Evolus, and Burt’s Bees. She is the CEO of Skin Type Solutions Inc., a company that independently tests skin care products and makes recommendations to physicians on which skin care technologies are best. Write to her at dermnews@mdedge.com.
References
1. Papadopoulou A et al. Biogerontology. 2020 Dec;21(6):695-708.
2. López-Otin C et al. Cell. 2013 June 6;153, 1194–217.
3. Yoon J E et al. Theranostics. 2018 Sep 9;8(17):4620-32.
4. Rodier F, Campisi J. J Cell Biol. 2011 Feb 21;192(4):547-56.
5. Dutta D et al. Circ Res. 2012 Apr 13;110(8):1125-38.
6. Terlecki-Zaniewicz L et al. J Invest Dermatol. 2019 Dec;139(12):2425-36.e5.
7. Campisi J et al. Nat Rev Mol Cell Biol. 2007 Sep;8(9):729-40.
8. Franceschi C and Campisi J. J Gerontol A Biol Sci Med Sci. 2014 Jun;69 Suppl 1:S4-9.
9. Nelson G et al. Aging Cell. 2012 Apr;11(2):345-9.
10. Passos JF et al. PLoS Biol. 2007 May;5(5):e110.
11. Hiebert P et al. Dev Cell. 2018 Jul 16;46(2):145-61.e10.
12. Baker DJ et al. Nature. 2016 Feb 11:530(7589):184-9.
13. Mavrogonatou E et al. Matrix Biol. 2019 Jan;75-76:27-42.
14. Park JT et al. Ageing Res Rev. 2018 Nov;47:176-82.
15. Levine B and Kroemer G. Cell. 2019 Jan 10;176(1-2):11-42.
16. Morselli E et al. Cell Death Dis. 2010;1(1):e10.
17. Lee JH et al. Oncotarget. 2016 Mar 15;7(11):12075-88.
I just completed the third edition of my Cosmetic Dermatology textbook (McGraw Hill), which will come out later this year. Although writing it is a huge effort, I really enjoy all the basic science. While I was working on the book, I was most surprised by the .
Right now, it is too early, and we don’t know enough yet, to have cosmeceuticals that affect cellular senescence and autophagy. But, it’s not too early to learn about this research, to avoid falling prey to any pseudoscience that invariably ends up affecting cosmeceuticals on the market. The following is a brief primer on cellular senescence, skin aging, and cosmeceuticals; it represents what we currently know.
Cell phases
Keratinocytes and fibroblasts go through five different phases: stem, proliferation, differentiation, senescence, and apoptosis. The difference between apoptotic cells and senescent cells is that apoptotic cells are not viable and are eliminated, while senescent cells, even though they have gone into cell cycle arrest, remain functional and are not eliminated from the skin.
What are senescent cells?
Senescent cells have lost the ability to proliferate but have not undergone apoptosis. Senescent human skin fibroblasts in cell culture lose the youthful spindlelike shape and become enlarged and flattened.1 Their lysosomes and mitochondria lose functionality.2 The presence of senescent cells is associated with increased aging and seems to speed aging.

Senescent cells and skin aging
Senescent cells are increased in the age-related phenotype3 because of an age-related decline of senescent cell removal systems, such as the immune system4 and the autophagy-lysosomal pathway.5 Senescent cells are deleterious because they develop into a senescence-associated secretory phenotype (SASP), which is believed to be one of the major causes of aging. SASP cells communicate with nearby cells using proinflammatory cytokines, which include catabolic modulators such as Matrix metalloproteinases. They are known to release growth factors, cytokines, chemokines, matrix-modeling enzymes, lipids, and extracellular vesicles. The last are lipid bilayer-lined vesicles that can transport functional RNA and microRNA and facilitate other modes of communication between cells.6
The SASP is likely a natural tumor suppressive mode employed by cells to prevent cells with cancerous mutations from undergoing replication;7 however, when it comes to aging, the deleterious effects of SASP outweigh the beneficial effects. For example, SASP contributes to a prolonged state of inflammation, known as “inflammaging,”8 which is detrimental to the skin’s appearance. Human fibroblasts that have assumed the SASP secrete proinflammatory cytokines and MMPs and release reactive oxygen species,9,10 resulting in degradation of the surrounding extracellular matrix (ECM). Loss of the ECM leads to fibroblast compaction and reduced DNA synthesis, all caused by SASPs.9
What causes cellular senescence?
Activation of the nuclear factor-erythroid 2-related transcription factor 2 (NRF2) induces cellular senescence via direct targeting of certain ECM genes. NRF2 is a key regulator of the skin’s antioxidant defense system, which controls the transcription of genes encoding reactive oxygen species–detoxifying enzymes and various other antioxidant proteins.11 Loss of mitochondrial autophagy also induces senescence, as do activation of the TP53 gene, inactivity of SIRT-1, and short telomeres.
Cellular senescence and skin aging
Timely clearance of senescent cells before they create too much damage postpones the onset and severity of age-related diseases and extends the life span of mice.12,6 Antiaging treatments should focus on decreasing the number of senescent cells and reverting senescent cells to the more juvenile forms: proliferating or differentiating cells as an approach to prevent skin aging.13 Restoration of the lysosomal-mitochondrial axis has been shown to revert SASP back to a juvenile status. Normalization of the lysosomal-mitochondrial axis is a prerequisite to reverse senescence.14
Cellular senescence, autophagy, the lysosomal-mitochondrial axis, and cosmeceuticals
Autophagy is the important process of organelles, like mitochondria,15 self-digesting their cytoplasmic material into lysosomes for degradation. Mitochondrial autophagy is very important in slowing the aging process because damaged mitochondria generate free radicals. As you can imagine, much research is focused on this area, but it is too early for any research to translate to efficacious cosmeceuticals.
Conclusion
To summarize, activation of sirtuin-1 (SIRT-1) has been shown to extend the lifespan of mammals, as does caloric restriction.16 This extension occurs because SIRT-1 decreases senescence and activates autophagy.
Although we do not yet know whether topical skincare products could affect senescence or autophagy, there are data to show that oral resveratrol16 and melatonin17 activate SIRT-1 and increase autophagy. I am closely watching this research and will let you know if there are any similar data on topical cosmeceuticals targeting senescence or autophagy. Stay tuned!
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann has written two textbooks and a New York Times Best Sellers book for consumers. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Revance, Evolus, and Burt’s Bees. She is the CEO of Skin Type Solutions Inc., a company that independently tests skin care products and makes recommendations to physicians on which skin care technologies are best. Write to her at dermnews@mdedge.com.
References
1. Papadopoulou A et al. Biogerontology. 2020 Dec;21(6):695-708.
2. López-Otin C et al. Cell. 2013 June 6;153, 1194–217.
3. Yoon J E et al. Theranostics. 2018 Sep 9;8(17):4620-32.
4. Rodier F, Campisi J. J Cell Biol. 2011 Feb 21;192(4):547-56.
5. Dutta D et al. Circ Res. 2012 Apr 13;110(8):1125-38.
6. Terlecki-Zaniewicz L et al. J Invest Dermatol. 2019 Dec;139(12):2425-36.e5.
7. Campisi J et al. Nat Rev Mol Cell Biol. 2007 Sep;8(9):729-40.
8. Franceschi C and Campisi J. J Gerontol A Biol Sci Med Sci. 2014 Jun;69 Suppl 1:S4-9.
9. Nelson G et al. Aging Cell. 2012 Apr;11(2):345-9.
10. Passos JF et al. PLoS Biol. 2007 May;5(5):e110.
11. Hiebert P et al. Dev Cell. 2018 Jul 16;46(2):145-61.e10.
12. Baker DJ et al. Nature. 2016 Feb 11:530(7589):184-9.
13. Mavrogonatou E et al. Matrix Biol. 2019 Jan;75-76:27-42.
14. Park JT et al. Ageing Res Rev. 2018 Nov;47:176-82.
15. Levine B and Kroemer G. Cell. 2019 Jan 10;176(1-2):11-42.
16. Morselli E et al. Cell Death Dis. 2010;1(1):e10.
17. Lee JH et al. Oncotarget. 2016 Mar 15;7(11):12075-88.
I just completed the third edition of my Cosmetic Dermatology textbook (McGraw Hill), which will come out later this year. Although writing it is a huge effort, I really enjoy all the basic science. While I was working on the book, I was most surprised by the .
Right now, it is too early, and we don’t know enough yet, to have cosmeceuticals that affect cellular senescence and autophagy. But, it’s not too early to learn about this research, to avoid falling prey to any pseudoscience that invariably ends up affecting cosmeceuticals on the market. The following is a brief primer on cellular senescence, skin aging, and cosmeceuticals; it represents what we currently know.
Cell phases
Keratinocytes and fibroblasts go through five different phases: stem, proliferation, differentiation, senescence, and apoptosis. The difference between apoptotic cells and senescent cells is that apoptotic cells are not viable and are eliminated, while senescent cells, even though they have gone into cell cycle arrest, remain functional and are not eliminated from the skin.
What are senescent cells?
Senescent cells have lost the ability to proliferate but have not undergone apoptosis. Senescent human skin fibroblasts in cell culture lose the youthful spindlelike shape and become enlarged and flattened.1 Their lysosomes and mitochondria lose functionality.2 The presence of senescent cells is associated with increased aging and seems to speed aging.

Senescent cells and skin aging
Senescent cells are increased in the age-related phenotype3 because of an age-related decline of senescent cell removal systems, such as the immune system4 and the autophagy-lysosomal pathway.5 Senescent cells are deleterious because they develop into a senescence-associated secretory phenotype (SASP), which is believed to be one of the major causes of aging. SASP cells communicate with nearby cells using proinflammatory cytokines, which include catabolic modulators such as Matrix metalloproteinases. They are known to release growth factors, cytokines, chemokines, matrix-modeling enzymes, lipids, and extracellular vesicles. The last are lipid bilayer-lined vesicles that can transport functional RNA and microRNA and facilitate other modes of communication between cells.6
The SASP is likely a natural tumor suppressive mode employed by cells to prevent cells with cancerous mutations from undergoing replication;7 however, when it comes to aging, the deleterious effects of SASP outweigh the beneficial effects. For example, SASP contributes to a prolonged state of inflammation, known as “inflammaging,”8 which is detrimental to the skin’s appearance. Human fibroblasts that have assumed the SASP secrete proinflammatory cytokines and MMPs and release reactive oxygen species,9,10 resulting in degradation of the surrounding extracellular matrix (ECM). Loss of the ECM leads to fibroblast compaction and reduced DNA synthesis, all caused by SASPs.9
What causes cellular senescence?
Activation of the nuclear factor-erythroid 2-related transcription factor 2 (NRF2) induces cellular senescence via direct targeting of certain ECM genes. NRF2 is a key regulator of the skin’s antioxidant defense system, which controls the transcription of genes encoding reactive oxygen species–detoxifying enzymes and various other antioxidant proteins.11 Loss of mitochondrial autophagy also induces senescence, as do activation of the TP53 gene, inactivity of SIRT-1, and short telomeres.
Cellular senescence and skin aging
Timely clearance of senescent cells before they create too much damage postpones the onset and severity of age-related diseases and extends the life span of mice.12,6 Antiaging treatments should focus on decreasing the number of senescent cells and reverting senescent cells to the more juvenile forms: proliferating or differentiating cells as an approach to prevent skin aging.13 Restoration of the lysosomal-mitochondrial axis has been shown to revert SASP back to a juvenile status. Normalization of the lysosomal-mitochondrial axis is a prerequisite to reverse senescence.14
Cellular senescence, autophagy, the lysosomal-mitochondrial axis, and cosmeceuticals
Autophagy is the important process of organelles, like mitochondria,15 self-digesting their cytoplasmic material into lysosomes for degradation. Mitochondrial autophagy is very important in slowing the aging process because damaged mitochondria generate free radicals. As you can imagine, much research is focused on this area, but it is too early for any research to translate to efficacious cosmeceuticals.
Conclusion
To summarize, activation of sirtuin-1 (SIRT-1) has been shown to extend the lifespan of mammals, as does caloric restriction.16 This extension occurs because SIRT-1 decreases senescence and activates autophagy.
Although we do not yet know whether topical skincare products could affect senescence or autophagy, there are data to show that oral resveratrol16 and melatonin17 activate SIRT-1 and increase autophagy. I am closely watching this research and will let you know if there are any similar data on topical cosmeceuticals targeting senescence or autophagy. Stay tuned!
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann has written two textbooks and a New York Times Best Sellers book for consumers. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Revance, Evolus, and Burt’s Bees. She is the CEO of Skin Type Solutions Inc., a company that independently tests skin care products and makes recommendations to physicians on which skin care technologies are best. Write to her at dermnews@mdedge.com.
References
1. Papadopoulou A et al. Biogerontology. 2020 Dec;21(6):695-708.
2. López-Otin C et al. Cell. 2013 June 6;153, 1194–217.
3. Yoon J E et al. Theranostics. 2018 Sep 9;8(17):4620-32.
4. Rodier F, Campisi J. J Cell Biol. 2011 Feb 21;192(4):547-56.
5. Dutta D et al. Circ Res. 2012 Apr 13;110(8):1125-38.
6. Terlecki-Zaniewicz L et al. J Invest Dermatol. 2019 Dec;139(12):2425-36.e5.
7. Campisi J et al. Nat Rev Mol Cell Biol. 2007 Sep;8(9):729-40.
8. Franceschi C and Campisi J. J Gerontol A Biol Sci Med Sci. 2014 Jun;69 Suppl 1:S4-9.
9. Nelson G et al. Aging Cell. 2012 Apr;11(2):345-9.
10. Passos JF et al. PLoS Biol. 2007 May;5(5):e110.
11. Hiebert P et al. Dev Cell. 2018 Jul 16;46(2):145-61.e10.
12. Baker DJ et al. Nature. 2016 Feb 11:530(7589):184-9.
13. Mavrogonatou E et al. Matrix Biol. 2019 Jan;75-76:27-42.
14. Park JT et al. Ageing Res Rev. 2018 Nov;47:176-82.
15. Levine B and Kroemer G. Cell. 2019 Jan 10;176(1-2):11-42.
16. Morselli E et al. Cell Death Dis. 2010;1(1):e10.
17. Lee JH et al. Oncotarget. 2016 Mar 15;7(11):12075-88.

