User login
Ready for post-acute care?
The definition of “hospitalist,” according to the SHM website, is a clinician “dedicated to delivering comprehensive medical care to hospitalized patients.” For years, the hospital setting was the specialties’ identifier. But as hospitalists’ scope has expanded, and post-acute care (PAC) in the United States has grown, more hospitalists are extending their roles into this space.
PAC today is more than the traditional nursing home, according to Manoj K. Mathew, MD, SFHM, national medical director of Agilon Health in Los Angeles.
Many of those expanded settings Dr. Mathew describes emerged as a result of the Affordable Care Act. Since its enactment in 2010, the ACA has heightened providers’ focus on the “Triple Aim” of improving the patient experience (including quality and satisfaction), improving the health of populations, and reducing the per capita cost of healthcare.1 Vishal Kuchaculla, MD, New England regional post-acute medical director of Knoxville,Tenn.-based TeamHealth, says new service lines also developed as Medicare clamped down on long-term inpatient hospital stays by giving financial impetus to discharge patients as soon as possible.
“Over the last few years, there’s been a major shift from fee-for-service to risk-based payment models,” Dr. Kuchaculla says. “The government’s financial incentives are driving outcomes to improve performance initiatives.”
“Today, LTACHs can be used as substitutes for short-term acute care,” says Sean R. Muldoon, MD, MPH, FCCP, chief medical officer of Kindred Healthcare in Louisville, Ky., and former chair of SHM’s Post-Acute Care Committee. “This means that a patient can be directly admitted from their home to an LTACH. In fact, many hospice and home-care patients are referred from physicians’ offices without a preceding hospitalization.”

Hospitalists can fill a need

More hospitalists are working in PACs for a number of reasons. Dr. Mathew says PAC facilities and services have “typically lacked the clinical structure and processes to obtain the results that patients and payors expect.
“These deficits needed to be quickly remedied as patients discharged from hospitals have increased acuity and higher disease burdens,” he adds. “Hospitalists were the natural choice to fill roles requiring their expertise and experience.”
Dr. Muldoon considers the expanded scope of practice into PACs an additional layer to hospital medicine’s value proposition to the healthcare system.
“As experts in the management of inpatient populations, it’s natural for hospitalists to expand to other facilities with inpatient-like populations,” he says, noting SNFs are the most popular choice, with IRFs and LTACHs also being common places to work. Few hospitalists work in home care or hospice.
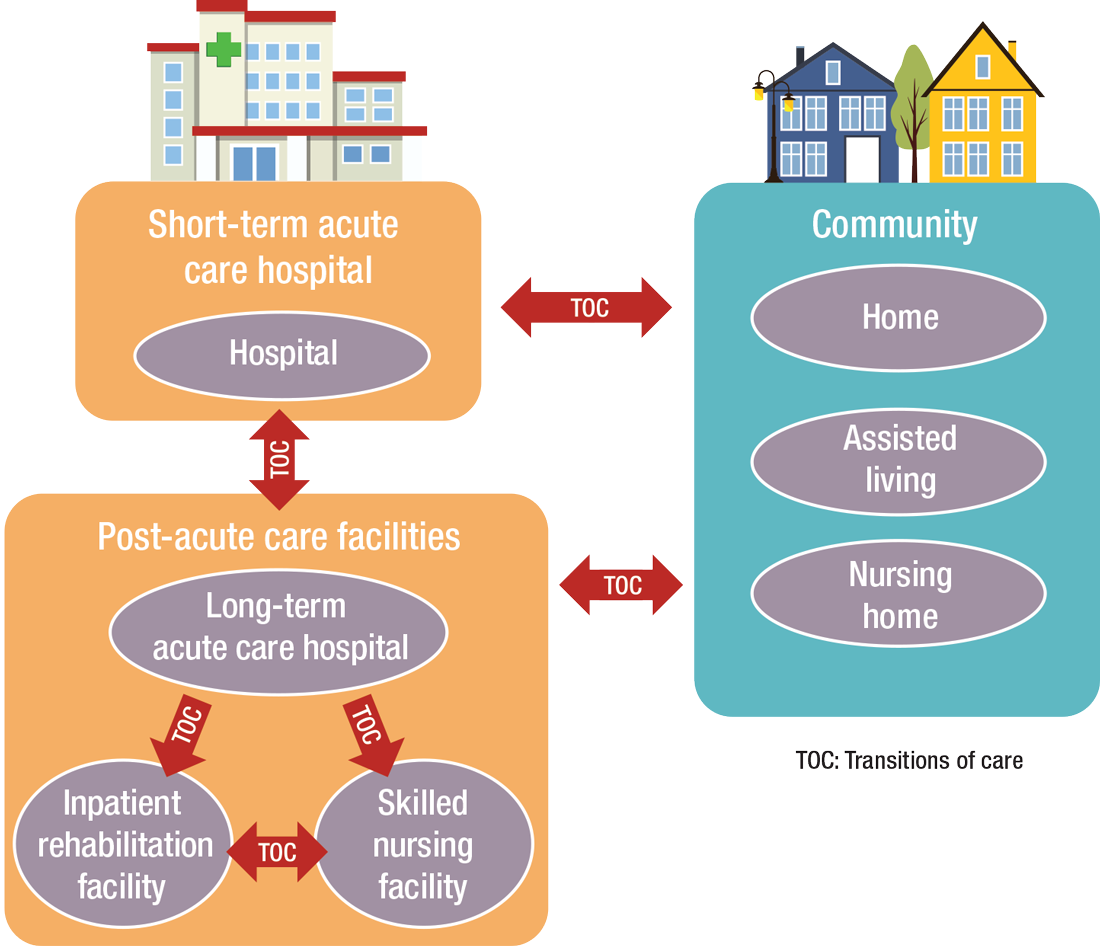
PAC settings are designed to help patients who are transitioning from an inpatient setting back to their home or other setting.
“Many patients go home after a SNF stay, while others will move to a nursing home or other longer-term care setting for the first time,” says Tiffany Radcliff, PhD, a health economist in the department of health policy and management at Texas A&M University School of Public Health in College Station. “With this in mind, hospitalists working in PAC have the opportunity to address each patient’s ongoing care needs and prepare them for their next setting. Hospitalists can manage medication or other care regimen changes that resulted from an inpatient stay, reinforce discharge instructions to the patient and their caregivers, and identify any other issues with continuing care that need to be addressed before discharge to the next care setting.”
Transitioning Care
Even if a hospitalist is not employed at a PAC, it’s important that they know something about them.
“As patients are moved downstream earlier, hospitalists are being asked to help make a judgment regarding when and where an inpatient is transitioned,” Dr. Muldoon says. As organizations move toward becoming fully risk capable, it is necessary to develop referral networks of high-quality PAC providers to achieve the best clinical outcomes, reduce readmissions, and lower costs.2“Therefore, hospitalists should have a working knowledge of the different sites of service as well as some opinion on the suitability of available options in their community,” Dr. Muldoon says. “The hospitalist can also help to educate the hospitalized patient on what to expect at a PAC.”
If a patient is inappropriately prepared for the PAC setting, it could lead to incomplete management of their condition, which ultimately could lead to readmission.
“When hospitalists know how care is provided in a PAC setting, they are better able to ensure a smoother transition of care between settings,” says Tochi Iroku-Malize, MD, MPH, MBA, FAAFP, SFHM, chair of family medicine at Northwell Health in Long Island, N.Y. “This will ultimately prevent unnecessary readmissions.”
Further, the quality metrics that hospitals and thereby hospitalists are judged by no longer end at the hospital’s exit.
“The ownership of acute-care outcomes requires extending the accountability to outside of the institution’s four walls,” Dr. Mathew says. “The inpatient team needs to place great importance on the transition of care and the subsequent quality of that care when the patient is discharged.”
Robert W. Harrington Jr., MD, SFHM, chief medical officer of Plano, Texas–based Reliant Post-Acute Care Solutions and former SHM president, says the health system landscapes are pushing HM beyond the hospitals’ walls.
How PAC settings differ from hospitals
Practicing in PAC has some important nuances that hospitalists from short-term acute care need to get accustomed to, Dr. Muldoon says. Primarily, the diagnostic capabilities are much more limited, as is the presence of high-level staffing. Further, patients are less resilient to medication changes and interventions, so changes need to be done gradually.
“Hospitalists who try to practice acute-care medicine in a PAC setting may become frustrated by the length of time it takes to do a work-up, get a consultation, and respond to a patient’s change of condition,” Dr. Muldoon says. “Nonetheless, hospitalists can overcome this once recognizing this mind shift.”
According to Dr. Harrington, another challenge hospitalists may face is the inability of the hospital’s and PAC facility’s IT platforms to exchange electronic information.
“The major vendors on both sides need to figure out an interoperability strategy,” he says. “Currently, it often takes 1-3 days to receive a new patient’s discharge summary. The summary may consist of a stack of paper that takes significant time to sort through and requires the PAC facility to perform duplicate data entry. It’s a very highly inefficient process that opens up the doors to mistakes and errors of omission and commission that can result in bad patient outcomes.”
Arif Nazir, MD, CMD, FACP, AGSF, chief medical officer of Signature HealthCARE and president of SHC Medical Partners, both in Louisville, Ky., cites additional reasons the lack of seamless communication between a hospital and PAC facility is problematic. “I see physicians order laboratory tests and investigations that were already done in the hospital because they didn’t know they were already performed or never received the results,” he says. “Similarly, I see patients continue to take medications prescribed in the hospital long term even though they were only supposed to take them short term. I’ve also seen patients come to a PAC setting from a hospital without any formal understanding of their rehabilitative period and expectations for recovery.”
What’s ahead?
Looking to the future, Surafel Tsega, MD, clinical instructor at Mount Sinai Hospital in New York, says he thinks there will be a move toward greater collaboration among inpatient and PAC facilities, particularly in the discharge process, given that hospitals have an added incentive to ensure safe transitions because reimbursement from the Centers for Medicare & Medicaid Services is tied to readmissions and there are penalties for readmission. This involves more comprehensive planning regarding “warm handoffs” (e.g., real-time discussions with PAC providers about a patient’s hospital course and plan of care upon discharge), transferring of information, and so forth.
And while it can still be challenging to identify high-risk patients or determine the intensity and duration of their care, Dr. Mathew says risk-stratification tools and care pathways are continually being refined to maximize value with the limited resources available. In addition, with an increased emphasis on employing a team approach to care, there will be better integration of non-medical services to address the social determinants of health, which play significant roles in overall health and healing.
“Working with community-based organizations for this purpose will be a valuable tool for any of the population health–based initiatives,” he says.
Dr. Muldoon says he believes healthcare reform will increasingly view an inpatient admission as something to be avoided.
“If hospitalization can’t be avoided, then it should be shortened as much as possible,” he says. “This will shift inpatient care into LTACHs, SNFs, and IRFs. Hospitalists would be wise to follow patients into those settings as traditional inpatient census is reduced. This will take a few years, so hospitalists should start now in preparing for that downstream transition of individuals who were previously inpatients.”
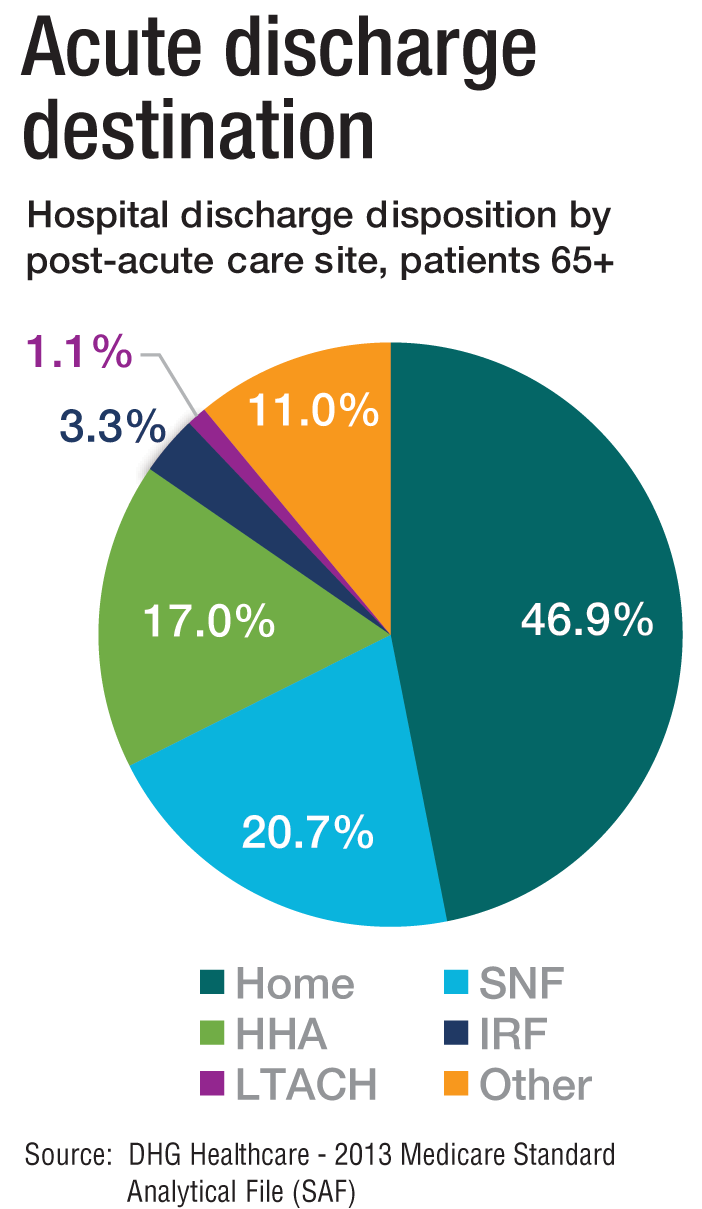
The cost of care, and other PAC facts and figures
The amount of money that Medicare spends on post-acute care (PAC) has been increasing. In 2012, 12.6% of Medicare beneficiaries used some form of PAC, costing $62 billion.2 That amounts to the Centers for Medicare & Medicaid Services spending close to 25% of Medicare beneficiary expenses on PAC, a 133% increase from 2001 to 2012. Among the different types, $30.4 billion was spent on skilled nursing facilities (SNFs), $18.6 billion on home health, and $13.1 billion on long-term acute care (LTAC) and acute-care rehabilitation.2
It’s also been reported that after short-term acute-care hospitalization, about one in five Medicare beneficiaries requires continued specialized treatment in one of the three typical Medicare PAC settings: inpatient rehabilitation facilities (IRFs), LTAC hospitals, and SNFs.3
What’s more, hospital readmission nearly doubles the cost of an episode, so the financial implications for organizations operating in risk-bearing arrangements are significant. In 2013, 2,213 hospitals were charged $280 million in readmission penalties.2
References
1. The role of post-acute care in new care delivery models. American Hospital Association website. Available at: http://www.aha.org/research/reports/tw/15dec-tw-postacute.pdf. Accessed Nov. 7, 2016.
2. Post-acute care integration: Today and in the future. DHG Healthcare website. Available at: http://www2.dhgllp.com/res_pubs/HCG-Post-Acute-Care-Integration.pdf. Accessed Nov. 7, 2016.
3. Overview: Post-acute care transitions toolkit. Society for Hospital Medicine website. Available at: http://www.hospitalmedicine.org/Web/Quality___Innovation/Implementation_Toolkit/pact/Overview_PACT.aspx?hkey=dea3da3c-8620-46db-a00f-89f07f021958. Accessed Nov. 10, 2016.
The definition of “hospitalist,” according to the SHM website, is a clinician “dedicated to delivering comprehensive medical care to hospitalized patients.” For years, the hospital setting was the specialties’ identifier. But as hospitalists’ scope has expanded, and post-acute care (PAC) in the United States has grown, more hospitalists are extending their roles into this space.
PAC today is more than the traditional nursing home, according to Manoj K. Mathew, MD, SFHM, national medical director of Agilon Health in Los Angeles.
Many of those expanded settings Dr. Mathew describes emerged as a result of the Affordable Care Act. Since its enactment in 2010, the ACA has heightened providers’ focus on the “Triple Aim” of improving the patient experience (including quality and satisfaction), improving the health of populations, and reducing the per capita cost of healthcare.1 Vishal Kuchaculla, MD, New England regional post-acute medical director of Knoxville,Tenn.-based TeamHealth, says new service lines also developed as Medicare clamped down on long-term inpatient hospital stays by giving financial impetus to discharge patients as soon as possible.
“Over the last few years, there’s been a major shift from fee-for-service to risk-based payment models,” Dr. Kuchaculla says. “The government’s financial incentives are driving outcomes to improve performance initiatives.”
“Today, LTACHs can be used as substitutes for short-term acute care,” says Sean R. Muldoon, MD, MPH, FCCP, chief medical officer of Kindred Healthcare in Louisville, Ky., and former chair of SHM’s Post-Acute Care Committee. “This means that a patient can be directly admitted from their home to an LTACH. In fact, many hospice and home-care patients are referred from physicians’ offices without a preceding hospitalization.”

Hospitalists can fill a need

More hospitalists are working in PACs for a number of reasons. Dr. Mathew says PAC facilities and services have “typically lacked the clinical structure and processes to obtain the results that patients and payors expect.
“These deficits needed to be quickly remedied as patients discharged from hospitals have increased acuity and higher disease burdens,” he adds. “Hospitalists were the natural choice to fill roles requiring their expertise and experience.”
Dr. Muldoon considers the expanded scope of practice into PACs an additional layer to hospital medicine’s value proposition to the healthcare system.
“As experts in the management of inpatient populations, it’s natural for hospitalists to expand to other facilities with inpatient-like populations,” he says, noting SNFs are the most popular choice, with IRFs and LTACHs also being common places to work. Few hospitalists work in home care or hospice.
PAC settings are designed to help patients who are transitioning from an inpatient setting back to their home or other setting.
“Many patients go home after a SNF stay, while others will move to a nursing home or other longer-term care setting for the first time,” says Tiffany Radcliff, PhD, a health economist in the department of health policy and management at Texas A&M University School of Public Health in College Station. “With this in mind, hospitalists working in PAC have the opportunity to address each patient’s ongoing care needs and prepare them for their next setting. Hospitalists can manage medication or other care regimen changes that resulted from an inpatient stay, reinforce discharge instructions to the patient and their caregivers, and identify any other issues with continuing care that need to be addressed before discharge to the next care setting.”
Transitioning Care
Even if a hospitalist is not employed at a PAC, it’s important that they know something about them.
“As patients are moved downstream earlier, hospitalists are being asked to help make a judgment regarding when and where an inpatient is transitioned,” Dr. Muldoon says. As organizations move toward becoming fully risk capable, it is necessary to develop referral networks of high-quality PAC providers to achieve the best clinical outcomes, reduce readmissions, and lower costs.2“Therefore, hospitalists should have a working knowledge of the different sites of service as well as some opinion on the suitability of available options in their community,” Dr. Muldoon says. “The hospitalist can also help to educate the hospitalized patient on what to expect at a PAC.”
If a patient is inappropriately prepared for the PAC setting, it could lead to incomplete management of their condition, which ultimately could lead to readmission.
“When hospitalists know how care is provided in a PAC setting, they are better able to ensure a smoother transition of care between settings,” says Tochi Iroku-Malize, MD, MPH, MBA, FAAFP, SFHM, chair of family medicine at Northwell Health in Long Island, N.Y. “This will ultimately prevent unnecessary readmissions.”
Further, the quality metrics that hospitals and thereby hospitalists are judged by no longer end at the hospital’s exit.
“The ownership of acute-care outcomes requires extending the accountability to outside of the institution’s four walls,” Dr. Mathew says. “The inpatient team needs to place great importance on the transition of care and the subsequent quality of that care when the patient is discharged.”
Robert W. Harrington Jr., MD, SFHM, chief medical officer of Plano, Texas–based Reliant Post-Acute Care Solutions and former SHM president, says the health system landscapes are pushing HM beyond the hospitals’ walls.
How PAC settings differ from hospitals
Practicing in PAC has some important nuances that hospitalists from short-term acute care need to get accustomed to, Dr. Muldoon says. Primarily, the diagnostic capabilities are much more limited, as is the presence of high-level staffing. Further, patients are less resilient to medication changes and interventions, so changes need to be done gradually.
“Hospitalists who try to practice acute-care medicine in a PAC setting may become frustrated by the length of time it takes to do a work-up, get a consultation, and respond to a patient’s change of condition,” Dr. Muldoon says. “Nonetheless, hospitalists can overcome this once recognizing this mind shift.”
According to Dr. Harrington, another challenge hospitalists may face is the inability of the hospital’s and PAC facility’s IT platforms to exchange electronic information.
“The major vendors on both sides need to figure out an interoperability strategy,” he says. “Currently, it often takes 1-3 days to receive a new patient’s discharge summary. The summary may consist of a stack of paper that takes significant time to sort through and requires the PAC facility to perform duplicate data entry. It’s a very highly inefficient process that opens up the doors to mistakes and errors of omission and commission that can result in bad patient outcomes.”
Arif Nazir, MD, CMD, FACP, AGSF, chief medical officer of Signature HealthCARE and president of SHC Medical Partners, both in Louisville, Ky., cites additional reasons the lack of seamless communication between a hospital and PAC facility is problematic. “I see physicians order laboratory tests and investigations that were already done in the hospital because they didn’t know they were already performed or never received the results,” he says. “Similarly, I see patients continue to take medications prescribed in the hospital long term even though they were only supposed to take them short term. I’ve also seen patients come to a PAC setting from a hospital without any formal understanding of their rehabilitative period and expectations for recovery.”
What’s ahead?
Looking to the future, Surafel Tsega, MD, clinical instructor at Mount Sinai Hospital in New York, says he thinks there will be a move toward greater collaboration among inpatient and PAC facilities, particularly in the discharge process, given that hospitals have an added incentive to ensure safe transitions because reimbursement from the Centers for Medicare & Medicaid Services is tied to readmissions and there are penalties for readmission. This involves more comprehensive planning regarding “warm handoffs” (e.g., real-time discussions with PAC providers about a patient’s hospital course and plan of care upon discharge), transferring of information, and so forth.
And while it can still be challenging to identify high-risk patients or determine the intensity and duration of their care, Dr. Mathew says risk-stratification tools and care pathways are continually being refined to maximize value with the limited resources available. In addition, with an increased emphasis on employing a team approach to care, there will be better integration of non-medical services to address the social determinants of health, which play significant roles in overall health and healing.
“Working with community-based organizations for this purpose will be a valuable tool for any of the population health–based initiatives,” he says.
Dr. Muldoon says he believes healthcare reform will increasingly view an inpatient admission as something to be avoided.
“If hospitalization can’t be avoided, then it should be shortened as much as possible,” he says. “This will shift inpatient care into LTACHs, SNFs, and IRFs. Hospitalists would be wise to follow patients into those settings as traditional inpatient census is reduced. This will take a few years, so hospitalists should start now in preparing for that downstream transition of individuals who were previously inpatients.”
The cost of care, and other PAC facts and figures
The amount of money that Medicare spends on post-acute care (PAC) has been increasing. In 2012, 12.6% of Medicare beneficiaries used some form of PAC, costing $62 billion.2 That amounts to the Centers for Medicare & Medicaid Services spending close to 25% of Medicare beneficiary expenses on PAC, a 133% increase from 2001 to 2012. Among the different types, $30.4 billion was spent on skilled nursing facilities (SNFs), $18.6 billion on home health, and $13.1 billion on long-term acute care (LTAC) and acute-care rehabilitation.2
It’s also been reported that after short-term acute-care hospitalization, about one in five Medicare beneficiaries requires continued specialized treatment in one of the three typical Medicare PAC settings: inpatient rehabilitation facilities (IRFs), LTAC hospitals, and SNFs.3
What’s more, hospital readmission nearly doubles the cost of an episode, so the financial implications for organizations operating in risk-bearing arrangements are significant. In 2013, 2,213 hospitals were charged $280 million in readmission penalties.2
References
1. The role of post-acute care in new care delivery models. American Hospital Association website. Available at: http://www.aha.org/research/reports/tw/15dec-tw-postacute.pdf. Accessed Nov. 7, 2016.
2. Post-acute care integration: Today and in the future. DHG Healthcare website. Available at: http://www2.dhgllp.com/res_pubs/HCG-Post-Acute-Care-Integration.pdf. Accessed Nov. 7, 2016.
3. Overview: Post-acute care transitions toolkit. Society for Hospital Medicine website. Available at: http://www.hospitalmedicine.org/Web/Quality___Innovation/Implementation_Toolkit/pact/Overview_PACT.aspx?hkey=dea3da3c-8620-46db-a00f-89f07f021958. Accessed Nov. 10, 2016.
The definition of “hospitalist,” according to the SHM website, is a clinician “dedicated to delivering comprehensive medical care to hospitalized patients.” For years, the hospital setting was the specialties’ identifier. But as hospitalists’ scope has expanded, and post-acute care (PAC) in the United States has grown, more hospitalists are extending their roles into this space.
PAC today is more than the traditional nursing home, according to Manoj K. Mathew, MD, SFHM, national medical director of Agilon Health in Los Angeles.
Many of those expanded settings Dr. Mathew describes emerged as a result of the Affordable Care Act. Since its enactment in 2010, the ACA has heightened providers’ focus on the “Triple Aim” of improving the patient experience (including quality and satisfaction), improving the health of populations, and reducing the per capita cost of healthcare.1 Vishal Kuchaculla, MD, New England regional post-acute medical director of Knoxville,Tenn.-based TeamHealth, says new service lines also developed as Medicare clamped down on long-term inpatient hospital stays by giving financial impetus to discharge patients as soon as possible.
“Over the last few years, there’s been a major shift from fee-for-service to risk-based payment models,” Dr. Kuchaculla says. “The government’s financial incentives are driving outcomes to improve performance initiatives.”
“Today, LTACHs can be used as substitutes for short-term acute care,” says Sean R. Muldoon, MD, MPH, FCCP, chief medical officer of Kindred Healthcare in Louisville, Ky., and former chair of SHM’s Post-Acute Care Committee. “This means that a patient can be directly admitted from their home to an LTACH. In fact, many hospice and home-care patients are referred from physicians’ offices without a preceding hospitalization.”

Hospitalists can fill a need

More hospitalists are working in PACs for a number of reasons. Dr. Mathew says PAC facilities and services have “typically lacked the clinical structure and processes to obtain the results that patients and payors expect.
“These deficits needed to be quickly remedied as patients discharged from hospitals have increased acuity and higher disease burdens,” he adds. “Hospitalists were the natural choice to fill roles requiring their expertise and experience.”
Dr. Muldoon considers the expanded scope of practice into PACs an additional layer to hospital medicine’s value proposition to the healthcare system.
“As experts in the management of inpatient populations, it’s natural for hospitalists to expand to other facilities with inpatient-like populations,” he says, noting SNFs are the most popular choice, with IRFs and LTACHs also being common places to work. Few hospitalists work in home care or hospice.
PAC settings are designed to help patients who are transitioning from an inpatient setting back to their home or other setting.
“Many patients go home after a SNF stay, while others will move to a nursing home or other longer-term care setting for the first time,” says Tiffany Radcliff, PhD, a health economist in the department of health policy and management at Texas A&M University School of Public Health in College Station. “With this in mind, hospitalists working in PAC have the opportunity to address each patient’s ongoing care needs and prepare them for their next setting. Hospitalists can manage medication or other care regimen changes that resulted from an inpatient stay, reinforce discharge instructions to the patient and their caregivers, and identify any other issues with continuing care that need to be addressed before discharge to the next care setting.”
Transitioning Care
Even if a hospitalist is not employed at a PAC, it’s important that they know something about them.
“As patients are moved downstream earlier, hospitalists are being asked to help make a judgment regarding when and where an inpatient is transitioned,” Dr. Muldoon says. As organizations move toward becoming fully risk capable, it is necessary to develop referral networks of high-quality PAC providers to achieve the best clinical outcomes, reduce readmissions, and lower costs.2“Therefore, hospitalists should have a working knowledge of the different sites of service as well as some opinion on the suitability of available options in their community,” Dr. Muldoon says. “The hospitalist can also help to educate the hospitalized patient on what to expect at a PAC.”
If a patient is inappropriately prepared for the PAC setting, it could lead to incomplete management of their condition, which ultimately could lead to readmission.
“When hospitalists know how care is provided in a PAC setting, they are better able to ensure a smoother transition of care between settings,” says Tochi Iroku-Malize, MD, MPH, MBA, FAAFP, SFHM, chair of family medicine at Northwell Health in Long Island, N.Y. “This will ultimately prevent unnecessary readmissions.”
Further, the quality metrics that hospitals and thereby hospitalists are judged by no longer end at the hospital’s exit.
“The ownership of acute-care outcomes requires extending the accountability to outside of the institution’s four walls,” Dr. Mathew says. “The inpatient team needs to place great importance on the transition of care and the subsequent quality of that care when the patient is discharged.”
Robert W. Harrington Jr., MD, SFHM, chief medical officer of Plano, Texas–based Reliant Post-Acute Care Solutions and former SHM president, says the health system landscapes are pushing HM beyond the hospitals’ walls.
How PAC settings differ from hospitals
Practicing in PAC has some important nuances that hospitalists from short-term acute care need to get accustomed to, Dr. Muldoon says. Primarily, the diagnostic capabilities are much more limited, as is the presence of high-level staffing. Further, patients are less resilient to medication changes and interventions, so changes need to be done gradually.
“Hospitalists who try to practice acute-care medicine in a PAC setting may become frustrated by the length of time it takes to do a work-up, get a consultation, and respond to a patient’s change of condition,” Dr. Muldoon says. “Nonetheless, hospitalists can overcome this once recognizing this mind shift.”
According to Dr. Harrington, another challenge hospitalists may face is the inability of the hospital’s and PAC facility’s IT platforms to exchange electronic information.
“The major vendors on both sides need to figure out an interoperability strategy,” he says. “Currently, it often takes 1-3 days to receive a new patient’s discharge summary. The summary may consist of a stack of paper that takes significant time to sort through and requires the PAC facility to perform duplicate data entry. It’s a very highly inefficient process that opens up the doors to mistakes and errors of omission and commission that can result in bad patient outcomes.”
Arif Nazir, MD, CMD, FACP, AGSF, chief medical officer of Signature HealthCARE and president of SHC Medical Partners, both in Louisville, Ky., cites additional reasons the lack of seamless communication between a hospital and PAC facility is problematic. “I see physicians order laboratory tests and investigations that were already done in the hospital because they didn’t know they were already performed or never received the results,” he says. “Similarly, I see patients continue to take medications prescribed in the hospital long term even though they were only supposed to take them short term. I’ve also seen patients come to a PAC setting from a hospital without any formal understanding of their rehabilitative period and expectations for recovery.”
What’s ahead?
Looking to the future, Surafel Tsega, MD, clinical instructor at Mount Sinai Hospital in New York, says he thinks there will be a move toward greater collaboration among inpatient and PAC facilities, particularly in the discharge process, given that hospitals have an added incentive to ensure safe transitions because reimbursement from the Centers for Medicare & Medicaid Services is tied to readmissions and there are penalties for readmission. This involves more comprehensive planning regarding “warm handoffs” (e.g., real-time discussions with PAC providers about a patient’s hospital course and plan of care upon discharge), transferring of information, and so forth.
And while it can still be challenging to identify high-risk patients or determine the intensity and duration of their care, Dr. Mathew says risk-stratification tools and care pathways are continually being refined to maximize value with the limited resources available. In addition, with an increased emphasis on employing a team approach to care, there will be better integration of non-medical services to address the social determinants of health, which play significant roles in overall health and healing.
“Working with community-based organizations for this purpose will be a valuable tool for any of the population health–based initiatives,” he says.
Dr. Muldoon says he believes healthcare reform will increasingly view an inpatient admission as something to be avoided.
“If hospitalization can’t be avoided, then it should be shortened as much as possible,” he says. “This will shift inpatient care into LTACHs, SNFs, and IRFs. Hospitalists would be wise to follow patients into those settings as traditional inpatient census is reduced. This will take a few years, so hospitalists should start now in preparing for that downstream transition of individuals who were previously inpatients.”
The cost of care, and other PAC facts and figures
The amount of money that Medicare spends on post-acute care (PAC) has been increasing. In 2012, 12.6% of Medicare beneficiaries used some form of PAC, costing $62 billion.2 That amounts to the Centers for Medicare & Medicaid Services spending close to 25% of Medicare beneficiary expenses on PAC, a 133% increase from 2001 to 2012. Among the different types, $30.4 billion was spent on skilled nursing facilities (SNFs), $18.6 billion on home health, and $13.1 billion on long-term acute care (LTAC) and acute-care rehabilitation.2
It’s also been reported that after short-term acute-care hospitalization, about one in five Medicare beneficiaries requires continued specialized treatment in one of the three typical Medicare PAC settings: inpatient rehabilitation facilities (IRFs), LTAC hospitals, and SNFs.3
What’s more, hospital readmission nearly doubles the cost of an episode, so the financial implications for organizations operating in risk-bearing arrangements are significant. In 2013, 2,213 hospitals were charged $280 million in readmission penalties.2
References
1. The role of post-acute care in new care delivery models. American Hospital Association website. Available at: http://www.aha.org/research/reports/tw/15dec-tw-postacute.pdf. Accessed Nov. 7, 2016.
2. Post-acute care integration: Today and in the future. DHG Healthcare website. Available at: http://www2.dhgllp.com/res_pubs/HCG-Post-Acute-Care-Integration.pdf. Accessed Nov. 7, 2016.
3. Overview: Post-acute care transitions toolkit. Society for Hospital Medicine website. Available at: http://www.hospitalmedicine.org/Web/Quality___Innovation/Implementation_Toolkit/pact/Overview_PACT.aspx?hkey=dea3da3c-8620-46db-a00f-89f07f021958. Accessed Nov. 10, 2016.
Study IDs Immune Abnormality Possibly Causing Long COVID
Swiss scientists have identified immune system abnormalities in patients with long COVID that might open the door to new diagnostic tests and treatments.
The researchers found that a group of proteins in the blood that are part of the body’s immune response called the “complement system” are not working properly in patients with long COVID.
Blood samples turned up important differences between those who recovered from COVID and those who did not. These differences might be used as biomarkers to diagnose long COVID and might even point the way to new treatments for the condition, the researchers said.
By testing for 6500 blood proteins in about 300 patients, the Swiss researchers found that dysfunctional complement system proteins could possibly explain fatigue and “smoldering inflammation,” said Onur Boyman, MD, a professor of immunology from University Hospital Zurich in Zurich, Switzerland.
Long COVID has been linked to hundreds of symptoms including brain fog, chronic fatigue, pain, and digestive issues. Various factors drive the condition and likely work with one another other, said David Putrino, PhD, from the Icahn School of Medicine at Mount Sinai in New York City. The Swiss study is useful because “we’re trying to best understand how we can explain all of this far-reaching pathobiology,” he said.
Testing Across Continents
Dr. Boyman’s team collected blood samples from people with COVID in Europe and New York and tracked them. They compared those who developed long COVID with those who did not. One protein that was most unique to patients with long COVID is a blood complement that activates the immune system, Dr. Boyman said. But in people with long COVID, the immune response stays activated after the virus is gone. He described the response as “smoldering inflammation” in multiple organs, including the lungs and the gastrointestinal system.
The complement system also plays a role in clearing the body of dead cells. If the cells “lie around too much,” they can trigger an immune response, he said.
That may explain exercise intolerance in people with long COVID, Dr. Boyman said. Some people with long COVID have inflammation in the epithelium — the inner layer of their blood vessels. This would make it harder for the circulatory systems to recover from exercise, Dr. Boyman said.
“We think this regulated complement system is actually quite a central piece of the puzzle,” he said.
The Microclot Connection
The findings also support past research linking blood clots to long COVID. He suggested that clinicians and researchers consider testing drugs that regulate or inhibit the complementary system as a treatment of long COVID. Dr. Boyman said they are currently used for rare immune diseases.
Resia Pretorius, PhD, a professor of physiological sciences at Stellenbosch University in Stellenbosch, South Africa, said scientists studying the role of microclots in patients with long COVID often see complementary proteins inside the clots, so it has already been associated with long COVID. But she likened this clotting process to a garbage can that “just rolls along and collects everything that gets in its way. I think they are actively driving inflammation and disease.”
One factor complicating long COVID diagnosis and treatment is that it is a complex condition that involves multiple organ systems. That’s why the latest research suggests an underlying driver for the multiple symptoms of long COVID, Dr. Putrino said.
“Not every person has every symptom; not every person has every organ system affected,” Dr. Putrino said. “Whatever is happening is decided across the whole body.”
Research Offers New Direction
The Swiss paper contributes to the effort to identify systemic issues contributing to long COVID. It gives researchers one more thing to test for and link to specific, long COVID symptoms, opening the door to new treatments, Dr. Putrino said.
He doesn’t think the study supports treating the complement dysfunction if researchers don’t know what’s driving it. It may be complicated by the body’s failure to clear the virus completely, he said.
Dr. Pretorius recommended doctors test patients with long COVID for specific symptoms that may be treated using existing therapies. “If you think your patient had vascular pathology, you can test for it,” she said.
Some patients have found certain supplements and over-the-counter products helpful, she said. Among them: Coenzyme Q 10 and clot-busters such as streptokinase and Nattokinase (though she noted some doctors may not be comfortable with supplements).
“It’s the only thing we have until we’ve got trials,” she said.
Dr. Putrino said more research is needed to identify potential root causes and symptoms. A common refrain, but the only thing that will lead to specific treatments.
A version of this article appeared on Medscape.com.
Swiss scientists have identified immune system abnormalities in patients with long COVID that might open the door to new diagnostic tests and treatments.
The researchers found that a group of proteins in the blood that are part of the body’s immune response called the “complement system” are not working properly in patients with long COVID.
Blood samples turned up important differences between those who recovered from COVID and those who did not. These differences might be used as biomarkers to diagnose long COVID and might even point the way to new treatments for the condition, the researchers said.
By testing for 6500 blood proteins in about 300 patients, the Swiss researchers found that dysfunctional complement system proteins could possibly explain fatigue and “smoldering inflammation,” said Onur Boyman, MD, a professor of immunology from University Hospital Zurich in Zurich, Switzerland.
Long COVID has been linked to hundreds of symptoms including brain fog, chronic fatigue, pain, and digestive issues. Various factors drive the condition and likely work with one another other, said David Putrino, PhD, from the Icahn School of Medicine at Mount Sinai in New York City. The Swiss study is useful because “we’re trying to best understand how we can explain all of this far-reaching pathobiology,” he said.
Testing Across Continents
Dr. Boyman’s team collected blood samples from people with COVID in Europe and New York and tracked them. They compared those who developed long COVID with those who did not. One protein that was most unique to patients with long COVID is a blood complement that activates the immune system, Dr. Boyman said. But in people with long COVID, the immune response stays activated after the virus is gone. He described the response as “smoldering inflammation” in multiple organs, including the lungs and the gastrointestinal system.
The complement system also plays a role in clearing the body of dead cells. If the cells “lie around too much,” they can trigger an immune response, he said.
That may explain exercise intolerance in people with long COVID, Dr. Boyman said. Some people with long COVID have inflammation in the epithelium — the inner layer of their blood vessels. This would make it harder for the circulatory systems to recover from exercise, Dr. Boyman said.
“We think this regulated complement system is actually quite a central piece of the puzzle,” he said.
The Microclot Connection
The findings also support past research linking blood clots to long COVID. He suggested that clinicians and researchers consider testing drugs that regulate or inhibit the complementary system as a treatment of long COVID. Dr. Boyman said they are currently used for rare immune diseases.
Resia Pretorius, PhD, a professor of physiological sciences at Stellenbosch University in Stellenbosch, South Africa, said scientists studying the role of microclots in patients with long COVID often see complementary proteins inside the clots, so it has already been associated with long COVID. But she likened this clotting process to a garbage can that “just rolls along and collects everything that gets in its way. I think they are actively driving inflammation and disease.”
One factor complicating long COVID diagnosis and treatment is that it is a complex condition that involves multiple organ systems. That’s why the latest research suggests an underlying driver for the multiple symptoms of long COVID, Dr. Putrino said.
“Not every person has every symptom; not every person has every organ system affected,” Dr. Putrino said. “Whatever is happening is decided across the whole body.”
Research Offers New Direction
The Swiss paper contributes to the effort to identify systemic issues contributing to long COVID. It gives researchers one more thing to test for and link to specific, long COVID symptoms, opening the door to new treatments, Dr. Putrino said.
He doesn’t think the study supports treating the complement dysfunction if researchers don’t know what’s driving it. It may be complicated by the body’s failure to clear the virus completely, he said.
Dr. Pretorius recommended doctors test patients with long COVID for specific symptoms that may be treated using existing therapies. “If you think your patient had vascular pathology, you can test for it,” she said.
Some patients have found certain supplements and over-the-counter products helpful, she said. Among them: Coenzyme Q 10 and clot-busters such as streptokinase and Nattokinase (though she noted some doctors may not be comfortable with supplements).
“It’s the only thing we have until we’ve got trials,” she said.
Dr. Putrino said more research is needed to identify potential root causes and symptoms. A common refrain, but the only thing that will lead to specific treatments.
A version of this article appeared on Medscape.com.
Swiss scientists have identified immune system abnormalities in patients with long COVID that might open the door to new diagnostic tests and treatments.
The researchers found that a group of proteins in the blood that are part of the body’s immune response called the “complement system” are not working properly in patients with long COVID.
Blood samples turned up important differences between those who recovered from COVID and those who did not. These differences might be used as biomarkers to diagnose long COVID and might even point the way to new treatments for the condition, the researchers said.
By testing for 6500 blood proteins in about 300 patients, the Swiss researchers found that dysfunctional complement system proteins could possibly explain fatigue and “smoldering inflammation,” said Onur Boyman, MD, a professor of immunology from University Hospital Zurich in Zurich, Switzerland.
Long COVID has been linked to hundreds of symptoms including brain fog, chronic fatigue, pain, and digestive issues. Various factors drive the condition and likely work with one another other, said David Putrino, PhD, from the Icahn School of Medicine at Mount Sinai in New York City. The Swiss study is useful because “we’re trying to best understand how we can explain all of this far-reaching pathobiology,” he said.
Testing Across Continents
Dr. Boyman’s team collected blood samples from people with COVID in Europe and New York and tracked them. They compared those who developed long COVID with those who did not. One protein that was most unique to patients with long COVID is a blood complement that activates the immune system, Dr. Boyman said. But in people with long COVID, the immune response stays activated after the virus is gone. He described the response as “smoldering inflammation” in multiple organs, including the lungs and the gastrointestinal system.
The complement system also plays a role in clearing the body of dead cells. If the cells “lie around too much,” they can trigger an immune response, he said.
That may explain exercise intolerance in people with long COVID, Dr. Boyman said. Some people with long COVID have inflammation in the epithelium — the inner layer of their blood vessels. This would make it harder for the circulatory systems to recover from exercise, Dr. Boyman said.
“We think this regulated complement system is actually quite a central piece of the puzzle,” he said.
The Microclot Connection
The findings also support past research linking blood clots to long COVID. He suggested that clinicians and researchers consider testing drugs that regulate or inhibit the complementary system as a treatment of long COVID. Dr. Boyman said they are currently used for rare immune diseases.
Resia Pretorius, PhD, a professor of physiological sciences at Stellenbosch University in Stellenbosch, South Africa, said scientists studying the role of microclots in patients with long COVID often see complementary proteins inside the clots, so it has already been associated with long COVID. But she likened this clotting process to a garbage can that “just rolls along and collects everything that gets in its way. I think they are actively driving inflammation and disease.”
One factor complicating long COVID diagnosis and treatment is that it is a complex condition that involves multiple organ systems. That’s why the latest research suggests an underlying driver for the multiple symptoms of long COVID, Dr. Putrino said.
“Not every person has every symptom; not every person has every organ system affected,” Dr. Putrino said. “Whatever is happening is decided across the whole body.”
Research Offers New Direction
The Swiss paper contributes to the effort to identify systemic issues contributing to long COVID. It gives researchers one more thing to test for and link to specific, long COVID symptoms, opening the door to new treatments, Dr. Putrino said.
He doesn’t think the study supports treating the complement dysfunction if researchers don’t know what’s driving it. It may be complicated by the body’s failure to clear the virus completely, he said.
Dr. Pretorius recommended doctors test patients with long COVID for specific symptoms that may be treated using existing therapies. “If you think your patient had vascular pathology, you can test for it,” she said.
Some patients have found certain supplements and over-the-counter products helpful, she said. Among them: Coenzyme Q 10 and clot-busters such as streptokinase and Nattokinase (though she noted some doctors may not be comfortable with supplements).
“It’s the only thing we have until we’ve got trials,” she said.
Dr. Putrino said more research is needed to identify potential root causes and symptoms. A common refrain, but the only thing that will lead to specific treatments.
A version of this article appeared on Medscape.com.
Monoclonal Antibodies: A New Treatment for Long COVID?
A treatment used to treat acute COVID-19 infection has also been found to be effective against long COVID, a new small study has found. The research, which assessed the benefits of monoclonal antibodies, suggests relief may finally be ahead for millions of Americans with long COVID for whom treatment has remained elusive.
The study, published in the American Journal of Emergency Medicine, found
“We were struck by how rapid and complete the remissions were,” said study coauthor Paul Pepe, MD, MPH, a professor of management, policy, and community health at the School of Public Health at the University of Texas Health Sciences Center. “We found that no matter how long the patients were sick for — whether it was 5, 8, or 18 months — within 5 days, they appeared to be completely cured.”
All three patients had been initially infected with COVID-19 early in the pandemic, in 2020 or the first half of 2021. They were given Regeneron either after a reinfection or exposure to COVID-19, as a preventative, at state-run COVID clinics in Florida.
“In each case, the infusions were given to help prevent their long COVID from worsening,” said Dr. Pepe.
The researchers collected medical histories for all three patients, asking about symptoms such as physical fatigue, exercise intolerance, chest pain, heart palpitations, shortness of breath, cognitive fatigue, and memory problems. They asked patients to rate symptoms pre-COVID (baseline), during the long COVID phase, post-vaccine, and finally a week after their monoclonal antibody treatment. They also interviewed family members.
They found that across the board, symptoms improved significantly and often completely vanished. Their loved ones corroborated these reports as well.
One of the patients, a 63-year-old Floridian woman, came down with a mild case of COVID-19 at the start of the pandemic in March 2020 that lasted about 2 weeks. But several weeks later, she developed extreme, debilitating fatigue, along with chest pain and shortness of breath.
“I was chasing my 6-pound Yorkie one day after she got loose, and I was struck with such intense chest pain I fell down,” the woman, asking not to be identified, said in an interview.
Her symptoms progressed to the point where she no longer felt safe babysitting her grandchildren or driving to the grocery store.
“My short-term memory was completely gone. I couldn’t even read more than a paragraph at a time,” she said.
When she was exposed to COVID-19 in October 2021, her doctor suggested Regeneron as a preventative. She agreed to it.
“I was terrified that a second round would leave me permanently disabled and stuck in bed for the rest of my life,” she said.
About 4 days after her monoclonal antibody treatment, she noticed that some of the brain fog that had persisted after COVID was lifting.
“By day 5, it felt almost like a heavy-weighted blanket had been lifted off of me,” she recalled. “I was able to take my dog for a walk and go to the grocery store. It felt like I had gone from 0 to 100. As quickly as I went downhill, I quickly went back up.”
Reasons for Recovery
Researchers have come up with a few theories about why monoclonal antibodies may help treat long COVID, said study coauthor Aileen Marty, MD, professor of translational medicine at the Herbert Wertheim College of Medicine at Florida International University. Among them:
- It stimulates the body to fight off any residual virus. “We suspect that many of these patients simply have levels of virus that are so low they can’t be picked up by conventional testing,” said Dr. Marty. “The virus lingers in their body and causes long COVID symptoms. The monoclonal antibodies can zero in on them and knock them out.” This may also help explain why some patients with long COVID reported a temporary improvement of symptoms after their COVID-19 vaccination.
- It combats dysfunctional antibodies. Another theory is that people with long COVID have symptoms “not because of residual virus but because of junky antibodies,” said Dr. Marty. These antibodies go into overdrive and attack your own cells, which is what causes long COVID symptoms. “This may be why monoclonal antibodies work because they displace the dysfunctional antibodies that are attached to a patient’s cells,” she explained.
- Reactivation of other viruses. Long COVID is very similar to chronic fatigue syndrome, which is often thought to be triggered by reactivation of viruses like the Epstein-Barr virus, noted coauthor Nancy Klimas, MD, director of the Institute for Neuro-Immune Medicine at Nova Southeastern University in Fort Lauderdale. “It may not explain all of the cases of long COVID, but it could make up a subgroup,” she said. It’s thought that the monoclonal antibodies may perhaps neutralize this reactivation.
Where Research Is Headed
While Regeneron worked well in all three patients, it may be because they developed long COVID from either the initial virus or from early variants like Alpha, Beta, and Delta, said Dr. Pepe. As a result, it’s unclear whether this treatment would work for patients who developed long COVID from newer strains like Omicron.
“What concerns me is I believe there may be many people walking around with mild long COVID from these strains who don’t realize it,” he said. “They may assume that if they have difficulty walking upstairs, or forget why they went into another room, that it’s age related.”
The next step, the researchers said, is to create a registry of volunteer patients with severe long COVID. Dr. Klimas plans to enroll 20 volunteers who were infected before September 2022 to see how they respond to another monoclonal antibody initially used to treat COVID-19, bebtelovimab. (Like Regeneron, bebtelovimab is no longer approved for use against COVID-19 by the US Food and Drug Administration because it is no longer effective against variants of the virus circulating today.)
As for patients who developed long COVID after September 2022, research is ongoing to see if they respond to other monoclonal antibodies that are in development. One such study is currently enrolling participants at the University of California San Francisco. The center is recruiting 30 patients with long COVID to try a monoclonal antibody developed by Aerium Therapeutics.
“They created an investigational monoclonal antibody to treat acute COVID, but it proved less effective against variants that emerged in late 2022,” said lead investigator Michael Peluso, MD, an assistant professor of medicine in the Division of HIV, Infectious Diseases, and Global Medicine at the University of California San Francisco. The hope is it may still work to fight long COVID among patients infected with those variants.
In the meantime, the three patients with long COVID who responded to Regeneron have resumed life as they knew it pre-COVID. Although two subsequently became infected with COVID again, they recovered quickly and did not see symptoms return, something which, for them, seems nothing short of miraculous.
“I had prepared myself to be disabled for life,” said one of the patients, a 46-year-old Floridian woman who developed long COVID after an infection in January 2021. “I had crippling fatigue and dizziness so intense I felt like I was walking on a trampoline. My brain fog was so pronounced I had to write everything down constantly. Otherwise, I’d forget.”
When she became infected with COVID again in September 2021, “I thought I was going to die because I had no idea how I could possibly get worse,” she recalled. Her doctors recommended Regeneron infusion treatment. Forty-eight hours later, her symptoms improved significantly.
“I was able to go out to a cocktail party and dinner for the first time in months,” she said. “I would not have been able to do either of those things a week before.”
It’s also profoundly affected her husband, who had had to take over running the household and raising their five children, aged 11-22 years, for months.
“I can’t tell you how many school events and sports games I missed because I physically didn’t have the strength to get to them,” she noted. “To this day, my husband gets upset whenever we talk about that time. Long COVID literally took over all of our lives. It was devastating to me, but it’s just as devastating for loved ones, too. My family is just grateful to have me back.”
A version of this article first appeared on Medscape.com.
A treatment used to treat acute COVID-19 infection has also been found to be effective against long COVID, a new small study has found. The research, which assessed the benefits of monoclonal antibodies, suggests relief may finally be ahead for millions of Americans with long COVID for whom treatment has remained elusive.
The study, published in the American Journal of Emergency Medicine, found
“We were struck by how rapid and complete the remissions were,” said study coauthor Paul Pepe, MD, MPH, a professor of management, policy, and community health at the School of Public Health at the University of Texas Health Sciences Center. “We found that no matter how long the patients were sick for — whether it was 5, 8, or 18 months — within 5 days, they appeared to be completely cured.”
All three patients had been initially infected with COVID-19 early in the pandemic, in 2020 or the first half of 2021. They were given Regeneron either after a reinfection or exposure to COVID-19, as a preventative, at state-run COVID clinics in Florida.
“In each case, the infusions were given to help prevent their long COVID from worsening,” said Dr. Pepe.
The researchers collected medical histories for all three patients, asking about symptoms such as physical fatigue, exercise intolerance, chest pain, heart palpitations, shortness of breath, cognitive fatigue, and memory problems. They asked patients to rate symptoms pre-COVID (baseline), during the long COVID phase, post-vaccine, and finally a week after their monoclonal antibody treatment. They also interviewed family members.
They found that across the board, symptoms improved significantly and often completely vanished. Their loved ones corroborated these reports as well.
One of the patients, a 63-year-old Floridian woman, came down with a mild case of COVID-19 at the start of the pandemic in March 2020 that lasted about 2 weeks. But several weeks later, she developed extreme, debilitating fatigue, along with chest pain and shortness of breath.
“I was chasing my 6-pound Yorkie one day after she got loose, and I was struck with such intense chest pain I fell down,” the woman, asking not to be identified, said in an interview.
Her symptoms progressed to the point where she no longer felt safe babysitting her grandchildren or driving to the grocery store.
“My short-term memory was completely gone. I couldn’t even read more than a paragraph at a time,” she said.
When she was exposed to COVID-19 in October 2021, her doctor suggested Regeneron as a preventative. She agreed to it.
“I was terrified that a second round would leave me permanently disabled and stuck in bed for the rest of my life,” she said.
About 4 days after her monoclonal antibody treatment, she noticed that some of the brain fog that had persisted after COVID was lifting.
“By day 5, it felt almost like a heavy-weighted blanket had been lifted off of me,” she recalled. “I was able to take my dog for a walk and go to the grocery store. It felt like I had gone from 0 to 100. As quickly as I went downhill, I quickly went back up.”
Reasons for Recovery
Researchers have come up with a few theories about why monoclonal antibodies may help treat long COVID, said study coauthor Aileen Marty, MD, professor of translational medicine at the Herbert Wertheim College of Medicine at Florida International University. Among them:
- It stimulates the body to fight off any residual virus. “We suspect that many of these patients simply have levels of virus that are so low they can’t be picked up by conventional testing,” said Dr. Marty. “The virus lingers in their body and causes long COVID symptoms. The monoclonal antibodies can zero in on them and knock them out.” This may also help explain why some patients with long COVID reported a temporary improvement of symptoms after their COVID-19 vaccination.
- It combats dysfunctional antibodies. Another theory is that people with long COVID have symptoms “not because of residual virus but because of junky antibodies,” said Dr. Marty. These antibodies go into overdrive and attack your own cells, which is what causes long COVID symptoms. “This may be why monoclonal antibodies work because they displace the dysfunctional antibodies that are attached to a patient’s cells,” she explained.
- Reactivation of other viruses. Long COVID is very similar to chronic fatigue syndrome, which is often thought to be triggered by reactivation of viruses like the Epstein-Barr virus, noted coauthor Nancy Klimas, MD, director of the Institute for Neuro-Immune Medicine at Nova Southeastern University in Fort Lauderdale. “It may not explain all of the cases of long COVID, but it could make up a subgroup,” she said. It’s thought that the monoclonal antibodies may perhaps neutralize this reactivation.
Where Research Is Headed
While Regeneron worked well in all three patients, it may be because they developed long COVID from either the initial virus or from early variants like Alpha, Beta, and Delta, said Dr. Pepe. As a result, it’s unclear whether this treatment would work for patients who developed long COVID from newer strains like Omicron.
“What concerns me is I believe there may be many people walking around with mild long COVID from these strains who don’t realize it,” he said. “They may assume that if they have difficulty walking upstairs, or forget why they went into another room, that it’s age related.”
The next step, the researchers said, is to create a registry of volunteer patients with severe long COVID. Dr. Klimas plans to enroll 20 volunteers who were infected before September 2022 to see how they respond to another monoclonal antibody initially used to treat COVID-19, bebtelovimab. (Like Regeneron, bebtelovimab is no longer approved for use against COVID-19 by the US Food and Drug Administration because it is no longer effective against variants of the virus circulating today.)
As for patients who developed long COVID after September 2022, research is ongoing to see if they respond to other monoclonal antibodies that are in development. One such study is currently enrolling participants at the University of California San Francisco. The center is recruiting 30 patients with long COVID to try a monoclonal antibody developed by Aerium Therapeutics.
“They created an investigational monoclonal antibody to treat acute COVID, but it proved less effective against variants that emerged in late 2022,” said lead investigator Michael Peluso, MD, an assistant professor of medicine in the Division of HIV, Infectious Diseases, and Global Medicine at the University of California San Francisco. The hope is it may still work to fight long COVID among patients infected with those variants.
In the meantime, the three patients with long COVID who responded to Regeneron have resumed life as they knew it pre-COVID. Although two subsequently became infected with COVID again, they recovered quickly and did not see symptoms return, something which, for them, seems nothing short of miraculous.
“I had prepared myself to be disabled for life,” said one of the patients, a 46-year-old Floridian woman who developed long COVID after an infection in January 2021. “I had crippling fatigue and dizziness so intense I felt like I was walking on a trampoline. My brain fog was so pronounced I had to write everything down constantly. Otherwise, I’d forget.”
When she became infected with COVID again in September 2021, “I thought I was going to die because I had no idea how I could possibly get worse,” she recalled. Her doctors recommended Regeneron infusion treatment. Forty-eight hours later, her symptoms improved significantly.
“I was able to go out to a cocktail party and dinner for the first time in months,” she said. “I would not have been able to do either of those things a week before.”
It’s also profoundly affected her husband, who had had to take over running the household and raising their five children, aged 11-22 years, for months.
“I can’t tell you how many school events and sports games I missed because I physically didn’t have the strength to get to them,” she noted. “To this day, my husband gets upset whenever we talk about that time. Long COVID literally took over all of our lives. It was devastating to me, but it’s just as devastating for loved ones, too. My family is just grateful to have me back.”
A version of this article first appeared on Medscape.com.
A treatment used to treat acute COVID-19 infection has also been found to be effective against long COVID, a new small study has found. The research, which assessed the benefits of monoclonal antibodies, suggests relief may finally be ahead for millions of Americans with long COVID for whom treatment has remained elusive.
The study, published in the American Journal of Emergency Medicine, found
“We were struck by how rapid and complete the remissions were,” said study coauthor Paul Pepe, MD, MPH, a professor of management, policy, and community health at the School of Public Health at the University of Texas Health Sciences Center. “We found that no matter how long the patients were sick for — whether it was 5, 8, or 18 months — within 5 days, they appeared to be completely cured.”
All three patients had been initially infected with COVID-19 early in the pandemic, in 2020 or the first half of 2021. They were given Regeneron either after a reinfection or exposure to COVID-19, as a preventative, at state-run COVID clinics in Florida.
“In each case, the infusions were given to help prevent their long COVID from worsening,” said Dr. Pepe.
The researchers collected medical histories for all three patients, asking about symptoms such as physical fatigue, exercise intolerance, chest pain, heart palpitations, shortness of breath, cognitive fatigue, and memory problems. They asked patients to rate symptoms pre-COVID (baseline), during the long COVID phase, post-vaccine, and finally a week after their monoclonal antibody treatment. They also interviewed family members.
They found that across the board, symptoms improved significantly and often completely vanished. Their loved ones corroborated these reports as well.
One of the patients, a 63-year-old Floridian woman, came down with a mild case of COVID-19 at the start of the pandemic in March 2020 that lasted about 2 weeks. But several weeks later, she developed extreme, debilitating fatigue, along with chest pain and shortness of breath.
“I was chasing my 6-pound Yorkie one day after she got loose, and I was struck with such intense chest pain I fell down,” the woman, asking not to be identified, said in an interview.
Her symptoms progressed to the point where she no longer felt safe babysitting her grandchildren or driving to the grocery store.
“My short-term memory was completely gone. I couldn’t even read more than a paragraph at a time,” she said.
When she was exposed to COVID-19 in October 2021, her doctor suggested Regeneron as a preventative. She agreed to it.
“I was terrified that a second round would leave me permanently disabled and stuck in bed for the rest of my life,” she said.
About 4 days after her monoclonal antibody treatment, she noticed that some of the brain fog that had persisted after COVID was lifting.
“By day 5, it felt almost like a heavy-weighted blanket had been lifted off of me,” she recalled. “I was able to take my dog for a walk and go to the grocery store. It felt like I had gone from 0 to 100. As quickly as I went downhill, I quickly went back up.”
Reasons for Recovery
Researchers have come up with a few theories about why monoclonal antibodies may help treat long COVID, said study coauthor Aileen Marty, MD, professor of translational medicine at the Herbert Wertheim College of Medicine at Florida International University. Among them:
- It stimulates the body to fight off any residual virus. “We suspect that many of these patients simply have levels of virus that are so low they can’t be picked up by conventional testing,” said Dr. Marty. “The virus lingers in their body and causes long COVID symptoms. The monoclonal antibodies can zero in on them and knock them out.” This may also help explain why some patients with long COVID reported a temporary improvement of symptoms after their COVID-19 vaccination.
- It combats dysfunctional antibodies. Another theory is that people with long COVID have symptoms “not because of residual virus but because of junky antibodies,” said Dr. Marty. These antibodies go into overdrive and attack your own cells, which is what causes long COVID symptoms. “This may be why monoclonal antibodies work because they displace the dysfunctional antibodies that are attached to a patient’s cells,” she explained.
- Reactivation of other viruses. Long COVID is very similar to chronic fatigue syndrome, which is often thought to be triggered by reactivation of viruses like the Epstein-Barr virus, noted coauthor Nancy Klimas, MD, director of the Institute for Neuro-Immune Medicine at Nova Southeastern University in Fort Lauderdale. “It may not explain all of the cases of long COVID, but it could make up a subgroup,” she said. It’s thought that the monoclonal antibodies may perhaps neutralize this reactivation.
Where Research Is Headed
While Regeneron worked well in all three patients, it may be because they developed long COVID from either the initial virus or from early variants like Alpha, Beta, and Delta, said Dr. Pepe. As a result, it’s unclear whether this treatment would work for patients who developed long COVID from newer strains like Omicron.
“What concerns me is I believe there may be many people walking around with mild long COVID from these strains who don’t realize it,” he said. “They may assume that if they have difficulty walking upstairs, or forget why they went into another room, that it’s age related.”
The next step, the researchers said, is to create a registry of volunteer patients with severe long COVID. Dr. Klimas plans to enroll 20 volunteers who were infected before September 2022 to see how they respond to another monoclonal antibody initially used to treat COVID-19, bebtelovimab. (Like Regeneron, bebtelovimab is no longer approved for use against COVID-19 by the US Food and Drug Administration because it is no longer effective against variants of the virus circulating today.)
As for patients who developed long COVID after September 2022, research is ongoing to see if they respond to other monoclonal antibodies that are in development. One such study is currently enrolling participants at the University of California San Francisco. The center is recruiting 30 patients with long COVID to try a monoclonal antibody developed by Aerium Therapeutics.
“They created an investigational monoclonal antibody to treat acute COVID, but it proved less effective against variants that emerged in late 2022,” said lead investigator Michael Peluso, MD, an assistant professor of medicine in the Division of HIV, Infectious Diseases, and Global Medicine at the University of California San Francisco. The hope is it may still work to fight long COVID among patients infected with those variants.
In the meantime, the three patients with long COVID who responded to Regeneron have resumed life as they knew it pre-COVID. Although two subsequently became infected with COVID again, they recovered quickly and did not see symptoms return, something which, for them, seems nothing short of miraculous.
“I had prepared myself to be disabled for life,” said one of the patients, a 46-year-old Floridian woman who developed long COVID after an infection in January 2021. “I had crippling fatigue and dizziness so intense I felt like I was walking on a trampoline. My brain fog was so pronounced I had to write everything down constantly. Otherwise, I’d forget.”
When she became infected with COVID again in September 2021, “I thought I was going to die because I had no idea how I could possibly get worse,” she recalled. Her doctors recommended Regeneron infusion treatment. Forty-eight hours later, her symptoms improved significantly.
“I was able to go out to a cocktail party and dinner for the first time in months,” she said. “I would not have been able to do either of those things a week before.”
It’s also profoundly affected her husband, who had had to take over running the household and raising their five children, aged 11-22 years, for months.
“I can’t tell you how many school events and sports games I missed because I physically didn’t have the strength to get to them,” she noted. “To this day, my husband gets upset whenever we talk about that time. Long COVID literally took over all of our lives. It was devastating to me, but it’s just as devastating for loved ones, too. My family is just grateful to have me back.”
A version of this article first appeared on Medscape.com.
FROM THE AMERICAN JOURNAL OF EMERGENCY MEDICINe
COVID, no matter the severity, linked with urologic effects in men
SARS-CoV-2 infection is linked in men with increased incidence of urinary retention, urinary tract infection (UTI), and blood in the urine, a new study finds.
Authors of the study, led by Alex Qinyang Liu, of S.H. Ho Urology Centre, at The Chinese University of Hong Kong, highlighted the clinical implications.
“Clinicians should be aware of the significantly higher incidence of LUTS [lower urinary tract symptoms] complications with COVID-19 in this patient group and understand that these urological manifestations can occur regardless of COVID-19 severity,” the authors wrote.
Findings were published online in the Journal of Internal Medicine.
They explained that current literature has included only small case series and observational studies assessing the connection between COVID-19 and male LUTS.
Nearly 18,000 patients in study
Included in this study were all male patients who used the public health care system in Hong Kong who received alpha-blocker monotherapy for LUTS from 2021 to 2022. After propensity score matching, 17,986 patients were included. Half had polymerase chain reaction–confirmed SARS-CoV-2 infection (n = 8,993).
The retrospective study compared urologic outcomes, including male benign prostatic hyperplasia (BPH) complications, and changes in medical treatment in the two groups. They compared male patients with SARS-CoV-2 infection who were taking baseline alpha blocker monotherapy for LUTS with a control group who had no SARS-CoV-2 infection.
They found that, compared with controls, the SARS-CoV-2–infected group had significantly higher incidence of retention of urine (4.55% vs. 0.86%, P < .001), hematuria (1.36% vs. 0.41%, P < .001), clinical UTI (4.31% vs. 1.49%, P < .001), culture-proven bacteriuria (9.02% vs. 1.97%, P < .001), and addition of 5-alpha reductase inhibitors (0.50% vs. 0.02%, P < .001).
Similar side effects even with asymptomatic infection
The researchers pointed out that similar incidence of retention of urine, hematuria, and addition of medication were seen even when patients had asymptomatic infection.
They added that their findings have biological plausibility because the coexpression of the proteins ACE2 and TMPRSS2 in the prostate makes it a target for SARS-CoV-2, which leads to inflammation and may help explain the primary outcomes.
“Given the high infectivity and unprecedented scale of the COVID-19 pandemic, these urological symptoms and complications represent a significant clinical burden that clinicians and urologists should be aware of,” the authors wrote.
The authors noted that the prevalence of BPH and LUTS rises with age and are among the most common urologic conditions affecting older men. “Incidentally, male patients of advanced age are also more significantly affected by COVID-19.”
The authors declare no relevant financial relationships.
SARS-CoV-2 infection is linked in men with increased incidence of urinary retention, urinary tract infection (UTI), and blood in the urine, a new study finds.
Authors of the study, led by Alex Qinyang Liu, of S.H. Ho Urology Centre, at The Chinese University of Hong Kong, highlighted the clinical implications.
“Clinicians should be aware of the significantly higher incidence of LUTS [lower urinary tract symptoms] complications with COVID-19 in this patient group and understand that these urological manifestations can occur regardless of COVID-19 severity,” the authors wrote.
Findings were published online in the Journal of Internal Medicine.
They explained that current literature has included only small case series and observational studies assessing the connection between COVID-19 and male LUTS.
Nearly 18,000 patients in study
Included in this study were all male patients who used the public health care system in Hong Kong who received alpha-blocker monotherapy for LUTS from 2021 to 2022. After propensity score matching, 17,986 patients were included. Half had polymerase chain reaction–confirmed SARS-CoV-2 infection (n = 8,993).
The retrospective study compared urologic outcomes, including male benign prostatic hyperplasia (BPH) complications, and changes in medical treatment in the two groups. They compared male patients with SARS-CoV-2 infection who were taking baseline alpha blocker monotherapy for LUTS with a control group who had no SARS-CoV-2 infection.
They found that, compared with controls, the SARS-CoV-2–infected group had significantly higher incidence of retention of urine (4.55% vs. 0.86%, P < .001), hematuria (1.36% vs. 0.41%, P < .001), clinical UTI (4.31% vs. 1.49%, P < .001), culture-proven bacteriuria (9.02% vs. 1.97%, P < .001), and addition of 5-alpha reductase inhibitors (0.50% vs. 0.02%, P < .001).
Similar side effects even with asymptomatic infection
The researchers pointed out that similar incidence of retention of urine, hematuria, and addition of medication were seen even when patients had asymptomatic infection.
They added that their findings have biological plausibility because the coexpression of the proteins ACE2 and TMPRSS2 in the prostate makes it a target for SARS-CoV-2, which leads to inflammation and may help explain the primary outcomes.
“Given the high infectivity and unprecedented scale of the COVID-19 pandemic, these urological symptoms and complications represent a significant clinical burden that clinicians and urologists should be aware of,” the authors wrote.
The authors noted that the prevalence of BPH and LUTS rises with age and are among the most common urologic conditions affecting older men. “Incidentally, male patients of advanced age are also more significantly affected by COVID-19.”
The authors declare no relevant financial relationships.
SARS-CoV-2 infection is linked in men with increased incidence of urinary retention, urinary tract infection (UTI), and blood in the urine, a new study finds.
Authors of the study, led by Alex Qinyang Liu, of S.H. Ho Urology Centre, at The Chinese University of Hong Kong, highlighted the clinical implications.
“Clinicians should be aware of the significantly higher incidence of LUTS [lower urinary tract symptoms] complications with COVID-19 in this patient group and understand that these urological manifestations can occur regardless of COVID-19 severity,” the authors wrote.
Findings were published online in the Journal of Internal Medicine.
They explained that current literature has included only small case series and observational studies assessing the connection between COVID-19 and male LUTS.
Nearly 18,000 patients in study
Included in this study were all male patients who used the public health care system in Hong Kong who received alpha-blocker monotherapy for LUTS from 2021 to 2022. After propensity score matching, 17,986 patients were included. Half had polymerase chain reaction–confirmed SARS-CoV-2 infection (n = 8,993).
The retrospective study compared urologic outcomes, including male benign prostatic hyperplasia (BPH) complications, and changes in medical treatment in the two groups. They compared male patients with SARS-CoV-2 infection who were taking baseline alpha blocker monotherapy for LUTS with a control group who had no SARS-CoV-2 infection.
They found that, compared with controls, the SARS-CoV-2–infected group had significantly higher incidence of retention of urine (4.55% vs. 0.86%, P < .001), hematuria (1.36% vs. 0.41%, P < .001), clinical UTI (4.31% vs. 1.49%, P < .001), culture-proven bacteriuria (9.02% vs. 1.97%, P < .001), and addition of 5-alpha reductase inhibitors (0.50% vs. 0.02%, P < .001).
Similar side effects even with asymptomatic infection
The researchers pointed out that similar incidence of retention of urine, hematuria, and addition of medication were seen even when patients had asymptomatic infection.
They added that their findings have biological plausibility because the coexpression of the proteins ACE2 and TMPRSS2 in the prostate makes it a target for SARS-CoV-2, which leads to inflammation and may help explain the primary outcomes.
“Given the high infectivity and unprecedented scale of the COVID-19 pandemic, these urological symptoms and complications represent a significant clinical burden that clinicians and urologists should be aware of,” the authors wrote.
The authors noted that the prevalence of BPH and LUTS rises with age and are among the most common urologic conditions affecting older men. “Incidentally, male patients of advanced age are also more significantly affected by COVID-19.”
The authors declare no relevant financial relationships.
FROM THE JOURNAL OF INTERNAL MEDICINE
Paxlovid tied to benefits in high-risk patients with COVID
In a cohort study from British Columbia that included nearly 7,000 patients with COVID-19, nirmatrelvir-ritonavir was associated with a 2.5% reduction in risk for death or emergency hospitalization in clinically extremely vulnerable (CEV) patients who were severely immunocompromised. No significant benefit was observed in patients who were not immunocompromised.
“This finding could help substantially limit unnecessary use of nirmatrelvir and ritonavir in older, otherwise healthy individuals,” lead author Colin R. Dormuth, ScD, associate professor of anesthesiology, pharmacology, and therapeutics at the University of British Columbia, Vancouver, told this news organization. “Another finding that was surprising and might help place the role of nirmatrelvir and ritonavir in context is that even in severely immunocompromised individuals who did not take [the drug], the risk of death or hospitalization with COVID-19 was less than 4% in our study population.”
The study was published online in JAMA Network Open.
Who benefits?
The investigators analyzed medical records for 6,866 patients in British Columbia (median age, 70 years; 57% women) who presented between Feb. 1, 2022, and Feb. 3, 2023. Eligible patients belonged to one of four higher-risk groups who received priority for COVID-19 vaccination.
Two groups included CEV patients who were severely (CEV1) or moderately (CEV2) immunocompromised. The CEV3 group was not immunocompromised but had medical conditions associated with a high risk for complications from COVID-19. A fourth expanded eligibility (EXEL) group included higher-risk patients who were not in one of the other groups, such as unvaccinated patients older than age 70 years.
The investigators matched treated patients to untreated patients in the same vulnerability group according to age, sex, and month of infection. The primary outcome was death from any cause or emergency hospitalization with COVID-19 within 28 days.
Treatment with nirmatrelvir-ritonavir was associated with statistically significant relative reductions in the primary outcome, compared with no treatment, for patients in the CEV1 (risk difference, −2.5%) and CEV2 (RD, −1.7%) groups. In the CEV3 group, the RD of −1.3% was not statistically significant. In the EXEL group, treatment was associated with a higher risk for the primary outcome (RD, 1.0%), but the result was not statistically significant.
The results were “robust across sex and older vs. younger age,” the authors note. “No reduction in the primary outcome was observed in lower-risk individuals, including those aged 70 years or older without serious comorbidities.”
The combination of nirmatrelvir-ritonavir was approved for use in Canada based on interim efficacy and safety data from the Evaluation of Inhibition for COVID-19 in High-Risk Patients (EPIC-HR) trial, said Dr. Dormuth.
British Columbia’s eligibility criteria for nirmatrelvir-ritonavir coverage differ substantially from the criteria for participants in the EPIC-HR trial, he noted. Those patients were unvaccinated, had no natural immunity from a previous COVID-19 infection, and were infected with COVID-19 variants that were different from those now circulating. The current study was prompted by the need to look at a broader population of individuals in British Columbia with varying risks of complications from COVID-19 infection.
Before the study, a common view was that patients aged 70 and older would benefit from the drug, said Dr. Dormuth. “Our study, which accounted for medical conditions related to an individual’s vulnerability to complications, showed that older age on its own was not a reason to use nirmatrelvir and ritonavir once relevant medical conditions were taken into consideration.”
The researchers are working on a study to identify with greater specificity which comorbid conditions are most associated with nirmatrelvir-ritonavir effectiveness, he added. “It could be that a relatively small number of conditions can be used to identify most individuals who would benefit from the drug.”
‘Signal toward benefit’
Commenting on the findings for this news organization, Abhijit Duggal, MD, vice chair of critical care at the Cleveland Clinic, who was not involved in this study, said, “I’m always very wary when we look at observational data and we start saying the effectiveness is not really as high as was seen in other studies. We are seeing an effect with all these studies that seems to be in the right direction.
“Having said that,” he added, “is the effect going to be potentially more in patients at higher risk? Absolutely. I think these postmarket studies are really showing that after vaccination, if someone does get infected, this is a secondary option available to us that can prevent progression of the disease, which would likely be more severe in immunocompromised patients.”
Dr. Duggal was a coinvestigator on a recent study of more than 68,000 patients that showed that nirmatrelvir-ritonavir or molnupiravir was associated with reductions in mortality and hospitalization in nonhospitalized patients infected with the Omicron variant, regardless of age, race and ethnicity, virus strain, vaccination status, previous infection status, or coexisting conditions.
“In all groups, there was a signal toward benefit,” said Dr. Duggal. “These studies tell us that these drugs do remain valid options. But their use needs to be discussed on a case-by-case basis with patients we feel are deteriorating or at a higher risk because of underlying disease processes.”
The study was supported by funding from the British Columbia Ministry of Health. Dr. Dormuth and Dr. Duggal report no relevant financial relationships.
A version of this article appeared on Medscape.com.
In a cohort study from British Columbia that included nearly 7,000 patients with COVID-19, nirmatrelvir-ritonavir was associated with a 2.5% reduction in risk for death or emergency hospitalization in clinically extremely vulnerable (CEV) patients who were severely immunocompromised. No significant benefit was observed in patients who were not immunocompromised.
“This finding could help substantially limit unnecessary use of nirmatrelvir and ritonavir in older, otherwise healthy individuals,” lead author Colin R. Dormuth, ScD, associate professor of anesthesiology, pharmacology, and therapeutics at the University of British Columbia, Vancouver, told this news organization. “Another finding that was surprising and might help place the role of nirmatrelvir and ritonavir in context is that even in severely immunocompromised individuals who did not take [the drug], the risk of death or hospitalization with COVID-19 was less than 4% in our study population.”
The study was published online in JAMA Network Open.
Who benefits?
The investigators analyzed medical records for 6,866 patients in British Columbia (median age, 70 years; 57% women) who presented between Feb. 1, 2022, and Feb. 3, 2023. Eligible patients belonged to one of four higher-risk groups who received priority for COVID-19 vaccination.
Two groups included CEV patients who were severely (CEV1) or moderately (CEV2) immunocompromised. The CEV3 group was not immunocompromised but had medical conditions associated with a high risk for complications from COVID-19. A fourth expanded eligibility (EXEL) group included higher-risk patients who were not in one of the other groups, such as unvaccinated patients older than age 70 years.
The investigators matched treated patients to untreated patients in the same vulnerability group according to age, sex, and month of infection. The primary outcome was death from any cause or emergency hospitalization with COVID-19 within 28 days.
Treatment with nirmatrelvir-ritonavir was associated with statistically significant relative reductions in the primary outcome, compared with no treatment, for patients in the CEV1 (risk difference, −2.5%) and CEV2 (RD, −1.7%) groups. In the CEV3 group, the RD of −1.3% was not statistically significant. In the EXEL group, treatment was associated with a higher risk for the primary outcome (RD, 1.0%), but the result was not statistically significant.
The results were “robust across sex and older vs. younger age,” the authors note. “No reduction in the primary outcome was observed in lower-risk individuals, including those aged 70 years or older without serious comorbidities.”
The combination of nirmatrelvir-ritonavir was approved for use in Canada based on interim efficacy and safety data from the Evaluation of Inhibition for COVID-19 in High-Risk Patients (EPIC-HR) trial, said Dr. Dormuth.
British Columbia’s eligibility criteria for nirmatrelvir-ritonavir coverage differ substantially from the criteria for participants in the EPIC-HR trial, he noted. Those patients were unvaccinated, had no natural immunity from a previous COVID-19 infection, and were infected with COVID-19 variants that were different from those now circulating. The current study was prompted by the need to look at a broader population of individuals in British Columbia with varying risks of complications from COVID-19 infection.
Before the study, a common view was that patients aged 70 and older would benefit from the drug, said Dr. Dormuth. “Our study, which accounted for medical conditions related to an individual’s vulnerability to complications, showed that older age on its own was not a reason to use nirmatrelvir and ritonavir once relevant medical conditions were taken into consideration.”
The researchers are working on a study to identify with greater specificity which comorbid conditions are most associated with nirmatrelvir-ritonavir effectiveness, he added. “It could be that a relatively small number of conditions can be used to identify most individuals who would benefit from the drug.”
‘Signal toward benefit’
Commenting on the findings for this news organization, Abhijit Duggal, MD, vice chair of critical care at the Cleveland Clinic, who was not involved in this study, said, “I’m always very wary when we look at observational data and we start saying the effectiveness is not really as high as was seen in other studies. We are seeing an effect with all these studies that seems to be in the right direction.
“Having said that,” he added, “is the effect going to be potentially more in patients at higher risk? Absolutely. I think these postmarket studies are really showing that after vaccination, if someone does get infected, this is a secondary option available to us that can prevent progression of the disease, which would likely be more severe in immunocompromised patients.”
Dr. Duggal was a coinvestigator on a recent study of more than 68,000 patients that showed that nirmatrelvir-ritonavir or molnupiravir was associated with reductions in mortality and hospitalization in nonhospitalized patients infected with the Omicron variant, regardless of age, race and ethnicity, virus strain, vaccination status, previous infection status, or coexisting conditions.
“In all groups, there was a signal toward benefit,” said Dr. Duggal. “These studies tell us that these drugs do remain valid options. But their use needs to be discussed on a case-by-case basis with patients we feel are deteriorating or at a higher risk because of underlying disease processes.”
The study was supported by funding from the British Columbia Ministry of Health. Dr. Dormuth and Dr. Duggal report no relevant financial relationships.
A version of this article appeared on Medscape.com.
In a cohort study from British Columbia that included nearly 7,000 patients with COVID-19, nirmatrelvir-ritonavir was associated with a 2.5% reduction in risk for death or emergency hospitalization in clinically extremely vulnerable (CEV) patients who were severely immunocompromised. No significant benefit was observed in patients who were not immunocompromised.
“This finding could help substantially limit unnecessary use of nirmatrelvir and ritonavir in older, otherwise healthy individuals,” lead author Colin R. Dormuth, ScD, associate professor of anesthesiology, pharmacology, and therapeutics at the University of British Columbia, Vancouver, told this news organization. “Another finding that was surprising and might help place the role of nirmatrelvir and ritonavir in context is that even in severely immunocompromised individuals who did not take [the drug], the risk of death or hospitalization with COVID-19 was less than 4% in our study population.”
The study was published online in JAMA Network Open.
Who benefits?
The investigators analyzed medical records for 6,866 patients in British Columbia (median age, 70 years; 57% women) who presented between Feb. 1, 2022, and Feb. 3, 2023. Eligible patients belonged to one of four higher-risk groups who received priority for COVID-19 vaccination.
Two groups included CEV patients who were severely (CEV1) or moderately (CEV2) immunocompromised. The CEV3 group was not immunocompromised but had medical conditions associated with a high risk for complications from COVID-19. A fourth expanded eligibility (EXEL) group included higher-risk patients who were not in one of the other groups, such as unvaccinated patients older than age 70 years.
The investigators matched treated patients to untreated patients in the same vulnerability group according to age, sex, and month of infection. The primary outcome was death from any cause or emergency hospitalization with COVID-19 within 28 days.
Treatment with nirmatrelvir-ritonavir was associated with statistically significant relative reductions in the primary outcome, compared with no treatment, for patients in the CEV1 (risk difference, −2.5%) and CEV2 (RD, −1.7%) groups. In the CEV3 group, the RD of −1.3% was not statistically significant. In the EXEL group, treatment was associated with a higher risk for the primary outcome (RD, 1.0%), but the result was not statistically significant.
The results were “robust across sex and older vs. younger age,” the authors note. “No reduction in the primary outcome was observed in lower-risk individuals, including those aged 70 years or older without serious comorbidities.”
The combination of nirmatrelvir-ritonavir was approved for use in Canada based on interim efficacy and safety data from the Evaluation of Inhibition for COVID-19 in High-Risk Patients (EPIC-HR) trial, said Dr. Dormuth.
British Columbia’s eligibility criteria for nirmatrelvir-ritonavir coverage differ substantially from the criteria for participants in the EPIC-HR trial, he noted. Those patients were unvaccinated, had no natural immunity from a previous COVID-19 infection, and were infected with COVID-19 variants that were different from those now circulating. The current study was prompted by the need to look at a broader population of individuals in British Columbia with varying risks of complications from COVID-19 infection.
Before the study, a common view was that patients aged 70 and older would benefit from the drug, said Dr. Dormuth. “Our study, which accounted for medical conditions related to an individual’s vulnerability to complications, showed that older age on its own was not a reason to use nirmatrelvir and ritonavir once relevant medical conditions were taken into consideration.”
The researchers are working on a study to identify with greater specificity which comorbid conditions are most associated with nirmatrelvir-ritonavir effectiveness, he added. “It could be that a relatively small number of conditions can be used to identify most individuals who would benefit from the drug.”
‘Signal toward benefit’
Commenting on the findings for this news organization, Abhijit Duggal, MD, vice chair of critical care at the Cleveland Clinic, who was not involved in this study, said, “I’m always very wary when we look at observational data and we start saying the effectiveness is not really as high as was seen in other studies. We are seeing an effect with all these studies that seems to be in the right direction.
“Having said that,” he added, “is the effect going to be potentially more in patients at higher risk? Absolutely. I think these postmarket studies are really showing that after vaccination, if someone does get infected, this is a secondary option available to us that can prevent progression of the disease, which would likely be more severe in immunocompromised patients.”
Dr. Duggal was a coinvestigator on a recent study of more than 68,000 patients that showed that nirmatrelvir-ritonavir or molnupiravir was associated with reductions in mortality and hospitalization in nonhospitalized patients infected with the Omicron variant, regardless of age, race and ethnicity, virus strain, vaccination status, previous infection status, or coexisting conditions.
“In all groups, there was a signal toward benefit,” said Dr. Duggal. “These studies tell us that these drugs do remain valid options. But their use needs to be discussed on a case-by-case basis with patients we feel are deteriorating or at a higher risk because of underlying disease processes.”
The study was supported by funding from the British Columbia Ministry of Health. Dr. Dormuth and Dr. Duggal report no relevant financial relationships.
A version of this article appeared on Medscape.com.
Preparing for the viral trifecta: RSV, influenza, and COVID-19
New armamentaria available to fight an old disease.
In July 2023, nirsevimab (Beyfortus), a monoclonal antibody, was approved by the Food and Drug Administration for the prevention of respiratory syncytial virus (RSV) disease in infants and children younger than 2 years of age. On Aug. 3, 2023, the Advisory Committee on Immunization Practices (ACIP) of the Centers for Disease Control and Prevention recommended routine use of it for all infants younger than 8 months of age born during or entering their first RSV season. Its use is also recommended for certain children 8-19 months of age who are at increased risk for severe RSV disease at the start of their second RSV season. Hearing the approval, I immediately had a flashback to residency, recalling the multiple infants admitted each fall and winter exhibiting classic symptoms including cough, rhinorrhea, nasal flaring, retractions, and wheezing with many having oxygen requirements and others needing intubation. Only supportive care was available.
RSV is the leading cause of infant hospitalizations. Annually, the CDC estimates there are 50,000-80,000 RSV hospitalizations and 100-300 RSV-related deaths in the United States in persons younger than 5 years of age. While premature infants have the highest rates of hospitalization (three times a term infant) about 79% of hospitalized children younger than 2 years have no underlying medical risks.1 The majority of children will experience RSV as an upper respiratory infection within the first 2 years of life. However, severe disease requiring hospitalization is more likely to occur in premature infants and children younger than 6 months; children younger than 2 with congenital heart disease and/or chronic lung disease; children with severe cystic fibrosis; as well as the immunocompromised child and individuals with neuromuscular disorders that preclude clearing mucous secretions or have difficulty swallowing.
Palivizumab (Synagis), the first monoclonal antibody to prevent RSV in infants was licensed in 1998. Its use was limited to infants meeting specific criteria developed by the American Academy of Pediatrics. Only 5% of infants had access to it. It was a short-acting agent requiring monthly injections, which were very costly ($1,661-$2,584 per dose). Eligible infants could receive up to five injections per season. Several studies proved its use was not cost beneficial.
What are the advantages of nirsevimab? It’s a long-acting monoclonal antibody. Only one dose is required per season. Costs will significantly diminish. It is recommended for all infants younger than 8 months of age born during RSV season. Those children 8-19 months at risk for severe RSV disease can receive it prior to the start of their second RSV season. During RSV season (October 1 to March 31), the initial dose should be administered to newborns just prior to hospital discharge. Older infants and newborns who did not receive it prior to hospital discharge can receive it at their medical home. Newborns should receive it within the first week of life. It is covered by the Vaccine for Children Program. Simultaneous administration with routine childhood immunizations is recommended. Finally, RSV season may vary in tropical areas (Southern Florida, Puerto Rico. etc.) and Alaska. The timing of nirsevimab administration should be based on local RSV activity provided by state and local authorities.
In addition, the FDA approved an RSV vaccine (Abrysvo) for use in adults at least 60 years of age and in pregnant women at 32-36 weeks’ gestation. The latter is administered to prevent lower respiratory tract infection in infants from birth to 6 months. Recommendations have been published for administration in nonpregnant adults. Specific information is forthcoming in terms timing of administration of nirsevimab in infants whose mothers receive Abrysvo.
RSV season is quickly approaching. Detailed recommendations for administration and FAQ questions related to nirsevimab and palivizumab can be found at https://www.aap.org or https://www.cdc.gov/vaccines/hcp/acip-recs/index.html.
Influenza
So, what about influenza? Vaccine composition has been tweaked to match the circulating viruses but the recommended age for annual routine administration remains unchanged. All persons at least 6 months of age should be vaccinated. Children between 6 months and 8 years need two doses at least 4 weeks apart when receiving vaccine for the first time. Immunizing everyone in the household is encouraged especially if there are household contacts at risk for developing severe disease, including infants too young to be vaccinated. Keep in mind children may be coinfected with multiple viruses. Adams and colleagues reviewed the prevalence of coinfection of influenza and Sars-CoV-2 in persons younger than 18 years reported to three CDC surveillance platforms during the 2021-2022 season.2 Thirty-two of 575 hospitalized (6%) coinfections were analyzed and 7 of 44 (16%) deaths. Compared with patients without coinfections, the coinfected patients were more likely to require mechanical ventilation (13% vs. 4%) or CPAP (16% vs. 6%). Only 4 of 23 who were influenza vaccine eligible were vaccinated. Of seven coinfected children who died, none had received influenza vaccine and only one received an antiviral. Only 5 of 31 (16%) infected only with influenza were vaccinated.3
Influenza activity was lower than usual during the 2021-2022 season. However, this report revealed underuse of both influenza vaccine and antiviral therapy, both of which are routinely recommended.
COVID-19
What’s new with COVID-19? On Sept. 12, 2023, ACIP recommended that everyone at least 6 months of age receive the 2023-2024 (monovalent, XBB containing) COVID-19 vaccines. Children at least 5 years of age need one dose and those younger need one or two doses depending on the number of doses previously received. Why the change? Circulating variants continue to change. There is a current uptick in cases including hospitalizations (7.7%) and deaths (4.5%) and it’s just the beginning of the season.4 Symptoms, risk groups and complications have not changed. The primary goal is to prevent infection, hospitalization, long term complications, and death.
We are now armed with the most up-to-date interventions to help prevent the acquisition of these three viruses. Our next step is recommending and delivering them to our patients.
Dr. Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She reported no relevant financial disclosures.
References
1.Suh M et al. J Infect Dis. 2022;226(Suppl 2):S154-36. doi: 10.1093/infdis/jiac120.
2. Adams K et al. MMWR Morb Mortal Wkly Rep. 2022;71:1589-96. doi: http://dx.doi.org/10.15585/mmwr.mm7150a4.
3. Pingali C et al. MMWR Morb Mortal Wkly Rep. 2023 Aug 25;72:912-9. doi: http://dx.doi.org/10.15585/mmwr.mm7234a3.
4. CDC Covid Data Tracker.
New armamentaria available to fight an old disease.
New armamentaria available to fight an old disease.
In July 2023, nirsevimab (Beyfortus), a monoclonal antibody, was approved by the Food and Drug Administration for the prevention of respiratory syncytial virus (RSV) disease in infants and children younger than 2 years of age. On Aug. 3, 2023, the Advisory Committee on Immunization Practices (ACIP) of the Centers for Disease Control and Prevention recommended routine use of it for all infants younger than 8 months of age born during or entering their first RSV season. Its use is also recommended for certain children 8-19 months of age who are at increased risk for severe RSV disease at the start of their second RSV season. Hearing the approval, I immediately had a flashback to residency, recalling the multiple infants admitted each fall and winter exhibiting classic symptoms including cough, rhinorrhea, nasal flaring, retractions, and wheezing with many having oxygen requirements and others needing intubation. Only supportive care was available.
RSV is the leading cause of infant hospitalizations. Annually, the CDC estimates there are 50,000-80,000 RSV hospitalizations and 100-300 RSV-related deaths in the United States in persons younger than 5 years of age. While premature infants have the highest rates of hospitalization (three times a term infant) about 79% of hospitalized children younger than 2 years have no underlying medical risks.1 The majority of children will experience RSV as an upper respiratory infection within the first 2 years of life. However, severe disease requiring hospitalization is more likely to occur in premature infants and children younger than 6 months; children younger than 2 with congenital heart disease and/or chronic lung disease; children with severe cystic fibrosis; as well as the immunocompromised child and individuals with neuromuscular disorders that preclude clearing mucous secretions or have difficulty swallowing.
Palivizumab (Synagis), the first monoclonal antibody to prevent RSV in infants was licensed in 1998. Its use was limited to infants meeting specific criteria developed by the American Academy of Pediatrics. Only 5% of infants had access to it. It was a short-acting agent requiring monthly injections, which were very costly ($1,661-$2,584 per dose). Eligible infants could receive up to five injections per season. Several studies proved its use was not cost beneficial.
What are the advantages of nirsevimab? It’s a long-acting monoclonal antibody. Only one dose is required per season. Costs will significantly diminish. It is recommended for all infants younger than 8 months of age born during RSV season. Those children 8-19 months at risk for severe RSV disease can receive it prior to the start of their second RSV season. During RSV season (October 1 to March 31), the initial dose should be administered to newborns just prior to hospital discharge. Older infants and newborns who did not receive it prior to hospital discharge can receive it at their medical home. Newborns should receive it within the first week of life. It is covered by the Vaccine for Children Program. Simultaneous administration with routine childhood immunizations is recommended. Finally, RSV season may vary in tropical areas (Southern Florida, Puerto Rico. etc.) and Alaska. The timing of nirsevimab administration should be based on local RSV activity provided by state and local authorities.
In addition, the FDA approved an RSV vaccine (Abrysvo) for use in adults at least 60 years of age and in pregnant women at 32-36 weeks’ gestation. The latter is administered to prevent lower respiratory tract infection in infants from birth to 6 months. Recommendations have been published for administration in nonpregnant adults. Specific information is forthcoming in terms timing of administration of nirsevimab in infants whose mothers receive Abrysvo.
RSV season is quickly approaching. Detailed recommendations for administration and FAQ questions related to nirsevimab and palivizumab can be found at https://www.aap.org or https://www.cdc.gov/vaccines/hcp/acip-recs/index.html.
Influenza
So, what about influenza? Vaccine composition has been tweaked to match the circulating viruses but the recommended age for annual routine administration remains unchanged. All persons at least 6 months of age should be vaccinated. Children between 6 months and 8 years need two doses at least 4 weeks apart when receiving vaccine for the first time. Immunizing everyone in the household is encouraged especially if there are household contacts at risk for developing severe disease, including infants too young to be vaccinated. Keep in mind children may be coinfected with multiple viruses. Adams and colleagues reviewed the prevalence of coinfection of influenza and Sars-CoV-2 in persons younger than 18 years reported to three CDC surveillance platforms during the 2021-2022 season.2 Thirty-two of 575 hospitalized (6%) coinfections were analyzed and 7 of 44 (16%) deaths. Compared with patients without coinfections, the coinfected patients were more likely to require mechanical ventilation (13% vs. 4%) or CPAP (16% vs. 6%). Only 4 of 23 who were influenza vaccine eligible were vaccinated. Of seven coinfected children who died, none had received influenza vaccine and only one received an antiviral. Only 5 of 31 (16%) infected only with influenza were vaccinated.3
Influenza activity was lower than usual during the 2021-2022 season. However, this report revealed underuse of both influenza vaccine and antiviral therapy, both of which are routinely recommended.
COVID-19
What’s new with COVID-19? On Sept. 12, 2023, ACIP recommended that everyone at least 6 months of age receive the 2023-2024 (monovalent, XBB containing) COVID-19 vaccines. Children at least 5 years of age need one dose and those younger need one or two doses depending on the number of doses previously received. Why the change? Circulating variants continue to change. There is a current uptick in cases including hospitalizations (7.7%) and deaths (4.5%) and it’s just the beginning of the season.4 Symptoms, risk groups and complications have not changed. The primary goal is to prevent infection, hospitalization, long term complications, and death.
We are now armed with the most up-to-date interventions to help prevent the acquisition of these three viruses. Our next step is recommending and delivering them to our patients.
Dr. Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She reported no relevant financial disclosures.
References
1.Suh M et al. J Infect Dis. 2022;226(Suppl 2):S154-36. doi: 10.1093/infdis/jiac120.
2. Adams K et al. MMWR Morb Mortal Wkly Rep. 2022;71:1589-96. doi: http://dx.doi.org/10.15585/mmwr.mm7150a4.
3. Pingali C et al. MMWR Morb Mortal Wkly Rep. 2023 Aug 25;72:912-9. doi: http://dx.doi.org/10.15585/mmwr.mm7234a3.
4. CDC Covid Data Tracker.
In July 2023, nirsevimab (Beyfortus), a monoclonal antibody, was approved by the Food and Drug Administration for the prevention of respiratory syncytial virus (RSV) disease in infants and children younger than 2 years of age. On Aug. 3, 2023, the Advisory Committee on Immunization Practices (ACIP) of the Centers for Disease Control and Prevention recommended routine use of it for all infants younger than 8 months of age born during or entering their first RSV season. Its use is also recommended for certain children 8-19 months of age who are at increased risk for severe RSV disease at the start of their second RSV season. Hearing the approval, I immediately had a flashback to residency, recalling the multiple infants admitted each fall and winter exhibiting classic symptoms including cough, rhinorrhea, nasal flaring, retractions, and wheezing with many having oxygen requirements and others needing intubation. Only supportive care was available.
RSV is the leading cause of infant hospitalizations. Annually, the CDC estimates there are 50,000-80,000 RSV hospitalizations and 100-300 RSV-related deaths in the United States in persons younger than 5 years of age. While premature infants have the highest rates of hospitalization (three times a term infant) about 79% of hospitalized children younger than 2 years have no underlying medical risks.1 The majority of children will experience RSV as an upper respiratory infection within the first 2 years of life. However, severe disease requiring hospitalization is more likely to occur in premature infants and children younger than 6 months; children younger than 2 with congenital heart disease and/or chronic lung disease; children with severe cystic fibrosis; as well as the immunocompromised child and individuals with neuromuscular disorders that preclude clearing mucous secretions or have difficulty swallowing.
Palivizumab (Synagis), the first monoclonal antibody to prevent RSV in infants was licensed in 1998. Its use was limited to infants meeting specific criteria developed by the American Academy of Pediatrics. Only 5% of infants had access to it. It was a short-acting agent requiring monthly injections, which were very costly ($1,661-$2,584 per dose). Eligible infants could receive up to five injections per season. Several studies proved its use was not cost beneficial.
What are the advantages of nirsevimab? It’s a long-acting monoclonal antibody. Only one dose is required per season. Costs will significantly diminish. It is recommended for all infants younger than 8 months of age born during RSV season. Those children 8-19 months at risk for severe RSV disease can receive it prior to the start of their second RSV season. During RSV season (October 1 to March 31), the initial dose should be administered to newborns just prior to hospital discharge. Older infants and newborns who did not receive it prior to hospital discharge can receive it at their medical home. Newborns should receive it within the first week of life. It is covered by the Vaccine for Children Program. Simultaneous administration with routine childhood immunizations is recommended. Finally, RSV season may vary in tropical areas (Southern Florida, Puerto Rico. etc.) and Alaska. The timing of nirsevimab administration should be based on local RSV activity provided by state and local authorities.
In addition, the FDA approved an RSV vaccine (Abrysvo) for use in adults at least 60 years of age and in pregnant women at 32-36 weeks’ gestation. The latter is administered to prevent lower respiratory tract infection in infants from birth to 6 months. Recommendations have been published for administration in nonpregnant adults. Specific information is forthcoming in terms timing of administration of nirsevimab in infants whose mothers receive Abrysvo.
RSV season is quickly approaching. Detailed recommendations for administration and FAQ questions related to nirsevimab and palivizumab can be found at https://www.aap.org or https://www.cdc.gov/vaccines/hcp/acip-recs/index.html.
Influenza
So, what about influenza? Vaccine composition has been tweaked to match the circulating viruses but the recommended age for annual routine administration remains unchanged. All persons at least 6 months of age should be vaccinated. Children between 6 months and 8 years need two doses at least 4 weeks apart when receiving vaccine for the first time. Immunizing everyone in the household is encouraged especially if there are household contacts at risk for developing severe disease, including infants too young to be vaccinated. Keep in mind children may be coinfected with multiple viruses. Adams and colleagues reviewed the prevalence of coinfection of influenza and Sars-CoV-2 in persons younger than 18 years reported to three CDC surveillance platforms during the 2021-2022 season.2 Thirty-two of 575 hospitalized (6%) coinfections were analyzed and 7 of 44 (16%) deaths. Compared with patients without coinfections, the coinfected patients were more likely to require mechanical ventilation (13% vs. 4%) or CPAP (16% vs. 6%). Only 4 of 23 who were influenza vaccine eligible were vaccinated. Of seven coinfected children who died, none had received influenza vaccine and only one received an antiviral. Only 5 of 31 (16%) infected only with influenza were vaccinated.3
Influenza activity was lower than usual during the 2021-2022 season. However, this report revealed underuse of both influenza vaccine and antiviral therapy, both of which are routinely recommended.
COVID-19
What’s new with COVID-19? On Sept. 12, 2023, ACIP recommended that everyone at least 6 months of age receive the 2023-2024 (monovalent, XBB containing) COVID-19 vaccines. Children at least 5 years of age need one dose and those younger need one or two doses depending on the number of doses previously received. Why the change? Circulating variants continue to change. There is a current uptick in cases including hospitalizations (7.7%) and deaths (4.5%) and it’s just the beginning of the season.4 Symptoms, risk groups and complications have not changed. The primary goal is to prevent infection, hospitalization, long term complications, and death.
We are now armed with the most up-to-date interventions to help prevent the acquisition of these three viruses. Our next step is recommending and delivering them to our patients.
Dr. Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She reported no relevant financial disclosures.
References
1.Suh M et al. J Infect Dis. 2022;226(Suppl 2):S154-36. doi: 10.1093/infdis/jiac120.
2. Adams K et al. MMWR Morb Mortal Wkly Rep. 2022;71:1589-96. doi: http://dx.doi.org/10.15585/mmwr.mm7150a4.
3. Pingali C et al. MMWR Morb Mortal Wkly Rep. 2023 Aug 25;72:912-9. doi: http://dx.doi.org/10.15585/mmwr.mm7234a3.
4. CDC Covid Data Tracker.
Creatine may improve key long COVID symptoms: Small study
Taking creatine as a supplement for 6 months appears to significantly improve clinical features of post–COVID-19 fatigue syndrome (PVFS or long COVID), a small randomized, placebo-controlled, double-blinded study suggests.
Researchers, led by Jelena Slankamenac, with Applied Bioenergetics Lab, Faculty of Sport and PE, University of Novi Sad, Serbia, published their findings in Food, Science & Nutrition .
“This is the first human study known to the authors that evaluated the efficacy and safety of supplemental creatine for fatigue, tissue bioenergetics, and patient-reported outcomes in patients with post–COVID-19 fatigue syndrome,” the authors write.
They say the findings may be attributed to creatine’s “energy-replenishing and neuroprotective activity.”
Significant reductions in symptoms
Researchers randomized the 12 participants into two groups of 6 each. The creatine group received 4 g creatine monohydrate per day, while the placebo group received the same amount of inulin.
At 3 months, dietary creatine supplements produced a significant reduction in fatigue, compared with baseline values ( P = .04) and significantly improved scores for several long COVID–related symptoms, including loss of taste, breathing difficulties, body aches, headache, and difficulties concentrating) ( P < .05), the researchers report.
Intervention effect sizes were assessed by Cohen statistics, with a d of at least 0.8 indicating a large effect.
Among highlights of the results were that patients reported a significant 77.8% drop in scores for concentration difficulties at the 3-month follow-up (Cohen’s effect, d = 1.19) and no concentration difficulties at the 6-month follow-up (Cohen’s effect, d = 2.46).
Total creatine levels increased in several locations across the brain (as much as 33% for right parietal white matter). No changes in tissue creatine levels were found in the placebo group during the trial.
“Since PVFS is characterized by impaired tissue bioenergetics ..., supplemental creatine might be an effective dietary intervention to uphold brain creatine in post–COVID-19 fatigue syndrome,” the authors write.
The authors add that creatine supplements for long COVID patients could benefit organs beyond the brain as participants saw “a significant drop in lung and body pain after the intervention.”
Unanswered questions
Some experts said the results should be interpreted with caution.
“This research paper is very interesting,” says Nisha Viswanathan, MD, director of the long COVID program at University of California, Los Angeles, “but the limited number of patients makes the results difficult to generalize.”
Dr. Viswanathan, who was not part of the study, pointed out that the patients included in this study had a recent COVID infection (under 3 months).
“Acute COVID infection can take up to 3 months to resolve,” she says. “We define patients with long COVID as those with symptoms lasting greater than 3 months. Therefore, these patients could have had improvements in their fatigue due to the natural course of the illness rather than creatine supplementation.”
Alba Azola, MD, assistant professor in the department of physical medicine and rehabilitation at Johns Hopkins University, Baltimore, said she also was troubled by the window of 3 months for recent COVID infection.
She said she would like to see results for patients who have ongoing symptoms for at least 6 months after infection, especially given creatine supplements’ history in research.
Creatine supplements for other conditions, such as fibromyalgia and chronic fatigue syndrome, have been tested for nearly 2 decades, she pointed out, with conflicting findings, something the authors acknowledge in the paper.
“I think it’s premature to say (creatine) is the key,” she says. She added that the small sample size is important to consider given the heterogeneity of patients with long COVID.
That said, Dr. Azola says, she applauds all efforts to find treatments for long COVID, especially randomized, controlled studies like this one.
No major side effects
No major side effects were reported for either intervention, except for transient mild nausea reported by one patient after taking creatine.
Compliance with the intervention was 90.6% ± 3.5% in the creatine group and 95.3% ± 5.0% in the control group (P = .04).
Participants were eligible for inclusion if they were 18-65 years old, had a positive COVID test within the last 3 months (documented by a valid polymerase chain reaction [PCR] or antigen test performed in a COVID-19–certified lab); had moderate to severe fatigue; and at least one additional COVID-related symptom, including loss of taste or smell, breathing trouble, lung pain, body aches, headaches, or difficulties concentrating.
The authors acknowledge that they selected a sample of young to middle-aged adults experiencing moderate long COVID symptoms, and it’s unknown whether creatine is equally effective in other PVFS populations, such as elderly people, children, or patients with less or more severe disease.
Senior author Dr. Sergei Ostojic serves as a member of the Scientific Advisory Board on creatine in health and medicine (AlzChem LLC). He co-owns a patent for “Supplements Based on Liquid Creatine” at the European Patent Office. He has received research support related to creatine during the past 36 months from the Serbian Ministry of Education, Science, and Technological Development; Provincial Secretariat for Higher Education and Scientific Research; Alzchem GmbH; ThermoLife International; and Hueston Hennigan LLP. He does not own stocks and shares in any organization. Other authors declare no known relevant financial interests. Dr. Viswanathan and Dr. Azola report no relevant financial relationships.
Taking creatine as a supplement for 6 months appears to significantly improve clinical features of post–COVID-19 fatigue syndrome (PVFS or long COVID), a small randomized, placebo-controlled, double-blinded study suggests.
Researchers, led by Jelena Slankamenac, with Applied Bioenergetics Lab, Faculty of Sport and PE, University of Novi Sad, Serbia, published their findings in Food, Science & Nutrition .
“This is the first human study known to the authors that evaluated the efficacy and safety of supplemental creatine for fatigue, tissue bioenergetics, and patient-reported outcomes in patients with post–COVID-19 fatigue syndrome,” the authors write.
They say the findings may be attributed to creatine’s “energy-replenishing and neuroprotective activity.”
Significant reductions in symptoms
Researchers randomized the 12 participants into two groups of 6 each. The creatine group received 4 g creatine monohydrate per day, while the placebo group received the same amount of inulin.
At 3 months, dietary creatine supplements produced a significant reduction in fatigue, compared with baseline values ( P = .04) and significantly improved scores for several long COVID–related symptoms, including loss of taste, breathing difficulties, body aches, headache, and difficulties concentrating) ( P < .05), the researchers report.
Intervention effect sizes were assessed by Cohen statistics, with a d of at least 0.8 indicating a large effect.
Among highlights of the results were that patients reported a significant 77.8% drop in scores for concentration difficulties at the 3-month follow-up (Cohen’s effect, d = 1.19) and no concentration difficulties at the 6-month follow-up (Cohen’s effect, d = 2.46).
Total creatine levels increased in several locations across the brain (as much as 33% for right parietal white matter). No changes in tissue creatine levels were found in the placebo group during the trial.
“Since PVFS is characterized by impaired tissue bioenergetics ..., supplemental creatine might be an effective dietary intervention to uphold brain creatine in post–COVID-19 fatigue syndrome,” the authors write.
The authors add that creatine supplements for long COVID patients could benefit organs beyond the brain as participants saw “a significant drop in lung and body pain after the intervention.”
Unanswered questions
Some experts said the results should be interpreted with caution.
“This research paper is very interesting,” says Nisha Viswanathan, MD, director of the long COVID program at University of California, Los Angeles, “but the limited number of patients makes the results difficult to generalize.”
Dr. Viswanathan, who was not part of the study, pointed out that the patients included in this study had a recent COVID infection (under 3 months).
“Acute COVID infection can take up to 3 months to resolve,” she says. “We define patients with long COVID as those with symptoms lasting greater than 3 months. Therefore, these patients could have had improvements in their fatigue due to the natural course of the illness rather than creatine supplementation.”
Alba Azola, MD, assistant professor in the department of physical medicine and rehabilitation at Johns Hopkins University, Baltimore, said she also was troubled by the window of 3 months for recent COVID infection.
She said she would like to see results for patients who have ongoing symptoms for at least 6 months after infection, especially given creatine supplements’ history in research.
Creatine supplements for other conditions, such as fibromyalgia and chronic fatigue syndrome, have been tested for nearly 2 decades, she pointed out, with conflicting findings, something the authors acknowledge in the paper.
“I think it’s premature to say (creatine) is the key,” she says. She added that the small sample size is important to consider given the heterogeneity of patients with long COVID.
That said, Dr. Azola says, she applauds all efforts to find treatments for long COVID, especially randomized, controlled studies like this one.
No major side effects
No major side effects were reported for either intervention, except for transient mild nausea reported by one patient after taking creatine.
Compliance with the intervention was 90.6% ± 3.5% in the creatine group and 95.3% ± 5.0% in the control group (P = .04).
Participants were eligible for inclusion if they were 18-65 years old, had a positive COVID test within the last 3 months (documented by a valid polymerase chain reaction [PCR] or antigen test performed in a COVID-19–certified lab); had moderate to severe fatigue; and at least one additional COVID-related symptom, including loss of taste or smell, breathing trouble, lung pain, body aches, headaches, or difficulties concentrating.
The authors acknowledge that they selected a sample of young to middle-aged adults experiencing moderate long COVID symptoms, and it’s unknown whether creatine is equally effective in other PVFS populations, such as elderly people, children, or patients with less or more severe disease.
Senior author Dr. Sergei Ostojic serves as a member of the Scientific Advisory Board on creatine in health and medicine (AlzChem LLC). He co-owns a patent for “Supplements Based on Liquid Creatine” at the European Patent Office. He has received research support related to creatine during the past 36 months from the Serbian Ministry of Education, Science, and Technological Development; Provincial Secretariat for Higher Education and Scientific Research; Alzchem GmbH; ThermoLife International; and Hueston Hennigan LLP. He does not own stocks and shares in any organization. Other authors declare no known relevant financial interests. Dr. Viswanathan and Dr. Azola report no relevant financial relationships.
Taking creatine as a supplement for 6 months appears to significantly improve clinical features of post–COVID-19 fatigue syndrome (PVFS or long COVID), a small randomized, placebo-controlled, double-blinded study suggests.
Researchers, led by Jelena Slankamenac, with Applied Bioenergetics Lab, Faculty of Sport and PE, University of Novi Sad, Serbia, published their findings in Food, Science & Nutrition .
“This is the first human study known to the authors that evaluated the efficacy and safety of supplemental creatine for fatigue, tissue bioenergetics, and patient-reported outcomes in patients with post–COVID-19 fatigue syndrome,” the authors write.
They say the findings may be attributed to creatine’s “energy-replenishing and neuroprotective activity.”
Significant reductions in symptoms
Researchers randomized the 12 participants into two groups of 6 each. The creatine group received 4 g creatine monohydrate per day, while the placebo group received the same amount of inulin.
At 3 months, dietary creatine supplements produced a significant reduction in fatigue, compared with baseline values ( P = .04) and significantly improved scores for several long COVID–related symptoms, including loss of taste, breathing difficulties, body aches, headache, and difficulties concentrating) ( P < .05), the researchers report.
Intervention effect sizes were assessed by Cohen statistics, with a d of at least 0.8 indicating a large effect.
Among highlights of the results were that patients reported a significant 77.8% drop in scores for concentration difficulties at the 3-month follow-up (Cohen’s effect, d = 1.19) and no concentration difficulties at the 6-month follow-up (Cohen’s effect, d = 2.46).
Total creatine levels increased in several locations across the brain (as much as 33% for right parietal white matter). No changes in tissue creatine levels were found in the placebo group during the trial.
“Since PVFS is characterized by impaired tissue bioenergetics ..., supplemental creatine might be an effective dietary intervention to uphold brain creatine in post–COVID-19 fatigue syndrome,” the authors write.
The authors add that creatine supplements for long COVID patients could benefit organs beyond the brain as participants saw “a significant drop in lung and body pain after the intervention.”
Unanswered questions
Some experts said the results should be interpreted with caution.
“This research paper is very interesting,” says Nisha Viswanathan, MD, director of the long COVID program at University of California, Los Angeles, “but the limited number of patients makes the results difficult to generalize.”
Dr. Viswanathan, who was not part of the study, pointed out that the patients included in this study had a recent COVID infection (under 3 months).
“Acute COVID infection can take up to 3 months to resolve,” she says. “We define patients with long COVID as those with symptoms lasting greater than 3 months. Therefore, these patients could have had improvements in their fatigue due to the natural course of the illness rather than creatine supplementation.”
Alba Azola, MD, assistant professor in the department of physical medicine and rehabilitation at Johns Hopkins University, Baltimore, said she also was troubled by the window of 3 months for recent COVID infection.
She said she would like to see results for patients who have ongoing symptoms for at least 6 months after infection, especially given creatine supplements’ history in research.
Creatine supplements for other conditions, such as fibromyalgia and chronic fatigue syndrome, have been tested for nearly 2 decades, she pointed out, with conflicting findings, something the authors acknowledge in the paper.
“I think it’s premature to say (creatine) is the key,” she says. She added that the small sample size is important to consider given the heterogeneity of patients with long COVID.
That said, Dr. Azola says, she applauds all efforts to find treatments for long COVID, especially randomized, controlled studies like this one.
No major side effects
No major side effects were reported for either intervention, except for transient mild nausea reported by one patient after taking creatine.
Compliance with the intervention was 90.6% ± 3.5% in the creatine group and 95.3% ± 5.0% in the control group (P = .04).
Participants were eligible for inclusion if they were 18-65 years old, had a positive COVID test within the last 3 months (documented by a valid polymerase chain reaction [PCR] or antigen test performed in a COVID-19–certified lab); had moderate to severe fatigue; and at least one additional COVID-related symptom, including loss of taste or smell, breathing trouble, lung pain, body aches, headaches, or difficulties concentrating.
The authors acknowledge that they selected a sample of young to middle-aged adults experiencing moderate long COVID symptoms, and it’s unknown whether creatine is equally effective in other PVFS populations, such as elderly people, children, or patients with less or more severe disease.
Senior author Dr. Sergei Ostojic serves as a member of the Scientific Advisory Board on creatine in health and medicine (AlzChem LLC). He co-owns a patent for “Supplements Based on Liquid Creatine” at the European Patent Office. He has received research support related to creatine during the past 36 months from the Serbian Ministry of Education, Science, and Technological Development; Provincial Secretariat for Higher Education and Scientific Research; Alzchem GmbH; ThermoLife International; and Hueston Hennigan LLP. He does not own stocks and shares in any organization. Other authors declare no known relevant financial interests. Dr. Viswanathan and Dr. Azola report no relevant financial relationships.
FROM FOOD, SCIENCE & NUTRITION
Paxlovid and Lagevrio benefit COVID outpatients in Omicron era
The American College of Physicians has issued an updated version of its living, rapid practice point guideline on the best treatment options for outpatients with confirmed COVID-19 in the era of the dominant Omicron variant of SARS-CoV-2. The recommendations in version 2 apply to persons presenting with mild to moderate infection and symptom onset in the past 5 days who are at high risk for progression to severe disease and potential hospitalization or death.
Version 1 appeared in late 2022.
While outpatient management is appropriate for most patients, treatment should be personalized and based on careful risk stratification and informed decision-making, said the guideline authors, led by Amir Qaseem, MD, PhD, MHA, vice president of clinical policy and the Center for Evidence Reviews at the ACP in Philadelphia.
Practice points
- Consider the oral antivirals nirmatrelvir-ritonavir (Paxlovid) or molnupiravir (Lagevrio) for symptomatic outpatients with confirmed mild to moderate COVID-19 who are within 5 days of the onset of symptoms and at high risk for progressing to severe disease.
New evidence for the Omicron variant suggests a possible net benefit of the antiviral molnupiravir versus standard or no treatment in terms of reducing recovery time if treatment is initiated within 5 days of symptom onset. Nirmatrelvir-ritonavir was associated with reductions in COVID-19 hospitalization and all-cause mortality.
“The practice points only address [whether] treatments work compared to placebo, no treatment, or usual care,” cautioned Linda L. Humphrey, MD, MPH, MACP, chair of the ACP’s Population Health and Medical Science Committee and a professor of medicine at Oregon Health and Science University VA Portland Health Care System. The ACP continues to monitor the evidence. “Once enough evidence has emerged, it will be possible to compare treatments to each other. Until that time we are unable to determine if there is an advantage to using one treatment over another.”
- Do not use the antiparasitic ivermectin (Stromectol) or the monoclonal antibody sotrovimab (Xevudy) to treat this patient population. “It is not expected to be effective against the Omicron variant,” Dr. Humphrey said.
There was no evidence to support the use of medications such as corticosteroids, antibiotics, antihistamines, SSRIs, and multiple other agents.
“The guideline is not a departure from previous knowledge and reflects what appears in other guidelines and is already being done generally in practice,” said Mirella Salvatore, MD, an associate professor of medicine and population health sciences at Weill Cornell Medicine, New York, who was not involved in the ACP statement. It is therefore unlikely the recommendations will trigger controversy or negative feedback, added Dr. Salvatore, who is also a spokesperson for the Infectious Diseases Society of America. “We believe that our evidence-based approach, which considers the balance of benefits and harms of various treatments, will be embraced by the physician community,” Dr. Humphrey said.
The updated recommendations are based on new data from the evidence review of multiple treatments, which concluded that both nirmatrelvir-ritonavir and molnupiravir likely improve outcomes for outpatients with mild to moderate COVID-19. The review was conducted after the emergence of the Omicron variant by the ACP Center for Evidence Reviews at Cochrane Austria/University for Continuing Education Krems (Austria).
Review details
Inclusion criteria were modified to focus on the Omicron variant by limiting eligible studies to only those enrolling patients on or after Nov. 26, 2021. The investigators included two randomized controlled trials and six retrospective cohort studies and ranked quality of evidence for the effectiveness of the following treatments, compared with usual care or no treatment: azithromycin, camostat mesylate, chloroquine-hydroxychloroquine, chlorpheniramine, colchicine, convalescent plasma, corticosteroids, ensitrelvir, favipiravir, fluvoxamine, ivermectin, lopinavir-ritonavir, molnupiravir, neutralizing monoclonal antibodies, metformin, niclosamide, nitazoxanide, nirmatrelvir-ritonavir, and remdesivir.
It compared results for all-cause and COVID-specific mortality, recovery, time to recovery, COVID hospitalization, and adverse and serious adverse events.
Nirmatrelvir-ritonavir was associated with a reduction in hospitalization caused by COVID-19 of 0.7% versus 1.2% (moderate certainty of evidence [COE]) and a reduction in all-cause mortality of less than 0.1% versus 0.2% (moderate COE).
Molnupiravir led to a higher recovery rate of 31.8% versus 22.6% (moderate COE) and a reduced time to recovery of 9 versus 15 median days (moderate COE). It had no effect, however, on all-cause mortality: 0.02% versus 0.04% (moderate COE). Nor did it affect the incidence of serious adverse events: 0.4% versus 0.3% (moderate COE).
“There have been no head-to-head comparative studies of these two treatments, but nirmatrelvir-ritonavir appears to be the preferred treatment,” Dr. Salvatore said. She noted that molnupiravir cannot be used in pregnant women or young persons under age 18, while nirmatrelvir-ritonavir carries the risk of drug interactions. Viral rebound and recurrence of symptoms have been reported in some patients receiving nirmatrelvir-ritonavir.
In other review findings, ivermectin had no effect on time to recovery (moderate COE) and adverse events versus placebo (low COE). Sotrovimab resulted in no difference in all-cause mortality, compared with no treatment (low COE). There were no eligible studies for all of the other treatments of interest nor were there any that specifically evaluated the benefits and harms of treatments for the Omicron variant.
The panel pointed to the need for more evaluation of the efficacy, effectiveness, and comparative effectiveness, as well as harms of pharmacologic and biologic treatments of COVID-19 in the outpatient setting, particularly in the context of changing dominant SARS-CoV-2 variants and subvariants.
Another area requiring further research is the effectiveness of retreatment in patients with previous COVID-19 infection. Subgroup analyses are also needed to assess whether the efficacy and effectiveness of outpatient treatments vary by age, sex, socioeconomic status, and comorbid conditions – or by SARS-CoV-2 variant, immunity status (prior SARS-CoV-2 infection, vaccination status, or time since infection or vaccination), symptom duration, or disease severity.
Dr. Salvatore agreed that more research is needed in special convalescent groups. “For instance, those with cancer who are immunocompromised may need longer treatment and adjunctive treatment with convalescent plasma. But is difficult to find a large enough study with 5,000 immunocompromised patients.”
Financial support for the development of the practice points came exclusively from the ACP operating budget. The evidence review was funded by the ACP. The authors disclosed no relevant high-level competing interests with regard to this guidance, although several authors reported intellectual interests in various areas of research. Dr. Salvatore disclosed no conflicts of interest relevant to her comments but is engaged in influenza research for Genentech.
The American College of Physicians has issued an updated version of its living, rapid practice point guideline on the best treatment options for outpatients with confirmed COVID-19 in the era of the dominant Omicron variant of SARS-CoV-2. The recommendations in version 2 apply to persons presenting with mild to moderate infection and symptom onset in the past 5 days who are at high risk for progression to severe disease and potential hospitalization or death.
Version 1 appeared in late 2022.
While outpatient management is appropriate for most patients, treatment should be personalized and based on careful risk stratification and informed decision-making, said the guideline authors, led by Amir Qaseem, MD, PhD, MHA, vice president of clinical policy and the Center for Evidence Reviews at the ACP in Philadelphia.
Practice points
- Consider the oral antivirals nirmatrelvir-ritonavir (Paxlovid) or molnupiravir (Lagevrio) for symptomatic outpatients with confirmed mild to moderate COVID-19 who are within 5 days of the onset of symptoms and at high risk for progressing to severe disease.
New evidence for the Omicron variant suggests a possible net benefit of the antiviral molnupiravir versus standard or no treatment in terms of reducing recovery time if treatment is initiated within 5 days of symptom onset. Nirmatrelvir-ritonavir was associated with reductions in COVID-19 hospitalization and all-cause mortality.
“The practice points only address [whether] treatments work compared to placebo, no treatment, or usual care,” cautioned Linda L. Humphrey, MD, MPH, MACP, chair of the ACP’s Population Health and Medical Science Committee and a professor of medicine at Oregon Health and Science University VA Portland Health Care System. The ACP continues to monitor the evidence. “Once enough evidence has emerged, it will be possible to compare treatments to each other. Until that time we are unable to determine if there is an advantage to using one treatment over another.”
- Do not use the antiparasitic ivermectin (Stromectol) or the monoclonal antibody sotrovimab (Xevudy) to treat this patient population. “It is not expected to be effective against the Omicron variant,” Dr. Humphrey said.
There was no evidence to support the use of medications such as corticosteroids, antibiotics, antihistamines, SSRIs, and multiple other agents.
“The guideline is not a departure from previous knowledge and reflects what appears in other guidelines and is already being done generally in practice,” said Mirella Salvatore, MD, an associate professor of medicine and population health sciences at Weill Cornell Medicine, New York, who was not involved in the ACP statement. It is therefore unlikely the recommendations will trigger controversy or negative feedback, added Dr. Salvatore, who is also a spokesperson for the Infectious Diseases Society of America. “We believe that our evidence-based approach, which considers the balance of benefits and harms of various treatments, will be embraced by the physician community,” Dr. Humphrey said.
The updated recommendations are based on new data from the evidence review of multiple treatments, which concluded that both nirmatrelvir-ritonavir and molnupiravir likely improve outcomes for outpatients with mild to moderate COVID-19. The review was conducted after the emergence of the Omicron variant by the ACP Center for Evidence Reviews at Cochrane Austria/University for Continuing Education Krems (Austria).
Review details
Inclusion criteria were modified to focus on the Omicron variant by limiting eligible studies to only those enrolling patients on or after Nov. 26, 2021. The investigators included two randomized controlled trials and six retrospective cohort studies and ranked quality of evidence for the effectiveness of the following treatments, compared with usual care or no treatment: azithromycin, camostat mesylate, chloroquine-hydroxychloroquine, chlorpheniramine, colchicine, convalescent plasma, corticosteroids, ensitrelvir, favipiravir, fluvoxamine, ivermectin, lopinavir-ritonavir, molnupiravir, neutralizing monoclonal antibodies, metformin, niclosamide, nitazoxanide, nirmatrelvir-ritonavir, and remdesivir.
It compared results for all-cause and COVID-specific mortality, recovery, time to recovery, COVID hospitalization, and adverse and serious adverse events.
Nirmatrelvir-ritonavir was associated with a reduction in hospitalization caused by COVID-19 of 0.7% versus 1.2% (moderate certainty of evidence [COE]) and a reduction in all-cause mortality of less than 0.1% versus 0.2% (moderate COE).
Molnupiravir led to a higher recovery rate of 31.8% versus 22.6% (moderate COE) and a reduced time to recovery of 9 versus 15 median days (moderate COE). It had no effect, however, on all-cause mortality: 0.02% versus 0.04% (moderate COE). Nor did it affect the incidence of serious adverse events: 0.4% versus 0.3% (moderate COE).
“There have been no head-to-head comparative studies of these two treatments, but nirmatrelvir-ritonavir appears to be the preferred treatment,” Dr. Salvatore said. She noted that molnupiravir cannot be used in pregnant women or young persons under age 18, while nirmatrelvir-ritonavir carries the risk of drug interactions. Viral rebound and recurrence of symptoms have been reported in some patients receiving nirmatrelvir-ritonavir.
In other review findings, ivermectin had no effect on time to recovery (moderate COE) and adverse events versus placebo (low COE). Sotrovimab resulted in no difference in all-cause mortality, compared with no treatment (low COE). There were no eligible studies for all of the other treatments of interest nor were there any that specifically evaluated the benefits and harms of treatments for the Omicron variant.
The panel pointed to the need for more evaluation of the efficacy, effectiveness, and comparative effectiveness, as well as harms of pharmacologic and biologic treatments of COVID-19 in the outpatient setting, particularly in the context of changing dominant SARS-CoV-2 variants and subvariants.
Another area requiring further research is the effectiveness of retreatment in patients with previous COVID-19 infection. Subgroup analyses are also needed to assess whether the efficacy and effectiveness of outpatient treatments vary by age, sex, socioeconomic status, and comorbid conditions – or by SARS-CoV-2 variant, immunity status (prior SARS-CoV-2 infection, vaccination status, or time since infection or vaccination), symptom duration, or disease severity.
Dr. Salvatore agreed that more research is needed in special convalescent groups. “For instance, those with cancer who are immunocompromised may need longer treatment and adjunctive treatment with convalescent plasma. But is difficult to find a large enough study with 5,000 immunocompromised patients.”
Financial support for the development of the practice points came exclusively from the ACP operating budget. The evidence review was funded by the ACP. The authors disclosed no relevant high-level competing interests with regard to this guidance, although several authors reported intellectual interests in various areas of research. Dr. Salvatore disclosed no conflicts of interest relevant to her comments but is engaged in influenza research for Genentech.
The American College of Physicians has issued an updated version of its living, rapid practice point guideline on the best treatment options for outpatients with confirmed COVID-19 in the era of the dominant Omicron variant of SARS-CoV-2. The recommendations in version 2 apply to persons presenting with mild to moderate infection and symptom onset in the past 5 days who are at high risk for progression to severe disease and potential hospitalization or death.
Version 1 appeared in late 2022.
While outpatient management is appropriate for most patients, treatment should be personalized and based on careful risk stratification and informed decision-making, said the guideline authors, led by Amir Qaseem, MD, PhD, MHA, vice president of clinical policy and the Center for Evidence Reviews at the ACP in Philadelphia.
Practice points
- Consider the oral antivirals nirmatrelvir-ritonavir (Paxlovid) or molnupiravir (Lagevrio) for symptomatic outpatients with confirmed mild to moderate COVID-19 who are within 5 days of the onset of symptoms and at high risk for progressing to severe disease.
New evidence for the Omicron variant suggests a possible net benefit of the antiviral molnupiravir versus standard or no treatment in terms of reducing recovery time if treatment is initiated within 5 days of symptom onset. Nirmatrelvir-ritonavir was associated with reductions in COVID-19 hospitalization and all-cause mortality.
“The practice points only address [whether] treatments work compared to placebo, no treatment, or usual care,” cautioned Linda L. Humphrey, MD, MPH, MACP, chair of the ACP’s Population Health and Medical Science Committee and a professor of medicine at Oregon Health and Science University VA Portland Health Care System. The ACP continues to monitor the evidence. “Once enough evidence has emerged, it will be possible to compare treatments to each other. Until that time we are unable to determine if there is an advantage to using one treatment over another.”
- Do not use the antiparasitic ivermectin (Stromectol) or the monoclonal antibody sotrovimab (Xevudy) to treat this patient population. “It is not expected to be effective against the Omicron variant,” Dr. Humphrey said.
There was no evidence to support the use of medications such as corticosteroids, antibiotics, antihistamines, SSRIs, and multiple other agents.
“The guideline is not a departure from previous knowledge and reflects what appears in other guidelines and is already being done generally in practice,” said Mirella Salvatore, MD, an associate professor of medicine and population health sciences at Weill Cornell Medicine, New York, who was not involved in the ACP statement. It is therefore unlikely the recommendations will trigger controversy or negative feedback, added Dr. Salvatore, who is also a spokesperson for the Infectious Diseases Society of America. “We believe that our evidence-based approach, which considers the balance of benefits and harms of various treatments, will be embraced by the physician community,” Dr. Humphrey said.
The updated recommendations are based on new data from the evidence review of multiple treatments, which concluded that both nirmatrelvir-ritonavir and molnupiravir likely improve outcomes for outpatients with mild to moderate COVID-19. The review was conducted after the emergence of the Omicron variant by the ACP Center for Evidence Reviews at Cochrane Austria/University for Continuing Education Krems (Austria).
Review details
Inclusion criteria were modified to focus on the Omicron variant by limiting eligible studies to only those enrolling patients on or after Nov. 26, 2021. The investigators included two randomized controlled trials and six retrospective cohort studies and ranked quality of evidence for the effectiveness of the following treatments, compared with usual care or no treatment: azithromycin, camostat mesylate, chloroquine-hydroxychloroquine, chlorpheniramine, colchicine, convalescent plasma, corticosteroids, ensitrelvir, favipiravir, fluvoxamine, ivermectin, lopinavir-ritonavir, molnupiravir, neutralizing monoclonal antibodies, metformin, niclosamide, nitazoxanide, nirmatrelvir-ritonavir, and remdesivir.
It compared results for all-cause and COVID-specific mortality, recovery, time to recovery, COVID hospitalization, and adverse and serious adverse events.
Nirmatrelvir-ritonavir was associated with a reduction in hospitalization caused by COVID-19 of 0.7% versus 1.2% (moderate certainty of evidence [COE]) and a reduction in all-cause mortality of less than 0.1% versus 0.2% (moderate COE).
Molnupiravir led to a higher recovery rate of 31.8% versus 22.6% (moderate COE) and a reduced time to recovery of 9 versus 15 median days (moderate COE). It had no effect, however, on all-cause mortality: 0.02% versus 0.04% (moderate COE). Nor did it affect the incidence of serious adverse events: 0.4% versus 0.3% (moderate COE).
“There have been no head-to-head comparative studies of these two treatments, but nirmatrelvir-ritonavir appears to be the preferred treatment,” Dr. Salvatore said. She noted that molnupiravir cannot be used in pregnant women or young persons under age 18, while nirmatrelvir-ritonavir carries the risk of drug interactions. Viral rebound and recurrence of symptoms have been reported in some patients receiving nirmatrelvir-ritonavir.
In other review findings, ivermectin had no effect on time to recovery (moderate COE) and adverse events versus placebo (low COE). Sotrovimab resulted in no difference in all-cause mortality, compared with no treatment (low COE). There were no eligible studies for all of the other treatments of interest nor were there any that specifically evaluated the benefits and harms of treatments for the Omicron variant.
The panel pointed to the need for more evaluation of the efficacy, effectiveness, and comparative effectiveness, as well as harms of pharmacologic and biologic treatments of COVID-19 in the outpatient setting, particularly in the context of changing dominant SARS-CoV-2 variants and subvariants.
Another area requiring further research is the effectiveness of retreatment in patients with previous COVID-19 infection. Subgroup analyses are also needed to assess whether the efficacy and effectiveness of outpatient treatments vary by age, sex, socioeconomic status, and comorbid conditions – or by SARS-CoV-2 variant, immunity status (prior SARS-CoV-2 infection, vaccination status, or time since infection or vaccination), symptom duration, or disease severity.
Dr. Salvatore agreed that more research is needed in special convalescent groups. “For instance, those with cancer who are immunocompromised may need longer treatment and adjunctive treatment with convalescent plasma. But is difficult to find a large enough study with 5,000 immunocompromised patients.”
Financial support for the development of the practice points came exclusively from the ACP operating budget. The evidence review was funded by the ACP. The authors disclosed no relevant high-level competing interests with regard to this guidance, although several authors reported intellectual interests in various areas of research. Dr. Salvatore disclosed no conflicts of interest relevant to her comments but is engaged in influenza research for Genentech.
FROM ANNALS OF INTERNAL MEDICINE
Long COVID patients turn to doctors for help with disability claims
As millions of Americans face another year of long COVID, some are finding they are unable to return to work or cannot work as they did before they got sick and are turning to doctors for help with documenting their disability.
For those who can return to work, a doctor’s diagnosis of long COVID is key to gaining access to workplace accommodations, such as working flex hours or remotely. For those who cannot work, a note from the doctor is the first step to collecting disability payments.
With no definitive blood tests or scans for long COVID that could confirm a diagnosis, some say doctors may feel uncomfortable in this role, which puts them in a tough spot, said Wes Ely, MD, MPH, codirector of the critical illness, brain dysfunction and survivorship center at Vanderbilt University, Nashville, Tenn.
Doctors typically are not taught to deal with vagueness in diagnostics.
“Long COVID falls straight into the gray zone,” he said. There are no tests and a long list of common symptoms. “It makes a lot of doctors feel super insecure,” he said.
Now, patients and their advocates are calling for doctors to be more open-minded about how they assess those with long COVID and other chronic illnesses. Although their disability may not be visible, many with long COVID struggle to function. If they need help, they say, they need a doctor to confirm their limitations – test results or no test results.
Better documentation of patient-reported symptoms would go a long way, according to a perspective published in The New England Journal of Medicine.
“There’s a long history of people with disabilities being forced to ask doctors to legitimize their symptoms,” said study author Zackary Berger, MD, PhD, Johns Hopkins University, Baltimore, Md. Dr. Berger believes doctors should learn to listen more closely to patients, turn their narratives into patient notes, and use the new International Classification of Diseases 10 (ICD-10) code, a worldwide system for identifying and generating data on diseases, when they diagnose long COVID. He also thinks doctors should become advocates for their patients.
The Americans With Disabilities Act allows employers to request medical proof of disability, “and thereby assigns physicians the gate-keeping role of determining patients’ eligibility for reasonable accommodations,” according to the analysis. Those accommodations may mean a handicapped parking space or extra days working remotely.
Without a definitive diagnostic test, long COVID joins fibromyalgia and ME/CFS (myalgic encephalomyelitis/chronic fatigue syndrome), which lack biomarkers or imaging tests to support a diagnosis, they write.
“These diagnoses are therefore contentious, and government agencies, employers, and many physicians do not accept these conditions as real,” they write.
Physicians make a good faith effort in trying to understand long COVID, but both doctors and the courts like to see evidence, said Michael Ashley Stein, JD, PHD, director of the Harvard Law School Project on Disability. Dr. Stein and others say that doctors should listen closely to their patients’ descriptions of their symptoms.
“In the absence of agreed-upon biomarkers, doctors need to listen to their patients and look for other [indications] and other consistent evidence of conditions, and then work from there rather than dismiss the existence of these conditions,” he said.
Dr. Ely said he and others were taught in medical school that if it doesn’t come up on a diagnostic test, there’s no problem. “I am absolutely complicit,” he said. “I’m part of the community that did that for so many years.”
Dr. Ely agreed that the demand for clinical test results does not work for long COVID and chronic diseases such as ME/CFS. People come in with complaints and they get a typical medical workup with labs, he said, and the labs look normal on paper.
“And [the doctor is] thinking: ‘I don’t know what is wrong with this person and there’s nothing on paper I can treat. I don’t know if I even believe in long COVID.’ ”
At the same time, patients might need support from a doctor to get accommodations at work under the ADA, such as flexible hours. Or doctors’ notes may be required if a patient is trying to collect private disability insurance, workers compensation, or federal disability payments through Social Security.
The U.S. Centers for Disease Control and Prevention guidelines on diagnosing long COVID, updated last December, point out that normal laboratory or imaging findings do not rule out long COVID.
In addition, 12 key symptoms of long COVID were identified in May by scientists working with the RECOVER Initiative, the federal government’s long COVID research program. These symptoms include fatigue, brain fog, dizziness, gastrointestinal symptoms, loss of or change in smell or taste, chest pain, and abnormal movements.
Still, patients with long COVID seeking help also face the “disability con,” a term coined by the second author of the NEJM article, Doron Dorfman, a professor at Seton Hall Law School in Newark, N.J.
“Nowadays, when people think disability, they immediately think fraud,” he said.
Prof. Dorfman thinks the perception that many people are faking disabilities to gain an unfair advantage is the biggest barrier for anyone seeking help. The disability system is “preventing people who deserve legal rights from actually obtaining them,” he said.
He urged doctors to believe their patients. One way is to try to “translate the person’s narrative into medical language.”
His coauthor Dr. Berger did not agree with the argument that doctors cannot diagnose without tests.
“Any clinician knows that lab tests are not everything,” he said. “There are conditions that don’t have specific biomarkers that we diagnose all the time.” He cited acquired pneumonia and urinary tract infections as examples.
Benefits lawyers have taken note of the complexities for people with long COVID who seek help through the ADA and federal disability program.
One law firm noted: “The government safety net is not designed to help an emerging disease with no clear diagnosis or treatment plans. Insurance carriers are denying claims, and long-term disability benefits are being denied.”
About 16 million working-age Americans have long COVID, according to an update of a 2022 report by the Brookings Institute. Up to 4 million of these people are out of work because of the condition, the study found. The research is based on newly collected U.S. Census Bureau data that show 24% of those with long COVID report “significant activity limitations.”
Dr. Ely said he sees progress in this area. Many of these issues have come up at the committee convened by the National Academy of Science to look at the working definition of long COVID. NAS, a Washington research group, held a public meeting on their findings on June 22.
A version of this article first appeared on Medscape.com.
As millions of Americans face another year of long COVID, some are finding they are unable to return to work or cannot work as they did before they got sick and are turning to doctors for help with documenting their disability.
For those who can return to work, a doctor’s diagnosis of long COVID is key to gaining access to workplace accommodations, such as working flex hours or remotely. For those who cannot work, a note from the doctor is the first step to collecting disability payments.
With no definitive blood tests or scans for long COVID that could confirm a diagnosis, some say doctors may feel uncomfortable in this role, which puts them in a tough spot, said Wes Ely, MD, MPH, codirector of the critical illness, brain dysfunction and survivorship center at Vanderbilt University, Nashville, Tenn.
Doctors typically are not taught to deal with vagueness in diagnostics.
“Long COVID falls straight into the gray zone,” he said. There are no tests and a long list of common symptoms. “It makes a lot of doctors feel super insecure,” he said.
Now, patients and their advocates are calling for doctors to be more open-minded about how they assess those with long COVID and other chronic illnesses. Although their disability may not be visible, many with long COVID struggle to function. If they need help, they say, they need a doctor to confirm their limitations – test results or no test results.
Better documentation of patient-reported symptoms would go a long way, according to a perspective published in The New England Journal of Medicine.
“There’s a long history of people with disabilities being forced to ask doctors to legitimize their symptoms,” said study author Zackary Berger, MD, PhD, Johns Hopkins University, Baltimore, Md. Dr. Berger believes doctors should learn to listen more closely to patients, turn their narratives into patient notes, and use the new International Classification of Diseases 10 (ICD-10) code, a worldwide system for identifying and generating data on diseases, when they diagnose long COVID. He also thinks doctors should become advocates for their patients.
The Americans With Disabilities Act allows employers to request medical proof of disability, “and thereby assigns physicians the gate-keeping role of determining patients’ eligibility for reasonable accommodations,” according to the analysis. Those accommodations may mean a handicapped parking space or extra days working remotely.
Without a definitive diagnostic test, long COVID joins fibromyalgia and ME/CFS (myalgic encephalomyelitis/chronic fatigue syndrome), which lack biomarkers or imaging tests to support a diagnosis, they write.
“These diagnoses are therefore contentious, and government agencies, employers, and many physicians do not accept these conditions as real,” they write.
Physicians make a good faith effort in trying to understand long COVID, but both doctors and the courts like to see evidence, said Michael Ashley Stein, JD, PHD, director of the Harvard Law School Project on Disability. Dr. Stein and others say that doctors should listen closely to their patients’ descriptions of their symptoms.
“In the absence of agreed-upon biomarkers, doctors need to listen to their patients and look for other [indications] and other consistent evidence of conditions, and then work from there rather than dismiss the existence of these conditions,” he said.
Dr. Ely said he and others were taught in medical school that if it doesn’t come up on a diagnostic test, there’s no problem. “I am absolutely complicit,” he said. “I’m part of the community that did that for so many years.”
Dr. Ely agreed that the demand for clinical test results does not work for long COVID and chronic diseases such as ME/CFS. People come in with complaints and they get a typical medical workup with labs, he said, and the labs look normal on paper.
“And [the doctor is] thinking: ‘I don’t know what is wrong with this person and there’s nothing on paper I can treat. I don’t know if I even believe in long COVID.’ ”
At the same time, patients might need support from a doctor to get accommodations at work under the ADA, such as flexible hours. Or doctors’ notes may be required if a patient is trying to collect private disability insurance, workers compensation, or federal disability payments through Social Security.
The U.S. Centers for Disease Control and Prevention guidelines on diagnosing long COVID, updated last December, point out that normal laboratory or imaging findings do not rule out long COVID.
In addition, 12 key symptoms of long COVID were identified in May by scientists working with the RECOVER Initiative, the federal government’s long COVID research program. These symptoms include fatigue, brain fog, dizziness, gastrointestinal symptoms, loss of or change in smell or taste, chest pain, and abnormal movements.
Still, patients with long COVID seeking help also face the “disability con,” a term coined by the second author of the NEJM article, Doron Dorfman, a professor at Seton Hall Law School in Newark, N.J.
“Nowadays, when people think disability, they immediately think fraud,” he said.
Prof. Dorfman thinks the perception that many people are faking disabilities to gain an unfair advantage is the biggest barrier for anyone seeking help. The disability system is “preventing people who deserve legal rights from actually obtaining them,” he said.
He urged doctors to believe their patients. One way is to try to “translate the person’s narrative into medical language.”
His coauthor Dr. Berger did not agree with the argument that doctors cannot diagnose without tests.
“Any clinician knows that lab tests are not everything,” he said. “There are conditions that don’t have specific biomarkers that we diagnose all the time.” He cited acquired pneumonia and urinary tract infections as examples.
Benefits lawyers have taken note of the complexities for people with long COVID who seek help through the ADA and federal disability program.
One law firm noted: “The government safety net is not designed to help an emerging disease with no clear diagnosis or treatment plans. Insurance carriers are denying claims, and long-term disability benefits are being denied.”
About 16 million working-age Americans have long COVID, according to an update of a 2022 report by the Brookings Institute. Up to 4 million of these people are out of work because of the condition, the study found. The research is based on newly collected U.S. Census Bureau data that show 24% of those with long COVID report “significant activity limitations.”
Dr. Ely said he sees progress in this area. Many of these issues have come up at the committee convened by the National Academy of Science to look at the working definition of long COVID. NAS, a Washington research group, held a public meeting on their findings on June 22.
A version of this article first appeared on Medscape.com.
As millions of Americans face another year of long COVID, some are finding they are unable to return to work or cannot work as they did before they got sick and are turning to doctors for help with documenting their disability.
For those who can return to work, a doctor’s diagnosis of long COVID is key to gaining access to workplace accommodations, such as working flex hours or remotely. For those who cannot work, a note from the doctor is the first step to collecting disability payments.
With no definitive blood tests or scans for long COVID that could confirm a diagnosis, some say doctors may feel uncomfortable in this role, which puts them in a tough spot, said Wes Ely, MD, MPH, codirector of the critical illness, brain dysfunction and survivorship center at Vanderbilt University, Nashville, Tenn.
Doctors typically are not taught to deal with vagueness in diagnostics.
“Long COVID falls straight into the gray zone,” he said. There are no tests and a long list of common symptoms. “It makes a lot of doctors feel super insecure,” he said.
Now, patients and their advocates are calling for doctors to be more open-minded about how they assess those with long COVID and other chronic illnesses. Although their disability may not be visible, many with long COVID struggle to function. If they need help, they say, they need a doctor to confirm their limitations – test results or no test results.
Better documentation of patient-reported symptoms would go a long way, according to a perspective published in The New England Journal of Medicine.
“There’s a long history of people with disabilities being forced to ask doctors to legitimize their symptoms,” said study author Zackary Berger, MD, PhD, Johns Hopkins University, Baltimore, Md. Dr. Berger believes doctors should learn to listen more closely to patients, turn their narratives into patient notes, and use the new International Classification of Diseases 10 (ICD-10) code, a worldwide system for identifying and generating data on diseases, when they diagnose long COVID. He also thinks doctors should become advocates for their patients.
The Americans With Disabilities Act allows employers to request medical proof of disability, “and thereby assigns physicians the gate-keeping role of determining patients’ eligibility for reasonable accommodations,” according to the analysis. Those accommodations may mean a handicapped parking space or extra days working remotely.
Without a definitive diagnostic test, long COVID joins fibromyalgia and ME/CFS (myalgic encephalomyelitis/chronic fatigue syndrome), which lack biomarkers or imaging tests to support a diagnosis, they write.
“These diagnoses are therefore contentious, and government agencies, employers, and many physicians do not accept these conditions as real,” they write.
Physicians make a good faith effort in trying to understand long COVID, but both doctors and the courts like to see evidence, said Michael Ashley Stein, JD, PHD, director of the Harvard Law School Project on Disability. Dr. Stein and others say that doctors should listen closely to their patients’ descriptions of their symptoms.
“In the absence of agreed-upon biomarkers, doctors need to listen to their patients and look for other [indications] and other consistent evidence of conditions, and then work from there rather than dismiss the existence of these conditions,” he said.
Dr. Ely said he and others were taught in medical school that if it doesn’t come up on a diagnostic test, there’s no problem. “I am absolutely complicit,” he said. “I’m part of the community that did that for so many years.”
Dr. Ely agreed that the demand for clinical test results does not work for long COVID and chronic diseases such as ME/CFS. People come in with complaints and they get a typical medical workup with labs, he said, and the labs look normal on paper.
“And [the doctor is] thinking: ‘I don’t know what is wrong with this person and there’s nothing on paper I can treat. I don’t know if I even believe in long COVID.’ ”
At the same time, patients might need support from a doctor to get accommodations at work under the ADA, such as flexible hours. Or doctors’ notes may be required if a patient is trying to collect private disability insurance, workers compensation, or federal disability payments through Social Security.
The U.S. Centers for Disease Control and Prevention guidelines on diagnosing long COVID, updated last December, point out that normal laboratory or imaging findings do not rule out long COVID.
In addition, 12 key symptoms of long COVID were identified in May by scientists working with the RECOVER Initiative, the federal government’s long COVID research program. These symptoms include fatigue, brain fog, dizziness, gastrointestinal symptoms, loss of or change in smell or taste, chest pain, and abnormal movements.
Still, patients with long COVID seeking help also face the “disability con,” a term coined by the second author of the NEJM article, Doron Dorfman, a professor at Seton Hall Law School in Newark, N.J.
“Nowadays, when people think disability, they immediately think fraud,” he said.
Prof. Dorfman thinks the perception that many people are faking disabilities to gain an unfair advantage is the biggest barrier for anyone seeking help. The disability system is “preventing people who deserve legal rights from actually obtaining them,” he said.
He urged doctors to believe their patients. One way is to try to “translate the person’s narrative into medical language.”
His coauthor Dr. Berger did not agree with the argument that doctors cannot diagnose without tests.
“Any clinician knows that lab tests are not everything,” he said. “There are conditions that don’t have specific biomarkers that we diagnose all the time.” He cited acquired pneumonia and urinary tract infections as examples.
Benefits lawyers have taken note of the complexities for people with long COVID who seek help through the ADA and federal disability program.
One law firm noted: “The government safety net is not designed to help an emerging disease with no clear diagnosis or treatment plans. Insurance carriers are denying claims, and long-term disability benefits are being denied.”
About 16 million working-age Americans have long COVID, according to an update of a 2022 report by the Brookings Institute. Up to 4 million of these people are out of work because of the condition, the study found. The research is based on newly collected U.S. Census Bureau data that show 24% of those with long COVID report “significant activity limitations.”
Dr. Ely said he sees progress in this area. Many of these issues have come up at the committee convened by the National Academy of Science to look at the working definition of long COVID. NAS, a Washington research group, held a public meeting on their findings on June 22.
A version of this article first appeared on Medscape.com.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Skin Diseases Associated With COVID-19: A Narrative Review
COVID-19 is a potentially severe systemic disease caused by SARS-CoV-2. SARS-CoV and Middle East respiratory syndrome (MERS-CoV) caused fatal epidemics in Asia in 2002 to 2003 and in the Arabian Peninsula in 2012, respectively. In 2019, SARS-CoV-2 was detected in patients with severe, sometimes fatal pneumonia of previously unknown origin; it rapidly spread around the world, and the World Health Organization declared the disease a pandemic on March 11, 2020. SARS-CoV-2 is a β-coronavirus that is genetically related to the bat coronavirus and SARS-CoV; it is a single-stranded RNA virus of which several variants and subvariants exist. The SARS-CoV-2 viral particles bind via their surface spike protein (S protein) to the angiotensin-converting enzyme 2 receptor present on the membrane of several cell types, including epidermal and adnexal keratinocytes.1,2 The α and δ variants, predominant from 2020 to 2021, mainly affected the lower respiratory tract and caused severe, potentially fatal pneumonia, especially in patients older than 65 years and/or with comorbidities, such as obesity, hypertension, diabetes, and (iatrogenic) immunosuppression. The ο variant, which appeared in late 2021, is more contagious than the initial variants, but it causes a less severe disease preferentially affecting the upper respiratory airways.3 As of April 5, 2023, more than 762,000,000 confirmed cases of COVID-19 have been recorded worldwide, causing more than 6,800,000 deaths.4
Early studies from China describing the symptoms of COVID-19 reported a low frequency of skin manifestations (0.2%), probably because they were focused on the most severe disease symptoms.5 Subsequently, when COVID-19 spread to the rest of the world, an increasing number of skin manifestations were reported in association with the disease. After the first publication from northern Italy in spring 2020, which was specifically devoted to skin manifestations of COVID-19,6 an explosive number of publications reported a large number of skin manifestations, and national registries were established in several countries to record these manifestations, such as the American Academy of Dermatology and the International League of Dermatological Societies registry,7,8 the COVIDSKIN registry of the French Dermatology Society,9 and the Italian registry.10 Highlighting the unprecedented number of scientific articles published on this new disease, a PubMed search of articles indexed for MEDLINE search using the terms SARS-CoV-2 or COVID-19, on April 6, 2023, revealed 351,596 articles; that is more than 300 articles published every day in this database alone, with a large number of them concerning the skin.
SKIN DISEASSES ASSOCIATED WITH COVID-19
There are several types of COVID-19–related skin manifestations, depending on the circumstances of onset and the evolution of the pandemic.
Skin Manifestations Associated With SARS-CoV-2 Infection
The estimated incidence varies greatly according to the published series of patients, possibly depending on the geographic location. The estimated incidence seems lower in Asian countries, such as China (0.2%)5 and Japan (0.56%),11 compared with Europe (up to 20%).6 Skin manifestations associated with SARS-CoV-2 infection affect individuals of all ages, slightly more females, and are clinically polymorphous; some of them are associated with the severity of the infection.12 They may precede, accompany, or appear after the symptoms of COVID-19, most often within a month of the infection, of which they rarely are the only manifestation; however, their precise relationship to SARS-CoV-2 is not always well known. They have been classified according to their clinical presentation into several forms.13-15
Morbilliform Maculopapular Eruption—Representing 16% to 53% of skin manifestations, morbilliform and maculopapular eruptions usually appear within 15 days of infection; they manifest with more or less confluent erythematous macules that may be hemorrhagic/petechial, and usually are asymptomatic and rarely pruritic. The rash mainly affects the trunk and limbs, sparing the face, palmoplantar regions, and mucous membranes; it appears concomitantly with or a few days after the first symptoms of COVID-19 (eg, fever, respiratory symptoms), regresses within a few days, and does not appear to be associated with disease severity. The distinction from maculopapular drug eruptions may be subtle. Histologically, the rash manifests with a spongiform dermatitis (ie, variable parakeratosis; spongiosis; and a mixed dermal perivascular infiltrate of lymphocytes, eosinophils and histiocytes, depending on the lesion age)(Figure 1). The etiopathogenesis is unknown; it may involve immune complexes to SARS-CoV-2 deposited on skin vessels. Treatment is not mandatory; if necessary, local or systemic corticosteroids may be used.
Vesicular (Pseudovaricella) Rash—This rash accounts for 11% to 18% of all skin manifestations and usually appears within 15 days of COVID-19 onset. It manifests with small monomorphous or varicellalike (pseudopolymorphic) vesicles appearing on the trunk, usually in young patients. The vesicles may be herpetiform, hemorrhagic, or pruritic, and appear before or within 3 days of the onset of mild COVID-19 symptoms; they regress within a few days without scarring. Histologically, the lesions show basal cell vacuolization; multinucleated, dyskeratotic/apoptotic or ballooning/acantholytic epidermal keratinocytes; reticular degeneration of the epidermis; intraepidermal vesicles sometimes resembling herpetic vesicular infections or Grover disease; and mild dermal inflammation. There is no specific treatment.
Urticaria—Urticarial rash, or urticaria, represents 5% to 16% of skin manifestations; usually appears within 15 days of disease onset; and manifests with pruritic, migratory, edematous papules appearing mainly on the trunk and occasionally the face and limbs. The urticarial rash tends to be associated with more severe forms of the disease and regresses within a week, responding to antihistamines. Of note, clinically similar rashes can be caused by drugs. Histologically, the lesions show dermal edema and a mild perivascular lymphocytic infiltrate, sometimes admixed with eosinophils.
Chilblainlike Lesions—Chilblainlike lesions (CBLLs) account for 19% of skin manifestations associated with COVID-1913 and present as erythematous-purplish, edematous lesions that can be mildly pruritic or painful, appearing on the toes—COVID toes—and more rarely the fingers (Figure 2). They were seen epidemically during the first pandemic wave (2020 lockdown) in several countries, and clinically are very similar to, if not indistinguishable from, idiopathic chilblains, but are not necessarily associated with cold exposure. They appear in young, generally healthy patients or those with mild COVID-19 symptoms 2 to 4 weeks after symptom onset. They regress spontaneously or under local corticosteroid treatment within a few days or weeks. Histologically, CBLLs are indistinguishable from chilblains of other origins, namely idiopathic (seasonal) ones. They manifest with necrosis of epidermal keratinocytes; dermal edema that may be severe, leading to the development of subepidermal pseudobullae; a rather dense perivascular and perieccrine gland lymphocytic infiltrate; and sometimes with vascular lesions (eg, edema of endothelial cells, microthromboses of dermal capillaries and venules, fibrinoid deposits within the wall of dermal venules)(Figure 3).16-18 Most patients (>80%) with CBLLs have negative serologic or polymerase chain reaction tests for SARS-CoV-2,19 which generated a lively debate about the role of SARS-CoV-2 in the genesis of CBLLs. According to some authors, SARS-CoV-2 plays no direct role, and CBLLs would occur in young people who sit or walk barefoot on cold floors at home during confinement.20-23 Remarkably, CBLLs appeared in patients with no history of chilblains during a season that was not particularly cold, namely in France or in southern California, where their incidence was much higher compared to the same time period of prior years. Some reports have supported a direct role for the virus based on questionable observations of the virus within skin lesions (eg, sweat glands, endothelial cells) by immunohistochemistry, electron microscopy, and/or in situ hybridization.17,24,25 A more satisfactory hypothesis would involve the role of a strong innate immunity leading to elimination of the virus before the development of specific antibodies via the increased production of type 1 interferon (IFN-1); this would affect the vessels, causing CBLLs. This mechanism would be similar to the one observed in some interferonopathies (eg, Aicardi-Goutières syndrome), also characterized by IFN-1 hypersecretion and chilblains.26-29 According to this hypothesis, CBLLs should be considered a paraviral rash similar to other skin manifestations associated with COVID-19.30
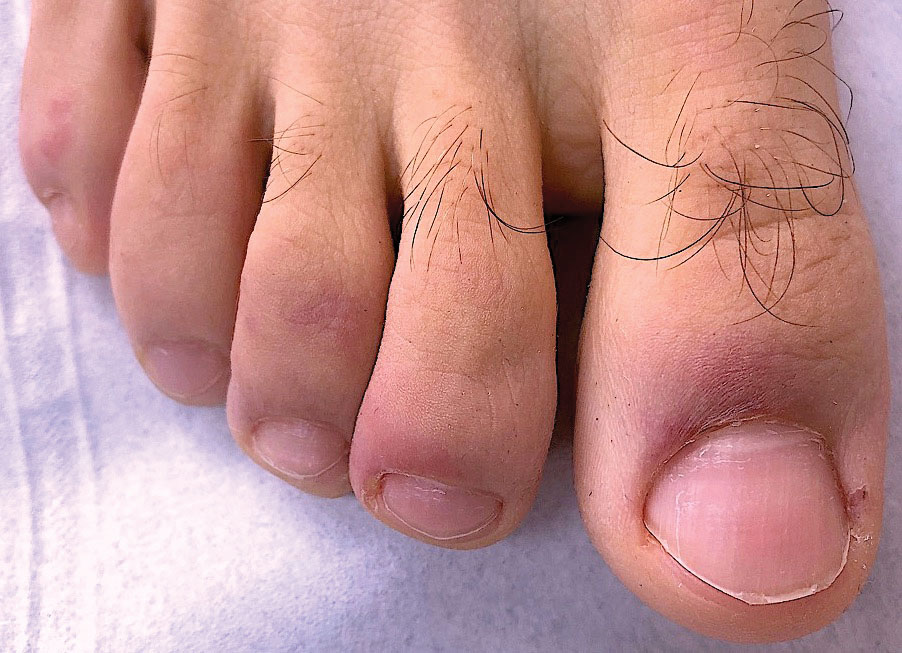
Acro-ischemia—Acro-ischemia livedoid lesions account for 1% to 6% of skin manifestations and comprise lesions of livedo (either reticulated or racemosa); necrotic acral bullae; and gangrenous necrosis of the extremities, especially the toes. The livedoid lesions most often appear within 15 days of COVID-19 symptom onset, and the purpuric lesions somewhat later (2–4 weeks); they mainly affect adult patients, last about 10 days, and are the hallmark of severe infection, presumably related to microthromboses of the cutaneous capillaries (endothelial dysfunction, prothrombotic state, elevated D-dimers). Histologically, they show capillary thrombosis and dermoepidermal necrosis (Figure 4).
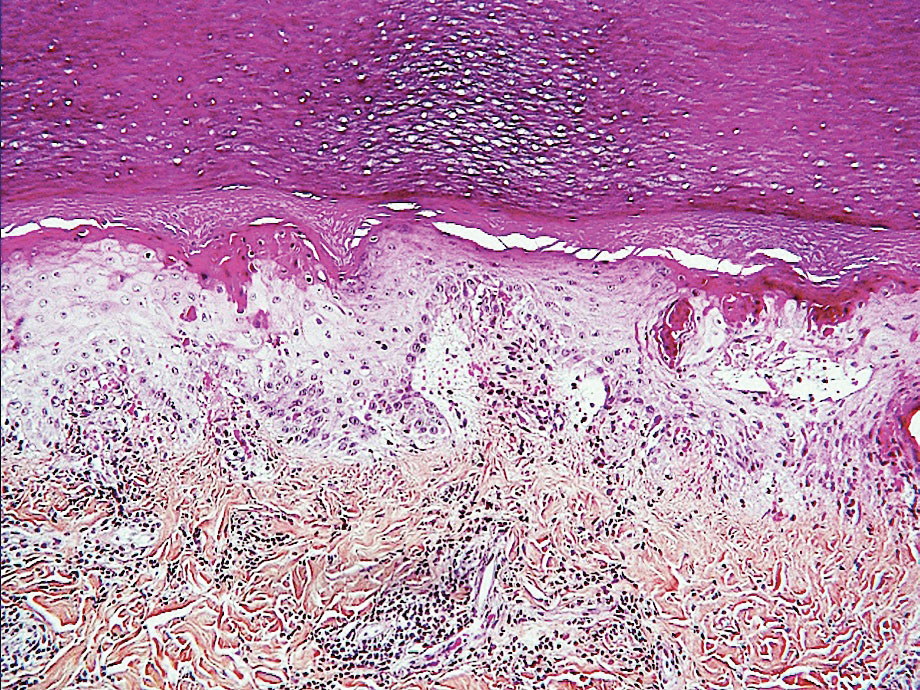
Other Reported Polymorphic or Atypical Rashes—Erythema multiforme–like eruptions may appear before other COVID-19 symptoms and manifest as reddish-purple, nearly symmetric, diffuse, occasionally targetoid bullous or necrotic macules. The eruptions mainly affect adults and most often are seen on the palms, elbows, knees, and sometimes the mucous membranes. The rash regresses in 1 to 3 weeks without scarring and represents a delayed cutaneous hypersensitivity reaction. Histologically, the lesions show vacuolization of basal epidermal keratinocytes, keratinocyte necrosis, dermoepidermal detachment, a variably dense dermal T-lymphocytic infiltrate, and red blood cell extravasation (Figure 5).
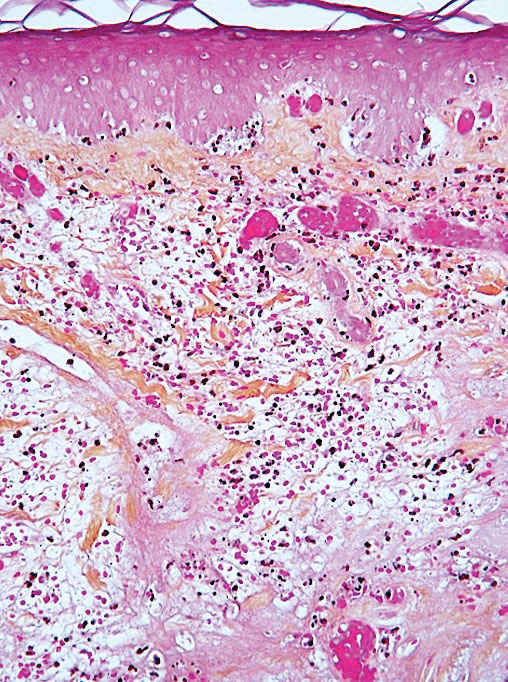
Leukocytoclastic vasculitis may be generalized or localized. It manifests clinically by petechial/purpuric maculopapules, especially on the legs, mainly in elderly patients with COVID-19. Histologically, the lesions show necrotizing changes of dermal postcapillary venules, neutrophilic perivascular inflammation, red blood cell extravasation, and occasionally vascular IgA deposits by direct immunofluorescence examination. The course usually is benign.
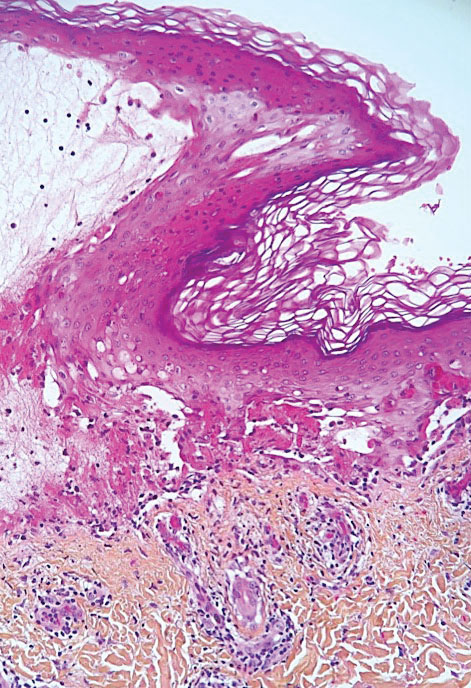
The incidence of pityriasis rosea and of clinically similar rashes (referred to as “pityriasis rosea–like”) increased 5-fold during the COVID-19 pandemic.31,32 These dermatoses manifest with erythematous, scaly, circinate plaques, typically with an initial herald lesion followed a few days later by smaller erythematous macules. Histologically, the lesions comprise a spongiform dermatitis with intraepidermal exocytosis of red blood cells and a mild to moderate dermal lymphocytic infiltrate.
Erythrodysesthesia, or hand-foot syndrome, manifests with edematous erythema and palmoplantar desquamation accompanied by a burning sensation or pain. This syndrome is known as an adverse effect of some chemotherapies because of the associated drug toxicity and sweat gland inflammation; it was observed in 40% of 666 COVID-19–positive patients with mild to moderate pneumonitis.33
“COVID nose” is a rare cutaneous manifestation characterized by nasal pigmentation comprising multiple coalescent frecklelike macules on the tip and wings of the nose and sometimes the malar areas. These lesions predominantly appear in women aged 25 to 65 years and show on average 23 days after onset of COVID-19, which is usually mild. This pigmentation is similar to pigmentary changes after infection with chikungunya; it can be treated with depigmenting products such as azelaic acid and hydroquinone cream with sunscreen use, and it regresses in 2 to 4 months.34
Telogen effluvium (excessive and temporary shedding of normal telogen club hairs of the entire scalp due to the disturbance of the hair cycle) is reportedly frequent in patients (48%) 1 month after COVID-19 infection, but it may appear later (after 12 weeks).35 Alopecia also is frequently reported during long (or postacute) COVID-19 (ie, the symptomatic disease phase past the acute 4 weeks’ stage of the infection) and shows a female predominance36; it likely represents the telogen effluvium seen 90 days after a severe illness. Trichodynia (pruritus, burning, pain, or paresthesia of the scalp) also is reportedly common (developing in more than 58% of patients) and is associated with telogen effluvium in 44% of cases. Several cases of alopecia areata (AA) triggered or aggravated by COVID-19 also have been reported37,38; they could be explained by the “cytokine storm” triggered by the infection, involving T and B lymphocytes; plasmacytoid dendritic cells; natural killer cells with oversecretion of IL-6, IL-4, tumor necrosis factor α, and IFN type I; and a cytotoxic reaction associated with loss of the immune privilege of hair follicles.
Nail Manifestations
The red half-moon nail sign is an asymptomatic purplish-red band around the distal margin of the lunula that affects some adult patients with COVID-19.39 It appears shortly after onset of symptoms, likely the manifestation of vascular inflammation in the nail bed, and regresses slowly after approximately 1 week.40 Beau lines are transverse grooves in the nail plate due to the temporary arrest of the proximal nail matrix growth accompanying systemic illnesses; they appear approximately 2 to 3 weeks after the onset of COVID-19.41 Furthermore, nail alterations can be caused by drugs used to treat COVID-19, such as longitudinal melanonychia due to treatment with hydroxychloroquine or fluorescence of the lunula or nail plate due to treatment with favipiravir.42
Multisystem Inflammatory Syndrome
Multisystem inflammatory syndrome (MIS) is clinically similar to Kawasaki disease; it typically affects children43 and more rarely adults with COVID-19. It manifests with fever, weakness, and biological inflammation and also frequently with skin lesions (72%), which are polymorphous and include morbilliform rash (27%); urticaria (24%); periorbital edema (24%); nonspecific erythema (21.2%); retiform purpura (18%); targetoid lesions (15%); malar rash (15.2%); and periareolar erythema (6%).44 Compared to Kawasaki disease, MIS affects slightly older children (mean age, 8.5 vs 3 years) and more frequently includes cardiac and gastrointestinal manifestations; the mortality rate also is slightly higher (2% vs 0.17%).45
Confirmed COVID-19 Infection
At the beginning of the pandemic, skin manifestations were reported in patients who were suspected of having COVID-19 but did not always have biological confirmation of SARS-CoV-2 infection due to the unavailability of diagnostic tests or the physical impossibility of testing. However, subsequent studies have confirmed that most of these dermatoses were indeed associated with COVID-19 infection.9,46 For example, a study of 655 patients with confirmed COVID-19 infection reported maculopapular (38%), vascular (22%), urticarial (15%), and vesicular (15%) rashes; erythema multiforme or Stevens-Johnson–like syndrome (3%, often related to the use of hydroxychloroquine); generalized pruritus (1%); and MIS (0.5%). The study confirmed that CBLLs were mostly seen in young patients with mild disease, whereas livedo (fixed rash) and retiform purpura occurred in older patients with a guarded prognosis.46
Remarkably, most dermatoses associated with SARS-CoV-2 infection were reported during the initial waves of the pandemic, which were due to the α and δ viral variants. These manifestations were reported more rarely when the ο variant was predominant, even though most patients (63%) who developed CBLLs in the first wave also developed them during the second pandemic wave.47 This decrease in the incidence of COVID-19–associated dermatoses could be because of the lower pathogenicity of the o variant,3 a lower tropism for the skin, and variations in SARS-CoV-2 antigenicity that would induce a different immunologic response, combined with an increasingly stronger herd immunity compared to the first pandemic waves achieved through vaccination and spontaneous infections in the population. Additional reasons may include different baseline characteristics in patients hospitalized with COVID-19 (regarding comorbidities, disease severity, and received treatments), and the possibility that some of the initially reported COVID-19–associated skin manifestations could have been produced by different etiologic agents.48 In the last 2 years, COVID-19–related skin manifestations have been reported mainly as adverse events to COVID-19 vaccination.
CUTANEOUS ADVERSE EFFECTS OF DRUGS USED TO TREAT COVID-19
Prior to the advent of vaccines and specific treatments for SARS-CoV-2, various drugs were used—namely hydroxychloroquine, ivermectin, and tocilizumab—that did not prove efficacious and caused diverse adverse effects, including cutaneous eruptions such as urticaria, maculopapular eruptions, erythema multiforme or Stevens-Johnson syndrome, vasculitis, longitudinal melanonychia, and acute generalized exanthematous pustulosis.49,50 Nirmatrelvir 150 mg–ritonavir 100 mg, which was authorized for emergency use by the US Food and Drug Administration for the treatment of COVID-19, is a viral protease inhibitor blocking the replication of the virus. Ritonavir can induce pruritus, maculopapular rash, acne, Stevens-Johnson syndrome, and toxic epidermal necrolysis; of note, these effects have been observed following administration of ritonavir for treatment of HIV at higher daily doses and for much longer periods of time compared with treatment of COVID-19 (600–1200 mg vs 200 mg/d, respectively). These cutaneous drug side effects are clinically similar to the manifestations caused either directly or indirectly by SARS-CoV-2 infection; therefore, it may be difficult to differentiate them.
DERMATOSES DUE TO PROTECTIVE DEVICES
Dermatoses due to personal protective equipment such as masks or face shields affected the general population and mostly health care professionals51; 54.4% of 879 health care professionals in one study reported such events.52 These dermatoses mainly include contact dermatitis of the face (nose, forehead, and cheeks) of irritant or allergic nature (eg, from preservatives releasing formaldehyde contained in masks and protective goggles). They manifest with skin dryness; desquamation; maceration; fissures; or erosions or ulcerations of the cheeks, forehead, and nose. Cases of pressure urticaria also have been reported. Irritant dermatitis induced by the frequent use of disinfectants (eg, soaps, hydroalcoholic sanitizing gels) also can affect the hands. Allergic hand dermatitis can be caused by medical gloves.
The term maskne (or mask acne) refers to a variety of mechanical acne due to the prolonged use of surgical masks (>4 hours per day for ≥6 weeks); it includes cases of de novo acne and cases of pre-existing acne aggravated by wearing a mask. Maskne is characterized by acne lesions located on the facial area covered by the mask (Figure 6). It is caused by follicular occlusion; increased sebum secretion; mechanical stress (pressure, friction); and dysbiosis of the microbiome induced by changes in heat, pH, and humidity. Preventive measures include application of noncomedogenic moisturizers or gauze before wearing the mask as well as facial cleansing with appropriate nonalcoholic products. Similar to acne, rosacea often is aggravated by prolonged wearing of surgical masks (mask rosacea).53,54
DERMATOSES REVEALED OR AGGRAVATED BY COVID-19
Exacerbation of various skin diseases has been reported after infection with SARS-CoV-2.55 Psoriasis and acrodermatitis continua of Hallopeau,56 which may progress into generalized, pustular, or erythrodermic forms,57 have been reported; the role of hydroxychloroquine and oral corticosteroids used for the treatment of COVID-19 has been suspected.57 Atopic dermatitis patients—26% to 43%—have experienced worsening of their disease after symptomatic COVID-19 infection.58 The incidence of herpesvirus infections, including herpes zoster, increased during the pandemic.59 Alopecia areata relapses occurred in 42.5% of 392 patients with preexisting disease within 2 months of COVID-19 onset in one study,60 possibly favored by the psychological stress; however, some studies have not confirmed the aggravating role of COVID-19 on alopecia areata.61 Lupus erythematosus, which may relapse in the form of Rowell syndrome,62 and livedoid vasculopathy63 also have been reported following COVID-19 infection.
SKIN MANIFESTATIONS ASSOCIATED WITH COVID-19 VACCINES
In parallel with the rapid spread of COVID-19 vaccination,4 an increasing number of skin manifestations has been observed following vaccination; these dermatoses now are more frequently reported than those related to natural SARS-CoV-2 infection.64-70 Vaccine-induced skin manifestations have a reported incidence of approximately 4% and show a female predominance.65 Most of them (79%) have been reported in association with messenger RNA (mRNA)–based vaccines, which have been the most widely used; however, the frequency of side effects would be lower after mRNA vaccines than after inactivated virus-based vaccines. Eighteen percent occurred after the adenoviral vector vaccine, and 3% after the inactivated virus vaccine.70 Fifty-nine percent were observed after the first dose. They are clinically polymorphous and generally benign, regressing spontaneously after a few days, and they should not constitute a contraindication to vaccination.Interestingly, many skin manifestations are similar to those associated with natural SARS-CoV-2 infection; however, their frequency and severity does not seem to depend on whether the patients had developed skin reactions during prior SARS-CoV-2 infection. These reactions have been classified into several types:
• Immediate local reactions at the injection site: pain, erythema, or edema represent the vast majority (96%) of reactions to vaccines. They appear within 7 days after vaccination (average, 1 day), slightly more frequently (59%) after the first dose. They concern mostly young patients and are benign, regressing in 2 to 3 days.70
• Delayed local reactions: characterized by pain or pruritus, erythema, and skin induration mimicking cellulitis (COVID arm) and represent 1.7% of postvaccination reactions. They correspond to a delayed hypersensitivity reaction and appear approximately 7 days after vaccination, most often after the first vaccine dose (75% of cases), which is almost invariably mRNA based.70
• Urticarial reactions corresponding to an immediate (type 1) hypersensitivity reaction: constitute 1% of postvaccination reactions, probably due to an allergy to vaccine ingredients. They appear on average 1 day after vaccination, almost always with mRNA vaccines.70
• Angioedema: characterized by mucosal or subcutaneous edema and constitutes 0.5% of postvaccination reactions. It is a potentially serious reaction that appears on average 12 hours after vaccination, always with an mRNA-based vaccine.70
• Morbilliform rash: represents delayed hypersensitivity reactions (0.1% of postvaccination reactions) that appear mostly after the first dose (72%), on average 3 days after vaccination, always with an mRNA-based vaccine.70
• Herpes zoster: usually develops after the first vaccine dose in elderly patients (69% of cases) on average 4 days after vaccination and constitutes 0.1% of postvaccination reactions.71
• Bullous diseases: mainly bullous pemphigoid (90%) and more rarely pemphigus (5%) or bullous erythema pigmentosum (5%). They appear in elderly patients on average 7 days after vaccination and constitute 0.04% of postvaccination reactions.72
• Chilblainlike lesions: several such cases have been reported so far73; they constitute 0.03% of postvaccination reactions.70 Clinically, they are similar to those associated with natural COVID-19; they appear mostly after the first dose (64%), on average 5 days after vaccination with the mRNA or adenovirus vaccine, and show a female predominance. The appearance of these lesions in vaccinated patients, who are a priori not carriers of the virus, strongly suggests that CBLLs are due to the immune reaction against SARS-CoV-2 rather than to a direct effect of this virus on the skin, which also is a likely scenario with regards to other skin manifestations seen during the successive COVID-19 epidemic waves.73-75
• Reactions to hyaluronic acid–containing cosmetic fillers: erythema, edema, and potentially painful induration at the filler injection sites. They constitute 0.04% of postvaccination skin reactions and appear 24 hours after vaccination with mRNA-based vaccines, equally after the first or second dose.76
• Pityriasis rosea–like rash: most occur after the second dose of mRNA-based vaccines (0.023% of postvaccination skin reactions).70
• Severe reactions: these include acute generalized exanthematous pustulosis77 and Stevens-Johnson syndrome.78 One case of each has been reported after the adenoviral vector vaccine 3 days after vaccination.
Other more rarely observed manifestations include reactivation/aggravation or de novo appearance of inflammatory dermatoses such as psoriasis,79,80 leukocytoclastic vasculitis,81,82 lymphocytic83 or urticarial84 vasculitis, Sweet syndrome,85 lupus erythematosus, dermatomyositis,86,87 alopecia,37,88 infection with Trichophyton rubrum,89 Grover disease,90 and lymphomatoid reactions (such as recurrences of cutaneous T-cell lymphomas [CD30+], and de novo development of lymphomatoid papulosis).91
FINAL THOUGHTS
COVID-19 is associated with several skin manifestations, even though the causative role of SARS-CoV-2 has remained elusive. These dermatoses are highly polymorphous, mostly benign, and usually spontaneously regressive, but some of them reflect severe infection. They mostly were described during the first pandemic waves, reported in several national and international registries, which allowed for their morphological classification. Currently, cutaneous adverse effects of vaccines are the most frequently reported dermatoses associated with SARS-CoV-2, and it is likely that they will continue to be observed while COVID-19 vaccination lasts. Hopefully the end of the COVID-19 pandemic is near. In January 2023, the International Health Regulations Emergency Committee of the World Health Organization acknowledged that the COVID-19 pandemic may be approaching an inflexion point, and even though the event continues to constitute a public health emergency of international concern, the higher levels of population immunity achieved globally through infection and/or vaccination may limit the impact of SARS-CoV-2 on morbidity and mortality. However, there is little doubt that this virus will remain a permanently established pathogen in humans and animals for the foreseeable future.92 Therefore, physicians—especially dermatologists—should be aware of the various skin manifestations associated with COVID-19 so they can more efficiently manage their patients.
- Ashraf UM, Abokor AA, Edwards JM, et al. SARS-CoV-2, ACE2 expression, and systemic organ invasion. Physiol Genomics. 2021;53:51-60.
- Ganier C, Harun N, Peplow I, et al. Angiotensin-converting enzyme 2 expression is detectable in keratinocytes, cutaneous appendages, and blood vessels by multiplex RNA in situ hybridization. Adv Skin Wound Care. 2022;35:219-223.
- Ulloa AC, Buchan SA, Daneman N, et al. Estimates of SARS-CoV-2 omicron variant severity in Ontario, Canada. JAMA. 2022;327:1286-1288.
- World Health Organization. Coronavirus (COVID-19) Dashboard. Accessed April 6, 2023. https://covid19.who.int
- Guan WJ, Ni ZY, Hu Y, et al; China Medical Treatment Expert Group for COVID-19. clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708-1720.
- Recalcati S. Cutaneous manifestations in COVID-9: a first perspective. J Eur Acad Dermatol Venereol. 2020;34:E212-E213.
- Freeman EE, McMahon DE, Lipoff JB, et al. The spectrum of COVID-19-associated dermatologic manifestations: an international registry of 716 patients from 31 countries. J Am Acad Dermatol. 2020;83:1118-1129.
- Freeman EE, Chamberlin GC, McMahon DE, et al. Dermatology COVID-19 registries: updates and future directions. Dermatol Clin. 2021;39:575-585.
- Guelimi R, Salle R, Dousset L, et al. Non-acral skin manifestations during the COVID-19 epidemic: COVIDSKIN study by the French Society of Dermatology. J Eur Acad Dermatol Venereol. 2021;35:E539-E541.
- Marzano AV, Genovese G, Moltrasio C, et al; Italian Skin COVID-19 Network of the Italian Society of Dermatology and Sexually Transmitted Diseases. The clinical spectrum of COVID-19 associated cutaneous manifestations: an Italian multicenter study of 200 adult patients. J Am Acad Dermatol. 2021;84:1356-1363.
- Sugai T, Fujita Y, Inamura E, et al. Prevalence and patterns of cutaneous manifestations in 1245 COVID-19 patients in Japan: a single-centre study. J Eur Acad Dermatol Venereol. 2022;36:E522-E524.
- Holmes Z, Courtney A, Lincoln M, et al. Rash morphology as a predictor of COVID‐19 severity: a systematic review of the cutaneous manifestations of COVID‐19. Skin Health Dis. 2022;2:E120. doi:10.1002/ski2.120
- Galván Casas C, Català A, Carretero Hernández G, et al. Classification of the cutaneous manifestations of COVID-19: a rapid prospective nationwide consensus study in Spain with 375 cases. Br J Dermatol. 2020;183:71-77.
- Garduño‑Soto M, Choreño-Parra, Cazarin-Barrientos Dermatological aspects of SARS‑CoV‑2 infection: mechanisms and manifestations. Arch Dermatol Res. 2021;313:611-622.
- Huynh T, Sanchez-Flores X, Yau J, et al. Cutaneous manifestations of SARS-CoV-2 Infection. Am J Clin Dermatol. 2022;23:277-286.
- Kanitakis J, Lesort C, Danset M, et al.
Chilblain-like acral lesions during the COVID-19 pandemic (“COVID toes”): histologic, immunofluorescence, and immunohistochemical study of 17 cases. J Am Acad Dermatol. 2020; 83:870-875. - Kolivras A, Thompson C, Pastushenko I, et al. A clinicopathological description of COVID-19-induced chilblains (COVID-toes) correlated with a published literature review. J Cutan Pathol. 2022;49:17-28.
- Roca-Ginés J, Torres-Navarro I, Sánchez-Arráez J, et al. Assessment of acute acral lesions in a case series of children and adolescents during the COVID-19 pandemic. 2020;156:992-997.
- Le Cleach L, Dousset L, Assier H, et al; French Society of Dermatology. Most chilblains observed during the COVID-19 outbreak occur in patients who are negative for COVID-19 on polymerase chain reaction and serology testing. Br J Dermatol. 2020;183:866-874.
- Discepolo V, Catzola A, Pierri L, et al. Bilateral chilblain-like lesions of the toes characterized by microvascular remodeling in adolescents during the COVID-19 pandemic. JAMA Netw Open. 2021;4:E2111369.
- Gehlhausen JR, Little AJ, Ko CJ, et al. Lack of association between pandemic chilblains and SARS-CoV-2 infection. Proc Natl Acad Sci U S A. 2022;119:e2122090119.
- Neri, Virdi, Corsini, et al Major cluster of paediatric ‘true’ primary chilblains during the COVID-19 pandemic: a consequence of lifestyle changes due to lockdown. J Eur Acad Dermatol Venereol. 2020;34:2630-2635.
- De Greef A, Choteau M, Herman A, et al. Chilblains observed during the COVID-19 pandemic cannot be distinguished from the classic, cold-related chilblains. Eur J Dermatol. 2022;32:377-383.
- Colmenero I, Santonja C, Alonso-Riaño M, et al. SARS-CoV-2 endothelial infection causes COVID-19 chilblains: histopathological, immunohistochemical and ultrastructural study of seven paediatric cases. Br J Dermatol. 2020;183:729-737.
- Quintero-Bustos G, Aguilar-Leon D, Saeb-Lima M. Histopathological and immunohistochemical characterization of skin biopsies from 41 SARS-CoV-2 (+) patients: experience in a Mexican concentration institute: a case series and literature review. Am J Dermatopathol. 2022;44:327-337.
- Arkin LM, Moon JJ, Tran JM, et al; COVID Human Genetic Effort. From your nose to your toes: a review of severe acute respiratory syndrome coronavirus 2 pandemic-associated pernio. J Invest Dermatol. 2021;141:2791-2796.
- Frumholtz L, Bouaziz JD, Battistella M, et al; Saint-Louis CORE (COvid REsearch). Type I interferon response and vascular alteration in chilblain-like lesions during the COVID-19 outbreak. Br J Dermatol. 2021;185:1176-1185.
- Hubiche T, Cardot-Leccia N, Le Duff F, et al. Clinical, laboratory, and interferon-alpha response characteristics of patients with chilblain-like lesions during the COVID-19 pandemic. JAMA Dermatol. 2021;157:202-206.
- Lesort C, Kanitakis J, Villani A, et al. COVID-19 and outbreak of chilblains: are they related? J Eur Acad Dermatol Venereol. 2020;34:E757-E758.
- Sanchez A, Sohier P, Benghanem S, et al. Digitate papulosquamous eruption associated with severe acute respiratory syndrome coronavirus 2 infection. JAMA Dermatol. 2020;156:819-820.
- Drago F, Broccolo F, Ciccarese G. Pityriasis rosea, pityriasis rosea-like eruptions, and herpes zoster in the setting of COVID-19 and COVID-19 vaccination. Clin Dermatol. 2022;S0738-081X(22)00002-5.
- Dursun R, Temiz SA. The clinics of HHV-6 infection in COVID-19 pandemic: pityriasis rosea and Kawasaki disease. Dermatol Ther. 2020;33:E13730.
- Nuno-Gonzalez A, Magaletsky K, Feito Rodríguez M, et al. Palmoplantar erythrodysesthesia: a diagnostic sign of COVID-19. J Eur Acad Dermatol Venereol. 2021;35:e247-e249.
- Sil A, Panigrahi A, Chandra A, et al. “COVID nose”: a unique post-COVID pigmentary sequelae reminiscing Chik sign: a descriptive case series. J Eur Acad Dermatol Venereol. 2022;36:E419-E421.
- Starace M, Iorizzo M, Sechi A, et al. Trichodynia and telogen effluvium in COVID-19 patients: results of an international expert opinion survey on diagnosis and management. JAAD Int. 2021;5:11-18.
- Wong-Chew RM, Rodríguez Cabrera EX, Rodríguez Valdez CA, et al. Symptom cluster analysis of long COVID-19 in patients discharged from the Temporary COVID-19 Hospital in Mexico City. Ther Adv Infect Dis. 2022;9:20499361211069264.
- Bardazzi F, Guglielmo A, Abbenante D, et al. New insights into alopecia areata during COVID-19 pandemic: when infection or vaccination could play a role. J Cosmet Dermatol. 2022;21:1796-1798.
- Christensen RE, Jafferany M. Association between alopecia areata and COVID-19: a systematic review. JAAD Int. 2022;7:57-61.
- Wollina U, Kanitakis J, Baran R. Nails and COVID-19: a comprehensive review of clinical findings and treatment. Dermatol Ther. 2021;34:E15100.
- Méndez-Flores S, Zaladonis A, Valdes-Rodriguez R. COVID-19 and nail manifestation: be on the lookout for the red half-moon nail sign. Int J Dermatol. 2020;59:1414.
- Alobaida S, Lam JM. Beau lines associated with COVID-19. CMAJ. 2020;192:E1040.
- Durmaz EÖ, Demirciog˘lu D. Fluorescence in the sclera, nails, and teeth secondary to favipiravir use for COVID-19 infections. J Clin Aesthet Dermatol. 2022;15:35-37.
- Brumfiel CM, DiLorenzo AM, Petronic-Rosic VM. Dermatologic manifestations of COVID-19-associated multisystem inflammatory syndrome in children. Clin Dermatol. 2021;39:329-333.
- Akçay N, Topkarcı Z, Menentog˘lu ME, et al. New dermatological findings of MIS-C: can mucocutaneous involvement be associated with severe disease course? Australas J Dermatol. 2022;63:228-234. doi:10.1111/ajd.13819
- Vogel TP, Top KA, Karatzios C, et al. Multisystem inflammatory syndrome in children and adults (MIS-C/A): case definition & guidelines for data collection, analysis, and presentation of immunization safety data. Vaccine. 2021;39:3037-3049.
- Conforti C, Dianzani C, Agozzino M, et al. Cutaneous manifestations in confirmed COVID-19 patients: a systematic review. Biology (Basel). 2020;9:449.
- Hubiche T, Le Duff F, Fontas E, et al. Relapse of chilblain-like lesions during the second wave of the COVID-19 pandemic: a cohort follow-up. Br J Dermatol. 2021;185:858-859.
- Fernandez-Nieto, Ortega-Quijano, Suarez-Valle, et al Lack of skin manifestations in COVID-19 hospitalized patients during the second epidemic wave in Spain: a possible association with a novel SARS-CoV-2 variant: a cross-sectional study. J Eur Acad Dermatol Venereol. 2021;35:E183-E185.
- Martinez-LopezA, Cuenca-Barrales, Montero-Vilchezet al Review of adverse cutaneous reactions of pharmacologic interventions for COVID-19: a guide for the dermatologist. J Am Acad Dermatol. 2020;83:1738-1748.
- Cutaneous side-effects of the potential COVID-19 drugs. Dermatol Ther. 2020;33:E13476.
- Mawhirt SL, Frankel D, Diaz AM. Cutaneous manifestations in adult patients with COVID-19 and dermatologic conditions related to the COVID-19 pandemic in health care workers. Curr Allerg Asthma Rep. 2020;20:75.
- Nguyen C, Young FG, McElroy D, et al. Personal protective equipment and adverse dermatological reactions among healthcare workers: survey observations from the COVID-19 pandemic. Medicine (Baltimore). 2022;101:E29003.
- Rathi SK, Dsouza JM. Maskne: a new acne variant in COVID-19 era. Indian J Dermatol. 2022;67:552-555.
- Damiani G, Girono L, Grada A, et al. COVID-19 related masks increase severity of both acne (maskne) and rosacea (mask rosacea): multi-center, real-life, telemedical, and observational prospective study. Dermatol Ther. 2021;34:E14848.
- Aram K, Patil A, Goldust M, et al. COVID-19 and exacerbation of dermatological diseases: a review of the available literature. Dermatol Ther. 2021;34:E15113.
- Samotij D, Gawron E, Szcze˛ch J, et al. Acrodermatitis continua of Hallopeau evolving into generalized pustular psoriasis following COVID-19: a case report of a successful treatment with infliximab in combination with acitretin. Biologics. 2021;15:107-113.
- Demiri J, Abdo M, Tsianakas A. Erythrodermic psoriasis after COVID-19 [in German]. Hautarzt. 2022;73:156-159.
- de Wijs LEM, Joustra MM, Olydam JI, et al. COVID-19 in patients with cutaneous immune-mediated diseases in the Netherlands: real-world observational data. J Eur Acad Dermatol Venereol. 2021;35:E173-E176.
- Marques NP, Maia CMF, Marques NCT, et al. Continuous increase of herpes zoster cases in Brazil during the COVID-19 pandemic. Oral Surg Oral Med Oral Pathol Oral Radiol. 2022;133:612-614.
- Rinaldi F, Trink A, Giuliani G, et al. Italian survey for the evaluation of the effects of coronavirus disease 2019 (COVID-19) pandemic on alopecia areata recurrence. Dermatol Ther (Heidelb). 2021;11:339-345.
- Rudnicka L, Rakowska A, Waskiel-Burnat A, et al. Mild-to-moderate COVID-19 is not associated with worsening of alopecia areata: a retrospective analysis of 32 patients. J Am Acad Dermatol. 2021;85:723-725.
- Drenovska K, Shahid M, Mateeva V, et al. Case report: Rowell syndrome-like flare of cutaneous lupus erythematosus following COVID-19 infection. Front Med (Lausanne). 2022;9:815743.
- Kawabe R, Tonomura K, Kotobuki Y, et al. Exacerbation of livedoid vasculopathy after coronavirus disease 2019. Eur J Dermatol. 2022;32:129-131. doi:10.1684/ejd.2022.4200
- McMahon DE, Kovarik CL, Damsky W, et al. Clinical and pathologic correlation of cutaneous COVID-19 vaccine reactions including V-REPP: a registry-based study. J Am Acad Dermatol. 2022;86:113-121.
- Avallone G, Quaglino P, Cavallo F, et al. SARS-CoV-2 vaccine-related cutaneous manifestations: a systematic review. Int J Dermatol. 2022;61:1187-1204. doi:10.1111/ijd.16063
- Gambichler T, Boms S, Susok L, et al. Cutaneous findings following COVID-19 vaccination: review of world literature and own experience. J Eur Acad Dermatol Venereol. 2022;36:172-180.
- Kroumpouzos G, Paroikaki ME, Yumeen S, et al. Cutaneous complications of mRNA and AZD1222 COVID-19 vaccines: a worldwide review. Microorganisms. 2022;10:624.
- Robinson L,Fu X,Hashimoto D, et al. Incidence of cutaneous reactions after messenger RNA COVID-19 vaccines. 2021;
- Wollina U, Chiriac A, Kocic H, et al. Cutaneous and hypersensitivity reactions associated with COVID-19 vaccination: a narrative review. Wien Med Wochenschr. 2022;172:63-69.
- Wei TS. Cutaneous reactions to COVID-19 vaccines: a review. JAAD Int. 2022;7:178-186.
- Katsikas Triantafyllidis K, Giannos P, Mian IT, et al. Varicella zoster virus reactivation following COVID-19 vaccination: a systematic review of case reports. Vaccines (Basel). 2021;9:1013.
- Maronese CA, Caproni M, Moltrasio C, et al. Bullous pemphigoid associated with COVID-19 vaccines: an Italian multicentre study. Front Med (Lausanne). 2022;9:841506.
- Cavazos A, Deb A, Sharma U, et al. COVID toes following vaccination. Proc (Bayl Univ Med Cent). 2022;35:476-479.
- Lesort C, Kanitakis J, Danset M, et al. Chilblain-like lesions after BNT162b2 mRNA COVID-19 vaccine: a case report suggesting that ‘COVID toes’ are due to the immune reaction to SARS-CoV-2. J Eur Acad Dermatol Venereol. 2021;35:E630-E632.
- Russo R, Cozzani E, Micalizzi C, et al. Chilblain-like lesions after COVID-19 vaccination: a case series. Acta Derm Venereol. 2022;102:adv00711. doi:10.2340/actadv.v102.2076
- Ortigosa LCM, Lenzoni FC, Suárez MV, et al. Hypersensitivity reaction to hyaluronic acid dermal filler after COVID-19 vaccination: a series of cases in São Paulo, Brazil. Int J Infect Dis. 2022;116:268-270.
- Agaronov A, Makdesi C, Hall CS. Acute generalized exanthematous pustulosis induced by Moderna COVID-19 messenger RNA vaccine. JAAD Case Rep. 2021;16:96-97.
- Dash S, Sirka CS, Mishra S, et al. COVID-19 vaccine-induced Stevens-Johnson syndrome. Clin Exp Dermatol. 2021;46:1615-1617.
- Huang Y, Tsai TF. Exacerbation of psoriasis following COVID-19 vaccination: report from a single center. Front Med (Lausanne). 2021;8:812010.
- Elamin S, Hinds F, Tolland J. De novo generalized pustular psoriasis following Oxford-AstraZeneca COVID-19 vaccine. Clin Exp Dermatol 2022;47:153-155.
- Abdelmaksoud A, Wollina U, Temiz SA, et al. SARS-CoV-2 vaccination-induced cutaneous vasculitis: report of two new cases and literature review. Dermatol Ther. 2022;35:E15458.
- Fritzen M, Funchal GDG, Luiz MO, et al. Leukocytoclastic vasculitis after exposure to COVID-19 vaccine. An Bras Dermatol. 2022;97:118-121.
- Vassallo C, Boveri E, Brazzelli V, et al. Cutaneous lymphocytic vasculitis after administration of COVID-19 mRNA vaccine. Dermatol Ther. 2021;34:E15076.
- Nazzaro G, Maronese CA. Urticarial vasculitis following mRNA anti-COVID-19 vaccine. Dermatol Ther. 2022;35:E15282.
- Hoshina D, Orita A. Sweet syndrome after severe acute respiratory syndrome coronavirus 2 mRNA vaccine: a case report and literature review. J Dermatol. 2022;49:E175-E176.
- Lemoine C, Padilla C, Krampe N, et al. Systemic lupus erythematous after Pfizer COVID-19 vaccine: a case report. Clin Rheumatol. 2022;41:1597-1601.
- Nguyen B, Lalama MJ, Gamret AC, et al. Cutaneous symptoms of connective tissue diseases after COVID-19 vaccination: a systematic review. Int J Dermatol. 2022;61:E238-E241.
- Gallo G, Mastorino L, Tonella L, et al. Alopecia areata after COVID-19 vaccination. Clin Exp Vaccine Res. 2022;11:129-132.
- Norimatsu Y, Norimatsu Y. A severe case of Trichophyton rubrum-caused dermatomycosis exacerbated after COVID-19 vaccination that had to be differentiated from pustular psoriasis. Med Mycol Case Rep. 2022;36:19-22.
- Yang K, Prussick L, Hartman R, et al. Acantholytic dyskeratosis post-COVID vaccination. Am J Dermatopathol. 2022;44:E61-E63.
- Koumaki D, Marinos L, Nikolaou V, et al. Lymphomatoid papulosis (LyP) after AZD1222 and BNT162b2 COVID-19 vaccines. Int J Dermatol. 2022;61:900-902.
- World Health Organization. Statement on the fourteenth meeting of the International Health Regulations (2005) Emergency Committee regarding the coronavirus disease (COVID-19) pandemic. Published January 30, 2023. Accessed April 12, 2023. https://www.who.int/news/item/30-01-2023-statement-on-the-fourteenth-meeting-of-the-international-health-regulations-(2005)-emergency-committee-regarding-the-coronavirus-disease-(covid-19)-pandemic
COVID-19 is a potentially severe systemic disease caused by SARS-CoV-2. SARS-CoV and Middle East respiratory syndrome (MERS-CoV) caused fatal epidemics in Asia in 2002 to 2003 and in the Arabian Peninsula in 2012, respectively. In 2019, SARS-CoV-2 was detected in patients with severe, sometimes fatal pneumonia of previously unknown origin; it rapidly spread around the world, and the World Health Organization declared the disease a pandemic on March 11, 2020. SARS-CoV-2 is a β-coronavirus that is genetically related to the bat coronavirus and SARS-CoV; it is a single-stranded RNA virus of which several variants and subvariants exist. The SARS-CoV-2 viral particles bind via their surface spike protein (S protein) to the angiotensin-converting enzyme 2 receptor present on the membrane of several cell types, including epidermal and adnexal keratinocytes.1,2 The α and δ variants, predominant from 2020 to 2021, mainly affected the lower respiratory tract and caused severe, potentially fatal pneumonia, especially in patients older than 65 years and/or with comorbidities, such as obesity, hypertension, diabetes, and (iatrogenic) immunosuppression. The ο variant, which appeared in late 2021, is more contagious than the initial variants, but it causes a less severe disease preferentially affecting the upper respiratory airways.3 As of April 5, 2023, more than 762,000,000 confirmed cases of COVID-19 have been recorded worldwide, causing more than 6,800,000 deaths.4
Early studies from China describing the symptoms of COVID-19 reported a low frequency of skin manifestations (0.2%), probably because they were focused on the most severe disease symptoms.5 Subsequently, when COVID-19 spread to the rest of the world, an increasing number of skin manifestations were reported in association with the disease. After the first publication from northern Italy in spring 2020, which was specifically devoted to skin manifestations of COVID-19,6 an explosive number of publications reported a large number of skin manifestations, and national registries were established in several countries to record these manifestations, such as the American Academy of Dermatology and the International League of Dermatological Societies registry,7,8 the COVIDSKIN registry of the French Dermatology Society,9 and the Italian registry.10 Highlighting the unprecedented number of scientific articles published on this new disease, a PubMed search of articles indexed for MEDLINE search using the terms SARS-CoV-2 or COVID-19, on April 6, 2023, revealed 351,596 articles; that is more than 300 articles published every day in this database alone, with a large number of them concerning the skin.
SKIN DISEASSES ASSOCIATED WITH COVID-19
There are several types of COVID-19–related skin manifestations, depending on the circumstances of onset and the evolution of the pandemic.
Skin Manifestations Associated With SARS-CoV-2 Infection
The estimated incidence varies greatly according to the published series of patients, possibly depending on the geographic location. The estimated incidence seems lower in Asian countries, such as China (0.2%)5 and Japan (0.56%),11 compared with Europe (up to 20%).6 Skin manifestations associated with SARS-CoV-2 infection affect individuals of all ages, slightly more females, and are clinically polymorphous; some of them are associated with the severity of the infection.12 They may precede, accompany, or appear after the symptoms of COVID-19, most often within a month of the infection, of which they rarely are the only manifestation; however, their precise relationship to SARS-CoV-2 is not always well known. They have been classified according to their clinical presentation into several forms.13-15
Morbilliform Maculopapular Eruption—Representing 16% to 53% of skin manifestations, morbilliform and maculopapular eruptions usually appear within 15 days of infection; they manifest with more or less confluent erythematous macules that may be hemorrhagic/petechial, and usually are asymptomatic and rarely pruritic. The rash mainly affects the trunk and limbs, sparing the face, palmoplantar regions, and mucous membranes; it appears concomitantly with or a few days after the first symptoms of COVID-19 (eg, fever, respiratory symptoms), regresses within a few days, and does not appear to be associated with disease severity. The distinction from maculopapular drug eruptions may be subtle. Histologically, the rash manifests with a spongiform dermatitis (ie, variable parakeratosis; spongiosis; and a mixed dermal perivascular infiltrate of lymphocytes, eosinophils and histiocytes, depending on the lesion age)(Figure 1). The etiopathogenesis is unknown; it may involve immune complexes to SARS-CoV-2 deposited on skin vessels. Treatment is not mandatory; if necessary, local or systemic corticosteroids may be used.

Vesicular (Pseudovaricella) Rash—This rash accounts for 11% to 18% of all skin manifestations and usually appears within 15 days of COVID-19 onset. It manifests with small monomorphous or varicellalike (pseudopolymorphic) vesicles appearing on the trunk, usually in young patients. The vesicles may be herpetiform, hemorrhagic, or pruritic, and appear before or within 3 days of the onset of mild COVID-19 symptoms; they regress within a few days without scarring. Histologically, the lesions show basal cell vacuolization; multinucleated, dyskeratotic/apoptotic or ballooning/acantholytic epidermal keratinocytes; reticular degeneration of the epidermis; intraepidermal vesicles sometimes resembling herpetic vesicular infections or Grover disease; and mild dermal inflammation. There is no specific treatment.
Urticaria—Urticarial rash, or urticaria, represents 5% to 16% of skin manifestations; usually appears within 15 days of disease onset; and manifests with pruritic, migratory, edematous papules appearing mainly on the trunk and occasionally the face and limbs. The urticarial rash tends to be associated with more severe forms of the disease and regresses within a week, responding to antihistamines. Of note, clinically similar rashes can be caused by drugs. Histologically, the lesions show dermal edema and a mild perivascular lymphocytic infiltrate, sometimes admixed with eosinophils.
Chilblainlike Lesions—Chilblainlike lesions (CBLLs) account for 19% of skin manifestations associated with COVID-1913 and present as erythematous-purplish, edematous lesions that can be mildly pruritic or painful, appearing on the toes—COVID toes—and more rarely the fingers (Figure 2). They were seen epidemically during the first pandemic wave (2020 lockdown) in several countries, and clinically are very similar to, if not indistinguishable from, idiopathic chilblains, but are not necessarily associated with cold exposure. They appear in young, generally healthy patients or those with mild COVID-19 symptoms 2 to 4 weeks after symptom onset. They regress spontaneously or under local corticosteroid treatment within a few days or weeks. Histologically, CBLLs are indistinguishable from chilblains of other origins, namely idiopathic (seasonal) ones. They manifest with necrosis of epidermal keratinocytes; dermal edema that may be severe, leading to the development of subepidermal pseudobullae; a rather dense perivascular and perieccrine gland lymphocytic infiltrate; and sometimes with vascular lesions (eg, edema of endothelial cells, microthromboses of dermal capillaries and venules, fibrinoid deposits within the wall of dermal venules)(Figure 3).16-18 Most patients (>80%) with CBLLs have negative serologic or polymerase chain reaction tests for SARS-CoV-2,19 which generated a lively debate about the role of SARS-CoV-2 in the genesis of CBLLs. According to some authors, SARS-CoV-2 plays no direct role, and CBLLs would occur in young people who sit or walk barefoot on cold floors at home during confinement.20-23 Remarkably, CBLLs appeared in patients with no history of chilblains during a season that was not particularly cold, namely in France or in southern California, where their incidence was much higher compared to the same time period of prior years. Some reports have supported a direct role for the virus based on questionable observations of the virus within skin lesions (eg, sweat glands, endothelial cells) by immunohistochemistry, electron microscopy, and/or in situ hybridization.17,24,25 A more satisfactory hypothesis would involve the role of a strong innate immunity leading to elimination of the virus before the development of specific antibodies via the increased production of type 1 interferon (IFN-1); this would affect the vessels, causing CBLLs. This mechanism would be similar to the one observed in some interferonopathies (eg, Aicardi-Goutières syndrome), also characterized by IFN-1 hypersecretion and chilblains.26-29 According to this hypothesis, CBLLs should be considered a paraviral rash similar to other skin manifestations associated with COVID-19.30

Acro-ischemia—Acro-ischemia livedoid lesions account for 1% to 6% of skin manifestations and comprise lesions of livedo (either reticulated or racemosa); necrotic acral bullae; and gangrenous necrosis of the extremities, especially the toes. The livedoid lesions most often appear within 15 days of COVID-19 symptom onset, and the purpuric lesions somewhat later (2–4 weeks); they mainly affect adult patients, last about 10 days, and are the hallmark of severe infection, presumably related to microthromboses of the cutaneous capillaries (endothelial dysfunction, prothrombotic state, elevated D-dimers). Histologically, they show capillary thrombosis and dermoepidermal necrosis (Figure 4).

Other Reported Polymorphic or Atypical Rashes—Erythema multiforme–like eruptions may appear before other COVID-19 symptoms and manifest as reddish-purple, nearly symmetric, diffuse, occasionally targetoid bullous or necrotic macules. The eruptions mainly affect adults and most often are seen on the palms, elbows, knees, and sometimes the mucous membranes. The rash regresses in 1 to 3 weeks without scarring and represents a delayed cutaneous hypersensitivity reaction. Histologically, the lesions show vacuolization of basal epidermal keratinocytes, keratinocyte necrosis, dermoepidermal detachment, a variably dense dermal T-lymphocytic infiltrate, and red blood cell extravasation (Figure 5).

Leukocytoclastic vasculitis may be generalized or localized. It manifests clinically by petechial/purpuric maculopapules, especially on the legs, mainly in elderly patients with COVID-19. Histologically, the lesions show necrotizing changes of dermal postcapillary venules, neutrophilic perivascular inflammation, red blood cell extravasation, and occasionally vascular IgA deposits by direct immunofluorescence examination. The course usually is benign.

The incidence of pityriasis rosea and of clinically similar rashes (referred to as “pityriasis rosea–like”) increased 5-fold during the COVID-19 pandemic.31,32 These dermatoses manifest with erythematous, scaly, circinate plaques, typically with an initial herald lesion followed a few days later by smaller erythematous macules. Histologically, the lesions comprise a spongiform dermatitis with intraepidermal exocytosis of red blood cells and a mild to moderate dermal lymphocytic infiltrate.
Erythrodysesthesia, or hand-foot syndrome, manifests with edematous erythema and palmoplantar desquamation accompanied by a burning sensation or pain. This syndrome is known as an adverse effect of some chemotherapies because of the associated drug toxicity and sweat gland inflammation; it was observed in 40% of 666 COVID-19–positive patients with mild to moderate pneumonitis.33
“COVID nose” is a rare cutaneous manifestation characterized by nasal pigmentation comprising multiple coalescent frecklelike macules on the tip and wings of the nose and sometimes the malar areas. These lesions predominantly appear in women aged 25 to 65 years and show on average 23 days after onset of COVID-19, which is usually mild. This pigmentation is similar to pigmentary changes after infection with chikungunya; it can be treated with depigmenting products such as azelaic acid and hydroquinone cream with sunscreen use, and it regresses in 2 to 4 months.34
Telogen effluvium (excessive and temporary shedding of normal telogen club hairs of the entire scalp due to the disturbance of the hair cycle) is reportedly frequent in patients (48%) 1 month after COVID-19 infection, but it may appear later (after 12 weeks).35 Alopecia also is frequently reported during long (or postacute) COVID-19 (ie, the symptomatic disease phase past the acute 4 weeks’ stage of the infection) and shows a female predominance36; it likely represents the telogen effluvium seen 90 days after a severe illness. Trichodynia (pruritus, burning, pain, or paresthesia of the scalp) also is reportedly common (developing in more than 58% of patients) and is associated with telogen effluvium in 44% of cases. Several cases of alopecia areata (AA) triggered or aggravated by COVID-19 also have been reported37,38; they could be explained by the “cytokine storm” triggered by the infection, involving T and B lymphocytes; plasmacytoid dendritic cells; natural killer cells with oversecretion of IL-6, IL-4, tumor necrosis factor α, and IFN type I; and a cytotoxic reaction associated with loss of the immune privilege of hair follicles.
Nail Manifestations
The red half-moon nail sign is an asymptomatic purplish-red band around the distal margin of the lunula that affects some adult patients with COVID-19.39 It appears shortly after onset of symptoms, likely the manifestation of vascular inflammation in the nail bed, and regresses slowly after approximately 1 week.40 Beau lines are transverse grooves in the nail plate due to the temporary arrest of the proximal nail matrix growth accompanying systemic illnesses; they appear approximately 2 to 3 weeks after the onset of COVID-19.41 Furthermore, nail alterations can be caused by drugs used to treat COVID-19, such as longitudinal melanonychia due to treatment with hydroxychloroquine or fluorescence of the lunula or nail plate due to treatment with favipiravir.42
Multisystem Inflammatory Syndrome
Multisystem inflammatory syndrome (MIS) is clinically similar to Kawasaki disease; it typically affects children43 and more rarely adults with COVID-19. It manifests with fever, weakness, and biological inflammation and also frequently with skin lesions (72%), which are polymorphous and include morbilliform rash (27%); urticaria (24%); periorbital edema (24%); nonspecific erythema (21.2%); retiform purpura (18%); targetoid lesions (15%); malar rash (15.2%); and periareolar erythema (6%).44 Compared to Kawasaki disease, MIS affects slightly older children (mean age, 8.5 vs 3 years) and more frequently includes cardiac and gastrointestinal manifestations; the mortality rate also is slightly higher (2% vs 0.17%).45
Confirmed COVID-19 Infection
At the beginning of the pandemic, skin manifestations were reported in patients who were suspected of having COVID-19 but did not always have biological confirmation of SARS-CoV-2 infection due to the unavailability of diagnostic tests or the physical impossibility of testing. However, subsequent studies have confirmed that most of these dermatoses were indeed associated with COVID-19 infection.9,46 For example, a study of 655 patients with confirmed COVID-19 infection reported maculopapular (38%), vascular (22%), urticarial (15%), and vesicular (15%) rashes; erythema multiforme or Stevens-Johnson–like syndrome (3%, often related to the use of hydroxychloroquine); generalized pruritus (1%); and MIS (0.5%). The study confirmed that CBLLs were mostly seen in young patients with mild disease, whereas livedo (fixed rash) and retiform purpura occurred in older patients with a guarded prognosis.46
Remarkably, most dermatoses associated with SARS-CoV-2 infection were reported during the initial waves of the pandemic, which were due to the α and δ viral variants. These manifestations were reported more rarely when the ο variant was predominant, even though most patients (63%) who developed CBLLs in the first wave also developed them during the second pandemic wave.47 This decrease in the incidence of COVID-19–associated dermatoses could be because of the lower pathogenicity of the o variant,3 a lower tropism for the skin, and variations in SARS-CoV-2 antigenicity that would induce a different immunologic response, combined with an increasingly stronger herd immunity compared to the first pandemic waves achieved through vaccination and spontaneous infections in the population. Additional reasons may include different baseline characteristics in patients hospitalized with COVID-19 (regarding comorbidities, disease severity, and received treatments), and the possibility that some of the initially reported COVID-19–associated skin manifestations could have been produced by different etiologic agents.48 In the last 2 years, COVID-19–related skin manifestations have been reported mainly as adverse events to COVID-19 vaccination.
CUTANEOUS ADVERSE EFFECTS OF DRUGS USED TO TREAT COVID-19
Prior to the advent of vaccines and specific treatments for SARS-CoV-2, various drugs were used—namely hydroxychloroquine, ivermectin, and tocilizumab—that did not prove efficacious and caused diverse adverse effects, including cutaneous eruptions such as urticaria, maculopapular eruptions, erythema multiforme or Stevens-Johnson syndrome, vasculitis, longitudinal melanonychia, and acute generalized exanthematous pustulosis.49,50 Nirmatrelvir 150 mg–ritonavir 100 mg, which was authorized for emergency use by the US Food and Drug Administration for the treatment of COVID-19, is a viral protease inhibitor blocking the replication of the virus. Ritonavir can induce pruritus, maculopapular rash, acne, Stevens-Johnson syndrome, and toxic epidermal necrolysis; of note, these effects have been observed following administration of ritonavir for treatment of HIV at higher daily doses and for much longer periods of time compared with treatment of COVID-19 (600–1200 mg vs 200 mg/d, respectively). These cutaneous drug side effects are clinically similar to the manifestations caused either directly or indirectly by SARS-CoV-2 infection; therefore, it may be difficult to differentiate them.
DERMATOSES DUE TO PROTECTIVE DEVICES
Dermatoses due to personal protective equipment such as masks or face shields affected the general population and mostly health care professionals51; 54.4% of 879 health care professionals in one study reported such events.52 These dermatoses mainly include contact dermatitis of the face (nose, forehead, and cheeks) of irritant or allergic nature (eg, from preservatives releasing formaldehyde contained in masks and protective goggles). They manifest with skin dryness; desquamation; maceration; fissures; or erosions or ulcerations of the cheeks, forehead, and nose. Cases of pressure urticaria also have been reported. Irritant dermatitis induced by the frequent use of disinfectants (eg, soaps, hydroalcoholic sanitizing gels) also can affect the hands. Allergic hand dermatitis can be caused by medical gloves.
The term maskne (or mask acne) refers to a variety of mechanical acne due to the prolonged use of surgical masks (>4 hours per day for ≥6 weeks); it includes cases of de novo acne and cases of pre-existing acne aggravated by wearing a mask. Maskne is characterized by acne lesions located on the facial area covered by the mask (Figure 6). It is caused by follicular occlusion; increased sebum secretion; mechanical stress (pressure, friction); and dysbiosis of the microbiome induced by changes in heat, pH, and humidity. Preventive measures include application of noncomedogenic moisturizers or gauze before wearing the mask as well as facial cleansing with appropriate nonalcoholic products. Similar to acne, rosacea often is aggravated by prolonged wearing of surgical masks (mask rosacea).53,54

DERMATOSES REVEALED OR AGGRAVATED BY COVID-19
Exacerbation of various skin diseases has been reported after infection with SARS-CoV-2.55 Psoriasis and acrodermatitis continua of Hallopeau,56 which may progress into generalized, pustular, or erythrodermic forms,57 have been reported; the role of hydroxychloroquine and oral corticosteroids used for the treatment of COVID-19 has been suspected.57 Atopic dermatitis patients—26% to 43%—have experienced worsening of their disease after symptomatic COVID-19 infection.58 The incidence of herpesvirus infections, including herpes zoster, increased during the pandemic.59 Alopecia areata relapses occurred in 42.5% of 392 patients with preexisting disease within 2 months of COVID-19 onset in one study,60 possibly favored by the psychological stress; however, some studies have not confirmed the aggravating role of COVID-19 on alopecia areata.61 Lupus erythematosus, which may relapse in the form of Rowell syndrome,62 and livedoid vasculopathy63 also have been reported following COVID-19 infection.
SKIN MANIFESTATIONS ASSOCIATED WITH COVID-19 VACCINES
In parallel with the rapid spread of COVID-19 vaccination,4 an increasing number of skin manifestations has been observed following vaccination; these dermatoses now are more frequently reported than those related to natural SARS-CoV-2 infection.64-70 Vaccine-induced skin manifestations have a reported incidence of approximately 4% and show a female predominance.65 Most of them (79%) have been reported in association with messenger RNA (mRNA)–based vaccines, which have been the most widely used; however, the frequency of side effects would be lower after mRNA vaccines than after inactivated virus-based vaccines. Eighteen percent occurred after the adenoviral vector vaccine, and 3% after the inactivated virus vaccine.70 Fifty-nine percent were observed after the first dose. They are clinically polymorphous and generally benign, regressing spontaneously after a few days, and they should not constitute a contraindication to vaccination.Interestingly, many skin manifestations are similar to those associated with natural SARS-CoV-2 infection; however, their frequency and severity does not seem to depend on whether the patients had developed skin reactions during prior SARS-CoV-2 infection. These reactions have been classified into several types:
• Immediate local reactions at the injection site: pain, erythema, or edema represent the vast majority (96%) of reactions to vaccines. They appear within 7 days after vaccination (average, 1 day), slightly more frequently (59%) after the first dose. They concern mostly young patients and are benign, regressing in 2 to 3 days.70
• Delayed local reactions: characterized by pain or pruritus, erythema, and skin induration mimicking cellulitis (COVID arm) and represent 1.7% of postvaccination reactions. They correspond to a delayed hypersensitivity reaction and appear approximately 7 days after vaccination, most often after the first vaccine dose (75% of cases), which is almost invariably mRNA based.70
• Urticarial reactions corresponding to an immediate (type 1) hypersensitivity reaction: constitute 1% of postvaccination reactions, probably due to an allergy to vaccine ingredients. They appear on average 1 day after vaccination, almost always with mRNA vaccines.70
• Angioedema: characterized by mucosal or subcutaneous edema and constitutes 0.5% of postvaccination reactions. It is a potentially serious reaction that appears on average 12 hours after vaccination, always with an mRNA-based vaccine.70
• Morbilliform rash: represents delayed hypersensitivity reactions (0.1% of postvaccination reactions) that appear mostly after the first dose (72%), on average 3 days after vaccination, always with an mRNA-based vaccine.70
• Herpes zoster: usually develops after the first vaccine dose in elderly patients (69% of cases) on average 4 days after vaccination and constitutes 0.1% of postvaccination reactions.71
• Bullous diseases: mainly bullous pemphigoid (90%) and more rarely pemphigus (5%) or bullous erythema pigmentosum (5%). They appear in elderly patients on average 7 days after vaccination and constitute 0.04% of postvaccination reactions.72
• Chilblainlike lesions: several such cases have been reported so far73; they constitute 0.03% of postvaccination reactions.70 Clinically, they are similar to those associated with natural COVID-19; they appear mostly after the first dose (64%), on average 5 days after vaccination with the mRNA or adenovirus vaccine, and show a female predominance. The appearance of these lesions in vaccinated patients, who are a priori not carriers of the virus, strongly suggests that CBLLs are due to the immune reaction against SARS-CoV-2 rather than to a direct effect of this virus on the skin, which also is a likely scenario with regards to other skin manifestations seen during the successive COVID-19 epidemic waves.73-75
• Reactions to hyaluronic acid–containing cosmetic fillers: erythema, edema, and potentially painful induration at the filler injection sites. They constitute 0.04% of postvaccination skin reactions and appear 24 hours after vaccination with mRNA-based vaccines, equally after the first or second dose.76
• Pityriasis rosea–like rash: most occur after the second dose of mRNA-based vaccines (0.023% of postvaccination skin reactions).70
• Severe reactions: these include acute generalized exanthematous pustulosis77 and Stevens-Johnson syndrome.78 One case of each has been reported after the adenoviral vector vaccine 3 days after vaccination.
Other more rarely observed manifestations include reactivation/aggravation or de novo appearance of inflammatory dermatoses such as psoriasis,79,80 leukocytoclastic vasculitis,81,82 lymphocytic83 or urticarial84 vasculitis, Sweet syndrome,85 lupus erythematosus, dermatomyositis,86,87 alopecia,37,88 infection with Trichophyton rubrum,89 Grover disease,90 and lymphomatoid reactions (such as recurrences of cutaneous T-cell lymphomas [CD30+], and de novo development of lymphomatoid papulosis).91
FINAL THOUGHTS
COVID-19 is associated with several skin manifestations, even though the causative role of SARS-CoV-2 has remained elusive. These dermatoses are highly polymorphous, mostly benign, and usually spontaneously regressive, but some of them reflect severe infection. They mostly were described during the first pandemic waves, reported in several national and international registries, which allowed for their morphological classification. Currently, cutaneous adverse effects of vaccines are the most frequently reported dermatoses associated with SARS-CoV-2, and it is likely that they will continue to be observed while COVID-19 vaccination lasts. Hopefully the end of the COVID-19 pandemic is near. In January 2023, the International Health Regulations Emergency Committee of the World Health Organization acknowledged that the COVID-19 pandemic may be approaching an inflexion point, and even though the event continues to constitute a public health emergency of international concern, the higher levels of population immunity achieved globally through infection and/or vaccination may limit the impact of SARS-CoV-2 on morbidity and mortality. However, there is little doubt that this virus will remain a permanently established pathogen in humans and animals for the foreseeable future.92 Therefore, physicians—especially dermatologists—should be aware of the various skin manifestations associated with COVID-19 so they can more efficiently manage their patients.
COVID-19 is a potentially severe systemic disease caused by SARS-CoV-2. SARS-CoV and Middle East respiratory syndrome (MERS-CoV) caused fatal epidemics in Asia in 2002 to 2003 and in the Arabian Peninsula in 2012, respectively. In 2019, SARS-CoV-2 was detected in patients with severe, sometimes fatal pneumonia of previously unknown origin; it rapidly spread around the world, and the World Health Organization declared the disease a pandemic on March 11, 2020. SARS-CoV-2 is a β-coronavirus that is genetically related to the bat coronavirus and SARS-CoV; it is a single-stranded RNA virus of which several variants and subvariants exist. The SARS-CoV-2 viral particles bind via their surface spike protein (S protein) to the angiotensin-converting enzyme 2 receptor present on the membrane of several cell types, including epidermal and adnexal keratinocytes.1,2 The α and δ variants, predominant from 2020 to 2021, mainly affected the lower respiratory tract and caused severe, potentially fatal pneumonia, especially in patients older than 65 years and/or with comorbidities, such as obesity, hypertension, diabetes, and (iatrogenic) immunosuppression. The ο variant, which appeared in late 2021, is more contagious than the initial variants, but it causes a less severe disease preferentially affecting the upper respiratory airways.3 As of April 5, 2023, more than 762,000,000 confirmed cases of COVID-19 have been recorded worldwide, causing more than 6,800,000 deaths.4
Early studies from China describing the symptoms of COVID-19 reported a low frequency of skin manifestations (0.2%), probably because they were focused on the most severe disease symptoms.5 Subsequently, when COVID-19 spread to the rest of the world, an increasing number of skin manifestations were reported in association with the disease. After the first publication from northern Italy in spring 2020, which was specifically devoted to skin manifestations of COVID-19,6 an explosive number of publications reported a large number of skin manifestations, and national registries were established in several countries to record these manifestations, such as the American Academy of Dermatology and the International League of Dermatological Societies registry,7,8 the COVIDSKIN registry of the French Dermatology Society,9 and the Italian registry.10 Highlighting the unprecedented number of scientific articles published on this new disease, a PubMed search of articles indexed for MEDLINE search using the terms SARS-CoV-2 or COVID-19, on April 6, 2023, revealed 351,596 articles; that is more than 300 articles published every day in this database alone, with a large number of them concerning the skin.
SKIN DISEASSES ASSOCIATED WITH COVID-19
There are several types of COVID-19–related skin manifestations, depending on the circumstances of onset and the evolution of the pandemic.
Skin Manifestations Associated With SARS-CoV-2 Infection
The estimated incidence varies greatly according to the published series of patients, possibly depending on the geographic location. The estimated incidence seems lower in Asian countries, such as China (0.2%)5 and Japan (0.56%),11 compared with Europe (up to 20%).6 Skin manifestations associated with SARS-CoV-2 infection affect individuals of all ages, slightly more females, and are clinically polymorphous; some of them are associated with the severity of the infection.12 They may precede, accompany, or appear after the symptoms of COVID-19, most often within a month of the infection, of which they rarely are the only manifestation; however, their precise relationship to SARS-CoV-2 is not always well known. They have been classified according to their clinical presentation into several forms.13-15
Morbilliform Maculopapular Eruption—Representing 16% to 53% of skin manifestations, morbilliform and maculopapular eruptions usually appear within 15 days of infection; they manifest with more or less confluent erythematous macules that may be hemorrhagic/petechial, and usually are asymptomatic and rarely pruritic. The rash mainly affects the trunk and limbs, sparing the face, palmoplantar regions, and mucous membranes; it appears concomitantly with or a few days after the first symptoms of COVID-19 (eg, fever, respiratory symptoms), regresses within a few days, and does not appear to be associated with disease severity. The distinction from maculopapular drug eruptions may be subtle. Histologically, the rash manifests with a spongiform dermatitis (ie, variable parakeratosis; spongiosis; and a mixed dermal perivascular infiltrate of lymphocytes, eosinophils and histiocytes, depending on the lesion age)(Figure 1). The etiopathogenesis is unknown; it may involve immune complexes to SARS-CoV-2 deposited on skin vessels. Treatment is not mandatory; if necessary, local or systemic corticosteroids may be used.

Vesicular (Pseudovaricella) Rash—This rash accounts for 11% to 18% of all skin manifestations and usually appears within 15 days of COVID-19 onset. It manifests with small monomorphous or varicellalike (pseudopolymorphic) vesicles appearing on the trunk, usually in young patients. The vesicles may be herpetiform, hemorrhagic, or pruritic, and appear before or within 3 days of the onset of mild COVID-19 symptoms; they regress within a few days without scarring. Histologically, the lesions show basal cell vacuolization; multinucleated, dyskeratotic/apoptotic or ballooning/acantholytic epidermal keratinocytes; reticular degeneration of the epidermis; intraepidermal vesicles sometimes resembling herpetic vesicular infections or Grover disease; and mild dermal inflammation. There is no specific treatment.
Urticaria—Urticarial rash, or urticaria, represents 5% to 16% of skin manifestations; usually appears within 15 days of disease onset; and manifests with pruritic, migratory, edematous papules appearing mainly on the trunk and occasionally the face and limbs. The urticarial rash tends to be associated with more severe forms of the disease and regresses within a week, responding to antihistamines. Of note, clinically similar rashes can be caused by drugs. Histologically, the lesions show dermal edema and a mild perivascular lymphocytic infiltrate, sometimes admixed with eosinophils.
Chilblainlike Lesions—Chilblainlike lesions (CBLLs) account for 19% of skin manifestations associated with COVID-1913 and present as erythematous-purplish, edematous lesions that can be mildly pruritic or painful, appearing on the toes—COVID toes—and more rarely the fingers (Figure 2). They were seen epidemically during the first pandemic wave (2020 lockdown) in several countries, and clinically are very similar to, if not indistinguishable from, idiopathic chilblains, but are not necessarily associated with cold exposure. They appear in young, generally healthy patients or those with mild COVID-19 symptoms 2 to 4 weeks after symptom onset. They regress spontaneously or under local corticosteroid treatment within a few days or weeks. Histologically, CBLLs are indistinguishable from chilblains of other origins, namely idiopathic (seasonal) ones. They manifest with necrosis of epidermal keratinocytes; dermal edema that may be severe, leading to the development of subepidermal pseudobullae; a rather dense perivascular and perieccrine gland lymphocytic infiltrate; and sometimes with vascular lesions (eg, edema of endothelial cells, microthromboses of dermal capillaries and venules, fibrinoid deposits within the wall of dermal venules)(Figure 3).16-18 Most patients (>80%) with CBLLs have negative serologic or polymerase chain reaction tests for SARS-CoV-2,19 which generated a lively debate about the role of SARS-CoV-2 in the genesis of CBLLs. According to some authors, SARS-CoV-2 plays no direct role, and CBLLs would occur in young people who sit or walk barefoot on cold floors at home during confinement.20-23 Remarkably, CBLLs appeared in patients with no history of chilblains during a season that was not particularly cold, namely in France or in southern California, where their incidence was much higher compared to the same time period of prior years. Some reports have supported a direct role for the virus based on questionable observations of the virus within skin lesions (eg, sweat glands, endothelial cells) by immunohistochemistry, electron microscopy, and/or in situ hybridization.17,24,25 A more satisfactory hypothesis would involve the role of a strong innate immunity leading to elimination of the virus before the development of specific antibodies via the increased production of type 1 interferon (IFN-1); this would affect the vessels, causing CBLLs. This mechanism would be similar to the one observed in some interferonopathies (eg, Aicardi-Goutières syndrome), also characterized by IFN-1 hypersecretion and chilblains.26-29 According to this hypothesis, CBLLs should be considered a paraviral rash similar to other skin manifestations associated with COVID-19.30

Acro-ischemia—Acro-ischemia livedoid lesions account for 1% to 6% of skin manifestations and comprise lesions of livedo (either reticulated or racemosa); necrotic acral bullae; and gangrenous necrosis of the extremities, especially the toes. The livedoid lesions most often appear within 15 days of COVID-19 symptom onset, and the purpuric lesions somewhat later (2–4 weeks); they mainly affect adult patients, last about 10 days, and are the hallmark of severe infection, presumably related to microthromboses of the cutaneous capillaries (endothelial dysfunction, prothrombotic state, elevated D-dimers). Histologically, they show capillary thrombosis and dermoepidermal necrosis (Figure 4).

Other Reported Polymorphic or Atypical Rashes—Erythema multiforme–like eruptions may appear before other COVID-19 symptoms and manifest as reddish-purple, nearly symmetric, diffuse, occasionally targetoid bullous or necrotic macules. The eruptions mainly affect adults and most often are seen on the palms, elbows, knees, and sometimes the mucous membranes. The rash regresses in 1 to 3 weeks without scarring and represents a delayed cutaneous hypersensitivity reaction. Histologically, the lesions show vacuolization of basal epidermal keratinocytes, keratinocyte necrosis, dermoepidermal detachment, a variably dense dermal T-lymphocytic infiltrate, and red blood cell extravasation (Figure 5).

Leukocytoclastic vasculitis may be generalized or localized. It manifests clinically by petechial/purpuric maculopapules, especially on the legs, mainly in elderly patients with COVID-19. Histologically, the lesions show necrotizing changes of dermal postcapillary venules, neutrophilic perivascular inflammation, red blood cell extravasation, and occasionally vascular IgA deposits by direct immunofluorescence examination. The course usually is benign.

The incidence of pityriasis rosea and of clinically similar rashes (referred to as “pityriasis rosea–like”) increased 5-fold during the COVID-19 pandemic.31,32 These dermatoses manifest with erythematous, scaly, circinate plaques, typically with an initial herald lesion followed a few days later by smaller erythematous macules. Histologically, the lesions comprise a spongiform dermatitis with intraepidermal exocytosis of red blood cells and a mild to moderate dermal lymphocytic infiltrate.
Erythrodysesthesia, or hand-foot syndrome, manifests with edematous erythema and palmoplantar desquamation accompanied by a burning sensation or pain. This syndrome is known as an adverse effect of some chemotherapies because of the associated drug toxicity and sweat gland inflammation; it was observed in 40% of 666 COVID-19–positive patients with mild to moderate pneumonitis.33
“COVID nose” is a rare cutaneous manifestation characterized by nasal pigmentation comprising multiple coalescent frecklelike macules on the tip and wings of the nose and sometimes the malar areas. These lesions predominantly appear in women aged 25 to 65 years and show on average 23 days after onset of COVID-19, which is usually mild. This pigmentation is similar to pigmentary changes after infection with chikungunya; it can be treated with depigmenting products such as azelaic acid and hydroquinone cream with sunscreen use, and it regresses in 2 to 4 months.34
Telogen effluvium (excessive and temporary shedding of normal telogen club hairs of the entire scalp due to the disturbance of the hair cycle) is reportedly frequent in patients (48%) 1 month after COVID-19 infection, but it may appear later (after 12 weeks).35 Alopecia also is frequently reported during long (or postacute) COVID-19 (ie, the symptomatic disease phase past the acute 4 weeks’ stage of the infection) and shows a female predominance36; it likely represents the telogen effluvium seen 90 days after a severe illness. Trichodynia (pruritus, burning, pain, or paresthesia of the scalp) also is reportedly common (developing in more than 58% of patients) and is associated with telogen effluvium in 44% of cases. Several cases of alopecia areata (AA) triggered or aggravated by COVID-19 also have been reported37,38; they could be explained by the “cytokine storm” triggered by the infection, involving T and B lymphocytes; plasmacytoid dendritic cells; natural killer cells with oversecretion of IL-6, IL-4, tumor necrosis factor α, and IFN type I; and a cytotoxic reaction associated with loss of the immune privilege of hair follicles.
Nail Manifestations
The red half-moon nail sign is an asymptomatic purplish-red band around the distal margin of the lunula that affects some adult patients with COVID-19.39 It appears shortly after onset of symptoms, likely the manifestation of vascular inflammation in the nail bed, and regresses slowly after approximately 1 week.40 Beau lines are transverse grooves in the nail plate due to the temporary arrest of the proximal nail matrix growth accompanying systemic illnesses; they appear approximately 2 to 3 weeks after the onset of COVID-19.41 Furthermore, nail alterations can be caused by drugs used to treat COVID-19, such as longitudinal melanonychia due to treatment with hydroxychloroquine or fluorescence of the lunula or nail plate due to treatment with favipiravir.42
Multisystem Inflammatory Syndrome
Multisystem inflammatory syndrome (MIS) is clinically similar to Kawasaki disease; it typically affects children43 and more rarely adults with COVID-19. It manifests with fever, weakness, and biological inflammation and also frequently with skin lesions (72%), which are polymorphous and include morbilliform rash (27%); urticaria (24%); periorbital edema (24%); nonspecific erythema (21.2%); retiform purpura (18%); targetoid lesions (15%); malar rash (15.2%); and periareolar erythema (6%).44 Compared to Kawasaki disease, MIS affects slightly older children (mean age, 8.5 vs 3 years) and more frequently includes cardiac and gastrointestinal manifestations; the mortality rate also is slightly higher (2% vs 0.17%).45
Confirmed COVID-19 Infection
At the beginning of the pandemic, skin manifestations were reported in patients who were suspected of having COVID-19 but did not always have biological confirmation of SARS-CoV-2 infection due to the unavailability of diagnostic tests or the physical impossibility of testing. However, subsequent studies have confirmed that most of these dermatoses were indeed associated with COVID-19 infection.9,46 For example, a study of 655 patients with confirmed COVID-19 infection reported maculopapular (38%), vascular (22%), urticarial (15%), and vesicular (15%) rashes; erythema multiforme or Stevens-Johnson–like syndrome (3%, often related to the use of hydroxychloroquine); generalized pruritus (1%); and MIS (0.5%). The study confirmed that CBLLs were mostly seen in young patients with mild disease, whereas livedo (fixed rash) and retiform purpura occurred in older patients with a guarded prognosis.46
Remarkably, most dermatoses associated with SARS-CoV-2 infection were reported during the initial waves of the pandemic, which were due to the α and δ viral variants. These manifestations were reported more rarely when the ο variant was predominant, even though most patients (63%) who developed CBLLs in the first wave also developed them during the second pandemic wave.47 This decrease in the incidence of COVID-19–associated dermatoses could be because of the lower pathogenicity of the o variant,3 a lower tropism for the skin, and variations in SARS-CoV-2 antigenicity that would induce a different immunologic response, combined with an increasingly stronger herd immunity compared to the first pandemic waves achieved through vaccination and spontaneous infections in the population. Additional reasons may include different baseline characteristics in patients hospitalized with COVID-19 (regarding comorbidities, disease severity, and received treatments), and the possibility that some of the initially reported COVID-19–associated skin manifestations could have been produced by different etiologic agents.48 In the last 2 years, COVID-19–related skin manifestations have been reported mainly as adverse events to COVID-19 vaccination.
CUTANEOUS ADVERSE EFFECTS OF DRUGS USED TO TREAT COVID-19
Prior to the advent of vaccines and specific treatments for SARS-CoV-2, various drugs were used—namely hydroxychloroquine, ivermectin, and tocilizumab—that did not prove efficacious and caused diverse adverse effects, including cutaneous eruptions such as urticaria, maculopapular eruptions, erythema multiforme or Stevens-Johnson syndrome, vasculitis, longitudinal melanonychia, and acute generalized exanthematous pustulosis.49,50 Nirmatrelvir 150 mg–ritonavir 100 mg, which was authorized for emergency use by the US Food and Drug Administration for the treatment of COVID-19, is a viral protease inhibitor blocking the replication of the virus. Ritonavir can induce pruritus, maculopapular rash, acne, Stevens-Johnson syndrome, and toxic epidermal necrolysis; of note, these effects have been observed following administration of ritonavir for treatment of HIV at higher daily doses and for much longer periods of time compared with treatment of COVID-19 (600–1200 mg vs 200 mg/d, respectively). These cutaneous drug side effects are clinically similar to the manifestations caused either directly or indirectly by SARS-CoV-2 infection; therefore, it may be difficult to differentiate them.
DERMATOSES DUE TO PROTECTIVE DEVICES
Dermatoses due to personal protective equipment such as masks or face shields affected the general population and mostly health care professionals51; 54.4% of 879 health care professionals in one study reported such events.52 These dermatoses mainly include contact dermatitis of the face (nose, forehead, and cheeks) of irritant or allergic nature (eg, from preservatives releasing formaldehyde contained in masks and protective goggles). They manifest with skin dryness; desquamation; maceration; fissures; or erosions or ulcerations of the cheeks, forehead, and nose. Cases of pressure urticaria also have been reported. Irritant dermatitis induced by the frequent use of disinfectants (eg, soaps, hydroalcoholic sanitizing gels) also can affect the hands. Allergic hand dermatitis can be caused by medical gloves.
The term maskne (or mask acne) refers to a variety of mechanical acne due to the prolonged use of surgical masks (>4 hours per day for ≥6 weeks); it includes cases of de novo acne and cases of pre-existing acne aggravated by wearing a mask. Maskne is characterized by acne lesions located on the facial area covered by the mask (Figure 6). It is caused by follicular occlusion; increased sebum secretion; mechanical stress (pressure, friction); and dysbiosis of the microbiome induced by changes in heat, pH, and humidity. Preventive measures include application of noncomedogenic moisturizers or gauze before wearing the mask as well as facial cleansing with appropriate nonalcoholic products. Similar to acne, rosacea often is aggravated by prolonged wearing of surgical masks (mask rosacea).53,54

DERMATOSES REVEALED OR AGGRAVATED BY COVID-19
Exacerbation of various skin diseases has been reported after infection with SARS-CoV-2.55 Psoriasis and acrodermatitis continua of Hallopeau,56 which may progress into generalized, pustular, or erythrodermic forms,57 have been reported; the role of hydroxychloroquine and oral corticosteroids used for the treatment of COVID-19 has been suspected.57 Atopic dermatitis patients—26% to 43%—have experienced worsening of their disease after symptomatic COVID-19 infection.58 The incidence of herpesvirus infections, including herpes zoster, increased during the pandemic.59 Alopecia areata relapses occurred in 42.5% of 392 patients with preexisting disease within 2 months of COVID-19 onset in one study,60 possibly favored by the psychological stress; however, some studies have not confirmed the aggravating role of COVID-19 on alopecia areata.61 Lupus erythematosus, which may relapse in the form of Rowell syndrome,62 and livedoid vasculopathy63 also have been reported following COVID-19 infection.
SKIN MANIFESTATIONS ASSOCIATED WITH COVID-19 VACCINES
In parallel with the rapid spread of COVID-19 vaccination,4 an increasing number of skin manifestations has been observed following vaccination; these dermatoses now are more frequently reported than those related to natural SARS-CoV-2 infection.64-70 Vaccine-induced skin manifestations have a reported incidence of approximately 4% and show a female predominance.65 Most of them (79%) have been reported in association with messenger RNA (mRNA)–based vaccines, which have been the most widely used; however, the frequency of side effects would be lower after mRNA vaccines than after inactivated virus-based vaccines. Eighteen percent occurred after the adenoviral vector vaccine, and 3% after the inactivated virus vaccine.70 Fifty-nine percent were observed after the first dose. They are clinically polymorphous and generally benign, regressing spontaneously after a few days, and they should not constitute a contraindication to vaccination.Interestingly, many skin manifestations are similar to those associated with natural SARS-CoV-2 infection; however, their frequency and severity does not seem to depend on whether the patients had developed skin reactions during prior SARS-CoV-2 infection. These reactions have been classified into several types:
• Immediate local reactions at the injection site: pain, erythema, or edema represent the vast majority (96%) of reactions to vaccines. They appear within 7 days after vaccination (average, 1 day), slightly more frequently (59%) after the first dose. They concern mostly young patients and are benign, regressing in 2 to 3 days.70
• Delayed local reactions: characterized by pain or pruritus, erythema, and skin induration mimicking cellulitis (COVID arm) and represent 1.7% of postvaccination reactions. They correspond to a delayed hypersensitivity reaction and appear approximately 7 days after vaccination, most often after the first vaccine dose (75% of cases), which is almost invariably mRNA based.70
• Urticarial reactions corresponding to an immediate (type 1) hypersensitivity reaction: constitute 1% of postvaccination reactions, probably due to an allergy to vaccine ingredients. They appear on average 1 day after vaccination, almost always with mRNA vaccines.70
• Angioedema: characterized by mucosal or subcutaneous edema and constitutes 0.5% of postvaccination reactions. It is a potentially serious reaction that appears on average 12 hours after vaccination, always with an mRNA-based vaccine.70
• Morbilliform rash: represents delayed hypersensitivity reactions (0.1% of postvaccination reactions) that appear mostly after the first dose (72%), on average 3 days after vaccination, always with an mRNA-based vaccine.70
• Herpes zoster: usually develops after the first vaccine dose in elderly patients (69% of cases) on average 4 days after vaccination and constitutes 0.1% of postvaccination reactions.71
• Bullous diseases: mainly bullous pemphigoid (90%) and more rarely pemphigus (5%) or bullous erythema pigmentosum (5%). They appear in elderly patients on average 7 days after vaccination and constitute 0.04% of postvaccination reactions.72
• Chilblainlike lesions: several such cases have been reported so far73; they constitute 0.03% of postvaccination reactions.70 Clinically, they are similar to those associated with natural COVID-19; they appear mostly after the first dose (64%), on average 5 days after vaccination with the mRNA or adenovirus vaccine, and show a female predominance. The appearance of these lesions in vaccinated patients, who are a priori not carriers of the virus, strongly suggests that CBLLs are due to the immune reaction against SARS-CoV-2 rather than to a direct effect of this virus on the skin, which also is a likely scenario with regards to other skin manifestations seen during the successive COVID-19 epidemic waves.73-75
• Reactions to hyaluronic acid–containing cosmetic fillers: erythema, edema, and potentially painful induration at the filler injection sites. They constitute 0.04% of postvaccination skin reactions and appear 24 hours after vaccination with mRNA-based vaccines, equally after the first or second dose.76
• Pityriasis rosea–like rash: most occur after the second dose of mRNA-based vaccines (0.023% of postvaccination skin reactions).70
• Severe reactions: these include acute generalized exanthematous pustulosis77 and Stevens-Johnson syndrome.78 One case of each has been reported after the adenoviral vector vaccine 3 days after vaccination.
Other more rarely observed manifestations include reactivation/aggravation or de novo appearance of inflammatory dermatoses such as psoriasis,79,80 leukocytoclastic vasculitis,81,82 lymphocytic83 or urticarial84 vasculitis, Sweet syndrome,85 lupus erythematosus, dermatomyositis,86,87 alopecia,37,88 infection with Trichophyton rubrum,89 Grover disease,90 and lymphomatoid reactions (such as recurrences of cutaneous T-cell lymphomas [CD30+], and de novo development of lymphomatoid papulosis).91
FINAL THOUGHTS
COVID-19 is associated with several skin manifestations, even though the causative role of SARS-CoV-2 has remained elusive. These dermatoses are highly polymorphous, mostly benign, and usually spontaneously regressive, but some of them reflect severe infection. They mostly were described during the first pandemic waves, reported in several national and international registries, which allowed for their morphological classification. Currently, cutaneous adverse effects of vaccines are the most frequently reported dermatoses associated with SARS-CoV-2, and it is likely that they will continue to be observed while COVID-19 vaccination lasts. Hopefully the end of the COVID-19 pandemic is near. In January 2023, the International Health Regulations Emergency Committee of the World Health Organization acknowledged that the COVID-19 pandemic may be approaching an inflexion point, and even though the event continues to constitute a public health emergency of international concern, the higher levels of population immunity achieved globally through infection and/or vaccination may limit the impact of SARS-CoV-2 on morbidity and mortality. However, there is little doubt that this virus will remain a permanently established pathogen in humans and animals for the foreseeable future.92 Therefore, physicians—especially dermatologists—should be aware of the various skin manifestations associated with COVID-19 so they can more efficiently manage their patients.
- Ashraf UM, Abokor AA, Edwards JM, et al. SARS-CoV-2, ACE2 expression, and systemic organ invasion. Physiol Genomics. 2021;53:51-60.
- Ganier C, Harun N, Peplow I, et al. Angiotensin-converting enzyme 2 expression is detectable in keratinocytes, cutaneous appendages, and blood vessels by multiplex RNA in situ hybridization. Adv Skin Wound Care. 2022;35:219-223.
- Ulloa AC, Buchan SA, Daneman N, et al. Estimates of SARS-CoV-2 omicron variant severity in Ontario, Canada. JAMA. 2022;327:1286-1288.
- World Health Organization. Coronavirus (COVID-19) Dashboard. Accessed April 6, 2023. https://covid19.who.int
- Guan WJ, Ni ZY, Hu Y, et al; China Medical Treatment Expert Group for COVID-19. clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708-1720.
- Recalcati S. Cutaneous manifestations in COVID-9: a first perspective. J Eur Acad Dermatol Venereol. 2020;34:E212-E213.
- Freeman EE, McMahon DE, Lipoff JB, et al. The spectrum of COVID-19-associated dermatologic manifestations: an international registry of 716 patients from 31 countries. J Am Acad Dermatol. 2020;83:1118-1129.
- Freeman EE, Chamberlin GC, McMahon DE, et al. Dermatology COVID-19 registries: updates and future directions. Dermatol Clin. 2021;39:575-585.
- Guelimi R, Salle R, Dousset L, et al. Non-acral skin manifestations during the COVID-19 epidemic: COVIDSKIN study by the French Society of Dermatology. J Eur Acad Dermatol Venereol. 2021;35:E539-E541.
- Marzano AV, Genovese G, Moltrasio C, et al; Italian Skin COVID-19 Network of the Italian Society of Dermatology and Sexually Transmitted Diseases. The clinical spectrum of COVID-19 associated cutaneous manifestations: an Italian multicenter study of 200 adult patients. J Am Acad Dermatol. 2021;84:1356-1363.
- Sugai T, Fujita Y, Inamura E, et al. Prevalence and patterns of cutaneous manifestations in 1245 COVID-19 patients in Japan: a single-centre study. J Eur Acad Dermatol Venereol. 2022;36:E522-E524.
- Holmes Z, Courtney A, Lincoln M, et al. Rash morphology as a predictor of COVID‐19 severity: a systematic review of the cutaneous manifestations of COVID‐19. Skin Health Dis. 2022;2:E120. doi:10.1002/ski2.120
- Galván Casas C, Català A, Carretero Hernández G, et al. Classification of the cutaneous manifestations of COVID-19: a rapid prospective nationwide consensus study in Spain with 375 cases. Br J Dermatol. 2020;183:71-77.
- Garduño‑Soto M, Choreño-Parra, Cazarin-Barrientos Dermatological aspects of SARS‑CoV‑2 infection: mechanisms and manifestations. Arch Dermatol Res. 2021;313:611-622.
- Huynh T, Sanchez-Flores X, Yau J, et al. Cutaneous manifestations of SARS-CoV-2 Infection. Am J Clin Dermatol. 2022;23:277-286.
- Kanitakis J, Lesort C, Danset M, et al.
Chilblain-like acral lesions during the COVID-19 pandemic (“COVID toes”): histologic, immunofluorescence, and immunohistochemical study of 17 cases. J Am Acad Dermatol. 2020; 83:870-875. - Kolivras A, Thompson C, Pastushenko I, et al. A clinicopathological description of COVID-19-induced chilblains (COVID-toes) correlated with a published literature review. J Cutan Pathol. 2022;49:17-28.
- Roca-Ginés J, Torres-Navarro I, Sánchez-Arráez J, et al. Assessment of acute acral lesions in a case series of children and adolescents during the COVID-19 pandemic. 2020;156:992-997.
- Le Cleach L, Dousset L, Assier H, et al; French Society of Dermatology. Most chilblains observed during the COVID-19 outbreak occur in patients who are negative for COVID-19 on polymerase chain reaction and serology testing. Br J Dermatol. 2020;183:866-874.
- Discepolo V, Catzola A, Pierri L, et al. Bilateral chilblain-like lesions of the toes characterized by microvascular remodeling in adolescents during the COVID-19 pandemic. JAMA Netw Open. 2021;4:E2111369.
- Gehlhausen JR, Little AJ, Ko CJ, et al. Lack of association between pandemic chilblains and SARS-CoV-2 infection. Proc Natl Acad Sci U S A. 2022;119:e2122090119.
- Neri, Virdi, Corsini, et al Major cluster of paediatric ‘true’ primary chilblains during the COVID-19 pandemic: a consequence of lifestyle changes due to lockdown. J Eur Acad Dermatol Venereol. 2020;34:2630-2635.
- De Greef A, Choteau M, Herman A, et al. Chilblains observed during the COVID-19 pandemic cannot be distinguished from the classic, cold-related chilblains. Eur J Dermatol. 2022;32:377-383.
- Colmenero I, Santonja C, Alonso-Riaño M, et al. SARS-CoV-2 endothelial infection causes COVID-19 chilblains: histopathological, immunohistochemical and ultrastructural study of seven paediatric cases. Br J Dermatol. 2020;183:729-737.
- Quintero-Bustos G, Aguilar-Leon D, Saeb-Lima M. Histopathological and immunohistochemical characterization of skin biopsies from 41 SARS-CoV-2 (+) patients: experience in a Mexican concentration institute: a case series and literature review. Am J Dermatopathol. 2022;44:327-337.
- Arkin LM, Moon JJ, Tran JM, et al; COVID Human Genetic Effort. From your nose to your toes: a review of severe acute respiratory syndrome coronavirus 2 pandemic-associated pernio. J Invest Dermatol. 2021;141:2791-2796.
- Frumholtz L, Bouaziz JD, Battistella M, et al; Saint-Louis CORE (COvid REsearch). Type I interferon response and vascular alteration in chilblain-like lesions during the COVID-19 outbreak. Br J Dermatol. 2021;185:1176-1185.
- Hubiche T, Cardot-Leccia N, Le Duff F, et al. Clinical, laboratory, and interferon-alpha response characteristics of patients with chilblain-like lesions during the COVID-19 pandemic. JAMA Dermatol. 2021;157:202-206.
- Lesort C, Kanitakis J, Villani A, et al. COVID-19 and outbreak of chilblains: are they related? J Eur Acad Dermatol Venereol. 2020;34:E757-E758.
- Sanchez A, Sohier P, Benghanem S, et al. Digitate papulosquamous eruption associated with severe acute respiratory syndrome coronavirus 2 infection. JAMA Dermatol. 2020;156:819-820.
- Drago F, Broccolo F, Ciccarese G. Pityriasis rosea, pityriasis rosea-like eruptions, and herpes zoster in the setting of COVID-19 and COVID-19 vaccination. Clin Dermatol. 2022;S0738-081X(22)00002-5.
- Dursun R, Temiz SA. The clinics of HHV-6 infection in COVID-19 pandemic: pityriasis rosea and Kawasaki disease. Dermatol Ther. 2020;33:E13730.
- Nuno-Gonzalez A, Magaletsky K, Feito Rodríguez M, et al. Palmoplantar erythrodysesthesia: a diagnostic sign of COVID-19. J Eur Acad Dermatol Venereol. 2021;35:e247-e249.
- Sil A, Panigrahi A, Chandra A, et al. “COVID nose”: a unique post-COVID pigmentary sequelae reminiscing Chik sign: a descriptive case series. J Eur Acad Dermatol Venereol. 2022;36:E419-E421.
- Starace M, Iorizzo M, Sechi A, et al. Trichodynia and telogen effluvium in COVID-19 patients: results of an international expert opinion survey on diagnosis and management. JAAD Int. 2021;5:11-18.
- Wong-Chew RM, Rodríguez Cabrera EX, Rodríguez Valdez CA, et al. Symptom cluster analysis of long COVID-19 in patients discharged from the Temporary COVID-19 Hospital in Mexico City. Ther Adv Infect Dis. 2022;9:20499361211069264.
- Bardazzi F, Guglielmo A, Abbenante D, et al. New insights into alopecia areata during COVID-19 pandemic: when infection or vaccination could play a role. J Cosmet Dermatol. 2022;21:1796-1798.
- Christensen RE, Jafferany M. Association between alopecia areata and COVID-19: a systematic review. JAAD Int. 2022;7:57-61.
- Wollina U, Kanitakis J, Baran R. Nails and COVID-19: a comprehensive review of clinical findings and treatment. Dermatol Ther. 2021;34:E15100.
- Méndez-Flores S, Zaladonis A, Valdes-Rodriguez R. COVID-19 and nail manifestation: be on the lookout for the red half-moon nail sign. Int J Dermatol. 2020;59:1414.
- Alobaida S, Lam JM. Beau lines associated with COVID-19. CMAJ. 2020;192:E1040.
- Durmaz EÖ, Demirciog˘lu D. Fluorescence in the sclera, nails, and teeth secondary to favipiravir use for COVID-19 infections. J Clin Aesthet Dermatol. 2022;15:35-37.
- Brumfiel CM, DiLorenzo AM, Petronic-Rosic VM. Dermatologic manifestations of COVID-19-associated multisystem inflammatory syndrome in children. Clin Dermatol. 2021;39:329-333.
- Akçay N, Topkarcı Z, Menentog˘lu ME, et al. New dermatological findings of MIS-C: can mucocutaneous involvement be associated with severe disease course? Australas J Dermatol. 2022;63:228-234. doi:10.1111/ajd.13819
- Vogel TP, Top KA, Karatzios C, et al. Multisystem inflammatory syndrome in children and adults (MIS-C/A): case definition & guidelines for data collection, analysis, and presentation of immunization safety data. Vaccine. 2021;39:3037-3049.
- Conforti C, Dianzani C, Agozzino M, et al. Cutaneous manifestations in confirmed COVID-19 patients: a systematic review. Biology (Basel). 2020;9:449.
- Hubiche T, Le Duff F, Fontas E, et al. Relapse of chilblain-like lesions during the second wave of the COVID-19 pandemic: a cohort follow-up. Br J Dermatol. 2021;185:858-859.
- Fernandez-Nieto, Ortega-Quijano, Suarez-Valle, et al Lack of skin manifestations in COVID-19 hospitalized patients during the second epidemic wave in Spain: a possible association with a novel SARS-CoV-2 variant: a cross-sectional study. J Eur Acad Dermatol Venereol. 2021;35:E183-E185.
- Martinez-LopezA, Cuenca-Barrales, Montero-Vilchezet al Review of adverse cutaneous reactions of pharmacologic interventions for COVID-19: a guide for the dermatologist. J Am Acad Dermatol. 2020;83:1738-1748.
- Cutaneous side-effects of the potential COVID-19 drugs. Dermatol Ther. 2020;33:E13476.
- Mawhirt SL, Frankel D, Diaz AM. Cutaneous manifestations in adult patients with COVID-19 and dermatologic conditions related to the COVID-19 pandemic in health care workers. Curr Allerg Asthma Rep. 2020;20:75.
- Nguyen C, Young FG, McElroy D, et al. Personal protective equipment and adverse dermatological reactions among healthcare workers: survey observations from the COVID-19 pandemic. Medicine (Baltimore). 2022;101:E29003.
- Rathi SK, Dsouza JM. Maskne: a new acne variant in COVID-19 era. Indian J Dermatol. 2022;67:552-555.
- Damiani G, Girono L, Grada A, et al. COVID-19 related masks increase severity of both acne (maskne) and rosacea (mask rosacea): multi-center, real-life, telemedical, and observational prospective study. Dermatol Ther. 2021;34:E14848.
- Aram K, Patil A, Goldust M, et al. COVID-19 and exacerbation of dermatological diseases: a review of the available literature. Dermatol Ther. 2021;34:E15113.
- Samotij D, Gawron E, Szcze˛ch J, et al. Acrodermatitis continua of Hallopeau evolving into generalized pustular psoriasis following COVID-19: a case report of a successful treatment with infliximab in combination with acitretin. Biologics. 2021;15:107-113.
- Demiri J, Abdo M, Tsianakas A. Erythrodermic psoriasis after COVID-19 [in German]. Hautarzt. 2022;73:156-159.
- de Wijs LEM, Joustra MM, Olydam JI, et al. COVID-19 in patients with cutaneous immune-mediated diseases in the Netherlands: real-world observational data. J Eur Acad Dermatol Venereol. 2021;35:E173-E176.
- Marques NP, Maia CMF, Marques NCT, et al. Continuous increase of herpes zoster cases in Brazil during the COVID-19 pandemic. Oral Surg Oral Med Oral Pathol Oral Radiol. 2022;133:612-614.
- Rinaldi F, Trink A, Giuliani G, et al. Italian survey for the evaluation of the effects of coronavirus disease 2019 (COVID-19) pandemic on alopecia areata recurrence. Dermatol Ther (Heidelb). 2021;11:339-345.
- Rudnicka L, Rakowska A, Waskiel-Burnat A, et al. Mild-to-moderate COVID-19 is not associated with worsening of alopecia areata: a retrospective analysis of 32 patients. J Am Acad Dermatol. 2021;85:723-725.
- Drenovska K, Shahid M, Mateeva V, et al. Case report: Rowell syndrome-like flare of cutaneous lupus erythematosus following COVID-19 infection. Front Med (Lausanne). 2022;9:815743.
- Kawabe R, Tonomura K, Kotobuki Y, et al. Exacerbation of livedoid vasculopathy after coronavirus disease 2019. Eur J Dermatol. 2022;32:129-131. doi:10.1684/ejd.2022.4200
- McMahon DE, Kovarik CL, Damsky W, et al. Clinical and pathologic correlation of cutaneous COVID-19 vaccine reactions including V-REPP: a registry-based study. J Am Acad Dermatol. 2022;86:113-121.
- Avallone G, Quaglino P, Cavallo F, et al. SARS-CoV-2 vaccine-related cutaneous manifestations: a systematic review. Int J Dermatol. 2022;61:1187-1204. doi:10.1111/ijd.16063
- Gambichler T, Boms S, Susok L, et al. Cutaneous findings following COVID-19 vaccination: review of world literature and own experience. J Eur Acad Dermatol Venereol. 2022;36:172-180.
- Kroumpouzos G, Paroikaki ME, Yumeen S, et al. Cutaneous complications of mRNA and AZD1222 COVID-19 vaccines: a worldwide review. Microorganisms. 2022;10:624.
- Robinson L,Fu X,Hashimoto D, et al. Incidence of cutaneous reactions after messenger RNA COVID-19 vaccines. 2021;
- Wollina U, Chiriac A, Kocic H, et al. Cutaneous and hypersensitivity reactions associated with COVID-19 vaccination: a narrative review. Wien Med Wochenschr. 2022;172:63-69.
- Wei TS. Cutaneous reactions to COVID-19 vaccines: a review. JAAD Int. 2022;7:178-186.
- Katsikas Triantafyllidis K, Giannos P, Mian IT, et al. Varicella zoster virus reactivation following COVID-19 vaccination: a systematic review of case reports. Vaccines (Basel). 2021;9:1013.
- Maronese CA, Caproni M, Moltrasio C, et al. Bullous pemphigoid associated with COVID-19 vaccines: an Italian multicentre study. Front Med (Lausanne). 2022;9:841506.
- Cavazos A, Deb A, Sharma U, et al. COVID toes following vaccination. Proc (Bayl Univ Med Cent). 2022;35:476-479.
- Lesort C, Kanitakis J, Danset M, et al. Chilblain-like lesions after BNT162b2 mRNA COVID-19 vaccine: a case report suggesting that ‘COVID toes’ are due to the immune reaction to SARS-CoV-2. J Eur Acad Dermatol Venereol. 2021;35:E630-E632.
- Russo R, Cozzani E, Micalizzi C, et al. Chilblain-like lesions after COVID-19 vaccination: a case series. Acta Derm Venereol. 2022;102:adv00711. doi:10.2340/actadv.v102.2076
- Ortigosa LCM, Lenzoni FC, Suárez MV, et al. Hypersensitivity reaction to hyaluronic acid dermal filler after COVID-19 vaccination: a series of cases in São Paulo, Brazil. Int J Infect Dis. 2022;116:268-270.
- Agaronov A, Makdesi C, Hall CS. Acute generalized exanthematous pustulosis induced by Moderna COVID-19 messenger RNA vaccine. JAAD Case Rep. 2021;16:96-97.
- Dash S, Sirka CS, Mishra S, et al. COVID-19 vaccine-induced Stevens-Johnson syndrome. Clin Exp Dermatol. 2021;46:1615-1617.
- Huang Y, Tsai TF. Exacerbation of psoriasis following COVID-19 vaccination: report from a single center. Front Med (Lausanne). 2021;8:812010.
- Elamin S, Hinds F, Tolland J. De novo generalized pustular psoriasis following Oxford-AstraZeneca COVID-19 vaccine. Clin Exp Dermatol 2022;47:153-155.
- Abdelmaksoud A, Wollina U, Temiz SA, et al. SARS-CoV-2 vaccination-induced cutaneous vasculitis: report of two new cases and literature review. Dermatol Ther. 2022;35:E15458.
- Fritzen M, Funchal GDG, Luiz MO, et al. Leukocytoclastic vasculitis after exposure to COVID-19 vaccine. An Bras Dermatol. 2022;97:118-121.
- Vassallo C, Boveri E, Brazzelli V, et al. Cutaneous lymphocytic vasculitis after administration of COVID-19 mRNA vaccine. Dermatol Ther. 2021;34:E15076.
- Nazzaro G, Maronese CA. Urticarial vasculitis following mRNA anti-COVID-19 vaccine. Dermatol Ther. 2022;35:E15282.
- Hoshina D, Orita A. Sweet syndrome after severe acute respiratory syndrome coronavirus 2 mRNA vaccine: a case report and literature review. J Dermatol. 2022;49:E175-E176.
- Lemoine C, Padilla C, Krampe N, et al. Systemic lupus erythematous after Pfizer COVID-19 vaccine: a case report. Clin Rheumatol. 2022;41:1597-1601.
- Nguyen B, Lalama MJ, Gamret AC, et al. Cutaneous symptoms of connective tissue diseases after COVID-19 vaccination: a systematic review. Int J Dermatol. 2022;61:E238-E241.
- Gallo G, Mastorino L, Tonella L, et al. Alopecia areata after COVID-19 vaccination. Clin Exp Vaccine Res. 2022;11:129-132.
- Norimatsu Y, Norimatsu Y. A severe case of Trichophyton rubrum-caused dermatomycosis exacerbated after COVID-19 vaccination that had to be differentiated from pustular psoriasis. Med Mycol Case Rep. 2022;36:19-22.
- Yang K, Prussick L, Hartman R, et al. Acantholytic dyskeratosis post-COVID vaccination. Am J Dermatopathol. 2022;44:E61-E63.
- Koumaki D, Marinos L, Nikolaou V, et al. Lymphomatoid papulosis (LyP) after AZD1222 and BNT162b2 COVID-19 vaccines. Int J Dermatol. 2022;61:900-902.
- World Health Organization. Statement on the fourteenth meeting of the International Health Regulations (2005) Emergency Committee regarding the coronavirus disease (COVID-19) pandemic. Published January 30, 2023. Accessed April 12, 2023. https://www.who.int/news/item/30-01-2023-statement-on-the-fourteenth-meeting-of-the-international-health-regulations-(2005)-emergency-committee-regarding-the-coronavirus-disease-(covid-19)-pandemic
- Ashraf UM, Abokor AA, Edwards JM, et al. SARS-CoV-2, ACE2 expression, and systemic organ invasion. Physiol Genomics. 2021;53:51-60.
- Ganier C, Harun N, Peplow I, et al. Angiotensin-converting enzyme 2 expression is detectable in keratinocytes, cutaneous appendages, and blood vessels by multiplex RNA in situ hybridization. Adv Skin Wound Care. 2022;35:219-223.
- Ulloa AC, Buchan SA, Daneman N, et al. Estimates of SARS-CoV-2 omicron variant severity in Ontario, Canada. JAMA. 2022;327:1286-1288.
- World Health Organization. Coronavirus (COVID-19) Dashboard. Accessed April 6, 2023. https://covid19.who.int
- Guan WJ, Ni ZY, Hu Y, et al; China Medical Treatment Expert Group for COVID-19. clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708-1720.
- Recalcati S. Cutaneous manifestations in COVID-9: a first perspective. J Eur Acad Dermatol Venereol. 2020;34:E212-E213.
- Freeman EE, McMahon DE, Lipoff JB, et al. The spectrum of COVID-19-associated dermatologic manifestations: an international registry of 716 patients from 31 countries. J Am Acad Dermatol. 2020;83:1118-1129.
- Freeman EE, Chamberlin GC, McMahon DE, et al. Dermatology COVID-19 registries: updates and future directions. Dermatol Clin. 2021;39:575-585.
- Guelimi R, Salle R, Dousset L, et al. Non-acral skin manifestations during the COVID-19 epidemic: COVIDSKIN study by the French Society of Dermatology. J Eur Acad Dermatol Venereol. 2021;35:E539-E541.
- Marzano AV, Genovese G, Moltrasio C, et al; Italian Skin COVID-19 Network of the Italian Society of Dermatology and Sexually Transmitted Diseases. The clinical spectrum of COVID-19 associated cutaneous manifestations: an Italian multicenter study of 200 adult patients. J Am Acad Dermatol. 2021;84:1356-1363.
- Sugai T, Fujita Y, Inamura E, et al. Prevalence and patterns of cutaneous manifestations in 1245 COVID-19 patients in Japan: a single-centre study. J Eur Acad Dermatol Venereol. 2022;36:E522-E524.
- Holmes Z, Courtney A, Lincoln M, et al. Rash morphology as a predictor of COVID‐19 severity: a systematic review of the cutaneous manifestations of COVID‐19. Skin Health Dis. 2022;2:E120. doi:10.1002/ski2.120
- Galván Casas C, Català A, Carretero Hernández G, et al. Classification of the cutaneous manifestations of COVID-19: a rapid prospective nationwide consensus study in Spain with 375 cases. Br J Dermatol. 2020;183:71-77.
- Garduño‑Soto M, Choreño-Parra, Cazarin-Barrientos Dermatological aspects of SARS‑CoV‑2 infection: mechanisms and manifestations. Arch Dermatol Res. 2021;313:611-622.
- Huynh T, Sanchez-Flores X, Yau J, et al. Cutaneous manifestations of SARS-CoV-2 Infection. Am J Clin Dermatol. 2022;23:277-286.
- Kanitakis J, Lesort C, Danset M, et al.
Chilblain-like acral lesions during the COVID-19 pandemic (“COVID toes”): histologic, immunofluorescence, and immunohistochemical study of 17 cases. J Am Acad Dermatol. 2020; 83:870-875. - Kolivras A, Thompson C, Pastushenko I, et al. A clinicopathological description of COVID-19-induced chilblains (COVID-toes) correlated with a published literature review. J Cutan Pathol. 2022;49:17-28.
- Roca-Ginés J, Torres-Navarro I, Sánchez-Arráez J, et al. Assessment of acute acral lesions in a case series of children and adolescents during the COVID-19 pandemic. 2020;156:992-997.
- Le Cleach L, Dousset L, Assier H, et al; French Society of Dermatology. Most chilblains observed during the COVID-19 outbreak occur in patients who are negative for COVID-19 on polymerase chain reaction and serology testing. Br J Dermatol. 2020;183:866-874.
- Discepolo V, Catzola A, Pierri L, et al. Bilateral chilblain-like lesions of the toes characterized by microvascular remodeling in adolescents during the COVID-19 pandemic. JAMA Netw Open. 2021;4:E2111369.
- Gehlhausen JR, Little AJ, Ko CJ, et al. Lack of association between pandemic chilblains and SARS-CoV-2 infection. Proc Natl Acad Sci U S A. 2022;119:e2122090119.
- Neri, Virdi, Corsini, et al Major cluster of paediatric ‘true’ primary chilblains during the COVID-19 pandemic: a consequence of lifestyle changes due to lockdown. J Eur Acad Dermatol Venereol. 2020;34:2630-2635.
- De Greef A, Choteau M, Herman A, et al. Chilblains observed during the COVID-19 pandemic cannot be distinguished from the classic, cold-related chilblains. Eur J Dermatol. 2022;32:377-383.
- Colmenero I, Santonja C, Alonso-Riaño M, et al. SARS-CoV-2 endothelial infection causes COVID-19 chilblains: histopathological, immunohistochemical and ultrastructural study of seven paediatric cases. Br J Dermatol. 2020;183:729-737.
- Quintero-Bustos G, Aguilar-Leon D, Saeb-Lima M. Histopathological and immunohistochemical characterization of skin biopsies from 41 SARS-CoV-2 (+) patients: experience in a Mexican concentration institute: a case series and literature review. Am J Dermatopathol. 2022;44:327-337.
- Arkin LM, Moon JJ, Tran JM, et al; COVID Human Genetic Effort. From your nose to your toes: a review of severe acute respiratory syndrome coronavirus 2 pandemic-associated pernio. J Invest Dermatol. 2021;141:2791-2796.
- Frumholtz L, Bouaziz JD, Battistella M, et al; Saint-Louis CORE (COvid REsearch). Type I interferon response and vascular alteration in chilblain-like lesions during the COVID-19 outbreak. Br J Dermatol. 2021;185:1176-1185.
- Hubiche T, Cardot-Leccia N, Le Duff F, et al. Clinical, laboratory, and interferon-alpha response characteristics of patients with chilblain-like lesions during the COVID-19 pandemic. JAMA Dermatol. 2021;157:202-206.
- Lesort C, Kanitakis J, Villani A, et al. COVID-19 and outbreak of chilblains: are they related? J Eur Acad Dermatol Venereol. 2020;34:E757-E758.
- Sanchez A, Sohier P, Benghanem S, et al. Digitate papulosquamous eruption associated with severe acute respiratory syndrome coronavirus 2 infection. JAMA Dermatol. 2020;156:819-820.
- Drago F, Broccolo F, Ciccarese G. Pityriasis rosea, pityriasis rosea-like eruptions, and herpes zoster in the setting of COVID-19 and COVID-19 vaccination. Clin Dermatol. 2022;S0738-081X(22)00002-5.
- Dursun R, Temiz SA. The clinics of HHV-6 infection in COVID-19 pandemic: pityriasis rosea and Kawasaki disease. Dermatol Ther. 2020;33:E13730.
- Nuno-Gonzalez A, Magaletsky K, Feito Rodríguez M, et al. Palmoplantar erythrodysesthesia: a diagnostic sign of COVID-19. J Eur Acad Dermatol Venereol. 2021;35:e247-e249.
- Sil A, Panigrahi A, Chandra A, et al. “COVID nose”: a unique post-COVID pigmentary sequelae reminiscing Chik sign: a descriptive case series. J Eur Acad Dermatol Venereol. 2022;36:E419-E421.
- Starace M, Iorizzo M, Sechi A, et al. Trichodynia and telogen effluvium in COVID-19 patients: results of an international expert opinion survey on diagnosis and management. JAAD Int. 2021;5:11-18.
- Wong-Chew RM, Rodríguez Cabrera EX, Rodríguez Valdez CA, et al. Symptom cluster analysis of long COVID-19 in patients discharged from the Temporary COVID-19 Hospital in Mexico City. Ther Adv Infect Dis. 2022;9:20499361211069264.
- Bardazzi F, Guglielmo A, Abbenante D, et al. New insights into alopecia areata during COVID-19 pandemic: when infection or vaccination could play a role. J Cosmet Dermatol. 2022;21:1796-1798.
- Christensen RE, Jafferany M. Association between alopecia areata and COVID-19: a systematic review. JAAD Int. 2022;7:57-61.
- Wollina U, Kanitakis J, Baran R. Nails and COVID-19: a comprehensive review of clinical findings and treatment. Dermatol Ther. 2021;34:E15100.
- Méndez-Flores S, Zaladonis A, Valdes-Rodriguez R. COVID-19 and nail manifestation: be on the lookout for the red half-moon nail sign. Int J Dermatol. 2020;59:1414.
- Alobaida S, Lam JM. Beau lines associated with COVID-19. CMAJ. 2020;192:E1040.
- Durmaz EÖ, Demirciog˘lu D. Fluorescence in the sclera, nails, and teeth secondary to favipiravir use for COVID-19 infections. J Clin Aesthet Dermatol. 2022;15:35-37.
- Brumfiel CM, DiLorenzo AM, Petronic-Rosic VM. Dermatologic manifestations of COVID-19-associated multisystem inflammatory syndrome in children. Clin Dermatol. 2021;39:329-333.
- Akçay N, Topkarcı Z, Menentog˘lu ME, et al. New dermatological findings of MIS-C: can mucocutaneous involvement be associated with severe disease course? Australas J Dermatol. 2022;63:228-234. doi:10.1111/ajd.13819
- Vogel TP, Top KA, Karatzios C, et al. Multisystem inflammatory syndrome in children and adults (MIS-C/A): case definition & guidelines for data collection, analysis, and presentation of immunization safety data. Vaccine. 2021;39:3037-3049.
- Conforti C, Dianzani C, Agozzino M, et al. Cutaneous manifestations in confirmed COVID-19 patients: a systematic review. Biology (Basel). 2020;9:449.
- Hubiche T, Le Duff F, Fontas E, et al. Relapse of chilblain-like lesions during the second wave of the COVID-19 pandemic: a cohort follow-up. Br J Dermatol. 2021;185:858-859.
- Fernandez-Nieto, Ortega-Quijano, Suarez-Valle, et al Lack of skin manifestations in COVID-19 hospitalized patients during the second epidemic wave in Spain: a possible association with a novel SARS-CoV-2 variant: a cross-sectional study. J Eur Acad Dermatol Venereol. 2021;35:E183-E185.
- Martinez-LopezA, Cuenca-Barrales, Montero-Vilchezet al Review of adverse cutaneous reactions of pharmacologic interventions for COVID-19: a guide for the dermatologist. J Am Acad Dermatol. 2020;83:1738-1748.
- Cutaneous side-effects of the potential COVID-19 drugs. Dermatol Ther. 2020;33:E13476.
- Mawhirt SL, Frankel D, Diaz AM. Cutaneous manifestations in adult patients with COVID-19 and dermatologic conditions related to the COVID-19 pandemic in health care workers. Curr Allerg Asthma Rep. 2020;20:75.
- Nguyen C, Young FG, McElroy D, et al. Personal protective equipment and adverse dermatological reactions among healthcare workers: survey observations from the COVID-19 pandemic. Medicine (Baltimore). 2022;101:E29003.
- Rathi SK, Dsouza JM. Maskne: a new acne variant in COVID-19 era. Indian J Dermatol. 2022;67:552-555.
- Damiani G, Girono L, Grada A, et al. COVID-19 related masks increase severity of both acne (maskne) and rosacea (mask rosacea): multi-center, real-life, telemedical, and observational prospective study. Dermatol Ther. 2021;34:E14848.
- Aram K, Patil A, Goldust M, et al. COVID-19 and exacerbation of dermatological diseases: a review of the available literature. Dermatol Ther. 2021;34:E15113.
- Samotij D, Gawron E, Szcze˛ch J, et al. Acrodermatitis continua of Hallopeau evolving into generalized pustular psoriasis following COVID-19: a case report of a successful treatment with infliximab in combination with acitretin. Biologics. 2021;15:107-113.
- Demiri J, Abdo M, Tsianakas A. Erythrodermic psoriasis after COVID-19 [in German]. Hautarzt. 2022;73:156-159.
- de Wijs LEM, Joustra MM, Olydam JI, et al. COVID-19 in patients with cutaneous immune-mediated diseases in the Netherlands: real-world observational data. J Eur Acad Dermatol Venereol. 2021;35:E173-E176.
- Marques NP, Maia CMF, Marques NCT, et al. Continuous increase of herpes zoster cases in Brazil during the COVID-19 pandemic. Oral Surg Oral Med Oral Pathol Oral Radiol. 2022;133:612-614.
- Rinaldi F, Trink A, Giuliani G, et al. Italian survey for the evaluation of the effects of coronavirus disease 2019 (COVID-19) pandemic on alopecia areata recurrence. Dermatol Ther (Heidelb). 2021;11:339-345.
- Rudnicka L, Rakowska A, Waskiel-Burnat A, et al. Mild-to-moderate COVID-19 is not associated with worsening of alopecia areata: a retrospective analysis of 32 patients. J Am Acad Dermatol. 2021;85:723-725.
- Drenovska K, Shahid M, Mateeva V, et al. Case report: Rowell syndrome-like flare of cutaneous lupus erythematosus following COVID-19 infection. Front Med (Lausanne). 2022;9:815743.
- Kawabe R, Tonomura K, Kotobuki Y, et al. Exacerbation of livedoid vasculopathy after coronavirus disease 2019. Eur J Dermatol. 2022;32:129-131. doi:10.1684/ejd.2022.4200
- McMahon DE, Kovarik CL, Damsky W, et al. Clinical and pathologic correlation of cutaneous COVID-19 vaccine reactions including V-REPP: a registry-based study. J Am Acad Dermatol. 2022;86:113-121.
- Avallone G, Quaglino P, Cavallo F, et al. SARS-CoV-2 vaccine-related cutaneous manifestations: a systematic review. Int J Dermatol. 2022;61:1187-1204. doi:10.1111/ijd.16063
- Gambichler T, Boms S, Susok L, et al. Cutaneous findings following COVID-19 vaccination: review of world literature and own experience. J Eur Acad Dermatol Venereol. 2022;36:172-180.
- Kroumpouzos G, Paroikaki ME, Yumeen S, et al. Cutaneous complications of mRNA and AZD1222 COVID-19 vaccines: a worldwide review. Microorganisms. 2022;10:624.
- Robinson L,Fu X,Hashimoto D, et al. Incidence of cutaneous reactions after messenger RNA COVID-19 vaccines. 2021;
- Wollina U, Chiriac A, Kocic H, et al. Cutaneous and hypersensitivity reactions associated with COVID-19 vaccination: a narrative review. Wien Med Wochenschr. 2022;172:63-69.
- Wei TS. Cutaneous reactions to COVID-19 vaccines: a review. JAAD Int. 2022;7:178-186.
- Katsikas Triantafyllidis K, Giannos P, Mian IT, et al. Varicella zoster virus reactivation following COVID-19 vaccination: a systematic review of case reports. Vaccines (Basel). 2021;9:1013.
- Maronese CA, Caproni M, Moltrasio C, et al. Bullous pemphigoid associated with COVID-19 vaccines: an Italian multicentre study. Front Med (Lausanne). 2022;9:841506.
- Cavazos A, Deb A, Sharma U, et al. COVID toes following vaccination. Proc (Bayl Univ Med Cent). 2022;35:476-479.
- Lesort C, Kanitakis J, Danset M, et al. Chilblain-like lesions after BNT162b2 mRNA COVID-19 vaccine: a case report suggesting that ‘COVID toes’ are due to the immune reaction to SARS-CoV-2. J Eur Acad Dermatol Venereol. 2021;35:E630-E632.
- Russo R, Cozzani E, Micalizzi C, et al. Chilblain-like lesions after COVID-19 vaccination: a case series. Acta Derm Venereol. 2022;102:adv00711. doi:10.2340/actadv.v102.2076
- Ortigosa LCM, Lenzoni FC, Suárez MV, et al. Hypersensitivity reaction to hyaluronic acid dermal filler after COVID-19 vaccination: a series of cases in São Paulo, Brazil. Int J Infect Dis. 2022;116:268-270.
- Agaronov A, Makdesi C, Hall CS. Acute generalized exanthematous pustulosis induced by Moderna COVID-19 messenger RNA vaccine. JAAD Case Rep. 2021;16:96-97.
- Dash S, Sirka CS, Mishra S, et al. COVID-19 vaccine-induced Stevens-Johnson syndrome. Clin Exp Dermatol. 2021;46:1615-1617.
- Huang Y, Tsai TF. Exacerbation of psoriasis following COVID-19 vaccination: report from a single center. Front Med (Lausanne). 2021;8:812010.
- Elamin S, Hinds F, Tolland J. De novo generalized pustular psoriasis following Oxford-AstraZeneca COVID-19 vaccine. Clin Exp Dermatol 2022;47:153-155.
- Abdelmaksoud A, Wollina U, Temiz SA, et al. SARS-CoV-2 vaccination-induced cutaneous vasculitis: report of two new cases and literature review. Dermatol Ther. 2022;35:E15458.
- Fritzen M, Funchal GDG, Luiz MO, et al. Leukocytoclastic vasculitis after exposure to COVID-19 vaccine. An Bras Dermatol. 2022;97:118-121.
- Vassallo C, Boveri E, Brazzelli V, et al. Cutaneous lymphocytic vasculitis after administration of COVID-19 mRNA vaccine. Dermatol Ther. 2021;34:E15076.
- Nazzaro G, Maronese CA. Urticarial vasculitis following mRNA anti-COVID-19 vaccine. Dermatol Ther. 2022;35:E15282.
- Hoshina D, Orita A. Sweet syndrome after severe acute respiratory syndrome coronavirus 2 mRNA vaccine: a case report and literature review. J Dermatol. 2022;49:E175-E176.
- Lemoine C, Padilla C, Krampe N, et al. Systemic lupus erythematous after Pfizer COVID-19 vaccine: a case report. Clin Rheumatol. 2022;41:1597-1601.
- Nguyen B, Lalama MJ, Gamret AC, et al. Cutaneous symptoms of connective tissue diseases after COVID-19 vaccination: a systematic review. Int J Dermatol. 2022;61:E238-E241.
- Gallo G, Mastorino L, Tonella L, et al. Alopecia areata after COVID-19 vaccination. Clin Exp Vaccine Res. 2022;11:129-132.
- Norimatsu Y, Norimatsu Y. A severe case of Trichophyton rubrum-caused dermatomycosis exacerbated after COVID-19 vaccination that had to be differentiated from pustular psoriasis. Med Mycol Case Rep. 2022;36:19-22.
- Yang K, Prussick L, Hartman R, et al. Acantholytic dyskeratosis post-COVID vaccination. Am J Dermatopathol. 2022;44:E61-E63.
- Koumaki D, Marinos L, Nikolaou V, et al. Lymphomatoid papulosis (LyP) after AZD1222 and BNT162b2 COVID-19 vaccines. Int J Dermatol. 2022;61:900-902.
- World Health Organization. Statement on the fourteenth meeting of the International Health Regulations (2005) Emergency Committee regarding the coronavirus disease (COVID-19) pandemic. Published January 30, 2023. Accessed April 12, 2023. https://www.who.int/news/item/30-01-2023-statement-on-the-fourteenth-meeting-of-the-international-health-regulations-(2005)-emergency-committee-regarding-the-coronavirus-disease-(covid-19)-pandemic
Practice Points
- During the COVID-19 pandemic, several skin diseases were reported in association with this new infectious disease and were classified mainly according to their morphologic aspect. However, the pathogenetic mechanisms often are unclear and the causal link of the culprit virus (SARS-CoV-2) not always well established.
- Currently, most skin manifestations related to COVID-19 are reported after vaccination against COVID-19; remarkably, many of them are similar to those attributed to the natural infection.