User login
Point/Counterpoint: Is endograft PAA repair durable?
Endovascular repair is durable
Endovascular repair of popliteal artery aneurysms is vastly superior to all other previous techniques of popliteal aneurysm repair. Half of all popliteal artery aneurysms are bilateral, and 40% are associated with abdominal aortic aneurysm; 1%-2% of patients with abdominal aortic aneurysm have a popliteal aneurysm (ANZ J Surg. 2006 Oct;76[10]:912-5). Less than 0.01% of hospitalized patients have popliteal artery aneurysms, and men are 20 times more prone to them than women are.
Traditional treatment involves either bypass with interval ligation or a direct posterior approach with an interposition graft, but surgery is not without its problems. I think of the retired anesthesiologist who came to me with a popliteal artery aneurysm (PAA) that his primary care doctor diagnosed. “I’m not having any damn femoral popliteal bypass operation,” he told me. “Every single one of those patients dies.”
Endograft repair is a technique that is reaching its prime, as a growing number of reports have shown – although none of these studies has large numbers because the volume just isn’t available. One recent paper compared 52 open and 23 endovascular PAA repairs (Ann Vasc Surg. 2016 Jan;30:253-7) and found both had similarly high rates of reintervention – 50% at 4 years. But it is noteworthy that the endovascular results improved with time.
A University of Pittsburgh study of 186 open and endovascular repairs found that patients with acute presentations of embolization or aneurysm thrombosis did better with open surgery. In addition, while open repair had superior patency initially after surgery, midterm secondary patency and amputation rates of open and endovascular repair were similar (J Vasc Surg. 2016 Jan;63[1]:70-6).
A Netherlands study of 72 PAA treated with endografting showed that 84% had primary patency at 1 year, and 74% had assisted primary patency at 3 years (Eur J Vasc Endovasc Surg. 2016 Jul;52[1]:99-104). Among these patients, 13 had late occlusions, 7 were converted to bypass, and 2 required thrombolysis; but none required limb amputation.
A meta-analysis of 540 patients found no statistically significant difference in outcomes between endovascular and open repair for PAA (Eur J Vasc Endovasc Surg. 2015 Sep;50(3):351-9). Another systematic review and meta-analysis of 14 studies and 514 patients also found no difference in pooled primary and secondary patency at 5 years (J Endovasc Ther. 2015 Jun;22[3]:330-7).
There certainly are contradictory studies, such as one by Dr. Alik Farber’s group in Boston that showed open repair is superior to endovascular surgery (J Vasc Surg. 2015 Mar;61[3]:663-9); but retrospective database mining certainly has its limitations. Their retrospective study queried the Vascular Quality Initiative database and found that 95% of patients who had open elective popliteal aneurysm repair were free from major adverse limb events, vs. 80% for endovascular treatments.
The best outcomes of open repair happen with autologous vein, but there is precious little of that around now. Emergency patients would probably do better with open surgery, but in elective repair there is no clear differential data.
So, if that’s the case, I’m going to take the small incision.
Peter Rossi, MD, FACS, is an associate professor of surgery and radiology, and the clinical director of vascular surgery, at the Medical College of Wisconsin, Milwaukee. He is also on staff at Clement J. Zablocki Veterans Affairs Medical Center in Milwaukee. Dr. Rossi had no financial relationships to disclose.
Endovascular repair may not be durable
Debating the durability of elective endovascular repair of popliteal artery aneurysm raises a question: Who determines durability anyway?
Is it the patients who only want the Band-Aid and no incision? I don’t think so. Is it the interventionalist who only does endovascular repairs? I don’t think so. I’m sure it’s not the insurance companies, who only worry about cost containment, either.
So, who should determine durability of endovascular popliteal artery aneurysm (PAA) repair?
So, the question is, do we have such data?
There are multiple reports looking at how well open repair works. It has been done for decades. In 2008, a Veterans Affairs study of 583 open PAA repairs reported low death rates and excellent rates of limb salvage at 2 years, even in high-risk patients (J Vasc Surg. 2008 Oct;48[4]:845-51). Open surgical repair has excellent documented durability, and that is not the question at hand.
Endovascular repair has some presumed advantages. It’s less invasive and involves less postoperative pain and a quicker recovery. But it is not without problems – graft thrombosis and occlusion, endoleaks, distal limb ischemia, and stent fractures among them.
Surgery, to be clear, is not perfect, either. One of my patients who years ago presented with an occluded PAA underwent open bypass repair – but then went on later to have a pseudoaneurysm of the proximal anastomosis. I repaired this with an endograft, and he has done quite well. So, we all do endograft repairs, walk out, chest bump the Gore rep, and send the patient home that day.
Is it durable, though?
Most of the data on endovascular repair are from single-center studies dating back to 2003. There’s only one prospective trial comparing endovascular vs. open repair (J Vasc Surg. 2005 Aug;42[2]:185-93), but it was a single-center trial with a severe power limitation, because it involved only 30 patients. It found endovascular repair was comparable to open surgery. Also, I suspect a great deal of selection bias is involved in studies of endovascular repair.
A number of studies have found endovascular repair is not inferior to surgical repair. For example, a study by Dr. Audra Duncan, at Mayo Clinic, and her colleagues found that primary and secondary patency rates of elective and emergent stenting were excellent – but the study results only extended out to 2 years (J Vasc Surg. 2013 May;57[5]:1299-305). I don’t think we could hang our hat on that.
A Swedish study that compared open and endovascular surgery in 592 patients reported that endovascular repair has “significantly inferior results compared with open repair,” particularly in those who present with acute ischemia (Eur J Vasc Endovasc Surg. 2015 Sep;50[3]:342-50). A close look at the data shows that primary patency rates were 89% for open repair and 67.4% for stent graft.
Referencing the systematic review and meta-analysis that Dr. Rossi cited, the primary patency of endovascular repair was only 69% and the secondary patency rate was 77% at 5 years (J Endovasc Ther. 2015 Jun;22[3]:330-7). As physicians, I submit that we can do better.
A Netherlands study investigated stent fractures, finding that 17% (13 out of 78 cases) had circumferential fractures (J Vasc Surg. 2010 Jun;51[6]:1413-8). This study only included circumferential stent fractures and excluded localized strut fractures. I think these studies show that endovascular repair is not always durable.
I want to remind you that we are vascular surgeons, so it is appropriate for us to embrace surgical bypass and its known durability, especially when the durability of endovascular repair is still not known.
Patrick Muck, MD, is chief of vascular surgery and director of vascular residency and fellowship at Good Samaritan Hospital, Cincinnati. He is also on staff at Bethesda North Hospital, Cincinnati, and is affiliated with TriHealth Heart Institute in southwestern Ohio. Dr. Muck had no financial relationships to disclose.
Endovascular repair is durable
Endovascular repair of popliteal artery aneurysms is vastly superior to all other previous techniques of popliteal aneurysm repair. Half of all popliteal artery aneurysms are bilateral, and 40% are associated with abdominal aortic aneurysm; 1%-2% of patients with abdominal aortic aneurysm have a popliteal aneurysm (ANZ J Surg. 2006 Oct;76[10]:912-5). Less than 0.01% of hospitalized patients have popliteal artery aneurysms, and men are 20 times more prone to them than women are.
Traditional treatment involves either bypass with interval ligation or a direct posterior approach with an interposition graft, but surgery is not without its problems. I think of the retired anesthesiologist who came to me with a popliteal artery aneurysm (PAA) that his primary care doctor diagnosed. “I’m not having any damn femoral popliteal bypass operation,” he told me. “Every single one of those patients dies.”
Endograft repair is a technique that is reaching its prime, as a growing number of reports have shown – although none of these studies has large numbers because the volume just isn’t available. One recent paper compared 52 open and 23 endovascular PAA repairs (Ann Vasc Surg. 2016 Jan;30:253-7) and found both had similarly high rates of reintervention – 50% at 4 years. But it is noteworthy that the endovascular results improved with time.
A University of Pittsburgh study of 186 open and endovascular repairs found that patients with acute presentations of embolization or aneurysm thrombosis did better with open surgery. In addition, while open repair had superior patency initially after surgery, midterm secondary patency and amputation rates of open and endovascular repair were similar (J Vasc Surg. 2016 Jan;63[1]:70-6).
A Netherlands study of 72 PAA treated with endografting showed that 84% had primary patency at 1 year, and 74% had assisted primary patency at 3 years (Eur J Vasc Endovasc Surg. 2016 Jul;52[1]:99-104). Among these patients, 13 had late occlusions, 7 were converted to bypass, and 2 required thrombolysis; but none required limb amputation.
A meta-analysis of 540 patients found no statistically significant difference in outcomes between endovascular and open repair for PAA (Eur J Vasc Endovasc Surg. 2015 Sep;50(3):351-9). Another systematic review and meta-analysis of 14 studies and 514 patients also found no difference in pooled primary and secondary patency at 5 years (J Endovasc Ther. 2015 Jun;22[3]:330-7).
There certainly are contradictory studies, such as one by Dr. Alik Farber’s group in Boston that showed open repair is superior to endovascular surgery (J Vasc Surg. 2015 Mar;61[3]:663-9); but retrospective database mining certainly has its limitations. Their retrospective study queried the Vascular Quality Initiative database and found that 95% of patients who had open elective popliteal aneurysm repair were free from major adverse limb events, vs. 80% for endovascular treatments.
The best outcomes of open repair happen with autologous vein, but there is precious little of that around now. Emergency patients would probably do better with open surgery, but in elective repair there is no clear differential data.
So, if that’s the case, I’m going to take the small incision.
Peter Rossi, MD, FACS, is an associate professor of surgery and radiology, and the clinical director of vascular surgery, at the Medical College of Wisconsin, Milwaukee. He is also on staff at Clement J. Zablocki Veterans Affairs Medical Center in Milwaukee. Dr. Rossi had no financial relationships to disclose.
Endovascular repair may not be durable
Debating the durability of elective endovascular repair of popliteal artery aneurysm raises a question: Who determines durability anyway?
Is it the patients who only want the Band-Aid and no incision? I don’t think so. Is it the interventionalist who only does endovascular repairs? I don’t think so. I’m sure it’s not the insurance companies, who only worry about cost containment, either.
So, who should determine durability of endovascular popliteal artery aneurysm (PAA) repair?
So, the question is, do we have such data?
There are multiple reports looking at how well open repair works. It has been done for decades. In 2008, a Veterans Affairs study of 583 open PAA repairs reported low death rates and excellent rates of limb salvage at 2 years, even in high-risk patients (J Vasc Surg. 2008 Oct;48[4]:845-51). Open surgical repair has excellent documented durability, and that is not the question at hand.
Endovascular repair has some presumed advantages. It’s less invasive and involves less postoperative pain and a quicker recovery. But it is not without problems – graft thrombosis and occlusion, endoleaks, distal limb ischemia, and stent fractures among them.
Surgery, to be clear, is not perfect, either. One of my patients who years ago presented with an occluded PAA underwent open bypass repair – but then went on later to have a pseudoaneurysm of the proximal anastomosis. I repaired this with an endograft, and he has done quite well. So, we all do endograft repairs, walk out, chest bump the Gore rep, and send the patient home that day.
Is it durable, though?
Most of the data on endovascular repair are from single-center studies dating back to 2003. There’s only one prospective trial comparing endovascular vs. open repair (J Vasc Surg. 2005 Aug;42[2]:185-93), but it was a single-center trial with a severe power limitation, because it involved only 30 patients. It found endovascular repair was comparable to open surgery. Also, I suspect a great deal of selection bias is involved in studies of endovascular repair.
A number of studies have found endovascular repair is not inferior to surgical repair. For example, a study by Dr. Audra Duncan, at Mayo Clinic, and her colleagues found that primary and secondary patency rates of elective and emergent stenting were excellent – but the study results only extended out to 2 years (J Vasc Surg. 2013 May;57[5]:1299-305). I don’t think we could hang our hat on that.
A Swedish study that compared open and endovascular surgery in 592 patients reported that endovascular repair has “significantly inferior results compared with open repair,” particularly in those who present with acute ischemia (Eur J Vasc Endovasc Surg. 2015 Sep;50[3]:342-50). A close look at the data shows that primary patency rates were 89% for open repair and 67.4% for stent graft.
Referencing the systematic review and meta-analysis that Dr. Rossi cited, the primary patency of endovascular repair was only 69% and the secondary patency rate was 77% at 5 years (J Endovasc Ther. 2015 Jun;22[3]:330-7). As physicians, I submit that we can do better.
A Netherlands study investigated stent fractures, finding that 17% (13 out of 78 cases) had circumferential fractures (J Vasc Surg. 2010 Jun;51[6]:1413-8). This study only included circumferential stent fractures and excluded localized strut fractures. I think these studies show that endovascular repair is not always durable.
I want to remind you that we are vascular surgeons, so it is appropriate for us to embrace surgical bypass and its known durability, especially when the durability of endovascular repair is still not known.
Patrick Muck, MD, is chief of vascular surgery and director of vascular residency and fellowship at Good Samaritan Hospital, Cincinnati. He is also on staff at Bethesda North Hospital, Cincinnati, and is affiliated with TriHealth Heart Institute in southwestern Ohio. Dr. Muck had no financial relationships to disclose.
Endovascular repair is durable
Endovascular repair of popliteal artery aneurysms is vastly superior to all other previous techniques of popliteal aneurysm repair. Half of all popliteal artery aneurysms are bilateral, and 40% are associated with abdominal aortic aneurysm; 1%-2% of patients with abdominal aortic aneurysm have a popliteal aneurysm (ANZ J Surg. 2006 Oct;76[10]:912-5). Less than 0.01% of hospitalized patients have popliteal artery aneurysms, and men are 20 times more prone to them than women are.
Traditional treatment involves either bypass with interval ligation or a direct posterior approach with an interposition graft, but surgery is not without its problems. I think of the retired anesthesiologist who came to me with a popliteal artery aneurysm (PAA) that his primary care doctor diagnosed. “I’m not having any damn femoral popliteal bypass operation,” he told me. “Every single one of those patients dies.”
Endograft repair is a technique that is reaching its prime, as a growing number of reports have shown – although none of these studies has large numbers because the volume just isn’t available. One recent paper compared 52 open and 23 endovascular PAA repairs (Ann Vasc Surg. 2016 Jan;30:253-7) and found both had similarly high rates of reintervention – 50% at 4 years. But it is noteworthy that the endovascular results improved with time.
A University of Pittsburgh study of 186 open and endovascular repairs found that patients with acute presentations of embolization or aneurysm thrombosis did better with open surgery. In addition, while open repair had superior patency initially after surgery, midterm secondary patency and amputation rates of open and endovascular repair were similar (J Vasc Surg. 2016 Jan;63[1]:70-6).
A Netherlands study of 72 PAA treated with endografting showed that 84% had primary patency at 1 year, and 74% had assisted primary patency at 3 years (Eur J Vasc Endovasc Surg. 2016 Jul;52[1]:99-104). Among these patients, 13 had late occlusions, 7 were converted to bypass, and 2 required thrombolysis; but none required limb amputation.
A meta-analysis of 540 patients found no statistically significant difference in outcomes between endovascular and open repair for PAA (Eur J Vasc Endovasc Surg. 2015 Sep;50(3):351-9). Another systematic review and meta-analysis of 14 studies and 514 patients also found no difference in pooled primary and secondary patency at 5 years (J Endovasc Ther. 2015 Jun;22[3]:330-7).
There certainly are contradictory studies, such as one by Dr. Alik Farber’s group in Boston that showed open repair is superior to endovascular surgery (J Vasc Surg. 2015 Mar;61[3]:663-9); but retrospective database mining certainly has its limitations. Their retrospective study queried the Vascular Quality Initiative database and found that 95% of patients who had open elective popliteal aneurysm repair were free from major adverse limb events, vs. 80% for endovascular treatments.
The best outcomes of open repair happen with autologous vein, but there is precious little of that around now. Emergency patients would probably do better with open surgery, but in elective repair there is no clear differential data.
So, if that’s the case, I’m going to take the small incision.
Peter Rossi, MD, FACS, is an associate professor of surgery and radiology, and the clinical director of vascular surgery, at the Medical College of Wisconsin, Milwaukee. He is also on staff at Clement J. Zablocki Veterans Affairs Medical Center in Milwaukee. Dr. Rossi had no financial relationships to disclose.
Endovascular repair may not be durable
Debating the durability of elective endovascular repair of popliteal artery aneurysm raises a question: Who determines durability anyway?
Is it the patients who only want the Band-Aid and no incision? I don’t think so. Is it the interventionalist who only does endovascular repairs? I don’t think so. I’m sure it’s not the insurance companies, who only worry about cost containment, either.
So, who should determine durability of endovascular popliteal artery aneurysm (PAA) repair?
So, the question is, do we have such data?
There are multiple reports looking at how well open repair works. It has been done for decades. In 2008, a Veterans Affairs study of 583 open PAA repairs reported low death rates and excellent rates of limb salvage at 2 years, even in high-risk patients (J Vasc Surg. 2008 Oct;48[4]:845-51). Open surgical repair has excellent documented durability, and that is not the question at hand.
Endovascular repair has some presumed advantages. It’s less invasive and involves less postoperative pain and a quicker recovery. But it is not without problems – graft thrombosis and occlusion, endoleaks, distal limb ischemia, and stent fractures among them.
Surgery, to be clear, is not perfect, either. One of my patients who years ago presented with an occluded PAA underwent open bypass repair – but then went on later to have a pseudoaneurysm of the proximal anastomosis. I repaired this with an endograft, and he has done quite well. So, we all do endograft repairs, walk out, chest bump the Gore rep, and send the patient home that day.
Is it durable, though?
Most of the data on endovascular repair are from single-center studies dating back to 2003. There’s only one prospective trial comparing endovascular vs. open repair (J Vasc Surg. 2005 Aug;42[2]:185-93), but it was a single-center trial with a severe power limitation, because it involved only 30 patients. It found endovascular repair was comparable to open surgery. Also, I suspect a great deal of selection bias is involved in studies of endovascular repair.
A number of studies have found endovascular repair is not inferior to surgical repair. For example, a study by Dr. Audra Duncan, at Mayo Clinic, and her colleagues found that primary and secondary patency rates of elective and emergent stenting were excellent – but the study results only extended out to 2 years (J Vasc Surg. 2013 May;57[5]:1299-305). I don’t think we could hang our hat on that.
A Swedish study that compared open and endovascular surgery in 592 patients reported that endovascular repair has “significantly inferior results compared with open repair,” particularly in those who present with acute ischemia (Eur J Vasc Endovasc Surg. 2015 Sep;50[3]:342-50). A close look at the data shows that primary patency rates were 89% for open repair and 67.4% for stent graft.
Referencing the systematic review and meta-analysis that Dr. Rossi cited, the primary patency of endovascular repair was only 69% and the secondary patency rate was 77% at 5 years (J Endovasc Ther. 2015 Jun;22[3]:330-7). As physicians, I submit that we can do better.
A Netherlands study investigated stent fractures, finding that 17% (13 out of 78 cases) had circumferential fractures (J Vasc Surg. 2010 Jun;51[6]:1413-8). This study only included circumferential stent fractures and excluded localized strut fractures. I think these studies show that endovascular repair is not always durable.
I want to remind you that we are vascular surgeons, so it is appropriate for us to embrace surgical bypass and its known durability, especially when the durability of endovascular repair is still not known.
Patrick Muck, MD, is chief of vascular surgery and director of vascular residency and fellowship at Good Samaritan Hospital, Cincinnati. He is also on staff at Bethesda North Hospital, Cincinnati, and is affiliated with TriHealth Heart Institute in southwestern Ohio. Dr. Muck had no financial relationships to disclose.
AT MIDWESTERN VASCULAR 2016
Point/Counterpoint: Is limb salvage always best in diabetes?
Salvage limbs at all costs
Aggressive limb salvage in people with diabetes leads to an overall reduction in cost not only economically, but also from the patient’s perspective. The vast majority of diabetic patients with critical ischemia are actually good candidates for limb salvage. Tragically, many of these patients are never referred for evaluation for limb salvage because of misconceptions about the pathophysiology of the disease.
An argument against limb salvage is that primary amputation prevents or shortens the course of wound care and enables patients to become ambulatory, albeit with a prosthesis, faster. However, in the modern era of vascular surgery, revascularization can be performed successfully with minimal mortality and excellent rates of limb salvage, especially when it’s done within a team-based approach.
The mortality in primary amputation is shockingly high, anywhere from 5% to 23% higher than revascularization alone, and the major complication rate of amputation associated with diabetes is also unacceptably high – up to 37%. This is in contrast to a 17% rate in major nonamputation vascular surgery and 1%-5% in endovascular procedures (BMC Nephrol. 2005;6:3).
We can’t ignore the economic burden this places on the country. In 2014, primary amputations cost the health care system $11 billion annually, and that is expected to grow to more than $25 billion in the next several years, according to the SAGE Group. It’s important to keep in mind that Medicare covers over 80% of this cost.
A number of studies have shown that conservative management with wound care and amputation is more cost effective than primary amputation in ambulatory, independent adults. Data can be difficult to interpret because of different recording strategies for all the costs associated with amputation, but a single-institution study concluded that revascularization costs almost $5,280 more than expectant management, but $33,900 less than primary amputation alone (Cardiovasc Surg. 1999;7;62-9).
We must also consider the costs of revision after primary amputation; above-the-knee amputation has a 12% in-hospital revision rate, and below-the-knee amputation about 20%. Endovascular interventions, on the other hand, have a 1%-9% in-hospital revision rate, and only 2%-4% of these patients will go on to require an amputation during the same admission (Eur J Vasc Endovasc Surg. 2006;32:484-90; Arch Phys Med Rehabil. 2005;86:480-6).This does not include the costs of those complications as well as other indirect costs of amputation, such as nursing home care and living situation modification (Int J Behav Med. 2016;23:714-21; Pak J Med Sci. 2014; 30:1044-9). They quickly add up to that $25 billion.
The proponents of primary amputation tell us that it leads to quicker recovery time and an earlier time to ambulation. However, only 47% of patients will actually ambulate after amputation, in contrast to 97% who will ambulate after limb salvage as a primary procedure. In a nonambulatory cohort, 21% of those patients go on to regain functional status that was lost prior to surgery (J Vasc Surg. 1997;25;287-95).
Many question if our success with vascular surgery over the past few decades can translate to helping the most difficult subset of patients. An Italian study reported on a cohort of diabetic vs. nondiabetic patients and determined both groups have similar amputation-free rates after infrainguinal arterial reconstruction for critical limb ischemia, with excellent primary and secondary patency rates and a limb salvage rate of 88% at 5 years (J Vasc Surg. 2014;59:708-19). This tells us that we do have the skill set necessary to save these limbs.
A multidisciplinary limb preservation team is paramount to the success of any limb salvage program. A revascularization team should be in place which uses early intervention to achieve the highest limb salvage rates possible. Wound care needs to be an integrated part of it. Advanced podiatric reconstructive surgery also is key because this can provide complex foot reconstructions and help ambulatory patients return home.
Dr. Trissa A. Babrowski is an assistant professor of surgery, specializing in vascular surgery and endovascular therapy, at the University of Chicago Heart and Vascular Center. She had no financial relationships to disclose.
Primary amputation can be OK
I am not an amputationalist. I do practice limb salvage. In fact I’m probably the most aggressive limb salvage surgeon in my hospital. But primary amputation is a completely acceptable option for a selected group of patients with diabetes. We should not try to do limb salvage “at all costs.”
I do not find this to be a contradictory position. In fact, I think it adds credence to my support of limb salvage that I think primary amputation can be OK. In all honesty, there are very few things in life that should be done at all costs.
A study out of Loma Linda University involving patients with CLI compared primary amputation vs. revascularization; 43% of patients had a primary amputation (Ann Vasc Surg. 2007;21:458-63). A multivariate analysis showed that patients with major tissue loss, end-stage renal disease (ESRD), diabetes and nonambulatory status were more likely to undergo primary amputation rather than revascularization.
While major tissue loss (Rutherford category 6) is certainly an indication for primary amputation, ambulatory status can represent a gray area in determining the best course. ESRD and diabetes are much more nonspecific factors; probably more than 10% of the patients that we see with CLI have ESRD. Also, 50%-70% of these patients with CLI, and in some series even higher percentages, have diabetes. Thus, these factors by themselves do not assist us in determining which patients potentially should be offered primary amputation vs. revascularization.
In general, we know that we can get good results in limb bypass or revascularization in patients with CLI: The PREVENT III multicenter trial, with the use of the vein as the conduit, showed 1-year limb salvage rates of 88% in these high-risk patients (J Vasc Surg. 2006;43:742-51). However, one of the major risk factors that adversely affected outcome was ESRD.
We know that ESRD is a significant predictor of lowering our chances of saving a limb successfully. Knowing the cost of multiple continued episodes of revascularization in these patients prior to proceeding with an amputation, it’s intuitive that these patients would benefit from a more precise process in their treatment from the beginning. A number of papers have concluded that a primary amputation may be the preferred approach in patients with ESRD.
Can we preoperatively predict which patients with CLI will fail operative revascularization? Data from the New England Vascular Quality Initiative identified eight variables associated with failure of revascularization, among them age younger than 59, ESRD, diabetes, CLI, conduit requiring venovenostomy, tarsal target, and nursing home residence (Ann Vasc Surg. 2010;24:57-68). The presence of three or more risk factors has a 27.7% risk of limb loss and/or graft thrombosis within 1 year.
Postponing amputation is a major cost issue. Direct costs of bypass for critical limb ischemia were $3.6 billion in 2004 (J Vasc Surg. 2011;54:1021-31), and we know that a functional outcome can be problematic in this patient group. Factors associated with a poor functional outcome include dementia, dependent-living situation preoperatively and nonambulatory status.
Unfortunately, there are not a lot of data that deal with quality of life outcomes for patients with CLI who have undergone bypass. Using a point system comprised of dialysis (4 points), tissue loss (3 points), age above 75 (2 points), hematocrit less than or equal to 30 (2 points), and coronary artery disease (1 point), a follow-up study of patients in the PREVENT III trial found that a high-risk group (greater than or equal to 8 points) had an amputation-free survival of only 45% (J Vasc Surg. 2009;50:769-75). Again, these results do not justify the effort and costs of limb salvage in this high-risk patient group.
We should consider the following options carefully in selecting a cost-effective patient-focused approach in patients with CLI: wound care, primary amputation, bypass revascularization, or endovascular revascularization. I would argue that the vascular surgeon who is qualified as an expert in all of the above is best positioned to select an appropriate plan of treatment based upon the patient’s risk factors, wound factors, ambulatory ability, pattern of disease, severity of ischemia, and living status.
Thus, upon presentation, a patient with CLI should undergo confirmatory tests and optimize his or her risk factors. The vascular surgeon then has the option, in discussion with the patient and family, to pursue an appropriate treatment plan inclusive of primary amputation – not one of limb salvage “at all costs.”
Primary amputation should be used in situations where there is dementia and nonambulatory status, and in patients who are poor candidates for revascularization because of high risk of failure and limited life expectancy. The recently developed WIfI (wound, ischemia, and foot infection) classification can also be utilized, as stage 4 WIfI classification is associated with high risk of limb loss – 38%-40% at 1 year.
Primary amputation is an option that can result in better care overall, and it is a cost-effective approach for a selected group of patients. We should not try to do limb salvage at all cost. Primary amputation, in selected patients, is OK.
Dr. Timothy J. Nypaver is head of vascular surgery at Henry Ford Hospital, Detroit. He had no financial relationships to disclose.
Salvage limbs at all costs
Aggressive limb salvage in people with diabetes leads to an overall reduction in cost not only economically, but also from the patient’s perspective. The vast majority of diabetic patients with critical ischemia are actually good candidates for limb salvage. Tragically, many of these patients are never referred for evaluation for limb salvage because of misconceptions about the pathophysiology of the disease.
An argument against limb salvage is that primary amputation prevents or shortens the course of wound care and enables patients to become ambulatory, albeit with a prosthesis, faster. However, in the modern era of vascular surgery, revascularization can be performed successfully with minimal mortality and excellent rates of limb salvage, especially when it’s done within a team-based approach.
The mortality in primary amputation is shockingly high, anywhere from 5% to 23% higher than revascularization alone, and the major complication rate of amputation associated with diabetes is also unacceptably high – up to 37%. This is in contrast to a 17% rate in major nonamputation vascular surgery and 1%-5% in endovascular procedures (BMC Nephrol. 2005;6:3).
We can’t ignore the economic burden this places on the country. In 2014, primary amputations cost the health care system $11 billion annually, and that is expected to grow to more than $25 billion in the next several years, according to the SAGE Group. It’s important to keep in mind that Medicare covers over 80% of this cost.
A number of studies have shown that conservative management with wound care and amputation is more cost effective than primary amputation in ambulatory, independent adults. Data can be difficult to interpret because of different recording strategies for all the costs associated with amputation, but a single-institution study concluded that revascularization costs almost $5,280 more than expectant management, but $33,900 less than primary amputation alone (Cardiovasc Surg. 1999;7;62-9).
We must also consider the costs of revision after primary amputation; above-the-knee amputation has a 12% in-hospital revision rate, and below-the-knee amputation about 20%. Endovascular interventions, on the other hand, have a 1%-9% in-hospital revision rate, and only 2%-4% of these patients will go on to require an amputation during the same admission (Eur J Vasc Endovasc Surg. 2006;32:484-90; Arch Phys Med Rehabil. 2005;86:480-6).This does not include the costs of those complications as well as other indirect costs of amputation, such as nursing home care and living situation modification (Int J Behav Med. 2016;23:714-21; Pak J Med Sci. 2014; 30:1044-9). They quickly add up to that $25 billion.
The proponents of primary amputation tell us that it leads to quicker recovery time and an earlier time to ambulation. However, only 47% of patients will actually ambulate after amputation, in contrast to 97% who will ambulate after limb salvage as a primary procedure. In a nonambulatory cohort, 21% of those patients go on to regain functional status that was lost prior to surgery (J Vasc Surg. 1997;25;287-95).
Many question if our success with vascular surgery over the past few decades can translate to helping the most difficult subset of patients. An Italian study reported on a cohort of diabetic vs. nondiabetic patients and determined both groups have similar amputation-free rates after infrainguinal arterial reconstruction for critical limb ischemia, with excellent primary and secondary patency rates and a limb salvage rate of 88% at 5 years (J Vasc Surg. 2014;59:708-19). This tells us that we do have the skill set necessary to save these limbs.
A multidisciplinary limb preservation team is paramount to the success of any limb salvage program. A revascularization team should be in place which uses early intervention to achieve the highest limb salvage rates possible. Wound care needs to be an integrated part of it. Advanced podiatric reconstructive surgery also is key because this can provide complex foot reconstructions and help ambulatory patients return home.
Dr. Trissa A. Babrowski is an assistant professor of surgery, specializing in vascular surgery and endovascular therapy, at the University of Chicago Heart and Vascular Center. She had no financial relationships to disclose.
Primary amputation can be OK
I am not an amputationalist. I do practice limb salvage. In fact I’m probably the most aggressive limb salvage surgeon in my hospital. But primary amputation is a completely acceptable option for a selected group of patients with diabetes. We should not try to do limb salvage “at all costs.”
I do not find this to be a contradictory position. In fact, I think it adds credence to my support of limb salvage that I think primary amputation can be OK. In all honesty, there are very few things in life that should be done at all costs.
A study out of Loma Linda University involving patients with CLI compared primary amputation vs. revascularization; 43% of patients had a primary amputation (Ann Vasc Surg. 2007;21:458-63). A multivariate analysis showed that patients with major tissue loss, end-stage renal disease (ESRD), diabetes and nonambulatory status were more likely to undergo primary amputation rather than revascularization.
While major tissue loss (Rutherford category 6) is certainly an indication for primary amputation, ambulatory status can represent a gray area in determining the best course. ESRD and diabetes are much more nonspecific factors; probably more than 10% of the patients that we see with CLI have ESRD. Also, 50%-70% of these patients with CLI, and in some series even higher percentages, have diabetes. Thus, these factors by themselves do not assist us in determining which patients potentially should be offered primary amputation vs. revascularization.
In general, we know that we can get good results in limb bypass or revascularization in patients with CLI: The PREVENT III multicenter trial, with the use of the vein as the conduit, showed 1-year limb salvage rates of 88% in these high-risk patients (J Vasc Surg. 2006;43:742-51). However, one of the major risk factors that adversely affected outcome was ESRD.
We know that ESRD is a significant predictor of lowering our chances of saving a limb successfully. Knowing the cost of multiple continued episodes of revascularization in these patients prior to proceeding with an amputation, it’s intuitive that these patients would benefit from a more precise process in their treatment from the beginning. A number of papers have concluded that a primary amputation may be the preferred approach in patients with ESRD.
Can we preoperatively predict which patients with CLI will fail operative revascularization? Data from the New England Vascular Quality Initiative identified eight variables associated with failure of revascularization, among them age younger than 59, ESRD, diabetes, CLI, conduit requiring venovenostomy, tarsal target, and nursing home residence (Ann Vasc Surg. 2010;24:57-68). The presence of three or more risk factors has a 27.7% risk of limb loss and/or graft thrombosis within 1 year.
Postponing amputation is a major cost issue. Direct costs of bypass for critical limb ischemia were $3.6 billion in 2004 (J Vasc Surg. 2011;54:1021-31), and we know that a functional outcome can be problematic in this patient group. Factors associated with a poor functional outcome include dementia, dependent-living situation preoperatively and nonambulatory status.
Unfortunately, there are not a lot of data that deal with quality of life outcomes for patients with CLI who have undergone bypass. Using a point system comprised of dialysis (4 points), tissue loss (3 points), age above 75 (2 points), hematocrit less than or equal to 30 (2 points), and coronary artery disease (1 point), a follow-up study of patients in the PREVENT III trial found that a high-risk group (greater than or equal to 8 points) had an amputation-free survival of only 45% (J Vasc Surg. 2009;50:769-75). Again, these results do not justify the effort and costs of limb salvage in this high-risk patient group.
We should consider the following options carefully in selecting a cost-effective patient-focused approach in patients with CLI: wound care, primary amputation, bypass revascularization, or endovascular revascularization. I would argue that the vascular surgeon who is qualified as an expert in all of the above is best positioned to select an appropriate plan of treatment based upon the patient’s risk factors, wound factors, ambulatory ability, pattern of disease, severity of ischemia, and living status.
Thus, upon presentation, a patient with CLI should undergo confirmatory tests and optimize his or her risk factors. The vascular surgeon then has the option, in discussion with the patient and family, to pursue an appropriate treatment plan inclusive of primary amputation – not one of limb salvage “at all costs.”
Primary amputation should be used in situations where there is dementia and nonambulatory status, and in patients who are poor candidates for revascularization because of high risk of failure and limited life expectancy. The recently developed WIfI (wound, ischemia, and foot infection) classification can also be utilized, as stage 4 WIfI classification is associated with high risk of limb loss – 38%-40% at 1 year.
Primary amputation is an option that can result in better care overall, and it is a cost-effective approach for a selected group of patients. We should not try to do limb salvage at all cost. Primary amputation, in selected patients, is OK.
Dr. Timothy J. Nypaver is head of vascular surgery at Henry Ford Hospital, Detroit. He had no financial relationships to disclose.
Salvage limbs at all costs
Aggressive limb salvage in people with diabetes leads to an overall reduction in cost not only economically, but also from the patient’s perspective. The vast majority of diabetic patients with critical ischemia are actually good candidates for limb salvage. Tragically, many of these patients are never referred for evaluation for limb salvage because of misconceptions about the pathophysiology of the disease.
An argument against limb salvage is that primary amputation prevents or shortens the course of wound care and enables patients to become ambulatory, albeit with a prosthesis, faster. However, in the modern era of vascular surgery, revascularization can be performed successfully with minimal mortality and excellent rates of limb salvage, especially when it’s done within a team-based approach.
The mortality in primary amputation is shockingly high, anywhere from 5% to 23% higher than revascularization alone, and the major complication rate of amputation associated with diabetes is also unacceptably high – up to 37%. This is in contrast to a 17% rate in major nonamputation vascular surgery and 1%-5% in endovascular procedures (BMC Nephrol. 2005;6:3).
We can’t ignore the economic burden this places on the country. In 2014, primary amputations cost the health care system $11 billion annually, and that is expected to grow to more than $25 billion in the next several years, according to the SAGE Group. It’s important to keep in mind that Medicare covers over 80% of this cost.
A number of studies have shown that conservative management with wound care and amputation is more cost effective than primary amputation in ambulatory, independent adults. Data can be difficult to interpret because of different recording strategies for all the costs associated with amputation, but a single-institution study concluded that revascularization costs almost $5,280 more than expectant management, but $33,900 less than primary amputation alone (Cardiovasc Surg. 1999;7;62-9).
We must also consider the costs of revision after primary amputation; above-the-knee amputation has a 12% in-hospital revision rate, and below-the-knee amputation about 20%. Endovascular interventions, on the other hand, have a 1%-9% in-hospital revision rate, and only 2%-4% of these patients will go on to require an amputation during the same admission (Eur J Vasc Endovasc Surg. 2006;32:484-90; Arch Phys Med Rehabil. 2005;86:480-6).This does not include the costs of those complications as well as other indirect costs of amputation, such as nursing home care and living situation modification (Int J Behav Med. 2016;23:714-21; Pak J Med Sci. 2014; 30:1044-9). They quickly add up to that $25 billion.
The proponents of primary amputation tell us that it leads to quicker recovery time and an earlier time to ambulation. However, only 47% of patients will actually ambulate after amputation, in contrast to 97% who will ambulate after limb salvage as a primary procedure. In a nonambulatory cohort, 21% of those patients go on to regain functional status that was lost prior to surgery (J Vasc Surg. 1997;25;287-95).
Many question if our success with vascular surgery over the past few decades can translate to helping the most difficult subset of patients. An Italian study reported on a cohort of diabetic vs. nondiabetic patients and determined both groups have similar amputation-free rates after infrainguinal arterial reconstruction for critical limb ischemia, with excellent primary and secondary patency rates and a limb salvage rate of 88% at 5 years (J Vasc Surg. 2014;59:708-19). This tells us that we do have the skill set necessary to save these limbs.
A multidisciplinary limb preservation team is paramount to the success of any limb salvage program. A revascularization team should be in place which uses early intervention to achieve the highest limb salvage rates possible. Wound care needs to be an integrated part of it. Advanced podiatric reconstructive surgery also is key because this can provide complex foot reconstructions and help ambulatory patients return home.
Dr. Trissa A. Babrowski is an assistant professor of surgery, specializing in vascular surgery and endovascular therapy, at the University of Chicago Heart and Vascular Center. She had no financial relationships to disclose.
Primary amputation can be OK
I am not an amputationalist. I do practice limb salvage. In fact I’m probably the most aggressive limb salvage surgeon in my hospital. But primary amputation is a completely acceptable option for a selected group of patients with diabetes. We should not try to do limb salvage “at all costs.”
I do not find this to be a contradictory position. In fact, I think it adds credence to my support of limb salvage that I think primary amputation can be OK. In all honesty, there are very few things in life that should be done at all costs.
A study out of Loma Linda University involving patients with CLI compared primary amputation vs. revascularization; 43% of patients had a primary amputation (Ann Vasc Surg. 2007;21:458-63). A multivariate analysis showed that patients with major tissue loss, end-stage renal disease (ESRD), diabetes and nonambulatory status were more likely to undergo primary amputation rather than revascularization.
While major tissue loss (Rutherford category 6) is certainly an indication for primary amputation, ambulatory status can represent a gray area in determining the best course. ESRD and diabetes are much more nonspecific factors; probably more than 10% of the patients that we see with CLI have ESRD. Also, 50%-70% of these patients with CLI, and in some series even higher percentages, have diabetes. Thus, these factors by themselves do not assist us in determining which patients potentially should be offered primary amputation vs. revascularization.
In general, we know that we can get good results in limb bypass or revascularization in patients with CLI: The PREVENT III multicenter trial, with the use of the vein as the conduit, showed 1-year limb salvage rates of 88% in these high-risk patients (J Vasc Surg. 2006;43:742-51). However, one of the major risk factors that adversely affected outcome was ESRD.
We know that ESRD is a significant predictor of lowering our chances of saving a limb successfully. Knowing the cost of multiple continued episodes of revascularization in these patients prior to proceeding with an amputation, it’s intuitive that these patients would benefit from a more precise process in their treatment from the beginning. A number of papers have concluded that a primary amputation may be the preferred approach in patients with ESRD.
Can we preoperatively predict which patients with CLI will fail operative revascularization? Data from the New England Vascular Quality Initiative identified eight variables associated with failure of revascularization, among them age younger than 59, ESRD, diabetes, CLI, conduit requiring venovenostomy, tarsal target, and nursing home residence (Ann Vasc Surg. 2010;24:57-68). The presence of three or more risk factors has a 27.7% risk of limb loss and/or graft thrombosis within 1 year.
Postponing amputation is a major cost issue. Direct costs of bypass for critical limb ischemia were $3.6 billion in 2004 (J Vasc Surg. 2011;54:1021-31), and we know that a functional outcome can be problematic in this patient group. Factors associated with a poor functional outcome include dementia, dependent-living situation preoperatively and nonambulatory status.
Unfortunately, there are not a lot of data that deal with quality of life outcomes for patients with CLI who have undergone bypass. Using a point system comprised of dialysis (4 points), tissue loss (3 points), age above 75 (2 points), hematocrit less than or equal to 30 (2 points), and coronary artery disease (1 point), a follow-up study of patients in the PREVENT III trial found that a high-risk group (greater than or equal to 8 points) had an amputation-free survival of only 45% (J Vasc Surg. 2009;50:769-75). Again, these results do not justify the effort and costs of limb salvage in this high-risk patient group.
We should consider the following options carefully in selecting a cost-effective patient-focused approach in patients with CLI: wound care, primary amputation, bypass revascularization, or endovascular revascularization. I would argue that the vascular surgeon who is qualified as an expert in all of the above is best positioned to select an appropriate plan of treatment based upon the patient’s risk factors, wound factors, ambulatory ability, pattern of disease, severity of ischemia, and living status.
Thus, upon presentation, a patient with CLI should undergo confirmatory tests and optimize his or her risk factors. The vascular surgeon then has the option, in discussion with the patient and family, to pursue an appropriate treatment plan inclusive of primary amputation – not one of limb salvage “at all costs.”
Primary amputation should be used in situations where there is dementia and nonambulatory status, and in patients who are poor candidates for revascularization because of high risk of failure and limited life expectancy. The recently developed WIfI (wound, ischemia, and foot infection) classification can also be utilized, as stage 4 WIfI classification is associated with high risk of limb loss – 38%-40% at 1 year.
Primary amputation is an option that can result in better care overall, and it is a cost-effective approach for a selected group of patients. We should not try to do limb salvage at all cost. Primary amputation, in selected patients, is OK.
Dr. Timothy J. Nypaver is head of vascular surgery at Henry Ford Hospital, Detroit. He had no financial relationships to disclose.
Is regional anesthesia safer in CEA?
COLUMBUS, OHIO – General anesthesia during carotid endarterectomy carries almost twice the risk of complications and unplanned intubation as regional anesthesia, but the latter approach, which is not available in all hospitals, has its own issues, an analysis of procedures from a statewide database in Michigan found.
“This study is timely because of CMS [Center for Medicare & Medicaid Services] initiatives tying reimbursement to specific quality measures,” Ahmad S Hussain, MD, of Wayne State University in Detroit said in reporting the study results at the annual meeting of the Midwestern Vascular Surgery Society.
Regional anesthesia in CEA emerged in the 1990s, Dr. Hussain said, and allows for more reliable neurologic monitoring and more direct evaluation of the need for stenting during CEA than general anesthesia, which requires continuous monitoring of cerebral perfusion with carotid stump pressures, electroencephalogram, and transcranial doppler.
The researchers retrospectively analyzed 4,558 patients who had CEA at hospitals participating in the Michigan Surgical Quality Cooperative from 2012 to 2014 – 4,008 of whom had general anesthesia and 550 regional anesthesia.
“Advocates for carotid endarterectomy with regional anesthesia cite a reduction in hemodynamic instability and the ability for neurological monitoring, but many still prefer general anesthesia because the benefits of regional anesthesia have not been clearly demonstrated, allowing that regional anesthesia may not be available in all centers and allowing that a certain amount of patient movement during the procedure may not be uniformly tolerated,” Dr. Hussain said.
General anesthesia patients in the study had more than twice the rate of any morbidity at 30 days than those who had regional, 8.7% vs. 4.2%, and significantly higher rates of unplanned intervention, 2.1% vs. 0.6%. Dr. Hussain said. However, the study could not determine differences in 30-day mortality or other key outcomes, such as rates of pneumonia, sepsis, deep vein thrombosis, or pulmonary embolism, becauseof insufficient sample sizes, Dr. Hussain said
The study found less significant differences between general and regional anesthesia techniques, respectively, in rates of extended length of stay, 12.1% vs. 9.5%; readmissions, 9.2% vs. 6.1%; and reoperation, 4.5% vs. 3%.
The retrospective study used two models to analyze odds ratios: Model 1 adjusted for case mix; and model 2 adjusted for case mix as fixed effects and site as a random effect. While the retrospective nature of the study may be a limitation, the findings support the use of regional anesthesia for CEA when available, Dr. Hussain said.
Dr. Hussain had no relationships to disclose.
COLUMBUS, OHIO – General anesthesia during carotid endarterectomy carries almost twice the risk of complications and unplanned intubation as regional anesthesia, but the latter approach, which is not available in all hospitals, has its own issues, an analysis of procedures from a statewide database in Michigan found.
“This study is timely because of CMS [Center for Medicare & Medicaid Services] initiatives tying reimbursement to specific quality measures,” Ahmad S Hussain, MD, of Wayne State University in Detroit said in reporting the study results at the annual meeting of the Midwestern Vascular Surgery Society.
Regional anesthesia in CEA emerged in the 1990s, Dr. Hussain said, and allows for more reliable neurologic monitoring and more direct evaluation of the need for stenting during CEA than general anesthesia, which requires continuous monitoring of cerebral perfusion with carotid stump pressures, electroencephalogram, and transcranial doppler.
The researchers retrospectively analyzed 4,558 patients who had CEA at hospitals participating in the Michigan Surgical Quality Cooperative from 2012 to 2014 – 4,008 of whom had general anesthesia and 550 regional anesthesia.
“Advocates for carotid endarterectomy with regional anesthesia cite a reduction in hemodynamic instability and the ability for neurological monitoring, but many still prefer general anesthesia because the benefits of regional anesthesia have not been clearly demonstrated, allowing that regional anesthesia may not be available in all centers and allowing that a certain amount of patient movement during the procedure may not be uniformly tolerated,” Dr. Hussain said.
General anesthesia patients in the study had more than twice the rate of any morbidity at 30 days than those who had regional, 8.7% vs. 4.2%, and significantly higher rates of unplanned intervention, 2.1% vs. 0.6%. Dr. Hussain said. However, the study could not determine differences in 30-day mortality or other key outcomes, such as rates of pneumonia, sepsis, deep vein thrombosis, or pulmonary embolism, becauseof insufficient sample sizes, Dr. Hussain said
The study found less significant differences between general and regional anesthesia techniques, respectively, in rates of extended length of stay, 12.1% vs. 9.5%; readmissions, 9.2% vs. 6.1%; and reoperation, 4.5% vs. 3%.
The retrospective study used two models to analyze odds ratios: Model 1 adjusted for case mix; and model 2 adjusted for case mix as fixed effects and site as a random effect. While the retrospective nature of the study may be a limitation, the findings support the use of regional anesthesia for CEA when available, Dr. Hussain said.
Dr. Hussain had no relationships to disclose.
COLUMBUS, OHIO – General anesthesia during carotid endarterectomy carries almost twice the risk of complications and unplanned intubation as regional anesthesia, but the latter approach, which is not available in all hospitals, has its own issues, an analysis of procedures from a statewide database in Michigan found.
“This study is timely because of CMS [Center for Medicare & Medicaid Services] initiatives tying reimbursement to specific quality measures,” Ahmad S Hussain, MD, of Wayne State University in Detroit said in reporting the study results at the annual meeting of the Midwestern Vascular Surgery Society.
Regional anesthesia in CEA emerged in the 1990s, Dr. Hussain said, and allows for more reliable neurologic monitoring and more direct evaluation of the need for stenting during CEA than general anesthesia, which requires continuous monitoring of cerebral perfusion with carotid stump pressures, electroencephalogram, and transcranial doppler.
The researchers retrospectively analyzed 4,558 patients who had CEA at hospitals participating in the Michigan Surgical Quality Cooperative from 2012 to 2014 – 4,008 of whom had general anesthesia and 550 regional anesthesia.
“Advocates for carotid endarterectomy with regional anesthesia cite a reduction in hemodynamic instability and the ability for neurological monitoring, but many still prefer general anesthesia because the benefits of regional anesthesia have not been clearly demonstrated, allowing that regional anesthesia may not be available in all centers and allowing that a certain amount of patient movement during the procedure may not be uniformly tolerated,” Dr. Hussain said.
General anesthesia patients in the study had more than twice the rate of any morbidity at 30 days than those who had regional, 8.7% vs. 4.2%, and significantly higher rates of unplanned intervention, 2.1% vs. 0.6%. Dr. Hussain said. However, the study could not determine differences in 30-day mortality or other key outcomes, such as rates of pneumonia, sepsis, deep vein thrombosis, or pulmonary embolism, becauseof insufficient sample sizes, Dr. Hussain said
The study found less significant differences between general and regional anesthesia techniques, respectively, in rates of extended length of stay, 12.1% vs. 9.5%; readmissions, 9.2% vs. 6.1%; and reoperation, 4.5% vs. 3%.
The retrospective study used two models to analyze odds ratios: Model 1 adjusted for case mix; and model 2 adjusted for case mix as fixed effects and site as a random effect. While the retrospective nature of the study may be a limitation, the findings support the use of regional anesthesia for CEA when available, Dr. Hussain said.
Dr. Hussain had no relationships to disclose.
AT MIDWESTERN VASCULAR 2016
Key clinical point: General anesthesia for carotid endarterectomy carries a higher risk of complications and readmissions than regional anesthesia.
Major finding: Any morbidity after CEA with general anesthesia was 8.7% vs. 4.2% for regional anesthesia, and readmissions rates were 9.2% vs. 6.1%.
Data source: Retrospective analysis of 4,558 patients who had CEA between 2012 and 2014 at hospitals participating in the Michigan Surgical Quality Collaborative database.
Disclosures: Dr. Hussain reported having no financial disclosures.
CEA risk models fit for an app
COLUMBUS, OHIO – Carotid endarterectomy is an effective treatment for people with asymptomatic carotid artery disease when stroke rates are low and they survive long enough to benefit from the treatment. But determining who those patients are can be a challenge for vascular surgeons. A team of vascular specialists from around the country have developed risk prediction models to help surgeons better select asymptomatic patients for the procedure, Randall DeMartino, MD, said at the annual meeting of the Midwestern Vascular Surgical Society.
“These models will be used for mobile apps and web-based applications for point of care patient risk assessment,” said Dr. DeMartino of the Mayo Clinic in Rochester, Minn. He is the lead researcher for the study, which uses data from the Vascular Quality Initiative (VQI).
In developing the models, the researchers sampled asymptomatic patients in the VQI who had first-time elective CEA. There were 31,939 patients in the stroke analysis who had CEA from 2010-2015, and 24,086 patients in the mortality analysis who had procedures from 2010-2014. Dr. DeMartino and his colleagues evaluated all preoperative patient and surgeon characteristics, then used an algorithm to optimize the variables that were selected for the final logistic model.
The researchers also evaluated 30-day stroke rates and 1-year mortality at participating centers and found wide variability: an average of 0.9% for stroke, with a range of 0-8.3%; and 3.2% for mortality, with a range of 0-20%. “Actually, 22% of centers had a 1-year mortality rate that exceeded 5%,” Dr. DeMartino said.
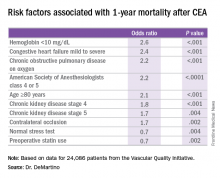
The model for 1-year mortality identified the following variables associated with the highest risk of death 1 year after CEA: age greater than or equal to 80 years; a preoperative hemoglobin less than 10 mg/dL; oxygen-dependent chronic obstructive pulmonary disease; mild to severe congestive heart failure; American Society of Anesthesiologists classification of IV or V; stage 4 or 5 chronic kidney disease; and a contralateral occlusion.
“Conversely, a normal stress test, when performed, and preoperative statin use were associated with reduced risk of death over a year,” Dr. DeMartino said.
“These data have been used to provide Center Opportunity for Improvement reports through VQI where centers can identify if they are selecting patients with risk factors for stroke or mortality more often compared to other centers,” Dr. DeMartino said. “This allows centers to see where opportunities for improvement exist.”
Also, physicians can see the proportion of patients they select with a predicted mortality risk over 5% at one year – “a group of patients who may gain little benefit from prophylactic CEA,” he said. “Physicians can compare their patient selection to those in their region or nationally.”
Dr. DeMartino had no relationships to disclose.
COLUMBUS, OHIO – Carotid endarterectomy is an effective treatment for people with asymptomatic carotid artery disease when stroke rates are low and they survive long enough to benefit from the treatment. But determining who those patients are can be a challenge for vascular surgeons. A team of vascular specialists from around the country have developed risk prediction models to help surgeons better select asymptomatic patients for the procedure, Randall DeMartino, MD, said at the annual meeting of the Midwestern Vascular Surgical Society.
“These models will be used for mobile apps and web-based applications for point of care patient risk assessment,” said Dr. DeMartino of the Mayo Clinic in Rochester, Minn. He is the lead researcher for the study, which uses data from the Vascular Quality Initiative (VQI).
In developing the models, the researchers sampled asymptomatic patients in the VQI who had first-time elective CEA. There were 31,939 patients in the stroke analysis who had CEA from 2010-2015, and 24,086 patients in the mortality analysis who had procedures from 2010-2014. Dr. DeMartino and his colleagues evaluated all preoperative patient and surgeon characteristics, then used an algorithm to optimize the variables that were selected for the final logistic model.
The researchers also evaluated 30-day stroke rates and 1-year mortality at participating centers and found wide variability: an average of 0.9% for stroke, with a range of 0-8.3%; and 3.2% for mortality, with a range of 0-20%. “Actually, 22% of centers had a 1-year mortality rate that exceeded 5%,” Dr. DeMartino said.
The model for 1-year mortality identified the following variables associated with the highest risk of death 1 year after CEA: age greater than or equal to 80 years; a preoperative hemoglobin less than 10 mg/dL; oxygen-dependent chronic obstructive pulmonary disease; mild to severe congestive heart failure; American Society of Anesthesiologists classification of IV or V; stage 4 or 5 chronic kidney disease; and a contralateral occlusion.
“Conversely, a normal stress test, when performed, and preoperative statin use were associated with reduced risk of death over a year,” Dr. DeMartino said.
“These data have been used to provide Center Opportunity for Improvement reports through VQI where centers can identify if they are selecting patients with risk factors for stroke or mortality more often compared to other centers,” Dr. DeMartino said. “This allows centers to see where opportunities for improvement exist.”
Also, physicians can see the proportion of patients they select with a predicted mortality risk over 5% at one year – “a group of patients who may gain little benefit from prophylactic CEA,” he said. “Physicians can compare their patient selection to those in their region or nationally.”
Dr. DeMartino had no relationships to disclose.
COLUMBUS, OHIO – Carotid endarterectomy is an effective treatment for people with asymptomatic carotid artery disease when stroke rates are low and they survive long enough to benefit from the treatment. But determining who those patients are can be a challenge for vascular surgeons. A team of vascular specialists from around the country have developed risk prediction models to help surgeons better select asymptomatic patients for the procedure, Randall DeMartino, MD, said at the annual meeting of the Midwestern Vascular Surgical Society.
“These models will be used for mobile apps and web-based applications for point of care patient risk assessment,” said Dr. DeMartino of the Mayo Clinic in Rochester, Minn. He is the lead researcher for the study, which uses data from the Vascular Quality Initiative (VQI).
In developing the models, the researchers sampled asymptomatic patients in the VQI who had first-time elective CEA. There were 31,939 patients in the stroke analysis who had CEA from 2010-2015, and 24,086 patients in the mortality analysis who had procedures from 2010-2014. Dr. DeMartino and his colleagues evaluated all preoperative patient and surgeon characteristics, then used an algorithm to optimize the variables that were selected for the final logistic model.
The researchers also evaluated 30-day stroke rates and 1-year mortality at participating centers and found wide variability: an average of 0.9% for stroke, with a range of 0-8.3%; and 3.2% for mortality, with a range of 0-20%. “Actually, 22% of centers had a 1-year mortality rate that exceeded 5%,” Dr. DeMartino said.
The model for 1-year mortality identified the following variables associated with the highest risk of death 1 year after CEA: age greater than or equal to 80 years; a preoperative hemoglobin less than 10 mg/dL; oxygen-dependent chronic obstructive pulmonary disease; mild to severe congestive heart failure; American Society of Anesthesiologists classification of IV or V; stage 4 or 5 chronic kidney disease; and a contralateral occlusion.
“Conversely, a normal stress test, when performed, and preoperative statin use were associated with reduced risk of death over a year,” Dr. DeMartino said.
“These data have been used to provide Center Opportunity for Improvement reports through VQI where centers can identify if they are selecting patients with risk factors for stroke or mortality more often compared to other centers,” Dr. DeMartino said. “This allows centers to see where opportunities for improvement exist.”
Also, physicians can see the proportion of patients they select with a predicted mortality risk over 5% at one year – “a group of patients who may gain little benefit from prophylactic CEA,” he said. “Physicians can compare their patient selection to those in their region or nationally.”
Dr. DeMartino had no relationships to disclose.
AT MIDWESTERN VASCULAR 2016
Key clinical point: Risk-prediction models may identify patients at greatest risk of stroke and 1-year death after carotid endarterectomy (CEA).
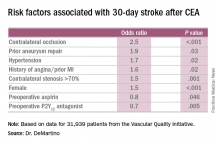
Major finding: Contralateral occlusion has odds ratios of 2.5 for 30-day stroke after CEA and 1.7 for death at 1 year.
Data source: Sampling of patients from the Vascular Quality Initiative who had first-time CEA: 31,939 in the stroke analysis and 24,086 in the mortality analysis.
Disclosures: Dr. DeMartino reported having no financial disclosures.
Infection, readmission linked after open lower-extremity procedures
COLUMBUS, OHIO – Infections account for more than one-third of readmissions after endovascular lower-extremity procedures, but an analysis of these procedures over a 6-year period has identified a handful of factors, including an extended hospital stay, that may help vascular surgeons identify patients at greatest risk and reduce infection-related readmissions.
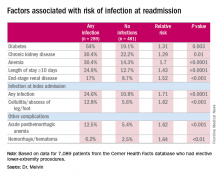
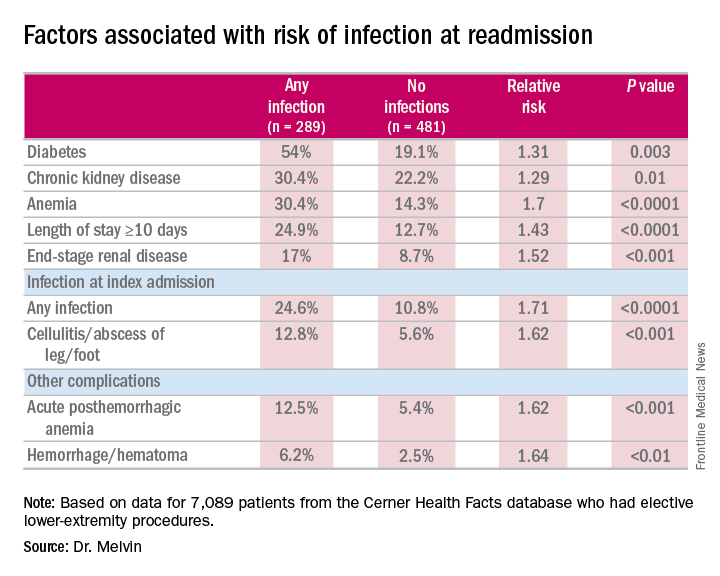
“Of a little over 7,000 patients that we evaluated with peripheral artery disease who underwent an elective lower-extremity procedure, we found an overall readmission rate of 10.9%; about 9.5% for those who underwent an open procedure and just over 12% for those who underwent an endovascular procedure,” Joseph C. Melvin, MD, of the University of Missouri Hospitals & Clinics in Columbia said at the annual meeting of the Midwestern Vascular Surgery Society.
While the readmission rate for open operations was lower, the infection rate at readmission was higher for open procedures: 45.5% (157 of 345 readmissions) vs. 31.1% (132 of 425 readmissions), Dr. Melvin said.
“The risk factors for diagnosis of infection at readmission we found to be significant were anemia, chronic kidney disease, and end-stage renal disease, any infection at the time of the index admission, specifically cellulitis or abscess of the lower extremity given the patient’s peripheral artery disease status, diabetes, and then complications including posthemorrhagic anemia,” Dr. Melvin said. Laboratory testing values at the time of index admissions confirmed the risk factors.
The investigators also used multivariable logistic regression models in the analysis and found that factors most predictive of an infection-related readmission were length of stay, having the procedure at a teaching facility, anemia, and infection at the index admission, Dr. Melvin said.
The surgical site was the most common source of the infection, and Staphylococcus “not surprisingly” accounted for 25% of pathogens, Dr. Melvin said. “But what we did find to be interesting was that just over 40% of patients were found to have a gram-negative bacteria isolated, which would come into play with our decision with regards to antibiotic treatment,” he said.
The data suggest that further evaluation of ways to decrease postoperative infections and use of broad-spectrum antibiotics during readmissions may improve outcomes after open lower-extremity procedures, Dr. Melvin said.
Dr. Melvin had no financial relationships to disclose.
COLUMBUS, OHIO – Infections account for more than one-third of readmissions after endovascular lower-extremity procedures, but an analysis of these procedures over a 6-year period has identified a handful of factors, including an extended hospital stay, that may help vascular surgeons identify patients at greatest risk and reduce infection-related readmissions.
“Of a little over 7,000 patients that we evaluated with peripheral artery disease who underwent an elective lower-extremity procedure, we found an overall readmission rate of 10.9%; about 9.5% for those who underwent an open procedure and just over 12% for those who underwent an endovascular procedure,” Joseph C. Melvin, MD, of the University of Missouri Hospitals & Clinics in Columbia said at the annual meeting of the Midwestern Vascular Surgery Society.
While the readmission rate for open operations was lower, the infection rate at readmission was higher for open procedures: 45.5% (157 of 345 readmissions) vs. 31.1% (132 of 425 readmissions), Dr. Melvin said.
“The risk factors for diagnosis of infection at readmission we found to be significant were anemia, chronic kidney disease, and end-stage renal disease, any infection at the time of the index admission, specifically cellulitis or abscess of the lower extremity given the patient’s peripheral artery disease status, diabetes, and then complications including posthemorrhagic anemia,” Dr. Melvin said. Laboratory testing values at the time of index admissions confirmed the risk factors.
The investigators also used multivariable logistic regression models in the analysis and found that factors most predictive of an infection-related readmission were length of stay, having the procedure at a teaching facility, anemia, and infection at the index admission, Dr. Melvin said.
The surgical site was the most common source of the infection, and Staphylococcus “not surprisingly” accounted for 25% of pathogens, Dr. Melvin said. “But what we did find to be interesting was that just over 40% of patients were found to have a gram-negative bacteria isolated, which would come into play with our decision with regards to antibiotic treatment,” he said.
The data suggest that further evaluation of ways to decrease postoperative infections and use of broad-spectrum antibiotics during readmissions may improve outcomes after open lower-extremity procedures, Dr. Melvin said.
Dr. Melvin had no financial relationships to disclose.
COLUMBUS, OHIO – Infections account for more than one-third of readmissions after endovascular lower-extremity procedures, but an analysis of these procedures over a 6-year period has identified a handful of factors, including an extended hospital stay, that may help vascular surgeons identify patients at greatest risk and reduce infection-related readmissions.
“Of a little over 7,000 patients that we evaluated with peripheral artery disease who underwent an elective lower-extremity procedure, we found an overall readmission rate of 10.9%; about 9.5% for those who underwent an open procedure and just over 12% for those who underwent an endovascular procedure,” Joseph C. Melvin, MD, of the University of Missouri Hospitals & Clinics in Columbia said at the annual meeting of the Midwestern Vascular Surgery Society.
While the readmission rate for open operations was lower, the infection rate at readmission was higher for open procedures: 45.5% (157 of 345 readmissions) vs. 31.1% (132 of 425 readmissions), Dr. Melvin said.
“The risk factors for diagnosis of infection at readmission we found to be significant were anemia, chronic kidney disease, and end-stage renal disease, any infection at the time of the index admission, specifically cellulitis or abscess of the lower extremity given the patient’s peripheral artery disease status, diabetes, and then complications including posthemorrhagic anemia,” Dr. Melvin said. Laboratory testing values at the time of index admissions confirmed the risk factors.
The investigators also used multivariable logistic regression models in the analysis and found that factors most predictive of an infection-related readmission were length of stay, having the procedure at a teaching facility, anemia, and infection at the index admission, Dr. Melvin said.
The surgical site was the most common source of the infection, and Staphylococcus “not surprisingly” accounted for 25% of pathogens, Dr. Melvin said. “But what we did find to be interesting was that just over 40% of patients were found to have a gram-negative bacteria isolated, which would come into play with our decision with regards to antibiotic treatment,” he said.
The data suggest that further evaluation of ways to decrease postoperative infections and use of broad-spectrum antibiotics during readmissions may improve outcomes after open lower-extremity procedures, Dr. Melvin said.
Dr. Melvin had no financial relationships to disclose.
AT MIDWESTERN VASCULAR 2016
Key clinical point: Extended hospital stay and other factors can help identify patients at greatest risk for readmission due to infection.
Major finding: More than one-third of readmissions from lower-extremity procedures are the result of infections.
Data source: 7,089 elective lower extremity procedures selected from the Cerner Health Facts database.
Disclosures: Dr. Melvin reported having no financial disclosures.
Can carotid interventions affect cognitive function?
COLUMBUS, OHIO – The primary goal of carotid artery revascularization is to prevent stroke, heart attack or death, but carotid artery stenting and carotid endarterectomy may also cause changes in cognitive skills, according Raghu Motaganahalli, MD, of the Indiana University, Indianapolis.
“What about cognitive dysfunction as a result of carotid artery stenting (CAS) or carotid endarterectomy (CEA)?” Dr. Motaganahalli asked at the annual meeting of the Midwestern Vascular Surgical Society. “I think this is real, that there’s some truth to the matter. The question is how much and what domains of cognitive functions are affected?”
“Cerebrovascular hemodynamics status plays a role in cognitive function, but we need a better understanding of cerebrovascular hemodynamic failure and either improvement or decline of cognitive function after CAS or CEA,” he said.
A review of published trials shows that 10%-20% of patients who have either CAS or CEA have some degree of cognitive dysfunction as early as a day after the procedure. “It’s not a small number, compared to stoke, risk of myocardial infarction and death,” he said.
Some series have reported up to 40% of patients showed some cognitive dysfunction, and post–carotid endarterectomy cognitive dysfunction has been associated with early death, Dr. Motaganahalli said.
Cognitive dysfunction manifests in various forms, ranging from level of consciousness and memory to mood and ability to make calculations. Although the Mini-Mental State Examination Global Cognitive Assessment tool provides a method for evaluating cognitive function, “There is no uniformly accepted neurocognition test,” Dr. Motaganahalli said. That explains the wide variability of findings among published studies.
Vascular surgeons take a somewhat casual approach to their patients’ cognitive abilities after carotid revascularization, Dr. Motaganahalli said. “We don’t evaluate their memory and their cognitive functions on post-op day one; we just look to see whether they have neurologic dysfunction up front and that they’re capable of going home after that.”
But predicting in advance which patients are predisposed to cognitive decline after the procedures is difficult, he said. He cited a systematic review of 32 studies published between 1990-2007 that showed variable results (Stroke. 2008;39:3116-27): 11 studies during 1990-2005 suggested cognition actually improved after CEA; 9 studies during 1994-2006 suggested the opposite; 4 trials during 1992-2005 suggested no change in cognition after CEA; 5 studies during 2003-2007 showed improvement in cognition after CAS; and 3 trials comparing CAS and CEA and cognition found no differences in how the two procedures affect cognition.
Dr. Motaganahalli also cited a systematic review of 37 studies, 18 of which examined CEA, 12 CAS and seven compared CEA and CAS, found that either cognitive improvement or impairment for CEA and CAS separately were 10–15% of patients (Cerebrovasc Dis Extra. 2014;4:132-48).
“We have 69 papers that looked at cognitive function alone, but unfortunately, we don’t know whether cognitive function really improved based on this data set,” he said. “None of them are making the argument so clearly that there is cognitive improvement after revascularization.”
The variability in study findings can be due to differences in methodologies, the types of psychometric tests used, statistical analyses and the timing of cognitive assessments, Dr. Motaganahalli said.
Cognitive impairment after stroke caused by carotid disease is better understood than is cognitive impairment in the absence of a major stroke, Dr. Motaganahalli said.
“The mechanisms of how carotid disease can cause the cognitive impairment are threefold: It could be microembolism and hypoperfusion, which together can cause white matter disease and thereby some cognitive dysfunction in the long term,” he said (Neuroimaging Clin N Am. 2007 Aug;17:313-24).
Functional neurons may be a biomarker of cognitive outcome, he said. Hypoperfusion of functional neurons may lead to hypofunctional neurons, which can increase cerebral blood flow and cerebral metabolic rate for oxygen (CMRO2), and thus improve cognition. However, when additional variables are introduced to the hypofunctional neurons – such as microembolism, white matter disease, and prolonged hypoperfusion – that can lead to neuronal infarction that, while increasing cerebral blood flow, causes no change in CMRO2 and, thus, no cognitive improvement. The interval between hypofunctional neurons and neuronal infarction “is the time to do the revascularization, as long as you can demonstrate that there may be some truth to matter that it influences cognition,” Dr. Motaganahalli said.
While vascular surgeons may not be able to predict who will have cognitive decline after carotid interventions, “There are some pointers for possibly picking those patients who may benefit,” Dr. Motaganahalli said.
That choice of patients revolves around recognizing that chronic ischemia induces and increases the severity of cognitive dysfunction. Therefore, incorporating the pathophysiology of chronic ischemia into the algorithm for carotid artery disease may provide an opportunity to extend the goals of carotid artery revascularization to include preventing or reversing cognitive decline, he said.
Dr. Motaganahalli disclosed he is a consultant to Silk Road Medical.
COLUMBUS, OHIO – The primary goal of carotid artery revascularization is to prevent stroke, heart attack or death, but carotid artery stenting and carotid endarterectomy may also cause changes in cognitive skills, according Raghu Motaganahalli, MD, of the Indiana University, Indianapolis.
“What about cognitive dysfunction as a result of carotid artery stenting (CAS) or carotid endarterectomy (CEA)?” Dr. Motaganahalli asked at the annual meeting of the Midwestern Vascular Surgical Society. “I think this is real, that there’s some truth to the matter. The question is how much and what domains of cognitive functions are affected?”
“Cerebrovascular hemodynamics status plays a role in cognitive function, but we need a better understanding of cerebrovascular hemodynamic failure and either improvement or decline of cognitive function after CAS or CEA,” he said.
A review of published trials shows that 10%-20% of patients who have either CAS or CEA have some degree of cognitive dysfunction as early as a day after the procedure. “It’s not a small number, compared to stoke, risk of myocardial infarction and death,” he said.
Some series have reported up to 40% of patients showed some cognitive dysfunction, and post–carotid endarterectomy cognitive dysfunction has been associated with early death, Dr. Motaganahalli said.
Cognitive dysfunction manifests in various forms, ranging from level of consciousness and memory to mood and ability to make calculations. Although the Mini-Mental State Examination Global Cognitive Assessment tool provides a method for evaluating cognitive function, “There is no uniformly accepted neurocognition test,” Dr. Motaganahalli said. That explains the wide variability of findings among published studies.
Vascular surgeons take a somewhat casual approach to their patients’ cognitive abilities after carotid revascularization, Dr. Motaganahalli said. “We don’t evaluate their memory and their cognitive functions on post-op day one; we just look to see whether they have neurologic dysfunction up front and that they’re capable of going home after that.”
But predicting in advance which patients are predisposed to cognitive decline after the procedures is difficult, he said. He cited a systematic review of 32 studies published between 1990-2007 that showed variable results (Stroke. 2008;39:3116-27): 11 studies during 1990-2005 suggested cognition actually improved after CEA; 9 studies during 1994-2006 suggested the opposite; 4 trials during 1992-2005 suggested no change in cognition after CEA; 5 studies during 2003-2007 showed improvement in cognition after CAS; and 3 trials comparing CAS and CEA and cognition found no differences in how the two procedures affect cognition.
Dr. Motaganahalli also cited a systematic review of 37 studies, 18 of which examined CEA, 12 CAS and seven compared CEA and CAS, found that either cognitive improvement or impairment for CEA and CAS separately were 10–15% of patients (Cerebrovasc Dis Extra. 2014;4:132-48).
“We have 69 papers that looked at cognitive function alone, but unfortunately, we don’t know whether cognitive function really improved based on this data set,” he said. “None of them are making the argument so clearly that there is cognitive improvement after revascularization.”
The variability in study findings can be due to differences in methodologies, the types of psychometric tests used, statistical analyses and the timing of cognitive assessments, Dr. Motaganahalli said.
Cognitive impairment after stroke caused by carotid disease is better understood than is cognitive impairment in the absence of a major stroke, Dr. Motaganahalli said.
“The mechanisms of how carotid disease can cause the cognitive impairment are threefold: It could be microembolism and hypoperfusion, which together can cause white matter disease and thereby some cognitive dysfunction in the long term,” he said (Neuroimaging Clin N Am. 2007 Aug;17:313-24).
Functional neurons may be a biomarker of cognitive outcome, he said. Hypoperfusion of functional neurons may lead to hypofunctional neurons, which can increase cerebral blood flow and cerebral metabolic rate for oxygen (CMRO2), and thus improve cognition. However, when additional variables are introduced to the hypofunctional neurons – such as microembolism, white matter disease, and prolonged hypoperfusion – that can lead to neuronal infarction that, while increasing cerebral blood flow, causes no change in CMRO2 and, thus, no cognitive improvement. The interval between hypofunctional neurons and neuronal infarction “is the time to do the revascularization, as long as you can demonstrate that there may be some truth to matter that it influences cognition,” Dr. Motaganahalli said.
While vascular surgeons may not be able to predict who will have cognitive decline after carotid interventions, “There are some pointers for possibly picking those patients who may benefit,” Dr. Motaganahalli said.
That choice of patients revolves around recognizing that chronic ischemia induces and increases the severity of cognitive dysfunction. Therefore, incorporating the pathophysiology of chronic ischemia into the algorithm for carotid artery disease may provide an opportunity to extend the goals of carotid artery revascularization to include preventing or reversing cognitive decline, he said.
Dr. Motaganahalli disclosed he is a consultant to Silk Road Medical.
COLUMBUS, OHIO – The primary goal of carotid artery revascularization is to prevent stroke, heart attack or death, but carotid artery stenting and carotid endarterectomy may also cause changes in cognitive skills, according Raghu Motaganahalli, MD, of the Indiana University, Indianapolis.
“What about cognitive dysfunction as a result of carotid artery stenting (CAS) or carotid endarterectomy (CEA)?” Dr. Motaganahalli asked at the annual meeting of the Midwestern Vascular Surgical Society. “I think this is real, that there’s some truth to the matter. The question is how much and what domains of cognitive functions are affected?”
“Cerebrovascular hemodynamics status plays a role in cognitive function, but we need a better understanding of cerebrovascular hemodynamic failure and either improvement or decline of cognitive function after CAS or CEA,” he said.
A review of published trials shows that 10%-20% of patients who have either CAS or CEA have some degree of cognitive dysfunction as early as a day after the procedure. “It’s not a small number, compared to stoke, risk of myocardial infarction and death,” he said.
Some series have reported up to 40% of patients showed some cognitive dysfunction, and post–carotid endarterectomy cognitive dysfunction has been associated with early death, Dr. Motaganahalli said.
Cognitive dysfunction manifests in various forms, ranging from level of consciousness and memory to mood and ability to make calculations. Although the Mini-Mental State Examination Global Cognitive Assessment tool provides a method for evaluating cognitive function, “There is no uniformly accepted neurocognition test,” Dr. Motaganahalli said. That explains the wide variability of findings among published studies.
Vascular surgeons take a somewhat casual approach to their patients’ cognitive abilities after carotid revascularization, Dr. Motaganahalli said. “We don’t evaluate their memory and their cognitive functions on post-op day one; we just look to see whether they have neurologic dysfunction up front and that they’re capable of going home after that.”
But predicting in advance which patients are predisposed to cognitive decline after the procedures is difficult, he said. He cited a systematic review of 32 studies published between 1990-2007 that showed variable results (Stroke. 2008;39:3116-27): 11 studies during 1990-2005 suggested cognition actually improved after CEA; 9 studies during 1994-2006 suggested the opposite; 4 trials during 1992-2005 suggested no change in cognition after CEA; 5 studies during 2003-2007 showed improvement in cognition after CAS; and 3 trials comparing CAS and CEA and cognition found no differences in how the two procedures affect cognition.
Dr. Motaganahalli also cited a systematic review of 37 studies, 18 of which examined CEA, 12 CAS and seven compared CEA and CAS, found that either cognitive improvement or impairment for CEA and CAS separately were 10–15% of patients (Cerebrovasc Dis Extra. 2014;4:132-48).
“We have 69 papers that looked at cognitive function alone, but unfortunately, we don’t know whether cognitive function really improved based on this data set,” he said. “None of them are making the argument so clearly that there is cognitive improvement after revascularization.”
The variability in study findings can be due to differences in methodologies, the types of psychometric tests used, statistical analyses and the timing of cognitive assessments, Dr. Motaganahalli said.
Cognitive impairment after stroke caused by carotid disease is better understood than is cognitive impairment in the absence of a major stroke, Dr. Motaganahalli said.
“The mechanisms of how carotid disease can cause the cognitive impairment are threefold: It could be microembolism and hypoperfusion, which together can cause white matter disease and thereby some cognitive dysfunction in the long term,” he said (Neuroimaging Clin N Am. 2007 Aug;17:313-24).
Functional neurons may be a biomarker of cognitive outcome, he said. Hypoperfusion of functional neurons may lead to hypofunctional neurons, which can increase cerebral blood flow and cerebral metabolic rate for oxygen (CMRO2), and thus improve cognition. However, when additional variables are introduced to the hypofunctional neurons – such as microembolism, white matter disease, and prolonged hypoperfusion – that can lead to neuronal infarction that, while increasing cerebral blood flow, causes no change in CMRO2 and, thus, no cognitive improvement. The interval between hypofunctional neurons and neuronal infarction “is the time to do the revascularization, as long as you can demonstrate that there may be some truth to matter that it influences cognition,” Dr. Motaganahalli said.
While vascular surgeons may not be able to predict who will have cognitive decline after carotid interventions, “There are some pointers for possibly picking those patients who may benefit,” Dr. Motaganahalli said.
That choice of patients revolves around recognizing that chronic ischemia induces and increases the severity of cognitive dysfunction. Therefore, incorporating the pathophysiology of chronic ischemia into the algorithm for carotid artery disease may provide an opportunity to extend the goals of carotid artery revascularization to include preventing or reversing cognitive decline, he said.
Dr. Motaganahalli disclosed he is a consultant to Silk Road Medical.
AT THE ANNUAL MEETING OF THE MIDWESTERN VASCULAR SURGERY SOCIETY
Key clinical point: Incorporating the pathophysiology of chronic ischemia into the algorithm for carotid artery disease could expand the goals of revascularization to encompass cognitive decline.
Major finding: Cerebrovascular hemodynamic status plays a role in cognitive function after carotid artery interventions, but the mechanisms of either improvement or decline need better understanding.
Data source: Systematic review of 32 papers on neurocognition after carotid interventions published between 1990-2007 and analysis of 37 studies of CAS or CEA or both published since 2007.
Disclosures: Dr. Motaganahalli disclosed he is a consultant to Silk Road Medical Inc.
Study identifies SSI risk factors after open LEB
COLUMBUS, OHIO – A study of vascular procedures at 35 Michigan hospitals has identified three risk factors for surgical site infection after lower-extremity bypass that hospitals and vascular surgery teams may be able to modify.
“Patients who had iodine-only skin antiseptic preparation, a high-peak intraoperative glucose, or long operative times were more likely to have substantially increased risk for surgical site infection (SSI),” Frank Davis, MD, of the University of Michigan said in reporting the study results at the annual meeting of the Midwestern Vascular Surgical Society. Those risk factors are modifiable, Dr. Davis said.
“Specific attention needs to be served moving forward in attempts to decrease the risk of SSI for lower-extremity bypass,” Dr. Davis said. “The incidence of SSI in our cohort across the state of Michigan was approximately 9.2%, and for those who did develop a SSI, there was a substantial increase in 30-day morbidity.”
Patients who had an SSI were more than three times more likely to have a major amputation (9% vs. 2.3%) than those without, and more than five times more likely to have a reoperation (3.9% vs. 0.7%), Dr. Davis said.
“With regard to preoperative symptomatology, those with lower peripheral artery questionnaire scores, resting pain, or acute ischemia were more likely to develop SSI postoperatively,” Dr. Davis said. “Patients who underwent an interim coronal bypass had a significant increase of SSI in comparison to all other bypass configurations.”
He also noted that major teaching hospitals or hospitals with 500 or fewer beds had higher rates of SSI.
“Targeted improvements in preoperative care may decrease complications and improve vascular patient outcomes,” Dr. Davis said.
Dr. Davis had no relationships to disclose.
COLUMBUS, OHIO – A study of vascular procedures at 35 Michigan hospitals has identified three risk factors for surgical site infection after lower-extremity bypass that hospitals and vascular surgery teams may be able to modify.
“Patients who had iodine-only skin antiseptic preparation, a high-peak intraoperative glucose, or long operative times were more likely to have substantially increased risk for surgical site infection (SSI),” Frank Davis, MD, of the University of Michigan said in reporting the study results at the annual meeting of the Midwestern Vascular Surgical Society. Those risk factors are modifiable, Dr. Davis said.
“Specific attention needs to be served moving forward in attempts to decrease the risk of SSI for lower-extremity bypass,” Dr. Davis said. “The incidence of SSI in our cohort across the state of Michigan was approximately 9.2%, and for those who did develop a SSI, there was a substantial increase in 30-day morbidity.”
Patients who had an SSI were more than three times more likely to have a major amputation (9% vs. 2.3%) than those without, and more than five times more likely to have a reoperation (3.9% vs. 0.7%), Dr. Davis said.
“With regard to preoperative symptomatology, those with lower peripheral artery questionnaire scores, resting pain, or acute ischemia were more likely to develop SSI postoperatively,” Dr. Davis said. “Patients who underwent an interim coronal bypass had a significant increase of SSI in comparison to all other bypass configurations.”
He also noted that major teaching hospitals or hospitals with 500 or fewer beds had higher rates of SSI.
“Targeted improvements in preoperative care may decrease complications and improve vascular patient outcomes,” Dr. Davis said.
Dr. Davis had no relationships to disclose.
COLUMBUS, OHIO – A study of vascular procedures at 35 Michigan hospitals has identified three risk factors for surgical site infection after lower-extremity bypass that hospitals and vascular surgery teams may be able to modify.
“Patients who had iodine-only skin antiseptic preparation, a high-peak intraoperative glucose, or long operative times were more likely to have substantially increased risk for surgical site infection (SSI),” Frank Davis, MD, of the University of Michigan said in reporting the study results at the annual meeting of the Midwestern Vascular Surgical Society. Those risk factors are modifiable, Dr. Davis said.
“Specific attention needs to be served moving forward in attempts to decrease the risk of SSI for lower-extremity bypass,” Dr. Davis said. “The incidence of SSI in our cohort across the state of Michigan was approximately 9.2%, and for those who did develop a SSI, there was a substantial increase in 30-day morbidity.”
Patients who had an SSI were more than three times more likely to have a major amputation (9% vs. 2.3%) than those without, and more than five times more likely to have a reoperation (3.9% vs. 0.7%), Dr. Davis said.
“With regard to preoperative symptomatology, those with lower peripheral artery questionnaire scores, resting pain, or acute ischemia were more likely to develop SSI postoperatively,” Dr. Davis said. “Patients who underwent an interim coronal bypass had a significant increase of SSI in comparison to all other bypass configurations.”
He also noted that major teaching hospitals or hospitals with 500 or fewer beds had higher rates of SSI.
“Targeted improvements in preoperative care may decrease complications and improve vascular patient outcomes,” Dr. Davis said.
Dr. Davis had no relationships to disclose.
AT MIDWESTERN VASCULAR 2016
Key clinical point: Study identified three key modifiable risk factors in surgical site infection (SSI) open after lower-extremity bypass (LEB).
Major finding: Incidence of SSI was 9.2% in the study cohort.
Data source: Blue Cross Blue Shield Michigan Vascular Intervention Collaborative database of 3,992 open LEB operations at 35 centers from January 2012 to June 2015.
Disclosures: Dr. Davis reported having no financial disclosures.
Blacks found to have higher amputation rates than whites
COLUMBUS, OHIO – The disparity in amputation rates between blacks and non-Hispanic whites in Chicago was higher than the national average and up to five times greater on the predominantly black South Side than on the predominantly white North Side of the city.
The reasons for this disparity are unclear, but a recent study of white and black Chicagoans has shown that the rates at which the races self-report claudication do not differ, suggesting that better utilization of public health resources in the black community could potentially reduce this disparity.
Reporting at the annual meeting of the Midwestern Vascular Surgery Society, lead investigator Samantha Minc, MD, of Rush University in Chicago, said the difference in amputation rates may be traced to later recognition of disease in nonwhites.
“Possible reasons for late recognition of disease include lack of access to care, communication issues with doctors, education issues, or the patient and/or caregiver and primary care provider having difficulty accessing specialists,” Dr. Minc said. “Another often-cited explanation for the late recognition is the possibility of differences in disease symptom manifestations in nonwhites, which is what we chose to study.”
Investigators from Rush University analyzed data from a combined cohort of Chicago residents age 65 years and older from two studies of aging: The Rush Memory and Aging Project and the Minority Aging Research Study.
Because of the segregated nature of Chicago – the North Side is mostly white, the South Side mostly black and the West Side mostly Hispanic – prior research has coupled census data with the Illinois Department of Public Health database to analyze care patterns. This data showed that the disparity in amputation rates among groups in Chicago was higher than the national average and up to five times greater on the South Side, compared with the North, Dr. Minc said.
The latest study matched blacks and whites from both studies 1:2 based on age, education, gender, and length of follow-up. In all, a cohort of 2,487 subjects was generated: 801 blacks and 1,686 non-Hispanic whites. Among blacks, 6.5% reported claudication at baseline, compared with 5.9% of whites, and at any point of the study 19.5% of blacks and 19.6% of non-Hispanic whites reported claudication, differences that were not statistically significant.
Blacks did have higher rates of diabetes, 27% vs. 12.6%, were more likely to have used tobacco, more likely to be female, and were slightly younger on average, but other demographic factors – such as income or rates of congestive heart failure and coronary artery disease – were not significantly different, Dr. Minc said.
The study findings will inform the Chicago Department of Public Health’s Healthy Chicago 2.0 initiative to reduce the disparity in amputation rates between whites and blacks by 10% by 2020.
Dr. Minc acknowledged limitations of the study, among them that participants were volunteers, aged 65 years and older, and 72% female, that Hispanics were excluded, and that the use of the Modified Rose/World Health Organization questionnaire to detect claudication is “specific, but not very sensitive.”
“Our findings suggest that the delay in recognition in disease in nonwhites is not due to a racial differences in disease manifestation or symptomatology and that addressing socioeconomic issues, such as access to care, communication, and education may reduce the racial disparity in amputation rates,” Dr. Minc said. “Public health resources should focus on early recognition of the disease in higher-risk groups to decrease this disparity.”
Dr. Minc and her coauthors had no relationships to disclose.
COLUMBUS, OHIO – The disparity in amputation rates between blacks and non-Hispanic whites in Chicago was higher than the national average and up to five times greater on the predominantly black South Side than on the predominantly white North Side of the city.
The reasons for this disparity are unclear, but a recent study of white and black Chicagoans has shown that the rates at which the races self-report claudication do not differ, suggesting that better utilization of public health resources in the black community could potentially reduce this disparity.
Reporting at the annual meeting of the Midwestern Vascular Surgery Society, lead investigator Samantha Minc, MD, of Rush University in Chicago, said the difference in amputation rates may be traced to later recognition of disease in nonwhites.
“Possible reasons for late recognition of disease include lack of access to care, communication issues with doctors, education issues, or the patient and/or caregiver and primary care provider having difficulty accessing specialists,” Dr. Minc said. “Another often-cited explanation for the late recognition is the possibility of differences in disease symptom manifestations in nonwhites, which is what we chose to study.”
Investigators from Rush University analyzed data from a combined cohort of Chicago residents age 65 years and older from two studies of aging: The Rush Memory and Aging Project and the Minority Aging Research Study.
Because of the segregated nature of Chicago – the North Side is mostly white, the South Side mostly black and the West Side mostly Hispanic – prior research has coupled census data with the Illinois Department of Public Health database to analyze care patterns. This data showed that the disparity in amputation rates among groups in Chicago was higher than the national average and up to five times greater on the South Side, compared with the North, Dr. Minc said.
The latest study matched blacks and whites from both studies 1:2 based on age, education, gender, and length of follow-up. In all, a cohort of 2,487 subjects was generated: 801 blacks and 1,686 non-Hispanic whites. Among blacks, 6.5% reported claudication at baseline, compared with 5.9% of whites, and at any point of the study 19.5% of blacks and 19.6% of non-Hispanic whites reported claudication, differences that were not statistically significant.
Blacks did have higher rates of diabetes, 27% vs. 12.6%, were more likely to have used tobacco, more likely to be female, and were slightly younger on average, but other demographic factors – such as income or rates of congestive heart failure and coronary artery disease – were not significantly different, Dr. Minc said.
The study findings will inform the Chicago Department of Public Health’s Healthy Chicago 2.0 initiative to reduce the disparity in amputation rates between whites and blacks by 10% by 2020.
Dr. Minc acknowledged limitations of the study, among them that participants were volunteers, aged 65 years and older, and 72% female, that Hispanics were excluded, and that the use of the Modified Rose/World Health Organization questionnaire to detect claudication is “specific, but not very sensitive.”
“Our findings suggest that the delay in recognition in disease in nonwhites is not due to a racial differences in disease manifestation or symptomatology and that addressing socioeconomic issues, such as access to care, communication, and education may reduce the racial disparity in amputation rates,” Dr. Minc said. “Public health resources should focus on early recognition of the disease in higher-risk groups to decrease this disparity.”
Dr. Minc and her coauthors had no relationships to disclose.
COLUMBUS, OHIO – The disparity in amputation rates between blacks and non-Hispanic whites in Chicago was higher than the national average and up to five times greater on the predominantly black South Side than on the predominantly white North Side of the city.
The reasons for this disparity are unclear, but a recent study of white and black Chicagoans has shown that the rates at which the races self-report claudication do not differ, suggesting that better utilization of public health resources in the black community could potentially reduce this disparity.
Reporting at the annual meeting of the Midwestern Vascular Surgery Society, lead investigator Samantha Minc, MD, of Rush University in Chicago, said the difference in amputation rates may be traced to later recognition of disease in nonwhites.
“Possible reasons for late recognition of disease include lack of access to care, communication issues with doctors, education issues, or the patient and/or caregiver and primary care provider having difficulty accessing specialists,” Dr. Minc said. “Another often-cited explanation for the late recognition is the possibility of differences in disease symptom manifestations in nonwhites, which is what we chose to study.”
Investigators from Rush University analyzed data from a combined cohort of Chicago residents age 65 years and older from two studies of aging: The Rush Memory and Aging Project and the Minority Aging Research Study.
Because of the segregated nature of Chicago – the North Side is mostly white, the South Side mostly black and the West Side mostly Hispanic – prior research has coupled census data with the Illinois Department of Public Health database to analyze care patterns. This data showed that the disparity in amputation rates among groups in Chicago was higher than the national average and up to five times greater on the South Side, compared with the North, Dr. Minc said.
The latest study matched blacks and whites from both studies 1:2 based on age, education, gender, and length of follow-up. In all, a cohort of 2,487 subjects was generated: 801 blacks and 1,686 non-Hispanic whites. Among blacks, 6.5% reported claudication at baseline, compared with 5.9% of whites, and at any point of the study 19.5% of blacks and 19.6% of non-Hispanic whites reported claudication, differences that were not statistically significant.
Blacks did have higher rates of diabetes, 27% vs. 12.6%, were more likely to have used tobacco, more likely to be female, and were slightly younger on average, but other demographic factors – such as income or rates of congestive heart failure and coronary artery disease – were not significantly different, Dr. Minc said.
The study findings will inform the Chicago Department of Public Health’s Healthy Chicago 2.0 initiative to reduce the disparity in amputation rates between whites and blacks by 10% by 2020.
Dr. Minc acknowledged limitations of the study, among them that participants were volunteers, aged 65 years and older, and 72% female, that Hispanics were excluded, and that the use of the Modified Rose/World Health Organization questionnaire to detect claudication is “specific, but not very sensitive.”
“Our findings suggest that the delay in recognition in disease in nonwhites is not due to a racial differences in disease manifestation or symptomatology and that addressing socioeconomic issues, such as access to care, communication, and education may reduce the racial disparity in amputation rates,” Dr. Minc said. “Public health resources should focus on early recognition of the disease in higher-risk groups to decrease this disparity.”
Dr. Minc and her coauthors had no relationships to disclose.
AT MIDWESTERN VASCULAR 2016
Key clinical point: Amputation rates in Chicago were up to five times greater on the predominantly black South Side of the city than on the predominantly white North Side of the city.
Major finding: Disparities in amputation rates suggest a need to address access to care and improve communication and education with regard to amputation risks in the nonwhite communities.
Data source: 2,487 subjects from the Rush Memory and Aging Project and the Minority Aging Research Study.
Disclosures: Dr. Minc and her coauthors had no relationships to disclose.