User login
Improving ILD diagnosis in primary care settings
Interstitial lung diseases (ILDs), with their many ubiquitous symptoms, are often hard to diagnose in patients. That’s why Amirahwaty Abdullah, MBBS, and Kavitha Selvan, MD, see value in educating clinicians on how to identify and diagnose ILD.
Both Dr. Abdullah and Dr. Selvan received quality improvement grants from CHEST in October 2023 to do just that. Their projects put the ILD Clinician Toolkit into practice, created as a part of CHEST’s Bridging Specialties™: Timely Diagnosis for ILD initiative aimed at shortening the time to diagnosis.
Recently, CHEST caught up with the two grant recipients to see how their projects were progressing.
Combating the prevalence of ILDs in West Virginia
Dr. Abdullah and Co-Principal Investigator, Haroon Ahmed, MD, are two of the staff members supporting West Virginia University ILD Clinic, where the ILD Clinician Toolkit is being utilized.
“ILD is so prevalent here, so we thought it would be an excellent opportunity to do the quality improvement project here because we really do need the resources to improve the care of our [patients with ILD],” Dr. Abdullah said. “For the entire state of West Virginia, we’re the only ILD center.”
In West Virginia, many factors are at play making ILD prevalent, with a recent study showing that the state has the second highest rate of interstitial pulmonary fibrosis, Dr. Abdullah said. This is all due to the economy of the state, the rurality of the population, and the occupational hazards with common jobs like coal mining and farming.
“This topic about bridging the gap and early diagnosis really resonated with us because we see all these patients who end up seeing us after they’ve been on oxygen for years, when they can’t do anything else,” Dr. Ahmed said. “There was a big gap here, and we saw that every day in our clinical practice.”
Since implementing the ILD Clinician Toolkit into their program, the two have started providing these resources to primary care physicians in an effort to help expedite their workups when they see patients with common ILD symptoms. This was done through grand rounds and educational conferences for those practicing in family medicine, internal medicine, and pediatric medicine. And more education is planned for the future.
They have also created working relationships with these departments and have encouraged them to send patients to the ILD Clinic, so patients don’t have to be referred to multiple different physicians.
The next step for the project is to implement telemedicine capabilities, which will allow the team to roll out the patient questionnaire from the toolkit. The questionnaire helps physicians gather a detailed history about their patients, including their past and current medications, surgeries, occupational and environmental exposures, and known comorbidities.
“We definitely want to reach out via telemedicine to patients because, at this point in time, some of our patients travel 3, 4 hours one way just to come see us. So, if we can make it more accessible, we will,” Dr. Abdullah said.
Their plan is to provide iPads that are equipped with the questionnaire and other toolkit resources to the providers. Through this method, the team will be able to see how often the questionnaire is used.
“We are very thankful for this grant because I do think we are saving lives,” Dr. Abdullah said. “Any little thing that we can do to improve the outcomes of these patients who have a rare but difficult-to-treat disease—it’s crucial. This gives us the ability to reach out and help patients who are out of our physical bounds.”
Diagnosing ILD among underrepresented minority populations in Chicago
Dr. Kavitha Selvan is conducting her quality improvement research project at the University of Chicago, alongside her team.
“The community we serve in South Chicago houses a significant number of underrepresented [minority populations],” she said.
“We know that Black patients with ILD experience higher rates of hospitalization, compared with White patients,” she said. “And they are hospitalized, require lung transplants, and die at a younger age too.”
In clinical practice, Dr. Selvan has noticed a trend of patients being referred for evaluation of possible ILD later and later. In some cases, several years go by before the appropriate work for ILD is initiated, and that valuable time lost leads to irreversible loss of lung function.
Dr. Selvan’s plan is to partner with primary care providers who are seeing these patients first in order to expedite the work-up and referral of ILD when appropriate and, ultimately, reduce the amount of time that passes between symptom onset and definitive diagnosis.
“To me, this grant and the resources it provided represented an important opportunity to improve outcomes in the high-risk patients we care for through early disease recognition and treatment,” she said.
Dr. Selvan’s project began with educating members of the Primary Care Group at UChicago on risk factors and exam findings that may suggest ILD in patients coming to clinic with nonspecific respiratory complaints. Then they had to equip providers with the ILD Clinician Toolkit and reach patients by handing out the patient questionnaire in primary care clinic.
The next step for this project is going to be dissecting the answers to a postintervention survey that was sent out to understand the practices and comfort of primary care providers in evaluating suspected ILD and the utility of the additional resources created by Dr. Selvan’s team.
“My hope is that we can utilize this partnership with the Primary Care Group to provide the education that’s needed both on the patient side and the provider side, like knowing not to ignore respiratory symptoms, knowing which patients warrant ILD-specific testing, and knowing what the appropriate tests are. In doing so, we can get patients into ILD clinic earlier, confirm their diagnosis, and get them initiated on the appropriate therapies sooner,” she said.
Dr. Selvan credits the quality improvement grant as being fundamental in her success as a fellow and early faculty at UChicago Medicine and believes that this project has created a culture of awareness that wouldn’t have been possible without funding.
“I truly believe that early detection and risk factor modification is the most critical aspect of interstitial lung disease care, and the mechanism for how we’re going to actually improve patient outcomes in our community,” Dr. Selvan said. “Building the infrastructure to accomplish that requires time, resources, and support from institutions and philanthropic foundations that believe in that mission too."
Opportunities like this are made possible by generous contributions from our donors. Make a gift to CHEST and select “Clinical Research” to help aid future research into interstitial lung disease and much more.
Interstitial lung diseases (ILDs), with their many ubiquitous symptoms, are often hard to diagnose in patients. That’s why Amirahwaty Abdullah, MBBS, and Kavitha Selvan, MD, see value in educating clinicians on how to identify and diagnose ILD.
Both Dr. Abdullah and Dr. Selvan received quality improvement grants from CHEST in October 2023 to do just that. Their projects put the ILD Clinician Toolkit into practice, created as a part of CHEST’s Bridging Specialties™: Timely Diagnosis for ILD initiative aimed at shortening the time to diagnosis.
Recently, CHEST caught up with the two grant recipients to see how their projects were progressing.
Combating the prevalence of ILDs in West Virginia
Dr. Abdullah and Co-Principal Investigator, Haroon Ahmed, MD, are two of the staff members supporting West Virginia University ILD Clinic, where the ILD Clinician Toolkit is being utilized.
“ILD is so prevalent here, so we thought it would be an excellent opportunity to do the quality improvement project here because we really do need the resources to improve the care of our [patients with ILD],” Dr. Abdullah said. “For the entire state of West Virginia, we’re the only ILD center.”
In West Virginia, many factors are at play making ILD prevalent, with a recent study showing that the state has the second highest rate of interstitial pulmonary fibrosis, Dr. Abdullah said. This is all due to the economy of the state, the rurality of the population, and the occupational hazards with common jobs like coal mining and farming.
“This topic about bridging the gap and early diagnosis really resonated with us because we see all these patients who end up seeing us after they’ve been on oxygen for years, when they can’t do anything else,” Dr. Ahmed said. “There was a big gap here, and we saw that every day in our clinical practice.”
Since implementing the ILD Clinician Toolkit into their program, the two have started providing these resources to primary care physicians in an effort to help expedite their workups when they see patients with common ILD symptoms. This was done through grand rounds and educational conferences for those practicing in family medicine, internal medicine, and pediatric medicine. And more education is planned for the future.
They have also created working relationships with these departments and have encouraged them to send patients to the ILD Clinic, so patients don’t have to be referred to multiple different physicians.
The next step for the project is to implement telemedicine capabilities, which will allow the team to roll out the patient questionnaire from the toolkit. The questionnaire helps physicians gather a detailed history about their patients, including their past and current medications, surgeries, occupational and environmental exposures, and known comorbidities.
“We definitely want to reach out via telemedicine to patients because, at this point in time, some of our patients travel 3, 4 hours one way just to come see us. So, if we can make it more accessible, we will,” Dr. Abdullah said.
Their plan is to provide iPads that are equipped with the questionnaire and other toolkit resources to the providers. Through this method, the team will be able to see how often the questionnaire is used.
“We are very thankful for this grant because I do think we are saving lives,” Dr. Abdullah said. “Any little thing that we can do to improve the outcomes of these patients who have a rare but difficult-to-treat disease—it’s crucial. This gives us the ability to reach out and help patients who are out of our physical bounds.”
Diagnosing ILD among underrepresented minority populations in Chicago
Dr. Kavitha Selvan is conducting her quality improvement research project at the University of Chicago, alongside her team.
“The community we serve in South Chicago houses a significant number of underrepresented [minority populations],” she said.
“We know that Black patients with ILD experience higher rates of hospitalization, compared with White patients,” she said. “And they are hospitalized, require lung transplants, and die at a younger age too.”
In clinical practice, Dr. Selvan has noticed a trend of patients being referred for evaluation of possible ILD later and later. In some cases, several years go by before the appropriate work for ILD is initiated, and that valuable time lost leads to irreversible loss of lung function.
Dr. Selvan’s plan is to partner with primary care providers who are seeing these patients first in order to expedite the work-up and referral of ILD when appropriate and, ultimately, reduce the amount of time that passes between symptom onset and definitive diagnosis.
“To me, this grant and the resources it provided represented an important opportunity to improve outcomes in the high-risk patients we care for through early disease recognition and treatment,” she said.
Dr. Selvan’s project began with educating members of the Primary Care Group at UChicago on risk factors and exam findings that may suggest ILD in patients coming to clinic with nonspecific respiratory complaints. Then they had to equip providers with the ILD Clinician Toolkit and reach patients by handing out the patient questionnaire in primary care clinic.
The next step for this project is going to be dissecting the answers to a postintervention survey that was sent out to understand the practices and comfort of primary care providers in evaluating suspected ILD and the utility of the additional resources created by Dr. Selvan’s team.
“My hope is that we can utilize this partnership with the Primary Care Group to provide the education that’s needed both on the patient side and the provider side, like knowing not to ignore respiratory symptoms, knowing which patients warrant ILD-specific testing, and knowing what the appropriate tests are. In doing so, we can get patients into ILD clinic earlier, confirm their diagnosis, and get them initiated on the appropriate therapies sooner,” she said.
Dr. Selvan credits the quality improvement grant as being fundamental in her success as a fellow and early faculty at UChicago Medicine and believes that this project has created a culture of awareness that wouldn’t have been possible without funding.
“I truly believe that early detection and risk factor modification is the most critical aspect of interstitial lung disease care, and the mechanism for how we’re going to actually improve patient outcomes in our community,” Dr. Selvan said. “Building the infrastructure to accomplish that requires time, resources, and support from institutions and philanthropic foundations that believe in that mission too."
Opportunities like this are made possible by generous contributions from our donors. Make a gift to CHEST and select “Clinical Research” to help aid future research into interstitial lung disease and much more.
Interstitial lung diseases (ILDs), with their many ubiquitous symptoms, are often hard to diagnose in patients. That’s why Amirahwaty Abdullah, MBBS, and Kavitha Selvan, MD, see value in educating clinicians on how to identify and diagnose ILD.
Both Dr. Abdullah and Dr. Selvan received quality improvement grants from CHEST in October 2023 to do just that. Their projects put the ILD Clinician Toolkit into practice, created as a part of CHEST’s Bridging Specialties™: Timely Diagnosis for ILD initiative aimed at shortening the time to diagnosis.
Recently, CHEST caught up with the two grant recipients to see how their projects were progressing.
Combating the prevalence of ILDs in West Virginia
Dr. Abdullah and Co-Principal Investigator, Haroon Ahmed, MD, are two of the staff members supporting West Virginia University ILD Clinic, where the ILD Clinician Toolkit is being utilized.
“ILD is so prevalent here, so we thought it would be an excellent opportunity to do the quality improvement project here because we really do need the resources to improve the care of our [patients with ILD],” Dr. Abdullah said. “For the entire state of West Virginia, we’re the only ILD center.”
In West Virginia, many factors are at play making ILD prevalent, with a recent study showing that the state has the second highest rate of interstitial pulmonary fibrosis, Dr. Abdullah said. This is all due to the economy of the state, the rurality of the population, and the occupational hazards with common jobs like coal mining and farming.
“This topic about bridging the gap and early diagnosis really resonated with us because we see all these patients who end up seeing us after they’ve been on oxygen for years, when they can’t do anything else,” Dr. Ahmed said. “There was a big gap here, and we saw that every day in our clinical practice.”
Since implementing the ILD Clinician Toolkit into their program, the two have started providing these resources to primary care physicians in an effort to help expedite their workups when they see patients with common ILD symptoms. This was done through grand rounds and educational conferences for those practicing in family medicine, internal medicine, and pediatric medicine. And more education is planned for the future.
They have also created working relationships with these departments and have encouraged them to send patients to the ILD Clinic, so patients don’t have to be referred to multiple different physicians.
The next step for the project is to implement telemedicine capabilities, which will allow the team to roll out the patient questionnaire from the toolkit. The questionnaire helps physicians gather a detailed history about their patients, including their past and current medications, surgeries, occupational and environmental exposures, and known comorbidities.
“We definitely want to reach out via telemedicine to patients because, at this point in time, some of our patients travel 3, 4 hours one way just to come see us. So, if we can make it more accessible, we will,” Dr. Abdullah said.
Their plan is to provide iPads that are equipped with the questionnaire and other toolkit resources to the providers. Through this method, the team will be able to see how often the questionnaire is used.
“We are very thankful for this grant because I do think we are saving lives,” Dr. Abdullah said. “Any little thing that we can do to improve the outcomes of these patients who have a rare but difficult-to-treat disease—it’s crucial. This gives us the ability to reach out and help patients who are out of our physical bounds.”
Diagnosing ILD among underrepresented minority populations in Chicago
Dr. Kavitha Selvan is conducting her quality improvement research project at the University of Chicago, alongside her team.
“The community we serve in South Chicago houses a significant number of underrepresented [minority populations],” she said.
“We know that Black patients with ILD experience higher rates of hospitalization, compared with White patients,” she said. “And they are hospitalized, require lung transplants, and die at a younger age too.”
In clinical practice, Dr. Selvan has noticed a trend of patients being referred for evaluation of possible ILD later and later. In some cases, several years go by before the appropriate work for ILD is initiated, and that valuable time lost leads to irreversible loss of lung function.
Dr. Selvan’s plan is to partner with primary care providers who are seeing these patients first in order to expedite the work-up and referral of ILD when appropriate and, ultimately, reduce the amount of time that passes between symptom onset and definitive diagnosis.
“To me, this grant and the resources it provided represented an important opportunity to improve outcomes in the high-risk patients we care for through early disease recognition and treatment,” she said.
Dr. Selvan’s project began with educating members of the Primary Care Group at UChicago on risk factors and exam findings that may suggest ILD in patients coming to clinic with nonspecific respiratory complaints. Then they had to equip providers with the ILD Clinician Toolkit and reach patients by handing out the patient questionnaire in primary care clinic.
The next step for this project is going to be dissecting the answers to a postintervention survey that was sent out to understand the practices and comfort of primary care providers in evaluating suspected ILD and the utility of the additional resources created by Dr. Selvan’s team.
“My hope is that we can utilize this partnership with the Primary Care Group to provide the education that’s needed both on the patient side and the provider side, like knowing not to ignore respiratory symptoms, knowing which patients warrant ILD-specific testing, and knowing what the appropriate tests are. In doing so, we can get patients into ILD clinic earlier, confirm their diagnosis, and get them initiated on the appropriate therapies sooner,” she said.
Dr. Selvan credits the quality improvement grant as being fundamental in her success as a fellow and early faculty at UChicago Medicine and believes that this project has created a culture of awareness that wouldn’t have been possible without funding.
“I truly believe that early detection and risk factor modification is the most critical aspect of interstitial lung disease care, and the mechanism for how we’re going to actually improve patient outcomes in our community,” Dr. Selvan said. “Building the infrastructure to accomplish that requires time, resources, and support from institutions and philanthropic foundations that believe in that mission too."
Opportunities like this are made possible by generous contributions from our donors. Make a gift to CHEST and select “Clinical Research” to help aid future research into interstitial lung disease and much more.
Top reads from the CHEST journal portfolio
Studying endotypes in sleep apnea, diagnostic testing in ILD, and inhaled corticosteroid use in children
Journal CHEST®
By Xiaoting Wang, MM, and colleagues
This study from the Shanghai Sleep Health Study cohort enhances our understanding of physiologic endotypes in sleep apnea—nonpositional OSA (NPOSA) and positional OSA (POSA). The study found that nonanatomic traits play a more significant role in POSA severity. Patients with POSA exhibited lower arousal thresholds and greater ventilatory compensation, while anatomical abnormalities were more prominent in NPOSA. This research underscores the need for personalized treatment strategies based on specific endotypes, especially with the increasing emergence of alternatives to CPAP therapy, such as mandibular advancement therapy and hypoglossal nerve stimulation. Patients with obesity with NPOSA were found to have elevated loop gain, which is known to be impacted by weight loss. This is particularly relevant in light of recent studies showing that new GLP-1 agonists can dramatically reduce sleep apnea severity. Future research should focus on refining these personalized approaches and integrating alternative treatments to provide a more individualized approach to managing sleep apnea.
– Commentary by Shyam Subramanian, MD, FCCP, Member of the CHEST Physician Editorial Board
CHEST® Pulmonary
By Fayez Kheir, MD, and colleagues
The novel mRNA genomic array, Envisia Genomic Classifier (EGC), used in this study has been shown to be highly specific for a histopathologic diagnosis of usual interstitial pneumonia (UIP). A diagnosis of UIP carries significant prognostic import as patients exhibit a higher risk for progressive fibrosis and death compared with other interstitial lung diseases (ILDs). In this retrospective study, the combination of bronchoscopic lung cryobiopsy and EGC testing was associated with a significant change in clinical management of those living with an indeterminate ILD. Participants were more likely to be started on antifibrotic therapy following this intervention. This study emphasizes the need for prospective trials on the diagnostic yield of molecular phenotyping with bronchoscopic lung biopsy compared with the traditional surgical approach for the undifferentiated ILD.
– Commentary by Michael Marll, MD, Member of the CHEST Physician Editorial Board
CHEST® Critical Care
By Elizabeth Landzberg, MD, and colleagues
Respiratory illness is the most common reason for both hospitalization and ICU admission in children. Studies suggest inhaled corticosteroids (ICSs) may protect adults with direct lung injury (DLI) from developing respiratory failure. However, there is a paucity of literature on this therapeutic intervention within the pediatric population. This retrospective, large, single-center study identified children seeking treatment at the emergency department (ED) with DLI before hospitalization. ICS use before hospitalization was associated with one-half the odds of escalation to intubation and noninvasive respiratory support (NRS) in pediatric patients seeking treatment in the ED with DLI. The protective effect was greatest in patients with a history of asthma. According to the authors, this is the first study to assess ICS exposure before hospitalization and progression to respiratory failure in all-cause pediatric DLI. It highlights the need for future studies to evaluate whether a role exists for early prophylactic ICS use in pediatric DLI without status asthmaticus, stratified by history of asthma.
– Commentary by Anne C. Coates, MD, FCCP, Member of the CHEST Physician Editorial Board
Studying endotypes in sleep apnea, diagnostic testing in ILD, and inhaled corticosteroid use in children
Studying endotypes in sleep apnea, diagnostic testing in ILD, and inhaled corticosteroid use in children
Journal CHEST®
By Xiaoting Wang, MM, and colleagues
This study from the Shanghai Sleep Health Study cohort enhances our understanding of physiologic endotypes in sleep apnea—nonpositional OSA (NPOSA) and positional OSA (POSA). The study found that nonanatomic traits play a more significant role in POSA severity. Patients with POSA exhibited lower arousal thresholds and greater ventilatory compensation, while anatomical abnormalities were more prominent in NPOSA. This research underscores the need for personalized treatment strategies based on specific endotypes, especially with the increasing emergence of alternatives to CPAP therapy, such as mandibular advancement therapy and hypoglossal nerve stimulation. Patients with obesity with NPOSA were found to have elevated loop gain, which is known to be impacted by weight loss. This is particularly relevant in light of recent studies showing that new GLP-1 agonists can dramatically reduce sleep apnea severity. Future research should focus on refining these personalized approaches and integrating alternative treatments to provide a more individualized approach to managing sleep apnea.
– Commentary by Shyam Subramanian, MD, FCCP, Member of the CHEST Physician Editorial Board
CHEST® Pulmonary
By Fayez Kheir, MD, and colleagues
The novel mRNA genomic array, Envisia Genomic Classifier (EGC), used in this study has been shown to be highly specific for a histopathologic diagnosis of usual interstitial pneumonia (UIP). A diagnosis of UIP carries significant prognostic import as patients exhibit a higher risk for progressive fibrosis and death compared with other interstitial lung diseases (ILDs). In this retrospective study, the combination of bronchoscopic lung cryobiopsy and EGC testing was associated with a significant change in clinical management of those living with an indeterminate ILD. Participants were more likely to be started on antifibrotic therapy following this intervention. This study emphasizes the need for prospective trials on the diagnostic yield of molecular phenotyping with bronchoscopic lung biopsy compared with the traditional surgical approach for the undifferentiated ILD.
– Commentary by Michael Marll, MD, Member of the CHEST Physician Editorial Board
CHEST® Critical Care
By Elizabeth Landzberg, MD, and colleagues
Respiratory illness is the most common reason for both hospitalization and ICU admission in children. Studies suggest inhaled corticosteroids (ICSs) may protect adults with direct lung injury (DLI) from developing respiratory failure. However, there is a paucity of literature on this therapeutic intervention within the pediatric population. This retrospective, large, single-center study identified children seeking treatment at the emergency department (ED) with DLI before hospitalization. ICS use before hospitalization was associated with one-half the odds of escalation to intubation and noninvasive respiratory support (NRS) in pediatric patients seeking treatment in the ED with DLI. The protective effect was greatest in patients with a history of asthma. According to the authors, this is the first study to assess ICS exposure before hospitalization and progression to respiratory failure in all-cause pediatric DLI. It highlights the need for future studies to evaluate whether a role exists for early prophylactic ICS use in pediatric DLI without status asthmaticus, stratified by history of asthma.
– Commentary by Anne C. Coates, MD, FCCP, Member of the CHEST Physician Editorial Board
Journal CHEST®
By Xiaoting Wang, MM, and colleagues
This study from the Shanghai Sleep Health Study cohort enhances our understanding of physiologic endotypes in sleep apnea—nonpositional OSA (NPOSA) and positional OSA (POSA). The study found that nonanatomic traits play a more significant role in POSA severity. Patients with POSA exhibited lower arousal thresholds and greater ventilatory compensation, while anatomical abnormalities were more prominent in NPOSA. This research underscores the need for personalized treatment strategies based on specific endotypes, especially with the increasing emergence of alternatives to CPAP therapy, such as mandibular advancement therapy and hypoglossal nerve stimulation. Patients with obesity with NPOSA were found to have elevated loop gain, which is known to be impacted by weight loss. This is particularly relevant in light of recent studies showing that new GLP-1 agonists can dramatically reduce sleep apnea severity. Future research should focus on refining these personalized approaches and integrating alternative treatments to provide a more individualized approach to managing sleep apnea.
– Commentary by Shyam Subramanian, MD, FCCP, Member of the CHEST Physician Editorial Board
CHEST® Pulmonary
By Fayez Kheir, MD, and colleagues
The novel mRNA genomic array, Envisia Genomic Classifier (EGC), used in this study has been shown to be highly specific for a histopathologic diagnosis of usual interstitial pneumonia (UIP). A diagnosis of UIP carries significant prognostic import as patients exhibit a higher risk for progressive fibrosis and death compared with other interstitial lung diseases (ILDs). In this retrospective study, the combination of bronchoscopic lung cryobiopsy and EGC testing was associated with a significant change in clinical management of those living with an indeterminate ILD. Participants were more likely to be started on antifibrotic therapy following this intervention. This study emphasizes the need for prospective trials on the diagnostic yield of molecular phenotyping with bronchoscopic lung biopsy compared with the traditional surgical approach for the undifferentiated ILD.
– Commentary by Michael Marll, MD, Member of the CHEST Physician Editorial Board
CHEST® Critical Care
By Elizabeth Landzberg, MD, and colleagues
Respiratory illness is the most common reason for both hospitalization and ICU admission in children. Studies suggest inhaled corticosteroids (ICSs) may protect adults with direct lung injury (DLI) from developing respiratory failure. However, there is a paucity of literature on this therapeutic intervention within the pediatric population. This retrospective, large, single-center study identified children seeking treatment at the emergency department (ED) with DLI before hospitalization. ICS use before hospitalization was associated with one-half the odds of escalation to intubation and noninvasive respiratory support (NRS) in pediatric patients seeking treatment in the ED with DLI. The protective effect was greatest in patients with a history of asthma. According to the authors, this is the first study to assess ICS exposure before hospitalization and progression to respiratory failure in all-cause pediatric DLI. It highlights the need for future studies to evaluate whether a role exists for early prophylactic ICS use in pediatric DLI without status asthmaticus, stratified by history of asthma.
– Commentary by Anne C. Coates, MD, FCCP, Member of the CHEST Physician Editorial Board
APPs and POCUS: Overcoming credentialing challenges
APP INTERSECTION
Advanced practice providers (APPs) play an integral role in the care and management of patients both in the ICU and across the spectrum of health care. Due to reduced residency hours and the coming physician shortage, APPs are playing, and will continue to play, a greater role in the care and management of critically ill patients.
Point of care ultrasound (POCUS) is a key diagnostic modality that promotes rapid diagnosis, shortens time to key interventions, and allows for interval reassessment. However, the benefit relies heavily on operator skill to obtain good quality images, interpret this within the context of the patient, and know what interventions, if any, to make. It is imperative that APPs, given their role within critical care, become not only familiar with POCUS but demonstrate proficiency in practice.
Before this can become standard, there are a number of challenges to overcome. Educational institutions are behind on integrating POCUS into the APP curriculum.1 Additionally, APPs have few opportunities to develop these skills as residencies and fellowships are not required prior to entering the workforce. Instead, for a majority of APPs, POCUS training relies on nonstandardized on-the-job training or an (typically expensive) off-site training, for which they may not receive reimbursement. Other barriers include cultural practices that limit POCUS use by APPs; inability to upload and document images obtained; lack of willing, skilled mentors with time to provide feedback and assess for clinical competence; and even accessibility of ultrasound machines.2
Despite these challenges, the clinical benefits of utilizing ultrasound in practice necessitates overcoming these barriers. Institutionally, when APPs perform POCUS, it can lead to more rapid diagnosis and triage of patients, provide billing opportunities, and may reduce overall costs of care. From an APP perspective, having formalized training and credentialing can lead to more consistent POCUS assessment between providers, comprehensive patient care, security in one’s skills and knowledge base, and a tangible way of communicating one’s credibility between institutions.
While some institutions have overcome these barriers and APPs are trained and credentialed in POCUS, this is not always the norm. For this to come to fruition, we need educators to incorporate this into curriculum, colleagues who are willing to mentor learners, cultural changes to allow APPs to develop and utilize necessary skills for practice, clear structure for obtaining and maintaining competency, physicians to champion these efforts, and institutions to credential skilled APPs. Until then, it is important that we as APPs seek to facilitate these institutional changes and continue to set and maintain high quality standards for ourselves.
CHEST 2024 is for APPs too
With more than 300 educational sessions on the CHEST 2024 program, it can be overwhelming to figure out which ones you should put on your schedule. That’s why CHEST asked Danielle McCamey, DNP, CRNP, ACNP-BC, FCCP, and LaDonna Brown, DNP, CRNA, of DNPs of Color (DOC), as well as Corinne Young, MSN, FNP-C, FCCP, of the Association of Pulmonary Advanced Practice Providers (APAPP), for their recommendations on sessions that advanced practice providers shouldn’t miss. Visit chestnet.org/annual-meeting-apps for all of their recommendations.
References
1. Rudy S, Widmar B, Wilbeck J. Ultrasound education for nurse practitioner students: strategies for curricular integration. J Nurse Pract. 2024;20(6):104986.
2. Resnyk J, Weichold A. Barriers to learning and performing point-of-care ultrasound (POCUS): An integrative review. J Prof Nursing. 2024;54:54-62.
APP INTERSECTION
Advanced practice providers (APPs) play an integral role in the care and management of patients both in the ICU and across the spectrum of health care. Due to reduced residency hours and the coming physician shortage, APPs are playing, and will continue to play, a greater role in the care and management of critically ill patients.
Point of care ultrasound (POCUS) is a key diagnostic modality that promotes rapid diagnosis, shortens time to key interventions, and allows for interval reassessment. However, the benefit relies heavily on operator skill to obtain good quality images, interpret this within the context of the patient, and know what interventions, if any, to make. It is imperative that APPs, given their role within critical care, become not only familiar with POCUS but demonstrate proficiency in practice.
Before this can become standard, there are a number of challenges to overcome. Educational institutions are behind on integrating POCUS into the APP curriculum.1 Additionally, APPs have few opportunities to develop these skills as residencies and fellowships are not required prior to entering the workforce. Instead, for a majority of APPs, POCUS training relies on nonstandardized on-the-job training or an (typically expensive) off-site training, for which they may not receive reimbursement. Other barriers include cultural practices that limit POCUS use by APPs; inability to upload and document images obtained; lack of willing, skilled mentors with time to provide feedback and assess for clinical competence; and even accessibility of ultrasound machines.2
Despite these challenges, the clinical benefits of utilizing ultrasound in practice necessitates overcoming these barriers. Institutionally, when APPs perform POCUS, it can lead to more rapid diagnosis and triage of patients, provide billing opportunities, and may reduce overall costs of care. From an APP perspective, having formalized training and credentialing can lead to more consistent POCUS assessment between providers, comprehensive patient care, security in one’s skills and knowledge base, and a tangible way of communicating one’s credibility between institutions.
While some institutions have overcome these barriers and APPs are trained and credentialed in POCUS, this is not always the norm. For this to come to fruition, we need educators to incorporate this into curriculum, colleagues who are willing to mentor learners, cultural changes to allow APPs to develop and utilize necessary skills for practice, clear structure for obtaining and maintaining competency, physicians to champion these efforts, and institutions to credential skilled APPs. Until then, it is important that we as APPs seek to facilitate these institutional changes and continue to set and maintain high quality standards for ourselves.
CHEST 2024 is for APPs too
With more than 300 educational sessions on the CHEST 2024 program, it can be overwhelming to figure out which ones you should put on your schedule. That’s why CHEST asked Danielle McCamey, DNP, CRNP, ACNP-BC, FCCP, and LaDonna Brown, DNP, CRNA, of DNPs of Color (DOC), as well as Corinne Young, MSN, FNP-C, FCCP, of the Association of Pulmonary Advanced Practice Providers (APAPP), for their recommendations on sessions that advanced practice providers shouldn’t miss. Visit chestnet.org/annual-meeting-apps for all of their recommendations.
References
1. Rudy S, Widmar B, Wilbeck J. Ultrasound education for nurse practitioner students: strategies for curricular integration. J Nurse Pract. 2024;20(6):104986.
2. Resnyk J, Weichold A. Barriers to learning and performing point-of-care ultrasound (POCUS): An integrative review. J Prof Nursing. 2024;54:54-62.
APP INTERSECTION
Advanced practice providers (APPs) play an integral role in the care and management of patients both in the ICU and across the spectrum of health care. Due to reduced residency hours and the coming physician shortage, APPs are playing, and will continue to play, a greater role in the care and management of critically ill patients.
Point of care ultrasound (POCUS) is a key diagnostic modality that promotes rapid diagnosis, shortens time to key interventions, and allows for interval reassessment. However, the benefit relies heavily on operator skill to obtain good quality images, interpret this within the context of the patient, and know what interventions, if any, to make. It is imperative that APPs, given their role within critical care, become not only familiar with POCUS but demonstrate proficiency in practice.
Before this can become standard, there are a number of challenges to overcome. Educational institutions are behind on integrating POCUS into the APP curriculum.1 Additionally, APPs have few opportunities to develop these skills as residencies and fellowships are not required prior to entering the workforce. Instead, for a majority of APPs, POCUS training relies on nonstandardized on-the-job training or an (typically expensive) off-site training, for which they may not receive reimbursement. Other barriers include cultural practices that limit POCUS use by APPs; inability to upload and document images obtained; lack of willing, skilled mentors with time to provide feedback and assess for clinical competence; and even accessibility of ultrasound machines.2
Despite these challenges, the clinical benefits of utilizing ultrasound in practice necessitates overcoming these barriers. Institutionally, when APPs perform POCUS, it can lead to more rapid diagnosis and triage of patients, provide billing opportunities, and may reduce overall costs of care. From an APP perspective, having formalized training and credentialing can lead to more consistent POCUS assessment between providers, comprehensive patient care, security in one’s skills and knowledge base, and a tangible way of communicating one’s credibility between institutions.
While some institutions have overcome these barriers and APPs are trained and credentialed in POCUS, this is not always the norm. For this to come to fruition, we need educators to incorporate this into curriculum, colleagues who are willing to mentor learners, cultural changes to allow APPs to develop and utilize necessary skills for practice, clear structure for obtaining and maintaining competency, physicians to champion these efforts, and institutions to credential skilled APPs. Until then, it is important that we as APPs seek to facilitate these institutional changes and continue to set and maintain high quality standards for ourselves.
CHEST 2024 is for APPs too
With more than 300 educational sessions on the CHEST 2024 program, it can be overwhelming to figure out which ones you should put on your schedule. That’s why CHEST asked Danielle McCamey, DNP, CRNP, ACNP-BC, FCCP, and LaDonna Brown, DNP, CRNA, of DNPs of Color (DOC), as well as Corinne Young, MSN, FNP-C, FCCP, of the Association of Pulmonary Advanced Practice Providers (APAPP), for their recommendations on sessions that advanced practice providers shouldn’t miss. Visit chestnet.org/annual-meeting-apps for all of their recommendations.
References
1. Rudy S, Widmar B, Wilbeck J. Ultrasound education for nurse practitioner students: strategies for curricular integration. J Nurse Pract. 2024;20(6):104986.
2. Resnyk J, Weichold A. Barriers to learning and performing point-of-care ultrasound (POCUS): An integrative review. J Prof Nursing. 2024;54:54-62.
In memoriam: Dr. James E. Dalen
CHEST was recently informed of the death of former CHEST President, James E. Dalen, MD, MPH, Master FCCP. He served as CHEST President from 1985 to 1986.
Dr. Dalen was a graduate of Washington State University. (His undergraduate education was temporarily interrupted when he served as a Corpsman in the Navy and Marines.) He held a master’s degree from the University of Michigan, an MD from the University of Washington, an MPH from Harvard University, and an honorary Doctor of Science degree from the University of Massachusetts. He was internationally recognized as a medical visionary and esteemed cardiologist.
Dr. Dalen’s academic career spanned 3 medical schools. During the time he spent at each, he achieved great things and nurtured great ideas that helped transform medicine. From 1967 to 1975, he was on the faculty of Harvard Medical School (and the Peter Bent Brigham Hospital). From 1975 to 1988, he was a faculty member at the University of Massachusetts Medical School where he served as Chairman of Cardiovascular Medicine from 1975 to 1977 and as Chairman of Medicine from 1977 to 1988. From 1986 to 1987, he served as Interim Chancellor of the University of Massachusetts at the Worcester Campus. Dr. Dalen left Massachusetts in 1988 to become the Dean of the University of Arizona College of Medicine.
During his tenure as Dean and Vice-President at the University of Arizona, Dr. Dalen oversaw the establishment of the Zuckerman College of Public Health, the Arizona Center for Integrative Medicine, and the Arizona Telemedicine Program. Successful fundraising under his leadership led to the establishment of new research facilities, including the Children’s Research Center, the Sarver Heart Center, the Arizona Arthritis Center, and a major expansion of the Arizona Cancer Center. While at the University of Arizona College of Medicine, Dr. Dalen recognized the importance of integrative medicine and the need to promote integrative medicine in the academic environment. With the active participation of Dr. Andrew Weil, this led to the creation of the Academic Consortium for Integrative Medicine and Health and the Arizona Center for Integrative Medicine.
Dr. Dalen received many teaching awards. In 1987, he received the Distinguished Public Service Award from the University of Massachusetts. In 1988, he was named the University of Washington Distinguished Medical Alumnus of the Year and received the Alumni Achievement Award from Washington State University. In 2000, he received the College Medal from CHEST and was named a Master Fellow. In 2010, he was awarded the Harvard School of Public Health’s highest honor for its alumni: the 2010 Alumni Award of Merit. In 2012, he was named a Master Fellow of the American College of Physicians and was awarded an honorary Doctor of Science degree by the University of Massachusetts in 2013. In 2015, he received the Bravewell Distinguished Service Award from the Academic Consortium for Integrative Medicine and Health for his role as one of the founders of the consortium and as one of the founders of the Arizona Center for Integrative Medicine at the University of Arizona.
In his spare time, he enjoyed reading, gardening, sailing, art, and music and was a lifelong student with a passion for the pursuit of knowledge. Dr. Dalen enjoyed watching basketball, particularly the Arizona Wildcats. His favorite place to spend time was by the ocean in Coronado, California. Dr. Dalen was an advocate for public health, social justice, and health care reform throughout his life and career. He mentored thousands of medical students and championed access to health care for populations who were underserved. He touched many lives as a leading physician and caring friend.
Dr. Dalen is survived by his devoted wife, Priscilla M. Dalen (Dunton); loving children, James E. Dalen, Jr, and Angela M. Snodgrass (Dalen); daughter-in-law, Suzan M. Dalen; son-in-law, Daniel N. Snodgrass; and many step-grandchildren. We remember our colleague and extend our sincere condolences.
CHEST was recently informed of the death of former CHEST President, James E. Dalen, MD, MPH, Master FCCP. He served as CHEST President from 1985 to 1986.
Dr. Dalen was a graduate of Washington State University. (His undergraduate education was temporarily interrupted when he served as a Corpsman in the Navy and Marines.) He held a master’s degree from the University of Michigan, an MD from the University of Washington, an MPH from Harvard University, and an honorary Doctor of Science degree from the University of Massachusetts. He was internationally recognized as a medical visionary and esteemed cardiologist.
Dr. Dalen’s academic career spanned 3 medical schools. During the time he spent at each, he achieved great things and nurtured great ideas that helped transform medicine. From 1967 to 1975, he was on the faculty of Harvard Medical School (and the Peter Bent Brigham Hospital). From 1975 to 1988, he was a faculty member at the University of Massachusetts Medical School where he served as Chairman of Cardiovascular Medicine from 1975 to 1977 and as Chairman of Medicine from 1977 to 1988. From 1986 to 1987, he served as Interim Chancellor of the University of Massachusetts at the Worcester Campus. Dr. Dalen left Massachusetts in 1988 to become the Dean of the University of Arizona College of Medicine.
During his tenure as Dean and Vice-President at the University of Arizona, Dr. Dalen oversaw the establishment of the Zuckerman College of Public Health, the Arizona Center for Integrative Medicine, and the Arizona Telemedicine Program. Successful fundraising under his leadership led to the establishment of new research facilities, including the Children’s Research Center, the Sarver Heart Center, the Arizona Arthritis Center, and a major expansion of the Arizona Cancer Center. While at the University of Arizona College of Medicine, Dr. Dalen recognized the importance of integrative medicine and the need to promote integrative medicine in the academic environment. With the active participation of Dr. Andrew Weil, this led to the creation of the Academic Consortium for Integrative Medicine and Health and the Arizona Center for Integrative Medicine.
Dr. Dalen received many teaching awards. In 1987, he received the Distinguished Public Service Award from the University of Massachusetts. In 1988, he was named the University of Washington Distinguished Medical Alumnus of the Year and received the Alumni Achievement Award from Washington State University. In 2000, he received the College Medal from CHEST and was named a Master Fellow. In 2010, he was awarded the Harvard School of Public Health’s highest honor for its alumni: the 2010 Alumni Award of Merit. In 2012, he was named a Master Fellow of the American College of Physicians and was awarded an honorary Doctor of Science degree by the University of Massachusetts in 2013. In 2015, he received the Bravewell Distinguished Service Award from the Academic Consortium for Integrative Medicine and Health for his role as one of the founders of the consortium and as one of the founders of the Arizona Center for Integrative Medicine at the University of Arizona.
In his spare time, he enjoyed reading, gardening, sailing, art, and music and was a lifelong student with a passion for the pursuit of knowledge. Dr. Dalen enjoyed watching basketball, particularly the Arizona Wildcats. His favorite place to spend time was by the ocean in Coronado, California. Dr. Dalen was an advocate for public health, social justice, and health care reform throughout his life and career. He mentored thousands of medical students and championed access to health care for populations who were underserved. He touched many lives as a leading physician and caring friend.
Dr. Dalen is survived by his devoted wife, Priscilla M. Dalen (Dunton); loving children, James E. Dalen, Jr, and Angela M. Snodgrass (Dalen); daughter-in-law, Suzan M. Dalen; son-in-law, Daniel N. Snodgrass; and many step-grandchildren. We remember our colleague and extend our sincere condolences.
CHEST was recently informed of the death of former CHEST President, James E. Dalen, MD, MPH, Master FCCP. He served as CHEST President from 1985 to 1986.
Dr. Dalen was a graduate of Washington State University. (His undergraduate education was temporarily interrupted when he served as a Corpsman in the Navy and Marines.) He held a master’s degree from the University of Michigan, an MD from the University of Washington, an MPH from Harvard University, and an honorary Doctor of Science degree from the University of Massachusetts. He was internationally recognized as a medical visionary and esteemed cardiologist.
Dr. Dalen’s academic career spanned 3 medical schools. During the time he spent at each, he achieved great things and nurtured great ideas that helped transform medicine. From 1967 to 1975, he was on the faculty of Harvard Medical School (and the Peter Bent Brigham Hospital). From 1975 to 1988, he was a faculty member at the University of Massachusetts Medical School where he served as Chairman of Cardiovascular Medicine from 1975 to 1977 and as Chairman of Medicine from 1977 to 1988. From 1986 to 1987, he served as Interim Chancellor of the University of Massachusetts at the Worcester Campus. Dr. Dalen left Massachusetts in 1988 to become the Dean of the University of Arizona College of Medicine.
During his tenure as Dean and Vice-President at the University of Arizona, Dr. Dalen oversaw the establishment of the Zuckerman College of Public Health, the Arizona Center for Integrative Medicine, and the Arizona Telemedicine Program. Successful fundraising under his leadership led to the establishment of new research facilities, including the Children’s Research Center, the Sarver Heart Center, the Arizona Arthritis Center, and a major expansion of the Arizona Cancer Center. While at the University of Arizona College of Medicine, Dr. Dalen recognized the importance of integrative medicine and the need to promote integrative medicine in the academic environment. With the active participation of Dr. Andrew Weil, this led to the creation of the Academic Consortium for Integrative Medicine and Health and the Arizona Center for Integrative Medicine.
Dr. Dalen received many teaching awards. In 1987, he received the Distinguished Public Service Award from the University of Massachusetts. In 1988, he was named the University of Washington Distinguished Medical Alumnus of the Year and received the Alumni Achievement Award from Washington State University. In 2000, he received the College Medal from CHEST and was named a Master Fellow. In 2010, he was awarded the Harvard School of Public Health’s highest honor for its alumni: the 2010 Alumni Award of Merit. In 2012, he was named a Master Fellow of the American College of Physicians and was awarded an honorary Doctor of Science degree by the University of Massachusetts in 2013. In 2015, he received the Bravewell Distinguished Service Award from the Academic Consortium for Integrative Medicine and Health for his role as one of the founders of the consortium and as one of the founders of the Arizona Center for Integrative Medicine at the University of Arizona.
In his spare time, he enjoyed reading, gardening, sailing, art, and music and was a lifelong student with a passion for the pursuit of knowledge. Dr. Dalen enjoyed watching basketball, particularly the Arizona Wildcats. His favorite place to spend time was by the ocean in Coronado, California. Dr. Dalen was an advocate for public health, social justice, and health care reform throughout his life and career. He mentored thousands of medical students and championed access to health care for populations who were underserved. He touched many lives as a leading physician and caring friend.
Dr. Dalen is survived by his devoted wife, Priscilla M. Dalen (Dunton); loving children, James E. Dalen, Jr, and Angela M. Snodgrass (Dalen); daughter-in-law, Suzan M. Dalen; son-in-law, Daniel N. Snodgrass; and many step-grandchildren. We remember our colleague and extend our sincere condolences.
‘Psychological Weight’ Crucial in Patients With Obesity
Increasingly recognized as a multifactorial disease, obesity demands an approach that involves multiple healthcare professionals. For psychologist Andréa Levy, coordinator and founder of the nongovernmental organization Obesity Brazil, addressing the patient’s “psychological weight” is crucial.
In an interview with this news organization, Ms. Levy, who was one of the speakers at the International Congress on Obesity in 2024, emphasized the importance of integrating emotional and behavioral aspects into treatment, because these factors often influence eating habits and weight gain.
She also highlighted the essential collaboration between endocrinologists, nutritionists, psychiatrists, and psychologists, who must work together to provide comprehensive and effective care to patients.
How do psychological factors affect the treatment of obesity?
Psychological factors are important triggers for weight gain. As the degree of obesity increases, so does the predisposition to mental health problems such as anxiety, mood disorders, personality disorders, and eating disorders. Understanding these factors is important because accurate psychodiagnosis is essential for effective disease treatment.
Without a proper diagnosis, the treatment may be incomplete and omit relevant factors. For example, a person with undiagnosed depression who is starting treatment for weight loss may feel discouraged and low on energy. He or she may wrongly attribute these symptoms to the diet or surgery. Similarly, someone undergoing bariatric surgery may confuse malnutrition symptoms with depression, resulting in inadequate treatment with antidepressants and possible iatrogenic complications.
Furthermore, psychotherapy and psychological follow-up are essential to help the individual organize better and understand the treatment and the disease itself. This is especially important in stigmatized diseases and those subject to prejudice such as obesity, where understanding and acceptance are often challenging, which affects treatment adherence.
Is the collaboration between psychologist and psychiatrist always necessary?
Often, it is necessary to have the support of both a psychologist and a psychiatrist. The process generally begins with a good psychodiagnosis. Initially, there may not be a case that requires treatment, but it is important to perform this evaluation to rule out any issues.
The follow-up, unlike weekly psychotherapy, can be monthly or at an interval agreed on with the patient. It is crucial to help him or her navigate the various stages of obesity treatment. For example, the patient may be going through a period of mourning or separation, or a happier moment, such as the beginning of a relationship or the birth of a child in the family. These moments affect eating habits and need to be well managed.
Depending on the degree of the pathology, such as depression, severe binge-eating disorder, or personality disorders, the psychologist works in conjunction with the psychiatrist. When we talk about obesity, we are possibly also talking about a psychiatric population because it is a disease that, besides being highly recurrent, involves many other factors, such as the gaze of others, difficulty with dressing, body pains, mobility, and relationships. Therefore, having this disease alone is already a trigger for disorders such as depression.
What is the main evidence regarding the psychological follow-up of patients with obesity?
Several studies have investigated the relationship between obesity and mental health. Research indicates that the greater the obesity, the higher the likelihood of a positive diagnosis for a psychiatric disorder. Additionally, there is evidence of the benefits of psychological treatment for patients with obesity.
A study published in the Journal of Clinical Endocrinology and Metabolism addressed the impact of cognitive-behavioral therapy (CBT), which helps patients manage goals and treat maladaptive behaviors such as binge-eating disorders. CBT has a modest effect on weight loss, but its integration as part of a lifestyle modification amplifies the results of this loss.
Recent research also shows that weight loss through bariatric surgery offers significant psychological benefits. In the past, it was believed that this procedure could cause depression and other severe psychiatric disorders, but it is now more than proven that weight loss, when done properly and without misconduct or malnutrition, improves psychological and psychiatric issues.
How does psychological follow-up affect the use of medication during obesity treatment?
Many people who take medications, such as corticosteroids for chronic pain or psychiatric medications, may experience weight gain. It is essential to discuss these issues with the psychiatrist because if the patient already has a predisposition to weight gain, medication X should be chosen instead of medication Y, or the dosage should be adjusted. The psychiatrist needs to understand obesity to medicate correctly. Other types of medication, such as chemotherapeutics, may also cause weight gain, often resulting in more abdominal obesity.
There is also lipedema, a hormone-dependent disease that is different from obesity. In this disease, the person gains weight mainly in the legs and arms. In this case, bariatric surgery may result in weight loss only in specific areas, causing disproportionality and difficulty in understanding for the patient. Therefore, when treating obesity, it is important to analyze the patient from all angles: psychological, physiologic, and physical, considering the diversity of the body, its functioning, and hormonal reactions.
Although psychologists do not prescribe medications, they often explain their functioning to the patient. For example, if a patient is taking a glucagon-like peptide 1 analog and experiences initial nausea, he or she may stop using the treatment because the wrong dose had been started. In this case, the psychologist can explain how the medication works and encourage the patient to discuss adjustments with the doctor, avoiding premature discontinuation.
How has the mental health follow-up of patients with obesity evolved over the years?
I started working with people with obesity 25 years ago, when I myself underwent bariatric surgery. At that time, surgeons were used to “solving” the problem and sending the person home. Often, the patient did not even return for surgical follow-up because, in theory, the problem was solved.
Over time, I believe that surgeons learned to talk to the patient, understanding that there is a whole process that even involves creating a bond with the individual who underwent the surgical procedure. Within this process, the importance of the mental health of patients was recognized, and how common it is to confuse a degree of malnutrition with a mental disorder.
Even though I am not a nutritionist, I need to know the difference between a case of malnutrition and depression. So, it is a whole set of factors that needs to be worked on like an orchestra. It is not necessary for this work to be done in the same physical space, but dialogue is important.
Of course, there are things that the patient will only share with the psychologist or with the surgeon, but there are also pieces of information that need to be shared for positive management. I have had patients who were afraid to go back to the nutritionist because they did not lose weight. If they are afraid, it is because the professional is guiding them incorrectly.
What tips would you give to clinicians regarding the psychological approach to people with obesity?
Accessibility is crucial. When someone tells me they are dealing with obesity and depression, I usually ask, “Did you know you have two chronic diseases?” It is essential to explain these concepts because the patient may often think they are free after a successful diet and weight loss, which is not true because of the high relapse associated with obesity. Depression and anxiety follow similar patterns. If the same person wears prescription glasses, I interact by saying, “Did you know you have three chronic diseases?” This question often causes surprise. “I hadn’t thought of that.”
It is essential to use accessible language for the patient to understand the functioning of the disease. More important than choosing a treatment approach is understanding the pathophysiology of obesity and its psychological impact. This avoids a one-size-fits-all approach for all patients.
For example, the impact on someone who developed obesity in childhood after suffering physical, moral, or sexual abuse will probably be deeper than on someone in a healthy family who gained weight after becoming sedentary. Each life story requires a personalized approach.
Sometimes, a patient with mild obesity (grade 1) may not seem to need specific interventions at first glance, but it is crucial to listen to his or her story. Similarly, patients with severe obesity (grades 3 or 4) who resist surgery are entitled to other treatment options, and this is perfectly valid. Therefore, it is always important to ask, “Who is this person? What does obesity represent in their story?” Then propose the most appropriate treatment.
Ms. Levy reported having no relevant financial relationships.
This story was translated from the Medscape Portuguese edition using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Increasingly recognized as a multifactorial disease, obesity demands an approach that involves multiple healthcare professionals. For psychologist Andréa Levy, coordinator and founder of the nongovernmental organization Obesity Brazil, addressing the patient’s “psychological weight” is crucial.
In an interview with this news organization, Ms. Levy, who was one of the speakers at the International Congress on Obesity in 2024, emphasized the importance of integrating emotional and behavioral aspects into treatment, because these factors often influence eating habits and weight gain.
She also highlighted the essential collaboration between endocrinologists, nutritionists, psychiatrists, and psychologists, who must work together to provide comprehensive and effective care to patients.
How do psychological factors affect the treatment of obesity?
Psychological factors are important triggers for weight gain. As the degree of obesity increases, so does the predisposition to mental health problems such as anxiety, mood disorders, personality disorders, and eating disorders. Understanding these factors is important because accurate psychodiagnosis is essential for effective disease treatment.
Without a proper diagnosis, the treatment may be incomplete and omit relevant factors. For example, a person with undiagnosed depression who is starting treatment for weight loss may feel discouraged and low on energy. He or she may wrongly attribute these symptoms to the diet or surgery. Similarly, someone undergoing bariatric surgery may confuse malnutrition symptoms with depression, resulting in inadequate treatment with antidepressants and possible iatrogenic complications.
Furthermore, psychotherapy and psychological follow-up are essential to help the individual organize better and understand the treatment and the disease itself. This is especially important in stigmatized diseases and those subject to prejudice such as obesity, where understanding and acceptance are often challenging, which affects treatment adherence.
Is the collaboration between psychologist and psychiatrist always necessary?
Often, it is necessary to have the support of both a psychologist and a psychiatrist. The process generally begins with a good psychodiagnosis. Initially, there may not be a case that requires treatment, but it is important to perform this evaluation to rule out any issues.
The follow-up, unlike weekly psychotherapy, can be monthly or at an interval agreed on with the patient. It is crucial to help him or her navigate the various stages of obesity treatment. For example, the patient may be going through a period of mourning or separation, or a happier moment, such as the beginning of a relationship or the birth of a child in the family. These moments affect eating habits and need to be well managed.
Depending on the degree of the pathology, such as depression, severe binge-eating disorder, or personality disorders, the psychologist works in conjunction with the psychiatrist. When we talk about obesity, we are possibly also talking about a psychiatric population because it is a disease that, besides being highly recurrent, involves many other factors, such as the gaze of others, difficulty with dressing, body pains, mobility, and relationships. Therefore, having this disease alone is already a trigger for disorders such as depression.
What is the main evidence regarding the psychological follow-up of patients with obesity?
Several studies have investigated the relationship between obesity and mental health. Research indicates that the greater the obesity, the higher the likelihood of a positive diagnosis for a psychiatric disorder. Additionally, there is evidence of the benefits of psychological treatment for patients with obesity.
A study published in the Journal of Clinical Endocrinology and Metabolism addressed the impact of cognitive-behavioral therapy (CBT), which helps patients manage goals and treat maladaptive behaviors such as binge-eating disorders. CBT has a modest effect on weight loss, but its integration as part of a lifestyle modification amplifies the results of this loss.
Recent research also shows that weight loss through bariatric surgery offers significant psychological benefits. In the past, it was believed that this procedure could cause depression and other severe psychiatric disorders, but it is now more than proven that weight loss, when done properly and without misconduct or malnutrition, improves psychological and psychiatric issues.
How does psychological follow-up affect the use of medication during obesity treatment?
Many people who take medications, such as corticosteroids for chronic pain or psychiatric medications, may experience weight gain. It is essential to discuss these issues with the psychiatrist because if the patient already has a predisposition to weight gain, medication X should be chosen instead of medication Y, or the dosage should be adjusted. The psychiatrist needs to understand obesity to medicate correctly. Other types of medication, such as chemotherapeutics, may also cause weight gain, often resulting in more abdominal obesity.
There is also lipedema, a hormone-dependent disease that is different from obesity. In this disease, the person gains weight mainly in the legs and arms. In this case, bariatric surgery may result in weight loss only in specific areas, causing disproportionality and difficulty in understanding for the patient. Therefore, when treating obesity, it is important to analyze the patient from all angles: psychological, physiologic, and physical, considering the diversity of the body, its functioning, and hormonal reactions.
Although psychologists do not prescribe medications, they often explain their functioning to the patient. For example, if a patient is taking a glucagon-like peptide 1 analog and experiences initial nausea, he or she may stop using the treatment because the wrong dose had been started. In this case, the psychologist can explain how the medication works and encourage the patient to discuss adjustments with the doctor, avoiding premature discontinuation.
How has the mental health follow-up of patients with obesity evolved over the years?
I started working with people with obesity 25 years ago, when I myself underwent bariatric surgery. At that time, surgeons were used to “solving” the problem and sending the person home. Often, the patient did not even return for surgical follow-up because, in theory, the problem was solved.
Over time, I believe that surgeons learned to talk to the patient, understanding that there is a whole process that even involves creating a bond with the individual who underwent the surgical procedure. Within this process, the importance of the mental health of patients was recognized, and how common it is to confuse a degree of malnutrition with a mental disorder.
Even though I am not a nutritionist, I need to know the difference between a case of malnutrition and depression. So, it is a whole set of factors that needs to be worked on like an orchestra. It is not necessary for this work to be done in the same physical space, but dialogue is important.
Of course, there are things that the patient will only share with the psychologist or with the surgeon, but there are also pieces of information that need to be shared for positive management. I have had patients who were afraid to go back to the nutritionist because they did not lose weight. If they are afraid, it is because the professional is guiding them incorrectly.
What tips would you give to clinicians regarding the psychological approach to people with obesity?
Accessibility is crucial. When someone tells me they are dealing with obesity and depression, I usually ask, “Did you know you have two chronic diseases?” It is essential to explain these concepts because the patient may often think they are free after a successful diet and weight loss, which is not true because of the high relapse associated with obesity. Depression and anxiety follow similar patterns. If the same person wears prescription glasses, I interact by saying, “Did you know you have three chronic diseases?” This question often causes surprise. “I hadn’t thought of that.”
It is essential to use accessible language for the patient to understand the functioning of the disease. More important than choosing a treatment approach is understanding the pathophysiology of obesity and its psychological impact. This avoids a one-size-fits-all approach for all patients.
For example, the impact on someone who developed obesity in childhood after suffering physical, moral, or sexual abuse will probably be deeper than on someone in a healthy family who gained weight after becoming sedentary. Each life story requires a personalized approach.
Sometimes, a patient with mild obesity (grade 1) may not seem to need specific interventions at first glance, but it is crucial to listen to his or her story. Similarly, patients with severe obesity (grades 3 or 4) who resist surgery are entitled to other treatment options, and this is perfectly valid. Therefore, it is always important to ask, “Who is this person? What does obesity represent in their story?” Then propose the most appropriate treatment.
Ms. Levy reported having no relevant financial relationships.
This story was translated from the Medscape Portuguese edition using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Increasingly recognized as a multifactorial disease, obesity demands an approach that involves multiple healthcare professionals. For psychologist Andréa Levy, coordinator and founder of the nongovernmental organization Obesity Brazil, addressing the patient’s “psychological weight” is crucial.
In an interview with this news organization, Ms. Levy, who was one of the speakers at the International Congress on Obesity in 2024, emphasized the importance of integrating emotional and behavioral aspects into treatment, because these factors often influence eating habits and weight gain.
She also highlighted the essential collaboration between endocrinologists, nutritionists, psychiatrists, and psychologists, who must work together to provide comprehensive and effective care to patients.
How do psychological factors affect the treatment of obesity?
Psychological factors are important triggers for weight gain. As the degree of obesity increases, so does the predisposition to mental health problems such as anxiety, mood disorders, personality disorders, and eating disorders. Understanding these factors is important because accurate psychodiagnosis is essential for effective disease treatment.
Without a proper diagnosis, the treatment may be incomplete and omit relevant factors. For example, a person with undiagnosed depression who is starting treatment for weight loss may feel discouraged and low on energy. He or she may wrongly attribute these symptoms to the diet or surgery. Similarly, someone undergoing bariatric surgery may confuse malnutrition symptoms with depression, resulting in inadequate treatment with antidepressants and possible iatrogenic complications.
Furthermore, psychotherapy and psychological follow-up are essential to help the individual organize better and understand the treatment and the disease itself. This is especially important in stigmatized diseases and those subject to prejudice such as obesity, where understanding and acceptance are often challenging, which affects treatment adherence.
Is the collaboration between psychologist and psychiatrist always necessary?
Often, it is necessary to have the support of both a psychologist and a psychiatrist. The process generally begins with a good psychodiagnosis. Initially, there may not be a case that requires treatment, but it is important to perform this evaluation to rule out any issues.
The follow-up, unlike weekly psychotherapy, can be monthly or at an interval agreed on with the patient. It is crucial to help him or her navigate the various stages of obesity treatment. For example, the patient may be going through a period of mourning or separation, or a happier moment, such as the beginning of a relationship or the birth of a child in the family. These moments affect eating habits and need to be well managed.
Depending on the degree of the pathology, such as depression, severe binge-eating disorder, or personality disorders, the psychologist works in conjunction with the psychiatrist. When we talk about obesity, we are possibly also talking about a psychiatric population because it is a disease that, besides being highly recurrent, involves many other factors, such as the gaze of others, difficulty with dressing, body pains, mobility, and relationships. Therefore, having this disease alone is already a trigger for disorders such as depression.
What is the main evidence regarding the psychological follow-up of patients with obesity?
Several studies have investigated the relationship between obesity and mental health. Research indicates that the greater the obesity, the higher the likelihood of a positive diagnosis for a psychiatric disorder. Additionally, there is evidence of the benefits of psychological treatment for patients with obesity.
A study published in the Journal of Clinical Endocrinology and Metabolism addressed the impact of cognitive-behavioral therapy (CBT), which helps patients manage goals and treat maladaptive behaviors such as binge-eating disorders. CBT has a modest effect on weight loss, but its integration as part of a lifestyle modification amplifies the results of this loss.
Recent research also shows that weight loss through bariatric surgery offers significant psychological benefits. In the past, it was believed that this procedure could cause depression and other severe psychiatric disorders, but it is now more than proven that weight loss, when done properly and without misconduct or malnutrition, improves psychological and psychiatric issues.
How does psychological follow-up affect the use of medication during obesity treatment?
Many people who take medications, such as corticosteroids for chronic pain or psychiatric medications, may experience weight gain. It is essential to discuss these issues with the psychiatrist because if the patient already has a predisposition to weight gain, medication X should be chosen instead of medication Y, or the dosage should be adjusted. The psychiatrist needs to understand obesity to medicate correctly. Other types of medication, such as chemotherapeutics, may also cause weight gain, often resulting in more abdominal obesity.
There is also lipedema, a hormone-dependent disease that is different from obesity. In this disease, the person gains weight mainly in the legs and arms. In this case, bariatric surgery may result in weight loss only in specific areas, causing disproportionality and difficulty in understanding for the patient. Therefore, when treating obesity, it is important to analyze the patient from all angles: psychological, physiologic, and physical, considering the diversity of the body, its functioning, and hormonal reactions.
Although psychologists do not prescribe medications, they often explain their functioning to the patient. For example, if a patient is taking a glucagon-like peptide 1 analog and experiences initial nausea, he or she may stop using the treatment because the wrong dose had been started. In this case, the psychologist can explain how the medication works and encourage the patient to discuss adjustments with the doctor, avoiding premature discontinuation.
How has the mental health follow-up of patients with obesity evolved over the years?
I started working with people with obesity 25 years ago, when I myself underwent bariatric surgery. At that time, surgeons were used to “solving” the problem and sending the person home. Often, the patient did not even return for surgical follow-up because, in theory, the problem was solved.
Over time, I believe that surgeons learned to talk to the patient, understanding that there is a whole process that even involves creating a bond with the individual who underwent the surgical procedure. Within this process, the importance of the mental health of patients was recognized, and how common it is to confuse a degree of malnutrition with a mental disorder.
Even though I am not a nutritionist, I need to know the difference between a case of malnutrition and depression. So, it is a whole set of factors that needs to be worked on like an orchestra. It is not necessary for this work to be done in the same physical space, but dialogue is important.
Of course, there are things that the patient will only share with the psychologist or with the surgeon, but there are also pieces of information that need to be shared for positive management. I have had patients who were afraid to go back to the nutritionist because they did not lose weight. If they are afraid, it is because the professional is guiding them incorrectly.
What tips would you give to clinicians regarding the psychological approach to people with obesity?
Accessibility is crucial. When someone tells me they are dealing with obesity and depression, I usually ask, “Did you know you have two chronic diseases?” It is essential to explain these concepts because the patient may often think they are free after a successful diet and weight loss, which is not true because of the high relapse associated with obesity. Depression and anxiety follow similar patterns. If the same person wears prescription glasses, I interact by saying, “Did you know you have three chronic diseases?” This question often causes surprise. “I hadn’t thought of that.”
It is essential to use accessible language for the patient to understand the functioning of the disease. More important than choosing a treatment approach is understanding the pathophysiology of obesity and its psychological impact. This avoids a one-size-fits-all approach for all patients.
For example, the impact on someone who developed obesity in childhood after suffering physical, moral, or sexual abuse will probably be deeper than on someone in a healthy family who gained weight after becoming sedentary. Each life story requires a personalized approach.
Sometimes, a patient with mild obesity (grade 1) may not seem to need specific interventions at first glance, but it is crucial to listen to his or her story. Similarly, patients with severe obesity (grades 3 or 4) who resist surgery are entitled to other treatment options, and this is perfectly valid. Therefore, it is always important to ask, “Who is this person? What does obesity represent in their story?” Then propose the most appropriate treatment.
Ms. Levy reported having no relevant financial relationships.
This story was translated from the Medscape Portuguese edition using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Elevated Estradiol Linked to Bulging Cornea in Premenopausal Women
TOPLINE:
Elevated blood levels of estradiol are associated with an increased risk for corneal ectasia, characterized by thinning and outward bulging of the tissue, in premenopausal women.
METHODOLOGY:
- Researchers conducted an observational case-control study of premenopausal women with naturally occurring corneal ectasia, named keratoconus (n = 36), or those who developed ectasia after refractive surgery (n = 29) from an eye clinic in Israel between 2019 and 2022, and healthy women from hospital staff (n = 31).
- The median age of the participants was 29, 33, and 31 years, respectively.
- Levels of estradiol in the study participants were measured on the second day of their menstrual cycles.
TAKEAWAY:
- The mean levels of estradiol were 38.0 pg/mL in patients with keratoconus, 43.4 pg/mL in those who developed ectasia after surgery, and 28.6 pg/mL in the healthy controls (all P = .001).
- Increased levels of estradiol were associated with corneal ectasia (adjusted odds ratio, 2.44; P < .001).
- Age and use of oral contraceptives were not associated with the risk for corneal ectasia.
IN PRACTICE:
“Our results could have an impact on patient selection for refractive surgery and on better management of patients with keratoconus,” the authors wrote.
SOURCE:
The study was led by Nir Stanescu, MD, of the Department of Ophthalmology at Assuta Samson Hospital, in Ashdod, Israel. It was published online in the European Journal of Ophthalmology.
LIMITATIONS:
The sample size of the study was relatively small. Causality could not be established due to cross-sectional design. The control group consisting of hospital staff may have led to selection bias. The study did not account for factors such as body mass index, diet, smoking status, alcohol consumption, and exercise, which may affect the circulating levels of estradiol.
DISCLOSURES:
The study did not receive any financial support. The authors declared no conflicts of interests.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
Elevated blood levels of estradiol are associated with an increased risk for corneal ectasia, characterized by thinning and outward bulging of the tissue, in premenopausal women.
METHODOLOGY:
- Researchers conducted an observational case-control study of premenopausal women with naturally occurring corneal ectasia, named keratoconus (n = 36), or those who developed ectasia after refractive surgery (n = 29) from an eye clinic in Israel between 2019 and 2022, and healthy women from hospital staff (n = 31).
- The median age of the participants was 29, 33, and 31 years, respectively.
- Levels of estradiol in the study participants were measured on the second day of their menstrual cycles.
TAKEAWAY:
- The mean levels of estradiol were 38.0 pg/mL in patients with keratoconus, 43.4 pg/mL in those who developed ectasia after surgery, and 28.6 pg/mL in the healthy controls (all P = .001).
- Increased levels of estradiol were associated with corneal ectasia (adjusted odds ratio, 2.44; P < .001).
- Age and use of oral contraceptives were not associated with the risk for corneal ectasia.
IN PRACTICE:
“Our results could have an impact on patient selection for refractive surgery and on better management of patients with keratoconus,” the authors wrote.
SOURCE:
The study was led by Nir Stanescu, MD, of the Department of Ophthalmology at Assuta Samson Hospital, in Ashdod, Israel. It was published online in the European Journal of Ophthalmology.
LIMITATIONS:
The sample size of the study was relatively small. Causality could not be established due to cross-sectional design. The control group consisting of hospital staff may have led to selection bias. The study did not account for factors such as body mass index, diet, smoking status, alcohol consumption, and exercise, which may affect the circulating levels of estradiol.
DISCLOSURES:
The study did not receive any financial support. The authors declared no conflicts of interests.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
Elevated blood levels of estradiol are associated with an increased risk for corneal ectasia, characterized by thinning and outward bulging of the tissue, in premenopausal women.
METHODOLOGY:
- Researchers conducted an observational case-control study of premenopausal women with naturally occurring corneal ectasia, named keratoconus (n = 36), or those who developed ectasia after refractive surgery (n = 29) from an eye clinic in Israel between 2019 and 2022, and healthy women from hospital staff (n = 31).
- The median age of the participants was 29, 33, and 31 years, respectively.
- Levels of estradiol in the study participants were measured on the second day of their menstrual cycles.
TAKEAWAY:
- The mean levels of estradiol were 38.0 pg/mL in patients with keratoconus, 43.4 pg/mL in those who developed ectasia after surgery, and 28.6 pg/mL in the healthy controls (all P = .001).
- Increased levels of estradiol were associated with corneal ectasia (adjusted odds ratio, 2.44; P < .001).
- Age and use of oral contraceptives were not associated with the risk for corneal ectasia.
IN PRACTICE:
“Our results could have an impact on patient selection for refractive surgery and on better management of patients with keratoconus,” the authors wrote.
SOURCE:
The study was led by Nir Stanescu, MD, of the Department of Ophthalmology at Assuta Samson Hospital, in Ashdod, Israel. It was published online in the European Journal of Ophthalmology.
LIMITATIONS:
The sample size of the study was relatively small. Causality could not be established due to cross-sectional design. The control group consisting of hospital staff may have led to selection bias. The study did not account for factors such as body mass index, diet, smoking status, alcohol consumption, and exercise, which may affect the circulating levels of estradiol.
DISCLOSURES:
The study did not receive any financial support. The authors declared no conflicts of interests.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
Is Parenthood Losing Its Appeal?
A recent survey by the Pew Research Center has found that among adults younger than 50, the percentage who say they are unlikely to have children rose from 37% to 47%. With this trend freshly etched in my consciousness, I stumbled across an interview with Anastasia Berg, an assistant professor of philosophy at the University of California, Irvine. Professor Berg and Rachel Wiseman have just published What Are Children For? On Ambivalence and Choice. How could a pediatrician with time on his hands ignore a provocative title like that?
I was immediately drawn to Professor Berg’s observations about the “concerns, anxieties, and lines of reasoning people encounter when considering whether or not they should have children.” Prior to the 1960s, motherhood seemed to just be a natural progression from marriage. That’s the way my wife and I approached it when we had our first child while I was in my last year of medical school in 1971. There was no discussion of the pros and cons, except maybe that financially waiting until the eve of my first professional paycheck seemed to make sense.
However, as Professor Berg points out, from the 1960s up until well into the 1980s, as feminist thought gained a higher profile, there were anti-motherhood factions. There were others who wanted to see motherhood reformed and adapted so it “could once again be a legitimate source of meaning and value in life.” However, both camps agreed that the choice to have children was a decision that “women should make completely on their own.”
Now, well into the new millennium, we are looking at a completely different landscape. In the past, having children was woven into the fabric of human life in which we had a past, a present, and a role in creating the future. Professor Berg observes that currently, having children is often considered a project, not unlike our other projects such as “career choice or travel plans.” What are the pluses and minuses?
The Pew Survey found that 60% of adults younger than 50 who don’t have children said that not having children made it less difficult to be successful and have an active social life. Many felt that being a parent would improve the chances of having someone to care for you as you aged.
When my wife and I considered the financial costs of motherhood more than 50 years ago, our calculation was primarily about the timing. The decision to have a second child focused our concern around our ability to balance our attention between two siblings. A third child just sorta happened without any discussion.
Professor Berg echoes the Pew findings when she observes that currently woman are considering the cost in terms of their identities. Will motherhood transform me? Will there be a cost not only to my career but also to all the associations, interests, and activities I have accumulated? These costs are likely to be greater the longer the decision to have a child is put off. She adds that viewing motherhood as a transformation can make the decision to have children scarier than it needs to be. My wife and I, at age 26 and 27, were still in the early stages of building our identities. My wife had a 2-year college degree and no career plans on the horizon. Having a child was one of those things that was built into who we became.
But to compare our experiences in the 1970s to the realities of the first quarter of the 21st century ignores the concerns facing today’s adults who are facing the cloud of uncertainty hanging over all of us. Despite their claims to fix the situation, both sides of the political spectrum are leveraging fear to gain our support. Even climate change skeptics must have some concern in the spate of natural disasters we are experiencing. Not to mention the pandemic. Anxiety in this country is at an all time high. Optimism doesn’t seem to fit into today’s journalists’ lexicon, as they chose to focus on conflict instead of cooperation. It’s hard to question any adult who harbors serious doubts on taking on the challenge of parenthood and bringing a child into a world that feels unsettled.
However, based on her research and her own experience as a parent, Professor Berg offers some advice. She encourages people to think and discuss the decision to have children earlier in their life trajectory, before they have made decisions that may eventually limit their options. Second, she discourages making a list of pros and cons. Finally, she advises taking a long view and ask yourself whether you “choose to take a direct part in ushering in the next generation.”
Sounds like advice that will optimize the chances of making the good decision about having a child. I’m just thankful to have lived at time and in a situation when having child was just the thing most married couples did.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at pdnews@mdedge.com.
A recent survey by the Pew Research Center has found that among adults younger than 50, the percentage who say they are unlikely to have children rose from 37% to 47%. With this trend freshly etched in my consciousness, I stumbled across an interview with Anastasia Berg, an assistant professor of philosophy at the University of California, Irvine. Professor Berg and Rachel Wiseman have just published What Are Children For? On Ambivalence and Choice. How could a pediatrician with time on his hands ignore a provocative title like that?
I was immediately drawn to Professor Berg’s observations about the “concerns, anxieties, and lines of reasoning people encounter when considering whether or not they should have children.” Prior to the 1960s, motherhood seemed to just be a natural progression from marriage. That’s the way my wife and I approached it when we had our first child while I was in my last year of medical school in 1971. There was no discussion of the pros and cons, except maybe that financially waiting until the eve of my first professional paycheck seemed to make sense.
However, as Professor Berg points out, from the 1960s up until well into the 1980s, as feminist thought gained a higher profile, there were anti-motherhood factions. There were others who wanted to see motherhood reformed and adapted so it “could once again be a legitimate source of meaning and value in life.” However, both camps agreed that the choice to have children was a decision that “women should make completely on their own.”
Now, well into the new millennium, we are looking at a completely different landscape. In the past, having children was woven into the fabric of human life in which we had a past, a present, and a role in creating the future. Professor Berg observes that currently, having children is often considered a project, not unlike our other projects such as “career choice or travel plans.” What are the pluses and minuses?
The Pew Survey found that 60% of adults younger than 50 who don’t have children said that not having children made it less difficult to be successful and have an active social life. Many felt that being a parent would improve the chances of having someone to care for you as you aged.
When my wife and I considered the financial costs of motherhood more than 50 years ago, our calculation was primarily about the timing. The decision to have a second child focused our concern around our ability to balance our attention between two siblings. A third child just sorta happened without any discussion.
Professor Berg echoes the Pew findings when she observes that currently woman are considering the cost in terms of their identities. Will motherhood transform me? Will there be a cost not only to my career but also to all the associations, interests, and activities I have accumulated? These costs are likely to be greater the longer the decision to have a child is put off. She adds that viewing motherhood as a transformation can make the decision to have children scarier than it needs to be. My wife and I, at age 26 and 27, were still in the early stages of building our identities. My wife had a 2-year college degree and no career plans on the horizon. Having a child was one of those things that was built into who we became.
But to compare our experiences in the 1970s to the realities of the first quarter of the 21st century ignores the concerns facing today’s adults who are facing the cloud of uncertainty hanging over all of us. Despite their claims to fix the situation, both sides of the political spectrum are leveraging fear to gain our support. Even climate change skeptics must have some concern in the spate of natural disasters we are experiencing. Not to mention the pandemic. Anxiety in this country is at an all time high. Optimism doesn’t seem to fit into today’s journalists’ lexicon, as they chose to focus on conflict instead of cooperation. It’s hard to question any adult who harbors serious doubts on taking on the challenge of parenthood and bringing a child into a world that feels unsettled.
However, based on her research and her own experience as a parent, Professor Berg offers some advice. She encourages people to think and discuss the decision to have children earlier in their life trajectory, before they have made decisions that may eventually limit their options. Second, she discourages making a list of pros and cons. Finally, she advises taking a long view and ask yourself whether you “choose to take a direct part in ushering in the next generation.”
Sounds like advice that will optimize the chances of making the good decision about having a child. I’m just thankful to have lived at time and in a situation when having child was just the thing most married couples did.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at pdnews@mdedge.com.
A recent survey by the Pew Research Center has found that among adults younger than 50, the percentage who say they are unlikely to have children rose from 37% to 47%. With this trend freshly etched in my consciousness, I stumbled across an interview with Anastasia Berg, an assistant professor of philosophy at the University of California, Irvine. Professor Berg and Rachel Wiseman have just published What Are Children For? On Ambivalence and Choice. How could a pediatrician with time on his hands ignore a provocative title like that?
I was immediately drawn to Professor Berg’s observations about the “concerns, anxieties, and lines of reasoning people encounter when considering whether or not they should have children.” Prior to the 1960s, motherhood seemed to just be a natural progression from marriage. That’s the way my wife and I approached it when we had our first child while I was in my last year of medical school in 1971. There was no discussion of the pros and cons, except maybe that financially waiting until the eve of my first professional paycheck seemed to make sense.
However, as Professor Berg points out, from the 1960s up until well into the 1980s, as feminist thought gained a higher profile, there were anti-motherhood factions. There were others who wanted to see motherhood reformed and adapted so it “could once again be a legitimate source of meaning and value in life.” However, both camps agreed that the choice to have children was a decision that “women should make completely on their own.”
Now, well into the new millennium, we are looking at a completely different landscape. In the past, having children was woven into the fabric of human life in which we had a past, a present, and a role in creating the future. Professor Berg observes that currently, having children is often considered a project, not unlike our other projects such as “career choice or travel plans.” What are the pluses and minuses?
The Pew Survey found that 60% of adults younger than 50 who don’t have children said that not having children made it less difficult to be successful and have an active social life. Many felt that being a parent would improve the chances of having someone to care for you as you aged.
When my wife and I considered the financial costs of motherhood more than 50 years ago, our calculation was primarily about the timing. The decision to have a second child focused our concern around our ability to balance our attention between two siblings. A third child just sorta happened without any discussion.
Professor Berg echoes the Pew findings when she observes that currently woman are considering the cost in terms of their identities. Will motherhood transform me? Will there be a cost not only to my career but also to all the associations, interests, and activities I have accumulated? These costs are likely to be greater the longer the decision to have a child is put off. She adds that viewing motherhood as a transformation can make the decision to have children scarier than it needs to be. My wife and I, at age 26 and 27, were still in the early stages of building our identities. My wife had a 2-year college degree and no career plans on the horizon. Having a child was one of those things that was built into who we became.
But to compare our experiences in the 1970s to the realities of the first quarter of the 21st century ignores the concerns facing today’s adults who are facing the cloud of uncertainty hanging over all of us. Despite their claims to fix the situation, both sides of the political spectrum are leveraging fear to gain our support. Even climate change skeptics must have some concern in the spate of natural disasters we are experiencing. Not to mention the pandemic. Anxiety in this country is at an all time high. Optimism doesn’t seem to fit into today’s journalists’ lexicon, as they chose to focus on conflict instead of cooperation. It’s hard to question any adult who harbors serious doubts on taking on the challenge of parenthood and bringing a child into a world that feels unsettled.
However, based on her research and her own experience as a parent, Professor Berg offers some advice. She encourages people to think and discuss the decision to have children earlier in their life trajectory, before they have made decisions that may eventually limit their options. Second, she discourages making a list of pros and cons. Finally, she advises taking a long view and ask yourself whether you “choose to take a direct part in ushering in the next generation.”
Sounds like advice that will optimize the chances of making the good decision about having a child. I’m just thankful to have lived at time and in a situation when having child was just the thing most married couples did.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at pdnews@mdedge.com.
In Search of a Hobby
I need a hobby. Any suggestions?
Due to the annual summertime slowdown, I find myself with less to do and catch up on during weekends. My kids are grown. Nowadays, when I have free time, I have no idea what to do with myself.
That’s not to say I don’t do things to relax. Jigsaw puzzles, reading P.G. Wodehouse ... but there’s only so long I can sit there, maybe 30 minutes, before I get bored. Then I go back to my desk, check email, log in to see if any prescription refills need to be addressed ...
I look online for ideas. No, I don’t want to collect things. Or start gardening. Or learn an instrument. Or paint. Or take up photography. The last thing I want is a hobby that involves a significant financial outlay for stuff I may be selling on eBay in 3 months.
I like writing, but also spend most of my day at the computer typing up patient notes one after another. Not sure I want to spend even more time at my computer than I already do.
Maybe walking. Is that a hobby? Or just exercise? I’ve never been much of a gym rat, as my scale can tell you. I’m definitely not a golfer, aside from the occasional trip to the windmill course when my kids were younger.
I’d love to travel more, but right now my wife’s job and my practice responsibilities make that difficult.
I sit here and wonder, what is a good hobby for an early 21st century doctor?
Then I went online to check something on UpToDate for next week, and suddenly it occurred to me: Being a neurologist IS my hobby. It’s what I enjoy.
Is that a bad thing? I have no idea. They say “do what you love, love what you do.”
Of course, I can’t always be a neurologist. Sooner or later the day will come when I walk away from this.
Between now and then I have some thinking to do.
Dr. Block has a solo neurology practice in Scottsdale, Arizona.
I need a hobby. Any suggestions?
Due to the annual summertime slowdown, I find myself with less to do and catch up on during weekends. My kids are grown. Nowadays, when I have free time, I have no idea what to do with myself.
That’s not to say I don’t do things to relax. Jigsaw puzzles, reading P.G. Wodehouse ... but there’s only so long I can sit there, maybe 30 minutes, before I get bored. Then I go back to my desk, check email, log in to see if any prescription refills need to be addressed ...
I look online for ideas. No, I don’t want to collect things. Or start gardening. Or learn an instrument. Or paint. Or take up photography. The last thing I want is a hobby that involves a significant financial outlay for stuff I may be selling on eBay in 3 months.
I like writing, but also spend most of my day at the computer typing up patient notes one after another. Not sure I want to spend even more time at my computer than I already do.
Maybe walking. Is that a hobby? Or just exercise? I’ve never been much of a gym rat, as my scale can tell you. I’m definitely not a golfer, aside from the occasional trip to the windmill course when my kids were younger.
I’d love to travel more, but right now my wife’s job and my practice responsibilities make that difficult.
I sit here and wonder, what is a good hobby for an early 21st century doctor?
Then I went online to check something on UpToDate for next week, and suddenly it occurred to me: Being a neurologist IS my hobby. It’s what I enjoy.
Is that a bad thing? I have no idea. They say “do what you love, love what you do.”
Of course, I can’t always be a neurologist. Sooner or later the day will come when I walk away from this.
Between now and then I have some thinking to do.
Dr. Block has a solo neurology practice in Scottsdale, Arizona.
I need a hobby. Any suggestions?
Due to the annual summertime slowdown, I find myself with less to do and catch up on during weekends. My kids are grown. Nowadays, when I have free time, I have no idea what to do with myself.
That’s not to say I don’t do things to relax. Jigsaw puzzles, reading P.G. Wodehouse ... but there’s only so long I can sit there, maybe 30 minutes, before I get bored. Then I go back to my desk, check email, log in to see if any prescription refills need to be addressed ...
I look online for ideas. No, I don’t want to collect things. Or start gardening. Or learn an instrument. Or paint. Or take up photography. The last thing I want is a hobby that involves a significant financial outlay for stuff I may be selling on eBay in 3 months.
I like writing, but also spend most of my day at the computer typing up patient notes one after another. Not sure I want to spend even more time at my computer than I already do.
Maybe walking. Is that a hobby? Or just exercise? I’ve never been much of a gym rat, as my scale can tell you. I’m definitely not a golfer, aside from the occasional trip to the windmill course when my kids were younger.
I’d love to travel more, but right now my wife’s job and my practice responsibilities make that difficult.
I sit here and wonder, what is a good hobby for an early 21st century doctor?
Then I went online to check something on UpToDate for next week, and suddenly it occurred to me: Being a neurologist IS my hobby. It’s what I enjoy.
Is that a bad thing? I have no idea. They say “do what you love, love what you do.”
Of course, I can’t always be a neurologist. Sooner or later the day will come when I walk away from this.
Between now and then I have some thinking to do.
Dr. Block has a solo neurology practice in Scottsdale, Arizona.
A Paradigm Shift in Evaluating and Investigating the Etiology of Bloating
Introduction
Abdominal bloating is a common condition affecting up to 3.5% of people globally (4.6% in women and 2.4% in men),1 with 13.9% of the US population reporting bloating in the past 7 days.2 The prevalence of bloating and distention exceeds 50% when linked to disorders of gut-brain interaction (DGBIs) such as irritable bowel syndrome (IBS), constipation, gastroparesis, and functional dyspepsia (FD).3,4 According to the Rome IV criteria, functional bloating and distention (FABD) patients are characterized by recurrent symptoms of abdominal fullness or pressure (bloating), or a visible increase in abdominal girth (distention) occurring at least 1 day per week for 3 consecutive months with an onset of 6 months and without predominant pain or altered bowel habits.5
Prolonged abdominal bloating and distention (ABD) can significantly impact quality of life and work productivity and can lead to increased medical consultations.2 Multiple pathophysiological mechanisms are involved in ABD that complicate the clinical management.4 There is an unmet need to understand the underlying mechanisms that lead to the development of ABD such as, food intolerance, abnormal viscerosomatic reflex, visceral hypersensitivity, and gut microbial dysbiosis. Recent advancements and acceptance of a multidisciplinary management of ABD have shifted the paradigm from merely treating symptoms to subtyping the condition and identifying overlaps with other DGBIs in order to individualize treatment that addresses the underlying pathophysiological mechanism. The recent American Gastroenterological Association (AGA) clinical update provided insights into the best practice advice for evaluating and managing ABD based on a review of current literature and on expert opinion of coauthors.6 This article aims to deliberate a practical approach to diagnostic strategies and treatment options based on etiology to refine clinical care of patients with ABD.
Pathophysiological Mechanisms
ABD can result from various pathophysiological mechanisms. This section highlights the major causes (illustrated in Figure 1).
Food intolerances
Understanding food intolerances is crucial for diagnosing and managing patients with ABD. Disaccharidase deficiency is common (e.g., lactase deficiency is found in 35%-40% of adults).7 It can be undiagnosed in patients presenting with IBS symptoms, given the overlap in presentation with a prevalence of 9% of pan-disaccharidase deficiency. Sucrase-deficient patients must often adjust sugar and carbohydrate/starch intake to relieve symptoms.7 Deficiencies in lactase and sucrase activity, along with the consumption of some artificial sweeteners (e.g., sugar alcohols and sorbitol) and fructans can lead to bloating and distention. These substances increase osmotic load, fluid retention, microbial fermentation, and visceral hypersensitivity, leading to gas production and abdominal distention. One prospective study of symptomatic patients with various DGBIs (n = 1372) reported a prevalence of lactose intolerance and malabsorption at 51% and 32%, respectively.8 Furthermore, fructose intolerance and malabsorption prevalence were 60% and 45%, respectively.8 Notably, lactase deficiency does not always cause ABD, as not all individuals with lactase deficiency experience these symptoms after consuming lactose. Patients with celiac disease (CD), non-celiac gluten sensitivity (NCGS), and gluten intolerance can also experience bloating and distention, with or without changes in bowel habits.9 In some patients with self-reported NCGS, symptoms may be due to fructans in gluten-rich foods rather than gluten itself, thus recommending the elimination of fructans may help improve symptoms.9
Visceral hypersensitivity
Visceral hypersensitivity is explained by an increased perception of gut mechano-chemical stimulation, which typically manifests in an aggravated feeling of pain, nausea, distension, and ABD.10 In the gut, food particles and gut bacteria and their derived molecules interact with neuroimmune and enteroendocrine cells causing visceral sensitivity by the proximity of gut’s neurons to immune cells activated by them and leading to inflammatory reactions (Figure 1). 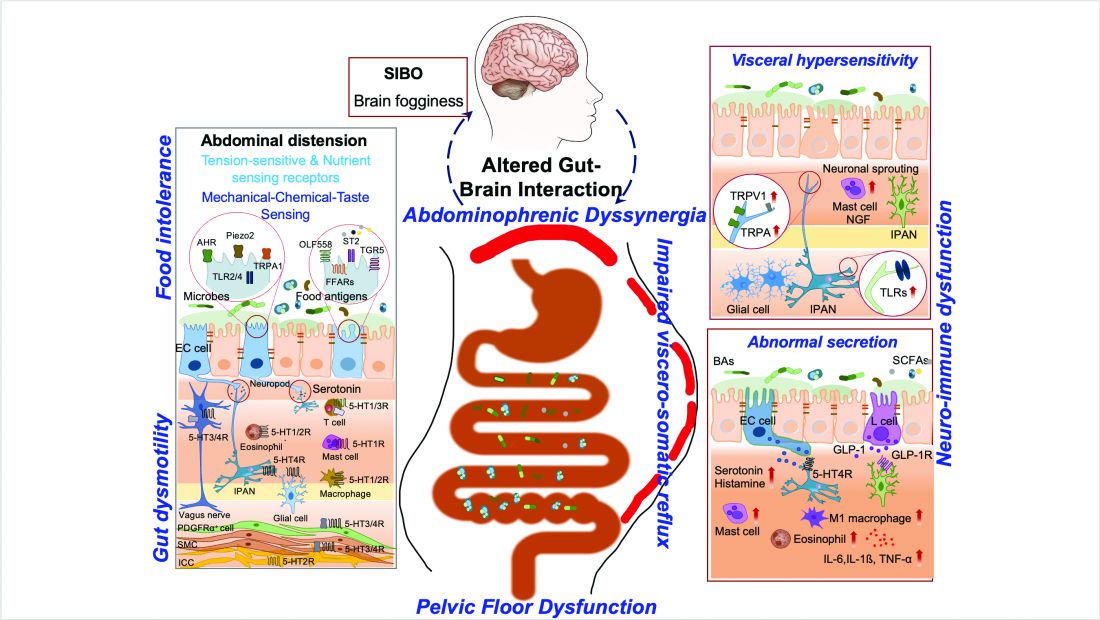
Pelvic floor dysfunction
Patients with anorectal motor dysfunction often experience difficulty in effectively evacuating both gas and stool, leading to ABD.12 Impaired ability to expel gas and stool results in prolonged balloon expulsion times, which correlates with symptoms of distention in patients with constipation.
Abdominophrenic dyssynergia
Abdominophrenic dyssynergia is characterized as a paradoxical viscerosomatic reflex response to minimal gaseous distention in individuals with FABD.13 In this condition, the diaphragm contracts (descends), and the anterior abdominal wall muscles relax in response to the presence of gas. This response is opposite to the normal physiological response to increased intraluminal gas, where the diaphragm relaxes and the anterior abdominal muscles contract to increase the craniocaudal capacity of the abdominal cavity without causing abdominal protrusion.13 Patients with FABD exhibit significant abdominal wall protrusion and diaphragmatic descent even with relatively small increases in intraluminal gas.11 Understanding the role of abdominophrenic dyssynergia in abdominal bloating and distention is essential for effective diagnosis and management of the patients.
Gut dysmotility
Gut dysmotility is a crucial factor that can contribute to FABD. Gut dysmotility affects the movement of contents through the GI tract, accumulating gas and stool, directly contributing to bloating and distention. A prospective study involving over 2000 patients with functional constipation and constipation predominant-IBS (IBS-C) found that more than 90% of these patients reported symptoms of bloating.14 Furthermore, in IBS-C patients, those with prolonged colonic transit exhibited greater abdominal distention compared to those with normal gut transit times. In patients with gastroparesis, delayed gastric emptying resulting in prolonged retention of stomach contents is the main factor in the generation of bloating symptoms.4
Small intestinal bacterial overgrowth (SIBO)
SIBO is overrepresented in various conditions, including IBS, FD, diabetes, gastrointestinal (GI) surgery patients and obesity, and can play an important role in generating ABD. Excess bacteria in the small intestine ferment carbohydrates, producing gas that stretches and distends the small intestine, leading to these symptoms. Additionally, altered sensation and abnormal viscerosomatic reflexes may contribute to SIBO-related bloating.4 One recent study noted decreased duodenal phylogenetic diversity in individuals who developed postprandial bloating.15 Increased methane levels caused by intestinal methanogen overgrowth, primarily the archaea Methanobrevibacter smithii, is possibly responsible for ABD in patients with IBS-C.16 Testing for SIBO in patients with ABD is generally only recommended if there are clear risk factors or severe symptoms warranting a test-and-treat approach.
Practical Diagnosis
Diagnosing ABD typically does not require extensive laboratory testing, imaging, or endoscopy unless there are alarm features or significant changes in symptoms. Here is the AGA clinical update on best practice advice6 for when to conduct further testing:
Diagnostic tests should be considered if patients exhibit:
- Recent onset or worsening of dyspepsia or abdominal pain
- Vomiting
- GI bleeding
- Unintentional weight loss exceeding 10% of body weight
- Chronic diarrhea
- Family history of GI malignancy, celiac disease, or inflammatory bowel disease
Physical examination
If visible abdominal distention is present, a thorough abdominal examination can help identify potential issues:
- Tympany to percussion suggests bowel dilation.
- Abnormal bowel sounds may indicate obstruction or ileus.
- A succussion splash could indicate the presence of ascites and obstruction.
- Any abnormalities discovered during the physical exam should prompt further investigation with imaging, such as a computed tomography (CT) scan or ultrasound, to evaluate for ascites, masses, or increased bowel gas due to ileus, obstruction, or pseudo-obstruction.
Radiologic imaging, laboratory testing and endoscopy
- An abdominal x-ray may reveal an increased stool burden, suggesting the need for further evaluation of slow transit constipation or a pelvic floor disorder, particularly in patients with functional constipation, IBS-mixed, or IBS-C.
- Hyperglycemia, weight gain, and bloating can be a presenting sign of ovarian cancer therefore all women should continue pelvic exams as dictated by the gynecologic societies. The need for an annual pelvic exam should be discussed with health care professionals especially in those with family history of ovarian cancer.
- An upper endoscopy may be warranted for patients over 40 years old with dyspeptic symptoms and abdominal bloating or distention, especially in regions with a high prevalence of Helicobacter pylori.
- Chronic pancreatitis, indicated by bloating and pain, may necessitate fecal elastase testing to assess pancreatic function.
The expert review in the AGA clinical update provides step-by-step advice regarding the best practices6 for diagnosis and identifying who to test for ABD.
Treatment Options
The following sections highlight recent best practice advice on therapeutic approaches for treating ABD.
Dietary interventions
Specific foods may trigger bloating and abdominal distention, especially in patients with overlapping DGBIs. However, only a few studies have evaluated dietary restriction specifically for patients with primary ABD. Restricting non-absorbable sugars led to symptomatic improvement in 81% of patients with FABD who had documented sugar malabsorption.17 Two studies have shown that IBS patients treated with a low-fermentable, oligo-, di-, and monosaccharides (FODMAP) diet noted improvement in ABD and that restricting fructans initially may be the most optimal.18 A recent study showed that the Mediterranean diet improved IBS symptoms, including abdominal pain and bloating.19 It should be noted restrictive diets are efficacious but come with short- and long-term challenges. If empiric treatment and/or therapeutic testing do not resolve symptoms, a referral to a dietitian can be useful. Dietitians can provide tailored dietary advice, ensuring patients avoid trigger foods while maintaining a balanced and nutritious diet.
Prokinetics and laxatives
Prokinetic agents are used to treat symptoms of FD, gastroparesis, chronic idiopathic constipation (CIC), and IBS. A meta-analysis of 13 trials found all constipation medications superior to placebo for treating abdominal bloating in patients with IBS-C.20
Probiotics
Treatment with probiotics is recommended for bloating or distention. One double-blind placebo-controlled trial with two separate probiotics, Bifidobacterium lactis and Lactobacillus acidophilus, showed improvements in global GI symptoms of patients with DGBI at 8 weeks versus placebo, with improvements in bloating symptoms.21
Antibiotics
The most commonly studied antibiotic for treating bloating is rifaximin.22 Global symptomatic improvement in IBS patients treated with antibiotics has correlated with the normalization of hydrogen levels in lactulose hydrogen breath tests.22 Patients with non-constipation IBS randomized to rifaximin 550 mg three times daily for 14 days had a greater proportion of relief of IBS-related bloating compared to placebo for at least 2 of the first 4 weeks after treatment.22 Future research warrants use of narrow-spectrum antibiotics study for FABD as the use of broad-spectrum antibiotics may deplete commensals forever, resulting in metabolic disorders.
Biofeedback therapy
Anorectal biofeedback therapy may help with ABD, particularly in patients with IBS-C and chronic constipation. One study noted that post-biofeedback therapy, myoelectric activity of the intercostals and diaphragm decreased, and internal oblique myoelectric activity increased.23 This study also showed ascent of the diaphragm and decreased girth, improving distention.
Central neuromodulators
As bloating results from multiple disturbed mechanisms, including altered gut-brain interaction, these symptoms can be amplified by psychological states such as anxiety, depression, or somatization. Central neuromodulators reduce the perception of visceral signals, re-regulate brain-gut control mechanisms, and improve psychological comorbidities.6 A large study of FD patients demonstrated that both amitriptyline (50 mg daily) and escitalopram (10 mg daily) significantly improved postprandial bloating compared to placebo.24 Antidepressants that activate noradrenergic and serotonergic pathways, including tricyclic antidepressants (e.g., amitriptyline) and serotonin-norepinephrine reuptake inhibitors (e.g., duloxetine and venlafaxine), show the greatest benefit in reducing visceral sensations.6
Brain-gut behavioral therapies
A recent multidisciplinary consensus report supports a myriad of potential brain-gut behavioral therapies (BGBTs) for treating DGBI.25 These therapies, including hypnotherapy, cognitive behavioral therapy (CBT), and other modalities, may be combined with central neuromodulators and other GI treatments in a safe, noninvasive, and complementary fashion. BGBTs do not need to be symptom-specific, as they improve overall quality of life, anxiety, stress, and the burden associated with DGBIs. To date, none of the BGBTs have focused exclusively on FABD; however, prescription-based psychological therapies are now FDA-approved for use on smart apps, improving global symptoms that include bloating in IBS and FD.
Recent AGA clinical update best practices should be considered for the clinical care of patients with ABD.6
Conclusion and Future Perspectives
ABD are highly prevalent and significantly impact patients with various GI and metabolic disorders. Although our understanding of these symptoms is still evolving, evidence increasingly points to the dysregulation of the gut-brain axis and supports the application of the biopsychosocial model in treatment. This model addresses diet, motility, visceral sensitivity, pelvic floor disorders and psychosocial factors, providing a comprehensive approach to patient care.
Physician-scientists around the globe face numerous challenges when evaluating patients with these symptoms. However, the recent AGA clinical update on the best practice guidelines offers step-by-step diagnostic tests and treatment options to assist physicians in making informed decisions. . More comprehensive, large-scale, and longitudinal studies using metabolomics, capsule technologies for discovery of dysbiosis, mass spectrometry, and imaging data are needed to identify the exact contributors to disease pathogenesis, particularly those that can be targeted with pharmacologic agents. Collaborative work between gastroenterologists, dietitians, gut-brain behavioral therapists, endocrinologists, is crucial for clinical care of patients with ABD.
Careful attention to the patient’s primary symptoms and physical examination, combined with advancements in targeted diagnostics like the analysis of microbial markers, metabolites, and molecular signals, can significantly enhance patient clinical outcomes. Additionally, education and effective communication using a patient-centered care model are essential for guiding practical evaluation and individualized treatment.
Dr. Singh is assistant professor (research) at the University of Nevada, Reno, School of Medicine. Dr. Moshiree is director of motility at Atrium Health, and clinical professor of medicine, Wake Forest Medical University, Charlotte, North Carolina.
References
1. Ballou S et al. Prevalence and associated factors of bloating: Results from the Rome Foundation Global Epidemiology Study. Gastroenterology. 2023 June. doi: 10.1053/j.gastro.2023.05.049.
2. Oh JE et al. Abdominal bloating in the United States: Results of a survey of 88,795 Americans examining prevalence and healthcare seeking. Clin Gastroenterol Hepatol. 2023 Aug. doi: 10.1016/j.cgh.2022.10.031.
3. Drossman DA et al. Neuromodulators for functional gastrointestinal disorders (disorders of gut-brain interaction): A Rome Foundation Working Team Report. Gastroenterology. 2018 Mar. doi: 10.1053/j.gastro.2017.11.279.
4. Lacy BE et al. Management of chronic abdominal distension and bloating. Clin Gastroenterol Hepatol. 2021 Feb. doi: 10.1016/j.cgh.2020.03.056.
5. Mearin F et al. Bowel disorders. Gastroenterology. 2016 Feb. doi: 10.1053/j.gastro.2016.02.031.
6. Moshiree B et al. AGA Clinical Practice Update on evaluation and management of belching, abdominal bloating, and distention: expert review. Gastroenterology. 2023 Sep. doi: 10.1053/j.gastro.2023.04.039.
7. Viswanathan L and Rao SS. Intestinal disaccharidase deficiency in adults: evaluation and treatment. Curr Gastroenterol Rep 2023 May. doi: 10.1007/s11894-023-00870-z.
8. Wilder-Smith CH et al. Fructose and lactose intolerance and malabsorption testing: the relationship with symptoms in functional gastrointestinal disorders. Aliment Pharmacol Ther. 2013 Jun. doi: 10.1111/apt.12306.
9. Skodje GI et al. Fructan, rather than gluten, induces symptoms in patients with self-reported non-celiac gluten sensitivity. Gastroenterology. 2018 Feb. doi: 10.1053/j.gastro.2017.10.040.
10. Singh R et al. Current treatment options and therapeutic insights for gastrointestinal dysmotility and functional gastrointestinal disorders. Front Pharmacol. 2022 Jan. doi: 10.3389/fphar.2022.808195.
11. Accarino A et al. Abdominal distention results from caudo-ventral redistribution of contents. Gastroenterology 2009 May. doi: 10.1053/j.gastro.2009.01.067.
12. Shim L et al. Prolonged balloon expulsion is predictive of abdominal distension in bloating. Am J Gastroenterol. 2010 Apr. doi: 10.1038/ajg.2010.54.
13. Villoria A et al. Abdomino-phrenic dyssynergia in patients with abdominal bloating and distension. Am J Gastroenterol. 2011 May. doi: 10.1038/ajg.2010.408.
14. Neri L and Iovino P. Laxative Inadequate Relief Survey Group. Bloating is associated with worse quality of life, treatment satisfaction, and treatment responsiveness among patients with constipation-predominant irritable bowel syndrome and functional constipation. Neurogastroenterol Motil. 2016 Apr. doi: 10.1111/nmo.12758.
15. Saffouri GB et al. Small intestinal microbial dysbiosis underlies symptoms associated with functional gastrointestinal disorders. Nat Commun. 2019 May. doi: 10.1038/s41467-019-09964-7.
16. Villanueva-Millan MJ et al. Methanogens and hydrogen sulfide producing bacteria guide distinct gut microbe profiles and irritable bowel syndrome subtypes. Am J Gastroenterol. 2022 Dec. doi: 10.14309/ajg.0000000000001997.
17. Fernández-Bañares F et al. Sugar malabsorption in functional abdominal bloating: a pilot study on the long-term effect of dietary treatment. Clin Nutr. 2006 Oct. doi: 10.1016/j.clnu.2005.11.010.
18. Böhn L et al. Diet low in FODMAPs reduces symptoms of irritable bowel syndrome as well as traditional dietary advice: a randomized controlled trial. Gastroenterology. 2015 Nov. doi: 10.1053/j.gastro.2015.07.054.
19. Staudacher HM et al. Clinical trial: A Mediterranean diet is feasible and improves gastrointestinal and psychological symptoms in irritable bowel syndrome. Aliment Pharmacol Ther. 2024 Feb. doi: 10.1111/apt.17791.
20. Nelson AD et al. Systematic review and network meta-analysis: efficacy of licensed drugs for abdominal bloating in irritable bowel syndrome with constipation. Aliment Pharmacol Ther. 2021 Jul. doi: 10.1111/apt.16437.
21. Ringel-Kulka T et al. Probiotic bacteria Lactobacillus acidophilus NCFM and Bifidobacterium lactis Bi-07 versus placebo for the symptoms of bloating in patients with functional bowel disorders: a double-blind study. J Clin Gastroenterol. 2011 Jul. doi: 10.1097/MCG.0b013e31820ca4d6.
22. Pimentel M et al. Rifaximin therapy for patients with irritable bowel syndrome without constipation. N Engl J Med. 2011 Jan. doi: 10.1056/NEJMoa1004409.
23. Iovino P et al. Pelvic floor biofeedback is an effective treatment for severe bloating in disorders of gut-brain interaction with outlet dysfunction. Neurogastroenterol Motil 2022 May. doi: 10.1111/nmo.14264.
24. Talley NJ et al. Effect of amitriptyline and escitalopram on functional dyspepsia: A multicenter, randomized controlled study. Gastroenterology. 2015 Aug. doi: 10.1053/j.gastro.2015.04.020.
25. Keefer L et al. A Rome Working Team Report on brain-gut behavior therapies for disorders of gut-brain interaction. Gastroenterology. 2022 Jan. doi: 10.1053/j.gastro.2021.09.015.
Introduction
Abdominal bloating is a common condition affecting up to 3.5% of people globally (4.6% in women and 2.4% in men),1 with 13.9% of the US population reporting bloating in the past 7 days.2 The prevalence of bloating and distention exceeds 50% when linked to disorders of gut-brain interaction (DGBIs) such as irritable bowel syndrome (IBS), constipation, gastroparesis, and functional dyspepsia (FD).3,4 According to the Rome IV criteria, functional bloating and distention (FABD) patients are characterized by recurrent symptoms of abdominal fullness or pressure (bloating), or a visible increase in abdominal girth (distention) occurring at least 1 day per week for 3 consecutive months with an onset of 6 months and without predominant pain or altered bowel habits.5
Prolonged abdominal bloating and distention (ABD) can significantly impact quality of life and work productivity and can lead to increased medical consultations.2 Multiple pathophysiological mechanisms are involved in ABD that complicate the clinical management.4 There is an unmet need to understand the underlying mechanisms that lead to the development of ABD such as, food intolerance, abnormal viscerosomatic reflex, visceral hypersensitivity, and gut microbial dysbiosis. Recent advancements and acceptance of a multidisciplinary management of ABD have shifted the paradigm from merely treating symptoms to subtyping the condition and identifying overlaps with other DGBIs in order to individualize treatment that addresses the underlying pathophysiological mechanism. The recent American Gastroenterological Association (AGA) clinical update provided insights into the best practice advice for evaluating and managing ABD based on a review of current literature and on expert opinion of coauthors.6 This article aims to deliberate a practical approach to diagnostic strategies and treatment options based on etiology to refine clinical care of patients with ABD.
Pathophysiological Mechanisms
ABD can result from various pathophysiological mechanisms. This section highlights the major causes (illustrated in Figure 1).
Food intolerances
Understanding food intolerances is crucial for diagnosing and managing patients with ABD. Disaccharidase deficiency is common (e.g., lactase deficiency is found in 35%-40% of adults).7 It can be undiagnosed in patients presenting with IBS symptoms, given the overlap in presentation with a prevalence of 9% of pan-disaccharidase deficiency. Sucrase-deficient patients must often adjust sugar and carbohydrate/starch intake to relieve symptoms.7 Deficiencies in lactase and sucrase activity, along with the consumption of some artificial sweeteners (e.g., sugar alcohols and sorbitol) and fructans can lead to bloating and distention. These substances increase osmotic load, fluid retention, microbial fermentation, and visceral hypersensitivity, leading to gas production and abdominal distention. One prospective study of symptomatic patients with various DGBIs (n = 1372) reported a prevalence of lactose intolerance and malabsorption at 51% and 32%, respectively.8 Furthermore, fructose intolerance and malabsorption prevalence were 60% and 45%, respectively.8 Notably, lactase deficiency does not always cause ABD, as not all individuals with lactase deficiency experience these symptoms after consuming lactose. Patients with celiac disease (CD), non-celiac gluten sensitivity (NCGS), and gluten intolerance can also experience bloating and distention, with or without changes in bowel habits.9 In some patients with self-reported NCGS, symptoms may be due to fructans in gluten-rich foods rather than gluten itself, thus recommending the elimination of fructans may help improve symptoms.9
Visceral hypersensitivity
Visceral hypersensitivity is explained by an increased perception of gut mechano-chemical stimulation, which typically manifests in an aggravated feeling of pain, nausea, distension, and ABD.10 In the gut, food particles and gut bacteria and their derived molecules interact with neuroimmune and enteroendocrine cells causing visceral sensitivity by the proximity of gut’s neurons to immune cells activated by them and leading to inflammatory reactions (Figure 1). 
Pelvic floor dysfunction
Patients with anorectal motor dysfunction often experience difficulty in effectively evacuating both gas and stool, leading to ABD.12 Impaired ability to expel gas and stool results in prolonged balloon expulsion times, which correlates with symptoms of distention in patients with constipation.
Abdominophrenic dyssynergia
Abdominophrenic dyssynergia is characterized as a paradoxical viscerosomatic reflex response to minimal gaseous distention in individuals with FABD.13 In this condition, the diaphragm contracts (descends), and the anterior abdominal wall muscles relax in response to the presence of gas. This response is opposite to the normal physiological response to increased intraluminal gas, where the diaphragm relaxes and the anterior abdominal muscles contract to increase the craniocaudal capacity of the abdominal cavity without causing abdominal protrusion.13 Patients with FABD exhibit significant abdominal wall protrusion and diaphragmatic descent even with relatively small increases in intraluminal gas.11 Understanding the role of abdominophrenic dyssynergia in abdominal bloating and distention is essential for effective diagnosis and management of the patients.
Gut dysmotility
Gut dysmotility is a crucial factor that can contribute to FABD. Gut dysmotility affects the movement of contents through the GI tract, accumulating gas and stool, directly contributing to bloating and distention. A prospective study involving over 2000 patients with functional constipation and constipation predominant-IBS (IBS-C) found that more than 90% of these patients reported symptoms of bloating.14 Furthermore, in IBS-C patients, those with prolonged colonic transit exhibited greater abdominal distention compared to those with normal gut transit times. In patients with gastroparesis, delayed gastric emptying resulting in prolonged retention of stomach contents is the main factor in the generation of bloating symptoms.4
Small intestinal bacterial overgrowth (SIBO)
SIBO is overrepresented in various conditions, including IBS, FD, diabetes, gastrointestinal (GI) surgery patients and obesity, and can play an important role in generating ABD. Excess bacteria in the small intestine ferment carbohydrates, producing gas that stretches and distends the small intestine, leading to these symptoms. Additionally, altered sensation and abnormal viscerosomatic reflexes may contribute to SIBO-related bloating.4 One recent study noted decreased duodenal phylogenetic diversity in individuals who developed postprandial bloating.15 Increased methane levels caused by intestinal methanogen overgrowth, primarily the archaea Methanobrevibacter smithii, is possibly responsible for ABD in patients with IBS-C.16 Testing for SIBO in patients with ABD is generally only recommended if there are clear risk factors or severe symptoms warranting a test-and-treat approach.
Practical Diagnosis
Diagnosing ABD typically does not require extensive laboratory testing, imaging, or endoscopy unless there are alarm features or significant changes in symptoms. Here is the AGA clinical update on best practice advice6 for when to conduct further testing:
Diagnostic tests should be considered if patients exhibit:
- Recent onset or worsening of dyspepsia or abdominal pain
- Vomiting
- GI bleeding
- Unintentional weight loss exceeding 10% of body weight
- Chronic diarrhea
- Family history of GI malignancy, celiac disease, or inflammatory bowel disease
Physical examination
If visible abdominal distention is present, a thorough abdominal examination can help identify potential issues:
- Tympany to percussion suggests bowel dilation.
- Abnormal bowel sounds may indicate obstruction or ileus.
- A succussion splash could indicate the presence of ascites and obstruction.
- Any abnormalities discovered during the physical exam should prompt further investigation with imaging, such as a computed tomography (CT) scan or ultrasound, to evaluate for ascites, masses, or increased bowel gas due to ileus, obstruction, or pseudo-obstruction.
Radiologic imaging, laboratory testing and endoscopy
- An abdominal x-ray may reveal an increased stool burden, suggesting the need for further evaluation of slow transit constipation or a pelvic floor disorder, particularly in patients with functional constipation, IBS-mixed, or IBS-C.
- Hyperglycemia, weight gain, and bloating can be a presenting sign of ovarian cancer therefore all women should continue pelvic exams as dictated by the gynecologic societies. The need for an annual pelvic exam should be discussed with health care professionals especially in those with family history of ovarian cancer.
- An upper endoscopy may be warranted for patients over 40 years old with dyspeptic symptoms and abdominal bloating or distention, especially in regions with a high prevalence of Helicobacter pylori.
- Chronic pancreatitis, indicated by bloating and pain, may necessitate fecal elastase testing to assess pancreatic function.
The expert review in the AGA clinical update provides step-by-step advice regarding the best practices6 for diagnosis and identifying who to test for ABD.
Treatment Options
The following sections highlight recent best practice advice on therapeutic approaches for treating ABD.
Dietary interventions
Specific foods may trigger bloating and abdominal distention, especially in patients with overlapping DGBIs. However, only a few studies have evaluated dietary restriction specifically for patients with primary ABD. Restricting non-absorbable sugars led to symptomatic improvement in 81% of patients with FABD who had documented sugar malabsorption.17 Two studies have shown that IBS patients treated with a low-fermentable, oligo-, di-, and monosaccharides (FODMAP) diet noted improvement in ABD and that restricting fructans initially may be the most optimal.18 A recent study showed that the Mediterranean diet improved IBS symptoms, including abdominal pain and bloating.19 It should be noted restrictive diets are efficacious but come with short- and long-term challenges. If empiric treatment and/or therapeutic testing do not resolve symptoms, a referral to a dietitian can be useful. Dietitians can provide tailored dietary advice, ensuring patients avoid trigger foods while maintaining a balanced and nutritious diet.
Prokinetics and laxatives
Prokinetic agents are used to treat symptoms of FD, gastroparesis, chronic idiopathic constipation (CIC), and IBS. A meta-analysis of 13 trials found all constipation medications superior to placebo for treating abdominal bloating in patients with IBS-C.20
Probiotics
Treatment with probiotics is recommended for bloating or distention. One double-blind placebo-controlled trial with two separate probiotics, Bifidobacterium lactis and Lactobacillus acidophilus, showed improvements in global GI symptoms of patients with DGBI at 8 weeks versus placebo, with improvements in bloating symptoms.21
Antibiotics
The most commonly studied antibiotic for treating bloating is rifaximin.22 Global symptomatic improvement in IBS patients treated with antibiotics has correlated with the normalization of hydrogen levels in lactulose hydrogen breath tests.22 Patients with non-constipation IBS randomized to rifaximin 550 mg three times daily for 14 days had a greater proportion of relief of IBS-related bloating compared to placebo for at least 2 of the first 4 weeks after treatment.22 Future research warrants use of narrow-spectrum antibiotics study for FABD as the use of broad-spectrum antibiotics may deplete commensals forever, resulting in metabolic disorders.
Biofeedback therapy
Anorectal biofeedback therapy may help with ABD, particularly in patients with IBS-C and chronic constipation. One study noted that post-biofeedback therapy, myoelectric activity of the intercostals and diaphragm decreased, and internal oblique myoelectric activity increased.23 This study also showed ascent of the diaphragm and decreased girth, improving distention.
Central neuromodulators
As bloating results from multiple disturbed mechanisms, including altered gut-brain interaction, these symptoms can be amplified by psychological states such as anxiety, depression, or somatization. Central neuromodulators reduce the perception of visceral signals, re-regulate brain-gut control mechanisms, and improve psychological comorbidities.6 A large study of FD patients demonstrated that both amitriptyline (50 mg daily) and escitalopram (10 mg daily) significantly improved postprandial bloating compared to placebo.24 Antidepressants that activate noradrenergic and serotonergic pathways, including tricyclic antidepressants (e.g., amitriptyline) and serotonin-norepinephrine reuptake inhibitors (e.g., duloxetine and venlafaxine), show the greatest benefit in reducing visceral sensations.6
Brain-gut behavioral therapies
A recent multidisciplinary consensus report supports a myriad of potential brain-gut behavioral therapies (BGBTs) for treating DGBI.25 These therapies, including hypnotherapy, cognitive behavioral therapy (CBT), and other modalities, may be combined with central neuromodulators and other GI treatments in a safe, noninvasive, and complementary fashion. BGBTs do not need to be symptom-specific, as they improve overall quality of life, anxiety, stress, and the burden associated with DGBIs. To date, none of the BGBTs have focused exclusively on FABD; however, prescription-based psychological therapies are now FDA-approved for use on smart apps, improving global symptoms that include bloating in IBS and FD.
Recent AGA clinical update best practices should be considered for the clinical care of patients with ABD.6
Conclusion and Future Perspectives
ABD are highly prevalent and significantly impact patients with various GI and metabolic disorders. Although our understanding of these symptoms is still evolving, evidence increasingly points to the dysregulation of the gut-brain axis and supports the application of the biopsychosocial model in treatment. This model addresses diet, motility, visceral sensitivity, pelvic floor disorders and psychosocial factors, providing a comprehensive approach to patient care.
Physician-scientists around the globe face numerous challenges when evaluating patients with these symptoms. However, the recent AGA clinical update on the best practice guidelines offers step-by-step diagnostic tests and treatment options to assist physicians in making informed decisions. . More comprehensive, large-scale, and longitudinal studies using metabolomics, capsule technologies for discovery of dysbiosis, mass spectrometry, and imaging data are needed to identify the exact contributors to disease pathogenesis, particularly those that can be targeted with pharmacologic agents. Collaborative work between gastroenterologists, dietitians, gut-brain behavioral therapists, endocrinologists, is crucial for clinical care of patients with ABD.
Careful attention to the patient’s primary symptoms and physical examination, combined with advancements in targeted diagnostics like the analysis of microbial markers, metabolites, and molecular signals, can significantly enhance patient clinical outcomes. Additionally, education and effective communication using a patient-centered care model are essential for guiding practical evaluation and individualized treatment.
Dr. Singh is assistant professor (research) at the University of Nevada, Reno, School of Medicine. Dr. Moshiree is director of motility at Atrium Health, and clinical professor of medicine, Wake Forest Medical University, Charlotte, North Carolina.
References
1. Ballou S et al. Prevalence and associated factors of bloating: Results from the Rome Foundation Global Epidemiology Study. Gastroenterology. 2023 June. doi: 10.1053/j.gastro.2023.05.049.
2. Oh JE et al. Abdominal bloating in the United States: Results of a survey of 88,795 Americans examining prevalence and healthcare seeking. Clin Gastroenterol Hepatol. 2023 Aug. doi: 10.1016/j.cgh.2022.10.031.
3. Drossman DA et al. Neuromodulators for functional gastrointestinal disorders (disorders of gut-brain interaction): A Rome Foundation Working Team Report. Gastroenterology. 2018 Mar. doi: 10.1053/j.gastro.2017.11.279.
4. Lacy BE et al. Management of chronic abdominal distension and bloating. Clin Gastroenterol Hepatol. 2021 Feb. doi: 10.1016/j.cgh.2020.03.056.
5. Mearin F et al. Bowel disorders. Gastroenterology. 2016 Feb. doi: 10.1053/j.gastro.2016.02.031.
6. Moshiree B et al. AGA Clinical Practice Update on evaluation and management of belching, abdominal bloating, and distention: expert review. Gastroenterology. 2023 Sep. doi: 10.1053/j.gastro.2023.04.039.
7. Viswanathan L and Rao SS. Intestinal disaccharidase deficiency in adults: evaluation and treatment. Curr Gastroenterol Rep 2023 May. doi: 10.1007/s11894-023-00870-z.
8. Wilder-Smith CH et al. Fructose and lactose intolerance and malabsorption testing: the relationship with symptoms in functional gastrointestinal disorders. Aliment Pharmacol Ther. 2013 Jun. doi: 10.1111/apt.12306.
9. Skodje GI et al. Fructan, rather than gluten, induces symptoms in patients with self-reported non-celiac gluten sensitivity. Gastroenterology. 2018 Feb. doi: 10.1053/j.gastro.2017.10.040.
10. Singh R et al. Current treatment options and therapeutic insights for gastrointestinal dysmotility and functional gastrointestinal disorders. Front Pharmacol. 2022 Jan. doi: 10.3389/fphar.2022.808195.
11. Accarino A et al. Abdominal distention results from caudo-ventral redistribution of contents. Gastroenterology 2009 May. doi: 10.1053/j.gastro.2009.01.067.
12. Shim L et al. Prolonged balloon expulsion is predictive of abdominal distension in bloating. Am J Gastroenterol. 2010 Apr. doi: 10.1038/ajg.2010.54.
13. Villoria A et al. Abdomino-phrenic dyssynergia in patients with abdominal bloating and distension. Am J Gastroenterol. 2011 May. doi: 10.1038/ajg.2010.408.
14. Neri L and Iovino P. Laxative Inadequate Relief Survey Group. Bloating is associated with worse quality of life, treatment satisfaction, and treatment responsiveness among patients with constipation-predominant irritable bowel syndrome and functional constipation. Neurogastroenterol Motil. 2016 Apr. doi: 10.1111/nmo.12758.
15. Saffouri GB et al. Small intestinal microbial dysbiosis underlies symptoms associated with functional gastrointestinal disorders. Nat Commun. 2019 May. doi: 10.1038/s41467-019-09964-7.
16. Villanueva-Millan MJ et al. Methanogens and hydrogen sulfide producing bacteria guide distinct gut microbe profiles and irritable bowel syndrome subtypes. Am J Gastroenterol. 2022 Dec. doi: 10.14309/ajg.0000000000001997.
17. Fernández-Bañares F et al. Sugar malabsorption in functional abdominal bloating: a pilot study on the long-term effect of dietary treatment. Clin Nutr. 2006 Oct. doi: 10.1016/j.clnu.2005.11.010.
18. Böhn L et al. Diet low in FODMAPs reduces symptoms of irritable bowel syndrome as well as traditional dietary advice: a randomized controlled trial. Gastroenterology. 2015 Nov. doi: 10.1053/j.gastro.2015.07.054.
19. Staudacher HM et al. Clinical trial: A Mediterranean diet is feasible and improves gastrointestinal and psychological symptoms in irritable bowel syndrome. Aliment Pharmacol Ther. 2024 Feb. doi: 10.1111/apt.17791.
20. Nelson AD et al. Systematic review and network meta-analysis: efficacy of licensed drugs for abdominal bloating in irritable bowel syndrome with constipation. Aliment Pharmacol Ther. 2021 Jul. doi: 10.1111/apt.16437.
21. Ringel-Kulka T et al. Probiotic bacteria Lactobacillus acidophilus NCFM and Bifidobacterium lactis Bi-07 versus placebo for the symptoms of bloating in patients with functional bowel disorders: a double-blind study. J Clin Gastroenterol. 2011 Jul. doi: 10.1097/MCG.0b013e31820ca4d6.
22. Pimentel M et al. Rifaximin therapy for patients with irritable bowel syndrome without constipation. N Engl J Med. 2011 Jan. doi: 10.1056/NEJMoa1004409.
23. Iovino P et al. Pelvic floor biofeedback is an effective treatment for severe bloating in disorders of gut-brain interaction with outlet dysfunction. Neurogastroenterol Motil 2022 May. doi: 10.1111/nmo.14264.
24. Talley NJ et al. Effect of amitriptyline and escitalopram on functional dyspepsia: A multicenter, randomized controlled study. Gastroenterology. 2015 Aug. doi: 10.1053/j.gastro.2015.04.020.
25. Keefer L et al. A Rome Working Team Report on brain-gut behavior therapies for disorders of gut-brain interaction. Gastroenterology. 2022 Jan. doi: 10.1053/j.gastro.2021.09.015.
Introduction
Abdominal bloating is a common condition affecting up to 3.5% of people globally (4.6% in women and 2.4% in men),1 with 13.9% of the US population reporting bloating in the past 7 days.2 The prevalence of bloating and distention exceeds 50% when linked to disorders of gut-brain interaction (DGBIs) such as irritable bowel syndrome (IBS), constipation, gastroparesis, and functional dyspepsia (FD).3,4 According to the Rome IV criteria, functional bloating and distention (FABD) patients are characterized by recurrent symptoms of abdominal fullness or pressure (bloating), or a visible increase in abdominal girth (distention) occurring at least 1 day per week for 3 consecutive months with an onset of 6 months and without predominant pain or altered bowel habits.5
Prolonged abdominal bloating and distention (ABD) can significantly impact quality of life and work productivity and can lead to increased medical consultations.2 Multiple pathophysiological mechanisms are involved in ABD that complicate the clinical management.4 There is an unmet need to understand the underlying mechanisms that lead to the development of ABD such as, food intolerance, abnormal viscerosomatic reflex, visceral hypersensitivity, and gut microbial dysbiosis. Recent advancements and acceptance of a multidisciplinary management of ABD have shifted the paradigm from merely treating symptoms to subtyping the condition and identifying overlaps with other DGBIs in order to individualize treatment that addresses the underlying pathophysiological mechanism. The recent American Gastroenterological Association (AGA) clinical update provided insights into the best practice advice for evaluating and managing ABD based on a review of current literature and on expert opinion of coauthors.6 This article aims to deliberate a practical approach to diagnostic strategies and treatment options based on etiology to refine clinical care of patients with ABD.
Pathophysiological Mechanisms
ABD can result from various pathophysiological mechanisms. This section highlights the major causes (illustrated in Figure 1).
Food intolerances
Understanding food intolerances is crucial for diagnosing and managing patients with ABD. Disaccharidase deficiency is common (e.g., lactase deficiency is found in 35%-40% of adults).7 It can be undiagnosed in patients presenting with IBS symptoms, given the overlap in presentation with a prevalence of 9% of pan-disaccharidase deficiency. Sucrase-deficient patients must often adjust sugar and carbohydrate/starch intake to relieve symptoms.7 Deficiencies in lactase and sucrase activity, along with the consumption of some artificial sweeteners (e.g., sugar alcohols and sorbitol) and fructans can lead to bloating and distention. These substances increase osmotic load, fluid retention, microbial fermentation, and visceral hypersensitivity, leading to gas production and abdominal distention. One prospective study of symptomatic patients with various DGBIs (n = 1372) reported a prevalence of lactose intolerance and malabsorption at 51% and 32%, respectively.8 Furthermore, fructose intolerance and malabsorption prevalence were 60% and 45%, respectively.8 Notably, lactase deficiency does not always cause ABD, as not all individuals with lactase deficiency experience these symptoms after consuming lactose. Patients with celiac disease (CD), non-celiac gluten sensitivity (NCGS), and gluten intolerance can also experience bloating and distention, with or without changes in bowel habits.9 In some patients with self-reported NCGS, symptoms may be due to fructans in gluten-rich foods rather than gluten itself, thus recommending the elimination of fructans may help improve symptoms.9
Visceral hypersensitivity
Visceral hypersensitivity is explained by an increased perception of gut mechano-chemical stimulation, which typically manifests in an aggravated feeling of pain, nausea, distension, and ABD.10 In the gut, food particles and gut bacteria and their derived molecules interact with neuroimmune and enteroendocrine cells causing visceral sensitivity by the proximity of gut’s neurons to immune cells activated by them and leading to inflammatory reactions (Figure 1). 
Pelvic floor dysfunction
Patients with anorectal motor dysfunction often experience difficulty in effectively evacuating both gas and stool, leading to ABD.12 Impaired ability to expel gas and stool results in prolonged balloon expulsion times, which correlates with symptoms of distention in patients with constipation.
Abdominophrenic dyssynergia
Abdominophrenic dyssynergia is characterized as a paradoxical viscerosomatic reflex response to minimal gaseous distention in individuals with FABD.13 In this condition, the diaphragm contracts (descends), and the anterior abdominal wall muscles relax in response to the presence of gas. This response is opposite to the normal physiological response to increased intraluminal gas, where the diaphragm relaxes and the anterior abdominal muscles contract to increase the craniocaudal capacity of the abdominal cavity without causing abdominal protrusion.13 Patients with FABD exhibit significant abdominal wall protrusion and diaphragmatic descent even with relatively small increases in intraluminal gas.11 Understanding the role of abdominophrenic dyssynergia in abdominal bloating and distention is essential for effective diagnosis and management of the patients.
Gut dysmotility
Gut dysmotility is a crucial factor that can contribute to FABD. Gut dysmotility affects the movement of contents through the GI tract, accumulating gas and stool, directly contributing to bloating and distention. A prospective study involving over 2000 patients with functional constipation and constipation predominant-IBS (IBS-C) found that more than 90% of these patients reported symptoms of bloating.14 Furthermore, in IBS-C patients, those with prolonged colonic transit exhibited greater abdominal distention compared to those with normal gut transit times. In patients with gastroparesis, delayed gastric emptying resulting in prolonged retention of stomach contents is the main factor in the generation of bloating symptoms.4
Small intestinal bacterial overgrowth (SIBO)
SIBO is overrepresented in various conditions, including IBS, FD, diabetes, gastrointestinal (GI) surgery patients and obesity, and can play an important role in generating ABD. Excess bacteria in the small intestine ferment carbohydrates, producing gas that stretches and distends the small intestine, leading to these symptoms. Additionally, altered sensation and abnormal viscerosomatic reflexes may contribute to SIBO-related bloating.4 One recent study noted decreased duodenal phylogenetic diversity in individuals who developed postprandial bloating.15 Increased methane levels caused by intestinal methanogen overgrowth, primarily the archaea Methanobrevibacter smithii, is possibly responsible for ABD in patients with IBS-C.16 Testing for SIBO in patients with ABD is generally only recommended if there are clear risk factors or severe symptoms warranting a test-and-treat approach.
Practical Diagnosis
Diagnosing ABD typically does not require extensive laboratory testing, imaging, or endoscopy unless there are alarm features or significant changes in symptoms. Here is the AGA clinical update on best practice advice6 for when to conduct further testing:
Diagnostic tests should be considered if patients exhibit:
- Recent onset or worsening of dyspepsia or abdominal pain
- Vomiting
- GI bleeding
- Unintentional weight loss exceeding 10% of body weight
- Chronic diarrhea
- Family history of GI malignancy, celiac disease, or inflammatory bowel disease
Physical examination
If visible abdominal distention is present, a thorough abdominal examination can help identify potential issues:
- Tympany to percussion suggests bowel dilation.
- Abnormal bowel sounds may indicate obstruction or ileus.
- A succussion splash could indicate the presence of ascites and obstruction.
- Any abnormalities discovered during the physical exam should prompt further investigation with imaging, such as a computed tomography (CT) scan or ultrasound, to evaluate for ascites, masses, or increased bowel gas due to ileus, obstruction, or pseudo-obstruction.
Radiologic imaging, laboratory testing and endoscopy
- An abdominal x-ray may reveal an increased stool burden, suggesting the need for further evaluation of slow transit constipation or a pelvic floor disorder, particularly in patients with functional constipation, IBS-mixed, or IBS-C.
- Hyperglycemia, weight gain, and bloating can be a presenting sign of ovarian cancer therefore all women should continue pelvic exams as dictated by the gynecologic societies. The need for an annual pelvic exam should be discussed with health care professionals especially in those with family history of ovarian cancer.
- An upper endoscopy may be warranted for patients over 40 years old with dyspeptic symptoms and abdominal bloating or distention, especially in regions with a high prevalence of Helicobacter pylori.
- Chronic pancreatitis, indicated by bloating and pain, may necessitate fecal elastase testing to assess pancreatic function.
The expert review in the AGA clinical update provides step-by-step advice regarding the best practices6 for diagnosis and identifying who to test for ABD.
Treatment Options
The following sections highlight recent best practice advice on therapeutic approaches for treating ABD.
Dietary interventions
Specific foods may trigger bloating and abdominal distention, especially in patients with overlapping DGBIs. However, only a few studies have evaluated dietary restriction specifically for patients with primary ABD. Restricting non-absorbable sugars led to symptomatic improvement in 81% of patients with FABD who had documented sugar malabsorption.17 Two studies have shown that IBS patients treated with a low-fermentable, oligo-, di-, and monosaccharides (FODMAP) diet noted improvement in ABD and that restricting fructans initially may be the most optimal.18 A recent study showed that the Mediterranean diet improved IBS symptoms, including abdominal pain and bloating.19 It should be noted restrictive diets are efficacious but come with short- and long-term challenges. If empiric treatment and/or therapeutic testing do not resolve symptoms, a referral to a dietitian can be useful. Dietitians can provide tailored dietary advice, ensuring patients avoid trigger foods while maintaining a balanced and nutritious diet.
Prokinetics and laxatives
Prokinetic agents are used to treat symptoms of FD, gastroparesis, chronic idiopathic constipation (CIC), and IBS. A meta-analysis of 13 trials found all constipation medications superior to placebo for treating abdominal bloating in patients with IBS-C.20
Probiotics
Treatment with probiotics is recommended for bloating or distention. One double-blind placebo-controlled trial with two separate probiotics, Bifidobacterium lactis and Lactobacillus acidophilus, showed improvements in global GI symptoms of patients with DGBI at 8 weeks versus placebo, with improvements in bloating symptoms.21
Antibiotics
The most commonly studied antibiotic for treating bloating is rifaximin.22 Global symptomatic improvement in IBS patients treated with antibiotics has correlated with the normalization of hydrogen levels in lactulose hydrogen breath tests.22 Patients with non-constipation IBS randomized to rifaximin 550 mg three times daily for 14 days had a greater proportion of relief of IBS-related bloating compared to placebo for at least 2 of the first 4 weeks after treatment.22 Future research warrants use of narrow-spectrum antibiotics study for FABD as the use of broad-spectrum antibiotics may deplete commensals forever, resulting in metabolic disorders.
Biofeedback therapy
Anorectal biofeedback therapy may help with ABD, particularly in patients with IBS-C and chronic constipation. One study noted that post-biofeedback therapy, myoelectric activity of the intercostals and diaphragm decreased, and internal oblique myoelectric activity increased.23 This study also showed ascent of the diaphragm and decreased girth, improving distention.
Central neuromodulators
As bloating results from multiple disturbed mechanisms, including altered gut-brain interaction, these symptoms can be amplified by psychological states such as anxiety, depression, or somatization. Central neuromodulators reduce the perception of visceral signals, re-regulate brain-gut control mechanisms, and improve psychological comorbidities.6 A large study of FD patients demonstrated that both amitriptyline (50 mg daily) and escitalopram (10 mg daily) significantly improved postprandial bloating compared to placebo.24 Antidepressants that activate noradrenergic and serotonergic pathways, including tricyclic antidepressants (e.g., amitriptyline) and serotonin-norepinephrine reuptake inhibitors (e.g., duloxetine and venlafaxine), show the greatest benefit in reducing visceral sensations.6
Brain-gut behavioral therapies
A recent multidisciplinary consensus report supports a myriad of potential brain-gut behavioral therapies (BGBTs) for treating DGBI.25 These therapies, including hypnotherapy, cognitive behavioral therapy (CBT), and other modalities, may be combined with central neuromodulators and other GI treatments in a safe, noninvasive, and complementary fashion. BGBTs do not need to be symptom-specific, as they improve overall quality of life, anxiety, stress, and the burden associated with DGBIs. To date, none of the BGBTs have focused exclusively on FABD; however, prescription-based psychological therapies are now FDA-approved for use on smart apps, improving global symptoms that include bloating in IBS and FD.
Recent AGA clinical update best practices should be considered for the clinical care of patients with ABD.6
Conclusion and Future Perspectives
ABD are highly prevalent and significantly impact patients with various GI and metabolic disorders. Although our understanding of these symptoms is still evolving, evidence increasingly points to the dysregulation of the gut-brain axis and supports the application of the biopsychosocial model in treatment. This model addresses diet, motility, visceral sensitivity, pelvic floor disorders and psychosocial factors, providing a comprehensive approach to patient care.
Physician-scientists around the globe face numerous challenges when evaluating patients with these symptoms. However, the recent AGA clinical update on the best practice guidelines offers step-by-step diagnostic tests and treatment options to assist physicians in making informed decisions. . More comprehensive, large-scale, and longitudinal studies using metabolomics, capsule technologies for discovery of dysbiosis, mass spectrometry, and imaging data are needed to identify the exact contributors to disease pathogenesis, particularly those that can be targeted with pharmacologic agents. Collaborative work between gastroenterologists, dietitians, gut-brain behavioral therapists, endocrinologists, is crucial for clinical care of patients with ABD.
Careful attention to the patient’s primary symptoms and physical examination, combined with advancements in targeted diagnostics like the analysis of microbial markers, metabolites, and molecular signals, can significantly enhance patient clinical outcomes. Additionally, education and effective communication using a patient-centered care model are essential for guiding practical evaluation and individualized treatment.
Dr. Singh is assistant professor (research) at the University of Nevada, Reno, School of Medicine. Dr. Moshiree is director of motility at Atrium Health, and clinical professor of medicine, Wake Forest Medical University, Charlotte, North Carolina.
References
1. Ballou S et al. Prevalence and associated factors of bloating: Results from the Rome Foundation Global Epidemiology Study. Gastroenterology. 2023 June. doi: 10.1053/j.gastro.2023.05.049.
2. Oh JE et al. Abdominal bloating in the United States: Results of a survey of 88,795 Americans examining prevalence and healthcare seeking. Clin Gastroenterol Hepatol. 2023 Aug. doi: 10.1016/j.cgh.2022.10.031.
3. Drossman DA et al. Neuromodulators for functional gastrointestinal disorders (disorders of gut-brain interaction): A Rome Foundation Working Team Report. Gastroenterology. 2018 Mar. doi: 10.1053/j.gastro.2017.11.279.
4. Lacy BE et al. Management of chronic abdominal distension and bloating. Clin Gastroenterol Hepatol. 2021 Feb. doi: 10.1016/j.cgh.2020.03.056.
5. Mearin F et al. Bowel disorders. Gastroenterology. 2016 Feb. doi: 10.1053/j.gastro.2016.02.031.
6. Moshiree B et al. AGA Clinical Practice Update on evaluation and management of belching, abdominal bloating, and distention: expert review. Gastroenterology. 2023 Sep. doi: 10.1053/j.gastro.2023.04.039.
7. Viswanathan L and Rao SS. Intestinal disaccharidase deficiency in adults: evaluation and treatment. Curr Gastroenterol Rep 2023 May. doi: 10.1007/s11894-023-00870-z.
8. Wilder-Smith CH et al. Fructose and lactose intolerance and malabsorption testing: the relationship with symptoms in functional gastrointestinal disorders. Aliment Pharmacol Ther. 2013 Jun. doi: 10.1111/apt.12306.
9. Skodje GI et al. Fructan, rather than gluten, induces symptoms in patients with self-reported non-celiac gluten sensitivity. Gastroenterology. 2018 Feb. doi: 10.1053/j.gastro.2017.10.040.
10. Singh R et al. Current treatment options and therapeutic insights for gastrointestinal dysmotility and functional gastrointestinal disorders. Front Pharmacol. 2022 Jan. doi: 10.3389/fphar.2022.808195.
11. Accarino A et al. Abdominal distention results from caudo-ventral redistribution of contents. Gastroenterology 2009 May. doi: 10.1053/j.gastro.2009.01.067.
12. Shim L et al. Prolonged balloon expulsion is predictive of abdominal distension in bloating. Am J Gastroenterol. 2010 Apr. doi: 10.1038/ajg.2010.54.
13. Villoria A et al. Abdomino-phrenic dyssynergia in patients with abdominal bloating and distension. Am J Gastroenterol. 2011 May. doi: 10.1038/ajg.2010.408.
14. Neri L and Iovino P. Laxative Inadequate Relief Survey Group. Bloating is associated with worse quality of life, treatment satisfaction, and treatment responsiveness among patients with constipation-predominant irritable bowel syndrome and functional constipation. Neurogastroenterol Motil. 2016 Apr. doi: 10.1111/nmo.12758.
15. Saffouri GB et al. Small intestinal microbial dysbiosis underlies symptoms associated with functional gastrointestinal disorders. Nat Commun. 2019 May. doi: 10.1038/s41467-019-09964-7.
16. Villanueva-Millan MJ et al. Methanogens and hydrogen sulfide producing bacteria guide distinct gut microbe profiles and irritable bowel syndrome subtypes. Am J Gastroenterol. 2022 Dec. doi: 10.14309/ajg.0000000000001997.
17. Fernández-Bañares F et al. Sugar malabsorption in functional abdominal bloating: a pilot study on the long-term effect of dietary treatment. Clin Nutr. 2006 Oct. doi: 10.1016/j.clnu.2005.11.010.
18. Böhn L et al. Diet low in FODMAPs reduces symptoms of irritable bowel syndrome as well as traditional dietary advice: a randomized controlled trial. Gastroenterology. 2015 Nov. doi: 10.1053/j.gastro.2015.07.054.
19. Staudacher HM et al. Clinical trial: A Mediterranean diet is feasible and improves gastrointestinal and psychological symptoms in irritable bowel syndrome. Aliment Pharmacol Ther. 2024 Feb. doi: 10.1111/apt.17791.
20. Nelson AD et al. Systematic review and network meta-analysis: efficacy of licensed drugs for abdominal bloating in irritable bowel syndrome with constipation. Aliment Pharmacol Ther. 2021 Jul. doi: 10.1111/apt.16437.
21. Ringel-Kulka T et al. Probiotic bacteria Lactobacillus acidophilus NCFM and Bifidobacterium lactis Bi-07 versus placebo for the symptoms of bloating in patients with functional bowel disorders: a double-blind study. J Clin Gastroenterol. 2011 Jul. doi: 10.1097/MCG.0b013e31820ca4d6.
22. Pimentel M et al. Rifaximin therapy for patients with irritable bowel syndrome without constipation. N Engl J Med. 2011 Jan. doi: 10.1056/NEJMoa1004409.
23. Iovino P et al. Pelvic floor biofeedback is an effective treatment for severe bloating in disorders of gut-brain interaction with outlet dysfunction. Neurogastroenterol Motil 2022 May. doi: 10.1111/nmo.14264.
24. Talley NJ et al. Effect of amitriptyline and escitalopram on functional dyspepsia: A multicenter, randomized controlled study. Gastroenterology. 2015 Aug. doi: 10.1053/j.gastro.2015.04.020.
25. Keefer L et al. A Rome Working Team Report on brain-gut behavior therapies for disorders of gut-brain interaction. Gastroenterology. 2022 Jan. doi: 10.1053/j.gastro.2021.09.015.
Physician-Scientist Taps into Microbiome to Fight Cancer
The lowest point in the nascent career of Neelendu Dey, MD, helped seal his fate as a physician-scientist.
He had just started his first year as a resident at University of California, San Francisco. One of his patients was a 30-year-old woman who was dying of metastatic colorectal cancer. “I was in my mid-20s interacting with an individual just a few years older than I am, going through one of the most terrible health outcomes one could imagine,” Dr. Dey said.
He remembers asking the patient what he could do for her, how he could make her feel more comfortable. “That feeling of helplessness, particularly as we think about young people developing cancer, it really stuck with me through the years,” he said.
In an interview, he talked about his dual role as a physician and scientist, and how those two interests are guiding his research in precancerous conditions of the colon.
Cases like that of the young woman with colon cancer “really help drive the urgency of the work we do, and the research questions we ask, as we try to move the ball forward and help folks at earlier stages,” he said.
Q: Why did you choose GI?
When you think about what sorts of chronic diseases really impact your quality of life, gut health is one of the chief contributors among various aspects of health. And that really appealed to me — the ability to take someone who is essentially handicapped by a series of illnesses and symptoms that derive from the GI tract and enable them to return to the person they want to be, to be productive in the way that they want to be, and have a rewarding life.
As I thought about how I wanted to contribute to the future of medicine, one of the ways in which I’ve always thought that I would do that is through research. When I considered the fields that really appealed to me, both from that clinical standpoint and research standpoint, GI was one that really stood out. There has been a lot of exciting research going on in GI. My lab currently studies the microbiome, and I feel like this is an area in which we can contribute.
Q: What role does digestive health play in overall health?
Obviously, the direct answer is gut health is so critical in something like nutritional intake. Some GI symptoms, if your gut health has gone awry, can really be detrimental in terms of quality of life. But one less obvious role that digestive health plays is its long-term effects. We’re starting to appreciate that gut health, the gut microbiome, and gut immune education are probably long-term players. Some experiences in early life might shape our immunity in ways that have consequences for us much later in life. Whether we get early life antibiotics, for example, may potentially contribute to colorectal cancer down the line. Thinking about the long-term players is more challenging, but it’s also an appealing opportunity as we think about how we can shape medicine moving forward.
Q: What practice challenges have you faced in your career?
First, being a physician-scientist. It’s challenging to be either a physician alone or to be a researcher alone. And trying to do both includes the challenges of both individual worlds. It just takes more time to get all the prerequisite training. And second, there are just challenges with getting the opportunities to contribute in the ways that you want — to get the research funding, to get the papers out, things like that.
Q: Tell me about the work you’ve been doing in your lab to develop microbiome-based strategies for preventing and treating cancer.
The microbiome presents several opportunities when it comes to cancer prevention. One is identifying markers of cancer risk, or of general good health down the line. Some of those biomarkers could — potentially — feed directly into personalized risk assessment and maybe even inform a future screening strategy. The second opportunity the microbiome presents is if we identify a microbe that influences your cancer risk, can we then understand and exploit, or utilize, that mechanism to mitigate cancer risk in the future? Our lab has done work looking at subspecies levels of microbes that track with health or cancer. We’ve done some work to identify what these subspecies groupings are and have identified some links to certain precancerous changes in the colon. We think that there’s an opportunity here for future interventions.
Q: Have you published other papers?
We recently published another paper describing how some microbes can interact with a tumor suppressor gene and are influenced in a sex-biased manner to drive tumorigenesis in a mouse model. We think, based on what we’re seeing in human data, that there may be some relationships and we’re exploring that now as well.
Q: What is your vision for the future in GI, and in your career?
The vision that I have is to create clinical tools that can expand our reach and our effectiveness and cancer prevention. I think that there are opportunities for leveraging microbiome research to accomplish this. And one outcome I could imagine is leveraging some of these insights to expand noninvasive screening at even earlier ages than we do now. I mean, we just dialed back the recommended age for colonoscopy for average risk individuals to 45. But I could envision a future in which noninvasive screening starts earlier, in which the first stool-based tests that we deploy to assess personalized risk are used in the pediatric clinic.
Lightning Round
Texting or talking?
Talking
Favorite city in the United States besides the one you live in?
St. Louis
Cat or dog person?
Both
If you weren’t a GI, what would you be?
Musician
Best place you went on vacation?
Borneo
Favorite sport?
Soccer
Favorite ice cream?
Cashew-based salted caramel
What song do you have to sing along with when you hear it?
Sweet Child of Mine
Favorite movie or TV show?
25th Hour or Shawshank Redemption
Optimist or Pessimist?
Optimist
The lowest point in the nascent career of Neelendu Dey, MD, helped seal his fate as a physician-scientist.
He had just started his first year as a resident at University of California, San Francisco. One of his patients was a 30-year-old woman who was dying of metastatic colorectal cancer. “I was in my mid-20s interacting with an individual just a few years older than I am, going through one of the most terrible health outcomes one could imagine,” Dr. Dey said.
He remembers asking the patient what he could do for her, how he could make her feel more comfortable. “That feeling of helplessness, particularly as we think about young people developing cancer, it really stuck with me through the years,” he said.
In an interview, he talked about his dual role as a physician and scientist, and how those two interests are guiding his research in precancerous conditions of the colon.
Cases like that of the young woman with colon cancer “really help drive the urgency of the work we do, and the research questions we ask, as we try to move the ball forward and help folks at earlier stages,” he said.
Q: Why did you choose GI?
When you think about what sorts of chronic diseases really impact your quality of life, gut health is one of the chief contributors among various aspects of health. And that really appealed to me — the ability to take someone who is essentially handicapped by a series of illnesses and symptoms that derive from the GI tract and enable them to return to the person they want to be, to be productive in the way that they want to be, and have a rewarding life.
As I thought about how I wanted to contribute to the future of medicine, one of the ways in which I’ve always thought that I would do that is through research. When I considered the fields that really appealed to me, both from that clinical standpoint and research standpoint, GI was one that really stood out. There has been a lot of exciting research going on in GI. My lab currently studies the microbiome, and I feel like this is an area in which we can contribute.
Q: What role does digestive health play in overall health?
Obviously, the direct answer is gut health is so critical in something like nutritional intake. Some GI symptoms, if your gut health has gone awry, can really be detrimental in terms of quality of life. But one less obvious role that digestive health plays is its long-term effects. We’re starting to appreciate that gut health, the gut microbiome, and gut immune education are probably long-term players. Some experiences in early life might shape our immunity in ways that have consequences for us much later in life. Whether we get early life antibiotics, for example, may potentially contribute to colorectal cancer down the line. Thinking about the long-term players is more challenging, but it’s also an appealing opportunity as we think about how we can shape medicine moving forward.
Q: What practice challenges have you faced in your career?
First, being a physician-scientist. It’s challenging to be either a physician alone or to be a researcher alone. And trying to do both includes the challenges of both individual worlds. It just takes more time to get all the prerequisite training. And second, there are just challenges with getting the opportunities to contribute in the ways that you want — to get the research funding, to get the papers out, things like that.
Q: Tell me about the work you’ve been doing in your lab to develop microbiome-based strategies for preventing and treating cancer.
The microbiome presents several opportunities when it comes to cancer prevention. One is identifying markers of cancer risk, or of general good health down the line. Some of those biomarkers could — potentially — feed directly into personalized risk assessment and maybe even inform a future screening strategy. The second opportunity the microbiome presents is if we identify a microbe that influences your cancer risk, can we then understand and exploit, or utilize, that mechanism to mitigate cancer risk in the future? Our lab has done work looking at subspecies levels of microbes that track with health or cancer. We’ve done some work to identify what these subspecies groupings are and have identified some links to certain precancerous changes in the colon. We think that there’s an opportunity here for future interventions.
Q: Have you published other papers?
We recently published another paper describing how some microbes can interact with a tumor suppressor gene and are influenced in a sex-biased manner to drive tumorigenesis in a mouse model. We think, based on what we’re seeing in human data, that there may be some relationships and we’re exploring that now as well.
Q: What is your vision for the future in GI, and in your career?
The vision that I have is to create clinical tools that can expand our reach and our effectiveness and cancer prevention. I think that there are opportunities for leveraging microbiome research to accomplish this. And one outcome I could imagine is leveraging some of these insights to expand noninvasive screening at even earlier ages than we do now. I mean, we just dialed back the recommended age for colonoscopy for average risk individuals to 45. But I could envision a future in which noninvasive screening starts earlier, in which the first stool-based tests that we deploy to assess personalized risk are used in the pediatric clinic.
Lightning Round
Texting or talking?
Talking
Favorite city in the United States besides the one you live in?
St. Louis
Cat or dog person?
Both
If you weren’t a GI, what would you be?
Musician
Best place you went on vacation?
Borneo
Favorite sport?
Soccer
Favorite ice cream?
Cashew-based salted caramel
What song do you have to sing along with when you hear it?
Sweet Child of Mine
Favorite movie or TV show?
25th Hour or Shawshank Redemption
Optimist or Pessimist?
Optimist
The lowest point in the nascent career of Neelendu Dey, MD, helped seal his fate as a physician-scientist.
He had just started his first year as a resident at University of California, San Francisco. One of his patients was a 30-year-old woman who was dying of metastatic colorectal cancer. “I was in my mid-20s interacting with an individual just a few years older than I am, going through one of the most terrible health outcomes one could imagine,” Dr. Dey said.
He remembers asking the patient what he could do for her, how he could make her feel more comfortable. “That feeling of helplessness, particularly as we think about young people developing cancer, it really stuck with me through the years,” he said.
In an interview, he talked about his dual role as a physician and scientist, and how those two interests are guiding his research in precancerous conditions of the colon.
Cases like that of the young woman with colon cancer “really help drive the urgency of the work we do, and the research questions we ask, as we try to move the ball forward and help folks at earlier stages,” he said.
Q: Why did you choose GI?
When you think about what sorts of chronic diseases really impact your quality of life, gut health is one of the chief contributors among various aspects of health. And that really appealed to me — the ability to take someone who is essentially handicapped by a series of illnesses and symptoms that derive from the GI tract and enable them to return to the person they want to be, to be productive in the way that they want to be, and have a rewarding life.
As I thought about how I wanted to contribute to the future of medicine, one of the ways in which I’ve always thought that I would do that is through research. When I considered the fields that really appealed to me, both from that clinical standpoint and research standpoint, GI was one that really stood out. There has been a lot of exciting research going on in GI. My lab currently studies the microbiome, and I feel like this is an area in which we can contribute.
Q: What role does digestive health play in overall health?
Obviously, the direct answer is gut health is so critical in something like nutritional intake. Some GI symptoms, if your gut health has gone awry, can really be detrimental in terms of quality of life. But one less obvious role that digestive health plays is its long-term effects. We’re starting to appreciate that gut health, the gut microbiome, and gut immune education are probably long-term players. Some experiences in early life might shape our immunity in ways that have consequences for us much later in life. Whether we get early life antibiotics, for example, may potentially contribute to colorectal cancer down the line. Thinking about the long-term players is more challenging, but it’s also an appealing opportunity as we think about how we can shape medicine moving forward.
Q: What practice challenges have you faced in your career?
First, being a physician-scientist. It’s challenging to be either a physician alone or to be a researcher alone. And trying to do both includes the challenges of both individual worlds. It just takes more time to get all the prerequisite training. And second, there are just challenges with getting the opportunities to contribute in the ways that you want — to get the research funding, to get the papers out, things like that.
Q: Tell me about the work you’ve been doing in your lab to develop microbiome-based strategies for preventing and treating cancer.
The microbiome presents several opportunities when it comes to cancer prevention. One is identifying markers of cancer risk, or of general good health down the line. Some of those biomarkers could — potentially — feed directly into personalized risk assessment and maybe even inform a future screening strategy. The second opportunity the microbiome presents is if we identify a microbe that influences your cancer risk, can we then understand and exploit, or utilize, that mechanism to mitigate cancer risk in the future? Our lab has done work looking at subspecies levels of microbes that track with health or cancer. We’ve done some work to identify what these subspecies groupings are and have identified some links to certain precancerous changes in the colon. We think that there’s an opportunity here for future interventions.
Q: Have you published other papers?
We recently published another paper describing how some microbes can interact with a tumor suppressor gene and are influenced in a sex-biased manner to drive tumorigenesis in a mouse model. We think, based on what we’re seeing in human data, that there may be some relationships and we’re exploring that now as well.
Q: What is your vision for the future in GI, and in your career?
The vision that I have is to create clinical tools that can expand our reach and our effectiveness and cancer prevention. I think that there are opportunities for leveraging microbiome research to accomplish this. And one outcome I could imagine is leveraging some of these insights to expand noninvasive screening at even earlier ages than we do now. I mean, we just dialed back the recommended age for colonoscopy for average risk individuals to 45. But I could envision a future in which noninvasive screening starts earlier, in which the first stool-based tests that we deploy to assess personalized risk are used in the pediatric clinic.
Lightning Round
Texting or talking?
Talking
Favorite city in the United States besides the one you live in?
St. Louis
Cat or dog person?
Both
If you weren’t a GI, what would you be?
Musician
Best place you went on vacation?
Borneo
Favorite sport?
Soccer
Favorite ice cream?
Cashew-based salted caramel
What song do you have to sing along with when you hear it?
Sweet Child of Mine
Favorite movie or TV show?
25th Hour or Shawshank Redemption
Optimist or Pessimist?
Optimist




















