User login
Neal R. Axon, MD
Ed note: This article is the second in a series of interviews with members of Team Hospitalist: 12 hospital medicine experts who are serving a two-year term as special editorial consultants to our magazine.
Ever consider working as an academic hospitalist? Here to give you the scoop on what it’s like is “Team Hospitalist” member R. Neal Axon, MD, assistant professor of internal medicine and pediatrics at the Medical University of South Carolina (MUSC) in Charleston.
Dr. Axon completed his residency at Duke University Medical Center and received his medical degree from the University of Alabama School of Medicine in 2000.
Why is it important to conduct research in hospital medicine?
We haven’t perfected medicine just yet, and until we do we have to work to make it better. Even though hospital medicine research is different from clinical medicine, we need to have people who are working to make the systems of care better.
What attracted you to academic medicine?
I love teaching residents and medical students, and I missed doing it when I entered in private practice. I just completed my master’s at MUSC in clinical research. My department was very supportive and even paid my tuition.
Is it difficult to balance research work with shift work?
It’s definitely a challenge. Fortunately for me, my group does not have shift work in the traditional sense. We do have a night shift, but it’s something we do on an infrequent basis. It would be extremely difficult to do in a seven-on, seven-off schedule that most hospitalists have.
What type of research are you working on?
I’m currently doing some work with hypertension. One of the projects is doing survey work where we access the attitudes of providers (doctors and house staff) on what to do with patients who have hypertension. My observation has been that, in many cases, when patients are admitted to a hospital, they also have high blood pressure that may equate with hypertension in the outpatient setting. It’s not clear when that should be addressed--or how. This survey would help us understand that.
What do you like about what you do?
I worry more about what the department chief thinks than what the CEO of the hospital thinks. At community and non-teaching hospitals, the focus is much more on the bottom line.
So is it impossible to do research if you work at a non-teaching hospital?
I think it’s likely to be more difficult--in that setting--to be a pure clinical researcher, but I do think there are opportunities out there for every day hospitalists to participate in research. This is one of the things I’m currently working on as a member of the SHM Research Committee. One deliverable we’re excited about is the fact that there will be sessions at the [2009] annual meeting in Chicago that will specifically address how hospitalists can do research.
Another thing I hope can evolve is practice-based research networks, which exist in the primary care setting, but not so much in hospital medicine. These networks include groups of community doctors who band together to do clinical research projects. Central leadership helps the members of the group come up with research questions. This is something I’m working on in my state to develop, but this type of setup does exist in other areas.
What advice do you have for hospitalists who are interested in research?
The most important piece of advice is to find a good mentor.
The second thing is that most medical schools have master’s degree programs that teach you the skills that will get you started in clinical research. I went to medical school, but didn’t learn anything about biostatistics or trial design. TH
Ed note: This article is the second in a series of interviews with members of Team Hospitalist: 12 hospital medicine experts who are serving a two-year term as special editorial consultants to our magazine.
Ever consider working as an academic hospitalist? Here to give you the scoop on what it’s like is “Team Hospitalist” member R. Neal Axon, MD, assistant professor of internal medicine and pediatrics at the Medical University of South Carolina (MUSC) in Charleston.
Dr. Axon completed his residency at Duke University Medical Center and received his medical degree from the University of Alabama School of Medicine in 2000.
Why is it important to conduct research in hospital medicine?
We haven’t perfected medicine just yet, and until we do we have to work to make it better. Even though hospital medicine research is different from clinical medicine, we need to have people who are working to make the systems of care better.
What attracted you to academic medicine?
I love teaching residents and medical students, and I missed doing it when I entered in private practice. I just completed my master’s at MUSC in clinical research. My department was very supportive and even paid my tuition.
Is it difficult to balance research work with shift work?
It’s definitely a challenge. Fortunately for me, my group does not have shift work in the traditional sense. We do have a night shift, but it’s something we do on an infrequent basis. It would be extremely difficult to do in a seven-on, seven-off schedule that most hospitalists have.
What type of research are you working on?
I’m currently doing some work with hypertension. One of the projects is doing survey work where we access the attitudes of providers (doctors and house staff) on what to do with patients who have hypertension. My observation has been that, in many cases, when patients are admitted to a hospital, they also have high blood pressure that may equate with hypertension in the outpatient setting. It’s not clear when that should be addressed--or how. This survey would help us understand that.
What do you like about what you do?
I worry more about what the department chief thinks than what the CEO of the hospital thinks. At community and non-teaching hospitals, the focus is much more on the bottom line.
So is it impossible to do research if you work at a non-teaching hospital?
I think it’s likely to be more difficult--in that setting--to be a pure clinical researcher, but I do think there are opportunities out there for every day hospitalists to participate in research. This is one of the things I’m currently working on as a member of the SHM Research Committee. One deliverable we’re excited about is the fact that there will be sessions at the [2009] annual meeting in Chicago that will specifically address how hospitalists can do research.
Another thing I hope can evolve is practice-based research networks, which exist in the primary care setting, but not so much in hospital medicine. These networks include groups of community doctors who band together to do clinical research projects. Central leadership helps the members of the group come up with research questions. This is something I’m working on in my state to develop, but this type of setup does exist in other areas.
What advice do you have for hospitalists who are interested in research?
The most important piece of advice is to find a good mentor.
The second thing is that most medical schools have master’s degree programs that teach you the skills that will get you started in clinical research. I went to medical school, but didn’t learn anything about biostatistics or trial design. TH
Ed note: This article is the second in a series of interviews with members of Team Hospitalist: 12 hospital medicine experts who are serving a two-year term as special editorial consultants to our magazine.
Ever consider working as an academic hospitalist? Here to give you the scoop on what it’s like is “Team Hospitalist” member R. Neal Axon, MD, assistant professor of internal medicine and pediatrics at the Medical University of South Carolina (MUSC) in Charleston.
Dr. Axon completed his residency at Duke University Medical Center and received his medical degree from the University of Alabama School of Medicine in 2000.
Why is it important to conduct research in hospital medicine?
We haven’t perfected medicine just yet, and until we do we have to work to make it better. Even though hospital medicine research is different from clinical medicine, we need to have people who are working to make the systems of care better.
What attracted you to academic medicine?
I love teaching residents and medical students, and I missed doing it when I entered in private practice. I just completed my master’s at MUSC in clinical research. My department was very supportive and even paid my tuition.
Is it difficult to balance research work with shift work?
It’s definitely a challenge. Fortunately for me, my group does not have shift work in the traditional sense. We do have a night shift, but it’s something we do on an infrequent basis. It would be extremely difficult to do in a seven-on, seven-off schedule that most hospitalists have.
What type of research are you working on?
I’m currently doing some work with hypertension. One of the projects is doing survey work where we access the attitudes of providers (doctors and house staff) on what to do with patients who have hypertension. My observation has been that, in many cases, when patients are admitted to a hospital, they also have high blood pressure that may equate with hypertension in the outpatient setting. It’s not clear when that should be addressed--or how. This survey would help us understand that.
What do you like about what you do?
I worry more about what the department chief thinks than what the CEO of the hospital thinks. At community and non-teaching hospitals, the focus is much more on the bottom line.
So is it impossible to do research if you work at a non-teaching hospital?
I think it’s likely to be more difficult--in that setting--to be a pure clinical researcher, but I do think there are opportunities out there for every day hospitalists to participate in research. This is one of the things I’m currently working on as a member of the SHM Research Committee. One deliverable we’re excited about is the fact that there will be sessions at the [2009] annual meeting in Chicago that will specifically address how hospitalists can do research.
Another thing I hope can evolve is practice-based research networks, which exist in the primary care setting, but not so much in hospital medicine. These networks include groups of community doctors who band together to do clinical research projects. Central leadership helps the members of the group come up with research questions. This is something I’m working on in my state to develop, but this type of setup does exist in other areas.
What advice do you have for hospitalists who are interested in research?
The most important piece of advice is to find a good mentor.
The second thing is that most medical schools have master’s degree programs that teach you the skills that will get you started in clinical research. I went to medical school, but didn’t learn anything about biostatistics or trial design. TH
SHM Invests in 'Champions' and SHM's Future
Not content with the status quo, SHM has taken significant steps to ensure the society’s reach grows in line with the exponential expansion of hospital medicine. These investments put SHM’s premier educational content into the hands of more hospitalists and increase the society’s ability to hear firsthand—and more quickly react to—the challenges of the field.
One key initiative is SHM’s Champions Program, which identifies hospitalists on the ground to serve as information conduits. These “champions” share feedback with SHM’s staff and leadership about the state of hospital medicine locally, and react to SHM plans and proposals. They also help SHM disseminate news and resources to hospitalists in their communities.
In the Champions Program’s short tenure, it already has proven its value. Champions in 26 key markets are engaged and providing valuable feedback in a wide variety of areas, through participation on conference calls, individual surveys, and even a private breakfast with SHM CEO Larry Wellikson.
On top of that, the program has enabled SHM to expand its pool of identified hospitalists by nearly 60%. This expansion not only means SHM’s education and quality improvement resources will reach more communities, but that the society will gain clout and credibility in all facets of healthcare from divergent groups, such as MedPAC and Congress.
Champions are leaders in their communities, dedicated to making a difference in hospital medicine. If this describes you, consider becoming a Champion. Committing to this vital program means helping to steer the course of hospital medicine locally and globally. If you aspire to become a leader within the society, the Champions Program is a great way to begin.
If you’re interested in becoming a Champion or want to suggest someone in your community who would be a good fit, call Cathy Peduzzi, SHM’s manager of membership outreach programs at (215) 351-2584 or e-mail her at cpeduzzi@hospitalmedicine.org.
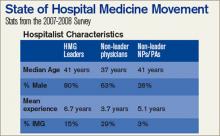
The SHM Bi-Annual Survey on the State of the Hospital Medicine Movement has quickly become a must-have resource for hospital medicine group leaders, administrators, and hospitalists. This survey provides baseline information on topics such as hospital medicine group financial support, compensation and employment models, and productivity.
Whether you’re an experienced hospitalist or you are just starting out in the specialty, the survey has information that will benefit you. Here’s a sampling of the frequently asked questions that can be answered using information found in the survey:
Q: I’m recruiting hospitalists for a hospital medicine group on the East Coast. What’s the average salary for a hospitalist in my area?
A: The average salary for the hospitalist on the East Coast is $189,400, compared to the national average of $193,300.
Q: As a hospital medicine group leader, should I expect financial support for my program?
A: According to the survey, 91% of HMGs receive some kind of financial backing for their program, with an average of $97,275 in support per FTE physician.
Q: How do hospital medicine groups handle night coverage?
A: Currently, 53% of HMGs have an on-site provider, 27% have a hospitalist on-call at home, 16% have a combination of on-site and on-call coverage, and 3% have no night coverage at all.
To purchase the “2007-2008 Bi-Annual Survey on the State of the Hospital Medicine Movement,” visit www.hospital medicine.org/survey.
Not content with the status quo, SHM has taken significant steps to ensure the society’s reach grows in line with the exponential expansion of hospital medicine. These investments put SHM’s premier educational content into the hands of more hospitalists and increase the society’s ability to hear firsthand—and more quickly react to—the challenges of the field.
One key initiative is SHM’s Champions Program, which identifies hospitalists on the ground to serve as information conduits. These “champions” share feedback with SHM’s staff and leadership about the state of hospital medicine locally, and react to SHM plans and proposals. They also help SHM disseminate news and resources to hospitalists in their communities.
In the Champions Program’s short tenure, it already has proven its value. Champions in 26 key markets are engaged and providing valuable feedback in a wide variety of areas, through participation on conference calls, individual surveys, and even a private breakfast with SHM CEO Larry Wellikson.
On top of that, the program has enabled SHM to expand its pool of identified hospitalists by nearly 60%. This expansion not only means SHM’s education and quality improvement resources will reach more communities, but that the society will gain clout and credibility in all facets of healthcare from divergent groups, such as MedPAC and Congress.
Champions are leaders in their communities, dedicated to making a difference in hospital medicine. If this describes you, consider becoming a Champion. Committing to this vital program means helping to steer the course of hospital medicine locally and globally. If you aspire to become a leader within the society, the Champions Program is a great way to begin.
If you’re interested in becoming a Champion or want to suggest someone in your community who would be a good fit, call Cathy Peduzzi, SHM’s manager of membership outreach programs at (215) 351-2584 or e-mail her at cpeduzzi@hospitalmedicine.org.
The SHM Bi-Annual Survey on the State of the Hospital Medicine Movement has quickly become a must-have resource for hospital medicine group leaders, administrators, and hospitalists. This survey provides baseline information on topics such as hospital medicine group financial support, compensation and employment models, and productivity.
Whether you’re an experienced hospitalist or you are just starting out in the specialty, the survey has information that will benefit you. Here’s a sampling of the frequently asked questions that can be answered using information found in the survey:
Q: I’m recruiting hospitalists for a hospital medicine group on the East Coast. What’s the average salary for a hospitalist in my area?
A: The average salary for the hospitalist on the East Coast is $189,400, compared to the national average of $193,300.
Q: As a hospital medicine group leader, should I expect financial support for my program?
A: According to the survey, 91% of HMGs receive some kind of financial backing for their program, with an average of $97,275 in support per FTE physician.
Q: How do hospital medicine groups handle night coverage?
A: Currently, 53% of HMGs have an on-site provider, 27% have a hospitalist on-call at home, 16% have a combination of on-site and on-call coverage, and 3% have no night coverage at all.
To purchase the “2007-2008 Bi-Annual Survey on the State of the Hospital Medicine Movement,” visit www.hospital medicine.org/survey.
Not content with the status quo, SHM has taken significant steps to ensure the society’s reach grows in line with the exponential expansion of hospital medicine. These investments put SHM’s premier educational content into the hands of more hospitalists and increase the society’s ability to hear firsthand—and more quickly react to—the challenges of the field.
One key initiative is SHM’s Champions Program, which identifies hospitalists on the ground to serve as information conduits. These “champions” share feedback with SHM’s staff and leadership about the state of hospital medicine locally, and react to SHM plans and proposals. They also help SHM disseminate news and resources to hospitalists in their communities.
In the Champions Program’s short tenure, it already has proven its value. Champions in 26 key markets are engaged and providing valuable feedback in a wide variety of areas, through participation on conference calls, individual surveys, and even a private breakfast with SHM CEO Larry Wellikson.
On top of that, the program has enabled SHM to expand its pool of identified hospitalists by nearly 60%. This expansion not only means SHM’s education and quality improvement resources will reach more communities, but that the society will gain clout and credibility in all facets of healthcare from divergent groups, such as MedPAC and Congress.
Champions are leaders in their communities, dedicated to making a difference in hospital medicine. If this describes you, consider becoming a Champion. Committing to this vital program means helping to steer the course of hospital medicine locally and globally. If you aspire to become a leader within the society, the Champions Program is a great way to begin.
If you’re interested in becoming a Champion or want to suggest someone in your community who would be a good fit, call Cathy Peduzzi, SHM’s manager of membership outreach programs at (215) 351-2584 or e-mail her at cpeduzzi@hospitalmedicine.org.
The SHM Bi-Annual Survey on the State of the Hospital Medicine Movement has quickly become a must-have resource for hospital medicine group leaders, administrators, and hospitalists. This survey provides baseline information on topics such as hospital medicine group financial support, compensation and employment models, and productivity.
Whether you’re an experienced hospitalist or you are just starting out in the specialty, the survey has information that will benefit you. Here’s a sampling of the frequently asked questions that can be answered using information found in the survey:
Q: I’m recruiting hospitalists for a hospital medicine group on the East Coast. What’s the average salary for a hospitalist in my area?
A: The average salary for the hospitalist on the East Coast is $189,400, compared to the national average of $193,300.
Q: As a hospital medicine group leader, should I expect financial support for my program?
A: According to the survey, 91% of HMGs receive some kind of financial backing for their program, with an average of $97,275 in support per FTE physician.
Q: How do hospital medicine groups handle night coverage?
A: Currently, 53% of HMGs have an on-site provider, 27% have a hospitalist on-call at home, 16% have a combination of on-site and on-call coverage, and 3% have no night coverage at all.
To purchase the “2007-2008 Bi-Annual Survey on the State of the Hospital Medicine Movement,” visit www.hospital medicine.org/survey.
A Bumpy Bundling Road Ahead
Patrick J. Torcson, MD, MMM, FACP, laughs when he recalls his initial reaction to the proposal to bundle Medicare payments to hospitals: “If this passes legislation, I’m moving to Dubai.”
Dr. Torcson, chairman of SHM’s Performance and Standards Committee, and medical director of the hospitalist program at St. Tammany Parish Hospital in Covington, La., has since tempered his thinking. Like many physicians, he understands the need for Medicare to address growing costs. Nevertheless, he is wary about the bundling proposal in June’s Medicare Payment Advisory Commission (MedPAC) report to Congress.
Dr. Torcson’s opinion about reforming the nation’s healthcare delivery system points to the difficult dichotomy facing hospitalists and other physicians: they agree change is necessary, but worry about the consequences of bundling payments.
Under the new model, rather than pay for each service provided, Medicare would reimburse a lump sum for all treatment linked to an episode of care for conditions such as congestive heart failure, chronic obstructive pulmonary disease or cardiac bypass surgery. In addition, the Centers for Medicare & Medicaid Services (CMS) would provide hospitals and physicians with reports detailing their resource use and readmission rates for specific episodes of inpatient care. After two years, the providers’ reports would become public.
Another proposal would cut payments to hospitals with high risk-adjusted readmission rates for select conditions while urging Congress to ease gainsharing restrictions to financially reward physicians helping hospitals improve readmission rates and overall patient care.
—Eric Siegal, MD, chairman of SHM’s Public Policy Committee and regional medical director for Cogent Healthcare
The switch to bundling, Dr. Torcson says, could entice hospitalists to encourage a healthcare delivery model that fosters collective accountability. He and other physicians warn the system could just as easily create imbalances in power, provide incentives for withholding care and spell disaster for rural physicians and ill-prepared networks.
“Philosophically, it’s a nice idea, but I don’t think it’s realistic and I don’t think hospitals that have a small budget will be able to survive it,” says Rachel Lovins, MD, director of the hospitalist program at Waterbury Hospital in Connecticut. Dividing a bundled payment equally amongst hospital departments “will be close to impossible,” she says, and struggling hospitals will fall further into debt. The new system also may leave providers with inadequate resources and lead to angry outpatient doctors who refuse to accept Medicare patients.
Part of the problem, according to Eric Siegal, MD, chairman of SHM’s Public Policy Committee and regional medical director for Cogent Healthcare, is how little physicians know about the effects of the new plan. “Everyone understands that this is a dramatic paradigm shift,” he says. “Bundling is a really radical change. It’s going to generate all kinds of consequences—intended and unintended—and no one really has a handle on what’s going to happen.”
The Status Quo Must Change
One of few points of agreement is that the status quo is untenable. A recent summary of MedPAC’s report in The New England Journal of Medicine blamed the fee-for-service model for fueling negative aspects of the current healthcare system and warned of an escalation in Medicare spending. “Fee-for-service payment spurs spending growth, supports a fragmented and compartmentalized delivery system and does nothing to reward quality or value,” the MedPAC authors write.
Though some physicians remain cautiously optimistic about bundling, Dr. Siegal doubts the model is ready for primetime. “Bundling says, ‘Let’s create accountability for outcomes by not paying for single services but for an entire episode of care,’” he says. But many questions remain unanswered. What constitutes an episode? Who controls the allocation of the Medicare payment? If an episode is defined as 30 days from when a patient enters the hospital for a specific procedure, are other health providers accountable for addressing unrelated complaints within the same episode window?
“There are an enormous number of ways you can contort and twist this so you can create scenarios where people come out of it feeling like they have been penalized for behavior well outside their control,” Dr. Siegal says. “I think those are the big risks: imbalance of power and how do you create lines of accountability that actually make sense?”
But, he says, a system that promotes collective responsibility for patients could create a compelling incentive for more collaboration between physicians and consultants. “I think if ‘done right,’ and I say that in quotations because nobody knows what ‘done right’ means,” Dr. Siegal says, “bundling could actually be huge for hospitalists.”
Mary Dallas, MD, medical information officer at Presbyterian Healthcare Services in Albuquerque, N.M., says she’s seen first hand how collaboration among a health plan, a hospital and physicians can improve quality and balance finances. “Those efforts were really spawned out of a forced alignment between all groups in order to focus on common goals,” she says. Bundling could force a similar convergence of priorities.
Transition Troubles
If “alignment” has become the favorite watchword in bundling discussions, “gainsharing” as a concept has been greeted by far more ambivalence. “I oppose gainsharing that puts money directly into the physician’s pocket,” Dr. Dallas says. “This is something that occurs frequently in the free-market world in other industries, and to pay a few for the success that demands participation of many is just wrong in my book.”
Instead, Dr. Dallas supports the idea of directing monetary rewards toward improving infrastructure and the overall healthcare delivery process. As examples, she suggests using the money to buy time from physicians willing to be involved in pilot projects aimed at improving the delivery process, or to add more resources for better patient continuity between the hospital and the community.
Even with a more equitable distribution of resources, she and other hospitalists concede any transition to bundling could be bumpy. “If I were an independent physician, and my personal payment from Medicare was dependent on or tied to the hospital’s performance,” Dr. Dallas says, “there would be a lot of work to prepare me for this.”
As several hospitalists warn, a bundling system could trigger ratcheting down of care—and a whole new set of headaches. “The concern for everyone is that it is going to incentivize physicians to give less services,” says Jonathan Lovins, MD, director of hospitalist and midlevel practitioner services at the Hospital of Central Connecticut, and the brother of Waterbury Hospital’s Dr. Rachel Lovins.
Several hospitalists say the problem could be similar to what happened with Medicare’s capitation system, which gained traction in the 1980s and peaked in the mid-1990s before waning because of a backlash by both providers and patients. This fixed pre-payment reimbursement system, Dr. Lovins says, created an inherent conflict of interest for primary caregivers because referring patients to specialists for tests lowered profits whereas delivering fewer services did not.
Bradley Flansbaum, DO, MPH, FACP, director of hospitalist services at Lenox Hill Hospital in New York City, goes a step further, calling the bundling plan capitation on steroids, and cautions that a one-size-fits-all system is bound to fail. “Having technology and having the intellectual firepower to figure out how this system is going to manage the bundled payment is an advantage,” he says, adding that larger hospitals are more likely to have this advantage. For rural physicians or those within inefficient networks, bundling payments could be disastrous. “CMS may just say, ‘We’re turning on the lights tomorrow and tough,’ but it’s going to be a hell of a mess if they do,” he says.
A Demonstration Project
A demonstration project slated to begin in four states next year may show just how steep that learning curve is. The Acute Care Episode (ACE) Demonstration would bundle virtually all payments of certain orthopedic and cardiovascular inpatient procedures at participating hospitals in Texas, Oklahoma, New Mexico, and Colorado. Hospitals and physicians still would receive separate fee-for-service payments, but a confidential report would detail their resource use. High-resource providers would incur penalties while low-resource providers would receive bonuses.
Dr. Siegal says it makes sense to begin a bundling pilot project with procedures that have defined treatment windows, such as hip replacements or open heart surgery. Other conditions will be far more difficult to contain within a neatly defined episode. “Clearly, we’re going to have to figure out what to do with heart failure and pneumonia and stroke because those are huge consumers of dollars,” he says.
Despite all the caveats and unknowns, hospitalists still may have much to gain if bundling follows in the footsteps of the successful diagnosis-related group (DRG) payment system. “I think that enlightened hospital CEOs are going to be looking to their hospitalists as their champions to really pull this off and make this work,” Dr. Torcson says, particularly as the stakes for hospitals increase.
Medicare’s Physician Quality Reporting Initiative, established by the 2006 Tax Relief and Health Care Act, already links a 1.5% financial incentive to increased performance. With bundled payments adjusted up or down by the proposed gainsharing and penalties for higher readmission rates, Dr. Torcson says, the equivalent of 3% to 5% of DRG payments could be at risk.
“I think it could be something potentially very beneficial to those hospitalist groups that get it right,” he says. “It’s kind of like before the Ice Age comes, if you’ve got your animal skins ready, those are the tribes that survive.” TH
Gretchen Henkel is a medical writer based in California.
Patrick J. Torcson, MD, MMM, FACP, laughs when he recalls his initial reaction to the proposal to bundle Medicare payments to hospitals: “If this passes legislation, I’m moving to Dubai.”
Dr. Torcson, chairman of SHM’s Performance and Standards Committee, and medical director of the hospitalist program at St. Tammany Parish Hospital in Covington, La., has since tempered his thinking. Like many physicians, he understands the need for Medicare to address growing costs. Nevertheless, he is wary about the bundling proposal in June’s Medicare Payment Advisory Commission (MedPAC) report to Congress.
Dr. Torcson’s opinion about reforming the nation’s healthcare delivery system points to the difficult dichotomy facing hospitalists and other physicians: they agree change is necessary, but worry about the consequences of bundling payments.
Under the new model, rather than pay for each service provided, Medicare would reimburse a lump sum for all treatment linked to an episode of care for conditions such as congestive heart failure, chronic obstructive pulmonary disease or cardiac bypass surgery. In addition, the Centers for Medicare & Medicaid Services (CMS) would provide hospitals and physicians with reports detailing their resource use and readmission rates for specific episodes of inpatient care. After two years, the providers’ reports would become public.
Another proposal would cut payments to hospitals with high risk-adjusted readmission rates for select conditions while urging Congress to ease gainsharing restrictions to financially reward physicians helping hospitals improve readmission rates and overall patient care.
—Eric Siegal, MD, chairman of SHM’s Public Policy Committee and regional medical director for Cogent Healthcare
The switch to bundling, Dr. Torcson says, could entice hospitalists to encourage a healthcare delivery model that fosters collective accountability. He and other physicians warn the system could just as easily create imbalances in power, provide incentives for withholding care and spell disaster for rural physicians and ill-prepared networks.
“Philosophically, it’s a nice idea, but I don’t think it’s realistic and I don’t think hospitals that have a small budget will be able to survive it,” says Rachel Lovins, MD, director of the hospitalist program at Waterbury Hospital in Connecticut. Dividing a bundled payment equally amongst hospital departments “will be close to impossible,” she says, and struggling hospitals will fall further into debt. The new system also may leave providers with inadequate resources and lead to angry outpatient doctors who refuse to accept Medicare patients.
Part of the problem, according to Eric Siegal, MD, chairman of SHM’s Public Policy Committee and regional medical director for Cogent Healthcare, is how little physicians know about the effects of the new plan. “Everyone understands that this is a dramatic paradigm shift,” he says. “Bundling is a really radical change. It’s going to generate all kinds of consequences—intended and unintended—and no one really has a handle on what’s going to happen.”
The Status Quo Must Change
One of few points of agreement is that the status quo is untenable. A recent summary of MedPAC’s report in The New England Journal of Medicine blamed the fee-for-service model for fueling negative aspects of the current healthcare system and warned of an escalation in Medicare spending. “Fee-for-service payment spurs spending growth, supports a fragmented and compartmentalized delivery system and does nothing to reward quality or value,” the MedPAC authors write.
Though some physicians remain cautiously optimistic about bundling, Dr. Siegal doubts the model is ready for primetime. “Bundling says, ‘Let’s create accountability for outcomes by not paying for single services but for an entire episode of care,’” he says. But many questions remain unanswered. What constitutes an episode? Who controls the allocation of the Medicare payment? If an episode is defined as 30 days from when a patient enters the hospital for a specific procedure, are other health providers accountable for addressing unrelated complaints within the same episode window?
“There are an enormous number of ways you can contort and twist this so you can create scenarios where people come out of it feeling like they have been penalized for behavior well outside their control,” Dr. Siegal says. “I think those are the big risks: imbalance of power and how do you create lines of accountability that actually make sense?”
But, he says, a system that promotes collective responsibility for patients could create a compelling incentive for more collaboration between physicians and consultants. “I think if ‘done right,’ and I say that in quotations because nobody knows what ‘done right’ means,” Dr. Siegal says, “bundling could actually be huge for hospitalists.”
Mary Dallas, MD, medical information officer at Presbyterian Healthcare Services in Albuquerque, N.M., says she’s seen first hand how collaboration among a health plan, a hospital and physicians can improve quality and balance finances. “Those efforts were really spawned out of a forced alignment between all groups in order to focus on common goals,” she says. Bundling could force a similar convergence of priorities.
Transition Troubles
If “alignment” has become the favorite watchword in bundling discussions, “gainsharing” as a concept has been greeted by far more ambivalence. “I oppose gainsharing that puts money directly into the physician’s pocket,” Dr. Dallas says. “This is something that occurs frequently in the free-market world in other industries, and to pay a few for the success that demands participation of many is just wrong in my book.”
Instead, Dr. Dallas supports the idea of directing monetary rewards toward improving infrastructure and the overall healthcare delivery process. As examples, she suggests using the money to buy time from physicians willing to be involved in pilot projects aimed at improving the delivery process, or to add more resources for better patient continuity between the hospital and the community.
Even with a more equitable distribution of resources, she and other hospitalists concede any transition to bundling could be bumpy. “If I were an independent physician, and my personal payment from Medicare was dependent on or tied to the hospital’s performance,” Dr. Dallas says, “there would be a lot of work to prepare me for this.”
As several hospitalists warn, a bundling system could trigger ratcheting down of care—and a whole new set of headaches. “The concern for everyone is that it is going to incentivize physicians to give less services,” says Jonathan Lovins, MD, director of hospitalist and midlevel practitioner services at the Hospital of Central Connecticut, and the brother of Waterbury Hospital’s Dr. Rachel Lovins.
Several hospitalists say the problem could be similar to what happened with Medicare’s capitation system, which gained traction in the 1980s and peaked in the mid-1990s before waning because of a backlash by both providers and patients. This fixed pre-payment reimbursement system, Dr. Lovins says, created an inherent conflict of interest for primary caregivers because referring patients to specialists for tests lowered profits whereas delivering fewer services did not.
Bradley Flansbaum, DO, MPH, FACP, director of hospitalist services at Lenox Hill Hospital in New York City, goes a step further, calling the bundling plan capitation on steroids, and cautions that a one-size-fits-all system is bound to fail. “Having technology and having the intellectual firepower to figure out how this system is going to manage the bundled payment is an advantage,” he says, adding that larger hospitals are more likely to have this advantage. For rural physicians or those within inefficient networks, bundling payments could be disastrous. “CMS may just say, ‘We’re turning on the lights tomorrow and tough,’ but it’s going to be a hell of a mess if they do,” he says.
A Demonstration Project
A demonstration project slated to begin in four states next year may show just how steep that learning curve is. The Acute Care Episode (ACE) Demonstration would bundle virtually all payments of certain orthopedic and cardiovascular inpatient procedures at participating hospitals in Texas, Oklahoma, New Mexico, and Colorado. Hospitals and physicians still would receive separate fee-for-service payments, but a confidential report would detail their resource use. High-resource providers would incur penalties while low-resource providers would receive bonuses.
Dr. Siegal says it makes sense to begin a bundling pilot project with procedures that have defined treatment windows, such as hip replacements or open heart surgery. Other conditions will be far more difficult to contain within a neatly defined episode. “Clearly, we’re going to have to figure out what to do with heart failure and pneumonia and stroke because those are huge consumers of dollars,” he says.
Despite all the caveats and unknowns, hospitalists still may have much to gain if bundling follows in the footsteps of the successful diagnosis-related group (DRG) payment system. “I think that enlightened hospital CEOs are going to be looking to their hospitalists as their champions to really pull this off and make this work,” Dr. Torcson says, particularly as the stakes for hospitals increase.
Medicare’s Physician Quality Reporting Initiative, established by the 2006 Tax Relief and Health Care Act, already links a 1.5% financial incentive to increased performance. With bundled payments adjusted up or down by the proposed gainsharing and penalties for higher readmission rates, Dr. Torcson says, the equivalent of 3% to 5% of DRG payments could be at risk.
“I think it could be something potentially very beneficial to those hospitalist groups that get it right,” he says. “It’s kind of like before the Ice Age comes, if you’ve got your animal skins ready, those are the tribes that survive.” TH
Gretchen Henkel is a medical writer based in California.
Patrick J. Torcson, MD, MMM, FACP, laughs when he recalls his initial reaction to the proposal to bundle Medicare payments to hospitals: “If this passes legislation, I’m moving to Dubai.”
Dr. Torcson, chairman of SHM’s Performance and Standards Committee, and medical director of the hospitalist program at St. Tammany Parish Hospital in Covington, La., has since tempered his thinking. Like many physicians, he understands the need for Medicare to address growing costs. Nevertheless, he is wary about the bundling proposal in June’s Medicare Payment Advisory Commission (MedPAC) report to Congress.
Dr. Torcson’s opinion about reforming the nation’s healthcare delivery system points to the difficult dichotomy facing hospitalists and other physicians: they agree change is necessary, but worry about the consequences of bundling payments.
Under the new model, rather than pay for each service provided, Medicare would reimburse a lump sum for all treatment linked to an episode of care for conditions such as congestive heart failure, chronic obstructive pulmonary disease or cardiac bypass surgery. In addition, the Centers for Medicare & Medicaid Services (CMS) would provide hospitals and physicians with reports detailing their resource use and readmission rates for specific episodes of inpatient care. After two years, the providers’ reports would become public.
Another proposal would cut payments to hospitals with high risk-adjusted readmission rates for select conditions while urging Congress to ease gainsharing restrictions to financially reward physicians helping hospitals improve readmission rates and overall patient care.
—Eric Siegal, MD, chairman of SHM’s Public Policy Committee and regional medical director for Cogent Healthcare
The switch to bundling, Dr. Torcson says, could entice hospitalists to encourage a healthcare delivery model that fosters collective accountability. He and other physicians warn the system could just as easily create imbalances in power, provide incentives for withholding care and spell disaster for rural physicians and ill-prepared networks.
“Philosophically, it’s a nice idea, but I don’t think it’s realistic and I don’t think hospitals that have a small budget will be able to survive it,” says Rachel Lovins, MD, director of the hospitalist program at Waterbury Hospital in Connecticut. Dividing a bundled payment equally amongst hospital departments “will be close to impossible,” she says, and struggling hospitals will fall further into debt. The new system also may leave providers with inadequate resources and lead to angry outpatient doctors who refuse to accept Medicare patients.
Part of the problem, according to Eric Siegal, MD, chairman of SHM’s Public Policy Committee and regional medical director for Cogent Healthcare, is how little physicians know about the effects of the new plan. “Everyone understands that this is a dramatic paradigm shift,” he says. “Bundling is a really radical change. It’s going to generate all kinds of consequences—intended and unintended—and no one really has a handle on what’s going to happen.”
The Status Quo Must Change
One of few points of agreement is that the status quo is untenable. A recent summary of MedPAC’s report in The New England Journal of Medicine blamed the fee-for-service model for fueling negative aspects of the current healthcare system and warned of an escalation in Medicare spending. “Fee-for-service payment spurs spending growth, supports a fragmented and compartmentalized delivery system and does nothing to reward quality or value,” the MedPAC authors write.
Though some physicians remain cautiously optimistic about bundling, Dr. Siegal doubts the model is ready for primetime. “Bundling says, ‘Let’s create accountability for outcomes by not paying for single services but for an entire episode of care,’” he says. But many questions remain unanswered. What constitutes an episode? Who controls the allocation of the Medicare payment? If an episode is defined as 30 days from when a patient enters the hospital for a specific procedure, are other health providers accountable for addressing unrelated complaints within the same episode window?
“There are an enormous number of ways you can contort and twist this so you can create scenarios where people come out of it feeling like they have been penalized for behavior well outside their control,” Dr. Siegal says. “I think those are the big risks: imbalance of power and how do you create lines of accountability that actually make sense?”
But, he says, a system that promotes collective responsibility for patients could create a compelling incentive for more collaboration between physicians and consultants. “I think if ‘done right,’ and I say that in quotations because nobody knows what ‘done right’ means,” Dr. Siegal says, “bundling could actually be huge for hospitalists.”
Mary Dallas, MD, medical information officer at Presbyterian Healthcare Services in Albuquerque, N.M., says she’s seen first hand how collaboration among a health plan, a hospital and physicians can improve quality and balance finances. “Those efforts were really spawned out of a forced alignment between all groups in order to focus on common goals,” she says. Bundling could force a similar convergence of priorities.
Transition Troubles
If “alignment” has become the favorite watchword in bundling discussions, “gainsharing” as a concept has been greeted by far more ambivalence. “I oppose gainsharing that puts money directly into the physician’s pocket,” Dr. Dallas says. “This is something that occurs frequently in the free-market world in other industries, and to pay a few for the success that demands participation of many is just wrong in my book.”
Instead, Dr. Dallas supports the idea of directing monetary rewards toward improving infrastructure and the overall healthcare delivery process. As examples, she suggests using the money to buy time from physicians willing to be involved in pilot projects aimed at improving the delivery process, or to add more resources for better patient continuity between the hospital and the community.
Even with a more equitable distribution of resources, she and other hospitalists concede any transition to bundling could be bumpy. “If I were an independent physician, and my personal payment from Medicare was dependent on or tied to the hospital’s performance,” Dr. Dallas says, “there would be a lot of work to prepare me for this.”
As several hospitalists warn, a bundling system could trigger ratcheting down of care—and a whole new set of headaches. “The concern for everyone is that it is going to incentivize physicians to give less services,” says Jonathan Lovins, MD, director of hospitalist and midlevel practitioner services at the Hospital of Central Connecticut, and the brother of Waterbury Hospital’s Dr. Rachel Lovins.
Several hospitalists say the problem could be similar to what happened with Medicare’s capitation system, which gained traction in the 1980s and peaked in the mid-1990s before waning because of a backlash by both providers and patients. This fixed pre-payment reimbursement system, Dr. Lovins says, created an inherent conflict of interest for primary caregivers because referring patients to specialists for tests lowered profits whereas delivering fewer services did not.
Bradley Flansbaum, DO, MPH, FACP, director of hospitalist services at Lenox Hill Hospital in New York City, goes a step further, calling the bundling plan capitation on steroids, and cautions that a one-size-fits-all system is bound to fail. “Having technology and having the intellectual firepower to figure out how this system is going to manage the bundled payment is an advantage,” he says, adding that larger hospitals are more likely to have this advantage. For rural physicians or those within inefficient networks, bundling payments could be disastrous. “CMS may just say, ‘We’re turning on the lights tomorrow and tough,’ but it’s going to be a hell of a mess if they do,” he says.
A Demonstration Project
A demonstration project slated to begin in four states next year may show just how steep that learning curve is. The Acute Care Episode (ACE) Demonstration would bundle virtually all payments of certain orthopedic and cardiovascular inpatient procedures at participating hospitals in Texas, Oklahoma, New Mexico, and Colorado. Hospitals and physicians still would receive separate fee-for-service payments, but a confidential report would detail their resource use. High-resource providers would incur penalties while low-resource providers would receive bonuses.
Dr. Siegal says it makes sense to begin a bundling pilot project with procedures that have defined treatment windows, such as hip replacements or open heart surgery. Other conditions will be far more difficult to contain within a neatly defined episode. “Clearly, we’re going to have to figure out what to do with heart failure and pneumonia and stroke because those are huge consumers of dollars,” he says.
Despite all the caveats and unknowns, hospitalists still may have much to gain if bundling follows in the footsteps of the successful diagnosis-related group (DRG) payment system. “I think that enlightened hospital CEOs are going to be looking to their hospitalists as their champions to really pull this off and make this work,” Dr. Torcson says, particularly as the stakes for hospitals increase.
Medicare’s Physician Quality Reporting Initiative, established by the 2006 Tax Relief and Health Care Act, already links a 1.5% financial incentive to increased performance. With bundled payments adjusted up or down by the proposed gainsharing and penalties for higher readmission rates, Dr. Torcson says, the equivalent of 3% to 5% of DRG payments could be at risk.
“I think it could be something potentially very beneficial to those hospitalist groups that get it right,” he says. “It’s kind of like before the Ice Age comes, if you’ve got your animal skins ready, those are the tribes that survive.” TH
Gretchen Henkel is a medical writer based in California.
The New Intensivists
As critical care workforce shortages continue, and as Medicare enrollment swells—a number slated to increase an estimated 50% by 2030—hospitalists are increasingly filling in the gaps in their institutions’ intensive care units.1-2 In SHM’s 2005-06 survey, “The Authoritative Source on the State of the Hospital Medicine Movement,” for example, 75% of participants reported caring for patients in the ICU.3
The Committee on Manpower for Pulmonary and Critical Care Societies (COMPACCS) has predicted a 22-35% The Committee on Manpower for Pulmonary and Critical Care Societies (COMPACCS) has predicted a 22-25% shortfall of needed critical care physicians (also called “intensivists”) by 2030. Are hospitalists a viable option to fill the void created by the shortage of intensivists? What is the practice scope of hospitalists in the ICU? Which models work for effective co-management of ICUs and can hospitalists help to deliver round-the-clock coverage in the ICU that the Leapfrog Group safety standards have stipulated should be provided by intensivists?4 According to academic and community-based hospitalists and intensivists, much depends on local demographics and each hospital’s ICU model.
Two Models
Michael A. Gropper, MD, PhD, believes hospitalists are well suited to help manage patients in the critical care unit. At the University of California, San Francisco (UCSF), where Gropper is a professor, vice chair of the Department of Anesthesia and Perioperative Care, and the Medical Center’s director of critical care medicine, the ICU uses a co-management system.
This high-intensity model mandates an intensivist consultation for every patient. In addition, the intensivists conduct procedures, such as ventilator management, placement of central lines, and sedation. The hospital medicine service then handles patient medical management. Hospitalists write orders for antibiotics, nutrition, and fluid management. Splitting the patient management with their hospitalist colleagues allows the intensivists to care for more patients than in a completely “closed” ICU. (In “closed” systems, only intensivists are authorized to care for ICU patients.)
For this model to be effective, however, participating hospitalists should have experience and feel comfortable working in the ICU. “I don’t think you would want to pluck the average hospitalist and throw him into the ICU,” cautions Dr. Gropper. “But a hospitalist who started to consistently spend some time in the ICU would be very good. In collaboration with an intensivist, I think it’s a model that allows high-quality patient care.”
Inpatient Physician Associates, a privately owned hospital medicine group in Lincoln, Neb., headed by Brian Bossard, MD, found a slightly different way to collaborate with a group of intensivists to deliver high-quality care at the city’s two community hospitals, BryanLGH Medical Center and St. Elizabeth Regional Medical Center. Dr. Bossard and Bill Johnson, MD, a pulmonologist certified in intensive care, and director of the ICU at both hospitals, crafted an open ICU model. Although intensivists conduct multidisciplinary rounds at Bryan and St. Elizabeth, consults are not mandatory. Hospitalists provide 24/7 coverage, often placing central lines and doing other procedures. Intensivists are available for consultations and more complicated critical care procedures, such as chest tube placement, Swan-Ganz catheters, and difficult ventilator management.
This system evolved out of necessity; the hospitalist program predated the intensivist program at BryanLGH. “When I started the group, we needed docs who were comfortable in the ICU,” explains Dr. Bossard. With the open ICU model, that requirement still applies today.
When recruiting new hospitalists, Dr. Bossard looks for those who function well in the ICU environment, understand evidence-based practice, and have an aptitude for learning procedures. He also seeks out doctors comfortable with cognitive critical care.
The system seems to work.
“There are many patients who are admitted and discharged from the ICU who don’t require an intensivist’s care,” Dr. Bossard says. Hospitalists in his group cooperatively manage most patients, with intensivist consultation.
“We have a very good collaborative approach here,” says Dr. Johnson, adding that it’s difficult to have a closed ICU at a community hospital because of intensivist shortages and resistance from primary care physicians who want access to their patients. The key to the success of the program in Lincoln is that all physicians know their limits. “We don’t force ICU consults upon anybody,” Dr. Johnson says. “But I think the hospitalists do recognize when it benefits them to have the intensivist involved.”
The proof is in the proverbial pudding. Since the co-managed ICU program began in 2006 in Lincoln, the ICU mortality rate has dropped 50%, and there have been no ventilator-acquired pneumonias or central line-related infections for two years.
Ideals Versus Reality
The Leapfrog Group identified around-the-clock coverage of surgical and medical intensive care units by intensivists as one of its three safety standards.5 “In an ideal world,” says Bradley A. Sharpe, MD, associate division chief in the Division of Hospital Medicine at UCSF, “every critically ill patient would be seen, managed, or co-managed by a critical care specialist.”
David A. Hoffmann, DO, agrees certain standards in the ICU should exist, but says community hospitals will never be able to reproduce the academic model. “They don’t have the labor from residencies,” says Dr. Hoffman, medical director of Hospitalists of Franklin County, an HM group at Chambersburg Hospital, a community hospital in Chambersburg, Pa.
He says his hospitalists fill a crucial gap that intensivists can’t: “We’re the only doctors who are here in the hospital 24 hours a day—besides the ER doctors.”
Dr. Hoffman believes it’s more important to focus on outcomes than to adhere to strict Leapfrog standards. His HM group, comprised of half family medicine-trained, half internal medicine-trained hospitalists, emphasizes teamwork, evidence-based protocols, and bonuses tied to quality outcomes and patient satisfaction.
In many smaller community hospitals, HM groups must do what works to simply provide coverage. Richard Rohr, MD, vice president for medical affairs at Cortland Regional Medical Center in Cortland, N.Y, who also works as a hospitalist, says, “The people who are here around the clock are the hospitalists, so they also do the ICU management.” Last summer an intensivist who joined his hospitalist team provided ICU coverage five days a week.
Dr. Rohr believes hospitalists must acquire skills in mechanical ventilation and placement of central lines, and have high-level knowledge of infectious disease. For most ICU patients, however, this type of care is “basically internal medicine pushed to extremes,” he says.
Advantages and Disadvantages of Shared Responsibility
Under a co-managed ICU model, hospitalists can offer benefits beyond their direct time on the unit, says Hugo Quinny Cheng, MD, associate clinical professor in the Division of Hospital Medicine at the University of California at San Francisco. Dr. Cheng says his colleagues can provide more continuous care to patients because they rotate less frequently than do intensivists. In addition, hospitalists may have a broader view of hospital-wide systems and often can maximize ancillary services, such as physical therapy or nutrition, when it’s most appropriate for the patient, Dr. Sharpe adds.
One possible downside to a co-managed ICU, however, is confusion about responsibility. “In a critical care setting, ambiguity can lead to bad outcomes,” says Dr. Sharpe. To avoid this, make all ICU policies and procedures collaborative and involve all providers, including ancillary staff, in the process. “The clearer those guidelines and boundaries are, the easier it is for everyone,” he emphasizes.
For the most part, offering ICU rotations is a useful recruiting, hiring, and retention tool.
“Many hospitalists enjoy critical care and enjoy the opportunity to take care of very ill patients as part of their day-to-day practice, as long as they’re not in over their heads,” Dr. Sharpe says.
Preparation for the Future
Physicians have differing ideas about how intensivist-hospitalist relations will look in the future and what role hospitalists should play in the ICU. R. Neal Axon, MD, assistant professor in the departments of internal medicine and pediatrics at the Medical University of South Carolina in Charleston and a Team Hospitalist member, has worked in both academic and community settings. In the former, a pulmonary critical care specialist with a team of fellows, residents, and students ran the high-intensity ICU. In a local hospital where he worked as an attending, “there was no critical care team, per se. The hospitalists were the critical care team,” he says. “The difference in the care setting was pretty dramatic.”
Dr. Axon believes it might be a better long-term solution (in light of continuing critical care workforce shortages) to pursue formation of a fellowship program that combines advanced training in hospital medicine and critical care medicine.
Robert M. Wachter, MD, professor and chief of the Division of Hospital Medicine, associate chairman of the Department of Medicine, and Lynne and Marc Benioff Endowed Chair in Hospital Medicine at the University of California at San Francisco, and author of the “Wachter’s World” blog (www.the-hospitalist.org) says it’s a matter of whether hospitalists have enough intensive care training to work in the ICU.
“My own bias is that they’re probably close enough that they don’t need an extra year of training, but they’re not quite there,” he says.
As a result, UCSF’s HM division developed these strategies to augment hospitalists’ ICU skills:
- A hospitalist mini-college: a small group, hands-on experience, with one full day in the ICU, added to its annual CME course in October (www.ucsfcme. com/2009/MDM09P01A.pdf); and
- The creation of a critical care/hos-pital medicine fellowship that will launch in 2009.
By improving their ICU skills, hospitalists can form collaborative partnerships with their intensivist colleagues—both on the unit and in the critical care committees. This team approach can help their hospitals achieve the attributes of successful intensive care units.
“We have to acknowledge there’s no magic in being a hospitalist or a critical care specialist,” Dr. Axon says. “Individual decisions for individual patients, and the ways in which we all work together to systematize care, are the real differences that affect outcomes.” TH
Gretchen Henkel is a freelance writer based in California and a frequent contributor to The Hospitalist.
References
- Angus DC, Kelley MA, Schmitz RJ, et al. Current and projected workforce requirements for care of the critically ill and patients with pulmonary disease: Can we meet the requirements of an aging population? JAMA December, 2006;284(21):2762-2770.
- White J. Uses and abuses of long-term Medicare cost estimates. Health Aff (Millwood). 1999;18:63-79.
- The Society of Hospital Medicine 2005-2006 Survey: The Authoritative Source on the State of the Hospital Medicine Movement.
- Mello MM, Studdert DM, and Brennan TA. The Leapfrog Standards: Ready to jump from marketplace to courtroom? Health Aff 2003;22(2):46-59.
- Leapfrog Group, The Leapfrog Group Fact Sheet, May 2002. Available at www.leapfroggroup.org/FactSheets/LF_FactSheet.pdf. Last accessed May 28, 2008.
As critical care workforce shortages continue, and as Medicare enrollment swells—a number slated to increase an estimated 50% by 2030—hospitalists are increasingly filling in the gaps in their institutions’ intensive care units.1-2 In SHM’s 2005-06 survey, “The Authoritative Source on the State of the Hospital Medicine Movement,” for example, 75% of participants reported caring for patients in the ICU.3
The Committee on Manpower for Pulmonary and Critical Care Societies (COMPACCS) has predicted a 22-35% The Committee on Manpower for Pulmonary and Critical Care Societies (COMPACCS) has predicted a 22-25% shortfall of needed critical care physicians (also called “intensivists”) by 2030. Are hospitalists a viable option to fill the void created by the shortage of intensivists? What is the practice scope of hospitalists in the ICU? Which models work for effective co-management of ICUs and can hospitalists help to deliver round-the-clock coverage in the ICU that the Leapfrog Group safety standards have stipulated should be provided by intensivists?4 According to academic and community-based hospitalists and intensivists, much depends on local demographics and each hospital’s ICU model.
Two Models
Michael A. Gropper, MD, PhD, believes hospitalists are well suited to help manage patients in the critical care unit. At the University of California, San Francisco (UCSF), where Gropper is a professor, vice chair of the Department of Anesthesia and Perioperative Care, and the Medical Center’s director of critical care medicine, the ICU uses a co-management system.
This high-intensity model mandates an intensivist consultation for every patient. In addition, the intensivists conduct procedures, such as ventilator management, placement of central lines, and sedation. The hospital medicine service then handles patient medical management. Hospitalists write orders for antibiotics, nutrition, and fluid management. Splitting the patient management with their hospitalist colleagues allows the intensivists to care for more patients than in a completely “closed” ICU. (In “closed” systems, only intensivists are authorized to care for ICU patients.)
For this model to be effective, however, participating hospitalists should have experience and feel comfortable working in the ICU. “I don’t think you would want to pluck the average hospitalist and throw him into the ICU,” cautions Dr. Gropper. “But a hospitalist who started to consistently spend some time in the ICU would be very good. In collaboration with an intensivist, I think it’s a model that allows high-quality patient care.”
Inpatient Physician Associates, a privately owned hospital medicine group in Lincoln, Neb., headed by Brian Bossard, MD, found a slightly different way to collaborate with a group of intensivists to deliver high-quality care at the city’s two community hospitals, BryanLGH Medical Center and St. Elizabeth Regional Medical Center. Dr. Bossard and Bill Johnson, MD, a pulmonologist certified in intensive care, and director of the ICU at both hospitals, crafted an open ICU model. Although intensivists conduct multidisciplinary rounds at Bryan and St. Elizabeth, consults are not mandatory. Hospitalists provide 24/7 coverage, often placing central lines and doing other procedures. Intensivists are available for consultations and more complicated critical care procedures, such as chest tube placement, Swan-Ganz catheters, and difficult ventilator management.
This system evolved out of necessity; the hospitalist program predated the intensivist program at BryanLGH. “When I started the group, we needed docs who were comfortable in the ICU,” explains Dr. Bossard. With the open ICU model, that requirement still applies today.
When recruiting new hospitalists, Dr. Bossard looks for those who function well in the ICU environment, understand evidence-based practice, and have an aptitude for learning procedures. He also seeks out doctors comfortable with cognitive critical care.
The system seems to work.
“There are many patients who are admitted and discharged from the ICU who don’t require an intensivist’s care,” Dr. Bossard says. Hospitalists in his group cooperatively manage most patients, with intensivist consultation.
“We have a very good collaborative approach here,” says Dr. Johnson, adding that it’s difficult to have a closed ICU at a community hospital because of intensivist shortages and resistance from primary care physicians who want access to their patients. The key to the success of the program in Lincoln is that all physicians know their limits. “We don’t force ICU consults upon anybody,” Dr. Johnson says. “But I think the hospitalists do recognize when it benefits them to have the intensivist involved.”
The proof is in the proverbial pudding. Since the co-managed ICU program began in 2006 in Lincoln, the ICU mortality rate has dropped 50%, and there have been no ventilator-acquired pneumonias or central line-related infections for two years.
Ideals Versus Reality
The Leapfrog Group identified around-the-clock coverage of surgical and medical intensive care units by intensivists as one of its three safety standards.5 “In an ideal world,” says Bradley A. Sharpe, MD, associate division chief in the Division of Hospital Medicine at UCSF, “every critically ill patient would be seen, managed, or co-managed by a critical care specialist.”
David A. Hoffmann, DO, agrees certain standards in the ICU should exist, but says community hospitals will never be able to reproduce the academic model. “They don’t have the labor from residencies,” says Dr. Hoffman, medical director of Hospitalists of Franklin County, an HM group at Chambersburg Hospital, a community hospital in Chambersburg, Pa.
He says his hospitalists fill a crucial gap that intensivists can’t: “We’re the only doctors who are here in the hospital 24 hours a day—besides the ER doctors.”
Dr. Hoffman believes it’s more important to focus on outcomes than to adhere to strict Leapfrog standards. His HM group, comprised of half family medicine-trained, half internal medicine-trained hospitalists, emphasizes teamwork, evidence-based protocols, and bonuses tied to quality outcomes and patient satisfaction.
In many smaller community hospitals, HM groups must do what works to simply provide coverage. Richard Rohr, MD, vice president for medical affairs at Cortland Regional Medical Center in Cortland, N.Y, who also works as a hospitalist, says, “The people who are here around the clock are the hospitalists, so they also do the ICU management.” Last summer an intensivist who joined his hospitalist team provided ICU coverage five days a week.
Dr. Rohr believes hospitalists must acquire skills in mechanical ventilation and placement of central lines, and have high-level knowledge of infectious disease. For most ICU patients, however, this type of care is “basically internal medicine pushed to extremes,” he says.
Advantages and Disadvantages of Shared Responsibility
Under a co-managed ICU model, hospitalists can offer benefits beyond their direct time on the unit, says Hugo Quinny Cheng, MD, associate clinical professor in the Division of Hospital Medicine at the University of California at San Francisco. Dr. Cheng says his colleagues can provide more continuous care to patients because they rotate less frequently than do intensivists. In addition, hospitalists may have a broader view of hospital-wide systems and often can maximize ancillary services, such as physical therapy or nutrition, when it’s most appropriate for the patient, Dr. Sharpe adds.
One possible downside to a co-managed ICU, however, is confusion about responsibility. “In a critical care setting, ambiguity can lead to bad outcomes,” says Dr. Sharpe. To avoid this, make all ICU policies and procedures collaborative and involve all providers, including ancillary staff, in the process. “The clearer those guidelines and boundaries are, the easier it is for everyone,” he emphasizes.
For the most part, offering ICU rotations is a useful recruiting, hiring, and retention tool.
“Many hospitalists enjoy critical care and enjoy the opportunity to take care of very ill patients as part of their day-to-day practice, as long as they’re not in over their heads,” Dr. Sharpe says.
Preparation for the Future
Physicians have differing ideas about how intensivist-hospitalist relations will look in the future and what role hospitalists should play in the ICU. R. Neal Axon, MD, assistant professor in the departments of internal medicine and pediatrics at the Medical University of South Carolina in Charleston and a Team Hospitalist member, has worked in both academic and community settings. In the former, a pulmonary critical care specialist with a team of fellows, residents, and students ran the high-intensity ICU. In a local hospital where he worked as an attending, “there was no critical care team, per se. The hospitalists were the critical care team,” he says. “The difference in the care setting was pretty dramatic.”
Dr. Axon believes it might be a better long-term solution (in light of continuing critical care workforce shortages) to pursue formation of a fellowship program that combines advanced training in hospital medicine and critical care medicine.
Robert M. Wachter, MD, professor and chief of the Division of Hospital Medicine, associate chairman of the Department of Medicine, and Lynne and Marc Benioff Endowed Chair in Hospital Medicine at the University of California at San Francisco, and author of the “Wachter’s World” blog (www.the-hospitalist.org) says it’s a matter of whether hospitalists have enough intensive care training to work in the ICU.
“My own bias is that they’re probably close enough that they don’t need an extra year of training, but they’re not quite there,” he says.
As a result, UCSF’s HM division developed these strategies to augment hospitalists’ ICU skills:
- A hospitalist mini-college: a small group, hands-on experience, with one full day in the ICU, added to its annual CME course in October (www.ucsfcme. com/2009/MDM09P01A.pdf); and
- The creation of a critical care/hos-pital medicine fellowship that will launch in 2009.
By improving their ICU skills, hospitalists can form collaborative partnerships with their intensivist colleagues—both on the unit and in the critical care committees. This team approach can help their hospitals achieve the attributes of successful intensive care units.
“We have to acknowledge there’s no magic in being a hospitalist or a critical care specialist,” Dr. Axon says. “Individual decisions for individual patients, and the ways in which we all work together to systematize care, are the real differences that affect outcomes.” TH
Gretchen Henkel is a freelance writer based in California and a frequent contributor to The Hospitalist.
References
- Angus DC, Kelley MA, Schmitz RJ, et al. Current and projected workforce requirements for care of the critically ill and patients with pulmonary disease: Can we meet the requirements of an aging population? JAMA December, 2006;284(21):2762-2770.
- White J. Uses and abuses of long-term Medicare cost estimates. Health Aff (Millwood). 1999;18:63-79.
- The Society of Hospital Medicine 2005-2006 Survey: The Authoritative Source on the State of the Hospital Medicine Movement.
- Mello MM, Studdert DM, and Brennan TA. The Leapfrog Standards: Ready to jump from marketplace to courtroom? Health Aff 2003;22(2):46-59.
- Leapfrog Group, The Leapfrog Group Fact Sheet, May 2002. Available at www.leapfroggroup.org/FactSheets/LF_FactSheet.pdf. Last accessed May 28, 2008.
As critical care workforce shortages continue, and as Medicare enrollment swells—a number slated to increase an estimated 50% by 2030—hospitalists are increasingly filling in the gaps in their institutions’ intensive care units.1-2 In SHM’s 2005-06 survey, “The Authoritative Source on the State of the Hospital Medicine Movement,” for example, 75% of participants reported caring for patients in the ICU.3
The Committee on Manpower for Pulmonary and Critical Care Societies (COMPACCS) has predicted a 22-35% The Committee on Manpower for Pulmonary and Critical Care Societies (COMPACCS) has predicted a 22-25% shortfall of needed critical care physicians (also called “intensivists”) by 2030. Are hospitalists a viable option to fill the void created by the shortage of intensivists? What is the practice scope of hospitalists in the ICU? Which models work for effective co-management of ICUs and can hospitalists help to deliver round-the-clock coverage in the ICU that the Leapfrog Group safety standards have stipulated should be provided by intensivists?4 According to academic and community-based hospitalists and intensivists, much depends on local demographics and each hospital’s ICU model.
Two Models
Michael A. Gropper, MD, PhD, believes hospitalists are well suited to help manage patients in the critical care unit. At the University of California, San Francisco (UCSF), where Gropper is a professor, vice chair of the Department of Anesthesia and Perioperative Care, and the Medical Center’s director of critical care medicine, the ICU uses a co-management system.
This high-intensity model mandates an intensivist consultation for every patient. In addition, the intensivists conduct procedures, such as ventilator management, placement of central lines, and sedation. The hospital medicine service then handles patient medical management. Hospitalists write orders for antibiotics, nutrition, and fluid management. Splitting the patient management with their hospitalist colleagues allows the intensivists to care for more patients than in a completely “closed” ICU. (In “closed” systems, only intensivists are authorized to care for ICU patients.)
For this model to be effective, however, participating hospitalists should have experience and feel comfortable working in the ICU. “I don’t think you would want to pluck the average hospitalist and throw him into the ICU,” cautions Dr. Gropper. “But a hospitalist who started to consistently spend some time in the ICU would be very good. In collaboration with an intensivist, I think it’s a model that allows high-quality patient care.”
Inpatient Physician Associates, a privately owned hospital medicine group in Lincoln, Neb., headed by Brian Bossard, MD, found a slightly different way to collaborate with a group of intensivists to deliver high-quality care at the city’s two community hospitals, BryanLGH Medical Center and St. Elizabeth Regional Medical Center. Dr. Bossard and Bill Johnson, MD, a pulmonologist certified in intensive care, and director of the ICU at both hospitals, crafted an open ICU model. Although intensivists conduct multidisciplinary rounds at Bryan and St. Elizabeth, consults are not mandatory. Hospitalists provide 24/7 coverage, often placing central lines and doing other procedures. Intensivists are available for consultations and more complicated critical care procedures, such as chest tube placement, Swan-Ganz catheters, and difficult ventilator management.
This system evolved out of necessity; the hospitalist program predated the intensivist program at BryanLGH. “When I started the group, we needed docs who were comfortable in the ICU,” explains Dr. Bossard. With the open ICU model, that requirement still applies today.
When recruiting new hospitalists, Dr. Bossard looks for those who function well in the ICU environment, understand evidence-based practice, and have an aptitude for learning procedures. He also seeks out doctors comfortable with cognitive critical care.
The system seems to work.
“There are many patients who are admitted and discharged from the ICU who don’t require an intensivist’s care,” Dr. Bossard says. Hospitalists in his group cooperatively manage most patients, with intensivist consultation.
“We have a very good collaborative approach here,” says Dr. Johnson, adding that it’s difficult to have a closed ICU at a community hospital because of intensivist shortages and resistance from primary care physicians who want access to their patients. The key to the success of the program in Lincoln is that all physicians know their limits. “We don’t force ICU consults upon anybody,” Dr. Johnson says. “But I think the hospitalists do recognize when it benefits them to have the intensivist involved.”
The proof is in the proverbial pudding. Since the co-managed ICU program began in 2006 in Lincoln, the ICU mortality rate has dropped 50%, and there have been no ventilator-acquired pneumonias or central line-related infections for two years.
Ideals Versus Reality
The Leapfrog Group identified around-the-clock coverage of surgical and medical intensive care units by intensivists as one of its three safety standards.5 “In an ideal world,” says Bradley A. Sharpe, MD, associate division chief in the Division of Hospital Medicine at UCSF, “every critically ill patient would be seen, managed, or co-managed by a critical care specialist.”
David A. Hoffmann, DO, agrees certain standards in the ICU should exist, but says community hospitals will never be able to reproduce the academic model. “They don’t have the labor from residencies,” says Dr. Hoffman, medical director of Hospitalists of Franklin County, an HM group at Chambersburg Hospital, a community hospital in Chambersburg, Pa.
He says his hospitalists fill a crucial gap that intensivists can’t: “We’re the only doctors who are here in the hospital 24 hours a day—besides the ER doctors.”
Dr. Hoffman believes it’s more important to focus on outcomes than to adhere to strict Leapfrog standards. His HM group, comprised of half family medicine-trained, half internal medicine-trained hospitalists, emphasizes teamwork, evidence-based protocols, and bonuses tied to quality outcomes and patient satisfaction.
In many smaller community hospitals, HM groups must do what works to simply provide coverage. Richard Rohr, MD, vice president for medical affairs at Cortland Regional Medical Center in Cortland, N.Y, who also works as a hospitalist, says, “The people who are here around the clock are the hospitalists, so they also do the ICU management.” Last summer an intensivist who joined his hospitalist team provided ICU coverage five days a week.
Dr. Rohr believes hospitalists must acquire skills in mechanical ventilation and placement of central lines, and have high-level knowledge of infectious disease. For most ICU patients, however, this type of care is “basically internal medicine pushed to extremes,” he says.
Advantages and Disadvantages of Shared Responsibility
Under a co-managed ICU model, hospitalists can offer benefits beyond their direct time on the unit, says Hugo Quinny Cheng, MD, associate clinical professor in the Division of Hospital Medicine at the University of California at San Francisco. Dr. Cheng says his colleagues can provide more continuous care to patients because they rotate less frequently than do intensivists. In addition, hospitalists may have a broader view of hospital-wide systems and often can maximize ancillary services, such as physical therapy or nutrition, when it’s most appropriate for the patient, Dr. Sharpe adds.
One possible downside to a co-managed ICU, however, is confusion about responsibility. “In a critical care setting, ambiguity can lead to bad outcomes,” says Dr. Sharpe. To avoid this, make all ICU policies and procedures collaborative and involve all providers, including ancillary staff, in the process. “The clearer those guidelines and boundaries are, the easier it is for everyone,” he emphasizes.
For the most part, offering ICU rotations is a useful recruiting, hiring, and retention tool.
“Many hospitalists enjoy critical care and enjoy the opportunity to take care of very ill patients as part of their day-to-day practice, as long as they’re not in over their heads,” Dr. Sharpe says.
Preparation for the Future
Physicians have differing ideas about how intensivist-hospitalist relations will look in the future and what role hospitalists should play in the ICU. R. Neal Axon, MD, assistant professor in the departments of internal medicine and pediatrics at the Medical University of South Carolina in Charleston and a Team Hospitalist member, has worked in both academic and community settings. In the former, a pulmonary critical care specialist with a team of fellows, residents, and students ran the high-intensity ICU. In a local hospital where he worked as an attending, “there was no critical care team, per se. The hospitalists were the critical care team,” he says. “The difference in the care setting was pretty dramatic.”
Dr. Axon believes it might be a better long-term solution (in light of continuing critical care workforce shortages) to pursue formation of a fellowship program that combines advanced training in hospital medicine and critical care medicine.
Robert M. Wachter, MD, professor and chief of the Division of Hospital Medicine, associate chairman of the Department of Medicine, and Lynne and Marc Benioff Endowed Chair in Hospital Medicine at the University of California at San Francisco, and author of the “Wachter’s World” blog (www.the-hospitalist.org) says it’s a matter of whether hospitalists have enough intensive care training to work in the ICU.
“My own bias is that they’re probably close enough that they don’t need an extra year of training, but they’re not quite there,” he says.
As a result, UCSF’s HM division developed these strategies to augment hospitalists’ ICU skills:
- A hospitalist mini-college: a small group, hands-on experience, with one full day in the ICU, added to its annual CME course in October (www.ucsfcme. com/2009/MDM09P01A.pdf); and
- The creation of a critical care/hos-pital medicine fellowship that will launch in 2009.
By improving their ICU skills, hospitalists can form collaborative partnerships with their intensivist colleagues—both on the unit and in the critical care committees. This team approach can help their hospitals achieve the attributes of successful intensive care units.
“We have to acknowledge there’s no magic in being a hospitalist or a critical care specialist,” Dr. Axon says. “Individual decisions for individual patients, and the ways in which we all work together to systematize care, are the real differences that affect outcomes.” TH
Gretchen Henkel is a freelance writer based in California and a frequent contributor to The Hospitalist.
References
- Angus DC, Kelley MA, Schmitz RJ, et al. Current and projected workforce requirements for care of the critically ill and patients with pulmonary disease: Can we meet the requirements of an aging population? JAMA December, 2006;284(21):2762-2770.
- White J. Uses and abuses of long-term Medicare cost estimates. Health Aff (Millwood). 1999;18:63-79.
- The Society of Hospital Medicine 2005-2006 Survey: The Authoritative Source on the State of the Hospital Medicine Movement.
- Mello MM, Studdert DM, and Brennan TA. The Leapfrog Standards: Ready to jump from marketplace to courtroom? Health Aff 2003;22(2):46-59.
- Leapfrog Group, The Leapfrog Group Fact Sheet, May 2002. Available at www.leapfroggroup.org/FactSheets/LF_FactSheet.pdf. Last accessed May 28, 2008.
Nonalcoholic fatty liver disease: A manifestation of the metabolic syndrome
As the nation gets heavier, our livers will get fattier. The prevalence of nonalcoholic fatty liver disease (NAFLD) has been rising in tandem with the rise in obesity ever since the term nonalcoholic steatohepatitis (NASH, a subtype of NAFLD) was coined by Ludwig in 1980.1 Yet, despite an explosion of research on NAFLD and gains in understanding its epidemiology and pathogenesis, a number of issues remain unresolved, including how to treat it.
NAFLD IS A SPECTRUM
NAFLD is a spectrum. The mildest form is simple fatty liver, or simple steatosis. Next is NASH, or fatty liver with inflammation and evidence of damage to hepatocytes (liver cells). Still more severe is cirrhosis, and in its most extreme form NAFLD can progress to hepatocellular carcinoma or liver failure. The distinction between simple steatosis and NASH is important because their prognoses and management are different.
NAFLD IS COMMON AND LINKED TO OBESITY
NAFLD is the most common cause of elevated liver enzymes and also one of the most common forms of liver disease in the world. It is now estimated to affect about 20% to 30% of people in the United States and other Western countries. In contrast, the prevalence of chronic hepatitis C virus infection is estimated at 3% of the world’s population. In comparison to the prevalence of NAFLD, the prevalence of NASH is much lower: 2% to 3% in the United States.2 The incidence of NAFLD is expected to rise further with the increase in obesity in the United States.
NAFLD is even more common in people who are morbidly obese, ie, who have a body mass index greater than 40 kg/m2. In a series of studies of morbidly obese patients undergoing bariatric surgery (N = 1,620), the prevalence of hepatic steatosis was 91% (range 85%–98%), and the prevalence of NASH was 37% (range 24%–98%). NASH was not predicted by age or body mass index, but it was more common in men, people with diabetes, and people with insulin resistance.3
Obesity is also increasing in prevalence in children. Since liver biopsies were not done in most pediatric studies, the pediatric prevalence data are based on elevated aminotransferase levels and on ultrasonographic findings of echogenic livers. The overall prevalence of NAFLD in children is estimated at 3% to 10%, but it may be much higher in obese children.4
Arun et al5 found that the prevalence of NASH in morbidly obese men was almost twice as high as in morbidly obese women (60.3% vs 30.9%). In contrast, earlier studies suggested that NAFLD was more prevalent in women. This higher incidence of NASH may also reflect the higher incidence of metabolic syndrome in morbidly obese men (91.4% vs 76.2%).
Less common in African Americans
In the United States, African Americans have consistently been found to have the lowest prevalence of NAFLD. In a California population study of 159 newly diagnosed NAFLD cases, non-Hispanic whites accounted for 45%, followed by Hispanics (28%), Asians (18%), and African Americans (3%). After controlling for the ethnic composition of the entire cohort, Hispanics had the highest rate of NAFLD and African Americans the lowest.6 In Eastern countries such as Japan, the prevalence of NAFLD is estimated to be about 9.3%. Interestingly, about half of the people with NAFLD in Japan were not overweight.7
The difference in prevalence of NAFLD in different ethnic groups may be explained by their different rates of metabolic syndrome (21.6% in African Americans vs 23.8% in whites vs 31.9% in Mexican Americans8) as well as other genetic and environmental factors.
NAFLD IS USUALLY CLINICALLY SILENT
NAFLD is usually clinically silent, and its impact has most likely been underestimated. Symptoms, if present, are minimal and non-specific, such as fatigue and right upper quadrant discomfort. Most findings on physical examination are also normal. Most patients seek care because of an incidental finding of elevated aminotransferase levels or radiographic studies suggesting the liver is fatty.9
The estimated prevalence of aminotransferase elevations in the general population from the third National Health and Nutrition Examination Survey data is 7.9%,10 with about two-thirds of cases unexplained. Of the unexplained cases, most are strongly associated with metabolic syndrome and probably represent underlying NAFLD.10
Yet aminotransferase levels are typically normal or elevated by less than five times the upper limit of normal (usually < 250 IU/L).9 In contrast to those with alcoholic hepatitis, most patients with NAFLD have a ratio of aspartate aminotransferase to alanine aminotransferase of less than 1. As the disease progresses, the aspartate aminotransferase level increases more than the alanine aminotransferase level, so if the ratio is more than 1, more advanced liver disease may be suspected.11
Levels of other liver enzymes such as alkaline phosphatase and of acute-phase reactants such as ferritin may also be elevated. Ferritin is believed to reflect hepatic injury, inflammation, or insulin resistance.
A DIAGNOSIS OF EXCLUSION
Excessive alcohol consumption must especially be excluded. Most studies defined excessive alcohol consumption as more than 20 to 40 g/day.2 Recently, this threshold has been lowered to 20 g/day (roughly two drinks) in men and 10 g/day in women.
Insulin resistance should be estimated, given the close relationship between NAFLD and insulin resistance and the metabolic syndrome. Insulin resistance can be measured accurately in a number of ways. The Homeostasis Model Assessment is an easy method that provides an estimate of insulin resistance based on fasting serum glucose and serum insulin levels.13
Serologic tests can rule out hepatitis B and hepatitis C. In those with negative results, especially in those with components of the metabolic syndrome or insulin resistance, NAFLD is responsible for most cases of persistently elevated serum liver enzymes.
Imaging tests
Radiographic evaluation is another noninvasive way to diagnose fatty liver. The sensitivity of either ultrasonography or computed tomography for detecting hepatic steatosis is between 93% and 100% when there is more than 33% fat in the hepatic parenchyma.14 None of the radiographic methods, including magnetic resonance imaging, can accurately differentiate between nonprogressive simple steatosis and NASH, but the technology is advancing. Contrast ultrasonography and magnetic resonance spectroscopy have shown promise and may become useful in the future.
Other noninvasive tests
Ultrasonographic elastrography (FibroScan), a noninvasive way to measure liver stiffness, has also been used in patients with hepatitis C. Although the preliminary data in NAFLD are interesting, additional validation is needed.
Serum biomarkers, including markers of fibrosis (eg, FibroSURE), apoptosis, and adipocytokines have been used to diagnose NASH. The markers of apoptosis are especially interesting but need further validation.
Liver biopsy remains the gold standard
Because we lack a fully validated noninvasive biomarker of NASH, liver biopsy remains the gold standard for diagnosing it. The minimum histologic criteria for establishing the diagnosis of NASH have been debated; most pathologists require at least 5% hepatic steatosis, mixed lobular inflammation, and hepatocellular ballooning.
In a study of 354 liver biopsies of patients with negative results on serologic tests, NASH was found in 34% and fatty liver in 32%. In the same study, the findings on liver biopsy led to alterations in patient management in 18% of cases.15
Some clinicians doubt the value of liver biopsy in patients with suspected NASH, in view of possible sampling error in the biopsy specimens (the distribution can be patchy, and if the specimen is taken from an unaffected area, the results can be falsely negative) and because there is no established effective therapy for NAFLD. However, liver biopsy is the only test that can accurately establish the diagnosis of NASH and tell us the stage of liver disease, which has important prognostic implications. Most experts agree that liver biopsy should be considered for patients at risk of advanced liver disease, such as those with persistently elevated liver enzyme levels despite intervention to reverse conditions associated with metabolic syndrome.16
PATHOGENESIS: THE MULTIPLE-HIT HYPOTHESIS
NAFLD is closely linked to obesity, insulin resistance, and metabolic syndrome.13 Insulin allows free fatty esterification and triglyceride fat storage in adipose tissues. When insulin resistance develops, free fatty acids are inappropriately shifted to nonadipose tissues, including the liver. Insulin resistance increases free fatty acid flux to the liver by decreased inhibition of lipolysis and also increased de novo lipogenesis.17
Insulin resistance and visceral obesity also result in decreased levels of a “protective adipokine,” adiponectin. Adiponectin inhibits liver gluconeogenesis and suppresses lipogenesis. Thus, decreased adiponectin hinders fatty acid oxidation and increases fat accumulation in the liver. Other adipocytokines that are important in NAFLD are resistin, leptin, visfatin, tumor necrosis factor alpha, and interleukin 6.
Apoptosis and oxidative stress may also contribute to the development and progression of NASH. In this context, the “multiple-hit hypothesis” for the pathogenesis of NASH has become quite popular.18 An in-depth review of the pathogenesis of NAFLD is beyond the scope of this paper; readers are referred to a recently published review on this subject.19
STEATOSIS IS BENIGN, BUT NASH CAN PROGRESS
Simple steatosis by itself generally has a benign prognosis. In a 1995 cohort study with a median follow-up of 11 years, there was no progression of simple steatosis to NASH or cirrhosis,20 and recent reviews estimate that only a small portion of patients with simple steatosis develop steatohepatitis. The validity of these data is still being debated.
On the other hand, once patients have progressed to NASH, histologic progression has been noted in about 32% to 41% of patients over a median follow-up of 4.3 to 13.7 years.21,22 This would mean that approximately 9% of patients with NASH may develop cirrhosis.21
People with cirrhosis due to NAFLD are at risk of developing liver-related morbidity and of death. In one of the longest follow-up cohort studies (mean follow-up of 13.7 years), end-stage liver disease developed in 5.4%, and hepatocellular carcinoma developed in about 2%. About 20% of the patients died, with more than 70% of the deaths in patients who had NASH at baseline. The survival rate was lower in patients with NASH, whereas no difference in survival was seen in the group with simple steatosis.22
A number of studies have assessed independent predictors of advanced fibrosis. Most studies suggest that elevated liver enzymes, metabolic syndrome, or type 2 diabetes is associated with advanced liver disease. Although noninvasive biomarkers of fibrosis have been developed for hepatitis C, to date, a fully validated, noninvasive biomarker of fibrosis for NAFLD does not exist.
As noted, the spectrum of NAFLD also includes hepatocellular carcinoma, and in a series of 105 patients with hepatocellular carcinoma, hepatitis C virus accounted for 51% and cryptogenic liver disease accounted for another 29%. Since cases of cryptogenic cirrhosis in the United States are considered to be “burned out NASH,” approximately 13% of patients with hepatocellular carcinoma may have had underlying NAFLD as the cause of their liver disease.23 These data suggest that, similar to other cirrhotic patients, NAFLD patients with cirrhosis should be screened for hepatocellular carcinoma.
NO CONSENSUS ON TREATMENT
Weight loss
Modest weight loss—less than 2 pounds (1 kg) per week—is associated with a decrease in the incidence of metabolic syndrome and can also improve the histologic features of NASH in more than 80% of cases.24 Loss of as little as 4% to 5% of body weight is also associated with lowering of aminotransferase and fasting insulin levels.25
The mechanism of benefit is via loss of adipose tissue, which decreases insulin resistance. Weight loss by any means, including bariatric surgery for morbid obesity or use of weight-reducing agents, has been correlated with improvement in liver enzyme levels, liver histologic findings, or both.24,26
However, the traditional low-calorie, low-fat diet may not be optimal for NAFLD patients. In one study,27 patients consuming more than 54% of their calories from carbohydrates compared with those consuming less than 35% had an odds ratio of 6.5 for hepatic inflammation. This finding is not surprising in light of prior research in which high carbohydrate intake increased hepatic de novo lipogenesis. On the other hand, there was no association between total caloric or protein intake and hepatic steatosis or fibrosis. Contrary to traditional beliefs, patients with higher fat intake had less inflammation, steatosis, and fibrosis.
Insulin sensitizers
Given that insulin resistance seems to be the main pathophysiologic culprit in NAFLD, two classes of insulin sensitizers have been studied:
Biguanides act mainly by increasing hepatic insulin sensitivity and reversing insulin resistance induced by tumor necrosis factor alpha.
Glitazones improve insulin sensitivity in both diabetic and euglycemic patients by activating the nuclear transcription factor called peroxisome proliferator-activated receptor (PPAR) gamma.
Both biguanides and glitazones have been found to lower liver enzyme levels, decrease insulin resistance, and improve histopathologic findings. However, the effects of glitazones do not persist after the drugs are stopped, and these drugs and are also associated with an average weight gain of 3 to 6 kg.28,29
Although these data are encouraging, they are preliminary, and more evidence is needed to establish the safety and efficacy of these drugs in treating patients with NASH.
Antioxidants
Antioxidants such as vitamin E, n-acetyl-l-cysteine, s-adenosylmethionine (SAMe), and betaine have been investigated in the treatment of NAFLD.
Vitamin E has been most widely studied. Being fat-soluble, vitamin E can stabilize mitochondrial function and is theorized to inhibit lipid peroxidation and subsequent free radical reactions. Smaller, nonrandomized trials have found that vitamin E improves biochemical markers of liver inflammation. However, in one of the largest randomized controlled trials (with 45 patients), patients taking vitamin E showed improvement in their fibrosis scores but no differences in their necroinflammatory activity or alanine aminotransferase levels.30 Most studies of antioxidants show at least mild improvement in biochemical or histologic signs of NAFLD.31
SAMe and betaine are important antioxidants. However, most studies of SAMe and betaine have been small and inconclusive.
Two large phase III clinical trials are under way at the National Institute of Diabetes and Digestive and Kidney Diseases. They should clarify the role of these agents in the treatment of NASH. The PIVENS (Pioglitazone vs Vitamin E vs Placebo for the Treatment of Non-Diabetic Patients With Nonalcoholic Steatohepatitis) study has completed enrollment of 240 patients, but the final data are not available. The second study, TONIC (Treatment of Nonalcoholic Fatty Liver Disease in Children) will be one of the largest studies of NAFLD in children; it will be looking at vitamin E, metformin, or placebo over a 2-year follow-up. The TONIC study is still under way, so the final data are not yet available.
Ursodeoxycholic acid, another cytoprotective agent, has traditionally been used for primary biliary cirrhosis, but the data are conflicting on its efficacy in NAFLD. Of note, some bile acids are hepatotoxic and facilitate apoptosis via a Fas ligand-mediated pathway. On the other hand, ursodeoxycholic acid is a hydrophilic bile acid that may act to displace the hepatotoxic hydrophobic endogenous bile acids and potentially has an antiapoptotic and cytoprotective effect in NAFLD. Although liver enzyme levels declined in a few of the studies of ursodeoxycholic acid in patients with NAFLD, a large randomized clinical trial (in 166 patients) did not show any significant difference from placebo in liver enzyme levels or liver histologic findings.32
Lipid-lowering drugs
Lipid-lowering drugs target the high levels of triglycerides and low levels of high-density lipoprotein cholesterol that often occur in insulin resistance and metabolic syndrome associated with NAFLD. A few small studies found that aminotransferase levels fell with both statins and gemfibrozil (Lopid).33 Even if liver enzyme levels are abnormal, most experts believe that statins are relatively safe to use in patients with NAFLD who need cholesterol-lowering agents. Nevertheless, clinical monitoring of these patients for potential hepatic toxicity is recommended.
Other medications
Other medications, such as pentoxifylline (Pentoxil, Trental), probiotics, and angiotensin-converting enzyme inhibitors, have been used in small studies of patients with NASH, with encouraging but inconclusive results.
Although a number of pilot studies of agents for treating NAFLD have been proposed, they are small and open-label. With the tremendous recent gains in clinical investigation, functional genomics, and proteomics, it is expected that our understanding of NASH and its treatment will be broadened.
In summary, despite the relatively large number of agents tested for the treatment of NAFLD, most of the data are preliminary. Thus, in 2008, there is no established, evidence-based treatment for patients with NASH.
- Ludwig J, Viggiano TR, McGill DB, Ott BJ. Nonalcoholic steatohepatitis: Mayo Clinic experiences with a hitherto unnamed disease. Mayo Clin Proc. 1980; 55:434–438.
- Neuschwander-Tetri BA, Caldwell SH. Nonalcoholic steatohepatitis: summary of an AASLD single topic conference. Hepatology. 2003; 37:1202–1209.
- Machado M, Marques-Vidal P, Cortez-Pinto H. Hepatic histology in obese patients undergoing bariatric surgery. J Hepatol. 2006; 45:600–606.
- Shneider BL, Gonzalez-Peralta R, Roberts EA. Controversies in the management of pediatric liver disease: hepatitis B, C, and NAFLD: summary of a single topic conference. Hepatology. 2006; 44:1344–1354.
- Arun J, Clements RH, Lazenby AJ, Leeth RR, Abrams GA. The prevalence of nonalcoholic steatohepatitis is greater in morbidly obese men compared to women. Obes Surg. 2006; 16:1351–1358.
- Weston SR, Leyden W, Murphy R, et al. Racial and ethnic distribution of nonalcoholic fatty liver in persons with newly diagnosed chronic liver disease. Hepatology. 2005; 41:372–379.
- Omagari K, Kadokawa Y, Masuda JI, et al. Fatty liver in non-alcoholic non-overweight Japanese adults: incidence and clinical characteristics. J Gastroenterol Hepatol. 2002; 17:1098–1105.
- Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults. JAMA. 2002; 287:356–359.
- Ramesh S, Sanyal AJ. Evaluation and management of non-alcoholic steatohepatitis. J Hepatol 2005; 42:S2–S12.
- Clark JM, Brancati FL, Diehl AM. The prevalence and etiology of elevated aminotransferase levels in the United States. Am J Gastroenterol. 2003; 98:960–967.
- Angulo P, Keach JC, Batts KP, Lindor KD. Independent predictors of liver fibrosis in patients with nonalcoholic steatohepatitis. Hepatology. 1999; 30:1356–1362.
- Angulo P. Nonalcoholic fatty liver disease. N Engl J Med. 2002; 346:1221–1231.
- Marchesini G, Brizi M, Morselli-Labate AM, et al. Association of nonalcoholic fatty liver disease with insulin resistance. Am J Med. 1999; 107:450–455.
- Saadeh S, Younossi ZM, Remer EM, et al. The utility of radiological imaging in nonalcoholic fatty liver disease. Gastroenterology 2002; 123:745–750.
- Skelly MM, James PD, Ryder SD. Findings on liver biopsy to investigate abnormal liver function tests in the absence of diagnostic serology. J Hepatol. 2001; 35:195–199.
- Collantes R, Ong JP, Younossi ZM. Nonalcoholic fatty liver disease and the epidemic of obesity. Cleve Clin J Med. 2004; 71:657–664.
- Utzschneider KM, Kahn SE. Review: the role of insulin resistance in nonalcoholic fatty liver disease. J Clin Endocrinol Metab. 2006; 91:4753–4761.
- Hui JM, Hodge A, Farrell GC, Kench JG, Kriketos A, George J. Beyond insulin resistance in NASH: TNF-alpha or adiponectin? Hepatology 2004; 40:46–54.
- Edmison J, McCullough AJ. Pathogenesis of non-alcoholic steatohepatitis: human data. Clin Liver Dis. 2007; 11:75–104.
- Teli MR, James OFW, Burt AD, Bennett MK, Day CP. The natural history of nonalcoholic fatty liver: a follow-up study. Hepatology. 1995; 22:1714–1719.
- Harrison SA, Torgerson S, Hayashi PH. The natural history of nonalcoholic fatty liver disease: a clinical histopathological study. Am J Gastroenterol. 2003; 98:2042–2047.
- Ekstedt M, Franzen LE, Mathiesen UL, et al. Long-term follow-up of patients with NAFLD and elevated liver enzymes. Hepatology. 2006; 44:865–873.
- Marrero JA, Fontana RJ, Su GL, Conjeevaram HS, Emick DM, Lok AS. NAFLD may be a common underlying liver disease in patients with hepatocellular carcinoma in the United States. Hepatology. 2003; 36:1349–1354.
- Dixon JB, Bhathal PS, O’Brien PE. Weight loss and non-alcoholic fatty liver disease: falls in gamma-glutamyl transferase concentrations are associated with histologic improvement. Obes Surg. 2006; 16:1278–1286.
- Hickman IJ, Jonsson JR, Prins JB, et al. Modest weight loss and physical activity in overweight patients with chronic liver disease results in sustained improvements in alanine aminotransferase, fasting insulin, and quality of life. Gut 2004: 53:413–419.
- Zelber-Sagi S, Kessler A, Brazowsky E, et al. A double-blind randomized placebo-controlled trial of orlistat for the treatment of nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2006; 4:639–644.
- Solgas S, Alkhuraishe AR, Clark JM, et al. Dietary composition and nonalcoholic fatty liver disease. Dig Dis Sci. 2004; 49:1578–1583.
- Bugianesi E, Gentilcore E, Manini R, et al. A randomized controlled trial of metformin versus vitamin E or prescriptive diet in nonalcoholic fatty liver disease. Am J Gastroenterol. 2005; 100:1082–1090.
- Neuschwander-Tetri BA, Brunt EM, Wehmeier KR, Oliver D, Bacon BR. Improved nonalcoholic steatohepatitis after 48 weeks of treatment with the PPAR-gamma ligand rosiglitazone. Hepatology. 2003; 38:1008–1017.
- Harrison SA, Torgerson S, Hayashi P, Ward J, Schenker S. Vitamin E and vitamin C treatment improves fibrosis in patients with nonalcoholic steatohepatitis. Am J Gastroenterol. 2003; 98:2485–2490.
- Chang CY, Argo CK, Al-Osaimi AMS, Caldwell SH. Therapy of NAFLD, antioxidants and cytoprotective agents. J Clin Gastroenterol 2006; 40:S51–S60.
- Lindor KD, Kowdley KV, Heathcote EJ, et al. Ursodeoxycholic acid for treatment of nonalcoholic steatohepatitis: results of a randomized trial. Hepatology. 2004; 39:770–778.
- Adams LA, Angulo P. Treatment of non-alcoholic fatty liver disease. Postgrad Med J. 2006; 82:315–322.
As the nation gets heavier, our livers will get fattier. The prevalence of nonalcoholic fatty liver disease (NAFLD) has been rising in tandem with the rise in obesity ever since the term nonalcoholic steatohepatitis (NASH, a subtype of NAFLD) was coined by Ludwig in 1980.1 Yet, despite an explosion of research on NAFLD and gains in understanding its epidemiology and pathogenesis, a number of issues remain unresolved, including how to treat it.
NAFLD IS A SPECTRUM
NAFLD is a spectrum. The mildest form is simple fatty liver, or simple steatosis. Next is NASH, or fatty liver with inflammation and evidence of damage to hepatocytes (liver cells). Still more severe is cirrhosis, and in its most extreme form NAFLD can progress to hepatocellular carcinoma or liver failure. The distinction between simple steatosis and NASH is important because their prognoses and management are different.
NAFLD IS COMMON AND LINKED TO OBESITY
NAFLD is the most common cause of elevated liver enzymes and also one of the most common forms of liver disease in the world. It is now estimated to affect about 20% to 30% of people in the United States and other Western countries. In contrast, the prevalence of chronic hepatitis C virus infection is estimated at 3% of the world’s population. In comparison to the prevalence of NAFLD, the prevalence of NASH is much lower: 2% to 3% in the United States.2 The incidence of NAFLD is expected to rise further with the increase in obesity in the United States.
NAFLD is even more common in people who are morbidly obese, ie, who have a body mass index greater than 40 kg/m2. In a series of studies of morbidly obese patients undergoing bariatric surgery (N = 1,620), the prevalence of hepatic steatosis was 91% (range 85%–98%), and the prevalence of NASH was 37% (range 24%–98%). NASH was not predicted by age or body mass index, but it was more common in men, people with diabetes, and people with insulin resistance.3
Obesity is also increasing in prevalence in children. Since liver biopsies were not done in most pediatric studies, the pediatric prevalence data are based on elevated aminotransferase levels and on ultrasonographic findings of echogenic livers. The overall prevalence of NAFLD in children is estimated at 3% to 10%, but it may be much higher in obese children.4
Arun et al5 found that the prevalence of NASH in morbidly obese men was almost twice as high as in morbidly obese women (60.3% vs 30.9%). In contrast, earlier studies suggested that NAFLD was more prevalent in women. This higher incidence of NASH may also reflect the higher incidence of metabolic syndrome in morbidly obese men (91.4% vs 76.2%).
Less common in African Americans
In the United States, African Americans have consistently been found to have the lowest prevalence of NAFLD. In a California population study of 159 newly diagnosed NAFLD cases, non-Hispanic whites accounted for 45%, followed by Hispanics (28%), Asians (18%), and African Americans (3%). After controlling for the ethnic composition of the entire cohort, Hispanics had the highest rate of NAFLD and African Americans the lowest.6 In Eastern countries such as Japan, the prevalence of NAFLD is estimated to be about 9.3%. Interestingly, about half of the people with NAFLD in Japan were not overweight.7
The difference in prevalence of NAFLD in different ethnic groups may be explained by their different rates of metabolic syndrome (21.6% in African Americans vs 23.8% in whites vs 31.9% in Mexican Americans8) as well as other genetic and environmental factors.
NAFLD IS USUALLY CLINICALLY SILENT
NAFLD is usually clinically silent, and its impact has most likely been underestimated. Symptoms, if present, are minimal and non-specific, such as fatigue and right upper quadrant discomfort. Most findings on physical examination are also normal. Most patients seek care because of an incidental finding of elevated aminotransferase levels or radiographic studies suggesting the liver is fatty.9
The estimated prevalence of aminotransferase elevations in the general population from the third National Health and Nutrition Examination Survey data is 7.9%,10 with about two-thirds of cases unexplained. Of the unexplained cases, most are strongly associated with metabolic syndrome and probably represent underlying NAFLD.10
Yet aminotransferase levels are typically normal or elevated by less than five times the upper limit of normal (usually < 250 IU/L).9 In contrast to those with alcoholic hepatitis, most patients with NAFLD have a ratio of aspartate aminotransferase to alanine aminotransferase of less than 1. As the disease progresses, the aspartate aminotransferase level increases more than the alanine aminotransferase level, so if the ratio is more than 1, more advanced liver disease may be suspected.11
Levels of other liver enzymes such as alkaline phosphatase and of acute-phase reactants such as ferritin may also be elevated. Ferritin is believed to reflect hepatic injury, inflammation, or insulin resistance.
A DIAGNOSIS OF EXCLUSION
Excessive alcohol consumption must especially be excluded. Most studies defined excessive alcohol consumption as more than 20 to 40 g/day.2 Recently, this threshold has been lowered to 20 g/day (roughly two drinks) in men and 10 g/day in women.
Insulin resistance should be estimated, given the close relationship between NAFLD and insulin resistance and the metabolic syndrome. Insulin resistance can be measured accurately in a number of ways. The Homeostasis Model Assessment is an easy method that provides an estimate of insulin resistance based on fasting serum glucose and serum insulin levels.13
Serologic tests can rule out hepatitis B and hepatitis C. In those with negative results, especially in those with components of the metabolic syndrome or insulin resistance, NAFLD is responsible for most cases of persistently elevated serum liver enzymes.
Imaging tests
Radiographic evaluation is another noninvasive way to diagnose fatty liver. The sensitivity of either ultrasonography or computed tomography for detecting hepatic steatosis is between 93% and 100% when there is more than 33% fat in the hepatic parenchyma.14 None of the radiographic methods, including magnetic resonance imaging, can accurately differentiate between nonprogressive simple steatosis and NASH, but the technology is advancing. Contrast ultrasonography and magnetic resonance spectroscopy have shown promise and may become useful in the future.
Other noninvasive tests
Ultrasonographic elastrography (FibroScan), a noninvasive way to measure liver stiffness, has also been used in patients with hepatitis C. Although the preliminary data in NAFLD are interesting, additional validation is needed.
Serum biomarkers, including markers of fibrosis (eg, FibroSURE), apoptosis, and adipocytokines have been used to diagnose NASH. The markers of apoptosis are especially interesting but need further validation.
Liver biopsy remains the gold standard
Because we lack a fully validated noninvasive biomarker of NASH, liver biopsy remains the gold standard for diagnosing it. The minimum histologic criteria for establishing the diagnosis of NASH have been debated; most pathologists require at least 5% hepatic steatosis, mixed lobular inflammation, and hepatocellular ballooning.
In a study of 354 liver biopsies of patients with negative results on serologic tests, NASH was found in 34% and fatty liver in 32%. In the same study, the findings on liver biopsy led to alterations in patient management in 18% of cases.15
Some clinicians doubt the value of liver biopsy in patients with suspected NASH, in view of possible sampling error in the biopsy specimens (the distribution can be patchy, and if the specimen is taken from an unaffected area, the results can be falsely negative) and because there is no established effective therapy for NAFLD. However, liver biopsy is the only test that can accurately establish the diagnosis of NASH and tell us the stage of liver disease, which has important prognostic implications. Most experts agree that liver biopsy should be considered for patients at risk of advanced liver disease, such as those with persistently elevated liver enzyme levels despite intervention to reverse conditions associated with metabolic syndrome.16
PATHOGENESIS: THE MULTIPLE-HIT HYPOTHESIS
NAFLD is closely linked to obesity, insulin resistance, and metabolic syndrome.13 Insulin allows free fatty esterification and triglyceride fat storage in adipose tissues. When insulin resistance develops, free fatty acids are inappropriately shifted to nonadipose tissues, including the liver. Insulin resistance increases free fatty acid flux to the liver by decreased inhibition of lipolysis and also increased de novo lipogenesis.17
Insulin resistance and visceral obesity also result in decreased levels of a “protective adipokine,” adiponectin. Adiponectin inhibits liver gluconeogenesis and suppresses lipogenesis. Thus, decreased adiponectin hinders fatty acid oxidation and increases fat accumulation in the liver. Other adipocytokines that are important in NAFLD are resistin, leptin, visfatin, tumor necrosis factor alpha, and interleukin 6.
Apoptosis and oxidative stress may also contribute to the development and progression of NASH. In this context, the “multiple-hit hypothesis” for the pathogenesis of NASH has become quite popular.18 An in-depth review of the pathogenesis of NAFLD is beyond the scope of this paper; readers are referred to a recently published review on this subject.19
STEATOSIS IS BENIGN, BUT NASH CAN PROGRESS
Simple steatosis by itself generally has a benign prognosis. In a 1995 cohort study with a median follow-up of 11 years, there was no progression of simple steatosis to NASH or cirrhosis,20 and recent reviews estimate that only a small portion of patients with simple steatosis develop steatohepatitis. The validity of these data is still being debated.
On the other hand, once patients have progressed to NASH, histologic progression has been noted in about 32% to 41% of patients over a median follow-up of 4.3 to 13.7 years.21,22 This would mean that approximately 9% of patients with NASH may develop cirrhosis.21
People with cirrhosis due to NAFLD are at risk of developing liver-related morbidity and of death. In one of the longest follow-up cohort studies (mean follow-up of 13.7 years), end-stage liver disease developed in 5.4%, and hepatocellular carcinoma developed in about 2%. About 20% of the patients died, with more than 70% of the deaths in patients who had NASH at baseline. The survival rate was lower in patients with NASH, whereas no difference in survival was seen in the group with simple steatosis.22
A number of studies have assessed independent predictors of advanced fibrosis. Most studies suggest that elevated liver enzymes, metabolic syndrome, or type 2 diabetes is associated with advanced liver disease. Although noninvasive biomarkers of fibrosis have been developed for hepatitis C, to date, a fully validated, noninvasive biomarker of fibrosis for NAFLD does not exist.
As noted, the spectrum of NAFLD also includes hepatocellular carcinoma, and in a series of 105 patients with hepatocellular carcinoma, hepatitis C virus accounted for 51% and cryptogenic liver disease accounted for another 29%. Since cases of cryptogenic cirrhosis in the United States are considered to be “burned out NASH,” approximately 13% of patients with hepatocellular carcinoma may have had underlying NAFLD as the cause of their liver disease.23 These data suggest that, similar to other cirrhotic patients, NAFLD patients with cirrhosis should be screened for hepatocellular carcinoma.
NO CONSENSUS ON TREATMENT
Weight loss
Modest weight loss—less than 2 pounds (1 kg) per week—is associated with a decrease in the incidence of metabolic syndrome and can also improve the histologic features of NASH in more than 80% of cases.24 Loss of as little as 4% to 5% of body weight is also associated with lowering of aminotransferase and fasting insulin levels.25
The mechanism of benefit is via loss of adipose tissue, which decreases insulin resistance. Weight loss by any means, including bariatric surgery for morbid obesity or use of weight-reducing agents, has been correlated with improvement in liver enzyme levels, liver histologic findings, or both.24,26
However, the traditional low-calorie, low-fat diet may not be optimal for NAFLD patients. In one study,27 patients consuming more than 54% of their calories from carbohydrates compared with those consuming less than 35% had an odds ratio of 6.5 for hepatic inflammation. This finding is not surprising in light of prior research in which high carbohydrate intake increased hepatic de novo lipogenesis. On the other hand, there was no association between total caloric or protein intake and hepatic steatosis or fibrosis. Contrary to traditional beliefs, patients with higher fat intake had less inflammation, steatosis, and fibrosis.
Insulin sensitizers
Given that insulin resistance seems to be the main pathophysiologic culprit in NAFLD, two classes of insulin sensitizers have been studied:
Biguanides act mainly by increasing hepatic insulin sensitivity and reversing insulin resistance induced by tumor necrosis factor alpha.
Glitazones improve insulin sensitivity in both diabetic and euglycemic patients by activating the nuclear transcription factor called peroxisome proliferator-activated receptor (PPAR) gamma.
Both biguanides and glitazones have been found to lower liver enzyme levels, decrease insulin resistance, and improve histopathologic findings. However, the effects of glitazones do not persist after the drugs are stopped, and these drugs and are also associated with an average weight gain of 3 to 6 kg.28,29
Although these data are encouraging, they are preliminary, and more evidence is needed to establish the safety and efficacy of these drugs in treating patients with NASH.
Antioxidants
Antioxidants such as vitamin E, n-acetyl-l-cysteine, s-adenosylmethionine (SAMe), and betaine have been investigated in the treatment of NAFLD.
Vitamin E has been most widely studied. Being fat-soluble, vitamin E can stabilize mitochondrial function and is theorized to inhibit lipid peroxidation and subsequent free radical reactions. Smaller, nonrandomized trials have found that vitamin E improves biochemical markers of liver inflammation. However, in one of the largest randomized controlled trials (with 45 patients), patients taking vitamin E showed improvement in their fibrosis scores but no differences in their necroinflammatory activity or alanine aminotransferase levels.30 Most studies of antioxidants show at least mild improvement in biochemical or histologic signs of NAFLD.31
SAMe and betaine are important antioxidants. However, most studies of SAMe and betaine have been small and inconclusive.
Two large phase III clinical trials are under way at the National Institute of Diabetes and Digestive and Kidney Diseases. They should clarify the role of these agents in the treatment of NASH. The PIVENS (Pioglitazone vs Vitamin E vs Placebo for the Treatment of Non-Diabetic Patients With Nonalcoholic Steatohepatitis) study has completed enrollment of 240 patients, but the final data are not available. The second study, TONIC (Treatment of Nonalcoholic Fatty Liver Disease in Children) will be one of the largest studies of NAFLD in children; it will be looking at vitamin E, metformin, or placebo over a 2-year follow-up. The TONIC study is still under way, so the final data are not yet available.
Ursodeoxycholic acid, another cytoprotective agent, has traditionally been used for primary biliary cirrhosis, but the data are conflicting on its efficacy in NAFLD. Of note, some bile acids are hepatotoxic and facilitate apoptosis via a Fas ligand-mediated pathway. On the other hand, ursodeoxycholic acid is a hydrophilic bile acid that may act to displace the hepatotoxic hydrophobic endogenous bile acids and potentially has an antiapoptotic and cytoprotective effect in NAFLD. Although liver enzyme levels declined in a few of the studies of ursodeoxycholic acid in patients with NAFLD, a large randomized clinical trial (in 166 patients) did not show any significant difference from placebo in liver enzyme levels or liver histologic findings.32
Lipid-lowering drugs
Lipid-lowering drugs target the high levels of triglycerides and low levels of high-density lipoprotein cholesterol that often occur in insulin resistance and metabolic syndrome associated with NAFLD. A few small studies found that aminotransferase levels fell with both statins and gemfibrozil (Lopid).33 Even if liver enzyme levels are abnormal, most experts believe that statins are relatively safe to use in patients with NAFLD who need cholesterol-lowering agents. Nevertheless, clinical monitoring of these patients for potential hepatic toxicity is recommended.
Other medications
Other medications, such as pentoxifylline (Pentoxil, Trental), probiotics, and angiotensin-converting enzyme inhibitors, have been used in small studies of patients with NASH, with encouraging but inconclusive results.
Although a number of pilot studies of agents for treating NAFLD have been proposed, they are small and open-label. With the tremendous recent gains in clinical investigation, functional genomics, and proteomics, it is expected that our understanding of NASH and its treatment will be broadened.
In summary, despite the relatively large number of agents tested for the treatment of NAFLD, most of the data are preliminary. Thus, in 2008, there is no established, evidence-based treatment for patients with NASH.
As the nation gets heavier, our livers will get fattier. The prevalence of nonalcoholic fatty liver disease (NAFLD) has been rising in tandem with the rise in obesity ever since the term nonalcoholic steatohepatitis (NASH, a subtype of NAFLD) was coined by Ludwig in 1980.1 Yet, despite an explosion of research on NAFLD and gains in understanding its epidemiology and pathogenesis, a number of issues remain unresolved, including how to treat it.
NAFLD IS A SPECTRUM
NAFLD is a spectrum. The mildest form is simple fatty liver, or simple steatosis. Next is NASH, or fatty liver with inflammation and evidence of damage to hepatocytes (liver cells). Still more severe is cirrhosis, and in its most extreme form NAFLD can progress to hepatocellular carcinoma or liver failure. The distinction between simple steatosis and NASH is important because their prognoses and management are different.
NAFLD IS COMMON AND LINKED TO OBESITY
NAFLD is the most common cause of elevated liver enzymes and also one of the most common forms of liver disease in the world. It is now estimated to affect about 20% to 30% of people in the United States and other Western countries. In contrast, the prevalence of chronic hepatitis C virus infection is estimated at 3% of the world’s population. In comparison to the prevalence of NAFLD, the prevalence of NASH is much lower: 2% to 3% in the United States.2 The incidence of NAFLD is expected to rise further with the increase in obesity in the United States.
NAFLD is even more common in people who are morbidly obese, ie, who have a body mass index greater than 40 kg/m2. In a series of studies of morbidly obese patients undergoing bariatric surgery (N = 1,620), the prevalence of hepatic steatosis was 91% (range 85%–98%), and the prevalence of NASH was 37% (range 24%–98%). NASH was not predicted by age or body mass index, but it was more common in men, people with diabetes, and people with insulin resistance.3
Obesity is also increasing in prevalence in children. Since liver biopsies were not done in most pediatric studies, the pediatric prevalence data are based on elevated aminotransferase levels and on ultrasonographic findings of echogenic livers. The overall prevalence of NAFLD in children is estimated at 3% to 10%, but it may be much higher in obese children.4
Arun et al5 found that the prevalence of NASH in morbidly obese men was almost twice as high as in morbidly obese women (60.3% vs 30.9%). In contrast, earlier studies suggested that NAFLD was more prevalent in women. This higher incidence of NASH may also reflect the higher incidence of metabolic syndrome in morbidly obese men (91.4% vs 76.2%).
Less common in African Americans
In the United States, African Americans have consistently been found to have the lowest prevalence of NAFLD. In a California population study of 159 newly diagnosed NAFLD cases, non-Hispanic whites accounted for 45%, followed by Hispanics (28%), Asians (18%), and African Americans (3%). After controlling for the ethnic composition of the entire cohort, Hispanics had the highest rate of NAFLD and African Americans the lowest.6 In Eastern countries such as Japan, the prevalence of NAFLD is estimated to be about 9.3%. Interestingly, about half of the people with NAFLD in Japan were not overweight.7
The difference in prevalence of NAFLD in different ethnic groups may be explained by their different rates of metabolic syndrome (21.6% in African Americans vs 23.8% in whites vs 31.9% in Mexican Americans8) as well as other genetic and environmental factors.
NAFLD IS USUALLY CLINICALLY SILENT
NAFLD is usually clinically silent, and its impact has most likely been underestimated. Symptoms, if present, are minimal and non-specific, such as fatigue and right upper quadrant discomfort. Most findings on physical examination are also normal. Most patients seek care because of an incidental finding of elevated aminotransferase levels or radiographic studies suggesting the liver is fatty.9
The estimated prevalence of aminotransferase elevations in the general population from the third National Health and Nutrition Examination Survey data is 7.9%,10 with about two-thirds of cases unexplained. Of the unexplained cases, most are strongly associated with metabolic syndrome and probably represent underlying NAFLD.10
Yet aminotransferase levels are typically normal or elevated by less than five times the upper limit of normal (usually < 250 IU/L).9 In contrast to those with alcoholic hepatitis, most patients with NAFLD have a ratio of aspartate aminotransferase to alanine aminotransferase of less than 1. As the disease progresses, the aspartate aminotransferase level increases more than the alanine aminotransferase level, so if the ratio is more than 1, more advanced liver disease may be suspected.11
Levels of other liver enzymes such as alkaline phosphatase and of acute-phase reactants such as ferritin may also be elevated. Ferritin is believed to reflect hepatic injury, inflammation, or insulin resistance.
A DIAGNOSIS OF EXCLUSION
Excessive alcohol consumption must especially be excluded. Most studies defined excessive alcohol consumption as more than 20 to 40 g/day.2 Recently, this threshold has been lowered to 20 g/day (roughly two drinks) in men and 10 g/day in women.
Insulin resistance should be estimated, given the close relationship between NAFLD and insulin resistance and the metabolic syndrome. Insulin resistance can be measured accurately in a number of ways. The Homeostasis Model Assessment is an easy method that provides an estimate of insulin resistance based on fasting serum glucose and serum insulin levels.13
Serologic tests can rule out hepatitis B and hepatitis C. In those with negative results, especially in those with components of the metabolic syndrome or insulin resistance, NAFLD is responsible for most cases of persistently elevated serum liver enzymes.
Imaging tests
Radiographic evaluation is another noninvasive way to diagnose fatty liver. The sensitivity of either ultrasonography or computed tomography for detecting hepatic steatosis is between 93% and 100% when there is more than 33% fat in the hepatic parenchyma.14 None of the radiographic methods, including magnetic resonance imaging, can accurately differentiate between nonprogressive simple steatosis and NASH, but the technology is advancing. Contrast ultrasonography and magnetic resonance spectroscopy have shown promise and may become useful in the future.
Other noninvasive tests
Ultrasonographic elastrography (FibroScan), a noninvasive way to measure liver stiffness, has also been used in patients with hepatitis C. Although the preliminary data in NAFLD are interesting, additional validation is needed.
Serum biomarkers, including markers of fibrosis (eg, FibroSURE), apoptosis, and adipocytokines have been used to diagnose NASH. The markers of apoptosis are especially interesting but need further validation.
Liver biopsy remains the gold standard
Because we lack a fully validated noninvasive biomarker of NASH, liver biopsy remains the gold standard for diagnosing it. The minimum histologic criteria for establishing the diagnosis of NASH have been debated; most pathologists require at least 5% hepatic steatosis, mixed lobular inflammation, and hepatocellular ballooning.
In a study of 354 liver biopsies of patients with negative results on serologic tests, NASH was found in 34% and fatty liver in 32%. In the same study, the findings on liver biopsy led to alterations in patient management in 18% of cases.15
Some clinicians doubt the value of liver biopsy in patients with suspected NASH, in view of possible sampling error in the biopsy specimens (the distribution can be patchy, and if the specimen is taken from an unaffected area, the results can be falsely negative) and because there is no established effective therapy for NAFLD. However, liver biopsy is the only test that can accurately establish the diagnosis of NASH and tell us the stage of liver disease, which has important prognostic implications. Most experts agree that liver biopsy should be considered for patients at risk of advanced liver disease, such as those with persistently elevated liver enzyme levels despite intervention to reverse conditions associated with metabolic syndrome.16
PATHOGENESIS: THE MULTIPLE-HIT HYPOTHESIS
NAFLD is closely linked to obesity, insulin resistance, and metabolic syndrome.13 Insulin allows free fatty esterification and triglyceride fat storage in adipose tissues. When insulin resistance develops, free fatty acids are inappropriately shifted to nonadipose tissues, including the liver. Insulin resistance increases free fatty acid flux to the liver by decreased inhibition of lipolysis and also increased de novo lipogenesis.17
Insulin resistance and visceral obesity also result in decreased levels of a “protective adipokine,” adiponectin. Adiponectin inhibits liver gluconeogenesis and suppresses lipogenesis. Thus, decreased adiponectin hinders fatty acid oxidation and increases fat accumulation in the liver. Other adipocytokines that are important in NAFLD are resistin, leptin, visfatin, tumor necrosis factor alpha, and interleukin 6.
Apoptosis and oxidative stress may also contribute to the development and progression of NASH. In this context, the “multiple-hit hypothesis” for the pathogenesis of NASH has become quite popular.18 An in-depth review of the pathogenesis of NAFLD is beyond the scope of this paper; readers are referred to a recently published review on this subject.19
STEATOSIS IS BENIGN, BUT NASH CAN PROGRESS
Simple steatosis by itself generally has a benign prognosis. In a 1995 cohort study with a median follow-up of 11 years, there was no progression of simple steatosis to NASH or cirrhosis,20 and recent reviews estimate that only a small portion of patients with simple steatosis develop steatohepatitis. The validity of these data is still being debated.
On the other hand, once patients have progressed to NASH, histologic progression has been noted in about 32% to 41% of patients over a median follow-up of 4.3 to 13.7 years.21,22 This would mean that approximately 9% of patients with NASH may develop cirrhosis.21
People with cirrhosis due to NAFLD are at risk of developing liver-related morbidity and of death. In one of the longest follow-up cohort studies (mean follow-up of 13.7 years), end-stage liver disease developed in 5.4%, and hepatocellular carcinoma developed in about 2%. About 20% of the patients died, with more than 70% of the deaths in patients who had NASH at baseline. The survival rate was lower in patients with NASH, whereas no difference in survival was seen in the group with simple steatosis.22
A number of studies have assessed independent predictors of advanced fibrosis. Most studies suggest that elevated liver enzymes, metabolic syndrome, or type 2 diabetes is associated with advanced liver disease. Although noninvasive biomarkers of fibrosis have been developed for hepatitis C, to date, a fully validated, noninvasive biomarker of fibrosis for NAFLD does not exist.
As noted, the spectrum of NAFLD also includes hepatocellular carcinoma, and in a series of 105 patients with hepatocellular carcinoma, hepatitis C virus accounted for 51% and cryptogenic liver disease accounted for another 29%. Since cases of cryptogenic cirrhosis in the United States are considered to be “burned out NASH,” approximately 13% of patients with hepatocellular carcinoma may have had underlying NAFLD as the cause of their liver disease.23 These data suggest that, similar to other cirrhotic patients, NAFLD patients with cirrhosis should be screened for hepatocellular carcinoma.
NO CONSENSUS ON TREATMENT
Weight loss
Modest weight loss—less than 2 pounds (1 kg) per week—is associated with a decrease in the incidence of metabolic syndrome and can also improve the histologic features of NASH in more than 80% of cases.24 Loss of as little as 4% to 5% of body weight is also associated with lowering of aminotransferase and fasting insulin levels.25
The mechanism of benefit is via loss of adipose tissue, which decreases insulin resistance. Weight loss by any means, including bariatric surgery for morbid obesity or use of weight-reducing agents, has been correlated with improvement in liver enzyme levels, liver histologic findings, or both.24,26
However, the traditional low-calorie, low-fat diet may not be optimal for NAFLD patients. In one study,27 patients consuming more than 54% of their calories from carbohydrates compared with those consuming less than 35% had an odds ratio of 6.5 for hepatic inflammation. This finding is not surprising in light of prior research in which high carbohydrate intake increased hepatic de novo lipogenesis. On the other hand, there was no association between total caloric or protein intake and hepatic steatosis or fibrosis. Contrary to traditional beliefs, patients with higher fat intake had less inflammation, steatosis, and fibrosis.
Insulin sensitizers
Given that insulin resistance seems to be the main pathophysiologic culprit in NAFLD, two classes of insulin sensitizers have been studied:
Biguanides act mainly by increasing hepatic insulin sensitivity and reversing insulin resistance induced by tumor necrosis factor alpha.
Glitazones improve insulin sensitivity in both diabetic and euglycemic patients by activating the nuclear transcription factor called peroxisome proliferator-activated receptor (PPAR) gamma.
Both biguanides and glitazones have been found to lower liver enzyme levels, decrease insulin resistance, and improve histopathologic findings. However, the effects of glitazones do not persist after the drugs are stopped, and these drugs and are also associated with an average weight gain of 3 to 6 kg.28,29
Although these data are encouraging, they are preliminary, and more evidence is needed to establish the safety and efficacy of these drugs in treating patients with NASH.
Antioxidants
Antioxidants such as vitamin E, n-acetyl-l-cysteine, s-adenosylmethionine (SAMe), and betaine have been investigated in the treatment of NAFLD.
Vitamin E has been most widely studied. Being fat-soluble, vitamin E can stabilize mitochondrial function and is theorized to inhibit lipid peroxidation and subsequent free radical reactions. Smaller, nonrandomized trials have found that vitamin E improves biochemical markers of liver inflammation. However, in one of the largest randomized controlled trials (with 45 patients), patients taking vitamin E showed improvement in their fibrosis scores but no differences in their necroinflammatory activity or alanine aminotransferase levels.30 Most studies of antioxidants show at least mild improvement in biochemical or histologic signs of NAFLD.31
SAMe and betaine are important antioxidants. However, most studies of SAMe and betaine have been small and inconclusive.
Two large phase III clinical trials are under way at the National Institute of Diabetes and Digestive and Kidney Diseases. They should clarify the role of these agents in the treatment of NASH. The PIVENS (Pioglitazone vs Vitamin E vs Placebo for the Treatment of Non-Diabetic Patients With Nonalcoholic Steatohepatitis) study has completed enrollment of 240 patients, but the final data are not available. The second study, TONIC (Treatment of Nonalcoholic Fatty Liver Disease in Children) will be one of the largest studies of NAFLD in children; it will be looking at vitamin E, metformin, or placebo over a 2-year follow-up. The TONIC study is still under way, so the final data are not yet available.
Ursodeoxycholic acid, another cytoprotective agent, has traditionally been used for primary biliary cirrhosis, but the data are conflicting on its efficacy in NAFLD. Of note, some bile acids are hepatotoxic and facilitate apoptosis via a Fas ligand-mediated pathway. On the other hand, ursodeoxycholic acid is a hydrophilic bile acid that may act to displace the hepatotoxic hydrophobic endogenous bile acids and potentially has an antiapoptotic and cytoprotective effect in NAFLD. Although liver enzyme levels declined in a few of the studies of ursodeoxycholic acid in patients with NAFLD, a large randomized clinical trial (in 166 patients) did not show any significant difference from placebo in liver enzyme levels or liver histologic findings.32
Lipid-lowering drugs
Lipid-lowering drugs target the high levels of triglycerides and low levels of high-density lipoprotein cholesterol that often occur in insulin resistance and metabolic syndrome associated with NAFLD. A few small studies found that aminotransferase levels fell with both statins and gemfibrozil (Lopid).33 Even if liver enzyme levels are abnormal, most experts believe that statins are relatively safe to use in patients with NAFLD who need cholesterol-lowering agents. Nevertheless, clinical monitoring of these patients for potential hepatic toxicity is recommended.
Other medications
Other medications, such as pentoxifylline (Pentoxil, Trental), probiotics, and angiotensin-converting enzyme inhibitors, have been used in small studies of patients with NASH, with encouraging but inconclusive results.
Although a number of pilot studies of agents for treating NAFLD have been proposed, they are small and open-label. With the tremendous recent gains in clinical investigation, functional genomics, and proteomics, it is expected that our understanding of NASH and its treatment will be broadened.
In summary, despite the relatively large number of agents tested for the treatment of NAFLD, most of the data are preliminary. Thus, in 2008, there is no established, evidence-based treatment for patients with NASH.
- Ludwig J, Viggiano TR, McGill DB, Ott BJ. Nonalcoholic steatohepatitis: Mayo Clinic experiences with a hitherto unnamed disease. Mayo Clin Proc. 1980; 55:434–438.
- Neuschwander-Tetri BA, Caldwell SH. Nonalcoholic steatohepatitis: summary of an AASLD single topic conference. Hepatology. 2003; 37:1202–1209.
- Machado M, Marques-Vidal P, Cortez-Pinto H. Hepatic histology in obese patients undergoing bariatric surgery. J Hepatol. 2006; 45:600–606.
- Shneider BL, Gonzalez-Peralta R, Roberts EA. Controversies in the management of pediatric liver disease: hepatitis B, C, and NAFLD: summary of a single topic conference. Hepatology. 2006; 44:1344–1354.
- Arun J, Clements RH, Lazenby AJ, Leeth RR, Abrams GA. The prevalence of nonalcoholic steatohepatitis is greater in morbidly obese men compared to women. Obes Surg. 2006; 16:1351–1358.
- Weston SR, Leyden W, Murphy R, et al. Racial and ethnic distribution of nonalcoholic fatty liver in persons with newly diagnosed chronic liver disease. Hepatology. 2005; 41:372–379.
- Omagari K, Kadokawa Y, Masuda JI, et al. Fatty liver in non-alcoholic non-overweight Japanese adults: incidence and clinical characteristics. J Gastroenterol Hepatol. 2002; 17:1098–1105.
- Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults. JAMA. 2002; 287:356–359.
- Ramesh S, Sanyal AJ. Evaluation and management of non-alcoholic steatohepatitis. J Hepatol 2005; 42:S2–S12.
- Clark JM, Brancati FL, Diehl AM. The prevalence and etiology of elevated aminotransferase levels in the United States. Am J Gastroenterol. 2003; 98:960–967.
- Angulo P, Keach JC, Batts KP, Lindor KD. Independent predictors of liver fibrosis in patients with nonalcoholic steatohepatitis. Hepatology. 1999; 30:1356–1362.
- Angulo P. Nonalcoholic fatty liver disease. N Engl J Med. 2002; 346:1221–1231.
- Marchesini G, Brizi M, Morselli-Labate AM, et al. Association of nonalcoholic fatty liver disease with insulin resistance. Am J Med. 1999; 107:450–455.
- Saadeh S, Younossi ZM, Remer EM, et al. The utility of radiological imaging in nonalcoholic fatty liver disease. Gastroenterology 2002; 123:745–750.
- Skelly MM, James PD, Ryder SD. Findings on liver biopsy to investigate abnormal liver function tests in the absence of diagnostic serology. J Hepatol. 2001; 35:195–199.
- Collantes R, Ong JP, Younossi ZM. Nonalcoholic fatty liver disease and the epidemic of obesity. Cleve Clin J Med. 2004; 71:657–664.
- Utzschneider KM, Kahn SE. Review: the role of insulin resistance in nonalcoholic fatty liver disease. J Clin Endocrinol Metab. 2006; 91:4753–4761.
- Hui JM, Hodge A, Farrell GC, Kench JG, Kriketos A, George J. Beyond insulin resistance in NASH: TNF-alpha or adiponectin? Hepatology 2004; 40:46–54.
- Edmison J, McCullough AJ. Pathogenesis of non-alcoholic steatohepatitis: human data. Clin Liver Dis. 2007; 11:75–104.
- Teli MR, James OFW, Burt AD, Bennett MK, Day CP. The natural history of nonalcoholic fatty liver: a follow-up study. Hepatology. 1995; 22:1714–1719.
- Harrison SA, Torgerson S, Hayashi PH. The natural history of nonalcoholic fatty liver disease: a clinical histopathological study. Am J Gastroenterol. 2003; 98:2042–2047.
- Ekstedt M, Franzen LE, Mathiesen UL, et al. Long-term follow-up of patients with NAFLD and elevated liver enzymes. Hepatology. 2006; 44:865–873.
- Marrero JA, Fontana RJ, Su GL, Conjeevaram HS, Emick DM, Lok AS. NAFLD may be a common underlying liver disease in patients with hepatocellular carcinoma in the United States. Hepatology. 2003; 36:1349–1354.
- Dixon JB, Bhathal PS, O’Brien PE. Weight loss and non-alcoholic fatty liver disease: falls in gamma-glutamyl transferase concentrations are associated with histologic improvement. Obes Surg. 2006; 16:1278–1286.
- Hickman IJ, Jonsson JR, Prins JB, et al. Modest weight loss and physical activity in overweight patients with chronic liver disease results in sustained improvements in alanine aminotransferase, fasting insulin, and quality of life. Gut 2004: 53:413–419.
- Zelber-Sagi S, Kessler A, Brazowsky E, et al. A double-blind randomized placebo-controlled trial of orlistat for the treatment of nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2006; 4:639–644.
- Solgas S, Alkhuraishe AR, Clark JM, et al. Dietary composition and nonalcoholic fatty liver disease. Dig Dis Sci. 2004; 49:1578–1583.
- Bugianesi E, Gentilcore E, Manini R, et al. A randomized controlled trial of metformin versus vitamin E or prescriptive diet in nonalcoholic fatty liver disease. Am J Gastroenterol. 2005; 100:1082–1090.
- Neuschwander-Tetri BA, Brunt EM, Wehmeier KR, Oliver D, Bacon BR. Improved nonalcoholic steatohepatitis after 48 weeks of treatment with the PPAR-gamma ligand rosiglitazone. Hepatology. 2003; 38:1008–1017.
- Harrison SA, Torgerson S, Hayashi P, Ward J, Schenker S. Vitamin E and vitamin C treatment improves fibrosis in patients with nonalcoholic steatohepatitis. Am J Gastroenterol. 2003; 98:2485–2490.
- Chang CY, Argo CK, Al-Osaimi AMS, Caldwell SH. Therapy of NAFLD, antioxidants and cytoprotective agents. J Clin Gastroenterol 2006; 40:S51–S60.
- Lindor KD, Kowdley KV, Heathcote EJ, et al. Ursodeoxycholic acid for treatment of nonalcoholic steatohepatitis: results of a randomized trial. Hepatology. 2004; 39:770–778.
- Adams LA, Angulo P. Treatment of non-alcoholic fatty liver disease. Postgrad Med J. 2006; 82:315–322.
- Ludwig J, Viggiano TR, McGill DB, Ott BJ. Nonalcoholic steatohepatitis: Mayo Clinic experiences with a hitherto unnamed disease. Mayo Clin Proc. 1980; 55:434–438.
- Neuschwander-Tetri BA, Caldwell SH. Nonalcoholic steatohepatitis: summary of an AASLD single topic conference. Hepatology. 2003; 37:1202–1209.
- Machado M, Marques-Vidal P, Cortez-Pinto H. Hepatic histology in obese patients undergoing bariatric surgery. J Hepatol. 2006; 45:600–606.
- Shneider BL, Gonzalez-Peralta R, Roberts EA. Controversies in the management of pediatric liver disease: hepatitis B, C, and NAFLD: summary of a single topic conference. Hepatology. 2006; 44:1344–1354.
- Arun J, Clements RH, Lazenby AJ, Leeth RR, Abrams GA. The prevalence of nonalcoholic steatohepatitis is greater in morbidly obese men compared to women. Obes Surg. 2006; 16:1351–1358.
- Weston SR, Leyden W, Murphy R, et al. Racial and ethnic distribution of nonalcoholic fatty liver in persons with newly diagnosed chronic liver disease. Hepatology. 2005; 41:372–379.
- Omagari K, Kadokawa Y, Masuda JI, et al. Fatty liver in non-alcoholic non-overweight Japanese adults: incidence and clinical characteristics. J Gastroenterol Hepatol. 2002; 17:1098–1105.
- Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults. JAMA. 2002; 287:356–359.
- Ramesh S, Sanyal AJ. Evaluation and management of non-alcoholic steatohepatitis. J Hepatol 2005; 42:S2–S12.
- Clark JM, Brancati FL, Diehl AM. The prevalence and etiology of elevated aminotransferase levels in the United States. Am J Gastroenterol. 2003; 98:960–967.
- Angulo P, Keach JC, Batts KP, Lindor KD. Independent predictors of liver fibrosis in patients with nonalcoholic steatohepatitis. Hepatology. 1999; 30:1356–1362.
- Angulo P. Nonalcoholic fatty liver disease. N Engl J Med. 2002; 346:1221–1231.
- Marchesini G, Brizi M, Morselli-Labate AM, et al. Association of nonalcoholic fatty liver disease with insulin resistance. Am J Med. 1999; 107:450–455.
- Saadeh S, Younossi ZM, Remer EM, et al. The utility of radiological imaging in nonalcoholic fatty liver disease. Gastroenterology 2002; 123:745–750.
- Skelly MM, James PD, Ryder SD. Findings on liver biopsy to investigate abnormal liver function tests in the absence of diagnostic serology. J Hepatol. 2001; 35:195–199.
- Collantes R, Ong JP, Younossi ZM. Nonalcoholic fatty liver disease and the epidemic of obesity. Cleve Clin J Med. 2004; 71:657–664.
- Utzschneider KM, Kahn SE. Review: the role of insulin resistance in nonalcoholic fatty liver disease. J Clin Endocrinol Metab. 2006; 91:4753–4761.
- Hui JM, Hodge A, Farrell GC, Kench JG, Kriketos A, George J. Beyond insulin resistance in NASH: TNF-alpha or adiponectin? Hepatology 2004; 40:46–54.
- Edmison J, McCullough AJ. Pathogenesis of non-alcoholic steatohepatitis: human data. Clin Liver Dis. 2007; 11:75–104.
- Teli MR, James OFW, Burt AD, Bennett MK, Day CP. The natural history of nonalcoholic fatty liver: a follow-up study. Hepatology. 1995; 22:1714–1719.
- Harrison SA, Torgerson S, Hayashi PH. The natural history of nonalcoholic fatty liver disease: a clinical histopathological study. Am J Gastroenterol. 2003; 98:2042–2047.
- Ekstedt M, Franzen LE, Mathiesen UL, et al. Long-term follow-up of patients with NAFLD and elevated liver enzymes. Hepatology. 2006; 44:865–873.
- Marrero JA, Fontana RJ, Su GL, Conjeevaram HS, Emick DM, Lok AS. NAFLD may be a common underlying liver disease in patients with hepatocellular carcinoma in the United States. Hepatology. 2003; 36:1349–1354.
- Dixon JB, Bhathal PS, O’Brien PE. Weight loss and non-alcoholic fatty liver disease: falls in gamma-glutamyl transferase concentrations are associated with histologic improvement. Obes Surg. 2006; 16:1278–1286.
- Hickman IJ, Jonsson JR, Prins JB, et al. Modest weight loss and physical activity in overweight patients with chronic liver disease results in sustained improvements in alanine aminotransferase, fasting insulin, and quality of life. Gut 2004: 53:413–419.
- Zelber-Sagi S, Kessler A, Brazowsky E, et al. A double-blind randomized placebo-controlled trial of orlistat for the treatment of nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2006; 4:639–644.
- Solgas S, Alkhuraishe AR, Clark JM, et al. Dietary composition and nonalcoholic fatty liver disease. Dig Dis Sci. 2004; 49:1578–1583.
- Bugianesi E, Gentilcore E, Manini R, et al. A randomized controlled trial of metformin versus vitamin E or prescriptive diet in nonalcoholic fatty liver disease. Am J Gastroenterol. 2005; 100:1082–1090.
- Neuschwander-Tetri BA, Brunt EM, Wehmeier KR, Oliver D, Bacon BR. Improved nonalcoholic steatohepatitis after 48 weeks of treatment with the PPAR-gamma ligand rosiglitazone. Hepatology. 2003; 38:1008–1017.
- Harrison SA, Torgerson S, Hayashi P, Ward J, Schenker S. Vitamin E and vitamin C treatment improves fibrosis in patients with nonalcoholic steatohepatitis. Am J Gastroenterol. 2003; 98:2485–2490.
- Chang CY, Argo CK, Al-Osaimi AMS, Caldwell SH. Therapy of NAFLD, antioxidants and cytoprotective agents. J Clin Gastroenterol 2006; 40:S51–S60.
- Lindor KD, Kowdley KV, Heathcote EJ, et al. Ursodeoxycholic acid for treatment of nonalcoholic steatohepatitis: results of a randomized trial. Hepatology. 2004; 39:770–778.
- Adams LA, Angulo P. Treatment of non-alcoholic fatty liver disease. Postgrad Med J. 2006; 82:315–322.
KEY POINTS
- The clinical spectrum of NAFLD ranges from simple steatosis to nonalcoholic steatohepatitis, cirrhosis, and hepatocellular carcinoma.
- NAFLD is closely associated with metabolic syndrome, insulin resistance, and obesity.
- Weight loss and treating components of the metabolic syndrome are central to the treatment of NAFLD. Insulin sensitizers such as biguanides and glitazones, antioxidants such as vitamin E, and lipid-lowering agents have shown promise in small clinical trials, but the evidence remains preliminary.
Which patients benefit from carotid stenting? What recent trials show
Whether carotid stenting has any advantage over carotid surgery (endarterectomy)—and for which patients—is still a topic of study and debate.
Treatment of carotid atherosclerosis and stenosis is important in preventing stroke and its comorbidities. Today, three main treatments exist: medical management (lipid-lowering, antihypertensive, and antiplatelet therapy), surgery, and, more recently, carotid angioplasty and stenting. The rationale for these treatments is to decrease the risk of cerebral infarction by stabilizing or removing plaque and improving blood flow.
Surgery has proven beneficial in patients with symptomatic carotid stenosis greater than 50% or asymptomatic stenosis greater than 60%, but it is risky in some patients. Stenting has evolved in part from the success of surgery and the need for alternative treatments for patients who are at unacceptable risk of perioperative complications. However, it does not have a clear advantage over surgery in patients at average risk. Further, its use in patients with asymptomatic stenosis of any severity is still controversial.
In this paper we review the major trials of carotid endarterectomy and stenting and summarize what we know today about who should undergo these therapies.
NOT ALL STROKES ARE DUE TO CAROTID ATHEROSCLEROSIS
Depending on the institution’s referral pattern and population served, between 80% and 90% of strokes are ischemic (the rest being hemorrhagic).1 Atherosclerosis of large arteries (typically defined as more than 50% stenosis of a major brain artery or branch cortical artery2) is just one cause of ischemic stroke, but it is an important one. Other identifiable causes of ischemic stroke include cardioembolism and small-artery occlusion (lacunar stroke), and some cases are idiopathic.
Large-artery atherosclerotic disease can damage the brain gradually, with carotid stenosis resulting in hypoperfusion and subsequent cerebral infarction. More commonly, however, the carotid plaque often seen in large-artery atherosclerotic disease can ulcerate and occlude the vessel acutely or generate platelet aggregates that may embolize, resulting in cerebral infarction or transient ischemic attack.
In the Lausanne Stroke Registry,3 the rate of ischemic stroke in patients with a greater than 50% large-artery stenosis ranged from 27% in 1979 to 17% in 2003, the decline likely being due to therapeutic advances.
SURGERY BEATS MEDICAL THERAPY FOR CAROTID ATHEROSCLEROSIS
Four landmark trials provided substantial evidence that carotid endarterectomy is better than medical management in patients with symptomatic or asymptomatic high-grade stenosis. These trials indirectly paved the way for carotid stenting.
The North American Symptomatic Carotid Endarterectomy Trial (NASCET)
Patients at 50 clinical centers who had had a hemispheric or retinal transient ischemic attack or a nondisabling stroke were randomized to undergo surgery (carotid endarterectomy) or no surgery. All patients received maximal medical management consisting of blood pressure control, lipid management if indicated, and antiplatelet therapy with aspirin. At baseline, 37% of patients were taking 650 mg or more of aspirin per day, and 11% were taking less than 325 mg per day. The patients were stratified into two prespecified groups on the basis of the severity of carotid stenosis: those with narrowing of 30% to 69% and those with narrowing of 70% to 99%.
Results in high-grade stenosis. In August 1991, the investigators published their results in patients with symptomatic high-grade (70%–99%) stenosis.4 Surgical treatment was more beneficial than medical management alone: the cumulative risk of any ipsilateral stroke at 2 years was 26% in the medical group and 9% in the surgical group, an absolute risk reduction of 17%. The benefit of endarterectomy was still apparent at 8 years of follow-up.5
Results in moderate stenosis. In 1998, the investigators published their results in patients with symptomatic moderate (< 70%) stenosis.5 Surgery was more beneficial than medical therapy in this subgroup as well: at 5 years, the rate of any ipsilateral stroke in patients with 50% to 69% stenosis was 15.7% in those treated surgically and 22.2% in those treated medically (P = .045). In patients with less than 50% stenosis, the 5-year stroke rate was not significantly lower with endarterectomy than with medical therapy.
The European Carotid Surgery Trial (ECST)
The ECST,6 published in 1998, corroborated the NASCET findings. This multicenter, randomized, controlled trial enrolled 3,024 patients with symptoms of at least one transient ischemic attack in the distribution of one or both carotid arteries.
Results. In patients with stenosis of greater than 80% (60% by the NASCET criteria for calculating angiographic stenosis), the frequency of major stroke or death at 3 years was 26.5% in the control group and 14.9% in the surgery group, an absolute difference of 11.6%.
The Endarterectomy for Asymptomatic Carotid Artery Stenosis (ACAS) trial
The NASCET and ECST studies made it clear that select groups of patients with symptomatic carotid stenosis benefit from carotid endarterectomy. But what about patients with stenosis but no prior stroke?
The ACAS trial aimed to find out.7 In this pivotal study, 1,662 patients with asymptomatic carotid artery stenosis greater than 60% were randomized to receive either medical therapy alone or medical plus surgical therapy.
Results were published in 2004. After a median follow-up of 2.7 years, the aggregate 5-year risk of ipsilateral stroke, any perioperative stroke, or death was estimated to be 5.1% in the surgical group and 11.0% in the medical group, a relative risk reduction of 53%. However, for surgery to be beneficial, the rate of perioperative death and other serious complications had to be less than 3%, and the expected patient survival had to be at least 5 years.
Of note, the benefit of carotid endarterectomy in this study was predominantly in men, with less of a benefit for women and diabetic patients. Furthermore, even though endarterectomy was beneficial in this asymptomatic cohort, the overall benefit in terms of stroke risk reduction was small compared with that in NASCET and ECST, in which patients had symptomatic disease.
The Asymptomatic Carotid Surgery Trial (ACST)
In this European version of ACAS, published in 2004, 3,120 patients with asymptomatic carotid narrowing on ultrasonography were randomized to undergo surgery or medical therapy.
Results. The risk of stroke or death within 30 days of carotid endarterectomy was 3.1%. In patients younger than 75 years who had carotid narrowing of 70% or more, immediate surgery decreased the net 5-year stroke risk from 12% to 6%.8
WHO SHOULD NOT UNDERGO CAROTID ENDARTERECTOMY?
From these studies, we can conclude that patients with symptomatic carotid stenosis of 50% or greater and patients with asymptomatic stenosis of 60% or greater benefit from carotid endarterectomy, but only if the perioperative rate of death and other serious complications is less than 3%.7
What are the risk factors for complications during this surgery? In 2006, Cremonesi et al,9 in a consensus paper, defined patients as being at high risk if they had any of the following:
- Contralateral laryngeal nerve palsy
- Radiation therapy to the neck
- Previous carotid endarterectomy with recurrent stenosis
- Lesions high in the cervical internal carotid artery or below the clavicle in the common carotid artery
- Severe tandem lesions
- Age greater than 80 years
- Severe pulmonary disease
- Congestive heart failure (New York Heart
- Association class 3 or 4) or known severe left ventricular dysfunction
- Open heart surgery needed within 6 weeks
- Myocardial infarction within the past 4 weeks
- Unstable angina
- Contralateral carotid occlusion.
Could endovascular treatment be the answer for these patients at high risk who should not undergo carotid endarterectomy? Indeed, the procedure is being studied extensively and performed more frequently. We summarize the major studies below.
STUDIES OF CAROTID STENTING VS ENDARTERECTOMY
The Carotid and Vertebral Artery Transluminal Angioplasty Study (CAVATAS)
This study, published in 2001,10 was the first randomized, multicenter trial to compare the risks and benefits of endovascular treatment (angioplasty with or without stenting) of carotid and vertebral artery stenosis with those of conventional surgery.
To be included, patients had to have carotid artery stenosis (symptomatic or asymptomatic) that was suitable for either carotid endarterectomy or endovascular treatment. Patients were not grouped on the basis of the severity of their stenosis, but the mean stenosis in randomized patients was 86%.
A total of 504 patients were enrolled, of whom 251 were randomized to undergo endovascular treatment. Most patients in this group underwent angioplasty alone, but 26% also received stents because of suboptimal vessel dilatation or at the discretion of the intervening physician.
The primary end point was any disabling stroke or death. Secondary end points were any ipsilateral stroke lasting longer than 7 days and the combination of death or disabling ipsilateral stroke.
The results showed no significant difference between endovascular treatment and surgery in any of these end points at 3 years. However, the overall rates of procedural stroke and death were nearly double those seen in NASCET and ECST. The investigators could not determine the reason for this higher risk, but they hypothesized that CAVATAS included patients at higher risk.
The restenosis rate was higher in the endovascular therapy group (14%) than in the surgery group (4%; P < .001). On the other hand, the surgery group had a higher rate of minor complications, including cranial nerve palsies and neck hematomas.
Carotid Revascularization With Endarterectomy or Stenting Systems (CARESS)
This prospective, multicenter, phase 2 trial, published in 2003, compared the outcomes of standard carotid endarterectomy vs carotid artery stenting using distal embolic protection devices.11 All the patients in this study had at least 50% symptomatic stenosis or 75% asymptomatic stenosis.
Results. At 30 days, 7 (2.4%) of 254 patients in the endarterectomy group had had strokes, and one of the 7 patients with stroke died, so the combined rate of stroke or death (the primary end point) was 2.4%. In the stenting group, 3 (2.1%) of 143 patients had strokes and no patients died. Overall, there was no significant difference in the composite of death, stroke, or myocardial infarction (the secondary end point): 3% for carotid endarterectomy and 2% for stenting patients.
The Stenting and Angioplasty With Protection in Patients at High Risk for Endarterectomy (SAPPHIRE) trial
In this trial,12 published in 2004, patients had to have either symptomatic carotid disease with 50% stenosis or greater or asymptomatic stenosis of 80% or greater, determined by ultrasonography. Further, all patients had to have at least one comorbid condition that increased their perioperative risk. Up until this point, no trial had strictly defined patients at increased risk for complications after carotid endarterectomy and assessed subsequent outcomes. The risk factors included severe cardiac or pulmonary disease, age greater than 80, postendarterectomy carotid stenosis, previous neck surgery, previous neck radiation, contralateral recurrent laryngeal nerve palsy, and contralateral carotid occlusion.
Patients were randomized to undergo carotid artery stenting with distal protection or carotid endarterectomy.
The primary end points of this study were the cumulative incidence of major cardiovascular events at 1 year; death, stroke, or myocardial infarction within 30 days of intervention; and ipsilateral stroke between 31 days and 1 year. Secondary outcomes measured were the rates of target-vessel recanalization at 1 year, cranial nerve palsy, and surgical site complications.
Results. The rate of stroke or death was similar in both groups. The stenting group had fewer adverse cardiac events (mainly non-Q-wave myocardial infarction) than the surgery group. At 1 year the rate of major ipsilateral stroke was 3.3% in the endarterectomy group vs 0% in the stenting group (the difference was not significant), and the cardiovascular event rates continued to be higher in the endarterectomy group.
The investigators noted that myocardial infarction was included as a primary end point because patients with atherosclerotic vascular disease who undergo either stenting or endarterectomy are at a substantial risk of myocardial infarction, and a Q-wave or a non-Q-wave myocardial infarction in the perioperative period increases the risk of future complications and death. A perioperative non-Q-wave infarction increases the risk of death by a factor of 6 and increases the risk of myocardial infarction by a factor of 27 in the subsequent 6 months.
Overall, this study presents evidence that stenting, using distal embolic protection devices, is not inferior to endarterectomy and has fewer cardiovascular complications in patients who have at least one risk factor.
The Endarterectomy Versus Stenting in Patients With Symptomatic Severe Carotid Stenosis (EVA-3S) study
This recent multicenter, randomized study13 was designed to determine if stenting is as good as (not inferior to) carotid endarterectomy in patients with symptomatic carotid stenosis of at least 60%. The primary end point was to be the incidence of stroke or death within 30 days after treatment. However, the trial was stopped early after the inclusion of 527 patients for reasons of safety and futility.
Results. The 30-day incidence of any stroke or death was higher in the stenting group (9.6% vs 3.9%). The relative risk of any stroke or death after stenting as compared with endarterectomy was 2.5. The 30-day incidence of disabling stroke or death was also higher in the stenting group (3.4% vs 1.5%; relative risk 2.2). At 6 months, the incidence of any stroke or death was 6.1% after endarterectomy and 11.7% after stenting (P = .02). There was a trend toward more major local complications after stenting and systemic complications after endarterectomy. Cranial-nerve injury was more common after endarterectomy than after stenting (as expected). Overall, death and stroke rates were lower at 1 month and 6 months with endarterectomy than with stenting.
The Stent-Protected Angioplasty Versus Carotid Endarterectomy (SPACE) trial
This randomized, multicenter study,14 published in 2006, was also designed to compare the safety and efficacy of carotid stenting and endarterectomy. Some 1,200 patients with symptomatic carotid artery stenosis confirmed by ultrasonography were randomly assigned within 180 days of a transient ischemic attack or moderate stroke to undergo carotid artery stenting (n = 605) or carotid endarterectomy (n = 595). The primary end point was ipsilateral ischemic stroke or death 30 days after the procedure. A total of 1,183 patients were included in the analysis.
Results. The rate of the primary end point was 6.84% with stenting and 6.34% with endarterectomy. The study failed to prove the noninferiority of carotid artery stenting compared with carotid endarterectomy for the periprocedural complication rate. Results at 6 to 24 months are awaited.
The Carotid Revascularization Endarterectomy Versus Stenting (CREST) trial
Perhaps the most anxiously awaited results are those of the CREST trial,15 funded by the National Institutes of Health. This is a prospective, randomized, parallel, two-arm, multicenter clinical trial with blinded end point evaluation. Anticipated enrollment will include 2,500 patients. Patients are eligible for enrollment if they have symptoms of carotid stenosis within 180 days of a stroke or transient ischemic attack with ipsilateral carotid stenosis of at least 50% by angiography (70% by ultrasonography), or if they have asymptomatic carotid stenosis of at least 60% by angiography (70% by ultrasonography).
Patients are being randomized to undergo either carotid artery stenting or carotid endarterectomy. All receive aspirin as anti-platelet therapy, treatment for hypertension, and management of other stroke risk factors. Follow-up will last 4 years, with clinic visits at 1, 6, 12, 18, 24, 30, 36, 42, and 48 months. Primary outcome measures will be rates of death, stroke, or myocardial infarction at 30 days postoperatively, and ipsilateral stroke at 30 days postoperatively.
As of February 2007, 1,506 patients had been enrolled and 1,453 had been randomized at 94 sites in North America.
MEDICAID AND MEDICARE NOW PAY FOR THESE THERAPIES
An important practical consideration for patients and physicians is whether Medicaid and Medicare will pay for these therapies.
In July 2001, Medicare began to cover percutaneous transluminal angioplasty of the carotid artery with concurrent stent placement, when furnished in accordance with US Food and Drug Administration (FDA) protocols governing Category B (nonexperimental) investigational device exemption clinical trials.16 Angioplasty of the carotid artery, when provided solely for the purpose of carotid artery dilation concurrent with carotid stent placement, is considered to be a reasonable and necessary service when provided in the context of clinical trials.
In March 2005, Medicare began to provide coverage for percutaneous transluminal angioplasty of the carotid artery concurrent with the placement of an FDA-approved carotid stent with embolic protection for the following groups of patients:
- Those who would be at high risk during carotid endarterectomy and who also have symptomatic carotid artery stenosis of 70% or greater. Coverage is limited to procedures performed using FDA-approved carotid artery stenting systems and embolic protection devices.
- Those who would be at high risk during endarterectomy and who have symptomatic carotid artery stenosis of 50% to 70%, in accordance with the Category B Investigational Device Exemption clinical trials regulation, as a routine cost under the clinical trials policy, or in accordance with the national coverage determination on carotid artery stenting post-approval.
- Those who would be at high risk during carotid endarterectomy and have asymptomatic carotid artery stenosis greater than 80%, in accordance with the Category B Investigational Device Exemption clinical trials regulation, as a routine cost under the clinical trials policy, or in accordance with the national coverage determination on carotid artery stenting postapproval studies.
As noted above, Medicare and Medicaid will only cover carotid stenting if the stent system is FDA-approved, with concomitant use of a distal embolic protection device. However, in view of conflicting data from stenting trials to date, including EVA-3S13 and SPACE,14 it remains to be seen if emboli protection devices significantly reduce periprocedural stroke rates. The FDA recommends that if it is not technically possible to use one of these devices, then the procedure should be aborted due to safety issues.
These coverage decisions are an important practical aspect of carotid stenting and they should be familiar to physicians when they see and refer patients with carotid disease.
WHAT CAN WE SAY AT THIS POINT?
Given the multiple recent and ongoing trials of stenting vs endarterectomy in carotid stenosis, debate continues as to what the role of stenting will be in the future. What can we say at this point?
In patients with asymptomatic carotid stenosis of greater than 60% or symptomatic carotid stenosis of greater than 50%, carotid endarterectomy has been proven to be superior to medical therapy alone.
The efficacy and safety of carotid stenting compared with carotid endarterectomy is still uncertain. In the trials reviewed above, carotid stenting did not appear to have a clear advantage over endarterectomy in patients at average surgical risk. Stenting may be most advantageous when used in patients with symptomatic carotid stenosis who would be at high operative risk, as indicated by the SAPPHIRE trial.
In patients with severe but asymptomatic carotid stenosis who are at high operative risk, the addition of carotid angioplasty and stenting to maximum medical therapy remains controversial. The periprocedural complication rate in these patients may actually exceed the rate of stroke in asymptomatic patients with greater than 60% stenosis who do not undergo stenting or surgery. In addition, subgroup analyses of patients with 70% to 99% symptomatic stenosis in various trials show that surgical benefit is greater in men than in women, and it remains to be seen whether there is any benefit in women with moderate stenoses, asymptomatic lesions, or both.17
Further experience and study are needed, and the results of the Carotid Stenting vs Surgery of Severe Carotid Artery Disease and Stroke Prevention in Asymptomatic Patients (ACT I) study (comparing stenting and surgery in asymptomatic carotid stenosis), and the ongoing CREST trial (comparing stenting and surgery in symptomatic and asymptomatic carotid stenosis) are eagerly awaited. Until then, clinicians should continue to weigh individual patient risks and benefits when referring patients for surgical treatment of carotid athero-sclerotic disease. Regardless of whether surgery is undertaken, maximal medical therapy with the use of antiplatelet agents, blood pressure control, and statin therapy remains the mainstay of treatment.
- Incidence and Prevalence 2006 Chart Book on Cardiovascular and Lung Diseases Bethesda, MD: National Heart, Lung, and Blood Institute; 2006.
- Adams HP, Bendixen BH, Kappelle LJ, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke. 1993; 24:35–41.
- Carrera E, Maeder-Ingvar M, Rossetti AO, Devuyst G, Bogousslavsky JLausanne Stroke Registry. Trends in risk factors, patterns and causes in hospitalized strokes over 25 years: The Lausanne Stroke Registry. Cerebrovasc Dis. 2007; 24:97–103.
- North American Symptomatic Carotid Endarterectomy Trial Collaborators. Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis. N Engl J Med. 1991; 325:445–453.
- Barnett HJ, Taylor DW, Eliasziw M, et al. Benefit of carotid endarterectomy in patients with symptomatic moderate or severe stenosis. North America Symptomatic Carotid Endarterectomy Trial Collaborators. N Engl J Med. 1998; 339:1415–1425.
- European Carotid Surgery Trialists’ Collaborative Group. Randomized trial of endarterectomy for recently symptomatic carotid stenosis: final results of the MRC European Carotid Surgery Trial (ECST). Lancet. 1998; 351:1379–1387.
- Halliday A, Mansfield A, Marro J, et al., MRC Asymptomatic Carotid Surgery Collaborative Group. Prevention of disabling and fatal strokes by successful carotid endarterectomy in patients without recent neurological symptoms: randomized controlled trial. Lancet. 2004; 363:1491–1502.
- Executive Committee for the Asymptomatic Carotid Atherosclerosis Study. Endarterectomy for asymptomatic carotid artery stenosis. JAMA. 1995; 273:1421–1428.
- Cremonesi A, Setacci C, Bignamini A, et al. Carotid artery stenting: first consensus document of the ICCS-SPREAD Joint Committee. Stroke. 2006; 37:2400–2409.
- CAVATAS Investigators. Endovascular versus surgical treatment in patients with carotid stenosis in the Carotid and Vertebral Artery Transluminal Angioplasty Study (CA-VATAS): a randomized trial. Lancet. 2001; 357:1729–1737.
- CARESS Steering Committee. Carotid revascularization using endarterectomy or stenting systems (CARESS): phase I clinical trial: J Endovasc Ther 2003; 10:1021–1030.
- Yadav JS, Wholey MD, Kuntz RE, et al; Stenting and Angioplasty with Protection in Patients at High Risk for Endarterectomy Investigators. Protected carotidartery stenting versus endarterectomy in high-risk patients, N Engl J Med 2004; 351:1493–1501.
- Mas JL, Chatellier G, Beyssen B, et al., EVA-3S Investigators. Endarterectomy versus stenting in patients with symptomatic severe carotid stenosis. N Engl J Med. 2006; 355:1660–1671.
- Ringleb PA, Allenberg J, Bruckmann H, et al., SPACE Collaborative Group. 30 day results from the SPACE trial of stent-protected angioplasty versus carotid endarterectomy in symptomatic patients: a randomised non-inferiority trial. Lancet. 2006; 368:1239–1247.
- CREST. Carotid Revascularization Endarterectomy vs Stent Trial. The Internet Stroke Center. www.strokecenter.org/trials/trialDetail.aspx?tid=80&search_string=crest.
- Center for Medicare and Medicaid Services. Expansion of coverage for percutaneous transluminal angioplasty (PTA). www.cms.hhs.gov/ContractorLearningResources/downloads/JA3811.pdf.
- Rothwell PM, Goldstein LB. Carotid endarterectomy for asymptomatic carotid stenosis: asymptomatic carotid surgery trial. Stroke. 2004; 35:2425–2427.
Whether carotid stenting has any advantage over carotid surgery (endarterectomy)—and for which patients—is still a topic of study and debate.
Treatment of carotid atherosclerosis and stenosis is important in preventing stroke and its comorbidities. Today, three main treatments exist: medical management (lipid-lowering, antihypertensive, and antiplatelet therapy), surgery, and, more recently, carotid angioplasty and stenting. The rationale for these treatments is to decrease the risk of cerebral infarction by stabilizing or removing plaque and improving blood flow.
Surgery has proven beneficial in patients with symptomatic carotid stenosis greater than 50% or asymptomatic stenosis greater than 60%, but it is risky in some patients. Stenting has evolved in part from the success of surgery and the need for alternative treatments for patients who are at unacceptable risk of perioperative complications. However, it does not have a clear advantage over surgery in patients at average risk. Further, its use in patients with asymptomatic stenosis of any severity is still controversial.
In this paper we review the major trials of carotid endarterectomy and stenting and summarize what we know today about who should undergo these therapies.
NOT ALL STROKES ARE DUE TO CAROTID ATHEROSCLEROSIS
Depending on the institution’s referral pattern and population served, between 80% and 90% of strokes are ischemic (the rest being hemorrhagic).1 Atherosclerosis of large arteries (typically defined as more than 50% stenosis of a major brain artery or branch cortical artery2) is just one cause of ischemic stroke, but it is an important one. Other identifiable causes of ischemic stroke include cardioembolism and small-artery occlusion (lacunar stroke), and some cases are idiopathic.
Large-artery atherosclerotic disease can damage the brain gradually, with carotid stenosis resulting in hypoperfusion and subsequent cerebral infarction. More commonly, however, the carotid plaque often seen in large-artery atherosclerotic disease can ulcerate and occlude the vessel acutely or generate platelet aggregates that may embolize, resulting in cerebral infarction or transient ischemic attack.
In the Lausanne Stroke Registry,3 the rate of ischemic stroke in patients with a greater than 50% large-artery stenosis ranged from 27% in 1979 to 17% in 2003, the decline likely being due to therapeutic advances.
SURGERY BEATS MEDICAL THERAPY FOR CAROTID ATHEROSCLEROSIS
Four landmark trials provided substantial evidence that carotid endarterectomy is better than medical management in patients with symptomatic or asymptomatic high-grade stenosis. These trials indirectly paved the way for carotid stenting.
The North American Symptomatic Carotid Endarterectomy Trial (NASCET)
Patients at 50 clinical centers who had had a hemispheric or retinal transient ischemic attack or a nondisabling stroke were randomized to undergo surgery (carotid endarterectomy) or no surgery. All patients received maximal medical management consisting of blood pressure control, lipid management if indicated, and antiplatelet therapy with aspirin. At baseline, 37% of patients were taking 650 mg or more of aspirin per day, and 11% were taking less than 325 mg per day. The patients were stratified into two prespecified groups on the basis of the severity of carotid stenosis: those with narrowing of 30% to 69% and those with narrowing of 70% to 99%.
Results in high-grade stenosis. In August 1991, the investigators published their results in patients with symptomatic high-grade (70%–99%) stenosis.4 Surgical treatment was more beneficial than medical management alone: the cumulative risk of any ipsilateral stroke at 2 years was 26% in the medical group and 9% in the surgical group, an absolute risk reduction of 17%. The benefit of endarterectomy was still apparent at 8 years of follow-up.5
Results in moderate stenosis. In 1998, the investigators published their results in patients with symptomatic moderate (< 70%) stenosis.5 Surgery was more beneficial than medical therapy in this subgroup as well: at 5 years, the rate of any ipsilateral stroke in patients with 50% to 69% stenosis was 15.7% in those treated surgically and 22.2% in those treated medically (P = .045). In patients with less than 50% stenosis, the 5-year stroke rate was not significantly lower with endarterectomy than with medical therapy.
The European Carotid Surgery Trial (ECST)
The ECST,6 published in 1998, corroborated the NASCET findings. This multicenter, randomized, controlled trial enrolled 3,024 patients with symptoms of at least one transient ischemic attack in the distribution of one or both carotid arteries.
Results. In patients with stenosis of greater than 80% (60% by the NASCET criteria for calculating angiographic stenosis), the frequency of major stroke or death at 3 years was 26.5% in the control group and 14.9% in the surgery group, an absolute difference of 11.6%.
The Endarterectomy for Asymptomatic Carotid Artery Stenosis (ACAS) trial
The NASCET and ECST studies made it clear that select groups of patients with symptomatic carotid stenosis benefit from carotid endarterectomy. But what about patients with stenosis but no prior stroke?
The ACAS trial aimed to find out.7 In this pivotal study, 1,662 patients with asymptomatic carotid artery stenosis greater than 60% were randomized to receive either medical therapy alone or medical plus surgical therapy.
Results were published in 2004. After a median follow-up of 2.7 years, the aggregate 5-year risk of ipsilateral stroke, any perioperative stroke, or death was estimated to be 5.1% in the surgical group and 11.0% in the medical group, a relative risk reduction of 53%. However, for surgery to be beneficial, the rate of perioperative death and other serious complications had to be less than 3%, and the expected patient survival had to be at least 5 years.
Of note, the benefit of carotid endarterectomy in this study was predominantly in men, with less of a benefit for women and diabetic patients. Furthermore, even though endarterectomy was beneficial in this asymptomatic cohort, the overall benefit in terms of stroke risk reduction was small compared with that in NASCET and ECST, in which patients had symptomatic disease.
The Asymptomatic Carotid Surgery Trial (ACST)
In this European version of ACAS, published in 2004, 3,120 patients with asymptomatic carotid narrowing on ultrasonography were randomized to undergo surgery or medical therapy.
Results. The risk of stroke or death within 30 days of carotid endarterectomy was 3.1%. In patients younger than 75 years who had carotid narrowing of 70% or more, immediate surgery decreased the net 5-year stroke risk from 12% to 6%.8
WHO SHOULD NOT UNDERGO CAROTID ENDARTERECTOMY?
From these studies, we can conclude that patients with symptomatic carotid stenosis of 50% or greater and patients with asymptomatic stenosis of 60% or greater benefit from carotid endarterectomy, but only if the perioperative rate of death and other serious complications is less than 3%.7
What are the risk factors for complications during this surgery? In 2006, Cremonesi et al,9 in a consensus paper, defined patients as being at high risk if they had any of the following:
- Contralateral laryngeal nerve palsy
- Radiation therapy to the neck
- Previous carotid endarterectomy with recurrent stenosis
- Lesions high in the cervical internal carotid artery or below the clavicle in the common carotid artery
- Severe tandem lesions
- Age greater than 80 years
- Severe pulmonary disease
- Congestive heart failure (New York Heart
- Association class 3 or 4) or known severe left ventricular dysfunction
- Open heart surgery needed within 6 weeks
- Myocardial infarction within the past 4 weeks
- Unstable angina
- Contralateral carotid occlusion.
Could endovascular treatment be the answer for these patients at high risk who should not undergo carotid endarterectomy? Indeed, the procedure is being studied extensively and performed more frequently. We summarize the major studies below.
STUDIES OF CAROTID STENTING VS ENDARTERECTOMY
The Carotid and Vertebral Artery Transluminal Angioplasty Study (CAVATAS)
This study, published in 2001,10 was the first randomized, multicenter trial to compare the risks and benefits of endovascular treatment (angioplasty with or without stenting) of carotid and vertebral artery stenosis with those of conventional surgery.
To be included, patients had to have carotid artery stenosis (symptomatic or asymptomatic) that was suitable for either carotid endarterectomy or endovascular treatment. Patients were not grouped on the basis of the severity of their stenosis, but the mean stenosis in randomized patients was 86%.
A total of 504 patients were enrolled, of whom 251 were randomized to undergo endovascular treatment. Most patients in this group underwent angioplasty alone, but 26% also received stents because of suboptimal vessel dilatation or at the discretion of the intervening physician.
The primary end point was any disabling stroke or death. Secondary end points were any ipsilateral stroke lasting longer than 7 days and the combination of death or disabling ipsilateral stroke.
The results showed no significant difference between endovascular treatment and surgery in any of these end points at 3 years. However, the overall rates of procedural stroke and death were nearly double those seen in NASCET and ECST. The investigators could not determine the reason for this higher risk, but they hypothesized that CAVATAS included patients at higher risk.
The restenosis rate was higher in the endovascular therapy group (14%) than in the surgery group (4%; P < .001). On the other hand, the surgery group had a higher rate of minor complications, including cranial nerve palsies and neck hematomas.
Carotid Revascularization With Endarterectomy or Stenting Systems (CARESS)
This prospective, multicenter, phase 2 trial, published in 2003, compared the outcomes of standard carotid endarterectomy vs carotid artery stenting using distal embolic protection devices.11 All the patients in this study had at least 50% symptomatic stenosis or 75% asymptomatic stenosis.
Results. At 30 days, 7 (2.4%) of 254 patients in the endarterectomy group had had strokes, and one of the 7 patients with stroke died, so the combined rate of stroke or death (the primary end point) was 2.4%. In the stenting group, 3 (2.1%) of 143 patients had strokes and no patients died. Overall, there was no significant difference in the composite of death, stroke, or myocardial infarction (the secondary end point): 3% for carotid endarterectomy and 2% for stenting patients.
The Stenting and Angioplasty With Protection in Patients at High Risk for Endarterectomy (SAPPHIRE) trial
In this trial,12 published in 2004, patients had to have either symptomatic carotid disease with 50% stenosis or greater or asymptomatic stenosis of 80% or greater, determined by ultrasonography. Further, all patients had to have at least one comorbid condition that increased their perioperative risk. Up until this point, no trial had strictly defined patients at increased risk for complications after carotid endarterectomy and assessed subsequent outcomes. The risk factors included severe cardiac or pulmonary disease, age greater than 80, postendarterectomy carotid stenosis, previous neck surgery, previous neck radiation, contralateral recurrent laryngeal nerve palsy, and contralateral carotid occlusion.
Patients were randomized to undergo carotid artery stenting with distal protection or carotid endarterectomy.
The primary end points of this study were the cumulative incidence of major cardiovascular events at 1 year; death, stroke, or myocardial infarction within 30 days of intervention; and ipsilateral stroke between 31 days and 1 year. Secondary outcomes measured were the rates of target-vessel recanalization at 1 year, cranial nerve palsy, and surgical site complications.
Results. The rate of stroke or death was similar in both groups. The stenting group had fewer adverse cardiac events (mainly non-Q-wave myocardial infarction) than the surgery group. At 1 year the rate of major ipsilateral stroke was 3.3% in the endarterectomy group vs 0% in the stenting group (the difference was not significant), and the cardiovascular event rates continued to be higher in the endarterectomy group.
The investigators noted that myocardial infarction was included as a primary end point because patients with atherosclerotic vascular disease who undergo either stenting or endarterectomy are at a substantial risk of myocardial infarction, and a Q-wave or a non-Q-wave myocardial infarction in the perioperative period increases the risk of future complications and death. A perioperative non-Q-wave infarction increases the risk of death by a factor of 6 and increases the risk of myocardial infarction by a factor of 27 in the subsequent 6 months.
Overall, this study presents evidence that stenting, using distal embolic protection devices, is not inferior to endarterectomy and has fewer cardiovascular complications in patients who have at least one risk factor.
The Endarterectomy Versus Stenting in Patients With Symptomatic Severe Carotid Stenosis (EVA-3S) study
This recent multicenter, randomized study13 was designed to determine if stenting is as good as (not inferior to) carotid endarterectomy in patients with symptomatic carotid stenosis of at least 60%. The primary end point was to be the incidence of stroke or death within 30 days after treatment. However, the trial was stopped early after the inclusion of 527 patients for reasons of safety and futility.
Results. The 30-day incidence of any stroke or death was higher in the stenting group (9.6% vs 3.9%). The relative risk of any stroke or death after stenting as compared with endarterectomy was 2.5. The 30-day incidence of disabling stroke or death was also higher in the stenting group (3.4% vs 1.5%; relative risk 2.2). At 6 months, the incidence of any stroke or death was 6.1% after endarterectomy and 11.7% after stenting (P = .02). There was a trend toward more major local complications after stenting and systemic complications after endarterectomy. Cranial-nerve injury was more common after endarterectomy than after stenting (as expected). Overall, death and stroke rates were lower at 1 month and 6 months with endarterectomy than with stenting.
The Stent-Protected Angioplasty Versus Carotid Endarterectomy (SPACE) trial
This randomized, multicenter study,14 published in 2006, was also designed to compare the safety and efficacy of carotid stenting and endarterectomy. Some 1,200 patients with symptomatic carotid artery stenosis confirmed by ultrasonography were randomly assigned within 180 days of a transient ischemic attack or moderate stroke to undergo carotid artery stenting (n = 605) or carotid endarterectomy (n = 595). The primary end point was ipsilateral ischemic stroke or death 30 days after the procedure. A total of 1,183 patients were included in the analysis.
Results. The rate of the primary end point was 6.84% with stenting and 6.34% with endarterectomy. The study failed to prove the noninferiority of carotid artery stenting compared with carotid endarterectomy for the periprocedural complication rate. Results at 6 to 24 months are awaited.
The Carotid Revascularization Endarterectomy Versus Stenting (CREST) trial
Perhaps the most anxiously awaited results are those of the CREST trial,15 funded by the National Institutes of Health. This is a prospective, randomized, parallel, two-arm, multicenter clinical trial with blinded end point evaluation. Anticipated enrollment will include 2,500 patients. Patients are eligible for enrollment if they have symptoms of carotid stenosis within 180 days of a stroke or transient ischemic attack with ipsilateral carotid stenosis of at least 50% by angiography (70% by ultrasonography), or if they have asymptomatic carotid stenosis of at least 60% by angiography (70% by ultrasonography).
Patients are being randomized to undergo either carotid artery stenting or carotid endarterectomy. All receive aspirin as anti-platelet therapy, treatment for hypertension, and management of other stroke risk factors. Follow-up will last 4 years, with clinic visits at 1, 6, 12, 18, 24, 30, 36, 42, and 48 months. Primary outcome measures will be rates of death, stroke, or myocardial infarction at 30 days postoperatively, and ipsilateral stroke at 30 days postoperatively.
As of February 2007, 1,506 patients had been enrolled and 1,453 had been randomized at 94 sites in North America.
MEDICAID AND MEDICARE NOW PAY FOR THESE THERAPIES
An important practical consideration for patients and physicians is whether Medicaid and Medicare will pay for these therapies.
In July 2001, Medicare began to cover percutaneous transluminal angioplasty of the carotid artery with concurrent stent placement, when furnished in accordance with US Food and Drug Administration (FDA) protocols governing Category B (nonexperimental) investigational device exemption clinical trials.16 Angioplasty of the carotid artery, when provided solely for the purpose of carotid artery dilation concurrent with carotid stent placement, is considered to be a reasonable and necessary service when provided in the context of clinical trials.
In March 2005, Medicare began to provide coverage for percutaneous transluminal angioplasty of the carotid artery concurrent with the placement of an FDA-approved carotid stent with embolic protection for the following groups of patients:
- Those who would be at high risk during carotid endarterectomy and who also have symptomatic carotid artery stenosis of 70% or greater. Coverage is limited to procedures performed using FDA-approved carotid artery stenting systems and embolic protection devices.
- Those who would be at high risk during endarterectomy and who have symptomatic carotid artery stenosis of 50% to 70%, in accordance with the Category B Investigational Device Exemption clinical trials regulation, as a routine cost under the clinical trials policy, or in accordance with the national coverage determination on carotid artery stenting post-approval.
- Those who would be at high risk during carotid endarterectomy and have asymptomatic carotid artery stenosis greater than 80%, in accordance with the Category B Investigational Device Exemption clinical trials regulation, as a routine cost under the clinical trials policy, or in accordance with the national coverage determination on carotid artery stenting postapproval studies.
As noted above, Medicare and Medicaid will only cover carotid stenting if the stent system is FDA-approved, with concomitant use of a distal embolic protection device. However, in view of conflicting data from stenting trials to date, including EVA-3S13 and SPACE,14 it remains to be seen if emboli protection devices significantly reduce periprocedural stroke rates. The FDA recommends that if it is not technically possible to use one of these devices, then the procedure should be aborted due to safety issues.
These coverage decisions are an important practical aspect of carotid stenting and they should be familiar to physicians when they see and refer patients with carotid disease.
WHAT CAN WE SAY AT THIS POINT?
Given the multiple recent and ongoing trials of stenting vs endarterectomy in carotid stenosis, debate continues as to what the role of stenting will be in the future. What can we say at this point?
In patients with asymptomatic carotid stenosis of greater than 60% or symptomatic carotid stenosis of greater than 50%, carotid endarterectomy has been proven to be superior to medical therapy alone.
The efficacy and safety of carotid stenting compared with carotid endarterectomy is still uncertain. In the trials reviewed above, carotid stenting did not appear to have a clear advantage over endarterectomy in patients at average surgical risk. Stenting may be most advantageous when used in patients with symptomatic carotid stenosis who would be at high operative risk, as indicated by the SAPPHIRE trial.
In patients with severe but asymptomatic carotid stenosis who are at high operative risk, the addition of carotid angioplasty and stenting to maximum medical therapy remains controversial. The periprocedural complication rate in these patients may actually exceed the rate of stroke in asymptomatic patients with greater than 60% stenosis who do not undergo stenting or surgery. In addition, subgroup analyses of patients with 70% to 99% symptomatic stenosis in various trials show that surgical benefit is greater in men than in women, and it remains to be seen whether there is any benefit in women with moderate stenoses, asymptomatic lesions, or both.17
Further experience and study are needed, and the results of the Carotid Stenting vs Surgery of Severe Carotid Artery Disease and Stroke Prevention in Asymptomatic Patients (ACT I) study (comparing stenting and surgery in asymptomatic carotid stenosis), and the ongoing CREST trial (comparing stenting and surgery in symptomatic and asymptomatic carotid stenosis) are eagerly awaited. Until then, clinicians should continue to weigh individual patient risks and benefits when referring patients for surgical treatment of carotid athero-sclerotic disease. Regardless of whether surgery is undertaken, maximal medical therapy with the use of antiplatelet agents, blood pressure control, and statin therapy remains the mainstay of treatment.
Whether carotid stenting has any advantage over carotid surgery (endarterectomy)—and for which patients—is still a topic of study and debate.
Treatment of carotid atherosclerosis and stenosis is important in preventing stroke and its comorbidities. Today, three main treatments exist: medical management (lipid-lowering, antihypertensive, and antiplatelet therapy), surgery, and, more recently, carotid angioplasty and stenting. The rationale for these treatments is to decrease the risk of cerebral infarction by stabilizing or removing plaque and improving blood flow.
Surgery has proven beneficial in patients with symptomatic carotid stenosis greater than 50% or asymptomatic stenosis greater than 60%, but it is risky in some patients. Stenting has evolved in part from the success of surgery and the need for alternative treatments for patients who are at unacceptable risk of perioperative complications. However, it does not have a clear advantage over surgery in patients at average risk. Further, its use in patients with asymptomatic stenosis of any severity is still controversial.
In this paper we review the major trials of carotid endarterectomy and stenting and summarize what we know today about who should undergo these therapies.
NOT ALL STROKES ARE DUE TO CAROTID ATHEROSCLEROSIS
Depending on the institution’s referral pattern and population served, between 80% and 90% of strokes are ischemic (the rest being hemorrhagic).1 Atherosclerosis of large arteries (typically defined as more than 50% stenosis of a major brain artery or branch cortical artery2) is just one cause of ischemic stroke, but it is an important one. Other identifiable causes of ischemic stroke include cardioembolism and small-artery occlusion (lacunar stroke), and some cases are idiopathic.
Large-artery atherosclerotic disease can damage the brain gradually, with carotid stenosis resulting in hypoperfusion and subsequent cerebral infarction. More commonly, however, the carotid plaque often seen in large-artery atherosclerotic disease can ulcerate and occlude the vessel acutely or generate platelet aggregates that may embolize, resulting in cerebral infarction or transient ischemic attack.
In the Lausanne Stroke Registry,3 the rate of ischemic stroke in patients with a greater than 50% large-artery stenosis ranged from 27% in 1979 to 17% in 2003, the decline likely being due to therapeutic advances.
SURGERY BEATS MEDICAL THERAPY FOR CAROTID ATHEROSCLEROSIS
Four landmark trials provided substantial evidence that carotid endarterectomy is better than medical management in patients with symptomatic or asymptomatic high-grade stenosis. These trials indirectly paved the way for carotid stenting.
The North American Symptomatic Carotid Endarterectomy Trial (NASCET)
Patients at 50 clinical centers who had had a hemispheric or retinal transient ischemic attack or a nondisabling stroke were randomized to undergo surgery (carotid endarterectomy) or no surgery. All patients received maximal medical management consisting of blood pressure control, lipid management if indicated, and antiplatelet therapy with aspirin. At baseline, 37% of patients were taking 650 mg or more of aspirin per day, and 11% were taking less than 325 mg per day. The patients were stratified into two prespecified groups on the basis of the severity of carotid stenosis: those with narrowing of 30% to 69% and those with narrowing of 70% to 99%.
Results in high-grade stenosis. In August 1991, the investigators published their results in patients with symptomatic high-grade (70%–99%) stenosis.4 Surgical treatment was more beneficial than medical management alone: the cumulative risk of any ipsilateral stroke at 2 years was 26% in the medical group and 9% in the surgical group, an absolute risk reduction of 17%. The benefit of endarterectomy was still apparent at 8 years of follow-up.5
Results in moderate stenosis. In 1998, the investigators published their results in patients with symptomatic moderate (< 70%) stenosis.5 Surgery was more beneficial than medical therapy in this subgroup as well: at 5 years, the rate of any ipsilateral stroke in patients with 50% to 69% stenosis was 15.7% in those treated surgically and 22.2% in those treated medically (P = .045). In patients with less than 50% stenosis, the 5-year stroke rate was not significantly lower with endarterectomy than with medical therapy.
The European Carotid Surgery Trial (ECST)
The ECST,6 published in 1998, corroborated the NASCET findings. This multicenter, randomized, controlled trial enrolled 3,024 patients with symptoms of at least one transient ischemic attack in the distribution of one or both carotid arteries.
Results. In patients with stenosis of greater than 80% (60% by the NASCET criteria for calculating angiographic stenosis), the frequency of major stroke or death at 3 years was 26.5% in the control group and 14.9% in the surgery group, an absolute difference of 11.6%.
The Endarterectomy for Asymptomatic Carotid Artery Stenosis (ACAS) trial
The NASCET and ECST studies made it clear that select groups of patients with symptomatic carotid stenosis benefit from carotid endarterectomy. But what about patients with stenosis but no prior stroke?
The ACAS trial aimed to find out.7 In this pivotal study, 1,662 patients with asymptomatic carotid artery stenosis greater than 60% were randomized to receive either medical therapy alone or medical plus surgical therapy.
Results were published in 2004. After a median follow-up of 2.7 years, the aggregate 5-year risk of ipsilateral stroke, any perioperative stroke, or death was estimated to be 5.1% in the surgical group and 11.0% in the medical group, a relative risk reduction of 53%. However, for surgery to be beneficial, the rate of perioperative death and other serious complications had to be less than 3%, and the expected patient survival had to be at least 5 years.
Of note, the benefit of carotid endarterectomy in this study was predominantly in men, with less of a benefit for women and diabetic patients. Furthermore, even though endarterectomy was beneficial in this asymptomatic cohort, the overall benefit in terms of stroke risk reduction was small compared with that in NASCET and ECST, in which patients had symptomatic disease.
The Asymptomatic Carotid Surgery Trial (ACST)
In this European version of ACAS, published in 2004, 3,120 patients with asymptomatic carotid narrowing on ultrasonography were randomized to undergo surgery or medical therapy.
Results. The risk of stroke or death within 30 days of carotid endarterectomy was 3.1%. In patients younger than 75 years who had carotid narrowing of 70% or more, immediate surgery decreased the net 5-year stroke risk from 12% to 6%.8
WHO SHOULD NOT UNDERGO CAROTID ENDARTERECTOMY?
From these studies, we can conclude that patients with symptomatic carotid stenosis of 50% or greater and patients with asymptomatic stenosis of 60% or greater benefit from carotid endarterectomy, but only if the perioperative rate of death and other serious complications is less than 3%.7
What are the risk factors for complications during this surgery? In 2006, Cremonesi et al,9 in a consensus paper, defined patients as being at high risk if they had any of the following:
- Contralateral laryngeal nerve palsy
- Radiation therapy to the neck
- Previous carotid endarterectomy with recurrent stenosis
- Lesions high in the cervical internal carotid artery or below the clavicle in the common carotid artery
- Severe tandem lesions
- Age greater than 80 years
- Severe pulmonary disease
- Congestive heart failure (New York Heart
- Association class 3 or 4) or known severe left ventricular dysfunction
- Open heart surgery needed within 6 weeks
- Myocardial infarction within the past 4 weeks
- Unstable angina
- Contralateral carotid occlusion.
Could endovascular treatment be the answer for these patients at high risk who should not undergo carotid endarterectomy? Indeed, the procedure is being studied extensively and performed more frequently. We summarize the major studies below.
STUDIES OF CAROTID STENTING VS ENDARTERECTOMY
The Carotid and Vertebral Artery Transluminal Angioplasty Study (CAVATAS)
This study, published in 2001,10 was the first randomized, multicenter trial to compare the risks and benefits of endovascular treatment (angioplasty with or without stenting) of carotid and vertebral artery stenosis with those of conventional surgery.
To be included, patients had to have carotid artery stenosis (symptomatic or asymptomatic) that was suitable for either carotid endarterectomy or endovascular treatment. Patients were not grouped on the basis of the severity of their stenosis, but the mean stenosis in randomized patients was 86%.
A total of 504 patients were enrolled, of whom 251 were randomized to undergo endovascular treatment. Most patients in this group underwent angioplasty alone, but 26% also received stents because of suboptimal vessel dilatation or at the discretion of the intervening physician.
The primary end point was any disabling stroke or death. Secondary end points were any ipsilateral stroke lasting longer than 7 days and the combination of death or disabling ipsilateral stroke.
The results showed no significant difference between endovascular treatment and surgery in any of these end points at 3 years. However, the overall rates of procedural stroke and death were nearly double those seen in NASCET and ECST. The investigators could not determine the reason for this higher risk, but they hypothesized that CAVATAS included patients at higher risk.
The restenosis rate was higher in the endovascular therapy group (14%) than in the surgery group (4%; P < .001). On the other hand, the surgery group had a higher rate of minor complications, including cranial nerve palsies and neck hematomas.
Carotid Revascularization With Endarterectomy or Stenting Systems (CARESS)
This prospective, multicenter, phase 2 trial, published in 2003, compared the outcomes of standard carotid endarterectomy vs carotid artery stenting using distal embolic protection devices.11 All the patients in this study had at least 50% symptomatic stenosis or 75% asymptomatic stenosis.
Results. At 30 days, 7 (2.4%) of 254 patients in the endarterectomy group had had strokes, and one of the 7 patients with stroke died, so the combined rate of stroke or death (the primary end point) was 2.4%. In the stenting group, 3 (2.1%) of 143 patients had strokes and no patients died. Overall, there was no significant difference in the composite of death, stroke, or myocardial infarction (the secondary end point): 3% for carotid endarterectomy and 2% for stenting patients.
The Stenting and Angioplasty With Protection in Patients at High Risk for Endarterectomy (SAPPHIRE) trial
In this trial,12 published in 2004, patients had to have either symptomatic carotid disease with 50% stenosis or greater or asymptomatic stenosis of 80% or greater, determined by ultrasonography. Further, all patients had to have at least one comorbid condition that increased their perioperative risk. Up until this point, no trial had strictly defined patients at increased risk for complications after carotid endarterectomy and assessed subsequent outcomes. The risk factors included severe cardiac or pulmonary disease, age greater than 80, postendarterectomy carotid stenosis, previous neck surgery, previous neck radiation, contralateral recurrent laryngeal nerve palsy, and contralateral carotid occlusion.
Patients were randomized to undergo carotid artery stenting with distal protection or carotid endarterectomy.
The primary end points of this study were the cumulative incidence of major cardiovascular events at 1 year; death, stroke, or myocardial infarction within 30 days of intervention; and ipsilateral stroke between 31 days and 1 year. Secondary outcomes measured were the rates of target-vessel recanalization at 1 year, cranial nerve palsy, and surgical site complications.
Results. The rate of stroke or death was similar in both groups. The stenting group had fewer adverse cardiac events (mainly non-Q-wave myocardial infarction) than the surgery group. At 1 year the rate of major ipsilateral stroke was 3.3% in the endarterectomy group vs 0% in the stenting group (the difference was not significant), and the cardiovascular event rates continued to be higher in the endarterectomy group.
The investigators noted that myocardial infarction was included as a primary end point because patients with atherosclerotic vascular disease who undergo either stenting or endarterectomy are at a substantial risk of myocardial infarction, and a Q-wave or a non-Q-wave myocardial infarction in the perioperative period increases the risk of future complications and death. A perioperative non-Q-wave infarction increases the risk of death by a factor of 6 and increases the risk of myocardial infarction by a factor of 27 in the subsequent 6 months.
Overall, this study presents evidence that stenting, using distal embolic protection devices, is not inferior to endarterectomy and has fewer cardiovascular complications in patients who have at least one risk factor.
The Endarterectomy Versus Stenting in Patients With Symptomatic Severe Carotid Stenosis (EVA-3S) study
This recent multicenter, randomized study13 was designed to determine if stenting is as good as (not inferior to) carotid endarterectomy in patients with symptomatic carotid stenosis of at least 60%. The primary end point was to be the incidence of stroke or death within 30 days after treatment. However, the trial was stopped early after the inclusion of 527 patients for reasons of safety and futility.
Results. The 30-day incidence of any stroke or death was higher in the stenting group (9.6% vs 3.9%). The relative risk of any stroke or death after stenting as compared with endarterectomy was 2.5. The 30-day incidence of disabling stroke or death was also higher in the stenting group (3.4% vs 1.5%; relative risk 2.2). At 6 months, the incidence of any stroke or death was 6.1% after endarterectomy and 11.7% after stenting (P = .02). There was a trend toward more major local complications after stenting and systemic complications after endarterectomy. Cranial-nerve injury was more common after endarterectomy than after stenting (as expected). Overall, death and stroke rates were lower at 1 month and 6 months with endarterectomy than with stenting.
The Stent-Protected Angioplasty Versus Carotid Endarterectomy (SPACE) trial
This randomized, multicenter study,14 published in 2006, was also designed to compare the safety and efficacy of carotid stenting and endarterectomy. Some 1,200 patients with symptomatic carotid artery stenosis confirmed by ultrasonography were randomly assigned within 180 days of a transient ischemic attack or moderate stroke to undergo carotid artery stenting (n = 605) or carotid endarterectomy (n = 595). The primary end point was ipsilateral ischemic stroke or death 30 days after the procedure. A total of 1,183 patients were included in the analysis.
Results. The rate of the primary end point was 6.84% with stenting and 6.34% with endarterectomy. The study failed to prove the noninferiority of carotid artery stenting compared with carotid endarterectomy for the periprocedural complication rate. Results at 6 to 24 months are awaited.
The Carotid Revascularization Endarterectomy Versus Stenting (CREST) trial
Perhaps the most anxiously awaited results are those of the CREST trial,15 funded by the National Institutes of Health. This is a prospective, randomized, parallel, two-arm, multicenter clinical trial with blinded end point evaluation. Anticipated enrollment will include 2,500 patients. Patients are eligible for enrollment if they have symptoms of carotid stenosis within 180 days of a stroke or transient ischemic attack with ipsilateral carotid stenosis of at least 50% by angiography (70% by ultrasonography), or if they have asymptomatic carotid stenosis of at least 60% by angiography (70% by ultrasonography).
Patients are being randomized to undergo either carotid artery stenting or carotid endarterectomy. All receive aspirin as anti-platelet therapy, treatment for hypertension, and management of other stroke risk factors. Follow-up will last 4 years, with clinic visits at 1, 6, 12, 18, 24, 30, 36, 42, and 48 months. Primary outcome measures will be rates of death, stroke, or myocardial infarction at 30 days postoperatively, and ipsilateral stroke at 30 days postoperatively.
As of February 2007, 1,506 patients had been enrolled and 1,453 had been randomized at 94 sites in North America.
MEDICAID AND MEDICARE NOW PAY FOR THESE THERAPIES
An important practical consideration for patients and physicians is whether Medicaid and Medicare will pay for these therapies.
In July 2001, Medicare began to cover percutaneous transluminal angioplasty of the carotid artery with concurrent stent placement, when furnished in accordance with US Food and Drug Administration (FDA) protocols governing Category B (nonexperimental) investigational device exemption clinical trials.16 Angioplasty of the carotid artery, when provided solely for the purpose of carotid artery dilation concurrent with carotid stent placement, is considered to be a reasonable and necessary service when provided in the context of clinical trials.
In March 2005, Medicare began to provide coverage for percutaneous transluminal angioplasty of the carotid artery concurrent with the placement of an FDA-approved carotid stent with embolic protection for the following groups of patients:
- Those who would be at high risk during carotid endarterectomy and who also have symptomatic carotid artery stenosis of 70% or greater. Coverage is limited to procedures performed using FDA-approved carotid artery stenting systems and embolic protection devices.
- Those who would be at high risk during endarterectomy and who have symptomatic carotid artery stenosis of 50% to 70%, in accordance with the Category B Investigational Device Exemption clinical trials regulation, as a routine cost under the clinical trials policy, or in accordance with the national coverage determination on carotid artery stenting post-approval.
- Those who would be at high risk during carotid endarterectomy and have asymptomatic carotid artery stenosis greater than 80%, in accordance with the Category B Investigational Device Exemption clinical trials regulation, as a routine cost under the clinical trials policy, or in accordance with the national coverage determination on carotid artery stenting postapproval studies.
As noted above, Medicare and Medicaid will only cover carotid stenting if the stent system is FDA-approved, with concomitant use of a distal embolic protection device. However, in view of conflicting data from stenting trials to date, including EVA-3S13 and SPACE,14 it remains to be seen if emboli protection devices significantly reduce periprocedural stroke rates. The FDA recommends that if it is not technically possible to use one of these devices, then the procedure should be aborted due to safety issues.
These coverage decisions are an important practical aspect of carotid stenting and they should be familiar to physicians when they see and refer patients with carotid disease.
WHAT CAN WE SAY AT THIS POINT?
Given the multiple recent and ongoing trials of stenting vs endarterectomy in carotid stenosis, debate continues as to what the role of stenting will be in the future. What can we say at this point?
In patients with asymptomatic carotid stenosis of greater than 60% or symptomatic carotid stenosis of greater than 50%, carotid endarterectomy has been proven to be superior to medical therapy alone.
The efficacy and safety of carotid stenting compared with carotid endarterectomy is still uncertain. In the trials reviewed above, carotid stenting did not appear to have a clear advantage over endarterectomy in patients at average surgical risk. Stenting may be most advantageous when used in patients with symptomatic carotid stenosis who would be at high operative risk, as indicated by the SAPPHIRE trial.
In patients with severe but asymptomatic carotid stenosis who are at high operative risk, the addition of carotid angioplasty and stenting to maximum medical therapy remains controversial. The periprocedural complication rate in these patients may actually exceed the rate of stroke in asymptomatic patients with greater than 60% stenosis who do not undergo stenting or surgery. In addition, subgroup analyses of patients with 70% to 99% symptomatic stenosis in various trials show that surgical benefit is greater in men than in women, and it remains to be seen whether there is any benefit in women with moderate stenoses, asymptomatic lesions, or both.17
Further experience and study are needed, and the results of the Carotid Stenting vs Surgery of Severe Carotid Artery Disease and Stroke Prevention in Asymptomatic Patients (ACT I) study (comparing stenting and surgery in asymptomatic carotid stenosis), and the ongoing CREST trial (comparing stenting and surgery in symptomatic and asymptomatic carotid stenosis) are eagerly awaited. Until then, clinicians should continue to weigh individual patient risks and benefits when referring patients for surgical treatment of carotid athero-sclerotic disease. Regardless of whether surgery is undertaken, maximal medical therapy with the use of antiplatelet agents, blood pressure control, and statin therapy remains the mainstay of treatment.
- Incidence and Prevalence 2006 Chart Book on Cardiovascular and Lung Diseases Bethesda, MD: National Heart, Lung, and Blood Institute; 2006.
- Adams HP, Bendixen BH, Kappelle LJ, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke. 1993; 24:35–41.
- Carrera E, Maeder-Ingvar M, Rossetti AO, Devuyst G, Bogousslavsky JLausanne Stroke Registry. Trends in risk factors, patterns and causes in hospitalized strokes over 25 years: The Lausanne Stroke Registry. Cerebrovasc Dis. 2007; 24:97–103.
- North American Symptomatic Carotid Endarterectomy Trial Collaborators. Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis. N Engl J Med. 1991; 325:445–453.
- Barnett HJ, Taylor DW, Eliasziw M, et al. Benefit of carotid endarterectomy in patients with symptomatic moderate or severe stenosis. North America Symptomatic Carotid Endarterectomy Trial Collaborators. N Engl J Med. 1998; 339:1415–1425.
- European Carotid Surgery Trialists’ Collaborative Group. Randomized trial of endarterectomy for recently symptomatic carotid stenosis: final results of the MRC European Carotid Surgery Trial (ECST). Lancet. 1998; 351:1379–1387.
- Halliday A, Mansfield A, Marro J, et al., MRC Asymptomatic Carotid Surgery Collaborative Group. Prevention of disabling and fatal strokes by successful carotid endarterectomy in patients without recent neurological symptoms: randomized controlled trial. Lancet. 2004; 363:1491–1502.
- Executive Committee for the Asymptomatic Carotid Atherosclerosis Study. Endarterectomy for asymptomatic carotid artery stenosis. JAMA. 1995; 273:1421–1428.
- Cremonesi A, Setacci C, Bignamini A, et al. Carotid artery stenting: first consensus document of the ICCS-SPREAD Joint Committee. Stroke. 2006; 37:2400–2409.
- CAVATAS Investigators. Endovascular versus surgical treatment in patients with carotid stenosis in the Carotid and Vertebral Artery Transluminal Angioplasty Study (CA-VATAS): a randomized trial. Lancet. 2001; 357:1729–1737.
- CARESS Steering Committee. Carotid revascularization using endarterectomy or stenting systems (CARESS): phase I clinical trial: J Endovasc Ther 2003; 10:1021–1030.
- Yadav JS, Wholey MD, Kuntz RE, et al; Stenting and Angioplasty with Protection in Patients at High Risk for Endarterectomy Investigators. Protected carotidartery stenting versus endarterectomy in high-risk patients, N Engl J Med 2004; 351:1493–1501.
- Mas JL, Chatellier G, Beyssen B, et al., EVA-3S Investigators. Endarterectomy versus stenting in patients with symptomatic severe carotid stenosis. N Engl J Med. 2006; 355:1660–1671.
- Ringleb PA, Allenberg J, Bruckmann H, et al., SPACE Collaborative Group. 30 day results from the SPACE trial of stent-protected angioplasty versus carotid endarterectomy in symptomatic patients: a randomised non-inferiority trial. Lancet. 2006; 368:1239–1247.
- CREST. Carotid Revascularization Endarterectomy vs Stent Trial. The Internet Stroke Center. www.strokecenter.org/trials/trialDetail.aspx?tid=80&search_string=crest.
- Center for Medicare and Medicaid Services. Expansion of coverage for percutaneous transluminal angioplasty (PTA). www.cms.hhs.gov/ContractorLearningResources/downloads/JA3811.pdf.
- Rothwell PM, Goldstein LB. Carotid endarterectomy for asymptomatic carotid stenosis: asymptomatic carotid surgery trial. Stroke. 2004; 35:2425–2427.
- Incidence and Prevalence 2006 Chart Book on Cardiovascular and Lung Diseases Bethesda, MD: National Heart, Lung, and Blood Institute; 2006.
- Adams HP, Bendixen BH, Kappelle LJ, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke. 1993; 24:35–41.
- Carrera E, Maeder-Ingvar M, Rossetti AO, Devuyst G, Bogousslavsky JLausanne Stroke Registry. Trends in risk factors, patterns and causes in hospitalized strokes over 25 years: The Lausanne Stroke Registry. Cerebrovasc Dis. 2007; 24:97–103.
- North American Symptomatic Carotid Endarterectomy Trial Collaborators. Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis. N Engl J Med. 1991; 325:445–453.
- Barnett HJ, Taylor DW, Eliasziw M, et al. Benefit of carotid endarterectomy in patients with symptomatic moderate or severe stenosis. North America Symptomatic Carotid Endarterectomy Trial Collaborators. N Engl J Med. 1998; 339:1415–1425.
- European Carotid Surgery Trialists’ Collaborative Group. Randomized trial of endarterectomy for recently symptomatic carotid stenosis: final results of the MRC European Carotid Surgery Trial (ECST). Lancet. 1998; 351:1379–1387.
- Halliday A, Mansfield A, Marro J, et al., MRC Asymptomatic Carotid Surgery Collaborative Group. Prevention of disabling and fatal strokes by successful carotid endarterectomy in patients without recent neurological symptoms: randomized controlled trial. Lancet. 2004; 363:1491–1502.
- Executive Committee for the Asymptomatic Carotid Atherosclerosis Study. Endarterectomy for asymptomatic carotid artery stenosis. JAMA. 1995; 273:1421–1428.
- Cremonesi A, Setacci C, Bignamini A, et al. Carotid artery stenting: first consensus document of the ICCS-SPREAD Joint Committee. Stroke. 2006; 37:2400–2409.
- CAVATAS Investigators. Endovascular versus surgical treatment in patients with carotid stenosis in the Carotid and Vertebral Artery Transluminal Angioplasty Study (CA-VATAS): a randomized trial. Lancet. 2001; 357:1729–1737.
- CARESS Steering Committee. Carotid revascularization using endarterectomy or stenting systems (CARESS): phase I clinical trial: J Endovasc Ther 2003; 10:1021–1030.
- Yadav JS, Wholey MD, Kuntz RE, et al; Stenting and Angioplasty with Protection in Patients at High Risk for Endarterectomy Investigators. Protected carotidartery stenting versus endarterectomy in high-risk patients, N Engl J Med 2004; 351:1493–1501.
- Mas JL, Chatellier G, Beyssen B, et al., EVA-3S Investigators. Endarterectomy versus stenting in patients with symptomatic severe carotid stenosis. N Engl J Med. 2006; 355:1660–1671.
- Ringleb PA, Allenberg J, Bruckmann H, et al., SPACE Collaborative Group. 30 day results from the SPACE trial of stent-protected angioplasty versus carotid endarterectomy in symptomatic patients: a randomised non-inferiority trial. Lancet. 2006; 368:1239–1247.
- CREST. Carotid Revascularization Endarterectomy vs Stent Trial. The Internet Stroke Center. www.strokecenter.org/trials/trialDetail.aspx?tid=80&search_string=crest.
- Center for Medicare and Medicaid Services. Expansion of coverage for percutaneous transluminal angioplasty (PTA). www.cms.hhs.gov/ContractorLearningResources/downloads/JA3811.pdf.
- Rothwell PM, Goldstein LB. Carotid endarterectomy for asymptomatic carotid stenosis: asymptomatic carotid surgery trial. Stroke. 2004; 35:2425–2427.
KEY POINTS
- In patients with asymptomatic carotid stenosis greater than 60% or symptomatic carotid stenosis greater than 50%, carotid endarterectomy has been proven to be superior to medical therapy alone.
- In clinical trials, carotid stenting did not appear to have a clear advantage over endarterectomy in patients at average surgical risk.
- Stenting may be most advantageous when used in patients with symptomatic carotid stenosis who would be at high risk of perioperative complications if they were to undergo carotid endarterectomy.
A judgment call
A 22-year-old African American man with sickle cell disease comes to the in his joints and chest—a presentation similar to those of his previous sickle cell crises. He is given intravenous fluids for dehydration and morphine sulfate for pain via a peripheral line, and he is admitted to the hospital.
Shortly afterward, the peripheral line becomes infiltrated. After failed attempts at peripheral cannulation, central venous cannulation via an internal jugular site is considered.
WHERE IS THE CATHETER TIP?
HAZARDS OF ABERRANT LINE PLACEMENT
Central venous catheters are commonly used to give various infusions (eg, parenteral nutrition), to draw blood, and to monitor the central venous pressure.1 The internal jugular vein is one of the preferred veins for central venous access.1,2 Normally, the anatomy of the jugular venous system and the design of the catheter facilitate proper insertion. Occasionally, however, despite proper technique, the tip of the catheter may not terminate at the desired level, resulting in aberrant placement in the internal thoracic vein, superior vena cava, azygos vein, accessory hemiazygos vein, or axillary vein.1–8
The use of chest radiographs to establish the correct placement of internal jugular and subclavian lines has been advocated and is routinely practiced.6,7 Obtaining a chest x-ray to confirm line placement is particularly important in patients with prior multiple and difficult catheterizations. The lateral view is seldom obtained when confirming central neck line placement, but when the anteroposterior view is not reassuring, it may be prudent to obtain this alternate view.
In a large retrospective analysis,9 cannulation of the azygos arch occurred in about 1.2% of insertions, and the rate was about seven times higher when the left jugular vein was used than when the right one was used. A smaller study gave the frequency of azygos arch cannulation as 0.7%.10
All procedure-related complications of central line insertion in the neck can also occur with aberrant azygos vein cannulation. These include infection, bacteremia, pneumothorax, hemothorax, arterial puncture, and various other mechanical complications. It should be noted that aberrant cannulation of the azygos arch is particularly hazardous, and that complication rates are typically higher. These complications are mainly due to the smaller vascular lumen and to the direction of blood flow in the azygos venous system.
KNOWING THE ANATOMY IS CRUCIAL
Knowledge of venous anatomy and its variants is crucial both for insertion and for ascertaining the correct placement of central venous lines.
The azygos vein has a much smaller lumen than the superior vena cava. Although the lumen size may vary significantly, the maximum diameter of the anterior arch of the azygos vein is about 6 to 7 mm,11 whereas the superior vena cava lumen is typically 1.5 to 2 cm in diameter.12 In addition, when a catheter is inserted into the superior vena cava, the direction of blood flow and the direction of the infusion are the same, but when the catheter is inserted into the azygos system, the directions of blood flow and infusion are opposite, contributing to local turbulence.
Both these factors increase the chance of puncturing the vein when the azygos arch is aberrantly cannulated for central venous access.9 Venous perforation has been reported in as many as 19% of cases in which the azygos arch was inadvertently cannulated. Venous perforation can lead to hemopericardium, hemomediastinum, and hemorrhagic pleural effusions, which can lead to significant morbidity and even death. Perforation, thrombosis, stenosis, and complete occlusion have been reported subsequent to catheter malposition in the azygos vein.13
Patients in whom the azygos vein is inadvertently cannulated may tolerate infusions and blood draws, but this does not mean that inadvertent azygos vein cannulation is acceptable, especially given the late complications of vascular perforation that can occur.9
The cannulation of the azygos vein in our patient was completely unintentional; nevertheless, the line was kept in and used for a short period for the initial rehydration and pain control and was subsequently removed without any complications.
WHEN IS CANNULATION OF THE AZYGOS VEIN AN OPTION?
In patients with previous multiple central vein cannulations, the rates of thrombosis and of fibrotic changes in these veins are high. In patients with thrombosis of both the superior vena cava and the inferior vena cava, direct insertion of a catheter into the azygos vein has been suggested as an alternate route to obtain access for dialysis.8 This approach has also been used successfully to administer total parenteral nutrition for a prolonged time in pediatric patients.14 In short, the azygos vein is never preferred for central venous access, but it can occasionally serve as an alternate route.5–9
TAKE-HOME POINTS
The radiographic assessment of an internal jugular or subclavian line may occasionally be deceptive if based solely on the anteroposterior view; confirmation may require a lateral view. We found no guidelines for using the azygos vein for central venous access. The options in cases of aberrant cannulation include leaving the line in, removing and reinserting it at the same or another site under fluoroscopy, and attempting to reposition it after changing the catheter over a guidewire.
The use of central lines found to be in an aberrant position should be driven by clinical judgment based on the urgency of the need of access, the availability or feasibility of other access options, and the intended use. The use of the azygos vein is fraught with procedural complications, as well as postprocedural complications related to vascular perforation. If the position of the catheter tip on the anteroposterior radiographic view is not satisfactory, obtaining a lateral view should be considered.
- McGee DC, Goud MK. Preventing complications of central venous catheterization. N Engl J Med. 2003; 348:1123–1133.
- Pittiruti M, Malerba M, Carriero C, Tazza L, Gui D. Which is the easiest and safest technique for central venous access? A retrospective survey of more than 5,400 cases. J Vasc Access. 2000; 1:100–107.
- Towers MJ. Preventing complications of central venous catheterization. N Engl J Med 2003; 348:2684–2686; author reply 2684–2686.
- Langston CS. The aberrant central venous catheter and its complications. Radiology. 1971; 100:55–59.
- Smith DC, Pop PM. Malposition of a total parenteral nutrition catheter in the accessory hemiazygos vein. JPEN J Parenter Enteral Nutr. 1983; 7:289–292.
- Abood GJ, Davis KA, Esposito TJ, Luchette FA, Gamelli RL. Comparison of routine chest radiograph versus clinician judgment to determine adequate central line placement in critically ill patients. J Trauma. 2007; 63:50–56.
- Gladwin MT, Slonim A, Landucci DL, Gutierrez DC, Cunnion RE. Cannulation of the internal jugular vein: is postprocedural chest radiography always necessary? Crit Care Med 1999; 27:1819–1823.
- Meranze SG, McLean GK, Stein EJ, Jordan HA. Catheter placement in the azygos system: an unusual approach to venous access. Am J Roentgenol. 1985; 144:1075–1076.
- Bankier AA, Mallek R, Wiesmayr MN, et al. Azygos arch cannulation by central venous catheters: radiographic detection of malposition and subsequent complications. J Thorac Imaging. 1997; 12:64–69.
- Langston CT. The aberrant central venous catheter and its complications. Radiology. 1971; 100:55–59.
- Heitzman ER. Radiologic appearance of the azygos vein in cardiovascular disease. Circulation. 1973; 47:628–634.
- McGowan AR, Pugatch RD. Partial obstruction of the superior vena cava. BrighamRAD. Available at: http://brighamrad.harvard.edu/Cases/bwh/hcache/58/full.html. Accessed 9/4/2008.
- Granata A, Figuera M, Castellino S, Logias F, Basile A. Azygos arch cannulation by central venous catheters for hemodialysis. J Vasc Access. 2006; 7:43–45.
- Malt RA, Kempster M. Direct azygos vein and superior vena cava cannulation for parenteral nutrition. JPEN J Parenter Enteral Nutr. 1983; 7:580–581.
A 22-year-old African American man with sickle cell disease comes to the in his joints and chest—a presentation similar to those of his previous sickle cell crises. He is given intravenous fluids for dehydration and morphine sulfate for pain via a peripheral line, and he is admitted to the hospital.
Shortly afterward, the peripheral line becomes infiltrated. After failed attempts at peripheral cannulation, central venous cannulation via an internal jugular site is considered.
WHERE IS THE CATHETER TIP?
HAZARDS OF ABERRANT LINE PLACEMENT
Central venous catheters are commonly used to give various infusions (eg, parenteral nutrition), to draw blood, and to monitor the central venous pressure.1 The internal jugular vein is one of the preferred veins for central venous access.1,2 Normally, the anatomy of the jugular venous system and the design of the catheter facilitate proper insertion. Occasionally, however, despite proper technique, the tip of the catheter may not terminate at the desired level, resulting in aberrant placement in the internal thoracic vein, superior vena cava, azygos vein, accessory hemiazygos vein, or axillary vein.1–8
The use of chest radiographs to establish the correct placement of internal jugular and subclavian lines has been advocated and is routinely practiced.6,7 Obtaining a chest x-ray to confirm line placement is particularly important in patients with prior multiple and difficult catheterizations. The lateral view is seldom obtained when confirming central neck line placement, but when the anteroposterior view is not reassuring, it may be prudent to obtain this alternate view.
In a large retrospective analysis,9 cannulation of the azygos arch occurred in about 1.2% of insertions, and the rate was about seven times higher when the left jugular vein was used than when the right one was used. A smaller study gave the frequency of azygos arch cannulation as 0.7%.10
All procedure-related complications of central line insertion in the neck can also occur with aberrant azygos vein cannulation. These include infection, bacteremia, pneumothorax, hemothorax, arterial puncture, and various other mechanical complications. It should be noted that aberrant cannulation of the azygos arch is particularly hazardous, and that complication rates are typically higher. These complications are mainly due to the smaller vascular lumen and to the direction of blood flow in the azygos venous system.
KNOWING THE ANATOMY IS CRUCIAL
Knowledge of venous anatomy and its variants is crucial both for insertion and for ascertaining the correct placement of central venous lines.
The azygos vein has a much smaller lumen than the superior vena cava. Although the lumen size may vary significantly, the maximum diameter of the anterior arch of the azygos vein is about 6 to 7 mm,11 whereas the superior vena cava lumen is typically 1.5 to 2 cm in diameter.12 In addition, when a catheter is inserted into the superior vena cava, the direction of blood flow and the direction of the infusion are the same, but when the catheter is inserted into the azygos system, the directions of blood flow and infusion are opposite, contributing to local turbulence.
Both these factors increase the chance of puncturing the vein when the azygos arch is aberrantly cannulated for central venous access.9 Venous perforation has been reported in as many as 19% of cases in which the azygos arch was inadvertently cannulated. Venous perforation can lead to hemopericardium, hemomediastinum, and hemorrhagic pleural effusions, which can lead to significant morbidity and even death. Perforation, thrombosis, stenosis, and complete occlusion have been reported subsequent to catheter malposition in the azygos vein.13
Patients in whom the azygos vein is inadvertently cannulated may tolerate infusions and blood draws, but this does not mean that inadvertent azygos vein cannulation is acceptable, especially given the late complications of vascular perforation that can occur.9
The cannulation of the azygos vein in our patient was completely unintentional; nevertheless, the line was kept in and used for a short period for the initial rehydration and pain control and was subsequently removed without any complications.
WHEN IS CANNULATION OF THE AZYGOS VEIN AN OPTION?
In patients with previous multiple central vein cannulations, the rates of thrombosis and of fibrotic changes in these veins are high. In patients with thrombosis of both the superior vena cava and the inferior vena cava, direct insertion of a catheter into the azygos vein has been suggested as an alternate route to obtain access for dialysis.8 This approach has also been used successfully to administer total parenteral nutrition for a prolonged time in pediatric patients.14 In short, the azygos vein is never preferred for central venous access, but it can occasionally serve as an alternate route.5–9
TAKE-HOME POINTS
The radiographic assessment of an internal jugular or subclavian line may occasionally be deceptive if based solely on the anteroposterior view; confirmation may require a lateral view. We found no guidelines for using the azygos vein for central venous access. The options in cases of aberrant cannulation include leaving the line in, removing and reinserting it at the same or another site under fluoroscopy, and attempting to reposition it after changing the catheter over a guidewire.
The use of central lines found to be in an aberrant position should be driven by clinical judgment based on the urgency of the need of access, the availability or feasibility of other access options, and the intended use. The use of the azygos vein is fraught with procedural complications, as well as postprocedural complications related to vascular perforation. If the position of the catheter tip on the anteroposterior radiographic view is not satisfactory, obtaining a lateral view should be considered.
A 22-year-old African American man with sickle cell disease comes to the in his joints and chest—a presentation similar to those of his previous sickle cell crises. He is given intravenous fluids for dehydration and morphine sulfate for pain via a peripheral line, and he is admitted to the hospital.
Shortly afterward, the peripheral line becomes infiltrated. After failed attempts at peripheral cannulation, central venous cannulation via an internal jugular site is considered.
WHERE IS THE CATHETER TIP?
HAZARDS OF ABERRANT LINE PLACEMENT
Central venous catheters are commonly used to give various infusions (eg, parenteral nutrition), to draw blood, and to monitor the central venous pressure.1 The internal jugular vein is one of the preferred veins for central venous access.1,2 Normally, the anatomy of the jugular venous system and the design of the catheter facilitate proper insertion. Occasionally, however, despite proper technique, the tip of the catheter may not terminate at the desired level, resulting in aberrant placement in the internal thoracic vein, superior vena cava, azygos vein, accessory hemiazygos vein, or axillary vein.1–8
The use of chest radiographs to establish the correct placement of internal jugular and subclavian lines has been advocated and is routinely practiced.6,7 Obtaining a chest x-ray to confirm line placement is particularly important in patients with prior multiple and difficult catheterizations. The lateral view is seldom obtained when confirming central neck line placement, but when the anteroposterior view is not reassuring, it may be prudent to obtain this alternate view.
In a large retrospective analysis,9 cannulation of the azygos arch occurred in about 1.2% of insertions, and the rate was about seven times higher when the left jugular vein was used than when the right one was used. A smaller study gave the frequency of azygos arch cannulation as 0.7%.10
All procedure-related complications of central line insertion in the neck can also occur with aberrant azygos vein cannulation. These include infection, bacteremia, pneumothorax, hemothorax, arterial puncture, and various other mechanical complications. It should be noted that aberrant cannulation of the azygos arch is particularly hazardous, and that complication rates are typically higher. These complications are mainly due to the smaller vascular lumen and to the direction of blood flow in the azygos venous system.
KNOWING THE ANATOMY IS CRUCIAL
Knowledge of venous anatomy and its variants is crucial both for insertion and for ascertaining the correct placement of central venous lines.
The azygos vein has a much smaller lumen than the superior vena cava. Although the lumen size may vary significantly, the maximum diameter of the anterior arch of the azygos vein is about 6 to 7 mm,11 whereas the superior vena cava lumen is typically 1.5 to 2 cm in diameter.12 In addition, when a catheter is inserted into the superior vena cava, the direction of blood flow and the direction of the infusion are the same, but when the catheter is inserted into the azygos system, the directions of blood flow and infusion are opposite, contributing to local turbulence.
Both these factors increase the chance of puncturing the vein when the azygos arch is aberrantly cannulated for central venous access.9 Venous perforation has been reported in as many as 19% of cases in which the azygos arch was inadvertently cannulated. Venous perforation can lead to hemopericardium, hemomediastinum, and hemorrhagic pleural effusions, which can lead to significant morbidity and even death. Perforation, thrombosis, stenosis, and complete occlusion have been reported subsequent to catheter malposition in the azygos vein.13
Patients in whom the azygos vein is inadvertently cannulated may tolerate infusions and blood draws, but this does not mean that inadvertent azygos vein cannulation is acceptable, especially given the late complications of vascular perforation that can occur.9
The cannulation of the azygos vein in our patient was completely unintentional; nevertheless, the line was kept in and used for a short period for the initial rehydration and pain control and was subsequently removed without any complications.
WHEN IS CANNULATION OF THE AZYGOS VEIN AN OPTION?
In patients with previous multiple central vein cannulations, the rates of thrombosis and of fibrotic changes in these veins are high. In patients with thrombosis of both the superior vena cava and the inferior vena cava, direct insertion of a catheter into the azygos vein has been suggested as an alternate route to obtain access for dialysis.8 This approach has also been used successfully to administer total parenteral nutrition for a prolonged time in pediatric patients.14 In short, the azygos vein is never preferred for central venous access, but it can occasionally serve as an alternate route.5–9
TAKE-HOME POINTS
The radiographic assessment of an internal jugular or subclavian line may occasionally be deceptive if based solely on the anteroposterior view; confirmation may require a lateral view. We found no guidelines for using the azygos vein for central venous access. The options in cases of aberrant cannulation include leaving the line in, removing and reinserting it at the same or another site under fluoroscopy, and attempting to reposition it after changing the catheter over a guidewire.
The use of central lines found to be in an aberrant position should be driven by clinical judgment based on the urgency of the need of access, the availability or feasibility of other access options, and the intended use. The use of the azygos vein is fraught with procedural complications, as well as postprocedural complications related to vascular perforation. If the position of the catheter tip on the anteroposterior radiographic view is not satisfactory, obtaining a lateral view should be considered.
- McGee DC, Goud MK. Preventing complications of central venous catheterization. N Engl J Med. 2003; 348:1123–1133.
- Pittiruti M, Malerba M, Carriero C, Tazza L, Gui D. Which is the easiest and safest technique for central venous access? A retrospective survey of more than 5,400 cases. J Vasc Access. 2000; 1:100–107.
- Towers MJ. Preventing complications of central venous catheterization. N Engl J Med 2003; 348:2684–2686; author reply 2684–2686.
- Langston CS. The aberrant central venous catheter and its complications. Radiology. 1971; 100:55–59.
- Smith DC, Pop PM. Malposition of a total parenteral nutrition catheter in the accessory hemiazygos vein. JPEN J Parenter Enteral Nutr. 1983; 7:289–292.
- Abood GJ, Davis KA, Esposito TJ, Luchette FA, Gamelli RL. Comparison of routine chest radiograph versus clinician judgment to determine adequate central line placement in critically ill patients. J Trauma. 2007; 63:50–56.
- Gladwin MT, Slonim A, Landucci DL, Gutierrez DC, Cunnion RE. Cannulation of the internal jugular vein: is postprocedural chest radiography always necessary? Crit Care Med 1999; 27:1819–1823.
- Meranze SG, McLean GK, Stein EJ, Jordan HA. Catheter placement in the azygos system: an unusual approach to venous access. Am J Roentgenol. 1985; 144:1075–1076.
- Bankier AA, Mallek R, Wiesmayr MN, et al. Azygos arch cannulation by central venous catheters: radiographic detection of malposition and subsequent complications. J Thorac Imaging. 1997; 12:64–69.
- Langston CT. The aberrant central venous catheter and its complications. Radiology. 1971; 100:55–59.
- Heitzman ER. Radiologic appearance of the azygos vein in cardiovascular disease. Circulation. 1973; 47:628–634.
- McGowan AR, Pugatch RD. Partial obstruction of the superior vena cava. BrighamRAD. Available at: http://brighamrad.harvard.edu/Cases/bwh/hcache/58/full.html. Accessed 9/4/2008.
- Granata A, Figuera M, Castellino S, Logias F, Basile A. Azygos arch cannulation by central venous catheters for hemodialysis. J Vasc Access. 2006; 7:43–45.
- Malt RA, Kempster M. Direct azygos vein and superior vena cava cannulation for parenteral nutrition. JPEN J Parenter Enteral Nutr. 1983; 7:580–581.
- McGee DC, Goud MK. Preventing complications of central venous catheterization. N Engl J Med. 2003; 348:1123–1133.
- Pittiruti M, Malerba M, Carriero C, Tazza L, Gui D. Which is the easiest and safest technique for central venous access? A retrospective survey of more than 5,400 cases. J Vasc Access. 2000; 1:100–107.
- Towers MJ. Preventing complications of central venous catheterization. N Engl J Med 2003; 348:2684–2686; author reply 2684–2686.
- Langston CS. The aberrant central venous catheter and its complications. Radiology. 1971; 100:55–59.
- Smith DC, Pop PM. Malposition of a total parenteral nutrition catheter in the accessory hemiazygos vein. JPEN J Parenter Enteral Nutr. 1983; 7:289–292.
- Abood GJ, Davis KA, Esposito TJ, Luchette FA, Gamelli RL. Comparison of routine chest radiograph versus clinician judgment to determine adequate central line placement in critically ill patients. J Trauma. 2007; 63:50–56.
- Gladwin MT, Slonim A, Landucci DL, Gutierrez DC, Cunnion RE. Cannulation of the internal jugular vein: is postprocedural chest radiography always necessary? Crit Care Med 1999; 27:1819–1823.
- Meranze SG, McLean GK, Stein EJ, Jordan HA. Catheter placement in the azygos system: an unusual approach to venous access. Am J Roentgenol. 1985; 144:1075–1076.
- Bankier AA, Mallek R, Wiesmayr MN, et al. Azygos arch cannulation by central venous catheters: radiographic detection of malposition and subsequent complications. J Thorac Imaging. 1997; 12:64–69.
- Langston CT. The aberrant central venous catheter and its complications. Radiology. 1971; 100:55–59.
- Heitzman ER. Radiologic appearance of the azygos vein in cardiovascular disease. Circulation. 1973; 47:628–634.
- McGowan AR, Pugatch RD. Partial obstruction of the superior vena cava. BrighamRAD. Available at: http://brighamrad.harvard.edu/Cases/bwh/hcache/58/full.html. Accessed 9/4/2008.
- Granata A, Figuera M, Castellino S, Logias F, Basile A. Azygos arch cannulation by central venous catheters for hemodialysis. J Vasc Access. 2006; 7:43–45.
- Malt RA, Kempster M. Direct azygos vein and superior vena cava cannulation for parenteral nutrition. JPEN J Parenter Enteral Nutr. 1983; 7:580–581.
When a quick sound bite won’t do
The sound bites about these trials in the news have confused physicians and patients alike. But, as we have all experienced during this election year, to understand complex problems requires an in-depth analysis instead of a sound bite.
I was troubled by the results of the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial,2 in which more patients who were treated with an intense hemoglobin A1c-lowering strategy died (mostly of macrovascular events) than those treated with a standard strategy. Older data showing a beneficial effect of glucose-lowering on the microvascular complications of diabetes are solid. I did not understand the mechanistic basis of the ACCORD results, unless the very aggressive therapy caused many hypoglycemic events with catecholamine surges, resulting in stroke or myocardial infarction, or whether a problem with a specific drug arose more often in the intensive-treatment group. There has been similar dialogue surrounding intensity of glucose control in critically ill inpatients3; here, the data suggest that hypoglycemic episodes may limit other benefits of aggressive treatment in the intensive care unit, such as reduced infection rates.
Not to be ignored is that the patients in all arms of the ACCORD trial fared far better than historical diabetic controls. The meticulous attention to management of blood pressure and LDL-C that all patients in the ACCORD trial received paid off. (If only we could do as well in our practices!) But what do we do about the sugar?
This large, well-done, ongoing trial deserves a detailed analysis for those of us who need to translate the conclusions regarding glucose control to our patients. This month in the Journal, I have invited Byron Hoogwerf, a clinical diabetologist, former internal medicine program director, well-published clinical trialist, and ACCORD investigator, to provide this analysis.4 His discussion is more detailed than what we often print purposefully, and it is well worth reading. Some issues simply can’t be understood as a sound bite.
- Kastelein JJ, Akdim F, Stroes ES, et alENHANCE Investigators. Simvastatin with or without ezetimibe in familial hypercholesterolemia. N Engl J Med. 2008; 358:1431–1443.
- Gerstein HC, Miller ME, Byington RP, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008; 358:2545–2559.
- Soylemez Wiener R, Wiener DC, Larson RJ. Benefits and risks of tight glucose control in critically ill adults: a meta-analysis. JAMA. 2008; 300:933–944.
- Hoogwerf BF. A clinician and clinical trialist’s perspective: does intensive therapy of type 2 diabetes help or harm? Seeking accord on ACCORD. Cleve Clin J Med. 2008; 75:729–737.
The sound bites about these trials in the news have confused physicians and patients alike. But, as we have all experienced during this election year, to understand complex problems requires an in-depth analysis instead of a sound bite.
I was troubled by the results of the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial,2 in which more patients who were treated with an intense hemoglobin A1c-lowering strategy died (mostly of macrovascular events) than those treated with a standard strategy. Older data showing a beneficial effect of glucose-lowering on the microvascular complications of diabetes are solid. I did not understand the mechanistic basis of the ACCORD results, unless the very aggressive therapy caused many hypoglycemic events with catecholamine surges, resulting in stroke or myocardial infarction, or whether a problem with a specific drug arose more often in the intensive-treatment group. There has been similar dialogue surrounding intensity of glucose control in critically ill inpatients3; here, the data suggest that hypoglycemic episodes may limit other benefits of aggressive treatment in the intensive care unit, such as reduced infection rates.
Not to be ignored is that the patients in all arms of the ACCORD trial fared far better than historical diabetic controls. The meticulous attention to management of blood pressure and LDL-C that all patients in the ACCORD trial received paid off. (If only we could do as well in our practices!) But what do we do about the sugar?
This large, well-done, ongoing trial deserves a detailed analysis for those of us who need to translate the conclusions regarding glucose control to our patients. This month in the Journal, I have invited Byron Hoogwerf, a clinical diabetologist, former internal medicine program director, well-published clinical trialist, and ACCORD investigator, to provide this analysis.4 His discussion is more detailed than what we often print purposefully, and it is well worth reading. Some issues simply can’t be understood as a sound bite.
The sound bites about these trials in the news have confused physicians and patients alike. But, as we have all experienced during this election year, to understand complex problems requires an in-depth analysis instead of a sound bite.
I was troubled by the results of the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial,2 in which more patients who were treated with an intense hemoglobin A1c-lowering strategy died (mostly of macrovascular events) than those treated with a standard strategy. Older data showing a beneficial effect of glucose-lowering on the microvascular complications of diabetes are solid. I did not understand the mechanistic basis of the ACCORD results, unless the very aggressive therapy caused many hypoglycemic events with catecholamine surges, resulting in stroke or myocardial infarction, or whether a problem with a specific drug arose more often in the intensive-treatment group. There has been similar dialogue surrounding intensity of glucose control in critically ill inpatients3; here, the data suggest that hypoglycemic episodes may limit other benefits of aggressive treatment in the intensive care unit, such as reduced infection rates.
Not to be ignored is that the patients in all arms of the ACCORD trial fared far better than historical diabetic controls. The meticulous attention to management of blood pressure and LDL-C that all patients in the ACCORD trial received paid off. (If only we could do as well in our practices!) But what do we do about the sugar?
This large, well-done, ongoing trial deserves a detailed analysis for those of us who need to translate the conclusions regarding glucose control to our patients. This month in the Journal, I have invited Byron Hoogwerf, a clinical diabetologist, former internal medicine program director, well-published clinical trialist, and ACCORD investigator, to provide this analysis.4 His discussion is more detailed than what we often print purposefully, and it is well worth reading. Some issues simply can’t be understood as a sound bite.
- Kastelein JJ, Akdim F, Stroes ES, et alENHANCE Investigators. Simvastatin with or without ezetimibe in familial hypercholesterolemia. N Engl J Med. 2008; 358:1431–1443.
- Gerstein HC, Miller ME, Byington RP, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008; 358:2545–2559.
- Soylemez Wiener R, Wiener DC, Larson RJ. Benefits and risks of tight glucose control in critically ill adults: a meta-analysis. JAMA. 2008; 300:933–944.
- Hoogwerf BF. A clinician and clinical trialist’s perspective: does intensive therapy of type 2 diabetes help or harm? Seeking accord on ACCORD. Cleve Clin J Med. 2008; 75:729–737.
- Kastelein JJ, Akdim F, Stroes ES, et alENHANCE Investigators. Simvastatin with or without ezetimibe in familial hypercholesterolemia. N Engl J Med. 2008; 358:1431–1443.
- Gerstein HC, Miller ME, Byington RP, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008; 358:2545–2559.
- Soylemez Wiener R, Wiener DC, Larson RJ. Benefits and risks of tight glucose control in critically ill adults: a meta-analysis. JAMA. 2008; 300:933–944.
- Hoogwerf BF. A clinician and clinical trialist’s perspective: does intensive therapy of type 2 diabetes help or harm? Seeking accord on ACCORD. Cleve Clin J Med. 2008; 75:729–737.
Does intensive therapy of type 2 diabetes help or harm? Seeking accord on ACCORD
The Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial1–5 was designed primarily to address, in patients with type 2 diabetes at high risk of cardiovascular events, whether intensive glucose control would result in a lower risk of atherosclerotic disease events or death than would standard treatment.
It was widely expected that intensive treatment would confer either modest benefit or, at worst, no benefit. However, the glucose-lowering arm of the trial was terminated early because of a higher mortality rate in the intensively treated group. (The ACCORD trial has two other arms, which concern blood pressure and lipid-lowering, and these are continuing.)
In earlier trials in type 2 diabetes, concerns had been raised about an increased risk of cardiovascular events and possibly death associated with glucose-lowering drugs, hypoglycemia itself, or both, and these were well known when ACCORD was convened. ACCORD was very carefully designed and included careful adjudication of each cardiovascular event and death, including whether hypoglycemia might have been a proximate cause of some sudden deaths.5
Therefore, the surprising result of the higher mortality rate with intensive glycemic control in ACCORD will be fodder for discussion in many arenas over the next several years, and it poses some challenges for physicians and patients in determining treatment goals, as well as for organizations that write clinical practice guidelines (and perhaps organizations involved in pay-for-performance based on these guidelines).
Still, I believe that the ACCORD results should not substantially change our approach to treatment goals in type 2 diabetes, although hemoglobin A1c targets below 6% may not have much added value for cardiovascular risk reduction. The low overall mortality rate in all the arms of the ACCORD trial emphasizes the importance of lifestyle modification, lipid and blood pressure therapy, and encouragement of aspirin use in all patients with type 2 diabetes.
This article reflects my views as a practicing diabetologist and clinical trialist (I was an investigator in the ACCORD trial) with a long-standing interest in clinical trials and in how the results influence clinical practice. The views I express herein may not reflect the views of other ACCORD investigators, the National Heart, Lung, and Blood Institute (NHLBI), the ACCORD trial coordinating center at Wake Forest University, or its data safety and monitoring board.
RISK OF CORONARY DISEASE INCREASES WITH GLUCOSE
Many observational studies6–10 have shown that the risk of cardiovascular disease, especially coronary heart disease, is two to five times higher in people with diabetes mellitus than in people without diabetes. The risk appears to be continuous, so the higher one’s glucose or hemoglobin A1c, the higher the risk.6 This risk even extends to glucose values well below the threshold values currently used to diagnose diabetes mellitus.6 Since there is no glucose threshold for coronary heart disease, the term dysglycemia (rather than hyperglycemia) has been proposed to note the relationship between glucose and coronary heart disease. (The glucose threshold for microvascular complications of diabetes, such as retinopathy and nephropathy, appears to be between 110 and 126 mg/dL).
The clustering of multiple coronary risk factors such as obesity, dyslipidemia, and hypertension has always raised the question of whether glucose is a culprit in coronary risk or whether it simply “runs in bad company.”
EARLIER CLINICAL TRIALS SUGGEST INTENSIVE TREATMENT RAISES RISK
Even though it has been widely believed that intensive glucose-lowering would reduce cardiovascular risk in type 2 diabetes, there have been hints in previous studies that some intensive-treatment regimens might increase risk.
Two large randomized clinical trials and one small one (discussed below) addressed whether glucose control would reduce the risk of atherosclerotic vascular disease events. In each of them, an increased risk of cardiovascular events and possibly of death was seen in at least one intensively treated group.
In the following discussion, I have calculated all of the death rates as the number of deaths per 1,000 patients per year, based on published study results. In this way, we can compare the rates in the various studies (including ACCORD), regardless of the trial duration.
The university group Diabetes Program: Controvery about tolbutamide therapy
The University Group Diabetes Program (UGDP)11–16 included about 1,000 participants randomized to five treatments: tolbutamide (Orinase, a sulfonylurea), insulin in a fixed dose based on body weight, insulin in adjusted doses based on fasting glucose levels, placebo, and (later) phenformin.
In the 1970s, when the UGDP was carried out, randomized clinical trials were uncommon. Like other trials from that era, the UGDP was underpowered by today’s standards and did not have a data safety and monitoring board.
Rates of cardiovascular events and deaths (per 1,000 patient-years):
- 25 (tolbutamide group)
- 12 (placebo group).
The two insulin groups did not differ from the placebo group in their rates of cardiovascular events or death.15 The tolbutamide arm was stopped, and the ensuing controversy about how to interpret the trial results lasted for more than a decade. It also resulted in a black-box warning for tolbutamide and all subsequent sulfonylureas.
United Kingdom Prospective Diabetes Study: Method of glucose-lowering an issue
The United Kingdom Prospective Diabetes Study (UKPDS)17–27 was launched in 1977. A cohort of 5,102 patients (mean age 54 years) with newly diagnosed type 2 diabetes mellitus followed a “prudent diet” for the first 3 to 4 months. Then, if their fasting glucose levels were in the range of 6.1 to 15 mmol/L (110–270 mg/dL), they were randomized to receive various treatments.
Patients who were not obese were randomized to receive either intensive treatment or conventional treatment. The intensive-treatment group received either insulin or a sulfonylurea (chlorpropamide [Diabinese], glibenclamide, or glipizide [Glucotrol]); the conventional-treatment group received diet therapy. The sulfonylurea arm was included partly to address the UGDP results.
Patients who were obese were randomized to receive one of three treatments: intensive treatment (with the agents listed above), conventional treatment, or metformin (Fortamet, Glucophage).
The mean in-trial hemoglobin A1c level in the intensive-treatment group was 7.0%, compared with 7.9% in the conventional-treatment group.
After a mean follow-up of more than 10 years, the incidence of myocardial infarction was 16% lower in the intensive-treatment group, but the difference was not statistically significant (P = .052).
Rates of death from all causes among nonobese subjects (per 1,000 patient-years):
- 18.2–20.5 (intensive-treatment group)
- 19.9 (conventional-treatment group).
In the obese patients who received metformin, the incidence of myocardial infarction was lower than in the conventional-treatment group but not the intensive-treatment group.
Rates of death among obese patients (per 1,000 patient-years):
- 13.5 (metformin group)
- 18.9 (intensive-treatment group)
- 20.6 (conventional-treatment group).
However, a small subset (n = 587) of the original group assigned to sulfonylurea therapy whose glycemic control deteriorated during the trial were rerandomized to continue to receive a sulfonylurea alone or to have metformin added. There was a statistically significantly higher rate of cardiovascular events and a nonsignificantly higher rate of total mortality in the metformin-plus-sulfonylurea group (30.3 per 1,000 patient-years) than in the sulfonylurea-only group (19.1 per 1,000 patient-years).
These data suggested that the way glucose-lowering was achieved might be as important as the glucose levels actually achieved. However, no definite conclusions could be drawn.
In an editorial on the UKPDS, Nathan26 made a comment that may have been prescient in terms of the ACCORD trial: “Professional organizations will now scramble to decide how to translate the UKPDS results … Whether the UKPDS firmly establishes the choice of any one therapy…or any combination of therapies for the long-term treatment of type 2 diabetes is more questionable.”26
Veterans Administration feasibility study
A Veterans Administration feasibility study28,29 included 153 men (mean age 60) with type 2 diabetes (mean duration 7.8 years) who received either conventional therapy (a single daily dose of insulin) or intensive therapy (multiple doses of insulin plus a sulfonylurea). Over a mean of 27 months, the intensive-therapy group achieved a hemoglobin A1c level that was 2 percentage points lower than in the conventional-therapy group.
At 2.25 years of follow-up, cardiovascular events had occurred in 24 (24%) of the intensive-therapy group and in 16 (20%) of the standard-therapy group (P = .10).
Rates of death from all causes (per 1,000 patient-years):
- 28.9 (intensive-treatment group)
- 17.5 (conventional-treatment group).
ACCORD TRIAL DESIGN
The primary outcome measured was the combined incidence of nonfatal myocardial infarction, nonfatal stroke, or death from cardiovascular causes. Secondary outcomes included death from any cause. The study is also evaluating the effect of intensive treatment on microvascular disease, hypoglycemia, cognition, quality of life, and cost-effectiveness.
The ACCORD study was designed to have 89% power to detect a 15% treatment effect of intensive glycemic control compared with standard glycemic control for the primary end point.
ACCORD RESULTS
Rates of death from any cause (per 1,000 patient-years):
- 14 (intensive-treatment group)
- 11 (standard-treatment group).
In the analyses available at the time that this study arm closed, the excess mortality was not attributable to any particular treatment regimen. In particular, rosiglitazone (Avandia) use did not contribute to the excess mortality. (Of note, 91.2% of the intensive-treatment group and 57.5% of the conventional-treatment group had been treated with rosiglitazone, with more than 19,000 patient-years of rosiglitazone exposure). The excess mortality was also not attributable to hypoglycemia immediately proximate to the death.
The ACCORD trial’s data safety and monitoring board recommended that this arm of the study be discontinued for safety reasons, and this recommendation was accepted by the NHLBI project office. All participants were notified by letter before the trial results were announced publicly, and all intensive-therapy group participants are now being treated by the protocol used in the standard-therapy group.1
FEWER DEATHS IN ACCORD THAN IN OTHER STUDIES IN DIABETES
The mortality rates in both arms of ACCORD were much lower than in other observational studies and clinical trials in type 2 diabetes.
The National Health and Nutrition Education Survey (NHANES),30 conducted from 1971 to 1975, included 14,374 people with diabetes between the ages of 25 and 74. Many of them were younger than the ACCORD patients, but two NHANES age-groups overlapped the ACCORD cohort. Rates of death from any cause at 22 years (per 1,000 patient-years):
- 39.7 (ages 45–64)
- 89.7 (ages 65–74).
The NHANES cohort would not have been treated as vigorously for coronary risk and other common causes of death.
UGDP, UKPDS. Death rates in the glucose-lowering trials of type 2 diabetes mellitus cited above were typically in the range of 20 deaths per 1,000 patient-years but were as high as 30 deaths per 1,000 patient-years in the UGDP tolbutamide group16 and the UK-PDS sulfonylurea-plus-metformin group.20,22,26
Steno-2.31 Half of 160 patients with type 2 diabetes were randomized to intensive strategies for controlling glucose, lipids, and blood pressure and for taking aspirin and angiotensin-converting enzyme inhibitors and following a healthy lifestyle. The other half received conventional therapy. Even in the intensive-treatment group, the mortality rate at 13 years was higher than in ACCORD. Rates of death from any cause (per 1,000 patient years):
- 22.5 (intensive-treatment group)
- 37.6 (conventional-treatment group).
After the ACCORD results were presented, two other trials addressing the question of whether lower hemoglobin A1c would reduce cardiovascular risk in type 2 diabetes have reported their outcomes:
The ADVANCE trial (Action in Diabetes and Vascular Disease: Preterax and Diamicron Modified Release Controlled Evaluation),32,33 with 11,140 patients, had a target hemoglobin A1c of 6.5% in an intensive-treatment group and 7.3% in a usual-treatment group. The intensive-treatment group showed no difference in the rates of major macrovascular events (HR 0.94, 95% CI 0.84–1.06, P = .32) or all-cause mortality (HR 0.93, 95% CI 0.83–1.06, P = .32). The overall death rate in ADVANCE (about 18 deaths per 1,000 patient-years) was higher than in ACCORD.
The Veterans Administration Diabetes Trial included 1,791 patients.34 Like the ADVANCE trial, it also found no difference in major cardiovascular outcomes (HR 0.868, P = .11) or cardiovascular mortality rates (HR 1.258, P = .36) with intensive therapy vs conventional therapy, ie, achieved hemoglobin A1c levels of 6.9% vs 8.4% (presented at the American Diabetes Association 2008 Scientific Sessions). Hypoglycemia was associated with an increased risk of death in the standard-treatment group.
An analysis suggested that patients with a shorter duration of diabetes may have had cardiovascular benefit from intensive glucose-lowering, while those who had had it longer may have had increased risk associated with the more intensive therapy. The rate of death from all causes appears to have been higher than in ACCORD, but this could not be determined accurately from the presentations.
Comment. Thus, the ACCORD cohort as a whole has had strikingly lower death rates than in these other studies. The fact that all participants had lower glucose levels on therapy than at baseline may possibly contribute to these lower death rates. In addition, all ACCORD participants in the lipid arm received a statin; all participants in the blood pressure arm had their blood pressure lowered to levels below those commonly seen in clinical practice; participants were encouraged to exercise regularly; most participants were given diet instruction; and other healthy behaviors such as aspirin use, regular follow-up with primary care physicians, and recommendations about smoking were encouraged throughout the study. These comprehensive strategies may represent better care and thus result in lower death rates than in other studies.
POSSIBLE EXPLANATIONS FOR THE ACCORD OUTCOMES
The ACCORD trial has already stimulated fierce debate about the reasons for the higher mortality rate in the intensive-treatment group. With longer follow-up, some new risk factors for death may be identified that are not evident in the analyses of the current 460 deaths. What follows are some of my thoughts, with the caveat that they are not confirmed (supported statistically) by any currently available analyses from ACCORD.
It seems unlikely that lower glucose values as reflected by lower hemoglobin A1c values in the intensive-treatment group are an a priori explanation for the observed differences in mortality rates—especially since the mortality rates were lower than in the NHANES and clinical trial data sets cited above. If we assume that a type 1 statistical error (finding a difference where no difference actually exists) does not explain the findings, then at least four reasonable postulates exist:
Hypoglycemia may have some adverse effect, either acutely or from recurrent events that trigger a catecholamine response with associated risk for arrhythmia or increased coronary heart disease risk. However, the investigators analyzed each death to determine whether hypoglycemia was a contributing cause, and they found no statistically significant relationship between hypoglycemia and death in the intensive-treatment group.
Weight gain is common with intensive therapy. Obesity may be associated with greater cytokine production, higher concentrations of clotting factors, higher levels of free fatty acids, and other potential contributors to the risk of coronary heart disease and death. Currently, the ACCORD analyses do not suggest that weight gain explains the higher death rate.
Medications such as rosiglitazone, sulfonylureas, and the combination of a sulfonylurea plus metformin have been previously associated with increased death rates in some observational and intervention trials. These studies had some serious methodologic limitations (eg, absence of risk adjustment, events not adjudicated, small study cohorts, wide variation in study cohort characteristics) and small numbers of events.11–13,16,26,35 ACCORD analyses have not shown that any single glucose-lowering agent—including rosiglitazone—or combination of agents explains the death rates.
The stress of maintaining glycemic control has been speculated to have in some way contributed to an increased risk. To achieve intensive control, patients had to have frequent contact with their health care providers, they were often told that their hemoglobin A1c values were “too high” even when they were well below those in the American Diabetes Association guidelines, and they had to follow complex glucose-lowering regimens.
Semiquantitative measures of overall attitudes about health exist (eg, the “Feeling Thermometer” scale), but stress was not measured quantitatively in the ACCORD trial.
IMPLICATIONS OF ACCORD
In practice, most clinicians believe that the target glucose level in patients with type 2 diabetes should be as low as safely possible. This approach does not need to be modified on the basis of current information from ACCORD.
To be safe, regimens should be associated with a low risk of hypoglycemia and a low risk of weight gain. Use of combinations of medications that work by different mechanisms is still prudent. Agents should be used that may have favorable effects on other cardiovascular risk factors (eg, lipids, blood pressure, visceral fat).
Hemoglobin A1c targets below 7% are not precluded in all patients on the basis of the ACCORD results, though values lower than 6% may not have much added benefit for cardiovascular risk reduction. We should note that hemoglobin A1c was reduced in all ACCORD participants and that death rates were lower than in many other type 2 diabetic cohorts. Pending data on other outcomes in ACCORD (nephropathy, retinopathy, dementia, fracture risk), I believe it is premature for organizations to change their proposed hemoglobin A1c targets,36,37 as none have proposed values as low as the target in the ACCORD intensive-treatment group. At present, no class of glucose-lowering agents needs to be excluded from consideration on the basis of the ACCORD data.
The overall low rates of death in this population at high risk of coronary heart disease deserve comment. Not only are they lower than in other glucose-lowering trials, but they are also lower than in a number of studies of mortality in diabetes cohorts. As noted above, multiple risk factors for coronary heart disease and death were (and are) addressed in the ACCORD study participants, including repeated recommendation for lifestyle modification, intervention arms with lipid and blood pressure therapy, encouragement of aspirin use, and regular follow-up with health care providers for risk factors not managed by the ACCORD trial protocol. It is likely that multiple approaches to reducing the risk of cardiovascular disease contributed to this low mortality rate and that similar approaches will reduce the risk of coronary disease and death in regular clinical practice.
The ACCORD lipid and blood pressure arms are continuing, with results expected in 2010. The future results from ACCORD as well as from several glucose-lowering trials currently in progress (ADVANCE,32,33 Veteran’s Administration,34 Bypass Angioplasty Revascularization Investigation 2 Diabetes [BARI-2D]38) will likely help refine our understanding of the effects of glucose-lowering, glucose-lowering strategies and targets, and multiple interventions on coronary events and all-cause mortality.
For now, any strategy that lowers glucose and is associated with a low risk of hypoglycemia and does not cause excessive weight gain should be considered appropriate in patients with type 2 diabetes.
- Gerstein HC, Miller ME, Byington RP, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008; 358:2545–2559.
- Goff DC, Gerstein HC, Ginsberg HN, et al. Prevention of cardiovascular disease in persons with type 2 diabetes mellitus: current knowledge and rationale for the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial. Am J Cardiol 2007; 99:4i–20i.
- Buse JB, Bigger JT, Byington RP, et al. Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial: design and methods. Am J Cardiol 2007; 99:21i–33i.
- Gerstein HC, Riddle MC, Kendall DM, et al. Glycemia treatment strategies in the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial. Am J Cardiol 2007; 99:34i–43i.
- Bonds DE, Kurashige EM, Bergenstal R, et al. Severe hypoglycemia monitoring and risk management procedures in the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial. Am J Cardiol 2007; 99:80i–89i.
- Gerstein HC. Dysglycemia, not just diabetes, is a continuous risk factor for cardiovascular disease. Evid Based Cardiovasc Med. 1997; 1:87–88.
- Gerstein HC, Pais P, Pogue J, Yusuf S. Relationship of glucose and insulin levels to the risk of myocardial infarction: a case-control study. J Am Coll Cardiol. 1999; 33:612–619.
- Gerstein HC, Capes SE. Dysglycemia: a key cardiovascular risk factor. Semin Vasc Med. 2002; 2:165–174.
- Gerstein HC, Santaguida P, Raina P, et al. Annual incidence and relative risk of diabetes in people with various categories of dysglycemia: a systematic overview and meta-analysis of prospective studies. Diabetes Res Clin Pract. 2007; 78:305–312.
- American Diabetes Association. Role of cardiovascular risk factors in prevention and treatment of macrovascular disease in diabetes. Diabetes Care. 1989; 12:573–579.
- Schor S. The University Group Diabetes Program. A statistician looks at the mortality results. JAMA. 1971; 217:1671–1675.
- Cornfield JThe University Group Diabetes Program. A further statistical analysis of the mortality findings. JAMA. 1971; 217:1676–1687.
- Feinstein AR. Clinical biostatistics. 8. An analytic appraisal of the University Group Diabetes Program (UGDP) study. Clin Pharmacol Ther. 1971; 12:167–191.
- The University Group Diabetes Program. A study of the effects of hypoglycemic agents on vascular complications in patients with adult-onset diabetes. V. Evaluation of pheniformin therapy. Diabetes 1975; 24( suppl 1):65–184.
- Knatterud GL, Klimt CR, Levin ME, Jacobson ME, Goldner MG. Effects of hypoglycemic agents on vascular complications in patients with adult-onset diabetes. VII. Mortality and selected nonfatal events with insulin treatment. JAMA. 1978; 240:37–42.
- Schwartz TB, Meinert CL. The UGDP controversy: thirty-four years of contentious ambiguity laid to rest. Perspect Biol Med. 2004; 47:564–574.
- Turner RC, Holman RR. Lessons from UK Prospective Diabetes Study. Diabetes Res Clin Pract 1995; 28( suppl):S151–S157.
- UKPDS Research Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998; 352:854–865.
- UKPDS Study Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998; 352:837–853.
- UK Prospective Diabetes Study Group. UKPDS 28: a randomized trial of efficacy of early addition of metformin in sulfonylurea-treated type 2 diabetes. Diabetes Care. 1998; 21:87–92.
- Bretzel RG, Voigt K, Schatz H. The United Kingdom Prospective Diabetes Study (UKPDS) implications for the pharmacotherapy of type 2 diabetes mellitus. Exp Clin Endocrinol Diabetes. 1998; 106:369–372.
- Turner RC, Cull CA, Frighi V, Holman RR. Glycemic control with diet, sulfonylurea, metformin, or insulin in patients with type 2 diabetes mellitus: progressive requirement for multiple therapies (UKPDS 49). UK Prospective Diabetes Study (UKPDS) Group. JAMA. 1999; 281:2005–2012.
- Leslie RD. United Kingdom prospective diabetes study (UKPDS): what now or so what? Diabetes Metab Res Rev 1999; 15:65–71.
- Stratton IM, Adler AI, Neil HA, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ. 2000; 321:405–412.
- Mooradian AD, Chehade J. Implications of the UK Prospective Diabetes Study: questions answered and issues remaining. Drugs Aging. 2000; 16:159–164.
- Nathan DM. Some answers, more controversy, from UKPDS. United Kingdom Prospective Diabetes Study. Lancet. 1998; 352:832–833.
- Srimanunthiphol J, Beddow R, Arakaki R. A review of the United Kingdom Prospective Diabetes Study (UKPDS) and a discussion of the implications for patient care. Hawaii Med J. 2000; 59:295–298.
- Duckworth WC, McCarren M, Abraira C. Glucose control and cardiovascular complications: the VA Diabetes Trial. Diabetes Care. 2001; 24:942–945.
- Abraira C, Colwell JA, Nuttall FQ, et al. Veterans Affairs Cooperative Study on glycemic control and complications in type II diabetes (VA CSDM). Results of the feasibility trial. Veterans Affairs Cooperative Study in Type II Diabetes. Diabetes Care. 1995; 18:1113–1123.
- Gu K, Cowie CC, Harris MI. Mortality in adults with and without diabetes in a national cohort of the U.S. population, 1971–1993. Diabetes Care. 1998; 21:1138–1145. NHANES
- Gaede P, Lund-Andersen H, Parving HH, Pedersen O. Effect of a multifactorial intervention on mortality in type 2 diabetes. N Engl J Med. 2008; 358:580–591.
- Patel A, MacMahon S, Chalmers J, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008; 358:2560–2572.
- Action in Diabetes and Vascular Disease: PreterAx and DiamicroN Modified-Release Controlled Evaluation. Rationale and design of the ADVANCE study: a randomised trial of blood pressure lowering and intensive glucose control in high-risk individuals with type 2 diabetes mellitus. J Hypertens 2001; 19(suppl):S21–S28.
- Abraira C, Duckworth W, McCarren M, et al. Design of the cooperative study on glycemic control and complications in diabetes mellitus type 2: Veterans Affairs Diabetes Trial. J Diabetes Complications. 2003; 17:314–322.
- Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med. 2007; 356:2457–2471.
- American Association of Clinical Endocrinologists. American Association of Clinical Endocrinologists medical guidelines for clinical practice for the management of diabetes mellitus. Endocr Pract 2007; 13(suppl 1):1–68.
- American Diabetes Association. Standards of medical care in diabetes—2008. Diabetes Care 2008; 31(suppl 1):S12–S54.
- Magee MF, Isley WL. Rationale, design, and methods for glycemic control in the Bypass Angioplasty Revascularization Investigation 2 Diabetes (BARI 2D) Trial. Am J Cardiol 2006; 97:20G–30G.
The Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial1–5 was designed primarily to address, in patients with type 2 diabetes at high risk of cardiovascular events, whether intensive glucose control would result in a lower risk of atherosclerotic disease events or death than would standard treatment.
It was widely expected that intensive treatment would confer either modest benefit or, at worst, no benefit. However, the glucose-lowering arm of the trial was terminated early because of a higher mortality rate in the intensively treated group. (The ACCORD trial has two other arms, which concern blood pressure and lipid-lowering, and these are continuing.)
In earlier trials in type 2 diabetes, concerns had been raised about an increased risk of cardiovascular events and possibly death associated with glucose-lowering drugs, hypoglycemia itself, or both, and these were well known when ACCORD was convened. ACCORD was very carefully designed and included careful adjudication of each cardiovascular event and death, including whether hypoglycemia might have been a proximate cause of some sudden deaths.5
Therefore, the surprising result of the higher mortality rate with intensive glycemic control in ACCORD will be fodder for discussion in many arenas over the next several years, and it poses some challenges for physicians and patients in determining treatment goals, as well as for organizations that write clinical practice guidelines (and perhaps organizations involved in pay-for-performance based on these guidelines).
Still, I believe that the ACCORD results should not substantially change our approach to treatment goals in type 2 diabetes, although hemoglobin A1c targets below 6% may not have much added value for cardiovascular risk reduction. The low overall mortality rate in all the arms of the ACCORD trial emphasizes the importance of lifestyle modification, lipid and blood pressure therapy, and encouragement of aspirin use in all patients with type 2 diabetes.
This article reflects my views as a practicing diabetologist and clinical trialist (I was an investigator in the ACCORD trial) with a long-standing interest in clinical trials and in how the results influence clinical practice. The views I express herein may not reflect the views of other ACCORD investigators, the National Heart, Lung, and Blood Institute (NHLBI), the ACCORD trial coordinating center at Wake Forest University, or its data safety and monitoring board.
RISK OF CORONARY DISEASE INCREASES WITH GLUCOSE
Many observational studies6–10 have shown that the risk of cardiovascular disease, especially coronary heart disease, is two to five times higher in people with diabetes mellitus than in people without diabetes. The risk appears to be continuous, so the higher one’s glucose or hemoglobin A1c, the higher the risk.6 This risk even extends to glucose values well below the threshold values currently used to diagnose diabetes mellitus.6 Since there is no glucose threshold for coronary heart disease, the term dysglycemia (rather than hyperglycemia) has been proposed to note the relationship between glucose and coronary heart disease. (The glucose threshold for microvascular complications of diabetes, such as retinopathy and nephropathy, appears to be between 110 and 126 mg/dL).
The clustering of multiple coronary risk factors such as obesity, dyslipidemia, and hypertension has always raised the question of whether glucose is a culprit in coronary risk or whether it simply “runs in bad company.”
EARLIER CLINICAL TRIALS SUGGEST INTENSIVE TREATMENT RAISES RISK
Even though it has been widely believed that intensive glucose-lowering would reduce cardiovascular risk in type 2 diabetes, there have been hints in previous studies that some intensive-treatment regimens might increase risk.
Two large randomized clinical trials and one small one (discussed below) addressed whether glucose control would reduce the risk of atherosclerotic vascular disease events. In each of them, an increased risk of cardiovascular events and possibly of death was seen in at least one intensively treated group.
In the following discussion, I have calculated all of the death rates as the number of deaths per 1,000 patients per year, based on published study results. In this way, we can compare the rates in the various studies (including ACCORD), regardless of the trial duration.
The university group Diabetes Program: Controvery about tolbutamide therapy
The University Group Diabetes Program (UGDP)11–16 included about 1,000 participants randomized to five treatments: tolbutamide (Orinase, a sulfonylurea), insulin in a fixed dose based on body weight, insulin in adjusted doses based on fasting glucose levels, placebo, and (later) phenformin.
In the 1970s, when the UGDP was carried out, randomized clinical trials were uncommon. Like other trials from that era, the UGDP was underpowered by today’s standards and did not have a data safety and monitoring board.
Rates of cardiovascular events and deaths (per 1,000 patient-years):
- 25 (tolbutamide group)
- 12 (placebo group).
The two insulin groups did not differ from the placebo group in their rates of cardiovascular events or death.15 The tolbutamide arm was stopped, and the ensuing controversy about how to interpret the trial results lasted for more than a decade. It also resulted in a black-box warning for tolbutamide and all subsequent sulfonylureas.
United Kingdom Prospective Diabetes Study: Method of glucose-lowering an issue
The United Kingdom Prospective Diabetes Study (UKPDS)17–27 was launched in 1977. A cohort of 5,102 patients (mean age 54 years) with newly diagnosed type 2 diabetes mellitus followed a “prudent diet” for the first 3 to 4 months. Then, if their fasting glucose levels were in the range of 6.1 to 15 mmol/L (110–270 mg/dL), they were randomized to receive various treatments.
Patients who were not obese were randomized to receive either intensive treatment or conventional treatment. The intensive-treatment group received either insulin or a sulfonylurea (chlorpropamide [Diabinese], glibenclamide, or glipizide [Glucotrol]); the conventional-treatment group received diet therapy. The sulfonylurea arm was included partly to address the UGDP results.
Patients who were obese were randomized to receive one of three treatments: intensive treatment (with the agents listed above), conventional treatment, or metformin (Fortamet, Glucophage).
The mean in-trial hemoglobin A1c level in the intensive-treatment group was 7.0%, compared with 7.9% in the conventional-treatment group.
After a mean follow-up of more than 10 years, the incidence of myocardial infarction was 16% lower in the intensive-treatment group, but the difference was not statistically significant (P = .052).
Rates of death from all causes among nonobese subjects (per 1,000 patient-years):
- 18.2–20.5 (intensive-treatment group)
- 19.9 (conventional-treatment group).
In the obese patients who received metformin, the incidence of myocardial infarction was lower than in the conventional-treatment group but not the intensive-treatment group.
Rates of death among obese patients (per 1,000 patient-years):
- 13.5 (metformin group)
- 18.9 (intensive-treatment group)
- 20.6 (conventional-treatment group).
However, a small subset (n = 587) of the original group assigned to sulfonylurea therapy whose glycemic control deteriorated during the trial were rerandomized to continue to receive a sulfonylurea alone or to have metformin added. There was a statistically significantly higher rate of cardiovascular events and a nonsignificantly higher rate of total mortality in the metformin-plus-sulfonylurea group (30.3 per 1,000 patient-years) than in the sulfonylurea-only group (19.1 per 1,000 patient-years).
These data suggested that the way glucose-lowering was achieved might be as important as the glucose levels actually achieved. However, no definite conclusions could be drawn.
In an editorial on the UKPDS, Nathan26 made a comment that may have been prescient in terms of the ACCORD trial: “Professional organizations will now scramble to decide how to translate the UKPDS results … Whether the UKPDS firmly establishes the choice of any one therapy…or any combination of therapies for the long-term treatment of type 2 diabetes is more questionable.”26
Veterans Administration feasibility study
A Veterans Administration feasibility study28,29 included 153 men (mean age 60) with type 2 diabetes (mean duration 7.8 years) who received either conventional therapy (a single daily dose of insulin) or intensive therapy (multiple doses of insulin plus a sulfonylurea). Over a mean of 27 months, the intensive-therapy group achieved a hemoglobin A1c level that was 2 percentage points lower than in the conventional-therapy group.
At 2.25 years of follow-up, cardiovascular events had occurred in 24 (24%) of the intensive-therapy group and in 16 (20%) of the standard-therapy group (P = .10).
Rates of death from all causes (per 1,000 patient-years):
- 28.9 (intensive-treatment group)
- 17.5 (conventional-treatment group).
ACCORD TRIAL DESIGN
The primary outcome measured was the combined incidence of nonfatal myocardial infarction, nonfatal stroke, or death from cardiovascular causes. Secondary outcomes included death from any cause. The study is also evaluating the effect of intensive treatment on microvascular disease, hypoglycemia, cognition, quality of life, and cost-effectiveness.
The ACCORD study was designed to have 89% power to detect a 15% treatment effect of intensive glycemic control compared with standard glycemic control for the primary end point.
ACCORD RESULTS
Rates of death from any cause (per 1,000 patient-years):
- 14 (intensive-treatment group)
- 11 (standard-treatment group).
In the analyses available at the time that this study arm closed, the excess mortality was not attributable to any particular treatment regimen. In particular, rosiglitazone (Avandia) use did not contribute to the excess mortality. (Of note, 91.2% of the intensive-treatment group and 57.5% of the conventional-treatment group had been treated with rosiglitazone, with more than 19,000 patient-years of rosiglitazone exposure). The excess mortality was also not attributable to hypoglycemia immediately proximate to the death.
The ACCORD trial’s data safety and monitoring board recommended that this arm of the study be discontinued for safety reasons, and this recommendation was accepted by the NHLBI project office. All participants were notified by letter before the trial results were announced publicly, and all intensive-therapy group participants are now being treated by the protocol used in the standard-therapy group.1
FEWER DEATHS IN ACCORD THAN IN OTHER STUDIES IN DIABETES
The mortality rates in both arms of ACCORD were much lower than in other observational studies and clinical trials in type 2 diabetes.
The National Health and Nutrition Education Survey (NHANES),30 conducted from 1971 to 1975, included 14,374 people with diabetes between the ages of 25 and 74. Many of them were younger than the ACCORD patients, but two NHANES age-groups overlapped the ACCORD cohort. Rates of death from any cause at 22 years (per 1,000 patient-years):
- 39.7 (ages 45–64)
- 89.7 (ages 65–74).
The NHANES cohort would not have been treated as vigorously for coronary risk and other common causes of death.
UGDP, UKPDS. Death rates in the glucose-lowering trials of type 2 diabetes mellitus cited above were typically in the range of 20 deaths per 1,000 patient-years but were as high as 30 deaths per 1,000 patient-years in the UGDP tolbutamide group16 and the UK-PDS sulfonylurea-plus-metformin group.20,22,26
Steno-2.31 Half of 160 patients with type 2 diabetes were randomized to intensive strategies for controlling glucose, lipids, and blood pressure and for taking aspirin and angiotensin-converting enzyme inhibitors and following a healthy lifestyle. The other half received conventional therapy. Even in the intensive-treatment group, the mortality rate at 13 years was higher than in ACCORD. Rates of death from any cause (per 1,000 patient years):
- 22.5 (intensive-treatment group)
- 37.6 (conventional-treatment group).
After the ACCORD results were presented, two other trials addressing the question of whether lower hemoglobin A1c would reduce cardiovascular risk in type 2 diabetes have reported their outcomes:
The ADVANCE trial (Action in Diabetes and Vascular Disease: Preterax and Diamicron Modified Release Controlled Evaluation),32,33 with 11,140 patients, had a target hemoglobin A1c of 6.5% in an intensive-treatment group and 7.3% in a usual-treatment group. The intensive-treatment group showed no difference in the rates of major macrovascular events (HR 0.94, 95% CI 0.84–1.06, P = .32) or all-cause mortality (HR 0.93, 95% CI 0.83–1.06, P = .32). The overall death rate in ADVANCE (about 18 deaths per 1,000 patient-years) was higher than in ACCORD.
The Veterans Administration Diabetes Trial included 1,791 patients.34 Like the ADVANCE trial, it also found no difference in major cardiovascular outcomes (HR 0.868, P = .11) or cardiovascular mortality rates (HR 1.258, P = .36) with intensive therapy vs conventional therapy, ie, achieved hemoglobin A1c levels of 6.9% vs 8.4% (presented at the American Diabetes Association 2008 Scientific Sessions). Hypoglycemia was associated with an increased risk of death in the standard-treatment group.
An analysis suggested that patients with a shorter duration of diabetes may have had cardiovascular benefit from intensive glucose-lowering, while those who had had it longer may have had increased risk associated with the more intensive therapy. The rate of death from all causes appears to have been higher than in ACCORD, but this could not be determined accurately from the presentations.
Comment. Thus, the ACCORD cohort as a whole has had strikingly lower death rates than in these other studies. The fact that all participants had lower glucose levels on therapy than at baseline may possibly contribute to these lower death rates. In addition, all ACCORD participants in the lipid arm received a statin; all participants in the blood pressure arm had their blood pressure lowered to levels below those commonly seen in clinical practice; participants were encouraged to exercise regularly; most participants were given diet instruction; and other healthy behaviors such as aspirin use, regular follow-up with primary care physicians, and recommendations about smoking were encouraged throughout the study. These comprehensive strategies may represent better care and thus result in lower death rates than in other studies.
POSSIBLE EXPLANATIONS FOR THE ACCORD OUTCOMES
The ACCORD trial has already stimulated fierce debate about the reasons for the higher mortality rate in the intensive-treatment group. With longer follow-up, some new risk factors for death may be identified that are not evident in the analyses of the current 460 deaths. What follows are some of my thoughts, with the caveat that they are not confirmed (supported statistically) by any currently available analyses from ACCORD.
It seems unlikely that lower glucose values as reflected by lower hemoglobin A1c values in the intensive-treatment group are an a priori explanation for the observed differences in mortality rates—especially since the mortality rates were lower than in the NHANES and clinical trial data sets cited above. If we assume that a type 1 statistical error (finding a difference where no difference actually exists) does not explain the findings, then at least four reasonable postulates exist:
Hypoglycemia may have some adverse effect, either acutely or from recurrent events that trigger a catecholamine response with associated risk for arrhythmia or increased coronary heart disease risk. However, the investigators analyzed each death to determine whether hypoglycemia was a contributing cause, and they found no statistically significant relationship between hypoglycemia and death in the intensive-treatment group.
Weight gain is common with intensive therapy. Obesity may be associated with greater cytokine production, higher concentrations of clotting factors, higher levels of free fatty acids, and other potential contributors to the risk of coronary heart disease and death. Currently, the ACCORD analyses do not suggest that weight gain explains the higher death rate.
Medications such as rosiglitazone, sulfonylureas, and the combination of a sulfonylurea plus metformin have been previously associated with increased death rates in some observational and intervention trials. These studies had some serious methodologic limitations (eg, absence of risk adjustment, events not adjudicated, small study cohorts, wide variation in study cohort characteristics) and small numbers of events.11–13,16,26,35 ACCORD analyses have not shown that any single glucose-lowering agent—including rosiglitazone—or combination of agents explains the death rates.
The stress of maintaining glycemic control has been speculated to have in some way contributed to an increased risk. To achieve intensive control, patients had to have frequent contact with their health care providers, they were often told that their hemoglobin A1c values were “too high” even when they were well below those in the American Diabetes Association guidelines, and they had to follow complex glucose-lowering regimens.
Semiquantitative measures of overall attitudes about health exist (eg, the “Feeling Thermometer” scale), but stress was not measured quantitatively in the ACCORD trial.
IMPLICATIONS OF ACCORD
In practice, most clinicians believe that the target glucose level in patients with type 2 diabetes should be as low as safely possible. This approach does not need to be modified on the basis of current information from ACCORD.
To be safe, regimens should be associated with a low risk of hypoglycemia and a low risk of weight gain. Use of combinations of medications that work by different mechanisms is still prudent. Agents should be used that may have favorable effects on other cardiovascular risk factors (eg, lipids, blood pressure, visceral fat).
Hemoglobin A1c targets below 7% are not precluded in all patients on the basis of the ACCORD results, though values lower than 6% may not have much added benefit for cardiovascular risk reduction. We should note that hemoglobin A1c was reduced in all ACCORD participants and that death rates were lower than in many other type 2 diabetic cohorts. Pending data on other outcomes in ACCORD (nephropathy, retinopathy, dementia, fracture risk), I believe it is premature for organizations to change their proposed hemoglobin A1c targets,36,37 as none have proposed values as low as the target in the ACCORD intensive-treatment group. At present, no class of glucose-lowering agents needs to be excluded from consideration on the basis of the ACCORD data.
The overall low rates of death in this population at high risk of coronary heart disease deserve comment. Not only are they lower than in other glucose-lowering trials, but they are also lower than in a number of studies of mortality in diabetes cohorts. As noted above, multiple risk factors for coronary heart disease and death were (and are) addressed in the ACCORD study participants, including repeated recommendation for lifestyle modification, intervention arms with lipid and blood pressure therapy, encouragement of aspirin use, and regular follow-up with health care providers for risk factors not managed by the ACCORD trial protocol. It is likely that multiple approaches to reducing the risk of cardiovascular disease contributed to this low mortality rate and that similar approaches will reduce the risk of coronary disease and death in regular clinical practice.
The ACCORD lipid and blood pressure arms are continuing, with results expected in 2010. The future results from ACCORD as well as from several glucose-lowering trials currently in progress (ADVANCE,32,33 Veteran’s Administration,34 Bypass Angioplasty Revascularization Investigation 2 Diabetes [BARI-2D]38) will likely help refine our understanding of the effects of glucose-lowering, glucose-lowering strategies and targets, and multiple interventions on coronary events and all-cause mortality.
For now, any strategy that lowers glucose and is associated with a low risk of hypoglycemia and does not cause excessive weight gain should be considered appropriate in patients with type 2 diabetes.
The Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial1–5 was designed primarily to address, in patients with type 2 diabetes at high risk of cardiovascular events, whether intensive glucose control would result in a lower risk of atherosclerotic disease events or death than would standard treatment.
It was widely expected that intensive treatment would confer either modest benefit or, at worst, no benefit. However, the glucose-lowering arm of the trial was terminated early because of a higher mortality rate in the intensively treated group. (The ACCORD trial has two other arms, which concern blood pressure and lipid-lowering, and these are continuing.)
In earlier trials in type 2 diabetes, concerns had been raised about an increased risk of cardiovascular events and possibly death associated with glucose-lowering drugs, hypoglycemia itself, or both, and these were well known when ACCORD was convened. ACCORD was very carefully designed and included careful adjudication of each cardiovascular event and death, including whether hypoglycemia might have been a proximate cause of some sudden deaths.5
Therefore, the surprising result of the higher mortality rate with intensive glycemic control in ACCORD will be fodder for discussion in many arenas over the next several years, and it poses some challenges for physicians and patients in determining treatment goals, as well as for organizations that write clinical practice guidelines (and perhaps organizations involved in pay-for-performance based on these guidelines).
Still, I believe that the ACCORD results should not substantially change our approach to treatment goals in type 2 diabetes, although hemoglobin A1c targets below 6% may not have much added value for cardiovascular risk reduction. The low overall mortality rate in all the arms of the ACCORD trial emphasizes the importance of lifestyle modification, lipid and blood pressure therapy, and encouragement of aspirin use in all patients with type 2 diabetes.
This article reflects my views as a practicing diabetologist and clinical trialist (I was an investigator in the ACCORD trial) with a long-standing interest in clinical trials and in how the results influence clinical practice. The views I express herein may not reflect the views of other ACCORD investigators, the National Heart, Lung, and Blood Institute (NHLBI), the ACCORD trial coordinating center at Wake Forest University, or its data safety and monitoring board.
RISK OF CORONARY DISEASE INCREASES WITH GLUCOSE
Many observational studies6–10 have shown that the risk of cardiovascular disease, especially coronary heart disease, is two to five times higher in people with diabetes mellitus than in people without diabetes. The risk appears to be continuous, so the higher one’s glucose or hemoglobin A1c, the higher the risk.6 This risk even extends to glucose values well below the threshold values currently used to diagnose diabetes mellitus.6 Since there is no glucose threshold for coronary heart disease, the term dysglycemia (rather than hyperglycemia) has been proposed to note the relationship between glucose and coronary heart disease. (The glucose threshold for microvascular complications of diabetes, such as retinopathy and nephropathy, appears to be between 110 and 126 mg/dL).
The clustering of multiple coronary risk factors such as obesity, dyslipidemia, and hypertension has always raised the question of whether glucose is a culprit in coronary risk or whether it simply “runs in bad company.”
EARLIER CLINICAL TRIALS SUGGEST INTENSIVE TREATMENT RAISES RISK
Even though it has been widely believed that intensive glucose-lowering would reduce cardiovascular risk in type 2 diabetes, there have been hints in previous studies that some intensive-treatment regimens might increase risk.
Two large randomized clinical trials and one small one (discussed below) addressed whether glucose control would reduce the risk of atherosclerotic vascular disease events. In each of them, an increased risk of cardiovascular events and possibly of death was seen in at least one intensively treated group.
In the following discussion, I have calculated all of the death rates as the number of deaths per 1,000 patients per year, based on published study results. In this way, we can compare the rates in the various studies (including ACCORD), regardless of the trial duration.
The university group Diabetes Program: Controvery about tolbutamide therapy
The University Group Diabetes Program (UGDP)11–16 included about 1,000 participants randomized to five treatments: tolbutamide (Orinase, a sulfonylurea), insulin in a fixed dose based on body weight, insulin in adjusted doses based on fasting glucose levels, placebo, and (later) phenformin.
In the 1970s, when the UGDP was carried out, randomized clinical trials were uncommon. Like other trials from that era, the UGDP was underpowered by today’s standards and did not have a data safety and monitoring board.
Rates of cardiovascular events and deaths (per 1,000 patient-years):
- 25 (tolbutamide group)
- 12 (placebo group).
The two insulin groups did not differ from the placebo group in their rates of cardiovascular events or death.15 The tolbutamide arm was stopped, and the ensuing controversy about how to interpret the trial results lasted for more than a decade. It also resulted in a black-box warning for tolbutamide and all subsequent sulfonylureas.
United Kingdom Prospective Diabetes Study: Method of glucose-lowering an issue
The United Kingdom Prospective Diabetes Study (UKPDS)17–27 was launched in 1977. A cohort of 5,102 patients (mean age 54 years) with newly diagnosed type 2 diabetes mellitus followed a “prudent diet” for the first 3 to 4 months. Then, if their fasting glucose levels were in the range of 6.1 to 15 mmol/L (110–270 mg/dL), they were randomized to receive various treatments.
Patients who were not obese were randomized to receive either intensive treatment or conventional treatment. The intensive-treatment group received either insulin or a sulfonylurea (chlorpropamide [Diabinese], glibenclamide, or glipizide [Glucotrol]); the conventional-treatment group received diet therapy. The sulfonylurea arm was included partly to address the UGDP results.
Patients who were obese were randomized to receive one of three treatments: intensive treatment (with the agents listed above), conventional treatment, or metformin (Fortamet, Glucophage).
The mean in-trial hemoglobin A1c level in the intensive-treatment group was 7.0%, compared with 7.9% in the conventional-treatment group.
After a mean follow-up of more than 10 years, the incidence of myocardial infarction was 16% lower in the intensive-treatment group, but the difference was not statistically significant (P = .052).
Rates of death from all causes among nonobese subjects (per 1,000 patient-years):
- 18.2–20.5 (intensive-treatment group)
- 19.9 (conventional-treatment group).
In the obese patients who received metformin, the incidence of myocardial infarction was lower than in the conventional-treatment group but not the intensive-treatment group.
Rates of death among obese patients (per 1,000 patient-years):
- 13.5 (metformin group)
- 18.9 (intensive-treatment group)
- 20.6 (conventional-treatment group).
However, a small subset (n = 587) of the original group assigned to sulfonylurea therapy whose glycemic control deteriorated during the trial were rerandomized to continue to receive a sulfonylurea alone or to have metformin added. There was a statistically significantly higher rate of cardiovascular events and a nonsignificantly higher rate of total mortality in the metformin-plus-sulfonylurea group (30.3 per 1,000 patient-years) than in the sulfonylurea-only group (19.1 per 1,000 patient-years).
These data suggested that the way glucose-lowering was achieved might be as important as the glucose levels actually achieved. However, no definite conclusions could be drawn.
In an editorial on the UKPDS, Nathan26 made a comment that may have been prescient in terms of the ACCORD trial: “Professional organizations will now scramble to decide how to translate the UKPDS results … Whether the UKPDS firmly establishes the choice of any one therapy…or any combination of therapies for the long-term treatment of type 2 diabetes is more questionable.”26
Veterans Administration feasibility study
A Veterans Administration feasibility study28,29 included 153 men (mean age 60) with type 2 diabetes (mean duration 7.8 years) who received either conventional therapy (a single daily dose of insulin) or intensive therapy (multiple doses of insulin plus a sulfonylurea). Over a mean of 27 months, the intensive-therapy group achieved a hemoglobin A1c level that was 2 percentage points lower than in the conventional-therapy group.
At 2.25 years of follow-up, cardiovascular events had occurred in 24 (24%) of the intensive-therapy group and in 16 (20%) of the standard-therapy group (P = .10).
Rates of death from all causes (per 1,000 patient-years):
- 28.9 (intensive-treatment group)
- 17.5 (conventional-treatment group).
ACCORD TRIAL DESIGN
The primary outcome measured was the combined incidence of nonfatal myocardial infarction, nonfatal stroke, or death from cardiovascular causes. Secondary outcomes included death from any cause. The study is also evaluating the effect of intensive treatment on microvascular disease, hypoglycemia, cognition, quality of life, and cost-effectiveness.
The ACCORD study was designed to have 89% power to detect a 15% treatment effect of intensive glycemic control compared with standard glycemic control for the primary end point.
ACCORD RESULTS
Rates of death from any cause (per 1,000 patient-years):
- 14 (intensive-treatment group)
- 11 (standard-treatment group).
In the analyses available at the time that this study arm closed, the excess mortality was not attributable to any particular treatment regimen. In particular, rosiglitazone (Avandia) use did not contribute to the excess mortality. (Of note, 91.2% of the intensive-treatment group and 57.5% of the conventional-treatment group had been treated with rosiglitazone, with more than 19,000 patient-years of rosiglitazone exposure). The excess mortality was also not attributable to hypoglycemia immediately proximate to the death.
The ACCORD trial’s data safety and monitoring board recommended that this arm of the study be discontinued for safety reasons, and this recommendation was accepted by the NHLBI project office. All participants were notified by letter before the trial results were announced publicly, and all intensive-therapy group participants are now being treated by the protocol used in the standard-therapy group.1
FEWER DEATHS IN ACCORD THAN IN OTHER STUDIES IN DIABETES
The mortality rates in both arms of ACCORD were much lower than in other observational studies and clinical trials in type 2 diabetes.
The National Health and Nutrition Education Survey (NHANES),30 conducted from 1971 to 1975, included 14,374 people with diabetes between the ages of 25 and 74. Many of them were younger than the ACCORD patients, but two NHANES age-groups overlapped the ACCORD cohort. Rates of death from any cause at 22 years (per 1,000 patient-years):
- 39.7 (ages 45–64)
- 89.7 (ages 65–74).
The NHANES cohort would not have been treated as vigorously for coronary risk and other common causes of death.
UGDP, UKPDS. Death rates in the glucose-lowering trials of type 2 diabetes mellitus cited above were typically in the range of 20 deaths per 1,000 patient-years but were as high as 30 deaths per 1,000 patient-years in the UGDP tolbutamide group16 and the UK-PDS sulfonylurea-plus-metformin group.20,22,26
Steno-2.31 Half of 160 patients with type 2 diabetes were randomized to intensive strategies for controlling glucose, lipids, and blood pressure and for taking aspirin and angiotensin-converting enzyme inhibitors and following a healthy lifestyle. The other half received conventional therapy. Even in the intensive-treatment group, the mortality rate at 13 years was higher than in ACCORD. Rates of death from any cause (per 1,000 patient years):
- 22.5 (intensive-treatment group)
- 37.6 (conventional-treatment group).
After the ACCORD results were presented, two other trials addressing the question of whether lower hemoglobin A1c would reduce cardiovascular risk in type 2 diabetes have reported their outcomes:
The ADVANCE trial (Action in Diabetes and Vascular Disease: Preterax and Diamicron Modified Release Controlled Evaluation),32,33 with 11,140 patients, had a target hemoglobin A1c of 6.5% in an intensive-treatment group and 7.3% in a usual-treatment group. The intensive-treatment group showed no difference in the rates of major macrovascular events (HR 0.94, 95% CI 0.84–1.06, P = .32) or all-cause mortality (HR 0.93, 95% CI 0.83–1.06, P = .32). The overall death rate in ADVANCE (about 18 deaths per 1,000 patient-years) was higher than in ACCORD.
The Veterans Administration Diabetes Trial included 1,791 patients.34 Like the ADVANCE trial, it also found no difference in major cardiovascular outcomes (HR 0.868, P = .11) or cardiovascular mortality rates (HR 1.258, P = .36) with intensive therapy vs conventional therapy, ie, achieved hemoglobin A1c levels of 6.9% vs 8.4% (presented at the American Diabetes Association 2008 Scientific Sessions). Hypoglycemia was associated with an increased risk of death in the standard-treatment group.
An analysis suggested that patients with a shorter duration of diabetes may have had cardiovascular benefit from intensive glucose-lowering, while those who had had it longer may have had increased risk associated with the more intensive therapy. The rate of death from all causes appears to have been higher than in ACCORD, but this could not be determined accurately from the presentations.
Comment. Thus, the ACCORD cohort as a whole has had strikingly lower death rates than in these other studies. The fact that all participants had lower glucose levels on therapy than at baseline may possibly contribute to these lower death rates. In addition, all ACCORD participants in the lipid arm received a statin; all participants in the blood pressure arm had their blood pressure lowered to levels below those commonly seen in clinical practice; participants were encouraged to exercise regularly; most participants were given diet instruction; and other healthy behaviors such as aspirin use, regular follow-up with primary care physicians, and recommendations about smoking were encouraged throughout the study. These comprehensive strategies may represent better care and thus result in lower death rates than in other studies.
POSSIBLE EXPLANATIONS FOR THE ACCORD OUTCOMES
The ACCORD trial has already stimulated fierce debate about the reasons for the higher mortality rate in the intensive-treatment group. With longer follow-up, some new risk factors for death may be identified that are not evident in the analyses of the current 460 deaths. What follows are some of my thoughts, with the caveat that they are not confirmed (supported statistically) by any currently available analyses from ACCORD.
It seems unlikely that lower glucose values as reflected by lower hemoglobin A1c values in the intensive-treatment group are an a priori explanation for the observed differences in mortality rates—especially since the mortality rates were lower than in the NHANES and clinical trial data sets cited above. If we assume that a type 1 statistical error (finding a difference where no difference actually exists) does not explain the findings, then at least four reasonable postulates exist:
Hypoglycemia may have some adverse effect, either acutely or from recurrent events that trigger a catecholamine response with associated risk for arrhythmia or increased coronary heart disease risk. However, the investigators analyzed each death to determine whether hypoglycemia was a contributing cause, and they found no statistically significant relationship between hypoglycemia and death in the intensive-treatment group.
Weight gain is common with intensive therapy. Obesity may be associated with greater cytokine production, higher concentrations of clotting factors, higher levels of free fatty acids, and other potential contributors to the risk of coronary heart disease and death. Currently, the ACCORD analyses do not suggest that weight gain explains the higher death rate.
Medications such as rosiglitazone, sulfonylureas, and the combination of a sulfonylurea plus metformin have been previously associated with increased death rates in some observational and intervention trials. These studies had some serious methodologic limitations (eg, absence of risk adjustment, events not adjudicated, small study cohorts, wide variation in study cohort characteristics) and small numbers of events.11–13,16,26,35 ACCORD analyses have not shown that any single glucose-lowering agent—including rosiglitazone—or combination of agents explains the death rates.
The stress of maintaining glycemic control has been speculated to have in some way contributed to an increased risk. To achieve intensive control, patients had to have frequent contact with their health care providers, they were often told that their hemoglobin A1c values were “too high” even when they were well below those in the American Diabetes Association guidelines, and they had to follow complex glucose-lowering regimens.
Semiquantitative measures of overall attitudes about health exist (eg, the “Feeling Thermometer” scale), but stress was not measured quantitatively in the ACCORD trial.
IMPLICATIONS OF ACCORD
In practice, most clinicians believe that the target glucose level in patients with type 2 diabetes should be as low as safely possible. This approach does not need to be modified on the basis of current information from ACCORD.
To be safe, regimens should be associated with a low risk of hypoglycemia and a low risk of weight gain. Use of combinations of medications that work by different mechanisms is still prudent. Agents should be used that may have favorable effects on other cardiovascular risk factors (eg, lipids, blood pressure, visceral fat).
Hemoglobin A1c targets below 7% are not precluded in all patients on the basis of the ACCORD results, though values lower than 6% may not have much added benefit for cardiovascular risk reduction. We should note that hemoglobin A1c was reduced in all ACCORD participants and that death rates were lower than in many other type 2 diabetic cohorts. Pending data on other outcomes in ACCORD (nephropathy, retinopathy, dementia, fracture risk), I believe it is premature for organizations to change their proposed hemoglobin A1c targets,36,37 as none have proposed values as low as the target in the ACCORD intensive-treatment group. At present, no class of glucose-lowering agents needs to be excluded from consideration on the basis of the ACCORD data.
The overall low rates of death in this population at high risk of coronary heart disease deserve comment. Not only are they lower than in other glucose-lowering trials, but they are also lower than in a number of studies of mortality in diabetes cohorts. As noted above, multiple risk factors for coronary heart disease and death were (and are) addressed in the ACCORD study participants, including repeated recommendation for lifestyle modification, intervention arms with lipid and blood pressure therapy, encouragement of aspirin use, and regular follow-up with health care providers for risk factors not managed by the ACCORD trial protocol. It is likely that multiple approaches to reducing the risk of cardiovascular disease contributed to this low mortality rate and that similar approaches will reduce the risk of coronary disease and death in regular clinical practice.
The ACCORD lipid and blood pressure arms are continuing, with results expected in 2010. The future results from ACCORD as well as from several glucose-lowering trials currently in progress (ADVANCE,32,33 Veteran’s Administration,34 Bypass Angioplasty Revascularization Investigation 2 Diabetes [BARI-2D]38) will likely help refine our understanding of the effects of glucose-lowering, glucose-lowering strategies and targets, and multiple interventions on coronary events and all-cause mortality.
For now, any strategy that lowers glucose and is associated with a low risk of hypoglycemia and does not cause excessive weight gain should be considered appropriate in patients with type 2 diabetes.
- Gerstein HC, Miller ME, Byington RP, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008; 358:2545–2559.
- Goff DC, Gerstein HC, Ginsberg HN, et al. Prevention of cardiovascular disease in persons with type 2 diabetes mellitus: current knowledge and rationale for the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial. Am J Cardiol 2007; 99:4i–20i.
- Buse JB, Bigger JT, Byington RP, et al. Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial: design and methods. Am J Cardiol 2007; 99:21i–33i.
- Gerstein HC, Riddle MC, Kendall DM, et al. Glycemia treatment strategies in the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial. Am J Cardiol 2007; 99:34i–43i.
- Bonds DE, Kurashige EM, Bergenstal R, et al. Severe hypoglycemia monitoring and risk management procedures in the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial. Am J Cardiol 2007; 99:80i–89i.
- Gerstein HC. Dysglycemia, not just diabetes, is a continuous risk factor for cardiovascular disease. Evid Based Cardiovasc Med. 1997; 1:87–88.
- Gerstein HC, Pais P, Pogue J, Yusuf S. Relationship of glucose and insulin levels to the risk of myocardial infarction: a case-control study. J Am Coll Cardiol. 1999; 33:612–619.
- Gerstein HC, Capes SE. Dysglycemia: a key cardiovascular risk factor. Semin Vasc Med. 2002; 2:165–174.
- Gerstein HC, Santaguida P, Raina P, et al. Annual incidence and relative risk of diabetes in people with various categories of dysglycemia: a systematic overview and meta-analysis of prospective studies. Diabetes Res Clin Pract. 2007; 78:305–312.
- American Diabetes Association. Role of cardiovascular risk factors in prevention and treatment of macrovascular disease in diabetes. Diabetes Care. 1989; 12:573–579.
- Schor S. The University Group Diabetes Program. A statistician looks at the mortality results. JAMA. 1971; 217:1671–1675.
- Cornfield JThe University Group Diabetes Program. A further statistical analysis of the mortality findings. JAMA. 1971; 217:1676–1687.
- Feinstein AR. Clinical biostatistics. 8. An analytic appraisal of the University Group Diabetes Program (UGDP) study. Clin Pharmacol Ther. 1971; 12:167–191.
- The University Group Diabetes Program. A study of the effects of hypoglycemic agents on vascular complications in patients with adult-onset diabetes. V. Evaluation of pheniformin therapy. Diabetes 1975; 24( suppl 1):65–184.
- Knatterud GL, Klimt CR, Levin ME, Jacobson ME, Goldner MG. Effects of hypoglycemic agents on vascular complications in patients with adult-onset diabetes. VII. Mortality and selected nonfatal events with insulin treatment. JAMA. 1978; 240:37–42.
- Schwartz TB, Meinert CL. The UGDP controversy: thirty-four years of contentious ambiguity laid to rest. Perspect Biol Med. 2004; 47:564–574.
- Turner RC, Holman RR. Lessons from UK Prospective Diabetes Study. Diabetes Res Clin Pract 1995; 28( suppl):S151–S157.
- UKPDS Research Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998; 352:854–865.
- UKPDS Study Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998; 352:837–853.
- UK Prospective Diabetes Study Group. UKPDS 28: a randomized trial of efficacy of early addition of metformin in sulfonylurea-treated type 2 diabetes. Diabetes Care. 1998; 21:87–92.
- Bretzel RG, Voigt K, Schatz H. The United Kingdom Prospective Diabetes Study (UKPDS) implications for the pharmacotherapy of type 2 diabetes mellitus. Exp Clin Endocrinol Diabetes. 1998; 106:369–372.
- Turner RC, Cull CA, Frighi V, Holman RR. Glycemic control with diet, sulfonylurea, metformin, or insulin in patients with type 2 diabetes mellitus: progressive requirement for multiple therapies (UKPDS 49). UK Prospective Diabetes Study (UKPDS) Group. JAMA. 1999; 281:2005–2012.
- Leslie RD. United Kingdom prospective diabetes study (UKPDS): what now or so what? Diabetes Metab Res Rev 1999; 15:65–71.
- Stratton IM, Adler AI, Neil HA, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ. 2000; 321:405–412.
- Mooradian AD, Chehade J. Implications of the UK Prospective Diabetes Study: questions answered and issues remaining. Drugs Aging. 2000; 16:159–164.
- Nathan DM. Some answers, more controversy, from UKPDS. United Kingdom Prospective Diabetes Study. Lancet. 1998; 352:832–833.
- Srimanunthiphol J, Beddow R, Arakaki R. A review of the United Kingdom Prospective Diabetes Study (UKPDS) and a discussion of the implications for patient care. Hawaii Med J. 2000; 59:295–298.
- Duckworth WC, McCarren M, Abraira C. Glucose control and cardiovascular complications: the VA Diabetes Trial. Diabetes Care. 2001; 24:942–945.
- Abraira C, Colwell JA, Nuttall FQ, et al. Veterans Affairs Cooperative Study on glycemic control and complications in type II diabetes (VA CSDM). Results of the feasibility trial. Veterans Affairs Cooperative Study in Type II Diabetes. Diabetes Care. 1995; 18:1113–1123.
- Gu K, Cowie CC, Harris MI. Mortality in adults with and without diabetes in a national cohort of the U.S. population, 1971–1993. Diabetes Care. 1998; 21:1138–1145. NHANES
- Gaede P, Lund-Andersen H, Parving HH, Pedersen O. Effect of a multifactorial intervention on mortality in type 2 diabetes. N Engl J Med. 2008; 358:580–591.
- Patel A, MacMahon S, Chalmers J, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008; 358:2560–2572.
- Action in Diabetes and Vascular Disease: PreterAx and DiamicroN Modified-Release Controlled Evaluation. Rationale and design of the ADVANCE study: a randomised trial of blood pressure lowering and intensive glucose control in high-risk individuals with type 2 diabetes mellitus. J Hypertens 2001; 19(suppl):S21–S28.
- Abraira C, Duckworth W, McCarren M, et al. Design of the cooperative study on glycemic control and complications in diabetes mellitus type 2: Veterans Affairs Diabetes Trial. J Diabetes Complications. 2003; 17:314–322.
- Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med. 2007; 356:2457–2471.
- American Association of Clinical Endocrinologists. American Association of Clinical Endocrinologists medical guidelines for clinical practice for the management of diabetes mellitus. Endocr Pract 2007; 13(suppl 1):1–68.
- American Diabetes Association. Standards of medical care in diabetes—2008. Diabetes Care 2008; 31(suppl 1):S12–S54.
- Magee MF, Isley WL. Rationale, design, and methods for glycemic control in the Bypass Angioplasty Revascularization Investigation 2 Diabetes (BARI 2D) Trial. Am J Cardiol 2006; 97:20G–30G.
- Gerstein HC, Miller ME, Byington RP, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008; 358:2545–2559.
- Goff DC, Gerstein HC, Ginsberg HN, et al. Prevention of cardiovascular disease in persons with type 2 diabetes mellitus: current knowledge and rationale for the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial. Am J Cardiol 2007; 99:4i–20i.
- Buse JB, Bigger JT, Byington RP, et al. Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial: design and methods. Am J Cardiol 2007; 99:21i–33i.
- Gerstein HC, Riddle MC, Kendall DM, et al. Glycemia treatment strategies in the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial. Am J Cardiol 2007; 99:34i–43i.
- Bonds DE, Kurashige EM, Bergenstal R, et al. Severe hypoglycemia monitoring and risk management procedures in the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial. Am J Cardiol 2007; 99:80i–89i.
- Gerstein HC. Dysglycemia, not just diabetes, is a continuous risk factor for cardiovascular disease. Evid Based Cardiovasc Med. 1997; 1:87–88.
- Gerstein HC, Pais P, Pogue J, Yusuf S. Relationship of glucose and insulin levels to the risk of myocardial infarction: a case-control study. J Am Coll Cardiol. 1999; 33:612–619.
- Gerstein HC, Capes SE. Dysglycemia: a key cardiovascular risk factor. Semin Vasc Med. 2002; 2:165–174.
- Gerstein HC, Santaguida P, Raina P, et al. Annual incidence and relative risk of diabetes in people with various categories of dysglycemia: a systematic overview and meta-analysis of prospective studies. Diabetes Res Clin Pract. 2007; 78:305–312.
- American Diabetes Association. Role of cardiovascular risk factors in prevention and treatment of macrovascular disease in diabetes. Diabetes Care. 1989; 12:573–579.
- Schor S. The University Group Diabetes Program. A statistician looks at the mortality results. JAMA. 1971; 217:1671–1675.
- Cornfield JThe University Group Diabetes Program. A further statistical analysis of the mortality findings. JAMA. 1971; 217:1676–1687.
- Feinstein AR. Clinical biostatistics. 8. An analytic appraisal of the University Group Diabetes Program (UGDP) study. Clin Pharmacol Ther. 1971; 12:167–191.
- The University Group Diabetes Program. A study of the effects of hypoglycemic agents on vascular complications in patients with adult-onset diabetes. V. Evaluation of pheniformin therapy. Diabetes 1975; 24( suppl 1):65–184.
- Knatterud GL, Klimt CR, Levin ME, Jacobson ME, Goldner MG. Effects of hypoglycemic agents on vascular complications in patients with adult-onset diabetes. VII. Mortality and selected nonfatal events with insulin treatment. JAMA. 1978; 240:37–42.
- Schwartz TB, Meinert CL. The UGDP controversy: thirty-four years of contentious ambiguity laid to rest. Perspect Biol Med. 2004; 47:564–574.
- Turner RC, Holman RR. Lessons from UK Prospective Diabetes Study. Diabetes Res Clin Pract 1995; 28( suppl):S151–S157.
- UKPDS Research Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998; 352:854–865.
- UKPDS Study Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998; 352:837–853.
- UK Prospective Diabetes Study Group. UKPDS 28: a randomized trial of efficacy of early addition of metformin in sulfonylurea-treated type 2 diabetes. Diabetes Care. 1998; 21:87–92.
- Bretzel RG, Voigt K, Schatz H. The United Kingdom Prospective Diabetes Study (UKPDS) implications for the pharmacotherapy of type 2 diabetes mellitus. Exp Clin Endocrinol Diabetes. 1998; 106:369–372.
- Turner RC, Cull CA, Frighi V, Holman RR. Glycemic control with diet, sulfonylurea, metformin, or insulin in patients with type 2 diabetes mellitus: progressive requirement for multiple therapies (UKPDS 49). UK Prospective Diabetes Study (UKPDS) Group. JAMA. 1999; 281:2005–2012.
- Leslie RD. United Kingdom prospective diabetes study (UKPDS): what now or so what? Diabetes Metab Res Rev 1999; 15:65–71.
- Stratton IM, Adler AI, Neil HA, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ. 2000; 321:405–412.
- Mooradian AD, Chehade J. Implications of the UK Prospective Diabetes Study: questions answered and issues remaining. Drugs Aging. 2000; 16:159–164.
- Nathan DM. Some answers, more controversy, from UKPDS. United Kingdom Prospective Diabetes Study. Lancet. 1998; 352:832–833.
- Srimanunthiphol J, Beddow R, Arakaki R. A review of the United Kingdom Prospective Diabetes Study (UKPDS) and a discussion of the implications for patient care. Hawaii Med J. 2000; 59:295–298.
- Duckworth WC, McCarren M, Abraira C. Glucose control and cardiovascular complications: the VA Diabetes Trial. Diabetes Care. 2001; 24:942–945.
- Abraira C, Colwell JA, Nuttall FQ, et al. Veterans Affairs Cooperative Study on glycemic control and complications in type II diabetes (VA CSDM). Results of the feasibility trial. Veterans Affairs Cooperative Study in Type II Diabetes. Diabetes Care. 1995; 18:1113–1123.
- Gu K, Cowie CC, Harris MI. Mortality in adults with and without diabetes in a national cohort of the U.S. population, 1971–1993. Diabetes Care. 1998; 21:1138–1145. NHANES
- Gaede P, Lund-Andersen H, Parving HH, Pedersen O. Effect of a multifactorial intervention on mortality in type 2 diabetes. N Engl J Med. 2008; 358:580–591.
- Patel A, MacMahon S, Chalmers J, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008; 358:2560–2572.
- Action in Diabetes and Vascular Disease: PreterAx and DiamicroN Modified-Release Controlled Evaluation. Rationale and design of the ADVANCE study: a randomised trial of blood pressure lowering and intensive glucose control in high-risk individuals with type 2 diabetes mellitus. J Hypertens 2001; 19(suppl):S21–S28.
- Abraira C, Duckworth W, McCarren M, et al. Design of the cooperative study on glycemic control and complications in diabetes mellitus type 2: Veterans Affairs Diabetes Trial. J Diabetes Complications. 2003; 17:314–322.
- Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med. 2007; 356:2457–2471.
- American Association of Clinical Endocrinologists. American Association of Clinical Endocrinologists medical guidelines for clinical practice for the management of diabetes mellitus. Endocr Pract 2007; 13(suppl 1):1–68.
- American Diabetes Association. Standards of medical care in diabetes—2008. Diabetes Care 2008; 31(suppl 1):S12–S54.
- Magee MF, Isley WL. Rationale, design, and methods for glycemic control in the Bypass Angioplasty Revascularization Investigation 2 Diabetes (BARI 2D) Trial. Am J Cardiol 2006; 97:20G–30G.
KEY POINTS
- No obvious cause, including hypoglycemia proximate to death or the use of any particular medication, clearly explained the excess deaths, although hypoglycemia occurred more often in intensively treated participants.
- The death rates in ACCORD were lower than in population studies and in other intervention trials. It is likely that multiple approaches to reducing the risk of cardiovascular disease contributed to this low mortality rate.