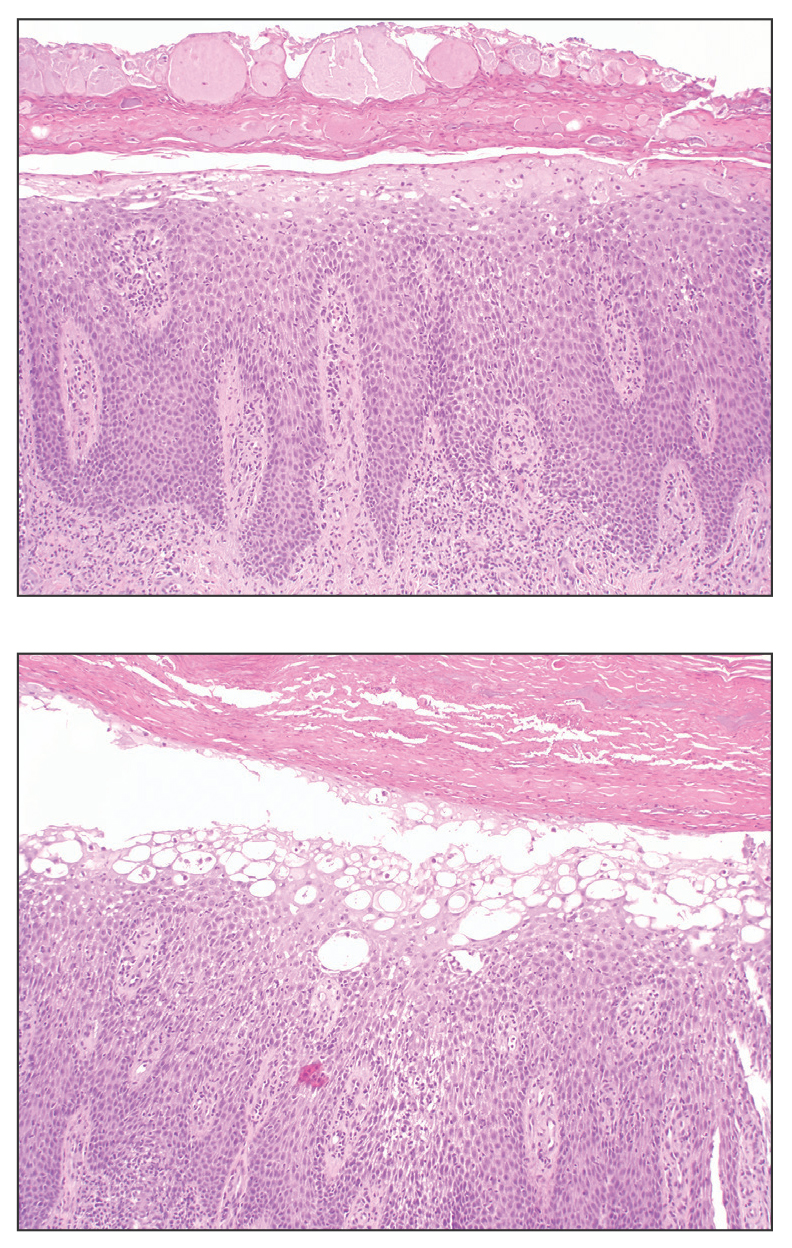
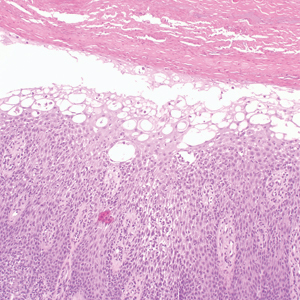
User login
EULAR 2024 Preview: Therapeutics in Development Take Center Stage
The European Alliance of Associations for Rheumatology (EULAR) 2024 European Congress of Rheumatology annual meeting is about to take place in Vienna, Austria. From June 12 to 15, some of the world’s leading researchers and clinicians will convene to present and learn about data on some of the new and innovative treatments for people with rheumatic and musculoskeletal diseases (RMDs) as well as to discuss how to use and optimize existing approaches.
Ahead of the Congress, this news organization asked the Congress Committee’s Scientific Programme Chair Caroline Ospelt, MD, PhD, and Abstract Chair Christian Dejaco, MD, PhD, MBA, to discuss some of their highlights of this year’s meeting.
From Bench to Bedside
“For me, the beauty at EULAR is really that you have the latest on basic research, how this can be translated in clinical trials, and then the last step would be how EULAR recommends it to be used in clinical practice,” Dr. Ospelt, professor of experimental rheumatology at University Hospital Zurich, said in an interview.
“So, if you go to EULAR continuously, you can actually follow the whole story of how novelty comes into clinical practice,” she added.
In a separate interview, Dr. Dejaco, a consultant rheumatologist and associate professor at the Medical University of Graz in Austria, said: “There are several new drug trials that are going to be presented.”
One of his highlights on the use of new drugs for the treatment of giant cell arteritis will be the phase 3 SELECT-GCA trial of the Janus kinase (JAK) inhibitor upadacitinib (LBA0001).
“It’s a trial that hopefully will lead to the approval of this drug in this indication,” Dr. Dejaco said.
Late-Breaking Abstracts
Dr. Ospelt noted: “We had a lot of good late-breaking abstracts this year.”
Some of these include:
- Real-world data on the comparative effectiveness of five different classes of drugs used to treat psoriatic arthritis (PsA; LBA0002)
- The 16-week results of a phase 2b/3 study with the novel interleukin (IL)–17A inhibitor izokibep in people with PsA (LBA0005)
- Data from the COSPIRIT-JIA trial on the efficacy and safety of ixekizumab (Taltz) in juvenile idiopathic arthritis (LBA0009)
- Phase 2 data on the safety and efficacy of the CD38-targeting monoclonal antibody daratumumab in systemic lupus erythematosus (LBA0007)
- Results of the phase 2 DAHLIAS study of the anti–neonatal Fc receptor monoclonal antibody nipocalimab in people with primary Sjögren disease (LBA0010)
- Safety and immunogenicity data from a phase 1 study of an active anti–IL-6 immunotherapy in people with knee osteoarthritis (LBA0011)
The latter is “really interesting,” Dr. Ospelt said. As of now, there is no approved treatment for osteoarthritis, and there is no immunotherapy, “so this would be the first.”
But it’s not just the late-breaker abstracts to look out for. Dr. Dejaco highlighted two abstracts that will be presented during the Abstract Plenary:
- A phase 3 study of a new selective JAK1 inhibitor, SHR0302, in rheumatoid arthritis (OP0037)
- A multi-omics analysis and targeted gene-editing study in people with , which causes inflammatory and hematologic changes (OP0073)
Of the latter, he said, “this disease is still incompletely understood, and this abstract really helps to better understand the mechanisms underlying this disease.”
One to Watch: CAR T-Cell Therapy
Dr. Ospelt said that the scientific program is about 80% clinical and 20% basic science overall. However, more sessions are being held jointly because data are starting to move from the bench to bedside.
One of the basic science areas that has had “a real buzz” around it and is now producing results in the clinic is the use of chimeric antigen receptor (CAR) T cells. In one of the first, and perhaps aptly titled What Is New, or WIN, sessions of the congress, Georg Schett, MD, vice president of research at Friedrich-Alexander-Universität Erlangen-Nüremberg in Germany, will discuss the use of CAR T-cell therapy for inflammatory RMDs. There are also multiple abstract presentations on this topic.
In-depth tissue analysis and prediction of treatment response is another interesting approach, Dr. Ospelt said. “I think that’s the way to go, that we come from the blood, we go into the tissue.” A “very nice” example of this approach will be presented during the Abstract Plenary session on Wednesday, June 12, looking at how synovial tissue macrophages may be able to give information on likely treatment response in treatment-naive rheumatoid arthritis (OP0062). There are also some further findings related to the tissue biopsy–driven treatment trial R4RA that are being presented at the meeting (OP0218, OP0242, and POS0351).
EULAR Highlighted Sessions
Among the highlighted sessions on the EULAR 2024 website is one on axial involvement in PsA and spondyloarthritis (SpA).
“Axial involvement in psoriatic arthritis and peripheral involvement in axial spondyloarthritis is quite a hot topic at the moment,” Dr. Ospelt said. There are lots of questions: “How connected are they? How different are they? Do we need different treatment for axial involvement compared to peripheral involvement?”
Another EULAR highlighted session is the 75th anniversary of glucocorticoid treatment, during which Past President of EULAR and Emeritus Professor of Rheumatology Josef S. Smolen, MD, will overview the “past, present, and future” of glucocorticoids in RMDs. Consultant rheumatologist Frank Buttgereit, MD, from the German Rheumatism Research Center in Berlin, will discuss the practicalities of using these drugs in clinical practice.
Dr. Dejaco noted: “Glucocorticoids have been one of the most important treatments for a very long time, and they’re still the most important treatment for the acute treatment of systemic inflammatory diseases.”
For a long time, there was no alternative to using steroids, he added, but steroid-sparing options now exist, and there will be data presented on a new type of drug that could potentially be used to control cortisol levels in the body (OP0335).
Recommendations and More
Dr. Ospelt and Dr. Dejaco both pointed out other sessions that are likely to be very popular, such as the first and second EULAR Recommendations sessions, a session on rheumatoid arthritis prevention, as well as the many presentations and sessions on digital health and nonpharmacologic interventions such as exercise.
With over 5242 submitted abstracts, there is going to be no shortage of data being presented at EULAR 2024. Alongside the traditional abstract submission categories, this year there is a new clinical case reports category.
“We had about 578 submissions for that category,” Dr. Dejaco said. There were 3315 abstracts submitted for the clinical research category, 812 for the basic and translational research category, 283 from health professionals in rheumatology, 152 from patient groups, and 102 in the field of pediatric rheumatology.
Join in On-Site, Watch on Demand
EULAR 2024 reverts to an on-site–only meeting this year. Some of the more lighthearted yet educational elements of the program for those attending include the second edition of the EMEUNET Rheumatology Quiz and, new for this year, two escape rooms. These rooms will provide an interactive experience where small teams will have to solve rheumatologic conundrums in order to escape the room within the hour, Dr. Dejaco explained. There will also be a morning run on Friday, June 14. “It’s not a race, it’s simply to meet and run together,” Dr. Dejaco said.
But if you cannot make the congress in person, the EULAR 2024 Livestream will be broadcasting throughout the congress. Anyone registered by June 30 will have on-demand access to the recorded content from June 17 until December 31, 2024.
Abstracts for the meeting will be published as a supplement to Annals of the Rheumatic Diseases, the official journal of EULAR.
Dr. Ospelt reported no relevant financial relationships. Dr. Dejaco has received consulting/speaker fees from AbbVie, Eli Lilly, Janssen, Sparrow, Novartis, Pfizer, Roche, Galapagos, and Sanofi.
A version of this article appeared on Medscape.com.
The European Alliance of Associations for Rheumatology (EULAR) 2024 European Congress of Rheumatology annual meeting is about to take place in Vienna, Austria. From June 12 to 15, some of the world’s leading researchers and clinicians will convene to present and learn about data on some of the new and innovative treatments for people with rheumatic and musculoskeletal diseases (RMDs) as well as to discuss how to use and optimize existing approaches.
Ahead of the Congress, this news organization asked the Congress Committee’s Scientific Programme Chair Caroline Ospelt, MD, PhD, and Abstract Chair Christian Dejaco, MD, PhD, MBA, to discuss some of their highlights of this year’s meeting.
From Bench to Bedside
“For me, the beauty at EULAR is really that you have the latest on basic research, how this can be translated in clinical trials, and then the last step would be how EULAR recommends it to be used in clinical practice,” Dr. Ospelt, professor of experimental rheumatology at University Hospital Zurich, said in an interview.
“So, if you go to EULAR continuously, you can actually follow the whole story of how novelty comes into clinical practice,” she added.
In a separate interview, Dr. Dejaco, a consultant rheumatologist and associate professor at the Medical University of Graz in Austria, said: “There are several new drug trials that are going to be presented.”
One of his highlights on the use of new drugs for the treatment of giant cell arteritis will be the phase 3 SELECT-GCA trial of the Janus kinase (JAK) inhibitor upadacitinib (LBA0001).
“It’s a trial that hopefully will lead to the approval of this drug in this indication,” Dr. Dejaco said.
Late-Breaking Abstracts
Dr. Ospelt noted: “We had a lot of good late-breaking abstracts this year.”
Some of these include:
- Real-world data on the comparative effectiveness of five different classes of drugs used to treat psoriatic arthritis (PsA; LBA0002)
- The 16-week results of a phase 2b/3 study with the novel interleukin (IL)–17A inhibitor izokibep in people with PsA (LBA0005)
- Data from the COSPIRIT-JIA trial on the efficacy and safety of ixekizumab (Taltz) in juvenile idiopathic arthritis (LBA0009)
- Phase 2 data on the safety and efficacy of the CD38-targeting monoclonal antibody daratumumab in systemic lupus erythematosus (LBA0007)
- Results of the phase 2 DAHLIAS study of the anti–neonatal Fc receptor monoclonal antibody nipocalimab in people with primary Sjögren disease (LBA0010)
- Safety and immunogenicity data from a phase 1 study of an active anti–IL-6 immunotherapy in people with knee osteoarthritis (LBA0011)
The latter is “really interesting,” Dr. Ospelt said. As of now, there is no approved treatment for osteoarthritis, and there is no immunotherapy, “so this would be the first.”
But it’s not just the late-breaker abstracts to look out for. Dr. Dejaco highlighted two abstracts that will be presented during the Abstract Plenary:
- A phase 3 study of a new selective JAK1 inhibitor, SHR0302, in rheumatoid arthritis (OP0037)
- A multi-omics analysis and targeted gene-editing study in people with , which causes inflammatory and hematologic changes (OP0073)
Of the latter, he said, “this disease is still incompletely understood, and this abstract really helps to better understand the mechanisms underlying this disease.”
One to Watch: CAR T-Cell Therapy
Dr. Ospelt said that the scientific program is about 80% clinical and 20% basic science overall. However, more sessions are being held jointly because data are starting to move from the bench to bedside.
One of the basic science areas that has had “a real buzz” around it and is now producing results in the clinic is the use of chimeric antigen receptor (CAR) T cells. In one of the first, and perhaps aptly titled What Is New, or WIN, sessions of the congress, Georg Schett, MD, vice president of research at Friedrich-Alexander-Universität Erlangen-Nüremberg in Germany, will discuss the use of CAR T-cell therapy for inflammatory RMDs. There are also multiple abstract presentations on this topic.
In-depth tissue analysis and prediction of treatment response is another interesting approach, Dr. Ospelt said. “I think that’s the way to go, that we come from the blood, we go into the tissue.” A “very nice” example of this approach will be presented during the Abstract Plenary session on Wednesday, June 12, looking at how synovial tissue macrophages may be able to give information on likely treatment response in treatment-naive rheumatoid arthritis (OP0062). There are also some further findings related to the tissue biopsy–driven treatment trial R4RA that are being presented at the meeting (OP0218, OP0242, and POS0351).
EULAR Highlighted Sessions
Among the highlighted sessions on the EULAR 2024 website is one on axial involvement in PsA and spondyloarthritis (SpA).
“Axial involvement in psoriatic arthritis and peripheral involvement in axial spondyloarthritis is quite a hot topic at the moment,” Dr. Ospelt said. There are lots of questions: “How connected are they? How different are they? Do we need different treatment for axial involvement compared to peripheral involvement?”
Another EULAR highlighted session is the 75th anniversary of glucocorticoid treatment, during which Past President of EULAR and Emeritus Professor of Rheumatology Josef S. Smolen, MD, will overview the “past, present, and future” of glucocorticoids in RMDs. Consultant rheumatologist Frank Buttgereit, MD, from the German Rheumatism Research Center in Berlin, will discuss the practicalities of using these drugs in clinical practice.
Dr. Dejaco noted: “Glucocorticoids have been one of the most important treatments for a very long time, and they’re still the most important treatment for the acute treatment of systemic inflammatory diseases.”
For a long time, there was no alternative to using steroids, he added, but steroid-sparing options now exist, and there will be data presented on a new type of drug that could potentially be used to control cortisol levels in the body (OP0335).
Recommendations and More
Dr. Ospelt and Dr. Dejaco both pointed out other sessions that are likely to be very popular, such as the first and second EULAR Recommendations sessions, a session on rheumatoid arthritis prevention, as well as the many presentations and sessions on digital health and nonpharmacologic interventions such as exercise.
With over 5242 submitted abstracts, there is going to be no shortage of data being presented at EULAR 2024. Alongside the traditional abstract submission categories, this year there is a new clinical case reports category.
“We had about 578 submissions for that category,” Dr. Dejaco said. There were 3315 abstracts submitted for the clinical research category, 812 for the basic and translational research category, 283 from health professionals in rheumatology, 152 from patient groups, and 102 in the field of pediatric rheumatology.
Join in On-Site, Watch on Demand
EULAR 2024 reverts to an on-site–only meeting this year. Some of the more lighthearted yet educational elements of the program for those attending include the second edition of the EMEUNET Rheumatology Quiz and, new for this year, two escape rooms. These rooms will provide an interactive experience where small teams will have to solve rheumatologic conundrums in order to escape the room within the hour, Dr. Dejaco explained. There will also be a morning run on Friday, June 14. “It’s not a race, it’s simply to meet and run together,” Dr. Dejaco said.
But if you cannot make the congress in person, the EULAR 2024 Livestream will be broadcasting throughout the congress. Anyone registered by June 30 will have on-demand access to the recorded content from June 17 until December 31, 2024.
Abstracts for the meeting will be published as a supplement to Annals of the Rheumatic Diseases, the official journal of EULAR.
Dr. Ospelt reported no relevant financial relationships. Dr. Dejaco has received consulting/speaker fees from AbbVie, Eli Lilly, Janssen, Sparrow, Novartis, Pfizer, Roche, Galapagos, and Sanofi.
A version of this article appeared on Medscape.com.
The European Alliance of Associations for Rheumatology (EULAR) 2024 European Congress of Rheumatology annual meeting is about to take place in Vienna, Austria. From June 12 to 15, some of the world’s leading researchers and clinicians will convene to present and learn about data on some of the new and innovative treatments for people with rheumatic and musculoskeletal diseases (RMDs) as well as to discuss how to use and optimize existing approaches.
Ahead of the Congress, this news organization asked the Congress Committee’s Scientific Programme Chair Caroline Ospelt, MD, PhD, and Abstract Chair Christian Dejaco, MD, PhD, MBA, to discuss some of their highlights of this year’s meeting.
From Bench to Bedside
“For me, the beauty at EULAR is really that you have the latest on basic research, how this can be translated in clinical trials, and then the last step would be how EULAR recommends it to be used in clinical practice,” Dr. Ospelt, professor of experimental rheumatology at University Hospital Zurich, said in an interview.
“So, if you go to EULAR continuously, you can actually follow the whole story of how novelty comes into clinical practice,” she added.
In a separate interview, Dr. Dejaco, a consultant rheumatologist and associate professor at the Medical University of Graz in Austria, said: “There are several new drug trials that are going to be presented.”
One of his highlights on the use of new drugs for the treatment of giant cell arteritis will be the phase 3 SELECT-GCA trial of the Janus kinase (JAK) inhibitor upadacitinib (LBA0001).
“It’s a trial that hopefully will lead to the approval of this drug in this indication,” Dr. Dejaco said.
Late-Breaking Abstracts
Dr. Ospelt noted: “We had a lot of good late-breaking abstracts this year.”
Some of these include:
- Real-world data on the comparative effectiveness of five different classes of drugs used to treat psoriatic arthritis (PsA; LBA0002)
- The 16-week results of a phase 2b/3 study with the novel interleukin (IL)–17A inhibitor izokibep in people with PsA (LBA0005)
- Data from the COSPIRIT-JIA trial on the efficacy and safety of ixekizumab (Taltz) in juvenile idiopathic arthritis (LBA0009)
- Phase 2 data on the safety and efficacy of the CD38-targeting monoclonal antibody daratumumab in systemic lupus erythematosus (LBA0007)
- Results of the phase 2 DAHLIAS study of the anti–neonatal Fc receptor monoclonal antibody nipocalimab in people with primary Sjögren disease (LBA0010)
- Safety and immunogenicity data from a phase 1 study of an active anti–IL-6 immunotherapy in people with knee osteoarthritis (LBA0011)
The latter is “really interesting,” Dr. Ospelt said. As of now, there is no approved treatment for osteoarthritis, and there is no immunotherapy, “so this would be the first.”
But it’s not just the late-breaker abstracts to look out for. Dr. Dejaco highlighted two abstracts that will be presented during the Abstract Plenary:
- A phase 3 study of a new selective JAK1 inhibitor, SHR0302, in rheumatoid arthritis (OP0037)
- A multi-omics analysis and targeted gene-editing study in people with , which causes inflammatory and hematologic changes (OP0073)
Of the latter, he said, “this disease is still incompletely understood, and this abstract really helps to better understand the mechanisms underlying this disease.”
One to Watch: CAR T-Cell Therapy
Dr. Ospelt said that the scientific program is about 80% clinical and 20% basic science overall. However, more sessions are being held jointly because data are starting to move from the bench to bedside.
One of the basic science areas that has had “a real buzz” around it and is now producing results in the clinic is the use of chimeric antigen receptor (CAR) T cells. In one of the first, and perhaps aptly titled What Is New, or WIN, sessions of the congress, Georg Schett, MD, vice president of research at Friedrich-Alexander-Universität Erlangen-Nüremberg in Germany, will discuss the use of CAR T-cell therapy for inflammatory RMDs. There are also multiple abstract presentations on this topic.
In-depth tissue analysis and prediction of treatment response is another interesting approach, Dr. Ospelt said. “I think that’s the way to go, that we come from the blood, we go into the tissue.” A “very nice” example of this approach will be presented during the Abstract Plenary session on Wednesday, June 12, looking at how synovial tissue macrophages may be able to give information on likely treatment response in treatment-naive rheumatoid arthritis (OP0062). There are also some further findings related to the tissue biopsy–driven treatment trial R4RA that are being presented at the meeting (OP0218, OP0242, and POS0351).
EULAR Highlighted Sessions
Among the highlighted sessions on the EULAR 2024 website is one on axial involvement in PsA and spondyloarthritis (SpA).
“Axial involvement in psoriatic arthritis and peripheral involvement in axial spondyloarthritis is quite a hot topic at the moment,” Dr. Ospelt said. There are lots of questions: “How connected are they? How different are they? Do we need different treatment for axial involvement compared to peripheral involvement?”
Another EULAR highlighted session is the 75th anniversary of glucocorticoid treatment, during which Past President of EULAR and Emeritus Professor of Rheumatology Josef S. Smolen, MD, will overview the “past, present, and future” of glucocorticoids in RMDs. Consultant rheumatologist Frank Buttgereit, MD, from the German Rheumatism Research Center in Berlin, will discuss the practicalities of using these drugs in clinical practice.
Dr. Dejaco noted: “Glucocorticoids have been one of the most important treatments for a very long time, and they’re still the most important treatment for the acute treatment of systemic inflammatory diseases.”
For a long time, there was no alternative to using steroids, he added, but steroid-sparing options now exist, and there will be data presented on a new type of drug that could potentially be used to control cortisol levels in the body (OP0335).
Recommendations and More
Dr. Ospelt and Dr. Dejaco both pointed out other sessions that are likely to be very popular, such as the first and second EULAR Recommendations sessions, a session on rheumatoid arthritis prevention, as well as the many presentations and sessions on digital health and nonpharmacologic interventions such as exercise.
With over 5242 submitted abstracts, there is going to be no shortage of data being presented at EULAR 2024. Alongside the traditional abstract submission categories, this year there is a new clinical case reports category.
“We had about 578 submissions for that category,” Dr. Dejaco said. There were 3315 abstracts submitted for the clinical research category, 812 for the basic and translational research category, 283 from health professionals in rheumatology, 152 from patient groups, and 102 in the field of pediatric rheumatology.
Join in On-Site, Watch on Demand
EULAR 2024 reverts to an on-site–only meeting this year. Some of the more lighthearted yet educational elements of the program for those attending include the second edition of the EMEUNET Rheumatology Quiz and, new for this year, two escape rooms. These rooms will provide an interactive experience where small teams will have to solve rheumatologic conundrums in order to escape the room within the hour, Dr. Dejaco explained. There will also be a morning run on Friday, June 14. “It’s not a race, it’s simply to meet and run together,” Dr. Dejaco said.
But if you cannot make the congress in person, the EULAR 2024 Livestream will be broadcasting throughout the congress. Anyone registered by June 30 will have on-demand access to the recorded content from June 17 until December 31, 2024.
Abstracts for the meeting will be published as a supplement to Annals of the Rheumatic Diseases, the official journal of EULAR.
Dr. Ospelt reported no relevant financial relationships. Dr. Dejaco has received consulting/speaker fees from AbbVie, Eli Lilly, Janssen, Sparrow, Novartis, Pfizer, Roche, Galapagos, and Sanofi.
A version of this article appeared on Medscape.com.
Autonomous AI Outperforms Humans in Optical Diagnosis of Colorectal Polyps
, while providing greater alignment with pathology-based surveillance intervals, based on a randomized controlled trial.
These findings suggest that autonomous AI may one day replace histologic assessment of diminutive polyps, reported lead author Roupen Djinbachian, MD, of the Montreal University Hospital Research Center, Montreal, Quebec, Canada, and colleagues.Optical diagnosis of diminutive colorectal polyps has been proposed as a cost-effective alternative to histologic diagnosis, but its implementation in general clinical practice has been hindered by endoscopists’ concerns about incorrect diagnoses, the investigators wrote in Gastroenterology.“AI-based systems (CADx) have been proposed as a solution to these barriers to implementation, with studies showing high adherence to Preservation and Incorporation of Valuable Endoscopic Innovations (PIVI) thresholds when using AI-H,” they wrote. “However, the efficacy and safety of autonomous AI-based diagnostic platforms have not yet been evaluated.”
To address this knowledge gap, Dr. Djinbachian and colleagues conducted a randomized controlled noninferiority trial involving 467 patients, all of whom underwent elective colonoscopies at a single academic institution.
Participants were randomly assigned to one of two groups. The first group received an optical diagnosis of diminutive (1-5 mm) colorectal polyps using an autonomous AI-based CADx system without any human input. The second group had diagnoses performed by endoscopists who used AI-H to make their optical diagnoses.
The primary outcome was the accuracy of optical diagnosis compared with the gold standard of histologic evaluation. Secondarily, the investigators explored associations between pathology-based surveillance intervals and various measures of accuracy, including sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV).
The results showed that the accuracy of optical diagnosis for diminutive polyps was similar between the two groups, supporting noninferiority. Autonomous AI achieved an accuracy rate of 77.2%, while the AI-H group had an accuracy of 72.1%, which was not statistically significant (P = .86).
But when it came to pathology-based surveillance intervals, autonomous AI showed a clear advantage; the autonomous AI system achieved a 91.5% agreement rate, compared with 82.1% for the AI-H group (P = .016).
“These findings indicate that autonomous AI not only matches but also surpasses AI-H in accuracy for determining surveillance intervals,” the investigators wrote, noting that this finding highlights the “complexities of human interaction with AI modules where human intervention could lead to worse outcomes.”
Further analysis revealed that the sensitivity of autonomous AI for identifying adenomas was 84.8%, slightly higher than the 83.6% sensitivity of the AI-H group. Specificity was 64.4% for autonomous AI vs 63.8% for AI-H. While PPV was higher in the autonomous AI group (85.6%), compared with the AI-H group (78.6%), NPV was lower for autonomous AI than AI-H (63.0% vs 71.0%).
Dr. Djinbachian and colleagues suggested that future research should focus on larger, multicenter trials to validate these findings and further explore the integration of autonomous AI systems in clinical practice. They also noted that improving AI algorithms to accurately diagnose sessile serrated lesions could enhance the overall effectiveness of AI-based optical diagnosis.
“The performance of autonomous AI in accurately diagnosing diminutive polyps and determining appropriate surveillance intervals suggests that it could play a crucial role in streamlining colorectal cancer screening processes, reducing the burden on pathologists, and potentially lowering healthcare costs,” the investigators concluded.The study was supported by Fujifilm, which had no role in the study design or data analysis. Dr. von Renteln reported additional research funding from Vantage and Fujifilm.
In the era of computer vision for endoscopy and colonoscopy, current paradigms rely on AI as a co-pilot or second observer, with the physician serving as the final arbiter in procedure-related decision-making. This study by Djinbachian and Haumesser et al brings up the interesting wrinkle of autonomous AI as a potentially superior (or noninferior) option in narrow, task-specific use cases.
In this study, human input from the endoscopist after CADx diagnosis led to lower agreement between the AI-predicted diagnosis and corresponding surveillance intervals; human oversight more often incorrectly changed the resultant diagnosis and led to shorter than recommended surveillance intervals.
This study offers a small but very important update to the growing body of literature on CADx in colonoscopy. So far, prospective validation of CADx compared with the human eye for in-situ diagnosis of polyps has provided mixed results. This study is one of the first to examine the potential role of “automatic” CADx without additional human input and sheds light on the importance of the AI-human hybrid in medical care. How do the ways in which humans interact with the user interface and output of AI lead to changes in outcome? How can we optimize the AI-human interaction in order to provide optimal results?
Jeremy R. Glissen Brown is an assistant professor in the Department of Internal Medicine and Division of Gastroenterology at Duke University Medical Center, Durham, North Carolina. He has served as a consultant for Medtronic and Olympus, and on the advisory board for Odin Vision.
In the era of computer vision for endoscopy and colonoscopy, current paradigms rely on AI as a co-pilot or second observer, with the physician serving as the final arbiter in procedure-related decision-making. This study by Djinbachian and Haumesser et al brings up the interesting wrinkle of autonomous AI as a potentially superior (or noninferior) option in narrow, task-specific use cases.
In this study, human input from the endoscopist after CADx diagnosis led to lower agreement between the AI-predicted diagnosis and corresponding surveillance intervals; human oversight more often incorrectly changed the resultant diagnosis and led to shorter than recommended surveillance intervals.
This study offers a small but very important update to the growing body of literature on CADx in colonoscopy. So far, prospective validation of CADx compared with the human eye for in-situ diagnosis of polyps has provided mixed results. This study is one of the first to examine the potential role of “automatic” CADx without additional human input and sheds light on the importance of the AI-human hybrid in medical care. How do the ways in which humans interact with the user interface and output of AI lead to changes in outcome? How can we optimize the AI-human interaction in order to provide optimal results?
Jeremy R. Glissen Brown is an assistant professor in the Department of Internal Medicine and Division of Gastroenterology at Duke University Medical Center, Durham, North Carolina. He has served as a consultant for Medtronic and Olympus, and on the advisory board for Odin Vision.
In the era of computer vision for endoscopy and colonoscopy, current paradigms rely on AI as a co-pilot or second observer, with the physician serving as the final arbiter in procedure-related decision-making. This study by Djinbachian and Haumesser et al brings up the interesting wrinkle of autonomous AI as a potentially superior (or noninferior) option in narrow, task-specific use cases.
In this study, human input from the endoscopist after CADx diagnosis led to lower agreement between the AI-predicted diagnosis and corresponding surveillance intervals; human oversight more often incorrectly changed the resultant diagnosis and led to shorter than recommended surveillance intervals.
This study offers a small but very important update to the growing body of literature on CADx in colonoscopy. So far, prospective validation of CADx compared with the human eye for in-situ diagnosis of polyps has provided mixed results. This study is one of the first to examine the potential role of “automatic” CADx without additional human input and sheds light on the importance of the AI-human hybrid in medical care. How do the ways in which humans interact with the user interface and output of AI lead to changes in outcome? How can we optimize the AI-human interaction in order to provide optimal results?
Jeremy R. Glissen Brown is an assistant professor in the Department of Internal Medicine and Division of Gastroenterology at Duke University Medical Center, Durham, North Carolina. He has served as a consultant for Medtronic and Olympus, and on the advisory board for Odin Vision.
, while providing greater alignment with pathology-based surveillance intervals, based on a randomized controlled trial.
These findings suggest that autonomous AI may one day replace histologic assessment of diminutive polyps, reported lead author Roupen Djinbachian, MD, of the Montreal University Hospital Research Center, Montreal, Quebec, Canada, and colleagues.Optical diagnosis of diminutive colorectal polyps has been proposed as a cost-effective alternative to histologic diagnosis, but its implementation in general clinical practice has been hindered by endoscopists’ concerns about incorrect diagnoses, the investigators wrote in Gastroenterology.“AI-based systems (CADx) have been proposed as a solution to these barriers to implementation, with studies showing high adherence to Preservation and Incorporation of Valuable Endoscopic Innovations (PIVI) thresholds when using AI-H,” they wrote. “However, the efficacy and safety of autonomous AI-based diagnostic platforms have not yet been evaluated.”
To address this knowledge gap, Dr. Djinbachian and colleagues conducted a randomized controlled noninferiority trial involving 467 patients, all of whom underwent elective colonoscopies at a single academic institution.
Participants were randomly assigned to one of two groups. The first group received an optical diagnosis of diminutive (1-5 mm) colorectal polyps using an autonomous AI-based CADx system without any human input. The second group had diagnoses performed by endoscopists who used AI-H to make their optical diagnoses.
The primary outcome was the accuracy of optical diagnosis compared with the gold standard of histologic evaluation. Secondarily, the investigators explored associations between pathology-based surveillance intervals and various measures of accuracy, including sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV).
The results showed that the accuracy of optical diagnosis for diminutive polyps was similar between the two groups, supporting noninferiority. Autonomous AI achieved an accuracy rate of 77.2%, while the AI-H group had an accuracy of 72.1%, which was not statistically significant (P = .86).
But when it came to pathology-based surveillance intervals, autonomous AI showed a clear advantage; the autonomous AI system achieved a 91.5% agreement rate, compared with 82.1% for the AI-H group (P = .016).
“These findings indicate that autonomous AI not only matches but also surpasses AI-H in accuracy for determining surveillance intervals,” the investigators wrote, noting that this finding highlights the “complexities of human interaction with AI modules where human intervention could lead to worse outcomes.”
Further analysis revealed that the sensitivity of autonomous AI for identifying adenomas was 84.8%, slightly higher than the 83.6% sensitivity of the AI-H group. Specificity was 64.4% for autonomous AI vs 63.8% for AI-H. While PPV was higher in the autonomous AI group (85.6%), compared with the AI-H group (78.6%), NPV was lower for autonomous AI than AI-H (63.0% vs 71.0%).
Dr. Djinbachian and colleagues suggested that future research should focus on larger, multicenter trials to validate these findings and further explore the integration of autonomous AI systems in clinical practice. They also noted that improving AI algorithms to accurately diagnose sessile serrated lesions could enhance the overall effectiveness of AI-based optical diagnosis.
“The performance of autonomous AI in accurately diagnosing diminutive polyps and determining appropriate surveillance intervals suggests that it could play a crucial role in streamlining colorectal cancer screening processes, reducing the burden on pathologists, and potentially lowering healthcare costs,” the investigators concluded.The study was supported by Fujifilm, which had no role in the study design or data analysis. Dr. von Renteln reported additional research funding from Vantage and Fujifilm.
, while providing greater alignment with pathology-based surveillance intervals, based on a randomized controlled trial.
These findings suggest that autonomous AI may one day replace histologic assessment of diminutive polyps, reported lead author Roupen Djinbachian, MD, of the Montreal University Hospital Research Center, Montreal, Quebec, Canada, and colleagues.Optical diagnosis of diminutive colorectal polyps has been proposed as a cost-effective alternative to histologic diagnosis, but its implementation in general clinical practice has been hindered by endoscopists’ concerns about incorrect diagnoses, the investigators wrote in Gastroenterology.“AI-based systems (CADx) have been proposed as a solution to these barriers to implementation, with studies showing high adherence to Preservation and Incorporation of Valuable Endoscopic Innovations (PIVI) thresholds when using AI-H,” they wrote. “However, the efficacy and safety of autonomous AI-based diagnostic platforms have not yet been evaluated.”
To address this knowledge gap, Dr. Djinbachian and colleagues conducted a randomized controlled noninferiority trial involving 467 patients, all of whom underwent elective colonoscopies at a single academic institution.
Participants were randomly assigned to one of two groups. The first group received an optical diagnosis of diminutive (1-5 mm) colorectal polyps using an autonomous AI-based CADx system without any human input. The second group had diagnoses performed by endoscopists who used AI-H to make their optical diagnoses.
The primary outcome was the accuracy of optical diagnosis compared with the gold standard of histologic evaluation. Secondarily, the investigators explored associations between pathology-based surveillance intervals and various measures of accuracy, including sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV).
The results showed that the accuracy of optical diagnosis for diminutive polyps was similar between the two groups, supporting noninferiority. Autonomous AI achieved an accuracy rate of 77.2%, while the AI-H group had an accuracy of 72.1%, which was not statistically significant (P = .86).
But when it came to pathology-based surveillance intervals, autonomous AI showed a clear advantage; the autonomous AI system achieved a 91.5% agreement rate, compared with 82.1% for the AI-H group (P = .016).
“These findings indicate that autonomous AI not only matches but also surpasses AI-H in accuracy for determining surveillance intervals,” the investigators wrote, noting that this finding highlights the “complexities of human interaction with AI modules where human intervention could lead to worse outcomes.”
Further analysis revealed that the sensitivity of autonomous AI for identifying adenomas was 84.8%, slightly higher than the 83.6% sensitivity of the AI-H group. Specificity was 64.4% for autonomous AI vs 63.8% for AI-H. While PPV was higher in the autonomous AI group (85.6%), compared with the AI-H group (78.6%), NPV was lower for autonomous AI than AI-H (63.0% vs 71.0%).
Dr. Djinbachian and colleagues suggested that future research should focus on larger, multicenter trials to validate these findings and further explore the integration of autonomous AI systems in clinical practice. They also noted that improving AI algorithms to accurately diagnose sessile serrated lesions could enhance the overall effectiveness of AI-based optical diagnosis.
“The performance of autonomous AI in accurately diagnosing diminutive polyps and determining appropriate surveillance intervals suggests that it could play a crucial role in streamlining colorectal cancer screening processes, reducing the burden on pathologists, and potentially lowering healthcare costs,” the investigators concluded.The study was supported by Fujifilm, which had no role in the study design or data analysis. Dr. von Renteln reported additional research funding from Vantage and Fujifilm.
FROM GASTROENTEROLOGY
Inflammatory Bowel Disease Highlights From Digestive Disease Week 2024
Highlights in ulcerative colitis (UC) and Crohn's disease (CD) from Digestive Disease Week® (DDW) 2024 are reported on by Dr. Andres Yarur from Cedars Sinai Medical Center in Los Angeles.
Dr. Yarur opens by discussing two phase 3 studies focused on risankizumab (RZB), which is currently approved for treatment of CD and has shown efficacy in UC. The first showed an induction period extended from 12 to 24 weeks resulted in clinical response in more than half of patients with UC.
The second study compared maintenance therapy with RZB to ustekinumab in patients with CD and found that RZB resulted in a higher rate of remission.
Dr. Yarur next looks at a study that explored use of darvadstrocel, an allogeneic stem cell therapy, in a subset of patients with CD and complex perianal fistulas. The disappointing results of the ADMIRE-CD II trial showed no benefit over placebo.
Patients hospitalized with UC, a population with few therapeutic options, were the focus of the next study. The TRIUMPH study explored use of the Janus kinase inhibitor tofacitinib for these patients and found that clinical response was achieved by 58.3% of them by day 7.
The final study addressed a clinical challenge: devising the optimal vaccination strategy for patients on immunosuppressive or anti–tumor necrosis factor therapies. Dr. Yarur reports that the study found an intensified pneumococcal vaccine regimen was more immunogenic and provided immunity for a longer duration than did the standard regimen.
--
Andres J. Yarur, MD, Associate Professor of Medicine, Cedars Sinai Medical Center, Los Angeles, California
Andres J. Yarur, MD, has disclosed the following relevant financial relationships:
Serve(d) as a consultant for: Takeda; Pfizer; Arena; AbbVie; Bristol Myers Squibb; Boehringer Ingelheim; Celltrion
Highlights in ulcerative colitis (UC) and Crohn's disease (CD) from Digestive Disease Week® (DDW) 2024 are reported on by Dr. Andres Yarur from Cedars Sinai Medical Center in Los Angeles.
Dr. Yarur opens by discussing two phase 3 studies focused on risankizumab (RZB), which is currently approved for treatment of CD and has shown efficacy in UC. The first showed an induction period extended from 12 to 24 weeks resulted in clinical response in more than half of patients with UC.
The second study compared maintenance therapy with RZB to ustekinumab in patients with CD and found that RZB resulted in a higher rate of remission.
Dr. Yarur next looks at a study that explored use of darvadstrocel, an allogeneic stem cell therapy, in a subset of patients with CD and complex perianal fistulas. The disappointing results of the ADMIRE-CD II trial showed no benefit over placebo.
Patients hospitalized with UC, a population with few therapeutic options, were the focus of the next study. The TRIUMPH study explored use of the Janus kinase inhibitor tofacitinib for these patients and found that clinical response was achieved by 58.3% of them by day 7.
The final study addressed a clinical challenge: devising the optimal vaccination strategy for patients on immunosuppressive or anti–tumor necrosis factor therapies. Dr. Yarur reports that the study found an intensified pneumococcal vaccine regimen was more immunogenic and provided immunity for a longer duration than did the standard regimen.
--
Andres J. Yarur, MD, Associate Professor of Medicine, Cedars Sinai Medical Center, Los Angeles, California
Andres J. Yarur, MD, has disclosed the following relevant financial relationships:
Serve(d) as a consultant for: Takeda; Pfizer; Arena; AbbVie; Bristol Myers Squibb; Boehringer Ingelheim; Celltrion
Highlights in ulcerative colitis (UC) and Crohn's disease (CD) from Digestive Disease Week® (DDW) 2024 are reported on by Dr. Andres Yarur from Cedars Sinai Medical Center in Los Angeles.
Dr. Yarur opens by discussing two phase 3 studies focused on risankizumab (RZB), which is currently approved for treatment of CD and has shown efficacy in UC. The first showed an induction period extended from 12 to 24 weeks resulted in clinical response in more than half of patients with UC.
The second study compared maintenance therapy with RZB to ustekinumab in patients with CD and found that RZB resulted in a higher rate of remission.
Dr. Yarur next looks at a study that explored use of darvadstrocel, an allogeneic stem cell therapy, in a subset of patients with CD and complex perianal fistulas. The disappointing results of the ADMIRE-CD II trial showed no benefit over placebo.
Patients hospitalized with UC, a population with few therapeutic options, were the focus of the next study. The TRIUMPH study explored use of the Janus kinase inhibitor tofacitinib for these patients and found that clinical response was achieved by 58.3% of them by day 7.
The final study addressed a clinical challenge: devising the optimal vaccination strategy for patients on immunosuppressive or anti–tumor necrosis factor therapies. Dr. Yarur reports that the study found an intensified pneumococcal vaccine regimen was more immunogenic and provided immunity for a longer duration than did the standard regimen.
--
Andres J. Yarur, MD, Associate Professor of Medicine, Cedars Sinai Medical Center, Los Angeles, California
Andres J. Yarur, MD, has disclosed the following relevant financial relationships:
Serve(d) as a consultant for: Takeda; Pfizer; Arena; AbbVie; Bristol Myers Squibb; Boehringer Ingelheim; Celltrion

COPD Highlights From ATS 2024
The latest research on treatment of patients with COPD presented at the American Thoracic Society (ATS) 2024 annual meeting is reported on by Diego J. Maselli, MD, FCCP, CHEST Physician Editorial Board Member, from UT Health San Antonio in Texas.
Dr. Maselli discusses the phase 2a COURSE study, which looked at patients with moderate to severe COPD to determine whether novel tezepelumab would help reduce exacerbations over 52 weeks. The study reached a nonsignificant numerical reduction in the annual rate vs placebo, but Dr. Maselli suggests that outcomes in patients. with high blood eosinophil counts merit further study.
Next, Dr. Maselli discusses the phase 3 NOTUS trial, looking at the efficacy and safety of the monoclonal antibody dupilumab in patients with moderate to severe COPD. The researchers found a 34% reduction in exacerbations in the dupilumab group vs placebo after 52 weeks.
He then details a 272-patient study looking at nebulized ensifentrine, a dual inhibitor of PDE3 and PDE4. The study demonstrated improved lung function as well as a reduction in exacerbation rate to patients with moderate to severe COPD treated with ensifentrined added to long-acting beta agonists-inhaled corticosteroid maintenance therapy.
Finally, Dr. Maselli highlights the MAZI study, a large retrospective analysis comparing the mortality rate in patients with COPD taking single-inhaler triple therapy (SITT) vs multiple-inhaler triple therapy (MITT). The researchers found that SITT was superior to MITT.
--
Diego J. Maselli, MD, FCCP, Professor, Chief, Division of Pulmonary Diseases & Critical Care, UT Health San Antonio, Texas
Diego J. Maselli, MD, FCCP has disclosed the following relevant financial relationships:
Serve(d) as a speaker or a member of a speakers bureau for: GSK; AstraZeneca; Sanofi/Regeneron; Amgen
Received research grant from: Gates Foundation; COPD Foundation; NIH
The latest research on treatment of patients with COPD presented at the American Thoracic Society (ATS) 2024 annual meeting is reported on by Diego J. Maselli, MD, FCCP, CHEST Physician Editorial Board Member, from UT Health San Antonio in Texas.
Dr. Maselli discusses the phase 2a COURSE study, which looked at patients with moderate to severe COPD to determine whether novel tezepelumab would help reduce exacerbations over 52 weeks. The study reached a nonsignificant numerical reduction in the annual rate vs placebo, but Dr. Maselli suggests that outcomes in patients. with high blood eosinophil counts merit further study.
Next, Dr. Maselli discusses the phase 3 NOTUS trial, looking at the efficacy and safety of the monoclonal antibody dupilumab in patients with moderate to severe COPD. The researchers found a 34% reduction in exacerbations in the dupilumab group vs placebo after 52 weeks.
He then details a 272-patient study looking at nebulized ensifentrine, a dual inhibitor of PDE3 and PDE4. The study demonstrated improved lung function as well as a reduction in exacerbation rate to patients with moderate to severe COPD treated with ensifentrined added to long-acting beta agonists-inhaled corticosteroid maintenance therapy.
Finally, Dr. Maselli highlights the MAZI study, a large retrospective analysis comparing the mortality rate in patients with COPD taking single-inhaler triple therapy (SITT) vs multiple-inhaler triple therapy (MITT). The researchers found that SITT was superior to MITT.
--
Diego J. Maselli, MD, FCCP, Professor, Chief, Division of Pulmonary Diseases & Critical Care, UT Health San Antonio, Texas
Diego J. Maselli, MD, FCCP has disclosed the following relevant financial relationships:
Serve(d) as a speaker or a member of a speakers bureau for: GSK; AstraZeneca; Sanofi/Regeneron; Amgen
Received research grant from: Gates Foundation; COPD Foundation; NIH
The latest research on treatment of patients with COPD presented at the American Thoracic Society (ATS) 2024 annual meeting is reported on by Diego J. Maselli, MD, FCCP, CHEST Physician Editorial Board Member, from UT Health San Antonio in Texas.
Dr. Maselli discusses the phase 2a COURSE study, which looked at patients with moderate to severe COPD to determine whether novel tezepelumab would help reduce exacerbations over 52 weeks. The study reached a nonsignificant numerical reduction in the annual rate vs placebo, but Dr. Maselli suggests that outcomes in patients. with high blood eosinophil counts merit further study.
Next, Dr. Maselli discusses the phase 3 NOTUS trial, looking at the efficacy and safety of the monoclonal antibody dupilumab in patients with moderate to severe COPD. The researchers found a 34% reduction in exacerbations in the dupilumab group vs placebo after 52 weeks.
He then details a 272-patient study looking at nebulized ensifentrine, a dual inhibitor of PDE3 and PDE4. The study demonstrated improved lung function as well as a reduction in exacerbation rate to patients with moderate to severe COPD treated with ensifentrined added to long-acting beta agonists-inhaled corticosteroid maintenance therapy.
Finally, Dr. Maselli highlights the MAZI study, a large retrospective analysis comparing the mortality rate in patients with COPD taking single-inhaler triple therapy (SITT) vs multiple-inhaler triple therapy (MITT). The researchers found that SITT was superior to MITT.
--
Diego J. Maselli, MD, FCCP, Professor, Chief, Division of Pulmonary Diseases & Critical Care, UT Health San Antonio, Texas
Diego J. Maselli, MD, FCCP has disclosed the following relevant financial relationships:
Serve(d) as a speaker or a member of a speakers bureau for: GSK; AstraZeneca; Sanofi/Regeneron; Amgen
Received research grant from: Gates Foundation; COPD Foundation; NIH

Narcolepsy an Independent Cardiovascular Disease Risk Factor
HOUSTON — Narcolepsy is associated with a significantly increased risk for cardiovascular disease (CVD) and major adverse cardiac events (MACEs), independent of common comorbid conditions and medications commonly used to treat the chronic sleep disorder, according to two new studies.
A nationwide analysis revealed that people with narcolepsy had a 77% higher risk for CVD and an 82% higher risk for MACE than those without the disorder.
“These findings indicate that it is important for clinicians to regularly monitor patients for cardiovascular disease and take this into consideration when recommending specific treatments for narcolepsy,” study investigators Christopher Kaufmann, PhD; Munaza Riaz, PharmD, MPhil; and Rakesh Bhattacharjee, MD, told this news organization.
“Additionally, physicians should consider monitoring the presence of other health conditions as contributing factors to the risk of CVD,” they said. Dr. Kaufmann and Dr. Riaz are with the University of Florida, Gainesville, Florida, and Dr. Bhattacharjee is with the University of California, San Diego.
They presented their research at SLEEP 2024: 38th Annual Meeting of the Associated Professional Sleep Societies.
Independent Risk Factor
The National Institute of Neurological Disorders and Stroke reports an estimated 125,000 to 200,000 people in the United States live with narcolepsy. The condition often coexists with other common health conditions including obstructive sleep apnea (OSA), diabetes, and other comorbidities, which can all contribute to the risk for CVD.
This raises doubt as to whether narcolepsy itself directly leads to CVD or if it is the result of these comorbid health conditions. Additionally, some medications used to treat narcolepsy carry their own cardiovascular risks.
Using the IBM MarketScan Commercial and Medicare supplemental databases, the researchers identified 34,562 adults with a diagnosis of narcolepsy and a propensity-matched comparison cohort of 100,405 adults without narcolepsy. The patients had a mean age of 40 years, and 62% were women.
Compared with adults without narcolepsy, those with the chronic sleep disorder that causes overwhelming daytime drowsiness had a 77% increased risk for any CVD (hazard ratio [HR], 1.77) and an 82% increased risk for MACE (HR, 1.82).
They also had an increased risk for stroke (HR, 2.04), heart failure or myocardial infarction (MI; HR, 1.64), and atrial fibrillation (HR, 1.58).
The results were similar in a separate analysis of the same population that also controlled for baseline use of stimulants, oxybates, and wake-promoting agents — medications commonly used to treat excessive daytime sleepiness associated with narcolepsy.
In this analysis, narcolepsy was associated with an 89% higher risk for CVD (HR, 1.89) and a 95% increased risk for MACE (HR, 1.95). The risk for any stroke (HR, 2.06), heart failure (HR, 1.90), atrial fibrillation (HR, 1.66), and MI (HR, 1.93) was also higher in those with narcolepsy.
“Our study found that even after considering the presence of health conditions like obstructive sleep apnea, diabetes, hypertension, hyperlipidemia, and even depression, as well as medication use, there still appears to be an independent relationship between narcolepsy and CVD,” the investigators said.
They cautioned that the mechanisms explaining the link between CVD and narcolepsy are unclear and warrant further study.
“Sleep fragmentation is a hallmark of narcolepsy, and it is speculated that this fragmentation, which may trigger disturbances in autonomic function, predisposes individuals to CVD. In rodent models, a possible link has been observed between hypocretin — a central neurotransmitter that is reduced or deficient in patients with narcolepsy — and atherosclerosis.
“However, it remains uncertain whether this is the primary mechanism related to CVD,” they commented.
Compelling Evidence for Higher CVD
Commenting on the findings for this news organization, Shaheen Lakhan, MD, a neurologist and researcher based in Miami, Florida, called for narcolepsy to be recognized as a significant contributor to higher CVD risk.
“Given the compelling evidence linking narcolepsy to a higher incidence of cardiovascular disease, it is crucial that narcolepsy be included in clinical guidelines and risk assessment tools alongside other known risk factors,” said Dr. Lakhan, who was not involved in this research.
“Physicians and health care providers should proactively address the increased cardiovascular risk associated with narcolepsy by incorporating preventive strategies and interventions into the management of patients with this condition,” Dr. Lakhan suggested.
Regular CVD screening, a healthier lifestyle, and targeted therapies could all decrease cardiac risk, Dr. Lakhan added.
“Ultimately, novel disease-modifying therapies for narcolepsy should target the core mechanisms driving the increased cardiovascular risk associated with this condition. By elucidating the specific biological pathways and developing targeted therapies that address the unique challenges faced by narcolepsy patients, we can effectively mitigate the risk,” Dr. Lakhan said.
The studies were funded by the Sleep Research Society Foundation. The authors and Dr. Lakhan had no relevant disclosures.
A version of this article appeared on Medscape.com.
HOUSTON — Narcolepsy is associated with a significantly increased risk for cardiovascular disease (CVD) and major adverse cardiac events (MACEs), independent of common comorbid conditions and medications commonly used to treat the chronic sleep disorder, according to two new studies.
A nationwide analysis revealed that people with narcolepsy had a 77% higher risk for CVD and an 82% higher risk for MACE than those without the disorder.
“These findings indicate that it is important for clinicians to regularly monitor patients for cardiovascular disease and take this into consideration when recommending specific treatments for narcolepsy,” study investigators Christopher Kaufmann, PhD; Munaza Riaz, PharmD, MPhil; and Rakesh Bhattacharjee, MD, told this news organization.
“Additionally, physicians should consider monitoring the presence of other health conditions as contributing factors to the risk of CVD,” they said. Dr. Kaufmann and Dr. Riaz are with the University of Florida, Gainesville, Florida, and Dr. Bhattacharjee is with the University of California, San Diego.
They presented their research at SLEEP 2024: 38th Annual Meeting of the Associated Professional Sleep Societies.
Independent Risk Factor
The National Institute of Neurological Disorders and Stroke reports an estimated 125,000 to 200,000 people in the United States live with narcolepsy. The condition often coexists with other common health conditions including obstructive sleep apnea (OSA), diabetes, and other comorbidities, which can all contribute to the risk for CVD.
This raises doubt as to whether narcolepsy itself directly leads to CVD or if it is the result of these comorbid health conditions. Additionally, some medications used to treat narcolepsy carry their own cardiovascular risks.
Using the IBM MarketScan Commercial and Medicare supplemental databases, the researchers identified 34,562 adults with a diagnosis of narcolepsy and a propensity-matched comparison cohort of 100,405 adults without narcolepsy. The patients had a mean age of 40 years, and 62% were women.
Compared with adults without narcolepsy, those with the chronic sleep disorder that causes overwhelming daytime drowsiness had a 77% increased risk for any CVD (hazard ratio [HR], 1.77) and an 82% increased risk for MACE (HR, 1.82).
They also had an increased risk for stroke (HR, 2.04), heart failure or myocardial infarction (MI; HR, 1.64), and atrial fibrillation (HR, 1.58).
The results were similar in a separate analysis of the same population that also controlled for baseline use of stimulants, oxybates, and wake-promoting agents — medications commonly used to treat excessive daytime sleepiness associated with narcolepsy.
In this analysis, narcolepsy was associated with an 89% higher risk for CVD (HR, 1.89) and a 95% increased risk for MACE (HR, 1.95). The risk for any stroke (HR, 2.06), heart failure (HR, 1.90), atrial fibrillation (HR, 1.66), and MI (HR, 1.93) was also higher in those with narcolepsy.
“Our study found that even after considering the presence of health conditions like obstructive sleep apnea, diabetes, hypertension, hyperlipidemia, and even depression, as well as medication use, there still appears to be an independent relationship between narcolepsy and CVD,” the investigators said.
They cautioned that the mechanisms explaining the link between CVD and narcolepsy are unclear and warrant further study.
“Sleep fragmentation is a hallmark of narcolepsy, and it is speculated that this fragmentation, which may trigger disturbances in autonomic function, predisposes individuals to CVD. In rodent models, a possible link has been observed between hypocretin — a central neurotransmitter that is reduced or deficient in patients with narcolepsy — and atherosclerosis.
“However, it remains uncertain whether this is the primary mechanism related to CVD,” they commented.
Compelling Evidence for Higher CVD
Commenting on the findings for this news organization, Shaheen Lakhan, MD, a neurologist and researcher based in Miami, Florida, called for narcolepsy to be recognized as a significant contributor to higher CVD risk.
“Given the compelling evidence linking narcolepsy to a higher incidence of cardiovascular disease, it is crucial that narcolepsy be included in clinical guidelines and risk assessment tools alongside other known risk factors,” said Dr. Lakhan, who was not involved in this research.
“Physicians and health care providers should proactively address the increased cardiovascular risk associated with narcolepsy by incorporating preventive strategies and interventions into the management of patients with this condition,” Dr. Lakhan suggested.
Regular CVD screening, a healthier lifestyle, and targeted therapies could all decrease cardiac risk, Dr. Lakhan added.
“Ultimately, novel disease-modifying therapies for narcolepsy should target the core mechanisms driving the increased cardiovascular risk associated with this condition. By elucidating the specific biological pathways and developing targeted therapies that address the unique challenges faced by narcolepsy patients, we can effectively mitigate the risk,” Dr. Lakhan said.
The studies were funded by the Sleep Research Society Foundation. The authors and Dr. Lakhan had no relevant disclosures.
A version of this article appeared on Medscape.com.
HOUSTON — Narcolepsy is associated with a significantly increased risk for cardiovascular disease (CVD) and major adverse cardiac events (MACEs), independent of common comorbid conditions and medications commonly used to treat the chronic sleep disorder, according to two new studies.
A nationwide analysis revealed that people with narcolepsy had a 77% higher risk for CVD and an 82% higher risk for MACE than those without the disorder.
“These findings indicate that it is important for clinicians to regularly monitor patients for cardiovascular disease and take this into consideration when recommending specific treatments for narcolepsy,” study investigators Christopher Kaufmann, PhD; Munaza Riaz, PharmD, MPhil; and Rakesh Bhattacharjee, MD, told this news organization.
“Additionally, physicians should consider monitoring the presence of other health conditions as contributing factors to the risk of CVD,” they said. Dr. Kaufmann and Dr. Riaz are with the University of Florida, Gainesville, Florida, and Dr. Bhattacharjee is with the University of California, San Diego.
They presented their research at SLEEP 2024: 38th Annual Meeting of the Associated Professional Sleep Societies.
Independent Risk Factor
The National Institute of Neurological Disorders and Stroke reports an estimated 125,000 to 200,000 people in the United States live with narcolepsy. The condition often coexists with other common health conditions including obstructive sleep apnea (OSA), diabetes, and other comorbidities, which can all contribute to the risk for CVD.
This raises doubt as to whether narcolepsy itself directly leads to CVD or if it is the result of these comorbid health conditions. Additionally, some medications used to treat narcolepsy carry their own cardiovascular risks.
Using the IBM MarketScan Commercial and Medicare supplemental databases, the researchers identified 34,562 adults with a diagnosis of narcolepsy and a propensity-matched comparison cohort of 100,405 adults without narcolepsy. The patients had a mean age of 40 years, and 62% were women.
Compared with adults without narcolepsy, those with the chronic sleep disorder that causes overwhelming daytime drowsiness had a 77% increased risk for any CVD (hazard ratio [HR], 1.77) and an 82% increased risk for MACE (HR, 1.82).
They also had an increased risk for stroke (HR, 2.04), heart failure or myocardial infarction (MI; HR, 1.64), and atrial fibrillation (HR, 1.58).
The results were similar in a separate analysis of the same population that also controlled for baseline use of stimulants, oxybates, and wake-promoting agents — medications commonly used to treat excessive daytime sleepiness associated with narcolepsy.
In this analysis, narcolepsy was associated with an 89% higher risk for CVD (HR, 1.89) and a 95% increased risk for MACE (HR, 1.95). The risk for any stroke (HR, 2.06), heart failure (HR, 1.90), atrial fibrillation (HR, 1.66), and MI (HR, 1.93) was also higher in those with narcolepsy.
“Our study found that even after considering the presence of health conditions like obstructive sleep apnea, diabetes, hypertension, hyperlipidemia, and even depression, as well as medication use, there still appears to be an independent relationship between narcolepsy and CVD,” the investigators said.
They cautioned that the mechanisms explaining the link between CVD and narcolepsy are unclear and warrant further study.
“Sleep fragmentation is a hallmark of narcolepsy, and it is speculated that this fragmentation, which may trigger disturbances in autonomic function, predisposes individuals to CVD. In rodent models, a possible link has been observed between hypocretin — a central neurotransmitter that is reduced or deficient in patients with narcolepsy — and atherosclerosis.
“However, it remains uncertain whether this is the primary mechanism related to CVD,” they commented.
Compelling Evidence for Higher CVD
Commenting on the findings for this news organization, Shaheen Lakhan, MD, a neurologist and researcher based in Miami, Florida, called for narcolepsy to be recognized as a significant contributor to higher CVD risk.
“Given the compelling evidence linking narcolepsy to a higher incidence of cardiovascular disease, it is crucial that narcolepsy be included in clinical guidelines and risk assessment tools alongside other known risk factors,” said Dr. Lakhan, who was not involved in this research.
“Physicians and health care providers should proactively address the increased cardiovascular risk associated with narcolepsy by incorporating preventive strategies and interventions into the management of patients with this condition,” Dr. Lakhan suggested.
Regular CVD screening, a healthier lifestyle, and targeted therapies could all decrease cardiac risk, Dr. Lakhan added.
“Ultimately, novel disease-modifying therapies for narcolepsy should target the core mechanisms driving the increased cardiovascular risk associated with this condition. By elucidating the specific biological pathways and developing targeted therapies that address the unique challenges faced by narcolepsy patients, we can effectively mitigate the risk,” Dr. Lakhan said.
The studies were funded by the Sleep Research Society Foundation. The authors and Dr. Lakhan had no relevant disclosures.
A version of this article appeared on Medscape.com.
FROM SLEEP 2024
Helping Patients Cut Down on Sodium: Useful Substitutes and Strategies
Humans have used salt for centuries, to preserve or cure food before refrigeration was readily available, and even as currency in some cultures. Though modern food preservation efforts have decreased our reliance on salt, we still heavily incorporate it as a flavor enhancer.
It’s only relatively recently that we’ve begun limiting salt in our diets, as research has linked high sodium intake with chronic, preventable conditions like hypertension, heart disease, and kidney disease.
How to Recommend Restriction in a Helpful Way
The US Department of Agriculture’s Dietary Guidelines for Americans recommends intake of no more than 2300 mg of sodium daily for adults and children aged 14 years or older. This echoes similar recommendations for people at risk for heart disease, kidney disease, and hypertension. However, the sodium intake of the average American still sits at a whopping 3400 mg daily.
High sodium intake is primarily the result of modern commercial food processing. Food prepared outside the home accounts for up to 70% of sodium intake in the United States, whereas only about 10% comes from salt that is added to food either during or after cooking. For this reason, I hesitate to recommend withholding salt as a primary focus when counseling on a low-sodium diet.
To many people, certain foods just taste better with salt. Many of my patients in the southern United States simply will not eat foods like eggs and tomatoes if they cannot salt them. We can spend every moment of patient interaction time explaining why excess sodium is unhealthy, but the fact remains that humans prefer food that tastes good. This is why I try to avoid counseling a “no-added-salt” diet; instead, I recommend a low-sodium diet with a focus on fresh, whole foods and limiting salt to only a few food items.
Patients should be counseled to slowly restrict their salt intake and be made aware that doing so may increase the time it takes for their sensitivity to the taste of less salty foods to return. But it is also important for them to know that it will return. The surest way to kill progress is for an unprepared patient to believe that their food will taste bland forever. A prepared patient understands that their food may taste different for a couple of weeks, but that the change will not last forever.
Types of Salt
I have often worked with patients who insist that their sodium intake is acceptable because they are using sea salt instead of table salt. This is the result of exceptional marketing and misinformation.
Specialty salts like sea salt and Himalayan pink salt contain about 560 mg and 590 mg of sodium, respectively, per quarter teaspoon. These products do have a slightly different mineral content, with sea salt typically having a negligible amount of calcium, magnesium, or potassium. The very small amount of these minerals offers no obvious health benefits compared with more affordable table salt.
The sodium content of iodized table salt is comparable to these products, with about 590 mg of sodium per quarter teaspoon. Though its high sodium content will put some practitioners off, it is also an excellent source of iodine, at about 75 mg per serving. It has been estimated that upward of 35% of the US population has iodine deficiency, most commonly due to pregnancy, avoidance of dairy products, increasing rates of vegetarianism, intake of highly processed foods, and avoidance of added salt. For this reason, and its relative affordability, I find table salt to be far more appropriate for the average American than specialty salts.
Salt Substitutes
Monosodium glutamate (MSG). MSG was previously at the center of public health concern owing to reports of “Chinese restaurant syndrome” that have since been debunked. I often recommend MSG to people trying to decrease sodium intake because the US Food and Drug Administration has designated it as GRAS (“generally recognized as safe”), and it has about one quarter of the sodium content of table salt at 125 mg per quarter teaspoon. Its crystalline structure makes it a lower-sodium salt substitute in savory applications like soups, stews, and gravies.
Hot sauce. These sauces are generally composed of peppers, vinegar, salt, and sugar. There may be some variation and occasionally added ingredients depending upon the brand. However, I find most hot sauces to be a low-sodium seasoning option that works especially well on proteins like eggs, chicken, and pork.
Potassium-based substitutes. Salt alternatives such as Nu-Salt and Morton Salt Substitute are sodium-free options with a significant amount of potassium, at 525 mg per quarter-teaspoon serving. These alternatives may not be ideal for patients with kidney problems, but they can be very helpful for those with potassium deficiency.
Herb-based seasonings. Garlic and onion powder are both sodium-free seasonings that many of my patients have found help to increase palatability while decreasing salt use. Black pepper; lemon and lime juice; salt-free herb mixes like Mrs. Dash; and spices like cumin, paprika, dill, chili powder, and ginger are also sodium-free or low-sodium alternatives that can help to alleviate blandness for someone new to a low-sodium diet. I recommend them often and use them in my own cooking at home.
Plant-based diet. If the goal of care is to improve cardiovascular or kidney health, then I find that working with patients to increase intake of plant foods to be a helpful option. This way of eating encourages replacing highly processed foods that may be high in sodium and sugar with plants that tend to be higher in potassium and calcium. The Dietary Approaches to Stop Hypertension (DASH), Mediterranean, and other plant-based diets have been shown to increase cardiovascular and metabolic health by significantly decreasing serum lipids, blood pressure, and hemoglobin A1c and promoting weight loss. They have also been shown to increase the gut microbiome and promote increased cognitive function.
I rarely encourage the use of added salt. However, research shows that putting down the salt shaker is probably not the most effective option to restrict sodium intake. For those who can cut back, these options can help keep food flavorful and patients compliant.
Ms. Winfree is a renal dietitian in private practice in Mary Esther, Florida. She has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
Humans have used salt for centuries, to preserve or cure food before refrigeration was readily available, and even as currency in some cultures. Though modern food preservation efforts have decreased our reliance on salt, we still heavily incorporate it as a flavor enhancer.
It’s only relatively recently that we’ve begun limiting salt in our diets, as research has linked high sodium intake with chronic, preventable conditions like hypertension, heart disease, and kidney disease.
How to Recommend Restriction in a Helpful Way
The US Department of Agriculture’s Dietary Guidelines for Americans recommends intake of no more than 2300 mg of sodium daily for adults and children aged 14 years or older. This echoes similar recommendations for people at risk for heart disease, kidney disease, and hypertension. However, the sodium intake of the average American still sits at a whopping 3400 mg daily.
High sodium intake is primarily the result of modern commercial food processing. Food prepared outside the home accounts for up to 70% of sodium intake in the United States, whereas only about 10% comes from salt that is added to food either during or after cooking. For this reason, I hesitate to recommend withholding salt as a primary focus when counseling on a low-sodium diet.
To many people, certain foods just taste better with salt. Many of my patients in the southern United States simply will not eat foods like eggs and tomatoes if they cannot salt them. We can spend every moment of patient interaction time explaining why excess sodium is unhealthy, but the fact remains that humans prefer food that tastes good. This is why I try to avoid counseling a “no-added-salt” diet; instead, I recommend a low-sodium diet with a focus on fresh, whole foods and limiting salt to only a few food items.
Patients should be counseled to slowly restrict their salt intake and be made aware that doing so may increase the time it takes for their sensitivity to the taste of less salty foods to return. But it is also important for them to know that it will return. The surest way to kill progress is for an unprepared patient to believe that their food will taste bland forever. A prepared patient understands that their food may taste different for a couple of weeks, but that the change will not last forever.
Types of Salt
I have often worked with patients who insist that their sodium intake is acceptable because they are using sea salt instead of table salt. This is the result of exceptional marketing and misinformation.
Specialty salts like sea salt and Himalayan pink salt contain about 560 mg and 590 mg of sodium, respectively, per quarter teaspoon. These products do have a slightly different mineral content, with sea salt typically having a negligible amount of calcium, magnesium, or potassium. The very small amount of these minerals offers no obvious health benefits compared with more affordable table salt.
The sodium content of iodized table salt is comparable to these products, with about 590 mg of sodium per quarter teaspoon. Though its high sodium content will put some practitioners off, it is also an excellent source of iodine, at about 75 mg per serving. It has been estimated that upward of 35% of the US population has iodine deficiency, most commonly due to pregnancy, avoidance of dairy products, increasing rates of vegetarianism, intake of highly processed foods, and avoidance of added salt. For this reason, and its relative affordability, I find table salt to be far more appropriate for the average American than specialty salts.
Salt Substitutes
Monosodium glutamate (MSG). MSG was previously at the center of public health concern owing to reports of “Chinese restaurant syndrome” that have since been debunked. I often recommend MSG to people trying to decrease sodium intake because the US Food and Drug Administration has designated it as GRAS (“generally recognized as safe”), and it has about one quarter of the sodium content of table salt at 125 mg per quarter teaspoon. Its crystalline structure makes it a lower-sodium salt substitute in savory applications like soups, stews, and gravies.
Hot sauce. These sauces are generally composed of peppers, vinegar, salt, and sugar. There may be some variation and occasionally added ingredients depending upon the brand. However, I find most hot sauces to be a low-sodium seasoning option that works especially well on proteins like eggs, chicken, and pork.
Potassium-based substitutes. Salt alternatives such as Nu-Salt and Morton Salt Substitute are sodium-free options with a significant amount of potassium, at 525 mg per quarter-teaspoon serving. These alternatives may not be ideal for patients with kidney problems, but they can be very helpful for those with potassium deficiency.
Herb-based seasonings. Garlic and onion powder are both sodium-free seasonings that many of my patients have found help to increase palatability while decreasing salt use. Black pepper; lemon and lime juice; salt-free herb mixes like Mrs. Dash; and spices like cumin, paprika, dill, chili powder, and ginger are also sodium-free or low-sodium alternatives that can help to alleviate blandness for someone new to a low-sodium diet. I recommend them often and use them in my own cooking at home.
Plant-based diet. If the goal of care is to improve cardiovascular or kidney health, then I find that working with patients to increase intake of plant foods to be a helpful option. This way of eating encourages replacing highly processed foods that may be high in sodium and sugar with plants that tend to be higher in potassium and calcium. The Dietary Approaches to Stop Hypertension (DASH), Mediterranean, and other plant-based diets have been shown to increase cardiovascular and metabolic health by significantly decreasing serum lipids, blood pressure, and hemoglobin A1c and promoting weight loss. They have also been shown to increase the gut microbiome and promote increased cognitive function.
I rarely encourage the use of added salt. However, research shows that putting down the salt shaker is probably not the most effective option to restrict sodium intake. For those who can cut back, these options can help keep food flavorful and patients compliant.
Ms. Winfree is a renal dietitian in private practice in Mary Esther, Florida. She has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
Humans have used salt for centuries, to preserve or cure food before refrigeration was readily available, and even as currency in some cultures. Though modern food preservation efforts have decreased our reliance on salt, we still heavily incorporate it as a flavor enhancer.
It’s only relatively recently that we’ve begun limiting salt in our diets, as research has linked high sodium intake with chronic, preventable conditions like hypertension, heart disease, and kidney disease.
How to Recommend Restriction in a Helpful Way
The US Department of Agriculture’s Dietary Guidelines for Americans recommends intake of no more than 2300 mg of sodium daily for adults and children aged 14 years or older. This echoes similar recommendations for people at risk for heart disease, kidney disease, and hypertension. However, the sodium intake of the average American still sits at a whopping 3400 mg daily.
High sodium intake is primarily the result of modern commercial food processing. Food prepared outside the home accounts for up to 70% of sodium intake in the United States, whereas only about 10% comes from salt that is added to food either during or after cooking. For this reason, I hesitate to recommend withholding salt as a primary focus when counseling on a low-sodium diet.
To many people, certain foods just taste better with salt. Many of my patients in the southern United States simply will not eat foods like eggs and tomatoes if they cannot salt them. We can spend every moment of patient interaction time explaining why excess sodium is unhealthy, but the fact remains that humans prefer food that tastes good. This is why I try to avoid counseling a “no-added-salt” diet; instead, I recommend a low-sodium diet with a focus on fresh, whole foods and limiting salt to only a few food items.
Patients should be counseled to slowly restrict their salt intake and be made aware that doing so may increase the time it takes for their sensitivity to the taste of less salty foods to return. But it is also important for them to know that it will return. The surest way to kill progress is for an unprepared patient to believe that their food will taste bland forever. A prepared patient understands that their food may taste different for a couple of weeks, but that the change will not last forever.
Types of Salt
I have often worked with patients who insist that their sodium intake is acceptable because they are using sea salt instead of table salt. This is the result of exceptional marketing and misinformation.
Specialty salts like sea salt and Himalayan pink salt contain about 560 mg and 590 mg of sodium, respectively, per quarter teaspoon. These products do have a slightly different mineral content, with sea salt typically having a negligible amount of calcium, magnesium, or potassium. The very small amount of these minerals offers no obvious health benefits compared with more affordable table salt.
The sodium content of iodized table salt is comparable to these products, with about 590 mg of sodium per quarter teaspoon. Though its high sodium content will put some practitioners off, it is also an excellent source of iodine, at about 75 mg per serving. It has been estimated that upward of 35% of the US population has iodine deficiency, most commonly due to pregnancy, avoidance of dairy products, increasing rates of vegetarianism, intake of highly processed foods, and avoidance of added salt. For this reason, and its relative affordability, I find table salt to be far more appropriate for the average American than specialty salts.
Salt Substitutes
Monosodium glutamate (MSG). MSG was previously at the center of public health concern owing to reports of “Chinese restaurant syndrome” that have since been debunked. I often recommend MSG to people trying to decrease sodium intake because the US Food and Drug Administration has designated it as GRAS (“generally recognized as safe”), and it has about one quarter of the sodium content of table salt at 125 mg per quarter teaspoon. Its crystalline structure makes it a lower-sodium salt substitute in savory applications like soups, stews, and gravies.
Hot sauce. These sauces are generally composed of peppers, vinegar, salt, and sugar. There may be some variation and occasionally added ingredients depending upon the brand. However, I find most hot sauces to be a low-sodium seasoning option that works especially well on proteins like eggs, chicken, and pork.
Potassium-based substitutes. Salt alternatives such as Nu-Salt and Morton Salt Substitute are sodium-free options with a significant amount of potassium, at 525 mg per quarter-teaspoon serving. These alternatives may not be ideal for patients with kidney problems, but they can be very helpful for those with potassium deficiency.
Herb-based seasonings. Garlic and onion powder are both sodium-free seasonings that many of my patients have found help to increase palatability while decreasing salt use. Black pepper; lemon and lime juice; salt-free herb mixes like Mrs. Dash; and spices like cumin, paprika, dill, chili powder, and ginger are also sodium-free or low-sodium alternatives that can help to alleviate blandness for someone new to a low-sodium diet. I recommend them often and use them in my own cooking at home.
Plant-based diet. If the goal of care is to improve cardiovascular or kidney health, then I find that working with patients to increase intake of plant foods to be a helpful option. This way of eating encourages replacing highly processed foods that may be high in sodium and sugar with plants that tend to be higher in potassium and calcium. The Dietary Approaches to Stop Hypertension (DASH), Mediterranean, and other plant-based diets have been shown to increase cardiovascular and metabolic health by significantly decreasing serum lipids, blood pressure, and hemoglobin A1c and promoting weight loss. They have also been shown to increase the gut microbiome and promote increased cognitive function.
I rarely encourage the use of added salt. However, research shows that putting down the salt shaker is probably not the most effective option to restrict sodium intake. For those who can cut back, these options can help keep food flavorful and patients compliant.
Ms. Winfree is a renal dietitian in private practice in Mary Esther, Florida. She has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
Myeloma: VRd Plus Isatuximab Improves Outcomes
Patients who took isatuximab (Sarclisa) plus bortezomib, lenalidomide, and dexamethasone (VRd) reached higher estimated progression-free survival at a median 59.7 months vs. those who took VRd alone (63.2% vs. 45.2%, respectively, 98.5% CI, hazard ratio [HR] = 0.60, P < .001), reported Thierry Facon, MD, professor of hematology at Lille University Hospital, France, and colleagues at the annual meeting of the American Society of Clinical Oncology in Chicago. The study was simultaneously published in The New England Journal of Medicine.
“The significant progression-free benefit observed with Sarclisa with combination therapy compared to VRd is important and encouraging for patients with newly diagnosed multiple myeloma,” Dr. Facon said in an interview. The findings demonstrated the VRd-isatuximab’s potential as “a first-in-class combination to address gaps in care for newly diagnosed multiple myeloma transplant-ineligible patients,” he said.
According to Dr. Facon, more than 180,000 people worldwide are diagnosed with MM each year, he said, making it the second-most common hematologic malignancy.
“There is a need for new frontline therapeutic options for all MM patients,” he said. “Effective frontline therapy has the potential to modify the course of the disease, which is a key outcome for transplant-ineligible patients who often face high rates of attrition in later lines of therapy.”
For the industry-funded IMROZ study, researchers recruited patients aged 18-80 at 93 sites in 21 nations from 2017-2019. All were ineligible for transplant due to comorbidities or being aged 65 or older. Exclusions included Eastern Cooperative Oncology Group (ECOG) performance status scores of more than 2.
The subjects were randomly assigned in a 3-to-2 ratio to isatuximab-VRd (n = 265) or VRd alone (n = 181) and received four induction cycles (6 weeks per cycle) followed by 4-week cycles of continuous treatment until disease progression, unacceptable adverse event, or other criteria for discontinuation. If progression occurred, patients could be switched from the VRd-only group to the isatuximab-VRd group.
The median age in both the isatuximab-VRd and VRd groups was 72. The percentages of women were 46.0% and 48.1%, respectively, and 72.5% and 72.4%, respectively, were White. The next largest race/ethnic group was Asian (11.7% and 9.4%, respectively). Almost all had ECOG status of 0 or 1 (88.7% and 89.5%, respectively).
At study cut-off in September 2023, the percentages of subjects in the isatuximab-VRd and VRd groups who were still receiving treatment were 47.2% and 24.3%, respectively.
An intention-to-treat analysis found that the two groups had similar rates of overall response (91.3% for isatuximab-VRd vs. 92.3% for VRd), but the isatuximab-VRd group had higher complete or better response (74.7% vs. 64.1%, P = .01).
The percentage of patients who were minimal residual disease (MRD)-negative and had a complete response was also higher in the VRd-isatuximab group vs. the VRd group (55.5% vs. 40.9%, respectively, P = .003). A total of 26.0% of patients in the VRd-isatuximab group died vs. 32.6% in the VRd group; the estimated overall survival rates at 60 months were 72.3% and 66.3%, respectively, HR = 0.78, 99.97% CI).
As for adverse events, grade 5 events were more common in the VRd-isatuximab group (11.0% vs. 5.5%), as were deaths within the first 60 days of treatment (1.5% vs. 0.6%). “The difference was driven in part by different treatment exposures,” the researchers reported. Treatment-emergent events led to treatment discontinuation in 22.8% and 26.0% of patients, respectively.
“The safety and tolerability of Sarclisa observed was consistent with the established safety profile of Sarclisa and VRd with no new safety signals observed,” Dr. Facon said.
In an interview, Zandra Klippel, MD, global product head for multiple myeloma at Sanofi — the maker of isatuximab and funder of the study — said the Food and Drug Administration has accepted a priority review application for the investigational use of isatuximab in combination with VRd for the treatment of patients with transplant-ineligible, newly diagnosed MM.
“Our FDA approval date is expected on September 27, 2024,” Dr. Klippel said. “If all goes well, we anticipate launching as early as 2024 in the US and rolling out in other key countries starting in 2025 and continuing through 2026.”
Dr. Klippel added that isatuximab “continues to be evaluated in multiple ongoing phase 3 clinical trials in combination with current standard treatments across the MM treatment continuum.”
In an interview, Sagar Lonial, MD, chair and professor of hematology and medical oncology and chief medical officer at Winship Cancer Institute at Emory University in Atlanta, said the study is “important.”
However, Dr. Lonial, who is familiar with the findings but didn’t take part in the study, said it’s difficult to understand the impact of the treatment on frail patients. It appears that the combination treatment may be good for frail patients, he said, “but I need to better understand the magnitude of the benefit in that subset a little more.”
As for adverse events, he said “they are what would be expected for a trial like this.”
Pneumonia and COVID-19 infections were higher in the VRd-isatuximab group, he said, and “we know in general that vaccine responses are blocked by CD38 antibodies.” This can be managed, he said, via intravenous immunoglobulin support.
Manni Mohyuddin, MD, assistant professor at Huntsman Cancer Institute in Utah, said in an interview that the findings suggest that in older, fit patients, “you can get fairly good outcomes without use of transplant.”
In the United States, many more patients in the cohort would have been considered transplant-eligible, he said, and not eliminated from consideration for transplant due to age over 65. However, as patients age, “you get more diminishing returns for transplants,” said Dr. Mohyuddin, who is familiar with the study findings but didn’t take part in the research.
All the drugs in the new combination are FDA approved, he said, although the combination isn’t. “I suspect this will make it to our guidelines very soon and then be reimbursed by insurance companies and Medicare.”
The study was funded by Sanofi and an M.D. Anderson Cancer Center support grant. Dr. Facon has no disclosures. Other study authors report multiple ties relationships with various drug makers. Dr. Lonial disclosed ties with Takeda, Amgen, Novartis, BMS, GSK, AbbVie, Genentech, Pfizer, Regeneron, Janssen, AstraZeneca, and TG Therapeutics). Dr. Mohyuddin disclosed a relationship with Janssen.
Patients who took isatuximab (Sarclisa) plus bortezomib, lenalidomide, and dexamethasone (VRd) reached higher estimated progression-free survival at a median 59.7 months vs. those who took VRd alone (63.2% vs. 45.2%, respectively, 98.5% CI, hazard ratio [HR] = 0.60, P < .001), reported Thierry Facon, MD, professor of hematology at Lille University Hospital, France, and colleagues at the annual meeting of the American Society of Clinical Oncology in Chicago. The study was simultaneously published in The New England Journal of Medicine.
“The significant progression-free benefit observed with Sarclisa with combination therapy compared to VRd is important and encouraging for patients with newly diagnosed multiple myeloma,” Dr. Facon said in an interview. The findings demonstrated the VRd-isatuximab’s potential as “a first-in-class combination to address gaps in care for newly diagnosed multiple myeloma transplant-ineligible patients,” he said.
According to Dr. Facon, more than 180,000 people worldwide are diagnosed with MM each year, he said, making it the second-most common hematologic malignancy.
“There is a need for new frontline therapeutic options for all MM patients,” he said. “Effective frontline therapy has the potential to modify the course of the disease, which is a key outcome for transplant-ineligible patients who often face high rates of attrition in later lines of therapy.”
For the industry-funded IMROZ study, researchers recruited patients aged 18-80 at 93 sites in 21 nations from 2017-2019. All were ineligible for transplant due to comorbidities or being aged 65 or older. Exclusions included Eastern Cooperative Oncology Group (ECOG) performance status scores of more than 2.
The subjects were randomly assigned in a 3-to-2 ratio to isatuximab-VRd (n = 265) or VRd alone (n = 181) and received four induction cycles (6 weeks per cycle) followed by 4-week cycles of continuous treatment until disease progression, unacceptable adverse event, or other criteria for discontinuation. If progression occurred, patients could be switched from the VRd-only group to the isatuximab-VRd group.
The median age in both the isatuximab-VRd and VRd groups was 72. The percentages of women were 46.0% and 48.1%, respectively, and 72.5% and 72.4%, respectively, were White. The next largest race/ethnic group was Asian (11.7% and 9.4%, respectively). Almost all had ECOG status of 0 or 1 (88.7% and 89.5%, respectively).
At study cut-off in September 2023, the percentages of subjects in the isatuximab-VRd and VRd groups who were still receiving treatment were 47.2% and 24.3%, respectively.
An intention-to-treat analysis found that the two groups had similar rates of overall response (91.3% for isatuximab-VRd vs. 92.3% for VRd), but the isatuximab-VRd group had higher complete or better response (74.7% vs. 64.1%, P = .01).
The percentage of patients who were minimal residual disease (MRD)-negative and had a complete response was also higher in the VRd-isatuximab group vs. the VRd group (55.5% vs. 40.9%, respectively, P = .003). A total of 26.0% of patients in the VRd-isatuximab group died vs. 32.6% in the VRd group; the estimated overall survival rates at 60 months were 72.3% and 66.3%, respectively, HR = 0.78, 99.97% CI).
As for adverse events, grade 5 events were more common in the VRd-isatuximab group (11.0% vs. 5.5%), as were deaths within the first 60 days of treatment (1.5% vs. 0.6%). “The difference was driven in part by different treatment exposures,” the researchers reported. Treatment-emergent events led to treatment discontinuation in 22.8% and 26.0% of patients, respectively.
“The safety and tolerability of Sarclisa observed was consistent with the established safety profile of Sarclisa and VRd with no new safety signals observed,” Dr. Facon said.
In an interview, Zandra Klippel, MD, global product head for multiple myeloma at Sanofi — the maker of isatuximab and funder of the study — said the Food and Drug Administration has accepted a priority review application for the investigational use of isatuximab in combination with VRd for the treatment of patients with transplant-ineligible, newly diagnosed MM.
“Our FDA approval date is expected on September 27, 2024,” Dr. Klippel said. “If all goes well, we anticipate launching as early as 2024 in the US and rolling out in other key countries starting in 2025 and continuing through 2026.”
Dr. Klippel added that isatuximab “continues to be evaluated in multiple ongoing phase 3 clinical trials in combination with current standard treatments across the MM treatment continuum.”
In an interview, Sagar Lonial, MD, chair and professor of hematology and medical oncology and chief medical officer at Winship Cancer Institute at Emory University in Atlanta, said the study is “important.”
However, Dr. Lonial, who is familiar with the findings but didn’t take part in the study, said it’s difficult to understand the impact of the treatment on frail patients. It appears that the combination treatment may be good for frail patients, he said, “but I need to better understand the magnitude of the benefit in that subset a little more.”
As for adverse events, he said “they are what would be expected for a trial like this.”
Pneumonia and COVID-19 infections were higher in the VRd-isatuximab group, he said, and “we know in general that vaccine responses are blocked by CD38 antibodies.” This can be managed, he said, via intravenous immunoglobulin support.
Manni Mohyuddin, MD, assistant professor at Huntsman Cancer Institute in Utah, said in an interview that the findings suggest that in older, fit patients, “you can get fairly good outcomes without use of transplant.”
In the United States, many more patients in the cohort would have been considered transplant-eligible, he said, and not eliminated from consideration for transplant due to age over 65. However, as patients age, “you get more diminishing returns for transplants,” said Dr. Mohyuddin, who is familiar with the study findings but didn’t take part in the research.
All the drugs in the new combination are FDA approved, he said, although the combination isn’t. “I suspect this will make it to our guidelines very soon and then be reimbursed by insurance companies and Medicare.”
The study was funded by Sanofi and an M.D. Anderson Cancer Center support grant. Dr. Facon has no disclosures. Other study authors report multiple ties relationships with various drug makers. Dr. Lonial disclosed ties with Takeda, Amgen, Novartis, BMS, GSK, AbbVie, Genentech, Pfizer, Regeneron, Janssen, AstraZeneca, and TG Therapeutics). Dr. Mohyuddin disclosed a relationship with Janssen.
Patients who took isatuximab (Sarclisa) plus bortezomib, lenalidomide, and dexamethasone (VRd) reached higher estimated progression-free survival at a median 59.7 months vs. those who took VRd alone (63.2% vs. 45.2%, respectively, 98.5% CI, hazard ratio [HR] = 0.60, P < .001), reported Thierry Facon, MD, professor of hematology at Lille University Hospital, France, and colleagues at the annual meeting of the American Society of Clinical Oncology in Chicago. The study was simultaneously published in The New England Journal of Medicine.
“The significant progression-free benefit observed with Sarclisa with combination therapy compared to VRd is important and encouraging for patients with newly diagnosed multiple myeloma,” Dr. Facon said in an interview. The findings demonstrated the VRd-isatuximab’s potential as “a first-in-class combination to address gaps in care for newly diagnosed multiple myeloma transplant-ineligible patients,” he said.
According to Dr. Facon, more than 180,000 people worldwide are diagnosed with MM each year, he said, making it the second-most common hematologic malignancy.
“There is a need for new frontline therapeutic options for all MM patients,” he said. “Effective frontline therapy has the potential to modify the course of the disease, which is a key outcome for transplant-ineligible patients who often face high rates of attrition in later lines of therapy.”
For the industry-funded IMROZ study, researchers recruited patients aged 18-80 at 93 sites in 21 nations from 2017-2019. All were ineligible for transplant due to comorbidities or being aged 65 or older. Exclusions included Eastern Cooperative Oncology Group (ECOG) performance status scores of more than 2.
The subjects were randomly assigned in a 3-to-2 ratio to isatuximab-VRd (n = 265) or VRd alone (n = 181) and received four induction cycles (6 weeks per cycle) followed by 4-week cycles of continuous treatment until disease progression, unacceptable adverse event, or other criteria for discontinuation. If progression occurred, patients could be switched from the VRd-only group to the isatuximab-VRd group.
The median age in both the isatuximab-VRd and VRd groups was 72. The percentages of women were 46.0% and 48.1%, respectively, and 72.5% and 72.4%, respectively, were White. The next largest race/ethnic group was Asian (11.7% and 9.4%, respectively). Almost all had ECOG status of 0 or 1 (88.7% and 89.5%, respectively).
At study cut-off in September 2023, the percentages of subjects in the isatuximab-VRd and VRd groups who were still receiving treatment were 47.2% and 24.3%, respectively.
An intention-to-treat analysis found that the two groups had similar rates of overall response (91.3% for isatuximab-VRd vs. 92.3% for VRd), but the isatuximab-VRd group had higher complete or better response (74.7% vs. 64.1%, P = .01).
The percentage of patients who were minimal residual disease (MRD)-negative and had a complete response was also higher in the VRd-isatuximab group vs. the VRd group (55.5% vs. 40.9%, respectively, P = .003). A total of 26.0% of patients in the VRd-isatuximab group died vs. 32.6% in the VRd group; the estimated overall survival rates at 60 months were 72.3% and 66.3%, respectively, HR = 0.78, 99.97% CI).
As for adverse events, grade 5 events were more common in the VRd-isatuximab group (11.0% vs. 5.5%), as were deaths within the first 60 days of treatment (1.5% vs. 0.6%). “The difference was driven in part by different treatment exposures,” the researchers reported. Treatment-emergent events led to treatment discontinuation in 22.8% and 26.0% of patients, respectively.
“The safety and tolerability of Sarclisa observed was consistent with the established safety profile of Sarclisa and VRd with no new safety signals observed,” Dr. Facon said.
In an interview, Zandra Klippel, MD, global product head for multiple myeloma at Sanofi — the maker of isatuximab and funder of the study — said the Food and Drug Administration has accepted a priority review application for the investigational use of isatuximab in combination with VRd for the treatment of patients with transplant-ineligible, newly diagnosed MM.
“Our FDA approval date is expected on September 27, 2024,” Dr. Klippel said. “If all goes well, we anticipate launching as early as 2024 in the US and rolling out in other key countries starting in 2025 and continuing through 2026.”
Dr. Klippel added that isatuximab “continues to be evaluated in multiple ongoing phase 3 clinical trials in combination with current standard treatments across the MM treatment continuum.”
In an interview, Sagar Lonial, MD, chair and professor of hematology and medical oncology and chief medical officer at Winship Cancer Institute at Emory University in Atlanta, said the study is “important.”
However, Dr. Lonial, who is familiar with the findings but didn’t take part in the study, said it’s difficult to understand the impact of the treatment on frail patients. It appears that the combination treatment may be good for frail patients, he said, “but I need to better understand the magnitude of the benefit in that subset a little more.”
As for adverse events, he said “they are what would be expected for a trial like this.”
Pneumonia and COVID-19 infections were higher in the VRd-isatuximab group, he said, and “we know in general that vaccine responses are blocked by CD38 antibodies.” This can be managed, he said, via intravenous immunoglobulin support.
Manni Mohyuddin, MD, assistant professor at Huntsman Cancer Institute in Utah, said in an interview that the findings suggest that in older, fit patients, “you can get fairly good outcomes without use of transplant.”
In the United States, many more patients in the cohort would have been considered transplant-eligible, he said, and not eliminated from consideration for transplant due to age over 65. However, as patients age, “you get more diminishing returns for transplants,” said Dr. Mohyuddin, who is familiar with the study findings but didn’t take part in the research.
All the drugs in the new combination are FDA approved, he said, although the combination isn’t. “I suspect this will make it to our guidelines very soon and then be reimbursed by insurance companies and Medicare.”
The study was funded by Sanofi and an M.D. Anderson Cancer Center support grant. Dr. Facon has no disclosures. Other study authors report multiple ties relationships with various drug makers. Dr. Lonial disclosed ties with Takeda, Amgen, Novartis, BMS, GSK, AbbVie, Genentech, Pfizer, Regeneron, Janssen, AstraZeneca, and TG Therapeutics). Dr. Mohyuddin disclosed a relationship with Janssen.
FROM ASCO 2024
Melatonin May Cut Risk for Age-Related Eye Disease
TOPLINE:
Melatonin supplementation is linked to a reduced risk for developing age-related macular degeneration (AMD) and slowing its progression, suggesting potential as a preventive therapy.
METHODOLOGY:
- Researchers analyzed data from the TriNetX database, covering electronic medical records across the United States from December 2023 to March 2024.
- The retrospective study included patients aged ≥ 50 years, divided into groups based on their history of AMD and melatonin medication codes between November 2008 and November 2023.
- Propensity score matching was used to compare melatonin users and nonusers for the risk for developing any form of AMD or the progression to exudative AMD from the nonexudative form of the condition.
TAKEAWAY:
- Use of melatonin was associated with a 58% reduction in the risk for developing AMD, according to the researchers.
- In people with nonexudative AMD, use of the supplement was linked to a 56% lower risk for progression to exudative AMD.
- The findings were consistent across age groups, suggesting melatonin’s benefits may extend to older populations at higher risk for AMD, the researchers reported.
IN PRACTICE:
“In this cohort study of 121,523 patients with no history of AMD aged ≥ 50 years, taking melatonin was associated with a decreased risk of developing AMD,” the authors of the study wrote. “Likewise, among 66,253 patients with preexisting nonexudative AMD, melatonin supplementation was negatively associated with the rate of progression to exudative AMD.”
Studies in animals and humans have shown melatonin may be a potent antioxidant and anti-inflammatory agent and have both antiangiogenic and mitochondrial-preserving properties, the authors noted. The new findings “provide a rationale for expanding clinical research on the potential therapeutic efficacy of melatonin in preventing AMD development or its progression,” they added.
SOURCE:
The study was led by Hejin Jeong, Case Western Reserve University School of Medicine, Cleveland, and was published online in JAMA Ophthalmology.
LIMITATIONS:
The study’s reliance on diagnostic codes may have limited the accuracy of identifying AMD progression. Variations in coding practices and the reporting of over-the-counter medications like melatonin could have influenced the results. The study did not control for all modifiable risk factors for AMD, which may have introduced healthy user bias.
DISCLOSURES:
The authors reported various potential conflicts of interest, including receiving personal fees and grants from various pharmaceutical companies. The study was funded by grants from the National Institutes of Health and the Cleveland Eye Bank Foundation.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article appeared on Medscape.com.
TOPLINE:
Melatonin supplementation is linked to a reduced risk for developing age-related macular degeneration (AMD) and slowing its progression, suggesting potential as a preventive therapy.
METHODOLOGY:
- Researchers analyzed data from the TriNetX database, covering electronic medical records across the United States from December 2023 to March 2024.
- The retrospective study included patients aged ≥ 50 years, divided into groups based on their history of AMD and melatonin medication codes between November 2008 and November 2023.
- Propensity score matching was used to compare melatonin users and nonusers for the risk for developing any form of AMD or the progression to exudative AMD from the nonexudative form of the condition.
TAKEAWAY:
- Use of melatonin was associated with a 58% reduction in the risk for developing AMD, according to the researchers.
- In people with nonexudative AMD, use of the supplement was linked to a 56% lower risk for progression to exudative AMD.
- The findings were consistent across age groups, suggesting melatonin’s benefits may extend to older populations at higher risk for AMD, the researchers reported.
IN PRACTICE:
“In this cohort study of 121,523 patients with no history of AMD aged ≥ 50 years, taking melatonin was associated with a decreased risk of developing AMD,” the authors of the study wrote. “Likewise, among 66,253 patients with preexisting nonexudative AMD, melatonin supplementation was negatively associated with the rate of progression to exudative AMD.”
Studies in animals and humans have shown melatonin may be a potent antioxidant and anti-inflammatory agent and have both antiangiogenic and mitochondrial-preserving properties, the authors noted. The new findings “provide a rationale for expanding clinical research on the potential therapeutic efficacy of melatonin in preventing AMD development or its progression,” they added.
SOURCE:
The study was led by Hejin Jeong, Case Western Reserve University School of Medicine, Cleveland, and was published online in JAMA Ophthalmology.
LIMITATIONS:
The study’s reliance on diagnostic codes may have limited the accuracy of identifying AMD progression. Variations in coding practices and the reporting of over-the-counter medications like melatonin could have influenced the results. The study did not control for all modifiable risk factors for AMD, which may have introduced healthy user bias.
DISCLOSURES:
The authors reported various potential conflicts of interest, including receiving personal fees and grants from various pharmaceutical companies. The study was funded by grants from the National Institutes of Health and the Cleveland Eye Bank Foundation.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article appeared on Medscape.com.
TOPLINE:
Melatonin supplementation is linked to a reduced risk for developing age-related macular degeneration (AMD) and slowing its progression, suggesting potential as a preventive therapy.
METHODOLOGY:
- Researchers analyzed data from the TriNetX database, covering electronic medical records across the United States from December 2023 to March 2024.
- The retrospective study included patients aged ≥ 50 years, divided into groups based on their history of AMD and melatonin medication codes between November 2008 and November 2023.
- Propensity score matching was used to compare melatonin users and nonusers for the risk for developing any form of AMD or the progression to exudative AMD from the nonexudative form of the condition.
TAKEAWAY:
- Use of melatonin was associated with a 58% reduction in the risk for developing AMD, according to the researchers.
- In people with nonexudative AMD, use of the supplement was linked to a 56% lower risk for progression to exudative AMD.
- The findings were consistent across age groups, suggesting melatonin’s benefits may extend to older populations at higher risk for AMD, the researchers reported.
IN PRACTICE:
“In this cohort study of 121,523 patients with no history of AMD aged ≥ 50 years, taking melatonin was associated with a decreased risk of developing AMD,” the authors of the study wrote. “Likewise, among 66,253 patients with preexisting nonexudative AMD, melatonin supplementation was negatively associated with the rate of progression to exudative AMD.”
Studies in animals and humans have shown melatonin may be a potent antioxidant and anti-inflammatory agent and have both antiangiogenic and mitochondrial-preserving properties, the authors noted. The new findings “provide a rationale for expanding clinical research on the potential therapeutic efficacy of melatonin in preventing AMD development or its progression,” they added.
SOURCE:
The study was led by Hejin Jeong, Case Western Reserve University School of Medicine, Cleveland, and was published online in JAMA Ophthalmology.
LIMITATIONS:
The study’s reliance on diagnostic codes may have limited the accuracy of identifying AMD progression. Variations in coding practices and the reporting of over-the-counter medications like melatonin could have influenced the results. The study did not control for all modifiable risk factors for AMD, which may have introduced healthy user bias.
DISCLOSURES:
The authors reported various potential conflicts of interest, including receiving personal fees and grants from various pharmaceutical companies. The study was funded by grants from the National Institutes of Health and the Cleveland Eye Bank Foundation.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article appeared on Medscape.com.
SCD: Delaying Transition to Adult Care Poses Risks
TOPLINE:
METHODOLOGY:
- Guidelines have recommended that young adults with SCD transfer from pediatric care within 6 months, but many transfers take longer — sometimes up to a year.
- Researchers evaluated the impact of prolonged transition gaps on health outcomes in 183 young adults who completed pediatric care between 2012 and 2018 and were transitioned to an adult care program. Patients were followed for 2-8 years from their first adult care visit.
TAKEAWAY:
- Approximately 88% of patients transferred to adult health care within 6 months, with a median transfer gap of 1.4 months. At 2 years of adult care, patients with a transition gap of 6 months or longer were 89% (relative risk, 1.89) more likely to have an inpatient visit and 75% (RR, 1.75) more likely to have ED visits.
- Those with transfer gaps of 6 months or longer had twice the rate of inpatient visits (rate ratio, 2.01) at 8 years of follow-up, compared with those who transitioned within 2 months.
- However, fewer adult care outpatient visits were seen (5.1 vs 6.7 visits per year) for young adults transferred in 6 or more months versus those within 6 months.
IN PRACTICE:
According to the authors, “longer delays in establishing adult health care following pediatric care were associated with greater acute health care resource utilization and fewer health care maintenance (ie, outpatient) SCD visits. These findings emphasize the importance of swift transfer from pediatric to adult care among young adults with SCD.”
SOURCE:
The study was led by Kristen E. Howell, of the Department of Epidemiology and Biostatistics, Texas A&M School of Public Health, College Station, Texas, and was published online in Blood Advances.
LIMITATIONS:
Data was available only for patients within a specific health care system, limiting the generalizability of the findings. Involving only one pediatric and two adult programs could impact findings. Insurance loss or changes due to low income were not accounted for.
DISCLOSURES:
The study was funded by U1EMC19331 and the American Lebanese Syrian Associated Charities. The authors declared no relevant conflicts of interest.
TOPLINE:
METHODOLOGY:
- Guidelines have recommended that young adults with SCD transfer from pediatric care within 6 months, but many transfers take longer — sometimes up to a year.
- Researchers evaluated the impact of prolonged transition gaps on health outcomes in 183 young adults who completed pediatric care between 2012 and 2018 and were transitioned to an adult care program. Patients were followed for 2-8 years from their first adult care visit.
TAKEAWAY:
- Approximately 88% of patients transferred to adult health care within 6 months, with a median transfer gap of 1.4 months. At 2 years of adult care, patients with a transition gap of 6 months or longer were 89% (relative risk, 1.89) more likely to have an inpatient visit and 75% (RR, 1.75) more likely to have ED visits.
- Those with transfer gaps of 6 months or longer had twice the rate of inpatient visits (rate ratio, 2.01) at 8 years of follow-up, compared with those who transitioned within 2 months.
- However, fewer adult care outpatient visits were seen (5.1 vs 6.7 visits per year) for young adults transferred in 6 or more months versus those within 6 months.
IN PRACTICE:
According to the authors, “longer delays in establishing adult health care following pediatric care were associated with greater acute health care resource utilization and fewer health care maintenance (ie, outpatient) SCD visits. These findings emphasize the importance of swift transfer from pediatric to adult care among young adults with SCD.”
SOURCE:
The study was led by Kristen E. Howell, of the Department of Epidemiology and Biostatistics, Texas A&M School of Public Health, College Station, Texas, and was published online in Blood Advances.
LIMITATIONS:
Data was available only for patients within a specific health care system, limiting the generalizability of the findings. Involving only one pediatric and two adult programs could impact findings. Insurance loss or changes due to low income were not accounted for.
DISCLOSURES:
The study was funded by U1EMC19331 and the American Lebanese Syrian Associated Charities. The authors declared no relevant conflicts of interest.
TOPLINE:
METHODOLOGY:
- Guidelines have recommended that young adults with SCD transfer from pediatric care within 6 months, but many transfers take longer — sometimes up to a year.
- Researchers evaluated the impact of prolonged transition gaps on health outcomes in 183 young adults who completed pediatric care between 2012 and 2018 and were transitioned to an adult care program. Patients were followed for 2-8 years from their first adult care visit.
TAKEAWAY:
- Approximately 88% of patients transferred to adult health care within 6 months, with a median transfer gap of 1.4 months. At 2 years of adult care, patients with a transition gap of 6 months or longer were 89% (relative risk, 1.89) more likely to have an inpatient visit and 75% (RR, 1.75) more likely to have ED visits.
- Those with transfer gaps of 6 months or longer had twice the rate of inpatient visits (rate ratio, 2.01) at 8 years of follow-up, compared with those who transitioned within 2 months.
- However, fewer adult care outpatient visits were seen (5.1 vs 6.7 visits per year) for young adults transferred in 6 or more months versus those within 6 months.
IN PRACTICE:
According to the authors, “longer delays in establishing adult health care following pediatric care were associated with greater acute health care resource utilization and fewer health care maintenance (ie, outpatient) SCD visits. These findings emphasize the importance of swift transfer from pediatric to adult care among young adults with SCD.”
SOURCE:
The study was led by Kristen E. Howell, of the Department of Epidemiology and Biostatistics, Texas A&M School of Public Health, College Station, Texas, and was published online in Blood Advances.
LIMITATIONS:
Data was available only for patients within a specific health care system, limiting the generalizability of the findings. Involving only one pediatric and two adult programs could impact findings. Insurance loss or changes due to low income were not accounted for.
DISCLOSURES:
The study was funded by U1EMC19331 and the American Lebanese Syrian Associated Charities. The authors declared no relevant conflicts of interest.
Erythematous Flaky Rash on the Toe
The Diagnosis: Necrolytic Migratory Erythema
Necrolytic migratory erythema (NME) is a waxing and waning rash associated with rare pancreatic neuroendocrine tumors called glucagonomas. It is characterized by pruritic and painful, well-demarcated, erythematous plaques that manifest in the intertriginous areas and on the perineum and buttocks.1 Due to the evolving nature of the rash, the histopathologic findings in NME vary depending on the stage of the cutaneous lesions at the time of biopsy.2 Multiple dyskeratotic keratinocytes spanning all epidermal layers may be a diagnostic clue in early lesions of NME.3 Typical features of longstanding lesions include confluent parakeratosis, psoriasiform hyperplasia with mild or absent spongiosis, and upper epidermal necrosis with keratinocyte vacuolization and pallor.4 Morphologic features that are present prior to the development of epidermal vacuolation and necrosis frequently are misattributed to psoriasis or eczema. Long-standing lesions also may develop a neutrophilic infiltrate with subcorneal and intraepidermal pustules.2 Other common features include a discrete perivascular lymphocytic infiltrate and an erosive or encrusted epidermis.5 Although direct immunofluorescence typically is negative, nonspecific findings can be seen, including apoptotic keratinocytes labeling with fibrinogen and C3, as well as scattered, clumped, IgM-positive cytoid bodies present at the dermal-epidermal junction (DEJ).6 Biopsies also have shown scattered, clumped, IgM-positive cytoid bodies present at the DEJ.5
Psoriasis is a chronic relapsing papulosquamous disorder characterized by scaly erythematous plaques often overlying the extensor surfaces of the extremities. Histopathology shows a psoriasiform pattern of inflammation with thinning of the suprapapillary plates and elongation of the rete ridges. Further diagnostic clues of psoriasis include regular acanthosis, characteristic Munro microabscesses with neutrophils in a hyperkeratotic stratum corneum (Figure 1), hypogranulosis, and neutrophilic spongiform pustules of Kogoj in the stratum spinosum. Generally, there is a lack of the epidermal necrosis seen with NME.7,8
Lichen simplex chronicus manifests as pruritic, often hyperpigmented, well-defined, lichenified plaques with excoriation following repetitive mechanical trauma, commonly on the lower lateral legs, posterior neck, and flexural areas.9 The histologic landscape is marked by well-developed lesions evolving to show compact orthokeratosis, hypergranulosis, irregularly elongated rete ridges (ie, irregular acanthosis), and papillary dermal fibrosis with vertical streaking of collagen (Figure 2).9,10
Subacute cutaneous lupus erythematosus (SCLE) is recognized clinically by scaly/psoriasiform and annular lesions with mild or absent systemic involvement. Common histopathologic findings include epidermal atrophy, vacuolar interface dermatitis with hydropic degeneration of the basal layer, a subepidermal lymphocytic infiltrate, and a periadnexal and perivascular infiltrate (Figure 3).11 Upper dermal edema, spotty necrosis of individual cells in the epidermis, dermal-epidermal separation caused by prominent basal cell degeneration, and accumulation of acid mucopolysaccharides (mucin) are other histologic features associated with SCLE.12,13
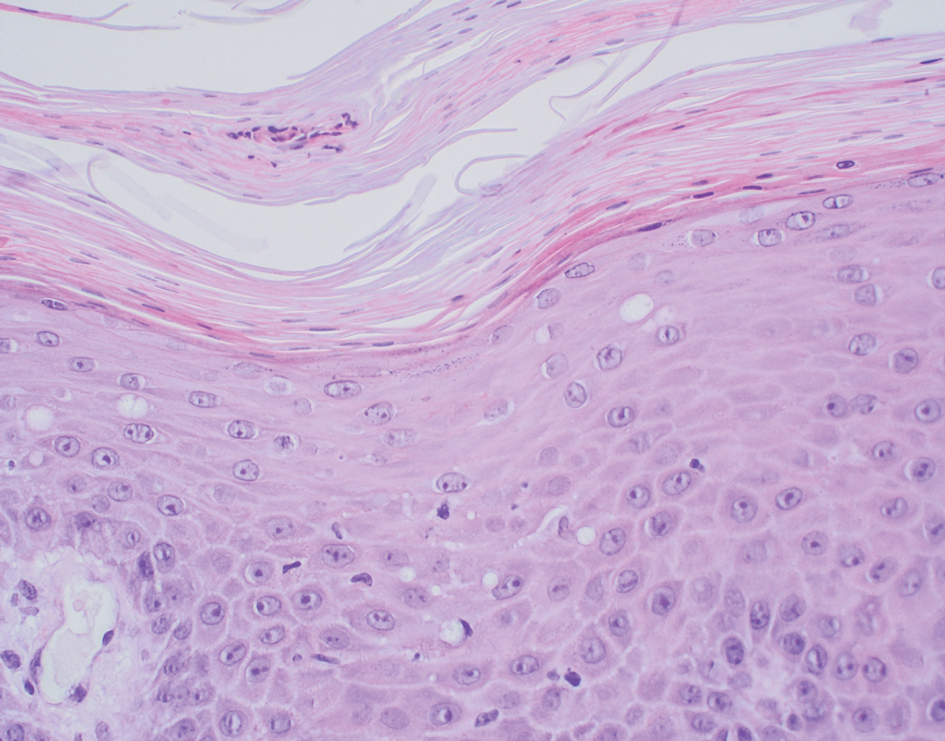
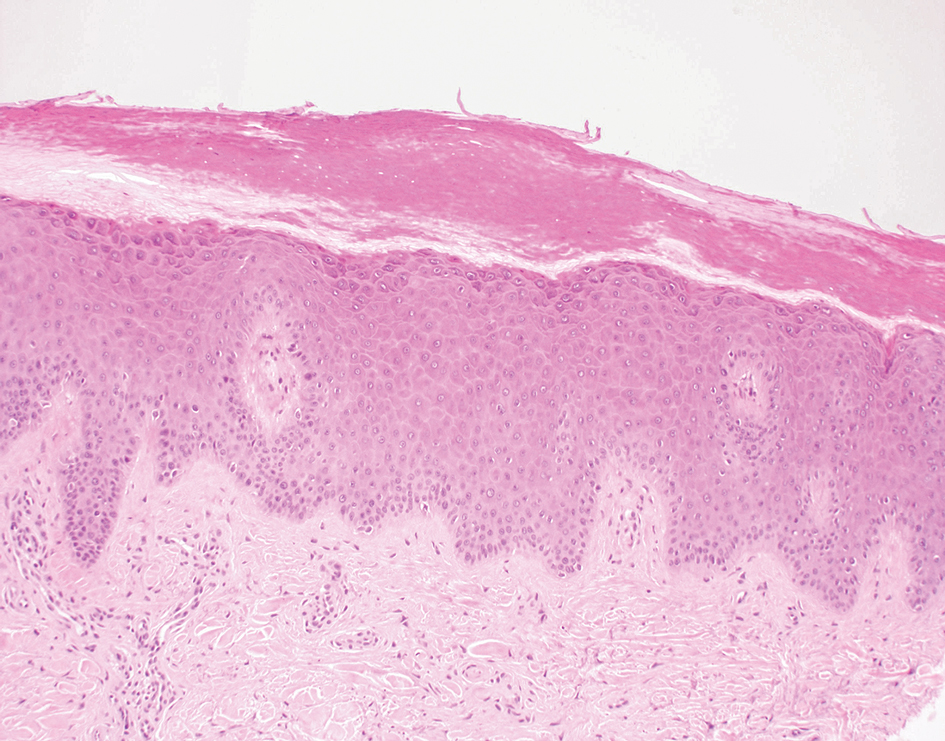
The immunofluorescence pattern in SCLE features dustlike particles of IgG deposition in the epidermis, subepidermal region, and dermal cellular infiltrate. Lesions also may have granular deposition of immunoreactions at the DEJ.11,13
The manifestation of drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome (also known as drug-induced hypersensitivity syndrome) is variable, with a morbilliform rash that spreads from the face to the entire body, urticaria, atypical target lesions, purpuriform lesions, lymphadenopathy, and exfoliative dermatitis.14 The nonspecific morphologic features of DRESS syndrome lesions are associated with variable histologic features, which include focal interface changes with vacuolar alteration of the basal layer; atypical lymphocytes with hyperchromic nuclei; and a superficial, inconsistently dense, perivascular lymphocytic infiltrate. Other relatively common histopathologic patterns include an upper dermis with dilated blood vessels, spongiosis with exocytosis of lymphocytes (Figure 4), and necrotic keratinocytes. Although peripheral eosinophilia is an important diagnostic criterion and is observed consistently, eosinophils are variably present on skin biopsy.15,16 Given the histopathologic variability and nonspecific findings, clinical correlation is required when diagnosing DRESS syndrome.
- Halvorson SA, Gilbert E, Hopkins RS, et al. Putting the pieces together: necrolytic migratory erythema and the glucagonoma syndrome. J Gen Intern Med. 2013;28:1525-1529. doi:10.1007 /s11606-013-2490-5
- Toberer F, Hartschuh W, Wiedemeyer K. Glucagonoma-associated necrolytic migratory erythema: the broad spectrum of the clinical and histopathological findings and clues to the diagnosis. Am J Dermatopathol. 2019;41:E29-E32. doi:10.1097DAD .0000000000001219
- Hunt SJ, Narus VT, Abell E. Necrolytic migratory erythema: dyskeratotic dermatitis, a clue to early diagnosis. J Am Acad Dermatol. 1991; 24:473-477. doi:10.1016/0190-9622(91)70076-e
- van Beek AP, de Haas ER, van Vloten WA, et al. The glucagonoma syndrome and necrolytic migratory erythema: a clinical review. Eur J Endocrinol. 2004;151:531-537. doi:10.1530/eje.0.1510531
- Pujol RM, Wang C-Y E, el-Azhary RA, et al. Necrolytic migratory erythema: clinicopathologic study of 13 cases. Int J Dermatol. 2004;43:12- 18. doi:10.1111/j.1365-4632.2004.01844.x
- Johnson SM, Smoller BR, Lamps LW, et al. Necrolytic migratory erythema as the only presenting sign of a glucagonoma. J Am Acad Dermatol. 2003;49:325-328. doi:10.1067/s0190-9622(02)61774-8
- De Rosa G, Mignogna C. The histopathology of psoriasis. Reumatismo. 2007;59(suppl 1):46-48. doi:10.4081/reumatismo.2007.1s.46
- Kimmel GW, Lebwohl M. Psoriasis: overview and diagnosis. In: Bhutani T, Liao W, Nakamura M, eds. Evidence-Based Psoriasis. Springer; 2018:1-16. doi:10.1007/978-3-319-90107-7_1
- Balan R, Grigoras¸ A, Popovici D, et al. The histopathological landscape of the major psoriasiform dermatoses. Arch Clin Cases. 2021;6:59-68. doi:10.22551/2019.24.0603.10155
- O’Keefe RJ, Scurry JP, Dennerstein G, et al. Audit of 114 nonneoplastic vulvar biopsies. Br J Obstet Gynaecol. 1995;102:780-786. doi:10.1111/j.1471-0528.1995.tb10842.x
- Parodi A, Caproni M, Cardinali C, et al P. Clinical, histological and immunopathological features of 58 patients with subacute cutaneous lupus erythematosus. Dermatology. 2000;200:6-10. doi:10.1159/000018307
- Lyon CC, Blewitt R, Harrison PV. Subacute cutaneous lupus erythematosus: two cases of delayed diagnosis. Acta Derm Venereol. 1998;78:57-59. doi:10.1080/00015559850135869
- David-Bajar KM. Subacute cutaneous lupus erythematosus. J Invest Dermatol. 1993;100:2S-8S. doi:10.1111/1523-1747.ep12355164
- Paulmann M, Mockenhaupt M. Severe drug-induced skin reactions: clinical features, diagnosis, etiology, and therapy. J Dtsch Dermatol Ges. 2015;13:625-643. doi:10.1111/ddg.12747
- Borroni G, Torti S, Pezzini C, et al. Histopathologic spectrum of drug reaction with eosinophilia and systemic symptoms (DRESS): a diagnosis that needs clinico-pathological correlation. G Ital Dermatol Venereol. 2014;149:291-300.
- Ortonne N, Valeyrie-Allanore L, Bastuji-Garin S, et al. Histopathology of drug rash with eosinophilia and systemic symptoms syndrome: a morphological and phenotypical study. Br J Dermatol. 2015;173:50-58. doi:10.1111/bjd.13683
The Diagnosis: Necrolytic Migratory Erythema
Necrolytic migratory erythema (NME) is a waxing and waning rash associated with rare pancreatic neuroendocrine tumors called glucagonomas. It is characterized by pruritic and painful, well-demarcated, erythematous plaques that manifest in the intertriginous areas and on the perineum and buttocks.1 Due to the evolving nature of the rash, the histopathologic findings in NME vary depending on the stage of the cutaneous lesions at the time of biopsy.2 Multiple dyskeratotic keratinocytes spanning all epidermal layers may be a diagnostic clue in early lesions of NME.3 Typical features of longstanding lesions include confluent parakeratosis, psoriasiform hyperplasia with mild or absent spongiosis, and upper epidermal necrosis with keratinocyte vacuolization and pallor.4 Morphologic features that are present prior to the development of epidermal vacuolation and necrosis frequently are misattributed to psoriasis or eczema. Long-standing lesions also may develop a neutrophilic infiltrate with subcorneal and intraepidermal pustules.2 Other common features include a discrete perivascular lymphocytic infiltrate and an erosive or encrusted epidermis.5 Although direct immunofluorescence typically is negative, nonspecific findings can be seen, including apoptotic keratinocytes labeling with fibrinogen and C3, as well as scattered, clumped, IgM-positive cytoid bodies present at the dermal-epidermal junction (DEJ).6 Biopsies also have shown scattered, clumped, IgM-positive cytoid bodies present at the DEJ.5
Psoriasis is a chronic relapsing papulosquamous disorder characterized by scaly erythematous plaques often overlying the extensor surfaces of the extremities. Histopathology shows a psoriasiform pattern of inflammation with thinning of the suprapapillary plates and elongation of the rete ridges. Further diagnostic clues of psoriasis include regular acanthosis, characteristic Munro microabscesses with neutrophils in a hyperkeratotic stratum corneum (Figure 1), hypogranulosis, and neutrophilic spongiform pustules of Kogoj in the stratum spinosum. Generally, there is a lack of the epidermal necrosis seen with NME.7,8
Lichen simplex chronicus manifests as pruritic, often hyperpigmented, well-defined, lichenified plaques with excoriation following repetitive mechanical trauma, commonly on the lower lateral legs, posterior neck, and flexural areas.9 The histologic landscape is marked by well-developed lesions evolving to show compact orthokeratosis, hypergranulosis, irregularly elongated rete ridges (ie, irregular acanthosis), and papillary dermal fibrosis with vertical streaking of collagen (Figure 2).9,10
Subacute cutaneous lupus erythematosus (SCLE) is recognized clinically by scaly/psoriasiform and annular lesions with mild or absent systemic involvement. Common histopathologic findings include epidermal atrophy, vacuolar interface dermatitis with hydropic degeneration of the basal layer, a subepidermal lymphocytic infiltrate, and a periadnexal and perivascular infiltrate (Figure 3).11 Upper dermal edema, spotty necrosis of individual cells in the epidermis, dermal-epidermal separation caused by prominent basal cell degeneration, and accumulation of acid mucopolysaccharides (mucin) are other histologic features associated with SCLE.12,13


The immunofluorescence pattern in SCLE features dustlike particles of IgG deposition in the epidermis, subepidermal region, and dermal cellular infiltrate. Lesions also may have granular deposition of immunoreactions at the DEJ.11,13


The manifestation of drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome (also known as drug-induced hypersensitivity syndrome) is variable, with a morbilliform rash that spreads from the face to the entire body, urticaria, atypical target lesions, purpuriform lesions, lymphadenopathy, and exfoliative dermatitis.14 The nonspecific morphologic features of DRESS syndrome lesions are associated with variable histologic features, which include focal interface changes with vacuolar alteration of the basal layer; atypical lymphocytes with hyperchromic nuclei; and a superficial, inconsistently dense, perivascular lymphocytic infiltrate. Other relatively common histopathologic patterns include an upper dermis with dilated blood vessels, spongiosis with exocytosis of lymphocytes (Figure 4), and necrotic keratinocytes. Although peripheral eosinophilia is an important diagnostic criterion and is observed consistently, eosinophils are variably present on skin biopsy.15,16 Given the histopathologic variability and nonspecific findings, clinical correlation is required when diagnosing DRESS syndrome.
The Diagnosis: Necrolytic Migratory Erythema
Necrolytic migratory erythema (NME) is a waxing and waning rash associated with rare pancreatic neuroendocrine tumors called glucagonomas. It is characterized by pruritic and painful, well-demarcated, erythematous plaques that manifest in the intertriginous areas and on the perineum and buttocks.1 Due to the evolving nature of the rash, the histopathologic findings in NME vary depending on the stage of the cutaneous lesions at the time of biopsy.2 Multiple dyskeratotic keratinocytes spanning all epidermal layers may be a diagnostic clue in early lesions of NME.3 Typical features of longstanding lesions include confluent parakeratosis, psoriasiform hyperplasia with mild or absent spongiosis, and upper epidermal necrosis with keratinocyte vacuolization and pallor.4 Morphologic features that are present prior to the development of epidermal vacuolation and necrosis frequently are misattributed to psoriasis or eczema. Long-standing lesions also may develop a neutrophilic infiltrate with subcorneal and intraepidermal pustules.2 Other common features include a discrete perivascular lymphocytic infiltrate and an erosive or encrusted epidermis.5 Although direct immunofluorescence typically is negative, nonspecific findings can be seen, including apoptotic keratinocytes labeling with fibrinogen and C3, as well as scattered, clumped, IgM-positive cytoid bodies present at the dermal-epidermal junction (DEJ).6 Biopsies also have shown scattered, clumped, IgM-positive cytoid bodies present at the DEJ.5
Psoriasis is a chronic relapsing papulosquamous disorder characterized by scaly erythematous plaques often overlying the extensor surfaces of the extremities. Histopathology shows a psoriasiform pattern of inflammation with thinning of the suprapapillary plates and elongation of the rete ridges. Further diagnostic clues of psoriasis include regular acanthosis, characteristic Munro microabscesses with neutrophils in a hyperkeratotic stratum corneum (Figure 1), hypogranulosis, and neutrophilic spongiform pustules of Kogoj in the stratum spinosum. Generally, there is a lack of the epidermal necrosis seen with NME.7,8
Lichen simplex chronicus manifests as pruritic, often hyperpigmented, well-defined, lichenified plaques with excoriation following repetitive mechanical trauma, commonly on the lower lateral legs, posterior neck, and flexural areas.9 The histologic landscape is marked by well-developed lesions evolving to show compact orthokeratosis, hypergranulosis, irregularly elongated rete ridges (ie, irregular acanthosis), and papillary dermal fibrosis with vertical streaking of collagen (Figure 2).9,10
Subacute cutaneous lupus erythematosus (SCLE) is recognized clinically by scaly/psoriasiform and annular lesions with mild or absent systemic involvement. Common histopathologic findings include epidermal atrophy, vacuolar interface dermatitis with hydropic degeneration of the basal layer, a subepidermal lymphocytic infiltrate, and a periadnexal and perivascular infiltrate (Figure 3).11 Upper dermal edema, spotty necrosis of individual cells in the epidermis, dermal-epidermal separation caused by prominent basal cell degeneration, and accumulation of acid mucopolysaccharides (mucin) are other histologic features associated with SCLE.12,13


The immunofluorescence pattern in SCLE features dustlike particles of IgG deposition in the epidermis, subepidermal region, and dermal cellular infiltrate. Lesions also may have granular deposition of immunoreactions at the DEJ.11,13


The manifestation of drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome (also known as drug-induced hypersensitivity syndrome) is variable, with a morbilliform rash that spreads from the face to the entire body, urticaria, atypical target lesions, purpuriform lesions, lymphadenopathy, and exfoliative dermatitis.14 The nonspecific morphologic features of DRESS syndrome lesions are associated with variable histologic features, which include focal interface changes with vacuolar alteration of the basal layer; atypical lymphocytes with hyperchromic nuclei; and a superficial, inconsistently dense, perivascular lymphocytic infiltrate. Other relatively common histopathologic patterns include an upper dermis with dilated blood vessels, spongiosis with exocytosis of lymphocytes (Figure 4), and necrotic keratinocytes. Although peripheral eosinophilia is an important diagnostic criterion and is observed consistently, eosinophils are variably present on skin biopsy.15,16 Given the histopathologic variability and nonspecific findings, clinical correlation is required when diagnosing DRESS syndrome.
- Halvorson SA, Gilbert E, Hopkins RS, et al. Putting the pieces together: necrolytic migratory erythema and the glucagonoma syndrome. J Gen Intern Med. 2013;28:1525-1529. doi:10.1007 /s11606-013-2490-5
- Toberer F, Hartschuh W, Wiedemeyer K. Glucagonoma-associated necrolytic migratory erythema: the broad spectrum of the clinical and histopathological findings and clues to the diagnosis. Am J Dermatopathol. 2019;41:E29-E32. doi:10.1097DAD .0000000000001219
- Hunt SJ, Narus VT, Abell E. Necrolytic migratory erythema: dyskeratotic dermatitis, a clue to early diagnosis. J Am Acad Dermatol. 1991; 24:473-477. doi:10.1016/0190-9622(91)70076-e
- van Beek AP, de Haas ER, van Vloten WA, et al. The glucagonoma syndrome and necrolytic migratory erythema: a clinical review. Eur J Endocrinol. 2004;151:531-537. doi:10.1530/eje.0.1510531
- Pujol RM, Wang C-Y E, el-Azhary RA, et al. Necrolytic migratory erythema: clinicopathologic study of 13 cases. Int J Dermatol. 2004;43:12- 18. doi:10.1111/j.1365-4632.2004.01844.x
- Johnson SM, Smoller BR, Lamps LW, et al. Necrolytic migratory erythema as the only presenting sign of a glucagonoma. J Am Acad Dermatol. 2003;49:325-328. doi:10.1067/s0190-9622(02)61774-8
- De Rosa G, Mignogna C. The histopathology of psoriasis. Reumatismo. 2007;59(suppl 1):46-48. doi:10.4081/reumatismo.2007.1s.46
- Kimmel GW, Lebwohl M. Psoriasis: overview and diagnosis. In: Bhutani T, Liao W, Nakamura M, eds. Evidence-Based Psoriasis. Springer; 2018:1-16. doi:10.1007/978-3-319-90107-7_1
- Balan R, Grigoras¸ A, Popovici D, et al. The histopathological landscape of the major psoriasiform dermatoses. Arch Clin Cases. 2021;6:59-68. doi:10.22551/2019.24.0603.10155
- O’Keefe RJ, Scurry JP, Dennerstein G, et al. Audit of 114 nonneoplastic vulvar biopsies. Br J Obstet Gynaecol. 1995;102:780-786. doi:10.1111/j.1471-0528.1995.tb10842.x
- Parodi A, Caproni M, Cardinali C, et al P. Clinical, histological and immunopathological features of 58 patients with subacute cutaneous lupus erythematosus. Dermatology. 2000;200:6-10. doi:10.1159/000018307
- Lyon CC, Blewitt R, Harrison PV. Subacute cutaneous lupus erythematosus: two cases of delayed diagnosis. Acta Derm Venereol. 1998;78:57-59. doi:10.1080/00015559850135869
- David-Bajar KM. Subacute cutaneous lupus erythematosus. J Invest Dermatol. 1993;100:2S-8S. doi:10.1111/1523-1747.ep12355164
- Paulmann M, Mockenhaupt M. Severe drug-induced skin reactions: clinical features, diagnosis, etiology, and therapy. J Dtsch Dermatol Ges. 2015;13:625-643. doi:10.1111/ddg.12747
- Borroni G, Torti S, Pezzini C, et al. Histopathologic spectrum of drug reaction with eosinophilia and systemic symptoms (DRESS): a diagnosis that needs clinico-pathological correlation. G Ital Dermatol Venereol. 2014;149:291-300.
- Ortonne N, Valeyrie-Allanore L, Bastuji-Garin S, et al. Histopathology of drug rash with eosinophilia and systemic symptoms syndrome: a morphological and phenotypical study. Br J Dermatol. 2015;173:50-58. doi:10.1111/bjd.13683
- Halvorson SA, Gilbert E, Hopkins RS, et al. Putting the pieces together: necrolytic migratory erythema and the glucagonoma syndrome. J Gen Intern Med. 2013;28:1525-1529. doi:10.1007 /s11606-013-2490-5
- Toberer F, Hartschuh W, Wiedemeyer K. Glucagonoma-associated necrolytic migratory erythema: the broad spectrum of the clinical and histopathological findings and clues to the diagnosis. Am J Dermatopathol. 2019;41:E29-E32. doi:10.1097DAD .0000000000001219
- Hunt SJ, Narus VT, Abell E. Necrolytic migratory erythema: dyskeratotic dermatitis, a clue to early diagnosis. J Am Acad Dermatol. 1991; 24:473-477. doi:10.1016/0190-9622(91)70076-e
- van Beek AP, de Haas ER, van Vloten WA, et al. The glucagonoma syndrome and necrolytic migratory erythema: a clinical review. Eur J Endocrinol. 2004;151:531-537. doi:10.1530/eje.0.1510531
- Pujol RM, Wang C-Y E, el-Azhary RA, et al. Necrolytic migratory erythema: clinicopathologic study of 13 cases. Int J Dermatol. 2004;43:12- 18. doi:10.1111/j.1365-4632.2004.01844.x
- Johnson SM, Smoller BR, Lamps LW, et al. Necrolytic migratory erythema as the only presenting sign of a glucagonoma. J Am Acad Dermatol. 2003;49:325-328. doi:10.1067/s0190-9622(02)61774-8
- De Rosa G, Mignogna C. The histopathology of psoriasis. Reumatismo. 2007;59(suppl 1):46-48. doi:10.4081/reumatismo.2007.1s.46
- Kimmel GW, Lebwohl M. Psoriasis: overview and diagnosis. In: Bhutani T, Liao W, Nakamura M, eds. Evidence-Based Psoriasis. Springer; 2018:1-16. doi:10.1007/978-3-319-90107-7_1
- Balan R, Grigoras¸ A, Popovici D, et al. The histopathological landscape of the major psoriasiform dermatoses. Arch Clin Cases. 2021;6:59-68. doi:10.22551/2019.24.0603.10155
- O’Keefe RJ, Scurry JP, Dennerstein G, et al. Audit of 114 nonneoplastic vulvar biopsies. Br J Obstet Gynaecol. 1995;102:780-786. doi:10.1111/j.1471-0528.1995.tb10842.x
- Parodi A, Caproni M, Cardinali C, et al P. Clinical, histological and immunopathological features of 58 patients with subacute cutaneous lupus erythematosus. Dermatology. 2000;200:6-10. doi:10.1159/000018307
- Lyon CC, Blewitt R, Harrison PV. Subacute cutaneous lupus erythematosus: two cases of delayed diagnosis. Acta Derm Venereol. 1998;78:57-59. doi:10.1080/00015559850135869
- David-Bajar KM. Subacute cutaneous lupus erythematosus. J Invest Dermatol. 1993;100:2S-8S. doi:10.1111/1523-1747.ep12355164
- Paulmann M, Mockenhaupt M. Severe drug-induced skin reactions: clinical features, diagnosis, etiology, and therapy. J Dtsch Dermatol Ges. 2015;13:625-643. doi:10.1111/ddg.12747
- Borroni G, Torti S, Pezzini C, et al. Histopathologic spectrum of drug reaction with eosinophilia and systemic symptoms (DRESS): a diagnosis that needs clinico-pathological correlation. G Ital Dermatol Venereol. 2014;149:291-300.
- Ortonne N, Valeyrie-Allanore L, Bastuji-Garin S, et al. Histopathology of drug rash with eosinophilia and systemic symptoms syndrome: a morphological and phenotypical study. Br J Dermatol. 2015;173:50-58. doi:10.1111/bjd.13683
A 62-year-old man presented with an erythematous flaky rash associated with burning pain on the right medial second toe that persisted for several months. Prior treatment with econazole, ciclopirox, and oral amoxicillin had failed. A shave biopsy was performed.