User login
Sequential dermoscopy imaging helps find melanomas early
WAIKOLOA, HAWAII – Sequential dermoscopy imaging (SDI) is a valuable strategy for diagnosing melanomas early and with better sensitivity and specificity, compared with biopsy decisions based solely on the ugly duckling sign, the ABCDs of melanoma, or other aspects of lesion morphology, Michael A. Marchetti, MD, said at the Hawaii Dermatology Seminar provided by the Global Academy for Medical Education/Skin Disease Education Foundation.
SDI entails obtaining repeated dermoscopy images over time in order to detect subtle changes. It is typically done short term, over the course of 3-4 months, or longer term, over a period of 6 months to years, with long-term SDI being reserved for monitoring of less suspicious lesions, often in patients with an atypical mole syndrome.
SDI improves diagnostic specificity by dramatically reducing excision of benign pigmented lesions: in one large Belgian study, by up to 75% (Br J Dermatol. 2012 Oct;167[4]:778-86).
Short-term SDI also improves diagnostic sensitivity. That’s because it enables early identification of clinically featureless melanomas that are detected solely based upon change over a 3-month follow-up period. The operative principle here is that 93%-96% of melanomas will show change on dermoscopy within 3 months, while 99% of unchanged melanocytic lesions are benign. Since 16% of benign nevi will change within 3 months, that means 10%-30% of changed lesions are melanomas.
“If there is any change – it doesn’t matter what the change is, but the two images look different – that should lead to a biopsy,” explained Dr. Marchetti, a dermatologist at Memorial Sloan Kettering Cancer Center, New York.
As a result of this improved sensitivity and specificity, SDI has been shown to reduce the cost per melanoma diagnosis by about 40% (PLoS One. 2014 Oct 14;9[10]:e109339. doi: 10.1371/journal.pone.0109339).
Dr. Marchetti considers SDI a second-level diagnostic test for individual equivocal lesions. His first-level diagnostic tool is total-body photography.
SDI needs to be done by scrupulous examination of digital photographic images side-by-side on a computer monitor. A basic rule of SDI is that it should never be used to monitor raised or palpable lesions.
“The only thing you can monitor is something that’s flat,” he stressed.
Nor should SDI be used to monitor lesions with a peripheral globular pattern. And very slow-growing melanomas could potentially be missed by short-term SDI, so suspected lentigo maligna should be monitored for a minimum of 12 months, according to Dr. Marchetti.
Not every patient with an equivocal melanocytic lesion is a good candidate for SDI. It’s a monitoring strategy that should be reserved for reliable patients who will come back in 3 months. “If a patient doesn’t come back I take that very seriously. We call or send a letter,” Dr. Marchetti said.
Moreover, even in a patient who is a good candidate for SDI, he always offers the option of biopsy today rather than short-term monitoring.
SDI employed in conjunction with total-body photography is an extremely effective means of monitoring patients at very high risk for melanoma, Dr. Marchetti said. The power of this combination was illustrated in a prospective Australian study of 311 patients with a history of invasive melanoma plus either a high-risk genetic mutation or a strong family history. During a median follow-up of 3.5 years, 75 melanomas were detected, 14 of them at the baseline visit. The median thickness of melanomas detected post baseline was in situ. Thirty-nine percent of melanomas were detected using SDI and 38% via total body photography. Roughly one in five biopsied melanocytic lesions proved to be melanoma. Of note, five of the melanomas were more than 1 mm in Breslow thickness: Three of them were histologically desmoplastic, and the other two had nodular components (JAMA Dermatol. 2014 Aug;150(8):819-27).
For dermatologists who need to brush up on their dermoscopy skills, Dr. Marchetti recommended dermoscopedia as a useful, free resource.
Legal implications of monitoring via photography
“People often get worked up about this, but I’m not aware of a lawsuit alleging missed melanoma using baseline photography as evidence. And patients, in my experience, are universally appreciative of the use of imaging, although admittedly my experience is biased because people generally come to me for imaging,” Dr. Marchetti said.
He makes a point of telling every patient who opts for short-term SDI that, although the lesion has no features of concern now, it’s important to return promptly for reexamination should any changes occur.
Dr. Marchetti reported having no financial conflicts regarding his presentation.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
WAIKOLOA, HAWAII – Sequential dermoscopy imaging (SDI) is a valuable strategy for diagnosing melanomas early and with better sensitivity and specificity, compared with biopsy decisions based solely on the ugly duckling sign, the ABCDs of melanoma, or other aspects of lesion morphology, Michael A. Marchetti, MD, said at the Hawaii Dermatology Seminar provided by the Global Academy for Medical Education/Skin Disease Education Foundation.
SDI entails obtaining repeated dermoscopy images over time in order to detect subtle changes. It is typically done short term, over the course of 3-4 months, or longer term, over a period of 6 months to years, with long-term SDI being reserved for monitoring of less suspicious lesions, often in patients with an atypical mole syndrome.
SDI improves diagnostic specificity by dramatically reducing excision of benign pigmented lesions: in one large Belgian study, by up to 75% (Br J Dermatol. 2012 Oct;167[4]:778-86).
Short-term SDI also improves diagnostic sensitivity. That’s because it enables early identification of clinically featureless melanomas that are detected solely based upon change over a 3-month follow-up period. The operative principle here is that 93%-96% of melanomas will show change on dermoscopy within 3 months, while 99% of unchanged melanocytic lesions are benign. Since 16% of benign nevi will change within 3 months, that means 10%-30% of changed lesions are melanomas.
“If there is any change – it doesn’t matter what the change is, but the two images look different – that should lead to a biopsy,” explained Dr. Marchetti, a dermatologist at Memorial Sloan Kettering Cancer Center, New York.
As a result of this improved sensitivity and specificity, SDI has been shown to reduce the cost per melanoma diagnosis by about 40% (PLoS One. 2014 Oct 14;9[10]:e109339. doi: 10.1371/journal.pone.0109339).
Dr. Marchetti considers SDI a second-level diagnostic test for individual equivocal lesions. His first-level diagnostic tool is total-body photography.
SDI needs to be done by scrupulous examination of digital photographic images side-by-side on a computer monitor. A basic rule of SDI is that it should never be used to monitor raised or palpable lesions.
“The only thing you can monitor is something that’s flat,” he stressed.
Nor should SDI be used to monitor lesions with a peripheral globular pattern. And very slow-growing melanomas could potentially be missed by short-term SDI, so suspected lentigo maligna should be monitored for a minimum of 12 months, according to Dr. Marchetti.
Not every patient with an equivocal melanocytic lesion is a good candidate for SDI. It’s a monitoring strategy that should be reserved for reliable patients who will come back in 3 months. “If a patient doesn’t come back I take that very seriously. We call or send a letter,” Dr. Marchetti said.
Moreover, even in a patient who is a good candidate for SDI, he always offers the option of biopsy today rather than short-term monitoring.
SDI employed in conjunction with total-body photography is an extremely effective means of monitoring patients at very high risk for melanoma, Dr. Marchetti said. The power of this combination was illustrated in a prospective Australian study of 311 patients with a history of invasive melanoma plus either a high-risk genetic mutation or a strong family history. During a median follow-up of 3.5 years, 75 melanomas were detected, 14 of them at the baseline visit. The median thickness of melanomas detected post baseline was in situ. Thirty-nine percent of melanomas were detected using SDI and 38% via total body photography. Roughly one in five biopsied melanocytic lesions proved to be melanoma. Of note, five of the melanomas were more than 1 mm in Breslow thickness: Three of them were histologically desmoplastic, and the other two had nodular components (JAMA Dermatol. 2014 Aug;150(8):819-27).
For dermatologists who need to brush up on their dermoscopy skills, Dr. Marchetti recommended dermoscopedia as a useful, free resource.
Legal implications of monitoring via photography
“People often get worked up about this, but I’m not aware of a lawsuit alleging missed melanoma using baseline photography as evidence. And patients, in my experience, are universally appreciative of the use of imaging, although admittedly my experience is biased because people generally come to me for imaging,” Dr. Marchetti said.
He makes a point of telling every patient who opts for short-term SDI that, although the lesion has no features of concern now, it’s important to return promptly for reexamination should any changes occur.
Dr. Marchetti reported having no financial conflicts regarding his presentation.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
WAIKOLOA, HAWAII – Sequential dermoscopy imaging (SDI) is a valuable strategy for diagnosing melanomas early and with better sensitivity and specificity, compared with biopsy decisions based solely on the ugly duckling sign, the ABCDs of melanoma, or other aspects of lesion morphology, Michael A. Marchetti, MD, said at the Hawaii Dermatology Seminar provided by the Global Academy for Medical Education/Skin Disease Education Foundation.
SDI entails obtaining repeated dermoscopy images over time in order to detect subtle changes. It is typically done short term, over the course of 3-4 months, or longer term, over a period of 6 months to years, with long-term SDI being reserved for monitoring of less suspicious lesions, often in patients with an atypical mole syndrome.
SDI improves diagnostic specificity by dramatically reducing excision of benign pigmented lesions: in one large Belgian study, by up to 75% (Br J Dermatol. 2012 Oct;167[4]:778-86).
Short-term SDI also improves diagnostic sensitivity. That’s because it enables early identification of clinically featureless melanomas that are detected solely based upon change over a 3-month follow-up period. The operative principle here is that 93%-96% of melanomas will show change on dermoscopy within 3 months, while 99% of unchanged melanocytic lesions are benign. Since 16% of benign nevi will change within 3 months, that means 10%-30% of changed lesions are melanomas.
“If there is any change – it doesn’t matter what the change is, but the two images look different – that should lead to a biopsy,” explained Dr. Marchetti, a dermatologist at Memorial Sloan Kettering Cancer Center, New York.
As a result of this improved sensitivity and specificity, SDI has been shown to reduce the cost per melanoma diagnosis by about 40% (PLoS One. 2014 Oct 14;9[10]:e109339. doi: 10.1371/journal.pone.0109339).
Dr. Marchetti considers SDI a second-level diagnostic test for individual equivocal lesions. His first-level diagnostic tool is total-body photography.
SDI needs to be done by scrupulous examination of digital photographic images side-by-side on a computer monitor. A basic rule of SDI is that it should never be used to monitor raised or palpable lesions.
“The only thing you can monitor is something that’s flat,” he stressed.
Nor should SDI be used to monitor lesions with a peripheral globular pattern. And very slow-growing melanomas could potentially be missed by short-term SDI, so suspected lentigo maligna should be monitored for a minimum of 12 months, according to Dr. Marchetti.
Not every patient with an equivocal melanocytic lesion is a good candidate for SDI. It’s a monitoring strategy that should be reserved for reliable patients who will come back in 3 months. “If a patient doesn’t come back I take that very seriously. We call or send a letter,” Dr. Marchetti said.
Moreover, even in a patient who is a good candidate for SDI, he always offers the option of biopsy today rather than short-term monitoring.
SDI employed in conjunction with total-body photography is an extremely effective means of monitoring patients at very high risk for melanoma, Dr. Marchetti said. The power of this combination was illustrated in a prospective Australian study of 311 patients with a history of invasive melanoma plus either a high-risk genetic mutation or a strong family history. During a median follow-up of 3.5 years, 75 melanomas were detected, 14 of them at the baseline visit. The median thickness of melanomas detected post baseline was in situ. Thirty-nine percent of melanomas were detected using SDI and 38% via total body photography. Roughly one in five biopsied melanocytic lesions proved to be melanoma. Of note, five of the melanomas were more than 1 mm in Breslow thickness: Three of them were histologically desmoplastic, and the other two had nodular components (JAMA Dermatol. 2014 Aug;150(8):819-27).
For dermatologists who need to brush up on their dermoscopy skills, Dr. Marchetti recommended dermoscopedia as a useful, free resource.
Legal implications of monitoring via photography
“People often get worked up about this, but I’m not aware of a lawsuit alleging missed melanoma using baseline photography as evidence. And patients, in my experience, are universally appreciative of the use of imaging, although admittedly my experience is biased because people generally come to me for imaging,” Dr. Marchetti said.
He makes a point of telling every patient who opts for short-term SDI that, although the lesion has no features of concern now, it’s important to return promptly for reexamination should any changes occur.
Dr. Marchetti reported having no financial conflicts regarding his presentation.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
REPORTING FROM SDEF HAWAII DERMATOLOGY SEMINAR
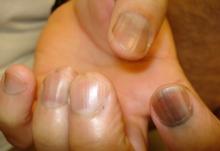
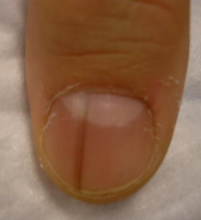
Five rules for evaluating melanonychia
WAIKOLOA, HAWAII – Many dermatologists find melanonychia to be intimidating. The clinical features are ambiguous, and the prospect of doing a painful nail apparatus biopsy can be daunting for the inexperienced. As a result, the biopsy gets delayed and melanoma of the nail is often initially a missed diagnosis, not uncommonly for years, with devastating consequences.
Here are five at the Hawaii Dermatology Seminar provided by the Global Academy for Medical Education/Skin Disease Education Foundation.
Rule #1: Always look beyond the nail
When a light-skinned person presents with more than one nail with pigmentation, the likelihood that one of them is melanoma is much less than if there is only one nail with melanonychia, according to Dr. Jellinek, a dermatologist in private practice in East Greenwich, R.I.
Also, be sure to look at the skin and mucosa. Consider the medications the patients may be taking: For example, cyclophosphamide (Cytoxan) is notorious for causing nail changes as a side effect. A past medical history of lichen planus, carpal tunnel syndrome, Addison disease, or other conditions may explain the melanonychia.
Laugier-Hunziker syndrome is a condition worth getting to know. It’s an acquired disorder characterized longitudinal melanonychia and other pigmentary changes, which may include diffuse hyperpigmentation of the orolabial mucosa, ocular pigment, and/or pigmented palmoplantar lesions. It’s said to be rare, but Dr. Jellinek disagrees.
“Learn this one if you don’t know it. I see a case about every 2 weeks. It’s not heritable and not associated with any other medical condition,” he said.
Rule #2: Your dermatoscope is great for nails
What Dr. Jellinek considers to be among the all-time best papers on the value of dermoscopy for nail pigmentation was authored by French investigators. They analyzed 148 consecutive cases of longitudinal melanonychia and concluded that the dermoscopic combination of a brown background coupled with irregular longitudinal lines in terms of color, spacing, diameter, and/or lack of parallelism strongly suggests melanoma. A micro-Hutchinson’s sign, while a rare finding, occurred only in melanoma, where it represented periungual spread of a radial growth phase malignancy (Arch Dermatol. 2002 Oct;138[10]:1327-33).
“I think nail dermoscopy is most helpful for subungual hemorrhage. I average one referral per week for hemorrhage under the nail. On dermoscopy it’s as if someone took paint and threw it at the nail. Purple to brown blood spots, with no background color. This should be a doorway diagnosis of hemorrhage,” Dr. Jellinek said.
Rule #3: Know when you don’t know
“This is really the key for me,” the dermatologist commented. “There are automatic cases for biopsy, and more commonly routine cases for reassurance. But the gray zone, when you know you don’t know, is the key decision making moment.”
When something just doesn’t feel right, there’s absolutely nothing wrong with getting a second opinion, he stressed.
“It’s worthwhile getting to know people whose opinions you trust. There’s a saying I like to teach our fellows: ‘Never worry alone.’ So if you’re worried about someone, listen to that inner voice. There’s no shame in getting a second opinion. It’s great! Patients are never upset, either. They feel really well taken care of,” he said.
Rule #4: Don’t wimp out when a biopsy is warranted
Many dermatologists hem and haw about doing a biopsy for a concerning lesion on the nail, when they wouldn’t hesitate to biopsy a similarly suspicious lesion on the face.
But it’s essential to biopsy the right area, he added. For longitudinal melanonychia, that’s the matrix. The nail plate is the wrong place; a biopsy obtained there will result in an inappropriate benign diagnosis.
“The starter set is to do a punch biopsy. This is your gateway drug to the world of nail surgery. Lots of dermatologists are intimidated by nail surgery, but if you can do any minor surgery, you can do a punch of the matrix. All it takes is a little practice. And if all you can do is punch biopsies, you’re good for your career. If you can do that, you’re golden. There are people who’ve just done punch biopsies for their whole career and they don’t miss melanomas,” he said.
Step one is to undermine the proximal nail fold using a pediatric elevator, which costs only about $30. “If you’re going to do a lot of nail surgery, they’re really helpful,” he said.
There’s no need at all to evulse the nail. Just make oblique incisions in the proximal nail fold in order to reflect it and look at the matrix. A 3-mm punch is standard, directed right over the origin of the pigment. Resist the temptation to force or squeeze the specimen in order to extract it. Instead, use really fine-tipped scissors to nibble at the base of the specimen, then gently pull it out, making an effort to keep the nail plate attached to the digit and avoid getting it stuck up in the punch.
Rule #5: Have dermatopathologists extensively experienced with nail pathology on your Rolodex
The histopathologic findings present in early subungual melanoma in situ are often too subtle for general dermatopathologists to appreciate, in Dr. Jellinek’s experience. He cited other investigators’ study of 18 cases of subungual melanoma in situ, all marked by longitudinal melanonychia. Only half showed the classic giveaway on the original nail matrix biopsy, consisting of a significantly increased number of atypical melanocytes with marked nuclear atypia. Blatant pagetoid spread was infrequent. However, all 18 cases displayed a novel, more subtle, and previously undescribed finding: haphazard and uneven distribution of atypical solitary melanocytes with variably sized and shaped hyperchromatic nuclei (J Cutan Pathol. 2016 Jan;43[1]:41-52).
Dr. Jellinek reported having no financial conflicts regarding his presentation. SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
WAIKOLOA, HAWAII – Many dermatologists find melanonychia to be intimidating. The clinical features are ambiguous, and the prospect of doing a painful nail apparatus biopsy can be daunting for the inexperienced. As a result, the biopsy gets delayed and melanoma of the nail is often initially a missed diagnosis, not uncommonly for years, with devastating consequences.
Here are five at the Hawaii Dermatology Seminar provided by the Global Academy for Medical Education/Skin Disease Education Foundation.
Rule #1: Always look beyond the nail
When a light-skinned person presents with more than one nail with pigmentation, the likelihood that one of them is melanoma is much less than if there is only one nail with melanonychia, according to Dr. Jellinek, a dermatologist in private practice in East Greenwich, R.I.
Also, be sure to look at the skin and mucosa. Consider the medications the patients may be taking: For example, cyclophosphamide (Cytoxan) is notorious for causing nail changes as a side effect. A past medical history of lichen planus, carpal tunnel syndrome, Addison disease, or other conditions may explain the melanonychia.
Laugier-Hunziker syndrome is a condition worth getting to know. It’s an acquired disorder characterized longitudinal melanonychia and other pigmentary changes, which may include diffuse hyperpigmentation of the orolabial mucosa, ocular pigment, and/or pigmented palmoplantar lesions. It’s said to be rare, but Dr. Jellinek disagrees.
“Learn this one if you don’t know it. I see a case about every 2 weeks. It’s not heritable and not associated with any other medical condition,” he said.
Rule #2: Your dermatoscope is great for nails
What Dr. Jellinek considers to be among the all-time best papers on the value of dermoscopy for nail pigmentation was authored by French investigators. They analyzed 148 consecutive cases of longitudinal melanonychia and concluded that the dermoscopic combination of a brown background coupled with irregular longitudinal lines in terms of color, spacing, diameter, and/or lack of parallelism strongly suggests melanoma. A micro-Hutchinson’s sign, while a rare finding, occurred only in melanoma, where it represented periungual spread of a radial growth phase malignancy (Arch Dermatol. 2002 Oct;138[10]:1327-33).
“I think nail dermoscopy is most helpful for subungual hemorrhage. I average one referral per week for hemorrhage under the nail. On dermoscopy it’s as if someone took paint and threw it at the nail. Purple to brown blood spots, with no background color. This should be a doorway diagnosis of hemorrhage,” Dr. Jellinek said.
Rule #3: Know when you don’t know
“This is really the key for me,” the dermatologist commented. “There are automatic cases for biopsy, and more commonly routine cases for reassurance. But the gray zone, when you know you don’t know, is the key decision making moment.”
When something just doesn’t feel right, there’s absolutely nothing wrong with getting a second opinion, he stressed.
“It’s worthwhile getting to know people whose opinions you trust. There’s a saying I like to teach our fellows: ‘Never worry alone.’ So if you’re worried about someone, listen to that inner voice. There’s no shame in getting a second opinion. It’s great! Patients are never upset, either. They feel really well taken care of,” he said.
Rule #4: Don’t wimp out when a biopsy is warranted
Many dermatologists hem and haw about doing a biopsy for a concerning lesion on the nail, when they wouldn’t hesitate to biopsy a similarly suspicious lesion on the face.
But it’s essential to biopsy the right area, he added. For longitudinal melanonychia, that’s the matrix. The nail plate is the wrong place; a biopsy obtained there will result in an inappropriate benign diagnosis.
“The starter set is to do a punch biopsy. This is your gateway drug to the world of nail surgery. Lots of dermatologists are intimidated by nail surgery, but if you can do any minor surgery, you can do a punch of the matrix. All it takes is a little practice. And if all you can do is punch biopsies, you’re good for your career. If you can do that, you’re golden. There are people who’ve just done punch biopsies for their whole career and they don’t miss melanomas,” he said.
Step one is to undermine the proximal nail fold using a pediatric elevator, which costs only about $30. “If you’re going to do a lot of nail surgery, they’re really helpful,” he said.
There’s no need at all to evulse the nail. Just make oblique incisions in the proximal nail fold in order to reflect it and look at the matrix. A 3-mm punch is standard, directed right over the origin of the pigment. Resist the temptation to force or squeeze the specimen in order to extract it. Instead, use really fine-tipped scissors to nibble at the base of the specimen, then gently pull it out, making an effort to keep the nail plate attached to the digit and avoid getting it stuck up in the punch.
Rule #5: Have dermatopathologists extensively experienced with nail pathology on your Rolodex
The histopathologic findings present in early subungual melanoma in situ are often too subtle for general dermatopathologists to appreciate, in Dr. Jellinek’s experience. He cited other investigators’ study of 18 cases of subungual melanoma in situ, all marked by longitudinal melanonychia. Only half showed the classic giveaway on the original nail matrix biopsy, consisting of a significantly increased number of atypical melanocytes with marked nuclear atypia. Blatant pagetoid spread was infrequent. However, all 18 cases displayed a novel, more subtle, and previously undescribed finding: haphazard and uneven distribution of atypical solitary melanocytes with variably sized and shaped hyperchromatic nuclei (J Cutan Pathol. 2016 Jan;43[1]:41-52).
Dr. Jellinek reported having no financial conflicts regarding his presentation. SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
WAIKOLOA, HAWAII – Many dermatologists find melanonychia to be intimidating. The clinical features are ambiguous, and the prospect of doing a painful nail apparatus biopsy can be daunting for the inexperienced. As a result, the biopsy gets delayed and melanoma of the nail is often initially a missed diagnosis, not uncommonly for years, with devastating consequences.
Here are five at the Hawaii Dermatology Seminar provided by the Global Academy for Medical Education/Skin Disease Education Foundation.
Rule #1: Always look beyond the nail
When a light-skinned person presents with more than one nail with pigmentation, the likelihood that one of them is melanoma is much less than if there is only one nail with melanonychia, according to Dr. Jellinek, a dermatologist in private practice in East Greenwich, R.I.
Also, be sure to look at the skin and mucosa. Consider the medications the patients may be taking: For example, cyclophosphamide (Cytoxan) is notorious for causing nail changes as a side effect. A past medical history of lichen planus, carpal tunnel syndrome, Addison disease, or other conditions may explain the melanonychia.
Laugier-Hunziker syndrome is a condition worth getting to know. It’s an acquired disorder characterized longitudinal melanonychia and other pigmentary changes, which may include diffuse hyperpigmentation of the orolabial mucosa, ocular pigment, and/or pigmented palmoplantar lesions. It’s said to be rare, but Dr. Jellinek disagrees.
“Learn this one if you don’t know it. I see a case about every 2 weeks. It’s not heritable and not associated with any other medical condition,” he said.
Rule #2: Your dermatoscope is great for nails
What Dr. Jellinek considers to be among the all-time best papers on the value of dermoscopy for nail pigmentation was authored by French investigators. They analyzed 148 consecutive cases of longitudinal melanonychia and concluded that the dermoscopic combination of a brown background coupled with irregular longitudinal lines in terms of color, spacing, diameter, and/or lack of parallelism strongly suggests melanoma. A micro-Hutchinson’s sign, while a rare finding, occurred only in melanoma, where it represented periungual spread of a radial growth phase malignancy (Arch Dermatol. 2002 Oct;138[10]:1327-33).
“I think nail dermoscopy is most helpful for subungual hemorrhage. I average one referral per week for hemorrhage under the nail. On dermoscopy it’s as if someone took paint and threw it at the nail. Purple to brown blood spots, with no background color. This should be a doorway diagnosis of hemorrhage,” Dr. Jellinek said.
Rule #3: Know when you don’t know
“This is really the key for me,” the dermatologist commented. “There are automatic cases for biopsy, and more commonly routine cases for reassurance. But the gray zone, when you know you don’t know, is the key decision making moment.”
When something just doesn’t feel right, there’s absolutely nothing wrong with getting a second opinion, he stressed.
“It’s worthwhile getting to know people whose opinions you trust. There’s a saying I like to teach our fellows: ‘Never worry alone.’ So if you’re worried about someone, listen to that inner voice. There’s no shame in getting a second opinion. It’s great! Patients are never upset, either. They feel really well taken care of,” he said.
Rule #4: Don’t wimp out when a biopsy is warranted
Many dermatologists hem and haw about doing a biopsy for a concerning lesion on the nail, when they wouldn’t hesitate to biopsy a similarly suspicious lesion on the face.
But it’s essential to biopsy the right area, he added. For longitudinal melanonychia, that’s the matrix. The nail plate is the wrong place; a biopsy obtained there will result in an inappropriate benign diagnosis.
“The starter set is to do a punch biopsy. This is your gateway drug to the world of nail surgery. Lots of dermatologists are intimidated by nail surgery, but if you can do any minor surgery, you can do a punch of the matrix. All it takes is a little practice. And if all you can do is punch biopsies, you’re good for your career. If you can do that, you’re golden. There are people who’ve just done punch biopsies for their whole career and they don’t miss melanomas,” he said.
Step one is to undermine the proximal nail fold using a pediatric elevator, which costs only about $30. “If you’re going to do a lot of nail surgery, they’re really helpful,” he said.
There’s no need at all to evulse the nail. Just make oblique incisions in the proximal nail fold in order to reflect it and look at the matrix. A 3-mm punch is standard, directed right over the origin of the pigment. Resist the temptation to force or squeeze the specimen in order to extract it. Instead, use really fine-tipped scissors to nibble at the base of the specimen, then gently pull it out, making an effort to keep the nail plate attached to the digit and avoid getting it stuck up in the punch.
Rule #5: Have dermatopathologists extensively experienced with nail pathology on your Rolodex
The histopathologic findings present in early subungual melanoma in situ are often too subtle for general dermatopathologists to appreciate, in Dr. Jellinek’s experience. He cited other investigators’ study of 18 cases of subungual melanoma in situ, all marked by longitudinal melanonychia. Only half showed the classic giveaway on the original nail matrix biopsy, consisting of a significantly increased number of atypical melanocytes with marked nuclear atypia. Blatant pagetoid spread was infrequent. However, all 18 cases displayed a novel, more subtle, and previously undescribed finding: haphazard and uneven distribution of atypical solitary melanocytes with variably sized and shaped hyperchromatic nuclei (J Cutan Pathol. 2016 Jan;43[1]:41-52).
Dr. Jellinek reported having no financial conflicts regarding his presentation. SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
EXPERT ANALYSIS FROM SDEF HAWAII DERMATOLOGY SEMINAR
Patch testing in atopic dermatitis: when and how
WAIKOLOA, HAWAII – The according to Jonathan I. Silverberg, MD, PhD.
“What are atopic dermatitis patients allergic to? It’s all coming from their personal care products and the things being used to treat their atopic dermatitis,” Dr. Silverberg said at the Hawaii Dermatology Seminar provided by the Global Academy for Medical Education/Skin Disease Education Foundation.
Dr. Silverberg, of the department of dermatology at Northwestern University, Chicago, coauthored a systematic review and meta-analysis that examined the association between AD and contact sensitization. In their examination of 74 published studies, the investigators found that the likelihood of allergic contact dermatitis was 1.5-fold greater in adults and children with AD than in healthy individuals from the general population (J Am Acad Dermatol. 2017 Jul;77[1]:70-8).
This finding is at odds with an earlier widespread belief that AD patients should not be at increased risk because the immune profile of their primarily Th2-mediated disease would have a suppressant effect on Th1-mediated hypersensitivity.
“Recent data are calling into question old dogmas and reshaping the way we think about this. And this is not just an academic exercise, this is highly clinically relevant,” the dermatologist asserted.
The results of the meta-analysis prompted Dr. Silverberg and colleagues to conduct a retrospective study of more than 500 adults patch tested to an expanded allergen series at Northwestern’s patch test clinic with the purpose of identifying the common offending allergens in patients with AD. The key finding: The patients with AD were significantly more likely to have positive patch test reactions to ingredients in their repetitively used personal care products, topical corticosteroids, and topical antibiotics than the individuals without AD. The probable explanation for this results is that the skin barrier disruption inherent in AD allows for easier passage of weak allergens through the skin (J Am Acad Dermatol. 2018 Dec;79[6]:1028-33.e6).
Lanolin was identified as a particularly common allergen in the AD group. “Lanolin is found in one of the most commonly used moisturizers we recommend to patients: Aquaphor. It’s also found in tons of lip balms and emollients. Pretty much every soft soap out there contains lanolin, and it’s in a variety of other personal care products,” Dr. Silverberg noted.
Other common offenders in the AD population included fragrance mix II, cinnamal, quaternium-15, budesonide, tixocortol, carba mix, neomycin, bacitracin, rubber mix, and chlorhexidine. Relevance was established in more than 90% of the positive reactions.
“You can patch test them directly to their personal care products and make that connection beautifully and see how they’re reacting to them,” he said.
When to patch test atopic dermatitis patients
Dr. Silverberg was a coauthor of multidisciplinary expert consensus guidelines on when to consider patch testing in AD (Dermatitis. 2016 Jul-Aug;27[4]:186-92). “We had to go consensus because we don’t have nearly enough studies to provide true evidence-based recommendations,” he explained.
Because allergic contact dermatitis is a potentially curable comorbid condition in AD patients, it’s important to recognize the scenarios in which patch testing should be considered. These include AD refractory to topical therapy; adolescent- or adult-onset atopic dermatitis; and in AD patients with an atypical or evolving lesional distribution, such as localized dermatitis on the eyelids, head and neck, or hands and feet. Patch testing is also warranted before initiating systemic therapy for AD.
“If you’re about to put a patient on a biologic or phototherapy and step them up to a whole new class of risk of adverse events, that’s an ideal time to think about reversible options,” Dr. Silverberg advised.
Another situation in which he considers patch testing advisable, although this one isn’t covered in the consensus guidelines, is in AD patients with prominent nummular eczema lesions. “Widespread nummular eczema lesions may be a sign of allergic contact dermatitis in atopic dermatitis patients. I’m not saying everyone with nummular lesions is going to have a positive patch test, but it’s definitely a situation you want to think about,” he said.
How to patch test atopic dermatitis patients
Most of the common topical allergens in AD patients are not included in the T.R.U.E. Test. An expanded allergen series, such as the American Contact Dermatitis Society core 80 series, is the better way to go.
Once the dermatologist determines that a patient’s positive patch test reaction is relevant, it’s important to recommend the use of personal care products that are “pretty clean,” Dr. Silverberg said.
“Clean in my opinion is not a matter of ‘It should be all organic and all natural,’ ” he emphasized. “I’m not anti- any of that, but clean means having the fewest ingredients possible and trying to steer clear of those really common allergens that patients are highly likely to have been exposed to and potentially sensitized to over the many years of their tenure of atopic dermatitis.”
Dr. Silverberg reported receiving research grants from Galderma and GlaxoSmithKline and serving as a consultant to more than a dozen pharmaceutical companies.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
WAIKOLOA, HAWAII – The according to Jonathan I. Silverberg, MD, PhD.
“What are atopic dermatitis patients allergic to? It’s all coming from their personal care products and the things being used to treat their atopic dermatitis,” Dr. Silverberg said at the Hawaii Dermatology Seminar provided by the Global Academy for Medical Education/Skin Disease Education Foundation.
Dr. Silverberg, of the department of dermatology at Northwestern University, Chicago, coauthored a systematic review and meta-analysis that examined the association between AD and contact sensitization. In their examination of 74 published studies, the investigators found that the likelihood of allergic contact dermatitis was 1.5-fold greater in adults and children with AD than in healthy individuals from the general population (J Am Acad Dermatol. 2017 Jul;77[1]:70-8).
This finding is at odds with an earlier widespread belief that AD patients should not be at increased risk because the immune profile of their primarily Th2-mediated disease would have a suppressant effect on Th1-mediated hypersensitivity.
“Recent data are calling into question old dogmas and reshaping the way we think about this. And this is not just an academic exercise, this is highly clinically relevant,” the dermatologist asserted.
The results of the meta-analysis prompted Dr. Silverberg and colleagues to conduct a retrospective study of more than 500 adults patch tested to an expanded allergen series at Northwestern’s patch test clinic with the purpose of identifying the common offending allergens in patients with AD. The key finding: The patients with AD were significantly more likely to have positive patch test reactions to ingredients in their repetitively used personal care products, topical corticosteroids, and topical antibiotics than the individuals without AD. The probable explanation for this results is that the skin barrier disruption inherent in AD allows for easier passage of weak allergens through the skin (J Am Acad Dermatol. 2018 Dec;79[6]:1028-33.e6).
Lanolin was identified as a particularly common allergen in the AD group. “Lanolin is found in one of the most commonly used moisturizers we recommend to patients: Aquaphor. It’s also found in tons of lip balms and emollients. Pretty much every soft soap out there contains lanolin, and it’s in a variety of other personal care products,” Dr. Silverberg noted.
Other common offenders in the AD population included fragrance mix II, cinnamal, quaternium-15, budesonide, tixocortol, carba mix, neomycin, bacitracin, rubber mix, and chlorhexidine. Relevance was established in more than 90% of the positive reactions.
“You can patch test them directly to their personal care products and make that connection beautifully and see how they’re reacting to them,” he said.
When to patch test atopic dermatitis patients
Dr. Silverberg was a coauthor of multidisciplinary expert consensus guidelines on when to consider patch testing in AD (Dermatitis. 2016 Jul-Aug;27[4]:186-92). “We had to go consensus because we don’t have nearly enough studies to provide true evidence-based recommendations,” he explained.
Because allergic contact dermatitis is a potentially curable comorbid condition in AD patients, it’s important to recognize the scenarios in which patch testing should be considered. These include AD refractory to topical therapy; adolescent- or adult-onset atopic dermatitis; and in AD patients with an atypical or evolving lesional distribution, such as localized dermatitis on the eyelids, head and neck, or hands and feet. Patch testing is also warranted before initiating systemic therapy for AD.
“If you’re about to put a patient on a biologic or phototherapy and step them up to a whole new class of risk of adverse events, that’s an ideal time to think about reversible options,” Dr. Silverberg advised.
Another situation in which he considers patch testing advisable, although this one isn’t covered in the consensus guidelines, is in AD patients with prominent nummular eczema lesions. “Widespread nummular eczema lesions may be a sign of allergic contact dermatitis in atopic dermatitis patients. I’m not saying everyone with nummular lesions is going to have a positive patch test, but it’s definitely a situation you want to think about,” he said.
How to patch test atopic dermatitis patients
Most of the common topical allergens in AD patients are not included in the T.R.U.E. Test. An expanded allergen series, such as the American Contact Dermatitis Society core 80 series, is the better way to go.
Once the dermatologist determines that a patient’s positive patch test reaction is relevant, it’s important to recommend the use of personal care products that are “pretty clean,” Dr. Silverberg said.
“Clean in my opinion is not a matter of ‘It should be all organic and all natural,’ ” he emphasized. “I’m not anti- any of that, but clean means having the fewest ingredients possible and trying to steer clear of those really common allergens that patients are highly likely to have been exposed to and potentially sensitized to over the many years of their tenure of atopic dermatitis.”
Dr. Silverberg reported receiving research grants from Galderma and GlaxoSmithKline and serving as a consultant to more than a dozen pharmaceutical companies.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
WAIKOLOA, HAWAII – The according to Jonathan I. Silverberg, MD, PhD.
“What are atopic dermatitis patients allergic to? It’s all coming from their personal care products and the things being used to treat their atopic dermatitis,” Dr. Silverberg said at the Hawaii Dermatology Seminar provided by the Global Academy for Medical Education/Skin Disease Education Foundation.
Dr. Silverberg, of the department of dermatology at Northwestern University, Chicago, coauthored a systematic review and meta-analysis that examined the association between AD and contact sensitization. In their examination of 74 published studies, the investigators found that the likelihood of allergic contact dermatitis was 1.5-fold greater in adults and children with AD than in healthy individuals from the general population (J Am Acad Dermatol. 2017 Jul;77[1]:70-8).
This finding is at odds with an earlier widespread belief that AD patients should not be at increased risk because the immune profile of their primarily Th2-mediated disease would have a suppressant effect on Th1-mediated hypersensitivity.
“Recent data are calling into question old dogmas and reshaping the way we think about this. And this is not just an academic exercise, this is highly clinically relevant,” the dermatologist asserted.
The results of the meta-analysis prompted Dr. Silverberg and colleagues to conduct a retrospective study of more than 500 adults patch tested to an expanded allergen series at Northwestern’s patch test clinic with the purpose of identifying the common offending allergens in patients with AD. The key finding: The patients with AD were significantly more likely to have positive patch test reactions to ingredients in their repetitively used personal care products, topical corticosteroids, and topical antibiotics than the individuals without AD. The probable explanation for this results is that the skin barrier disruption inherent in AD allows for easier passage of weak allergens through the skin (J Am Acad Dermatol. 2018 Dec;79[6]:1028-33.e6).
Lanolin was identified as a particularly common allergen in the AD group. “Lanolin is found in one of the most commonly used moisturizers we recommend to patients: Aquaphor. It’s also found in tons of lip balms and emollients. Pretty much every soft soap out there contains lanolin, and it’s in a variety of other personal care products,” Dr. Silverberg noted.
Other common offenders in the AD population included fragrance mix II, cinnamal, quaternium-15, budesonide, tixocortol, carba mix, neomycin, bacitracin, rubber mix, and chlorhexidine. Relevance was established in more than 90% of the positive reactions.
“You can patch test them directly to their personal care products and make that connection beautifully and see how they’re reacting to them,” he said.
When to patch test atopic dermatitis patients
Dr. Silverberg was a coauthor of multidisciplinary expert consensus guidelines on when to consider patch testing in AD (Dermatitis. 2016 Jul-Aug;27[4]:186-92). “We had to go consensus because we don’t have nearly enough studies to provide true evidence-based recommendations,” he explained.
Because allergic contact dermatitis is a potentially curable comorbid condition in AD patients, it’s important to recognize the scenarios in which patch testing should be considered. These include AD refractory to topical therapy; adolescent- or adult-onset atopic dermatitis; and in AD patients with an atypical or evolving lesional distribution, such as localized dermatitis on the eyelids, head and neck, or hands and feet. Patch testing is also warranted before initiating systemic therapy for AD.
“If you’re about to put a patient on a biologic or phototherapy and step them up to a whole new class of risk of adverse events, that’s an ideal time to think about reversible options,” Dr. Silverberg advised.
Another situation in which he considers patch testing advisable, although this one isn’t covered in the consensus guidelines, is in AD patients with prominent nummular eczema lesions. “Widespread nummular eczema lesions may be a sign of allergic contact dermatitis in atopic dermatitis patients. I’m not saying everyone with nummular lesions is going to have a positive patch test, but it’s definitely a situation you want to think about,” he said.
How to patch test atopic dermatitis patients
Most of the common topical allergens in AD patients are not included in the T.R.U.E. Test. An expanded allergen series, such as the American Contact Dermatitis Society core 80 series, is the better way to go.
Once the dermatologist determines that a patient’s positive patch test reaction is relevant, it’s important to recommend the use of personal care products that are “pretty clean,” Dr. Silverberg said.
“Clean in my opinion is not a matter of ‘It should be all organic and all natural,’ ” he emphasized. “I’m not anti- any of that, but clean means having the fewest ingredients possible and trying to steer clear of those really common allergens that patients are highly likely to have been exposed to and potentially sensitized to over the many years of their tenure of atopic dermatitis.”
Dr. Silverberg reported receiving research grants from Galderma and GlaxoSmithKline and serving as a consultant to more than a dozen pharmaceutical companies.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
EXPERT ANALYSIS FROM SDEF HAWAII DERMATOLOGY SEMINAR
Consider 9-mm surgical margins for MIS
WAIKOLOA, HAWAII – The widely utilized 5-mm surgical margins for excision of melanoma in situ are inadequate in many cases, Christopher B. Zachary, MD, said at the Hawaii Dermatology Seminar provided by the Global Academy for Medical Education/Skin Disease Education Foundation.
“You probably should be considering more like 9- or 10-mm margins for melanoma in situ,” advised Dr. Zachary, professor and chair of the department of dermatology at the University of California, Irvine.
This has been a controversial matter. The recommendation for the long-standard 5-mm margins for excision of melanoma in situ (MIS) date back to a 1992 consensus opinion. Since then, however, persuasive data have emerged showing that 5-mm margins are often inadequate for clearance, and the latest American Academy of Dermatology guidelines for the management of primary cutaneous melanoma recommend margins of 5-10 mm (J Am Acad Dermatol. 2019 Jan;80[1]:208-50).
Dr. Zachary’s advice to go on the high side of that 5- to 10-mm zone is based in large part on studies led by John A Zitelli, MD, of the University of Pittsburgh. More than 20 years ago, Dr. Zitelli and his coinvestigators published a provocative prospective series of 535 patients whose melanomas – in situ or invasive – were excised via Mohs micrographic surgery with frozen section examination of the margins. A 9-mm margin successfully removed 95% of the melanomas, a 12-mm margin removed 97%, and a 6-mm margin successfully excised only 83% of the lesions (J Am Acad Dermatol. 1997 Sep;37(3 Pt 1):422-9).
In a follow-up study, Dr. Zitelli and his colleagues reported on a prospective series of 1,072 patients with 1,120 MIS, all excised by Mohs micrographic surgery with frozen sections (J Am Acad Dermatol. 2012 Mar;66[3]:438-44). They determined that 86% of the MIS were completely cleared using a 6-mm margin, compared with 98.9% excised with a 9 mm margin, a statistically significant difference (P less than .001).
Support for Dr. Zitelli’s stance that 5-mm margins for MIS are inadequate was provided by dermatologic surgeons at the Mayo Clinic in Scottsdale, Ariz. Of 46 patients who underwent Mohs micrographic surgery with immunostaining for excision of MIS, margins of 6 mm achieved clearance in only half of them. Surgical excision margins of 15 mm were required to successfully clear 96% of the MIS (Dermatol Surg. 2000 Aug;26[8]:771-84).
Quite a few hands shot up when Dr. Zachary asked how many members of his audience utilize 5-mm margins for surgical excision of MIS.
“That had been my practice as well until quite recently,” he said.
Dr. Zachary reported having no financial conflicts of interest regarding his presentation.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
WAIKOLOA, HAWAII – The widely utilized 5-mm surgical margins for excision of melanoma in situ are inadequate in many cases, Christopher B. Zachary, MD, said at the Hawaii Dermatology Seminar provided by the Global Academy for Medical Education/Skin Disease Education Foundation.
“You probably should be considering more like 9- or 10-mm margins for melanoma in situ,” advised Dr. Zachary, professor and chair of the department of dermatology at the University of California, Irvine.
This has been a controversial matter. The recommendation for the long-standard 5-mm margins for excision of melanoma in situ (MIS) date back to a 1992 consensus opinion. Since then, however, persuasive data have emerged showing that 5-mm margins are often inadequate for clearance, and the latest American Academy of Dermatology guidelines for the management of primary cutaneous melanoma recommend margins of 5-10 mm (J Am Acad Dermatol. 2019 Jan;80[1]:208-50).
Dr. Zachary’s advice to go on the high side of that 5- to 10-mm zone is based in large part on studies led by John A Zitelli, MD, of the University of Pittsburgh. More than 20 years ago, Dr. Zitelli and his coinvestigators published a provocative prospective series of 535 patients whose melanomas – in situ or invasive – were excised via Mohs micrographic surgery with frozen section examination of the margins. A 9-mm margin successfully removed 95% of the melanomas, a 12-mm margin removed 97%, and a 6-mm margin successfully excised only 83% of the lesions (J Am Acad Dermatol. 1997 Sep;37(3 Pt 1):422-9).
In a follow-up study, Dr. Zitelli and his colleagues reported on a prospective series of 1,072 patients with 1,120 MIS, all excised by Mohs micrographic surgery with frozen sections (J Am Acad Dermatol. 2012 Mar;66[3]:438-44). They determined that 86% of the MIS were completely cleared using a 6-mm margin, compared with 98.9% excised with a 9 mm margin, a statistically significant difference (P less than .001).
Support for Dr. Zitelli’s stance that 5-mm margins for MIS are inadequate was provided by dermatologic surgeons at the Mayo Clinic in Scottsdale, Ariz. Of 46 patients who underwent Mohs micrographic surgery with immunostaining for excision of MIS, margins of 6 mm achieved clearance in only half of them. Surgical excision margins of 15 mm were required to successfully clear 96% of the MIS (Dermatol Surg. 2000 Aug;26[8]:771-84).
Quite a few hands shot up when Dr. Zachary asked how many members of his audience utilize 5-mm margins for surgical excision of MIS.
“That had been my practice as well until quite recently,” he said.
Dr. Zachary reported having no financial conflicts of interest regarding his presentation.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
WAIKOLOA, HAWAII – The widely utilized 5-mm surgical margins for excision of melanoma in situ are inadequate in many cases, Christopher B. Zachary, MD, said at the Hawaii Dermatology Seminar provided by the Global Academy for Medical Education/Skin Disease Education Foundation.
“You probably should be considering more like 9- or 10-mm margins for melanoma in situ,” advised Dr. Zachary, professor and chair of the department of dermatology at the University of California, Irvine.
This has been a controversial matter. The recommendation for the long-standard 5-mm margins for excision of melanoma in situ (MIS) date back to a 1992 consensus opinion. Since then, however, persuasive data have emerged showing that 5-mm margins are often inadequate for clearance, and the latest American Academy of Dermatology guidelines for the management of primary cutaneous melanoma recommend margins of 5-10 mm (J Am Acad Dermatol. 2019 Jan;80[1]:208-50).
Dr. Zachary’s advice to go on the high side of that 5- to 10-mm zone is based in large part on studies led by John A Zitelli, MD, of the University of Pittsburgh. More than 20 years ago, Dr. Zitelli and his coinvestigators published a provocative prospective series of 535 patients whose melanomas – in situ or invasive – were excised via Mohs micrographic surgery with frozen section examination of the margins. A 9-mm margin successfully removed 95% of the melanomas, a 12-mm margin removed 97%, and a 6-mm margin successfully excised only 83% of the lesions (J Am Acad Dermatol. 1997 Sep;37(3 Pt 1):422-9).
In a follow-up study, Dr. Zitelli and his colleagues reported on a prospective series of 1,072 patients with 1,120 MIS, all excised by Mohs micrographic surgery with frozen sections (J Am Acad Dermatol. 2012 Mar;66[3]:438-44). They determined that 86% of the MIS were completely cleared using a 6-mm margin, compared with 98.9% excised with a 9 mm margin, a statistically significant difference (P less than .001).
Support for Dr. Zitelli’s stance that 5-mm margins for MIS are inadequate was provided by dermatologic surgeons at the Mayo Clinic in Scottsdale, Ariz. Of 46 patients who underwent Mohs micrographic surgery with immunostaining for excision of MIS, margins of 6 mm achieved clearance in only half of them. Surgical excision margins of 15 mm were required to successfully clear 96% of the MIS (Dermatol Surg. 2000 Aug;26[8]:771-84).
Quite a few hands shot up when Dr. Zachary asked how many members of his audience utilize 5-mm margins for surgical excision of MIS.
“That had been my practice as well until quite recently,” he said.
Dr. Zachary reported having no financial conflicts of interest regarding his presentation.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
REPORTING FROM SDEF HAWAII DERMATOLOGY SEMINAR
Use time-appropriate scar improvement sequencing
WAIKOLOA, HAWAII – , according to American Academy of Dermatology President-elect George J. Hruza, MD – and he’s got a raft of them.
“There are going to be situations where your scars aren’t going to be as wonderful as you’d like, or even if they’re pretty good, you might improve them further if you do some modifications,” he observed at the Hawaii Dermatology Seminar provided by the Global Academy for Medical Education/Skin Disease Education Foundation.
He became convinced of the importance of having a large toolbox for scar improvement in part as a result of an Australian prospective study of 576 patients surveyed 6-9 months following skin cancer surgery. Far and away the most important factor influencing patients’ overall perception of their experience wasn’t the cost, pain, quality of nursing care, complications, wait time prior to surgery, or gratitude that they’d successfully had a cancer removed. It was their perception of the scar (J Am Acad Dermatol. 2007 Sep;57[3]:445-53).
To be effective, interventions for scar improvement need to be timed in sync with the three phases of cellular activity involved in wound healing. For example, neurotoxin injections are effective during the first few days of the initial acute inflammation period, when cellular migration is active. Silicone and taping are of value when employed long term, starting at about 1 month and continuing for 3-6 months, throughout the neovascularization/granulation phase, then the time of fibroblast proliferation and matrix formation that follows, and even beyond. Pulsed dye and fractionated ablative lasers are best utilized to reshape matrix formation, starting at about 2 weeks. Intervention using dermabrasion or fillers has to wait for the scar to be a bit more mature, at about 2 months; utilized earlier these can cause dehiscence, explained Dr. Hruza of St. Louis University.
He shared what he called his “scar improvement hierarchy,” the sequence of interventions he turns to from the most to least often. But he began with prevention, noting that more than 2 decades ago, he and his coinvestigators demonstrated that running horizontal mattress sutures for primary closures of facial wounds provide better cosmetic results, with a final scar that’s smoother and flatter than the more commonly used simple running sutures (Dermatol Surg. 2005 Oct;31[10]:1313-6).
Scar improvement sequence
Massage. “I recommend this to almost every patient. I have them start at about 6 weeks and do it for several months. It’s really more like kneading dough, not rubbing. You want the skin pressing on the bone underneath,” according to Dr. Hruza. Various investigators have suggested that scar massage works by increasing hydration and capillary proliferation, while promoting desensitization, but the evidence is really anecdotal.
“I think it’s mainly tincture of time. Scars get better on their own,” he observed. Regardless, massage allows patients the satisfaction of actively participating in their own recovery.
Intralesional triamcinolone. Dr. Hruza calls this “our big friend.”
“I find that 90% of the time when you look at a thickened scar and you think, ‘Oh gee, I’m going to have to do some scar revision, the intralesional triamcinolone takes care of the problem,” he said. He usually injects the site at about 6 weeks post surgery using 10 mg/mL. If the response is inadequate he reinjects about a month later using 20 mg/mL. He generally avoids going to 40 mg/mL for facial scars. The goal is to make therapeutic use of the steroid’s major side effect – atrophy – to shrink the thickened scar. But because this can be a tricky business, of late he has turned increasingly to intralesional triamcinolone and 5-fluorouracil (5-FU).
Intralesional triamcinolone plus 5-FU. This combination causes less atrophy, hypopigmentation, and telangiectasias than full-on triamcinolone. He injects 0.9 mL of 5-FU at 50 mg/mL and 0.1 mL of triamcinolone at 40 mg/mL into and under the scar. The 5-FU inhibits fibroblast proliferation. It is rated pregnancy category D, so he avoids using it in women of childbearing age.
Spot dermabrasion. “To me, this is the go-to. After my intralesional steroids, if the scar hasn’t fully smoothed out, then I go to dermabrasion or the spot CO2 laser,” Dr. Hruza said.
“Dermabrasion is an old technology, but it’s actually still very useful,” he continued. “Do it at 6-10 weeks; that’s the sweet spot. Do it sooner and you can get into problems with dehiscence. And if you do it later than 10 weeks the improvement is much less because everything is stabilized and the collagen is set.” He uses a diamond fraise to abrade and sculpt, rather than sandpaper, which doesn’t allow him to go sufficiently deep once bleeding starts and the sandpaper gets wet.
Spot conventional CO2 or Er:YAG laser resurfacing. “I really find in my hands these ablative techniques are much more effective than using a fractionated laser, which only gives you a little bit of improvement,” he said.
Pulsed dye laser. Very effective for red, thickened scars. Dr. Hruza does two to four treatments at 4-week intervals. At wavelengths of 585-595 nm, a pulse of 0.5-1.5 millisecs, and 4-5 Joules/cm2, there is only minimal purpura.
The pulsed dye laser can also be employed preventively starting at the time of suture removal and then again at 4-6 weeks in order to reduce hypertrophy. “It’s something to consider in areas like the chest, upper back, and shoulders, where you’re trying to prevent problems. The only danger is occasionally patients have dehiscence,” according to the dermatologic surgeon.
Fractionated nonablative laser. Four or five treatments are typically required in order to achieve significant resurfacing.
Micropore tape. Dr. Hruza finds this works just as well as topical silicone gel sheets, rolls, and gels, all of which are quite expensive. A roll of micropore tape costs only a few dollars and will last a patient for a couple months. Patients are taught to apply the tape at the time of suture removal in a line parallel to the suture line, replacing the tape when it begins to peel off. As with the vastly more expensive silicone products, the tape needs to be left on 12-24 hours per day for 3-6 months in order to achieve a flat white scar. The benefit is thought to come from relief of mechanical stress coupled with occlusion.
Botulinum toxin A and other neurotoxins. Inject into muscle near the wound edges right after closing the wound, using 1-3 units at 1- to 3-cm intervals in order to prevent scar formation, Dr. Hruza advised. If the wound is on one side of the face, the other side needs to receive injections as well in order to spare the patient from several months of cosmetically undesirable asymmetry. However, Dr. Hruza rarely utilizes neurotoxin injections. “It’s a cost issue. I’m in the Midwest, where a lot of insurers are unwilling to pay for it,” he explained.
Flap defatting. Here the surgeon opens the flap and keeps digging with scalpel and scissors until the scar is slightly depressed, since there is likely to be some recurrence. Then it’s time to resuture the flap.
Technical scar revision procedures. The simplest of these is Z-plasty, which entails making two skin incisions to create a Z-shaped incision, then flipping the two sides to reorient the scar. The Z-plasty has two major uses: correction of a retracted lip or medial canthus webbing. “If you get either of these, Z-plasty is the way to go,” Dr. Hruza said.
Fillers for atrophic scars. “To me, this is the last thing to go to. The reason is that, if a patient has skin cancer surgery, they don’t expect to pay extra to improve that scar. And I can do dermabrasion with no incremental product cost to the practice,” he commented. The technique entails making a subcision to create a pocket for the filler. The products marketed as Restylane Silk, Belotero, and Radiesse all yield good results, he said.
Dr. Hruza reported having no financial conflicts of interest regarding his presentation.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
WAIKOLOA, HAWAII – , according to American Academy of Dermatology President-elect George J. Hruza, MD – and he’s got a raft of them.
“There are going to be situations where your scars aren’t going to be as wonderful as you’d like, or even if they’re pretty good, you might improve them further if you do some modifications,” he observed at the Hawaii Dermatology Seminar provided by the Global Academy for Medical Education/Skin Disease Education Foundation.
He became convinced of the importance of having a large toolbox for scar improvement in part as a result of an Australian prospective study of 576 patients surveyed 6-9 months following skin cancer surgery. Far and away the most important factor influencing patients’ overall perception of their experience wasn’t the cost, pain, quality of nursing care, complications, wait time prior to surgery, or gratitude that they’d successfully had a cancer removed. It was their perception of the scar (J Am Acad Dermatol. 2007 Sep;57[3]:445-53).
To be effective, interventions for scar improvement need to be timed in sync with the three phases of cellular activity involved in wound healing. For example, neurotoxin injections are effective during the first few days of the initial acute inflammation period, when cellular migration is active. Silicone and taping are of value when employed long term, starting at about 1 month and continuing for 3-6 months, throughout the neovascularization/granulation phase, then the time of fibroblast proliferation and matrix formation that follows, and even beyond. Pulsed dye and fractionated ablative lasers are best utilized to reshape matrix formation, starting at about 2 weeks. Intervention using dermabrasion or fillers has to wait for the scar to be a bit more mature, at about 2 months; utilized earlier these can cause dehiscence, explained Dr. Hruza of St. Louis University.
He shared what he called his “scar improvement hierarchy,” the sequence of interventions he turns to from the most to least often. But he began with prevention, noting that more than 2 decades ago, he and his coinvestigators demonstrated that running horizontal mattress sutures for primary closures of facial wounds provide better cosmetic results, with a final scar that’s smoother and flatter than the more commonly used simple running sutures (Dermatol Surg. 2005 Oct;31[10]:1313-6).
Scar improvement sequence
Massage. “I recommend this to almost every patient. I have them start at about 6 weeks and do it for several months. It’s really more like kneading dough, not rubbing. You want the skin pressing on the bone underneath,” according to Dr. Hruza. Various investigators have suggested that scar massage works by increasing hydration and capillary proliferation, while promoting desensitization, but the evidence is really anecdotal.
“I think it’s mainly tincture of time. Scars get better on their own,” he observed. Regardless, massage allows patients the satisfaction of actively participating in their own recovery.
Intralesional triamcinolone. Dr. Hruza calls this “our big friend.”
“I find that 90% of the time when you look at a thickened scar and you think, ‘Oh gee, I’m going to have to do some scar revision, the intralesional triamcinolone takes care of the problem,” he said. He usually injects the site at about 6 weeks post surgery using 10 mg/mL. If the response is inadequate he reinjects about a month later using 20 mg/mL. He generally avoids going to 40 mg/mL for facial scars. The goal is to make therapeutic use of the steroid’s major side effect – atrophy – to shrink the thickened scar. But because this can be a tricky business, of late he has turned increasingly to intralesional triamcinolone and 5-fluorouracil (5-FU).
Intralesional triamcinolone plus 5-FU. This combination causes less atrophy, hypopigmentation, and telangiectasias than full-on triamcinolone. He injects 0.9 mL of 5-FU at 50 mg/mL and 0.1 mL of triamcinolone at 40 mg/mL into and under the scar. The 5-FU inhibits fibroblast proliferation. It is rated pregnancy category D, so he avoids using it in women of childbearing age.
Spot dermabrasion. “To me, this is the go-to. After my intralesional steroids, if the scar hasn’t fully smoothed out, then I go to dermabrasion or the spot CO2 laser,” Dr. Hruza said.
“Dermabrasion is an old technology, but it’s actually still very useful,” he continued. “Do it at 6-10 weeks; that’s the sweet spot. Do it sooner and you can get into problems with dehiscence. And if you do it later than 10 weeks the improvement is much less because everything is stabilized and the collagen is set.” He uses a diamond fraise to abrade and sculpt, rather than sandpaper, which doesn’t allow him to go sufficiently deep once bleeding starts and the sandpaper gets wet.
Spot conventional CO2 or Er:YAG laser resurfacing. “I really find in my hands these ablative techniques are much more effective than using a fractionated laser, which only gives you a little bit of improvement,” he said.
Pulsed dye laser. Very effective for red, thickened scars. Dr. Hruza does two to four treatments at 4-week intervals. At wavelengths of 585-595 nm, a pulse of 0.5-1.5 millisecs, and 4-5 Joules/cm2, there is only minimal purpura.
The pulsed dye laser can also be employed preventively starting at the time of suture removal and then again at 4-6 weeks in order to reduce hypertrophy. “It’s something to consider in areas like the chest, upper back, and shoulders, where you’re trying to prevent problems. The only danger is occasionally patients have dehiscence,” according to the dermatologic surgeon.
Fractionated nonablative laser. Four or five treatments are typically required in order to achieve significant resurfacing.
Micropore tape. Dr. Hruza finds this works just as well as topical silicone gel sheets, rolls, and gels, all of which are quite expensive. A roll of micropore tape costs only a few dollars and will last a patient for a couple months. Patients are taught to apply the tape at the time of suture removal in a line parallel to the suture line, replacing the tape when it begins to peel off. As with the vastly more expensive silicone products, the tape needs to be left on 12-24 hours per day for 3-6 months in order to achieve a flat white scar. The benefit is thought to come from relief of mechanical stress coupled with occlusion.
Botulinum toxin A and other neurotoxins. Inject into muscle near the wound edges right after closing the wound, using 1-3 units at 1- to 3-cm intervals in order to prevent scar formation, Dr. Hruza advised. If the wound is on one side of the face, the other side needs to receive injections as well in order to spare the patient from several months of cosmetically undesirable asymmetry. However, Dr. Hruza rarely utilizes neurotoxin injections. “It’s a cost issue. I’m in the Midwest, where a lot of insurers are unwilling to pay for it,” he explained.
Flap defatting. Here the surgeon opens the flap and keeps digging with scalpel and scissors until the scar is slightly depressed, since there is likely to be some recurrence. Then it’s time to resuture the flap.
Technical scar revision procedures. The simplest of these is Z-plasty, which entails making two skin incisions to create a Z-shaped incision, then flipping the two sides to reorient the scar. The Z-plasty has two major uses: correction of a retracted lip or medial canthus webbing. “If you get either of these, Z-plasty is the way to go,” Dr. Hruza said.
Fillers for atrophic scars. “To me, this is the last thing to go to. The reason is that, if a patient has skin cancer surgery, they don’t expect to pay extra to improve that scar. And I can do dermabrasion with no incremental product cost to the practice,” he commented. The technique entails making a subcision to create a pocket for the filler. The products marketed as Restylane Silk, Belotero, and Radiesse all yield good results, he said.
Dr. Hruza reported having no financial conflicts of interest regarding his presentation.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
WAIKOLOA, HAWAII – , according to American Academy of Dermatology President-elect George J. Hruza, MD – and he’s got a raft of them.
“There are going to be situations where your scars aren’t going to be as wonderful as you’d like, or even if they’re pretty good, you might improve them further if you do some modifications,” he observed at the Hawaii Dermatology Seminar provided by the Global Academy for Medical Education/Skin Disease Education Foundation.
He became convinced of the importance of having a large toolbox for scar improvement in part as a result of an Australian prospective study of 576 patients surveyed 6-9 months following skin cancer surgery. Far and away the most important factor influencing patients’ overall perception of their experience wasn’t the cost, pain, quality of nursing care, complications, wait time prior to surgery, or gratitude that they’d successfully had a cancer removed. It was their perception of the scar (J Am Acad Dermatol. 2007 Sep;57[3]:445-53).
To be effective, interventions for scar improvement need to be timed in sync with the three phases of cellular activity involved in wound healing. For example, neurotoxin injections are effective during the first few days of the initial acute inflammation period, when cellular migration is active. Silicone and taping are of value when employed long term, starting at about 1 month and continuing for 3-6 months, throughout the neovascularization/granulation phase, then the time of fibroblast proliferation and matrix formation that follows, and even beyond. Pulsed dye and fractionated ablative lasers are best utilized to reshape matrix formation, starting at about 2 weeks. Intervention using dermabrasion or fillers has to wait for the scar to be a bit more mature, at about 2 months; utilized earlier these can cause dehiscence, explained Dr. Hruza of St. Louis University.
He shared what he called his “scar improvement hierarchy,” the sequence of interventions he turns to from the most to least often. But he began with prevention, noting that more than 2 decades ago, he and his coinvestigators demonstrated that running horizontal mattress sutures for primary closures of facial wounds provide better cosmetic results, with a final scar that’s smoother and flatter than the more commonly used simple running sutures (Dermatol Surg. 2005 Oct;31[10]:1313-6).
Scar improvement sequence
Massage. “I recommend this to almost every patient. I have them start at about 6 weeks and do it for several months. It’s really more like kneading dough, not rubbing. You want the skin pressing on the bone underneath,” according to Dr. Hruza. Various investigators have suggested that scar massage works by increasing hydration and capillary proliferation, while promoting desensitization, but the evidence is really anecdotal.
“I think it’s mainly tincture of time. Scars get better on their own,” he observed. Regardless, massage allows patients the satisfaction of actively participating in their own recovery.
Intralesional triamcinolone. Dr. Hruza calls this “our big friend.”
“I find that 90% of the time when you look at a thickened scar and you think, ‘Oh gee, I’m going to have to do some scar revision, the intralesional triamcinolone takes care of the problem,” he said. He usually injects the site at about 6 weeks post surgery using 10 mg/mL. If the response is inadequate he reinjects about a month later using 20 mg/mL. He generally avoids going to 40 mg/mL for facial scars. The goal is to make therapeutic use of the steroid’s major side effect – atrophy – to shrink the thickened scar. But because this can be a tricky business, of late he has turned increasingly to intralesional triamcinolone and 5-fluorouracil (5-FU).
Intralesional triamcinolone plus 5-FU. This combination causes less atrophy, hypopigmentation, and telangiectasias than full-on triamcinolone. He injects 0.9 mL of 5-FU at 50 mg/mL and 0.1 mL of triamcinolone at 40 mg/mL into and under the scar. The 5-FU inhibits fibroblast proliferation. It is rated pregnancy category D, so he avoids using it in women of childbearing age.
Spot dermabrasion. “To me, this is the go-to. After my intralesional steroids, if the scar hasn’t fully smoothed out, then I go to dermabrasion or the spot CO2 laser,” Dr. Hruza said.
“Dermabrasion is an old technology, but it’s actually still very useful,” he continued. “Do it at 6-10 weeks; that’s the sweet spot. Do it sooner and you can get into problems with dehiscence. And if you do it later than 10 weeks the improvement is much less because everything is stabilized and the collagen is set.” He uses a diamond fraise to abrade and sculpt, rather than sandpaper, which doesn’t allow him to go sufficiently deep once bleeding starts and the sandpaper gets wet.
Spot conventional CO2 or Er:YAG laser resurfacing. “I really find in my hands these ablative techniques are much more effective than using a fractionated laser, which only gives you a little bit of improvement,” he said.
Pulsed dye laser. Very effective for red, thickened scars. Dr. Hruza does two to four treatments at 4-week intervals. At wavelengths of 585-595 nm, a pulse of 0.5-1.5 millisecs, and 4-5 Joules/cm2, there is only minimal purpura.
The pulsed dye laser can also be employed preventively starting at the time of suture removal and then again at 4-6 weeks in order to reduce hypertrophy. “It’s something to consider in areas like the chest, upper back, and shoulders, where you’re trying to prevent problems. The only danger is occasionally patients have dehiscence,” according to the dermatologic surgeon.
Fractionated nonablative laser. Four or five treatments are typically required in order to achieve significant resurfacing.
Micropore tape. Dr. Hruza finds this works just as well as topical silicone gel sheets, rolls, and gels, all of which are quite expensive. A roll of micropore tape costs only a few dollars and will last a patient for a couple months. Patients are taught to apply the tape at the time of suture removal in a line parallel to the suture line, replacing the tape when it begins to peel off. As with the vastly more expensive silicone products, the tape needs to be left on 12-24 hours per day for 3-6 months in order to achieve a flat white scar. The benefit is thought to come from relief of mechanical stress coupled with occlusion.
Botulinum toxin A and other neurotoxins. Inject into muscle near the wound edges right after closing the wound, using 1-3 units at 1- to 3-cm intervals in order to prevent scar formation, Dr. Hruza advised. If the wound is on one side of the face, the other side needs to receive injections as well in order to spare the patient from several months of cosmetically undesirable asymmetry. However, Dr. Hruza rarely utilizes neurotoxin injections. “It’s a cost issue. I’m in the Midwest, where a lot of insurers are unwilling to pay for it,” he explained.
Flap defatting. Here the surgeon opens the flap and keeps digging with scalpel and scissors until the scar is slightly depressed, since there is likely to be some recurrence. Then it’s time to resuture the flap.
Technical scar revision procedures. The simplest of these is Z-plasty, which entails making two skin incisions to create a Z-shaped incision, then flipping the two sides to reorient the scar. The Z-plasty has two major uses: correction of a retracted lip or medial canthus webbing. “If you get either of these, Z-plasty is the way to go,” Dr. Hruza said.
Fillers for atrophic scars. “To me, this is the last thing to go to. The reason is that, if a patient has skin cancer surgery, they don’t expect to pay extra to improve that scar. And I can do dermabrasion with no incremental product cost to the practice,” he commented. The technique entails making a subcision to create a pocket for the filler. The products marketed as Restylane Silk, Belotero, and Radiesse all yield good results, he said.
Dr. Hruza reported having no financial conflicts of interest regarding his presentation.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
REPORTING FROM SDEF HAWAII DERMATOLOGY SEMINAR
Don’t miss baby scabies
WAIKOLOA, HAWAII –
“It’s really important to think of scabies in any widespread rash that a baby presents with,” said Andrea Zaenglein, MD, professor of dermatology and pediatric dermatology at Penn State University, Hershey. It’s often missed in the ED because it’s not recognized.
While lesions might be limited to the webbing of the hands in older patients, infants generally have a widespread rash with many different lesion types involving the armpits, trunk, and even the scalp. “In older kids, we always think of itch as our primary criteria, but for infants with scabies, that’s not always the case. The younger the kid, the less able they’re to manifest the itch in a way that we recognize,” she said in an interview at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
Standard treatment for infants with scabies is permethrin cream, which, Dr. Zaenglein advises, should be applied from head to toe. “And make sure to treat all family members, even if they’re not demonstrating any symptoms. It’s really important, because that baby had to get scabies from somebody,” she said. Although permethrin isn’t approved for use under 2 months old, she said she has no problem with it in younger, otherwise healthy infants, but cases below 2 months are uncommon. Even if infants are exposed at birth, it takes several weeks for scabies to manifest.
Topical corticosteroids are useful as well to speed healing and help with itch. Ivermectin is held in reserve for older patients, especially in institutional settings where many people have to be treated at a time, or when permethrin cream is not effective.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
WAIKOLOA, HAWAII –
“It’s really important to think of scabies in any widespread rash that a baby presents with,” said Andrea Zaenglein, MD, professor of dermatology and pediatric dermatology at Penn State University, Hershey. It’s often missed in the ED because it’s not recognized.
While lesions might be limited to the webbing of the hands in older patients, infants generally have a widespread rash with many different lesion types involving the armpits, trunk, and even the scalp. “In older kids, we always think of itch as our primary criteria, but for infants with scabies, that’s not always the case. The younger the kid, the less able they’re to manifest the itch in a way that we recognize,” she said in an interview at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
Standard treatment for infants with scabies is permethrin cream, which, Dr. Zaenglein advises, should be applied from head to toe. “And make sure to treat all family members, even if they’re not demonstrating any symptoms. It’s really important, because that baby had to get scabies from somebody,” she said. Although permethrin isn’t approved for use under 2 months old, she said she has no problem with it in younger, otherwise healthy infants, but cases below 2 months are uncommon. Even if infants are exposed at birth, it takes several weeks for scabies to manifest.
Topical corticosteroids are useful as well to speed healing and help with itch. Ivermectin is held in reserve for older patients, especially in institutional settings where many people have to be treated at a time, or when permethrin cream is not effective.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
WAIKOLOA, HAWAII –
“It’s really important to think of scabies in any widespread rash that a baby presents with,” said Andrea Zaenglein, MD, professor of dermatology and pediatric dermatology at Penn State University, Hershey. It’s often missed in the ED because it’s not recognized.
While lesions might be limited to the webbing of the hands in older patients, infants generally have a widespread rash with many different lesion types involving the armpits, trunk, and even the scalp. “In older kids, we always think of itch as our primary criteria, but for infants with scabies, that’s not always the case. The younger the kid, the less able they’re to manifest the itch in a way that we recognize,” she said in an interview at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
Standard treatment for infants with scabies is permethrin cream, which, Dr. Zaenglein advises, should be applied from head to toe. “And make sure to treat all family members, even if they’re not demonstrating any symptoms. It’s really important, because that baby had to get scabies from somebody,” she said. Although permethrin isn’t approved for use under 2 months old, she said she has no problem with it in younger, otherwise healthy infants, but cases below 2 months are uncommon. Even if infants are exposed at birth, it takes several weeks for scabies to manifest.
Topical corticosteroids are useful as well to speed healing and help with itch. Ivermectin is held in reserve for older patients, especially in institutional settings where many people have to be treated at a time, or when permethrin cream is not effective.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
EXPERT ANALYSIS FROM THE SDEF HAWAII DERMATOLOGY SEMINAR
How to manage infected eczema in children
WAIKOLOA, HAWAII – , according to Lawrence Eichenfield, MD, chief of pediatric and adolescent dermatology at the University of California, San Diego.
It’s the breach in skin integrity that gives skin flora – most commonly Staphylococcus or Streptococcus – an opening for infection.
Dr. Eichenfield shared his treatment approach in a pearl-filled interview at the Hawaii Dermatology Seminar, provided by Global Academy for Medical Education/Skin Disease Education Foundation.
“Many times, anti-inflammatories are going to be the effective therapy,” whether topical steroids or systemic therapies. But with a secondary infection, systemic antibiotics are in order, too, but topical ones aren’t much use, he said.
Dr. Eichenfield gets a lot of questions about steroid-sparing options for very young children, since topical calcineurin inhibitors and the like aren’t approved in children under 2 years old, and insurance coverage can be a problem. He noted, however, that various guidelines support their use even in the very young, “so I tell my physicians to fight for them ... If you need a steroid-sparing agent, push for it, because it may be the right thing for your patient,” he said.
He’s finding in his area that infected eczema often is resistant to clindamycin, which has been used heavily because of concerns about methicillin-resistant Staphylococcus aureus (MRSA). But it often will “respond to what we considered to be wimpier antibiotics in the past, such as cephalosporin ... So I’ll use cephalosporin or an extended-spectrum penicillin as my first-line agent, and then I’ll culture, depending on history, to see if I have to be concerned about methicillin resistance,” he said.
Be on the lookout for cutaneous herpes and show parents pictures of what it looks like so they recognize it and know to come in right away. It is a dangerous infection, but can be shut down quickly with oral acyclovir and similar agents, Dr. Eichenfield added.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
WAIKOLOA, HAWAII – , according to Lawrence Eichenfield, MD, chief of pediatric and adolescent dermatology at the University of California, San Diego.
It’s the breach in skin integrity that gives skin flora – most commonly Staphylococcus or Streptococcus – an opening for infection.
Dr. Eichenfield shared his treatment approach in a pearl-filled interview at the Hawaii Dermatology Seminar, provided by Global Academy for Medical Education/Skin Disease Education Foundation.
“Many times, anti-inflammatories are going to be the effective therapy,” whether topical steroids or systemic therapies. But with a secondary infection, systemic antibiotics are in order, too, but topical ones aren’t much use, he said.
Dr. Eichenfield gets a lot of questions about steroid-sparing options for very young children, since topical calcineurin inhibitors and the like aren’t approved in children under 2 years old, and insurance coverage can be a problem. He noted, however, that various guidelines support their use even in the very young, “so I tell my physicians to fight for them ... If you need a steroid-sparing agent, push for it, because it may be the right thing for your patient,” he said.
He’s finding in his area that infected eczema often is resistant to clindamycin, which has been used heavily because of concerns about methicillin-resistant Staphylococcus aureus (MRSA). But it often will “respond to what we considered to be wimpier antibiotics in the past, such as cephalosporin ... So I’ll use cephalosporin or an extended-spectrum penicillin as my first-line agent, and then I’ll culture, depending on history, to see if I have to be concerned about methicillin resistance,” he said.
Be on the lookout for cutaneous herpes and show parents pictures of what it looks like so they recognize it and know to come in right away. It is a dangerous infection, but can be shut down quickly with oral acyclovir and similar agents, Dr. Eichenfield added.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
WAIKOLOA, HAWAII – , according to Lawrence Eichenfield, MD, chief of pediatric and adolescent dermatology at the University of California, San Diego.
It’s the breach in skin integrity that gives skin flora – most commonly Staphylococcus or Streptococcus – an opening for infection.
Dr. Eichenfield shared his treatment approach in a pearl-filled interview at the Hawaii Dermatology Seminar, provided by Global Academy for Medical Education/Skin Disease Education Foundation.
“Many times, anti-inflammatories are going to be the effective therapy,” whether topical steroids or systemic therapies. But with a secondary infection, systemic antibiotics are in order, too, but topical ones aren’t much use, he said.
Dr. Eichenfield gets a lot of questions about steroid-sparing options for very young children, since topical calcineurin inhibitors and the like aren’t approved in children under 2 years old, and insurance coverage can be a problem. He noted, however, that various guidelines support their use even in the very young, “so I tell my physicians to fight for them ... If you need a steroid-sparing agent, push for it, because it may be the right thing for your patient,” he said.
He’s finding in his area that infected eczema often is resistant to clindamycin, which has been used heavily because of concerns about methicillin-resistant Staphylococcus aureus (MRSA). But it often will “respond to what we considered to be wimpier antibiotics in the past, such as cephalosporin ... So I’ll use cephalosporin or an extended-spectrum penicillin as my first-line agent, and then I’ll culture, depending on history, to see if I have to be concerned about methicillin resistance,” he said.
Be on the lookout for cutaneous herpes and show parents pictures of what it looks like so they recognize it and know to come in right away. It is a dangerous infection, but can be shut down quickly with oral acyclovir and similar agents, Dr. Eichenfield added.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
EXPERT ANALYSIS FROM SDEF HAWAII DERMATOLOGY SEMINAR
Derm MOC reboot welcomed
WAIKOLOA, HAWAII – The and the revised program has received rave reviews in pilot testing, Erik J. Stratman, MD, announced at the Hawaii Dermatology Seminar provided by the Global Academy for Medical Education/Skin Disease Education Foundation.
“Especially if you look at the last 2 years, you’ll see that things in MOC are a lot simpler, they’re quicker, and they’re cheaper than what they’ve ever been before,” according to Dr. Stratman, current president of the American Board of Dermatology (ABD) and chairman of the department of dermatology at the Marshfield (Wisc.) Clinic.
The first version of MOC for dermatology started in 2006 and ran for a decade. Unhappiness abounded. Major changes were introduced during the 2016-2018 pilot program. A second pilot program starts in March 2019, with a full launch expected in 2020.
Dr. Stratman came to the Hawaii Dermatology Seminar to convey the message that dermatologists’ complaining about the first version of MOC “was listened to very intently,” and that the second-generation MOC addresses all four of their major complaints.
“It’s very, very rare that the first iteration of anything is perfect,” he observed.
He also bore a second message about the new MOC: “There are some very, very simple things that every one of us ought to be doing that make it so much easier, cheaper, and so much quicker.”
First off, he recommended logging into the ABD website and clicking on “My MOC” to see a personalized table that provides a snapshot of where the user stands in the MOC process. Of note, the “Patient Safety” component is soon going to be eliminated because the ABD has recognized that patient safety is already embedded in other elements of MOC. So there’s no point in wasting time trying to seek out Patient Safety credits. On the My MOC table, the dermatologist can click a box attesting that he or she possesses an unrestricted medical license and another box attesting to the total CME credits accrued for the year. It’s also possible to securely pay the $150 annual MOC fee electronically on that site.
Why the $150 per year? Traditionally, dermatologists with time-limited certification have had to take the dreaded high-stakes, standalone recertification exam once every 10 years at a cost of $1,500, plus the expense incurred in closing the practice and traveling to a test site. The ABD decided to lessen the financial blow by breaking that expense up into 10 yearly payments of $150 for MOC, with no additional charge for the 10-year exam. All the educational materials required to meet the MOC requirements are included in that $150 per year; dermatologists should not feel obligated to purchase any additional materials.
Also, the ABD is one of seven members of the American Board of Medical Specialties (ABMS) piloting a novel longitudinal method of physician assessment known as CertLink. Instead of taking an anxiety-producing, all-or-nothing recertification exam once every 10 years, CertLink, a web-based platform developed by ABMS, involves longitudinal assessment coupled with education on an ongoing basis.
“It’s something you can do at home or work at your own pace using a your desktop or mobile device,” Dr. Stratman explained.
Participating dermatologists will receive small batches of questions electronically, a total of 40-50 per year. Before submitting their answer, they rate the relevance of the question to their own practice and their confidence in their response. After they answer a question they receive immediate focused feedback and an evidence-based explanation as to why their answer was correct or incorrect. And if they have prospectively rated the question as high relevance and then gotten the answer wrong, they can expect that question to come back later in the process.
Dr. Stratman’s first big time-saving MOC tip was directed to the 98% of practicing dermatologists who are members of the American Academy of Dermatology. Go to the academy website, he urged, and sign up to participate in the academy’s CME transcript program. Then check the box that allows everything on that CME transcript to be auto-updated to the ABD My MOC table.
His second major tip, both a time- and money-saver, is to sign up on the AAD website for the self-assessment question of the week. The question, delivered electronically, typically involves a clinical scenario with a photo and perhaps a pathological finding, followed by a question as to the most likely diagnosis or the therapy of choice. Concise feedback is provided about the medical condition and why the right answer was right and the other answers were wrong. And each question that’s completed earns the dermatologist 1 point of ABD MOC self-assessment credit, regardless of whether the answer provided was right or wrong.
“I’d say this is incredibly important. It’s part of your member benefits. It doesn’t cost you any additional money beyond your AAD membership fee. And it’s a very easy way to get your ABD MOC self-assessment elements [a required 100 points every 3 years] met,” Dr. Stratman noted.
An AAD survey showed that the question of the week is the single most successful AAD educational activity ever, with nearly 300,000 total MOC credits claimed and more than 74,000 total CME credits awarded in the first year and a half of the program.
The survey also showed that dermatologists spent an average of 45 seconds from clicking on the question of the week to submitting an answer. That works out to a mere 22 minutes and 30 seconds per year in order to meet the MOC self-assessment elements, simply by participating in the question of the week.
“Not too shabby,” Dr. Stratman commented.
He is especially pleased by this work-time analysis because one of the four major criticisms repeatedly leveled at the ABD regarding the first iteration of MOC was that it’s too time-consuming.
Another major gripe was that MOC is too expensive. But now every MOC requirement can be completed at no additional cost beyond AAD membership and the MOC participation fee, the dermatologist noted.
The third major criticism of MOC was that traditional CME should be adequate to demonstrate proficiency. Not so, according to Dr. Stratman, who cited two Cochrane reviews that concluded traditional CME activities don’t change physician performance or improve health care outcomes, which are the stated purposes of MOC.
The fourth criticism digested by Dr. Stratman and his fellow board members was that MOC wasn’t relevant to clinical practice. So here, major changes have been made in the ABD-approved Practice Improvement activity component. And to accomplish this, the ABD turned to the specialty of ob.gyn.
“We came up with a concept we borrowed from ACOG [the American College of Obstetricians and Gynecologists]. When you talk to people from the ob.gyn. world, they’re like the one specialty that almost never complains about MOC. It makes you ask, what is it that’s so different that they like what they’re doing? Because we want to have diplomates that are very happy with their MOC as well. And it came down to ob.gyns. being asked to participate in very focused quality improvement projects, where they’re looking at 1 thing to be changed, not 35,” the dermatologist explained.
So now, once every 5 years, dermatologists pick from a menu of roughly 50 focused Practice Improvement modules that address every area of dermatology. Some examples include “contraceptive education prior to starting isotretinoin,” “the importance of completing a delayed patch test reading,” or “choosing a step in the skin biopsy pathway to assess in your office.” And no more than three questions are asked about one patient’s chart. “This is what the majority of people are doing, and they’re really finding it worthwhile, quick, and relevant,” Dr. Stratman said.
Indeed, during the 2-year pilot in 2016-2018, more than 6,000 ABD diplomates completed more than 7,500 Practice Improvement modules. And in anonymous surveys, participants gave the program enthusiastically positive reviews. About 98% reported that their focused Practice Improvement activity was relevant to their practice as a dermatologist, 20% reported a resultant improvement in the care they delivered, 21% indicated at least one patient experienced a better outcome as a result, and 97% said they would recommend these modules to a practice partner or other dermatologists, he noted.
One Hawaii audience member offered a personal testimonial: “I used to rail against the irrelevance of the process. But I took my first recertification exam and I give the ABD a lot of credit for listening to our bitching and moaning and then streamlining things and making everything more pertinent. A big thank you to you guys!”
More about CertLink: The questions will be of three types. There will be core questions about general dermatology and skin conditions that all dermatologists should recognize; concentration-based questions focused on whatever the participant considers areas of individual expertise, such as pediatric or cosmetic dermatology; and article-based questions. The article-based questions are based on input from the editors of the major dermatology journals as to what they consider to be the top five articles of the past year that should have the most influence on practice. The participant clicks on the article, reads it, and then launches the questions; the answers to which are embedded in the body of the article. The questions are answered on an unlimited time basis with the article in front of the participant.
“I think the article-based questions are going to be a very big deal because the purpose of MOC isn’t really about testing you, it’s about trying to keep you as right-now and fresh and on top of your game as possible,” Dr. Stratman said.
He encouraged dermatologists to contact the ABD with any MOC-related questions, addressing them to Pam Zuziak (pzuzuak1@hfhs.org).
Dr. Stratman noted that he and his fellow board members are still studying an extensive report recently submitted to the ABMS on recommendations to reform MOC. It’s possible that the ABD will need to tweak its pilot program in response. So he recommended checking the ABD website often during this period of change.
“If you have an inbox email from the ABD, it’s going to be something that you should open and read. It’s not going to be an advertisement,” he promised.
Dr. Stratman reported having created many MOC-related educational materials during the past dozen years.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
WAIKOLOA, HAWAII – The and the revised program has received rave reviews in pilot testing, Erik J. Stratman, MD, announced at the Hawaii Dermatology Seminar provided by the Global Academy for Medical Education/Skin Disease Education Foundation.
“Especially if you look at the last 2 years, you’ll see that things in MOC are a lot simpler, they’re quicker, and they’re cheaper than what they’ve ever been before,” according to Dr. Stratman, current president of the American Board of Dermatology (ABD) and chairman of the department of dermatology at the Marshfield (Wisc.) Clinic.
The first version of MOC for dermatology started in 2006 and ran for a decade. Unhappiness abounded. Major changes were introduced during the 2016-2018 pilot program. A second pilot program starts in March 2019, with a full launch expected in 2020.
Dr. Stratman came to the Hawaii Dermatology Seminar to convey the message that dermatologists’ complaining about the first version of MOC “was listened to very intently,” and that the second-generation MOC addresses all four of their major complaints.
“It’s very, very rare that the first iteration of anything is perfect,” he observed.
He also bore a second message about the new MOC: “There are some very, very simple things that every one of us ought to be doing that make it so much easier, cheaper, and so much quicker.”
First off, he recommended logging into the ABD website and clicking on “My MOC” to see a personalized table that provides a snapshot of where the user stands in the MOC process. Of note, the “Patient Safety” component is soon going to be eliminated because the ABD has recognized that patient safety is already embedded in other elements of MOC. So there’s no point in wasting time trying to seek out Patient Safety credits. On the My MOC table, the dermatologist can click a box attesting that he or she possesses an unrestricted medical license and another box attesting to the total CME credits accrued for the year. It’s also possible to securely pay the $150 annual MOC fee electronically on that site.
Why the $150 per year? Traditionally, dermatologists with time-limited certification have had to take the dreaded high-stakes, standalone recertification exam once every 10 years at a cost of $1,500, plus the expense incurred in closing the practice and traveling to a test site. The ABD decided to lessen the financial blow by breaking that expense up into 10 yearly payments of $150 for MOC, with no additional charge for the 10-year exam. All the educational materials required to meet the MOC requirements are included in that $150 per year; dermatologists should not feel obligated to purchase any additional materials.
Also, the ABD is one of seven members of the American Board of Medical Specialties (ABMS) piloting a novel longitudinal method of physician assessment known as CertLink. Instead of taking an anxiety-producing, all-or-nothing recertification exam once every 10 years, CertLink, a web-based platform developed by ABMS, involves longitudinal assessment coupled with education on an ongoing basis.
“It’s something you can do at home or work at your own pace using a your desktop or mobile device,” Dr. Stratman explained.
Participating dermatologists will receive small batches of questions electronically, a total of 40-50 per year. Before submitting their answer, they rate the relevance of the question to their own practice and their confidence in their response. After they answer a question they receive immediate focused feedback and an evidence-based explanation as to why their answer was correct or incorrect. And if they have prospectively rated the question as high relevance and then gotten the answer wrong, they can expect that question to come back later in the process.
Dr. Stratman’s first big time-saving MOC tip was directed to the 98% of practicing dermatologists who are members of the American Academy of Dermatology. Go to the academy website, he urged, and sign up to participate in the academy’s CME transcript program. Then check the box that allows everything on that CME transcript to be auto-updated to the ABD My MOC table.
His second major tip, both a time- and money-saver, is to sign up on the AAD website for the self-assessment question of the week. The question, delivered electronically, typically involves a clinical scenario with a photo and perhaps a pathological finding, followed by a question as to the most likely diagnosis or the therapy of choice. Concise feedback is provided about the medical condition and why the right answer was right and the other answers were wrong. And each question that’s completed earns the dermatologist 1 point of ABD MOC self-assessment credit, regardless of whether the answer provided was right or wrong.
“I’d say this is incredibly important. It’s part of your member benefits. It doesn’t cost you any additional money beyond your AAD membership fee. And it’s a very easy way to get your ABD MOC self-assessment elements [a required 100 points every 3 years] met,” Dr. Stratman noted.
An AAD survey showed that the question of the week is the single most successful AAD educational activity ever, with nearly 300,000 total MOC credits claimed and more than 74,000 total CME credits awarded in the first year and a half of the program.
The survey also showed that dermatologists spent an average of 45 seconds from clicking on the question of the week to submitting an answer. That works out to a mere 22 minutes and 30 seconds per year in order to meet the MOC self-assessment elements, simply by participating in the question of the week.
“Not too shabby,” Dr. Stratman commented.
He is especially pleased by this work-time analysis because one of the four major criticisms repeatedly leveled at the ABD regarding the first iteration of MOC was that it’s too time-consuming.
Another major gripe was that MOC is too expensive. But now every MOC requirement can be completed at no additional cost beyond AAD membership and the MOC participation fee, the dermatologist noted.
The third major criticism of MOC was that traditional CME should be adequate to demonstrate proficiency. Not so, according to Dr. Stratman, who cited two Cochrane reviews that concluded traditional CME activities don’t change physician performance or improve health care outcomes, which are the stated purposes of MOC.
The fourth criticism digested by Dr. Stratman and his fellow board members was that MOC wasn’t relevant to clinical practice. So here, major changes have been made in the ABD-approved Practice Improvement activity component. And to accomplish this, the ABD turned to the specialty of ob.gyn.
“We came up with a concept we borrowed from ACOG [the American College of Obstetricians and Gynecologists]. When you talk to people from the ob.gyn. world, they’re like the one specialty that almost never complains about MOC. It makes you ask, what is it that’s so different that they like what they’re doing? Because we want to have diplomates that are very happy with their MOC as well. And it came down to ob.gyns. being asked to participate in very focused quality improvement projects, where they’re looking at 1 thing to be changed, not 35,” the dermatologist explained.
So now, once every 5 years, dermatologists pick from a menu of roughly 50 focused Practice Improvement modules that address every area of dermatology. Some examples include “contraceptive education prior to starting isotretinoin,” “the importance of completing a delayed patch test reading,” or “choosing a step in the skin biopsy pathway to assess in your office.” And no more than three questions are asked about one patient’s chart. “This is what the majority of people are doing, and they’re really finding it worthwhile, quick, and relevant,” Dr. Stratman said.
Indeed, during the 2-year pilot in 2016-2018, more than 6,000 ABD diplomates completed more than 7,500 Practice Improvement modules. And in anonymous surveys, participants gave the program enthusiastically positive reviews. About 98% reported that their focused Practice Improvement activity was relevant to their practice as a dermatologist, 20% reported a resultant improvement in the care they delivered, 21% indicated at least one patient experienced a better outcome as a result, and 97% said they would recommend these modules to a practice partner or other dermatologists, he noted.
One Hawaii audience member offered a personal testimonial: “I used to rail against the irrelevance of the process. But I took my first recertification exam and I give the ABD a lot of credit for listening to our bitching and moaning and then streamlining things and making everything more pertinent. A big thank you to you guys!”
More about CertLink: The questions will be of three types. There will be core questions about general dermatology and skin conditions that all dermatologists should recognize; concentration-based questions focused on whatever the participant considers areas of individual expertise, such as pediatric or cosmetic dermatology; and article-based questions. The article-based questions are based on input from the editors of the major dermatology journals as to what they consider to be the top five articles of the past year that should have the most influence on practice. The participant clicks on the article, reads it, and then launches the questions; the answers to which are embedded in the body of the article. The questions are answered on an unlimited time basis with the article in front of the participant.
“I think the article-based questions are going to be a very big deal because the purpose of MOC isn’t really about testing you, it’s about trying to keep you as right-now and fresh and on top of your game as possible,” Dr. Stratman said.
He encouraged dermatologists to contact the ABD with any MOC-related questions, addressing them to Pam Zuziak (pzuzuak1@hfhs.org).
Dr. Stratman noted that he and his fellow board members are still studying an extensive report recently submitted to the ABMS on recommendations to reform MOC. It’s possible that the ABD will need to tweak its pilot program in response. So he recommended checking the ABD website often during this period of change.
“If you have an inbox email from the ABD, it’s going to be something that you should open and read. It’s not going to be an advertisement,” he promised.
Dr. Stratman reported having created many MOC-related educational materials during the past dozen years.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
WAIKOLOA, HAWAII – The and the revised program has received rave reviews in pilot testing, Erik J. Stratman, MD, announced at the Hawaii Dermatology Seminar provided by the Global Academy for Medical Education/Skin Disease Education Foundation.
“Especially if you look at the last 2 years, you’ll see that things in MOC are a lot simpler, they’re quicker, and they’re cheaper than what they’ve ever been before,” according to Dr. Stratman, current president of the American Board of Dermatology (ABD) and chairman of the department of dermatology at the Marshfield (Wisc.) Clinic.
The first version of MOC for dermatology started in 2006 and ran for a decade. Unhappiness abounded. Major changes were introduced during the 2016-2018 pilot program. A second pilot program starts in March 2019, with a full launch expected in 2020.
Dr. Stratman came to the Hawaii Dermatology Seminar to convey the message that dermatologists’ complaining about the first version of MOC “was listened to very intently,” and that the second-generation MOC addresses all four of their major complaints.
“It’s very, very rare that the first iteration of anything is perfect,” he observed.
He also bore a second message about the new MOC: “There are some very, very simple things that every one of us ought to be doing that make it so much easier, cheaper, and so much quicker.”
First off, he recommended logging into the ABD website and clicking on “My MOC” to see a personalized table that provides a snapshot of where the user stands in the MOC process. Of note, the “Patient Safety” component is soon going to be eliminated because the ABD has recognized that patient safety is already embedded in other elements of MOC. So there’s no point in wasting time trying to seek out Patient Safety credits. On the My MOC table, the dermatologist can click a box attesting that he or she possesses an unrestricted medical license and another box attesting to the total CME credits accrued for the year. It’s also possible to securely pay the $150 annual MOC fee electronically on that site.
Why the $150 per year? Traditionally, dermatologists with time-limited certification have had to take the dreaded high-stakes, standalone recertification exam once every 10 years at a cost of $1,500, plus the expense incurred in closing the practice and traveling to a test site. The ABD decided to lessen the financial blow by breaking that expense up into 10 yearly payments of $150 for MOC, with no additional charge for the 10-year exam. All the educational materials required to meet the MOC requirements are included in that $150 per year; dermatologists should not feel obligated to purchase any additional materials.
Also, the ABD is one of seven members of the American Board of Medical Specialties (ABMS) piloting a novel longitudinal method of physician assessment known as CertLink. Instead of taking an anxiety-producing, all-or-nothing recertification exam once every 10 years, CertLink, a web-based platform developed by ABMS, involves longitudinal assessment coupled with education on an ongoing basis.
“It’s something you can do at home or work at your own pace using a your desktop or mobile device,” Dr. Stratman explained.
Participating dermatologists will receive small batches of questions electronically, a total of 40-50 per year. Before submitting their answer, they rate the relevance of the question to their own practice and their confidence in their response. After they answer a question they receive immediate focused feedback and an evidence-based explanation as to why their answer was correct or incorrect. And if they have prospectively rated the question as high relevance and then gotten the answer wrong, they can expect that question to come back later in the process.
Dr. Stratman’s first big time-saving MOC tip was directed to the 98% of practicing dermatologists who are members of the American Academy of Dermatology. Go to the academy website, he urged, and sign up to participate in the academy’s CME transcript program. Then check the box that allows everything on that CME transcript to be auto-updated to the ABD My MOC table.
His second major tip, both a time- and money-saver, is to sign up on the AAD website for the self-assessment question of the week. The question, delivered electronically, typically involves a clinical scenario with a photo and perhaps a pathological finding, followed by a question as to the most likely diagnosis or the therapy of choice. Concise feedback is provided about the medical condition and why the right answer was right and the other answers were wrong. And each question that’s completed earns the dermatologist 1 point of ABD MOC self-assessment credit, regardless of whether the answer provided was right or wrong.
“I’d say this is incredibly important. It’s part of your member benefits. It doesn’t cost you any additional money beyond your AAD membership fee. And it’s a very easy way to get your ABD MOC self-assessment elements [a required 100 points every 3 years] met,” Dr. Stratman noted.
An AAD survey showed that the question of the week is the single most successful AAD educational activity ever, with nearly 300,000 total MOC credits claimed and more than 74,000 total CME credits awarded in the first year and a half of the program.
The survey also showed that dermatologists spent an average of 45 seconds from clicking on the question of the week to submitting an answer. That works out to a mere 22 minutes and 30 seconds per year in order to meet the MOC self-assessment elements, simply by participating in the question of the week.
“Not too shabby,” Dr. Stratman commented.
He is especially pleased by this work-time analysis because one of the four major criticisms repeatedly leveled at the ABD regarding the first iteration of MOC was that it’s too time-consuming.
Another major gripe was that MOC is too expensive. But now every MOC requirement can be completed at no additional cost beyond AAD membership and the MOC participation fee, the dermatologist noted.
The third major criticism of MOC was that traditional CME should be adequate to demonstrate proficiency. Not so, according to Dr. Stratman, who cited two Cochrane reviews that concluded traditional CME activities don’t change physician performance or improve health care outcomes, which are the stated purposes of MOC.
The fourth criticism digested by Dr. Stratman and his fellow board members was that MOC wasn’t relevant to clinical practice. So here, major changes have been made in the ABD-approved Practice Improvement activity component. And to accomplish this, the ABD turned to the specialty of ob.gyn.
“We came up with a concept we borrowed from ACOG [the American College of Obstetricians and Gynecologists]. When you talk to people from the ob.gyn. world, they’re like the one specialty that almost never complains about MOC. It makes you ask, what is it that’s so different that they like what they’re doing? Because we want to have diplomates that are very happy with their MOC as well. And it came down to ob.gyns. being asked to participate in very focused quality improvement projects, where they’re looking at 1 thing to be changed, not 35,” the dermatologist explained.
So now, once every 5 years, dermatologists pick from a menu of roughly 50 focused Practice Improvement modules that address every area of dermatology. Some examples include “contraceptive education prior to starting isotretinoin,” “the importance of completing a delayed patch test reading,” or “choosing a step in the skin biopsy pathway to assess in your office.” And no more than three questions are asked about one patient’s chart. “This is what the majority of people are doing, and they’re really finding it worthwhile, quick, and relevant,” Dr. Stratman said.
Indeed, during the 2-year pilot in 2016-2018, more than 6,000 ABD diplomates completed more than 7,500 Practice Improvement modules. And in anonymous surveys, participants gave the program enthusiastically positive reviews. About 98% reported that their focused Practice Improvement activity was relevant to their practice as a dermatologist, 20% reported a resultant improvement in the care they delivered, 21% indicated at least one patient experienced a better outcome as a result, and 97% said they would recommend these modules to a practice partner or other dermatologists, he noted.
One Hawaii audience member offered a personal testimonial: “I used to rail against the irrelevance of the process. But I took my first recertification exam and I give the ABD a lot of credit for listening to our bitching and moaning and then streamlining things and making everything more pertinent. A big thank you to you guys!”
More about CertLink: The questions will be of three types. There will be core questions about general dermatology and skin conditions that all dermatologists should recognize; concentration-based questions focused on whatever the participant considers areas of individual expertise, such as pediatric or cosmetic dermatology; and article-based questions. The article-based questions are based on input from the editors of the major dermatology journals as to what they consider to be the top five articles of the past year that should have the most influence on practice. The participant clicks on the article, reads it, and then launches the questions; the answers to which are embedded in the body of the article. The questions are answered on an unlimited time basis with the article in front of the participant.
“I think the article-based questions are going to be a very big deal because the purpose of MOC isn’t really about testing you, it’s about trying to keep you as right-now and fresh and on top of your game as possible,” Dr. Stratman said.
He encouraged dermatologists to contact the ABD with any MOC-related questions, addressing them to Pam Zuziak (pzuzuak1@hfhs.org).
Dr. Stratman noted that he and his fellow board members are still studying an extensive report recently submitted to the ABMS on recommendations to reform MOC. It’s possible that the ABD will need to tweak its pilot program in response. So he recommended checking the ABD website often during this period of change.
“If you have an inbox email from the ABD, it’s going to be something that you should open and read. It’s not going to be an advertisement,” he promised.
Dr. Stratman reported having created many MOC-related educational materials during the past dozen years.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
EXPERT ANALYSIS FROM SDEF HAWAII DERMATOLOGY SEMINAR
Rhymin’ pediatric dermatologist provides Demodex tips
WAIKOLOA, HAWAII – Pediatric dermatologist Andrea L. Zaenglein, MD, has composed a whimsical couplet to help physicians broaden their differential diagnosis for acneiform rashes in children.
Pustules on noses: Think demodicosis!
The classic teaching has been that Demodex carriage and its pathologic clinical expression, demodicosis, are rare in children. Not so, Dr. Zaenglein asserted at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
“One of the things that we never used to see in kids but now I see all the time, probably because I’m looking for it, is Demodex. You’re not born with Demodex. Your carriage rate will increase over the years and by the time you’re elderly, 95% of us will have it – but not me. I just decided I’m not having these. I’m not looking for them, and I don’t want to know if they’re there,” she quipped, referring to the creepiness factor surrounding these facial parasitic mites invisible to the naked eye.
The mites live in pilosebaceous units. In animals, the disease is called mange. In humans, demodicosis is caused by two species of mites: Demodex folliculorum and D. brevis. The diagnosis is made by microscopic examination of mineral oil skin scrapings.
Primary demodicosis can take the form of pityriasis folliculorum, also known as spinulate demodicosis, papulopustular perioral, periauricular, or periorbital demodicosis, or a nodulocystic/conglobate version.
More commonly, however, .
“Demodicosis can be associated with immunosuppression. Kids with Langerhans cell histiocytosis seem to be prone to it. But you can see it in kids who don’t have any underlying immunosuppression, and I think there are more and more cases of that as we go looking for it,” said Dr. Zaenglein, professor of dermatology and pediatrics at Penn State University, Hershey, and immediate past president of the Society for Pediatric Dermatology.
Look for Demodex in children with somewhat atypical versions of common acneiform eruptions: for example, the ‘pustules on noses’ variant.
“Finding Demodex can alter your treatment and make it easier in these cases,” she said.
Metronidazole and ivermectin are the systemic treatment options for pediatric demodicosis.
“I tend to use topical permethrin, just because it’s easy to get. I’ll treat them once a week for 3 weeks in a row. But also make sure to treat the underlying primary inflammatory disorder,” the pediatric dermatologist advised.
Other topical options include ivermectin cream, crotamiton cream, metronidazole gel, and salicylic acid cream.
Dr. Zaenglein reported having financial relationships with Sun Pharmaceuticals, Allergan, and Ortho Dermatologics.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
WAIKOLOA, HAWAII – Pediatric dermatologist Andrea L. Zaenglein, MD, has composed a whimsical couplet to help physicians broaden their differential diagnosis for acneiform rashes in children.
Pustules on noses: Think demodicosis!
The classic teaching has been that Demodex carriage and its pathologic clinical expression, demodicosis, are rare in children. Not so, Dr. Zaenglein asserted at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
“One of the things that we never used to see in kids but now I see all the time, probably because I’m looking for it, is Demodex. You’re not born with Demodex. Your carriage rate will increase over the years and by the time you’re elderly, 95% of us will have it – but not me. I just decided I’m not having these. I’m not looking for them, and I don’t want to know if they’re there,” she quipped, referring to the creepiness factor surrounding these facial parasitic mites invisible to the naked eye.
The mites live in pilosebaceous units. In animals, the disease is called mange. In humans, demodicosis is caused by two species of mites: Demodex folliculorum and D. brevis. The diagnosis is made by microscopic examination of mineral oil skin scrapings.
Primary demodicosis can take the form of pityriasis folliculorum, also known as spinulate demodicosis, papulopustular perioral, periauricular, or periorbital demodicosis, or a nodulocystic/conglobate version.
More commonly, however, .
“Demodicosis can be associated with immunosuppression. Kids with Langerhans cell histiocytosis seem to be prone to it. But you can see it in kids who don’t have any underlying immunosuppression, and I think there are more and more cases of that as we go looking for it,” said Dr. Zaenglein, professor of dermatology and pediatrics at Penn State University, Hershey, and immediate past president of the Society for Pediatric Dermatology.
Look for Demodex in children with somewhat atypical versions of common acneiform eruptions: for example, the ‘pustules on noses’ variant.
“Finding Demodex can alter your treatment and make it easier in these cases,” she said.
Metronidazole and ivermectin are the systemic treatment options for pediatric demodicosis.
“I tend to use topical permethrin, just because it’s easy to get. I’ll treat them once a week for 3 weeks in a row. But also make sure to treat the underlying primary inflammatory disorder,” the pediatric dermatologist advised.
Other topical options include ivermectin cream, crotamiton cream, metronidazole gel, and salicylic acid cream.
Dr. Zaenglein reported having financial relationships with Sun Pharmaceuticals, Allergan, and Ortho Dermatologics.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
WAIKOLOA, HAWAII – Pediatric dermatologist Andrea L. Zaenglein, MD, has composed a whimsical couplet to help physicians broaden their differential diagnosis for acneiform rashes in children.
Pustules on noses: Think demodicosis!
The classic teaching has been that Demodex carriage and its pathologic clinical expression, demodicosis, are rare in children. Not so, Dr. Zaenglein asserted at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
“One of the things that we never used to see in kids but now I see all the time, probably because I’m looking for it, is Demodex. You’re not born with Demodex. Your carriage rate will increase over the years and by the time you’re elderly, 95% of us will have it – but not me. I just decided I’m not having these. I’m not looking for them, and I don’t want to know if they’re there,” she quipped, referring to the creepiness factor surrounding these facial parasitic mites invisible to the naked eye.
The mites live in pilosebaceous units. In animals, the disease is called mange. In humans, demodicosis is caused by two species of mites: Demodex folliculorum and D. brevis. The diagnosis is made by microscopic examination of mineral oil skin scrapings.
Primary demodicosis can take the form of pityriasis folliculorum, also known as spinulate demodicosis, papulopustular perioral, periauricular, or periorbital demodicosis, or a nodulocystic/conglobate version.
More commonly, however, .
“Demodicosis can be associated with immunosuppression. Kids with Langerhans cell histiocytosis seem to be prone to it. But you can see it in kids who don’t have any underlying immunosuppression, and I think there are more and more cases of that as we go looking for it,” said Dr. Zaenglein, professor of dermatology and pediatrics at Penn State University, Hershey, and immediate past president of the Society for Pediatric Dermatology.
Look for Demodex in children with somewhat atypical versions of common acneiform eruptions: for example, the ‘pustules on noses’ variant.
“Finding Demodex can alter your treatment and make it easier in these cases,” she said.
Metronidazole and ivermectin are the systemic treatment options for pediatric demodicosis.
“I tend to use topical permethrin, just because it’s easy to get. I’ll treat them once a week for 3 weeks in a row. But also make sure to treat the underlying primary inflammatory disorder,” the pediatric dermatologist advised.
Other topical options include ivermectin cream, crotamiton cream, metronidazole gel, and salicylic acid cream.
Dr. Zaenglein reported having financial relationships with Sun Pharmaceuticals, Allergan, and Ortho Dermatologics.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
REPORTING FROM SDEF HAWAII DERMATOLOGY SEMINAR
TCA and punch excision are two options for icepick acne scars
WAIKOLOA, HAWAII – Dermatologists can certainly improve icepick acne scars, but they have to be careful not to make them worse, according to dermatologist Nazanin Saedi, MD, director of the Jefferson Laser Surgery and Cosmetic Dermatology Center at Thomas Jefferson University, Philadelphia.
.
After about three to five TCA treatments, most patients will have a better than 50% improvement, Dr. Saedi said, but the treatment isn’t for darker skin types – Fitzpatrick types V or VI – because of the risk of pigmentation changes. Dr. Saedi uses toothpicks to apply a small amount of 80% TCA to the base of the scar, and waits for the “frost” to appear. It’s important not to reapply the TCA. “A lot of people double dip and ... keep dipping into the scar,” which causes more damage.
For patients with darker skin types, or those who don’t want to go through a series of treatments, punch excision is an option, with nonablative laser treatment a week later when sutures are removed. “Some patients heal beautifully,” but some patients may have a spread scar or a small atrophic scar at the punch site, she noted. Options to treat atrophic scarring after treatment are laser treatments and fillers.
She offered her advice in an interview at the Hawaii Dermatology Seminar, provided by the Global Academy for Medical Education/Skin Disease Education Foundation. It’s important to set realistic expectations, Dr. Saedi said.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
WAIKOLOA, HAWAII – Dermatologists can certainly improve icepick acne scars, but they have to be careful not to make them worse, according to dermatologist Nazanin Saedi, MD, director of the Jefferson Laser Surgery and Cosmetic Dermatology Center at Thomas Jefferson University, Philadelphia.
.
After about three to five TCA treatments, most patients will have a better than 50% improvement, Dr. Saedi said, but the treatment isn’t for darker skin types – Fitzpatrick types V or VI – because of the risk of pigmentation changes. Dr. Saedi uses toothpicks to apply a small amount of 80% TCA to the base of the scar, and waits for the “frost” to appear. It’s important not to reapply the TCA. “A lot of people double dip and ... keep dipping into the scar,” which causes more damage.
For patients with darker skin types, or those who don’t want to go through a series of treatments, punch excision is an option, with nonablative laser treatment a week later when sutures are removed. “Some patients heal beautifully,” but some patients may have a spread scar or a small atrophic scar at the punch site, she noted. Options to treat atrophic scarring after treatment are laser treatments and fillers.
She offered her advice in an interview at the Hawaii Dermatology Seminar, provided by the Global Academy for Medical Education/Skin Disease Education Foundation. It’s important to set realistic expectations, Dr. Saedi said.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
WAIKOLOA, HAWAII – Dermatologists can certainly improve icepick acne scars, but they have to be careful not to make them worse, according to dermatologist Nazanin Saedi, MD, director of the Jefferson Laser Surgery and Cosmetic Dermatology Center at Thomas Jefferson University, Philadelphia.
.
After about three to five TCA treatments, most patients will have a better than 50% improvement, Dr. Saedi said, but the treatment isn’t for darker skin types – Fitzpatrick types V or VI – because of the risk of pigmentation changes. Dr. Saedi uses toothpicks to apply a small amount of 80% TCA to the base of the scar, and waits for the “frost” to appear. It’s important not to reapply the TCA. “A lot of people double dip and ... keep dipping into the scar,” which causes more damage.
For patients with darker skin types, or those who don’t want to go through a series of treatments, punch excision is an option, with nonablative laser treatment a week later when sutures are removed. “Some patients heal beautifully,” but some patients may have a spread scar or a small atrophic scar at the punch site, she noted. Options to treat atrophic scarring after treatment are laser treatments and fillers.
She offered her advice in an interview at the Hawaii Dermatology Seminar, provided by the Global Academy for Medical Education/Skin Disease Education Foundation. It’s important to set realistic expectations, Dr. Saedi said.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
EXPERT ANALYSIS FROM SDEF HAWAII DERMATOLOGY SEMINAR