User login
TNF blockers not associated with poorer pregnancy outcomes
SAN DIEGO – Continuing a tumor necrosis factor inhibitor (TNFi) during pregnancy does not increase risk of worse fetal or obstetric outcomes, according to new research presented at the annual meeting of the American College of Rheumatology.
Patients who continued a TNFi also had fewer severe infections requiring hospitalization, compared with those who stopped taking the medication during their pregnancy.
“The main message is that patients continuing were not doing worse than the patients stopping. It’s an important clinical message for rheumatologists who are not really confident in dealing with these drugs during pregnancy,” said Anna Moltó, MD, PhD, a rheumatologist at Cochin Hospital, Paris, who led the research. “It adds to the data that it seems to be safe,” she added in an interview.
Previous research, largely from pregnant patients with inflammatory bowel disease, suggests that taking a TNFi during pregnancy is safe, and 2020 ACR guidelines conditionally recommend continuing therapy prior to and during pregnancy; however, many people still stop taking the drugs during pregnancy for fear of potentially harming the fetus.
To better understand how TNFi use affected pregnancy outcomes, Dr. Moltó and colleagues analyzed data from a French nationwide health insurance database to identify adult women with chronic rheumatic inflammatory disease. All women included in the cohort had a singleton pregnancy between 2008 and 2017 and were taking a TNFi upon pregnancy diagnosis.
Patients who restarted TNFi after initially pausing because of pregnancy were included in the continuation group.
Researchers identified more than 2,000 pregnancies, including 1,503 in individuals with spondyloarthritis and 579 individuals with rheumatoid arthritis. Patients were, on average, 31 years old and were diagnosed with a rheumatic disease 4 years prior to their pregnancy.
About 72% (n = 1,497) discontinued TNFi after learning they were pregnant, and 584 individuals continued treatment. Dr. Moltó noted that data from more recent years might have captured lower discontinuation rates among pregnant individuals, but those data were not available for the study.
There was no difference in unfavorable obstetrical or infant outcomes, including spontaneous abortion, preeclampsia, gestational diabetes, major congenital malformation, and severe infection of the infant requiring hospitalization. Somewhat surprisingly, the data showed that women who discontinued a TNFi were more likely to be hospitalized for infection either during their pregnancy or up to 6 weeks after delivery, compared with those who continued therapy (1.3% vs. 0.2%, respectively).
Dr. Moltó is currently looking into what could be behind this counterintuitive result, but she hypothesizes that patients who had stopped TNFi may have been taking more glucocorticoids.
“At our institution, there is generally a comfort level with continuing TNF inhibitors during pregnancy, at least until about 36 weeks,” said Sara K. Tedeschi, MD, MPH, a rheumatologist at Brigham and Women’s Hospital and assistant professor of medicine at Harvard Medical School, both in Boston. Sometimes, there is concern for risk of infection to the infant, depending on the type of TNFi being used, she added during a press conference.
“I think that these are really informative and supportive data to let women know that they probably have a really good chance of doing very well during the pregnancy if they continue” their TNFi, said Dr. Tedeschi, who was not involved with the study.
TNF discontinuation on the decline
In a related study, researchers at McGill University, Montreal, found that TNFi discontinuation prior to pregnancy had decreased over time in individuals with chronic inflammatory diseases.
Using a database of U.S. insurance claims, they identified 3,372 women with RA, ankylosing spondylitis (AS), psoriasis/psoriatic arthritis (PsA), and/or inflammatory bowel disease (IBD) who previously used a TNFi and gave birth between 2011 and 2019. A patient was considered to have used a TNFi if she had filled a prescription or had an infusion procedure insurance claim within 12 weeks before the gestational period or anytime during pregnancy. Researchers did not have time-specific data to account for women who stopped treatment at pregnancy diagnosis.
Nearly half (47%) of all identified pregnancies were in individuals with IBD, and the rest included patients with RA (24%), psoriasis or PsA (16%), AS (3%), or more than one diagnosis (10%).
In total, 14% of women discontinued TNFi use in the 12 weeks before becoming pregnant and did not restart. From 2011 to 2013, 19% of patients stopped their TNFi, but this proportion decreased overtime, with 10% of patients stopping therapy from 2017 to 2019 (P < .0001).
This decline “possibly reflects the increase in real-world evidence about the safety of TNFi in pregnancy. That research, in turn, led to new guidelines recommending the continuation of TNFi during pregnancy,” first author Leah Flatman, a PhD candidate in epidemiology at McGill, said in an interview. “I think we can see this potentially as good news.”
More patients with RA, psoriasis/PsA, and AS discontinued TNFi therapy prior to conception (23%-25%), compared with those with IBD (5%).
Ms. Flatman noted that her study and Moltó’s study complement each other by providing data on individuals stopping TNFi prior to conception versus those stopping treatment after pregnancy diagnosis.
“These findings demonstrate that continuing TNFi during pregnancy appears not to be associated with an increase in adverse obstetrical or infant outcomes,” Ms. Flatman said of Dr. Moltó’s study. “As guidelines currently recommend continuing TNFi, studies like this help demonstrate that the guideline changes do not appear to be associated with an increase in adverse events.”
Dr. Moltó and Ms. Flatman disclosed no relevant financial relationships. Dr. Tedeschi has worked as a consultant for Novartis.
A version of this article appeared on Medscape.com.
SAN DIEGO – Continuing a tumor necrosis factor inhibitor (TNFi) during pregnancy does not increase risk of worse fetal or obstetric outcomes, according to new research presented at the annual meeting of the American College of Rheumatology.
Patients who continued a TNFi also had fewer severe infections requiring hospitalization, compared with those who stopped taking the medication during their pregnancy.
“The main message is that patients continuing were not doing worse than the patients stopping. It’s an important clinical message for rheumatologists who are not really confident in dealing with these drugs during pregnancy,” said Anna Moltó, MD, PhD, a rheumatologist at Cochin Hospital, Paris, who led the research. “It adds to the data that it seems to be safe,” she added in an interview.
Previous research, largely from pregnant patients with inflammatory bowel disease, suggests that taking a TNFi during pregnancy is safe, and 2020 ACR guidelines conditionally recommend continuing therapy prior to and during pregnancy; however, many people still stop taking the drugs during pregnancy for fear of potentially harming the fetus.
To better understand how TNFi use affected pregnancy outcomes, Dr. Moltó and colleagues analyzed data from a French nationwide health insurance database to identify adult women with chronic rheumatic inflammatory disease. All women included in the cohort had a singleton pregnancy between 2008 and 2017 and were taking a TNFi upon pregnancy diagnosis.
Patients who restarted TNFi after initially pausing because of pregnancy were included in the continuation group.
Researchers identified more than 2,000 pregnancies, including 1,503 in individuals with spondyloarthritis and 579 individuals with rheumatoid arthritis. Patients were, on average, 31 years old and were diagnosed with a rheumatic disease 4 years prior to their pregnancy.
About 72% (n = 1,497) discontinued TNFi after learning they were pregnant, and 584 individuals continued treatment. Dr. Moltó noted that data from more recent years might have captured lower discontinuation rates among pregnant individuals, but those data were not available for the study.
There was no difference in unfavorable obstetrical or infant outcomes, including spontaneous abortion, preeclampsia, gestational diabetes, major congenital malformation, and severe infection of the infant requiring hospitalization. Somewhat surprisingly, the data showed that women who discontinued a TNFi were more likely to be hospitalized for infection either during their pregnancy or up to 6 weeks after delivery, compared with those who continued therapy (1.3% vs. 0.2%, respectively).
Dr. Moltó is currently looking into what could be behind this counterintuitive result, but she hypothesizes that patients who had stopped TNFi may have been taking more glucocorticoids.
“At our institution, there is generally a comfort level with continuing TNF inhibitors during pregnancy, at least until about 36 weeks,” said Sara K. Tedeschi, MD, MPH, a rheumatologist at Brigham and Women’s Hospital and assistant professor of medicine at Harvard Medical School, both in Boston. Sometimes, there is concern for risk of infection to the infant, depending on the type of TNFi being used, she added during a press conference.
“I think that these are really informative and supportive data to let women know that they probably have a really good chance of doing very well during the pregnancy if they continue” their TNFi, said Dr. Tedeschi, who was not involved with the study.
TNF discontinuation on the decline
In a related study, researchers at McGill University, Montreal, found that TNFi discontinuation prior to pregnancy had decreased over time in individuals with chronic inflammatory diseases.
Using a database of U.S. insurance claims, they identified 3,372 women with RA, ankylosing spondylitis (AS), psoriasis/psoriatic arthritis (PsA), and/or inflammatory bowel disease (IBD) who previously used a TNFi and gave birth between 2011 and 2019. A patient was considered to have used a TNFi if she had filled a prescription or had an infusion procedure insurance claim within 12 weeks before the gestational period or anytime during pregnancy. Researchers did not have time-specific data to account for women who stopped treatment at pregnancy diagnosis.
Nearly half (47%) of all identified pregnancies were in individuals with IBD, and the rest included patients with RA (24%), psoriasis or PsA (16%), AS (3%), or more than one diagnosis (10%).
In total, 14% of women discontinued TNFi use in the 12 weeks before becoming pregnant and did not restart. From 2011 to 2013, 19% of patients stopped their TNFi, but this proportion decreased overtime, with 10% of patients stopping therapy from 2017 to 2019 (P < .0001).
This decline “possibly reflects the increase in real-world evidence about the safety of TNFi in pregnancy. That research, in turn, led to new guidelines recommending the continuation of TNFi during pregnancy,” first author Leah Flatman, a PhD candidate in epidemiology at McGill, said in an interview. “I think we can see this potentially as good news.”
More patients with RA, psoriasis/PsA, and AS discontinued TNFi therapy prior to conception (23%-25%), compared with those with IBD (5%).
Ms. Flatman noted that her study and Moltó’s study complement each other by providing data on individuals stopping TNFi prior to conception versus those stopping treatment after pregnancy diagnosis.
“These findings demonstrate that continuing TNFi during pregnancy appears not to be associated with an increase in adverse obstetrical or infant outcomes,” Ms. Flatman said of Dr. Moltó’s study. “As guidelines currently recommend continuing TNFi, studies like this help demonstrate that the guideline changes do not appear to be associated with an increase in adverse events.”
Dr. Moltó and Ms. Flatman disclosed no relevant financial relationships. Dr. Tedeschi has worked as a consultant for Novartis.
A version of this article appeared on Medscape.com.
SAN DIEGO – Continuing a tumor necrosis factor inhibitor (TNFi) during pregnancy does not increase risk of worse fetal or obstetric outcomes, according to new research presented at the annual meeting of the American College of Rheumatology.
Patients who continued a TNFi also had fewer severe infections requiring hospitalization, compared with those who stopped taking the medication during their pregnancy.
“The main message is that patients continuing were not doing worse than the patients stopping. It’s an important clinical message for rheumatologists who are not really confident in dealing with these drugs during pregnancy,” said Anna Moltó, MD, PhD, a rheumatologist at Cochin Hospital, Paris, who led the research. “It adds to the data that it seems to be safe,” she added in an interview.
Previous research, largely from pregnant patients with inflammatory bowel disease, suggests that taking a TNFi during pregnancy is safe, and 2020 ACR guidelines conditionally recommend continuing therapy prior to and during pregnancy; however, many people still stop taking the drugs during pregnancy for fear of potentially harming the fetus.
To better understand how TNFi use affected pregnancy outcomes, Dr. Moltó and colleagues analyzed data from a French nationwide health insurance database to identify adult women with chronic rheumatic inflammatory disease. All women included in the cohort had a singleton pregnancy between 2008 and 2017 and were taking a TNFi upon pregnancy diagnosis.
Patients who restarted TNFi after initially pausing because of pregnancy were included in the continuation group.
Researchers identified more than 2,000 pregnancies, including 1,503 in individuals with spondyloarthritis and 579 individuals with rheumatoid arthritis. Patients were, on average, 31 years old and were diagnosed with a rheumatic disease 4 years prior to their pregnancy.
About 72% (n = 1,497) discontinued TNFi after learning they were pregnant, and 584 individuals continued treatment. Dr. Moltó noted that data from more recent years might have captured lower discontinuation rates among pregnant individuals, but those data were not available for the study.
There was no difference in unfavorable obstetrical or infant outcomes, including spontaneous abortion, preeclampsia, gestational diabetes, major congenital malformation, and severe infection of the infant requiring hospitalization. Somewhat surprisingly, the data showed that women who discontinued a TNFi were more likely to be hospitalized for infection either during their pregnancy or up to 6 weeks after delivery, compared with those who continued therapy (1.3% vs. 0.2%, respectively).
Dr. Moltó is currently looking into what could be behind this counterintuitive result, but she hypothesizes that patients who had stopped TNFi may have been taking more glucocorticoids.
“At our institution, there is generally a comfort level with continuing TNF inhibitors during pregnancy, at least until about 36 weeks,” said Sara K. Tedeschi, MD, MPH, a rheumatologist at Brigham and Women’s Hospital and assistant professor of medicine at Harvard Medical School, both in Boston. Sometimes, there is concern for risk of infection to the infant, depending on the type of TNFi being used, she added during a press conference.
“I think that these are really informative and supportive data to let women know that they probably have a really good chance of doing very well during the pregnancy if they continue” their TNFi, said Dr. Tedeschi, who was not involved with the study.
TNF discontinuation on the decline
In a related study, researchers at McGill University, Montreal, found that TNFi discontinuation prior to pregnancy had decreased over time in individuals with chronic inflammatory diseases.
Using a database of U.S. insurance claims, they identified 3,372 women with RA, ankylosing spondylitis (AS), psoriasis/psoriatic arthritis (PsA), and/or inflammatory bowel disease (IBD) who previously used a TNFi and gave birth between 2011 and 2019. A patient was considered to have used a TNFi if she had filled a prescription or had an infusion procedure insurance claim within 12 weeks before the gestational period or anytime during pregnancy. Researchers did not have time-specific data to account for women who stopped treatment at pregnancy diagnosis.
Nearly half (47%) of all identified pregnancies were in individuals with IBD, and the rest included patients with RA (24%), psoriasis or PsA (16%), AS (3%), or more than one diagnosis (10%).
In total, 14% of women discontinued TNFi use in the 12 weeks before becoming pregnant and did not restart. From 2011 to 2013, 19% of patients stopped their TNFi, but this proportion decreased overtime, with 10% of patients stopping therapy from 2017 to 2019 (P < .0001).
This decline “possibly reflects the increase in real-world evidence about the safety of TNFi in pregnancy. That research, in turn, led to new guidelines recommending the continuation of TNFi during pregnancy,” first author Leah Flatman, a PhD candidate in epidemiology at McGill, said in an interview. “I think we can see this potentially as good news.”
More patients with RA, psoriasis/PsA, and AS discontinued TNFi therapy prior to conception (23%-25%), compared with those with IBD (5%).
Ms. Flatman noted that her study and Moltó’s study complement each other by providing data on individuals stopping TNFi prior to conception versus those stopping treatment after pregnancy diagnosis.
“These findings demonstrate that continuing TNFi during pregnancy appears not to be associated with an increase in adverse obstetrical or infant outcomes,” Ms. Flatman said of Dr. Moltó’s study. “As guidelines currently recommend continuing TNFi, studies like this help demonstrate that the guideline changes do not appear to be associated with an increase in adverse events.”
Dr. Moltó and Ms. Flatman disclosed no relevant financial relationships. Dr. Tedeschi has worked as a consultant for Novartis.
A version of this article appeared on Medscape.com.
AT ACR 2023
Caution raised on reduced-dose steroids in rare vasculitides GPA, MPA
SAN DIEGO – A real-world analysis linked the PEXIVAS reduced-dose glucocorticoid (GC) regimen to a higher likelihood of a group of poor outcomes such as death in patients with severe granulomatosis with polyangiitis (GPA) or microscopic polyangiitis (MPA).
The retrospective observational study, presented at the annual meeting of the American College of Rheumatology, aimed to see whether results from the landmark PEXIVAS trial would hold up in a real-world analysis given some important limitations of the PEXIVAS’s primary outcome and its relative lack of balance in choice of induction agents.
First author of the new study, Sophie Nagle, MD, of Cochin Hospital in Paris, and colleagues in the French Vasculitis Study Group noted that PEXIVAS “demonstrated noninferiority of reduced-dose GC regimen compared to standard dose for the incidence of death or end-stage kidney disease (ESKD), with a significant reduction in serious infections at 1 year. However, the primary endpoint did not include disease progression or relapse, the majority of patients received cyclophosphamide as induction therapy, and subgroup analysis showed a trend towards an increased risk of death or ESKD in [rituximab]-treated patients.”
The new findings “give us pause about using low-dose glucocorticoid regimens” and suggest that rheumatologists might be a little more conservative about their use than randomized controlled trials such as PEXIVAS might suggest, said Vanderbilt University vasculitis specialist Kevin W. Byram, MD, who’s familiar with the findings but did not take part in the study.
Dr. Nagle reported that among 234 patients with either GPA or MPA, 33.3% of 126 who received a reduced-dose GC regimen experienced one of the 12-month composite primary outcome’s events of death, disease relapse, ESKD, or disease progression before remission that required treatment modification, compared with 18.5% of 108 who received the standard GC regimen (P = .016).
In a propensity score analysis, the higher risk of poor outcomes in the reduced-dose group remained (hazard ratio, 1.57; 95% confidence interval, 0.93-2.64). A multivariate analysis also identified a higher risk for the composite primary outcome in the reduced-dose group (HR, 1.72; 95% CI, 1.08-2.74), although there was no association with an increased risk of death or ESKD.
The PEXIVAS study, published in 2020, supported lower GC doses in antineutrophil cytoplasmic antibody–associated vasculitis, potentially revolutionizing treatment. “Historically, we have used high doses of glucocorticoids on slower tapers to treat this disease, which itself is a strategy that leads to potential complications,” Dr. Byram said. “PEXIVAS suggested we could potentially use less glucocorticoids in these patients.”
For the retrospective, multicenter study, researchers tracked patients from 2018 to 2022, all aged 15 and above. They included 93 with MPA and 141 with GPA. Nearly half were female, and they had a mean age of 61 years. The patients had severe flare-ups treated with rituximab or cyclophosphamide induction and reduced-dose or standard GC regimen.
The standard care and reduced-dose groups were similar, Dr. Nagle said, although the standard group had significantly more patients with GPA (71% vs. 29% with MPA) than did the low-dose group (51% with GPA, 49% with MPA).
The researchers reported that in a reduced-dose subgroup, patients with creatinine levels above 300 micromol/L were more likely to meet the primary endpoint (relative risk, 2.14; 95% CI, 1.14-4.03). Those treated with the reduced-dose GC regimen were also more likely to reach the primary endpoint (HR, 1.61; 95% CI, 0.94-2.77) and die or develop ESKD (HR, 2.42; 95% CI, 1.04-5.66).
However, adverse events at 12 months were similar in both groups: The authors noted that those who received the reduced-dose GC regimen didn’t have higher risk of death, ESKD, or severe infections.
The authors highlighted that “increased vigilance is required when using the reduced-dose GC regimen especially in two subgroups of patients due to the risk of failure: Patients receiving rituximab as induction therapy [and] patients with severe initial kidney disease (serum creatinine > 300 micromol/L).”
The study authors note several limitations: The study is retrospective, and the standard dose group is heterogeneous.
“This study raises the idea that we need to be careful in using low-dose glucocorticoid regimens, but not avoid them all together,” Dr. Byram said. “The finding that those with worse kidney function fared worse lines up with my clinical experiences. There are clearly populations with this disease that could benefit from more steroid, and it tends to be the ones that are sicker at presentation, particularly those requiring ICU-level care.”
He advised colleagues to “not be dogmatic and use strict low-dose regimens ‘just because.’ ”
No study funding was reported. Dr. Nagle reported having no relevant financial relationships, and disclosures for other authors were not reported. Dr. Byram reports serving on the Vasculitis Foundation board of directors.
SAN DIEGO – A real-world analysis linked the PEXIVAS reduced-dose glucocorticoid (GC) regimen to a higher likelihood of a group of poor outcomes such as death in patients with severe granulomatosis with polyangiitis (GPA) or microscopic polyangiitis (MPA).
The retrospective observational study, presented at the annual meeting of the American College of Rheumatology, aimed to see whether results from the landmark PEXIVAS trial would hold up in a real-world analysis given some important limitations of the PEXIVAS’s primary outcome and its relative lack of balance in choice of induction agents.
First author of the new study, Sophie Nagle, MD, of Cochin Hospital in Paris, and colleagues in the French Vasculitis Study Group noted that PEXIVAS “demonstrated noninferiority of reduced-dose GC regimen compared to standard dose for the incidence of death or end-stage kidney disease (ESKD), with a significant reduction in serious infections at 1 year. However, the primary endpoint did not include disease progression or relapse, the majority of patients received cyclophosphamide as induction therapy, and subgroup analysis showed a trend towards an increased risk of death or ESKD in [rituximab]-treated patients.”
The new findings “give us pause about using low-dose glucocorticoid regimens” and suggest that rheumatologists might be a little more conservative about their use than randomized controlled trials such as PEXIVAS might suggest, said Vanderbilt University vasculitis specialist Kevin W. Byram, MD, who’s familiar with the findings but did not take part in the study.
Dr. Nagle reported that among 234 patients with either GPA or MPA, 33.3% of 126 who received a reduced-dose GC regimen experienced one of the 12-month composite primary outcome’s events of death, disease relapse, ESKD, or disease progression before remission that required treatment modification, compared with 18.5% of 108 who received the standard GC regimen (P = .016).
In a propensity score analysis, the higher risk of poor outcomes in the reduced-dose group remained (hazard ratio, 1.57; 95% confidence interval, 0.93-2.64). A multivariate analysis also identified a higher risk for the composite primary outcome in the reduced-dose group (HR, 1.72; 95% CI, 1.08-2.74), although there was no association with an increased risk of death or ESKD.
The PEXIVAS study, published in 2020, supported lower GC doses in antineutrophil cytoplasmic antibody–associated vasculitis, potentially revolutionizing treatment. “Historically, we have used high doses of glucocorticoids on slower tapers to treat this disease, which itself is a strategy that leads to potential complications,” Dr. Byram said. “PEXIVAS suggested we could potentially use less glucocorticoids in these patients.”
For the retrospective, multicenter study, researchers tracked patients from 2018 to 2022, all aged 15 and above. They included 93 with MPA and 141 with GPA. Nearly half were female, and they had a mean age of 61 years. The patients had severe flare-ups treated with rituximab or cyclophosphamide induction and reduced-dose or standard GC regimen.
The standard care and reduced-dose groups were similar, Dr. Nagle said, although the standard group had significantly more patients with GPA (71% vs. 29% with MPA) than did the low-dose group (51% with GPA, 49% with MPA).
The researchers reported that in a reduced-dose subgroup, patients with creatinine levels above 300 micromol/L were more likely to meet the primary endpoint (relative risk, 2.14; 95% CI, 1.14-4.03). Those treated with the reduced-dose GC regimen were also more likely to reach the primary endpoint (HR, 1.61; 95% CI, 0.94-2.77) and die or develop ESKD (HR, 2.42; 95% CI, 1.04-5.66).
However, adverse events at 12 months were similar in both groups: The authors noted that those who received the reduced-dose GC regimen didn’t have higher risk of death, ESKD, or severe infections.
The authors highlighted that “increased vigilance is required when using the reduced-dose GC regimen especially in two subgroups of patients due to the risk of failure: Patients receiving rituximab as induction therapy [and] patients with severe initial kidney disease (serum creatinine > 300 micromol/L).”
The study authors note several limitations: The study is retrospective, and the standard dose group is heterogeneous.
“This study raises the idea that we need to be careful in using low-dose glucocorticoid regimens, but not avoid them all together,” Dr. Byram said. “The finding that those with worse kidney function fared worse lines up with my clinical experiences. There are clearly populations with this disease that could benefit from more steroid, and it tends to be the ones that are sicker at presentation, particularly those requiring ICU-level care.”
He advised colleagues to “not be dogmatic and use strict low-dose regimens ‘just because.’ ”
No study funding was reported. Dr. Nagle reported having no relevant financial relationships, and disclosures for other authors were not reported. Dr. Byram reports serving on the Vasculitis Foundation board of directors.
SAN DIEGO – A real-world analysis linked the PEXIVAS reduced-dose glucocorticoid (GC) regimen to a higher likelihood of a group of poor outcomes such as death in patients with severe granulomatosis with polyangiitis (GPA) or microscopic polyangiitis (MPA).
The retrospective observational study, presented at the annual meeting of the American College of Rheumatology, aimed to see whether results from the landmark PEXIVAS trial would hold up in a real-world analysis given some important limitations of the PEXIVAS’s primary outcome and its relative lack of balance in choice of induction agents.
First author of the new study, Sophie Nagle, MD, of Cochin Hospital in Paris, and colleagues in the French Vasculitis Study Group noted that PEXIVAS “demonstrated noninferiority of reduced-dose GC regimen compared to standard dose for the incidence of death or end-stage kidney disease (ESKD), with a significant reduction in serious infections at 1 year. However, the primary endpoint did not include disease progression or relapse, the majority of patients received cyclophosphamide as induction therapy, and subgroup analysis showed a trend towards an increased risk of death or ESKD in [rituximab]-treated patients.”
The new findings “give us pause about using low-dose glucocorticoid regimens” and suggest that rheumatologists might be a little more conservative about their use than randomized controlled trials such as PEXIVAS might suggest, said Vanderbilt University vasculitis specialist Kevin W. Byram, MD, who’s familiar with the findings but did not take part in the study.
Dr. Nagle reported that among 234 patients with either GPA or MPA, 33.3% of 126 who received a reduced-dose GC regimen experienced one of the 12-month composite primary outcome’s events of death, disease relapse, ESKD, or disease progression before remission that required treatment modification, compared with 18.5% of 108 who received the standard GC regimen (P = .016).
In a propensity score analysis, the higher risk of poor outcomes in the reduced-dose group remained (hazard ratio, 1.57; 95% confidence interval, 0.93-2.64). A multivariate analysis also identified a higher risk for the composite primary outcome in the reduced-dose group (HR, 1.72; 95% CI, 1.08-2.74), although there was no association with an increased risk of death or ESKD.
The PEXIVAS study, published in 2020, supported lower GC doses in antineutrophil cytoplasmic antibody–associated vasculitis, potentially revolutionizing treatment. “Historically, we have used high doses of glucocorticoids on slower tapers to treat this disease, which itself is a strategy that leads to potential complications,” Dr. Byram said. “PEXIVAS suggested we could potentially use less glucocorticoids in these patients.”
For the retrospective, multicenter study, researchers tracked patients from 2018 to 2022, all aged 15 and above. They included 93 with MPA and 141 with GPA. Nearly half were female, and they had a mean age of 61 years. The patients had severe flare-ups treated with rituximab or cyclophosphamide induction and reduced-dose or standard GC regimen.
The standard care and reduced-dose groups were similar, Dr. Nagle said, although the standard group had significantly more patients with GPA (71% vs. 29% with MPA) than did the low-dose group (51% with GPA, 49% with MPA).
The researchers reported that in a reduced-dose subgroup, patients with creatinine levels above 300 micromol/L were more likely to meet the primary endpoint (relative risk, 2.14; 95% CI, 1.14-4.03). Those treated with the reduced-dose GC regimen were also more likely to reach the primary endpoint (HR, 1.61; 95% CI, 0.94-2.77) and die or develop ESKD (HR, 2.42; 95% CI, 1.04-5.66).
However, adverse events at 12 months were similar in both groups: The authors noted that those who received the reduced-dose GC regimen didn’t have higher risk of death, ESKD, or severe infections.
The authors highlighted that “increased vigilance is required when using the reduced-dose GC regimen especially in two subgroups of patients due to the risk of failure: Patients receiving rituximab as induction therapy [and] patients with severe initial kidney disease (serum creatinine > 300 micromol/L).”
The study authors note several limitations: The study is retrospective, and the standard dose group is heterogeneous.
“This study raises the idea that we need to be careful in using low-dose glucocorticoid regimens, but not avoid them all together,” Dr. Byram said. “The finding that those with worse kidney function fared worse lines up with my clinical experiences. There are clearly populations with this disease that could benefit from more steroid, and it tends to be the ones that are sicker at presentation, particularly those requiring ICU-level care.”
He advised colleagues to “not be dogmatic and use strict low-dose regimens ‘just because.’ ”
No study funding was reported. Dr. Nagle reported having no relevant financial relationships, and disclosures for other authors were not reported. Dr. Byram reports serving on the Vasculitis Foundation board of directors.
AT ACR 2023
Pregnancy in rheumatic disease quadruples risk of cardiovascular events
SAN DIEGO – Pregnant individuals with autoimmune rheumatic diseases (ARDs) are at least four times more likely to experience an acute cardiovascular event (CVE) than are pregnant individuals without these conditions, according to new research presented at the annual meeting of the American College of Rheumatology. Pregnant individuals with primary antiphospholipid syndrome (APS) had a 15-fold increase in CVE risk.
Patients who experienced CVEs were also more likely to experience preterm birth and other adverse pregnancy outcomes (APOs).
Rashmi Dhital, MD, a rheumatology fellow at the University of California, San Diego, and colleagues examined the medical records of pregnant individuals in California who had delivered singleton live-born infants from 2005 to 2020. Using data from the Study of Outcomes in Mothers and Infants (SOMI) database, an administrative population-based birth cohort in California, they identified more than 7 million individuals, 19,340 with ARDs and 7,758 with APS.
They then analyzed how many patients experienced an acute CVE during pregnancy and up to 6 weeks after giving birth.
CVEs occurred in 2.0% of patients with ARDs, 6.9% of individuals with APS, and 0.4% of women without these conditions. CVE risk was four times higher in the ARDs group (adjusted relative risk, 4.1; 95% confidence interval, 3.7-4.5) and nearly 15 times higher in the APS group (aRR, 14.7; 95% CI, 13.5-16.0) than in the comparison group. Patients with systemic lupus erythematosus (SLE) had a sixfold higher risk of CVE, which was further exacerbated by concomitant APS (18-fold higher risk) or lupus nephritis (15-fold higher risk).
Dr. Dhital also classified CVEs as either venous thromboembolism and non-VTE events. Pregnant patients with APS had a high risk for VTE-only CVE (40-fold greater) and a 3.7-fold higher risk of non-VTE events, compared with pregnant patients without these conditions. Patients with SLE along with lupus nephritis had a 20-fold increased risk of VTE-only CVE and an 11-fold higher risk of non-VTE CVE.
Although the study grouped rheumatic diseases together, “lupus is generally driving these results,” Sharon Kolasinski, MD, of the University of Pennsylvania, Philadelphia, noted in an interview. She moderated the plenary session where the research was presented. “If you take out lupus, then what is the risk? That would be an interesting question.”
Between 25% and 30% of all CVEs occurred in the postpartum period, highlighting the importance of close monitoring of cardiovascular risks and events in women with ARDs or APS both during pregnancy and postpartum, Dr. Dhital noted.
Recognizing these risks “can sometimes be challenging due to a lower suspicion of CVE in younger patients, and also symptoms overlap with normal pregnancy,” Dr. Dhital said during her plenary presentation. Working with other clinical teams could help physicians detect these risks in patients.
“It’s important for us to remember that there’s increased risk of cardiovascular events in pregnancy in our patients. It’s uncommon, but it’s not zero,” added Dr. Kolasinski, and this study highlighted when physicians should be more focused about that risk.
Dr. Dhital noted there were some limitations to the study that are inherent in using administrative databases for research that relies on ICD codes, including “the availability of information on disease activity, medications, and labs, which may restrict clinical interpretation.”
SOMI data reinforced by National Inpatient Sample study
The findings were complemented by a study using the National Inpatient Sample database to explore CVE risk in pregnant individuals with various rheumatic diseases. Lead author Karun Shrestha, MD, a resident physician at St. Barnabas Hospital in New York, and colleagues identified delivery hospitalizations from 2016 to 2019 for individuals with SLE, RA, and systemic vasculitis and looked for CVEs including preeclampsia, peripartum cardiomyopathy (PPCM), heart failure, stroke, cardiac arrhythmias, and VTE.
Out of over 3.4 million delivery hospitalizations, researchers identified 5,900 individuals with SLE, 4,895 with RA, and 325 with vasculitis. After adjusting for confounding factors such as race, age, insurance, and other comorbidities, SLE was identified as an independent risk factor for preeclampsia (odds ratio, 1.5; 95% CI, 1.1-2.1), arrhythmia (OR, 3.17; 95% CI, 1.73-5.79), and venous thrombosis (OR, 8.4; 95% CI, 2.9-22.1). Vasculitis was tied to increased risk for preeclampsia (OR, 4.7; 95% CI, 2-11.3), stroke (OR, 513.3; 95% CI, 114-2,284), heart failure (OR, 24.17; 95% CI, 4.68-124.6), and PPCM (OR, 66.7; 95% CI, 8.7-509.4). RA was tied to an increased risk for preeclampsia (OR, 1.5; 95% CI, 1.05-2.1).
Patients with SLE or vasculitis had longer, more costly hospital stays, compared with those without these conditions, and they experienced higher rates of in-hospital mortality. While previous research has demonstrated that patients with SLE have higher risk of cardiac events, there is less literature on CVE risk in pregnancies for vasculitis, Dr. Shrestha said in an interview.
“It’s something to work on,” he said.
Adverse pregnancy outcomes higher with ARDs, APS
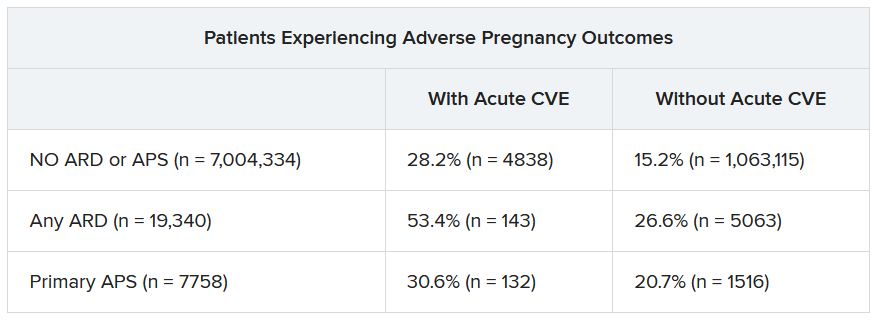
In a second abstract also led by Dr. Dhital using SOMI data, researchers found that pregnant individuals with ARDs or APS had a higher risk of experiencing an APO – preterm birth or small-for-gestational age – than individuals without these conditions. CVEs exacerbated that risk, regardless of underlying chronic health conditions.
Over half of patients with an ARD and a CVE during pregnancy experienced an APO – most commonly preterm birth. More than one in four pregnant individuals without ARD or APS who experienced a CVE also had an APO.
After differentiating CVEs as either VTE and non-VTE events, patients with ARD and a non-VTE CVE had a fivefold greater risk of early preterm birth (< 32 weeks) and a threefold higher risk of moderate preterm birth (32 to < 34 weeks).
“These findings highlight the need for close monitoring and management of pregnant women, not only for adverse outcomes, but also for cardiovascular risks and events, in order to identify those at the highest risk for adverse outcomes,” the authors wrote. “This need is particularly significant for individuals with ARDs, as 53.4% of our population with an ARD and CVE in pregnancy experienced an APO.”
Dr. Dhital, Dr. Kolasinski, and Dr. Shrestha disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
SAN DIEGO – Pregnant individuals with autoimmune rheumatic diseases (ARDs) are at least four times more likely to experience an acute cardiovascular event (CVE) than are pregnant individuals without these conditions, according to new research presented at the annual meeting of the American College of Rheumatology. Pregnant individuals with primary antiphospholipid syndrome (APS) had a 15-fold increase in CVE risk.
Patients who experienced CVEs were also more likely to experience preterm birth and other adverse pregnancy outcomes (APOs).
Rashmi Dhital, MD, a rheumatology fellow at the University of California, San Diego, and colleagues examined the medical records of pregnant individuals in California who had delivered singleton live-born infants from 2005 to 2020. Using data from the Study of Outcomes in Mothers and Infants (SOMI) database, an administrative population-based birth cohort in California, they identified more than 7 million individuals, 19,340 with ARDs and 7,758 with APS.
They then analyzed how many patients experienced an acute CVE during pregnancy and up to 6 weeks after giving birth.
CVEs occurred in 2.0% of patients with ARDs, 6.9% of individuals with APS, and 0.4% of women without these conditions. CVE risk was four times higher in the ARDs group (adjusted relative risk, 4.1; 95% confidence interval, 3.7-4.5) and nearly 15 times higher in the APS group (aRR, 14.7; 95% CI, 13.5-16.0) than in the comparison group. Patients with systemic lupus erythematosus (SLE) had a sixfold higher risk of CVE, which was further exacerbated by concomitant APS (18-fold higher risk) or lupus nephritis (15-fold higher risk).
Dr. Dhital also classified CVEs as either venous thromboembolism and non-VTE events. Pregnant patients with APS had a high risk for VTE-only CVE (40-fold greater) and a 3.7-fold higher risk of non-VTE events, compared with pregnant patients without these conditions. Patients with SLE along with lupus nephritis had a 20-fold increased risk of VTE-only CVE and an 11-fold higher risk of non-VTE CVE.
Although the study grouped rheumatic diseases together, “lupus is generally driving these results,” Sharon Kolasinski, MD, of the University of Pennsylvania, Philadelphia, noted in an interview. She moderated the plenary session where the research was presented. “If you take out lupus, then what is the risk? That would be an interesting question.”
Between 25% and 30% of all CVEs occurred in the postpartum period, highlighting the importance of close monitoring of cardiovascular risks and events in women with ARDs or APS both during pregnancy and postpartum, Dr. Dhital noted.
Recognizing these risks “can sometimes be challenging due to a lower suspicion of CVE in younger patients, and also symptoms overlap with normal pregnancy,” Dr. Dhital said during her plenary presentation. Working with other clinical teams could help physicians detect these risks in patients.
“It’s important for us to remember that there’s increased risk of cardiovascular events in pregnancy in our patients. It’s uncommon, but it’s not zero,” added Dr. Kolasinski, and this study highlighted when physicians should be more focused about that risk.
Dr. Dhital noted there were some limitations to the study that are inherent in using administrative databases for research that relies on ICD codes, including “the availability of information on disease activity, medications, and labs, which may restrict clinical interpretation.”
SOMI data reinforced by National Inpatient Sample study
The findings were complemented by a study using the National Inpatient Sample database to explore CVE risk in pregnant individuals with various rheumatic diseases. Lead author Karun Shrestha, MD, a resident physician at St. Barnabas Hospital in New York, and colleagues identified delivery hospitalizations from 2016 to 2019 for individuals with SLE, RA, and systemic vasculitis and looked for CVEs including preeclampsia, peripartum cardiomyopathy (PPCM), heart failure, stroke, cardiac arrhythmias, and VTE.
Out of over 3.4 million delivery hospitalizations, researchers identified 5,900 individuals with SLE, 4,895 with RA, and 325 with vasculitis. After adjusting for confounding factors such as race, age, insurance, and other comorbidities, SLE was identified as an independent risk factor for preeclampsia (odds ratio, 1.5; 95% CI, 1.1-2.1), arrhythmia (OR, 3.17; 95% CI, 1.73-5.79), and venous thrombosis (OR, 8.4; 95% CI, 2.9-22.1). Vasculitis was tied to increased risk for preeclampsia (OR, 4.7; 95% CI, 2-11.3), stroke (OR, 513.3; 95% CI, 114-2,284), heart failure (OR, 24.17; 95% CI, 4.68-124.6), and PPCM (OR, 66.7; 95% CI, 8.7-509.4). RA was tied to an increased risk for preeclampsia (OR, 1.5; 95% CI, 1.05-2.1).
Patients with SLE or vasculitis had longer, more costly hospital stays, compared with those without these conditions, and they experienced higher rates of in-hospital mortality. While previous research has demonstrated that patients with SLE have higher risk of cardiac events, there is less literature on CVE risk in pregnancies for vasculitis, Dr. Shrestha said in an interview.
“It’s something to work on,” he said.
Adverse pregnancy outcomes higher with ARDs, APS
In a second abstract also led by Dr. Dhital using SOMI data, researchers found that pregnant individuals with ARDs or APS had a higher risk of experiencing an APO – preterm birth or small-for-gestational age – than individuals without these conditions. CVEs exacerbated that risk, regardless of underlying chronic health conditions.
Over half of patients with an ARD and a CVE during pregnancy experienced an APO – most commonly preterm birth. More than one in four pregnant individuals without ARD or APS who experienced a CVE also had an APO.
After differentiating CVEs as either VTE and non-VTE events, patients with ARD and a non-VTE CVE had a fivefold greater risk of early preterm birth (< 32 weeks) and a threefold higher risk of moderate preterm birth (32 to < 34 weeks).
“These findings highlight the need for close monitoring and management of pregnant women, not only for adverse outcomes, but also for cardiovascular risks and events, in order to identify those at the highest risk for adverse outcomes,” the authors wrote. “This need is particularly significant for individuals with ARDs, as 53.4% of our population with an ARD and CVE in pregnancy experienced an APO.”
Dr. Dhital, Dr. Kolasinski, and Dr. Shrestha disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
SAN DIEGO – Pregnant individuals with autoimmune rheumatic diseases (ARDs) are at least four times more likely to experience an acute cardiovascular event (CVE) than are pregnant individuals without these conditions, according to new research presented at the annual meeting of the American College of Rheumatology. Pregnant individuals with primary antiphospholipid syndrome (APS) had a 15-fold increase in CVE risk.
Patients who experienced CVEs were also more likely to experience preterm birth and other adverse pregnancy outcomes (APOs).
Rashmi Dhital, MD, a rheumatology fellow at the University of California, San Diego, and colleagues examined the medical records of pregnant individuals in California who had delivered singleton live-born infants from 2005 to 2020. Using data from the Study of Outcomes in Mothers and Infants (SOMI) database, an administrative population-based birth cohort in California, they identified more than 7 million individuals, 19,340 with ARDs and 7,758 with APS.
They then analyzed how many patients experienced an acute CVE during pregnancy and up to 6 weeks after giving birth.
CVEs occurred in 2.0% of patients with ARDs, 6.9% of individuals with APS, and 0.4% of women without these conditions. CVE risk was four times higher in the ARDs group (adjusted relative risk, 4.1; 95% confidence interval, 3.7-4.5) and nearly 15 times higher in the APS group (aRR, 14.7; 95% CI, 13.5-16.0) than in the comparison group. Patients with systemic lupus erythematosus (SLE) had a sixfold higher risk of CVE, which was further exacerbated by concomitant APS (18-fold higher risk) or lupus nephritis (15-fold higher risk).
Dr. Dhital also classified CVEs as either venous thromboembolism and non-VTE events. Pregnant patients with APS had a high risk for VTE-only CVE (40-fold greater) and a 3.7-fold higher risk of non-VTE events, compared with pregnant patients without these conditions. Patients with SLE along with lupus nephritis had a 20-fold increased risk of VTE-only CVE and an 11-fold higher risk of non-VTE CVE.
Although the study grouped rheumatic diseases together, “lupus is generally driving these results,” Sharon Kolasinski, MD, of the University of Pennsylvania, Philadelphia, noted in an interview. She moderated the plenary session where the research was presented. “If you take out lupus, then what is the risk? That would be an interesting question.”
Between 25% and 30% of all CVEs occurred in the postpartum period, highlighting the importance of close monitoring of cardiovascular risks and events in women with ARDs or APS both during pregnancy and postpartum, Dr. Dhital noted.
Recognizing these risks “can sometimes be challenging due to a lower suspicion of CVE in younger patients, and also symptoms overlap with normal pregnancy,” Dr. Dhital said during her plenary presentation. Working with other clinical teams could help physicians detect these risks in patients.
“It’s important for us to remember that there’s increased risk of cardiovascular events in pregnancy in our patients. It’s uncommon, but it’s not zero,” added Dr. Kolasinski, and this study highlighted when physicians should be more focused about that risk.
Dr. Dhital noted there were some limitations to the study that are inherent in using administrative databases for research that relies on ICD codes, including “the availability of information on disease activity, medications, and labs, which may restrict clinical interpretation.”
SOMI data reinforced by National Inpatient Sample study
The findings were complemented by a study using the National Inpatient Sample database to explore CVE risk in pregnant individuals with various rheumatic diseases. Lead author Karun Shrestha, MD, a resident physician at St. Barnabas Hospital in New York, and colleagues identified delivery hospitalizations from 2016 to 2019 for individuals with SLE, RA, and systemic vasculitis and looked for CVEs including preeclampsia, peripartum cardiomyopathy (PPCM), heart failure, stroke, cardiac arrhythmias, and VTE.
Out of over 3.4 million delivery hospitalizations, researchers identified 5,900 individuals with SLE, 4,895 with RA, and 325 with vasculitis. After adjusting for confounding factors such as race, age, insurance, and other comorbidities, SLE was identified as an independent risk factor for preeclampsia (odds ratio, 1.5; 95% CI, 1.1-2.1), arrhythmia (OR, 3.17; 95% CI, 1.73-5.79), and venous thrombosis (OR, 8.4; 95% CI, 2.9-22.1). Vasculitis was tied to increased risk for preeclampsia (OR, 4.7; 95% CI, 2-11.3), stroke (OR, 513.3; 95% CI, 114-2,284), heart failure (OR, 24.17; 95% CI, 4.68-124.6), and PPCM (OR, 66.7; 95% CI, 8.7-509.4). RA was tied to an increased risk for preeclampsia (OR, 1.5; 95% CI, 1.05-2.1).
Patients with SLE or vasculitis had longer, more costly hospital stays, compared with those without these conditions, and they experienced higher rates of in-hospital mortality. While previous research has demonstrated that patients with SLE have higher risk of cardiac events, there is less literature on CVE risk in pregnancies for vasculitis, Dr. Shrestha said in an interview.
“It’s something to work on,” he said.
Adverse pregnancy outcomes higher with ARDs, APS
In a second abstract also led by Dr. Dhital using SOMI data, researchers found that pregnant individuals with ARDs or APS had a higher risk of experiencing an APO – preterm birth or small-for-gestational age – than individuals without these conditions. CVEs exacerbated that risk, regardless of underlying chronic health conditions.
Over half of patients with an ARD and a CVE during pregnancy experienced an APO – most commonly preterm birth. More than one in four pregnant individuals without ARD or APS who experienced a CVE also had an APO.
After differentiating CVEs as either VTE and non-VTE events, patients with ARD and a non-VTE CVE had a fivefold greater risk of early preterm birth (< 32 weeks) and a threefold higher risk of moderate preterm birth (32 to < 34 weeks).
“These findings highlight the need for close monitoring and management of pregnant women, not only for adverse outcomes, but also for cardiovascular risks and events, in order to identify those at the highest risk for adverse outcomes,” the authors wrote. “This need is particularly significant for individuals with ARDs, as 53.4% of our population with an ARD and CVE in pregnancy experienced an APO.”
Dr. Dhital, Dr. Kolasinski, and Dr. Shrestha disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
AT ACR 2023
Pregnancies with low anti-SSA/Ro autoantibody levels: Forgo fetal heart rhythm monitoring?
SAN DIEGO – Pregnant women with anti-SSA/Ro autoantibodies at titer levels of less than 1,000 ELISA units per mL are at minimal to no risk for fetal atrioventricular (AV) block and may be able to forgo traditional echocardiographic heart rhythm monitoring, results from an ongoing, prospective, multicenter trial demonstrated.
However, pregnant patients with higher titer antibodies seem to be at greatest risk for fetal AV block and may benefit from ambulatory fetal heart rhythm monitoring (FHRM), which can detect emergent AV block, according to the study findings. The findings were published online in Arthritis & Rheumatology and will be presented Nov. 13 at the American College of Rheumatology (ACR) 2023 Annual Meeting by Jill P. Buyon, MD, a rheumatologist who directs the division of rheumatology and the Lupus Center at NYU Langone Health in New York.
“While anti-Ro antibodies have been known to be associated with AV block for decades, it has become increasingly clear that antibody titers matter,” Dr. Buyon said in an interview.
For the investigation, which is the largest of its kind, researchers at 22 sites drew from the large multiracial national study of pregnant women, Surveillance To Prevent AV Block Likely to Occur Quickly (STOP BLOQ), to address the impact of anti-Ro titers and use of frequent ambulatory FHRM on outcomes in women with no previously affected children and those at risk for recurrence. Monitoring occurred during the second trimester of pregnancy (from 17 weeks through 26 weeks) and consisted of daily fetal home testing by mothers using handheld, commercially available Doppler devices.
These were followed up by weekly or biweekly echocardiograms, and ultrasound tests to evaluate fetal heart rhythm and function, as well as to show any structural problems. Three times per day, the pregnant women texted the Doppler sound recordings in real time to a pediatric cardiologist, who immediately ordered an additional echocardiogram in cases of irregular or slowing fetal heart rates. If second-degree heart block was detected, drug therapy was initiated.
No AV block seen with low anti-Ro titers
Dr. Buyon, who led the study with Bettina Cuneo, MD, clinical scholar and professor of surgery and pediatrics at the University of Arizona in Tucson, presented findings from 413 pregnant subjects with a mean age of 33 years who finished monitoring surveillance: 152 women had low titers of both anti-Ro60 and –Ro52 (defined as < 1,000 ELISA units per mL), and 261 women with titers above the threshold for either antibody (defined as ≥ 1,000 ELISA units per mL). Of the 152 women with low titers of both anti-Ro60 and –Ro52, none of the pregnancies past 26 weeks resulted in AV block. Of the 261 women with titers above the threshold for either antibody, 10 of the pregnancies resulted in AV block (3.8%). The incidence of AV block increased with higher antibody titer levels, reaching 7.7% for those in the top quartile for anti–60-kD SSA/Ro; this increased to 27.3% in study participants with a previous child who had AV block, although numbers in this category were small.
Analysis of cumulative FHRM recordings between surveillance echocardiograms revealed that no case of second-degree or third-degree AV block was missed. In addition, 70% of AV blocks detected by FHRM were second-degree and all occurred less than 12 hours from normal FHRM and within another 45 minutes to 4.5 hours to echocardiogram. The one case of second/third-degree and two cases of third-degree AV block were diagnosed by urgent echocardiogram more than 17 to 72 hours from a previously normal FHRM episode.
Other factors besides high anti-Ro titer likely play a role
“STOP BLOQ nicely demonstrates that low titer is associated with a very low risk AV block, and intense monitoring may not be needed,” Dr. Buyon told this news organization. “However, high titer is not the whole answer since even women with the very highest titers can have healthy babies. This report also shows that titers stay constant through pregnancies in the same mother, whether there is the complication of AV block or not. This suggests other factors contribute to AV block.”
She added that FHRM can be easily performed by the mother, but at this time is still best interpreted by a cardiologist. “FHRM detected all cases of AV block, which can happen in hours,” she said. “FHRM should decrease the need for frequent echocardiograms. Some mothers do have more difficulty in deciding whether the baby’s heart is beating irregularly. We need [to improve our teaching] and for how best to have a cardiologist or trained listener interpret. FHRM can be done by the mother but needs interpretation by a cardiologist until we develop a device which can identify abnormalities.”
She acknowledged certain limitations of the study, including the fact that a commercial test for anti-SSA/Ro antibody levels is not available to all clinicians. “Try to find a lab that measures high titer anti-Ro antibodies, but if not, then use one of the common commercial tests such as the BioPlex 2000 autoimmune panels and consider decreased surveillance if titer is < 8,” Dr. Buyon advised.
Vaneet K. Sandhu, MD, a rheumatologist with Loma Linda (Calif.) Medical Center, who was asked to comment on the work, said that the study not only justifies the limited use of FHRM in those with high titer antibodies (followed by urgent fetal echocardiography where indicated), but also risk stratification for fetal AV block.
“For years, we have recommended frequent fetal echocardiography testing in pregnant women with positive anti-SSA/Ro,” Dr. Sandhu said. “This study tells us we need to look deeper. On one hand, recognizing that low titer anti-Ro antibodies do not confer a risk of AV block is cost effective. On the other hand, while the titer of the antibody appears to contribute to fetal AV block, we need to delve deeper into additional factors contributing to fetal AV block risk in order to better navigate our surveillance methods.”
The study was supported by NIH grants from the National Institute of Child Health and Human Development and the National Institute of Arthritis and Musculoskeletal and Skin Diseases. Dr. Sandhu has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
SAN DIEGO – Pregnant women with anti-SSA/Ro autoantibodies at titer levels of less than 1,000 ELISA units per mL are at minimal to no risk for fetal atrioventricular (AV) block and may be able to forgo traditional echocardiographic heart rhythm monitoring, results from an ongoing, prospective, multicenter trial demonstrated.
However, pregnant patients with higher titer antibodies seem to be at greatest risk for fetal AV block and may benefit from ambulatory fetal heart rhythm monitoring (FHRM), which can detect emergent AV block, according to the study findings. The findings were published online in Arthritis & Rheumatology and will be presented Nov. 13 at the American College of Rheumatology (ACR) 2023 Annual Meeting by Jill P. Buyon, MD, a rheumatologist who directs the division of rheumatology and the Lupus Center at NYU Langone Health in New York.
“While anti-Ro antibodies have been known to be associated with AV block for decades, it has become increasingly clear that antibody titers matter,” Dr. Buyon said in an interview.
For the investigation, which is the largest of its kind, researchers at 22 sites drew from the large multiracial national study of pregnant women, Surveillance To Prevent AV Block Likely to Occur Quickly (STOP BLOQ), to address the impact of anti-Ro titers and use of frequent ambulatory FHRM on outcomes in women with no previously affected children and those at risk for recurrence. Monitoring occurred during the second trimester of pregnancy (from 17 weeks through 26 weeks) and consisted of daily fetal home testing by mothers using handheld, commercially available Doppler devices.
These were followed up by weekly or biweekly echocardiograms, and ultrasound tests to evaluate fetal heart rhythm and function, as well as to show any structural problems. Three times per day, the pregnant women texted the Doppler sound recordings in real time to a pediatric cardiologist, who immediately ordered an additional echocardiogram in cases of irregular or slowing fetal heart rates. If second-degree heart block was detected, drug therapy was initiated.
No AV block seen with low anti-Ro titers
Dr. Buyon, who led the study with Bettina Cuneo, MD, clinical scholar and professor of surgery and pediatrics at the University of Arizona in Tucson, presented findings from 413 pregnant subjects with a mean age of 33 years who finished monitoring surveillance: 152 women had low titers of both anti-Ro60 and –Ro52 (defined as < 1,000 ELISA units per mL), and 261 women with titers above the threshold for either antibody (defined as ≥ 1,000 ELISA units per mL). Of the 152 women with low titers of both anti-Ro60 and –Ro52, none of the pregnancies past 26 weeks resulted in AV block. Of the 261 women with titers above the threshold for either antibody, 10 of the pregnancies resulted in AV block (3.8%). The incidence of AV block increased with higher antibody titer levels, reaching 7.7% for those in the top quartile for anti–60-kD SSA/Ro; this increased to 27.3% in study participants with a previous child who had AV block, although numbers in this category were small.
Analysis of cumulative FHRM recordings between surveillance echocardiograms revealed that no case of second-degree or third-degree AV block was missed. In addition, 70% of AV blocks detected by FHRM were second-degree and all occurred less than 12 hours from normal FHRM and within another 45 minutes to 4.5 hours to echocardiogram. The one case of second/third-degree and two cases of third-degree AV block were diagnosed by urgent echocardiogram more than 17 to 72 hours from a previously normal FHRM episode.
Other factors besides high anti-Ro titer likely play a role
“STOP BLOQ nicely demonstrates that low titer is associated with a very low risk AV block, and intense monitoring may not be needed,” Dr. Buyon told this news organization. “However, high titer is not the whole answer since even women with the very highest titers can have healthy babies. This report also shows that titers stay constant through pregnancies in the same mother, whether there is the complication of AV block or not. This suggests other factors contribute to AV block.”
She added that FHRM can be easily performed by the mother, but at this time is still best interpreted by a cardiologist. “FHRM detected all cases of AV block, which can happen in hours,” she said. “FHRM should decrease the need for frequent echocardiograms. Some mothers do have more difficulty in deciding whether the baby’s heart is beating irregularly. We need [to improve our teaching] and for how best to have a cardiologist or trained listener interpret. FHRM can be done by the mother but needs interpretation by a cardiologist until we develop a device which can identify abnormalities.”
She acknowledged certain limitations of the study, including the fact that a commercial test for anti-SSA/Ro antibody levels is not available to all clinicians. “Try to find a lab that measures high titer anti-Ro antibodies, but if not, then use one of the common commercial tests such as the BioPlex 2000 autoimmune panels and consider decreased surveillance if titer is < 8,” Dr. Buyon advised.
Vaneet K. Sandhu, MD, a rheumatologist with Loma Linda (Calif.) Medical Center, who was asked to comment on the work, said that the study not only justifies the limited use of FHRM in those with high titer antibodies (followed by urgent fetal echocardiography where indicated), but also risk stratification for fetal AV block.
“For years, we have recommended frequent fetal echocardiography testing in pregnant women with positive anti-SSA/Ro,” Dr. Sandhu said. “This study tells us we need to look deeper. On one hand, recognizing that low titer anti-Ro antibodies do not confer a risk of AV block is cost effective. On the other hand, while the titer of the antibody appears to contribute to fetal AV block, we need to delve deeper into additional factors contributing to fetal AV block risk in order to better navigate our surveillance methods.”
The study was supported by NIH grants from the National Institute of Child Health and Human Development and the National Institute of Arthritis and Musculoskeletal and Skin Diseases. Dr. Sandhu has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
SAN DIEGO – Pregnant women with anti-SSA/Ro autoantibodies at titer levels of less than 1,000 ELISA units per mL are at minimal to no risk for fetal atrioventricular (AV) block and may be able to forgo traditional echocardiographic heart rhythm monitoring, results from an ongoing, prospective, multicenter trial demonstrated.
However, pregnant patients with higher titer antibodies seem to be at greatest risk for fetal AV block and may benefit from ambulatory fetal heart rhythm monitoring (FHRM), which can detect emergent AV block, according to the study findings. The findings were published online in Arthritis & Rheumatology and will be presented Nov. 13 at the American College of Rheumatology (ACR) 2023 Annual Meeting by Jill P. Buyon, MD, a rheumatologist who directs the division of rheumatology and the Lupus Center at NYU Langone Health in New York.
“While anti-Ro antibodies have been known to be associated with AV block for decades, it has become increasingly clear that antibody titers matter,” Dr. Buyon said in an interview.
For the investigation, which is the largest of its kind, researchers at 22 sites drew from the large multiracial national study of pregnant women, Surveillance To Prevent AV Block Likely to Occur Quickly (STOP BLOQ), to address the impact of anti-Ro titers and use of frequent ambulatory FHRM on outcomes in women with no previously affected children and those at risk for recurrence. Monitoring occurred during the second trimester of pregnancy (from 17 weeks through 26 weeks) and consisted of daily fetal home testing by mothers using handheld, commercially available Doppler devices.
These were followed up by weekly or biweekly echocardiograms, and ultrasound tests to evaluate fetal heart rhythm and function, as well as to show any structural problems. Three times per day, the pregnant women texted the Doppler sound recordings in real time to a pediatric cardiologist, who immediately ordered an additional echocardiogram in cases of irregular or slowing fetal heart rates. If second-degree heart block was detected, drug therapy was initiated.
No AV block seen with low anti-Ro titers
Dr. Buyon, who led the study with Bettina Cuneo, MD, clinical scholar and professor of surgery and pediatrics at the University of Arizona in Tucson, presented findings from 413 pregnant subjects with a mean age of 33 years who finished monitoring surveillance: 152 women had low titers of both anti-Ro60 and –Ro52 (defined as < 1,000 ELISA units per mL), and 261 women with titers above the threshold for either antibody (defined as ≥ 1,000 ELISA units per mL). Of the 152 women with low titers of both anti-Ro60 and –Ro52, none of the pregnancies past 26 weeks resulted in AV block. Of the 261 women with titers above the threshold for either antibody, 10 of the pregnancies resulted in AV block (3.8%). The incidence of AV block increased with higher antibody titer levels, reaching 7.7% for those in the top quartile for anti–60-kD SSA/Ro; this increased to 27.3% in study participants with a previous child who had AV block, although numbers in this category were small.
Analysis of cumulative FHRM recordings between surveillance echocardiograms revealed that no case of second-degree or third-degree AV block was missed. In addition, 70% of AV blocks detected by FHRM were second-degree and all occurred less than 12 hours from normal FHRM and within another 45 minutes to 4.5 hours to echocardiogram. The one case of second/third-degree and two cases of third-degree AV block were diagnosed by urgent echocardiogram more than 17 to 72 hours from a previously normal FHRM episode.
Other factors besides high anti-Ro titer likely play a role
“STOP BLOQ nicely demonstrates that low titer is associated with a very low risk AV block, and intense monitoring may not be needed,” Dr. Buyon told this news organization. “However, high titer is not the whole answer since even women with the very highest titers can have healthy babies. This report also shows that titers stay constant through pregnancies in the same mother, whether there is the complication of AV block or not. This suggests other factors contribute to AV block.”
She added that FHRM can be easily performed by the mother, but at this time is still best interpreted by a cardiologist. “FHRM detected all cases of AV block, which can happen in hours,” she said. “FHRM should decrease the need for frequent echocardiograms. Some mothers do have more difficulty in deciding whether the baby’s heart is beating irregularly. We need [to improve our teaching] and for how best to have a cardiologist or trained listener interpret. FHRM can be done by the mother but needs interpretation by a cardiologist until we develop a device which can identify abnormalities.”
She acknowledged certain limitations of the study, including the fact that a commercial test for anti-SSA/Ro antibody levels is not available to all clinicians. “Try to find a lab that measures high titer anti-Ro antibodies, but if not, then use one of the common commercial tests such as the BioPlex 2000 autoimmune panels and consider decreased surveillance if titer is < 8,” Dr. Buyon advised.
Vaneet K. Sandhu, MD, a rheumatologist with Loma Linda (Calif.) Medical Center, who was asked to comment on the work, said that the study not only justifies the limited use of FHRM in those with high titer antibodies (followed by urgent fetal echocardiography where indicated), but also risk stratification for fetal AV block.
“For years, we have recommended frequent fetal echocardiography testing in pregnant women with positive anti-SSA/Ro,” Dr. Sandhu said. “This study tells us we need to look deeper. On one hand, recognizing that low titer anti-Ro antibodies do not confer a risk of AV block is cost effective. On the other hand, while the titer of the antibody appears to contribute to fetal AV block, we need to delve deeper into additional factors contributing to fetal AV block risk in order to better navigate our surveillance methods.”
The study was supported by NIH grants from the National Institute of Child Health and Human Development and the National Institute of Arthritis and Musculoskeletal and Skin Diseases. Dr. Sandhu has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
AT ACR 2023
Obinutuzumab promotes renal preservation in lupus nephritis
TOPLINE:
Adults with lupus nephritis (LN) who received obinutuzumab (Gazyva) plus standard of care therapy experienced significantly improved kidney function and fewer flares compared with those given a placebo plus standard of care.
METHODOLOGY:
- Researchers conducted a post hoc analysis of the phase 2 NOBILITY study, a randomized trial in which 63 adults received 1,000 mg of obinutuzumab or placebo by infusion on day 1 and at weeks 2, 24, and 26.
- Outcomes were time to an unfavorable kidney outcome, defined by the first of any of the following events: treatment failure, doubling of serum creatinine, or death; researchers also measured LN flare outcomes including the first 30% and 40% declines in estimated glomerular filtration rate (eGFR) from baseline, chronic eGFR slope, and how many patients achieved complete renal response (CRR) on no more than 7.5 mg of prednisone.
TAKEAWAY:
- Adding obinutuzumab to the treatment of patients with LN reduced the risk of the composite outcome by 60% and reduced the risk for LN flare by 57%.
- The risk of first eGFR 30% and 40% decline was reduced by 80% and 91%, respectively, with obinutuzumab, and patients who took obinutuzumab had a significantly slower eGFR decline than with placebo (annualized eGFR slope advantage, 4.1 mL/min/1.73 m2 /year).
- At 76 weeks (1.5 years), 38% of patients receiving obinutuzumab achieved CRR on 7.5 mg or less of daily prednisone, compared with 16% of placebo patients, but this difference was not statistically significant at 104 weeks (2 years).
- The total numbers of unfavorable kidney outcomes for obinutuzumab vs. placebo were 12 vs. 24 for treatment failure, 1 vs. 6 for creatinine doubling, and 1 vs. 4 for death, respectively.
IN PRACTICE:
“By reducing flare risk, obinutuzumab should decrease the accumulation of chronic parenchymal kidney damage,” the authors wrote.
SOURCE:
The study was presented at the American College of Rheumatology (ACR) 2023 annual meeting and was published online in Arthritis & Rheumatology. The lead author was Brad H. Rovin, MD, of The Ohio State University in Columbus.
LIMITATIONS:
The analyses were post hoc and not prespecified, and the number of events was small, which prevented statistical confirmation, but the analyses are being repeated in an ongoing phase 3 study.
DISCLOSURES:
The study was supported by F. Hoffman–La Roche. Dr. Rovin reported receiving personal fees from F. Hoffman–La Roche during the conduct of the original trial. Several coauthors are F. Hoffman–La Roche employees.
A version of this article first appeared on Medscape.com.
TOPLINE:
Adults with lupus nephritis (LN) who received obinutuzumab (Gazyva) plus standard of care therapy experienced significantly improved kidney function and fewer flares compared with those given a placebo plus standard of care.
METHODOLOGY:
- Researchers conducted a post hoc analysis of the phase 2 NOBILITY study, a randomized trial in which 63 adults received 1,000 mg of obinutuzumab or placebo by infusion on day 1 and at weeks 2, 24, and 26.
- Outcomes were time to an unfavorable kidney outcome, defined by the first of any of the following events: treatment failure, doubling of serum creatinine, or death; researchers also measured LN flare outcomes including the first 30% and 40% declines in estimated glomerular filtration rate (eGFR) from baseline, chronic eGFR slope, and how many patients achieved complete renal response (CRR) on no more than 7.5 mg of prednisone.
TAKEAWAY:
- Adding obinutuzumab to the treatment of patients with LN reduced the risk of the composite outcome by 60% and reduced the risk for LN flare by 57%.
- The risk of first eGFR 30% and 40% decline was reduced by 80% and 91%, respectively, with obinutuzumab, and patients who took obinutuzumab had a significantly slower eGFR decline than with placebo (annualized eGFR slope advantage, 4.1 mL/min/1.73 m2 /year).
- At 76 weeks (1.5 years), 38% of patients receiving obinutuzumab achieved CRR on 7.5 mg or less of daily prednisone, compared with 16% of placebo patients, but this difference was not statistically significant at 104 weeks (2 years).
- The total numbers of unfavorable kidney outcomes for obinutuzumab vs. placebo were 12 vs. 24 for treatment failure, 1 vs. 6 for creatinine doubling, and 1 vs. 4 for death, respectively.
IN PRACTICE:
“By reducing flare risk, obinutuzumab should decrease the accumulation of chronic parenchymal kidney damage,” the authors wrote.
SOURCE:
The study was presented at the American College of Rheumatology (ACR) 2023 annual meeting and was published online in Arthritis & Rheumatology. The lead author was Brad H. Rovin, MD, of The Ohio State University in Columbus.
LIMITATIONS:
The analyses were post hoc and not prespecified, and the number of events was small, which prevented statistical confirmation, but the analyses are being repeated in an ongoing phase 3 study.
DISCLOSURES:
The study was supported by F. Hoffman–La Roche. Dr. Rovin reported receiving personal fees from F. Hoffman–La Roche during the conduct of the original trial. Several coauthors are F. Hoffman–La Roche employees.
A version of this article first appeared on Medscape.com.
TOPLINE:
Adults with lupus nephritis (LN) who received obinutuzumab (Gazyva) plus standard of care therapy experienced significantly improved kidney function and fewer flares compared with those given a placebo plus standard of care.
METHODOLOGY:
- Researchers conducted a post hoc analysis of the phase 2 NOBILITY study, a randomized trial in which 63 adults received 1,000 mg of obinutuzumab or placebo by infusion on day 1 and at weeks 2, 24, and 26.
- Outcomes were time to an unfavorable kidney outcome, defined by the first of any of the following events: treatment failure, doubling of serum creatinine, or death; researchers also measured LN flare outcomes including the first 30% and 40% declines in estimated glomerular filtration rate (eGFR) from baseline, chronic eGFR slope, and how many patients achieved complete renal response (CRR) on no more than 7.5 mg of prednisone.
TAKEAWAY:
- Adding obinutuzumab to the treatment of patients with LN reduced the risk of the composite outcome by 60% and reduced the risk for LN flare by 57%.
- The risk of first eGFR 30% and 40% decline was reduced by 80% and 91%, respectively, with obinutuzumab, and patients who took obinutuzumab had a significantly slower eGFR decline than with placebo (annualized eGFR slope advantage, 4.1 mL/min/1.73 m2 /year).
- At 76 weeks (1.5 years), 38% of patients receiving obinutuzumab achieved CRR on 7.5 mg or less of daily prednisone, compared with 16% of placebo patients, but this difference was not statistically significant at 104 weeks (2 years).
- The total numbers of unfavorable kidney outcomes for obinutuzumab vs. placebo were 12 vs. 24 for treatment failure, 1 vs. 6 for creatinine doubling, and 1 vs. 4 for death, respectively.
IN PRACTICE:
“By reducing flare risk, obinutuzumab should decrease the accumulation of chronic parenchymal kidney damage,” the authors wrote.
SOURCE:
The study was presented at the American College of Rheumatology (ACR) 2023 annual meeting and was published online in Arthritis & Rheumatology. The lead author was Brad H. Rovin, MD, of The Ohio State University in Columbus.
LIMITATIONS:
The analyses were post hoc and not prespecified, and the number of events was small, which prevented statistical confirmation, but the analyses are being repeated in an ongoing phase 3 study.
DISCLOSURES:
The study was supported by F. Hoffman–La Roche. Dr. Rovin reported receiving personal fees from F. Hoffman–La Roche during the conduct of the original trial. Several coauthors are F. Hoffman–La Roche employees.
A version of this article first appeared on Medscape.com.