User login
Neurology Reviews covers innovative and emerging news in neurology and neuroscience every month, with a focus on practical approaches to treating Parkinson's disease, epilepsy, headache, stroke, multiple sclerosis, Alzheimer's disease, and other neurologic disorders.
PML
Progressive multifocal leukoencephalopathy
Rituxan
The leading independent newspaper covering neurology news and commentary.
Boys may carry the weight, or overweight, of adults’ infertility
Overweight boy, infertile man?
When it comes to causes of infertility, history and science have generally focused on women. A lot of the research overlooks men, but some previous studies have suggested that male infertility contributes to about half of the cases of couple infertility. The reason for much of that male infertility, however, has been a mystery. Until now.
A group of Italian investigators looked at the declining trend in sperm counts over the past 40 years and the increase of childhood obesity. Is there a correlation? The researchers think so. Childhood obesity can be linked to multiple causes, but the researchers zeroed in on the effect that obesity has on metabolic rates and, therefore, testicular growth.
Collecting data on testicular volume, body mass index (BMI), and insulin resistance from 268 boys aged 2-18 years, the researchers discovered that those with normal weight and normal insulin levels had testicular volumes 1.5 times higher than their overweight counterparts and 1.5-2 times higher than those with hyperinsulinemia, building a case for obesity being a factor for infertility later in life.
Since low testicular volume is associated with lower sperm count and production as an adult, putting two and two together makes a compelling argument for childhood obesity being a major male infertility culprit. It also creates even more urgency for the health care industry and community decision makers to focus on childhood obesity.
It sure would be nice to be able to take one of the many risk factors for future human survival off the table. Maybe by taking something, like cake, off the table.
Fecal transplantation moves to the kitchen
Fecal microbiota transplantation is an effective way to treat Clostridioides difficile infection, but, in the end, it’s still a transplantation procedure involving a nasogastric or colorectal tube or rather large oral capsules with a demanding (30-40 capsules over 2 days) dosage. Please, Science, tell us there’s a better way.
Science, in the form of investigators at the University of Geneva and Lausanne University Hospital in Switzerland, has spoken, and there may be a better way. Presenting fecal beads: All the bacterial goodness of donor stool without the tubal insertions or massive quantities of giant capsules.
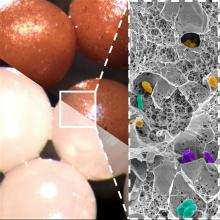
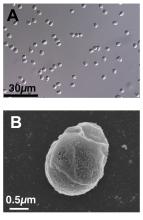
We know you’re scoffing out there, but it’s true. All you need is a little alginate, which is a “biocompatible polysaccharide isolated from brown algae” of the Phaeophyceae family. The donor feces is microencapsulated by mixing it with the alginate, dropping that mixture into water containing calcium chloride, turning it into a gel, and then freeze-drying the gel into small (just 2 mm), solid beads.
Sounds plausible enough, but what do you do with them? “These brownish beads can be easily dispersed in a liquid or food that is pleasant to eat. They also have no taste,” senior author Eric Allémann, PhD, said in a statement released by the University of Geneva.
Pleasant to eat? No taste? So which is it? If you really want to know, watch fecal beads week on the new season of “The Great British Baking Show,” when Paul and Prue judge poop baked into crumpets, crepes, and crostatas. Yum.
We’re on the low-oxygen diet
Nine out of ten doctors agree: Oxygen is more important to your continued well-being than food. After all, a human can go weeks without food, but just minutes without oxygen. However, ten out of ten doctors agree that the United States has an obesity problem. They all also agree that previous research has shown soldiers who train at high altitudes lose more weight than those training at lower altitudes.
So, on the one hand, we have a country full of overweight people, and on the other, we have low oxygen levels causing weight loss. The solution, then, is obvious: Stop breathing.
More specifically (and somewhat less facetiously), researchers from Louisiana have launched the Low Oxygen and Weight Status trial and are currently recruiting individuals with BMIs of 30-40 to, uh, suffocate themselves. No, no, it’s okay, it’s just when they’re sleeping.
Fine, straight face. Participants in the LOWS trial will undergo an 8-week period when they will consume a controlled weight-loss diet and spend their nights in a hypoxic sealed tent, where they will sleep in an environment with an oxygen level equivalent to 8,500 feet above sea level (roughly equivalent to Aspen, Colo.). They will be compared with people on the same diet who sleep in a normal, sea-level oxygen environment.
The study’s goal is to determine whether or not spending time in a low-oxygen environment will suppress appetite, increase energy expenditure, and improve weight loss and insulin sensitivity. Excessive weight loss in high-altitude environments isn’t a good thing for soldiers – they kind of need their muscles and body weight to do the whole soldiering thing – but it could be great for people struggling to lose those last few pounds. And it also may prove LOTME’s previous thesis: Air is not good.
Overweight boy, infertile man?
When it comes to causes of infertility, history and science have generally focused on women. A lot of the research overlooks men, but some previous studies have suggested that male infertility contributes to about half of the cases of couple infertility. The reason for much of that male infertility, however, has been a mystery. Until now.
A group of Italian investigators looked at the declining trend in sperm counts over the past 40 years and the increase of childhood obesity. Is there a correlation? The researchers think so. Childhood obesity can be linked to multiple causes, but the researchers zeroed in on the effect that obesity has on metabolic rates and, therefore, testicular growth.
Collecting data on testicular volume, body mass index (BMI), and insulin resistance from 268 boys aged 2-18 years, the researchers discovered that those with normal weight and normal insulin levels had testicular volumes 1.5 times higher than their overweight counterparts and 1.5-2 times higher than those with hyperinsulinemia, building a case for obesity being a factor for infertility later in life.
Since low testicular volume is associated with lower sperm count and production as an adult, putting two and two together makes a compelling argument for childhood obesity being a major male infertility culprit. It also creates even more urgency for the health care industry and community decision makers to focus on childhood obesity.
It sure would be nice to be able to take one of the many risk factors for future human survival off the table. Maybe by taking something, like cake, off the table.
Fecal transplantation moves to the kitchen
Fecal microbiota transplantation is an effective way to treat Clostridioides difficile infection, but, in the end, it’s still a transplantation procedure involving a nasogastric or colorectal tube or rather large oral capsules with a demanding (30-40 capsules over 2 days) dosage. Please, Science, tell us there’s a better way.
Science, in the form of investigators at the University of Geneva and Lausanne University Hospital in Switzerland, has spoken, and there may be a better way. Presenting fecal beads: All the bacterial goodness of donor stool without the tubal insertions or massive quantities of giant capsules.
We know you’re scoffing out there, but it’s true. All you need is a little alginate, which is a “biocompatible polysaccharide isolated from brown algae” of the Phaeophyceae family. The donor feces is microencapsulated by mixing it with the alginate, dropping that mixture into water containing calcium chloride, turning it into a gel, and then freeze-drying the gel into small (just 2 mm), solid beads.
Sounds plausible enough, but what do you do with them? “These brownish beads can be easily dispersed in a liquid or food that is pleasant to eat. They also have no taste,” senior author Eric Allémann, PhD, said in a statement released by the University of Geneva.
Pleasant to eat? No taste? So which is it? If you really want to know, watch fecal beads week on the new season of “The Great British Baking Show,” when Paul and Prue judge poop baked into crumpets, crepes, and crostatas. Yum.
We’re on the low-oxygen diet
Nine out of ten doctors agree: Oxygen is more important to your continued well-being than food. After all, a human can go weeks without food, but just minutes without oxygen. However, ten out of ten doctors agree that the United States has an obesity problem. They all also agree that previous research has shown soldiers who train at high altitudes lose more weight than those training at lower altitudes.
So, on the one hand, we have a country full of overweight people, and on the other, we have low oxygen levels causing weight loss. The solution, then, is obvious: Stop breathing.
More specifically (and somewhat less facetiously), researchers from Louisiana have launched the Low Oxygen and Weight Status trial and are currently recruiting individuals with BMIs of 30-40 to, uh, suffocate themselves. No, no, it’s okay, it’s just when they’re sleeping.
Fine, straight face. Participants in the LOWS trial will undergo an 8-week period when they will consume a controlled weight-loss diet and spend their nights in a hypoxic sealed tent, where they will sleep in an environment with an oxygen level equivalent to 8,500 feet above sea level (roughly equivalent to Aspen, Colo.). They will be compared with people on the same diet who sleep in a normal, sea-level oxygen environment.
The study’s goal is to determine whether or not spending time in a low-oxygen environment will suppress appetite, increase energy expenditure, and improve weight loss and insulin sensitivity. Excessive weight loss in high-altitude environments isn’t a good thing for soldiers – they kind of need their muscles and body weight to do the whole soldiering thing – but it could be great for people struggling to lose those last few pounds. And it also may prove LOTME’s previous thesis: Air is not good.
Overweight boy, infertile man?
When it comes to causes of infertility, history and science have generally focused on women. A lot of the research overlooks men, but some previous studies have suggested that male infertility contributes to about half of the cases of couple infertility. The reason for much of that male infertility, however, has been a mystery. Until now.
A group of Italian investigators looked at the declining trend in sperm counts over the past 40 years and the increase of childhood obesity. Is there a correlation? The researchers think so. Childhood obesity can be linked to multiple causes, but the researchers zeroed in on the effect that obesity has on metabolic rates and, therefore, testicular growth.
Collecting data on testicular volume, body mass index (BMI), and insulin resistance from 268 boys aged 2-18 years, the researchers discovered that those with normal weight and normal insulin levels had testicular volumes 1.5 times higher than their overweight counterparts and 1.5-2 times higher than those with hyperinsulinemia, building a case for obesity being a factor for infertility later in life.
Since low testicular volume is associated with lower sperm count and production as an adult, putting two and two together makes a compelling argument for childhood obesity being a major male infertility culprit. It also creates even more urgency for the health care industry and community decision makers to focus on childhood obesity.
It sure would be nice to be able to take one of the many risk factors for future human survival off the table. Maybe by taking something, like cake, off the table.
Fecal transplantation moves to the kitchen
Fecal microbiota transplantation is an effective way to treat Clostridioides difficile infection, but, in the end, it’s still a transplantation procedure involving a nasogastric or colorectal tube or rather large oral capsules with a demanding (30-40 capsules over 2 days) dosage. Please, Science, tell us there’s a better way.
Science, in the form of investigators at the University of Geneva and Lausanne University Hospital in Switzerland, has spoken, and there may be a better way. Presenting fecal beads: All the bacterial goodness of donor stool without the tubal insertions or massive quantities of giant capsules.
We know you’re scoffing out there, but it’s true. All you need is a little alginate, which is a “biocompatible polysaccharide isolated from brown algae” of the Phaeophyceae family. The donor feces is microencapsulated by mixing it with the alginate, dropping that mixture into water containing calcium chloride, turning it into a gel, and then freeze-drying the gel into small (just 2 mm), solid beads.
Sounds plausible enough, but what do you do with them? “These brownish beads can be easily dispersed in a liquid or food that is pleasant to eat. They also have no taste,” senior author Eric Allémann, PhD, said in a statement released by the University of Geneva.
Pleasant to eat? No taste? So which is it? If you really want to know, watch fecal beads week on the new season of “The Great British Baking Show,” when Paul and Prue judge poop baked into crumpets, crepes, and crostatas. Yum.
We’re on the low-oxygen diet
Nine out of ten doctors agree: Oxygen is more important to your continued well-being than food. After all, a human can go weeks without food, but just minutes without oxygen. However, ten out of ten doctors agree that the United States has an obesity problem. They all also agree that previous research has shown soldiers who train at high altitudes lose more weight than those training at lower altitudes.
So, on the one hand, we have a country full of overweight people, and on the other, we have low oxygen levels causing weight loss. The solution, then, is obvious: Stop breathing.
More specifically (and somewhat less facetiously), researchers from Louisiana have launched the Low Oxygen and Weight Status trial and are currently recruiting individuals with BMIs of 30-40 to, uh, suffocate themselves. No, no, it’s okay, it’s just when they’re sleeping.
Fine, straight face. Participants in the LOWS trial will undergo an 8-week period when they will consume a controlled weight-loss diet and spend their nights in a hypoxic sealed tent, where they will sleep in an environment with an oxygen level equivalent to 8,500 feet above sea level (roughly equivalent to Aspen, Colo.). They will be compared with people on the same diet who sleep in a normal, sea-level oxygen environment.
The study’s goal is to determine whether or not spending time in a low-oxygen environment will suppress appetite, increase energy expenditure, and improve weight loss and insulin sensitivity. Excessive weight loss in high-altitude environments isn’t a good thing for soldiers – they kind of need their muscles and body weight to do the whole soldiering thing – but it could be great for people struggling to lose those last few pounds. And it also may prove LOTME’s previous thesis: Air is not good.
Nurses: The unsung heroes
Try practicing inpatient medicine without nurses.
You can’t.
We blow in and out of the rooms, write notes, check results and vitals, then move on to the next person.
But the nurses are the ones who actually make this all happen. And, amazingly, can do all that work with a smile.
But in our current postpandemic world, we’re facing a serious shortage. A recent survey of registered nurses found that only 15% of hospital nurses were planning on being there in 1 year. Thirty percent said they were planning on changing careers entirely in the aftermath of the pandemic. Their job satisfaction scores have dropped 15% from 2019 to 2023. Their stress scores, and concerns that the job is affecting their health, have increased 15%-20%.
The problem reflects a combination of things intersecting at a bad time: Staffing shortages resulting in more patients per nurse, hospital administrators cutting corners on staffing and pay, and the ongoing state of incivility.
The last one is a particularly new issue. Difficult patients and their families are nothing new. We all encounter them, and learn to deal with them in our own way. It’s part of the territory.
But since 2020 it’s climbed to a new-level of in-your-face confrontation, rudeness, and aggression, sometimes leading to violence. Physical attacks on people in all jobs have increased, but health care workers are five times more likely to encounter workplace violence than any other field.
Underpaid, overworked, and a sitting duck for violence. Can you blame people for looking elsewhere?
All of this is coming at a time when a whole generation of nurses is retiring, another generation is starting to reach an age of needing more health care, and nursing schools are short on teaching staff, limiting the number of new people that can be trained. Nursing education, like medical school, isn’t a place to cut corners (neither is care, obviously).
These days we toss the word “burnout” around to the point that it’s become almost meaningless, but to those affected by it, the consequences are quite real. And when it causes a loss of staff and impairs the ability of all to provide quality medical care, it quickly becomes everyone’s problem.
Finding solutions for such things isn’t a can you just kick down the road, as governmental agencies have always been so good at doing. These are things that have real-world consequences for all involved, and solutions need to involve private, public, and educational sectors working together.
I don’t have any ideas, but I hope the people who can change this will sit down and work some out.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Try practicing inpatient medicine without nurses.
You can’t.
We blow in and out of the rooms, write notes, check results and vitals, then move on to the next person.
But the nurses are the ones who actually make this all happen. And, amazingly, can do all that work with a smile.
But in our current postpandemic world, we’re facing a serious shortage. A recent survey of registered nurses found that only 15% of hospital nurses were planning on being there in 1 year. Thirty percent said they were planning on changing careers entirely in the aftermath of the pandemic. Their job satisfaction scores have dropped 15% from 2019 to 2023. Their stress scores, and concerns that the job is affecting their health, have increased 15%-20%.
The problem reflects a combination of things intersecting at a bad time: Staffing shortages resulting in more patients per nurse, hospital administrators cutting corners on staffing and pay, and the ongoing state of incivility.
The last one is a particularly new issue. Difficult patients and their families are nothing new. We all encounter them, and learn to deal with them in our own way. It’s part of the territory.
But since 2020 it’s climbed to a new-level of in-your-face confrontation, rudeness, and aggression, sometimes leading to violence. Physical attacks on people in all jobs have increased, but health care workers are five times more likely to encounter workplace violence than any other field.
Underpaid, overworked, and a sitting duck for violence. Can you blame people for looking elsewhere?
All of this is coming at a time when a whole generation of nurses is retiring, another generation is starting to reach an age of needing more health care, and nursing schools are short on teaching staff, limiting the number of new people that can be trained. Nursing education, like medical school, isn’t a place to cut corners (neither is care, obviously).
These days we toss the word “burnout” around to the point that it’s become almost meaningless, but to those affected by it, the consequences are quite real. And when it causes a loss of staff and impairs the ability of all to provide quality medical care, it quickly becomes everyone’s problem.
Finding solutions for such things isn’t a can you just kick down the road, as governmental agencies have always been so good at doing. These are things that have real-world consequences for all involved, and solutions need to involve private, public, and educational sectors working together.
I don’t have any ideas, but I hope the people who can change this will sit down and work some out.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Try practicing inpatient medicine without nurses.
You can’t.
We blow in and out of the rooms, write notes, check results and vitals, then move on to the next person.
But the nurses are the ones who actually make this all happen. And, amazingly, can do all that work with a smile.
But in our current postpandemic world, we’re facing a serious shortage. A recent survey of registered nurses found that only 15% of hospital nurses were planning on being there in 1 year. Thirty percent said they were planning on changing careers entirely in the aftermath of the pandemic. Their job satisfaction scores have dropped 15% from 2019 to 2023. Their stress scores, and concerns that the job is affecting their health, have increased 15%-20%.
The problem reflects a combination of things intersecting at a bad time: Staffing shortages resulting in more patients per nurse, hospital administrators cutting corners on staffing and pay, and the ongoing state of incivility.
The last one is a particularly new issue. Difficult patients and their families are nothing new. We all encounter them, and learn to deal with them in our own way. It’s part of the territory.
But since 2020 it’s climbed to a new-level of in-your-face confrontation, rudeness, and aggression, sometimes leading to violence. Physical attacks on people in all jobs have increased, but health care workers are five times more likely to encounter workplace violence than any other field.
Underpaid, overworked, and a sitting duck for violence. Can you blame people for looking elsewhere?
All of this is coming at a time when a whole generation of nurses is retiring, another generation is starting to reach an age of needing more health care, and nursing schools are short on teaching staff, limiting the number of new people that can be trained. Nursing education, like medical school, isn’t a place to cut corners (neither is care, obviously).
These days we toss the word “burnout” around to the point that it’s become almost meaningless, but to those affected by it, the consequences are quite real. And when it causes a loss of staff and impairs the ability of all to provide quality medical care, it quickly becomes everyone’s problem.
Finding solutions for such things isn’t a can you just kick down the road, as governmental agencies have always been so good at doing. These are things that have real-world consequences for all involved, and solutions need to involve private, public, and educational sectors working together.
I don’t have any ideas, but I hope the people who can change this will sit down and work some out.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Hearing aids are a ‘powerful’ tool for reducing dementia risk
, new research confirms. A large observational study from the United Kingdom showed a 42% increased risk for dementia in people with hearing loss compared with their peers with no hearing trouble. In addition, there was no increased risk in those with hearing loss who used hearing aids.
“The evidence is building that hearing loss may be the most impactful modifiable risk factor for dementia in mid-life, but the effectiveness of hearing aid use on reducing the risk of dementia in the real world has remained unclear,” Dongshan Zhu, PhD, with Shandong University, Jinan, China, said in a news release.
“Our study provides the best evidence to date to suggest that hearing aids could be a minimally invasive, cost-effective treatment to mitigate the potential impact of hearing loss on dementia,” Dr. Zhu said.
The study, which was published online in Lancet Public Health, comes on the heels of the 2020 Lancet Commission report on dementia, which suggested hearing loss may be linked to approximately 8% of worldwide dementia cases.
‘Compelling’ evidence
For the study, investigators analyzed longitudinal data on 437,704 individuals, most of whom were White, from the UK Biobank (54% female; mean age at baseline, 56 years). Roughly three quarters of the cohort had no hearing loss and one quarter had some level of hearing loss, with 12% of these individuals using hearing aids.
After the researchers controlled for relevant cofactors, compared with people without hearing loss, those with hearing loss who were not using hearing aids had an increased risk for all-cause dementia (hazard ratio [HR], 1.42; 95% confidence interval [CI], 1.29-1.56).
No increased risk was seen in people with hearing loss who were using hearing aids (HR, 1.04; 95% CI, 0.98-1.10).
The positive association of hearing aid use was observed in all-cause dementia and cause-specific dementia subtypes, including Alzheimer’s disease, vascular dementia, and non–Alzheimer’s disease nonvascular dementia.
The data also suggest that the protection against dementia conferred by hearing aid use most likely stems from direct effects from hearing aids rather than indirect mediators, such as social isolation, loneliness, and low mood.
Dr. Zhu said the findings highlight the “urgent need” for the early use of hearing aids when an individual starts having trouble hearing.
“A group effort from across society is necessary, including raising awareness of hearing loss and the potential links with dementia; increasing accessibility to hearing aids by reducing cost; and more support for primary care workers to screen for hearing impairment, raise awareness, and deliver treatment such as fitting hearing aids,” Dr. Zhu said.
Writing in a linked comment, Gill Livingston, MD, and Sergi Costafreda, MD, PhD, with University College London, noted that with addition of this study, “the evidence that hearing aids are a powerful tool to reduce the risk of dementia in people with hearing loss, is as good as possible without randomized controlled trials, which might not be practically possible or ethical because people with hearing loss should not be stopped from using effective treatments.”
“The evidence is compelling that treating hearing loss is a promising way of reducing dementia risk. This is the time to increase awareness of and detection of hearing loss, as well as the acceptability and usability of hearing aids,” Dr. Livingston and Dr. Costafreda added.
High-quality evidence – with caveats
Several experts offered perspective on the analysis in a statement from the U.K.-based nonprofit Science Media Centre, which was not involved with the conduct of this study. Charles Marshall, MRCP, PhD, with Queen Mary University of London, said that the study provides “high-quality evidence” that those with hearing loss who use hearing aids are at lower risk for dementia than are those with hearing loss who do not use hearing aids.
“This raises the possibility that a proportion of dementia cases could be prevented by using hearing aids to correct hearing loss. However, the observational nature of this study makes it difficult to be sure that hearing aids are actually causing the reduced risk of dementia,” Dr. Marshall added.
“Hearing aids produce slightly distorted sound, and the brain has to adapt to this in order for hearing aids to be helpful,” he said. “People who are at risk of developing dementia in the future may have early changes in their brain that impair this adaptation, and this may lead to them choosing to not use hearing aids. This would confound the association, creating the appearance that hearing aids were reducing dementia risk, when actually their use was just identifying people with relatively healthy brains,” Dr. Marshall added.
Tara Spires-Jones, PhD, with the University of Edinburgh, said this “well-conducted” study confirms previous similar studies showing an association between hearing loss and dementia risk.
Echoing Dr. Marshall, Dr. Spires-Jones noted that this type of study cannot prove conclusively that hearing loss causes dementia.
“For example,” she said, “it is possible that people who are already in the very early stages of disease are less likely to seek help for hearing loss. However, on balance, this study and the rest of the data in the field indicate that keeping your brain healthy and engaged reduces dementia risk.”
Dr. Spires-Jones said that she agrees with the investigators that it’s “important to help people with hearing loss to get effective hearing aids to help keep their brains engaged through allowing richer social interactions.”
This study was funded by the National Natural Science Foundation of China and Shandong Province, Taishan Scholars Project, China Medical Board, and China Postdoctoral Science Foundation. Dr. Zhu, Dr. Livingston, Dr. Costafreda, Dr. Marshall, and Dr. Spires-Jones have no relevant disclosures.
A version of this article originally appeared on Medscape.com.
, new research confirms. A large observational study from the United Kingdom showed a 42% increased risk for dementia in people with hearing loss compared with their peers with no hearing trouble. In addition, there was no increased risk in those with hearing loss who used hearing aids.
“The evidence is building that hearing loss may be the most impactful modifiable risk factor for dementia in mid-life, but the effectiveness of hearing aid use on reducing the risk of dementia in the real world has remained unclear,” Dongshan Zhu, PhD, with Shandong University, Jinan, China, said in a news release.
“Our study provides the best evidence to date to suggest that hearing aids could be a minimally invasive, cost-effective treatment to mitigate the potential impact of hearing loss on dementia,” Dr. Zhu said.
The study, which was published online in Lancet Public Health, comes on the heels of the 2020 Lancet Commission report on dementia, which suggested hearing loss may be linked to approximately 8% of worldwide dementia cases.
‘Compelling’ evidence
For the study, investigators analyzed longitudinal data on 437,704 individuals, most of whom were White, from the UK Biobank (54% female; mean age at baseline, 56 years). Roughly three quarters of the cohort had no hearing loss and one quarter had some level of hearing loss, with 12% of these individuals using hearing aids.
After the researchers controlled for relevant cofactors, compared with people without hearing loss, those with hearing loss who were not using hearing aids had an increased risk for all-cause dementia (hazard ratio [HR], 1.42; 95% confidence interval [CI], 1.29-1.56).
No increased risk was seen in people with hearing loss who were using hearing aids (HR, 1.04; 95% CI, 0.98-1.10).
The positive association of hearing aid use was observed in all-cause dementia and cause-specific dementia subtypes, including Alzheimer’s disease, vascular dementia, and non–Alzheimer’s disease nonvascular dementia.
The data also suggest that the protection against dementia conferred by hearing aid use most likely stems from direct effects from hearing aids rather than indirect mediators, such as social isolation, loneliness, and low mood.
Dr. Zhu said the findings highlight the “urgent need” for the early use of hearing aids when an individual starts having trouble hearing.
“A group effort from across society is necessary, including raising awareness of hearing loss and the potential links with dementia; increasing accessibility to hearing aids by reducing cost; and more support for primary care workers to screen for hearing impairment, raise awareness, and deliver treatment such as fitting hearing aids,” Dr. Zhu said.
Writing in a linked comment, Gill Livingston, MD, and Sergi Costafreda, MD, PhD, with University College London, noted that with addition of this study, “the evidence that hearing aids are a powerful tool to reduce the risk of dementia in people with hearing loss, is as good as possible without randomized controlled trials, which might not be practically possible or ethical because people with hearing loss should not be stopped from using effective treatments.”
“The evidence is compelling that treating hearing loss is a promising way of reducing dementia risk. This is the time to increase awareness of and detection of hearing loss, as well as the acceptability and usability of hearing aids,” Dr. Livingston and Dr. Costafreda added.
High-quality evidence – with caveats
Several experts offered perspective on the analysis in a statement from the U.K.-based nonprofit Science Media Centre, which was not involved with the conduct of this study. Charles Marshall, MRCP, PhD, with Queen Mary University of London, said that the study provides “high-quality evidence” that those with hearing loss who use hearing aids are at lower risk for dementia than are those with hearing loss who do not use hearing aids.
“This raises the possibility that a proportion of dementia cases could be prevented by using hearing aids to correct hearing loss. However, the observational nature of this study makes it difficult to be sure that hearing aids are actually causing the reduced risk of dementia,” Dr. Marshall added.
“Hearing aids produce slightly distorted sound, and the brain has to adapt to this in order for hearing aids to be helpful,” he said. “People who are at risk of developing dementia in the future may have early changes in their brain that impair this adaptation, and this may lead to them choosing to not use hearing aids. This would confound the association, creating the appearance that hearing aids were reducing dementia risk, when actually their use was just identifying people with relatively healthy brains,” Dr. Marshall added.
Tara Spires-Jones, PhD, with the University of Edinburgh, said this “well-conducted” study confirms previous similar studies showing an association between hearing loss and dementia risk.
Echoing Dr. Marshall, Dr. Spires-Jones noted that this type of study cannot prove conclusively that hearing loss causes dementia.
“For example,” she said, “it is possible that people who are already in the very early stages of disease are less likely to seek help for hearing loss. However, on balance, this study and the rest of the data in the field indicate that keeping your brain healthy and engaged reduces dementia risk.”
Dr. Spires-Jones said that she agrees with the investigators that it’s “important to help people with hearing loss to get effective hearing aids to help keep their brains engaged through allowing richer social interactions.”
This study was funded by the National Natural Science Foundation of China and Shandong Province, Taishan Scholars Project, China Medical Board, and China Postdoctoral Science Foundation. Dr. Zhu, Dr. Livingston, Dr. Costafreda, Dr. Marshall, and Dr. Spires-Jones have no relevant disclosures.
A version of this article originally appeared on Medscape.com.
, new research confirms. A large observational study from the United Kingdom showed a 42% increased risk for dementia in people with hearing loss compared with their peers with no hearing trouble. In addition, there was no increased risk in those with hearing loss who used hearing aids.
“The evidence is building that hearing loss may be the most impactful modifiable risk factor for dementia in mid-life, but the effectiveness of hearing aid use on reducing the risk of dementia in the real world has remained unclear,” Dongshan Zhu, PhD, with Shandong University, Jinan, China, said in a news release.
“Our study provides the best evidence to date to suggest that hearing aids could be a minimally invasive, cost-effective treatment to mitigate the potential impact of hearing loss on dementia,” Dr. Zhu said.
The study, which was published online in Lancet Public Health, comes on the heels of the 2020 Lancet Commission report on dementia, which suggested hearing loss may be linked to approximately 8% of worldwide dementia cases.
‘Compelling’ evidence
For the study, investigators analyzed longitudinal data on 437,704 individuals, most of whom were White, from the UK Biobank (54% female; mean age at baseline, 56 years). Roughly three quarters of the cohort had no hearing loss and one quarter had some level of hearing loss, with 12% of these individuals using hearing aids.
After the researchers controlled for relevant cofactors, compared with people without hearing loss, those with hearing loss who were not using hearing aids had an increased risk for all-cause dementia (hazard ratio [HR], 1.42; 95% confidence interval [CI], 1.29-1.56).
No increased risk was seen in people with hearing loss who were using hearing aids (HR, 1.04; 95% CI, 0.98-1.10).
The positive association of hearing aid use was observed in all-cause dementia and cause-specific dementia subtypes, including Alzheimer’s disease, vascular dementia, and non–Alzheimer’s disease nonvascular dementia.
The data also suggest that the protection against dementia conferred by hearing aid use most likely stems from direct effects from hearing aids rather than indirect mediators, such as social isolation, loneliness, and low mood.
Dr. Zhu said the findings highlight the “urgent need” for the early use of hearing aids when an individual starts having trouble hearing.
“A group effort from across society is necessary, including raising awareness of hearing loss and the potential links with dementia; increasing accessibility to hearing aids by reducing cost; and more support for primary care workers to screen for hearing impairment, raise awareness, and deliver treatment such as fitting hearing aids,” Dr. Zhu said.
Writing in a linked comment, Gill Livingston, MD, and Sergi Costafreda, MD, PhD, with University College London, noted that with addition of this study, “the evidence that hearing aids are a powerful tool to reduce the risk of dementia in people with hearing loss, is as good as possible without randomized controlled trials, which might not be practically possible or ethical because people with hearing loss should not be stopped from using effective treatments.”
“The evidence is compelling that treating hearing loss is a promising way of reducing dementia risk. This is the time to increase awareness of and detection of hearing loss, as well as the acceptability and usability of hearing aids,” Dr. Livingston and Dr. Costafreda added.
High-quality evidence – with caveats
Several experts offered perspective on the analysis in a statement from the U.K.-based nonprofit Science Media Centre, which was not involved with the conduct of this study. Charles Marshall, MRCP, PhD, with Queen Mary University of London, said that the study provides “high-quality evidence” that those with hearing loss who use hearing aids are at lower risk for dementia than are those with hearing loss who do not use hearing aids.
“This raises the possibility that a proportion of dementia cases could be prevented by using hearing aids to correct hearing loss. However, the observational nature of this study makes it difficult to be sure that hearing aids are actually causing the reduced risk of dementia,” Dr. Marshall added.
“Hearing aids produce slightly distorted sound, and the brain has to adapt to this in order for hearing aids to be helpful,” he said. “People who are at risk of developing dementia in the future may have early changes in their brain that impair this adaptation, and this may lead to them choosing to not use hearing aids. This would confound the association, creating the appearance that hearing aids were reducing dementia risk, when actually their use was just identifying people with relatively healthy brains,” Dr. Marshall added.
Tara Spires-Jones, PhD, with the University of Edinburgh, said this “well-conducted” study confirms previous similar studies showing an association between hearing loss and dementia risk.
Echoing Dr. Marshall, Dr. Spires-Jones noted that this type of study cannot prove conclusively that hearing loss causes dementia.
“For example,” she said, “it is possible that people who are already in the very early stages of disease are less likely to seek help for hearing loss. However, on balance, this study and the rest of the data in the field indicate that keeping your brain healthy and engaged reduces dementia risk.”
Dr. Spires-Jones said that she agrees with the investigators that it’s “important to help people with hearing loss to get effective hearing aids to help keep their brains engaged through allowing richer social interactions.”
This study was funded by the National Natural Science Foundation of China and Shandong Province, Taishan Scholars Project, China Medical Board, and China Postdoctoral Science Foundation. Dr. Zhu, Dr. Livingston, Dr. Costafreda, Dr. Marshall, and Dr. Spires-Jones have no relevant disclosures.
A version of this article originally appeared on Medscape.com.
Stroke scale cutoff might not be ideal guide for ordering CTA and detecting large vessel occlusions
BOSTON – (LVO), according to large body of data presented at the 2023 annual meeting of the American Academy of Neurology.
If the goal is not to miss any LVOs, there is no NIHSS score below which these do not occur, according to Theresa Sevilis, DO, regional medical director, TeleSpecialists, Fort Myers, Fla.
For example, her evaluation of a large and nationally representative dataset shows that more than 10% of the LVOs eventually identified and accepted for intervention would be missed with a cutoff of NIHSS score of 6 or higher. Moving the cutoff NIHSS score to 4 or greater, 6% of LVOs among the 23,166 strokes evaluated would have gone undetected.
“The current guidelines do not address low NIHSS score largely due to a paucity of data,” according to Dr. Sevilis, who showed data indicating that there is great variation among institutions in regard to ordering computed tomography angiography (CTA). She indicated that CTA is the current imaging standard for detecting LVO.
Large prospective dataset
The data for this study were derived from the TeleCare database, which captures acute stroke consultations in the emergency departments in 227 facilities in 27 states. Stroke consultations over a 6-month period from July through December 2021 were evaluated. The prospectively collected data were subjected to a multivariate analysis to determine the odds ratio for a CTA performed and LVO found at each NIHSS score of 0 to 5. Scores 6 or above served as the reference.
“Only consults performed within 24 hours [of presentation] were included,” Dr. Sevilis said.
After excluding cases in which no NIHSS score was captured, which represented less than 1% of cases, more than 10,500 cases underwent CTA, providing a rate of 45.5%. The rate of CTA for the whole dataset was 45.5%. Of the study population, 24.6% had a NIHSS score of 6 or above.
“When you are discussing when to perform CTA in patients with a low NIHSS score, you are discussing the majority of patients,” Dr. Sevilis said.
Of those with a NIHSS stroke of 6 or below, 28.2% had a score of 0. Not surprisingly, these were the least likely to have a CTA performed on the basis of an odds ratio of 0.14 and the least likely to have a LVO detected (OR, 0.1). With the exception of a NIHSS stroke score of 1, the likelihood of CTA and LVO climbed incrementally with higher stroke scores. These odds ratios were, respectively, 0.16 and 0.09 for a score of 1; 0.27 and 0.16 for a score of 2; 0.33 and 0.14 for a score of 3; 0.49 and 0.24 for a score of 4; and 0.71 and 0.27 for a score of 5.
In the group with NIHSS score of 6 or above, 24.1% were found to have an LVO. Of these, the proportion accepted for a mechanical thrombectomy was less than half. The intervention acceptance rate for mechanical intervention among LVOs in patients with lower NIHSS scores again fell incrementally by score. The acceptance rate was about 35% among LVO patients with a NIHSS score of 3 or 4 and 25% for those with a score of 0-2.
The interpretation of these data “depends on goals,” Dr. Sevilis said. “If the goal is to not miss a single LVO, then it is important to consider the balance between benefits and risks.”
No consistent cutoff
In participating facilities, the protocol for considering CTA to detect and treat LVOs ranges from neurologist choice to cutoffs of NIHSS scores of 2, 4, and 6, according to Dr. Sevilis. Where the data suggest that a cutoff of 4 or above might be reasonable, she said that NIHSS scoring is not a useful tool for those “who do not want to miss any LVOs.”
These data are based on emergency room stroke consultations and not on confirmed strokes,” Dr. Sevilis emphasized. Indeed, she noted that the final discharge diagnosis was not available. Recognizing that the analysis was not performed on a population with confirmed strokes is particularly important for understanding the limited rate of CTAs performed even in those with relatively high NIHSS scores. She noted this could be explained by many different reasons, including suspicion of hemorrhage or clinical features that took the workup in a different direction.
Reconsidering protocols
Based on the large sample size, Dr. Sevilis contended that it is likely that these data are representative, but she considers this study a first step toward considering protocols and developing guidelines for addressing stroke alerts in the emergency department.
A more important step will be ongoing trials designed specifically to generate data to answer this question. Pascal Jabbour, MD, chief of the division of neurovascular and endovascular neurosurgery, Thomas Jefferson University Hospitals, Philadelphia, is participating in one of these trials. He agreed with the premise that better evidence-based criteria are needed when evaluating acute stroke patients with a potential LVO.
The trial in which he is a coinvestigator, called ENDOLOW, is testing the hypothesis that outcomes will be better if acute stroke patients with a LVO and a low baseline NIHSS score (< 5) are treated with immediate thrombectomy rather than medical management. If this hypothesis is confirmed in the randomized ENDOLOW, it will provide an evidence basis for an approach already being practiced at some centers.
“There should be a very low threshold for CTA,” said Dr. Jabbour in an interview. This imaging “takes less than 2 minutes and it can provide the basis for a life-saving endovascular thrombectomy if a LVO is found.”
It is already well known that LVO is not restricted only to patients with an elevated NIHSS score, he said.
For determining whether to order a CTA, “I do not agree with NIHSS score of 6 or above. There is no absolute number below which risk of missing a LVO is eliminated,” Dr. Jabbour said. He also argued against relying on NIHSS score without considering other clinical features, particularly cortical signs, which should raise suspicion of a LVO regardless of NIHSS score.
One problem is that NIHSS scores are not static. Decompensation can be rapid with the NIHSS score quickly climbing. When this happens, the delay in treatment might lead to a preventable adverse outcome.
“There is a change in the paradigm now that we have more evidence of a benefit from aggressive treatment in the right candidates,” according to Dr. Jabbour, referring to the recently published SELECT2 trial. In that trial, on which Dr. Jabbour served as a coauthor, patients with LVO and large territory infarct were randomized to thrombectomy or medical care within 24 hours of a stroke. It was stopped early for efficacy because of the increased functional independence (20% vs. 7%) in the surgical intervention group.
If the ongoing trials establish better criteria for ruling in or out the presence of LVO in patients with acute stroke, Dr. Jabbour predicted that guidelines will be written to standardize practice.
Dr. Sevilis reports no potential conflicts of interest. Dr. Jabbour has financial relationships with Cerenovus, Medtronic, and Microvention.
BOSTON – (LVO), according to large body of data presented at the 2023 annual meeting of the American Academy of Neurology.
If the goal is not to miss any LVOs, there is no NIHSS score below which these do not occur, according to Theresa Sevilis, DO, regional medical director, TeleSpecialists, Fort Myers, Fla.
For example, her evaluation of a large and nationally representative dataset shows that more than 10% of the LVOs eventually identified and accepted for intervention would be missed with a cutoff of NIHSS score of 6 or higher. Moving the cutoff NIHSS score to 4 or greater, 6% of LVOs among the 23,166 strokes evaluated would have gone undetected.
“The current guidelines do not address low NIHSS score largely due to a paucity of data,” according to Dr. Sevilis, who showed data indicating that there is great variation among institutions in regard to ordering computed tomography angiography (CTA). She indicated that CTA is the current imaging standard for detecting LVO.
Large prospective dataset
The data for this study were derived from the TeleCare database, which captures acute stroke consultations in the emergency departments in 227 facilities in 27 states. Stroke consultations over a 6-month period from July through December 2021 were evaluated. The prospectively collected data were subjected to a multivariate analysis to determine the odds ratio for a CTA performed and LVO found at each NIHSS score of 0 to 5. Scores 6 or above served as the reference.
“Only consults performed within 24 hours [of presentation] were included,” Dr. Sevilis said.
After excluding cases in which no NIHSS score was captured, which represented less than 1% of cases, more than 10,500 cases underwent CTA, providing a rate of 45.5%. The rate of CTA for the whole dataset was 45.5%. Of the study population, 24.6% had a NIHSS score of 6 or above.
“When you are discussing when to perform CTA in patients with a low NIHSS score, you are discussing the majority of patients,” Dr. Sevilis said.
Of those with a NIHSS stroke of 6 or below, 28.2% had a score of 0. Not surprisingly, these were the least likely to have a CTA performed on the basis of an odds ratio of 0.14 and the least likely to have a LVO detected (OR, 0.1). With the exception of a NIHSS stroke score of 1, the likelihood of CTA and LVO climbed incrementally with higher stroke scores. These odds ratios were, respectively, 0.16 and 0.09 for a score of 1; 0.27 and 0.16 for a score of 2; 0.33 and 0.14 for a score of 3; 0.49 and 0.24 for a score of 4; and 0.71 and 0.27 for a score of 5.
In the group with NIHSS score of 6 or above, 24.1% were found to have an LVO. Of these, the proportion accepted for a mechanical thrombectomy was less than half. The intervention acceptance rate for mechanical intervention among LVOs in patients with lower NIHSS scores again fell incrementally by score. The acceptance rate was about 35% among LVO patients with a NIHSS score of 3 or 4 and 25% for those with a score of 0-2.
The interpretation of these data “depends on goals,” Dr. Sevilis said. “If the goal is to not miss a single LVO, then it is important to consider the balance between benefits and risks.”
No consistent cutoff
In participating facilities, the protocol for considering CTA to detect and treat LVOs ranges from neurologist choice to cutoffs of NIHSS scores of 2, 4, and 6, according to Dr. Sevilis. Where the data suggest that a cutoff of 4 or above might be reasonable, she said that NIHSS scoring is not a useful tool for those “who do not want to miss any LVOs.”
These data are based on emergency room stroke consultations and not on confirmed strokes,” Dr. Sevilis emphasized. Indeed, she noted that the final discharge diagnosis was not available. Recognizing that the analysis was not performed on a population with confirmed strokes is particularly important for understanding the limited rate of CTAs performed even in those with relatively high NIHSS scores. She noted this could be explained by many different reasons, including suspicion of hemorrhage or clinical features that took the workup in a different direction.
Reconsidering protocols
Based on the large sample size, Dr. Sevilis contended that it is likely that these data are representative, but she considers this study a first step toward considering protocols and developing guidelines for addressing stroke alerts in the emergency department.
A more important step will be ongoing trials designed specifically to generate data to answer this question. Pascal Jabbour, MD, chief of the division of neurovascular and endovascular neurosurgery, Thomas Jefferson University Hospitals, Philadelphia, is participating in one of these trials. He agreed with the premise that better evidence-based criteria are needed when evaluating acute stroke patients with a potential LVO.
The trial in which he is a coinvestigator, called ENDOLOW, is testing the hypothesis that outcomes will be better if acute stroke patients with a LVO and a low baseline NIHSS score (< 5) are treated with immediate thrombectomy rather than medical management. If this hypothesis is confirmed in the randomized ENDOLOW, it will provide an evidence basis for an approach already being practiced at some centers.
“There should be a very low threshold for CTA,” said Dr. Jabbour in an interview. This imaging “takes less than 2 minutes and it can provide the basis for a life-saving endovascular thrombectomy if a LVO is found.”
It is already well known that LVO is not restricted only to patients with an elevated NIHSS score, he said.
For determining whether to order a CTA, “I do not agree with NIHSS score of 6 or above. There is no absolute number below which risk of missing a LVO is eliminated,” Dr. Jabbour said. He also argued against relying on NIHSS score without considering other clinical features, particularly cortical signs, which should raise suspicion of a LVO regardless of NIHSS score.
One problem is that NIHSS scores are not static. Decompensation can be rapid with the NIHSS score quickly climbing. When this happens, the delay in treatment might lead to a preventable adverse outcome.
“There is a change in the paradigm now that we have more evidence of a benefit from aggressive treatment in the right candidates,” according to Dr. Jabbour, referring to the recently published SELECT2 trial. In that trial, on which Dr. Jabbour served as a coauthor, patients with LVO and large territory infarct were randomized to thrombectomy or medical care within 24 hours of a stroke. It was stopped early for efficacy because of the increased functional independence (20% vs. 7%) in the surgical intervention group.
If the ongoing trials establish better criteria for ruling in or out the presence of LVO in patients with acute stroke, Dr. Jabbour predicted that guidelines will be written to standardize practice.
Dr. Sevilis reports no potential conflicts of interest. Dr. Jabbour has financial relationships with Cerenovus, Medtronic, and Microvention.
BOSTON – (LVO), according to large body of data presented at the 2023 annual meeting of the American Academy of Neurology.
If the goal is not to miss any LVOs, there is no NIHSS score below which these do not occur, according to Theresa Sevilis, DO, regional medical director, TeleSpecialists, Fort Myers, Fla.
For example, her evaluation of a large and nationally representative dataset shows that more than 10% of the LVOs eventually identified and accepted for intervention would be missed with a cutoff of NIHSS score of 6 or higher. Moving the cutoff NIHSS score to 4 or greater, 6% of LVOs among the 23,166 strokes evaluated would have gone undetected.
“The current guidelines do not address low NIHSS score largely due to a paucity of data,” according to Dr. Sevilis, who showed data indicating that there is great variation among institutions in regard to ordering computed tomography angiography (CTA). She indicated that CTA is the current imaging standard for detecting LVO.
Large prospective dataset
The data for this study were derived from the TeleCare database, which captures acute stroke consultations in the emergency departments in 227 facilities in 27 states. Stroke consultations over a 6-month period from July through December 2021 were evaluated. The prospectively collected data were subjected to a multivariate analysis to determine the odds ratio for a CTA performed and LVO found at each NIHSS score of 0 to 5. Scores 6 or above served as the reference.
“Only consults performed within 24 hours [of presentation] were included,” Dr. Sevilis said.
After excluding cases in which no NIHSS score was captured, which represented less than 1% of cases, more than 10,500 cases underwent CTA, providing a rate of 45.5%. The rate of CTA for the whole dataset was 45.5%. Of the study population, 24.6% had a NIHSS score of 6 or above.
“When you are discussing when to perform CTA in patients with a low NIHSS score, you are discussing the majority of patients,” Dr. Sevilis said.
Of those with a NIHSS stroke of 6 or below, 28.2% had a score of 0. Not surprisingly, these were the least likely to have a CTA performed on the basis of an odds ratio of 0.14 and the least likely to have a LVO detected (OR, 0.1). With the exception of a NIHSS stroke score of 1, the likelihood of CTA and LVO climbed incrementally with higher stroke scores. These odds ratios were, respectively, 0.16 and 0.09 for a score of 1; 0.27 and 0.16 for a score of 2; 0.33 and 0.14 for a score of 3; 0.49 and 0.24 for a score of 4; and 0.71 and 0.27 for a score of 5.
In the group with NIHSS score of 6 or above, 24.1% were found to have an LVO. Of these, the proportion accepted for a mechanical thrombectomy was less than half. The intervention acceptance rate for mechanical intervention among LVOs in patients with lower NIHSS scores again fell incrementally by score. The acceptance rate was about 35% among LVO patients with a NIHSS score of 3 or 4 and 25% for those with a score of 0-2.
The interpretation of these data “depends on goals,” Dr. Sevilis said. “If the goal is to not miss a single LVO, then it is important to consider the balance between benefits and risks.”
No consistent cutoff
In participating facilities, the protocol for considering CTA to detect and treat LVOs ranges from neurologist choice to cutoffs of NIHSS scores of 2, 4, and 6, according to Dr. Sevilis. Where the data suggest that a cutoff of 4 or above might be reasonable, she said that NIHSS scoring is not a useful tool for those “who do not want to miss any LVOs.”
These data are based on emergency room stroke consultations and not on confirmed strokes,” Dr. Sevilis emphasized. Indeed, she noted that the final discharge diagnosis was not available. Recognizing that the analysis was not performed on a population with confirmed strokes is particularly important for understanding the limited rate of CTAs performed even in those with relatively high NIHSS scores. She noted this could be explained by many different reasons, including suspicion of hemorrhage or clinical features that took the workup in a different direction.
Reconsidering protocols
Based on the large sample size, Dr. Sevilis contended that it is likely that these data are representative, but she considers this study a first step toward considering protocols and developing guidelines for addressing stroke alerts in the emergency department.
A more important step will be ongoing trials designed specifically to generate data to answer this question. Pascal Jabbour, MD, chief of the division of neurovascular and endovascular neurosurgery, Thomas Jefferson University Hospitals, Philadelphia, is participating in one of these trials. He agreed with the premise that better evidence-based criteria are needed when evaluating acute stroke patients with a potential LVO.
The trial in which he is a coinvestigator, called ENDOLOW, is testing the hypothesis that outcomes will be better if acute stroke patients with a LVO and a low baseline NIHSS score (< 5) are treated with immediate thrombectomy rather than medical management. If this hypothesis is confirmed in the randomized ENDOLOW, it will provide an evidence basis for an approach already being practiced at some centers.
“There should be a very low threshold for CTA,” said Dr. Jabbour in an interview. This imaging “takes less than 2 minutes and it can provide the basis for a life-saving endovascular thrombectomy if a LVO is found.”
It is already well known that LVO is not restricted only to patients with an elevated NIHSS score, he said.
For determining whether to order a CTA, “I do not agree with NIHSS score of 6 or above. There is no absolute number below which risk of missing a LVO is eliminated,” Dr. Jabbour said. He also argued against relying on NIHSS score without considering other clinical features, particularly cortical signs, which should raise suspicion of a LVO regardless of NIHSS score.
One problem is that NIHSS scores are not static. Decompensation can be rapid with the NIHSS score quickly climbing. When this happens, the delay in treatment might lead to a preventable adverse outcome.
“There is a change in the paradigm now that we have more evidence of a benefit from aggressive treatment in the right candidates,” according to Dr. Jabbour, referring to the recently published SELECT2 trial. In that trial, on which Dr. Jabbour served as a coauthor, patients with LVO and large territory infarct were randomized to thrombectomy or medical care within 24 hours of a stroke. It was stopped early for efficacy because of the increased functional independence (20% vs. 7%) in the surgical intervention group.
If the ongoing trials establish better criteria for ruling in or out the presence of LVO in patients with acute stroke, Dr. Jabbour predicted that guidelines will be written to standardize practice.
Dr. Sevilis reports no potential conflicts of interest. Dr. Jabbour has financial relationships with Cerenovus, Medtronic, and Microvention.
FROM AAN 2023
Teriflunomide delays MS symptoms in radiologically isolated syndrome
BOSTON – , according to a double-blind, phase 3 trial presented in the Emerging Science session of the 2023 annual meeting of the American Academy of Neurology.
“These data add to the evidence that early immunomodulation offers clinical benefit even in the presymptomatic phase of MS,” reported Christine Lebrun-Frenay, MD, PhD, head of inflammatory neurological disorders research unit, University of Nice, France. This is the second study to show a benefit from a disease-modifying therapy in asymptomatic RIS patients. The ARISE study, which was presented at the 2022 European Committee for Treatment and Research in MS and has now been published, compared 240 mg of twice-daily dimethyl fumarate with placebo. Dimethyl fumarate was associated with an 82% (hazard ratio, 0.18; P = .007) reduction in the risk of a first demyelinating event after 96 weeks of follow-up.
TERIS trial data
In the new study, called TERIS, the design and outcomes were similar to the ARISE study. Eighty-nine patients meeting standard criteria for RIS were randomized to 14 mg of once-daily teriflunomide or placebo. The majority (71%) were female, and the mean age was 39.8 years. At the time of RIS diagnosis, the mean age was 38 years. At study entry, standardized MRI studies were performed of the brain and spinal cord.
During 2 years of follow-up, 8 of 28 demyelinating events were observed in the active treatment group. The remaining 20 occurred in the placebo group. This translated to a 63% reduction (HR, 0.37; P = .018) in favor of teriflunomide. When graphed, the curves separated at about 6 months and then widened progressively over time.
Distinct from clinically isolated syndrome (CIS), which describes individuals who have a symptomatic episode consistent with a demyelinating event, RIS is based primarily on an MRI that shows lesions highly suggestive of MS. Neither confirms the MS diagnosis, but both are associated with a high likelihood of eventually meeting MS diagnostic criteria. The ARISE and TERIS studies now support therapy to delay demyelinating events.
“With more and more people having brain scans for various reasons, such as headache or head trauma, more of these cases are being discovered,” Dr. Lebrun-Frenay said.
Caution warranted when interpreting the findings
The data support the theory that treatment should begin early in patients with a high likelihood of developing symptomatic MS on the basis of brain lesions. It is logical to assume that preventing damage to the myelin will reduce or delay permanent symptoms and permanent neurologic impairment, but Dr. Lebrun-Frenay suggested that the available data from ARISE and TERIS are not practice changing even though both were multicenter double-blind trials.
“More data from larger groups of patients are needed to confirm the findings,” she said. She expressed concern about not adhering to strict criteria to diagnosis RIS.
“It is important that medical professionals are cautious,” she said, citing the risk of misdiagnosis of pathology of MRI that leads to treatment of patients with a low risk of developing symptomatic MS.
Teriflunomide and dimethyl fumarate, which have long been available as first-line therapies in relapsing-remitting MS, are generally well tolerated. In the TERIS and ARISE studies, mild or moderate events occurred more commonly in the active treatment than the placebo arms, but there were no serious adverse events. However, both can produce more serious adverse events, which, in the case of teriflunomide, include liver toxicity leading to injury and liver failure.
Challenging the traditional definition of MS
The author of the ARISE study, Darin T. Okuda, MD, a professor of neurology at the UT Southwestern Medical Center, Dallas, indicated that his study, now reinforced by the TERIS study, challenges the definition of MS.
“Both ARISE and TERIS demonstrated a significant reduction in seminal clinical event rates related to inflammatory demyelination,” Dr. Okuda said in an interview. They provide evidence that patients are at high risk of the demyelinating events that characterize MS. Given the potential difficulty for accessing therapies of benefit, “how we define multiple sclerosis is highly important.”
“Individuals of younger age with abnormal spinal cord MRI studies along with other paraclinical features related to risk for a first event may be the most ideal group to treat,” he said. However, he agreed with Dr. Lebrun-Frenay that it is not yet clear which RIS patients are the most appropriate candidates.
“Gaining a more refined sense of who we should treat will require more work,” he said.
These data are likely to change the orientation toward RIS, according to Melina Hosseiny, MD, department of radiology, University of California, Los Angeles, Medical Center. She noted that the relationship between RIS and increased risk of MS has long been recognized, and the risk increases with specific features on imaging.
“Studies have shown that spinal cord lesions are associated with a greater than 50% chance of converting to MS,” said Dr. Hosseiny, who was the lead author of a review article on RIS. “Identifying such imaging findings can help identify patients who may benefit from disease-modifying medications.”
Dr. Lebrun-Frenay reports no potential conflicts of interest. Dr. Okuda has financial relationships with Alexion, Biogen, Celgene, EMD Serono, Genzyme, TG Therapeutics, and VielaBio. Dr. Hosseiny reports no potential conflicts of interest.
BOSTON – , according to a double-blind, phase 3 trial presented in the Emerging Science session of the 2023 annual meeting of the American Academy of Neurology.
“These data add to the evidence that early immunomodulation offers clinical benefit even in the presymptomatic phase of MS,” reported Christine Lebrun-Frenay, MD, PhD, head of inflammatory neurological disorders research unit, University of Nice, France. This is the second study to show a benefit from a disease-modifying therapy in asymptomatic RIS patients. The ARISE study, which was presented at the 2022 European Committee for Treatment and Research in MS and has now been published, compared 240 mg of twice-daily dimethyl fumarate with placebo. Dimethyl fumarate was associated with an 82% (hazard ratio, 0.18; P = .007) reduction in the risk of a first demyelinating event after 96 weeks of follow-up.
TERIS trial data
In the new study, called TERIS, the design and outcomes were similar to the ARISE study. Eighty-nine patients meeting standard criteria for RIS were randomized to 14 mg of once-daily teriflunomide or placebo. The majority (71%) were female, and the mean age was 39.8 years. At the time of RIS diagnosis, the mean age was 38 years. At study entry, standardized MRI studies were performed of the brain and spinal cord.
During 2 years of follow-up, 8 of 28 demyelinating events were observed in the active treatment group. The remaining 20 occurred in the placebo group. This translated to a 63% reduction (HR, 0.37; P = .018) in favor of teriflunomide. When graphed, the curves separated at about 6 months and then widened progressively over time.
Distinct from clinically isolated syndrome (CIS), which describes individuals who have a symptomatic episode consistent with a demyelinating event, RIS is based primarily on an MRI that shows lesions highly suggestive of MS. Neither confirms the MS diagnosis, but both are associated with a high likelihood of eventually meeting MS diagnostic criteria. The ARISE and TERIS studies now support therapy to delay demyelinating events.
“With more and more people having brain scans for various reasons, such as headache or head trauma, more of these cases are being discovered,” Dr. Lebrun-Frenay said.
Caution warranted when interpreting the findings
The data support the theory that treatment should begin early in patients with a high likelihood of developing symptomatic MS on the basis of brain lesions. It is logical to assume that preventing damage to the myelin will reduce or delay permanent symptoms and permanent neurologic impairment, but Dr. Lebrun-Frenay suggested that the available data from ARISE and TERIS are not practice changing even though both were multicenter double-blind trials.
“More data from larger groups of patients are needed to confirm the findings,” she said. She expressed concern about not adhering to strict criteria to diagnosis RIS.
“It is important that medical professionals are cautious,” she said, citing the risk of misdiagnosis of pathology of MRI that leads to treatment of patients with a low risk of developing symptomatic MS.
Teriflunomide and dimethyl fumarate, which have long been available as first-line therapies in relapsing-remitting MS, are generally well tolerated. In the TERIS and ARISE studies, mild or moderate events occurred more commonly in the active treatment than the placebo arms, but there were no serious adverse events. However, both can produce more serious adverse events, which, in the case of teriflunomide, include liver toxicity leading to injury and liver failure.
Challenging the traditional definition of MS
The author of the ARISE study, Darin T. Okuda, MD, a professor of neurology at the UT Southwestern Medical Center, Dallas, indicated that his study, now reinforced by the TERIS study, challenges the definition of MS.
“Both ARISE and TERIS demonstrated a significant reduction in seminal clinical event rates related to inflammatory demyelination,” Dr. Okuda said in an interview. They provide evidence that patients are at high risk of the demyelinating events that characterize MS. Given the potential difficulty for accessing therapies of benefit, “how we define multiple sclerosis is highly important.”
“Individuals of younger age with abnormal spinal cord MRI studies along with other paraclinical features related to risk for a first event may be the most ideal group to treat,” he said. However, he agreed with Dr. Lebrun-Frenay that it is not yet clear which RIS patients are the most appropriate candidates.
“Gaining a more refined sense of who we should treat will require more work,” he said.
These data are likely to change the orientation toward RIS, according to Melina Hosseiny, MD, department of radiology, University of California, Los Angeles, Medical Center. She noted that the relationship between RIS and increased risk of MS has long been recognized, and the risk increases with specific features on imaging.
“Studies have shown that spinal cord lesions are associated with a greater than 50% chance of converting to MS,” said Dr. Hosseiny, who was the lead author of a review article on RIS. “Identifying such imaging findings can help identify patients who may benefit from disease-modifying medications.”
Dr. Lebrun-Frenay reports no potential conflicts of interest. Dr. Okuda has financial relationships with Alexion, Biogen, Celgene, EMD Serono, Genzyme, TG Therapeutics, and VielaBio. Dr. Hosseiny reports no potential conflicts of interest.
BOSTON – , according to a double-blind, phase 3 trial presented in the Emerging Science session of the 2023 annual meeting of the American Academy of Neurology.
“These data add to the evidence that early immunomodulation offers clinical benefit even in the presymptomatic phase of MS,” reported Christine Lebrun-Frenay, MD, PhD, head of inflammatory neurological disorders research unit, University of Nice, France. This is the second study to show a benefit from a disease-modifying therapy in asymptomatic RIS patients. The ARISE study, which was presented at the 2022 European Committee for Treatment and Research in MS and has now been published, compared 240 mg of twice-daily dimethyl fumarate with placebo. Dimethyl fumarate was associated with an 82% (hazard ratio, 0.18; P = .007) reduction in the risk of a first demyelinating event after 96 weeks of follow-up.
TERIS trial data
In the new study, called TERIS, the design and outcomes were similar to the ARISE study. Eighty-nine patients meeting standard criteria for RIS were randomized to 14 mg of once-daily teriflunomide or placebo. The majority (71%) were female, and the mean age was 39.8 years. At the time of RIS diagnosis, the mean age was 38 years. At study entry, standardized MRI studies were performed of the brain and spinal cord.
During 2 years of follow-up, 8 of 28 demyelinating events were observed in the active treatment group. The remaining 20 occurred in the placebo group. This translated to a 63% reduction (HR, 0.37; P = .018) in favor of teriflunomide. When graphed, the curves separated at about 6 months and then widened progressively over time.
Distinct from clinically isolated syndrome (CIS), which describes individuals who have a symptomatic episode consistent with a demyelinating event, RIS is based primarily on an MRI that shows lesions highly suggestive of MS. Neither confirms the MS diagnosis, but both are associated with a high likelihood of eventually meeting MS diagnostic criteria. The ARISE and TERIS studies now support therapy to delay demyelinating events.
“With more and more people having brain scans for various reasons, such as headache or head trauma, more of these cases are being discovered,” Dr. Lebrun-Frenay said.
Caution warranted when interpreting the findings
The data support the theory that treatment should begin early in patients with a high likelihood of developing symptomatic MS on the basis of brain lesions. It is logical to assume that preventing damage to the myelin will reduce or delay permanent symptoms and permanent neurologic impairment, but Dr. Lebrun-Frenay suggested that the available data from ARISE and TERIS are not practice changing even though both were multicenter double-blind trials.
“More data from larger groups of patients are needed to confirm the findings,” she said. She expressed concern about not adhering to strict criteria to diagnosis RIS.
“It is important that medical professionals are cautious,” she said, citing the risk of misdiagnosis of pathology of MRI that leads to treatment of patients with a low risk of developing symptomatic MS.
Teriflunomide and dimethyl fumarate, which have long been available as first-line therapies in relapsing-remitting MS, are generally well tolerated. In the TERIS and ARISE studies, mild or moderate events occurred more commonly in the active treatment than the placebo arms, but there were no serious adverse events. However, both can produce more serious adverse events, which, in the case of teriflunomide, include liver toxicity leading to injury and liver failure.
Challenging the traditional definition of MS
The author of the ARISE study, Darin T. Okuda, MD, a professor of neurology at the UT Southwestern Medical Center, Dallas, indicated that his study, now reinforced by the TERIS study, challenges the definition of MS.
“Both ARISE and TERIS demonstrated a significant reduction in seminal clinical event rates related to inflammatory demyelination,” Dr. Okuda said in an interview. They provide evidence that patients are at high risk of the demyelinating events that characterize MS. Given the potential difficulty for accessing therapies of benefit, “how we define multiple sclerosis is highly important.”
“Individuals of younger age with abnormal spinal cord MRI studies along with other paraclinical features related to risk for a first event may be the most ideal group to treat,” he said. However, he agreed with Dr. Lebrun-Frenay that it is not yet clear which RIS patients are the most appropriate candidates.
“Gaining a more refined sense of who we should treat will require more work,” he said.
These data are likely to change the orientation toward RIS, according to Melina Hosseiny, MD, department of radiology, University of California, Los Angeles, Medical Center. She noted that the relationship between RIS and increased risk of MS has long been recognized, and the risk increases with specific features on imaging.
“Studies have shown that spinal cord lesions are associated with a greater than 50% chance of converting to MS,” said Dr. Hosseiny, who was the lead author of a review article on RIS. “Identifying such imaging findings can help identify patients who may benefit from disease-modifying medications.”
Dr. Lebrun-Frenay reports no potential conflicts of interest. Dr. Okuda has financial relationships with Alexion, Biogen, Celgene, EMD Serono, Genzyme, TG Therapeutics, and VielaBio. Dr. Hosseiny reports no potential conflicts of interest.
AT AAN 2023
Plasma monitoring supports earlier osimertinib treatment in lung cancer patients
Previous studies have suggested that molecular progression of disease in patients with EGFR-mutant NSCLC, as measured by sequential plasma EGFR T790M, may precede radiological progression, as measured by Response Evaluation Criteria in Solid Tumors (RECIST).
However, the impact of these measures on timing of treatment changes and patient outcomes has not been examined, wrote Jordi Remon, MD, of Paris (France)–Saclay University and colleagues, in Annals of Oncology.
The European Organization for Research Treatment and Cancer Lung Cancer Group designed a phase 2 clinical trial known as APPLE to evaluate the use of sequential plasma EGFR T790M and determine the optimal sequencing for gefitinib and osimertinib in patients with EGFR-mutant NSCLC.
The researchers reported results from two randomized arms of the APPLE trial. In arm B, 52 patients received gefitinib until emergence of circulating tumor DNA (ctDNA) EGFR T790M mutation, based on the cobas EGFR test v2 (a real-time PCR test), or progression of disease based on Response Evaluation Criteria in Solid Tumors (RECIST). In arm C, 51 patients received gefitinib until disease progression based on RECIST. Both arms then switched to osimertinib. Patients randomized to a third arm (arm A) received osimertinib upfront until progression of disease based on RECIST, and they were not included in the current study.
The primary endpoint was progression-free survival (PFS) while receiving osimertinib at 18 months in patients who were originally randomized to gefitinib, then switched to osimertinib at the emergence of circulating tumor DNA. Secondary endpoints included PFS, overall response rate, overall survival, and brain PFS.
Patients entered the study between November 2017 and February 2020. A total of 75% and 65% of those in arms B and C, respectively, were female, approximately 65% had the mutation EGFR Del19, and approximately one-third had baseline brain metastases. In arm B, 17% of patients switched to osimertinib based on the emergence of ctDNA T790M mutation before progressive disease based on RECIST. The median time to molecular disease progression was 266 days.
More patients in arm B met the primary endpoint of PFS while receiving osimertinib at 18 months (67.2%) than in arm C (53.5%), after a median follow-up of 30 months.
As for secondary endpoints, the median PFS in the two arms was 22.0 months and 20.2 months, respectively. Median overall survival was 42.8 months in arm C and was not reached in arm B. The median brain PFS was 24.4 months for arm B and 21.4 months for arm C.
The benefits seen in the osimertinib patients may be due in part to the timing of the switch to correspond with molecular or radiological disease progression, the researchers wrote in their discussion.
In the future, more research is needed to determine whether molecular monitoring may impact patients’ outcomes, compared with monitoring based on radiological progression, they said.
The findings were limited by several factors, mainly the rapid evolution in the treatment landscape of EGFR-mutant NSCLC, the researchers noted.
Osimertinib is currently considered the preferred first-line treatment by most physicians, they said. “The APPLE trial is the first prospective study supporting the role of dynamic adaptive strategies based on ctDNA monitoring in patients with EGFR-mutant advanced NSCLC.”
The study was supported by AstraZeneca. Lead author Dr. Remon had no financial conflicts to disclose. Corresponding author Dr. Dziadziuszko disclosed honoraria for consultancy or lectures from AstraZeneca, Roche, Novartis, MSD, Takeda, Pfizer, Amgen, and Bristol-Myers Squibb.
Previous studies have suggested that molecular progression of disease in patients with EGFR-mutant NSCLC, as measured by sequential plasma EGFR T790M, may precede radiological progression, as measured by Response Evaluation Criteria in Solid Tumors (RECIST).
However, the impact of these measures on timing of treatment changes and patient outcomes has not been examined, wrote Jordi Remon, MD, of Paris (France)–Saclay University and colleagues, in Annals of Oncology.
The European Organization for Research Treatment and Cancer Lung Cancer Group designed a phase 2 clinical trial known as APPLE to evaluate the use of sequential plasma EGFR T790M and determine the optimal sequencing for gefitinib and osimertinib in patients with EGFR-mutant NSCLC.
The researchers reported results from two randomized arms of the APPLE trial. In arm B, 52 patients received gefitinib until emergence of circulating tumor DNA (ctDNA) EGFR T790M mutation, based on the cobas EGFR test v2 (a real-time PCR test), or progression of disease based on Response Evaluation Criteria in Solid Tumors (RECIST). In arm C, 51 patients received gefitinib until disease progression based on RECIST. Both arms then switched to osimertinib. Patients randomized to a third arm (arm A) received osimertinib upfront until progression of disease based on RECIST, and they were not included in the current study.
The primary endpoint was progression-free survival (PFS) while receiving osimertinib at 18 months in patients who were originally randomized to gefitinib, then switched to osimertinib at the emergence of circulating tumor DNA. Secondary endpoints included PFS, overall response rate, overall survival, and brain PFS.
Patients entered the study between November 2017 and February 2020. A total of 75% and 65% of those in arms B and C, respectively, were female, approximately 65% had the mutation EGFR Del19, and approximately one-third had baseline brain metastases. In arm B, 17% of patients switched to osimertinib based on the emergence of ctDNA T790M mutation before progressive disease based on RECIST. The median time to molecular disease progression was 266 days.
More patients in arm B met the primary endpoint of PFS while receiving osimertinib at 18 months (67.2%) than in arm C (53.5%), after a median follow-up of 30 months.
As for secondary endpoints, the median PFS in the two arms was 22.0 months and 20.2 months, respectively. Median overall survival was 42.8 months in arm C and was not reached in arm B. The median brain PFS was 24.4 months for arm B and 21.4 months for arm C.
The benefits seen in the osimertinib patients may be due in part to the timing of the switch to correspond with molecular or radiological disease progression, the researchers wrote in their discussion.
In the future, more research is needed to determine whether molecular monitoring may impact patients’ outcomes, compared with monitoring based on radiological progression, they said.
The findings were limited by several factors, mainly the rapid evolution in the treatment landscape of EGFR-mutant NSCLC, the researchers noted.
Osimertinib is currently considered the preferred first-line treatment by most physicians, they said. “The APPLE trial is the first prospective study supporting the role of dynamic adaptive strategies based on ctDNA monitoring in patients with EGFR-mutant advanced NSCLC.”
The study was supported by AstraZeneca. Lead author Dr. Remon had no financial conflicts to disclose. Corresponding author Dr. Dziadziuszko disclosed honoraria for consultancy or lectures from AstraZeneca, Roche, Novartis, MSD, Takeda, Pfizer, Amgen, and Bristol-Myers Squibb.
Previous studies have suggested that molecular progression of disease in patients with EGFR-mutant NSCLC, as measured by sequential plasma EGFR T790M, may precede radiological progression, as measured by Response Evaluation Criteria in Solid Tumors (RECIST).
However, the impact of these measures on timing of treatment changes and patient outcomes has not been examined, wrote Jordi Remon, MD, of Paris (France)–Saclay University and colleagues, in Annals of Oncology.
The European Organization for Research Treatment and Cancer Lung Cancer Group designed a phase 2 clinical trial known as APPLE to evaluate the use of sequential plasma EGFR T790M and determine the optimal sequencing for gefitinib and osimertinib in patients with EGFR-mutant NSCLC.
The researchers reported results from two randomized arms of the APPLE trial. In arm B, 52 patients received gefitinib until emergence of circulating tumor DNA (ctDNA) EGFR T790M mutation, based on the cobas EGFR test v2 (a real-time PCR test), or progression of disease based on Response Evaluation Criteria in Solid Tumors (RECIST). In arm C, 51 patients received gefitinib until disease progression based on RECIST. Both arms then switched to osimertinib. Patients randomized to a third arm (arm A) received osimertinib upfront until progression of disease based on RECIST, and they were not included in the current study.
The primary endpoint was progression-free survival (PFS) while receiving osimertinib at 18 months in patients who were originally randomized to gefitinib, then switched to osimertinib at the emergence of circulating tumor DNA. Secondary endpoints included PFS, overall response rate, overall survival, and brain PFS.
Patients entered the study between November 2017 and February 2020. A total of 75% and 65% of those in arms B and C, respectively, were female, approximately 65% had the mutation EGFR Del19, and approximately one-third had baseline brain metastases. In arm B, 17% of patients switched to osimertinib based on the emergence of ctDNA T790M mutation before progressive disease based on RECIST. The median time to molecular disease progression was 266 days.
More patients in arm B met the primary endpoint of PFS while receiving osimertinib at 18 months (67.2%) than in arm C (53.5%), after a median follow-up of 30 months.
As for secondary endpoints, the median PFS in the two arms was 22.0 months and 20.2 months, respectively. Median overall survival was 42.8 months in arm C and was not reached in arm B. The median brain PFS was 24.4 months for arm B and 21.4 months for arm C.
The benefits seen in the osimertinib patients may be due in part to the timing of the switch to correspond with molecular or radiological disease progression, the researchers wrote in their discussion.
In the future, more research is needed to determine whether molecular monitoring may impact patients’ outcomes, compared with monitoring based on radiological progression, they said.
The findings were limited by several factors, mainly the rapid evolution in the treatment landscape of EGFR-mutant NSCLC, the researchers noted.
Osimertinib is currently considered the preferred first-line treatment by most physicians, they said. “The APPLE trial is the first prospective study supporting the role of dynamic adaptive strategies based on ctDNA monitoring in patients with EGFR-mutant advanced NSCLC.”
The study was supported by AstraZeneca. Lead author Dr. Remon had no financial conflicts to disclose. Corresponding author Dr. Dziadziuszko disclosed honoraria for consultancy or lectures from AstraZeneca, Roche, Novartis, MSD, Takeda, Pfizer, Amgen, and Bristol-Myers Squibb.
FROM ANNALS OF ONCOLOGY
Novel levodopa delivery system promises continuous dosing without surgery or pump
BOSTON – , according to an early clinical experience described in the Emerging Science session at the 2023 annual meeting of the American Academy of Neurology.
On this device, the attenuation of levodopa fluctuations “translated into dramatic improvements in clinical behavior, including highly significant reductions in OFF time and an increase in ON time with no dyskinesias,” reported C. Warren Olanow, MD, who is a chairman emeritus of the department of neurology at the Icahn School of Medicine at Mount Sinai, New York, and now an employee of the company developing this new device.
A novel strategy
Numerous studies have demonstrated that reductions in the troughs of plasma levodopa associated with oral dosing result in longer ON time with fewer dyskinesias, according to Dr. Olanow, who explained this has led to strategies for numerous strategies to achieve continuous delivery. A device that delivers levodopa into the stomach through a surgically implanted catheter has already received regulatory approval. Other devices delivering levodopa subcutaneously are in development, but Dr. Olanow said each of these has had limitations.
“The problem with these approaches is they are associated with potentially serious side effects and they require the patient to wear a cumbersome device,” he explained. Relative to the subcutaneous delivery systems, which have been associated with injection site reactions that include painful nodules, and the surgically implanted devices, which also require an external pump, the latest strategy avoids both disadvantages.
Called DopaFuse, the experimental device is designed to deliver the levodopa and carbidopa into the mouth through a micropump within a wearable retainer. Dr. Olanow said that previous experimental studies demonstrated that small doses of levodopa delivered by mouth to the gastrointestinal system reduce levodopa plasma variability. This early clinical study supports that premise. Levodopa delivered into the mouth by way of a propellant in the retainer-mounted pump improved clinical endpoints.
Encouraging trial results
In the study, 16 patients between the ages of 30 and 75 with Parkinson’s disease were enrolled. On day 1, they received an oral dose of levodopa/carbidopa consistent with their current treatment. On day 2, levodopa/carbidopa was delivered through the retainer-mounted device at equivalent doses. On day 3, they received a single morning oral dose and the received the remainder of their levodopa/carbidopa regimen through the device. On days 4 to 14, they received treatment in the same schedule as day 3.
When pharmacokinetics of levodopa on day 3 were compared with those on day 1, the fluctuation index and coefficient of levodopa concentration variability was reduced to a degree that was highly statistically significant (P < .0001). This, in turn, correlated with “striking” reductions in OFF time with equally statistically significant improvement in ON time and ON time without dyskinesias, according to Dr. Olanow.
Relative to an OFF time of 3.2 hours on day 1, the OFF time of 1.6 hours on day 3 represented a 50% reduction (P < .0001). ON time improved from 12.8 hours to 14.5 hours (P < .001). ON time without dyskinesias improved numerically from 8.8 hours to 9.6 hours.
“There were also improvements in activities of daily living when patients were on DopaFuse, which is a hard endpoint to reach in a study with such a small sample size,” Dr. Olanow reported.
There were no serious adverse events. Three patients reported vomiting and two patients each reported headache, but these events were mild and all resolved within a day. Three patients reported buccal lesions, but these also resolved within a day.
“Some patients reported trouble with speaking in the beginning but at the end of the study, patients were reporting that it was easier to speak because of the motor improvements,” Dr. Olanow said.
Overall, the device was well tolerated by the subjects, providing the evidence for the next stages of clinical studies, reported Dr. Olanow.
“If this turns out to be what we hope it is, it will allow us to deliver levodopa without motor complications, without need for a surgical procedure, and without the risk of subcutaneous lesions,” Dr. Olanow said.
More delivery strategies are needed
This device is in an early phase of development, but several specialists in Parkinson’s disease agreed that there is a need for more strategies to provide continuous levodopa in patients with advancing symptoms. Stuart Isaacson, MD, director, Parkinson’s Disease and Movement Disorders Center of Boca Raton, Fla., is among them.
“Novel delivery devices that can provide more continuous levodopa delivery would be an important therapeutic advance,” Dr. Isaacson said. He called levodopa “the cornerstone of treatment through the course of Parkinson’s disease,” but more physiologic dosing in advancing disease has been a challenge.
“While there are many therapies currently available to manage OFF time, many people living with Parkinson’s disease continue to spend only half of their waking day with good ON time,” he added.
The currently approved method of delivering continuous levodopa through a surgically placed catheter into the gastrointestinal system is effective, but has limitations, according to Aaron L. Ellenbogen, MD, a neurologist at Beaumont Hospital, Farmington Hills, Mich.
“One of the challenges with the current treatment landscape of Parkinson’s disease is that medication can be absorbed variably through the gastrointestinal system,” he said. “As the disease progresses, this often becomes more troublesome.” Although this new device is likely to share this issue, Dr. Ellenbogen said that several devices might be useful to match patients with the one that works best for them.
Dr. Olanow is the founder and CEO of Clintrex Research Corporation, through which he also serves as chief medical officer of SynAgile, the company developing DopaFuse. Dr. Isaacson has financial relationships with more than 30 companies, including those that produce levodopa and levodopa delivery systems. Dr. Ellenbogen has financial relationships with Allergan, Acorda, Supernus, and Teva.
BOSTON – , according to an early clinical experience described in the Emerging Science session at the 2023 annual meeting of the American Academy of Neurology.
On this device, the attenuation of levodopa fluctuations “translated into dramatic improvements in clinical behavior, including highly significant reductions in OFF time and an increase in ON time with no dyskinesias,” reported C. Warren Olanow, MD, who is a chairman emeritus of the department of neurology at the Icahn School of Medicine at Mount Sinai, New York, and now an employee of the company developing this new device.
A novel strategy
Numerous studies have demonstrated that reductions in the troughs of plasma levodopa associated with oral dosing result in longer ON time with fewer dyskinesias, according to Dr. Olanow, who explained this has led to strategies for numerous strategies to achieve continuous delivery. A device that delivers levodopa into the stomach through a surgically implanted catheter has already received regulatory approval. Other devices delivering levodopa subcutaneously are in development, but Dr. Olanow said each of these has had limitations.
“The problem with these approaches is they are associated with potentially serious side effects and they require the patient to wear a cumbersome device,” he explained. Relative to the subcutaneous delivery systems, which have been associated with injection site reactions that include painful nodules, and the surgically implanted devices, which also require an external pump, the latest strategy avoids both disadvantages.
Called DopaFuse, the experimental device is designed to deliver the levodopa and carbidopa into the mouth through a micropump within a wearable retainer. Dr. Olanow said that previous experimental studies demonstrated that small doses of levodopa delivered by mouth to the gastrointestinal system reduce levodopa plasma variability. This early clinical study supports that premise. Levodopa delivered into the mouth by way of a propellant in the retainer-mounted pump improved clinical endpoints.
Encouraging trial results
In the study, 16 patients between the ages of 30 and 75 with Parkinson’s disease were enrolled. On day 1, they received an oral dose of levodopa/carbidopa consistent with their current treatment. On day 2, levodopa/carbidopa was delivered through the retainer-mounted device at equivalent doses. On day 3, they received a single morning oral dose and the received the remainder of their levodopa/carbidopa regimen through the device. On days 4 to 14, they received treatment in the same schedule as day 3.
When pharmacokinetics of levodopa on day 3 were compared with those on day 1, the fluctuation index and coefficient of levodopa concentration variability was reduced to a degree that was highly statistically significant (P < .0001). This, in turn, correlated with “striking” reductions in OFF time with equally statistically significant improvement in ON time and ON time without dyskinesias, according to Dr. Olanow.
Relative to an OFF time of 3.2 hours on day 1, the OFF time of 1.6 hours on day 3 represented a 50% reduction (P < .0001). ON time improved from 12.8 hours to 14.5 hours (P < .001). ON time without dyskinesias improved numerically from 8.8 hours to 9.6 hours.
“There were also improvements in activities of daily living when patients were on DopaFuse, which is a hard endpoint to reach in a study with such a small sample size,” Dr. Olanow reported.
There were no serious adverse events. Three patients reported vomiting and two patients each reported headache, but these events were mild and all resolved within a day. Three patients reported buccal lesions, but these also resolved within a day.
“Some patients reported trouble with speaking in the beginning but at the end of the study, patients were reporting that it was easier to speak because of the motor improvements,” Dr. Olanow said.
Overall, the device was well tolerated by the subjects, providing the evidence for the next stages of clinical studies, reported Dr. Olanow.
“If this turns out to be what we hope it is, it will allow us to deliver levodopa without motor complications, without need for a surgical procedure, and without the risk of subcutaneous lesions,” Dr. Olanow said.
More delivery strategies are needed
This device is in an early phase of development, but several specialists in Parkinson’s disease agreed that there is a need for more strategies to provide continuous levodopa in patients with advancing symptoms. Stuart Isaacson, MD, director, Parkinson’s Disease and Movement Disorders Center of Boca Raton, Fla., is among them.
“Novel delivery devices that can provide more continuous levodopa delivery would be an important therapeutic advance,” Dr. Isaacson said. He called levodopa “the cornerstone of treatment through the course of Parkinson’s disease,” but more physiologic dosing in advancing disease has been a challenge.
“While there are many therapies currently available to manage OFF time, many people living with Parkinson’s disease continue to spend only half of their waking day with good ON time,” he added.
The currently approved method of delivering continuous levodopa through a surgically placed catheter into the gastrointestinal system is effective, but has limitations, according to Aaron L. Ellenbogen, MD, a neurologist at Beaumont Hospital, Farmington Hills, Mich.
“One of the challenges with the current treatment landscape of Parkinson’s disease is that medication can be absorbed variably through the gastrointestinal system,” he said. “As the disease progresses, this often becomes more troublesome.” Although this new device is likely to share this issue, Dr. Ellenbogen said that several devices might be useful to match patients with the one that works best for them.
Dr. Olanow is the founder and CEO of Clintrex Research Corporation, through which he also serves as chief medical officer of SynAgile, the company developing DopaFuse. Dr. Isaacson has financial relationships with more than 30 companies, including those that produce levodopa and levodopa delivery systems. Dr. Ellenbogen has financial relationships with Allergan, Acorda, Supernus, and Teva.
BOSTON – , according to an early clinical experience described in the Emerging Science session at the 2023 annual meeting of the American Academy of Neurology.
On this device, the attenuation of levodopa fluctuations “translated into dramatic improvements in clinical behavior, including highly significant reductions in OFF time and an increase in ON time with no dyskinesias,” reported C. Warren Olanow, MD, who is a chairman emeritus of the department of neurology at the Icahn School of Medicine at Mount Sinai, New York, and now an employee of the company developing this new device.
A novel strategy
Numerous studies have demonstrated that reductions in the troughs of plasma levodopa associated with oral dosing result in longer ON time with fewer dyskinesias, according to Dr. Olanow, who explained this has led to strategies for numerous strategies to achieve continuous delivery. A device that delivers levodopa into the stomach through a surgically implanted catheter has already received regulatory approval. Other devices delivering levodopa subcutaneously are in development, but Dr. Olanow said each of these has had limitations.
“The problem with these approaches is they are associated with potentially serious side effects and they require the patient to wear a cumbersome device,” he explained. Relative to the subcutaneous delivery systems, which have been associated with injection site reactions that include painful nodules, and the surgically implanted devices, which also require an external pump, the latest strategy avoids both disadvantages.
Called DopaFuse, the experimental device is designed to deliver the levodopa and carbidopa into the mouth through a micropump within a wearable retainer. Dr. Olanow said that previous experimental studies demonstrated that small doses of levodopa delivered by mouth to the gastrointestinal system reduce levodopa plasma variability. This early clinical study supports that premise. Levodopa delivered into the mouth by way of a propellant in the retainer-mounted pump improved clinical endpoints.
Encouraging trial results
In the study, 16 patients between the ages of 30 and 75 with Parkinson’s disease were enrolled. On day 1, they received an oral dose of levodopa/carbidopa consistent with their current treatment. On day 2, levodopa/carbidopa was delivered through the retainer-mounted device at equivalent doses. On day 3, they received a single morning oral dose and the received the remainder of their levodopa/carbidopa regimen through the device. On days 4 to 14, they received treatment in the same schedule as day 3.
When pharmacokinetics of levodopa on day 3 were compared with those on day 1, the fluctuation index and coefficient of levodopa concentration variability was reduced to a degree that was highly statistically significant (P < .0001). This, in turn, correlated with “striking” reductions in OFF time with equally statistically significant improvement in ON time and ON time without dyskinesias, according to Dr. Olanow.
Relative to an OFF time of 3.2 hours on day 1, the OFF time of 1.6 hours on day 3 represented a 50% reduction (P < .0001). ON time improved from 12.8 hours to 14.5 hours (P < .001). ON time without dyskinesias improved numerically from 8.8 hours to 9.6 hours.
“There were also improvements in activities of daily living when patients were on DopaFuse, which is a hard endpoint to reach in a study with such a small sample size,” Dr. Olanow reported.
There were no serious adverse events. Three patients reported vomiting and two patients each reported headache, but these events were mild and all resolved within a day. Three patients reported buccal lesions, but these also resolved within a day.
“Some patients reported trouble with speaking in the beginning but at the end of the study, patients were reporting that it was easier to speak because of the motor improvements,” Dr. Olanow said.
Overall, the device was well tolerated by the subjects, providing the evidence for the next stages of clinical studies, reported Dr. Olanow.
“If this turns out to be what we hope it is, it will allow us to deliver levodopa without motor complications, without need for a surgical procedure, and without the risk of subcutaneous lesions,” Dr. Olanow said.
More delivery strategies are needed
This device is in an early phase of development, but several specialists in Parkinson’s disease agreed that there is a need for more strategies to provide continuous levodopa in patients with advancing symptoms. Stuart Isaacson, MD, director, Parkinson’s Disease and Movement Disorders Center of Boca Raton, Fla., is among them.
“Novel delivery devices that can provide more continuous levodopa delivery would be an important therapeutic advance,” Dr. Isaacson said. He called levodopa “the cornerstone of treatment through the course of Parkinson’s disease,” but more physiologic dosing in advancing disease has been a challenge.
“While there are many therapies currently available to manage OFF time, many people living with Parkinson’s disease continue to spend only half of their waking day with good ON time,” he added.
The currently approved method of delivering continuous levodopa through a surgically placed catheter into the gastrointestinal system is effective, but has limitations, according to Aaron L. Ellenbogen, MD, a neurologist at Beaumont Hospital, Farmington Hills, Mich.
“One of the challenges with the current treatment landscape of Parkinson’s disease is that medication can be absorbed variably through the gastrointestinal system,” he said. “As the disease progresses, this often becomes more troublesome.” Although this new device is likely to share this issue, Dr. Ellenbogen said that several devices might be useful to match patients with the one that works best for them.
Dr. Olanow is the founder and CEO of Clintrex Research Corporation, through which he also serves as chief medical officer of SynAgile, the company developing DopaFuse. Dr. Isaacson has financial relationships with more than 30 companies, including those that produce levodopa and levodopa delivery systems. Dr. Ellenbogen has financial relationships with Allergan, Acorda, Supernus, and Teva.
FROM AAN 2023
Medical-level empathy? Yup, ChatGPT can fake that
Caution: Robotic uprisings in the rearview mirror are closer than they appear
ChatGPT. If you’ve been even in the proximity of the Internet lately, you may have heard of it. It’s quite an incredible piece of technology, an artificial intelligence that really could up-end a lot of industries. And lest doctors believe they’re safe from robotic replacement, consider this: ChatGPT took a test commonly used as a study resource by ophthalmologists and scored a 46%. Obviously, that’s not a passing grade. Job safe, right?
A month later, the researchers tried again. This time, ChatGPT got a 58%. Still not passing, and ChatGPT did especially poorly on ophthalmology specialty questions (it got 80% of general medicine questions right), but still, the jump in quality after just a month is ... concerning. It’s not like an AI will forget things. That score can only go up, and it’ll go up faster than you think.
“Sure, the robot is smart,” the doctors out there are thinking, “but how can an AI compete with human compassion, understanding, and bedside manner?”
And they’d be right. When it comes to bedside manner, there’s no competition between man and bot. ChatGPT is already winning.
In another study, researchers sampled nearly 200 questions from the subreddit r/AskDocs, which received verified physician responses. The researchers fed ChatGPT the questions – without the doctor’s answer – and a panel of health care professionals evaluated both the human doctor and ChatGPT in terms of quality and empathy.
Perhaps not surprisingly, the robot did better when it came to quality, providing a high-quality response 79% of the time, versus 22% for the human. But empathy? It was a bloodbath. ChatGPT provided an empathetic or very empathetic response 45% of the time, while humans could only do so 4.6% of the time. So much for bedside manner.
The researchers were suspiciously quick to note that ChatGPT isn’t a legitimate replacement for physicians, but could represent a tool to better provide care for patients. But let’s be honest, given ChatGPT’s quick advancement, how long before some intrepid stockholder says: “Hey, instead of paying doctors, why don’t we just use the free robot instead?” We give it a week. Or 11 minutes.
This week, on ‘As the sperm turns’
We’ve got a lot of spermy ground to cover, so let’s get right to it, starting with the small and working our way up.
We’re all pretty familiar with the basic structure of a sperm cell, yes? Bulbous head that contains all the important genetic information and a tail-like flagellum to propel it to its ultimate destination. Not much to work with there, you’d think, but what if Mother Nature, who clearly has a robust sense of humor, had something else in mind?
We present exhibit A, Paramormyorps kingsleyae, also known as the electric elephantfish, which happens to be the only known vertebrate species with tailless sperm. Sounds crazy to us, too, but Jason Gallant, PhD, of
Michigan State University, Lansing, has a theory: “A general notion in biology is that sperm are cheap, and eggs are expensive – but these fish may be telling us that sperm are more expensive than we might think. They could be saving energy by cutting back on sperm tails.”
He and his team think that finding the gene that turns off development of the flagellum in the elephant fish could benefit humans, specifically those with a genetic disorder called primary ciliary dyskinesia, whose lack of normally functioning cilia and flagella leads to chronic respiratory infection, abnormally positioned organs, fluid on the brain, and infertility.
And that – with “that” being infertility – brings us to exhibit B, a 41-year-old Dutch man named Jonathan Meijer who clearly has too much time on his hands.
A court in the Netherlands recently ordered him, and not for the first time, to stop donating sperm to fertility clinics after it was discovered that he had fathered between 500 and 600 children around the world. He had been banned from donating to Dutch clinics in 2017, at which point he had already fathered 100 children, but managed a workaround by donating internationally and online, sometimes using another name.
The judge ordered Mr. Meijer to contact all of the clinics abroad and ask them to destroy any of his sperm they still had in stock and threatened to fine him over $100,000 for each future violation.
Okay, so here’s the thing. We have been, um, let’s call it ... warned, about the evils of tastelessness in journalism, so we’re going to do what Mr. Meijer should have done and abstain. And we can last for longer than 11 minutes.
The realm of lost luggage and lost sleep
It may be convenient to live near an airport if you’re a frequent flyer, but it really doesn’t help your sleep numbers.
The first look at how such a common sound affects sleep duration showed that people exposed to even 45 decibels of airplane noise were less likely to get the 7-9 hours of sleep needed for healthy functioning, investigators said in Environmental Health Perspectives.
How loud is 45 dB exactly? A normal conversation is about 50 dB, while a whisper is 30 dB, to give you an idea. Airplane noise at 45 dB? You might not even notice it amongst the other noises in daily life.
The researchers looked at data from about 35,000 participants in the Nurses’ Health Study who live around 90 major U.S. airports. They examined plane noise every 5 years between 1995 and 2005, focusing on estimates of nighttime and daytime levels. Short sleep was most common among the nurses who lived on the West Coast, near major cargo airports or large bodies of water, and also among those who reported no hearing loss.
The investigators noted, however, that there was no consistent association between airplane noise and quality of sleep and stopped short of making any policy recommendations. Still, sleep is a very important, yet slept-on (pun intended) factor for our overall health, so it’s good to know if anything has the potential to cause disruption.
Caution: Robotic uprisings in the rearview mirror are closer than they appear
ChatGPT. If you’ve been even in the proximity of the Internet lately, you may have heard of it. It’s quite an incredible piece of technology, an artificial intelligence that really could up-end a lot of industries. And lest doctors believe they’re safe from robotic replacement, consider this: ChatGPT took a test commonly used as a study resource by ophthalmologists and scored a 46%. Obviously, that’s not a passing grade. Job safe, right?
A month later, the researchers tried again. This time, ChatGPT got a 58%. Still not passing, and ChatGPT did especially poorly on ophthalmology specialty questions (it got 80% of general medicine questions right), but still, the jump in quality after just a month is ... concerning. It’s not like an AI will forget things. That score can only go up, and it’ll go up faster than you think.
“Sure, the robot is smart,” the doctors out there are thinking, “but how can an AI compete with human compassion, understanding, and bedside manner?”
And they’d be right. When it comes to bedside manner, there’s no competition between man and bot. ChatGPT is already winning.
In another study, researchers sampled nearly 200 questions from the subreddit r/AskDocs, which received verified physician responses. The researchers fed ChatGPT the questions – without the doctor’s answer – and a panel of health care professionals evaluated both the human doctor and ChatGPT in terms of quality and empathy.
Perhaps not surprisingly, the robot did better when it came to quality, providing a high-quality response 79% of the time, versus 22% for the human. But empathy? It was a bloodbath. ChatGPT provided an empathetic or very empathetic response 45% of the time, while humans could only do so 4.6% of the time. So much for bedside manner.
The researchers were suspiciously quick to note that ChatGPT isn’t a legitimate replacement for physicians, but could represent a tool to better provide care for patients. But let’s be honest, given ChatGPT’s quick advancement, how long before some intrepid stockholder says: “Hey, instead of paying doctors, why don’t we just use the free robot instead?” We give it a week. Or 11 minutes.
This week, on ‘As the sperm turns’
We’ve got a lot of spermy ground to cover, so let’s get right to it, starting with the small and working our way up.
We’re all pretty familiar with the basic structure of a sperm cell, yes? Bulbous head that contains all the important genetic information and a tail-like flagellum to propel it to its ultimate destination. Not much to work with there, you’d think, but what if Mother Nature, who clearly has a robust sense of humor, had something else in mind?
We present exhibit A, Paramormyorps kingsleyae, also known as the electric elephantfish, which happens to be the only known vertebrate species with tailless sperm. Sounds crazy to us, too, but Jason Gallant, PhD, of
Michigan State University, Lansing, has a theory: “A general notion in biology is that sperm are cheap, and eggs are expensive – but these fish may be telling us that sperm are more expensive than we might think. They could be saving energy by cutting back on sperm tails.”
He and his team think that finding the gene that turns off development of the flagellum in the elephant fish could benefit humans, specifically those with a genetic disorder called primary ciliary dyskinesia, whose lack of normally functioning cilia and flagella leads to chronic respiratory infection, abnormally positioned organs, fluid on the brain, and infertility.
And that – with “that” being infertility – brings us to exhibit B, a 41-year-old Dutch man named Jonathan Meijer who clearly has too much time on his hands.
A court in the Netherlands recently ordered him, and not for the first time, to stop donating sperm to fertility clinics after it was discovered that he had fathered between 500 and 600 children around the world. He had been banned from donating to Dutch clinics in 2017, at which point he had already fathered 100 children, but managed a workaround by donating internationally and online, sometimes using another name.
The judge ordered Mr. Meijer to contact all of the clinics abroad and ask them to destroy any of his sperm they still had in stock and threatened to fine him over $100,000 for each future violation.
Okay, so here’s the thing. We have been, um, let’s call it ... warned, about the evils of tastelessness in journalism, so we’re going to do what Mr. Meijer should have done and abstain. And we can last for longer than 11 minutes.
The realm of lost luggage and lost sleep
It may be convenient to live near an airport if you’re a frequent flyer, but it really doesn’t help your sleep numbers.
The first look at how such a common sound affects sleep duration showed that people exposed to even 45 decibels of airplane noise were less likely to get the 7-9 hours of sleep needed for healthy functioning, investigators said in Environmental Health Perspectives.
How loud is 45 dB exactly? A normal conversation is about 50 dB, while a whisper is 30 dB, to give you an idea. Airplane noise at 45 dB? You might not even notice it amongst the other noises in daily life.
The researchers looked at data from about 35,000 participants in the Nurses’ Health Study who live around 90 major U.S. airports. They examined plane noise every 5 years between 1995 and 2005, focusing on estimates of nighttime and daytime levels. Short sleep was most common among the nurses who lived on the West Coast, near major cargo airports or large bodies of water, and also among those who reported no hearing loss.
The investigators noted, however, that there was no consistent association between airplane noise and quality of sleep and stopped short of making any policy recommendations. Still, sleep is a very important, yet slept-on (pun intended) factor for our overall health, so it’s good to know if anything has the potential to cause disruption.
Caution: Robotic uprisings in the rearview mirror are closer than they appear
ChatGPT. If you’ve been even in the proximity of the Internet lately, you may have heard of it. It’s quite an incredible piece of technology, an artificial intelligence that really could up-end a lot of industries. And lest doctors believe they’re safe from robotic replacement, consider this: ChatGPT took a test commonly used as a study resource by ophthalmologists and scored a 46%. Obviously, that’s not a passing grade. Job safe, right?
A month later, the researchers tried again. This time, ChatGPT got a 58%. Still not passing, and ChatGPT did especially poorly on ophthalmology specialty questions (it got 80% of general medicine questions right), but still, the jump in quality after just a month is ... concerning. It’s not like an AI will forget things. That score can only go up, and it’ll go up faster than you think.
“Sure, the robot is smart,” the doctors out there are thinking, “but how can an AI compete with human compassion, understanding, and bedside manner?”
And they’d be right. When it comes to bedside manner, there’s no competition between man and bot. ChatGPT is already winning.
In another study, researchers sampled nearly 200 questions from the subreddit r/AskDocs, which received verified physician responses. The researchers fed ChatGPT the questions – without the doctor’s answer – and a panel of health care professionals evaluated both the human doctor and ChatGPT in terms of quality and empathy.
Perhaps not surprisingly, the robot did better when it came to quality, providing a high-quality response 79% of the time, versus 22% for the human. But empathy? It was a bloodbath. ChatGPT provided an empathetic or very empathetic response 45% of the time, while humans could only do so 4.6% of the time. So much for bedside manner.
The researchers were suspiciously quick to note that ChatGPT isn’t a legitimate replacement for physicians, but could represent a tool to better provide care for patients. But let’s be honest, given ChatGPT’s quick advancement, how long before some intrepid stockholder says: “Hey, instead of paying doctors, why don’t we just use the free robot instead?” We give it a week. Or 11 minutes.
This week, on ‘As the sperm turns’
We’ve got a lot of spermy ground to cover, so let’s get right to it, starting with the small and working our way up.
We’re all pretty familiar with the basic structure of a sperm cell, yes? Bulbous head that contains all the important genetic information and a tail-like flagellum to propel it to its ultimate destination. Not much to work with there, you’d think, but what if Mother Nature, who clearly has a robust sense of humor, had something else in mind?
We present exhibit A, Paramormyorps kingsleyae, also known as the electric elephantfish, which happens to be the only known vertebrate species with tailless sperm. Sounds crazy to us, too, but Jason Gallant, PhD, of
Michigan State University, Lansing, has a theory: “A general notion in biology is that sperm are cheap, and eggs are expensive – but these fish may be telling us that sperm are more expensive than we might think. They could be saving energy by cutting back on sperm tails.”
He and his team think that finding the gene that turns off development of the flagellum in the elephant fish could benefit humans, specifically those with a genetic disorder called primary ciliary dyskinesia, whose lack of normally functioning cilia and flagella leads to chronic respiratory infection, abnormally positioned organs, fluid on the brain, and infertility.
And that – with “that” being infertility – brings us to exhibit B, a 41-year-old Dutch man named Jonathan Meijer who clearly has too much time on his hands.
A court in the Netherlands recently ordered him, and not for the first time, to stop donating sperm to fertility clinics after it was discovered that he had fathered between 500 and 600 children around the world. He had been banned from donating to Dutch clinics in 2017, at which point he had already fathered 100 children, but managed a workaround by donating internationally and online, sometimes using another name.
The judge ordered Mr. Meijer to contact all of the clinics abroad and ask them to destroy any of his sperm they still had in stock and threatened to fine him over $100,000 for each future violation.
Okay, so here’s the thing. We have been, um, let’s call it ... warned, about the evils of tastelessness in journalism, so we’re going to do what Mr. Meijer should have done and abstain. And we can last for longer than 11 minutes.
The realm of lost luggage and lost sleep
It may be convenient to live near an airport if you’re a frequent flyer, but it really doesn’t help your sleep numbers.
The first look at how such a common sound affects sleep duration showed that people exposed to even 45 decibels of airplane noise were less likely to get the 7-9 hours of sleep needed for healthy functioning, investigators said in Environmental Health Perspectives.
How loud is 45 dB exactly? A normal conversation is about 50 dB, while a whisper is 30 dB, to give you an idea. Airplane noise at 45 dB? You might not even notice it amongst the other noises in daily life.
The researchers looked at data from about 35,000 participants in the Nurses’ Health Study who live around 90 major U.S. airports. They examined plane noise every 5 years between 1995 and 2005, focusing on estimates of nighttime and daytime levels. Short sleep was most common among the nurses who lived on the West Coast, near major cargo airports or large bodies of water, and also among those who reported no hearing loss.
The investigators noted, however, that there was no consistent association between airplane noise and quality of sleep and stopped short of making any policy recommendations. Still, sleep is a very important, yet slept-on (pun intended) factor for our overall health, so it’s good to know if anything has the potential to cause disruption.
Surprising brain activity moments before death
This transcript has been edited for clarity.
Welcome to Impact Factor, your weekly dose of commentary on a new medical study. I’m Dr F. Perry Wilson of the Yale School of Medicine.
All the participants in the study I am going to tell you about this week died. And three of them died twice. But their deaths provide us with a fascinating window into the complex electrochemistry of the dying brain. What we might be looking at, indeed, is the physiologic correlate of the near-death experience.
The concept of the near-death experience is culturally ubiquitous. And though the content seems to track along culture lines – Western Christians are more likely to report seeing guardian angels, while Hindus are more likely to report seeing messengers of the god of death – certain factors seem to transcend culture: an out-of-body experience; a feeling of peace; and, of course, the light at the end of the tunnel.
As a materialist, I won’t discuss the possibility that these commonalities reflect some metaphysical structure to the afterlife. More likely, it seems to me, is that the commonalities result from the fact that the experience is mediated by our brains, and our brains, when dying, may be more alike than different.
We are talking about this study, appearing in the Proceedings of the National Academy of Sciences, by Jimo Borjigin and her team.
Dr. Borjigin studies the neural correlates of consciousness, perhaps one of the biggest questions in all of science today. To wit,
The study in question follows four unconscious patients –comatose patients, really – as life-sustaining support was withdrawn, up until the moment of death. Three had suffered severe anoxic brain injury in the setting of prolonged cardiac arrest. Though the heart was restarted, the brain damage was severe. The fourth had a large brain hemorrhage. All four patients were thus comatose and, though not brain-dead, unresponsive – with the lowest possible Glasgow Coma Scale score. No response to outside stimuli.
The families had made the decision to withdraw life support – to remove the breathing tube – but agreed to enroll their loved one in the study.
The team applied EEG leads to the head, EKG leads to the chest, and other monitoring equipment to observe the physiologic changes that occurred as the comatose and unresponsive patient died.
As the heart rhythm evolved from this:
To this:
And eventually stopped.
But this is a study about the brain, not the heart.
Prior to the withdrawal of life support, the brain electrical signals looked like this:
What you see is the EEG power at various frequencies, with red being higher. All the red was down at the low frequencies. Consciousness, at least as we understand it, is a higher-frequency phenomenon.
Right after the breathing tube was removed, the power didn’t change too much, but you can see some increased activity at the higher frequencies.
But in two of the four patients, something really surprising happened. Watch what happens as the brain gets closer and closer to death.
Here, about 300 seconds before death, there was a power surge at the high gamma frequencies.
This spike in power occurred in the somatosensory cortex and the dorsolateral prefrontal cortex, areas that are associated with conscious experience. It seems that this patient, 5 minutes before death, was experiencing something.
But I know what you’re thinking. This is a brain that is not receiving oxygen. Cells are going to become disordered quickly and start firing randomly – a last gasp, so to speak, before the end. Meaningless noise.
But connectivity mapping tells a different story. The signals seem to have structure.
Those high-frequency power surges increased connectivity in the posterior cortical “hot zone,” an area of the brain many researchers feel is necessary for conscious perception. This figure is not a map of raw brain electrical output like the one I showed before, but of coherence between brain regions in the consciousness hot zone. Those red areas indicate cross-talk – not the disordered scream of dying neurons, but a last set of messages passing back and forth from the parietal and posterior temporal lobes.
In fact, the electrical patterns of the brains in these patients looked very similar to the patterns seen in dreaming humans, as well as in patients with epilepsy who report sensations of out-of-body experiences.
It’s critical to realize two things here. First, these signals of consciousness were not present before life support was withdrawn. These comatose patients had minimal brain activity; there was no evidence that they were experiencing anything before the process of dying began. These brains are behaving fundamentally differently near death.
But second, we must realize that, although the brains of these individuals, in their last moments, appeared to be acting in a way that conscious brains act, we have no way of knowing if the patients were truly having a conscious experience. As I said, all the patients in the study died. Short of those metaphysics I alluded to earlier, we will have no way to ask them how they experienced their final moments.
Let’s be clear: This study doesn’t answer the question of what happens when we die. It says nothing about life after death or the existence or persistence of the soul. But what it does do is shed light on an incredibly difficult problem in neuroscience: the problem of consciousness. And as studies like this move forward, we may discover that the root of consciousness comes not from the breath of God or the energy of a living universe, but from very specific parts of the very complicated machine that is the brain, acting together to produce something transcendent. And to me, that is no less sublime.
Dr. Wilson is an associate professor of medicine and director of Yale’s Clinical and Translational Research Accelerator, Yale University, New Haven, Conn. His science communication work can be found in the Huffington Post, on NPR, and on Medscape. He tweets @fperrywilson and his new book, How Medicine Works and When It Doesn’t, is available now. Dr. Wilson has disclosed no relevant financial relationships.
This transcript has been edited for clarity.
Welcome to Impact Factor, your weekly dose of commentary on a new medical study. I’m Dr F. Perry Wilson of the Yale School of Medicine.
All the participants in the study I am going to tell you about this week died. And three of them died twice. But their deaths provide us with a fascinating window into the complex electrochemistry of the dying brain. What we might be looking at, indeed, is the physiologic correlate of the near-death experience.
The concept of the near-death experience is culturally ubiquitous. And though the content seems to track along culture lines – Western Christians are more likely to report seeing guardian angels, while Hindus are more likely to report seeing messengers of the god of death – certain factors seem to transcend culture: an out-of-body experience; a feeling of peace; and, of course, the light at the end of the tunnel.
As a materialist, I won’t discuss the possibility that these commonalities reflect some metaphysical structure to the afterlife. More likely, it seems to me, is that the commonalities result from the fact that the experience is mediated by our brains, and our brains, when dying, may be more alike than different.
We are talking about this study, appearing in the Proceedings of the National Academy of Sciences, by Jimo Borjigin and her team.
Dr. Borjigin studies the neural correlates of consciousness, perhaps one of the biggest questions in all of science today. To wit,
The study in question follows four unconscious patients –comatose patients, really – as life-sustaining support was withdrawn, up until the moment of death. Three had suffered severe anoxic brain injury in the setting of prolonged cardiac arrest. Though the heart was restarted, the brain damage was severe. The fourth had a large brain hemorrhage. All four patients were thus comatose and, though not brain-dead, unresponsive – with the lowest possible Glasgow Coma Scale score. No response to outside stimuli.
The families had made the decision to withdraw life support – to remove the breathing tube – but agreed to enroll their loved one in the study.
The team applied EEG leads to the head, EKG leads to the chest, and other monitoring equipment to observe the physiologic changes that occurred as the comatose and unresponsive patient died.
As the heart rhythm evolved from this:
To this:
And eventually stopped.
But this is a study about the brain, not the heart.
Prior to the withdrawal of life support, the brain electrical signals looked like this:
What you see is the EEG power at various frequencies, with red being higher. All the red was down at the low frequencies. Consciousness, at least as we understand it, is a higher-frequency phenomenon.
Right after the breathing tube was removed, the power didn’t change too much, but you can see some increased activity at the higher frequencies.
But in two of the four patients, something really surprising happened. Watch what happens as the brain gets closer and closer to death.
Here, about 300 seconds before death, there was a power surge at the high gamma frequencies.
This spike in power occurred in the somatosensory cortex and the dorsolateral prefrontal cortex, areas that are associated with conscious experience. It seems that this patient, 5 minutes before death, was experiencing something.
But I know what you’re thinking. This is a brain that is not receiving oxygen. Cells are going to become disordered quickly and start firing randomly – a last gasp, so to speak, before the end. Meaningless noise.
But connectivity mapping tells a different story. The signals seem to have structure.
Those high-frequency power surges increased connectivity in the posterior cortical “hot zone,” an area of the brain many researchers feel is necessary for conscious perception. This figure is not a map of raw brain electrical output like the one I showed before, but of coherence between brain regions in the consciousness hot zone. Those red areas indicate cross-talk – not the disordered scream of dying neurons, but a last set of messages passing back and forth from the parietal and posterior temporal lobes.
In fact, the electrical patterns of the brains in these patients looked very similar to the patterns seen in dreaming humans, as well as in patients with epilepsy who report sensations of out-of-body experiences.
It’s critical to realize two things here. First, these signals of consciousness were not present before life support was withdrawn. These comatose patients had minimal brain activity; there was no evidence that they were experiencing anything before the process of dying began. These brains are behaving fundamentally differently near death.
But second, we must realize that, although the brains of these individuals, in their last moments, appeared to be acting in a way that conscious brains act, we have no way of knowing if the patients were truly having a conscious experience. As I said, all the patients in the study died. Short of those metaphysics I alluded to earlier, we will have no way to ask them how they experienced their final moments.
Let’s be clear: This study doesn’t answer the question of what happens when we die. It says nothing about life after death or the existence or persistence of the soul. But what it does do is shed light on an incredibly difficult problem in neuroscience: the problem of consciousness. And as studies like this move forward, we may discover that the root of consciousness comes not from the breath of God or the energy of a living universe, but from very specific parts of the very complicated machine that is the brain, acting together to produce something transcendent. And to me, that is no less sublime.
Dr. Wilson is an associate professor of medicine and director of Yale’s Clinical and Translational Research Accelerator, Yale University, New Haven, Conn. His science communication work can be found in the Huffington Post, on NPR, and on Medscape. He tweets @fperrywilson and his new book, How Medicine Works and When It Doesn’t, is available now. Dr. Wilson has disclosed no relevant financial relationships.
This transcript has been edited for clarity.
Welcome to Impact Factor, your weekly dose of commentary on a new medical study. I’m Dr F. Perry Wilson of the Yale School of Medicine.
All the participants in the study I am going to tell you about this week died. And three of them died twice. But their deaths provide us with a fascinating window into the complex electrochemistry of the dying brain. What we might be looking at, indeed, is the physiologic correlate of the near-death experience.
The concept of the near-death experience is culturally ubiquitous. And though the content seems to track along culture lines – Western Christians are more likely to report seeing guardian angels, while Hindus are more likely to report seeing messengers of the god of death – certain factors seem to transcend culture: an out-of-body experience; a feeling of peace; and, of course, the light at the end of the tunnel.
As a materialist, I won’t discuss the possibility that these commonalities reflect some metaphysical structure to the afterlife. More likely, it seems to me, is that the commonalities result from the fact that the experience is mediated by our brains, and our brains, when dying, may be more alike than different.
We are talking about this study, appearing in the Proceedings of the National Academy of Sciences, by Jimo Borjigin and her team.
Dr. Borjigin studies the neural correlates of consciousness, perhaps one of the biggest questions in all of science today. To wit,
The study in question follows four unconscious patients –comatose patients, really – as life-sustaining support was withdrawn, up until the moment of death. Three had suffered severe anoxic brain injury in the setting of prolonged cardiac arrest. Though the heart was restarted, the brain damage was severe. The fourth had a large brain hemorrhage. All four patients were thus comatose and, though not brain-dead, unresponsive – with the lowest possible Glasgow Coma Scale score. No response to outside stimuli.
The families had made the decision to withdraw life support – to remove the breathing tube – but agreed to enroll their loved one in the study.
The team applied EEG leads to the head, EKG leads to the chest, and other monitoring equipment to observe the physiologic changes that occurred as the comatose and unresponsive patient died.
As the heart rhythm evolved from this:
To this:
And eventually stopped.
But this is a study about the brain, not the heart.
Prior to the withdrawal of life support, the brain electrical signals looked like this:
What you see is the EEG power at various frequencies, with red being higher. All the red was down at the low frequencies. Consciousness, at least as we understand it, is a higher-frequency phenomenon.
Right after the breathing tube was removed, the power didn’t change too much, but you can see some increased activity at the higher frequencies.
But in two of the four patients, something really surprising happened. Watch what happens as the brain gets closer and closer to death.
Here, about 300 seconds before death, there was a power surge at the high gamma frequencies.
This spike in power occurred in the somatosensory cortex and the dorsolateral prefrontal cortex, areas that are associated with conscious experience. It seems that this patient, 5 minutes before death, was experiencing something.
But I know what you’re thinking. This is a brain that is not receiving oxygen. Cells are going to become disordered quickly and start firing randomly – a last gasp, so to speak, before the end. Meaningless noise.
But connectivity mapping tells a different story. The signals seem to have structure.
Those high-frequency power surges increased connectivity in the posterior cortical “hot zone,” an area of the brain many researchers feel is necessary for conscious perception. This figure is not a map of raw brain electrical output like the one I showed before, but of coherence between brain regions in the consciousness hot zone. Those red areas indicate cross-talk – not the disordered scream of dying neurons, but a last set of messages passing back and forth from the parietal and posterior temporal lobes.
In fact, the electrical patterns of the brains in these patients looked very similar to the patterns seen in dreaming humans, as well as in patients with epilepsy who report sensations of out-of-body experiences.
It’s critical to realize two things here. First, these signals of consciousness were not present before life support was withdrawn. These comatose patients had minimal brain activity; there was no evidence that they were experiencing anything before the process of dying began. These brains are behaving fundamentally differently near death.
But second, we must realize that, although the brains of these individuals, in their last moments, appeared to be acting in a way that conscious brains act, we have no way of knowing if the patients were truly having a conscious experience. As I said, all the patients in the study died. Short of those metaphysics I alluded to earlier, we will have no way to ask them how they experienced their final moments.
Let’s be clear: This study doesn’t answer the question of what happens when we die. It says nothing about life after death or the existence or persistence of the soul. But what it does do is shed light on an incredibly difficult problem in neuroscience: the problem of consciousness. And as studies like this move forward, we may discover that the root of consciousness comes not from the breath of God or the energy of a living universe, but from very specific parts of the very complicated machine that is the brain, acting together to produce something transcendent. And to me, that is no less sublime.
Dr. Wilson is an associate professor of medicine and director of Yale’s Clinical and Translational Research Accelerator, Yale University, New Haven, Conn. His science communication work can be found in the Huffington Post, on NPR, and on Medscape. He tweets @fperrywilson and his new book, How Medicine Works and When It Doesn’t, is available now. Dr. Wilson has disclosed no relevant financial relationships.
AHA backs screening for cognitive impairment after stroke
Screening for cognitive impairment should be part of multidisciplinary care for stroke survivors, the American Heart Association says in a new scientific statement.
“Cognitive impairment after stroke is very common, is associated with other post-stroke outcomes, and often has significant impact on the quality of life,” Nada El Husseini, MD, MHSc, chair of the scientific statement writing group, told this news organization.
“It is important to screen stroke survivors for cognitive impairment as well as for associated comorbidities such as mood and sleep disorders,” said Dr. El Husseini, associate professor of neurology at Duke University Medical Center in Durham, N.C.
The scientific statement was published online in Stroke. It’s the first to specifically focus on the cognitive impairment resulting from an overt stroke (ischemic or hemorrhagic).
‘Actionable’ considerations for care
The writing group performed a “scoping” review of the literature on the prevalence, diagnosis, and management of poststroke cognitive impairment (PSCI) to provide a framework for “actionable considerations” for clinical practice as well as to highlight gaps needing additional studies, Dr. El Husseini explained.
PSCI, ranging from mild to severe, occurs in up to 60% of stroke survivors in the first year after stroke; yet, it is often underreported and underdiagnosed, the writing group notes.
Up to 20% of stroke survivors who experience mild cognitive impairment fully recover cognitive function, and cognitive recovery is most likely within the first 6 months after a stroke.
However, improvement in cognitive impairment without return to prestroke levels is more frequent than is complete recovery. As many as one in three stroke survivors may develop dementia within 5 years of stroke.
The writing group also notes that PSCI is often associated with other conditions, including physical disability, sleep disorders, behavioral and personality changes, depression, and other neuropsychological changes – each of which may contribute to lower quality of life.
Currently, there is no “gold standard” for cognitive screening following stroke, but several brief cognitive screening tests, including the Mini–Mental State Examination and the Montreal Cognitive Assessment, are widely used to identify cognitive impairment after stroke.
The statement also highlights the importance of assessing cognitive changes over time after stroke. Stroke survivors who experience unexplained difficulties with cognitive-related activities of daily living, following care instructions, or providing a reliable health history may be candidates for additional cognitive screening.
Manage risk factors to prevent repeat stroke
“Anticipatory guidance regarding home and driving safety and, return to work (if applicable) along with interdisciplinary collaboration among different medical and ancillary specialists in the diagnosis and management of cognitive impairment is key for the holistic care of stroke survivors,” Dr. El Husseini told this news organization.
The multidisciplinary poststroke health care team could include neurologists, occupational therapists, speech therapists, nurses, neuropsychologists, gerontologists, and primary care providers.
“Because recurrent stroke is strongly associated with the development of cognitive impairment and dementia, prevention of recurrent strokes should be sought to decrease that risk,” Dr. El Husseini said. This includes addressing stroke risk factors, including high blood pressure, high cholesterol, type 2 diabetes, and atrial fibrillation.
The writing group says research is needed in the future to determine how cognitive impairment develops after stroke and the impact of nonbrain factors, including infection, frailty, and social factors.
Further research is also needed to determine best practices for cognitive screening after stroke, including the development and use of screening instruments that consider demographic, cultural, and linguistic factors in determining “normal” function.
“Perhaps the most pressing need, however, is the development of effective and culturally relevant treatments for poststroke cognitive impairment,” Dr. El Husseini said in a news release.
“We hope to see big enough clinical trials that assess various techniques, medications, and lifestyle changes in diverse groups of patients that may help improve cognitive function,” she added.
This scientific statement was prepared by the volunteer writing group on behalf of the AHA Stroke Council, the Council on Cardiovascular Radiology and Intervention, the Council on Hypertension, and the Council on Lifestyle and Cardiometabolic Health.
Screening for cognitive impairment should be part of multidisciplinary care for stroke survivors, the American Heart Association says in a new scientific statement.
“Cognitive impairment after stroke is very common, is associated with other post-stroke outcomes, and often has significant impact on the quality of life,” Nada El Husseini, MD, MHSc, chair of the scientific statement writing group, told this news organization.
“It is important to screen stroke survivors for cognitive impairment as well as for associated comorbidities such as mood and sleep disorders,” said Dr. El Husseini, associate professor of neurology at Duke University Medical Center in Durham, N.C.
The scientific statement was published online in Stroke. It’s the first to specifically focus on the cognitive impairment resulting from an overt stroke (ischemic or hemorrhagic).
‘Actionable’ considerations for care
The writing group performed a “scoping” review of the literature on the prevalence, diagnosis, and management of poststroke cognitive impairment (PSCI) to provide a framework for “actionable considerations” for clinical practice as well as to highlight gaps needing additional studies, Dr. El Husseini explained.
PSCI, ranging from mild to severe, occurs in up to 60% of stroke survivors in the first year after stroke; yet, it is often underreported and underdiagnosed, the writing group notes.
Up to 20% of stroke survivors who experience mild cognitive impairment fully recover cognitive function, and cognitive recovery is most likely within the first 6 months after a stroke.
However, improvement in cognitive impairment without return to prestroke levels is more frequent than is complete recovery. As many as one in three stroke survivors may develop dementia within 5 years of stroke.
The writing group also notes that PSCI is often associated with other conditions, including physical disability, sleep disorders, behavioral and personality changes, depression, and other neuropsychological changes – each of which may contribute to lower quality of life.
Currently, there is no “gold standard” for cognitive screening following stroke, but several brief cognitive screening tests, including the Mini–Mental State Examination and the Montreal Cognitive Assessment, are widely used to identify cognitive impairment after stroke.
The statement also highlights the importance of assessing cognitive changes over time after stroke. Stroke survivors who experience unexplained difficulties with cognitive-related activities of daily living, following care instructions, or providing a reliable health history may be candidates for additional cognitive screening.
Manage risk factors to prevent repeat stroke
“Anticipatory guidance regarding home and driving safety and, return to work (if applicable) along with interdisciplinary collaboration among different medical and ancillary specialists in the diagnosis and management of cognitive impairment is key for the holistic care of stroke survivors,” Dr. El Husseini told this news organization.
The multidisciplinary poststroke health care team could include neurologists, occupational therapists, speech therapists, nurses, neuropsychologists, gerontologists, and primary care providers.
“Because recurrent stroke is strongly associated with the development of cognitive impairment and dementia, prevention of recurrent strokes should be sought to decrease that risk,” Dr. El Husseini said. This includes addressing stroke risk factors, including high blood pressure, high cholesterol, type 2 diabetes, and atrial fibrillation.
The writing group says research is needed in the future to determine how cognitive impairment develops after stroke and the impact of nonbrain factors, including infection, frailty, and social factors.
Further research is also needed to determine best practices for cognitive screening after stroke, including the development and use of screening instruments that consider demographic, cultural, and linguistic factors in determining “normal” function.
“Perhaps the most pressing need, however, is the development of effective and culturally relevant treatments for poststroke cognitive impairment,” Dr. El Husseini said in a news release.
“We hope to see big enough clinical trials that assess various techniques, medications, and lifestyle changes in diverse groups of patients that may help improve cognitive function,” she added.
This scientific statement was prepared by the volunteer writing group on behalf of the AHA Stroke Council, the Council on Cardiovascular Radiology and Intervention, the Council on Hypertension, and the Council on Lifestyle and Cardiometabolic Health.
Screening for cognitive impairment should be part of multidisciplinary care for stroke survivors, the American Heart Association says in a new scientific statement.
“Cognitive impairment after stroke is very common, is associated with other post-stroke outcomes, and often has significant impact on the quality of life,” Nada El Husseini, MD, MHSc, chair of the scientific statement writing group, told this news organization.
“It is important to screen stroke survivors for cognitive impairment as well as for associated comorbidities such as mood and sleep disorders,” said Dr. El Husseini, associate professor of neurology at Duke University Medical Center in Durham, N.C.
The scientific statement was published online in Stroke. It’s the first to specifically focus on the cognitive impairment resulting from an overt stroke (ischemic or hemorrhagic).
‘Actionable’ considerations for care
The writing group performed a “scoping” review of the literature on the prevalence, diagnosis, and management of poststroke cognitive impairment (PSCI) to provide a framework for “actionable considerations” for clinical practice as well as to highlight gaps needing additional studies, Dr. El Husseini explained.
PSCI, ranging from mild to severe, occurs in up to 60% of stroke survivors in the first year after stroke; yet, it is often underreported and underdiagnosed, the writing group notes.
Up to 20% of stroke survivors who experience mild cognitive impairment fully recover cognitive function, and cognitive recovery is most likely within the first 6 months after a stroke.
However, improvement in cognitive impairment without return to prestroke levels is more frequent than is complete recovery. As many as one in three stroke survivors may develop dementia within 5 years of stroke.
The writing group also notes that PSCI is often associated with other conditions, including physical disability, sleep disorders, behavioral and personality changes, depression, and other neuropsychological changes – each of which may contribute to lower quality of life.
Currently, there is no “gold standard” for cognitive screening following stroke, but several brief cognitive screening tests, including the Mini–Mental State Examination and the Montreal Cognitive Assessment, are widely used to identify cognitive impairment after stroke.
The statement also highlights the importance of assessing cognitive changes over time after stroke. Stroke survivors who experience unexplained difficulties with cognitive-related activities of daily living, following care instructions, or providing a reliable health history may be candidates for additional cognitive screening.
Manage risk factors to prevent repeat stroke
“Anticipatory guidance regarding home and driving safety and, return to work (if applicable) along with interdisciplinary collaboration among different medical and ancillary specialists in the diagnosis and management of cognitive impairment is key for the holistic care of stroke survivors,” Dr. El Husseini told this news organization.
The multidisciplinary poststroke health care team could include neurologists, occupational therapists, speech therapists, nurses, neuropsychologists, gerontologists, and primary care providers.
“Because recurrent stroke is strongly associated with the development of cognitive impairment and dementia, prevention of recurrent strokes should be sought to decrease that risk,” Dr. El Husseini said. This includes addressing stroke risk factors, including high blood pressure, high cholesterol, type 2 diabetes, and atrial fibrillation.
The writing group says research is needed in the future to determine how cognitive impairment develops after stroke and the impact of nonbrain factors, including infection, frailty, and social factors.
Further research is also needed to determine best practices for cognitive screening after stroke, including the development and use of screening instruments that consider demographic, cultural, and linguistic factors in determining “normal” function.
“Perhaps the most pressing need, however, is the development of effective and culturally relevant treatments for poststroke cognitive impairment,” Dr. El Husseini said in a news release.
“We hope to see big enough clinical trials that assess various techniques, medications, and lifestyle changes in diverse groups of patients that may help improve cognitive function,” she added.
This scientific statement was prepared by the volunteer writing group on behalf of the AHA Stroke Council, the Council on Cardiovascular Radiology and Intervention, the Council on Hypertension, and the Council on Lifestyle and Cardiometabolic Health.