User login
SC Insulin Order Sets and Protocols
Inpatient glycemic control and hypoglycemia are issues with well deserved increased attention in recent years. Prominent guidelines and technical reviews have been published,13 and a recent, randomized controlled trial demonstrated the superiority of basal bolus insulin regimens compared to sliding‐scale regimens.4 Effective glycemic control for inpatients has remained elusive in most medical centers. Recent reports57 detail clinical inertia and the continued widespread use of sliding‐scale subcutaneous insulin regimens, as opposed to the anticipatory, physiologic basal‐nutrition‐correction dose insulin regimens endorsed by these reviews.
Inpatient glycemic control faces a number of barriers, including fears of inducing hypoglycemia, uneven knowledge and training among staff, and competing institutional and patient priorities. These barriers occur in the background of an inherently complex inpatient environment that poses unique challenges in maintaining safe glycemic control. Patients frequently move across a variety of care teams and geographic locations during a single inpatient stay, giving rise to multiple opportunities for failed communication, incomplete handoffs, and inconsistent treatment. In addition, insulin requirements may change dramatically due to variations in the stress of illness, exposure to medications that effect glucose levels, and varied forms of nutritional intake with frequent interruption. Although insulin is recognized as one of the medications most likely to be associated with adverse events in the hospital, many hospitals do not have protocols or order sets in place to standardize its use.
A Call to Action consensus conference,8, 9 hosted by the American Association of Clinical Endocrinologists (AACE) and the American Diabetes Association (ADA), brought together many thought leaders and organizations, including representation from the Society of Hospital Medicine (SHM), to address these barriers and to outline components necessary for successful implementation of a program to improve inpatient glycemic control in the face of these difficulties. Institutional insulin management protocols and standardized insulin order sets (supported by appropriate educational efforts) were identified as key interventions. It may be tempting to quickly deploy a generic insulin order set in an effort to improve care. This often results in mediocre results, due to inadequate incorporation of standardization and guidance into the order set and other documentation tools, and uneven use of the order set.
The SHM Glycemic Control Task Force (GCTF) recommends the following steps for developing and implementing successful protocols and order sets addressing the needs of the noncritical care inpatient with diabetes/hyperglycemia.
-
Form a steering committee for this work, and assess the current processes of care.
-
Identify best practices and preferred regimens to manage diabetes and hyperglycemia in the hospital.
-
Integrate best practices and preferred institutional choices into an inpatient glycemic control protocol. Crystallize your protocol into a one page summary.
-
Place guidance from your protocol into the flow of work, by integrating it into standardized subcutaneous insulin order sets and other documentation and treatment tools.
-
Monitor the use of your order sets and protocol. Intervene actively on nonadherents to your protocol and those with poor glycemic control, and revise your protocol/order sets as needed.
IDENTIFYING AND INCORPORATING KEY CONCEPTS AND BEST PRACTICES
A protocol is a document that endorses specific monitoring and treatment strategies in a given institution. This potentially extensive document should provide guidance for transitions, special situations (like steroids and total parenteral nutrition [TPN]) and should outline preferred insulin regimens for all of the most common nutritional situations. One of the most difficult parts of creating a protocol is the assimilation of all of the important information on which to base decisions. Your protocol and order set will be promoting a set of clinical practices. Fortunately, the current best practice for noncritical care hyperglycemic patients has been summarized by several authoritative sources,13, 811 including references from the SHM Glycemic Task Force published in this supplement.4, 12
Table 1 summarizes the key concepts that should be emphasized in a protocol for subcutaneous insulin management in the hospital. We recommend embedding guidance from your protocol into order sets, the medication administration record, and educational materials. Although the details contained in a protocol and order set might vary from one institution to another, the key concepts should not. The remainder of this article provides practical information about how these concepts and guidance for how preferred insulin regimens should be included in these tools. Appendices 1 and 2 give examples of an institutional one‐page summary protocol and subcutaneous insulin order set, respectively.
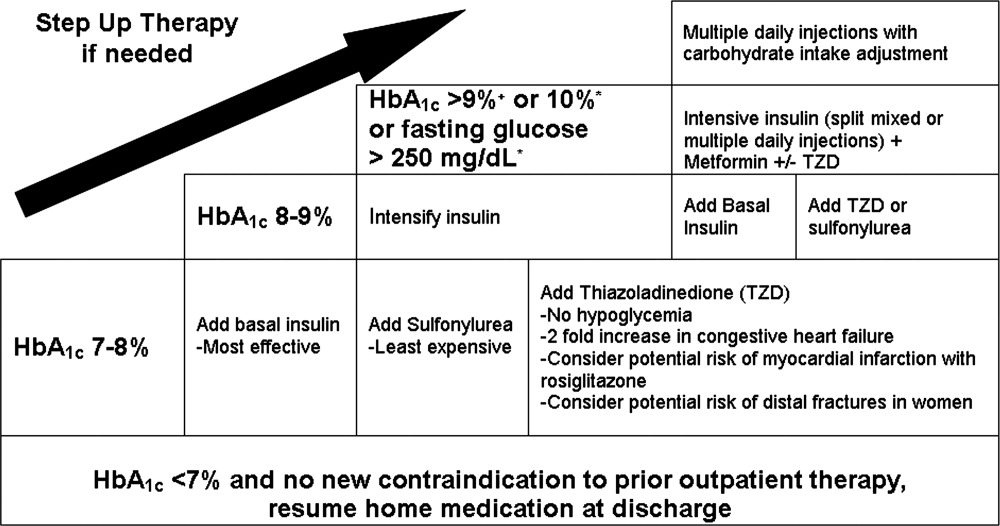
| 1. Establish a target range for blood glucose levels. |
| 2. Standardize monitoring of glucose levels and assessment of long‐term control (HbA1c). |
| 3. Incorporate nutritional management. |
| 4. Prompt clinicians to consider discontinuing oral antihyperglycemic medications. |
| 5. Prescribe physiologic (basal‐nutrition‐correction) insulin regimens. |
| a. Choose a total daily dose (TDD). |
| b. Divide the TDD into physiologic components of insulin therapy and provide basal and nutritional/correction separately. |
| c. Choose and dose a basal insulin. |
| d. Choose and dose a nutritional (prandial) insulin |
|
i. Match exactly to nutritional intake (see Table 2). |
| ii. Include standing orders to allow nurses to hold nutritional insulin for nutritional interruptions and to modify nutritional insulin depending on the actual nutritional intake. |
| e. Add correction insulin |
| i. Match to an estimate of the patients insulin sensitivity using prefabricated scales. |
| ii. Use the same insulin as nutritional insulin. |
| 6. Miscellaneous |
| a. Manage hypoglycemia in a standardized fashion and adjust regimen to prevent recurrences. |
| b. Provide diabetes education and appropriate consultation. |
| c. Coordinate glucose testing, nutrition delivery, and insulin administration. |
| d. Tailor discharge treatment regimens to the patient's individual circumstances and arrange for proper follow‐up. |
Standardize the Monitoring of Blood Glucose Values and Glucosylated Hemoglobin
Guidance for the coordination of glucose testing, nutrition delivery, and insulin administration, should be integrated into your protocols, and order sets. For noncritical care areas, the minimal frequency for blood glucose monitoring for patients who are eating is before meals and at bedtime. For the patient designated nothing by mouth (NPO) or the patient on continuous tube feeding, the type of nutritional/correction insulin used should drive the minimum frequency (every 4‐6 hours if rapid acting analog insulins [RAA‐I] are used, and every 6 hours if regular insulin is used). Directions for administering scheduled RAA‐I immediately before or immediately after nutrition delivery should be incorporated into protocols, order sets, and medication administration records. Unfortunately, having this guidance in the order sets and protocols does not automatically translate into its being carried out in the real world. Wide variability in the coordination of glucose monitoring, nutritional delivery, and insulin administration is common, so monitoring the process to make sure the protocol is followed is important.
Obtaining a glucosylated hemoglobin (HbA1c) level is important in gauging how well the patient's outpatient regimen is maintaining glycemic control, distinguishing stress hyperglycemia from established diabetes, and guiding the inpatient approach to glycemic control. ADA guidelines2, 3 endorse obtaining HbA1c levels of inpatients if these levels are not already available from the month prior to admission.
Establish a Target Range for Blood Glucose in NonCritical Care Areas
It is important to adopt a glycemic target that is institution‐wide, for critical care areas and noncritical care areas alike. Your glycemic target need not be identical to the ADA/AACE glycemic targets, but should be similar to them.
Examples of institutional glycemic targets for noncritical care areas:
-
Preprandial target 90‐130 mg/dL, maximum random glucose 180 mg/dL (ADA/AACE consensus target)
-
90‐150 mg/dL (a target used in some hospitals)
-
Preprandial target 90‐130 mg/dL for most patients, 100‐150 mg/dL if there are hypoglycemia risk factors, and 180 mg/dL if comfort‐care or end‐of‐life care (a more refined target, allowing for customization based on patient characteristics).
Your multidisciplinary glycemic control steering committee should pick the glycemic target it can most successfully implement and disseminate. It is fine to start with a conservative target and then ratchet down the goals as the environment becomes more accepting of the concept of tighter control of blood glucose in the hospital.
Although the choice of glycemic target is somewhat arbitrary, establishing an institutional glycemic target is critical to motivate clinical action. Your committee should design interventions, for instances when a patient's glycemic target is consistently not being met, including an assignment of responsibility.
Prompt Clinicians to Consider Discontinuing Oral Agents
Oral antihyperglycemic agents, in general, are difficult to quickly titrate to effect, and have side effects that limit their use in the hospital. In contrast, insulin acts rapidly and can be used in virtually all patients and clinical situations, making it the treatment of choice for treatment of hyperglycemia in the hospital.3, 11, 12 In certain circumstances, it may be entirely appropriate to continue a well‐controlled patient on his or her prior outpatient oral regimen. It is often also reasonable to resume oral agents in some patients when preparing for hospital discharge.
Incorporate Nutritional Management
Because diet is so integral to the management of diabetes and hyperglycemia, diet orders should be embedded in all diabetes or insulin‐related order sets. Diets with the same amount of carbohydrate with each meal should be the default rule for patients with diabetes. Nutritionist consultation should be considered and easy to access for patients with malnutrition, obesity, and other common conditions of the inpatient with diabetes.
Access Diabetes Education and Appropriate Consultation
Diabetes education should be offered to all hyperglycemic patients with normal mental status, complete with written materials, a listing of community resources, and survival skills. Consultation with physicians in internal medicine or endocrinology for difficult‐to‐control cases, or for cases in which the primary physician of record is not familiar with (or not adherent to) principles of inpatient glycemic management, should be very easy to obtain, or perhaps mandated, depending on your institution‐specific environment.
Prescribe Physiologic (Basal‐Nutritional‐Correction Dose) Insulin Regimens
Physiologic insulin use is the backbone of the recommended best practice for diabetes and hyperglycemia management in the hospital. The principles of such regimens are summarized elsewhere in this supplement.12 These principles will not be reiterated in detail here, but the major concepts that should be integrated into the protocols and order sets will be highlighted.
Choose a Total Daily Dose
Clinicians need guidance on how much subcutaneous insulin they should give a patient. These doses are well known from clinical experience and the published literature. The fear of hypoglycemia usually results in substantial underdosing of insulin, or total avoidance of scheduled insulin on admission. Your team should provide guidance for how much insulin to start a patient on when it is unclear from past experience how much insulin the patient needs. Waiting a few days to see how much insulin is required via sliding‐scale‐only regimens is a bad practice that should be discouraged for patients whose glucose values are substantially above the glycemic target. The total daily dose (TDD) can be estimated in several different ways (as demonstrated in Appendix 1 and 2), and protocols should make this step very clear for clinicians. Providing a specific location on the order set to declare the TDD may help ensure this step gets done more reliably. Some institutions with computer physician order entry (CPOE) provide assistance with calculating the TDD and the allocation of basal and nutritional components, based on data the ordering physician inputs into the system.
Select and Dose a Basal Insulin
Your protocol should describe how the TDD should be divided between basal and nutritional insulin. We generally recommend 50% of the TDD be given as basal insulin, with the other 50% administered on a scheduled basis to cover glycemic excursions from nutritional intake. The 50/50 rule is simple and generally works well, and should be widely promoted. However, there are exceptions to this rule that should be incorporated into your full protocol and educational programs. The order set should have separate steps for ordering basal insulin, nutritional insulin, and correction insulin. The advantage to providing these insulin components separately is that it allows them to be independently manipulated (eg, if a patient is unable to tolerate a meal, nutritional insulin is held, but basal insulin and correction insulin are continued).
The SHM GCTF specifically endorses long acting insulin (glargine and detemir) as the preferred basal insulin in the hospital setting, thus discouraging the use of neutral protamine Hagedorn (NPH) insulin and fixed combination insulin formulations (Table 2). In the absence of randomized controlled trials demonstrating superiority of the glargine or detemir to NPH insulin in the hospital, this endorsement deserves some further explanation. Although we believe that correctly dosed NPH containing insulin regimens can attain effective and safe glycemic control in the hospital setting, it is more difficult to standardize their use and adjust for fluctuations in nutritional intake. Glargine and detemir have much less pronounced spikes in their effect than NPH, rendering them relatively peakless in comparison. This pharmacokinetic profile allows for continued dosing with minimal or no correction when nutrition intake is variable, and allow for consistent reinforcement of the basal‐nutritional‐correction insulin concept.
| Nutritional situation | Necessary insulin components | Preferred regimen* |
|---|---|---|
| ||
| NPO (or clear liquids) | Basal insulin: 50% of TDD. Nutritional insulin: None. | Basal insulin: glargine given once daily or detemir given twice daily. Nutritional insulin: None. Correctional insulin: Regular insulin q 6 hours or RAA insulin q 4 hours. Other comments: Dextrose infusion (e.g., D5 containing solution at 75‐150 cc/hour) recommended when nutrition is held. An IV insulin infusion is preferred for management of prolonged fasts or fasting type 1 diabetes patients. |
| Eating meals | Basal insulin: 50% of TDD. Nutritional insulin: 50% of TDD, divided equally before each meal. | Basal insulin: glargine given once daily or detemir given twice daily. Nutritional insulin: RAA insulin with meals. Correctional insulin: RAA insulin q AC and HS (reduced dose at HS). |
| Bolus tube feeds | Basal insulin: 40% of TDD. Nutritional insulin: 60% of the TDD, divided equally before each bolus feed. | Basal insulin: glargine given once daily or detemir given twice daily. Nutritional insulin: RAA insulin with each bolus. Correctional insulin: RAA insulin with each bolus. |
| Continuous tube feeds | Basal insulin: 40% (conservative) of TDD. Nutritional insulin: 60% of the TDD in divided doses. | Basal insulin: glargine given once daily or detemir given twice daily. Nutritional insulin: RAA insulin q 4 hours or regular insulin q 6 hours. Correctional insulin: Should match nutritional insulin choice. |
| Parenteral nutrition | Insulin is usually given parenterally, with the nutrition | Initially, a separate insulin drip allows for accurate dose‐finding. Then, 80% of amount determined as TDD using drip is added to subsequent TPN bags as regular insulin. Use correctional subcutaneous insulin doses cautiously, in addition. |
There are some caveats to this general recommendation. First, patients who are well controlled on home regimens with NPH basal insulin can (and sometimes should) stay on the regimen that has worked well for them. However, extra vigilance in reducing the dose for reductions in nutrition is required, because NPH is generally used to cover both nutritional and basal requirements. Second, extensive experience with glargine and detemir are not available in obstetric populations. They are not U.S. Food and Drug Administration (FDA) approved for use in pregnant patients and formally carry a Class C rating, whereas NPH insulin has been used safely in obstetric populations for decades. Third, the insulin regimen used as an inpatient is not necessarily the preferred regimen to prescribe at discharge: cost, patient preferences, HbA1c level, and other factors should be considered in making this choice.
Select and Dose a Nutritional (Prandial) Insulin
The step for ordering nutritional insulin should assist the clinician in matching the insulin to the type of nutrition that the patient is receiving. For example, rapid‐acting insulin analogs are preferred over regular insulin in the eating patient, in view of their more physiologic profile, which averts the insulin stacking that can occur with regular insulin. If regular insulin is used as the preferred institutional choice for eating patients, the lunchtime dose should be reduced or eliminated altogether, to eliminate insulin stacking.
Table 2 outlines the SHM GCTF preferred regimens for different nutritional situations.
There should be a standing order for nutritional insulin to be held when nutrition is interrupted, whether intentional or unintentional. Patients with interrupted tube feedings could have standing orders for a dextrose infusion to replace the tube feeding carbohydrate load and prevent hypoglycemia. Ideally, there should also be a standing order allowing for real‐time management of the patient with uncertain nutritional intake. For example, when a patient's premeal assessment reveals that she may not tolerate the meal, the patient should be allowed to attempt to eat, and then the nutritional insulin should be given after the meal, in proportion to the amount of food that was eaten. This type of order will require significant nursing education and process redesign in many hospitals, but is essential for matching nutritional insulin to actual intake.
Add Correction Insulin
There is no convincing evidence for the benefit of correction (sliding‐scale) insulin in the inpatient setting, although a randomized trial demonstrating the superiority of basal/nutritional insulin regimens to sliding‐scale only regimens did incorporate a correction insulin scale as an adjunct to the superior basal/nutritional regimen.4 The SHM GCTF again emphasizes that control of hyperglycemia should be proactive and anticipatory of insulin needs, rather than reactive to hyperglycemia. Nonetheless, unexpected hyperglycemic excursions are common, and the use of correction insulin remains a pervasive and arguably logical practice. If correction insulin is used, it should be ordered as a separate step after considering basal and nutrition insulin needs. The doses of scheduled insulin should be adjusted regularly if correction insulin is consistently being required. Ideally, the prescriber should choose a preformatted corrective insulin scale, based on the patient's insulin sensitivity (Appendix 2). There should be a prompt to use the same type of insulin that is being used for nutritional insulin, and there should be instructions that this insulin is given in addition to the basal and nutritional insulin to correct for hyperglycemia. Nocturnal correction‐dose scales should be reduced in the eating patient.
Even after limiting insulin regimens to those in Table 2, multidisciplinary glycemic control teams are still left with several options within these SHM‐preferred regimens. We recommend that your team choose a single, institutionally‐preferred basal‐nutritional‐correction insulin combination for each situation.
Choosing one preferred option for these situations is advantageous because:
-
You can communicate preferred regimens more simply and succinctly to all staff.
-
You eliminate all inappropriate choices for insulin regimens for that situation, as well as some other less preferred, but acceptable choices.
-
You can encourage regimens that are most economical (by promoting the insulin regimens that reflect your hospital formulary choices).
-
Staff members can become very familiar with a few regimens, instead of being confused by a multitude of them. They can identify variations from your preferred choices and target these patients for extra scrutiny and actions should they fail to meet glycemic targets.
Although virtually every institution can provide specific guidance on insulin management in a protocol, there are tradeoffs inherent in how restrictive you can be in pushing these preferred choices in your order sets. Should you eliminate alternate basal or nutritional insulin choices from your order sets? As you integrate more and more of your preferred algorithm and regimens into your order set, you will gain incremental improvement in the standardization of inpatient insulin management. However, you reduce not only variability in ordering, but also the choices available to your prescribers and patients, and in effect you are pushing the providers to use an insulin regimen that often differs from the patient's outpatient regimen. If your institution is not yet ready to go with a single preferred insulin, simply listing your preferred insulin first with the annotation preferred can be enough to increase the use of the preferred insulin.
We endorse building the most protocol‐driven, proscriptive, insulin order set that the Glycemic Control Steering Committee believes their medical staff will accept. There are some caveats to this endorsement. First, there must be extra efforts on the backend of the admission, to ensure that the antihyperglycemic regimen is tailored to the unique needs of the patient (this is discussed further below). Second, a protocol‐driven approach is not a substitute for a good educational program for health care providers or well‐informed clinical judgment. Education should reinforce major concepts driving the protocol and should also highlight exceptions to the rule. Variance from the protocol endorsed choices should be allowed (and even encouraged) when the variance is driven by patient factors (as opposed to provider whim). Learning from this variance is a key concept in refining protocols. Education ideally should not be limited to only protocol‐endorsed choices, as staff should be familiar with the full range of antihyperglycemia regimens seen in inpatient and outpatient settings.
Special Situations
Most of the preferred regimens for different situations are outlined in Table 2 in a straightforward manner, and can be depicted in your protocols and order sets in the same way. Some conditions have enough complexity, however, that you will have difficulty placing all of the details into your one‐page protocol and order set. Details should be placed on your more detailed protocol, and educational programs should include the topics outlined below. Although insulin infusion is often the option that would provide the most reliable and expedient control of hyperglycemia in these special situations, it is an option not available in many noncritical care settings. Therefore, the discussion is limited to subcutaneous insulin control regimens.
Patient on Continuous Tube Feeding
The SHM GCTF endorses glargine or detemir as the basal insulin of choice for this setting. The nutritional and correction insulin of choice is either an RAA‐I every 4 hours (q4h), or regular insulin every 6 hours (q6h). We endorse this choice because it retains the basal‐nutritional‐correction dose concept, generally allows for continued basal insulin use if the tube feedings become interrupted, and is amenable to building a consistent institutional protocol.
There are some important caveats to this recommendation. First, realize that almost any regimen that provides a stable insulin supply would be acceptable, and many institutions will use glargine or detemir to cover both basal and nutritional needs. The downside to using large boluses of long‐acting insulin in this clinical situation is that any unexpected interruption of the feedings will necessitate prolonged infusions of dextrate 10% solution (D10) to avoid hypoglycemia
Second, because of the glycemic load inherent in tube feedings, maintenance of glycemic control in the setting of enteral feeding may be best managed by providing a higher percentage of the TDD as nutritional insulin. In these cases, ratios of basal to nutritional insulin of 40:60, or even less basal insulin, may be appropriate.
Glucocorticoid Therapy
High‐dose glucocorticoids are strongly associated with increased insulin requirements. The degree of hyperglycemia induced by steroids varies significantly from patient to patient, and the pattern of hyperglycemia will vary depending on the pattern of steroid administration. The general principle to keep in mind is that the hyperglycemia induced by a steroid dose will peak 8‐12 hours after it is given, so insulin regimens to address this should take this effect into account. For example, giving a long‐acting basal insulin like glargine to accommodate the hyperglycemic effect of a steroid bolus given in the morning would be inappropriate because the steroid effect would wane and then disappear overnight, leading to insulin‐induced hypoglycemia. NPH insulin can be ideal in this setting, either by itself, or by layering it on top of an existing regimen.
Another caveat: glucocorticoids exert their predominate effect on insulin sensitivity in muscle (as opposed to the liver), and as a result, have their most notable effect on postprandial glucose. For this reason, the best insulin regimens for this situation may use proportionally less basal insulin and more nutritional insulin. One common regimen calls for keeping the basal insulin dose the same as the preglucocorticoid dose, while escalating the RAA insulin dose at lunch and dinner.
Given the complexities of covering steroid‐induced hyperglycemia and its high prevalence in certain populations (such as transplantation patients and patients undergoing chemotherapy), this would be an excellent area on which to focus expertise. Examples include routine endocrinology consultation, intervention by a special glycemic control team, or incorporating routine glucose monitoring and triggers for initiating insulin infusion into the protocols for chemotherapy and transplantation patients.
Regiment the Management of Hypoglycemia
Hypoglycemia is defined by the ADA as a blood glucose of 70 mg/dL or less, based on the physiologic changes that can occur at this glucose level, even in subjectively asymptomatic patients.3 Protocols for management of hypoglycemia should be linked to your diabetes/hyperglycemia protocols. There are many hypoglycemia protocols available for review in the SHM Glycemic Control Resource Room and Glycemic Control Implementation Guide.10 Some common themes for effective implementation stand out. First, the protocols need to walk the balance between simplicity of use, and the need to provide instructions that will provide guidance in a variety of patient situations. Second, the protocols need to be nurse driven, so that nurses can initiate treatment without waiting for a physician order. Third, education and instruction regarding recognition of risk factors, and avoidance of hypoglycemia are needed to support a successful protocol. Importantly, any hypoglycemic event should lead to a reconsideration of the current anti‐hyperglycemic regimen so that future events can be prevented.
Plan for Discharge and Provide Guidance for the Transition
Your institution should have policies and procedures outlining all the steps needed to complete the important transition out of the hospital. At a minimum, this planning should include adequate education (including a learner assessment), appropriate follow‐up, referral to community resources, and a discharge glycemic control regimen that is tailored to the educational, financial, and motivational profile of a patient. The more your inpatient insulin management is driven by protocol, the more likely it is the patient will be on an inpatient treatment plan that differs from their outpatient regimen; therefore, it is even more important to plan this transition carefully and reliably.
Communicating the accurate hyperglycemia related diagnosis and related problems to the primary care provider is important for good care, perhaps even more so for patients who had hyperglycemia while hospitalized without a prior diagnosis of diabetes. Some centers place a prompt for hyperglycemia related diagnosis in the order set and/or discharge paperwork, to remind the clinician to convey the diagnosis to the primary provider, and to encourage more complete documentation. Improved documentation can also improve the business case for glycemic control, along with other strategies outlined elsewhere in this supplement.13
Transitions in care (including transitions out of the hospital and off of infusion insulin) are discussed in more detail14, 15 elsewhere in this supplement. The principles outlined in these references should be incorporated into your institutional protocol. Briefly, not all patients require or are capable of intensive basal‐bolus regimens upon discharge. The HbA1c can be very valuable in arriving at the optimal outpatient regimen.14 The capacities and preferences of the patient and the context of his or her outpatient care environment (including the preferences of the primary care provider) must be taken into consideration as an outpatient management program is planned.
PULLING IT ALL TOGETHER: MAKE SURE YOUR PROTOCOL/ORDER SET IS EASY TO USE AND WIDELY UTILIZED
When standardizing hospital management of diabetes and hyperglycemia, we recommend building the full protocol first, then crystallizing the protocol into a one‐page summary that can be widely disseminated. The protocol guidance is then incorporated into the order set and nursing medical administration record (MAR). Again, we recommend the most proscriptive and protocol‐driven order set feasible within the constraints of medical staff support. The example order set in Appendix 2 illustrates this approach along with other desirable features:
-
Check‐box simplicity on when to order appropriate glucose monitoring.
-
Prompt for the proper hyperglycemia‐related diagnosis.
-
Prompts to document diagnosis and to order HbA1c level.
-
Use of encouraged insulin terminology: basal, prandial (or nutritional), and correction. Language is a powerful thing, and just getting staff to use these terms goes a long way toward the more physiologic prescribing of insulin.
-
Statement/reminder of a glycemic goal.
-
Prompts and contact information for appropriate consultation.
-
Elimination of unapproved abbreviations (such as U for units).
-
Stating both generic and brand names of insulin preparations.
-
Important timing cues for administration of insulin.
-
Several correction‐dose scales suitable for different insulin sensitivities. One size does NOT fit all.
-
Incorporation of a simple hypoglycemia protocol into the order set.
-
Insulin dosing guidelines available at the point of care (in this case, on the back of the order set).
Additional nursing‐specific cues (such as an admonition to never mix glargine insulin with other types of insulin) can also be included in the MAR whenever glargine is ordered.
Once you have protocols and order sets to guide providers, you need to assure that they are used for the majority of hyperglycemic patients. Educational programs should introduce your interventions and the rationale for them. In order to make your method the default method of care, your team should survey all preprinted or CPOE insulin order sets of your institution. A review of postoperative, transfer, and admission order sets that all services use may reveal a half‐dozen or more embedded sliding‐scale insulin order sets that should be removed, with prompts to use the standardized insulin order set being placed in their stead.
Computerized order sets present both challenges and opportunities. Wording limitations and the scrolling nature can make concepts less clear, yet there is a capability for incorporating a hierarchical structure that allows for guiding the user through a more algorithmic approach. There is also a capacity to provide assistance with dosing calculations that do not exist in the paper world. Education remains of key importance for both methods.
MONITOR THE USE AND EFFECTIVENESS OF YOUR PROTOCOLS AND ORDER SETS
Creating and implementing protocols, order sets, and other tools is not the end of the journey to improve care. It is important to monitor order set utilization, insulin use patterns, and parameters measuring glycemic control and hypoglycemia, as outlined in more detail in another article in this supplement.16 In addition to summary data every month or so, we recommend daily reports that spur action in near real time. Triggers such as uncontrolled hyperglycemia, markedly elevated HbA1c levels, and nonphysiologic insulin regimens should initiate consultation, extra diabetes education, or referral to a glucose control team. If appropriate consultation is not readily available, the glycemic control steering group should lobby the administration to bolster this capability. Qualitative feedback from the frontline caregivers, as well as this quantitative data, can assist the local glycemic control champions in designing even more effective protocols, order sets, focused educational efforts, and concurrent mitigation of suboptimal care.
CONCLUSION
Diabetes, hyperglycemia, and iatrogenic hypoglycemia are common and important conditions affecting the noncritically ill inpatient. Interventional trials to validate the recommended noncritical care unit glycemic targets are needed. Although there is a growing consensus on best practices to care for these patients, numerous barriers and the complexity of caring for inpatients hamper the reliability of best practice delivery. Institutional protocols and protocol driven subcutaneous insulin orders, when implemented with the strategies outlined here, can be the key to delivering these best practices more reliably.
Appendix
- American College of Endocrinology Task Force on Inpatient Diabetes and Metabolic Control.American College of Endocrinology Position Statement on Inpatient Diabetes and Metabolic Control.Endocr Pract.2004;10:77–82.
- ,,,,,,.Management of diabetes and hyperglycemia in hospitals.Diabetes Care.2004;27:553–591.
- American Diabetes Association.Standards of Medical Carein Diabetes‐2006.Diabetes Care.2006;29(suppl 1):s4–s42.
- ,,, et al.Randomized study of basal‐bolus insulin therapy in the inpatient management of patients with type 2 diabetes (RABBIT 2 Trial).Diabetes Care.2007;30:2181–2186.
- ,,,,.Inpatient management of diabetes and hyperglycemia among general medicine patients at a large teaching hospital.J Hosp Med.2006;1:145–150.
- ,,, et al.Diabetes care in the hospital: is there clinical inertia?J Hosp Med.2006;1:151–160.
- ,,, et al.Diabetes care in hospitalized non‐critically ill patients: more evidence for clinical inertia and negative therapeutic momentum.JHosp Med.2007;2:203–211.
- Inpatient Diabetes and Glycemic Control: A Call to Action Conference. Position Statement. AACE, February2006. Available at: http://www.aace.com/meetings/consensus/IIDC/IDGC0207.pdf. Accessed October, 2006.
- Proceedings of the American College of Endocrinology and American Diabetes Association Consensus Conference, Washington, DC, January 30–31, 2006. Endocr Pract.2006; 12(suppl 3):3–13.
- Society of Hospital Medicine Glycemic Control Task Force. Implementation Guide: Improving Glycemic Control, Preventing Hypoglycemia, and Optimizing Care of the Inpatient with Hyperglycemia and Diabetes. Published January 2007 on the Society of Hospital Medicine website. Available at: http://www.hospitalmedicine.org. Accessed August,2007.
- .Management of hyperglycemia in the hospital setting.N Engl J Med.2006;355:1903–1911.
- ,,,,.Management of diabetes and hyperglycemia in the hospital: a practical guide to subcutaneous insulin use in the non‐critically ill adult patient.J Hosp Med.2008;3(5):S17–S28.
- ,.Practical strategies for developing the business case for hospital glycemic control teams.J Hosp Med2008;3(5):S76–S83.
- ,,,.Bridge over troubled waters: safe and effective transitions of the inpatient with hyperglycemia.J Hosp Med.2008;3(5):S55–S65.
- ,,,.Designing and implementing insulin infusion protocols and order sets.J Hosp Med.2008;3(5):S42–S54.
- ,,,,,.SHM Glycemic Control Task Force summary: practical recommendations for assessing the impact of glycemic control efforts.J Hosp Med.2008;3(5):S66–S75.
Inpatient glycemic control and hypoglycemia are issues with well deserved increased attention in recent years. Prominent guidelines and technical reviews have been published,13 and a recent, randomized controlled trial demonstrated the superiority of basal bolus insulin regimens compared to sliding‐scale regimens.4 Effective glycemic control for inpatients has remained elusive in most medical centers. Recent reports57 detail clinical inertia and the continued widespread use of sliding‐scale subcutaneous insulin regimens, as opposed to the anticipatory, physiologic basal‐nutrition‐correction dose insulin regimens endorsed by these reviews.
Inpatient glycemic control faces a number of barriers, including fears of inducing hypoglycemia, uneven knowledge and training among staff, and competing institutional and patient priorities. These barriers occur in the background of an inherently complex inpatient environment that poses unique challenges in maintaining safe glycemic control. Patients frequently move across a variety of care teams and geographic locations during a single inpatient stay, giving rise to multiple opportunities for failed communication, incomplete handoffs, and inconsistent treatment. In addition, insulin requirements may change dramatically due to variations in the stress of illness, exposure to medications that effect glucose levels, and varied forms of nutritional intake with frequent interruption. Although insulin is recognized as one of the medications most likely to be associated with adverse events in the hospital, many hospitals do not have protocols or order sets in place to standardize its use.
A Call to Action consensus conference,8, 9 hosted by the American Association of Clinical Endocrinologists (AACE) and the American Diabetes Association (ADA), brought together many thought leaders and organizations, including representation from the Society of Hospital Medicine (SHM), to address these barriers and to outline components necessary for successful implementation of a program to improve inpatient glycemic control in the face of these difficulties. Institutional insulin management protocols and standardized insulin order sets (supported by appropriate educational efforts) were identified as key interventions. It may be tempting to quickly deploy a generic insulin order set in an effort to improve care. This often results in mediocre results, due to inadequate incorporation of standardization and guidance into the order set and other documentation tools, and uneven use of the order set.
The SHM Glycemic Control Task Force (GCTF) recommends the following steps for developing and implementing successful protocols and order sets addressing the needs of the noncritical care inpatient with diabetes/hyperglycemia.
-
Form a steering committee for this work, and assess the current processes of care.
-
Identify best practices and preferred regimens to manage diabetes and hyperglycemia in the hospital.
-
Integrate best practices and preferred institutional choices into an inpatient glycemic control protocol. Crystallize your protocol into a one page summary.
-
Place guidance from your protocol into the flow of work, by integrating it into standardized subcutaneous insulin order sets and other documentation and treatment tools.
-
Monitor the use of your order sets and protocol. Intervene actively on nonadherents to your protocol and those with poor glycemic control, and revise your protocol/order sets as needed.
IDENTIFYING AND INCORPORATING KEY CONCEPTS AND BEST PRACTICES
A protocol is a document that endorses specific monitoring and treatment strategies in a given institution. This potentially extensive document should provide guidance for transitions, special situations (like steroids and total parenteral nutrition [TPN]) and should outline preferred insulin regimens for all of the most common nutritional situations. One of the most difficult parts of creating a protocol is the assimilation of all of the important information on which to base decisions. Your protocol and order set will be promoting a set of clinical practices. Fortunately, the current best practice for noncritical care hyperglycemic patients has been summarized by several authoritative sources,13, 811 including references from the SHM Glycemic Task Force published in this supplement.4, 12
Table 1 summarizes the key concepts that should be emphasized in a protocol for subcutaneous insulin management in the hospital. We recommend embedding guidance from your protocol into order sets, the medication administration record, and educational materials. Although the details contained in a protocol and order set might vary from one institution to another, the key concepts should not. The remainder of this article provides practical information about how these concepts and guidance for how preferred insulin regimens should be included in these tools. Appendices 1 and 2 give examples of an institutional one‐page summary protocol and subcutaneous insulin order set, respectively.
| 1. Establish a target range for blood glucose levels. |
| 2. Standardize monitoring of glucose levels and assessment of long‐term control (HbA1c). |
| 3. Incorporate nutritional management. |
| 4. Prompt clinicians to consider discontinuing oral antihyperglycemic medications. |
| 5. Prescribe physiologic (basal‐nutrition‐correction) insulin regimens. |
| a. Choose a total daily dose (TDD). |
| b. Divide the TDD into physiologic components of insulin therapy and provide basal and nutritional/correction separately. |
| c. Choose and dose a basal insulin. |
| d. Choose and dose a nutritional (prandial) insulin |
|
i. Match exactly to nutritional intake (see Table 2). |
| ii. Include standing orders to allow nurses to hold nutritional insulin for nutritional interruptions and to modify nutritional insulin depending on the actual nutritional intake. |
| e. Add correction insulin |
| i. Match to an estimate of the patients insulin sensitivity using prefabricated scales. |
| ii. Use the same insulin as nutritional insulin. |
| 6. Miscellaneous |
| a. Manage hypoglycemia in a standardized fashion and adjust regimen to prevent recurrences. |
| b. Provide diabetes education and appropriate consultation. |
| c. Coordinate glucose testing, nutrition delivery, and insulin administration. |
| d. Tailor discharge treatment regimens to the patient's individual circumstances and arrange for proper follow‐up. |
Standardize the Monitoring of Blood Glucose Values and Glucosylated Hemoglobin
Guidance for the coordination of glucose testing, nutrition delivery, and insulin administration, should be integrated into your protocols, and order sets. For noncritical care areas, the minimal frequency for blood glucose monitoring for patients who are eating is before meals and at bedtime. For the patient designated nothing by mouth (NPO) or the patient on continuous tube feeding, the type of nutritional/correction insulin used should drive the minimum frequency (every 4‐6 hours if rapid acting analog insulins [RAA‐I] are used, and every 6 hours if regular insulin is used). Directions for administering scheduled RAA‐I immediately before or immediately after nutrition delivery should be incorporated into protocols, order sets, and medication administration records. Unfortunately, having this guidance in the order sets and protocols does not automatically translate into its being carried out in the real world. Wide variability in the coordination of glucose monitoring, nutritional delivery, and insulin administration is common, so monitoring the process to make sure the protocol is followed is important.
Obtaining a glucosylated hemoglobin (HbA1c) level is important in gauging how well the patient's outpatient regimen is maintaining glycemic control, distinguishing stress hyperglycemia from established diabetes, and guiding the inpatient approach to glycemic control. ADA guidelines2, 3 endorse obtaining HbA1c levels of inpatients if these levels are not already available from the month prior to admission.
Establish a Target Range for Blood Glucose in NonCritical Care Areas
It is important to adopt a glycemic target that is institution‐wide, for critical care areas and noncritical care areas alike. Your glycemic target need not be identical to the ADA/AACE glycemic targets, but should be similar to them.
Examples of institutional glycemic targets for noncritical care areas:
-
Preprandial target 90‐130 mg/dL, maximum random glucose 180 mg/dL (ADA/AACE consensus target)
-
90‐150 mg/dL (a target used in some hospitals)
-
Preprandial target 90‐130 mg/dL for most patients, 100‐150 mg/dL if there are hypoglycemia risk factors, and 180 mg/dL if comfort‐care or end‐of‐life care (a more refined target, allowing for customization based on patient characteristics).
Your multidisciplinary glycemic control steering committee should pick the glycemic target it can most successfully implement and disseminate. It is fine to start with a conservative target and then ratchet down the goals as the environment becomes more accepting of the concept of tighter control of blood glucose in the hospital.
Although the choice of glycemic target is somewhat arbitrary, establishing an institutional glycemic target is critical to motivate clinical action. Your committee should design interventions, for instances when a patient's glycemic target is consistently not being met, including an assignment of responsibility.
Prompt Clinicians to Consider Discontinuing Oral Agents
Oral antihyperglycemic agents, in general, are difficult to quickly titrate to effect, and have side effects that limit their use in the hospital. In contrast, insulin acts rapidly and can be used in virtually all patients and clinical situations, making it the treatment of choice for treatment of hyperglycemia in the hospital.3, 11, 12 In certain circumstances, it may be entirely appropriate to continue a well‐controlled patient on his or her prior outpatient oral regimen. It is often also reasonable to resume oral agents in some patients when preparing for hospital discharge.
Incorporate Nutritional Management
Because diet is so integral to the management of diabetes and hyperglycemia, diet orders should be embedded in all diabetes or insulin‐related order sets. Diets with the same amount of carbohydrate with each meal should be the default rule for patients with diabetes. Nutritionist consultation should be considered and easy to access for patients with malnutrition, obesity, and other common conditions of the inpatient with diabetes.
Access Diabetes Education and Appropriate Consultation
Diabetes education should be offered to all hyperglycemic patients with normal mental status, complete with written materials, a listing of community resources, and survival skills. Consultation with physicians in internal medicine or endocrinology for difficult‐to‐control cases, or for cases in which the primary physician of record is not familiar with (or not adherent to) principles of inpatient glycemic management, should be very easy to obtain, or perhaps mandated, depending on your institution‐specific environment.
Prescribe Physiologic (Basal‐Nutritional‐Correction Dose) Insulin Regimens
Physiologic insulin use is the backbone of the recommended best practice for diabetes and hyperglycemia management in the hospital. The principles of such regimens are summarized elsewhere in this supplement.12 These principles will not be reiterated in detail here, but the major concepts that should be integrated into the protocols and order sets will be highlighted.
Choose a Total Daily Dose
Clinicians need guidance on how much subcutaneous insulin they should give a patient. These doses are well known from clinical experience and the published literature. The fear of hypoglycemia usually results in substantial underdosing of insulin, or total avoidance of scheduled insulin on admission. Your team should provide guidance for how much insulin to start a patient on when it is unclear from past experience how much insulin the patient needs. Waiting a few days to see how much insulin is required via sliding‐scale‐only regimens is a bad practice that should be discouraged for patients whose glucose values are substantially above the glycemic target. The total daily dose (TDD) can be estimated in several different ways (as demonstrated in Appendix 1 and 2), and protocols should make this step very clear for clinicians. Providing a specific location on the order set to declare the TDD may help ensure this step gets done more reliably. Some institutions with computer physician order entry (CPOE) provide assistance with calculating the TDD and the allocation of basal and nutritional components, based on data the ordering physician inputs into the system.
Select and Dose a Basal Insulin
Your protocol should describe how the TDD should be divided between basal and nutritional insulin. We generally recommend 50% of the TDD be given as basal insulin, with the other 50% administered on a scheduled basis to cover glycemic excursions from nutritional intake. The 50/50 rule is simple and generally works well, and should be widely promoted. However, there are exceptions to this rule that should be incorporated into your full protocol and educational programs. The order set should have separate steps for ordering basal insulin, nutritional insulin, and correction insulin. The advantage to providing these insulin components separately is that it allows them to be independently manipulated (eg, if a patient is unable to tolerate a meal, nutritional insulin is held, but basal insulin and correction insulin are continued).
The SHM GCTF specifically endorses long acting insulin (glargine and detemir) as the preferred basal insulin in the hospital setting, thus discouraging the use of neutral protamine Hagedorn (NPH) insulin and fixed combination insulin formulations (Table 2). In the absence of randomized controlled trials demonstrating superiority of the glargine or detemir to NPH insulin in the hospital, this endorsement deserves some further explanation. Although we believe that correctly dosed NPH containing insulin regimens can attain effective and safe glycemic control in the hospital setting, it is more difficult to standardize their use and adjust for fluctuations in nutritional intake. Glargine and detemir have much less pronounced spikes in their effect than NPH, rendering them relatively peakless in comparison. This pharmacokinetic profile allows for continued dosing with minimal or no correction when nutrition intake is variable, and allow for consistent reinforcement of the basal‐nutritional‐correction insulin concept.
| Nutritional situation | Necessary insulin components | Preferred regimen* |
|---|---|---|
| ||
| NPO (or clear liquids) | Basal insulin: 50% of TDD. Nutritional insulin: None. | Basal insulin: glargine given once daily or detemir given twice daily. Nutritional insulin: None. Correctional insulin: Regular insulin q 6 hours or RAA insulin q 4 hours. Other comments: Dextrose infusion (e.g., D5 containing solution at 75‐150 cc/hour) recommended when nutrition is held. An IV insulin infusion is preferred for management of prolonged fasts or fasting type 1 diabetes patients. |
| Eating meals | Basal insulin: 50% of TDD. Nutritional insulin: 50% of TDD, divided equally before each meal. | Basal insulin: glargine given once daily or detemir given twice daily. Nutritional insulin: RAA insulin with meals. Correctional insulin: RAA insulin q AC and HS (reduced dose at HS). |
| Bolus tube feeds | Basal insulin: 40% of TDD. Nutritional insulin: 60% of the TDD, divided equally before each bolus feed. | Basal insulin: glargine given once daily or detemir given twice daily. Nutritional insulin: RAA insulin with each bolus. Correctional insulin: RAA insulin with each bolus. |
| Continuous tube feeds | Basal insulin: 40% (conservative) of TDD. Nutritional insulin: 60% of the TDD in divided doses. | Basal insulin: glargine given once daily or detemir given twice daily. Nutritional insulin: RAA insulin q 4 hours or regular insulin q 6 hours. Correctional insulin: Should match nutritional insulin choice. |
| Parenteral nutrition | Insulin is usually given parenterally, with the nutrition | Initially, a separate insulin drip allows for accurate dose‐finding. Then, 80% of amount determined as TDD using drip is added to subsequent TPN bags as regular insulin. Use correctional subcutaneous insulin doses cautiously, in addition. |
There are some caveats to this general recommendation. First, patients who are well controlled on home regimens with NPH basal insulin can (and sometimes should) stay on the regimen that has worked well for them. However, extra vigilance in reducing the dose for reductions in nutrition is required, because NPH is generally used to cover both nutritional and basal requirements. Second, extensive experience with glargine and detemir are not available in obstetric populations. They are not U.S. Food and Drug Administration (FDA) approved for use in pregnant patients and formally carry a Class C rating, whereas NPH insulin has been used safely in obstetric populations for decades. Third, the insulin regimen used as an inpatient is not necessarily the preferred regimen to prescribe at discharge: cost, patient preferences, HbA1c level, and other factors should be considered in making this choice.
Select and Dose a Nutritional (Prandial) Insulin
The step for ordering nutritional insulin should assist the clinician in matching the insulin to the type of nutrition that the patient is receiving. For example, rapid‐acting insulin analogs are preferred over regular insulin in the eating patient, in view of their more physiologic profile, which averts the insulin stacking that can occur with regular insulin. If regular insulin is used as the preferred institutional choice for eating patients, the lunchtime dose should be reduced or eliminated altogether, to eliminate insulin stacking.
Table 2 outlines the SHM GCTF preferred regimens for different nutritional situations.
There should be a standing order for nutritional insulin to be held when nutrition is interrupted, whether intentional or unintentional. Patients with interrupted tube feedings could have standing orders for a dextrose infusion to replace the tube feeding carbohydrate load and prevent hypoglycemia. Ideally, there should also be a standing order allowing for real‐time management of the patient with uncertain nutritional intake. For example, when a patient's premeal assessment reveals that she may not tolerate the meal, the patient should be allowed to attempt to eat, and then the nutritional insulin should be given after the meal, in proportion to the amount of food that was eaten. This type of order will require significant nursing education and process redesign in many hospitals, but is essential for matching nutritional insulin to actual intake.
Add Correction Insulin
There is no convincing evidence for the benefit of correction (sliding‐scale) insulin in the inpatient setting, although a randomized trial demonstrating the superiority of basal/nutritional insulin regimens to sliding‐scale only regimens did incorporate a correction insulin scale as an adjunct to the superior basal/nutritional regimen.4 The SHM GCTF again emphasizes that control of hyperglycemia should be proactive and anticipatory of insulin needs, rather than reactive to hyperglycemia. Nonetheless, unexpected hyperglycemic excursions are common, and the use of correction insulin remains a pervasive and arguably logical practice. If correction insulin is used, it should be ordered as a separate step after considering basal and nutrition insulin needs. The doses of scheduled insulin should be adjusted regularly if correction insulin is consistently being required. Ideally, the prescriber should choose a preformatted corrective insulin scale, based on the patient's insulin sensitivity (Appendix 2). There should be a prompt to use the same type of insulin that is being used for nutritional insulin, and there should be instructions that this insulin is given in addition to the basal and nutritional insulin to correct for hyperglycemia. Nocturnal correction‐dose scales should be reduced in the eating patient.
Even after limiting insulin regimens to those in Table 2, multidisciplinary glycemic control teams are still left with several options within these SHM‐preferred regimens. We recommend that your team choose a single, institutionally‐preferred basal‐nutritional‐correction insulin combination for each situation.
Choosing one preferred option for these situations is advantageous because:
-
You can communicate preferred regimens more simply and succinctly to all staff.
-
You eliminate all inappropriate choices for insulin regimens for that situation, as well as some other less preferred, but acceptable choices.
-
You can encourage regimens that are most economical (by promoting the insulin regimens that reflect your hospital formulary choices).
-
Staff members can become very familiar with a few regimens, instead of being confused by a multitude of them. They can identify variations from your preferred choices and target these patients for extra scrutiny and actions should they fail to meet glycemic targets.
Although virtually every institution can provide specific guidance on insulin management in a protocol, there are tradeoffs inherent in how restrictive you can be in pushing these preferred choices in your order sets. Should you eliminate alternate basal or nutritional insulin choices from your order sets? As you integrate more and more of your preferred algorithm and regimens into your order set, you will gain incremental improvement in the standardization of inpatient insulin management. However, you reduce not only variability in ordering, but also the choices available to your prescribers and patients, and in effect you are pushing the providers to use an insulin regimen that often differs from the patient's outpatient regimen. If your institution is not yet ready to go with a single preferred insulin, simply listing your preferred insulin first with the annotation preferred can be enough to increase the use of the preferred insulin.
We endorse building the most protocol‐driven, proscriptive, insulin order set that the Glycemic Control Steering Committee believes their medical staff will accept. There are some caveats to this endorsement. First, there must be extra efforts on the backend of the admission, to ensure that the antihyperglycemic regimen is tailored to the unique needs of the patient (this is discussed further below). Second, a protocol‐driven approach is not a substitute for a good educational program for health care providers or well‐informed clinical judgment. Education should reinforce major concepts driving the protocol and should also highlight exceptions to the rule. Variance from the protocol endorsed choices should be allowed (and even encouraged) when the variance is driven by patient factors (as opposed to provider whim). Learning from this variance is a key concept in refining protocols. Education ideally should not be limited to only protocol‐endorsed choices, as staff should be familiar with the full range of antihyperglycemia regimens seen in inpatient and outpatient settings.
Special Situations
Most of the preferred regimens for different situations are outlined in Table 2 in a straightforward manner, and can be depicted in your protocols and order sets in the same way. Some conditions have enough complexity, however, that you will have difficulty placing all of the details into your one‐page protocol and order set. Details should be placed on your more detailed protocol, and educational programs should include the topics outlined below. Although insulin infusion is often the option that would provide the most reliable and expedient control of hyperglycemia in these special situations, it is an option not available in many noncritical care settings. Therefore, the discussion is limited to subcutaneous insulin control regimens.
Patient on Continuous Tube Feeding
The SHM GCTF endorses glargine or detemir as the basal insulin of choice for this setting. The nutritional and correction insulin of choice is either an RAA‐I every 4 hours (q4h), or regular insulin every 6 hours (q6h). We endorse this choice because it retains the basal‐nutritional‐correction dose concept, generally allows for continued basal insulin use if the tube feedings become interrupted, and is amenable to building a consistent institutional protocol.
There are some important caveats to this recommendation. First, realize that almost any regimen that provides a stable insulin supply would be acceptable, and many institutions will use glargine or detemir to cover both basal and nutritional needs. The downside to using large boluses of long‐acting insulin in this clinical situation is that any unexpected interruption of the feedings will necessitate prolonged infusions of dextrate 10% solution (D10) to avoid hypoglycemia
Second, because of the glycemic load inherent in tube feedings, maintenance of glycemic control in the setting of enteral feeding may be best managed by providing a higher percentage of the TDD as nutritional insulin. In these cases, ratios of basal to nutritional insulin of 40:60, or even less basal insulin, may be appropriate.
Glucocorticoid Therapy
High‐dose glucocorticoids are strongly associated with increased insulin requirements. The degree of hyperglycemia induced by steroids varies significantly from patient to patient, and the pattern of hyperglycemia will vary depending on the pattern of steroid administration. The general principle to keep in mind is that the hyperglycemia induced by a steroid dose will peak 8‐12 hours after it is given, so insulin regimens to address this should take this effect into account. For example, giving a long‐acting basal insulin like glargine to accommodate the hyperglycemic effect of a steroid bolus given in the morning would be inappropriate because the steroid effect would wane and then disappear overnight, leading to insulin‐induced hypoglycemia. NPH insulin can be ideal in this setting, either by itself, or by layering it on top of an existing regimen.
Another caveat: glucocorticoids exert their predominate effect on insulin sensitivity in muscle (as opposed to the liver), and as a result, have their most notable effect on postprandial glucose. For this reason, the best insulin regimens for this situation may use proportionally less basal insulin and more nutritional insulin. One common regimen calls for keeping the basal insulin dose the same as the preglucocorticoid dose, while escalating the RAA insulin dose at lunch and dinner.
Given the complexities of covering steroid‐induced hyperglycemia and its high prevalence in certain populations (such as transplantation patients and patients undergoing chemotherapy), this would be an excellent area on which to focus expertise. Examples include routine endocrinology consultation, intervention by a special glycemic control team, or incorporating routine glucose monitoring and triggers for initiating insulin infusion into the protocols for chemotherapy and transplantation patients.
Regiment the Management of Hypoglycemia
Hypoglycemia is defined by the ADA as a blood glucose of 70 mg/dL or less, based on the physiologic changes that can occur at this glucose level, even in subjectively asymptomatic patients.3 Protocols for management of hypoglycemia should be linked to your diabetes/hyperglycemia protocols. There are many hypoglycemia protocols available for review in the SHM Glycemic Control Resource Room and Glycemic Control Implementation Guide.10 Some common themes for effective implementation stand out. First, the protocols need to walk the balance between simplicity of use, and the need to provide instructions that will provide guidance in a variety of patient situations. Second, the protocols need to be nurse driven, so that nurses can initiate treatment without waiting for a physician order. Third, education and instruction regarding recognition of risk factors, and avoidance of hypoglycemia are needed to support a successful protocol. Importantly, any hypoglycemic event should lead to a reconsideration of the current anti‐hyperglycemic regimen so that future events can be prevented.
Plan for Discharge and Provide Guidance for the Transition
Your institution should have policies and procedures outlining all the steps needed to complete the important transition out of the hospital. At a minimum, this planning should include adequate education (including a learner assessment), appropriate follow‐up, referral to community resources, and a discharge glycemic control regimen that is tailored to the educational, financial, and motivational profile of a patient. The more your inpatient insulin management is driven by protocol, the more likely it is the patient will be on an inpatient treatment plan that differs from their outpatient regimen; therefore, it is even more important to plan this transition carefully and reliably.
Communicating the accurate hyperglycemia related diagnosis and related problems to the primary care provider is important for good care, perhaps even more so for patients who had hyperglycemia while hospitalized without a prior diagnosis of diabetes. Some centers place a prompt for hyperglycemia related diagnosis in the order set and/or discharge paperwork, to remind the clinician to convey the diagnosis to the primary provider, and to encourage more complete documentation. Improved documentation can also improve the business case for glycemic control, along with other strategies outlined elsewhere in this supplement.13
Transitions in care (including transitions out of the hospital and off of infusion insulin) are discussed in more detail14, 15 elsewhere in this supplement. The principles outlined in these references should be incorporated into your institutional protocol. Briefly, not all patients require or are capable of intensive basal‐bolus regimens upon discharge. The HbA1c can be very valuable in arriving at the optimal outpatient regimen.14 The capacities and preferences of the patient and the context of his or her outpatient care environment (including the preferences of the primary care provider) must be taken into consideration as an outpatient management program is planned.
PULLING IT ALL TOGETHER: MAKE SURE YOUR PROTOCOL/ORDER SET IS EASY TO USE AND WIDELY UTILIZED
When standardizing hospital management of diabetes and hyperglycemia, we recommend building the full protocol first, then crystallizing the protocol into a one‐page summary that can be widely disseminated. The protocol guidance is then incorporated into the order set and nursing medical administration record (MAR). Again, we recommend the most proscriptive and protocol‐driven order set feasible within the constraints of medical staff support. The example order set in Appendix 2 illustrates this approach along with other desirable features:
-
Check‐box simplicity on when to order appropriate glucose monitoring.
-
Prompt for the proper hyperglycemia‐related diagnosis.
-
Prompts to document diagnosis and to order HbA1c level.
-
Use of encouraged insulin terminology: basal, prandial (or nutritional), and correction. Language is a powerful thing, and just getting staff to use these terms goes a long way toward the more physiologic prescribing of insulin.
-
Statement/reminder of a glycemic goal.
-
Prompts and contact information for appropriate consultation.
-
Elimination of unapproved abbreviations (such as U for units).
-
Stating both generic and brand names of insulin preparations.
-
Important timing cues for administration of insulin.
-
Several correction‐dose scales suitable for different insulin sensitivities. One size does NOT fit all.
-
Incorporation of a simple hypoglycemia protocol into the order set.
-
Insulin dosing guidelines available at the point of care (in this case, on the back of the order set).
Additional nursing‐specific cues (such as an admonition to never mix glargine insulin with other types of insulin) can also be included in the MAR whenever glargine is ordered.
Once you have protocols and order sets to guide providers, you need to assure that they are used for the majority of hyperglycemic patients. Educational programs should introduce your interventions and the rationale for them. In order to make your method the default method of care, your team should survey all preprinted or CPOE insulin order sets of your institution. A review of postoperative, transfer, and admission order sets that all services use may reveal a half‐dozen or more embedded sliding‐scale insulin order sets that should be removed, with prompts to use the standardized insulin order set being placed in their stead.
Computerized order sets present both challenges and opportunities. Wording limitations and the scrolling nature can make concepts less clear, yet there is a capability for incorporating a hierarchical structure that allows for guiding the user through a more algorithmic approach. There is also a capacity to provide assistance with dosing calculations that do not exist in the paper world. Education remains of key importance for both methods.
MONITOR THE USE AND EFFECTIVENESS OF YOUR PROTOCOLS AND ORDER SETS
Creating and implementing protocols, order sets, and other tools is not the end of the journey to improve care. It is important to monitor order set utilization, insulin use patterns, and parameters measuring glycemic control and hypoglycemia, as outlined in more detail in another article in this supplement.16 In addition to summary data every month or so, we recommend daily reports that spur action in near real time. Triggers such as uncontrolled hyperglycemia, markedly elevated HbA1c levels, and nonphysiologic insulin regimens should initiate consultation, extra diabetes education, or referral to a glucose control team. If appropriate consultation is not readily available, the glycemic control steering group should lobby the administration to bolster this capability. Qualitative feedback from the frontline caregivers, as well as this quantitative data, can assist the local glycemic control champions in designing even more effective protocols, order sets, focused educational efforts, and concurrent mitigation of suboptimal care.
CONCLUSION
Diabetes, hyperglycemia, and iatrogenic hypoglycemia are common and important conditions affecting the noncritically ill inpatient. Interventional trials to validate the recommended noncritical care unit glycemic targets are needed. Although there is a growing consensus on best practices to care for these patients, numerous barriers and the complexity of caring for inpatients hamper the reliability of best practice delivery. Institutional protocols and protocol driven subcutaneous insulin orders, when implemented with the strategies outlined here, can be the key to delivering these best practices more reliably.
Appendix
Inpatient glycemic control and hypoglycemia are issues with well deserved increased attention in recent years. Prominent guidelines and technical reviews have been published,13 and a recent, randomized controlled trial demonstrated the superiority of basal bolus insulin regimens compared to sliding‐scale regimens.4 Effective glycemic control for inpatients has remained elusive in most medical centers. Recent reports57 detail clinical inertia and the continued widespread use of sliding‐scale subcutaneous insulin regimens, as opposed to the anticipatory, physiologic basal‐nutrition‐correction dose insulin regimens endorsed by these reviews.
Inpatient glycemic control faces a number of barriers, including fears of inducing hypoglycemia, uneven knowledge and training among staff, and competing institutional and patient priorities. These barriers occur in the background of an inherently complex inpatient environment that poses unique challenges in maintaining safe glycemic control. Patients frequently move across a variety of care teams and geographic locations during a single inpatient stay, giving rise to multiple opportunities for failed communication, incomplete handoffs, and inconsistent treatment. In addition, insulin requirements may change dramatically due to variations in the stress of illness, exposure to medications that effect glucose levels, and varied forms of nutritional intake with frequent interruption. Although insulin is recognized as one of the medications most likely to be associated with adverse events in the hospital, many hospitals do not have protocols or order sets in place to standardize its use.
A Call to Action consensus conference,8, 9 hosted by the American Association of Clinical Endocrinologists (AACE) and the American Diabetes Association (ADA), brought together many thought leaders and organizations, including representation from the Society of Hospital Medicine (SHM), to address these barriers and to outline components necessary for successful implementation of a program to improve inpatient glycemic control in the face of these difficulties. Institutional insulin management protocols and standardized insulin order sets (supported by appropriate educational efforts) were identified as key interventions. It may be tempting to quickly deploy a generic insulin order set in an effort to improve care. This often results in mediocre results, due to inadequate incorporation of standardization and guidance into the order set and other documentation tools, and uneven use of the order set.
The SHM Glycemic Control Task Force (GCTF) recommends the following steps for developing and implementing successful protocols and order sets addressing the needs of the noncritical care inpatient with diabetes/hyperglycemia.
-
Form a steering committee for this work, and assess the current processes of care.
-
Identify best practices and preferred regimens to manage diabetes and hyperglycemia in the hospital.
-
Integrate best practices and preferred institutional choices into an inpatient glycemic control protocol. Crystallize your protocol into a one page summary.
-
Place guidance from your protocol into the flow of work, by integrating it into standardized subcutaneous insulin order sets and other documentation and treatment tools.
-
Monitor the use of your order sets and protocol. Intervene actively on nonadherents to your protocol and those with poor glycemic control, and revise your protocol/order sets as needed.
IDENTIFYING AND INCORPORATING KEY CONCEPTS AND BEST PRACTICES
A protocol is a document that endorses specific monitoring and treatment strategies in a given institution. This potentially extensive document should provide guidance for transitions, special situations (like steroids and total parenteral nutrition [TPN]) and should outline preferred insulin regimens for all of the most common nutritional situations. One of the most difficult parts of creating a protocol is the assimilation of all of the important information on which to base decisions. Your protocol and order set will be promoting a set of clinical practices. Fortunately, the current best practice for noncritical care hyperglycemic patients has been summarized by several authoritative sources,13, 811 including references from the SHM Glycemic Task Force published in this supplement.4, 12
Table 1 summarizes the key concepts that should be emphasized in a protocol for subcutaneous insulin management in the hospital. We recommend embedding guidance from your protocol into order sets, the medication administration record, and educational materials. Although the details contained in a protocol and order set might vary from one institution to another, the key concepts should not. The remainder of this article provides practical information about how these concepts and guidance for how preferred insulin regimens should be included in these tools. Appendices 1 and 2 give examples of an institutional one‐page summary protocol and subcutaneous insulin order set, respectively.
| 1. Establish a target range for blood glucose levels. |
| 2. Standardize monitoring of glucose levels and assessment of long‐term control (HbA1c). |
| 3. Incorporate nutritional management. |
| 4. Prompt clinicians to consider discontinuing oral antihyperglycemic medications. |
| 5. Prescribe physiologic (basal‐nutrition‐correction) insulin regimens. |
| a. Choose a total daily dose (TDD). |
| b. Divide the TDD into physiologic components of insulin therapy and provide basal and nutritional/correction separately. |
| c. Choose and dose a basal insulin. |
| d. Choose and dose a nutritional (prandial) insulin |
|
i. Match exactly to nutritional intake (see Table 2). |
| ii. Include standing orders to allow nurses to hold nutritional insulin for nutritional interruptions and to modify nutritional insulin depending on the actual nutritional intake. |
| e. Add correction insulin |
| i. Match to an estimate of the patients insulin sensitivity using prefabricated scales. |
| ii. Use the same insulin as nutritional insulin. |
| 6. Miscellaneous |
| a. Manage hypoglycemia in a standardized fashion and adjust regimen to prevent recurrences. |
| b. Provide diabetes education and appropriate consultation. |
| c. Coordinate glucose testing, nutrition delivery, and insulin administration. |
| d. Tailor discharge treatment regimens to the patient's individual circumstances and arrange for proper follow‐up. |
Standardize the Monitoring of Blood Glucose Values and Glucosylated Hemoglobin
Guidance for the coordination of glucose testing, nutrition delivery, and insulin administration, should be integrated into your protocols, and order sets. For noncritical care areas, the minimal frequency for blood glucose monitoring for patients who are eating is before meals and at bedtime. For the patient designated nothing by mouth (NPO) or the patient on continuous tube feeding, the type of nutritional/correction insulin used should drive the minimum frequency (every 4‐6 hours if rapid acting analog insulins [RAA‐I] are used, and every 6 hours if regular insulin is used). Directions for administering scheduled RAA‐I immediately before or immediately after nutrition delivery should be incorporated into protocols, order sets, and medication administration records. Unfortunately, having this guidance in the order sets and protocols does not automatically translate into its being carried out in the real world. Wide variability in the coordination of glucose monitoring, nutritional delivery, and insulin administration is common, so monitoring the process to make sure the protocol is followed is important.
Obtaining a glucosylated hemoglobin (HbA1c) level is important in gauging how well the patient's outpatient regimen is maintaining glycemic control, distinguishing stress hyperglycemia from established diabetes, and guiding the inpatient approach to glycemic control. ADA guidelines2, 3 endorse obtaining HbA1c levels of inpatients if these levels are not already available from the month prior to admission.
Establish a Target Range for Blood Glucose in NonCritical Care Areas
It is important to adopt a glycemic target that is institution‐wide, for critical care areas and noncritical care areas alike. Your glycemic target need not be identical to the ADA/AACE glycemic targets, but should be similar to them.
Examples of institutional glycemic targets for noncritical care areas:
-
Preprandial target 90‐130 mg/dL, maximum random glucose 180 mg/dL (ADA/AACE consensus target)
-
90‐150 mg/dL (a target used in some hospitals)
-
Preprandial target 90‐130 mg/dL for most patients, 100‐150 mg/dL if there are hypoglycemia risk factors, and 180 mg/dL if comfort‐care or end‐of‐life care (a more refined target, allowing for customization based on patient characteristics).
Your multidisciplinary glycemic control steering committee should pick the glycemic target it can most successfully implement and disseminate. It is fine to start with a conservative target and then ratchet down the goals as the environment becomes more accepting of the concept of tighter control of blood glucose in the hospital.
Although the choice of glycemic target is somewhat arbitrary, establishing an institutional glycemic target is critical to motivate clinical action. Your committee should design interventions, for instances when a patient's glycemic target is consistently not being met, including an assignment of responsibility.
Prompt Clinicians to Consider Discontinuing Oral Agents
Oral antihyperglycemic agents, in general, are difficult to quickly titrate to effect, and have side effects that limit their use in the hospital. In contrast, insulin acts rapidly and can be used in virtually all patients and clinical situations, making it the treatment of choice for treatment of hyperglycemia in the hospital.3, 11, 12 In certain circumstances, it may be entirely appropriate to continue a well‐controlled patient on his or her prior outpatient oral regimen. It is often also reasonable to resume oral agents in some patients when preparing for hospital discharge.
Incorporate Nutritional Management
Because diet is so integral to the management of diabetes and hyperglycemia, diet orders should be embedded in all diabetes or insulin‐related order sets. Diets with the same amount of carbohydrate with each meal should be the default rule for patients with diabetes. Nutritionist consultation should be considered and easy to access for patients with malnutrition, obesity, and other common conditions of the inpatient with diabetes.
Access Diabetes Education and Appropriate Consultation
Diabetes education should be offered to all hyperglycemic patients with normal mental status, complete with written materials, a listing of community resources, and survival skills. Consultation with physicians in internal medicine or endocrinology for difficult‐to‐control cases, or for cases in which the primary physician of record is not familiar with (or not adherent to) principles of inpatient glycemic management, should be very easy to obtain, or perhaps mandated, depending on your institution‐specific environment.
Prescribe Physiologic (Basal‐Nutritional‐Correction Dose) Insulin Regimens
Physiologic insulin use is the backbone of the recommended best practice for diabetes and hyperglycemia management in the hospital. The principles of such regimens are summarized elsewhere in this supplement.12 These principles will not be reiterated in detail here, but the major concepts that should be integrated into the protocols and order sets will be highlighted.
Choose a Total Daily Dose
Clinicians need guidance on how much subcutaneous insulin they should give a patient. These doses are well known from clinical experience and the published literature. The fear of hypoglycemia usually results in substantial underdosing of insulin, or total avoidance of scheduled insulin on admission. Your team should provide guidance for how much insulin to start a patient on when it is unclear from past experience how much insulin the patient needs. Waiting a few days to see how much insulin is required via sliding‐scale‐only regimens is a bad practice that should be discouraged for patients whose glucose values are substantially above the glycemic target. The total daily dose (TDD) can be estimated in several different ways (as demonstrated in Appendix 1 and 2), and protocols should make this step very clear for clinicians. Providing a specific location on the order set to declare the TDD may help ensure this step gets done more reliably. Some institutions with computer physician order entry (CPOE) provide assistance with calculating the TDD and the allocation of basal and nutritional components, based on data the ordering physician inputs into the system.
Select and Dose a Basal Insulin
Your protocol should describe how the TDD should be divided between basal and nutritional insulin. We generally recommend 50% of the TDD be given as basal insulin, with the other 50% administered on a scheduled basis to cover glycemic excursions from nutritional intake. The 50/50 rule is simple and generally works well, and should be widely promoted. However, there are exceptions to this rule that should be incorporated into your full protocol and educational programs. The order set should have separate steps for ordering basal insulin, nutritional insulin, and correction insulin. The advantage to providing these insulin components separately is that it allows them to be independently manipulated (eg, if a patient is unable to tolerate a meal, nutritional insulin is held, but basal insulin and correction insulin are continued).
The SHM GCTF specifically endorses long acting insulin (glargine and detemir) as the preferred basal insulin in the hospital setting, thus discouraging the use of neutral protamine Hagedorn (NPH) insulin and fixed combination insulin formulations (Table 2). In the absence of randomized controlled trials demonstrating superiority of the glargine or detemir to NPH insulin in the hospital, this endorsement deserves some further explanation. Although we believe that correctly dosed NPH containing insulin regimens can attain effective and safe glycemic control in the hospital setting, it is more difficult to standardize their use and adjust for fluctuations in nutritional intake. Glargine and detemir have much less pronounced spikes in their effect than NPH, rendering them relatively peakless in comparison. This pharmacokinetic profile allows for continued dosing with minimal or no correction when nutrition intake is variable, and allow for consistent reinforcement of the basal‐nutritional‐correction insulin concept.
| Nutritional situation | Necessary insulin components | Preferred regimen* |
|---|---|---|
| ||
| NPO (or clear liquids) | Basal insulin: 50% of TDD. Nutritional insulin: None. | Basal insulin: glargine given once daily or detemir given twice daily. Nutritional insulin: None. Correctional insulin: Regular insulin q 6 hours or RAA insulin q 4 hours. Other comments: Dextrose infusion (e.g., D5 containing solution at 75‐150 cc/hour) recommended when nutrition is held. An IV insulin infusion is preferred for management of prolonged fasts or fasting type 1 diabetes patients. |
| Eating meals | Basal insulin: 50% of TDD. Nutritional insulin: 50% of TDD, divided equally before each meal. | Basal insulin: glargine given once daily or detemir given twice daily. Nutritional insulin: RAA insulin with meals. Correctional insulin: RAA insulin q AC and HS (reduced dose at HS). |
| Bolus tube feeds | Basal insulin: 40% of TDD. Nutritional insulin: 60% of the TDD, divided equally before each bolus feed. | Basal insulin: glargine given once daily or detemir given twice daily. Nutritional insulin: RAA insulin with each bolus. Correctional insulin: RAA insulin with each bolus. |
| Continuous tube feeds | Basal insulin: 40% (conservative) of TDD. Nutritional insulin: 60% of the TDD in divided doses. | Basal insulin: glargine given once daily or detemir given twice daily. Nutritional insulin: RAA insulin q 4 hours or regular insulin q 6 hours. Correctional insulin: Should match nutritional insulin choice. |
| Parenteral nutrition | Insulin is usually given parenterally, with the nutrition | Initially, a separate insulin drip allows for accurate dose‐finding. Then, 80% of amount determined as TDD using drip is added to subsequent TPN bags as regular insulin. Use correctional subcutaneous insulin doses cautiously, in addition. |
There are some caveats to this general recommendation. First, patients who are well controlled on home regimens with NPH basal insulin can (and sometimes should) stay on the regimen that has worked well for them. However, extra vigilance in reducing the dose for reductions in nutrition is required, because NPH is generally used to cover both nutritional and basal requirements. Second, extensive experience with glargine and detemir are not available in obstetric populations. They are not U.S. Food and Drug Administration (FDA) approved for use in pregnant patients and formally carry a Class C rating, whereas NPH insulin has been used safely in obstetric populations for decades. Third, the insulin regimen used as an inpatient is not necessarily the preferred regimen to prescribe at discharge: cost, patient preferences, HbA1c level, and other factors should be considered in making this choice.
Select and Dose a Nutritional (Prandial) Insulin
The step for ordering nutritional insulin should assist the clinician in matching the insulin to the type of nutrition that the patient is receiving. For example, rapid‐acting insulin analogs are preferred over regular insulin in the eating patient, in view of their more physiologic profile, which averts the insulin stacking that can occur with regular insulin. If regular insulin is used as the preferred institutional choice for eating patients, the lunchtime dose should be reduced or eliminated altogether, to eliminate insulin stacking.
Table 2 outlines the SHM GCTF preferred regimens for different nutritional situations.
There should be a standing order for nutritional insulin to be held when nutrition is interrupted, whether intentional or unintentional. Patients with interrupted tube feedings could have standing orders for a dextrose infusion to replace the tube feeding carbohydrate load and prevent hypoglycemia. Ideally, there should also be a standing order allowing for real‐time management of the patient with uncertain nutritional intake. For example, when a patient's premeal assessment reveals that she may not tolerate the meal, the patient should be allowed to attempt to eat, and then the nutritional insulin should be given after the meal, in proportion to the amount of food that was eaten. This type of order will require significant nursing education and process redesign in many hospitals, but is essential for matching nutritional insulin to actual intake.
Add Correction Insulin
There is no convincing evidence for the benefit of correction (sliding‐scale) insulin in the inpatient setting, although a randomized trial demonstrating the superiority of basal/nutritional insulin regimens to sliding‐scale only regimens did incorporate a correction insulin scale as an adjunct to the superior basal/nutritional regimen.4 The SHM GCTF again emphasizes that control of hyperglycemia should be proactive and anticipatory of insulin needs, rather than reactive to hyperglycemia. Nonetheless, unexpected hyperglycemic excursions are common, and the use of correction insulin remains a pervasive and arguably logical practice. If correction insulin is used, it should be ordered as a separate step after considering basal and nutrition insulin needs. The doses of scheduled insulin should be adjusted regularly if correction insulin is consistently being required. Ideally, the prescriber should choose a preformatted corrective insulin scale, based on the patient's insulin sensitivity (Appendix 2). There should be a prompt to use the same type of insulin that is being used for nutritional insulin, and there should be instructions that this insulin is given in addition to the basal and nutritional insulin to correct for hyperglycemia. Nocturnal correction‐dose scales should be reduced in the eating patient.
Even after limiting insulin regimens to those in Table 2, multidisciplinary glycemic control teams are still left with several options within these SHM‐preferred regimens. We recommend that your team choose a single, institutionally‐preferred basal‐nutritional‐correction insulin combination for each situation.
Choosing one preferred option for these situations is advantageous because:
-
You can communicate preferred regimens more simply and succinctly to all staff.
-
You eliminate all inappropriate choices for insulin regimens for that situation, as well as some other less preferred, but acceptable choices.
-
You can encourage regimens that are most economical (by promoting the insulin regimens that reflect your hospital formulary choices).
-
Staff members can become very familiar with a few regimens, instead of being confused by a multitude of them. They can identify variations from your preferred choices and target these patients for extra scrutiny and actions should they fail to meet glycemic targets.
Although virtually every institution can provide specific guidance on insulin management in a protocol, there are tradeoffs inherent in how restrictive you can be in pushing these preferred choices in your order sets. Should you eliminate alternate basal or nutritional insulin choices from your order sets? As you integrate more and more of your preferred algorithm and regimens into your order set, you will gain incremental improvement in the standardization of inpatient insulin management. However, you reduce not only variability in ordering, but also the choices available to your prescribers and patients, and in effect you are pushing the providers to use an insulin regimen that often differs from the patient's outpatient regimen. If your institution is not yet ready to go with a single preferred insulin, simply listing your preferred insulin first with the annotation preferred can be enough to increase the use of the preferred insulin.
We endorse building the most protocol‐driven, proscriptive, insulin order set that the Glycemic Control Steering Committee believes their medical staff will accept. There are some caveats to this endorsement. First, there must be extra efforts on the backend of the admission, to ensure that the antihyperglycemic regimen is tailored to the unique needs of the patient (this is discussed further below). Second, a protocol‐driven approach is not a substitute for a good educational program for health care providers or well‐informed clinical judgment. Education should reinforce major concepts driving the protocol and should also highlight exceptions to the rule. Variance from the protocol endorsed choices should be allowed (and even encouraged) when the variance is driven by patient factors (as opposed to provider whim). Learning from this variance is a key concept in refining protocols. Education ideally should not be limited to only protocol‐endorsed choices, as staff should be familiar with the full range of antihyperglycemia regimens seen in inpatient and outpatient settings.
Special Situations
Most of the preferred regimens for different situations are outlined in Table 2 in a straightforward manner, and can be depicted in your protocols and order sets in the same way. Some conditions have enough complexity, however, that you will have difficulty placing all of the details into your one‐page protocol and order set. Details should be placed on your more detailed protocol, and educational programs should include the topics outlined below. Although insulin infusion is often the option that would provide the most reliable and expedient control of hyperglycemia in these special situations, it is an option not available in many noncritical care settings. Therefore, the discussion is limited to subcutaneous insulin control regimens.
Patient on Continuous Tube Feeding
The SHM GCTF endorses glargine or detemir as the basal insulin of choice for this setting. The nutritional and correction insulin of choice is either an RAA‐I every 4 hours (q4h), or regular insulin every 6 hours (q6h). We endorse this choice because it retains the basal‐nutritional‐correction dose concept, generally allows for continued basal insulin use if the tube feedings become interrupted, and is amenable to building a consistent institutional protocol.
There are some important caveats to this recommendation. First, realize that almost any regimen that provides a stable insulin supply would be acceptable, and many institutions will use glargine or detemir to cover both basal and nutritional needs. The downside to using large boluses of long‐acting insulin in this clinical situation is that any unexpected interruption of the feedings will necessitate prolonged infusions of dextrate 10% solution (D10) to avoid hypoglycemia
Second, because of the glycemic load inherent in tube feedings, maintenance of glycemic control in the setting of enteral feeding may be best managed by providing a higher percentage of the TDD as nutritional insulin. In these cases, ratios of basal to nutritional insulin of 40:60, or even less basal insulin, may be appropriate.
Glucocorticoid Therapy
High‐dose glucocorticoids are strongly associated with increased insulin requirements. The degree of hyperglycemia induced by steroids varies significantly from patient to patient, and the pattern of hyperglycemia will vary depending on the pattern of steroid administration. The general principle to keep in mind is that the hyperglycemia induced by a steroid dose will peak 8‐12 hours after it is given, so insulin regimens to address this should take this effect into account. For example, giving a long‐acting basal insulin like glargine to accommodate the hyperglycemic effect of a steroid bolus given in the morning would be inappropriate because the steroid effect would wane and then disappear overnight, leading to insulin‐induced hypoglycemia. NPH insulin can be ideal in this setting, either by itself, or by layering it on top of an existing regimen.
Another caveat: glucocorticoids exert their predominate effect on insulin sensitivity in muscle (as opposed to the liver), and as a result, have their most notable effect on postprandial glucose. For this reason, the best insulin regimens for this situation may use proportionally less basal insulin and more nutritional insulin. One common regimen calls for keeping the basal insulin dose the same as the preglucocorticoid dose, while escalating the RAA insulin dose at lunch and dinner.
Given the complexities of covering steroid‐induced hyperglycemia and its high prevalence in certain populations (such as transplantation patients and patients undergoing chemotherapy), this would be an excellent area on which to focus expertise. Examples include routine endocrinology consultation, intervention by a special glycemic control team, or incorporating routine glucose monitoring and triggers for initiating insulin infusion into the protocols for chemotherapy and transplantation patients.
Regiment the Management of Hypoglycemia
Hypoglycemia is defined by the ADA as a blood glucose of 70 mg/dL or less, based on the physiologic changes that can occur at this glucose level, even in subjectively asymptomatic patients.3 Protocols for management of hypoglycemia should be linked to your diabetes/hyperglycemia protocols. There are many hypoglycemia protocols available for review in the SHM Glycemic Control Resource Room and Glycemic Control Implementation Guide.10 Some common themes for effective implementation stand out. First, the protocols need to walk the balance between simplicity of use, and the need to provide instructions that will provide guidance in a variety of patient situations. Second, the protocols need to be nurse driven, so that nurses can initiate treatment without waiting for a physician order. Third, education and instruction regarding recognition of risk factors, and avoidance of hypoglycemia are needed to support a successful protocol. Importantly, any hypoglycemic event should lead to a reconsideration of the current anti‐hyperglycemic regimen so that future events can be prevented.
Plan for Discharge and Provide Guidance for the Transition
Your institution should have policies and procedures outlining all the steps needed to complete the important transition out of the hospital. At a minimum, this planning should include adequate education (including a learner assessment), appropriate follow‐up, referral to community resources, and a discharge glycemic control regimen that is tailored to the educational, financial, and motivational profile of a patient. The more your inpatient insulin management is driven by protocol, the more likely it is the patient will be on an inpatient treatment plan that differs from their outpatient regimen; therefore, it is even more important to plan this transition carefully and reliably.
Communicating the accurate hyperglycemia related diagnosis and related problems to the primary care provider is important for good care, perhaps even more so for patients who had hyperglycemia while hospitalized without a prior diagnosis of diabetes. Some centers place a prompt for hyperglycemia related diagnosis in the order set and/or discharge paperwork, to remind the clinician to convey the diagnosis to the primary provider, and to encourage more complete documentation. Improved documentation can also improve the business case for glycemic control, along with other strategies outlined elsewhere in this supplement.13
Transitions in care (including transitions out of the hospital and off of infusion insulin) are discussed in more detail14, 15 elsewhere in this supplement. The principles outlined in these references should be incorporated into your institutional protocol. Briefly, not all patients require or are capable of intensive basal‐bolus regimens upon discharge. The HbA1c can be very valuable in arriving at the optimal outpatient regimen.14 The capacities and preferences of the patient and the context of his or her outpatient care environment (including the preferences of the primary care provider) must be taken into consideration as an outpatient management program is planned.
PULLING IT ALL TOGETHER: MAKE SURE YOUR PROTOCOL/ORDER SET IS EASY TO USE AND WIDELY UTILIZED
When standardizing hospital management of diabetes and hyperglycemia, we recommend building the full protocol first, then crystallizing the protocol into a one‐page summary that can be widely disseminated. The protocol guidance is then incorporated into the order set and nursing medical administration record (MAR). Again, we recommend the most proscriptive and protocol‐driven order set feasible within the constraints of medical staff support. The example order set in Appendix 2 illustrates this approach along with other desirable features:
-
Check‐box simplicity on when to order appropriate glucose monitoring.
-
Prompt for the proper hyperglycemia‐related diagnosis.
-
Prompts to document diagnosis and to order HbA1c level.
-
Use of encouraged insulin terminology: basal, prandial (or nutritional), and correction. Language is a powerful thing, and just getting staff to use these terms goes a long way toward the more physiologic prescribing of insulin.
-
Statement/reminder of a glycemic goal.
-
Prompts and contact information for appropriate consultation.
-
Elimination of unapproved abbreviations (such as U for units).
-
Stating both generic and brand names of insulin preparations.
-
Important timing cues for administration of insulin.
-
Several correction‐dose scales suitable for different insulin sensitivities. One size does NOT fit all.
-
Incorporation of a simple hypoglycemia protocol into the order set.
-
Insulin dosing guidelines available at the point of care (in this case, on the back of the order set).
Additional nursing‐specific cues (such as an admonition to never mix glargine insulin with other types of insulin) can also be included in the MAR whenever glargine is ordered.
Once you have protocols and order sets to guide providers, you need to assure that they are used for the majority of hyperglycemic patients. Educational programs should introduce your interventions and the rationale for them. In order to make your method the default method of care, your team should survey all preprinted or CPOE insulin order sets of your institution. A review of postoperative, transfer, and admission order sets that all services use may reveal a half‐dozen or more embedded sliding‐scale insulin order sets that should be removed, with prompts to use the standardized insulin order set being placed in their stead.
Computerized order sets present both challenges and opportunities. Wording limitations and the scrolling nature can make concepts less clear, yet there is a capability for incorporating a hierarchical structure that allows for guiding the user through a more algorithmic approach. There is also a capacity to provide assistance with dosing calculations that do not exist in the paper world. Education remains of key importance for both methods.
MONITOR THE USE AND EFFECTIVENESS OF YOUR PROTOCOLS AND ORDER SETS
Creating and implementing protocols, order sets, and other tools is not the end of the journey to improve care. It is important to monitor order set utilization, insulin use patterns, and parameters measuring glycemic control and hypoglycemia, as outlined in more detail in another article in this supplement.16 In addition to summary data every month or so, we recommend daily reports that spur action in near real time. Triggers such as uncontrolled hyperglycemia, markedly elevated HbA1c levels, and nonphysiologic insulin regimens should initiate consultation, extra diabetes education, or referral to a glucose control team. If appropriate consultation is not readily available, the glycemic control steering group should lobby the administration to bolster this capability. Qualitative feedback from the frontline caregivers, as well as this quantitative data, can assist the local glycemic control champions in designing even more effective protocols, order sets, focused educational efforts, and concurrent mitigation of suboptimal care.
CONCLUSION
Diabetes, hyperglycemia, and iatrogenic hypoglycemia are common and important conditions affecting the noncritically ill inpatient. Interventional trials to validate the recommended noncritical care unit glycemic targets are needed. Although there is a growing consensus on best practices to care for these patients, numerous barriers and the complexity of caring for inpatients hamper the reliability of best practice delivery. Institutional protocols and protocol driven subcutaneous insulin orders, when implemented with the strategies outlined here, can be the key to delivering these best practices more reliably.
Appendix
- American College of Endocrinology Task Force on Inpatient Diabetes and Metabolic Control.American College of Endocrinology Position Statement on Inpatient Diabetes and Metabolic Control.Endocr Pract.2004;10:77–82.
- ,,,,,,.Management of diabetes and hyperglycemia in hospitals.Diabetes Care.2004;27:553–591.
- American Diabetes Association.Standards of Medical Carein Diabetes‐2006.Diabetes Care.2006;29(suppl 1):s4–s42.
- ,,, et al.Randomized study of basal‐bolus insulin therapy in the inpatient management of patients with type 2 diabetes (RABBIT 2 Trial).Diabetes Care.2007;30:2181–2186.
- ,,,,.Inpatient management of diabetes and hyperglycemia among general medicine patients at a large teaching hospital.J Hosp Med.2006;1:145–150.
- ,,, et al.Diabetes care in the hospital: is there clinical inertia?J Hosp Med.2006;1:151–160.
- ,,, et al.Diabetes care in hospitalized non‐critically ill patients: more evidence for clinical inertia and negative therapeutic momentum.JHosp Med.2007;2:203–211.
- Inpatient Diabetes and Glycemic Control: A Call to Action Conference. Position Statement. AACE, February2006. Available at: http://www.aace.com/meetings/consensus/IIDC/IDGC0207.pdf. Accessed October, 2006.
- Proceedings of the American College of Endocrinology and American Diabetes Association Consensus Conference, Washington, DC, January 30–31, 2006. Endocr Pract.2006; 12(suppl 3):3–13.
- Society of Hospital Medicine Glycemic Control Task Force. Implementation Guide: Improving Glycemic Control, Preventing Hypoglycemia, and Optimizing Care of the Inpatient with Hyperglycemia and Diabetes. Published January 2007 on the Society of Hospital Medicine website. Available at: http://www.hospitalmedicine.org. Accessed August,2007.
- .Management of hyperglycemia in the hospital setting.N Engl J Med.2006;355:1903–1911.
- ,,,,.Management of diabetes and hyperglycemia in the hospital: a practical guide to subcutaneous insulin use in the non‐critically ill adult patient.J Hosp Med.2008;3(5):S17–S28.
- ,.Practical strategies for developing the business case for hospital glycemic control teams.J Hosp Med2008;3(5):S76–S83.
- ,,,.Bridge over troubled waters: safe and effective transitions of the inpatient with hyperglycemia.J Hosp Med.2008;3(5):S55–S65.
- ,,,.Designing and implementing insulin infusion protocols and order sets.J Hosp Med.2008;3(5):S42–S54.
- ,,,,,.SHM Glycemic Control Task Force summary: practical recommendations for assessing the impact of glycemic control efforts.J Hosp Med.2008;3(5):S66–S75.
- American College of Endocrinology Task Force on Inpatient Diabetes and Metabolic Control.American College of Endocrinology Position Statement on Inpatient Diabetes and Metabolic Control.Endocr Pract.2004;10:77–82.
- ,,,,,,.Management of diabetes and hyperglycemia in hospitals.Diabetes Care.2004;27:553–591.
- American Diabetes Association.Standards of Medical Carein Diabetes‐2006.Diabetes Care.2006;29(suppl 1):s4–s42.
- ,,, et al.Randomized study of basal‐bolus insulin therapy in the inpatient management of patients with type 2 diabetes (RABBIT 2 Trial).Diabetes Care.2007;30:2181–2186.
- ,,,,.Inpatient management of diabetes and hyperglycemia among general medicine patients at a large teaching hospital.J Hosp Med.2006;1:145–150.
- ,,, et al.Diabetes care in the hospital: is there clinical inertia?J Hosp Med.2006;1:151–160.
- ,,, et al.Diabetes care in hospitalized non‐critically ill patients: more evidence for clinical inertia and negative therapeutic momentum.JHosp Med.2007;2:203–211.
- Inpatient Diabetes and Glycemic Control: A Call to Action Conference. Position Statement. AACE, February2006. Available at: http://www.aace.com/meetings/consensus/IIDC/IDGC0207.pdf. Accessed October, 2006.
- Proceedings of the American College of Endocrinology and American Diabetes Association Consensus Conference, Washington, DC, January 30–31, 2006. Endocr Pract.2006; 12(suppl 3):3–13.
- Society of Hospital Medicine Glycemic Control Task Force. Implementation Guide: Improving Glycemic Control, Preventing Hypoglycemia, and Optimizing Care of the Inpatient with Hyperglycemia and Diabetes. Published January 2007 on the Society of Hospital Medicine website. Available at: http://www.hospitalmedicine.org. Accessed August,2007.
- .Management of hyperglycemia in the hospital setting.N Engl J Med.2006;355:1903–1911.
- ,,,,.Management of diabetes and hyperglycemia in the hospital: a practical guide to subcutaneous insulin use in the non‐critically ill adult patient.J Hosp Med.2008;3(5):S17–S28.
- ,.Practical strategies for developing the business case for hospital glycemic control teams.J Hosp Med2008;3(5):S76–S83.
- ,,,.Bridge over troubled waters: safe and effective transitions of the inpatient with hyperglycemia.J Hosp Med.2008;3(5):S55–S65.
- ,,,.Designing and implementing insulin infusion protocols and order sets.J Hosp Med.2008;3(5):S42–S54.
- ,,,,,.SHM Glycemic Control Task Force summary: practical recommendations for assessing the impact of glycemic control efforts.J Hosp Med.2008;3(5):S66–S75.
Transitions in Inpatient Hyperglycemia
Professional and patient safety organizations have recognized the importance of safe transitions as patients move through the health care system, and such attention is even more critical when attempting to achieve glycemic control.14 Since the publication of the Diabetes Control and Complications Trial (DCCT)5 and the United Kingdom Prospective Diabetes Study (UKPDS),6 we have known that intensive glycemic control in the ambulatory setting prevents complications in both type 1 and type 2 diabetes mellitus (DM). Despite the increased risk of hypoglycemia, these trials changed practice patterns in the outpatient settings in favor of intensification of diabetes therapy. In the same way, randomized, prospective trials using intravenous (IV) insulin therapy have revolutionized our thinking about inpatient care by showing that tight glycemic control in the critically ill7 and patients with acute myocardial infarction8 reduces mortality and morbidity. These, as well as additional observational studies associating hyperglycemia with poor outcomes in a variety of medical and surgical patients,915 have led to increased attention on glycemic control in all venues of care.16, 17 Concerns over excessive hypoglycemia and a nonsignificant increase in mortality in certain populations of medical intensive care unit (ICU) patients have raised questions over whether the initial studies can be reproduced or generalized to other groups of inpatients.18, 19 Additional studies are underway to clarify these questions but consensus exists that blood glucose values should at least be less than 180 mg/dL and that the traditional practice of ignoring hyperglycemia is no longer acceptable.
While a uniform focus on glycemic control will allow our patients to receive a consistent message about diabetes, the unique limitations inherent to each practice setting requires different therapeutic regimens and intentional focus on the risks as patients transition from one care area to another. This work addresses several areas of care transition that are particularly important in safely achieving glycemic control including: transition into the hospital for patients on a variety of home regimens, transitions within the hospital (related to changes in dietary intake, change from IV to subcutaneous [SC] therapy, and the perioperative setting), and the transition from the hospital to home or another healthcare facility.
TRANSITION INTO THE HOSPITAL
Until recently, most patients with diabetes admitted to the hospital were managed with sliding‐scale‐only regimens.20, 21 Unfortunately, this led to a variety of complications, including hyperglycemia, hypoglycemia, iatrogenic ketoacidosis, and an inconsistent message to patients on the importance of glycemic control.22 Some outpatient clinicians and patients combated this tradition by creating in‐hospital glucose control plans with orders, which patients would bring with them to the hospital.23 This practice continues to be a helpful way to guide inpatient therapy and is encouraged when available. Glycemic‐controlrelated documents from outpatient clinicians should include the most recent glycosylated hemoglobin (HbA1c) value, diagnosis and known complications, current names and doses of medications, and other patient‐specific preferences or needs (eg, compliance, financial, fear of needles). If the last HbA1c was performed more than 30 days before admission or is not available, one should be obtained upon hospital admission to help guide discharge therapy.24 By knowing the HbA1c, one can determine the level of diabetic control achieved with the current regimen and can help the inpatient team (clinician and patient) determine if a more aggressive glycemic control regimen is necessary at the time of discharge. It is important to note that if the patient has received a transfusion of red blood cells prior to HbA1c measurement or has a hemoglobinopathy, the HbA1c value may not be accurate.25, 26
In general, the outpatient regimen will need to be modified at admission to achieve the appropriate flexibility needed for the changing nutritional intake and insulin requirements that invariably accompany hospitalization. Sulfonylureas and dipeptidyl peptidase 4 inhibitors (DPP4), such as sitagliptin, have most of their effect immediately, but the other oral antihyperglycemic agents have a relatively long delay between treatment and effect, thus they are not a flexible enough method to achieve glycemic control in the hospital. Additionally, inpatients may have transient contraindications to their prior oral antihyperglycemic medications. Metformin is almost always on hold in the hospital setting, at least initially, due to concerns about lactic acidosis. Sulfonylureas can cause hypoglycemia in the setting of worsening renal function or reduced oral intake. Thiazoladinediones (TZDs) are often withheld due to concerns about fluid retention and should be avoided in patients admitted with heart failure. There is little experience in the hospital with the use of newer agents like exenatide, pramlintide, glinides, and DPP4 inhibitors.
Overall, it is generally recommended that oral antihyperglycemic agents be discontinued upon hospital admission and replaced with insulin infusions or scheduled SC insulin. An estimate of 0.4 to 0.5 units/kg of body weight provides a conservative starting point for the total daily dose of insulin (TDD) for most patients. This TDD should then be divided into basal and nutritional components to match the patients' caloric intake. Additional correction doses of insulin should be prescribed to cover episodes of hyperglycemia that develop despite the provision of anticipatory‐physiologic insulin. Further discussion of insulin dosing and SC regimens is available in detail elsewhere.27, 28 The recommendation for these insulin‐only regimens is made regardless of the glycemic control in the outpatient setting and is not meant to imply that they should be continued at discharge. In fact, most patients will return to their home regimen or to one that is intensified but less labor intensive than the basal‐nutritional‐correction insulin used in the hospital. The antihyperglycemic regimen planned for discharge should be anticipated as early as possible and clearly communicated to the patient and/or caregivers to allow for optimal education.
Outpatient insulin regimens that have a high percentage of basal insulin need to be modified during hospital admission to avoid hypoglycemia that may occur from variable nutritional intake. While hospitalized, the basal portion of the estimated TDD generally should not be more than 50% to 60%. The total number of units of all types of insulin used daily as an outpatient can be used as a starting point for determining the inpatient TDD by a 1:1 conversion. Adjustments up or down based on glycemic control, nutritional intake, and other factors are then necessary. If patients are on regimens with insulin plus oral agents at home, the inpatient TDD should either be the home insulin dose or the dose calculated based on their weight, whichever is greater. Patients who use carbohydrate counting to determine nutritional insulin doses as an outpatient might be continued on this regimen if they have a strong understanding of the methods, they are coherent enough to determine their doses, nursing staff are well educated, and dietary services provides the carbohydrate content for the hospital menu. If patients are on insulin pumps at home, these should be managed according to a uniform hospital policy to assure safety. If conversion to multiple daily injections is needed, the same 1:1 conversion is safe.29
Transitions Within the Hospital
General Issues
Within the hospital itself, there are several transitions that have important quality and safety implications regarding glycemic control. The handoffs between providers should follow a standardized format.4, 30, 31 Essential information will vary depending on the setting but should universally include recent hypoglycemia, insulin type and doses, and hypoglycemic risk factors such as changes in insulin doses, the development of renal insufficiency, inability of the patient to self‐report symptoms, tapering of steroids, and cessation or interruption of nutritional intake.32
One of the greatest risks for hypoglycemia in the hospital comes from the unpredictable nutritional interruptions that occur. Unplanned changes are best handled by nurses having an existing order to hold scheduled nutritional insulin if patients are classified nothing by mouth (NPO) or eat 50% of their meal. Additionally, nursing staff should have orders or policies that allow flexibility in the time of administering scheduled rapid‐acting nutritional insulin so that it may be given during or immediately following the meal in patients at higher risk for poor oral intake. Tube feedings also place patients at high risk for hypoglycemia because the tube may become dislodged or they may begin to have feeding intolerance. For these reasons, a measure of safety would be to have standing orders to substitute IV 10% dextrose in water (D10W) at the same rate as the prior tube feeds, hold nutritional insulin, and begin more frequent monitoring whenever tube feeds are stopped.33 Orders that rely on nursing staff to notify a physician when tube feedings are stopped are generally not directive enough because providers may be distracted by other changes or forget the patient is on long‐acting insulin. The need for this flexibility around nutritional dosing emphasizes the importance of avoiding excessive doses of basal insulin. If the total dose of basal insulin is 40% to 50% of the TDD, it can safely be continued at its usual dose despite changing nutritional intake. The only exception is neutral protamine Hagedorn (NPH) insulin, which should be reduced when patients are NPO due to its peak. Generally, a 50% reduction in NPH is recommended for morning doses, but bedtime doses may be given with little to no reduction. Because of the complexity of these issues, standardized order sets are the best way to reliably communicate all the necessary standing orders to nursing staff (Table 1).
|
| Nutritional insulin |
| Hold if patients are NPO or eat less than 50% of their meal. |
| Administer scheduled rapid acting nutritional insulin during or immediately following the meal if oral intake is questionable (ie, nausea, emesis, or newly advancing diet). |
| Tube feedings: When tube feeds are stopped unexpectedly |
| Start dextrose containing IV fluids (many institutions use D10W at the same rate as the prior tube feeds). |
| Hold scheduled nutritional insulin. |
| Notify physician. |
| Basal insulin |
| Continue if NPO. |
| Reduce morning dose of NPH by 50% if NPO and may need to reduce the dose of bedtime NPH. |
| IV to subcutaneous transition |
| Timing for discontinuing IV infusion in relation to first dose of subcutaneous insulin. |
| Prompts for verbal communication between ICU and general ward staff. |
Transitioning the Patient Off of IV Insulin
The strongest evidence for tight glycemic control derives from studies in the surgical ICU.7 Many hospitals have robust, effective IV‐insulin protocols. The frequency of monitoring and rapidity of action of IV insulin allow quick achievement of blood glucose control. As patients begin to eat, the layering of SC nutritional insulin on top of the insulin infusion may reduce the lability of the infusion rate and prevent excursions in glycemic control. When the patient is ready to leave the ICU or start a full oral diet, it is recommended that they transition off of the IV insulin to a basal‐nutritional‐correction regimen.33, 34
The amount of insulin needed with IV infusion is a useful estimate of the TDD of insulin.28, 33, 35, 36 There are important general steps to take when making this transition; but, due to the lack of conclusive data proving the advantage of one regimen over another, there are a variety of acceptable specific protocols (Table 2).3739 First, it should be determined if patients are expected to require ongoing scheduled SC insulin or not. Certainly, all patients with type 1 DM will require scheduled SC insulin, but patients with type 2 DM on low insulin infusion rates or some patients with new hyperglycemia can appropriately be managed with sliding‐scale alone. Next, the average hourly rate of the infusion over the preceding 6 to 8 hours should be determined because it most accurately reflects current insulin needs during the changing stress, nutrition, and medications in critical care patients. This hourly rate will then be converted to a TDD using a safety factor to anticipate decreasing insulin requirements. Some portion of this daily total will then be assigned to be basal insulin. As patients' clinical conditions approach baseline, so will their insulin requirements, and the dose will need to be revised.24
|
| Step 1: Is patient stable enough for transition? Hypotension, active sepsis, vasopressors, and intubation are contraindications to transition due to unreliable subcutaneous insulin absorption and continued need for the most flexible dosing due to frequently changing insulin requirements. |
| Step 2: Does this patient need a transition to scheduled subcutaneous (SC) insulin? |
| Yes |
| All patients with type 1 DM. |
| Type 2 DM patients on insulin as outpatient. |
| Type 2 DM patients with a recent mean infusion rate of 0.5 units/hour.* |
| No |
| Type 2 DM patients with infusion rate 0.5 units/hour.* |
| Stress hyperglycemia or previously unrecognized DM if infusion rate 1 unit/hour, or if HbA1c near normal. |
| Some institutions exclude all stress hyperglycemia patients from transition to a SC insulin regimen, regardless of drip rate. |
| Step 3: If transition is needed, calculate a total daily dose (TDD) of insulin. The TDD is an estimate of the 24‐hour insulin requirement when the patient is receiving full nutrition. |
| Determine mean insulin infusion rate from last 6 to 8 hours. |
| Calculate 24‐hour insulin dose based on this, and reduce this 24‐hour dose by some safety factor. There are several options for this step. |
| Multiply hourly rate by 24, then multiply by 0.7 or 0.8 to arrive at a safety‐adjusted 24 hour insulin dose. |
| OR |
| Multiply hourly infusion rate by 20 (80% of 24). |
| Determine if this total is the TDD or basal dose based on current nutrition. There are several options for this step for you or your institution to choose. |
| If infusion was serving basal AND nutritional needs of patient (such as a patient on 24‐hour tube feedings) this will be your TDD. |
| OR |
| If the infusion insulin was not covering significant nutrition, this could be the BASAL insulin dose. |
| Step 4: Construct a regimen tailored to the patient's nutritional situation, building in safeguards for any changes in nutritional intake and uncertainties about reliability of intake. Several options are again available. |
| Basal: should be ordered as basal glargine or detemir (these are preferred by SHM GCTF but NPH is also an option). |
| Dose is 40% to 50% of TDD. |
| OR |
| Adjusted 24‐hour IV requirement given all as basal. |
| Nutritional: The remainder of the TDD is scheduled nutritional insulin in divided doses. In general, these doses need to be adjusted down for 100% nutritional intake and the orders should allow for administering nutritional insulin just AFTER observed meals to allow an assessment of intake. There are several options for estimating the initial doses: |
| Use 50% of the TDD as nutritional coverage and divide this amount by 3 to determine the scheduled meal dose. Hold if they do not eat more than 50% of their meal. |
| Use a more conservative start of 10% to 20% of the basal dose scheduled with each meal. |
| Use carbohydrate counting to cover nutritional intake. |
| Step 5: Be sure to give SC insulin BEFORE the infusion stops |
| Basal glargine or detemir are ideally given at least 2 hours before infusion is discontinued. |
| Shorter lead times (30 minutes) are possible if rapid acting insulin is given with basal insulin. |
SC insulin should be given before the drip is discontinued to allow an overlap that takes into consideration the onset of action. The first dose of basal insulin should be given 2 hours before the insulin infusion is discontinued.24, 40 However, because this is not always feasible, (ie, the patient needs to leave the ICU sooner), another option is to turn off the drip and give 10% of the basal dose as rapid acting insulin along with the basal dose.39 The timing of subsequent doses will depend on the specific basal insulin that is ordered as well as institutional consideration of usual care delivery and nursing workflow. Given that there are several options to achieve this important overlap between IV and SC insulin, it is best for a multidisciplinary team to choose some preferred way that is the institutional standard. Having a standard allows targeted education and tracking of adherence to best practices.
Because conversion to SC insulin is a complex task and the opportunity may arise while physicians are busy with other clinical priorities, there are several options to assure that the necessary steps take place. Some institutions may build a protocol for this transition on paper or computerized order entry, build cues and dosing charts into order sets, and/or develop nursing documentation and nursing process to influence physician and nurse behavior. This critical juncture is also a good place to focus expertise with a glycemic control team, pharmacist, specially trained nurses, or some other dedicated team to take over this transition for all patients.36 The complexity and aggressiveness of the specific institutional protocol used will depend on the confidence and experience of those individuals responsible for determining the transition doses.
The transition from IV to SC insulin often coincides with a change in patient location, (ie, from the ICU to general medical ward). It is imperative that appropriate communication occurs between the transferring and receiving nurses and physicians to continue with the care plan for glycemic management. This communication can be encouraged through provider education and automated into the standardized order process.
Perioperative Transitions
Patients undergoing surgery present a special challenge. They are faced with not only the physiologic and mental stress of surgery but also the hazards of multiple handoffs across several care teams, all with different priorities and cultures. As in other areas, standardized protocols specific to this area of transition are important in assuring safe and effective perioperative glycemic control. Procedures should preferably be scheduled for the early morning to have the least impact on insulin dosing. Patients who are admitted only for the procedure will have to manage this transition on their own and need to be given specific instructions along with the general preoperative orders.24, 41 In general, the usual dose of glargine can be given the day prior to the procedure if it is approximately 50% of their TDD. This is an important caution because some outpatient regimens use large doses of glargine, which essentially provide both basal and nutritional coverage. In those patients, the glargine dose should be reduced by 20% to 50% to provide a safety margin. As with any patient who is NPO, the morning dose of NPH should be one‐half of the usual dose, scheduled nutritional insulin should be held, and the usual doses of correction insulin should be reduced. The appropriate preoperative dose adjustments also depend on whether the individual patient is ketosis‐prone and how tight their glycemic control is as an outpatient.
Upon arrival to the hospital or during the time that the inpatient is NPO, dextrose containing IV fluids should be administered to minimize the risk of hypoglycemia and prevent ketosis. Given the risks for wide variation, blood glucose monitoring should occur every 1 to 2 hours before, during, and initially after the procedure. Infusion insulin allows the most rapid titration and reliable delivery (compared with SC infusions or injections) and is therefore the preferred regimen for major surgery requiring prolonged NPO status or prolonged surgery in patients with type 1 diabetes. Basal‐nutritional‐correction SC insulin is preferred in other surgical inpatients because their nutritional intake is variable and the stress of surgery affects insulin requirements.
Oral antihyperglycemic agents should be held around the time of surgery. If patients are on an oral agent that can result in hypoglycemia, (ie, sulfonylurea or other insulin secretagogue), it should be held on the day of the procedure. Metformin must be held for safety concerns, given the possible decrease in renal function around surgery. It should be held beginning on the day of the procedure or the day before in the case of the sustained‐release formulation. It can then be resumed 48 hours postoperation after normal renal function is secured and the patient is discharged home. Alpha‐glucosidase inhibitors should be held whenever patients are NPO because they only work when taken with meals. Thiazoladinediones have a long duration of action and so can be continued or stopped around surgery. Finally, glucagon‐like peptide (GLP‐1) agonists (exenatide) should be held until the patient is eating normally and discharged home due to the high incidence of gastrointestinal side effects.
TRANSITIONING FROM THE HOSPITAL
The final but perhaps most important transition is the one from the hospital. With much attention on glycemic control in the hospital, it will become clear to many clinicians that the outpatient regimen needs to be modified. However, any changes in medications increase the chances of hypoglycemia and the possibility of error. The postdischarge time frame has been poorly studied and was specifically identified by the Association for Clinical Endocrinologists (ACE) and American Diabetes Association (ADA) as an area in need of future research.36
Patients may be discharged to a nursing home, hospice, or home, and numerous factors need to be considered to determine the optimal discharge regimen. Important considerations are the HbA1c at admission, home medications, medication interactions, current medical problems, nutritional status, physical disabilities, frequency of self‐monitoring, hypoglycemic risk factors, contraindications to oral medications, goals of care/life expectancy, and financial and other resources. If there are temporary physical or self‐care limitations, then a visiting nurse may need to be arranged to assure a safe transition home with the optimal therapy. If patients are going to a skilled nursing facility or other acute care hospital, the formulary, processes, and staffing issues of that facility will be additional important considerations in determining whether therapy is the same as in the hospital or more like what it will be at home.
An algorithm for outpatient therapy for type 2 DM was recommended in a consensus statement from the ADA and European Association for the Study of Diabetes.42, 43 This has been modified using additional recommendations from the AACE44 and is depicted in Figure 1. While the delineation of these steps is helpful, it must be emphasized that both the choice of regimen and dose will need to be individualized. Prescribing the ideal frequently falls short if there is no way for the patient to implement the recommendations. Intensive insulin therapy requires training in food intake/emnsulin matching, motivation of the patient and outpatient clinician, 4 times daily self‐monitoring of blood glucose, and considerable expense. Some patients may be temporarily continued on basal‐nutritional‐correction regimens as their insulin requirements are rapidly changing and later converted to regimens that involve less frequent insulin doses, (ie, twice daily premixed insulin or basal insulin with oral agents or oral agents alone).45, 46 Other patients who may be medically appropriate for intensive insulin therapy may first need to gain confidence with more simple insulin regimens. There are numerous additional resources on initiating insulin that the reader is referred to for more detail.4448
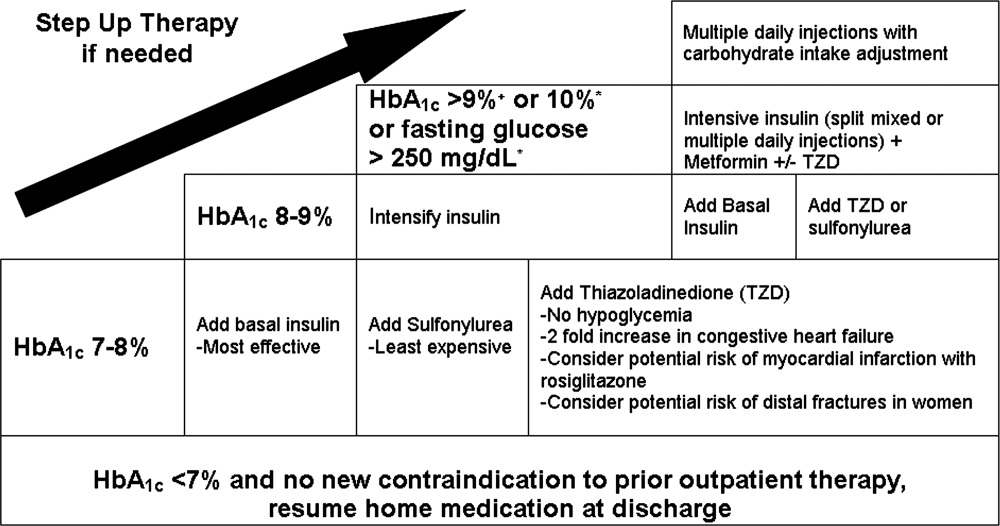
Oral antihyperglycemic drugs are usually held while a patient is admitted to the hospital but once medical conditions are improved, oral intake is established, and renal function stabilized, these drugs can be restarted. If a patient has a new contraindication to metformin or sulfonylureas but does not need insulin, a TZD or DPP4 inhibitor should be considered. Elderly patients and those with renal or liver disease are at increased risk for developing hypoglycemia.49, 50 Glyburide should be avoided, and doses of other sulfonylureas may need to be adjusted. Other options that may be considered in this situation include sitagliptin and exenatide.51 When patients will be discharged on oral diabetic medications alone, discontinue the basal insulin 12 to 24 hours before and the scheduled nutritional insulin at the same time oral agents are restarted. Sulfonylureas, metformin, DPP4 inhibitors, and exenatide will have most of their effect in the first day, but TZDs have a delayed onset and may not be a good bridge for immediate control at discharge.
If patients are going to be discharged on basal insulin in addition to oral agents, several options exist for determining the dose. Because of the risk of hypoglycemia after discharge, it is advised to either reduce the doses of oral agents or choose more conservative insulin starting doses.52 One possibility is to discontinue the nutritional and correction doses, continue the hospital dose of basal insulin, and restart the oral antidiabetes medications. If the dose of basal insulin was more than 50% of the TDD of insulin, it may need to be reduced. A more conservative option for patients at a higher risk of hypoglycemia is to start 0.2 units/kg or 10 units of NPH, glargine, or detemir at bedtime (Figure 2). Once discharged, blood glucose should be measured 1 to 4 times a day and the basal dose titrated by several different validated methods.53, 54 Appropriate orders for necessary supplies for insulin therapy include a meter with test strips, lancets, syringes, needles, and glucagon kit.55
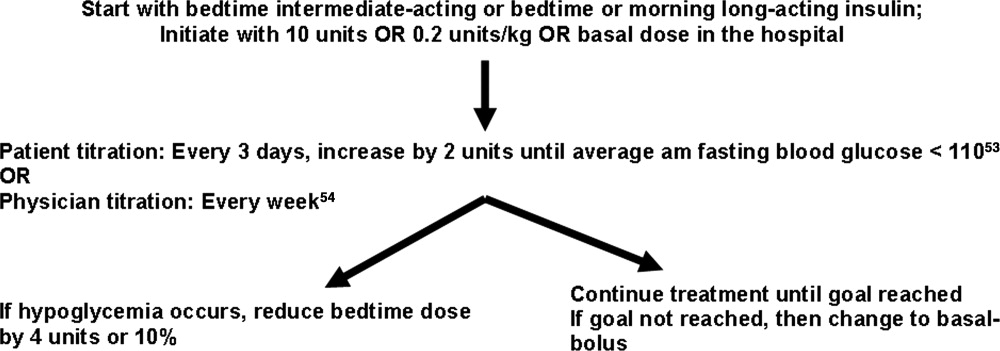
With a large number of patients with diabetes remaining undiagnosed, it is important to use the information available during hospitalization to identify previously unrecognized diabetes or prediabetes.24 Because there are no unique criteria for the diagnosis of DM in the stressed state, patients may have a presumptive diagnosis made in the hospital and/or follow‐up testing with fasting glucose or an oral glucose tolerance test. No ADA diagnostic thresholds for the HbA1c currently exist, but it can be a useful marker in making this distinction.56 Among patients with new hyperglycemia, an HbA1c of 6% or greater was 100% specific for predicting a future diagnosis of diabetes in the small prospective cohort study by Greci et al.,57 but many endocrinologists use a cutoff of 7%. For all hyperglycemic patients, lifestyle interventions that promote weight loss and increased activity levels should be encouraged. New hyperglycemia should be clearly identified as a diagnosis in discharge communication.
There are many barriers to diabetes self‐management education in the inpatient setting but there are also numerous resources and opportunities. New information will be available regarding patients' understanding of their disease and glycemic control and there may be plans for changes in the home medication regimen. Most of the focus of inpatient education sessions is on survival skills such as taking medications, performing blood glucose monitoring, basic meal planning, identification and treatment of hypoglycemia, sick‐day management, how to access further diabetes education as an outpatient, and when to call the healthcare team.58 The most effective way to accomplish all of this is to identify the discharge regimen early and include nurses and staff in a plan to educate all patients. An inpatient diabetes educator can provide additional help with newly‐diagnosed or uncontrolled patients. Dividing the material over the hospitalization makes it less overwhelming for patients, reinforces previously taught concepts, spreads the responsibility to more providers, and offers it in conjunction with the correlating clinical care. Throughout their hospital stay, patients can begin to practice new skills, including blood glucose monitoring and logbook use, drawing up and administering insulin, sharps disposal, basic diabetic diet information, and sick‐day management. The specific topics addressed in each session can be tracked as part of an interdisciplinary education record that allows coordination among the individuals involved in teaching.59 It is important to give patients the basics, support them with minimal written information, and provide them appropriate follow‐up diabetes education.60 Furthermore, the inpatient team should view the patient's glycemic control education as something that needs to continue across the continuum of care and develop communication strategies that connect with the follow‐up clinical team.
At the time of discharge, it is essential that written documentation and communication with outpatient care providers be completed.61, 62 The more standardized the inpatient insulin regimens are, the more likely the patient is to be on a much different glycemic control regimen than the one on admission; therefore, it is even more important to assure that the admission medication list is accurate and reconciled completely with the modified list at discharge. Discharge check lists and tools for assessing patient acceptance of the discharge plan help with this process.63 Follow‐up with the primary care physician should occur within 7 to 14 days if patients are new to insulin, had medication changes, or are elderly. An increased likelihood of keeping posthospitalization appointments with a diabetes specialty clinic has been associated with being discharged on insulin, a new diagnosis of diabetes, and direct referral.64 Additional attention should be paid to barriers to follow‐up, including lack of health insurance, prior difficulty with follow‐up, and transportation problems.65
SUMMARY
A variety of factors have contributed to difficulty in achieving inpatient and outpatient glucose control. These include care complexity, the lack of standardized protocols, limited knowledge about glucose control, and clinical inertia. Inpatient clinicians have a tendency toward keeping patients on their home regimen in hopes that they might test its effectiveness. Furthermore, there has been the notion of why optimize the glycemic regimen of inpatients because their diabetic needs will change in the outpatient setting. However, because the insulin requirements during acute illness are different and nutritional intake is variable, nearly all inpatients should be placed on multiple daily doses of scheduled insulin or IV insulin to allow the necessary flexibility for rapid titration and abrupt changes in nutrition. This intensive regimen is only appropriate for a minority of outpatients. This difference illustrates that a regimen that works perfectly in one clinical setting will not necessarily be optimal in the next. The patient's outpatient treatment regimen should be reassessed based on HbA1c, self‐monitoring prior to admission, and new contraindications based on medical issues. If a change is indicated and the inpatient physician is motivated, there are numerous helpful resources to aid in addressing all the necessary factors surrounding intensification of therapy.
Despite requiring different glycemic control regimens, the information gained from the needs in each setting guide the next, making communication and planning paramount. Important transitions that must be given attention are: (1) admission to the hospital; (2) in‐hospital transitions, including the perioperative period and IV‐to‐SC insulin; and (3) the hospital to outpatient transition. The complexity of such frequent transitions requires planning, education, and clear communication that are best handled with a systems approach and the development of standardized protocols and order sets. Hospitalists, endocrinologists, and other members of the healthcare team should take an aggressive role in developing systems and facilitating optimal transitions to maximize glycemic control. Further studies are needed to determine the best practices among the variety of options discussed in this article.
- ACE/ADA Task Force on Inpatient Diabetes.American College of Endocrinology and American Diabetes Association Consensus Statement on Inpatient Diabetes and Glycemic Control.Endocr Pract.2006;12:458–468.
- American Board of Internal Medicine Foundation Stepping Up to the Plate Alliance. Available at: http://www.abimfoundation.org/quality/suttp.shtm. Accessed November2007.
- National Transitions of Care Coalition. Available at: http://www.ntocc.org. Accessed November2007.
- JCAHO 2008 National Patient Safety Goals. Availableat: http://www.jointcommission.org/PatientSafety/NationalPatientSafetyGoals/08_hap_npsgs.htm. Accessed November2007.
- Diabetes Control and Complications Trial Research Group.The effect of intensive treatment of diabetes on the development and progression of long‐term complications in insulin‐dependent diabetes mellitus.N Engl J Med.1993;329:977–986.
- UK Prospective Diabetes Study (UKPDS) Group.Intensive blood‐glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes: UK Prospective Diabetes Study (UKPDS) Group.Lancet.1998;352:837–853.
- ,,, et al.,Intensive insulin therapy in critically ill patients.N Engl J Med.2001;345:1359–1367.
- ,,,.Glycometabolic state at admission: important risk marker of mortality in conventionally treated patients with diabetes mellitus and acute myocardial infarction: long‐term results from the Diabetes and Insulin‐Glucose Infusion in Acute Myocardial Infarction (DIGAMI) study.Circulation.1999;99:2626–2632.
- ,,,,.Relationship of early hyperglycemia to mortality in trauma patients.J Trauma.2004;56:1058–1062.
- ,,,,.Early peri‐operative glycaemic control and allograft rejection in patients with diabetes mellitus: a pilot study.Transplantation.2001;72:1321–1324.
- ,,, et al.Relation between the duration of remission and hyperglycemia in induction chemotherapy for acute lymphocytic leukemia with a hyperfractionated cyclophosphamide, vincristine, doxorubicin and dexamethasone/methotrexate cytarabine regimen.Cancer.2004;100:1179–1185.
- ,,,,.Stress hyperglycemia and prognosis of stroke in nondiabetic and diabetic patients: a systematic overview.Stroke.2001;32:2426–2432.
- ,,,,,.Diabetes and outcome of community‐acquired pneumococcal bacteriemia.Diabetes Care.2004;27:70–76.
- ,,.Early postoperative glucose control predicts nosocomial infection rate in diabetic patients.JPEN J Parenter Enteral Nutr.1998;22:77–81.
- ,,,,.Early post‐operative glucose levels are an independent risk factor for infection after peripheral vascular surgery. A retrospective study.Eur J Vasc Endovasc Surg.2004;28:520–525.
- American Diabetes Association.Standards of medical care in diabetes, 2006.Diabetes Care.2006;29(suppl 1):s4–s42.
- American College of Endocrinology Task Force on Inpatient Diabetes and Metabolic Control.American College of Endocrinology Position Statement on Inpatient Diabetes and Metabolic Control.Endocr Pract.2004;10:77–82.
- ,,, et al.Intensive insulin therapy in the medical ICU.N Engl J Med.2006;354:449–461
- ,,, et al.Intensive insulin therapy and pentastarch resuscitation in severe sepsis.N Engl J Med.2008;358(2):125–139.
- ,,,,.Inpatient management of diabetes and hyperglycemia among general medicine patients at a large teaching hospital.J Hosp Med.2006;1:145–150.
- ,,, et al.,Diabetes care in hospitalized noncritically ill patients: More evidence for clinical inertia and negative therapeutic momentum.J Hosp Med.2007;2:203–211.
- ,,.Glycemic control and sliding scale insulin use in medical inpatients with diabetes mellitus.Arch Intern Med.1997;157:545–552.
- ,.Hospital management of hyperglycemia.Clin Diabetes.2004;22:81–88.
- ,,, et al.Management of diabetes and hyperglycemia in hospitals. [Erratum appears in Diabetes Care. 2005; 28: 1990. Dosage error in text].Diabetes Care.2004;27:553–591.
- ,.Hemoglobinopathies and HbA(1c) measurement.Diabetes Care.2000;23(8):1197–1198.
- ,,,,,.Evaluation of HbA1c determination methods in patients with hemoglobinopathiesDiabetes Care.2000;23(3):339–344.
- ,,,.Subcutaneous insulin order sets and protocols: effective design and implementation strategies.J Hosp Med.2008;3.
- ,,,,.Management of diabetes and hyperglycemia in the hospital: a practical guide to subcutaneous insulin use in the non‐critically ill, adult patient.J Hosp Med.2008;3.PMID:8675920.
- ,,,.Switch to multiple daily injections with insulin glargine and insulin lispro from continuous subcutaneous insulin infusion with insulin lispro: a randomized, open‐label study using a continuous glucose monitoring system.Endocr Pract.2005;11:157–164.
- SBAR technique for communication: a situational briefing model. Available at: http://www.ihi.org/IHI/Topics/PatientSafety/SafetyGeneral/Tools/SBARTechniqueforCommunicationASituationalBriefingModel.htm. Accessed December2007.
- . Promising quality improvement initiatives: reports from the field. AHRQ Summit—Improving Health Care Quality for All Americans: Celebrating Success, Measuring Progress, Moving Forward 2004. Available at: http://www.ahrq.gov/qual/qsummit/qsummit4.htm#sentara. Accessed December2007.
- ,,, et al.Hospital hypoglycemia: not only treatment but also prevention.Endocr Pract.2004;10(suppl 2):89–99.
- American Association of Clinical Endocrinologists Inpatient Glycemic Control Resource Center. Available at: http://resources.aace.com/PDF/Section_05‐Final‐Inpatient_Non‐ICU/Hyperglycemia_Non‐ICU_Protocols/Transition_from_ Intravenous_to_Subcutaneous_Insulin.PDF. Accessed November2007.
- Recommendations for safe use of insulin in hospitals. American Society of Health System Pharmacists and the Hospital and Health System Association of Pennsylvania. 2005. Available at: http://www.premierinc.com/safety/safety‐share/01–06‐downloads/01‐safe‐use‐insulin‐ashp.pdf. Accessed December2007.
- ,,, for the Society of Hospital Medicine Glycemic Control Taskforce. Glycemic control resource room: improving reliability of care across transitions and in the perioperative setting. Available at: http://www.hospitalmedicine.org/ResourceRoomRedesign/html/07Layer_Inter/06_Transitions.cfm. Accessed August2008.
- ACE/ADA Task Force on Inpatient Diabetes American College of Endocrinology and American Diabetes Association Consensus Statement on Inpatient Diabetes and Glycemic Control: a call to action.Diabetes Care.2006;29:1955–1962.
- ,,, et al.Conversion of intravenous insulin infusions to subcutaneously administered insulin glargine in patients with hyperglycemia.Endocr Pract.2006;12:641–650.
- ,,,.Intravenous insulin infusion therapy: indications, methods, and transition to subcutaneous insulin therapy.Endocr Pract.2004;10(suppl 2):71–80.
- ,,, et al.Inpatient management of hyperglycemia: the northwestern experience.Endocr Pract.2006;12(5):491–505.
- American Diabetes Association.Position statement: standards of medical care in diabetes‐2007.Diabetes Care.2007;30(suppl 1):S4–S41.
- American Association of Clinical Endocrinologists Inpatient Glycemic Control Resource Center. Available at: http://resources.aace.com/PDF/Section_05‐Final‐Inpatient_Non‐ICU/Hyperglycemia_Non‐ICU_Protocols/Pre‐Operative_Instructions_for_Patients_with_Diabetes.PDF Accessed November2007.
- ,,, et al.Management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement from the American Diabetes Association and the European Association for the Study of Diabetes.Diabetes Care.2006;29:1963–1972.
- ,,, et al.Management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: update regarding thiazoladinediones.Diabetes Care.2008;31:173–175.
- American Association of Clinical Endocrinologists Inpatient Glycemic Control Resource Center. Challenges in Effective Discharge Planning for Hospitalized Patients with Diabetes. Available at: http://resources.aace.com/PDF/Section_07‐Final‐Transition‐Inpatient_to_Outpatient/Challenges_in_Effective_Discharge_for_Diabetes_Patients.PPT. Accessed December2007.
- ,,.Initiating insulin therapy in type 2 diabetes: a comparison of biphasic and basal insulin analogs.Diabetes Care.2005;28:260–265.
- ,,, et al.Addition of biphasic, prandial, or basal insulin to oral therapy in type 2 diabetes.N Engl J Med.2007;357:1716–1730.
- ,,.Narrative review: a rational approach to starting insulin therapy.Ann Intern Med.2006;145:125–134.
- ,,,,.A real‐world approach to insulin therapy in primary care practice.Clin Diabetes.2005;23:78–86.
- ,,,.Individual sulfonylureas and serious hypoglycemia in older persons.J Am Geriatr Soc.1996;44:751–755.
- ,,,.Incidence and risk factors for serious hypoglycemia in older persons using insulin or sulfonylureas.Arch Intern Med.1997;157(15):1681–1686.
- ,,, et al.Exenatide versus insulin glargine in patients with suboptimally controlled type 2 diabetes: a randomized trial.Ann Intern Med.2005;143:559–569.
- .The transition from insulin infusions to long‐term diabetes therapy: the argument for insulin analogs.Semin Thorac Cardiovasc Surg.2006;18:366–378.
- ,,,,.ATLANTUS Study Group. Improvement of glycemic control in subjects with poorly controlled type 2 diabetes.Diabetes Care.2005;28:1282–1288.
- ,,.Investigators Insulin Glargine 4002 Study. The Treat‐to Target trial: randomized addition of glargine or human NPH insulin to oral therapy of type 2 diabetes patients.Diabetes Care.2003;26:3080–3086.
- American Association of Clinical Endocrinologists Inpatient Glycemic Control Resource Center. Available at: http://resources.aace.com/PDF/Section_07‐Final‐Transition‐Inpatient_to_Outpatient/Effective_Discharge_Planning‐Sample_Discharge_Plans/Inpatient_Diabetes_Discharge_Prescription.PDF. Accessed November2007.
- American Diabetes Association.Diagnosis and classification of diabetes mellitus.Diabetes Care.2007;30(suppl):S42–S47.
- ,,, et al.Utility of HbA1c levels for diabetes case finding in hospitalized patients with hyperglycemia.Diabetes Care.2003;26:1064–1068.
- ,,, et al.National standards for diabetes self‐management education.Diabetes Care.2006;29(suppl 1):S78–S85.
- Society of Hospital Medicine Glycemic Control Task Force. Workbook for improvement: improving glycemic control, preventing hypoglycemia and optimizing care of the inpatient with diabetes and hyperglycemia. page 105. Available at: http://www.hospitalmedicine.org/ResourceRoomRedesign/pdf/GC_Workbook.pdf. Accessed December,2007.
- Joslin Diabetes Center. EZ Start Patient Information Handouts. Available at: http://www.joslin.org/ezstart. Accessed December2007.
- ,,,.Promoting effective transitions of care at hospital discharge: a review of key issues for hospitalists.J Hosp Med.2007;2:314–323.
- ,,,,,.Deficits in communication and information transfer between hospital‐based and primary care physicians: implications for patient safety and continuity of care.JAMA.2007;297:831–841.
- Society of Hospital Medicine On‐line Clinical Tools. Ideal discharge for the elderly patient: a hospitalist checklist. Available at: http://www.hospitalmedicine.org/AM/Template.cfm?Section=QI_Clinical_Toolsemplate=/CM/ContentDisplay.cfmContentID=10303. Accessed December2007.
- ,,, et al.Inpatient to outpatient transfer of care in urban patients with diabetes: patterns and determinants of immediate post‐discharge follow‐up.Arch Intern Med.2004;164:447–453.
- ,,,,,.Inpatient to outpatient transfer of diabetes care: perceptions of barriers to postdischarge follow‐up in urban African American patients.Ethn Dis.2007;17:238–243.
Professional and patient safety organizations have recognized the importance of safe transitions as patients move through the health care system, and such attention is even more critical when attempting to achieve glycemic control.14 Since the publication of the Diabetes Control and Complications Trial (DCCT)5 and the United Kingdom Prospective Diabetes Study (UKPDS),6 we have known that intensive glycemic control in the ambulatory setting prevents complications in both type 1 and type 2 diabetes mellitus (DM). Despite the increased risk of hypoglycemia, these trials changed practice patterns in the outpatient settings in favor of intensification of diabetes therapy. In the same way, randomized, prospective trials using intravenous (IV) insulin therapy have revolutionized our thinking about inpatient care by showing that tight glycemic control in the critically ill7 and patients with acute myocardial infarction8 reduces mortality and morbidity. These, as well as additional observational studies associating hyperglycemia with poor outcomes in a variety of medical and surgical patients,915 have led to increased attention on glycemic control in all venues of care.16, 17 Concerns over excessive hypoglycemia and a nonsignificant increase in mortality in certain populations of medical intensive care unit (ICU) patients have raised questions over whether the initial studies can be reproduced or generalized to other groups of inpatients.18, 19 Additional studies are underway to clarify these questions but consensus exists that blood glucose values should at least be less than 180 mg/dL and that the traditional practice of ignoring hyperglycemia is no longer acceptable.
While a uniform focus on glycemic control will allow our patients to receive a consistent message about diabetes, the unique limitations inherent to each practice setting requires different therapeutic regimens and intentional focus on the risks as patients transition from one care area to another. This work addresses several areas of care transition that are particularly important in safely achieving glycemic control including: transition into the hospital for patients on a variety of home regimens, transitions within the hospital (related to changes in dietary intake, change from IV to subcutaneous [SC] therapy, and the perioperative setting), and the transition from the hospital to home or another healthcare facility.
TRANSITION INTO THE HOSPITAL
Until recently, most patients with diabetes admitted to the hospital were managed with sliding‐scale‐only regimens.20, 21 Unfortunately, this led to a variety of complications, including hyperglycemia, hypoglycemia, iatrogenic ketoacidosis, and an inconsistent message to patients on the importance of glycemic control.22 Some outpatient clinicians and patients combated this tradition by creating in‐hospital glucose control plans with orders, which patients would bring with them to the hospital.23 This practice continues to be a helpful way to guide inpatient therapy and is encouraged when available. Glycemic‐controlrelated documents from outpatient clinicians should include the most recent glycosylated hemoglobin (HbA1c) value, diagnosis and known complications, current names and doses of medications, and other patient‐specific preferences or needs (eg, compliance, financial, fear of needles). If the last HbA1c was performed more than 30 days before admission or is not available, one should be obtained upon hospital admission to help guide discharge therapy.24 By knowing the HbA1c, one can determine the level of diabetic control achieved with the current regimen and can help the inpatient team (clinician and patient) determine if a more aggressive glycemic control regimen is necessary at the time of discharge. It is important to note that if the patient has received a transfusion of red blood cells prior to HbA1c measurement or has a hemoglobinopathy, the HbA1c value may not be accurate.25, 26
In general, the outpatient regimen will need to be modified at admission to achieve the appropriate flexibility needed for the changing nutritional intake and insulin requirements that invariably accompany hospitalization. Sulfonylureas and dipeptidyl peptidase 4 inhibitors (DPP4), such as sitagliptin, have most of their effect immediately, but the other oral antihyperglycemic agents have a relatively long delay between treatment and effect, thus they are not a flexible enough method to achieve glycemic control in the hospital. Additionally, inpatients may have transient contraindications to their prior oral antihyperglycemic medications. Metformin is almost always on hold in the hospital setting, at least initially, due to concerns about lactic acidosis. Sulfonylureas can cause hypoglycemia in the setting of worsening renal function or reduced oral intake. Thiazoladinediones (TZDs) are often withheld due to concerns about fluid retention and should be avoided in patients admitted with heart failure. There is little experience in the hospital with the use of newer agents like exenatide, pramlintide, glinides, and DPP4 inhibitors.
Overall, it is generally recommended that oral antihyperglycemic agents be discontinued upon hospital admission and replaced with insulin infusions or scheduled SC insulin. An estimate of 0.4 to 0.5 units/kg of body weight provides a conservative starting point for the total daily dose of insulin (TDD) for most patients. This TDD should then be divided into basal and nutritional components to match the patients' caloric intake. Additional correction doses of insulin should be prescribed to cover episodes of hyperglycemia that develop despite the provision of anticipatory‐physiologic insulin. Further discussion of insulin dosing and SC regimens is available in detail elsewhere.27, 28 The recommendation for these insulin‐only regimens is made regardless of the glycemic control in the outpatient setting and is not meant to imply that they should be continued at discharge. In fact, most patients will return to their home regimen or to one that is intensified but less labor intensive than the basal‐nutritional‐correction insulin used in the hospital. The antihyperglycemic regimen planned for discharge should be anticipated as early as possible and clearly communicated to the patient and/or caregivers to allow for optimal education.
Outpatient insulin regimens that have a high percentage of basal insulin need to be modified during hospital admission to avoid hypoglycemia that may occur from variable nutritional intake. While hospitalized, the basal portion of the estimated TDD generally should not be more than 50% to 60%. The total number of units of all types of insulin used daily as an outpatient can be used as a starting point for determining the inpatient TDD by a 1:1 conversion. Adjustments up or down based on glycemic control, nutritional intake, and other factors are then necessary. If patients are on regimens with insulin plus oral agents at home, the inpatient TDD should either be the home insulin dose or the dose calculated based on their weight, whichever is greater. Patients who use carbohydrate counting to determine nutritional insulin doses as an outpatient might be continued on this regimen if they have a strong understanding of the methods, they are coherent enough to determine their doses, nursing staff are well educated, and dietary services provides the carbohydrate content for the hospital menu. If patients are on insulin pumps at home, these should be managed according to a uniform hospital policy to assure safety. If conversion to multiple daily injections is needed, the same 1:1 conversion is safe.29
Transitions Within the Hospital
General Issues
Within the hospital itself, there are several transitions that have important quality and safety implications regarding glycemic control. The handoffs between providers should follow a standardized format.4, 30, 31 Essential information will vary depending on the setting but should universally include recent hypoglycemia, insulin type and doses, and hypoglycemic risk factors such as changes in insulin doses, the development of renal insufficiency, inability of the patient to self‐report symptoms, tapering of steroids, and cessation or interruption of nutritional intake.32
One of the greatest risks for hypoglycemia in the hospital comes from the unpredictable nutritional interruptions that occur. Unplanned changes are best handled by nurses having an existing order to hold scheduled nutritional insulin if patients are classified nothing by mouth (NPO) or eat 50% of their meal. Additionally, nursing staff should have orders or policies that allow flexibility in the time of administering scheduled rapid‐acting nutritional insulin so that it may be given during or immediately following the meal in patients at higher risk for poor oral intake. Tube feedings also place patients at high risk for hypoglycemia because the tube may become dislodged or they may begin to have feeding intolerance. For these reasons, a measure of safety would be to have standing orders to substitute IV 10% dextrose in water (D10W) at the same rate as the prior tube feeds, hold nutritional insulin, and begin more frequent monitoring whenever tube feeds are stopped.33 Orders that rely on nursing staff to notify a physician when tube feedings are stopped are generally not directive enough because providers may be distracted by other changes or forget the patient is on long‐acting insulin. The need for this flexibility around nutritional dosing emphasizes the importance of avoiding excessive doses of basal insulin. If the total dose of basal insulin is 40% to 50% of the TDD, it can safely be continued at its usual dose despite changing nutritional intake. The only exception is neutral protamine Hagedorn (NPH) insulin, which should be reduced when patients are NPO due to its peak. Generally, a 50% reduction in NPH is recommended for morning doses, but bedtime doses may be given with little to no reduction. Because of the complexity of these issues, standardized order sets are the best way to reliably communicate all the necessary standing orders to nursing staff (Table 1).
|
| Nutritional insulin |
| Hold if patients are NPO or eat less than 50% of their meal. |
| Administer scheduled rapid acting nutritional insulin during or immediately following the meal if oral intake is questionable (ie, nausea, emesis, or newly advancing diet). |
| Tube feedings: When tube feeds are stopped unexpectedly |
| Start dextrose containing IV fluids (many institutions use D10W at the same rate as the prior tube feeds). |
| Hold scheduled nutritional insulin. |
| Notify physician. |
| Basal insulin |
| Continue if NPO. |
| Reduce morning dose of NPH by 50% if NPO and may need to reduce the dose of bedtime NPH. |
| IV to subcutaneous transition |
| Timing for discontinuing IV infusion in relation to first dose of subcutaneous insulin. |
| Prompts for verbal communication between ICU and general ward staff. |
Transitioning the Patient Off of IV Insulin
The strongest evidence for tight glycemic control derives from studies in the surgical ICU.7 Many hospitals have robust, effective IV‐insulin protocols. The frequency of monitoring and rapidity of action of IV insulin allow quick achievement of blood glucose control. As patients begin to eat, the layering of SC nutritional insulin on top of the insulin infusion may reduce the lability of the infusion rate and prevent excursions in glycemic control. When the patient is ready to leave the ICU or start a full oral diet, it is recommended that they transition off of the IV insulin to a basal‐nutritional‐correction regimen.33, 34
The amount of insulin needed with IV infusion is a useful estimate of the TDD of insulin.28, 33, 35, 36 There are important general steps to take when making this transition; but, due to the lack of conclusive data proving the advantage of one regimen over another, there are a variety of acceptable specific protocols (Table 2).3739 First, it should be determined if patients are expected to require ongoing scheduled SC insulin or not. Certainly, all patients with type 1 DM will require scheduled SC insulin, but patients with type 2 DM on low insulin infusion rates or some patients with new hyperglycemia can appropriately be managed with sliding‐scale alone. Next, the average hourly rate of the infusion over the preceding 6 to 8 hours should be determined because it most accurately reflects current insulin needs during the changing stress, nutrition, and medications in critical care patients. This hourly rate will then be converted to a TDD using a safety factor to anticipate decreasing insulin requirements. Some portion of this daily total will then be assigned to be basal insulin. As patients' clinical conditions approach baseline, so will their insulin requirements, and the dose will need to be revised.24
|
| Step 1: Is patient stable enough for transition? Hypotension, active sepsis, vasopressors, and intubation are contraindications to transition due to unreliable subcutaneous insulin absorption and continued need for the most flexible dosing due to frequently changing insulin requirements. |
| Step 2: Does this patient need a transition to scheduled subcutaneous (SC) insulin? |
| Yes |
| All patients with type 1 DM. |
| Type 2 DM patients on insulin as outpatient. |
| Type 2 DM patients with a recent mean infusion rate of 0.5 units/hour.* |
| No |
| Type 2 DM patients with infusion rate 0.5 units/hour.* |
| Stress hyperglycemia or previously unrecognized DM if infusion rate 1 unit/hour, or if HbA1c near normal. |
| Some institutions exclude all stress hyperglycemia patients from transition to a SC insulin regimen, regardless of drip rate. |
| Step 3: If transition is needed, calculate a total daily dose (TDD) of insulin. The TDD is an estimate of the 24‐hour insulin requirement when the patient is receiving full nutrition. |
| Determine mean insulin infusion rate from last 6 to 8 hours. |
| Calculate 24‐hour insulin dose based on this, and reduce this 24‐hour dose by some safety factor. There are several options for this step. |
| Multiply hourly rate by 24, then multiply by 0.7 or 0.8 to arrive at a safety‐adjusted 24 hour insulin dose. |
| OR |
| Multiply hourly infusion rate by 20 (80% of 24). |
| Determine if this total is the TDD or basal dose based on current nutrition. There are several options for this step for you or your institution to choose. |
| If infusion was serving basal AND nutritional needs of patient (such as a patient on 24‐hour tube feedings) this will be your TDD. |
| OR |
| If the infusion insulin was not covering significant nutrition, this could be the BASAL insulin dose. |
| Step 4: Construct a regimen tailored to the patient's nutritional situation, building in safeguards for any changes in nutritional intake and uncertainties about reliability of intake. Several options are again available. |
| Basal: should be ordered as basal glargine or detemir (these are preferred by SHM GCTF but NPH is also an option). |
| Dose is 40% to 50% of TDD. |
| OR |
| Adjusted 24‐hour IV requirement given all as basal. |
| Nutritional: The remainder of the TDD is scheduled nutritional insulin in divided doses. In general, these doses need to be adjusted down for 100% nutritional intake and the orders should allow for administering nutritional insulin just AFTER observed meals to allow an assessment of intake. There are several options for estimating the initial doses: |
| Use 50% of the TDD as nutritional coverage and divide this amount by 3 to determine the scheduled meal dose. Hold if they do not eat more than 50% of their meal. |
| Use a more conservative start of 10% to 20% of the basal dose scheduled with each meal. |
| Use carbohydrate counting to cover nutritional intake. |
| Step 5: Be sure to give SC insulin BEFORE the infusion stops |
| Basal glargine or detemir are ideally given at least 2 hours before infusion is discontinued. |
| Shorter lead times (30 minutes) are possible if rapid acting insulin is given with basal insulin. |
SC insulin should be given before the drip is discontinued to allow an overlap that takes into consideration the onset of action. The first dose of basal insulin should be given 2 hours before the insulin infusion is discontinued.24, 40 However, because this is not always feasible, (ie, the patient needs to leave the ICU sooner), another option is to turn off the drip and give 10% of the basal dose as rapid acting insulin along with the basal dose.39 The timing of subsequent doses will depend on the specific basal insulin that is ordered as well as institutional consideration of usual care delivery and nursing workflow. Given that there are several options to achieve this important overlap between IV and SC insulin, it is best for a multidisciplinary team to choose some preferred way that is the institutional standard. Having a standard allows targeted education and tracking of adherence to best practices.
Because conversion to SC insulin is a complex task and the opportunity may arise while physicians are busy with other clinical priorities, there are several options to assure that the necessary steps take place. Some institutions may build a protocol for this transition on paper or computerized order entry, build cues and dosing charts into order sets, and/or develop nursing documentation and nursing process to influence physician and nurse behavior. This critical juncture is also a good place to focus expertise with a glycemic control team, pharmacist, specially trained nurses, or some other dedicated team to take over this transition for all patients.36 The complexity and aggressiveness of the specific institutional protocol used will depend on the confidence and experience of those individuals responsible for determining the transition doses.
The transition from IV to SC insulin often coincides with a change in patient location, (ie, from the ICU to general medical ward). It is imperative that appropriate communication occurs between the transferring and receiving nurses and physicians to continue with the care plan for glycemic management. This communication can be encouraged through provider education and automated into the standardized order process.
Perioperative Transitions
Patients undergoing surgery present a special challenge. They are faced with not only the physiologic and mental stress of surgery but also the hazards of multiple handoffs across several care teams, all with different priorities and cultures. As in other areas, standardized protocols specific to this area of transition are important in assuring safe and effective perioperative glycemic control. Procedures should preferably be scheduled for the early morning to have the least impact on insulin dosing. Patients who are admitted only for the procedure will have to manage this transition on their own and need to be given specific instructions along with the general preoperative orders.24, 41 In general, the usual dose of glargine can be given the day prior to the procedure if it is approximately 50% of their TDD. This is an important caution because some outpatient regimens use large doses of glargine, which essentially provide both basal and nutritional coverage. In those patients, the glargine dose should be reduced by 20% to 50% to provide a safety margin. As with any patient who is NPO, the morning dose of NPH should be one‐half of the usual dose, scheduled nutritional insulin should be held, and the usual doses of correction insulin should be reduced. The appropriate preoperative dose adjustments also depend on whether the individual patient is ketosis‐prone and how tight their glycemic control is as an outpatient.
Upon arrival to the hospital or during the time that the inpatient is NPO, dextrose containing IV fluids should be administered to minimize the risk of hypoglycemia and prevent ketosis. Given the risks for wide variation, blood glucose monitoring should occur every 1 to 2 hours before, during, and initially after the procedure. Infusion insulin allows the most rapid titration and reliable delivery (compared with SC infusions or injections) and is therefore the preferred regimen for major surgery requiring prolonged NPO status or prolonged surgery in patients with type 1 diabetes. Basal‐nutritional‐correction SC insulin is preferred in other surgical inpatients because their nutritional intake is variable and the stress of surgery affects insulin requirements.
Oral antihyperglycemic agents should be held around the time of surgery. If patients are on an oral agent that can result in hypoglycemia, (ie, sulfonylurea or other insulin secretagogue), it should be held on the day of the procedure. Metformin must be held for safety concerns, given the possible decrease in renal function around surgery. It should be held beginning on the day of the procedure or the day before in the case of the sustained‐release formulation. It can then be resumed 48 hours postoperation after normal renal function is secured and the patient is discharged home. Alpha‐glucosidase inhibitors should be held whenever patients are NPO because they only work when taken with meals. Thiazoladinediones have a long duration of action and so can be continued or stopped around surgery. Finally, glucagon‐like peptide (GLP‐1) agonists (exenatide) should be held until the patient is eating normally and discharged home due to the high incidence of gastrointestinal side effects.
TRANSITIONING FROM THE HOSPITAL
The final but perhaps most important transition is the one from the hospital. With much attention on glycemic control in the hospital, it will become clear to many clinicians that the outpatient regimen needs to be modified. However, any changes in medications increase the chances of hypoglycemia and the possibility of error. The postdischarge time frame has been poorly studied and was specifically identified by the Association for Clinical Endocrinologists (ACE) and American Diabetes Association (ADA) as an area in need of future research.36
Patients may be discharged to a nursing home, hospice, or home, and numerous factors need to be considered to determine the optimal discharge regimen. Important considerations are the HbA1c at admission, home medications, medication interactions, current medical problems, nutritional status, physical disabilities, frequency of self‐monitoring, hypoglycemic risk factors, contraindications to oral medications, goals of care/life expectancy, and financial and other resources. If there are temporary physical or self‐care limitations, then a visiting nurse may need to be arranged to assure a safe transition home with the optimal therapy. If patients are going to a skilled nursing facility or other acute care hospital, the formulary, processes, and staffing issues of that facility will be additional important considerations in determining whether therapy is the same as in the hospital or more like what it will be at home.
An algorithm for outpatient therapy for type 2 DM was recommended in a consensus statement from the ADA and European Association for the Study of Diabetes.42, 43 This has been modified using additional recommendations from the AACE44 and is depicted in Figure 1. While the delineation of these steps is helpful, it must be emphasized that both the choice of regimen and dose will need to be individualized. Prescribing the ideal frequently falls short if there is no way for the patient to implement the recommendations. Intensive insulin therapy requires training in food intake/emnsulin matching, motivation of the patient and outpatient clinician, 4 times daily self‐monitoring of blood glucose, and considerable expense. Some patients may be temporarily continued on basal‐nutritional‐correction regimens as their insulin requirements are rapidly changing and later converted to regimens that involve less frequent insulin doses, (ie, twice daily premixed insulin or basal insulin with oral agents or oral agents alone).45, 46 Other patients who may be medically appropriate for intensive insulin therapy may first need to gain confidence with more simple insulin regimens. There are numerous additional resources on initiating insulin that the reader is referred to for more detail.4448

Oral antihyperglycemic drugs are usually held while a patient is admitted to the hospital but once medical conditions are improved, oral intake is established, and renal function stabilized, these drugs can be restarted. If a patient has a new contraindication to metformin or sulfonylureas but does not need insulin, a TZD or DPP4 inhibitor should be considered. Elderly patients and those with renal or liver disease are at increased risk for developing hypoglycemia.49, 50 Glyburide should be avoided, and doses of other sulfonylureas may need to be adjusted. Other options that may be considered in this situation include sitagliptin and exenatide.51 When patients will be discharged on oral diabetic medications alone, discontinue the basal insulin 12 to 24 hours before and the scheduled nutritional insulin at the same time oral agents are restarted. Sulfonylureas, metformin, DPP4 inhibitors, and exenatide will have most of their effect in the first day, but TZDs have a delayed onset and may not be a good bridge for immediate control at discharge.
If patients are going to be discharged on basal insulin in addition to oral agents, several options exist for determining the dose. Because of the risk of hypoglycemia after discharge, it is advised to either reduce the doses of oral agents or choose more conservative insulin starting doses.52 One possibility is to discontinue the nutritional and correction doses, continue the hospital dose of basal insulin, and restart the oral antidiabetes medications. If the dose of basal insulin was more than 50% of the TDD of insulin, it may need to be reduced. A more conservative option for patients at a higher risk of hypoglycemia is to start 0.2 units/kg or 10 units of NPH, glargine, or detemir at bedtime (Figure 2). Once discharged, blood glucose should be measured 1 to 4 times a day and the basal dose titrated by several different validated methods.53, 54 Appropriate orders for necessary supplies for insulin therapy include a meter with test strips, lancets, syringes, needles, and glucagon kit.55

With a large number of patients with diabetes remaining undiagnosed, it is important to use the information available during hospitalization to identify previously unrecognized diabetes or prediabetes.24 Because there are no unique criteria for the diagnosis of DM in the stressed state, patients may have a presumptive diagnosis made in the hospital and/or follow‐up testing with fasting glucose or an oral glucose tolerance test. No ADA diagnostic thresholds for the HbA1c currently exist, but it can be a useful marker in making this distinction.56 Among patients with new hyperglycemia, an HbA1c of 6% or greater was 100% specific for predicting a future diagnosis of diabetes in the small prospective cohort study by Greci et al.,57 but many endocrinologists use a cutoff of 7%. For all hyperglycemic patients, lifestyle interventions that promote weight loss and increased activity levels should be encouraged. New hyperglycemia should be clearly identified as a diagnosis in discharge communication.
There are many barriers to diabetes self‐management education in the inpatient setting but there are also numerous resources and opportunities. New information will be available regarding patients' understanding of their disease and glycemic control and there may be plans for changes in the home medication regimen. Most of the focus of inpatient education sessions is on survival skills such as taking medications, performing blood glucose monitoring, basic meal planning, identification and treatment of hypoglycemia, sick‐day management, how to access further diabetes education as an outpatient, and when to call the healthcare team.58 The most effective way to accomplish all of this is to identify the discharge regimen early and include nurses and staff in a plan to educate all patients. An inpatient diabetes educator can provide additional help with newly‐diagnosed or uncontrolled patients. Dividing the material over the hospitalization makes it less overwhelming for patients, reinforces previously taught concepts, spreads the responsibility to more providers, and offers it in conjunction with the correlating clinical care. Throughout their hospital stay, patients can begin to practice new skills, including blood glucose monitoring and logbook use, drawing up and administering insulin, sharps disposal, basic diabetic diet information, and sick‐day management. The specific topics addressed in each session can be tracked as part of an interdisciplinary education record that allows coordination among the individuals involved in teaching.59 It is important to give patients the basics, support them with minimal written information, and provide them appropriate follow‐up diabetes education.60 Furthermore, the inpatient team should view the patient's glycemic control education as something that needs to continue across the continuum of care and develop communication strategies that connect with the follow‐up clinical team.
At the time of discharge, it is essential that written documentation and communication with outpatient care providers be completed.61, 62 The more standardized the inpatient insulin regimens are, the more likely the patient is to be on a much different glycemic control regimen than the one on admission; therefore, it is even more important to assure that the admission medication list is accurate and reconciled completely with the modified list at discharge. Discharge check lists and tools for assessing patient acceptance of the discharge plan help with this process.63 Follow‐up with the primary care physician should occur within 7 to 14 days if patients are new to insulin, had medication changes, or are elderly. An increased likelihood of keeping posthospitalization appointments with a diabetes specialty clinic has been associated with being discharged on insulin, a new diagnosis of diabetes, and direct referral.64 Additional attention should be paid to barriers to follow‐up, including lack of health insurance, prior difficulty with follow‐up, and transportation problems.65
SUMMARY
A variety of factors have contributed to difficulty in achieving inpatient and outpatient glucose control. These include care complexity, the lack of standardized protocols, limited knowledge about glucose control, and clinical inertia. Inpatient clinicians have a tendency toward keeping patients on their home regimen in hopes that they might test its effectiveness. Furthermore, there has been the notion of why optimize the glycemic regimen of inpatients because their diabetic needs will change in the outpatient setting. However, because the insulin requirements during acute illness are different and nutritional intake is variable, nearly all inpatients should be placed on multiple daily doses of scheduled insulin or IV insulin to allow the necessary flexibility for rapid titration and abrupt changes in nutrition. This intensive regimen is only appropriate for a minority of outpatients. This difference illustrates that a regimen that works perfectly in one clinical setting will not necessarily be optimal in the next. The patient's outpatient treatment regimen should be reassessed based on HbA1c, self‐monitoring prior to admission, and new contraindications based on medical issues. If a change is indicated and the inpatient physician is motivated, there are numerous helpful resources to aid in addressing all the necessary factors surrounding intensification of therapy.
Despite requiring different glycemic control regimens, the information gained from the needs in each setting guide the next, making communication and planning paramount. Important transitions that must be given attention are: (1) admission to the hospital; (2) in‐hospital transitions, including the perioperative period and IV‐to‐SC insulin; and (3) the hospital to outpatient transition. The complexity of such frequent transitions requires planning, education, and clear communication that are best handled with a systems approach and the development of standardized protocols and order sets. Hospitalists, endocrinologists, and other members of the healthcare team should take an aggressive role in developing systems and facilitating optimal transitions to maximize glycemic control. Further studies are needed to determine the best practices among the variety of options discussed in this article.
Professional and patient safety organizations have recognized the importance of safe transitions as patients move through the health care system, and such attention is even more critical when attempting to achieve glycemic control.14 Since the publication of the Diabetes Control and Complications Trial (DCCT)5 and the United Kingdom Prospective Diabetes Study (UKPDS),6 we have known that intensive glycemic control in the ambulatory setting prevents complications in both type 1 and type 2 diabetes mellitus (DM). Despite the increased risk of hypoglycemia, these trials changed practice patterns in the outpatient settings in favor of intensification of diabetes therapy. In the same way, randomized, prospective trials using intravenous (IV) insulin therapy have revolutionized our thinking about inpatient care by showing that tight glycemic control in the critically ill7 and patients with acute myocardial infarction8 reduces mortality and morbidity. These, as well as additional observational studies associating hyperglycemia with poor outcomes in a variety of medical and surgical patients,915 have led to increased attention on glycemic control in all venues of care.16, 17 Concerns over excessive hypoglycemia and a nonsignificant increase in mortality in certain populations of medical intensive care unit (ICU) patients have raised questions over whether the initial studies can be reproduced or generalized to other groups of inpatients.18, 19 Additional studies are underway to clarify these questions but consensus exists that blood glucose values should at least be less than 180 mg/dL and that the traditional practice of ignoring hyperglycemia is no longer acceptable.
While a uniform focus on glycemic control will allow our patients to receive a consistent message about diabetes, the unique limitations inherent to each practice setting requires different therapeutic regimens and intentional focus on the risks as patients transition from one care area to another. This work addresses several areas of care transition that are particularly important in safely achieving glycemic control including: transition into the hospital for patients on a variety of home regimens, transitions within the hospital (related to changes in dietary intake, change from IV to subcutaneous [SC] therapy, and the perioperative setting), and the transition from the hospital to home or another healthcare facility.
TRANSITION INTO THE HOSPITAL
Until recently, most patients with diabetes admitted to the hospital were managed with sliding‐scale‐only regimens.20, 21 Unfortunately, this led to a variety of complications, including hyperglycemia, hypoglycemia, iatrogenic ketoacidosis, and an inconsistent message to patients on the importance of glycemic control.22 Some outpatient clinicians and patients combated this tradition by creating in‐hospital glucose control plans with orders, which patients would bring with them to the hospital.23 This practice continues to be a helpful way to guide inpatient therapy and is encouraged when available. Glycemic‐controlrelated documents from outpatient clinicians should include the most recent glycosylated hemoglobin (HbA1c) value, diagnosis and known complications, current names and doses of medications, and other patient‐specific preferences or needs (eg, compliance, financial, fear of needles). If the last HbA1c was performed more than 30 days before admission or is not available, one should be obtained upon hospital admission to help guide discharge therapy.24 By knowing the HbA1c, one can determine the level of diabetic control achieved with the current regimen and can help the inpatient team (clinician and patient) determine if a more aggressive glycemic control regimen is necessary at the time of discharge. It is important to note that if the patient has received a transfusion of red blood cells prior to HbA1c measurement or has a hemoglobinopathy, the HbA1c value may not be accurate.25, 26
In general, the outpatient regimen will need to be modified at admission to achieve the appropriate flexibility needed for the changing nutritional intake and insulin requirements that invariably accompany hospitalization. Sulfonylureas and dipeptidyl peptidase 4 inhibitors (DPP4), such as sitagliptin, have most of their effect immediately, but the other oral antihyperglycemic agents have a relatively long delay between treatment and effect, thus they are not a flexible enough method to achieve glycemic control in the hospital. Additionally, inpatients may have transient contraindications to their prior oral antihyperglycemic medications. Metformin is almost always on hold in the hospital setting, at least initially, due to concerns about lactic acidosis. Sulfonylureas can cause hypoglycemia in the setting of worsening renal function or reduced oral intake. Thiazoladinediones (TZDs) are often withheld due to concerns about fluid retention and should be avoided in patients admitted with heart failure. There is little experience in the hospital with the use of newer agents like exenatide, pramlintide, glinides, and DPP4 inhibitors.
Overall, it is generally recommended that oral antihyperglycemic agents be discontinued upon hospital admission and replaced with insulin infusions or scheduled SC insulin. An estimate of 0.4 to 0.5 units/kg of body weight provides a conservative starting point for the total daily dose of insulin (TDD) for most patients. This TDD should then be divided into basal and nutritional components to match the patients' caloric intake. Additional correction doses of insulin should be prescribed to cover episodes of hyperglycemia that develop despite the provision of anticipatory‐physiologic insulin. Further discussion of insulin dosing and SC regimens is available in detail elsewhere.27, 28 The recommendation for these insulin‐only regimens is made regardless of the glycemic control in the outpatient setting and is not meant to imply that they should be continued at discharge. In fact, most patients will return to their home regimen or to one that is intensified but less labor intensive than the basal‐nutritional‐correction insulin used in the hospital. The antihyperglycemic regimen planned for discharge should be anticipated as early as possible and clearly communicated to the patient and/or caregivers to allow for optimal education.
Outpatient insulin regimens that have a high percentage of basal insulin need to be modified during hospital admission to avoid hypoglycemia that may occur from variable nutritional intake. While hospitalized, the basal portion of the estimated TDD generally should not be more than 50% to 60%. The total number of units of all types of insulin used daily as an outpatient can be used as a starting point for determining the inpatient TDD by a 1:1 conversion. Adjustments up or down based on glycemic control, nutritional intake, and other factors are then necessary. If patients are on regimens with insulin plus oral agents at home, the inpatient TDD should either be the home insulin dose or the dose calculated based on their weight, whichever is greater. Patients who use carbohydrate counting to determine nutritional insulin doses as an outpatient might be continued on this regimen if they have a strong understanding of the methods, they are coherent enough to determine their doses, nursing staff are well educated, and dietary services provides the carbohydrate content for the hospital menu. If patients are on insulin pumps at home, these should be managed according to a uniform hospital policy to assure safety. If conversion to multiple daily injections is needed, the same 1:1 conversion is safe.29
Transitions Within the Hospital
General Issues
Within the hospital itself, there are several transitions that have important quality and safety implications regarding glycemic control. The handoffs between providers should follow a standardized format.4, 30, 31 Essential information will vary depending on the setting but should universally include recent hypoglycemia, insulin type and doses, and hypoglycemic risk factors such as changes in insulin doses, the development of renal insufficiency, inability of the patient to self‐report symptoms, tapering of steroids, and cessation or interruption of nutritional intake.32
One of the greatest risks for hypoglycemia in the hospital comes from the unpredictable nutritional interruptions that occur. Unplanned changes are best handled by nurses having an existing order to hold scheduled nutritional insulin if patients are classified nothing by mouth (NPO) or eat 50% of their meal. Additionally, nursing staff should have orders or policies that allow flexibility in the time of administering scheduled rapid‐acting nutritional insulin so that it may be given during or immediately following the meal in patients at higher risk for poor oral intake. Tube feedings also place patients at high risk for hypoglycemia because the tube may become dislodged or they may begin to have feeding intolerance. For these reasons, a measure of safety would be to have standing orders to substitute IV 10% dextrose in water (D10W) at the same rate as the prior tube feeds, hold nutritional insulin, and begin more frequent monitoring whenever tube feeds are stopped.33 Orders that rely on nursing staff to notify a physician when tube feedings are stopped are generally not directive enough because providers may be distracted by other changes or forget the patient is on long‐acting insulin. The need for this flexibility around nutritional dosing emphasizes the importance of avoiding excessive doses of basal insulin. If the total dose of basal insulin is 40% to 50% of the TDD, it can safely be continued at its usual dose despite changing nutritional intake. The only exception is neutral protamine Hagedorn (NPH) insulin, which should be reduced when patients are NPO due to its peak. Generally, a 50% reduction in NPH is recommended for morning doses, but bedtime doses may be given with little to no reduction. Because of the complexity of these issues, standardized order sets are the best way to reliably communicate all the necessary standing orders to nursing staff (Table 1).
|
| Nutritional insulin |
| Hold if patients are NPO or eat less than 50% of their meal. |
| Administer scheduled rapid acting nutritional insulin during or immediately following the meal if oral intake is questionable (ie, nausea, emesis, or newly advancing diet). |
| Tube feedings: When tube feeds are stopped unexpectedly |
| Start dextrose containing IV fluids (many institutions use D10W at the same rate as the prior tube feeds). |
| Hold scheduled nutritional insulin. |
| Notify physician. |
| Basal insulin |
| Continue if NPO. |
| Reduce morning dose of NPH by 50% if NPO and may need to reduce the dose of bedtime NPH. |
| IV to subcutaneous transition |
| Timing for discontinuing IV infusion in relation to first dose of subcutaneous insulin. |
| Prompts for verbal communication between ICU and general ward staff. |
Transitioning the Patient Off of IV Insulin
The strongest evidence for tight glycemic control derives from studies in the surgical ICU.7 Many hospitals have robust, effective IV‐insulin protocols. The frequency of monitoring and rapidity of action of IV insulin allow quick achievement of blood glucose control. As patients begin to eat, the layering of SC nutritional insulin on top of the insulin infusion may reduce the lability of the infusion rate and prevent excursions in glycemic control. When the patient is ready to leave the ICU or start a full oral diet, it is recommended that they transition off of the IV insulin to a basal‐nutritional‐correction regimen.33, 34
The amount of insulin needed with IV infusion is a useful estimate of the TDD of insulin.28, 33, 35, 36 There are important general steps to take when making this transition; but, due to the lack of conclusive data proving the advantage of one regimen over another, there are a variety of acceptable specific protocols (Table 2).3739 First, it should be determined if patients are expected to require ongoing scheduled SC insulin or not. Certainly, all patients with type 1 DM will require scheduled SC insulin, but patients with type 2 DM on low insulin infusion rates or some patients with new hyperglycemia can appropriately be managed with sliding‐scale alone. Next, the average hourly rate of the infusion over the preceding 6 to 8 hours should be determined because it most accurately reflects current insulin needs during the changing stress, nutrition, and medications in critical care patients. This hourly rate will then be converted to a TDD using a safety factor to anticipate decreasing insulin requirements. Some portion of this daily total will then be assigned to be basal insulin. As patients' clinical conditions approach baseline, so will their insulin requirements, and the dose will need to be revised.24
|
| Step 1: Is patient stable enough for transition? Hypotension, active sepsis, vasopressors, and intubation are contraindications to transition due to unreliable subcutaneous insulin absorption and continued need for the most flexible dosing due to frequently changing insulin requirements. |
| Step 2: Does this patient need a transition to scheduled subcutaneous (SC) insulin? |
| Yes |
| All patients with type 1 DM. |
| Type 2 DM patients on insulin as outpatient. |
| Type 2 DM patients with a recent mean infusion rate of 0.5 units/hour.* |
| No |
| Type 2 DM patients with infusion rate 0.5 units/hour.* |
| Stress hyperglycemia or previously unrecognized DM if infusion rate 1 unit/hour, or if HbA1c near normal. |
| Some institutions exclude all stress hyperglycemia patients from transition to a SC insulin regimen, regardless of drip rate. |
| Step 3: If transition is needed, calculate a total daily dose (TDD) of insulin. The TDD is an estimate of the 24‐hour insulin requirement when the patient is receiving full nutrition. |
| Determine mean insulin infusion rate from last 6 to 8 hours. |
| Calculate 24‐hour insulin dose based on this, and reduce this 24‐hour dose by some safety factor. There are several options for this step. |
| Multiply hourly rate by 24, then multiply by 0.7 or 0.8 to arrive at a safety‐adjusted 24 hour insulin dose. |
| OR |
| Multiply hourly infusion rate by 20 (80% of 24). |
| Determine if this total is the TDD or basal dose based on current nutrition. There are several options for this step for you or your institution to choose. |
| If infusion was serving basal AND nutritional needs of patient (such as a patient on 24‐hour tube feedings) this will be your TDD. |
| OR |
| If the infusion insulin was not covering significant nutrition, this could be the BASAL insulin dose. |
| Step 4: Construct a regimen tailored to the patient's nutritional situation, building in safeguards for any changes in nutritional intake and uncertainties about reliability of intake. Several options are again available. |
| Basal: should be ordered as basal glargine or detemir (these are preferred by SHM GCTF but NPH is also an option). |
| Dose is 40% to 50% of TDD. |
| OR |
| Adjusted 24‐hour IV requirement given all as basal. |
| Nutritional: The remainder of the TDD is scheduled nutritional insulin in divided doses. In general, these doses need to be adjusted down for 100% nutritional intake and the orders should allow for administering nutritional insulin just AFTER observed meals to allow an assessment of intake. There are several options for estimating the initial doses: |
| Use 50% of the TDD as nutritional coverage and divide this amount by 3 to determine the scheduled meal dose. Hold if they do not eat more than 50% of their meal. |
| Use a more conservative start of 10% to 20% of the basal dose scheduled with each meal. |
| Use carbohydrate counting to cover nutritional intake. |
| Step 5: Be sure to give SC insulin BEFORE the infusion stops |
| Basal glargine or detemir are ideally given at least 2 hours before infusion is discontinued. |
| Shorter lead times (30 minutes) are possible if rapid acting insulin is given with basal insulin. |
SC insulin should be given before the drip is discontinued to allow an overlap that takes into consideration the onset of action. The first dose of basal insulin should be given 2 hours before the insulin infusion is discontinued.24, 40 However, because this is not always feasible, (ie, the patient needs to leave the ICU sooner), another option is to turn off the drip and give 10% of the basal dose as rapid acting insulin along with the basal dose.39 The timing of subsequent doses will depend on the specific basal insulin that is ordered as well as institutional consideration of usual care delivery and nursing workflow. Given that there are several options to achieve this important overlap between IV and SC insulin, it is best for a multidisciplinary team to choose some preferred way that is the institutional standard. Having a standard allows targeted education and tracking of adherence to best practices.
Because conversion to SC insulin is a complex task and the opportunity may arise while physicians are busy with other clinical priorities, there are several options to assure that the necessary steps take place. Some institutions may build a protocol for this transition on paper or computerized order entry, build cues and dosing charts into order sets, and/or develop nursing documentation and nursing process to influence physician and nurse behavior. This critical juncture is also a good place to focus expertise with a glycemic control team, pharmacist, specially trained nurses, or some other dedicated team to take over this transition for all patients.36 The complexity and aggressiveness of the specific institutional protocol used will depend on the confidence and experience of those individuals responsible for determining the transition doses.
The transition from IV to SC insulin often coincides with a change in patient location, (ie, from the ICU to general medical ward). It is imperative that appropriate communication occurs between the transferring and receiving nurses and physicians to continue with the care plan for glycemic management. This communication can be encouraged through provider education and automated into the standardized order process.
Perioperative Transitions
Patients undergoing surgery present a special challenge. They are faced with not only the physiologic and mental stress of surgery but also the hazards of multiple handoffs across several care teams, all with different priorities and cultures. As in other areas, standardized protocols specific to this area of transition are important in assuring safe and effective perioperative glycemic control. Procedures should preferably be scheduled for the early morning to have the least impact on insulin dosing. Patients who are admitted only for the procedure will have to manage this transition on their own and need to be given specific instructions along with the general preoperative orders.24, 41 In general, the usual dose of glargine can be given the day prior to the procedure if it is approximately 50% of their TDD. This is an important caution because some outpatient regimens use large doses of glargine, which essentially provide both basal and nutritional coverage. In those patients, the glargine dose should be reduced by 20% to 50% to provide a safety margin. As with any patient who is NPO, the morning dose of NPH should be one‐half of the usual dose, scheduled nutritional insulin should be held, and the usual doses of correction insulin should be reduced. The appropriate preoperative dose adjustments also depend on whether the individual patient is ketosis‐prone and how tight their glycemic control is as an outpatient.
Upon arrival to the hospital or during the time that the inpatient is NPO, dextrose containing IV fluids should be administered to minimize the risk of hypoglycemia and prevent ketosis. Given the risks for wide variation, blood glucose monitoring should occur every 1 to 2 hours before, during, and initially after the procedure. Infusion insulin allows the most rapid titration and reliable delivery (compared with SC infusions or injections) and is therefore the preferred regimen for major surgery requiring prolonged NPO status or prolonged surgery in patients with type 1 diabetes. Basal‐nutritional‐correction SC insulin is preferred in other surgical inpatients because their nutritional intake is variable and the stress of surgery affects insulin requirements.
Oral antihyperglycemic agents should be held around the time of surgery. If patients are on an oral agent that can result in hypoglycemia, (ie, sulfonylurea or other insulin secretagogue), it should be held on the day of the procedure. Metformin must be held for safety concerns, given the possible decrease in renal function around surgery. It should be held beginning on the day of the procedure or the day before in the case of the sustained‐release formulation. It can then be resumed 48 hours postoperation after normal renal function is secured and the patient is discharged home. Alpha‐glucosidase inhibitors should be held whenever patients are NPO because they only work when taken with meals. Thiazoladinediones have a long duration of action and so can be continued or stopped around surgery. Finally, glucagon‐like peptide (GLP‐1) agonists (exenatide) should be held until the patient is eating normally and discharged home due to the high incidence of gastrointestinal side effects.
TRANSITIONING FROM THE HOSPITAL
The final but perhaps most important transition is the one from the hospital. With much attention on glycemic control in the hospital, it will become clear to many clinicians that the outpatient regimen needs to be modified. However, any changes in medications increase the chances of hypoglycemia and the possibility of error. The postdischarge time frame has been poorly studied and was specifically identified by the Association for Clinical Endocrinologists (ACE) and American Diabetes Association (ADA) as an area in need of future research.36
Patients may be discharged to a nursing home, hospice, or home, and numerous factors need to be considered to determine the optimal discharge regimen. Important considerations are the HbA1c at admission, home medications, medication interactions, current medical problems, nutritional status, physical disabilities, frequency of self‐monitoring, hypoglycemic risk factors, contraindications to oral medications, goals of care/life expectancy, and financial and other resources. If there are temporary physical or self‐care limitations, then a visiting nurse may need to be arranged to assure a safe transition home with the optimal therapy. If patients are going to a skilled nursing facility or other acute care hospital, the formulary, processes, and staffing issues of that facility will be additional important considerations in determining whether therapy is the same as in the hospital or more like what it will be at home.
An algorithm for outpatient therapy for type 2 DM was recommended in a consensus statement from the ADA and European Association for the Study of Diabetes.42, 43 This has been modified using additional recommendations from the AACE44 and is depicted in Figure 1. While the delineation of these steps is helpful, it must be emphasized that both the choice of regimen and dose will need to be individualized. Prescribing the ideal frequently falls short if there is no way for the patient to implement the recommendations. Intensive insulin therapy requires training in food intake/emnsulin matching, motivation of the patient and outpatient clinician, 4 times daily self‐monitoring of blood glucose, and considerable expense. Some patients may be temporarily continued on basal‐nutritional‐correction regimens as their insulin requirements are rapidly changing and later converted to regimens that involve less frequent insulin doses, (ie, twice daily premixed insulin or basal insulin with oral agents or oral agents alone).45, 46 Other patients who may be medically appropriate for intensive insulin therapy may first need to gain confidence with more simple insulin regimens. There are numerous additional resources on initiating insulin that the reader is referred to for more detail.4448

Oral antihyperglycemic drugs are usually held while a patient is admitted to the hospital but once medical conditions are improved, oral intake is established, and renal function stabilized, these drugs can be restarted. If a patient has a new contraindication to metformin or sulfonylureas but does not need insulin, a TZD or DPP4 inhibitor should be considered. Elderly patients and those with renal or liver disease are at increased risk for developing hypoglycemia.49, 50 Glyburide should be avoided, and doses of other sulfonylureas may need to be adjusted. Other options that may be considered in this situation include sitagliptin and exenatide.51 When patients will be discharged on oral diabetic medications alone, discontinue the basal insulin 12 to 24 hours before and the scheduled nutritional insulin at the same time oral agents are restarted. Sulfonylureas, metformin, DPP4 inhibitors, and exenatide will have most of their effect in the first day, but TZDs have a delayed onset and may not be a good bridge for immediate control at discharge.
If patients are going to be discharged on basal insulin in addition to oral agents, several options exist for determining the dose. Because of the risk of hypoglycemia after discharge, it is advised to either reduce the doses of oral agents or choose more conservative insulin starting doses.52 One possibility is to discontinue the nutritional and correction doses, continue the hospital dose of basal insulin, and restart the oral antidiabetes medications. If the dose of basal insulin was more than 50% of the TDD of insulin, it may need to be reduced. A more conservative option for patients at a higher risk of hypoglycemia is to start 0.2 units/kg or 10 units of NPH, glargine, or detemir at bedtime (Figure 2). Once discharged, blood glucose should be measured 1 to 4 times a day and the basal dose titrated by several different validated methods.53, 54 Appropriate orders for necessary supplies for insulin therapy include a meter with test strips, lancets, syringes, needles, and glucagon kit.55

With a large number of patients with diabetes remaining undiagnosed, it is important to use the information available during hospitalization to identify previously unrecognized diabetes or prediabetes.24 Because there are no unique criteria for the diagnosis of DM in the stressed state, patients may have a presumptive diagnosis made in the hospital and/or follow‐up testing with fasting glucose or an oral glucose tolerance test. No ADA diagnostic thresholds for the HbA1c currently exist, but it can be a useful marker in making this distinction.56 Among patients with new hyperglycemia, an HbA1c of 6% or greater was 100% specific for predicting a future diagnosis of diabetes in the small prospective cohort study by Greci et al.,57 but many endocrinologists use a cutoff of 7%. For all hyperglycemic patients, lifestyle interventions that promote weight loss and increased activity levels should be encouraged. New hyperglycemia should be clearly identified as a diagnosis in discharge communication.
There are many barriers to diabetes self‐management education in the inpatient setting but there are also numerous resources and opportunities. New information will be available regarding patients' understanding of their disease and glycemic control and there may be plans for changes in the home medication regimen. Most of the focus of inpatient education sessions is on survival skills such as taking medications, performing blood glucose monitoring, basic meal planning, identification and treatment of hypoglycemia, sick‐day management, how to access further diabetes education as an outpatient, and when to call the healthcare team.58 The most effective way to accomplish all of this is to identify the discharge regimen early and include nurses and staff in a plan to educate all patients. An inpatient diabetes educator can provide additional help with newly‐diagnosed or uncontrolled patients. Dividing the material over the hospitalization makes it less overwhelming for patients, reinforces previously taught concepts, spreads the responsibility to more providers, and offers it in conjunction with the correlating clinical care. Throughout their hospital stay, patients can begin to practice new skills, including blood glucose monitoring and logbook use, drawing up and administering insulin, sharps disposal, basic diabetic diet information, and sick‐day management. The specific topics addressed in each session can be tracked as part of an interdisciplinary education record that allows coordination among the individuals involved in teaching.59 It is important to give patients the basics, support them with minimal written information, and provide them appropriate follow‐up diabetes education.60 Furthermore, the inpatient team should view the patient's glycemic control education as something that needs to continue across the continuum of care and develop communication strategies that connect with the follow‐up clinical team.
At the time of discharge, it is essential that written documentation and communication with outpatient care providers be completed.61, 62 The more standardized the inpatient insulin regimens are, the more likely the patient is to be on a much different glycemic control regimen than the one on admission; therefore, it is even more important to assure that the admission medication list is accurate and reconciled completely with the modified list at discharge. Discharge check lists and tools for assessing patient acceptance of the discharge plan help with this process.63 Follow‐up with the primary care physician should occur within 7 to 14 days if patients are new to insulin, had medication changes, or are elderly. An increased likelihood of keeping posthospitalization appointments with a diabetes specialty clinic has been associated with being discharged on insulin, a new diagnosis of diabetes, and direct referral.64 Additional attention should be paid to barriers to follow‐up, including lack of health insurance, prior difficulty with follow‐up, and transportation problems.65
SUMMARY
A variety of factors have contributed to difficulty in achieving inpatient and outpatient glucose control. These include care complexity, the lack of standardized protocols, limited knowledge about glucose control, and clinical inertia. Inpatient clinicians have a tendency toward keeping patients on their home regimen in hopes that they might test its effectiveness. Furthermore, there has been the notion of why optimize the glycemic regimen of inpatients because their diabetic needs will change in the outpatient setting. However, because the insulin requirements during acute illness are different and nutritional intake is variable, nearly all inpatients should be placed on multiple daily doses of scheduled insulin or IV insulin to allow the necessary flexibility for rapid titration and abrupt changes in nutrition. This intensive regimen is only appropriate for a minority of outpatients. This difference illustrates that a regimen that works perfectly in one clinical setting will not necessarily be optimal in the next. The patient's outpatient treatment regimen should be reassessed based on HbA1c, self‐monitoring prior to admission, and new contraindications based on medical issues. If a change is indicated and the inpatient physician is motivated, there are numerous helpful resources to aid in addressing all the necessary factors surrounding intensification of therapy.
Despite requiring different glycemic control regimens, the information gained from the needs in each setting guide the next, making communication and planning paramount. Important transitions that must be given attention are: (1) admission to the hospital; (2) in‐hospital transitions, including the perioperative period and IV‐to‐SC insulin; and (3) the hospital to outpatient transition. The complexity of such frequent transitions requires planning, education, and clear communication that are best handled with a systems approach and the development of standardized protocols and order sets. Hospitalists, endocrinologists, and other members of the healthcare team should take an aggressive role in developing systems and facilitating optimal transitions to maximize glycemic control. Further studies are needed to determine the best practices among the variety of options discussed in this article.
- ACE/ADA Task Force on Inpatient Diabetes.American College of Endocrinology and American Diabetes Association Consensus Statement on Inpatient Diabetes and Glycemic Control.Endocr Pract.2006;12:458–468.
- American Board of Internal Medicine Foundation Stepping Up to the Plate Alliance. Available at: http://www.abimfoundation.org/quality/suttp.shtm. Accessed November2007.
- National Transitions of Care Coalition. Available at: http://www.ntocc.org. Accessed November2007.
- JCAHO 2008 National Patient Safety Goals. Availableat: http://www.jointcommission.org/PatientSafety/NationalPatientSafetyGoals/08_hap_npsgs.htm. Accessed November2007.
- Diabetes Control and Complications Trial Research Group.The effect of intensive treatment of diabetes on the development and progression of long‐term complications in insulin‐dependent diabetes mellitus.N Engl J Med.1993;329:977–986.
- UK Prospective Diabetes Study (UKPDS) Group.Intensive blood‐glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes: UK Prospective Diabetes Study (UKPDS) Group.Lancet.1998;352:837–853.
- ,,, et al.,Intensive insulin therapy in critically ill patients.N Engl J Med.2001;345:1359–1367.
- ,,,.Glycometabolic state at admission: important risk marker of mortality in conventionally treated patients with diabetes mellitus and acute myocardial infarction: long‐term results from the Diabetes and Insulin‐Glucose Infusion in Acute Myocardial Infarction (DIGAMI) study.Circulation.1999;99:2626–2632.
- ,,,,.Relationship of early hyperglycemia to mortality in trauma patients.J Trauma.2004;56:1058–1062.
- ,,,,.Early peri‐operative glycaemic control and allograft rejection in patients with diabetes mellitus: a pilot study.Transplantation.2001;72:1321–1324.
- ,,, et al.Relation between the duration of remission and hyperglycemia in induction chemotherapy for acute lymphocytic leukemia with a hyperfractionated cyclophosphamide, vincristine, doxorubicin and dexamethasone/methotrexate cytarabine regimen.Cancer.2004;100:1179–1185.
- ,,,,.Stress hyperglycemia and prognosis of stroke in nondiabetic and diabetic patients: a systematic overview.Stroke.2001;32:2426–2432.
- ,,,,,.Diabetes and outcome of community‐acquired pneumococcal bacteriemia.Diabetes Care.2004;27:70–76.
- ,,.Early postoperative glucose control predicts nosocomial infection rate in diabetic patients.JPEN J Parenter Enteral Nutr.1998;22:77–81.
- ,,,,.Early post‐operative glucose levels are an independent risk factor for infection after peripheral vascular surgery. A retrospective study.Eur J Vasc Endovasc Surg.2004;28:520–525.
- American Diabetes Association.Standards of medical care in diabetes, 2006.Diabetes Care.2006;29(suppl 1):s4–s42.
- American College of Endocrinology Task Force on Inpatient Diabetes and Metabolic Control.American College of Endocrinology Position Statement on Inpatient Diabetes and Metabolic Control.Endocr Pract.2004;10:77–82.
- ,,, et al.Intensive insulin therapy in the medical ICU.N Engl J Med.2006;354:449–461
- ,,, et al.Intensive insulin therapy and pentastarch resuscitation in severe sepsis.N Engl J Med.2008;358(2):125–139.
- ,,,,.Inpatient management of diabetes and hyperglycemia among general medicine patients at a large teaching hospital.J Hosp Med.2006;1:145–150.
- ,,, et al.,Diabetes care in hospitalized noncritically ill patients: More evidence for clinical inertia and negative therapeutic momentum.J Hosp Med.2007;2:203–211.
- ,,.Glycemic control and sliding scale insulin use in medical inpatients with diabetes mellitus.Arch Intern Med.1997;157:545–552.
- ,.Hospital management of hyperglycemia.Clin Diabetes.2004;22:81–88.
- ,,, et al.Management of diabetes and hyperglycemia in hospitals. [Erratum appears in Diabetes Care. 2005; 28: 1990. Dosage error in text].Diabetes Care.2004;27:553–591.
- ,.Hemoglobinopathies and HbA(1c) measurement.Diabetes Care.2000;23(8):1197–1198.
- ,,,,,.Evaluation of HbA1c determination methods in patients with hemoglobinopathiesDiabetes Care.2000;23(3):339–344.
- ,,,.Subcutaneous insulin order sets and protocols: effective design and implementation strategies.J Hosp Med.2008;3.
- ,,,,.Management of diabetes and hyperglycemia in the hospital: a practical guide to subcutaneous insulin use in the non‐critically ill, adult patient.J Hosp Med.2008;3.PMID:8675920.
- ,,,.Switch to multiple daily injections with insulin glargine and insulin lispro from continuous subcutaneous insulin infusion with insulin lispro: a randomized, open‐label study using a continuous glucose monitoring system.Endocr Pract.2005;11:157–164.
- SBAR technique for communication: a situational briefing model. Available at: http://www.ihi.org/IHI/Topics/PatientSafety/SafetyGeneral/Tools/SBARTechniqueforCommunicationASituationalBriefingModel.htm. Accessed December2007.
- . Promising quality improvement initiatives: reports from the field. AHRQ Summit—Improving Health Care Quality for All Americans: Celebrating Success, Measuring Progress, Moving Forward 2004. Available at: http://www.ahrq.gov/qual/qsummit/qsummit4.htm#sentara. Accessed December2007.
- ,,, et al.Hospital hypoglycemia: not only treatment but also prevention.Endocr Pract.2004;10(suppl 2):89–99.
- American Association of Clinical Endocrinologists Inpatient Glycemic Control Resource Center. Available at: http://resources.aace.com/PDF/Section_05‐Final‐Inpatient_Non‐ICU/Hyperglycemia_Non‐ICU_Protocols/Transition_from_ Intravenous_to_Subcutaneous_Insulin.PDF. Accessed November2007.
- Recommendations for safe use of insulin in hospitals. American Society of Health System Pharmacists and the Hospital and Health System Association of Pennsylvania. 2005. Available at: http://www.premierinc.com/safety/safety‐share/01–06‐downloads/01‐safe‐use‐insulin‐ashp.pdf. Accessed December2007.
- ,,, for the Society of Hospital Medicine Glycemic Control Taskforce. Glycemic control resource room: improving reliability of care across transitions and in the perioperative setting. Available at: http://www.hospitalmedicine.org/ResourceRoomRedesign/html/07Layer_Inter/06_Transitions.cfm. Accessed August2008.
- ACE/ADA Task Force on Inpatient Diabetes American College of Endocrinology and American Diabetes Association Consensus Statement on Inpatient Diabetes and Glycemic Control: a call to action.Diabetes Care.2006;29:1955–1962.
- ,,, et al.Conversion of intravenous insulin infusions to subcutaneously administered insulin glargine in patients with hyperglycemia.Endocr Pract.2006;12:641–650.
- ,,,.Intravenous insulin infusion therapy: indications, methods, and transition to subcutaneous insulin therapy.Endocr Pract.2004;10(suppl 2):71–80.
- ,,, et al.Inpatient management of hyperglycemia: the northwestern experience.Endocr Pract.2006;12(5):491–505.
- American Diabetes Association.Position statement: standards of medical care in diabetes‐2007.Diabetes Care.2007;30(suppl 1):S4–S41.
- American Association of Clinical Endocrinologists Inpatient Glycemic Control Resource Center. Available at: http://resources.aace.com/PDF/Section_05‐Final‐Inpatient_Non‐ICU/Hyperglycemia_Non‐ICU_Protocols/Pre‐Operative_Instructions_for_Patients_with_Diabetes.PDF Accessed November2007.
- ,,, et al.Management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement from the American Diabetes Association and the European Association for the Study of Diabetes.Diabetes Care.2006;29:1963–1972.
- ,,, et al.Management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: update regarding thiazoladinediones.Diabetes Care.2008;31:173–175.
- American Association of Clinical Endocrinologists Inpatient Glycemic Control Resource Center. Challenges in Effective Discharge Planning for Hospitalized Patients with Diabetes. Available at: http://resources.aace.com/PDF/Section_07‐Final‐Transition‐Inpatient_to_Outpatient/Challenges_in_Effective_Discharge_for_Diabetes_Patients.PPT. Accessed December2007.
- ,,.Initiating insulin therapy in type 2 diabetes: a comparison of biphasic and basal insulin analogs.Diabetes Care.2005;28:260–265.
- ,,, et al.Addition of biphasic, prandial, or basal insulin to oral therapy in type 2 diabetes.N Engl J Med.2007;357:1716–1730.
- ,,.Narrative review: a rational approach to starting insulin therapy.Ann Intern Med.2006;145:125–134.
- ,,,,.A real‐world approach to insulin therapy in primary care practice.Clin Diabetes.2005;23:78–86.
- ,,,.Individual sulfonylureas and serious hypoglycemia in older persons.J Am Geriatr Soc.1996;44:751–755.
- ,,,.Incidence and risk factors for serious hypoglycemia in older persons using insulin or sulfonylureas.Arch Intern Med.1997;157(15):1681–1686.
- ,,, et al.Exenatide versus insulin glargine in patients with suboptimally controlled type 2 diabetes: a randomized trial.Ann Intern Med.2005;143:559–569.
- .The transition from insulin infusions to long‐term diabetes therapy: the argument for insulin analogs.Semin Thorac Cardiovasc Surg.2006;18:366–378.
- ,,,,.ATLANTUS Study Group. Improvement of glycemic control in subjects with poorly controlled type 2 diabetes.Diabetes Care.2005;28:1282–1288.
- ,,.Investigators Insulin Glargine 4002 Study. The Treat‐to Target trial: randomized addition of glargine or human NPH insulin to oral therapy of type 2 diabetes patients.Diabetes Care.2003;26:3080–3086.
- American Association of Clinical Endocrinologists Inpatient Glycemic Control Resource Center. Available at: http://resources.aace.com/PDF/Section_07‐Final‐Transition‐Inpatient_to_Outpatient/Effective_Discharge_Planning‐Sample_Discharge_Plans/Inpatient_Diabetes_Discharge_Prescription.PDF. Accessed November2007.
- American Diabetes Association.Diagnosis and classification of diabetes mellitus.Diabetes Care.2007;30(suppl):S42–S47.
- ,,, et al.Utility of HbA1c levels for diabetes case finding in hospitalized patients with hyperglycemia.Diabetes Care.2003;26:1064–1068.
- ,,, et al.National standards for diabetes self‐management education.Diabetes Care.2006;29(suppl 1):S78–S85.
- Society of Hospital Medicine Glycemic Control Task Force. Workbook for improvement: improving glycemic control, preventing hypoglycemia and optimizing care of the inpatient with diabetes and hyperglycemia. page 105. Available at: http://www.hospitalmedicine.org/ResourceRoomRedesign/pdf/GC_Workbook.pdf. Accessed December,2007.
- Joslin Diabetes Center. EZ Start Patient Information Handouts. Available at: http://www.joslin.org/ezstart. Accessed December2007.
- ,,,.Promoting effective transitions of care at hospital discharge: a review of key issues for hospitalists.J Hosp Med.2007;2:314–323.
- ,,,,,.Deficits in communication and information transfer between hospital‐based and primary care physicians: implications for patient safety and continuity of care.JAMA.2007;297:831–841.
- Society of Hospital Medicine On‐line Clinical Tools. Ideal discharge for the elderly patient: a hospitalist checklist. Available at: http://www.hospitalmedicine.org/AM/Template.cfm?Section=QI_Clinical_Toolsemplate=/CM/ContentDisplay.cfmContentID=10303. Accessed December2007.
- ,,, et al.Inpatient to outpatient transfer of care in urban patients with diabetes: patterns and determinants of immediate post‐discharge follow‐up.Arch Intern Med.2004;164:447–453.
- ,,,,,.Inpatient to outpatient transfer of diabetes care: perceptions of barriers to postdischarge follow‐up in urban African American patients.Ethn Dis.2007;17:238–243.
- ACE/ADA Task Force on Inpatient Diabetes.American College of Endocrinology and American Diabetes Association Consensus Statement on Inpatient Diabetes and Glycemic Control.Endocr Pract.2006;12:458–468.
- American Board of Internal Medicine Foundation Stepping Up to the Plate Alliance. Available at: http://www.abimfoundation.org/quality/suttp.shtm. Accessed November2007.
- National Transitions of Care Coalition. Available at: http://www.ntocc.org. Accessed November2007.
- JCAHO 2008 National Patient Safety Goals. Availableat: http://www.jointcommission.org/PatientSafety/NationalPatientSafetyGoals/08_hap_npsgs.htm. Accessed November2007.
- Diabetes Control and Complications Trial Research Group.The effect of intensive treatment of diabetes on the development and progression of long‐term complications in insulin‐dependent diabetes mellitus.N Engl J Med.1993;329:977–986.
- UK Prospective Diabetes Study (UKPDS) Group.Intensive blood‐glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes: UK Prospective Diabetes Study (UKPDS) Group.Lancet.1998;352:837–853.
- ,,, et al.,Intensive insulin therapy in critically ill patients.N Engl J Med.2001;345:1359–1367.
- ,,,.Glycometabolic state at admission: important risk marker of mortality in conventionally treated patients with diabetes mellitus and acute myocardial infarction: long‐term results from the Diabetes and Insulin‐Glucose Infusion in Acute Myocardial Infarction (DIGAMI) study.Circulation.1999;99:2626–2632.
- ,,,,.Relationship of early hyperglycemia to mortality in trauma patients.J Trauma.2004;56:1058–1062.
- ,,,,.Early peri‐operative glycaemic control and allograft rejection in patients with diabetes mellitus: a pilot study.Transplantation.2001;72:1321–1324.
- ,,, et al.Relation between the duration of remission and hyperglycemia in induction chemotherapy for acute lymphocytic leukemia with a hyperfractionated cyclophosphamide, vincristine, doxorubicin and dexamethasone/methotrexate cytarabine regimen.Cancer.2004;100:1179–1185.
- ,,,,.Stress hyperglycemia and prognosis of stroke in nondiabetic and diabetic patients: a systematic overview.Stroke.2001;32:2426–2432.
- ,,,,,.Diabetes and outcome of community‐acquired pneumococcal bacteriemia.Diabetes Care.2004;27:70–76.
- ,,.Early postoperative glucose control predicts nosocomial infection rate in diabetic patients.JPEN J Parenter Enteral Nutr.1998;22:77–81.
- ,,,,.Early post‐operative glucose levels are an independent risk factor for infection after peripheral vascular surgery. A retrospective study.Eur J Vasc Endovasc Surg.2004;28:520–525.
- American Diabetes Association.Standards of medical care in diabetes, 2006.Diabetes Care.2006;29(suppl 1):s4–s42.
- American College of Endocrinology Task Force on Inpatient Diabetes and Metabolic Control.American College of Endocrinology Position Statement on Inpatient Diabetes and Metabolic Control.Endocr Pract.2004;10:77–82.
- ,,, et al.Intensive insulin therapy in the medical ICU.N Engl J Med.2006;354:449–461
- ,,, et al.Intensive insulin therapy and pentastarch resuscitation in severe sepsis.N Engl J Med.2008;358(2):125–139.
- ,,,,.Inpatient management of diabetes and hyperglycemia among general medicine patients at a large teaching hospital.J Hosp Med.2006;1:145–150.
- ,,, et al.,Diabetes care in hospitalized noncritically ill patients: More evidence for clinical inertia and negative therapeutic momentum.J Hosp Med.2007;2:203–211.
- ,,.Glycemic control and sliding scale insulin use in medical inpatients with diabetes mellitus.Arch Intern Med.1997;157:545–552.
- ,.Hospital management of hyperglycemia.Clin Diabetes.2004;22:81–88.
- ,,, et al.Management of diabetes and hyperglycemia in hospitals. [Erratum appears in Diabetes Care. 2005; 28: 1990. Dosage error in text].Diabetes Care.2004;27:553–591.
- ,.Hemoglobinopathies and HbA(1c) measurement.Diabetes Care.2000;23(8):1197–1198.
- ,,,,,.Evaluation of HbA1c determination methods in patients with hemoglobinopathiesDiabetes Care.2000;23(3):339–344.
- ,,,.Subcutaneous insulin order sets and protocols: effective design and implementation strategies.J Hosp Med.2008;3.
- ,,,,.Management of diabetes and hyperglycemia in the hospital: a practical guide to subcutaneous insulin use in the non‐critically ill, adult patient.J Hosp Med.2008;3.PMID:8675920.
- ,,,.Switch to multiple daily injections with insulin glargine and insulin lispro from continuous subcutaneous insulin infusion with insulin lispro: a randomized, open‐label study using a continuous glucose monitoring system.Endocr Pract.2005;11:157–164.
- SBAR technique for communication: a situational briefing model. Available at: http://www.ihi.org/IHI/Topics/PatientSafety/SafetyGeneral/Tools/SBARTechniqueforCommunicationASituationalBriefingModel.htm. Accessed December2007.
- . Promising quality improvement initiatives: reports from the field. AHRQ Summit—Improving Health Care Quality for All Americans: Celebrating Success, Measuring Progress, Moving Forward 2004. Available at: http://www.ahrq.gov/qual/qsummit/qsummit4.htm#sentara. Accessed December2007.
- ,,, et al.Hospital hypoglycemia: not only treatment but also prevention.Endocr Pract.2004;10(suppl 2):89–99.
- American Association of Clinical Endocrinologists Inpatient Glycemic Control Resource Center. Available at: http://resources.aace.com/PDF/Section_05‐Final‐Inpatient_Non‐ICU/Hyperglycemia_Non‐ICU_Protocols/Transition_from_ Intravenous_to_Subcutaneous_Insulin.PDF. Accessed November2007.
- Recommendations for safe use of insulin in hospitals. American Society of Health System Pharmacists and the Hospital and Health System Association of Pennsylvania. 2005. Available at: http://www.premierinc.com/safety/safety‐share/01–06‐downloads/01‐safe‐use‐insulin‐ashp.pdf. Accessed December2007.
- ,,, for the Society of Hospital Medicine Glycemic Control Taskforce. Glycemic control resource room: improving reliability of care across transitions and in the perioperative setting. Available at: http://www.hospitalmedicine.org/ResourceRoomRedesign/html/07Layer_Inter/06_Transitions.cfm. Accessed August2008.
- ACE/ADA Task Force on Inpatient Diabetes American College of Endocrinology and American Diabetes Association Consensus Statement on Inpatient Diabetes and Glycemic Control: a call to action.Diabetes Care.2006;29:1955–1962.
- ,,, et al.Conversion of intravenous insulin infusions to subcutaneously administered insulin glargine in patients with hyperglycemia.Endocr Pract.2006;12:641–650.
- ,,,.Intravenous insulin infusion therapy: indications, methods, and transition to subcutaneous insulin therapy.Endocr Pract.2004;10(suppl 2):71–80.
- ,,, et al.Inpatient management of hyperglycemia: the northwestern experience.Endocr Pract.2006;12(5):491–505.
- American Diabetes Association.Position statement: standards of medical care in diabetes‐2007.Diabetes Care.2007;30(suppl 1):S4–S41.
- American Association of Clinical Endocrinologists Inpatient Glycemic Control Resource Center. Available at: http://resources.aace.com/PDF/Section_05‐Final‐Inpatient_Non‐ICU/Hyperglycemia_Non‐ICU_Protocols/Pre‐Operative_Instructions_for_Patients_with_Diabetes.PDF Accessed November2007.
- ,,, et al.Management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement from the American Diabetes Association and the European Association for the Study of Diabetes.Diabetes Care.2006;29:1963–1972.
- ,,, et al.Management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: update regarding thiazoladinediones.Diabetes Care.2008;31:173–175.
- American Association of Clinical Endocrinologists Inpatient Glycemic Control Resource Center. Challenges in Effective Discharge Planning for Hospitalized Patients with Diabetes. Available at: http://resources.aace.com/PDF/Section_07‐Final‐Transition‐Inpatient_to_Outpatient/Challenges_in_Effective_Discharge_for_Diabetes_Patients.PPT. Accessed December2007.
- ,,.Initiating insulin therapy in type 2 diabetes: a comparison of biphasic and basal insulin analogs.Diabetes Care.2005;28:260–265.
- ,,, et al.Addition of biphasic, prandial, or basal insulin to oral therapy in type 2 diabetes.N Engl J Med.2007;357:1716–1730.
- ,,.Narrative review: a rational approach to starting insulin therapy.Ann Intern Med.2006;145:125–134.
- ,,,,.A real‐world approach to insulin therapy in primary care practice.Clin Diabetes.2005;23:78–86.
- ,,,.Individual sulfonylureas and serious hypoglycemia in older persons.J Am Geriatr Soc.1996;44:751–755.
- ,,,.Incidence and risk factors for serious hypoglycemia in older persons using insulin or sulfonylureas.Arch Intern Med.1997;157(15):1681–1686.
- ,,, et al.Exenatide versus insulin glargine in patients with suboptimally controlled type 2 diabetes: a randomized trial.Ann Intern Med.2005;143:559–569.
- .The transition from insulin infusions to long‐term diabetes therapy: the argument for insulin analogs.Semin Thorac Cardiovasc Surg.2006;18:366–378.
- ,,,,.ATLANTUS Study Group. Improvement of glycemic control in subjects with poorly controlled type 2 diabetes.Diabetes Care.2005;28:1282–1288.
- ,,.Investigators Insulin Glargine 4002 Study. The Treat‐to Target trial: randomized addition of glargine or human NPH insulin to oral therapy of type 2 diabetes patients.Diabetes Care.2003;26:3080–3086.
- American Association of Clinical Endocrinologists Inpatient Glycemic Control Resource Center. Available at: http://resources.aace.com/PDF/Section_07‐Final‐Transition‐Inpatient_to_Outpatient/Effective_Discharge_Planning‐Sample_Discharge_Plans/Inpatient_Diabetes_Discharge_Prescription.PDF. Accessed November2007.
- American Diabetes Association.Diagnosis and classification of diabetes mellitus.Diabetes Care.2007;30(suppl):S42–S47.
- ,,, et al.Utility of HbA1c levels for diabetes case finding in hospitalized patients with hyperglycemia.Diabetes Care.2003;26:1064–1068.
- ,,, et al.National standards for diabetes self‐management education.Diabetes Care.2006;29(suppl 1):S78–S85.
- Society of Hospital Medicine Glycemic Control Task Force. Workbook for improvement: improving glycemic control, preventing hypoglycemia and optimizing care of the inpatient with diabetes and hyperglycemia. page 105. Available at: http://www.hospitalmedicine.org/ResourceRoomRedesign/pdf/GC_Workbook.pdf. Accessed December,2007.
- Joslin Diabetes Center. EZ Start Patient Information Handouts. Available at: http://www.joslin.org/ezstart. Accessed December2007.
- ,,,.Promoting effective transitions of care at hospital discharge: a review of key issues for hospitalists.J Hosp Med.2007;2:314–323.
- ,,,,,.Deficits in communication and information transfer between hospital‐based and primary care physicians: implications for patient safety and continuity of care.JAMA.2007;297:831–841.
- Society of Hospital Medicine On‐line Clinical Tools. Ideal discharge for the elderly patient: a hospitalist checklist. Available at: http://www.hospitalmedicine.org/AM/Template.cfm?Section=QI_Clinical_Toolsemplate=/CM/ContentDisplay.cfmContentID=10303. Accessed December2007.
- ,,, et al.Inpatient to outpatient transfer of care in urban patients with diabetes: patterns and determinants of immediate post‐discharge follow‐up.Arch Intern Med.2004;164:447–453.
- ,,,,,.Inpatient to outpatient transfer of diabetes care: perceptions of barriers to postdischarge follow‐up in urban African American patients.Ethn Dis.2007;17:238–243.
Supporting Inpatient Glycemic Control Programs Now
Medical centers are faced with multiple competing priorities when deciding how to focus their improvement efforts and meet the ever expanding menu of publicly reported and regulatory issues. In this article we expand on the rationale for supporting inpatient glycemic control programs as a priority that should be moved near the top of the list. We review the evidence for establishing glycemic range targets, and also review the limitations of this evidence, acknowledging, as does the American Diabetes Association (ADA), that in both the critical care and non‐critical care venue, glycemic goals must take into account the individual patient's situation as well as hospital system support for achieving these goals.1, 2 We emphasize that inpatient glycemic control programs are needed to address a wide variety of quality and safety issues surrounding the care of the inpatient with diabetes and hyperglycemia, and we wish to elevate the dialogue beyond arguments surrounding adoption of one glycemic target versus another. The Society of Hospital Medicine Glycemic Control Task Force members are not in unanimous agreement with the American Association of Clinical Endocrinologists (AACE)/ADA inpatient glycemic targets. However, we do agree on several other important points, which we will expand on in this article:
-
Uncontrolled hyperglycemia and iatrogenic hypoglycemia are common and potentially dangerous situations that are largely preventable with safe and proven methods.
-
The current state of care for our inpatients with hyperglycemia is unacceptably poor on a broad scale, with substandard education, communication, coordination, and treatment issues.
-
Concerted efforts with changes in the design of the process of care are needed to improve this state of affairs.
DIABETES AND HYPERGLYCEMIA ARE VERY COMMON INPATIENT CONDITIONS
Diabetes mellitus (DM) has reached epidemic proportions in the United States. A reported 9.3% of adults over 20 years of age have diabetes, representing over 20 million persons. Despite increasing awareness, diabetes remains undiagnosed in approximately 30% of these persons.3 Concurrent with the increasing prevalence of diabetes in the U.S. population from 1980 through 2003, the number of hospital discharges with diabetes as any listed diagnosis more than doubled, going from 2.2 to 5.1 million discharges.4 Hospital care for patients with diabetes and hyperglycemia poses a significant health economic burden in the United States, representing over 40 billion dollars in annual direct medical expenditures.5
Hyperglycemia in the hospital may be due to known diabetes, to previously unrecognized diabetes, to prediabetes, and/or to the stress of surgery or illness. Deterioration in glycemic control in the hospital setting is most commonly associated with one or more factors, including stress‐induced release of insulin counterregulatory hormones (catecholamines, cortisol, glucagon, and growth hormone), exogenous administration of high dose glucocorticoids, and suboptimal glycemic management strategies.68 In a Belgian medical intensive care unit (MICU) randomized controlled trial (RCT) of strict versus conventional glycemic control, mean blood glucose (BG) on admission to the unit in the intention to treat group was 162 70 mg/dL (n = 1200),9 and in this group's RCT of 1548 surgical intensive care unit (SICU) patients, BG > 110 mg/dL was observed in over 70% of subjects.10 Mean BG of >145 mg/dL has been reported in 39%11 and BG >200 mg/dL in anywhere from 11% to 31% of intensive care unit (ICU) patients.10, 12 For general medicine and surgery, 1 study of 2030 patients admitted to a teaching hospital revealed that 26% of admissions had a known history of DM and 12% had new hyperglycemia, as evidenced by an admission or in‐hospital fasting BG of 126 mg/dL or more or a random BG of 200 mg/dL or more on 2 or more determinations.13 National and regional estimates on hospital use maintained by the Agency for Healthcare Research and Quality include data concerning diabetes diagnoses alone, without hyperglycemia, and may be displayed by querying its Web site.14 In cardiovascular populations almost 70% of patients having a first myocardial infarction have been reported to have either known DM, previously unrecognized diabetes, or impaired glucose tolerance.15
THE EVIDENCE SUPPORTS INPATIENT GLYCEMIC CONTROL
Evidence: Physiology
The pathophysiologic mechanisms through which hyperglycemia is linked to suboptimal outcomes in the hospital are complex and multifactorial. Although it is beyond the scope of this article to discuss these mechanisms in detail, research has broadly focused in the following areas: (1) immune system dysfunction, associated with a proinflammatory state and impaired white blood cell function; (2) metabolic derangements leading to oxidative stress, release of free fatty acids, reduction in endogenous insulin secretion, and fluid and electrolyte imbalance; and (3) a wide variety of vascular system responses (eg, endothelial dysfunction with impairment of tissue perfusion, a prothrombotic state, increased platelet aggregation, and left ventricular dysfunction).8, 1618
Conversely administration of insulin suppresses or reverses many of these abnormalities including generation of reactive oxygen species (ROS) and activation of inflammatory mechanisms,19 and leads to a fall in C‐reactive protein, which accompanied the clinical benefit of intensive insulin therapy (IIT) in the Leuven, Belgium, ICU population,20 and prevents mitochondrial abnormalities in hepatocytes.21 In the same surgical ICU cohort, Langouche et al.22 report suppression of intracellular adhesion molecule‐1 (ICAM‐1) and E‐selectin, markers of inflammation, and reduction in plasma nitric oxide (NO) and innate nitric oxide (iNOS) expression with insulin administration in patients treated with intravenous (IV) IIT.22 These data further support the role of insulin infusion in suppressing inflammation and endothelial dysfunction. The authors suggest that maintaining normoglycemia with IIT during critical illness protects the endothelium, thereby contributing to prevention of organ failure and death.22 Based on accumulating data in the literature such as that cited above, it has been suggested that a new paradigm in which glucose and insulin are related not only through their metabolic action but also through inflammatory mechanisms offers important potential therapeutic opportunities.19
Evidence: Epidemiology/Observational Studies/Non‐RCT Interventional Studies
A strong association between hospital hyperglycemia and negative outcomes has been reported in numerous observational studies in diverse adult medical and surgical settings. In over 1800 hospital admissions, those with new hyperglycemia had an in‐hospital mortality rate of 16% compared with 3% mortality in patients with known diabetes and 1.7% in normoglycemic patients (P 0.01). These data suggest that hyperglycemia due to previously unrecognized diabetes may be an independent marker of in‐hospital mortality.13
Hyperglycemia has been linked to adverse outcomes in myocardial infarction, stroke,2328 postoperative nosocomial infection risk, pneumonia, renal transplant, cancer chemotherapy, percutaneous coronary interventions, and cardiac surgery.2938 These observational studies have the usual limitations inherent in their design. Demonstrating a strong association of hyperglycemia with adverse outcomes is not a guarantee that the hyperglycemia is the cause for the poor outcome, as hyperglycemia can reflect a patient under more stress who is at a higher risk for adverse outcome. By the same token, the strong association of hyperglycemia with the risk of poor outcomes seen in these studies does not guarantee that euglycemia would mitigate this risk.
Nonetheless, there are several factors that make the body of evidence for glycemic control more compelling. First, the association has a rational physiologic basis as described above. Second, the associations are consistent across a variety of patient populations and disease entities, and demonstrate a dose‐response relationship. Third, in studies that control for comorbidities and severity of illness, hyperglycemia persists as an independent risk factor for adverse outcomes, whether the patient has a preexisting diagnosis of diabetes or not. Last, non‐RCT interventional studies and RCTs largely reinforce these studies.
The Portland Diabetic Project has reported prospective, nonrandomized data over 17 years on the use of an IV insulin therapy protocol in cardiac surgery patients.38 This program has implemented stepped lowering of target BG, with the most recent data report implementing a goal BG 150 mg/dL.35 The current protocol uses a BG target of 70110 mg/dL, but results have not yet been published.39 Mortality and deep sternal wound infection rates for patients with diabetes who remain on the IV insulin protocol for 3 days have been lowered to levels equivalent to those for nondiabetic patients. This group has also reported reductions in length of stay and cost‐effectiveness of targeted glycemic control in the cardiac surgery population.35 Their data have to a large extent driven a nationwide movement to implement targeted BG control in cardiac surgery patients.
Another large ICU study (mixed medical‐surgical, n = 800 patients) also supports a benefit through targeted BG control (130.7 versus 152.3 mg/dL, P 0.001) when compared with historical controls. This study demonstrated reduction in in‐hospital mortality (relative risk reduction 29.3%, P = 0.002), duration of ICU stay (10.8%, P = 0.04), acute renal failure (75%, P = 0.03), and blood transfusions (18.7%, P = 0.002),40 representing a similar magnitude of effect as was demonstrated by the Belgian group.
Evidence: RCTs
Evidence is accumulating that demonstrates an advantage in terms of morbidity and mortality when targeted glycemic control using intravenous insulin infusion is implemented in the hospital. The most robust data have been reported from ICU and cardiac surgery settings. The largest randomized, controlled study to date enrolled 1548 patients in a surgical ICU in Leuven, Belgium who were randomized to either intensive (IT) or conventional (CT) insulin therapy. Mean glucose attained was 103 19 and 153 33 mg/dL in each arm, respectively. The intensive insulin group demonstrated a reduction in both ICU (4.6% versus 8.0%) and in‐hospital mortality (7.2% versus 10.9%), as well as bloodstream infections, acute renal failure, transfusions, and polyneuropathy, the latter being reflected by duration of mechanical ventilation (P 0.01 for all). Although a similar study in an MICU did not achieve statistical significance in the overall intention‐to‐treat analysis, it did demonstrate reductions in mortality (from 52.5% to 43.0%) in patients with at least 3 days of ICU treatment. It should also be noted that in this MICU population hypoglycemia rates were higher and level of glycemic control attained not as rigorous as in the same group's SICU cohort, factors which may have had an impact on observed outcomes. A meta‐analysis of these two Leuven, Belgium, studies demonstrated a reduction in mortality (23.6% versus 20.4%, absolute risk reduction [ARR] 3.2%, P = 0.004)) in all patients treated with IIT, with a larger reduction in mortality (37.9% versus 30.1%, ARR 7.8%, P = 0.002) observed in patients with at least 3 days of IIT, as well as substantial reductions in morbidity.9, 10, 41, 42
Several other studies must be mentioned in this context. A small (n = 61), randomized study in another SICU did not show a mortality benefit, perhaps because the number of subjects was not adequate to reach statistical significance, but did result in a significant reduction in nosocomial infections in patients receiving IIT (BG = 125 versus 179 mg/dL, P 0.001).43 Two international multicenter studies recently stopped enrollment due to excess rates of hypoglycemia. The Volume Substitution and Insulin Therapy in Severe Sepsis (VISEP) study, in a mixed medical and surgical sepsis population, showed no significant reduction in mortality in the intensively‐treated group. Serious adverse events were reported according to standard definitions. Enrollment was stopped before the full number of subjects had been randomized. Among the 537 evaluable cases, hypoglycemia (BG 40 mg/dL) was reported as 17.0% in the IT group and 4.1% (P 0.001) in the control group,44 and the rate of serious adverse events was higher in the IT group (10.9% versus 5.2%, P = 0.01). It is notable that the rate of hypoglycemia was comparable to the 18.7% rate seen in the IT group in the Leuven, Belgium, medical ICU study.9 The Glucontrol study enrolled 855 medical and surgical ICU patients and was similarly terminated because of hypoglycemia (BG 40 mg/dL) at a rate of 8.6% compared to 2.4% in the control group (P 0.001). Insulin infusion protocols and outcome data have not yet been published.42, 45
These studies with very high hypoglycemia rates each used an algorithm based on the Leuven, Belgium, protocol. The rates of severe hypoglycemia are 34 that reported by a variety of others achieving similar or identical glycemic targets. Hypoglycemia should not be construed as a reason to not use a standardized insulin infusion protocol. In comparing protocols that have been published, it is apparent that rates of hypoglycemia differ substantially and that performance results of some algorithms are not necessarily replicable across sites.46 Dose‐defining designs can be substantively more sophisticated than those used in the trials mentioned, in some cases incorporating principles of control engineering. The variability of hypoglycemia rates under differing insulin infusion protocols is a compelling reason to devote institutional effort to monitoring the efficacy and safety of the infusion protocols that are used.
High‐level evidence from randomized, controlled trials demonstrating outcomes benefit through targeted BG control outside the ICU is lacking at this point in time, but it must be noted that feasibility is suggested by a recent randomized control trial (RABBIT2) that demonstrated the superiority of basal bolus insulin regimens to sliding scale insulin in securing glycemic control, without any increase in hypoglycemia.47
Summing Up the Evidence
It is clear that hyperglycemia is associated with negative clinical outcomes throughout the hospital, and level A evidence is available to support tight glucose control in the SICU setting. However, in view of the imperfect and incomplete nature of the evidence, controversy persists around how stringent glycemic targets should be in the ICU, on whether glycemic targets should differ between SICU and MICU patients, and especially what the targets should be in the non‐ICU setting. There should be hesitancy to extrapolate glycemic targets to be applied beyond the populations that have been studied with RCTs or to assume benefit for medical conditions that have not been examined for the impact of interventions to control hyperglycemia. Institutions might justifiably choose more liberal targets than those promoted in national recommendations/guidelines2, 4850 until safe attainment of more moderate goals is demonstrated. However, even critics agree that uncontrolled hyperglycemia exceeding 180200 mg/dL in any acute care setting is undesirable. Moreover, strong observational data showing the hazards of hyperglycemia in noncritical care units (even after adjustment for severity of illness) combined with the high rate of adverse drug events associated with insulin use, argue strongly for a standardized approach to treating diabetes and hyperglycemia in the hospital. Even though no RCTs exist demonstrating outcomes benefits of achieving glycemic target on wards, the alternatives to control of hyperglycemia using scheduled insulin therapy are unacceptable. Oral agent therapy is potentially dangerous and within the necessary timeframe is likely to be ineffective; sliding scale management is inferior to basal‐bolus insulin therapy, as shown inan RCT,47 and is unsafe; and on the wards improved glycemic control can be achieved simultaneously with a reduction in hypoglycemia.51
INPATIENT GLYCEMIC CONTROL IS INCREASINGLY INCORPORATED INTO PUBLIC REPORTING, GUIDELINES, REGULATORY AGENCY, AND NATIONAL QUALITY INITIATIVE PRIORITIES
National quality initiatives, public reporting, pay‐for‐performance, and guideline‐based care continue to play an increasingly important role in the U.S. healthcare system. Over the years these initiatives have focused on various disease states (venous thromboembolism, congestive heart failure, community‐acquired pneumonia, etc.) in an attempt to standardize care and improve patient safety and quality. Inpatient hyperglycemic control is also increasingly being incorporated into public reporting, regulatory compliance, and national quality initiatives.
Professional organizations such as the ADA2 and AACE50 have published guidelines supporting improved glycemic control, the safe use of insulin, and other measures to improve care for hyperglycemic inpatients. The AACE has a Web site dedicated to hospital hyperglycemia.52 The Society of Hospital Medicine48 has created a resource room on its Web site and a workbook for improvement49 on optimizing the care of inpatients with hyperglycemia and diabetes. The guidelines and Web sites help raise awareness and educate physicians and healthcare workers in inpatient glucose management. The American Heart Association has incorporated specific recommendation regarding inpatient diabetic management in its Get With the Guidelines.53
The Joint Commission54 has developed an advanced disease‐specific certification on inpatient diabetes. Disease management programs are important components of complex healthcare systems that serve to coordinate chronic care, promote early detection and prevention, and reduce overall healthcare costs. Certification is increasingly important to providers, payers, and healthcare institutions because it demonstrates a commitment to quality and patient safety. The Joint Commission disease‐specific care certification is a patient‐centered model focusing on the delivery of clinical care and relationship between the practitioner and the patient. The evaluation and resulting certification by the Joint Commission is based on 3 core components: (1) an assessment of compliance with consensus‐based national standards; (2) the effective use of established clinical practice guidelines to manage and optimize care; and (3) an organized approach to performance measurement and improved activities.55 For inpatient diabetes, the Joint Commission program has 7 major elements following the ADA recommendations, including general recommendations regarding diabetic documentation, BG targets, preventing hypoglycemia, diabetes care providers, diabetes self‐management education, medical nutrition therapy, and BG monitoring.54 This mirrors the Call to Action Consensus Conference essential elements for successful glycemic control programs.1
Other organizations such as the Surgical Care Improvement Partnership (SCIP) and National Surgical Quality Improvement Program (NSQIP) have included perioperative glycemic control measures, as it impacts surgical wound infections. The University HealthSystem Consortium (UHC) has benchmarking data and endorses perioperative glycemic control measures, whereas the Institute for Healthcare Improvement (IHI) has focused on safe use of insulin practices in its 5 Million Lives campaign.
HOSPITALIZATION IS A MOMENT OF OPPORTUNITY TO ASSESS AND INTERVENE
The benefits of outpatient glycemic control and quality preventive care are well established, and the reduction of adverse consequences of uncontrolled diabetes are a high priority in ambulatory medicine.5658 Hospitalization provides an opportunity to identify previously undiagnosed diabetes or prediabetes and, for patients with known diabetes, to assess and impact upon the long term course of diabetes.
As a first step, unless a recent hemoglobin A1C (HbA1c) is known, among hospitalized hyperglycemic patients an HbA1C should be obtained upon admission. Greci et al.59 showed that an HbA1c level >6.0% was 100% specific (14/14) and 57% sensitive (12/21) for the diagnosis of diabetes. Among patients having known diabetes, an HbA1C elevation on admission may justify intensification of preadmission management at the time of discharge. If discharge and postdischarge adjustments of preadmission regimens are planned in response to admission A1C elevations, then the modified long‐term treatment strategy can improve the A1C in the ambulatory setting.60 Moreover, the event of hospitalization is the ideal teachable moment for patients and their caregivers to improve self‐care activities. Yet floor nurses may be overwhelmed by the tasks of patient education. For ideal patient education, both a nutritionist and a diabetes nurse educator are needed to assess compliance with medication, diet, and other aspects of care.6163 There also is need for outpatient follow‐up education. Finally, at the time of discharge, there is a duty and an opportunity for the diabetes provider to communicate with outpatient care providers about the patient's regimen and glycemic control, and also, based on information gathered during the admission, to convey any evidence that might support the need for a change of long‐term strategy.64 Unfortunately, the opportunity that hospitalization presents to assess, educate, and intervene frequently is underused.1, 8, 51, 65
LARGE GAPS EXIST BETWEEN CURRENT AND OPTIMAL CARE
Despite the evidence that inpatient glycemic control is important for patient outcomes, and despite guidelines recommending tighter inpatient glycemic control, clinical practice has been slow to change. In many institutions, inpatient glycemic management has not improved over the past decade, and large gaps remain between current practice and optimal practice.
Studies of individual institutions provide several insights into gaps in care. For example, Schnipper et al.66 examined practices on the general medicine service of an academic medical center in Boston in 2004. Among 107 prospectively identified patients with a known diagnosis of diabetes or at least 1 glucose reading >200 mg/dL (excluding patients with diabetic ketoacidosis, hyperglycemic hyperosmolar state, or pregnancy), they found scheduled long‐acting insulin prescribed in 43% of patients, scheduled short‐acting/rapid‐acting insulin in only 4% of patients, and 80 of 89 patients (90%) on the same sliding scale insulin regimen despite widely varying insulin requirements. Thirty‐one percent of glucose readings were >180 mg/dL compared with 1.2% of readings 60 mg/dL (but 11% of patients had at least 1 episode of hypoglycemia). Of the 75 patients with at least 1 episode of hyperglycemia or hypoglycemia, only 35% had any change to their insulin regimen during the first 5 days of the hospitalization.
Other studies have confirmed this concept of clinical inertia (ie, recognition of the problem but failure to act).67 A study by Cook et al.68 of all hospitalized non‐ICU patients with diabetes or hyperglycemia and length of stay of 3 days between 2001 and 2004 showed that 20% of patients had persistent hyperglycemia during the hospitalization (defined as a mean glucose >200 mg/dL). Forty‐six percent of patients whose average glucose was in the top tertile did not have their insulin regimen intensified to a combination of short‐acting/rapid‐acting and long‐acting insulin, and 35% of these patients either had no change in their total daily insulin dose or actually had a decrease in their dose when comparing the last 24 hours with the first 24 hours of hospitalization, a concept they term negative therapeutic momentum.
Perhaps the most well‐balanced view of the current state of medical practice comes from the UHC benchmarking project.69 UHC is an alliance of 90 academic health centers. For the diabetes project, each institution reviewed the records of 50 randomly selected patients over 18 years of age with at least a 72‐hour length of stay, 1 of 7 prespecified Diagnosis Related Group (DRG) codes, and at least 2 consecutive glucose readings >180 mg/dL or the receipt of insulin any time during the hospitalization. Patients with a history of pancreatic transplant, pregnant at the time of admission, receiving hospice or comfort care, or receiving insulin for a reason other than glucose management were excluded. The study showed widespread gaps in processes and outcomes (Table 1). Moreover, performance varied widely across hospitals. For example, the morning glucose in the ICU on the second measurement day was 110 mg/dL in 18% of patients for the median‐performing hospital, with a range of 0% to 67% across all 37 measured hospitals. In the non‐ICU setting on the second measurement day, 26% of patients had all BG measurements = 180 mg/dL in the median‐performing hospital, with a range of 7% to 48%. Of note, hypoglycemia was relatively uncommon: in the median hospital, 2.4% of patient‐days had 1 or more BG readings 50 mg/dL (range: 0%8.6%). Finally, in the median‐performing hospital, effective insulin therapy (defined as short‐acting/rapid‐acting and long‐acting subcutaneous insulin, continuous insulin infusion, or subcutaneous insulin pump therapy) was prescribed in 45% of patients, with a range of 12% to 77% across measured hospitals.
| Key Performance Measure | Results for Median‐Performing Hospital (%) |
|---|---|
| |
| Documentation of diabetes | 100 |
| Hob A1c assessment within 30 days | 36.1 |
| Glucose measurement within 8 hours of admission | 78.6 |
| Glucose monitoring 4 times a day | 85.4 |
| Median glucose reading > 200 mg/dL | 10.3 |
| Effective insulin therapy* | 44.7 |
| ICU day 2 morning glucose 110 mg/dL | 17.7 |
| Non‐ICU day 2 all glucose readings 180 mg/dL | 26.3 |
| Patient‐days with at least 1 glucose reading 50 mg/dL | 2.4 |
FREQUENT PROBLEMS WITH COMMUNICATION AND COORDINATION
Those who work closely with frontline practitioners striving to improve inpatient glycemic management have noticed other deficiencies in care.1, 70 These include: a lack of coordination between feeding, BG measurement, and insulin administration, leading to mistimed and incorrectly dosed insulin; frequent use of sliding‐scale only regimens despite evidence that they are useless at best and harmful at worst;6, 47, 60, 71 discharge summaries that often do not mention follow‐up plans for hyperglycemic management; incomplete patient educational programs; breakdowns in care at transition points; nursing and medical staffs that are unevenly educated about the proper use of insulin; and patients who are often angry or confused about their diabetes care in the hospital. Collectively, these gaps in care serve as prime targets for any glycemic control program.
HYPOGLYCEMIA IS A PROMINENT INPATIENT SAFETY CONCERN
Hypoglycemia is common in the inpatient setting and is a legitimate safety concern. In a recently reported series of 2174 hospitalized patients receiving antihyperglycemic agents, it was found that 9.5% of patients experienced a total 484 hypoglycemic episodes (defined as 60 mg/dL).72 Hypoglycemia often occurred in the setting of insulin therapy and frequently resulted from a failure to recognize trends in BG readings or other clues that a patient was at risk for developing hypoglycemia.73 A common thread is the risk created by interruption of carbohydrate intake, noted by Fischer et al.73 and once again in the recent ICU study by Vriesendorp et al.74 Sources of error include: lack of coordination between feeding and medication administration, leading to mistiming of insulin action; lack of sufficient frequency in BG monitoring; lack of clarity or uniformity in the writing of orders; failure to recognize changes in insulin requirements due to advanced age, renal failure, liver disease, or change in clinical status; steroid use with subsequent tapering or interruption; changes in feeding; failure to reconcile medications; inappropriate use of oral antihyperglycemic agents, and communication or handoff failures.
It has been difficult to sort out whether hypoglycemia is a marker of severity of illness or whether it is an independent factor leading to poor outcomes. Observational studies lend credibility to the concept that patients having congestive heart failure or myocardial infarction may be at risk for excessive mortality if their average BG resides in the low end of the normal range.7578 Sympathetic activation occurs as the threshold for hypoglycemia is approached, such as occurs at BG = 70 or 72 mg/dL.79 Patients living with BG levels observed to be in the low end of the normal range might experience more severe but unobserved and undocumented episodes of neuroglycopenia. Arrhythmia and fatality have been directly attributed to strict glycemic control.80, 81 We are confronted with the need to interpret well conducted observational studies, evaluating subgroups at risk, and using multivariate analysis to assess the impact of hypoglycemia upon outcomes.82 In such studies, we will need to examine high‐risk subgroups, including cardiac patients, in particular, for the possibility that there is a J‐shaped curve for mortality as a function of average BG.
Unfortunately, clinical inertia exists in response to hypoglycemia just as it does with hyperglycemia. One recent study examined 52 patients who received intravenous 50% dextrose solution for an episode of hypoglycemia.83 Changes to insulin regimens were subsequently made in only 21 patients (40%), and diabetes specialists agreed with the changes for 11 of these patients. The other 31 patients (60%) received no changes in treatment, and diabetes specialists agreed with that decision for only 10 of these patients.
Although some increase in hypoglycemia might be expected with initiation of tight glycemic control efforts, the solution is not to undertreat hyperglycemia. Hyperglycemia creates an unsafe setting for the treatment of illness and disease. Sliding‐scaleonly regimens are ineffective in securing glycemic control and can result in increases in hypoglycemia as well as hyperglycemic excursions.6, 66 Inappropriate withholding of insulin doses can lead to severe glycemic excursions and even iatrogenic diabetic ketoacidosis (DKA). Systems approaches to avoid the errors outlined above can minimize or even reverse the increased risk of hypoglycemia expected with tighter glycemic targets.51
A SYSTEMS APPROACH IS NEEDED FOR THESE MULTIPLE COMPLEX PROBLEMS
Care is of the hyperglycemic inpatient is inherently complex. Previously established treatments are often inappropriate under conditions of altered insulin resistance, changing patterns of nutrition and carbohydrate exposure, comorbidities, concomitant medications, and rapidly changing medical and surgical status. Patients frequently undergo changes in the route and amount of nutritional exposure, including discrete meals, continuous intravenous dextrose, nil per orem (nothing by mouth status; NPO) status, grazing on nutritional supplements or liquid diets with or without meals, bolus enteral feedings, overnight enteral feedings with daytime grazing, total parenteral nutrition, continuous peritoneal dialysis, and overnight cycling of peritoneal dialysis. Relying on individual expertise and vigilance to negotiate this complex terrain without safeguards, protocols, standardization of orders, and other systems change is impractical and unwise.
Transitions across care providers and locations lead to multiple opportunities for breakdown in the quality, consistency, and safety of care.64, 65 At the time of ward transfer or change of patient status, previous medication and monitoring orders sometimes are purged. At the time of discharge, there may be risk of continuation of anti‐hyperglycemic therapy, initiated to cover medical stress, in doses that will subsequently be unsafe.
In the face of this complexity, educational programs alone will not suffice to improve care. Institutional commitment and systems changes are essential.
MARKED IMPROVEMENT IS POSSIBLE AND TOOLS EXIST: A ROADMAP IS IN PLACE
Fortunately, a roadmap is in place to help us achieve better glycemic control, improve insulin management, and address the long list of current deficiencies in care. This is imperative to develop consistent processes in order to achieve maximum patient quality outcomes that effective glycemic management offers. This roadmap entails 4 components: (1) national awareness, (2) national guidelines, (3) consensus statements, and (4) effective tools. As mentioned above, the first two components of this roadmap are now in place.
As these national guidelines become more widely accepted, the next step will be the incorporation of this into programs like Pay‐for Performance and the Physician Quality Reporting Initiative (PQRI), which will impact reimbursement to both hospitals and providers.
Regarding the third component, a recent multidisciplinary consensus conference1 outlined the essential elements needed for successful implementation of an inpatient glycemic control program which include:
-
An appropriate level of administrative support.
-
Formation of a multidisciplinary steering committee to drive the development of initiatives and empowered to enact change.
-
Assessment of current processes, quality of care, and barriers to practice change.
-
Development and implementation of interventions including standardized order sets, protocols, policies and algorithms with associated educational programs.
-
Metrics for evaluation of glycemic control, hypoglycemia, insulin use patterns, and other aspects of care.
Finally, extensive resources and effective tools are now available to help institutions achieve better inpatient glucose control. The Society of Hospital Medicine (SHM), in conjunction with the ADA, AACE, the American College of Physicians (ACP), the Case Management Society of America (CMSA), the American Society of Consultant Pharmacists, nursing, and diabetic educators have all partnered to produce a comprehensive guide to effective implementation of glycemic control and preventing hypoglycemia.49 This comprehensive workbook is a proven performance improvement framework and is available on the SHM Web site.48 Details and examples of all essential elements are covered in this workbook along with opportunities for marked improvement bolstered by integration of high reliability design features and attention to effective implementation techniques. The remainder of this supplement crystallizes a substantial portion of this material. The AACE has also recently offered a valuable web‐based resource to encourage institutional glycemic control efforts.49
GLYCEMIC CONTROL INITIATIVES CAN BE COST‐EFFECTIVE
Achieving optimal glycemic control safely requires monitoring, education, and other measures, which can be expensive, labor intensive, and require coordination of the services of many hospital divisions. This incremental expense has been shown to be cost‐effective in a variety of settings.1, 84, 85 The costs of glycemic control initiatives have demonstrated a good return on investment via:
-
Improved LOS, readmission rates, morbidity, and mortality.
-
Improved documentation of patient acuity and related payment for acuity.
-
Income generated via incremental physician and allied health professional billing.
CONCLUSION AND SUMMARY
Evidence exists that appropriate management of hyperglycemia improves outcomes, whereas the current state of affairs is that most medical centers currently manage this suboptimally. This is concerning given the magnitude of diabetes and hyperglycemia in our inpatient setting in the United States. To bring awareness to this issue, multiple initiatives (guidelines, certification programs, workbooks, etc.) are available by various organizations including the ADA, AACE, SCIP, NSQIP, IHI, UHC, the Joint Commission, and SHM. However, this is not enough. Change occurs at the local level, and institutional prioritization and support is needed to empower a multidisciplinary steering committee, with appropriate administrative support, to standardize and improve systems in the face of substantial cultural issues and complex barriers. Improved data collection and reporting, incremental monitoring, creation of metrics, and improved documentation are an absolutely necessary necessity to achieve breakthrough levels of improvement.
Now the time is right to make an assertive effort to improve inpatient glycemic control and related issues, and push for appropriate support at your institution to help achieve this in the interest of patient safety and optimal outcomes.
- American College of Endocrinology and American Diabetes Association Consensus Statement on Inpatient Diabetes and Glycemic Control: A call to action.Diabetes Care.2006;29:1955–1962.
- Standards of medical care in diabetes‐‐2008.Diabetes Care.2008;31(Suppl 1):S12–S54.
- ,,, et al.Prevalence of diabetes and impaired fasting glucose in adults in the U.S. population: National Health And Nutrition Examination Survey 1999–2002.Diabetes Care.2006;29:1263–1268.
- Centers for Disease Control and Prevention.National Diabetes Fact Sheet: General Information and National Estimates on Diabetes in the United States, 2005.Atlanta, GA:U.S. Department of Health and Human Services, Centers for Disease Control and Prevention,2005. Available at: http://www.cdc.gov/diabetes/pubs/factsheet05.htm. Accessed September 2007.
- ,,.Economic costs of diabetes in the US in 2002.Diabetes Care.2003;26:917–932.
- ,,.Glycemic control and sliding scale insulin use in medical inpatients with diabetes mellitus.Arch Intern Med.1997;157:545–552.
- ,,.Inpatient management of diabetes mellitus.Am J Med.2002;113:317–323.
- ,,, et al.Management of diabetes and hyperglycemia in hospitals.Diabetes Care.2004;27:553–591.
- ,,, et al.Intensive insulin therapy in the medical ICU.N Engl J Med.2006;354:449–461.
- ,,, et al.Intensive insulin therapy in critically ill patients.N Engl J Med.2001;345:1359–1367.
- .Association between hyperglycemia and increased hospital mortality in a heterogeneous population of critically ill patients.Mayo Clin Proc.2003;78:1471–1478.
- ,,,,.Unrecognized diabetes among hospitalized patients.Diabetes Care.1998;21:246–249.
- ,,,,,.Hyperglycemia: an independent marker of in‐hospital mortality in patients with undiagnosed diabetes.J Clin Endocrinol Metab.2002;87:978–982.
- United States Department of Health and Human Services Agency for Healthcare Research and Quality.2007. Available at: http://hcupnet.ahrq.gov. Accessed December 2007.
- ,,, et al.Glucose metabolism in patients with acute myocardial infarction and no previous diagnosis of diabetes mellitus: a prospective study.Lancet.2002;359:2140–2144.
- .Mechanism by which hyperglycemia plays a role in the setting of acute cardiovascular illness.Rev Cardiovasc Med.2006;7(Suppl 2):S35–S43.
- ,,,,,, et al.Stress hyperglycaemia is an independent predictor of left ventricular remodelling after first anterior myocardial infarction in non‐diabetic patients.Eur Heart J.2007;28:546–552.
- ,.Implications and treatment of acute hyperglycemia in the setting of acute myocardial infarction.Circulation.2007;115:e436–e439.
- ,,,,.Insulin infusion in acute illness.J Clin Invest.2005;115:2069–2072.
- ,,,,.Intensive insulin therapy exerts antiinflammatory effects in critically ill patients and counteracts the adverse effect of low mannose‐gind lectin levels.J Clin Endocrinol Metab.2003;88:1082–1088.
- ,,,,,.Protection of hepatocyte mitochondrial ultrastructure and function by strict blood glucose control with insulin in critically ill patients.Lancet.2005;365:53–59.
- ,,, et al.Intensive insulin therapy protects the endothelium of critically ill patients.J Clin Invest.2005;115:2277–2286.
- ,,,.The association between hyperglycaemia on admission and 180‐day mortality in acute myocardial infarction patients with and without diabetes.Diabet Med.2005;22:1321–1325.
- ,,, et al.Admission glucose and mortality in elderly patients hospitalized with acute myocardial infarction: implications for patients with and without recognized diabetes.Circulation.2005;111:3078–3086.
- ,,,.Glycometabolic state at admission: important risk marker of mortality in conventionally treated patients with diabetes mellitus and acute myocardial infarction: long‐term results from the Diabetes and Insulin‐Glucose Infusion in Acute Myocardial Infarction (DIGAMI) study.Circulation.1999;99:2626–2632.
- ,,,.Stress hyperglycaemia and increased risk of death after myocardial infarction in patients with and without diabetes: a systematic overview.Lancet.2000;355:773–778.
- ,,.How important is hyperglycemia during acute brain infarction?Neurologist.2004;10:195–200.
- ,,,,.Stress hyperglycemia and prognosis of stroke in nondiabetic and diabetic patients: a systematic overview.Stroke.2001;32:2426–2432.
- ,,,.Perioperative glycemic control and the risk of infectious complications in a cohort of adults with diabetes.Diabetes Care.1999;22:1408–1414.
- ,,, et al.Early postoperative glucose control predicts nosocomial infection rate in diabetic patients.JPEN J Parenter Enteral Nutr.1998;22:77–81.
- ,,,,,.The relation between hyperglycemia and outcomes in 2,471 patients admitted to the hospital with community‐acquired pneumonia.Diabetes Care.2005;28:810–815.
- ,,,,.Early peri‐operative glycaemic control and allograft rejection in patients with diabetes mellitus: a pilot study.Transplantation.2001;72:1321–1324.
- ,,, et al.Relation between the duration of remission and hyperglycemia during induction chemotherapy for acute lymphocytic leukemia with a hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone/methotrexate‐cytarabine regimen.Cancer.2004;100:1179–1185.
- ,,, et al.Effect of fasting glucose levels on mortality rate in patients with and without diabetes mellitus and coronary artery disease undergoing percutaneous coronary intervention.Am Heart J.2003;146:351–358.
- ,,.Effect of hyperglycemia and continuous intravenous insulin infusions on outcomes of cardiac surgical procedures: the Portland diabetic project.Endocr Pract.2004;10(Suppl 2):21–33.
- ,,, et al.Intraoperative hyperglycemia and perioperative outcomes in cardiac surgery patients.Mayo Clin Proc.2005;80:862–866.
- ,,,,.The association of diabetes and glucose control with surgical‐site infections among cardiothoracic surgery patients.Infect Control Hosp Epidemiol.2001;22:607–612.
- ,,.Glucose control lowers the risk of wound infection in diabetics after open heart operations.Ann Thorac Surg.1997;63:356–361.
- The Portland Protocol. Available at: http://www.providence.org/oregon/grograms_and_services/heart/portlandprotocol/. Accessed September2007.
- .Effect of an intensive glucose management protocol on the mortality of critically ill adult patients.Mayo Clin Proc.2004;79:992–1000.
- ,,, et al.Intensive insulin therapy in mixed medical/surgical intensive care units: benefit versus harm.Diabetes.2006;55:3151–3159.
- ,,.Tight blood glucose control with insulin in the ICU: facts and controversies.Chest.2007;132:268–278.
- ,.Reduction of nosocomial infections in the surgical intensive‐care unit by strict glycemic control.Endocr Pract.2004;10(Suppl 2):46–52.
- ,,,,,, et al.Intensive insulin therapy and pentastarch resuscitation in severe sepsis.N Engl J Med.2008;358:125–139.
- ,.Current controversies around tight glucose control in critically ill patients.Curr Opin Clin Nutr Metab Care.2007;10:206–209.
- ,,,.Designing and implementing insulin infusion protocols and order sets.J Hosp Med.2008;3(5):S42–S54.
- ,,,,,, et al.Randomized study of basal‐bolus insulin therapy in the inpatient management of patients with type 2 diabetes (RABBIT 2 trial).Diabetes Care.2007;30:2181–2186.
- Society of Hospital Medicine. Glycemic control resource room. Available at: http://www.hospitalmedicine.org/ResourceRoomRedesign/GlycemicControl.cfm. Accessed November2007.
- Society of Hospital Medicine. Workbook for improvement: improving glycemic control, preventing hypoglycemia, and optimizing care of the inpatient with hyperglycemia and diabetes. Available at: http://www.hospitalmedicine.org/ResourceRoomRedesign/pdf/GC_Workbook.pdf. Accessed November2007.
- ,,, et al.American College of Endocrinology position statement on inpatient diabetes and metabolic control.Endocr Pract.2004;10:77–82.
- ,,,,.Improved inpatient use of basal insulin, reduced hypoglycemia, and improved glycemic control: effect of structured subcutaneous insulin orders and an insulin management algorithm.J Hosp Med.2008. In press.
- American Association of Clinical Endocrinologists Inpatient Glycemic Control Resource Center.2007. Available at: http://resources.aace.com/index.asp. Accessed December 2007.
- American Heart Association. Get With the Guidelines. Available at: http://www.americanheart.org/getwiththeguidelines. Accessed November2007.
- Joint Commission. Disease Specific‐Care Certification. Available at:http://www.jointcommission.org/CertificationPrograms. Accessed November2007.
- The Joint Commission Disease‐Specific Certification Program. Range JE. Oncology issues. July/August2007:40–41.
- Anonymous.The Diabetes Control and Complications Trial Research Group (DCCT). The effect of intensive treatment of diabetes on the development and progression of long‐term complications in insulin‐dependent diabetes mellitus.N Engl J Med.1993;329:977–986.
- Intensive blood‐glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type, 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group.Lancet.1998;352:837–853.
- ,,,.Intensified multifactorial intervention in patients with type 2 diabetes mellitus and microalbuminuria: the Steno type 2 randomised study.Lancet.1999;353:617–622.
- ,,, et al.Utility of HbA1c levels for diabetes case finding in hospitalized patients with hyperglycemia.Diabetes Care.2003;26:1064–1068.
- ,,,.Eliminating inpatient sliding‐scale insulin: a reeducation project with medical house staff.Diabetes Care.2005;28:1008–1011.
- ,.Advanced carbohydrate counting. In:Practical Carbohydrate Counting: A How‐to‐Teach Guide for Health Professionals.Alexandria, VA:American Diabetes Association;2001:26–28.
- ,,,,.The evidence for the effectiveness of medical nutrition therapy in diabetes management.Diabetes Care.2002;25:608–613.
- ,,, et al.Inpatient management of diabetes and hyperglycemia: implications for nutrition practice and the food and nutrition professional.J Am Diet Assoc.2007;107:105–111.
- .The transition from insulin infusions to long‐term diabetes therapy: the argument for insulin analogs.Semin Thorac Cardiovasc Surg.2006;18:366–378.
- .Transitions paper.J Hosp Med.2008.
- ,,,,.Inpatient management of diabetes and hyperglycemia among general medicine patients at a large teaching hospital.J Hosp Med.2006;1:145–150.
- ,,, et al.Clinical inertia.Ann Intern Med.2001;135:825–834.
- ,,, et al.Diabetes care in hospitalized noncritically ill patients: more evidence for clinical inertia and negative therapeutic momentum.J Hosp Med.2007;2:203–211.
- University HealthSystem Consortium.Glycemic control 2005 findings and conclusions. Presented at: Glycemic Control 2005 Knowledge Transfer Meeting; 2005 August 19,2005; Chicago, IL.
- ,.Glycemic chaos (not glycemic control) still the rule for inpatient care: how do we stop the insanity?J Hosp Med.2006;1:141–144.
- ,,,,.Management of diabetes mellitus in hospitalized patients: efficiency and effectiveness of sliding‐scale insulin therapy.Pharmacotherapy.2006;26:1421–1432.
- ,,,,,.Hypoglycemia in hospitalized patients treated with antihyperglycemic agents.J Hosp Med.2007;2:234–240.
- ,,.Hypoglycemia in hospitalized patients.N Engl J Med.1986;315:1245–1250.
- ,,, et al.Predisposing factors for hypoglycemia in the intensive care unit.Crit Care Med.2006;34:96–101.
- ,,,.Association between hyper‐ and hypoglycaemia and 2 year all‐cause mortality risk in diabetic patients with acute coronary events.Eur Heart J.2005;26:1255–1261.
- ,,, et al.U‐shaped relationship of blood glucose with adverse outcomes among patients with ST‐segment elevation myocardial infarction.J Am Coll Cardiol.2005;46:178–180.
- ,,.An unexpected inverse relationship between HbA1c levels and mortality in patients with diabetes and advanced systolic heart failure.Am Heart J.2006;151:91.
- ,,, et al.Glucometrics in patients hospitalized with acute myocardial infarction: defining the optimal outcomes‐based measure of risk.Circulation.2008;117:1018–1027.
- ,,.Hypoglycemia in diabetes.Diabetes Care.2003;26:1902–1912.
- ,,.Hypoglycemia and cardiac arrest in a critically ill patient on strict glycemic control.Anesth Analg.2006;102:549–551.
- ,,,,,.Tight glycemic control in critically injured trauma patients.Ann Surg.2007;246:605–610; discussion 10–12.
- ,.Severe hypoglycemia in critically ill patients: risk factors and outcomes.Crit Care Med.2007;35:2262–2267.
- ,,,.Provider response to insulin‐induced hypoglycemia in hospitalized patients.J Hosp Med.2007;2:258–260.
- ,.Financial implications of glycemic control: results of an inpatient diabetes management program.Endocr Pract.2006;12(Suppl 3):43–48.
- ,,,.Impact of endocrine and diabetes team consultation on hospital length of stay for patients with diabetes.Am J Med.1995;99:22–28.
Medical centers are faced with multiple competing priorities when deciding how to focus their improvement efforts and meet the ever expanding menu of publicly reported and regulatory issues. In this article we expand on the rationale for supporting inpatient glycemic control programs as a priority that should be moved near the top of the list. We review the evidence for establishing glycemic range targets, and also review the limitations of this evidence, acknowledging, as does the American Diabetes Association (ADA), that in both the critical care and non‐critical care venue, glycemic goals must take into account the individual patient's situation as well as hospital system support for achieving these goals.1, 2 We emphasize that inpatient glycemic control programs are needed to address a wide variety of quality and safety issues surrounding the care of the inpatient with diabetes and hyperglycemia, and we wish to elevate the dialogue beyond arguments surrounding adoption of one glycemic target versus another. The Society of Hospital Medicine Glycemic Control Task Force members are not in unanimous agreement with the American Association of Clinical Endocrinologists (AACE)/ADA inpatient glycemic targets. However, we do agree on several other important points, which we will expand on in this article:
-
Uncontrolled hyperglycemia and iatrogenic hypoglycemia are common and potentially dangerous situations that are largely preventable with safe and proven methods.
-
The current state of care for our inpatients with hyperglycemia is unacceptably poor on a broad scale, with substandard education, communication, coordination, and treatment issues.
-
Concerted efforts with changes in the design of the process of care are needed to improve this state of affairs.
DIABETES AND HYPERGLYCEMIA ARE VERY COMMON INPATIENT CONDITIONS
Diabetes mellitus (DM) has reached epidemic proportions in the United States. A reported 9.3% of adults over 20 years of age have diabetes, representing over 20 million persons. Despite increasing awareness, diabetes remains undiagnosed in approximately 30% of these persons.3 Concurrent with the increasing prevalence of diabetes in the U.S. population from 1980 through 2003, the number of hospital discharges with diabetes as any listed diagnosis more than doubled, going from 2.2 to 5.1 million discharges.4 Hospital care for patients with diabetes and hyperglycemia poses a significant health economic burden in the United States, representing over 40 billion dollars in annual direct medical expenditures.5
Hyperglycemia in the hospital may be due to known diabetes, to previously unrecognized diabetes, to prediabetes, and/or to the stress of surgery or illness. Deterioration in glycemic control in the hospital setting is most commonly associated with one or more factors, including stress‐induced release of insulin counterregulatory hormones (catecholamines, cortisol, glucagon, and growth hormone), exogenous administration of high dose glucocorticoids, and suboptimal glycemic management strategies.68 In a Belgian medical intensive care unit (MICU) randomized controlled trial (RCT) of strict versus conventional glycemic control, mean blood glucose (BG) on admission to the unit in the intention to treat group was 162 70 mg/dL (n = 1200),9 and in this group's RCT of 1548 surgical intensive care unit (SICU) patients, BG > 110 mg/dL was observed in over 70% of subjects.10 Mean BG of >145 mg/dL has been reported in 39%11 and BG >200 mg/dL in anywhere from 11% to 31% of intensive care unit (ICU) patients.10, 12 For general medicine and surgery, 1 study of 2030 patients admitted to a teaching hospital revealed that 26% of admissions had a known history of DM and 12% had new hyperglycemia, as evidenced by an admission or in‐hospital fasting BG of 126 mg/dL or more or a random BG of 200 mg/dL or more on 2 or more determinations.13 National and regional estimates on hospital use maintained by the Agency for Healthcare Research and Quality include data concerning diabetes diagnoses alone, without hyperglycemia, and may be displayed by querying its Web site.14 In cardiovascular populations almost 70% of patients having a first myocardial infarction have been reported to have either known DM, previously unrecognized diabetes, or impaired glucose tolerance.15
THE EVIDENCE SUPPORTS INPATIENT GLYCEMIC CONTROL
Evidence: Physiology
The pathophysiologic mechanisms through which hyperglycemia is linked to suboptimal outcomes in the hospital are complex and multifactorial. Although it is beyond the scope of this article to discuss these mechanisms in detail, research has broadly focused in the following areas: (1) immune system dysfunction, associated with a proinflammatory state and impaired white blood cell function; (2) metabolic derangements leading to oxidative stress, release of free fatty acids, reduction in endogenous insulin secretion, and fluid and electrolyte imbalance; and (3) a wide variety of vascular system responses (eg, endothelial dysfunction with impairment of tissue perfusion, a prothrombotic state, increased platelet aggregation, and left ventricular dysfunction).8, 1618
Conversely administration of insulin suppresses or reverses many of these abnormalities including generation of reactive oxygen species (ROS) and activation of inflammatory mechanisms,19 and leads to a fall in C‐reactive protein, which accompanied the clinical benefit of intensive insulin therapy (IIT) in the Leuven, Belgium, ICU population,20 and prevents mitochondrial abnormalities in hepatocytes.21 In the same surgical ICU cohort, Langouche et al.22 report suppression of intracellular adhesion molecule‐1 (ICAM‐1) and E‐selectin, markers of inflammation, and reduction in plasma nitric oxide (NO) and innate nitric oxide (iNOS) expression with insulin administration in patients treated with intravenous (IV) IIT.22 These data further support the role of insulin infusion in suppressing inflammation and endothelial dysfunction. The authors suggest that maintaining normoglycemia with IIT during critical illness protects the endothelium, thereby contributing to prevention of organ failure and death.22 Based on accumulating data in the literature such as that cited above, it has been suggested that a new paradigm in which glucose and insulin are related not only through their metabolic action but also through inflammatory mechanisms offers important potential therapeutic opportunities.19
Evidence: Epidemiology/Observational Studies/Non‐RCT Interventional Studies
A strong association between hospital hyperglycemia and negative outcomes has been reported in numerous observational studies in diverse adult medical and surgical settings. In over 1800 hospital admissions, those with new hyperglycemia had an in‐hospital mortality rate of 16% compared with 3% mortality in patients with known diabetes and 1.7% in normoglycemic patients (P 0.01). These data suggest that hyperglycemia due to previously unrecognized diabetes may be an independent marker of in‐hospital mortality.13
Hyperglycemia has been linked to adverse outcomes in myocardial infarction, stroke,2328 postoperative nosocomial infection risk, pneumonia, renal transplant, cancer chemotherapy, percutaneous coronary interventions, and cardiac surgery.2938 These observational studies have the usual limitations inherent in their design. Demonstrating a strong association of hyperglycemia with adverse outcomes is not a guarantee that the hyperglycemia is the cause for the poor outcome, as hyperglycemia can reflect a patient under more stress who is at a higher risk for adverse outcome. By the same token, the strong association of hyperglycemia with the risk of poor outcomes seen in these studies does not guarantee that euglycemia would mitigate this risk.
Nonetheless, there are several factors that make the body of evidence for glycemic control more compelling. First, the association has a rational physiologic basis as described above. Second, the associations are consistent across a variety of patient populations and disease entities, and demonstrate a dose‐response relationship. Third, in studies that control for comorbidities and severity of illness, hyperglycemia persists as an independent risk factor for adverse outcomes, whether the patient has a preexisting diagnosis of diabetes or not. Last, non‐RCT interventional studies and RCTs largely reinforce these studies.
The Portland Diabetic Project has reported prospective, nonrandomized data over 17 years on the use of an IV insulin therapy protocol in cardiac surgery patients.38 This program has implemented stepped lowering of target BG, with the most recent data report implementing a goal BG 150 mg/dL.35 The current protocol uses a BG target of 70110 mg/dL, but results have not yet been published.39 Mortality and deep sternal wound infection rates for patients with diabetes who remain on the IV insulin protocol for 3 days have been lowered to levels equivalent to those for nondiabetic patients. This group has also reported reductions in length of stay and cost‐effectiveness of targeted glycemic control in the cardiac surgery population.35 Their data have to a large extent driven a nationwide movement to implement targeted BG control in cardiac surgery patients.
Another large ICU study (mixed medical‐surgical, n = 800 patients) also supports a benefit through targeted BG control (130.7 versus 152.3 mg/dL, P 0.001) when compared with historical controls. This study demonstrated reduction in in‐hospital mortality (relative risk reduction 29.3%, P = 0.002), duration of ICU stay (10.8%, P = 0.04), acute renal failure (75%, P = 0.03), and blood transfusions (18.7%, P = 0.002),40 representing a similar magnitude of effect as was demonstrated by the Belgian group.
Evidence: RCTs
Evidence is accumulating that demonstrates an advantage in terms of morbidity and mortality when targeted glycemic control using intravenous insulin infusion is implemented in the hospital. The most robust data have been reported from ICU and cardiac surgery settings. The largest randomized, controlled study to date enrolled 1548 patients in a surgical ICU in Leuven, Belgium who were randomized to either intensive (IT) or conventional (CT) insulin therapy. Mean glucose attained was 103 19 and 153 33 mg/dL in each arm, respectively. The intensive insulin group demonstrated a reduction in both ICU (4.6% versus 8.0%) and in‐hospital mortality (7.2% versus 10.9%), as well as bloodstream infections, acute renal failure, transfusions, and polyneuropathy, the latter being reflected by duration of mechanical ventilation (P 0.01 for all). Although a similar study in an MICU did not achieve statistical significance in the overall intention‐to‐treat analysis, it did demonstrate reductions in mortality (from 52.5% to 43.0%) in patients with at least 3 days of ICU treatment. It should also be noted that in this MICU population hypoglycemia rates were higher and level of glycemic control attained not as rigorous as in the same group's SICU cohort, factors which may have had an impact on observed outcomes. A meta‐analysis of these two Leuven, Belgium, studies demonstrated a reduction in mortality (23.6% versus 20.4%, absolute risk reduction [ARR] 3.2%, P = 0.004)) in all patients treated with IIT, with a larger reduction in mortality (37.9% versus 30.1%, ARR 7.8%, P = 0.002) observed in patients with at least 3 days of IIT, as well as substantial reductions in morbidity.9, 10, 41, 42
Several other studies must be mentioned in this context. A small (n = 61), randomized study in another SICU did not show a mortality benefit, perhaps because the number of subjects was not adequate to reach statistical significance, but did result in a significant reduction in nosocomial infections in patients receiving IIT (BG = 125 versus 179 mg/dL, P 0.001).43 Two international multicenter studies recently stopped enrollment due to excess rates of hypoglycemia. The Volume Substitution and Insulin Therapy in Severe Sepsis (VISEP) study, in a mixed medical and surgical sepsis population, showed no significant reduction in mortality in the intensively‐treated group. Serious adverse events were reported according to standard definitions. Enrollment was stopped before the full number of subjects had been randomized. Among the 537 evaluable cases, hypoglycemia (BG 40 mg/dL) was reported as 17.0% in the IT group and 4.1% (P 0.001) in the control group,44 and the rate of serious adverse events was higher in the IT group (10.9% versus 5.2%, P = 0.01). It is notable that the rate of hypoglycemia was comparable to the 18.7% rate seen in the IT group in the Leuven, Belgium, medical ICU study.9 The Glucontrol study enrolled 855 medical and surgical ICU patients and was similarly terminated because of hypoglycemia (BG 40 mg/dL) at a rate of 8.6% compared to 2.4% in the control group (P 0.001). Insulin infusion protocols and outcome data have not yet been published.42, 45
These studies with very high hypoglycemia rates each used an algorithm based on the Leuven, Belgium, protocol. The rates of severe hypoglycemia are 34 that reported by a variety of others achieving similar or identical glycemic targets. Hypoglycemia should not be construed as a reason to not use a standardized insulin infusion protocol. In comparing protocols that have been published, it is apparent that rates of hypoglycemia differ substantially and that performance results of some algorithms are not necessarily replicable across sites.46 Dose‐defining designs can be substantively more sophisticated than those used in the trials mentioned, in some cases incorporating principles of control engineering. The variability of hypoglycemia rates under differing insulin infusion protocols is a compelling reason to devote institutional effort to monitoring the efficacy and safety of the infusion protocols that are used.
High‐level evidence from randomized, controlled trials demonstrating outcomes benefit through targeted BG control outside the ICU is lacking at this point in time, but it must be noted that feasibility is suggested by a recent randomized control trial (RABBIT2) that demonstrated the superiority of basal bolus insulin regimens to sliding scale insulin in securing glycemic control, without any increase in hypoglycemia.47
Summing Up the Evidence
It is clear that hyperglycemia is associated with negative clinical outcomes throughout the hospital, and level A evidence is available to support tight glucose control in the SICU setting. However, in view of the imperfect and incomplete nature of the evidence, controversy persists around how stringent glycemic targets should be in the ICU, on whether glycemic targets should differ between SICU and MICU patients, and especially what the targets should be in the non‐ICU setting. There should be hesitancy to extrapolate glycemic targets to be applied beyond the populations that have been studied with RCTs or to assume benefit for medical conditions that have not been examined for the impact of interventions to control hyperglycemia. Institutions might justifiably choose more liberal targets than those promoted in national recommendations/guidelines2, 4850 until safe attainment of more moderate goals is demonstrated. However, even critics agree that uncontrolled hyperglycemia exceeding 180200 mg/dL in any acute care setting is undesirable. Moreover, strong observational data showing the hazards of hyperglycemia in noncritical care units (even after adjustment for severity of illness) combined with the high rate of adverse drug events associated with insulin use, argue strongly for a standardized approach to treating diabetes and hyperglycemia in the hospital. Even though no RCTs exist demonstrating outcomes benefits of achieving glycemic target on wards, the alternatives to control of hyperglycemia using scheduled insulin therapy are unacceptable. Oral agent therapy is potentially dangerous and within the necessary timeframe is likely to be ineffective; sliding scale management is inferior to basal‐bolus insulin therapy, as shown inan RCT,47 and is unsafe; and on the wards improved glycemic control can be achieved simultaneously with a reduction in hypoglycemia.51
INPATIENT GLYCEMIC CONTROL IS INCREASINGLY INCORPORATED INTO PUBLIC REPORTING, GUIDELINES, REGULATORY AGENCY, AND NATIONAL QUALITY INITIATIVE PRIORITIES
National quality initiatives, public reporting, pay‐for‐performance, and guideline‐based care continue to play an increasingly important role in the U.S. healthcare system. Over the years these initiatives have focused on various disease states (venous thromboembolism, congestive heart failure, community‐acquired pneumonia, etc.) in an attempt to standardize care and improve patient safety and quality. Inpatient hyperglycemic control is also increasingly being incorporated into public reporting, regulatory compliance, and national quality initiatives.
Professional organizations such as the ADA2 and AACE50 have published guidelines supporting improved glycemic control, the safe use of insulin, and other measures to improve care for hyperglycemic inpatients. The AACE has a Web site dedicated to hospital hyperglycemia.52 The Society of Hospital Medicine48 has created a resource room on its Web site and a workbook for improvement49 on optimizing the care of inpatients with hyperglycemia and diabetes. The guidelines and Web sites help raise awareness and educate physicians and healthcare workers in inpatient glucose management. The American Heart Association has incorporated specific recommendation regarding inpatient diabetic management in its Get With the Guidelines.53
The Joint Commission54 has developed an advanced disease‐specific certification on inpatient diabetes. Disease management programs are important components of complex healthcare systems that serve to coordinate chronic care, promote early detection and prevention, and reduce overall healthcare costs. Certification is increasingly important to providers, payers, and healthcare institutions because it demonstrates a commitment to quality and patient safety. The Joint Commission disease‐specific care certification is a patient‐centered model focusing on the delivery of clinical care and relationship between the practitioner and the patient. The evaluation and resulting certification by the Joint Commission is based on 3 core components: (1) an assessment of compliance with consensus‐based national standards; (2) the effective use of established clinical practice guidelines to manage and optimize care; and (3) an organized approach to performance measurement and improved activities.55 For inpatient diabetes, the Joint Commission program has 7 major elements following the ADA recommendations, including general recommendations regarding diabetic documentation, BG targets, preventing hypoglycemia, diabetes care providers, diabetes self‐management education, medical nutrition therapy, and BG monitoring.54 This mirrors the Call to Action Consensus Conference essential elements for successful glycemic control programs.1
Other organizations such as the Surgical Care Improvement Partnership (SCIP) and National Surgical Quality Improvement Program (NSQIP) have included perioperative glycemic control measures, as it impacts surgical wound infections. The University HealthSystem Consortium (UHC) has benchmarking data and endorses perioperative glycemic control measures, whereas the Institute for Healthcare Improvement (IHI) has focused on safe use of insulin practices in its 5 Million Lives campaign.
HOSPITALIZATION IS A MOMENT OF OPPORTUNITY TO ASSESS AND INTERVENE
The benefits of outpatient glycemic control and quality preventive care are well established, and the reduction of adverse consequences of uncontrolled diabetes are a high priority in ambulatory medicine.5658 Hospitalization provides an opportunity to identify previously undiagnosed diabetes or prediabetes and, for patients with known diabetes, to assess and impact upon the long term course of diabetes.
As a first step, unless a recent hemoglobin A1C (HbA1c) is known, among hospitalized hyperglycemic patients an HbA1C should be obtained upon admission. Greci et al.59 showed that an HbA1c level >6.0% was 100% specific (14/14) and 57% sensitive (12/21) for the diagnosis of diabetes. Among patients having known diabetes, an HbA1C elevation on admission may justify intensification of preadmission management at the time of discharge. If discharge and postdischarge adjustments of preadmission regimens are planned in response to admission A1C elevations, then the modified long‐term treatment strategy can improve the A1C in the ambulatory setting.60 Moreover, the event of hospitalization is the ideal teachable moment for patients and their caregivers to improve self‐care activities. Yet floor nurses may be overwhelmed by the tasks of patient education. For ideal patient education, both a nutritionist and a diabetes nurse educator are needed to assess compliance with medication, diet, and other aspects of care.6163 There also is need for outpatient follow‐up education. Finally, at the time of discharge, there is a duty and an opportunity for the diabetes provider to communicate with outpatient care providers about the patient's regimen and glycemic control, and also, based on information gathered during the admission, to convey any evidence that might support the need for a change of long‐term strategy.64 Unfortunately, the opportunity that hospitalization presents to assess, educate, and intervene frequently is underused.1, 8, 51, 65
LARGE GAPS EXIST BETWEEN CURRENT AND OPTIMAL CARE
Despite the evidence that inpatient glycemic control is important for patient outcomes, and despite guidelines recommending tighter inpatient glycemic control, clinical practice has been slow to change. In many institutions, inpatient glycemic management has not improved over the past decade, and large gaps remain between current practice and optimal practice.
Studies of individual institutions provide several insights into gaps in care. For example, Schnipper et al.66 examined practices on the general medicine service of an academic medical center in Boston in 2004. Among 107 prospectively identified patients with a known diagnosis of diabetes or at least 1 glucose reading >200 mg/dL (excluding patients with diabetic ketoacidosis, hyperglycemic hyperosmolar state, or pregnancy), they found scheduled long‐acting insulin prescribed in 43% of patients, scheduled short‐acting/rapid‐acting insulin in only 4% of patients, and 80 of 89 patients (90%) on the same sliding scale insulin regimen despite widely varying insulin requirements. Thirty‐one percent of glucose readings were >180 mg/dL compared with 1.2% of readings 60 mg/dL (but 11% of patients had at least 1 episode of hypoglycemia). Of the 75 patients with at least 1 episode of hyperglycemia or hypoglycemia, only 35% had any change to their insulin regimen during the first 5 days of the hospitalization.
Other studies have confirmed this concept of clinical inertia (ie, recognition of the problem but failure to act).67 A study by Cook et al.68 of all hospitalized non‐ICU patients with diabetes or hyperglycemia and length of stay of 3 days between 2001 and 2004 showed that 20% of patients had persistent hyperglycemia during the hospitalization (defined as a mean glucose >200 mg/dL). Forty‐six percent of patients whose average glucose was in the top tertile did not have their insulin regimen intensified to a combination of short‐acting/rapid‐acting and long‐acting insulin, and 35% of these patients either had no change in their total daily insulin dose or actually had a decrease in their dose when comparing the last 24 hours with the first 24 hours of hospitalization, a concept they term negative therapeutic momentum.
Perhaps the most well‐balanced view of the current state of medical practice comes from the UHC benchmarking project.69 UHC is an alliance of 90 academic health centers. For the diabetes project, each institution reviewed the records of 50 randomly selected patients over 18 years of age with at least a 72‐hour length of stay, 1 of 7 prespecified Diagnosis Related Group (DRG) codes, and at least 2 consecutive glucose readings >180 mg/dL or the receipt of insulin any time during the hospitalization. Patients with a history of pancreatic transplant, pregnant at the time of admission, receiving hospice or comfort care, or receiving insulin for a reason other than glucose management were excluded. The study showed widespread gaps in processes and outcomes (Table 1). Moreover, performance varied widely across hospitals. For example, the morning glucose in the ICU on the second measurement day was 110 mg/dL in 18% of patients for the median‐performing hospital, with a range of 0% to 67% across all 37 measured hospitals. In the non‐ICU setting on the second measurement day, 26% of patients had all BG measurements = 180 mg/dL in the median‐performing hospital, with a range of 7% to 48%. Of note, hypoglycemia was relatively uncommon: in the median hospital, 2.4% of patient‐days had 1 or more BG readings 50 mg/dL (range: 0%8.6%). Finally, in the median‐performing hospital, effective insulin therapy (defined as short‐acting/rapid‐acting and long‐acting subcutaneous insulin, continuous insulin infusion, or subcutaneous insulin pump therapy) was prescribed in 45% of patients, with a range of 12% to 77% across measured hospitals.
| Key Performance Measure | Results for Median‐Performing Hospital (%) |
|---|---|
| |
| Documentation of diabetes | 100 |
| Hob A1c assessment within 30 days | 36.1 |
| Glucose measurement within 8 hours of admission | 78.6 |
| Glucose monitoring 4 times a day | 85.4 |
| Median glucose reading > 200 mg/dL | 10.3 |
| Effective insulin therapy* | 44.7 |
| ICU day 2 morning glucose 110 mg/dL | 17.7 |
| Non‐ICU day 2 all glucose readings 180 mg/dL | 26.3 |
| Patient‐days with at least 1 glucose reading 50 mg/dL | 2.4 |
FREQUENT PROBLEMS WITH COMMUNICATION AND COORDINATION
Those who work closely with frontline practitioners striving to improve inpatient glycemic management have noticed other deficiencies in care.1, 70 These include: a lack of coordination between feeding, BG measurement, and insulin administration, leading to mistimed and incorrectly dosed insulin; frequent use of sliding‐scale only regimens despite evidence that they are useless at best and harmful at worst;6, 47, 60, 71 discharge summaries that often do not mention follow‐up plans for hyperglycemic management; incomplete patient educational programs; breakdowns in care at transition points; nursing and medical staffs that are unevenly educated about the proper use of insulin; and patients who are often angry or confused about their diabetes care in the hospital. Collectively, these gaps in care serve as prime targets for any glycemic control program.
HYPOGLYCEMIA IS A PROMINENT INPATIENT SAFETY CONCERN
Hypoglycemia is common in the inpatient setting and is a legitimate safety concern. In a recently reported series of 2174 hospitalized patients receiving antihyperglycemic agents, it was found that 9.5% of patients experienced a total 484 hypoglycemic episodes (defined as 60 mg/dL).72 Hypoglycemia often occurred in the setting of insulin therapy and frequently resulted from a failure to recognize trends in BG readings or other clues that a patient was at risk for developing hypoglycemia.73 A common thread is the risk created by interruption of carbohydrate intake, noted by Fischer et al.73 and once again in the recent ICU study by Vriesendorp et al.74 Sources of error include: lack of coordination between feeding and medication administration, leading to mistiming of insulin action; lack of sufficient frequency in BG monitoring; lack of clarity or uniformity in the writing of orders; failure to recognize changes in insulin requirements due to advanced age, renal failure, liver disease, or change in clinical status; steroid use with subsequent tapering or interruption; changes in feeding; failure to reconcile medications; inappropriate use of oral antihyperglycemic agents, and communication or handoff failures.
It has been difficult to sort out whether hypoglycemia is a marker of severity of illness or whether it is an independent factor leading to poor outcomes. Observational studies lend credibility to the concept that patients having congestive heart failure or myocardial infarction may be at risk for excessive mortality if their average BG resides in the low end of the normal range.7578 Sympathetic activation occurs as the threshold for hypoglycemia is approached, such as occurs at BG = 70 or 72 mg/dL.79 Patients living with BG levels observed to be in the low end of the normal range might experience more severe but unobserved and undocumented episodes of neuroglycopenia. Arrhythmia and fatality have been directly attributed to strict glycemic control.80, 81 We are confronted with the need to interpret well conducted observational studies, evaluating subgroups at risk, and using multivariate analysis to assess the impact of hypoglycemia upon outcomes.82 In such studies, we will need to examine high‐risk subgroups, including cardiac patients, in particular, for the possibility that there is a J‐shaped curve for mortality as a function of average BG.
Unfortunately, clinical inertia exists in response to hypoglycemia just as it does with hyperglycemia. One recent study examined 52 patients who received intravenous 50% dextrose solution for an episode of hypoglycemia.83 Changes to insulin regimens were subsequently made in only 21 patients (40%), and diabetes specialists agreed with the changes for 11 of these patients. The other 31 patients (60%) received no changes in treatment, and diabetes specialists agreed with that decision for only 10 of these patients.
Although some increase in hypoglycemia might be expected with initiation of tight glycemic control efforts, the solution is not to undertreat hyperglycemia. Hyperglycemia creates an unsafe setting for the treatment of illness and disease. Sliding‐scaleonly regimens are ineffective in securing glycemic control and can result in increases in hypoglycemia as well as hyperglycemic excursions.6, 66 Inappropriate withholding of insulin doses can lead to severe glycemic excursions and even iatrogenic diabetic ketoacidosis (DKA). Systems approaches to avoid the errors outlined above can minimize or even reverse the increased risk of hypoglycemia expected with tighter glycemic targets.51
A SYSTEMS APPROACH IS NEEDED FOR THESE MULTIPLE COMPLEX PROBLEMS
Care is of the hyperglycemic inpatient is inherently complex. Previously established treatments are often inappropriate under conditions of altered insulin resistance, changing patterns of nutrition and carbohydrate exposure, comorbidities, concomitant medications, and rapidly changing medical and surgical status. Patients frequently undergo changes in the route and amount of nutritional exposure, including discrete meals, continuous intravenous dextrose, nil per orem (nothing by mouth status; NPO) status, grazing on nutritional supplements or liquid diets with or without meals, bolus enteral feedings, overnight enteral feedings with daytime grazing, total parenteral nutrition, continuous peritoneal dialysis, and overnight cycling of peritoneal dialysis. Relying on individual expertise and vigilance to negotiate this complex terrain without safeguards, protocols, standardization of orders, and other systems change is impractical and unwise.
Transitions across care providers and locations lead to multiple opportunities for breakdown in the quality, consistency, and safety of care.64, 65 At the time of ward transfer or change of patient status, previous medication and monitoring orders sometimes are purged. At the time of discharge, there may be risk of continuation of anti‐hyperglycemic therapy, initiated to cover medical stress, in doses that will subsequently be unsafe.
In the face of this complexity, educational programs alone will not suffice to improve care. Institutional commitment and systems changes are essential.
MARKED IMPROVEMENT IS POSSIBLE AND TOOLS EXIST: A ROADMAP IS IN PLACE
Fortunately, a roadmap is in place to help us achieve better glycemic control, improve insulin management, and address the long list of current deficiencies in care. This is imperative to develop consistent processes in order to achieve maximum patient quality outcomes that effective glycemic management offers. This roadmap entails 4 components: (1) national awareness, (2) national guidelines, (3) consensus statements, and (4) effective tools. As mentioned above, the first two components of this roadmap are now in place.
As these national guidelines become more widely accepted, the next step will be the incorporation of this into programs like Pay‐for Performance and the Physician Quality Reporting Initiative (PQRI), which will impact reimbursement to both hospitals and providers.
Regarding the third component, a recent multidisciplinary consensus conference1 outlined the essential elements needed for successful implementation of an inpatient glycemic control program which include:
-
An appropriate level of administrative support.
-
Formation of a multidisciplinary steering committee to drive the development of initiatives and empowered to enact change.
-
Assessment of current processes, quality of care, and barriers to practice change.
-
Development and implementation of interventions including standardized order sets, protocols, policies and algorithms with associated educational programs.
-
Metrics for evaluation of glycemic control, hypoglycemia, insulin use patterns, and other aspects of care.
Finally, extensive resources and effective tools are now available to help institutions achieve better inpatient glucose control. The Society of Hospital Medicine (SHM), in conjunction with the ADA, AACE, the American College of Physicians (ACP), the Case Management Society of America (CMSA), the American Society of Consultant Pharmacists, nursing, and diabetic educators have all partnered to produce a comprehensive guide to effective implementation of glycemic control and preventing hypoglycemia.49 This comprehensive workbook is a proven performance improvement framework and is available on the SHM Web site.48 Details and examples of all essential elements are covered in this workbook along with opportunities for marked improvement bolstered by integration of high reliability design features and attention to effective implementation techniques. The remainder of this supplement crystallizes a substantial portion of this material. The AACE has also recently offered a valuable web‐based resource to encourage institutional glycemic control efforts.49
GLYCEMIC CONTROL INITIATIVES CAN BE COST‐EFFECTIVE
Achieving optimal glycemic control safely requires monitoring, education, and other measures, which can be expensive, labor intensive, and require coordination of the services of many hospital divisions. This incremental expense has been shown to be cost‐effective in a variety of settings.1, 84, 85 The costs of glycemic control initiatives have demonstrated a good return on investment via:
-
Improved LOS, readmission rates, morbidity, and mortality.
-
Improved documentation of patient acuity and related payment for acuity.
-
Income generated via incremental physician and allied health professional billing.
CONCLUSION AND SUMMARY
Evidence exists that appropriate management of hyperglycemia improves outcomes, whereas the current state of affairs is that most medical centers currently manage this suboptimally. This is concerning given the magnitude of diabetes and hyperglycemia in our inpatient setting in the United States. To bring awareness to this issue, multiple initiatives (guidelines, certification programs, workbooks, etc.) are available by various organizations including the ADA, AACE, SCIP, NSQIP, IHI, UHC, the Joint Commission, and SHM. However, this is not enough. Change occurs at the local level, and institutional prioritization and support is needed to empower a multidisciplinary steering committee, with appropriate administrative support, to standardize and improve systems in the face of substantial cultural issues and complex barriers. Improved data collection and reporting, incremental monitoring, creation of metrics, and improved documentation are an absolutely necessary necessity to achieve breakthrough levels of improvement.
Now the time is right to make an assertive effort to improve inpatient glycemic control and related issues, and push for appropriate support at your institution to help achieve this in the interest of patient safety and optimal outcomes.
Medical centers are faced with multiple competing priorities when deciding how to focus their improvement efforts and meet the ever expanding menu of publicly reported and regulatory issues. In this article we expand on the rationale for supporting inpatient glycemic control programs as a priority that should be moved near the top of the list. We review the evidence for establishing glycemic range targets, and also review the limitations of this evidence, acknowledging, as does the American Diabetes Association (ADA), that in both the critical care and non‐critical care venue, glycemic goals must take into account the individual patient's situation as well as hospital system support for achieving these goals.1, 2 We emphasize that inpatient glycemic control programs are needed to address a wide variety of quality and safety issues surrounding the care of the inpatient with diabetes and hyperglycemia, and we wish to elevate the dialogue beyond arguments surrounding adoption of one glycemic target versus another. The Society of Hospital Medicine Glycemic Control Task Force members are not in unanimous agreement with the American Association of Clinical Endocrinologists (AACE)/ADA inpatient glycemic targets. However, we do agree on several other important points, which we will expand on in this article:
-
Uncontrolled hyperglycemia and iatrogenic hypoglycemia are common and potentially dangerous situations that are largely preventable with safe and proven methods.
-
The current state of care for our inpatients with hyperglycemia is unacceptably poor on a broad scale, with substandard education, communication, coordination, and treatment issues.
-
Concerted efforts with changes in the design of the process of care are needed to improve this state of affairs.
DIABETES AND HYPERGLYCEMIA ARE VERY COMMON INPATIENT CONDITIONS
Diabetes mellitus (DM) has reached epidemic proportions in the United States. A reported 9.3% of adults over 20 years of age have diabetes, representing over 20 million persons. Despite increasing awareness, diabetes remains undiagnosed in approximately 30% of these persons.3 Concurrent with the increasing prevalence of diabetes in the U.S. population from 1980 through 2003, the number of hospital discharges with diabetes as any listed diagnosis more than doubled, going from 2.2 to 5.1 million discharges.4 Hospital care for patients with diabetes and hyperglycemia poses a significant health economic burden in the United States, representing over 40 billion dollars in annual direct medical expenditures.5
Hyperglycemia in the hospital may be due to known diabetes, to previously unrecognized diabetes, to prediabetes, and/or to the stress of surgery or illness. Deterioration in glycemic control in the hospital setting is most commonly associated with one or more factors, including stress‐induced release of insulin counterregulatory hormones (catecholamines, cortisol, glucagon, and growth hormone), exogenous administration of high dose glucocorticoids, and suboptimal glycemic management strategies.68 In a Belgian medical intensive care unit (MICU) randomized controlled trial (RCT) of strict versus conventional glycemic control, mean blood glucose (BG) on admission to the unit in the intention to treat group was 162 70 mg/dL (n = 1200),9 and in this group's RCT of 1548 surgical intensive care unit (SICU) patients, BG > 110 mg/dL was observed in over 70% of subjects.10 Mean BG of >145 mg/dL has been reported in 39%11 and BG >200 mg/dL in anywhere from 11% to 31% of intensive care unit (ICU) patients.10, 12 For general medicine and surgery, 1 study of 2030 patients admitted to a teaching hospital revealed that 26% of admissions had a known history of DM and 12% had new hyperglycemia, as evidenced by an admission or in‐hospital fasting BG of 126 mg/dL or more or a random BG of 200 mg/dL or more on 2 or more determinations.13 National and regional estimates on hospital use maintained by the Agency for Healthcare Research and Quality include data concerning diabetes diagnoses alone, without hyperglycemia, and may be displayed by querying its Web site.14 In cardiovascular populations almost 70% of patients having a first myocardial infarction have been reported to have either known DM, previously unrecognized diabetes, or impaired glucose tolerance.15
THE EVIDENCE SUPPORTS INPATIENT GLYCEMIC CONTROL
Evidence: Physiology
The pathophysiologic mechanisms through which hyperglycemia is linked to suboptimal outcomes in the hospital are complex and multifactorial. Although it is beyond the scope of this article to discuss these mechanisms in detail, research has broadly focused in the following areas: (1) immune system dysfunction, associated with a proinflammatory state and impaired white blood cell function; (2) metabolic derangements leading to oxidative stress, release of free fatty acids, reduction in endogenous insulin secretion, and fluid and electrolyte imbalance; and (3) a wide variety of vascular system responses (eg, endothelial dysfunction with impairment of tissue perfusion, a prothrombotic state, increased platelet aggregation, and left ventricular dysfunction).8, 1618
Conversely administration of insulin suppresses or reverses many of these abnormalities including generation of reactive oxygen species (ROS) and activation of inflammatory mechanisms,19 and leads to a fall in C‐reactive protein, which accompanied the clinical benefit of intensive insulin therapy (IIT) in the Leuven, Belgium, ICU population,20 and prevents mitochondrial abnormalities in hepatocytes.21 In the same surgical ICU cohort, Langouche et al.22 report suppression of intracellular adhesion molecule‐1 (ICAM‐1) and E‐selectin, markers of inflammation, and reduction in plasma nitric oxide (NO) and innate nitric oxide (iNOS) expression with insulin administration in patients treated with intravenous (IV) IIT.22 These data further support the role of insulin infusion in suppressing inflammation and endothelial dysfunction. The authors suggest that maintaining normoglycemia with IIT during critical illness protects the endothelium, thereby contributing to prevention of organ failure and death.22 Based on accumulating data in the literature such as that cited above, it has been suggested that a new paradigm in which glucose and insulin are related not only through their metabolic action but also through inflammatory mechanisms offers important potential therapeutic opportunities.19
Evidence: Epidemiology/Observational Studies/Non‐RCT Interventional Studies
A strong association between hospital hyperglycemia and negative outcomes has been reported in numerous observational studies in diverse adult medical and surgical settings. In over 1800 hospital admissions, those with new hyperglycemia had an in‐hospital mortality rate of 16% compared with 3% mortality in patients with known diabetes and 1.7% in normoglycemic patients (P 0.01). These data suggest that hyperglycemia due to previously unrecognized diabetes may be an independent marker of in‐hospital mortality.13
Hyperglycemia has been linked to adverse outcomes in myocardial infarction, stroke,2328 postoperative nosocomial infection risk, pneumonia, renal transplant, cancer chemotherapy, percutaneous coronary interventions, and cardiac surgery.2938 These observational studies have the usual limitations inherent in their design. Demonstrating a strong association of hyperglycemia with adverse outcomes is not a guarantee that the hyperglycemia is the cause for the poor outcome, as hyperglycemia can reflect a patient under more stress who is at a higher risk for adverse outcome. By the same token, the strong association of hyperglycemia with the risk of poor outcomes seen in these studies does not guarantee that euglycemia would mitigate this risk.
Nonetheless, there are several factors that make the body of evidence for glycemic control more compelling. First, the association has a rational physiologic basis as described above. Second, the associations are consistent across a variety of patient populations and disease entities, and demonstrate a dose‐response relationship. Third, in studies that control for comorbidities and severity of illness, hyperglycemia persists as an independent risk factor for adverse outcomes, whether the patient has a preexisting diagnosis of diabetes or not. Last, non‐RCT interventional studies and RCTs largely reinforce these studies.
The Portland Diabetic Project has reported prospective, nonrandomized data over 17 years on the use of an IV insulin therapy protocol in cardiac surgery patients.38 This program has implemented stepped lowering of target BG, with the most recent data report implementing a goal BG 150 mg/dL.35 The current protocol uses a BG target of 70110 mg/dL, but results have not yet been published.39 Mortality and deep sternal wound infection rates for patients with diabetes who remain on the IV insulin protocol for 3 days have been lowered to levels equivalent to those for nondiabetic patients. This group has also reported reductions in length of stay and cost‐effectiveness of targeted glycemic control in the cardiac surgery population.35 Their data have to a large extent driven a nationwide movement to implement targeted BG control in cardiac surgery patients.
Another large ICU study (mixed medical‐surgical, n = 800 patients) also supports a benefit through targeted BG control (130.7 versus 152.3 mg/dL, P 0.001) when compared with historical controls. This study demonstrated reduction in in‐hospital mortality (relative risk reduction 29.3%, P = 0.002), duration of ICU stay (10.8%, P = 0.04), acute renal failure (75%, P = 0.03), and blood transfusions (18.7%, P = 0.002),40 representing a similar magnitude of effect as was demonstrated by the Belgian group.
Evidence: RCTs
Evidence is accumulating that demonstrates an advantage in terms of morbidity and mortality when targeted glycemic control using intravenous insulin infusion is implemented in the hospital. The most robust data have been reported from ICU and cardiac surgery settings. The largest randomized, controlled study to date enrolled 1548 patients in a surgical ICU in Leuven, Belgium who were randomized to either intensive (IT) or conventional (CT) insulin therapy. Mean glucose attained was 103 19 and 153 33 mg/dL in each arm, respectively. The intensive insulin group demonstrated a reduction in both ICU (4.6% versus 8.0%) and in‐hospital mortality (7.2% versus 10.9%), as well as bloodstream infections, acute renal failure, transfusions, and polyneuropathy, the latter being reflected by duration of mechanical ventilation (P 0.01 for all). Although a similar study in an MICU did not achieve statistical significance in the overall intention‐to‐treat analysis, it did demonstrate reductions in mortality (from 52.5% to 43.0%) in patients with at least 3 days of ICU treatment. It should also be noted that in this MICU population hypoglycemia rates were higher and level of glycemic control attained not as rigorous as in the same group's SICU cohort, factors which may have had an impact on observed outcomes. A meta‐analysis of these two Leuven, Belgium, studies demonstrated a reduction in mortality (23.6% versus 20.4%, absolute risk reduction [ARR] 3.2%, P = 0.004)) in all patients treated with IIT, with a larger reduction in mortality (37.9% versus 30.1%, ARR 7.8%, P = 0.002) observed in patients with at least 3 days of IIT, as well as substantial reductions in morbidity.9, 10, 41, 42
Several other studies must be mentioned in this context. A small (n = 61), randomized study in another SICU did not show a mortality benefit, perhaps because the number of subjects was not adequate to reach statistical significance, but did result in a significant reduction in nosocomial infections in patients receiving IIT (BG = 125 versus 179 mg/dL, P 0.001).43 Two international multicenter studies recently stopped enrollment due to excess rates of hypoglycemia. The Volume Substitution and Insulin Therapy in Severe Sepsis (VISEP) study, in a mixed medical and surgical sepsis population, showed no significant reduction in mortality in the intensively‐treated group. Serious adverse events were reported according to standard definitions. Enrollment was stopped before the full number of subjects had been randomized. Among the 537 evaluable cases, hypoglycemia (BG 40 mg/dL) was reported as 17.0% in the IT group and 4.1% (P 0.001) in the control group,44 and the rate of serious adverse events was higher in the IT group (10.9% versus 5.2%, P = 0.01). It is notable that the rate of hypoglycemia was comparable to the 18.7% rate seen in the IT group in the Leuven, Belgium, medical ICU study.9 The Glucontrol study enrolled 855 medical and surgical ICU patients and was similarly terminated because of hypoglycemia (BG 40 mg/dL) at a rate of 8.6% compared to 2.4% in the control group (P 0.001). Insulin infusion protocols and outcome data have not yet been published.42, 45
These studies with very high hypoglycemia rates each used an algorithm based on the Leuven, Belgium, protocol. The rates of severe hypoglycemia are 34 that reported by a variety of others achieving similar or identical glycemic targets. Hypoglycemia should not be construed as a reason to not use a standardized insulin infusion protocol. In comparing protocols that have been published, it is apparent that rates of hypoglycemia differ substantially and that performance results of some algorithms are not necessarily replicable across sites.46 Dose‐defining designs can be substantively more sophisticated than those used in the trials mentioned, in some cases incorporating principles of control engineering. The variability of hypoglycemia rates under differing insulin infusion protocols is a compelling reason to devote institutional effort to monitoring the efficacy and safety of the infusion protocols that are used.
High‐level evidence from randomized, controlled trials demonstrating outcomes benefit through targeted BG control outside the ICU is lacking at this point in time, but it must be noted that feasibility is suggested by a recent randomized control trial (RABBIT2) that demonstrated the superiority of basal bolus insulin regimens to sliding scale insulin in securing glycemic control, without any increase in hypoglycemia.47
Summing Up the Evidence
It is clear that hyperglycemia is associated with negative clinical outcomes throughout the hospital, and level A evidence is available to support tight glucose control in the SICU setting. However, in view of the imperfect and incomplete nature of the evidence, controversy persists around how stringent glycemic targets should be in the ICU, on whether glycemic targets should differ between SICU and MICU patients, and especially what the targets should be in the non‐ICU setting. There should be hesitancy to extrapolate glycemic targets to be applied beyond the populations that have been studied with RCTs or to assume benefit for medical conditions that have not been examined for the impact of interventions to control hyperglycemia. Institutions might justifiably choose more liberal targets than those promoted in national recommendations/guidelines2, 4850 until safe attainment of more moderate goals is demonstrated. However, even critics agree that uncontrolled hyperglycemia exceeding 180200 mg/dL in any acute care setting is undesirable. Moreover, strong observational data showing the hazards of hyperglycemia in noncritical care units (even after adjustment for severity of illness) combined with the high rate of adverse drug events associated with insulin use, argue strongly for a standardized approach to treating diabetes and hyperglycemia in the hospital. Even though no RCTs exist demonstrating outcomes benefits of achieving glycemic target on wards, the alternatives to control of hyperglycemia using scheduled insulin therapy are unacceptable. Oral agent therapy is potentially dangerous and within the necessary timeframe is likely to be ineffective; sliding scale management is inferior to basal‐bolus insulin therapy, as shown inan RCT,47 and is unsafe; and on the wards improved glycemic control can be achieved simultaneously with a reduction in hypoglycemia.51
INPATIENT GLYCEMIC CONTROL IS INCREASINGLY INCORPORATED INTO PUBLIC REPORTING, GUIDELINES, REGULATORY AGENCY, AND NATIONAL QUALITY INITIATIVE PRIORITIES
National quality initiatives, public reporting, pay‐for‐performance, and guideline‐based care continue to play an increasingly important role in the U.S. healthcare system. Over the years these initiatives have focused on various disease states (venous thromboembolism, congestive heart failure, community‐acquired pneumonia, etc.) in an attempt to standardize care and improve patient safety and quality. Inpatient hyperglycemic control is also increasingly being incorporated into public reporting, regulatory compliance, and national quality initiatives.
Professional organizations such as the ADA2 and AACE50 have published guidelines supporting improved glycemic control, the safe use of insulin, and other measures to improve care for hyperglycemic inpatients. The AACE has a Web site dedicated to hospital hyperglycemia.52 The Society of Hospital Medicine48 has created a resource room on its Web site and a workbook for improvement49 on optimizing the care of inpatients with hyperglycemia and diabetes. The guidelines and Web sites help raise awareness and educate physicians and healthcare workers in inpatient glucose management. The American Heart Association has incorporated specific recommendation regarding inpatient diabetic management in its Get With the Guidelines.53
The Joint Commission54 has developed an advanced disease‐specific certification on inpatient diabetes. Disease management programs are important components of complex healthcare systems that serve to coordinate chronic care, promote early detection and prevention, and reduce overall healthcare costs. Certification is increasingly important to providers, payers, and healthcare institutions because it demonstrates a commitment to quality and patient safety. The Joint Commission disease‐specific care certification is a patient‐centered model focusing on the delivery of clinical care and relationship between the practitioner and the patient. The evaluation and resulting certification by the Joint Commission is based on 3 core components: (1) an assessment of compliance with consensus‐based national standards; (2) the effective use of established clinical practice guidelines to manage and optimize care; and (3) an organized approach to performance measurement and improved activities.55 For inpatient diabetes, the Joint Commission program has 7 major elements following the ADA recommendations, including general recommendations regarding diabetic documentation, BG targets, preventing hypoglycemia, diabetes care providers, diabetes self‐management education, medical nutrition therapy, and BG monitoring.54 This mirrors the Call to Action Consensus Conference essential elements for successful glycemic control programs.1
Other organizations such as the Surgical Care Improvement Partnership (SCIP) and National Surgical Quality Improvement Program (NSQIP) have included perioperative glycemic control measures, as it impacts surgical wound infections. The University HealthSystem Consortium (UHC) has benchmarking data and endorses perioperative glycemic control measures, whereas the Institute for Healthcare Improvement (IHI) has focused on safe use of insulin practices in its 5 Million Lives campaign.
HOSPITALIZATION IS A MOMENT OF OPPORTUNITY TO ASSESS AND INTERVENE
The benefits of outpatient glycemic control and quality preventive care are well established, and the reduction of adverse consequences of uncontrolled diabetes are a high priority in ambulatory medicine.5658 Hospitalization provides an opportunity to identify previously undiagnosed diabetes or prediabetes and, for patients with known diabetes, to assess and impact upon the long term course of diabetes.
As a first step, unless a recent hemoglobin A1C (HbA1c) is known, among hospitalized hyperglycemic patients an HbA1C should be obtained upon admission. Greci et al.59 showed that an HbA1c level >6.0% was 100% specific (14/14) and 57% sensitive (12/21) for the diagnosis of diabetes. Among patients having known diabetes, an HbA1C elevation on admission may justify intensification of preadmission management at the time of discharge. If discharge and postdischarge adjustments of preadmission regimens are planned in response to admission A1C elevations, then the modified long‐term treatment strategy can improve the A1C in the ambulatory setting.60 Moreover, the event of hospitalization is the ideal teachable moment for patients and their caregivers to improve self‐care activities. Yet floor nurses may be overwhelmed by the tasks of patient education. For ideal patient education, both a nutritionist and a diabetes nurse educator are needed to assess compliance with medication, diet, and other aspects of care.6163 There also is need for outpatient follow‐up education. Finally, at the time of discharge, there is a duty and an opportunity for the diabetes provider to communicate with outpatient care providers about the patient's regimen and glycemic control, and also, based on information gathered during the admission, to convey any evidence that might support the need for a change of long‐term strategy.64 Unfortunately, the opportunity that hospitalization presents to assess, educate, and intervene frequently is underused.1, 8, 51, 65
LARGE GAPS EXIST BETWEEN CURRENT AND OPTIMAL CARE
Despite the evidence that inpatient glycemic control is important for patient outcomes, and despite guidelines recommending tighter inpatient glycemic control, clinical practice has been slow to change. In many institutions, inpatient glycemic management has not improved over the past decade, and large gaps remain between current practice and optimal practice.
Studies of individual institutions provide several insights into gaps in care. For example, Schnipper et al.66 examined practices on the general medicine service of an academic medical center in Boston in 2004. Among 107 prospectively identified patients with a known diagnosis of diabetes or at least 1 glucose reading >200 mg/dL (excluding patients with diabetic ketoacidosis, hyperglycemic hyperosmolar state, or pregnancy), they found scheduled long‐acting insulin prescribed in 43% of patients, scheduled short‐acting/rapid‐acting insulin in only 4% of patients, and 80 of 89 patients (90%) on the same sliding scale insulin regimen despite widely varying insulin requirements. Thirty‐one percent of glucose readings were >180 mg/dL compared with 1.2% of readings 60 mg/dL (but 11% of patients had at least 1 episode of hypoglycemia). Of the 75 patients with at least 1 episode of hyperglycemia or hypoglycemia, only 35% had any change to their insulin regimen during the first 5 days of the hospitalization.
Other studies have confirmed this concept of clinical inertia (ie, recognition of the problem but failure to act).67 A study by Cook et al.68 of all hospitalized non‐ICU patients with diabetes or hyperglycemia and length of stay of 3 days between 2001 and 2004 showed that 20% of patients had persistent hyperglycemia during the hospitalization (defined as a mean glucose >200 mg/dL). Forty‐six percent of patients whose average glucose was in the top tertile did not have their insulin regimen intensified to a combination of short‐acting/rapid‐acting and long‐acting insulin, and 35% of these patients either had no change in their total daily insulin dose or actually had a decrease in their dose when comparing the last 24 hours with the first 24 hours of hospitalization, a concept they term negative therapeutic momentum.
Perhaps the most well‐balanced view of the current state of medical practice comes from the UHC benchmarking project.69 UHC is an alliance of 90 academic health centers. For the diabetes project, each institution reviewed the records of 50 randomly selected patients over 18 years of age with at least a 72‐hour length of stay, 1 of 7 prespecified Diagnosis Related Group (DRG) codes, and at least 2 consecutive glucose readings >180 mg/dL or the receipt of insulin any time during the hospitalization. Patients with a history of pancreatic transplant, pregnant at the time of admission, receiving hospice or comfort care, or receiving insulin for a reason other than glucose management were excluded. The study showed widespread gaps in processes and outcomes (Table 1). Moreover, performance varied widely across hospitals. For example, the morning glucose in the ICU on the second measurement day was 110 mg/dL in 18% of patients for the median‐performing hospital, with a range of 0% to 67% across all 37 measured hospitals. In the non‐ICU setting on the second measurement day, 26% of patients had all BG measurements = 180 mg/dL in the median‐performing hospital, with a range of 7% to 48%. Of note, hypoglycemia was relatively uncommon: in the median hospital, 2.4% of patient‐days had 1 or more BG readings 50 mg/dL (range: 0%8.6%). Finally, in the median‐performing hospital, effective insulin therapy (defined as short‐acting/rapid‐acting and long‐acting subcutaneous insulin, continuous insulin infusion, or subcutaneous insulin pump therapy) was prescribed in 45% of patients, with a range of 12% to 77% across measured hospitals.
| Key Performance Measure | Results for Median‐Performing Hospital (%) |
|---|---|
| |
| Documentation of diabetes | 100 |
| Hob A1c assessment within 30 days | 36.1 |
| Glucose measurement within 8 hours of admission | 78.6 |
| Glucose monitoring 4 times a day | 85.4 |
| Median glucose reading > 200 mg/dL | 10.3 |
| Effective insulin therapy* | 44.7 |
| ICU day 2 morning glucose 110 mg/dL | 17.7 |
| Non‐ICU day 2 all glucose readings 180 mg/dL | 26.3 |
| Patient‐days with at least 1 glucose reading 50 mg/dL | 2.4 |
FREQUENT PROBLEMS WITH COMMUNICATION AND COORDINATION
Those who work closely with frontline practitioners striving to improve inpatient glycemic management have noticed other deficiencies in care.1, 70 These include: a lack of coordination between feeding, BG measurement, and insulin administration, leading to mistimed and incorrectly dosed insulin; frequent use of sliding‐scale only regimens despite evidence that they are useless at best and harmful at worst;6, 47, 60, 71 discharge summaries that often do not mention follow‐up plans for hyperglycemic management; incomplete patient educational programs; breakdowns in care at transition points; nursing and medical staffs that are unevenly educated about the proper use of insulin; and patients who are often angry or confused about their diabetes care in the hospital. Collectively, these gaps in care serve as prime targets for any glycemic control program.
HYPOGLYCEMIA IS A PROMINENT INPATIENT SAFETY CONCERN
Hypoglycemia is common in the inpatient setting and is a legitimate safety concern. In a recently reported series of 2174 hospitalized patients receiving antihyperglycemic agents, it was found that 9.5% of patients experienced a total 484 hypoglycemic episodes (defined as 60 mg/dL).72 Hypoglycemia often occurred in the setting of insulin therapy and frequently resulted from a failure to recognize trends in BG readings or other clues that a patient was at risk for developing hypoglycemia.73 A common thread is the risk created by interruption of carbohydrate intake, noted by Fischer et al.73 and once again in the recent ICU study by Vriesendorp et al.74 Sources of error include: lack of coordination between feeding and medication administration, leading to mistiming of insulin action; lack of sufficient frequency in BG monitoring; lack of clarity or uniformity in the writing of orders; failure to recognize changes in insulin requirements due to advanced age, renal failure, liver disease, or change in clinical status; steroid use with subsequent tapering or interruption; changes in feeding; failure to reconcile medications; inappropriate use of oral antihyperglycemic agents, and communication or handoff failures.
It has been difficult to sort out whether hypoglycemia is a marker of severity of illness or whether it is an independent factor leading to poor outcomes. Observational studies lend credibility to the concept that patients having congestive heart failure or myocardial infarction may be at risk for excessive mortality if their average BG resides in the low end of the normal range.7578 Sympathetic activation occurs as the threshold for hypoglycemia is approached, such as occurs at BG = 70 or 72 mg/dL.79 Patients living with BG levels observed to be in the low end of the normal range might experience more severe but unobserved and undocumented episodes of neuroglycopenia. Arrhythmia and fatality have been directly attributed to strict glycemic control.80, 81 We are confronted with the need to interpret well conducted observational studies, evaluating subgroups at risk, and using multivariate analysis to assess the impact of hypoglycemia upon outcomes.82 In such studies, we will need to examine high‐risk subgroups, including cardiac patients, in particular, for the possibility that there is a J‐shaped curve for mortality as a function of average BG.
Unfortunately, clinical inertia exists in response to hypoglycemia just as it does with hyperglycemia. One recent study examined 52 patients who received intravenous 50% dextrose solution for an episode of hypoglycemia.83 Changes to insulin regimens were subsequently made in only 21 patients (40%), and diabetes specialists agreed with the changes for 11 of these patients. The other 31 patients (60%) received no changes in treatment, and diabetes specialists agreed with that decision for only 10 of these patients.
Although some increase in hypoglycemia might be expected with initiation of tight glycemic control efforts, the solution is not to undertreat hyperglycemia. Hyperglycemia creates an unsafe setting for the treatment of illness and disease. Sliding‐scaleonly regimens are ineffective in securing glycemic control and can result in increases in hypoglycemia as well as hyperglycemic excursions.6, 66 Inappropriate withholding of insulin doses can lead to severe glycemic excursions and even iatrogenic diabetic ketoacidosis (DKA). Systems approaches to avoid the errors outlined above can minimize or even reverse the increased risk of hypoglycemia expected with tighter glycemic targets.51
A SYSTEMS APPROACH IS NEEDED FOR THESE MULTIPLE COMPLEX PROBLEMS
Care is of the hyperglycemic inpatient is inherently complex. Previously established treatments are often inappropriate under conditions of altered insulin resistance, changing patterns of nutrition and carbohydrate exposure, comorbidities, concomitant medications, and rapidly changing medical and surgical status. Patients frequently undergo changes in the route and amount of nutritional exposure, including discrete meals, continuous intravenous dextrose, nil per orem (nothing by mouth status; NPO) status, grazing on nutritional supplements or liquid diets with or without meals, bolus enteral feedings, overnight enteral feedings with daytime grazing, total parenteral nutrition, continuous peritoneal dialysis, and overnight cycling of peritoneal dialysis. Relying on individual expertise and vigilance to negotiate this complex terrain without safeguards, protocols, standardization of orders, and other systems change is impractical and unwise.
Transitions across care providers and locations lead to multiple opportunities for breakdown in the quality, consistency, and safety of care.64, 65 At the time of ward transfer or change of patient status, previous medication and monitoring orders sometimes are purged. At the time of discharge, there may be risk of continuation of anti‐hyperglycemic therapy, initiated to cover medical stress, in doses that will subsequently be unsafe.
In the face of this complexity, educational programs alone will not suffice to improve care. Institutional commitment and systems changes are essential.
MARKED IMPROVEMENT IS POSSIBLE AND TOOLS EXIST: A ROADMAP IS IN PLACE
Fortunately, a roadmap is in place to help us achieve better glycemic control, improve insulin management, and address the long list of current deficiencies in care. This is imperative to develop consistent processes in order to achieve maximum patient quality outcomes that effective glycemic management offers. This roadmap entails 4 components: (1) national awareness, (2) national guidelines, (3) consensus statements, and (4) effective tools. As mentioned above, the first two components of this roadmap are now in place.
As these national guidelines become more widely accepted, the next step will be the incorporation of this into programs like Pay‐for Performance and the Physician Quality Reporting Initiative (PQRI), which will impact reimbursement to both hospitals and providers.
Regarding the third component, a recent multidisciplinary consensus conference1 outlined the essential elements needed for successful implementation of an inpatient glycemic control program which include:
-
An appropriate level of administrative support.
-
Formation of a multidisciplinary steering committee to drive the development of initiatives and empowered to enact change.
-
Assessment of current processes, quality of care, and barriers to practice change.
-
Development and implementation of interventions including standardized order sets, protocols, policies and algorithms with associated educational programs.
-
Metrics for evaluation of glycemic control, hypoglycemia, insulin use patterns, and other aspects of care.
Finally, extensive resources and effective tools are now available to help institutions achieve better inpatient glucose control. The Society of Hospital Medicine (SHM), in conjunction with the ADA, AACE, the American College of Physicians (ACP), the Case Management Society of America (CMSA), the American Society of Consultant Pharmacists, nursing, and diabetic educators have all partnered to produce a comprehensive guide to effective implementation of glycemic control and preventing hypoglycemia.49 This comprehensive workbook is a proven performance improvement framework and is available on the SHM Web site.48 Details and examples of all essential elements are covered in this workbook along with opportunities for marked improvement bolstered by integration of high reliability design features and attention to effective implementation techniques. The remainder of this supplement crystallizes a substantial portion of this material. The AACE has also recently offered a valuable web‐based resource to encourage institutional glycemic control efforts.49
GLYCEMIC CONTROL INITIATIVES CAN BE COST‐EFFECTIVE
Achieving optimal glycemic control safely requires monitoring, education, and other measures, which can be expensive, labor intensive, and require coordination of the services of many hospital divisions. This incremental expense has been shown to be cost‐effective in a variety of settings.1, 84, 85 The costs of glycemic control initiatives have demonstrated a good return on investment via:
-
Improved LOS, readmission rates, morbidity, and mortality.
-
Improved documentation of patient acuity and related payment for acuity.
-
Income generated via incremental physician and allied health professional billing.
CONCLUSION AND SUMMARY
Evidence exists that appropriate management of hyperglycemia improves outcomes, whereas the current state of affairs is that most medical centers currently manage this suboptimally. This is concerning given the magnitude of diabetes and hyperglycemia in our inpatient setting in the United States. To bring awareness to this issue, multiple initiatives (guidelines, certification programs, workbooks, etc.) are available by various organizations including the ADA, AACE, SCIP, NSQIP, IHI, UHC, the Joint Commission, and SHM. However, this is not enough. Change occurs at the local level, and institutional prioritization and support is needed to empower a multidisciplinary steering committee, with appropriate administrative support, to standardize and improve systems in the face of substantial cultural issues and complex barriers. Improved data collection and reporting, incremental monitoring, creation of metrics, and improved documentation are an absolutely necessary necessity to achieve breakthrough levels of improvement.
Now the time is right to make an assertive effort to improve inpatient glycemic control and related issues, and push for appropriate support at your institution to help achieve this in the interest of patient safety and optimal outcomes.
- American College of Endocrinology and American Diabetes Association Consensus Statement on Inpatient Diabetes and Glycemic Control: A call to action.Diabetes Care.2006;29:1955–1962.
- Standards of medical care in diabetes‐‐2008.Diabetes Care.2008;31(Suppl 1):S12–S54.
- ,,, et al.Prevalence of diabetes and impaired fasting glucose in adults in the U.S. population: National Health And Nutrition Examination Survey 1999–2002.Diabetes Care.2006;29:1263–1268.
- Centers for Disease Control and Prevention.National Diabetes Fact Sheet: General Information and National Estimates on Diabetes in the United States, 2005.Atlanta, GA:U.S. Department of Health and Human Services, Centers for Disease Control and Prevention,2005. Available at: http://www.cdc.gov/diabetes/pubs/factsheet05.htm. Accessed September 2007.
- ,,.Economic costs of diabetes in the US in 2002.Diabetes Care.2003;26:917–932.
- ,,.Glycemic control and sliding scale insulin use in medical inpatients with diabetes mellitus.Arch Intern Med.1997;157:545–552.
- ,,.Inpatient management of diabetes mellitus.Am J Med.2002;113:317–323.
- ,,, et al.Management of diabetes and hyperglycemia in hospitals.Diabetes Care.2004;27:553–591.
- ,,, et al.Intensive insulin therapy in the medical ICU.N Engl J Med.2006;354:449–461.
- ,,, et al.Intensive insulin therapy in critically ill patients.N Engl J Med.2001;345:1359–1367.
- .Association between hyperglycemia and increased hospital mortality in a heterogeneous population of critically ill patients.Mayo Clin Proc.2003;78:1471–1478.
- ,,,,.Unrecognized diabetes among hospitalized patients.Diabetes Care.1998;21:246–249.
- ,,,,,.Hyperglycemia: an independent marker of in‐hospital mortality in patients with undiagnosed diabetes.J Clin Endocrinol Metab.2002;87:978–982.
- United States Department of Health and Human Services Agency for Healthcare Research and Quality.2007. Available at: http://hcupnet.ahrq.gov. Accessed December 2007.
- ,,, et al.Glucose metabolism in patients with acute myocardial infarction and no previous diagnosis of diabetes mellitus: a prospective study.Lancet.2002;359:2140–2144.
- .Mechanism by which hyperglycemia plays a role in the setting of acute cardiovascular illness.Rev Cardiovasc Med.2006;7(Suppl 2):S35–S43.
- ,,,,,, et al.Stress hyperglycaemia is an independent predictor of left ventricular remodelling after first anterior myocardial infarction in non‐diabetic patients.Eur Heart J.2007;28:546–552.
- ,.Implications and treatment of acute hyperglycemia in the setting of acute myocardial infarction.Circulation.2007;115:e436–e439.
- ,,,,.Insulin infusion in acute illness.J Clin Invest.2005;115:2069–2072.
- ,,,,.Intensive insulin therapy exerts antiinflammatory effects in critically ill patients and counteracts the adverse effect of low mannose‐gind lectin levels.J Clin Endocrinol Metab.2003;88:1082–1088.
- ,,,,,.Protection of hepatocyte mitochondrial ultrastructure and function by strict blood glucose control with insulin in critically ill patients.Lancet.2005;365:53–59.
- ,,, et al.Intensive insulin therapy protects the endothelium of critically ill patients.J Clin Invest.2005;115:2277–2286.
- ,,,.The association between hyperglycaemia on admission and 180‐day mortality in acute myocardial infarction patients with and without diabetes.Diabet Med.2005;22:1321–1325.
- ,,, et al.Admission glucose and mortality in elderly patients hospitalized with acute myocardial infarction: implications for patients with and without recognized diabetes.Circulation.2005;111:3078–3086.
- ,,,.Glycometabolic state at admission: important risk marker of mortality in conventionally treated patients with diabetes mellitus and acute myocardial infarction: long‐term results from the Diabetes and Insulin‐Glucose Infusion in Acute Myocardial Infarction (DIGAMI) study.Circulation.1999;99:2626–2632.
- ,,,.Stress hyperglycaemia and increased risk of death after myocardial infarction in patients with and without diabetes: a systematic overview.Lancet.2000;355:773–778.
- ,,.How important is hyperglycemia during acute brain infarction?Neurologist.2004;10:195–200.
- ,,,,.Stress hyperglycemia and prognosis of stroke in nondiabetic and diabetic patients: a systematic overview.Stroke.2001;32:2426–2432.
- ,,,.Perioperative glycemic control and the risk of infectious complications in a cohort of adults with diabetes.Diabetes Care.1999;22:1408–1414.
- ,,, et al.Early postoperative glucose control predicts nosocomial infection rate in diabetic patients.JPEN J Parenter Enteral Nutr.1998;22:77–81.
- ,,,,,.The relation between hyperglycemia and outcomes in 2,471 patients admitted to the hospital with community‐acquired pneumonia.Diabetes Care.2005;28:810–815.
- ,,,,.Early peri‐operative glycaemic control and allograft rejection in patients with diabetes mellitus: a pilot study.Transplantation.2001;72:1321–1324.
- ,,, et al.Relation between the duration of remission and hyperglycemia during induction chemotherapy for acute lymphocytic leukemia with a hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone/methotrexate‐cytarabine regimen.Cancer.2004;100:1179–1185.
- ,,, et al.Effect of fasting glucose levels on mortality rate in patients with and without diabetes mellitus and coronary artery disease undergoing percutaneous coronary intervention.Am Heart J.2003;146:351–358.
- ,,.Effect of hyperglycemia and continuous intravenous insulin infusions on outcomes of cardiac surgical procedures: the Portland diabetic project.Endocr Pract.2004;10(Suppl 2):21–33.
- ,,, et al.Intraoperative hyperglycemia and perioperative outcomes in cardiac surgery patients.Mayo Clin Proc.2005;80:862–866.
- ,,,,.The association of diabetes and glucose control with surgical‐site infections among cardiothoracic surgery patients.Infect Control Hosp Epidemiol.2001;22:607–612.
- ,,.Glucose control lowers the risk of wound infection in diabetics after open heart operations.Ann Thorac Surg.1997;63:356–361.
- The Portland Protocol. Available at: http://www.providence.org/oregon/grograms_and_services/heart/portlandprotocol/. Accessed September2007.
- .Effect of an intensive glucose management protocol on the mortality of critically ill adult patients.Mayo Clin Proc.2004;79:992–1000.
- ,,, et al.Intensive insulin therapy in mixed medical/surgical intensive care units: benefit versus harm.Diabetes.2006;55:3151–3159.
- ,,.Tight blood glucose control with insulin in the ICU: facts and controversies.Chest.2007;132:268–278.
- ,.Reduction of nosocomial infections in the surgical intensive‐care unit by strict glycemic control.Endocr Pract.2004;10(Suppl 2):46–52.
- ,,,,,, et al.Intensive insulin therapy and pentastarch resuscitation in severe sepsis.N Engl J Med.2008;358:125–139.
- ,.Current controversies around tight glucose control in critically ill patients.Curr Opin Clin Nutr Metab Care.2007;10:206–209.
- ,,,.Designing and implementing insulin infusion protocols and order sets.J Hosp Med.2008;3(5):S42–S54.
- ,,,,,, et al.Randomized study of basal‐bolus insulin therapy in the inpatient management of patients with type 2 diabetes (RABBIT 2 trial).Diabetes Care.2007;30:2181–2186.
- Society of Hospital Medicine. Glycemic control resource room. Available at: http://www.hospitalmedicine.org/ResourceRoomRedesign/GlycemicControl.cfm. Accessed November2007.
- Society of Hospital Medicine. Workbook for improvement: improving glycemic control, preventing hypoglycemia, and optimizing care of the inpatient with hyperglycemia and diabetes. Available at: http://www.hospitalmedicine.org/ResourceRoomRedesign/pdf/GC_Workbook.pdf. Accessed November2007.
- ,,, et al.American College of Endocrinology position statement on inpatient diabetes and metabolic control.Endocr Pract.2004;10:77–82.
- ,,,,.Improved inpatient use of basal insulin, reduced hypoglycemia, and improved glycemic control: effect of structured subcutaneous insulin orders and an insulin management algorithm.J Hosp Med.2008. In press.
- American Association of Clinical Endocrinologists Inpatient Glycemic Control Resource Center.2007. Available at: http://resources.aace.com/index.asp. Accessed December 2007.
- American Heart Association. Get With the Guidelines. Available at: http://www.americanheart.org/getwiththeguidelines. Accessed November2007.
- Joint Commission. Disease Specific‐Care Certification. Available at:http://www.jointcommission.org/CertificationPrograms. Accessed November2007.
- The Joint Commission Disease‐Specific Certification Program. Range JE. Oncology issues. July/August2007:40–41.
- Anonymous.The Diabetes Control and Complications Trial Research Group (DCCT). The effect of intensive treatment of diabetes on the development and progression of long‐term complications in insulin‐dependent diabetes mellitus.N Engl J Med.1993;329:977–986.
- Intensive blood‐glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type, 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group.Lancet.1998;352:837–853.
- ,,,.Intensified multifactorial intervention in patients with type 2 diabetes mellitus and microalbuminuria: the Steno type 2 randomised study.Lancet.1999;353:617–622.
- ,,, et al.Utility of HbA1c levels for diabetes case finding in hospitalized patients with hyperglycemia.Diabetes Care.2003;26:1064–1068.
- ,,,.Eliminating inpatient sliding‐scale insulin: a reeducation project with medical house staff.Diabetes Care.2005;28:1008–1011.
- ,.Advanced carbohydrate counting. In:Practical Carbohydrate Counting: A How‐to‐Teach Guide for Health Professionals.Alexandria, VA:American Diabetes Association;2001:26–28.
- ,,,,.The evidence for the effectiveness of medical nutrition therapy in diabetes management.Diabetes Care.2002;25:608–613.
- ,,, et al.Inpatient management of diabetes and hyperglycemia: implications for nutrition practice and the food and nutrition professional.J Am Diet Assoc.2007;107:105–111.
- .The transition from insulin infusions to long‐term diabetes therapy: the argument for insulin analogs.Semin Thorac Cardiovasc Surg.2006;18:366–378.
- .Transitions paper.J Hosp Med.2008.
- ,,,,.Inpatient management of diabetes and hyperglycemia among general medicine patients at a large teaching hospital.J Hosp Med.2006;1:145–150.
- ,,, et al.Clinical inertia.Ann Intern Med.2001;135:825–834.
- ,,, et al.Diabetes care in hospitalized noncritically ill patients: more evidence for clinical inertia and negative therapeutic momentum.J Hosp Med.2007;2:203–211.
- University HealthSystem Consortium.Glycemic control 2005 findings and conclusions. Presented at: Glycemic Control 2005 Knowledge Transfer Meeting; 2005 August 19,2005; Chicago, IL.
- ,.Glycemic chaos (not glycemic control) still the rule for inpatient care: how do we stop the insanity?J Hosp Med.2006;1:141–144.
- ,,,,.Management of diabetes mellitus in hospitalized patients: efficiency and effectiveness of sliding‐scale insulin therapy.Pharmacotherapy.2006;26:1421–1432.
- ,,,,,.Hypoglycemia in hospitalized patients treated with antihyperglycemic agents.J Hosp Med.2007;2:234–240.
- ,,.Hypoglycemia in hospitalized patients.N Engl J Med.1986;315:1245–1250.
- ,,, et al.Predisposing factors for hypoglycemia in the intensive care unit.Crit Care Med.2006;34:96–101.
- ,,,.Association between hyper‐ and hypoglycaemia and 2 year all‐cause mortality risk in diabetic patients with acute coronary events.Eur Heart J.2005;26:1255–1261.
- ,,, et al.U‐shaped relationship of blood glucose with adverse outcomes among patients with ST‐segment elevation myocardial infarction.J Am Coll Cardiol.2005;46:178–180.
- ,,.An unexpected inverse relationship between HbA1c levels and mortality in patients with diabetes and advanced systolic heart failure.Am Heart J.2006;151:91.
- ,,, et al.Glucometrics in patients hospitalized with acute myocardial infarction: defining the optimal outcomes‐based measure of risk.Circulation.2008;117:1018–1027.
- ,,.Hypoglycemia in diabetes.Diabetes Care.2003;26:1902–1912.
- ,,.Hypoglycemia and cardiac arrest in a critically ill patient on strict glycemic control.Anesth Analg.2006;102:549–551.
- ,,,,,.Tight glycemic control in critically injured trauma patients.Ann Surg.2007;246:605–610; discussion 10–12.
- ,.Severe hypoglycemia in critically ill patients: risk factors and outcomes.Crit Care Med.2007;35:2262–2267.
- ,,,.Provider response to insulin‐induced hypoglycemia in hospitalized patients.J Hosp Med.2007;2:258–260.
- ,.Financial implications of glycemic control: results of an inpatient diabetes management program.Endocr Pract.2006;12(Suppl 3):43–48.
- ,,,.Impact of endocrine and diabetes team consultation on hospital length of stay for patients with diabetes.Am J Med.1995;99:22–28.
- American College of Endocrinology and American Diabetes Association Consensus Statement on Inpatient Diabetes and Glycemic Control: A call to action.Diabetes Care.2006;29:1955–1962.
- Standards of medical care in diabetes‐‐2008.Diabetes Care.2008;31(Suppl 1):S12–S54.
- ,,, et al.Prevalence of diabetes and impaired fasting glucose in adults in the U.S. population: National Health And Nutrition Examination Survey 1999–2002.Diabetes Care.2006;29:1263–1268.
- Centers for Disease Control and Prevention.National Diabetes Fact Sheet: General Information and National Estimates on Diabetes in the United States, 2005.Atlanta, GA:U.S. Department of Health and Human Services, Centers for Disease Control and Prevention,2005. Available at: http://www.cdc.gov/diabetes/pubs/factsheet05.htm. Accessed September 2007.
- ,,.Economic costs of diabetes in the US in 2002.Diabetes Care.2003;26:917–932.
- ,,.Glycemic control and sliding scale insulin use in medical inpatients with diabetes mellitus.Arch Intern Med.1997;157:545–552.
- ,,.Inpatient management of diabetes mellitus.Am J Med.2002;113:317–323.
- ,,, et al.Management of diabetes and hyperglycemia in hospitals.Diabetes Care.2004;27:553–591.
- ,,, et al.Intensive insulin therapy in the medical ICU.N Engl J Med.2006;354:449–461.
- ,,, et al.Intensive insulin therapy in critically ill patients.N Engl J Med.2001;345:1359–1367.
- .Association between hyperglycemia and increased hospital mortality in a heterogeneous population of critically ill patients.Mayo Clin Proc.2003;78:1471–1478.
- ,,,,.Unrecognized diabetes among hospitalized patients.Diabetes Care.1998;21:246–249.
- ,,,,,.Hyperglycemia: an independent marker of in‐hospital mortality in patients with undiagnosed diabetes.J Clin Endocrinol Metab.2002;87:978–982.
- United States Department of Health and Human Services Agency for Healthcare Research and Quality.2007. Available at: http://hcupnet.ahrq.gov. Accessed December 2007.
- ,,, et al.Glucose metabolism in patients with acute myocardial infarction and no previous diagnosis of diabetes mellitus: a prospective study.Lancet.2002;359:2140–2144.
- .Mechanism by which hyperglycemia plays a role in the setting of acute cardiovascular illness.Rev Cardiovasc Med.2006;7(Suppl 2):S35–S43.
- ,,,,,, et al.Stress hyperglycaemia is an independent predictor of left ventricular remodelling after first anterior myocardial infarction in non‐diabetic patients.Eur Heart J.2007;28:546–552.
- ,.Implications and treatment of acute hyperglycemia in the setting of acute myocardial infarction.Circulation.2007;115:e436–e439.
- ,,,,.Insulin infusion in acute illness.J Clin Invest.2005;115:2069–2072.
- ,,,,.Intensive insulin therapy exerts antiinflammatory effects in critically ill patients and counteracts the adverse effect of low mannose‐gind lectin levels.J Clin Endocrinol Metab.2003;88:1082–1088.
- ,,,,,.Protection of hepatocyte mitochondrial ultrastructure and function by strict blood glucose control with insulin in critically ill patients.Lancet.2005;365:53–59.
- ,,, et al.Intensive insulin therapy protects the endothelium of critically ill patients.J Clin Invest.2005;115:2277–2286.
- ,,,.The association between hyperglycaemia on admission and 180‐day mortality in acute myocardial infarction patients with and without diabetes.Diabet Med.2005;22:1321–1325.
- ,,, et al.Admission glucose and mortality in elderly patients hospitalized with acute myocardial infarction: implications for patients with and without recognized diabetes.Circulation.2005;111:3078–3086.
- ,,,.Glycometabolic state at admission: important risk marker of mortality in conventionally treated patients with diabetes mellitus and acute myocardial infarction: long‐term results from the Diabetes and Insulin‐Glucose Infusion in Acute Myocardial Infarction (DIGAMI) study.Circulation.1999;99:2626–2632.
- ,,,.Stress hyperglycaemia and increased risk of death after myocardial infarction in patients with and without diabetes: a systematic overview.Lancet.2000;355:773–778.
- ,,.How important is hyperglycemia during acute brain infarction?Neurologist.2004;10:195–200.
- ,,,,.Stress hyperglycemia and prognosis of stroke in nondiabetic and diabetic patients: a systematic overview.Stroke.2001;32:2426–2432.
- ,,,.Perioperative glycemic control and the risk of infectious complications in a cohort of adults with diabetes.Diabetes Care.1999;22:1408–1414.
- ,,, et al.Early postoperative glucose control predicts nosocomial infection rate in diabetic patients.JPEN J Parenter Enteral Nutr.1998;22:77–81.
- ,,,,,.The relation between hyperglycemia and outcomes in 2,471 patients admitted to the hospital with community‐acquired pneumonia.Diabetes Care.2005;28:810–815.
- ,,,,.Early peri‐operative glycaemic control and allograft rejection in patients with diabetes mellitus: a pilot study.Transplantation.2001;72:1321–1324.
- ,,, et al.Relation between the duration of remission and hyperglycemia during induction chemotherapy for acute lymphocytic leukemia with a hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone/methotrexate‐cytarabine regimen.Cancer.2004;100:1179–1185.
- ,,, et al.Effect of fasting glucose levels on mortality rate in patients with and without diabetes mellitus and coronary artery disease undergoing percutaneous coronary intervention.Am Heart J.2003;146:351–358.
- ,,.Effect of hyperglycemia and continuous intravenous insulin infusions on outcomes of cardiac surgical procedures: the Portland diabetic project.Endocr Pract.2004;10(Suppl 2):21–33.
- ,,, et al.Intraoperative hyperglycemia and perioperative outcomes in cardiac surgery patients.Mayo Clin Proc.2005;80:862–866.
- ,,,,.The association of diabetes and glucose control with surgical‐site infections among cardiothoracic surgery patients.Infect Control Hosp Epidemiol.2001;22:607–612.
- ,,.Glucose control lowers the risk of wound infection in diabetics after open heart operations.Ann Thorac Surg.1997;63:356–361.
- The Portland Protocol. Available at: http://www.providence.org/oregon/grograms_and_services/heart/portlandprotocol/. Accessed September2007.
- .Effect of an intensive glucose management protocol on the mortality of critically ill adult patients.Mayo Clin Proc.2004;79:992–1000.
- ,,, et al.Intensive insulin therapy in mixed medical/surgical intensive care units: benefit versus harm.Diabetes.2006;55:3151–3159.
- ,,.Tight blood glucose control with insulin in the ICU: facts and controversies.Chest.2007;132:268–278.
- ,.Reduction of nosocomial infections in the surgical intensive‐care unit by strict glycemic control.Endocr Pract.2004;10(Suppl 2):46–52.
- ,,,,,, et al.Intensive insulin therapy and pentastarch resuscitation in severe sepsis.N Engl J Med.2008;358:125–139.
- ,.Current controversies around tight glucose control in critically ill patients.Curr Opin Clin Nutr Metab Care.2007;10:206–209.
- ,,,.Designing and implementing insulin infusion protocols and order sets.J Hosp Med.2008;3(5):S42–S54.
- ,,,,,, et al.Randomized study of basal‐bolus insulin therapy in the inpatient management of patients with type 2 diabetes (RABBIT 2 trial).Diabetes Care.2007;30:2181–2186.
- Society of Hospital Medicine. Glycemic control resource room. Available at: http://www.hospitalmedicine.org/ResourceRoomRedesign/GlycemicControl.cfm. Accessed November2007.
- Society of Hospital Medicine. Workbook for improvement: improving glycemic control, preventing hypoglycemia, and optimizing care of the inpatient with hyperglycemia and diabetes. Available at: http://www.hospitalmedicine.org/ResourceRoomRedesign/pdf/GC_Workbook.pdf. Accessed November2007.
- ,,, et al.American College of Endocrinology position statement on inpatient diabetes and metabolic control.Endocr Pract.2004;10:77–82.
- ,,,,.Improved inpatient use of basal insulin, reduced hypoglycemia, and improved glycemic control: effect of structured subcutaneous insulin orders and an insulin management algorithm.J Hosp Med.2008. In press.
- American Association of Clinical Endocrinologists Inpatient Glycemic Control Resource Center.2007. Available at: http://resources.aace.com/index.asp. Accessed December 2007.
- American Heart Association. Get With the Guidelines. Available at: http://www.americanheart.org/getwiththeguidelines. Accessed November2007.
- Joint Commission. Disease Specific‐Care Certification. Available at:http://www.jointcommission.org/CertificationPrograms. Accessed November2007.
- The Joint Commission Disease‐Specific Certification Program. Range JE. Oncology issues. July/August2007:40–41.
- Anonymous.The Diabetes Control and Complications Trial Research Group (DCCT). The effect of intensive treatment of diabetes on the development and progression of long‐term complications in insulin‐dependent diabetes mellitus.N Engl J Med.1993;329:977–986.
- Intensive blood‐glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type, 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group.Lancet.1998;352:837–853.
- ,,,.Intensified multifactorial intervention in patients with type 2 diabetes mellitus and microalbuminuria: the Steno type 2 randomised study.Lancet.1999;353:617–622.
- ,,, et al.Utility of HbA1c levels for diabetes case finding in hospitalized patients with hyperglycemia.Diabetes Care.2003;26:1064–1068.
- ,,,.Eliminating inpatient sliding‐scale insulin: a reeducation project with medical house staff.Diabetes Care.2005;28:1008–1011.
- ,.Advanced carbohydrate counting. In:Practical Carbohydrate Counting: A How‐to‐Teach Guide for Health Professionals.Alexandria, VA:American Diabetes Association;2001:26–28.
- ,,,,.The evidence for the effectiveness of medical nutrition therapy in diabetes management.Diabetes Care.2002;25:608–613.
- ,,, et al.Inpatient management of diabetes and hyperglycemia: implications for nutrition practice and the food and nutrition professional.J Am Diet Assoc.2007;107:105–111.
- .The transition from insulin infusions to long‐term diabetes therapy: the argument for insulin analogs.Semin Thorac Cardiovasc Surg.2006;18:366–378.
- .Transitions paper.J Hosp Med.2008.
- ,,,,.Inpatient management of diabetes and hyperglycemia among general medicine patients at a large teaching hospital.J Hosp Med.2006;1:145–150.
- ,,, et al.Clinical inertia.Ann Intern Med.2001;135:825–834.
- ,,, et al.Diabetes care in hospitalized noncritically ill patients: more evidence for clinical inertia and negative therapeutic momentum.J Hosp Med.2007;2:203–211.
- University HealthSystem Consortium.Glycemic control 2005 findings and conclusions. Presented at: Glycemic Control 2005 Knowledge Transfer Meeting; 2005 August 19,2005; Chicago, IL.
- ,.Glycemic chaos (not glycemic control) still the rule for inpatient care: how do we stop the insanity?J Hosp Med.2006;1:141–144.
- ,,,,.Management of diabetes mellitus in hospitalized patients: efficiency and effectiveness of sliding‐scale insulin therapy.Pharmacotherapy.2006;26:1421–1432.
- ,,,,,.Hypoglycemia in hospitalized patients treated with antihyperglycemic agents.J Hosp Med.2007;2:234–240.
- ,,.Hypoglycemia in hospitalized patients.N Engl J Med.1986;315:1245–1250.
- ,,, et al.Predisposing factors for hypoglycemia in the intensive care unit.Crit Care Med.2006;34:96–101.
- ,,,.Association between hyper‐ and hypoglycaemia and 2 year all‐cause mortality risk in diabetic patients with acute coronary events.Eur Heart J.2005;26:1255–1261.
- ,,, et al.U‐shaped relationship of blood glucose with adverse outcomes among patients with ST‐segment elevation myocardial infarction.J Am Coll Cardiol.2005;46:178–180.
- ,,.An unexpected inverse relationship between HbA1c levels and mortality in patients with diabetes and advanced systolic heart failure.Am Heart J.2006;151:91.
- ,,, et al.Glucometrics in patients hospitalized with acute myocardial infarction: defining the optimal outcomes‐based measure of risk.Circulation.2008;117:1018–1027.
- ,,.Hypoglycemia in diabetes.Diabetes Care.2003;26:1902–1912.
- ,,.Hypoglycemia and cardiac arrest in a critically ill patient on strict glycemic control.Anesth Analg.2006;102:549–551.
- ,,,,,.Tight glycemic control in critically injured trauma patients.Ann Surg.2007;246:605–610; discussion 10–12.
- ,.Severe hypoglycemia in critically ill patients: risk factors and outcomes.Crit Care Med.2007;35:2262–2267.
- ,,,.Provider response to insulin‐induced hypoglycemia in hospitalized patients.J Hosp Med.2007;2:258–260.
- ,.Financial implications of glycemic control: results of an inpatient diabetes management program.Endocr Pract.2006;12(Suppl 3):43–48.
- ,,,.Impact of endocrine and diabetes team consultation on hospital length of stay for patients with diabetes.Am J Med.1995;99:22–28.
Debates in Hospital Medicine
At a hospital at which I work, every patient who presents to the emergency department with a suspected stroke or transient ischemic attack is evaluated by the stroke team. Per protocol, the team rapidly assesses each patient, orders diagnostic and therapeutic interventions and then refers each and every patient to the hospitalist service for admission and medical comanagement. At no point is any consideration given to whether the patients actually have medical comorbidities, or if a hospitalist will have anything meaningful to add to the care. The firmly set expectation is that hospitalists admit all stroke patients for the purposes of comanagement, while the neurologists consult.
Comanagement has become a mainstay of hospital medicine.1 It is predicated upon the assumption that surgical and specialty patients benefit when their medical comorbidities are managed by hospitalists. It differs conceptually from traditional medical consultation in that hospitalists collaboratively manage patients with surgeons or specialists, sharing responsibility and authority. In practice, however, comanagement varies widely, ranging from a model of care indistinguishable from traditional medical consultation to one where hospitalists admit and assume primary responsibility for surgical and specialty patients. This variability makes it difficult to study and make generalizations about the role and impact of hospitalist comanagement. Nonetheless, recent evidence suggests that hospitalist consultation and comanagement may not be as effective as originally anticipated.
In a 2008 observational cohort study of patients undergoing surgery at an academic medical center, Auerbach et al demonstrated that medical consultation (provided by hospitalists) did not improve glycemic control or increase the likelihood of perioperative beta‐blockade and venous thromboembolism prophylaxis.2 Patients who received consultation had longer adjusted lengths of stay (12.98% longer; 95% confidence interval, 1.61%‐25.61%) and higher adjusted costs (24.36% higher; 95% confidence interval, 13.54%‐36.34%). Notwithstanding the limited generalizability of this study to community hospitals, it has raised concerns that hospitalist consultation does not automatically improve quality of care or cost effectiveness.3
Several other recent trials have also helped to define where hospitalist comanagement may work well and where it may not. In 2004, Huddleston et al published the Hospitalist Orthopedic Team (HOT) trial, the first randomized prospective trial comparing hospitalist‐surgical comanagement to standard care.4 A total of 526 patients undergoing elective hip or knee replacement surgery at the Mayo Clinic were randomized to either standard orthopedic care with consultation as needed, or immediate hospitalist comanagement. The outcomes were disappointing. Hospitalist comanagement reduced minor complications (such as incidence of urinary tract infections, fever, and hyponatremia) but had no effect on moderate or major complications. The HOT intervention modestly reduced adjusted length of stay (LOS), defined as the point at which patients were deemed stable for discharge, by 0.5 days, but had no impact on actual LOS or cost per case. Not surprisingly, orthopedic surgeons and nurses preferred the HOT model of care over the standard model. One year later, Phy et al analyzed outcomes for patients admitted with hip fracture at the same institution.5 This retrospective cohort study compared patients who were admitted to either a standard orthopedic service or to a hospitalist team. In contrast to the HOT trial, hospitalist comanagement of hip fracture patients decreased time to surgery and lowered LOS by 2.2 days without compromising patient outcomes.
How did two trials that occurred roughly simultaneously at the same hospital, involving the same hospitalists and orthopedic surgeons generate such different outcomes? A likely answer is patient selection. Patients who undergo elective joint replacement are usually relatively healthy. They are almost always ambulatory and their comorbidities, when present, are generally reasonably compensated. As a rule, they fare well postoperatively, as evidenced by the 1.3% major complication rate demonstrated in the HOT trial.3 In contrast, hip fracture patients are older, have greater comorbidity and are at remarkably high risk for developing perioperative delirium.3, 4, 6 By definition, their urgent/emergent hip surgery stratifies them to a higher operative risk category than patients who undergo elective joint replacement.7 Half of hip fracture patients do not return to premorbid levels of function, and the 1‐year mortality rate has been estimated to be as high as 25%.6, 8 Given these differences, it is not surprising that hip fracture patients are more likely than elective joint replacement patients to respond favorably to hospitalist comanagement.
In 2007, Simon et al published a retrospective study of 739 pediatric spinal fusion patients at Childrens' Hospital in Denver.9 Beginning in 2004, hospitalists comanaged selected, high‐risk surgical patients (14 of 115 spinal fusion patients, or 12%). Over the course of the study, the mean LOS for low‐risk patients decreased by 21% but the mean LOS for the high‐risk, hospitalist‐comanaged patients decreased by 28%; a 33% relative reduction favoring hospitalist‐managed patients. By targeting selected high‐risk patients, pediatric hospitalists were able to improve upon LOS reductions that occurred systemically across the entire spinal fusion program. Also in 2007, Southern et al compared outcomes for 2,913 patients admitted by full‐time teaching hospitalists vs 6,124 patients admitted by nonhospitalists at Montefiore Medical Center, Bronx, New York.10 Mean LOS for patients admitted to the hospitalist service was 5.01 days vs 5.87 days for the nonhospitalists. Subgroup analysis demonstrated the greatest LOS differentials for patients requiring close clinical monitoring (heart failure, stroke, asthma, or pneumonia) or complex discharge planning.
Although these studies, performed at large academic medical centers, may have limited generalizability, they support the common‐sense notion that hospitalists most benefit patients who are sick, frail, and medically or socially complex. As a corollary, hospitalists probably offer relatively little benefit to surgical and specialty patients who are young or have compensated medical comorbidities and/or straightforward disposition plans. The enormous variability across healthcare institutions makes it difficult if not impossible to define a patient acuity or complexity cutoff below which hospitalist comanagement is unlikely to be beneficial. Nonetheless, some degree of common sense can be applied. As a case in point, a hospitalist probably adds little value to the care of a basically healthy patient with a hemodynamically stable upper gastrointestinal bleed. Despite this, in many institutions, hospitalists admit or comanage all gastroenterology patients, irrespective of their diagnosis, acuity, or complexity.11
One can even hypothesize that hospitalist comanagement may potentially inject risk into patient care. Admitting that patient with a stable upper gastrointestinal bleed to a hospitalist service may delay the gastroenterologist's involvement and initiation of the necessary endoscopy. Having assumed that the hospitalist is running the show, the gastroenterologist may pay insufficient attention to the patient. The hospitalist and gastroenterologist may give conflicting orders and reports that confuse patients, families, and hospital staff, ultimately increasing the likelihood of medical errors.
Ultimately, the risks inherent in adding complexity into patient care must be balanced against the potential benefits. For patients who are sick, frail, or complicated, the risk‐benefit ratio probably tilts in favor of comanagement. However, for generally healthy patients, it is conceivable that adding complexity negates (or worse yet, exceeds) the putative benefits of comanagement.
Given the potential limitations of hospitalist comanagement, why are hospitalists admitting or managing broad and unselected populations of surgical and specialty patients? Hospital leaders have suggested that hospitalist comanagement may protect overstretched surgeons and specialists and extend their capacity. A hospital with only one neurosurgeon on staff might reasonably ask its hospitalists to primarily manage carefully selected low‐acuity neurosurgical patients, allowing the neurosurgeon to serve as a consultant. However, in communities where specialists and surgeons are abundant, this justification is less credible. In such cases, it is difficult not to suspect that the primary reason that hospitalists admit surgical and specialty patients is to enhance the income and quality of life of the surgeons and specialists.
Expanding hospitalist comanagement services for no other reason than to keep specialists and surgeons happy might be justifiable if hospital medicine was not faced with its own critical manpower shortage. Hospital medicine is expected to grow from approximately 20,000 current practitioners to more than 40,000 within a decade.12 The growing shortage of qualified hospitalists has become a preoccupation for hospitalist employers across the country.13 At its 2006 strategic planning retreat, the Board of Directors of the Society of Hospital Medicine identified this issue as one of the greatest threats to the future health of hospital medicine.14 Demand for hospitalists will not abate for at least a decade, which will leave many hospitalist programs significantly understaffed for the foreseeable future. Understaffing forces hospitalist programs to lower hiring standards, jeopardizes patient care, accelerates physician burnout, and may ultimately destabilize hospital medicine.15 Understaffed hospitalist programs should be very circumspect about how and where they expand their clinical coverage.
Another principle underlying hospitalist comanagement is that it improves care by allowing surgeons and specialists to focus on their areas of expertise. Surgeons and specialists who do not have to manage their patients' medical issues can presumably spend more time focusing on their own disciplines. Although this argument is conceptually appealing, there is no evidence that this actually occurs. In fact, it is equally conceivable that hospitalist comanagement could jeopardize care by disengaging surgeons and specialists from their patients' progress (or lack thereof). Furthermore, evidence suggests that hospitalists are underprepared to manage diagnoses that have historically been the purview of surgeons and specialists. Practicing hospitalists who manage acute neurological and neurosurgical conditions, orthopedic trauma, and acute psychiatric illnesses have reported relative undertraining in all of these disease states.10, 16 Generally, hospitalists are expected to deliver this care in the absence of any regime to assess their competence, provide targeted training to fill knowledge gaps, and monitor their progress. At minimum, this should raise concerns about the quality and consistency of care that hospitalists provide to nonmedical patients.
Finally, working collaboratively with other specialties should be a major professional benefit of comanagement. In well‐designed comanagement arrangements, hospitalists and specialists work equitably under clearly defined and mutually agreed upon rules of engagement. They share responsibility for patients, collaborate to improve care, and teach and learn from each other. Unfortunately, in many instances, the power structure becomes lopsided, with surgeons and specialists dictating how, when, and why hospitalists manage their patients.17 Emergency departments have learned to default surgical and specialty patient admissions to hospitalists when surgeons and specialists balk. Hospital administrations may tacitly or overtly expect their financially subsidized hospitalists to cheerfully accept any and all referrals, irrespective of how inappropriate they may be. Practicing hospitalists frequently complain about their subordinate status and inability to control their working conditions, both of which are identified risk factors for career dissatisfaction and burnout.14, 16, 18 Once again, as a specialty facing a critical manpower shortage, hospitalist programs should be very attuned to defining work conditions that foster career satisfaction and physician retention.
REFRAMING COMANAGEMENT
The history of healthcare is laden with examples of new ideas that were widely and indiscriminately adopted only to subsequently fail to withstand rigorous scrutiny.19, 20 The unchecked expansion of hospitalist comanagement has the potential to become another case in point. In the absence of clear definitions of comanagement and good evidence to define best practices, hospitalists are left to use their best judgment to define the parameters of their comanagement services. At minimum, hospitalist leaders should ask some basic questions as they ponder potential comanagement relationships:
-
Why are we being asked to provide this service?
-
Do the patients have comorbidities that require our input?
-
Is there a legitimate quality or efficiency case to be made to support our participation?
-
Do we have the manpower to provide the service? If not, what will suffer as a result?
-
Will the relationship be equitable?
-
What might go wrong?
Comanagement is an appealing construct that has grown to fill many niches of healthcare delivery.10 Given compelling reasons to be skeptical about the purported benefits of comanagement, hospitalists should be circumspect about how and where they offer such services. Comanagement should be applied carefully and methodically, paying close attention to the consequences, intended and unintended. Applying comanagement in a rational, evidence‐based, and sustainable fashion will ultimately better serve patients, the healthcare community, and hospital medicine.
- Society of Hospital Medicine. The Society of Hospital Medicine 2005–2006 Survey: The Authoritative Source on the State of the Hospital Medicine Movement. Published by the, 2006. Executive summary available at http://www.hospitalmedicine.org/AM/Template.cfm?Section=Surveys2167(21):2338–2344.
- .Exceed acceptable: new studies challenge hospitalists to prove our value.Hospitalist.2008;12(2):63.
- ,,, et al.Medical and surgical comanagement after elective hip and knee arthroplasty.Ann Intern Med.2004;141:28–38.
- ,,, et al.Effects of a hospitalist model on elderly patients with hip fracture.Arch Intern Med.2005;165:796–801.
- ,,,.Treatment and survival among elderly Americans with hip fractures: a population‐based study.Am J Public Health.1994;84:1287–1291.
- ,,, et al.Predicting cardiac complications in patients undergoing non‐cardiac surgery.J Gen Intern Med.1986;1:211–219.
- ,,,,.Predictors of functional recovery one year following hospital discharge for hip fracture: a prospective study.J Gerontol.1990;45(3):M101–M107.
- ,,,,,.Pediatric hospitalist comanagement of spinal fusion surgery patients.J Hosp Med.2007;2:23–29.
- ,,,,.Hospitalist care and length of stay in patients requiring complex discharge planning and close clinical monitoring.Arch Intern Med.2007;167:1869–1874.
- ,,,,.The spectrum of community‐based hospitalist practice, a call to tailor internal medicine residency training.Arch Intern Med.2007;167(7):727–728.
- Society of Hospital Medicine. Growth of Hospital Medicine Nationwide. http://www.hospitalmedicine.org/Content/NavigationMenu/Media/GrowthofHospitalMedicineNation wide/Growth_of_Hospital_M.htm. Accessed September 2,2008.
- ,,, et al.Rebuilding the future of the private practice of hospital medicine.The Phoenix Group, May2007.
- Society of Hospital Medicine Board of Directors Strategic Planning Retreat: November 28‐29,2006.
- ,,,,,,for the SGIM Career Satisfaction Study Group.Physician stress: results from the physician worklife study.Stress Health.2001;18(1):37–42.
- ,,, et al.Hospitalist's perceptions of their residency training needs: Results of a national survey.Am J Med.2001;111:247–254.
- .Feeling pressure to admit surgical patients? Hospitalists work to set limits on co‐management arrangements.Today's Hospitalist. January2008.
- Society of Hospital Medicine. Career Satisfaction White Paper. http://www.hospitalmedicine.org/AM/Template.cfm?Section=Practice_Resources321:406–412.
- .Pulmonary‐artery catheters—peace at last?N Engl J Med.2006;354(21):2273–2274.
At a hospital at which I work, every patient who presents to the emergency department with a suspected stroke or transient ischemic attack is evaluated by the stroke team. Per protocol, the team rapidly assesses each patient, orders diagnostic and therapeutic interventions and then refers each and every patient to the hospitalist service for admission and medical comanagement. At no point is any consideration given to whether the patients actually have medical comorbidities, or if a hospitalist will have anything meaningful to add to the care. The firmly set expectation is that hospitalists admit all stroke patients for the purposes of comanagement, while the neurologists consult.
Comanagement has become a mainstay of hospital medicine.1 It is predicated upon the assumption that surgical and specialty patients benefit when their medical comorbidities are managed by hospitalists. It differs conceptually from traditional medical consultation in that hospitalists collaboratively manage patients with surgeons or specialists, sharing responsibility and authority. In practice, however, comanagement varies widely, ranging from a model of care indistinguishable from traditional medical consultation to one where hospitalists admit and assume primary responsibility for surgical and specialty patients. This variability makes it difficult to study and make generalizations about the role and impact of hospitalist comanagement. Nonetheless, recent evidence suggests that hospitalist consultation and comanagement may not be as effective as originally anticipated.
In a 2008 observational cohort study of patients undergoing surgery at an academic medical center, Auerbach et al demonstrated that medical consultation (provided by hospitalists) did not improve glycemic control or increase the likelihood of perioperative beta‐blockade and venous thromboembolism prophylaxis.2 Patients who received consultation had longer adjusted lengths of stay (12.98% longer; 95% confidence interval, 1.61%‐25.61%) and higher adjusted costs (24.36% higher; 95% confidence interval, 13.54%‐36.34%). Notwithstanding the limited generalizability of this study to community hospitals, it has raised concerns that hospitalist consultation does not automatically improve quality of care or cost effectiveness.3
Several other recent trials have also helped to define where hospitalist comanagement may work well and where it may not. In 2004, Huddleston et al published the Hospitalist Orthopedic Team (HOT) trial, the first randomized prospective trial comparing hospitalist‐surgical comanagement to standard care.4 A total of 526 patients undergoing elective hip or knee replacement surgery at the Mayo Clinic were randomized to either standard orthopedic care with consultation as needed, or immediate hospitalist comanagement. The outcomes were disappointing. Hospitalist comanagement reduced minor complications (such as incidence of urinary tract infections, fever, and hyponatremia) but had no effect on moderate or major complications. The HOT intervention modestly reduced adjusted length of stay (LOS), defined as the point at which patients were deemed stable for discharge, by 0.5 days, but had no impact on actual LOS or cost per case. Not surprisingly, orthopedic surgeons and nurses preferred the HOT model of care over the standard model. One year later, Phy et al analyzed outcomes for patients admitted with hip fracture at the same institution.5 This retrospective cohort study compared patients who were admitted to either a standard orthopedic service or to a hospitalist team. In contrast to the HOT trial, hospitalist comanagement of hip fracture patients decreased time to surgery and lowered LOS by 2.2 days without compromising patient outcomes.
How did two trials that occurred roughly simultaneously at the same hospital, involving the same hospitalists and orthopedic surgeons generate such different outcomes? A likely answer is patient selection. Patients who undergo elective joint replacement are usually relatively healthy. They are almost always ambulatory and their comorbidities, when present, are generally reasonably compensated. As a rule, they fare well postoperatively, as evidenced by the 1.3% major complication rate demonstrated in the HOT trial.3 In contrast, hip fracture patients are older, have greater comorbidity and are at remarkably high risk for developing perioperative delirium.3, 4, 6 By definition, their urgent/emergent hip surgery stratifies them to a higher operative risk category than patients who undergo elective joint replacement.7 Half of hip fracture patients do not return to premorbid levels of function, and the 1‐year mortality rate has been estimated to be as high as 25%.6, 8 Given these differences, it is not surprising that hip fracture patients are more likely than elective joint replacement patients to respond favorably to hospitalist comanagement.
In 2007, Simon et al published a retrospective study of 739 pediatric spinal fusion patients at Childrens' Hospital in Denver.9 Beginning in 2004, hospitalists comanaged selected, high‐risk surgical patients (14 of 115 spinal fusion patients, or 12%). Over the course of the study, the mean LOS for low‐risk patients decreased by 21% but the mean LOS for the high‐risk, hospitalist‐comanaged patients decreased by 28%; a 33% relative reduction favoring hospitalist‐managed patients. By targeting selected high‐risk patients, pediatric hospitalists were able to improve upon LOS reductions that occurred systemically across the entire spinal fusion program. Also in 2007, Southern et al compared outcomes for 2,913 patients admitted by full‐time teaching hospitalists vs 6,124 patients admitted by nonhospitalists at Montefiore Medical Center, Bronx, New York.10 Mean LOS for patients admitted to the hospitalist service was 5.01 days vs 5.87 days for the nonhospitalists. Subgroup analysis demonstrated the greatest LOS differentials for patients requiring close clinical monitoring (heart failure, stroke, asthma, or pneumonia) or complex discharge planning.
Although these studies, performed at large academic medical centers, may have limited generalizability, they support the common‐sense notion that hospitalists most benefit patients who are sick, frail, and medically or socially complex. As a corollary, hospitalists probably offer relatively little benefit to surgical and specialty patients who are young or have compensated medical comorbidities and/or straightforward disposition plans. The enormous variability across healthcare institutions makes it difficult if not impossible to define a patient acuity or complexity cutoff below which hospitalist comanagement is unlikely to be beneficial. Nonetheless, some degree of common sense can be applied. As a case in point, a hospitalist probably adds little value to the care of a basically healthy patient with a hemodynamically stable upper gastrointestinal bleed. Despite this, in many institutions, hospitalists admit or comanage all gastroenterology patients, irrespective of their diagnosis, acuity, or complexity.11
One can even hypothesize that hospitalist comanagement may potentially inject risk into patient care. Admitting that patient with a stable upper gastrointestinal bleed to a hospitalist service may delay the gastroenterologist's involvement and initiation of the necessary endoscopy. Having assumed that the hospitalist is running the show, the gastroenterologist may pay insufficient attention to the patient. The hospitalist and gastroenterologist may give conflicting orders and reports that confuse patients, families, and hospital staff, ultimately increasing the likelihood of medical errors.
Ultimately, the risks inherent in adding complexity into patient care must be balanced against the potential benefits. For patients who are sick, frail, or complicated, the risk‐benefit ratio probably tilts in favor of comanagement. However, for generally healthy patients, it is conceivable that adding complexity negates (or worse yet, exceeds) the putative benefits of comanagement.
Given the potential limitations of hospitalist comanagement, why are hospitalists admitting or managing broad and unselected populations of surgical and specialty patients? Hospital leaders have suggested that hospitalist comanagement may protect overstretched surgeons and specialists and extend their capacity. A hospital with only one neurosurgeon on staff might reasonably ask its hospitalists to primarily manage carefully selected low‐acuity neurosurgical patients, allowing the neurosurgeon to serve as a consultant. However, in communities where specialists and surgeons are abundant, this justification is less credible. In such cases, it is difficult not to suspect that the primary reason that hospitalists admit surgical and specialty patients is to enhance the income and quality of life of the surgeons and specialists.
Expanding hospitalist comanagement services for no other reason than to keep specialists and surgeons happy might be justifiable if hospital medicine was not faced with its own critical manpower shortage. Hospital medicine is expected to grow from approximately 20,000 current practitioners to more than 40,000 within a decade.12 The growing shortage of qualified hospitalists has become a preoccupation for hospitalist employers across the country.13 At its 2006 strategic planning retreat, the Board of Directors of the Society of Hospital Medicine identified this issue as one of the greatest threats to the future health of hospital medicine.14 Demand for hospitalists will not abate for at least a decade, which will leave many hospitalist programs significantly understaffed for the foreseeable future. Understaffing forces hospitalist programs to lower hiring standards, jeopardizes patient care, accelerates physician burnout, and may ultimately destabilize hospital medicine.15 Understaffed hospitalist programs should be very circumspect about how and where they expand their clinical coverage.
Another principle underlying hospitalist comanagement is that it improves care by allowing surgeons and specialists to focus on their areas of expertise. Surgeons and specialists who do not have to manage their patients' medical issues can presumably spend more time focusing on their own disciplines. Although this argument is conceptually appealing, there is no evidence that this actually occurs. In fact, it is equally conceivable that hospitalist comanagement could jeopardize care by disengaging surgeons and specialists from their patients' progress (or lack thereof). Furthermore, evidence suggests that hospitalists are underprepared to manage diagnoses that have historically been the purview of surgeons and specialists. Practicing hospitalists who manage acute neurological and neurosurgical conditions, orthopedic trauma, and acute psychiatric illnesses have reported relative undertraining in all of these disease states.10, 16 Generally, hospitalists are expected to deliver this care in the absence of any regime to assess their competence, provide targeted training to fill knowledge gaps, and monitor their progress. At minimum, this should raise concerns about the quality and consistency of care that hospitalists provide to nonmedical patients.
Finally, working collaboratively with other specialties should be a major professional benefit of comanagement. In well‐designed comanagement arrangements, hospitalists and specialists work equitably under clearly defined and mutually agreed upon rules of engagement. They share responsibility for patients, collaborate to improve care, and teach and learn from each other. Unfortunately, in many instances, the power structure becomes lopsided, with surgeons and specialists dictating how, when, and why hospitalists manage their patients.17 Emergency departments have learned to default surgical and specialty patient admissions to hospitalists when surgeons and specialists balk. Hospital administrations may tacitly or overtly expect their financially subsidized hospitalists to cheerfully accept any and all referrals, irrespective of how inappropriate they may be. Practicing hospitalists frequently complain about their subordinate status and inability to control their working conditions, both of which are identified risk factors for career dissatisfaction and burnout.14, 16, 18 Once again, as a specialty facing a critical manpower shortage, hospitalist programs should be very attuned to defining work conditions that foster career satisfaction and physician retention.
REFRAMING COMANAGEMENT
The history of healthcare is laden with examples of new ideas that were widely and indiscriminately adopted only to subsequently fail to withstand rigorous scrutiny.19, 20 The unchecked expansion of hospitalist comanagement has the potential to become another case in point. In the absence of clear definitions of comanagement and good evidence to define best practices, hospitalists are left to use their best judgment to define the parameters of their comanagement services. At minimum, hospitalist leaders should ask some basic questions as they ponder potential comanagement relationships:
-
Why are we being asked to provide this service?
-
Do the patients have comorbidities that require our input?
-
Is there a legitimate quality or efficiency case to be made to support our participation?
-
Do we have the manpower to provide the service? If not, what will suffer as a result?
-
Will the relationship be equitable?
-
What might go wrong?
Comanagement is an appealing construct that has grown to fill many niches of healthcare delivery.10 Given compelling reasons to be skeptical about the purported benefits of comanagement, hospitalists should be circumspect about how and where they offer such services. Comanagement should be applied carefully and methodically, paying close attention to the consequences, intended and unintended. Applying comanagement in a rational, evidence‐based, and sustainable fashion will ultimately better serve patients, the healthcare community, and hospital medicine.
At a hospital at which I work, every patient who presents to the emergency department with a suspected stroke or transient ischemic attack is evaluated by the stroke team. Per protocol, the team rapidly assesses each patient, orders diagnostic and therapeutic interventions and then refers each and every patient to the hospitalist service for admission and medical comanagement. At no point is any consideration given to whether the patients actually have medical comorbidities, or if a hospitalist will have anything meaningful to add to the care. The firmly set expectation is that hospitalists admit all stroke patients for the purposes of comanagement, while the neurologists consult.
Comanagement has become a mainstay of hospital medicine.1 It is predicated upon the assumption that surgical and specialty patients benefit when their medical comorbidities are managed by hospitalists. It differs conceptually from traditional medical consultation in that hospitalists collaboratively manage patients with surgeons or specialists, sharing responsibility and authority. In practice, however, comanagement varies widely, ranging from a model of care indistinguishable from traditional medical consultation to one where hospitalists admit and assume primary responsibility for surgical and specialty patients. This variability makes it difficult to study and make generalizations about the role and impact of hospitalist comanagement. Nonetheless, recent evidence suggests that hospitalist consultation and comanagement may not be as effective as originally anticipated.
In a 2008 observational cohort study of patients undergoing surgery at an academic medical center, Auerbach et al demonstrated that medical consultation (provided by hospitalists) did not improve glycemic control or increase the likelihood of perioperative beta‐blockade and venous thromboembolism prophylaxis.2 Patients who received consultation had longer adjusted lengths of stay (12.98% longer; 95% confidence interval, 1.61%‐25.61%) and higher adjusted costs (24.36% higher; 95% confidence interval, 13.54%‐36.34%). Notwithstanding the limited generalizability of this study to community hospitals, it has raised concerns that hospitalist consultation does not automatically improve quality of care or cost effectiveness.3
Several other recent trials have also helped to define where hospitalist comanagement may work well and where it may not. In 2004, Huddleston et al published the Hospitalist Orthopedic Team (HOT) trial, the first randomized prospective trial comparing hospitalist‐surgical comanagement to standard care.4 A total of 526 patients undergoing elective hip or knee replacement surgery at the Mayo Clinic were randomized to either standard orthopedic care with consultation as needed, or immediate hospitalist comanagement. The outcomes were disappointing. Hospitalist comanagement reduced minor complications (such as incidence of urinary tract infections, fever, and hyponatremia) but had no effect on moderate or major complications. The HOT intervention modestly reduced adjusted length of stay (LOS), defined as the point at which patients were deemed stable for discharge, by 0.5 days, but had no impact on actual LOS or cost per case. Not surprisingly, orthopedic surgeons and nurses preferred the HOT model of care over the standard model. One year later, Phy et al analyzed outcomes for patients admitted with hip fracture at the same institution.5 This retrospective cohort study compared patients who were admitted to either a standard orthopedic service or to a hospitalist team. In contrast to the HOT trial, hospitalist comanagement of hip fracture patients decreased time to surgery and lowered LOS by 2.2 days without compromising patient outcomes.
How did two trials that occurred roughly simultaneously at the same hospital, involving the same hospitalists and orthopedic surgeons generate such different outcomes? A likely answer is patient selection. Patients who undergo elective joint replacement are usually relatively healthy. They are almost always ambulatory and their comorbidities, when present, are generally reasonably compensated. As a rule, they fare well postoperatively, as evidenced by the 1.3% major complication rate demonstrated in the HOT trial.3 In contrast, hip fracture patients are older, have greater comorbidity and are at remarkably high risk for developing perioperative delirium.3, 4, 6 By definition, their urgent/emergent hip surgery stratifies them to a higher operative risk category than patients who undergo elective joint replacement.7 Half of hip fracture patients do not return to premorbid levels of function, and the 1‐year mortality rate has been estimated to be as high as 25%.6, 8 Given these differences, it is not surprising that hip fracture patients are more likely than elective joint replacement patients to respond favorably to hospitalist comanagement.
In 2007, Simon et al published a retrospective study of 739 pediatric spinal fusion patients at Childrens' Hospital in Denver.9 Beginning in 2004, hospitalists comanaged selected, high‐risk surgical patients (14 of 115 spinal fusion patients, or 12%). Over the course of the study, the mean LOS for low‐risk patients decreased by 21% but the mean LOS for the high‐risk, hospitalist‐comanaged patients decreased by 28%; a 33% relative reduction favoring hospitalist‐managed patients. By targeting selected high‐risk patients, pediatric hospitalists were able to improve upon LOS reductions that occurred systemically across the entire spinal fusion program. Also in 2007, Southern et al compared outcomes for 2,913 patients admitted by full‐time teaching hospitalists vs 6,124 patients admitted by nonhospitalists at Montefiore Medical Center, Bronx, New York.10 Mean LOS for patients admitted to the hospitalist service was 5.01 days vs 5.87 days for the nonhospitalists. Subgroup analysis demonstrated the greatest LOS differentials for patients requiring close clinical monitoring (heart failure, stroke, asthma, or pneumonia) or complex discharge planning.
Although these studies, performed at large academic medical centers, may have limited generalizability, they support the common‐sense notion that hospitalists most benefit patients who are sick, frail, and medically or socially complex. As a corollary, hospitalists probably offer relatively little benefit to surgical and specialty patients who are young or have compensated medical comorbidities and/or straightforward disposition plans. The enormous variability across healthcare institutions makes it difficult if not impossible to define a patient acuity or complexity cutoff below which hospitalist comanagement is unlikely to be beneficial. Nonetheless, some degree of common sense can be applied. As a case in point, a hospitalist probably adds little value to the care of a basically healthy patient with a hemodynamically stable upper gastrointestinal bleed. Despite this, in many institutions, hospitalists admit or comanage all gastroenterology patients, irrespective of their diagnosis, acuity, or complexity.11
One can even hypothesize that hospitalist comanagement may potentially inject risk into patient care. Admitting that patient with a stable upper gastrointestinal bleed to a hospitalist service may delay the gastroenterologist's involvement and initiation of the necessary endoscopy. Having assumed that the hospitalist is running the show, the gastroenterologist may pay insufficient attention to the patient. The hospitalist and gastroenterologist may give conflicting orders and reports that confuse patients, families, and hospital staff, ultimately increasing the likelihood of medical errors.
Ultimately, the risks inherent in adding complexity into patient care must be balanced against the potential benefits. For patients who are sick, frail, or complicated, the risk‐benefit ratio probably tilts in favor of comanagement. However, for generally healthy patients, it is conceivable that adding complexity negates (or worse yet, exceeds) the putative benefits of comanagement.
Given the potential limitations of hospitalist comanagement, why are hospitalists admitting or managing broad and unselected populations of surgical and specialty patients? Hospital leaders have suggested that hospitalist comanagement may protect overstretched surgeons and specialists and extend their capacity. A hospital with only one neurosurgeon on staff might reasonably ask its hospitalists to primarily manage carefully selected low‐acuity neurosurgical patients, allowing the neurosurgeon to serve as a consultant. However, in communities where specialists and surgeons are abundant, this justification is less credible. In such cases, it is difficult not to suspect that the primary reason that hospitalists admit surgical and specialty patients is to enhance the income and quality of life of the surgeons and specialists.
Expanding hospitalist comanagement services for no other reason than to keep specialists and surgeons happy might be justifiable if hospital medicine was not faced with its own critical manpower shortage. Hospital medicine is expected to grow from approximately 20,000 current practitioners to more than 40,000 within a decade.12 The growing shortage of qualified hospitalists has become a preoccupation for hospitalist employers across the country.13 At its 2006 strategic planning retreat, the Board of Directors of the Society of Hospital Medicine identified this issue as one of the greatest threats to the future health of hospital medicine.14 Demand for hospitalists will not abate for at least a decade, which will leave many hospitalist programs significantly understaffed for the foreseeable future. Understaffing forces hospitalist programs to lower hiring standards, jeopardizes patient care, accelerates physician burnout, and may ultimately destabilize hospital medicine.15 Understaffed hospitalist programs should be very circumspect about how and where they expand their clinical coverage.
Another principle underlying hospitalist comanagement is that it improves care by allowing surgeons and specialists to focus on their areas of expertise. Surgeons and specialists who do not have to manage their patients' medical issues can presumably spend more time focusing on their own disciplines. Although this argument is conceptually appealing, there is no evidence that this actually occurs. In fact, it is equally conceivable that hospitalist comanagement could jeopardize care by disengaging surgeons and specialists from their patients' progress (or lack thereof). Furthermore, evidence suggests that hospitalists are underprepared to manage diagnoses that have historically been the purview of surgeons and specialists. Practicing hospitalists who manage acute neurological and neurosurgical conditions, orthopedic trauma, and acute psychiatric illnesses have reported relative undertraining in all of these disease states.10, 16 Generally, hospitalists are expected to deliver this care in the absence of any regime to assess their competence, provide targeted training to fill knowledge gaps, and monitor their progress. At minimum, this should raise concerns about the quality and consistency of care that hospitalists provide to nonmedical patients.
Finally, working collaboratively with other specialties should be a major professional benefit of comanagement. In well‐designed comanagement arrangements, hospitalists and specialists work equitably under clearly defined and mutually agreed upon rules of engagement. They share responsibility for patients, collaborate to improve care, and teach and learn from each other. Unfortunately, in many instances, the power structure becomes lopsided, with surgeons and specialists dictating how, when, and why hospitalists manage their patients.17 Emergency departments have learned to default surgical and specialty patient admissions to hospitalists when surgeons and specialists balk. Hospital administrations may tacitly or overtly expect their financially subsidized hospitalists to cheerfully accept any and all referrals, irrespective of how inappropriate they may be. Practicing hospitalists frequently complain about their subordinate status and inability to control their working conditions, both of which are identified risk factors for career dissatisfaction and burnout.14, 16, 18 Once again, as a specialty facing a critical manpower shortage, hospitalist programs should be very attuned to defining work conditions that foster career satisfaction and physician retention.
REFRAMING COMANAGEMENT
The history of healthcare is laden with examples of new ideas that were widely and indiscriminately adopted only to subsequently fail to withstand rigorous scrutiny.19, 20 The unchecked expansion of hospitalist comanagement has the potential to become another case in point. In the absence of clear definitions of comanagement and good evidence to define best practices, hospitalists are left to use their best judgment to define the parameters of their comanagement services. At minimum, hospitalist leaders should ask some basic questions as they ponder potential comanagement relationships:
-
Why are we being asked to provide this service?
-
Do the patients have comorbidities that require our input?
-
Is there a legitimate quality or efficiency case to be made to support our participation?
-
Do we have the manpower to provide the service? If not, what will suffer as a result?
-
Will the relationship be equitable?
-
What might go wrong?
Comanagement is an appealing construct that has grown to fill many niches of healthcare delivery.10 Given compelling reasons to be skeptical about the purported benefits of comanagement, hospitalists should be circumspect about how and where they offer such services. Comanagement should be applied carefully and methodically, paying close attention to the consequences, intended and unintended. Applying comanagement in a rational, evidence‐based, and sustainable fashion will ultimately better serve patients, the healthcare community, and hospital medicine.
- Society of Hospital Medicine. The Society of Hospital Medicine 2005–2006 Survey: The Authoritative Source on the State of the Hospital Medicine Movement. Published by the, 2006. Executive summary available at http://www.hospitalmedicine.org/AM/Template.cfm?Section=Surveys2167(21):2338–2344.
- .Exceed acceptable: new studies challenge hospitalists to prove our value.Hospitalist.2008;12(2):63.
- ,,, et al.Medical and surgical comanagement after elective hip and knee arthroplasty.Ann Intern Med.2004;141:28–38.
- ,,, et al.Effects of a hospitalist model on elderly patients with hip fracture.Arch Intern Med.2005;165:796–801.
- ,,,.Treatment and survival among elderly Americans with hip fractures: a population‐based study.Am J Public Health.1994;84:1287–1291.
- ,,, et al.Predicting cardiac complications in patients undergoing non‐cardiac surgery.J Gen Intern Med.1986;1:211–219.
- ,,,,.Predictors of functional recovery one year following hospital discharge for hip fracture: a prospective study.J Gerontol.1990;45(3):M101–M107.
- ,,,,,.Pediatric hospitalist comanagement of spinal fusion surgery patients.J Hosp Med.2007;2:23–29.
- ,,,,.Hospitalist care and length of stay in patients requiring complex discharge planning and close clinical monitoring.Arch Intern Med.2007;167:1869–1874.
- ,,,,.The spectrum of community‐based hospitalist practice, a call to tailor internal medicine residency training.Arch Intern Med.2007;167(7):727–728.
- Society of Hospital Medicine. Growth of Hospital Medicine Nationwide. http://www.hospitalmedicine.org/Content/NavigationMenu/Media/GrowthofHospitalMedicineNation wide/Growth_of_Hospital_M.htm. Accessed September 2,2008.
- ,,, et al.Rebuilding the future of the private practice of hospital medicine.The Phoenix Group, May2007.
- Society of Hospital Medicine Board of Directors Strategic Planning Retreat: November 28‐29,2006.
- ,,,,,,for the SGIM Career Satisfaction Study Group.Physician stress: results from the physician worklife study.Stress Health.2001;18(1):37–42.
- ,,, et al.Hospitalist's perceptions of their residency training needs: Results of a national survey.Am J Med.2001;111:247–254.
- .Feeling pressure to admit surgical patients? Hospitalists work to set limits on co‐management arrangements.Today's Hospitalist. January2008.
- Society of Hospital Medicine. Career Satisfaction White Paper. http://www.hospitalmedicine.org/AM/Template.cfm?Section=Practice_Resources321:406–412.
- .Pulmonary‐artery catheters—peace at last?N Engl J Med.2006;354(21):2273–2274.
- Society of Hospital Medicine. The Society of Hospital Medicine 2005–2006 Survey: The Authoritative Source on the State of the Hospital Medicine Movement. Published by the, 2006. Executive summary available at http://www.hospitalmedicine.org/AM/Template.cfm?Section=Surveys2167(21):2338–2344.
- .Exceed acceptable: new studies challenge hospitalists to prove our value.Hospitalist.2008;12(2):63.
- ,,, et al.Medical and surgical comanagement after elective hip and knee arthroplasty.Ann Intern Med.2004;141:28–38.
- ,,, et al.Effects of a hospitalist model on elderly patients with hip fracture.Arch Intern Med.2005;165:796–801.
- ,,,.Treatment and survival among elderly Americans with hip fractures: a population‐based study.Am J Public Health.1994;84:1287–1291.
- ,,, et al.Predicting cardiac complications in patients undergoing non‐cardiac surgery.J Gen Intern Med.1986;1:211–219.
- ,,,,.Predictors of functional recovery one year following hospital discharge for hip fracture: a prospective study.J Gerontol.1990;45(3):M101–M107.
- ,,,,,.Pediatric hospitalist comanagement of spinal fusion surgery patients.J Hosp Med.2007;2:23–29.
- ,,,,.Hospitalist care and length of stay in patients requiring complex discharge planning and close clinical monitoring.Arch Intern Med.2007;167:1869–1874.
- ,,,,.The spectrum of community‐based hospitalist practice, a call to tailor internal medicine residency training.Arch Intern Med.2007;167(7):727–728.
- Society of Hospital Medicine. Growth of Hospital Medicine Nationwide. http://www.hospitalmedicine.org/Content/NavigationMenu/Media/GrowthofHospitalMedicineNation wide/Growth_of_Hospital_M.htm. Accessed September 2,2008.
- ,,, et al.Rebuilding the future of the private practice of hospital medicine.The Phoenix Group, May2007.
- Society of Hospital Medicine Board of Directors Strategic Planning Retreat: November 28‐29,2006.
- ,,,,,,for the SGIM Career Satisfaction Study Group.Physician stress: results from the physician worklife study.Stress Health.2001;18(1):37–42.
- ,,, et al.Hospitalist's perceptions of their residency training needs: Results of a national survey.Am J Med.2001;111:247–254.
- .Feeling pressure to admit surgical patients? Hospitalists work to set limits on co‐management arrangements.Today's Hospitalist. January2008.
- Society of Hospital Medicine. Career Satisfaction White Paper. http://www.hospitalmedicine.org/AM/Template.cfm?Section=Practice_Resources321:406–412.
- .Pulmonary‐artery catheters—peace at last?N Engl J Med.2006;354(21):2273–2274.
Purple Like a Glove
A 90‐year‐old female nursing home resident was admitted for hyponatremia and altered mental status. A Foley catheter was placed on admission. On hospital day 2, the Foley catheter was found to be draining violet urine. Urinalysis showed a pH of 9.0, numerous white cells, leukocyte esterase, and bacteria. Urine culture grew Proteus mirabilis. Purple Urine Bag Syndrome (PUBS) is a rare phenomenon associated with alkaline urine due to a urinary tract infection. The patient was treated with ciprofloxacin, and her urine returned to a pale yellow color. While alarming to patients and providers alike, PUBS is a benign herald of urinary tract infection, often in an elderly woman with constipation. In normal individuals, tryptophan is metabolized to indole by gut flora, which is in turn conjugated to indoxyl sulfate (IS) by the liver. Urine excretion of IS varies by individual. Sulfatase‐containing bacteria, notably Providencia, Klebsiella, and Proteus species, then catabolize IS to indoxyl. In an alkaline environment indoxyl isomers interact to alternately yield indigo or indirubin which jointly create the urine's characteristic violet color.0
- ,,.Enzymatic degradation of urinary indoxyl sulfate by Providencia stuartii and Klebsiella pneumonia causes the purple urine bag syndrome.J Clin Microbiol.1988;26(10):2152–2156.
A 90‐year‐old female nursing home resident was admitted for hyponatremia and altered mental status. A Foley catheter was placed on admission. On hospital day 2, the Foley catheter was found to be draining violet urine. Urinalysis showed a pH of 9.0, numerous white cells, leukocyte esterase, and bacteria. Urine culture grew Proteus mirabilis. Purple Urine Bag Syndrome (PUBS) is a rare phenomenon associated with alkaline urine due to a urinary tract infection. The patient was treated with ciprofloxacin, and her urine returned to a pale yellow color. While alarming to patients and providers alike, PUBS is a benign herald of urinary tract infection, often in an elderly woman with constipation. In normal individuals, tryptophan is metabolized to indole by gut flora, which is in turn conjugated to indoxyl sulfate (IS) by the liver. Urine excretion of IS varies by individual. Sulfatase‐containing bacteria, notably Providencia, Klebsiella, and Proteus species, then catabolize IS to indoxyl. In an alkaline environment indoxyl isomers interact to alternately yield indigo or indirubin which jointly create the urine's characteristic violet color.0

A 90‐year‐old female nursing home resident was admitted for hyponatremia and altered mental status. A Foley catheter was placed on admission. On hospital day 2, the Foley catheter was found to be draining violet urine. Urinalysis showed a pH of 9.0, numerous white cells, leukocyte esterase, and bacteria. Urine culture grew Proteus mirabilis. Purple Urine Bag Syndrome (PUBS) is a rare phenomenon associated with alkaline urine due to a urinary tract infection. The patient was treated with ciprofloxacin, and her urine returned to a pale yellow color. While alarming to patients and providers alike, PUBS is a benign herald of urinary tract infection, often in an elderly woman with constipation. In normal individuals, tryptophan is metabolized to indole by gut flora, which is in turn conjugated to indoxyl sulfate (IS) by the liver. Urine excretion of IS varies by individual. Sulfatase‐containing bacteria, notably Providencia, Klebsiella, and Proteus species, then catabolize IS to indoxyl. In an alkaline environment indoxyl isomers interact to alternately yield indigo or indirubin which jointly create the urine's characteristic violet color.0

- ,,.Enzymatic degradation of urinary indoxyl sulfate by Providencia stuartii and Klebsiella pneumonia causes the purple urine bag syndrome.J Clin Microbiol.1988;26(10):2152–2156.
- ,,.Enzymatic degradation of urinary indoxyl sulfate by Providencia stuartii and Klebsiella pneumonia causes the purple urine bag syndrome.J Clin Microbiol.1988;26(10):2152–2156.
Ethics of Discharge Against Medical Advice
BACKGROUND
Discharges against medical advice (AMA) account for approximately 1% of discharges for general medical patients and up to 10% and 30% for patients afflicted with HIV disease and psychiatric disorders, respectively.17 Patients discharged AMA have higher rates of readmission, longer subsequent hospital stays, and worse health outcomes.3, 5, 811 Not unexpectedly, discharges AMA are associated with overall health costs of up to 50% greater than usual discharges.2
Patients who leave AMA are more likely to have poorer social supports, to abuse alcohol, heroin, and other substances, and often have weighty psychosocial or financial concerns.1218 They are also less likely to have an established relationship with a primary care physician.19 Although studies have found that rates of discharge AMA are higher among some ethnic minorities, one recent study suggests that other patient variables, such as level of income and type of insurance, may be more closely related.7, 20 Unfortunately, many patients who leave AMA have dual sources of distress: compelling personal concerns that fuel one's wish to leave and the illness that initially caused the patient to seek care.
Physicians are often distressed by the clinical and ethical challenges of discharges AMA. How should physicians manage their conflicted obligations to respect patients' choices and to prevent harms from befalling their patients? What are physicians' obligations to their patients who leave accepting only partial or inadequate treatment plans or no treatment at all? When should physicians call into question the decision‐making capacity of patients' who make seemingly unwise or clearly dangerous judgments to leave the hospital? In addition to these sorts of concerns, physicians who discharge patients AMA enjoy no definitive legal protection from the consequences of their patients' choices.2123 In fact, good clinical judgement and careful documentation provide the best liability protection.24
Clearly, discharges AMA are problematic for patients, stressful for physicians, and resource intensive for health facilities. Therefore, efforts to understand, better manage, and ultimately decrease discharges AMA will benefit all parties. Whereas the literature on discharge AMA tends to focus on psychiatric and substance abuse patients, this review examines the professional and ethical implications of discharge AMA more generally.
Does Discharge AMA Differ from Treatment Nonadherence Elsewhere in Health Care?
Patients' nonadherence to recommended treatment is often influenced by treatment side effects, costs, inconvenience, psychosocial burden, and the quality of the patient‐physician relationship. Not surprisingly, these same factors are often associated with discharge AMA.2528 In fact, nonadherence in discharge AMA and nonadherence elsewhere are fundamentally similar. Differences, where they exist, are often in the degree or imminency of health risk and in the ability of physicians to monitor the patient.
Discharges AMA tend to involve health risks that are more acute and more severe compared to general nonadherence. To illustrate, Patient A is diagnosed with the metabolic syndrome during an office visit. His physician recommends medical therapy, and the patient declines, thereby incurring a high risk of a cardiovascular event within the next 10 years. Patient B presents to the hospital with an acute coronary syndrome. He declines to remain in the hospital for an evaluation of ischemic burden despite a high risk of a myocardial infarction in the next few days. Patient A is motivated by the cost of medication and chooses to purchase his wife's medications, foregoing his own. Patient B is motivated by distress over leaving his frail wife alone at home and concerns of medical bills that he can not afford to pay. The patient in each of these cases is motivated by social and financial concerns. The consequence of each patient's choice is a higher risk of a cardiovascular event. A major difference is the temporal relationship between the decision to not accept treatment and the ensuing adverse event.
Of course, high‐risk situations are not exclusive to the inpatient setting. For example, a patient presents to a physician's office after having experienced substernal chest pain during the previous evening. The physician recommends hospitalization but the patient declines. Conversely, a hospitalized patient may pursue discharge AMA because the patient disagrees with the physician's stipulations for safe discharge plan including assistance at home. Yet, these concerns about custodial needs, if identified by the physician in an office setting, may not necessarily compel the physician to hospitalize the patient.
Another difference between discharge AMA and general nonadherence is that adherence is more readily and closely measured in the inpatient setting. Hospital‐based occurrences of nonadherence are immediately identified and addressed. To contrast, in the outpatient setting, adherence is far poorer with a 20% nonadherence rate considered to be good compliance.2931 Regardless of the setting for nonadherence, the variance between recommended and accepted treatments often stems from the fact that patients tend to make decisions based on values and broader interests whereas physicians tend to emphasize more circumscribed medical goals.32, 33
Informed and Voluntary Refusal of Treatment
A patient's intention to leave AMA may trigger physicians and other hospital staff to question the patient's decision‐making capacity.34 One's capacity to make decisions is specific to the decision at hand. For example, a patient with early dementia and an infected arterial insufficiency ulcer may not be able to fully appreciate all the consequences of premature discharge on her health, but may be able to reliably indicate her preferred health agent.
Clinicians commonly make implicit capacity determinations, and do so each time a patient's general consent for treatment is accepted. These assessments tend to be made more explicitly when the patient's decision appears to be grossly contrary to his or her welfare. Capacity to make decisions includes the ability to understand information germane to the decision, to deliberate, and to appreciate the consequences of choices.35 As with consent to treatment, a physician who accepts a patient's refusal for treatment has determined that the patient has adequate decision‐making capacity. However, physicians do not regularly document assessments of capacity in discharge AMA.3638
Writers on the subject suggest that patients who refuse low‐risk but high‐benefit treatments should be held to a higher standard of capacity.22 This notion could expose patients to incapacity determinations based on a physician's subjective assessment of net benefit or net harm. Rather, I contend that the standard itself should not vary. It should always require that the patient's level of cognitive function, insight, and deliberative abilities be appropriate to the decision at hand and sufficient for the patient to render an autonomous decision. The relative benefit of a treatment, in and of itself, is not relevant to the level of capacity required. Rather, net benefit is relevant to physicians' obligations to more carefully verify patients' understanding of the pertinent information and their perceptions of the consequences of their choices when declining high benefit/low harm treatments.
A capacitated patient's decision to leave AMA, however well informed, may nevertheless not be entirely voluntary. Voluntary decisions are those that are made with substantially free choice.39 Various controlling influences may impact a patient's decision to leave AMA, including social or emotional challenges such as a desperate concern about losing employment.9, 1315 Health professionals may view a patient's action under some controlling influences as meritorious, for example, leaving AMA to fulfill one's obligation to care for a demented spouse, whereas professionals may view acting on other controlling influences as contemptible, such as a leaving to satisfy a drug addiction. Physicians should view controlling influences, regardless of its moral valence, as affecting the voluntariness of a patient's decision. Moreover, physicians are positioned, through either support or coercion, to influence the degree to which a patient's decision about treatment is voluntary. To illustrate, physicians who support their substance abuse patients by providing adequate treatment of their withdrawal symptoms see lower rates of discharge AMA among these addicted patients.3, 5, 7 Regarding coercion, physicians of hospitalized patients may state their refusal to prescribe a beneficial but inferior outpatient treatment in order to compel their patients to accept standard inpatient treatment.
Physicians' Obligations in Discharge AMA
Broadly stated, physicians' obligations are to promote their patients' welfare and to respect their autonomy which is understood as serving the patient's self‐defined best interests including maintaining dignity.40 When discharging a patient AMA, physicians are sometimes limited in the ways in which they can fulfill these obligations. Physicians should attempt to promote informed decision‐making by discussing the likely harms of premature discharge, the likely harms and benefits of inpatient treatment, and alternatives to inpatient treatment, including medically inferior options where these exist.
Within this obligation to promote patients' welfare, physicians should render only objective and conservative assessments of harm and benefit. These assessments may directly reflect well‐established medical evidence (eg, use of statins in acute coronary syndromes), but may also be partly or even wholly dependent on clinical judgment (eg, interpreting and applying criteria for inpatient versus outpatient treatment of pneumonia). The process though which these clinical judgments are made is critical because it forms the basis of the medical advice that defines whether a patient's discharge is routine or AMA. Physicians, in addition to their obligation to objectively assess options for treatment, should be mindful of their fiduciary responsibilities in their position to influence patients' choices by the content, emphasis, and manner with which they communicate treatment options.4144
In addition to supporting patient autonomy through information and education, physicians can promote authenticity of choice by identifying patients' compelling reasons to leave AMA. Does the patient have a demented spouse alone at home? Does the patient have a cultural or religious requirement that they perceive cannot be met while hospitalized? Is the patient concerned about loss of employment? Does the patient have an important family obligation (eg, wedding, funeral) to fulfill? Ways in which these concerns can be mitigated should be explored, often through a multidisciplinary approach that may include social work and pastoral care.45
What are physicians' obligations to patients who are willing to accept only partial or inadequate treatment plans upon discharge AMA? Should physicians be complicit in treatments that are substandard, such as the writing of a prescription for an oral antibiotic for a patient whose clinical condition meets criteria for inpatient treatment of pneumonia? Should physicians be complicit in treatments that are somewhat effective, but clearly inadequate and potentially dangerous? An example of this is the providing of a prescription for an oral anti‐arrhythmic medication for a patient diagnosed in the emergency department (ED) with syncope from a tachyarrhythmia.
In considering these scenarios, physicians may need to focus primarily on their ethical obligations to not cause harms, because discharge AMA limits physicians' ability to actively promote patients' health.46 To illustrate, Patient C, a frequent abuser of alcohol, presents to the ED and is diagnosed with a pulmonary embolus. She wants only analgesic medication for her chest pain and states that she plans no outpatient follow up. What options should the ED physician consider? The physician should not discharge the patient with a prescription for warfarin, the use of which requires close and careful monitoring especially in the setting of alcohol consumption, because this treatment, along with this patient's social practices and disinclination for follow up, introduces risks similar in seriousness to her medical condition.47 Should the ED physician give her an injection of low molecular weight heparin before the patient exits? Although a single injection of heparin is not likely to meaningfully affect her disease course, there is little direct harm in providing it. However, one must also consider possible indirect harms. For example, the offer of heparin may harm Patient C if she construes it as a bona fide treatment alternative, thereby influencing her decision to leave AMA. In another scenario, Patient D presents to the ED with an upper gastrointestinal hemorrhage and orthostatic hypotension that responds quickly to intravenous fluids. The patient unconditionally refuses to undergo an endoscopy or to accept admission into the hospital. Should the ED physician administer a dose of intravenous proton pump inhibitor (PPI), and write a prescription for high‐dose oral PPI? Because the harms of PPIs are low and it may prevent rebleeding, providing such care does not violate the obligation to not cause disproportionate harms, and attends to the obligation to promote the patient's health. To summarize, physicians' obligations to provide treatment upon discharge AMA is determined by a complex evaluation of the likelihood and magnitude of each the harms and benefits associated with the outpatient treatment and the disease‐associated risks of morbidity and mortality. This assessment is outlined in Table 1.
| Disease Risk | Treatment Efficacy | Treatment Risk | Ethical Obligation |
|---|---|---|---|
| High | High | Low | Clear obligation to treat |
| High | Low | Low | Weak obligation to treat |
| Low | High | Low | Weak obligation to treat |
| High | High | High | No clear obligation to treat |
| High | Low | High | No clear obligation to treat |
| Low | High | High | No clear obligation to treat |
| Low | Low | Low | No clear obligation to treat |
| Low | Low | High | Clear obligation not to treat |
Do physicians have obligations for facilitating after‐care when discharging a patient AMA? The policy of some hospitals is that there are no such obligations.48 Arguably, providing resources for after‐care to these patients may benefit these patients with no additional medical risk, with the caveat that offering after‐care does not influence the patient's decision to leave AMA. Therefore, physicians are ethically obligated to offer this care. In fact, this is the practice of many physicians and consistent with a number of authorities in medicine and ethics.24, 36, 49, 50 There is little evidence to support the concern that providing patients with after‐care resources exposes physicians or institutions to greater legal liability. In fact the opposite may be true.51 For patients who habitually leave AMA and who repeatedly have not sought recommended after‐care, it should not be ethically obligatory for hospital staff to expend efforts to secure after‐care.
A corollary to physicians' obligations is the obligations of patients as users of health resources. There is an enormous literature on patients' rights, yet a relative dearth of discourse, let alone consensus, on patients' duties and responsibilities.52, 53 At a minimum, patients are obligated to honor commitments and to disclose relevant information in the interest of their personal health.54 Do patients discharged AMA have moral obligations to their fellow patients or to society in terms of responsible use of often costly and sometimes limited health resources? If so, what do these obligations require and which patients should be so obligated? These are important questions to consider, yet are beyond the scope of this discussion.
Summary and Conclusions
Clinicians caring for patients who seek discharge AMA are often faced with emotionally charged and time‐pressured treatment situations. These clinicians must weigh multiple considerations for the benefit of their patients, and maintain professional standards of clinical care. Clinicians presented with these situations should (1) evaluate patients' decision‐making capacity, (2) assess the degree to which their choices are influenced by controlling external influences and mitigate these factors where possible, and (3) encourage and facilitate after‐care (Table 2).
| 1. | Capacity | Assess patient's factual understanding, reasoning, and insight into consequences of decision |
| 2. | Voluntariness | Assess for controlling influences; physical, social, emotional, psychiatric, cultural |
| 3. | Mitigation | Multidisciplinary efforts to mitigate controlling influences |
| 4. | Treatment alternatives | Assess for medically appropriate outpatient treatment alternatives. (See table 1) |
| 5. | Aftercare | Encourage and facilitate after care |
Although discharge AMA accounts for only a small percentage of hospital discharges, its medical, emotional, and resource utilization consequences for patients as well as for physicians and hospitals is disproportionate. The clinical impacts of discharge AMA should be further investigated and specific strategies and interventions to mitigate its health effects should be validated.
- ,,.Factors associated with patients who leave acute‐care hospitals against medical advice.Am J Public Health.2007;97(12):2204–2208.
- .Discharge against medical advice: sociodemographic, clinical and financial perspectives.Int J Clin Pract.2002;56(5):325–327.
- ,,,,,.Leaving hospital against medical advice among HIV‐positive patients.CMAJ.2002;167(6):633–637.
- ,,.Leaving hospital against medical advice.J Qual Clin Pract.1996;16(3):157–164.
- ,,,,,.Predictors and outcome of discharge against medical advice from the psychiatric units of a general hospital.Psychiatr Serv.1998;49(9):1187–1192.
- ,.Discharges against medical advice at regional acute care hospitals.Am J Public Health.1991;81(2):212–215.
- ,,.Discharges against medical advice: are race/ethnicity predictors?J Gen Intern Med.2006;21(9):955–960.
- ,,,,.What happens to patients who leave hospital against medical advice?CMAJ.2003;168(4):417–420.
- ,,,,.Hospitalized patients with asthma who leave against medical advice: characteristics, reasons, and outcomes.J Allergy Clin Immunol.2007;19(4):924–929.
- ,,.Post partum discharge against medical advice: who leaves and does it matter?Matern Child Health J.2007;11(5):431–436.
- ,,,,.Uncompleted emergency department care: patients who leave against medical advice.Acad Emerg Med.2007;14(10):870–876.
- ,,, et al.HIV‐positive injection drug users who leave the hospital against medical advice: the mitigating role of methadone and social support.J Acquir Immune Defic Syndr.2004;35(1):56–59.
- ,,,,.Risk fctors for AMA discharge from VA inpatient alcoholism treatment programs.J Subst Abuse Treat.1994;11(3):239–245.
- ,.Interventions to improve the AMA‐discharge rate for opiate‐addicted patients.J Psychosoc Nurs Ment Health Serv.1994;32(8):36–40.
- ,,,.Why patients sign out against medical advice (AMA): factors motivating patients to sign out AMA.Am J Drug Alcohol Abuse.2004;30(2):489–493.
- ,.Diagnostic correlates of discharge against medical advice.Arch Gen Psychiatry.1977;34(2):153–155.
- ,,.Who leaves against medical advice?J Gen Intern Med.1995;10(7):403–405.
- ,,.Hospital discharge against medical advice after myocardial infarction: deaths and readmissions.Am J Med.2007;120(12):104–153.
- ,,.Patients discharged against medical advice from a general medicine service.J Gen Intern Med.1998;13(8):568–571.
- ,.Race and hospital discharge against medical advice.J Natl Med Assoc.1996;88(10):658–660.
- ,,.An examination of whether discharging patients against medical advice protects physicians from malpractice charges.Psychiatr Serv.2000;51(7):899–902.
- ,.Patients' rights and psychiatrists' duties: discharging patients against medical advice.Harv Rev Psychiatry.2003;11(6):333–343.
- ,,.Does identifying a discharge as “against medical advice” confer legal protection?J Fam Pract.2000;49(3):224–227.
- American College of Emergency Physicians Scientific Meeting. http://meetings.acep.org/NR/rdonlyres/3389C314–2395‐4FCE‐BD9A‐FAABFFC0DFB6/0/WE184.pdf. Accessed November 30,2007.
- ,,, et al.Posttraumatic stress, nonadherence, and adverse outcome in survivors of a myocardial infarction.Psychosom Med.2004;66(4):521–526.
- ,,,.The role of patient‐physician trust in moderating medication nonadherence due to cost pressures.Arch Intern Med.2005;165(15):1749–1755.
- ,,,.Factors associated with medication nonadherence in patients with COPD.Chest.2005;128(5):3198–3204.
- ,,,.Medication nonadherence and substance abuse in psychotic disorders: impact of depressive symptoms and social stability.J Nerv Ment Dis.2005;193(10):673–679.
- ,,,,,.Compliance with antihypertensive therapy among elderly medicaid enrollees: the roles of age, gender, and race.Am J Public Health.1996;86(12):1805–1808.
- ,,,,,.How well do patients report noncompliance with antihypertensive medications?: a comparison of self‐report versus filled prescriptions.Pharmacoepidemiol Drug Saf.2004;13(1):11–19.
- .Variations in patients' adherence to medical recommendations: a quantitative review of 50 years of research.Med Care.2004;42(3):200–209.
- ,.Physicians' evaluations of patients' decisions to refuse oncological treatment.J Med Ethics.2005;31(3):131–136.
- ,.Patient non‐compliance: deviance or reasoned decision‐making?Soc Sci Med.1992;34(5):507–513.
- ,,,,.Ten myths about decision‐making capacity.J Am Med Dir Assoc.2005;6(3 Suppl):S100–S104.
- ,,.The MacCAT‐T: a clinical tool to assess patients' capacities to make treatment decisions.Psychiatr Serv.1997;48(11):1415–1419.
- ,,.Emergency department discharges against medical advice.J Emerg Med.1992;10(4):513–516.
- ,.Discharges against medical advice: a community hospital's experience.Can J Rural Med.2004;9(3):148–153.
- ,.Patient self discharge from the emergency department: who is at Risk?Emergency Med J.2005;22(7):499–501.
- ,.Respect for Autonomy.Principles of Biomedical Ethics.Fifth ed.New York:Oxford University Press;2001. p.57–112.
- ,.Ethics Manual: fifth edition.Ann Intern Med.2005;142(7):560–582.
- ,.The effect of physician's explanations on patients' treatment preferences: five‐year survival data.Med Decis Making.1994;14(3):255–258.
- ,.How the manner of presentation of data influences older patients in determining their treatment preferences.J Am Geriatr Soc.1993;41(3):223–228.
- ,,,.The role of doctor's opinion in shared decision making: what does shared decision making really mean when considering invasive medical procedures?Health Expect.2005;8(2):97–102.
- ,,,.The influence of treatment descriptions on advance medical directive decisions.J Am Geriatr Soc.1992;40(12):1255–1260.
- ,,,.Patients who leave the hospital against medical advice: the role of the psychiatric consultant.Psychosomatics.1989;30(4):396–404.
- ,.Nonmaleficence.Principles of Biomedical Ethics.Fifth ed.New York:Oxford University Press;2001:113–164.
- ,,.Untreated patients with pulmonary embolism. Outcome, clinical, and laboratory assessment.Chest.1995;107(4):931–935.
- Memorial Hospital Pembroke, Pembroke Pines, Florida. Medical Staff Rules and Regulations. http://www.mhs.net/AboutUs/Physician_Bylaws/pdfs/mhp/MHP_Rules_and%20_Regs_2004.pdf. Accessed August 29,2008.
- ,.Nonabandonment: a central obligation for physicians.Ann Intern Med.1995;122(5):368–374.
- .Changing policy to reflect a concern for patients who sign out against medical advice.Am J Bioethic.2007;7(3):32–34.
- ,,,,,.Reducing risk with telephone follow‐up of patients who leave against medical advice of fail to complete an ED visit.J Emerg Nurs.2000;26(3):223–232.
- .Moral obligations of patients: a clinical view.J Med Philos.2005;30(2):139–152.
- ,.Patients' responsibilities in medical ethics.Bioethics.2002;16(4):335–352.
- .Patients' Responsibilities. In:Post SG, ed.Encyclopedia of Bioethics.Third ed.New York:Thompson Gale;2004. p.1990–1992.
BACKGROUND
Discharges against medical advice (AMA) account for approximately 1% of discharges for general medical patients and up to 10% and 30% for patients afflicted with HIV disease and psychiatric disorders, respectively.17 Patients discharged AMA have higher rates of readmission, longer subsequent hospital stays, and worse health outcomes.3, 5, 811 Not unexpectedly, discharges AMA are associated with overall health costs of up to 50% greater than usual discharges.2
Patients who leave AMA are more likely to have poorer social supports, to abuse alcohol, heroin, and other substances, and often have weighty psychosocial or financial concerns.1218 They are also less likely to have an established relationship with a primary care physician.19 Although studies have found that rates of discharge AMA are higher among some ethnic minorities, one recent study suggests that other patient variables, such as level of income and type of insurance, may be more closely related.7, 20 Unfortunately, many patients who leave AMA have dual sources of distress: compelling personal concerns that fuel one's wish to leave and the illness that initially caused the patient to seek care.
Physicians are often distressed by the clinical and ethical challenges of discharges AMA. How should physicians manage their conflicted obligations to respect patients' choices and to prevent harms from befalling their patients? What are physicians' obligations to their patients who leave accepting only partial or inadequate treatment plans or no treatment at all? When should physicians call into question the decision‐making capacity of patients' who make seemingly unwise or clearly dangerous judgments to leave the hospital? In addition to these sorts of concerns, physicians who discharge patients AMA enjoy no definitive legal protection from the consequences of their patients' choices.2123 In fact, good clinical judgement and careful documentation provide the best liability protection.24
Clearly, discharges AMA are problematic for patients, stressful for physicians, and resource intensive for health facilities. Therefore, efforts to understand, better manage, and ultimately decrease discharges AMA will benefit all parties. Whereas the literature on discharge AMA tends to focus on psychiatric and substance abuse patients, this review examines the professional and ethical implications of discharge AMA more generally.
Does Discharge AMA Differ from Treatment Nonadherence Elsewhere in Health Care?
Patients' nonadherence to recommended treatment is often influenced by treatment side effects, costs, inconvenience, psychosocial burden, and the quality of the patient‐physician relationship. Not surprisingly, these same factors are often associated with discharge AMA.2528 In fact, nonadherence in discharge AMA and nonadherence elsewhere are fundamentally similar. Differences, where they exist, are often in the degree or imminency of health risk and in the ability of physicians to monitor the patient.
Discharges AMA tend to involve health risks that are more acute and more severe compared to general nonadherence. To illustrate, Patient A is diagnosed with the metabolic syndrome during an office visit. His physician recommends medical therapy, and the patient declines, thereby incurring a high risk of a cardiovascular event within the next 10 years. Patient B presents to the hospital with an acute coronary syndrome. He declines to remain in the hospital for an evaluation of ischemic burden despite a high risk of a myocardial infarction in the next few days. Patient A is motivated by the cost of medication and chooses to purchase his wife's medications, foregoing his own. Patient B is motivated by distress over leaving his frail wife alone at home and concerns of medical bills that he can not afford to pay. The patient in each of these cases is motivated by social and financial concerns. The consequence of each patient's choice is a higher risk of a cardiovascular event. A major difference is the temporal relationship between the decision to not accept treatment and the ensuing adverse event.
Of course, high‐risk situations are not exclusive to the inpatient setting. For example, a patient presents to a physician's office after having experienced substernal chest pain during the previous evening. The physician recommends hospitalization but the patient declines. Conversely, a hospitalized patient may pursue discharge AMA because the patient disagrees with the physician's stipulations for safe discharge plan including assistance at home. Yet, these concerns about custodial needs, if identified by the physician in an office setting, may not necessarily compel the physician to hospitalize the patient.
Another difference between discharge AMA and general nonadherence is that adherence is more readily and closely measured in the inpatient setting. Hospital‐based occurrences of nonadherence are immediately identified and addressed. To contrast, in the outpatient setting, adherence is far poorer with a 20% nonadherence rate considered to be good compliance.2931 Regardless of the setting for nonadherence, the variance between recommended and accepted treatments often stems from the fact that patients tend to make decisions based on values and broader interests whereas physicians tend to emphasize more circumscribed medical goals.32, 33
Informed and Voluntary Refusal of Treatment
A patient's intention to leave AMA may trigger physicians and other hospital staff to question the patient's decision‐making capacity.34 One's capacity to make decisions is specific to the decision at hand. For example, a patient with early dementia and an infected arterial insufficiency ulcer may not be able to fully appreciate all the consequences of premature discharge on her health, but may be able to reliably indicate her preferred health agent.
Clinicians commonly make implicit capacity determinations, and do so each time a patient's general consent for treatment is accepted. These assessments tend to be made more explicitly when the patient's decision appears to be grossly contrary to his or her welfare. Capacity to make decisions includes the ability to understand information germane to the decision, to deliberate, and to appreciate the consequences of choices.35 As with consent to treatment, a physician who accepts a patient's refusal for treatment has determined that the patient has adequate decision‐making capacity. However, physicians do not regularly document assessments of capacity in discharge AMA.3638
Writers on the subject suggest that patients who refuse low‐risk but high‐benefit treatments should be held to a higher standard of capacity.22 This notion could expose patients to incapacity determinations based on a physician's subjective assessment of net benefit or net harm. Rather, I contend that the standard itself should not vary. It should always require that the patient's level of cognitive function, insight, and deliberative abilities be appropriate to the decision at hand and sufficient for the patient to render an autonomous decision. The relative benefit of a treatment, in and of itself, is not relevant to the level of capacity required. Rather, net benefit is relevant to physicians' obligations to more carefully verify patients' understanding of the pertinent information and their perceptions of the consequences of their choices when declining high benefit/low harm treatments.
A capacitated patient's decision to leave AMA, however well informed, may nevertheless not be entirely voluntary. Voluntary decisions are those that are made with substantially free choice.39 Various controlling influences may impact a patient's decision to leave AMA, including social or emotional challenges such as a desperate concern about losing employment.9, 1315 Health professionals may view a patient's action under some controlling influences as meritorious, for example, leaving AMA to fulfill one's obligation to care for a demented spouse, whereas professionals may view acting on other controlling influences as contemptible, such as a leaving to satisfy a drug addiction. Physicians should view controlling influences, regardless of its moral valence, as affecting the voluntariness of a patient's decision. Moreover, physicians are positioned, through either support or coercion, to influence the degree to which a patient's decision about treatment is voluntary. To illustrate, physicians who support their substance abuse patients by providing adequate treatment of their withdrawal symptoms see lower rates of discharge AMA among these addicted patients.3, 5, 7 Regarding coercion, physicians of hospitalized patients may state their refusal to prescribe a beneficial but inferior outpatient treatment in order to compel their patients to accept standard inpatient treatment.
Physicians' Obligations in Discharge AMA
Broadly stated, physicians' obligations are to promote their patients' welfare and to respect their autonomy which is understood as serving the patient's self‐defined best interests including maintaining dignity.40 When discharging a patient AMA, physicians are sometimes limited in the ways in which they can fulfill these obligations. Physicians should attempt to promote informed decision‐making by discussing the likely harms of premature discharge, the likely harms and benefits of inpatient treatment, and alternatives to inpatient treatment, including medically inferior options where these exist.
Within this obligation to promote patients' welfare, physicians should render only objective and conservative assessments of harm and benefit. These assessments may directly reflect well‐established medical evidence (eg, use of statins in acute coronary syndromes), but may also be partly or even wholly dependent on clinical judgment (eg, interpreting and applying criteria for inpatient versus outpatient treatment of pneumonia). The process though which these clinical judgments are made is critical because it forms the basis of the medical advice that defines whether a patient's discharge is routine or AMA. Physicians, in addition to their obligation to objectively assess options for treatment, should be mindful of their fiduciary responsibilities in their position to influence patients' choices by the content, emphasis, and manner with which they communicate treatment options.4144
In addition to supporting patient autonomy through information and education, physicians can promote authenticity of choice by identifying patients' compelling reasons to leave AMA. Does the patient have a demented spouse alone at home? Does the patient have a cultural or religious requirement that they perceive cannot be met while hospitalized? Is the patient concerned about loss of employment? Does the patient have an important family obligation (eg, wedding, funeral) to fulfill? Ways in which these concerns can be mitigated should be explored, often through a multidisciplinary approach that may include social work and pastoral care.45
What are physicians' obligations to patients who are willing to accept only partial or inadequate treatment plans upon discharge AMA? Should physicians be complicit in treatments that are substandard, such as the writing of a prescription for an oral antibiotic for a patient whose clinical condition meets criteria for inpatient treatment of pneumonia? Should physicians be complicit in treatments that are somewhat effective, but clearly inadequate and potentially dangerous? An example of this is the providing of a prescription for an oral anti‐arrhythmic medication for a patient diagnosed in the emergency department (ED) with syncope from a tachyarrhythmia.
In considering these scenarios, physicians may need to focus primarily on their ethical obligations to not cause harms, because discharge AMA limits physicians' ability to actively promote patients' health.46 To illustrate, Patient C, a frequent abuser of alcohol, presents to the ED and is diagnosed with a pulmonary embolus. She wants only analgesic medication for her chest pain and states that she plans no outpatient follow up. What options should the ED physician consider? The physician should not discharge the patient with a prescription for warfarin, the use of which requires close and careful monitoring especially in the setting of alcohol consumption, because this treatment, along with this patient's social practices and disinclination for follow up, introduces risks similar in seriousness to her medical condition.47 Should the ED physician give her an injection of low molecular weight heparin before the patient exits? Although a single injection of heparin is not likely to meaningfully affect her disease course, there is little direct harm in providing it. However, one must also consider possible indirect harms. For example, the offer of heparin may harm Patient C if she construes it as a bona fide treatment alternative, thereby influencing her decision to leave AMA. In another scenario, Patient D presents to the ED with an upper gastrointestinal hemorrhage and orthostatic hypotension that responds quickly to intravenous fluids. The patient unconditionally refuses to undergo an endoscopy or to accept admission into the hospital. Should the ED physician administer a dose of intravenous proton pump inhibitor (PPI), and write a prescription for high‐dose oral PPI? Because the harms of PPIs are low and it may prevent rebleeding, providing such care does not violate the obligation to not cause disproportionate harms, and attends to the obligation to promote the patient's health. To summarize, physicians' obligations to provide treatment upon discharge AMA is determined by a complex evaluation of the likelihood and magnitude of each the harms and benefits associated with the outpatient treatment and the disease‐associated risks of morbidity and mortality. This assessment is outlined in Table 1.
| Disease Risk | Treatment Efficacy | Treatment Risk | Ethical Obligation |
|---|---|---|---|
| High | High | Low | Clear obligation to treat |
| High | Low | Low | Weak obligation to treat |
| Low | High | Low | Weak obligation to treat |
| High | High | High | No clear obligation to treat |
| High | Low | High | No clear obligation to treat |
| Low | High | High | No clear obligation to treat |
| Low | Low | Low | No clear obligation to treat |
| Low | Low | High | Clear obligation not to treat |
Do physicians have obligations for facilitating after‐care when discharging a patient AMA? The policy of some hospitals is that there are no such obligations.48 Arguably, providing resources for after‐care to these patients may benefit these patients with no additional medical risk, with the caveat that offering after‐care does not influence the patient's decision to leave AMA. Therefore, physicians are ethically obligated to offer this care. In fact, this is the practice of many physicians and consistent with a number of authorities in medicine and ethics.24, 36, 49, 50 There is little evidence to support the concern that providing patients with after‐care resources exposes physicians or institutions to greater legal liability. In fact the opposite may be true.51 For patients who habitually leave AMA and who repeatedly have not sought recommended after‐care, it should not be ethically obligatory for hospital staff to expend efforts to secure after‐care.
A corollary to physicians' obligations is the obligations of patients as users of health resources. There is an enormous literature on patients' rights, yet a relative dearth of discourse, let alone consensus, on patients' duties and responsibilities.52, 53 At a minimum, patients are obligated to honor commitments and to disclose relevant information in the interest of their personal health.54 Do patients discharged AMA have moral obligations to their fellow patients or to society in terms of responsible use of often costly and sometimes limited health resources? If so, what do these obligations require and which patients should be so obligated? These are important questions to consider, yet are beyond the scope of this discussion.
Summary and Conclusions
Clinicians caring for patients who seek discharge AMA are often faced with emotionally charged and time‐pressured treatment situations. These clinicians must weigh multiple considerations for the benefit of their patients, and maintain professional standards of clinical care. Clinicians presented with these situations should (1) evaluate patients' decision‐making capacity, (2) assess the degree to which their choices are influenced by controlling external influences and mitigate these factors where possible, and (3) encourage and facilitate after‐care (Table 2).
| 1. | Capacity | Assess patient's factual understanding, reasoning, and insight into consequences of decision |
| 2. | Voluntariness | Assess for controlling influences; physical, social, emotional, psychiatric, cultural |
| 3. | Mitigation | Multidisciplinary efforts to mitigate controlling influences |
| 4. | Treatment alternatives | Assess for medically appropriate outpatient treatment alternatives. (See table 1) |
| 5. | Aftercare | Encourage and facilitate after care |
Although discharge AMA accounts for only a small percentage of hospital discharges, its medical, emotional, and resource utilization consequences for patients as well as for physicians and hospitals is disproportionate. The clinical impacts of discharge AMA should be further investigated and specific strategies and interventions to mitigate its health effects should be validated.
BACKGROUND
Discharges against medical advice (AMA) account for approximately 1% of discharges for general medical patients and up to 10% and 30% for patients afflicted with HIV disease and psychiatric disorders, respectively.17 Patients discharged AMA have higher rates of readmission, longer subsequent hospital stays, and worse health outcomes.3, 5, 811 Not unexpectedly, discharges AMA are associated with overall health costs of up to 50% greater than usual discharges.2
Patients who leave AMA are more likely to have poorer social supports, to abuse alcohol, heroin, and other substances, and often have weighty psychosocial or financial concerns.1218 They are also less likely to have an established relationship with a primary care physician.19 Although studies have found that rates of discharge AMA are higher among some ethnic minorities, one recent study suggests that other patient variables, such as level of income and type of insurance, may be more closely related.7, 20 Unfortunately, many patients who leave AMA have dual sources of distress: compelling personal concerns that fuel one's wish to leave and the illness that initially caused the patient to seek care.
Physicians are often distressed by the clinical and ethical challenges of discharges AMA. How should physicians manage their conflicted obligations to respect patients' choices and to prevent harms from befalling their patients? What are physicians' obligations to their patients who leave accepting only partial or inadequate treatment plans or no treatment at all? When should physicians call into question the decision‐making capacity of patients' who make seemingly unwise or clearly dangerous judgments to leave the hospital? In addition to these sorts of concerns, physicians who discharge patients AMA enjoy no definitive legal protection from the consequences of their patients' choices.2123 In fact, good clinical judgement and careful documentation provide the best liability protection.24
Clearly, discharges AMA are problematic for patients, stressful for physicians, and resource intensive for health facilities. Therefore, efforts to understand, better manage, and ultimately decrease discharges AMA will benefit all parties. Whereas the literature on discharge AMA tends to focus on psychiatric and substance abuse patients, this review examines the professional and ethical implications of discharge AMA more generally.
Does Discharge AMA Differ from Treatment Nonadherence Elsewhere in Health Care?
Patients' nonadherence to recommended treatment is often influenced by treatment side effects, costs, inconvenience, psychosocial burden, and the quality of the patient‐physician relationship. Not surprisingly, these same factors are often associated with discharge AMA.2528 In fact, nonadherence in discharge AMA and nonadherence elsewhere are fundamentally similar. Differences, where they exist, are often in the degree or imminency of health risk and in the ability of physicians to monitor the patient.
Discharges AMA tend to involve health risks that are more acute and more severe compared to general nonadherence. To illustrate, Patient A is diagnosed with the metabolic syndrome during an office visit. His physician recommends medical therapy, and the patient declines, thereby incurring a high risk of a cardiovascular event within the next 10 years. Patient B presents to the hospital with an acute coronary syndrome. He declines to remain in the hospital for an evaluation of ischemic burden despite a high risk of a myocardial infarction in the next few days. Patient A is motivated by the cost of medication and chooses to purchase his wife's medications, foregoing his own. Patient B is motivated by distress over leaving his frail wife alone at home and concerns of medical bills that he can not afford to pay. The patient in each of these cases is motivated by social and financial concerns. The consequence of each patient's choice is a higher risk of a cardiovascular event. A major difference is the temporal relationship between the decision to not accept treatment and the ensuing adverse event.
Of course, high‐risk situations are not exclusive to the inpatient setting. For example, a patient presents to a physician's office after having experienced substernal chest pain during the previous evening. The physician recommends hospitalization but the patient declines. Conversely, a hospitalized patient may pursue discharge AMA because the patient disagrees with the physician's stipulations for safe discharge plan including assistance at home. Yet, these concerns about custodial needs, if identified by the physician in an office setting, may not necessarily compel the physician to hospitalize the patient.
Another difference between discharge AMA and general nonadherence is that adherence is more readily and closely measured in the inpatient setting. Hospital‐based occurrences of nonadherence are immediately identified and addressed. To contrast, in the outpatient setting, adherence is far poorer with a 20% nonadherence rate considered to be good compliance.2931 Regardless of the setting for nonadherence, the variance between recommended and accepted treatments often stems from the fact that patients tend to make decisions based on values and broader interests whereas physicians tend to emphasize more circumscribed medical goals.32, 33
Informed and Voluntary Refusal of Treatment
A patient's intention to leave AMA may trigger physicians and other hospital staff to question the patient's decision‐making capacity.34 One's capacity to make decisions is specific to the decision at hand. For example, a patient with early dementia and an infected arterial insufficiency ulcer may not be able to fully appreciate all the consequences of premature discharge on her health, but may be able to reliably indicate her preferred health agent.
Clinicians commonly make implicit capacity determinations, and do so each time a patient's general consent for treatment is accepted. These assessments tend to be made more explicitly when the patient's decision appears to be grossly contrary to his or her welfare. Capacity to make decisions includes the ability to understand information germane to the decision, to deliberate, and to appreciate the consequences of choices.35 As with consent to treatment, a physician who accepts a patient's refusal for treatment has determined that the patient has adequate decision‐making capacity. However, physicians do not regularly document assessments of capacity in discharge AMA.3638
Writers on the subject suggest that patients who refuse low‐risk but high‐benefit treatments should be held to a higher standard of capacity.22 This notion could expose patients to incapacity determinations based on a physician's subjective assessment of net benefit or net harm. Rather, I contend that the standard itself should not vary. It should always require that the patient's level of cognitive function, insight, and deliberative abilities be appropriate to the decision at hand and sufficient for the patient to render an autonomous decision. The relative benefit of a treatment, in and of itself, is not relevant to the level of capacity required. Rather, net benefit is relevant to physicians' obligations to more carefully verify patients' understanding of the pertinent information and their perceptions of the consequences of their choices when declining high benefit/low harm treatments.
A capacitated patient's decision to leave AMA, however well informed, may nevertheless not be entirely voluntary. Voluntary decisions are those that are made with substantially free choice.39 Various controlling influences may impact a patient's decision to leave AMA, including social or emotional challenges such as a desperate concern about losing employment.9, 1315 Health professionals may view a patient's action under some controlling influences as meritorious, for example, leaving AMA to fulfill one's obligation to care for a demented spouse, whereas professionals may view acting on other controlling influences as contemptible, such as a leaving to satisfy a drug addiction. Physicians should view controlling influences, regardless of its moral valence, as affecting the voluntariness of a patient's decision. Moreover, physicians are positioned, through either support or coercion, to influence the degree to which a patient's decision about treatment is voluntary. To illustrate, physicians who support their substance abuse patients by providing adequate treatment of their withdrawal symptoms see lower rates of discharge AMA among these addicted patients.3, 5, 7 Regarding coercion, physicians of hospitalized patients may state their refusal to prescribe a beneficial but inferior outpatient treatment in order to compel their patients to accept standard inpatient treatment.
Physicians' Obligations in Discharge AMA
Broadly stated, physicians' obligations are to promote their patients' welfare and to respect their autonomy which is understood as serving the patient's self‐defined best interests including maintaining dignity.40 When discharging a patient AMA, physicians are sometimes limited in the ways in which they can fulfill these obligations. Physicians should attempt to promote informed decision‐making by discussing the likely harms of premature discharge, the likely harms and benefits of inpatient treatment, and alternatives to inpatient treatment, including medically inferior options where these exist.
Within this obligation to promote patients' welfare, physicians should render only objective and conservative assessments of harm and benefit. These assessments may directly reflect well‐established medical evidence (eg, use of statins in acute coronary syndromes), but may also be partly or even wholly dependent on clinical judgment (eg, interpreting and applying criteria for inpatient versus outpatient treatment of pneumonia). The process though which these clinical judgments are made is critical because it forms the basis of the medical advice that defines whether a patient's discharge is routine or AMA. Physicians, in addition to their obligation to objectively assess options for treatment, should be mindful of their fiduciary responsibilities in their position to influence patients' choices by the content, emphasis, and manner with which they communicate treatment options.4144
In addition to supporting patient autonomy through information and education, physicians can promote authenticity of choice by identifying patients' compelling reasons to leave AMA. Does the patient have a demented spouse alone at home? Does the patient have a cultural or religious requirement that they perceive cannot be met while hospitalized? Is the patient concerned about loss of employment? Does the patient have an important family obligation (eg, wedding, funeral) to fulfill? Ways in which these concerns can be mitigated should be explored, often through a multidisciplinary approach that may include social work and pastoral care.45
What are physicians' obligations to patients who are willing to accept only partial or inadequate treatment plans upon discharge AMA? Should physicians be complicit in treatments that are substandard, such as the writing of a prescription for an oral antibiotic for a patient whose clinical condition meets criteria for inpatient treatment of pneumonia? Should physicians be complicit in treatments that are somewhat effective, but clearly inadequate and potentially dangerous? An example of this is the providing of a prescription for an oral anti‐arrhythmic medication for a patient diagnosed in the emergency department (ED) with syncope from a tachyarrhythmia.
In considering these scenarios, physicians may need to focus primarily on their ethical obligations to not cause harms, because discharge AMA limits physicians' ability to actively promote patients' health.46 To illustrate, Patient C, a frequent abuser of alcohol, presents to the ED and is diagnosed with a pulmonary embolus. She wants only analgesic medication for her chest pain and states that she plans no outpatient follow up. What options should the ED physician consider? The physician should not discharge the patient with a prescription for warfarin, the use of which requires close and careful monitoring especially in the setting of alcohol consumption, because this treatment, along with this patient's social practices and disinclination for follow up, introduces risks similar in seriousness to her medical condition.47 Should the ED physician give her an injection of low molecular weight heparin before the patient exits? Although a single injection of heparin is not likely to meaningfully affect her disease course, there is little direct harm in providing it. However, one must also consider possible indirect harms. For example, the offer of heparin may harm Patient C if she construes it as a bona fide treatment alternative, thereby influencing her decision to leave AMA. In another scenario, Patient D presents to the ED with an upper gastrointestinal hemorrhage and orthostatic hypotension that responds quickly to intravenous fluids. The patient unconditionally refuses to undergo an endoscopy or to accept admission into the hospital. Should the ED physician administer a dose of intravenous proton pump inhibitor (PPI), and write a prescription for high‐dose oral PPI? Because the harms of PPIs are low and it may prevent rebleeding, providing such care does not violate the obligation to not cause disproportionate harms, and attends to the obligation to promote the patient's health. To summarize, physicians' obligations to provide treatment upon discharge AMA is determined by a complex evaluation of the likelihood and magnitude of each the harms and benefits associated with the outpatient treatment and the disease‐associated risks of morbidity and mortality. This assessment is outlined in Table 1.
| Disease Risk | Treatment Efficacy | Treatment Risk | Ethical Obligation |
|---|---|---|---|
| High | High | Low | Clear obligation to treat |
| High | Low | Low | Weak obligation to treat |
| Low | High | Low | Weak obligation to treat |
| High | High | High | No clear obligation to treat |
| High | Low | High | No clear obligation to treat |
| Low | High | High | No clear obligation to treat |
| Low | Low | Low | No clear obligation to treat |
| Low | Low | High | Clear obligation not to treat |
Do physicians have obligations for facilitating after‐care when discharging a patient AMA? The policy of some hospitals is that there are no such obligations.48 Arguably, providing resources for after‐care to these patients may benefit these patients with no additional medical risk, with the caveat that offering after‐care does not influence the patient's decision to leave AMA. Therefore, physicians are ethically obligated to offer this care. In fact, this is the practice of many physicians and consistent with a number of authorities in medicine and ethics.24, 36, 49, 50 There is little evidence to support the concern that providing patients with after‐care resources exposes physicians or institutions to greater legal liability. In fact the opposite may be true.51 For patients who habitually leave AMA and who repeatedly have not sought recommended after‐care, it should not be ethically obligatory for hospital staff to expend efforts to secure after‐care.
A corollary to physicians' obligations is the obligations of patients as users of health resources. There is an enormous literature on patients' rights, yet a relative dearth of discourse, let alone consensus, on patients' duties and responsibilities.52, 53 At a minimum, patients are obligated to honor commitments and to disclose relevant information in the interest of their personal health.54 Do patients discharged AMA have moral obligations to their fellow patients or to society in terms of responsible use of often costly and sometimes limited health resources? If so, what do these obligations require and which patients should be so obligated? These are important questions to consider, yet are beyond the scope of this discussion.
Summary and Conclusions
Clinicians caring for patients who seek discharge AMA are often faced with emotionally charged and time‐pressured treatment situations. These clinicians must weigh multiple considerations for the benefit of their patients, and maintain professional standards of clinical care. Clinicians presented with these situations should (1) evaluate patients' decision‐making capacity, (2) assess the degree to which their choices are influenced by controlling external influences and mitigate these factors where possible, and (3) encourage and facilitate after‐care (Table 2).
| 1. | Capacity | Assess patient's factual understanding, reasoning, and insight into consequences of decision |
| 2. | Voluntariness | Assess for controlling influences; physical, social, emotional, psychiatric, cultural |
| 3. | Mitigation | Multidisciplinary efforts to mitigate controlling influences |
| 4. | Treatment alternatives | Assess for medically appropriate outpatient treatment alternatives. (See table 1) |
| 5. | Aftercare | Encourage and facilitate after care |
Although discharge AMA accounts for only a small percentage of hospital discharges, its medical, emotional, and resource utilization consequences for patients as well as for physicians and hospitals is disproportionate. The clinical impacts of discharge AMA should be further investigated and specific strategies and interventions to mitigate its health effects should be validated.
- ,,.Factors associated with patients who leave acute‐care hospitals against medical advice.Am J Public Health.2007;97(12):2204–2208.
- .Discharge against medical advice: sociodemographic, clinical and financial perspectives.Int J Clin Pract.2002;56(5):325–327.
- ,,,,,.Leaving hospital against medical advice among HIV‐positive patients.CMAJ.2002;167(6):633–637.
- ,,.Leaving hospital against medical advice.J Qual Clin Pract.1996;16(3):157–164.
- ,,,,,.Predictors and outcome of discharge against medical advice from the psychiatric units of a general hospital.Psychiatr Serv.1998;49(9):1187–1192.
- ,.Discharges against medical advice at regional acute care hospitals.Am J Public Health.1991;81(2):212–215.
- ,,.Discharges against medical advice: are race/ethnicity predictors?J Gen Intern Med.2006;21(9):955–960.
- ,,,,.What happens to patients who leave hospital against medical advice?CMAJ.2003;168(4):417–420.
- ,,,,.Hospitalized patients with asthma who leave against medical advice: characteristics, reasons, and outcomes.J Allergy Clin Immunol.2007;19(4):924–929.
- ,,.Post partum discharge against medical advice: who leaves and does it matter?Matern Child Health J.2007;11(5):431–436.
- ,,,,.Uncompleted emergency department care: patients who leave against medical advice.Acad Emerg Med.2007;14(10):870–876.
- ,,, et al.HIV‐positive injection drug users who leave the hospital against medical advice: the mitigating role of methadone and social support.J Acquir Immune Defic Syndr.2004;35(1):56–59.
- ,,,,.Risk fctors for AMA discharge from VA inpatient alcoholism treatment programs.J Subst Abuse Treat.1994;11(3):239–245.
- ,.Interventions to improve the AMA‐discharge rate for opiate‐addicted patients.J Psychosoc Nurs Ment Health Serv.1994;32(8):36–40.
- ,,,.Why patients sign out against medical advice (AMA): factors motivating patients to sign out AMA.Am J Drug Alcohol Abuse.2004;30(2):489–493.
- ,.Diagnostic correlates of discharge against medical advice.Arch Gen Psychiatry.1977;34(2):153–155.
- ,,.Who leaves against medical advice?J Gen Intern Med.1995;10(7):403–405.
- ,,.Hospital discharge against medical advice after myocardial infarction: deaths and readmissions.Am J Med.2007;120(12):104–153.
- ,,.Patients discharged against medical advice from a general medicine service.J Gen Intern Med.1998;13(8):568–571.
- ,.Race and hospital discharge against medical advice.J Natl Med Assoc.1996;88(10):658–660.
- ,,.An examination of whether discharging patients against medical advice protects physicians from malpractice charges.Psychiatr Serv.2000;51(7):899–902.
- ,.Patients' rights and psychiatrists' duties: discharging patients against medical advice.Harv Rev Psychiatry.2003;11(6):333–343.
- ,,.Does identifying a discharge as “against medical advice” confer legal protection?J Fam Pract.2000;49(3):224–227.
- American College of Emergency Physicians Scientific Meeting. http://meetings.acep.org/NR/rdonlyres/3389C314–2395‐4FCE‐BD9A‐FAABFFC0DFB6/0/WE184.pdf. Accessed November 30,2007.
- ,,, et al.Posttraumatic stress, nonadherence, and adverse outcome in survivors of a myocardial infarction.Psychosom Med.2004;66(4):521–526.
- ,,,.The role of patient‐physician trust in moderating medication nonadherence due to cost pressures.Arch Intern Med.2005;165(15):1749–1755.
- ,,,.Factors associated with medication nonadherence in patients with COPD.Chest.2005;128(5):3198–3204.
- ,,,.Medication nonadherence and substance abuse in psychotic disorders: impact of depressive symptoms and social stability.J Nerv Ment Dis.2005;193(10):673–679.
- ,,,,,.Compliance with antihypertensive therapy among elderly medicaid enrollees: the roles of age, gender, and race.Am J Public Health.1996;86(12):1805–1808.
- ,,,,,.How well do patients report noncompliance with antihypertensive medications?: a comparison of self‐report versus filled prescriptions.Pharmacoepidemiol Drug Saf.2004;13(1):11–19.
- .Variations in patients' adherence to medical recommendations: a quantitative review of 50 years of research.Med Care.2004;42(3):200–209.
- ,.Physicians' evaluations of patients' decisions to refuse oncological treatment.J Med Ethics.2005;31(3):131–136.
- ,.Patient non‐compliance: deviance or reasoned decision‐making?Soc Sci Med.1992;34(5):507–513.
- ,,,,.Ten myths about decision‐making capacity.J Am Med Dir Assoc.2005;6(3 Suppl):S100–S104.
- ,,.The MacCAT‐T: a clinical tool to assess patients' capacities to make treatment decisions.Psychiatr Serv.1997;48(11):1415–1419.
- ,,.Emergency department discharges against medical advice.J Emerg Med.1992;10(4):513–516.
- ,.Discharges against medical advice: a community hospital's experience.Can J Rural Med.2004;9(3):148–153.
- ,.Patient self discharge from the emergency department: who is at Risk?Emergency Med J.2005;22(7):499–501.
- ,.Respect for Autonomy.Principles of Biomedical Ethics.Fifth ed.New York:Oxford University Press;2001. p.57–112.
- ,.Ethics Manual: fifth edition.Ann Intern Med.2005;142(7):560–582.
- ,.The effect of physician's explanations on patients' treatment preferences: five‐year survival data.Med Decis Making.1994;14(3):255–258.
- ,.How the manner of presentation of data influences older patients in determining their treatment preferences.J Am Geriatr Soc.1993;41(3):223–228.
- ,,,.The role of doctor's opinion in shared decision making: what does shared decision making really mean when considering invasive medical procedures?Health Expect.2005;8(2):97–102.
- ,,,.The influence of treatment descriptions on advance medical directive decisions.J Am Geriatr Soc.1992;40(12):1255–1260.
- ,,,.Patients who leave the hospital against medical advice: the role of the psychiatric consultant.Psychosomatics.1989;30(4):396–404.
- ,.Nonmaleficence.Principles of Biomedical Ethics.Fifth ed.New York:Oxford University Press;2001:113–164.
- ,,.Untreated patients with pulmonary embolism. Outcome, clinical, and laboratory assessment.Chest.1995;107(4):931–935.
- Memorial Hospital Pembroke, Pembroke Pines, Florida. Medical Staff Rules and Regulations. http://www.mhs.net/AboutUs/Physician_Bylaws/pdfs/mhp/MHP_Rules_and%20_Regs_2004.pdf. Accessed August 29,2008.
- ,.Nonabandonment: a central obligation for physicians.Ann Intern Med.1995;122(5):368–374.
- .Changing policy to reflect a concern for patients who sign out against medical advice.Am J Bioethic.2007;7(3):32–34.
- ,,,,,.Reducing risk with telephone follow‐up of patients who leave against medical advice of fail to complete an ED visit.J Emerg Nurs.2000;26(3):223–232.
- .Moral obligations of patients: a clinical view.J Med Philos.2005;30(2):139–152.
- ,.Patients' responsibilities in medical ethics.Bioethics.2002;16(4):335–352.
- .Patients' Responsibilities. In:Post SG, ed.Encyclopedia of Bioethics.Third ed.New York:Thompson Gale;2004. p.1990–1992.
- ,,.Factors associated with patients who leave acute‐care hospitals against medical advice.Am J Public Health.2007;97(12):2204–2208.
- .Discharge against medical advice: sociodemographic, clinical and financial perspectives.Int J Clin Pract.2002;56(5):325–327.
- ,,,,,.Leaving hospital against medical advice among HIV‐positive patients.CMAJ.2002;167(6):633–637.
- ,,.Leaving hospital against medical advice.J Qual Clin Pract.1996;16(3):157–164.
- ,,,,,.Predictors and outcome of discharge against medical advice from the psychiatric units of a general hospital.Psychiatr Serv.1998;49(9):1187–1192.
- ,.Discharges against medical advice at regional acute care hospitals.Am J Public Health.1991;81(2):212–215.
- ,,.Discharges against medical advice: are race/ethnicity predictors?J Gen Intern Med.2006;21(9):955–960.
- ,,,,.What happens to patients who leave hospital against medical advice?CMAJ.2003;168(4):417–420.
- ,,,,.Hospitalized patients with asthma who leave against medical advice: characteristics, reasons, and outcomes.J Allergy Clin Immunol.2007;19(4):924–929.
- ,,.Post partum discharge against medical advice: who leaves and does it matter?Matern Child Health J.2007;11(5):431–436.
- ,,,,.Uncompleted emergency department care: patients who leave against medical advice.Acad Emerg Med.2007;14(10):870–876.
- ,,, et al.HIV‐positive injection drug users who leave the hospital against medical advice: the mitigating role of methadone and social support.J Acquir Immune Defic Syndr.2004;35(1):56–59.
- ,,,,.Risk fctors for AMA discharge from VA inpatient alcoholism treatment programs.J Subst Abuse Treat.1994;11(3):239–245.
- ,.Interventions to improve the AMA‐discharge rate for opiate‐addicted patients.J Psychosoc Nurs Ment Health Serv.1994;32(8):36–40.
- ,,,.Why patients sign out against medical advice (AMA): factors motivating patients to sign out AMA.Am J Drug Alcohol Abuse.2004;30(2):489–493.
- ,.Diagnostic correlates of discharge against medical advice.Arch Gen Psychiatry.1977;34(2):153–155.
- ,,.Who leaves against medical advice?J Gen Intern Med.1995;10(7):403–405.
- ,,.Hospital discharge against medical advice after myocardial infarction: deaths and readmissions.Am J Med.2007;120(12):104–153.
- ,,.Patients discharged against medical advice from a general medicine service.J Gen Intern Med.1998;13(8):568–571.
- ,.Race and hospital discharge against medical advice.J Natl Med Assoc.1996;88(10):658–660.
- ,,.An examination of whether discharging patients against medical advice protects physicians from malpractice charges.Psychiatr Serv.2000;51(7):899–902.
- ,.Patients' rights and psychiatrists' duties: discharging patients against medical advice.Harv Rev Psychiatry.2003;11(6):333–343.
- ,,.Does identifying a discharge as “against medical advice” confer legal protection?J Fam Pract.2000;49(3):224–227.
- American College of Emergency Physicians Scientific Meeting. http://meetings.acep.org/NR/rdonlyres/3389C314–2395‐4FCE‐BD9A‐FAABFFC0DFB6/0/WE184.pdf. Accessed November 30,2007.
- ,,, et al.Posttraumatic stress, nonadherence, and adverse outcome in survivors of a myocardial infarction.Psychosom Med.2004;66(4):521–526.
- ,,,.The role of patient‐physician trust in moderating medication nonadherence due to cost pressures.Arch Intern Med.2005;165(15):1749–1755.
- ,,,.Factors associated with medication nonadherence in patients with COPD.Chest.2005;128(5):3198–3204.
- ,,,.Medication nonadherence and substance abuse in psychotic disorders: impact of depressive symptoms and social stability.J Nerv Ment Dis.2005;193(10):673–679.
- ,,,,,.Compliance with antihypertensive therapy among elderly medicaid enrollees: the roles of age, gender, and race.Am J Public Health.1996;86(12):1805–1808.
- ,,,,,.How well do patients report noncompliance with antihypertensive medications?: a comparison of self‐report versus filled prescriptions.Pharmacoepidemiol Drug Saf.2004;13(1):11–19.
- .Variations in patients' adherence to medical recommendations: a quantitative review of 50 years of research.Med Care.2004;42(3):200–209.
- ,.Physicians' evaluations of patients' decisions to refuse oncological treatment.J Med Ethics.2005;31(3):131–136.
- ,.Patient non‐compliance: deviance or reasoned decision‐making?Soc Sci Med.1992;34(5):507–513.
- ,,,,.Ten myths about decision‐making capacity.J Am Med Dir Assoc.2005;6(3 Suppl):S100–S104.
- ,,.The MacCAT‐T: a clinical tool to assess patients' capacities to make treatment decisions.Psychiatr Serv.1997;48(11):1415–1419.
- ,,.Emergency department discharges against medical advice.J Emerg Med.1992;10(4):513–516.
- ,.Discharges against medical advice: a community hospital's experience.Can J Rural Med.2004;9(3):148–153.
- ,.Patient self discharge from the emergency department: who is at Risk?Emergency Med J.2005;22(7):499–501.
- ,.Respect for Autonomy.Principles of Biomedical Ethics.Fifth ed.New York:Oxford University Press;2001. p.57–112.
- ,.Ethics Manual: fifth edition.Ann Intern Med.2005;142(7):560–582.
- ,.The effect of physician's explanations on patients' treatment preferences: five‐year survival data.Med Decis Making.1994;14(3):255–258.
- ,.How the manner of presentation of data influences older patients in determining their treatment preferences.J Am Geriatr Soc.1993;41(3):223–228.
- ,,,.The role of doctor's opinion in shared decision making: what does shared decision making really mean when considering invasive medical procedures?Health Expect.2005;8(2):97–102.
- ,,,.The influence of treatment descriptions on advance medical directive decisions.J Am Geriatr Soc.1992;40(12):1255–1260.
- ,,,.Patients who leave the hospital against medical advice: the role of the psychiatric consultant.Psychosomatics.1989;30(4):396–404.
- ,.Nonmaleficence.Principles of Biomedical Ethics.Fifth ed.New York:Oxford University Press;2001:113–164.
- ,,.Untreated patients with pulmonary embolism. Outcome, clinical, and laboratory assessment.Chest.1995;107(4):931–935.
- Memorial Hospital Pembroke, Pembroke Pines, Florida. Medical Staff Rules and Regulations. http://www.mhs.net/AboutUs/Physician_Bylaws/pdfs/mhp/MHP_Rules_and%20_Regs_2004.pdf. Accessed August 29,2008.
- ,.Nonabandonment: a central obligation for physicians.Ann Intern Med.1995;122(5):368–374.
- .Changing policy to reflect a concern for patients who sign out against medical advice.Am J Bioethic.2007;7(3):32–34.
- ,,,,,.Reducing risk with telephone follow‐up of patients who leave against medical advice of fail to complete an ED visit.J Emerg Nurs.2000;26(3):223–232.
- .Moral obligations of patients: a clinical view.J Med Philos.2005;30(2):139–152.
- ,.Patients' responsibilities in medical ethics.Bioethics.2002;16(4):335–352.
- .Patients' Responsibilities. In:Post SG, ed.Encyclopedia of Bioethics.Third ed.New York:Thompson Gale;2004. p.1990–1992.
ITP and Hyperthyroidism
The connection between idiopathic thrombocytopenic purpura (ITP) and Grave's disease is not well known in the Western hemisphere. The immunologic relationship between these 2 conditions is well reported15 but poorly defined in the literature. New‐onset hyperthyroidism in the setting of preexisting ITP can be overlooked and, if untreated, lead to worsening of the ITP, rendering it refractory to standard therapy. Early recognition and treatment of the hyperthyroid state with antithyroid medications can lead to significant improvement in the platelet count.1, 8 We report this rare but critical clinical relationship.
CASE REPORT
A 35‐year‐old Asian woman with a known history of stable ITP for 12 years (baseline platelet count of 40,000/mL) presented to her outpatient provider with a diffuse petechial rash, easy bruisability, and heavy menorrhagia for 2 weeks. Her new platelet count was 7000/mL. She was immediately started on prednisone at a dose of 1 mg/kg without any improvement in her platelet count. At the end of 4 weeks on prednisone, she developed fever, intractable nausea and vomiting, severe headache, hypotension, and tachycardia. She was subsequently hospitalized with the presumptive diagnosis of meningitis and sepsis syndrome. Her clinical syndrome was consistent with systemic inflammatory response syndrome. She was treated aggressively with intravenous fluids and a broad‐spectrum empirical antimicrobial regimen consisting of ceftriaxone, vancomycin, and acyclovir. Lumbar puncture was deferred because of her low platelet count. The sepsis workup, which included viral, fungal, and bacterial blood cultures, remained negative. Her peripheral smear did not show evidence of microangiopathic hemolytic anemia, therefore ruling out thrombotic thrombocytopenic purpura and disseminated intravascular coagulation. HIV and tuberculosis were also ruled out. After the initial sepsis workup turned out negative, she was started on solumedrol 125 mg IV every 6 hours. Over the next 2 weeks, she received an average of 4‐6 units of platelets per day and multiple blood transfusions to maintain her hemoglobin and platelet counts. The latter remained in the 1000‐5000 platelets/mL range throughout her hospitalization without any significant improvement. Her clinical course was further complicated by multiple small intracranial hemorrhages without major focal neurological deficits. A bone marrow biopsy was eventually done. It showed early dysplastic cells but no definite features of myelodysplasia and few large megakaryocytes. She received 1 dose of vincristine without response in the bone marrow after 2 weeks, and consideration was given to treatment with rituximab for refractory ITP. At that point, she informed her hematologist that 10 years ago, she had been treated for hyperthyroidism with antithyroid medications for 6 months, without further follow‐up. A thyroid panel was then ordered, and she was found to be hyperthyroid, with thyroid‐stimulating hormone (TSH) 0.01 mU/mL and free T4 of 3.1 ng/dL. She was subsequently started on propylthiouracil at 300 mg per day. Her platelet count dramatically improved and went up to the 50,000/mm3 range without further intervention over the next few months. After her discharge, an outpatient thyroid scan showed diffuse, homogeneous uptake of iodine, thereby confirming the diagnosis of Grave's disease. Retrospectively, her initial clinical syndrome of fever, hypotension, and tachycardia may have been the result of thyrotoxicosis or worsened by it.
DISCUSSION
The association between ITP and Grave's disease is poorly understood. Many hypotheses from observational data have been given in the literature. The leading theory to explain the coexistence of these 2 disorders is the presence of a common autoimmune pathway with production of 2 kinds of antibodies against platelets and TSH receptors. Indeed, autoimmune disorders tend to occur concurrently in individuals or families. Bizzaro et al. reported the coexistence of ITP and Grave's in 4 members of the same family.6 Hymes et al. found elevated levels of platelet‐bound IgG in 44% of 25 study patients with Grave's thyrotoxicosis.7 Most of these patients had easy bruising and/or bleeding, and 12% were thrombocytopenic. Panzer et al. reported the presence of antiplatelet IgG in patients with Grave's as well as improved platelet counts and increased mean platelet volume after successful antithyroid therapy.8
In addition to the coexistence of thyroid‐stimulatingimmunoglobulins (TSIs) and antiplatelet antibodies as a potential mechanism for Grave's‐associated thrombocytopenia, some have postulated that in Grave's patients, TSIs and other thyroid antibodies might actually bind to the platelets themselves. The postulated site for binding would be a truncated actin‐binding protein on the platelets that would link the high‐affinity Fc receptor of immunoglobulin G to the platelets' cytoskeleton, thereby accelerating their destruction.9
Another plausible mechanism is activation of the reticuloendothelial system by thyroid hormones, with increased clearance of platelets by the spleen in thyrotoxic states. This may explain the restoration of the platelet count when euthyroidism is reached.
Finally, thyrotoxicosis seems to alter platelet aggregation, partially by inhibition of myosin light‐chain kinase, and that also improves with restoration of euthyroidism.10
The coexistence of severe hyperthyroidism and thrombocytopenia can mimic severe sepsis in critically ill patients, and the hyperthyroid state in itself can worsen the thrombocytopenia of ITP. We suspect this patient's severe sepsis may actually have been an unrecognized severe thyrotoxicosis, with bone marrow dysfunction secondary to the hyperthyroidism, which might partially explain her lack of response to standard therapy.
CONCLUSIONS
This case underscores the importance of screening for and treating hyperthyroidism in patients with ITP, especially those resistant to steroid therapy, because the literature seems to indicate that treatment of the hyperthyroid state improves platelet count. This might help to prevent devastating clinical complications. Further research is necessary to define this empirical finding.
- ,,,,,.Improvement of idiopathic thrombocytopenic purpura by antithyroid therapy.Eur J Haematol.74:73–74.
- ,,,.Graves disease associated with autoimmune thrombocytopenic purpura.Arch Intern Med.1997;157:1033–1036.
- .The thyrotoxicosis/thrombocytopenia connection.Surgery.1983;94:966–968.
- ,,,.Idiopathic thrombocytopenic purpura and Graves disease.Am J Hematol.1982;12:69–72.
- ,.Autoimmune thrombocytopenic purpura associated with hyperthyroidism in a single individual.South Med J.1997;90:933–936.
- .Familial association of autoimmune thrombocytopenia and hyperthyroidism.Am J Hematol.1992;39:294–298.
- ,,, et al.Easy bruising, thrombocytopenia, and elevated platelet immunoglobulin G in Graves' disease and Hashimoto's thyroiditis.Ann Intern Med.1981;94:27–30.
- ,,.Platelets in hyperthyroidism: studies on platelet counts, mean platelet volume.111‐indium‐labeled platelet kinetics, and platelet associated immunoglobulins G and M.J Clin Endocrinol Metab.1990;70:491–496.
- ,.Coagulation disorders in thyroid diseases.Eur J Endocrinol.1997;136:1.
- ,,.Alteration of platelet aggregation in patients with thyroid disorders.Metabolism.1997;46:1128.
The connection between idiopathic thrombocytopenic purpura (ITP) and Grave's disease is not well known in the Western hemisphere. The immunologic relationship between these 2 conditions is well reported15 but poorly defined in the literature. New‐onset hyperthyroidism in the setting of preexisting ITP can be overlooked and, if untreated, lead to worsening of the ITP, rendering it refractory to standard therapy. Early recognition and treatment of the hyperthyroid state with antithyroid medications can lead to significant improvement in the platelet count.1, 8 We report this rare but critical clinical relationship.
CASE REPORT
A 35‐year‐old Asian woman with a known history of stable ITP for 12 years (baseline platelet count of 40,000/mL) presented to her outpatient provider with a diffuse petechial rash, easy bruisability, and heavy menorrhagia for 2 weeks. Her new platelet count was 7000/mL. She was immediately started on prednisone at a dose of 1 mg/kg without any improvement in her platelet count. At the end of 4 weeks on prednisone, she developed fever, intractable nausea and vomiting, severe headache, hypotension, and tachycardia. She was subsequently hospitalized with the presumptive diagnosis of meningitis and sepsis syndrome. Her clinical syndrome was consistent with systemic inflammatory response syndrome. She was treated aggressively with intravenous fluids and a broad‐spectrum empirical antimicrobial regimen consisting of ceftriaxone, vancomycin, and acyclovir. Lumbar puncture was deferred because of her low platelet count. The sepsis workup, which included viral, fungal, and bacterial blood cultures, remained negative. Her peripheral smear did not show evidence of microangiopathic hemolytic anemia, therefore ruling out thrombotic thrombocytopenic purpura and disseminated intravascular coagulation. HIV and tuberculosis were also ruled out. After the initial sepsis workup turned out negative, she was started on solumedrol 125 mg IV every 6 hours. Over the next 2 weeks, she received an average of 4‐6 units of platelets per day and multiple blood transfusions to maintain her hemoglobin and platelet counts. The latter remained in the 1000‐5000 platelets/mL range throughout her hospitalization without any significant improvement. Her clinical course was further complicated by multiple small intracranial hemorrhages without major focal neurological deficits. A bone marrow biopsy was eventually done. It showed early dysplastic cells but no definite features of myelodysplasia and few large megakaryocytes. She received 1 dose of vincristine without response in the bone marrow after 2 weeks, and consideration was given to treatment with rituximab for refractory ITP. At that point, she informed her hematologist that 10 years ago, she had been treated for hyperthyroidism with antithyroid medications for 6 months, without further follow‐up. A thyroid panel was then ordered, and she was found to be hyperthyroid, with thyroid‐stimulating hormone (TSH) 0.01 mU/mL and free T4 of 3.1 ng/dL. She was subsequently started on propylthiouracil at 300 mg per day. Her platelet count dramatically improved and went up to the 50,000/mm3 range without further intervention over the next few months. After her discharge, an outpatient thyroid scan showed diffuse, homogeneous uptake of iodine, thereby confirming the diagnosis of Grave's disease. Retrospectively, her initial clinical syndrome of fever, hypotension, and tachycardia may have been the result of thyrotoxicosis or worsened by it.
DISCUSSION
The association between ITP and Grave's disease is poorly understood. Many hypotheses from observational data have been given in the literature. The leading theory to explain the coexistence of these 2 disorders is the presence of a common autoimmune pathway with production of 2 kinds of antibodies against platelets and TSH receptors. Indeed, autoimmune disorders tend to occur concurrently in individuals or families. Bizzaro et al. reported the coexistence of ITP and Grave's in 4 members of the same family.6 Hymes et al. found elevated levels of platelet‐bound IgG in 44% of 25 study patients with Grave's thyrotoxicosis.7 Most of these patients had easy bruising and/or bleeding, and 12% were thrombocytopenic. Panzer et al. reported the presence of antiplatelet IgG in patients with Grave's as well as improved platelet counts and increased mean platelet volume after successful antithyroid therapy.8
In addition to the coexistence of thyroid‐stimulatingimmunoglobulins (TSIs) and antiplatelet antibodies as a potential mechanism for Grave's‐associated thrombocytopenia, some have postulated that in Grave's patients, TSIs and other thyroid antibodies might actually bind to the platelets themselves. The postulated site for binding would be a truncated actin‐binding protein on the platelets that would link the high‐affinity Fc receptor of immunoglobulin G to the platelets' cytoskeleton, thereby accelerating their destruction.9
Another plausible mechanism is activation of the reticuloendothelial system by thyroid hormones, with increased clearance of platelets by the spleen in thyrotoxic states. This may explain the restoration of the platelet count when euthyroidism is reached.
Finally, thyrotoxicosis seems to alter platelet aggregation, partially by inhibition of myosin light‐chain kinase, and that also improves with restoration of euthyroidism.10
The coexistence of severe hyperthyroidism and thrombocytopenia can mimic severe sepsis in critically ill patients, and the hyperthyroid state in itself can worsen the thrombocytopenia of ITP. We suspect this patient's severe sepsis may actually have been an unrecognized severe thyrotoxicosis, with bone marrow dysfunction secondary to the hyperthyroidism, which might partially explain her lack of response to standard therapy.
CONCLUSIONS
This case underscores the importance of screening for and treating hyperthyroidism in patients with ITP, especially those resistant to steroid therapy, because the literature seems to indicate that treatment of the hyperthyroid state improves platelet count. This might help to prevent devastating clinical complications. Further research is necessary to define this empirical finding.
The connection between idiopathic thrombocytopenic purpura (ITP) and Grave's disease is not well known in the Western hemisphere. The immunologic relationship between these 2 conditions is well reported15 but poorly defined in the literature. New‐onset hyperthyroidism in the setting of preexisting ITP can be overlooked and, if untreated, lead to worsening of the ITP, rendering it refractory to standard therapy. Early recognition and treatment of the hyperthyroid state with antithyroid medications can lead to significant improvement in the platelet count.1, 8 We report this rare but critical clinical relationship.
CASE REPORT
A 35‐year‐old Asian woman with a known history of stable ITP for 12 years (baseline platelet count of 40,000/mL) presented to her outpatient provider with a diffuse petechial rash, easy bruisability, and heavy menorrhagia for 2 weeks. Her new platelet count was 7000/mL. She was immediately started on prednisone at a dose of 1 mg/kg without any improvement in her platelet count. At the end of 4 weeks on prednisone, she developed fever, intractable nausea and vomiting, severe headache, hypotension, and tachycardia. She was subsequently hospitalized with the presumptive diagnosis of meningitis and sepsis syndrome. Her clinical syndrome was consistent with systemic inflammatory response syndrome. She was treated aggressively with intravenous fluids and a broad‐spectrum empirical antimicrobial regimen consisting of ceftriaxone, vancomycin, and acyclovir. Lumbar puncture was deferred because of her low platelet count. The sepsis workup, which included viral, fungal, and bacterial blood cultures, remained negative. Her peripheral smear did not show evidence of microangiopathic hemolytic anemia, therefore ruling out thrombotic thrombocytopenic purpura and disseminated intravascular coagulation. HIV and tuberculosis were also ruled out. After the initial sepsis workup turned out negative, she was started on solumedrol 125 mg IV every 6 hours. Over the next 2 weeks, she received an average of 4‐6 units of platelets per day and multiple blood transfusions to maintain her hemoglobin and platelet counts. The latter remained in the 1000‐5000 platelets/mL range throughout her hospitalization without any significant improvement. Her clinical course was further complicated by multiple small intracranial hemorrhages without major focal neurological deficits. A bone marrow biopsy was eventually done. It showed early dysplastic cells but no definite features of myelodysplasia and few large megakaryocytes. She received 1 dose of vincristine without response in the bone marrow after 2 weeks, and consideration was given to treatment with rituximab for refractory ITP. At that point, she informed her hematologist that 10 years ago, she had been treated for hyperthyroidism with antithyroid medications for 6 months, without further follow‐up. A thyroid panel was then ordered, and she was found to be hyperthyroid, with thyroid‐stimulating hormone (TSH) 0.01 mU/mL and free T4 of 3.1 ng/dL. She was subsequently started on propylthiouracil at 300 mg per day. Her platelet count dramatically improved and went up to the 50,000/mm3 range without further intervention over the next few months. After her discharge, an outpatient thyroid scan showed diffuse, homogeneous uptake of iodine, thereby confirming the diagnosis of Grave's disease. Retrospectively, her initial clinical syndrome of fever, hypotension, and tachycardia may have been the result of thyrotoxicosis or worsened by it.
DISCUSSION
The association between ITP and Grave's disease is poorly understood. Many hypotheses from observational data have been given in the literature. The leading theory to explain the coexistence of these 2 disorders is the presence of a common autoimmune pathway with production of 2 kinds of antibodies against platelets and TSH receptors. Indeed, autoimmune disorders tend to occur concurrently in individuals or families. Bizzaro et al. reported the coexistence of ITP and Grave's in 4 members of the same family.6 Hymes et al. found elevated levels of platelet‐bound IgG in 44% of 25 study patients with Grave's thyrotoxicosis.7 Most of these patients had easy bruising and/or bleeding, and 12% were thrombocytopenic. Panzer et al. reported the presence of antiplatelet IgG in patients with Grave's as well as improved platelet counts and increased mean platelet volume after successful antithyroid therapy.8
In addition to the coexistence of thyroid‐stimulatingimmunoglobulins (TSIs) and antiplatelet antibodies as a potential mechanism for Grave's‐associated thrombocytopenia, some have postulated that in Grave's patients, TSIs and other thyroid antibodies might actually bind to the platelets themselves. The postulated site for binding would be a truncated actin‐binding protein on the platelets that would link the high‐affinity Fc receptor of immunoglobulin G to the platelets' cytoskeleton, thereby accelerating their destruction.9
Another plausible mechanism is activation of the reticuloendothelial system by thyroid hormones, with increased clearance of platelets by the spleen in thyrotoxic states. This may explain the restoration of the platelet count when euthyroidism is reached.
Finally, thyrotoxicosis seems to alter platelet aggregation, partially by inhibition of myosin light‐chain kinase, and that also improves with restoration of euthyroidism.10
The coexistence of severe hyperthyroidism and thrombocytopenia can mimic severe sepsis in critically ill patients, and the hyperthyroid state in itself can worsen the thrombocytopenia of ITP. We suspect this patient's severe sepsis may actually have been an unrecognized severe thyrotoxicosis, with bone marrow dysfunction secondary to the hyperthyroidism, which might partially explain her lack of response to standard therapy.
CONCLUSIONS
This case underscores the importance of screening for and treating hyperthyroidism in patients with ITP, especially those resistant to steroid therapy, because the literature seems to indicate that treatment of the hyperthyroid state improves platelet count. This might help to prevent devastating clinical complications. Further research is necessary to define this empirical finding.
- ,,,,,.Improvement of idiopathic thrombocytopenic purpura by antithyroid therapy.Eur J Haematol.74:73–74.
- ,,,.Graves disease associated with autoimmune thrombocytopenic purpura.Arch Intern Med.1997;157:1033–1036.
- .The thyrotoxicosis/thrombocytopenia connection.Surgery.1983;94:966–968.
- ,,,.Idiopathic thrombocytopenic purpura and Graves disease.Am J Hematol.1982;12:69–72.
- ,.Autoimmune thrombocytopenic purpura associated with hyperthyroidism in a single individual.South Med J.1997;90:933–936.
- .Familial association of autoimmune thrombocytopenia and hyperthyroidism.Am J Hematol.1992;39:294–298.
- ,,, et al.Easy bruising, thrombocytopenia, and elevated platelet immunoglobulin G in Graves' disease and Hashimoto's thyroiditis.Ann Intern Med.1981;94:27–30.
- ,,.Platelets in hyperthyroidism: studies on platelet counts, mean platelet volume.111‐indium‐labeled platelet kinetics, and platelet associated immunoglobulins G and M.J Clin Endocrinol Metab.1990;70:491–496.
- ,.Coagulation disorders in thyroid diseases.Eur J Endocrinol.1997;136:1.
- ,,.Alteration of platelet aggregation in patients with thyroid disorders.Metabolism.1997;46:1128.
- ,,,,,.Improvement of idiopathic thrombocytopenic purpura by antithyroid therapy.Eur J Haematol.74:73–74.
- ,,,.Graves disease associated with autoimmune thrombocytopenic purpura.Arch Intern Med.1997;157:1033–1036.
- .The thyrotoxicosis/thrombocytopenia connection.Surgery.1983;94:966–968.
- ,,,.Idiopathic thrombocytopenic purpura and Graves disease.Am J Hematol.1982;12:69–72.
- ,.Autoimmune thrombocytopenic purpura associated with hyperthyroidism in a single individual.South Med J.1997;90:933–936.
- .Familial association of autoimmune thrombocytopenia and hyperthyroidism.Am J Hematol.1992;39:294–298.
- ,,, et al.Easy bruising, thrombocytopenia, and elevated platelet immunoglobulin G in Graves' disease and Hashimoto's thyroiditis.Ann Intern Med.1981;94:27–30.
- ,,.Platelets in hyperthyroidism: studies on platelet counts, mean platelet volume.111‐indium‐labeled platelet kinetics, and platelet associated immunoglobulins G and M.J Clin Endocrinol Metab.1990;70:491–496.
- ,.Coagulation disorders in thyroid diseases.Eur J Endocrinol.1997;136:1.
- ,,.Alteration of platelet aggregation in patients with thyroid disorders.Metabolism.1997;46:1128.
Editorial
Older Americans comprise approximately half the patients on inpatient medical wards. There are too few geriatricians to care for these patients, and few geriatricians practice hospital medicine. Hospitalists often provide the majority of inpatient geriatric care, and at teaching hospitals, hospitalists also play a pivotal role in educating residents and students to provide high‐quality care for hospitalized geriatric patients. Thus, hospitalists will be the primary clinicians educating many trainees to care for older patients, and the hospitalists must be skilled in addressing the clinical syndromes that are common in these patients, including delirium, dementia, falls, and infection.1 Generalists and geriatricians have anticipated a shortfall in clinicians prepared to educate trainees about geriatrics and called for faculty development for generalists in geriatrics.2, 3
In this issue of the Journal of Hospital Medicine, Podrazik and colleagues present initial results from a major initiative to enhance the quality and quantity of geriatric inpatient education for residents and students.4 The Curriculum for the Hospitalized Aging Medical Patient (CHAMP) at the University of Chicago represents a multifaceted faculty development effort funded in part by the Donald W. Reynolds and John A. Hartford Foundations. In 12 half‐day sessions offered weekly, hospitalist and general internist faculty members learned about four thematic areasthe frail older person, hazards of hospitalization, end‐of‐life issues, and transitions of carewhile also receiving training in engaging and effective teaching strategies. At each session, participants drew on their own experiences attending on the wards to generate clinical examples and test new teaching strategies. CHAMP incorporates the attributes of best practices for integrating geriatrics education into internal medicine residency training: it promotes model care for older hospital patients, uses a train‐the‐trainer model, addresses care transitions, and promotes interdisciplinary teamwork.5
CHAMP achieved its initial goals. Faculty participants were satisfied and CHAMP substantially increased participants' confidence in practicing and teaching geriatric care. Faculty participants also gained confidence in their teaching abilities and presumably learned teaching strategies that could be applied to other topics in inpatient medicine. Faculty participants demonstrated modest improvements in their knowledge of geriatric issues and more positive attitudes about geriatrics at the end of the course than at the beginning. It is worth noting that the hospitalist and general internist ward attending physicians who participated in CHAMP were volunteers and may have started the process with greater interest in learning geriatric care than other attendings. Thus, it is unknown whether CHAMP might have greater or lesser effect on other faculty.
The CHAMP train‐the‐trainer model offers the potential to impact future practitioners. Findings of the CHAMP investigators are consistent with the literature on faculty development programs for educators, which shows that faculty development on teaching yields high participant satisfaction, knowledge gains, and improved self‐assessment of the ability to implement changes in teaching practice.6 The use in CHAMP of a diverse menu of teaching strategies and active learning techniques such as case‐based discussions and the Objective Structured Teaching Exercise in a small group of colleagues should promote learning and retention.
Is the CHAMP curriculum worth the cost? The program requires resources to pay for 48 hours for each faculty participant and for instructors with expertise in geriatrics and teaching skills. We estimate that the cost for 12 faculty participants would be roughly $72,000. We believe this investment will likely pay off in terms of enhancing faculty skills, improving faculty job satisfaction, promoting faculty retention in academic or other teaching positions, and improving care provided by trainees. For example, if CHAMP were to lead to the retention and promotion of even 2 faculty for just 1 year, it would save recruitment costs that would exceed the direct program costs, and other benefits of CHAMP would only further add value. However, analysis of the benefits of CHAMP will require more in‐depth evaluation data of its impact. The program leaders currently contact former participants around the time of ward attending to reinforce teaching concepts and encourage implementation of CHAMP materials, through a Commitment to Change contract. The ultimate downstream educational goal would be that these faculty learners retain and apply this newly acquired knowledge and skills in their clinical practice and teaching activities. Ideally, evidence would confirm that these benefits improve patient care. The long‐term evaluation plan for CHAMP incorporates important additional outcome measures including resident and student geriatric knowledge as well as patient satisfaction and clinical outcomes. We commend the authors for aiming to expand their evaluation plan over time and aspiring for sustained changes in teaching practice. The literature on the impact of hospitalists has similarly evolved from early descriptions of hospitalists and the logistics of developing a hospitalist program to sophisticated analyses of the impact of hospitalists on clinical outcomes such as length of stay and mortality.7, 8
The feasibility of disseminating CHAMP is an open question. The University of Chicago model employs a time‐intensive curriculum that engages participants in part by releasing them from clinical duties for a half day per week. Release time was funded through combined support from external funding sources and the Department of Medicine. This model addresses the major barrier to faculty development in geriatrics for general internists: lack of time.2, 9 The investment in intensive, longitudinal faculty development may generate higher returns than periodic short faculty workshop sessions that do not build in the time for role‐playing, practice, and reinforcement of key concepts. This type of intervention may also be more feasible when done in conjunction with one of the approximately 50 Health Resources and Services Administration (HRSA)supported Geriatrics Education Centers, which can fund teachers and infrastructure for faculty development.
How is this article useful for hospitalist educators? Many hospitalists at academic centers serve important teaching functions, and some will aspire to advance their educational efforts through more scholarly activities such as curriculum design. The CHAMP curriculum represents a successful model for hospitalists aiming to follow a rigorous approach to curriculum design relevant to inpatient medicine, and the extensive CHAMP materials are available online.10 It serves as a practical model that could be applied to other clinical topics related to hospital medicine. Hospitalists are effective and respected teachers for residents and students, and they develop unique expertise in the content and process of inpatient medicine.11 The authors followed the 6 steps of effective curriculum design: problem identification, targeted needs assessment, goals and objectives, education methods, implementation, and evaluation.12
The CHAMP curriculum typifies a set of materials that aligns well with the Society of Hospital Medicine (SHM) Core Competencies.13 As part of their needs assessment, the authors also surveyed hospitalists at a regional SHM meeting to determine the geriatrics topics for which they perceived greatest educational need. The Core Competencies chapters on the care of the elderly patient, delirium and dementia, hospital‐acquired infections, and palliative care highlight the common learning goals shared by hospital medicine and geriatrics. Both disciplines also emphasize the team‐based, multidisciplinary approach to care, particularly during care transitions, that is highlighted in the CHAMP curriculum.
More generally, the CHAMP curriculum can be used to teach and assess the Accreditation Council for Graduate Medical Education (ACGME) competencies, which must be assessed in all ACGME‐accredited residency programs.14 In an initial session on Teaching on Today's Wards, CHAMP participants brainstorm about how to incorporate both geriatrics content and the ACGME competencies into their post‐call rounds. The emphasis in CHAMP on the health care system and interdisciplinary care is evident in topics such as end‐of‐life care and transitions in care, and provides opportunity for assessment of residents' performance in the ACGME competency of systems‐based practice. The organization of the curriculum by ACGME competency makes it more applicable today than some prior geriatric curricula that emphasized similar themes but without the emphasis on demonstrating competency as an outcome.15
Hospitalists partnering with the Donald W. Reynolds and John A. Hartford Foundations and other external organizations may find funding opportunities for educational projects. For example, the Hartford Foundation has partnered with SHM since 2002 to support hospitalists' efforts to improve care for older adults. Products of this collaboration include a Geriatric Toolbox that contains assessment tools designed for use with geriatric patients.16 The tools assess a range of parameters including nutritional, functional, and mental status, and the website supplies guidelines on the advantages and disadvantages and appropriate use of each assessment tool. With support from the Hartford Foundation, hospitalists have also conducted several workshops at SHM meetings on improving assessment and care of geriatric patients and developed a discharge‐planning checklist for older adults.
As hospitalist programs gain traction in academic centers, hospitalists will increasingly serve as key geriatric content educators for trainees. The CHAMP curriculum offers a model of intensive faculty development for hospitalists and general internists that clinician educators find engaging and empowering. The partnerships of geriatricians and hospitalists, and of the SHM with national geriatrics organizations, have the potential for widespread benefits for both learners and elderly patients.
Older Americans comprise approximately half the patients on inpatient medical wards. There are too few geriatricians to care for these patients, and few geriatricians practice hospital medicine. Hospitalists often provide the majority of inpatient geriatric care, and at teaching hospitals, hospitalists also play a pivotal role in educating residents and students to provide high‐quality care for hospitalized geriatric patients. Thus, hospitalists will be the primary clinicians educating many trainees to care for older patients, and the hospitalists must be skilled in addressing the clinical syndromes that are common in these patients, including delirium, dementia, falls, and infection.1 Generalists and geriatricians have anticipated a shortfall in clinicians prepared to educate trainees about geriatrics and called for faculty development for generalists in geriatrics.2, 3
In this issue of the Journal of Hospital Medicine, Podrazik and colleagues present initial results from a major initiative to enhance the quality and quantity of geriatric inpatient education for residents and students.4 The Curriculum for the Hospitalized Aging Medical Patient (CHAMP) at the University of Chicago represents a multifaceted faculty development effort funded in part by the Donald W. Reynolds and John A. Hartford Foundations. In 12 half‐day sessions offered weekly, hospitalist and general internist faculty members learned about four thematic areasthe frail older person, hazards of hospitalization, end‐of‐life issues, and transitions of carewhile also receiving training in engaging and effective teaching strategies. At each session, participants drew on their own experiences attending on the wards to generate clinical examples and test new teaching strategies. CHAMP incorporates the attributes of best practices for integrating geriatrics education into internal medicine residency training: it promotes model care for older hospital patients, uses a train‐the‐trainer model, addresses care transitions, and promotes interdisciplinary teamwork.5
CHAMP achieved its initial goals. Faculty participants were satisfied and CHAMP substantially increased participants' confidence in practicing and teaching geriatric care. Faculty participants also gained confidence in their teaching abilities and presumably learned teaching strategies that could be applied to other topics in inpatient medicine. Faculty participants demonstrated modest improvements in their knowledge of geriatric issues and more positive attitudes about geriatrics at the end of the course than at the beginning. It is worth noting that the hospitalist and general internist ward attending physicians who participated in CHAMP were volunteers and may have started the process with greater interest in learning geriatric care than other attendings. Thus, it is unknown whether CHAMP might have greater or lesser effect on other faculty.
The CHAMP train‐the‐trainer model offers the potential to impact future practitioners. Findings of the CHAMP investigators are consistent with the literature on faculty development programs for educators, which shows that faculty development on teaching yields high participant satisfaction, knowledge gains, and improved self‐assessment of the ability to implement changes in teaching practice.6 The use in CHAMP of a diverse menu of teaching strategies and active learning techniques such as case‐based discussions and the Objective Structured Teaching Exercise in a small group of colleagues should promote learning and retention.
Is the CHAMP curriculum worth the cost? The program requires resources to pay for 48 hours for each faculty participant and for instructors with expertise in geriatrics and teaching skills. We estimate that the cost for 12 faculty participants would be roughly $72,000. We believe this investment will likely pay off in terms of enhancing faculty skills, improving faculty job satisfaction, promoting faculty retention in academic or other teaching positions, and improving care provided by trainees. For example, if CHAMP were to lead to the retention and promotion of even 2 faculty for just 1 year, it would save recruitment costs that would exceed the direct program costs, and other benefits of CHAMP would only further add value. However, analysis of the benefits of CHAMP will require more in‐depth evaluation data of its impact. The program leaders currently contact former participants around the time of ward attending to reinforce teaching concepts and encourage implementation of CHAMP materials, through a Commitment to Change contract. The ultimate downstream educational goal would be that these faculty learners retain and apply this newly acquired knowledge and skills in their clinical practice and teaching activities. Ideally, evidence would confirm that these benefits improve patient care. The long‐term evaluation plan for CHAMP incorporates important additional outcome measures including resident and student geriatric knowledge as well as patient satisfaction and clinical outcomes. We commend the authors for aiming to expand their evaluation plan over time and aspiring for sustained changes in teaching practice. The literature on the impact of hospitalists has similarly evolved from early descriptions of hospitalists and the logistics of developing a hospitalist program to sophisticated analyses of the impact of hospitalists on clinical outcomes such as length of stay and mortality.7, 8
The feasibility of disseminating CHAMP is an open question. The University of Chicago model employs a time‐intensive curriculum that engages participants in part by releasing them from clinical duties for a half day per week. Release time was funded through combined support from external funding sources and the Department of Medicine. This model addresses the major barrier to faculty development in geriatrics for general internists: lack of time.2, 9 The investment in intensive, longitudinal faculty development may generate higher returns than periodic short faculty workshop sessions that do not build in the time for role‐playing, practice, and reinforcement of key concepts. This type of intervention may also be more feasible when done in conjunction with one of the approximately 50 Health Resources and Services Administration (HRSA)supported Geriatrics Education Centers, which can fund teachers and infrastructure for faculty development.
How is this article useful for hospitalist educators? Many hospitalists at academic centers serve important teaching functions, and some will aspire to advance their educational efforts through more scholarly activities such as curriculum design. The CHAMP curriculum represents a successful model for hospitalists aiming to follow a rigorous approach to curriculum design relevant to inpatient medicine, and the extensive CHAMP materials are available online.10 It serves as a practical model that could be applied to other clinical topics related to hospital medicine. Hospitalists are effective and respected teachers for residents and students, and they develop unique expertise in the content and process of inpatient medicine.11 The authors followed the 6 steps of effective curriculum design: problem identification, targeted needs assessment, goals and objectives, education methods, implementation, and evaluation.12
The CHAMP curriculum typifies a set of materials that aligns well with the Society of Hospital Medicine (SHM) Core Competencies.13 As part of their needs assessment, the authors also surveyed hospitalists at a regional SHM meeting to determine the geriatrics topics for which they perceived greatest educational need. The Core Competencies chapters on the care of the elderly patient, delirium and dementia, hospital‐acquired infections, and palliative care highlight the common learning goals shared by hospital medicine and geriatrics. Both disciplines also emphasize the team‐based, multidisciplinary approach to care, particularly during care transitions, that is highlighted in the CHAMP curriculum.
More generally, the CHAMP curriculum can be used to teach and assess the Accreditation Council for Graduate Medical Education (ACGME) competencies, which must be assessed in all ACGME‐accredited residency programs.14 In an initial session on Teaching on Today's Wards, CHAMP participants brainstorm about how to incorporate both geriatrics content and the ACGME competencies into their post‐call rounds. The emphasis in CHAMP on the health care system and interdisciplinary care is evident in topics such as end‐of‐life care and transitions in care, and provides opportunity for assessment of residents' performance in the ACGME competency of systems‐based practice. The organization of the curriculum by ACGME competency makes it more applicable today than some prior geriatric curricula that emphasized similar themes but without the emphasis on demonstrating competency as an outcome.15
Hospitalists partnering with the Donald W. Reynolds and John A. Hartford Foundations and other external organizations may find funding opportunities for educational projects. For example, the Hartford Foundation has partnered with SHM since 2002 to support hospitalists' efforts to improve care for older adults. Products of this collaboration include a Geriatric Toolbox that contains assessment tools designed for use with geriatric patients.16 The tools assess a range of parameters including nutritional, functional, and mental status, and the website supplies guidelines on the advantages and disadvantages and appropriate use of each assessment tool. With support from the Hartford Foundation, hospitalists have also conducted several workshops at SHM meetings on improving assessment and care of geriatric patients and developed a discharge‐planning checklist for older adults.
As hospitalist programs gain traction in academic centers, hospitalists will increasingly serve as key geriatric content educators for trainees. The CHAMP curriculum offers a model of intensive faculty development for hospitalists and general internists that clinician educators find engaging and empowering. The partnerships of geriatricians and hospitalists, and of the SHM with national geriatrics organizations, have the potential for widespread benefits for both learners and elderly patients.
Older Americans comprise approximately half the patients on inpatient medical wards. There are too few geriatricians to care for these patients, and few geriatricians practice hospital medicine. Hospitalists often provide the majority of inpatient geriatric care, and at teaching hospitals, hospitalists also play a pivotal role in educating residents and students to provide high‐quality care for hospitalized geriatric patients. Thus, hospitalists will be the primary clinicians educating many trainees to care for older patients, and the hospitalists must be skilled in addressing the clinical syndromes that are common in these patients, including delirium, dementia, falls, and infection.1 Generalists and geriatricians have anticipated a shortfall in clinicians prepared to educate trainees about geriatrics and called for faculty development for generalists in geriatrics.2, 3
In this issue of the Journal of Hospital Medicine, Podrazik and colleagues present initial results from a major initiative to enhance the quality and quantity of geriatric inpatient education for residents and students.4 The Curriculum for the Hospitalized Aging Medical Patient (CHAMP) at the University of Chicago represents a multifaceted faculty development effort funded in part by the Donald W. Reynolds and John A. Hartford Foundations. In 12 half‐day sessions offered weekly, hospitalist and general internist faculty members learned about four thematic areasthe frail older person, hazards of hospitalization, end‐of‐life issues, and transitions of carewhile also receiving training in engaging and effective teaching strategies. At each session, participants drew on their own experiences attending on the wards to generate clinical examples and test new teaching strategies. CHAMP incorporates the attributes of best practices for integrating geriatrics education into internal medicine residency training: it promotes model care for older hospital patients, uses a train‐the‐trainer model, addresses care transitions, and promotes interdisciplinary teamwork.5
CHAMP achieved its initial goals. Faculty participants were satisfied and CHAMP substantially increased participants' confidence in practicing and teaching geriatric care. Faculty participants also gained confidence in their teaching abilities and presumably learned teaching strategies that could be applied to other topics in inpatient medicine. Faculty participants demonstrated modest improvements in their knowledge of geriatric issues and more positive attitudes about geriatrics at the end of the course than at the beginning. It is worth noting that the hospitalist and general internist ward attending physicians who participated in CHAMP were volunteers and may have started the process with greater interest in learning geriatric care than other attendings. Thus, it is unknown whether CHAMP might have greater or lesser effect on other faculty.
The CHAMP train‐the‐trainer model offers the potential to impact future practitioners. Findings of the CHAMP investigators are consistent with the literature on faculty development programs for educators, which shows that faculty development on teaching yields high participant satisfaction, knowledge gains, and improved self‐assessment of the ability to implement changes in teaching practice.6 The use in CHAMP of a diverse menu of teaching strategies and active learning techniques such as case‐based discussions and the Objective Structured Teaching Exercise in a small group of colleagues should promote learning and retention.
Is the CHAMP curriculum worth the cost? The program requires resources to pay for 48 hours for each faculty participant and for instructors with expertise in geriatrics and teaching skills. We estimate that the cost for 12 faculty participants would be roughly $72,000. We believe this investment will likely pay off in terms of enhancing faculty skills, improving faculty job satisfaction, promoting faculty retention in academic or other teaching positions, and improving care provided by trainees. For example, if CHAMP were to lead to the retention and promotion of even 2 faculty for just 1 year, it would save recruitment costs that would exceed the direct program costs, and other benefits of CHAMP would only further add value. However, analysis of the benefits of CHAMP will require more in‐depth evaluation data of its impact. The program leaders currently contact former participants around the time of ward attending to reinforce teaching concepts and encourage implementation of CHAMP materials, through a Commitment to Change contract. The ultimate downstream educational goal would be that these faculty learners retain and apply this newly acquired knowledge and skills in their clinical practice and teaching activities. Ideally, evidence would confirm that these benefits improve patient care. The long‐term evaluation plan for CHAMP incorporates important additional outcome measures including resident and student geriatric knowledge as well as patient satisfaction and clinical outcomes. We commend the authors for aiming to expand their evaluation plan over time and aspiring for sustained changes in teaching practice. The literature on the impact of hospitalists has similarly evolved from early descriptions of hospitalists and the logistics of developing a hospitalist program to sophisticated analyses of the impact of hospitalists on clinical outcomes such as length of stay and mortality.7, 8
The feasibility of disseminating CHAMP is an open question. The University of Chicago model employs a time‐intensive curriculum that engages participants in part by releasing them from clinical duties for a half day per week. Release time was funded through combined support from external funding sources and the Department of Medicine. This model addresses the major barrier to faculty development in geriatrics for general internists: lack of time.2, 9 The investment in intensive, longitudinal faculty development may generate higher returns than periodic short faculty workshop sessions that do not build in the time for role‐playing, practice, and reinforcement of key concepts. This type of intervention may also be more feasible when done in conjunction with one of the approximately 50 Health Resources and Services Administration (HRSA)supported Geriatrics Education Centers, which can fund teachers and infrastructure for faculty development.
How is this article useful for hospitalist educators? Many hospitalists at academic centers serve important teaching functions, and some will aspire to advance their educational efforts through more scholarly activities such as curriculum design. The CHAMP curriculum represents a successful model for hospitalists aiming to follow a rigorous approach to curriculum design relevant to inpatient medicine, and the extensive CHAMP materials are available online.10 It serves as a practical model that could be applied to other clinical topics related to hospital medicine. Hospitalists are effective and respected teachers for residents and students, and they develop unique expertise in the content and process of inpatient medicine.11 The authors followed the 6 steps of effective curriculum design: problem identification, targeted needs assessment, goals and objectives, education methods, implementation, and evaluation.12
The CHAMP curriculum typifies a set of materials that aligns well with the Society of Hospital Medicine (SHM) Core Competencies.13 As part of their needs assessment, the authors also surveyed hospitalists at a regional SHM meeting to determine the geriatrics topics for which they perceived greatest educational need. The Core Competencies chapters on the care of the elderly patient, delirium and dementia, hospital‐acquired infections, and palliative care highlight the common learning goals shared by hospital medicine and geriatrics. Both disciplines also emphasize the team‐based, multidisciplinary approach to care, particularly during care transitions, that is highlighted in the CHAMP curriculum.
More generally, the CHAMP curriculum can be used to teach and assess the Accreditation Council for Graduate Medical Education (ACGME) competencies, which must be assessed in all ACGME‐accredited residency programs.14 In an initial session on Teaching on Today's Wards, CHAMP participants brainstorm about how to incorporate both geriatrics content and the ACGME competencies into their post‐call rounds. The emphasis in CHAMP on the health care system and interdisciplinary care is evident in topics such as end‐of‐life care and transitions in care, and provides opportunity for assessment of residents' performance in the ACGME competency of systems‐based practice. The organization of the curriculum by ACGME competency makes it more applicable today than some prior geriatric curricula that emphasized similar themes but without the emphasis on demonstrating competency as an outcome.15
Hospitalists partnering with the Donald W. Reynolds and John A. Hartford Foundations and other external organizations may find funding opportunities for educational projects. For example, the Hartford Foundation has partnered with SHM since 2002 to support hospitalists' efforts to improve care for older adults. Products of this collaboration include a Geriatric Toolbox that contains assessment tools designed for use with geriatric patients.16 The tools assess a range of parameters including nutritional, functional, and mental status, and the website supplies guidelines on the advantages and disadvantages and appropriate use of each assessment tool. With support from the Hartford Foundation, hospitalists have also conducted several workshops at SHM meetings on improving assessment and care of geriatric patients and developed a discharge‐planning checklist for older adults.
As hospitalist programs gain traction in academic centers, hospitalists will increasingly serve as key geriatric content educators for trainees. The CHAMP curriculum offers a model of intensive faculty development for hospitalists and general internists that clinician educators find engaging and empowering. The partnerships of geriatricians and hospitalists, and of the SHM with national geriatrics organizations, have the potential for widespread benefits for both learners and elderly patients.
An Unconventional Living Will
Her name was Mrs. Carberry, but her readers knew her as Mary Margaret. Two months earlier, she suffered a debilitating stroke that took away her vibrant life. Previously a prolific writer of advice columns and opinion pieces, the blockage of blood to her brain dammed the steady flow of wisdom to her innumerable fans. They never again would benefit from the words of this intelligent, feisty, and self‐proclaimed Cranky Catholic. Unfortunately, I would not know Mary Margaret for her words, but only her numbers: her vital signs, her urine output, her tube feed residuals.
Not able to communicate with us, we wondered did Mrs. Carberry want this tracheostomy that was placed? Did she want this peg tube that fed her continuously? And even if she would have initially agreed to them, would she still want them now? Especially given she was not improving and had little hope for a meaningful recovery.
We posed these difficult questions to her two sons. One son believed that his mother would want an end to these aggressive measures. The other son disagreed; he said his mother would want to make every effort to stay alive.
In the end, Mary Margaret's voice found its way to us and provided us with the answer. She did not tell us in the conventional manner via a lawyer or a living will. Her friends did not come forth and let us know about serious conversations during their afternoon lunches. Instead, Mary Margaret penned it to her audience of devoted readers in a newspaper column written 14 years earlier. Mary Margaret's poignant words were revealed to her doctors by her son who understood its undeniable significance.
Mary Margaret was an essayist in Chicago whose pieces filled many major newspapers and magazines. She had strong opinions on matters large and small, writing articles addressing topics ranging from her Catholic beliefs to gender‐based inequities in the workplace. One article in particular addressed sickness and death and provided her sons the answer they sought. Having witnessed illness strike two loved ones, it was only natural for Mary Margaret to write about it. Her very personal essay was entitled Tough Questions on Life, Death and a Dog Named Bamboo. The words, resurfacing years later, and now having direct meaning to her own life and death, may have been some of the most profound and prophetic words of her career:
Tough Questions on Life, Death and a Dog Named Bamboo
I sat with her that evening while she was dyingrubbing her back, smoothing her head, whispering that I loved her, trying to be of some small comfort as she snuggled closer, looking up with her mysteriously accepting, somehow understanding brown eyes.
Adjusting herself once more, she half rose, then toppled sideways and simply stopped breathing. She died as a lady ought to be able to diequietly, as easily as possible, in her own bed. In my bed actually. She was Bamboo, my Shar‐pei, and it is difficult to write this even several months later without tears starting to roll.
It was not just that last day, of course. For a bit more than a week, Bamboo had been giving signs of a serious problema heavy doggie cough indicating severe congestion and a firm, stubborn decision not to eat. The kitchen offered a parade of small bowls of her favorite people foods with which I hoped to restore her appetite when she determinedly ignored her regular dog food. She had her choice of cottage cheese, scrambled eggs, ground sirloin, cheddar chunks, ice cream, buttered rice and morea virtual buffet for ants.
After I set each dish out, she would go over to look and sniff admiringly, even wag her tail, but then rather reluctantly return to her favorite resting place, a small rug at the top of the stairs to the front door.
She would only drink a lot of water, bowl after bowl, in which she did also get her medicine, mashed and melted. Late on that last afternoon, however she stopped the drinking, too. I couldn't get her to sip even when I brought the water dish to her or offered the ice cubes she once loved to lick. In her own way, she was saying No.
Lately I have been reflecting again on the experience as a result of having heard some discussion about the death of a woman with whom I once shared friendly commuter chitchat as we trained together into the Loop.
Following a stroke, she had been unconscious, vegetative, tragically for almost as long as I had enjoyed the eight years of Bamboo's delightful companionship. Her husband, I learned, had ultimately gone to court and had been granted permission to remove the feeding tube and let nature take its course. A counteraction to prevent this was filed by some well‐intentioned people; but what I believe as good human and legal sense prevailed. So without the tube feeding, this nice long‐suffering woman finally slipped away to God.
People who oppose the dying being released this way argue that they are being starved to death without the feeding tubes. But I don't buy that, especially after having watched Bamboo decide by some deep natural instinct that it was time for her, first, to stop eating even the treats she loved and, finally, to stop drinking while she waited patiently for what was to come, what was inevitable.
There used to be an advertising slogan: It's not nice to fool Mother Nature. If you believe in God and the promise of eternity, perhaps it is equally not nice to fool dying human bodies into a semblance of living when nature is poised to move them beyond the rim of this life. Nature or God, I mean, and absolutely never, of course, a manipulative Dr. Death.
I don't think my puppy was starving those last few days so much as simply stopping. Simply letting herself be folded into an immutable process. At least this is something to ponder in terms of the will of God overwhelming the hopes of man. I am awed and rather apprehensive and yet somehow comfortable with this conclusion.
A couple of months tops with the tubes and no other reasonable hope, I think I'll tell my kids.
Mary Margaret's words struck a cord with both her sons. Her wishes, neatly laid out in a dusty newspaper, were respected. Mary Margaret entered hospice and died peacefully 2 weeks later.
(Tough Questions on Life, Death and a Dog Named Bamboo originally appeared in the The Catholic New World on July 16, 1993. The article was reprinted with their permission.)
Postscript: The author of the essay (my mother) passed away after a week in hospice care and 7 weeks in hospitals following a stroke. She was a published writer for more than 60 years. For her 61st birthday, my brother and I decided to buy her a wrinkly little puppy to keep her company and bark at anyone who came to her house. It ended up being a terrific watchdog as well as my mom's best friend. The last days with her puppy were translated into the essay, which also helped guide me during my mother's final days. My hope is that she and Bamboo are enjoying the promise of eternity in each other's company.
Patrick Carberry
Her name was Mrs. Carberry, but her readers knew her as Mary Margaret. Two months earlier, she suffered a debilitating stroke that took away her vibrant life. Previously a prolific writer of advice columns and opinion pieces, the blockage of blood to her brain dammed the steady flow of wisdom to her innumerable fans. They never again would benefit from the words of this intelligent, feisty, and self‐proclaimed Cranky Catholic. Unfortunately, I would not know Mary Margaret for her words, but only her numbers: her vital signs, her urine output, her tube feed residuals.
Not able to communicate with us, we wondered did Mrs. Carberry want this tracheostomy that was placed? Did she want this peg tube that fed her continuously? And even if she would have initially agreed to them, would she still want them now? Especially given she was not improving and had little hope for a meaningful recovery.
We posed these difficult questions to her two sons. One son believed that his mother would want an end to these aggressive measures. The other son disagreed; he said his mother would want to make every effort to stay alive.
In the end, Mary Margaret's voice found its way to us and provided us with the answer. She did not tell us in the conventional manner via a lawyer or a living will. Her friends did not come forth and let us know about serious conversations during their afternoon lunches. Instead, Mary Margaret penned it to her audience of devoted readers in a newspaper column written 14 years earlier. Mary Margaret's poignant words were revealed to her doctors by her son who understood its undeniable significance.
Mary Margaret was an essayist in Chicago whose pieces filled many major newspapers and magazines. She had strong opinions on matters large and small, writing articles addressing topics ranging from her Catholic beliefs to gender‐based inequities in the workplace. One article in particular addressed sickness and death and provided her sons the answer they sought. Having witnessed illness strike two loved ones, it was only natural for Mary Margaret to write about it. Her very personal essay was entitled Tough Questions on Life, Death and a Dog Named Bamboo. The words, resurfacing years later, and now having direct meaning to her own life and death, may have been some of the most profound and prophetic words of her career:
Tough Questions on Life, Death and a Dog Named Bamboo
I sat with her that evening while she was dyingrubbing her back, smoothing her head, whispering that I loved her, trying to be of some small comfort as she snuggled closer, looking up with her mysteriously accepting, somehow understanding brown eyes.
Adjusting herself once more, she half rose, then toppled sideways and simply stopped breathing. She died as a lady ought to be able to diequietly, as easily as possible, in her own bed. In my bed actually. She was Bamboo, my Shar‐pei, and it is difficult to write this even several months later without tears starting to roll.
It was not just that last day, of course. For a bit more than a week, Bamboo had been giving signs of a serious problema heavy doggie cough indicating severe congestion and a firm, stubborn decision not to eat. The kitchen offered a parade of small bowls of her favorite people foods with which I hoped to restore her appetite when she determinedly ignored her regular dog food. She had her choice of cottage cheese, scrambled eggs, ground sirloin, cheddar chunks, ice cream, buttered rice and morea virtual buffet for ants.
After I set each dish out, she would go over to look and sniff admiringly, even wag her tail, but then rather reluctantly return to her favorite resting place, a small rug at the top of the stairs to the front door.
She would only drink a lot of water, bowl after bowl, in which she did also get her medicine, mashed and melted. Late on that last afternoon, however she stopped the drinking, too. I couldn't get her to sip even when I brought the water dish to her or offered the ice cubes she once loved to lick. In her own way, she was saying No.
Lately I have been reflecting again on the experience as a result of having heard some discussion about the death of a woman with whom I once shared friendly commuter chitchat as we trained together into the Loop.
Following a stroke, she had been unconscious, vegetative, tragically for almost as long as I had enjoyed the eight years of Bamboo's delightful companionship. Her husband, I learned, had ultimately gone to court and had been granted permission to remove the feeding tube and let nature take its course. A counteraction to prevent this was filed by some well‐intentioned people; but what I believe as good human and legal sense prevailed. So without the tube feeding, this nice long‐suffering woman finally slipped away to God.
People who oppose the dying being released this way argue that they are being starved to death without the feeding tubes. But I don't buy that, especially after having watched Bamboo decide by some deep natural instinct that it was time for her, first, to stop eating even the treats she loved and, finally, to stop drinking while she waited patiently for what was to come, what was inevitable.
There used to be an advertising slogan: It's not nice to fool Mother Nature. If you believe in God and the promise of eternity, perhaps it is equally not nice to fool dying human bodies into a semblance of living when nature is poised to move them beyond the rim of this life. Nature or God, I mean, and absolutely never, of course, a manipulative Dr. Death.
I don't think my puppy was starving those last few days so much as simply stopping. Simply letting herself be folded into an immutable process. At least this is something to ponder in terms of the will of God overwhelming the hopes of man. I am awed and rather apprehensive and yet somehow comfortable with this conclusion.
A couple of months tops with the tubes and no other reasonable hope, I think I'll tell my kids.
Mary Margaret's words struck a cord with both her sons. Her wishes, neatly laid out in a dusty newspaper, were respected. Mary Margaret entered hospice and died peacefully 2 weeks later.
(Tough Questions on Life, Death and a Dog Named Bamboo originally appeared in the The Catholic New World on July 16, 1993. The article was reprinted with their permission.)
Postscript: The author of the essay (my mother) passed away after a week in hospice care and 7 weeks in hospitals following a stroke. She was a published writer for more than 60 years. For her 61st birthday, my brother and I decided to buy her a wrinkly little puppy to keep her company and bark at anyone who came to her house. It ended up being a terrific watchdog as well as my mom's best friend. The last days with her puppy were translated into the essay, which also helped guide me during my mother's final days. My hope is that she and Bamboo are enjoying the promise of eternity in each other's company.
Patrick Carberry
Her name was Mrs. Carberry, but her readers knew her as Mary Margaret. Two months earlier, she suffered a debilitating stroke that took away her vibrant life. Previously a prolific writer of advice columns and opinion pieces, the blockage of blood to her brain dammed the steady flow of wisdom to her innumerable fans. They never again would benefit from the words of this intelligent, feisty, and self‐proclaimed Cranky Catholic. Unfortunately, I would not know Mary Margaret for her words, but only her numbers: her vital signs, her urine output, her tube feed residuals.
Not able to communicate with us, we wondered did Mrs. Carberry want this tracheostomy that was placed? Did she want this peg tube that fed her continuously? And even if she would have initially agreed to them, would she still want them now? Especially given she was not improving and had little hope for a meaningful recovery.
We posed these difficult questions to her two sons. One son believed that his mother would want an end to these aggressive measures. The other son disagreed; he said his mother would want to make every effort to stay alive.
In the end, Mary Margaret's voice found its way to us and provided us with the answer. She did not tell us in the conventional manner via a lawyer or a living will. Her friends did not come forth and let us know about serious conversations during their afternoon lunches. Instead, Mary Margaret penned it to her audience of devoted readers in a newspaper column written 14 years earlier. Mary Margaret's poignant words were revealed to her doctors by her son who understood its undeniable significance.
Mary Margaret was an essayist in Chicago whose pieces filled many major newspapers and magazines. She had strong opinions on matters large and small, writing articles addressing topics ranging from her Catholic beliefs to gender‐based inequities in the workplace. One article in particular addressed sickness and death and provided her sons the answer they sought. Having witnessed illness strike two loved ones, it was only natural for Mary Margaret to write about it. Her very personal essay was entitled Tough Questions on Life, Death and a Dog Named Bamboo. The words, resurfacing years later, and now having direct meaning to her own life and death, may have been some of the most profound and prophetic words of her career:
Tough Questions on Life, Death and a Dog Named Bamboo
I sat with her that evening while she was dyingrubbing her back, smoothing her head, whispering that I loved her, trying to be of some small comfort as she snuggled closer, looking up with her mysteriously accepting, somehow understanding brown eyes.
Adjusting herself once more, she half rose, then toppled sideways and simply stopped breathing. She died as a lady ought to be able to diequietly, as easily as possible, in her own bed. In my bed actually. She was Bamboo, my Shar‐pei, and it is difficult to write this even several months later without tears starting to roll.
It was not just that last day, of course. For a bit more than a week, Bamboo had been giving signs of a serious problema heavy doggie cough indicating severe congestion and a firm, stubborn decision not to eat. The kitchen offered a parade of small bowls of her favorite people foods with which I hoped to restore her appetite when she determinedly ignored her regular dog food. She had her choice of cottage cheese, scrambled eggs, ground sirloin, cheddar chunks, ice cream, buttered rice and morea virtual buffet for ants.
After I set each dish out, she would go over to look and sniff admiringly, even wag her tail, but then rather reluctantly return to her favorite resting place, a small rug at the top of the stairs to the front door.
She would only drink a lot of water, bowl after bowl, in which she did also get her medicine, mashed and melted. Late on that last afternoon, however she stopped the drinking, too. I couldn't get her to sip even when I brought the water dish to her or offered the ice cubes she once loved to lick. In her own way, she was saying No.
Lately I have been reflecting again on the experience as a result of having heard some discussion about the death of a woman with whom I once shared friendly commuter chitchat as we trained together into the Loop.
Following a stroke, she had been unconscious, vegetative, tragically for almost as long as I had enjoyed the eight years of Bamboo's delightful companionship. Her husband, I learned, had ultimately gone to court and had been granted permission to remove the feeding tube and let nature take its course. A counteraction to prevent this was filed by some well‐intentioned people; but what I believe as good human and legal sense prevailed. So without the tube feeding, this nice long‐suffering woman finally slipped away to God.
People who oppose the dying being released this way argue that they are being starved to death without the feeding tubes. But I don't buy that, especially after having watched Bamboo decide by some deep natural instinct that it was time for her, first, to stop eating even the treats she loved and, finally, to stop drinking while she waited patiently for what was to come, what was inevitable.
There used to be an advertising slogan: It's not nice to fool Mother Nature. If you believe in God and the promise of eternity, perhaps it is equally not nice to fool dying human bodies into a semblance of living when nature is poised to move them beyond the rim of this life. Nature or God, I mean, and absolutely never, of course, a manipulative Dr. Death.
I don't think my puppy was starving those last few days so much as simply stopping. Simply letting herself be folded into an immutable process. At least this is something to ponder in terms of the will of God overwhelming the hopes of man. I am awed and rather apprehensive and yet somehow comfortable with this conclusion.
A couple of months tops with the tubes and no other reasonable hope, I think I'll tell my kids.
Mary Margaret's words struck a cord with both her sons. Her wishes, neatly laid out in a dusty newspaper, were respected. Mary Margaret entered hospice and died peacefully 2 weeks later.
(Tough Questions on Life, Death and a Dog Named Bamboo originally appeared in the The Catholic New World on July 16, 1993. The article was reprinted with their permission.)
Postscript: The author of the essay (my mother) passed away after a week in hospice care and 7 weeks in hospitals following a stroke. She was a published writer for more than 60 years. For her 61st birthday, my brother and I decided to buy her a wrinkly little puppy to keep her company and bark at anyone who came to her house. It ended up being a terrific watchdog as well as my mom's best friend. The last days with her puppy were translated into the essay, which also helped guide me during my mother's final days. My hope is that she and Bamboo are enjoying the promise of eternity in each other's company.
Patrick Carberry
Differences in End‐of‐Life Hospital Care for Children
More than 53,000 children 19 years of age or younger died in 2004,1 and more than 40% of these children died while hospitalized.25 Recently, pediatric end‐of‐life (EOL) issues have gained clinical and research attention, primarily focused on children with chronic conditions, ethical dilemmas surrounding childhood death and dying, and the need for interdisciplinary palliative care efforts for dying children and their families.2, 3, 69
Much remains unknown about patterns of EOL hospital care at the national level for all children, both with and without complex chronic conditions. Because a large proportion of childhood mortality occurs during hospitalization, the inpatient setting is a crucial arena for patients and families facing EOL issues. However, little is known about how insurance status and interhospital transfer are associated with patterns of hospitalization and mortality for children while hospitalized, or about hospital charges and lengths of stay for children who die as inpatients versus those who survive to discharge. In addition, although spending on EOL health care in the United States has attracted considerable attention in recent years, the published literature focuses almost exclusively on adult populations.1012
Illuminating the patterns of childhood mortality in hospital settings may inform expanding institutional efforts to address death and dying for children and their families. We conducted an analysis of national patterns of hospitalization over a span of a decade (19922002), in order to characterize sociodemographic and health care factors associated with inpatient mortality, and to examine patterns of hospital resource use related to EOL care. We hypothesized that resource use would be higher for children who died versus those who survived, and would be higher for uninsured versus insured children.13 We also hypothesized that children admitted upon transfer from another hospital would have higher risk of mortality.14
METHODS
Our data source was the National Inpatient Sample (NIS), which is a component of the Healthcare Cost and Utilization Project (HCUP) sponsored by the Agency for Healthcare Research and Quality. The HCUP is a set of databases developed through partnership among health care institutions and federal and state governments.15 The NIS is the largest publicly available all‐payer inpatient database in the United States, and contains de‐identified, patient‐level clinical data included in a typical discharge abstract. For each year, these data reflect hospital stays from between 800 and 1000 institutions sampled to approximate a 20% stratified sample of nonfederal community hospitals, including public hospitals, children's hospitals, and academic medical centers but excluding long‐term hospitals, psychiatric hospitals, and chemical dependency treatment facilities.
We chose the NIS for this analysis because we were interested in the most common diagnoses for hospitalized children. An alternative database, such as the KID (Kids Inpatient Database), is optimal for less commonly seen discharge diagnoses and did not permit a full decade of retrospective analysis.
In order to characterize changes in mortality and health resource utilization related to our research questions, we conducted a comparative cross‐sectional analysis of 3 years of the NIS over the years 1992, 1997, and 2002. For each year of NIS data, discharge‐level weights were provided to permit calculation of national estimates of hospitalization rates standardized to the concurrent national population.15 All inpatient hospital stays of children aged 17 years and younger were selected.
Discharge data were analyzed based on age, sex, payer status, and transfer status on admission. Although transfer status is not often considered in studies of mortality, we expected that it would be associated with mortality, as a potential indicator of disease severity.14 We included only interhospital transfers, and excluded patients transferred from other locations such as long‐term care facilities. We categorized discharges into 5 age groups: newborns, whose hospitalization began at birth; infants up to 1 year of age who were not born during hospitalization; 15 years; 610 years; and 1117 years. This stratification allowed us to separate infants who were admitted from home or from another hospital versus those who were born during hospitalization. Payer groups included Medicaid, private insurance, and uninsured. Medicare and other payers were analyzed, but were present in very small numbers and are not reported.
Outcomes included weighted inpatient mortality rate, weighted mean of length of stay (in days), and weighted mean total hospital charges. For nationally weighted data, lengths of stay and hospital charges are typically reported as means because weighted medians cannot be estimated.16 We compared mortality patterns for patients who were transferred between hospitals versus those who were not, using multivariable logistic regression to identify factors associated with in‐hospital mortality. Of note, transfer status was evaluated from the standpoint of the receiving hospital as children who were admitted upon transfer from another hospital. Thus, our estimates likely underestimate the effects attributed to interhospital transfer, because this evaluation is unilateral and does not include the transferring hospital. The 5 most common principal Diagnosis‐Related Groups (DRGs) upon discharge were compiled for each of the study years for both survivors and decedents. In order to interpret the analyses of discharge‐related hospital charges in constant dollars, we standardized all hospital charges to 2002 US dollars using the Consumer Price Index.17
Statistical analyses included bivariate comparisons of sociodemographic characteristics and the study outcomes, for each of the study years. We also conducted multivariable regression analyses of mortality, comparing effects of sociodemographic variables and transfer status. We conducted all analyses using Stata, version 8 (Stata Corp., College Station, TX), with which we incorporated sample weights to account for the complex stratified sampling of hospitals that comprise the NIS, and to generate variance estimates with which we derived 95% confidence intervals (95% CI). NIS samples included weighted data for 6.2 million discharges in 1992, 7.1 million discharges in 1997, and 7.9 million discharges in 2002. All results are presented using weighted values. The study was funded internally and all analyses were conducted by the authors. The authors had no financial interest in the outcome. The study was exempt from human subjects review as an analysis of de‐identified secondary data.
RESULTS
Study Sample
NIS samples represented between 35 million and 37.8 million discharges nationally in each of the study years. Distributions of discharges across age group, gender, and payer group were similar across the study years (Table 1).
| Characteristic | 1992 N = 6,722,647 | 1997 N = 6,365,886 | 2002 N = 6,456,077 |
|---|---|---|---|
| |||
| Age (%) | |||
| Newborn | 60.0 | 63.0 | 65.0 |
| Admitted as transfer* | 1.3 | 1.1 | 1.2 |
| 0‐<1 year | 8.7 | 8.0 | 8.6 |
| Admitted as transfer* | 7.6 | 7.2 | 8.8 |
| 1‐5 years | 11.9 | 11.0 | 9.2 |
| Admitted as transfer* | 5.1 | 4.5 | 5.6 |
| 6‐10 years | 5.5 | 5.0 | 5.0 |
| Admitted as transfer* | 4.9 | 4.7 | 5.3 |
| 11‐17 years | 13.9 | 13.0 | 12.2 |
| Admitted as transfer* | 3.1 | 4.2 | 4.8 |
| Gender (%) | |||
| Female | 49.0 | 49.0 | 49.0 |
| Payer (%) | |||
| Medicaid | 37.0 | 36.0 | 39.0 |
| Admitted as transfer* | 3.3 | 3.0 | 3.4 |
| Private | 52.0 | 55.0 | 53.0 |
| Admitted as transfer* | 2.3 | 2.3 | 2.4 |
| Uninsured | 7.0 | 5.0 | 5.0 |
| Admitted as transfer* | 2.4 | 2.4 | 2.4 |
The proportions of patients admitted as transfers between hospitals are shown for each age group, as well as by payer. Non‐newborn infants had the highest rate of transfer for each year studied, compared with the other age groups. Across the study years, transfer status was fairly uniform across payers.
Patterns of Inpatient Mortality
During the study period, overall pediatric inpatient mortality decreased from 32,941 children (0.49% of all child discharges) in 1992 to 25,824 children (0.40%) in 2002, although this was not a statistically significant change. The inpatient mortality rate across all years studied was significantly higher for the non‐newborn infants (<1 years) than for all other age groups in all study years (P <.005) (Table 2). The newborn age group had the second highest mortality rate in all years, and the remaining 3 groups had similar mortality rates.
| Age Groups* | Annual Inpatient Mortality Rate | ||
|---|---|---|---|
| 1992 N = 6,722,647 | 1997 N = 6,365,886 | 2002 N = 6,456,077 | |
| |||
| Overall | 0.49% | 0.41% | 0.40% |
| Newborn | 0.50% | 0.41% | 0.40% |
| 0‐<1 year | 0.77% | 0.64% | 0.52% |
| 1‐5 years | 0.43% | 0.34% | 0.33% |
| 6‐10 years | 0.41% | 0.34% | 0.34% |
| 11‐17 years | 0.35% | 0.34% | 0.36% |
| Payer groups | |||
| Medicaid | 0.51% | 0.44% | 0.45% |
| Private | 0.38% | 0.34% | 0.33% |
| Uninsured | 0.69% | 0.69% | 0.58% |
However, because the majority of child hospitalizations are for newborns, the overall burden of mortality was greatest for newborns in all years studied. In 2002, 68.6% of pediatric inpatient deaths were newborns, 8.2% were non‐newborn infants, 7.7% were 15 years old, 4.2% were 610 years old, and 11.3% were 1117 years old. These findings were similarly distributed across age groups in 1992 and 1997 as well (data not shown).
Inpatient mortality rates also differed significantly by payer in all study years (Table 2). In each year, uninsured children had the highest mortality rates followed by children with Medicaid coverage and children with private health plans. Given the proportions of discharges with coverage by Medicaid versus private plans and the differences in mortality rates, the overall burden of mortality was greatest for children with private coverage in 1992 and 1997, and was equivalent to that of Medicaid (11,292 versus 11,330, respectively) in 2002.
Table 3 presents inpatient mortality rate by age and transfer status. Patients who were admitted on transfer from another acute care hospital had a significantly greater mortality rate for all age groups, compared with patients admitted not on transfer, within the same age group. The strong association of mortality with transfer status remained in multivariable regression analyses, adjusted for age and payer status (data not shown).
| Mortality Rate (% of Discharges) | |||
|---|---|---|---|
| Age Group and Transfer Status | 1992 (95% CI) | 1997 (95% CI) | 2002 (95% CI) |
| Newborn | |||
| Admitted as transfer | 4.57 (3.56, 5.59) | 4.22 (3.44, 5.00) | 4.75 (3.80, 5.93) |
| Admitted not on transfer | 0.45 (0.40, 0.51) | 0.37 (0.33, 0.40) | 0.36 (0.32, 0.40) |
| 0‐<1 year | |||
| Admitted as transfer | 5.05 (3.83, 6.28) | 4.38 (3.59, 5.17) | 2.86 (2.32, 3.53) |
| Admitted not on transfer | 0.43 (0.34, 0.50) | 0.35 (0.28, 0.43) | 0.30 (0.23, 0.40) |
| 1‐5 years | |||
| Admitted as transfer | 2.26 (1.61, 2.19) | 1.59 (1.20, 1.98) | 1.33 (0.97, 1.83) |
| Admitted not on transfer | 0.33 (0.25, 0.40) | 0.27 (0.22, 0.33) | 0.27 (0.22, 0.33) |
| 6‐10 years | |||
| Admitted as transfer | 2.01 (1.23, 2.96) | 1.48 (0.92, 2.03) | 1.11 (0.83, 1.49) |
| Admitted not on transfer | 0.32 (0.26, 0.39) | 0.28 (0.22, 0.34) | 0.29 (0.24, 0.36) |
| 11‐17 years | |||
| Admitted as transfer | 1.87 (1.42, 2.33) | 1.09 (0.81, 1.38) | 1.33 (1.02, 1.73) |
| Admitted not on transfer | 0.30 (0.25, 0.35) | 0.30 (0.25, 0.34) | 0.32 (0.27, 0.37) |
DRGs were evaluated based on transfer status, mortality, and study year. The most common DRGs for survivors were generally consistent across years and transfer status: neonate, bronchitis and asthma, pneumonia, esophagitis/gastroenteritis, nutritional and metabolic disturbances, and vaginal delivery. Among decedents, the primary diagnoses also included neonate, but in contrast with survivors were more likely to include traumatic injury, cardiothoracic surgery/medical care (ie, for congenital cardiac/valve disease), respiratory diagnosis with ventilatory support, and craniotomy. DRGs for decedents were consistent across years and transfer status (data available upon request to the authors).
DRGs were also evaluated based by payer status across all 3 study years (data not shown). The most common DRGs showed no meaningful differences in the types of conditions for children who were transferred versus not, across all payer types (including uninsured children).
Length of Stay and Hospital Charges, by Survival, Payer, and Transfer Status
Table 4 illustrates the national patterns of mean length of stay by age, survival, and transfer status. Data for 2002 are shown; the other study years had very similar findings and are available from the authors.
| Admitted on Transfer (95% CI) | Admitted Not on Transfer (95% CI) | |||
|---|---|---|---|---|
| Alive | Died | Alive | Died | |
| Age | ||||
| Newborn | 16.9 (14.7‐19.0) | 19.6 (15.1‐24.0) | 3.2 (3.0‐3.3) | 8.3 (6.9‐9.7) |
| 0‐<1year | 11.3 (9.1‐13.0) | 24.8 (18.8‐30.8) | 3.5 (3.2‐3.8) | 20.1 (12.8‐27.5) |
| 1‐5 years | 4.8 (4.2‐5.6) | 16.0 (8.5‐23.4) | 3.0 (3.4‐4.0) | 12.7 (7.2‐18.2) |
| 6‐10 years | 6.4 (4.7‐8.2) | 12.9 (4.9‐20.8) | 3.7 (3.4‐4.0) | 13.8 (9.7‐17.8) |
| 11‐17 years | 8.0 (6.0‐10.0) | 8.8 (5.8‐11.7) | 4.0 (3.7‐4.3) | 10.2 (6.4‐14.0) |
| Payer | ||||
| Medicaid | 11.4 (9.7‐13.1) | 21.8 (16.2‐27.4) | 3.5 (3.4‐3.7) | 11.2 (9.2‐13.3) |
| Private | 9.7 (8.6‐10.7) | 17.1 (13.5‐20.7) | 3.1 (3.0‐3.2) | 9.3 (7.4‐11.1) |
| Uninsured | 7.0 (4.8‐9.2) | 5.3 (1.1‐9.5) | 2.8 (2.6‐3.1) | 3.1 (1.2‐5.0) |
Length of stay differed significantly by transfer and survival status, and also varied significantly by insurance coverage. In 2002, among children who were admitted not on transfer, those who died had significantly longer mean length stay than those who survived. Among children admitted as a transfer, for all but non‐newborn infants and those 15 years of age, length of stay did not differ significantly by survival status.
For children covered by Medicaid and private insurance, decedents had significantly longer length of stay compared to survivors, regardless of transfer status. However, this was not the case for uninsured children, for whom those who died and those who survived had statistically indistinguishable lengths of stay, within the transfer/non‐ transfer groups. Findings for 1997 and 1992 were similar (data not shown).
Mean hospital charges are presented in Table 5. For children covered by Medicaid and private insurance, among patients who were admitted not on transfer, those who died had more than 8‐fold greater charges than those who survived. A similar trend was seen for patients admitted on transfer who were covered by Medicaid and private insurance, with more than 3‐fold greater charges for those who died versus those who survived. In contrast, for uninsured children, those who were admitted not on transfer and died had only 3.5‐fold greater charges compared to survivors, and those who were admitted on transfer and died had only 2‐fold greater charges compared to survivors.
| Admitted on Transfer (95% CI) | Admitted Not on Transfer (95% CI) | |||
|---|---|---|---|---|
| Alive | Died | Alive | Died | |
| Payer | ||||
| Medicaid | 43,123 (34,570‐51,675) | 141,280 (104,881‐177,679) | 8,456 (7,3489‐9,564) | 73,798 (59,71‐87,884) |
| Private | 41,037 (33,420‐48,653) | 142,739 (110,122‐175,355) | 7,519 (6,597‐8,441) | 62,195 (50,722‐73,667) |
| Uninsured | 21,228 (15,389‐27,068) | 48,036 (28,974‐67,099) | 5,591 (4,372‐6,810) | 19,910 (13,342‐26,479) |
DISCUSSION
Children's Inpatient Mortality
This is the first study of which we are aware that examines EOL hospitalization patterns for children in a national sample, spanning a decade. Our data revealed that the pediatric inpatient mortality rate is consistently highest among children in the non‐newborn infant age group over this time period, and that the burden of mortality is persistently greatest among newborns. These age‐specific findings are consistent with vital statistics published separately for each of the study years regarding overall childhood mortality.1820
This study highlights what many health care providers may not recognize: to meet the needs of the greatest numbers of families with gravely ill children, EOL care efforts must focus on the very youngest. Many of these children may not have chronic conditions, which have been a central focus of many pediatric EOL efforts to date. In fact, the parents of most gravely ill children in the hospital may have had just a few days or hours to prepare to face the loss of their children.
In addition, children admitted on interhospital transfer are significantly more likely to die while hospitalized. This pattern likely represents referral of severely ill children to medical centers that offer tertiary and quaternary specialty care, rather than risks associated with the transfer event itself. Some parents and their children may be far away from home and their closest networks of social support.7 Overall, these findings strongly indicate that EOL efforts will meet the needs of greater proportions of parents if they actively incorporate considerations of age and transfer status as institutions reach out to families in need of support.
Of note, this analysis does not capture children who were discharged into hospice, or long‐term care facilities, or who may have been discharged to home and may have died thereafter. Discharge disposition is known to vary by age, with older children with chronic conditions being more likely to use hospice services compared with infants.8 A recent study suggests that deaths outside the hospital have become increasingly common for older children over time, with the expansion of EOL supportive services in communities to meet the needs of families with gravely ill children.8
Length of Stay, Hospital Charges, and Mortality Related to Insurance Status
In this study, insured children who were admitted and died had significantly longer hospital stays compared to uninsured children who were admitted and died. DRG diagnoses by payer were very similar among children who died, although it is possible that differences in length of stay by payer status may reflect differences in severity of illness at admission and/or processes of care during hospitalization, which could not be fully accounted for using diagnostic codes. Hospitalizations that ended in death were significantly more expensive than hospitalizations in which children survived to discharge, regardless of age, payer status, or transfer status. However, incremental differences in spending for those who died versus those who survived were much greater for children with health insurance than for children without, suggesting greater resource utilization for children with coverage. Resource utilization is reflected largely in length of stay, which explains why our findings for differences in length of stay were echoed so strongly in our findings regarding differences in hospital charges.
Several studies of EOL care for adults have indicated that uninsured patients sustained higher inpatient mortality and lower hospital resource use versus insured adults, across similar diagnoses.13, 2123 Among children, Braveman and colleagues found differences in hospital resource allocation among sick newborns according to insurance coverage that are echoed in the findings of our study.24 Sick newborns without insurance received fewer inpatient services, with statistically significant shorter length of stay and total charges compared to insured newborns. In our study, disparities related to insurance coverage were consistent over the decade considered, and likely indicate ongoing challenges of broad disparities in access to care for children related to insurance coverage in the US health care system. Perhaps the greatest disparity was in mortality itself, which was highest among the uninsured, although the gap in mortality rates by insurance status appeared narrower in 2002 than in the prior study years.
Mortality Rates by Transfer Status
Mortality rates stratified by transfer status revealed that children transferred between hospitals had a significantly higher mortality rate, compared to children admitted not on transfer. Literature evaluating adult intensive care units found that transferred patients have more comorbid conditions, greater severity of illness, and 1.4‐fold to 2.5‐fold higher hospital mortality rates compared to direct admissions.25 Similar challenges face pediatric patients who are transferred to intensive care settings, where children at higher clinical risk have a higher morality rate and utilize greater resources compared with less critically ill children.14 Hospital EOL support personnel must be cognizant of the high mortality rate for transferred patients, and services may need to be adjusted to address the needs of these families. Additionally, further research is needed to better understand and remedy these potential disparities in care for children based on insurance status.
Limitations
This study is potentially limited by the accuracy of hospital discharge data, which may have influenced our outcomes. Further, not all states participate in the NIS; 11 states participated in 1992, 22 states participated in 1997, and 35 states participated in 2002. Although NIS data are weighted to be nationally representative in each year, it is possible that the participating states may have differed in systematic ways from nonparticipating states. However, the external validity of our data with regard to patterns of mortality by age and diagnoses, and the stability of patterns across a span of several years, suggest strongly that our findings are likely robust to these potential biases in this dataset.
As with any hospital resource use data, we are mindful that the distribution of data regarding length of stay and charges are typically right‐skewed, and therefore mean values should be interpreted with caution. In using mean values to test our hypotheses, we have followed the standard method of comparison for nationally weighted data.16
CONCLUSION
This national study of inpatient mortality patterns among US children over the span of a decade presents a new framework of challenges to clinicians and investigators regarding EOL care for children. As health care providers and institutions expand their efforts to meet the needs of severely ill children and their families, such efforts must be cognizant of the high burden of mortality among the youngest children, as well as those who are transferred between hospitals, and children without insurance coverage. These children and their families may require expanded EOL care and support services, beyond those typically available in most hospitals and communities.
APPENDIX
DIAGNOSIS‐RELATED GROUPS BY TRANSFER AND SURVIVAL STATUS
| 1992 | % | 1997 | % | 2002 | % |
|---|---|---|---|---|---|
| |||||
| Transferred ‐ Survived | |||||
| Neonate* | 26.2 | Neonate* | 23.2 | Neonate* | 24.6 |
| Bronchitis and Asthma | 6.4 | Bronchitis and Asthma | 7.4 | Bronchitis and Asthma | 8.0 |
| Seizure and Headache | 3.7 | Simple Pneumonia | 3.3 | Seizure and Headache | 4.2 |
| Simple Pneumonia | 3.4 | Seizure and Headache | 3.2 | Simple Pneumonia | 3.7 |
| Esophagitis and Gastroenteritis | 3.0 | Psychoses | 3.2 | Esophagitis and Gastroenteritis | 3.0 |
| Transferred ‐ Died | |||||
| Neonate | 35.1 | Neonate | 38.2 | Neonate | 40.5 |
| Cardiac Disease and/or Cardiothoracic surgery | 9.6 | Cardiac Disease and/or Cardiothoracic surgery | 12.2 | Cardiac Disease and/or Cardiothoracic surgery | 10.9 |
| Respiratory diagnosis with ventilatory support | 6.8 | Respiratory diagnosis with ventilatory support | 7.7 | Respiratory diagnosis with ventilatory support | 7.0 |
| Craniotomy | 3.5 | Septicemia | 2.8 | Injury, Poisoning | 2.4 |
| Injury, Poisoning | 3.3 | Tracheostomy with ventilatory support | 2.8 | Craniotomy | 2.2 |
| Not Transferred ‐ Survived | |||||
| Neonate* | 60.6 | Neonate* | 63 | Neonate* | 66.4 |
| Bronchitis and Asthma | 4.9 | Bronchitis and Asthma | 5.3 | Bronchitis and Asthma | 4.7 |
| Esophagitis and Gastroenteritis | 3.1 | Simple Pneumonia | 2.9 | Simple Pneumonia | 2.5 |
| Simple Pneumonia | 2.7 | Esophagitis and Gastroenteritis | 2.6 | Esophagitis and Gastroenteritis | 2.0 |
| Vaginal Delivery | 2.2 | Vaginal Delivery | 2.3 | Nutritional and Metabolic Disorder | 1.8 |
| Not Transferred ‐ Died | |||||
| Neonate | 61.5 | Neonate | 66.2 | Neonate | 69.0 |
| Traumatic Coma or Operative Procedure for Traumatic Injury | 3.3 | Traumatic Coma or Operative Procedure for Traumatic Injury | 4.8 | Traumatic Coma or Operative Procedure for Traumatic Injury | 4.7 |
| Cardiac Disease and/or Cardiothoracic surgery | 2.9 | Cardiac Disease and/or Cardiothoracic surgery | 2.7 | Respiratory diagnosis with ventilatory support | 2.7 |
| Craniotomy | 2.3 | Respiratory diagnosis with ventilatory support | 2.5 | Craniotomy | 2.4 |
| Respiratory diagnosis with ventilatory support | 2.0 | Septicemia | 1.4 | Septicemia | 1.2 |
- ,,,,,.Annual summary of vital statistics: 2005.Pediatrics.2007;119(2):345–360.
- ,,,,,.Circumstances surrounding the deaths of hospitalized children: opportunities for pediatric palliative care.Pediatrics.2004;114(3):e361–e366.
- ,,,,,.Characteristics of deaths occurring in children's hospitals: implications for supportive care services.Pediatrics.2002;109(5):887–893.
- ,,,,,.Declining severity adjusted mortality: evidence of improving neonatal intensive care.Pediatrics.1998;102(4):893–899.
- ,,, et al.Use of intensive care at the end of life in the United States: an epidemiologic study.Crit Care Med.2004;32(3):638–643.
- ,,,,.Cancer‐related deaths in children and adolescents.J Palliat Med.2005;8(1):86–95.
- ,,.Where do children with complex chronic conditions die? Patterns in Washington State, 1980‐1998.Pediatrics.2002;109(4):656–660.
- ,,,,,.Deaths attributed to pediatric complex chronic conditions: national trends and implications for supportive care services.Pediatrics.2001;107(6):e99.
- ,,, et al.Medical end‐of‐life decisions for children in the Netherlands.Arch Pediatr Adolescent Med.005;159(9):802–809.
- ,,,.Medicare beneficiaries' costs of care in the last year of life.Health Affairs.2001;20(4):188–195.
- ,,,,.Medicare Beneficiaries' Costs and Use of Care in the Last Year of Life.Washington, DC:MedPAC;2000.
- ,.Trends in Medicare payments in the last year of life.N Engl J Med.1993;328(15):1092–1096.
- ,,.Comparing uninsured and privately insured hospital patients: admission severity, health outcomes and resource use.Health Serv Manage Res.2001;14(3):203–210.
- ,,, et al.Characteristics and outcomes of interhospital transfers from level II to level I pediatric intensive care units.Pediatr Crit Care Med.2006;7(6):536–540.
- Agency for Healthcare Research and Quality. National Inpatient Sample (NIS). Healthcare Cost and Utilization Project (HCUP). http://www.hcup‐us.ahrq.gov/nisoverview.jsp. Accessed August 26,2008.
- Agency for Healthcare Research and Quality,. Healthcare Cost and Utilization Project H CUP. Care of Children and Adolescents in U.S. Hospitals. HCUP Fact Book No. 4, Publication No. 04‐0004. http://www.ahrq.gov/data/hcup/factbk4/. Accessed August 26,2008.
- U.S. Department of Labor, Bureau of Labor Statistics. Consumer Price Index.ftp://ftp.bls.gov/pub/special.requests/cpi/cpiai.txt. Accessed August 26,2008.
- ,,,.Annual summary of vital statistics‐‐2002.Pediatrics.2003;112(6):1215–1230.
- ,,,,.Annual summary of vital statistics‐‐1996.Pediatrics.1997;100(6):905–918.
- .Annual summary of vital statistics‐‐1992.Pediatrics.1993;92(6):743–754.
- ,.Acutely injured patients with trauma in Massachusetts: differences in care and mortality, by insurance status.Am J Publ Health.1994;84(10):1605–1608.
- ,,.Comparison of uninsured and privately insured hospital patients. Condition on admission, resource use, and outcome.JAMA.1991;265(3):374–379.
- ,.Inequities in hospital care, the Massachusetts experience.Inquiry.1991;28(3):255–262.
- ,,,.Differences in hospital resource allocation among sick newborns according to insurance coverage.JAMA.1991;266(23):3300–3308.
- ,,,,.Accepting critically ill transfer patients: adverse effect on a referral center's outcome and benchmark measures. [summary for patients in Ann Intern Med. 2003;138(11):I42; PMID: 12779311].Ann Intern Med.2003;138(11):882–890.
More than 53,000 children 19 years of age or younger died in 2004,1 and more than 40% of these children died while hospitalized.25 Recently, pediatric end‐of‐life (EOL) issues have gained clinical and research attention, primarily focused on children with chronic conditions, ethical dilemmas surrounding childhood death and dying, and the need for interdisciplinary palliative care efforts for dying children and their families.2, 3, 69
Much remains unknown about patterns of EOL hospital care at the national level for all children, both with and without complex chronic conditions. Because a large proportion of childhood mortality occurs during hospitalization, the inpatient setting is a crucial arena for patients and families facing EOL issues. However, little is known about how insurance status and interhospital transfer are associated with patterns of hospitalization and mortality for children while hospitalized, or about hospital charges and lengths of stay for children who die as inpatients versus those who survive to discharge. In addition, although spending on EOL health care in the United States has attracted considerable attention in recent years, the published literature focuses almost exclusively on adult populations.1012
Illuminating the patterns of childhood mortality in hospital settings may inform expanding institutional efforts to address death and dying for children and their families. We conducted an analysis of national patterns of hospitalization over a span of a decade (19922002), in order to characterize sociodemographic and health care factors associated with inpatient mortality, and to examine patterns of hospital resource use related to EOL care. We hypothesized that resource use would be higher for children who died versus those who survived, and would be higher for uninsured versus insured children.13 We also hypothesized that children admitted upon transfer from another hospital would have higher risk of mortality.14
METHODS
Our data source was the National Inpatient Sample (NIS), which is a component of the Healthcare Cost and Utilization Project (HCUP) sponsored by the Agency for Healthcare Research and Quality. The HCUP is a set of databases developed through partnership among health care institutions and federal and state governments.15 The NIS is the largest publicly available all‐payer inpatient database in the United States, and contains de‐identified, patient‐level clinical data included in a typical discharge abstract. For each year, these data reflect hospital stays from between 800 and 1000 institutions sampled to approximate a 20% stratified sample of nonfederal community hospitals, including public hospitals, children's hospitals, and academic medical centers but excluding long‐term hospitals, psychiatric hospitals, and chemical dependency treatment facilities.
We chose the NIS for this analysis because we were interested in the most common diagnoses for hospitalized children. An alternative database, such as the KID (Kids Inpatient Database), is optimal for less commonly seen discharge diagnoses and did not permit a full decade of retrospective analysis.
In order to characterize changes in mortality and health resource utilization related to our research questions, we conducted a comparative cross‐sectional analysis of 3 years of the NIS over the years 1992, 1997, and 2002. For each year of NIS data, discharge‐level weights were provided to permit calculation of national estimates of hospitalization rates standardized to the concurrent national population.15 All inpatient hospital stays of children aged 17 years and younger were selected.
Discharge data were analyzed based on age, sex, payer status, and transfer status on admission. Although transfer status is not often considered in studies of mortality, we expected that it would be associated with mortality, as a potential indicator of disease severity.14 We included only interhospital transfers, and excluded patients transferred from other locations such as long‐term care facilities. We categorized discharges into 5 age groups: newborns, whose hospitalization began at birth; infants up to 1 year of age who were not born during hospitalization; 15 years; 610 years; and 1117 years. This stratification allowed us to separate infants who were admitted from home or from another hospital versus those who were born during hospitalization. Payer groups included Medicaid, private insurance, and uninsured. Medicare and other payers were analyzed, but were present in very small numbers and are not reported.
Outcomes included weighted inpatient mortality rate, weighted mean of length of stay (in days), and weighted mean total hospital charges. For nationally weighted data, lengths of stay and hospital charges are typically reported as means because weighted medians cannot be estimated.16 We compared mortality patterns for patients who were transferred between hospitals versus those who were not, using multivariable logistic regression to identify factors associated with in‐hospital mortality. Of note, transfer status was evaluated from the standpoint of the receiving hospital as children who were admitted upon transfer from another hospital. Thus, our estimates likely underestimate the effects attributed to interhospital transfer, because this evaluation is unilateral and does not include the transferring hospital. The 5 most common principal Diagnosis‐Related Groups (DRGs) upon discharge were compiled for each of the study years for both survivors and decedents. In order to interpret the analyses of discharge‐related hospital charges in constant dollars, we standardized all hospital charges to 2002 US dollars using the Consumer Price Index.17
Statistical analyses included bivariate comparisons of sociodemographic characteristics and the study outcomes, for each of the study years. We also conducted multivariable regression analyses of mortality, comparing effects of sociodemographic variables and transfer status. We conducted all analyses using Stata, version 8 (Stata Corp., College Station, TX), with which we incorporated sample weights to account for the complex stratified sampling of hospitals that comprise the NIS, and to generate variance estimates with which we derived 95% confidence intervals (95% CI). NIS samples included weighted data for 6.2 million discharges in 1992, 7.1 million discharges in 1997, and 7.9 million discharges in 2002. All results are presented using weighted values. The study was funded internally and all analyses were conducted by the authors. The authors had no financial interest in the outcome. The study was exempt from human subjects review as an analysis of de‐identified secondary data.
RESULTS
Study Sample
NIS samples represented between 35 million and 37.8 million discharges nationally in each of the study years. Distributions of discharges across age group, gender, and payer group were similar across the study years (Table 1).
| Characteristic | 1992 N = 6,722,647 | 1997 N = 6,365,886 | 2002 N = 6,456,077 |
|---|---|---|---|
| |||
| Age (%) | |||
| Newborn | 60.0 | 63.0 | 65.0 |
| Admitted as transfer* | 1.3 | 1.1 | 1.2 |
| 0‐<1 year | 8.7 | 8.0 | 8.6 |
| Admitted as transfer* | 7.6 | 7.2 | 8.8 |
| 1‐5 years | 11.9 | 11.0 | 9.2 |
| Admitted as transfer* | 5.1 | 4.5 | 5.6 |
| 6‐10 years | 5.5 | 5.0 | 5.0 |
| Admitted as transfer* | 4.9 | 4.7 | 5.3 |
| 11‐17 years | 13.9 | 13.0 | 12.2 |
| Admitted as transfer* | 3.1 | 4.2 | 4.8 |
| Gender (%) | |||
| Female | 49.0 | 49.0 | 49.0 |
| Payer (%) | |||
| Medicaid | 37.0 | 36.0 | 39.0 |
| Admitted as transfer* | 3.3 | 3.0 | 3.4 |
| Private | 52.0 | 55.0 | 53.0 |
| Admitted as transfer* | 2.3 | 2.3 | 2.4 |
| Uninsured | 7.0 | 5.0 | 5.0 |
| Admitted as transfer* | 2.4 | 2.4 | 2.4 |
The proportions of patients admitted as transfers between hospitals are shown for each age group, as well as by payer. Non‐newborn infants had the highest rate of transfer for each year studied, compared with the other age groups. Across the study years, transfer status was fairly uniform across payers.
Patterns of Inpatient Mortality
During the study period, overall pediatric inpatient mortality decreased from 32,941 children (0.49% of all child discharges) in 1992 to 25,824 children (0.40%) in 2002, although this was not a statistically significant change. The inpatient mortality rate across all years studied was significantly higher for the non‐newborn infants (<1 years) than for all other age groups in all study years (P <.005) (Table 2). The newborn age group had the second highest mortality rate in all years, and the remaining 3 groups had similar mortality rates.
| Age Groups* | Annual Inpatient Mortality Rate | ||
|---|---|---|---|
| 1992 N = 6,722,647 | 1997 N = 6,365,886 | 2002 N = 6,456,077 | |
| |||
| Overall | 0.49% | 0.41% | 0.40% |
| Newborn | 0.50% | 0.41% | 0.40% |
| 0‐<1 year | 0.77% | 0.64% | 0.52% |
| 1‐5 years | 0.43% | 0.34% | 0.33% |
| 6‐10 years | 0.41% | 0.34% | 0.34% |
| 11‐17 years | 0.35% | 0.34% | 0.36% |
| Payer groups | |||
| Medicaid | 0.51% | 0.44% | 0.45% |
| Private | 0.38% | 0.34% | 0.33% |
| Uninsured | 0.69% | 0.69% | 0.58% |
However, because the majority of child hospitalizations are for newborns, the overall burden of mortality was greatest for newborns in all years studied. In 2002, 68.6% of pediatric inpatient deaths were newborns, 8.2% were non‐newborn infants, 7.7% were 15 years old, 4.2% were 610 years old, and 11.3% were 1117 years old. These findings were similarly distributed across age groups in 1992 and 1997 as well (data not shown).
Inpatient mortality rates also differed significantly by payer in all study years (Table 2). In each year, uninsured children had the highest mortality rates followed by children with Medicaid coverage and children with private health plans. Given the proportions of discharges with coverage by Medicaid versus private plans and the differences in mortality rates, the overall burden of mortality was greatest for children with private coverage in 1992 and 1997, and was equivalent to that of Medicaid (11,292 versus 11,330, respectively) in 2002.
Table 3 presents inpatient mortality rate by age and transfer status. Patients who were admitted on transfer from another acute care hospital had a significantly greater mortality rate for all age groups, compared with patients admitted not on transfer, within the same age group. The strong association of mortality with transfer status remained in multivariable regression analyses, adjusted for age and payer status (data not shown).
| Mortality Rate (% of Discharges) | |||
|---|---|---|---|
| Age Group and Transfer Status | 1992 (95% CI) | 1997 (95% CI) | 2002 (95% CI) |
| Newborn | |||
| Admitted as transfer | 4.57 (3.56, 5.59) | 4.22 (3.44, 5.00) | 4.75 (3.80, 5.93) |
| Admitted not on transfer | 0.45 (0.40, 0.51) | 0.37 (0.33, 0.40) | 0.36 (0.32, 0.40) |
| 0‐<1 year | |||
| Admitted as transfer | 5.05 (3.83, 6.28) | 4.38 (3.59, 5.17) | 2.86 (2.32, 3.53) |
| Admitted not on transfer | 0.43 (0.34, 0.50) | 0.35 (0.28, 0.43) | 0.30 (0.23, 0.40) |
| 1‐5 years | |||
| Admitted as transfer | 2.26 (1.61, 2.19) | 1.59 (1.20, 1.98) | 1.33 (0.97, 1.83) |
| Admitted not on transfer | 0.33 (0.25, 0.40) | 0.27 (0.22, 0.33) | 0.27 (0.22, 0.33) |
| 6‐10 years | |||
| Admitted as transfer | 2.01 (1.23, 2.96) | 1.48 (0.92, 2.03) | 1.11 (0.83, 1.49) |
| Admitted not on transfer | 0.32 (0.26, 0.39) | 0.28 (0.22, 0.34) | 0.29 (0.24, 0.36) |
| 11‐17 years | |||
| Admitted as transfer | 1.87 (1.42, 2.33) | 1.09 (0.81, 1.38) | 1.33 (1.02, 1.73) |
| Admitted not on transfer | 0.30 (0.25, 0.35) | 0.30 (0.25, 0.34) | 0.32 (0.27, 0.37) |
DRGs were evaluated based on transfer status, mortality, and study year. The most common DRGs for survivors were generally consistent across years and transfer status: neonate, bronchitis and asthma, pneumonia, esophagitis/gastroenteritis, nutritional and metabolic disturbances, and vaginal delivery. Among decedents, the primary diagnoses also included neonate, but in contrast with survivors were more likely to include traumatic injury, cardiothoracic surgery/medical care (ie, for congenital cardiac/valve disease), respiratory diagnosis with ventilatory support, and craniotomy. DRGs for decedents were consistent across years and transfer status (data available upon request to the authors).
DRGs were also evaluated based by payer status across all 3 study years (data not shown). The most common DRGs showed no meaningful differences in the types of conditions for children who were transferred versus not, across all payer types (including uninsured children).
Length of Stay and Hospital Charges, by Survival, Payer, and Transfer Status
Table 4 illustrates the national patterns of mean length of stay by age, survival, and transfer status. Data for 2002 are shown; the other study years had very similar findings and are available from the authors.
| Admitted on Transfer (95% CI) | Admitted Not on Transfer (95% CI) | |||
|---|---|---|---|---|
| Alive | Died | Alive | Died | |
| Age | ||||
| Newborn | 16.9 (14.7‐19.0) | 19.6 (15.1‐24.0) | 3.2 (3.0‐3.3) | 8.3 (6.9‐9.7) |
| 0‐<1year | 11.3 (9.1‐13.0) | 24.8 (18.8‐30.8) | 3.5 (3.2‐3.8) | 20.1 (12.8‐27.5) |
| 1‐5 years | 4.8 (4.2‐5.6) | 16.0 (8.5‐23.4) | 3.0 (3.4‐4.0) | 12.7 (7.2‐18.2) |
| 6‐10 years | 6.4 (4.7‐8.2) | 12.9 (4.9‐20.8) | 3.7 (3.4‐4.0) | 13.8 (9.7‐17.8) |
| 11‐17 years | 8.0 (6.0‐10.0) | 8.8 (5.8‐11.7) | 4.0 (3.7‐4.3) | 10.2 (6.4‐14.0) |
| Payer | ||||
| Medicaid | 11.4 (9.7‐13.1) | 21.8 (16.2‐27.4) | 3.5 (3.4‐3.7) | 11.2 (9.2‐13.3) |
| Private | 9.7 (8.6‐10.7) | 17.1 (13.5‐20.7) | 3.1 (3.0‐3.2) | 9.3 (7.4‐11.1) |
| Uninsured | 7.0 (4.8‐9.2) | 5.3 (1.1‐9.5) | 2.8 (2.6‐3.1) | 3.1 (1.2‐5.0) |
Length of stay differed significantly by transfer and survival status, and also varied significantly by insurance coverage. In 2002, among children who were admitted not on transfer, those who died had significantly longer mean length stay than those who survived. Among children admitted as a transfer, for all but non‐newborn infants and those 15 years of age, length of stay did not differ significantly by survival status.
For children covered by Medicaid and private insurance, decedents had significantly longer length of stay compared to survivors, regardless of transfer status. However, this was not the case for uninsured children, for whom those who died and those who survived had statistically indistinguishable lengths of stay, within the transfer/non‐ transfer groups. Findings for 1997 and 1992 were similar (data not shown).
Mean hospital charges are presented in Table 5. For children covered by Medicaid and private insurance, among patients who were admitted not on transfer, those who died had more than 8‐fold greater charges than those who survived. A similar trend was seen for patients admitted on transfer who were covered by Medicaid and private insurance, with more than 3‐fold greater charges for those who died versus those who survived. In contrast, for uninsured children, those who were admitted not on transfer and died had only 3.5‐fold greater charges compared to survivors, and those who were admitted on transfer and died had only 2‐fold greater charges compared to survivors.
| Admitted on Transfer (95% CI) | Admitted Not on Transfer (95% CI) | |||
|---|---|---|---|---|
| Alive | Died | Alive | Died | |
| Payer | ||||
| Medicaid | 43,123 (34,570‐51,675) | 141,280 (104,881‐177,679) | 8,456 (7,3489‐9,564) | 73,798 (59,71‐87,884) |
| Private | 41,037 (33,420‐48,653) | 142,739 (110,122‐175,355) | 7,519 (6,597‐8,441) | 62,195 (50,722‐73,667) |
| Uninsured | 21,228 (15,389‐27,068) | 48,036 (28,974‐67,099) | 5,591 (4,372‐6,810) | 19,910 (13,342‐26,479) |
DISCUSSION
Children's Inpatient Mortality
This is the first study of which we are aware that examines EOL hospitalization patterns for children in a national sample, spanning a decade. Our data revealed that the pediatric inpatient mortality rate is consistently highest among children in the non‐newborn infant age group over this time period, and that the burden of mortality is persistently greatest among newborns. These age‐specific findings are consistent with vital statistics published separately for each of the study years regarding overall childhood mortality.1820
This study highlights what many health care providers may not recognize: to meet the needs of the greatest numbers of families with gravely ill children, EOL care efforts must focus on the very youngest. Many of these children may not have chronic conditions, which have been a central focus of many pediatric EOL efforts to date. In fact, the parents of most gravely ill children in the hospital may have had just a few days or hours to prepare to face the loss of their children.
In addition, children admitted on interhospital transfer are significantly more likely to die while hospitalized. This pattern likely represents referral of severely ill children to medical centers that offer tertiary and quaternary specialty care, rather than risks associated with the transfer event itself. Some parents and their children may be far away from home and their closest networks of social support.7 Overall, these findings strongly indicate that EOL efforts will meet the needs of greater proportions of parents if they actively incorporate considerations of age and transfer status as institutions reach out to families in need of support.
Of note, this analysis does not capture children who were discharged into hospice, or long‐term care facilities, or who may have been discharged to home and may have died thereafter. Discharge disposition is known to vary by age, with older children with chronic conditions being more likely to use hospice services compared with infants.8 A recent study suggests that deaths outside the hospital have become increasingly common for older children over time, with the expansion of EOL supportive services in communities to meet the needs of families with gravely ill children.8
Length of Stay, Hospital Charges, and Mortality Related to Insurance Status
In this study, insured children who were admitted and died had significantly longer hospital stays compared to uninsured children who were admitted and died. DRG diagnoses by payer were very similar among children who died, although it is possible that differences in length of stay by payer status may reflect differences in severity of illness at admission and/or processes of care during hospitalization, which could not be fully accounted for using diagnostic codes. Hospitalizations that ended in death were significantly more expensive than hospitalizations in which children survived to discharge, regardless of age, payer status, or transfer status. However, incremental differences in spending for those who died versus those who survived were much greater for children with health insurance than for children without, suggesting greater resource utilization for children with coverage. Resource utilization is reflected largely in length of stay, which explains why our findings for differences in length of stay were echoed so strongly in our findings regarding differences in hospital charges.
Several studies of EOL care for adults have indicated that uninsured patients sustained higher inpatient mortality and lower hospital resource use versus insured adults, across similar diagnoses.13, 2123 Among children, Braveman and colleagues found differences in hospital resource allocation among sick newborns according to insurance coverage that are echoed in the findings of our study.24 Sick newborns without insurance received fewer inpatient services, with statistically significant shorter length of stay and total charges compared to insured newborns. In our study, disparities related to insurance coverage were consistent over the decade considered, and likely indicate ongoing challenges of broad disparities in access to care for children related to insurance coverage in the US health care system. Perhaps the greatest disparity was in mortality itself, which was highest among the uninsured, although the gap in mortality rates by insurance status appeared narrower in 2002 than in the prior study years.
Mortality Rates by Transfer Status
Mortality rates stratified by transfer status revealed that children transferred between hospitals had a significantly higher mortality rate, compared to children admitted not on transfer. Literature evaluating adult intensive care units found that transferred patients have more comorbid conditions, greater severity of illness, and 1.4‐fold to 2.5‐fold higher hospital mortality rates compared to direct admissions.25 Similar challenges face pediatric patients who are transferred to intensive care settings, where children at higher clinical risk have a higher morality rate and utilize greater resources compared with less critically ill children.14 Hospital EOL support personnel must be cognizant of the high mortality rate for transferred patients, and services may need to be adjusted to address the needs of these families. Additionally, further research is needed to better understand and remedy these potential disparities in care for children based on insurance status.
Limitations
This study is potentially limited by the accuracy of hospital discharge data, which may have influenced our outcomes. Further, not all states participate in the NIS; 11 states participated in 1992, 22 states participated in 1997, and 35 states participated in 2002. Although NIS data are weighted to be nationally representative in each year, it is possible that the participating states may have differed in systematic ways from nonparticipating states. However, the external validity of our data with regard to patterns of mortality by age and diagnoses, and the stability of patterns across a span of several years, suggest strongly that our findings are likely robust to these potential biases in this dataset.
As with any hospital resource use data, we are mindful that the distribution of data regarding length of stay and charges are typically right‐skewed, and therefore mean values should be interpreted with caution. In using mean values to test our hypotheses, we have followed the standard method of comparison for nationally weighted data.16
CONCLUSION
This national study of inpatient mortality patterns among US children over the span of a decade presents a new framework of challenges to clinicians and investigators regarding EOL care for children. As health care providers and institutions expand their efforts to meet the needs of severely ill children and their families, such efforts must be cognizant of the high burden of mortality among the youngest children, as well as those who are transferred between hospitals, and children without insurance coverage. These children and their families may require expanded EOL care and support services, beyond those typically available in most hospitals and communities.
APPENDIX
DIAGNOSIS‐RELATED GROUPS BY TRANSFER AND SURVIVAL STATUS
| 1992 | % | 1997 | % | 2002 | % |
|---|---|---|---|---|---|
| |||||
| Transferred ‐ Survived | |||||
| Neonate* | 26.2 | Neonate* | 23.2 | Neonate* | 24.6 |
| Bronchitis and Asthma | 6.4 | Bronchitis and Asthma | 7.4 | Bronchitis and Asthma | 8.0 |
| Seizure and Headache | 3.7 | Simple Pneumonia | 3.3 | Seizure and Headache | 4.2 |
| Simple Pneumonia | 3.4 | Seizure and Headache | 3.2 | Simple Pneumonia | 3.7 |
| Esophagitis and Gastroenteritis | 3.0 | Psychoses | 3.2 | Esophagitis and Gastroenteritis | 3.0 |
| Transferred ‐ Died | |||||
| Neonate | 35.1 | Neonate | 38.2 | Neonate | 40.5 |
| Cardiac Disease and/or Cardiothoracic surgery | 9.6 | Cardiac Disease and/or Cardiothoracic surgery | 12.2 | Cardiac Disease and/or Cardiothoracic surgery | 10.9 |
| Respiratory diagnosis with ventilatory support | 6.8 | Respiratory diagnosis with ventilatory support | 7.7 | Respiratory diagnosis with ventilatory support | 7.0 |
| Craniotomy | 3.5 | Septicemia | 2.8 | Injury, Poisoning | 2.4 |
| Injury, Poisoning | 3.3 | Tracheostomy with ventilatory support | 2.8 | Craniotomy | 2.2 |
| Not Transferred ‐ Survived | |||||
| Neonate* | 60.6 | Neonate* | 63 | Neonate* | 66.4 |
| Bronchitis and Asthma | 4.9 | Bronchitis and Asthma | 5.3 | Bronchitis and Asthma | 4.7 |
| Esophagitis and Gastroenteritis | 3.1 | Simple Pneumonia | 2.9 | Simple Pneumonia | 2.5 |
| Simple Pneumonia | 2.7 | Esophagitis and Gastroenteritis | 2.6 | Esophagitis and Gastroenteritis | 2.0 |
| Vaginal Delivery | 2.2 | Vaginal Delivery | 2.3 | Nutritional and Metabolic Disorder | 1.8 |
| Not Transferred ‐ Died | |||||
| Neonate | 61.5 | Neonate | 66.2 | Neonate | 69.0 |
| Traumatic Coma or Operative Procedure for Traumatic Injury | 3.3 | Traumatic Coma or Operative Procedure for Traumatic Injury | 4.8 | Traumatic Coma or Operative Procedure for Traumatic Injury | 4.7 |
| Cardiac Disease and/or Cardiothoracic surgery | 2.9 | Cardiac Disease and/or Cardiothoracic surgery | 2.7 | Respiratory diagnosis with ventilatory support | 2.7 |
| Craniotomy | 2.3 | Respiratory diagnosis with ventilatory support | 2.5 | Craniotomy | 2.4 |
| Respiratory diagnosis with ventilatory support | 2.0 | Septicemia | 1.4 | Septicemia | 1.2 |
More than 53,000 children 19 years of age or younger died in 2004,1 and more than 40% of these children died while hospitalized.25 Recently, pediatric end‐of‐life (EOL) issues have gained clinical and research attention, primarily focused on children with chronic conditions, ethical dilemmas surrounding childhood death and dying, and the need for interdisciplinary palliative care efforts for dying children and their families.2, 3, 69
Much remains unknown about patterns of EOL hospital care at the national level for all children, both with and without complex chronic conditions. Because a large proportion of childhood mortality occurs during hospitalization, the inpatient setting is a crucial arena for patients and families facing EOL issues. However, little is known about how insurance status and interhospital transfer are associated with patterns of hospitalization and mortality for children while hospitalized, or about hospital charges and lengths of stay for children who die as inpatients versus those who survive to discharge. In addition, although spending on EOL health care in the United States has attracted considerable attention in recent years, the published literature focuses almost exclusively on adult populations.1012
Illuminating the patterns of childhood mortality in hospital settings may inform expanding institutional efforts to address death and dying for children and their families. We conducted an analysis of national patterns of hospitalization over a span of a decade (19922002), in order to characterize sociodemographic and health care factors associated with inpatient mortality, and to examine patterns of hospital resource use related to EOL care. We hypothesized that resource use would be higher for children who died versus those who survived, and would be higher for uninsured versus insured children.13 We also hypothesized that children admitted upon transfer from another hospital would have higher risk of mortality.14
METHODS
Our data source was the National Inpatient Sample (NIS), which is a component of the Healthcare Cost and Utilization Project (HCUP) sponsored by the Agency for Healthcare Research and Quality. The HCUP is a set of databases developed through partnership among health care institutions and federal and state governments.15 The NIS is the largest publicly available all‐payer inpatient database in the United States, and contains de‐identified, patient‐level clinical data included in a typical discharge abstract. For each year, these data reflect hospital stays from between 800 and 1000 institutions sampled to approximate a 20% stratified sample of nonfederal community hospitals, including public hospitals, children's hospitals, and academic medical centers but excluding long‐term hospitals, psychiatric hospitals, and chemical dependency treatment facilities.
We chose the NIS for this analysis because we were interested in the most common diagnoses for hospitalized children. An alternative database, such as the KID (Kids Inpatient Database), is optimal for less commonly seen discharge diagnoses and did not permit a full decade of retrospective analysis.
In order to characterize changes in mortality and health resource utilization related to our research questions, we conducted a comparative cross‐sectional analysis of 3 years of the NIS over the years 1992, 1997, and 2002. For each year of NIS data, discharge‐level weights were provided to permit calculation of national estimates of hospitalization rates standardized to the concurrent national population.15 All inpatient hospital stays of children aged 17 years and younger were selected.
Discharge data were analyzed based on age, sex, payer status, and transfer status on admission. Although transfer status is not often considered in studies of mortality, we expected that it would be associated with mortality, as a potential indicator of disease severity.14 We included only interhospital transfers, and excluded patients transferred from other locations such as long‐term care facilities. We categorized discharges into 5 age groups: newborns, whose hospitalization began at birth; infants up to 1 year of age who were not born during hospitalization; 15 years; 610 years; and 1117 years. This stratification allowed us to separate infants who were admitted from home or from another hospital versus those who were born during hospitalization. Payer groups included Medicaid, private insurance, and uninsured. Medicare and other payers were analyzed, but were present in very small numbers and are not reported.
Outcomes included weighted inpatient mortality rate, weighted mean of length of stay (in days), and weighted mean total hospital charges. For nationally weighted data, lengths of stay and hospital charges are typically reported as means because weighted medians cannot be estimated.16 We compared mortality patterns for patients who were transferred between hospitals versus those who were not, using multivariable logistic regression to identify factors associated with in‐hospital mortality. Of note, transfer status was evaluated from the standpoint of the receiving hospital as children who were admitted upon transfer from another hospital. Thus, our estimates likely underestimate the effects attributed to interhospital transfer, because this evaluation is unilateral and does not include the transferring hospital. The 5 most common principal Diagnosis‐Related Groups (DRGs) upon discharge were compiled for each of the study years for both survivors and decedents. In order to interpret the analyses of discharge‐related hospital charges in constant dollars, we standardized all hospital charges to 2002 US dollars using the Consumer Price Index.17
Statistical analyses included bivariate comparisons of sociodemographic characteristics and the study outcomes, for each of the study years. We also conducted multivariable regression analyses of mortality, comparing effects of sociodemographic variables and transfer status. We conducted all analyses using Stata, version 8 (Stata Corp., College Station, TX), with which we incorporated sample weights to account for the complex stratified sampling of hospitals that comprise the NIS, and to generate variance estimates with which we derived 95% confidence intervals (95% CI). NIS samples included weighted data for 6.2 million discharges in 1992, 7.1 million discharges in 1997, and 7.9 million discharges in 2002. All results are presented using weighted values. The study was funded internally and all analyses were conducted by the authors. The authors had no financial interest in the outcome. The study was exempt from human subjects review as an analysis of de‐identified secondary data.
RESULTS
Study Sample
NIS samples represented between 35 million and 37.8 million discharges nationally in each of the study years. Distributions of discharges across age group, gender, and payer group were similar across the study years (Table 1).
| Characteristic | 1992 N = 6,722,647 | 1997 N = 6,365,886 | 2002 N = 6,456,077 |
|---|---|---|---|
| |||
| Age (%) | |||
| Newborn | 60.0 | 63.0 | 65.0 |
| Admitted as transfer* | 1.3 | 1.1 | 1.2 |
| 0‐<1 year | 8.7 | 8.0 | 8.6 |
| Admitted as transfer* | 7.6 | 7.2 | 8.8 |
| 1‐5 years | 11.9 | 11.0 | 9.2 |
| Admitted as transfer* | 5.1 | 4.5 | 5.6 |
| 6‐10 years | 5.5 | 5.0 | 5.0 |
| Admitted as transfer* | 4.9 | 4.7 | 5.3 |
| 11‐17 years | 13.9 | 13.0 | 12.2 |
| Admitted as transfer* | 3.1 | 4.2 | 4.8 |
| Gender (%) | |||
| Female | 49.0 | 49.0 | 49.0 |
| Payer (%) | |||
| Medicaid | 37.0 | 36.0 | 39.0 |
| Admitted as transfer* | 3.3 | 3.0 | 3.4 |
| Private | 52.0 | 55.0 | 53.0 |
| Admitted as transfer* | 2.3 | 2.3 | 2.4 |
| Uninsured | 7.0 | 5.0 | 5.0 |
| Admitted as transfer* | 2.4 | 2.4 | 2.4 |
The proportions of patients admitted as transfers between hospitals are shown for each age group, as well as by payer. Non‐newborn infants had the highest rate of transfer for each year studied, compared with the other age groups. Across the study years, transfer status was fairly uniform across payers.
Patterns of Inpatient Mortality
During the study period, overall pediatric inpatient mortality decreased from 32,941 children (0.49% of all child discharges) in 1992 to 25,824 children (0.40%) in 2002, although this was not a statistically significant change. The inpatient mortality rate across all years studied was significantly higher for the non‐newborn infants (<1 years) than for all other age groups in all study years (P <.005) (Table 2). The newborn age group had the second highest mortality rate in all years, and the remaining 3 groups had similar mortality rates.
| Age Groups* | Annual Inpatient Mortality Rate | ||
|---|---|---|---|
| 1992 N = 6,722,647 | 1997 N = 6,365,886 | 2002 N = 6,456,077 | |
| |||
| Overall | 0.49% | 0.41% | 0.40% |
| Newborn | 0.50% | 0.41% | 0.40% |
| 0‐<1 year | 0.77% | 0.64% | 0.52% |
| 1‐5 years | 0.43% | 0.34% | 0.33% |
| 6‐10 years | 0.41% | 0.34% | 0.34% |
| 11‐17 years | 0.35% | 0.34% | 0.36% |
| Payer groups | |||
| Medicaid | 0.51% | 0.44% | 0.45% |
| Private | 0.38% | 0.34% | 0.33% |
| Uninsured | 0.69% | 0.69% | 0.58% |
However, because the majority of child hospitalizations are for newborns, the overall burden of mortality was greatest for newborns in all years studied. In 2002, 68.6% of pediatric inpatient deaths were newborns, 8.2% were non‐newborn infants, 7.7% were 15 years old, 4.2% were 610 years old, and 11.3% were 1117 years old. These findings were similarly distributed across age groups in 1992 and 1997 as well (data not shown).
Inpatient mortality rates also differed significantly by payer in all study years (Table 2). In each year, uninsured children had the highest mortality rates followed by children with Medicaid coverage and children with private health plans. Given the proportions of discharges with coverage by Medicaid versus private plans and the differences in mortality rates, the overall burden of mortality was greatest for children with private coverage in 1992 and 1997, and was equivalent to that of Medicaid (11,292 versus 11,330, respectively) in 2002.
Table 3 presents inpatient mortality rate by age and transfer status. Patients who were admitted on transfer from another acute care hospital had a significantly greater mortality rate for all age groups, compared with patients admitted not on transfer, within the same age group. The strong association of mortality with transfer status remained in multivariable regression analyses, adjusted for age and payer status (data not shown).
| Mortality Rate (% of Discharges) | |||
|---|---|---|---|
| Age Group and Transfer Status | 1992 (95% CI) | 1997 (95% CI) | 2002 (95% CI) |
| Newborn | |||
| Admitted as transfer | 4.57 (3.56, 5.59) | 4.22 (3.44, 5.00) | 4.75 (3.80, 5.93) |
| Admitted not on transfer | 0.45 (0.40, 0.51) | 0.37 (0.33, 0.40) | 0.36 (0.32, 0.40) |
| 0‐<1 year | |||
| Admitted as transfer | 5.05 (3.83, 6.28) | 4.38 (3.59, 5.17) | 2.86 (2.32, 3.53) |
| Admitted not on transfer | 0.43 (0.34, 0.50) | 0.35 (0.28, 0.43) | 0.30 (0.23, 0.40) |
| 1‐5 years | |||
| Admitted as transfer | 2.26 (1.61, 2.19) | 1.59 (1.20, 1.98) | 1.33 (0.97, 1.83) |
| Admitted not on transfer | 0.33 (0.25, 0.40) | 0.27 (0.22, 0.33) | 0.27 (0.22, 0.33) |
| 6‐10 years | |||
| Admitted as transfer | 2.01 (1.23, 2.96) | 1.48 (0.92, 2.03) | 1.11 (0.83, 1.49) |
| Admitted not on transfer | 0.32 (0.26, 0.39) | 0.28 (0.22, 0.34) | 0.29 (0.24, 0.36) |
| 11‐17 years | |||
| Admitted as transfer | 1.87 (1.42, 2.33) | 1.09 (0.81, 1.38) | 1.33 (1.02, 1.73) |
| Admitted not on transfer | 0.30 (0.25, 0.35) | 0.30 (0.25, 0.34) | 0.32 (0.27, 0.37) |
DRGs were evaluated based on transfer status, mortality, and study year. The most common DRGs for survivors were generally consistent across years and transfer status: neonate, bronchitis and asthma, pneumonia, esophagitis/gastroenteritis, nutritional and metabolic disturbances, and vaginal delivery. Among decedents, the primary diagnoses also included neonate, but in contrast with survivors were more likely to include traumatic injury, cardiothoracic surgery/medical care (ie, for congenital cardiac/valve disease), respiratory diagnosis with ventilatory support, and craniotomy. DRGs for decedents were consistent across years and transfer status (data available upon request to the authors).
DRGs were also evaluated based by payer status across all 3 study years (data not shown). The most common DRGs showed no meaningful differences in the types of conditions for children who were transferred versus not, across all payer types (including uninsured children).
Length of Stay and Hospital Charges, by Survival, Payer, and Transfer Status
Table 4 illustrates the national patterns of mean length of stay by age, survival, and transfer status. Data for 2002 are shown; the other study years had very similar findings and are available from the authors.
| Admitted on Transfer (95% CI) | Admitted Not on Transfer (95% CI) | |||
|---|---|---|---|---|
| Alive | Died | Alive | Died | |
| Age | ||||
| Newborn | 16.9 (14.7‐19.0) | 19.6 (15.1‐24.0) | 3.2 (3.0‐3.3) | 8.3 (6.9‐9.7) |
| 0‐<1year | 11.3 (9.1‐13.0) | 24.8 (18.8‐30.8) | 3.5 (3.2‐3.8) | 20.1 (12.8‐27.5) |
| 1‐5 years | 4.8 (4.2‐5.6) | 16.0 (8.5‐23.4) | 3.0 (3.4‐4.0) | 12.7 (7.2‐18.2) |
| 6‐10 years | 6.4 (4.7‐8.2) | 12.9 (4.9‐20.8) | 3.7 (3.4‐4.0) | 13.8 (9.7‐17.8) |
| 11‐17 years | 8.0 (6.0‐10.0) | 8.8 (5.8‐11.7) | 4.0 (3.7‐4.3) | 10.2 (6.4‐14.0) |
| Payer | ||||
| Medicaid | 11.4 (9.7‐13.1) | 21.8 (16.2‐27.4) | 3.5 (3.4‐3.7) | 11.2 (9.2‐13.3) |
| Private | 9.7 (8.6‐10.7) | 17.1 (13.5‐20.7) | 3.1 (3.0‐3.2) | 9.3 (7.4‐11.1) |
| Uninsured | 7.0 (4.8‐9.2) | 5.3 (1.1‐9.5) | 2.8 (2.6‐3.1) | 3.1 (1.2‐5.0) |
Length of stay differed significantly by transfer and survival status, and also varied significantly by insurance coverage. In 2002, among children who were admitted not on transfer, those who died had significantly longer mean length stay than those who survived. Among children admitted as a transfer, for all but non‐newborn infants and those 15 years of age, length of stay did not differ significantly by survival status.
For children covered by Medicaid and private insurance, decedents had significantly longer length of stay compared to survivors, regardless of transfer status. However, this was not the case for uninsured children, for whom those who died and those who survived had statistically indistinguishable lengths of stay, within the transfer/non‐ transfer groups. Findings for 1997 and 1992 were similar (data not shown).
Mean hospital charges are presented in Table 5. For children covered by Medicaid and private insurance, among patients who were admitted not on transfer, those who died had more than 8‐fold greater charges than those who survived. A similar trend was seen for patients admitted on transfer who were covered by Medicaid and private insurance, with more than 3‐fold greater charges for those who died versus those who survived. In contrast, for uninsured children, those who were admitted not on transfer and died had only 3.5‐fold greater charges compared to survivors, and those who were admitted on transfer and died had only 2‐fold greater charges compared to survivors.
| Admitted on Transfer (95% CI) | Admitted Not on Transfer (95% CI) | |||
|---|---|---|---|---|
| Alive | Died | Alive | Died | |
| Payer | ||||
| Medicaid | 43,123 (34,570‐51,675) | 141,280 (104,881‐177,679) | 8,456 (7,3489‐9,564) | 73,798 (59,71‐87,884) |
| Private | 41,037 (33,420‐48,653) | 142,739 (110,122‐175,355) | 7,519 (6,597‐8,441) | 62,195 (50,722‐73,667) |
| Uninsured | 21,228 (15,389‐27,068) | 48,036 (28,974‐67,099) | 5,591 (4,372‐6,810) | 19,910 (13,342‐26,479) |
DISCUSSION
Children's Inpatient Mortality
This is the first study of which we are aware that examines EOL hospitalization patterns for children in a national sample, spanning a decade. Our data revealed that the pediatric inpatient mortality rate is consistently highest among children in the non‐newborn infant age group over this time period, and that the burden of mortality is persistently greatest among newborns. These age‐specific findings are consistent with vital statistics published separately for each of the study years regarding overall childhood mortality.1820
This study highlights what many health care providers may not recognize: to meet the needs of the greatest numbers of families with gravely ill children, EOL care efforts must focus on the very youngest. Many of these children may not have chronic conditions, which have been a central focus of many pediatric EOL efforts to date. In fact, the parents of most gravely ill children in the hospital may have had just a few days or hours to prepare to face the loss of their children.
In addition, children admitted on interhospital transfer are significantly more likely to die while hospitalized. This pattern likely represents referral of severely ill children to medical centers that offer tertiary and quaternary specialty care, rather than risks associated with the transfer event itself. Some parents and their children may be far away from home and their closest networks of social support.7 Overall, these findings strongly indicate that EOL efforts will meet the needs of greater proportions of parents if they actively incorporate considerations of age and transfer status as institutions reach out to families in need of support.
Of note, this analysis does not capture children who were discharged into hospice, or long‐term care facilities, or who may have been discharged to home and may have died thereafter. Discharge disposition is known to vary by age, with older children with chronic conditions being more likely to use hospice services compared with infants.8 A recent study suggests that deaths outside the hospital have become increasingly common for older children over time, with the expansion of EOL supportive services in communities to meet the needs of families with gravely ill children.8
Length of Stay, Hospital Charges, and Mortality Related to Insurance Status
In this study, insured children who were admitted and died had significantly longer hospital stays compared to uninsured children who were admitted and died. DRG diagnoses by payer were very similar among children who died, although it is possible that differences in length of stay by payer status may reflect differences in severity of illness at admission and/or processes of care during hospitalization, which could not be fully accounted for using diagnostic codes. Hospitalizations that ended in death were significantly more expensive than hospitalizations in which children survived to discharge, regardless of age, payer status, or transfer status. However, incremental differences in spending for those who died versus those who survived were much greater for children with health insurance than for children without, suggesting greater resource utilization for children with coverage. Resource utilization is reflected largely in length of stay, which explains why our findings for differences in length of stay were echoed so strongly in our findings regarding differences in hospital charges.
Several studies of EOL care for adults have indicated that uninsured patients sustained higher inpatient mortality and lower hospital resource use versus insured adults, across similar diagnoses.13, 2123 Among children, Braveman and colleagues found differences in hospital resource allocation among sick newborns according to insurance coverage that are echoed in the findings of our study.24 Sick newborns without insurance received fewer inpatient services, with statistically significant shorter length of stay and total charges compared to insured newborns. In our study, disparities related to insurance coverage were consistent over the decade considered, and likely indicate ongoing challenges of broad disparities in access to care for children related to insurance coverage in the US health care system. Perhaps the greatest disparity was in mortality itself, which was highest among the uninsured, although the gap in mortality rates by insurance status appeared narrower in 2002 than in the prior study years.
Mortality Rates by Transfer Status
Mortality rates stratified by transfer status revealed that children transferred between hospitals had a significantly higher mortality rate, compared to children admitted not on transfer. Literature evaluating adult intensive care units found that transferred patients have more comorbid conditions, greater severity of illness, and 1.4‐fold to 2.5‐fold higher hospital mortality rates compared to direct admissions.25 Similar challenges face pediatric patients who are transferred to intensive care settings, where children at higher clinical risk have a higher morality rate and utilize greater resources compared with less critically ill children.14 Hospital EOL support personnel must be cognizant of the high mortality rate for transferred patients, and services may need to be adjusted to address the needs of these families. Additionally, further research is needed to better understand and remedy these potential disparities in care for children based on insurance status.
Limitations
This study is potentially limited by the accuracy of hospital discharge data, which may have influenced our outcomes. Further, not all states participate in the NIS; 11 states participated in 1992, 22 states participated in 1997, and 35 states participated in 2002. Although NIS data are weighted to be nationally representative in each year, it is possible that the participating states may have differed in systematic ways from nonparticipating states. However, the external validity of our data with regard to patterns of mortality by age and diagnoses, and the stability of patterns across a span of several years, suggest strongly that our findings are likely robust to these potential biases in this dataset.
As with any hospital resource use data, we are mindful that the distribution of data regarding length of stay and charges are typically right‐skewed, and therefore mean values should be interpreted with caution. In using mean values to test our hypotheses, we have followed the standard method of comparison for nationally weighted data.16
CONCLUSION
This national study of inpatient mortality patterns among US children over the span of a decade presents a new framework of challenges to clinicians and investigators regarding EOL care for children. As health care providers and institutions expand their efforts to meet the needs of severely ill children and their families, such efforts must be cognizant of the high burden of mortality among the youngest children, as well as those who are transferred between hospitals, and children without insurance coverage. These children and their families may require expanded EOL care and support services, beyond those typically available in most hospitals and communities.
APPENDIX
DIAGNOSIS‐RELATED GROUPS BY TRANSFER AND SURVIVAL STATUS
| 1992 | % | 1997 | % | 2002 | % |
|---|---|---|---|---|---|
| |||||
| Transferred ‐ Survived | |||||
| Neonate* | 26.2 | Neonate* | 23.2 | Neonate* | 24.6 |
| Bronchitis and Asthma | 6.4 | Bronchitis and Asthma | 7.4 | Bronchitis and Asthma | 8.0 |
| Seizure and Headache | 3.7 | Simple Pneumonia | 3.3 | Seizure and Headache | 4.2 |
| Simple Pneumonia | 3.4 | Seizure and Headache | 3.2 | Simple Pneumonia | 3.7 |
| Esophagitis and Gastroenteritis | 3.0 | Psychoses | 3.2 | Esophagitis and Gastroenteritis | 3.0 |
| Transferred ‐ Died | |||||
| Neonate | 35.1 | Neonate | 38.2 | Neonate | 40.5 |
| Cardiac Disease and/or Cardiothoracic surgery | 9.6 | Cardiac Disease and/or Cardiothoracic surgery | 12.2 | Cardiac Disease and/or Cardiothoracic surgery | 10.9 |
| Respiratory diagnosis with ventilatory support | 6.8 | Respiratory diagnosis with ventilatory support | 7.7 | Respiratory diagnosis with ventilatory support | 7.0 |
| Craniotomy | 3.5 | Septicemia | 2.8 | Injury, Poisoning | 2.4 |
| Injury, Poisoning | 3.3 | Tracheostomy with ventilatory support | 2.8 | Craniotomy | 2.2 |
| Not Transferred ‐ Survived | |||||
| Neonate* | 60.6 | Neonate* | 63 | Neonate* | 66.4 |
| Bronchitis and Asthma | 4.9 | Bronchitis and Asthma | 5.3 | Bronchitis and Asthma | 4.7 |
| Esophagitis and Gastroenteritis | 3.1 | Simple Pneumonia | 2.9 | Simple Pneumonia | 2.5 |
| Simple Pneumonia | 2.7 | Esophagitis and Gastroenteritis | 2.6 | Esophagitis and Gastroenteritis | 2.0 |
| Vaginal Delivery | 2.2 | Vaginal Delivery | 2.3 | Nutritional and Metabolic Disorder | 1.8 |
| Not Transferred ‐ Died | |||||
| Neonate | 61.5 | Neonate | 66.2 | Neonate | 69.0 |
| Traumatic Coma or Operative Procedure for Traumatic Injury | 3.3 | Traumatic Coma or Operative Procedure for Traumatic Injury | 4.8 | Traumatic Coma or Operative Procedure for Traumatic Injury | 4.7 |
| Cardiac Disease and/or Cardiothoracic surgery | 2.9 | Cardiac Disease and/or Cardiothoracic surgery | 2.7 | Respiratory diagnosis with ventilatory support | 2.7 |
| Craniotomy | 2.3 | Respiratory diagnosis with ventilatory support | 2.5 | Craniotomy | 2.4 |
| Respiratory diagnosis with ventilatory support | 2.0 | Septicemia | 1.4 | Septicemia | 1.2 |
- ,,,,,.Annual summary of vital statistics: 2005.Pediatrics.2007;119(2):345–360.
- ,,,,,.Circumstances surrounding the deaths of hospitalized children: opportunities for pediatric palliative care.Pediatrics.2004;114(3):e361–e366.
- ,,,,,.Characteristics of deaths occurring in children's hospitals: implications for supportive care services.Pediatrics.2002;109(5):887–893.
- ,,,,,.Declining severity adjusted mortality: evidence of improving neonatal intensive care.Pediatrics.1998;102(4):893–899.
- ,,, et al.Use of intensive care at the end of life in the United States: an epidemiologic study.Crit Care Med.2004;32(3):638–643.
- ,,,,.Cancer‐related deaths in children and adolescents.J Palliat Med.2005;8(1):86–95.
- ,,.Where do children with complex chronic conditions die? Patterns in Washington State, 1980‐1998.Pediatrics.2002;109(4):656–660.
- ,,,,,.Deaths attributed to pediatric complex chronic conditions: national trends and implications for supportive care services.Pediatrics.2001;107(6):e99.
- ,,, et al.Medical end‐of‐life decisions for children in the Netherlands.Arch Pediatr Adolescent Med.005;159(9):802–809.
- ,,,.Medicare beneficiaries' costs of care in the last year of life.Health Affairs.2001;20(4):188–195.
- ,,,,.Medicare Beneficiaries' Costs and Use of Care in the Last Year of Life.Washington, DC:MedPAC;2000.
- ,.Trends in Medicare payments in the last year of life.N Engl J Med.1993;328(15):1092–1096.
- ,,.Comparing uninsured and privately insured hospital patients: admission severity, health outcomes and resource use.Health Serv Manage Res.2001;14(3):203–210.
- ,,, et al.Characteristics and outcomes of interhospital transfers from level II to level I pediatric intensive care units.Pediatr Crit Care Med.2006;7(6):536–540.
- Agency for Healthcare Research and Quality. National Inpatient Sample (NIS). Healthcare Cost and Utilization Project (HCUP). http://www.hcup‐us.ahrq.gov/nisoverview.jsp. Accessed August 26,2008.
- Agency for Healthcare Research and Quality,. Healthcare Cost and Utilization Project H CUP. Care of Children and Adolescents in U.S. Hospitals. HCUP Fact Book No. 4, Publication No. 04‐0004. http://www.ahrq.gov/data/hcup/factbk4/. Accessed August 26,2008.
- U.S. Department of Labor, Bureau of Labor Statistics. Consumer Price Index.ftp://ftp.bls.gov/pub/special.requests/cpi/cpiai.txt. Accessed August 26,2008.
- ,,,.Annual summary of vital statistics‐‐2002.Pediatrics.2003;112(6):1215–1230.
- ,,,,.Annual summary of vital statistics‐‐1996.Pediatrics.1997;100(6):905–918.
- .Annual summary of vital statistics‐‐1992.Pediatrics.1993;92(6):743–754.
- ,.Acutely injured patients with trauma in Massachusetts: differences in care and mortality, by insurance status.Am J Publ Health.1994;84(10):1605–1608.
- ,,.Comparison of uninsured and privately insured hospital patients. Condition on admission, resource use, and outcome.JAMA.1991;265(3):374–379.
- ,.Inequities in hospital care, the Massachusetts experience.Inquiry.1991;28(3):255–262.
- ,,,.Differences in hospital resource allocation among sick newborns according to insurance coverage.JAMA.1991;266(23):3300–3308.
- ,,,,.Accepting critically ill transfer patients: adverse effect on a referral center's outcome and benchmark measures. [summary for patients in Ann Intern Med. 2003;138(11):I42; PMID: 12779311].Ann Intern Med.2003;138(11):882–890.
- ,,,,,.Annual summary of vital statistics: 2005.Pediatrics.2007;119(2):345–360.
- ,,,,,.Circumstances surrounding the deaths of hospitalized children: opportunities for pediatric palliative care.Pediatrics.2004;114(3):e361–e366.
- ,,,,,.Characteristics of deaths occurring in children's hospitals: implications for supportive care services.Pediatrics.2002;109(5):887–893.
- ,,,,,.Declining severity adjusted mortality: evidence of improving neonatal intensive care.Pediatrics.1998;102(4):893–899.
- ,,, et al.Use of intensive care at the end of life in the United States: an epidemiologic study.Crit Care Med.2004;32(3):638–643.
- ,,,,.Cancer‐related deaths in children and adolescents.J Palliat Med.2005;8(1):86–95.
- ,,.Where do children with complex chronic conditions die? Patterns in Washington State, 1980‐1998.Pediatrics.2002;109(4):656–660.
- ,,,,,.Deaths attributed to pediatric complex chronic conditions: national trends and implications for supportive care services.Pediatrics.2001;107(6):e99.
- ,,, et al.Medical end‐of‐life decisions for children in the Netherlands.Arch Pediatr Adolescent Med.005;159(9):802–809.
- ,,,.Medicare beneficiaries' costs of care in the last year of life.Health Affairs.2001;20(4):188–195.
- ,,,,.Medicare Beneficiaries' Costs and Use of Care in the Last Year of Life.Washington, DC:MedPAC;2000.
- ,.Trends in Medicare payments in the last year of life.N Engl J Med.1993;328(15):1092–1096.
- ,,.Comparing uninsured and privately insured hospital patients: admission severity, health outcomes and resource use.Health Serv Manage Res.2001;14(3):203–210.
- ,,, et al.Characteristics and outcomes of interhospital transfers from level II to level I pediatric intensive care units.Pediatr Crit Care Med.2006;7(6):536–540.
- Agency for Healthcare Research and Quality. National Inpatient Sample (NIS). Healthcare Cost and Utilization Project (HCUP). http://www.hcup‐us.ahrq.gov/nisoverview.jsp. Accessed August 26,2008.
- Agency for Healthcare Research and Quality,. Healthcare Cost and Utilization Project H CUP. Care of Children and Adolescents in U.S. Hospitals. HCUP Fact Book No. 4, Publication No. 04‐0004. http://www.ahrq.gov/data/hcup/factbk4/. Accessed August 26,2008.
- U.S. Department of Labor, Bureau of Labor Statistics. Consumer Price Index.ftp://ftp.bls.gov/pub/special.requests/cpi/cpiai.txt. Accessed August 26,2008.
- ,,,.Annual summary of vital statistics‐‐2002.Pediatrics.2003;112(6):1215–1230.
- ,,,,.Annual summary of vital statistics‐‐1996.Pediatrics.1997;100(6):905–918.
- .Annual summary of vital statistics‐‐1992.Pediatrics.1993;92(6):743–754.
- ,.Acutely injured patients with trauma in Massachusetts: differences in care and mortality, by insurance status.Am J Publ Health.1994;84(10):1605–1608.
- ,,.Comparison of uninsured and privately insured hospital patients. Condition on admission, resource use, and outcome.JAMA.1991;265(3):374–379.
- ,.Inequities in hospital care, the Massachusetts experience.Inquiry.1991;28(3):255–262.
- ,,,.Differences in hospital resource allocation among sick newborns according to insurance coverage.JAMA.1991;266(23):3300–3308.
- ,,,,.Accepting critically ill transfer patients: adverse effect on a referral center's outcome and benchmark measures. [summary for patients in Ann Intern Med. 2003;138(11):I42; PMID: 12779311].Ann Intern Med.2003;138(11):882–890.
Copyright © 2008 Society of Hospital Medicine