User login
Patient-centered care in clinic
Almost 30 years ago a young woman made an appointment to see me. I had just started my internal medicine practice and almost all the patients who saw me were new to me. I assumed she was establishing care with me. Her first words to me were “Hello Dr. Paauw, I would like to interview you to see if you will be a good fit as my doctor.” We talked for the 40-minute appointment time. I asked her about her health, her life, and what she wanted out of both. We shared with each other that we both were parents of young children. When the appointment was over, she said she would really like for me to be her doctor. She told me that the main thing she appreciated about me was that I listened, and that her previous physician never sat down at her appointments and often had his hand on the door handle for much of the visit. Physicians and patients both agree that compassionate care is essential for good patient care, yet about half of patients and 60% of doctors believe it is lacking in our medical system.1
Remember the golden first minutes
I often start by asking the patient to give me an update on how they are doing. This lets me know what is important to them. I do not touch the computer until after this initial check-in.
Use the computer as a bond to strengthen your patient relationship
Many studies have shown patients find the computer gets between the doctor and patient. It is especially problematic if it breaks eye contact with the patient. People are less likely to share scary, sensitive, or embarrassing information if someone is looking at a computer and typing. As you look up tests, radiology reports, or consultant notes, let the patient in on what you are doing. Explain why you are searching in the record, and if it helps make an important point, show your findings to the patient. Offer to print out results, so they have something to carry with them.
Explain what you are looking for and what you find on the physical exam
Being a patient is scary. We all want reassurance that our fears are not true. When you find normal findings on exam, share those with the patient. Hearing “your heart sounds good, your pulses are strong” really helps patients. Explaining what we are doing when we examine is also helpful. Explain why you are feeling for lymph nodes in the neck, why we percuss the abdomen. Patients are often fascinated by getting a window into how we are thinking. I usually have medical students with me, which offers another avenue to explaining the how and why behind the exam. In asking and explaining to students, the patient is also taught why we do what we do.
Make sure that we cover what they are afraid of, not just what their symptom is
Patients come in not just to get symptom relief but to rest their mind from their fears of what it could be. I find it helpful to ask the patient what they think is the cause of the problem, or if they are worried about any specific diagnosis. With certain symptoms this is particularly important (for example, headaches, fatigue, or abdominal pain).
None of these suggestions are easy to do in busy, time-pressured clinic visits. I have found though that when patients feel cared about, listened to and can have their fears addressed they value our advice more, and less time is needed to negotiate the plan, as it has been developed together.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and serves as third-year medical student clerkship director at the University of Washington. Contact Dr. Paauw at fpnews@mdedge.com.
Reference
Lown BA et al. Health Aff (Millwood). 2011 Sep;30(9):1772-8.
Almost 30 years ago a young woman made an appointment to see me. I had just started my internal medicine practice and almost all the patients who saw me were new to me. I assumed she was establishing care with me. Her first words to me were “Hello Dr. Paauw, I would like to interview you to see if you will be a good fit as my doctor.” We talked for the 40-minute appointment time. I asked her about her health, her life, and what she wanted out of both. We shared with each other that we both were parents of young children. When the appointment was over, she said she would really like for me to be her doctor. She told me that the main thing she appreciated about me was that I listened, and that her previous physician never sat down at her appointments and often had his hand on the door handle for much of the visit. Physicians and patients both agree that compassionate care is essential for good patient care, yet about half of patients and 60% of doctors believe it is lacking in our medical system.1
Remember the golden first minutes
I often start by asking the patient to give me an update on how they are doing. This lets me know what is important to them. I do not touch the computer until after this initial check-in.
Use the computer as a bond to strengthen your patient relationship
Many studies have shown patients find the computer gets between the doctor and patient. It is especially problematic if it breaks eye contact with the patient. People are less likely to share scary, sensitive, or embarrassing information if someone is looking at a computer and typing. As you look up tests, radiology reports, or consultant notes, let the patient in on what you are doing. Explain why you are searching in the record, and if it helps make an important point, show your findings to the patient. Offer to print out results, so they have something to carry with them.
Explain what you are looking for and what you find on the physical exam
Being a patient is scary. We all want reassurance that our fears are not true. When you find normal findings on exam, share those with the patient. Hearing “your heart sounds good, your pulses are strong” really helps patients. Explaining what we are doing when we examine is also helpful. Explain why you are feeling for lymph nodes in the neck, why we percuss the abdomen. Patients are often fascinated by getting a window into how we are thinking. I usually have medical students with me, which offers another avenue to explaining the how and why behind the exam. In asking and explaining to students, the patient is also taught why we do what we do.
Make sure that we cover what they are afraid of, not just what their symptom is
Patients come in not just to get symptom relief but to rest their mind from their fears of what it could be. I find it helpful to ask the patient what they think is the cause of the problem, or if they are worried about any specific diagnosis. With certain symptoms this is particularly important (for example, headaches, fatigue, or abdominal pain).
None of these suggestions are easy to do in busy, time-pressured clinic visits. I have found though that when patients feel cared about, listened to and can have their fears addressed they value our advice more, and less time is needed to negotiate the plan, as it has been developed together.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and serves as third-year medical student clerkship director at the University of Washington. Contact Dr. Paauw at fpnews@mdedge.com.
Reference
Lown BA et al. Health Aff (Millwood). 2011 Sep;30(9):1772-8.
Almost 30 years ago a young woman made an appointment to see me. I had just started my internal medicine practice and almost all the patients who saw me were new to me. I assumed she was establishing care with me. Her first words to me were “Hello Dr. Paauw, I would like to interview you to see if you will be a good fit as my doctor.” We talked for the 40-minute appointment time. I asked her about her health, her life, and what she wanted out of both. We shared with each other that we both were parents of young children. When the appointment was over, she said she would really like for me to be her doctor. She told me that the main thing she appreciated about me was that I listened, and that her previous physician never sat down at her appointments and often had his hand on the door handle for much of the visit. Physicians and patients both agree that compassionate care is essential for good patient care, yet about half of patients and 60% of doctors believe it is lacking in our medical system.1
Remember the golden first minutes
I often start by asking the patient to give me an update on how they are doing. This lets me know what is important to them. I do not touch the computer until after this initial check-in.
Use the computer as a bond to strengthen your patient relationship
Many studies have shown patients find the computer gets between the doctor and patient. It is especially problematic if it breaks eye contact with the patient. People are less likely to share scary, sensitive, or embarrassing information if someone is looking at a computer and typing. As you look up tests, radiology reports, or consultant notes, let the patient in on what you are doing. Explain why you are searching in the record, and if it helps make an important point, show your findings to the patient. Offer to print out results, so they have something to carry with them.
Explain what you are looking for and what you find on the physical exam
Being a patient is scary. We all want reassurance that our fears are not true. When you find normal findings on exam, share those with the patient. Hearing “your heart sounds good, your pulses are strong” really helps patients. Explaining what we are doing when we examine is also helpful. Explain why you are feeling for lymph nodes in the neck, why we percuss the abdomen. Patients are often fascinated by getting a window into how we are thinking. I usually have medical students with me, which offers another avenue to explaining the how and why behind the exam. In asking and explaining to students, the patient is also taught why we do what we do.
Make sure that we cover what they are afraid of, not just what their symptom is
Patients come in not just to get symptom relief but to rest their mind from their fears of what it could be. I find it helpful to ask the patient what they think is the cause of the problem, or if they are worried about any specific diagnosis. With certain symptoms this is particularly important (for example, headaches, fatigue, or abdominal pain).
None of these suggestions are easy to do in busy, time-pressured clinic visits. I have found though that when patients feel cared about, listened to and can have their fears addressed they value our advice more, and less time is needed to negotiate the plan, as it has been developed together.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and serves as third-year medical student clerkship director at the University of Washington. Contact Dr. Paauw at fpnews@mdedge.com.
Reference
Lown BA et al. Health Aff (Millwood). 2011 Sep;30(9):1772-8.
Consider varying generational needs, preferences in the workplace and with patients
NASHVILLE, TENN. – By 2020, millennials will comprise more than a third of individuals in the workplace, and that has important implications for employment, communication, and education, according to Patrice M. Weiss, MD.
Each generation brings a unique set of experiences and expectations. Millennials – or members of “Generation Y,” who comprised 18% of the workforce in 2018 and 0% in 2008 – tend to prefer flexible work hours and communication via technology, she said during a session on navigating generational differences in the workplace during the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists.
They, along with members of “Generation X” (generally those born between 1962 and 1981) and “Generation Z” (generally those born from 1987 on), tend to be technology savvy, whereas the “Silent Generation” (generally those born between 1925 and 1942) and older members of the “Baby Boomer Generation” (generally those born between 1943 and 1961), may prefer printed communication and phone calls, said Dr. Weiss, chief medical officer at the Carilion Clinic in Roanoke, Va.
“It’s not good, it’s not bad – it’s just the way things are changing,” she said, adding that it’s important to look at the strengths that each generation brings to the workplace.
Importantly, the she said.
In this video interview, she further discusses how generational differences should be considered in medical practice, and how clinicians can adapt to the changing expectations and different needs of patients from different generations.
“Gen Ys want to communicate with us through technology. They don’t want to pick up the phone and schedule an appointment, they want to be able to go online ... through an app and self-schedule an appointment,” she said. “And they want health care when they want it.
“We as health care providers and health care organizations, we need to meet the needs of each generation ... so what we need to do is really identify what are the needs of all the generations as patients.”
During her presentation, Dr. Weiss further noted that these generational differences present a major challenge with respect to teaching, learning, and communicating.
“Rather than becoming frustrated ... let’s hope that we can ... reach across generations, identify what their strengths are, capitalize on those, and then, as health care providers, be more user and consumer friendly to the generations, particularly the millennials [so] that they have access to us and to information.”
Dr. Weiss said she had no relevant financial disclosures.
NASHVILLE, TENN. – By 2020, millennials will comprise more than a third of individuals in the workplace, and that has important implications for employment, communication, and education, according to Patrice M. Weiss, MD.
Each generation brings a unique set of experiences and expectations. Millennials – or members of “Generation Y,” who comprised 18% of the workforce in 2018 and 0% in 2008 – tend to prefer flexible work hours and communication via technology, she said during a session on navigating generational differences in the workplace during the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists.
They, along with members of “Generation X” (generally those born between 1962 and 1981) and “Generation Z” (generally those born from 1987 on), tend to be technology savvy, whereas the “Silent Generation” (generally those born between 1925 and 1942) and older members of the “Baby Boomer Generation” (generally those born between 1943 and 1961), may prefer printed communication and phone calls, said Dr. Weiss, chief medical officer at the Carilion Clinic in Roanoke, Va.
“It’s not good, it’s not bad – it’s just the way things are changing,” she said, adding that it’s important to look at the strengths that each generation brings to the workplace.
Importantly, the she said.
In this video interview, she further discusses how generational differences should be considered in medical practice, and how clinicians can adapt to the changing expectations and different needs of patients from different generations.
“Gen Ys want to communicate with us through technology. They don’t want to pick up the phone and schedule an appointment, they want to be able to go online ... through an app and self-schedule an appointment,” she said. “And they want health care when they want it.
“We as health care providers and health care organizations, we need to meet the needs of each generation ... so what we need to do is really identify what are the needs of all the generations as patients.”
During her presentation, Dr. Weiss further noted that these generational differences present a major challenge with respect to teaching, learning, and communicating.
“Rather than becoming frustrated ... let’s hope that we can ... reach across generations, identify what their strengths are, capitalize on those, and then, as health care providers, be more user and consumer friendly to the generations, particularly the millennials [so] that they have access to us and to information.”
Dr. Weiss said she had no relevant financial disclosures.
NASHVILLE, TENN. – By 2020, millennials will comprise more than a third of individuals in the workplace, and that has important implications for employment, communication, and education, according to Patrice M. Weiss, MD.
Each generation brings a unique set of experiences and expectations. Millennials – or members of “Generation Y,” who comprised 18% of the workforce in 2018 and 0% in 2008 – tend to prefer flexible work hours and communication via technology, she said during a session on navigating generational differences in the workplace during the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists.
They, along with members of “Generation X” (generally those born between 1962 and 1981) and “Generation Z” (generally those born from 1987 on), tend to be technology savvy, whereas the “Silent Generation” (generally those born between 1925 and 1942) and older members of the “Baby Boomer Generation” (generally those born between 1943 and 1961), may prefer printed communication and phone calls, said Dr. Weiss, chief medical officer at the Carilion Clinic in Roanoke, Va.
“It’s not good, it’s not bad – it’s just the way things are changing,” she said, adding that it’s important to look at the strengths that each generation brings to the workplace.
Importantly, the she said.
In this video interview, she further discusses how generational differences should be considered in medical practice, and how clinicians can adapt to the changing expectations and different needs of patients from different generations.
“Gen Ys want to communicate with us through technology. They don’t want to pick up the phone and schedule an appointment, they want to be able to go online ... through an app and self-schedule an appointment,” she said. “And they want health care when they want it.
“We as health care providers and health care organizations, we need to meet the needs of each generation ... so what we need to do is really identify what are the needs of all the generations as patients.”
During her presentation, Dr. Weiss further noted that these generational differences present a major challenge with respect to teaching, learning, and communicating.
“Rather than becoming frustrated ... let’s hope that we can ... reach across generations, identify what their strengths are, capitalize on those, and then, as health care providers, be more user and consumer friendly to the generations, particularly the millennials [so] that they have access to us and to information.”
Dr. Weiss said she had no relevant financial disclosures.
EXPERT ANALYSIS FROM ACOG 2019
It’s time to start asking all patients about intimate partner violence
Intimate partner violence (IPV) is a serious public health problem with considerable harmful health consequences. Decades of research have been dedicated to improving the identification of women in abusive heterosexual relationships and interventions that support healthier outcomes. A result of this work has been the recommendation of the US Preventive Services Task Force that all women of childbearing age be screened for IPV and provided with intervention or referral.1
The problem extends further, however: Epidemiologic studies and comprehensive reviews show: 1) a high rate of IPV victimization among heterosexual men and lesbian, gay, bisexual, and transsexual (LGBT) men and women2,3; 2) significant harmful effects on health and greater expectations of prejudice and discrimination among these populations4-6; and 3) evidence that screening and referral for IPV are likely to confer similar benefits for these populations.7 We argue that it is reasonable to ask all patients about abuse in their relationships while the research literature progresses.
We intend this article to serve a number of purposes:
- support national standards for IPV screening of female patients
- highlight the need for piloting universal IPV screening for all patients (ie, male and female, across the lifespan)
- offer recommendations for navigating the process from IPV screening to referral, using insights gained from the substance abuse literature.
We also provide supplemental materials that facilitate establishment of screening and referral protocols for physicians across practice settings.
What is intimate partner violence? How can you identify it?
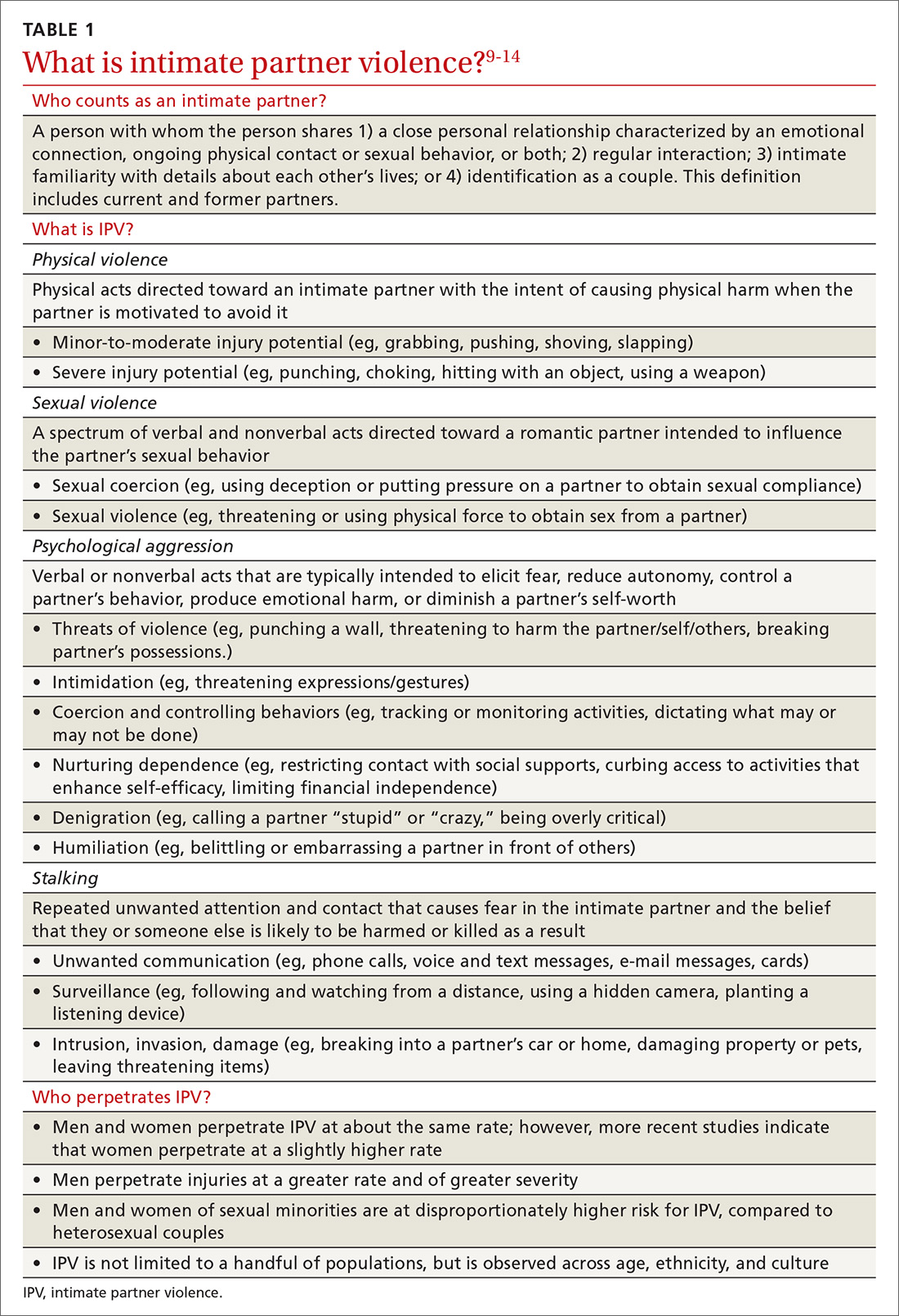
Intimate partner violence includes physical and sexual violence and nonphysical forms of abuse, such as psychological aggression and emotional abuse, perpetrated by a current or former intimate partner.8 TABLE 19-14 provides definitions for each of these behavior categories and example behaviors. Nearly 25% of women and 20% of men report having experienced physical violence from a romantic partner and even higher rates of nonphysical IPV.15 Consequences of IPV victimization include acute and chronic medical illness, injury, and psychological problems, including depression, anxiety, and poor self-esteem.16
Intimate partner violence is heterogeneous, with differences in severity (eg, frequency and intensity of violence) and laterality (ie, is one partner violent? are both partners violent?). A recent comprehensive review of the literature revealed that, for 49.2%-69.7% of partner-violent couples across diverse samples, IPV is perpetrated by both partners.17 Furthermore, this bidirectionality is not due entirely to aggression perpetrated in self-defense; rather, across diverse patient samples, that is the case for fewer than one-quarter of males and no more than approximately one-third of females.18 In the remaining cases, bidirectionality may be attributed to other motivations, such as a maladaptive emotional expression or a means by which to get a partner’s attention.18
Women are disproportionately susceptible to harmful outcomes as a result of severe violence, including physical injury, psychological distress (eg, depression and anxiety), and substance abuse.16,19 Some patients in unidirectionally violent relationships experience severe physical violence that may be, or become, life-threatening (0.4%-2.4% of couples in community samples)20—victimization that is traditionally known as “battering.”21
Continue to: These tools can facilitate screening for IPV
These tools can facilitate screening for IPV
Physicians might have reservations asking about IPV because of 1) concern whether there is sufficient time during an office visit to interview, screen, and refer, 2) feelings of powerlessness to stop violence by or toward a patient, and 3) general discomfort with the topic.22 Additionally, mandated reporting laws regarding IPV vary by state, making it crucial to know one’s own state laws on this issue to protect the safety of the patient and those around them.
Research has shown that some patients prefer that their health care providers ask about relationship violence directly23; others are more willing to acknowledge IPV if asked using a paper-and-pencil measure, rather than face-to-face questions.24 Either way, screening increases the likelihood of engaging the patient in supportive services, thus decreasing the isolation that is typical of abuse.25 Based on this research, screening that utilizes face-valid items embedded within paperwork completed in the waiting room is recommended as an important first step toward identifying and helping patients who are experiencing IPV. Even under these conditions, however, heterosexual men and sexual minorities might be less willing than heterosexual women to admit experiencing IPV.26,27
A brief vignette that depicts how quickly the screening and referral process can be applied is presented in “IPV screening and referral: A real-world vignette." The vignette is a de-identified composite of heterosexual men experiencing IPV whom we have counseled.
SIDEBAR
IPV screening and referral: A real-world vignette
Physician: Before we wrap up: I noticed on your screening that you have been hurt and threatened a fair amount in the past year. Would it be OK if we spoke about that more?
Patient: My wife is emotional. Sometimes she gets really stressed out and just starts screaming and punching me. That’s just how she is.
Physician: Do you ever feel concerned for your safety?
Patient: Not really. She’s smaller than me and I can generally calm her down. I keep the guns locked up, so she can’t grab those any more. Mostly she just screams at me.
Physician: This may or may not fit with your perception but, based on what you are reporting, your relationship is what is called “at risk”—meaning you are at risk for having your physical or mental health negatively impacted. This actually happens to a lot of men, and there’s a brochure I can give you that has a lot more information about the risks and consequences of being hurt or threatened by a partner. Would you be willing to take a look at it?
Patient: I guess so.
Physician: OK. I’ll have the nurse bring you that brochure, and we can talk more about it next time you come in for an appointment. Would it be OK if we get you back in here 6 months from now?
Patient: Yeah, that could work.
Physician: Great. Let’s do that. Don’t hesitate to give me a call if your situation changes in any way in the meantime.
One model that provides a useful framework for IPV assessment is the Screening, Brief Intervention, and Referral to Treatment (SBIRT) model, which was developed to facilitate assessment of, and referral for, substance abuse—another heavily stigmatized health care problem. The SBIRT approach for substance abuse screening is associated with significant reduction in alcohol and drug abuse 6 months postintervention, as well as improvements in well-being, mental health, and functioning across gender, race and ethnicity, and age.28
IPASSPRT. Inspired by the SBIRT model for substance abuse, we created the Intimate Partner Aggression Screening, Safety Planning, and Referral to Treatment, or IPASSPRT (spoken as “i-passport”) project to provide tools that make IPV screening and referral accessible to a range of health care providers. These tools include a script and safety plan that guide providers through screening, safety planning, and referral in a manner that is collaborative and grounded in the spirit of motivational interviewing. We have made these tools available on the Web for ease of distribution (http://bit.ly/ipassprt; open by linking through “IPASSPRT-Script”).
Continue to: The IPASSPRT script appears lengthy...
The IPASSPRT script appears lengthy, but progress through its sections is directed by patient need; most patients will not require that all parts be completed. For example, a patient whose screen for IPV is negative and who feels safe in their relationship does not need assessment beyond page 2; on the other hand, the physician might need more information from a patient who is at greater risk for IPV. This response-based progression through the script makes the screening process dynamic, data-driven, and tailored to the patient’s needs—an approach that aids rapport and optimizes the physician’s limited time during the appointment.
In the sections that follow, we describe key components of this script.
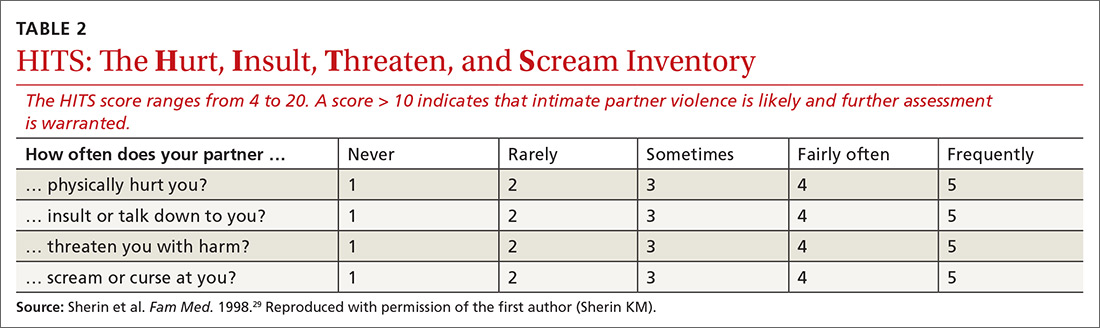
What aggression, if any, is present? From whom? The Hurt, Insult, Threaten, and Scream inventory (HITS) (TABLE 2)29 is a widely used screen for IPV that has been validated for use in family medicine. A 4-item scale asks patients to report how often their partner physically hurts, insults, threatens, and screams at them using a 5-point scale (1 point, “never,” to 5 points, “frequently”). Although a score > 10 is indicative of IPV, item-level analysis is encouraged. Attending to which items the patient acknowledges and how often these behaviors occur yields a richer assessment than a summary score. In regard to simply asking a patient, “Do you feel safe at home?” (sensitivity of this question, 8.8%; specificity, 91.2%), the HITS better detects IPV with male and female patient populations in family practice and emergency care settings (sensitivity, 30%-100%; specificity, 86%-99%).27,30
What contextual factors and related concerns are present? It is important to understand proximal factors that might influence IPV risk to determine what kind of referral or treatment is appropriate—particularly for patients experiencing or engaging in infrequent, noninjurious, and bidirectional forms of IPV. Environmental and contextual stressors, such as financial hardship, unemployment, pregnancy, and discussion of divorce, can increase the risk for IPV.31,32 Situational influences, such as alcohol and drug intoxication, can also increase the risk for IPV. Victims of partner violence are at greater risk for mental health problems, including depression, anxiety, trauma- and stressor-related disorders, and substance use disorders. Risk goes both ways, however: Mental illness predicts subsequent IPV perpetration or victimization, and vice versa.31
Does the patient feel safe? Assessing the situation. Patient perception of safety in the relationship provides important information about the necessity of referral. Asking a patient if they feel unsafe because of the behavior of a current or former partner sheds light on the need for further safety assessment and immediate connection with appropriate resources.
Continue to: The Danger Assessment-5...
The Danger Assessment-5 (DA-5) (TABLE 333) is a useful 5-item tool for quickly assessing the risk for severe IPV.33 Patients respond to whether:
- the frequency or severity of violence has increased in the past year
- the partner has ever used, or threatened to use, a weapon
- the patient believes the partner is capable of killing her (him)
- the partner has ever tried to choke or strangle her (him)
- the partner is violently and constantly jealous.
Sensitivity and specificity analyses with a high-risk female sample suggested that 3 affirmative responses indicate a high risk for severe IPV and a need for adequate safety planning.
Brief motivational enhancement intervention. There are 3 components to this intervention.
- Assess interest in making changes or seeking help. IPV is paradoxical: Many factors complicate the decision to leave or stay, and patients across the spectrum of victimization might have some motivation to stay with their partner. It is important to assess the patient’s motivation to make changes in their relationship.4,34
- Provide feedback on screening. Sharing the results of screening with patients makes the assessment and referral process collaborative and transparent; collaborative engagement helps patients feel in control and invested in the follow-through.35 In the spirit of this endeavor, physicians are encouraged to refrain from providing raw or total scores from the measures; instead, share the interpretation of those scores, based on the participant’s responses to the screening items, in a matter-of-fact manner. At this point, elicit the patient’s response to this information, listen empathically, and answer questions before proceeding.
Consistent with screening for other serious health problems, we recommend that all patients be provided with information about abuse in romantic relationships. The National Center for Injury Prevention and Control Division of Violence Prevention has published a useful, easy-to-understand fact sheet (www.cdc.gov/violenceprevention/pdf/ipv-factsheet.pdf) that provides an overview of IPV-related behavior, how it influences health outcomes, who is at risk for IPV, and sources for support.
Continue to: Our IPASSPRT interview script...
Our IPASSPRT interview script (http://bit.ly/ipassprt) outlines how this information can be presented to patients as a typical part of the screening process. Providers are encouraged to share and review the information from the fact sheet with all patients and present it as part of the normal screening process to mitigate the potential for defensiveness on the part of the patient. For patients who screen positive for IPV, it might be important to brainstorm ideas for a safe, secure place to store this fact sheet and other resources from the brief intervention and referral process below (eg, a safety plan and specific referral information) so that the patient can access them quickly and easily, if needed.
For patients who screen negative for IPV, their screen and interview conclude at this point.
- Provide recommendations based on the screen. Evidence suggests that collaborating with the patient on safety planning and referral can increase the likelihood of their engagement.7 Furthermore, failure to tailor the referral to the needs of the patient can be detrimental36—ie, overshooting the level of intervention might decrease the patient’s future treatment-seeking behavior and undermine their internal coping strategies, increasing the likelihood of future victimization. For that reason, we provide the following guidance on navigating the referral process for patients who screen positive for IPV.
Screening-based referral: A delicate and collaborative process
Referral for IPV victimization. Individual counseling, with or without an IPV focus, might be appropriate for patients at lower levels of risk; immediate connection with local IPV resources is strongly encouraged for patients at higher risk. This is a delicate, collaborative process, in which the physician offers recommendations for referral commensurate to the patient’s risk but must, ultimately, respect the patient’s autonomy by identifying referrals that fit the patient’s goals. We encourage providers to provide risk-informed recommendations and to elicit the patient’s thoughts about that information.
Several online resources are available to help physicians locate and connect with IPV-related resources in their community, including the National Health Resource Center on Domestic Violence (http://ipvhealth.org/), which provides a step-by-step guide to making such connections. We encourage physicians to develop these collaborative partnerships early to facilitate warm handoffs and increase the likelihood that a patient will follow through with the referral after screening.37
Referral for related concerns. As we’ve noted, IPV has numerous physical and mental health consequences, including depression, low self-esteem, trauma- and non-trauma-related anxiety, and substance abuse. In general, cognitive behavioral therapies appear most efficacious for treating these IPV-related consequences, but evidence is limited that such interventions diminish the likelihood of re-victimization.38 Intervention programs that foster problem-solving, solution-seeking, and cognitive restructuring for self-critical thoughts and misconceptions seem to produce the best physical and mental health outcomes.39 For patients who have a substance use disorder, treatment programs that target substance use have demonstrated a reduction in the rate of IPV recidivism.40 These findings indicate that establishing multiple treatment targets might reduce the risk for future aggression in relationships.
Continue to: The Substance Abuse and Mental Health Services Administration...
The Substance Abuse and Mental Health Services Administration of the US Department of Health and Human Services provides a useful online tool (https://findtreatment.samhsa.gov/) for locating local referrals that address behavioral health and substance-related concerns. The agency also provides a hotline (1-800-662-HELP [4357]) as an alternative resource for information and treatment referrals.
Safety planning can improve outcomes
For a patient who screens above low risk, safety planning with the patient is an important part of improving outcomes and can take several forms. Online resources, such as the Path to Safety interactive Web page (www.thehotline.org/help/path-to-safety/) maintained by The National Domestic Violence Hotline ([800]799-SAFE [7233]), provide information regarding important considerations for safety planning when:
- living with an abusive partner
- children are in the home
- the patient is pregnant
- pets are involved.
The Web site also provides information regarding legal options and resources related to IPV (eg, an order of protection) and steps for improving safety when leaving an abusive relationship. Patients at risk for IPV can explore the online tool and call the hotline.
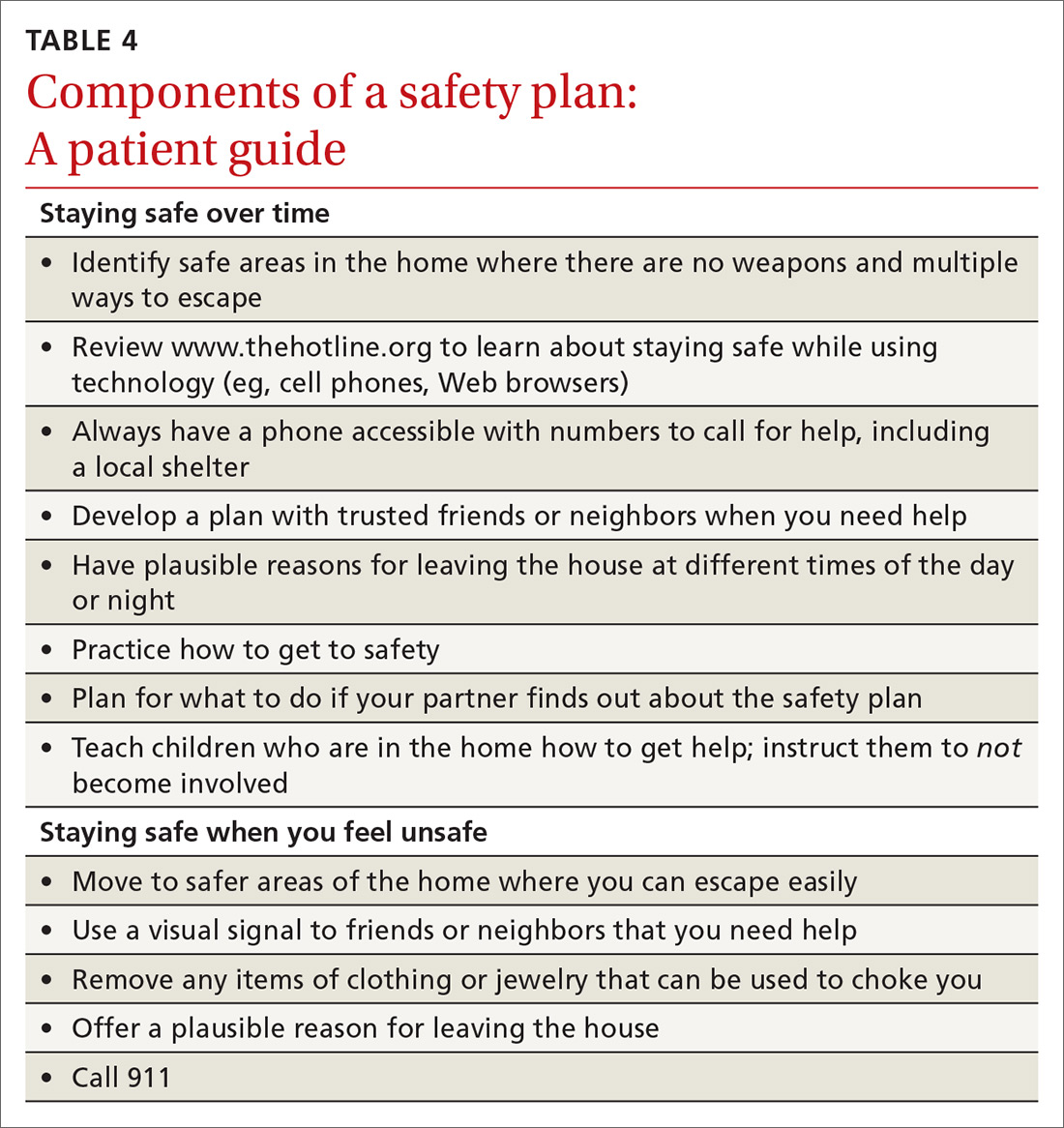
For physicians who want to engage in provider-assisted safety planning, we’ve provided further guidance in the IPASSPRT screening script and safety plan (http://bit.ly/ipassprt) (TABLE 4).
Goal: Affirm patients’ strengths and reinforce hope
Psychological aggression is the most common form of relationship aggression; repeated denigration might leave a person with little confidence in their ability to change their relationship or seek out identified resources. That’s why it’s useful to inquire—with genuine curiosity—about a time in the past when the patient accomplished something challenging. The physician’s enthusiastic reflection on this achievement can be a means of highlighting the patient’s ability to accomplish a meaningful goal; of reinforcing their hope; and of eliciting important resources within and around the patient that can facilitate action on their safety plan. (See “IPV-related resources for physicians and patients.”)
SIDEBAR
IPV-related resources for physicians and patients
Intimate Partner Aggression Screening, Safety Planning, and Referral to Treatment (IPASSPRT) Project
› http://bit.ly/ipassprt
Online resource with tools designed by the authors, including an SBIRT-inspired script and safety plan template for IPV screening, safety planning, and referral
National Center for Injury Prevention and Control Division of Violence Prevention
› www.cdc.gov/violenceprevention/pdf/ipv-factsheet.pdf
Overview of IPV-related behavior, influence on health outcomes, people at risk of IPV, and sources of support, all in a format easily understood by patients
National Health Resource Center on Domestic Violence
› http://ipvhealth.org/
Includes guidance on connecting with IPV-related community resources; establishing such connections can facilitate warm handoffs and improve the likelihood that patients will follow through
Path to Safety, a service of The National Domestic Violence Hotline
› www.thehotline.org/help/path-to-safety/
Extensive primer on safety plans for patients intending to stay in (or leave) an abusive relationship; includes important considerations for children, pets, and pregnancy, as well as emotional safety and legal options
The National Domestic Violence Hotline
› (800) 799-SAFE (7233)
Substance Abuse and Mental Health Services Administration
› www.samhsa.gov/sbirt
Learning resources for the SBIRT protocol for substance abuse
› https://findtreatment.samhsa.gov/
Search engine and resources for locating local referrals
› (800) 662-HELP (4357)
Hotline for information and assistance with locating local treatment referral
IPV, intimate partner violence; SBIRT, screening, brief intervention, and referral to treatment.
Continue to: Closing the screen and making a referral
Closing the screen and making a referral
The end of the interview should consist of a summary of topics discussed, including:
- changes that the patient wants to make (if any)
- their stated reasons for making those changes
- the patient’s plan for accomplishing changes.
Physicians should also include their own role in next steps—whether providing a warm handoff to a local IPV referral, agreeing to a follow-up schedule with the patient, or making a call as a mandated reporter. To close out the interview, it is important to affirm respect for the patient’s autonomy in executing the plan.
It’s important to screen all patients—here’s why
A major impetus for this article has been to raise awareness about the need for expanded IPV screening across primary care settings. As mentioned, much of the literature on IPV victimization has focused on women; however, the few epidemiological investigations of victimization rates among men and members of LGBT couples show a high rate of victimization and considerable harmful health outcomes. Driven by stigma surrounding IPV, sex, and sexual minority status, patients might have expectations that they will be judged by a provider or “outed.”
Such barriers can lead many to suffer in silence until the problem can no longer be hidden or the danger becomes more emergent. Compassionate, nonjudgmental screening and collaborative safety planning—such as the approach we describe in this article—help ease the concerns of LGBT victims of IPV and improve the likelihood that conversations you have with them will occur earlier, rather than later, in care.*
Underassessment of IPV (ie, underreporting as well as under-inquiry) because of stigma, misconception, and other factors obscures an accurate estimate of the rate of partner violence and its consequences for all couples. As a consequence, we know little about the dynamics of IPV, best practices for screening, and appropriate referral for couples from these populations. Furthermore, few resources are available to these understudied and underserved groups (eg, shelters for men and for transgender people).
Continue to: Although our immediate approach to IPV screening...
Although our immediate approach to IPV screening, safety planning, and referral with understudied patient populations might be informed by what we have learned from the experiences of heterosexual women in abusive relationships, such a practice is unsustainable. Unless we expand our scope of screening to all patients, it is unlikely that we will develop the evidence base necessary to 1) warrant stronger IPV screening recommendations for patient groups apart from women of childbearing age, let alone 2) demonstrate the need for additional community resources, and 3) provide comprehensive care in family practice of comparable quality.
The benefits of screening go beyond the individual patient
Screening for violence in the relationship does not take long; the value of asking about its presence in a relationship might offer benefits beyond the individual patient by raising awareness and providing the field of study with more data to increase attention and resources for under-researched and underserved populations. Screening might also combat the stigma that perpetuates the silence of many who deserve access to care.
CORRESPONDENCE
Joel G. Sprunger, PhD, Department of Psychiatry and Behavioral Neuroscience, University of Cincinnati College of Medicine, 260 Stetson St, Suite 3200, Cincinnati OH 45219; joel.sprunger@UC.edu.
ACKNOWLEDGMENTS
The authors thank Jeffrey M. Girard, PhD, and Daniel C. Williams, PhD, for their input on the design and content, respectively, of the IPASSPRT screening materials; the authors of the DA-5 and the HITS screening tools, particularly Jacquelyn Campbell, PhD, RN, FAAN, and Kevin Sherin, MD, MPH, MBA, respectively, for permission to include these measures in this article and for their support of its goals; and The Journal of Family Practice’s peer reviewers for their thoughtful feedback throughout the prepublication process.
1. Campos-Outcalt D. USPSTF: What’s recommended, what’s not. J Fam Pract. 2014;63:265-269.
2. Black MC, Basile KC, Breiding MJ, et al. National Intimate Partner and Sexual Violence Survey: 2010 Summary Report. Atlanta, GA: National Center for Injury Prevention and Control, Centers for Disease Control and Prevention; 2011:113. www.cdc.gov/violenceprevention/pdf/NISVS_Report2010-a.pdf. Accessed February 20, 2019.
3. West CM. Partner abuse in ethnic minority and gay, lesbian, bisexual, and transgender populations. Partner Abuse. 2012;3:336-357.
4. Hines DA, Malley-Morrison K. Psychological effects of partner abuse against men: a neglected research area. Psychology of Men & Masculinities. 2001;2:75-85.
5. Houston E, McKirnan DJ. Intimate partner abuse among gay and bisexual men: risk correlates and health outcomes. J Urban Health. 2007;84:681-690.
6. Carvalho AF, Lewis RJ, Derlega VJ, et al. Internalized sexual minority stressors and same-sex intimate partner violence. J Fam Violence. 2011;26:501-509.
7. Nicholls TL, Pritchard MM, Reeves KA, et al. Risk assessment in intimate partner violence: a systematic review of contemporary approaches. Partner Abuse. 2013;4:76-168.
8. Intimate partner violence: definitions. Atlanta, GA: National Center for Injury Prevention and Control, Division of Violence Prevention, Centers for Disease Control and Prevention, August 22, 2017. www.cdc.gov/violenceprevention/intimatepartnerviolence/definitions.html. Accessed February 20, 2019.
9. Archer J. Sex differences in aggression between heterosexual partners: a meta-analytic review. Psychol Bull. 2000;126:651-680.
10. Baron RA, Richardson DR. Human Aggression. New York, NY: Springer Science+Business Media; 2004.
11. Breiding MJ, Basile KC, Smith SG, et al. Intimate Partner Violence Surveillance: Uniform Definitions and Recommended Data Elements, Version 2.0. Atlanta, GA: National Center for Injury Prevention and Control, Centers for Disease Control and Prevention; 2015.
12. Murphy CM, Eckhardt CI. Treating the Abusive Partner: An Individualized Cognitive-Behavioral Approach. New York, NY: Guilford Press; 2005.
13. Straus MA, Hamby SL, Boney-McCoy S, et al. The revised Conflict Tactics Scales (CTS2): development and preliminary psychometric data. J Fam Issues. 1996;17:283-316.
14. West CM. Partner abuse in ethnic minority and gay, lesbian, bisexual, and transgender populations. Partner Abuse. 2012;3:336-357.
15. Desmarais SL, Reeves KA, Nicholls TL, et al. Prevalence of physical violence in intimate relationships. Part 1: rates of male and female victimization. Partner Abuse. 2012;3:140-169.
16. Lawrence E, Orengo-Aguayo R, Langer A, et al. The impact and consequences of partner abuse on partners. Partner Abuse. 2012;3:406-428.
17. Langhinrichsen-Rohling J, Selwyn C, Rohling ML. Rates of bidirectional versus unidirectional intimate partner violence across samples, sexual orientations, and race/ethnicities: a comprehensive review. Partner Abuse. 2012;3:199-230.
18. Langhinrichsen-Rohling J, McCullars A, Misra TA. Motivations for men and women’s intimate partner violence perpetration: a comprehensive review. Partner Abuse. 2012;3:429-468.
19. Anderson CA, Bushman BJ. Human aggression. Annu Rev Psychol. 2002;53:27-51.
20. Straus MA, Gozjolko KL. “Intimate terrorism” and gender differences in injury of dating partners by male and female university students. J Fam Violence. 2014;29:51-65.
21. Ferraro KJ, Johnson JM. How women experience battering: the process of victimization. Soc Probl. 1983;30:325-339.
22. Sugg NK, Inui T. Primary care physicians’ response to domestic violence: opening Pandora’s box. JAMA. 1992;267:3157-3160.
23. Morgan KJ, Williamson E, Hester M, et al. Asking men about domestic violence and abuse in a family medicine context: help seeking and views on the general practitioner role. Aggress Violent Behav. 2014;19:637-642.
24. MacMillan HL, Wathen CN, Jamieson E, et al; McMaster Violence Against Women Research Group. Approaches to screening for intimate partner violence in health care settings: a randomized trial. JAMA. 2006;296:530-536.
25. Thompson RS, Rivara FP, Thompson DC, et al. Identification and management of domestic violence: a randomized trial. Am J Prev Med. 2000;19:253-263.
26. Ard KL, Makadon HJ. Addressing intimate partner violence in lesbian, gay, bisexual, and transgender patients. J Gen Intern Med. 2011;26:930-933.
27. Rabin RF, Jennings JM, Campbell JC, et al. Intimate partner violence screening tools: a systematic review. Am J Prev Med. 2009;36:439-445.e4.
28. Madras BK, Compton WM, Avula D, et al. Screening, brief interventions, referral to treatment (SBIRT) for illicit drug and alcohol use at multiple healthcare sites: comparison at intake and 6 months later. Drug Alcohol Depend. 2009;99:280-295.
29. Sherin KM, Sinacore JM, Li XQ, et al. HITS: A short domestic violence screening tool for use in a family practice setting. Fam Med. 1998;30:508-512.
30. Peralta RL, Fleming MF. Screening for intimate partner violence in a primary care setting: the validity of “feeling safe at home” and prevalence results. J Am Board Fam Pract. 2003;16:525-532.
31. Capaldi DM, Knoble NB, Shortt JW, et al. A systematic review of risk factors for intimate partner violence. Partner Abuse. 2012;3:231-280.
32. Brownridge DA, Taillieu TL, Tyler KA, et al. Pregnancy and intimate partner violence: risk factors, severity, and health effects. Violence Against Women. 2011;17:858-881.
33. Messing JT, Campbell JC, Snider C. Validation and adaptation of the danger assessment-5: a brief intimate partner violence risk assessment. J Adv Nurs. 2017;73:3220-3230.
34. Grigsby N, Hartman BR. The Barriers Model: an integrated strategy for intervention with battered women. Psychotherapy: Theory, Research, Practice, Training. 1997;34:485-497.
35. Moyers TB, Rollnick S. A motivational interviewing perspective on resistance in psychotherapy. J Clin Psychol. 2002;58:185-193.
36. Belfrage H, Strand S, Storey JE, et al. Assessment and management of risk for intimate partner violence by police officers using the Spousal Assault Risk Assessment Guide. Law Hum Behav. 2012;36:60-67.
37. McCloskey LA, Lichter E, Williams C, et al. Assessing intimate partner violence in health care settings leads to women’s receipt of interventions and improved health. Publ Health Rep. 2006;121:435-444.
38. Eckhardt CI, Murphy CM, Whitaker DJ, et al. The effectiveness of intervention programs for perpetrators and victims of intimate partner violence. Partner Abuse. 2013;4:196-231.
39. Trabold N, McMahon J, Alsobrooks S, et al. A systematic review of intimate partner violence interventions: state of the field and implications for practitioners. Trauma Violence Abuse. January 2018:1524838018767934.
40. Kraanen FL, Vedel E, Scholing A, et al. The comparative effectiveness of Integrated treatment for Substance abuse and Partner violence (I-StoP) and substance abuse treatment alone: a randomized controlled trial. BMC Psychiatry. 2013;13:189.
Intimate partner violence (IPV) is a serious public health problem with considerable harmful health consequences. Decades of research have been dedicated to improving the identification of women in abusive heterosexual relationships and interventions that support healthier outcomes. A result of this work has been the recommendation of the US Preventive Services Task Force that all women of childbearing age be screened for IPV and provided with intervention or referral.1
The problem extends further, however: Epidemiologic studies and comprehensive reviews show: 1) a high rate of IPV victimization among heterosexual men and lesbian, gay, bisexual, and transsexual (LGBT) men and women2,3; 2) significant harmful effects on health and greater expectations of prejudice and discrimination among these populations4-6; and 3) evidence that screening and referral for IPV are likely to confer similar benefits for these populations.7 We argue that it is reasonable to ask all patients about abuse in their relationships while the research literature progresses.
We intend this article to serve a number of purposes:
- support national standards for IPV screening of female patients
- highlight the need for piloting universal IPV screening for all patients (ie, male and female, across the lifespan)
- offer recommendations for navigating the process from IPV screening to referral, using insights gained from the substance abuse literature.
We also provide supplemental materials that facilitate establishment of screening and referral protocols for physicians across practice settings.
What is intimate partner violence? How can you identify it?
Intimate partner violence includes physical and sexual violence and nonphysical forms of abuse, such as psychological aggression and emotional abuse, perpetrated by a current or former intimate partner.8 TABLE 19-14 provides definitions for each of these behavior categories and example behaviors. Nearly 25% of women and 20% of men report having experienced physical violence from a romantic partner and even higher rates of nonphysical IPV.15 Consequences of IPV victimization include acute and chronic medical illness, injury, and psychological problems, including depression, anxiety, and poor self-esteem.16
Intimate partner violence is heterogeneous, with differences in severity (eg, frequency and intensity of violence) and laterality (ie, is one partner violent? are both partners violent?). A recent comprehensive review of the literature revealed that, for 49.2%-69.7% of partner-violent couples across diverse samples, IPV is perpetrated by both partners.17 Furthermore, this bidirectionality is not due entirely to aggression perpetrated in self-defense; rather, across diverse patient samples, that is the case for fewer than one-quarter of males and no more than approximately one-third of females.18 In the remaining cases, bidirectionality may be attributed to other motivations, such as a maladaptive emotional expression or a means by which to get a partner’s attention.18
Women are disproportionately susceptible to harmful outcomes as a result of severe violence, including physical injury, psychological distress (eg, depression and anxiety), and substance abuse.16,19 Some patients in unidirectionally violent relationships experience severe physical violence that may be, or become, life-threatening (0.4%-2.4% of couples in community samples)20—victimization that is traditionally known as “battering.”21
Continue to: These tools can facilitate screening for IPV
These tools can facilitate screening for IPV
Physicians might have reservations asking about IPV because of 1) concern whether there is sufficient time during an office visit to interview, screen, and refer, 2) feelings of powerlessness to stop violence by or toward a patient, and 3) general discomfort with the topic.22 Additionally, mandated reporting laws regarding IPV vary by state, making it crucial to know one’s own state laws on this issue to protect the safety of the patient and those around them.
Research has shown that some patients prefer that their health care providers ask about relationship violence directly23; others are more willing to acknowledge IPV if asked using a paper-and-pencil measure, rather than face-to-face questions.24 Either way, screening increases the likelihood of engaging the patient in supportive services, thus decreasing the isolation that is typical of abuse.25 Based on this research, screening that utilizes face-valid items embedded within paperwork completed in the waiting room is recommended as an important first step toward identifying and helping patients who are experiencing IPV. Even under these conditions, however, heterosexual men and sexual minorities might be less willing than heterosexual women to admit experiencing IPV.26,27
A brief vignette that depicts how quickly the screening and referral process can be applied is presented in “IPV screening and referral: A real-world vignette." The vignette is a de-identified composite of heterosexual men experiencing IPV whom we have counseled.
SIDEBAR
IPV screening and referral: A real-world vignette
Physician: Before we wrap up: I noticed on your screening that you have been hurt and threatened a fair amount in the past year. Would it be OK if we spoke about that more?
Patient: My wife is emotional. Sometimes she gets really stressed out and just starts screaming and punching me. That’s just how she is.
Physician: Do you ever feel concerned for your safety?
Patient: Not really. She’s smaller than me and I can generally calm her down. I keep the guns locked up, so she can’t grab those any more. Mostly she just screams at me.
Physician: This may or may not fit with your perception but, based on what you are reporting, your relationship is what is called “at risk”—meaning you are at risk for having your physical or mental health negatively impacted. This actually happens to a lot of men, and there’s a brochure I can give you that has a lot more information about the risks and consequences of being hurt or threatened by a partner. Would you be willing to take a look at it?
Patient: I guess so.
Physician: OK. I’ll have the nurse bring you that brochure, and we can talk more about it next time you come in for an appointment. Would it be OK if we get you back in here 6 months from now?
Patient: Yeah, that could work.
Physician: Great. Let’s do that. Don’t hesitate to give me a call if your situation changes in any way in the meantime.
One model that provides a useful framework for IPV assessment is the Screening, Brief Intervention, and Referral to Treatment (SBIRT) model, which was developed to facilitate assessment of, and referral for, substance abuse—another heavily stigmatized health care problem. The SBIRT approach for substance abuse screening is associated with significant reduction in alcohol and drug abuse 6 months postintervention, as well as improvements in well-being, mental health, and functioning across gender, race and ethnicity, and age.28
IPASSPRT. Inspired by the SBIRT model for substance abuse, we created the Intimate Partner Aggression Screening, Safety Planning, and Referral to Treatment, or IPASSPRT (spoken as “i-passport”) project to provide tools that make IPV screening and referral accessible to a range of health care providers. These tools include a script and safety plan that guide providers through screening, safety planning, and referral in a manner that is collaborative and grounded in the spirit of motivational interviewing. We have made these tools available on the Web for ease of distribution (http://bit.ly/ipassprt; open by linking through “IPASSPRT-Script”).
Continue to: The IPASSPRT script appears lengthy...
The IPASSPRT script appears lengthy, but progress through its sections is directed by patient need; most patients will not require that all parts be completed. For example, a patient whose screen for IPV is negative and who feels safe in their relationship does not need assessment beyond page 2; on the other hand, the physician might need more information from a patient who is at greater risk for IPV. This response-based progression through the script makes the screening process dynamic, data-driven, and tailored to the patient’s needs—an approach that aids rapport and optimizes the physician’s limited time during the appointment.
In the sections that follow, we describe key components of this script.
What aggression, if any, is present? From whom? The Hurt, Insult, Threaten, and Scream inventory (HITS) (TABLE 2)29 is a widely used screen for IPV that has been validated for use in family medicine. A 4-item scale asks patients to report how often their partner physically hurts, insults, threatens, and screams at them using a 5-point scale (1 point, “never,” to 5 points, “frequently”). Although a score > 10 is indicative of IPV, item-level analysis is encouraged. Attending to which items the patient acknowledges and how often these behaviors occur yields a richer assessment than a summary score. In regard to simply asking a patient, “Do you feel safe at home?” (sensitivity of this question, 8.8%; specificity, 91.2%), the HITS better detects IPV with male and female patient populations in family practice and emergency care settings (sensitivity, 30%-100%; specificity, 86%-99%).27,30
What contextual factors and related concerns are present? It is important to understand proximal factors that might influence IPV risk to determine what kind of referral or treatment is appropriate—particularly for patients experiencing or engaging in infrequent, noninjurious, and bidirectional forms of IPV. Environmental and contextual stressors, such as financial hardship, unemployment, pregnancy, and discussion of divorce, can increase the risk for IPV.31,32 Situational influences, such as alcohol and drug intoxication, can also increase the risk for IPV. Victims of partner violence are at greater risk for mental health problems, including depression, anxiety, trauma- and stressor-related disorders, and substance use disorders. Risk goes both ways, however: Mental illness predicts subsequent IPV perpetration or victimization, and vice versa.31
Does the patient feel safe? Assessing the situation. Patient perception of safety in the relationship provides important information about the necessity of referral. Asking a patient if they feel unsafe because of the behavior of a current or former partner sheds light on the need for further safety assessment and immediate connection with appropriate resources.
Continue to: The Danger Assessment-5...
The Danger Assessment-5 (DA-5) (TABLE 333) is a useful 5-item tool for quickly assessing the risk for severe IPV.33 Patients respond to whether:
- the frequency or severity of violence has increased in the past year
- the partner has ever used, or threatened to use, a weapon
- the patient believes the partner is capable of killing her (him)
- the partner has ever tried to choke or strangle her (him)
- the partner is violently and constantly jealous.
Sensitivity and specificity analyses with a high-risk female sample suggested that 3 affirmative responses indicate a high risk for severe IPV and a need for adequate safety planning.
Brief motivational enhancement intervention. There are 3 components to this intervention.
- Assess interest in making changes or seeking help. IPV is paradoxical: Many factors complicate the decision to leave or stay, and patients across the spectrum of victimization might have some motivation to stay with their partner. It is important to assess the patient’s motivation to make changes in their relationship.4,34
- Provide feedback on screening. Sharing the results of screening with patients makes the assessment and referral process collaborative and transparent; collaborative engagement helps patients feel in control and invested in the follow-through.35 In the spirit of this endeavor, physicians are encouraged to refrain from providing raw or total scores from the measures; instead, share the interpretation of those scores, based on the participant’s responses to the screening items, in a matter-of-fact manner. At this point, elicit the patient’s response to this information, listen empathically, and answer questions before proceeding.
Consistent with screening for other serious health problems, we recommend that all patients be provided with information about abuse in romantic relationships. The National Center for Injury Prevention and Control Division of Violence Prevention has published a useful, easy-to-understand fact sheet (www.cdc.gov/violenceprevention/pdf/ipv-factsheet.pdf) that provides an overview of IPV-related behavior, how it influences health outcomes, who is at risk for IPV, and sources for support.
Continue to: Our IPASSPRT interview script...
Our IPASSPRT interview script (http://bit.ly/ipassprt) outlines how this information can be presented to patients as a typical part of the screening process. Providers are encouraged to share and review the information from the fact sheet with all patients and present it as part of the normal screening process to mitigate the potential for defensiveness on the part of the patient. For patients who screen positive for IPV, it might be important to brainstorm ideas for a safe, secure place to store this fact sheet and other resources from the brief intervention and referral process below (eg, a safety plan and specific referral information) so that the patient can access them quickly and easily, if needed.
For patients who screen negative for IPV, their screen and interview conclude at this point.
- Provide recommendations based on the screen. Evidence suggests that collaborating with the patient on safety planning and referral can increase the likelihood of their engagement.7 Furthermore, failure to tailor the referral to the needs of the patient can be detrimental36—ie, overshooting the level of intervention might decrease the patient’s future treatment-seeking behavior and undermine their internal coping strategies, increasing the likelihood of future victimization. For that reason, we provide the following guidance on navigating the referral process for patients who screen positive for IPV.
Screening-based referral: A delicate and collaborative process
Referral for IPV victimization. Individual counseling, with or without an IPV focus, might be appropriate for patients at lower levels of risk; immediate connection with local IPV resources is strongly encouraged for patients at higher risk. This is a delicate, collaborative process, in which the physician offers recommendations for referral commensurate to the patient’s risk but must, ultimately, respect the patient’s autonomy by identifying referrals that fit the patient’s goals. We encourage providers to provide risk-informed recommendations and to elicit the patient’s thoughts about that information.
Several online resources are available to help physicians locate and connect with IPV-related resources in their community, including the National Health Resource Center on Domestic Violence (http://ipvhealth.org/), which provides a step-by-step guide to making such connections. We encourage physicians to develop these collaborative partnerships early to facilitate warm handoffs and increase the likelihood that a patient will follow through with the referral after screening.37
Referral for related concerns. As we’ve noted, IPV has numerous physical and mental health consequences, including depression, low self-esteem, trauma- and non-trauma-related anxiety, and substance abuse. In general, cognitive behavioral therapies appear most efficacious for treating these IPV-related consequences, but evidence is limited that such interventions diminish the likelihood of re-victimization.38 Intervention programs that foster problem-solving, solution-seeking, and cognitive restructuring for self-critical thoughts and misconceptions seem to produce the best physical and mental health outcomes.39 For patients who have a substance use disorder, treatment programs that target substance use have demonstrated a reduction in the rate of IPV recidivism.40 These findings indicate that establishing multiple treatment targets might reduce the risk for future aggression in relationships.
Continue to: The Substance Abuse and Mental Health Services Administration...
The Substance Abuse and Mental Health Services Administration of the US Department of Health and Human Services provides a useful online tool (https://findtreatment.samhsa.gov/) for locating local referrals that address behavioral health and substance-related concerns. The agency also provides a hotline (1-800-662-HELP [4357]) as an alternative resource for information and treatment referrals.
Safety planning can improve outcomes
For a patient who screens above low risk, safety planning with the patient is an important part of improving outcomes and can take several forms. Online resources, such as the Path to Safety interactive Web page (www.thehotline.org/help/path-to-safety/) maintained by The National Domestic Violence Hotline ([800]799-SAFE [7233]), provide information regarding important considerations for safety planning when:
- living with an abusive partner
- children are in the home
- the patient is pregnant
- pets are involved.
The Web site also provides information regarding legal options and resources related to IPV (eg, an order of protection) and steps for improving safety when leaving an abusive relationship. Patients at risk for IPV can explore the online tool and call the hotline.
For physicians who want to engage in provider-assisted safety planning, we’ve provided further guidance in the IPASSPRT screening script and safety plan (http://bit.ly/ipassprt) (TABLE 4).
Goal: Affirm patients’ strengths and reinforce hope
Psychological aggression is the most common form of relationship aggression; repeated denigration might leave a person with little confidence in their ability to change their relationship or seek out identified resources. That’s why it’s useful to inquire—with genuine curiosity—about a time in the past when the patient accomplished something challenging. The physician’s enthusiastic reflection on this achievement can be a means of highlighting the patient’s ability to accomplish a meaningful goal; of reinforcing their hope; and of eliciting important resources within and around the patient that can facilitate action on their safety plan. (See “IPV-related resources for physicians and patients.”)
SIDEBAR
IPV-related resources for physicians and patients
Intimate Partner Aggression Screening, Safety Planning, and Referral to Treatment (IPASSPRT) Project
› http://bit.ly/ipassprt
Online resource with tools designed by the authors, including an SBIRT-inspired script and safety plan template for IPV screening, safety planning, and referral
National Center for Injury Prevention and Control Division of Violence Prevention
› www.cdc.gov/violenceprevention/pdf/ipv-factsheet.pdf
Overview of IPV-related behavior, influence on health outcomes, people at risk of IPV, and sources of support, all in a format easily understood by patients
National Health Resource Center on Domestic Violence
› http://ipvhealth.org/
Includes guidance on connecting with IPV-related community resources; establishing such connections can facilitate warm handoffs and improve the likelihood that patients will follow through
Path to Safety, a service of The National Domestic Violence Hotline
› www.thehotline.org/help/path-to-safety/
Extensive primer on safety plans for patients intending to stay in (or leave) an abusive relationship; includes important considerations for children, pets, and pregnancy, as well as emotional safety and legal options
The National Domestic Violence Hotline
› (800) 799-SAFE (7233)
Substance Abuse and Mental Health Services Administration
› www.samhsa.gov/sbirt
Learning resources for the SBIRT protocol for substance abuse
› https://findtreatment.samhsa.gov/
Search engine and resources for locating local referrals
› (800) 662-HELP (4357)
Hotline for information and assistance with locating local treatment referral
IPV, intimate partner violence; SBIRT, screening, brief intervention, and referral to treatment.
Continue to: Closing the screen and making a referral
Closing the screen and making a referral
The end of the interview should consist of a summary of topics discussed, including:
- changes that the patient wants to make (if any)
- their stated reasons for making those changes
- the patient’s plan for accomplishing changes.
Physicians should also include their own role in next steps—whether providing a warm handoff to a local IPV referral, agreeing to a follow-up schedule with the patient, or making a call as a mandated reporter. To close out the interview, it is important to affirm respect for the patient’s autonomy in executing the plan.
It’s important to screen all patients—here’s why
A major impetus for this article has been to raise awareness about the need for expanded IPV screening across primary care settings. As mentioned, much of the literature on IPV victimization has focused on women; however, the few epidemiological investigations of victimization rates among men and members of LGBT couples show a high rate of victimization and considerable harmful health outcomes. Driven by stigma surrounding IPV, sex, and sexual minority status, patients might have expectations that they will be judged by a provider or “outed.”
Such barriers can lead many to suffer in silence until the problem can no longer be hidden or the danger becomes more emergent. Compassionate, nonjudgmental screening and collaborative safety planning—such as the approach we describe in this article—help ease the concerns of LGBT victims of IPV and improve the likelihood that conversations you have with them will occur earlier, rather than later, in care.*
Underassessment of IPV (ie, underreporting as well as under-inquiry) because of stigma, misconception, and other factors obscures an accurate estimate of the rate of partner violence and its consequences for all couples. As a consequence, we know little about the dynamics of IPV, best practices for screening, and appropriate referral for couples from these populations. Furthermore, few resources are available to these understudied and underserved groups (eg, shelters for men and for transgender people).
Continue to: Although our immediate approach to IPV screening...
Although our immediate approach to IPV screening, safety planning, and referral with understudied patient populations might be informed by what we have learned from the experiences of heterosexual women in abusive relationships, such a practice is unsustainable. Unless we expand our scope of screening to all patients, it is unlikely that we will develop the evidence base necessary to 1) warrant stronger IPV screening recommendations for patient groups apart from women of childbearing age, let alone 2) demonstrate the need for additional community resources, and 3) provide comprehensive care in family practice of comparable quality.
The benefits of screening go beyond the individual patient
Screening for violence in the relationship does not take long; the value of asking about its presence in a relationship might offer benefits beyond the individual patient by raising awareness and providing the field of study with more data to increase attention and resources for under-researched and underserved populations. Screening might also combat the stigma that perpetuates the silence of many who deserve access to care.
CORRESPONDENCE
Joel G. Sprunger, PhD, Department of Psychiatry and Behavioral Neuroscience, University of Cincinnati College of Medicine, 260 Stetson St, Suite 3200, Cincinnati OH 45219; joel.sprunger@UC.edu.
ACKNOWLEDGMENTS
The authors thank Jeffrey M. Girard, PhD, and Daniel C. Williams, PhD, for their input on the design and content, respectively, of the IPASSPRT screening materials; the authors of the DA-5 and the HITS screening tools, particularly Jacquelyn Campbell, PhD, RN, FAAN, and Kevin Sherin, MD, MPH, MBA, respectively, for permission to include these measures in this article and for their support of its goals; and The Journal of Family Practice’s peer reviewers for their thoughtful feedback throughout the prepublication process.
Intimate partner violence (IPV) is a serious public health problem with considerable harmful health consequences. Decades of research have been dedicated to improving the identification of women in abusive heterosexual relationships and interventions that support healthier outcomes. A result of this work has been the recommendation of the US Preventive Services Task Force that all women of childbearing age be screened for IPV and provided with intervention or referral.1
The problem extends further, however: Epidemiologic studies and comprehensive reviews show: 1) a high rate of IPV victimization among heterosexual men and lesbian, gay, bisexual, and transsexual (LGBT) men and women2,3; 2) significant harmful effects on health and greater expectations of prejudice and discrimination among these populations4-6; and 3) evidence that screening and referral for IPV are likely to confer similar benefits for these populations.7 We argue that it is reasonable to ask all patients about abuse in their relationships while the research literature progresses.
We intend this article to serve a number of purposes:
- support national standards for IPV screening of female patients
- highlight the need for piloting universal IPV screening for all patients (ie, male and female, across the lifespan)
- offer recommendations for navigating the process from IPV screening to referral, using insights gained from the substance abuse literature.
We also provide supplemental materials that facilitate establishment of screening and referral protocols for physicians across practice settings.
What is intimate partner violence? How can you identify it?
Intimate partner violence includes physical and sexual violence and nonphysical forms of abuse, such as psychological aggression and emotional abuse, perpetrated by a current or former intimate partner.8 TABLE 19-14 provides definitions for each of these behavior categories and example behaviors. Nearly 25% of women and 20% of men report having experienced physical violence from a romantic partner and even higher rates of nonphysical IPV.15 Consequences of IPV victimization include acute and chronic medical illness, injury, and psychological problems, including depression, anxiety, and poor self-esteem.16
Intimate partner violence is heterogeneous, with differences in severity (eg, frequency and intensity of violence) and laterality (ie, is one partner violent? are both partners violent?). A recent comprehensive review of the literature revealed that, for 49.2%-69.7% of partner-violent couples across diverse samples, IPV is perpetrated by both partners.17 Furthermore, this bidirectionality is not due entirely to aggression perpetrated in self-defense; rather, across diverse patient samples, that is the case for fewer than one-quarter of males and no more than approximately one-third of females.18 In the remaining cases, bidirectionality may be attributed to other motivations, such as a maladaptive emotional expression or a means by which to get a partner’s attention.18
Women are disproportionately susceptible to harmful outcomes as a result of severe violence, including physical injury, psychological distress (eg, depression and anxiety), and substance abuse.16,19 Some patients in unidirectionally violent relationships experience severe physical violence that may be, or become, life-threatening (0.4%-2.4% of couples in community samples)20—victimization that is traditionally known as “battering.”21
Continue to: These tools can facilitate screening for IPV
These tools can facilitate screening for IPV
Physicians might have reservations asking about IPV because of 1) concern whether there is sufficient time during an office visit to interview, screen, and refer, 2) feelings of powerlessness to stop violence by or toward a patient, and 3) general discomfort with the topic.22 Additionally, mandated reporting laws regarding IPV vary by state, making it crucial to know one’s own state laws on this issue to protect the safety of the patient and those around them.
Research has shown that some patients prefer that their health care providers ask about relationship violence directly23; others are more willing to acknowledge IPV if asked using a paper-and-pencil measure, rather than face-to-face questions.24 Either way, screening increases the likelihood of engaging the patient in supportive services, thus decreasing the isolation that is typical of abuse.25 Based on this research, screening that utilizes face-valid items embedded within paperwork completed in the waiting room is recommended as an important first step toward identifying and helping patients who are experiencing IPV. Even under these conditions, however, heterosexual men and sexual minorities might be less willing than heterosexual women to admit experiencing IPV.26,27
A brief vignette that depicts how quickly the screening and referral process can be applied is presented in “IPV screening and referral: A real-world vignette." The vignette is a de-identified composite of heterosexual men experiencing IPV whom we have counseled.
SIDEBAR
IPV screening and referral: A real-world vignette
Physician: Before we wrap up: I noticed on your screening that you have been hurt and threatened a fair amount in the past year. Would it be OK if we spoke about that more?
Patient: My wife is emotional. Sometimes she gets really stressed out and just starts screaming and punching me. That’s just how she is.
Physician: Do you ever feel concerned for your safety?
Patient: Not really. She’s smaller than me and I can generally calm her down. I keep the guns locked up, so she can’t grab those any more. Mostly she just screams at me.
Physician: This may or may not fit with your perception but, based on what you are reporting, your relationship is what is called “at risk”—meaning you are at risk for having your physical or mental health negatively impacted. This actually happens to a lot of men, and there’s a brochure I can give you that has a lot more information about the risks and consequences of being hurt or threatened by a partner. Would you be willing to take a look at it?
Patient: I guess so.
Physician: OK. I’ll have the nurse bring you that brochure, and we can talk more about it next time you come in for an appointment. Would it be OK if we get you back in here 6 months from now?
Patient: Yeah, that could work.
Physician: Great. Let’s do that. Don’t hesitate to give me a call if your situation changes in any way in the meantime.
One model that provides a useful framework for IPV assessment is the Screening, Brief Intervention, and Referral to Treatment (SBIRT) model, which was developed to facilitate assessment of, and referral for, substance abuse—another heavily stigmatized health care problem. The SBIRT approach for substance abuse screening is associated with significant reduction in alcohol and drug abuse 6 months postintervention, as well as improvements in well-being, mental health, and functioning across gender, race and ethnicity, and age.28
IPASSPRT. Inspired by the SBIRT model for substance abuse, we created the Intimate Partner Aggression Screening, Safety Planning, and Referral to Treatment, or IPASSPRT (spoken as “i-passport”) project to provide tools that make IPV screening and referral accessible to a range of health care providers. These tools include a script and safety plan that guide providers through screening, safety planning, and referral in a manner that is collaborative and grounded in the spirit of motivational interviewing. We have made these tools available on the Web for ease of distribution (http://bit.ly/ipassprt; open by linking through “IPASSPRT-Script”).
Continue to: The IPASSPRT script appears lengthy...
The IPASSPRT script appears lengthy, but progress through its sections is directed by patient need; most patients will not require that all parts be completed. For example, a patient whose screen for IPV is negative and who feels safe in their relationship does not need assessment beyond page 2; on the other hand, the physician might need more information from a patient who is at greater risk for IPV. This response-based progression through the script makes the screening process dynamic, data-driven, and tailored to the patient’s needs—an approach that aids rapport and optimizes the physician’s limited time during the appointment.
In the sections that follow, we describe key components of this script.
What aggression, if any, is present? From whom? The Hurt, Insult, Threaten, and Scream inventory (HITS) (TABLE 2)29 is a widely used screen for IPV that has been validated for use in family medicine. A 4-item scale asks patients to report how often their partner physically hurts, insults, threatens, and screams at them using a 5-point scale (1 point, “never,” to 5 points, “frequently”). Although a score > 10 is indicative of IPV, item-level analysis is encouraged. Attending to which items the patient acknowledges and how often these behaviors occur yields a richer assessment than a summary score. In regard to simply asking a patient, “Do you feel safe at home?” (sensitivity of this question, 8.8%; specificity, 91.2%), the HITS better detects IPV with male and female patient populations in family practice and emergency care settings (sensitivity, 30%-100%; specificity, 86%-99%).27,30
What contextual factors and related concerns are present? It is important to understand proximal factors that might influence IPV risk to determine what kind of referral or treatment is appropriate—particularly for patients experiencing or engaging in infrequent, noninjurious, and bidirectional forms of IPV. Environmental and contextual stressors, such as financial hardship, unemployment, pregnancy, and discussion of divorce, can increase the risk for IPV.31,32 Situational influences, such as alcohol and drug intoxication, can also increase the risk for IPV. Victims of partner violence are at greater risk for mental health problems, including depression, anxiety, trauma- and stressor-related disorders, and substance use disorders. Risk goes both ways, however: Mental illness predicts subsequent IPV perpetration or victimization, and vice versa.31
Does the patient feel safe? Assessing the situation. Patient perception of safety in the relationship provides important information about the necessity of referral. Asking a patient if they feel unsafe because of the behavior of a current or former partner sheds light on the need for further safety assessment and immediate connection with appropriate resources.
Continue to: The Danger Assessment-5...
The Danger Assessment-5 (DA-5) (TABLE 333) is a useful 5-item tool for quickly assessing the risk for severe IPV.33 Patients respond to whether:
- the frequency or severity of violence has increased in the past year
- the partner has ever used, or threatened to use, a weapon
- the patient believes the partner is capable of killing her (him)
- the partner has ever tried to choke or strangle her (him)
- the partner is violently and constantly jealous.
Sensitivity and specificity analyses with a high-risk female sample suggested that 3 affirmative responses indicate a high risk for severe IPV and a need for adequate safety planning.
Brief motivational enhancement intervention. There are 3 components to this intervention.
- Assess interest in making changes or seeking help. IPV is paradoxical: Many factors complicate the decision to leave or stay, and patients across the spectrum of victimization might have some motivation to stay with their partner. It is important to assess the patient’s motivation to make changes in their relationship.4,34
- Provide feedback on screening. Sharing the results of screening with patients makes the assessment and referral process collaborative and transparent; collaborative engagement helps patients feel in control and invested in the follow-through.35 In the spirit of this endeavor, physicians are encouraged to refrain from providing raw or total scores from the measures; instead, share the interpretation of those scores, based on the participant’s responses to the screening items, in a matter-of-fact manner. At this point, elicit the patient’s response to this information, listen empathically, and answer questions before proceeding.
Consistent with screening for other serious health problems, we recommend that all patients be provided with information about abuse in romantic relationships. The National Center for Injury Prevention and Control Division of Violence Prevention has published a useful, easy-to-understand fact sheet (www.cdc.gov/violenceprevention/pdf/ipv-factsheet.pdf) that provides an overview of IPV-related behavior, how it influences health outcomes, who is at risk for IPV, and sources for support.
Continue to: Our IPASSPRT interview script...
Our IPASSPRT interview script (http://bit.ly/ipassprt) outlines how this information can be presented to patients as a typical part of the screening process. Providers are encouraged to share and review the information from the fact sheet with all patients and present it as part of the normal screening process to mitigate the potential for defensiveness on the part of the patient. For patients who screen positive for IPV, it might be important to brainstorm ideas for a safe, secure place to store this fact sheet and other resources from the brief intervention and referral process below (eg, a safety plan and specific referral information) so that the patient can access them quickly and easily, if needed.
For patients who screen negative for IPV, their screen and interview conclude at this point.
- Provide recommendations based on the screen. Evidence suggests that collaborating with the patient on safety planning and referral can increase the likelihood of their engagement.7 Furthermore, failure to tailor the referral to the needs of the patient can be detrimental36—ie, overshooting the level of intervention might decrease the patient’s future treatment-seeking behavior and undermine their internal coping strategies, increasing the likelihood of future victimization. For that reason, we provide the following guidance on navigating the referral process for patients who screen positive for IPV.
Screening-based referral: A delicate and collaborative process
Referral for IPV victimization. Individual counseling, with or without an IPV focus, might be appropriate for patients at lower levels of risk; immediate connection with local IPV resources is strongly encouraged for patients at higher risk. This is a delicate, collaborative process, in which the physician offers recommendations for referral commensurate to the patient’s risk but must, ultimately, respect the patient’s autonomy by identifying referrals that fit the patient’s goals. We encourage providers to provide risk-informed recommendations and to elicit the patient’s thoughts about that information.
Several online resources are available to help physicians locate and connect with IPV-related resources in their community, including the National Health Resource Center on Domestic Violence (http://ipvhealth.org/), which provides a step-by-step guide to making such connections. We encourage physicians to develop these collaborative partnerships early to facilitate warm handoffs and increase the likelihood that a patient will follow through with the referral after screening.37
Referral for related concerns. As we’ve noted, IPV has numerous physical and mental health consequences, including depression, low self-esteem, trauma- and non-trauma-related anxiety, and substance abuse. In general, cognitive behavioral therapies appear most efficacious for treating these IPV-related consequences, but evidence is limited that such interventions diminish the likelihood of re-victimization.38 Intervention programs that foster problem-solving, solution-seeking, and cognitive restructuring for self-critical thoughts and misconceptions seem to produce the best physical and mental health outcomes.39 For patients who have a substance use disorder, treatment programs that target substance use have demonstrated a reduction in the rate of IPV recidivism.40 These findings indicate that establishing multiple treatment targets might reduce the risk for future aggression in relationships.
Continue to: The Substance Abuse and Mental Health Services Administration...
The Substance Abuse and Mental Health Services Administration of the US Department of Health and Human Services provides a useful online tool (https://findtreatment.samhsa.gov/) for locating local referrals that address behavioral health and substance-related concerns. The agency also provides a hotline (1-800-662-HELP [4357]) as an alternative resource for information and treatment referrals.
Safety planning can improve outcomes
For a patient who screens above low risk, safety planning with the patient is an important part of improving outcomes and can take several forms. Online resources, such as the Path to Safety interactive Web page (www.thehotline.org/help/path-to-safety/) maintained by The National Domestic Violence Hotline ([800]799-SAFE [7233]), provide information regarding important considerations for safety planning when:
- living with an abusive partner
- children are in the home
- the patient is pregnant
- pets are involved.
The Web site also provides information regarding legal options and resources related to IPV (eg, an order of protection) and steps for improving safety when leaving an abusive relationship. Patients at risk for IPV can explore the online tool and call the hotline.
For physicians who want to engage in provider-assisted safety planning, we’ve provided further guidance in the IPASSPRT screening script and safety plan (http://bit.ly/ipassprt) (TABLE 4).
Goal: Affirm patients’ strengths and reinforce hope
Psychological aggression is the most common form of relationship aggression; repeated denigration might leave a person with little confidence in their ability to change their relationship or seek out identified resources. That’s why it’s useful to inquire—with genuine curiosity—about a time in the past when the patient accomplished something challenging. The physician’s enthusiastic reflection on this achievement can be a means of highlighting the patient’s ability to accomplish a meaningful goal; of reinforcing their hope; and of eliciting important resources within and around the patient that can facilitate action on their safety plan. (See “IPV-related resources for physicians and patients.”)
SIDEBAR
IPV-related resources for physicians and patients
Intimate Partner Aggression Screening, Safety Planning, and Referral to Treatment (IPASSPRT) Project
› http://bit.ly/ipassprt
Online resource with tools designed by the authors, including an SBIRT-inspired script and safety plan template for IPV screening, safety planning, and referral
National Center for Injury Prevention and Control Division of Violence Prevention
› www.cdc.gov/violenceprevention/pdf/ipv-factsheet.pdf
Overview of IPV-related behavior, influence on health outcomes, people at risk of IPV, and sources of support, all in a format easily understood by patients
National Health Resource Center on Domestic Violence
› http://ipvhealth.org/
Includes guidance on connecting with IPV-related community resources; establishing such connections can facilitate warm handoffs and improve the likelihood that patients will follow through
Path to Safety, a service of The National Domestic Violence Hotline
› www.thehotline.org/help/path-to-safety/
Extensive primer on safety plans for patients intending to stay in (or leave) an abusive relationship; includes important considerations for children, pets, and pregnancy, as well as emotional safety and legal options
The National Domestic Violence Hotline
› (800) 799-SAFE (7233)
Substance Abuse and Mental Health Services Administration
› www.samhsa.gov/sbirt
Learning resources for the SBIRT protocol for substance abuse
› https://findtreatment.samhsa.gov/
Search engine and resources for locating local referrals
› (800) 662-HELP (4357)
Hotline for information and assistance with locating local treatment referral
IPV, intimate partner violence; SBIRT, screening, brief intervention, and referral to treatment.
Continue to: Closing the screen and making a referral
Closing the screen and making a referral
The end of the interview should consist of a summary of topics discussed, including:
- changes that the patient wants to make (if any)
- their stated reasons for making those changes
- the patient’s plan for accomplishing changes.
Physicians should also include their own role in next steps—whether providing a warm handoff to a local IPV referral, agreeing to a follow-up schedule with the patient, or making a call as a mandated reporter. To close out the interview, it is important to affirm respect for the patient’s autonomy in executing the plan.
It’s important to screen all patients—here’s why
A major impetus for this article has been to raise awareness about the need for expanded IPV screening across primary care settings. As mentioned, much of the literature on IPV victimization has focused on women; however, the few epidemiological investigations of victimization rates among men and members of LGBT couples show a high rate of victimization and considerable harmful health outcomes. Driven by stigma surrounding IPV, sex, and sexual minority status, patients might have expectations that they will be judged by a provider or “outed.”
Such barriers can lead many to suffer in silence until the problem can no longer be hidden or the danger becomes more emergent. Compassionate, nonjudgmental screening and collaborative safety planning—such as the approach we describe in this article—help ease the concerns of LGBT victims of IPV and improve the likelihood that conversations you have with them will occur earlier, rather than later, in care.*
Underassessment of IPV (ie, underreporting as well as under-inquiry) because of stigma, misconception, and other factors obscures an accurate estimate of the rate of partner violence and its consequences for all couples. As a consequence, we know little about the dynamics of IPV, best practices for screening, and appropriate referral for couples from these populations. Furthermore, few resources are available to these understudied and underserved groups (eg, shelters for men and for transgender people).
Continue to: Although our immediate approach to IPV screening...
Although our immediate approach to IPV screening, safety planning, and referral with understudied patient populations might be informed by what we have learned from the experiences of heterosexual women in abusive relationships, such a practice is unsustainable. Unless we expand our scope of screening to all patients, it is unlikely that we will develop the evidence base necessary to 1) warrant stronger IPV screening recommendations for patient groups apart from women of childbearing age, let alone 2) demonstrate the need for additional community resources, and 3) provide comprehensive care in family practice of comparable quality.
The benefits of screening go beyond the individual patient
Screening for violence in the relationship does not take long; the value of asking about its presence in a relationship might offer benefits beyond the individual patient by raising awareness and providing the field of study with more data to increase attention and resources for under-researched and underserved populations. Screening might also combat the stigma that perpetuates the silence of many who deserve access to care.
CORRESPONDENCE
Joel G. Sprunger, PhD, Department of Psychiatry and Behavioral Neuroscience, University of Cincinnati College of Medicine, 260 Stetson St, Suite 3200, Cincinnati OH 45219; joel.sprunger@UC.edu.
ACKNOWLEDGMENTS
The authors thank Jeffrey M. Girard, PhD, and Daniel C. Williams, PhD, for their input on the design and content, respectively, of the IPASSPRT screening materials; the authors of the DA-5 and the HITS screening tools, particularly Jacquelyn Campbell, PhD, RN, FAAN, and Kevin Sherin, MD, MPH, MBA, respectively, for permission to include these measures in this article and for their support of its goals; and The Journal of Family Practice’s peer reviewers for their thoughtful feedback throughout the prepublication process.
1. Campos-Outcalt D. USPSTF: What’s recommended, what’s not. J Fam Pract. 2014;63:265-269.
2. Black MC, Basile KC, Breiding MJ, et al. National Intimate Partner and Sexual Violence Survey: 2010 Summary Report. Atlanta, GA: National Center for Injury Prevention and Control, Centers for Disease Control and Prevention; 2011:113. www.cdc.gov/violenceprevention/pdf/NISVS_Report2010-a.pdf. Accessed February 20, 2019.
3. West CM. Partner abuse in ethnic minority and gay, lesbian, bisexual, and transgender populations. Partner Abuse. 2012;3:336-357.
4. Hines DA, Malley-Morrison K. Psychological effects of partner abuse against men: a neglected research area. Psychology of Men & Masculinities. 2001;2:75-85.
5. Houston E, McKirnan DJ. Intimate partner abuse among gay and bisexual men: risk correlates and health outcomes. J Urban Health. 2007;84:681-690.
6. Carvalho AF, Lewis RJ, Derlega VJ, et al. Internalized sexual minority stressors and same-sex intimate partner violence. J Fam Violence. 2011;26:501-509.
7. Nicholls TL, Pritchard MM, Reeves KA, et al. Risk assessment in intimate partner violence: a systematic review of contemporary approaches. Partner Abuse. 2013;4:76-168.
8. Intimate partner violence: definitions. Atlanta, GA: National Center for Injury Prevention and Control, Division of Violence Prevention, Centers for Disease Control and Prevention, August 22, 2017. www.cdc.gov/violenceprevention/intimatepartnerviolence/definitions.html. Accessed February 20, 2019.
9. Archer J. Sex differences in aggression between heterosexual partners: a meta-analytic review. Psychol Bull. 2000;126:651-680.
10. Baron RA, Richardson DR. Human Aggression. New York, NY: Springer Science+Business Media; 2004.
11. Breiding MJ, Basile KC, Smith SG, et al. Intimate Partner Violence Surveillance: Uniform Definitions and Recommended Data Elements, Version 2.0. Atlanta, GA: National Center for Injury Prevention and Control, Centers for Disease Control and Prevention; 2015.
12. Murphy CM, Eckhardt CI. Treating the Abusive Partner: An Individualized Cognitive-Behavioral Approach. New York, NY: Guilford Press; 2005.
13. Straus MA, Hamby SL, Boney-McCoy S, et al. The revised Conflict Tactics Scales (CTS2): development and preliminary psychometric data. J Fam Issues. 1996;17:283-316.
14. West CM. Partner abuse in ethnic minority and gay, lesbian, bisexual, and transgender populations. Partner Abuse. 2012;3:336-357.
15. Desmarais SL, Reeves KA, Nicholls TL, et al. Prevalence of physical violence in intimate relationships. Part 1: rates of male and female victimization. Partner Abuse. 2012;3:140-169.
16. Lawrence E, Orengo-Aguayo R, Langer A, et al. The impact and consequences of partner abuse on partners. Partner Abuse. 2012;3:406-428.
17. Langhinrichsen-Rohling J, Selwyn C, Rohling ML. Rates of bidirectional versus unidirectional intimate partner violence across samples, sexual orientations, and race/ethnicities: a comprehensive review. Partner Abuse. 2012;3:199-230.
18. Langhinrichsen-Rohling J, McCullars A, Misra TA. Motivations for men and women’s intimate partner violence perpetration: a comprehensive review. Partner Abuse. 2012;3:429-468.
19. Anderson CA, Bushman BJ. Human aggression. Annu Rev Psychol. 2002;53:27-51.
20. Straus MA, Gozjolko KL. “Intimate terrorism” and gender differences in injury of dating partners by male and female university students. J Fam Violence. 2014;29:51-65.
21. Ferraro KJ, Johnson JM. How women experience battering: the process of victimization. Soc Probl. 1983;30:325-339.
22. Sugg NK, Inui T. Primary care physicians’ response to domestic violence: opening Pandora’s box. JAMA. 1992;267:3157-3160.
23. Morgan KJ, Williamson E, Hester M, et al. Asking men about domestic violence and abuse in a family medicine context: help seeking and views on the general practitioner role. Aggress Violent Behav. 2014;19:637-642.
24. MacMillan HL, Wathen CN, Jamieson E, et al; McMaster Violence Against Women Research Group. Approaches to screening for intimate partner violence in health care settings: a randomized trial. JAMA. 2006;296:530-536.
25. Thompson RS, Rivara FP, Thompson DC, et al. Identification and management of domestic violence: a randomized trial. Am J Prev Med. 2000;19:253-263.
26. Ard KL, Makadon HJ. Addressing intimate partner violence in lesbian, gay, bisexual, and transgender patients. J Gen Intern Med. 2011;26:930-933.
27. Rabin RF, Jennings JM, Campbell JC, et al. Intimate partner violence screening tools: a systematic review. Am J Prev Med. 2009;36:439-445.e4.
28. Madras BK, Compton WM, Avula D, et al. Screening, brief interventions, referral to treatment (SBIRT) for illicit drug and alcohol use at multiple healthcare sites: comparison at intake and 6 months later. Drug Alcohol Depend. 2009;99:280-295.
29. Sherin KM, Sinacore JM, Li XQ, et al. HITS: A short domestic violence screening tool for use in a family practice setting. Fam Med. 1998;30:508-512.
30. Peralta RL, Fleming MF. Screening for intimate partner violence in a primary care setting: the validity of “feeling safe at home” and prevalence results. J Am Board Fam Pract. 2003;16:525-532.
31. Capaldi DM, Knoble NB, Shortt JW, et al. A systematic review of risk factors for intimate partner violence. Partner Abuse. 2012;3:231-280.
32. Brownridge DA, Taillieu TL, Tyler KA, et al. Pregnancy and intimate partner violence: risk factors, severity, and health effects. Violence Against Women. 2011;17:858-881.
33. Messing JT, Campbell JC, Snider C. Validation and adaptation of the danger assessment-5: a brief intimate partner violence risk assessment. J Adv Nurs. 2017;73:3220-3230.
34. Grigsby N, Hartman BR. The Barriers Model: an integrated strategy for intervention with battered women. Psychotherapy: Theory, Research, Practice, Training. 1997;34:485-497.
35. Moyers TB, Rollnick S. A motivational interviewing perspective on resistance in psychotherapy. J Clin Psychol. 2002;58:185-193.
36. Belfrage H, Strand S, Storey JE, et al. Assessment and management of risk for intimate partner violence by police officers using the Spousal Assault Risk Assessment Guide. Law Hum Behav. 2012;36:60-67.
37. McCloskey LA, Lichter E, Williams C, et al. Assessing intimate partner violence in health care settings leads to women’s receipt of interventions and improved health. Publ Health Rep. 2006;121:435-444.
38. Eckhardt CI, Murphy CM, Whitaker DJ, et al. The effectiveness of intervention programs for perpetrators and victims of intimate partner violence. Partner Abuse. 2013;4:196-231.
39. Trabold N, McMahon J, Alsobrooks S, et al. A systematic review of intimate partner violence interventions: state of the field and implications for practitioners. Trauma Violence Abuse. January 2018:1524838018767934.
40. Kraanen FL, Vedel E, Scholing A, et al. The comparative effectiveness of Integrated treatment for Substance abuse and Partner violence (I-StoP) and substance abuse treatment alone: a randomized controlled trial. BMC Psychiatry. 2013;13:189.
1. Campos-Outcalt D. USPSTF: What’s recommended, what’s not. J Fam Pract. 2014;63:265-269.
2. Black MC, Basile KC, Breiding MJ, et al. National Intimate Partner and Sexual Violence Survey: 2010 Summary Report. Atlanta, GA: National Center for Injury Prevention and Control, Centers for Disease Control and Prevention; 2011:113. www.cdc.gov/violenceprevention/pdf/NISVS_Report2010-a.pdf. Accessed February 20, 2019.
3. West CM. Partner abuse in ethnic minority and gay, lesbian, bisexual, and transgender populations. Partner Abuse. 2012;3:336-357.
4. Hines DA, Malley-Morrison K. Psychological effects of partner abuse against men: a neglected research area. Psychology of Men & Masculinities. 2001;2:75-85.
5. Houston E, McKirnan DJ. Intimate partner abuse among gay and bisexual men: risk correlates and health outcomes. J Urban Health. 2007;84:681-690.
6. Carvalho AF, Lewis RJ, Derlega VJ, et al. Internalized sexual minority stressors and same-sex intimate partner violence. J Fam Violence. 2011;26:501-509.
7. Nicholls TL, Pritchard MM, Reeves KA, et al. Risk assessment in intimate partner violence: a systematic review of contemporary approaches. Partner Abuse. 2013;4:76-168.
8. Intimate partner violence: definitions. Atlanta, GA: National Center for Injury Prevention and Control, Division of Violence Prevention, Centers for Disease Control and Prevention, August 22, 2017. www.cdc.gov/violenceprevention/intimatepartnerviolence/definitions.html. Accessed February 20, 2019.
9. Archer J. Sex differences in aggression between heterosexual partners: a meta-analytic review. Psychol Bull. 2000;126:651-680.
10. Baron RA, Richardson DR. Human Aggression. New York, NY: Springer Science+Business Media; 2004.
11. Breiding MJ, Basile KC, Smith SG, et al. Intimate Partner Violence Surveillance: Uniform Definitions and Recommended Data Elements, Version 2.0. Atlanta, GA: National Center for Injury Prevention and Control, Centers for Disease Control and Prevention; 2015.
12. Murphy CM, Eckhardt CI. Treating the Abusive Partner: An Individualized Cognitive-Behavioral Approach. New York, NY: Guilford Press; 2005.
13. Straus MA, Hamby SL, Boney-McCoy S, et al. The revised Conflict Tactics Scales (CTS2): development and preliminary psychometric data. J Fam Issues. 1996;17:283-316.
14. West CM. Partner abuse in ethnic minority and gay, lesbian, bisexual, and transgender populations. Partner Abuse. 2012;3:336-357.
15. Desmarais SL, Reeves KA, Nicholls TL, et al. Prevalence of physical violence in intimate relationships. Part 1: rates of male and female victimization. Partner Abuse. 2012;3:140-169.
16. Lawrence E, Orengo-Aguayo R, Langer A, et al. The impact and consequences of partner abuse on partners. Partner Abuse. 2012;3:406-428.
17. Langhinrichsen-Rohling J, Selwyn C, Rohling ML. Rates of bidirectional versus unidirectional intimate partner violence across samples, sexual orientations, and race/ethnicities: a comprehensive review. Partner Abuse. 2012;3:199-230.
18. Langhinrichsen-Rohling J, McCullars A, Misra TA. Motivations for men and women’s intimate partner violence perpetration: a comprehensive review. Partner Abuse. 2012;3:429-468.
19. Anderson CA, Bushman BJ. Human aggression. Annu Rev Psychol. 2002;53:27-51.
20. Straus MA, Gozjolko KL. “Intimate terrorism” and gender differences in injury of dating partners by male and female university students. J Fam Violence. 2014;29:51-65.
21. Ferraro KJ, Johnson JM. How women experience battering: the process of victimization. Soc Probl. 1983;30:325-339.
22. Sugg NK, Inui T. Primary care physicians’ response to domestic violence: opening Pandora’s box. JAMA. 1992;267:3157-3160.
23. Morgan KJ, Williamson E, Hester M, et al. Asking men about domestic violence and abuse in a family medicine context: help seeking and views on the general practitioner role. Aggress Violent Behav. 2014;19:637-642.
24. MacMillan HL, Wathen CN, Jamieson E, et al; McMaster Violence Against Women Research Group. Approaches to screening for intimate partner violence in health care settings: a randomized trial. JAMA. 2006;296:530-536.
25. Thompson RS, Rivara FP, Thompson DC, et al. Identification and management of domestic violence: a randomized trial. Am J Prev Med. 2000;19:253-263.
26. Ard KL, Makadon HJ. Addressing intimate partner violence in lesbian, gay, bisexual, and transgender patients. J Gen Intern Med. 2011;26:930-933.
27. Rabin RF, Jennings JM, Campbell JC, et al. Intimate partner violence screening tools: a systematic review. Am J Prev Med. 2009;36:439-445.e4.
28. Madras BK, Compton WM, Avula D, et al. Screening, brief interventions, referral to treatment (SBIRT) for illicit drug and alcohol use at multiple healthcare sites: comparison at intake and 6 months later. Drug Alcohol Depend. 2009;99:280-295.
29. Sherin KM, Sinacore JM, Li XQ, et al. HITS: A short domestic violence screening tool for use in a family practice setting. Fam Med. 1998;30:508-512.
30. Peralta RL, Fleming MF. Screening for intimate partner violence in a primary care setting: the validity of “feeling safe at home” and prevalence results. J Am Board Fam Pract. 2003;16:525-532.
31. Capaldi DM, Knoble NB, Shortt JW, et al. A systematic review of risk factors for intimate partner violence. Partner Abuse. 2012;3:231-280.
32. Brownridge DA, Taillieu TL, Tyler KA, et al. Pregnancy and intimate partner violence: risk factors, severity, and health effects. Violence Against Women. 2011;17:858-881.
33. Messing JT, Campbell JC, Snider C. Validation and adaptation of the danger assessment-5: a brief intimate partner violence risk assessment. J Adv Nurs. 2017;73:3220-3230.
34. Grigsby N, Hartman BR. The Barriers Model: an integrated strategy for intervention with battered women. Psychotherapy: Theory, Research, Practice, Training. 1997;34:485-497.
35. Moyers TB, Rollnick S. A motivational interviewing perspective on resistance in psychotherapy. J Clin Psychol. 2002;58:185-193.
36. Belfrage H, Strand S, Storey JE, et al. Assessment and management of risk for intimate partner violence by police officers using the Spousal Assault Risk Assessment Guide. Law Hum Behav. 2012;36:60-67.
37. McCloskey LA, Lichter E, Williams C, et al. Assessing intimate partner violence in health care settings leads to women’s receipt of interventions and improved health. Publ Health Rep. 2006;121:435-444.
38. Eckhardt CI, Murphy CM, Whitaker DJ, et al. The effectiveness of intervention programs for perpetrators and victims of intimate partner violence. Partner Abuse. 2013;4:196-231.
39. Trabold N, McMahon J, Alsobrooks S, et al. A systematic review of intimate partner violence interventions: state of the field and implications for practitioners. Trauma Violence Abuse. January 2018:1524838018767934.
40. Kraanen FL, Vedel E, Scholing A, et al. The comparative effectiveness of Integrated treatment for Substance abuse and Partner violence (I-StoP) and substance abuse treatment alone: a randomized controlled trial. BMC Psychiatry. 2013;13:189.
PRACTICE RECOMMENDATIONS
› Perform annual screening for intimate partner violence of all female patients of childbearing age; strongly consider a pilot program of universal screening (all male and female patients, across the lifespan). B
› Establish a protocol for intimate partner violence screening and referral—possibly the most effective means of identifying intimate partner violence at early and severe stages. B
› Collaborate with the patient in the safety planning and referral process; benefits include improved likelihood that the patient will adhere to a safety plan and follow through with the referral. B
› Utilize online resources to 1) ease the process of establishing relationships with local intimate partner violence referrals and 2) facilitate warm handoffs to increase the likelihood of patient engagement. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Screening and counseling interventions to prevent peripartum depression: A practical approach
Perinatal depression is an episode of major or minor depression that occurs during pregnancy or in the 12 months after birth; it affects about 10% of new mothers.1 Perinatal depression adversely impacts mothers, children, and their families. Pregnant women with depression are at increased risk for preterm birth and low birth weight.2 Infants of mothers with postpartum depression have reduced bonding, lower rates of breastfeeding, delayed cognitive and social development, and an increased risk of future mental health issues.3 Timely treatment of perinatal depression can improve health outcomes for the woman, her children, and their family.
Clinicians follow current screening recommendations
The American College of Obstetricians and Gynecologists (ACOG) currently recommends that ObGynsscreen all pregnant women for depression and anxiety symptoms at least once during the perinatal period.1 Many practices use the Edinburgh Postnatal Depression Scale (EPDS) during pregnancy and postpartum. Women who screen positive are referred to mental health clinicians or have treatment initiated by their primary obstetrician.
Clinicians have been phenomenally successful in screening for perinatal depression. In a recent study from Kaiser Permanente Northern California, 98% of pregnant women were screened for perinatal depression, and a diagnosis of depression was made in 12%.4 Of note, only 47% of women who screened positive for depression initiated treatment, although 82% of women with the most severe symptoms initiated treatment. These data demonstrate that ObGyns consistently screen pregnant women for depression but, due to patient and system issues, treatment of all screen-positive women remains a yet unattained goal.5,6
New USPSTF guideline: Identify women at risk for perinatal depression and refer for counseling
In 2016 the United States Preventive Services Task Force (USPSTF) recommended that pregnant and postpartum women be screened for depression with adequate systems in place to ensure diagnosis, effective treatment, and follow-up.7 The 2016 USPSTF recommendation was consistent with prior guidelines from both the American Academy of Pediatrics in 20108 and ACOG in 2015.9
Now, the USPSTF is making a bold new recommendation, jumping ahead of professional societies: screen pregnant women to identify those at risk for perinatal depression and refer them for counseling (B recommendation; net benefit is moderate).10,11 The USPSTF recommendation is based on growing literature that shows counseling women at risk for perinatal depression reduces the risk of having an episode of major depression by 40%.11 Both interpersonal psychotherapy and cognitive behavioral therapy have been reported to be effective for preventing perinatal depression.12,13
As an example of the relevant literature, in one trial performed in Rhode Island, women who were 20 to 35 weeks pregnant with a high score (≥27) on the Cooper Survey Questionnaire and on public assistance were randomized to counseling or usual care. The counseling intervention involved 4 small group (2 to 5 women) sessions of 90 minutes and one individual session of 50 minutes.14 The treatment focused on managing the transition to motherhood, developing a support system, improving communication skills to manage conflict, goal setting, and identifying psychosocial supports for new mothers. At 6 months after birth, a depressive episode had occurred in 31% of the control women and 16% of the women who had experienced the intervention (P = .041). At 12 months after birth, a depressive episode had occurred in 40% of control women and 26% of women in the intervention group (P = .052).
Of note, most cases of postpartum depression were diagnosed more than 3 months after birth, a time when new mothers generally no longer are receiving regular postpartum care by an obstetrician. The timing of the diagnosis of perinatal depression indicates that an effective handoff between the obstetrician and primary care and/or mental health clinicians is of great importance. The investigators concluded that pregnant women at very high risk for perinatal depression who receive interpersonal therapy have a lower rate of a postpartum depressive episode than women receiving usual care.14
Pregnancy, delivery, and the first year following birth are stressful for many women and their families. Women who are young, poor, and with minimal social supports are at especially high risk for developing perinatal depression. However, it will be challenging for obstetric practices to rapidly implement the new USPSTF recommendations because there is no professional consensus on how to screen women to identify those at high risk for perinatal depression, and mental health resources to care for the screen-positive women are not sufficient.

Continue to: Challenges to implementing new USPSTF guideline...
Challenges to implementing new USPSTF guideline
Obstetricians have had great success in screening for perinatal depression because validated screening tools are available. Professional societies need to reach a consensus on recommending a specific screening tool for perinatal depression risk that can be used in all obstetric practices.
- personal history of depression
- current depressive symptoms that do not reach a diagnostic threshold
- low income
- all adolescents
- all single mothers
- recent exposure to intimate partner violence
- elevated anxiety symptoms
- a history of significant negative life events.
For many obstetricians, most of their pregnant patients meet the USPSTF criteria for being at high risk for perinatal depression and, per the guideline, these women should have a counseling intervention.
For many health systems, the resources available to provide mental health services are very limited. If most pregnant women need a counseling intervention, the health system must evolve to meet this need. In addition, risk factors for perinatal depression are also risk factors for having difficulty in participating in mental health interventions due to limitations, such as lack of transportation, social support, and money.4
Fortunately, clinicians from many backgrounds, including psychologists, social workers, nurse practitioners, and public health workers have the experience and/or training to provide the counseling interventions that have been shown to reduce the risk of perinatal depression. Health systems will need to tap all these resources to accommodate the large numbers of pregnant women who will be referred for counseling interventions. Pilot projects using electronic interventions, including telephone counseling, smartphone apps, and internet programs show promise.15,16 Electronic interventions have the potential to reach many pregnant women without over-taxing limited mental health resources.
A practical approach
Identify women at the greatest risk for perinatal depression and focus counseling interventions on this group. In my opinion, implementation of the USPSTF recommendation will take time. A practical approach would be to implement them in a staged sequence, focusing first on the women at highest risk, later extending the program to women at lesser risk. The two factors that confer the greatest risk of perinatal depression are a personal history of depression and high depression symptoms that do not meet criteria for depression.17 Many women with depression who take antidepressants discontinue their medications during pregnancy. These women are at very high risk for perinatal depression and deserve extra attention.18
Continue to: To identify women with a prior personal history of depression...
To identify women with a prior personal history of depression, it may be helpful to ask open-ended questions about a past diagnosis of depression or a mood disorder or use of antidepressant medications. To identify women with the greatest depression symptoms, utilize a lower cut-off for screening positive in the Edinburgh questionnaire. Practices that use an EPDS screen-positive score of 13 or greater could reduce the cut-off to 10 or 11, which would increase the number of women referred for evaluation and treatment.19
Clinical judgment and screening
Screening for prevalent depression and screening for women at increased risk for perinatal depression is challenging. ACOG highlights two important clinical issues1:
“Women with current depression or anxiety, a history of perinatal mood disorders, risk factors for perinatal mood disorders or suicidal thoughts warrant particularly close monitoring, evaluation and assessment.”
When screening for perinatal depression, screening test results should be interpreted within the clinical context. “A normal score for a tearful patient with a flat affect does not exclude depression; an elevated score in the context of an acute stressful event may resolve with close follow-up.”
In addition, women who screen-positive for prevalent depression and are subsequently evaluated by a mental health specialist may be identified as having mental health problems such as an anxiety disorder, substance misuse, or borderline personality disorder.20
Policy changes that support pregnant women and mothers could help to reduce the stress of pregnancy, birth, and childrearing, thereby reducing the risk of perinatal depression. The United States stands alone among rich nations in not providing paid parental leave. Paid maternity and parental leave would help many families respond more effectively to the initial stresses of parenthood.21 For women and families living in poverty, improved social support, including secure housing, protection from abusive partners, transportation resources, and access to healthy foods likely will reduce both stress and the risk of depression.
The ultimate goal: A healthy pregnancy
Clinicians have been phenomenally successful in screening for perinatal depression. The new USPSTF recommendation adds the prevention of perinatal depression to the goals of a healthy pregnancy. This recommendation builds upon the foundation of screening for acute illness (depression), pivoting to the public health perspective of disease prevention.
- American College of Obstetricians and Gynecologists. Screening for perinatal depression. ACOG Committee Opinion No 757. Obstet Gynecol. 2018;132:e208-e212.
- Grote NK, Bridge JA, Gavin AR, et al. A meta-analysis of depression during pregnancy and the risk of preterm birth, low birth weight and intrauterine growth restriction. Arch Gen Psychiatry. 2010;67:1012-1024.
- Pearlstein T, Howard M, Salisbury A, et al. Postpartum depression. Am J Obstet Gynecol. 2009;200:357-364.
- Avalos LA, Raine-Bennett T, Chen H, et al. Improved perinatal depression screening, treatment and outcomes with a universal obstetric program. Obstet Gynecol. 2016;127:917-925.
- Cox EQ, Sowa NA, Meltzer-Brody SE, et al. The perinatal depression treatment cascade: baby steps toward improving outcomes. J Clin Psychiatry. 2016;77:1189-1200.
- Byatt N, Simas TA, Lundquist RS, et al. Strategies for improving perinatal depression treatment in North American outpatient obstetric settings. J Psychosom Obstet Gynaecol. 2012;33:143-161.
- Siu AL, Bibbins-Domingo K, Grossman DC, et al. US Preventive Services Task Force (USPSTF). Screening for depression in adults. JAMA. 2016;315:380-387.
- Earls MF. Committee on Psychological Aspects of Child and Family Health. American Academy of Pediatrics. Incorporating recognition and management of perinatal and postpartum depression into pediatric practice. Pediatrics. 2010;126:1032-1039.
- The American College of Obstetricians and Gynecologists. Committee on Obstetric Practice. Committee Opinion No 630. Screening for perinatal depression. Obstet Gynecol. 2015;125:1268-1271.
- US Preventive Services Task Force. Interventions to prevent perinatal depression: US Preventive Services Task Force recommendations statement. JAMA. 2019;321:580-587.
- O’Connor E, Senger CA, Henninger ML, et al. Interventions to prevent perinatal depression: evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2019;321:588-601.
- Sockol LE. A systematic review and meta-analysis of interpersonal psychotherapy for perinatal women. J Affective Disorders. 2018;232:316-328.
- Sockol LE. A systematic review of the efficacy of cognitive behavioral therapy for treating and preventing perinatal depression. J Affective Disorders. 2015;177:7-21.
- Zlotnick C, Tzilos G, Miller I, et al. Randomized controlled trial to prevent postpartum depression in mothers on public assistance. J Affective Disorders. 2016;189:263-268.
- Haga SM, Drozd F, Lisoy C, et al. Mamma Mia—a randomized controlled trial of an internet-based intervention for perinatal depression. Psycholog Med. 2018;1-9.
- Shorey S, Ng YM, Ng ED, et al. Effectiveness of a technology-based supportive educational parenting program on parent outcomes (Part 1): Randomized controlled trial. J Med Internet Res. 2019;21:e10816.
- Cohen LS, Altshuler LL, Harlow BL, et al. Relapse of major depression during pregnancy in women who maintain or discontinue antidepressant treatment. JAMA. 2006;295:499-507.
- Goodman JH. Women’s attitudes, preferences and perceived barriers to treatment for perinatal depression. Birth. 2009;36:60-69.
- Smith-Nielsen J, Matthey S, Lange T, Vaever MS. Validation of the Edinburgh Postnatal Depression Scale against both DSM-5 and ICD-10 diagnostic criteria for depression. BMC Psychiatry. 2018;18:393.
- Judd F, Lorimer S, Thomson RH, et al. Screening for depression with the Edinburgh Postnatal Depression Scale and finding borderline personality disorder. Aust N Z J Psychiatry. 2018;Epub Oct 12. doi: 10.1177/0004867418804067.
- Diamond R. Promoting sensible parenting policies. Leading by example. JAMA. 2019;321:645- 646.
Perinatal depression is an episode of major or minor depression that occurs during pregnancy or in the 12 months after birth; it affects about 10% of new mothers.1 Perinatal depression adversely impacts mothers, children, and their families. Pregnant women with depression are at increased risk for preterm birth and low birth weight.2 Infants of mothers with postpartum depression have reduced bonding, lower rates of breastfeeding, delayed cognitive and social development, and an increased risk of future mental health issues.3 Timely treatment of perinatal depression can improve health outcomes for the woman, her children, and their family.
Clinicians follow current screening recommendations
The American College of Obstetricians and Gynecologists (ACOG) currently recommends that ObGynsscreen all pregnant women for depression and anxiety symptoms at least once during the perinatal period.1 Many practices use the Edinburgh Postnatal Depression Scale (EPDS) during pregnancy and postpartum. Women who screen positive are referred to mental health clinicians or have treatment initiated by their primary obstetrician.
Clinicians have been phenomenally successful in screening for perinatal depression. In a recent study from Kaiser Permanente Northern California, 98% of pregnant women were screened for perinatal depression, and a diagnosis of depression was made in 12%.4 Of note, only 47% of women who screened positive for depression initiated treatment, although 82% of women with the most severe symptoms initiated treatment. These data demonstrate that ObGyns consistently screen pregnant women for depression but, due to patient and system issues, treatment of all screen-positive women remains a yet unattained goal.5,6
New USPSTF guideline: Identify women at risk for perinatal depression and refer for counseling
In 2016 the United States Preventive Services Task Force (USPSTF) recommended that pregnant and postpartum women be screened for depression with adequate systems in place to ensure diagnosis, effective treatment, and follow-up.7 The 2016 USPSTF recommendation was consistent with prior guidelines from both the American Academy of Pediatrics in 20108 and ACOG in 2015.9
Now, the USPSTF is making a bold new recommendation, jumping ahead of professional societies: screen pregnant women to identify those at risk for perinatal depression and refer them for counseling (B recommendation; net benefit is moderate).10,11 The USPSTF recommendation is based on growing literature that shows counseling women at risk for perinatal depression reduces the risk of having an episode of major depression by 40%.11 Both interpersonal psychotherapy and cognitive behavioral therapy have been reported to be effective for preventing perinatal depression.12,13
As an example of the relevant literature, in one trial performed in Rhode Island, women who were 20 to 35 weeks pregnant with a high score (≥27) on the Cooper Survey Questionnaire and on public assistance were randomized to counseling or usual care. The counseling intervention involved 4 small group (2 to 5 women) sessions of 90 minutes and one individual session of 50 minutes.14 The treatment focused on managing the transition to motherhood, developing a support system, improving communication skills to manage conflict, goal setting, and identifying psychosocial supports for new mothers. At 6 months after birth, a depressive episode had occurred in 31% of the control women and 16% of the women who had experienced the intervention (P = .041). At 12 months after birth, a depressive episode had occurred in 40% of control women and 26% of women in the intervention group (P = .052).
Of note, most cases of postpartum depression were diagnosed more than 3 months after birth, a time when new mothers generally no longer are receiving regular postpartum care by an obstetrician. The timing of the diagnosis of perinatal depression indicates that an effective handoff between the obstetrician and primary care and/or mental health clinicians is of great importance. The investigators concluded that pregnant women at very high risk for perinatal depression who receive interpersonal therapy have a lower rate of a postpartum depressive episode than women receiving usual care.14
Pregnancy, delivery, and the first year following birth are stressful for many women and their families. Women who are young, poor, and with minimal social supports are at especially high risk for developing perinatal depression. However, it will be challenging for obstetric practices to rapidly implement the new USPSTF recommendations because there is no professional consensus on how to screen women to identify those at high risk for perinatal depression, and mental health resources to care for the screen-positive women are not sufficient.

Continue to: Challenges to implementing new USPSTF guideline...
Challenges to implementing new USPSTF guideline
Obstetricians have had great success in screening for perinatal depression because validated screening tools are available. Professional societies need to reach a consensus on recommending a specific screening tool for perinatal depression risk that can be used in all obstetric practices.
- personal history of depression
- current depressive symptoms that do not reach a diagnostic threshold
- low income
- all adolescents
- all single mothers
- recent exposure to intimate partner violence
- elevated anxiety symptoms
- a history of significant negative life events.
For many obstetricians, most of their pregnant patients meet the USPSTF criteria for being at high risk for perinatal depression and, per the guideline, these women should have a counseling intervention.
For many health systems, the resources available to provide mental health services are very limited. If most pregnant women need a counseling intervention, the health system must evolve to meet this need. In addition, risk factors for perinatal depression are also risk factors for having difficulty in participating in mental health interventions due to limitations, such as lack of transportation, social support, and money.4
Fortunately, clinicians from many backgrounds, including psychologists, social workers, nurse practitioners, and public health workers have the experience and/or training to provide the counseling interventions that have been shown to reduce the risk of perinatal depression. Health systems will need to tap all these resources to accommodate the large numbers of pregnant women who will be referred for counseling interventions. Pilot projects using electronic interventions, including telephone counseling, smartphone apps, and internet programs show promise.15,16 Electronic interventions have the potential to reach many pregnant women without over-taxing limited mental health resources.
A practical approach
Identify women at the greatest risk for perinatal depression and focus counseling interventions on this group. In my opinion, implementation of the USPSTF recommendation will take time. A practical approach would be to implement them in a staged sequence, focusing first on the women at highest risk, later extending the program to women at lesser risk. The two factors that confer the greatest risk of perinatal depression are a personal history of depression and high depression symptoms that do not meet criteria for depression.17 Many women with depression who take antidepressants discontinue their medications during pregnancy. These women are at very high risk for perinatal depression and deserve extra attention.18
Continue to: To identify women with a prior personal history of depression...
To identify women with a prior personal history of depression, it may be helpful to ask open-ended questions about a past diagnosis of depression or a mood disorder or use of antidepressant medications. To identify women with the greatest depression symptoms, utilize a lower cut-off for screening positive in the Edinburgh questionnaire. Practices that use an EPDS screen-positive score of 13 or greater could reduce the cut-off to 10 or 11, which would increase the number of women referred for evaluation and treatment.19
Clinical judgment and screening
Screening for prevalent depression and screening for women at increased risk for perinatal depression is challenging. ACOG highlights two important clinical issues1:
“Women with current depression or anxiety, a history of perinatal mood disorders, risk factors for perinatal mood disorders or suicidal thoughts warrant particularly close monitoring, evaluation and assessment.”
When screening for perinatal depression, screening test results should be interpreted within the clinical context. “A normal score for a tearful patient with a flat affect does not exclude depression; an elevated score in the context of an acute stressful event may resolve with close follow-up.”
In addition, women who screen-positive for prevalent depression and are subsequently evaluated by a mental health specialist may be identified as having mental health problems such as an anxiety disorder, substance misuse, or borderline personality disorder.20
Policy changes that support pregnant women and mothers could help to reduce the stress of pregnancy, birth, and childrearing, thereby reducing the risk of perinatal depression. The United States stands alone among rich nations in not providing paid parental leave. Paid maternity and parental leave would help many families respond more effectively to the initial stresses of parenthood.21 For women and families living in poverty, improved social support, including secure housing, protection from abusive partners, transportation resources, and access to healthy foods likely will reduce both stress and the risk of depression.
The ultimate goal: A healthy pregnancy
Clinicians have been phenomenally successful in screening for perinatal depression. The new USPSTF recommendation adds the prevention of perinatal depression to the goals of a healthy pregnancy. This recommendation builds upon the foundation of screening for acute illness (depression), pivoting to the public health perspective of disease prevention.
Perinatal depression is an episode of major or minor depression that occurs during pregnancy or in the 12 months after birth; it affects about 10% of new mothers.1 Perinatal depression adversely impacts mothers, children, and their families. Pregnant women with depression are at increased risk for preterm birth and low birth weight.2 Infants of mothers with postpartum depression have reduced bonding, lower rates of breastfeeding, delayed cognitive and social development, and an increased risk of future mental health issues.3 Timely treatment of perinatal depression can improve health outcomes for the woman, her children, and their family.
Clinicians follow current screening recommendations
The American College of Obstetricians and Gynecologists (ACOG) currently recommends that ObGynsscreen all pregnant women for depression and anxiety symptoms at least once during the perinatal period.1 Many practices use the Edinburgh Postnatal Depression Scale (EPDS) during pregnancy and postpartum. Women who screen positive are referred to mental health clinicians or have treatment initiated by their primary obstetrician.
Clinicians have been phenomenally successful in screening for perinatal depression. In a recent study from Kaiser Permanente Northern California, 98% of pregnant women were screened for perinatal depression, and a diagnosis of depression was made in 12%.4 Of note, only 47% of women who screened positive for depression initiated treatment, although 82% of women with the most severe symptoms initiated treatment. These data demonstrate that ObGyns consistently screen pregnant women for depression but, due to patient and system issues, treatment of all screen-positive women remains a yet unattained goal.5,6
New USPSTF guideline: Identify women at risk for perinatal depression and refer for counseling
In 2016 the United States Preventive Services Task Force (USPSTF) recommended that pregnant and postpartum women be screened for depression with adequate systems in place to ensure diagnosis, effective treatment, and follow-up.7 The 2016 USPSTF recommendation was consistent with prior guidelines from both the American Academy of Pediatrics in 20108 and ACOG in 2015.9
Now, the USPSTF is making a bold new recommendation, jumping ahead of professional societies: screen pregnant women to identify those at risk for perinatal depression and refer them for counseling (B recommendation; net benefit is moderate).10,11 The USPSTF recommendation is based on growing literature that shows counseling women at risk for perinatal depression reduces the risk of having an episode of major depression by 40%.11 Both interpersonal psychotherapy and cognitive behavioral therapy have been reported to be effective for preventing perinatal depression.12,13
As an example of the relevant literature, in one trial performed in Rhode Island, women who were 20 to 35 weeks pregnant with a high score (≥27) on the Cooper Survey Questionnaire and on public assistance were randomized to counseling or usual care. The counseling intervention involved 4 small group (2 to 5 women) sessions of 90 minutes and one individual session of 50 minutes.14 The treatment focused on managing the transition to motherhood, developing a support system, improving communication skills to manage conflict, goal setting, and identifying psychosocial supports for new mothers. At 6 months after birth, a depressive episode had occurred in 31% of the control women and 16% of the women who had experienced the intervention (P = .041). At 12 months after birth, a depressive episode had occurred in 40% of control women and 26% of women in the intervention group (P = .052).
Of note, most cases of postpartum depression were diagnosed more than 3 months after birth, a time when new mothers generally no longer are receiving regular postpartum care by an obstetrician. The timing of the diagnosis of perinatal depression indicates that an effective handoff between the obstetrician and primary care and/or mental health clinicians is of great importance. The investigators concluded that pregnant women at very high risk for perinatal depression who receive interpersonal therapy have a lower rate of a postpartum depressive episode than women receiving usual care.14
Pregnancy, delivery, and the first year following birth are stressful for many women and their families. Women who are young, poor, and with minimal social supports are at especially high risk for developing perinatal depression. However, it will be challenging for obstetric practices to rapidly implement the new USPSTF recommendations because there is no professional consensus on how to screen women to identify those at high risk for perinatal depression, and mental health resources to care for the screen-positive women are not sufficient.

Continue to: Challenges to implementing new USPSTF guideline...
Challenges to implementing new USPSTF guideline
Obstetricians have had great success in screening for perinatal depression because validated screening tools are available. Professional societies need to reach a consensus on recommending a specific screening tool for perinatal depression risk that can be used in all obstetric practices.
- personal history of depression
- current depressive symptoms that do not reach a diagnostic threshold
- low income
- all adolescents
- all single mothers
- recent exposure to intimate partner violence
- elevated anxiety symptoms
- a history of significant negative life events.
For many obstetricians, most of their pregnant patients meet the USPSTF criteria for being at high risk for perinatal depression and, per the guideline, these women should have a counseling intervention.
For many health systems, the resources available to provide mental health services are very limited. If most pregnant women need a counseling intervention, the health system must evolve to meet this need. In addition, risk factors for perinatal depression are also risk factors for having difficulty in participating in mental health interventions due to limitations, such as lack of transportation, social support, and money.4
Fortunately, clinicians from many backgrounds, including psychologists, social workers, nurse practitioners, and public health workers have the experience and/or training to provide the counseling interventions that have been shown to reduce the risk of perinatal depression. Health systems will need to tap all these resources to accommodate the large numbers of pregnant women who will be referred for counseling interventions. Pilot projects using electronic interventions, including telephone counseling, smartphone apps, and internet programs show promise.15,16 Electronic interventions have the potential to reach many pregnant women without over-taxing limited mental health resources.
A practical approach
Identify women at the greatest risk for perinatal depression and focus counseling interventions on this group. In my opinion, implementation of the USPSTF recommendation will take time. A practical approach would be to implement them in a staged sequence, focusing first on the women at highest risk, later extending the program to women at lesser risk. The two factors that confer the greatest risk of perinatal depression are a personal history of depression and high depression symptoms that do not meet criteria for depression.17 Many women with depression who take antidepressants discontinue their medications during pregnancy. These women are at very high risk for perinatal depression and deserve extra attention.18
Continue to: To identify women with a prior personal history of depression...
To identify women with a prior personal history of depression, it may be helpful to ask open-ended questions about a past diagnosis of depression or a mood disorder or use of antidepressant medications. To identify women with the greatest depression symptoms, utilize a lower cut-off for screening positive in the Edinburgh questionnaire. Practices that use an EPDS screen-positive score of 13 or greater could reduce the cut-off to 10 or 11, which would increase the number of women referred for evaluation and treatment.19
Clinical judgment and screening
Screening for prevalent depression and screening for women at increased risk for perinatal depression is challenging. ACOG highlights two important clinical issues1:
“Women with current depression or anxiety, a history of perinatal mood disorders, risk factors for perinatal mood disorders or suicidal thoughts warrant particularly close monitoring, evaluation and assessment.”
When screening for perinatal depression, screening test results should be interpreted within the clinical context. “A normal score for a tearful patient with a flat affect does not exclude depression; an elevated score in the context of an acute stressful event may resolve with close follow-up.”
In addition, women who screen-positive for prevalent depression and are subsequently evaluated by a mental health specialist may be identified as having mental health problems such as an anxiety disorder, substance misuse, or borderline personality disorder.20
Policy changes that support pregnant women and mothers could help to reduce the stress of pregnancy, birth, and childrearing, thereby reducing the risk of perinatal depression. The United States stands alone among rich nations in not providing paid parental leave. Paid maternity and parental leave would help many families respond more effectively to the initial stresses of parenthood.21 For women and families living in poverty, improved social support, including secure housing, protection from abusive partners, transportation resources, and access to healthy foods likely will reduce both stress and the risk of depression.
The ultimate goal: A healthy pregnancy
Clinicians have been phenomenally successful in screening for perinatal depression. The new USPSTF recommendation adds the prevention of perinatal depression to the goals of a healthy pregnancy. This recommendation builds upon the foundation of screening for acute illness (depression), pivoting to the public health perspective of disease prevention.
- American College of Obstetricians and Gynecologists. Screening for perinatal depression. ACOG Committee Opinion No 757. Obstet Gynecol. 2018;132:e208-e212.
- Grote NK, Bridge JA, Gavin AR, et al. A meta-analysis of depression during pregnancy and the risk of preterm birth, low birth weight and intrauterine growth restriction. Arch Gen Psychiatry. 2010;67:1012-1024.
- Pearlstein T, Howard M, Salisbury A, et al. Postpartum depression. Am J Obstet Gynecol. 2009;200:357-364.
- Avalos LA, Raine-Bennett T, Chen H, et al. Improved perinatal depression screening, treatment and outcomes with a universal obstetric program. Obstet Gynecol. 2016;127:917-925.
- Cox EQ, Sowa NA, Meltzer-Brody SE, et al. The perinatal depression treatment cascade: baby steps toward improving outcomes. J Clin Psychiatry. 2016;77:1189-1200.
- Byatt N, Simas TA, Lundquist RS, et al. Strategies for improving perinatal depression treatment in North American outpatient obstetric settings. J Psychosom Obstet Gynaecol. 2012;33:143-161.
- Siu AL, Bibbins-Domingo K, Grossman DC, et al. US Preventive Services Task Force (USPSTF). Screening for depression in adults. JAMA. 2016;315:380-387.
- Earls MF. Committee on Psychological Aspects of Child and Family Health. American Academy of Pediatrics. Incorporating recognition and management of perinatal and postpartum depression into pediatric practice. Pediatrics. 2010;126:1032-1039.
- The American College of Obstetricians and Gynecologists. Committee on Obstetric Practice. Committee Opinion No 630. Screening for perinatal depression. Obstet Gynecol. 2015;125:1268-1271.
- US Preventive Services Task Force. Interventions to prevent perinatal depression: US Preventive Services Task Force recommendations statement. JAMA. 2019;321:580-587.
- O’Connor E, Senger CA, Henninger ML, et al. Interventions to prevent perinatal depression: evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2019;321:588-601.
- Sockol LE. A systematic review and meta-analysis of interpersonal psychotherapy for perinatal women. J Affective Disorders. 2018;232:316-328.
- Sockol LE. A systematic review of the efficacy of cognitive behavioral therapy for treating and preventing perinatal depression. J Affective Disorders. 2015;177:7-21.
- Zlotnick C, Tzilos G, Miller I, et al. Randomized controlled trial to prevent postpartum depression in mothers on public assistance. J Affective Disorders. 2016;189:263-268.
- Haga SM, Drozd F, Lisoy C, et al. Mamma Mia—a randomized controlled trial of an internet-based intervention for perinatal depression. Psycholog Med. 2018;1-9.
- Shorey S, Ng YM, Ng ED, et al. Effectiveness of a technology-based supportive educational parenting program on parent outcomes (Part 1): Randomized controlled trial. J Med Internet Res. 2019;21:e10816.
- Cohen LS, Altshuler LL, Harlow BL, et al. Relapse of major depression during pregnancy in women who maintain or discontinue antidepressant treatment. JAMA. 2006;295:499-507.
- Goodman JH. Women’s attitudes, preferences and perceived barriers to treatment for perinatal depression. Birth. 2009;36:60-69.
- Smith-Nielsen J, Matthey S, Lange T, Vaever MS. Validation of the Edinburgh Postnatal Depression Scale against both DSM-5 and ICD-10 diagnostic criteria for depression. BMC Psychiatry. 2018;18:393.
- Judd F, Lorimer S, Thomson RH, et al. Screening for depression with the Edinburgh Postnatal Depression Scale and finding borderline personality disorder. Aust N Z J Psychiatry. 2018;Epub Oct 12. doi: 10.1177/0004867418804067.
- Diamond R. Promoting sensible parenting policies. Leading by example. JAMA. 2019;321:645- 646.
- American College of Obstetricians and Gynecologists. Screening for perinatal depression. ACOG Committee Opinion No 757. Obstet Gynecol. 2018;132:e208-e212.
- Grote NK, Bridge JA, Gavin AR, et al. A meta-analysis of depression during pregnancy and the risk of preterm birth, low birth weight and intrauterine growth restriction. Arch Gen Psychiatry. 2010;67:1012-1024.
- Pearlstein T, Howard M, Salisbury A, et al. Postpartum depression. Am J Obstet Gynecol. 2009;200:357-364.
- Avalos LA, Raine-Bennett T, Chen H, et al. Improved perinatal depression screening, treatment and outcomes with a universal obstetric program. Obstet Gynecol. 2016;127:917-925.
- Cox EQ, Sowa NA, Meltzer-Brody SE, et al. The perinatal depression treatment cascade: baby steps toward improving outcomes. J Clin Psychiatry. 2016;77:1189-1200.
- Byatt N, Simas TA, Lundquist RS, et al. Strategies for improving perinatal depression treatment in North American outpatient obstetric settings. J Psychosom Obstet Gynaecol. 2012;33:143-161.
- Siu AL, Bibbins-Domingo K, Grossman DC, et al. US Preventive Services Task Force (USPSTF). Screening for depression in adults. JAMA. 2016;315:380-387.
- Earls MF. Committee on Psychological Aspects of Child and Family Health. American Academy of Pediatrics. Incorporating recognition and management of perinatal and postpartum depression into pediatric practice. Pediatrics. 2010;126:1032-1039.
- The American College of Obstetricians and Gynecologists. Committee on Obstetric Practice. Committee Opinion No 630. Screening for perinatal depression. Obstet Gynecol. 2015;125:1268-1271.
- US Preventive Services Task Force. Interventions to prevent perinatal depression: US Preventive Services Task Force recommendations statement. JAMA. 2019;321:580-587.
- O’Connor E, Senger CA, Henninger ML, et al. Interventions to prevent perinatal depression: evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2019;321:588-601.
- Sockol LE. A systematic review and meta-analysis of interpersonal psychotherapy for perinatal women. J Affective Disorders. 2018;232:316-328.
- Sockol LE. A systematic review of the efficacy of cognitive behavioral therapy for treating and preventing perinatal depression. J Affective Disorders. 2015;177:7-21.
- Zlotnick C, Tzilos G, Miller I, et al. Randomized controlled trial to prevent postpartum depression in mothers on public assistance. J Affective Disorders. 2016;189:263-268.
- Haga SM, Drozd F, Lisoy C, et al. Mamma Mia—a randomized controlled trial of an internet-based intervention for perinatal depression. Psycholog Med. 2018;1-9.
- Shorey S, Ng YM, Ng ED, et al. Effectiveness of a technology-based supportive educational parenting program on parent outcomes (Part 1): Randomized controlled trial. J Med Internet Res. 2019;21:e10816.
- Cohen LS, Altshuler LL, Harlow BL, et al. Relapse of major depression during pregnancy in women who maintain or discontinue antidepressant treatment. JAMA. 2006;295:499-507.
- Goodman JH. Women’s attitudes, preferences and perceived barriers to treatment for perinatal depression. Birth. 2009;36:60-69.
- Smith-Nielsen J, Matthey S, Lange T, Vaever MS. Validation of the Edinburgh Postnatal Depression Scale against both DSM-5 and ICD-10 diagnostic criteria for depression. BMC Psychiatry. 2018;18:393.
- Judd F, Lorimer S, Thomson RH, et al. Screening for depression with the Edinburgh Postnatal Depression Scale and finding borderline personality disorder. Aust N Z J Psychiatry. 2018;Epub Oct 12. doi: 10.1177/0004867418804067.
- Diamond R. Promoting sensible parenting policies. Leading by example. JAMA. 2019;321:645- 646.
‘Difficult’ discussions reduced anxiety, depression in life-limiting cancer patients
A program to encourage difficult discussions between seriously ill patients and their oncologists reduced anxiety and depression in a recent randomized trial, but its impact on patient-centered outcomes were uncertain.
Goal-concordant care and peacefulness at the end of life, the coprimary study outcomes, were not significantly different between patients who received the quality improvement intervention and controls in the study, which included 91 clinicians providing care for 278 patients with advanced cancer.
However, it’s not clear whether the intervention, known as the Serious Illness Care Program (SICP), failed to improve those outcomes, or if there simply weren’t enough patients in the trial to detect a meaningful difference, according to investigators led by Rachelle Bernacki, MD, of Brigham and Women’s Hospital and the Harvard School of Public Health, Boston.
“Our challenges reflect the need in our field for patient-centered measures of communication that are agreed upon, validated, and demonstrably sensitive to communication interventions,” wrote Dr. Bernacki and her coinvestigators in a report on the study published in JAMA Internal Medicine.
However, the SICP intervention did clearly result in a larger number of serious-illness conversations that occurred earlier and were of higher quality, the investigators wrote in a separate report published in JAMA Oncology. In medical records reviewed after the patients’ deaths, 96% of those who received the intervention had a documented serious-illness conversation with their oncology clinician, compared with 79% of controls (P = .005), according to that report.
The conversations among SICP recipients occurred a median of 2.4 months earlier than controls, and had a greater focus on values and goals, prognosis and understanding of illness, and treatment preferences.
These outcomes are reassuring, since patients “want, require, and deserve” conversations about serious illness, regardless of their impact on measurable outcomes, the authors of an editorial published in JAMA Oncology wrote.
The SICP intervention included a communication guide for clinicians, who also participated in a 2.5-hour training session designed to improve their serious-illness conversation skills. Other aspects of the program for clinicians included email reminders before outpatient visits, a specialized EMR template, and personal coaching. The program also included patient tools, including a letter introducing the intervention and a guide for continuing the conversation with their family.
The study did not demonstrate a significant difference in peacefulness, as measured by the validated Peace, Equanimity, and Acceptance in the Cancer Experience questionnaire, or in goal-concordant care, which was measured by asking patients to select goals of importance, and then asking caregivers to rate whether those goals had been met at the end of life.
However, patients in the SICP group reported less anxiety and depression 14 weeks into the trial, according to the investigators. The proportion of patients reporting moderate to severe anxiety at that time point was 10.2% for the intervention group versus 5.0% for controls (P = .05), while the proportion reporting depression symptoms was 20.8% for the intervention versus 10.6% for controls (P = .04).
The anxiety reduction was maintained at 24 weeks, though the depression reduction was not, the investigators wrote, adding that there were no differences in survival between arms.
Taken together, these results suggest that oncology clinicians can discuss difficult topics without causing harm, and with potential benefit, the investigators wrote in a discussion of their results.
“Further development of serious illness communication interventions will require more reliable and well-accepted patient-centered outcome measures and additional testing of the effect on patients throughout their illness trajectory,” they concluded.
Dr. Bernacki reported no disclosures. Coauthor Susan D. Block, MD, reported compensation from Up to Date and Atul A. Gawande, MD, MPH, reported receiving compensation from health care writing and media and is employed by a health care venture formed by Amazon, Berkshire Hathaway, and JPMorgan Chase.
SOURCE: Bernacki R et al. JAMA Intern Med. 2018 Mar 14. doi: 10.1001/jamainternmed.2019.0077.
While results of this rigorous and innovative clinical trial are disappointing because of an apparent lack of impact on the primary outcomes of care, oncologists still must initiate serious illness conversations with advanced cancer patients at risk of dying in the foreseeable future, according to the authors of an editorial.
Doing so is important “not because this will necessarily improve outcomes, but because patients want, require, and deserve to know what is coming,” wrote Belinda E. Kiely, MBBS, PhD, FRACP, and Martin R. Stockler, MBBS, MSc, FRACP.
Those difficult conversations should not stop at discussing the limits of care, but should include a discussion of the patient’s preferences, priorities, and values, Dr. Kiely and Dr. Stockler wrote, adding that they should be documented in the EMR to ensure they are accessible to other health care providers.
“If nothing else, oncologists should be reassured that having these conversations is unlikely to increase anxiety or depression in their patients,” wrote the editorial authors, referencing the significantly reduced incidence of those secondary endpoints in the study.
However, conversations alone may not be enough to improve other patient-centered outcomes, based on the inability of this trial to demonstrate significant improvements in goal-centered care or peacefulness at the end of life.
Moreover, building this Serious Illness Care Program intervention into a health system could be complicated and may require significant resources.
“Simple, pragmatic, and effective tactics are needed to ensure greater generalizability and widespread applicability of such programs,” the authors concluded.
Dr. Kiely and Dr. Stockler are with the National Health and Medical Research Council Clinical Trials Centre at the University of Sydney. Their editorial appears in JAMA Oncology. Dr. Stockler reported grants outside the submitted work from Astellas, Amgen, AstraZeneca, Cancer Australia, Celgene, Bionomics, Bayer, Medivation, Merck, National Health and Medical Research Council Australia, Pfizer, Roche, Sanofi, and Tilray.
While results of this rigorous and innovative clinical trial are disappointing because of an apparent lack of impact on the primary outcomes of care, oncologists still must initiate serious illness conversations with advanced cancer patients at risk of dying in the foreseeable future, according to the authors of an editorial.
Doing so is important “not because this will necessarily improve outcomes, but because patients want, require, and deserve to know what is coming,” wrote Belinda E. Kiely, MBBS, PhD, FRACP, and Martin R. Stockler, MBBS, MSc, FRACP.
Those difficult conversations should not stop at discussing the limits of care, but should include a discussion of the patient’s preferences, priorities, and values, Dr. Kiely and Dr. Stockler wrote, adding that they should be documented in the EMR to ensure they are accessible to other health care providers.
“If nothing else, oncologists should be reassured that having these conversations is unlikely to increase anxiety or depression in their patients,” wrote the editorial authors, referencing the significantly reduced incidence of those secondary endpoints in the study.
However, conversations alone may not be enough to improve other patient-centered outcomes, based on the inability of this trial to demonstrate significant improvements in goal-centered care or peacefulness at the end of life.
Moreover, building this Serious Illness Care Program intervention into a health system could be complicated and may require significant resources.
“Simple, pragmatic, and effective tactics are needed to ensure greater generalizability and widespread applicability of such programs,” the authors concluded.
Dr. Kiely and Dr. Stockler are with the National Health and Medical Research Council Clinical Trials Centre at the University of Sydney. Their editorial appears in JAMA Oncology. Dr. Stockler reported grants outside the submitted work from Astellas, Amgen, AstraZeneca, Cancer Australia, Celgene, Bionomics, Bayer, Medivation, Merck, National Health and Medical Research Council Australia, Pfizer, Roche, Sanofi, and Tilray.
While results of this rigorous and innovative clinical trial are disappointing because of an apparent lack of impact on the primary outcomes of care, oncologists still must initiate serious illness conversations with advanced cancer patients at risk of dying in the foreseeable future, according to the authors of an editorial.
Doing so is important “not because this will necessarily improve outcomes, but because patients want, require, and deserve to know what is coming,” wrote Belinda E. Kiely, MBBS, PhD, FRACP, and Martin R. Stockler, MBBS, MSc, FRACP.
Those difficult conversations should not stop at discussing the limits of care, but should include a discussion of the patient’s preferences, priorities, and values, Dr. Kiely and Dr. Stockler wrote, adding that they should be documented in the EMR to ensure they are accessible to other health care providers.
“If nothing else, oncologists should be reassured that having these conversations is unlikely to increase anxiety or depression in their patients,” wrote the editorial authors, referencing the significantly reduced incidence of those secondary endpoints in the study.
However, conversations alone may not be enough to improve other patient-centered outcomes, based on the inability of this trial to demonstrate significant improvements in goal-centered care or peacefulness at the end of life.
Moreover, building this Serious Illness Care Program intervention into a health system could be complicated and may require significant resources.
“Simple, pragmatic, and effective tactics are needed to ensure greater generalizability and widespread applicability of such programs,” the authors concluded.
Dr. Kiely and Dr. Stockler are with the National Health and Medical Research Council Clinical Trials Centre at the University of Sydney. Their editorial appears in JAMA Oncology. Dr. Stockler reported grants outside the submitted work from Astellas, Amgen, AstraZeneca, Cancer Australia, Celgene, Bionomics, Bayer, Medivation, Merck, National Health and Medical Research Council Australia, Pfizer, Roche, Sanofi, and Tilray.
A program to encourage difficult discussions between seriously ill patients and their oncologists reduced anxiety and depression in a recent randomized trial, but its impact on patient-centered outcomes were uncertain.
Goal-concordant care and peacefulness at the end of life, the coprimary study outcomes, were not significantly different between patients who received the quality improvement intervention and controls in the study, which included 91 clinicians providing care for 278 patients with advanced cancer.
However, it’s not clear whether the intervention, known as the Serious Illness Care Program (SICP), failed to improve those outcomes, or if there simply weren’t enough patients in the trial to detect a meaningful difference, according to investigators led by Rachelle Bernacki, MD, of Brigham and Women’s Hospital and the Harvard School of Public Health, Boston.
“Our challenges reflect the need in our field for patient-centered measures of communication that are agreed upon, validated, and demonstrably sensitive to communication interventions,” wrote Dr. Bernacki and her coinvestigators in a report on the study published in JAMA Internal Medicine.
However, the SICP intervention did clearly result in a larger number of serious-illness conversations that occurred earlier and were of higher quality, the investigators wrote in a separate report published in JAMA Oncology. In medical records reviewed after the patients’ deaths, 96% of those who received the intervention had a documented serious-illness conversation with their oncology clinician, compared with 79% of controls (P = .005), according to that report.
The conversations among SICP recipients occurred a median of 2.4 months earlier than controls, and had a greater focus on values and goals, prognosis and understanding of illness, and treatment preferences.
These outcomes are reassuring, since patients “want, require, and deserve” conversations about serious illness, regardless of their impact on measurable outcomes, the authors of an editorial published in JAMA Oncology wrote.
The SICP intervention included a communication guide for clinicians, who also participated in a 2.5-hour training session designed to improve their serious-illness conversation skills. Other aspects of the program for clinicians included email reminders before outpatient visits, a specialized EMR template, and personal coaching. The program also included patient tools, including a letter introducing the intervention and a guide for continuing the conversation with their family.
The study did not demonstrate a significant difference in peacefulness, as measured by the validated Peace, Equanimity, and Acceptance in the Cancer Experience questionnaire, or in goal-concordant care, which was measured by asking patients to select goals of importance, and then asking caregivers to rate whether those goals had been met at the end of life.
However, patients in the SICP group reported less anxiety and depression 14 weeks into the trial, according to the investigators. The proportion of patients reporting moderate to severe anxiety at that time point was 10.2% for the intervention group versus 5.0% for controls (P = .05), while the proportion reporting depression symptoms was 20.8% for the intervention versus 10.6% for controls (P = .04).
The anxiety reduction was maintained at 24 weeks, though the depression reduction was not, the investigators wrote, adding that there were no differences in survival between arms.
Taken together, these results suggest that oncology clinicians can discuss difficult topics without causing harm, and with potential benefit, the investigators wrote in a discussion of their results.
“Further development of serious illness communication interventions will require more reliable and well-accepted patient-centered outcome measures and additional testing of the effect on patients throughout their illness trajectory,” they concluded.
Dr. Bernacki reported no disclosures. Coauthor Susan D. Block, MD, reported compensation from Up to Date and Atul A. Gawande, MD, MPH, reported receiving compensation from health care writing and media and is employed by a health care venture formed by Amazon, Berkshire Hathaway, and JPMorgan Chase.
SOURCE: Bernacki R et al. JAMA Intern Med. 2018 Mar 14. doi: 10.1001/jamainternmed.2019.0077.
A program to encourage difficult discussions between seriously ill patients and their oncologists reduced anxiety and depression in a recent randomized trial, but its impact on patient-centered outcomes were uncertain.
Goal-concordant care and peacefulness at the end of life, the coprimary study outcomes, were not significantly different between patients who received the quality improvement intervention and controls in the study, which included 91 clinicians providing care for 278 patients with advanced cancer.
However, it’s not clear whether the intervention, known as the Serious Illness Care Program (SICP), failed to improve those outcomes, or if there simply weren’t enough patients in the trial to detect a meaningful difference, according to investigators led by Rachelle Bernacki, MD, of Brigham and Women’s Hospital and the Harvard School of Public Health, Boston.
“Our challenges reflect the need in our field for patient-centered measures of communication that are agreed upon, validated, and demonstrably sensitive to communication interventions,” wrote Dr. Bernacki and her coinvestigators in a report on the study published in JAMA Internal Medicine.
However, the SICP intervention did clearly result in a larger number of serious-illness conversations that occurred earlier and were of higher quality, the investigators wrote in a separate report published in JAMA Oncology. In medical records reviewed after the patients’ deaths, 96% of those who received the intervention had a documented serious-illness conversation with their oncology clinician, compared with 79% of controls (P = .005), according to that report.
The conversations among SICP recipients occurred a median of 2.4 months earlier than controls, and had a greater focus on values and goals, prognosis and understanding of illness, and treatment preferences.
These outcomes are reassuring, since patients “want, require, and deserve” conversations about serious illness, regardless of their impact on measurable outcomes, the authors of an editorial published in JAMA Oncology wrote.
The SICP intervention included a communication guide for clinicians, who also participated in a 2.5-hour training session designed to improve their serious-illness conversation skills. Other aspects of the program for clinicians included email reminders before outpatient visits, a specialized EMR template, and personal coaching. The program also included patient tools, including a letter introducing the intervention and a guide for continuing the conversation with their family.
The study did not demonstrate a significant difference in peacefulness, as measured by the validated Peace, Equanimity, and Acceptance in the Cancer Experience questionnaire, or in goal-concordant care, which was measured by asking patients to select goals of importance, and then asking caregivers to rate whether those goals had been met at the end of life.
However, patients in the SICP group reported less anxiety and depression 14 weeks into the trial, according to the investigators. The proportion of patients reporting moderate to severe anxiety at that time point was 10.2% for the intervention group versus 5.0% for controls (P = .05), while the proportion reporting depression symptoms was 20.8% for the intervention versus 10.6% for controls (P = .04).
The anxiety reduction was maintained at 24 weeks, though the depression reduction was not, the investigators wrote, adding that there were no differences in survival between arms.
Taken together, these results suggest that oncology clinicians can discuss difficult topics without causing harm, and with potential benefit, the investigators wrote in a discussion of their results.
“Further development of serious illness communication interventions will require more reliable and well-accepted patient-centered outcome measures and additional testing of the effect on patients throughout their illness trajectory,” they concluded.
Dr. Bernacki reported no disclosures. Coauthor Susan D. Block, MD, reported compensation from Up to Date and Atul A. Gawande, MD, MPH, reported receiving compensation from health care writing and media and is employed by a health care venture formed by Amazon, Berkshire Hathaway, and JPMorgan Chase.
SOURCE: Bernacki R et al. JAMA Intern Med. 2018 Mar 14. doi: 10.1001/jamainternmed.2019.0077.
FROM JAMA INTERNAL MEDICINE
Getting that Dx right, keeping patients safe
Even the best physicians make mistakes.
Each of us can recall a patient for whom our initial diagnosis was incorrect, or conversely, where we uncovered the correct diagnosis. My memorable mistake was missing an obvious case of hypothyroidism, and my happier encounter was correcting an incorrect diagnosis of asthma when the patient actually had primary pulmonary fibrosis. These patients came to mind as I read this month’s cover story, “COPD and asthma: Diagnostic accuracy requires spirometry.”
In a previous editorial, “When our biases derail the diagnosis,”1 I discussed types of cognitive bias that can lead us to the wrong conclusion. Today, I want to address diagnostic errors in medicine as a patient safety issue.
The patient safety movement gained traction in 1999 with the publication of the Institute of Medicine (IOM, now National Academy of Medicine) report, To Err is Human: Building a Safer Health System. That report focused on health care system issues and had little to offer regarding improving diagnoses. It was not until 2015, with the publication of the IOM report Improving Diagnosis in Health Care, that serious national attention was directed to accurate diagnosis. It is worth reading the summary of this report, which includes 8 goals and is available at nas.edu/improvingdiagnosis.
The recommendations most pertinent to family physicians are to: 1) facilitate more effective teamwork among health care professionals, patients, and families, 2) teach health care professionals about the diagnostic process, 3) ensure that technology supports proper diagnosis, 4) establish a work culture that supports diagnostic processes, and 5) identify diagnostic errors and learn from them.
Teamwork. The first recommendation is intriguing because the focus on teamwork includes patients and their families. I have found that listening closely to the patient’s and family’s concerns can lead me in a direction other than my initial impression—especially when they are insistent about a particular diagnosis.
Technology. Despite all of their “warts,” electronic health records are gradually incorporating clinical decision support tools that really do help steer us in the right direction. I have found that electronic point-of-care references can be very helpful in establishing an accurate diagnosis in the exam room—and can help convince patients that my diagnosis is correct when they read it for themselves!
Continue to: Finally, discussing our mistakes...
Finally, discussing our mistakes openly with our colleagues helps us—and them—to avoid that mistake in the future. Let’s be sure to keep that dialogue open. And let’s continue to refine our diagnostic skills so that we can continue to keep our patients safe.
1. Hickner J. When our biases derail the diagnosis. J Fam Pract. 2018;67:334.
Even the best physicians make mistakes.
Each of us can recall a patient for whom our initial diagnosis was incorrect, or conversely, where we uncovered the correct diagnosis. My memorable mistake was missing an obvious case of hypothyroidism, and my happier encounter was correcting an incorrect diagnosis of asthma when the patient actually had primary pulmonary fibrosis. These patients came to mind as I read this month’s cover story, “COPD and asthma: Diagnostic accuracy requires spirometry.”
In a previous editorial, “When our biases derail the diagnosis,”1 I discussed types of cognitive bias that can lead us to the wrong conclusion. Today, I want to address diagnostic errors in medicine as a patient safety issue.
The patient safety movement gained traction in 1999 with the publication of the Institute of Medicine (IOM, now National Academy of Medicine) report, To Err is Human: Building a Safer Health System. That report focused on health care system issues and had little to offer regarding improving diagnoses. It was not until 2015, with the publication of the IOM report Improving Diagnosis in Health Care, that serious national attention was directed to accurate diagnosis. It is worth reading the summary of this report, which includes 8 goals and is available at nas.edu/improvingdiagnosis.
The recommendations most pertinent to family physicians are to: 1) facilitate more effective teamwork among health care professionals, patients, and families, 2) teach health care professionals about the diagnostic process, 3) ensure that technology supports proper diagnosis, 4) establish a work culture that supports diagnostic processes, and 5) identify diagnostic errors and learn from them.
Teamwork. The first recommendation is intriguing because the focus on teamwork includes patients and their families. I have found that listening closely to the patient’s and family’s concerns can lead me in a direction other than my initial impression—especially when they are insistent about a particular diagnosis.
Technology. Despite all of their “warts,” electronic health records are gradually incorporating clinical decision support tools that really do help steer us in the right direction. I have found that electronic point-of-care references can be very helpful in establishing an accurate diagnosis in the exam room—and can help convince patients that my diagnosis is correct when they read it for themselves!
Continue to: Finally, discussing our mistakes...
Finally, discussing our mistakes openly with our colleagues helps us—and them—to avoid that mistake in the future. Let’s be sure to keep that dialogue open. And let’s continue to refine our diagnostic skills so that we can continue to keep our patients safe.
Even the best physicians make mistakes.
Each of us can recall a patient for whom our initial diagnosis was incorrect, or conversely, where we uncovered the correct diagnosis. My memorable mistake was missing an obvious case of hypothyroidism, and my happier encounter was correcting an incorrect diagnosis of asthma when the patient actually had primary pulmonary fibrosis. These patients came to mind as I read this month’s cover story, “COPD and asthma: Diagnostic accuracy requires spirometry.”
In a previous editorial, “When our biases derail the diagnosis,”1 I discussed types of cognitive bias that can lead us to the wrong conclusion. Today, I want to address diagnostic errors in medicine as a patient safety issue.
The patient safety movement gained traction in 1999 with the publication of the Institute of Medicine (IOM, now National Academy of Medicine) report, To Err is Human: Building a Safer Health System. That report focused on health care system issues and had little to offer regarding improving diagnoses. It was not until 2015, with the publication of the IOM report Improving Diagnosis in Health Care, that serious national attention was directed to accurate diagnosis. It is worth reading the summary of this report, which includes 8 goals and is available at nas.edu/improvingdiagnosis.
The recommendations most pertinent to family physicians are to: 1) facilitate more effective teamwork among health care professionals, patients, and families, 2) teach health care professionals about the diagnostic process, 3) ensure that technology supports proper diagnosis, 4) establish a work culture that supports diagnostic processes, and 5) identify diagnostic errors and learn from them.
Teamwork. The first recommendation is intriguing because the focus on teamwork includes patients and their families. I have found that listening closely to the patient’s and family’s concerns can lead me in a direction other than my initial impression—especially when they are insistent about a particular diagnosis.
Technology. Despite all of their “warts,” electronic health records are gradually incorporating clinical decision support tools that really do help steer us in the right direction. I have found that electronic point-of-care references can be very helpful in establishing an accurate diagnosis in the exam room—and can help convince patients that my diagnosis is correct when they read it for themselves!
Continue to: Finally, discussing our mistakes...
Finally, discussing our mistakes openly with our colleagues helps us—and them—to avoid that mistake in the future. Let’s be sure to keep that dialogue open. And let’s continue to refine our diagnostic skills so that we can continue to keep our patients safe.
1. Hickner J. When our biases derail the diagnosis. J Fam Pract. 2018;67:334.
1. Hickner J. When our biases derail the diagnosis. J Fam Pract. 2018;67:334.
A look at new guidelines for HIV treatment and prevention
An International Antiviral Society-USA Panel recently published an updated set of recommendations on using antiviral drugs to treat and prevent human immunodeficiency virus (HIV) infection1—a rapidly changing and complex topic. This new guideline updates the society’s 2016 publication.2 It contains recommendations on when to start antiretroviral therapy for those who are HIV positive and advice on suitable combinations of antiretroviral drugs. It also details pre- and post-exposure prophylaxis strategies for preventing HIV infection in those at risk.
This Practice Alert highlights the most important recommendations on treating those newly diagnosed as HIV positive and on preventing infection. Physicians who provide care for those who are HIV positive should familiarize themselves with the entire guideline.
Initiating treatment in those newly diagnosed as HIV positive
The panel now recommends starting antiretroviral therapy (ART) as soon as possible after HIV infection is confirmed; immediately if a patient is ready to commit to starting and continuing treatment. Any patient with an opportunistic infection should begin ART within 2 weeks of its diagnosis. Patients being treated for tuberculosis (TB) should begin ART within 2 weeks of starting TB treatment if their CD4 cell count is <50/mcL; those whose count is ≥50/mcL should begin ART within 2 to 8 weeks.
The panel recommends one of 3 ART combinations (TABLE 11), all of which contain an integrase strand transfer inhibitor (INSTI). ART started immediately should not include a nonnucleoside reverse transcriptase inhibitor (NNRTI) because of possible viral resistance. The guideline recommends 6 other ART combinations if none of the first 3 options can be used.1
An initial set of laboratory tests (TABLE 21) should be conducted on each individual receiving ART, although treatment can start before the results are returned. Ongoing laboratory monitoring, described in detail in the guideline, depends on the ART regimen chosen and the patient’s response to therapy. The only routinely recommended prophylaxis for opportunistic infections is for Pneumocystis pneumonia if the CD4 count is <200/mcL.
Preventing HIV with prEP
Consider prescribing daily pre-exposure prophylaxis (PrEP) with emtricitabine/tenofovir disoproxil fumarate (Truvada) for men and women who are at risk from sexual exposure to HIV or who inject illicit drugs. It takes about 1 week for protective tissue levels to be achieved. Testing to rule out HIV infection is recommended before starting PrEP, as is testing for serum creatinine level, estimated glomerular filtration rate, and hepatitis B surface antigen. Tenofovir disoproxil fumarate is not recommended for those with creatinine clearance of less than 60 mL/min/1.73 m2. For patients taking PrEP, emphasize other preventive measures such as using condoms to protect against both HIV and other sexually-transmitted diseases (STDs), using clean needles and syringes when injecting drugs, or entering a drug rehabilitation program. After initiating PrEP, schedule the first follow-up visit for 30 days later to repeat the HIV test and to assess adverse reactions and PrEP adherence.
For men who have sex with men (MSM), there is an alternative form of PrEP when sexual exposure is infrequent. “On-demand” or “event-driven” PrEP involves 4 doses of emtricitabine/tenofovir disoproxil fumarate; 2 doses given with food 2 to 24 hours before sex (the closer to 24 the better), one dose 24 hours after the first and one 24 hours after the second. This is referred to as 2-1-1 dosing. This option has only been tested in MSM with sexual exposure. It is not recommended at this time for others at risk for HIV or for MSM with chronic or active hepatitis B infection.
Continue to: Preventing HIV infection with post-exposure prophylaxis
Preventing HIV infection with post-exposure prophylaxis
Post-exposure prophylaxis (PEP) for HIV infection is divided into 2 categories: occupational PEP (oPEP) and non-occupational PEP (nPEP). Recommendations for oPEP are described elsewhere3 and are not covered in this Practice Alert. Summarized below are the recommendations for nPEP after sex, injection drug use, and other nonoccupational exposures, which are also described on the Centers for Disease Control and Prevention (CDC) Web site.4
Assess the need for nPEP if high-risk exposure (TABLE 34) occurred ≤72 hours earlier. Before starting nPEP, perform a rapid HIV blood test. If rapid testing is unavailable, start nPEP, which can be discontinued if the patient is later determined to have HIV infection. Repeat HIV testing at 4 to 6 weeks and 3 months following initiation of nPEP. Approved HIV tests are described on the CDC Web site at http://www.cdc.gov/hiv/testing/laboratorytests.html. Oral HIV tests are not recommended for HIV testing before initiating nPEP.
nPEP is not recommended when an individual’s risk of exposure to HIV is not high, or if the exposure occurred more than 72 hours before presentation. An algorithm is available to assist with assessing whether nPEP is recommended (FIGURE4).
Specific nPEP regimens. For otherwise healthy adults and adolescents, preferred nPEP consists of a 28-day course of a 3-drug combination: tenofovir disoproxil fumarate 300 mg once daily; emtricitabine 200 mg once daily; and raltegravir, 400 mg twice daily, or dolutegravir 50 mg once daily. Alternative regimens for adults and adolescents are described in the guideline, as are options for children, those with decreased renal function, and pregnant women. Those who receive more than one course of nPEP within a 12-month period should consider PrEP.
When additional vaccination is needed. For victims of sexual assault, offer prophylaxis against STD (TABLE 44) and hepatitis B virus (HBV). Those who have not been vaccinated against HBV should receive the first dose at the initial visit. If the exposure source is known to be HBsAg-positive, give the unvaccinated patient both hepatitis B vaccine and hepatitis B immune globulin at the first visit. The full hepatitis B vaccine series should then be completed according to the recommended schedule and the vaccine product used. Those who have completed hepatitis B vaccination but who were not tested with a post-vaccine titer should receive a single dose of hepatitis B vaccine.
Continue to: Victims of sexual assault...
Victims of sexual assault can benefit from referral to professionals with expertise in post-assault counseling. Sexual Assault Nurse Examiner programs are listed at http://www.sane-sart.com.
Financial assistance for patients. Anti-retroviral drugs are expensive, and those who need nPEP may not have a payer source. Many pharmaceutical manufacturers offer medication assistance programs, and processes are set up to handle time-sensitive requests. Information for specific medications can be found at http://www.pparx.org/en/prescription_assistance_programs/list_of_participating_programs. Those who are prescribed nPEP after a sexual assault can receive reimbursement for medications and health care costs through state Crime Victim Compensation Programs funded by the Department of Justice. State-specific contact information is available at http://www.nacvcb.org/index.asp?sid=6.
1. Saag MS, Benson CA, Gandhi RT, et al. Antiretroviral drugs for treatment and prevention of HIV infection in adults: 2018 recommendations of the International Antiviral Society-USA Panel. JAMA. 2018;320:379-396.
2. Günthard HF, Saag MS, Benson CA, et al. Antiretroviral drugs for treatment and prevention of HIV infection in adults: 2016 recommendations of the International Antiviral Society-USA Panel. JAMA. 2016;316:191-210.
3. Kuhar DT, Henderson DK, Struble KA, et al; US Public Health Service Working Group. Updated US Public Health Service guidelines for the management of occupational exposures to human immunodeficiency virus and recommendations for postexposure prophylaxis. Infect Control Hosp Epidemiol. 2013;34:875-892.
4. CDC. Updated guidelines for antiretroviral postexposure prophylaxis after sexual, injection drug use, or other nonoccupational exposure to HIV—United States, 2016. https://www-cdc-gov.ezproxy3.library.arizona.edu/hiv/pdf/programresources/cdc-hiv-npep-guidelines.pdf. Accessed October 11, 2018.
An International Antiviral Society-USA Panel recently published an updated set of recommendations on using antiviral drugs to treat and prevent human immunodeficiency virus (HIV) infection1—a rapidly changing and complex topic. This new guideline updates the society’s 2016 publication.2 It contains recommendations on when to start antiretroviral therapy for those who are HIV positive and advice on suitable combinations of antiretroviral drugs. It also details pre- and post-exposure prophylaxis strategies for preventing HIV infection in those at risk.
This Practice Alert highlights the most important recommendations on treating those newly diagnosed as HIV positive and on preventing infection. Physicians who provide care for those who are HIV positive should familiarize themselves with the entire guideline.
Initiating treatment in those newly diagnosed as HIV positive
The panel now recommends starting antiretroviral therapy (ART) as soon as possible after HIV infection is confirmed; immediately if a patient is ready to commit to starting and continuing treatment. Any patient with an opportunistic infection should begin ART within 2 weeks of its diagnosis. Patients being treated for tuberculosis (TB) should begin ART within 2 weeks of starting TB treatment if their CD4 cell count is <50/mcL; those whose count is ≥50/mcL should begin ART within 2 to 8 weeks.
The panel recommends one of 3 ART combinations (TABLE 11), all of which contain an integrase strand transfer inhibitor (INSTI). ART started immediately should not include a nonnucleoside reverse transcriptase inhibitor (NNRTI) because of possible viral resistance. The guideline recommends 6 other ART combinations if none of the first 3 options can be used.1
An initial set of laboratory tests (TABLE 21) should be conducted on each individual receiving ART, although treatment can start before the results are returned. Ongoing laboratory monitoring, described in detail in the guideline, depends on the ART regimen chosen and the patient’s response to therapy. The only routinely recommended prophylaxis for opportunistic infections is for Pneumocystis pneumonia if the CD4 count is <200/mcL.
Preventing HIV with prEP
Consider prescribing daily pre-exposure prophylaxis (PrEP) with emtricitabine/tenofovir disoproxil fumarate (Truvada) for men and women who are at risk from sexual exposure to HIV or who inject illicit drugs. It takes about 1 week for protective tissue levels to be achieved. Testing to rule out HIV infection is recommended before starting PrEP, as is testing for serum creatinine level, estimated glomerular filtration rate, and hepatitis B surface antigen. Tenofovir disoproxil fumarate is not recommended for those with creatinine clearance of less than 60 mL/min/1.73 m2. For patients taking PrEP, emphasize other preventive measures such as using condoms to protect against both HIV and other sexually-transmitted diseases (STDs), using clean needles and syringes when injecting drugs, or entering a drug rehabilitation program. After initiating PrEP, schedule the first follow-up visit for 30 days later to repeat the HIV test and to assess adverse reactions and PrEP adherence.
For men who have sex with men (MSM), there is an alternative form of PrEP when sexual exposure is infrequent. “On-demand” or “event-driven” PrEP involves 4 doses of emtricitabine/tenofovir disoproxil fumarate; 2 doses given with food 2 to 24 hours before sex (the closer to 24 the better), one dose 24 hours after the first and one 24 hours after the second. This is referred to as 2-1-1 dosing. This option has only been tested in MSM with sexual exposure. It is not recommended at this time for others at risk for HIV or for MSM with chronic or active hepatitis B infection.
Continue to: Preventing HIV infection with post-exposure prophylaxis
Preventing HIV infection with post-exposure prophylaxis
Post-exposure prophylaxis (PEP) for HIV infection is divided into 2 categories: occupational PEP (oPEP) and non-occupational PEP (nPEP). Recommendations for oPEP are described elsewhere3 and are not covered in this Practice Alert. Summarized below are the recommendations for nPEP after sex, injection drug use, and other nonoccupational exposures, which are also described on the Centers for Disease Control and Prevention (CDC) Web site.4
Assess the need for nPEP if high-risk exposure (TABLE 34) occurred ≤72 hours earlier. Before starting nPEP, perform a rapid HIV blood test. If rapid testing is unavailable, start nPEP, which can be discontinued if the patient is later determined to have HIV infection. Repeat HIV testing at 4 to 6 weeks and 3 months following initiation of nPEP. Approved HIV tests are described on the CDC Web site at http://www.cdc.gov/hiv/testing/laboratorytests.html. Oral HIV tests are not recommended for HIV testing before initiating nPEP.
nPEP is not recommended when an individual’s risk of exposure to HIV is not high, or if the exposure occurred more than 72 hours before presentation. An algorithm is available to assist with assessing whether nPEP is recommended (FIGURE4).
Specific nPEP regimens. For otherwise healthy adults and adolescents, preferred nPEP consists of a 28-day course of a 3-drug combination: tenofovir disoproxil fumarate 300 mg once daily; emtricitabine 200 mg once daily; and raltegravir, 400 mg twice daily, or dolutegravir 50 mg once daily. Alternative regimens for adults and adolescents are described in the guideline, as are options for children, those with decreased renal function, and pregnant women. Those who receive more than one course of nPEP within a 12-month period should consider PrEP.
When additional vaccination is needed. For victims of sexual assault, offer prophylaxis against STD (TABLE 44) and hepatitis B virus (HBV). Those who have not been vaccinated against HBV should receive the first dose at the initial visit. If the exposure source is known to be HBsAg-positive, give the unvaccinated patient both hepatitis B vaccine and hepatitis B immune globulin at the first visit. The full hepatitis B vaccine series should then be completed according to the recommended schedule and the vaccine product used. Those who have completed hepatitis B vaccination but who were not tested with a post-vaccine titer should receive a single dose of hepatitis B vaccine.
Continue to: Victims of sexual assault...
Victims of sexual assault can benefit from referral to professionals with expertise in post-assault counseling. Sexual Assault Nurse Examiner programs are listed at http://www.sane-sart.com.
Financial assistance for patients. Anti-retroviral drugs are expensive, and those who need nPEP may not have a payer source. Many pharmaceutical manufacturers offer medication assistance programs, and processes are set up to handle time-sensitive requests. Information for specific medications can be found at http://www.pparx.org/en/prescription_assistance_programs/list_of_participating_programs. Those who are prescribed nPEP after a sexual assault can receive reimbursement for medications and health care costs through state Crime Victim Compensation Programs funded by the Department of Justice. State-specific contact information is available at http://www.nacvcb.org/index.asp?sid=6.
An International Antiviral Society-USA Panel recently published an updated set of recommendations on using antiviral drugs to treat and prevent human immunodeficiency virus (HIV) infection1—a rapidly changing and complex topic. This new guideline updates the society’s 2016 publication.2 It contains recommendations on when to start antiretroviral therapy for those who are HIV positive and advice on suitable combinations of antiretroviral drugs. It also details pre- and post-exposure prophylaxis strategies for preventing HIV infection in those at risk.
This Practice Alert highlights the most important recommendations on treating those newly diagnosed as HIV positive and on preventing infection. Physicians who provide care for those who are HIV positive should familiarize themselves with the entire guideline.
Initiating treatment in those newly diagnosed as HIV positive
The panel now recommends starting antiretroviral therapy (ART) as soon as possible after HIV infection is confirmed; immediately if a patient is ready to commit to starting and continuing treatment. Any patient with an opportunistic infection should begin ART within 2 weeks of its diagnosis. Patients being treated for tuberculosis (TB) should begin ART within 2 weeks of starting TB treatment if their CD4 cell count is <50/mcL; those whose count is ≥50/mcL should begin ART within 2 to 8 weeks.
The panel recommends one of 3 ART combinations (TABLE 11), all of which contain an integrase strand transfer inhibitor (INSTI). ART started immediately should not include a nonnucleoside reverse transcriptase inhibitor (NNRTI) because of possible viral resistance. The guideline recommends 6 other ART combinations if none of the first 3 options can be used.1
An initial set of laboratory tests (TABLE 21) should be conducted on each individual receiving ART, although treatment can start before the results are returned. Ongoing laboratory monitoring, described in detail in the guideline, depends on the ART regimen chosen and the patient’s response to therapy. The only routinely recommended prophylaxis for opportunistic infections is for Pneumocystis pneumonia if the CD4 count is <200/mcL.
Preventing HIV with prEP
Consider prescribing daily pre-exposure prophylaxis (PrEP) with emtricitabine/tenofovir disoproxil fumarate (Truvada) for men and women who are at risk from sexual exposure to HIV or who inject illicit drugs. It takes about 1 week for protective tissue levels to be achieved. Testing to rule out HIV infection is recommended before starting PrEP, as is testing for serum creatinine level, estimated glomerular filtration rate, and hepatitis B surface antigen. Tenofovir disoproxil fumarate is not recommended for those with creatinine clearance of less than 60 mL/min/1.73 m2. For patients taking PrEP, emphasize other preventive measures such as using condoms to protect against both HIV and other sexually-transmitted diseases (STDs), using clean needles and syringes when injecting drugs, or entering a drug rehabilitation program. After initiating PrEP, schedule the first follow-up visit for 30 days later to repeat the HIV test and to assess adverse reactions and PrEP adherence.
For men who have sex with men (MSM), there is an alternative form of PrEP when sexual exposure is infrequent. “On-demand” or “event-driven” PrEP involves 4 doses of emtricitabine/tenofovir disoproxil fumarate; 2 doses given with food 2 to 24 hours before sex (the closer to 24 the better), one dose 24 hours after the first and one 24 hours after the second. This is referred to as 2-1-1 dosing. This option has only been tested in MSM with sexual exposure. It is not recommended at this time for others at risk for HIV or for MSM with chronic or active hepatitis B infection.
Continue to: Preventing HIV infection with post-exposure prophylaxis
Preventing HIV infection with post-exposure prophylaxis
Post-exposure prophylaxis (PEP) for HIV infection is divided into 2 categories: occupational PEP (oPEP) and non-occupational PEP (nPEP). Recommendations for oPEP are described elsewhere3 and are not covered in this Practice Alert. Summarized below are the recommendations for nPEP after sex, injection drug use, and other nonoccupational exposures, which are also described on the Centers for Disease Control and Prevention (CDC) Web site.4
Assess the need for nPEP if high-risk exposure (TABLE 34) occurred ≤72 hours earlier. Before starting nPEP, perform a rapid HIV blood test. If rapid testing is unavailable, start nPEP, which can be discontinued if the patient is later determined to have HIV infection. Repeat HIV testing at 4 to 6 weeks and 3 months following initiation of nPEP. Approved HIV tests are described on the CDC Web site at http://www.cdc.gov/hiv/testing/laboratorytests.html. Oral HIV tests are not recommended for HIV testing before initiating nPEP.
nPEP is not recommended when an individual’s risk of exposure to HIV is not high, or if the exposure occurred more than 72 hours before presentation. An algorithm is available to assist with assessing whether nPEP is recommended (FIGURE4).
Specific nPEP regimens. For otherwise healthy adults and adolescents, preferred nPEP consists of a 28-day course of a 3-drug combination: tenofovir disoproxil fumarate 300 mg once daily; emtricitabine 200 mg once daily; and raltegravir, 400 mg twice daily, or dolutegravir 50 mg once daily. Alternative regimens for adults and adolescents are described in the guideline, as are options for children, those with decreased renal function, and pregnant women. Those who receive more than one course of nPEP within a 12-month period should consider PrEP.
When additional vaccination is needed. For victims of sexual assault, offer prophylaxis against STD (TABLE 44) and hepatitis B virus (HBV). Those who have not been vaccinated against HBV should receive the first dose at the initial visit. If the exposure source is known to be HBsAg-positive, give the unvaccinated patient both hepatitis B vaccine and hepatitis B immune globulin at the first visit. The full hepatitis B vaccine series should then be completed according to the recommended schedule and the vaccine product used. Those who have completed hepatitis B vaccination but who were not tested with a post-vaccine titer should receive a single dose of hepatitis B vaccine.
Continue to: Victims of sexual assault...
Victims of sexual assault can benefit from referral to professionals with expertise in post-assault counseling. Sexual Assault Nurse Examiner programs are listed at http://www.sane-sart.com.
Financial assistance for patients. Anti-retroviral drugs are expensive, and those who need nPEP may not have a payer source. Many pharmaceutical manufacturers offer medication assistance programs, and processes are set up to handle time-sensitive requests. Information for specific medications can be found at http://www.pparx.org/en/prescription_assistance_programs/list_of_participating_programs. Those who are prescribed nPEP after a sexual assault can receive reimbursement for medications and health care costs through state Crime Victim Compensation Programs funded by the Department of Justice. State-specific contact information is available at http://www.nacvcb.org/index.asp?sid=6.
1. Saag MS, Benson CA, Gandhi RT, et al. Antiretroviral drugs for treatment and prevention of HIV infection in adults: 2018 recommendations of the International Antiviral Society-USA Panel. JAMA. 2018;320:379-396.
2. Günthard HF, Saag MS, Benson CA, et al. Antiretroviral drugs for treatment and prevention of HIV infection in adults: 2016 recommendations of the International Antiviral Society-USA Panel. JAMA. 2016;316:191-210.
3. Kuhar DT, Henderson DK, Struble KA, et al; US Public Health Service Working Group. Updated US Public Health Service guidelines for the management of occupational exposures to human immunodeficiency virus and recommendations for postexposure prophylaxis. Infect Control Hosp Epidemiol. 2013;34:875-892.
4. CDC. Updated guidelines for antiretroviral postexposure prophylaxis after sexual, injection drug use, or other nonoccupational exposure to HIV—United States, 2016. https://www-cdc-gov.ezproxy3.library.arizona.edu/hiv/pdf/programresources/cdc-hiv-npep-guidelines.pdf. Accessed October 11, 2018.
1. Saag MS, Benson CA, Gandhi RT, et al. Antiretroviral drugs for treatment and prevention of HIV infection in adults: 2018 recommendations of the International Antiviral Society-USA Panel. JAMA. 2018;320:379-396.
2. Günthard HF, Saag MS, Benson CA, et al. Antiretroviral drugs for treatment and prevention of HIV infection in adults: 2016 recommendations of the International Antiviral Society-USA Panel. JAMA. 2016;316:191-210.
3. Kuhar DT, Henderson DK, Struble KA, et al; US Public Health Service Working Group. Updated US Public Health Service guidelines for the management of occupational exposures to human immunodeficiency virus and recommendations for postexposure prophylaxis. Infect Control Hosp Epidemiol. 2013;34:875-892.
4. CDC. Updated guidelines for antiretroviral postexposure prophylaxis after sexual, injection drug use, or other nonoccupational exposure to HIV—United States, 2016. https://www-cdc-gov.ezproxy3.library.arizona.edu/hiv/pdf/programresources/cdc-hiv-npep-guidelines.pdf. Accessed October 11, 2018.
Discussing immunization with vaccine-hesitant parents requires caring, individualized approach
ORLANDO – Putting parents at ease, making vaccination the default option during discussions, appealing to their identity as a good parent, and focusing on positive word choice during discussions are the techniques two pediatricians have recommended using to get vaccine-hesitant parents to immunize their children.
“Your goal is to get parents to immunize their kids,” Katrina Saba, MD, of the Permanente Medical Group in Oakland, Calif., said during an interactive group panel at the annual meeting of the American Academy of Pediatrics. “Our goal is not to win a debate. You don’t have to correct every mistaken idea.”
“And really importantly, as we know, belief trumps science,” she added. “Their belief is so much stronger than our proof, and their belief will not be changed by evidence.”
Many parents who are vaccine- hesitant also belong to a social network that forms or reinforces their beliefs, and attacking those beliefs is the same as attacking their identity, Dr. Saba noted. “When you attack someone’s identity, you are immediately seen as not like them, and if you’re not like them, you’ve lost your strength in persuading them.”
Dr. Saba; Kenneth Hempstead, MD; and other pediatricians and educators in the Permanente Medical Group developed a framework for pediatricians and educators to talk with their patients about immunization at their center after California passed a law in 2013 that required health care professionals to discuss vaccines with patients and sign off that they had that discussion.
“We felt that, if we were going to be by law required to have that discussion, maybe we needed some new tools to have [the discussion] more effectively,” Dr. Saba said. “Because clearly, [what we were doing ] wasn’t working or there wouldn’t have been a need for that law.”
Dr. Hempstead explained the concerns of three major categories of vaccine-hesitant parents: those patients who are unsure of whether they should vaccinate, parents who wish to delay vaccination, and parents who refuse vaccination of their children.
Each parent requires a different approach for discussing the importance of vaccination based on their level of vaccine hesitancy, he said. For parents who are unsure, they may require general information about the safety and importance of vaccines.
Parents who delay immunization may have less trust in vaccines, have done research in their own social networks, and may present alternatives to a standard immunization schedule or want to omit certain vaccines from their child’s immunization schedule, he noted. Using the analogy of a car seat is one approach to identify the importance of vaccination to these parents: “Waiting to give the shots is like putting your baby in the car seat after you’ve already arrived at the store – the protection isn’t there for the most important part of the journey!”
In cases where parents refuse vaccination, you should not expect to change a parent’s mind in a single visit, but instead focus on building the patient-provider bond. However, presenting information the parent may have already seen, such as vaccination data from the Food and Drug Administration or Centers for Disease Control and Prevention, may alienate parents who identify with groups that share vaccine-hesitant viewpoints and erode your ability to persuade a parent’s intent to vaccinate.
A study by Nyhan et al. randomized parents to receive one of four pieces of interventions about the MMR vaccine: information from the CDC explaining the lack of evidence linking autism and the vaccine, information about the dangers of the diseases prevented by the vaccine, images of children who have had diseases prevented by the vaccine, and a “dramatic narrative” from a CDC fact sheet about a child who nearly died of measles. The researchers found no informational intervention helped in persuading to vaccinate in parents who had the “least favorable” attitudes toward the vaccine. And in the case of the dramatic narrative, there was an increased misperception about the MMR vaccine (Pediatrics. 2014;133[4]:e835-e842).
Dr. Hempstead and Dr. Saba outlined four rhetorical devices to include in conversations with patients about vaccination: cognitive ease, natural assumption, appeal to identity, and using advantageous terms.
Cognitive ease
Cognitive ease means creating an environment in which the patient is relaxed, comfortable, and more likely to be agreeable. Recognize when the tone shifts, and strive to maintain this calm and comfortable environment throughout the discussion. “If your blood pressure is coming up, that means theirs is, too,” Dr. Hempstead said.
Natural assumption
How you are offering the vaccination also matters, he added. Rather than asking whether a patient wants to vaccinate (“Have you thought about your flu vaccine this year?”), instead frame the discussion with vaccination as the default option (“Is your child due for a flu vaccination this year? Yes, he is. Let’s get that taken care of today”). Equating inaction with vaccination prevents the risk fallacy phenomenon from occurring in which, when given multiple options, people give equal weight to each option and may choose not to vaccinate, Dr. Hempstead noted.
Dr. Saba cited research that backed this approach. In a study by Opel et al., using a “presumptive” approach instead of a “participatory” approach when discussing a provider’s recommendation to vaccinate helped: The presumptive conversations had an odds ratio of 17.5, compared with the participatory approach. In cases in which parents resisted the provider’s recommendations, 50% of providers persisted with their original recommendations, and 47% of parents who initially resisted the recommendations agreed to vaccinate (Pediatrics. 2013;132[6]:1037-46).
Appeal to identity
Another strategy to use is appealing to the patient’s identity as a good parent and link the concept of vaccination with the good parent identity. Forging a new common identity with the parents through common beliefs – such as recognizing that networks to which parents belong are an important part of his or her identify – and reemphasizing the mutual desire to protect the child is another strategy.
Using advantageous terms
Positive terms, such as “protection,” “health,” “safety,” and “what’s best,” are much better words to use in conversation with parents and have more staying power than negative terms, like “autism” and “side effects,” Dr. Hempstead said.
“Stay with positive messaging,” he said. “Immediately coming back to the positive impact of this vaccine, why we care so much, why we’re doing this vaccine, is absolutely critical.”
Dr. Hempstead and Dr. Saba reported no relevant conflicts of interest.
ORLANDO – Putting parents at ease, making vaccination the default option during discussions, appealing to their identity as a good parent, and focusing on positive word choice during discussions are the techniques two pediatricians have recommended using to get vaccine-hesitant parents to immunize their children.
“Your goal is to get parents to immunize their kids,” Katrina Saba, MD, of the Permanente Medical Group in Oakland, Calif., said during an interactive group panel at the annual meeting of the American Academy of Pediatrics. “Our goal is not to win a debate. You don’t have to correct every mistaken idea.”
“And really importantly, as we know, belief trumps science,” she added. “Their belief is so much stronger than our proof, and their belief will not be changed by evidence.”
Many parents who are vaccine- hesitant also belong to a social network that forms or reinforces their beliefs, and attacking those beliefs is the same as attacking their identity, Dr. Saba noted. “When you attack someone’s identity, you are immediately seen as not like them, and if you’re not like them, you’ve lost your strength in persuading them.”
Dr. Saba; Kenneth Hempstead, MD; and other pediatricians and educators in the Permanente Medical Group developed a framework for pediatricians and educators to talk with their patients about immunization at their center after California passed a law in 2013 that required health care professionals to discuss vaccines with patients and sign off that they had that discussion.
“We felt that, if we were going to be by law required to have that discussion, maybe we needed some new tools to have [the discussion] more effectively,” Dr. Saba said. “Because clearly, [what we were doing ] wasn’t working or there wouldn’t have been a need for that law.”
Dr. Hempstead explained the concerns of three major categories of vaccine-hesitant parents: those patients who are unsure of whether they should vaccinate, parents who wish to delay vaccination, and parents who refuse vaccination of their children.
Each parent requires a different approach for discussing the importance of vaccination based on their level of vaccine hesitancy, he said. For parents who are unsure, they may require general information about the safety and importance of vaccines.
Parents who delay immunization may have less trust in vaccines, have done research in their own social networks, and may present alternatives to a standard immunization schedule or want to omit certain vaccines from their child’s immunization schedule, he noted. Using the analogy of a car seat is one approach to identify the importance of vaccination to these parents: “Waiting to give the shots is like putting your baby in the car seat after you’ve already arrived at the store – the protection isn’t there for the most important part of the journey!”
In cases where parents refuse vaccination, you should not expect to change a parent’s mind in a single visit, but instead focus on building the patient-provider bond. However, presenting information the parent may have already seen, such as vaccination data from the Food and Drug Administration or Centers for Disease Control and Prevention, may alienate parents who identify with groups that share vaccine-hesitant viewpoints and erode your ability to persuade a parent’s intent to vaccinate.
A study by Nyhan et al. randomized parents to receive one of four pieces of interventions about the MMR vaccine: information from the CDC explaining the lack of evidence linking autism and the vaccine, information about the dangers of the diseases prevented by the vaccine, images of children who have had diseases prevented by the vaccine, and a “dramatic narrative” from a CDC fact sheet about a child who nearly died of measles. The researchers found no informational intervention helped in persuading to vaccinate in parents who had the “least favorable” attitudes toward the vaccine. And in the case of the dramatic narrative, there was an increased misperception about the MMR vaccine (Pediatrics. 2014;133[4]:e835-e842).
Dr. Hempstead and Dr. Saba outlined four rhetorical devices to include in conversations with patients about vaccination: cognitive ease, natural assumption, appeal to identity, and using advantageous terms.
Cognitive ease
Cognitive ease means creating an environment in which the patient is relaxed, comfortable, and more likely to be agreeable. Recognize when the tone shifts, and strive to maintain this calm and comfortable environment throughout the discussion. “If your blood pressure is coming up, that means theirs is, too,” Dr. Hempstead said.
Natural assumption
How you are offering the vaccination also matters, he added. Rather than asking whether a patient wants to vaccinate (“Have you thought about your flu vaccine this year?”), instead frame the discussion with vaccination as the default option (“Is your child due for a flu vaccination this year? Yes, he is. Let’s get that taken care of today”). Equating inaction with vaccination prevents the risk fallacy phenomenon from occurring in which, when given multiple options, people give equal weight to each option and may choose not to vaccinate, Dr. Hempstead noted.
Dr. Saba cited research that backed this approach. In a study by Opel et al., using a “presumptive” approach instead of a “participatory” approach when discussing a provider’s recommendation to vaccinate helped: The presumptive conversations had an odds ratio of 17.5, compared with the participatory approach. In cases in which parents resisted the provider’s recommendations, 50% of providers persisted with their original recommendations, and 47% of parents who initially resisted the recommendations agreed to vaccinate (Pediatrics. 2013;132[6]:1037-46).
Appeal to identity
Another strategy to use is appealing to the patient’s identity as a good parent and link the concept of vaccination with the good parent identity. Forging a new common identity with the parents through common beliefs – such as recognizing that networks to which parents belong are an important part of his or her identify – and reemphasizing the mutual desire to protect the child is another strategy.
Using advantageous terms
Positive terms, such as “protection,” “health,” “safety,” and “what’s best,” are much better words to use in conversation with parents and have more staying power than negative terms, like “autism” and “side effects,” Dr. Hempstead said.
“Stay with positive messaging,” he said. “Immediately coming back to the positive impact of this vaccine, why we care so much, why we’re doing this vaccine, is absolutely critical.”
Dr. Hempstead and Dr. Saba reported no relevant conflicts of interest.
ORLANDO – Putting parents at ease, making vaccination the default option during discussions, appealing to their identity as a good parent, and focusing on positive word choice during discussions are the techniques two pediatricians have recommended using to get vaccine-hesitant parents to immunize their children.
“Your goal is to get parents to immunize their kids,” Katrina Saba, MD, of the Permanente Medical Group in Oakland, Calif., said during an interactive group panel at the annual meeting of the American Academy of Pediatrics. “Our goal is not to win a debate. You don’t have to correct every mistaken idea.”
“And really importantly, as we know, belief trumps science,” she added. “Their belief is so much stronger than our proof, and their belief will not be changed by evidence.”
Many parents who are vaccine- hesitant also belong to a social network that forms or reinforces their beliefs, and attacking those beliefs is the same as attacking their identity, Dr. Saba noted. “When you attack someone’s identity, you are immediately seen as not like them, and if you’re not like them, you’ve lost your strength in persuading them.”
Dr. Saba; Kenneth Hempstead, MD; and other pediatricians and educators in the Permanente Medical Group developed a framework for pediatricians and educators to talk with their patients about immunization at their center after California passed a law in 2013 that required health care professionals to discuss vaccines with patients and sign off that they had that discussion.
“We felt that, if we were going to be by law required to have that discussion, maybe we needed some new tools to have [the discussion] more effectively,” Dr. Saba said. “Because clearly, [what we were doing ] wasn’t working or there wouldn’t have been a need for that law.”
Dr. Hempstead explained the concerns of three major categories of vaccine-hesitant parents: those patients who are unsure of whether they should vaccinate, parents who wish to delay vaccination, and parents who refuse vaccination of their children.
Each parent requires a different approach for discussing the importance of vaccination based on their level of vaccine hesitancy, he said. For parents who are unsure, they may require general information about the safety and importance of vaccines.
Parents who delay immunization may have less trust in vaccines, have done research in their own social networks, and may present alternatives to a standard immunization schedule or want to omit certain vaccines from their child’s immunization schedule, he noted. Using the analogy of a car seat is one approach to identify the importance of vaccination to these parents: “Waiting to give the shots is like putting your baby in the car seat after you’ve already arrived at the store – the protection isn’t there for the most important part of the journey!”
In cases where parents refuse vaccination, you should not expect to change a parent’s mind in a single visit, but instead focus on building the patient-provider bond. However, presenting information the parent may have already seen, such as vaccination data from the Food and Drug Administration or Centers for Disease Control and Prevention, may alienate parents who identify with groups that share vaccine-hesitant viewpoints and erode your ability to persuade a parent’s intent to vaccinate.
A study by Nyhan et al. randomized parents to receive one of four pieces of interventions about the MMR vaccine: information from the CDC explaining the lack of evidence linking autism and the vaccine, information about the dangers of the diseases prevented by the vaccine, images of children who have had diseases prevented by the vaccine, and a “dramatic narrative” from a CDC fact sheet about a child who nearly died of measles. The researchers found no informational intervention helped in persuading to vaccinate in parents who had the “least favorable” attitudes toward the vaccine. And in the case of the dramatic narrative, there was an increased misperception about the MMR vaccine (Pediatrics. 2014;133[4]:e835-e842).
Dr. Hempstead and Dr. Saba outlined four rhetorical devices to include in conversations with patients about vaccination: cognitive ease, natural assumption, appeal to identity, and using advantageous terms.
Cognitive ease
Cognitive ease means creating an environment in which the patient is relaxed, comfortable, and more likely to be agreeable. Recognize when the tone shifts, and strive to maintain this calm and comfortable environment throughout the discussion. “If your blood pressure is coming up, that means theirs is, too,” Dr. Hempstead said.
Natural assumption
How you are offering the vaccination also matters, he added. Rather than asking whether a patient wants to vaccinate (“Have you thought about your flu vaccine this year?”), instead frame the discussion with vaccination as the default option (“Is your child due for a flu vaccination this year? Yes, he is. Let’s get that taken care of today”). Equating inaction with vaccination prevents the risk fallacy phenomenon from occurring in which, when given multiple options, people give equal weight to each option and may choose not to vaccinate, Dr. Hempstead noted.
Dr. Saba cited research that backed this approach. In a study by Opel et al., using a “presumptive” approach instead of a “participatory” approach when discussing a provider’s recommendation to vaccinate helped: The presumptive conversations had an odds ratio of 17.5, compared with the participatory approach. In cases in which parents resisted the provider’s recommendations, 50% of providers persisted with their original recommendations, and 47% of parents who initially resisted the recommendations agreed to vaccinate (Pediatrics. 2013;132[6]:1037-46).
Appeal to identity
Another strategy to use is appealing to the patient’s identity as a good parent and link the concept of vaccination with the good parent identity. Forging a new common identity with the parents through common beliefs – such as recognizing that networks to which parents belong are an important part of his or her identify – and reemphasizing the mutual desire to protect the child is another strategy.
Using advantageous terms
Positive terms, such as “protection,” “health,” “safety,” and “what’s best,” are much better words to use in conversation with parents and have more staying power than negative terms, like “autism” and “side effects,” Dr. Hempstead said.
“Stay with positive messaging,” he said. “Immediately coming back to the positive impact of this vaccine, why we care so much, why we’re doing this vaccine, is absolutely critical.”
Dr. Hempstead and Dr. Saba reported no relevant conflicts of interest.
EXPERT ANALYSIS FROM AAP 18
Caregiver Health Promotion in Pediatrics: A Novel Opportunity to Enhance Adult and Child Health
From the Division of General Internal Medicine (Dr. Venkataramani), and the Department of Pediatrics (Dr. Venkataramani and Dr. Solomon), Johns Hopkins University School of Medicine, Baltimore, MD.
In 2003, the American Academy of Pediatrics (AAP) published the recommendations of its Task Force on the Family, an initiative borne of the recognition that pediatricians have an important role in promoting well-functioning families as a means of ultimately promoting pediatric health.1 Among the various facets of “family pediatrics” discussed in these recommendations was the practice of addressing caregiver health or health behaviors which directly impact children’s health. “Pediatricians have both opportunity and reason to take note of the health of their young patients’ parents,” declared the Task Force.1
Benefits Beyond Pediatric Preventive Care
Drawing upon evidence showing that caregiver health or health behaviors impact children’s health (the “reason” to intervene), current guidelines identify several caregiver-related issues on which pediatric providers are encouraged to focus their caregiver health promot
Efforts have been made to expand the framework to other issues with similar potential to impact current and future generations of children, such as caregiver family planning.2,9,10 And there exist still other issues which may be particularly well-suited to being addressed through the caregiver health promotion framework, such as follow-up care for mothers with gestational diabetes. These mothers are at high-risk for the development of type 2 diabetes and having subsequent pregnancies affected by poor glycemic control, but traditionally have had poor follow-up rates in the postpartum period and beyond.11 Their regular interactions with pediatric providers resulting from the frequent visits required for their infants presents an important, and as yet untapped, opportunity to re-engage them in recommended medical care and prevent adverse outcomes for their future children as well as themselves.
The maternal gestational diabetes example highlights an important point: caregiver health promotion in pediatric settings can have direct health benefits for caregivers. As such, there are arguably additional reasons for health systems and adult providers to support the practice of caregiver health promotion in pediatric settings. First, it may represent one of the only exposures to the health care setting and health promotion activities for certain caregivers. Caregivers are often younger adults, an age-group that is less likely to have a usual source of care or access preventive services, and low-income caregivers of any age are more likely to have limited health care access. Given the frequency of routine care (12 health maintenance visits in the child’s first 3 years of life),2 caregivers are likely to have more consistent access with the pediatric health care system than with the adult health care system. Therefore, pediatric visits represent an important touchpoint for these adults that could be leveraged to deliver services and further engage them with the adult health care system. Improving the reach of these services is particularly important in the era of population health where health systems, and particularly accountable care organizations, assume responsibility for the health-related outcomes of communities at large.
Second, studies exploring caregiver perspectives on pediatricians addressing their depression or tobacco use suggest that caregivers appreciate and welcome pediatrician engagement in their care.12,13 Thus, supporting these efforts enables patient-centered care delivery. And third, caregivers may be more motivated to address their own health issues or behaviors (such as substance use) when counseled on the implications of their actions on their children’s health. To the extent such counseling is more routinely (and effectively) delivered in the pediatric setting, supporting pediatrics-based counseling efforts is also in the best interest of adult health care providers.
Challenges to Caregiver Health Promotion in Pediatric Settings
Studies suggest that a fairly broad scope of caregiver health promotion activities do occur in pediatric practice. In our survey of a nationally representative sample of children’s primary care physicians (including pediatricians, family medicine physicians, and medicine-pediatrics physicians), over three-quarters of respondents reported addressing at least 3 caregiver health issues (including maternal depression, tobacco use, family planning, influenza immunization status, intimate partner violence exposure, and caregiver health insurance status) during well-infant or well-child visits.14 At the same time, we found limited depth in practice in terms of the regularity with which caregiver issues are addressed at visits or, when applicable, services beyond screening are offered to caregivers. For example, we found that only 36% of physicians addressed caregiver exposure to intimate partner violence in at least half of the well-infant or well-child visits they conducted.14 And while the vast majority of our respondents addressed parental tobacco use with some regularity, less than 15% reported assisting parents with cessation efforts by prescribing cessation therapies. Other studies exploring practices surrounding maternal depression, intimate partner violence screening, or tobacco cessation counseling have revealed similar patterns with regards to the reach of caregiver health promotion in practices across the country.15-18
Such variability in practice seems to stem primarily from structural and/or organizational barriers to caregiver health promotion in pediatric primary care settings, such as limited time, inability to bill for services provided to caregivers, and lack of efficient systems to refer caregivers to adult providers or services. These structural barriers could lead to attitudinal barriers (ie, pediatric physicians’ reluctance to address caregiver health). Attitudinal or physician-associated barriers may arise in instances when the caregiver health issue’s relevance to child health is less clear or expected actions are perceived as being beyond the scope of pediatric practice, raising concerns about personal effectiveness and liability. But it appears that when caregiver health issues clearly impact child health, and the role of the pediatrician is to screen and counsel in the context of pediatric implications of caregiver health or health behaviors, the majority of pediatric providers do endorse a sense of personal responsibility to address these issues. In our survey, for example, the vast majority of pediatric primary care physicians endorsed maternal depression and caregiver tobacco use as relevant to child well-being, and also endorsed a sense of personal responsibility to address these issues.19
Structural or organizational barriers thus appear to play a larger role overall in influencing caregiver health promotion practices. Various studies have characterized these barriers as they relate to caregiver health promotion, and lack of time is a paramount concern.14,20 This is not surprising, given the multiple competing interests for a pediatrician’s time during already time-constrained well-child visits (which include growth and development assessment, anticipatory guidance provision, delivery of children’s preventive care services, and addressing any acute concerns). The time constraints may be even more acutely felt if the results of screening necessitate additional action, such as referral to relevant services. We found that a lack of referral resources or complex referral mechanisms were cited by over half of children’s primary care physicians as general barriers to caregiver health promotion, and in particular by pediatricians (versus medicine-pediatrics or family medicine physicians).14
This highlights the key difference between family medicine and caregiver health promotion in pediatrics: the latter involves addressing adult health issues in a setting where care for adults is often not provided. While some practices that see children may provide care to adults (such as family medicine or medicine-pediatrics clinics) or are co-located with adult health care providers, most pediatric practices are not integrated with adult health care settings. As a result, the “next steps” in caregiver health promotion can prove challenging to pursue, thereby limiting the beneficial impact of these activities on both child and adult health. For example, in the absence of such integration, pediatricians may find it challenging to connect mothers with positive depression screens to appropriate mental health care or parents who smoke to tobacco cessation services. In addition to leading to missed opportunities to comprehensively address caregiver health issues, such obstacles may also discourage pediatric providers from pursuing caregiver health promotion activities to begin with.
The Way Forward
How can health systems and adult health care providers support the caregiver promotion activities of pediatric primary care providers? There are several ways to enhance integration with adult practices and adult health care services. The co-location and integration of relevant caregiver-related auxiliary services at pediatric clinics is one way. In fact, when asked to identify facilitators to caregiver health promotion, pediatricians who responded to our survey most frequently endorsed the co-location of relevant providers, such as mental health professionals or social workers, as facilitators for addressing caregiver depression or intimate partner violence.14 For example, at the Harriet Lane Clinic at Johns Hopkins, the integration of a comprehensive maternal mental health team (including a part-time licensed therapist, part-time psychiatrist from an affiliated psychiatric practice, and full-time maternal case manager) has proven to be an effective, patient-oriented approach to providing services for mothers with depression.21 The role of health systems and adult health care providers/practices in advancing such models of care delivery is two-fold: to provide necessary staff and financial support. The latter is particularly important as many of the relevant caregiver-related services (eg, social work or case manager visits) may not generate the revenue required to support their sustained presence at pediatric sites.
Pediatric practices would also benefit from enhanced mechanisms for referral to appropriate services that are not co-located, such as tobacco cessation “quitlines.” Adopting protocolized interventions that focus on connecting parents with existing resources for their own health, such as the CEASE intervention developed for parental tobacco control in pediatrics,22,23 is one way to streamline the referral process for pediatric practices. Another is by advancing a truly integrated electronic medical record (EMR), which enables caregiver health screenings and referral to additional services to be completed during pediatric encounters.
Finally, while only a relative minority of physicians we surveyed suggested that a lack of reimbursement for their activities served as a general barrier to caregiver health promotion, ensuring that pediatric providers are adequately compensated for their efforts on behalf of parents and guardians would undoubtedly help support their activities. Integrated EMRs could be one way to support this, particularly for services that are traditionally billed for (eg, depression screening or tobacco cessation counseling). Novel ways to reimburse pediatric providers for their contribution to adult health indicators could also be considered; for example, to the extent caregiver health promotion activities contribute to adult quality indicators (eg, postpartum depression screening rates and completion of postpartum visits) that are associated with financial rewards, health systems could consider sharing these “bonuses” among pediatric providers.
From Family Pediatrics to Family-Oriented Care
While caregiver health promotion has long been considered part of the practice of “family pediatrics,” it should more accurately be seen as an integral component of the delivery of family-oriented primary care, as it represents a novel opportunity to advance the health of not only children, but also their caregivers. Following existing preventive care guidelines, pediatricians currently engage in a variety of activities to promote child and caregiver health, but require support to more consistently and effectively address issues such as caregiver tobacco use or maternal depression. The barriers faced by pediatricians could be most effectively addressed with the engagement of adult health care providers and health systems; this includes the development of an integrated EMR that would support screening activities and referral to connect caregivers with necessary follow-up resources. Further characterizing the barriers faced in pediatric settings, and exploring how health systems could provide the necessary support to address these barriers, is crucial to realizing the potential of caregiver health promotion to have multi-generational impacts on well-being.
Corresponding author: Maya Venkataramani, MD, MPH, 2024 E. Monument St., Suite 2-502, Baltimore, MD 21287; mvenkat2@jhmi.edu.
Financial disclosures: None.
1. Schor EL, American Academy of Pediatrics Task Force on the Family. Family pediatrics: report of the Task Force on the Family. Pediatrics. 2003;111:1541-1571.
2. Hagan JF, Shaw JS, Duncan PM, eds. Bright Futures: Guidelines for Health Supervision of Infants, Children, and Adolescents. 4th ed. Elk Grove Village, IL: American Academy of Pediatrics; 2017. 4
3. Best D, Committee on Environmental Health, Committee on Native American Child Health, Committee on Adolescence. From the American Academy of Pediatrics: Technical report--Secondhand and prenatal tobacco smoke exposure. Pediatrics. 2009; 124:e1017-1044.
4. Treyster Z, Gitterman B. Second hand smoke exposure in children: environmental factors, physiological effects, and interventions within pediatrics. Rev Environ Health. 2011;26:187-195.
5. American Medical Association. H-490.917: physician responsibilities for tobacco cessation. Adopted by House of Delegates, Chicago, IL: American Medical Association.
6. Committee on Environmental Health, Committee on Substance Abuse, Committee on Adolescence, and Committee on Native American Child Health. Tobacco use: a pediatric disorder. Pediatrics. 2009;124;1474. http://pediatrics.aappublications.org/content/pediatrics/124/5/1474.full.pdf. Accessed October 9, 2018.
7. Earls MF, Committee on Psychosocial Aspects of Child and Family Health American Academy of Pediatrics. Incorporating recognition and management of perinatal and postpartum depression into pediatric practice. Pediatrics. 2010;126:1032-1039.
8. Yogman M, Garfield CF, Committee on Psychological Aspects of Child and Family Health. Pediatrics. 2016;138(1):e20161128.
9. Cheng TL, Kotelchuck M, Guyer B. Preconception women’s health and pediatrics: an Opportunity to address infant mortality and family health. Acad Pediatr. 2012;12:357-359.
10. Zuckerman B, Nathan S, Mate K. Preventing unintended pregnancy: a pediatric opportunity. Pediatrics. 2014;133:181-183.
11. McCloskey L, Bernstein J, Winter M, et al. Follow-up of gestational diabetes mellitus in an urban safety net hospital: missed opportunities to launch preventive care for women. J Womens Health. 2014;23:327-334.
12. Groner J, Ahijevych K, Grossman L, Rich L. Smoking behaviors of women whose children attend an urban pediatric primary care clinic. Women Health. 1998;28:19-32.
13. Kahn RS, Wise P, Finkelstein MD, et al. The scope of unmet maternal health needs in pediatric settings. Pediatrics. 1999;103:576-581.
14. Venkataramani M, Cheng TL, Solomon BS, Pollack CE. Caregiver health promotion in pediatric primary care settings: results of a national survey. J Pediatr. 2017;181:254-260.e2.
15. Kerker BD, Storfer-Isser A, Stein RE, et al. Identifying maternal depression in pediatric primary care: changes over a decade. J Dev Behav Pediatr. 2016;37:113-120.
16. Collins BN, Levin KP, Bryant-Stephens T. Pediatricians’ practices and attitudes about environmental tobacco smoke and parental smoking. J Pediatr. 2007;150:547-552.
17. Borowsky IW, Ireland M. Parental screening for intimate partner violence by pediatricians and family physicians. Pediatrics. 2002;110:509-516.
18. Olson AL, Kemper KJ, Kelleher KJ, et al. Primary care pediatricians’ roles and perceived responsibilities in the identification and management of maternal depression. Pediatrics. 2002;110:1169-1176.
19. Venkataramani M, Cheng TL, Solomon BS, Pollack CE. Addressing parental health in pediatrics: physician perceptions of relevance and responsibility. Clin Pediatr. 2017;56:953-958.
20. Horwitz SM, Kelleher KJ, Stein RE, et al. Barriers to the identification and management of psychosocial issues in children and maternal depression. Pediatrics. 2007;119:e208-218.
21. Kimmel MC, Platt RE, Steinberg DN, et al. Integrating maternal mental health care in the pediatric medical home: treatment engagement and child outcomes. Clin Pediatric. 2017;56:1148-1156.
22. Winickoff JP, Nabi-Burza E, Chang Y, et al. Implementation of a parental tobacco control interventionin pediatric practice. Pediatrics. 2013;132:109-117.
23. Winickoff JP, Nabi-Burza E, Chang Y, et al. Sustainability of a parental tobacco control intervention in pediatric practice. Pediatrics. 2014;134:933-941.
From the Division of General Internal Medicine (Dr. Venkataramani), and the Department of Pediatrics (Dr. Venkataramani and Dr. Solomon), Johns Hopkins University School of Medicine, Baltimore, MD.
In 2003, the American Academy of Pediatrics (AAP) published the recommendations of its Task Force on the Family, an initiative borne of the recognition that pediatricians have an important role in promoting well-functioning families as a means of ultimately promoting pediatric health.1 Among the various facets of “family pediatrics” discussed in these recommendations was the practice of addressing caregiver health or health behaviors which directly impact children’s health. “Pediatricians have both opportunity and reason to take note of the health of their young patients’ parents,” declared the Task Force.1
Benefits Beyond Pediatric Preventive Care
Drawing upon evidence showing that caregiver health or health behaviors impact children’s health (the “reason” to intervene), current guidelines identify several caregiver-related issues on which pediatric providers are encouraged to focus their caregiver health promot
Efforts have been made to expand the framework to other issues with similar potential to impact current and future generations of children, such as caregiver family planning.2,9,10 And there exist still other issues which may be particularly well-suited to being addressed through the caregiver health promotion framework, such as follow-up care for mothers with gestational diabetes. These mothers are at high-risk for the development of type 2 diabetes and having subsequent pregnancies affected by poor glycemic control, but traditionally have had poor follow-up rates in the postpartum period and beyond.11 Their regular interactions with pediatric providers resulting from the frequent visits required for their infants presents an important, and as yet untapped, opportunity to re-engage them in recommended medical care and prevent adverse outcomes for their future children as well as themselves.
The maternal gestational diabetes example highlights an important point: caregiver health promotion in pediatric settings can have direct health benefits for caregivers. As such, there are arguably additional reasons for health systems and adult providers to support the practice of caregiver health promotion in pediatric settings. First, it may represent one of the only exposures to the health care setting and health promotion activities for certain caregivers. Caregivers are often younger adults, an age-group that is less likely to have a usual source of care or access preventive services, and low-income caregivers of any age are more likely to have limited health care access. Given the frequency of routine care (12 health maintenance visits in the child’s first 3 years of life),2 caregivers are likely to have more consistent access with the pediatric health care system than with the adult health care system. Therefore, pediatric visits represent an important touchpoint for these adults that could be leveraged to deliver services and further engage them with the adult health care system. Improving the reach of these services is particularly important in the era of population health where health systems, and particularly accountable care organizations, assume responsibility for the health-related outcomes of communities at large.
Second, studies exploring caregiver perspectives on pediatricians addressing their depression or tobacco use suggest that caregivers appreciate and welcome pediatrician engagement in their care.12,13 Thus, supporting these efforts enables patient-centered care delivery. And third, caregivers may be more motivated to address their own health issues or behaviors (such as substance use) when counseled on the implications of their actions on their children’s health. To the extent such counseling is more routinely (and effectively) delivered in the pediatric setting, supporting pediatrics-based counseling efforts is also in the best interest of adult health care providers.
Challenges to Caregiver Health Promotion in Pediatric Settings
Studies suggest that a fairly broad scope of caregiver health promotion activities do occur in pediatric practice. In our survey of a nationally representative sample of children’s primary care physicians (including pediatricians, family medicine physicians, and medicine-pediatrics physicians), over three-quarters of respondents reported addressing at least 3 caregiver health issues (including maternal depression, tobacco use, family planning, influenza immunization status, intimate partner violence exposure, and caregiver health insurance status) during well-infant or well-child visits.14 At the same time, we found limited depth in practice in terms of the regularity with which caregiver issues are addressed at visits or, when applicable, services beyond screening are offered to caregivers. For example, we found that only 36% of physicians addressed caregiver exposure to intimate partner violence in at least half of the well-infant or well-child visits they conducted.14 And while the vast majority of our respondents addressed parental tobacco use with some regularity, less than 15% reported assisting parents with cessation efforts by prescribing cessation therapies. Other studies exploring practices surrounding maternal depression, intimate partner violence screening, or tobacco cessation counseling have revealed similar patterns with regards to the reach of caregiver health promotion in practices across the country.15-18
Such variability in practice seems to stem primarily from structural and/or organizational barriers to caregiver health promotion in pediatric primary care settings, such as limited time, inability to bill for services provided to caregivers, and lack of efficient systems to refer caregivers to adult providers or services. These structural barriers could lead to attitudinal barriers (ie, pediatric physicians’ reluctance to address caregiver health). Attitudinal or physician-associated barriers may arise in instances when the caregiver health issue’s relevance to child health is less clear or expected actions are perceived as being beyond the scope of pediatric practice, raising concerns about personal effectiveness and liability. But it appears that when caregiver health issues clearly impact child health, and the role of the pediatrician is to screen and counsel in the context of pediatric implications of caregiver health or health behaviors, the majority of pediatric providers do endorse a sense of personal responsibility to address these issues. In our survey, for example, the vast majority of pediatric primary care physicians endorsed maternal depression and caregiver tobacco use as relevant to child well-being, and also endorsed a sense of personal responsibility to address these issues.19
Structural or organizational barriers thus appear to play a larger role overall in influencing caregiver health promotion practices. Various studies have characterized these barriers as they relate to caregiver health promotion, and lack of time is a paramount concern.14,20 This is not surprising, given the multiple competing interests for a pediatrician’s time during already time-constrained well-child visits (which include growth and development assessment, anticipatory guidance provision, delivery of children’s preventive care services, and addressing any acute concerns). The time constraints may be even more acutely felt if the results of screening necessitate additional action, such as referral to relevant services. We found that a lack of referral resources or complex referral mechanisms were cited by over half of children’s primary care physicians as general barriers to caregiver health promotion, and in particular by pediatricians (versus medicine-pediatrics or family medicine physicians).14
This highlights the key difference between family medicine and caregiver health promotion in pediatrics: the latter involves addressing adult health issues in a setting where care for adults is often not provided. While some practices that see children may provide care to adults (such as family medicine or medicine-pediatrics clinics) or are co-located with adult health care providers, most pediatric practices are not integrated with adult health care settings. As a result, the “next steps” in caregiver health promotion can prove challenging to pursue, thereby limiting the beneficial impact of these activities on both child and adult health. For example, in the absence of such integration, pediatricians may find it challenging to connect mothers with positive depression screens to appropriate mental health care or parents who smoke to tobacco cessation services. In addition to leading to missed opportunities to comprehensively address caregiver health issues, such obstacles may also discourage pediatric providers from pursuing caregiver health promotion activities to begin with.
The Way Forward
How can health systems and adult health care providers support the caregiver promotion activities of pediatric primary care providers? There are several ways to enhance integration with adult practices and adult health care services. The co-location and integration of relevant caregiver-related auxiliary services at pediatric clinics is one way. In fact, when asked to identify facilitators to caregiver health promotion, pediatricians who responded to our survey most frequently endorsed the co-location of relevant providers, such as mental health professionals or social workers, as facilitators for addressing caregiver depression or intimate partner violence.14 For example, at the Harriet Lane Clinic at Johns Hopkins, the integration of a comprehensive maternal mental health team (including a part-time licensed therapist, part-time psychiatrist from an affiliated psychiatric practice, and full-time maternal case manager) has proven to be an effective, patient-oriented approach to providing services for mothers with depression.21 The role of health systems and adult health care providers/practices in advancing such models of care delivery is two-fold: to provide necessary staff and financial support. The latter is particularly important as many of the relevant caregiver-related services (eg, social work or case manager visits) may not generate the revenue required to support their sustained presence at pediatric sites.
Pediatric practices would also benefit from enhanced mechanisms for referral to appropriate services that are not co-located, such as tobacco cessation “quitlines.” Adopting protocolized interventions that focus on connecting parents with existing resources for their own health, such as the CEASE intervention developed for parental tobacco control in pediatrics,22,23 is one way to streamline the referral process for pediatric practices. Another is by advancing a truly integrated electronic medical record (EMR), which enables caregiver health screenings and referral to additional services to be completed during pediatric encounters.
Finally, while only a relative minority of physicians we surveyed suggested that a lack of reimbursement for their activities served as a general barrier to caregiver health promotion, ensuring that pediatric providers are adequately compensated for their efforts on behalf of parents and guardians would undoubtedly help support their activities. Integrated EMRs could be one way to support this, particularly for services that are traditionally billed for (eg, depression screening or tobacco cessation counseling). Novel ways to reimburse pediatric providers for their contribution to adult health indicators could also be considered; for example, to the extent caregiver health promotion activities contribute to adult quality indicators (eg, postpartum depression screening rates and completion of postpartum visits) that are associated with financial rewards, health systems could consider sharing these “bonuses” among pediatric providers.
From Family Pediatrics to Family-Oriented Care
While caregiver health promotion has long been considered part of the practice of “family pediatrics,” it should more accurately be seen as an integral component of the delivery of family-oriented primary care, as it represents a novel opportunity to advance the health of not only children, but also their caregivers. Following existing preventive care guidelines, pediatricians currently engage in a variety of activities to promote child and caregiver health, but require support to more consistently and effectively address issues such as caregiver tobacco use or maternal depression. The barriers faced by pediatricians could be most effectively addressed with the engagement of adult health care providers and health systems; this includes the development of an integrated EMR that would support screening activities and referral to connect caregivers with necessary follow-up resources. Further characterizing the barriers faced in pediatric settings, and exploring how health systems could provide the necessary support to address these barriers, is crucial to realizing the potential of caregiver health promotion to have multi-generational impacts on well-being.
Corresponding author: Maya Venkataramani, MD, MPH, 2024 E. Monument St., Suite 2-502, Baltimore, MD 21287; mvenkat2@jhmi.edu.
Financial disclosures: None.
From the Division of General Internal Medicine (Dr. Venkataramani), and the Department of Pediatrics (Dr. Venkataramani and Dr. Solomon), Johns Hopkins University School of Medicine, Baltimore, MD.
In 2003, the American Academy of Pediatrics (AAP) published the recommendations of its Task Force on the Family, an initiative borne of the recognition that pediatricians have an important role in promoting well-functioning families as a means of ultimately promoting pediatric health.1 Among the various facets of “family pediatrics” discussed in these recommendations was the practice of addressing caregiver health or health behaviors which directly impact children’s health. “Pediatricians have both opportunity and reason to take note of the health of their young patients’ parents,” declared the Task Force.1
Benefits Beyond Pediatric Preventive Care
Drawing upon evidence showing that caregiver health or health behaviors impact children’s health (the “reason” to intervene), current guidelines identify several caregiver-related issues on which pediatric providers are encouraged to focus their caregiver health promot
Efforts have been made to expand the framework to other issues with similar potential to impact current and future generations of children, such as caregiver family planning.2,9,10 And there exist still other issues which may be particularly well-suited to being addressed through the caregiver health promotion framework, such as follow-up care for mothers with gestational diabetes. These mothers are at high-risk for the development of type 2 diabetes and having subsequent pregnancies affected by poor glycemic control, but traditionally have had poor follow-up rates in the postpartum period and beyond.11 Their regular interactions with pediatric providers resulting from the frequent visits required for their infants presents an important, and as yet untapped, opportunity to re-engage them in recommended medical care and prevent adverse outcomes for their future children as well as themselves.
The maternal gestational diabetes example highlights an important point: caregiver health promotion in pediatric settings can have direct health benefits for caregivers. As such, there are arguably additional reasons for health systems and adult providers to support the practice of caregiver health promotion in pediatric settings. First, it may represent one of the only exposures to the health care setting and health promotion activities for certain caregivers. Caregivers are often younger adults, an age-group that is less likely to have a usual source of care or access preventive services, and low-income caregivers of any age are more likely to have limited health care access. Given the frequency of routine care (12 health maintenance visits in the child’s first 3 years of life),2 caregivers are likely to have more consistent access with the pediatric health care system than with the adult health care system. Therefore, pediatric visits represent an important touchpoint for these adults that could be leveraged to deliver services and further engage them with the adult health care system. Improving the reach of these services is particularly important in the era of population health where health systems, and particularly accountable care organizations, assume responsibility for the health-related outcomes of communities at large.
Second, studies exploring caregiver perspectives on pediatricians addressing their depression or tobacco use suggest that caregivers appreciate and welcome pediatrician engagement in their care.12,13 Thus, supporting these efforts enables patient-centered care delivery. And third, caregivers may be more motivated to address their own health issues or behaviors (such as substance use) when counseled on the implications of their actions on their children’s health. To the extent such counseling is more routinely (and effectively) delivered in the pediatric setting, supporting pediatrics-based counseling efforts is also in the best interest of adult health care providers.
Challenges to Caregiver Health Promotion in Pediatric Settings
Studies suggest that a fairly broad scope of caregiver health promotion activities do occur in pediatric practice. In our survey of a nationally representative sample of children’s primary care physicians (including pediatricians, family medicine physicians, and medicine-pediatrics physicians), over three-quarters of respondents reported addressing at least 3 caregiver health issues (including maternal depression, tobacco use, family planning, influenza immunization status, intimate partner violence exposure, and caregiver health insurance status) during well-infant or well-child visits.14 At the same time, we found limited depth in practice in terms of the regularity with which caregiver issues are addressed at visits or, when applicable, services beyond screening are offered to caregivers. For example, we found that only 36% of physicians addressed caregiver exposure to intimate partner violence in at least half of the well-infant or well-child visits they conducted.14 And while the vast majority of our respondents addressed parental tobacco use with some regularity, less than 15% reported assisting parents with cessation efforts by prescribing cessation therapies. Other studies exploring practices surrounding maternal depression, intimate partner violence screening, or tobacco cessation counseling have revealed similar patterns with regards to the reach of caregiver health promotion in practices across the country.15-18
Such variability in practice seems to stem primarily from structural and/or organizational barriers to caregiver health promotion in pediatric primary care settings, such as limited time, inability to bill for services provided to caregivers, and lack of efficient systems to refer caregivers to adult providers or services. These structural barriers could lead to attitudinal barriers (ie, pediatric physicians’ reluctance to address caregiver health). Attitudinal or physician-associated barriers may arise in instances when the caregiver health issue’s relevance to child health is less clear or expected actions are perceived as being beyond the scope of pediatric practice, raising concerns about personal effectiveness and liability. But it appears that when caregiver health issues clearly impact child health, and the role of the pediatrician is to screen and counsel in the context of pediatric implications of caregiver health or health behaviors, the majority of pediatric providers do endorse a sense of personal responsibility to address these issues. In our survey, for example, the vast majority of pediatric primary care physicians endorsed maternal depression and caregiver tobacco use as relevant to child well-being, and also endorsed a sense of personal responsibility to address these issues.19
Structural or organizational barriers thus appear to play a larger role overall in influencing caregiver health promotion practices. Various studies have characterized these barriers as they relate to caregiver health promotion, and lack of time is a paramount concern.14,20 This is not surprising, given the multiple competing interests for a pediatrician’s time during already time-constrained well-child visits (which include growth and development assessment, anticipatory guidance provision, delivery of children’s preventive care services, and addressing any acute concerns). The time constraints may be even more acutely felt if the results of screening necessitate additional action, such as referral to relevant services. We found that a lack of referral resources or complex referral mechanisms were cited by over half of children’s primary care physicians as general barriers to caregiver health promotion, and in particular by pediatricians (versus medicine-pediatrics or family medicine physicians).14
This highlights the key difference between family medicine and caregiver health promotion in pediatrics: the latter involves addressing adult health issues in a setting where care for adults is often not provided. While some practices that see children may provide care to adults (such as family medicine or medicine-pediatrics clinics) or are co-located with adult health care providers, most pediatric practices are not integrated with adult health care settings. As a result, the “next steps” in caregiver health promotion can prove challenging to pursue, thereby limiting the beneficial impact of these activities on both child and adult health. For example, in the absence of such integration, pediatricians may find it challenging to connect mothers with positive depression screens to appropriate mental health care or parents who smoke to tobacco cessation services. In addition to leading to missed opportunities to comprehensively address caregiver health issues, such obstacles may also discourage pediatric providers from pursuing caregiver health promotion activities to begin with.
The Way Forward
How can health systems and adult health care providers support the caregiver promotion activities of pediatric primary care providers? There are several ways to enhance integration with adult practices and adult health care services. The co-location and integration of relevant caregiver-related auxiliary services at pediatric clinics is one way. In fact, when asked to identify facilitators to caregiver health promotion, pediatricians who responded to our survey most frequently endorsed the co-location of relevant providers, such as mental health professionals or social workers, as facilitators for addressing caregiver depression or intimate partner violence.14 For example, at the Harriet Lane Clinic at Johns Hopkins, the integration of a comprehensive maternal mental health team (including a part-time licensed therapist, part-time psychiatrist from an affiliated psychiatric practice, and full-time maternal case manager) has proven to be an effective, patient-oriented approach to providing services for mothers with depression.21 The role of health systems and adult health care providers/practices in advancing such models of care delivery is two-fold: to provide necessary staff and financial support. The latter is particularly important as many of the relevant caregiver-related services (eg, social work or case manager visits) may not generate the revenue required to support their sustained presence at pediatric sites.
Pediatric practices would also benefit from enhanced mechanisms for referral to appropriate services that are not co-located, such as tobacco cessation “quitlines.” Adopting protocolized interventions that focus on connecting parents with existing resources for their own health, such as the CEASE intervention developed for parental tobacco control in pediatrics,22,23 is one way to streamline the referral process for pediatric practices. Another is by advancing a truly integrated electronic medical record (EMR), which enables caregiver health screenings and referral to additional services to be completed during pediatric encounters.
Finally, while only a relative minority of physicians we surveyed suggested that a lack of reimbursement for their activities served as a general barrier to caregiver health promotion, ensuring that pediatric providers are adequately compensated for their efforts on behalf of parents and guardians would undoubtedly help support their activities. Integrated EMRs could be one way to support this, particularly for services that are traditionally billed for (eg, depression screening or tobacco cessation counseling). Novel ways to reimburse pediatric providers for their contribution to adult health indicators could also be considered; for example, to the extent caregiver health promotion activities contribute to adult quality indicators (eg, postpartum depression screening rates and completion of postpartum visits) that are associated with financial rewards, health systems could consider sharing these “bonuses” among pediatric providers.
From Family Pediatrics to Family-Oriented Care
While caregiver health promotion has long been considered part of the practice of “family pediatrics,” it should more accurately be seen as an integral component of the delivery of family-oriented primary care, as it represents a novel opportunity to advance the health of not only children, but also their caregivers. Following existing preventive care guidelines, pediatricians currently engage in a variety of activities to promote child and caregiver health, but require support to more consistently and effectively address issues such as caregiver tobacco use or maternal depression. The barriers faced by pediatricians could be most effectively addressed with the engagement of adult health care providers and health systems; this includes the development of an integrated EMR that would support screening activities and referral to connect caregivers with necessary follow-up resources. Further characterizing the barriers faced in pediatric settings, and exploring how health systems could provide the necessary support to address these barriers, is crucial to realizing the potential of caregiver health promotion to have multi-generational impacts on well-being.
Corresponding author: Maya Venkataramani, MD, MPH, 2024 E. Monument St., Suite 2-502, Baltimore, MD 21287; mvenkat2@jhmi.edu.
Financial disclosures: None.
1. Schor EL, American Academy of Pediatrics Task Force on the Family. Family pediatrics: report of the Task Force on the Family. Pediatrics. 2003;111:1541-1571.
2. Hagan JF, Shaw JS, Duncan PM, eds. Bright Futures: Guidelines for Health Supervision of Infants, Children, and Adolescents. 4th ed. Elk Grove Village, IL: American Academy of Pediatrics; 2017. 4
3. Best D, Committee on Environmental Health, Committee on Native American Child Health, Committee on Adolescence. From the American Academy of Pediatrics: Technical report--Secondhand and prenatal tobacco smoke exposure. Pediatrics. 2009; 124:e1017-1044.
4. Treyster Z, Gitterman B. Second hand smoke exposure in children: environmental factors, physiological effects, and interventions within pediatrics. Rev Environ Health. 2011;26:187-195.
5. American Medical Association. H-490.917: physician responsibilities for tobacco cessation. Adopted by House of Delegates, Chicago, IL: American Medical Association.
6. Committee on Environmental Health, Committee on Substance Abuse, Committee on Adolescence, and Committee on Native American Child Health. Tobacco use: a pediatric disorder. Pediatrics. 2009;124;1474. http://pediatrics.aappublications.org/content/pediatrics/124/5/1474.full.pdf. Accessed October 9, 2018.
7. Earls MF, Committee on Psychosocial Aspects of Child and Family Health American Academy of Pediatrics. Incorporating recognition and management of perinatal and postpartum depression into pediatric practice. Pediatrics. 2010;126:1032-1039.
8. Yogman M, Garfield CF, Committee on Psychological Aspects of Child and Family Health. Pediatrics. 2016;138(1):e20161128.
9. Cheng TL, Kotelchuck M, Guyer B. Preconception women’s health and pediatrics: an Opportunity to address infant mortality and family health. Acad Pediatr. 2012;12:357-359.
10. Zuckerman B, Nathan S, Mate K. Preventing unintended pregnancy: a pediatric opportunity. Pediatrics. 2014;133:181-183.
11. McCloskey L, Bernstein J, Winter M, et al. Follow-up of gestational diabetes mellitus in an urban safety net hospital: missed opportunities to launch preventive care for women. J Womens Health. 2014;23:327-334.
12. Groner J, Ahijevych K, Grossman L, Rich L. Smoking behaviors of women whose children attend an urban pediatric primary care clinic. Women Health. 1998;28:19-32.
13. Kahn RS, Wise P, Finkelstein MD, et al. The scope of unmet maternal health needs in pediatric settings. Pediatrics. 1999;103:576-581.
14. Venkataramani M, Cheng TL, Solomon BS, Pollack CE. Caregiver health promotion in pediatric primary care settings: results of a national survey. J Pediatr. 2017;181:254-260.e2.
15. Kerker BD, Storfer-Isser A, Stein RE, et al. Identifying maternal depression in pediatric primary care: changes over a decade. J Dev Behav Pediatr. 2016;37:113-120.
16. Collins BN, Levin KP, Bryant-Stephens T. Pediatricians’ practices and attitudes about environmental tobacco smoke and parental smoking. J Pediatr. 2007;150:547-552.
17. Borowsky IW, Ireland M. Parental screening for intimate partner violence by pediatricians and family physicians. Pediatrics. 2002;110:509-516.
18. Olson AL, Kemper KJ, Kelleher KJ, et al. Primary care pediatricians’ roles and perceived responsibilities in the identification and management of maternal depression. Pediatrics. 2002;110:1169-1176.
19. Venkataramani M, Cheng TL, Solomon BS, Pollack CE. Addressing parental health in pediatrics: physician perceptions of relevance and responsibility. Clin Pediatr. 2017;56:953-958.
20. Horwitz SM, Kelleher KJ, Stein RE, et al. Barriers to the identification and management of psychosocial issues in children and maternal depression. Pediatrics. 2007;119:e208-218.
21. Kimmel MC, Platt RE, Steinberg DN, et al. Integrating maternal mental health care in the pediatric medical home: treatment engagement and child outcomes. Clin Pediatric. 2017;56:1148-1156.
22. Winickoff JP, Nabi-Burza E, Chang Y, et al. Implementation of a parental tobacco control interventionin pediatric practice. Pediatrics. 2013;132:109-117.
23. Winickoff JP, Nabi-Burza E, Chang Y, et al. Sustainability of a parental tobacco control intervention in pediatric practice. Pediatrics. 2014;134:933-941.
1. Schor EL, American Academy of Pediatrics Task Force on the Family. Family pediatrics: report of the Task Force on the Family. Pediatrics. 2003;111:1541-1571.
2. Hagan JF, Shaw JS, Duncan PM, eds. Bright Futures: Guidelines for Health Supervision of Infants, Children, and Adolescents. 4th ed. Elk Grove Village, IL: American Academy of Pediatrics; 2017. 4
3. Best D, Committee on Environmental Health, Committee on Native American Child Health, Committee on Adolescence. From the American Academy of Pediatrics: Technical report--Secondhand and prenatal tobacco smoke exposure. Pediatrics. 2009; 124:e1017-1044.
4. Treyster Z, Gitterman B. Second hand smoke exposure in children: environmental factors, physiological effects, and interventions within pediatrics. Rev Environ Health. 2011;26:187-195.
5. American Medical Association. H-490.917: physician responsibilities for tobacco cessation. Adopted by House of Delegates, Chicago, IL: American Medical Association.
6. Committee on Environmental Health, Committee on Substance Abuse, Committee on Adolescence, and Committee on Native American Child Health. Tobacco use: a pediatric disorder. Pediatrics. 2009;124;1474. http://pediatrics.aappublications.org/content/pediatrics/124/5/1474.full.pdf. Accessed October 9, 2018.
7. Earls MF, Committee on Psychosocial Aspects of Child and Family Health American Academy of Pediatrics. Incorporating recognition and management of perinatal and postpartum depression into pediatric practice. Pediatrics. 2010;126:1032-1039.
8. Yogman M, Garfield CF, Committee on Psychological Aspects of Child and Family Health. Pediatrics. 2016;138(1):e20161128.
9. Cheng TL, Kotelchuck M, Guyer B. Preconception women’s health and pediatrics: an Opportunity to address infant mortality and family health. Acad Pediatr. 2012;12:357-359.
10. Zuckerman B, Nathan S, Mate K. Preventing unintended pregnancy: a pediatric opportunity. Pediatrics. 2014;133:181-183.
11. McCloskey L, Bernstein J, Winter M, et al. Follow-up of gestational diabetes mellitus in an urban safety net hospital: missed opportunities to launch preventive care for women. J Womens Health. 2014;23:327-334.
12. Groner J, Ahijevych K, Grossman L, Rich L. Smoking behaviors of women whose children attend an urban pediatric primary care clinic. Women Health. 1998;28:19-32.
13. Kahn RS, Wise P, Finkelstein MD, et al. The scope of unmet maternal health needs in pediatric settings. Pediatrics. 1999;103:576-581.
14. Venkataramani M, Cheng TL, Solomon BS, Pollack CE. Caregiver health promotion in pediatric primary care settings: results of a national survey. J Pediatr. 2017;181:254-260.e2.
15. Kerker BD, Storfer-Isser A, Stein RE, et al. Identifying maternal depression in pediatric primary care: changes over a decade. J Dev Behav Pediatr. 2016;37:113-120.
16. Collins BN, Levin KP, Bryant-Stephens T. Pediatricians’ practices and attitudes about environmental tobacco smoke and parental smoking. J Pediatr. 2007;150:547-552.
17. Borowsky IW, Ireland M. Parental screening for intimate partner violence by pediatricians and family physicians. Pediatrics. 2002;110:509-516.
18. Olson AL, Kemper KJ, Kelleher KJ, et al. Primary care pediatricians’ roles and perceived responsibilities in the identification and management of maternal depression. Pediatrics. 2002;110:1169-1176.
19. Venkataramani M, Cheng TL, Solomon BS, Pollack CE. Addressing parental health in pediatrics: physician perceptions of relevance and responsibility. Clin Pediatr. 2017;56:953-958.
20. Horwitz SM, Kelleher KJ, Stein RE, et al. Barriers to the identification and management of psychosocial issues in children and maternal depression. Pediatrics. 2007;119:e208-218.
21. Kimmel MC, Platt RE, Steinberg DN, et al. Integrating maternal mental health care in the pediatric medical home: treatment engagement and child outcomes. Clin Pediatric. 2017;56:1148-1156.
22. Winickoff JP, Nabi-Burza E, Chang Y, et al. Implementation of a parental tobacco control interventionin pediatric practice. Pediatrics. 2013;132:109-117.
23. Winickoff JP, Nabi-Burza E, Chang Y, et al. Sustainability of a parental tobacco control intervention in pediatric practice. Pediatrics. 2014;134:933-941.
When is the right time to stop treatment?
Martha Boxer (not her real name) had mentally prepared herself that this could happen, but the news still hit hard. Her doctors on the leukemia service broke the facts as gently as we could: The chemotherapy she had been suffering through for the last 2 weeks hadn’t worked. The results of her latest bone marrow biopsy showed it remained packed with cancer cells.
As Martha absorbed the news quietly, her son, sitting next to her bedside with his hand on hers, spoke first. “What now?”
I looked at my attending and nodded, as we were fully ready to answer this question. From the outset, we knew that Martha’s leukemia carried a genetic mutation that unfortunately put her in a high-risk category. The chances of her cancer responding to the first round of chemotherapy were low. When this happens, what we typically do next is reinduction, we explained. It’s a different combination of chemotherapy drugs, with a somewhat different side effect profile. But it would give her the best chance of response, we believed. We could start the new chemotherapy as early as today, we said.
Martha took this in. “Okay,” she said pensively. “I’ve been thinking. And I think maybe … I won’t do chemotherapy anymore.”
Her words caught me off guard because, frankly, they seemed premature. Her leukemia had not budged with the first round of treatment. But we still had an option B, and then an option C. It was usually at a later, more dire stage – when multiple lines of treatment had not worked, and instead had only caused harm – or, when the decision was forced by the medical system’s admission that we had nothing left to offer – that I’d heard patients express similar preferences. It was then that I’d seen patients and their loved ones flip a mental switch and choose to focus the time they had left on what really mattered to them.
It didn’t feel like we were at that point.
And so, as we debriefed outside her room, my first instinct was to convince her otherwise.
However, Martha had other priorities, as I would come to learn. Above all else, she hated the hospital. She hated feeling trapped in a strange room that wasn’t hers; she hated how the chemotherapy stole her energy and made her feel too weak to even shower. She wanted to be in her own home. She wanted to eat her own food, sleep in her own bed, and be surrounded by what she recognized.
But, she also wanted to live. Two paths lay ahead of her. It was a trade-off of more suffering with a small chance at remission, versus accepting no chance of cure but feeling well for as long as she could. She soon clarified that she wasn’t definitely against chemotherapy. She couldn’t decide. She needed more information from us to make this decision, the hardest of her life.
Over the next few days, I watched as our attending physician expertly provided just that. There were actually three options, she laid out. There was aggressive chemotherapy, entailing at least 3 more weeks in the hospital and coming with significant risk of infection, nausea, vomiting, and fatigue. The chances of inducing a remission were about one in three to one in two, and that remission would likely last between several months and 2 years before the leukemia would relapse. The second option was a chemotherapy pill she could take at home, an option with fewer side effects but no longer aimed at cure. The third option was home hospice support, focused on symptoms, without any anticancer medication.
I noticed a few things during those conversations. I noticed how my attending took a navigator role, not pushing Martha in one direction or another, but rather imparting all the relevant information to empower Martha to decide for herself. I noticed how she provided realistic estimates, not hedging away from numbers, but giving the honest, nitty-gritty facts, as best as she could predict. I noticed how she took the time and never rushed, even in spite of external pressures to discharge the patient from the hospital.
There was no right or wrong answer. I no longer felt that we had something in our grasp – a clear-cut, best decision – to persuade Martha toward. Is one in three good odds, or bad odds? Is 2 years a long period of time, or a short one? Of course, there is no actual answer to these questions; the answer is as elusive and personal as if we had asked Martha: What do you think?
What I learned from Martha is that, with a devastating diagnosis, there isn’t a right time to make this decision. There isn’t one defining moment where we flip a switch and change course. It isn’t only when we run out of treatment options that the choice to forgo it makes sense. That option is on a flexible line, different for every person and priority. As my attending later said, with Martha’s diagnosis and her values, it wouldn’t have been unreasonable to decide against chemotherapy from the start.
The language we use can sometimes mask that reality. As doctors, we may casually slip in words like “need” and “have to” in response to patients’ questions about what to do next. “You need more chemotherapy,” we might say. “We’d have to treat it.” We have “treatment,” after all, and so we go down the line of offering what’s next in the medical algorithm. That word, too, can be deceivingly tempting, enticing down a road that makes it seem like the obvious answer – or the only one. If the choices are treatment versus not, who wouldn’t want the treatment? But the details are where things get murky. What does that treatment involve? What are the chances it will work, and for how long?
Surreptitiously missing from this language is the fact that there’s a choice. There’s always a choice, and it’s on the table at any point. You can start chemotherapy without committing to stick it out until the end. You can go home, if that is what’s important to you. The best treatment option is the one the patient wants.
After 4 days, Martha decided to go home with palliative chemotherapy and a bridge to hospice. Each member of our team hugged her goodbye and wished her luck. She was nervous. But she packed her hospital room, and she left.
I recently pulled up her medical chart, bracing myself for bad news. But the interesting thing about hospice is that even though the focus is no longer on prolonging life, people sometimes live longer.
She felt well, the most recent palliative note said. She was spending her time writing, getting her finances in order, and finishing a legacy project for her grandchildren.
For Martha, it seemed to be the right choice.
Dr. Yurkiewicz is a fellow in hematology and oncology at Stanford (Calif.) University. Follow her on Twitter @ilanayurkiewicz.
Martha Boxer (not her real name) had mentally prepared herself that this could happen, but the news still hit hard. Her doctors on the leukemia service broke the facts as gently as we could: The chemotherapy she had been suffering through for the last 2 weeks hadn’t worked. The results of her latest bone marrow biopsy showed it remained packed with cancer cells.
As Martha absorbed the news quietly, her son, sitting next to her bedside with his hand on hers, spoke first. “What now?”
I looked at my attending and nodded, as we were fully ready to answer this question. From the outset, we knew that Martha’s leukemia carried a genetic mutation that unfortunately put her in a high-risk category. The chances of her cancer responding to the first round of chemotherapy were low. When this happens, what we typically do next is reinduction, we explained. It’s a different combination of chemotherapy drugs, with a somewhat different side effect profile. But it would give her the best chance of response, we believed. We could start the new chemotherapy as early as today, we said.
Martha took this in. “Okay,” she said pensively. “I’ve been thinking. And I think maybe … I won’t do chemotherapy anymore.”
Her words caught me off guard because, frankly, they seemed premature. Her leukemia had not budged with the first round of treatment. But we still had an option B, and then an option C. It was usually at a later, more dire stage – when multiple lines of treatment had not worked, and instead had only caused harm – or, when the decision was forced by the medical system’s admission that we had nothing left to offer – that I’d heard patients express similar preferences. It was then that I’d seen patients and their loved ones flip a mental switch and choose to focus the time they had left on what really mattered to them.
It didn’t feel like we were at that point.
And so, as we debriefed outside her room, my first instinct was to convince her otherwise.
However, Martha had other priorities, as I would come to learn. Above all else, she hated the hospital. She hated feeling trapped in a strange room that wasn’t hers; she hated how the chemotherapy stole her energy and made her feel too weak to even shower. She wanted to be in her own home. She wanted to eat her own food, sleep in her own bed, and be surrounded by what she recognized.
But, she also wanted to live. Two paths lay ahead of her. It was a trade-off of more suffering with a small chance at remission, versus accepting no chance of cure but feeling well for as long as she could. She soon clarified that she wasn’t definitely against chemotherapy. She couldn’t decide. She needed more information from us to make this decision, the hardest of her life.
Over the next few days, I watched as our attending physician expertly provided just that. There were actually three options, she laid out. There was aggressive chemotherapy, entailing at least 3 more weeks in the hospital and coming with significant risk of infection, nausea, vomiting, and fatigue. The chances of inducing a remission were about one in three to one in two, and that remission would likely last between several months and 2 years before the leukemia would relapse. The second option was a chemotherapy pill she could take at home, an option with fewer side effects but no longer aimed at cure. The third option was home hospice support, focused on symptoms, without any anticancer medication.
I noticed a few things during those conversations. I noticed how my attending took a navigator role, not pushing Martha in one direction or another, but rather imparting all the relevant information to empower Martha to decide for herself. I noticed how she provided realistic estimates, not hedging away from numbers, but giving the honest, nitty-gritty facts, as best as she could predict. I noticed how she took the time and never rushed, even in spite of external pressures to discharge the patient from the hospital.
There was no right or wrong answer. I no longer felt that we had something in our grasp – a clear-cut, best decision – to persuade Martha toward. Is one in three good odds, or bad odds? Is 2 years a long period of time, or a short one? Of course, there is no actual answer to these questions; the answer is as elusive and personal as if we had asked Martha: What do you think?
What I learned from Martha is that, with a devastating diagnosis, there isn’t a right time to make this decision. There isn’t one defining moment where we flip a switch and change course. It isn’t only when we run out of treatment options that the choice to forgo it makes sense. That option is on a flexible line, different for every person and priority. As my attending later said, with Martha’s diagnosis and her values, it wouldn’t have been unreasonable to decide against chemotherapy from the start.
The language we use can sometimes mask that reality. As doctors, we may casually slip in words like “need” and “have to” in response to patients’ questions about what to do next. “You need more chemotherapy,” we might say. “We’d have to treat it.” We have “treatment,” after all, and so we go down the line of offering what’s next in the medical algorithm. That word, too, can be deceivingly tempting, enticing down a road that makes it seem like the obvious answer – or the only one. If the choices are treatment versus not, who wouldn’t want the treatment? But the details are where things get murky. What does that treatment involve? What are the chances it will work, and for how long?
Surreptitiously missing from this language is the fact that there’s a choice. There’s always a choice, and it’s on the table at any point. You can start chemotherapy without committing to stick it out until the end. You can go home, if that is what’s important to you. The best treatment option is the one the patient wants.
After 4 days, Martha decided to go home with palliative chemotherapy and a bridge to hospice. Each member of our team hugged her goodbye and wished her luck. She was nervous. But she packed her hospital room, and she left.
I recently pulled up her medical chart, bracing myself for bad news. But the interesting thing about hospice is that even though the focus is no longer on prolonging life, people sometimes live longer.
She felt well, the most recent palliative note said. She was spending her time writing, getting her finances in order, and finishing a legacy project for her grandchildren.
For Martha, it seemed to be the right choice.
Dr. Yurkiewicz is a fellow in hematology and oncology at Stanford (Calif.) University. Follow her on Twitter @ilanayurkiewicz.
Martha Boxer (not her real name) had mentally prepared herself that this could happen, but the news still hit hard. Her doctors on the leukemia service broke the facts as gently as we could: The chemotherapy she had been suffering through for the last 2 weeks hadn’t worked. The results of her latest bone marrow biopsy showed it remained packed with cancer cells.
As Martha absorbed the news quietly, her son, sitting next to her bedside with his hand on hers, spoke first. “What now?”
I looked at my attending and nodded, as we were fully ready to answer this question. From the outset, we knew that Martha’s leukemia carried a genetic mutation that unfortunately put her in a high-risk category. The chances of her cancer responding to the first round of chemotherapy were low. When this happens, what we typically do next is reinduction, we explained. It’s a different combination of chemotherapy drugs, with a somewhat different side effect profile. But it would give her the best chance of response, we believed. We could start the new chemotherapy as early as today, we said.
Martha took this in. “Okay,” she said pensively. “I’ve been thinking. And I think maybe … I won’t do chemotherapy anymore.”
Her words caught me off guard because, frankly, they seemed premature. Her leukemia had not budged with the first round of treatment. But we still had an option B, and then an option C. It was usually at a later, more dire stage – when multiple lines of treatment had not worked, and instead had only caused harm – or, when the decision was forced by the medical system’s admission that we had nothing left to offer – that I’d heard patients express similar preferences. It was then that I’d seen patients and their loved ones flip a mental switch and choose to focus the time they had left on what really mattered to them.
It didn’t feel like we were at that point.
And so, as we debriefed outside her room, my first instinct was to convince her otherwise.
However, Martha had other priorities, as I would come to learn. Above all else, she hated the hospital. She hated feeling trapped in a strange room that wasn’t hers; she hated how the chemotherapy stole her energy and made her feel too weak to even shower. She wanted to be in her own home. She wanted to eat her own food, sleep in her own bed, and be surrounded by what she recognized.
But, she also wanted to live. Two paths lay ahead of her. It was a trade-off of more suffering with a small chance at remission, versus accepting no chance of cure but feeling well for as long as she could. She soon clarified that she wasn’t definitely against chemotherapy. She couldn’t decide. She needed more information from us to make this decision, the hardest of her life.
Over the next few days, I watched as our attending physician expertly provided just that. There were actually three options, she laid out. There was aggressive chemotherapy, entailing at least 3 more weeks in the hospital and coming with significant risk of infection, nausea, vomiting, and fatigue. The chances of inducing a remission were about one in three to one in two, and that remission would likely last between several months and 2 years before the leukemia would relapse. The second option was a chemotherapy pill she could take at home, an option with fewer side effects but no longer aimed at cure. The third option was home hospice support, focused on symptoms, without any anticancer medication.
I noticed a few things during those conversations. I noticed how my attending took a navigator role, not pushing Martha in one direction or another, but rather imparting all the relevant information to empower Martha to decide for herself. I noticed how she provided realistic estimates, not hedging away from numbers, but giving the honest, nitty-gritty facts, as best as she could predict. I noticed how she took the time and never rushed, even in spite of external pressures to discharge the patient from the hospital.
There was no right or wrong answer. I no longer felt that we had something in our grasp – a clear-cut, best decision – to persuade Martha toward. Is one in three good odds, or bad odds? Is 2 years a long period of time, or a short one? Of course, there is no actual answer to these questions; the answer is as elusive and personal as if we had asked Martha: What do you think?
What I learned from Martha is that, with a devastating diagnosis, there isn’t a right time to make this decision. There isn’t one defining moment where we flip a switch and change course. It isn’t only when we run out of treatment options that the choice to forgo it makes sense. That option is on a flexible line, different for every person and priority. As my attending later said, with Martha’s diagnosis and her values, it wouldn’t have been unreasonable to decide against chemotherapy from the start.
The language we use can sometimes mask that reality. As doctors, we may casually slip in words like “need” and “have to” in response to patients’ questions about what to do next. “You need more chemotherapy,” we might say. “We’d have to treat it.” We have “treatment,” after all, and so we go down the line of offering what’s next in the medical algorithm. That word, too, can be deceivingly tempting, enticing down a road that makes it seem like the obvious answer – or the only one. If the choices are treatment versus not, who wouldn’t want the treatment? But the details are where things get murky. What does that treatment involve? What are the chances it will work, and for how long?
Surreptitiously missing from this language is the fact that there’s a choice. There’s always a choice, and it’s on the table at any point. You can start chemotherapy without committing to stick it out until the end. You can go home, if that is what’s important to you. The best treatment option is the one the patient wants.
After 4 days, Martha decided to go home with palliative chemotherapy and a bridge to hospice. Each member of our team hugged her goodbye and wished her luck. She was nervous. But she packed her hospital room, and she left.
I recently pulled up her medical chart, bracing myself for bad news. But the interesting thing about hospice is that even though the focus is no longer on prolonging life, people sometimes live longer.
She felt well, the most recent palliative note said. She was spending her time writing, getting her finances in order, and finishing a legacy project for her grandchildren.
For Martha, it seemed to be the right choice.
Dr. Yurkiewicz is a fellow in hematology and oncology at Stanford (Calif.) University. Follow her on Twitter @ilanayurkiewicz.